User login
Clinical Guideline Highlights for the Hospitalist: Therapeutic Monitoring of Vancomycin
Vancomycin, a glycopeptide antibiotic, has been used for decades, yet knowledge gaps remain regarding the most appropriate dosing approach to optimize therapeutic effect while avoiding adverse effects in all patient populations. A committee composed of members of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists reviewed data available since publication of the original 2009 vancomycin dosing guidelines to provide new recommendations regarding vancomycin dosing and serum concentration–monitoring in the empiric treatment of presumed or confirmed methicillin-resistant Staphylococcus aureus (MRSA) infections.1
The new guidelines provide 25 recommendations encompassing the following topics: vancomycin dosing and monitoring in adult, pediatric, and neonate care; vancomycin minimum inhibitory concentration (MIC) susceptibility testing; continuous infusion vs intermittent infusion; loading doses; dosing in obesity; and dosing in patients on hemodialysis and continuous renal replacement therapy. Because hospitalists in pediatric and adult care frequently prescribe vancomycin for empiric and targeted treatment of serious infections, they have a vested interest in ensuring optimal vancomycin outcomes (ie, best efficacy with least toxicity) with use of therapeutic drug monitoring and personalized dosing of vancomycin. Thus, it is important for hospitalists to be aware of the updated guideline and pivotal changes regarding therapeutic drug monitoring. In this guideline review, we will focus on the major differences from the 2009 guideline, specifically regarding therapeutic monitoring in adults and children.
The guideline includes pharmacology language and terminology with which many clinicians may not be familiar. To better understand the rationale for the guideline changes, a few concepts will be reviewed. Overall, antibiotics are dosed based on preclinical studies to determine the needed drug exposure for optimal efficacy. β-Lactams, for example, are optimally dosed with longer drug exposure time above the MIC of the infectious organism. Alternatively, area under the concentration time curve (AUC) describes the efficacy and toxicity of many other antibiotics. Since AUC is derived from products of concentration (mg/L) and time (hours), the units are often mg × h/L. For vancomycin, both drug exposure (ie, AUC) and organism susceptibility (ie, MIC) are incorporated to determine optimal drug exposure, with the ratio of AUC to MIC being the ideal marker. Therapeutic drug monitoring of vancomycin has classically been conducted with trough concentration monitoring, but with the updated guideline, there will be a transition to AUC monitoring that will affect patient care and experience.
KEY RECOMMENDATIONS FOR HOSPITALISTS TREATING ADULTS
The following is a summary of recommendations 1 to 6:
- In adults, the optimal drug exposure for vancomycin should be an AUC to MIC ratio of 400 to 600 for MRSA, with the assumption of MIC to be 1 mg/L (evidence quality: A-II).
- The preferred method to monitor AUC is with a clinical statistical software that uses two blood samples (1 to 2 hours after completion of infusion and at the end of a dosing interval [ie, trough]) (evidence quality: A-II).
- An alternative approach would be to use first-order pharmacokinetic equations at steady state with a peak and trough (evidence quality: A-II).
- These approaches replace the previously recommended trough-only monitoring. AUC-targeted exposure should generally be achieved within 48 hours; severity of infection does not justify higher AUC goals. Once the goal AUC is achieved, once-weekly monitoring is recommended for hemodynamically stable patients, but more frequent or daily monitoring is advised in patients at high risk of nephrotoxicity or who are hemodynamically unstable (evidence quality: B-II).
The currently accepted optimal drug exposure for vancomycin is an AUC to MIC ratio of 400 to 600 to maximize efficacy and minimize nephrotoxicity.2 Due to clinical inconvenience of performing AUC-based monitoring for vancomycin in the past, previous guidelines recommended using trough concentrations as a surrogate marker for an AUC to MIC ratio, with the goal trough being 15 to 20 mg/L for serious MRSA infections.3 However, trough values may not correlate well with AUC. For example, a trough of 15 mg/L may represent an AUC ranging from 400 to 1000 mg × h/L over 24 hours. Without knowing an accurate AUC, there is risk for ineffective bactericidal activity with low AUCs or nephrotoxicity with high AUCs. Compared with trough-only monitoring, AUC-guided dosing is associated with decreased risk of acute kidney injury.4,5 Therefore, the recommendation to transition to two-sample collection with a peak and trough was included.
Software programs are now readily available to compute the AUC and work best with peak and trough values rather than a single trough value because computing with two concentrations will rely more on specific patient data than it does on previously published vancomycin models. Trough-only monitoring (and without the support of clinical software) may still be possible when the exposures needed are further from the toxic range. To this end, trough-only monitoring may be reasonable when infections are not MRSA and are less invasive (eg, cellulitis) since the guideline found insufficient evidence for AUC monitoring in these scenarios. While specific targets are not provided, a plethora of historical literature demonstrated low kidney injury rates when troughs were maintained between 5 to 10 mg/L.
KEY RECOMMENDATIONS FOR PEDIATRIC HOSPITALISTS
The following is a summary of recommendations 18 to 20:
- In pediatric care, based on a target AUC to MIC ratio of 400 to 600 with the assumption of MIC to be 1 mg/L, initial vancomycin dosage for MRSA is as follows (evidence quality: A-II) :
- 60 to 80 mg/kg per day, divided into four doses, each given 6 hours apart, for children 3 months and older but younger than 12 years
- 60 to 70 mg/kg per day, divided into four doses, each given 6 hours apart, for children 12 years and older
- As recommended in adults, use of a statistical software program to measure AUC is the optimal approach in pediatric care because it can account for age, weight, and renal function, which should be monitored closely. Monitoring should begin within 48 hours of therapy. Vancomycin AUC and trough concentrations should be less than 800 µg × h/mL over 24 hours and 15 µg/mL, respectively, to minimize acute kidney injury (evidence quality: A-II).
All the recommendations for pediatrics are new for the updated guideline. Pediatric data to support these recommendations are fewer in comparison with adult literature. Given MRSA infections are felt to be similar in adults and children, many pediatric recommendations are extrapolated from adult data and recommendations. The strongest level of evidence in children is the association of acute kidney injury with higher vancomycin exposure, especially with troughs exceeding 15 to 20 mg/L.6 In addition, one pediatric study found an AUC exposure of greater than 800 mg × h/L over 24 hours was strongly associated with risk for acute kidney injury.7 These findings suggest that high vancomycin exposure correlates with nephrotoxicity, so with AUC monitoring, the goal exposure should be less than 800 mg × hr/L over 24 hours.
Only one study has evaluated statistical software and prediction of AUC in pediatrics.8 A two-concentration approach (peak and trough) outperformed trough-only monitoring for accuracy and precision in determining AUC. While limited to one study, the results are similar to the studies completed in adults, thereby leading to the recommendation of the two-concentration technique in children.
Prospective outcome data are lacking, but multiple retrospective studies have examined S aureus bacteremia in children. Thus far, there have been no studies that have determined the optimal vancomycin exposure required for successful outcomes.9,10 The proven risks of toxicity are the primary driver for the pediatric guideline change with the outcomes extrapolated from adult data.
CRITIQUE
Methods in Preparing Guideline
The main strength of the guideline is that the committee was represented by multiple organizations, which created a multidisciplinary panel of pharmacists and infectious disease physicians with clinical and research expertise in vancomycin dosing. Evidence was graded using an adaptation from the Canadian Task Force on the Periodic Health Examination.11 The draft was peer-reviewed by the society organizations and allowed for comments, suggestions, and recommendations.
Sources of Potential Conflict of Interest or Bias
Disclosures of all authors were reported and identified in the guideline. While many members are involved with pharmaceutical companies through research or speakers’ roles, vancomycin, a generic drug, should have minimal conflicts of interest or bias from this involvement.
Generalizability
Implementation of vancomycin AUC dosing will be hospital dependent due to the implementation-related increase in human resources and the cost of clinical software; many hospital systems do not already have the software integrated into their clinical practice. Local guidelines will have to be developed to help clinicians determine which clinical situations require AUC-based dosing vs trough-only monitoring. Pharmacists at many hospitals are primarily responsible for vancomycin monitoring and provide dosing recommendations to physicians. Depending on a hospital system’s decision, the workload to determine the optimal vancomycin dose may increase, and it will be important to have close collaboration between hospitalists, pharmacists, and infectious diseases clinicians to appropriately educate clinicians who might be required to dose/monitor vancomycin. One potential way to decrease the burden of monitoring with two concentrations is to use specialized software that can perform complex assessments with only a single concentration. These software applications will still require serious collaboration of the aforementioned practitioners to implement. The variation in guideline adoption will likely be even more significant in pediatrics because the literature is extrapolated and the increased blood draws can be more problematic in pediatric patients.
Furthermore, clinicians should understand the dosing guideline is specifically addressing treatment of MRSA infections and extrapolation to other organisms such as coagulase-negative staphylococcal or methicillin-susceptible S aureus infections should be cautioned. Another caveat to note is that, when the MRSA isolate has an MIC of 2 mg/L or higher, these infections are associated with poor outcomes when vancomycin is used and alternative agents are recommended.
AREAS IN NEED OF FUTURE STUDY
Research gaps still remain with appropriate vancomycin drug exposure. In pediatrics, determining the appropriate AUC target will be important given that current recommendations extrapolate from adult data. Future studies can focus on prospective outcome data in both pediatric and adult patients for infections outside of bacteremia or pneumonia, notably central nervous system and osteomyelitis infections. Thresholds for kidney injury will need to be more clearly defined for both adult and pediatric patients. There should also be research emphasis on the appropriate dosing for other non-MRSA invasive infections, notably coagulase-negative staphylococcal infections.
Disclosures
Dr Scheetz reported personal fees for consulting for Achaogen, SIGA technologies, and for serving on an advisory board for Paratek; grants from Merck and Co, Allecra, Nevakar, and SuperTrans Medical; personal fees from Hall, Booth, Smith, PC, and Chambless, Higdon, Richardson, Katz & Griggs, LLP, for consulting and expert testimony, outside the submitted work. In addition, Dr. Scheetz has patent US 2019 / 0099500 A1 pending. Dr Murphy reported having received fees from Becton Dickinson for participation to review IDSA guidelines on gastroenteritis. Dr Tang Girdwood has nothing to disclose.
Funding
Dr Murphy and Dr Tang Girdwood are supported by the National Institute of Child Health and Development Cincinnati Pediatric Clinical Pharmacology Postdoctoral Training Program (5T32HD069054-09). Dr Tang Girdwood is also supported by the Cincinnati Children’s Hospital Medical Center Arnold W Strauss Fellow Award and Cincinnati Children’s Hospital Medical Center Hospital Medicine Fellow Award. Dr Scheetz is supported in part by the National Institute of Allergy and Infectious Diseases award (R21AI149026). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
1. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835-864. https://doi.org/10.1093/ajhp/zxaa036
2. Men P, Li HB, Zhai SD, Zhao RS. Association between the AUC0-24/MIC ratio of vancomycin and its clinical effectiveness: a systematic review and meta-analysis. PLoS One. 2016;11(1):e0146224. https://doi.org/10.1371/journal.pone.0146224
3. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82-98. https://doi.org/10.2146/ajhp080434
4. Finch NA, Zasowski EJ, Murray KP, et al. A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycin-associated nephrotoxicity. Antimicrob Agents Chemother. 2017;61(12):e01293-17. https://doi.org/10.1128/aac.01293-17
5. Neely MN, Kato L, Youn G, et al. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother. 2018;62(2):e02042-17. https://doi.org/10.1128/aac.02042-17
6. Fiorito TM, Luther MK, Dennehy PH, LaPlante KL, Matson KL. Nephrotoxicity with vancomycin in the pediatric population: a systematic review and meta-analysis. Pediatr Infect Dis J. 2018;37(7):654-661. https://doi.org/10.1097/inf.0000000000001882
7. Le J, Ny P, Capparelli E, et al. Pharmacodynamic characteristics of nephrotoxicity associated with vancomycin use in children. J Pediatric Infect Dis Soc. 2015;4(4):e109-e116. https://doi.org/10.1093/jpids/piu110
8. Le J, Ngu B, Bradley JS, et al. Vancomycin monitoring in children using bayesian estimation. Ther Drug Monit. 2014;36(4):510-518. https://doi.org/10.1097/ftd.0000000000000039
9. Hahn A, Frenck RW Jr, Allen-Staat M, Zou Y, Vinks AA. Evaluation of target attainment of vancomycin area under the curve in children with methicillin-resistant Staphylococcus aureus bacteremia. Ther Drug Monit. 2015;37(5):619-625. https://doi.org/10.1097/ftd.0000000000000190
10. McNeil JC, Kok EY, Forbes AR, et al. Healthcare-associated Staphylococcus aureus bacteremia in children: evidence for reverse vancomycin creep and impact of vancomycin trough values on outcome. Pediatr Infect Dis J. 2016;35(3):263-268. https://doi.org/10.1097/inf.0000000000000991
11. The periodic health examination. Canadian Task Force on the Periodic Health Examination. Can Med Assoc J. 1979;121(9):1193-1254.
Vancomycin, a glycopeptide antibiotic, has been used for decades, yet knowledge gaps remain regarding the most appropriate dosing approach to optimize therapeutic effect while avoiding adverse effects in all patient populations. A committee composed of members of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists reviewed data available since publication of the original 2009 vancomycin dosing guidelines to provide new recommendations regarding vancomycin dosing and serum concentration–monitoring in the empiric treatment of presumed or confirmed methicillin-resistant Staphylococcus aureus (MRSA) infections.1
The new guidelines provide 25 recommendations encompassing the following topics: vancomycin dosing and monitoring in adult, pediatric, and neonate care; vancomycin minimum inhibitory concentration (MIC) susceptibility testing; continuous infusion vs intermittent infusion; loading doses; dosing in obesity; and dosing in patients on hemodialysis and continuous renal replacement therapy. Because hospitalists in pediatric and adult care frequently prescribe vancomycin for empiric and targeted treatment of serious infections, they have a vested interest in ensuring optimal vancomycin outcomes (ie, best efficacy with least toxicity) with use of therapeutic drug monitoring and personalized dosing of vancomycin. Thus, it is important for hospitalists to be aware of the updated guideline and pivotal changes regarding therapeutic drug monitoring. In this guideline review, we will focus on the major differences from the 2009 guideline, specifically regarding therapeutic monitoring in adults and children.
The guideline includes pharmacology language and terminology with which many clinicians may not be familiar. To better understand the rationale for the guideline changes, a few concepts will be reviewed. Overall, antibiotics are dosed based on preclinical studies to determine the needed drug exposure for optimal efficacy. β-Lactams, for example, are optimally dosed with longer drug exposure time above the MIC of the infectious organism. Alternatively, area under the concentration time curve (AUC) describes the efficacy and toxicity of many other antibiotics. Since AUC is derived from products of concentration (mg/L) and time (hours), the units are often mg × h/L. For vancomycin, both drug exposure (ie, AUC) and organism susceptibility (ie, MIC) are incorporated to determine optimal drug exposure, with the ratio of AUC to MIC being the ideal marker. Therapeutic drug monitoring of vancomycin has classically been conducted with trough concentration monitoring, but with the updated guideline, there will be a transition to AUC monitoring that will affect patient care and experience.
KEY RECOMMENDATIONS FOR HOSPITALISTS TREATING ADULTS
The following is a summary of recommendations 1 to 6:
- In adults, the optimal drug exposure for vancomycin should be an AUC to MIC ratio of 400 to 600 for MRSA, with the assumption of MIC to be 1 mg/L (evidence quality: A-II).
- The preferred method to monitor AUC is with a clinical statistical software that uses two blood samples (1 to 2 hours after completion of infusion and at the end of a dosing interval [ie, trough]) (evidence quality: A-II).
- An alternative approach would be to use first-order pharmacokinetic equations at steady state with a peak and trough (evidence quality: A-II).
- These approaches replace the previously recommended trough-only monitoring. AUC-targeted exposure should generally be achieved within 48 hours; severity of infection does not justify higher AUC goals. Once the goal AUC is achieved, once-weekly monitoring is recommended for hemodynamically stable patients, but more frequent or daily monitoring is advised in patients at high risk of nephrotoxicity or who are hemodynamically unstable (evidence quality: B-II).
The currently accepted optimal drug exposure for vancomycin is an AUC to MIC ratio of 400 to 600 to maximize efficacy and minimize nephrotoxicity.2 Due to clinical inconvenience of performing AUC-based monitoring for vancomycin in the past, previous guidelines recommended using trough concentrations as a surrogate marker for an AUC to MIC ratio, with the goal trough being 15 to 20 mg/L for serious MRSA infections.3 However, trough values may not correlate well with AUC. For example, a trough of 15 mg/L may represent an AUC ranging from 400 to 1000 mg × h/L over 24 hours. Without knowing an accurate AUC, there is risk for ineffective bactericidal activity with low AUCs or nephrotoxicity with high AUCs. Compared with trough-only monitoring, AUC-guided dosing is associated with decreased risk of acute kidney injury.4,5 Therefore, the recommendation to transition to two-sample collection with a peak and trough was included.
Software programs are now readily available to compute the AUC and work best with peak and trough values rather than a single trough value because computing with two concentrations will rely more on specific patient data than it does on previously published vancomycin models. Trough-only monitoring (and without the support of clinical software) may still be possible when the exposures needed are further from the toxic range. To this end, trough-only monitoring may be reasonable when infections are not MRSA and are less invasive (eg, cellulitis) since the guideline found insufficient evidence for AUC monitoring in these scenarios. While specific targets are not provided, a plethora of historical literature demonstrated low kidney injury rates when troughs were maintained between 5 to 10 mg/L.
KEY RECOMMENDATIONS FOR PEDIATRIC HOSPITALISTS
The following is a summary of recommendations 18 to 20:
- In pediatric care, based on a target AUC to MIC ratio of 400 to 600 with the assumption of MIC to be 1 mg/L, initial vancomycin dosage for MRSA is as follows (evidence quality: A-II) :
- 60 to 80 mg/kg per day, divided into four doses, each given 6 hours apart, for children 3 months and older but younger than 12 years
- 60 to 70 mg/kg per day, divided into four doses, each given 6 hours apart, for children 12 years and older
- As recommended in adults, use of a statistical software program to measure AUC is the optimal approach in pediatric care because it can account for age, weight, and renal function, which should be monitored closely. Monitoring should begin within 48 hours of therapy. Vancomycin AUC and trough concentrations should be less than 800 µg × h/mL over 24 hours and 15 µg/mL, respectively, to minimize acute kidney injury (evidence quality: A-II).
