User login
Parabens – friend or foe?
Parabens were named nonallergen of the year! It is time that we help consumers understand that the substitutes for parabens are often worse than parabens, and parabens are not as sensitizing as we thought. Preservatives are essential parts of most cosmetics and cosmeceuticals. (I say “most” because many organic products do not have them and consequently have shorter shelf lives.) Without them, products are vulnerable to rapid decomposition and infiltration by bacteria, fungi, and molds. The preservatives that are used in the place of parabens often are sensitizers. What do we tell our patients about the safety of parabens with all of these conflicting reports? This column will focus on current thoughts regarding the safety of parabens used as preservatives. I would love to hear your thoughts.
Background
Parabens are alkyl esters of p-hydroxybenzoic acid and have been used as a class of preservatives since the late 1920s and early 1930s. Parabens are found naturally in raspberries, blackberries, carrots, and cucumbers and are common ingredients in food and pharmaceuticals. They are still widely used in skin, hair, and body care products, despite the public outcry against them.1-4
There are many kinds of parabens such as butylparaben, isobutylparaben, ethylparaben, methylparaben, propylparaben, isopropylparaben, and benzylparaben, each with its own characteristics.5 Parabens are considered ideal preservative ingredients because they exhibit a broad spectrum of antimicrobial activity, stability over a large pH and temperature range, have no odor, do not change color, and are water soluble enough to yield an effective concentration in a hydrophilic formulation.3 As the alkyl chain length of parabens increases, they become less water soluble and more oil soluble. Parabens penetrate the skin barrier in inverse relation to its ester chain length.6 Often, several parabens will be combined to take advantage of each paraben’s solubility characteristics.
Many patients avoid parabens because of “health risks.” Now other preservatives are being substituted for parabens, even though these ingredients may be less studied or even less safe than parabens. It is important not to lump all parabens together as they each have different characteristics. Methylparaben and propylparaben are the most commonly used parabens in skin care products.7 Combinations of parabens are notably more effective than the use of single parabens.3,8 High concentrations of any type of paraben can cause an irritant reaction on the skin, but those with longer ester chain lengths are more likely to cause irritation.
Methylparaben
The methyl ester of p-hydroxybenzoic acid is found in many skin care products. It is readily absorbed through the skin and gastrointestinal tract. It is quickly hydrolyzed and excreted in the urine and does not accumulate in the body. Studies have shown it is nontoxic, nonirritating, and nonsensitizing. It is not teratogenic, embryotoxic, or carcinogenic. Methylparaben, because of its shorter side chain groups and greater lipophilicity, has been shown to be more readily absorbed by the skin than other paraben chemicals.8,9 It is also on the low order of ingredients provoking acute and chronic toxicity.3
Propylparaben
Propylparaben is the ester form of p-hydroxybenzoic acid that has been esterified with n-propanol. It is the most commonly used antimicrobial preservative in foods, cosmetics, and drugs. It is readily absorbed through the skin and GI tract. It is quickly hydrolyzed and excreted in the urine and does not accumulate in the body.
Estrogenic activity of parabens
In a 2004 study, Darbre et al. reported on the discovery of parabens-like substances in breast tissue and published these findings in the Journal of Applied Toxicology.10 The media and public panicked, saying that parabens have estrogenic activity and can cause breast cancer. However, many studies have shown that certain parabens do not have estrogenic activity. Although some parabens have been shown to impart estrogenic effects in vitro, these are very weak. The four most commonly used parabens in cosmetic products are 10,000-fold or less potent than 17beta-estradiol.11 The potential to result in an adverse effect mediated via an estrogen mode of action has not been established in humans.6 Paraben exposure differs geographically. No correlation has been found between the amount of parabens in a geographic location and the incidence of breast cancer. Current scientific knowledge is insufficient to demonstrate a clear cancer risk caused by the topical application of cosmetics that contain parabens on normal intact skin.11
Parabens and contact dermatitis
Paraben compounds are capable of minimal penetrance through intact skin.12 When they are able to penetrate the skin – a capacity that varies among the class – parabens are rapidly metabolized to p-hydroxybenzoic acid and promptly excreted in the urine.3,11 Parabens for many years were thought to cause contact dermatitis, and there are many reports of this. However, the incidence is much lower than previously thought. In fact, parabens were named “Nonallergen of the Year in 2018” because of the low incidence of reactions in patch tests.13 Higher concentrations of parabens applied topically to skin – especially “nonintact” skin – have been shown to cause mild irritant reactions. It is likely that many of these reported cases of “contact dermatitis” were actually irritant dermatitis. Longstanding concerns about the allergenicity of parabens in relation to the skin have been rendered insignificant, as the wealth of evidence reveals little to no support for the cutaneous toxicity of these substances.11 Yim et al. add that parabens remain far less sensitizing than agents newly introduced for use in personal care products.4
Daily average exposure to parabens
It is estimated that parabens are found in 10% of personal care products. In most cases, these products contain 1% or less of parabens. If the average patient uses 50 g of personal care products a day, then the average daily exposure to parabens topically is 0.05 g. Parabens also are found in food and drugs, so the total paraben exposure per day is assumed to be about 1 mg/day. (See the 2002 Food and Chemical Toxicology article for details of how this was calculated.)7 When food, personal care products, and drug exposure rates are added, the average person is exposed to 1.29 mg/kg per day or 77.5 mg/day for a 60-kg individual. You can see that personal care products account for a fraction of exposure, as most paraben exposure comes from food.
Government opinion on the safety of parabens for the skin
Parabens long have been assessed as safe for use in cosmetic products in many countries. The European Commission stipulated a maximum concentration of 0.4% for each paraben and 0.8% for total mixture of paraben esters.4,6 While the Federal Food, Drug, and Cosmetic Act of 1938 prohibits the Food and Drug Administration from ruling on cosmetic ingredients, the industry-sponsored Cosmetic Ingredient Review expert panel has endorsed the European guidelines.4,6 Further, the North American Contact Dermatitis Group has pointed out that parabens continue to demonstrate the lowest prevalence of positivity (0.6%) of any major preservative available on the North American market, which includes over 10,000 cosmetic and personal care products, and remain one of the safest classes of preservatives for the skin.14 Further, the FDA has listed or classified parabens as generally regarded as safe.8
Safety of parabens
Parabens do not accumulate in tissues or organs for any appreciable length of time.6,8 In addition, carcinogenicity, cytotoxicity, or mutagenicity has not been proven in relation to parabens.8 Indeed, classical assays have shown no activity from parabens in terms of mutagenicity or carcinogenicity.11,15 Some estrogenic effects or activity that mimics estrogen have been associated with parabens in vitro, but this activity has been noted as very weak and there are no established reports of human cases in which parabens have elicited an estrogen-mediated adverse event.6,11
Concerns about a possible link between parabens and breast cancer have been largely diminished or relegated to the status of unknown and difficult to ascertain.13 Further, present knowledge provides no established link between the topical application of parabens-containing skin care formulations on healthy skin and cancer risk.10 Only intact skin should come in touch with products containing parabens to prevent irritant reactions.
Conclusion
Despite the fearful hype and reaction to one report 15 years ago, parabens continue to be safely used in numerous topical formulations. Their widespread use and lack of association with adverse events are a testament to their safety. From a dermatologic perspective, this nonallergen of the year deserves a better reputation.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected]
References
1. “Goodman and Gilman’s The Pharmacological Basis of Therapeutics,” 6th ed. (New York: Macmillan, 1980, p. 969).
2. Toxicity: The Butyl, Ethyl, Methyl, and Propyl Esters have been found to promote allergic sensitization in humans, in “Dangerous Properties of Industrial Materials,” 4th ed. (New York: Van Nostrand Reinhold, 1975, p. 929).
3. Food Chem Toxicol. 2001 Jun;39(6):513-32.
4. Dermatitis. 2014 Sep-Oct;25(5):215-31.
5. Crit Rev Toxicol. 2005 Jun;35(5):435-58.
6. Int J Toxicol. 2008;27 Suppl 4:1-82.
7. Food Chem Toxicol. 2002 Oct;40(10):1335-73.
8. Dermatitis. 2019 Jan/Feb;30(1):3-31.
9. Exp Dermatol. 2007 Oct;16(10):830-6.
10. J Appl Toxicol. 2004 Jan-Feb;24(1):5-13.
11. Dermatitis. 2019 Jan/Feb;30(1):32-45.
12. Food Chem Toxicol. 2005 Feb;43(2):279-91.
13. Dermatitis. 2018 Dec 18. doi: 10.1097/DER.0000000000000429.
14. Dermatitis. 2018 Nov/Dec;29(6):297-309.
15. Food Chem Toxicol. 2005 Jul;43(7):985-1015.
Parabens were named nonallergen of the year! It is time that we help consumers understand that the substitutes for parabens are often worse than parabens, and parabens are not as sensitizing as we thought. Preservatives are essential parts of most cosmetics and cosmeceuticals. (I say “most” because many organic products do not have them and consequently have shorter shelf lives.) Without them, products are vulnerable to rapid decomposition and infiltration by bacteria, fungi, and molds. The preservatives that are used in the place of parabens often are sensitizers. What do we tell our patients about the safety of parabens with all of these conflicting reports? This column will focus on current thoughts regarding the safety of parabens used as preservatives. I would love to hear your thoughts.
Background
Parabens are alkyl esters of p-hydroxybenzoic acid and have been used as a class of preservatives since the late 1920s and early 1930s. Parabens are found naturally in raspberries, blackberries, carrots, and cucumbers and are common ingredients in food and pharmaceuticals. They are still widely used in skin, hair, and body care products, despite the public outcry against them.1-4
There are many kinds of parabens such as butylparaben, isobutylparaben, ethylparaben, methylparaben, propylparaben, isopropylparaben, and benzylparaben, each with its own characteristics.5 Parabens are considered ideal preservative ingredients because they exhibit a broad spectrum of antimicrobial activity, stability over a large pH and temperature range, have no odor, do not change color, and are water soluble enough to yield an effective concentration in a hydrophilic formulation.3 As the alkyl chain length of parabens increases, they become less water soluble and more oil soluble. Parabens penetrate the skin barrier in inverse relation to its ester chain length.6 Often, several parabens will be combined to take advantage of each paraben’s solubility characteristics.
Many patients avoid parabens because of “health risks.” Now other preservatives are being substituted for parabens, even though these ingredients may be less studied or even less safe than parabens. It is important not to lump all parabens together as they each have different characteristics. Methylparaben and propylparaben are the most commonly used parabens in skin care products.7 Combinations of parabens are notably more effective than the use of single parabens.3,8 High concentrations of any type of paraben can cause an irritant reaction on the skin, but those with longer ester chain lengths are more likely to cause irritation.
Methylparaben
The methyl ester of p-hydroxybenzoic acid is found in many skin care products. It is readily absorbed through the skin and gastrointestinal tract. It is quickly hydrolyzed and excreted in the urine and does not accumulate in the body. Studies have shown it is nontoxic, nonirritating, and nonsensitizing. It is not teratogenic, embryotoxic, or carcinogenic. Methylparaben, because of its shorter side chain groups and greater lipophilicity, has been shown to be more readily absorbed by the skin than other paraben chemicals.8,9 It is also on the low order of ingredients provoking acute and chronic toxicity.3
Propylparaben
Propylparaben is the ester form of p-hydroxybenzoic acid that has been esterified with n-propanol. It is the most commonly used antimicrobial preservative in foods, cosmetics, and drugs. It is readily absorbed through the skin and GI tract. It is quickly hydrolyzed and excreted in the urine and does not accumulate in the body.
Estrogenic activity of parabens
In a 2004 study, Darbre et al. reported on the discovery of parabens-like substances in breast tissue and published these findings in the Journal of Applied Toxicology.10 The media and public panicked, saying that parabens have estrogenic activity and can cause breast cancer. However, many studies have shown that certain parabens do not have estrogenic activity. Although some parabens have been shown to impart estrogenic effects in vitro, these are very weak. The four most commonly used parabens in cosmetic products are 10,000-fold or less potent than 17beta-estradiol.11 The potential to result in an adverse effect mediated via an estrogen mode of action has not been established in humans.6 Paraben exposure differs geographically. No correlation has been found between the amount of parabens in a geographic location and the incidence of breast cancer. Current scientific knowledge is insufficient to demonstrate a clear cancer risk caused by the topical application of cosmetics that contain parabens on normal intact skin.11
Parabens and contact dermatitis
Paraben compounds are capable of minimal penetrance through intact skin.12 When they are able to penetrate the skin – a capacity that varies among the class – parabens are rapidly metabolized to p-hydroxybenzoic acid and promptly excreted in the urine.3,11 Parabens for many years were thought to cause contact dermatitis, and there are many reports of this. However, the incidence is much lower than previously thought. In fact, parabens were named “Nonallergen of the Year in 2018” because of the low incidence of reactions in patch tests.13 Higher concentrations of parabens applied topically to skin – especially “nonintact” skin – have been shown to cause mild irritant reactions. It is likely that many of these reported cases of “contact dermatitis” were actually irritant dermatitis. Longstanding concerns about the allergenicity of parabens in relation to the skin have been rendered insignificant, as the wealth of evidence reveals little to no support for the cutaneous toxicity of these substances.11 Yim et al. add that parabens remain far less sensitizing than agents newly introduced for use in personal care products.4
Daily average exposure to parabens
It is estimated that parabens are found in 10% of personal care products. In most cases, these products contain 1% or less of parabens. If the average patient uses 50 g of personal care products a day, then the average daily exposure to parabens topically is 0.05 g. Parabens also are found in food and drugs, so the total paraben exposure per day is assumed to be about 1 mg/day. (See the 2002 Food and Chemical Toxicology article for details of how this was calculated.)7 When food, personal care products, and drug exposure rates are added, the average person is exposed to 1.29 mg/kg per day or 77.5 mg/day for a 60-kg individual. You can see that personal care products account for a fraction of exposure, as most paraben exposure comes from food.
Government opinion on the safety of parabens for the skin
Parabens long have been assessed as safe for use in cosmetic products in many countries. The European Commission stipulated a maximum concentration of 0.4% for each paraben and 0.8% for total mixture of paraben esters.4,6 While the Federal Food, Drug, and Cosmetic Act of 1938 prohibits the Food and Drug Administration from ruling on cosmetic ingredients, the industry-sponsored Cosmetic Ingredient Review expert panel has endorsed the European guidelines.4,6 Further, the North American Contact Dermatitis Group has pointed out that parabens continue to demonstrate the lowest prevalence of positivity (0.6%) of any major preservative available on the North American market, which includes over 10,000 cosmetic and personal care products, and remain one of the safest classes of preservatives for the skin.14 Further, the FDA has listed or classified parabens as generally regarded as safe.8
Safety of parabens
Parabens do not accumulate in tissues or organs for any appreciable length of time.6,8 In addition, carcinogenicity, cytotoxicity, or mutagenicity has not been proven in relation to parabens.8 Indeed, classical assays have shown no activity from parabens in terms of mutagenicity or carcinogenicity.11,15 Some estrogenic effects or activity that mimics estrogen have been associated with parabens in vitro, but this activity has been noted as very weak and there are no established reports of human cases in which parabens have elicited an estrogen-mediated adverse event.6,11
Concerns about a possible link between parabens and breast cancer have been largely diminished or relegated to the status of unknown and difficult to ascertain.13 Further, present knowledge provides no established link between the topical application of parabens-containing skin care formulations on healthy skin and cancer risk.10 Only intact skin should come in touch with products containing parabens to prevent irritant reactions.
Conclusion
Despite the fearful hype and reaction to one report 15 years ago, parabens continue to be safely used in numerous topical formulations. Their widespread use and lack of association with adverse events are a testament to their safety. From a dermatologic perspective, this nonallergen of the year deserves a better reputation.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected]
References
1. “Goodman and Gilman’s The Pharmacological Basis of Therapeutics,” 6th ed. (New York: Macmillan, 1980, p. 969).
2. Toxicity: The Butyl, Ethyl, Methyl, and Propyl Esters have been found to promote allergic sensitization in humans, in “Dangerous Properties of Industrial Materials,” 4th ed. (New York: Van Nostrand Reinhold, 1975, p. 929).
3. Food Chem Toxicol. 2001 Jun;39(6):513-32.
4. Dermatitis. 2014 Sep-Oct;25(5):215-31.
5. Crit Rev Toxicol. 2005 Jun;35(5):435-58.
6. Int J Toxicol. 2008;27 Suppl 4:1-82.
7. Food Chem Toxicol. 2002 Oct;40(10):1335-73.
8. Dermatitis. 2019 Jan/Feb;30(1):3-31.
9. Exp Dermatol. 2007 Oct;16(10):830-6.
10. J Appl Toxicol. 2004 Jan-Feb;24(1):5-13.
11. Dermatitis. 2019 Jan/Feb;30(1):32-45.
12. Food Chem Toxicol. 2005 Feb;43(2):279-91.
13. Dermatitis. 2018 Dec 18. doi: 10.1097/DER.0000000000000429.
14. Dermatitis. 2018 Nov/Dec;29(6):297-309.
15. Food Chem Toxicol. 2005 Jul;43(7):985-1015.
Parabens were named nonallergen of the year! It is time that we help consumers understand that the substitutes for parabens are often worse than parabens, and parabens are not as sensitizing as we thought. Preservatives are essential parts of most cosmetics and cosmeceuticals. (I say “most” because many organic products do not have them and consequently have shorter shelf lives.) Without them, products are vulnerable to rapid decomposition and infiltration by bacteria, fungi, and molds. The preservatives that are used in the place of parabens often are sensitizers. What do we tell our patients about the safety of parabens with all of these conflicting reports? This column will focus on current thoughts regarding the safety of parabens used as preservatives. I would love to hear your thoughts.
Background
Parabens are alkyl esters of p-hydroxybenzoic acid and have been used as a class of preservatives since the late 1920s and early 1930s. Parabens are found naturally in raspberries, blackberries, carrots, and cucumbers and are common ingredients in food and pharmaceuticals. They are still widely used in skin, hair, and body care products, despite the public outcry against them.1-4
There are many kinds of parabens such as butylparaben, isobutylparaben, ethylparaben, methylparaben, propylparaben, isopropylparaben, and benzylparaben, each with its own characteristics.5 Parabens are considered ideal preservative ingredients because they exhibit a broad spectrum of antimicrobial activity, stability over a large pH and temperature range, have no odor, do not change color, and are water soluble enough to yield an effective concentration in a hydrophilic formulation.3 As the alkyl chain length of parabens increases, they become less water soluble and more oil soluble. Parabens penetrate the skin barrier in inverse relation to its ester chain length.6 Often, several parabens will be combined to take advantage of each paraben’s solubility characteristics.
Many patients avoid parabens because of “health risks.” Now other preservatives are being substituted for parabens, even though these ingredients may be less studied or even less safe than parabens. It is important not to lump all parabens together as they each have different characteristics. Methylparaben and propylparaben are the most commonly used parabens in skin care products.7 Combinations of parabens are notably more effective than the use of single parabens.3,8 High concentrations of any type of paraben can cause an irritant reaction on the skin, but those with longer ester chain lengths are more likely to cause irritation.
Methylparaben
The methyl ester of p-hydroxybenzoic acid is found in many skin care products. It is readily absorbed through the skin and gastrointestinal tract. It is quickly hydrolyzed and excreted in the urine and does not accumulate in the body. Studies have shown it is nontoxic, nonirritating, and nonsensitizing. It is not teratogenic, embryotoxic, or carcinogenic. Methylparaben, because of its shorter side chain groups and greater lipophilicity, has been shown to be more readily absorbed by the skin than other paraben chemicals.8,9 It is also on the low order of ingredients provoking acute and chronic toxicity.3
Propylparaben
Propylparaben is the ester form of p-hydroxybenzoic acid that has been esterified with n-propanol. It is the most commonly used antimicrobial preservative in foods, cosmetics, and drugs. It is readily absorbed through the skin and GI tract. It is quickly hydrolyzed and excreted in the urine and does not accumulate in the body.
Estrogenic activity of parabens
In a 2004 study, Darbre et al. reported on the discovery of parabens-like substances in breast tissue and published these findings in the Journal of Applied Toxicology.10 The media and public panicked, saying that parabens have estrogenic activity and can cause breast cancer. However, many studies have shown that certain parabens do not have estrogenic activity. Although some parabens have been shown to impart estrogenic effects in vitro, these are very weak. The four most commonly used parabens in cosmetic products are 10,000-fold or less potent than 17beta-estradiol.11 The potential to result in an adverse effect mediated via an estrogen mode of action has not been established in humans.6 Paraben exposure differs geographically. No correlation has been found between the amount of parabens in a geographic location and the incidence of breast cancer. Current scientific knowledge is insufficient to demonstrate a clear cancer risk caused by the topical application of cosmetics that contain parabens on normal intact skin.11
Parabens and contact dermatitis
Paraben compounds are capable of minimal penetrance through intact skin.12 When they are able to penetrate the skin – a capacity that varies among the class – parabens are rapidly metabolized to p-hydroxybenzoic acid and promptly excreted in the urine.3,11 Parabens for many years were thought to cause contact dermatitis, and there are many reports of this. However, the incidence is much lower than previously thought. In fact, parabens were named “Nonallergen of the Year in 2018” because of the low incidence of reactions in patch tests.13 Higher concentrations of parabens applied topically to skin – especially “nonintact” skin – have been shown to cause mild irritant reactions. It is likely that many of these reported cases of “contact dermatitis” were actually irritant dermatitis. Longstanding concerns about the allergenicity of parabens in relation to the skin have been rendered insignificant, as the wealth of evidence reveals little to no support for the cutaneous toxicity of these substances.11 Yim et al. add that parabens remain far less sensitizing than agents newly introduced for use in personal care products.4
Daily average exposure to parabens
It is estimated that parabens are found in 10% of personal care products. In most cases, these products contain 1% or less of parabens. If the average patient uses 50 g of personal care products a day, then the average daily exposure to parabens topically is 0.05 g. Parabens also are found in food and drugs, so the total paraben exposure per day is assumed to be about 1 mg/day. (See the 2002 Food and Chemical Toxicology article for details of how this was calculated.)7 When food, personal care products, and drug exposure rates are added, the average person is exposed to 1.29 mg/kg per day or 77.5 mg/day for a 60-kg individual. You can see that personal care products account for a fraction of exposure, as most paraben exposure comes from food.
Government opinion on the safety of parabens for the skin
Parabens long have been assessed as safe for use in cosmetic products in many countries. The European Commission stipulated a maximum concentration of 0.4% for each paraben and 0.8% for total mixture of paraben esters.4,6 While the Federal Food, Drug, and Cosmetic Act of 1938 prohibits the Food and Drug Administration from ruling on cosmetic ingredients, the industry-sponsored Cosmetic Ingredient Review expert panel has endorsed the European guidelines.4,6 Further, the North American Contact Dermatitis Group has pointed out that parabens continue to demonstrate the lowest prevalence of positivity (0.6%) of any major preservative available on the North American market, which includes over 10,000 cosmetic and personal care products, and remain one of the safest classes of preservatives for the skin.14 Further, the FDA has listed or classified parabens as generally regarded as safe.8
Safety of parabens
Parabens do not accumulate in tissues or organs for any appreciable length of time.6,8 In addition, carcinogenicity, cytotoxicity, or mutagenicity has not been proven in relation to parabens.8 Indeed, classical assays have shown no activity from parabens in terms of mutagenicity or carcinogenicity.11,15 Some estrogenic effects or activity that mimics estrogen have been associated with parabens in vitro, but this activity has been noted as very weak and there are no established reports of human cases in which parabens have elicited an estrogen-mediated adverse event.6,11
Concerns about a possible link between parabens and breast cancer have been largely diminished or relegated to the status of unknown and difficult to ascertain.13 Further, present knowledge provides no established link between the topical application of parabens-containing skin care formulations on healthy skin and cancer risk.10 Only intact skin should come in touch with products containing parabens to prevent irritant reactions.
Conclusion
Despite the fearful hype and reaction to one report 15 years ago, parabens continue to be safely used in numerous topical formulations. Their widespread use and lack of association with adverse events are a testament to their safety. From a dermatologic perspective, this nonallergen of the year deserves a better reputation.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected]
References
1. “Goodman and Gilman’s The Pharmacological Basis of Therapeutics,” 6th ed. (New York: Macmillan, 1980, p. 969).
2. Toxicity: The Butyl, Ethyl, Methyl, and Propyl Esters have been found to promote allergic sensitization in humans, in “Dangerous Properties of Industrial Materials,” 4th ed. (New York: Van Nostrand Reinhold, 1975, p. 929).
3. Food Chem Toxicol. 2001 Jun;39(6):513-32.
4. Dermatitis. 2014 Sep-Oct;25(5):215-31.
5. Crit Rev Toxicol. 2005 Jun;35(5):435-58.
6. Int J Toxicol. 2008;27 Suppl 4:1-82.
7. Food Chem Toxicol. 2002 Oct;40(10):1335-73.
8. Dermatitis. 2019 Jan/Feb;30(1):3-31.
9. Exp Dermatol. 2007 Oct;16(10):830-6.
10. J Appl Toxicol. 2004 Jan-Feb;24(1):5-13.
11. Dermatitis. 2019 Jan/Feb;30(1):32-45.
12. Food Chem Toxicol. 2005 Feb;43(2):279-91.
13. Dermatitis. 2018 Dec 18. doi: 10.1097/DER.0000000000000429.
14. Dermatitis. 2018 Nov/Dec;29(6):297-309.
15. Food Chem Toxicol. 2005 Jul;43(7):985-1015.
Bakuchiol
Bakuchiol [(1E,3S)-3-ethenyl-3,7-dimethyl-1,6-octadien-1-yl]phenol, a prenylated phenolic monoterpene found in the seeds and leaves of various plants, particularly Psoralea corylifolia, has been used to treat a broad array of disorders, including skin conditions, in the traditional medical practices of China, Japan, and Korea, as well as Ayurvedic medicine in India.1-6 Specifically, the seeds of as well as cardiovascular diseases, nephritis, osteoporosis, and cancer.7-9
This primary active ingredient is reputed to exert antioxidant, antibacterial, anti-inflammatory, antiaging, and estrogen-like functions, and recent data suggest anticancer activity, including activity against skin cancer. Its antiaging properties manifest via preservation of cutaneous collagen.4 The plant itself has displayed a wide range of biological functions, such as antibacterial, anticancer, cytotoxic, cardiac, diaphoretic, diuretic, stimulant, aphrodisiac, and tonifying activities.8,9 A 2016 quantitative analysis of Psoralea corylifolia and seven of its standard constituents (psoralen, angelicin, neobavaisoflavone, psoralidin, isobavachalcone, bavachinin, and bakuchiol) using high-performance liquid chromatography revealed that bakuchiol is the strongest phytochemical ingredient in the plant, which the investigators found also confers neuroprotective and antineuroinflammatory benefits.3
Other species contain bakuchiol, and its biological activities have been harnessed in other folk medical traditions. The monoterpene is an important constituent found in Ulmus davidiana var. japonica, which is used for its anti-inflammatory properties in traditional Korean medicine.10 Further, bakuchiol and 3-hydroxy-bakuchiol have been identified as key components isolated from Psoralea glandulosa, which is a shrub used in Chilean folk medicine to treat cutaneous disorders engendered by bacteria and fungus.11 Topical applications of bakuchiol have been demonstrated to confer antiaging benefits.12 This column briefly identifies some of the various uses emerging for this compelling botanical agent.
Antiaging activities
In 2014, Yu et al. found that bakuchiol may impart antiaging benefits by supporting the cellular activity of the expression level of human skin fibroblasts (ESF-1), as well as production of collagen types I and III, while reducing the matrix metalloproteinase-1 mRNA expression.13
The same year, Chaudhuri et al. compared the skin care–related activities of retinol and bakuchiol, finding their gene expression profiles very similar. In addition, they observed that bakuchiol up-regulated collagen types I and IV in a DNA microarray study and stimulated type III collagen production in a model of mature fibroblasts. Further, the investigators formulated bakuchiol into a skin care product and tested it clinically, with twice daily applications over 12 weeks yielding significant amelioration in lines and wrinkles, pigmentation, elasticity, and firmness, as well as overall diminished photodamage without provoking redness. They concluded that bakuchiol can act as an antiaging agent through regulation of gene expression comparable to retinol.1
Retinoids without reactions?
In 2017, Ma et al. set out to synthesize and test in psoriatic cytokine–treated cultures of keratinocytes and organotypic skin substitutes a new substance created by combining two skin-active compounds (bakuchiol and salicylic acid) into bakuchiol salicylate (bakusylan), with the intention of rendering a novel functional retinoid. The researchers reported that the gene expression profile showed elimination of various retinoid-like proinflammatory responses, without a loss of normalizing activity. They concluded that their work may result in a new class of functional retinoids.14
Early this year, Dhaliwal et al. reported on a randomized, double-blind, 12-week study of 44 patients who applied either bakuchiol 0.5% cream twice daily or retinol 0.5% cream daily. Facial photographs were evaluated at baseline, 4, 8, and 12 weeks, and a blinded dermatologist rated pigmentation and erythema. Side effects were also noted by subjects in tolerability assessment questionnaires. Both compounds significantly reduced wrinkles and hyperpigmentation, with no statistical variance found between the two. More facial skin scaling and stinging was experienced by the retinol group. The investigators concluded that bakuchiol exhibits photoaging activity comparable with retinol and appears to be an emerging alternative to retinol because it is better tolerated.12 Notably, there is one report to date of an allergic reaction to topical bakuchiol.15
Topical combination therapies for hyperpigmentation, photodamage, and acne
Bakuchiol was a key ingredient incorporated into a 0.5% retinol treatment evaluated in a 12-week, open-label, single-center clinical-usage trial of 44 women with mild to moderate hyperpigmentation and photodamaged facial skin who took a dual product regimen. This 2016 study showed that the retinol and vitamin C facial regimen yielded a statistically significant amelioration in clinical grading of all parameters.16
A 2015 randomized controlled clinical trial in 111 subjects evaluated the use of adapalene 0.1% gel and a formulation containing bakuchiol, Ginkgo biloba extract, and mannitol in patients with acne. Patients were randomized to the adapalene and botanical formulation or adapalene and vehicle cream for 2 months. Both treatment groups experienced improvements according to all measured outcomes. The botanical formulation was associated with a statistically significant edge over the vehicle combination in reducing inflammatory lesions, investigator global assessment, and intensity of seborrhea. Quality of life was also perceived to be better with the combination of adapalene and the bakuchiol-containing product, which was deemed to be safe with good local tolerability.17
A subsequent evaluation by a different team also considered the antibacterial, anti-inflammatory, and antioxidative potential of this combination product via in vitro, ex vivo, and clinical studies. The work by Trompezinski et al. revealed that bakuchiol displays nearly twice the antioxidative potential asthat of vitamin E. The bakuchiol-containing cream was shown in acne patients to successfully regulate sebum composition by raising linolenic and sapienic acid levels while lowering oleic acid levels. Its efficacy against Propionibacterium acnes was also suggested by a decrease in the number of skin surface porphyrins. The investigators concluded that the formulation serves as an effective adjuvant acne treatment by attacking inflammation, dysseborrhea, and proliferation of Propionibacterium acnes.18
Anticancer activity
In 2016, Kim et al. demonstrated that bakuchiol exhibits chemopreventive activity by hindering epidermal growth factor (EGF)–induced neoplastic cell transformation. In what was the first mechanistic study to reveal molecular targets for the anticancer activity of this substance, the investigators found that bakuchiol also reduced the viability and suppressed anchorage-independent growth of A431 human epithelial carcinoma cells. They identified Hck, Blk, and p38 MAPK as the molecular targets of what they identified as a potent anticancer compound.2
Skin-whitening potential
In 2010, Ohno et al. found that bakuchiol, along with other ingredients, isolated from Piper longum demonstrated strong suppressive activity against melanin production in B16 mouse melanoma cells and may have potential to affect melanin synthesis in human skin.19 Further, with use of a new method for screening tyrosinase, Cheng et al. found in 2017 that four substances used in traditional Chinese medicine (quercetin, kaempferol, bavachinin, and bakuchiol) displayed the potential for inhibiting tyrosinase.20
Conclusion
A compound that acts like a retinoid – yielding antiacne and antiaging effects – without provoking irritation? Most dermatologists and their patients would say, sign me up. Bakuchiol, an active ingredient in various plants, especially Psoralea corylifolia, seems to present that kind of profile. While more research is necessary, experience with this herbal ingredient in traditional medicine and an increasing body of research, including clinical results, provides reasons for optimism that this ingredient may have a versatile role to play in topical skin care, particularly in its retinoid-like functions.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected].
