User login
Personalizing depression treatment: 2 clinical tools
Approximately one-half of patients with major depressive disorder (MDD) will have partial or nonresponse to first-line antidepressant monotherapy, despite receiving an adequate dosage and sufficient duration of treatment.1 This has led to the definition of treatment-resistant depression (TRD) as a depressive episode that has shown insufficient response to ≥1 trial of an antidepressant that has demonstrated efficacy in clinical trials.1 Depressed patients should be treated to full remission because absence of complete remission is associated with:
- a more recurrent and chronic illness course2,3
- increased medical and psychiatric comorbidities
- greater functional burden
- increased social and economic costs linked with impaired social functioning.4
Clinicians need to properly identify MDD and treatment resistance to guide optimal treatment choices. Additional tools are necessary to accurately identify, document, and communicate about symptoms commonly experienced by depressed patients but not fully characterized by DSM-IV-TR MDD criteria.5 Finally, in many cases, trait or situational factors might obfuscate accurate diagnosis and the natural course of illness, and tools that can be implemented practically will help identify patients with MDD.
Our group has created and implemented 2 clinician-administered tools—the SAFER Interview and the Antidepressant Treatment Response Questionnaire (ATRQ)—to enrich the qualitative assessment of MDD and treatment resistance.
SAFER: Assessing the diagnosis, symptom severity
The SAFER interview refines the diagnosis of depressed patients by assessing the state vs trait nature of the symptoms, their assessability, their face and ecological validity, and if they pass the rule of the 3 Ps: pervasiveness, persistence, and pathological nature of the current MDD episode (Table 1 and Table 2).6 This reliable assessment of the patient’s diagnosis and symptom severity is made in a way that reflects the illness in a real-world setting.
Clinical application of SAFER. Implementing SAFER in clinical settings promotes a personalized, dimensional approach by taking into account a varying degree of symptom severity in depressed patients, in contrast to relying on symptom lists as found in the DSM-IV-TR. Using the SAFER interview deepens the typical psychiatric diagnostic process, allows for a more precise understanding of the patient’s situation, and may help clinicians select effective treatments that target specific symptoms, thus resulting in more rapid alleviation of MDD.6
Table 1
The SAFER interview: Assessing depression in a real-world setting
State vs trait nature of the symptoms
|
Assessability
|
Face validity
|
Ecological validity
|
Rule of the 3 Ps
|
| © Massachusetts General Hospital Source: Reference 6 |
Table 2
The SAFER criteria: Rule of the 3 Ps
| Pervasive—Do the major symptoms affect the patient across multiple arenas of life (work, relationships, school, chores, etc.)? |
| Persistent—Do the main symptoms occur most days, most of the day during the current episode? |
| Pathological—Do the symptoms of the present episode interfere with functioning? |
| © Massachusetts General Hospital Source: Reference 6 |
CASE REPORT: Worsening symptoms
Ms. Y, age 53, has been depressed for 30 years. She hardly remembers a time in her life when she felt good for more than a few days. However, 2 months ago she noted her symptoms got worse. She presents with many MDD symptoms as assessed by the Hamilton Depression Rating Scale, eg, ongoing depressive mood, feelings of guilt, major sleep disturbances, and work impairments.
Using SAFER to evaluate Ms. Y, a clinician would ask: Does she have symptoms that are present primarily during an episode of acute illness? Does the episode constitute a measurable exacerbation of preexisting symptoms? This clinical vignette illustrates the importance of the first SAFER criterion, state vs trait nature of the symptoms. Ms. Y is a SAFER “pass”—meaning consistent with a major depressive episode—because exacerbation of preexisting symptoms is measurable. However, if her symptoms represented a chronic, long-standing trait, she would be a SAFER “fail” based on this criterion, and her symptoms likely would not improve during a brief pharmacologic intervention. For such patients, SAFER would have oriented the clinician toward alternative therapies such as psychotherapy or a combination of longer, more complex pharmacologic treatment and psychotherapy.
By helping clinicians refine MDD diagnoses, SAFER draws attention to specific depression entities (eg, MDD or dysthymia) and directs them toward appropriate guidelines, treatment algorithms, and precautions (eg, when treated with pharmacotherapy, dysthymic patients are particularly sensitive to unwanted effects and adherence is a serious issue).7 The SAFER interview also can help identify when what appears to be a depressive episode clearly is precipitated by situational factors, and is more likely to be influenced by changes in the patient’s life than by treatment. For such patients, first consider nonpharmacologic interventions to avoid unnecessary medication exposure.
ATRQ: Efficacy and adequacy
The ATRQ examines the efficacy (improvement from 0% [not improved at all] to 100% [completely improved]), and adequacy (adequate duration and dose) of any antidepressant treatment in a step-by-step procedure.1,8,9 For a copy of the ATRQ, click here.
While conducting the interview, clinicians ask about treatment adherence to each medication trial. A treatment-resistant patient may go through many types of trials, from monotherapy to combination to augmentation.10 For each trial, the ATRQ systematically reviews 4 strategies to enhance treatment response:
- increasing the initial antidepressant dosage11
- combining the initial antidepressant with another antidepressant, typically from another class12
- augmenting the initial antidepressant with a nonantidepressant12
- switching from the initial antidepressant to another antidepressant.13
These strategies also are applied in cases of lost sustained antidepressant efficacy or depressive relapse/recurrence, although empirical evidence supporting these strategies is lacking, with the possible exception of dose increase.14,15
In the convention our group adopted, an adequate antidepressant trial must be ≥6 weeks in total length, with a dose within an adequate range as specified in the medication’s package insert. In addition, for the purposes of conducting TRD trials, we have considered a patient treatment-resistant if response to adequate dose and duration is <50%. On the ATRQ, 50% improvement refers to 50% symptom reduction from baseline without achieving remission. In an initial clinical trial that lasts ≥6 weeks, any dose increase (for ≥4 weeks) represents optimization and is not considered a new or separate trial, whereas augmentation or combination therapy (for ≥3 weeks) or a switch to another antidepressant (for ≥6 weeks) are considered new trials/treatments.
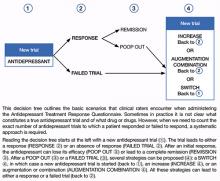
Decision tree for ATRQ. Because antidepressant treatment always is constrained by personal (eg, adherence, insurance coverage, etc.) and clinical (eg, contraindications due to comorbid conditions, side effects, etc.) considerations, we propose a decision tree to help clinicians determine the number of failed antidepressant trials their patient experienced (Figure). Although this tree does not represent all treatment scenarios, we hope it could help clinicians implement TRD treatment strategies because it highlights proper assessment of treatment duration, dosage, and changes before applying a TRD diagnosis.
The ATRQ meticulously examines patients’ antidepressant history to identify:
- pseudo-resistance, to guide adequate dosing and/or duration, and
- resistance, to propose next-step treatment.
Pseudo-resistance refers to treatment failures that can be attributed to factors such as inadequate treatment dosing or duration, atypical pharmacokinetics that reduce agents’ effectiveness, patient nonadherence (eg, due to adverse effects), or misdiagnosis of the primary disorder (ie, other mood disorders or depressive subsets such as dysthymia or minor depression mistreated as unipolar depression).16,17 Studies show that many patients with MDD referred to specialty settings are undertreated and receive inadequate antidepressant doses,18 which suggests that many referrals for TRD are in fact pseudo-resistance.19 Despite the lack of consensus on criteria for TRD,1 standardization of what constitutes treatment adequacy during antidepressant trials (eg, adherence, dose, duration) is indispensable.
Clinical application of the ATRQ. Because TRD may require specific interventions, we first need to properly identify treatment resistance. Also, systematic use of a classification system enhances the ability of clinicians and patients to provide meaningful descriptions of antidepressant resistance.
In clinical practice, choice of treatment strategy is based on factors that include partial or nonresponse, tolerability, avoiding withdrawal symptoms, the need to target side effects of a current antidepressant by administering another drug, cost, avoiding drug-drug interactions, and patient preference. Because a treatment-resistant patient may go through many types of trials, it is essential to obtain information about all treatments to determine the number of failed clinical trials a patient may have had for the current MDD episode and lifetime episodes. The importance of asking about adherence to each trial cannot be overemphasized.
Figure: Using the Massachusetts General Hospital Antidepressant Treatment History Questionnaire: A decision tree
CASE REPORT: Limited improvement
Mr. T, age 45, reported that his current depressive episode started several years ago. The first antidepressant trial he received, sertraline, 100 mg/d for 3 months, resulted in 0% improvement. Next he received citalopram, 20 mg/d, for 1 month, without any improvement. The next trial, venlafaxine, 75 mg/d, for 7 weeks, resulted in a 30% response. More recently Mr. B tried duloxetine, up to 90 mg/d, for 2 years with an 80% improvement during the first 3 months and then a decrease of response to <40%. He then received aripiprazole, 10 mg/d, in combination with duloxetine for approximately 4 weeks with no response.
Using the ATRQ to evaluate Mr. B, a clinician would consider the sertraline trial as a failed trial. The citalopram treatment would not be considered an adequate trial because it was too short. The venlafaxine course wouldn’t count as an adequate trial because the dosage was too low. The trial with duloxetine would count as a response (80% improvement) followed by a “poop out” or tachyphylaxis (40% improvement). The fifth trial with the combination of duloxetine and aripiprazole would count as a failed trial. The ATRQ highlights which drugs have been used for too short a duration or at too low a dosage. In Mr. B’s case, using the ATRQ revealed that of 5 trials, only 2 showed antidepressant resistance.
The ATRQ and decision tree are meant to provide clinicians with user-friendly tools to more precisely determine the number of failed antidepressant trials a patient experienced. By assessing if an antidepressant trial had an adequate dose and duration, the ATRQ can help suggest the next treatment options. For example, if a trial was inadequate in dose and/or duration but the patient tolerated the medication, then optimizing treatment with the current drug would be a logical next step. If a patient does not respond to an adequate trial, clinicians have several options, such as switching to another antidepressant, using a combination of medications or an augmentation strategy, or increasing the dose of the original antidepressant.
Limitations of the ATRQ. Historical rating of treatment is not as accurate as a prospective trial. Another limitation of the ATRQ is that the minimum effective dose is accepted as adequate; many clinicians would suggest that such a dose is inadequate. Also, the duration specified for augmentation, dose increase, and monotherapy are based on expert consensus1 rather than systematic research. Nonetheless, this method of documenting prior trials and treatment adequacy is an important advance.
The ATRQ lacks a place to indicate discontinuation due to intolerance. Knowing if adverse events caused treatment nonadherence or discontinuation is relevant to selecting treatment.
The ATRQ considers only pharmacotherapy and electroconvulsive therapy. Comprehensive assessment of treatment resistance requires asking about depression-specific, evidence-based psychotherapies, including cognitive-behavioral therapy and interpersonal therapy. Another precaution is that the ATRQ and SAFER should be used in conjunction with structured or semi-structured clinical interviews and other clinical tools to rule out other primary diagnoses (eg, bipolar disorder, posttraumatic stress disorder, obsessive-compulsive disorder, or surreptitious substance abuse).20
Using SAFER and ATRQ allows clinicians to make a proper MDD diagnosis with an accurate, reliable assessment of symptom severity and a precise count of antidepressant trials of adequate duration and dose. SAFER helps differentiate MDD from other depressive disorders and can be used to separate MDD from a similar presentation of a reaction to external circumstances that may remit if these circumstances change.
These 2 clinical tools focus on MDD and TRD. Compared with treatment resistance in MDD, treatment resistance in other forms of depression, such as minor depressive disorder or dysthymia, has been inadequately researched21,22 and should be addressed in large studies. However, to help patients achieve remission of depressive disorders—especially MDD—clinicians should use patient information gathered via measurement-based care in combination with algorithm recommendations.23 Persistence of clinical care of MDD is essential because several treatment steps might be necessary for some patients to achieve remission. Throughout treatment, patients’ symptoms, adverse events secondary to ongoing treatment, and medication adherence should be assessed with appropriate tools. ATRQ and SAFER offer a clinician support in assessing treatment resistance history and a patient’s likelihood to respond to pharmacologic treatment.
Related Resources
- Desseilles M, Witte J, Chang TE, et al. Assessing the adequacy of past antidepressant trials: a clinician’s guide to the Antidepressant Treatment Response Questionnaire (ATRQ). J Clin Psychiatry. 2011;72(8):1152-1154.
- Targum SD, Pollack MH, Fava M. Redefining affective disorders: relevance for drug development. CNS Neurosci Ther. 2008;14(1):2-9.
- Depression Clinical and Research Program. www.massgeneral.org/psychiatry/services/dcrp_home.aspx.
Drug Brand Names
- Aripiprazole • Abilify
- Citalopram • Celexa
- Duloxetine • Cymbalta
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosures
Dr. Desseilles was supported by the National Funds for Scientific Research of Belgium. Additional support came from the Belgian American Educational Foundation.
Dr. Fava receives grant/research support from, is a consultant to, and/or is a speaker for Abbott Laboratories, Adamed, Advanced Meeting Partners, Affectis Pharmaceuticals, Alkermes, Amarin Pharma, American Psychiatric Association, American Society of Clinical Psychopharmacology, Aspect Medical Systems, AstraZeneca, Auspex Pharmaceuticals, Bayer, Belvoir Media Group, Best Practice Project Management, BioMarin, BioResearch, Biovail, Boehringer Ingelheim, BrainCells, Bristol-Myers Squibb, CeNeRx BioPharma, Cephalon, Clinical Trials Solutions, Clintara, CME Institute/Physicians Postgraduate Press, CNS Response, Compellis Pharmaceuticals, Covance, Covidien, Cypress Pharmaceutical, DiagnoSearch Life Sciences, Dainippon Sumitomo Pharma, Dov Pharmaceuticals, Edgemont Pharmaceuticals, Eisai, Eli Lilly and Company, EnVivo Pharmaceuticals, ePharmaSolutions, EPIX Pharmaceuticals, Euthymics Bioscience, Fabre-Kramer Pharmaceuticals, Forest Pharmaceuticals, Ganeden Biotech, GenOmind, GlaxoSmithKline, Grünenthal GmbH, Icon Clinical Research, Imedex, i3 Innovus/Ingenix, Janssen Pharmaceutica, Johnson & Johnson Pharmaceutical Research & Development, Knoll Pharmaceuticals, Labopharm, Lichtwer Pharma GmbH, Lorex Pharmaceuticals, Lundbeck, MedAvante, Merck, MGH Psychiatry Academy/Primedia, MGH Psychiatry Academy/Reed Elsevier, MSI Methylation Sciences, National Alliance for Research on Schizophrenia & Depression, National Center for Complementary and Alternative Medicine, National Institute of Drug Abuse, National Institute of Mental Health, Naurex, Neuronetics, NextWave Pharmaceuticals, Novartis, Nutrition 21, Orexigen Therapeutics, Organon Pharmaceuticals, Otsuka Pharmaceuticals, PamLab, Pfizer, PharmaStar, Pharmavite®, PharmoRx Therapeutics, Photothera, Precision Human Biolaboratory, Prexa Pharmaceuticals, Puretech Ventures, PsychoGenics, Psylin Neurosciences, RCT Logic, Rexahn Pharmaceuticals, Ridge Diagnostics, Roche Pharmaceuticals, sanofi-aventis US, Schering-Plough, Sepracor, Servier Laboratories, Shire, Solvay Pharmaceuticals, Somaxon Pharmaceuticals, Somerset Pharmaceuticals, Sunovion Pharmaceuticals, Supernus Pharmaceuticals, Synthelabo, Takeda Pharmaceutical, Tal Medical, Tetragenex Pharmaceuticals, Transcept Pharmaceuticals, TransForm Pharmaceuticals, United BioSource, Vanda Pharmaceuticals, and Wyeth-Ayerst Laboratories.
Over the past year, Dr. Mischoulon has received research support from the Bowman Family Foundation, FisherWallace, Ganeden, and Nordic Naturals. He has received honoraria for speaking from Nordic Naturals. He has received royalties from Lippincott Williams & Wilkins for the book Natural medications for psychiatric disorders: Considering the alternatives. No payment has exceeded $10,000.
Dr. Freeman has received research support from Eli Lilly and Company, Forest Laboratories, and GlaxoSmithKline, and served on a one-time advisory board to Bristol-Myers Squibb.
Acknowledgment
The authors acknowledge Janet Witte, MD, MPH, Trina E. Chang, MD, MPH, Nadia Iovieno, MD, PhD, Christina Dording, MD, Heidi Ashih, MD, PhD, Maren Nyer, PhD, and Marasha-Fiona De Jong, MD from the Clinical Trials Network and Institute at Massachusetts General Hospital, Boston, MA.
1. Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53(8):649-659.
2. Judd LL, Akiskal HS, Maser JD, et al. Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J Affect Disord. 1998;50(2-3):97-108.
3. Van Londen L, Molenaar RP, Goekoop JG, et al. Three- to 5-year prospective follow-up of outcome in major depression. Psychol Med. 1998;28(3):731-735.
4. Paykel ES. Achieving gains beyond response. Acta Psychiatr Scand Suppl. 2002;(415):12-17.
5. Cassano P, Fava M. Depression and public health: an overview. J Psychosom Res. 2002;53(4):849-857.
6. Targum SD, Pollack MH, Fava M. Redefining affective disorders: relevance for drug development. CNS Neurosci Ther. 2008;14(1):2-9.
7. Versiani M, Nardi AE, Figueira I. Pharmacotherapy of dysthymia: review and new findings. Eur Psychiatry. 1998;13(4):203-209.
8. Chandler GM, Iosifescu DV, Pollack MH, et al. RESEARCH: validation of the Massachusetts General Hospital Antidepressant Treatment History Questionnaire (ATRQ). CNS Neurosci Ther. 2010;16(5):322-325.
9. Desseilles M, Witte J, Chang TE, et al. Assessing the adequacy of past antidepressant trials: a clinician’s guide to the antidepressant treatment response questionnaire. J Clin Psychiatry. 2011;72(8):1152-1154.
10. Fava M. Augmentation and combination strategies for complicated depression. J Clin Psychiatry. 2009;70(11):e40.-
11. Adli M, Baethge C, Heinz A, et al. Is dose escalation of antidepressants a rational strategy after a medium-dose treatment has failed? A systematic review. Eur Arch Psychiatry Clin Neurosci. 2005;255(6):387-400.
12. Fava M, Rush AJ. Current status of augmentation and combination treatments for major depressive disorder: a literature review and a proposal for a novel approach to improve practice. Psychother Psychosom. 2006;75(3):139-153.
13. Wohlreich MM, Mallinckrodt CH, Watkin JG, et al. Immediate switching of antidepressant therapy: results from a clinical trial of duloxetine. Ann Clin Psychiatry. 2005;17(4):259-268.
14. Schmidt ME, Fava M, Zhang S, et al. Treatment approaches to major depressive disorder relapse. Part 1: dose increase. Psychother Psychosom. 2002;71(4):190-194.
15. Fava M, Detke MJ, Balestrieri M, et al. Management of depression relapse: re-initiation of duloxetine treatment or dose increase. J Psychiatr Res. 2006;40(4):328-336.
16. Kornstein SG, Schneider RK. Clinical features of treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl 16):18-25.
17. Souery D, Papakostas GI, Trivedi MH. Treatment-resistant depression. J Clin Psychiatry. 2006;67(suppl 6):16-22.
18. Hirschfeld RM, Keller MB, Panico S, et al. The National Depressive and Manic-Depressive Association consensus statement on the undertreatment of depression. JAMA. 1997;277(4):333-340.
19. Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl 16):10-17.
20. Parker G, Malhi GS, Crawford JG, et al. Identifying “paradigm failures” contributing to treatment-resistant depression. J Affect Disord. 2005;87(2-3):185-191.
21. Harris SJ, Parent M. Patient with chronic and apparently treatment-resistant dysthymia. Am J Psychiatry. 1986;143(2):260-261.
22. Amsterdam J, Hornig M, Nierenberg AA. Treatment-resistant mood disorders. Cambridge United Kingdom: Cambridge University Press; 2001.
23. Trivedi MH. Tools and strategies for ongoing assessment of depression: a measurement-based approach to remission. J Clin Psychiatry. 2009;70(suppl 6):26-31.
Approximately one-half of patients with major depressive disorder (MDD) will have partial or nonresponse to first-line antidepressant monotherapy, despite receiving an adequate dosage and sufficient duration of treatment.1 This has led to the definition of treatment-resistant depression (TRD) as a depressive episode that has shown insufficient response to ≥1 trial of an antidepressant that has demonstrated efficacy in clinical trials.1 Depressed patients should be treated to full remission because absence of complete remission is associated with:
- a more recurrent and chronic illness course2,3
- increased medical and psychiatric comorbidities
- greater functional burden
- increased social and economic costs linked with impaired social functioning.4
Clinicians need to properly identify MDD and treatment resistance to guide optimal treatment choices. Additional tools are necessary to accurately identify, document, and communicate about symptoms commonly experienced by depressed patients but not fully characterized by DSM-IV-TR MDD criteria.5 Finally, in many cases, trait or situational factors might obfuscate accurate diagnosis and the natural course of illness, and tools that can be implemented practically will help identify patients with MDD.
Our group has created and implemented 2 clinician-administered tools—the SAFER Interview and the Antidepressant Treatment Response Questionnaire (ATRQ)—to enrich the qualitative assessment of MDD and treatment resistance.
SAFER: Assessing the diagnosis, symptom severity
The SAFER interview refines the diagnosis of depressed patients by assessing the state vs trait nature of the symptoms, their assessability, their face and ecological validity, and if they pass the rule of the 3 Ps: pervasiveness, persistence, and pathological nature of the current MDD episode (Table 1 and Table 2).6 This reliable assessment of the patient’s diagnosis and symptom severity is made in a way that reflects the illness in a real-world setting.
Clinical application of SAFER. Implementing SAFER in clinical settings promotes a personalized, dimensional approach by taking into account a varying degree of symptom severity in depressed patients, in contrast to relying on symptom lists as found in the DSM-IV-TR. Using the SAFER interview deepens the typical psychiatric diagnostic process, allows for a more precise understanding of the patient’s situation, and may help clinicians select effective treatments that target specific symptoms, thus resulting in more rapid alleviation of MDD.6
Table 1
The SAFER interview: Assessing depression in a real-world setting
State vs trait nature of the symptoms
|
Assessability
|
Face validity
|
Ecological validity
|
Rule of the 3 Ps
|
| © Massachusetts General Hospital Source: Reference 6 |
Table 2
The SAFER criteria: Rule of the 3 Ps
| Pervasive—Do the major symptoms affect the patient across multiple arenas of life (work, relationships, school, chores, etc.)? |
| Persistent—Do the main symptoms occur most days, most of the day during the current episode? |
| Pathological—Do the symptoms of the present episode interfere with functioning? |
| © Massachusetts General Hospital Source: Reference 6 |
CASE REPORT: Worsening symptoms
Ms. Y, age 53, has been depressed for 30 years. She hardly remembers a time in her life when she felt good for more than a few days. However, 2 months ago she noted her symptoms got worse. She presents with many MDD symptoms as assessed by the Hamilton Depression Rating Scale, eg, ongoing depressive mood, feelings of guilt, major sleep disturbances, and work impairments.
Using SAFER to evaluate Ms. Y, a clinician would ask: Does she have symptoms that are present primarily during an episode of acute illness? Does the episode constitute a measurable exacerbation of preexisting symptoms? This clinical vignette illustrates the importance of the first SAFER criterion, state vs trait nature of the symptoms. Ms. Y is a SAFER “pass”—meaning consistent with a major depressive episode—because exacerbation of preexisting symptoms is measurable. However, if her symptoms represented a chronic, long-standing trait, she would be a SAFER “fail” based on this criterion, and her symptoms likely would not improve during a brief pharmacologic intervention. For such patients, SAFER would have oriented the clinician toward alternative therapies such as psychotherapy or a combination of longer, more complex pharmacologic treatment and psychotherapy.
By helping clinicians refine MDD diagnoses, SAFER draws attention to specific depression entities (eg, MDD or dysthymia) and directs them toward appropriate guidelines, treatment algorithms, and precautions (eg, when treated with pharmacotherapy, dysthymic patients are particularly sensitive to unwanted effects and adherence is a serious issue).7 The SAFER interview also can help identify when what appears to be a depressive episode clearly is precipitated by situational factors, and is more likely to be influenced by changes in the patient’s life than by treatment. For such patients, first consider nonpharmacologic interventions to avoid unnecessary medication exposure.
ATRQ: Efficacy and adequacy
The ATRQ examines the efficacy (improvement from 0% [not improved at all] to 100% [completely improved]), and adequacy (adequate duration and dose) of any antidepressant treatment in a step-by-step procedure.1,8,9 For a copy of the ATRQ, click here.
While conducting the interview, clinicians ask about treatment adherence to each medication trial. A treatment-resistant patient may go through many types of trials, from monotherapy to combination to augmentation.10 For each trial, the ATRQ systematically reviews 4 strategies to enhance treatment response:
- increasing the initial antidepressant dosage11
- combining the initial antidepressant with another antidepressant, typically from another class12
- augmenting the initial antidepressant with a nonantidepressant12
- switching from the initial antidepressant to another antidepressant.13
These strategies also are applied in cases of lost sustained antidepressant efficacy or depressive relapse/recurrence, although empirical evidence supporting these strategies is lacking, with the possible exception of dose increase.14,15
In the convention our group adopted, an adequate antidepressant trial must be ≥6 weeks in total length, with a dose within an adequate range as specified in the medication’s package insert. In addition, for the purposes of conducting TRD trials, we have considered a patient treatment-resistant if response to adequate dose and duration is <50%. On the ATRQ, 50% improvement refers to 50% symptom reduction from baseline without achieving remission. In an initial clinical trial that lasts ≥6 weeks, any dose increase (for ≥4 weeks) represents optimization and is not considered a new or separate trial, whereas augmentation or combination therapy (for ≥3 weeks) or a switch to another antidepressant (for ≥6 weeks) are considered new trials/treatments.
Decision tree for ATRQ. Because antidepressant treatment always is constrained by personal (eg, adherence, insurance coverage, etc.) and clinical (eg, contraindications due to comorbid conditions, side effects, etc.) considerations, we propose a decision tree to help clinicians determine the number of failed antidepressant trials their patient experienced (Figure). Although this tree does not represent all treatment scenarios, we hope it could help clinicians implement TRD treatment strategies because it highlights proper assessment of treatment duration, dosage, and changes before applying a TRD diagnosis.
The ATRQ meticulously examines patients’ antidepressant history to identify:
- pseudo-resistance, to guide adequate dosing and/or duration, and
- resistance, to propose next-step treatment.
Pseudo-resistance refers to treatment failures that can be attributed to factors such as inadequate treatment dosing or duration, atypical pharmacokinetics that reduce agents’ effectiveness, patient nonadherence (eg, due to adverse effects), or misdiagnosis of the primary disorder (ie, other mood disorders or depressive subsets such as dysthymia or minor depression mistreated as unipolar depression).16,17 Studies show that many patients with MDD referred to specialty settings are undertreated and receive inadequate antidepressant doses,18 which suggests that many referrals for TRD are in fact pseudo-resistance.19 Despite the lack of consensus on criteria for TRD,1 standardization of what constitutes treatment adequacy during antidepressant trials (eg, adherence, dose, duration) is indispensable.
Clinical application of the ATRQ. Because TRD may require specific interventions, we first need to properly identify treatment resistance. Also, systematic use of a classification system enhances the ability of clinicians and patients to provide meaningful descriptions of antidepressant resistance.
In clinical practice, choice of treatment strategy is based on factors that include partial or nonresponse, tolerability, avoiding withdrawal symptoms, the need to target side effects of a current antidepressant by administering another drug, cost, avoiding drug-drug interactions, and patient preference. Because a treatment-resistant patient may go through many types of trials, it is essential to obtain information about all treatments to determine the number of failed clinical trials a patient may have had for the current MDD episode and lifetime episodes. The importance of asking about adherence to each trial cannot be overemphasized.
Figure: Using the Massachusetts General Hospital Antidepressant Treatment History Questionnaire: A decision tree
CASE REPORT: Limited improvement
Mr. T, age 45, reported that his current depressive episode started several years ago. The first antidepressant trial he received, sertraline, 100 mg/d for 3 months, resulted in 0% improvement. Next he received citalopram, 20 mg/d, for 1 month, without any improvement. The next trial, venlafaxine, 75 mg/d, for 7 weeks, resulted in a 30% response. More recently Mr. B tried duloxetine, up to 90 mg/d, for 2 years with an 80% improvement during the first 3 months and then a decrease of response to <40%. He then received aripiprazole, 10 mg/d, in combination with duloxetine for approximately 4 weeks with no response.
Using the ATRQ to evaluate Mr. B, a clinician would consider the sertraline trial as a failed trial. The citalopram treatment would not be considered an adequate trial because it was too short. The venlafaxine course wouldn’t count as an adequate trial because the dosage was too low. The trial with duloxetine would count as a response (80% improvement) followed by a “poop out” or tachyphylaxis (40% improvement). The fifth trial with the combination of duloxetine and aripiprazole would count as a failed trial. The ATRQ highlights which drugs have been used for too short a duration or at too low a dosage. In Mr. B’s case, using the ATRQ revealed that of 5 trials, only 2 showed antidepressant resistance.
The ATRQ and decision tree are meant to provide clinicians with user-friendly tools to more precisely determine the number of failed antidepressant trials a patient experienced. By assessing if an antidepressant trial had an adequate dose and duration, the ATRQ can help suggest the next treatment options. For example, if a trial was inadequate in dose and/or duration but the patient tolerated the medication, then optimizing treatment with the current drug would be a logical next step. If a patient does not respond to an adequate trial, clinicians have several options, such as switching to another antidepressant, using a combination of medications or an augmentation strategy, or increasing the dose of the original antidepressant.
Limitations of the ATRQ. Historical rating of treatment is not as accurate as a prospective trial. Another limitation of the ATRQ is that the minimum effective dose is accepted as adequate; many clinicians would suggest that such a dose is inadequate. Also, the duration specified for augmentation, dose increase, and monotherapy are based on expert consensus1 rather than systematic research. Nonetheless, this method of documenting prior trials and treatment adequacy is an important advance.
The ATRQ lacks a place to indicate discontinuation due to intolerance. Knowing if adverse events caused treatment nonadherence or discontinuation is relevant to selecting treatment.
The ATRQ considers only pharmacotherapy and electroconvulsive therapy. Comprehensive assessment of treatment resistance requires asking about depression-specific, evidence-based psychotherapies, including cognitive-behavioral therapy and interpersonal therapy. Another precaution is that the ATRQ and SAFER should be used in conjunction with structured or semi-structured clinical interviews and other clinical tools to rule out other primary diagnoses (eg, bipolar disorder, posttraumatic stress disorder, obsessive-compulsive disorder, or surreptitious substance abuse).20
Using SAFER and ATRQ allows clinicians to make a proper MDD diagnosis with an accurate, reliable assessment of symptom severity and a precise count of antidepressant trials of adequate duration and dose. SAFER helps differentiate MDD from other depressive disorders and can be used to separate MDD from a similar presentation of a reaction to external circumstances that may remit if these circumstances change.
These 2 clinical tools focus on MDD and TRD. Compared with treatment resistance in MDD, treatment resistance in other forms of depression, such as minor depressive disorder or dysthymia, has been inadequately researched21,22 and should be addressed in large studies. However, to help patients achieve remission of depressive disorders—especially MDD—clinicians should use patient information gathered via measurement-based care in combination with algorithm recommendations.23 Persistence of clinical care of MDD is essential because several treatment steps might be necessary for some patients to achieve remission. Throughout treatment, patients’ symptoms, adverse events secondary to ongoing treatment, and medication adherence should be assessed with appropriate tools. ATRQ and SAFER offer a clinician support in assessing treatment resistance history and a patient’s likelihood to respond to pharmacologic treatment.
Related Resources
- Desseilles M, Witte J, Chang TE, et al. Assessing the adequacy of past antidepressant trials: a clinician’s guide to the Antidepressant Treatment Response Questionnaire (ATRQ). J Clin Psychiatry. 2011;72(8):1152-1154.
- Targum SD, Pollack MH, Fava M. Redefining affective disorders: relevance for drug development. CNS Neurosci Ther. 2008;14(1):2-9.
- Depression Clinical and Research Program. www.massgeneral.org/psychiatry/services/dcrp_home.aspx.
Drug Brand Names
- Aripiprazole • Abilify
- Citalopram • Celexa
- Duloxetine • Cymbalta
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosures
Dr. Desseilles was supported by the National Funds for Scientific Research of Belgium. Additional support came from the Belgian American Educational Foundation.
Dr. Fava receives grant/research support from, is a consultant to, and/or is a speaker for Abbott Laboratories, Adamed, Advanced Meeting Partners, Affectis Pharmaceuticals, Alkermes, Amarin Pharma, American Psychiatric Association, American Society of Clinical Psychopharmacology, Aspect Medical Systems, AstraZeneca, Auspex Pharmaceuticals, Bayer, Belvoir Media Group, Best Practice Project Management, BioMarin, BioResearch, Biovail, Boehringer Ingelheim, BrainCells, Bristol-Myers Squibb, CeNeRx BioPharma, Cephalon, Clinical Trials Solutions, Clintara, CME Institute/Physicians Postgraduate Press, CNS Response, Compellis Pharmaceuticals, Covance, Covidien, Cypress Pharmaceutical, DiagnoSearch Life Sciences, Dainippon Sumitomo Pharma, Dov Pharmaceuticals, Edgemont Pharmaceuticals, Eisai, Eli Lilly and Company, EnVivo Pharmaceuticals, ePharmaSolutions, EPIX Pharmaceuticals, Euthymics Bioscience, Fabre-Kramer Pharmaceuticals, Forest Pharmaceuticals, Ganeden Biotech, GenOmind, GlaxoSmithKline, Grünenthal GmbH, Icon Clinical Research, Imedex, i3 Innovus/Ingenix, Janssen Pharmaceutica, Johnson & Johnson Pharmaceutical Research & Development, Knoll Pharmaceuticals, Labopharm, Lichtwer Pharma GmbH, Lorex Pharmaceuticals, Lundbeck, MedAvante, Merck, MGH Psychiatry Academy/Primedia, MGH Psychiatry Academy/Reed Elsevier, MSI Methylation Sciences, National Alliance for Research on Schizophrenia & Depression, National Center for Complementary and Alternative Medicine, National Institute of Drug Abuse, National Institute of Mental Health, Naurex, Neuronetics, NextWave Pharmaceuticals, Novartis, Nutrition 21, Orexigen Therapeutics, Organon Pharmaceuticals, Otsuka Pharmaceuticals, PamLab, Pfizer, PharmaStar, Pharmavite®, PharmoRx Therapeutics, Photothera, Precision Human Biolaboratory, Prexa Pharmaceuticals, Puretech Ventures, PsychoGenics, Psylin Neurosciences, RCT Logic, Rexahn Pharmaceuticals, Ridge Diagnostics, Roche Pharmaceuticals, sanofi-aventis US, Schering-Plough, Sepracor, Servier Laboratories, Shire, Solvay Pharmaceuticals, Somaxon Pharmaceuticals, Somerset Pharmaceuticals, Sunovion Pharmaceuticals, Supernus Pharmaceuticals, Synthelabo, Takeda Pharmaceutical, Tal Medical, Tetragenex Pharmaceuticals, Transcept Pharmaceuticals, TransForm Pharmaceuticals, United BioSource, Vanda Pharmaceuticals, and Wyeth-Ayerst Laboratories.
Over the past year, Dr. Mischoulon has received research support from the Bowman Family Foundation, FisherWallace, Ganeden, and Nordic Naturals. He has received honoraria for speaking from Nordic Naturals. He has received royalties from Lippincott Williams & Wilkins for the book Natural medications for psychiatric disorders: Considering the alternatives. No payment has exceeded $10,000.
Dr. Freeman has received research support from Eli Lilly and Company, Forest Laboratories, and GlaxoSmithKline, and served on a one-time advisory board to Bristol-Myers Squibb.
Acknowledgment
The authors acknowledge Janet Witte, MD, MPH, Trina E. Chang, MD, MPH, Nadia Iovieno, MD, PhD, Christina Dording, MD, Heidi Ashih, MD, PhD, Maren Nyer, PhD, and Marasha-Fiona De Jong, MD from the Clinical Trials Network and Institute at Massachusetts General Hospital, Boston, MA.
Approximately one-half of patients with major depressive disorder (MDD) will have partial or nonresponse to first-line antidepressant monotherapy, despite receiving an adequate dosage and sufficient duration of treatment.1 This has led to the definition of treatment-resistant depression (TRD) as a depressive episode that has shown insufficient response to ≥1 trial of an antidepressant that has demonstrated efficacy in clinical trials.1 Depressed patients should be treated to full remission because absence of complete remission is associated with:
- a more recurrent and chronic illness course2,3
- increased medical and psychiatric comorbidities
- greater functional burden
- increased social and economic costs linked with impaired social functioning.4
Clinicians need to properly identify MDD and treatment resistance to guide optimal treatment choices. Additional tools are necessary to accurately identify, document, and communicate about symptoms commonly experienced by depressed patients but not fully characterized by DSM-IV-TR MDD criteria.5 Finally, in many cases, trait or situational factors might obfuscate accurate diagnosis and the natural course of illness, and tools that can be implemented practically will help identify patients with MDD.
Our group has created and implemented 2 clinician-administered tools—the SAFER Interview and the Antidepressant Treatment Response Questionnaire (ATRQ)—to enrich the qualitative assessment of MDD and treatment resistance.
SAFER: Assessing the diagnosis, symptom severity
The SAFER interview refines the diagnosis of depressed patients by assessing the state vs trait nature of the symptoms, their assessability, their face and ecological validity, and if they pass the rule of the 3 Ps: pervasiveness, persistence, and pathological nature of the current MDD episode (Table 1 and Table 2).6 This reliable assessment of the patient’s diagnosis and symptom severity is made in a way that reflects the illness in a real-world setting.
Clinical application of SAFER. Implementing SAFER in clinical settings promotes a personalized, dimensional approach by taking into account a varying degree of symptom severity in depressed patients, in contrast to relying on symptom lists as found in the DSM-IV-TR. Using the SAFER interview deepens the typical psychiatric diagnostic process, allows for a more precise understanding of the patient’s situation, and may help clinicians select effective treatments that target specific symptoms, thus resulting in more rapid alleviation of MDD.6
Table 1
The SAFER interview: Assessing depression in a real-world setting
State vs trait nature of the symptoms
|
Assessability
|
Face validity
|
Ecological validity
|
Rule of the 3 Ps
|
| © Massachusetts General Hospital Source: Reference 6 |
Table 2
The SAFER criteria: Rule of the 3 Ps
| Pervasive—Do the major symptoms affect the patient across multiple arenas of life (work, relationships, school, chores, etc.)? |
| Persistent—Do the main symptoms occur most days, most of the day during the current episode? |
| Pathological—Do the symptoms of the present episode interfere with functioning? |
| © Massachusetts General Hospital Source: Reference 6 |
CASE REPORT: Worsening symptoms
Ms. Y, age 53, has been depressed for 30 years. She hardly remembers a time in her life when she felt good for more than a few days. However, 2 months ago she noted her symptoms got worse. She presents with many MDD symptoms as assessed by the Hamilton Depression Rating Scale, eg, ongoing depressive mood, feelings of guilt, major sleep disturbances, and work impairments.
Using SAFER to evaluate Ms. Y, a clinician would ask: Does she have symptoms that are present primarily during an episode of acute illness? Does the episode constitute a measurable exacerbation of preexisting symptoms? This clinical vignette illustrates the importance of the first SAFER criterion, state vs trait nature of the symptoms. Ms. Y is a SAFER “pass”—meaning consistent with a major depressive episode—because exacerbation of preexisting symptoms is measurable. However, if her symptoms represented a chronic, long-standing trait, she would be a SAFER “fail” based on this criterion, and her symptoms likely would not improve during a brief pharmacologic intervention. For such patients, SAFER would have oriented the clinician toward alternative therapies such as psychotherapy or a combination of longer, more complex pharmacologic treatment and psychotherapy.
By helping clinicians refine MDD diagnoses, SAFER draws attention to specific depression entities (eg, MDD or dysthymia) and directs them toward appropriate guidelines, treatment algorithms, and precautions (eg, when treated with pharmacotherapy, dysthymic patients are particularly sensitive to unwanted effects and adherence is a serious issue).7 The SAFER interview also can help identify when what appears to be a depressive episode clearly is precipitated by situational factors, and is more likely to be influenced by changes in the patient’s life than by treatment. For such patients, first consider nonpharmacologic interventions to avoid unnecessary medication exposure.
ATRQ: Efficacy and adequacy
The ATRQ examines the efficacy (improvement from 0% [not improved at all] to 100% [completely improved]), and adequacy (adequate duration and dose) of any antidepressant treatment in a step-by-step procedure.1,8,9 For a copy of the ATRQ, click here.
While conducting the interview, clinicians ask about treatment adherence to each medication trial. A treatment-resistant patient may go through many types of trials, from monotherapy to combination to augmentation.10 For each trial, the ATRQ systematically reviews 4 strategies to enhance treatment response:
- increasing the initial antidepressant dosage11
- combining the initial antidepressant with another antidepressant, typically from another class12
- augmenting the initial antidepressant with a nonantidepressant12
- switching from the initial antidepressant to another antidepressant.13
These strategies also are applied in cases of lost sustained antidepressant efficacy or depressive relapse/recurrence, although empirical evidence supporting these strategies is lacking, with the possible exception of dose increase.14,15
In the convention our group adopted, an adequate antidepressant trial must be ≥6 weeks in total length, with a dose within an adequate range as specified in the medication’s package insert. In addition, for the purposes of conducting TRD trials, we have considered a patient treatment-resistant if response to adequate dose and duration is <50%. On the ATRQ, 50% improvement refers to 50% symptom reduction from baseline without achieving remission. In an initial clinical trial that lasts ≥6 weeks, any dose increase (for ≥4 weeks) represents optimization and is not considered a new or separate trial, whereas augmentation or combination therapy (for ≥3 weeks) or a switch to another antidepressant (for ≥6 weeks) are considered new trials/treatments.
Decision tree for ATRQ. Because antidepressant treatment always is constrained by personal (eg, adherence, insurance coverage, etc.) and clinical (eg, contraindications due to comorbid conditions, side effects, etc.) considerations, we propose a decision tree to help clinicians determine the number of failed antidepressant trials their patient experienced (Figure). Although this tree does not represent all treatment scenarios, we hope it could help clinicians implement TRD treatment strategies because it highlights proper assessment of treatment duration, dosage, and changes before applying a TRD diagnosis.
The ATRQ meticulously examines patients’ antidepressant history to identify:
- pseudo-resistance, to guide adequate dosing and/or duration, and
- resistance, to propose next-step treatment.
Pseudo-resistance refers to treatment failures that can be attributed to factors such as inadequate treatment dosing or duration, atypical pharmacokinetics that reduce agents’ effectiveness, patient nonadherence (eg, due to adverse effects), or misdiagnosis of the primary disorder (ie, other mood disorders or depressive subsets such as dysthymia or minor depression mistreated as unipolar depression).16,17 Studies show that many patients with MDD referred to specialty settings are undertreated and receive inadequate antidepressant doses,18 which suggests that many referrals for TRD are in fact pseudo-resistance.19 Despite the lack of consensus on criteria for TRD,1 standardization of what constitutes treatment adequacy during antidepressant trials (eg, adherence, dose, duration) is indispensable.