All the recommendations for pediatrics are new for the updated guideline. Pediatric data to support these recommendations are fewer in comparison with adult literature. Given MRSA infections are felt to be similar in adults and children, many pediatric recommendations are extrapolated from adult data and recommendations. The strongest level of evidence in children is the association of acute kidney injury with higher vancomycin exposure, especially with troughs exceeding 15 to 20 mg/L.6 In addition, one pediatric study found an AUC exposure of greater than 800 mg × h/L over 24 hours was strongly associated with risk for acute kidney injury.7 These findings suggest that high vancomycin exposure correlates with nephrotoxicity, so with AUC monitoring, the goal exposure should be less than 800 mg × hr/L over 24 hours.
Only one study has evaluated statistical software and prediction of AUC in pediatrics.8 A two-concentration approach (peak and trough) outperformed trough-only monitoring for accuracy and precision in determining AUC. While limited to one study, the results are similar to the studies completed in adults, thereby leading to the recommendation of the two-concentration technique in children.
Prospective outcome data are lacking, but multiple retrospective studies have examined S aureus bacteremia in children. Thus far, there have been no studies that have determined the optimal vancomycin exposure required for successful outcomes.9,10 The proven risks of toxicity are the primary driver for the pediatric guideline change with the outcomes extrapolated from adult data.
CRITIQUE
Methods in Preparing Guideline
The main strength of the guideline is that the committee was represented by multiple organizations, which created a multidisciplinary panel of pharmacists and infectious disease physicians with clinical and research expertise in vancomycin dosing. Evidence was graded using an adaptation from the Canadian Task Force on the Periodic Health Examination.11 The draft was peer-reviewed by the society organizations and allowed for comments, suggestions, and recommendations.
Sources of Potential Conflict of Interest or Bias
Disclosures of all authors were reported and identified in the guideline. While many members are involved with pharmaceutical companies through research or speakers’ roles, vancomycin, a generic drug, should have minimal conflicts of interest or bias from this involvement.
Generalizability
Implementation of vancomycin AUC dosing will be hospital dependent due to the implementation-related increase in human resources and the cost of clinical software; many hospital systems do not already have the software integrated into their clinical practice. Local guidelines will have to be developed to help clinicians determine which clinical situations require AUC-based dosing vs trough-only monitoring. Pharmacists at many hospitals are primarily responsible for vancomycin monitoring and provide dosing recommendations to physicians. Depending on a hospital system’s decision, the workload to determine the optimal vancomycin dose may increase, and it will be important to have close collaboration between hospitalists, pharmacists, and infectious diseases clinicians to appropriately educate clinicians who might be required to dose/monitor vancomycin. One potential way to decrease the burden of monitoring with two concentrations is to use specialized software that can perform complex assessments with only a single concentration. These software applications will still require serious collaboration of the aforementioned practitioners to implement. The variation in guideline adoption will likely be even more significant in pediatrics because the literature is extrapolated and the increased blood draws can be more problematic in pediatric patients.
Furthermore, clinicians should understand the dosing guideline is specifically addressing treatment of MRSA infections and extrapolation to other organisms such as coagulase-negative staphylococcal or methicillin-susceptible S aureus infections should be cautioned. Another caveat to note is that, when the MRSA isolate has an MIC of 2 mg/L or higher, these infections are associated with poor outcomes when vancomycin is used and alternative agents are recommended.
AREAS IN NEED OF FUTURE STUDY
Research gaps still remain with appropriate vancomycin drug exposure. In pediatrics, determining the appropriate AUC target will be important given that current recommendations extrapolate from adult data. Future studies can focus on prospective outcome data in both pediatric and adult patients for infections outside of bacteremia or pneumonia, notably central nervous system and osteomyelitis infections. Thresholds for kidney injury will need to be more clearly defined for both adult and pediatric patients. There should also be research emphasis on the appropriate dosing for other non-MRSA invasive infections, notably coagulase-negative staphylococcal infections.
Disclosures
Dr Scheetz reported personal fees for consulting for Achaogen, SIGA technologies, and for serving on an advisory board for Paratek; grants from Merck and Co, Allecra, Nevakar, and SuperTrans Medical; personal fees from Hall, Booth, Smith, PC, and Chambless, Higdon, Richardson, Katz & Griggs, LLP, for consulting and expert testimony, outside the submitted work. In addition, Dr. Scheetz has patent US 2019 / 0099500 A1 pending. Dr Murphy reported having received fees from Becton Dickinson for participation to review IDSA guidelines on gastroenteritis. Dr Tang Girdwood has nothing to disclose.
Funding
Dr Murphy and Dr Tang Girdwood are supported by the National Institute of Child Health and Development Cincinnati Pediatric Clinical Pharmacology Postdoctoral Training Program (5T32HD069054-09). Dr Tang Girdwood is also supported by the Cincinnati Children’s Hospital Medical Center Arnold W Strauss Fellow Award and Cincinnati Children’s Hospital Medical Center Hospital Medicine Fellow Award. Dr Scheetz is supported in part by the National Institute of Allergy and Infectious Diseases award (R21AI149026). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Vancomycin, a glycopeptide antibiotic, has been used for decades, yet knowledge gaps remain regarding the most appropriate dosing approach to optimize therapeutic effect while avoiding adverse effects in all patient populations. A committee composed of members of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists reviewed data available since publication of the original 2009 vancomycin dosing guidelines to provide new recommendations regarding vancomycin dosing and serum concentration–monitoring in the empiric treatment of presumed or confirmed methicillin-resistant Staphylococcus aureus (MRSA) infections.1
The new guidelines provide 25 recommendations encompassing the following topics: vancomycin dosing and monitoring in adult, pediatric, and neonate care; vancomycin minimum inhibitory concentration (MIC) susceptibility testing; continuous infusion vs intermittent infusion; loading doses; dosing in obesity; and dosing in patients on hemodialysis and continuous renal replacement therapy. Because hospitalists in pediatric and adult care frequently prescribe vancomycin for empiric and targeted treatment of serious infections, they have a vested interest in ensuring optimal vancomycin outcomes (ie, best efficacy with least toxicity) with use of therapeutic drug monitoring and personalized dosing of vancomycin. Thus, it is important for hospitalists to be aware of the updated guideline and pivotal changes regarding therapeutic drug monitoring. In this guideline review, we will focus on the major differences from the 2009 guideline, specifically regarding therapeutic monitoring in adults and children.
The guideline includes pharmacology language and terminology with which many clinicians may not be familiar. To better understand the rationale for the guideline changes, a few concepts will be reviewed. Overall, antibiotics are dosed based on preclinical studies to determine the needed drug exposure for optimal efficacy. β-Lactams, for example, are optimally dosed with longer drug exposure time above the MIC of the infectious organism. Alternatively, area under the concentration time curve (AUC) describes the efficacy and toxicity of many other antibiotics. Since AUC is derived from products of concentration (mg/L) and time (hours), the units are often mg × h/L. For vancomycin, both drug exposure (ie, AUC) and organism susceptibility (ie, MIC) are incorporated to determine optimal drug exposure, with the ratio of AUC to MIC being the ideal marker. Therapeutic drug monitoring of vancomycin has classically been conducted with trough concentration monitoring, but with the updated guideline, there will be a transition to AUC monitoring that will affect patient care and experience.
KEY RECOMMENDATIONS FOR HOSPITALISTS TREATING ADULTS
The following is a summary of recommendations 1 to 6:
- In adults, the optimal drug exposure for vancomycin should be an AUC to MIC ratio of 400 to 600 for MRSA, with the assumption of MIC to be 1 mg/L (evidence quality: A-II).
- The preferred method to monitor AUC is with a clinical statistical software that uses two blood samples (1 to 2 hours after completion of infusion and at the end of a dosing interval [ie, trough]) (evidence quality: A-II).
- An alternative approach would be to use first-order pharmacokinetic equations at steady state with a peak and trough (evidence quality: A-II).
- These approaches replace the previously recommended trough-only monitoring. AUC-targeted exposure should generally be achieved within 48 hours; severity of infection does not justify higher AUC goals. Once the goal AUC is achieved, once-weekly monitoring is recommended for hemodynamically stable patients, but more frequent or daily monitoring is advised in patients at high risk of nephrotoxicity or who are hemodynamically unstable (evidence quality: B-II).
The currently accepted optimal drug exposure for vancomycin is an AUC to MIC ratio of 400 to 600 to maximize efficacy and minimize nephrotoxicity.2 Due to clinical inconvenience of performing AUC-based monitoring for vancomycin in the past, previous guidelines recommended using trough concentrations as a surrogate marker for an AUC to MIC ratio, with the goal trough being 15 to 20 mg/L for serious MRSA infections.3 However, trough values may not correlate well with AUC. For example, a trough of 15 mg/L may represent an AUC ranging from 400 to 1000 mg × h/L over 24 hours. Without knowing an accurate AUC, there is risk for ineffective bactericidal activity with low AUCs or nephrotoxicity with high AUCs. Compared with trough-only monitoring, AUC-guided dosing is associated with decreased risk of acute kidney injury.4,5 Therefore, the recommendation to transition to two-sample collection with a peak and trough was included.
Software programs are now readily available to compute the AUC and work best with peak and trough values rather than a single trough value because computing with two concentrations will rely more on specific patient data than it does on previously published vancomycin models. Trough-only monitoring (and without the support of clinical software) may still be possible when the exposures needed are further from the toxic range. To this end, trough-only monitoring may be reasonable when infections are not MRSA and are less invasive (eg, cellulitis) since the guideline found insufficient evidence for AUC monitoring in these scenarios. While specific targets are not provided, a plethora of historical literature demonstrated low kidney injury rates when troughs were maintained between 5 to 10 mg/L.
KEY RECOMMENDATIONS FOR PEDIATRIC HOSPITALISTS
The following is a summary of recommendations 18 to 20:
- In pediatric care, based on a target AUC to MIC ratio of 400 to 600 with the assumption of MIC to be 1 mg/L, initial vancomycin dosage for MRSA is as follows (evidence quality: A-II) :
- 60 to 80 mg/kg per day, divided into four doses, each given 6 hours apart, for children 3 months and older but younger than 12 years
- 60 to 70 mg/kg per day, divided into four doses, each given 6 hours apart, for children 12 years and older
- As recommended in adults, use of a statistical software program to measure AUC is the optimal approach in pediatric care because it can account for age, weight, and renal function, which should be monitored closely. Monitoring should begin within 48 hours of therapy. Vancomycin AUC and trough concentrations should be less than 800 µg × h/mL over 24 hours and 15 µg/mL, respectively, to minimize acute kidney injury (evidence quality: A-II).
All the recommendations for pediatrics are new for the updated guideline. Pediatric data to support these recommendations are fewer in comparison with adult literature. Given MRSA infections are felt to be similar in adults and children, many pediatric recommendations are extrapolated from adult data and recommendations. The strongest level of evidence in children is the association of acute kidney injury with higher vancomycin exposure, especially with troughs exceeding 15 to 20 mg/L.6 In addition, one pediatric study found an AUC exposure of greater than 800 mg × h/L over 24 hours was strongly associated with risk for acute kidney injury.7 These findings suggest that high vancomycin exposure correlates with nephrotoxicity, so with AUC monitoring, the goal exposure should be less than 800 mg × hr/L over 24 hours.
Only one study has evaluated statistical software and prediction of AUC in pediatrics.8 A two-concentration approach (peak and trough) outperformed trough-only monitoring for accuracy and precision in determining AUC. While limited to one study, the results are similar to the studies completed in adults, thereby leading to the recommendation of the two-concentration technique in children.
Prospective outcome data are lacking, but multiple retrospective studies have examined S aureus bacteremia in children. Thus far, there have been no studies that have determined the optimal vancomycin exposure required for successful outcomes.9,10 The proven risks of toxicity are the primary driver for the pediatric guideline change with the outcomes extrapolated from adult data.
CRITIQUE
Methods in Preparing Guideline
The main strength of the guideline is that the committee was represented by multiple organizations, which created a multidisciplinary panel of pharmacists and infectious disease physicians with clinical and research expertise in vancomycin dosing. Evidence was graded using an adaptation from the Canadian Task Force on the Periodic Health Examination.11 The draft was peer-reviewed by the society organizations and allowed for comments, suggestions, and recommendations.
Sources of Potential Conflict of Interest or Bias
Disclosures of all authors were reported and identified in the guideline. While many members are involved with pharmaceutical companies through research or speakers’ roles, vancomycin, a generic drug, should have minimal conflicts of interest or bias from this involvement.
Generalizability
Implementation of vancomycin AUC dosing will be hospital dependent due to the implementation-related increase in human resources and the cost of clinical software; many hospital systems do not already have the software integrated into their clinical practice. Local guidelines will have to be developed to help clinicians determine which clinical situations require AUC-based dosing vs trough-only monitoring. Pharmacists at many hospitals are primarily responsible for vancomycin monitoring and provide dosing recommendations to physicians. Depending on a hospital system’s decision, the workload to determine the optimal vancomycin dose may increase, and it will be important to have close collaboration between hospitalists, pharmacists, and infectious diseases clinicians to appropriately educate clinicians who might be required to dose/monitor vancomycin. One potential way to decrease the burden of monitoring with two concentrations is to use specialized software that can perform complex assessments with only a single concentration. These software applications will still require serious collaboration of the aforementioned practitioners to implement. The variation in guideline adoption will likely be even more significant in pediatrics because the literature is extrapolated and the increased blood draws can be more problematic in pediatric patients.
Furthermore, clinicians should understand the dosing guideline is specifically addressing treatment of MRSA infections and extrapolation to other organisms such as coagulase-negative staphylococcal or methicillin-susceptible S aureus infections should be cautioned. Another caveat to note is that, when the MRSA isolate has an MIC of 2 mg/L or higher, these infections are associated with poor outcomes when vancomycin is used and alternative agents are recommended.
AREAS IN NEED OF FUTURE STUDY
Research gaps still remain with appropriate vancomycin drug exposure. In pediatrics, determining the appropriate AUC target will be important given that current recommendations extrapolate from adult data. Future studies can focus on prospective outcome data in both pediatric and adult patients for infections outside of bacteremia or pneumonia, notably central nervous system and osteomyelitis infections. Thresholds for kidney injury will need to be more clearly defined for both adult and pediatric patients. There should also be research emphasis on the appropriate dosing for other non-MRSA invasive infections, notably coagulase-negative staphylococcal infections.
Disclosures
Dr Scheetz reported personal fees for consulting for Achaogen, SIGA technologies, and for serving on an advisory board for Paratek; grants from Merck and Co, Allecra, Nevakar, and SuperTrans Medical; personal fees from Hall, Booth, Smith, PC, and Chambless, Higdon, Richardson, Katz & Griggs, LLP, for consulting and expert testimony, outside the submitted work. In addition, Dr. Scheetz has patent US 2019 / 0099500 A1 pending. Dr Murphy reported having received fees from Becton Dickinson for participation to review IDSA guidelines on gastroenteritis. Dr Tang Girdwood has nothing to disclose.
Funding
Dr Murphy and Dr Tang Girdwood are supported by the National Institute of Child Health and Development Cincinnati Pediatric Clinical Pharmacology Postdoctoral Training Program (5T32HD069054-09). Dr Tang Girdwood is also supported by the Cincinnati Children’s Hospital Medical Center Arnold W Strauss Fellow Award and Cincinnati Children’s Hospital Medical Center Hospital Medicine Fellow Award. Dr Scheetz is supported in part by the National Institute of Allergy and Infectious Diseases award (R21AI149026). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
1. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835-864. https://doi.org/10.1093/ajhp/zxaa036
2. Men P, Li HB, Zhai SD, Zhao RS. Association between the AUC0-24/MIC ratio of vancomycin and its clinical effectiveness: a systematic review and meta-analysis. PLoS One. 2016;11(1):e0146224. https://doi.org/10.1371/journal.pone.0146224
3. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82-98. https://doi.org/10.2146/ajhp080434
4. Finch NA, Zasowski EJ, Murray KP, et al. A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycin-associated nephrotoxicity. Antimicrob Agents Chemother. 2017;61(12):e01293-17. https://doi.org/10.1128/aac.01293-17
5. Neely MN, Kato L, Youn G, et al. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother. 2018;62(2):e02042-17. https://doi.org/10.1128/aac.02042-17
6. Fiorito TM, Luther MK, Dennehy PH, LaPlante KL, Matson KL. Nephrotoxicity with vancomycin in the pediatric population: a systematic review and meta-analysis. Pediatr Infect Dis J. 2018;37(7):654-661. https://doi.org/10.1097/inf.0000000000001882
7. Le J, Ny P, Capparelli E, et al. Pharmacodynamic characteristics of nephrotoxicity associated with vancomycin use in children. J Pediatric Infect Dis Soc. 2015;4(4):e109-e116. https://doi.org/10.1093/jpids/piu110
8. Le J, Ngu B, Bradley JS, et al. Vancomycin monitoring in children using bayesian estimation. Ther Drug Monit. 2014;36(4):510-518. https://doi.org/10.1097/ftd.0000000000000039
9. Hahn A, Frenck RW Jr, Allen-Staat M, Zou Y, Vinks AA. Evaluation of target attainment of vancomycin area under the curve in children with methicillin-resistant Staphylococcus aureus bacteremia. Ther Drug Monit. 2015;37(5):619-625. https://doi.org/10.1097/ftd.0000000000000190
10. McNeil JC, Kok EY, Forbes AR, et al. Healthcare-associated Staphylococcus aureus bacteremia in children: evidence for reverse vancomycin creep and impact of vancomycin trough values on outcome. Pediatr Infect Dis J. 2016;35(3):263-268. https://doi.org/10.1097/inf.0000000000000991
11. The periodic health examination. Canadian Task Force on the Periodic Health Examination. Can Med Assoc J. 1979;121(9):1193-1254.
1. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835-864. https://doi.org/10.1093/ajhp/zxaa036
2. Men P, Li HB, Zhai SD, Zhao RS. Association between the AUC0-24/MIC ratio of vancomycin and its clinical effectiveness: a systematic review and meta-analysis. PLoS One. 2016;11(1):e0146224. https://doi.org/10.1371/journal.pone.0146224
3. Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66(1):82-98. https://doi.org/10.2146/ajhp080434
4. Finch NA, Zasowski EJ, Murray KP, et al. A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycin-associated nephrotoxicity. Antimicrob Agents Chemother. 2017;61(12):e01293-17. https://doi.org/10.1128/aac.01293-17
5. Neely MN, Kato L, Youn G, et al. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother. 2018;62(2):e02042-17. https://doi.org/10.1128/aac.02042-17
6. Fiorito TM, Luther MK, Dennehy PH, LaPlante KL, Matson KL. Nephrotoxicity with vancomycin in the pediatric population: a systematic review and meta-analysis. Pediatr Infect Dis J. 2018;37(7):654-661. https://doi.org/10.1097/inf.0000000000001882
7. Le J, Ny P, Capparelli E, et al. Pharmacodynamic characteristics of nephrotoxicity associated with vancomycin use in children. J Pediatric Infect Dis Soc. 2015;4(4):e109-e116. https://doi.org/10.1093/jpids/piu110
8. Le J, Ngu B, Bradley JS, et al. Vancomycin monitoring in children using bayesian estimation. Ther Drug Monit. 2014;36(4):510-518. https://doi.org/10.1097/ftd.0000000000000039
9. Hahn A, Frenck RW Jr, Allen-Staat M, Zou Y, Vinks AA. Evaluation of target attainment of vancomycin area under the curve in children with methicillin-resistant Staphylococcus aureus bacteremia. Ther Drug Monit. 2015;37(5):619-625. https://doi.org/10.1097/ftd.0000000000000190
10. McNeil JC, Kok EY, Forbes AR, et al. Healthcare-associated Staphylococcus aureus bacteremia in children: evidence for reverse vancomycin creep and impact of vancomycin trough values on outcome. Pediatr Infect Dis J. 2016;35(3):263-268. https://doi.org/10.1097/inf.0000000000000991
11. The periodic health examination. Canadian Task Force on the Periodic Health Examination. Can Med Assoc J. 1979;121(9):1193-1254.
© 2020 Society of Hospital Medicine
Clinical Guideline Highlights for the Hospitalist: 2019 American Thoracic Society/Infectious Diseases Society of America Update on Community-Acquired Pneumonia
Community-acquired pneumonia (CAP) is the second most common cause of hospitalization in the United States, with over 1.5 million unique hospitalizations annually.1 CAP is also the most common infectious cause of death in US adults.2 The 2019 CAP guideline from the American Thoracic Society (ATS) and the Infectious Diseases Society of America (IDSA) provides recommendations on the diagnosis and management of CAP. The guideline provides 16 recommendations, which we have consolidated to highlight practice changing updates in diagnostic testing, risk stratification, and treatment.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Diagnostic Testing
Recommendation 1. In patients with CAP, routine blood cultures, sputum cultures, and urinary antigen tests are not routinely recommended unless severe CAP (Table), history of methicillin-resistant Staphylococcus aureus (MRSA) and/or Pseudomonas infection, or prior hospitalization for which intravenous antibiotics were administered. (Strong recommendation; very low quality of evidence)
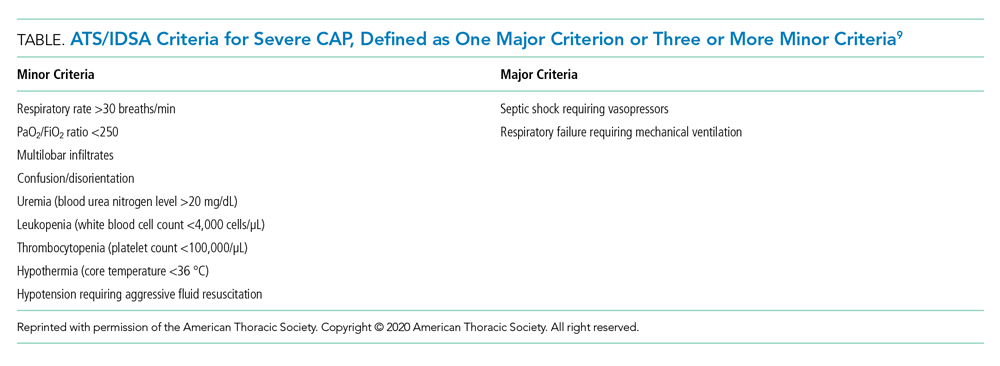
The guideline emphasizes that the diagnostic yield of blood/sputum cultures and urinary antigen testing is low. Additionally, high-quality data showing improved clinical outcomes with routine testing of blood cultures and urinary antigens are lacking. Instead, the guideline suggests obtaining blood cultures, urinary antigens, and sputum gram stain and culture only for patients with severe CAP and those being treated for or having prior infection with MRSA or P aeruginosa. They recommend narrowing therapy as appropriate if cultures are negative for either of these two organisms or previous hospitalization with intravenous antibiotics.
Risk Stratification
Recommendation 2. In patients with CAP, Pneumonia Severity Index (PSI) or CURB-65 (tool based on confusion, urea level, respiratory rate, blood pressure, and age 65 years or older) scores should not be used to determine general medical ward vs intensive care unit care (ICU). (Strong recommendation; low quality of evidence)
The PSI and CURB-65 scores are not validated to determine location of hospital care. Multiple prognostic models have been studied to predict the need for ICU-level care including SMART-COP (systolic blood pressure, multilobar chest radiography involvement, albumin level, respiratory rate, tachycardia, confusion, oxygenation, and arterial pH), SCAPA (Study of Community Acquired Pneumonia Aetiology), and the ATS/IDSA criteria (Table). The positive and negative likelihood ratios for needing ICU admission in CAP with either one major or three or more minor ATS/IDSA criteria are 3.28 and 0.21, respectively.
Treatment
Recommendation 3a. In patients with nonsevere CAP and no risk factors for MRSA or Pseudomonas infection, empiric treatment with a ß-lactam plus macrolide or monotherapy with fluoroquinolones is recommended. (Strong recommendation; high quality of evidence)
Recommendation 3b. In patients with severe CAP and no risk factors for MRSA or Pseudomonas infection, empiric treatment with a ß-lactam plus either a macrolide or fluoroquinolone is recommended. (Strong recommendation; low to moderate quality of evidence)
Microbiologic risk assessment is critical. Risk factors for MRSA or P aeruginosa pneumonia include isolation of these agents in culture and recent hospitalization with receipt of parenteral antibiotics. ß-Lactam monotherapy is not recommended because previous randomized clinical trials (RCTs) demonstrated inferiority of ß-lactam monotherapy to combination therapy for resolution of CAP. The recommended combination therapy for patients with severe CAP without risk factors for MRSA or P aeruginosa infection is a ß-lactam plus either a macrolide or a respiratory fluoroquinolone.
Recommendation 4. In patients with suspected aspiration pneumonia, additional anaerobic coverage is not routinely recommended. (Conditional recommendation; very low quality of evidence)
Aspiration often causes a self-limited pneumonitis that will resolve in 24 to 48 hours with supportive care. Use of additional anaerobic coverage in these patients increases risk for complications (eg, Clostridioides difficile infection) without improving outcomes.
Recommendation 5. In patients with nonsevere CAP, corticosteroids are not routinely recommended. (Conditional recommendation; moderate quality of evidence)
There is no direct evidence that steroids reduce mortality or organ failure in nonsevere CAP. Additionally, the use of steroids in CAP can come with considerable risks (eg, secondary infection, hyperglycemia).
Recommendation 6. In hospitalized patients with CAP, empiric coverage for MRSA or P aeruginosa should be limited to patients meeting specific criteria. (Strong recommendation; moderate quality of evidence)
The guideline highlights the current overuse of extended spectrum antibiotics in patients meeting the previous definition of healthcare-associated pneumonia (HCAP). HCAP was defined by the presence of new chest x-ray infiltrates in patients with various exposures to healthcare settings (eg, chronic dialysis, infusion centers, emergency rooms). Antimicrobial therapy covering MRSA or P aeruginosa should be reserved for patients at risk for MRSA or P aeruginosa infection unless microbiologic testing is negative. Empiric antibiotic selection should incorporate local resistance patterns guided by hospital antibiograms.
Recommendation 7. In adults with CAP, antibiotics should be continued for no less than 5 days with documented clinical stability. (Strong recommendation; moderate quality of evidence)
Hospitalists often determine the length of antibiotic therapy for CAP. Recent studies show extended antibiotic treatment for pneumonia increases risk for adverse events without improving outcomes. Studies also demonstrate patients who receive 5 days of antibiotics total after achieving clinical stability by day 3 do no worse than patients receiving 8 or more days of antibiotics.
CRITIQUE
This guideline was created by a panel of pulmonologists, infectious disease specialists, general internists, and methodologists using the GRADE (Grading of Recommendations Assessment, Development and Evaluations) approach to draft recommendations. Conflicts of interest were disclosed by all panel members according to the ATS and IDSA policies, and ultimately, two panel members recused themselves owing to conflicts of interest. The inclusion of a large number of RCTs, observational studies, and meta-analyses provides for good generalizability of the guideline published by this group.
Equal support was given in the guideline to all ß-lactams listed, including ampicillin/sulbactam, cefotaxime, ceftriaxone, and ceftaroline, regardless of MRSA risk factors. As the authors explicitly state in the guideline, one of the major reasons for abandoning the HCAP classification was to correct the overuse of anti-MRSA and antipseudomonal therapy.3 It is surprising, then, that the authors would include ceftaroline, a broad-spectrum cephalosporin that covers MRSA, as first-line therapy for patients without risk factors for MRSA.
The guideline also supported the use of a respiratory fluoroquinolone or a ß-lactam with macrolide equally. Although most RCTs have found equal efficacy between these two regimens,4 there is growing concern about the safety of fluoroquinolones.5 While the authors do encourage clinicians to consider these side effects in the main body of the text, a stronger statement could have been made more prominently to warn clinicians of safety concerns with fluoroquinolones.
Finally, while monotherapy with a ß-lactam was supported for the treatment of nonsevere outpatient CAP, it was not included in the recommendations for the treatment of hospitalized patients. There is conflicting data on this topic. One RCT failed to show noninferiority of monotherapy, but this was most pronounced among patients with severe pneumonia (PSI category IV) or cases with proven atypical infections.6 Another RCT found monotherapy to be noninferior to combination therapy for hospitalized patients not admitted to the ICU.7 There is also evidence suggesting that many patients hospitalized with pneumonia have viral rather than bacterial infections,8 which brings into question the need for antibiotics in this subset entirely. When these findings are considered from a stewardship perspective and patient safety profile, monotherapy with a ß-lactam for hospitalized patients without severe pneumonia could have been considered.
AREAS IN NEED OF FUTURE STUDY
Future research should track the effects of this guideline’s recommendation to narrow empiric therapy on patients empirically treated for MRSA or P aeruginosa infection once sputum and blood cultures are negative, particularly with respect to reduction of time on broad spectrum antimicrobials and clinical outcomes. Similarly, better definitions of which patients require empiric MRSA and P aeruginosa antimicrobial coverage are needed. Ideally, further research will facilitate rapid, cost-effective, and individualized therapy, particularly with growing concerns for antimicrobial resistance and safety.
Disclosures
The authors have no relevant financial conflicts of interest to disclose.
1. Ramirez JA, Wiemken TL, Peyrani P, et al. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65(11):1806-1812. https://doi.org/10.1093/cid/cix647
2. Xu J, Murphy SL, Kochanek KD, Bastian BA. Deaths: final data for 2013. Natl Vital Stat Rep. 2016;64(2):1-119.
3. Jones BE, Jones MM, Huttner B, et al. Trends in antibiotic use and nosocomial pathogens in hospitalized veterans with pneumonia at 128 medical centers, 2006-2010. Clin Infect Dis. 2015;61(9):1403-1410. https://doi.org/10.1093/cid/civ629
4. Fogarty C, Siami G, Kohler R, et al. Multicenter, open-label, randomized study to compare the safety and efficacy of levofloxacin versus ceftriaxone sodium and erythromycin followed by clarithromycin and amoxicillin-clavulanate in the treatment of serious community-acquired pneumonia in adults. Clin Infect Dis. 2004;38(Suppl 1):S16-S23.
5. U.S. Food and Drug Administration. Fluoroquinolone antimicrobial drugs information. Accessed February 4, 2020. https://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm346750.htm
6. Garin N, Genné D, Carballo S, et al. ß-Lactam monotherapy vs ß-lactam-macrolide combination treatment in moderately severe community-acquired pneumonia: a randomized noninferiority trial. JAMA Intern Med. 2014;174(12):1894-1901. https://doi.org/10.1001/jamainternmed.2014.4887
7. Postma DF, van Werkhoven CH, van Elden LJ, et al. Antibiotic treatment strategies for community-acquired pneumonia in adults. N Engl J Med. 2015;372(14):1312-1323. https://doi.org/10.1056/nejmoa1406330
8. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415-427. https://doi.org/10.1056/nejmoa1500245
9. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia: an official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67. https://doi.org/10.1164/rccm.201908-1581st
Community-acquired pneumonia (CAP) is the second most common cause of hospitalization in the United States, with over 1.5 million unique hospitalizations annually.1 CAP is also the most common infectious cause of death in US adults.2 The 2019 CAP guideline from the American Thoracic Society (ATS) and the Infectious Diseases Society of America (IDSA) provides recommendations on the diagnosis and management of CAP. The guideline provides 16 recommendations, which we have consolidated to highlight practice changing updates in diagnostic testing, risk stratification, and treatment.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Diagnostic Testing
Recommendation 1. In patients with CAP, routine blood cultures, sputum cultures, and urinary antigen tests are not routinely recommended unless severe CAP (Table), history of methicillin-resistant Staphylococcus aureus (MRSA) and/or Pseudomonas infection, or prior hospitalization for which intravenous antibiotics were administered. (Strong recommendation; very low quality of evidence)

The guideline emphasizes that the diagnostic yield of blood/sputum cultures and urinary antigen testing is low. Additionally, high-quality data showing improved clinical outcomes with routine testing of blood cultures and urinary antigens are lacking. Instead, the guideline suggests obtaining blood cultures, urinary antigens, and sputum gram stain and culture only for patients with severe CAP and those being treated for or having prior infection with MRSA or P aeruginosa. They recommend narrowing therapy as appropriate if cultures are negative for either of these two organisms or previous hospitalization with intravenous antibiotics.
Risk Stratification
Recommendation 2. In patients with CAP, Pneumonia Severity Index (PSI) or CURB-65 (tool based on confusion, urea level, respiratory rate, blood pressure, and age 65 years or older) scores should not be used to determine general medical ward vs intensive care unit care (ICU). (Strong recommendation; low quality of evidence)
The PSI and CURB-65 scores are not validated to determine location of hospital care. Multiple prognostic models have been studied to predict the need for ICU-level care including SMART-COP (systolic blood pressure, multilobar chest radiography involvement, albumin level, respiratory rate, tachycardia, confusion, oxygenation, and arterial pH), SCAPA (Study of Community Acquired Pneumonia Aetiology), and the ATS/IDSA criteria (Table). The positive and negative likelihood ratios for needing ICU admission in CAP with either one major or three or more minor ATS/IDSA criteria are 3.28 and 0.21, respectively.
Treatment
Recommendation 3a. In patients with nonsevere CAP and no risk factors for MRSA or Pseudomonas infection, empiric treatment with a ß-lactam plus macrolide or monotherapy with fluoroquinolones is recommended. (Strong recommendation; high quality of evidence)
Recommendation 3b. In patients with severe CAP and no risk factors for MRSA or Pseudomonas infection, empiric treatment with a ß-lactam plus either a macrolide or fluoroquinolone is recommended. (Strong recommendation; low to moderate quality of evidence)
Microbiologic risk assessment is critical. Risk factors for MRSA or P aeruginosa pneumonia include isolation of these agents in culture and recent hospitalization with receipt of parenteral antibiotics. ß-Lactam monotherapy is not recommended because previous randomized clinical trials (RCTs) demonstrated inferiority of ß-lactam monotherapy to combination therapy for resolution of CAP. The recommended combination therapy for patients with severe CAP without risk factors for MRSA or P aeruginosa infection is a ß-lactam plus either a macrolide or a respiratory fluoroquinolone.
Recommendation 4. In patients with suspected aspiration pneumonia, additional anaerobic coverage is not routinely recommended. (Conditional recommendation; very low quality of evidence)
Aspiration often causes a self-limited pneumonitis that will resolve in 24 to 48 hours with supportive care. Use of additional anaerobic coverage in these patients increases risk for complications (eg, Clostridioides difficile infection) without improving outcomes.
Recommendation 5. In patients with nonsevere CAP, corticosteroids are not routinely recommended. (Conditional recommendation; moderate quality of evidence)
There is no direct evidence that steroids reduce mortality or organ failure in nonsevere CAP. Additionally, the use of steroids in CAP can come with considerable risks (eg, secondary infection, hyperglycemia).
Recommendation 6. In hospitalized patients with CAP, empiric coverage for MRSA or P aeruginosa should be limited to patients meeting specific criteria. (Strong recommendation; moderate quality of evidence)
The guideline highlights the current overuse of extended spectrum antibiotics in patients meeting the previous definition of healthcare-associated pneumonia (HCAP). HCAP was defined by the presence of new chest x-ray infiltrates in patients with various exposures to healthcare settings (eg, chronic dialysis, infusion centers, emergency rooms). Antimicrobial therapy covering MRSA or P aeruginosa should be reserved for patients at risk for MRSA or P aeruginosa infection unless microbiologic testing is negative. Empiric antibiotic selection should incorporate local resistance patterns guided by hospital antibiograms.
Recommendation 7. In adults with CAP, antibiotics should be continued for no less than 5 days with documented clinical stability. (Strong recommendation; moderate quality of evidence)
Hospitalists often determine the length of antibiotic therapy for CAP. Recent studies show extended antibiotic treatment for pneumonia increases risk for adverse events without improving outcomes. Studies also demonstrate patients who receive 5 days of antibiotics total after achieving clinical stability by day 3 do no worse than patients receiving 8 or more days of antibiotics.
