User login
Is it time to reconsider Rh testing and Rh D immune globulin treatment for miscarriage and abortion care in early pregnancy?
All obstetrician-gynecologists know that pregnant patients who are Rh negative and exposed to a sufficient quantity of fetal red blood cells expressing Rh D antigen may become sensitized, producing Rh D antibodies that adversely impact future pregnancies with an Rh D-positive fetus, potentially causing hemolytic disease of the fetus and newborn. In countries where Rh D immune globulin is available, there is a consensus recommendation to administer Rh D immune globulin to Rh-negative pregnant patients at approximately 28 weeks’ gestation and at birth in order to decrease the risk of alloimmunization and hemolytic disease of the fetus and newborn in future pregnancies.1 In contrast to this global consensus, there is no worldwide agreement about how to manage Rh testing and Rh D immune globulin administration in cases of early pregnancy loss or abortion care before 12 weeks’ gestation. This editorial examines the evolving guidelines of major professional societies.
Guidelines consistent with the routine use of Rh D immune globulin in all cases of early pregnancy loss and abortion care
As of the publication date of this editorial, the American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin on prevention of Rh D alloimmunization provides the following guidance based on consensus and expert opinion2:
- “Although the risk of alloimmunization is low, the consequences can be significant, and administration of Rh D immune globulin should be considered in cases of spontaneous first trimester miscarriage, especially those that are later in the first trimester.”
- “Because of the higher risk of alloimmunization, Rh D-negative women who have instrumentation for their miscarriage should receive Rh D immune globulin prophylaxis.”
- “Rh D immune globulin should be given to Rh D-negative women who have pregnancy termination either medical or surgical.”
The Society of Obstetricians and Gynaecologists of Canada (SOGC) recommends that, “After miscarriage or threatened abortion or induced abortion during the first 12 weeks of gestation, non-sensitized D-negative women should be given a minimum anti-D of 120 µg.”3
The liberal use of Rh D immune globulin in all cases of early pregnancy loss and abortion care is based, in part, on the following considerations:
- the recognized safety of Rh D immune globulin administration2,3
- the report that fetal megaloblasts may express Rh antigen as early as 38 days of gestation4
- the observation that 0.1 mL of Rh D-positive red cells may provoke an immune response in some Rh D-negative patients5-7
- the estimate that in some patients with threatened miscarriage a significant quantity of fetal blood may enter the maternal circulation.8
Guidelines that suggest restricted use of Rh D immune globulin before 7 to 8 weeks’ gestation
The Reproductive Care Program of Nova Scotia guideline from 2022 notes that “the benefits of administering Rh immune globulin before 8 weeks gestation have not been demonstrated.” Given the burden of Rh testing and Rh D immune globulin administration they suggest that clinicians may withhold Rh testing and Rh D immune globulin administration in cases less than 8 weeks’ gestation (less than 56 days) for spontaneous, threatened, or medication abortions if there is reliable pregnancy dating.9
The Dutch Association of Abortion Specialists guidelines from 2018 suggest to not provide Rh D immune globulin treatment in the following clinical situations: patients under 10 weeks’ gestation with spontaneous miscarriage or patients under 7 weeks’ gestation having an induced abortion.10
Continue to: Guidelines that suggest restricted use of Rh D immune globulin before 10 to 12 weeks’ gestation...
Guidelines that suggest restricted use of Rh D immune globulin before 10 to 12 weeks’ gestation
There are a growing number of guidelines that recommend restricting the use of Rh testing and Rh D immune globulin treatment in the management of early miscarriage and induced abortion. In 2019, the United Kingdom’s National Institute for Health and Care Excellence (NICE) recommended that for patients having a spontaneous miscarriage, Rh testing and Rh D immune globulin are not necessary before 10 weeks 0 days of gestation.11 In addition, NICE recommends, “Do not offer anti-D prophylaxis to women who are having a medical abortion up to and including 10+0 weeks’ gestation.…Consider anti-D prophylaxis for women who are rhesus D negative and are having a surgical abortion up to and including 10+0 weeks’ gestation.”11
In 2019, the National Abortion Federation (NAF) Clinical Policies Committee recommended that “…it is reasonable to forgo Rh testing and anti-D immunoglobulin for women having any type of induced abortion before 8 weeks from the last menstrual period. Prior to 8 weeks, the likelihood of fetal-maternal hemorrhage adequate to cause sensitization is negligible. Given that medication abortion is more similar to spontaneous abortion with less risk of fetal-maternal hemorrhage, forgoing Rh testing and anti-D immunoglobulin for medication abortion under 10 weeks may be considered.”12 In 2022, NAF noted, “Emerging epidemiologic and clinical evidence indicates that the risk of maternal-fetal hemorrhage caused by early abortion is negligible and Rh testing and provision of Rh immune globulin may not be necessary. It is reasonable to forego Rh testing and anti-D immunoglobulin for people having any type of abortion before 56 days and medication abortion before 70 days since the last menstrual period. The pregnancy dating at which people need Rh testing and anti-D immunoglobulin is not well established. Foregoing Rh testing and anti-D immunoglobulinfor those using medication abortion through 11 to 12 weeks may be considered.”13
In 2020 the International Federation of Gynaecology and Obstetrics (FIGO) Committee for Safe Motherhood and Newborn Health recommended, “The risk for sensitization is most probably extremely low for spontaneous abortions before 10 gestational weeks; however, data are scarce. Based on the clinical expertise of the guideline committee from the UK’s National Institute for Health and Care Excellence (NICE), it is suggested that prophylaxis should be given only to women who are having a spontaneous abortion or medical management of miscarriage after 10 and 0/7 gestational weeks. Moreover, for women who have surgical management, prophylaxis may also be considered before 10 gestational weeks.”14
In 2022 the Royal College of Obstetricians and Gynaecologists recommended that for induced abortion, medication or surgical, “a determination of Rhesus blood status may be considered if the duration of pregnancy is over 12 weeks and anti-D is available.”15 “If available, anti-D should be offered to non-sensitised RhD-negative individuals from 12 weeks of pregnancy and provided within 72 hours of the abortion.”15
In 2022, the Society of Family Planning recommended that “Rh testing and administration are not recommended prior to 12 weeks gestation for patients undergoing spontaneous, medication or uterine aspiration abortion.” “For patients under 12 weeks gestation, although not recommended, Rh testing and Rh D immune globulin administration may be considered at patient request as part of a shared decision making process.”16
In 2022, the World Health Organization (WHO) reported “There are few studies examining Rh isoimmunization in unsensitized Rh-negative individuals seeking abortion before 12 weeks of gestation.” “The evidence on the effectiveness of the intervention may favor the intervention, because fewer women in the intervention group (anti-D administration) had antibody formation after the initial pregnancy compared to women in the comparison group (no anti-D) and no harms (undesirable effects) of the intervention were noted.”17 The evidence referenced for these statements are two low-quality studies from 1972.18,19 The WHO continues, “…after consideration of the resources required, cost-effectiveness and feasibility of administering anti-D, as well as the very low certainty of evidence on effectiveness, the expert panel concluded that overall, the evidence does not favor the intervention and decided to recommend against it for gestational ages < 12 weeks, rather than < 9 weeks, as mentioned in the 2012 guidance.”17 In conclusion, the WHO recommended that “for both medical and surgical abortion at < 12 weeks: Recommend against anti-D immunoglobulin administration.”17
Guidelines that recommend restricted use of Rh D immune globulin during the first trimester, are based, in part, on the following considerations:
- there are no high-quality clinical trials demonstrating the benefit of Rh D immune globulin treatment in first trimester miscarriage and abortion care
- the Kleihauer-Betke technique cannot distinguish between maternal red blood cells expressing fetal hemoglobin (maternal F cells) and fetal cells, which has resulted in the over-estimation of the number of fetal cells in the maternal circulation20
- using a dual-label flow cytometry method that distinguishes maternal F cells and fetal red blood cells, maternal F cells usually far outnumber fetal red blood cells in the maternal circulation in the first trimester20
- among women in the first trimester undergoing uterine aspiration, the number of fetal cells in the maternal circulation is very low both before and after the procedure20
- Rh testing and Rh immune globulin administration is burdensome and expensive.16
Implications for your practice
The fundamental reason for the proliferation of divergent guidelines is that there is no evidence from high-quality randomized clinical trials demonstrating that Rh testing and Rh D immune globulin treatment in early pregnancy miscarriage or induced abortion care reduces the risk of hemolytic disease of the fetus and newborn. The Cochrane review on Rh D immune globulin administration for preventing alloimmunization among patients with spontaneous miscarriage concluded, “There are insufficient data available to evaluate the practice of anti-D administration in an unsensitized Rh-negative mother after spontaneous miscarriage.”21
Given divergent guidelines, obstetrician-gynecologists must decide on which guideline to use in their practice. Clinicians may conclude that absent high-quality evidence from clinical trials, they will continue to use the ACOG/SOGC guidelines2,3 in their practice, providing universal Rh testing and Rh D immune globulin treatment for all miscarriages and abortions, regardless of the gestational age. Other clinicians may conclude that Rh testing and Rh D immune globulin is not warranted before 8 to 12 weeks’ gestation, because the number of fetal red blood cells in the maternal circulation in cases of miscarriage and induced abortion is too low in early pregnancy to induce a maternal immune response.22 Based on recent studies demonstrating a low number of fetal red blood cells in the maternal circulation in the first trimester, family planning specialists are reducing the use of Rh testing and Rh immune globulin administration in both early pregnancy medication abortion and uterine aspiration abortion.16 With regard to Rh testing and Rh D immune globulin treatment, the future will definitely be different than the past. It is likely that many clinicians will reduce the use of Rh testing and Rh D immune globulin treatment in patients with miscarriage or induced abortion in early pregnancy. ●
- Sperling JD, Dahlke JD, Sutton D, et al. Prevention of Rh D alloimmunization: a comparison of four national guidelines. Am J Perinatol. 2018;35:110-119.
- Prevention of Rh D alloimmunization. Practice Bulletin No. 181. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2017;130:e57-e70.
- Fung KFK, Eason E. No. 133-Prevention of Rh alloimmunization. J Obstet Gynaecol Can. 2018;40: e1-e10.
- Bergstrom H, Nilsson LA, Nilsson L, et al. Demonstration of Rh antigens in a 38-day-old fetus. Am J Obstet Gynecol. 1967;99:130-133.
- Bowman JM. The prevention of Rh Immunization. Transfus Med Rev. 1988;2:129-150.
- Zipursky A, Israels LG. The pathogenesis and prevention of Rh immunization. Can Med Assoc J. 1967;97:1245-1257.
- Pollack W, Ascari WQ, Kochesky RJ, et al. Studies on Rh prophylaxis. 1. Relationship between doses of anti-Rh and size of antigenic stimulus. Transfusion. 1971;11:333-339.
- Von Stein GA, Munsick RA, Stiver K, et al. Feto-maternal hemorrhage in threatened abortion. Obstet Gynecol. 1992;79:383-386.
- Rh Program of Nova Scotia. Guideline for Rh prophylaxis before 8 weeks (56 days) gestation for Early Pregnancy Complications and Medical Abortions. http://rcp.nshealth.ca/sites/default /files/rh/RhIg%20before%208%20weeks%20 Guideline_%20Jun2022_Final_2page.pdf. Accessed January 24, 2023.
- Wiebe ER, Campbell M, Aiken ARA, et al. Can we safety stop testing for Rh Status and immunizing Rh-negative women having early abortions? A comparison of Rh alloimmunization in Canada and the Netherlands. Contraception. 2019;100001. https://doi.org/10.1016/j.conx.2018.100001.
- Abortion care. National Institute for Health and Care Excellence. https://www.nice.org .uk/guidance/ng140/resources/abortion-care -pdf-66141773098693. Accessed January 24, 2023.
- Mark A, Foster AM, Grossman D. Foregoing Rh testing and anti-D immunoglobulin for women presenting for early abortion: a recommendation from the National Abortion Federation’s Clinical Policies Committee. Contraception. 2019;99:265-266.
- National Abortion Federation. 2022 Clinical Policy Guidelines for Abortion Care. https: //prochoice.org/wp-content/uploads/2022 -CPGs.pdf. Accessed January 24, 2023.
- Visser GHA, Thommesen T, Di Renzo GC, et al. FIGO Safe Motherhood and Newborn Health Committee. Int J Gynecol Obstet. 2021;152: 144-147.
- Making abortion safe: RCOG’s global initiative to advocate for women’s health. https://www .rcog.org.uk/media/geify5bx/abortion-care-best -practice-paper-april-2022.pdf. Accessed January 24, 2023.
- Horvath S, Goyal V, Traxler S, et al. Society of Family Planning committee consensus on Rh testing in early pregnancy. Contraception. 2022;114:1-5.
- World Health Organization. Abortion care guideline. https://www.who.int/publications/i/ item/9789240039483. Accessed January 24, 2023.
- Gavin P. Rhesus sensitization in abortion. Obstet Gynecol. 1972;39:37-40.
- Goldman J, Eckerling B. Rh immunization in spontaneous abortion. Acta Eur Fertil. 1972;3:253254.
- Horvath S, Tsao P, Huang ZY, et al. The concentration of fetal red blood cells in first-trimester pregnant women undergoing uterine aspiration is below the calculated threshold for Rh sensitization. Contraception. 2020;102:1-6.
- Karanth L, Jaafar SH, Kanagasabai S, et al. Anti-D administration after spontaneous miscarriage for preventing Rhesus alloimmunization. Cochrane Database Syst Rev. 2023;CD009617.
- Gilmore E, Sonalkar S, Schreiber CA. Use of Rh immune globulin in first-trimester abortion and miscarriage. Obstet Gynecol. 2023;141:219-222.
All obstetrician-gynecologists know that pregnant patients who are Rh negative and exposed to a sufficient quantity of fetal red blood cells expressing Rh D antigen may become sensitized, producing Rh D antibodies that adversely impact future pregnancies with an Rh D-positive fetus, potentially causing hemolytic disease of the fetus and newborn. In countries where Rh D immune globulin is available, there is a consensus recommendation to administer Rh D immune globulin to Rh-negative pregnant patients at approximately 28 weeks’ gestation and at birth in order to decrease the risk of alloimmunization and hemolytic disease of the fetus and newborn in future pregnancies.1 In contrast to this global consensus, there is no worldwide agreement about how to manage Rh testing and Rh D immune globulin administration in cases of early pregnancy loss or abortion care before 12 weeks’ gestation. This editorial examines the evolving guidelines of major professional societies.
Guidelines consistent with the routine use of Rh D immune globulin in all cases of early pregnancy loss and abortion care
As of the publication date of this editorial, the American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin on prevention of Rh D alloimmunization provides the following guidance based on consensus and expert opinion2:
- “Although the risk of alloimmunization is low, the consequences can be significant, and administration of Rh D immune globulin should be considered in cases of spontaneous first trimester miscarriage, especially those that are later in the first trimester.”
- “Because of the higher risk of alloimmunization, Rh D-negative women who have instrumentation for their miscarriage should receive Rh D immune globulin prophylaxis.”
- “Rh D immune globulin should be given to Rh D-negative women who have pregnancy termination either medical or surgical.”
The Society of Obstetricians and Gynaecologists of Canada (SOGC) recommends that, “After miscarriage or threatened abortion or induced abortion during the first 12 weeks of gestation, non-sensitized D-negative women should be given a minimum anti-D of 120 µg.”3
The liberal use of Rh D immune globulin in all cases of early pregnancy loss and abortion care is based, in part, on the following considerations:
- the recognized safety of Rh D immune globulin administration2,3
- the report that fetal megaloblasts may express Rh antigen as early as 38 days of gestation4
- the observation that 0.1 mL of Rh D-positive red cells may provoke an immune response in some Rh D-negative patients5-7
- the estimate that in some patients with threatened miscarriage a significant quantity of fetal blood may enter the maternal circulation.8
Guidelines that suggest restricted use of Rh D immune globulin before 7 to 8 weeks’ gestation
The Reproductive Care Program of Nova Scotia guideline from 2022 notes that “the benefits of administering Rh immune globulin before 8 weeks gestation have not been demonstrated.” Given the burden of Rh testing and Rh D immune globulin administration they suggest that clinicians may withhold Rh testing and Rh D immune globulin administration in cases less than 8 weeks’ gestation (less than 56 days) for spontaneous, threatened, or medication abortions if there is reliable pregnancy dating.9
The Dutch Association of Abortion Specialists guidelines from 2018 suggest to not provide Rh D immune globulin treatment in the following clinical situations: patients under 10 weeks’ gestation with spontaneous miscarriage or patients under 7 weeks’ gestation having an induced abortion.10
Continue to: Guidelines that suggest restricted use of Rh D immune globulin before 10 to 12 weeks’ gestation...
Guidelines that suggest restricted use of Rh D immune globulin before 10 to 12 weeks’ gestation
There are a growing number of guidelines that recommend restricting the use of Rh testing and Rh D immune globulin treatment in the management of early miscarriage and induced abortion. In 2019, the United Kingdom’s National Institute for Health and Care Excellence (NICE) recommended that for patients having a spontaneous miscarriage, Rh testing and Rh D immune globulin are not necessary before 10 weeks 0 days of gestation.11 In addition, NICE recommends, “Do not offer anti-D prophylaxis to women who are having a medical abortion up to and including 10+0 weeks’ gestation.…Consider anti-D prophylaxis for women who are rhesus D negative and are having a surgical abortion up to and including 10+0 weeks’ gestation.”11
In 2019, the National Abortion Federation (NAF) Clinical Policies Committee recommended that “…it is reasonable to forgo Rh testing and anti-D immunoglobulin for women having any type of induced abortion before 8 weeks from the last menstrual period. Prior to 8 weeks, the likelihood of fetal-maternal hemorrhage adequate to cause sensitization is negligible. Given that medication abortion is more similar to spontaneous abortion with less risk of fetal-maternal hemorrhage, forgoing Rh testing and anti-D immunoglobulin for medication abortion under 10 weeks may be considered.”12 In 2022, NAF noted, “Emerging epidemiologic and clinical evidence indicates that the risk of maternal-fetal hemorrhage caused by early abortion is negligible and Rh testing and provision of Rh immune globulin may not be necessary. It is reasonable to forego Rh testing and anti-D immunoglobulin for people having any type of abortion before 56 days and medication abortion before 70 days since the last menstrual period. The pregnancy dating at which people need Rh testing and anti-D immunoglobulin is not well established. Foregoing Rh testing and anti-D immunoglobulinfor those using medication abortion through 11 to 12 weeks may be considered.”13
In 2020 the International Federation of Gynaecology and Obstetrics (FIGO) Committee for Safe Motherhood and Newborn Health recommended, “The risk for sensitization is most probably extremely low for spontaneous abortions before 10 gestational weeks; however, data are scarce. Based on the clinical expertise of the guideline committee from the UK’s National Institute for Health and Care Excellence (NICE), it is suggested that prophylaxis should be given only to women who are having a spontaneous abortion or medical management of miscarriage after 10 and 0/7 gestational weeks. Moreover, for women who have surgical management, prophylaxis may also be considered before 10 gestational weeks.”14
In 2022 the Royal College of Obstetricians and Gynaecologists recommended that for induced abortion, medication or surgical, “a determination of Rhesus blood status may be considered if the duration of pregnancy is over 12 weeks and anti-D is available.”15 “If available, anti-D should be offered to non-sensitised RhD-negative individuals from 12 weeks of pregnancy and provided within 72 hours of the abortion.”15
In 2022, the Society of Family Planning recommended that “Rh testing and administration are not recommended prior to 12 weeks gestation for patients undergoing spontaneous, medication or uterine aspiration abortion.” “For patients under 12 weeks gestation, although not recommended, Rh testing and Rh D immune globulin administration may be considered at patient request as part of a shared decision making process.”16
In 2022, the World Health Organization (WHO) reported “There are few studies examining Rh isoimmunization in unsensitized Rh-negative individuals seeking abortion before 12 weeks of gestation.” “The evidence on the effectiveness of the intervention may favor the intervention, because fewer women in the intervention group (anti-D administration) had antibody formation after the initial pregnancy compared to women in the comparison group (no anti-D) and no harms (undesirable effects) of the intervention were noted.”17 The evidence referenced for these statements are two low-quality studies from 1972.18,19 The WHO continues, “…after consideration of the resources required, cost-effectiveness and feasibility of administering anti-D, as well as the very low certainty of evidence on effectiveness, the expert panel concluded that overall, the evidence does not favor the intervention and decided to recommend against it for gestational ages < 12 weeks, rather than < 9 weeks, as mentioned in the 2012 guidance.”17 In conclusion, the WHO recommended that “for both medical and surgical abortion at < 12 weeks: Recommend against anti-D immunoglobulin administration.”17
Guidelines that recommend restricted use of Rh D immune globulin during the first trimester, are based, in part, on the following considerations:
- there are no high-quality clinical trials demonstrating the benefit of Rh D immune globulin treatment in first trimester miscarriage and abortion care
- the Kleihauer-Betke technique cannot distinguish between maternal red blood cells expressing fetal hemoglobin (maternal F cells) and fetal cells, which has resulted in the over-estimation of the number of fetal cells in the maternal circulation20
- using a dual-label flow cytometry method that distinguishes maternal F cells and fetal red blood cells, maternal F cells usually far outnumber fetal red blood cells in the maternal circulation in the first trimester20
- among women in the first trimester undergoing uterine aspiration, the number of fetal cells in the maternal circulation is very low both before and after the procedure20
- Rh testing and Rh immune globulin administration is burdensome and expensive.16
Implications for your practice
The fundamental reason for the proliferation of divergent guidelines is that there is no evidence from high-quality randomized clinical trials demonstrating that Rh testing and Rh D immune globulin treatment in early pregnancy miscarriage or induced abortion care reduces the risk of hemolytic disease of the fetus and newborn. The Cochrane review on Rh D immune globulin administration for preventing alloimmunization among patients with spontaneous miscarriage concluded, “There are insufficient data available to evaluate the practice of anti-D administration in an unsensitized Rh-negative mother after spontaneous miscarriage.”21
Given divergent guidelines, obstetrician-gynecologists must decide on which guideline to use in their practice. Clinicians may conclude that absent high-quality evidence from clinical trials, they will continue to use the ACOG/SOGC guidelines2,3 in their practice, providing universal Rh testing and Rh D immune globulin treatment for all miscarriages and abortions, regardless of the gestational age. Other clinicians may conclude that Rh testing and Rh D immune globulin is not warranted before 8 to 12 weeks’ gestation, because the number of fetal red blood cells in the maternal circulation in cases of miscarriage and induced abortion is too low in early pregnancy to induce a maternal immune response.22 Based on recent studies demonstrating a low number of fetal red blood cells in the maternal circulation in the first trimester, family planning specialists are reducing the use of Rh testing and Rh immune globulin administration in both early pregnancy medication abortion and uterine aspiration abortion.16 With regard to Rh testing and Rh D immune globulin treatment, the future will definitely be different than the past. It is likely that many clinicians will reduce the use of Rh testing and Rh D immune globulin treatment in patients with miscarriage or induced abortion in early pregnancy. ●
All obstetrician-gynecologists know that pregnant patients who are Rh negative and exposed to a sufficient quantity of fetal red blood cells expressing Rh D antigen may become sensitized, producing Rh D antibodies that adversely impact future pregnancies with an Rh D-positive fetus, potentially causing hemolytic disease of the fetus and newborn. In countries where Rh D immune globulin is available, there is a consensus recommendation to administer Rh D immune globulin to Rh-negative pregnant patients at approximately 28 weeks’ gestation and at birth in order to decrease the risk of alloimmunization and hemolytic disease of the fetus and newborn in future pregnancies.1 In contrast to this global consensus, there is no worldwide agreement about how to manage Rh testing and Rh D immune globulin administration in cases of early pregnancy loss or abortion care before 12 weeks’ gestation. This editorial examines the evolving guidelines of major professional societies.