Clinical application of the ATRQ. Because TRD may require specific interventions, we first need to properly identify treatment resistance. Also, systematic use of a classification system enhances the ability of clinicians and patients to provide meaningful descriptions of antidepressant resistance.
In clinical practice, choice of treatment strategy is based on factors that include partial or nonresponse, tolerability, avoiding withdrawal symptoms, the need to target side effects of a current antidepressant by administering another drug, cost, avoiding drug-drug interactions, and patient preference. Because a treatment-resistant patient may go through many types of trials, it is essential to obtain information about all treatments to determine the number of failed clinical trials a patient may have had for the current MDD episode and lifetime episodes. The importance of asking about adherence to each trial cannot be overemphasized.
Figure: Using the Massachusetts General Hospital Antidepressant Treatment History Questionnaire: A decision tree
CASE REPORT: Limited improvement
Mr. T, age 45, reported that his current depressive episode started several years ago. The first antidepressant trial he received, sertraline, 100 mg/d for 3 months, resulted in 0% improvement. Next he received citalopram, 20 mg/d, for 1 month, without any improvement. The next trial, venlafaxine, 75 mg/d, for 7 weeks, resulted in a 30% response. More recently Mr. B tried duloxetine, up to 90 mg/d, for 2 years with an 80% improvement during the first 3 months and then a decrease of response to <40%. He then received aripiprazole, 10 mg/d, in combination with duloxetine for approximately 4 weeks with no response.
Using the ATRQ to evaluate Mr. B, a clinician would consider the sertraline trial as a failed trial. The citalopram treatment would not be considered an adequate trial because it was too short. The venlafaxine course wouldn’t count as an adequate trial because the dosage was too low. The trial with duloxetine would count as a response (80% improvement) followed by a “poop out” or tachyphylaxis (40% improvement). The fifth trial with the combination of duloxetine and aripiprazole would count as a failed trial. The ATRQ highlights which drugs have been used for too short a duration or at too low a dosage. In Mr. B’s case, using the ATRQ revealed that of 5 trials, only 2 showed antidepressant resistance.
The ATRQ and decision tree are meant to provide clinicians with user-friendly tools to more precisely determine the number of failed antidepressant trials a patient experienced. By assessing if an antidepressant trial had an adequate dose and duration, the ATRQ can help suggest the next treatment options. For example, if a trial was inadequate in dose and/or duration but the patient tolerated the medication, then optimizing treatment with the current drug would be a logical next step. If a patient does not respond to an adequate trial, clinicians have several options, such as switching to another antidepressant, using a combination of medications or an augmentation strategy, or increasing the dose of the original antidepressant.
Limitations of the ATRQ. Historical rating of treatment is not as accurate as a prospective trial. Another limitation of the ATRQ is that the minimum effective dose is accepted as adequate; many clinicians would suggest that such a dose is inadequate. Also, the duration specified for augmentation, dose increase, and monotherapy are based on expert consensus1 rather than systematic research. Nonetheless, this method of documenting prior trials and treatment adequacy is an important advance.
The ATRQ lacks a place to indicate discontinuation due to intolerance. Knowing if adverse events caused treatment nonadherence or discontinuation is relevant to selecting treatment.
The ATRQ considers only pharmacotherapy and electroconvulsive therapy. Comprehensive assessment of treatment resistance requires asking about depression-specific, evidence-based psychotherapies, including cognitive-behavioral therapy and interpersonal therapy. Another precaution is that the ATRQ and SAFER should be used in conjunction with structured or semi-structured clinical interviews and other clinical tools to rule out other primary diagnoses (eg, bipolar disorder, posttraumatic stress disorder, obsessive-compulsive disorder, or surreptitious substance abuse).20
Using SAFER and ATRQ allows clinicians to make a proper MDD diagnosis with an accurate, reliable assessment of symptom severity and a precise count of antidepressant trials of adequate duration and dose. SAFER helps differentiate MDD from other depressive disorders and can be used to separate MDD from a similar presentation of a reaction to external circumstances that may remit if these circumstances change.
These 2 clinical tools focus on MDD and TRD. Compared with treatment resistance in MDD, treatment resistance in other forms of depression, such as minor depressive disorder or dysthymia, has been inadequately researched21,22 and should be addressed in large studies. However, to help patients achieve remission of depressive disorders—especially MDD—clinicians should use patient information gathered via measurement-based care in combination with algorithm recommendations.23 Persistence of clinical care of MDD is essential because several treatment steps might be necessary for some patients to achieve remission. Throughout treatment, patients’ symptoms, adverse events secondary to ongoing treatment, and medication adherence should be assessed with appropriate tools. ATRQ and SAFER offer a clinician support in assessing treatment resistance history and a patient’s likelihood to respond to pharmacologic treatment.
Related Resources
- Desseilles M, Witte J, Chang TE, et al. Assessing the adequacy of past antidepressant trials: a clinician’s guide to the Antidepressant Treatment Response Questionnaire (ATRQ). J Clin Psychiatry. 2011;72(8):1152-1154.
- Targum SD, Pollack MH, Fava M. Redefining affective disorders: relevance for drug development. CNS Neurosci Ther. 2008;14(1):2-9.
- Depression Clinical and Research Program. www.massgeneral.org/psychiatry/services/dcrp_home.aspx.
Drug Brand Names
- Aripiprazole • Abilify
- Citalopram • Celexa
- Duloxetine • Cymbalta
- Sertraline • Zoloft
- Venlafaxine • Effexor
Disclosures
Dr. Desseilles was supported by the National Funds for Scientific Research of Belgium. Additional support came from the Belgian American Educational Foundation.
Dr. Fava receives grant/research support from, is a consultant to, and/or is a speaker for Abbott Laboratories, Adamed, Advanced Meeting Partners, Affectis Pharmaceuticals, Alkermes, Amarin Pharma, American Psychiatric Association, American Society of Clinical Psychopharmacology, Aspect Medical Systems, AstraZeneca, Auspex Pharmaceuticals, Bayer, Belvoir Media Group, Best Practice Project Management, BioMarin, BioResearch, Biovail, Boehringer Ingelheim, BrainCells, Bristol-Myers Squibb, CeNeRx BioPharma, Cephalon, Clinical Trials Solutions, Clintara, CME Institute/Physicians Postgraduate Press, CNS Response, Compellis Pharmaceuticals, Covance, Covidien, Cypress Pharmaceutical, DiagnoSearch Life Sciences, Dainippon Sumitomo Pharma, Dov Pharmaceuticals, Edgemont Pharmaceuticals, Eisai, Eli Lilly and Company, EnVivo Pharmaceuticals, ePharmaSolutions, EPIX Pharmaceuticals, Euthymics Bioscience, Fabre-Kramer Pharmaceuticals, Forest Pharmaceuticals, Ganeden Biotech, GenOmind, GlaxoSmithKline, Grünenthal GmbH, Icon Clinical Research, Imedex, i3 Innovus/Ingenix, Janssen Pharmaceutica, Johnson & Johnson Pharmaceutical Research & Development, Knoll Pharmaceuticals, Labopharm, Lichtwer Pharma GmbH, Lorex Pharmaceuticals, Lundbeck, MedAvante, Merck, MGH Psychiatry Academy/Primedia, MGH Psychiatry Academy/Reed Elsevier, MSI Methylation Sciences, National Alliance for Research on Schizophrenia & Depression, National Center for Complementary and Alternative Medicine, National Institute of Drug Abuse, National Institute of Mental Health, Naurex, Neuronetics, NextWave Pharmaceuticals, Novartis, Nutrition 21, Orexigen Therapeutics, Organon Pharmaceuticals, Otsuka Pharmaceuticals, PamLab, Pfizer, PharmaStar, Pharmavite®, PharmoRx Therapeutics, Photothera, Precision Human Biolaboratory, Prexa Pharmaceuticals, Puretech Ventures, PsychoGenics, Psylin Neurosciences, RCT Logic, Rexahn Pharmaceuticals, Ridge Diagnostics, Roche Pharmaceuticals, sanofi-aventis US, Schering-Plough, Sepracor, Servier Laboratories, Shire, Solvay Pharmaceuticals, Somaxon Pharmaceuticals, Somerset Pharmaceuticals, Sunovion Pharmaceuticals, Supernus Pharmaceuticals, Synthelabo, Takeda Pharmaceutical, Tal Medical, Tetragenex Pharmaceuticals, Transcept Pharmaceuticals, TransForm Pharmaceuticals, United BioSource, Vanda Pharmaceuticals, and Wyeth-Ayerst Laboratories.
Over the past year, Dr. Mischoulon has received research support from the Bowman Family Foundation, FisherWallace, Ganeden, and Nordic Naturals. He has received honoraria for speaking from Nordic Naturals. He has received royalties from Lippincott Williams & Wilkins for the book Natural medications for psychiatric disorders: Considering the alternatives. No payment has exceeded $10,000.
Dr. Freeman has received research support from Eli Lilly and Company, Forest Laboratories, and GlaxoSmithKline, and served on a one-time advisory board to Bristol-Myers Squibb.
Acknowledgment
The authors acknowledge Janet Witte, MD, MPH, Trina E. Chang, MD, MPH, Nadia Iovieno, MD, PhD, Christina Dording, MD, Heidi Ashih, MD, PhD, Maren Nyer, PhD, and Marasha-Fiona De Jong, MD from the Clinical Trials Network and Institute at Massachusetts General Hospital, Boston, MA.
1. Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53(8):649-659.
2. Judd LL, Akiskal HS, Maser JD, et al. Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J Affect Disord. 1998;50(2-3):97-108.
3. Van Londen L, Molenaar RP, Goekoop JG, et al. Three- to 5-year prospective follow-up of outcome in major depression. Psychol Med. 1998;28(3):731-735.
4. Paykel ES. Achieving gains beyond response. Acta Psychiatr Scand Suppl. 2002;(415):12-17.
5. Cassano P, Fava M. Depression and public health: an overview. J Psychosom Res. 2002;53(4):849-857.
6. Targum SD, Pollack MH, Fava M. Redefining affective disorders: relevance for drug development. CNS Neurosci Ther. 2008;14(1):2-9.
7. Versiani M, Nardi AE, Figueira I. Pharmacotherapy of dysthymia: review and new findings. Eur Psychiatry. 1998;13(4):203-209.
8. Chandler GM, Iosifescu DV, Pollack MH, et al. RESEARCH: validation of the Massachusetts General Hospital Antidepressant Treatment History Questionnaire (ATRQ). CNS Neurosci Ther. 2010;16(5):322-325.
9. Desseilles M, Witte J, Chang TE, et al. Assessing the adequacy of past antidepressant trials: a clinician’s guide to the antidepressant treatment response questionnaire. J Clin Psychiatry. 2011;72(8):1152-1154.
10. Fava M. Augmentation and combination strategies for complicated depression. J Clin Psychiatry. 2009;70(11):e40.-
11. Adli M, Baethge C, Heinz A, et al. Is dose escalation of antidepressants a rational strategy after a medium-dose treatment has failed? A systematic review. Eur Arch Psychiatry Clin Neurosci. 2005;255(6):387-400.
12. Fava M, Rush AJ. Current status of augmentation and combination treatments for major depressive disorder: a literature review and a proposal for a novel approach to improve practice. Psychother Psychosom. 2006;75(3):139-153.
13. Wohlreich MM, Mallinckrodt CH, Watkin JG, et al. Immediate switching of antidepressant therapy: results from a clinical trial of duloxetine. Ann Clin Psychiatry. 2005;17(4):259-268.
14. Schmidt ME, Fava M, Zhang S, et al. Treatment approaches to major depressive disorder relapse. Part 1: dose increase. Psychother Psychosom. 2002;71(4):190-194.
15. Fava M, Detke MJ, Balestrieri M, et al. Management of depression relapse: re-initiation of duloxetine treatment or dose increase. J Psychiatr Res. 2006;40(4):328-336.
16. Kornstein SG, Schneider RK. Clinical features of treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl 16):18-25.
17. Souery D, Papakostas GI, Trivedi MH. Treatment-resistant depression. J Clin Psychiatry. 2006;67(suppl 6):16-22.
18. Hirschfeld RM, Keller MB, Panico S, et al. The National Depressive and Manic-Depressive Association consensus statement on the undertreatment of depression. JAMA. 1997;277(4):333-340.
19. Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl 16):10-17.
20. Parker G, Malhi GS, Crawford JG, et al. Identifying “paradigm failures” contributing to treatment-resistant depression. J Affect Disord. 2005;87(2-3):185-191.
21. Harris SJ, Parent M. Patient with chronic and apparently treatment-resistant dysthymia. Am J Psychiatry. 1986;143(2):260-261.
22. Amsterdam J, Hornig M, Nierenberg AA. Treatment-resistant mood disorders. Cambridge United Kingdom: Cambridge University Press; 2001.
23. Trivedi MH. Tools and strategies for ongoing assessment of depression: a measurement-based approach to remission. J Clin Psychiatry. 2009;70(suppl 6):26-31.
1. Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53(8):649-659.
2. Judd LL, Akiskal HS, Maser JD, et al. Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J Affect Disord. 1998;50(2-3):97-108.
3. Van Londen L, Molenaar RP, Goekoop JG, et al. Three- to 5-year prospective follow-up of outcome in major depression. Psychol Med. 1998;28(3):731-735.
4. Paykel ES. Achieving gains beyond response. Acta Psychiatr Scand Suppl. 2002;(415):12-17.
5. Cassano P, Fava M. Depression and public health: an overview. J Psychosom Res. 2002;53(4):849-857.
6. Targum SD, Pollack MH, Fava M. Redefining affective disorders: relevance for drug development. CNS Neurosci Ther. 2008;14(1):2-9.
7. Versiani M, Nardi AE, Figueira I. Pharmacotherapy of dysthymia: review and new findings. Eur Psychiatry. 1998;13(4):203-209.
8. Chandler GM, Iosifescu DV, Pollack MH, et al. RESEARCH: validation of the Massachusetts General Hospital Antidepressant Treatment History Questionnaire (ATRQ). CNS Neurosci Ther. 2010;16(5):322-325.
9. Desseilles M, Witte J, Chang TE, et al. Assessing the adequacy of past antidepressant trials: a clinician’s guide to the antidepressant treatment response questionnaire. J Clin Psychiatry. 2011;72(8):1152-1154.
10. Fava M. Augmentation and combination strategies for complicated depression. J Clin Psychiatry. 2009;70(11):e40.-
11. Adli M, Baethge C, Heinz A, et al. Is dose escalation of antidepressants a rational strategy after a medium-dose treatment has failed? A systematic review. Eur Arch Psychiatry Clin Neurosci. 2005;255(6):387-400.
12. Fava M, Rush AJ. Current status of augmentation and combination treatments for major depressive disorder: a literature review and a proposal for a novel approach to improve practice. Psychother Psychosom. 2006;75(3):139-153.
13. Wohlreich MM, Mallinckrodt CH, Watkin JG, et al. Immediate switching of antidepressant therapy: results from a clinical trial of duloxetine. Ann Clin Psychiatry. 2005;17(4):259-268.
14. Schmidt ME, Fava M, Zhang S, et al. Treatment approaches to major depressive disorder relapse. Part 1: dose increase. Psychother Psychosom. 2002;71(4):190-194.
15. Fava M, Detke MJ, Balestrieri M, et al. Management of depression relapse: re-initiation of duloxetine treatment or dose increase. J Psychiatr Res. 2006;40(4):328-336.
16. Kornstein SG, Schneider RK. Clinical features of treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl 16):18-25.
17. Souery D, Papakostas GI, Trivedi MH. Treatment-resistant depression. J Clin Psychiatry. 2006;67(suppl 6):16-22.
18. Hirschfeld RM, Keller MB, Panico S, et al. The National Depressive and Manic-Depressive Association consensus statement on the undertreatment of depression. JAMA. 1997;277(4):333-340.
19. Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(suppl 16):10-17.
20. Parker G, Malhi GS, Crawford JG, et al. Identifying “paradigm failures” contributing to treatment-resistant depression. J Affect Disord. 2005;87(2-3):185-191.
21. Harris SJ, Parent M. Patient with chronic and apparently treatment-resistant dysthymia. Am J Psychiatry. 1986;143(2):260-261.
22. Amsterdam J, Hornig M, Nierenberg AA. Treatment-resistant mood disorders. Cambridge United Kingdom: Cambridge University Press; 2001.
23. Trivedi MH. Tools and strategies for ongoing assessment of depression: a measurement-based approach to remission. J Clin Psychiatry. 2009;70(suppl 6):26-31.
New Alzheimer’s disease guidelines: Implications for clinicians
Discuss this article at www.facebook.com/CurrentPsychiatry
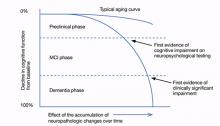
In 2011, a workgroup of experts from the Alzheimer’s Association and the National Institute on Aging published new criteria and guidelines for diagnosing Alzheimer’s disease (AD), the first new AD guidelines since 1984.1-4 These criteria reflect data that suggest AD is not synonymous with dementia of the Alzheimer’s type (DAT) but is a disease that slowly develops over many years as a result of accumulated neuropathologic changes, with dementia representing only the final phase of the disease (Figure).1-4
Figure: Cognitive decline in AD over time
AD: Alzheimer’s disease; MCI: mild cognitive impairment
Source: Adapted from reference 2
This article highlights the similarities and differences of the 1984 and 2011 AD diagnosis guidelines. We also discuss the new guidelines’ limitations and clinical implications.
The 1984 AD criteria
Both the 1984 AD criteria5 and DSM-IV-TR criteria6 rely on the concept that AD is a clinical diagnosis made after a patient develops dementia. That is, diagnosis rests on the physician’s clinical judgment about the etiology of the patient’s symptoms, taking into account reports from the patient, family, and friends, as well as results of neurocognitive testing and mental status evaluation. The 1984 criteria were developed with the expectation that if a patient who met clinical criteria for AD were to undergo an autopsy, he or she likely would have evidence of AD pathology as the underlying etiology. These criteria were developed before researchers discovered that in AD, pathologic changes occur over many years and clinical dementia is the end product of accumulated pathology. The 1984 criteria did not address important phases that precede clinical dementia—such as mild cognitive impairment (MCI). See the Table for a summary of the 1984 AD criteria.
Table
The 1984 NINCDS-ADRDA criteria for clinical diagnosis of AD
|
| AD: Alzheimer’s disease; NINCDS-ADRDA: National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer’s Disease and Related Disorders Association Source: McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939-944 |
The 2011 AD criteria
The new AD criteria differ from the 1984 criteria in 2 major ways:
- expansion of AD into 3 phases, only 1 of which is characterized by dementia
- incorporation of biomarkers to provide information regarding pathophysiologic changes underlying the disease state (Table 1).1-5
The 3 phases. The 2011 criteria expand the definition of AD to include an asymptomatic, preclinical phase; a symptomatic, pre-dementia phase; and a dementia phase. In the initial phase, neuronal toxins such as beta-amyloid (Aβ) plaques and elevated tau first become detectable. Patients in this phase are asymptomatic or have subtle symptoms. This phase should be viewed as part of a continuum and includes patients who may, for instance, develop Aβ plaques but do not progress to further neurodegeneration.2 The diagnostic criteria of this phase are intended for research purposes only.1,2
Patients in the symptomatic, pre-dementia phase—also known as the MCI phase—exhibit mild decline in memory, attention, and thinking. Although this decline is more than what is expected for the patient’s age and education, it does not compromise everyday activity and functioning.
A patient who develops cognitive or behavioral problems that interfere with his or her ability to function at work or in everyday activities has entered the dementia phase. Similar to the 1984 guidelines, the 2011 criteria classify patients into probable and possible AD dementia. All patients who would have satisfied criteria for probable AD under the 1984 guidelines will satisfy criteria for probable AD dementia under the 2011 criteria.4 The same is not true for possible AD dementia. The 2011 criteria include 2 other major categories for patients with AD dementia: probable and possible AD dementia with evidence of the AD pathophysiological process. These categories are intended for research purposes only, whereas the criteria for the MCI and dementia phases are intended to guide diagnosis in the clinical setting.
By incorporating phases of AD that precede dementia into the disease spectrum, the new guidelines are designed to move clinicians toward earlier diagnosis and treatment.1-3 Similar to how early, pre-symptomatic detection and treatment of conditions such as diabetes and cancer can reduce mortality, improving diagnosis of AD in its early phases may allow clinicians to better test potential therapies and eventually use them to treat at-risk individuals.2,3 Most pharmacotherapies for AD are FDA-approved only for patients diagnosed with clinical dementia. Furthermore, current pharmacotherapies do not alter the course of AD; they have a modest effect in slowing cognitive and functional decline.7,8 If patients in the earlier phases of AD could be recruited for research studies, we may be able to develop new treatments to stop or reverse AD pathology and its clinical manifestations.
Biomarkers. The new criteria incorporate biomarkers to provide information about pathophysiologic changes underlying the disease process. These criteria define biomarkers as physiologic, biochemical, or anatomic parameters that can be measured in vivo and reflect specific features of disease-related pathophysiologic processes.1 Presently, there are no cutoff values to demarcate “normal” levels from “abnormal,” and biomarkers are proposed primarily as research tools because they have not been studied adequately in community settings and laboratory techniques to measure biomarkers have not been standardized.1-4,9
The 5 biomarkers incorporated into the new criteria are divided into 2 categories: biomarkers of Aβ accumulation and those of neuronal degeneration or injury (Table 2).1-4 In the initial, preclinical phase, biomarkers are used to detect changes in the brain—such as amyloid accumulation and nerve cell degeneration—that may already be in process in an individual whose clinical symptoms are subtle or not yet evident.1,2 In this phase, progressive evidence of biomarkers, such as both Aβ accumulation and neuronal injury rather than Aβ accumulation alone, may increase the probability that a patient will decline quickly into the MCI phase.2 Biomarkers of neuronal degeneration or injury especially correlate with the likelihood that the disease will progress to clinical dementia.1 Subtle cognitive symptoms in the preclinical phase also might predict rapid decline into MCI.2
In the MCI and dementia phases, biomarkers are used to determine the level of certainty that AD is responsible for the patient’s symptoms.1,3,4 For example, a patient could meet criteria for a non-AD dementia such as dementia with Lewy bodies, but also meet pathologic criteria for AD on autopsy.3 The diagnostic category of possible AD dementia with evidence of the AD pathophysiologic process is intended for this type of scenario.4 For the MCI phase, the criteria propose levels of certainty that a patient’s MCI syndrome is caused by AD, ranging from MCI due to AD-high likelihood to MCI-unlikely due to AD.3
Research has demonstrated that a patient’s clinical picture doesn’t necessarily reflect the extent of the underlying pathology. For example, a patient could have extensive AD pathology, such as diffuse amyloid plaques, without any obvious clinical symptoms.3 Conversely, although both Aβ deposition and elevated tau are hallmarks of AD, variations in these proteins can be seen in neuropsychiatric disorders other than AD.10 That said, it appears that worsening of clinical symptoms often parallels worsening of neurodegenerative biomarkers.1
Under the 2011 guidelines, biomarkers would not be used to diagnose or exclude AD or MCI, but instead would help improve diagnostic accuracy in individuals with cognitive decline.1,3,4 In other words, AD remains a clinical diagnosis, but these biomarkers could raise or lower the positive predictive value of a clinician’s judgment about the etiology of a patient’s symptoms.
See the Box for a description of the potential risks and benefits of using the new diagnostic criteria.
Table 1
Comparing the 1984 and 2011 AD criteria
| 1984 criteria | 2011 criteria |
|---|---|
| AD is a clinical diagnosis | AD remains a clinical diagnosis but biomarkers serve to improve the accuracy of diagnosis of the disease |
| There is only 1 phase of AD—dementia. | AD is expanded into 3 phases: an asymptomatic, preclinical phase; a symptomatic, pre-dementia phase; and a dementia phase |
| A patient who meets the clinical criteria for AD would be expected to have AD pathology as the underlying etiology were he/she to undergo a brain autopsy | Presently, biomarkers are proposed as research tools only and are not intended to be applied in the clinical setting. However, eventually clinicians will be able to diagnose AD in all 3 phases, as biomarker testing becomes standardized and reliable enough to be accurately applied in clinical settings |
| Little consideration is given to specific neuropathologic changes underlying the disease process | Biomarkers provide information regarding the pathophysiologic changes underlying the disease state |
| Little consideration is given to the idea that pathologic changes occur over many years | Inherent in dividing AD into 3 phases is the concept that AD develops slowly over many years and has a long prodromal phase that is clinically silent |
| AD: Alzheimer’s disease Source: References 1-5 | |
Table 2
5 biomarkers incorporated into the 2011 AD criteria
| Category | Biomarkers |
|---|---|
| Biomarkers of Aβ accumulation | Abnormal tracer retention on amyloid PET imaging |
| Low CSF Aβ42 | |
| Biomarkers of neuronal degeneration or injury | Elevated CSF tau (total and phosphorylated tau) |
| Decreased fluorodeoxyglucose uptake on PET | |
| Atrophy on structural magnetic resonance imaging | |
| Aβ: beta-amyloid; AD: Alzheimer’s disease; CSF: cerebrospinal fluid; PET: positron emission tomography Source: References 1-4 | |
The earlier an Alzheimer’s disease (AD) diagnosis is made, the less certain it is AD.a Biomarkers typically found in individuals with AD also can be found in patients with dementia not caused by AD, such as vascular dementia, as well as in individuals who may never develop dementia.b Additionally, there is no certainty that a patient in an early phase of AD will develop clinical dementia. Falsely diagnosing a patient with AD may lead the individual and their family to feel helpless, hopeless, depressed, anxious, or ashamed and to spend money and other resources preparing for a prognosis that may never come to fruition. Clinicians may feel compelled to assess for biomarkers using expensive, invasive tests that are not yet standardized in an attempt to support the AD diagnosis.
Early diagnosis of AD has many benefits that should not be overlooked, however. It provides patients and their families an opportunity to become familiar with the disease course, which may help some patients cope with the diagnosis. Patients diagnosed in the early stages would be able to make important decisions regarding health care, social, and financial planning before they develop pathology that limits their executive planning abilities or become functionally impaired.
Diagnosing an illness when there are no disease-modifying therapies available is not futile. Some patients with newly diagnosed AD in the pre-dementia phases may want to participate in clinical research trials to help develop therapies for AD. Some data suggest that AD treatment appears to provide the greatest benefit when initiated early in the disease course and maintained over a long duration.c Eventually, we may be able to tailor specific AD treatments in different phases of the disease. For instance, we may discover treatments for patients who show evidence of beta-amyloid plaques but not neuronal injury, or vice versa. Patients also may benefit from education on nonpharmacologic treatments, including reducing vascular risk factors to help improve brain aging,d reducing stress, and learning cognitive strategies such as using mnemonics to aid memory.
In many clinical settings, patients are being clinically diagnosed with mild cognitive impairment (MCI). Research indicates that patients with MCI are at near-term risk of developing dementia, particularly dementia of the Alzheimer’s type.d,e Presently, no definite transition points demarcate MCI from dementia; this progression is based upon clinical judgment.
In the last decade, researchers have begun to describe a syndrome of subjective cognitive impairment (SCI), which may be a phase that precedes the MCI phase of AD.f Patients with SCI report cognitive deficits (eg, forgetfulness and word-finding difficulties) but have no objective evidence of cognitive impairment on neuropsychological tests. Cognitive problems associated with SCI do not cause functional decline.g SCI may reflect the minimal cognitive complaints mentioned in the research criteria for the preclinical phase of AD. Eventually, biomarkers may be able to help clinicians more accurately predict which patients with SCI are most likely to progress to the MCI or dementia phase of AD.
References
- Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-279.
- Galasko D. Biomarkers in non-Alzheimer dementias. Clinical Neuroscience Research. 2004;3(6):375-381.
- Geldmacher DS. Treatment guidelines for Alzheimer’s disease: redefining perceptions in primary care. Prim Care Companion J Clin Psychiatry. 2007;9(2):113-121.
- Chertkow H, Massoud F, Nasreddine Z, et al. Diagnosis and treatment of dementia: 3. Mild cognitive impairment and cognitive impairment without dementia. CMAJ. 2008;178(10):1273-1285.
- Rosenberg PB, Lyketsos C. Mild cognitive impairment: searching for the prodrome of Alzheimer’s disease. World Psychiatry. 2008;7(2):72-78.
- Reisberg B, Shulman MB, Torossian C, et al. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement. 2010;6(1):11-24.
- Desai AK, Schwarz L. Subjective cognitive impairment: when to be concerned about ‘senior moments.’ Current Psychiatry. 2011;10(4):31-44.
Clinical applications
Although pharmacologic therapies for the early phases of AD are not yet available, research supports implementing nonpharmacologic modalities in older adults with MCI as well as those without any cognitive impairment (Table 3).8,11 Growing evidence suggests physicians should encourage patients to lead an active and socially integrated lifestyle that includes leisure activities, cognitive stimulation, meditation, a balanced diet, and daily exercise.8 Practitioners should treat vascular risk factors in geriatric patients with and without cognitive impairment to optimize healthy brain aging and reduce the risk of cardiovascular disease and stroke.11 By raising awareness of available treatments for early phases of AD, we may be able to reduce the anxiety and sense of helplessness or hopelessness that may accompany an AD diagnosis.
Depression and AD. Having depression nearly doubles one’s risk of developing AD later in life, and depression may exacerbate AD.12 Although the precise mechanism linking depression to AD is unclear, depression seems to exert a toxic effect on the hippocampus.13 Treating depression may prevent or mitigate the rate of memory impairment and overall AD severity and improve a patient’s quality of life, overall health, and ability to function.
Almost one-third of family caregivers become depressed while helping a family member with DAT.14 Directing caregivers to peer support groups and providing them with tips on how to take care of themselves physically, emotionally, and psychologically can be extremely beneficial. Data suggest that improving the psychological and emotional well-being of caretakers may delay nursing home placement of patients with DAT.15 Delaying nursing home placement can substantially improve quality of life and reduce the financial strain on patients and caregivers.
Patients and families often turn to clinicians for advice on what problems they or their loved ones may encounter if they suffer from cognitive impairment. One benefit of the new guidelines is that they can help us become educated about the early phases of AD as well as the long and often difficult course of the disease. In turn, we can better educate our patients and their families about the disease.
As early screening of AD improves, patients in the early phases will have an opportunity to take part in clinical trials for potential pharmacologic treatments of the disease. Our role as clinicians will be to guide patients and their families to such trials and give them the opportunity to help change our understanding of and approach to treating AD. It is important to keep in mind that the new guidelines should not be considered final, but rather as a work in progress that periodically will be revised as AD research progresses.3
Table 3
Promoting healthy brain aging
| Healthy diet (eg, Mediterranean diet) |
| Adequate sleep |
| Daily exercise |
| Smoking cessation |
| Active, socially integrated lifestyle |
| Leisure activities |
| Cognitive stimulation |
| Optimize treatment of depression and other mental illnesses |
| Meditation and other mindfulness strategies (eg, yoga) |
| Spiritual activities |
| Controlling vascular risk factors (hypertension, diabetes, dyslipidemia, and obesity) |
| Source: References 8,11 |
Related Resources
- Alzheimer’s Association. www.alz.org.
- National Institute on Aging. Alzheimer’s disease education and referral center. www.nia.nih.gov/alzheimers.
Disclosures
Drs. Kimchi and Desai report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Grossberg is a consultant to Baxter, Forest Laboratories, Merck, Otsuka, and Novartis.
1. Jack CR Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):257-262.
2. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280-292.
3. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-279.
4. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269.
5. McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939-944.
6. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
7. Ihl R, Frölich L, Winblad B, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of Alzheimer’s disease and other dementias. World J Biol Psychiatry. 2011;12(1):2-32.
8. Chertkow H, Massoud F, Nasreddine Z, et al. Diagnosis and treatment of dementia: 3. Mild cognitive impairment and cognitive impairment without dementia. CMAJ. 2008;178(10):1273-1285.
9. McKhann GM. Changing concepts of Alzheimer disease. JAMA. 2011;305(23):2458-2459.
10. Galasko D. Biomarkers in non-Alzheimer’s dementias. Clinical Neuroscience Research. 2004;3(6):375-381.
11. Desai AK, Grossberg GT, Chibnall JT. Healthy brain aging: a road map. Clin Geriatr Med. 2010;26(1):1-16.
12. Wilson RS, Hoganson GM, Rajan KB, et al. Temporal course of depressive symptoms during the development of Alzheimer disease. Neurology. 2010;75(1):21-26.
13. Bremner JD, Narayan M, Anderson ER, et al. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157(1):115-118.
14. Yaffe K, Fox P, Newcomer R, et al. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA. 2002;287(16):2090-2097.
15. Mittelman MS, Haley WE, Clay OJ, et al. Improving caregiver well-being delays nursing home placement of patients with Alzheimer disease. Neurology. 2006;67(9):1592-1599.
Discuss this article at www.facebook.com/CurrentPsychiatry
In 2011, a workgroup of experts from the Alzheimer’s Association and the National Institute on Aging published new criteria and guidelines for diagnosing Alzheimer’s disease (AD), the first new AD guidelines since 1984.1-4 These criteria reflect data that suggest AD is not synonymous with dementia of the Alzheimer’s type (DAT) but is a disease that slowly develops over many years as a result of accumulated neuropathologic changes, with dementia representing only the final phase of the disease (Figure).1-4
Figure: Cognitive decline in AD over time
AD: Alzheimer’s disease; MCI: mild cognitive impairment
Source: Adapted from reference 2
This article highlights the similarities and differences of the 1984 and 2011 AD diagnosis guidelines. We also discuss the new guidelines’ limitations and clinical implications.
The 1984 AD criteria
Both the 1984 AD criteria5 and DSM-IV-TR criteria6 rely on the concept that AD is a clinical diagnosis made after a patient develops dementia. That is, diagnosis rests on the physician’s clinical judgment about the etiology of the patient’s symptoms, taking into account reports from the patient, family, and friends, as well as results of neurocognitive testing and mental status evaluation. The 1984 criteria were developed with the expectation that if a patient who met clinical criteria for AD were to undergo an autopsy, he or she likely would have evidence of AD pathology as the underlying etiology. These criteria were developed before researchers discovered that in AD, pathologic changes occur over many years and clinical dementia is the end product of accumulated pathology. The 1984 criteria did not address important phases that precede clinical dementia—such as mild cognitive impairment (MCI). See the Table for a summary of the 1984 AD criteria.
Table
The 1984 NINCDS-ADRDA criteria for clinical diagnosis of AD
|
| AD: Alzheimer’s disease; NINCDS-ADRDA: National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer’s Disease and Related Disorders Association Source: McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939-944 |
The 2011 AD criteria
The new AD criteria differ from the 1984 criteria in 2 major ways:
- expansion of AD into 3 phases, only 1 of which is characterized by dementia
- incorporation of biomarkers to provide information regarding pathophysiologic changes underlying the disease state (Table 1).1-5
The 3 phases. The 2011 criteria expand the definition of AD to include an asymptomatic, preclinical phase; a symptomatic, pre-dementia phase; and a dementia phase. In the initial phase, neuronal toxins such as beta-amyloid (Aβ) plaques and elevated tau first become detectable. Patients in this phase are asymptomatic or have subtle symptoms. This phase should be viewed as part of a continuum and includes patients who may, for instance, develop Aβ plaques but do not progress to further neurodegeneration.2 The diagnostic criteria of this phase are intended for research purposes only.1,2
Patients in the symptomatic, pre-dementia phase—also known as the MCI phase—exhibit mild decline in memory, attention, and thinking. Although this decline is more than what is expected for the patient’s age and education, it does not compromise everyday activity and functioning.
A patient who develops cognitive or behavioral problems that interfere with his or her ability to function at work or in everyday activities has entered the dementia phase. Similar to the 1984 guidelines, the 2011 criteria classify patients into probable and possible AD dementia. All patients who would have satisfied criteria for probable AD under the 1984 guidelines will satisfy criteria for probable AD dementia under the 2011 criteria.4 The same is not true for possible AD dementia. The 2011 criteria include 2 other major categories for patients with AD dementia: probable and possible AD dementia with evidence of the AD pathophysiological process. These categories are intended for research purposes only, whereas the criteria for the MCI and dementia phases are intended to guide diagnosis in the clinical setting.
By incorporating phases of AD that precede dementia into the disease spectrum, the new guidelines are designed to move clinicians toward earlier diagnosis and treatment.1-3 Similar to how early, pre-symptomatic detection and treatment of conditions such as diabetes and cancer can reduce mortality, improving diagnosis of AD in its early phases may allow clinicians to better test potential therapies and eventually use them to treat at-risk individuals.2,3 Most pharmacotherapies for AD are FDA-approved only for patients diagnosed with clinical dementia. Furthermore, current pharmacotherapies do not alter the course of AD; they have a modest effect in slowing cognitive and functional decline.7,8 If patients in the earlier phases of AD could be recruited for research studies, we may be able to develop new treatments to stop or reverse AD pathology and its clinical manifestations.
Biomarkers. The new criteria incorporate biomarkers to provide information about pathophysiologic changes underlying the disease process. These criteria define biomarkers as physiologic, biochemical, or anatomic parameters that can be measured in vivo and reflect specific features of disease-related pathophysiologic processes.1 Presently, there are no cutoff values to demarcate “normal” levels from “abnormal,” and biomarkers are proposed primarily as research tools because they have not been studied adequately in community settings and laboratory techniques to measure biomarkers have not been standardized.1-4,9
The 5 biomarkers incorporated into the new criteria are divided into 2 categories: biomarkers of Aβ accumulation and those of neuronal degeneration or injury (Table 2).1-4 In the initial, preclinical phase, biomarkers are used to detect changes in the brain—such as amyloid accumulation and nerve cell degeneration—that may already be in process in an individual whose clinical symptoms are subtle or not yet evident.1,2 In this phase, progressive evidence of biomarkers, such as both Aβ accumulation and neuronal injury rather than Aβ accumulation alone, may increase the probability that a patient will decline quickly into the MCI phase.2 Biomarkers of neuronal degeneration or injury especially correlate with the likelihood that the disease will progress to clinical dementia.1 Subtle cognitive symptoms in the preclinical phase also might predict rapid decline into MCI.2
In the MCI and dementia phases, biomarkers are used to determine the level of certainty that AD is responsible for the patient’s symptoms.1,3,4 For example, a patient could meet criteria for a non-AD dementia such as dementia with Lewy bodies, but also meet pathologic criteria for AD on autopsy.3 The diagnostic category of possible AD dementia with evidence of the AD pathophysiologic process is intended for this type of scenario.4 For the MCI phase, the criteria propose levels of certainty that a patient’s MCI syndrome is caused by AD, ranging from MCI due to AD-high likelihood to MCI-unlikely due to AD.3
Research has demonstrated that a patient’s clinical picture doesn’t necessarily reflect the extent of the underlying pathology. For example, a patient could have extensive AD pathology, such as diffuse amyloid plaques, without any obvious clinical symptoms.3 Conversely, although both Aβ deposition and elevated tau are hallmarks of AD, variations in these proteins can be seen in neuropsychiatric disorders other than AD.10 That said, it appears that worsening of clinical symptoms often parallels worsening of neurodegenerative biomarkers.1
Under the 2011 guidelines, biomarkers would not be used to diagnose or exclude AD or MCI, but instead would help improve diagnostic accuracy in individuals with cognitive decline.1,3,4 In other words, AD remains a clinical diagnosis, but these biomarkers could raise or lower the positive predictive value of a clinician’s judgment about the etiology of a patient’s symptoms.
See the Box for a description of the potential risks and benefits of using the new diagnostic criteria.
Table 1
Comparing the 1984 and 2011 AD criteria
| 1984 criteria | 2011 criteria |
|---|---|
| AD is a clinical diagnosis | AD remains a clinical diagnosis but biomarkers serve to improve the accuracy of diagnosis of the disease |
| There is only 1 phase of AD—dementia. | AD is expanded into 3 phases: an asymptomatic, preclinical phase; a symptomatic, pre-dementia phase; and a dementia phase |
| A patient who meets the clinical criteria for AD would be expected to have AD pathology as the underlying etiology were he/she to undergo a brain autopsy | Presently, biomarkers are proposed as research tools only and are not intended to be applied in the clinical setting. However, eventually clinicians will be able to diagnose AD in all 3 phases, as biomarker testing becomes standardized and reliable enough to be accurately applied in clinical settings |
| Little consideration is given to specific neuropathologic changes underlying the disease process | Biomarkers provide information regarding the pathophysiologic changes underlying the disease state |
| Little consideration is given to the idea that pathologic changes occur over many years | Inherent in dividing AD into 3 phases is the concept that AD develops slowly over many years and has a long prodromal phase that is clinically silent |
| AD: Alzheimer’s disease Source: References 1-5 | |
Table 2
5 biomarkers incorporated into the 2011 AD criteria
| Category | Biomarkers |
|---|---|
| Biomarkers of Aβ accumulation | Abnormal tracer retention on amyloid PET imaging |
| Low CSF Aβ42 | |
| Biomarkers of neuronal degeneration or injury | Elevated CSF tau (total and phosphorylated tau) |
| Decreased fluorodeoxyglucose uptake on PET | |
| Atrophy on structural magnetic resonance imaging | |
| Aβ: beta-amyloid; AD: Alzheimer’s disease; CSF: cerebrospinal fluid; PET: positron emission tomography Source: References 1-4 | |
The earlier an Alzheimer’s disease (AD) diagnosis is made, the less certain it is AD.a Biomarkers typically found in individuals with AD also can be found in patients with dementia not caused by AD, such as vascular dementia, as well as in individuals who may never develop dementia.b Additionally, there is no certainty that a patient in an early phase of AD will develop clinical dementia. Falsely diagnosing a patient with AD may lead the individual and their family to feel helpless, hopeless, depressed, anxious, or ashamed and to spend money and other resources preparing for a prognosis that may never come to fruition. Clinicians may feel compelled to assess for biomarkers using expensive, invasive tests that are not yet standardized in an attempt to support the AD diagnosis.
Early diagnosis of AD has many benefits that should not be overlooked, however. It provides patients and their families an opportunity to become familiar with the disease course, which may help some patients cope with the diagnosis. Patients diagnosed in the early stages would be able to make important decisions regarding health care, social, and financial planning before they develop pathology that limits their executive planning abilities or become functionally impaired.
Diagnosing an illness when there are no disease-modifying therapies available is not futile. Some patients with newly diagnosed AD in the pre-dementia phases may want to participate in clinical research trials to help develop therapies for AD. Some data suggest that AD treatment appears to provide the greatest benefit when initiated early in the disease course and maintained over a long duration.c Eventually, we may be able to tailor specific AD treatments in different phases of the disease. For instance, we may discover treatments for patients who show evidence of beta-amyloid plaques but not neuronal injury, or vice versa. Patients also may benefit from education on nonpharmacologic treatments, including reducing vascular risk factors to help improve brain aging,d reducing stress, and learning cognitive strategies such as using mnemonics to aid memory.
In many clinical settings, patients are being clinically diagnosed with mild cognitive impairment (MCI). Research indicates that patients with MCI are at near-term risk of developing dementia, particularly dementia of the Alzheimer’s type.d,e Presently, no definite transition points demarcate MCI from dementia; this progression is based upon clinical judgment.
In the last decade, researchers have begun to describe a syndrome of subjective cognitive impairment (SCI), which may be a phase that precedes the MCI phase of AD.f Patients with SCI report cognitive deficits (eg, forgetfulness and word-finding difficulties) but have no objective evidence of cognitive impairment on neuropsychological tests. Cognitive problems associated with SCI do not cause functional decline.g SCI may reflect the minimal cognitive complaints mentioned in the research criteria for the preclinical phase of AD. Eventually, biomarkers may be able to help clinicians more accurately predict which patients with SCI are most likely to progress to the MCI or dementia phase of AD.