References
1. Chaudhuri RK et al. Int J Cosmet Sci. 2014 Jun;36(3):221-30.
2. Kim JE et al. Oncotarget. 2016 Mar 22;7(12):14616-27.
3. Kim YJ et al. Molecules. 2016 Aug 17. doi: 10.3390/molecules21081076.
4. Xin Z et al. Pharmacol Res. 2019 Mar;141:208-13.
5. Lev-Tov H. Br J Dermatol. 2019 Feb;180(2):253-4.
6. Shrestha S et al. J Ayurveda Integr Med. 2018 Jul - Sep; 9(3):209-12.
7. Li CC et al. Evid Based Complement Alternat Med. 2016. doi: 10.1155/2016/8108643.
8. Hu C et al. Fitoterapia. 2015 Oct;106:129-34.
9. Yan DM et al. J Ethnopharmacol. 2010 Apr 21;128(3):697-702.
10. Choi SY et al. J Med Food. 2010 Aug;13(4):1019-23.
11. Madrid A et al. J Ethnopharmacol. 2012 Dec 18;144(3):809-11.
12. Dhaliwal S et al. Br J Dermatol. 2019 Feb;180(2):289-96.
13. Yu Q et al. Zhong Yao Cai. 2014 Apr;37(4):632-5.
14. Ma S et al. Clin Exp Dermatol. 2017 Apr;42(3):251-60.
15. Malinauskiene L et al. Contact Dermatitis. 2019 Jun;80(6):398-9.
16. Herndon JH Jr, et al. J Drugs Dermatol. 2016 Apr;15(4):476-82.
17. Poláková K et al. Clin Cosmet Investig Dermatol. 2015 Apr 10;8:187-91.
18. Trompezinski S et al. Clin Cosmet Investig Dermatol. 2016 Aug 31;9:233-9.
19. Ohno O et al. Biosci Biotechnol Biochem. 2010;74(7):1504-6.
20. Cheng M et al. Electrophoresis. 2017 Feb;38(3-4):486-93.
Bakuchiol [(1E,3S)-3-ethenyl-3,7-dimethyl-1,6-octadien-1-yl]phenol, a prenylated phenolic monoterpene found in the seeds and leaves of various plants, particularly Psoralea corylifolia, has been used to treat a broad array of disorders, including skin conditions, in the traditional medical practices of China, Japan, and Korea, as well as Ayurvedic medicine in India.1-6 Specifically, the seeds of as well as cardiovascular diseases, nephritis, osteoporosis, and cancer.7-9
This primary active ingredient is reputed to exert antioxidant, antibacterial, anti-inflammatory, antiaging, and estrogen-like functions, and recent data suggest anticancer activity, including activity against skin cancer. Its antiaging properties manifest via preservation of cutaneous collagen.4 The plant itself has displayed a wide range of biological functions, such as antibacterial, anticancer, cytotoxic, cardiac, diaphoretic, diuretic, stimulant, aphrodisiac, and tonifying activities.8,9 A 2016 quantitative analysis of Psoralea corylifolia and seven of its standard constituents (psoralen, angelicin, neobavaisoflavone, psoralidin, isobavachalcone, bavachinin, and bakuchiol) using high-performance liquid chromatography revealed that bakuchiol is the strongest phytochemical ingredient in the plant, which the investigators found also confers neuroprotective and antineuroinflammatory benefits.3
Other species contain bakuchiol, and its biological activities have been harnessed in other folk medical traditions. The monoterpene is an important constituent found in Ulmus davidiana var. japonica, which is used for its anti-inflammatory properties in traditional Korean medicine.10 Further, bakuchiol and 3-hydroxy-bakuchiol have been identified as key components isolated from Psoralea glandulosa, which is a shrub used in Chilean folk medicine to treat cutaneous disorders engendered by bacteria and fungus.11 Topical applications of bakuchiol have been demonstrated to confer antiaging benefits.12 This column briefly identifies some of the various uses emerging for this compelling botanical agent.
Antiaging activities
In 2014, Yu et al. found that bakuchiol may impart antiaging benefits by supporting the cellular activity of the expression level of human skin fibroblasts (ESF-1), as well as production of collagen types I and III, while reducing the matrix metalloproteinase-1 mRNA expression.13
The same year, Chaudhuri et al. compared the skin care–related activities of retinol and bakuchiol, finding their gene expression profiles very similar. In addition, they observed that bakuchiol up-regulated collagen types I and IV in a DNA microarray study and stimulated type III collagen production in a model of mature fibroblasts. Further, the investigators formulated bakuchiol into a skin care product and tested it clinically, with twice daily applications over 12 weeks yielding significant amelioration in lines and wrinkles, pigmentation, elasticity, and firmness, as well as overall diminished photodamage without provoking redness. They concluded that bakuchiol can act as an antiaging agent through regulation of gene expression comparable to retinol.1
Retinoids without reactions?
In 2017, Ma et al. set out to synthesize and test in psoriatic cytokine–treated cultures of keratinocytes and organotypic skin substitutes a new substance created by combining two skin-active compounds (bakuchiol and salicylic acid) into bakuchiol salicylate (bakusylan), with the intention of rendering a novel functional retinoid. The researchers reported that the gene expression profile showed elimination of various retinoid-like proinflammatory responses, without a loss of normalizing activity. They concluded that their work may result in a new class of functional retinoids.14
Early this year, Dhaliwal et al. reported on a randomized, double-blind, 12-week study of 44 patients who applied either bakuchiol 0.5% cream twice daily or retinol 0.5% cream daily. Facial photographs were evaluated at baseline, 4, 8, and 12 weeks, and a blinded dermatologist rated pigmentation and erythema. Side effects were also noted by subjects in tolerability assessment questionnaires. Both compounds significantly reduced wrinkles and hyperpigmentation, with no statistical variance found between the two. More facial skin scaling and stinging was experienced by the retinol group. The investigators concluded that bakuchiol exhibits photoaging activity comparable with retinol and appears to be an emerging alternative to retinol because it is better tolerated.12 Notably, there is one report to date of an allergic reaction to topical bakuchiol.15
Topical combination therapies for hyperpigmentation, photodamage, and acne
Bakuchiol was a key ingredient incorporated into a 0.5% retinol treatment evaluated in a 12-week, open-label, single-center clinical-usage trial of 44 women with mild to moderate hyperpigmentation and photodamaged facial skin who took a dual product regimen. This 2016 study showed that the retinol and vitamin C facial regimen yielded a statistically significant amelioration in clinical grading of all parameters.16
A 2015 randomized controlled clinical trial in 111 subjects evaluated the use of adapalene 0.1% gel and a formulation containing bakuchiol, Ginkgo biloba extract, and mannitol in patients with acne. Patients were randomized to the adapalene and botanical formulation or adapalene and vehicle cream for 2 months. Both treatment groups experienced improvements according to all measured outcomes. The botanical formulation was associated with a statistically significant edge over the vehicle combination in reducing inflammatory lesions, investigator global assessment, and intensity of seborrhea. Quality of life was also perceived to be better with the combination of adapalene and the bakuchiol-containing product, which was deemed to be safe with good local tolerability.17
A subsequent evaluation by a different team also considered the antibacterial, anti-inflammatory, and antioxidative potential of this combination product via in vitro, ex vivo, and clinical studies. The work by Trompezinski et al. revealed that bakuchiol displays nearly twice the antioxidative potential asthat of vitamin E. The bakuchiol-containing cream was shown in acne patients to successfully regulate sebum composition by raising linolenic and sapienic acid levels while lowering oleic acid levels. Its efficacy against Propionibacterium acnes was also suggested by a decrease in the number of skin surface porphyrins. The investigators concluded that the formulation serves as an effective adjuvant acne treatment by attacking inflammation, dysseborrhea, and proliferation of Propionibacterium acnes.18
Anticancer activity
In 2016, Kim et al. demonstrated that bakuchiol exhibits chemopreventive activity by hindering epidermal growth factor (EGF)–induced neoplastic cell transformation. In what was the first mechanistic study to reveal molecular targets for the anticancer activity of this substance, the investigators found that bakuchiol also reduced the viability and suppressed anchorage-independent growth of A431 human epithelial carcinoma cells. They identified Hck, Blk, and p38 MAPK as the molecular targets of what they identified as a potent anticancer compound.2
Skin-whitening potential
In 2010, Ohno et al. found that bakuchiol, along with other ingredients, isolated from Piper longum demonstrated strong suppressive activity against melanin production in B16 mouse melanoma cells and may have potential to affect melanin synthesis in human skin.19 Further, with use of a new method for screening tyrosinase, Cheng et al. found in 2017 that four substances used in traditional Chinese medicine (quercetin, kaempferol, bavachinin, and bakuchiol) displayed the potential for inhibiting tyrosinase.20
Conclusion
A compound that acts like a retinoid – yielding antiacne and antiaging effects – without provoking irritation? Most dermatologists and their patients would say, sign me up. Bakuchiol, an active ingredient in various plants, especially Psoralea corylifolia, seems to present that kind of profile. While more research is necessary, experience with this herbal ingredient in traditional medicine and an increasing body of research, including clinical results, provides reasons for optimism that this ingredient may have a versatile role to play in topical skin care, particularly in its retinoid-like functions.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected].
References
1. Chaudhuri RK et al. Int J Cosmet Sci. 2014 Jun;36(3):221-30.
2. Kim JE et al. Oncotarget. 2016 Mar 22;7(12):14616-27.
3. Kim YJ et al. Molecules. 2016 Aug 17. doi: 10.3390/molecules21081076.
4. Xin Z et al. Pharmacol Res. 2019 Mar;141:208-13.
5. Lev-Tov H. Br J Dermatol. 2019 Feb;180(2):253-4.
6. Shrestha S et al. J Ayurveda Integr Med. 2018 Jul - Sep; 9(3):209-12.
7. Li CC et al. Evid Based Complement Alternat Med. 2016. doi: 10.1155/2016/8108643.
8. Hu C et al. Fitoterapia. 2015 Oct;106:129-34.
9. Yan DM et al. J Ethnopharmacol. 2010 Apr 21;128(3):697-702.
10. Choi SY et al. J Med Food. 2010 Aug;13(4):1019-23.
11. Madrid A et al. J Ethnopharmacol. 2012 Dec 18;144(3):809-11.
12. Dhaliwal S et al. Br J Dermatol. 2019 Feb;180(2):289-96.
13. Yu Q et al. Zhong Yao Cai. 2014 Apr;37(4):632-5.
14. Ma S et al. Clin Exp Dermatol. 2017 Apr;42(3):251-60.
15. Malinauskiene L et al. Contact Dermatitis. 2019 Jun;80(6):398-9.
16. Herndon JH Jr, et al. J Drugs Dermatol. 2016 Apr;15(4):476-82.
17. Poláková K et al. Clin Cosmet Investig Dermatol. 2015 Apr 10;8:187-91.
18. Trompezinski S et al. Clin Cosmet Investig Dermatol. 2016 Aug 31;9:233-9.
19. Ohno O et al. Biosci Biotechnol Biochem. 2010;74(7):1504-6.
20. Cheng M et al. Electrophoresis. 2017 Feb;38(3-4):486-93.
Bakuchiol [(1E,3S)-3-ethenyl-3,7-dimethyl-1,6-octadien-1-yl]phenol, a prenylated phenolic monoterpene found in the seeds and leaves of various plants, particularly Psoralea corylifolia, has been used to treat a broad array of disorders, including skin conditions, in the traditional medical practices of China, Japan, and Korea, as well as Ayurvedic medicine in India.1-6 Specifically, the seeds of as well as cardiovascular diseases, nephritis, osteoporosis, and cancer.7-9
This primary active ingredient is reputed to exert antioxidant, antibacterial, anti-inflammatory, antiaging, and estrogen-like functions, and recent data suggest anticancer activity, including activity against skin cancer. Its antiaging properties manifest via preservation of cutaneous collagen.4 The plant itself has displayed a wide range of biological functions, such as antibacterial, anticancer, cytotoxic, cardiac, diaphoretic, diuretic, stimulant, aphrodisiac, and tonifying activities.8,9 A 2016 quantitative analysis of Psoralea corylifolia and seven of its standard constituents (psoralen, angelicin, neobavaisoflavone, psoralidin, isobavachalcone, bavachinin, and bakuchiol) using high-performance liquid chromatography revealed that bakuchiol is the strongest phytochemical ingredient in the plant, which the investigators found also confers neuroprotective and antineuroinflammatory benefits.3
Other species contain bakuchiol, and its biological activities have been harnessed in other folk medical traditions. The monoterpene is an important constituent found in Ulmus davidiana var. japonica, which is used for its anti-inflammatory properties in traditional Korean medicine.10 Further, bakuchiol and 3-hydroxy-bakuchiol have been identified as key components isolated from Psoralea glandulosa, which is a shrub used in Chilean folk medicine to treat cutaneous disorders engendered by bacteria and fungus.11 Topical applications of bakuchiol have been demonstrated to confer antiaging benefits.12 This column briefly identifies some of the various uses emerging for this compelling botanical agent.
Antiaging activities
In 2014, Yu et al. found that bakuchiol may impart antiaging benefits by supporting the cellular activity of the expression level of human skin fibroblasts (ESF-1), as well as production of collagen types I and III, while reducing the matrix metalloproteinase-1 mRNA expression.13
The same year, Chaudhuri et al. compared the skin care–related activities of retinol and bakuchiol, finding their gene expression profiles very similar. In addition, they observed that bakuchiol up-regulated collagen types I and IV in a DNA microarray study and stimulated type III collagen production in a model of mature fibroblasts. Further, the investigators formulated bakuchiol into a skin care product and tested it clinically, with twice daily applications over 12 weeks yielding significant amelioration in lines and wrinkles, pigmentation, elasticity, and firmness, as well as overall diminished photodamage without provoking redness. They concluded that bakuchiol can act as an antiaging agent through regulation of gene expression comparable to retinol.1
Retinoids without reactions?
In 2017, Ma et al. set out to synthesize and test in psoriatic cytokine–treated cultures of keratinocytes and organotypic skin substitutes a new substance created by combining two skin-active compounds (bakuchiol and salicylic acid) into bakuchiol salicylate (bakusylan), with the intention of rendering a novel functional retinoid. The researchers reported that the gene expression profile showed elimination of various retinoid-like proinflammatory responses, without a loss of normalizing activity. They concluded that their work may result in a new class of functional retinoids.14
Early this year, Dhaliwal et al. reported on a randomized, double-blind, 12-week study of 44 patients who applied either bakuchiol 0.5% cream twice daily or retinol 0.5% cream daily. Facial photographs were evaluated at baseline, 4, 8, and 12 weeks, and a blinded dermatologist rated pigmentation and erythema. Side effects were also noted by subjects in tolerability assessment questionnaires. Both compounds significantly reduced wrinkles and hyperpigmentation, with no statistical variance found between the two. More facial skin scaling and stinging was experienced by the retinol group. The investigators concluded that bakuchiol exhibits photoaging activity comparable with retinol and appears to be an emerging alternative to retinol because it is better tolerated.12 Notably, there is one report to date of an allergic reaction to topical bakuchiol.15
Topical combination therapies for hyperpigmentation, photodamage, and acne
Bakuchiol was a key ingredient incorporated into a 0.5% retinol treatment evaluated in a 12-week, open-label, single-center clinical-usage trial of 44 women with mild to moderate hyperpigmentation and photodamaged facial skin who took a dual product regimen. This 2016 study showed that the retinol and vitamin C facial regimen yielded a statistically significant amelioration in clinical grading of all parameters.16
A 2015 randomized controlled clinical trial in 111 subjects evaluated the use of adapalene 0.1% gel and a formulation containing bakuchiol, Ginkgo biloba extract, and mannitol in patients with acne. Patients were randomized to the adapalene and botanical formulation or adapalene and vehicle cream for 2 months. Both treatment groups experienced improvements according to all measured outcomes. The botanical formulation was associated with a statistically significant edge over the vehicle combination in reducing inflammatory lesions, investigator global assessment, and intensity of seborrhea. Quality of life was also perceived to be better with the combination of adapalene and the bakuchiol-containing product, which was deemed to be safe with good local tolerability.17
A subsequent evaluation by a different team also considered the antibacterial, anti-inflammatory, and antioxidative potential of this combination product via in vitro, ex vivo, and clinical studies. The work by Trompezinski et al. revealed that bakuchiol displays nearly twice the antioxidative potential asthat of vitamin E. The bakuchiol-containing cream was shown in acne patients to successfully regulate sebum composition by raising linolenic and sapienic acid levels while lowering oleic acid levels. Its efficacy against Propionibacterium acnes was also suggested by a decrease in the number of skin surface porphyrins. The investigators concluded that the formulation serves as an effective adjuvant acne treatment by attacking inflammation, dysseborrhea, and proliferation of Propionibacterium acnes.18
Anticancer activity
In 2016, Kim et al. demonstrated that bakuchiol exhibits chemopreventive activity by hindering epidermal growth factor (EGF)–induced neoplastic cell transformation. In what was the first mechanistic study to reveal molecular targets for the anticancer activity of this substance, the investigators found that bakuchiol also reduced the viability and suppressed anchorage-independent growth of A431 human epithelial carcinoma cells. They identified Hck, Blk, and p38 MAPK as the molecular targets of what they identified as a potent anticancer compound.2
Skin-whitening potential
In 2010, Ohno et al. found that bakuchiol, along with other ingredients, isolated from Piper longum demonstrated strong suppressive activity against melanin production in B16 mouse melanoma cells and may have potential to affect melanin synthesis in human skin.19 Further, with use of a new method for screening tyrosinase, Cheng et al. found in 2017 that four substances used in traditional Chinese medicine (quercetin, kaempferol, bavachinin, and bakuchiol) displayed the potential for inhibiting tyrosinase.20
Conclusion
A compound that acts like a retinoid – yielding antiacne and antiaging effects – without provoking irritation? Most dermatologists and their patients would say, sign me up. Bakuchiol, an active ingredient in various plants, especially Psoralea corylifolia, seems to present that kind of profile. While more research is necessary, experience with this herbal ingredient in traditional medicine and an increasing body of research, including clinical results, provides reasons for optimism that this ingredient may have a versatile role to play in topical skin care, particularly in its retinoid-like functions.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected].
References
1. Chaudhuri RK et al. Int J Cosmet Sci. 2014 Jun;36(3):221-30.
2. Kim JE et al. Oncotarget. 2016 Mar 22;7(12):14616-27.
3. Kim YJ et al. Molecules. 2016 Aug 17. doi: 10.3390/molecules21081076.
4. Xin Z et al. Pharmacol Res. 2019 Mar;141:208-13.
5. Lev-Tov H. Br J Dermatol. 2019 Feb;180(2):253-4.
6. Shrestha S et al. J Ayurveda Integr Med. 2018 Jul - Sep; 9(3):209-12.
7. Li CC et al. Evid Based Complement Alternat Med. 2016. doi: 10.1155/2016/8108643.
8. Hu C et al. Fitoterapia. 2015 Oct;106:129-34.
9. Yan DM et al. J Ethnopharmacol. 2010 Apr 21;128(3):697-702.
10. Choi SY et al. J Med Food. 2010 Aug;13(4):1019-23.
11. Madrid A et al. J Ethnopharmacol. 2012 Dec 18;144(3):809-11.
12. Dhaliwal S et al. Br J Dermatol. 2019 Feb;180(2):289-96.
13. Yu Q et al. Zhong Yao Cai. 2014 Apr;37(4):632-5.
14. Ma S et al. Clin Exp Dermatol. 2017 Apr;42(3):251-60.
15. Malinauskiene L et al. Contact Dermatitis. 2019 Jun;80(6):398-9.
16. Herndon JH Jr, et al. J Drugs Dermatol. 2016 Apr;15(4):476-82.
17. Poláková K et al. Clin Cosmet Investig Dermatol. 2015 Apr 10;8:187-91.
18. Trompezinski S et al. Clin Cosmet Investig Dermatol. 2016 Aug 31;9:233-9.
19. Ohno O et al. Biosci Biotechnol Biochem. 2010;74(7):1504-6.
20. Cheng M et al. Electrophoresis. 2017 Feb;38(3-4):486-93.
A primer on cannabis for cosmeceuticals: Research and treatments for particular skin conditions
The relatively recent discovery of the endogenous cannabinoid system and the quickly evolving, yet still convoluted, legal status of cannabis in the United States has spurred excitement over expanded research opportunities. Despite its checkered legal history, marijuana – derived from Cannabis sativa and Cannabis indica – has long been used for medical purposes and is one of the most widely used drugs throughout the world.1 Modern medicine has deployed this dynamic plant to treat chronic pain, glaucoma, and nausea, and continues to investigate its application in a broad array of conditions: anorexia, spasticity, atherosclerosis, autoimmune disorders, inflammatory bowel disease, multiple sclerosis, spasticity, tumorigenesis, and multiple cutaneous disorders, including acne, eczematous disorders, lichen simplex, melanoma and nonmelanoma skin cancer, melasma, prurigo, pruritus, psoriasis, scleroderma and systemic sclerosis, and seborrheic dermatitis.1-4 This .

Acne
Oláh et al. have demonstrated that the nonpsychotropic phytocannabinoid ((-)-cannabidiol [CBD]) imparts anti-acne benefits by diminishing sebaceous lipid synthesis, decreasing proliferation, and easing inflammation in human SZ95 sebocytes.5 In additional investigations of nonpsychotropic phytocannabinoids and their effects on human sebocyte function, they reported in 2016 that the phytocannabinoids (-)-cannabigerol [CBG] and (-)-cannabigerovarin (CBGV) appear to exhibit promise in treating xerotic and seborrheic skin, and ((-)-cannabichromene [CBC], (-)-cannabidivarin [CBDV], and (-)-delta9-tetrahydrocannabivarin [THCV], in particular, display notable potential as anti-acne ingredients. The investigators added that these compounds, due to their substantial anti-inflammatory effects, warrant consideration for use in treating skin inflammation.5 Previously, Ali and Akhtar conducted a single-blinded, 12-week comparative study in healthy male volunteers to evaluate the effects of twice-daily application of 3% cannabis seed extract cream on human cheek skin. The researchers found the base with 3% cannabis seed extract to be safe and effective, with skin sebum and erythema content on the treated side reduced significantly compared with the side treated only with the control base. They concluded that this well-tolerated formulation could be indicated for the treatment of acne and seborrhea to enhance facial appearance.6
Psoriasis
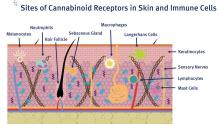
The endocannabinoid system itself is thought to play a potentially important role in the treatment of psoriasis, as interactions between the immune and nervous systems via cholinergic anti-inflammatory pathways are considered to be key in psoriasis etiology and the endocannabinoid system interacts with both systems through the cannabinoid (CB) receptors CB1 and CB2.7 Compared with normal human skin, psoriatic skin is characterized by fewer CB receptors.8
In 2007, Wilkinson and Williamson used a keratinocyte proliferation assay to study the phytocannabinoids delta9-tetrahydrocannabinol (THC), CBD, CBG, and cannabinol (CNB) to assess their capacity to halt the growth of a hyper-proliferating human keratinocyte cell line with an eye toward potential use in treating psoriasis. CB1 and CB2 receptors were confirmed present by Western blot and RT-PCR analyses. All cannabinoids investigated concentration-dependently hindered keratinocyte proliferation, as the authors concluded that these compounds show potential for use in psoriasis treatment.9
In 2013, Ramot et al. found that treating human skin culture with the CB1-specific agonist arachidonoyl-chloro-ethanolamide reduced the expression of keratins K6 and K16 in vitro and in situ, which may have implications for psoriasis treatment as K6 and K16 are upregulated in that disorder.10 The same team has also recently shown that the CB1 agonist arachidonyl-2’-chloroethylamide upregulated K10 protein expression in human epidermis and reduced K1 in human skin culture thus suggesting its potential as a treatment for epidermolytic ichthyosis.11
Notably, the synthetic cannabinoid JWH-133, known for its potent antiangiogenic and anti-inflammatory properties, has been shown in vivo and in vitro to suppress various inflammatory cytokines and angiogenic growth factors involved in psoriasis pathogenesis, including hypoxia inducible factor-1 alpha (HIF-1 alpha), vascular endothelial growth factor (VEGF), matrix metalloproteinases, basic fibroblast growth factor (bFGF), angiopoietin-2, interleukin-8 (IL-8), IL-17, and IL-2. While more research is necessary to determine the safety and efficacy of this product, it appears promising as an anti-psoriatic agent.12
Pruritus
Stimulation of the CB1 receptor has been demonstrated to inhibit histamine-induced pruritus.8
In 2005, Szepietowski et al. conducted a preliminary study to ascertain the efficacy and tolerance of a cream with structured physiological lipids and endogenous cannabinoids in managing pruritus in 21 patients on maintenance dialysis. For 3 weeks, the patients with uremic pruritus applied the test cream twice daily, with eight patients experiencing full eradication of pruritus at the end of this period. Further, xerosis was completely eliminated in 17 patients after the study, and significantly decreased during the 3-week period. The investigators suggested that while more research was needed, the well-tolerated product is thought to have been enhanced by the addition of endocannabinoids.13
A year later, Ständer et al. assessed the effects of the use of the topical cannabinoid agonist N-palmitoyl ethanolamine (PEA), which stimulates the endocannabinoid arachidonoyl ethanolamide (AEA) to activate CB1, in an open application study with 22 patients with prurigo, lichen simplex, and pruritus. Antipruritic benefits were seen in 14 patients, with an average decrease in itch of 86.4%. The treatment was reported to be well tolerated, as no patients complained of adverse effects such as contact dermatitis or a burning sensation.14
Eczematic dermatoses
Atopic dermatitis
In a small pilot study on pediatric atopic dermatitis in 2007, Pulvirenti et al. evaluated the safety and efficacy of the twice-daily application of a topical emulsion containing a synthetic aliamide (adelmidrol 2%), comparable to its parent substance PEA, in the treatment of 11 males and 9 females with atopic dermatitis (AD), whose mean age was 8 years. Among the 20 pediatric patients, 16 experienced complete resolution of symptoms after 4 weeks of treatment and had no relapses at the 8-week follow-up assessment. No improvement was noted in the six patches of AD in six patients with several untreated lesions that served as controls.15 Also in 2007, Del Rosso reported on a trial in which a PEA-containing nonsteroidal cream significantly lowered the mean time between flares in pediatric and adult AD patients.16
One year later, Eberlein et al. evaluated an emollient containing PEA in AD patients, finding that itch severity and sleep loss were improved by an average of 60%, with 38% of participants stopping oral antihistamines, 33.6% discontinuing topical steroid regimens, and 20% ending their use of topical immunomodulators as the study concluded.4,17
In 2018, Río et al. suggested that targeted manipulation of the endocannabinoid system at various AD stages might rein in the inflammatory and immune responses and ensuing alterations in keratinocytes, thus helping to preserve epidermal barrier function.18 As Trusler et al. noted, though, no control groups were used in the latter two studies, so it is unknown what effect the application of the vehicle alone would have had on the pruritus in these patients.19
Allergic contact dermatitis
In 2007, Karsak et al. demonstrated that mice lacking CB1/2 receptors exhibited aggravated contact hypersensitivity, whereas mice with higher levels of AEA evinced lower cutaneous allergic responses.20
Recently, Petrosino et al. provided the first evidence that the nonpsychotropic cannabinoid cannabidiol conferred anti-inflammatory activity in an experimental in vitro model of allergic contact dermatitis.21
Dermatomyositis
Robinson et al. have found that treating blood samples of patients with dermatomyositis with the nonpsychoactive cannabinoid ajulemic acid appears to limit the production of pathogenic cytokines. They suggest that oral administration of this cannabinoid merits consideration for dermatomyositis.22
Skin cancer
In 2015, Glodde et al. used a mouse model to investigate the role of cannabinoids in skin cancer pathogenesis. They considered THC, which binds to CB1 and CB2, and the endogenous cannabinoid system. The researchers found that in a CB receptor-dependent fashion THC significantly hindered the tumor growth of HCmel12 melanomas in vivo, verifying the merit of exogenous cannabinoids in melanoma treatment. They did not identify a role of the endogenous cannabinoid system in skin cancer pathogenesis.23
Additional studies suggest that endocannabinoids, phytocannabinoids, and synthetic cannabinoids diminish skin cancer growth (melanoma and nonmelanoma) in vitro and in vivo through CB receptor-dependent and -independent pathways, though in vivo human studies have not yet been conducted.8,24
Epidermolysis bullosa
In a promising observational study in 2018, Chelliah et al. reported on three cases of self-initiated topical cannabidiol use in patients with epidermolysis bullosa. Each patient experienced more rapid wound healing, less blistering, and reduced pain as a result of cannabidiol treatment, and one was able to discontinue oral opioids. The authors were encouraged by such findings, but cautioned that randomized, double-blind clinical trials are needed to establish cannabidiol as an effective therapy.25
This seems particularly important given the climate of expanding legalization of medical and recreational cannabis use, as well as the increasing use of topical cannabinoids among dermatology patients.26 Nevertheless, it is important to be cognizant of one’s own state laws as topical cannabinoids may be restricted; these products are marketed for pain and pruritus on the Internet but are unavailable by prescription unless the physician has a special license.4
Attitudes about cannabinoid use in dermatology
In an intriguing study last year about the knowledge, cognizance, and perceptions of cannabinoids among dermatologists, Robinson et al. created a 20-question online survey that netted a response rate of 21% (n = 531). In terms of awareness, 29% of respondents did not know that THC is psychoactive and a significant majority (64%) did not know that CBD is not psychoactive. Nevertheless, the majority thought that cannabinoids should be legal for medical treatment (86%), and even more (94%) support researching dermatologic applications of cannabinoids. More responders (86%) would prescribe a Food and Drug Administration–approved cannabinoid-containing topical formulation than an oral product (71%). In also noting that 55% revealed at least one conversation about cannabinoids initiated by a patient in the previous year, while 48% expressed concern about a possible stigma associated with suggesting cannabinoid treatments to patients, Robinson et al. call for further education about the benefits and risks of cutaneous cannabinoids for dermatologists.27
Conclusion
It is important that we educate ourselves as to the effects of orally administered and topical products containing cannabis so that we are prepared for questions from patients. Data on psoriasis, pruritus, eczema, and acne warrant optimism and much additional research. Now that the FDA is allowing research sites to enroll for a special license to investigate schedule I drugs, we stand to learn much more about the various effects on the health benefits of cannabis. Despite the longstanding traditional use of C. sativa and C. indica, we are in the early stages of research on the impact of phytocannabinoids and synthetic cannabinoids on human health and the role that the endocannabinoid system plays. The extant findings provide reasons to consider the endocannabinoid system as a target for therapeutic intervention for various cutaneous disorders as research continues.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. She has no relevant disclosures related to this column. Write to her at [email protected].