Guidelines consistent with the routine use of Rh D immune globulin in all cases of early pregnancy loss and abortion care
As of the publication date of this editorial, the American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin on prevention of Rh D alloimmunization provides the following guidance based on consensus and expert opinion2:
- “Although the risk of alloimmunization is low, the consequences can be significant, and administration of Rh D immune globulin should be considered in cases of spontaneous first trimester miscarriage, especially those that are later in the first trimester.”
- “Because of the higher risk of alloimmunization, Rh D-negative women who have instrumentation for their miscarriage should receive Rh D immune globulin prophylaxis.”
- “Rh D immune globulin should be given to Rh D-negative women who have pregnancy termination either medical or surgical.”
The Society of Obstetricians and Gynaecologists of Canada (SOGC) recommends that, “After miscarriage or threatened abortion or induced abortion during the first 12 weeks of gestation, non-sensitized D-negative women should be given a minimum anti-D of 120 µg.”3
The liberal use of Rh D immune globulin in all cases of early pregnancy loss and abortion care is based, in part, on the following considerations:
- the recognized safety of Rh D immune globulin administration2,3
- the report that fetal megaloblasts may express Rh antigen as early as 38 days of gestation4
- the observation that 0.1 mL of Rh D-positive red cells may provoke an immune response in some Rh D-negative patients5-7
- the estimate that in some patients with threatened miscarriage a significant quantity of fetal blood may enter the maternal circulation.8
Guidelines that suggest restricted use of Rh D immune globulin before 7 to 8 weeks’ gestation
The Reproductive Care Program of Nova Scotia guideline from 2022 notes that “the benefits of administering Rh immune globulin before 8 weeks gestation have not been demonstrated.” Given the burden of Rh testing and Rh D immune globulin administration they suggest that clinicians may withhold Rh testing and Rh D immune globulin administration in cases less than 8 weeks’ gestation (less than 56 days) for spontaneous, threatened, or medication abortions if there is reliable pregnancy dating.9
The Dutch Association of Abortion Specialists guidelines from 2018 suggest to not provide Rh D immune globulin treatment in the following clinical situations: patients under 10 weeks’ gestation with spontaneous miscarriage or patients under 7 weeks’ gestation having an induced abortion.10
Continue to: Guidelines that suggest restricted use of Rh D immune globulin before 10 to 12 weeks’ gestation...
Guidelines that suggest restricted use of Rh D immune globulin before 10 to 12 weeks’ gestation
There are a growing number of guidelines that recommend restricting the use of Rh testing and Rh D immune globulin treatment in the management of early miscarriage and induced abortion. In 2019, the United Kingdom’s National Institute for Health and Care Excellence (NICE) recommended that for patients having a spontaneous miscarriage, Rh testing and Rh D immune globulin are not necessary before 10 weeks 0 days of gestation.11 In addition, NICE recommends, “Do not offer anti-D prophylaxis to women who are having a medical abortion up to and including 10+0 weeks’ gestation.…Consider anti-D prophylaxis for women who are rhesus D negative and are having a surgical abortion up to and including 10+0 weeks’ gestation.”11
In 2019, the National Abortion Federation (NAF) Clinical Policies Committee recommended that “…it is reasonable to forgo Rh testing and anti-D immunoglobulin for women having any type of induced abortion before 8 weeks from the last menstrual period. Prior to 8 weeks, the likelihood of fetal-maternal hemorrhage adequate to cause sensitization is negligible. Given that medication abortion is more similar to spontaneous abortion with less risk of fetal-maternal hemorrhage, forgoing Rh testing and anti-D immunoglobulin for medication abortion under 10 weeks may be considered.”12 In 2022, NAF noted, “Emerging epidemiologic and clinical evidence indicates that the risk of maternal-fetal hemorrhage caused by early abortion is negligible and Rh testing and provision of Rh immune globulin may not be necessary. It is reasonable to forego Rh testing and anti-D immunoglobulin for people having any type of abortion before 56 days and medication abortion before 70 days since the last menstrual period. The pregnancy dating at which people need Rh testing and anti-D immunoglobulin is not well established. Foregoing Rh testing and anti-D immunoglobulinfor those using medication abortion through 11 to 12 weeks may be considered.”13
In 2020 the International Federation of Gynaecology and Obstetrics (FIGO) Committee for Safe Motherhood and Newborn Health recommended, “The risk for sensitization is most probably extremely low for spontaneous abortions before 10 gestational weeks; however, data are scarce. Based on the clinical expertise of the guideline committee from the UK’s National Institute for Health and Care Excellence (NICE), it is suggested that prophylaxis should be given only to women who are having a spontaneous abortion or medical management of miscarriage after 10 and 0/7 gestational weeks. Moreover, for women who have surgical management, prophylaxis may also be considered before 10 gestational weeks.”14
In 2022 the Royal College of Obstetricians and Gynaecologists recommended that for induced abortion, medication or surgical, “a determination of Rhesus blood status may be considered if the duration of pregnancy is over 12 weeks and anti-D is available.”15 “If available, anti-D should be offered to non-sensitised RhD-negative individuals from 12 weeks of pregnancy and provided within 72 hours of the abortion.”15
In 2022, the Society of Family Planning recommended that “Rh testing and administration are not recommended prior to 12 weeks gestation for patients undergoing spontaneous, medication or uterine aspiration abortion.” “For patients under 12 weeks gestation, although not recommended, Rh testing and Rh D immune globulin administration may be considered at patient request as part of a shared decision making process.”16
In 2022, the World Health Organization (WHO) reported “There are few studies examining Rh isoimmunization in unsensitized Rh-negative individuals seeking abortion before 12 weeks of gestation.” “The evidence on the effectiveness of the intervention may favor the intervention, because fewer women in the intervention group (anti-D administration) had antibody formation after the initial pregnancy compared to women in the comparison group (no anti-D) and no harms (undesirable effects) of the intervention were noted.”17 The evidence referenced for these statements are two low-quality studies from 1972.18,19 The WHO continues, “…after consideration of the resources required, cost-effectiveness and feasibility of administering anti-D, as well as the very low certainty of evidence on effectiveness, the expert panel concluded that overall, the evidence does not favor the intervention and decided to recommend against it for gestational ages < 12 weeks, rather than < 9 weeks, as mentioned in the 2012 guidance.”17 In conclusion, the WHO recommended that “for both medical and surgical abortion at < 12 weeks: Recommend against anti-D immunoglobulin administration.”17
Guidelines that recommend restricted use of Rh D immune globulin during the first trimester, are based, in part, on the following considerations:
- there are no high-quality clinical trials demonstrating the benefit of Rh D immune globulin treatment in first trimester miscarriage and abortion care
- the Kleihauer-Betke technique cannot distinguish between maternal red blood cells expressing fetal hemoglobin (maternal F cells) and fetal cells, which has resulted in the over-estimation of the number of fetal cells in the maternal circulation20
- using a dual-label flow cytometry method that distinguishes maternal F cells and fetal red blood cells, maternal F cells usually far outnumber fetal red blood cells in the maternal circulation in the first trimester20
- among women in the first trimester undergoing uterine aspiration, the number of fetal cells in the maternal circulation is very low both before and after the procedure20
- Rh testing and Rh immune globulin administration is burdensome and expensive.16
Implications for your practice
The fundamental reason for the proliferation of divergent guidelines is that there is no evidence from high-quality randomized clinical trials demonstrating that Rh testing and Rh D immune globulin treatment in early pregnancy miscarriage or induced abortion care reduces the risk of hemolytic disease of the fetus and newborn. The Cochrane review on Rh D immune globulin administration for preventing alloimmunization among patients with spontaneous miscarriage concluded, “There are insufficient data available to evaluate the practice of anti-D administration in an unsensitized Rh-negative mother after spontaneous miscarriage.”21
Given divergent guidelines, obstetrician-gynecologists must decide on which guideline to use in their practice. Clinicians may conclude that absent high-quality evidence from clinical trials, they will continue to use the ACOG/SOGC guidelines2,3 in their practice, providing universal Rh testing and Rh D immune globulin treatment for all miscarriages and abortions, regardless of the gestational age. Other clinicians may conclude that Rh testing and Rh D immune globulin is not warranted before 8 to 12 weeks’ gestation, because the number of fetal red blood cells in the maternal circulation in cases of miscarriage and induced abortion is too low in early pregnancy to induce a maternal immune response.22 Based on recent studies demonstrating a low number of fetal red blood cells in the maternal circulation in the first trimester, family planning specialists are reducing the use of Rh testing and Rh immune globulin administration in both early pregnancy medication abortion and uterine aspiration abortion.16 With regard to Rh testing and Rh D immune globulin treatment, the future will definitely be different than the past. It is likely that many clinicians will reduce the use of Rh testing and Rh D immune globulin treatment in patients with miscarriage or induced abortion in early pregnancy. ●
- Sperling JD, Dahlke JD, Sutton D, et al. Prevention of Rh D alloimmunization: a comparison of four national guidelines. Am J Perinatol. 2018;35:110-119.
- Prevention of Rh D alloimmunization. Practice Bulletin No. 181. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2017;130:e57-e70.
- Fung KFK, Eason E. No. 133-Prevention of Rh alloimmunization. J Obstet Gynaecol Can. 2018;40: e1-e10.
- Bergstrom H, Nilsson LA, Nilsson L, et al. Demonstration of Rh antigens in a 38-day-old fetus. Am J Obstet Gynecol. 1967;99:130-133.
- Bowman JM. The prevention of Rh Immunization. Transfus Med Rev. 1988;2:129-150.
- Zipursky A, Israels LG. The pathogenesis and prevention of Rh immunization. Can Med Assoc J. 1967;97:1245-1257.
- Pollack W, Ascari WQ, Kochesky RJ, et al. Studies on Rh prophylaxis. 1. Relationship between doses of anti-Rh and size of antigenic stimulus. Transfusion. 1971;11:333-339.
- Von Stein GA, Munsick RA, Stiver K, et al. Feto-maternal hemorrhage in threatened abortion. Obstet Gynecol. 1992;79:383-386.
- Rh Program of Nova Scotia. Guideline for Rh prophylaxis before 8 weeks (56 days) gestation for Early Pregnancy Complications and Medical Abortions. http://rcp.nshealth.ca/sites/default /files/rh/RhIg%20before%208%20weeks%20 Guideline_%20Jun2022_Final_2page.pdf. Accessed January 24, 2023.
- Wiebe ER, Campbell M, Aiken ARA, et al. Can we safety stop testing for Rh Status and immunizing Rh-negative women having early abortions? A comparison of Rh alloimmunization in Canada and the Netherlands. Contraception. 2019;100001. https://doi.org/10.1016/j.conx.2018.100001.
- Abortion care. National Institute for Health and Care Excellence. https://www.nice.org .uk/guidance/ng140/resources/abortion-care -pdf-66141773098693. Accessed January 24, 2023.
- Mark A, Foster AM, Grossman D. Foregoing Rh testing and anti-D immunoglobulin for women presenting for early abortion: a recommendation from the National Abortion Federation’s Clinical Policies Committee. Contraception. 2019;99:265-266.
- National Abortion Federation. 2022 Clinical Policy Guidelines for Abortion Care. https: //prochoice.org/wp-content/uploads/2022 -CPGs.pdf. Accessed January 24, 2023.
- Visser GHA, Thommesen T, Di Renzo GC, et al. FIGO Safe Motherhood and Newborn Health Committee. Int J Gynecol Obstet. 2021;152: 144-147.
- Making abortion safe: RCOG’s global initiative to advocate for women’s health. https://www .rcog.org.uk/media/geify5bx/abortion-care-best -practice-paper-april-2022.pdf. Accessed January 24, 2023.
- Horvath S, Goyal V, Traxler S, et al. Society of Family Planning committee consensus on Rh testing in early pregnancy. Contraception. 2022;114:1-5.
- World Health Organization. Abortion care guideline. https://www.who.int/publications/i/ item/9789240039483. Accessed January 24, 2023.
- Gavin P. Rhesus sensitization in abortion. Obstet Gynecol. 1972;39:37-40.
- Goldman J, Eckerling B. Rh immunization in spontaneous abortion. Acta Eur Fertil. 1972;3:253254.
- Horvath S, Tsao P, Huang ZY, et al. The concentration of fetal red blood cells in first-trimester pregnant women undergoing uterine aspiration is below the calculated threshold for Rh sensitization. Contraception. 2020;102:1-6.
- Karanth L, Jaafar SH, Kanagasabai S, et al. Anti-D administration after spontaneous miscarriage for preventing Rhesus alloimmunization. Cochrane Database Syst Rev. 2023;CD009617.
- Gilmore E, Sonalkar S, Schreiber CA. Use of Rh immune globulin in first-trimester abortion and miscarriage. Obstet Gynecol. 2023;141:219-222.
- Sperling JD, Dahlke JD, Sutton D, et al. Prevention of Rh D alloimmunization: a comparison of four national guidelines. Am J Perinatol. 2018;35:110-119.
- Prevention of Rh D alloimmunization. Practice Bulletin No. 181. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2017;130:e57-e70.
- Fung KFK, Eason E. No. 133-Prevention of Rh alloimmunization. J Obstet Gynaecol Can. 2018;40: e1-e10.
- Bergstrom H, Nilsson LA, Nilsson L, et al. Demonstration of Rh antigens in a 38-day-old fetus. Am J Obstet Gynecol. 1967;99:130-133.
- Bowman JM. The prevention of Rh Immunization. Transfus Med Rev. 1988;2:129-150.
- Zipursky A, Israels LG. The pathogenesis and prevention of Rh immunization. Can Med Assoc J. 1967;97:1245-1257.
- Pollack W, Ascari WQ, Kochesky RJ, et al. Studies on Rh prophylaxis. 1. Relationship between doses of anti-Rh and size of antigenic stimulus. Transfusion. 1971;11:333-339.
- Von Stein GA, Munsick RA, Stiver K, et al. Feto-maternal hemorrhage in threatened abortion. Obstet Gynecol. 1992;79:383-386.
- Rh Program of Nova Scotia. Guideline for Rh prophylaxis before 8 weeks (56 days) gestation for Early Pregnancy Complications and Medical Abortions. http://rcp.nshealth.ca/sites/default /files/rh/RhIg%20before%208%20weeks%20 Guideline_%20Jun2022_Final_2page.pdf. Accessed January 24, 2023.
- Wiebe ER, Campbell M, Aiken ARA, et al. Can we safety stop testing for Rh Status and immunizing Rh-negative women having early abortions? A comparison of Rh alloimmunization in Canada and the Netherlands. Contraception. 2019;100001. https://doi.org/10.1016/j.conx.2018.100001.
- Abortion care. National Institute for Health and Care Excellence. https://www.nice.org .uk/guidance/ng140/resources/abortion-care -pdf-66141773098693. Accessed January 24, 2023.
- Mark A, Foster AM, Grossman D. Foregoing Rh testing and anti-D immunoglobulin for women presenting for early abortion: a recommendation from the National Abortion Federation’s Clinical Policies Committee. Contraception. 2019;99:265-266.
- National Abortion Federation. 2022 Clinical Policy Guidelines for Abortion Care. https: //prochoice.org/wp-content/uploads/2022 -CPGs.pdf. Accessed January 24, 2023.
- Visser GHA, Thommesen T, Di Renzo GC, et al. FIGO Safe Motherhood and Newborn Health Committee. Int J Gynecol Obstet. 2021;152: 144-147.
- Making abortion safe: RCOG’s global initiative to advocate for women’s health. https://www .rcog.org.uk/media/geify5bx/abortion-care-best -practice-paper-april-2022.pdf. Accessed January 24, 2023.
- Horvath S, Goyal V, Traxler S, et al. Society of Family Planning committee consensus on Rh testing in early pregnancy. Contraception. 2022;114:1-5.
- World Health Organization. Abortion care guideline. https://www.who.int/publications/i/ item/9789240039483. Accessed January 24, 2023.
- Gavin P. Rhesus sensitization in abortion. Obstet Gynecol. 1972;39:37-40.
- Goldman J, Eckerling B. Rh immunization in spontaneous abortion. Acta Eur Fertil. 1972;3:253254.
- Horvath S, Tsao P, Huang ZY, et al. The concentration of fetal red blood cells in first-trimester pregnant women undergoing uterine aspiration is below the calculated threshold for Rh sensitization. Contraception. 2020;102:1-6.
- Karanth L, Jaafar SH, Kanagasabai S, et al. Anti-D administration after spontaneous miscarriage for preventing Rhesus alloimmunization. Cochrane Database Syst Rev. 2023;CD009617.
- Gilmore E, Sonalkar S, Schreiber CA. Use of Rh immune globulin in first-trimester abortion and miscarriage. Obstet Gynecol. 2023;141:219-222.
Depression and schizophrenia: Many biological and clinical similarities
Clinicians generally regard major depressive disorder (MDD) and schizophrenia as 2 separate and distinct psychiatric brain disorders. However, despite some differences, those 2 psychiatric syndromes have numerous similarities across clinical features and neurobiologic parameters.