References
- Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-279.
- Galasko D. Biomarkers in non-Alzheimer dementias. Clinical Neuroscience Research. 2004;3(6):375-381.
- Geldmacher DS. Treatment guidelines for Alzheimer’s disease: redefining perceptions in primary care. Prim Care Companion J Clin Psychiatry. 2007;9(2):113-121.
- Chertkow H, Massoud F, Nasreddine Z, et al. Diagnosis and treatment of dementia: 3. Mild cognitive impairment and cognitive impairment without dementia. CMAJ. 2008;178(10):1273-1285.
- Rosenberg PB, Lyketsos C. Mild cognitive impairment: searching for the prodrome of Alzheimer’s disease. World Psychiatry. 2008;7(2):72-78.
- Reisberg B, Shulman MB, Torossian C, et al. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement. 2010;6(1):11-24.
- Desai AK, Schwarz L. Subjective cognitive impairment: when to be concerned about ‘senior moments.’ Current Psychiatry. 2011;10(4):31-44.
Clinical applications
Although pharmacologic therapies for the early phases of AD are not yet available, research supports implementing nonpharmacologic modalities in older adults with MCI as well as those without any cognitive impairment (Table 3).8,11 Growing evidence suggests physicians should encourage patients to lead an active and socially integrated lifestyle that includes leisure activities, cognitive stimulation, meditation, a balanced diet, and daily exercise.8 Practitioners should treat vascular risk factors in geriatric patients with and without cognitive impairment to optimize healthy brain aging and reduce the risk of cardiovascular disease and stroke.11 By raising awareness of available treatments for early phases of AD, we may be able to reduce the anxiety and sense of helplessness or hopelessness that may accompany an AD diagnosis.
Depression and AD. Having depression nearly doubles one’s risk of developing AD later in life, and depression may exacerbate AD.12 Although the precise mechanism linking depression to AD is unclear, depression seems to exert a toxic effect on the hippocampus.13 Treating depression may prevent or mitigate the rate of memory impairment and overall AD severity and improve a patient’s quality of life, overall health, and ability to function.
Almost one-third of family caregivers become depressed while helping a family member with DAT.14 Directing caregivers to peer support groups and providing them with tips on how to take care of themselves physically, emotionally, and psychologically can be extremely beneficial. Data suggest that improving the psychological and emotional well-being of caretakers may delay nursing home placement of patients with DAT.15 Delaying nursing home placement can substantially improve quality of life and reduce the financial strain on patients and caregivers.
Patients and families often turn to clinicians for advice on what problems they or their loved ones may encounter if they suffer from cognitive impairment. One benefit of the new guidelines is that they can help us become educated about the early phases of AD as well as the long and often difficult course of the disease. In turn, we can better educate our patients and their families about the disease.
As early screening of AD improves, patients in the early phases will have an opportunity to take part in clinical trials for potential pharmacologic treatments of the disease. Our role as clinicians will be to guide patients and their families to such trials and give them the opportunity to help change our understanding of and approach to treating AD. It is important to keep in mind that the new guidelines should not be considered final, but rather as a work in progress that periodically will be revised as AD research progresses.3
Table 3
Promoting healthy brain aging
| Healthy diet (eg, Mediterranean diet) |
| Adequate sleep |
| Daily exercise |
| Smoking cessation |
| Active, socially integrated lifestyle |
| Leisure activities |
| Cognitive stimulation |
| Optimize treatment of depression and other mental illnesses |
| Meditation and other mindfulness strategies (eg, yoga) |
| Spiritual activities |
| Controlling vascular risk factors (hypertension, diabetes, dyslipidemia, and obesity) |
| Source: References 8,11 |
Related Resources
- Alzheimer’s Association. www.alz.org.
- National Institute on Aging. Alzheimer’s disease education and referral center. www.nia.nih.gov/alzheimers.
Disclosures
Drs. Kimchi and Desai report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Grossberg is a consultant to Baxter, Forest Laboratories, Merck, Otsuka, and Novartis.
Discuss this article at www.facebook.com/CurrentPsychiatry
In 2011, a workgroup of experts from the Alzheimer’s Association and the National Institute on Aging published new criteria and guidelines for diagnosing Alzheimer’s disease (AD), the first new AD guidelines since 1984.1-4 These criteria reflect data that suggest AD is not synonymous with dementia of the Alzheimer’s type (DAT) but is a disease that slowly develops over many years as a result of accumulated neuropathologic changes, with dementia representing only the final phase of the disease (Figure).1-4
Figure: Cognitive decline in AD over time
AD: Alzheimer’s disease; MCI: mild cognitive impairment
Source: Adapted from reference 2
This article highlights the similarities and differences of the 1984 and 2011 AD diagnosis guidelines. We also discuss the new guidelines’ limitations and clinical implications.
The 1984 AD criteria
Both the 1984 AD criteria5 and DSM-IV-TR criteria6 rely on the concept that AD is a clinical diagnosis made after a patient develops dementia. That is, diagnosis rests on the physician’s clinical judgment about the etiology of the patient’s symptoms, taking into account reports from the patient, family, and friends, as well as results of neurocognitive testing and mental status evaluation. The 1984 criteria were developed with the expectation that if a patient who met clinical criteria for AD were to undergo an autopsy, he or she likely would have evidence of AD pathology as the underlying etiology. These criteria were developed before researchers discovered that in AD, pathologic changes occur over many years and clinical dementia is the end product of accumulated pathology. The 1984 criteria did not address important phases that precede clinical dementia—such as mild cognitive impairment (MCI). See the Table for a summary of the 1984 AD criteria.
Table
The 1984 NINCDS-ADRDA criteria for clinical diagnosis of AD
|
| AD: Alzheimer’s disease; NINCDS-ADRDA: National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer’s Disease and Related Disorders Association Source: McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939-944 |
The 2011 AD criteria
The new AD criteria differ from the 1984 criteria in 2 major ways:
- expansion of AD into 3 phases, only 1 of which is characterized by dementia
- incorporation of biomarkers to provide information regarding pathophysiologic changes underlying the disease state (Table 1).1-5
The 3 phases. The 2011 criteria expand the definition of AD to include an asymptomatic, preclinical phase; a symptomatic, pre-dementia phase; and a dementia phase. In the initial phase, neuronal toxins such as beta-amyloid (Aβ) plaques and elevated tau first become detectable. Patients in this phase are asymptomatic or have subtle symptoms. This phase should be viewed as part of a continuum and includes patients who may, for instance, develop Aβ plaques but do not progress to further neurodegeneration.2 The diagnostic criteria of this phase are intended for research purposes only.1,2
Patients in the symptomatic, pre-dementia phase—also known as the MCI phase—exhibit mild decline in memory, attention, and thinking. Although this decline is more than what is expected for the patient’s age and education, it does not compromise everyday activity and functioning.
A patient who develops cognitive or behavioral problems that interfere with his or her ability to function at work or in everyday activities has entered the dementia phase. Similar to the 1984 guidelines, the 2011 criteria classify patients into probable and possible AD dementia. All patients who would have satisfied criteria for probable AD under the 1984 guidelines will satisfy criteria for probable AD dementia under the 2011 criteria.4 The same is not true for possible AD dementia. The 2011 criteria include 2 other major categories for patients with AD dementia: probable and possible AD dementia with evidence of the AD pathophysiological process. These categories are intended for research purposes only, whereas the criteria for the MCI and dementia phases are intended to guide diagnosis in the clinical setting.
By incorporating phases of AD that precede dementia into the disease spectrum, the new guidelines are designed to move clinicians toward earlier diagnosis and treatment.1-3 Similar to how early, pre-symptomatic detection and treatment of conditions such as diabetes and cancer can reduce mortality, improving diagnosis of AD in its early phases may allow clinicians to better test potential therapies and eventually use them to treat at-risk individuals.2,3 Most pharmacotherapies for AD are FDA-approved only for patients diagnosed with clinical dementia. Furthermore, current pharmacotherapies do not alter the course of AD; they have a modest effect in slowing cognitive and functional decline.7,8 If patients in the earlier phases of AD could be recruited for research studies, we may be able to develop new treatments to stop or reverse AD pathology and its clinical manifestations.
Biomarkers. The new criteria incorporate biomarkers to provide information about pathophysiologic changes underlying the disease process. These criteria define biomarkers as physiologic, biochemical, or anatomic parameters that can be measured in vivo and reflect specific features of disease-related pathophysiologic processes.1 Presently, there are no cutoff values to demarcate “normal” levels from “abnormal,” and biomarkers are proposed primarily as research tools because they have not been studied adequately in community settings and laboratory techniques to measure biomarkers have not been standardized.1-4,9
The 5 biomarkers incorporated into the new criteria are divided into 2 categories: biomarkers of Aβ accumulation and those of neuronal degeneration or injury (Table 2).1-4 In the initial, preclinical phase, biomarkers are used to detect changes in the brain—such as amyloid accumulation and nerve cell degeneration—that may already be in process in an individual whose clinical symptoms are subtle or not yet evident.1,2 In this phase, progressive evidence of biomarkers, such as both Aβ accumulation and neuronal injury rather than Aβ accumulation alone, may increase the probability that a patient will decline quickly into the MCI phase.2 Biomarkers of neuronal degeneration or injury especially correlate with the likelihood that the disease will progress to clinical dementia.1 Subtle cognitive symptoms in the preclinical phase also might predict rapid decline into MCI.2
In the MCI and dementia phases, biomarkers are used to determine the level of certainty that AD is responsible for the patient’s symptoms.1,3,4 For example, a patient could meet criteria for a non-AD dementia such as dementia with Lewy bodies, but also meet pathologic criteria for AD on autopsy.3 The diagnostic category of possible AD dementia with evidence of the AD pathophysiologic process is intended for this type of scenario.4 For the MCI phase, the criteria propose levels of certainty that a patient’s MCI syndrome is caused by AD, ranging from MCI due to AD-high likelihood to MCI-unlikely due to AD.3
Research has demonstrated that a patient’s clinical picture doesn’t necessarily reflect the extent of the underlying pathology. For example, a patient could have extensive AD pathology, such as diffuse amyloid plaques, without any obvious clinical symptoms.3 Conversely, although both Aβ deposition and elevated tau are hallmarks of AD, variations in these proteins can be seen in neuropsychiatric disorders other than AD.10 That said, it appears that worsening of clinical symptoms often parallels worsening of neurodegenerative biomarkers.1
Under the 2011 guidelines, biomarkers would not be used to diagnose or exclude AD or MCI, but instead would help improve diagnostic accuracy in individuals with cognitive decline.1,3,4 In other words, AD remains a clinical diagnosis, but these biomarkers could raise or lower the positive predictive value of a clinician’s judgment about the etiology of a patient’s symptoms.
See the Box for a description of the potential risks and benefits of using the new diagnostic criteria.
Table 1
Comparing the 1984 and 2011 AD criteria
| 1984 criteria | 2011 criteria |
|---|---|
| AD is a clinical diagnosis | AD remains a clinical diagnosis but biomarkers serve to improve the accuracy of diagnosis of the disease |
| There is only 1 phase of AD—dementia. | AD is expanded into 3 phases: an asymptomatic, preclinical phase; a symptomatic, pre-dementia phase; and a dementia phase |
| A patient who meets the clinical criteria for AD would be expected to have AD pathology as the underlying etiology were he/she to undergo a brain autopsy | Presently, biomarkers are proposed as research tools only and are not intended to be applied in the clinical setting. However, eventually clinicians will be able to diagnose AD in all 3 phases, as biomarker testing becomes standardized and reliable enough to be accurately applied in clinical settings |
| Little consideration is given to specific neuropathologic changes underlying the disease process | Biomarkers provide information regarding the pathophysiologic changes underlying the disease state |
| Little consideration is given to the idea that pathologic changes occur over many years | Inherent in dividing AD into 3 phases is the concept that AD develops slowly over many years and has a long prodromal phase that is clinically silent |
| AD: Alzheimer’s disease Source: References 1-5 | |
Table 2
5 biomarkers incorporated into the 2011 AD criteria
| Category | Biomarkers |
|---|---|
| Biomarkers of Aβ accumulation | Abnormal tracer retention on amyloid PET imaging |
| Low CSF Aβ42 | |
| Biomarkers of neuronal degeneration or injury | Elevated CSF tau (total and phosphorylated tau) |
| Decreased fluorodeoxyglucose uptake on PET | |
| Atrophy on structural magnetic resonance imaging | |
| Aβ: beta-amyloid; AD: Alzheimer’s disease; CSF: cerebrospinal fluid; PET: positron emission tomography Source: References 1-4 | |
The earlier an Alzheimer’s disease (AD) diagnosis is made, the less certain it is AD.a Biomarkers typically found in individuals with AD also can be found in patients with dementia not caused by AD, such as vascular dementia, as well as in individuals who may never develop dementia.b Additionally, there is no certainty that a patient in an early phase of AD will develop clinical dementia. Falsely diagnosing a patient with AD may lead the individual and their family to feel helpless, hopeless, depressed, anxious, or ashamed and to spend money and other resources preparing for a prognosis that may never come to fruition. Clinicians may feel compelled to assess for biomarkers using expensive, invasive tests that are not yet standardized in an attempt to support the AD diagnosis.
Early diagnosis of AD has many benefits that should not be overlooked, however. It provides patients and their families an opportunity to become familiar with the disease course, which may help some patients cope with the diagnosis. Patients diagnosed in the early stages would be able to make important decisions regarding health care, social, and financial planning before they develop pathology that limits their executive planning abilities or become functionally impaired.
Diagnosing an illness when there are no disease-modifying therapies available is not futile. Some patients with newly diagnosed AD in the pre-dementia phases may want to participate in clinical research trials to help develop therapies for AD. Some data suggest that AD treatment appears to provide the greatest benefit when initiated early in the disease course and maintained over a long duration.c Eventually, we may be able to tailor specific AD treatments in different phases of the disease. For instance, we may discover treatments for patients who show evidence of beta-amyloid plaques but not neuronal injury, or vice versa. Patients also may benefit from education on nonpharmacologic treatments, including reducing vascular risk factors to help improve brain aging,d reducing stress, and learning cognitive strategies such as using mnemonics to aid memory.
In many clinical settings, patients are being clinically diagnosed with mild cognitive impairment (MCI). Research indicates that patients with MCI are at near-term risk of developing dementia, particularly dementia of the Alzheimer’s type.d,e Presently, no definite transition points demarcate MCI from dementia; this progression is based upon clinical judgment.
In the last decade, researchers have begun to describe a syndrome of subjective cognitive impairment (SCI), which may be a phase that precedes the MCI phase of AD.f Patients with SCI report cognitive deficits (eg, forgetfulness and word-finding difficulties) but have no objective evidence of cognitive impairment on neuropsychological tests. Cognitive problems associated with SCI do not cause functional decline.g SCI may reflect the minimal cognitive complaints mentioned in the research criteria for the preclinical phase of AD. Eventually, biomarkers may be able to help clinicians more accurately predict which patients with SCI are most likely to progress to the MCI or dementia phase of AD.
References
- Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-279.
- Galasko D. Biomarkers in non-Alzheimer dementias. Clinical Neuroscience Research. 2004;3(6):375-381.
- Geldmacher DS. Treatment guidelines for Alzheimer’s disease: redefining perceptions in primary care. Prim Care Companion J Clin Psychiatry. 2007;9(2):113-121.
- Chertkow H, Massoud F, Nasreddine Z, et al. Diagnosis and treatment of dementia: 3. Mild cognitive impairment and cognitive impairment without dementia. CMAJ. 2008;178(10):1273-1285.
- Rosenberg PB, Lyketsos C. Mild cognitive impairment: searching for the prodrome of Alzheimer’s disease. World Psychiatry. 2008;7(2):72-78.
- Reisberg B, Shulman MB, Torossian C, et al. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement. 2010;6(1):11-24.
- Desai AK, Schwarz L. Subjective cognitive impairment: when to be concerned about ‘senior moments.’ Current Psychiatry. 2011;10(4):31-44.
Clinical applications
Although pharmacologic therapies for the early phases of AD are not yet available, research supports implementing nonpharmacologic modalities in older adults with MCI as well as those without any cognitive impairment (Table 3).8,11 Growing evidence suggests physicians should encourage patients to lead an active and socially integrated lifestyle that includes leisure activities, cognitive stimulation, meditation, a balanced diet, and daily exercise.8 Practitioners should treat vascular risk factors in geriatric patients with and without cognitive impairment to optimize healthy brain aging and reduce the risk of cardiovascular disease and stroke.11 By raising awareness of available treatments for early phases of AD, we may be able to reduce the anxiety and sense of helplessness or hopelessness that may accompany an AD diagnosis.
Depression and AD. Having depression nearly doubles one’s risk of developing AD later in life, and depression may exacerbate AD.12 Although the precise mechanism linking depression to AD is unclear, depression seems to exert a toxic effect on the hippocampus.13 Treating depression may prevent or mitigate the rate of memory impairment and overall AD severity and improve a patient’s quality of life, overall health, and ability to function.
Almost one-third of family caregivers become depressed while helping a family member with DAT.14 Directing caregivers to peer support groups and providing them with tips on how to take care of themselves physically, emotionally, and psychologically can be extremely beneficial. Data suggest that improving the psychological and emotional well-being of caretakers may delay nursing home placement of patients with DAT.15 Delaying nursing home placement can substantially improve quality of life and reduce the financial strain on patients and caregivers.
Patients and families often turn to clinicians for advice on what problems they or their loved ones may encounter if they suffer from cognitive impairment. One benefit of the new guidelines is that they can help us become educated about the early phases of AD as well as the long and often difficult course of the disease. In turn, we can better educate our patients and their families about the disease.
As early screening of AD improves, patients in the early phases will have an opportunity to take part in clinical trials for potential pharmacologic treatments of the disease. Our role as clinicians will be to guide patients and their families to such trials and give them the opportunity to help change our understanding of and approach to treating AD. It is important to keep in mind that the new guidelines should not be considered final, but rather as a work in progress that periodically will be revised as AD research progresses.3
Table 3
Promoting healthy brain aging
| Healthy diet (eg, Mediterranean diet) |
| Adequate sleep |
| Daily exercise |
| Smoking cessation |
| Active, socially integrated lifestyle |
| Leisure activities |
| Cognitive stimulation |
| Optimize treatment of depression and other mental illnesses |
| Meditation and other mindfulness strategies (eg, yoga) |
| Spiritual activities |
| Controlling vascular risk factors (hypertension, diabetes, dyslipidemia, and obesity) |
| Source: References 8,11 |
Related Resources
- Alzheimer’s Association. www.alz.org.
- National Institute on Aging. Alzheimer’s disease education and referral center. www.nia.nih.gov/alzheimers.
Disclosures
Drs. Kimchi and Desai report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Grossberg is a consultant to Baxter, Forest Laboratories, Merck, Otsuka, and Novartis.
1. Jack CR Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):257-262.
2. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280-292.
3. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-279.
4. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269.
5. McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939-944.
6. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
7. Ihl R, Frölich L, Winblad B, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of Alzheimer’s disease and other dementias. World J Biol Psychiatry. 2011;12(1):2-32.
8. Chertkow H, Massoud F, Nasreddine Z, et al. Diagnosis and treatment of dementia: 3. Mild cognitive impairment and cognitive impairment without dementia. CMAJ. 2008;178(10):1273-1285.
9. McKhann GM. Changing concepts of Alzheimer disease. JAMA. 2011;305(23):2458-2459.
10. Galasko D. Biomarkers in non-Alzheimer’s dementias. Clinical Neuroscience Research. 2004;3(6):375-381.
11. Desai AK, Grossberg GT, Chibnall JT. Healthy brain aging: a road map. Clin Geriatr Med. 2010;26(1):1-16.
12. Wilson RS, Hoganson GM, Rajan KB, et al. Temporal course of depressive symptoms during the development of Alzheimer disease. Neurology. 2010;75(1):21-26.
13. Bremner JD, Narayan M, Anderson ER, et al. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157(1):115-118.
14. Yaffe K, Fox P, Newcomer R, et al. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA. 2002;287(16):2090-2097.
15. Mittelman MS, Haley WE, Clay OJ, et al. Improving caregiver well-being delays nursing home placement of patients with Alzheimer disease. Neurology. 2006;67(9):1592-1599.
1. Jack CR Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):257-262.
2. Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280-292.
3. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-279.
4. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269.
5. McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939-944.
6. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
7. Ihl R, Frölich L, Winblad B, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of Alzheimer’s disease and other dementias. World J Biol Psychiatry. 2011;12(1):2-32.
8. Chertkow H, Massoud F, Nasreddine Z, et al. Diagnosis and treatment of dementia: 3. Mild cognitive impairment and cognitive impairment without dementia. CMAJ. 2008;178(10):1273-1285.
9. McKhann GM. Changing concepts of Alzheimer disease. JAMA. 2011;305(23):2458-2459.
10. Galasko D. Biomarkers in non-Alzheimer’s dementias. Clinical Neuroscience Research. 2004;3(6):375-381.
11. Desai AK, Grossberg GT, Chibnall JT. Healthy brain aging: a road map. Clin Geriatr Med. 2010;26(1):1-16.
12. Wilson RS, Hoganson GM, Rajan KB, et al. Temporal course of depressive symptoms during the development of Alzheimer disease. Neurology. 2010;75(1):21-26.
13. Bremner JD, Narayan M, Anderson ER, et al. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157(1):115-118.
14. Yaffe K, Fox P, Newcomer R, et al. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA. 2002;287(16):2090-2097.
15. Mittelman MS, Haley WE, Clay OJ, et al. Improving caregiver well-being delays nursing home placement of patients with Alzheimer disease. Neurology. 2006;67(9):1592-1599.
Getting ready for DSM-5: Part 1
Discuss this article at www.facebook.com/CurrentPsychiatry
Work on the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5)—scheduled to be published in May 2013—has been ongoing for more than a decade. Momentous advances in genetics and brain imaging since publication of DSM-IV in 1994 have generated optimism that an improved understanding of the neurobiologic underpinnings of psychiatric disorders might lead to a paradigm shift from the current descriptive classification system to a more scientific etiopathophysiological system similar to that used by other medical specialities.1
Some fear that any changes to our current classification system may be premature and could make an already complex system even more unwieldy.2 Scores of articles about the content and process of DSM-5 and several critiques and commentaries on the topic have been published. The American Psychiatric Association (APA) has made the DSM-5 process transparent by posting frequent updates to the DSM-5 Development Web site (www.dsm5.org), seeking feedback from the psychiatric community and the public, and presenting progress reports by members of the DSM-5 Task Force at scientific meetings.
There have been few discussions on the implications of DSM-5 from the practicing clinician’s vantage point, which I seek to present in this series of articles, the remainder of which will be published here, at CurrentPsychiatry.com. In this article, I:
- provide a brief history of psychiatric classification, focusing on the origins and evolution of the DSM system
- summarize the limitations of DSM-IV
- note the challenges and tensions in the construction of DSM-5
- review the DSM-5 process
- outline its current status
- discuss the organization and content of future articles in this series.
Although I am a member of the DSM-5 Psychotic Disorders Work Group, I am solely responsible for the content and any opinions that I offer in this article and series. All details of DSM-5 that I discuss are publicly available at www.dsm5.org. I’ve been a clinician and clinical researcher for >25 years, and my opinions are colored by the need for clarity, rigor, clinical relevance, and a disdain for overly speculative thinking.
Evolution of DSM
A nosological system (system of classification of disease) enables clinicians to provide specific treatments for medical causes of human disease and/or disability with precise and predictable effects and guide patients and families about the likely course and outcome. Such classification systems also are used by:
- researchers, to learn more about the nature of the conditions being classified and develop better treatments for them
- health care systems, to provide optimal health care and track its appropriate provision
- insurance companies, to provide appropriate reimbursement for health care
- health product developers, including pharmaceutical companies, to develop health care products and promote their appropriate utilization
- government agencies, to determine health priorities and apportionment of health care resources
- public health agencies, to track the distribution of health and disease in communities around the world.
An ideal classification system would meet all constituents’ needs while perfectly mapping natural disease entities with distinct etiology and pathophysiology (validity), consistently allow all users to reach the same diagnosis (reliability), and provide clinicians with clear guidance about treatment and likely course for each of the entities (utility), with the list of entities being mutually exclusive and collectively exhaustive (coverage).
The current nosological system for psychiatric disorders originated in the late 19th and early 20th centuries and culminated in the first edition of the Diagnostic and Statistical Manual of Mental Disorders3 released in 1952 and a section related to mental disorders (section V) in the sixth revision of the International Classification of Disease (ICD).4 Whereas DSM focuses exclusively on mental disorders, the ICD is a general medical classification system that began covering mental disorders with its sixth revision in 1949. In subsequent revisions (ICD-7 through -10 and DSM-II through -IV), substantial changes in diagnostic criteria have been made, although the systems’ basic structure has been retained. Table 1 describes major changes from DSM-I through DSM-IV-TR.3,5-9 DSM and ICD both are being revised; DSM-5 is scheduled to be released in 2013 and ICD-11 is to be finalized by 2016.
Table 1
Conceptual development of DSM-I to DSM-IV-TR
| Version | Comments |
|---|---|
| DSM-I (1952)3 | Presumed etiology. 106 diagnoses |
| DSM-II (1968)5 | Glossary definitions. 185 diagnoses |
| DSM-III (1980)6 | Paradigm shift. Explicit criteria. Emphasis on reliability. 265 diagnoses |
| DSM-III-R (1987)7 | Modest changes. Blunted hierarchies. Clarifications. 292 diagnoses |
| DSM-IV (1994)8 | Modest changes. More blunted hierarchies. 361 diagnoses |
| DSM-IV-TR (2000)9 | Only text revision. 361 diagnostic conditions |
What do clinicians need?
Similar to ICD-10, DSM-IV is marked by considerable complexity, variable validity, limited clinical and research utility, and problems of burgeoning comorbidity.10 Efforts to revise DSM seek to address these limitations. From a clinician’s perspective, the most challenging aspects of DSM-IV derive from its complexity—which makes clinical application difficult—and its limited clinical utility, which is exemplified by artificial comorbidity,11 frequent use of “not otherwise specified” (NOS), and relative treatment nonspecificity with reference to diagnosis.
As clinicians, we want a nosological system that is easy to use, can guide treatment decisions, provides useful information about likely disease course and outcomes, and allows us to easily communicate about disease nature with patients, families, payers, and health care administrators. Additionally, although good validity and reliability are desirable for clinicians, adequate coverage of psychiatric disease—the listed conditions should be collectively exhaustive and mutually exclusive—is particularly valued. Finally, we want a diagnostic system that allows us to explain the reasoning behind psychiatric diagnoses and related treatment in lay terms to patients and their families.
DSM-5 development to date
DSM-5 development has been a collaborative effort led by the APA and involves the National Institute of Mental Health, the National Institute on Drug Abuse, the National Institute on Alcohol Abuse and Alcoholism, and the World Health Organization (WHO). Between 1999 and 2002, 3 work conferences resulted in a series of white papers that identified gaps and research needs.1 Between 2003 and 2008, the American Psychiatric Institute for Research and Education, the National Institute of Health, and the WHO organized 13 international conferences to review a wide range of nosologic issues; the proceedings have been compiled into 13 monographs (11 published and 2 in press) and >125 scientific articles that serve as key reference sources for the DSM-5 process. For a continually updated list of these publications, see www.dsm5.org/Research.
In 2006, DSM-5 Task Force Chair David J. Kupfer, MD and Vice Chair Darrel A. Regier, MD, MPH were appointed and began selecting members of the DSM-5 Task Force, a process that was completed in 2007. Members of the 13 diagnostic area Work Groups (Table 2) were selected and the Work Groups were constituted in 2008. All 168 Task Force and Work Group members were vetted to ensure that they met standards of minimum conflict of interest and broad representation. Membership includes diverse professional representation from academia and mental health; 75% of members are from the United States. Six cross-cutting study groups have deliberated on a range of common issues, including:
- spectrum disorders
- lifespan and development
- gender and cross-cultural
- psychiatric/general medicine interface
- impairment and disability assessment
- diagnostic measurement and assessment.
Additionally, >300 external advisors with special expertise have participated in the process. Since 2008, each of the Work Groups has conducted extensive literature reviews of all assigned disorders, evaluated what works and what doesn’t work in DSM-IV-TR, assessed new research developments and clinical issues that have arisen since publication of DSM-IV-TR in 1994, and developed research plans to investigate critical issues utilizing systematic reviews and secondary data analyses. Based on these analyses, each Work Group proposed draft diagnostic criteria for its disorders, using a strict protocol for criteria revisions such as addition or deletion of disorders and changes to existing diagnostic criteria. These draft diagnostic criteria were first presented on www.dsm5.org in late 2009 through early 2010. Based on input from other Work Groups, the Task Force, several external groups, and the public, the Work Groups revised these criteria and prioritized necessary field trials to evaluate key recommendations. Phase I of the field trials began in 2010.12 Results of these field trials are being compiled and analyzed.
The DSM-5 Work Groups have met via teleconference 1 to 2 times a month and in-person twice a year, with significant communication between meetings. Work Group chairs are members of the Task Force, which has equally frequent meetings. Reports of DSM-5 deliberations have been presented at hundreds of professional meetings and described in >200 scientific publications. Comprehensive information and ongoing updates on DSM-5 and a list of publications and meetings are provided at www.dsm5.org.
Public input has been sought and the Work Groups have received and processed >10,000 comments. In 2010, the APA Board of Trustees appointed a Scientific Review Committee to evaluate the scientific merit and clinical impact of the Work Group recommendations and comment on the strength of the evidence advanced in support of each proposed revision. In 2011, the Board of Trustees appointed a Clinical and Public Health Committee to evaluate the clinical utility and public health significance of the proposed revisions. The APA and WHO have shared information and assessments in an effort to harmonize diagnostic criteria between DSM-5 and ICD-11.
Initial hopes that DSM-5 could represent a paradigm shift toward an etiopathophysiological classification of psychiatric disorders have been tempered by recognition of the limitations of our current neurobiologic understanding of psychiatric disorders. Therefore, the focus for DSM-5 has shifted from validity enhancements to improved clinical utility while building a framework that better lends itself to a future etiopathophysiological nosology.13-18 Whereas dimensional assessments are likely to be added across various diagnostic categories, a primarily categorical nosology will be retained and the proposed criterion changes are relatively modest. The results of our enhanced knowledge about the neurobiologic underpinnings of psychiatric disorders will not be reflected in diagnostic criteria, but in the significant revisions to the DSM text.
Our DSM-5 series
Subsequent articles in this series—which will be published here, at CurrentPsychiatry.com—will discuss specific proposed DSM-5 changes in 13 groups of disorders (Table 2) and their clinical implications. These articles also will address the relationship of DSM to ICD, issues with dimensional classification, and the importance of and challenges in precise diagnostic measurement.
Table 2
DSM-5 Work Groups
| Attention-deficit/hyperactivity disorder and disruptive behaviors |
| Anxiety, obsessive-compulsive, posttraumatic, and dissociative disorders |
| Disorders in childhood and adolescence |
| Eating disorders |
| Mood disorders |
| Neurocognitive disorders |
| Neurodevelopmental disorders |
| Personality and personality disorders |
| Psychotic disorders |
| Sexual and gender identity disorders |
| Sleep-wake disorders |
| Somatic distress disorders |
| Substance-related disorders |
Related Resources
- American Psychiatric Association. DSM-5 Development. www.dsm5.org.
- Black DW, Zimmerman M. Redefining personality disorders: Proposed revisions for DSM-5. Current Psychiatry. 2011;10(9):26-38.
Disclosure
Dr. Tandon is a member of the DSM-5 Psychotic Disorders Work Group. He reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kupfer DJ, First MB, Regier DA. eds. A research agenda for DSM-V. Washington, DC: American Psychiatric Association; 2002.
2. Frances A. Whither DSM-V? Br J Psychiatry. 2009;195(5):391-392.
3. Diagnostic and statistical manual of mental disorders, 1st ed. Washington DC: American Psychiatric Association; 1952.
4. World Health Organization. Manual of the international statistical classification of diseases injuries and causes of death, 6th revision (ICD-6). Geneva, Switzerland: World Health Organization; 1949.
5. Diagnostic and statistical manual of mental disorders, 2nd ed. Washington DC: American Psychiatric Association; 1968.
6. Diagnostic and statistical manual of mental disorders, 3rd ed. Washington DC: American Psychiatric Association; 1980.
7. Diagnostic and statistical manual of mental disorders, 3rd ed, rev. Washington, DC: American Psychiatric Association; 1987.
8. Diagnostic and statistical manual of mental disorders, 4th ed. Washington DC: American Psychiatric Association; 1994.
9. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
10. Kendell RE, Jablensky A. Distinguishing between the validity and utility of psychiatric diagnoses. Am J Psychiatry. 2003;160(1):4-12.
11. Maj M. “Psychiatric comorbidity”: an artifact of current diagnostic systems? Br J Psychiatry. 2005;186:182-184.
12. Kraemer HC, Kupfer DJ, Narrow WE, et al. Moving toward DSM-5: the field trials. Am J Psychiatry. 2010;167(10):1158-1160.
13. Hyman SE. The diagnosis of mental disorders: the problem of reification. Annu Rev Clin Psychol. 2010;6:155-179.
14. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia “just the facts” 4. Clinical features and conceptualization. Schizophr Res. 2009;110(1-3):1-23.
15. Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748-751.
16. Kendler KS, First MB. Alternative futures for the DSM revision process: iteration versus paradigm shift. Br J Psychiatry. 2010;197(4):263-265.
17. Kupfer DJ, Regier DA. Neuroscience clinical evidence, and the future of psychiatric classification in DSM-5. Am J Psychiatry. 2011;168(7):672-674.
18. Regier DA, Kuhl EA, Narrow WE, et al. Research planning for the future of psychiatric diagnosis [published online ahead of print June 13, 2011]. Eur Psychiatry. doi:10.1016/j.eurpsy.2009.11.013.
Discuss this article at www.facebook.com/CurrentPsychiatry
Work on the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5)—scheduled to be published in May 2013—has been ongoing for more than a decade. Momentous advances in genetics and brain imaging since publication of DSM-IV in 1994 have generated optimism that an improved understanding of the neurobiologic underpinnings of psychiatric disorders might lead to a paradigm shift from the current descriptive classification system to a more scientific etiopathophysiological system similar to that used by other medical specialities.1
Some fear that any changes to our current classification system may be premature and could make an already complex system even more unwieldy.2 Scores of articles about the content and process of DSM-5 and several critiques and commentaries on the topic have been published. The American Psychiatric Association (APA) has made the DSM-5 process transparent by posting frequent updates to the DSM-5 Development Web site (www.dsm5.org), seeking feedback from the psychiatric community and the public, and presenting progress reports by members of the DSM-5 Task Force at scientific meetings.
There have been few discussions on the implications of DSM-5 from the practicing clinician’s vantage point, which I seek to present in this series of articles, the remainder of which will be published here, at CurrentPsychiatry.com. In this article, I:
- provide a brief history of psychiatric classification, focusing on the origins and evolution of the DSM system
- summarize the limitations of DSM-IV
- note the challenges and tensions in the construction of DSM-5
- review the DSM-5 process
- outline its current status
- discuss the organization and content of future articles in this series.
Although I am a member of the DSM-5 Psychotic Disorders Work Group, I am solely responsible for the content and any opinions that I offer in this article and series. All details of DSM-5 that I discuss are publicly available at www.dsm5.org. I’ve been a clinician and clinical researcher for >25 years, and my opinions are colored by the need for clarity, rigor, clinical relevance, and a disdain for overly speculative thinking.
Evolution of DSM
A nosological system (system of classification of disease) enables clinicians to provide specific treatments for medical causes of human disease and/or disability with precise and predictable effects and guide patients and families about the likely course and outcome. Such classification systems also are used by:
- researchers, to learn more about the nature of the conditions being classified and develop better treatments for them
- health care systems, to provide optimal health care and track its appropriate provision
- insurance companies, to provide appropriate reimbursement for health care
- health product developers, including pharmaceutical companies, to develop health care products and promote their appropriate utilization
- government agencies, to determine health priorities and apportionment of health care resources
- public health agencies, to track the distribution of health and disease in communities around the world.
An ideal classification system would meet all constituents’ needs while perfectly mapping natural disease entities with distinct etiology and pathophysiology (validity), consistently allow all users to reach the same diagnosis (reliability), and provide clinicians with clear guidance about treatment and likely course for each of the entities (utility), with the list of entities being mutually exclusive and collectively exhaustive (coverage).
The current nosological system for psychiatric disorders originated in the late 19th and early 20th centuries and culminated in the first edition of the Diagnostic and Statistical Manual of Mental Disorders3 released in 1952 and a section related to mental disorders (section V) in the sixth revision of the International Classification of Disease (ICD).4 Whereas DSM focuses exclusively on mental disorders, the ICD is a general medical classification system that began covering mental disorders with its sixth revision in 1949. In subsequent revisions (ICD-7 through -10 and DSM-II through -IV), substantial changes in diagnostic criteria have been made, although the systems’ basic structure has been retained. Table 1 describes major changes from DSM-I through DSM-IV-TR.3,5-9 DSM and ICD both are being revised; DSM-5 is scheduled to be released in 2013 and ICD-11 is to be finalized by 2016.
Table 1
Conceptual development of DSM-I to DSM-IV-TR
| Version | Comments |
|---|---|
| DSM-I (1952)3 | Presumed etiology. 106 diagnoses |
| DSM-II (1968)5 | Glossary definitions. 185 diagnoses |
| DSM-III (1980)6 | Paradigm shift. Explicit criteria. Emphasis on reliability. 265 diagnoses |
| DSM-III-R (1987)7 | Modest changes. Blunted hierarchies. Clarifications. 292 diagnoses |
| DSM-IV (1994)8 | Modest changes. More blunted hierarchies. 361 diagnoses |
| DSM-IV-TR (2000)9 | Only text revision. 361 diagnostic conditions |
What do clinicians need?
Similar to ICD-10, DSM-IV is marked by considerable complexity, variable validity, limited clinical and research utility, and problems of burgeoning comorbidity.10 Efforts to revise DSM seek to address these limitations. From a clinician’s perspective, the most challenging aspects of DSM-IV derive from its complexity—which makes clinical application difficult—and its limited clinical utility, which is exemplified by artificial comorbidity,11 frequent use of “not otherwise specified” (NOS), and relative treatment nonspecificity with reference to diagnosis.
As clinicians, we want a nosological system that is easy to use, can guide treatment decisions, provides useful information about likely disease course and outcomes, and allows us to easily communicate about disease nature with patients, families, payers, and health care administrators. Additionally, although good validity and reliability are desirable for clinicians, adequate coverage of psychiatric disease—the listed conditions should be collectively exhaustive and mutually exclusive—is particularly valued. Finally, we want a diagnostic system that allows us to explain the reasoning behind psychiatric diagnoses and related treatment in lay terms to patients and their families.
DSM-5 development to date
DSM-5 development has been a collaborative effort led by the APA and involves the National Institute of Mental Health, the National Institute on Drug Abuse, the National Institute on Alcohol Abuse and Alcoholism, and the World Health Organization (WHO). Between 1999 and 2002, 3 work conferences resulted in a series of white papers that identified gaps and research needs.1 Between 2003 and 2008, the American Psychiatric Institute for Research and Education, the National Institute of Health, and the WHO organized 13 international conferences to review a wide range of nosologic issues; the proceedings have been compiled into 13 monographs (11 published and 2 in press) and >125 scientific articles that serve as key reference sources for the DSM-5 process. For a continually updated list of these publications, see www.dsm5.org/Research.
In 2006, DSM-5 Task Force Chair David J. Kupfer, MD and Vice Chair Darrel A. Regier, MD, MPH were appointed and began selecting members of the DSM-5 Task Force, a process that was completed in 2007. Members of the 13 diagnostic area Work Groups (Table 2) were selected and the Work Groups were constituted in 2008. All 168 Task Force and Work Group members were vetted to ensure that they met standards of minimum conflict of interest and broad representation. Membership includes diverse professional representation from academia and mental health; 75% of members are from the United States. Six cross-cutting study groups have deliberated on a range of common issues, including:
- spectrum disorders
- lifespan and development
- gender and cross-cultural
- psychiatric/general medicine interface
- impairment and disability assessment
- diagnostic measurement and assessment.
Additionally, >300 external advisors with special expertise have participated in the process. Since 2008, each of the Work Groups has conducted extensive literature reviews of all assigned disorders, evaluated what works and what doesn’t work in DSM-IV-TR, assessed new research developments and clinical issues that have arisen since publication of DSM-IV-TR in 1994, and developed research plans to investigate critical issues utilizing systematic reviews and secondary data analyses. Based on these analyses, each Work Group proposed draft diagnostic criteria for its disorders, using a strict protocol for criteria revisions such as addition or deletion of disorders and changes to existing diagnostic criteria. These draft diagnostic criteria were first presented on www.dsm5.org in late 2009 through early 2010. Based on input from other Work Groups, the Task Force, several external groups, and the public, the Work Groups revised these criteria and prioritized necessary field trials to evaluate key recommendations. Phase I of the field trials began in 2010.12 Results of these field trials are being compiled and analyzed.
The DSM-5 Work Groups have met via teleconference 1 to 2 times a month and in-person twice a year, with significant communication between meetings. Work Group chairs are members of the Task Force, which has equally frequent meetings. Reports of DSM-5 deliberations have been presented at hundreds of professional meetings and described in >200 scientific publications. Comprehensive information and ongoing updates on DSM-5 and a list of publications and meetings are provided at www.dsm5.org.
Public input has been sought and the Work Groups have received and processed >10,000 comments. In 2010, the APA Board of Trustees appointed a Scientific Review Committee to evaluate the scientific merit and clinical impact of the Work Group recommendations and comment on the strength of the evidence advanced in support of each proposed revision. In 2011, the Board of Trustees appointed a Clinical and Public Health Committee to evaluate the clinical utility and public health significance of the proposed revisions. The APA and WHO have shared information and assessments in an effort to harmonize diagnostic criteria between DSM-5 and ICD-11.
Initial hopes that DSM-5 could represent a paradigm shift toward an etiopathophysiological classification of psychiatric disorders have been tempered by recognition of the limitations of our current neurobiologic understanding of psychiatric disorders. Therefore, the focus for DSM-5 has shifted from validity enhancements to improved clinical utility while building a framework that better lends itself to a future etiopathophysiological nosology.13-18 Whereas dimensional assessments are likely to be added across various diagnostic categories, a primarily categorical nosology will be retained and the proposed criterion changes are relatively modest. The results of our enhanced knowledge about the neurobiologic underpinnings of psychiatric disorders will not be reflected in diagnostic criteria, but in the significant revisions to the DSM text.
Our DSM-5 series
Subsequent articles in this series—which will be published here, at CurrentPsychiatry.com—will discuss specific proposed DSM-5 changes in 13 groups of disorders (Table 2) and their clinical implications. These articles also will address the relationship of DSM to ICD, issues with dimensional classification, and the importance of and challenges in precise diagnostic measurement.
Table 2
DSM-5 Work Groups
| Attention-deficit/hyperactivity disorder and disruptive behaviors |
| Anxiety, obsessive-compulsive, posttraumatic, and dissociative disorders |
| Disorders in childhood and adolescence |
| Eating disorders |
| Mood disorders |
| Neurocognitive disorders |
| Neurodevelopmental disorders |
| Personality and personality disorders |
| Psychotic disorders |
| Sexual and gender identity disorders |
| Sleep-wake disorders |
| Somatic distress disorders |
| Substance-related disorders |
Related Resources
- American Psychiatric Association. DSM-5 Development. www.dsm5.org.