CRITIQUE
This guideline was created by a panel of pulmonologists, infectious disease specialists, general internists, and methodologists using the GRADE (Grading of Recommendations Assessment, Development and Evaluations) approach to draft recommendations. Conflicts of interest were disclosed by all panel members according to the ATS and IDSA policies, and ultimately, two panel members recused themselves owing to conflicts of interest. The inclusion of a large number of RCTs, observational studies, and meta-analyses provides for good generalizability of the guideline published by this group.
Equal support was given in the guideline to all ß-lactams listed, including ampicillin/sulbactam, cefotaxime, ceftriaxone, and ceftaroline, regardless of MRSA risk factors. As the authors explicitly state in the guideline, one of the major reasons for abandoning the HCAP classification was to correct the overuse of anti-MRSA and antipseudomonal therapy.3 It is surprising, then, that the authors would include ceftaroline, a broad-spectrum cephalosporin that covers MRSA, as first-line therapy for patients without risk factors for MRSA.
The guideline also supported the use of a respiratory fluoroquinolone or a ß-lactam with macrolide equally. Although most RCTs have found equal efficacy between these two regimens,4 there is growing concern about the safety of fluoroquinolones.5 While the authors do encourage clinicians to consider these side effects in the main body of the text, a stronger statement could have been made more prominently to warn clinicians of safety concerns with fluoroquinolones.
Finally, while monotherapy with a ß-lactam was supported for the treatment of nonsevere outpatient CAP, it was not included in the recommendations for the treatment of hospitalized patients. There is conflicting data on this topic. One RCT failed to show noninferiority of monotherapy, but this was most pronounced among patients with severe pneumonia (PSI category IV) or cases with proven atypical infections.6 Another RCT found monotherapy to be noninferior to combination therapy for hospitalized patients not admitted to the ICU.7 There is also evidence suggesting that many patients hospitalized with pneumonia have viral rather than bacterial infections,8 which brings into question the need for antibiotics in this subset entirely. When these findings are considered from a stewardship perspective and patient safety profile, monotherapy with a ß-lactam for hospitalized patients without severe pneumonia could have been considered.
AREAS IN NEED OF FUTURE STUDY
Future research should track the effects of this guideline’s recommendation to narrow empiric therapy on patients empirically treated for MRSA or P aeruginosa infection once sputum and blood cultures are negative, particularly with respect to reduction of time on broad spectrum antimicrobials and clinical outcomes. Similarly, better definitions of which patients require empiric MRSA and P aeruginosa antimicrobial coverage are needed. Ideally, further research will facilitate rapid, cost-effective, and individualized therapy, particularly with growing concerns for antimicrobial resistance and safety.
Disclosures
The authors have no relevant financial conflicts of interest to disclose.
Community-acquired pneumonia (CAP) is the second most common cause of hospitalization in the United States, with over 1.5 million unique hospitalizations annually.1 CAP is also the most common infectious cause of death in US adults.2 The 2019 CAP guideline from the American Thoracic Society (ATS) and the Infectious Diseases Society of America (IDSA) provides recommendations on the diagnosis and management of CAP. The guideline provides 16 recommendations, which we have consolidated to highlight practice changing updates in diagnostic testing, risk stratification, and treatment.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Diagnostic Testing
Recommendation 1. In patients with CAP, routine blood cultures, sputum cultures, and urinary antigen tests are not routinely recommended unless severe CAP (Table), history of methicillin-resistant Staphylococcus aureus (MRSA) and/or Pseudomonas infection, or prior hospitalization for which intravenous antibiotics were administered. (Strong recommendation; very low quality of evidence)

The guideline emphasizes that the diagnostic yield of blood/sputum cultures and urinary antigen testing is low. Additionally, high-quality data showing improved clinical outcomes with routine testing of blood cultures and urinary antigens are lacking. Instead, the guideline suggests obtaining blood cultures, urinary antigens, and sputum gram stain and culture only for patients with severe CAP and those being treated for or having prior infection with MRSA or P aeruginosa. They recommend narrowing therapy as appropriate if cultures are negative for either of these two organisms or previous hospitalization with intravenous antibiotics.
Risk Stratification
Recommendation 2. In patients with CAP, Pneumonia Severity Index (PSI) or CURB-65 (tool based on confusion, urea level, respiratory rate, blood pressure, and age 65 years or older) scores should not be used to determine general medical ward vs intensive care unit care (ICU). (Strong recommendation; low quality of evidence)
The PSI and CURB-65 scores are not validated to determine location of hospital care. Multiple prognostic models have been studied to predict the need for ICU-level care including SMART-COP (systolic blood pressure, multilobar chest radiography involvement, albumin level, respiratory rate, tachycardia, confusion, oxygenation, and arterial pH), SCAPA (Study of Community Acquired Pneumonia Aetiology), and the ATS/IDSA criteria (Table). The positive and negative likelihood ratios for needing ICU admission in CAP with either one major or three or more minor ATS/IDSA criteria are 3.28 and 0.21, respectively.
Treatment
Recommendation 3a. In patients with nonsevere CAP and no risk factors for MRSA or Pseudomonas infection, empiric treatment with a ß-lactam plus macrolide or monotherapy with fluoroquinolones is recommended. (Strong recommendation; high quality of evidence)
Recommendation 3b. In patients with severe CAP and no risk factors for MRSA or Pseudomonas infection, empiric treatment with a ß-lactam plus either a macrolide or fluoroquinolone is recommended. (Strong recommendation; low to moderate quality of evidence)
Microbiologic risk assessment is critical. Risk factors for MRSA or P aeruginosa pneumonia include isolation of these agents in culture and recent hospitalization with receipt of parenteral antibiotics. ß-Lactam monotherapy is not recommended because previous randomized clinical trials (RCTs) demonstrated inferiority of ß-lactam monotherapy to combination therapy for resolution of CAP. The recommended combination therapy for patients with severe CAP without risk factors for MRSA or P aeruginosa infection is a ß-lactam plus either a macrolide or a respiratory fluoroquinolone.
Recommendation 4. In patients with suspected aspiration pneumonia, additional anaerobic coverage is not routinely recommended. (Conditional recommendation; very low quality of evidence)
Aspiration often causes a self-limited pneumonitis that will resolve in 24 to 48 hours with supportive care. Use of additional anaerobic coverage in these patients increases risk for complications (eg, Clostridioides difficile infection) without improving outcomes.
Recommendation 5. In patients with nonsevere CAP, corticosteroids are not routinely recommended. (Conditional recommendation; moderate quality of evidence)
There is no direct evidence that steroids reduce mortality or organ failure in nonsevere CAP. Additionally, the use of steroids in CAP can come with considerable risks (eg, secondary infection, hyperglycemia).
Recommendation 6. In hospitalized patients with CAP, empiric coverage for MRSA or P aeruginosa should be limited to patients meeting specific criteria. (Strong recommendation; moderate quality of evidence)
The guideline highlights the current overuse of extended spectrum antibiotics in patients meeting the previous definition of healthcare-associated pneumonia (HCAP). HCAP was defined by the presence of new chest x-ray infiltrates in patients with various exposures to healthcare settings (eg, chronic dialysis, infusion centers, emergency rooms). Antimicrobial therapy covering MRSA or P aeruginosa should be reserved for patients at risk for MRSA or P aeruginosa infection unless microbiologic testing is negative. Empiric antibiotic selection should incorporate local resistance patterns guided by hospital antibiograms.
Recommendation 7. In adults with CAP, antibiotics should be continued for no less than 5 days with documented clinical stability. (Strong recommendation; moderate quality of evidence)
Hospitalists often determine the length of antibiotic therapy for CAP. Recent studies show extended antibiotic treatment for pneumonia increases risk for adverse events without improving outcomes. Studies also demonstrate patients who receive 5 days of antibiotics total after achieving clinical stability by day 3 do no worse than patients receiving 8 or more days of antibiotics.
CRITIQUE
This guideline was created by a panel of pulmonologists, infectious disease specialists, general internists, and methodologists using the GRADE (Grading of Recommendations Assessment, Development and Evaluations) approach to draft recommendations. Conflicts of interest were disclosed by all panel members according to the ATS and IDSA policies, and ultimately, two panel members recused themselves owing to conflicts of interest. The inclusion of a large number of RCTs, observational studies, and meta-analyses provides for good generalizability of the guideline published by this group.
Equal support was given in the guideline to all ß-lactams listed, including ampicillin/sulbactam, cefotaxime, ceftriaxone, and ceftaroline, regardless of MRSA risk factors. As the authors explicitly state in the guideline, one of the major reasons for abandoning the HCAP classification was to correct the overuse of anti-MRSA and antipseudomonal therapy.3 It is surprising, then, that the authors would include ceftaroline, a broad-spectrum cephalosporin that covers MRSA, as first-line therapy for patients without risk factors for MRSA.
The guideline also supported the use of a respiratory fluoroquinolone or a ß-lactam with macrolide equally. Although most RCTs have found equal efficacy between these two regimens,4 there is growing concern about the safety of fluoroquinolones.5 While the authors do encourage clinicians to consider these side effects in the main body of the text, a stronger statement could have been made more prominently to warn clinicians of safety concerns with fluoroquinolones.
Finally, while monotherapy with a ß-lactam was supported for the treatment of nonsevere outpatient CAP, it was not included in the recommendations for the treatment of hospitalized patients. There is conflicting data on this topic. One RCT failed to show noninferiority of monotherapy, but this was most pronounced among patients with severe pneumonia (PSI category IV) or cases with proven atypical infections.6 Another RCT found monotherapy to be noninferior to combination therapy for hospitalized patients not admitted to the ICU.7 There is also evidence suggesting that many patients hospitalized with pneumonia have viral rather than bacterial infections,8 which brings into question the need for antibiotics in this subset entirely. When these findings are considered from a stewardship perspective and patient safety profile, monotherapy with a ß-lactam for hospitalized patients without severe pneumonia could have been considered.
AREAS IN NEED OF FUTURE STUDY
Future research should track the effects of this guideline’s recommendation to narrow empiric therapy on patients empirically treated for MRSA or P aeruginosa infection once sputum and blood cultures are negative, particularly with respect to reduction of time on broad spectrum antimicrobials and clinical outcomes. Similarly, better definitions of which patients require empiric MRSA and P aeruginosa antimicrobial coverage are needed. Ideally, further research will facilitate rapid, cost-effective, and individualized therapy, particularly with growing concerns for antimicrobial resistance and safety.
Disclosures
The authors have no relevant financial conflicts of interest to disclose.
1. Ramirez JA, Wiemken TL, Peyrani P, et al. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65(11):1806-1812. https://doi.org/10.1093/cid/cix647
2. Xu J, Murphy SL, Kochanek KD, Bastian BA. Deaths: final data for 2013. Natl Vital Stat Rep. 2016;64(2):1-119.
3. Jones BE, Jones MM, Huttner B, et al. Trends in antibiotic use and nosocomial pathogens in hospitalized veterans with pneumonia at 128 medical centers, 2006-2010. Clin Infect Dis. 2015;61(9):1403-1410. https://doi.org/10.1093/cid/civ629
4. Fogarty C, Siami G, Kohler R, et al. Multicenter, open-label, randomized study to compare the safety and efficacy of levofloxacin versus ceftriaxone sodium and erythromycin followed by clarithromycin and amoxicillin-clavulanate in the treatment of serious community-acquired pneumonia in adults. Clin Infect Dis. 2004;38(Suppl 1):S16-S23.
5. U.S. Food and Drug Administration. Fluoroquinolone antimicrobial drugs information. Accessed February 4, 2020. https://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm346750.htm
6. Garin N, Genné D, Carballo S, et al. ß-Lactam monotherapy vs ß-lactam-macrolide combination treatment in moderately severe community-acquired pneumonia: a randomized noninferiority trial. JAMA Intern Med. 2014;174(12):1894-1901. https://doi.org/10.1001/jamainternmed.2014.4887
7. Postma DF, van Werkhoven CH, van Elden LJ, et al. Antibiotic treatment strategies for community-acquired pneumonia in adults. N Engl J Med. 2015;372(14):1312-1323. https://doi.org/10.1056/nejmoa1406330
8. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415-427. https://doi.org/10.1056/nejmoa1500245
9. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia: an official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67. https://doi.org/10.1164/rccm.201908-1581st
1. Ramirez JA, Wiemken TL, Peyrani P, et al. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65(11):1806-1812. https://doi.org/10.1093/cid/cix647
2. Xu J, Murphy SL, Kochanek KD, Bastian BA. Deaths: final data for 2013. Natl Vital Stat Rep. 2016;64(2):1-119.
3. Jones BE, Jones MM, Huttner B, et al. Trends in antibiotic use and nosocomial pathogens in hospitalized veterans with pneumonia at 128 medical centers, 2006-2010. Clin Infect Dis. 2015;61(9):1403-1410. https://doi.org/10.1093/cid/civ629
4. Fogarty C, Siami G, Kohler R, et al. Multicenter, open-label, randomized study to compare the safety and efficacy of levofloxacin versus ceftriaxone sodium and erythromycin followed by clarithromycin and amoxicillin-clavulanate in the treatment of serious community-acquired pneumonia in adults. Clin Infect Dis. 2004;38(Suppl 1):S16-S23.
5. U.S. Food and Drug Administration. Fluoroquinolone antimicrobial drugs information. Accessed February 4, 2020. https://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm346750.htm
6. Garin N, Genné D, Carballo S, et al. ß-Lactam monotherapy vs ß-lactam-macrolide combination treatment in moderately severe community-acquired pneumonia: a randomized noninferiority trial. JAMA Intern Med. 2014;174(12):1894-1901. https://doi.org/10.1001/jamainternmed.2014.4887
7. Postma DF, van Werkhoven CH, van Elden LJ, et al. Antibiotic treatment strategies for community-acquired pneumonia in adults. N Engl J Med. 2015;372(14):1312-1323. https://doi.org/10.1056/nejmoa1406330
8. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415-427. https://doi.org/10.1056/nejmoa1500245
9. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia: an official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67. https://doi.org/10.1164/rccm.201908-1581st
© 2020 Society of Hospital Medicine
Clinical Guideline Highlights for the Hospitalist: The GOLD and NICE Guidelines for the Management of COPD
Chronic obstructive pulmonary disease (COPD), projected to be the third leading cause of death by 2020, accounts for 6% of deaths globally.3 Hospitalization for COPD exacerbations is common and impacts patients’ disease trajectory, and mortality, with fewer than half of patients hospitalized for exacerbation surviving 5 years.4 Hospitalization provides an opportunity to optimize care. Due to recent practice-changing evidence, the National Institute for Health and Care Excellence (NICE) and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) published updated guidelines.
KEY RECOMMENDATIONS
These are selected recommendations relevant to adult hospitalists. The GOLD guidelines grade recommendations by evidence strength from category A (randomized control trial data) to category D (expert consensus). The NICE guidelines relay strength of evidence through terminology referring to the presence or absence of a strong recommendation. Recommendations without evidence level specified are NS.
Diagnosis and Classification of COPD Severity
Recommendation 1. In patients with risk factors for and symptoms of COPD, spirometry is required to confirm the diagnosis, defined as a postbronchodilator FEV1/FVC ratio of <0.7 (NS, NICE, GOLD). The Global Lung Function Initiative (GLI) 2012 reference ranges5 are recommended (NS, NICE). Recommendation 2. Severity of airflow obstruction should be assessed according to reduction in the postbronchodilator FEV1 as: Stage I, Mild: FEV1 ≥80%; Stage II, Moderate: FEV1 = 50-79%; Stage III, Severe FEV1 = 30%-49%; Stage IV, FEV1<30% (NS, NICE, GOLD). Recommendation 3. Reversibility testing (aka bronchodilator response) does not indicate long-term response to therapy (NS, NICE, GOLD). Recommendation 4. The combined COPD assessment to classify patient symptoms and disease severity in one of four groups (A, B, C, or D) based on exacerbation history and daily symptom control (NS, GOLD). Use the Medical Research Council dyspnea scale to classify symptoms (strong, NICE).
Pharmacologic COPD Management
Recommendation 5. Short-acting inhaled bronchodilators such as short-acting beta2 agonists (SABAs) or short-acting muscarinic antagonists (SAMAs) improve FEV1 and symptoms. Combining SABA/SAMA is superior to monotherapy (A, GOLD). Recommendation 6. Long-acting bronchodilators, such as long-acting antimuscarinics (LAMAs) or long-acting beta2 agonists (LABAs), improve lung function and dyspnea and reduce exacerbations. Combination therapy (LABA/LAMA) is superior to using a single agent (LABA or LAMA) for improving FEV1 and reducing exacerbations (A, GOLD). Recommendation 7. Triple therapy of inhaled corticosteroid ICS/LAMA/LABA is more effective than the individual components in reducing exacerbations in the case of moderate to severe COPD (A, GOLD). Recommendation 8. Treatment with an ICS increases pneumonia risk (A, GOLD). Discuss these side effects (Strong, NICE). Recommendation 9. Use SABAs and SAMAs as initial treatment for patients with COPD (Strong, NICE). LABAs and LAMAs are preferred over short-acting agents except for patients with mild symptoms (A, GOLD). Recommendation 10. For symptomatic patients on long-acting monotherapy, escalate to combination LABA/LAMA, or if asthmatic features or elevated eosinophils (≥300 cells/µL) are present, combination LABA/ICS (A, GOLD; Strong, NICE). Recommendation 11. Assess and correct patient inhaler technique (NS, GOLD; Strong, NICE).
Nonpharmacologic COPD Management
Oxygen. Recommendation 12. Long-term oxygen supplementation increases survival in patients with resting arterial hypoxemia (PaO2<55 mm Hg) or hypoxemia (PaO2<60 mm Hg) with cor pulmonale (A, GOLD). Recommendation 13. In patients with moderate resting (89%-93%) or exercise-induced arterial desaturation (80%-90%), long-term oxygen does not improve outcomes (A, GOLD).6 Recommendation 14. Consider long-term oxygan after a risk assessment of fall and burn risk. Do not offer oxygen to those who continue to smoke (Strong, NICE).