Biological similarities
Both disorders share the following variables:
- Highly genetic in etiology but with environmental influences and epigenetics
- Associated with childhood maltreatment, abuse, or neglect
- Disrupted neuroplasticity, especially shrinkage in hippocampal volume
- Significant drop in brain-derived neurotrophic factor resulting in decreased neurogenesis
- Extensive white matter pathology across interhemispheric and intrahemispheric bundles
- Increased levels of serum cortisol, a stress hormone and inflammatory biomarker
- Hypofrontal cerebral blood flow during acute episodes of both MDD and schizophrenia
- Reduced dendritic spines (in number and size) and impaired experiential neuroplasticity
- Neuroinflammation (eg, cytokines, tumor necrosis factor-alpha, C-reactive protein) during acute episodes
- Elevated oxidative stress biomarkers, indicating an increase in free radicals
- Overactive default mode network associated with ruminations in MDD and “daydreaming” in schizophrenia
- Decrease in gamma-aminobutyric acid (GABA) and its inhibitory activity, translating into dysregulation of glutamatergic pathways and other neurotransmitters
- Immune dysregulation and comorbid autoimmune disorders
Clinical similarities
- Psychotic symptoms, especially delusional thinking such as paranoia in schizophrenia and severe self-deprecation in MDD
- Significantly elevated lifetime suicide risk
- Cognitive impairment (more severe in schizophrenia across several cognitive functions)
- Similarity of depressive and negative symptoms (especially anhedonia, apathy, restricted facial expression, social withdrawal)
- Antidepressant medications im-prove depressive and negative symptoms (though not completely in the case of negative symptoms of schizophrenia)
- Both have treatment-resistant subtypes that fail to respond to standard therapies
- Both are associated with comorbid generalized anxiety disorder
- Both are associated with comorbid obsessive-compulsive disorder
- Both are associated with serious alcohol and drug use
- Early mortality from general medical conditions, especially cardiovascular risks due to obesity, diabetes, hypertension, dyslipidemia
- Elevated risk of dementia with aging compared to the unaffected general population
- Opioids improve MDD and psychosis (buprenorphine in MDD and morphine in schizophrenia)
- Several second-generation antipsychotic medications are approved for both MDD and schizophrenia
- Electroconvulsive therapy is effective when pharmacotherapy fails in both MDD and schizophrenia
Biological differences
- Glutamate N-methyl-D-aspartate receptor antagonists (eg, ketamine) improve MDD but worsen schizophrenia
- Muscarinic agonists improve psychosis but worsen depression
- High pain threshold in schizophrenia (pain insensitivity) and low threshold in MDD (in which pain is a common comorbidity)
- Cortical thinning more severe in schizophrenia
- Hippocampal atrophy is reversible with successful treatment in MDD but not in schizophrenia
- Hypofrontality is reversible with remission in MDD but not in schizophrenia
Clinical differences
- Auditory and visual hallucinations are more common in schizophrenia than in MDD
- Anosognosia is common in schizophrenia but not in MDD
- Implausible delusions are more common in schizophrenia than in MDD
- Mood-congruent delusions are more common in MDD than in schizophrenia
- Sadness, crying, pessimism, and self-deprecation are common in MDD but not in schizophrenia
- Achieving full remission is more common in MDD than in schizophrenia
- Long-acting injectable medications are available for schizophrenia but not for MDD
- Evidence-based psychotherapy, without pharmacotherapy, is more likely to be effective in MDD than in schizophrenia
A transdiagnostic model of psychopathology
The significant overlap between MDD and schizophrenia should not be surprising. They are both generated by the same organ, the human brain, with disrupted neurochemical and physiological circuits in the brain.
The overlap is also consistent with the emerging transdiagnostic model of psychopathology.1-9 This model proposes that there is a “core” genetic risk for psychopathology with different iterations. The transdiagnostic model is in stark contrast to the prevailing DSM-5, which categorizes psychiatric disorders in “silos,” as if they are completely independent from each other despite many shared features. This is highly debatable according to the substantial evidence that multiple psychiatric disorders share many genes that influence brain development in utero and predispose individuals to a variety of clinical symptoms in adolescence and young adulthood.
The origin of mental illness is being disentangled by emerging research, which is identifying the common links among the various disorders currently listed in DSM-5.10 However, the evolution of psychiatric diagnosis has come full circle from a single entity before DSM, to multiple entities with DSM, and now back to a unified transdiagnostic model that is rapidly emerging.11 This has implications for the FDA’s persistent dogma that clinical trials for new drugs must be targeted for 1 of the DSM-5 categories, a flawed and narrow assumption. Given the accelerating body of evidence for a unified, transdiagnostic model, it makes much more sense for the FDA to approve medications that target a psychiatric symptom that is shared by multiple psychiatric conditions within a transdiagnostic clinical system. When medications are approved for a symptom regardless of a DSM diagnosis, the term “off-label” and its “stigma” will then fade into history, along with the malignant preauthorization racket that was invented by greedy insurance companies that exploit the off-label use of medications (even when an FDA-approved medication for the patient’s condition does not yet exist) simply to deny coverage, lower their expenses, and fatten their profits.
1. Goodkind M, Eickhoff SB, Oathes DJ, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72(4):305-315.
2. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
3. Krueger RF, Easton NR. Transdiagnostic factors in mental disorders. World Psychiatry. 2015;14(1):27-29.
4. Hyman SE. New evidence for shared risk architecture for mental disorders. JAMA Psychiatry. 2019;76(3):235-236.
5. Selzam S, Coleman JRI, Caspi A, et al. A polygenic p factor for major psychiatric disorders. Translational Psychiatry. 2018;8(1):205.
6. Barch DM. What it means to be transdiagnostic and how do we know? Am J Psychiatry. 2020;177(5):370-372.
7. Nasrallah HA. Is there only 1 neurobiologic psychiatric disorder with different clinical expressions? Current Psychiatry. 2015;14(7):10-12.
8. Nasrallah HA. Pleiotropy of psychiatric disorders will reinvent DSM. Current Psychiatry. 2013;12(4):6-7.
9. Nasrallah HA. Beyond DSM-5: clinical and biological features shared by major psychiatric syndromes. Current Psychiatry. 2017;16(10):4-7.
10. Marshall M. Roots of mental illness: researchers are beginning to untangle the common biology that links supposedly distinct psychiatric conditions. Nature. 2020;581:19-21.
11. Kendler KS. From many to one to many--the search for causes of psychiatric illness. JAMA Psychiatry. 2019;76(10):1085-1091.
Clinicians generally regard major depressive disorder (MDD) and schizophrenia as 2 separate and distinct psychiatric brain disorders. However, despite some differences, those 2 psychiatric syndromes have numerous similarities across clinical features and neurobiologic parameters.
Biological similarities
Both disorders share the following variables:
- Highly genetic in etiology but with environmental influences and epigenetics
- Associated with childhood maltreatment, abuse, or neglect
- Disrupted neuroplasticity, especially shrinkage in hippocampal volume
- Significant drop in brain-derived neurotrophic factor resulting in decreased neurogenesis
- Extensive white matter pathology across interhemispheric and intrahemispheric bundles
- Increased levels of serum cortisol, a stress hormone and inflammatory biomarker
- Hypofrontal cerebral blood flow during acute episodes of both MDD and schizophrenia
- Reduced dendritic spines (in number and size) and impaired experiential neuroplasticity
- Neuroinflammation (eg, cytokines, tumor necrosis factor-alpha, C-reactive protein) during acute episodes
- Elevated oxidative stress biomarkers, indicating an increase in free radicals
- Overactive default mode network associated with ruminations in MDD and “daydreaming” in schizophrenia
- Decrease in gamma-aminobutyric acid (GABA) and its inhibitory activity, translating into dysregulation of glutamatergic pathways and other neurotransmitters
- Immune dysregulation and comorbid autoimmune disorders
Clinical similarities
- Psychotic symptoms, especially delusional thinking such as paranoia in schizophrenia and severe self-deprecation in MDD
- Significantly elevated lifetime suicide risk
- Cognitive impairment (more severe in schizophrenia across several cognitive functions)
- Similarity of depressive and negative symptoms (especially anhedonia, apathy, restricted facial expression, social withdrawal)
- Antidepressant medications im-prove depressive and negative symptoms (though not completely in the case of negative symptoms of schizophrenia)
- Both have treatment-resistant subtypes that fail to respond to standard therapies
- Both are associated with comorbid generalized anxiety disorder
- Both are associated with comorbid obsessive-compulsive disorder
- Both are associated with serious alcohol and drug use
- Early mortality from general medical conditions, especially cardiovascular risks due to obesity, diabetes, hypertension, dyslipidemia
- Elevated risk of dementia with aging compared to the unaffected general population
- Opioids improve MDD and psychosis (buprenorphine in MDD and morphine in schizophrenia)
- Several second-generation antipsychotic medications are approved for both MDD and schizophrenia
- Electroconvulsive therapy is effective when pharmacotherapy fails in both MDD and schizophrenia
Biological differences
- Glutamate N-methyl-D-aspartate receptor antagonists (eg, ketamine) improve MDD but worsen schizophrenia
- Muscarinic agonists improve psychosis but worsen depression
- High pain threshold in schizophrenia (pain insensitivity) and low threshold in MDD (in which pain is a common comorbidity)
- Cortical thinning more severe in schizophrenia
- Hippocampal atrophy is reversible with successful treatment in MDD but not in schizophrenia
- Hypofrontality is reversible with remission in MDD but not in schizophrenia
Clinical differences
- Auditory and visual hallucinations are more common in schizophrenia than in MDD
- Anosognosia is common in schizophrenia but not in MDD
- Implausible delusions are more common in schizophrenia than in MDD
- Mood-congruent delusions are more common in MDD than in schizophrenia
- Sadness, crying, pessimism, and self-deprecation are common in MDD but not in schizophrenia
- Achieving full remission is more common in MDD than in schizophrenia
- Long-acting injectable medications are available for schizophrenia but not for MDD
- Evidence-based psychotherapy, without pharmacotherapy, is more likely to be effective in MDD than in schizophrenia
A transdiagnostic model of psychopathology
The significant overlap between MDD and schizophrenia should not be surprising. They are both generated by the same organ, the human brain, with disrupted neurochemical and physiological circuits in the brain.
The overlap is also consistent with the emerging transdiagnostic model of psychopathology.1-9 This model proposes that there is a “core” genetic risk for psychopathology with different iterations. The transdiagnostic model is in stark contrast to the prevailing DSM-5, which categorizes psychiatric disorders in “silos,” as if they are completely independent from each other despite many shared features. This is highly debatable according to the substantial evidence that multiple psychiatric disorders share many genes that influence brain development in utero and predispose individuals to a variety of clinical symptoms in adolescence and young adulthood.
The origin of mental illness is being disentangled by emerging research, which is identifying the common links among the various disorders currently listed in DSM-5.10 However, the evolution of psychiatric diagnosis has come full circle from a single entity before DSM, to multiple entities with DSM, and now back to a unified transdiagnostic model that is rapidly emerging.11 This has implications for the FDA’s persistent dogma that clinical trials for new drugs must be targeted for 1 of the DSM-5 categories, a flawed and narrow assumption. Given the accelerating body of evidence for a unified, transdiagnostic model, it makes much more sense for the FDA to approve medications that target a psychiatric symptom that is shared by multiple psychiatric conditions within a transdiagnostic clinical system. When medications are approved for a symptom regardless of a DSM diagnosis, the term “off-label” and its “stigma” will then fade into history, along with the malignant preauthorization racket that was invented by greedy insurance companies that exploit the off-label use of medications (even when an FDA-approved medication for the patient’s condition does not yet exist) simply to deny coverage, lower their expenses, and fatten their profits.
Clinicians generally regard major depressive disorder (MDD) and schizophrenia as 2 separate and distinct psychiatric brain disorders. However, despite some differences, those 2 psychiatric syndromes have numerous similarities across clinical features and neurobiologic parameters.
Biological similarities
Both disorders share the following variables:
- Highly genetic in etiology but with environmental influences and epigenetics
- Associated with childhood maltreatment, abuse, or neglect
- Disrupted neuroplasticity, especially shrinkage in hippocampal volume
- Significant drop in brain-derived neurotrophic factor resulting in decreased neurogenesis
- Extensive white matter pathology across interhemispheric and intrahemispheric bundles
- Increased levels of serum cortisol, a stress hormone and inflammatory biomarker
- Hypofrontal cerebral blood flow during acute episodes of both MDD and schizophrenia
- Reduced dendritic spines (in number and size) and impaired experiential neuroplasticity
- Neuroinflammation (eg, cytokines, tumor necrosis factor-alpha, C-reactive protein) during acute episodes
- Elevated oxidative stress biomarkers, indicating an increase in free radicals
- Overactive default mode network associated with ruminations in MDD and “daydreaming” in schizophrenia
- Decrease in gamma-aminobutyric acid (GABA) and its inhibitory activity, translating into dysregulation of glutamatergic pathways and other neurotransmitters
- Immune dysregulation and comorbid autoimmune disorders
Clinical similarities
- Psychotic symptoms, especially delusional thinking such as paranoia in schizophrenia and severe self-deprecation in MDD
- Significantly elevated lifetime suicide risk
- Cognitive impairment (more severe in schizophrenia across several cognitive functions)
- Similarity of depressive and negative symptoms (especially anhedonia, apathy, restricted facial expression, social withdrawal)
- Antidepressant medications im-prove depressive and negative symptoms (though not completely in the case of negative symptoms of schizophrenia)
- Both have treatment-resistant subtypes that fail to respond to standard therapies
- Both are associated with comorbid generalized anxiety disorder
- Both are associated with comorbid obsessive-compulsive disorder
- Both are associated with serious alcohol and drug use
- Early mortality from general medical conditions, especially cardiovascular risks due to obesity, diabetes, hypertension, dyslipidemia
- Elevated risk of dementia with aging compared to the unaffected general population
- Opioids improve MDD and psychosis (buprenorphine in MDD and morphine in schizophrenia)
- Several second-generation antipsychotic medications are approved for both MDD and schizophrenia
- Electroconvulsive therapy is effective when pharmacotherapy fails in both MDD and schizophrenia
Biological differences
- Glutamate N-methyl-D-aspartate receptor antagonists (eg, ketamine) improve MDD but worsen schizophrenia
- Muscarinic agonists improve psychosis but worsen depression
- High pain threshold in schizophrenia (pain insensitivity) and low threshold in MDD (in which pain is a common comorbidity)
- Cortical thinning more severe in schizophrenia
- Hippocampal atrophy is reversible with successful treatment in MDD but not in schizophrenia
- Hypofrontality is reversible with remission in MDD but not in schizophrenia
Clinical differences
- Auditory and visual hallucinations are more common in schizophrenia than in MDD
- Anosognosia is common in schizophrenia but not in MDD
- Implausible delusions are more common in schizophrenia than in MDD
- Mood-congruent delusions are more common in MDD than in schizophrenia
- Sadness, crying, pessimism, and self-deprecation are common in MDD but not in schizophrenia
- Achieving full remission is more common in MDD than in schizophrenia
- Long-acting injectable medications are available for schizophrenia but not for MDD
- Evidence-based psychotherapy, without pharmacotherapy, is more likely to be effective in MDD than in schizophrenia
A transdiagnostic model of psychopathology
The significant overlap between MDD and schizophrenia should not be surprising. They are both generated by the same organ, the human brain, with disrupted neurochemical and physiological circuits in the brain.
The overlap is also consistent with the emerging transdiagnostic model of psychopathology.1-9 This model proposes that there is a “core” genetic risk for psychopathology with different iterations. The transdiagnostic model is in stark contrast to the prevailing DSM-5, which categorizes psychiatric disorders in “silos,” as if they are completely independent from each other despite many shared features. This is highly debatable according to the substantial evidence that multiple psychiatric disorders share many genes that influence brain development in utero and predispose individuals to a variety of clinical symptoms in adolescence and young adulthood.
The origin of mental illness is being disentangled by emerging research, which is identifying the common links among the various disorders currently listed in DSM-5.10 However, the evolution of psychiatric diagnosis has come full circle from a single entity before DSM, to multiple entities with DSM, and now back to a unified transdiagnostic model that is rapidly emerging.11 This has implications for the FDA’s persistent dogma that clinical trials for new drugs must be targeted for 1 of the DSM-5 categories, a flawed and narrow assumption. Given the accelerating body of evidence for a unified, transdiagnostic model, it makes much more sense for the FDA to approve medications that target a psychiatric symptom that is shared by multiple psychiatric conditions within a transdiagnostic clinical system. When medications are approved for a symptom regardless of a DSM diagnosis, the term “off-label” and its “stigma” will then fade into history, along with the malignant preauthorization racket that was invented by greedy insurance companies that exploit the off-label use of medications (even when an FDA-approved medication for the patient’s condition does not yet exist) simply to deny coverage, lower their expenses, and fatten their profits.
1. Goodkind M, Eickhoff SB, Oathes DJ, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72(4):305-315.
2. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
3. Krueger RF, Easton NR. Transdiagnostic factors in mental disorders. World Psychiatry. 2015;14(1):27-29.
4. Hyman SE. New evidence for shared risk architecture for mental disorders. JAMA Psychiatry. 2019;76(3):235-236.
5. Selzam S, Coleman JRI, Caspi A, et al. A polygenic p factor for major psychiatric disorders. Translational Psychiatry. 2018;8(1):205.
6. Barch DM. What it means to be transdiagnostic and how do we know? Am J Psychiatry. 2020;177(5):370-372.
7. Nasrallah HA. Is there only 1 neurobiologic psychiatric disorder with different clinical expressions? Current Psychiatry. 2015;14(7):10-12.
8. Nasrallah HA. Pleiotropy of psychiatric disorders will reinvent DSM. Current Psychiatry. 2013;12(4):6-7.
9. Nasrallah HA. Beyond DSM-5: clinical and biological features shared by major psychiatric syndromes. Current Psychiatry. 2017;16(10):4-7.
10. Marshall M. Roots of mental illness: researchers are beginning to untangle the common biology that links supposedly distinct psychiatric conditions. Nature. 2020;581:19-21.
11. Kendler KS. From many to one to many--the search for causes of psychiatric illness. JAMA Psychiatry. 2019;76(10):1085-1091.
1. Goodkind M, Eickhoff SB, Oathes DJ, et al. Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry. 2015;72(4):305-315.
2. Caspi A, Moffitt TE. All for one and one for all: mental disorders in one dimension. Am J Psychiatry. 2018;175(9):831-844.
3. Krueger RF, Easton NR. Transdiagnostic factors in mental disorders. World Psychiatry. 2015;14(1):27-29.
4. Hyman SE. New evidence for shared risk architecture for mental disorders. JAMA Psychiatry. 2019;76(3):235-236.
5. Selzam S, Coleman JRI, Caspi A, et al. A polygenic p factor for major psychiatric disorders. Translational Psychiatry. 2018;8(1):205.
6. Barch DM. What it means to be transdiagnostic and how do we know? Am J Psychiatry. 2020;177(5):370-372.
7. Nasrallah HA. Is there only 1 neurobiologic psychiatric disorder with different clinical expressions? Current Psychiatry. 2015;14(7):10-12.
8. Nasrallah HA. Pleiotropy of psychiatric disorders will reinvent DSM. Current Psychiatry. 2013;12(4):6-7.
9. Nasrallah HA. Beyond DSM-5: clinical and biological features shared by major psychiatric syndromes. Current Psychiatry. 2017;16(10):4-7.
10. Marshall M. Roots of mental illness: researchers are beginning to untangle the common biology that links supposedly distinct psychiatric conditions. Nature. 2020;581:19-21.
11. Kendler KS. From many to one to many--the search for causes of psychiatric illness. JAMA Psychiatry. 2019;76(10):1085-1091.
35 years in service to you, our community of reproductive health care clinicians
The mission of OBG
OBG
We wish all our readers a wonderful New Year and the best health possible for our patients.
Arnold P. Advincula, MD
I serve on the executive board that oversees the Fellowships in Minimally Invasive Gynecologic Surgery (FMIGS), and in January 2023 will transition into the role of President. I bring to this leadership role nearly 25 years of surgical experience, both as a clinician educator and inventor. My goal during the next 2 years will be to move toward subspecialty recognition of Complex Gynecology.
Linda D. Bradley, MD
My passion is diagnostic and operative hysteroscopy, simple procedures that can both evaluate and treat intrauterine pathology. Recently, I was thrilled to coauthor an article on office hysteroscopy for Obstetrics & Gynecology (September 2022). I will have a chapter on operative hysteroscopy in the 2023 edition of TeLinde’s Textbook of Gynecology, and I am an author for the topic Office and Operative Hysteroscopy in UpToDate. Locally, I am known as the “foodie gynecologist”—I travel, take cooking classes, and I have more cookbooks than gynecology textbooks. Since Covid, I have embraced biking and just completed a riverboat biking cruise from Salamanca, Spain, to Lisbon, Portugal.
Amy L. Garcia, MD
I am fellowship trained as a minimally invasive gynecologic surgeon (MIGS) and have had a private surgical practice since 2005. I am involved with The American College of Obstetricians and Gynecologists (ACOG), AAGL, and international surgical education for office hysteroscopy and related practice management. I am passionate about working with start-up companies in the gynecologic medical device arena and innovation in gynecologic surgery.
Steven R. Goldstein, MD, NCMP, CCD
I just completed my term as President of the International Menopause Society. This culminated in the society’s 18th World Congress in Lisbon, attended by over 1,700 health care providers from 76 countries. I delivered the Pieter van Keep Memorial Lecture, named for one of the society’s founders who died prematurely of pancreatic cancer. I was further honored by receiving the society’s Distinguished Service Award. I am very proud to have previously received the NAMS Thomas B. Clarkson award for Outstanding Clinical and Basic Science Research in Menopause. I also have one foot in the gynecologic ultrasound world and was given the Joseph H. Holmes Pioneer Award and was the 2023 recipient of the William J. Fry Memorial Lecture Award, both from the American Institute of Ultrasound in Medicine, having written the second book ever on vaginal ultrasonography.
On a personal level, I love to play golf (in spite of my foot drop and 14 orthopedic surgeries). My season tickets show some diversity—the New York City Ballet and St. John’s basketball.
Cheryl B. Iglesia, MD
I am the 49th president of the Society of Gynecologic Surgeons, the 5th woman to hold this position, and the first of Filipino-American descent. I recognize that it is only through extraordinary mentorship and support from other giants in gynecology, like Drs. Andrew Kaunitz (fellow OBG
PS—In the spirit of continually learning, I want to add the Argentine tango to my dancing repertoire and go on an African safari; both are on my bucket list as the pandemic eases.