- Black DW, Zimmerman M. Redefining personality disorders: Proposed revisions for DSM-5. Current Psychiatry. 2011;10(9):26-38.
Disclosure
Dr. Tandon is a member of the DSM-5 Psychotic Disorders Work Group. He reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Work on the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5)—scheduled to be published in May 2013—has been ongoing for more than a decade. Momentous advances in genetics and brain imaging since publication of DSM-IV in 1994 have generated optimism that an improved understanding of the neurobiologic underpinnings of psychiatric disorders might lead to a paradigm shift from the current descriptive classification system to a more scientific etiopathophysiological system similar to that used by other medical specialities.1
Some fear that any changes to our current classification system may be premature and could make an already complex system even more unwieldy.2 Scores of articles about the content and process of DSM-5 and several critiques and commentaries on the topic have been published. The American Psychiatric Association (APA) has made the DSM-5 process transparent by posting frequent updates to the DSM-5 Development Web site (www.dsm5.org), seeking feedback from the psychiatric community and the public, and presenting progress reports by members of the DSM-5 Task Force at scientific meetings.
There have been few discussions on the implications of DSM-5 from the practicing clinician’s vantage point, which I seek to present in this series of articles, the remainder of which will be published here, at CurrentPsychiatry.com. In this article, I:
- provide a brief history of psychiatric classification, focusing on the origins and evolution of the DSM system
- summarize the limitations of DSM-IV
- note the challenges and tensions in the construction of DSM-5
- review the DSM-5 process
- outline its current status
- discuss the organization and content of future articles in this series.
Although I am a member of the DSM-5 Psychotic Disorders Work Group, I am solely responsible for the content and any opinions that I offer in this article and series. All details of DSM-5 that I discuss are publicly available at www.dsm5.org. I’ve been a clinician and clinical researcher for >25 years, and my opinions are colored by the need for clarity, rigor, clinical relevance, and a disdain for overly speculative thinking.
Evolution of DSM
A nosological system (system of classification of disease) enables clinicians to provide specific treatments for medical causes of human disease and/or disability with precise and predictable effects and guide patients and families about the likely course and outcome. Such classification systems also are used by:
- researchers, to learn more about the nature of the conditions being classified and develop better treatments for them
- health care systems, to provide optimal health care and track its appropriate provision
- insurance companies, to provide appropriate reimbursement for health care
- health product developers, including pharmaceutical companies, to develop health care products and promote their appropriate utilization
- government agencies, to determine health priorities and apportionment of health care resources
- public health agencies, to track the distribution of health and disease in communities around the world.
An ideal classification system would meet all constituents’ needs while perfectly mapping natural disease entities with distinct etiology and pathophysiology (validity), consistently allow all users to reach the same diagnosis (reliability), and provide clinicians with clear guidance about treatment and likely course for each of the entities (utility), with the list of entities being mutually exclusive and collectively exhaustive (coverage).
The current nosological system for psychiatric disorders originated in the late 19th and early 20th centuries and culminated in the first edition of the Diagnostic and Statistical Manual of Mental Disorders3 released in 1952 and a section related to mental disorders (section V) in the sixth revision of the International Classification of Disease (ICD).4 Whereas DSM focuses exclusively on mental disorders, the ICD is a general medical classification system that began covering mental disorders with its sixth revision in 1949. In subsequent revisions (ICD-7 through -10 and DSM-II through -IV), substantial changes in diagnostic criteria have been made, although the systems’ basic structure has been retained. Table 1 describes major changes from DSM-I through DSM-IV-TR.3,5-9 DSM and ICD both are being revised; DSM-5 is scheduled to be released in 2013 and ICD-11 is to be finalized by 2016.
Table 1
Conceptual development of DSM-I to DSM-IV-TR
| Version | Comments |
|---|---|
| DSM-I (1952)3 | Presumed etiology. 106 diagnoses |
| DSM-II (1968)5 | Glossary definitions. 185 diagnoses |
| DSM-III (1980)6 | Paradigm shift. Explicit criteria. Emphasis on reliability. 265 diagnoses |
| DSM-III-R (1987)7 | Modest changes. Blunted hierarchies. Clarifications. 292 diagnoses |
| DSM-IV (1994)8 | Modest changes. More blunted hierarchies. 361 diagnoses |
| DSM-IV-TR (2000)9 | Only text revision. 361 diagnostic conditions |
What do clinicians need?
Similar to ICD-10, DSM-IV is marked by considerable complexity, variable validity, limited clinical and research utility, and problems of burgeoning comorbidity.10 Efforts to revise DSM seek to address these limitations. From a clinician’s perspective, the most challenging aspects of DSM-IV derive from its complexity—which makes clinical application difficult—and its limited clinical utility, which is exemplified by artificial comorbidity,11 frequent use of “not otherwise specified” (NOS), and relative treatment nonspecificity with reference to diagnosis.
As clinicians, we want a nosological system that is easy to use, can guide treatment decisions, provides useful information about likely disease course and outcomes, and allows us to easily communicate about disease nature with patients, families, payers, and health care administrators. Additionally, although good validity and reliability are desirable for clinicians, adequate coverage of psychiatric disease—the listed conditions should be collectively exhaustive and mutually exclusive—is particularly valued. Finally, we want a diagnostic system that allows us to explain the reasoning behind psychiatric diagnoses and related treatment in lay terms to patients and their families.
DSM-5 development to date
DSM-5 development has been a collaborative effort led by the APA and involves the National Institute of Mental Health, the National Institute on Drug Abuse, the National Institute on Alcohol Abuse and Alcoholism, and the World Health Organization (WHO). Between 1999 and 2002, 3 work conferences resulted in a series of white papers that identified gaps and research needs.1 Between 2003 and 2008, the American Psychiatric Institute for Research and Education, the National Institute of Health, and the WHO organized 13 international conferences to review a wide range of nosologic issues; the proceedings have been compiled into 13 monographs (11 published and 2 in press) and >125 scientific articles that serve as key reference sources for the DSM-5 process. For a continually updated list of these publications, see www.dsm5.org/Research.
In 2006, DSM-5 Task Force Chair David J. Kupfer, MD and Vice Chair Darrel A. Regier, MD, MPH were appointed and began selecting members of the DSM-5 Task Force, a process that was completed in 2007. Members of the 13 diagnostic area Work Groups (Table 2) were selected and the Work Groups were constituted in 2008. All 168 Task Force and Work Group members were vetted to ensure that they met standards of minimum conflict of interest and broad representation. Membership includes diverse professional representation from academia and mental health; 75% of members are from the United States. Six cross-cutting study groups have deliberated on a range of common issues, including:
- spectrum disorders
- lifespan and development
- gender and cross-cultural
- psychiatric/general medicine interface
- impairment and disability assessment
- diagnostic measurement and assessment.
Additionally, >300 external advisors with special expertise have participated in the process. Since 2008, each of the Work Groups has conducted extensive literature reviews of all assigned disorders, evaluated what works and what doesn’t work in DSM-IV-TR, assessed new research developments and clinical issues that have arisen since publication of DSM-IV-TR in 1994, and developed research plans to investigate critical issues utilizing systematic reviews and secondary data analyses. Based on these analyses, each Work Group proposed draft diagnostic criteria for its disorders, using a strict protocol for criteria revisions such as addition or deletion of disorders and changes to existing diagnostic criteria. These draft diagnostic criteria were first presented on www.dsm5.org in late 2009 through early 2010. Based on input from other Work Groups, the Task Force, several external groups, and the public, the Work Groups revised these criteria and prioritized necessary field trials to evaluate key recommendations. Phase I of the field trials began in 2010.12 Results of these field trials are being compiled and analyzed.
The DSM-5 Work Groups have met via teleconference 1 to 2 times a month and in-person twice a year, with significant communication between meetings. Work Group chairs are members of the Task Force, which has equally frequent meetings. Reports of DSM-5 deliberations have been presented at hundreds of professional meetings and described in >200 scientific publications. Comprehensive information and ongoing updates on DSM-5 and a list of publications and meetings are provided at www.dsm5.org.
Public input has been sought and the Work Groups have received and processed >10,000 comments. In 2010, the APA Board of Trustees appointed a Scientific Review Committee to evaluate the scientific merit and clinical impact of the Work Group recommendations and comment on the strength of the evidence advanced in support of each proposed revision. In 2011, the Board of Trustees appointed a Clinical and Public Health Committee to evaluate the clinical utility and public health significance of the proposed revisions. The APA and WHO have shared information and assessments in an effort to harmonize diagnostic criteria between DSM-5 and ICD-11.
Initial hopes that DSM-5 could represent a paradigm shift toward an etiopathophysiological classification of psychiatric disorders have been tempered by recognition of the limitations of our current neurobiologic understanding of psychiatric disorders. Therefore, the focus for DSM-5 has shifted from validity enhancements to improved clinical utility while building a framework that better lends itself to a future etiopathophysiological nosology.13-18 Whereas dimensional assessments are likely to be added across various diagnostic categories, a primarily categorical nosology will be retained and the proposed criterion changes are relatively modest. The results of our enhanced knowledge about the neurobiologic underpinnings of psychiatric disorders will not be reflected in diagnostic criteria, but in the significant revisions to the DSM text.
Our DSM-5 series
Subsequent articles in this series—which will be published here, at CurrentPsychiatry.com—will discuss specific proposed DSM-5 changes in 13 groups of disorders (Table 2) and their clinical implications. These articles also will address the relationship of DSM to ICD, issues with dimensional classification, and the importance of and challenges in precise diagnostic measurement.
Table 2
DSM-5 Work Groups
| Attention-deficit/hyperactivity disorder and disruptive behaviors |
| Anxiety, obsessive-compulsive, posttraumatic, and dissociative disorders |
| Disorders in childhood and adolescence |
| Eating disorders |
| Mood disorders |
| Neurocognitive disorders |
| Neurodevelopmental disorders |
| Personality and personality disorders |
| Psychotic disorders |
| Sexual and gender identity disorders |
| Sleep-wake disorders |
| Somatic distress disorders |
| Substance-related disorders |
Related Resources
- American Psychiatric Association. DSM-5 Development. www.dsm5.org.
- Black DW, Zimmerman M. Redefining personality disorders: Proposed revisions for DSM-5. Current Psychiatry. 2011;10(9):26-38.
Disclosure
Dr. Tandon is a member of the DSM-5 Psychotic Disorders Work Group. He reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kupfer DJ, First MB, Regier DA. eds. A research agenda for DSM-V. Washington, DC: American Psychiatric Association; 2002.
2. Frances A. Whither DSM-V? Br J Psychiatry. 2009;195(5):391-392.
3. Diagnostic and statistical manual of mental disorders, 1st ed. Washington DC: American Psychiatric Association; 1952.
4. World Health Organization. Manual of the international statistical classification of diseases injuries and causes of death, 6th revision (ICD-6). Geneva, Switzerland: World Health Organization; 1949.
5. Diagnostic and statistical manual of mental disorders, 2nd ed. Washington DC: American Psychiatric Association; 1968.
6. Diagnostic and statistical manual of mental disorders, 3rd ed. Washington DC: American Psychiatric Association; 1980.
7. Diagnostic and statistical manual of mental disorders, 3rd ed, rev. Washington, DC: American Psychiatric Association; 1987.
8. Diagnostic and statistical manual of mental disorders, 4th ed. Washington DC: American Psychiatric Association; 1994.
9. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
10. Kendell RE, Jablensky A. Distinguishing between the validity and utility of psychiatric diagnoses. Am J Psychiatry. 2003;160(1):4-12.
11. Maj M. “Psychiatric comorbidity”: an artifact of current diagnostic systems? Br J Psychiatry. 2005;186:182-184.
12. Kraemer HC, Kupfer DJ, Narrow WE, et al. Moving toward DSM-5: the field trials. Am J Psychiatry. 2010;167(10):1158-1160.
13. Hyman SE. The diagnosis of mental disorders: the problem of reification. Annu Rev Clin Psychol. 2010;6:155-179.
14. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia “just the facts” 4. Clinical features and conceptualization. Schizophr Res. 2009;110(1-3):1-23.
15. Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748-751.
16. Kendler KS, First MB. Alternative futures for the DSM revision process: iteration versus paradigm shift. Br J Psychiatry. 2010;197(4):263-265.
17. Kupfer DJ, Regier DA. Neuroscience clinical evidence, and the future of psychiatric classification in DSM-5. Am J Psychiatry. 2011;168(7):672-674.
18. Regier DA, Kuhl EA, Narrow WE, et al. Research planning for the future of psychiatric diagnosis [published online ahead of print June 13, 2011]. Eur Psychiatry. doi:10.1016/j.eurpsy.2009.11.013.
1. Kupfer DJ, First MB, Regier DA. eds. A research agenda for DSM-V. Washington, DC: American Psychiatric Association; 2002.
2. Frances A. Whither DSM-V? Br J Psychiatry. 2009;195(5):391-392.
3. Diagnostic and statistical manual of mental disorders, 1st ed. Washington DC: American Psychiatric Association; 1952.
4. World Health Organization. Manual of the international statistical classification of diseases injuries and causes of death, 6th revision (ICD-6). Geneva, Switzerland: World Health Organization; 1949.
5. Diagnostic and statistical manual of mental disorders, 2nd ed. Washington DC: American Psychiatric Association; 1968.
6. Diagnostic and statistical manual of mental disorders, 3rd ed. Washington DC: American Psychiatric Association; 1980.
7. Diagnostic and statistical manual of mental disorders, 3rd ed, rev. Washington, DC: American Psychiatric Association; 1987.
8. Diagnostic and statistical manual of mental disorders, 4th ed. Washington DC: American Psychiatric Association; 1994.
9. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
10. Kendell RE, Jablensky A. Distinguishing between the validity and utility of psychiatric diagnoses. Am J Psychiatry. 2003;160(1):4-12.
11. Maj M. “Psychiatric comorbidity”: an artifact of current diagnostic systems? Br J Psychiatry. 2005;186:182-184.
12. Kraemer HC, Kupfer DJ, Narrow WE, et al. Moving toward DSM-5: the field trials. Am J Psychiatry. 2010;167(10):1158-1160.
13. Hyman SE. The diagnosis of mental disorders: the problem of reification. Annu Rev Clin Psychol. 2010;6:155-179.
14. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia “just the facts” 4. Clinical features and conceptualization. Schizophr Res. 2009;110(1-3):1-23.
15. Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748-751.
16. Kendler KS, First MB. Alternative futures for the DSM revision process: iteration versus paradigm shift. Br J Psychiatry. 2010;197(4):263-265.
17. Kupfer DJ, Regier DA. Neuroscience clinical evidence, and the future of psychiatric classification in DSM-5. Am J Psychiatry. 2011;168(7):672-674.
18. Regier DA, Kuhl EA, Narrow WE, et al. Research planning for the future of psychiatric diagnosis [published online ahead of print June 13, 2011]. Eur Psychiatry. doi:10.1016/j.eurpsy.2009.11.013.
Psychiatric illness during pregnancy
Perinatal psychopathology is a common and undertreated problem with wide-ranging consequences for both mother and child.1-4 Women at risk for psychopathology are more likely to engage in unhealthy behaviors such as smoking and substance abuse and have difficulty engaging in treatment and attending psychiatric and obstetrics appointments.5 In addition, many of these women have trouble attaching to and caring for their infants and struggle with everyday stressors during pregnancy and postpartum.6
Routine prenatal screening for mental illness coupled with non-judgmental, collaborative, and individualized care delivered by a multidisciplinary team is critical for treatment engagement and adherence. Providers should be aware of risk factors for perinatal psychiatric illness—including a history of mental illness, stressful life events, and interpersonal conflict—and should be versed in current treatment guidelines.
CASE REPORT: Difficulty coping
Ms. A, age 28, is referred to our High Risk Perinatal Team by her obstetrician when she is approximately 6 weeks pregnant. She is single, has 3 other children (age 10, 4, and 2), a history of depression, and chronic pain related to an auto accident 3 years ago. She reports that this pregnancy likely is the result of a sexual assault, but she has decided to keep the baby. Ms. A describes severe depressive symptoms, including insomnia, low appetite, feelings of worthlessness, and thoughts of harming herself. In addition, she has incapacitating panic attacks and constantly worries about her children’s safety when she is not with them. She schedules an appointment with the perinatal team, but does not show up twice.
When our team finally sees Ms. A, she is well into her second trimester and brings her 2 youngest children with her. She says she recently was fired from her job as a cashier because she missed too many days of work, and is applying for Medicaid. Recently, her back and shoulder pain have worsened, and she is running out of her prescription for acetaminophen/hydrocodone. Ms. A’s affect is flat, her mood depressed, and she has difficulty explaining her history because her 2-year-old son interrupts the interview. She has never been in psychotherapy, and is reluctant to take antidepressants. Despite a difficult first visit, she engages with the clinician and agrees to schedule a second appointment.
What complicates pregnancy?
Women are at higher risk for developing depression during puberty, the perinatal period (ie, pregnancy and first year postpartum), and perimenopause.7 These times often are fraught with unfamiliar hormonal fluctuations, role transitions, emotional upheaval, and physical changes. However, because these times are expected to be stressful, serious mood changes often go unnoticed by patients and untreated by clinicians.8 Women are expected to celebrate, thrive, and “glow” during pregnancy, and those who suffer from depression and anxiety frequently do so in silence. Social stigma surrounding perinatal depression or anxiety leads many women to believe they are alone in their struggle and hesitant to seek help.9
Most pregnant women who develop psychiatric illness do not present for treatment.10 One study found that 86% of pregnant women who screened positive for depression in an obstetrics (OB) setting did not receive treatment.11 Some women are reluctant to take antidepressants out of concern for their infant’s safety,8 and psychotherapy or alternative approaches are not available in all areas.12 Transportation, childcare issues, or ongoing life stressors may prevent women from seeking help (Box 1).9
Diagnostic uncertainty among professionals may aggravate undertreatment. Clinicians who are unfamiliar with the presentation of perinatal mental illness may mislabel depressive features—such as irritability, loss of interest in activities, low energy, increased anxiety, difficulty sleeping, or appetite dysregulation—as normative experiences during pregnancy or adjustment after childbirth. Concerned about fetal exposure to potentially teratogenic compounds, clinicians may under-dose otherwise effective medications, which can lead to treatment resistance. Even if treated aggressively, depression in pregnancy may persist because of other factors, such as comorbid anxiety, somatization, pain, substance use/dependence, undiagnosed bipolar illness, or the presence of severe psychosocial stress or trauma.
Maternal suicide and/or harm to the infant—the most severe result of untreated perinatal psychopathology—is rare.13 Common negative outcomes of untreated depression or anxiety in pregnant women include inadequate weight gain, preeclampsia, difficulty bonding with their unborn baby, premature labor, and lack of follow through with prenatal care.14,15 Symptoms become harder to treat when aggravated by psychosocial stressors such as poor social support, ambivalence about the pregnancy, and/or substance abuse.
The key to successful intervention is finding a balance between managing psychiatric concerns, facilitating adequate coping with psychosocial stressors, and, if necessary, aggressively treating pregnancy-related physical illnesses. Successful treatment response depends on early detection and initiating individualized care as soon as possible.
Lack of insurance, childcare, or transportation can make it difficult for a pregnant woman to receive psychiatric treatment. All pregnant women are eligible for Medicaid if private insurance is unavailable to them, and clinicians can help patients apply for assistance. Some programs—for example, Michigan’s state-funded Maternal Infant Health Programs—offer help with transportation to appointments, such as cabs and reimbursement for gas, in addition to nutrition guidance, counseling, home visits, and referrals to community resources such as childbirth classes, infant mental health specialists, and/or substance abuse treatment (see Related Resources ).
Offering childcare during psychotherapy sessions can be particularly helpful, and may provide valuable experience for a student or resident interested in working with at-risk children. Women may be more likely to engage in care if psychotherapy sessions are conducted by phone or in their homes. A positive experience with mental health care during pregnancy may increase the likelihood that women will remain engaged in treatment after childbirth, therefore lessening the negative effects of perinatal psychopathology on mother and child.
Early detection. Women’s health care providers play a fundamental role in guiding decision-making about mental health care, providing referrals, and most important, allowing women to talk about perinatal psychopathology without fear of stigma.
When a woman becomes pregnant, it is critical to determine if she is at risk for developing psychopathology or presents with active illness. Many OB clinics screen for depression several times during pregnancy and early postpartum. The most commonly used screening tool is the Edinburgh Postpartum Depression Scale (EPDS),16 a 10-item self-report measure that is sensitive to cognitive and affective symptoms of depression. If a woman scores >15 during pregnancy or >13 postpartum, further assessment is indicated.17 The anxiety subscale (items 5 and 6) of the EPDS has been validated for screening perinatal anxiety using a cut-off score >4.18 Depression can be quickly assessed using the 2-question Patient Health Questionnaire (PHQ-2) or the 9-question PHQ-9.19,20 All 3 scales are free and available on the Internet (Table 1).21
These screening tools offer clinicians an opportunity to assess for risk factors that may increase the likelihood of illness onset or worsened prognosis (Table 2).5,22 All women who present with pregnancy-related medical illness, such as preeclampsia or gestational diabetes, should be screened for co-occurring depression or anxiety because psychiatric comorbidity is common.
Individualized care. Have an open mind about the kind of care to offer and collaborate with the patient when discussing treatment options.5 Some pregnant women may reject traditional treatments, such as pharmacotherapy or psychotherapy, because of concern about harm to the unborn baby or reluctance to work through past or present conflicts in therapy during a vulnerable time.9 Women may assume that medication will be the only treatment offered, or even fear that they will be forced to take antidepressants. Women often do not follow through on mental health referrals, even when they are appropriately screened and identified to be at risk, and an OB nurse explains the risks of untreated psychopathology.11
A multidisciplinary, collaborative care model is vital for positive pregnancy outcomes. Connecting obstetricians and midwives with psychologists, psychiatrists, social workers, and infant mental health specialists to coordinate treatment ensures that at-risk pregnant and postpartum women get the care they need. A nonjudgmental approach is essential to engage pregnant women in care. Assure women that pharmacotherapy is not required when receiving mental health treatment, but is an option they can choose.
Table 1
Screening for psychiatric illness during pregnancy
| Screening tool | Sensitivity/specificity | Administration | Availability |
|---|---|---|---|
| Edinburgh Postpartum Depression Scale | Sensitivity = 0.86 Specificity = 0.78 Positive screen: >10 | Self-administered in 5 to 10 minutes. Could be self-scored | http://bit.ly/PPDscale |
| Patient Health Questionnaire-2 (PHQ-2) | Sensitivity = 0.83 Specificity = 0.92 Positive screen: >3 | Self- or clinician-administered in <1 minute | www.phqscreeners.com The 2 questions from the PHQ-9 for mood and anhedonia are used |
| Patient Health Questionnaire-9 (PHQ-9) | Sensitivity = 0.88 Specificity = 0.88 Positive screen: >10 | Self-administered and self-scored, 5 to 10 minutes | www.phqscreeners.com |
| Source: Reference 21 | |||
Treatment choices
Pharmacotherapy. If a woman has only mild symptoms or has been symptom-free for ≥6 months, it may be safe to decrease or discontinue antidepressants during pregnancy or while trying to conceive, but such patients should be monitored closely for signs of relapse.23 In a study of 201 depressed pregnant women, 68% of those who discontinued medication experienced symptom relapse compared with 26% of those who continued medication.24 If a depressed woman has a history of relapse or severe symptoms, including suicide attempts and inpatient psychiatric admissions, it is recommended that she remain on antidepressants or mood stabilizers, regardless of pregnancy status.25 If medications are necessary during pregnancy— ie, the benefits to the mother outweigh the risks to the unborn baby—the following precautions could help decrease fetal exposure:23
- keep the medication regimen simple and at the lowest effective dose
- use monotherapy when appropriate
- if possible, do not change medications during pregnancy.
When considering pharmacotherapy, evaluate each woman’s risk for disease exacerbation and consequences for pregnancy and neonatal outcomes, and ask the woman how she views reproductive risk vs disease benefit.
Developing fetuses are exposed to either the effects of the mother’s untreated mental illness or the medication.26 A recent study comparing birth and neonatal outcomes among women with untreated depression vs those taking selective serotonin reuptake inhibitors (SSRIs) found similar adverse outcomes.27 Babies continously exposed to either prenatal depression or SSRIs were more likely to be born prematurely, but partial exposure to either condition did not increase this risk.27 In addition, women who were not taking SSRIs had more depressive symptoms and more trouble functioning, which can interfere with bonding between mother and baby, both in-utero and postpartum.6,27 Neither SSRIs nor depression exposure increased risk for minor physical anomalies.27
A careful process of informed consent and documentation is essential when prescribing medications during pregnancy. Women should understand the risks of pharmacotherapy as well as the risks of undertreated illness.
Electroconvulsive therapy can safely help pregnant women with treatment-resistant, life-threatening, or psychotic depression.28,29
Table 2
Risk factors for perinatal psychopathology
| Pregnancy during adolescence |
| Previous diagnosis of depression, anxiety, psychosis, or bipolar disorder |
| Trauma history, including physical, emotional, or sexual abuse |
| Current or past substance abuse/dependence, including cigarette smoking |
| Lack of social support |
| Single parenthood |
| Low socioeconomic status |
| History of sexual assault or domestic violence |
| Unstable home environment |
| Stopping antidepressants during pregnancy |
| Financial problems |
| Ambivalence about pregnancy |
| Source: References 5,22 |
Psychotherapy. The American College of Obstetricians and Gynecologists treatment guidelines22 favor psychotherapy over medication for women with mild depressive symptoms and no loss of function, suicidality, or psychotic experiences; pharmacotherapy is suggested for women who have moderate to severely impaired functioning, recurrent depressive symptoms, or suicidal thinking (Table 3).22
Interpersonal psychotherapy or cognitive-behavioral therapy can be safe and effective during pregnancy.30,31 Other psychotherapeutic modalities and alternative/complementary treatments offer potential benefit without substantial risk, and could help prevent relapse when discontinuing mood stabilizers or antidepressants after conception (Box 2).32-35
Table 3
ACOG guidelines for treating depression during pregnancy
| Women who are thinking about getting pregnant |
| For women on medication with mild or no symptoms for ≥6 months, it may be appropriate to taper and discontinue medication before becoming pregnant |
| Medication discontinuation may not be appropriate in women with a history of severe, recurrent depression or who have psychosis, bipolar disorder, other psychiatric illness requiring medication, or a history of suicide attempts |
| Pregnant women currently taking medication for depression |
| Psychiatrically stable women who prefer to stay on medication may be able to do so after consultation between their psychiatrist and obstetrician to discuss risks and benefits |
| Women who want to discontinue medication may attempt to taper and discontinue if they are not experiencing symptoms, depending on their psychiatric history. Women with a history of recurrent depression are at a high risk of relapse if medication is discontinued |
| Women with recurrent depression or who have symptoms despite medication may benefit from psychotherapy to replace or augment medication |
| Women with severe depression (with suicide attempts, functional incapacitation, or weight loss) should remain on medication. If a patient refuses medication, alternative treatment and monitoring should be in place, preferably before discontinuation |
| Pregnant and not currently on medication for depression |
| Psychotherapy may be beneficial for women who prefer to avoid antidepressants |
| For women who want to take medication, risks and benefits of treatment choices should be evaluated and discussed, including factors such as stage of gestation, symptoms, history of depression, and other conditions and circumstances (eg, smoking, difficulty gaining weight) |
| All pregnant women |
| Regardless of circumstances, a woman with suicidal or psychotic symptoms should immediately see a psychiatrist |
| ACOG: American College of Obstetricians and Gynecologists Source: Reference 22 |
Mind-body approaches such as mindfulness-based stress reduction, yoga, and progressive relaxation and supplements such as fish oil may be good adjuncts to psychotherapy. Many pregnant women prefer mindfulness yoga to other mind-body techniques.32 A pilot study found that mindfulness yoga significantly decreased depressive symptoms and increased maternal-fetal attachment, particularly in mildly depressed women.33 For women who do not wish to engage in traditional treatments, alternative approaches such as progressive relaxation are easily taught and can help reduce depressive symptoms.34 Regular exercise may improve self-esteem and reduce symptoms of depression and anxiety in pregnant women.35
CASE CONTINUED: Healthy baby boy
Ms. A either doesn’t show up or cancels her weekly appointments about once a month, but seems to be making progress. Her therapist makes accommodations for Ms. A, such as offering childcare in an adjacent room during sessions, conducting brief sessions by phone when Ms. A is unable to come to the clinic, and helping her enroll in the state’s Maternal Infant Health Program. Ms. A’s therapist has referred her to a specialized OB clinic that can manage her pain medication and monitor for signs of abuse and keeps in regular contact with her obstetrician.
At 26 weeks gestation, Ms. A still is reluctant to try psychotropics, so her therapist works with her to integrate psychotherapy with alternative approaches such as mindfulness meditation and yoga. During therapy, Ms. A learns ways to manage her depressive symptoms, improve her social functioning, adjust to role transitions, and work through her traumatic experiences. Ms. A enrolls in a prenatal yoga class with a mindfulness focus, which allows her to interact with other pregnant women at risk for psychopathology and learn new ways to cope with her depressed mood and chronic pain.
Ms. A delivers a healthy boy at 38 weeks gestation. During labor, she uses many of the yoga poses she learned to manage pain, but elects to have an epidural after 30 hours of labor. Her baby tests positive for hydrocodone, which can cause ongoing mild irritability and occasional jitteriness. He is observed in the hospital for signs of withdrawal for 48 hours and then discharged home with his mother. Ms. A starts breast-feeding in the hospital and plans to continue at home.
Ms. A’s therapist continues to stay in touch with her by phone until she schedules another appointment and assists with referrals to other community resources.
- University of Michigan Department of Psychiatry Depression Center Women’s Mental Health and Infants Program. Women and depression. www.psych.med.umich.edu/wimhc.
- Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403-413.
- Michigan Families Medicaid Project. Year 1 final report. Includes a list of maternal and child health services similar to Michigan’s Maternal Infant Health Program, for all 50 states. http://1.usa.gov/yCzdca.
Drug Brand Name
- Acetaminophen/hydrocodone • Vicodin
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Steer RA, Scholl TO, Hediger ML, et al. Self-reported depression and negative pregnancy outcomes. J Clin Epidemiol. 1992;45(10):1093-1099.
2. Zuckerman B, Bauchner H, Parker S, et al. Maternal depressive symptoms during pregnancy, and newborn irritability. J Dev Behav Pediatr. 1990;11(4):190-194.
3. Field T, Healy B, Goldstein S, et al. Infants of depressed mothers show depressed behavior even with non-depressed adults. Child Dev. 1988;59(6):1569-1579.
4. Coghill SR, Caplan HL, Alexandra H, et al. Impact of maternal postnatal depression on cognitive development of young children. Br Med J (Clin Res Ed). 1986;292(6529):1165-1167.
5. Muzik M, Marcus S, Heringhausen J, et al. When depression complicates childbearing: guidelines for screening and treatment during antenatal and postpartum obstetric care. Obstet Gynecol Clin North Am. 2009;36(4):771-788, ix–x.
6. Bifulco A, Figueiredo B, Guedeney N, et al. Maternal attachment style and depression associated with childbirth: preliminary results from a European and US cross-cultural study. Br J Psychiatry Suppl. 2004;46:s31-s37.
7. National Institute of Mental Health. Women and depression: discovering hope. http://www.nimh.nih.gov/health/publications/women-and-depression-discovering-hope/complete-index.shtml. Accessed December 27 2011.
8. Kumar R, Robson KM. A prospective study of emotional disorders in childbearing women. Br J Psychiatry. 1984;144:35-47.
9. O’Mahen HA, Flynn HA. P and perceived barriers to treatment for depression during the perinatal period. J Womens Health (Larchmt). 2008;17(8):1301-1309.
10. Flynn HA, O’Mahen HA, Massey L, et al. The impact of a brief obstetrics clinic based intervention on treatment use for perinatal depression. J Womens Health (Larchmt). 2006;15(10):1195-1204.
11. Marcus SM, Flynn HA, Blow FC, et al. Depressive symptoms among pregnant women screened in obstetrics settings. J Womens Health (Larchmt). 2003;12(4):373-380.
12. Olfson M, Marcus SC. National trends in outpatient psychotherapy. Am J Psychiatry. 2010;167(12):1456-1463.
13. Schiff MA, Grossman DC. Adverse perinatal outcomes and risk for postpartum suicide attempt in Washington state 1987-2001. Pediatrics. 2006;118(3):669-675.
14. Kurki T, Hiilesmaa V, Raitasalo R, et al. Depression and anxiety in early pregnancy and risk for preeclampsia. Obstet Gynecol. 2000;95:(4)487-490.
15. McKee MD, Cunningham M, Jankowski KR, et al. Health-related functional status in pregnancy: relationship to depression and social support in a multiethnic population. Obstet Gynecol. 2001;97(6):988-993.
16. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
17. Matthey S, Henshaw C, Elliott S, et al. Variability in use of cut-off scores and formats on the Edinburgh Postnatal Depression Scale: implications for clinical and research practice. Arch Womens Ment Health. 2006;9(6):309-315.
18. Chaudron LH, Szilagyi PG, Tang W, et al. Accuracy of depression screening tools for identifying postpartum depression among urban mothers. Pediatrics. 2010;125(3):609-617.
19. Ross AS, Hall RW, Frost K, et al. Antenatal and neonatal guidelines, education and learning system. J Ark Med Soc. 2006;102(12):328-330.
20. Muzik M, Klier CK, Rosenblum KL, et al. Are commonly used self-report inventories suitable for screening postpartum depression and anxiety disorders? Acta Psychiatr Scand. 2000;102(1):71-73.
21. Muzik M, Thelen K, Rosenblum KL. Perinatal depression: detection and treatment. Neuropsychiatry. 2011;1(2):179-195.
22. Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403-413.
23. ACOG Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin. Clinical management guidelines for obstetrician-gynecologists number 92. Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111(4):1001-1020.
24. Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499-507.
25. Gonsalves L, Schuermeyer I. Treating depression in pregnancy: practical suggestions. Cleve Clin J Med. 2006;73(12):1098-1104.
26. Misri S, Kendrick K. Treatment of perinatal mood and anxiety disorders: a review. Can J Psychiatry. 2007;52(8):489-498.
27. Wisner KL, Sit DY, Hanusa BH, et al. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry. 2009;166(5):557-566.
28. Anderson EL, Reti IM. ECT in pregnancy: a review of the literature from 1941 to 2007. Psychosom Med. 2009;71(2):235-242.
29. Miller LJ. Use of electroconvulsive therapy during pregnancy. Hosp Community Psychiatry. 1994;45(5):444-450.
30. O’Hara MW, Stuart S, Gorman LL, et al. Efficacy of interpersonal psychotherapy for postpartum depression. Arch Gen Psychiatry. 2000;57(11):1039-1045.
31. Bhatia SC, Bhatia SK. Depression in women: diagnostic and treatment considerations. Am Fam Physician. 1999;60(1):225-240.
32. Battle CL, Uebelacker LA, Howard M, et al. Prenatal yoga and depression during pregnancy. Birth. 2010;37(4):353-354.
33. Muzik M, Hamilton SE, Waxler EG, et al. Mindfulness yoga during pregnancy for women with depression and PTSD: preliminary results from a pilot feasibility study. Paper presented at: 36th Annual Meeting of the Association for Women in Psychology; March 5, 2011; Philadelphia, PA.
34. Deligiannidis KM, Freeman MP. Complementary and alternative medicine for the treatment of depressive disorders in women. Psychiatr Clin North Am. 2010;33(2):441-463.
35. Shivakumar G, Brandon AR, Snell PG, et al. Antenatal depression: a rationale for studying exercise. Depress Anxiety. 2011;28(3):234-242.
Perinatal psychopathology is a common and undertreated problem with wide-ranging consequences for both mother and child.1-4 Women at risk for psychopathology are more likely to engage in unhealthy behaviors such as smoking and substance abuse and have difficulty engaging in treatment and attending psychiatric and obstetrics appointments.5 In addition, many of these women have trouble attaching to and caring for their infants and struggle with everyday stressors during pregnancy and postpartum.6
Routine prenatal screening for mental illness coupled with non-judgmental, collaborative, and individualized care delivered by a multidisciplinary team is critical for treatment engagement and adherence. Providers should be aware of risk factors for perinatal psychiatric illness—including a history of mental illness, stressful life events, and interpersonal conflict—and should be versed in current treatment guidelines.
CASE REPORT: Difficulty coping
Ms. A, age 28, is referred to our High Risk Perinatal Team by her obstetrician when she is approximately 6 weeks pregnant. She is single, has 3 other children (age 10, 4, and 2), a history of depression, and chronic pain related to an auto accident 3 years ago. She reports that this pregnancy likely is the result of a sexual assault, but she has decided to keep the baby. Ms. A describes severe depressive symptoms, including insomnia, low appetite, feelings of worthlessness, and thoughts of harming herself. In addition, she has incapacitating panic attacks and constantly worries about her children’s safety when she is not with them. She schedules an appointment with the perinatal team, but does not show up twice.
When our team finally sees Ms. A, she is well into her second trimester and brings her 2 youngest children with her. She says she recently was fired from her job as a cashier because she missed too many days of work, and is applying for Medicaid. Recently, her back and shoulder pain have worsened, and she is running out of her prescription for acetaminophen/hydrocodone. Ms. A’s affect is flat, her mood depressed, and she has difficulty explaining her history because her 2-year-old son interrupts the interview. She has never been in psychotherapy, and is reluctant to take antidepressants. Despite a difficult first visit, she engages with the clinician and agrees to schedule a second appointment.
What complicates pregnancy?
Women are at higher risk for developing depression during puberty, the perinatal period (ie, pregnancy and first year postpartum), and perimenopause.7 These times often are fraught with unfamiliar hormonal fluctuations, role transitions, emotional upheaval, and physical changes. However, because these times are expected to be stressful, serious mood changes often go unnoticed by patients and untreated by clinicians.8 Women are expected to celebrate, thrive, and “glow” during pregnancy, and those who suffer from depression and anxiety frequently do so in silence. Social stigma surrounding perinatal depression or anxiety leads many women to believe they are alone in their struggle and hesitant to seek help.9
Most pregnant women who develop psychiatric illness do not present for treatment.10 One study found that 86% of pregnant women who screened positive for depression in an obstetrics (OB) setting did not receive treatment.11 Some women are reluctant to take antidepressants out of concern for their infant’s safety,8 and psychotherapy or alternative approaches are not available in all areas.12 Transportation, childcare issues, or ongoing life stressors may prevent women from seeking help (Box 1).9
Diagnostic uncertainty among professionals may aggravate undertreatment. Clinicians who are unfamiliar with the presentation of perinatal mental illness may mislabel depressive features—such as irritability, loss of interest in activities, low energy, increased anxiety, difficulty sleeping, or appetite dysregulation—as normative experiences during pregnancy or adjustment after childbirth. Concerned about fetal exposure to potentially teratogenic compounds, clinicians may under-dose otherwise effective medications, which can lead to treatment resistance. Even if treated aggressively, depression in pregnancy may persist because of other factors, such as comorbid anxiety, somatization, pain, substance use/dependence, undiagnosed bipolar illness, or the presence of severe psychosocial stress or trauma.
Maternal suicide and/or harm to the infant—the most severe result of untreated perinatal psychopathology—is rare.13 Common negative outcomes of untreated depression or anxiety in pregnant women include inadequate weight gain, preeclampsia, difficulty bonding with their unborn baby, premature labor, and lack of follow through with prenatal care.14,15 Symptoms become harder to treat when aggravated by psychosocial stressors such as poor social support, ambivalence about the pregnancy, and/or substance abuse.
The key to successful intervention is finding a balance between managing psychiatric concerns, facilitating adequate coping with psychosocial stressors, and, if necessary, aggressively treating pregnancy-related physical illnesses. Successful treatment response depends on early detection and initiating individualized care as soon as possible.
Lack of insurance, childcare, or transportation can make it difficult for a pregnant woman to receive psychiatric treatment. All pregnant women are eligible for Medicaid if private insurance is unavailable to them, and clinicians can help patients apply for assistance. Some programs—for example, Michigan’s state-funded Maternal Infant Health Programs—offer help with transportation to appointments, such as cabs and reimbursement for gas, in addition to nutrition guidance, counseling, home visits, and referrals to community resources such as childbirth classes, infant mental health specialists, and/or substance abuse treatment (see Related Resources ).
Offering childcare during psychotherapy sessions can be particularly helpful, and may provide valuable experience for a student or resident interested in working with at-risk children. Women may be more likely to engage in care if psychotherapy sessions are conducted by phone or in their homes. A positive experience with mental health care during pregnancy may increase the likelihood that women will remain engaged in treatment after childbirth, therefore lessening the negative effects of perinatal psychopathology on mother and child.
Early detection. Women’s health care providers play a fundamental role in guiding decision-making about mental health care, providing referrals, and most important, allowing women to talk about perinatal psychopathology without fear of stigma.
When a woman becomes pregnant, it is critical to determine if she is at risk for developing psychopathology or presents with active illness. Many OB clinics screen for depression several times during pregnancy and early postpartum. The most commonly used screening tool is the Edinburgh Postpartum Depression Scale (EPDS),16 a 10-item self-report measure that is sensitive to cognitive and affective symptoms of depression. If a woman scores >15 during pregnancy or >13 postpartum, further assessment is indicated.17 The anxiety subscale (items 5 and 6) of the EPDS has been validated for screening perinatal anxiety using a cut-off score >4.18 Depression can be quickly assessed using the 2-question Patient Health Questionnaire (PHQ-2) or the 9-question PHQ-9.19,20 All 3 scales are free and available on the Internet (Table 1).21
These screening tools offer clinicians an opportunity to assess for risk factors that may increase the likelihood of illness onset or worsened prognosis (Table 2).5,22 All women who present with pregnancy-related medical illness, such as preeclampsia or gestational diabetes, should be screened for co-occurring depression or anxiety because psychiatric comorbidity is common.
Individualized care. Have an open mind about the kind of care to offer and collaborate with the patient when discussing treatment options.5 Some pregnant women may reject traditional treatments, such as pharmacotherapy or psychotherapy, because of concern about harm to the unborn baby or reluctance to work through past or present conflicts in therapy during a vulnerable time.9 Women may assume that medication will be the only treatment offered, or even fear that they will be forced to take antidepressants. Women often do not follow through on mental health referrals, even when they are appropriately screened and identified to be at risk, and an OB nurse explains the risks of untreated psychopathology.11
A multidisciplinary, collaborative care model is vital for positive pregnancy outcomes. Connecting obstetricians and midwives with psychologists, psychiatrists, social workers, and infant mental health specialists to coordinate treatment ensures that at-risk pregnant and postpartum women get the care they need. A nonjudgmental approach is essential to engage pregnant women in care. Assure women that pharmacotherapy is not required when receiving mental health treatment, but is an option they can choose.