References
1. Russo EB. Chem Biodivers. 2007 Aug;4(8):1614-48.
2. Goldenberg M et al. Drug Alcohol Depend. 2017 May 1;174:80-90.
3. Mounessa JS et al. J Am Acad Dermatol. 2017 Jul;77(1):188-90.
4. Shalaby M et al. Pract Dermatol. 2018;68-70.
5. Oláh A et al. Exp Dermatol. 2016 Sep;25(9):701-7.
6. Ali A et al. Pak J Pharm Sci. 2015 Jul;28(4):1389-95.
7. Derakhshan N et al. Curr Clin Pharmacol. 2016;11(2):146-7.
8. Singh D et al. Clin Dermatol. 2018 May-Jun;36(3):399-419.
9. Wilkinson JD et al. J Dermatol Sci. 2007 Feb;45(2):87-92.
10. Ramot Y et al. PeerJ. 2013 Feb 19;1:e40.
11. Ramot Y et al. Br J Dermatol. 2018 Jun;178(6):1469.
12. Norooznezhad AH et al. Med Hypotheses. 2017 Feb;99:15-18.
13. Szepietowski JC et al. Acta Dermatovenerol Croat. 2005;13(2):97-103.
14. Ständer S et al. Hautarzt. 2006 Sep;57(9):801-7.
15. Pulvirenti N et al. Acta Dermatovenerol Croat. 2007;15(2):80-3.
16. Del Rosso JQ. Cosmetic Dermatol. 2007 Apr; 20(4):208-211.
17. Eberlein B et al. J Eur Acad Dermatol Venereol. 2008 Jan;22(1):73-82.
18. Del Río CD et al. Biochem Pharmacol. 2018 Nov;157:122-133.
19. Trusler AR et al. Dermatitis. 2017 Jan/Feb;28(1):22-32.
20. Karsak M et al. Science. 2007 Jun 8;316(5830):1494-7.
21. Petrosino S et al. J Pharmacol Exp Ther. 2018 Jun;365(3):652-63.
22. Robinson ES et al. J Invest Dermatol. 2017 Nov;137(11):2445-7.
23. Glodde N et al. Life Sci. 2015 Oct 1;138:35-40.
24. Soliman E. et al. J Dermatol Clin Res. 2016;4(2):1069-76.
25. Chelliah MP et al. Pediatr Dermatol. 2018 Jul;35(4):e224-e227.
26. Hashim PW et al. Cutis. 2017 Jul;100(1):50-52.
27. Robinson E et al. J Drugs Dermatol. 2018 Dec 1;17(12):1273-8.
The relatively recent discovery of the endogenous cannabinoid system and the quickly evolving, yet still convoluted, legal status of cannabis in the United States has spurred excitement over expanded research opportunities. Despite its checkered legal history, marijuana – derived from Cannabis sativa and Cannabis indica – has long been used for medical purposes and is one of the most widely used drugs throughout the world.1 Modern medicine has deployed this dynamic plant to treat chronic pain, glaucoma, and nausea, and continues to investigate its application in a broad array of conditions: anorexia, spasticity, atherosclerosis, autoimmune disorders, inflammatory bowel disease, multiple sclerosis, spasticity, tumorigenesis, and multiple cutaneous disorders, including acne, eczematous disorders, lichen simplex, melanoma and nonmelanoma skin cancer, melasma, prurigo, pruritus, psoriasis, scleroderma and systemic sclerosis, and seborrheic dermatitis.1-4 This .

Acne
Oláh et al. have demonstrated that the nonpsychotropic phytocannabinoid ((-)-cannabidiol [CBD]) imparts anti-acne benefits by diminishing sebaceous lipid synthesis, decreasing proliferation, and easing inflammation in human SZ95 sebocytes.5 In additional investigations of nonpsychotropic phytocannabinoids and their effects on human sebocyte function, they reported in 2016 that the phytocannabinoids (-)-cannabigerol [CBG] and (-)-cannabigerovarin (CBGV) appear to exhibit promise in treating xerotic and seborrheic skin, and ((-)-cannabichromene [CBC], (-)-cannabidivarin [CBDV], and (-)-delta9-tetrahydrocannabivarin [THCV], in particular, display notable potential as anti-acne ingredients. The investigators added that these compounds, due to their substantial anti-inflammatory effects, warrant consideration for use in treating skin inflammation.5 Previously, Ali and Akhtar conducted a single-blinded, 12-week comparative study in healthy male volunteers to evaluate the effects of twice-daily application of 3% cannabis seed extract cream on human cheek skin. The researchers found the base with 3% cannabis seed extract to be safe and effective, with skin sebum and erythema content on the treated side reduced significantly compared with the side treated only with the control base. They concluded that this well-tolerated formulation could be indicated for the treatment of acne and seborrhea to enhance facial appearance.6
Psoriasis
The endocannabinoid system itself is thought to play a potentially important role in the treatment of psoriasis, as interactions between the immune and nervous systems via cholinergic anti-inflammatory pathways are considered to be key in psoriasis etiology and the endocannabinoid system interacts with both systems through the cannabinoid (CB) receptors CB1 and CB2.7 Compared with normal human skin, psoriatic skin is characterized by fewer CB receptors.8
In 2007, Wilkinson and Williamson used a keratinocyte proliferation assay to study the phytocannabinoids delta9-tetrahydrocannabinol (THC), CBD, CBG, and cannabinol (CNB) to assess their capacity to halt the growth of a hyper-proliferating human keratinocyte cell line with an eye toward potential use in treating psoriasis. CB1 and CB2 receptors were confirmed present by Western blot and RT-PCR analyses. All cannabinoids investigated concentration-dependently hindered keratinocyte proliferation, as the authors concluded that these compounds show potential for use in psoriasis treatment.9
In 2013, Ramot et al. found that treating human skin culture with the CB1-specific agonist arachidonoyl-chloro-ethanolamide reduced the expression of keratins K6 and K16 in vitro and in situ, which may have implications for psoriasis treatment as K6 and K16 are upregulated in that disorder.10 The same team has also recently shown that the CB1 agonist arachidonyl-2’-chloroethylamide upregulated K10 protein expression in human epidermis and reduced K1 in human skin culture thus suggesting its potential as a treatment for epidermolytic ichthyosis.11
Notably, the synthetic cannabinoid JWH-133, known for its potent antiangiogenic and anti-inflammatory properties, has been shown in vivo and in vitro to suppress various inflammatory cytokines and angiogenic growth factors involved in psoriasis pathogenesis, including hypoxia inducible factor-1 alpha (HIF-1 alpha), vascular endothelial growth factor (VEGF), matrix metalloproteinases, basic fibroblast growth factor (bFGF), angiopoietin-2, interleukin-8 (IL-8), IL-17, and IL-2. While more research is necessary to determine the safety and efficacy of this product, it appears promising as an anti-psoriatic agent.12
Pruritus
Stimulation of the CB1 receptor has been demonstrated to inhibit histamine-induced pruritus.8
In 2005, Szepietowski et al. conducted a preliminary study to ascertain the efficacy and tolerance of a cream with structured physiological lipids and endogenous cannabinoids in managing pruritus in 21 patients on maintenance dialysis. For 3 weeks, the patients with uremic pruritus applied the test cream twice daily, with eight patients experiencing full eradication of pruritus at the end of this period. Further, xerosis was completely eliminated in 17 patients after the study, and significantly decreased during the 3-week period. The investigators suggested that while more research was needed, the well-tolerated product is thought to have been enhanced by the addition of endocannabinoids.13
A year later, Ständer et al. assessed the effects of the use of the topical cannabinoid agonist N-palmitoyl ethanolamine (PEA), which stimulates the endocannabinoid arachidonoyl ethanolamide (AEA) to activate CB1, in an open application study with 22 patients with prurigo, lichen simplex, and pruritus. Antipruritic benefits were seen in 14 patients, with an average decrease in itch of 86.4%. The treatment was reported to be well tolerated, as no patients complained of adverse effects such as contact dermatitis or a burning sensation.14
Eczematic dermatoses
Atopic dermatitis
In a small pilot study on pediatric atopic dermatitis in 2007, Pulvirenti et al. evaluated the safety and efficacy of the twice-daily application of a topical emulsion containing a synthetic aliamide (adelmidrol 2%), comparable to its parent substance PEA, in the treatment of 11 males and 9 females with atopic dermatitis (AD), whose mean age was 8 years. Among the 20 pediatric patients, 16 experienced complete resolution of symptoms after 4 weeks of treatment and had no relapses at the 8-week follow-up assessment. No improvement was noted in the six patches of AD in six patients with several untreated lesions that served as controls.15 Also in 2007, Del Rosso reported on a trial in which a PEA-containing nonsteroidal cream significantly lowered the mean time between flares in pediatric and adult AD patients.16
One year later, Eberlein et al. evaluated an emollient containing PEA in AD patients, finding that itch severity and sleep loss were improved by an average of 60%, with 38% of participants stopping oral antihistamines, 33.6% discontinuing topical steroid regimens, and 20% ending their use of topical immunomodulators as the study concluded.4,17
In 2018, Río et al. suggested that targeted manipulation of the endocannabinoid system at various AD stages might rein in the inflammatory and immune responses and ensuing alterations in keratinocytes, thus helping to preserve epidermal barrier function.18 As Trusler et al. noted, though, no control groups were used in the latter two studies, so it is unknown what effect the application of the vehicle alone would have had on the pruritus in these patients.19
Allergic contact dermatitis
In 2007, Karsak et al. demonstrated that mice lacking CB1/2 receptors exhibited aggravated contact hypersensitivity, whereas mice with higher levels of AEA evinced lower cutaneous allergic responses.20
Recently, Petrosino et al. provided the first evidence that the nonpsychotropic cannabinoid cannabidiol conferred anti-inflammatory activity in an experimental in vitro model of allergic contact dermatitis.21
Dermatomyositis
Robinson et al. have found that treating blood samples of patients with dermatomyositis with the nonpsychoactive cannabinoid ajulemic acid appears to limit the production of pathogenic cytokines. They suggest that oral administration of this cannabinoid merits consideration for dermatomyositis.22
Skin cancer
In 2015, Glodde et al. used a mouse model to investigate the role of cannabinoids in skin cancer pathogenesis. They considered THC, which binds to CB1 and CB2, and the endogenous cannabinoid system. The researchers found that in a CB receptor-dependent fashion THC significantly hindered the tumor growth of HCmel12 melanomas in vivo, verifying the merit of exogenous cannabinoids in melanoma treatment. They did not identify a role of the endogenous cannabinoid system in skin cancer pathogenesis.23
Additional studies suggest that endocannabinoids, phytocannabinoids, and synthetic cannabinoids diminish skin cancer growth (melanoma and nonmelanoma) in vitro and in vivo through CB receptor-dependent and -independent pathways, though in vivo human studies have not yet been conducted.8,24
Epidermolysis bullosa
In a promising observational study in 2018, Chelliah et al. reported on three cases of self-initiated topical cannabidiol use in patients with epidermolysis bullosa. Each patient experienced more rapid wound healing, less blistering, and reduced pain as a result of cannabidiol treatment, and one was able to discontinue oral opioids. The authors were encouraged by such findings, but cautioned that randomized, double-blind clinical trials are needed to establish cannabidiol as an effective therapy.25
This seems particularly important given the climate of expanding legalization of medical and recreational cannabis use, as well as the increasing use of topical cannabinoids among dermatology patients.26 Nevertheless, it is important to be cognizant of one’s own state laws as topical cannabinoids may be restricted; these products are marketed for pain and pruritus on the Internet but are unavailable by prescription unless the physician has a special license.4
Attitudes about cannabinoid use in dermatology
In an intriguing study last year about the knowledge, cognizance, and perceptions of cannabinoids among dermatologists, Robinson et al. created a 20-question online survey that netted a response rate of 21% (n = 531). In terms of awareness, 29% of respondents did not know that THC is psychoactive and a significant majority (64%) did not know that CBD is not psychoactive. Nevertheless, the majority thought that cannabinoids should be legal for medical treatment (86%), and even more (94%) support researching dermatologic applications of cannabinoids. More responders (86%) would prescribe a Food and Drug Administration–approved cannabinoid-containing topical formulation than an oral product (71%). In also noting that 55% revealed at least one conversation about cannabinoids initiated by a patient in the previous year, while 48% expressed concern about a possible stigma associated with suggesting cannabinoid treatments to patients, Robinson et al. call for further education about the benefits and risks of cutaneous cannabinoids for dermatologists.27
Conclusion
It is important that we educate ourselves as to the effects of orally administered and topical products containing cannabis so that we are prepared for questions from patients. Data on psoriasis, pruritus, eczema, and acne warrant optimism and much additional research. Now that the FDA is allowing research sites to enroll for a special license to investigate schedule I drugs, we stand to learn much more about the various effects on the health benefits of cannabis. Despite the longstanding traditional use of C. sativa and C. indica, we are in the early stages of research on the impact of phytocannabinoids and synthetic cannabinoids on human health and the role that the endocannabinoid system plays. The extant findings provide reasons to consider the endocannabinoid system as a target for therapeutic intervention for various cutaneous disorders as research continues.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. She has no relevant disclosures related to this column. Write to her at [email protected].
References
1. Russo EB. Chem Biodivers. 2007 Aug;4(8):1614-48.
2. Goldenberg M et al. Drug Alcohol Depend. 2017 May 1;174:80-90.
3. Mounessa JS et al. J Am Acad Dermatol. 2017 Jul;77(1):188-90.
4. Shalaby M et al. Pract Dermatol. 2018;68-70.
5. Oláh A et al. Exp Dermatol. 2016 Sep;25(9):701-7.
6. Ali A et al. Pak J Pharm Sci. 2015 Jul;28(4):1389-95.
7. Derakhshan N et al. Curr Clin Pharmacol. 2016;11(2):146-7.
8. Singh D et al. Clin Dermatol. 2018 May-Jun;36(3):399-419.
9. Wilkinson JD et al. J Dermatol Sci. 2007 Feb;45(2):87-92.
10. Ramot Y et al. PeerJ. 2013 Feb 19;1:e40.
11. Ramot Y et al. Br J Dermatol. 2018 Jun;178(6):1469.
12. Norooznezhad AH et al. Med Hypotheses. 2017 Feb;99:15-18.
13. Szepietowski JC et al. Acta Dermatovenerol Croat. 2005;13(2):97-103.
14. Ständer S et al. Hautarzt. 2006 Sep;57(9):801-7.
15. Pulvirenti N et al. Acta Dermatovenerol Croat. 2007;15(2):80-3.
16. Del Rosso JQ. Cosmetic Dermatol. 2007 Apr; 20(4):208-211.
17. Eberlein B et al. J Eur Acad Dermatol Venereol. 2008 Jan;22(1):73-82.
18. Del Río CD et al. Biochem Pharmacol. 2018 Nov;157:122-133.
19. Trusler AR et al. Dermatitis. 2017 Jan/Feb;28(1):22-32.
20. Karsak M et al. Science. 2007 Jun 8;316(5830):1494-7.
21. Petrosino S et al. J Pharmacol Exp Ther. 2018 Jun;365(3):652-63.
22. Robinson ES et al. J Invest Dermatol. 2017 Nov;137(11):2445-7.
23. Glodde N et al. Life Sci. 2015 Oct 1;138:35-40.
24. Soliman E. et al. J Dermatol Clin Res. 2016;4(2):1069-76.
25. Chelliah MP et al. Pediatr Dermatol. 2018 Jul;35(4):e224-e227.
26. Hashim PW et al. Cutis. 2017 Jul;100(1):50-52.
27. Robinson E et al. J Drugs Dermatol. 2018 Dec 1;17(12):1273-8.
The relatively recent discovery of the endogenous cannabinoid system and the quickly evolving, yet still convoluted, legal status of cannabis in the United States has spurred excitement over expanded research opportunities. Despite its checkered legal history, marijuana – derived from Cannabis sativa and Cannabis indica – has long been used for medical purposes and is one of the most widely used drugs throughout the world.1 Modern medicine has deployed this dynamic plant to treat chronic pain, glaucoma, and nausea, and continues to investigate its application in a broad array of conditions: anorexia, spasticity, atherosclerosis, autoimmune disorders, inflammatory bowel disease, multiple sclerosis, spasticity, tumorigenesis, and multiple cutaneous disorders, including acne, eczematous disorders, lichen simplex, melanoma and nonmelanoma skin cancer, melasma, prurigo, pruritus, psoriasis, scleroderma and systemic sclerosis, and seborrheic dermatitis.1-4 This .

Acne
Oláh et al. have demonstrated that the nonpsychotropic phytocannabinoid ((-)-cannabidiol [CBD]) imparts anti-acne benefits by diminishing sebaceous lipid synthesis, decreasing proliferation, and easing inflammation in human SZ95 sebocytes.5 In additional investigations of nonpsychotropic phytocannabinoids and their effects on human sebocyte function, they reported in 2016 that the phytocannabinoids (-)-cannabigerol [CBG] and (-)-cannabigerovarin (CBGV) appear to exhibit promise in treating xerotic and seborrheic skin, and ((-)-cannabichromene [CBC], (-)-cannabidivarin [CBDV], and (-)-delta9-tetrahydrocannabivarin [THCV], in particular, display notable potential as anti-acne ingredients. The investigators added that these compounds, due to their substantial anti-inflammatory effects, warrant consideration for use in treating skin inflammation.5 Previously, Ali and Akhtar conducted a single-blinded, 12-week comparative study in healthy male volunteers to evaluate the effects of twice-daily application of 3% cannabis seed extract cream on human cheek skin. The researchers found the base with 3% cannabis seed extract to be safe and effective, with skin sebum and erythema content on the treated side reduced significantly compared with the side treated only with the control base. They concluded that this well-tolerated formulation could be indicated for the treatment of acne and seborrhea to enhance facial appearance.6
Psoriasis
The endocannabinoid system itself is thought to play a potentially important role in the treatment of psoriasis, as interactions between the immune and nervous systems via cholinergic anti-inflammatory pathways are considered to be key in psoriasis etiology and the endocannabinoid system interacts with both systems through the cannabinoid (CB) receptors CB1 and CB2.7 Compared with normal human skin, psoriatic skin is characterized by fewer CB receptors.8
In 2007, Wilkinson and Williamson used a keratinocyte proliferation assay to study the phytocannabinoids delta9-tetrahydrocannabinol (THC), CBD, CBG, and cannabinol (CNB) to assess their capacity to halt the growth of a hyper-proliferating human keratinocyte cell line with an eye toward potential use in treating psoriasis. CB1 and CB2 receptors were confirmed present by Western blot and RT-PCR analyses. All cannabinoids investigated concentration-dependently hindered keratinocyte proliferation, as the authors concluded that these compounds show potential for use in psoriasis treatment.9
In 2013, Ramot et al. found that treating human skin culture with the CB1-specific agonist arachidonoyl-chloro-ethanolamide reduced the expression of keratins K6 and K16 in vitro and in situ, which may have implications for psoriasis treatment as K6 and K16 are upregulated in that disorder.10 The same team has also recently shown that the CB1 agonist arachidonyl-2’-chloroethylamide upregulated K10 protein expression in human epidermis and reduced K1 in human skin culture thus suggesting its potential as a treatment for epidermolytic ichthyosis.11
Notably, the synthetic cannabinoid JWH-133, known for its potent antiangiogenic and anti-inflammatory properties, has been shown in vivo and in vitro to suppress various inflammatory cytokines and angiogenic growth factors involved in psoriasis pathogenesis, including hypoxia inducible factor-1 alpha (HIF-1 alpha), vascular endothelial growth factor (VEGF), matrix metalloproteinases, basic fibroblast growth factor (bFGF), angiopoietin-2, interleukin-8 (IL-8), IL-17, and IL-2. While more research is necessary to determine the safety and efficacy of this product, it appears promising as an anti-psoriatic agent.12
Pruritus
Stimulation of the CB1 receptor has been demonstrated to inhibit histamine-induced pruritus.8
In 2005, Szepietowski et al. conducted a preliminary study to ascertain the efficacy and tolerance of a cream with structured physiological lipids and endogenous cannabinoids in managing pruritus in 21 patients on maintenance dialysis. For 3 weeks, the patients with uremic pruritus applied the test cream twice daily, with eight patients experiencing full eradication of pruritus at the end of this period. Further, xerosis was completely eliminated in 17 patients after the study, and significantly decreased during the 3-week period. The investigators suggested that while more research was needed, the well-tolerated product is thought to have been enhanced by the addition of endocannabinoids.13
A year later, Ständer et al. assessed the effects of the use of the topical cannabinoid agonist N-palmitoyl ethanolamine (PEA), which stimulates the endocannabinoid arachidonoyl ethanolamide (AEA) to activate CB1, in an open application study with 22 patients with prurigo, lichen simplex, and pruritus. Antipruritic benefits were seen in 14 patients, with an average decrease in itch of 86.4%. The treatment was reported to be well tolerated, as no patients complained of adverse effects such as contact dermatitis or a burning sensation.14
Eczematic dermatoses
Atopic dermatitis
In a small pilot study on pediatric atopic dermatitis in 2007, Pulvirenti et al. evaluated the safety and efficacy of the twice-daily application of a topical emulsion containing a synthetic aliamide (adelmidrol 2%), comparable to its parent substance PEA, in the treatment of 11 males and 9 females with atopic dermatitis (AD), whose mean age was 8 years. Among the 20 pediatric patients, 16 experienced complete resolution of symptoms after 4 weeks of treatment and had no relapses at the 8-week follow-up assessment. No improvement was noted in the six patches of AD in six patients with several untreated lesions that served as controls.15 Also in 2007, Del Rosso reported on a trial in which a PEA-containing nonsteroidal cream significantly lowered the mean time between flares in pediatric and adult AD patients.16
One year later, Eberlein et al. evaluated an emollient containing PEA in AD patients, finding that itch severity and sleep loss were improved by an average of 60%, with 38% of participants stopping oral antihistamines, 33.6% discontinuing topical steroid regimens, and 20% ending their use of topical immunomodulators as the study concluded.4,17
In 2018, Río et al. suggested that targeted manipulation of the endocannabinoid system at various AD stages might rein in the inflammatory and immune responses and ensuing alterations in keratinocytes, thus helping to preserve epidermal barrier function.18 As Trusler et al. noted, though, no control groups were used in the latter two studies, so it is unknown what effect the application of the vehicle alone would have had on the pruritus in these patients.19
Allergic contact dermatitis
In 2007, Karsak et al. demonstrated that mice lacking CB1/2 receptors exhibited aggravated contact hypersensitivity, whereas mice with higher levels of AEA evinced lower cutaneous allergic responses.20
Recently, Petrosino et al. provided the first evidence that the nonpsychotropic cannabinoid cannabidiol conferred anti-inflammatory activity in an experimental in vitro model of allergic contact dermatitis.21
Dermatomyositis
Robinson et al. have found that treating blood samples of patients with dermatomyositis with the nonpsychoactive cannabinoid ajulemic acid appears to limit the production of pathogenic cytokines. They suggest that oral administration of this cannabinoid merits consideration for dermatomyositis.22
Skin cancer
In 2015, Glodde et al. used a mouse model to investigate the role of cannabinoids in skin cancer pathogenesis. They considered THC, which binds to CB1 and CB2, and the endogenous cannabinoid system. The researchers found that in a CB receptor-dependent fashion THC significantly hindered the tumor growth of HCmel12 melanomas in vivo, verifying the merit of exogenous cannabinoids in melanoma treatment. They did not identify a role of the endogenous cannabinoid system in skin cancer pathogenesis.23
Additional studies suggest that endocannabinoids, phytocannabinoids, and synthetic cannabinoids diminish skin cancer growth (melanoma and nonmelanoma) in vitro and in vivo through CB receptor-dependent and -independent pathways, though in vivo human studies have not yet been conducted.8,24
Epidermolysis bullosa
In a promising observational study in 2018, Chelliah et al. reported on three cases of self-initiated topical cannabidiol use in patients with epidermolysis bullosa. Each patient experienced more rapid wound healing, less blistering, and reduced pain as a result of cannabidiol treatment, and one was able to discontinue oral opioids. The authors were encouraged by such findings, but cautioned that randomized, double-blind clinical trials are needed to establish cannabidiol as an effective therapy.25
This seems particularly important given the climate of expanding legalization of medical and recreational cannabis use, as well as the increasing use of topical cannabinoids among dermatology patients.26 Nevertheless, it is important to be cognizant of one’s own state laws as topical cannabinoids may be restricted; these products are marketed for pain and pruritus on the Internet but are unavailable by prescription unless the physician has a special license.4
Attitudes about cannabinoid use in dermatology
In an intriguing study last year about the knowledge, cognizance, and perceptions of cannabinoids among dermatologists, Robinson et al. created a 20-question online survey that netted a response rate of 21% (n = 531). In terms of awareness, 29% of respondents did not know that THC is psychoactive and a significant majority (64%) did not know that CBD is not psychoactive. Nevertheless, the majority thought that cannabinoids should be legal for medical treatment (86%), and even more (94%) support researching dermatologic applications of cannabinoids. More responders (86%) would prescribe a Food and Drug Administration–approved cannabinoid-containing topical formulation than an oral product (71%). In also noting that 55% revealed at least one conversation about cannabinoids initiated by a patient in the previous year, while 48% expressed concern about a possible stigma associated with suggesting cannabinoid treatments to patients, Robinson et al. call for further education about the benefits and risks of cutaneous cannabinoids for dermatologists.27
Conclusion
It is important that we educate ourselves as to the effects of orally administered and topical products containing cannabis so that we are prepared for questions from patients. Data on psoriasis, pruritus, eczema, and acne warrant optimism and much additional research. Now that the FDA is allowing research sites to enroll for a special license to investigate schedule I drugs, we stand to learn much more about the various effects on the health benefits of cannabis. Despite the longstanding traditional use of C. sativa and C. indica, we are in the early stages of research on the impact of phytocannabinoids and synthetic cannabinoids on human health and the role that the endocannabinoid system plays. The extant findings provide reasons to consider the endocannabinoid system as a target for therapeutic intervention for various cutaneous disorders as research continues.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. She has no relevant disclosures related to this column. Write to her at [email protected].
References
1. Russo EB. Chem Biodivers. 2007 Aug;4(8):1614-48.
2. Goldenberg M et al. Drug Alcohol Depend. 2017 May 1;174:80-90.
3. Mounessa JS et al. J Am Acad Dermatol. 2017 Jul;77(1):188-90.
4. Shalaby M et al. Pract Dermatol. 2018;68-70.
5. Oláh A et al. Exp Dermatol. 2016 Sep;25(9):701-7.
6. Ali A et al. Pak J Pharm Sci. 2015 Jul;28(4):1389-95.
7. Derakhshan N et al. Curr Clin Pharmacol. 2016;11(2):146-7.
8. Singh D et al. Clin Dermatol. 2018 May-Jun;36(3):399-419.
9. Wilkinson JD et al. J Dermatol Sci. 2007 Feb;45(2):87-92.
10. Ramot Y et al. PeerJ. 2013 Feb 19;1:e40.
11. Ramot Y et al. Br J Dermatol. 2018 Jun;178(6):1469.
12. Norooznezhad AH et al. Med Hypotheses. 2017 Feb;99:15-18.
13. Szepietowski JC et al. Acta Dermatovenerol Croat. 2005;13(2):97-103.
14. Ständer S et al. Hautarzt. 2006 Sep;57(9):801-7.
15. Pulvirenti N et al. Acta Dermatovenerol Croat. 2007;15(2):80-3.
16. Del Rosso JQ. Cosmetic Dermatol. 2007 Apr; 20(4):208-211.
17. Eberlein B et al. J Eur Acad Dermatol Venereol. 2008 Jan;22(1):73-82.
18. Del Río CD et al. Biochem Pharmacol. 2018 Nov;157:122-133.
19. Trusler AR et al. Dermatitis. 2017 Jan/Feb;28(1):22-32.
20. Karsak M et al. Science. 2007 Jun 8;316(5830):1494-7.
21. Petrosino S et al. J Pharmacol Exp Ther. 2018 Jun;365(3):652-63.
22. Robinson ES et al. J Invest Dermatol. 2017 Nov;137(11):2445-7.
23. Glodde N et al. Life Sci. 2015 Oct 1;138:35-40.
24. Soliman E. et al. J Dermatol Clin Res. 2016;4(2):1069-76.
25. Chelliah MP et al. Pediatr Dermatol. 2018 Jul;35(4):e224-e227.
26. Hashim PW et al. Cutis. 2017 Jul;100(1):50-52.
27. Robinson E et al. J Drugs Dermatol. 2018 Dec 1;17(12):1273-8.
A primer on cannabis for cosmeceuticals: The endocannabinoid system
In the United States, 31 states, the District of Columbia, Puerto Rico, and Guam have legalized medical marijuana, which is also permitted for recreational use in 9 states, as well as in the District of Columbia. However, marijuana, derived from Cannabis sativa and Cannabis indica, is regulated as a schedule I drug in the United States at the federal level. (Some believe that the federal status may change in the coming year as a result of the Democratic Party’s takeover in the House of Representatives.1)
Cannabis species contain hundreds of various substances, of which the cannabinoids are the most studied. More than 113 biologically active chemical compounds are found within the class of cannabinoids and their derivatives,2 which have been used for centuries in natural medicine.3 The legal status of marijuana has long hampered scientific research of cannabinoids. Nevertheless, the number of studies focusing on the therapeutic potential of these compounds has steadily risen as the legal landscape of marijuana has evolved.
Findings over the last 20 years have shown that cannabinoids present in C. sativa exhibit anti-inflammatory activity and suppress the proliferation of multiple tumorigenic cell lines, some of which are moderated through cannabinoid (CB) receptors.4 In addition to anti-inflammatory properties, .3 Recent research has demonstrated that CB receptors are present in human skin.4
The endocannabinoid system has emerged as an intriguing area of research, as we’ve come to learn about its convoluted role in human anatomy and health. It features a pervasive network of endogenous ligands, enzymes, and receptors, which exogenous substances (including phytocannabinoids and synthetic cannabinoids) can activate.5 Data from recent studies indicate that the endocannabinoid system plays a significant role in cutaneous homeostasis, as it regulates proliferation, differentiation, and inflammatory mediator release.5 Further, psoriasis, atopic dermatitis, pruritus, and wound healing have been identified in recent research as cutaneous concerns in which the use of cannabinoids may be of benefit.6,7 We must also consider reports that cannabinoids can slow human hair growth and that some constituents may spur the synthesis of pro-inflammatory cytokines.8,9This column will briefly address potential confusion over the psychoactive aspects of cannabis, which are related to particular constituents of cannabis and specific CB receptors, and focus on the endocannabinoid system.
Psychoactive or not?