Andrew M. Kaunitz, MD, NCMP
Since starting with the University of Florida College of Medicine-Jacksonville in 1984, I have enjoyed caring for patients, training residents and medical students, and being involved with publications and research. My areas of focus are menopause, contraception, gyn ultrasound and evaluation/management of women with abnormal uterine bleeding. In 2020, I received the North American Menopause Society/Leon Speroff Outstanding Educator Award. In 2021, I received the ACOG Distinguished Service Award. I enjoy spending time with my family, neighborhood bicycling, and searching for sharks’ teeth at the beach.
Barbara Levy, MD
I have been privileged to serve on the OBG
Continue to: David G. Mutch, MD...
David G. Mutch, MD
I am ending my 6-year term as Chair of the National Cancer Institute’s (NCI) gynecologic cancer steering committee. That is the committee that vets all NCI-sponsored clinical trials in gynecologic oncology. I am on the International Federation of Gynecology and Obstetrics (FIGO) Cancer committee, Co-Chair of the American Joint Committee on Cancer gyn staging committee and on the Reproductive Scientist Development Program selection committee. I also am completing my term as Chair of the Foundation for Women’s Cancer; this is the C3, charitable arm, of the Society of Gynecologic Oncology. We have distributed more than $3.5 million to young investigators to help start their research careers in gynecologic oncology.
Errol R. Norwitz, MD, PhD, MBA
I am a physician-scientist with subspecialty training in high-risk obstetrics (maternal-fetal medicine). I was born and raised in Cape Town, South Africa, and I have trained/practiced in 5 countries on 3 continents. My research interests include the pathophysiology, prediction, prevention, and management of pregnancy complications, primarily preterm birth and preeclampsia. I am a member of the Board of Scientific Counselors of the National Institute of Child Health and Human Development. I am currently President & CEO of Newton-Wellesley Hospital, a comprehensive community-based academic medical center and a member of the Mass General Brigham health care system in Boston, Massachusetts.
Jaimey Pauli, MD
I am the Division Chief and Professor of Maternal-Fetal Medicine (MFM) at the Penn State College of Medicine and Penn State Health Milton S. Hershey Medical Center. I had exceptional mentoring throughout my medical career, particularly by a former member of the Editorial Board, Dr. John T. Repke. One of the biggest perks of my job is that our division provides full-scope MFM care. While I often serve as the more traditional MFM consultant and academic educator, I also provide longitudinal prenatal care and deliver many of my own patients, often through subsequent pregnancies. Serving as a member of the Editorial Board combines my passion for clinical obstetrical care with my talents (as a former English major) of reading, writing, and editing. I believe that the work we do provides accessible, evidence-based, and practical guidance for our colleagues so they can provide excellence in obstetrical care.
JoAnn Pinkerton, MD, NCMP
I am a Professor of Obstetrics and Gynecology and Division Chief of Midlife Health at the University of Virginia (UVA) Health. Passionate about menopause, I am an executive director emeritus of The North American Menopause Society (NAMS) and past-President of NAMS (2008-2009). Within the past few years, I have served as an expert advisor for the recent ACOG Clinical Practice Guidelines on Osteoporosis, the NAMS Position Statements on Hormone Therapy and Osteoporosis, and the Global Consensus on Menopause and Androgen Therapy. I received the 2022 South Atlantic Association of Obstetricians and Gynecologists Lifetime Achievement Award for my expertise and work in menopause and the NAMS 2020 Ann Voda Community Service Award for my biannual community educational symposiums. I remain active in research, currently the lead and UVA principal investigator for the Oasis 2 multicenter clinical trial, which is testing a neurokinin receptor antagonist as a nonhormone therapy for the relief of hot flashes. Serving on the OBG
Joseph S. Sanfilippo, MD, MBA
I feel honored and privileged to have received the Golden Apple Teaching Award from the Universityof Pittsburgh School of Medicine. I am also fortunate to be the recipient of the Faculty Educator of the Month Award for resident teaching. I have been named Top Doctor 20 years in a row. My current academic activities include, since 2007, Program Director for Reproductive Endocrinology & Infertility Fellowship at the University of Pittsburgh and Chair of the Mentor-Mentee Program at University of Pittsburgh Department of Obstetrics, Gynecology & Reproductive Sciences. I am Guest Editor for the medical malpractice section of the journal Clinical Obstetrics and Gynecology. Recently, I completed a patient-focused book, “Experts Guide to Fertility,” which will be published in May 2023 by J Hopkins University Publisher and is designed for patients going through infertility treatment. Regarding outside events, I enjoy climbing steep hills and riding far and wide on my “electric bike.” Highly recommend it!
James Simon, MD, CCD, IF, NCMP
It’s been an honor serving on the OBG
I asked our distinguished Board of Editors to identify the most important changes that they believe will occur over the next 5 years, influencing the practice of obstetrics and gynecology. Their expert predictions are summarized below.
Arnold Advincula, MD
As one of the world’s most experienced gynecologic robotic surgeons, the role of this technology will become even more refined over the next 2-5 years with the introduction of sophisticated image guidance, “smart molecules,” and artificial intelligence. All of this will transform both the patient and surgeon experience as well as impact how we train future surgeons.
Linda Bradley, MD
My hope is that a partnership with industry and hysteroscopy thought leaders will enable new developments/technology in performing hysteroscopic sterilization. Conquering the tubal ostia for sterilization in an office setting would profoundly improve contraceptive options for women. Conquering the tubal ostia is the last frontier in gynecology.
Amy Garcia, MD
I predict that new technologies will allow for a significant increase in the number of gynecologists who perform in-office hysteroscopy and that a paradigm shift will occur to replace blind biopsy with hysteroscopy-directed biopsy and evaluation of the uterine cavity.
Steven Goldstein, MD, NCMP, CCD
Among the most important changes in the next 5 years, in my opinion, will be in the arenas of precision medicine, genetic advancement, and artificial intelligence. In addition, unfortunately, there will be an even greater movement toward guidelines utilizing algorithms and clinical pathways. I leave you with the following quote:
“Neither evidence nor clinical judgement alone is sufficient. Evidence without judgement can be applied by a technician. Judgement without evidence can be applied by a friend. But the integration of evidence and judgement is what the healthcare provider does in order to dispense the best clinical care.” —Hertzel Gerstein, MD
Cheryl Iglesia, MD
Technology related to minimally invasive surgery will continue to change our practice, and I predict that surgery will be more centralized to high volume practices. Reimbursements for these procedures may remain a hot button issue, however. The materials used for pelvic reconstruction will be derived from autologous stem cells and advancements made in regenerative medicine.
Andrew Kaunitz, MD, NCMP
As use of contraceptive implants and intrauterine devices continues to grow, I anticipate the incidence of unintended pregnancies will continue to decline. As the novel gonadotropin-releasing hormone (GnRH) antagonists combined with estrogen-progestin add-back grow in use, I anticipate this will provide our patients with more nonsurgical options for managing abnormal uterine bleeding, including that associated with uterine fibroids.
Barbara Levy, MD
Quality will be redefined by patient-defined outcome measures that assess what matters to the people we serve. Real-world evidence will be incorporated to support those measures and provide data on patient outcomes in populations not studied in the randomized controlled trials on which we have created guidelines. This will help to refine guidelines and support more equitable and accessible care.
David Mutch, MD
Over the next 5 years, our expanding insights into the molecular biology of cancer will lead to targeted therapies that will yield better responses with less toxicity.
Errol R. Norwitz, MD, PhD, MBA
In the near future we will use predictive AI algorithms to: 1) identify patients at risk of adverse pregnancy events; 2) stratify patients into high-, average-, and low-risk; and 3) design a personalized obstetric care journey for each patient based on their individualized risk stratification with a view to improving safety and quality outcome metrics, addressing health care disparity, and lowering the cost of care.
Jaimey Pauli, MD
I predict (and fervently hope) that breakthroughs will occur in the prevention of two of the most devastating diseases to affect obstetric patients and their families—preterm birth and preeclampsia.
JoAnn Pinkerton, MD, NCMP
New nonhormone management therapies will be available to treat hot flashes and the genitourinary syndrome of menopause. These treatments will be especially welcomed by patients who cannot or choose not to take hormone therapy. We should not allow new technology to overshadow the patient. We must remember to treat the patient with the condition, not just the disease. Consider what is important to the individual woman, her quality of life, and her ability to function, and keep that in mind when deciding what therapy to suggest.
Joseph S. Sanfilippo, MD, MBA
Artificial intelligence will change the way we educate and provide patient care. Three-dimensional perspectives will cross a number of horizons, some of which include:
- advances in assisted reproductive technology (IVF), offering the next level of “in vitro maturation” of oocytes for patients heretofore unable to conceive. They can progress to having a baby with decreased ovarian reserve or in association with “life after cancer.”
- biogenic engineering and bioinformatics will allow correction of genetic defects in embryos prior to implantation
- the surgical arena will incorporate direct robotic initiated procedures and bring robotic surgery to the next level
- with regard to medical education, at all levels, virtual reality, computer-generated 3-dimensional imaging will provide innovative tools.
James Simon, MD, CCD, IF, NCMP
Medicine’s near-term future portends the realization of truly personalized medicine based upon one’s genetic predisposition to disease, and intentional genetic manipulation to mitigate it. Such advances are here already, simply pending regulatory and ethical approval. My concern going forward is that such individualization, and an algorithm-driven decision-making process will result in taking the personal out of personalized medicine. We humans are more than the collected downstream impact of our genes. In our quest for advances, let’s not forget the balance between nature (our genes) and nurture (environment). The risk of forgetting this aphorism, like the electronic health record, gives me heartburn, or worse, burnout!
The mission of OBG
OBG
We wish all our readers a wonderful New Year and the best health possible for our patients.
Arnold P. Advincula, MD
I serve on the executive board that oversees the Fellowships in Minimally Invasive Gynecologic Surgery (FMIGS), and in January 2023 will transition into the role of President. I bring to this leadership role nearly 25 years of surgical experience, both as a clinician educator and inventor. My goal during the next 2 years will be to move toward subspecialty recognition of Complex Gynecology.
Linda D. Bradley, MD
My passion is diagnostic and operative hysteroscopy, simple procedures that can both evaluate and treat intrauterine pathology. Recently, I was thrilled to coauthor an article on office hysteroscopy for Obstetrics & Gynecology (September 2022). I will have a chapter on operative hysteroscopy in the 2023 edition of TeLinde’s Textbook of Gynecology, and I am an author for the topic Office and Operative Hysteroscopy in UpToDate. Locally, I am known as the “foodie gynecologist”—I travel, take cooking classes, and I have more cookbooks than gynecology textbooks. Since Covid, I have embraced biking and just completed a riverboat biking cruise from Salamanca, Spain, to Lisbon, Portugal.
Amy L. Garcia, MD
I am fellowship trained as a minimally invasive gynecologic surgeon (MIGS) and have had a private surgical practice since 2005. I am involved with The American College of Obstetricians and Gynecologists (ACOG), AAGL, and international surgical education for office hysteroscopy and related practice management. I am passionate about working with start-up companies in the gynecologic medical device arena and innovation in gynecologic surgery.
Steven R. Goldstein, MD, NCMP, CCD
I just completed my term as President of the International Menopause Society. This culminated in the society’s 18th World Congress in Lisbon, attended by over 1,700 health care providers from 76 countries. I delivered the Pieter van Keep Memorial Lecture, named for one of the society’s founders who died prematurely of pancreatic cancer. I was further honored by receiving the society’s Distinguished Service Award. I am very proud to have previously received the NAMS Thomas B. Clarkson award for Outstanding Clinical and Basic Science Research in Menopause. I also have one foot in the gynecologic ultrasound world and was given the Joseph H. Holmes Pioneer Award and was the 2023 recipient of the William J. Fry Memorial Lecture Award, both from the American Institute of Ultrasound in Medicine, having written the second book ever on vaginal ultrasonography.
On a personal level, I love to play golf (in spite of my foot drop and 14 orthopedic surgeries). My season tickets show some diversity—the New York City Ballet and St. John’s basketball.
Cheryl B. Iglesia, MD
I am the 49th president of the Society of Gynecologic Surgeons, the 5th woman to hold this position, and the first of Filipino-American descent. I recognize that it is only through extraordinary mentorship and support from other giants in gynecology, like Drs. Andrew Kaunitz (fellow OBG
PS—In the spirit of continually learning, I want to add the Argentine tango to my dancing repertoire and go on an African safari; both are on my bucket list as the pandemic eases.
Andrew M. Kaunitz, MD, NCMP
Since starting with the University of Florida College of Medicine-Jacksonville in 1984, I have enjoyed caring for patients, training residents and medical students, and being involved with publications and research. My areas of focus are menopause, contraception, gyn ultrasound and evaluation/management of women with abnormal uterine bleeding. In 2020, I received the North American Menopause Society/Leon Speroff Outstanding Educator Award. In 2021, I received the ACOG Distinguished Service Award. I enjoy spending time with my family, neighborhood bicycling, and searching for sharks’ teeth at the beach.
Barbara Levy, MD
I have been privileged to serve on the OBG
Continue to: David G. Mutch, MD...
David G. Mutch, MD
I am ending my 6-year term as Chair of the National Cancer Institute’s (NCI) gynecologic cancer steering committee. That is the committee that vets all NCI-sponsored clinical trials in gynecologic oncology. I am on the International Federation of Gynecology and Obstetrics (FIGO) Cancer committee, Co-Chair of the American Joint Committee on Cancer gyn staging committee and on the Reproductive Scientist Development Program selection committee. I also am completing my term as Chair of the Foundation for Women’s Cancer; this is the C3, charitable arm, of the Society of Gynecologic Oncology. We have distributed more than $3.5 million to young investigators to help start their research careers in gynecologic oncology.
Errol R. Norwitz, MD, PhD, MBA
I am a physician-scientist with subspecialty training in high-risk obstetrics (maternal-fetal medicine). I was born and raised in Cape Town, South Africa, and I have trained/practiced in 5 countries on 3 continents. My research interests include the pathophysiology, prediction, prevention, and management of pregnancy complications, primarily preterm birth and preeclampsia. I am a member of the Board of Scientific Counselors of the National Institute of Child Health and Human Development. I am currently President & CEO of Newton-Wellesley Hospital, a comprehensive community-based academic medical center and a member of the Mass General Brigham health care system in Boston, Massachusetts.
Jaimey Pauli, MD
I am the Division Chief and Professor of Maternal-Fetal Medicine (MFM) at the Penn State College of Medicine and Penn State Health Milton S. Hershey Medical Center. I had exceptional mentoring throughout my medical career, particularly by a former member of the Editorial Board, Dr. John T. Repke. One of the biggest perks of my job is that our division provides full-scope MFM care. While I often serve as the more traditional MFM consultant and academic educator, I also provide longitudinal prenatal care and deliver many of my own patients, often through subsequent pregnancies. Serving as a member of the Editorial Board combines my passion for clinical obstetrical care with my talents (as a former English major) of reading, writing, and editing. I believe that the work we do provides accessible, evidence-based, and practical guidance for our colleagues so they can provide excellence in obstetrical care.
JoAnn Pinkerton, MD, NCMP
I am a Professor of Obstetrics and Gynecology and Division Chief of Midlife Health at the University of Virginia (UVA) Health. Passionate about menopause, I am an executive director emeritus of The North American Menopause Society (NAMS) and past-President of NAMS (2008-2009). Within the past few years, I have served as an expert advisor for the recent ACOG Clinical Practice Guidelines on Osteoporosis, the NAMS Position Statements on Hormone Therapy and Osteoporosis, and the Global Consensus on Menopause and Androgen Therapy. I received the 2022 South Atlantic Association of Obstetricians and Gynecologists Lifetime Achievement Award for my expertise and work in menopause and the NAMS 2020 Ann Voda Community Service Award for my biannual community educational symposiums. I remain active in research, currently the lead and UVA principal investigator for the Oasis 2 multicenter clinical trial, which is testing a neurokinin receptor antagonist as a nonhormone therapy for the relief of hot flashes. Serving on the OBG
Joseph S. Sanfilippo, MD, MBA
I feel honored and privileged to have received the Golden Apple Teaching Award from the Universityof Pittsburgh School of Medicine. I am also fortunate to be the recipient of the Faculty Educator of the Month Award for resident teaching. I have been named Top Doctor 20 years in a row. My current academic activities include, since 2007, Program Director for Reproductive Endocrinology & Infertility Fellowship at the University of Pittsburgh and Chair of the Mentor-Mentee Program at University of Pittsburgh Department of Obstetrics, Gynecology & Reproductive Sciences. I am Guest Editor for the medical malpractice section of the journal Clinical Obstetrics and Gynecology. Recently, I completed a patient-focused book, “Experts Guide to Fertility,” which will be published in May 2023 by J Hopkins University Publisher and is designed for patients going through infertility treatment. Regarding outside events, I enjoy climbing steep hills and riding far and wide on my “electric bike.” Highly recommend it!
James Simon, MD, CCD, IF, NCMP
It’s been an honor serving on the OBG
I asked our distinguished Board of Editors to identify the most important changes that they believe will occur over the next 5 years, influencing the practice of obstetrics and gynecology. Their expert predictions are summarized below.
Arnold Advincula, MD
As one of the world’s most experienced gynecologic robotic surgeons, the role of this technology will become even more refined over the next 2-5 years with the introduction of sophisticated image guidance, “smart molecules,” and artificial intelligence. All of this will transform both the patient and surgeon experience as well as impact how we train future surgeons.
Linda Bradley, MD
My hope is that a partnership with industry and hysteroscopy thought leaders will enable new developments/technology in performing hysteroscopic sterilization. Conquering the tubal ostia for sterilization in an office setting would profoundly improve contraceptive options for women. Conquering the tubal ostia is the last frontier in gynecology.
Amy Garcia, MD
I predict that new technologies will allow for a significant increase in the number of gynecologists who perform in-office hysteroscopy and that a paradigm shift will occur to replace blind biopsy with hysteroscopy-directed biopsy and evaluation of the uterine cavity.
Steven Goldstein, MD, NCMP, CCD
Among the most important changes in the next 5 years, in my opinion, will be in the arenas of precision medicine, genetic advancement, and artificial intelligence. In addition, unfortunately, there will be an even greater movement toward guidelines utilizing algorithms and clinical pathways. I leave you with the following quote:
“Neither evidence nor clinical judgement alone is sufficient. Evidence without judgement can be applied by a technician. Judgement without evidence can be applied by a friend. But the integration of evidence and judgement is what the healthcare provider does in order to dispense the best clinical care.” —Hertzel Gerstein, MD
Cheryl Iglesia, MD
Technology related to minimally invasive surgery will continue to change our practice, and I predict that surgery will be more centralized to high volume practices. Reimbursements for these procedures may remain a hot button issue, however. The materials used for pelvic reconstruction will be derived from autologous stem cells and advancements made in regenerative medicine.
Andrew Kaunitz, MD, NCMP
As use of contraceptive implants and intrauterine devices continues to grow, I anticipate the incidence of unintended pregnancies will continue to decline. As the novel gonadotropin-releasing hormone (GnRH) antagonists combined with estrogen-progestin add-back grow in use, I anticipate this will provide our patients with more nonsurgical options for managing abnormal uterine bleeding, including that associated with uterine fibroids.
Barbara Levy, MD
Quality will be redefined by patient-defined outcome measures that assess what matters to the people we serve. Real-world evidence will be incorporated to support those measures and provide data on patient outcomes in populations not studied in the randomized controlled trials on which we have created guidelines. This will help to refine guidelines and support more equitable and accessible care.
David Mutch, MD
Over the next 5 years, our expanding insights into the molecular biology of cancer will lead to targeted therapies that will yield better responses with less toxicity.
Errol R. Norwitz, MD, PhD, MBA
In the near future we will use predictive AI algorithms to: 1) identify patients at risk of adverse pregnancy events; 2) stratify patients into high-, average-, and low-risk; and 3) design a personalized obstetric care journey for each patient based on their individualized risk stratification with a view to improving safety and quality outcome metrics, addressing health care disparity, and lowering the cost of care.
Jaimey Pauli, MD
I predict (and fervently hope) that breakthroughs will occur in the prevention of two of the most devastating diseases to affect obstetric patients and their families—preterm birth and preeclampsia.
JoAnn Pinkerton, MD, NCMP
New nonhormone management therapies will be available to treat hot flashes and the genitourinary syndrome of menopause. These treatments will be especially welcomed by patients who cannot or choose not to take hormone therapy. We should not allow new technology to overshadow the patient. We must remember to treat the patient with the condition, not just the disease. Consider what is important to the individual woman, her quality of life, and her ability to function, and keep that in mind when deciding what therapy to suggest.
Joseph S. Sanfilippo, MD, MBA
Artificial intelligence will change the way we educate and provide patient care. Three-dimensional perspectives will cross a number of horizons, some of which include:
- advances in assisted reproductive technology (IVF), offering the next level of “in vitro maturation” of oocytes for patients heretofore unable to conceive. They can progress to having a baby with decreased ovarian reserve or in association with “life after cancer.”
- biogenic engineering and bioinformatics will allow correction of genetic defects in embryos prior to implantation
- the surgical arena will incorporate direct robotic initiated procedures and bring robotic surgery to the next level
- with regard to medical education, at all levels, virtual reality, computer-generated 3-dimensional imaging will provide innovative tools.