Table 1
Screening for psychiatric illness during pregnancy
| Screening tool | Sensitivity/specificity | Administration | Availability |
|---|---|---|---|
| Edinburgh Postpartum Depression Scale | Sensitivity = 0.86 Specificity = 0.78 Positive screen: >10 | Self-administered in 5 to 10 minutes. Could be self-scored | http://bit.ly/PPDscale |
| Patient Health Questionnaire-2 (PHQ-2) | Sensitivity = 0.83 Specificity = 0.92 Positive screen: >3 | Self- or clinician-administered in <1 minute | www.phqscreeners.com The 2 questions from the PHQ-9 for mood and anhedonia are used |
| Patient Health Questionnaire-9 (PHQ-9) | Sensitivity = 0.88 Specificity = 0.88 Positive screen: >10 | Self-administered and self-scored, 5 to 10 minutes | www.phqscreeners.com |
| Source: Reference 21 | |||
Treatment choices
Pharmacotherapy. If a woman has only mild symptoms or has been symptom-free for ≥6 months, it may be safe to decrease or discontinue antidepressants during pregnancy or while trying to conceive, but such patients should be monitored closely for signs of relapse.23 In a study of 201 depressed pregnant women, 68% of those who discontinued medication experienced symptom relapse compared with 26% of those who continued medication.24 If a depressed woman has a history of relapse or severe symptoms, including suicide attempts and inpatient psychiatric admissions, it is recommended that she remain on antidepressants or mood stabilizers, regardless of pregnancy status.25 If medications are necessary during pregnancy— ie, the benefits to the mother outweigh the risks to the unborn baby—the following precautions could help decrease fetal exposure:23
- keep the medication regimen simple and at the lowest effective dose
- use monotherapy when appropriate
- if possible, do not change medications during pregnancy.
When considering pharmacotherapy, evaluate each woman’s risk for disease exacerbation and consequences for pregnancy and neonatal outcomes, and ask the woman how she views reproductive risk vs disease benefit.
Developing fetuses are exposed to either the effects of the mother’s untreated mental illness or the medication.26 A recent study comparing birth and neonatal outcomes among women with untreated depression vs those taking selective serotonin reuptake inhibitors (SSRIs) found similar adverse outcomes.27 Babies continously exposed to either prenatal depression or SSRIs were more likely to be born prematurely, but partial exposure to either condition did not increase this risk.27 In addition, women who were not taking SSRIs had more depressive symptoms and more trouble functioning, which can interfere with bonding between mother and baby, both in-utero and postpartum.6,27 Neither SSRIs nor depression exposure increased risk for minor physical anomalies.27
A careful process of informed consent and documentation is essential when prescribing medications during pregnancy. Women should understand the risks of pharmacotherapy as well as the risks of undertreated illness.
Electroconvulsive therapy can safely help pregnant women with treatment-resistant, life-threatening, or psychotic depression.28,29
Table 2
Risk factors for perinatal psychopathology
| Pregnancy during adolescence |
| Previous diagnosis of depression, anxiety, psychosis, or bipolar disorder |
| Trauma history, including physical, emotional, or sexual abuse |
| Current or past substance abuse/dependence, including cigarette smoking |
| Lack of social support |
| Single parenthood |
| Low socioeconomic status |
| History of sexual assault or domestic violence |
| Unstable home environment |
| Stopping antidepressants during pregnancy |
| Financial problems |
| Ambivalence about pregnancy |
| Source: References 5,22 |
Psychotherapy. The American College of Obstetricians and Gynecologists treatment guidelines22 favor psychotherapy over medication for women with mild depressive symptoms and no loss of function, suicidality, or psychotic experiences; pharmacotherapy is suggested for women who have moderate to severely impaired functioning, recurrent depressive symptoms, or suicidal thinking (Table 3).22
Interpersonal psychotherapy or cognitive-behavioral therapy can be safe and effective during pregnancy.30,31 Other psychotherapeutic modalities and alternative/complementary treatments offer potential benefit without substantial risk, and could help prevent relapse when discontinuing mood stabilizers or antidepressants after conception (Box 2).32-35
Table 3
ACOG guidelines for treating depression during pregnancy
| Women who are thinking about getting pregnant |
| For women on medication with mild or no symptoms for ≥6 months, it may be appropriate to taper and discontinue medication before becoming pregnant |
| Medication discontinuation may not be appropriate in women with a history of severe, recurrent depression or who have psychosis, bipolar disorder, other psychiatric illness requiring medication, or a history of suicide attempts |
| Pregnant women currently taking medication for depression |
| Psychiatrically stable women who prefer to stay on medication may be able to do so after consultation between their psychiatrist and obstetrician to discuss risks and benefits |
| Women who want to discontinue medication may attempt to taper and discontinue if they are not experiencing symptoms, depending on their psychiatric history. Women with a history of recurrent depression are at a high risk of relapse if medication is discontinued |
| Women with recurrent depression or who have symptoms despite medication may benefit from psychotherapy to replace or augment medication |
| Women with severe depression (with suicide attempts, functional incapacitation, or weight loss) should remain on medication. If a patient refuses medication, alternative treatment and monitoring should be in place, preferably before discontinuation |
| Pregnant and not currently on medication for depression |
| Psychotherapy may be beneficial for women who prefer to avoid antidepressants |
| For women who want to take medication, risks and benefits of treatment choices should be evaluated and discussed, including factors such as stage of gestation, symptoms, history of depression, and other conditions and circumstances (eg, smoking, difficulty gaining weight) |
| All pregnant women |
| Regardless of circumstances, a woman with suicidal or psychotic symptoms should immediately see a psychiatrist |
| ACOG: American College of Obstetricians and Gynecologists Source: Reference 22 |
Mind-body approaches such as mindfulness-based stress reduction, yoga, and progressive relaxation and supplements such as fish oil may be good adjuncts to psychotherapy. Many pregnant women prefer mindfulness yoga to other mind-body techniques.32 A pilot study found that mindfulness yoga significantly decreased depressive symptoms and increased maternal-fetal attachment, particularly in mildly depressed women.33 For women who do not wish to engage in traditional treatments, alternative approaches such as progressive relaxation are easily taught and can help reduce depressive symptoms.34 Regular exercise may improve self-esteem and reduce symptoms of depression and anxiety in pregnant women.35
CASE CONTINUED: Healthy baby boy
Ms. A either doesn’t show up or cancels her weekly appointments about once a month, but seems to be making progress. Her therapist makes accommodations for Ms. A, such as offering childcare in an adjacent room during sessions, conducting brief sessions by phone when Ms. A is unable to come to the clinic, and helping her enroll in the state’s Maternal Infant Health Program. Ms. A’s therapist has referred her to a specialized OB clinic that can manage her pain medication and monitor for signs of abuse and keeps in regular contact with her obstetrician.
At 26 weeks gestation, Ms. A still is reluctant to try psychotropics, so her therapist works with her to integrate psychotherapy with alternative approaches such as mindfulness meditation and yoga. During therapy, Ms. A learns ways to manage her depressive symptoms, improve her social functioning, adjust to role transitions, and work through her traumatic experiences. Ms. A enrolls in a prenatal yoga class with a mindfulness focus, which allows her to interact with other pregnant women at risk for psychopathology and learn new ways to cope with her depressed mood and chronic pain.
Ms. A delivers a healthy boy at 38 weeks gestation. During labor, she uses many of the yoga poses she learned to manage pain, but elects to have an epidural after 30 hours of labor. Her baby tests positive for hydrocodone, which can cause ongoing mild irritability and occasional jitteriness. He is observed in the hospital for signs of withdrawal for 48 hours and then discharged home with his mother. Ms. A starts breast-feeding in the hospital and plans to continue at home.
Ms. A’s therapist continues to stay in touch with her by phone until she schedules another appointment and assists with referrals to other community resources.
- University of Michigan Department of Psychiatry Depression Center Women’s Mental Health and Infants Program. Women and depression. www.psych.med.umich.edu/wimhc.
- Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403-413.
- Michigan Families Medicaid Project. Year 1 final report. Includes a list of maternal and child health services similar to Michigan’s Maternal Infant Health Program, for all 50 states. http://1.usa.gov/yCzdca.
Drug Brand Name
- Acetaminophen/hydrocodone • Vicodin
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Perinatal psychopathology is a common and undertreated problem with wide-ranging consequences for both mother and child.1-4 Women at risk for psychopathology are more likely to engage in unhealthy behaviors such as smoking and substance abuse and have difficulty engaging in treatment and attending psychiatric and obstetrics appointments.5 In addition, many of these women have trouble attaching to and caring for their infants and struggle with everyday stressors during pregnancy and postpartum.6
Routine prenatal screening for mental illness coupled with non-judgmental, collaborative, and individualized care delivered by a multidisciplinary team is critical for treatment engagement and adherence. Providers should be aware of risk factors for perinatal psychiatric illness—including a history of mental illness, stressful life events, and interpersonal conflict—and should be versed in current treatment guidelines.
CASE REPORT: Difficulty coping
Ms. A, age 28, is referred to our High Risk Perinatal Team by her obstetrician when she is approximately 6 weeks pregnant. She is single, has 3 other children (age 10, 4, and 2), a history of depression, and chronic pain related to an auto accident 3 years ago. She reports that this pregnancy likely is the result of a sexual assault, but she has decided to keep the baby. Ms. A describes severe depressive symptoms, including insomnia, low appetite, feelings of worthlessness, and thoughts of harming herself. In addition, she has incapacitating panic attacks and constantly worries about her children’s safety when she is not with them. She schedules an appointment with the perinatal team, but does not show up twice.
When our team finally sees Ms. A, she is well into her second trimester and brings her 2 youngest children with her. She says she recently was fired from her job as a cashier because she missed too many days of work, and is applying for Medicaid. Recently, her back and shoulder pain have worsened, and she is running out of her prescription for acetaminophen/hydrocodone. Ms. A’s affect is flat, her mood depressed, and she has difficulty explaining her history because her 2-year-old son interrupts the interview. She has never been in psychotherapy, and is reluctant to take antidepressants. Despite a difficult first visit, she engages with the clinician and agrees to schedule a second appointment.
What complicates pregnancy?
Women are at higher risk for developing depression during puberty, the perinatal period (ie, pregnancy and first year postpartum), and perimenopause.7 These times often are fraught with unfamiliar hormonal fluctuations, role transitions, emotional upheaval, and physical changes. However, because these times are expected to be stressful, serious mood changes often go unnoticed by patients and untreated by clinicians.8 Women are expected to celebrate, thrive, and “glow” during pregnancy, and those who suffer from depression and anxiety frequently do so in silence. Social stigma surrounding perinatal depression or anxiety leads many women to believe they are alone in their struggle and hesitant to seek help.9
Most pregnant women who develop psychiatric illness do not present for treatment.10 One study found that 86% of pregnant women who screened positive for depression in an obstetrics (OB) setting did not receive treatment.11 Some women are reluctant to take antidepressants out of concern for their infant’s safety,8 and psychotherapy or alternative approaches are not available in all areas.12 Transportation, childcare issues, or ongoing life stressors may prevent women from seeking help (Box 1).9
Diagnostic uncertainty among professionals may aggravate undertreatment. Clinicians who are unfamiliar with the presentation of perinatal mental illness may mislabel depressive features—such as irritability, loss of interest in activities, low energy, increased anxiety, difficulty sleeping, or appetite dysregulation—as normative experiences during pregnancy or adjustment after childbirth. Concerned about fetal exposure to potentially teratogenic compounds, clinicians may under-dose otherwise effective medications, which can lead to treatment resistance. Even if treated aggressively, depression in pregnancy may persist because of other factors, such as comorbid anxiety, somatization, pain, substance use/dependence, undiagnosed bipolar illness, or the presence of severe psychosocial stress or trauma.
Maternal suicide and/or harm to the infant—the most severe result of untreated perinatal psychopathology—is rare.13 Common negative outcomes of untreated depression or anxiety in pregnant women include inadequate weight gain, preeclampsia, difficulty bonding with their unborn baby, premature labor, and lack of follow through with prenatal care.14,15 Symptoms become harder to treat when aggravated by psychosocial stressors such as poor social support, ambivalence about the pregnancy, and/or substance abuse.
The key to successful intervention is finding a balance between managing psychiatric concerns, facilitating adequate coping with psychosocial stressors, and, if necessary, aggressively treating pregnancy-related physical illnesses. Successful treatment response depends on early detection and initiating individualized care as soon as possible.
Lack of insurance, childcare, or transportation can make it difficult for a pregnant woman to receive psychiatric treatment. All pregnant women are eligible for Medicaid if private insurance is unavailable to them, and clinicians can help patients apply for assistance. Some programs—for example, Michigan’s state-funded Maternal Infant Health Programs—offer help with transportation to appointments, such as cabs and reimbursement for gas, in addition to nutrition guidance, counseling, home visits, and referrals to community resources such as childbirth classes, infant mental health specialists, and/or substance abuse treatment (see Related Resources ).
Offering childcare during psychotherapy sessions can be particularly helpful, and may provide valuable experience for a student or resident interested in working with at-risk children. Women may be more likely to engage in care if psychotherapy sessions are conducted by phone or in their homes. A positive experience with mental health care during pregnancy may increase the likelihood that women will remain engaged in treatment after childbirth, therefore lessening the negative effects of perinatal psychopathology on mother and child.
Early detection. Women’s health care providers play a fundamental role in guiding decision-making about mental health care, providing referrals, and most important, allowing women to talk about perinatal psychopathology without fear of stigma.
When a woman becomes pregnant, it is critical to determine if she is at risk for developing psychopathology or presents with active illness. Many OB clinics screen for depression several times during pregnancy and early postpartum. The most commonly used screening tool is the Edinburgh Postpartum Depression Scale (EPDS),16 a 10-item self-report measure that is sensitive to cognitive and affective symptoms of depression. If a woman scores >15 during pregnancy or >13 postpartum, further assessment is indicated.17 The anxiety subscale (items 5 and 6) of the EPDS has been validated for screening perinatal anxiety using a cut-off score >4.18 Depression can be quickly assessed using the 2-question Patient Health Questionnaire (PHQ-2) or the 9-question PHQ-9.19,20 All 3 scales are free and available on the Internet (Table 1).21
These screening tools offer clinicians an opportunity to assess for risk factors that may increase the likelihood of illness onset or worsened prognosis (Table 2).5,22 All women who present with pregnancy-related medical illness, such as preeclampsia or gestational diabetes, should be screened for co-occurring depression or anxiety because psychiatric comorbidity is common.
Individualized care. Have an open mind about the kind of care to offer and collaborate with the patient when discussing treatment options.5 Some pregnant women may reject traditional treatments, such as pharmacotherapy or psychotherapy, because of concern about harm to the unborn baby or reluctance to work through past or present conflicts in therapy during a vulnerable time.9 Women may assume that medication will be the only treatment offered, or even fear that they will be forced to take antidepressants. Women often do not follow through on mental health referrals, even when they are appropriately screened and identified to be at risk, and an OB nurse explains the risks of untreated psychopathology.11
A multidisciplinary, collaborative care model is vital for positive pregnancy outcomes. Connecting obstetricians and midwives with psychologists, psychiatrists, social workers, and infant mental health specialists to coordinate treatment ensures that at-risk pregnant and postpartum women get the care they need. A nonjudgmental approach is essential to engage pregnant women in care. Assure women that pharmacotherapy is not required when receiving mental health treatment, but is an option they can choose.
Table 1
Screening for psychiatric illness during pregnancy
| Screening tool | Sensitivity/specificity | Administration | Availability |
|---|---|---|---|
| Edinburgh Postpartum Depression Scale | Sensitivity = 0.86 Specificity = 0.78 Positive screen: >10 | Self-administered in 5 to 10 minutes. Could be self-scored | http://bit.ly/PPDscale |
| Patient Health Questionnaire-2 (PHQ-2) | Sensitivity = 0.83 Specificity = 0.92 Positive screen: >3 | Self- or clinician-administered in <1 minute | www.phqscreeners.com The 2 questions from the PHQ-9 for mood and anhedonia are used |
| Patient Health Questionnaire-9 (PHQ-9) | Sensitivity = 0.88 Specificity = 0.88 Positive screen: >10 | Self-administered and self-scored, 5 to 10 minutes | www.phqscreeners.com |
| Source: Reference 21 | |||
Treatment choices
Pharmacotherapy. If a woman has only mild symptoms or has been symptom-free for ≥6 months, it may be safe to decrease or discontinue antidepressants during pregnancy or while trying to conceive, but such patients should be monitored closely for signs of relapse.23 In a study of 201 depressed pregnant women, 68% of those who discontinued medication experienced symptom relapse compared with 26% of those who continued medication.24 If a depressed woman has a history of relapse or severe symptoms, including suicide attempts and inpatient psychiatric admissions, it is recommended that she remain on antidepressants or mood stabilizers, regardless of pregnancy status.25 If medications are necessary during pregnancy— ie, the benefits to the mother outweigh the risks to the unborn baby—the following precautions could help decrease fetal exposure:23
- keep the medication regimen simple and at the lowest effective dose
- use monotherapy when appropriate
- if possible, do not change medications during pregnancy.
When considering pharmacotherapy, evaluate each woman’s risk for disease exacerbation and consequences for pregnancy and neonatal outcomes, and ask the woman how she views reproductive risk vs disease benefit.
Developing fetuses are exposed to either the effects of the mother’s untreated mental illness or the medication.26 A recent study comparing birth and neonatal outcomes among women with untreated depression vs those taking selective serotonin reuptake inhibitors (SSRIs) found similar adverse outcomes.27 Babies continously exposed to either prenatal depression or SSRIs were more likely to be born prematurely, but partial exposure to either condition did not increase this risk.27 In addition, women who were not taking SSRIs had more depressive symptoms and more trouble functioning, which can interfere with bonding between mother and baby, both in-utero and postpartum.6,27 Neither SSRIs nor depression exposure increased risk for minor physical anomalies.27
A careful process of informed consent and documentation is essential when prescribing medications during pregnancy. Women should understand the risks of pharmacotherapy as well as the risks of undertreated illness.
Electroconvulsive therapy can safely help pregnant women with treatment-resistant, life-threatening, or psychotic depression.28,29
Table 2
Risk factors for perinatal psychopathology
| Pregnancy during adolescence |
| Previous diagnosis of depression, anxiety, psychosis, or bipolar disorder |
| Trauma history, including physical, emotional, or sexual abuse |
| Current or past substance abuse/dependence, including cigarette smoking |
| Lack of social support |
| Single parenthood |
| Low socioeconomic status |
| History of sexual assault or domestic violence |
| Unstable home environment |
| Stopping antidepressants during pregnancy |
| Financial problems |
| Ambivalence about pregnancy |
| Source: References 5,22 |
Psychotherapy. The American College of Obstetricians and Gynecologists treatment guidelines22 favor psychotherapy over medication for women with mild depressive symptoms and no loss of function, suicidality, or psychotic experiences; pharmacotherapy is suggested for women who have moderate to severely impaired functioning, recurrent depressive symptoms, or suicidal thinking (Table 3).22
Interpersonal psychotherapy or cognitive-behavioral therapy can be safe and effective during pregnancy.30,31 Other psychotherapeutic modalities and alternative/complementary treatments offer potential benefit without substantial risk, and could help prevent relapse when discontinuing mood stabilizers or antidepressants after conception (Box 2).32-35
Table 3
ACOG guidelines for treating depression during pregnancy
| Women who are thinking about getting pregnant |
| For women on medication with mild or no symptoms for ≥6 months, it may be appropriate to taper and discontinue medication before becoming pregnant |
| Medication discontinuation may not be appropriate in women with a history of severe, recurrent depression or who have psychosis, bipolar disorder, other psychiatric illness requiring medication, or a history of suicide attempts |
| Pregnant women currently taking medication for depression |
| Psychiatrically stable women who prefer to stay on medication may be able to do so after consultation between their psychiatrist and obstetrician to discuss risks and benefits |
| Women who want to discontinue medication may attempt to taper and discontinue if they are not experiencing symptoms, depending on their psychiatric history. Women with a history of recurrent depression are at a high risk of relapse if medication is discontinued |
| Women with recurrent depression or who have symptoms despite medication may benefit from psychotherapy to replace or augment medication |
| Women with severe depression (with suicide attempts, functional incapacitation, or weight loss) should remain on medication. If a patient refuses medication, alternative treatment and monitoring should be in place, preferably before discontinuation |
| Pregnant and not currently on medication for depression |
| Psychotherapy may be beneficial for women who prefer to avoid antidepressants |
| For women who want to take medication, risks and benefits of treatment choices should be evaluated and discussed, including factors such as stage of gestation, symptoms, history of depression, and other conditions and circumstances (eg, smoking, difficulty gaining weight) |
| All pregnant women |
| Regardless of circumstances, a woman with suicidal or psychotic symptoms should immediately see a psychiatrist |
| ACOG: American College of Obstetricians and Gynecologists Source: Reference 22 |
Mind-body approaches such as mindfulness-based stress reduction, yoga, and progressive relaxation and supplements such as fish oil may be good adjuncts to psychotherapy. Many pregnant women prefer mindfulness yoga to other mind-body techniques.32 A pilot study found that mindfulness yoga significantly decreased depressive symptoms and increased maternal-fetal attachment, particularly in mildly depressed women.33 For women who do not wish to engage in traditional treatments, alternative approaches such as progressive relaxation are easily taught and can help reduce depressive symptoms.34 Regular exercise may improve self-esteem and reduce symptoms of depression and anxiety in pregnant women.35
CASE CONTINUED: Healthy baby boy
Ms. A either doesn’t show up or cancels her weekly appointments about once a month, but seems to be making progress. Her therapist makes accommodations for Ms. A, such as offering childcare in an adjacent room during sessions, conducting brief sessions by phone when Ms. A is unable to come to the clinic, and helping her enroll in the state’s Maternal Infant Health Program. Ms. A’s therapist has referred her to a specialized OB clinic that can manage her pain medication and monitor for signs of abuse and keeps in regular contact with her obstetrician.
At 26 weeks gestation, Ms. A still is reluctant to try psychotropics, so her therapist works with her to integrate psychotherapy with alternative approaches such as mindfulness meditation and yoga. During therapy, Ms. A learns ways to manage her depressive symptoms, improve her social functioning, adjust to role transitions, and work through her traumatic experiences. Ms. A enrolls in a prenatal yoga class with a mindfulness focus, which allows her to interact with other pregnant women at risk for psychopathology and learn new ways to cope with her depressed mood and chronic pain.
Ms. A delivers a healthy boy at 38 weeks gestation. During labor, she uses many of the yoga poses she learned to manage pain, but elects to have an epidural after 30 hours of labor. Her baby tests positive for hydrocodone, which can cause ongoing mild irritability and occasional jitteriness. He is observed in the hospital for signs of withdrawal for 48 hours and then discharged home with his mother. Ms. A starts breast-feeding in the hospital and plans to continue at home.
Ms. A’s therapist continues to stay in touch with her by phone until she schedules another appointment and assists with referrals to other community resources.
- University of Michigan Department of Psychiatry Depression Center Women’s Mental Health and Infants Program. Women and depression. www.psych.med.umich.edu/wimhc.
- Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403-413.
- Michigan Families Medicaid Project. Year 1 final report. Includes a list of maternal and child health services similar to Michigan’s Maternal Infant Health Program, for all 50 states. http://1.usa.gov/yCzdca.
Drug Brand Name
- Acetaminophen/hydrocodone • Vicodin
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Steer RA, Scholl TO, Hediger ML, et al. Self-reported depression and negative pregnancy outcomes. J Clin Epidemiol. 1992;45(10):1093-1099.
2. Zuckerman B, Bauchner H, Parker S, et al. Maternal depressive symptoms during pregnancy, and newborn irritability. J Dev Behav Pediatr. 1990;11(4):190-194.
3. Field T, Healy B, Goldstein S, et al. Infants of depressed mothers show depressed behavior even with non-depressed adults. Child Dev. 1988;59(6):1569-1579.
4. Coghill SR, Caplan HL, Alexandra H, et al. Impact of maternal postnatal depression on cognitive development of young children. Br Med J (Clin Res Ed). 1986;292(6529):1165-1167.
5. Muzik M, Marcus S, Heringhausen J, et al. When depression complicates childbearing: guidelines for screening and treatment during antenatal and postpartum obstetric care. Obstet Gynecol Clin North Am. 2009;36(4):771-788, ix–x.
6. Bifulco A, Figueiredo B, Guedeney N, et al. Maternal attachment style and depression associated with childbirth: preliminary results from a European and US cross-cultural study. Br J Psychiatry Suppl. 2004;46:s31-s37.
7. National Institute of Mental Health. Women and depression: discovering hope. http://www.nimh.nih.gov/health/publications/women-and-depression-discovering-hope/complete-index.shtml. Accessed December 27 2011.
8. Kumar R, Robson KM. A prospective study of emotional disorders in childbearing women. Br J Psychiatry. 1984;144:35-47.
9. O’Mahen HA, Flynn HA. P and perceived barriers to treatment for depression during the perinatal period. J Womens Health (Larchmt). 2008;17(8):1301-1309.
10. Flynn HA, O’Mahen HA, Massey L, et al. The impact of a brief obstetrics clinic based intervention on treatment use for perinatal depression. J Womens Health (Larchmt). 2006;15(10):1195-1204.
11. Marcus SM, Flynn HA, Blow FC, et al. Depressive symptoms among pregnant women screened in obstetrics settings. J Womens Health (Larchmt). 2003;12(4):373-380.
12. Olfson M, Marcus SC. National trends in outpatient psychotherapy. Am J Psychiatry. 2010;167(12):1456-1463.
13. Schiff MA, Grossman DC. Adverse perinatal outcomes and risk for postpartum suicide attempt in Washington state 1987-2001. Pediatrics. 2006;118(3):669-675.
14. Kurki T, Hiilesmaa V, Raitasalo R, et al. Depression and anxiety in early pregnancy and risk for preeclampsia. Obstet Gynecol. 2000;95:(4)487-490.
15. McKee MD, Cunningham M, Jankowski KR, et al. Health-related functional status in pregnancy: relationship to depression and social support in a multiethnic population. Obstet Gynecol. 2001;97(6):988-993.
16. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
17. Matthey S, Henshaw C, Elliott S, et al. Variability in use of cut-off scores and formats on the Edinburgh Postnatal Depression Scale: implications for clinical and research practice. Arch Womens Ment Health. 2006;9(6):309-315.
18. Chaudron LH, Szilagyi PG, Tang W, et al. Accuracy of depression screening tools for identifying postpartum depression among urban mothers. Pediatrics. 2010;125(3):609-617.
19. Ross AS, Hall RW, Frost K, et al. Antenatal and neonatal guidelines, education and learning system. J Ark Med Soc. 2006;102(12):328-330.
20. Muzik M, Klier CK, Rosenblum KL, et al. Are commonly used self-report inventories suitable for screening postpartum depression and anxiety disorders? Acta Psychiatr Scand. 2000;102(1):71-73.
21. Muzik M, Thelen K, Rosenblum KL. Perinatal depression: detection and treatment. Neuropsychiatry. 2011;1(2):179-195.
22. Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403-413.
23. ACOG Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin. Clinical management guidelines for obstetrician-gynecologists number 92. Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111(4):1001-1020.
24. Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499-507.
25. Gonsalves L, Schuermeyer I. Treating depression in pregnancy: practical suggestions. Cleve Clin J Med. 2006;73(12):1098-1104.
26. Misri S, Kendrick K. Treatment of perinatal mood and anxiety disorders: a review. Can J Psychiatry. 2007;52(8):489-498.
27. Wisner KL, Sit DY, Hanusa BH, et al. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry. 2009;166(5):557-566.
28. Anderson EL, Reti IM. ECT in pregnancy: a review of the literature from 1941 to 2007. Psychosom Med. 2009;71(2):235-242.
29. Miller LJ. Use of electroconvulsive therapy during pregnancy. Hosp Community Psychiatry. 1994;45(5):444-450.
30. O’Hara MW, Stuart S, Gorman LL, et al. Efficacy of interpersonal psychotherapy for postpartum depression. Arch Gen Psychiatry. 2000;57(11):1039-1045.
31. Bhatia SC, Bhatia SK. Depression in women: diagnostic and treatment considerations. Am Fam Physician. 1999;60(1):225-240.
32. Battle CL, Uebelacker LA, Howard M, et al. Prenatal yoga and depression during pregnancy. Birth. 2010;37(4):353-354.
33. Muzik M, Hamilton SE, Waxler EG, et al. Mindfulness yoga during pregnancy for women with depression and PTSD: preliminary results from a pilot feasibility study. Paper presented at: 36th Annual Meeting of the Association for Women in Psychology; March 5, 2011; Philadelphia, PA.
34. Deligiannidis KM, Freeman MP. Complementary and alternative medicine for the treatment of depressive disorders in women. Psychiatr Clin North Am. 2010;33(2):441-463.
35. Shivakumar G, Brandon AR, Snell PG, et al. Antenatal depression: a rationale for studying exercise. Depress Anxiety. 2011;28(3):234-242.
1. Steer RA, Scholl TO, Hediger ML, et al. Self-reported depression and negative pregnancy outcomes. J Clin Epidemiol. 1992;45(10):1093-1099.
2. Zuckerman B, Bauchner H, Parker S, et al. Maternal depressive symptoms during pregnancy, and newborn irritability. J Dev Behav Pediatr. 1990;11(4):190-194.
3. Field T, Healy B, Goldstein S, et al. Infants of depressed mothers show depressed behavior even with non-depressed adults. Child Dev. 1988;59(6):1569-1579.
4. Coghill SR, Caplan HL, Alexandra H, et al. Impact of maternal postnatal depression on cognitive development of young children. Br Med J (Clin Res Ed). 1986;292(6529):1165-1167.
5. Muzik M, Marcus S, Heringhausen J, et al. When depression complicates childbearing: guidelines for screening and treatment during antenatal and postpartum obstetric care. Obstet Gynecol Clin North Am. 2009;36(4):771-788, ix–x.
6. Bifulco A, Figueiredo B, Guedeney N, et al. Maternal attachment style and depression associated with childbirth: preliminary results from a European and US cross-cultural study. Br J Psychiatry Suppl. 2004;46:s31-s37.
7. National Institute of Mental Health. Women and depression: discovering hope. http://www.nimh.nih.gov/health/publications/women-and-depression-discovering-hope/complete-index.shtml. Accessed December 27 2011.
8. Kumar R, Robson KM. A prospective study of emotional disorders in childbearing women. Br J Psychiatry. 1984;144:35-47.
9. O’Mahen HA, Flynn HA. P and perceived barriers to treatment for depression during the perinatal period. J Womens Health (Larchmt). 2008;17(8):1301-1309.
10. Flynn HA, O’Mahen HA, Massey L, et al. The impact of a brief obstetrics clinic based intervention on treatment use for perinatal depression. J Womens Health (Larchmt). 2006;15(10):1195-1204.
11. Marcus SM, Flynn HA, Blow FC, et al. Depressive symptoms among pregnant women screened in obstetrics settings. J Womens Health (Larchmt). 2003;12(4):373-380.
12. Olfson M, Marcus SC. National trends in outpatient psychotherapy. Am J Psychiatry. 2010;167(12):1456-1463.
13. Schiff MA, Grossman DC. Adverse perinatal outcomes and risk for postpartum suicide attempt in Washington state 1987-2001. Pediatrics. 2006;118(3):669-675.
14. Kurki T, Hiilesmaa V, Raitasalo R, et al. Depression and anxiety in early pregnancy and risk for preeclampsia. Obstet Gynecol. 2000;95:(4)487-490.
15. McKee MD, Cunningham M, Jankowski KR, et al. Health-related functional status in pregnancy: relationship to depression and social support in a multiethnic population. Obstet Gynecol. 2001;97(6):988-993.
16. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782-786.
17. Matthey S, Henshaw C, Elliott S, et al. Variability in use of cut-off scores and formats on the Edinburgh Postnatal Depression Scale: implications for clinical and research practice. Arch Womens Ment Health. 2006;9(6):309-315.
18. Chaudron LH, Szilagyi PG, Tang W, et al. Accuracy of depression screening tools for identifying postpartum depression among urban mothers. Pediatrics. 2010;125(3):609-617.
19. Ross AS, Hall RW, Frost K, et al. Antenatal and neonatal guidelines, education and learning system. J Ark Med Soc. 2006;102(12):328-330.
20. Muzik M, Klier CK, Rosenblum KL, et al. Are commonly used self-report inventories suitable for screening postpartum depression and anxiety disorders? Acta Psychiatr Scand. 2000;102(1):71-73.
21. Muzik M, Thelen K, Rosenblum KL. Perinatal depression: detection and treatment. Neuropsychiatry. 2011;1(2):179-195.
22. Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403-413.
23. ACOG Committee on Practice Bulletins–Obstetrics. ACOG practice bulletin. Clinical management guidelines for obstetrician-gynecologists number 92. Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111(4):1001-1020.
24. Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499-507.
25. Gonsalves L, Schuermeyer I. Treating depression in pregnancy: practical suggestions. Cleve Clin J Med. 2006;73(12):1098-1104.
26. Misri S, Kendrick K. Treatment of perinatal mood and anxiety disorders: a review. Can J Psychiatry. 2007;52(8):489-498.
27. Wisner KL, Sit DY, Hanusa BH, et al. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry. 2009;166(5):557-566.
28. Anderson EL, Reti IM. ECT in pregnancy: a review of the literature from 1941 to 2007. Psychosom Med. 2009;71(2):235-242.
29. Miller LJ. Use of electroconvulsive therapy during pregnancy. Hosp Community Psychiatry. 1994;45(5):444-450.
30. O’Hara MW, Stuart S, Gorman LL, et al. Efficacy of interpersonal psychotherapy for postpartum depression. Arch Gen Psychiatry. 2000;57(11):1039-1045.
31. Bhatia SC, Bhatia SK. Depression in women: diagnostic and treatment considerations. Am Fam Physician. 1999;60(1):225-240.
32. Battle CL, Uebelacker LA, Howard M, et al. Prenatal yoga and depression during pregnancy. Birth. 2010;37(4):353-354.
33. Muzik M, Hamilton SE, Waxler EG, et al. Mindfulness yoga during pregnancy for women with depression and PTSD: preliminary results from a pilot feasibility study. Paper presented at: 36th Annual Meeting of the Association for Women in Psychology; March 5, 2011; Philadelphia, PA.
34. Deligiannidis KM, Freeman MP. Complementary and alternative medicine for the treatment of depressive disorders in women. Psychiatr Clin North Am. 2010;33(2):441-463.
35. Shivakumar G, Brandon AR, Snell PG, et al. Antenatal depression: a rationale for studying exercise. Depress Anxiety. 2011;28(3):234-242.
Suicide assessment: Targeting acute risk factors
At his wife’s urging, Mr. L, age 34, presents to the local emergency room (ER). Approximately 1 week ago, he woke up in the middle of the night and told her he was afraid he would die because he had heart palpitations, a choking sensation, dizziness, and shortness of breath.
The ER physician rules out an acute medical illness and requests a psychiatric consultation. Mr. L is reluctant to talk to the psychiatrist, saying he has just had a difficult couple of weeks because of problems at work. With Mr. L’s permission, the psychiatrist speaks with his wife and learns that for several weeks Mr. L has been having problems falling asleep and has been waking up early. Mrs. L noticed her husband is unable to sit still, not enjoying his favorite television shows, and drinking more alcohol at night.
The clinical picture became clearer after Mr. L tells the psychiatrist that approximately 1 month ago, he lost his appetite, had low energy and concentration, and began to feel depressed. He denies having suicidal thoughts or plans, but says his suffering is increasing and he doesn’t know what to do.
Suicide is our worst outcome; at times it can seem like we are helpless to change its frequency or evaluate its likelihood. As clinicians, we are not expected to predict who will commit suicide, but are expected to perform an adequate suicide risk assessment and determine who is at high risk. We need to clearly document a patient’s suicide risk level in his or her chart, and our subsequent actions need to be consistent with that assessment. For instance, arranging for additional supports—including psychiatric hospitalization when necessary—for a patient deemed to be at high risk for suicide is considered the standard of care. In this article, I:
- discuss demographic factors related to suicide
- explore the importance of time-related suicide risk factors and the few treatments shown to reduce suicide risk
- review protective and preventive factors.
Sobering statistics
Over the past decade, suicide rates in the United States have remained fixed at slightly more than 30,000 per year. In 2009—the most recent year for which statistics are available from the Centers for Disease Control and Prevention—there were 36,547 suicides in the United States, making it the 10th leading cause of death.1 The rates of suicide completions and attempts vary by sex and age. Males complete suicide 4 times more often than females, whereas females attempt suicide 3 times more often. Among individuals age 15 to 24, 86% of those who completed suicide were male; in older persons (age >65), 85% were male.2 Although rates of completed suicide are highest among older adults, rates of suicide attempts are greatest among young persons. The ratio of attempted-to-completed suicide is 100 to 200:1 in individuals age 15 to 24 but 4:1 in those age >65.2
Whites and Native Americans have the highest suicide rates (12.3 and 12.9 per 100,000, respectively).2 Guns are the most common method of completed suicide in all age groups in the United States: they are used in 53% of all suicides and 76% of those among persons age >70.3 In >90% of completed suicides, the decedent had been diagnosed with ≥1 psychiatric disorder.3 By far, the most common psychiatric illness is major depressive disorder, present in 75% of those who commit suicide.3
Understanding intent
Many physicians believe that patients will tell them if they are feeling worse and are starting to think more seriously about suicide. There is no better example of this than the “contract for safety” or “no-harm contract,” in which a patient signs a paper agreeing to notify a clinician if he begins to develop more intense suicidal feelings. Studies have shown that these “no-harm contracts” do not prevent suicide; this makes sense because if a patient decides to kill himself, telling a clinician puts up an obstacle.4-6
Patients who commit suicide often communicate their suicidal intent, but usually tell family members rather than clinicians. In 1 study, 78% of patients who committed suicide on an inpatient unit denied suicidal ideation at their last communication with staff; although 60% told their spouse and 50% told other relatives, only 18% told their physician.7 In this study, precautions provided a false sense of security: 51% of patients were receiving 15-minute suicide checks or 1-to-1 observation at the time of suicide.7
Who is at risk?
The most recent American Psychiatric Association Task Force Report on Suicide identified 57 risk factors for suicide.8,9 This has led to confusion among clinicians and may have led some clinicians to repeatedly ask patients about suicidal ideation rather than conduct a suicide risk assessment.
Although a history of suicide attempts and a family history of suicide are well-established risk factors,9 these are not acute factors. It is important to differentiate between suicide attempts and suicide completions. Although many suicide attempts are accurate substitutes for actual suicides, there is a spectrum of intent in suicide attempts that differentiates them in terms of lethality.10 Clinicians need a more thorough understanding of who is at acute risk for suicide, which will help them make decisions about patients’ imminent risk to themselves.
In the only study that examined time-related predictors of suicide, Fawcett et al11 used the Schedule for Affective Disorders and Schizophrenia (SADS) to evaluate 954 patients with major affective disorders over 10 years. Raters were blinded to treatment, and clinicians could use any combination of psychotherapy or pharmacotherapy. These researchers found that acute risk factors—those associated with suicide within 1 year—were psychic anxiety, anhedonia, diminished concentration, insomnia, panic attacks, and active alcohol abuse (Table 1).11 These factors were present in the context of an underlying depressive disorder. Hopelessness, suicidal ideation, and a history of suicide attempts were linked to suicide between 2 and 10 years.
Busch et al12 performed a retrospective study on an inpatient unit using the SADS to evaluate symptoms present the week before patients’ suicides. They found that 79% of patients had extreme psychic anxiety, agitation, or both, and that 54% had active psychosis. The same authors studied an additional 12 cases of inpatient suicide and found 9 patients had severe anxiety, agitation, or both, and insomnia. The median time to suicide from admission was 3.5 days and none of the 12 patients had been started on an antidepressant, antipsychotic, or anxiolytic. This underscores the need to initiate symptomatic treatment quickly, even before reaching a definitive diagnosis.
The Columbia Suicide Severity Rating Scale (C-SSRS), which evaluates suicide ideation and behavior in the past week and lifetime, has predictive validity in determining those at highest risk for making a suicide attempt within up to 24 weeks of follow-up.13 A limitation of the C-SSRS is that it has predictive validity for suicide attempts only, and not suicide completions.
Table 1
Acute suicide risk factors: 3 A’s + 3 P’s
| Alcohol abuse |
| Attention (or concentration) impairment |
| Awake (insomnia) |
| Panic attacks |
| Pleasure (diminished) |
| Psychic anxiety |
| Source: Reference 11 |
Treatments to lower risk
Although identifying risk factors such as older age, being unmarried, male sex, experiencing a recent loss, a family history of completed suicide, and being white or Native American are helpful in evaluating a patient’s suicide risk, they are not time-sensitive or modifiable, which limits their value.
In contrast, most of the acute risk factors identified by Fawcett et al potentially are treatable. Psychic anxiety, insomnia, and panic attacks can be treated with benzodiazepines or other anxiolytics and sedative/hypnotics. Active psychosis, which Busch et al identified as a risk factor for inpatient suicide, may respond to antipsychotics.
Other medications have been identified as modifying suicide risk (Table 2).14-20 Among patients with major affective disorders, lithium has been shown to reduce suicidal acts by 93%, suicide attempts by 93%, and suicide completions by 82%.14 Lithium produces the largest suicide risk reduction in unipolar depression, at 100%, followed by bipolar II disorder (82%) and bipolar I disorder (67%).15 Several studies have demonstrated that lithium can reduce the mortality rate from suicide for patients with affective disorders, and that this effect persists.16,17
Clozapine has been associated with reduced rates of suicide attempts and completed suicides in patients with chronic psychosis. In a meta-analysis, long-term clozapine treatment was associated with an approximately 3-fold overall reduction of risk of suicidal behaviors,18 although a prospective study found no reduction in risk of completed suicide in patients with schizophrenia treated with clozapine.19
In one study, electroconvulsive therapy (ECT) reduced suicidal thoughts and acts by 38% after 1 week and 80% overall.20 There have been reports of amelioration of suicidal thoughts after just 1 ECT treatment.21 There are no published studies that show a reduction in suicide completions with ECT; however, this may be due to the relatively small number of patients who receive ECT and the infrequency of completed suicides.
Protective factors. The balance between protective factors and risk factors determines appropriate clinical decision making when attempting to evaluate a patient’s suicide risk. Perhaps the best measure of protective factors is the Reasons for Living Inventory, developed by Linehan et al,22 which has been validated in some populations, including adolescents and young adults.23 This inventory delineates protective factors against suicidal ideation and behavior rather than completed suicides.
Similar to suicide protection, suicide prevention focuses on factors that can serve as obstacles to a patient’s desire or ability to commit suicide. A large systematic literature review by Mann et al24 found that only primary care physician education and restricting access to lethal means prevented suicide. When working to remove lethal means from a suicidal patient’s home, it is critical to verify that this has been done rather than merely making a suggestion to a family member. It is necessary to follow up with a phone call and document the completion of this task.
When a patient commits suicide, it is common for psychiatrists to feel like there must have been something they could have done to prevent such a tragedy. Although typically that is not the case, there is more we can do to improve our suicide risk assessment skills. Focusing on acute, modifiable suicide risk factors may help us lower a patient’s risk. Also, shortening the time frame now considered acute (within 1 year) to hours and days and looking for additional risk factors may improve mental health professionals’ ability to accurately assess acute suicide risk.
Table 2
Treatments to lower suicide risk
| Acute |
| Benzodiazepines—to diminish panic, anxiety, insomnia |
| Antipsychotics—if acute psychosis is present |
| Trazodone (or non-benzodiazepine hypnotics)—if insomnia is present without daytime anxiety |
| Diagnosis–specific |
| Clozapine—for patients with schizophrenia and high suicide risk |
| Lithium—for patients with bipolar disorder (if not contraindicated); consider for patients with refractory unipolar depression at high suicide risk |
| Electroconvulsive therapy—for patients with severe depression and high suicide risk |
| Source: References 14-20 |
CASE CONTINUED: Hospitalization and improvement
The psychiatrist determines Mr. L is at high risk for suicide and recommends psychiatric hospitalization. She starts him on citalopram, 10 mg/d, and clonazepam, 0.5 mg twice daily and 1 mg at bedtime, to help with anxiety and insomnia. After 3 days, Mr. L tolerates the medications, sleeps better, and feels more hopeful about the future. The psychiatrist increases citalopram to 20 mg/d.