Tobacco
Pulmonary Rehabilitation. Recommendation 17. Provide rehabilitation to patients with high exacerbation risk and relevant symptoms (A, GOLD). Offer pulmonary rehabilitation to patients with recent hospitalizations and/or severe dyspnea (Strong, NICE).
Immunizations. Recommendation 18. Influenza and pneumococcal vaccinations (PPSV23 as well as PCV13 when age ≥ 65 years) are recommended for patients with COPD (NS, GOLD; Strong, NICE).
Palliative Care. Recommendation 19. For patients with end-stage COPD or poorly controlled symptoms, provide access to palliative care (NS, GOLD; Strong, NICE).
Management of COPD Exacerbations and Patients at high risk for Exacerbations
Recommendation 20. Use SABAs with or without SAMAs as initial bronchodilators to treat acute exacerbations (C, GOLD). Recommendation 21. Systemic corticosteroids for exacerbations improve lung function, oxygenation, and recovery time. Recommend 5 to 7 days of therapy (A, GOLD; Strong, NICE). Recommendation 22. Antibiotics shorten recovery time and reduce treatment failure and rehospitalization. Treatment should be 5 to 7 days (B, GOLD). Consider antibiotics while balancing the severity of symptoms and hospitalization need (Conditional, NICE). Recommendation 23. Noninvasive mechanical ventilation is the preferred mode of ventilation for COPD patients with acute respiratory failure without acute contraindications (A, GOLD). Recommendation 24. Avoid long-term oral corticosteroids therapy (A, GOLD). Recommendation 25. Consider roflumilast for patients with exacerbations despite LABA/ICS or LABA/LAMA/ICS, and seek respiratory medicine consultation (B, GOLD; Strong, NICE). For former smokers with exacerbations despite appropriate therapy, consider azithromycin (B, GOLD; Strong, NICE).
CRITIQUE
GOLD is an International committee of experts who compile the report based on scientific literature review. NICE is an independent organization funded by Department of Health and Social Care in the United Kingdom responsible for evidence-based guidance on healthcare determined by an expert committee through scientific review and a transparent process that details committee formation and framework (GRADE) used and stakeholder input. While both guidelines review current publications, practice-influencing clinical trials of recent publication may be missed.
On the GOLD Science committee, 17/20 members have pharmaceutical relationships, with no mitigation plan provided. The NICE guidelines detail a panel with few industry ties and a mitigation plan for potential conflicts of interest.
These recommendations comprehensively cover outpatient and inpatient COPD management. The GOLD and NICE guidelines are similar with the exception of recommendations surrounding use of oxygen. The NICE guidelines, based on the adverse events documented in the recent Long-Term Oxygen Treatment Trial,6 recommend against oxygen use by patients who smoke because of the risk of fire-related injuries;7 GOLD guidelines do not differentiate oxygen recommendation by patient population.
Differences in the strength of NICE and GOLD recommendations highlight areas for further study. Investigations determining distinct COPD phenotypes will likely influence future guidelines. More discriminative multidimensional prognostication tools are needed to improve precision surrounding prognosis.
1. NICE. Overview. Chronic obstructive pulmonary disease in over 16s: Diagnosis and management, Guidance. https://www.nice.org.uk/guidance/ng115. Accessed November 21, 2019
2. GOLD Reports for Personal Use. Global Initiative for Chronic Obstructive Lung Disease - GOLD. https://goldcopd.org/gold-reports/. Accessed September 17, 2019.
3. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095-128. https://doi.org/10.1016/S0140-6736(12)61728-0.
4. Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: Severe exacerbations and mortality. Thorax. 2012;67(11):957-63. https://doi.org/10.1136/thoraxjnl-2011-201518.
5. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: The global lung function 2012 equations. Eur Respir J. 2012;40(6):1324-43. https://doi.org/10.1183/09031936.00080312.
6. Albert RK, Au DH, Blackford AL, et al. Long-term oxygen treatment trial research group. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016;375(17):1617-27. https://doi.org/10.1056/NEJMoa1604344.
7. National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management [B} Oxygen therapy in people with stable COPD. https://www.nice.org.uk/guidance/ng115/evidence/b-oxygen-therapy-in-people-with-stable-copd-pdf-6602768751. Accessed November 21, 2019.
Chronic obstructive pulmonary disease (COPD), projected to be the third leading cause of death by 2020, accounts for 6% of deaths globally.3 Hospitalization for COPD exacerbations is common and impacts patients’ disease trajectory, and mortality, with fewer than half of patients hospitalized for exacerbation surviving 5 years.4 Hospitalization provides an opportunity to optimize care. Due to recent practice-changing evidence, the National Institute for Health and Care Excellence (NICE) and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) published updated guidelines.
KEY RECOMMENDATIONS
These are selected recommendations relevant to adult hospitalists. The GOLD guidelines grade recommendations by evidence strength from category A (randomized control trial data) to category D (expert consensus). The NICE guidelines relay strength of evidence through terminology referring to the presence or absence of a strong recommendation. Recommendations without evidence level specified are NS.
Diagnosis and Classification of COPD Severity
Recommendation 1. In patients with risk factors for and symptoms of COPD, spirometry is required to confirm the diagnosis, defined as a postbronchodilator FEV1/FVC ratio of <0.7 (NS, NICE, GOLD). The Global Lung Function Initiative (GLI) 2012 reference ranges5 are recommended (NS, NICE). Recommendation 2. Severity of airflow obstruction should be assessed according to reduction in the postbronchodilator FEV1 as: Stage I, Mild: FEV1 ≥80%; Stage II, Moderate: FEV1 = 50-79%; Stage III, Severe FEV1 = 30%-49%; Stage IV, FEV1<30% (NS, NICE, GOLD). Recommendation 3. Reversibility testing (aka bronchodilator response) does not indicate long-term response to therapy (NS, NICE, GOLD). Recommendation 4. The combined COPD assessment to classify patient symptoms and disease severity in one of four groups (A, B, C, or D) based on exacerbation history and daily symptom control (NS, GOLD). Use the Medical Research Council dyspnea scale to classify symptoms (strong, NICE).
Pharmacologic COPD Management
Recommendation 5. Short-acting inhaled bronchodilators such as short-acting beta2 agonists (SABAs) or short-acting muscarinic antagonists (SAMAs) improve FEV1 and symptoms. Combining SABA/SAMA is superior to monotherapy (A, GOLD). Recommendation 6. Long-acting bronchodilators, such as long-acting antimuscarinics (LAMAs) or long-acting beta2 agonists (LABAs), improve lung function and dyspnea and reduce exacerbations. Combination therapy (LABA/LAMA) is superior to using a single agent (LABA or LAMA) for improving FEV1 and reducing exacerbations (A, GOLD). Recommendation 7. Triple therapy of inhaled corticosteroid ICS/LAMA/LABA is more effective than the individual components in reducing exacerbations in the case of moderate to severe COPD (A, GOLD). Recommendation 8. Treatment with an ICS increases pneumonia risk (A, GOLD). Discuss these side effects (Strong, NICE). Recommendation 9. Use SABAs and SAMAs as initial treatment for patients with COPD (Strong, NICE). LABAs and LAMAs are preferred over short-acting agents except for patients with mild symptoms (A, GOLD). Recommendation 10. For symptomatic patients on long-acting monotherapy, escalate to combination LABA/LAMA, or if asthmatic features or elevated eosinophils (≥300 cells/µL) are present, combination LABA/ICS (A, GOLD; Strong, NICE). Recommendation 11. Assess and correct patient inhaler technique (NS, GOLD; Strong, NICE).
Nonpharmacologic COPD Management
Oxygen. Recommendation 12. Long-term oxygen supplementation increases survival in patients with resting arterial hypoxemia (PaO2<55 mm Hg) or hypoxemia (PaO2<60 mm Hg) with cor pulmonale (A, GOLD). Recommendation 13. In patients with moderate resting (89%-93%) or exercise-induced arterial desaturation (80%-90%), long-term oxygen does not improve outcomes (A, GOLD).6 Recommendation 14. Consider long-term oxygan after a risk assessment of fall and burn risk. Do not offer oxygen to those who continue to smoke (Strong, NICE).
Tobacco
Pulmonary Rehabilitation. Recommendation 17. Provide rehabilitation to patients with high exacerbation risk and relevant symptoms (A, GOLD). Offer pulmonary rehabilitation to patients with recent hospitalizations and/or severe dyspnea (Strong, NICE).
Immunizations. Recommendation 18. Influenza and pneumococcal vaccinations (PPSV23 as well as PCV13 when age ≥ 65 years) are recommended for patients with COPD (NS, GOLD; Strong, NICE).
Palliative Care. Recommendation 19. For patients with end-stage COPD or poorly controlled symptoms, provide access to palliative care (NS, GOLD; Strong, NICE).
Management of COPD Exacerbations and Patients at high risk for Exacerbations
Recommendation 20. Use SABAs with or without SAMAs as initial bronchodilators to treat acute exacerbations (C, GOLD). Recommendation 21. Systemic corticosteroids for exacerbations improve lung function, oxygenation, and recovery time. Recommend 5 to 7 days of therapy (A, GOLD; Strong, NICE). Recommendation 22. Antibiotics shorten recovery time and reduce treatment failure and rehospitalization. Treatment should be 5 to 7 days (B, GOLD). Consider antibiotics while balancing the severity of symptoms and hospitalization need (Conditional, NICE). Recommendation 23. Noninvasive mechanical ventilation is the preferred mode of ventilation for COPD patients with acute respiratory failure without acute contraindications (A, GOLD). Recommendation 24. Avoid long-term oral corticosteroids therapy (A, GOLD). Recommendation 25. Consider roflumilast for patients with exacerbations despite LABA/ICS or LABA/LAMA/ICS, and seek respiratory medicine consultation (B, GOLD; Strong, NICE). For former smokers with exacerbations despite appropriate therapy, consider azithromycin (B, GOLD; Strong, NICE).
CRITIQUE
GOLD is an International committee of experts who compile the report based on scientific literature review. NICE is an independent organization funded by Department of Health and Social Care in the United Kingdom responsible for evidence-based guidance on healthcare determined by an expert committee through scientific review and a transparent process that details committee formation and framework (GRADE) used and stakeholder input. While both guidelines review current publications, practice-influencing clinical trials of recent publication may be missed.
On the GOLD Science committee, 17/20 members have pharmaceutical relationships, with no mitigation plan provided. The NICE guidelines detail a panel with few industry ties and a mitigation plan for potential conflicts of interest.
These recommendations comprehensively cover outpatient and inpatient COPD management. The GOLD and NICE guidelines are similar with the exception of recommendations surrounding use of oxygen. The NICE guidelines, based on the adverse events documented in the recent Long-Term Oxygen Treatment Trial,6 recommend against oxygen use by patients who smoke because of the risk of fire-related injuries;7 GOLD guidelines do not differentiate oxygen recommendation by patient population.
Differences in the strength of NICE and GOLD recommendations highlight areas for further study. Investigations determining distinct COPD phenotypes will likely influence future guidelines. More discriminative multidimensional prognostication tools are needed to improve precision surrounding prognosis.
Chronic obstructive pulmonary disease (COPD), projected to be the third leading cause of death by 2020, accounts for 6% of deaths globally.3 Hospitalization for COPD exacerbations is common and impacts patients’ disease trajectory, and mortality, with fewer than half of patients hospitalized for exacerbation surviving 5 years.4 Hospitalization provides an opportunity to optimize care. Due to recent practice-changing evidence, the National Institute for Health and Care Excellence (NICE) and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) published updated guidelines.
KEY RECOMMENDATIONS
These are selected recommendations relevant to adult hospitalists. The GOLD guidelines grade recommendations by evidence strength from category A (randomized control trial data) to category D (expert consensus). The NICE guidelines relay strength of evidence through terminology referring to the presence or absence of a strong recommendation. Recommendations without evidence level specified are NS.
Diagnosis and Classification of COPD Severity
Recommendation 1. In patients with risk factors for and symptoms of COPD, spirometry is required to confirm the diagnosis, defined as a postbronchodilator FEV1/FVC ratio of <0.7 (NS, NICE, GOLD). The Global Lung Function Initiative (GLI) 2012 reference ranges5 are recommended (NS, NICE). Recommendation 2. Severity of airflow obstruction should be assessed according to reduction in the postbronchodilator FEV1 as: Stage I, Mild: FEV1 ≥80%; Stage II, Moderate: FEV1 = 50-79%; Stage III, Severe FEV1 = 30%-49%; Stage IV, FEV1<30% (NS, NICE, GOLD). Recommendation 3. Reversibility testing (aka bronchodilator response) does not indicate long-term response to therapy (NS, NICE, GOLD). Recommendation 4. The combined COPD assessment to classify patient symptoms and disease severity in one of four groups (A, B, C, or D) based on exacerbation history and daily symptom control (NS, GOLD). Use the Medical Research Council dyspnea scale to classify symptoms (strong, NICE).
Pharmacologic COPD Management
Recommendation 5. Short-acting inhaled bronchodilators such as short-acting beta2 agonists (SABAs) or short-acting muscarinic antagonists (SAMAs) improve FEV1 and symptoms. Combining SABA/SAMA is superior to monotherapy (A, GOLD). Recommendation 6. Long-acting bronchodilators, such as long-acting antimuscarinics (LAMAs) or long-acting beta2 agonists (LABAs), improve lung function and dyspnea and reduce exacerbations. Combination therapy (LABA/LAMA) is superior to using a single agent (LABA or LAMA) for improving FEV1 and reducing exacerbations (A, GOLD). Recommendation 7. Triple therapy of inhaled corticosteroid ICS/LAMA/LABA is more effective than the individual components in reducing exacerbations in the case of moderate to severe COPD (A, GOLD). Recommendation 8. Treatment with an ICS increases pneumonia risk (A, GOLD). Discuss these side effects (Strong, NICE). Recommendation 9. Use SABAs and SAMAs as initial treatment for patients with COPD (Strong, NICE). LABAs and LAMAs are preferred over short-acting agents except for patients with mild symptoms (A, GOLD). Recommendation 10. For symptomatic patients on long-acting monotherapy, escalate to combination LABA/LAMA, or if asthmatic features or elevated eosinophils (≥300 cells/µL) are present, combination LABA/ICS (A, GOLD; Strong, NICE). Recommendation 11. Assess and correct patient inhaler technique (NS, GOLD; Strong, NICE).
Nonpharmacologic COPD Management
Oxygen. Recommendation 12. Long-term oxygen supplementation increases survival in patients with resting arterial hypoxemia (PaO2<55 mm Hg) or hypoxemia (PaO2<60 mm Hg) with cor pulmonale (A, GOLD). Recommendation 13. In patients with moderate resting (89%-93%) or exercise-induced arterial desaturation (80%-90%), long-term oxygen does not improve outcomes (A, GOLD).6 Recommendation 14. Consider long-term oxygan after a risk assessment of fall and burn risk. Do not offer oxygen to those who continue to smoke (Strong, NICE).
Tobacco
Pulmonary Rehabilitation. Recommendation 17. Provide rehabilitation to patients with high exacerbation risk and relevant symptoms (A, GOLD). Offer pulmonary rehabilitation to patients with recent hospitalizations and/or severe dyspnea (Strong, NICE).
Immunizations. Recommendation 18. Influenza and pneumococcal vaccinations (PPSV23 as well as PCV13 when age ≥ 65 years) are recommended for patients with COPD (NS, GOLD; Strong, NICE).
Palliative Care. Recommendation 19. For patients with end-stage COPD or poorly controlled symptoms, provide access to palliative care (NS, GOLD; Strong, NICE).
Management of COPD Exacerbations and Patients at high risk for Exacerbations
Recommendation 20. Use SABAs with or without SAMAs as initial bronchodilators to treat acute exacerbations (C, GOLD). Recommendation 21. Systemic corticosteroids for exacerbations improve lung function, oxygenation, and recovery time. Recommend 5 to 7 days of therapy (A, GOLD; Strong, NICE). Recommendation 22. Antibiotics shorten recovery time and reduce treatment failure and rehospitalization. Treatment should be 5 to 7 days (B, GOLD). Consider antibiotics while balancing the severity of symptoms and hospitalization need (Conditional, NICE). Recommendation 23. Noninvasive mechanical ventilation is the preferred mode of ventilation for COPD patients with acute respiratory failure without acute contraindications (A, GOLD). Recommendation 24. Avoid long-term oral corticosteroids therapy (A, GOLD). Recommendation 25. Consider roflumilast for patients with exacerbations despite LABA/ICS or LABA/LAMA/ICS, and seek respiratory medicine consultation (B, GOLD; Strong, NICE). For former smokers with exacerbations despite appropriate therapy, consider azithromycin (B, GOLD; Strong, NICE).
CRITIQUE
GOLD is an International committee of experts who compile the report based on scientific literature review. NICE is an independent organization funded by Department of Health and Social Care in the United Kingdom responsible for evidence-based guidance on healthcare determined by an expert committee through scientific review and a transparent process that details committee formation and framework (GRADE) used and stakeholder input. While both guidelines review current publications, practice-influencing clinical trials of recent publication may be missed.
On the GOLD Science committee, 17/20 members have pharmaceutical relationships, with no mitigation plan provided. The NICE guidelines detail a panel with few industry ties and a mitigation plan for potential conflicts of interest.
These recommendations comprehensively cover outpatient and inpatient COPD management. The GOLD and NICE guidelines are similar with the exception of recommendations surrounding use of oxygen. The NICE guidelines, based on the adverse events documented in the recent Long-Term Oxygen Treatment Trial,6 recommend against oxygen use by patients who smoke because of the risk of fire-related injuries;7 GOLD guidelines do not differentiate oxygen recommendation by patient population.
Differences in the strength of NICE and GOLD recommendations highlight areas for further study. Investigations determining distinct COPD phenotypes will likely influence future guidelines. More discriminative multidimensional prognostication tools are needed to improve precision surrounding prognosis.
1. NICE. Overview. Chronic obstructive pulmonary disease in over 16s: Diagnosis and management, Guidance. https://www.nice.org.uk/guidance/ng115. Accessed November 21, 2019
2. GOLD Reports for Personal Use. Global Initiative for Chronic Obstructive Lung Disease - GOLD. https://goldcopd.org/gold-reports/. Accessed September 17, 2019.
3. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095-128. https://doi.org/10.1016/S0140-6736(12)61728-0.
4. Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: Severe exacerbations and mortality. Thorax. 2012;67(11):957-63. https://doi.org/10.1136/thoraxjnl-2011-201518.
5. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: The global lung function 2012 equations. Eur Respir J. 2012;40(6):1324-43. https://doi.org/10.1183/09031936.00080312.
6. Albert RK, Au DH, Blackford AL, et al. Long-term oxygen treatment trial research group. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016;375(17):1617-27. https://doi.org/10.1056/NEJMoa1604344.
7. National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management [B} Oxygen therapy in people with stable COPD. https://www.nice.org.uk/guidance/ng115/evidence/b-oxygen-therapy-in-people-with-stable-copd-pdf-6602768751. Accessed November 21, 2019.
1. NICE. Overview. Chronic obstructive pulmonary disease in over 16s: Diagnosis and management, Guidance. https://www.nice.org.uk/guidance/ng115. Accessed November 21, 2019
2. GOLD Reports for Personal Use. Global Initiative for Chronic Obstructive Lung Disease - GOLD. https://goldcopd.org/gold-reports/. Accessed September 17, 2019.
3. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095-128. https://doi.org/10.1016/S0140-6736(12)61728-0.
4. Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: Severe exacerbations and mortality. Thorax. 2012;67(11):957-63. https://doi.org/10.1136/thoraxjnl-2011-201518.
5. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: The global lung function 2012 equations. Eur Respir J. 2012;40(6):1324-43. https://doi.org/10.1183/09031936.00080312.
6. Albert RK, Au DH, Blackford AL, et al. Long-term oxygen treatment trial research group. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016;375(17):1617-27. https://doi.org/10.1056/NEJMoa1604344.
7. National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management [B} Oxygen therapy in people with stable COPD. https://www.nice.org.uk/guidance/ng115/evidence/b-oxygen-therapy-in-people-with-stable-copd-pdf-6602768751. Accessed November 21, 2019.
© 2020 Society of Hospital Medicine
Clinical Guideline Highlights for the Hospitalist: Diagnosis and Management of Measles
Measles is a highly contagious acute respiratory illness that can cause complications in multiple organ systems. Measles was declared eliminated in the United States in 2000; however, outbreaks still occur, especially in unvaccinated populations. The Centers for Disease Control and Prevention (CDC) reported that as of October 3, 2019, 1,250 cases of measles had been confirmed in 31 states in 2019, which represents the greatest number of cases reported in the US since 1992.1 Although the disease is often self-limited, infected individuals can also develop complications requiring hospitalization, which occurred in 10% of confirmed cases this year.1 In February 2018, the CDC updated their recommendations about measles diagnosis and treatment on their website,2 adding an interim update in July 2019 to include new guidelines about infection control and prevention.3 This highlight reviews those recommendations most relevant to hospitalists, who can play a critical role in the diagnosis and management of patients with suspected and/or confirmed measles.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1. Healthcare providers should consider measles in patients presenting with febrile rash illness and clinically compatible measles symptoms, especially if the person recently traveled internationally or was exposed to a person with febrile rash illness. Healthcare providers should report suspected measles cases to their local health department within 24 hours.
Measles is an acute febrile illness that begins with a prodrome of fever, followed by one or more of the following three “C’s”: cough, coryza (rhinitis), and conjunctivitis. Koplik spots, a pathognomonic buccal enanthem consisting of white lesions on an erythematous base, can appear shortly thereafter. An erythematous, maculopapular rash develops three to four days after the onset of the fever. The rash starts on the face and then spreads over the next few days to the trunk and extremities. Clinical recovery generally occurs within one week of rash onset in uncomplicated measles. Complications can affect almost any organ system. The most common complications are pneumonia, often caused by secondary viral or bacterial pathogens, diarrhea, otitis media, and laryngotracheobronchitis. Rare but serious complications include acute encephalitis and subacute sclerosing panencephalitis. Groups at the highest risk for serious disease include children aged <5 years, adults aged >20 years, pregnant women, and immunocompromised individuals.
When encountering patients with a febrile rash and compatible symptoms, clinicians should also have a high index of suspicion for measles in patients who are unvaccinated or undervaccinated, since the majority of measles cases have occurred in the unvaccinated population. Providers should contact their local health department and infectious diseases/infection control team as soon as suspected measles cases are identified. Laboratory confirmation is necessary for all suspected cases and should typically consist of measles IgM antibody testing from serum and real-time polymerase chain reaction (RT-PCR) from respiratory and urine specimens.
Recommendation 2. Adhere to airborne precautions for anyone with known or suspected measles.
Measles is highly contagious, and infectious particles can remain in the air for up to two hours after a person with measles leaves a room. From 2001 to 2014, 6% (78/1,318) of nonimported measles cases in the US were transmitted in healthcare settings.4 Key steps in preventing the spread of measles within hospitals include ensuring that all healthcare personnel have evidence of immunity to measles and rapid identification and isolation of suspect cases. Patients with suspected measles should be given a facemask and moved immediately into a single-patient airborne infection isolation room. Personnel, even those with presumptive evidence of immunity, should use N95 respirators or the equivalent when caring for patients with suspected or confirmed measles. Patients with measles are contagious from four days before to four days after rash onset; therefore, airborne precautions should be continued for four days following the onset of rash in immunocompetent patients. For immunocompromised patients, airborne precautions should be continued for the duration of the illness based on data suggesting prolonged shedding, particularly in the setting of altered T-cell immunity.4
Recommendation 3. People exposed to measles who cannot readily show that they have evidence of immunity against measles should be offered postexposure prophylaxis (PEP) or be excluded from the setting (school, hospital, childcare). To potentially provide protection or modify the clinical course of disease among susceptible persons, either administer a measles, mumps, and rubella (MMR) vaccine within 72 hours of initial measles exposure or immunoglobulin (IG) within six days of exposure.
MMR vaccine is recommended for vaccine-eligible, exposed individuals aged ≥6 months within 72 hours of measles exposure. IG, which contains measles antibody due to widespread immunization in the US, is recommended for individuals at high risk for serious illness, including infants aged ≤12 months, pregnant women without evidence of measles immunity, and severely immunocompromised patients regardless of vaccination status. For infants aged 6-11 months, MMR vaccine can be given in place of IG if done within 72 hours of exposure. PEP for children during the 2013 New York City outbreak reduced the risk of measles by 83.4% (95% CI: 34.4%-95.8%) in recipients of MMR vaccine and by 100% (95% CI: 56.2%-99.8%) in recipients of IG compared with those without prophylaxis.5 A 2014 Cochrane Review found that IG reduced the risk of measles by 83% (95% CI: 64%-92%).6
Recommendation 4. Severe measles cases among children, such as those who are hospitalized, should be treated with vitamin A. Vitamin A should be administered immediately on diagnosis and repeated the next day.
In children, vitamin A deficiency, even if clinically inapparent, leads to increased measles severity, and randomized controlled trial data suggest that supplementation reduces measles-related morbidity and mortality.4 Even in high-income countries, children with measles have high rates of vitamin A deficiency, which is associated with increased morbidity.7 A Cochrane review found that two-dose regimens of vitamin A reduced the overall mortality (RR 0.21; 95% CI: 0.07-0.66) in children with measles aged <2 years.8 World Health Organization guidelines suggest vitamin A therapy for all children with acute measles infection, and the AAP Committee on Infectious Diseases recommends vitamin A for severe (ie, hospitalized) cases. Vitamin A is given orally once daily for two days at the following doses: 50,000 international units (IU) for infants aged <6 months, 100,000 IU for infants aged 6-11 months, and 200,000 IU for children aged ≥12 months. A third dose can be given two to four weeks later for children with signs and symptoms of vitamin A deficiency (eg, corneal clouding or conjunctival plaques).
CRITIQUE
In outbreak settings, hospitalists may find challenges with having a sufficient number of single negative-pressure rooms for patients with suspected or confirmed measles and providing IG prophylaxis given the recent national shortages of intravenous immunoglobulin. Collaboration with the infection control team, pharmacy, and the local public health department is essential to appropriately address these challenges. With regard to treatment recommendations, randomized studies on the impact of vitamin A treatment in children have been primarily conducted in resource-limited settings.8 However, these data, in combination with observational data from resource-rich settings,7 support its use given the favorable risk-benefit profile. The role of vitamin A therapy in adults with measles infection is considerably less clear, although there are reports of its use in severe cases.
AREAS OF FUTURE STUDY
Much of our knowledge regarding measles complications and treatment outcomes comes from resource-limited settings or from older data before widespread vaccination. Data suggest that prophylactic antibiotics may prevent complications; however, currently available data are insufficient to support routine use.9 Coordination and collaboration between public health, infectious diseases, and hospital medicine would enhance the ability to conduct detailed epidemiologic studies during outbreak situations. Further studies examining treatment and outcomes in hospitalized patients, including the role of prophylactic antibiotics in the prevention of complications, would provide valuable guidance for hospitalists caring for patients with severe measles.
1. Centers for Disease Control and Prevention. Measles Cases and Outbreaks. 2019; https://www.cdc.gov/measles/cases-outbreaks.html. Accessed October 14, 2019.
2. Centers for Disease Control and Prevention. Measles (Rubeola): For Healthcare Professionals. 2019; https://www.cdc.gov/measles/hcp/index.html. Accessed October 14, 2019.
3. Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Measles in Healthcare Settings. 2019.
4. Fiebelkorn AP, Redd SB, Kuhar DT. Measles in healthcare facilities in the United States during the postelimination era, 2001-2014. Clin Infect Dis. 2015;61(4):615-618. https://doi.org/10.1093/cid/civ387.
5. Arciuolo RJ, Jablonski RR, Zucker JR, Rosen JB. Effectiveness of measles vaccination and immune globulin post-exposure prophylaxis in an outbreak setting-New York City, 2013. Clin Infect Dis. 2017;65(11):1843-1847. https://doi.org/10.1093/cid/cix639.
6. Young MK, Nimmo GR, Cripps AW, Jones MA. Post-exposure passive immunisation for preventing measles. Cochrane Database Syst Rev. 2014;(4):Cd010056. https://doi.org/10.1002/14651858.CD010056.pub2.
7. Frieden TR, Sowell AL, Henning KJ, Huff DL, Gunn RA. Vitamin A levels and severity of measles. New York City. Am J Dis Child. 1992;146(2):182-186. https://doi.org/10.1001/archpedi.1992.02160140048019.
8. Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database Syst Rev. 2005(4):Cd001479. https://doi.org/10.1002/14651858.CD001479.pub3.
9. Kabra SK, Lodha R. Antibiotics for preventing complications in children with measles. Cochrane Database Syst Rev. 2013(8):Cd001477. https://doi.org/10.1002/14651858.CD001477.pub3.
Measles is a highly contagious acute respiratory illness that can cause complications in multiple organ systems. Measles was declared eliminated in the United States in 2000; however, outbreaks still occur, especially in unvaccinated populations. The Centers for Disease Control and Prevention (CDC) reported that as of October 3, 2019, 1,250 cases of measles had been confirmed in 31 states in 2019, which represents the greatest number of cases reported in the US since 1992.1 Although the disease is often self-limited, infected individuals can also develop complications requiring hospitalization, which occurred in 10% of confirmed cases this year.1 In February 2018, the CDC updated their recommendations about measles diagnosis and treatment on their website,2 adding an interim update in July 2019 to include new guidelines about infection control and prevention.3 This highlight reviews those recommendations most relevant to hospitalists, who can play a critical role in the diagnosis and management of patients with suspected and/or confirmed measles.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1. Healthcare providers should consider measles in patients presenting with febrile rash illness and clinically compatible measles symptoms, especially if the person recently traveled internationally or was exposed to a person with febrile rash illness. Healthcare providers should report suspected measles cases to their local health department within 24 hours.
Measles is an acute febrile illness that begins with a prodrome of fever, followed by one or more of the following three “C’s”: cough, coryza (rhinitis), and conjunctivitis. Koplik spots, a pathognomonic buccal enanthem consisting of white lesions on an erythematous base, can appear shortly thereafter. An erythematous, maculopapular rash develops three to four days after the onset of the fever. The rash starts on the face and then spreads over the next few days to the trunk and extremities. Clinical recovery generally occurs within one week of rash onset in uncomplicated measles. Complications can affect almost any organ system. The most common complications are pneumonia, often caused by secondary viral or bacterial pathogens, diarrhea, otitis media, and laryngotracheobronchitis. Rare but serious complications include acute encephalitis and subacute sclerosing panencephalitis. Groups at the highest risk for serious disease include children aged <5 years, adults aged >20 years, pregnant women, and immunocompromised individuals.
When encountering patients with a febrile rash and compatible symptoms, clinicians should also have a high index of suspicion for measles in patients who are unvaccinated or undervaccinated, since the majority of measles cases have occurred in the unvaccinated population. Providers should contact their local health department and infectious diseases/infection control team as soon as suspected measles cases are identified. Laboratory confirmation is necessary for all suspected cases and should typically consist of measles IgM antibody testing from serum and real-time polymerase chain reaction (RT-PCR) from respiratory and urine specimens.
Recommendation 2. Adhere to airborne precautions for anyone with known or suspected measles.
Measles is highly contagious, and infectious particles can remain in the air for up to two hours after a person with measles leaves a room. From 2001 to 2014, 6% (78/1,318) of nonimported measles cases in the US were transmitted in healthcare settings.4 Key steps in preventing the spread of measles within hospitals include ensuring that all healthcare personnel have evidence of immunity to measles and rapid identification and isolation of suspect cases. Patients with suspected measles should be given a facemask and moved immediately into a single-patient airborne infection isolation room. Personnel, even those with presumptive evidence of immunity, should use N95 respirators or the equivalent when caring for patients with suspected or confirmed measles. Patients with measles are contagious from four days before to four days after rash onset; therefore, airborne precautions should be continued for four days following the onset of rash in immunocompetent patients. For immunocompromised patients, airborne precautions should be continued for the duration of the illness based on data suggesting prolonged shedding, particularly in the setting of altered T-cell immunity.4
Recommendation 3. People exposed to measles who cannot readily show that they have evidence of immunity against measles should be offered postexposure prophylaxis (PEP) or be excluded from the setting (school, hospital, childcare). To potentially provide protection or modify the clinical course of disease among susceptible persons, either administer a measles, mumps, and rubella (MMR) vaccine within 72 hours of initial measles exposure or immunoglobulin (IG) within six days of exposure.
MMR vaccine is recommended for vaccine-eligible, exposed individuals aged ≥6 months within 72 hours of measles exposure. IG, which contains measles antibody due to widespread immunization in the US, is recommended for individuals at high risk for serious illness, including infants aged ≤12 months, pregnant women without evidence of measles immunity, and severely immunocompromised patients regardless of vaccination status. For infants aged 6-11 months, MMR vaccine can be given in place of IG if done within 72 hours of exposure. PEP for children during the 2013 New York City outbreak reduced the risk of measles by 83.4% (95% CI: 34.4%-95.8%) in recipients of MMR vaccine and by 100% (95% CI: 56.2%-99.8%) in recipients of IG compared with those without prophylaxis.5 A 2014 Cochrane Review found that IG reduced the risk of measles by 83% (95% CI: 64%-92%).6
Recommendation 4. Severe measles cases among children, such as those who are hospitalized, should be treated with vitamin A. Vitamin A should be administered immediately on diagnosis and repeated the next day.
In children, vitamin A deficiency, even if clinically inapparent, leads to increased measles severity, and randomized controlled trial data suggest that supplementation reduces measles-related morbidity and mortality.4 Even in high-income countries, children with measles have high rates of vitamin A deficiency, which is associated with increased morbidity.7 A Cochrane review found that two-dose regimens of vitamin A reduced the overall mortality (RR 0.21; 95% CI: 0.07-0.66) in children with measles aged <2 years.8 World Health Organization guidelines suggest vitamin A therapy for all children with acute measles infection, and the AAP Committee on Infectious Diseases recommends vitamin A for severe (ie, hospitalized) cases. Vitamin A is given orally once daily for two days at the following doses: 50,000 international units (IU) for infants aged <6 months, 100,000 IU for infants aged 6-11 months, and 200,000 IU for children aged ≥12 months. A third dose can be given two to four weeks later for children with signs and symptoms of vitamin A deficiency (eg, corneal clouding or conjunctival plaques).
CRITIQUE
In outbreak settings, hospitalists may find challenges with having a sufficient number of single negative-pressure rooms for patients with suspected or confirmed measles and providing IG prophylaxis given the recent national shortages of intravenous immunoglobulin. Collaboration with the infection control team, pharmacy, and the local public health department is essential to appropriately address these challenges. With regard to treatment recommendations, randomized studies on the impact of vitamin A treatment in children have been primarily conducted in resource-limited settings.8 However, these data, in combination with observational data from resource-rich settings,7 support its use given the favorable risk-benefit profile. The role of vitamin A therapy in adults with measles infection is considerably less clear, although there are reports of its use in severe cases.
AREAS OF FUTURE STUDY
Much of our knowledge regarding measles complications and treatment outcomes comes from resource-limited settings or from older data before widespread vaccination. Data suggest that prophylactic antibiotics may prevent complications; however, currently available data are insufficient to support routine use.9 Coordination and collaboration between public health, infectious diseases, and hospital medicine would enhance the ability to conduct detailed epidemiologic studies during outbreak situations. Further studies examining treatment and outcomes in hospitalized patients, including the role of prophylactic antibiotics in the prevention of complications, would provide valuable guidance for hospitalists caring for patients with severe measles.
Measles is a highly contagious acute respiratory illness that can cause complications in multiple organ systems. Measles was declared eliminated in the United States in 2000; however, outbreaks still occur, especially in unvaccinated populations. The Centers for Disease Control and Prevention (CDC) reported that as of October 3, 2019, 1,250 cases of measles had been confirmed in 31 states in 2019, which represents the greatest number of cases reported in the US since 1992.1 Although the disease is often self-limited, infected individuals can also develop complications requiring hospitalization, which occurred in 10% of confirmed cases this year.1 In February 2018, the CDC updated their recommendations about measles diagnosis and treatment on their website,2 adding an interim update in July 2019 to include new guidelines about infection control and prevention.3 This highlight reviews those recommendations most relevant to hospitalists, who can play a critical role in the diagnosis and management of patients with suspected and/or confirmed measles.