James Simon, MD, CCD, IF, NCMP
Medicine’s near-term future portends the realization of truly personalized medicine based upon one’s genetic predisposition to disease, and intentional genetic manipulation to mitigate it. Such advances are here already, simply pending regulatory and ethical approval. My concern going forward is that such individualization, and an algorithm-driven decision-making process will result in taking the personal out of personalized medicine. We humans are more than the collected downstream impact of our genes. In our quest for advances, let’s not forget the balance between nature (our genes) and nurture (environment). The risk of forgetting this aphorism, like the electronic health record, gives me heartburn, or worse, burnout!
The mission of OBG
OBG
We wish all our readers a wonderful New Year and the best health possible for our patients.
Arnold P. Advincula, MD
I serve on the executive board that oversees the Fellowships in Minimally Invasive Gynecologic Surgery (FMIGS), and in January 2023 will transition into the role of President. I bring to this leadership role nearly 25 years of surgical experience, both as a clinician educator and inventor. My goal during the next 2 years will be to move toward subspecialty recognition of Complex Gynecology.
Linda D. Bradley, MD
My passion is diagnostic and operative hysteroscopy, simple procedures that can both evaluate and treat intrauterine pathology. Recently, I was thrilled to coauthor an article on office hysteroscopy for Obstetrics & Gynecology (September 2022). I will have a chapter on operative hysteroscopy in the 2023 edition of TeLinde’s Textbook of Gynecology, and I am an author for the topic Office and Operative Hysteroscopy in UpToDate. Locally, I am known as the “foodie gynecologist”—I travel, take cooking classes, and I have more cookbooks than gynecology textbooks. Since Covid, I have embraced biking and just completed a riverboat biking cruise from Salamanca, Spain, to Lisbon, Portugal.
Amy L. Garcia, MD
I am fellowship trained as a minimally invasive gynecologic surgeon (MIGS) and have had a private surgical practice since 2005. I am involved with The American College of Obstetricians and Gynecologists (ACOG), AAGL, and international surgical education for office hysteroscopy and related practice management. I am passionate about working with start-up companies in the gynecologic medical device arena and innovation in gynecologic surgery.
Steven R. Goldstein, MD, NCMP, CCD
I just completed my term as President of the International Menopause Society. This culminated in the society’s 18th World Congress in Lisbon, attended by over 1,700 health care providers from 76 countries. I delivered the Pieter van Keep Memorial Lecture, named for one of the society’s founders who died prematurely of pancreatic cancer. I was further honored by receiving the society’s Distinguished Service Award. I am very proud to have previously received the NAMS Thomas B. Clarkson award for Outstanding Clinical and Basic Science Research in Menopause. I also have one foot in the gynecologic ultrasound world and was given the Joseph H. Holmes Pioneer Award and was the 2023 recipient of the William J. Fry Memorial Lecture Award, both from the American Institute of Ultrasound in Medicine, having written the second book ever on vaginal ultrasonography.
On a personal level, I love to play golf (in spite of my foot drop and 14 orthopedic surgeries). My season tickets show some diversity—the New York City Ballet and St. John’s basketball.
Cheryl B. Iglesia, MD
I am the 49th president of the Society of Gynecologic Surgeons, the 5th woman to hold this position, and the first of Filipino-American descent. I recognize that it is only through extraordinary mentorship and support from other giants in gynecology, like Drs. Andrew Kaunitz (fellow OBG
PS—In the spirit of continually learning, I want to add the Argentine tango to my dancing repertoire and go on an African safari; both are on my bucket list as the pandemic eases.
Andrew M. Kaunitz, MD, NCMP
Since starting with the University of Florida College of Medicine-Jacksonville in 1984, I have enjoyed caring for patients, training residents and medical students, and being involved with publications and research. My areas of focus are menopause, contraception, gyn ultrasound and evaluation/management of women with abnormal uterine bleeding. In 2020, I received the North American Menopause Society/Leon Speroff Outstanding Educator Award. In 2021, I received the ACOG Distinguished Service Award. I enjoy spending time with my family, neighborhood bicycling, and searching for sharks’ teeth at the beach.
Barbara Levy, MD
I have been privileged to serve on the OBG
Continue to: David G. Mutch, MD...
David G. Mutch, MD
I am ending my 6-year term as Chair of the National Cancer Institute’s (NCI) gynecologic cancer steering committee. That is the committee that vets all NCI-sponsored clinical trials in gynecologic oncology. I am on the International Federation of Gynecology and Obstetrics (FIGO) Cancer committee, Co-Chair of the American Joint Committee on Cancer gyn staging committee and on the Reproductive Scientist Development Program selection committee. I also am completing my term as Chair of the Foundation for Women’s Cancer; this is the C3, charitable arm, of the Society of Gynecologic Oncology. We have distributed more than $3.5 million to young investigators to help start their research careers in gynecologic oncology.
Errol R. Norwitz, MD, PhD, MBA
I am a physician-scientist with subspecialty training in high-risk obstetrics (maternal-fetal medicine). I was born and raised in Cape Town, South Africa, and I have trained/practiced in 5 countries on 3 continents. My research interests include the pathophysiology, prediction, prevention, and management of pregnancy complications, primarily preterm birth and preeclampsia. I am a member of the Board of Scientific Counselors of the National Institute of Child Health and Human Development. I am currently President & CEO of Newton-Wellesley Hospital, a comprehensive community-based academic medical center and a member of the Mass General Brigham health care system in Boston, Massachusetts.
Jaimey Pauli, MD
I am the Division Chief and Professor of Maternal-Fetal Medicine (MFM) at the Penn State College of Medicine and Penn State Health Milton S. Hershey Medical Center. I had exceptional mentoring throughout my medical career, particularly by a former member of the Editorial Board, Dr. John T. Repke. One of the biggest perks of my job is that our division provides full-scope MFM care. While I often serve as the more traditional MFM consultant and academic educator, I also provide longitudinal prenatal care and deliver many of my own patients, often through subsequent pregnancies. Serving as a member of the Editorial Board combines my passion for clinical obstetrical care with my talents (as a former English major) of reading, writing, and editing. I believe that the work we do provides accessible, evidence-based, and practical guidance for our colleagues so they can provide excellence in obstetrical care.
JoAnn Pinkerton, MD, NCMP
I am a Professor of Obstetrics and Gynecology and Division Chief of Midlife Health at the University of Virginia (UVA) Health. Passionate about menopause, I am an executive director emeritus of The North American Menopause Society (NAMS) and past-President of NAMS (2008-2009). Within the past few years, I have served as an expert advisor for the recent ACOG Clinical Practice Guidelines on Osteoporosis, the NAMS Position Statements on Hormone Therapy and Osteoporosis, and the Global Consensus on Menopause and Androgen Therapy. I received the 2022 South Atlantic Association of Obstetricians and Gynecologists Lifetime Achievement Award for my expertise and work in menopause and the NAMS 2020 Ann Voda Community Service Award for my biannual community educational symposiums. I remain active in research, currently the lead and UVA principal investigator for the Oasis 2 multicenter clinical trial, which is testing a neurokinin receptor antagonist as a nonhormone therapy for the relief of hot flashes. Serving on the OBG
Joseph S. Sanfilippo, MD, MBA
I feel honored and privileged to have received the Golden Apple Teaching Award from the Universityof Pittsburgh School of Medicine. I am also fortunate to be the recipient of the Faculty Educator of the Month Award for resident teaching. I have been named Top Doctor 20 years in a row. My current academic activities include, since 2007, Program Director for Reproductive Endocrinology & Infertility Fellowship at the University of Pittsburgh and Chair of the Mentor-Mentee Program at University of Pittsburgh Department of Obstetrics, Gynecology & Reproductive Sciences. I am Guest Editor for the medical malpractice section of the journal Clinical Obstetrics and Gynecology. Recently, I completed a patient-focused book, “Experts Guide to Fertility,” which will be published in May 2023 by J Hopkins University Publisher and is designed for patients going through infertility treatment. Regarding outside events, I enjoy climbing steep hills and riding far and wide on my “electric bike.” Highly recommend it!
James Simon, MD, CCD, IF, NCMP
It’s been an honor serving on the OBG
I asked our distinguished Board of Editors to identify the most important changes that they believe will occur over the next 5 years, influencing the practice of obstetrics and gynecology. Their expert predictions are summarized below.
Arnold Advincula, MD
As one of the world’s most experienced gynecologic robotic surgeons, the role of this technology will become even more refined over the next 2-5 years with the introduction of sophisticated image guidance, “smart molecules,” and artificial intelligence. All of this will transform both the patient and surgeon experience as well as impact how we train future surgeons.
Linda Bradley, MD
My hope is that a partnership with industry and hysteroscopy thought leaders will enable new developments/technology in performing hysteroscopic sterilization. Conquering the tubal ostia for sterilization in an office setting would profoundly improve contraceptive options for women. Conquering the tubal ostia is the last frontier in gynecology.
Amy Garcia, MD
I predict that new technologies will allow for a significant increase in the number of gynecologists who perform in-office hysteroscopy and that a paradigm shift will occur to replace blind biopsy with hysteroscopy-directed biopsy and evaluation of the uterine cavity.
Steven Goldstein, MD, NCMP, CCD
Among the most important changes in the next 5 years, in my opinion, will be in the arenas of precision medicine, genetic advancement, and artificial intelligence. In addition, unfortunately, there will be an even greater movement toward guidelines utilizing algorithms and clinical pathways. I leave you with the following quote:
“Neither evidence nor clinical judgement alone is sufficient. Evidence without judgement can be applied by a technician. Judgement without evidence can be applied by a friend. But the integration of evidence and judgement is what the healthcare provider does in order to dispense the best clinical care.” —Hertzel Gerstein, MD
Cheryl Iglesia, MD
Technology related to minimally invasive surgery will continue to change our practice, and I predict that surgery will be more centralized to high volume practices. Reimbursements for these procedures may remain a hot button issue, however. The materials used for pelvic reconstruction will be derived from autologous stem cells and advancements made in regenerative medicine.
Andrew Kaunitz, MD, NCMP
As use of contraceptive implants and intrauterine devices continues to grow, I anticipate the incidence of unintended pregnancies will continue to decline. As the novel gonadotropin-releasing hormone (GnRH) antagonists combined with estrogen-progestin add-back grow in use, I anticipate this will provide our patients with more nonsurgical options for managing abnormal uterine bleeding, including that associated with uterine fibroids.
Barbara Levy, MD
Quality will be redefined by patient-defined outcome measures that assess what matters to the people we serve. Real-world evidence will be incorporated to support those measures and provide data on patient outcomes in populations not studied in the randomized controlled trials on which we have created guidelines. This will help to refine guidelines and support more equitable and accessible care.
David Mutch, MD
Over the next 5 years, our expanding insights into the molecular biology of cancer will lead to targeted therapies that will yield better responses with less toxicity.
Errol R. Norwitz, MD, PhD, MBA
In the near future we will use predictive AI algorithms to: 1) identify patients at risk of adverse pregnancy events; 2) stratify patients into high-, average-, and low-risk; and 3) design a personalized obstetric care journey for each patient based on their individualized risk stratification with a view to improving safety and quality outcome metrics, addressing health care disparity, and lowering the cost of care.
Jaimey Pauli, MD
I predict (and fervently hope) that breakthroughs will occur in the prevention of two of the most devastating diseases to affect obstetric patients and their families—preterm birth and preeclampsia.
JoAnn Pinkerton, MD, NCMP
New nonhormone management therapies will be available to treat hot flashes and the genitourinary syndrome of menopause. These treatments will be especially welcomed by patients who cannot or choose not to take hormone therapy. We should not allow new technology to overshadow the patient. We must remember to treat the patient with the condition, not just the disease. Consider what is important to the individual woman, her quality of life, and her ability to function, and keep that in mind when deciding what therapy to suggest.
Joseph S. Sanfilippo, MD, MBA
Artificial intelligence will change the way we educate and provide patient care. Three-dimensional perspectives will cross a number of horizons, some of which include:
- advances in assisted reproductive technology (IVF), offering the next level of “in vitro maturation” of oocytes for patients heretofore unable to conceive. They can progress to having a baby with decreased ovarian reserve or in association with “life after cancer.”
- biogenic engineering and bioinformatics will allow correction of genetic defects in embryos prior to implantation
- the surgical arena will incorporate direct robotic initiated procedures and bring robotic surgery to the next level
- with regard to medical education, at all levels, virtual reality, computer-generated 3-dimensional imaging will provide innovative tools.
James Simon, MD, CCD, IF, NCMP
Medicine’s near-term future portends the realization of truly personalized medicine based upon one’s genetic predisposition to disease, and intentional genetic manipulation to mitigate it. Such advances are here already, simply pending regulatory and ethical approval. My concern going forward is that such individualization, and an algorithm-driven decision-making process will result in taking the personal out of personalized medicine. We humans are more than the collected downstream impact of our genes. In our quest for advances, let’s not forget the balance between nature (our genes) and nurture (environment). The risk of forgetting this aphorism, like the electronic health record, gives me heartburn, or worse, burnout!
From debate to stalemate and hate: An epidemic of intellectual constipation
Groupthink is hazardous, especially when perfused with religious fervor. It can lead to adopting irrational thinking1 and aversion to new ideas or facts. Tenaciously clinging to 1 ideology as “the absolute truth” precludes an open-minded, constructive debate with any other point of view.
Three historical examples come to mind:
- The discovery of chlorpromazine in 1952 was a scientifically and clinically seismic and transformational event for the treatment of psychosis, which for centuries had been dogmatically deemed irreversible. Jean Delay, MD, the French psychiatrist and co-discoverer of chlorpromazine, was the first physician to witness the magical and dazzling dissolution of delusions and hallucinations in chronically institutionalized patients with psychosis.2 He published his landmark clinical observations and then traveled to the United States to share the great news and present his findings at a large psychiatric conference, hoping to enthrall American psychiatrists with the historic breakthrough in treating psychosis. This was an era in which psychoanalysis dominated American psychiatry (despite its dearth of empirical evidence). Dr. Delay was shocked when the audience of psychoanalysts booed him for saying that psychosis can be treated with a medication instead of with psychoanalysis (which, in the most intense groupthink in the history of psychiatry, they all believed was the only therapy for psychosis). Deeply disheartened, Dr. Delay returned to France and never returned to the United States. This groupthink was a prime example of intellectual constipation. Since then, not surprisingly, psychopharmacology grew meteorically while psychoanalysis declined precipitously.
- The monoamine hypothesis of depression, first propagated 60 years ago, became a groupthink dogma among psychiatric researchers for the next several decades, stultifying broader antidepressant medication development by focusing only on monoamines (eg, serotonin, norepinephrine, and dopamine). More recently, researchers have become more open-minded, and the monoamine hypothesis has taken a backseat to innovative new models of antidepressant therapy based on advances in the pathophysiology of depression, such as glutamatergic, opioid, and sigma pathways as well as neuroplasticity models.3 The consequence of groupthink in antidepressant research was a half-century delay in the development of effective alternative treatments that could have helped millions of patients recover from a life-threatening brain disorder such as major depressive disorder.
- Peptic ulcer and its serious gastritis were long believed to be due to stress and increased stomach acidity. So the groupthink gastroenterologists mocked 2 Australian researchers, Barry Marshall and Robin Warren, when they proposed that peptic ulcer may be due to an infection with a bacterium called Helicobacter pylori, and published their data demonstrating it.4 Marshall and Warren had the last laugh when they were awarded the 2005 Nobel Prize in Medicine and Physiology. It is ironic that even gastroenterologists are not immune to the affliction of intellectual constipation!
Intellectual constipation’s effects on youth
The principle of a civilized debate of contrarian ideas must be inculcated early, especially during college years. Youth should be mentored about not cowering into an ideological cocoon and shun listening to different or opposing points of view.5 Institutions of higher learning are incubators of future leaders. They must provide their young students with a wide diversity of ideas and philosophies and encourage them to critique those ideas, not “shelter” or isolate them from any ideas. Youth need to recognize that the complex societies in which we all live and work are not placid or unidimensional but a hotbed of clashing ideas and perspectives. An open-minded approach to education will inoculate young minds from developing intellectual constipation in adulthood.
Avoiding or insulating oneself from the ideas of others—no matter how disagreeable—leads to cognitive cowardice and behavioral intolerance. Healthy and vibrant debate is necessary as an inoculation against extremism, hate, paranoia, and, ultimately, violence. Psychiatrists help patients to self-reflect, gain insight, and consider changing their view of themselves and the world to help them grow into mature and resilient individuals. But for the millions of people with intellectual constipation, a potent cerebral enema comprised of a salubrious concoction of insight, common sense, and compromise may be the prescription to forestall lethal intellectual ileus.
1. Nasrallah HA. Irrational beliefs: a ubiquitous human trait. Current Psychiatry. 2007;6(2):15-16.
2. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
3. Boku S, Nakagawa S, Toda H, et al. Neural basis of major depressive disorder: beyond monoamine hypothesis. Psychiatry Clin Neurosci. 2018;72(1):3-12.
4. Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1(8336):1273-1275.
5. Lukianoff G, Haidt J. The Coddling of the American Mind: How Good Intentions and Bad Ideas Are Setting Up a Generation for Failure. Penguin Books; 2018.
Groupthink is hazardous, especially when perfused with religious fervor. It can lead to adopting irrational thinking1 and aversion to new ideas or facts. Tenaciously clinging to 1 ideology as “the absolute truth” precludes an open-minded, constructive debate with any other point of view.
Three historical examples come to mind:
- The discovery of chlorpromazine in 1952 was a scientifically and clinically seismic and transformational event for the treatment of psychosis, which for centuries had been dogmatically deemed irreversible. Jean Delay, MD, the French psychiatrist and co-discoverer of chlorpromazine, was the first physician to witness the magical and dazzling dissolution of delusions and hallucinations in chronically institutionalized patients with psychosis.2 He published his landmark clinical observations and then traveled to the United States to share the great news and present his findings at a large psychiatric conference, hoping to enthrall American psychiatrists with the historic breakthrough in treating psychosis. This was an era in which psychoanalysis dominated American psychiatry (despite its dearth of empirical evidence). Dr. Delay was shocked when the audience of psychoanalysts booed him for saying that psychosis can be treated with a medication instead of with psychoanalysis (which, in the most intense groupthink in the history of psychiatry, they all believed was the only therapy for psychosis). Deeply disheartened, Dr. Delay returned to France and never returned to the United States. This groupthink was a prime example of intellectual constipation. Since then, not surprisingly, psychopharmacology grew meteorically while psychoanalysis declined precipitously.
- The monoamine hypothesis of depression, first propagated 60 years ago, became a groupthink dogma among psychiatric researchers for the next several decades, stultifying broader antidepressant medication development by focusing only on monoamines (eg, serotonin, norepinephrine, and dopamine). More recently, researchers have become more open-minded, and the monoamine hypothesis has taken a backseat to innovative new models of antidepressant therapy based on advances in the pathophysiology of depression, such as glutamatergic, opioid, and sigma pathways as well as neuroplasticity models.3 The consequence of groupthink in antidepressant research was a half-century delay in the development of effective alternative treatments that could have helped millions of patients recover from a life-threatening brain disorder such as major depressive disorder.
- Peptic ulcer and its serious gastritis were long believed to be due to stress and increased stomach acidity. So the groupthink gastroenterologists mocked 2 Australian researchers, Barry Marshall and Robin Warren, when they proposed that peptic ulcer may be due to an infection with a bacterium called Helicobacter pylori, and published their data demonstrating it.4 Marshall and Warren had the last laugh when they were awarded the 2005 Nobel Prize in Medicine and Physiology. It is ironic that even gastroenterologists are not immune to the affliction of intellectual constipation!
Intellectual constipation’s effects on youth
The principle of a civilized debate of contrarian ideas must be inculcated early, especially during college years. Youth should be mentored about not cowering into an ideological cocoon and shun listening to different or opposing points of view.5 Institutions of higher learning are incubators of future leaders. They must provide their young students with a wide diversity of ideas and philosophies and encourage them to critique those ideas, not “shelter” or isolate them from any ideas. Youth need to recognize that the complex societies in which we all live and work are not placid or unidimensional but a hotbed of clashing ideas and perspectives. An open-minded approach to education will inoculate young minds from developing intellectual constipation in adulthood.
Avoiding or insulating oneself from the ideas of others—no matter how disagreeable—leads to cognitive cowardice and behavioral intolerance. Healthy and vibrant debate is necessary as an inoculation against extremism, hate, paranoia, and, ultimately, violence. Psychiatrists help patients to self-reflect, gain insight, and consider changing their view of themselves and the world to help them grow into mature and resilient individuals. But for the millions of people with intellectual constipation, a potent cerebral enema comprised of a salubrious concoction of insight, common sense, and compromise may be the prescription to forestall lethal intellectual ileus.
Groupthink is hazardous, especially when perfused with religious fervor. It can lead to adopting irrational thinking1 and aversion to new ideas or facts. Tenaciously clinging to 1 ideology as “the absolute truth” precludes an open-minded, constructive debate with any other point of view.