Four days later, Mr. L is eating better, can concentrate, and denies further episodes of dizziness or anxiety. The inpatient psychiatrist assesses his acute suicide risk as low and discharges him to a week-long partial hospitalization program.
Related Resources
- American Association of Suicidology. www.suicidology.org.
- Harvard School of Public Health. Means Matter. www.hsph.harvard.edu/means-matter.
- Simon RI. Preventing patient suicide: clinical assessment and management. Arlington, VA: American Psychiatric Publishing; 2011.
Drug Brand Names
- Citalopram • Celexa
- Clonazepam • Klonopin
- Clozapine • Clozaril
- Lithium • Eskalith, Lithobid
- Trazodone • Desyrel, Oleptro
Disclosure
Dr. Freeman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Centers for Disease Control and Prevention. US death rate falls for 10th straight year. http://www.cdc.gov/media/releases/2011/p0316_deathrate.html. Published March 16 2011. Accessed November 22, 2011.
2. Centers for Disease Control and Prevention. Suicide: facts at a glance. http://www.cdc.gov/violenceprevention/suicide. Published Summer 2010. Accessed November 17 2011.
3. Karch DL, Dahlberg LL, Patel N. Surveillance for violent deaths—National Violent Death Reporting System 16 states, 2007. MMWR Surveill Summ. 2010;59(4):1-50.
4. Resnick PJ. Recognizing that the suicidal patient views you as an ‘adversary.’ Current Psychiatry. 2002;1(1):8.-
5. Stanford EJ, Goetz RR, Bloom JD. The No Harm Contract in the emergency assessment of suicidal risk. J Clin Psychiatry. 1994;55(8):344-348.
6. Edwards SJ, Sachmann MD. No-suicide contracts no-suicide agreements, and no-suicide assurances: a study of their nature, utilization, perceived effectiveness, and potential to cause harm. Crisis. 2010;31(6):290-302.
7. Busch KA, Fawcett J, Jacobs DG. Clinical correlates of inpatient suicide. J Clin Psychiatry. 2003;64(1):14-19.
8. American Psychiatric Association. Practice guideline for the assessment and treatment of patients with suicidal behaviors. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1673332#56008. Published November 2003. Accessed November 22 2011.
9. Jacobs DG, Brewer ML. Application of the APA practice guidelines on suicide to clinical practice. CNS Spectr. 2006;11(6):447-454.
10. Nasser EH, Overholser JC. Assessing varying degrees of lethality in depressed adolescent suicide attempters. Acta Psychiatr Scand. 1999;99(6):423-431.
11. Fawcett J, Scheftner WA, Fogg L, et al. Time-related predictors of suicide in major affective disorder. Am J Psychiatry. 1990;147(9):1189-1194.
12. Busch KA, Fawcett J. A fine-grained study of patients who commit suicide. Psychiatric Ann. 2004;34(5):357-364.
13. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale (C-SSRS): initial validity and internal consistency findings from three multi-site studies with adolescents and adults. Am J Psychiatry. 2011;168:1266-1277.
14. Müller-Oerlinghausen B, Berghöfer A, Ahrens B. The antisuicidal and mortality-reducing effect of lithium prophylaxis: consequences for guidelines in clinical psychiatry. Can J Psychiatry. 2003;48(7):433-439.
15. Tondo L, Hennen J, Baldessarini RJ. Lower suicide risk with long-term lithium treatment in major affective illness: a meta-analysis. Acta Psychiatr Scand. 2001;104(3):163-172.
16. Kessing LV, Søndergård L, Kvist K, et al. Suicide risk in patients treated with lithium. Arch Gen Psychiatry. 2005;62(8):860-866.
17. Baldessarini RJ, Tondo L, Hennen J. Lithium treatment and suicide risk in major affective disorders: update and new findings. J Clin Psychiatry. 2003;64(suppl 5):44-52.
18. Hennen J, Baldessarini RJ. Suicidal risk during treatment with clozapine: a meta-analysis. Schizophr Res. 2005;73(2-3):139-145.
19. Kuo CJ, Tsai SY, Lo CH, et al. Risk factors for completed suicide in schizophrenia. J Clin Psychiatry. 2005;66(5):579-585.
20. Kobeissi J, Aloysi A, Tobias K, et al. Resolution of severe suicidality with a single electroconvulsive therapy. J ECT. 2011;27(1):86-88.
21. Kellner CH, Fink M, Knapp R, et al. Relief of expressed suicidal intent by ECT: a consortium for research in ECT study. Am J Psychiatry. 2005;162(5):977-982.
22. Linehan MM, Goodstein JL, Nielsen SL, et al. Reasons for staying alive when you are thinking of killing yourself: the reasons for living inventory. J Consult Clin Psychol. 1983;51(2):276-286.
23. Cole DA. Validation of the reasons for living inventory in general and delinquent adolescent samples. J Abnorm Child Psychol. 1989;17(1):13-27.
24. Mann JJ, Apter A, Bertolote J, et al. Suicide prevention strategies: a systematic review. JAMA. 2005;294(16):2064-2074.
At his wife’s urging, Mr. L, age 34, presents to the local emergency room (ER). Approximately 1 week ago, he woke up in the middle of the night and told her he was afraid he would die because he had heart palpitations, a choking sensation, dizziness, and shortness of breath.
The ER physician rules out an acute medical illness and requests a psychiatric consultation. Mr. L is reluctant to talk to the psychiatrist, saying he has just had a difficult couple of weeks because of problems at work. With Mr. L’s permission, the psychiatrist speaks with his wife and learns that for several weeks Mr. L has been having problems falling asleep and has been waking up early. Mrs. L noticed her husband is unable to sit still, not enjoying his favorite television shows, and drinking more alcohol at night.
The clinical picture became clearer after Mr. L tells the psychiatrist that approximately 1 month ago, he lost his appetite, had low energy and concentration, and began to feel depressed. He denies having suicidal thoughts or plans, but says his suffering is increasing and he doesn’t know what to do.
Suicide is our worst outcome; at times it can seem like we are helpless to change its frequency or evaluate its likelihood. As clinicians, we are not expected to predict who will commit suicide, but are expected to perform an adequate suicide risk assessment and determine who is at high risk. We need to clearly document a patient’s suicide risk level in his or her chart, and our subsequent actions need to be consistent with that assessment. For instance, arranging for additional supports—including psychiatric hospitalization when necessary—for a patient deemed to be at high risk for suicide is considered the standard of care. In this article, I:
- discuss demographic factors related to suicide
- explore the importance of time-related suicide risk factors and the few treatments shown to reduce suicide risk
- review protective and preventive factors.
Sobering statistics
Over the past decade, suicide rates in the United States have remained fixed at slightly more than 30,000 per year. In 2009—the most recent year for which statistics are available from the Centers for Disease Control and Prevention—there were 36,547 suicides in the United States, making it the 10th leading cause of death.1 The rates of suicide completions and attempts vary by sex and age. Males complete suicide 4 times more often than females, whereas females attempt suicide 3 times more often. Among individuals age 15 to 24, 86% of those who completed suicide were male; in older persons (age >65), 85% were male.2 Although rates of completed suicide are highest among older adults, rates of suicide attempts are greatest among young persons. The ratio of attempted-to-completed suicide is 100 to 200:1 in individuals age 15 to 24 but 4:1 in those age >65.2
Whites and Native Americans have the highest suicide rates (12.3 and 12.9 per 100,000, respectively).2 Guns are the most common method of completed suicide in all age groups in the United States: they are used in 53% of all suicides and 76% of those among persons age >70.3 In >90% of completed suicides, the decedent had been diagnosed with ≥1 psychiatric disorder.3 By far, the most common psychiatric illness is major depressive disorder, present in 75% of those who commit suicide.3
Understanding intent
Many physicians believe that patients will tell them if they are feeling worse and are starting to think more seriously about suicide. There is no better example of this than the “contract for safety” or “no-harm contract,” in which a patient signs a paper agreeing to notify a clinician if he begins to develop more intense suicidal feelings. Studies have shown that these “no-harm contracts” do not prevent suicide; this makes sense because if a patient decides to kill himself, telling a clinician puts up an obstacle.4-6
Patients who commit suicide often communicate their suicidal intent, but usually tell family members rather than clinicians. In 1 study, 78% of patients who committed suicide on an inpatient unit denied suicidal ideation at their last communication with staff; although 60% told their spouse and 50% told other relatives, only 18% told their physician.7 In this study, precautions provided a false sense of security: 51% of patients were receiving 15-minute suicide checks or 1-to-1 observation at the time of suicide.7
Who is at risk?
The most recent American Psychiatric Association Task Force Report on Suicide identified 57 risk factors for suicide.8,9 This has led to confusion among clinicians and may have led some clinicians to repeatedly ask patients about suicidal ideation rather than conduct a suicide risk assessment.
Although a history of suicide attempts and a family history of suicide are well-established risk factors,9 these are not acute factors. It is important to differentiate between suicide attempts and suicide completions. Although many suicide attempts are accurate substitutes for actual suicides, there is a spectrum of intent in suicide attempts that differentiates them in terms of lethality.10 Clinicians need a more thorough understanding of who is at acute risk for suicide, which will help them make decisions about patients’ imminent risk to themselves.
In the only study that examined time-related predictors of suicide, Fawcett et al11 used the Schedule for Affective Disorders and Schizophrenia (SADS) to evaluate 954 patients with major affective disorders over 10 years. Raters were blinded to treatment, and clinicians could use any combination of psychotherapy or pharmacotherapy. These researchers found that acute risk factors—those associated with suicide within 1 year—were psychic anxiety, anhedonia, diminished concentration, insomnia, panic attacks, and active alcohol abuse (Table 1).11 These factors were present in the context of an underlying depressive disorder. Hopelessness, suicidal ideation, and a history of suicide attempts were linked to suicide between 2 and 10 years.
Busch et al12 performed a retrospective study on an inpatient unit using the SADS to evaluate symptoms present the week before patients’ suicides. They found that 79% of patients had extreme psychic anxiety, agitation, or both, and that 54% had active psychosis. The same authors studied an additional 12 cases of inpatient suicide and found 9 patients had severe anxiety, agitation, or both, and insomnia. The median time to suicide from admission was 3.5 days and none of the 12 patients had been started on an antidepressant, antipsychotic, or anxiolytic. This underscores the need to initiate symptomatic treatment quickly, even before reaching a definitive diagnosis.
The Columbia Suicide Severity Rating Scale (C-SSRS), which evaluates suicide ideation and behavior in the past week and lifetime, has predictive validity in determining those at highest risk for making a suicide attempt within up to 24 weeks of follow-up.13 A limitation of the C-SSRS is that it has predictive validity for suicide attempts only, and not suicide completions.
Table 1
Acute suicide risk factors: 3 A’s + 3 P’s
| Alcohol abuse |
| Attention (or concentration) impairment |
| Awake (insomnia) |
| Panic attacks |
| Pleasure (diminished) |
| Psychic anxiety |
| Source: Reference 11 |
Treatments to lower risk
Although identifying risk factors such as older age, being unmarried, male sex, experiencing a recent loss, a family history of completed suicide, and being white or Native American are helpful in evaluating a patient’s suicide risk, they are not time-sensitive or modifiable, which limits their value.
In contrast, most of the acute risk factors identified by Fawcett et al potentially are treatable. Psychic anxiety, insomnia, and panic attacks can be treated with benzodiazepines or other anxiolytics and sedative/hypnotics. Active psychosis, which Busch et al identified as a risk factor for inpatient suicide, may respond to antipsychotics.
Other medications have been identified as modifying suicide risk (Table 2).14-20 Among patients with major affective disorders, lithium has been shown to reduce suicidal acts by 93%, suicide attempts by 93%, and suicide completions by 82%.14 Lithium produces the largest suicide risk reduction in unipolar depression, at 100%, followed by bipolar II disorder (82%) and bipolar I disorder (67%).15 Several studies have demonstrated that lithium can reduce the mortality rate from suicide for patients with affective disorders, and that this effect persists.16,17
Clozapine has been associated with reduced rates of suicide attempts and completed suicides in patients with chronic psychosis. In a meta-analysis, long-term clozapine treatment was associated with an approximately 3-fold overall reduction of risk of suicidal behaviors,18 although a prospective study found no reduction in risk of completed suicide in patients with schizophrenia treated with clozapine.19
In one study, electroconvulsive therapy (ECT) reduced suicidal thoughts and acts by 38% after 1 week and 80% overall.20 There have been reports of amelioration of suicidal thoughts after just 1 ECT treatment.21 There are no published studies that show a reduction in suicide completions with ECT; however, this may be due to the relatively small number of patients who receive ECT and the infrequency of completed suicides.
Protective factors. The balance between protective factors and risk factors determines appropriate clinical decision making when attempting to evaluate a patient’s suicide risk. Perhaps the best measure of protective factors is the Reasons for Living Inventory, developed by Linehan et al,22 which has been validated in some populations, including adolescents and young adults.23 This inventory delineates protective factors against suicidal ideation and behavior rather than completed suicides.
Similar to suicide protection, suicide prevention focuses on factors that can serve as obstacles to a patient’s desire or ability to commit suicide. A large systematic literature review by Mann et al24 found that only primary care physician education and restricting access to lethal means prevented suicide. When working to remove lethal means from a suicidal patient’s home, it is critical to verify that this has been done rather than merely making a suggestion to a family member. It is necessary to follow up with a phone call and document the completion of this task.
When a patient commits suicide, it is common for psychiatrists to feel like there must have been something they could have done to prevent such a tragedy. Although typically that is not the case, there is more we can do to improve our suicide risk assessment skills. Focusing on acute, modifiable suicide risk factors may help us lower a patient’s risk. Also, shortening the time frame now considered acute (within 1 year) to hours and days and looking for additional risk factors may improve mental health professionals’ ability to accurately assess acute suicide risk.
Table 2
Treatments to lower suicide risk
| Acute |
| Benzodiazepines—to diminish panic, anxiety, insomnia |
| Antipsychotics—if acute psychosis is present |
| Trazodone (or non-benzodiazepine hypnotics)—if insomnia is present without daytime anxiety |
| Diagnosis–specific |
| Clozapine—for patients with schizophrenia and high suicide risk |
| Lithium—for patients with bipolar disorder (if not contraindicated); consider for patients with refractory unipolar depression at high suicide risk |
| Electroconvulsive therapy—for patients with severe depression and high suicide risk |
| Source: References 14-20 |
CASE CONTINUED: Hospitalization and improvement
The psychiatrist determines Mr. L is at high risk for suicide and recommends psychiatric hospitalization. She starts him on citalopram, 10 mg/d, and clonazepam, 0.5 mg twice daily and 1 mg at bedtime, to help with anxiety and insomnia. After 3 days, Mr. L tolerates the medications, sleeps better, and feels more hopeful about the future. The psychiatrist increases citalopram to 20 mg/d.
Four days later, Mr. L is eating better, can concentrate, and denies further episodes of dizziness or anxiety. The inpatient psychiatrist assesses his acute suicide risk as low and discharges him to a week-long partial hospitalization program.
Related Resources
- American Association of Suicidology. www.suicidology.org.
- Harvard School of Public Health. Means Matter. www.hsph.harvard.edu/means-matter.
- Simon RI. Preventing patient suicide: clinical assessment and management. Arlington, VA: American Psychiatric Publishing; 2011.
Drug Brand Names
- Citalopram • Celexa
- Clonazepam • Klonopin
- Clozapine • Clozaril
- Lithium • Eskalith, Lithobid
- Trazodone • Desyrel, Oleptro
Disclosure
Dr. Freeman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
At his wife’s urging, Mr. L, age 34, presents to the local emergency room (ER). Approximately 1 week ago, he woke up in the middle of the night and told her he was afraid he would die because he had heart palpitations, a choking sensation, dizziness, and shortness of breath.
The ER physician rules out an acute medical illness and requests a psychiatric consultation. Mr. L is reluctant to talk to the psychiatrist, saying he has just had a difficult couple of weeks because of problems at work. With Mr. L’s permission, the psychiatrist speaks with his wife and learns that for several weeks Mr. L has been having problems falling asleep and has been waking up early. Mrs. L noticed her husband is unable to sit still, not enjoying his favorite television shows, and drinking more alcohol at night.
The clinical picture became clearer after Mr. L tells the psychiatrist that approximately 1 month ago, he lost his appetite, had low energy and concentration, and began to feel depressed. He denies having suicidal thoughts or plans, but says his suffering is increasing and he doesn’t know what to do.
Suicide is our worst outcome; at times it can seem like we are helpless to change its frequency or evaluate its likelihood. As clinicians, we are not expected to predict who will commit suicide, but are expected to perform an adequate suicide risk assessment and determine who is at high risk. We need to clearly document a patient’s suicide risk level in his or her chart, and our subsequent actions need to be consistent with that assessment. For instance, arranging for additional supports—including psychiatric hospitalization when necessary—for a patient deemed to be at high risk for suicide is considered the standard of care. In this article, I:
- discuss demographic factors related to suicide
- explore the importance of time-related suicide risk factors and the few treatments shown to reduce suicide risk
- review protective and preventive factors.
Sobering statistics
Over the past decade, suicide rates in the United States have remained fixed at slightly more than 30,000 per year. In 2009—the most recent year for which statistics are available from the Centers for Disease Control and Prevention—there were 36,547 suicides in the United States, making it the 10th leading cause of death.1 The rates of suicide completions and attempts vary by sex and age. Males complete suicide 4 times more often than females, whereas females attempt suicide 3 times more often. Among individuals age 15 to 24, 86% of those who completed suicide were male; in older persons (age >65), 85% were male.2 Although rates of completed suicide are highest among older adults, rates of suicide attempts are greatest among young persons. The ratio of attempted-to-completed suicide is 100 to 200:1 in individuals age 15 to 24 but 4:1 in those age >65.2
Whites and Native Americans have the highest suicide rates (12.3 and 12.9 per 100,000, respectively).2 Guns are the most common method of completed suicide in all age groups in the United States: they are used in 53% of all suicides and 76% of those among persons age >70.3 In >90% of completed suicides, the decedent had been diagnosed with ≥1 psychiatric disorder.3 By far, the most common psychiatric illness is major depressive disorder, present in 75% of those who commit suicide.3
Understanding intent
Many physicians believe that patients will tell them if they are feeling worse and are starting to think more seriously about suicide. There is no better example of this than the “contract for safety” or “no-harm contract,” in which a patient signs a paper agreeing to notify a clinician if he begins to develop more intense suicidal feelings. Studies have shown that these “no-harm contracts” do not prevent suicide; this makes sense because if a patient decides to kill himself, telling a clinician puts up an obstacle.4-6
Patients who commit suicide often communicate their suicidal intent, but usually tell family members rather than clinicians. In 1 study, 78% of patients who committed suicide on an inpatient unit denied suicidal ideation at their last communication with staff; although 60% told their spouse and 50% told other relatives, only 18% told their physician.7 In this study, precautions provided a false sense of security: 51% of patients were receiving 15-minute suicide checks or 1-to-1 observation at the time of suicide.7
Who is at risk?
The most recent American Psychiatric Association Task Force Report on Suicide identified 57 risk factors for suicide.8,9 This has led to confusion among clinicians and may have led some clinicians to repeatedly ask patients about suicidal ideation rather than conduct a suicide risk assessment.
Although a history of suicide attempts and a family history of suicide are well-established risk factors,9 these are not acute factors. It is important to differentiate between suicide attempts and suicide completions. Although many suicide attempts are accurate substitutes for actual suicides, there is a spectrum of intent in suicide attempts that differentiates them in terms of lethality.10 Clinicians need a more thorough understanding of who is at acute risk for suicide, which will help them make decisions about patients’ imminent risk to themselves.
In the only study that examined time-related predictors of suicide, Fawcett et al11 used the Schedule for Affective Disorders and Schizophrenia (SADS) to evaluate 954 patients with major affective disorders over 10 years. Raters were blinded to treatment, and clinicians could use any combination of psychotherapy or pharmacotherapy. These researchers found that acute risk factors—those associated with suicide within 1 year—were psychic anxiety, anhedonia, diminished concentration, insomnia, panic attacks, and active alcohol abuse (Table 1).11 These factors were present in the context of an underlying depressive disorder. Hopelessness, suicidal ideation, and a history of suicide attempts were linked to suicide between 2 and 10 years.
Busch et al12 performed a retrospective study on an inpatient unit using the SADS to evaluate symptoms present the week before patients’ suicides. They found that 79% of patients had extreme psychic anxiety, agitation, or both, and that 54% had active psychosis. The same authors studied an additional 12 cases of inpatient suicide and found 9 patients had severe anxiety, agitation, or both, and insomnia. The median time to suicide from admission was 3.5 days and none of the 12 patients had been started on an antidepressant, antipsychotic, or anxiolytic. This underscores the need to initiate symptomatic treatment quickly, even before reaching a definitive diagnosis.
The Columbia Suicide Severity Rating Scale (C-SSRS), which evaluates suicide ideation and behavior in the past week and lifetime, has predictive validity in determining those at highest risk for making a suicide attempt within up to 24 weeks of follow-up.13 A limitation of the C-SSRS is that it has predictive validity for suicide attempts only, and not suicide completions.
Table 1
Acute suicide risk factors: 3 A’s + 3 P’s
| Alcohol abuse |
| Attention (or concentration) impairment |
| Awake (insomnia) |
| Panic attacks |
| Pleasure (diminished) |
| Psychic anxiety |
| Source: Reference 11 |
Treatments to lower risk
Although identifying risk factors such as older age, being unmarried, male sex, experiencing a recent loss, a family history of completed suicide, and being white or Native American are helpful in evaluating a patient’s suicide risk, they are not time-sensitive or modifiable, which limits their value.
In contrast, most of the acute risk factors identified by Fawcett et al potentially are treatable. Psychic anxiety, insomnia, and panic attacks can be treated with benzodiazepines or other anxiolytics and sedative/hypnotics. Active psychosis, which Busch et al identified as a risk factor for inpatient suicide, may respond to antipsychotics.
Other medications have been identified as modifying suicide risk (Table 2).14-20 Among patients with major affective disorders, lithium has been shown to reduce suicidal acts by 93%, suicide attempts by 93%, and suicide completions by 82%.14 Lithium produces the largest suicide risk reduction in unipolar depression, at 100%, followed by bipolar II disorder (82%) and bipolar I disorder (67%).15 Several studies have demonstrated that lithium can reduce the mortality rate from suicide for patients with affective disorders, and that this effect persists.16,17
Clozapine has been associated with reduced rates of suicide attempts and completed suicides in patients with chronic psychosis. In a meta-analysis, long-term clozapine treatment was associated with an approximately 3-fold overall reduction of risk of suicidal behaviors,18 although a prospective study found no reduction in risk of completed suicide in patients with schizophrenia treated with clozapine.19
In one study, electroconvulsive therapy (ECT) reduced suicidal thoughts and acts by 38% after 1 week and 80% overall.20 There have been reports of amelioration of suicidal thoughts after just 1 ECT treatment.21 There are no published studies that show a reduction in suicide completions with ECT; however, this may be due to the relatively small number of patients who receive ECT and the infrequency of completed suicides.
Protective factors. The balance between protective factors and risk factors determines appropriate clinical decision making when attempting to evaluate a patient’s suicide risk. Perhaps the best measure of protective factors is the Reasons for Living Inventory, developed by Linehan et al,22 which has been validated in some populations, including adolescents and young adults.23 This inventory delineates protective factors against suicidal ideation and behavior rather than completed suicides.
Similar to suicide protection, suicide prevention focuses on factors that can serve as obstacles to a patient’s desire or ability to commit suicide. A large systematic literature review by Mann et al24 found that only primary care physician education and restricting access to lethal means prevented suicide. When working to remove lethal means from a suicidal patient’s home, it is critical to verify that this has been done rather than merely making a suggestion to a family member. It is necessary to follow up with a phone call and document the completion of this task.
When a patient commits suicide, it is common for psychiatrists to feel like there must have been something they could have done to prevent such a tragedy. Although typically that is not the case, there is more we can do to improve our suicide risk assessment skills. Focusing on acute, modifiable suicide risk factors may help us lower a patient’s risk. Also, shortening the time frame now considered acute (within 1 year) to hours and days and looking for additional risk factors may improve mental health professionals’ ability to accurately assess acute suicide risk.
Table 2
Treatments to lower suicide risk
| Acute |
| Benzodiazepines—to diminish panic, anxiety, insomnia |
| Antipsychotics—if acute psychosis is present |
| Trazodone (or non-benzodiazepine hypnotics)—if insomnia is present without daytime anxiety |
| Diagnosis–specific |
| Clozapine—for patients with schizophrenia and high suicide risk |
| Lithium—for patients with bipolar disorder (if not contraindicated); consider for patients with refractory unipolar depression at high suicide risk |
| Electroconvulsive therapy—for patients with severe depression and high suicide risk |
| Source: References 14-20 |
CASE CONTINUED: Hospitalization and improvement
The psychiatrist determines Mr. L is at high risk for suicide and recommends psychiatric hospitalization. She starts him on citalopram, 10 mg/d, and clonazepam, 0.5 mg twice daily and 1 mg at bedtime, to help with anxiety and insomnia. After 3 days, Mr. L tolerates the medications, sleeps better, and feels more hopeful about the future. The psychiatrist increases citalopram to 20 mg/d.
Four days later, Mr. L is eating better, can concentrate, and denies further episodes of dizziness or anxiety. The inpatient psychiatrist assesses his acute suicide risk as low and discharges him to a week-long partial hospitalization program.
Related Resources
- American Association of Suicidology. www.suicidology.org.
- Harvard School of Public Health. Means Matter. www.hsph.harvard.edu/means-matter.
- Simon RI. Preventing patient suicide: clinical assessment and management. Arlington, VA: American Psychiatric Publishing; 2011.
Drug Brand Names
- Citalopram • Celexa
- Clonazepam • Klonopin
- Clozapine • Clozaril
- Lithium • Eskalith, Lithobid
- Trazodone • Desyrel, Oleptro
Disclosure
Dr. Freeman reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Centers for Disease Control and Prevention. US death rate falls for 10th straight year. http://www.cdc.gov/media/releases/2011/p0316_deathrate.html. Published March 16 2011. Accessed November 22, 2011.
2. Centers for Disease Control and Prevention. Suicide: facts at a glance. http://www.cdc.gov/violenceprevention/suicide. Published Summer 2010. Accessed November 17 2011.
3. Karch DL, Dahlberg LL, Patel N. Surveillance for violent deaths—National Violent Death Reporting System 16 states, 2007. MMWR Surveill Summ. 2010;59(4):1-50.
4. Resnick PJ. Recognizing that the suicidal patient views you as an ‘adversary.’ Current Psychiatry. 2002;1(1):8.-
5. Stanford EJ, Goetz RR, Bloom JD. The No Harm Contract in the emergency assessment of suicidal risk. J Clin Psychiatry. 1994;55(8):344-348.
6. Edwards SJ, Sachmann MD. No-suicide contracts no-suicide agreements, and no-suicide assurances: a study of their nature, utilization, perceived effectiveness, and potential to cause harm. Crisis. 2010;31(6):290-302.
7. Busch KA, Fawcett J, Jacobs DG. Clinical correlates of inpatient suicide. J Clin Psychiatry. 2003;64(1):14-19.
8. American Psychiatric Association. Practice guideline for the assessment and treatment of patients with suicidal behaviors. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1673332#56008. Published November 2003. Accessed November 22 2011.
9. Jacobs DG, Brewer ML. Application of the APA practice guidelines on suicide to clinical practice. CNS Spectr. 2006;11(6):447-454.
10. Nasser EH, Overholser JC. Assessing varying degrees of lethality in depressed adolescent suicide attempters. Acta Psychiatr Scand. 1999;99(6):423-431.
11. Fawcett J, Scheftner WA, Fogg L, et al. Time-related predictors of suicide in major affective disorder. Am J Psychiatry. 1990;147(9):1189-1194.
12. Busch KA, Fawcett J. A fine-grained study of patients who commit suicide. Psychiatric Ann. 2004;34(5):357-364.
13. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale (C-SSRS): initial validity and internal consistency findings from three multi-site studies with adolescents and adults. Am J Psychiatry. 2011;168:1266-1277.
14. Müller-Oerlinghausen B, Berghöfer A, Ahrens B. The antisuicidal and mortality-reducing effect of lithium prophylaxis: consequences for guidelines in clinical psychiatry. Can J Psychiatry. 2003;48(7):433-439.
15. Tondo L, Hennen J, Baldessarini RJ. Lower suicide risk with long-term lithium treatment in major affective illness: a meta-analysis. Acta Psychiatr Scand. 2001;104(3):163-172.
16. Kessing LV, Søndergård L, Kvist K, et al. Suicide risk in patients treated with lithium. Arch Gen Psychiatry. 2005;62(8):860-866.
17. Baldessarini RJ, Tondo L, Hennen J. Lithium treatment and suicide risk in major affective disorders: update and new findings. J Clin Psychiatry. 2003;64(suppl 5):44-52.
18. Hennen J, Baldessarini RJ. Suicidal risk during treatment with clozapine: a meta-analysis. Schizophr Res. 2005;73(2-3):139-145.
19. Kuo CJ, Tsai SY, Lo CH, et al. Risk factors for completed suicide in schizophrenia. J Clin Psychiatry. 2005;66(5):579-585.
20. Kobeissi J, Aloysi A, Tobias K, et al. Resolution of severe suicidality with a single electroconvulsive therapy. J ECT. 2011;27(1):86-88.
21. Kellner CH, Fink M, Knapp R, et al. Relief of expressed suicidal intent by ECT: a consortium for research in ECT study. Am J Psychiatry. 2005;162(5):977-982.
22. Linehan MM, Goodstein JL, Nielsen SL, et al. Reasons for staying alive when you are thinking of killing yourself: the reasons for living inventory. J Consult Clin Psychol. 1983;51(2):276-286.
23. Cole DA. Validation of the reasons for living inventory in general and delinquent adolescent samples. J Abnorm Child Psychol. 1989;17(1):13-27.
24. Mann JJ, Apter A, Bertolote J, et al. Suicide prevention strategies: a systematic review. JAMA. 2005;294(16):2064-2074.
1. Centers for Disease Control and Prevention. US death rate falls for 10th straight year. http://www.cdc.gov/media/releases/2011/p0316_deathrate.html. Published March 16 2011. Accessed November 22, 2011.
2. Centers for Disease Control and Prevention. Suicide: facts at a glance. http://www.cdc.gov/violenceprevention/suicide. Published Summer 2010. Accessed November 17 2011.
3. Karch DL, Dahlberg LL, Patel N. Surveillance for violent deaths—National Violent Death Reporting System 16 states, 2007. MMWR Surveill Summ. 2010;59(4):1-50.
4. Resnick PJ. Recognizing that the suicidal patient views you as an ‘adversary.’ Current Psychiatry. 2002;1(1):8.-
5. Stanford EJ, Goetz RR, Bloom JD. The No Harm Contract in the emergency assessment of suicidal risk. J Clin Psychiatry. 1994;55(8):344-348.
6. Edwards SJ, Sachmann MD. No-suicide contracts no-suicide agreements, and no-suicide assurances: a study of their nature, utilization, perceived effectiveness, and potential to cause harm. Crisis. 2010;31(6):290-302.
7. Busch KA, Fawcett J, Jacobs DG. Clinical correlates of inpatient suicide. J Clin Psychiatry. 2003;64(1):14-19.
8. American Psychiatric Association. Practice guideline for the assessment and treatment of patients with suicidal behaviors. http://psychiatryonline.org/content.aspx?bookid=28§ionid=1673332#56008. Published November 2003. Accessed November 22 2011.
9. Jacobs DG, Brewer ML. Application of the APA practice guidelines on suicide to clinical practice. CNS Spectr. 2006;11(6):447-454.
10. Nasser EH, Overholser JC. Assessing varying degrees of lethality in depressed adolescent suicide attempters. Acta Psychiatr Scand. 1999;99(6):423-431.
11. Fawcett J, Scheftner WA, Fogg L, et al. Time-related predictors of suicide in major affective disorder. Am J Psychiatry. 1990;147(9):1189-1194.
12. Busch KA, Fawcett J. A fine-grained study of patients who commit suicide. Psychiatric Ann. 2004;34(5):357-364.
13. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale (C-SSRS): initial validity and internal consistency findings from three multi-site studies with adolescents and adults. Am J Psychiatry. 2011;168:1266-1277.
14. Müller-Oerlinghausen B, Berghöfer A, Ahrens B. The antisuicidal and mortality-reducing effect of lithium prophylaxis: consequences for guidelines in clinical psychiatry. Can J Psychiatry. 2003;48(7):433-439.
15. Tondo L, Hennen J, Baldessarini RJ. Lower suicide risk with long-term lithium treatment in major affective illness: a meta-analysis. Acta Psychiatr Scand. 2001;104(3):163-172.
16. Kessing LV, Søndergård L, Kvist K, et al. Suicide risk in patients treated with lithium. Arch Gen Psychiatry. 2005;62(8):860-866.
17. Baldessarini RJ, Tondo L, Hennen J. Lithium treatment and suicide risk in major affective disorders: update and new findings. J Clin Psychiatry. 2003;64(suppl 5):44-52.
18. Hennen J, Baldessarini RJ. Suicidal risk during treatment with clozapine: a meta-analysis. Schizophr Res. 2005;73(2-3):139-145.
19. Kuo CJ, Tsai SY, Lo CH, et al. Risk factors for completed suicide in schizophrenia. J Clin Psychiatry. 2005;66(5):579-585.
20. Kobeissi J, Aloysi A, Tobias K, et al. Resolution of severe suicidality with a single electroconvulsive therapy. J ECT. 2011;27(1):86-88.
21. Kellner CH, Fink M, Knapp R, et al. Relief of expressed suicidal intent by ECT: a consortium for research in ECT study. Am J Psychiatry. 2005;162(5):977-982.
22. Linehan MM, Goodstein JL, Nielsen SL, et al. Reasons for staying alive when you are thinking of killing yourself: the reasons for living inventory. J Consult Clin Psychol. 1983;51(2):276-286.
23. Cole DA. Validation of the reasons for living inventory in general and delinquent adolescent samples. J Abnorm Child Psychol. 1989;17(1):13-27.
24. Mann JJ, Apter A, Bertolote J, et al. Suicide prevention strategies: a systematic review. JAMA. 2005;294(16):2064-2074.
Psychostimulants for older adults
Discuss this article at www.facebook.com/CurrentPsychiatry
Psychostimulants are recognized for their role in managing attention-deficit/hyperactivity disorder (ADHD), but also have found a treatment niche in conditions such as apathy, fatigue, and depression.1 Psychostimulants—methylphenidate, amphetamines, and their respective isomers—are known to promote wakefulness, increase energy, and help improve attention. Although these medications can provide much-needed relief to many older patients, clinicians need to be mindful of possible side effects and safety concerns when prescribing psychostimulants for geriatric patients.
Most psychostimulant research has evaluated children and younger adults; however, geriatric patients (age >65) deserve special consideration. Although these patients’ changing physiology often presents treatment challenges and may predispose individuals to adverse events, emerging evidence suggests that psychostimulants are valuable in treating motivational and attentional symptoms that do not respond to other treatments. Older adults’ diminished treatment response to antidepressants, fatigue, and comorbid medical illness make stimulants an attractive treatment option. However, there is a paucity of research addressing psychostimulant use in geriatric patients. Moreover, psychostimulants should be used in older patients only after carefully considering potential side effects and general medical safety.
This article will focus on clinical scenarios in late life—such as apathy, ADHD, and depression in medically ill patients—when treatment with psychostimulants may be useful. Psychostimulants are FDA-approved primarily for use in ADHD and other uses are considered off-label. We will highlight research in this population and use case vignettes as examples to present a sensible approach to treating geriatric patients with psychostimulants (Table).
Table
Using psychostimulants in older adults
| Category | Comment(s) |
|---|---|
| Clinical utility | Apathy, ADHD, fatigue, depression in medically ill patients |
| Starting dosage | Methylphenidate: 10 mg/d (typical dose is 20 mg/d) Consider a 5 mg/d starting dosage for frail patients Give the second dose mid-afternoon to avoid insomnia Dextroamphetamine: 10 mg/d (typical dose is 20 mg/d) Consider a 5 mg/d starting dosage for frail patients Give the second dose mid-afternoon to avoid insomnia |
| Comorbid medical conditions that warrant concern | Cardiac or glaucoma history |
| Possible drug-drug interactions | MAOIs: Serotonin syndrome, hypertensive crisis TCAs: Increased antidepressant levels Warfarin: Increased warfarin levels |
| Safety monitoring | Heart rate, blood pressure, weight |
| ADHD: attention-deficit/hyperactivity disorder; MAOIs: monoamine oxidase inhibitors; TCAs: tricyclic antidepressants | |
Stimulants and apathy
Apathy is a loss of motivation, interest, or initiative that is not attributable to cognitive impairment, diminished consciousness, or emotional suffering.2 Considered a distinct entity from depression, apathy is common late in life, particularly in persons with dementia of the Alzheimer’s type (DAT); 70% to 90% of patients may experience apathy at some stage of dementia.3 Apathy is linked to impairment in activities of daily living and needing more assistance from caregivers, which increases caregiver burden. Treating apathetic symptoms may improve quality of life for the patient and caregivers. For a case study of an older patient with apathy treated with a psychostimulant, see Box 1.
Apathy has been treated successfully with a variety of stimulant medications. In an open-label study, patients with DAT who received methylphenidate, 10 to 20 mg/d, showed significant improvement in Apathy Evaluation Scale (AES) scores.4 Similarly, Herrmann et al5 also demonstrated improvements in AES scores in DAT patients taking methylphenidate, 20 mg/d, compared with placebo. Although methylphenidate appears to have the strongest evidence for treating apathy, dextroamphetamine also has been shown to produce modest improvements in apathy scale measures.6 A double-blind, placebo-controlled crossover study showed that dextroamphetamine, 20 mg/d, significantly improved scores on neuropsychiatric inventory scales that were driven by apathy subscales.6 However, this trial was small (N = 8).
Preliminary evidence indicates that psychostimulants may improve apathetic symptoms in patients with dementia. In Mr. A’s case (Box 1), he experienced apathy symptoms that affected his quality of life and that of those around him. He showed a clear lack of interest and motivation and indifference. This scenario is common among geriatric patients and may be misinterpreted as depression. Although the overlap may be considerable, screening for apathy may help determine a treatment course with psychostimulants instead of antidepressants, thus avoiding unnecessary medication trials.
Mr. A, age 76, has dementia of the Alzheimer’s type. His family brings him to a psychiatrist because Mr. A exhibits a generalized loss of interest. His history reveals a gradual onset of memory problems with steady decline. Current deficits include problems with forgetfulness, misplacing items, increasing difficulty with names, occasional repetitiveness, and mild word finding difficulty. His family complains that Mr. A does not take care of himself, sits all day long, is not interested in his favorite TV shows, is indifferent to his physical health, is not interested in catching up with friends, and has been doing very little from day to day. He does not seek food but cleans his plate when served. His family became concerned when Mr. A showed no excitement in going to his grandson’s baseball game, which he had previously enjoyed. Mr. A denies any concerns and scores a 3 out of 15 on the Geriatric Depression Scale. Mr. A’s family rated him 4 on the same scale.
On the Apathy Evaluation Scale (AES), he scores 46 (moderate severity). We start methylphenidate, 5 mg administered in the morning and early evening (5 pm). Subsequent conversations 2 weeks later with Mr. A’s family revealed Mr. A’s interest levels have improved and reported no side effects. We increase methylphenidate to 10 mg twice a day. Mr. A has remarkably improved hygiene 1 month later and is more engaged in the interview. He scored a 32 (mild severity) on the AES and the family notes that he is interested in watching his grandson play baseball. During this treatment, we did not change Mr. A’s other medications—donepezil, 10 mg/d, and bupropion, 150 mg/d.
Stimulants for ADHD
ADHD is a neurobehavioral disorder that is identified in approximately 8% of children and persists in 4% of adults.7 ADHD is characterized by impulsivity, motor restlessness, and inattention; the latter feature generally is more prominent with advancing age.8 If left untreated, ADHD has societal burdens, such as educational and occupational impairments.9 There is little data on ADHD in older adults and no placebo-controlled trials. For a case study of an older patient with ADHD treated with stimulants, see Box 2.10
Psychostimulants are considered the mainstay of ADHD treatment. First-line treatments include methylphenidate and amphetamines. A meta-analysis found a significant improvement in ADHD symptoms in adult patients taking psychostimulants compared with placebo, with no difference between immediate-release and long-acting formulations.11 Although these findings were reported in younger adults, they may be relevant for older persons as well. Wetzel and Burke12 described how ADHD presents in older adults and argued that the benefits of treating ADHD in this age group often outweigh the risks associated with psychostimulants, which can be diminished through careful screening.
Individuals who present with ADHD symptoms in late life often appear to be high functioning. Some may describe achieving academic and professional success, but may report chronic problems associated with inefficient learning and distraction compared with their peers because of untreated inattention symptoms. Faraone et al13 argue that similar to other illnesses, ADHD is represented by a spectrum of disease, which may be diagnosed in late life or as subthreshold ADHD. Individuals who did not meet diagnostic criteria in childhood or were not evaluated or treated may experience unremitting symptoms that contribute to functional impairment, persistent discouragement, and distress. Frustrations with distractibility, disorganization, and incompletion of tasks may have a psychological impact reflected by low self-esteem and irritability, and be a chronic source of occupational and relationship dysfunction. Diagnosing and treating ADHD in late life can improve longstanding functional impairments and overall quality of life.
Mr. J, age 66, is an attorney who presents for evaluation after he identified common features in friends who have attention-deficit/hyperactivity disorder (ADHD). In grade school, Mr. J’s teachers told him that he employed very little effort and was not meeting his potential, although he performed exceptionally well. He reports similar experiences throughout his education and says he was careful to select classes that were interesting, but did not require demanding projects or burdensome homework. In law school, he felt academically challenged for the first time but realized he had limited study skills. Mr. J graduated in the top 26th percentile of his class using “an unbelievable amount of effort compared with other students.”
Mr. J describes significant impairment in organizational skills and ability to keep track of time, procrastination, incompletion of tasks, and substantial distractibility during conferences. He says he has difficulty reading briefs depending on his emotional connection to the subject matter. Family history revealed that his mother likely had undiagnosed ADHD. He recently married and his wife encouraged him to seek treatment for “forgetfulness.” Mr. J maintains a busy, successful law practice but has become increasingly frustrated by his inability to follow through on simple tasks that could help grow the practice and generate revenue.
Mr. J has an elevated score on the Adult ADHD Symptom Rating Scale.10 He is referred to his primary care physician to evaluate his general health before beginning medication. At follow-up, Mr. J was started on lisdexamfetamine, 20 mg/d, titrated to 40 mg/d. On subsequent visits he reports improved symptoms without side effects. His vital signs are normal and he reports feeling more productive in his work and achieving significant improvement in the day-to-day operations of his practice.