C. sativa confers biological activity through its influence on the G-protein-coupled receptor types CB1 and CB2,10 which pervade human skin epithelium.11 CB1 receptors are found in greatest supply in the central nervous system, especially the basal ganglia, cerebellum, hippocampus, and prefrontal cortex, where their activation yields psychoactivity.2,5,12,13 Stimulation of CB1 receptors in the skin – where they are present in differentiated keratinocytes, hair follicle cells, immune cells, sebaceous glands, and sensory neurons14 – diminishes pain and pruritus, controls keratinocyte differentiation and proliferation, inhibits hair follicle growth, and regulates the release of damage-induced keratins and inflammatory mediators to maintain cutaneous homeostasis.11,14,15
CB2 receptors are expressed in the immune system, particularly monocytes, macrophages, as well as B and T cells, and in peripheral tissues including the spleen, tonsils, thymus gland, bone, and, notably, the skin.2,16 Stimulation of CB2 receptors in the skin – where they are found in keratinocytes, immune cells, sebaceous glands, and sensory neurons – fosters sebum production, regulates pain sensation, hinders keratinocyte differentiation and proliferation, and suppresses cutaneous inflammatory responses.14,15
The best known, or most notorious, component of exogenous cannabinoids is delta9-tetrahydrocannabinol (delta9-THC or simply THC), which is a natural psychoactive constituent in marijuana.3 In fact, of the five primary cannabinoids derived from marijuana, including cannabidiol (CBD), cannabichromene (CBC), cannabigerol (CBG), cannabinol (CBN), and THC, only THC imparts psychoactive effects.17
CBD is thought to exhibit anti-inflammatory and analgesic activities.18 THC has been found to have the capacity to induce cancer cell apoptosis and block angiogenesis,19 and is thought to have immunomodulatory potential, partly acting through the G-protein-coupled CB1 and CB2 receptors but also yielding effects not related to these receptors.20In a 2014 survey of medical cannabis users, a statistically significant preference for C. indica (which contains higher CBD and lower THC levels) was observed for pain management, sedation, and sleep, while C. sativa was associated with euphoria and improving energy.21
The endocannabinoid system and skin health
The endogenous cannabinoid or endocannabinoid system includes cannabinoid receptors, associated endogenous ligands (such as arachidonoyl ethanolamide [anandamide or AEA], 2-arachidonoyl glycerol [2-AG], and N-palmitoylethanolamide [PEA], a fatty acid amide that enhances AEA activity),2 and enzymes involved in endocannabinoid production and decay.11,15,22,23 Research in recent years appears to support the notion that the endocannabinoid system plays an important role in skin health, as its dysregulation has been linked to atopic dermatitis, psoriasis, scleroderma, and skin cancer. Data indicate that exogenous and endogenous cannabinoids influence the endocannabinoid system through cannabinoid receptors, transient receptor potential channels (TRPs), and peroxisome proliferator–activated receptors (PPARs). Río et al. suggest that the dynamism of the endocannabinoid system buttresses the targeting of multiple endpoints for therapeutic success with cannabinoids rather than the one-disease-one-target approach.24 Endogenous cannabinoids, such as arachidonoyl ethanolamide and 2-arachidonoylglycerol, are now thought to be significant mediators in the skin.3 Further, endocannabinoids have been shown to deliver analgesia to the skin, at the spinal and supraspinal levels.25
Anti-inflammatory activity
In 2010, Tubaro et al. used the Croton oil mouse ear dermatitis assay to study the in vivo topical anti-inflammatory effects of seven phytocannabinoids and their related cannabivarins (nonpsychoactive cannabinoids). They found that anti-inflammatory activity was derived from the involvement of the cannabinoid receptors as well as the inflammatory endpoints that the phytocannabinoids targeted.26
In 2013, Gaffal et al. explored the anti-inflammatory activity of topical THC in dinitrofluorobenzene-mediated allergic contact dermatitis independent of CB1/2 receptors by using wild-type and CB1/2 receptor-deficient mice. The researchers found that topically applied THC reduced contact allergic ear edema and myeloid immune cell infiltration in both groups of mice. They concluded that such a decline in inflammation resulted from mitigating the keratinocyte-derived proinflammatory mediators that direct myeloid immune cell infiltration independent of CB1/2 receptors, and positions cannabinoids well for future use in treating inflammatory cutaneous conditions.20
Literature reviews
In a 2018 literature review on the uses of cannabinoids for cutaneous disorders, Eagleston et al. determined that preclinical data on cannabinoids reveal the potential to treat acne, allergic contact dermatitis, asteatotic dermatitis, atopic dermatitis, hidradenitis suppurativa, Kaposi sarcoma, pruritus, psoriasis, skin cancer, and the skin symptoms of systemic sclerosis. They caution, though, that more preclinical work is necessary along with randomized, controlled trials with sufficiently large sample sizes to establish the safety and efficacy of cannabinoids to treat skin conditions.27
A literature review by Marks and Friedman published later that year on the therapeutic potential of phytocannabinoids, endocannabinoids, and synthetic cannabinoids in managing skin disorders revealed the same findings regarding the cutaneous conditions associated with these compounds. The authors noted, though, that while the preponderance of articles highlight the efficacy of cannabinoids in treating inflammatory and neoplastic cutaneous conditions, some reports indicate proinflammatory and proneoplastic activities of cannabinoids. Like Eagleston et al., they call for additional studies.28
Conclusion
As in many botanical agents that I cover in this column, cannabis is associated with numerous medical benefits. I am encouraged to see expanding legalization of medical marijuana and increased research into its reputedly broad potential to improve human health. Anecdotally, I have heard stunning reports from patients about amelioration of joint and back pain as well as relief from other inflammatory symptoms. Discovery and elucidation of the endogenous cannabinoid system is a recent development. Research on its functions and roles in cutaneous health has followed suit and is steadily increasing. Particular skin conditions for which cannabis and cannabinoids may be indicated will be the focus of the next column.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected]
References
1. Higdon J. Why 2019 could be marijuana’s biggest year yet. Politico Magazine. Jan 21, 2019.
2. Singh D et al. Clin Dermatol. 2018 May-Jun;36(3):399-419.
3. Kupczyk P et al. Exp Dermatol. 2009 Aug;18(8):669-79.
4. Wilkinson JD et al. J Dermatol Sci. 2007 Feb;45(2):87-92.
5. Milando R et al. Am J Clin Dermatol. 2019 April;20(2):167-80.
6. Robinson E et al. J Drugs Dermatol. 2018 Dec 1;17(12):1273-8.
7. Mounessa JS et al. J Am Acad Dermatol. 2017 Jul;77(1):188-90.
8. Liszewski W et al. J Am Acad Dermatol. 2017 Sep;77(3):e87-e88.
9. Telek A et al. FASEB J. 2007 Nov;21(13):3534-41.
10. Wollenberg A et al. Br J Dermatol. 2014 Jul;170 Suppl 1:7-11.
11. Ramot Y et al. PeerJ. 2013 Feb 19;1:e40.
12. Schlicker E et al. Trends Pharmacol Sci. 2001 Nov;22(11):565-72.
13. Christie MJ et al. Nature. 2001 Mar 29;410(6828):527-30.
14. Ibid.
15. Bíró T et al. Trends Pharmacol Sci. 2009 Aug;30(8):411-20.
16. Pacher P et al. Pharmacol Rev. 2006 Sep;58(3):389-462.
17. Shalaby M et al. Pract Dermatol. 2018 Jan;68-70.
18. Chelliah MP et al. Pediatr Dermatol. 2018 Jul;35(4):e224-e227.
19. Glodde N et al. Life Sci. 2015 Oct 1;138:35-40.
20. Gaffal E et al. Allergy. 2013 Aug;68(8):994-1000.
21. Pearce DD et al. J Altern Complement Med. 2014 Oct;20(10):787:91.
22. Leonti M et al. Biochem Pharmacol. 2010 Jun 15;79(12):1815-26.
23. Trusler AR et al. Dermatitis. 2017 Jan/Feb;28(1):22-32.
24. Río CD et al. Biochem Pharmacol. 2018 Nov;157:122-133.
25. Chuquilin M et al. J Am Acad Dermatol. 2016 Feb;74(2):197-212.
26. Tubaro A et al. Fitoterapia. 2010 Oct;81(7):816-9.
27. Eagleston LRM et al. Dermatol Online J. 2018 Jun 15;24(6).
28. Marks DH et al. Skin Therapy Lett. 2018 Nov;23(6):1-5.
In the United States, 31 states, the District of Columbia, Puerto Rico, and Guam have legalized medical marijuana, which is also permitted for recreational use in 9 states, as well as in the District of Columbia. However, marijuana, derived from Cannabis sativa and Cannabis indica, is regulated as a schedule I drug in the United States at the federal level. (Some believe that the federal status may change in the coming year as a result of the Democratic Party’s takeover in the House of Representatives.1)
Cannabis species contain hundreds of various substances, of which the cannabinoids are the most studied. More than 113 biologically active chemical compounds are found within the class of cannabinoids and their derivatives,2 which have been used for centuries in natural medicine.3 The legal status of marijuana has long hampered scientific research of cannabinoids. Nevertheless, the number of studies focusing on the therapeutic potential of these compounds has steadily risen as the legal landscape of marijuana has evolved.
Findings over the last 20 years have shown that cannabinoids present in C. sativa exhibit anti-inflammatory activity and suppress the proliferation of multiple tumorigenic cell lines, some of which are moderated through cannabinoid (CB) receptors.4 In addition to anti-inflammatory properties, .3 Recent research has demonstrated that CB receptors are present in human skin.4
The endocannabinoid system has emerged as an intriguing area of research, as we’ve come to learn about its convoluted role in human anatomy and health. It features a pervasive network of endogenous ligands, enzymes, and receptors, which exogenous substances (including phytocannabinoids and synthetic cannabinoids) can activate.5 Data from recent studies indicate that the endocannabinoid system plays a significant role in cutaneous homeostasis, as it regulates proliferation, differentiation, and inflammatory mediator release.5 Further, psoriasis, atopic dermatitis, pruritus, and wound healing have been identified in recent research as cutaneous concerns in which the use of cannabinoids may be of benefit.6,7 We must also consider reports that cannabinoids can slow human hair growth and that some constituents may spur the synthesis of pro-inflammatory cytokines.8,9This column will briefly address potential confusion over the psychoactive aspects of cannabis, which are related to particular constituents of cannabis and specific CB receptors, and focus on the endocannabinoid system.
Psychoactive or not?
C. sativa confers biological activity through its influence on the G-protein-coupled receptor types CB1 and CB2,10 which pervade human skin epithelium.11 CB1 receptors are found in greatest supply in the central nervous system, especially the basal ganglia, cerebellum, hippocampus, and prefrontal cortex, where their activation yields psychoactivity.2,5,12,13 Stimulation of CB1 receptors in the skin – where they are present in differentiated keratinocytes, hair follicle cells, immune cells, sebaceous glands, and sensory neurons14 – diminishes pain and pruritus, controls keratinocyte differentiation and proliferation, inhibits hair follicle growth, and regulates the release of damage-induced keratins and inflammatory mediators to maintain cutaneous homeostasis.11,14,15
CB2 receptors are expressed in the immune system, particularly monocytes, macrophages, as well as B and T cells, and in peripheral tissues including the spleen, tonsils, thymus gland, bone, and, notably, the skin.2,16 Stimulation of CB2 receptors in the skin – where they are found in keratinocytes, immune cells, sebaceous glands, and sensory neurons – fosters sebum production, regulates pain sensation, hinders keratinocyte differentiation and proliferation, and suppresses cutaneous inflammatory responses.14,15
The best known, or most notorious, component of exogenous cannabinoids is delta9-tetrahydrocannabinol (delta9-THC or simply THC), which is a natural psychoactive constituent in marijuana.3 In fact, of the five primary cannabinoids derived from marijuana, including cannabidiol (CBD), cannabichromene (CBC), cannabigerol (CBG), cannabinol (CBN), and THC, only THC imparts psychoactive effects.17
CBD is thought to exhibit anti-inflammatory and analgesic activities.18 THC has been found to have the capacity to induce cancer cell apoptosis and block angiogenesis,19 and is thought to have immunomodulatory potential, partly acting through the G-protein-coupled CB1 and CB2 receptors but also yielding effects not related to these receptors.20In a 2014 survey of medical cannabis users, a statistically significant preference for C. indica (which contains higher CBD and lower THC levels) was observed for pain management, sedation, and sleep, while C. sativa was associated with euphoria and improving energy.21
The endocannabinoid system and skin health
The endogenous cannabinoid or endocannabinoid system includes cannabinoid receptors, associated endogenous ligands (such as arachidonoyl ethanolamide [anandamide or AEA], 2-arachidonoyl glycerol [2-AG], and N-palmitoylethanolamide [PEA], a fatty acid amide that enhances AEA activity),2 and enzymes involved in endocannabinoid production and decay.11,15,22,23 Research in recent years appears to support the notion that the endocannabinoid system plays an important role in skin health, as its dysregulation has been linked to atopic dermatitis, psoriasis, scleroderma, and skin cancer. Data indicate that exogenous and endogenous cannabinoids influence the endocannabinoid system through cannabinoid receptors, transient receptor potential channels (TRPs), and peroxisome proliferator–activated receptors (PPARs). Río et al. suggest that the dynamism of the endocannabinoid system buttresses the targeting of multiple endpoints for therapeutic success with cannabinoids rather than the one-disease-one-target approach.24 Endogenous cannabinoids, such as arachidonoyl ethanolamide and 2-arachidonoylglycerol, are now thought to be significant mediators in the skin.3 Further, endocannabinoids have been shown to deliver analgesia to the skin, at the spinal and supraspinal levels.25
Anti-inflammatory activity
In 2010, Tubaro et al. used the Croton oil mouse ear dermatitis assay to study the in vivo topical anti-inflammatory effects of seven phytocannabinoids and their related cannabivarins (nonpsychoactive cannabinoids). They found that anti-inflammatory activity was derived from the involvement of the cannabinoid receptors as well as the inflammatory endpoints that the phytocannabinoids targeted.26
In 2013, Gaffal et al. explored the anti-inflammatory activity of topical THC in dinitrofluorobenzene-mediated allergic contact dermatitis independent of CB1/2 receptors by using wild-type and CB1/2 receptor-deficient mice. The researchers found that topically applied THC reduced contact allergic ear edema and myeloid immune cell infiltration in both groups of mice. They concluded that such a decline in inflammation resulted from mitigating the keratinocyte-derived proinflammatory mediators that direct myeloid immune cell infiltration independent of CB1/2 receptors, and positions cannabinoids well for future use in treating inflammatory cutaneous conditions.20
Literature reviews
In a 2018 literature review on the uses of cannabinoids for cutaneous disorders, Eagleston et al. determined that preclinical data on cannabinoids reveal the potential to treat acne, allergic contact dermatitis, asteatotic dermatitis, atopic dermatitis, hidradenitis suppurativa, Kaposi sarcoma, pruritus, psoriasis, skin cancer, and the skin symptoms of systemic sclerosis. They caution, though, that more preclinical work is necessary along with randomized, controlled trials with sufficiently large sample sizes to establish the safety and efficacy of cannabinoids to treat skin conditions.27
A literature review by Marks and Friedman published later that year on the therapeutic potential of phytocannabinoids, endocannabinoids, and synthetic cannabinoids in managing skin disorders revealed the same findings regarding the cutaneous conditions associated with these compounds. The authors noted, though, that while the preponderance of articles highlight the efficacy of cannabinoids in treating inflammatory and neoplastic cutaneous conditions, some reports indicate proinflammatory and proneoplastic activities of cannabinoids. Like Eagleston et al., they call for additional studies.28
Conclusion
As in many botanical agents that I cover in this column, cannabis is associated with numerous medical benefits. I am encouraged to see expanding legalization of medical marijuana and increased research into its reputedly broad potential to improve human health. Anecdotally, I have heard stunning reports from patients about amelioration of joint and back pain as well as relief from other inflammatory symptoms. Discovery and elucidation of the endogenous cannabinoid system is a recent development. Research on its functions and roles in cutaneous health has followed suit and is steadily increasing. Particular skin conditions for which cannabis and cannabinoids may be indicated will be the focus of the next column.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected]
References
1. Higdon J. Why 2019 could be marijuana’s biggest year yet. Politico Magazine. Jan 21, 2019.
2. Singh D et al. Clin Dermatol. 2018 May-Jun;36(3):399-419.
3. Kupczyk P et al. Exp Dermatol. 2009 Aug;18(8):669-79.
4. Wilkinson JD et al. J Dermatol Sci. 2007 Feb;45(2):87-92.
5. Milando R et al. Am J Clin Dermatol. 2019 April;20(2):167-80.
6. Robinson E et al. J Drugs Dermatol. 2018 Dec 1;17(12):1273-8.
7. Mounessa JS et al. J Am Acad Dermatol. 2017 Jul;77(1):188-90.
8. Liszewski W et al. J Am Acad Dermatol. 2017 Sep;77(3):e87-e88.
9. Telek A et al. FASEB J. 2007 Nov;21(13):3534-41.
10. Wollenberg A et al. Br J Dermatol. 2014 Jul;170 Suppl 1:7-11.
11. Ramot Y et al. PeerJ. 2013 Feb 19;1:e40.
12. Schlicker E et al. Trends Pharmacol Sci. 2001 Nov;22(11):565-72.
13. Christie MJ et al. Nature. 2001 Mar 29;410(6828):527-30.
14. Ibid.
15. Bíró T et al. Trends Pharmacol Sci. 2009 Aug;30(8):411-20.
16. Pacher P et al. Pharmacol Rev. 2006 Sep;58(3):389-462.
17. Shalaby M et al. Pract Dermatol. 2018 Jan;68-70.
18. Chelliah MP et al. Pediatr Dermatol. 2018 Jul;35(4):e224-e227.
19. Glodde N et al. Life Sci. 2015 Oct 1;138:35-40.
20. Gaffal E et al. Allergy. 2013 Aug;68(8):994-1000.
21. Pearce DD et al. J Altern Complement Med. 2014 Oct;20(10):787:91.
22. Leonti M et al. Biochem Pharmacol. 2010 Jun 15;79(12):1815-26.
23. Trusler AR et al. Dermatitis. 2017 Jan/Feb;28(1):22-32.
24. Río CD et al. Biochem Pharmacol. 2018 Nov;157:122-133.
25. Chuquilin M et al. J Am Acad Dermatol. 2016 Feb;74(2):197-212.
26. Tubaro A et al. Fitoterapia. 2010 Oct;81(7):816-9.
27. Eagleston LRM et al. Dermatol Online J. 2018 Jun 15;24(6).
28. Marks DH et al. Skin Therapy Lett. 2018 Nov;23(6):1-5.
In the United States, 31 states, the District of Columbia, Puerto Rico, and Guam have legalized medical marijuana, which is also permitted for recreational use in 9 states, as well as in the District of Columbia. However, marijuana, derived from Cannabis sativa and Cannabis indica, is regulated as a schedule I drug in the United States at the federal level. (Some believe that the federal status may change in the coming year as a result of the Democratic Party’s takeover in the House of Representatives.1)
Cannabis species contain hundreds of various substances, of which the cannabinoids are the most studied. More than 113 biologically active chemical compounds are found within the class of cannabinoids and their derivatives,2 which have been used for centuries in natural medicine.3 The legal status of marijuana has long hampered scientific research of cannabinoids. Nevertheless, the number of studies focusing on the therapeutic potential of these compounds has steadily risen as the legal landscape of marijuana has evolved.
Findings over the last 20 years have shown that cannabinoids present in C. sativa exhibit anti-inflammatory activity and suppress the proliferation of multiple tumorigenic cell lines, some of which are moderated through cannabinoid (CB) receptors.4 In addition to anti-inflammatory properties, .3 Recent research has demonstrated that CB receptors are present in human skin.4
The endocannabinoid system has emerged as an intriguing area of research, as we’ve come to learn about its convoluted role in human anatomy and health. It features a pervasive network of endogenous ligands, enzymes, and receptors, which exogenous substances (including phytocannabinoids and synthetic cannabinoids) can activate.5 Data from recent studies indicate that the endocannabinoid system plays a significant role in cutaneous homeostasis, as it regulates proliferation, differentiation, and inflammatory mediator release.5 Further, psoriasis, atopic dermatitis, pruritus, and wound healing have been identified in recent research as cutaneous concerns in which the use of cannabinoids may be of benefit.6,7 We must also consider reports that cannabinoids can slow human hair growth and that some constituents may spur the synthesis of pro-inflammatory cytokines.8,9This column will briefly address potential confusion over the psychoactive aspects of cannabis, which are related to particular constituents of cannabis and specific CB receptors, and focus on the endocannabinoid system.
Psychoactive or not?
C. sativa confers biological activity through its influence on the G-protein-coupled receptor types CB1 and CB2,10 which pervade human skin epithelium.11 CB1 receptors are found in greatest supply in the central nervous system, especially the basal ganglia, cerebellum, hippocampus, and prefrontal cortex, where their activation yields psychoactivity.2,5,12,13 Stimulation of CB1 receptors in the skin – where they are present in differentiated keratinocytes, hair follicle cells, immune cells, sebaceous glands, and sensory neurons14 – diminishes pain and pruritus, controls keratinocyte differentiation and proliferation, inhibits hair follicle growth, and regulates the release of damage-induced keratins and inflammatory mediators to maintain cutaneous homeostasis.11,14,15
CB2 receptors are expressed in the immune system, particularly monocytes, macrophages, as well as B and T cells, and in peripheral tissues including the spleen, tonsils, thymus gland, bone, and, notably, the skin.2,16 Stimulation of CB2 receptors in the skin – where they are found in keratinocytes, immune cells, sebaceous glands, and sensory neurons – fosters sebum production, regulates pain sensation, hinders keratinocyte differentiation and proliferation, and suppresses cutaneous inflammatory responses.14,15
The best known, or most notorious, component of exogenous cannabinoids is delta9-tetrahydrocannabinol (delta9-THC or simply THC), which is a natural psychoactive constituent in marijuana.3 In fact, of the five primary cannabinoids derived from marijuana, including cannabidiol (CBD), cannabichromene (CBC), cannabigerol (CBG), cannabinol (CBN), and THC, only THC imparts psychoactive effects.17
CBD is thought to exhibit anti-inflammatory and analgesic activities.18 THC has been found to have the capacity to induce cancer cell apoptosis and block angiogenesis,19 and is thought to have immunomodulatory potential, partly acting through the G-protein-coupled CB1 and CB2 receptors but also yielding effects not related to these receptors.20In a 2014 survey of medical cannabis users, a statistically significant preference for C. indica (which contains higher CBD and lower THC levels) was observed for pain management, sedation, and sleep, while C. sativa was associated with euphoria and improving energy.21
The endocannabinoid system and skin health
The endogenous cannabinoid or endocannabinoid system includes cannabinoid receptors, associated endogenous ligands (such as arachidonoyl ethanolamide [anandamide or AEA], 2-arachidonoyl glycerol [2-AG], and N-palmitoylethanolamide [PEA], a fatty acid amide that enhances AEA activity),2 and enzymes involved in endocannabinoid production and decay.11,15,22,23 Research in recent years appears to support the notion that the endocannabinoid system plays an important role in skin health, as its dysregulation has been linked to atopic dermatitis, psoriasis, scleroderma, and skin cancer. Data indicate that exogenous and endogenous cannabinoids influence the endocannabinoid system through cannabinoid receptors, transient receptor potential channels (TRPs), and peroxisome proliferator–activated receptors (PPARs). Río et al. suggest that the dynamism of the endocannabinoid system buttresses the targeting of multiple endpoints for therapeutic success with cannabinoids rather than the one-disease-one-target approach.24 Endogenous cannabinoids, such as arachidonoyl ethanolamide and 2-arachidonoylglycerol, are now thought to be significant mediators in the skin.3 Further, endocannabinoids have been shown to deliver analgesia to the skin, at the spinal and supraspinal levels.25
Anti-inflammatory activity
In 2010, Tubaro et al. used the Croton oil mouse ear dermatitis assay to study the in vivo topical anti-inflammatory effects of seven phytocannabinoids and their related cannabivarins (nonpsychoactive cannabinoids). They found that anti-inflammatory activity was derived from the involvement of the cannabinoid receptors as well as the inflammatory endpoints that the phytocannabinoids targeted.26
In 2013, Gaffal et al. explored the anti-inflammatory activity of topical THC in dinitrofluorobenzene-mediated allergic contact dermatitis independent of CB1/2 receptors by using wild-type and CB1/2 receptor-deficient mice. The researchers found that topically applied THC reduced contact allergic ear edema and myeloid immune cell infiltration in both groups of mice. They concluded that such a decline in inflammation resulted from mitigating the keratinocyte-derived proinflammatory mediators that direct myeloid immune cell infiltration independent of CB1/2 receptors, and positions cannabinoids well for future use in treating inflammatory cutaneous conditions.20
Literature reviews
In a 2018 literature review on the uses of cannabinoids for cutaneous disorders, Eagleston et al. determined that preclinical data on cannabinoids reveal the potential to treat acne, allergic contact dermatitis, asteatotic dermatitis, atopic dermatitis, hidradenitis suppurativa, Kaposi sarcoma, pruritus, psoriasis, skin cancer, and the skin symptoms of systemic sclerosis. They caution, though, that more preclinical work is necessary along with randomized, controlled trials with sufficiently large sample sizes to establish the safety and efficacy of cannabinoids to treat skin conditions.27
A literature review by Marks and Friedman published later that year on the therapeutic potential of phytocannabinoids, endocannabinoids, and synthetic cannabinoids in managing skin disorders revealed the same findings regarding the cutaneous conditions associated with these compounds. The authors noted, though, that while the preponderance of articles highlight the efficacy of cannabinoids in treating inflammatory and neoplastic cutaneous conditions, some reports indicate proinflammatory and proneoplastic activities of cannabinoids. Like Eagleston et al., they call for additional studies.28
Conclusion
As in many botanical agents that I cover in this column, cannabis is associated with numerous medical benefits. I am encouraged to see expanding legalization of medical marijuana and increased research into its reputedly broad potential to improve human health. Anecdotally, I have heard stunning reports from patients about amelioration of joint and back pain as well as relief from other inflammatory symptoms. Discovery and elucidation of the endogenous cannabinoid system is a recent development. Research on its functions and roles in cutaneous health has followed suit and is steadily increasing. Particular skin conditions for which cannabis and cannabinoids may be indicated will be the focus of the next column.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected]
References
1. Higdon J. Why 2019 could be marijuana’s biggest year yet. Politico Magazine. Jan 21, 2019.
2. Singh D et al. Clin Dermatol. 2018 May-Jun;36(3):399-419.
3. Kupczyk P et al. Exp Dermatol. 2009 Aug;18(8):669-79.
4. Wilkinson JD et al. J Dermatol Sci. 2007 Feb;45(2):87-92.
5. Milando R et al. Am J Clin Dermatol. 2019 April;20(2):167-80.
6. Robinson E et al. J Drugs Dermatol. 2018 Dec 1;17(12):1273-8.
7. Mounessa JS et al. J Am Acad Dermatol. 2017 Jul;77(1):188-90.
8. Liszewski W et al. J Am Acad Dermatol. 2017 Sep;77(3):e87-e88.
9. Telek A et al. FASEB J. 2007 Nov;21(13):3534-41.
10. Wollenberg A et al. Br J Dermatol. 2014 Jul;170 Suppl 1:7-11.
11. Ramot Y et al. PeerJ. 2013 Feb 19;1:e40.
12. Schlicker E et al. Trends Pharmacol Sci. 2001 Nov;22(11):565-72.
13. Christie MJ et al. Nature. 2001 Mar 29;410(6828):527-30.
14. Ibid.
15. Bíró T et al. Trends Pharmacol Sci. 2009 Aug;30(8):411-20.
16. Pacher P et al. Pharmacol Rev. 2006 Sep;58(3):389-462.
17. Shalaby M et al. Pract Dermatol. 2018 Jan;68-70.
18. Chelliah MP et al. Pediatr Dermatol. 2018 Jul;35(4):e224-e227.
19. Glodde N et al. Life Sci. 2015 Oct 1;138:35-40.
20. Gaffal E et al. Allergy. 2013 Aug;68(8):994-1000.
21. Pearce DD et al. J Altern Complement Med. 2014 Oct;20(10):787:91.
22. Leonti M et al. Biochem Pharmacol. 2010 Jun 15;79(12):1815-26.
23. Trusler AR et al. Dermatitis. 2017 Jan/Feb;28(1):22-32.
24. Río CD et al. Biochem Pharmacol. 2018 Nov;157:122-133.
25. Chuquilin M et al. J Am Acad Dermatol. 2016 Feb;74(2):197-212.
26. Tubaro A et al. Fitoterapia. 2010 Oct;81(7):816-9.
27. Eagleston LRM et al. Dermatol Online J. 2018 Jun 15;24(6).
28. Marks DH et al. Skin Therapy Lett. 2018 Nov;23(6):1-5.
Lactobionic acid
Lactobionic acid (4-O-beta-galactopyranosyl-D-gluconic acid), a disaccharide formed from gluconic acid and galactose, has been established as a potent antioxidant well suited for use in solutions intended to preserve organs stored for transplantation.1,2 This polyhydroxy bionic acid is used as an excipient agent in some pharmaceutical products and has been the object of increasing interest and use in cosmetics and cosmeceuticals.3 It is included in skin care formulations for its strong humectant and antiaging effects.3,4 Lactobionic acid has been shown to suppress the synthesis of hydroxyl radicals by dint of iron-chelating activity and hinders the production of matrix metalloproteinases (MMPs), which promote photoaging.2,3,5 It may also present an advantage over the class of alpha-hydroxy acids used to treat photoaging by engendering less or no irritation, because of its larger molecular size and corresponding slower penetration rate.6 This column will focus on some recent research on the application of this strong antioxidant in dermatologic practice.
Lactobionic acid as an ingredient and vehicle
In 2010, Tasic-Kostov et al. compared the efficacy and irritation potential of lactobionic and glycolic acids (in gel and emulsion vehicles). In 77 healthy volunteers, the investigators found that , insofar as the former caused no irritation or skin barrier damage. In a second part to the study, they determined that efficacy of the acids was improved through the use of vehicles based on the natural emulsifier, alkyl polyglucoside (APG). They concluded that lactobionic acid in a 6% concentration in an APG vehicle warranted consideration as a low-molecular option in cosmeceutical products.6
In a subsequent study, the same team found supportive evidence that APG-based emulsions are safe cosmetic/dermopharmaceutical vehicles and carriers for extremely acidic and hygroscopic AHAs, particularly lactobionic acid. They did note, however, that lactobionic acid markedly affected the colloidal structure of the emulsion and fostered the development of lamellar structures, which could influence water distribution within the cream. They concluded, therefore, that such an emulsion, which was stabilized by lamellar liquid crystalline structures, would not be a viable carrier for the hygroscopic actives to achieve optimal moisturizing potential.7More recently, Tasic-Kostov et al. investigated the antioxidant and moisturizing traits of lactobionic acid in solution as well as in a natural APG emulsifier–based system using 1,1-diphenyl-2-picrylhydrazyl free radical scavenging and lipid peroxidation inhibition assays. The researchers found that lactobionic acid exhibited suitable physical stability (though it exerted notable impact on the colloidal structure of the vehicle) as well as antioxidant activity in both formats, suggesting its application as a versatile cosmeceutical agent for treating photoaged skin.2
In 2017, Chaouat et al. found that lactobionic acid was a key component in a green microparticle carrier system for cosmetics also containing chitosan and linoleic acid (as the skin penetration–enhancing constituent). Chitosan and lactobionic acid made up the shell surrounding the linoleic acid core. The carrier system, in an aqueous solution, was found to be stable and able to encapsulate the hydrophobic skin lightener phenylethyl resorcinol.8
Potential in atopic dermatitis treatment
Using an oxazolone-induced, atopic dermatitis–like murine dermatitis model, Sakai et al. demonstrated in 2016 that the coapplication of a PAR2 inhibitor and lactobionic acid, which maintained stratum corneum acidity, could target skin barrier abnormality and allergic inflammation, the key mechanisms in atopic dermatitis etiology.9
Lactobionic acid in chemical peels
Early this year, Algiert-Zielinska et al. reported on the results of a split-face study with 20 white women in which the effects of a 20% lactobionic acid peel were compared with those of the 20% peel combined with aluminum oxide crystal microdermabrasion. Treatments were administered weekly over 6 weeks, with the peel alone performed on the left side and the combination therapy on the right. The combination was found to achieve a significantly higher hydration level as well as skin elasticity measurements. There were no statistically significant differences between the tested therapies in transepidermal water loss, which decreased for both approaches. Both the lactobionic acid peel and combination procedure delivered notable moisturizing effects.10
Previously, this team performed a comparative evaluation of the skin-moisturizing activities of lactobionic acid in 10% and 30% concentrations in 10 white subjects between 26 and 73 years old. In this split-face study, 10% lactobionic acid was applied on the left side and 30% on the right on a weekly basis through eight treatments. A 5% lactobionic acid cream was supplied for overnight use. Skin hydration levels were measured before each weekly treatment. Although any differences between cutaneous hydration between the lactobionic acid preparations could not be ascertained, the investigators identified a statistically significant enhancement of hydration levels for both concentrations after the full series of treatments. They concluded that lactobionic is a potent moisturizing compound.11The same authors also conducted a literature review on the moisturizing properties of lactobionic and lactic acids, noting that both acids are capable of binding copious amounts of water and display robust chelating characteristics, as well as antioxidant activity, by suppressing MMPs. The authors added that both act as strong moisturizing substances, helping to maintain epidermal barrier integrity, and are suitable for sensitive skin.3
Conclusion
Greater capacity to moisturize and deliver antiaging benefits while causing less or no irritation are desirable qualities in a dermatologic agent. Evidence is limited, but the data available seem to suggest that lactobionic acid exhibits such qualities in comparison to alpha-hydroxy acids. Much more research is needed, though, to determine the most appropriate ways to use this promising compound.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Annu Rev Med. 1995;46:235-47.
2. Int J Cosmet Sci. 2012 Oct;34(5):424-34.
3. Int J Dermatol. 2019 Mar;58(3):374-79.
4. Clin Dermatol. 2009 Sep-Oct;27(5):495-501.
5.The next generation hydroxy acids, in “Cosmeceuticals” (New York: Elsevier Saunders, 2005, pp. 205-11).