Three historical examples come to mind:
- The discovery of chlorpromazine in 1952 was a scientifically and clinically seismic and transformational event for the treatment of psychosis, which for centuries had been dogmatically deemed irreversible. Jean Delay, MD, the French psychiatrist and co-discoverer of chlorpromazine, was the first physician to witness the magical and dazzling dissolution of delusions and hallucinations in chronically institutionalized patients with psychosis.2 He published his landmark clinical observations and then traveled to the United States to share the great news and present his findings at a large psychiatric conference, hoping to enthrall American psychiatrists with the historic breakthrough in treating psychosis. This was an era in which psychoanalysis dominated American psychiatry (despite its dearth of empirical evidence). Dr. Delay was shocked when the audience of psychoanalysts booed him for saying that psychosis can be treated with a medication instead of with psychoanalysis (which, in the most intense groupthink in the history of psychiatry, they all believed was the only therapy for psychosis). Deeply disheartened, Dr. Delay returned to France and never returned to the United States. This groupthink was a prime example of intellectual constipation. Since then, not surprisingly, psychopharmacology grew meteorically while psychoanalysis declined precipitously.
- The monoamine hypothesis of depression, first propagated 60 years ago, became a groupthink dogma among psychiatric researchers for the next several decades, stultifying broader antidepressant medication development by focusing only on monoamines (eg, serotonin, norepinephrine, and dopamine). More recently, researchers have become more open-minded, and the monoamine hypothesis has taken a backseat to innovative new models of antidepressant therapy based on advances in the pathophysiology of depression, such as glutamatergic, opioid, and sigma pathways as well as neuroplasticity models.3 The consequence of groupthink in antidepressant research was a half-century delay in the development of effective alternative treatments that could have helped millions of patients recover from a life-threatening brain disorder such as major depressive disorder.
- Peptic ulcer and its serious gastritis were long believed to be due to stress and increased stomach acidity. So the groupthink gastroenterologists mocked 2 Australian researchers, Barry Marshall and Robin Warren, when they proposed that peptic ulcer may be due to an infection with a bacterium called Helicobacter pylori, and published their data demonstrating it.4 Marshall and Warren had the last laugh when they were awarded the 2005 Nobel Prize in Medicine and Physiology. It is ironic that even gastroenterologists are not immune to the affliction of intellectual constipation!
Intellectual constipation’s effects on youth
The principle of a civilized debate of contrarian ideas must be inculcated early, especially during college years. Youth should be mentored about not cowering into an ideological cocoon and shun listening to different or opposing points of view.5 Institutions of higher learning are incubators of future leaders. They must provide their young students with a wide diversity of ideas and philosophies and encourage them to critique those ideas, not “shelter” or isolate them from any ideas. Youth need to recognize that the complex societies in which we all live and work are not placid or unidimensional but a hotbed of clashing ideas and perspectives. An open-minded approach to education will inoculate young minds from developing intellectual constipation in adulthood.
Avoiding or insulating oneself from the ideas of others—no matter how disagreeable—leads to cognitive cowardice and behavioral intolerance. Healthy and vibrant debate is necessary as an inoculation against extremism, hate, paranoia, and, ultimately, violence. Psychiatrists help patients to self-reflect, gain insight, and consider changing their view of themselves and the world to help them grow into mature and resilient individuals. But for the millions of people with intellectual constipation, a potent cerebral enema comprised of a salubrious concoction of insight, common sense, and compromise may be the prescription to forestall lethal intellectual ileus.
1. Nasrallah HA. Irrational beliefs: a ubiquitous human trait. Current Psychiatry. 2007;6(2):15-16.
2. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
3. Boku S, Nakagawa S, Toda H, et al. Neural basis of major depressive disorder: beyond monoamine hypothesis. Psychiatry Clin Neurosci. 2018;72(1):3-12.
4. Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1(8336):1273-1275.
5. Lukianoff G, Haidt J. The Coddling of the American Mind: How Good Intentions and Bad Ideas Are Setting Up a Generation for Failure. Penguin Books; 2018.
1. Nasrallah HA. Irrational beliefs: a ubiquitous human trait. Current Psychiatry. 2007;6(2):15-16.
2. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
3. Boku S, Nakagawa S, Toda H, et al. Neural basis of major depressive disorder: beyond monoamine hypothesis. Psychiatry Clin Neurosci. 2018;72(1):3-12.
4. Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1(8336):1273-1275.
5. Lukianoff G, Haidt J. The Coddling of the American Mind: How Good Intentions and Bad Ideas Are Setting Up a Generation for Failure. Penguin Books; 2018.
Simplify your approach to the diagnosis and treatment of PCOS
PCOS is a common problem, with a prevalence of 6% to 10% among women of reproductive age.1 Patients with PCOS often present with hirsutism, acne, female androgenetic alopecia, oligomenorrhea (also known as infrequent menstrual bleeding), amenorrhea, infertility, overweight, or obesity. In addition, many patients with PCOS have insulin resistance, dyslipidemia, metabolic syndrome, and an increased risk for developing type 2 diabetes mellitus (DM).2 A simplified approach to the diagnosis of PCOS will save health care resources by reducing the use of low-value diagnostic tests. A simplified approach to the treatment of PCOS will support patient medication adherence and improve health outcomes.
Simplify the diagnosis of PCOS
Simplify PCOS diagnosis by focusing on the core criteria of hyperandrogenism and oligo-ovulation. There are 3 major approaches to diagnosis:
- the 1990 National Institutes of Health (NIH) criteria3
- the 2003 Rotterdam criteria4,5
- the 2008 Androgen Excess and PCOS Society (AES) criteria.6
Using the 1990 NIH approach, the diagnosis of PCOS is made by the presence of 2 core criteria: hyperandrogenism and oligo-ovulation, typically manifested as oligomenorrhea. In addition, other causes of hyperandrogenism should be excluded, including nonclassical adrenal hyperplasia (NCAH) due to 21-hydroxylase deficiency.3 Using the 1990 NIH criteria, PCOS can be diagnosed based on history (oligomenorrhea) and physical examination (assessment of the severity of hirsutism), but laboratory tests including total testosterone are often ordered.7
The Rotterdam approach to the diagnosis added a third criteria, the detection by ultrasonography of a multifollicular ovary and/or increased ovarian volume.4,5 Using the Rotterdam approach, PCOS is diagnosed in the presence of any 2 of the following 3 criteria: hyperandrogenism, oligo-ovulation, or ultrasound imaging showing the presence of a multifollicular ovary, identified by ≥ 12 antral follicles (2 to 9 mm in diameter) in each ovary or increased ovarian volume (> 10 mL).4,5
The Rotterdam approach using ovarian ultrasound as a criterion to diagnose PCOS is rife with serious problems, including:
- The number of small antral follicles in the normal ovary is age dependent, and many ovulatory and nonhirsute patients have ≥ 12 small antral follicles in each ovary.8,9
- There is no consensus on the number of small antral follicles needed to diagnose a multifollicular ovary, with recommendations to use thresholds of 124,5 or 20 follicles10 as the diagnostic cut-off.
- Accurate counting of the number of small ovarian follicles requires transvaginal ultrasound, which is not appropriate for many young adolescent patients.
- The process of counting ovarian follicles is operator-dependent.
- The high cost of ultrasound assessment of ovarian follicles (≥ $500 per examination).
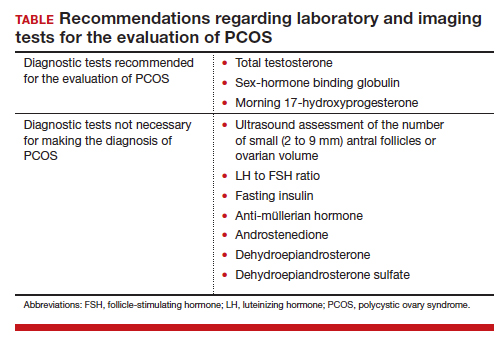
The Rotterdam approach supports the diagnosis of PCOS in a patient with oligo-ovulation plus an ultrasound showing a multifollicular ovary in the absence of any clinical or laboratory evidence of hyperandrogenism.3,4,5 This approach to the diagnosis of PCOS is rejected by both the 1990 NIH3 and AES6 recommendations, which require the presence of hyperandrogenism as the sine qua non in the diagnosis of PCOS. I recommend against diagnosing PCOS in a non-hyperandrogenic patient with oligo-ovulation and a multifollicular ovary because other diagnoses are also possible, such as functional hypothalamic oligo-ovulation, especially in young patients. The Rotterdam approach also supports the diagnosis of PCOS in a patient with hyperandrogenism, an ultrasound showing a multifollicular ovary, and normal ovulation and menses.3,4 For most patients with normal, regular ovulation and menses, the testosterone concentration is normal and the only evidence of hyperandrogenism is hirsutism. Patients with normal, regular ovulation and menses plus hirsutism usually have idiopathic hirsutism. Idiopathic hirsutism is a problem caused by excessive 5-alpha-reductase activity in the hair pilosebaceous unit, which catalyzes the conversion of weak androgens into dihydrotestosterone, a potent intracellular androgen that stimulates terminal hair growth.11 In my opinion, the Rotterdam approach to diagnosing PCOS has created unnecessary confusion and complexity for both clinicians and patients. I believe we should simplify the diagnosis of PCOS and return to the 1990 NIH criteria.3
On occasion, a patient presents for a consultation and has already had an ovarian ultrasound to assess for a multifollicular ovary. I carefully read the report and, if a multifollicular ovary has been identified, I consider it as a secondary supporting finding of PCOS in my clinical assessment. But I do not base my diagnosis on the ultrasound finding. Patients often present with other laboratory tests that are secondary supporting findings of PCOS, which I carefully consider but do not use to make a diagnosis of PCOS. Secondary supporting laboratory findings consistent with PCOS include: 1) a markedly elevated anti-müllerian hormone (AMH) level,12 2) an elevated fasting insulin level,2,13 and 3) an elevated luteinizing hormone (LH) to follicle-stimulating hormone (FSH) ratio.13,14 But it is not necessary to measure AMH, fasting insulin, LH, and FSH levels. To conserve health care resources, I recommend against measuring those analytes to diagnose PCOS.
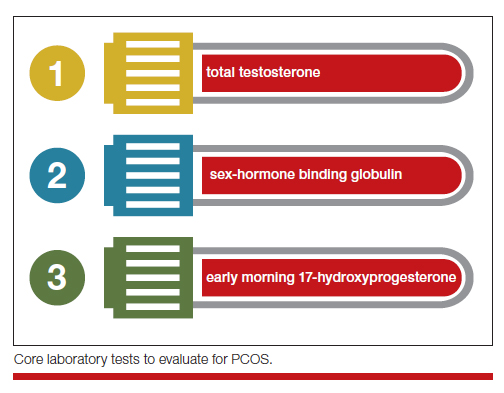
Continue to: Simplify the core laboratory tests...
Simplify the core laboratory tests
Simplify the testing used to support the diagnosis of PCOS by measuring total testosterone, sex-hormone binding globulin (SHBG) and early morning 17-hydroxyprogesterone (17-OH Prog).
The core criteria for diagnosis of PCOS are hyperandrogenism and oligo-ovulation, typically manifested as oligomenorrhea or amenorrhea. Hyperandrogenism can be clinically diagnosed by assessing for the presence of hirsutism.7 Elevated levels of total testosterone, free testosterone, androstenedione, and/or dehydroepiandrosterone sulfate (DHEAS) suggest the presence of hyperandrogenism. In clinical practice, the laboratory approach to the diagnosis of hyperandrogenism can be simplified to the measurement of total testosterone, SHBG, and 17-OH Prog. By measuring total testosterone and SHBG, an estimate of free testosterone can be made. If the total testosterone is elevated, it is highly likely that the free testosterone is elevated. If the SHBG is abnormally low and the total testosterone level is in the upper limit of the normal range, the free testosterone is likely to be elevated.15 Using this approach, either an elevated total testosterone or an abnormally low SHBG indicate elevated free testosterone. For patients with hyperandrogenism and oligo-ovulation, an early morning (8 to 9 AM) 17-OH Prog level ≤ 2 ng/mL rules out the presence of NCAH due to a 21-hydroxylase deficiency.16 In my practice, the core laboratory tests I order when considering the diagnosis of PCOS are a total testosterone, SHBG, and 17-OH Prog.
Additional laboratory tests may be warranted to assess the patient diagnosed with PCOS. For example, if the patient has amenorrhea due to anovulation, tests for prolactin, FSH, and thyroid-stimulating hormone levels are warranted to assess for the presence of a prolactinoma, primary ovarian insufficiency, or thyroid disease, respectively. If the patient has a body mass index (BMI) ≥ 25 kg/m2, a hemoglobin A1c concentration is warranted to assess for the presence of prediabetes or DM.2 Many patients with PCOS have dyslipidemia, manifested through low high-density lipoprotein cholesterol levels and elevated low-density lipoprotein cholesterol levels, and a lipid panel assessment may be indicated. Among patients with PCOS, the most common lipid abnormality is a low high-density lipoprotein cholesterol level.17
Simplify the treatment of PCOS
Simplify treatment by counseling about lifestyle changes and prescribing an estrogen-progestin contraceptive, spironolactone, and metformin.
Most patients with PCOS have dysfunction in reproductive, metabolic, and dermatologic systems. For patients who are overweight or obese, lifestyle changes, including diet and exercise, that result in a 5% to 10% decrease in weight can improve metabolic balance, reduce circulating androgens, and increase menstrual frequency.18 For patients with PCOS and weight issues, referral to nutrition counseling or a full-service weight loss program can be very beneficial. In addition to lifestyle changes, patients with PCOS benefit from treatment with estrogen-progestin medications, spironolactone, and metformin.
Combination estrogen-progestin medications will lower LH secretion, decrease ovarian androgen production, increase SHBG production, decrease free testosterone levels and, if given cyclically, cause regular withdrawal bleeding.19 Spironolactone is an antiandrogen, which blocks the intracellular action of dihydrotestosterone and improves hirsutism and acne. Spironolactone also modestly decreases circulating levels of testosterone and DHEAS.20 For patients with metabolic problems, including insulin resistance and obesity, weight loss and/or treatment with metformin can help improve metabolic balance, which may result in restoration of ovulatory menses.21,22 Metformin can be effective in restoring ovulatory menses in both obese and lean patients with PCOS.22 The most common dermatologic problem caused by PCOS are hirsutism and acne. Both combination estrogen-progestin medications and spironolactone are effective treatments for hirsutism and acne.23
Estrogen-progestin hormones, spironolactone, and metformin are low-cost medications for the treatment of PCOS. Additional high-cost options for treatment of PCOS in obese patients include bariatric surgery and glucagon-like peptide (GLP-1) agonist medications (liraglutide and exenatide). For patients with PCOS and a body mass index (BMI) ≥ 35 kg/m2, bariatric surgery often results in sufficient weight loss to resolve the patient’s hyperandrogenism and oligo-ovulation, restoring spontaneous ovulatory cycles.24 In a study of more than 1,000 patients with: PCOS; mean BMI, 44 kg/m2; mean age, 31 years who were followed post-bariatric surgery for 5 years, > 90% of patients reported reductions in hirsutism and resumption of regular menses.25 For patients with PCOS seeking fertility, bariatric surgery often results in spontaneous pregnancy and live birth.26 GLP-1 agonists, including liraglutide or exenatide with or without metformin are effective in reducing weight, decreasing androgen levels, and restoring ovulatory menses.27,28
In my practice, I often prescribe 2 or 3 core medications for a patient with PCOS: 1) combination estrogen-progestin used cyclically or continuously, 2) spironolactone, and 3) metformin.19 Any estrogen-progestin contraceptive will suppress LH and ovarian androgen production; however, in the treatment of patients with PCOS, I prefer to use an estrogen-progestin combination that does not contain the androgenic progestin levonorgestrel.29 For the treatment of PCOS, I prefer to use an estrogen-progestin contraceptive with a non-androgenic progestin such as drospirenone, desogestrel, or gestodene. I routinely prescribe spironolactone at a dose of 100 mg, once daily, a dose near the top of the dose-response curve. A daily dose ≤ 50 mg of spironolactone is subtherapeutic for the treatment of hirsutism. A daily dose of 200 mg of spironolactone may cause bothersome breakthrough bleeding. When prescribing metformin, I usually recommend the extended-release formulation, at a dose of 750 mg with dinner. If well tolerated, I will increase the dose to 1,500 mg with dinner. Most of my patients with PCOS are taking a combination of 2 medications, either an estrogen-progestin contraceptive plus spironolactone or an estrogen-progestin contraceptive plus metformin.19 Some of my patients are taking all 3 medications. All 3 medications are very low cost.
For patients with PCOS and anovulatory infertility, letrozole treatment often results in ovulatory cycles and pregnancy with live birth. In obese PCOS patients, compared with clomiphene, letrozole results in superior live birth rates.30 Unlike clomiphene, letrozole is not approved by the US Food and Drug Administration for the treatment of anovulatory infertility.
The diagnosis of PCOS is often delayed due to confusion about how to make the diagnosis.31 To simplify the diagnosis of PCOS and improve patient encounters for PCOS, I focus on 2 core criteria: hyperandrogenism and oligo-ovulation. I recommend against ordering ultrasound imaging to assess for the presence of a multifollicular ovary. To simplify the treatment of PCOS I frequently prescribe an estrogen-progestin contraceptive, spironolactone, and metformin. By simplifying the diagnosis and treatment of PCOS, ObGyns will reduce patient confusion, improve outcomes, and save health care resources. ●
PCOS and adolescent patients
It is difficult to diagnose polycystic ovary syndrome (PCOS) in adolescents because oligo-ovulation is a common physiological feature of adolescence. Based on consensus among experts, PCOS should not be diagnosed within the first 2 years following menarche because the prevalence of oligo-ovulation is common at this stage of pubertal development. Two years after menarche, if an adolescent has a cycle length that is routinely > 45 days, it is likely that the pattern will persist, suggesting the presence of oligo-ovulation. Hyperandrogenism can be diagnosed based on the presence of moderate to severe hirsutism and/or an elevated testosterone or abnormally low sex-hormone binding globulin (SHBG) concentration. Two years after menarche the presence of oligo-ovulation and hyperandrogenism establishes the diagnosis of PCOS.1
PCOS and thrombophilia or migraine with aura
For patients with PCOS and a Factor V Leiden allele, where an estrogen-progestin contraceptive is contraindicated because of an increased risk of a venous thrombus, I prescribe spironolactone plus a levonorgestrel-intrauterine device. A low-dose oral progestin also may be considered because it will modestly suppress LH and ovarian androgen production. Similarly for patients with migraine with aura, where an estrogen-progestin contraceptive is contraindicated because of an increased of stroke, spironolactone plus a levonorgesterel intrauterine device may be effective in the treatment of hirsutism.
Androgen secreting tumors
Occasionally during the evaluation of a patient with hyperandrogenism and oligo-ovulation, measurement of total testosterone levels will reveal a value > 1.5 ng/mL. Most patients with PCOS have a total testosterone level ≤ 1.5 ng/mL. A total testosterone concentration > 1.5 ng/mL may be caused by ovarian stromal hyperthecosis or an androgen-producing tumor.2
Strongly-held patient perspectives on PCOS
At the first consultation visit, some patients are fearful and not receptive to a diagnosis of PCOS. If a clinician senses that the patient is not prepared to hear that they have PCOS, the clinician can be supportive of the patient’s perspective and focus on the patient’s chief health concerns, which may include abnormal cycle length, hirsutism, and/or overweight or obesity. During follow-up visits, as the patient builds trust with the clinician, the patient will be better prepared to discuss the diagnosis of PCOS. At the first consultation visit, some patients present with a strong belief that they have PCOS but have seen clinicians who conclude that they do not have PCOS. The diagnosis of PCOS is confusing because of competing diagnostic frameworks (NIH, Rotterdam, and AES). I avoid engaging in an argument with a patient who strongly believes that they have PCOS. In these situations, I focus on identifying the patient’s chief health concerns and discussing interventions to support their health goals.
References
1. Rosenfield RL. Perspectives on the international recommendations for the diagnosis and treatment of polycystic ovary syndrome in adolescence. J Pediatr Adolesc Gynecol. 2020;33:445-447.
2. Meczekalski B, Szeliga A, Maciejewska-Jeske M, et al. Hyperthecosis: an underestimated nontumorous cause of hyperandrogenism. Gynecol Endocrinol. 2021;37:677-682.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Livadas S, Anagnostis P, Bosdou JK, et al. Polycystic ovary syndrome and type 2 diabetes mellitus: a state-of-the-art review. World J Diabetes. 2022;13:5-26.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Polycystic Ovary Syndrome. Current Issues in Endocrinology and Metabolism. Dunaif A, Givens JR, Haseltine FP, Merriam GE (eds.). Blackwell Scientific Inc. Boston, Massachusetts; 1992:377.
- Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Human Reprod. 2004;19:41-47.
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592.
- Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456-488.
- Hatch R, Rosenfield RS, Kim MH, et al. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981;140:815-830.
- Johnstone EB, Rosen MP, Neril R, et al. The polycystic ovary post-Rotterdam: a common age-dependent finding in ovulatory women without metabolic significance. J Clin Endocrinol Metab. 2010;95:4965-4972.
- Alsamarai S, Adams JM, Murphy MK, et al. Criteria for polycystic ovarian morphology in polycystic ovary syndrome as a function of age. J Clin Endocrinol Metab. 2009;94:4961-4970.
- Teede HJ, Misso ML, Costello MF, et al. International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110:364-379.
- Serafini P, Lobo RA. Increased 5 alpha-reductase activity in idiopathic hirsutism. Fertil Steril. 1985;43:74-78.