Other uses
Depression. Although not a first-line treatment, psychostimulants have shown benefit for treating depressive disorders, particularly when patients require immediate improvement. These scenarios are common among medically ill patients, such as those with cancer, stroke, or human immunodeficiency virus (HIV), when it is urgent for patients to participate in their treatment plan. A double-blind, placebo-controlled, randomized study that looked at older depressed patients with medical comorbidities found that methylphenidate was well tolerated, worked quickly, and effectively treated depression.14 However, these results must be interpreted cautiously because the entire study was conducted in 8 days, which included a crossover design that administered methylphenidate 10 mg/d and 20 mg/d for 2 days each. A review of stimulant effectiveness in patients whose depression was associated with HIV, stroke, or cancer and in medically ill patients argued that although benefits have been reported, they must be interpreted tentatively because of a lack of randomized trials.15 However, limited evidence supports an effect of stimulants in treating fatigue, anorexia, pain, and sedation in these populations.15
Stimulants’ immediate onset of action may be particularly useful in terminally ill patients who suffer from fatigue or depression. A double-blind, placebo-controlled, randomized study demonstrated that augmenting citalopram, 20 to 40 mg/d, with methylphenidate, mean dose 15 mg/d, for 3 weeks in older depressed patients significantly improved treatment response and accelerated time to remission compared with citalopram and placebo.16 However, a recent Cochrane review did not show clear efficacy for psychostimulants to treat depression.17
Fatigue. Along with depression, fatigue frequently is seen in older patients with medical illnesses. Mood disorders, medical comorbidities, and sleep disturbances are linked to fatigue. Underlying medical causes such as hypothyroidism, anemia, and electrolyte imbalances should be ruled out before starting a psychostimulant. A review by Minton et al18 that looked at cancer-related fatigue suggested that methylphenidate can be beneficial, although the evidence is mixed.
Interferon-alpha treatment for hepatitis C can cause depression and fatigue, and psychostimulants may help treat fatigue-related side effects.19 Fatigue may present as an isolated symptom in interferon-alpha treatment and psychostimulant use may prevent patients from taking an additional medication, therefore decreasing the risk of further side effects.
Fall risk. Some evidence supports using psychostimulants to lower the risk of falling and hypoactive delirium. A recent review by Elie et al20 concluded that stimulants could improve cognitive function in end-of-life hypoactive delirium. Additionally, a randomized, placebo-controlled, double-blind study that evaluated fall risk concluded that methylphenidate, 20 mg/d, might improve some aspects of executive function and gait stability in older adults.21 The authors hypothesized that improved cognition associated with psychostimulant use may play a role in improving fall risk.
Safety concerns
Clinicians should be aware of safety considerations and possible side effects when prescribing psychostimulants. Psychostimulants are controlled substances and are subject to restrictions (Box 3).22 In 2007, the FDA issued warnings regarding an association between psychostimulant use and sudden death, myocardial infarction, and stroke in patients with preexisting cardiac abnormalities or heart problems.23 Also, some evidence indicates that psychostimulants can increase heart rate and systolic blood pressure. These parameters should be monitored during treatment. Reducing or stopping psychostimulants generally reverses cardiovascular effects. Although the evidence to support these events appears sparse, perform a thorough history before beginning a stimulant and make appropriate referrals as indicated. In November 2011, the FDA reported that psychostimulant use in children and young adults is not associated with adverse cardiovascular events, including stroke, heart attack, and sudden cardiac death.24
Less common side effects reported with psychostimulants include anxiety, insomnia, hallucinations, anorexia, delirium, palpitations, and headache. A meta-analysis of studies of adults with ADHD found that adverse events related to psychostimulants were relatively rare; the most common side effects were diminished appetite and difficulty sleeping.25 Sleep-related side effects can be avoided by dosing these medications earlier in the day, typically before 5 pm. Herrmann et al5 reported 2 cases of apparent delirium and 1 with irregular heartbeat in patients with DAT taking methylphenidate vs placebo. However, most patients in this study experienced mild or no adverse events.
Other safety concerns involve using methylphenidate in patients with glaucoma. In theory, stimulants could exacerbate an acute attack of glaucoma in patients with narrow-angle glaucoma. Patients at risk should be referred to an ophthalmologist for an assessment.
Review other medications the patient is taking and assess for possible drug-drug interactions. Combining monoamine oxidase inhibitors (MAOIs) and methylphenidate warrants caution because of the risk of serotonin syndrome and hypertensive crisis. However, there are case reports of successful MAOI/methylphenidate therapy.26 Additionally, methylphenidate increases levels of warfarin and tricyclic antidepressants when taken with these agents.27 Psychostimulants generally are well tolerated by most individuals and taking a careful history may help prevent adverse events.
As schedule II controlled substances, psychostimulants are subject to prescribing limitations. The current Drug Enforcement Administration (DEA) policy on schedule II controlled substances allows for the equivalent of a 90-day supply of medication to be written with multiple prescriptions. DEA requirements for multiple prescriptions include:
- Each prescription issued is for a legitimate medical purpose by an individual practitioner acting in the usual course of his/her professional practice
- The individual practitioner must provide written instructions on each prescription indicating the earliest date on which a pharmacy may fill each prescription
- The issuance of multiple prescriptions is permissible under applicable state laws
- The individual practitioner fully complies with all other applicable requirements under the Controlled Substances Act and implementing regulations, as well as any additional requirements under state law.22
Related Resources
- Kessler RC, Adler L, Ames M, et al. The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med. 2005;35(2):245-256.
- Alexopoulos GS, Salzman C. Treatment of depression with heterocyclic antidepressants, monoamine oxidase inhibitors, and psychomotor stimulants. In: Salzman C, ed. Clinical geriatric psychopharmacology. Baltimore, MD: Williams and Wilkins; 1998:184-244.
Drug Brand Names
- Bupropion • Wellbutrin, Zyban
- Citalopram • Celexa
- Dexmethylphenidate • Focalin
- Dextroamphetamine • Dexedrine
- Donepezil • Aricept
- Lisdexamfetamine • Vyvanse
- Methamphetamine • Desoxyn
- Methylphenidate • Ritalin, Concerta
- Mixed amphetamine salts • Adderall
- Warfarin • Coumadin
Disclosures
Dr. Padala receives research funding from the Department of Veterans Health Administration and the Alzheimer’s Association.
Drs. Franzen, Wetzel, and Burke report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Ballas C, Evans D, Dinges D. Psychostimulants and wakefulness promoting agents. In: Schatzberg A Nemeroff C, eds. The American Psychiatric Publishing textbook of psychopharmacology. 4th ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2010: 843-860.
2. Marin RS. Apathy: a neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci. 1991;3(3):243-254.
3. Dolder CR, Davis LN, McKinsey J. Use of psychostimulants in patients with dementia. Ann Pharmacother. 2010;44(10):1624-1632.
4. Padala PR, Burke WJ, Shostrom VK, et al. Methylphenidate for apathy and functional status in dementia of the Alzheimer type. Am J Geriatr Psychiatry. 2010;18(4):371-374.
5. Herrmann N, Rothenburg LS, Black SE, et al. Methylphenidate for the treatment of apathy in Alzheimer disease: prediction of response using dextroamphetamine challenge. J Clin Psychopharmacol. 2008;28(3):296-301.
6. Huey ED, Garcia C, Wassermann EM, et al. Stimulant treatment of frontotemporal dementia in 8 patients. J Clin Psychiatry. 2008;69(12):1981-1982.
7. Centers for Disease Control and Prevention (CDC). Mental health in the United States: prevalence of diagnosis and medication treatment for attention-deficit/hyperactivity disorder—United States 2003. MMWR Morb Wkly Rep. 2005;54(34):842-847.
8. Biederman J, Mick E, Faraone SV. Age-dependent decline of attention deficit hyperactivity disorder: impact of remission definition and symptom type. Am J Psychiatry. 2000;157(5):816-818.
9. Biederman J, Faraone SV. The effects of attention-deficit/hyperactivity disorder on employment and household income. Med Gen Med. 2006;8(13):12.-
10. Adult ADHD Self-Report Scale (ASRS-v1. 1) symptom checklist instructions. http://www.hcp.med.harvard.edu/ncs/ftpdir/adhd/18%20Question%20ADHD-ASRS-v1-1.pdf. Accessed November 15 2011.
11. Faraone SV, Glatt SJ. A comparison of the efficacy of medications for adult attention-deficit/hyperactivity disorder using meta-analysis of effect sizes. J Clin Psychiatry. 2010;71(6):754-763.
12. Wetzel MW, Burke WJ. Addressing attention-deficit/hyperactivity disorder in late adulthood. Clinical Geriatrics. 2008;16(11):33-39.
13. Faraone SV, Kunwar A, Adamson J, et al. Personality traits among ADHD adults: implications of late-onset and subthreshold diagnoses. Psychol Med. 2009;39(4):685-693.
14. Wallace AE, Kofoed LL, West AN. Double-blind placebo-controlled trial of methylphenidate in older, depressed, medically ill patients. Am J Psychiatry. 1995;152(6):929-931.
15. Orr K, Taylor D. Psychostimulants in the treatment of depression: a review of the evidence. CNS Drugs. 2007;21(3):239-257.
16. Lavretsky H, Park S, Siddarth P, et al. Methylphenidate-enhanced antidepressant response to citalopram in the elderly: a double-blind, placebo-controlled pilot trial. Am J Geriatr Psychiatry. 2006;14(2):181-185.
17. Candy M, Jones L, Williams R, et al. Psychostimulants for depression. Cochrane Database Syst Rev. 2008;16(2):CD006722.-
18. Minton O, Richardson A, Sharpe M, et al. Psychostimulants for the management of cancer-related fatigue: a systematic review and meta-analysis. J Pain Symptom Manage. 2011;41(4):761-767.
19. Raison CL, Demetrashvili M, Capuron L, et al. Neuropsychiatric adverse events of interferon-α: recognition and management. CNS Drugs. 2005;19(2):105-123.
20. Elie D, Gagnon P, Gagnon B, et al. Using psychostimulants in end-of-life patients with hypoactive delirium and cognitive disorders: a literature review [in French]. Can J Psychiatry. 2010;55:386-393.
21. Ben-Itzhak R, Giladi N, Gruendlinger L, et al. Can methylphenidate reduce fall risk in community-living older adults? A double-blind, single-dose cross-over study. J Am Geriatr Soc. 2008;56(4):695-700.
22. U.S. Department of Justice Drug Enforcement Administration. Issuance of multiple prescriptions for Schedule II controlled substances. http://www.deadiversion.usdoj.gov/faq/mult_rx_faq.htm. Accessed November 1, 2011.
23. U.S. Food and Drug Administration. FDA directs ADHD drug manufacturers to notify patients about cardiovascular adverse events and psychiatric adverse events. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucm108849.htm. Accessed November 1, 2011.
24. U.S. Food and Drug Administration. FDA Drug Safety Communication: safety review update of medications used to treat attention-deficit/hyperactivity disorder (ADHD) in children and young adults. http://www.fda.gov/Drugs/DrugSafety/ucm277770.htm. Accessed November 2, 2011.
25. Peterson K, McDonagh MS, Fu R. Comparative benefits and harms of competing medications for adults with attention-deficit hyperactivity disorder: a systematic review and indirect comparison meta-analysis. Psychopharmacology (Berl). 2008;197(1):1-11.
26. Leonard BE, McCartan D, White J, et al. Methylphenidate: a review of its neuropharmacological, neuropsychological and adverse effects. Hum Psychopharmacol. 2004;19(3):151-180.
27. Howard P, Shuster J, Twycross R, et al. Psychostimulants. J Pain Symptom Manage. 2010;40(5):789-795.
Discuss this article at www.facebook.com/CurrentPsychiatry
Psychostimulants are recognized for their role in managing attention-deficit/hyperactivity disorder (ADHD), but also have found a treatment niche in conditions such as apathy, fatigue, and depression.1 Psychostimulants—methylphenidate, amphetamines, and their respective isomers—are known to promote wakefulness, increase energy, and help improve attention. Although these medications can provide much-needed relief to many older patients, clinicians need to be mindful of possible side effects and safety concerns when prescribing psychostimulants for geriatric patients.
Most psychostimulant research has evaluated children and younger adults; however, geriatric patients (age >65) deserve special consideration. Although these patients’ changing physiology often presents treatment challenges and may predispose individuals to adverse events, emerging evidence suggests that psychostimulants are valuable in treating motivational and attentional symptoms that do not respond to other treatments. Older adults’ diminished treatment response to antidepressants, fatigue, and comorbid medical illness make stimulants an attractive treatment option. However, there is a paucity of research addressing psychostimulant use in geriatric patients. Moreover, psychostimulants should be used in older patients only after carefully considering potential side effects and general medical safety.
This article will focus on clinical scenarios in late life—such as apathy, ADHD, and depression in medically ill patients—when treatment with psychostimulants may be useful. Psychostimulants are FDA-approved primarily for use in ADHD and other uses are considered off-label. We will highlight research in this population and use case vignettes as examples to present a sensible approach to treating geriatric patients with psychostimulants (Table).
Table
Using psychostimulants in older adults
| Category | Comment(s) |
|---|---|
| Clinical utility | Apathy, ADHD, fatigue, depression in medically ill patients |
| Starting dosage | Methylphenidate: 10 mg/d (typical dose is 20 mg/d) Consider a 5 mg/d starting dosage for frail patients Give the second dose mid-afternoon to avoid insomnia Dextroamphetamine: 10 mg/d (typical dose is 20 mg/d) Consider a 5 mg/d starting dosage for frail patients Give the second dose mid-afternoon to avoid insomnia |
| Comorbid medical conditions that warrant concern | Cardiac or glaucoma history |
| Possible drug-drug interactions | MAOIs: Serotonin syndrome, hypertensive crisis TCAs: Increased antidepressant levels Warfarin: Increased warfarin levels |
| Safety monitoring | Heart rate, blood pressure, weight |
| ADHD: attention-deficit/hyperactivity disorder; MAOIs: monoamine oxidase inhibitors; TCAs: tricyclic antidepressants | |
Stimulants and apathy
Apathy is a loss of motivation, interest, or initiative that is not attributable to cognitive impairment, diminished consciousness, or emotional suffering.2 Considered a distinct entity from depression, apathy is common late in life, particularly in persons with dementia of the Alzheimer’s type (DAT); 70% to 90% of patients may experience apathy at some stage of dementia.3 Apathy is linked to impairment in activities of daily living and needing more assistance from caregivers, which increases caregiver burden. Treating apathetic symptoms may improve quality of life for the patient and caregivers. For a case study of an older patient with apathy treated with a psychostimulant, see Box 1.
Apathy has been treated successfully with a variety of stimulant medications. In an open-label study, patients with DAT who received methylphenidate, 10 to 20 mg/d, showed significant improvement in Apathy Evaluation Scale (AES) scores.4 Similarly, Herrmann et al5 also demonstrated improvements in AES scores in DAT patients taking methylphenidate, 20 mg/d, compared with placebo. Although methylphenidate appears to have the strongest evidence for treating apathy, dextroamphetamine also has been shown to produce modest improvements in apathy scale measures.6 A double-blind, placebo-controlled crossover study showed that dextroamphetamine, 20 mg/d, significantly improved scores on neuropsychiatric inventory scales that were driven by apathy subscales.6 However, this trial was small (N = 8).
Preliminary evidence indicates that psychostimulants may improve apathetic symptoms in patients with dementia. In Mr. A’s case (Box 1), he experienced apathy symptoms that affected his quality of life and that of those around him. He showed a clear lack of interest and motivation and indifference. This scenario is common among geriatric patients and may be misinterpreted as depression. Although the overlap may be considerable, screening for apathy may help determine a treatment course with psychostimulants instead of antidepressants, thus avoiding unnecessary medication trials.
Mr. A, age 76, has dementia of the Alzheimer’s type. His family brings him to a psychiatrist because Mr. A exhibits a generalized loss of interest. His history reveals a gradual onset of memory problems with steady decline. Current deficits include problems with forgetfulness, misplacing items, increasing difficulty with names, occasional repetitiveness, and mild word finding difficulty. His family complains that Mr. A does not take care of himself, sits all day long, is not interested in his favorite TV shows, is indifferent to his physical health, is not interested in catching up with friends, and has been doing very little from day to day. He does not seek food but cleans his plate when served. His family became concerned when Mr. A showed no excitement in going to his grandson’s baseball game, which he had previously enjoyed. Mr. A denies any concerns and scores a 3 out of 15 on the Geriatric Depression Scale. Mr. A’s family rated him 4 on the same scale.
On the Apathy Evaluation Scale (AES), he scores 46 (moderate severity). We start methylphenidate, 5 mg administered in the morning and early evening (5 pm). Subsequent conversations 2 weeks later with Mr. A’s family revealed Mr. A’s interest levels have improved and reported no side effects. We increase methylphenidate to 10 mg twice a day. Mr. A has remarkably improved hygiene 1 month later and is more engaged in the interview. He scored a 32 (mild severity) on the AES and the family notes that he is interested in watching his grandson play baseball. During this treatment, we did not change Mr. A’s other medications—donepezil, 10 mg/d, and bupropion, 150 mg/d.
Stimulants for ADHD
ADHD is a neurobehavioral disorder that is identified in approximately 8% of children and persists in 4% of adults.7 ADHD is characterized by impulsivity, motor restlessness, and inattention; the latter feature generally is more prominent with advancing age.8 If left untreated, ADHD has societal burdens, such as educational and occupational impairments.9 There is little data on ADHD in older adults and no placebo-controlled trials. For a case study of an older patient with ADHD treated with stimulants, see Box 2.10
Psychostimulants are considered the mainstay of ADHD treatment. First-line treatments include methylphenidate and amphetamines. A meta-analysis found a significant improvement in ADHD symptoms in adult patients taking psychostimulants compared with placebo, with no difference between immediate-release and long-acting formulations.11 Although these findings were reported in younger adults, they may be relevant for older persons as well. Wetzel and Burke12 described how ADHD presents in older adults and argued that the benefits of treating ADHD in this age group often outweigh the risks associated with psychostimulants, which can be diminished through careful screening.
Individuals who present with ADHD symptoms in late life often appear to be high functioning. Some may describe achieving academic and professional success, but may report chronic problems associated with inefficient learning and distraction compared with their peers because of untreated inattention symptoms. Faraone et al13 argue that similar to other illnesses, ADHD is represented by a spectrum of disease, which may be diagnosed in late life or as subthreshold ADHD. Individuals who did not meet diagnostic criteria in childhood or were not evaluated or treated may experience unremitting symptoms that contribute to functional impairment, persistent discouragement, and distress. Frustrations with distractibility, disorganization, and incompletion of tasks may have a psychological impact reflected by low self-esteem and irritability, and be a chronic source of occupational and relationship dysfunction. Diagnosing and treating ADHD in late life can improve longstanding functional impairments and overall quality of life.
Mr. J, age 66, is an attorney who presents for evaluation after he identified common features in friends who have attention-deficit/hyperactivity disorder (ADHD). In grade school, Mr. J’s teachers told him that he employed very little effort and was not meeting his potential, although he performed exceptionally well. He reports similar experiences throughout his education and says he was careful to select classes that were interesting, but did not require demanding projects or burdensome homework. In law school, he felt academically challenged for the first time but realized he had limited study skills. Mr. J graduated in the top 26th percentile of his class using “an unbelievable amount of effort compared with other students.”
Mr. J describes significant impairment in organizational skills and ability to keep track of time, procrastination, incompletion of tasks, and substantial distractibility during conferences. He says he has difficulty reading briefs depending on his emotional connection to the subject matter. Family history revealed that his mother likely had undiagnosed ADHD. He recently married and his wife encouraged him to seek treatment for “forgetfulness.” Mr. J maintains a busy, successful law practice but has become increasingly frustrated by his inability to follow through on simple tasks that could help grow the practice and generate revenue.
Mr. J has an elevated score on the Adult ADHD Symptom Rating Scale.10 He is referred to his primary care physician to evaluate his general health before beginning medication. At follow-up, Mr. J was started on lisdexamfetamine, 20 mg/d, titrated to 40 mg/d. On subsequent visits he reports improved symptoms without side effects. His vital signs are normal and he reports feeling more productive in his work and achieving significant improvement in the day-to-day operations of his practice.
Other uses
Depression. Although not a first-line treatment, psychostimulants have shown benefit for treating depressive disorders, particularly when patients require immediate improvement. These scenarios are common among medically ill patients, such as those with cancer, stroke, or human immunodeficiency virus (HIV), when it is urgent for patients to participate in their treatment plan. A double-blind, placebo-controlled, randomized study that looked at older depressed patients with medical comorbidities found that methylphenidate was well tolerated, worked quickly, and effectively treated depression.14 However, these results must be interpreted cautiously because the entire study was conducted in 8 days, which included a crossover design that administered methylphenidate 10 mg/d and 20 mg/d for 2 days each. A review of stimulant effectiveness in patients whose depression was associated with HIV, stroke, or cancer and in medically ill patients argued that although benefits have been reported, they must be interpreted tentatively because of a lack of randomized trials.15 However, limited evidence supports an effect of stimulants in treating fatigue, anorexia, pain, and sedation in these populations.15
Stimulants’ immediate onset of action may be particularly useful in terminally ill patients who suffer from fatigue or depression. A double-blind, placebo-controlled, randomized study demonstrated that augmenting citalopram, 20 to 40 mg/d, with methylphenidate, mean dose 15 mg/d, for 3 weeks in older depressed patients significantly improved treatment response and accelerated time to remission compared with citalopram and placebo.16 However, a recent Cochrane review did not show clear efficacy for psychostimulants to treat depression.17
Fatigue. Along with depression, fatigue frequently is seen in older patients with medical illnesses. Mood disorders, medical comorbidities, and sleep disturbances are linked to fatigue. Underlying medical causes such as hypothyroidism, anemia, and electrolyte imbalances should be ruled out before starting a psychostimulant. A review by Minton et al18 that looked at cancer-related fatigue suggested that methylphenidate can be beneficial, although the evidence is mixed.
Interferon-alpha treatment for hepatitis C can cause depression and fatigue, and psychostimulants may help treat fatigue-related side effects.19 Fatigue may present as an isolated symptom in interferon-alpha treatment and psychostimulant use may prevent patients from taking an additional medication, therefore decreasing the risk of further side effects.
Fall risk. Some evidence supports using psychostimulants to lower the risk of falling and hypoactive delirium. A recent review by Elie et al20 concluded that stimulants could improve cognitive function in end-of-life hypoactive delirium. Additionally, a randomized, placebo-controlled, double-blind study that evaluated fall risk concluded that methylphenidate, 20 mg/d, might improve some aspects of executive function and gait stability in older adults.21 The authors hypothesized that improved cognition associated with psychostimulant use may play a role in improving fall risk.
Safety concerns
Clinicians should be aware of safety considerations and possible side effects when prescribing psychostimulants. Psychostimulants are controlled substances and are subject to restrictions (Box 3).22 In 2007, the FDA issued warnings regarding an association between psychostimulant use and sudden death, myocardial infarction, and stroke in patients with preexisting cardiac abnormalities or heart problems.23 Also, some evidence indicates that psychostimulants can increase heart rate and systolic blood pressure. These parameters should be monitored during treatment. Reducing or stopping psychostimulants generally reverses cardiovascular effects. Although the evidence to support these events appears sparse, perform a thorough history before beginning a stimulant and make appropriate referrals as indicated. In November 2011, the FDA reported that psychostimulant use in children and young adults is not associated with adverse cardiovascular events, including stroke, heart attack, and sudden cardiac death.24
Less common side effects reported with psychostimulants include anxiety, insomnia, hallucinations, anorexia, delirium, palpitations, and headache. A meta-analysis of studies of adults with ADHD found that adverse events related to psychostimulants were relatively rare; the most common side effects were diminished appetite and difficulty sleeping.25 Sleep-related side effects can be avoided by dosing these medications earlier in the day, typically before 5 pm. Herrmann et al5 reported 2 cases of apparent delirium and 1 with irregular heartbeat in patients with DAT taking methylphenidate vs placebo. However, most patients in this study experienced mild or no adverse events.
Other safety concerns involve using methylphenidate in patients with glaucoma. In theory, stimulants could exacerbate an acute attack of glaucoma in patients with narrow-angle glaucoma. Patients at risk should be referred to an ophthalmologist for an assessment.
Review other medications the patient is taking and assess for possible drug-drug interactions. Combining monoamine oxidase inhibitors (MAOIs) and methylphenidate warrants caution because of the risk of serotonin syndrome and hypertensive crisis. However, there are case reports of successful MAOI/methylphenidate therapy.26 Additionally, methylphenidate increases levels of warfarin and tricyclic antidepressants when taken with these agents.27 Psychostimulants generally are well tolerated by most individuals and taking a careful history may help prevent adverse events.
As schedule II controlled substances, psychostimulants are subject to prescribing limitations. The current Drug Enforcement Administration (DEA) policy on schedule II controlled substances allows for the equivalent of a 90-day supply of medication to be written with multiple prescriptions. DEA requirements for multiple prescriptions include:
- Each prescription issued is for a legitimate medical purpose by an individual practitioner acting in the usual course of his/her professional practice
- The individual practitioner must provide written instructions on each prescription indicating the earliest date on which a pharmacy may fill each prescription
- The issuance of multiple prescriptions is permissible under applicable state laws
- The individual practitioner fully complies with all other applicable requirements under the Controlled Substances Act and implementing regulations, as well as any additional requirements under state law.22
Related Resources
- Kessler RC, Adler L, Ames M, et al. The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med. 2005;35(2):245-256.
- Alexopoulos GS, Salzman C. Treatment of depression with heterocyclic antidepressants, monoamine oxidase inhibitors, and psychomotor stimulants. In: Salzman C, ed. Clinical geriatric psychopharmacology. Baltimore, MD: Williams and Wilkins; 1998:184-244.
Drug Brand Names
- Bupropion • Wellbutrin, Zyban
- Citalopram • Celexa
- Dexmethylphenidate • Focalin
- Dextroamphetamine • Dexedrine
- Donepezil • Aricept
- Lisdexamfetamine • Vyvanse
- Methamphetamine • Desoxyn
- Methylphenidate • Ritalin, Concerta
- Mixed amphetamine salts • Adderall
- Warfarin • Coumadin
Disclosures
Dr. Padala receives research funding from the Department of Veterans Health Administration and the Alzheimer’s Association.
Drs. Franzen, Wetzel, and Burke report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at www.facebook.com/CurrentPsychiatry
Psychostimulants are recognized for their role in managing attention-deficit/hyperactivity disorder (ADHD), but also have found a treatment niche in conditions such as apathy, fatigue, and depression.1 Psychostimulants—methylphenidate, amphetamines, and their respective isomers—are known to promote wakefulness, increase energy, and help improve attention. Although these medications can provide much-needed relief to many older patients, clinicians need to be mindful of possible side effects and safety concerns when prescribing psychostimulants for geriatric patients.
Most psychostimulant research has evaluated children and younger adults; however, geriatric patients (age >65) deserve special consideration. Although these patients’ changing physiology often presents treatment challenges and may predispose individuals to adverse events, emerging evidence suggests that psychostimulants are valuable in treating motivational and attentional symptoms that do not respond to other treatments. Older adults’ diminished treatment response to antidepressants, fatigue, and comorbid medical illness make stimulants an attractive treatment option. However, there is a paucity of research addressing psychostimulant use in geriatric patients. Moreover, psychostimulants should be used in older patients only after carefully considering potential side effects and general medical safety.
This article will focus on clinical scenarios in late life—such as apathy, ADHD, and depression in medically ill patients—when treatment with psychostimulants may be useful. Psychostimulants are FDA-approved primarily for use in ADHD and other uses are considered off-label. We will highlight research in this population and use case vignettes as examples to present a sensible approach to treating geriatric patients with psychostimulants (Table).
Table
Using psychostimulants in older adults
| Category | Comment(s) |
|---|---|
| Clinical utility | Apathy, ADHD, fatigue, depression in medically ill patients |
| Starting dosage | Methylphenidate: 10 mg/d (typical dose is 20 mg/d) Consider a 5 mg/d starting dosage for frail patients Give the second dose mid-afternoon to avoid insomnia Dextroamphetamine: 10 mg/d (typical dose is 20 mg/d) Consider a 5 mg/d starting dosage for frail patients Give the second dose mid-afternoon to avoid insomnia |
| Comorbid medical conditions that warrant concern | Cardiac or glaucoma history |
| Possible drug-drug interactions | MAOIs: Serotonin syndrome, hypertensive crisis TCAs: Increased antidepressant levels Warfarin: Increased warfarin levels |
| Safety monitoring | Heart rate, blood pressure, weight |
| ADHD: attention-deficit/hyperactivity disorder; MAOIs: monoamine oxidase inhibitors; TCAs: tricyclic antidepressants | |
Stimulants and apathy
Apathy is a loss of motivation, interest, or initiative that is not attributable to cognitive impairment, diminished consciousness, or emotional suffering.2 Considered a distinct entity from depression, apathy is common late in life, particularly in persons with dementia of the Alzheimer’s type (DAT); 70% to 90% of patients may experience apathy at some stage of dementia.3 Apathy is linked to impairment in activities of daily living and needing more assistance from caregivers, which increases caregiver burden. Treating apathetic symptoms may improve quality of life for the patient and caregivers. For a case study of an older patient with apathy treated with a psychostimulant, see Box 1.
Apathy has been treated successfully with a variety of stimulant medications. In an open-label study, patients with DAT who received methylphenidate, 10 to 20 mg/d, showed significant improvement in Apathy Evaluation Scale (AES) scores.4 Similarly, Herrmann et al5 also demonstrated improvements in AES scores in DAT patients taking methylphenidate, 20 mg/d, compared with placebo. Although methylphenidate appears to have the strongest evidence for treating apathy, dextroamphetamine also has been shown to produce modest improvements in apathy scale measures.6 A double-blind, placebo-controlled crossover study showed that dextroamphetamine, 20 mg/d, significantly improved scores on neuropsychiatric inventory scales that were driven by apathy subscales.6 However, this trial was small (N = 8).
Preliminary evidence indicates that psychostimulants may improve apathetic symptoms in patients with dementia. In Mr. A’s case (Box 1), he experienced apathy symptoms that affected his quality of life and that of those around him. He showed a clear lack of interest and motivation and indifference. This scenario is common among geriatric patients and may be misinterpreted as depression. Although the overlap may be considerable, screening for apathy may help determine a treatment course with psychostimulants instead of antidepressants, thus avoiding unnecessary medication trials.
Mr. A, age 76, has dementia of the Alzheimer’s type. His family brings him to a psychiatrist because Mr. A exhibits a generalized loss of interest. His history reveals a gradual onset of memory problems with steady decline. Current deficits include problems with forgetfulness, misplacing items, increasing difficulty with names, occasional repetitiveness, and mild word finding difficulty. His family complains that Mr. A does not take care of himself, sits all day long, is not interested in his favorite TV shows, is indifferent to his physical health, is not interested in catching up with friends, and has been doing very little from day to day. He does not seek food but cleans his plate when served. His family became concerned when Mr. A showed no excitement in going to his grandson’s baseball game, which he had previously enjoyed. Mr. A denies any concerns and scores a 3 out of 15 on the Geriatric Depression Scale. Mr. A’s family rated him 4 on the same scale.
On the Apathy Evaluation Scale (AES), he scores 46 (moderate severity). We start methylphenidate, 5 mg administered in the morning and early evening (5 pm). Subsequent conversations 2 weeks later with Mr. A’s family revealed Mr. A’s interest levels have improved and reported no side effects. We increase methylphenidate to 10 mg twice a day. Mr. A has remarkably improved hygiene 1 month later and is more engaged in the interview. He scored a 32 (mild severity) on the AES and the family notes that he is interested in watching his grandson play baseball. During this treatment, we did not change Mr. A’s other medications—donepezil, 10 mg/d, and bupropion, 150 mg/d.
Stimulants for ADHD
ADHD is a neurobehavioral disorder that is identified in approximately 8% of children and persists in 4% of adults.7 ADHD is characterized by impulsivity, motor restlessness, and inattention; the latter feature generally is more prominent with advancing age.8 If left untreated, ADHD has societal burdens, such as educational and occupational impairments.9 There is little data on ADHD in older adults and no placebo-controlled trials. For a case study of an older patient with ADHD treated with stimulants, see Box 2.10
Psychostimulants are considered the mainstay of ADHD treatment. First-line treatments include methylphenidate and amphetamines. A meta-analysis found a significant improvement in ADHD symptoms in adult patients taking psychostimulants compared with placebo, with no difference between immediate-release and long-acting formulations.11 Although these findings were reported in younger adults, they may be relevant for older persons as well. Wetzel and Burke12 described how ADHD presents in older adults and argued that the benefits of treating ADHD in this age group often outweigh the risks associated with psychostimulants, which can be diminished through careful screening.
Individuals who present with ADHD symptoms in late life often appear to be high functioning. Some may describe achieving academic and professional success, but may report chronic problems associated with inefficient learning and distraction compared with their peers because of untreated inattention symptoms. Faraone et al13 argue that similar to other illnesses, ADHD is represented by a spectrum of disease, which may be diagnosed in late life or as subthreshold ADHD. Individuals who did not meet diagnostic criteria in childhood or were not evaluated or treated may experience unremitting symptoms that contribute to functional impairment, persistent discouragement, and distress. Frustrations with distractibility, disorganization, and incompletion of tasks may have a psychological impact reflected by low self-esteem and irritability, and be a chronic source of occupational and relationship dysfunction. Diagnosing and treating ADHD in late life can improve longstanding functional impairments and overall quality of life.
Mr. J, age 66, is an attorney who presents for evaluation after he identified common features in friends who have attention-deficit/hyperactivity disorder (ADHD). In grade school, Mr. J’s teachers told him that he employed very little effort and was not meeting his potential, although he performed exceptionally well. He reports similar experiences throughout his education and says he was careful to select classes that were interesting, but did not require demanding projects or burdensome homework. In law school, he felt academically challenged for the first time but realized he had limited study skills. Mr. J graduated in the top 26th percentile of his class using “an unbelievable amount of effort compared with other students.”
Mr. J describes significant impairment in organizational skills and ability to keep track of time, procrastination, incompletion of tasks, and substantial distractibility during conferences. He says he has difficulty reading briefs depending on his emotional connection to the subject matter. Family history revealed that his mother likely had undiagnosed ADHD. He recently married and his wife encouraged him to seek treatment for “forgetfulness.” Mr. J maintains a busy, successful law practice but has become increasingly frustrated by his inability to follow through on simple tasks that could help grow the practice and generate revenue.
Mr. J has an elevated score on the Adult ADHD Symptom Rating Scale.10 He is referred to his primary care physician to evaluate his general health before beginning medication. At follow-up, Mr. J was started on lisdexamfetamine, 20 mg/d, titrated to 40 mg/d. On subsequent visits he reports improved symptoms without side effects. His vital signs are normal and he reports feeling more productive in his work and achieving significant improvement in the day-to-day operations of his practice.
Other uses
Depression. Although not a first-line treatment, psychostimulants have shown benefit for treating depressive disorders, particularly when patients require immediate improvement. These scenarios are common among medically ill patients, such as those with cancer, stroke, or human immunodeficiency virus (HIV), when it is urgent for patients to participate in their treatment plan. A double-blind, placebo-controlled, randomized study that looked at older depressed patients with medical comorbidities found that methylphenidate was well tolerated, worked quickly, and effectively treated depression.14 However, these results must be interpreted cautiously because the entire study was conducted in 8 days, which included a crossover design that administered methylphenidate 10 mg/d and 20 mg/d for 2 days each. A review of stimulant effectiveness in patients whose depression was associated with HIV, stroke, or cancer and in medically ill patients argued that although benefits have been reported, they must be interpreted tentatively because of a lack of randomized trials.15 However, limited evidence supports an effect of stimulants in treating fatigue, anorexia, pain, and sedation in these populations.15
Stimulants’ immediate onset of action may be particularly useful in terminally ill patients who suffer from fatigue or depression. A double-blind, placebo-controlled, randomized study demonstrated that augmenting citalopram, 20 to 40 mg/d, with methylphenidate, mean dose 15 mg/d, for 3 weeks in older depressed patients significantly improved treatment response and accelerated time to remission compared with citalopram and placebo.16 However, a recent Cochrane review did not show clear efficacy for psychostimulants to treat depression.17
Fatigue. Along with depression, fatigue frequently is seen in older patients with medical illnesses. Mood disorders, medical comorbidities, and sleep disturbances are linked to fatigue. Underlying medical causes such as hypothyroidism, anemia, and electrolyte imbalances should be ruled out before starting a psychostimulant. A review by Minton et al18 that looked at cancer-related fatigue suggested that methylphenidate can be beneficial, although the evidence is mixed.
Interferon-alpha treatment for hepatitis C can cause depression and fatigue, and psychostimulants may help treat fatigue-related side effects.19 Fatigue may present as an isolated symptom in interferon-alpha treatment and psychostimulant use may prevent patients from taking an additional medication, therefore decreasing the risk of further side effects.
Fall risk. Some evidence supports using psychostimulants to lower the risk of falling and hypoactive delirium. A recent review by Elie et al20 concluded that stimulants could improve cognitive function in end-of-life hypoactive delirium. Additionally, a randomized, placebo-controlled, double-blind study that evaluated fall risk concluded that methylphenidate, 20 mg/d, might improve some aspects of executive function and gait stability in older adults.21 The authors hypothesized that improved cognition associated with psychostimulant use may play a role in improving fall risk.
Safety concerns
Clinicians should be aware of safety considerations and possible side effects when prescribing psychostimulants. Psychostimulants are controlled substances and are subject to restrictions (Box 3).22 In 2007, the FDA issued warnings regarding an association between psychostimulant use and sudden death, myocardial infarction, and stroke in patients with preexisting cardiac abnormalities or heart problems.23 Also, some evidence indicates that psychostimulants can increase heart rate and systolic blood pressure. These parameters should be monitored during treatment. Reducing or stopping psychostimulants generally reverses cardiovascular effects. Although the evidence to support these events appears sparse, perform a thorough history before beginning a stimulant and make appropriate referrals as indicated. In November 2011, the FDA reported that psychostimulant use in children and young adults is not associated with adverse cardiovascular events, including stroke, heart attack, and sudden cardiac death.24
Less common side effects reported with psychostimulants include anxiety, insomnia, hallucinations, anorexia, delirium, palpitations, and headache. A meta-analysis of studies of adults with ADHD found that adverse events related to psychostimulants were relatively rare; the most common side effects were diminished appetite and difficulty sleeping.25 Sleep-related side effects can be avoided by dosing these medications earlier in the day, typically before 5 pm. Herrmann et al5 reported 2 cases of apparent delirium and 1 with irregular heartbeat in patients with DAT taking methylphenidate vs placebo. However, most patients in this study experienced mild or no adverse events.
Other safety concerns involve using methylphenidate in patients with glaucoma. In theory, stimulants could exacerbate an acute attack of glaucoma in patients with narrow-angle glaucoma. Patients at risk should be referred to an ophthalmologist for an assessment.
Review other medications the patient is taking and assess for possible drug-drug interactions. Combining monoamine oxidase inhibitors (MAOIs) and methylphenidate warrants caution because of the risk of serotonin syndrome and hypertensive crisis. However, there are case reports of successful MAOI/methylphenidate therapy.26 Additionally, methylphenidate increases levels of warfarin and tricyclic antidepressants when taken with these agents.27 Psychostimulants generally are well tolerated by most individuals and taking a careful history may help prevent adverse events.
As schedule II controlled substances, psychostimulants are subject to prescribing limitations. The current Drug Enforcement Administration (DEA) policy on schedule II controlled substances allows for the equivalent of a 90-day supply of medication to be written with multiple prescriptions. DEA requirements for multiple prescriptions include:
- Each prescription issued is for a legitimate medical purpose by an individual practitioner acting in the usual course of his/her professional practice
- The individual practitioner must provide written instructions on each prescription indicating the earliest date on which a pharmacy may fill each prescription
- The issuance of multiple prescriptions is permissible under applicable state laws
- The individual practitioner fully complies with all other applicable requirements under the Controlled Substances Act and implementing regulations, as well as any additional requirements under state law.22
Related Resources
- Kessler RC, Adler L, Ames M, et al. The World Health Organization Adult ADHD Self-Report Scale (ASRS): a short screening scale for use in the general population. Psychol Med. 2005;35(2):245-256.
- Alexopoulos GS, Salzman C. Treatment of depression with heterocyclic antidepressants, monoamine oxidase inhibitors, and psychomotor stimulants. In: Salzman C, ed. Clinical geriatric psychopharmacology. Baltimore, MD: Williams and Wilkins; 1998:184-244.
Drug Brand Names
- Bupropion • Wellbutrin, Zyban
- Citalopram • Celexa
- Dexmethylphenidate • Focalin
- Dextroamphetamine • Dexedrine
- Donepezil • Aricept
- Lisdexamfetamine • Vyvanse
- Methamphetamine • Desoxyn
- Methylphenidate • Ritalin, Concerta
- Mixed amphetamine salts • Adderall
- Warfarin • Coumadin
Disclosures
Dr. Padala receives research funding from the Department of Veterans Health Administration and the Alzheimer’s Association.
Drs. Franzen, Wetzel, and Burke report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Ballas C, Evans D, Dinges D. Psychostimulants and wakefulness promoting agents. In: Schatzberg A Nemeroff C, eds. The American Psychiatric Publishing textbook of psychopharmacology. 4th ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2010: 843-860.
2. Marin RS. Apathy: a neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci. 1991;3(3):243-254.
3. Dolder CR, Davis LN, McKinsey J. Use of psychostimulants in patients with dementia. Ann Pharmacother. 2010;44(10):1624-1632.
4. Padala PR, Burke WJ, Shostrom VK, et al. Methylphenidate for apathy and functional status in dementia of the Alzheimer type. Am J Geriatr Psychiatry. 2010;18(4):371-374.
5. Herrmann N, Rothenburg LS, Black SE, et al. Methylphenidate for the treatment of apathy in Alzheimer disease: prediction of response using dextroamphetamine challenge. J Clin Psychopharmacol. 2008;28(3):296-301.
6. Huey ED, Garcia C, Wassermann EM, et al. Stimulant treatment of frontotemporal dementia in 8 patients. J Clin Psychiatry. 2008;69(12):1981-1982.
7. Centers for Disease Control and Prevention (CDC). Mental health in the United States: prevalence of diagnosis and medication treatment for attention-deficit/hyperactivity disorder—United States 2003. MMWR Morb Wkly Rep. 2005;54(34):842-847.
8. Biederman J, Mick E, Faraone SV. Age-dependent decline of attention deficit hyperactivity disorder: impact of remission definition and symptom type. Am J Psychiatry. 2000;157(5):816-818.
9. Biederman J, Faraone SV. The effects of attention-deficit/hyperactivity disorder on employment and household income. Med Gen Med. 2006;8(13):12.-
10. Adult ADHD Self-Report Scale (ASRS-v1. 1) symptom checklist instructions. http://www.hcp.med.harvard.edu/ncs/ftpdir/adhd/18%20Question%20ADHD-ASRS-v1-1.pdf. Accessed November 15 2011.
11. Faraone SV, Glatt SJ. A comparison of the efficacy of medications for adult attention-deficit/hyperactivity disorder using meta-analysis of effect sizes. J Clin Psychiatry. 2010;71(6):754-763.
12. Wetzel MW, Burke WJ. Addressing attention-deficit/hyperactivity disorder in late adulthood. Clinical Geriatrics. 2008;16(11):33-39.
13. Faraone SV, Kunwar A, Adamson J, et al. Personality traits among ADHD adults: implications of late-onset and subthreshold diagnoses. Psychol Med. 2009;39(4):685-693.
14. Wallace AE, Kofoed LL, West AN. Double-blind placebo-controlled trial of methylphenidate in older, depressed, medically ill patients. Am J Psychiatry. 1995;152(6):929-931.