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Recommendation 1. Healthcare providers should consider measles in patients presenting with febrile rash illness and clinically compatible measles symptoms, especially if the person recently traveled internationally or was exposed to a person with febrile rash illness. Healthcare providers should report suspected measles cases to their local health department within 24 hours.
Measles is an acute febrile illness that begins with a prodrome of fever, followed by one or more of the following three “C’s”: cough, coryza (rhinitis), and conjunctivitis. Koplik spots, a pathognomonic buccal enanthem consisting of white lesions on an erythematous base, can appear shortly thereafter. An erythematous, maculopapular rash develops three to four days after the onset of the fever. The rash starts on the face and then spreads over the next few days to the trunk and extremities. Clinical recovery generally occurs within one week of rash onset in uncomplicated measles. Complications can affect almost any organ system. The most common complications are pneumonia, often caused by secondary viral or bacterial pathogens, diarrhea, otitis media, and laryngotracheobronchitis. Rare but serious complications include acute encephalitis and subacute sclerosing panencephalitis. Groups at the highest risk for serious disease include children aged <5 years, adults aged >20 years, pregnant women, and immunocompromised individuals.
When encountering patients with a febrile rash and compatible symptoms, clinicians should also have a high index of suspicion for measles in patients who are unvaccinated or undervaccinated, since the majority of measles cases have occurred in the unvaccinated population. Providers should contact their local health department and infectious diseases/infection control team as soon as suspected measles cases are identified. Laboratory confirmation is necessary for all suspected cases and should typically consist of measles IgM antibody testing from serum and real-time polymerase chain reaction (RT-PCR) from respiratory and urine specimens.
Recommendation 2. Adhere to airborne precautions for anyone with known or suspected measles.
Measles is highly contagious, and infectious particles can remain in the air for up to two hours after a person with measles leaves a room. From 2001 to 2014, 6% (78/1,318) of nonimported measles cases in the US were transmitted in healthcare settings.4 Key steps in preventing the spread of measles within hospitals include ensuring that all healthcare personnel have evidence of immunity to measles and rapid identification and isolation of suspect cases. Patients with suspected measles should be given a facemask and moved immediately into a single-patient airborne infection isolation room. Personnel, even those with presumptive evidence of immunity, should use N95 respirators or the equivalent when caring for patients with suspected or confirmed measles. Patients with measles are contagious from four days before to four days after rash onset; therefore, airborne precautions should be continued for four days following the onset of rash in immunocompetent patients. For immunocompromised patients, airborne precautions should be continued for the duration of the illness based on data suggesting prolonged shedding, particularly in the setting of altered T-cell immunity.4
Recommendation 3. People exposed to measles who cannot readily show that they have evidence of immunity against measles should be offered postexposure prophylaxis (PEP) or be excluded from the setting (school, hospital, childcare). To potentially provide protection or modify the clinical course of disease among susceptible persons, either administer a measles, mumps, and rubella (MMR) vaccine within 72 hours of initial measles exposure or immunoglobulin (IG) within six days of exposure.
MMR vaccine is recommended for vaccine-eligible, exposed individuals aged ≥6 months within 72 hours of measles exposure. IG, which contains measles antibody due to widespread immunization in the US, is recommended for individuals at high risk for serious illness, including infants aged ≤12 months, pregnant women without evidence of measles immunity, and severely immunocompromised patients regardless of vaccination status. For infants aged 6-11 months, MMR vaccine can be given in place of IG if done within 72 hours of exposure. PEP for children during the 2013 New York City outbreak reduced the risk of measles by 83.4% (95% CI: 34.4%-95.8%) in recipients of MMR vaccine and by 100% (95% CI: 56.2%-99.8%) in recipients of IG compared with those without prophylaxis.5 A 2014 Cochrane Review found that IG reduced the risk of measles by 83% (95% CI: 64%-92%).6
Recommendation 4. Severe measles cases among children, such as those who are hospitalized, should be treated with vitamin A. Vitamin A should be administered immediately on diagnosis and repeated the next day.
In children, vitamin A deficiency, even if clinically inapparent, leads to increased measles severity, and randomized controlled trial data suggest that supplementation reduces measles-related morbidity and mortality.4 Even in high-income countries, children with measles have high rates of vitamin A deficiency, which is associated with increased morbidity.7 A Cochrane review found that two-dose regimens of vitamin A reduced the overall mortality (RR 0.21; 95% CI: 0.07-0.66) in children with measles aged <2 years.8 World Health Organization guidelines suggest vitamin A therapy for all children with acute measles infection, and the AAP Committee on Infectious Diseases recommends vitamin A for severe (ie, hospitalized) cases. Vitamin A is given orally once daily for two days at the following doses: 50,000 international units (IU) for infants aged <6 months, 100,000 IU for infants aged 6-11 months, and 200,000 IU for children aged ≥12 months. A third dose can be given two to four weeks later for children with signs and symptoms of vitamin A deficiency (eg, corneal clouding or conjunctival plaques).
CRITIQUE
In outbreak settings, hospitalists may find challenges with having a sufficient number of single negative-pressure rooms for patients with suspected or confirmed measles and providing IG prophylaxis given the recent national shortages of intravenous immunoglobulin. Collaboration with the infection control team, pharmacy, and the local public health department is essential to appropriately address these challenges. With regard to treatment recommendations, randomized studies on the impact of vitamin A treatment in children have been primarily conducted in resource-limited settings.8 However, these data, in combination with observational data from resource-rich settings,7 support its use given the favorable risk-benefit profile. The role of vitamin A therapy in adults with measles infection is considerably less clear, although there are reports of its use in severe cases.
AREAS OF FUTURE STUDY
Much of our knowledge regarding measles complications and treatment outcomes comes from resource-limited settings or from older data before widespread vaccination. Data suggest that prophylactic antibiotics may prevent complications; however, currently available data are insufficient to support routine use.9 Coordination and collaboration between public health, infectious diseases, and hospital medicine would enhance the ability to conduct detailed epidemiologic studies during outbreak situations. Further studies examining treatment and outcomes in hospitalized patients, including the role of prophylactic antibiotics in the prevention of complications, would provide valuable guidance for hospitalists caring for patients with severe measles.
1. Centers for Disease Control and Prevention. Measles Cases and Outbreaks. 2019; https://www.cdc.gov/measles/cases-outbreaks.html. Accessed October 14, 2019.
2. Centers for Disease Control and Prevention. Measles (Rubeola): For Healthcare Professionals. 2019; https://www.cdc.gov/measles/hcp/index.html. Accessed October 14, 2019.
3. Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Measles in Healthcare Settings. 2019.
4. Fiebelkorn AP, Redd SB, Kuhar DT. Measles in healthcare facilities in the United States during the postelimination era, 2001-2014. Clin Infect Dis. 2015;61(4):615-618. https://doi.org/10.1093/cid/civ387.
5. Arciuolo RJ, Jablonski RR, Zucker JR, Rosen JB. Effectiveness of measles vaccination and immune globulin post-exposure prophylaxis in an outbreak setting-New York City, 2013. Clin Infect Dis. 2017;65(11):1843-1847. https://doi.org/10.1093/cid/cix639.
6. Young MK, Nimmo GR, Cripps AW, Jones MA. Post-exposure passive immunisation for preventing measles. Cochrane Database Syst Rev. 2014;(4):Cd010056. https://doi.org/10.1002/14651858.CD010056.pub2.
7. Frieden TR, Sowell AL, Henning KJ, Huff DL, Gunn RA. Vitamin A levels and severity of measles. New York City. Am J Dis Child. 1992;146(2):182-186. https://doi.org/10.1001/archpedi.1992.02160140048019.
8. Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database Syst Rev. 2005(4):Cd001479. https://doi.org/10.1002/14651858.CD001479.pub3.
9. Kabra SK, Lodha R. Antibiotics for preventing complications in children with measles. Cochrane Database Syst Rev. 2013(8):Cd001477. https://doi.org/10.1002/14651858.CD001477.pub3.
1. Centers for Disease Control and Prevention. Measles Cases and Outbreaks. 2019; https://www.cdc.gov/measles/cases-outbreaks.html. Accessed October 14, 2019.
2. Centers for Disease Control and Prevention. Measles (Rubeola): For Healthcare Professionals. 2019; https://www.cdc.gov/measles/hcp/index.html. Accessed October 14, 2019.
3. Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Measles in Healthcare Settings. 2019.
4. Fiebelkorn AP, Redd SB, Kuhar DT. Measles in healthcare facilities in the United States during the postelimination era, 2001-2014. Clin Infect Dis. 2015;61(4):615-618. https://doi.org/10.1093/cid/civ387.
5. Arciuolo RJ, Jablonski RR, Zucker JR, Rosen JB. Effectiveness of measles vaccination and immune globulin post-exposure prophylaxis in an outbreak setting-New York City, 2013. Clin Infect Dis. 2017;65(11):1843-1847. https://doi.org/10.1093/cid/cix639.
6. Young MK, Nimmo GR, Cripps AW, Jones MA. Post-exposure passive immunisation for preventing measles. Cochrane Database Syst Rev. 2014;(4):Cd010056. https://doi.org/10.1002/14651858.CD010056.pub2.
7. Frieden TR, Sowell AL, Henning KJ, Huff DL, Gunn RA. Vitamin A levels and severity of measles. New York City. Am J Dis Child. 1992;146(2):182-186. https://doi.org/10.1001/archpedi.1992.02160140048019.
8. Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database Syst Rev. 2005(4):Cd001479. https://doi.org/10.1002/14651858.CD001479.pub3.
9. Kabra SK, Lodha R. Antibiotics for preventing complications in children with measles. Cochrane Database Syst Rev. 2013(8):Cd001477. https://doi.org/10.1002/14651858.CD001477.pub3.
© 2020 Society of Hospital Medicine
Clinical Guideline Highlights for the Hospitalist: Initial Management of Acute Pancreatitis in the Hospitalized Adult
Acute pancreatitis (AP) is the most common gastrointestinal discharge diagnosis in the United States, with a mortality rate of 1%-5%.1 Recent data demonstrate increasing AP-related admissions, making AP management of utmost importance to hospitalists.1 The American Gastroenterological Association (AGA) guideline specifically addresses AP management in the initial 48-72 hours of admission, during which management decisions can alter disease course and length of stay. AP requires two of the following three criteria for diagnosis: characteristic abdominal pain, elevation of lipase or amylase ≥3 times the upper limit of normal, and/or radiographic evidence of pancreatitis on cross-sectional imaging. The guideline provides eight recommendations, which we consolidated to highlight practice changing recommendations: fluids, nutrition, management of the most common causes, and prophylactic antibiotics.2,3
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Fluids
Recommendation 1. In patients with AP, use goal-directed isotonic crystalloids for fluid management (conditional recommendation, very low-quality evidence).
The guideline emphasizes goal-directed fluid management despite low-quality, heterogeneous evidence and does not recommend Ringer’s lactate over normal saline. “Goal-directed” fluid management involves the use of crystalloid infusions titrated to improve physiologic and biochemical markers, but no target volume is specified by the guideline. Frequent reassessments should look for signs of volume overload, the primary risk of harm with fluid therapy. Despite failure to reduce mortality or morbidities such as pancreatic necrosis or persistent multi-organ failure, the AGA cites the mortality benefit of goal-directed therapy in sepsis as justification for this approach in AP, given the similar physiologic abnormalities.
Nutrition
Recommendation 2. Begin feeding early in patients with AP regardless of predicted severity. If oral nutrition is not tolerated, enteral feeding with either a nasogastric or nasojejunal tube is preferred to parenteral nutrition (strong recommendation, moderate-quality evidence).
Early feeding (ie, within 24 hours) is recommended regardless of AP severity. This represents a change from prior practices of bowel rest, theorized to prevent continued stimulation of an inflamed pancreas. Although early feeding has not been linked to improved mortality, it has demonstrated lower rates of multi-organ failure and infected pancreatic necrosis, possibly due to maintenance of the gut mucosal barrier and reduced bacterial translocation. When oral feeding is not tolerated, enteral nutrition is preferred over parenteral nutrition due to less risks. The preferred dietary composition guidance for patients with persistent pain or ileus is not addressed.
Management of the Most Common Causes of AP in Adults
Recommendation 3. Patients with mild acute biliary pancreatitis should have cholecystectomy during the initial admission (strong recommendation, moderate-quality evidence).
All patients with suspected biliary pancreatitis should receive a surgical consultation for cholecystectomy during the index admission. At the time of the guideline release, only one trial was available to support the recommendation of early cholecystectomy; however, newer studies similarly support cholecystectomy during index admission by demonstrating reductions in composite outcomes of mortality and gallstone-related complications, readmission for pancreatitis, and other pancreatobiliary complications.4 A Cochrane review included in the guideline found no differences in complication rates even in patients with severe biliary pancreatitis. In the absence of cholangitis, urgent endoscopic retrograde cholangiography (ERCP) is not indicated as most stones causing biliary pancreatitis pass spontaneously.
Recommendation 4. In patients with acute alcoholic pancreatitis, brief alcohol intervention should occur during admission (strong recommendation, moderate-quality evidence).
Ongoing alcohol consumption is a risk factor for recurrent acute and chronic pancreatitis. Only one trial assessed the impact of inpatient alcohol cessation counseling on recurrent AP, noting a trend toward reduced readmissions.5 However, indirect evidence from similar interventions in ambulatory settings demonstrates reductions in alcohol intake, leading to the AGA recommendation for inpatients with alcohol-induced AP.3
Antibiotics
Recommendation 5. Avoid empiric antibiotics in patients with AP who otherwise lack an indication, regardless of predicted severity (conditional recommendation, low-quality evidence).
Since 2002, well performed trials have consistently failed to demonstrate improvement in outcomes such as multi-organ failure or length of stay with use of prophylactic antibiotics for AP, even severe AP and pancreatic necrosis. Therefore, the AGA recommends against prophylactic antibiotics in initial management of AP regardless of disease severity. Lack of blinding in the majority of trial designs conducted before 2002 contributed to the overall assessment of low-quality evidence. The guideline does not address acute biliary pancreatitis with cholangitis, for which antibiotics and ERCP for decompression are critical.
CRITIQUE
The AGA Institute supported this guideline development and employed the rigorous and standardized GRADE (Grading of Recommendations Assessment, Development and Evaluation) methodology. This approach allowed the guideline panel members to account not only for evidence quality, but also the benefits and harms of an intervention and resource utilization. None of the authors had any stated conflicts of interest.
The guideline heavily weighted results from randomized control trials, most of which excluded key populations cared for by hospitalists (eg, patients older than 75 years, with end-stage renal disease). Particular areas where this creates challenges for clinicians and patients alike include goal-directed fluid therapy and when to consider more invasive interventions such as ERCP and early cholecystectomy. For example, patients considered to be poor surgical candidates may benefit from ERCP with biliary sphincterotomy to reduce the risk of recurrent biliary pancreatitis.
Lack of specificity in the guidelines for goal-directed fluid management and enteral feeding regimens makes it challenging to standardize hospitalists’ approach to the early care of patients with AP. Interestingly, the 2013 American College of Gastroenterology (ACG) Guideline for the Management of AP included strong recommendations for the use of Ringer’s lactate and volume targets in the initial management of AP.6 Evidence supporting the use of Ringer’s lactate versus normal saline is based largely upon improved inflammatory markers, theoretical potentiation of pancreatic enzyme activation with hypercholemic metabolic acidosis, and small studies demonstrating trends toward improved mortality.7 The ACG guideline was released prior to mounting evidence suggesting that goal-directed fluid therapy in sepsis does not improve mortality versus usual care.8 The growing uncertainty regarding the efficacy of goal-directed fluids for septic shock, as well limitations of studies on AP, may contribute to the differences between the AGA and ACG recommendations.
Finally, as the guideline covers the initial therapeutic management of AP, no recommendations are made for diagnostic studies such as right upper quadrant ultrasound. This noninvasive and readily available test plays a critical role in evaluating for presence of gallstones and other potential etiologies of abdominal pain.
AREAS IN NEED OF FUTURE STUDY
Additional research is needed to better understand goal-directed fluid therapy with respect to the fluid type, amount, and target outcomes. Similarly, determining the optimal enteral feeding regimens for patients failing oral intake would help clinicians meet the recommendation for early nutrition. Finally, clarification on the roles and timing of endoscopic and surgical procedures for patients with severe biliary pancreatitis, as well as geriatric and medically complex populations, would help hospitalists advocate for a multidisciplinary approach to this common and often serious disease.
Disclosures
The authors have nothing to disclose.
1. Krishna SG, Kamboj AK, Hart PA, Hinton A, Conwell DL. The changing epidemiology of acute pancreatitis hospitalizations: a decade of trends and the impact of chronic pancreatitis. Pancreas. 2017;46(4):482-488. https://doi.org/10.1097/MPA.0000000000000783.
2. Crockett SD, Wani S, Gardner TB, et al. American Gastroenterological Association Institute Guideline on initial management of acute pancreatitis. Gastroenterology. 2018;154(4):1096-1101. https://doi.org/10.1053/j.gastro.2018.01.032.
3. Vege SS, DiMagno MJ, Forsmark CE, Martel M, Barkun AN. Initial medical treatment of acute pancreatitis: American Gastroenterological Association Institute technical review. Gastroenterology. 2018;154(4):1103-1139. https://doi.org/10.1053/j.gastro.2018.01.031.
4 Noel R, Arnelo U, Lundell L, et al. Index versus delayed cholecystectomy in mild gallstone pancreatitis: results of a randomized controlled trial. HPB (Oxford). 2018;20(10):932-938. https://doi.org/10.1016/j.hpb.2018.03.016.
5. Kaner EF, Beyer F, Dickinson HO, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev. 2007:CD004148. https://doi.org/10.1002/14651858.CD004148.pub3.
6. Tenner S, Baillie J, DeWitt J, Vege SS. American College of Gastroenterology guideline: Management of acute pancreatitis. Am J Gastroenterol. 2013;108(9):1400-1415. https://doi.org/10.1038/ajg.2013.218.