- Pigny P, Jonard S, Robert Y, et al. Serum anti-Müllerian hormone as a surrogate for antral follicle count for definition of the polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:941-945.
- Randeva HS, Tan BK, Weickert MO, et al. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev. 2012;33:812-841.
- Kumar N, Agarwal H. Early clinical, biochemical and radiologic features in obese and non-obese young women with polycystic ovarian syndrome: a comparative study. Horm Metab Res. 2022;54:620-624.
- Lim SS, Norman RJ, Davies MJ, et al. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013;14:95-109.
- Nordenstrom A, Falhammar H. Management of endocrine disease: diagnosis and management of the patient with non-classic CAH due to 21-hydroxylase deficiency. Eur J Endocrinol. 2019;180:R127-145.
- Guo F, Gong Z, Fernando T, et al. The lipid profiles in different characteristics of women with PCOS and the interaction between dyslipidemia and metabolic disorder states: a retrospective study in Chinese population. Front Endocrinol. 2022;13:892125.
- Dietz de Loos ALP, Jiskoot G, Timman R, et al. Improvements in PCOS characteristics and phenotype severity during a randomized controlled lifestyle intervention. Reprod Biomed Online. 2021;43:298-309.
- Ezeh U, Huang A, Landay M, et al. Long-term response of hirsutism and other hyperandrogenic symptoms to combination therapy in polycystic ovary syndrome. J Women’s Health. 2018;27:892-902.
- Ashraf Ganie M, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Pasquali R, Gambineri A, Cavazza C, et al. Heterogeneity in the responsiveness to long-term lifestyle intervention and predictability in obese women with polycystic ovary syndrome. Eur J Endocrinol. 2011;164:53-60.
- Yang PK, Hsu CY, Chen MJ, et al. The efficacy of 24-month metformin for improving menses, hormones and metabolic profiles in polycystic ovary syndrome. J Clin Endocrinol Metab. 2018;103:890-899.
- Garg V, Choi J, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355.
- Hu L, Ma L, Ying T, et al. Efficacy of bariatric surgery in the treatment of women with obesity and polycystic ovary syndrome. J Clin Endocrinol Metab. 2022;107:e3217-3229.
- Bhandari M, Kosta S, Bhandari M, et al. Effects of bariatric surgery on people with obesity and polycystic ovary syndrome: a large single center study from India. Obes Surg. 2022;32:3305-3312.
- Benito E, Gomez-Martin JM, Vega-Pinero B, et al. Fertility and pregnancy outcomes in women with polycystic ovary syndrome following bariatric surgery. J Clin Endocrinol Metab. 2020;105:e3384-3391.
- Xing C, Li C, He B. Insulin sensitizers for improving the endocrine and metabolic profile in overweight women with PCOS. J Clin Endocrinol Metab. 2020;105:2950-2963.
- Elkind-Hirsch KE, Chappell N, Shaler D, et al. Liraglutide 3 mg on weight, body composition and hormonal and metabolic parameters in women with obesity and polycystic ovary syndrome: a randomized placebo-controlled-phase 3 study. Fertil Steril. 2022;118:371-381.
- Amiri M, Nahidi F, Bidhendi-Yarandi R, et al. A comparison of the effects of oral contraceptives on the clinical and biochemical manifestations of polycystic ovary syndrome: a crossover randomized controlled trial. Hum Reprod. 2020;35:175-186.
- Legro RS, Brzyski RG, Diamond NP, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371:119-129.
- Gibson-Helm M, Teede H, Dunaif A, et al. Delayed diagnosis and lack of information associated with dissatisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102:604-612.
PCOS is a common problem, with a prevalence of 6% to 10% among women of reproductive age.1 Patients with PCOS often present with hirsutism, acne, female androgenetic alopecia, oligomenorrhea (also known as infrequent menstrual bleeding), amenorrhea, infertility, overweight, or obesity. In addition, many patients with PCOS have insulin resistance, dyslipidemia, metabolic syndrome, and an increased risk for developing type 2 diabetes mellitus (DM).2 A simplified approach to the diagnosis of PCOS will save health care resources by reducing the use of low-value diagnostic tests. A simplified approach to the treatment of PCOS will support patient medication adherence and improve health outcomes.
Simplify the diagnosis of PCOS
Simplify PCOS diagnosis by focusing on the core criteria of hyperandrogenism and oligo-ovulation. There are 3 major approaches to diagnosis:
- the 1990 National Institutes of Health (NIH) criteria3
- the 2003 Rotterdam criteria4,5
- the 2008 Androgen Excess and PCOS Society (AES) criteria.6
Using the 1990 NIH approach, the diagnosis of PCOS is made by the presence of 2 core criteria: hyperandrogenism and oligo-ovulation, typically manifested as oligomenorrhea. In addition, other causes of hyperandrogenism should be excluded, including nonclassical adrenal hyperplasia (NCAH) due to 21-hydroxylase deficiency.3 Using the 1990 NIH criteria, PCOS can be diagnosed based on history (oligomenorrhea) and physical examination (assessment of the severity of hirsutism), but laboratory tests including total testosterone are often ordered.7
The Rotterdam approach to the diagnosis added a third criteria, the detection by ultrasonography of a multifollicular ovary and/or increased ovarian volume.4,5 Using the Rotterdam approach, PCOS is diagnosed in the presence of any 2 of the following 3 criteria: hyperandrogenism, oligo-ovulation, or ultrasound imaging showing the presence of a multifollicular ovary, identified by ≥ 12 antral follicles (2 to 9 mm in diameter) in each ovary or increased ovarian volume (> 10 mL).4,5
The Rotterdam approach using ovarian ultrasound as a criterion to diagnose PCOS is rife with serious problems, including:
- The number of small antral follicles in the normal ovary is age dependent, and many ovulatory and nonhirsute patients have ≥ 12 small antral follicles in each ovary.8,9
- There is no consensus on the number of small antral follicles needed to diagnose a multifollicular ovary, with recommendations to use thresholds of 124,5 or 20 follicles10 as the diagnostic cut-off.
- Accurate counting of the number of small ovarian follicles requires transvaginal ultrasound, which is not appropriate for many young adolescent patients.
- The process of counting ovarian follicles is operator-dependent.
- The high cost of ultrasound assessment of ovarian follicles (≥ $500 per examination).

The Rotterdam approach supports the diagnosis of PCOS in a patient with oligo-ovulation plus an ultrasound showing a multifollicular ovary in the absence of any clinical or laboratory evidence of hyperandrogenism.3,4,5 This approach to the diagnosis of PCOS is rejected by both the 1990 NIH3 and AES6 recommendations, which require the presence of hyperandrogenism as the sine qua non in the diagnosis of PCOS. I recommend against diagnosing PCOS in a non-hyperandrogenic patient with oligo-ovulation and a multifollicular ovary because other diagnoses are also possible, such as functional hypothalamic oligo-ovulation, especially in young patients. The Rotterdam approach also supports the diagnosis of PCOS in a patient with hyperandrogenism, an ultrasound showing a multifollicular ovary, and normal ovulation and menses.3,4 For most patients with normal, regular ovulation and menses, the testosterone concentration is normal and the only evidence of hyperandrogenism is hirsutism. Patients with normal, regular ovulation and menses plus hirsutism usually have idiopathic hirsutism. Idiopathic hirsutism is a problem caused by excessive 5-alpha-reductase activity in the hair pilosebaceous unit, which catalyzes the conversion of weak androgens into dihydrotestosterone, a potent intracellular androgen that stimulates terminal hair growth.11 In my opinion, the Rotterdam approach to diagnosing PCOS has created unnecessary confusion and complexity for both clinicians and patients. I believe we should simplify the diagnosis of PCOS and return to the 1990 NIH criteria.3
On occasion, a patient presents for a consultation and has already had an ovarian ultrasound to assess for a multifollicular ovary. I carefully read the report and, if a multifollicular ovary has been identified, I consider it as a secondary supporting finding of PCOS in my clinical assessment. But I do not base my diagnosis on the ultrasound finding. Patients often present with other laboratory tests that are secondary supporting findings of PCOS, which I carefully consider but do not use to make a diagnosis of PCOS. Secondary supporting laboratory findings consistent with PCOS include: 1) a markedly elevated anti-müllerian hormone (AMH) level,12 2) an elevated fasting insulin level,2,13 and 3) an elevated luteinizing hormone (LH) to follicle-stimulating hormone (FSH) ratio.13,14 But it is not necessary to measure AMH, fasting insulin, LH, and FSH levels. To conserve health care resources, I recommend against measuring those analytes to diagnose PCOS.

Continue to: Simplify the core laboratory tests...
Simplify the core laboratory tests
Simplify the testing used to support the diagnosis of PCOS by measuring total testosterone, sex-hormone binding globulin (SHBG) and early morning 17-hydroxyprogesterone (17-OH Prog).
The core criteria for diagnosis of PCOS are hyperandrogenism and oligo-ovulation, typically manifested as oligomenorrhea or amenorrhea. Hyperandrogenism can be clinically diagnosed by assessing for the presence of hirsutism.7 Elevated levels of total testosterone, free testosterone, androstenedione, and/or dehydroepiandrosterone sulfate (DHEAS) suggest the presence of hyperandrogenism. In clinical practice, the laboratory approach to the diagnosis of hyperandrogenism can be simplified to the measurement of total testosterone, SHBG, and 17-OH Prog. By measuring total testosterone and SHBG, an estimate of free testosterone can be made. If the total testosterone is elevated, it is highly likely that the free testosterone is elevated. If the SHBG is abnormally low and the total testosterone level is in the upper limit of the normal range, the free testosterone is likely to be elevated.15 Using this approach, either an elevated total testosterone or an abnormally low SHBG indicate elevated free testosterone. For patients with hyperandrogenism and oligo-ovulation, an early morning (8 to 9 AM) 17-OH Prog level ≤ 2 ng/mL rules out the presence of NCAH due to a 21-hydroxylase deficiency.16 In my practice, the core laboratory tests I order when considering the diagnosis of PCOS are a total testosterone, SHBG, and 17-OH Prog.
Additional laboratory tests may be warranted to assess the patient diagnosed with PCOS. For example, if the patient has amenorrhea due to anovulation, tests for prolactin, FSH, and thyroid-stimulating hormone levels are warranted to assess for the presence of a prolactinoma, primary ovarian insufficiency, or thyroid disease, respectively. If the patient has a body mass index (BMI) ≥ 25 kg/m2, a hemoglobin A1c concentration is warranted to assess for the presence of prediabetes or DM.2 Many patients with PCOS have dyslipidemia, manifested through low high-density lipoprotein cholesterol levels and elevated low-density lipoprotein cholesterol levels, and a lipid panel assessment may be indicated. Among patients with PCOS, the most common lipid abnormality is a low high-density lipoprotein cholesterol level.17
Simplify the treatment of PCOS
Simplify treatment by counseling about lifestyle changes and prescribing an estrogen-progestin contraceptive, spironolactone, and metformin.
Most patients with PCOS have dysfunction in reproductive, metabolic, and dermatologic systems. For patients who are overweight or obese, lifestyle changes, including diet and exercise, that result in a 5% to 10% decrease in weight can improve metabolic balance, reduce circulating androgens, and increase menstrual frequency.18 For patients with PCOS and weight issues, referral to nutrition counseling or a full-service weight loss program can be very beneficial. In addition to lifestyle changes, patients with PCOS benefit from treatment with estrogen-progestin medications, spironolactone, and metformin.
Combination estrogen-progestin medications will lower LH secretion, decrease ovarian androgen production, increase SHBG production, decrease free testosterone levels and, if given cyclically, cause regular withdrawal bleeding.19 Spironolactone is an antiandrogen, which blocks the intracellular action of dihydrotestosterone and improves hirsutism and acne. Spironolactone also modestly decreases circulating levels of testosterone and DHEAS.20 For patients with metabolic problems, including insulin resistance and obesity, weight loss and/or treatment with metformin can help improve metabolic balance, which may result in restoration of ovulatory menses.21,22 Metformin can be effective in restoring ovulatory menses in both obese and lean patients with PCOS.22 The most common dermatologic problem caused by PCOS are hirsutism and acne. Both combination estrogen-progestin medications and spironolactone are effective treatments for hirsutism and acne.23
Estrogen-progestin hormones, spironolactone, and metformin are low-cost medications for the treatment of PCOS. Additional high-cost options for treatment of PCOS in obese patients include bariatric surgery and glucagon-like peptide (GLP-1) agonist medications (liraglutide and exenatide). For patients with PCOS and a body mass index (BMI) ≥ 35 kg/m2, bariatric surgery often results in sufficient weight loss to resolve the patient’s hyperandrogenism and oligo-ovulation, restoring spontaneous ovulatory cycles.24 In a study of more than 1,000 patients with: PCOS; mean BMI, 44 kg/m2; mean age, 31 years who were followed post-bariatric surgery for 5 years, > 90% of patients reported reductions in hirsutism and resumption of regular menses.25 For patients with PCOS seeking fertility, bariatric surgery often results in spontaneous pregnancy and live birth.26 GLP-1 agonists, including liraglutide or exenatide with or without metformin are effective in reducing weight, decreasing androgen levels, and restoring ovulatory menses.27,28
In my practice, I often prescribe 2 or 3 core medications for a patient with PCOS: 1) combination estrogen-progestin used cyclically or continuously, 2) spironolactone, and 3) metformin.19 Any estrogen-progestin contraceptive will suppress LH and ovarian androgen production; however, in the treatment of patients with PCOS, I prefer to use an estrogen-progestin combination that does not contain the androgenic progestin levonorgestrel.29 For the treatment of PCOS, I prefer to use an estrogen-progestin contraceptive with a non-androgenic progestin such as drospirenone, desogestrel, or gestodene. I routinely prescribe spironolactone at a dose of 100 mg, once daily, a dose near the top of the dose-response curve. A daily dose ≤ 50 mg of spironolactone is subtherapeutic for the treatment of hirsutism. A daily dose of 200 mg of spironolactone may cause bothersome breakthrough bleeding. When prescribing metformin, I usually recommend the extended-release formulation, at a dose of 750 mg with dinner. If well tolerated, I will increase the dose to 1,500 mg with dinner. Most of my patients with PCOS are taking a combination of 2 medications, either an estrogen-progestin contraceptive plus spironolactone or an estrogen-progestin contraceptive plus metformin.19 Some of my patients are taking all 3 medications. All 3 medications are very low cost.
For patients with PCOS and anovulatory infertility, letrozole treatment often results in ovulatory cycles and pregnancy with live birth. In obese PCOS patients, compared with clomiphene, letrozole results in superior live birth rates.30 Unlike clomiphene, letrozole is not approved by the US Food and Drug Administration for the treatment of anovulatory infertility.
The diagnosis of PCOS is often delayed due to confusion about how to make the diagnosis.31 To simplify the diagnosis of PCOS and improve patient encounters for PCOS, I focus on 2 core criteria: hyperandrogenism and oligo-ovulation. I recommend against ordering ultrasound imaging to assess for the presence of a multifollicular ovary. To simplify the treatment of PCOS I frequently prescribe an estrogen-progestin contraceptive, spironolactone, and metformin. By simplifying the diagnosis and treatment of PCOS, ObGyns will reduce patient confusion, improve outcomes, and save health care resources. ●
PCOS and adolescent patients
It is difficult to diagnose polycystic ovary syndrome (PCOS) in adolescents because oligo-ovulation is a common physiological feature of adolescence. Based on consensus among experts, PCOS should not be diagnosed within the first 2 years following menarche because the prevalence of oligo-ovulation is common at this stage of pubertal development. Two years after menarche, if an adolescent has a cycle length that is routinely > 45 days, it is likely that the pattern will persist, suggesting the presence of oligo-ovulation. Hyperandrogenism can be diagnosed based on the presence of moderate to severe hirsutism and/or an elevated testosterone or abnormally low sex-hormone binding globulin (SHBG) concentration. Two years after menarche the presence of oligo-ovulation and hyperandrogenism establishes the diagnosis of PCOS.1
PCOS and thrombophilia or migraine with aura
For patients with PCOS and a Factor V Leiden allele, where an estrogen-progestin contraceptive is contraindicated because of an increased risk of a venous thrombus, I prescribe spironolactone plus a levonorgestrel-intrauterine device. A low-dose oral progestin also may be considered because it will modestly suppress LH and ovarian androgen production. Similarly for patients with migraine with aura, where an estrogen-progestin contraceptive is contraindicated because of an increased of stroke, spironolactone plus a levonorgesterel intrauterine device may be effective in the treatment of hirsutism.
Androgen secreting tumors
Occasionally during the evaluation of a patient with hyperandrogenism and oligo-ovulation, measurement of total testosterone levels will reveal a value > 1.5 ng/mL. Most patients with PCOS have a total testosterone level ≤ 1.5 ng/mL. A total testosterone concentration > 1.5 ng/mL may be caused by ovarian stromal hyperthecosis or an androgen-producing tumor.2
Strongly-held patient perspectives on PCOS
At the first consultation visit, some patients are fearful and not receptive to a diagnosis of PCOS. If a clinician senses that the patient is not prepared to hear that they have PCOS, the clinician can be supportive of the patient’s perspective and focus on the patient’s chief health concerns, which may include abnormal cycle length, hirsutism, and/or overweight or obesity. During follow-up visits, as the patient builds trust with the clinician, the patient will be better prepared to discuss the diagnosis of PCOS. At the first consultation visit, some patients present with a strong belief that they have PCOS but have seen clinicians who conclude that they do not have PCOS. The diagnosis of PCOS is confusing because of competing diagnostic frameworks (NIH, Rotterdam, and AES). I avoid engaging in an argument with a patient who strongly believes that they have PCOS. In these situations, I focus on identifying the patient’s chief health concerns and discussing interventions to support their health goals.
References
1. Rosenfield RL. Perspectives on the international recommendations for the diagnosis and treatment of polycystic ovary syndrome in adolescence. J Pediatr Adolesc Gynecol. 2020;33:445-447.
2. Meczekalski B, Szeliga A, Maciejewska-Jeske M, et al. Hyperthecosis: an underestimated nontumorous cause of hyperandrogenism. Gynecol Endocrinol. 2021;37:677-682.
PCOS is a common problem, with a prevalence of 6% to 10% among women of reproductive age.1 Patients with PCOS often present with hirsutism, acne, female androgenetic alopecia, oligomenorrhea (also known as infrequent menstrual bleeding), amenorrhea, infertility, overweight, or obesity. In addition, many patients with PCOS have insulin resistance, dyslipidemia, metabolic syndrome, and an increased risk for developing type 2 diabetes mellitus (DM).2 A simplified approach to the diagnosis of PCOS will save health care resources by reducing the use of low-value diagnostic tests. A simplified approach to the treatment of PCOS will support patient medication adherence and improve health outcomes.
Simplify the diagnosis of PCOS
Simplify PCOS diagnosis by focusing on the core criteria of hyperandrogenism and oligo-ovulation. There are 3 major approaches to diagnosis:
- the 1990 National Institutes of Health (NIH) criteria3
- the 2003 Rotterdam criteria4,5
- the 2008 Androgen Excess and PCOS Society (AES) criteria.6
Using the 1990 NIH approach, the diagnosis of PCOS is made by the presence of 2 core criteria: hyperandrogenism and oligo-ovulation, typically manifested as oligomenorrhea. In addition, other causes of hyperandrogenism should be excluded, including nonclassical adrenal hyperplasia (NCAH) due to 21-hydroxylase deficiency.3 Using the 1990 NIH criteria, PCOS can be diagnosed based on history (oligomenorrhea) and physical examination (assessment of the severity of hirsutism), but laboratory tests including total testosterone are often ordered.7
The Rotterdam approach to the diagnosis added a third criteria, the detection by ultrasonography of a multifollicular ovary and/or increased ovarian volume.4,5 Using the Rotterdam approach, PCOS is diagnosed in the presence of any 2 of the following 3 criteria: hyperandrogenism, oligo-ovulation, or ultrasound imaging showing the presence of a multifollicular ovary, identified by ≥ 12 antral follicles (2 to 9 mm in diameter) in each ovary or increased ovarian volume (> 10 mL).4,5
The Rotterdam approach using ovarian ultrasound as a criterion to diagnose PCOS is rife with serious problems, including:
- The number of small antral follicles in the normal ovary is age dependent, and many ovulatory and nonhirsute patients have ≥ 12 small antral follicles in each ovary.8,9
- There is no consensus on the number of small antral follicles needed to diagnose a multifollicular ovary, with recommendations to use thresholds of 124,5 or 20 follicles10 as the diagnostic cut-off.
- Accurate counting of the number of small ovarian follicles requires transvaginal ultrasound, which is not appropriate for many young adolescent patients.
- The process of counting ovarian follicles is operator-dependent.
- The high cost of ultrasound assessment of ovarian follicles (≥ $500 per examination).