6. J Cosmet Dermatol. 2010 Mar;9(1):3-10.
7. Pharmazie. 2011 Nov;66(11):862-70.
8. J Microencapsul. 2017 Mar;34(2):162-70.
9. J Invest Dermatol. 2016 Feb;136(2):538-41.
10. J Cosmet Dermatol. 2019 Jan 20. doi: 10.1111/jocd.12859. [Epub ahead of print].
11. J Cosmet Dermatol. 2018 Dec;17(6):1096-1100.
Lactobionic acid (4-O-beta-galactopyranosyl-D-gluconic acid), a disaccharide formed from gluconic acid and galactose, has been established as a potent antioxidant well suited for use in solutions intended to preserve organs stored for transplantation.1,2 This polyhydroxy bionic acid is used as an excipient agent in some pharmaceutical products and has been the object of increasing interest and use in cosmetics and cosmeceuticals.3 It is included in skin care formulations for its strong humectant and antiaging effects.3,4 Lactobionic acid has been shown to suppress the synthesis of hydroxyl radicals by dint of iron-chelating activity and hinders the production of matrix metalloproteinases (MMPs), which promote photoaging.2,3,5 It may also present an advantage over the class of alpha-hydroxy acids used to treat photoaging by engendering less or no irritation, because of its larger molecular size and corresponding slower penetration rate.6 This column will focus on some recent research on the application of this strong antioxidant in dermatologic practice.
Lactobionic acid as an ingredient and vehicle
In 2010, Tasic-Kostov et al. compared the efficacy and irritation potential of lactobionic and glycolic acids (in gel and emulsion vehicles). In 77 healthy volunteers, the investigators found that , insofar as the former caused no irritation or skin barrier damage. In a second part to the study, they determined that efficacy of the acids was improved through the use of vehicles based on the natural emulsifier, alkyl polyglucoside (APG). They concluded that lactobionic acid in a 6% concentration in an APG vehicle warranted consideration as a low-molecular option in cosmeceutical products.6
In a subsequent study, the same team found supportive evidence that APG-based emulsions are safe cosmetic/dermopharmaceutical vehicles and carriers for extremely acidic and hygroscopic AHAs, particularly lactobionic acid. They did note, however, that lactobionic acid markedly affected the colloidal structure of the emulsion and fostered the development of lamellar structures, which could influence water distribution within the cream. They concluded, therefore, that such an emulsion, which was stabilized by lamellar liquid crystalline structures, would not be a viable carrier for the hygroscopic actives to achieve optimal moisturizing potential.7More recently, Tasic-Kostov et al. investigated the antioxidant and moisturizing traits of lactobionic acid in solution as well as in a natural APG emulsifier–based system using 1,1-diphenyl-2-picrylhydrazyl free radical scavenging and lipid peroxidation inhibition assays. The researchers found that lactobionic acid exhibited suitable physical stability (though it exerted notable impact on the colloidal structure of the vehicle) as well as antioxidant activity in both formats, suggesting its application as a versatile cosmeceutical agent for treating photoaged skin.2
In 2017, Chaouat et al. found that lactobionic acid was a key component in a green microparticle carrier system for cosmetics also containing chitosan and linoleic acid (as the skin penetration–enhancing constituent). Chitosan and lactobionic acid made up the shell surrounding the linoleic acid core. The carrier system, in an aqueous solution, was found to be stable and able to encapsulate the hydrophobic skin lightener phenylethyl resorcinol.8
Potential in atopic dermatitis treatment
Using an oxazolone-induced, atopic dermatitis–like murine dermatitis model, Sakai et al. demonstrated in 2016 that the coapplication of a PAR2 inhibitor and lactobionic acid, which maintained stratum corneum acidity, could target skin barrier abnormality and allergic inflammation, the key mechanisms in atopic dermatitis etiology.9
Lactobionic acid in chemical peels
Early this year, Algiert-Zielinska et al. reported on the results of a split-face study with 20 white women in which the effects of a 20% lactobionic acid peel were compared with those of the 20% peel combined with aluminum oxide crystal microdermabrasion. Treatments were administered weekly over 6 weeks, with the peel alone performed on the left side and the combination therapy on the right. The combination was found to achieve a significantly higher hydration level as well as skin elasticity measurements. There were no statistically significant differences between the tested therapies in transepidermal water loss, which decreased for both approaches. Both the lactobionic acid peel and combination procedure delivered notable moisturizing effects.10
Previously, this team performed a comparative evaluation of the skin-moisturizing activities of lactobionic acid in 10% and 30% concentrations in 10 white subjects between 26 and 73 years old. In this split-face study, 10% lactobionic acid was applied on the left side and 30% on the right on a weekly basis through eight treatments. A 5% lactobionic acid cream was supplied for overnight use. Skin hydration levels were measured before each weekly treatment. Although any differences between cutaneous hydration between the lactobionic acid preparations could not be ascertained, the investigators identified a statistically significant enhancement of hydration levels for both concentrations after the full series of treatments. They concluded that lactobionic is a potent moisturizing compound.11The same authors also conducted a literature review on the moisturizing properties of lactobionic and lactic acids, noting that both acids are capable of binding copious amounts of water and display robust chelating characteristics, as well as antioxidant activity, by suppressing MMPs. The authors added that both act as strong moisturizing substances, helping to maintain epidermal barrier integrity, and are suitable for sensitive skin.3
Conclusion
Greater capacity to moisturize and deliver antiaging benefits while causing less or no irritation are desirable qualities in a dermatologic agent. Evidence is limited, but the data available seem to suggest that lactobionic acid exhibits such qualities in comparison to alpha-hydroxy acids. Much more research is needed, though, to determine the most appropriate ways to use this promising compound.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Annu Rev Med. 1995;46:235-47.
2. Int J Cosmet Sci. 2012 Oct;34(5):424-34.
3. Int J Dermatol. 2019 Mar;58(3):374-79.
4. Clin Dermatol. 2009 Sep-Oct;27(5):495-501.
5.The next generation hydroxy acids, in “Cosmeceuticals” (New York: Elsevier Saunders, 2005, pp. 205-11).
6. J Cosmet Dermatol. 2010 Mar;9(1):3-10.
7. Pharmazie. 2011 Nov;66(11):862-70.
8. J Microencapsul. 2017 Mar;34(2):162-70.
9. J Invest Dermatol. 2016 Feb;136(2):538-41.
10. J Cosmet Dermatol. 2019 Jan 20. doi: 10.1111/jocd.12859. [Epub ahead of print].
11. J Cosmet Dermatol. 2018 Dec;17(6):1096-1100.
Lactobionic acid (4-O-beta-galactopyranosyl-D-gluconic acid), a disaccharide formed from gluconic acid and galactose, has been established as a potent antioxidant well suited for use in solutions intended to preserve organs stored for transplantation.1,2 This polyhydroxy bionic acid is used as an excipient agent in some pharmaceutical products and has been the object of increasing interest and use in cosmetics and cosmeceuticals.3 It is included in skin care formulations for its strong humectant and antiaging effects.3,4 Lactobionic acid has been shown to suppress the synthesis of hydroxyl radicals by dint of iron-chelating activity and hinders the production of matrix metalloproteinases (MMPs), which promote photoaging.2,3,5 It may also present an advantage over the class of alpha-hydroxy acids used to treat photoaging by engendering less or no irritation, because of its larger molecular size and corresponding slower penetration rate.6 This column will focus on some recent research on the application of this strong antioxidant in dermatologic practice.
Lactobionic acid as an ingredient and vehicle
In 2010, Tasic-Kostov et al. compared the efficacy and irritation potential of lactobionic and glycolic acids (in gel and emulsion vehicles). In 77 healthy volunteers, the investigators found that , insofar as the former caused no irritation or skin barrier damage. In a second part to the study, they determined that efficacy of the acids was improved through the use of vehicles based on the natural emulsifier, alkyl polyglucoside (APG). They concluded that lactobionic acid in a 6% concentration in an APG vehicle warranted consideration as a low-molecular option in cosmeceutical products.6
In a subsequent study, the same team found supportive evidence that APG-based emulsions are safe cosmetic/dermopharmaceutical vehicles and carriers for extremely acidic and hygroscopic AHAs, particularly lactobionic acid. They did note, however, that lactobionic acid markedly affected the colloidal structure of the emulsion and fostered the development of lamellar structures, which could influence water distribution within the cream. They concluded, therefore, that such an emulsion, which was stabilized by lamellar liquid crystalline structures, would not be a viable carrier for the hygroscopic actives to achieve optimal moisturizing potential.7More recently, Tasic-Kostov et al. investigated the antioxidant and moisturizing traits of lactobionic acid in solution as well as in a natural APG emulsifier–based system using 1,1-diphenyl-2-picrylhydrazyl free radical scavenging and lipid peroxidation inhibition assays. The researchers found that lactobionic acid exhibited suitable physical stability (though it exerted notable impact on the colloidal structure of the vehicle) as well as antioxidant activity in both formats, suggesting its application as a versatile cosmeceutical agent for treating photoaged skin.2
In 2017, Chaouat et al. found that lactobionic acid was a key component in a green microparticle carrier system for cosmetics also containing chitosan and linoleic acid (as the skin penetration–enhancing constituent). Chitosan and lactobionic acid made up the shell surrounding the linoleic acid core. The carrier system, in an aqueous solution, was found to be stable and able to encapsulate the hydrophobic skin lightener phenylethyl resorcinol.8
Potential in atopic dermatitis treatment
Using an oxazolone-induced, atopic dermatitis–like murine dermatitis model, Sakai et al. demonstrated in 2016 that the coapplication of a PAR2 inhibitor and lactobionic acid, which maintained stratum corneum acidity, could target skin barrier abnormality and allergic inflammation, the key mechanisms in atopic dermatitis etiology.9
Lactobionic acid in chemical peels
Early this year, Algiert-Zielinska et al. reported on the results of a split-face study with 20 white women in which the effects of a 20% lactobionic acid peel were compared with those of the 20% peel combined with aluminum oxide crystal microdermabrasion. Treatments were administered weekly over 6 weeks, with the peel alone performed on the left side and the combination therapy on the right. The combination was found to achieve a significantly higher hydration level as well as skin elasticity measurements. There were no statistically significant differences between the tested therapies in transepidermal water loss, which decreased for both approaches. Both the lactobionic acid peel and combination procedure delivered notable moisturizing effects.10
Previously, this team performed a comparative evaluation of the skin-moisturizing activities of lactobionic acid in 10% and 30% concentrations in 10 white subjects between 26 and 73 years old. In this split-face study, 10% lactobionic acid was applied on the left side and 30% on the right on a weekly basis through eight treatments. A 5% lactobionic acid cream was supplied for overnight use. Skin hydration levels were measured before each weekly treatment. Although any differences between cutaneous hydration between the lactobionic acid preparations could not be ascertained, the investigators identified a statistically significant enhancement of hydration levels for both concentrations after the full series of treatments. They concluded that lactobionic is a potent moisturizing compound.11The same authors also conducted a literature review on the moisturizing properties of lactobionic and lactic acids, noting that both acids are capable of binding copious amounts of water and display robust chelating characteristics, as well as antioxidant activity, by suppressing MMPs. The authors added that both act as strong moisturizing substances, helping to maintain epidermal barrier integrity, and are suitable for sensitive skin.3
Conclusion
Greater capacity to moisturize and deliver antiaging benefits while causing less or no irritation are desirable qualities in a dermatologic agent. Evidence is limited, but the data available seem to suggest that lactobionic acid exhibits such qualities in comparison to alpha-hydroxy acids. Much more research is needed, though, to determine the most appropriate ways to use this promising compound.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Annu Rev Med. 1995;46:235-47.
2. Int J Cosmet Sci. 2012 Oct;34(5):424-34.
3. Int J Dermatol. 2019 Mar;58(3):374-79.
4. Clin Dermatol. 2009 Sep-Oct;27(5):495-501.
5.The next generation hydroxy acids, in “Cosmeceuticals” (New York: Elsevier Saunders, 2005, pp. 205-11).
6. J Cosmet Dermatol. 2010 Mar;9(1):3-10.
7. Pharmazie. 2011 Nov;66(11):862-70.
8. J Microencapsul. 2017 Mar;34(2):162-70.
9. J Invest Dermatol. 2016 Feb;136(2):538-41.
10. J Cosmet Dermatol. 2019 Jan 20. doi: 10.1111/jocd.12859. [Epub ahead of print].
11. J Cosmet Dermatol. 2018 Dec;17(6):1096-1100.
Melatonin update, Part 2
Recall that melatonin displays multiple biological functions, acting as an antioxidant, cytokine, neurotransmitter, and global regulator of the circadian clock, the latter for which it is best known.1-3 At the cutaneous level, melatonin exhibits antioxidant (direct, as a radical scavenger; indirect, through upregulating antioxidant enzymes), anti-inflammatory, photoprotective, tissue regenerative, and cytoprotective activity, particularly in its capacity to preserve mitochondrial function.4-8
Melatonin also protects skin homeostasis,6 and, consequently, is believed to act against carcinogenesis and potentially other deleterious dysfunctions such as hyperproliferative/inflammatory conditions.5 Notably, , further buttressing the critical role that melatonin plays in skin health.5 Melatonin also displays immunomodulatory, thermoregulatory, and antitumor functions.9 The topical application of melatonin has been demonstrated to diminish markers of reactive oxygen species as well as reverse manifestations of cutaneous aging.5
Melatonin is both produced by and metabolized in the skin. The hormone and its metabolites (6-hydroxymelatonin, N1-acetyl-N2-formyl-5-methoxykynuramine [AFMK], N-acetyl-serotonin, and 5-methoxytryptamine) reduce UVB-induced oxidative cell damage in human keratinocytes and melanocytes, and also act as radioprotectors.6
Melatonin has been shown to protect human dermal fibroblasts from UVA- and UVB-induced damage.9 In addition, melatonin and its metabolites have been demonstrated to suppress the growth of cultured human melanomas, and high doses of melatonin used in clinical trials in late metastatic melanoma stages have enhanced the efficacy of or diminished the side effects of chemotherapy/chemo-immunotherapy.9
UVB and melatonin in the lab
In a 2018 hairless mouse study in which animals were irradiated by UVB for 8 weeks, Park et al. showed that melatonin displays anti-wrinkle activity by suppressing reactive oxygen species- and sonic hedgehog-mediated inflammatory proteins. Melatonin also protected against transepidermal water loss and prevented epidermal thickness as well as dermal collagen degradation.10
Also that year, Skobowiat et al. found that the topical application of melatonin and its active derivatives (N1-acetyl-N2-formyl-5-methoxykynurenine and N-acetylserotonin) yielded photoprotective effects pre- and post-UVB treatment in human and porcine skin ex vivo. They concluded that their results justify additional investigation of the clinical applications of melatonin and its metabolites for its potential to exert protective effects against UVB in human subjects.8
Although the preponderance of previous work identifies melatonin as a strong antioxidant, Kocyigit et al. reported in 2018 on new in vitro studies suggesting that melatonin dose-dependently exerts cytotoxic and apoptotic activity on several cell types, including both human epidermoid carcinoma and normal skin fibroblasts. Their findings showed that melatonin exhibited proliferative effects on cancerous and normal cells at low doses and cytotoxic effects at high doses.11
Melatonin as a sunscreen ingredient
Further supporting its use in the topical armamentarium for skin health, melatonin is a key ingredient in a sunscreen formulation, the creation of which was driven by the need to protect the skin of military personnel facing lengthy UV exposure. Specifically, the formulation containing avobenzone, octinoxate, oxybenzone, and titanium dioxide along with melatonin and pumpkin seed oil underwent a preclinical safety evaluation in 2017, as reported by Bora et al. The formulation was found to be nonmutagenic, nontoxic, and safe in animal models and is deemed ready to test for its efficacy in humans.12 Melatonin is also among a host of systemic treatment options for skin lightening.13
Oral and topical melatonin in human studies
In a 2017 study on the impact of melatonin treatment on the skin of former smokers, Sagan et al. assessed oxidative damage to membrane lipids in blood serum and in epidermis exfoliated during microdermabrasion (at baseline, 2 weeks after, and 4 weeks after treatment) in postmenopausal women. Never smokers (n = 44) and former smokers (n = 46) were divided into control, melatonin topical, antioxidant topical, and melatonin oral treatment groups. The investigators found that after only 2 weeks, melatonin oral treatment significantly reversed the elevated serum lipid peroxidation in former smokers. Oral melatonin increased elasticity, moisture, and sebum levels after 4 weeks of treatment and topical melatonin increased sebum level. They concluded that the use of exogenous melatonin reverses the effects of oxidative damage to membrane lipids and ameliorates cutaneous biophysical traits in postmenopausal women who once smoked. The researchers added that melatonin use for all former smokers is warranted and that topically applied melatonin merits consideration for improving the effects of facial microdermabrasion.14
In a systematic literature review in 2017, Scheuer identified 20 studies (4 human and 16 experimental) indicating that melatonin exerts a protective effect against artificial UV-induced erythema when applied pre-exposure.7 Also that year, Scheuer and colleagues conducted randomized, double-blind, placebo-controlled work demonstrating that topical melatonin (12.5%) significantly reduced erythema resulting from natural sunlight, and in a separate randomized, double-blind, placebo-controlled crossover study that the same concentration of a full body application of melatonin exhibited no significant impact on cognition and should be considered safe for dermal application.7 Scheuer added that additional longitudinal research is needed to ascertain effects of topical melatonin usage over time.
Early in 2018, Milani and Sparavigna reported on a randomized, split-face, assessor-blinded, prospective 3-month study of 22 women (mean age 55 years) with moderate to severe facial skin aging; the study was designed to test the efficacy of melatonin-based day and night creams. All of the women completed the proof-of-concept trial in which crow’s feet were found to be significantly diminished on the sides of the face treated with the creams compared with the nontreated skin.
Both well-tolerated melatonin formulations were associated with significant improvements in surface microrelief, skin profilometry, tonicity, and dryness. With marked enhancement of skin hydration and reduction of roughness noted, the investigators concluded that their results supported the notion that the tested melatonin topical formulations yielded antiaging effects.4
Conclusion
The majority of research on the potent hormone melatonin over nearly the last quarter century indicates that this dynamic substance provides multifaceted benefits in performing several biological functions. Topical melatonin is available over the counter. Its expanded use in skin care warrants greater attention as we learn more about this versatile endogenous substance.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Zmijewski MA et al. Dermatoendocrinol. 2011 Jan;3(1):3-10.
2. Slominski A et al. Trends Endocrinol Metab. 2008 Jan;19(1):17-24.
3. Slominski A et al. J Cell Physiol. 2003 Jul;196(1):144-53.
4. Milani M et al. Clin Cosmet Investig Dermatol. 2018 Jan 24;11:51-7.
5. Day D et al. J Drugs Dermatol. 2018 Sep 1;17(9):966-9.
6. Slominski AT et al. Cell Mol Life Sci. 2017 Nov;74(21):3913-25.
7. Scheuer C. Dan Med J. 2017 Jun;64(6). pii:B5358.
8. Skobowiat C et al. J Pineal Res. 2018 Sep;65(2):e12501.
9. Slominski AT et al. J Invest Dermatol. 2018 Mar;138(3):490-9.
10. Park EK et al. Int J Mol Sci. 2018 Jul 8;19(7). pii: E1995.
11. Kocyigit A et al. Mutat Res. 2018 May-Jun;829-30:50-60.
12. Bora NS et al. Regul Toxicol Pharmacol. 2017 Oct;89:1-12.
13. Juhasz MLW et al. J Cosmet Dermatol. 2018 Dec;17(6):1144-57.
14. Sagan D et al. Ann Agric Environ Med. 2017 Dec 23;24(4):659-66.
Recall that melatonin displays multiple biological functions, acting as an antioxidant, cytokine, neurotransmitter, and global regulator of the circadian clock, the latter for which it is best known.1-3 At the cutaneous level, melatonin exhibits antioxidant (direct, as a radical scavenger; indirect, through upregulating antioxidant enzymes), anti-inflammatory, photoprotective, tissue regenerative, and cytoprotective activity, particularly in its capacity to preserve mitochondrial function.4-8
Melatonin also protects skin homeostasis,6 and, consequently, is believed to act against carcinogenesis and potentially other deleterious dysfunctions such as hyperproliferative/inflammatory conditions.5 Notably, , further buttressing the critical role that melatonin plays in skin health.5 Melatonin also displays immunomodulatory, thermoregulatory, and antitumor functions.9 The topical application of melatonin has been demonstrated to diminish markers of reactive oxygen species as well as reverse manifestations of cutaneous aging.5
Melatonin is both produced by and metabolized in the skin. The hormone and its metabolites (6-hydroxymelatonin, N1-acetyl-N2-formyl-5-methoxykynuramine [AFMK], N-acetyl-serotonin, and 5-methoxytryptamine) reduce UVB-induced oxidative cell damage in human keratinocytes and melanocytes, and also act as radioprotectors.6
Melatonin has been shown to protect human dermal fibroblasts from UVA- and UVB-induced damage.9 In addition, melatonin and its metabolites have been demonstrated to suppress the growth of cultured human melanomas, and high doses of melatonin used in clinical trials in late metastatic melanoma stages have enhanced the efficacy of or diminished the side effects of chemotherapy/chemo-immunotherapy.9
UVB and melatonin in the lab
In a 2018 hairless mouse study in which animals were irradiated by UVB for 8 weeks, Park et al. showed that melatonin displays anti-wrinkle activity by suppressing reactive oxygen species- and sonic hedgehog-mediated inflammatory proteins. Melatonin also protected against transepidermal water loss and prevented epidermal thickness as well as dermal collagen degradation.10
Also that year, Skobowiat et al. found that the topical application of melatonin and its active derivatives (N1-acetyl-N2-formyl-5-methoxykynurenine and N-acetylserotonin) yielded photoprotective effects pre- and post-UVB treatment in human and porcine skin ex vivo. They concluded that their results justify additional investigation of the clinical applications of melatonin and its metabolites for its potential to exert protective effects against UVB in human subjects.8
Although the preponderance of previous work identifies melatonin as a strong antioxidant, Kocyigit et al. reported in 2018 on new in vitro studies suggesting that melatonin dose-dependently exerts cytotoxic and apoptotic activity on several cell types, including both human epidermoid carcinoma and normal skin fibroblasts. Their findings showed that melatonin exhibited proliferative effects on cancerous and normal cells at low doses and cytotoxic effects at high doses.11
Melatonin as a sunscreen ingredient
Further supporting its use in the topical armamentarium for skin health, melatonin is a key ingredient in a sunscreen formulation, the creation of which was driven by the need to protect the skin of military personnel facing lengthy UV exposure. Specifically, the formulation containing avobenzone, octinoxate, oxybenzone, and titanium dioxide along with melatonin and pumpkin seed oil underwent a preclinical safety evaluation in 2017, as reported by Bora et al. The formulation was found to be nonmutagenic, nontoxic, and safe in animal models and is deemed ready to test for its efficacy in humans.12 Melatonin is also among a host of systemic treatment options for skin lightening.13
Oral and topical melatonin in human studies
In a 2017 study on the impact of melatonin treatment on the skin of former smokers, Sagan et al. assessed oxidative damage to membrane lipids in blood serum and in epidermis exfoliated during microdermabrasion (at baseline, 2 weeks after, and 4 weeks after treatment) in postmenopausal women. Never smokers (n = 44) and former smokers (n = 46) were divided into control, melatonin topical, antioxidant topical, and melatonin oral treatment groups. The investigators found that after only 2 weeks, melatonin oral treatment significantly reversed the elevated serum lipid peroxidation in former smokers. Oral melatonin increased elasticity, moisture, and sebum levels after 4 weeks of treatment and topical melatonin increased sebum level. They concluded that the use of exogenous melatonin reverses the effects of oxidative damage to membrane lipids and ameliorates cutaneous biophysical traits in postmenopausal women who once smoked. The researchers added that melatonin use for all former smokers is warranted and that topically applied melatonin merits consideration for improving the effects of facial microdermabrasion.14
In a systematic literature review in 2017, Scheuer identified 20 studies (4 human and 16 experimental) indicating that melatonin exerts a protective effect against artificial UV-induced erythema when applied pre-exposure.7 Also that year, Scheuer and colleagues conducted randomized, double-blind, placebo-controlled work demonstrating that topical melatonin (12.5%) significantly reduced erythema resulting from natural sunlight, and in a separate randomized, double-blind, placebo-controlled crossover study that the same concentration of a full body application of melatonin exhibited no significant impact on cognition and should be considered safe for dermal application.7 Scheuer added that additional longitudinal research is needed to ascertain effects of topical melatonin usage over time.
Early in 2018, Milani and Sparavigna reported on a randomized, split-face, assessor-blinded, prospective 3-month study of 22 women (mean age 55 years) with moderate to severe facial skin aging; the study was designed to test the efficacy of melatonin-based day and night creams. All of the women completed the proof-of-concept trial in which crow’s feet were found to be significantly diminished on the sides of the face treated with the creams compared with the nontreated skin.
Both well-tolerated melatonin formulations were associated with significant improvements in surface microrelief, skin profilometry, tonicity, and dryness. With marked enhancement of skin hydration and reduction of roughness noted, the investigators concluded that their results supported the notion that the tested melatonin topical formulations yielded antiaging effects.4
Conclusion
The majority of research on the potent hormone melatonin over nearly the last quarter century indicates that this dynamic substance provides multifaceted benefits in performing several biological functions. Topical melatonin is available over the counter. Its expanded use in skin care warrants greater attention as we learn more about this versatile endogenous substance.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Zmijewski MA et al. Dermatoendocrinol. 2011 Jan;3(1):3-10.
2. Slominski A et al. Trends Endocrinol Metab. 2008 Jan;19(1):17-24.
3. Slominski A et al. J Cell Physiol. 2003 Jul;196(1):144-53.
4. Milani M et al. Clin Cosmet Investig Dermatol. 2018 Jan 24;11:51-7.
5. Day D et al. J Drugs Dermatol. 2018 Sep 1;17(9):966-9.
6. Slominski AT et al. Cell Mol Life Sci. 2017 Nov;74(21):3913-25.
7. Scheuer C. Dan Med J. 2017 Jun;64(6). pii:B5358.
8. Skobowiat C et al. J Pineal Res. 2018 Sep;65(2):e12501.
9. Slominski AT et al. J Invest Dermatol. 2018 Mar;138(3):490-9.
10. Park EK et al. Int J Mol Sci. 2018 Jul 8;19(7). pii: E1995.
11. Kocyigit A et al. Mutat Res. 2018 May-Jun;829-30:50-60.
12. Bora NS et al. Regul Toxicol Pharmacol. 2017 Oct;89:1-12.
13. Juhasz MLW et al. J Cosmet Dermatol. 2018 Dec;17(6):1144-57.
14. Sagan D et al. Ann Agric Environ Med. 2017 Dec 23;24(4):659-66.
Recall that melatonin displays multiple biological functions, acting as an antioxidant, cytokine, neurotransmitter, and global regulator of the circadian clock, the latter for which it is best known.1-3 At the cutaneous level, melatonin exhibits antioxidant (direct, as a radical scavenger; indirect, through upregulating antioxidant enzymes), anti-inflammatory, photoprotective, tissue regenerative, and cytoprotective activity, particularly in its capacity to preserve mitochondrial function.4-8
Melatonin also protects skin homeostasis,6 and, consequently, is believed to act against carcinogenesis and potentially other deleterious dysfunctions such as hyperproliferative/inflammatory conditions.5 Notably, , further buttressing the critical role that melatonin plays in skin health.5 Melatonin also displays immunomodulatory, thermoregulatory, and antitumor functions.9 The topical application of melatonin has been demonstrated to diminish markers of reactive oxygen species as well as reverse manifestations of cutaneous aging.5
Melatonin is both produced by and metabolized in the skin. The hormone and its metabolites (6-hydroxymelatonin, N1-acetyl-N2-formyl-5-methoxykynuramine [AFMK], N-acetyl-serotonin, and 5-methoxytryptamine) reduce UVB-induced oxidative cell damage in human keratinocytes and melanocytes, and also act as radioprotectors.6
Melatonin has been shown to protect human dermal fibroblasts from UVA- and UVB-induced damage.9 In addition, melatonin and its metabolites have been demonstrated to suppress the growth of cultured human melanomas, and high doses of melatonin used in clinical trials in late metastatic melanoma stages have enhanced the efficacy of or diminished the side effects of chemotherapy/chemo-immunotherapy.9
UVB and melatonin in the lab
In a 2018 hairless mouse study in which animals were irradiated by UVB for 8 weeks, Park et al. showed that melatonin displays anti-wrinkle activity by suppressing reactive oxygen species- and sonic hedgehog-mediated inflammatory proteins. Melatonin also protected against transepidermal water loss and prevented epidermal thickness as well as dermal collagen degradation.10
Also that year, Skobowiat et al. found that the topical application of melatonin and its active derivatives (N1-acetyl-N2-formyl-5-methoxykynurenine and N-acetylserotonin) yielded photoprotective effects pre- and post-UVB treatment in human and porcine skin ex vivo. They concluded that their results justify additional investigation of the clinical applications of melatonin and its metabolites for its potential to exert protective effects against UVB in human subjects.8
Although the preponderance of previous work identifies melatonin as a strong antioxidant, Kocyigit et al. reported in 2018 on new in vitro studies suggesting that melatonin dose-dependently exerts cytotoxic and apoptotic activity on several cell types, including both human epidermoid carcinoma and normal skin fibroblasts. Their findings showed that melatonin exhibited proliferative effects on cancerous and normal cells at low doses and cytotoxic effects at high doses.11
Melatonin as a sunscreen ingredient
Further supporting its use in the topical armamentarium for skin health, melatonin is a key ingredient in a sunscreen formulation, the creation of which was driven by the need to protect the skin of military personnel facing lengthy UV exposure. Specifically, the formulation containing avobenzone, octinoxate, oxybenzone, and titanium dioxide along with melatonin and pumpkin seed oil underwent a preclinical safety evaluation in 2017, as reported by Bora et al. The formulation was found to be nonmutagenic, nontoxic, and safe in animal models and is deemed ready to test for its efficacy in humans.12 Melatonin is also among a host of systemic treatment options for skin lightening.13
Oral and topical melatonin in human studies
In a 2017 study on the impact of melatonin treatment on the skin of former smokers, Sagan et al. assessed oxidative damage to membrane lipids in blood serum and in epidermis exfoliated during microdermabrasion (at baseline, 2 weeks after, and 4 weeks after treatment) in postmenopausal women. Never smokers (n = 44) and former smokers (n = 46) were divided into control, melatonin topical, antioxidant topical, and melatonin oral treatment groups. The investigators found that after only 2 weeks, melatonin oral treatment significantly reversed the elevated serum lipid peroxidation in former smokers. Oral melatonin increased elasticity, moisture, and sebum levels after 4 weeks of treatment and topical melatonin increased sebum level. They concluded that the use of exogenous melatonin reverses the effects of oxidative damage to membrane lipids and ameliorates cutaneous biophysical traits in postmenopausal women who once smoked. The researchers added that melatonin use for all former smokers is warranted and that topically applied melatonin merits consideration for improving the effects of facial microdermabrasion.14
In a systematic literature review in 2017, Scheuer identified 20 studies (4 human and 16 experimental) indicating that melatonin exerts a protective effect against artificial UV-induced erythema when applied pre-exposure.7 Also that year, Scheuer and colleagues conducted randomized, double-blind, placebo-controlled work demonstrating that topical melatonin (12.5%) significantly reduced erythema resulting from natural sunlight, and in a separate randomized, double-blind, placebo-controlled crossover study that the same concentration of a full body application of melatonin exhibited no significant impact on cognition and should be considered safe for dermal application.7 Scheuer added that additional longitudinal research is needed to ascertain effects of topical melatonin usage over time.
Early in 2018, Milani and Sparavigna reported on a randomized, split-face, assessor-blinded, prospective 3-month study of 22 women (mean age 55 years) with moderate to severe facial skin aging; the study was designed to test the efficacy of melatonin-based day and night creams. All of the women completed the proof-of-concept trial in which crow’s feet were found to be significantly diminished on the sides of the face treated with the creams compared with the nontreated skin.
Both well-tolerated melatonin formulations were associated with significant improvements in surface microrelief, skin profilometry, tonicity, and dryness. With marked enhancement of skin hydration and reduction of roughness noted, the investigators concluded that their results supported the notion that the tested melatonin topical formulations yielded antiaging effects.4
Conclusion
The majority of research on the potent hormone melatonin over nearly the last quarter century indicates that this dynamic substance provides multifaceted benefits in performing several biological functions. Topical melatonin is available over the counter. Its expanded use in skin care warrants greater attention as we learn more about this versatile endogenous substance.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Zmijewski MA et al. Dermatoendocrinol. 2011 Jan;3(1):3-10.