7. de-Madaria E, Herrera-Marante I, González-Camacho V, et al. Fluid resuscitation with lactated Ringer’s solution vs normal saline in acute pancreatitis: a triple-blind, randomized, controlled trial. United European Gastroenterol J. 2018;6(1):63-72. https://doi.org/10.1177/2050640617707864
8. The PRISM Investigators. Early, goal-directed therapy for septic shock — a patient-level meta-analysis. New Engl J Med. 2017;376(23):2223-2234. https://doi.org/10.1056/NEJMoa1701380.
Acute pancreatitis (AP) is the most common gastrointestinal discharge diagnosis in the United States, with a mortality rate of 1%-5%.1 Recent data demonstrate increasing AP-related admissions, making AP management of utmost importance to hospitalists.1 The American Gastroenterological Association (AGA) guideline specifically addresses AP management in the initial 48-72 hours of admission, during which management decisions can alter disease course and length of stay. AP requires two of the following three criteria for diagnosis: characteristic abdominal pain, elevation of lipase or amylase ≥3 times the upper limit of normal, and/or radiographic evidence of pancreatitis on cross-sectional imaging. The guideline provides eight recommendations, which we consolidated to highlight practice changing recommendations: fluids, nutrition, management of the most common causes, and prophylactic antibiotics.2,3
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Fluids
Recommendation 1. In patients with AP, use goal-directed isotonic crystalloids for fluid management (conditional recommendation, very low-quality evidence).
The guideline emphasizes goal-directed fluid management despite low-quality, heterogeneous evidence and does not recommend Ringer’s lactate over normal saline. “Goal-directed” fluid management involves the use of crystalloid infusions titrated to improve physiologic and biochemical markers, but no target volume is specified by the guideline. Frequent reassessments should look for signs of volume overload, the primary risk of harm with fluid therapy. Despite failure to reduce mortality or morbidities such as pancreatic necrosis or persistent multi-organ failure, the AGA cites the mortality benefit of goal-directed therapy in sepsis as justification for this approach in AP, given the similar physiologic abnormalities.
Nutrition
Recommendation 2. Begin feeding early in patients with AP regardless of predicted severity. If oral nutrition is not tolerated, enteral feeding with either a nasogastric or nasojejunal tube is preferred to parenteral nutrition (strong recommendation, moderate-quality evidence).
Early feeding (ie, within 24 hours) is recommended regardless of AP severity. This represents a change from prior practices of bowel rest, theorized to prevent continued stimulation of an inflamed pancreas. Although early feeding has not been linked to improved mortality, it has demonstrated lower rates of multi-organ failure and infected pancreatic necrosis, possibly due to maintenance of the gut mucosal barrier and reduced bacterial translocation. When oral feeding is not tolerated, enteral nutrition is preferred over parenteral nutrition due to less risks. The preferred dietary composition guidance for patients with persistent pain or ileus is not addressed.
Management of the Most Common Causes of AP in Adults
Recommendation 3. Patients with mild acute biliary pancreatitis should have cholecystectomy during the initial admission (strong recommendation, moderate-quality evidence).
All patients with suspected biliary pancreatitis should receive a surgical consultation for cholecystectomy during the index admission. At the time of the guideline release, only one trial was available to support the recommendation of early cholecystectomy; however, newer studies similarly support cholecystectomy during index admission by demonstrating reductions in composite outcomes of mortality and gallstone-related complications, readmission for pancreatitis, and other pancreatobiliary complications.4 A Cochrane review included in the guideline found no differences in complication rates even in patients with severe biliary pancreatitis. In the absence of cholangitis, urgent endoscopic retrograde cholangiography (ERCP) is not indicated as most stones causing biliary pancreatitis pass spontaneously.
Recommendation 4. In patients with acute alcoholic pancreatitis, brief alcohol intervention should occur during admission (strong recommendation, moderate-quality evidence).
Ongoing alcohol consumption is a risk factor for recurrent acute and chronic pancreatitis. Only one trial assessed the impact of inpatient alcohol cessation counseling on recurrent AP, noting a trend toward reduced readmissions.5 However, indirect evidence from similar interventions in ambulatory settings demonstrates reductions in alcohol intake, leading to the AGA recommendation for inpatients with alcohol-induced AP.3
Antibiotics
Recommendation 5. Avoid empiric antibiotics in patients with AP who otherwise lack an indication, regardless of predicted severity (conditional recommendation, low-quality evidence).
Since 2002, well performed trials have consistently failed to demonstrate improvement in outcomes such as multi-organ failure or length of stay with use of prophylactic antibiotics for AP, even severe AP and pancreatic necrosis. Therefore, the AGA recommends against prophylactic antibiotics in initial management of AP regardless of disease severity. Lack of blinding in the majority of trial designs conducted before 2002 contributed to the overall assessment of low-quality evidence. The guideline does not address acute biliary pancreatitis with cholangitis, for which antibiotics and ERCP for decompression are critical.
CRITIQUE
The AGA Institute supported this guideline development and employed the rigorous and standardized GRADE (Grading of Recommendations Assessment, Development and Evaluation) methodology. This approach allowed the guideline panel members to account not only for evidence quality, but also the benefits and harms of an intervention and resource utilization. None of the authors had any stated conflicts of interest.
The guideline heavily weighted results from randomized control trials, most of which excluded key populations cared for by hospitalists (eg, patients older than 75 years, with end-stage renal disease). Particular areas where this creates challenges for clinicians and patients alike include goal-directed fluid therapy and when to consider more invasive interventions such as ERCP and early cholecystectomy. For example, patients considered to be poor surgical candidates may benefit from ERCP with biliary sphincterotomy to reduce the risk of recurrent biliary pancreatitis.
Lack of specificity in the guidelines for goal-directed fluid management and enteral feeding regimens makes it challenging to standardize hospitalists’ approach to the early care of patients with AP. Interestingly, the 2013 American College of Gastroenterology (ACG) Guideline for the Management of AP included strong recommendations for the use of Ringer’s lactate and volume targets in the initial management of AP.6 Evidence supporting the use of Ringer’s lactate versus normal saline is based largely upon improved inflammatory markers, theoretical potentiation of pancreatic enzyme activation with hypercholemic metabolic acidosis, and small studies demonstrating trends toward improved mortality.7 The ACG guideline was released prior to mounting evidence suggesting that goal-directed fluid therapy in sepsis does not improve mortality versus usual care.8 The growing uncertainty regarding the efficacy of goal-directed fluids for septic shock, as well limitations of studies on AP, may contribute to the differences between the AGA and ACG recommendations.
Finally, as the guideline covers the initial therapeutic management of AP, no recommendations are made for diagnostic studies such as right upper quadrant ultrasound. This noninvasive and readily available test plays a critical role in evaluating for presence of gallstones and other potential etiologies of abdominal pain.
AREAS IN NEED OF FUTURE STUDY
Additional research is needed to better understand goal-directed fluid therapy with respect to the fluid type, amount, and target outcomes. Similarly, determining the optimal enteral feeding regimens for patients failing oral intake would help clinicians meet the recommendation for early nutrition. Finally, clarification on the roles and timing of endoscopic and surgical procedures for patients with severe biliary pancreatitis, as well as geriatric and medically complex populations, would help hospitalists advocate for a multidisciplinary approach to this common and often serious disease.
Disclosures
The authors have nothing to disclose.
Acute pancreatitis (AP) is the most common gastrointestinal discharge diagnosis in the United States, with a mortality rate of 1%-5%.1 Recent data demonstrate increasing AP-related admissions, making AP management of utmost importance to hospitalists.1 The American Gastroenterological Association (AGA) guideline specifically addresses AP management in the initial 48-72 hours of admission, during which management decisions can alter disease course and length of stay. AP requires two of the following three criteria for diagnosis: characteristic abdominal pain, elevation of lipase or amylase ≥3 times the upper limit of normal, and/or radiographic evidence of pancreatitis on cross-sectional imaging. The guideline provides eight recommendations, which we consolidated to highlight practice changing recommendations: fluids, nutrition, management of the most common causes, and prophylactic antibiotics.2,3
KEY RECOMMENDATIONS FOR THE HOSPITALIST
Fluids
Recommendation 1. In patients with AP, use goal-directed isotonic crystalloids for fluid management (conditional recommendation, very low-quality evidence).
The guideline emphasizes goal-directed fluid management despite low-quality, heterogeneous evidence and does not recommend Ringer’s lactate over normal saline. “Goal-directed” fluid management involves the use of crystalloid infusions titrated to improve physiologic and biochemical markers, but no target volume is specified by the guideline. Frequent reassessments should look for signs of volume overload, the primary risk of harm with fluid therapy. Despite failure to reduce mortality or morbidities such as pancreatic necrosis or persistent multi-organ failure, the AGA cites the mortality benefit of goal-directed therapy in sepsis as justification for this approach in AP, given the similar physiologic abnormalities.
Nutrition
Recommendation 2. Begin feeding early in patients with AP regardless of predicted severity. If oral nutrition is not tolerated, enteral feeding with either a nasogastric or nasojejunal tube is preferred to parenteral nutrition (strong recommendation, moderate-quality evidence).
Early feeding (ie, within 24 hours) is recommended regardless of AP severity. This represents a change from prior practices of bowel rest, theorized to prevent continued stimulation of an inflamed pancreas. Although early feeding has not been linked to improved mortality, it has demonstrated lower rates of multi-organ failure and infected pancreatic necrosis, possibly due to maintenance of the gut mucosal barrier and reduced bacterial translocation. When oral feeding is not tolerated, enteral nutrition is preferred over parenteral nutrition due to less risks. The preferred dietary composition guidance for patients with persistent pain or ileus is not addressed.
Management of the Most Common Causes of AP in Adults
Recommendation 3. Patients with mild acute biliary pancreatitis should have cholecystectomy during the initial admission (strong recommendation, moderate-quality evidence).
All patients with suspected biliary pancreatitis should receive a surgical consultation for cholecystectomy during the index admission. At the time of the guideline release, only one trial was available to support the recommendation of early cholecystectomy; however, newer studies similarly support cholecystectomy during index admission by demonstrating reductions in composite outcomes of mortality and gallstone-related complications, readmission for pancreatitis, and other pancreatobiliary complications.4 A Cochrane review included in the guideline found no differences in complication rates even in patients with severe biliary pancreatitis. In the absence of cholangitis, urgent endoscopic retrograde cholangiography (ERCP) is not indicated as most stones causing biliary pancreatitis pass spontaneously.
Recommendation 4. In patients with acute alcoholic pancreatitis, brief alcohol intervention should occur during admission (strong recommendation, moderate-quality evidence).
Ongoing alcohol consumption is a risk factor for recurrent acute and chronic pancreatitis. Only one trial assessed the impact of inpatient alcohol cessation counseling on recurrent AP, noting a trend toward reduced readmissions.5 However, indirect evidence from similar interventions in ambulatory settings demonstrates reductions in alcohol intake, leading to the AGA recommendation for inpatients with alcohol-induced AP.3
Antibiotics
Recommendation 5. Avoid empiric antibiotics in patients with AP who otherwise lack an indication, regardless of predicted severity (conditional recommendation, low-quality evidence).
Since 2002, well performed trials have consistently failed to demonstrate improvement in outcomes such as multi-organ failure or length of stay with use of prophylactic antibiotics for AP, even severe AP and pancreatic necrosis. Therefore, the AGA recommends against prophylactic antibiotics in initial management of AP regardless of disease severity. Lack of blinding in the majority of trial designs conducted before 2002 contributed to the overall assessment of low-quality evidence. The guideline does not address acute biliary pancreatitis with cholangitis, for which antibiotics and ERCP for decompression are critical.
CRITIQUE
The AGA Institute supported this guideline development and employed the rigorous and standardized GRADE (Grading of Recommendations Assessment, Development and Evaluation) methodology. This approach allowed the guideline panel members to account not only for evidence quality, but also the benefits and harms of an intervention and resource utilization. None of the authors had any stated conflicts of interest.
The guideline heavily weighted results from randomized control trials, most of which excluded key populations cared for by hospitalists (eg, patients older than 75 years, with end-stage renal disease). Particular areas where this creates challenges for clinicians and patients alike include goal-directed fluid therapy and when to consider more invasive interventions such as ERCP and early cholecystectomy. For example, patients considered to be poor surgical candidates may benefit from ERCP with biliary sphincterotomy to reduce the risk of recurrent biliary pancreatitis.
Lack of specificity in the guidelines for goal-directed fluid management and enteral feeding regimens makes it challenging to standardize hospitalists’ approach to the early care of patients with AP. Interestingly, the 2013 American College of Gastroenterology (ACG) Guideline for the Management of AP included strong recommendations for the use of Ringer’s lactate and volume targets in the initial management of AP.6 Evidence supporting the use of Ringer’s lactate versus normal saline is based largely upon improved inflammatory markers, theoretical potentiation of pancreatic enzyme activation with hypercholemic metabolic acidosis, and small studies demonstrating trends toward improved mortality.7 The ACG guideline was released prior to mounting evidence suggesting that goal-directed fluid therapy in sepsis does not improve mortality versus usual care.8 The growing uncertainty regarding the efficacy of goal-directed fluids for septic shock, as well limitations of studies on AP, may contribute to the differences between the AGA and ACG recommendations.
Finally, as the guideline covers the initial therapeutic management of AP, no recommendations are made for diagnostic studies such as right upper quadrant ultrasound. This noninvasive and readily available test plays a critical role in evaluating for presence of gallstones and other potential etiologies of abdominal pain.
AREAS IN NEED OF FUTURE STUDY
Additional research is needed to better understand goal-directed fluid therapy with respect to the fluid type, amount, and target outcomes. Similarly, determining the optimal enteral feeding regimens for patients failing oral intake would help clinicians meet the recommendation for early nutrition. Finally, clarification on the roles and timing of endoscopic and surgical procedures for patients with severe biliary pancreatitis, as well as geriatric and medically complex populations, would help hospitalists advocate for a multidisciplinary approach to this common and often serious disease.
Disclosures
The authors have nothing to disclose.
1. Krishna SG, Kamboj AK, Hart PA, Hinton A, Conwell DL. The changing epidemiology of acute pancreatitis hospitalizations: a decade of trends and the impact of chronic pancreatitis. Pancreas. 2017;46(4):482-488. https://doi.org/10.1097/MPA.0000000000000783.
2. Crockett SD, Wani S, Gardner TB, et al. American Gastroenterological Association Institute Guideline on initial management of acute pancreatitis. Gastroenterology. 2018;154(4):1096-1101. https://doi.org/10.1053/j.gastro.2018.01.032.
3. Vege SS, DiMagno MJ, Forsmark CE, Martel M, Barkun AN. Initial medical treatment of acute pancreatitis: American Gastroenterological Association Institute technical review. Gastroenterology. 2018;154(4):1103-1139. https://doi.org/10.1053/j.gastro.2018.01.031.
4 Noel R, Arnelo U, Lundell L, et al. Index versus delayed cholecystectomy in mild gallstone pancreatitis: results of a randomized controlled trial. HPB (Oxford). 2018;20(10):932-938. https://doi.org/10.1016/j.hpb.2018.03.016.
5. Kaner EF, Beyer F, Dickinson HO, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev. 2007:CD004148. https://doi.org/10.1002/14651858.CD004148.pub3.
6. Tenner S, Baillie J, DeWitt J, Vege SS. American College of Gastroenterology guideline: Management of acute pancreatitis. Am J Gastroenterol. 2013;108(9):1400-1415. https://doi.org/10.1038/ajg.2013.218.
7. de-Madaria E, Herrera-Marante I, González-Camacho V, et al. Fluid resuscitation with lactated Ringer’s solution vs normal saline in acute pancreatitis: a triple-blind, randomized, controlled trial. United European Gastroenterol J. 2018;6(1):63-72. https://doi.org/10.1177/2050640617707864
8. The PRISM Investigators. Early, goal-directed therapy for septic shock — a patient-level meta-analysis. New Engl J Med. 2017;376(23):2223-2234. https://doi.org/10.1056/NEJMoa1701380.
1. Krishna SG, Kamboj AK, Hart PA, Hinton A, Conwell DL. The changing epidemiology of acute pancreatitis hospitalizations: a decade of trends and the impact of chronic pancreatitis. Pancreas. 2017;46(4):482-488. https://doi.org/10.1097/MPA.0000000000000783.
2. Crockett SD, Wani S, Gardner TB, et al. American Gastroenterological Association Institute Guideline on initial management of acute pancreatitis. Gastroenterology. 2018;154(4):1096-1101. https://doi.org/10.1053/j.gastro.2018.01.032.
3. Vege SS, DiMagno MJ, Forsmark CE, Martel M, Barkun AN. Initial medical treatment of acute pancreatitis: American Gastroenterological Association Institute technical review. Gastroenterology. 2018;154(4):1103-1139. https://doi.org/10.1053/j.gastro.2018.01.031.
4 Noel R, Arnelo U, Lundell L, et al. Index versus delayed cholecystectomy in mild gallstone pancreatitis: results of a randomized controlled trial. HPB (Oxford). 2018;20(10):932-938. https://doi.org/10.1016/j.hpb.2018.03.016.
5. Kaner EF, Beyer F, Dickinson HO, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev. 2007:CD004148. https://doi.org/10.1002/14651858.CD004148.pub3.
6. Tenner S, Baillie J, DeWitt J, Vege SS. American College of Gastroenterology guideline: Management of acute pancreatitis. Am J Gastroenterol. 2013;108(9):1400-1415. https://doi.org/10.1038/ajg.2013.218.
7. de-Madaria E, Herrera-Marante I, González-Camacho V, et al. Fluid resuscitation with lactated Ringer’s solution vs normal saline in acute pancreatitis: a triple-blind, randomized, controlled trial. United European Gastroenterol J. 2018;6(1):63-72. https://doi.org/10.1177/2050640617707864
8. The PRISM Investigators. Early, goal-directed therapy for septic shock — a patient-level meta-analysis. New Engl J Med. 2017;376(23):2223-2234. https://doi.org/10.1056/NEJMoa1701380.
© 2019 Society of Hospital Medicine