The Rotterdam approach supports the diagnosis of PCOS in a patient with oligo-ovulation plus an ultrasound showing a multifollicular ovary in the absence of any clinical or laboratory evidence of hyperandrogenism.3,4,5 This approach to the diagnosis of PCOS is rejected by both the 1990 NIH3 and AES6 recommendations, which require the presence of hyperandrogenism as the sine qua non in the diagnosis of PCOS. I recommend against diagnosing PCOS in a non-hyperandrogenic patient with oligo-ovulation and a multifollicular ovary because other diagnoses are also possible, such as functional hypothalamic oligo-ovulation, especially in young patients. The Rotterdam approach also supports the diagnosis of PCOS in a patient with hyperandrogenism, an ultrasound showing a multifollicular ovary, and normal ovulation and menses.3,4 For most patients with normal, regular ovulation and menses, the testosterone concentration is normal and the only evidence of hyperandrogenism is hirsutism. Patients with normal, regular ovulation and menses plus hirsutism usually have idiopathic hirsutism. Idiopathic hirsutism is a problem caused by excessive 5-alpha-reductase activity in the hair pilosebaceous unit, which catalyzes the conversion of weak androgens into dihydrotestosterone, a potent intracellular androgen that stimulates terminal hair growth.11 In my opinion, the Rotterdam approach to diagnosing PCOS has created unnecessary confusion and complexity for both clinicians and patients. I believe we should simplify the diagnosis of PCOS and return to the 1990 NIH criteria.3
On occasion, a patient presents for a consultation and has already had an ovarian ultrasound to assess for a multifollicular ovary. I carefully read the report and, if a multifollicular ovary has been identified, I consider it as a secondary supporting finding of PCOS in my clinical assessment. But I do not base my diagnosis on the ultrasound finding. Patients often present with other laboratory tests that are secondary supporting findings of PCOS, which I carefully consider but do not use to make a diagnosis of PCOS. Secondary supporting laboratory findings consistent with PCOS include: 1) a markedly elevated anti-müllerian hormone (AMH) level,12 2) an elevated fasting insulin level,2,13 and 3) an elevated luteinizing hormone (LH) to follicle-stimulating hormone (FSH) ratio.13,14 But it is not necessary to measure AMH, fasting insulin, LH, and FSH levels. To conserve health care resources, I recommend against measuring those analytes to diagnose PCOS.

Continue to: Simplify the core laboratory tests...
Simplify the core laboratory tests
Simplify the testing used to support the diagnosis of PCOS by measuring total testosterone, sex-hormone binding globulin (SHBG) and early morning 17-hydroxyprogesterone (17-OH Prog).
The core criteria for diagnosis of PCOS are hyperandrogenism and oligo-ovulation, typically manifested as oligomenorrhea or amenorrhea. Hyperandrogenism can be clinically diagnosed by assessing for the presence of hirsutism.7 Elevated levels of total testosterone, free testosterone, androstenedione, and/or dehydroepiandrosterone sulfate (DHEAS) suggest the presence of hyperandrogenism. In clinical practice, the laboratory approach to the diagnosis of hyperandrogenism can be simplified to the measurement of total testosterone, SHBG, and 17-OH Prog. By measuring total testosterone and SHBG, an estimate of free testosterone can be made. If the total testosterone is elevated, it is highly likely that the free testosterone is elevated. If the SHBG is abnormally low and the total testosterone level is in the upper limit of the normal range, the free testosterone is likely to be elevated.15 Using this approach, either an elevated total testosterone or an abnormally low SHBG indicate elevated free testosterone. For patients with hyperandrogenism and oligo-ovulation, an early morning (8 to 9 AM) 17-OH Prog level ≤ 2 ng/mL rules out the presence of NCAH due to a 21-hydroxylase deficiency.16 In my practice, the core laboratory tests I order when considering the diagnosis of PCOS are a total testosterone, SHBG, and 17-OH Prog.
Additional laboratory tests may be warranted to assess the patient diagnosed with PCOS. For example, if the patient has amenorrhea due to anovulation, tests for prolactin, FSH, and thyroid-stimulating hormone levels are warranted to assess for the presence of a prolactinoma, primary ovarian insufficiency, or thyroid disease, respectively. If the patient has a body mass index (BMI) ≥ 25 kg/m2, a hemoglobin A1c concentration is warranted to assess for the presence of prediabetes or DM.2 Many patients with PCOS have dyslipidemia, manifested through low high-density lipoprotein cholesterol levels and elevated low-density lipoprotein cholesterol levels, and a lipid panel assessment may be indicated. Among patients with PCOS, the most common lipid abnormality is a low high-density lipoprotein cholesterol level.17
Simplify the treatment of PCOS
Simplify treatment by counseling about lifestyle changes and prescribing an estrogen-progestin contraceptive, spironolactone, and metformin.
Most patients with PCOS have dysfunction in reproductive, metabolic, and dermatologic systems. For patients who are overweight or obese, lifestyle changes, including diet and exercise, that result in a 5% to 10% decrease in weight can improve metabolic balance, reduce circulating androgens, and increase menstrual frequency.18 For patients with PCOS and weight issues, referral to nutrition counseling or a full-service weight loss program can be very beneficial. In addition to lifestyle changes, patients with PCOS benefit from treatment with estrogen-progestin medications, spironolactone, and metformin.
Combination estrogen-progestin medications will lower LH secretion, decrease ovarian androgen production, increase SHBG production, decrease free testosterone levels and, if given cyclically, cause regular withdrawal bleeding.19 Spironolactone is an antiandrogen, which blocks the intracellular action of dihydrotestosterone and improves hirsutism and acne. Spironolactone also modestly decreases circulating levels of testosterone and DHEAS.20 For patients with metabolic problems, including insulin resistance and obesity, weight loss and/or treatment with metformin can help improve metabolic balance, which may result in restoration of ovulatory menses.21,22 Metformin can be effective in restoring ovulatory menses in both obese and lean patients with PCOS.22 The most common dermatologic problem caused by PCOS are hirsutism and acne. Both combination estrogen-progestin medications and spironolactone are effective treatments for hirsutism and acne.23
Estrogen-progestin hormones, spironolactone, and metformin are low-cost medications for the treatment of PCOS. Additional high-cost options for treatment of PCOS in obese patients include bariatric surgery and glucagon-like peptide (GLP-1) agonist medications (liraglutide and exenatide). For patients with PCOS and a body mass index (BMI) ≥ 35 kg/m2, bariatric surgery often results in sufficient weight loss to resolve the patient’s hyperandrogenism and oligo-ovulation, restoring spontaneous ovulatory cycles.24 In a study of more than 1,000 patients with: PCOS; mean BMI, 44 kg/m2; mean age, 31 years who were followed post-bariatric surgery for 5 years, > 90% of patients reported reductions in hirsutism and resumption of regular menses.25 For patients with PCOS seeking fertility, bariatric surgery often results in spontaneous pregnancy and live birth.26 GLP-1 agonists, including liraglutide or exenatide with or without metformin are effective in reducing weight, decreasing androgen levels, and restoring ovulatory menses.27,28
In my practice, I often prescribe 2 or 3 core medications for a patient with PCOS: 1) combination estrogen-progestin used cyclically or continuously, 2) spironolactone, and 3) metformin.19 Any estrogen-progestin contraceptive will suppress LH and ovarian androgen production; however, in the treatment of patients with PCOS, I prefer to use an estrogen-progestin combination that does not contain the androgenic progestin levonorgestrel.29 For the treatment of PCOS, I prefer to use an estrogen-progestin contraceptive with a non-androgenic progestin such as drospirenone, desogestrel, or gestodene. I routinely prescribe spironolactone at a dose of 100 mg, once daily, a dose near the top of the dose-response curve. A daily dose ≤ 50 mg of spironolactone is subtherapeutic for the treatment of hirsutism. A daily dose of 200 mg of spironolactone may cause bothersome breakthrough bleeding. When prescribing metformin, I usually recommend the extended-release formulation, at a dose of 750 mg with dinner. If well tolerated, I will increase the dose to 1,500 mg with dinner. Most of my patients with PCOS are taking a combination of 2 medications, either an estrogen-progestin contraceptive plus spironolactone or an estrogen-progestin contraceptive plus metformin.19 Some of my patients are taking all 3 medications. All 3 medications are very low cost.
For patients with PCOS and anovulatory infertility, letrozole treatment often results in ovulatory cycles and pregnancy with live birth. In obese PCOS patients, compared with clomiphene, letrozole results in superior live birth rates.30 Unlike clomiphene, letrozole is not approved by the US Food and Drug Administration for the treatment of anovulatory infertility.
The diagnosis of PCOS is often delayed due to confusion about how to make the diagnosis.31 To simplify the diagnosis of PCOS and improve patient encounters for PCOS, I focus on 2 core criteria: hyperandrogenism and oligo-ovulation. I recommend against ordering ultrasound imaging to assess for the presence of a multifollicular ovary. To simplify the treatment of PCOS I frequently prescribe an estrogen-progestin contraceptive, spironolactone, and metformin. By simplifying the diagnosis and treatment of PCOS, ObGyns will reduce patient confusion, improve outcomes, and save health care resources. ●
PCOS and adolescent patients
It is difficult to diagnose polycystic ovary syndrome (PCOS) in adolescents because oligo-ovulation is a common physiological feature of adolescence. Based on consensus among experts, PCOS should not be diagnosed within the first 2 years following menarche because the prevalence of oligo-ovulation is common at this stage of pubertal development. Two years after menarche, if an adolescent has a cycle length that is routinely > 45 days, it is likely that the pattern will persist, suggesting the presence of oligo-ovulation. Hyperandrogenism can be diagnosed based on the presence of moderate to severe hirsutism and/or an elevated testosterone or abnormally low sex-hormone binding globulin (SHBG) concentration. Two years after menarche the presence of oligo-ovulation and hyperandrogenism establishes the diagnosis of PCOS.1
PCOS and thrombophilia or migraine with aura
For patients with PCOS and a Factor V Leiden allele, where an estrogen-progestin contraceptive is contraindicated because of an increased risk of a venous thrombus, I prescribe spironolactone plus a levonorgestrel-intrauterine device. A low-dose oral progestin also may be considered because it will modestly suppress LH and ovarian androgen production. Similarly for patients with migraine with aura, where an estrogen-progestin contraceptive is contraindicated because of an increased of stroke, spironolactone plus a levonorgesterel intrauterine device may be effective in the treatment of hirsutism.
Androgen secreting tumors
Occasionally during the evaluation of a patient with hyperandrogenism and oligo-ovulation, measurement of total testosterone levels will reveal a value > 1.5 ng/mL. Most patients with PCOS have a total testosterone level ≤ 1.5 ng/mL. A total testosterone concentration > 1.5 ng/mL may be caused by ovarian stromal hyperthecosis or an androgen-producing tumor.2
Strongly-held patient perspectives on PCOS
At the first consultation visit, some patients are fearful and not receptive to a diagnosis of PCOS. If a clinician senses that the patient is not prepared to hear that they have PCOS, the clinician can be supportive of the patient’s perspective and focus on the patient’s chief health concerns, which may include abnormal cycle length, hirsutism, and/or overweight or obesity. During follow-up visits, as the patient builds trust with the clinician, the patient will be better prepared to discuss the diagnosis of PCOS. At the first consultation visit, some patients present with a strong belief that they have PCOS but have seen clinicians who conclude that they do not have PCOS. The diagnosis of PCOS is confusing because of competing diagnostic frameworks (NIH, Rotterdam, and AES). I avoid engaging in an argument with a patient who strongly believes that they have PCOS. In these situations, I focus on identifying the patient’s chief health concerns and discussing interventions to support their health goals.
References
1. Rosenfield RL. Perspectives on the international recommendations for the diagnosis and treatment of polycystic ovary syndrome in adolescence. J Pediatr Adolesc Gynecol. 2020;33:445-447.
2. Meczekalski B, Szeliga A, Maciejewska-Jeske M, et al. Hyperthecosis: an underestimated nontumorous cause of hyperandrogenism. Gynecol Endocrinol. 2021;37:677-682.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Livadas S, Anagnostis P, Bosdou JK, et al. Polycystic ovary syndrome and type 2 diabetes mellitus: a state-of-the-art review. World J Diabetes. 2022;13:5-26.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Polycystic Ovary Syndrome. Current Issues in Endocrinology and Metabolism. Dunaif A, Givens JR, Haseltine FP, Merriam GE (eds.). Blackwell Scientific Inc. Boston, Massachusetts; 1992:377.
- Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Human Reprod. 2004;19:41-47.
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592.
- Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456-488.
- Hatch R, Rosenfield RS, Kim MH, et al. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981;140:815-830.
- Johnstone EB, Rosen MP, Neril R, et al. The polycystic ovary post-Rotterdam: a common age-dependent finding in ovulatory women without metabolic significance. J Clin Endocrinol Metab. 2010;95:4965-4972.
- Alsamarai S, Adams JM, Murphy MK, et al. Criteria for polycystic ovarian morphology in polycystic ovary syndrome as a function of age. J Clin Endocrinol Metab. 2009;94:4961-4970.
- Teede HJ, Misso ML, Costello MF, et al. International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110:364-379.
- Serafini P, Lobo RA. Increased 5 alpha-reductase activity in idiopathic hirsutism. Fertil Steril. 1985;43:74-78.
- Pigny P, Jonard S, Robert Y, et al. Serum anti-Müllerian hormone as a surrogate for antral follicle count for definition of the polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:941-945.
- Randeva HS, Tan BK, Weickert MO, et al. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev. 2012;33:812-841.
- Kumar N, Agarwal H. Early clinical, biochemical and radiologic features in obese and non-obese young women with polycystic ovarian syndrome: a comparative study. Horm Metab Res. 2022;54:620-624.
- Lim SS, Norman RJ, Davies MJ, et al. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013;14:95-109.
- Nordenstrom A, Falhammar H. Management of endocrine disease: diagnosis and management of the patient with non-classic CAH due to 21-hydroxylase deficiency. Eur J Endocrinol. 2019;180:R127-145.
- Guo F, Gong Z, Fernando T, et al. The lipid profiles in different characteristics of women with PCOS and the interaction between dyslipidemia and metabolic disorder states: a retrospective study in Chinese population. Front Endocrinol. 2022;13:892125.
- Dietz de Loos ALP, Jiskoot G, Timman R, et al. Improvements in PCOS characteristics and phenotype severity during a randomized controlled lifestyle intervention. Reprod Biomed Online. 2021;43:298-309.
- Ezeh U, Huang A, Landay M, et al. Long-term response of hirsutism and other hyperandrogenic symptoms to combination therapy in polycystic ovary syndrome. J Women’s Health. 2018;27:892-902.
- Ashraf Ganie M, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Pasquali R, Gambineri A, Cavazza C, et al. Heterogeneity in the responsiveness to long-term lifestyle intervention and predictability in obese women with polycystic ovary syndrome. Eur J Endocrinol. 2011;164:53-60.
- Yang PK, Hsu CY, Chen MJ, et al. The efficacy of 24-month metformin for improving menses, hormones and metabolic profiles in polycystic ovary syndrome. J Clin Endocrinol Metab. 2018;103:890-899.
- Garg V, Choi J, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355.
- Hu L, Ma L, Ying T, et al. Efficacy of bariatric surgery in the treatment of women with obesity and polycystic ovary syndrome. J Clin Endocrinol Metab. 2022;107:e3217-3229.
- Bhandari M, Kosta S, Bhandari M, et al. Effects of bariatric surgery on people with obesity and polycystic ovary syndrome: a large single center study from India. Obes Surg. 2022;32:3305-3312.
- Benito E, Gomez-Martin JM, Vega-Pinero B, et al. Fertility and pregnancy outcomes in women with polycystic ovary syndrome following bariatric surgery. J Clin Endocrinol Metab. 2020;105:e3384-3391.
- Xing C, Li C, He B. Insulin sensitizers for improving the endocrine and metabolic profile in overweight women with PCOS. J Clin Endocrinol Metab. 2020;105:2950-2963.
- Elkind-Hirsch KE, Chappell N, Shaler D, et al. Liraglutide 3 mg on weight, body composition and hormonal and metabolic parameters in women with obesity and polycystic ovary syndrome: a randomized placebo-controlled-phase 3 study. Fertil Steril. 2022;118:371-381.
- Amiri M, Nahidi F, Bidhendi-Yarandi R, et al. A comparison of the effects of oral contraceptives on the clinical and biochemical manifestations of polycystic ovary syndrome: a crossover randomized controlled trial. Hum Reprod. 2020;35:175-186.
- Legro RS, Brzyski RG, Diamond NP, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371:119-129.
- Gibson-Helm M, Teede H, Dunaif A, et al. Delayed diagnosis and lack of information associated with dissatisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102:604-612.
- Bozdag G, Mumusoglu S, Zengin D, et al. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31:2841-2855.
- Livadas S, Anagnostis P, Bosdou JK, et al. Polycystic ovary syndrome and type 2 diabetes mellitus: a state-of-the-art review. World J Diabetes. 2022;13:5-26.
- Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Polycystic Ovary Syndrome. Current Issues in Endocrinology and Metabolism. Dunaif A, Givens JR, Haseltine FP, Merriam GE (eds.). Blackwell Scientific Inc. Boston, Massachusetts; 1992:377.
- Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Human Reprod. 2004;19:41-47.
- Legro RS, Arslanian SA, Ehrmann DA, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565-4592.
- Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456-488.
- Hatch R, Rosenfield RS, Kim MH, et al. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981;140:815-830.
- Johnstone EB, Rosen MP, Neril R, et al. The polycystic ovary post-Rotterdam: a common age-dependent finding in ovulatory women without metabolic significance. J Clin Endocrinol Metab. 2010;95:4965-4972.
- Alsamarai S, Adams JM, Murphy MK, et al. Criteria for polycystic ovarian morphology in polycystic ovary syndrome as a function of age. J Clin Endocrinol Metab. 2009;94:4961-4970.
- Teede HJ, Misso ML, Costello MF, et al. International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. 2018;110:364-379.
- Serafini P, Lobo RA. Increased 5 alpha-reductase activity in idiopathic hirsutism. Fertil Steril. 1985;43:74-78.
- Pigny P, Jonard S, Robert Y, et al. Serum anti-Müllerian hormone as a surrogate for antral follicle count for definition of the polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:941-945.
- Randeva HS, Tan BK, Weickert MO, et al. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev. 2012;33:812-841.
- Kumar N, Agarwal H. Early clinical, biochemical and radiologic features in obese and non-obese young women with polycystic ovarian syndrome: a comparative study. Horm Metab Res. 2022;54:620-624.
- Lim SS, Norman RJ, Davies MJ, et al. The effect of obesity on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Rev. 2013;14:95-109.
- Nordenstrom A, Falhammar H. Management of endocrine disease: diagnosis and management of the patient with non-classic CAH due to 21-hydroxylase deficiency. Eur J Endocrinol. 2019;180:R127-145.
- Guo F, Gong Z, Fernando T, et al. The lipid profiles in different characteristics of women with PCOS and the interaction between dyslipidemia and metabolic disorder states: a retrospective study in Chinese population. Front Endocrinol. 2022;13:892125.
- Dietz de Loos ALP, Jiskoot G, Timman R, et al. Improvements in PCOS characteristics and phenotype severity during a randomized controlled lifestyle intervention. Reprod Biomed Online. 2021;43:298-309.
- Ezeh U, Huang A, Landay M, et al. Long-term response of hirsutism and other hyperandrogenic symptoms to combination therapy in polycystic ovary syndrome. J Women’s Health. 2018;27:892-902.
- Ashraf Ganie M, Khurana ML, Eunice M, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: an open-labeled study. J Clin Endocrinol Metab. 2004;89:2756-2762.
- Pasquali R, Gambineri A, Cavazza C, et al. Heterogeneity in the responsiveness to long-term lifestyle intervention and predictability in obese women with polycystic ovary syndrome. Eur J Endocrinol. 2011;164:53-60.
- Yang PK, Hsu CY, Chen MJ, et al. The efficacy of 24-month metformin for improving menses, hormones and metabolic profiles in polycystic ovary syndrome. J Clin Endocrinol Metab. 2018;103:890-899.
- Garg V, Choi J, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355.
- Hu L, Ma L, Ying T, et al. Efficacy of bariatric surgery in the treatment of women with obesity and polycystic ovary syndrome. J Clin Endocrinol Metab. 2022;107:e3217-3229.
- Bhandari M, Kosta S, Bhandari M, et al. Effects of bariatric surgery on people with obesity and polycystic ovary syndrome: a large single center study from India. Obes Surg. 2022;32:3305-3312.
- Benito E, Gomez-Martin JM, Vega-Pinero B, et al. Fertility and pregnancy outcomes in women with polycystic ovary syndrome following bariatric surgery. J Clin Endocrinol Metab. 2020;105:e3384-3391.
- Xing C, Li C, He B. Insulin sensitizers for improving the endocrine and metabolic profile in overweight women with PCOS. J Clin Endocrinol Metab. 2020;105:2950-2963.
- Elkind-Hirsch KE, Chappell N, Shaler D, et al. Liraglutide 3 mg on weight, body composition and hormonal and metabolic parameters in women with obesity and polycystic ovary syndrome: a randomized placebo-controlled-phase 3 study. Fertil Steril. 2022;118:371-381.
- Amiri M, Nahidi F, Bidhendi-Yarandi R, et al. A comparison of the effects of oral contraceptives on the clinical and biochemical manifestations of polycystic ovary syndrome: a crossover randomized controlled trial. Hum Reprod. 2020;35:175-186.
- Legro RS, Brzyski RG, Diamond NP, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014;371:119-129.
- Gibson-Helm M, Teede H, Dunaif A, et al. Delayed diagnosis and lack of information associated with dissatisfaction in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2017;102:604-612.