2. Slominski A et al. Trends Endocrinol Metab. 2008 Jan;19(1):17-24.
3. Slominski A et al. J Cell Physiol. 2003 Jul;196(1):144-53.
4. Milani M et al. Clin Cosmet Investig Dermatol. 2018 Jan 24;11:51-7.
5. Day D et al. J Drugs Dermatol. 2018 Sep 1;17(9):966-9.
6. Slominski AT et al. Cell Mol Life Sci. 2017 Nov;74(21):3913-25.
7. Scheuer C. Dan Med J. 2017 Jun;64(6). pii:B5358.
8. Skobowiat C et al. J Pineal Res. 2018 Sep;65(2):e12501.
9. Slominski AT et al. J Invest Dermatol. 2018 Mar;138(3):490-9.
10. Park EK et al. Int J Mol Sci. 2018 Jul 8;19(7). pii: E1995.
11. Kocyigit A et al. Mutat Res. 2018 May-Jun;829-30:50-60.
12. Bora NS et al. Regul Toxicol Pharmacol. 2017 Oct;89:1-12.
13. Juhasz MLW et al. J Cosmet Dermatol. 2018 Dec;17(6):1144-57.
14. Sagan D et al. Ann Agric Environ Med. 2017 Dec 23;24(4):659-66.
Melatonin update, Part 1
Found in various plant and animal species, including humans, melatonin (N-acetyl-5-methoxytryptamine) is best known for its daily fluctuations in circulating levels that regulate circadian rhythms. But this ancient serotonin derivative, stimulated by beta-adrenergic receptors, is the primary neuroendocrine product of the pineal gland (discovered as such in 1917) in humans and a dynamic compound with diverse roles in human health levels of which decrease with age.1,2 Over the last quarter of a century, we have arrived at a much greater understanding of the varied biological functions of this highly lipophilic hormone, which is now recognized as the strongest endogenous antioxidant, particularly potent against hydroxyl radicals, the most harmful of reactive oxygen species, and known to protect mitochondria and DNA from direct oxidative harm.2-4 Directly or via its circadian impact, melatonin also affects skin as well as core body temperature.1 This column is a . Next month’s column will address some more of the activities of this dynamic hormone while concentrating on the interaction of melatonin and ultraviolet radiation.
Early studies
In the mid-1990s, Bangha et al. performed several studies in healthy human volunteers that demonstrated that topically applied melatonin suppressed UVB-induced erythema (with one study showing pre- and posttreatment as effective and a subsequent one showing only pretreatment as effective), and also found that melatonin appears to have the potential to accumulate in the stratum corneum with extended release into the blood system through cutaneous delivery.5-7
A randomized, double-blind study by Dreher et al. in 12 healthy adults (6 women and 6 men, all white, aged 29-49 years) considered the short-term photoprotective effects of topically applied vitamin C, vitamin E, and melatonin, alone or in combination, 30 minutes after UV exposure. A dose-dependent photoprotective effect was associated with melatonin, and photoprotective properties were enhanced when melatonin in was combined with vitamins C and E.8
The following year, Dreher et al. evaluated the short-term photoprotective effects of the same compounds in a randomized, double-blind, placebo-controlled human study. Each antioxidant was topically applied alone or in combination after UV exposure in a single application (immediately or 30 minutes after UV exposure) or in multiple applications 30 minutes, 1 hour, and 2 hours after UV exposure (totaling three applications). Interestingly, no photoprotective effects were seen. The researchers concluded that given the speed of cutaneous damage from UV radiation, antioxidants likely must be delivered at the appropriate site in sufficient doses at the outset of and during active oxidative harm.9
In 2004, Fischer et al. conducted a clinical study of 15 healthy volunteers to test the skin penetration activity of melatonin 0.01% in a cream and 0.01% and 0.03% in a solution. During a 24-hour period, researchers obtained blood samples for melatonin measurement prior to application at 9 a.m. as well as 1, 4, 8, and 24 hours after application. Preapplication serum melatonin levels ranged from 0.6 to 15.9 pg/mL. The mean serum value 24 hours later after application of the 0.01% melatonin cream was 9.0 pg/mL. For the 0.01% solution group, the mean melatonin level was 12.7 pg/mL 24 hours after application. Melatonin levels also substantially rose just 1 and 8 hours later in the 0.03% solution group, with cumulative melatonin measured as 7.1 pg/mL in the 0.01% cream group, 8.6 pg/mL in the 0.01% solution participants, and 15.7 pg/mL in the 0.03% group. The investigators concluded that as a strong lipophilic compound melatonin penetrates the skin with serum blood levels increasing in a dose- and galenic-dependent manner without prompting spikes above the physiological range.10
Wound healing and atopic dermatitis
In 2006, Sener et al. reported that topically applied and systemically administered melatonin was successful as a pressure ulcer treatment in rats.11 Four years later, in a study using a chronic wound model in rats with pinealectomy that suppressed basal melatonin, Ozler et al. found that systemic and topical melatonin treatment were equally effective in imparting wound healing effects.12
A study in mice conducted by Kim et al. at around the same time showed that topically applied melatonin, by reducing total IgE in serum and interleukin-4 and interferon-gamma production by activated CD4(+) T cells, inhibits atopic dermatitis–like skin lesion development engendered by 2,4-dinitrofluorobenzene (DNFB) treatment in NC/Nga mice.13
More recently, Abbaszadeh et al. have suggested that melatonin has the potential to enhance the therapeutic ratio in radiation oncology, and to be more effective at reducing skin damage in this setting when used in optimal and non-toxic doses.2
Pigmentation disorders
Melatonin and serotonin are thought to have potential to ameliorate or attenuate the spread of vitiligo.1 In addition, melatonin appears to have potential in the realm of hyperpigmentation treatment. Investigators have found that the combination of topical melatonin 5% and a daily dose of 3 g of oral melatonin over 120 days significantly reduces Melasma Area Severity Index scores in comparison to placebo; the improvement is attributed primarily to the use of topical melatonin.14,15
Androgenetic alopecia
In 2018, Hatem et al. designed nanostructured lipid carriers to better deliver melatonin in antioxidant oils to treat androgenic alopecia. They found that the carriers achieved a sustained release of 6 hours and raised the skin deposition of melatonin 4.5-fold in the stratum corneum, 7-fold in the epidermis, and 6.8-fold in the dermis compared with a melatonin solution. The nanostructured lipid carriers also improved on clinical results, compared to the melatonin formula, by increasing hair density and thickness and reducing hair loss in patients with androgenic alopecia.16
Conclusion
Studies in humans have shown that through systemic administration and, particularly, topical application. Demonstrated to be safe and effective, topically applied melatonin appears to warrant serious consideration as a skin-protective, anti-aging tool in the dermatologic armamentarium.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Slominski AT et al. J Invest Dermatol. 2018 Mar;138(3):490-9.
2. Abbaszadeh A et al. J Biomed Phys Eng. 2017 Jun;7(2):127-136.
3. Fischer T et al. Hautarzt. 1999 Jan;50(1):5-11.
4. Scheuer C. Dan Med J. 2017 Jun;64(6). pii:B5358.
5. Bangha E et al. Arch Dermatol Res. 1996 Aug;288(9):522-6.
6. Bangha E et al. Dermatology. 1997;195(3):248-52.
7. Bangha E et al. Skin Pharmacol. 1997;10(5-6):298-302.
8. Dreher F et al. Br J Dermatol. 1998 Aug;139(2):332-9.
9. Dreher F et al. Dermatology. 1999;198(1):52-5.
10. Fischer TW et al. Skin Pharmacol Physiol. 2004 Jul-Aug;17(4):190-4.
11. Sener G et al. J Pineal Res. 2006 Apr;40(3):280-7.
12. Ozler M et al. Scand J Clin Lab Invest. 2010 Oct;70(6):447-52.
13. Kim TH et al. J Pineal Res. 2009 Nov;47(4):324-9.
14. Juhasz MLW et al. J Cosmet Dermatol. 2018 Dec;17(6):1144-57.
15. Hamadi SA, Mohammed MM, Aljaf AN, et al. The role of topical and oral melatonin in management of melasma patients. J Arab Univ Basic Appl Sci. 2009;8:30‐42.
16. Hatem S et al. Expert Opin Drug Deliv. 2018 Oct;15(10):927-35.
Found in various plant and animal species, including humans, melatonin (N-acetyl-5-methoxytryptamine) is best known for its daily fluctuations in circulating levels that regulate circadian rhythms. But this ancient serotonin derivative, stimulated by beta-adrenergic receptors, is the primary neuroendocrine product of the pineal gland (discovered as such in 1917) in humans and a dynamic compound with diverse roles in human health levels of which decrease with age.1,2 Over the last quarter of a century, we have arrived at a much greater understanding of the varied biological functions of this highly lipophilic hormone, which is now recognized as the strongest endogenous antioxidant, particularly potent against hydroxyl radicals, the most harmful of reactive oxygen species, and known to protect mitochondria and DNA from direct oxidative harm.2-4 Directly or via its circadian impact, melatonin also affects skin as well as core body temperature.1 This column is a . Next month’s column will address some more of the activities of this dynamic hormone while concentrating on the interaction of melatonin and ultraviolet radiation.
Early studies
In the mid-1990s, Bangha et al. performed several studies in healthy human volunteers that demonstrated that topically applied melatonin suppressed UVB-induced erythema (with one study showing pre- and posttreatment as effective and a subsequent one showing only pretreatment as effective), and also found that melatonin appears to have the potential to accumulate in the stratum corneum with extended release into the blood system through cutaneous delivery.5-7
A randomized, double-blind study by Dreher et al. in 12 healthy adults (6 women and 6 men, all white, aged 29-49 years) considered the short-term photoprotective effects of topically applied vitamin C, vitamin E, and melatonin, alone or in combination, 30 minutes after UV exposure. A dose-dependent photoprotective effect was associated with melatonin, and photoprotective properties were enhanced when melatonin in was combined with vitamins C and E.8
The following year, Dreher et al. evaluated the short-term photoprotective effects of the same compounds in a randomized, double-blind, placebo-controlled human study. Each antioxidant was topically applied alone or in combination after UV exposure in a single application (immediately or 30 minutes after UV exposure) or in multiple applications 30 minutes, 1 hour, and 2 hours after UV exposure (totaling three applications). Interestingly, no photoprotective effects were seen. The researchers concluded that given the speed of cutaneous damage from UV radiation, antioxidants likely must be delivered at the appropriate site in sufficient doses at the outset of and during active oxidative harm.9
In 2004, Fischer et al. conducted a clinical study of 15 healthy volunteers to test the skin penetration activity of melatonin 0.01% in a cream and 0.01% and 0.03% in a solution. During a 24-hour period, researchers obtained blood samples for melatonin measurement prior to application at 9 a.m. as well as 1, 4, 8, and 24 hours after application. Preapplication serum melatonin levels ranged from 0.6 to 15.9 pg/mL. The mean serum value 24 hours later after application of the 0.01% melatonin cream was 9.0 pg/mL. For the 0.01% solution group, the mean melatonin level was 12.7 pg/mL 24 hours after application. Melatonin levels also substantially rose just 1 and 8 hours later in the 0.03% solution group, with cumulative melatonin measured as 7.1 pg/mL in the 0.01% cream group, 8.6 pg/mL in the 0.01% solution participants, and 15.7 pg/mL in the 0.03% group. The investigators concluded that as a strong lipophilic compound melatonin penetrates the skin with serum blood levels increasing in a dose- and galenic-dependent manner without prompting spikes above the physiological range.10
Wound healing and atopic dermatitis
In 2006, Sener et al. reported that topically applied and systemically administered melatonin was successful as a pressure ulcer treatment in rats.11 Four years later, in a study using a chronic wound model in rats with pinealectomy that suppressed basal melatonin, Ozler et al. found that systemic and topical melatonin treatment were equally effective in imparting wound healing effects.12
A study in mice conducted by Kim et al. at around the same time showed that topically applied melatonin, by reducing total IgE in serum and interleukin-4 and interferon-gamma production by activated CD4(+) T cells, inhibits atopic dermatitis–like skin lesion development engendered by 2,4-dinitrofluorobenzene (DNFB) treatment in NC/Nga mice.13
More recently, Abbaszadeh et al. have suggested that melatonin has the potential to enhance the therapeutic ratio in radiation oncology, and to be more effective at reducing skin damage in this setting when used in optimal and non-toxic doses.2
Pigmentation disorders
Melatonin and serotonin are thought to have potential to ameliorate or attenuate the spread of vitiligo.1 In addition, melatonin appears to have potential in the realm of hyperpigmentation treatment. Investigators have found that the combination of topical melatonin 5% and a daily dose of 3 g of oral melatonin over 120 days significantly reduces Melasma Area Severity Index scores in comparison to placebo; the improvement is attributed primarily to the use of topical melatonin.14,15
Androgenetic alopecia
In 2018, Hatem et al. designed nanostructured lipid carriers to better deliver melatonin in antioxidant oils to treat androgenic alopecia. They found that the carriers achieved a sustained release of 6 hours and raised the skin deposition of melatonin 4.5-fold in the stratum corneum, 7-fold in the epidermis, and 6.8-fold in the dermis compared with a melatonin solution. The nanostructured lipid carriers also improved on clinical results, compared to the melatonin formula, by increasing hair density and thickness and reducing hair loss in patients with androgenic alopecia.16
Conclusion
Studies in humans have shown that through systemic administration and, particularly, topical application. Demonstrated to be safe and effective, topically applied melatonin appears to warrant serious consideration as a skin-protective, anti-aging tool in the dermatologic armamentarium.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Slominski AT et al. J Invest Dermatol. 2018 Mar;138(3):490-9.
2. Abbaszadeh A et al. J Biomed Phys Eng. 2017 Jun;7(2):127-136.
3. Fischer T et al. Hautarzt. 1999 Jan;50(1):5-11.
4. Scheuer C. Dan Med J. 2017 Jun;64(6). pii:B5358.
5. Bangha E et al. Arch Dermatol Res. 1996 Aug;288(9):522-6.
6. Bangha E et al. Dermatology. 1997;195(3):248-52.
7. Bangha E et al. Skin Pharmacol. 1997;10(5-6):298-302.
8. Dreher F et al. Br J Dermatol. 1998 Aug;139(2):332-9.
9. Dreher F et al. Dermatology. 1999;198(1):52-5.
10. Fischer TW et al. Skin Pharmacol Physiol. 2004 Jul-Aug;17(4):190-4.
11. Sener G et al. J Pineal Res. 2006 Apr;40(3):280-7.
12. Ozler M et al. Scand J Clin Lab Invest. 2010 Oct;70(6):447-52.
13. Kim TH et al. J Pineal Res. 2009 Nov;47(4):324-9.
14. Juhasz MLW et al. J Cosmet Dermatol. 2018 Dec;17(6):1144-57.
15. Hamadi SA, Mohammed MM, Aljaf AN, et al. The role of topical and oral melatonin in management of melasma patients. J Arab Univ Basic Appl Sci. 2009;8:30‐42.
16. Hatem S et al. Expert Opin Drug Deliv. 2018 Oct;15(10):927-35.
Found in various plant and animal species, including humans, melatonin (N-acetyl-5-methoxytryptamine) is best known for its daily fluctuations in circulating levels that regulate circadian rhythms. But this ancient serotonin derivative, stimulated by beta-adrenergic receptors, is the primary neuroendocrine product of the pineal gland (discovered as such in 1917) in humans and a dynamic compound with diverse roles in human health levels of which decrease with age.1,2 Over the last quarter of a century, we have arrived at a much greater understanding of the varied biological functions of this highly lipophilic hormone, which is now recognized as the strongest endogenous antioxidant, particularly potent against hydroxyl radicals, the most harmful of reactive oxygen species, and known to protect mitochondria and DNA from direct oxidative harm.2-4 Directly or via its circadian impact, melatonin also affects skin as well as core body temperature.1 This column is a . Next month’s column will address some more of the activities of this dynamic hormone while concentrating on the interaction of melatonin and ultraviolet radiation.
Early studies
In the mid-1990s, Bangha et al. performed several studies in healthy human volunteers that demonstrated that topically applied melatonin suppressed UVB-induced erythema (with one study showing pre- and posttreatment as effective and a subsequent one showing only pretreatment as effective), and also found that melatonin appears to have the potential to accumulate in the stratum corneum with extended release into the blood system through cutaneous delivery.5-7
A randomized, double-blind study by Dreher et al. in 12 healthy adults (6 women and 6 men, all white, aged 29-49 years) considered the short-term photoprotective effects of topically applied vitamin C, vitamin E, and melatonin, alone or in combination, 30 minutes after UV exposure. A dose-dependent photoprotective effect was associated with melatonin, and photoprotective properties were enhanced when melatonin in was combined with vitamins C and E.8
The following year, Dreher et al. evaluated the short-term photoprotective effects of the same compounds in a randomized, double-blind, placebo-controlled human study. Each antioxidant was topically applied alone or in combination after UV exposure in a single application (immediately or 30 minutes after UV exposure) or in multiple applications 30 minutes, 1 hour, and 2 hours after UV exposure (totaling three applications). Interestingly, no photoprotective effects were seen. The researchers concluded that given the speed of cutaneous damage from UV radiation, antioxidants likely must be delivered at the appropriate site in sufficient doses at the outset of and during active oxidative harm.9
In 2004, Fischer et al. conducted a clinical study of 15 healthy volunteers to test the skin penetration activity of melatonin 0.01% in a cream and 0.01% and 0.03% in a solution. During a 24-hour period, researchers obtained blood samples for melatonin measurement prior to application at 9 a.m. as well as 1, 4, 8, and 24 hours after application. Preapplication serum melatonin levels ranged from 0.6 to 15.9 pg/mL. The mean serum value 24 hours later after application of the 0.01% melatonin cream was 9.0 pg/mL. For the 0.01% solution group, the mean melatonin level was 12.7 pg/mL 24 hours after application. Melatonin levels also substantially rose just 1 and 8 hours later in the 0.03% solution group, with cumulative melatonin measured as 7.1 pg/mL in the 0.01% cream group, 8.6 pg/mL in the 0.01% solution participants, and 15.7 pg/mL in the 0.03% group. The investigators concluded that as a strong lipophilic compound melatonin penetrates the skin with serum blood levels increasing in a dose- and galenic-dependent manner without prompting spikes above the physiological range.10
Wound healing and atopic dermatitis
In 2006, Sener et al. reported that topically applied and systemically administered melatonin was successful as a pressure ulcer treatment in rats.11 Four years later, in a study using a chronic wound model in rats with pinealectomy that suppressed basal melatonin, Ozler et al. found that systemic and topical melatonin treatment were equally effective in imparting wound healing effects.12
A study in mice conducted by Kim et al. at around the same time showed that topically applied melatonin, by reducing total IgE in serum and interleukin-4 and interferon-gamma production by activated CD4(+) T cells, inhibits atopic dermatitis–like skin lesion development engendered by 2,4-dinitrofluorobenzene (DNFB) treatment in NC/Nga mice.13
More recently, Abbaszadeh et al. have suggested that melatonin has the potential to enhance the therapeutic ratio in radiation oncology, and to be more effective at reducing skin damage in this setting when used in optimal and non-toxic doses.2
Pigmentation disorders
Melatonin and serotonin are thought to have potential to ameliorate or attenuate the spread of vitiligo.1 In addition, melatonin appears to have potential in the realm of hyperpigmentation treatment. Investigators have found that the combination of topical melatonin 5% and a daily dose of 3 g of oral melatonin over 120 days significantly reduces Melasma Area Severity Index scores in comparison to placebo; the improvement is attributed primarily to the use of topical melatonin.14,15
Androgenetic alopecia
In 2018, Hatem et al. designed nanostructured lipid carriers to better deliver melatonin in antioxidant oils to treat androgenic alopecia. They found that the carriers achieved a sustained release of 6 hours and raised the skin deposition of melatonin 4.5-fold in the stratum corneum, 7-fold in the epidermis, and 6.8-fold in the dermis compared with a melatonin solution. The nanostructured lipid carriers also improved on clinical results, compared to the melatonin formula, by increasing hair density and thickness and reducing hair loss in patients with androgenic alopecia.16
Conclusion
Studies in humans have shown that through systemic administration and, particularly, topical application. Demonstrated to be safe and effective, topically applied melatonin appears to warrant serious consideration as a skin-protective, anti-aging tool in the dermatologic armamentarium.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Slominski AT et al. J Invest Dermatol. 2018 Mar;138(3):490-9.
2. Abbaszadeh A et al. J Biomed Phys Eng. 2017 Jun;7(2):127-136.
3. Fischer T et al. Hautarzt. 1999 Jan;50(1):5-11.
4. Scheuer C. Dan Med J. 2017 Jun;64(6). pii:B5358.
5. Bangha E et al. Arch Dermatol Res. 1996 Aug;288(9):522-6.
6. Bangha E et al. Dermatology. 1997;195(3):248-52.
7. Bangha E et al. Skin Pharmacol. 1997;10(5-6):298-302.
8. Dreher F et al. Br J Dermatol. 1998 Aug;139(2):332-9.
9. Dreher F et al. Dermatology. 1999;198(1):52-5.
10. Fischer TW et al. Skin Pharmacol Physiol. 2004 Jul-Aug;17(4):190-4.
11. Sener G et al. J Pineal Res. 2006 Apr;40(3):280-7.
12. Ozler M et al. Scand J Clin Lab Invest. 2010 Oct;70(6):447-52.
13. Kim TH et al. J Pineal Res. 2009 Nov;47(4):324-9.
14. Juhasz MLW et al. J Cosmet Dermatol. 2018 Dec;17(6):1144-57.
15. Hamadi SA, Mohammed MM, Aljaf AN, et al. The role of topical and oral melatonin in management of melasma patients. J Arab Univ Basic Appl Sci. 2009;8:30‐42.
16. Hatem S et al. Expert Opin Drug Deliv. 2018 Oct;15(10):927-35.
The role of the skin microbiome in skin disease
The microbiome of the gut and skin can impact one another in health and disease. Numerous dermatologic disorders can be traced to gastrointestinal etiologic origins.1 Incorporating discussion of the latest findings on the cutaneous and gut microbiome expands our understanding of the origin of dermatologic disease. , but the gut microbiome also has effects on the skin microbiome that are just being elucidated. Although we do not yet know enough to give our patients definitive advice about probiotics, the knowledge in this field is rapidly expanding and is an exciting area to watch. Certainly, everything applied to the skin or ingested in the diet plays a role in the skin and gut microbiome. Therefore, the savvy dermatologist understands that personal care products, including cosmeceuticals, will affect the microbiome. At this point, we do not yet know what is beneficial, but we do know that diversity of organisms is important and is the preferred state as compared to having fewer types of organisms on the skin.
Acne
Acne has long been known to have a multifactorial etiologic pathway. It is increasingly thought that understanding the role of the skin (and possibly gut) microbiome in acne pathophysiology may lead to enhanced treatments.2 New gene sequencing technologies, particularly those based on recA and tly loci, are teaching us more about the anaerobic bacterium Propionibacterium acnes (now called Cutibacterium acnes).3
In 2017, Dréno et al. studied the skin microbiota in 26 subjects with mild to moderate acne. The microflora were characterized using a high‐throughput sequencing approach that targets a portion of the bacterial 16S rRNA gene. The samples were obtained before and after 28 days of treatment with erythromycin 4% or a cosmeceutical containing lipohydroxy acid, salicylic acid, linoleic acid, niacinamide, piroctone olamine, a ceramide, and thermal spring water. Upon conclusion of the study, Actinobacteria were reduced in both groups while staphylococci were reduced only in the dermocosmetic group.4 The interesting point of this study was that the cosmeceutical had a greater impact on staphylococci than did topical erythromycin, demonstrating that personal care products can have profound effects on the microbiome.
Early in 2018, Kelhälä et al. compared the impact of the systemic acne treatments isotretinoin and lymecycline on cutaneous microbiota in the cheeks, back, and axillae of mild to moderate acne patients using gene sequencing. They found that acne severity positively correlated with Propionibacterium acnes levels. P. acnes levels were decreased by both treatments, but isotretinoin resulted in a greater decrease. Increased microbiome diversity was seen on the cheek and back in all treated subjects, but diversity was highest in those treated with isotretinoin.5 The authors postulated that the diversity resulted from a decrease in P. acnes levels. To learn more about what to tell your patients about acne and the microbiome, read my blog
Atopic dermatitis
Atopic dermatitis (AD) is associated with dysbiosis of cutaneous microbiota and diminished diversity in microbial communities.6,7 There is also a robust epidemiologic relationship between the cutaneous and gut microbiomes and AD.8 Many studies have looked at the role of the microbiome in AD, including the role of Staphylococcus aureus, because it selectively colonizes the lesional skin of AD patients but is notably lacking on the skin of most healthy people.
In a 2017 literature review, Bjerre et al. found that while the data were not extensive, AD-affected skin was characterized by low bacterial diversity with S. aureus and Staphylococcus epidermidis more abundant. Also that year, Williams and Gallo reported on a prospective clinical trial in children that colonization by S. aureus occurred before the emergence of AD symptoms.9 In 2018, Clausen et al. reported on an observational case-control study of 45 adult healthy controls and 56 adult patients with AD between January and June 2015 to evaluate skin and nasal microbiome diversity and composition and to elucidate the relationship between disease severity and filaggrin gene mutations in AD patients. Next-generation sequencing targeting 16S ribosomal RNA was used to show that microbiome diversity was lower in the lesional skin, nonlesional skin, and nose in AD patients compared with controls. Such diversity was also found to be inversely correlated with disease severity, and microbiome composition in nonlesional AD skin was found to be associated with filaggrin gene mutations. The authors concluded that host genetics and skin microbiome may be connected in AD.10
However, the role of S. aureus in AD and the effect of its presence on microbiome diversity is still unclear. Marrs and Flohr note that the eradication of S. aureus does not appear to account for improvement in AD and increase in bacterial diversity after the use of antimicrobial and anti-inflammatory therapy.11
Rosacea
Rosacea is a chronic inflammatory skin condition long associated with Demodex mites (Demodex folliculorum and Demodex brevis).12 In rosacea-affected skin, Demodex mites are found to occur in greater density than in unaffected skin.13 Other microbiota-linked alterations have been detected on the skin and in the small intestines in cases of rosacea.14 One twin study showed that increased levels of Gordonia correlated with rosacea severity.15 A study in Korean women with rosacea demonstrated a reduction of Peptococcaceae, Methanobrevibacter, Slackia, Coprobacillus, Citrobacter (genus), and Desulfovibrio and an increased amount of Acidaminococcus, Megasphaera, and Lactobacillales in women with rosacea.16
Other studies have shown that treating bacterial overgrowth in the gut can improve rosacea.17 In my favorite recent study,18 complement appeared to affect microbial diversity and richness of the skin and the gut in mice, demonstrating that the immune system plays an important role in rosacea and the skin and gut microbiome. Certainly we have a lot to learn before we can make specific recommendations, but I feel certain that this area of research will unlock some of the mysteries of rosacea. To read more about what to tell your patients about the microbiome and rosacea visit the blog at STSfranchise.com.
Conclusion
In recent years, it has become increasingly clear that the cutaneous microbiome is a factor in various skin disorders. Some authors such as Egert et al. advocate the use of pre- and probiotics, including topical microbiome transplantation therapies, to treat acne, rosacea, and AD.14 I believe that we do not yet have enough data to support this approach or predict which ones may be effective. Stay tuned for more developments.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. O’Neill CA et al. Bioessays. 2016 Nov;38(11):1167-76.
2. Rocha MA et al. Arch Dermatol Res. 2018 Apr;310(3):181-5.
3. McDowell A. Microorganisms. 2017 Dec 21. doi: 10.3390/microorganisms6010001.
4. Dréno B et al. Exp Dermatol. 2017 Sep;26(9):798-803.
5. Kelhälä HL et al. Exp Dermatol. 2018 Jan;27(1):30-6.
6. Rodrigues Hoffmann A. Vet Dermatol. 2017 Feb;28(1):60-e15.
7. Bjerre RD et al. Br J Dermatol. 2017 Nov;177(5):1272-8.
8. Knaysi G et al. Curr Allergy Asthma Rep. 2017 Jan;17(1):7.
9. Williams MR et al. J Invest Dermatol. 2017 Dec;137(12):2460-1.
10. Clausen ML et al. JAMA Dermatol. 2018 Mar 1;154(3):293-300.
11. Marrs T et al. Br J Dermatol. 2016 Oct;175 Suppl 2:13-18.
12. Patra V et al. Front Microbiol. 2016 Aug 10. doi: 10.3389/fmicb.2016.01235.
13. Igawa S et al. Transl Res. 2017 Jun;184:68-76.
14. Egert Met al. Clin Pharmacol Ther. 2017;102(1):62-9.
15. Zaidi AK et al. Exp Dermatol. 2018 Mar;27(3):295-8.
16. Nam, JH et al. Exp Dermatol. 2018 Jan;27(1):37-42.
17. Porubsky CF et al. “The Role of Probiotics in Acne and Rosacea,” IntechOpen. 2018 Nov 5. doi: 10.5772/intechopen.79044.
18. Chehoud C et al. Proc Natl Acad Sci U S A. 2013 Sep 10;110(37):15061-6.
The microbiome of the gut and skin can impact one another in health and disease. Numerous dermatologic disorders can be traced to gastrointestinal etiologic origins.1 Incorporating discussion of the latest findings on the cutaneous and gut microbiome expands our understanding of the origin of dermatologic disease. , but the gut microbiome also has effects on the skin microbiome that are just being elucidated. Although we do not yet know enough to give our patients definitive advice about probiotics, the knowledge in this field is rapidly expanding and is an exciting area to watch. Certainly, everything applied to the skin or ingested in the diet plays a role in the skin and gut microbiome. Therefore, the savvy dermatologist understands that personal care products, including cosmeceuticals, will affect the microbiome. At this point, we do not yet know what is beneficial, but we do know that diversity of organisms is important and is the preferred state as compared to having fewer types of organisms on the skin.
Acne
Acne has long been known to have a multifactorial etiologic pathway. It is increasingly thought that understanding the role of the skin (and possibly gut) microbiome in acne pathophysiology may lead to enhanced treatments.2 New gene sequencing technologies, particularly those based on recA and tly loci, are teaching us more about the anaerobic bacterium Propionibacterium acnes (now called Cutibacterium acnes).3
In 2017, Dréno et al. studied the skin microbiota in 26 subjects with mild to moderate acne. The microflora were characterized using a high‐throughput sequencing approach that targets a portion of the bacterial 16S rRNA gene. The samples were obtained before and after 28 days of treatment with erythromycin 4% or a cosmeceutical containing lipohydroxy acid, salicylic acid, linoleic acid, niacinamide, piroctone olamine, a ceramide, and thermal spring water. Upon conclusion of the study, Actinobacteria were reduced in both groups while staphylococci were reduced only in the dermocosmetic group.4 The interesting point of this study was that the cosmeceutical had a greater impact on staphylococci than did topical erythromycin, demonstrating that personal care products can have profound effects on the microbiome.
Early in 2018, Kelhälä et al. compared the impact of the systemic acne treatments isotretinoin and lymecycline on cutaneous microbiota in the cheeks, back, and axillae of mild to moderate acne patients using gene sequencing. They found that acne severity positively correlated with Propionibacterium acnes levels. P. acnes levels were decreased by both treatments, but isotretinoin resulted in a greater decrease. Increased microbiome diversity was seen on the cheek and back in all treated subjects, but diversity was highest in those treated with isotretinoin.5 The authors postulated that the diversity resulted from a decrease in P. acnes levels. To learn more about what to tell your patients about acne and the microbiome, read my blog
Atopic dermatitis
Atopic dermatitis (AD) is associated with dysbiosis of cutaneous microbiota and diminished diversity in microbial communities.6,7 There is also a robust epidemiologic relationship between the cutaneous and gut microbiomes and AD.8 Many studies have looked at the role of the microbiome in AD, including the role of Staphylococcus aureus, because it selectively colonizes the lesional skin of AD patients but is notably lacking on the skin of most healthy people.
In a 2017 literature review, Bjerre et al. found that while the data were not extensive, AD-affected skin was characterized by low bacterial diversity with S. aureus and Staphylococcus epidermidis more abundant. Also that year, Williams and Gallo reported on a prospective clinical trial in children that colonization by S. aureus occurred before the emergence of AD symptoms.9 In 2018, Clausen et al. reported on an observational case-control study of 45 adult healthy controls and 56 adult patients with AD between January and June 2015 to evaluate skin and nasal microbiome diversity and composition and to elucidate the relationship between disease severity and filaggrin gene mutations in AD patients. Next-generation sequencing targeting 16S ribosomal RNA was used to show that microbiome diversity was lower in the lesional skin, nonlesional skin, and nose in AD patients compared with controls. Such diversity was also found to be inversely correlated with disease severity, and microbiome composition in nonlesional AD skin was found to be associated with filaggrin gene mutations. The authors concluded that host genetics and skin microbiome may be connected in AD.10
However, the role of S. aureus in AD and the effect of its presence on microbiome diversity is still unclear. Marrs and Flohr note that the eradication of S. aureus does not appear to account for improvement in AD and increase in bacterial diversity after the use of antimicrobial and anti-inflammatory therapy.11
Rosacea
Rosacea is a chronic inflammatory skin condition long associated with Demodex mites (Demodex folliculorum and Demodex brevis).12 In rosacea-affected skin, Demodex mites are found to occur in greater density than in unaffected skin.13 Other microbiota-linked alterations have been detected on the skin and in the small intestines in cases of rosacea.14 One twin study showed that increased levels of Gordonia correlated with rosacea severity.15 A study in Korean women with rosacea demonstrated a reduction of Peptococcaceae, Methanobrevibacter, Slackia, Coprobacillus, Citrobacter (genus), and Desulfovibrio and an increased amount of Acidaminococcus, Megasphaera, and Lactobacillales in women with rosacea.16
Other studies have shown that treating bacterial overgrowth in the gut can improve rosacea.17 In my favorite recent study,18 complement appeared to affect microbial diversity and richness of the skin and the gut in mice, demonstrating that the immune system plays an important role in rosacea and the skin and gut microbiome. Certainly we have a lot to learn before we can make specific recommendations, but I feel certain that this area of research will unlock some of the mysteries of rosacea. To read more about what to tell your patients about the microbiome and rosacea visit the blog at STSfranchise.com.
Conclusion
In recent years, it has become increasingly clear that the cutaneous microbiome is a factor in various skin disorders. Some authors such as Egert et al. advocate the use of pre- and probiotics, including topical microbiome transplantation therapies, to treat acne, rosacea, and AD.14 I believe that we do not yet have enough data to support this approach or predict which ones may be effective. Stay tuned for more developments.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. O’Neill CA et al. Bioessays. 2016 Nov;38(11):1167-76.
2. Rocha MA et al. Arch Dermatol Res. 2018 Apr;310(3):181-5.
3. McDowell A. Microorganisms. 2017 Dec 21. doi: 10.3390/microorganisms6010001.
4. Dréno B et al. Exp Dermatol. 2017 Sep;26(9):798-803.
5. Kelhälä HL et al. Exp Dermatol. 2018 Jan;27(1):30-6.
6. Rodrigues Hoffmann A. Vet Dermatol. 2017 Feb;28(1):60-e15.
7. Bjerre RD et al. Br J Dermatol. 2017 Nov;177(5):1272-8.
8. Knaysi G et al. Curr Allergy Asthma Rep. 2017 Jan;17(1):7.
9. Williams MR et al. J Invest Dermatol. 2017 Dec;137(12):2460-1.
10. Clausen ML et al. JAMA Dermatol. 2018 Mar 1;154(3):293-300.
11. Marrs T et al. Br J Dermatol. 2016 Oct;175 Suppl 2:13-18.
12. Patra V et al. Front Microbiol. 2016 Aug 10. doi: 10.3389/fmicb.2016.01235.
13. Igawa S et al. Transl Res. 2017 Jun;184:68-76.
14. Egert Met al. Clin Pharmacol Ther. 2017;102(1):62-9.
15. Zaidi AK et al. Exp Dermatol. 2018 Mar;27(3):295-8.
16. Nam, JH et al. Exp Dermatol. 2018 Jan;27(1):37-42.
17. Porubsky CF et al. “The Role of Probiotics in Acne and Rosacea,” IntechOpen. 2018 Nov 5. doi: 10.5772/intechopen.79044.
18. Chehoud C et al. Proc Natl Acad Sci U S A. 2013 Sep 10;110(37):15061-6.
The microbiome of the gut and skin can impact one another in health and disease. Numerous dermatologic disorders can be traced to gastrointestinal etiologic origins.1 Incorporating discussion of the latest findings on the cutaneous and gut microbiome expands our understanding of the origin of dermatologic disease. , but the gut microbiome also has effects on the skin microbiome that are just being elucidated. Although we do not yet know enough to give our patients definitive advice about probiotics, the knowledge in this field is rapidly expanding and is an exciting area to watch. Certainly, everything applied to the skin or ingested in the diet plays a role in the skin and gut microbiome. Therefore, the savvy dermatologist understands that personal care products, including cosmeceuticals, will affect the microbiome. At this point, we do not yet know what is beneficial, but we do know that diversity of organisms is important and is the preferred state as compared to having fewer types of organisms on the skin.
Acne
Acne has long been known to have a multifactorial etiologic pathway. It is increasingly thought that understanding the role of the skin (and possibly gut) microbiome in acne pathophysiology may lead to enhanced treatments.2 New gene sequencing technologies, particularly those based on recA and tly loci, are teaching us more about the anaerobic bacterium Propionibacterium acnes (now called Cutibacterium acnes).3
In 2017, Dréno et al. studied the skin microbiota in 26 subjects with mild to moderate acne. The microflora were characterized using a high‐throughput sequencing approach that targets a portion of the bacterial 16S rRNA gene. The samples were obtained before and after 28 days of treatment with erythromycin 4% or a cosmeceutical containing lipohydroxy acid, salicylic acid, linoleic acid, niacinamide, piroctone olamine, a ceramide, and thermal spring water. Upon conclusion of the study, Actinobacteria were reduced in both groups while staphylococci were reduced only in the dermocosmetic group.4 The interesting point of this study was that the cosmeceutical had a greater impact on staphylococci than did topical erythromycin, demonstrating that personal care products can have profound effects on the microbiome.
Early in 2018, Kelhälä et al. compared the impact of the systemic acne treatments isotretinoin and lymecycline on cutaneous microbiota in the cheeks, back, and axillae of mild to moderate acne patients using gene sequencing. They found that acne severity positively correlated with Propionibacterium acnes levels. P. acnes levels were decreased by both treatments, but isotretinoin resulted in a greater decrease. Increased microbiome diversity was seen on the cheek and back in all treated subjects, but diversity was highest in those treated with isotretinoin.5 The authors postulated that the diversity resulted from a decrease in P. acnes levels. To learn more about what to tell your patients about acne and the microbiome, read my blog
Atopic dermatitis
Atopic dermatitis (AD) is associated with dysbiosis of cutaneous microbiota and diminished diversity in microbial communities.6,7 There is also a robust epidemiologic relationship between the cutaneous and gut microbiomes and AD.8 Many studies have looked at the role of the microbiome in AD, including the role of Staphylococcus aureus, because it selectively colonizes the lesional skin of AD patients but is notably lacking on the skin of most healthy people.
In a 2017 literature review, Bjerre et al. found that while the data were not extensive, AD-affected skin was characterized by low bacterial diversity with S. aureus and Staphylococcus epidermidis more abundant. Also that year, Williams and Gallo reported on a prospective clinical trial in children that colonization by S. aureus occurred before the emergence of AD symptoms.9 In 2018, Clausen et al. reported on an observational case-control study of 45 adult healthy controls and 56 adult patients with AD between January and June 2015 to evaluate skin and nasal microbiome diversity and composition and to elucidate the relationship between disease severity and filaggrin gene mutations in AD patients. Next-generation sequencing targeting 16S ribosomal RNA was used to show that microbiome diversity was lower in the lesional skin, nonlesional skin, and nose in AD patients compared with controls. Such diversity was also found to be inversely correlated with disease severity, and microbiome composition in nonlesional AD skin was found to be associated with filaggrin gene mutations. The authors concluded that host genetics and skin microbiome may be connected in AD.10
However, the role of S. aureus in AD and the effect of its presence on microbiome diversity is still unclear. Marrs and Flohr note that the eradication of S. aureus does not appear to account for improvement in AD and increase in bacterial diversity after the use of antimicrobial and anti-inflammatory therapy.11
Rosacea
Rosacea is a chronic inflammatory skin condition long associated with Demodex mites (Demodex folliculorum and Demodex brevis).12 In rosacea-affected skin, Demodex mites are found to occur in greater density than in unaffected skin.13 Other microbiota-linked alterations have been detected on the skin and in the small intestines in cases of rosacea.14 One twin study showed that increased levels of Gordonia correlated with rosacea severity.15 A study in Korean women with rosacea demonstrated a reduction of Peptococcaceae, Methanobrevibacter, Slackia, Coprobacillus, Citrobacter (genus), and Desulfovibrio and an increased amount of Acidaminococcus, Megasphaera, and Lactobacillales in women with rosacea.16
Other studies have shown that treating bacterial overgrowth in the gut can improve rosacea.17 In my favorite recent study,18 complement appeared to affect microbial diversity and richness of the skin and the gut in mice, demonstrating that the immune system plays an important role in rosacea and the skin and gut microbiome. Certainly we have a lot to learn before we can make specific recommendations, but I feel certain that this area of research will unlock some of the mysteries of rosacea. To read more about what to tell your patients about the microbiome and rosacea visit the blog at STSfranchise.com.
Conclusion
In recent years, it has become increasingly clear that the cutaneous microbiome is a factor in various skin disorders. Some authors such as Egert et al. advocate the use of pre- and probiotics, including topical microbiome transplantation therapies, to treat acne, rosacea, and AD.14 I believe that we do not yet have enough data to support this approach or predict which ones may be effective. Stay tuned for more developments.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. O’Neill CA et al. Bioessays. 2016 Nov;38(11):1167-76.
2. Rocha MA et al. Arch Dermatol Res. 2018 Apr;310(3):181-5.
3. McDowell A. Microorganisms. 2017 Dec 21. doi: 10.3390/microorganisms6010001.
4. Dréno B et al. Exp Dermatol. 2017 Sep;26(9):798-803.
5. Kelhälä HL et al. Exp Dermatol. 2018 Jan;27(1):30-6.
6. Rodrigues Hoffmann A. Vet Dermatol. 2017 Feb;28(1):60-e15.
7. Bjerre RD et al. Br J Dermatol. 2017 Nov;177(5):1272-8.
8. Knaysi G et al. Curr Allergy Asthma Rep. 2017 Jan;17(1):7.
9. Williams MR et al. J Invest Dermatol. 2017 Dec;137(12):2460-1.
10. Clausen ML et al. JAMA Dermatol. 2018 Mar 1;154(3):293-300.
11. Marrs T et al. Br J Dermatol. 2016 Oct;175 Suppl 2:13-18.
12. Patra V et al. Front Microbiol. 2016 Aug 10. doi: 10.3389/fmicb.2016.01235.
13. Igawa S et al. Transl Res. 2017 Jun;184:68-76.
14. Egert Met al. Clin Pharmacol Ther. 2017;102(1):62-9.
15. Zaidi AK et al. Exp Dermatol. 2018 Mar;27(3):295-8.
16. Nam, JH et al. Exp Dermatol. 2018 Jan;27(1):37-42.
17. Porubsky CF et al. “The Role of Probiotics in Acne and Rosacea,” IntechOpen. 2018 Nov 5. doi: 10.5772/intechopen.79044.
18. Chehoud C et al. Proc Natl Acad Sci U S A. 2013 Sep 10;110(37):15061-6.
The role of the skin microbiome in skin care
It may not seem intuitive, but to understand some of the new skin care claims, you need to know a bit about the gut microbiome and its role in skin health. The The gut and skin play a balancing act between beneficial, neutral, and harmful flora that are interrelated with the innate and adaptive immune systems.1 The skin and gut seem to be intertwined and express several comorbidities.2 In this column, the focus is on the cutaneous microbiome’s role in skin health. To understand the cosmeceutical claims about pre- and probiotics, you first need to familiarize yourself with skin microbiome science. The skin-gut nexus will be discussed in next month’s column, which will address the role of the skin microbiome in skin diseases.
Why is the microbiome such a hot topic?
Genetic sequencing has spurred advances in the study of the microbiome and has provided intriguing clues that the gut and skin microbiome have influences on each other. Sequencing assays that focus on bacterial 16S ribosomal RNA genes have been used by investigators to distinguish and describe the wide variety of resident and transient microorganisms on the skin and elucidate their roles in skin health and disease.1 Genomic sequencing has identified species in the skin and gut that were not found previously by cultivating microbial isolates.3,4 Advances in technologies such as whole-genome shotgun sequencing, metagenomics, and functional metabolomics will further contribute to our understanding of the effects of the skin microbiome on skin health and skin type. Of course, many supplement and cosmeceutical companies have jumped on this bandwagon prematurely and claim that their products increase “good bacteria while diminishing bad bacteria.” While there are interesting data that have emerged, we still cannot say which bacteria are “good” and ‘bad” as far as the skin is concerned – with a few exceptions that we have known all along. For example, Cutibacterium acnes and Staphylococcus aureus still remain in the undesirable category. (P. acnes has been renamed and now is officially referred to as C. acnes.) While it is premature to recommend probiotic– or prebiotic–containing cosmeceuticals, your patients will ask you about them. New studies about rosacea and the microbiome have generated a lot of patient questions in my practice, so I am writing several blogs about how to answer patient questions, which can be found at STSFranchise.com/blog. I’m also educating consumers on Facebook and Instagram @skintypesolutions so that they will not be taken advantage of by the too early “pseudoscience.” So now that you have heard that it is too early to recommend pre- and probiotic skin care to target skin issues, let’s look at the science that does exist.
Terminology
- Microbiome: Microbes that live in a particular environment or biome.
- Microbiota: The collection of living microbes that live in or on an environment. This term includes the microorganisms only and not the characteristics of their environment.
- Prebiotics: A nondigestible food ingredient that promotes the growth of microorganisms in the intestines. These can promote the growth of beneficial or harmful microorganisms. Think of them as a type of “fertilizer” for the microbiome.
- Probiotics: Living microorganisms that can provide beneficial qualities when used orally or topically. What probiotics are not? Microbes naturally found in your body and on your skin; microbes that are no longer alive; fermented foods that contain an unknown amount of bacteria.
Skin surface area
Richard Gallo, MD, a dermatologist from the University of California, San Diego, who is a leader in the microbiome field of study, says that estimates of the cutaneous microbiome’s impact on human health via skin have failed to acknowledge the inner follicular surface, thus drastically undervaluing the potential of the cutaneous microbiome to influence systemic health.5 He suggests that the surface area of skin has been miscalculated as measuring 2 m2 because it is considered a flat surface. This ignores the plethora of hair follicles and sweat ducts that significantly broaden the epithelial surface to measure closer to 25 m2 and underscores that the expansive skin microbiome is much larger than previously recognized.5 Taking the hair follicle surface area into account, the skin has vast space to harbor various organisms and microbiome environments. What our patients use on their skin certainly influences these environments. The key is trying to figure out how to manipulate the microbiome to our patient’s advantage.
Microbes have environmental preferences
Different microbial species thrive on particular regions of the diverse topography of the expansive surface area and choose their preferred environments from among sebaceous or nonsebaceous, hairy or smooth, moist or dry, and creased or noncreased areas.6,7 Other host factors that affect which microorganisms colonize the skin include hair follicle thickness, age, sex, diet (especially high fat and sugar intake), climate, occupation, and personal hygiene.7-10 Gene sequencing has revealed that these variations are partially because of factors such as ultraviolet exposure, pH, and temperature.4,6,11 For example, C. acnes has been found to be more prevalent in highly sebaceous sites on the head and upper torso.4 In general, Propionibacteriaceae (Cutibacterium) prefer sebaceous areas, whereas Corynebacteriaceae and Staphylococcaceae prevail in moist regions, such as the navel or axilla. Dry areas host the widest diversity of microbes, including Corynebacterium, Staphylococcus, and Streptococcus species.1,7,12
Impact of sebum and skin hydration on microbiome
In 2016, Mukherjee et al. measured sebum and hydration from the forehead and cheeks of 30 healthy female volunteers in a study that tested the hypothesis that differences in sebum and hydration levels in specific facial areas account for interindividual variation in facial skin microbiome. They found that the most significant predictor of microbiome composition was cheek sebum level, followed by forehead hydration level, while cheek hydration and forehead sebum levels were not predictive. The prevalence of Actinobacteria/Propionibacterium rose, while microbiome diversity diminished with an increase in cheek sebum, with such trends reversed in relation to forehead hydration. The investigators concluded that site-specific sebum and water levels impact the nature and diversity of the facial skin microbiome.13
Lability of the cutaneous microbiome
The skin microbiome changes during various times of life. For example, in puberty, more lipophilic species such as Propionibacteriaceae and Cornebacteriaceae predominate, while prior to puberty there is a preponderance of Firmicutes, Bacteroidetes, and Proteobacteria.4,14 However, in the absence of lifestyle changes, cutaneous microbial communities have been found through longitudinal studies to be relatively stable over a 2-year period.6 A person’s skin microbiome is subject to influence from an adjacent skin microbiome, such as between cohabiting couples or the influence of breastfeeding mothers.15 It is never too early to consider the role of the microbiome in health and disease. For example, infant microbiomes play a role in eczema and the atopic march.16 For this reason, those of us who treat children need to be familiar with studies that have demonstrate how the cutaneous microbiome is affected by childbirth delivery method, breastfeeding, the mother’s diet antibiotic use during pregnancy and breastfeeding.4,17
Microbiome effects on skin function
The skin barrier, a bilayer lipid-laden membrane that surrounds keratinocytes and prevents transepidermal water loss, is affected by resident microbial communities and has been shown by research to be influenced by the volume and diversity of such microbes.18 Organisms on the skin’s surface play an important role in communicating with and educating the cutaneous arm of the immune system.19 In 2017, Maguire and Maguire reviewed recent studies of the gut and skin microbiomes and suggested that Nitrobacter, Lactobacillus, and Bifidobacterium can improve skin health and could be useful bacterial adjuvants in a probiotic and prebiotic strategy in homeostatic renormalization when skin health is compromised.20Nitrobacter has displayed antifungal activity against dermatophytes and Staphylococcus; Lactobacillus has exhibited anti-inflammatory effects and was shown to improve adult acne in a small study; Bifidobacterium combined with Lactobacillus lowered the incidence of atopic eczema in early childhood; and Bifidobacterium and the prebiotic galacto-oligosaccharide prevented hydration level losses in the stratum corneum among other beneficial effects in a double-blind, placebo-controlled, randomized trial.20
Microbiome diversity is key
Microbes interact, collaborate, and oppose one another while exerting influence and being affected by the host. Effective communication among the innate and adaptive parts of the immune system, epithelial cells, and cutaneous microbiota is essential for optimal functioning of the skin.6,7 Studies on subjects with atopic dermatitis showed a strong association between decreased diversity and increased disease severity. This suggests that a diverse microbiome is associated with skin health.21 For this reason, use of pre- and probiotics for skin issues is discouraged at this time. If we replace the normal diverse flora with one organism, we do not yet know the consequences. It is much more likely that successful treatments in the future will contain a diverse group of organisms.
Cosmeceutical effects on the skin microbiome
Cleansing and use of emollients certainly affect the skin biome, but we do not yet know to what extent. A study that looked at the effects of emollients on infants with atopic dermatitis showed that the emollient group has a lower skin pH and a more diverse microbiome.22 In a 2016 study on the impact of acute treatment with topical skin cleansers on the cutaneous microbiome, investigators evaluated multiple common skin cleansers in the washing of human forearms. Group A Streptococcus growth was reduced after washing with soaps infused with such antimicrobial compounds as benzalkonium chloride or triclocarban. The researchers stipulated that much more research is necessary to ascertain the effects of chronic washing as well as the that role skin care products may play in skin homeostasis or dysbiosis in some individuals.23
In a 2017 analysis of the effects of cosmetics on the skin microbiome of facial cheeks with high- and low-hydration levels over 4 weeks, Lee et al. found that bacterial diversity was higher in the low-hydration group, with increases in both observed after the use of cosmetics. The high-hydration group showed a greater supply of Propionibacterium. Cosmetic use was found not to have caused a shift in bacterial communities in the low-hydration group.24
Conclusion
We are in the early stages as we strive to learn more about the microbiome to leverage such knowledge to improve skin health. In the meantime, there is not enough evidence to suggest the use of any oral or topical prebiotics or probiotics to improve skin health. In fact, we may be causing harm by lessening diversity. The New York Times recently published an article called “The Problem with Probiotics” that referenced a JAMA Internal Medicine article entitled “Probiotic Safety – No Guarantees.”25 I recommend that you read those. Next month, I will look more closely at microbiome research pertaining to skin disease.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC.
References
1. Dréno B et al. J Eur Acad Dermatol Venereol. 2016 Dec;30(12):2038-47.
2. O’Neill CA et al. Bioessays. 2016 Nov;38(11):1167-76.
3. Kong HH. Trends Mol Med. 2011 Jun;17(6):320-8.
4. Kong HH et al. J Invest Dermatol. 2017 May;137(5):e119-22.
5. Gallo RL. J Invest Dermatol. 2017 Jun;137(6):1213-4.
6. Byrd AL et al. Nat Rev Microbiol. 2018 Mar;16(3):143-55.
7. Grice EA et al. Nat Rev Microbiol. 2011 Apr;9(4):244-53.
8. Rodrigues Hoffmann A. Vet Dermatol. 2017 Feb;28(1):60-e15.
9. Moestrup KS et al. J Invest Dermatol. 2018 May;138(5):1225-8.
10. Prescott SL et al. World Allergy Organ J. 2017 Aug 22;10(1):29.
11. Costello EK et al. Science. 2009 Dec 18;326(5960):1694-7.
12. Zeeuwen PL et al. Genome Biol. 2012 Nov 15;13(11):R101.
13. Mukherjee S et al. Sci Rep. 2016 Oct 27;6:36062.
14. Oh J et al. Genome Med. 2012 Oct 10;4(10):77.
15. Ross AA et al. mSystems. 2017 Jul 20;2(4).
16. Blázquez AB et al. Transl Res. 2017 Jan;179:199-203.
17. Rock R et al. Open Forum Infect Dis. 2017 Oct;4(1):S232.
18. Baldwin HE et al. J Drugs Dermatol. 2017 Jan 1;16(1):12-8.
19. Byrd AL et al. Nat Rev Microbiol. 2018 Mar;16(3):143-55.
20. Maguire M et al. Arch Dermatol Res. 2017 Aug;309(6):411-21.
21. Kong HH et al. Genome Res. 2012 May;22(5):850-9.
22. Glatz M et al. PLoS One. 2018 Feb 28;13(2):e0192443.
23. Two AM et al. J Invest Dermatol. 2016 Oct;136(10):1950-4.
24. Lee HJ et al. MicrobiologyOpen. 2018 Apr;7(2):e00557. doi: 10.1002/mbo3.557.
25. Cohen PA. JAMA Intern Med. 2018 Sep 17. doi: 10.1001/jamainternmed.2018.5403.
It may not seem intuitive, but to understand some of the new skin care claims, you need to know a bit about the gut microbiome and its role in skin health. The The gut and skin play a balancing act between beneficial, neutral, and harmful flora that are interrelated with the innate and adaptive immune systems.1 The skin and gut seem to be intertwined and express several comorbidities.2 In this column, the focus is on the cutaneous microbiome’s role in skin health. To understand the cosmeceutical claims about pre- and probiotics, you first need to familiarize yourself with skin microbiome science. The skin-gut nexus will be discussed in next month’s column, which will address the role of the skin microbiome in skin diseases.
Why is the microbiome such a hot topic?
Genetic sequencing has spurred advances in the study of the microbiome and has provided intriguing clues that the gut and skin microbiome have influences on each other. Sequencing assays that focus on bacterial 16S ribosomal RNA genes have been used by investigators to distinguish and describe the wide variety of resident and transient microorganisms on the skin and elucidate their roles in skin health and disease.1 Genomic sequencing has identified species in the skin and gut that were not found previously by cultivating microbial isolates.3,4 Advances in technologies such as whole-genome shotgun sequencing, metagenomics, and functional metabolomics will further contribute to our understanding of the effects of the skin microbiome on skin health and skin type. Of course, many supplement and cosmeceutical companies have jumped on this bandwagon prematurely and claim that their products increase “good bacteria while diminishing bad bacteria.” While there are interesting data that have emerged, we still cannot say which bacteria are “good” and ‘bad” as far as the skin is concerned – with a few exceptions that we have known all along. For example, Cutibacterium acnes and Staphylococcus aureus still remain in the undesirable category. (P. acnes has been renamed and now is officially referred to as C. acnes.) While it is premature to recommend probiotic– or prebiotic–containing cosmeceuticals, your patients will ask you about them. New studies about rosacea and the microbiome have generated a lot of patient questions in my practice, so I am writing several blogs about how to answer patient questions, which can be found at STSFranchise.com/blog. I’m also educating consumers on Facebook and Instagram @skintypesolutions so that they will not be taken advantage of by the too early “pseudoscience.” So now that you have heard that it is too early to recommend pre- and probiotic skin care to target skin issues, let’s look at the science that does exist.
Terminology
- Microbiome: Microbes that live in a particular environment or biome.
- Microbiota: The collection of living microbes that live in or on an environment. This term includes the microorganisms only and not the characteristics of their environment.
- Prebiotics: A nondigestible food ingredient that promotes the growth of microorganisms in the intestines. These can promote the growth of beneficial or harmful microorganisms. Think of them as a type of “fertilizer” for the microbiome.
- Probiotics: Living microorganisms that can provide beneficial qualities when used orally or topically. What probiotics are not? Microbes naturally found in your body and on your skin; microbes that are no longer alive; fermented foods that contain an unknown amount of bacteria.
Skin surface area
Richard Gallo, MD, a dermatologist from the University of California, San Diego, who is a leader in the microbiome field of study, says that estimates of the cutaneous microbiome’s impact on human health via skin have failed to acknowledge the inner follicular surface, thus drastically undervaluing the potential of the cutaneous microbiome to influence systemic health.5 He suggests that the surface area of skin has been miscalculated as measuring 2 m2 because it is considered a flat surface. This ignores the plethora of hair follicles and sweat ducts that significantly broaden the epithelial surface to measure closer to 25 m2 and underscores that the expansive skin microbiome is much larger than previously recognized.5 Taking the hair follicle surface area into account, the skin has vast space to harbor various organisms and microbiome environments. What our patients use on their skin certainly influences these environments. The key is trying to figure out how to manipulate the microbiome to our patient’s advantage.
Microbes have environmental preferences
Different microbial species thrive on particular regions of the diverse topography of the expansive surface area and choose their preferred environments from among sebaceous or nonsebaceous, hairy or smooth, moist or dry, and creased or noncreased areas.6,7 Other host factors that affect which microorganisms colonize the skin include hair follicle thickness, age, sex, diet (especially high fat and sugar intake), climate, occupation, and personal hygiene.7-10 Gene sequencing has revealed that these variations are partially because of factors such as ultraviolet exposure, pH, and temperature.4,6,11 For example, C. acnes has been found to be more prevalent in highly sebaceous sites on the head and upper torso.4 In general, Propionibacteriaceae (Cutibacterium) prefer sebaceous areas, whereas Corynebacteriaceae and Staphylococcaceae prevail in moist regions, such as the navel or axilla. Dry areas host the widest diversity of microbes, including Corynebacterium, Staphylococcus, and Streptococcus species.1,7,12
Impact of sebum and skin hydration on microbiome
In 2016, Mukherjee et al. measured sebum and hydration from the forehead and cheeks of 30 healthy female volunteers in a study that tested the hypothesis that differences in sebum and hydration levels in specific facial areas account for interindividual variation in facial skin microbiome. They found that the most significant predictor of microbiome composition was cheek sebum level, followed by forehead hydration level, while cheek hydration and forehead sebum levels were not predictive. The prevalence of Actinobacteria/Propionibacterium rose, while microbiome diversity diminished with an increase in cheek sebum, with such trends reversed in relation to forehead hydration. The investigators concluded that site-specific sebum and water levels impact the nature and diversity of the facial skin microbiome.13
Lability of the cutaneous microbiome
The skin microbiome changes during various times of life. For example, in puberty, more lipophilic species such as Propionibacteriaceae and Cornebacteriaceae predominate, while prior to puberty there is a preponderance of Firmicutes, Bacteroidetes, and Proteobacteria.4,14 However, in the absence of lifestyle changes, cutaneous microbial communities have been found through longitudinal studies to be relatively stable over a 2-year period.6 A person’s skin microbiome is subject to influence from an adjacent skin microbiome, such as between cohabiting couples or the influence of breastfeeding mothers.15 It is never too early to consider the role of the microbiome in health and disease. For example, infant microbiomes play a role in eczema and the atopic march.16 For this reason, those of us who treat children need to be familiar with studies that have demonstrate how the cutaneous microbiome is affected by childbirth delivery method, breastfeeding, the mother’s diet antibiotic use during pregnancy and breastfeeding.4,17
Microbiome effects on skin function
The skin barrier, a bilayer lipid-laden membrane that surrounds keratinocytes and prevents transepidermal water loss, is affected by resident microbial communities and has been shown by research to be influenced by the volume and diversity of such microbes.18 Organisms on the skin’s surface play an important role in communicating with and educating the cutaneous arm of the immune system.19 In 2017, Maguire and Maguire reviewed recent studies of the gut and skin microbiomes and suggested that Nitrobacter, Lactobacillus, and Bifidobacterium can improve skin health and could be useful bacterial adjuvants in a probiotic and prebiotic strategy in homeostatic renormalization when skin health is compromised.20Nitrobacter has displayed antifungal activity against dermatophytes and Staphylococcus; Lactobacillus has exhibited anti-inflammatory effects and was shown to improve adult acne in a small study; Bifidobacterium combined with Lactobacillus lowered the incidence of atopic eczema in early childhood; and Bifidobacterium and the prebiotic galacto-oligosaccharide prevented hydration level losses in the stratum corneum among other beneficial effects in a double-blind, placebo-controlled, randomized trial.20
Microbiome diversity is key
Microbes interact, collaborate, and oppose one another while exerting influence and being affected by the host. Effective communication among the innate and adaptive parts of the immune system, epithelial cells, and cutaneous microbiota is essential for optimal functioning of the skin.6,7 Studies on subjects with atopic dermatitis showed a strong association between decreased diversity and increased disease severity. This suggests that a diverse microbiome is associated with skin health.21 For this reason, use of pre- and probiotics for skin issues is discouraged at this time. If we replace the normal diverse flora with one organism, we do not yet know the consequences. It is much more likely that successful treatments in the future will contain a diverse group of organisms.
Cosmeceutical effects on the skin microbiome
Cleansing and use of emollients certainly affect the skin biome, but we do not yet know to what extent. A study that looked at the effects of emollients on infants with atopic dermatitis showed that the emollient group has a lower skin pH and a more diverse microbiome.22 In a 2016 study on the impact of acute treatment with topical skin cleansers on the cutaneous microbiome, investigators evaluated multiple common skin cleansers in the washing of human forearms. Group A Streptococcus growth was reduced after washing with soaps infused with such antimicrobial compounds as benzalkonium chloride or triclocarban. The researchers stipulated that much more research is necessary to ascertain the effects of chronic washing as well as the that role skin care products may play in skin homeostasis or dysbiosis in some individuals.23
In a 2017 analysis of the effects of cosmetics on the skin microbiome of facial cheeks with high- and low-hydration levels over 4 weeks, Lee et al. found that bacterial diversity was higher in the low-hydration group, with increases in both observed after the use of cosmetics. The high-hydration group showed a greater supply of Propionibacterium. Cosmetic use was found not to have caused a shift in bacterial communities in the low-hydration group.24
Conclusion
We are in the early stages as we strive to learn more about the microbiome to leverage such knowledge to improve skin health. In the meantime, there is not enough evidence to suggest the use of any oral or topical prebiotics or probiotics to improve skin health. In fact, we may be causing harm by lessening diversity. The New York Times recently published an article called “The Problem with Probiotics” that referenced a JAMA Internal Medicine article entitled “Probiotic Safety – No Guarantees.”25 I recommend that you read those. Next month, I will look more closely at microbiome research pertaining to skin disease.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC.
References
1. Dréno B et al. J Eur Acad Dermatol Venereol. 2016 Dec;30(12):2038-47.
2. O’Neill CA et al. Bioessays. 2016 Nov;38(11):1167-76.
3. Kong HH. Trends Mol Med. 2011 Jun;17(6):320-8.
4. Kong HH et al. J Invest Dermatol. 2017 May;137(5):e119-22.
5. Gallo RL. J Invest Dermatol. 2017 Jun;137(6):1213-4.
6. Byrd AL et al. Nat Rev Microbiol. 2018 Mar;16(3):143-55.
7. Grice EA et al. Nat Rev Microbiol. 2011 Apr;9(4):244-53.
8. Rodrigues Hoffmann A. Vet Dermatol. 2017 Feb;28(1):60-e15.
9. Moestrup KS et al. J Invest Dermatol. 2018 May;138(5):1225-8.
10. Prescott SL et al. World Allergy Organ J. 2017 Aug 22;10(1):29.
11. Costello EK et al. Science. 2009 Dec 18;326(5960):1694-7.
12. Zeeuwen PL et al. Genome Biol. 2012 Nov 15;13(11):R101.
13. Mukherjee S et al. Sci Rep. 2016 Oct 27;6:36062.
14. Oh J et al. Genome Med. 2012 Oct 10;4(10):77.
15. Ross AA et al. mSystems. 2017 Jul 20;2(4).
16. Blázquez AB et al. Transl Res. 2017 Jan;179:199-203.
17. Rock R et al. Open Forum Infect Dis. 2017 Oct;4(1):S232.
18. Baldwin HE et al. J Drugs Dermatol. 2017 Jan 1;16(1):12-8.
19. Byrd AL et al. Nat Rev Microbiol. 2018 Mar;16(3):143-55.
20. Maguire M et al. Arch Dermatol Res. 2017 Aug;309(6):411-21.
21. Kong HH et al. Genome Res. 2012 May;22(5):850-9.
22. Glatz M et al. PLoS One. 2018 Feb 28;13(2):e0192443.
23. Two AM et al. J Invest Dermatol. 2016 Oct;136(10):1950-4.
24. Lee HJ et al. MicrobiologyOpen. 2018 Apr;7(2):e00557. doi: 10.1002/mbo3.557.
25. Cohen PA. JAMA Intern Med. 2018 Sep 17. doi: 10.1001/jamainternmed.2018.5403.
It may not seem intuitive, but to understand some of the new skin care claims, you need to know a bit about the gut microbiome and its role in skin health. The The gut and skin play a balancing act between beneficial, neutral, and harmful flora that are interrelated with the innate and adaptive immune systems.1 The skin and gut seem to be intertwined and express several comorbidities.2 In this column, the focus is on the cutaneous microbiome’s role in skin health. To understand the cosmeceutical claims about pre- and probiotics, you first need to familiarize yourself with skin microbiome science. The skin-gut nexus will be discussed in next month’s column, which will address the role of the skin microbiome in skin diseases.
Why is the microbiome such a hot topic?
Genetic sequencing has spurred advances in the study of the microbiome and has provided intriguing clues that the gut and skin microbiome have influences on each other. Sequencing assays that focus on bacterial 16S ribosomal RNA genes have been used by investigators to distinguish and describe the wide variety of resident and transient microorganisms on the skin and elucidate their roles in skin health and disease.1 Genomic sequencing has identified species in the skin and gut that were not found previously by cultivating microbial isolates.3,4 Advances in technologies such as whole-genome shotgun sequencing, metagenomics, and functional metabolomics will further contribute to our understanding of the effects of the skin microbiome on skin health and skin type. Of course, many supplement and cosmeceutical companies have jumped on this bandwagon prematurely and claim that their products increase “good bacteria while diminishing bad bacteria.” While there are interesting data that have emerged, we still cannot say which bacteria are “good” and ‘bad” as far as the skin is concerned – with a few exceptions that we have known all along. For example, Cutibacterium acnes and Staphylococcus aureus still remain in the undesirable category. (P. acnes has been renamed and now is officially referred to as C. acnes.) While it is premature to recommend probiotic– or prebiotic–containing cosmeceuticals, your patients will ask you about them. New studies about rosacea and the microbiome have generated a lot of patient questions in my practice, so I am writing several blogs about how to answer patient questions, which can be found at STSFranchise.com/blog. I’m also educating consumers on Facebook and Instagram @skintypesolutions so that they will not be taken advantage of by the too early “pseudoscience.” So now that you have heard that it is too early to recommend pre- and probiotic skin care to target skin issues, let’s look at the science that does exist.
Terminology
- Microbiome: Microbes that live in a particular environment or biome.
- Microbiota: The collection of living microbes that live in or on an environment. This term includes the microorganisms only and not the characteristics of their environment.
- Prebiotics: A nondigestible food ingredient that promotes the growth of microorganisms in the intestines. These can promote the growth of beneficial or harmful microorganisms. Think of them as a type of “fertilizer” for the microbiome.
- Probiotics: Living microorganisms that can provide beneficial qualities when used orally or topically. What probiotics are not? Microbes naturally found in your body and on your skin; microbes that are no longer alive; fermented foods that contain an unknown amount of bacteria.
Skin surface area
Richard Gallo, MD, a dermatologist from the University of California, San Diego, who is a leader in the microbiome field of study, says that estimates of the cutaneous microbiome’s impact on human health via skin have failed to acknowledge the inner follicular surface, thus drastically undervaluing the potential of the cutaneous microbiome to influence systemic health.5 He suggests that the surface area of skin has been miscalculated as measuring 2 m2 because it is considered a flat surface. This ignores the plethora of hair follicles and sweat ducts that significantly broaden the epithelial surface to measure closer to 25 m2 and underscores that the expansive skin microbiome is much larger than previously recognized.5 Taking the hair follicle surface area into account, the skin has vast space to harbor various organisms and microbiome environments. What our patients use on their skin certainly influences these environments. The key is trying to figure out how to manipulate the microbiome to our patient’s advantage.
Microbes have environmental preferences
Different microbial species thrive on particular regions of the diverse topography of the expansive surface area and choose their preferred environments from among sebaceous or nonsebaceous, hairy or smooth, moist or dry, and creased or noncreased areas.6,7 Other host factors that affect which microorganisms colonize the skin include hair follicle thickness, age, sex, diet (especially high fat and sugar intake), climate, occupation, and personal hygiene.7-10 Gene sequencing has revealed that these variations are partially because of factors such as ultraviolet exposure, pH, and temperature.4,6,11 For example, C. acnes has been found to be more prevalent in highly sebaceous sites on the head and upper torso.4 In general, Propionibacteriaceae (Cutibacterium) prefer sebaceous areas, whereas Corynebacteriaceae and Staphylococcaceae prevail in moist regions, such as the navel or axilla. Dry areas host the widest diversity of microbes, including Corynebacterium, Staphylococcus, and Streptococcus species.1,7,12
Impact of sebum and skin hydration on microbiome
In 2016, Mukherjee et al. measured sebum and hydration from the forehead and cheeks of 30 healthy female volunteers in a study that tested the hypothesis that differences in sebum and hydration levels in specific facial areas account for interindividual variation in facial skin microbiome. They found that the most significant predictor of microbiome composition was cheek sebum level, followed by forehead hydration level, while cheek hydration and forehead sebum levels were not predictive. The prevalence of Actinobacteria/Propionibacterium rose, while microbiome diversity diminished with an increase in cheek sebum, with such trends reversed in relation to forehead hydration. The investigators concluded that site-specific sebum and water levels impact the nature and diversity of the facial skin microbiome.13
Lability of the cutaneous microbiome
The skin microbiome changes during various times of life. For example, in puberty, more lipophilic species such as Propionibacteriaceae and Cornebacteriaceae predominate, while prior to puberty there is a preponderance of Firmicutes, Bacteroidetes, and Proteobacteria.4,14 However, in the absence of lifestyle changes, cutaneous microbial communities have been found through longitudinal studies to be relatively stable over a 2-year period.6 A person’s skin microbiome is subject to influence from an adjacent skin microbiome, such as between cohabiting couples or the influence of breastfeeding mothers.15 It is never too early to consider the role of the microbiome in health and disease. For example, infant microbiomes play a role in eczema and the atopic march.16 For this reason, those of us who treat children need to be familiar with studies that have demonstrate how the cutaneous microbiome is affected by childbirth delivery method, breastfeeding, the mother’s diet antibiotic use during pregnancy and breastfeeding.4,17
Microbiome effects on skin function
The skin barrier, a bilayer lipid-laden membrane that surrounds keratinocytes and prevents transepidermal water loss, is affected by resident microbial communities and has been shown by research to be influenced by the volume and diversity of such microbes.18 Organisms on the skin’s surface play an important role in communicating with and educating the cutaneous arm of the immune system.19 In 2017, Maguire and Maguire reviewed recent studies of the gut and skin microbiomes and suggested that Nitrobacter, Lactobacillus, and Bifidobacterium can improve skin health and could be useful bacterial adjuvants in a probiotic and prebiotic strategy in homeostatic renormalization when skin health is compromised.20Nitrobacter has displayed antifungal activity against dermatophytes and Staphylococcus; Lactobacillus has exhibited anti-inflammatory effects and was shown to improve adult acne in a small study; Bifidobacterium combined with Lactobacillus lowered the incidence of atopic eczema in early childhood; and Bifidobacterium and the prebiotic galacto-oligosaccharide prevented hydration level losses in the stratum corneum among other beneficial effects in a double-blind, placebo-controlled, randomized trial.20
Microbiome diversity is key
Microbes interact, collaborate, and oppose one another while exerting influence and being affected by the host. Effective communication among the innate and adaptive parts of the immune system, epithelial cells, and cutaneous microbiota is essential for optimal functioning of the skin.6,7 Studies on subjects with atopic dermatitis showed a strong association between decreased diversity and increased disease severity. This suggests that a diverse microbiome is associated with skin health.21 For this reason, use of pre- and probiotics for skin issues is discouraged at this time. If we replace the normal diverse flora with one organism, we do not yet know the consequences. It is much more likely that successful treatments in the future will contain a diverse group of organisms.
Cosmeceutical effects on the skin microbiome
Cleansing and use of emollients certainly affect the skin biome, but we do not yet know to what extent. A study that looked at the effects of emollients on infants with atopic dermatitis showed that the emollient group has a lower skin pH and a more diverse microbiome.22 In a 2016 study on the impact of acute treatment with topical skin cleansers on the cutaneous microbiome, investigators evaluated multiple common skin cleansers in the washing of human forearms. Group A Streptococcus growth was reduced after washing with soaps infused with such antimicrobial compounds as benzalkonium chloride or triclocarban. The researchers stipulated that much more research is necessary to ascertain the effects of chronic washing as well as the that role skin care products may play in skin homeostasis or dysbiosis in some individuals.23
In a 2017 analysis of the effects of cosmetics on the skin microbiome of facial cheeks with high- and low-hydration levels over 4 weeks, Lee et al. found that bacterial diversity was higher in the low-hydration group, with increases in both observed after the use of cosmetics. The high-hydration group showed a greater supply of Propionibacterium. Cosmetic use was found not to have caused a shift in bacterial communities in the low-hydration group.24
Conclusion
We are in the early stages as we strive to learn more about the microbiome to leverage such knowledge to improve skin health. In the meantime, there is not enough evidence to suggest the use of any oral or topical prebiotics or probiotics to improve skin health. In fact, we may be causing harm by lessening diversity. The New York Times recently published an article called “The Problem with Probiotics” that referenced a JAMA Internal Medicine article entitled “Probiotic Safety – No Guarantees.”25 I recommend that you read those. Next month, I will look more closely at microbiome research pertaining to skin disease.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC.
References
1. Dréno B et al. J Eur Acad Dermatol Venereol. 2016 Dec;30(12):2038-47.
2. O’Neill CA et al. Bioessays. 2016 Nov;38(11):1167-76.
3. Kong HH. Trends Mol Med. 2011 Jun;17(6):320-8.
4. Kong HH et al. J Invest Dermatol. 2017 May;137(5):e119-22.
5. Gallo RL. J Invest Dermatol. 2017 Jun;137(6):1213-4.
6. Byrd AL et al. Nat Rev Microbiol. 2018 Mar;16(3):143-55.
7. Grice EA et al. Nat Rev Microbiol. 2011 Apr;9(4):244-53.
8. Rodrigues Hoffmann A. Vet Dermatol. 2017 Feb;28(1):60-e15.
9. Moestrup KS et al. J Invest Dermatol. 2018 May;138(5):1225-8.
10. Prescott SL et al. World Allergy Organ J. 2017 Aug 22;10(1):29.
11. Costello EK et al. Science. 2009 Dec 18;326(5960):1694-7.
12. Zeeuwen PL et al. Genome Biol. 2012 Nov 15;13(11):R101.
13. Mukherjee S et al. Sci Rep. 2016 Oct 27;6:36062.
14. Oh J et al. Genome Med. 2012 Oct 10;4(10):77.
15. Ross AA et al. mSystems. 2017 Jul 20;2(4).
16. Blázquez AB et al. Transl Res. 2017 Jan;179:199-203.
17. Rock R et al. Open Forum Infect Dis. 2017 Oct;4(1):S232.
18. Baldwin HE et al. J Drugs Dermatol. 2017 Jan 1;16(1):12-8.
19. Byrd AL et al. Nat Rev Microbiol. 2018 Mar;16(3):143-55.
20. Maguire M et al. Arch Dermatol Res. 2017 Aug;309(6):411-21.
21. Kong HH et al. Genome Res. 2012 May;22(5):850-9.
22. Glatz M et al. PLoS One. 2018 Feb 28;13(2):e0192443.
23. Two AM et al. J Invest Dermatol. 2016 Oct;136(10):1950-4.
24. Lee HJ et al. MicrobiologyOpen. 2018 Apr;7(2):e00557. doi: 10.1002/mbo3.557.
25. Cohen PA. JAMA Intern Med. 2018 Sep 17. doi: 10.1001/jamainternmed.2018.5403.
Taurine
Taurine, also known as 2-aminoethanesulfonic acid, is a naturally occurring beta-amino acid (which has a sulphonic acid group instead of carboxylic acid, differentiating it from other amino acids) yielded by methionine and cysteine metabolism in the liver.1,2 An important free beta-amino acid in mammals, it is often the free amino acid present in the greatest concentrations in several cell types in humans.1,2 Dietary intake of taurine also plays an important role in maintaining the body’s taurine levels because of mammals’ limited ability to synthesize it.1
Notably in terms of dermatologic treatment options, the combination product taurine bromamine is known to impart antioxidant, anti-inflammatory, and antibacterial activities.3 And taurine itself is associated with antioxidant, anti-inflammatory, antifibrotic, and immunomodulatory characteristics,1,4 and is noted for conferring antiaging benefits.5
Acne and other inflammatory conditions
The use of .6,7
In response to the problem of evolving antibiotic resistance, Marcinkiewicz reported in 2009 on the then-new therapeutic option of topical taurine bromamine for the treatment of inflammatory skin disorders such as acne. The author pointed out that Propionibacterium acnes is particularly sensitive to taurine bromamine, with the substance now known to suppress H2O2 production by activated neutrophils, likely contributing to moderating the severity and lowering the number of inflammatory acne lesions. In a 6-week double-blind pilot clinical study, Marcinkiewicz and his team compared the efficacy of 0.5% taurine bromamine cream with 1% clindamycin gel in 40 patients with mild to moderate acne. Treatments, which were randomly assigned, occurred twice daily through the study. Amelioration of acne symptoms was comparable in the two groups, with more than 90% of patients improving clinically and experiencing similar decreases in acne lesions (65% in the taurine bromamine group and 68% in the clindamycin group). Marcinkiewicz concluded that these results indicate the viability of taurine bromamine as an option for inflammatory acne therapy, particularly for patients who have shown antibiotic resistance.3
Wide-ranging protection potential
In 2003, Janeke et al. conducted analyses that showed that taurine accumulation defended cultured human keratinocytes from osmotically- and UV-induced apoptosis, suggesting the importance of taurine as an epidermal osmolyte necessary for maintaining keratinocyte hydration in a dry environment.2
Three years later, Collin et al. demonstrated the dynamic protective effects of taurine on the human hair follicle in an in vitro study in which taurine promoted hair survival and protected against TGF-beta1-induced damage.1
Taurine has also been found to stabilize and protect the catalytic activity of the hemoprotein cytochrome P450 3A4, which is a key enzyme responsible for metabolizing various endogenous as well as foreign substances, including drugs.8
Penetration enhancement
In 2016, Mueller et al. studied the effects of urea and taurine as hydrophilic penetration enhancers on stratum corneum lipid models as both substances are known to exert such effects. With inconclusive results as to the roots of such activity, they speculated that both entities enhance penetration through the introduction of copious water into the corneocytes, resulting from the robust water-binding capacity of urea and the consequent osmotic pressure related to taurine.9
Possible skin whitening and anti-aging roles and other promising lab results
Based on their previous work demonstrating that azelaic acid, a saturated dicarboxylic acid found naturally in wheat, rye, and barley, suppressed melanogenesis, Yu and Kim investigated the antimelanogenic activity of azelaic acid and taurine in B16F10 mouse melanoma cells in 2010. They found that the combination of the two substances exhibited a greater inhibitory effect in melanocytes than azelaic acid alone, with melanin production and tyrosinase activity suppressed without inducing cytotoxicity. The investigators concluded the combination of azelaic acid and taurine may be an effective approach for treating hyperpigmentation.10
In 2015, Ito et al. investigated the possible anti-aging role of taurine using a taurine transporter knockout mouse model. They noted that aging-related disorders affecting the skin, heart, skeletal muscle, and liver and resulting in a shorter lifespan have been correlated with tissue taurine depletion. The researchers proposed that proper protein folding allows endogenous taurine to perform as an antiaging molecule.5
Also in 2015, Kim et al. investigated potential mechanisms of the antiproliferative activity of taurine on murine B16F10 melanoma cells via the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and neutral red assays and microscopic analysis. They found that taurine prevented cell proliferation and engendered apoptosis in B16F10 cells, concluding that taurine may have a role to play as a chemotherapeutic agent for skin cancer.11
In 2014, Ashkani-Esfahani et al. studied the impact of taurine on cutaneous leishmaniasis wounds in a mouse model. Investigators induced 18 mice with wounds using L. major promastigotes, and divided them into a taurine injection group, taurine gel group, and no treatment group, performing treatments every 24 hours over 21 days. The taurine treatment groups exhibited significantly greater numerical fibroblast density, collagen bundle volume density, and vessel length densities compared with the nontreatment group. The taurine injection group displayed higher fibroblast numerical density than did the taurine gel group. The researchers concluded that taurine has the capacity to enhance wound healing and tissue regeneration but showed no direct anti-leishmaniasis effect.4
Conclusion
Taurine has been found over the last few decades to impart salutary effects for human health. This beta-amino acid that occurs naturally in humans and other mammals also appears to hold promising potential in the dermatologic realm, particularly for its anti-inflammatory and antioxidant effects. More research is needed to ascertain just how pivotal this compound can be for skin health.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Int J Cosmet Sci. 2006 Aug;28(4):289-98.
2. J Invest Dermatol. 2003 Aug;121(2):354-61.
3. Pol Arch Med Wewn. 2009 Oct;119(10):673-6.
4. Adv Biomed Res. 2014 Oct 7;3:204.
5. Adv Exp Med Biol. 2015;803:481-7.
6. Am J Clin Dermatol. 2012 Dec 1;13(6):357-64.
7. Eur J Dermatol. 2008 Jul-Aug;18(4):433-9.
8. Biochemistry (Mosc). 2015 Mar;80(3):366-73.
9. Biochim Biophys Acta. 2016 Sep;1858(9):2006-18.
10. J Biomed Sci. 2010 Aug 24;17 Suppl 1:S45.
11. Adv Exp Med Biol. 2015;803:167-77.
Taurine, also known as 2-aminoethanesulfonic acid, is a naturally occurring beta-amino acid (which has a sulphonic acid group instead of carboxylic acid, differentiating it from other amino acids) yielded by methionine and cysteine metabolism in the liver.1,2 An important free beta-amino acid in mammals, it is often the free amino acid present in the greatest concentrations in several cell types in humans.1,2 Dietary intake of taurine also plays an important role in maintaining the body’s taurine levels because of mammals’ limited ability to synthesize it.1
Notably in terms of dermatologic treatment options, the combination product taurine bromamine is known to impart antioxidant, anti-inflammatory, and antibacterial activities.3 And taurine itself is associated with antioxidant, anti-inflammatory, antifibrotic, and immunomodulatory characteristics,1,4 and is noted for conferring antiaging benefits.5
Acne and other inflammatory conditions
The use of .6,7
In response to the problem of evolving antibiotic resistance, Marcinkiewicz reported in 2009 on the then-new therapeutic option of topical taurine bromamine for the treatment of inflammatory skin disorders such as acne. The author pointed out that Propionibacterium acnes is particularly sensitive to taurine bromamine, with the substance now known to suppress H2O2 production by activated neutrophils, likely contributing to moderating the severity and lowering the number of inflammatory acne lesions. In a 6-week double-blind pilot clinical study, Marcinkiewicz and his team compared the efficacy of 0.5% taurine bromamine cream with 1% clindamycin gel in 40 patients with mild to moderate acne. Treatments, which were randomly assigned, occurred twice daily through the study. Amelioration of acne symptoms was comparable in the two groups, with more than 90% of patients improving clinically and experiencing similar decreases in acne lesions (65% in the taurine bromamine group and 68% in the clindamycin group). Marcinkiewicz concluded that these results indicate the viability of taurine bromamine as an option for inflammatory acne therapy, particularly for patients who have shown antibiotic resistance.3
Wide-ranging protection potential
In 2003, Janeke et al. conducted analyses that showed that taurine accumulation defended cultured human keratinocytes from osmotically- and UV-induced apoptosis, suggesting the importance of taurine as an epidermal osmolyte necessary for maintaining keratinocyte hydration in a dry environment.2
Three years later, Collin et al. demonstrated the dynamic protective effects of taurine on the human hair follicle in an in vitro study in which taurine promoted hair survival and protected against TGF-beta1-induced damage.1
Taurine has also been found to stabilize and protect the catalytic activity of the hemoprotein cytochrome P450 3A4, which is a key enzyme responsible for metabolizing various endogenous as well as foreign substances, including drugs.8
Penetration enhancement
In 2016, Mueller et al. studied the effects of urea and taurine as hydrophilic penetration enhancers on stratum corneum lipid models as both substances are known to exert such effects. With inconclusive results as to the roots of such activity, they speculated that both entities enhance penetration through the introduction of copious water into the corneocytes, resulting from the robust water-binding capacity of urea and the consequent osmotic pressure related to taurine.9
Possible skin whitening and anti-aging roles and other promising lab results
Based on their previous work demonstrating that azelaic acid, a saturated dicarboxylic acid found naturally in wheat, rye, and barley, suppressed melanogenesis, Yu and Kim investigated the antimelanogenic activity of azelaic acid and taurine in B16F10 mouse melanoma cells in 2010. They found that the combination of the two substances exhibited a greater inhibitory effect in melanocytes than azelaic acid alone, with melanin production and tyrosinase activity suppressed without inducing cytotoxicity. The investigators concluded the combination of azelaic acid and taurine may be an effective approach for treating hyperpigmentation.10
In 2015, Ito et al. investigated the possible anti-aging role of taurine using a taurine transporter knockout mouse model. They noted that aging-related disorders affecting the skin, heart, skeletal muscle, and liver and resulting in a shorter lifespan have been correlated with tissue taurine depletion. The researchers proposed that proper protein folding allows endogenous taurine to perform as an antiaging molecule.5
Also in 2015, Kim et al. investigated potential mechanisms of the antiproliferative activity of taurine on murine B16F10 melanoma cells via the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and neutral red assays and microscopic analysis. They found that taurine prevented cell proliferation and engendered apoptosis in B16F10 cells, concluding that taurine may have a role to play as a chemotherapeutic agent for skin cancer.11
In 2014, Ashkani-Esfahani et al. studied the impact of taurine on cutaneous leishmaniasis wounds in a mouse model. Investigators induced 18 mice with wounds using L. major promastigotes, and divided them into a taurine injection group, taurine gel group, and no treatment group, performing treatments every 24 hours over 21 days. The taurine treatment groups exhibited significantly greater numerical fibroblast density, collagen bundle volume density, and vessel length densities compared with the nontreatment group. The taurine injection group displayed higher fibroblast numerical density than did the taurine gel group. The researchers concluded that taurine has the capacity to enhance wound healing and tissue regeneration but showed no direct anti-leishmaniasis effect.4
Conclusion
Taurine has been found over the last few decades to impart salutary effects for human health. This beta-amino acid that occurs naturally in humans and other mammals also appears to hold promising potential in the dermatologic realm, particularly for its anti-inflammatory and antioxidant effects. More research is needed to ascertain just how pivotal this compound can be for skin health.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Int J Cosmet Sci. 2006 Aug;28(4):289-98.
2. J Invest Dermatol. 2003 Aug;121(2):354-61.
3. Pol Arch Med Wewn. 2009 Oct;119(10):673-6.
4. Adv Biomed Res. 2014 Oct 7;3:204.
5. Adv Exp Med Biol. 2015;803:481-7.
6. Am J Clin Dermatol. 2012 Dec 1;13(6):357-64.
7. Eur J Dermatol. 2008 Jul-Aug;18(4):433-9.
8. Biochemistry (Mosc). 2015 Mar;80(3):366-73.
9. Biochim Biophys Acta. 2016 Sep;1858(9):2006-18.
10. J Biomed Sci. 2010 Aug 24;17 Suppl 1:S45.
11. Adv Exp Med Biol. 2015;803:167-77.
Taurine, also known as 2-aminoethanesulfonic acid, is a naturally occurring beta-amino acid (which has a sulphonic acid group instead of carboxylic acid, differentiating it from other amino acids) yielded by methionine and cysteine metabolism in the liver.1,2 An important free beta-amino acid in mammals, it is often the free amino acid present in the greatest concentrations in several cell types in humans.1,2 Dietary intake of taurine also plays an important role in maintaining the body’s taurine levels because of mammals’ limited ability to synthesize it.1
Notably in terms of dermatologic treatment options, the combination product taurine bromamine is known to impart antioxidant, anti-inflammatory, and antibacterial activities.3 And taurine itself is associated with antioxidant, anti-inflammatory, antifibrotic, and immunomodulatory characteristics,1,4 and is noted for conferring antiaging benefits.5
Acne and other inflammatory conditions
The use of .6,7
In response to the problem of evolving antibiotic resistance, Marcinkiewicz reported in 2009 on the then-new therapeutic option of topical taurine bromamine for the treatment of inflammatory skin disorders such as acne. The author pointed out that Propionibacterium acnes is particularly sensitive to taurine bromamine, with the substance now known to suppress H2O2 production by activated neutrophils, likely contributing to moderating the severity and lowering the number of inflammatory acne lesions. In a 6-week double-blind pilot clinical study, Marcinkiewicz and his team compared the efficacy of 0.5% taurine bromamine cream with 1% clindamycin gel in 40 patients with mild to moderate acne. Treatments, which were randomly assigned, occurred twice daily through the study. Amelioration of acne symptoms was comparable in the two groups, with more than 90% of patients improving clinically and experiencing similar decreases in acne lesions (65% in the taurine bromamine group and 68% in the clindamycin group). Marcinkiewicz concluded that these results indicate the viability of taurine bromamine as an option for inflammatory acne therapy, particularly for patients who have shown antibiotic resistance.3
Wide-ranging protection potential
In 2003, Janeke et al. conducted analyses that showed that taurine accumulation defended cultured human keratinocytes from osmotically- and UV-induced apoptosis, suggesting the importance of taurine as an epidermal osmolyte necessary for maintaining keratinocyte hydration in a dry environment.2
Three years later, Collin et al. demonstrated the dynamic protective effects of taurine on the human hair follicle in an in vitro study in which taurine promoted hair survival and protected against TGF-beta1-induced damage.1
Taurine has also been found to stabilize and protect the catalytic activity of the hemoprotein cytochrome P450 3A4, which is a key enzyme responsible for metabolizing various endogenous as well as foreign substances, including drugs.8
Penetration enhancement
In 2016, Mueller et al. studied the effects of urea and taurine as hydrophilic penetration enhancers on stratum corneum lipid models as both substances are known to exert such effects. With inconclusive results as to the roots of such activity, they speculated that both entities enhance penetration through the introduction of copious water into the corneocytes, resulting from the robust water-binding capacity of urea and the consequent osmotic pressure related to taurine.9
Possible skin whitening and anti-aging roles and other promising lab results
Based on their previous work demonstrating that azelaic acid, a saturated dicarboxylic acid found naturally in wheat, rye, and barley, suppressed melanogenesis, Yu and Kim investigated the antimelanogenic activity of azelaic acid and taurine in B16F10 mouse melanoma cells in 2010. They found that the combination of the two substances exhibited a greater inhibitory effect in melanocytes than azelaic acid alone, with melanin production and tyrosinase activity suppressed without inducing cytotoxicity. The investigators concluded the combination of azelaic acid and taurine may be an effective approach for treating hyperpigmentation.10
In 2015, Ito et al. investigated the possible anti-aging role of taurine using a taurine transporter knockout mouse model. They noted that aging-related disorders affecting the skin, heart, skeletal muscle, and liver and resulting in a shorter lifespan have been correlated with tissue taurine depletion. The researchers proposed that proper protein folding allows endogenous taurine to perform as an antiaging molecule.5
Also in 2015, Kim et al. investigated potential mechanisms of the antiproliferative activity of taurine on murine B16F10 melanoma cells via the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and neutral red assays and microscopic analysis. They found that taurine prevented cell proliferation and engendered apoptosis in B16F10 cells, concluding that taurine may have a role to play as a chemotherapeutic agent for skin cancer.11
In 2014, Ashkani-Esfahani et al. studied the impact of taurine on cutaneous leishmaniasis wounds in a mouse model. Investigators induced 18 mice with wounds using L. major promastigotes, and divided them into a taurine injection group, taurine gel group, and no treatment group, performing treatments every 24 hours over 21 days. The taurine treatment groups exhibited significantly greater numerical fibroblast density, collagen bundle volume density, and vessel length densities compared with the nontreatment group. The taurine injection group displayed higher fibroblast numerical density than did the taurine gel group. The researchers concluded that taurine has the capacity to enhance wound healing and tissue regeneration but showed no direct anti-leishmaniasis effect.4
Conclusion
Taurine has been found over the last few decades to impart salutary effects for human health. This beta-amino acid that occurs naturally in humans and other mammals also appears to hold promising potential in the dermatologic realm, particularly for its anti-inflammatory and antioxidant effects. More research is needed to ascertain just how pivotal this compound can be for skin health.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Int J Cosmet Sci. 2006 Aug;28(4):289-98.
2. J Invest Dermatol. 2003 Aug;121(2):354-61.
3. Pol Arch Med Wewn. 2009 Oct;119(10):673-6.
4. Adv Biomed Res. 2014 Oct 7;3:204.
5. Adv Exp Med Biol. 2015;803:481-7.
6. Am J Clin Dermatol. 2012 Dec 1;13(6):357-64.
7. Eur J Dermatol. 2008 Jul-Aug;18(4):433-9.
8. Biochemistry (Mosc). 2015 Mar;80(3):366-73.
9. Biochim Biophys Acta. 2016 Sep;1858(9):2006-18.
10. J Biomed Sci. 2010 Aug 24;17 Suppl 1:S45.
11. Adv Exp Med Biol. 2015;803:167-77.