15. Orr K, Taylor D. Psychostimulants in the treatment of depression: a review of the evidence. CNS Drugs. 2007;21(3):239-257.
16. Lavretsky H, Park S, Siddarth P, et al. Methylphenidate-enhanced antidepressant response to citalopram in the elderly: a double-blind, placebo-controlled pilot trial. Am J Geriatr Psychiatry. 2006;14(2):181-185.
17. Candy M, Jones L, Williams R, et al. Psychostimulants for depression. Cochrane Database Syst Rev. 2008;16(2):CD006722.-
18. Minton O, Richardson A, Sharpe M, et al. Psychostimulants for the management of cancer-related fatigue: a systematic review and meta-analysis. J Pain Symptom Manage. 2011;41(4):761-767.
19. Raison CL, Demetrashvili M, Capuron L, et al. Neuropsychiatric adverse events of interferon-α: recognition and management. CNS Drugs. 2005;19(2):105-123.
20. Elie D, Gagnon P, Gagnon B, et al. Using psychostimulants in end-of-life patients with hypoactive delirium and cognitive disorders: a literature review [in French]. Can J Psychiatry. 2010;55:386-393.
21. Ben-Itzhak R, Giladi N, Gruendlinger L, et al. Can methylphenidate reduce fall risk in community-living older adults? A double-blind, single-dose cross-over study. J Am Geriatr Soc. 2008;56(4):695-700.
22. U.S. Department of Justice Drug Enforcement Administration. Issuance of multiple prescriptions for Schedule II controlled substances. http://www.deadiversion.usdoj.gov/faq/mult_rx_faq.htm. Accessed November 1, 2011.
23. U.S. Food and Drug Administration. FDA directs ADHD drug manufacturers to notify patients about cardiovascular adverse events and psychiatric adverse events. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucm108849.htm. Accessed November 1, 2011.
24. U.S. Food and Drug Administration. FDA Drug Safety Communication: safety review update of medications used to treat attention-deficit/hyperactivity disorder (ADHD) in children and young adults. http://www.fda.gov/Drugs/DrugSafety/ucm277770.htm. Accessed November 2, 2011.
25. Peterson K, McDonagh MS, Fu R. Comparative benefits and harms of competing medications for adults with attention-deficit hyperactivity disorder: a systematic review and indirect comparison meta-analysis. Psychopharmacology (Berl). 2008;197(1):1-11.
26. Leonard BE, McCartan D, White J, et al. Methylphenidate: a review of its neuropharmacological, neuropsychological and adverse effects. Hum Psychopharmacol. 2004;19(3):151-180.
27. Howard P, Shuster J, Twycross R, et al. Psychostimulants. J Pain Symptom Manage. 2010;40(5):789-795.
1. Ballas C, Evans D, Dinges D. Psychostimulants and wakefulness promoting agents. In: Schatzberg A Nemeroff C, eds. The American Psychiatric Publishing textbook of psychopharmacology. 4th ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2010: 843-860.
2. Marin RS. Apathy: a neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci. 1991;3(3):243-254.
3. Dolder CR, Davis LN, McKinsey J. Use of psychostimulants in patients with dementia. Ann Pharmacother. 2010;44(10):1624-1632.
4. Padala PR, Burke WJ, Shostrom VK, et al. Methylphenidate for apathy and functional status in dementia of the Alzheimer type. Am J Geriatr Psychiatry. 2010;18(4):371-374.
5. Herrmann N, Rothenburg LS, Black SE, et al. Methylphenidate for the treatment of apathy in Alzheimer disease: prediction of response using dextroamphetamine challenge. J Clin Psychopharmacol. 2008;28(3):296-301.
6. Huey ED, Garcia C, Wassermann EM, et al. Stimulant treatment of frontotemporal dementia in 8 patients. J Clin Psychiatry. 2008;69(12):1981-1982.
7. Centers for Disease Control and Prevention (CDC). Mental health in the United States: prevalence of diagnosis and medication treatment for attention-deficit/hyperactivity disorder—United States 2003. MMWR Morb Wkly Rep. 2005;54(34):842-847.
8. Biederman J, Mick E, Faraone SV. Age-dependent decline of attention deficit hyperactivity disorder: impact of remission definition and symptom type. Am J Psychiatry. 2000;157(5):816-818.
9. Biederman J, Faraone SV. The effects of attention-deficit/hyperactivity disorder on employment and household income. Med Gen Med. 2006;8(13):12.-
10. Adult ADHD Self-Report Scale (ASRS-v1. 1) symptom checklist instructions. http://www.hcp.med.harvard.edu/ncs/ftpdir/adhd/18%20Question%20ADHD-ASRS-v1-1.pdf. Accessed November 15 2011.
11. Faraone SV, Glatt SJ. A comparison of the efficacy of medications for adult attention-deficit/hyperactivity disorder using meta-analysis of effect sizes. J Clin Psychiatry. 2010;71(6):754-763.
12. Wetzel MW, Burke WJ. Addressing attention-deficit/hyperactivity disorder in late adulthood. Clinical Geriatrics. 2008;16(11):33-39.
13. Faraone SV, Kunwar A, Adamson J, et al. Personality traits among ADHD adults: implications of late-onset and subthreshold diagnoses. Psychol Med. 2009;39(4):685-693.
14. Wallace AE, Kofoed LL, West AN. Double-blind placebo-controlled trial of methylphenidate in older, depressed, medically ill patients. Am J Psychiatry. 1995;152(6):929-931.
15. Orr K, Taylor D. Psychostimulants in the treatment of depression: a review of the evidence. CNS Drugs. 2007;21(3):239-257.
16. Lavretsky H, Park S, Siddarth P, et al. Methylphenidate-enhanced antidepressant response to citalopram in the elderly: a double-blind, placebo-controlled pilot trial. Am J Geriatr Psychiatry. 2006;14(2):181-185.
17. Candy M, Jones L, Williams R, et al. Psychostimulants for depression. Cochrane Database Syst Rev. 2008;16(2):CD006722.-
18. Minton O, Richardson A, Sharpe M, et al. Psychostimulants for the management of cancer-related fatigue: a systematic review and meta-analysis. J Pain Symptom Manage. 2011;41(4):761-767.
19. Raison CL, Demetrashvili M, Capuron L, et al. Neuropsychiatric adverse events of interferon-α: recognition and management. CNS Drugs. 2005;19(2):105-123.
20. Elie D, Gagnon P, Gagnon B, et al. Using psychostimulants in end-of-life patients with hypoactive delirium and cognitive disorders: a literature review [in French]. Can J Psychiatry. 2010;55:386-393.
21. Ben-Itzhak R, Giladi N, Gruendlinger L, et al. Can methylphenidate reduce fall risk in community-living older adults? A double-blind, single-dose cross-over study. J Am Geriatr Soc. 2008;56(4):695-700.
22. U.S. Department of Justice Drug Enforcement Administration. Issuance of multiple prescriptions for Schedule II controlled substances. http://www.deadiversion.usdoj.gov/faq/mult_rx_faq.htm. Accessed November 1, 2011.
23. U.S. Food and Drug Administration. FDA directs ADHD drug manufacturers to notify patients about cardiovascular adverse events and psychiatric adverse events. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucm108849.htm. Accessed November 1, 2011.
24. U.S. Food and Drug Administration. FDA Drug Safety Communication: safety review update of medications used to treat attention-deficit/hyperactivity disorder (ADHD) in children and young adults. http://www.fda.gov/Drugs/DrugSafety/ucm277770.htm. Accessed November 2, 2011.
25. Peterson K, McDonagh MS, Fu R. Comparative benefits and harms of competing medications for adults with attention-deficit hyperactivity disorder: a systematic review and indirect comparison meta-analysis. Psychopharmacology (Berl). 2008;197(1):1-11.
26. Leonard BE, McCartan D, White J, et al. Methylphenidate: a review of its neuropharmacological, neuropsychological and adverse effects. Hum Psychopharmacol. 2004;19(3):151-180.
27. Howard P, Shuster J, Twycross R, et al. Psychostimulants. J Pain Symptom Manage. 2010;40(5):789-795.
How to care for patients who have delusions with religious content
Discuss this article at www.facebook.com/CurrentPsychiatry
Mr. D, a 72-year-old Christian with a long history of schizophrenia, presents to the emergency room with concerns about evil spirits in his home who have poisoned him. He has called for police assistance on numerous occasions and has tried to kill the evil spirits with his rifle, but states “they are bulletproof.” He is unable to sleep and is “fearful for my life every night because that is when the demons come out.” Mr. D also believes that God is “more powerful than the evil spirits.” Two elders at his church have prayed with him and encouraged him to go to the hospital.
Delusions with religious content (DRC) are associated with poorer clinical outcomes and dangerousness.1-6 Most mental health professionals will encounter patients with DRC because this type of delusion is relatively common in patients with symptoms of mania or psychosis. For example, in a study of 193 inpatients with schizophrenia, 24% had religious delusions.1 The prevalence of DRC varies considerably among populations and can be influenced by the local religion and culture.7-9 This article reviews clinical challenges and assessment and management strategies for patients with DRC.
A challenging course
In a UK study of 193 inpatients with schizophrenia, compared with patients with other types of delusions, those with DRC:
- had higher Positive and Negative Syndrome Scale scores and lower Global Assessment of Functioning scores
- waited longer before reengaging in treatment
- were prescribed more medications.1
In addition, compared with patients with other types of delusions, patients with DRC often hold these delusions with greater conviction,1,2 making them more challenging to treat.
Dangerousness in patients with DRC can manifest as self-harm or harm to others. Extreme examples include self-inflicted enucleation of the eye and autocastration. In a review of 9 cases of severe ocular self-injury, 4 patients had DRC.3 Genital self-mutilation associated with DRC is rare, but several cases of psychotic men who performed autocastration based on a literal, erroneous interpretation of a passage in the Bible (Matthew 19:12) have been reported.4,5 Patients with DRC have committed rape and murder because they believed they were the antichrist.6
In this article we use the phrase “delusions with religious content” instead of “religious delusions” because this distinction highlights that many subtypes of delusions can have a religious theme. Categories of delusions with religious themes include:
- persecutory (often involving Satan)
- grandiose (messianic delusions)
- guilt delusions.
Categorizing DRC is important because some are associated with more distress or dangerousness than others. For example, case studies of self-inflicted eye injuries found that most patients had guilt delusions with religious themes that referenced punishing transgressions, controlling unacceptable sexual impulses, and attaining prescience by destroying vision.3,10 In our example, Mr. D is experiencing a persecutory DRC. Also, using the label “religious delusion” can inadvertently pathologize religious experiences.
Tips for effective evaluation
DSM-IV-TR offers no specific guidelines for assessing DRC vs nondelusional religious beliefs.11 There is risk of pathologizing religious beliefs when listening to content alone.11-15 Instead, focus on the conviction, pervasiveness,2 uniqueness or bizarreness, and associated emotional distress of the delusion to the patient (Table 1).2,12,16-18
In the context of the patient’s spiritual history, deviations from conventional religious beliefs and practices are important factors in determining whether a religious belief is authentic or delusional. Involving family members and/or spiritual care professionals (eg, chaplains and clergy) can be especially helpful when making this differentiation.16,17 In the hospital, chaplains often are familiar with a variety of faith traditions and may provide important insight into the patient’s beliefs. In the community, clergy members from the patient’s faith also may provide valuable perspective.
Similar to how having a basic familiarity with a patient’s culture can improve care, a better understanding of a patient’s spiritual or religious beliefs and practices can build rapport and the therapeutic alliance.16,17 This is particularly important with patients with DRC because these individuals often have a poor therapeutic alliance and engagement with providers.19 Because many psychiatrists have limited time and may not be familiar with every patient’s spiritual or religious background, consultation with spiritual care professionals may be helpful.
Assess whether your patient has reservations about psychiatric treatment. Some may believe that seeking care from a doctor is evidence of weak faith, whereas others may feel that psychiatric treatment is forbidden or incompatible with their religious beliefs.19-22 Mental health clinicians need to consider their own religious biases that may cause them to minimize or pathologize a patient’s religiosity.20,23 Working collaboratively with spiritual care professionals may help reduce clinician biases or assumptions.24
Table 1
Assessing patients with DRC
| Use caution when making a diagnosis to decrease risk of pathologizing religious beliefs |
| Do not focus solely on the content of the delusion; instead look at conviction, pervasiveness, bizarreness, and associated distress |
| Look at the spiritual/religious context and deviations from conventional religious beliefs of the patient’s culture |
| Establish an open dialogue with the patient, the family, and individuals from the patient’s faith community to understand the psychosocial issues and any reservations about psychiatric care |
| Be aware of the categories of delusions, especially those associated with harm (eg, grandiose antichrist delusions, guilt delusions, and some persecutory delusions) |
| Perform a thorough safety assessment that includes previous self-harm, drug use, and severity of mental illness |
| Be vigilant for patients who are actively seeking evidence to support their misguided/dangerous beliefs |
| DRC: delusions with religious content Source: References 2,12,16-18 |
Evaluating safety
When constructing a differential diagnosis and evaluating patients for safety, remember that DRC are a feature of many psychiatric disorders (eg, persecutory DRC in schizophrenia, grandiose DRC in mania). Consider the course and severity of the patient’s illness, and determine if he or she has a history or evidence of self-injury or substance abuse. Be cognizant of the categories of delusions in the context of the diagnosis. For example, grandiose delusions that involve the antichrist can be associated with harm toward others.6 Patients who express extreme feelings of guilt or shame (as seen in psychotic depression) and the need to be physically punished may be at risk for self-harm. Finally, patients seeking evidence to support misguided and dangerous beliefs—for example, obsessing over a religious text regarding self-injury while in a delusional state—may be at high risk for self-harm.18
Researchers have suggested clinicians question patients to determine if they trust their delusions.25 Patients who trust their delusions may appear calm if they already have decided to act on their thoughts.25 Preventive measures for patients at risk of self-harm include close observation, hospitalization, and pharmacotherapy.
Pharmacotherapy for DRC
There are no clear recommendations on specific psychotropics or dosages for treating patients with DRC. When a patient with DRC is at high risk of self-harm or harming others, using antipsychotics, anxiolytics, hypnotics, or a combination of these agents sometimes is needed to quell agitation, along with close observation and restraints when necessary (Table 2).5,18,25,26 Mr. D benefited from risperidone, 3 mg at bedtime, and zolpidem, 10 mg as needed for insomnia.
Table 2
Treating patients with DRC
| If a patient is at risk for self-harm or harming others, take preventive measures such as hospitalization or close observation |
| Rapid tranquilization may be necessary to reduce risk of harm |
| Encourage positive religious coping and spiritual practices, when appropriate |
| DRC: delusions with religious content Source: References 5,18,25,26 |
Using spirituality to cope
Many persistently mentally ill patients identify themselves as religious and use religious activities or beliefs to cope with their illness.27,28 In a study of 1,824 seriously mentally ill patients, self-reports of religiousness were positively associated with psychological well-being and diminished psychiatric symptoms.29 Longitudinal research has shown that some aspects of spirituality and religion are associated with positive mental and physical health effects, whereas other aspects can worsen symptoms.30 Specifically, positive religious coping such as benevolent religious reappraisals (eg, “Jesus is my shield and savior”), collaborative religious coping, and spiritual support are associated with positive mental health.31 However, negative religious coping, such as punishing God reappraisals and reappraisals of God’s power (eg, “my illness is punishment for my sins”), are associated with distress and personal loss.32
For patients with psychotic disorders—and with schizophrenia in particular—religious beliefs can be a source of meaning, hope, strength, and recovery. In a study of 115 outpatients with psychosis, 71% used positive religious coping, compared with 14% who used negative religious coping.33 Among 38 patients with DRC, 45% used spirituality and religion to help cope with their illness, even though they received less support from religious communities than patients with other types of delusions.19 In this study, the authors suggest that positive religious coping among patients with DRC may alleviate delusion severity by decreasing levels of conviction and fear and preventing maladjusted behaviors.19 Religious beliefs and activities are associated with fewer hospitalizations among patients with persistent mental illness28 and are a significant protective factor against suicide in patients with psychotic disorders.34,35 However, some studies have found that intense, obsessive participation in spiritual activities can worsen psychiatric symptoms and undermine recovery.1,36,37
Addressing religion in treatment.
Although many studies have emphasized the importance of religion to patients with psychosis, evidence-based guidelines on how best to address religion/spirituality in the clinical setting in patients with psychosis have yet to be established. In a 2011 study, a spiritual assessment was well tolerated by 40 patients with psychotic disorders and improved patients’ appointment attendance compared with a control group who received traditional care only.26
Many mental health providers feel ill-equipped or are uncomfortable exploring spiritual or religious issues with patients. Enlisting the help of spiritual care professionals when assessing patients with DRC may improve evaluation and care (Table 3). Spiritual care professionals typically are experienced in exploring subjects associated with DRC, such as guilt, morality, conscience, repentance, and confession.24 Spiritual care professionals also may be able to assist patients with religious coping and provide comfort and support.
Finally, spiritual care professionals can help patients connect or reconnect to a spiritual or religious community. In Mr. D’s case, the hospital chaplain deterred him from focusing on the reason the evil spirits were trying to punish him and guided him toward positive religious coping. Mr. D felt we were listening to him on a deeper level and understanding his spiritual struggles. The chaplain’s involvement also enhanced Mr. D’s relationship with the psychiatrist.
Table 3
When to elicit help from spiritual care professionals
| To better understand the patient’s religious background |
| To reduce biases when the clinician comes from a different religious background or no religious background |
| To help identify positive and negative religious coping, and to reinforce positive coping |
| To connect or reconnect patients to members of their faith community or to help them find a religious community |
Related Resources
- Mohr S, Borras L, Betrisey C, et al. Delusions with religious content in patients with psychosis: how they interact with spiritual coping. Psychiatry. 2010;73(2):158-172.
- Huguelet P, Mohr S, Betrisey C, et al. A randomized trial of spiritual assessment of outpatients with schizophrenia: patients’ and clinicians’ experience. Psychiatr Serv. 2011;62(1):79-86.
Drug Brand Names
- Risperidone • Risperdal
- Zolpidem • Ambien
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgements
The authors acknowledge the support and guidance of Rev. Sean Doll O’Mahoney, Rev. Julie Hanada, Rev. Stephen King, PhD, Rev. George Fitchett, PhD, Patricia Murphy, PhD, LCPC, and Kevin Flannelly, PhD.
1. Siddle R, Haddock G, Tarrier N, et al. Religious delusions in patients admitted to hospital with schizophrenia. Soc Psychiatry Psychiatr Epidemiol. 2002;37(3):130-138.
2. Appelbaum PS, Robbins PC, Roth LH. Dimensional approach to delusions: comparison across types and diagnoses. Am J Psychiatry. 1999;156(12):1938-1943.
3. Field HL, Waldfogel S. Severe ocular self-injury. Gen Hosp Psychiatry. 1995;17(3):224-227.
4. Kushner AW. Two cases of auto-castration due to religious delusions. Br J Med Psychol. 1967;40(3):293-298.
5. Waugh AC. Autocastration and biblical delusions in schizophrenia. Br J Psychiatry. 1986;149:656-658.
6. Silva JA, Leong GB, Weinstock R. Violent behaviors associated with the antichrist delusion. J Forensic Sci. 1997;42(6):1058-1061.
7. Atallah SF, El-Dosoky AR, Coker EM, et al. A 22-year retrospective analysis of the changing frequency and patterns of religious symptoms among inpatients with psychotic illness in Egypt. Soc Psychiatry Psychiatr Epidemiol. 2001;36(8):407-415.
8. Bhavsar V, Bhugra D. Religious delusions: finding meanings in psychosis. Psychopathology. 2008;41(3):165-172.
9. Kim K, Hwu H, Zhang LD, et al. Schizophrenic delusions in Seoul, Shanghai and Taipei: a transcultural study. J Korean Med Sci. 2001;16(1):88-94.
10. Kennedy BL, Feldmann TB. Self-inflicted eye injuries: case presentations and a literature review. Hosp Community Psychiatry. 1994;45(5):470-474.
11. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
12. Sanderson S, Vandenberg B, Paese P. Authentic religious experience or insanity? J Clin Psychol. 1999;55(5):607-616.
13. O’Connor S, Vandenberg B. Psychosis of faith? Clinicians’ assessment of religious beliefs. J Consult Clin Psychol. 2005;73(4):610-616.
14. Spitzer M. On defining delusions. Compr Psychiatry. 1990;31(5):377-397.
15. Pierre JM. Faith or delusion? At the crossroads of religion and psychosis. J Psychiatr Pract. 2001;7(3):163-172.
16. Blass DM. A pragmatic approach to teaching psychiatry residents the assessment and treatment of religious patients. Acad Psychiatry. 2007;31(1):25-31.
17. Westermeyer J. Cultural factors in clinical assessment. J Consult Clin Psychol. 1987;55(4):471-478.
18. Clark RA. Self-mutilation accompanying religious delusions: a case report and review. J Clin Psychiatry. 1981;42(6):243-245.
19. Mohr S, Borras L, Betrisey C, et al. Delusions with religious content in patients with psychosis: how they interact with spiritual coping. Psychiatry. 2010;73(2):158-172.
20. Greenberg D, Witztum E. Problems in the treatment of religious patients. Am J Psychother. 1991;45(4):554-565.
21. Peteet JR. Issues in the treatment of religious patients. Am J Psychother. 1981;35(4):559-564.
22. Borras L, Mohr S, Brandt PY, et al. Religious beliefs in schizophrenia: their relevance for adherence to treatment. Schizophr Bull. 2007;33(5):1238-1246.
23. Ng F. The interface between religion and psychosis. Australas Psychiatry. 2007;15(1):62-66.
24. Sacks JM. Religious issues in psychotherapy. J Relig Health. 1985;24(1):26-30.
25. Shore D, Anderson DJ, Cutler NR. Prediction of self-mutilation in hospitalized schizophrenics. Am J Psychiatry. 1978;135(11):1406-1407.
26. Huguelet P, Mohr S, Betrisey C, et al. A randomized trial of spiritual assessment of outpatients with schizophrenia: patients’ and clinicians’ experience. Psychiatr Serv. 2011;62(1):79-86.
27. Kroll J, Sheehan W. Religious beliefs and practices among 52 psychiatric inpatients in Minnesota. Am J Psychiatry. 1989;146(1):67-72.
28. Tepper L, Rogers SA, Coleman EM, et al. The prevalence of religious coping among persons with persistent mental illness. Psychiatr Serv. 2001;52(5):660-665.
29. Corrigan P, McCorkle B, Schell B, et al. Religion and spirituality in the lives of people with serious mental illness. Community Ment Health J. 2003;39(6):487-499.
30. Koenig HG, McCullough ME, Larson DB. Handbook of religion and health. New York NY: Oxford University Press; 2001.
31. Pargament KI, Koenig HG, Perez LM. The many methods of religious coping: development and initial validation of the RCOPE. J Clin Psychol. 2000;56(4):519-543.
32. Phillips RE, III, Stein CH. God’s will God’s punishment, or God’s limitations? Religious coping strategies reported by young adults living with serious mental illness. J Clin Psychol. 2007;63(6):529-540.
33. Mohr S, Brandt PY, Borras L, et al. Toward an integration of spirituality and religiousness into the psychosocial dimension of schizophrenia. Am J Psychiatry. 2006;163(11):1952-1959.
34. Breier A, Astrachan BM. Characterization of schizophrenic patients who commit suicide. Am J Psychiatry. 1984;141(2):206-209.
35. Jarbin H, Von Knorring AL. Suicide and suicide attempts in adolescent-onset psychotic disorders. Nord J Psychiatry. 2004;58(2):115-123.
36. Brewerton TD. Hyperreligiosity in psychotic disorders. J Nerv Ment Dis. 1994;182(5):302-304.
37. Getz GE, Fleck DE, Strakowski SM. Frequency and severity of religious delusions in Christian patients with psychosis. Psychiatry Res. 2001;103(1):87-91.
Discuss this article at www.facebook.com/CurrentPsychiatry
Mr. D, a 72-year-old Christian with a long history of schizophrenia, presents to the emergency room with concerns about evil spirits in his home who have poisoned him. He has called for police assistance on numerous occasions and has tried to kill the evil spirits with his rifle, but states “they are bulletproof.” He is unable to sleep and is “fearful for my life every night because that is when the demons come out.” Mr. D also believes that God is “more powerful than the evil spirits.” Two elders at his church have prayed with him and encouraged him to go to the hospital.
Delusions with religious content (DRC) are associated with poorer clinical outcomes and dangerousness.1-6 Most mental health professionals will encounter patients with DRC because this type of delusion is relatively common in patients with symptoms of mania or psychosis. For example, in a study of 193 inpatients with schizophrenia, 24% had religious delusions.1 The prevalence of DRC varies considerably among populations and can be influenced by the local religion and culture.7-9 This article reviews clinical challenges and assessment and management strategies for patients with DRC.
A challenging course
In a UK study of 193 inpatients with schizophrenia, compared with patients with other types of delusions, those with DRC:
- had higher Positive and Negative Syndrome Scale scores and lower Global Assessment of Functioning scores
- waited longer before reengaging in treatment
- were prescribed more medications.1
In addition, compared with patients with other types of delusions, patients with DRC often hold these delusions with greater conviction,1,2 making them more challenging to treat.
Dangerousness in patients with DRC can manifest as self-harm or harm to others. Extreme examples include self-inflicted enucleation of the eye and autocastration. In a review of 9 cases of severe ocular self-injury, 4 patients had DRC.3 Genital self-mutilation associated with DRC is rare, but several cases of psychotic men who performed autocastration based on a literal, erroneous interpretation of a passage in the Bible (Matthew 19:12) have been reported.4,5 Patients with DRC have committed rape and murder because they believed they were the antichrist.6
In this article we use the phrase “delusions with religious content” instead of “religious delusions” because this distinction highlights that many subtypes of delusions can have a religious theme. Categories of delusions with religious themes include:
- persecutory (often involving Satan)
- grandiose (messianic delusions)
- guilt delusions.
Categorizing DRC is important because some are associated with more distress or dangerousness than others. For example, case studies of self-inflicted eye injuries found that most patients had guilt delusions with religious themes that referenced punishing transgressions, controlling unacceptable sexual impulses, and attaining prescience by destroying vision.3,10 In our example, Mr. D is experiencing a persecutory DRC. Also, using the label “religious delusion” can inadvertently pathologize religious experiences.
Tips for effective evaluation
DSM-IV-TR offers no specific guidelines for assessing DRC vs nondelusional religious beliefs.11 There is risk of pathologizing religious beliefs when listening to content alone.11-15 Instead, focus on the conviction, pervasiveness,2 uniqueness or bizarreness, and associated emotional distress of the delusion to the patient (Table 1).2,12,16-18
In the context of the patient’s spiritual history, deviations from conventional religious beliefs and practices are important factors in determining whether a religious belief is authentic or delusional. Involving family members and/or spiritual care professionals (eg, chaplains and clergy) can be especially helpful when making this differentiation.16,17 In the hospital, chaplains often are familiar with a variety of faith traditions and may provide important insight into the patient’s beliefs. In the community, clergy members from the patient’s faith also may provide valuable perspective.
Similar to how having a basic familiarity with a patient’s culture can improve care, a better understanding of a patient’s spiritual or religious beliefs and practices can build rapport and the therapeutic alliance.16,17 This is particularly important with patients with DRC because these individuals often have a poor therapeutic alliance and engagement with providers.19 Because many psychiatrists have limited time and may not be familiar with every patient’s spiritual or religious background, consultation with spiritual care professionals may be helpful.
Assess whether your patient has reservations about psychiatric treatment. Some may believe that seeking care from a doctor is evidence of weak faith, whereas others may feel that psychiatric treatment is forbidden or incompatible with their religious beliefs.19-22 Mental health clinicians need to consider their own religious biases that may cause them to minimize or pathologize a patient’s religiosity.20,23 Working collaboratively with spiritual care professionals may help reduce clinician biases or assumptions.24
Table 1
Assessing patients with DRC
| Use caution when making a diagnosis to decrease risk of pathologizing religious beliefs |
| Do not focus solely on the content of the delusion; instead look at conviction, pervasiveness, bizarreness, and associated distress |
| Look at the spiritual/religious context and deviations from conventional religious beliefs of the patient’s culture |
| Establish an open dialogue with the patient, the family, and individuals from the patient’s faith community to understand the psychosocial issues and any reservations about psychiatric care |
| Be aware of the categories of delusions, especially those associated with harm (eg, grandiose antichrist delusions, guilt delusions, and some persecutory delusions) |
| Perform a thorough safety assessment that includes previous self-harm, drug use, and severity of mental illness |
| Be vigilant for patients who are actively seeking evidence to support their misguided/dangerous beliefs |
| DRC: delusions with religious content Source: References 2,12,16-18 |
Evaluating safety
When constructing a differential diagnosis and evaluating patients for safety, remember that DRC are a feature of many psychiatric disorders (eg, persecutory DRC in schizophrenia, grandiose DRC in mania). Consider the course and severity of the patient’s illness, and determine if he or she has a history or evidence of self-injury or substance abuse. Be cognizant of the categories of delusions in the context of the diagnosis. For example, grandiose delusions that involve the antichrist can be associated with harm toward others.6 Patients who express extreme feelings of guilt or shame (as seen in psychotic depression) and the need to be physically punished may be at risk for self-harm. Finally, patients seeking evidence to support misguided and dangerous beliefs—for example, obsessing over a religious text regarding self-injury while in a delusional state—may be at high risk for self-harm.18
Researchers have suggested clinicians question patients to determine if they trust their delusions.25 Patients who trust their delusions may appear calm if they already have decided to act on their thoughts.25 Preventive measures for patients at risk of self-harm include close observation, hospitalization, and pharmacotherapy.
Pharmacotherapy for DRC
There are no clear recommendations on specific psychotropics or dosages for treating patients with DRC. When a patient with DRC is at high risk of self-harm or harming others, using antipsychotics, anxiolytics, hypnotics, or a combination of these agents sometimes is needed to quell agitation, along with close observation and restraints when necessary (Table 2).5,18,25,26 Mr. D benefited from risperidone, 3 mg at bedtime, and zolpidem, 10 mg as needed for insomnia.
Table 2
Treating patients with DRC
| If a patient is at risk for self-harm or harming others, take preventive measures such as hospitalization or close observation |
| Rapid tranquilization may be necessary to reduce risk of harm |
| Encourage positive religious coping and spiritual practices, when appropriate |
| DRC: delusions with religious content Source: References 5,18,25,26 |
Using spirituality to cope
Many persistently mentally ill patients identify themselves as religious and use religious activities or beliefs to cope with their illness.27,28 In a study of 1,824 seriously mentally ill patients, self-reports of religiousness were positively associated with psychological well-being and diminished psychiatric symptoms.29 Longitudinal research has shown that some aspects of spirituality and religion are associated with positive mental and physical health effects, whereas other aspects can worsen symptoms.30 Specifically, positive religious coping such as benevolent religious reappraisals (eg, “Jesus is my shield and savior”), collaborative religious coping, and spiritual support are associated with positive mental health.31 However, negative religious coping, such as punishing God reappraisals and reappraisals of God’s power (eg, “my illness is punishment for my sins”), are associated with distress and personal loss.32
For patients with psychotic disorders—and with schizophrenia in particular—religious beliefs can be a source of meaning, hope, strength, and recovery. In a study of 115 outpatients with psychosis, 71% used positive religious coping, compared with 14% who used negative religious coping.33 Among 38 patients with DRC, 45% used spirituality and religion to help cope with their illness, even though they received less support from religious communities than patients with other types of delusions.19 In this study, the authors suggest that positive religious coping among patients with DRC may alleviate delusion severity by decreasing levels of conviction and fear and preventing maladjusted behaviors.19 Religious beliefs and activities are associated with fewer hospitalizations among patients with persistent mental illness28 and are a significant protective factor against suicide in patients with psychotic disorders.34,35 However, some studies have found that intense, obsessive participation in spiritual activities can worsen psychiatric symptoms and undermine recovery.1,36,37
Addressing religion in treatment.
Although many studies have emphasized the importance of religion to patients with psychosis, evidence-based guidelines on how best to address religion/spirituality in the clinical setting in patients with psychosis have yet to be established. In a 2011 study, a spiritual assessment was well tolerated by 40 patients with psychotic disorders and improved patients’ appointment attendance compared with a control group who received traditional care only.26
Many mental health providers feel ill-equipped or are uncomfortable exploring spiritual or religious issues with patients. Enlisting the help of spiritual care professionals when assessing patients with DRC may improve evaluation and care (Table 3). Spiritual care professionals typically are experienced in exploring subjects associated with DRC, such as guilt, morality, conscience, repentance, and confession.24 Spiritual care professionals also may be able to assist patients with religious coping and provide comfort and support.
Finally, spiritual care professionals can help patients connect or reconnect to a spiritual or religious community. In Mr. D’s case, the hospital chaplain deterred him from focusing on the reason the evil spirits were trying to punish him and guided him toward positive religious coping. Mr. D felt we were listening to him on a deeper level and understanding his spiritual struggles. The chaplain’s involvement also enhanced Mr. D’s relationship with the psychiatrist.
Table 3
When to elicit help from spiritual care professionals
| To better understand the patient’s religious background |
| To reduce biases when the clinician comes from a different religious background or no religious background |
| To help identify positive and negative religious coping, and to reinforce positive coping |
| To connect or reconnect patients to members of their faith community or to help them find a religious community |
Related Resources
- Mohr S, Borras L, Betrisey C, et al. Delusions with religious content in patients with psychosis: how they interact with spiritual coping. Psychiatry. 2010;73(2):158-172.
- Huguelet P, Mohr S, Betrisey C, et al. A randomized trial of spiritual assessment of outpatients with schizophrenia: patients’ and clinicians’ experience. Psychiatr Serv. 2011;62(1):79-86.
Drug Brand Names
- Risperidone • Risperdal
- Zolpidem • Ambien
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgements
The authors acknowledge the support and guidance of Rev. Sean Doll O’Mahoney, Rev. Julie Hanada, Rev. Stephen King, PhD, Rev. George Fitchett, PhD, Patricia Murphy, PhD, LCPC, and Kevin Flannelly, PhD.
Discuss this article at www.facebook.com/CurrentPsychiatry
Mr. D, a 72-year-old Christian with a long history of schizophrenia, presents to the emergency room with concerns about evil spirits in his home who have poisoned him. He has called for police assistance on numerous occasions and has tried to kill the evil spirits with his rifle, but states “they are bulletproof.” He is unable to sleep and is “fearful for my life every night because that is when the demons come out.” Mr. D also believes that God is “more powerful than the evil spirits.” Two elders at his church have prayed with him and encouraged him to go to the hospital.
Delusions with religious content (DRC) are associated with poorer clinical outcomes and dangerousness.1-6 Most mental health professionals will encounter patients with DRC because this type of delusion is relatively common in patients with symptoms of mania or psychosis. For example, in a study of 193 inpatients with schizophrenia, 24% had religious delusions.1 The prevalence of DRC varies considerably among populations and can be influenced by the local religion and culture.7-9 This article reviews clinical challenges and assessment and management strategies for patients with DRC.
A challenging course
In a UK study of 193 inpatients with schizophrenia, compared with patients with other types of delusions, those with DRC:
- had higher Positive and Negative Syndrome Scale scores and lower Global Assessment of Functioning scores
- waited longer before reengaging in treatment
- were prescribed more medications.1
In addition, compared with patients with other types of delusions, patients with DRC often hold these delusions with greater conviction,1,2 making them more challenging to treat.
Dangerousness in patients with DRC can manifest as self-harm or harm to others. Extreme examples include self-inflicted enucleation of the eye and autocastration. In a review of 9 cases of severe ocular self-injury, 4 patients had DRC.3 Genital self-mutilation associated with DRC is rare, but several cases of psychotic men who performed autocastration based on a literal, erroneous interpretation of a passage in the Bible (Matthew 19:12) have been reported.4,5 Patients with DRC have committed rape and murder because they believed they were the antichrist.6
In this article we use the phrase “delusions with religious content” instead of “religious delusions” because this distinction highlights that many subtypes of delusions can have a religious theme. Categories of delusions with religious themes include:
- persecutory (often involving Satan)
- grandiose (messianic delusions)
- guilt delusions.
Categorizing DRC is important because some are associated with more distress or dangerousness than others. For example, case studies of self-inflicted eye injuries found that most patients had guilt delusions with religious themes that referenced punishing transgressions, controlling unacceptable sexual impulses, and attaining prescience by destroying vision.3,10 In our example, Mr. D is experiencing a persecutory DRC. Also, using the label “religious delusion” can inadvertently pathologize religious experiences.
Tips for effective evaluation
DSM-IV-TR offers no specific guidelines for assessing DRC vs nondelusional religious beliefs.11 There is risk of pathologizing religious beliefs when listening to content alone.11-15 Instead, focus on the conviction, pervasiveness,2 uniqueness or bizarreness, and associated emotional distress of the delusion to the patient (Table 1).2,12,16-18
In the context of the patient’s spiritual history, deviations from conventional religious beliefs and practices are important factors in determining whether a religious belief is authentic or delusional. Involving family members and/or spiritual care professionals (eg, chaplains and clergy) can be especially helpful when making this differentiation.16,17 In the hospital, chaplains often are familiar with a variety of faith traditions and may provide important insight into the patient’s beliefs. In the community, clergy members from the patient’s faith also may provide valuable perspective.
Similar to how having a basic familiarity with a patient’s culture can improve care, a better understanding of a patient’s spiritual or religious beliefs and practices can build rapport and the therapeutic alliance.16,17 This is particularly important with patients with DRC because these individuals often have a poor therapeutic alliance and engagement with providers.19 Because many psychiatrists have limited time and may not be familiar with every patient’s spiritual or religious background, consultation with spiritual care professionals may be helpful.
Assess whether your patient has reservations about psychiatric treatment. Some may believe that seeking care from a doctor is evidence of weak faith, whereas others may feel that psychiatric treatment is forbidden or incompatible with their religious beliefs.19-22 Mental health clinicians need to consider their own religious biases that may cause them to minimize or pathologize a patient’s religiosity.20,23 Working collaboratively with spiritual care professionals may help reduce clinician biases or assumptions.24
Table 1
Assessing patients with DRC
| Use caution when making a diagnosis to decrease risk of pathologizing religious beliefs |
| Do not focus solely on the content of the delusion; instead look at conviction, pervasiveness, bizarreness, and associated distress |
| Look at the spiritual/religious context and deviations from conventional religious beliefs of the patient’s culture |
| Establish an open dialogue with the patient, the family, and individuals from the patient’s faith community to understand the psychosocial issues and any reservations about psychiatric care |
| Be aware of the categories of delusions, especially those associated with harm (eg, grandiose antichrist delusions, guilt delusions, and some persecutory delusions) |
| Perform a thorough safety assessment that includes previous self-harm, drug use, and severity of mental illness |
| Be vigilant for patients who are actively seeking evidence to support their misguided/dangerous beliefs |
| DRC: delusions with religious content Source: References 2,12,16-18 |
Evaluating safety
When constructing a differential diagnosis and evaluating patients for safety, remember that DRC are a feature of many psychiatric disorders (eg, persecutory DRC in schizophrenia, grandiose DRC in mania). Consider the course and severity of the patient’s illness, and determine if he or she has a history or evidence of self-injury or substance abuse. Be cognizant of the categories of delusions in the context of the diagnosis. For example, grandiose delusions that involve the antichrist can be associated with harm toward others.6 Patients who express extreme feelings of guilt or shame (as seen in psychotic depression) and the need to be physically punished may be at risk for self-harm. Finally, patients seeking evidence to support misguided and dangerous beliefs—for example, obsessing over a religious text regarding self-injury while in a delusional state—may be at high risk for self-harm.18
Researchers have suggested clinicians question patients to determine if they trust their delusions.25 Patients who trust their delusions may appear calm if they already have decided to act on their thoughts.25 Preventive measures for patients at risk of self-harm include close observation, hospitalization, and pharmacotherapy.
Pharmacotherapy for DRC
There are no clear recommendations on specific psychotropics or dosages for treating patients with DRC. When a patient with DRC is at high risk of self-harm or harming others, using antipsychotics, anxiolytics, hypnotics, or a combination of these agents sometimes is needed to quell agitation, along with close observation and restraints when necessary (Table 2).5,18,25,26 Mr. D benefited from risperidone, 3 mg at bedtime, and zolpidem, 10 mg as needed for insomnia.
Table 2
Treating patients with DRC
| If a patient is at risk for self-harm or harming others, take preventive measures such as hospitalization or close observation |
| Rapid tranquilization may be necessary to reduce risk of harm |
| Encourage positive religious coping and spiritual practices, when appropriate |
| DRC: delusions with religious content Source: References 5,18,25,26 |
Using spirituality to cope
Many persistently mentally ill patients identify themselves as religious and use religious activities or beliefs to cope with their illness.27,28 In a study of 1,824 seriously mentally ill patients, self-reports of religiousness were positively associated with psychological well-being and diminished psychiatric symptoms.29 Longitudinal research has shown that some aspects of spirituality and religion are associated with positive mental and physical health effects, whereas other aspects can worsen symptoms.30 Specifically, positive religious coping such as benevolent religious reappraisals (eg, “Jesus is my shield and savior”), collaborative religious coping, and spiritual support are associated with positive mental health.31 However, negative religious coping, such as punishing God reappraisals and reappraisals of God’s power (eg, “my illness is punishment for my sins”), are associated with distress and personal loss.32
For patients with psychotic disorders—and with schizophrenia in particular—religious beliefs can be a source of meaning, hope, strength, and recovery. In a study of 115 outpatients with psychosis, 71% used positive religious coping, compared with 14% who used negative religious coping.33 Among 38 patients with DRC, 45% used spirituality and religion to help cope with their illness, even though they received less support from religious communities than patients with other types of delusions.19 In this study, the authors suggest that positive religious coping among patients with DRC may alleviate delusion severity by decreasing levels of conviction and fear and preventing maladjusted behaviors.19 Religious beliefs and activities are associated with fewer hospitalizations among patients with persistent mental illness28 and are a significant protective factor against suicide in patients with psychotic disorders.34,35 However, some studies have found that intense, obsessive participation in spiritual activities can worsen psychiatric symptoms and undermine recovery.1,36,37
Addressing religion in treatment.
Although many studies have emphasized the importance of religion to patients with psychosis, evidence-based guidelines on how best to address religion/spirituality in the clinical setting in patients with psychosis have yet to be established. In a 2011 study, a spiritual assessment was well tolerated by 40 patients with psychotic disorders and improved patients’ appointment attendance compared with a control group who received traditional care only.26
Many mental health providers feel ill-equipped or are uncomfortable exploring spiritual or religious issues with patients. Enlisting the help of spiritual care professionals when assessing patients with DRC may improve evaluation and care (Table 3). Spiritual care professionals typically are experienced in exploring subjects associated with DRC, such as guilt, morality, conscience, repentance, and confession.24 Spiritual care professionals also may be able to assist patients with religious coping and provide comfort and support.
Finally, spiritual care professionals can help patients connect or reconnect to a spiritual or religious community. In Mr. D’s case, the hospital chaplain deterred him from focusing on the reason the evil spirits were trying to punish him and guided him toward positive religious coping. Mr. D felt we were listening to him on a deeper level and understanding his spiritual struggles. The chaplain’s involvement also enhanced Mr. D’s relationship with the psychiatrist.
Table 3
When to elicit help from spiritual care professionals
| To better understand the patient’s religious background |
| To reduce biases when the clinician comes from a different religious background or no religious background |
| To help identify positive and negative religious coping, and to reinforce positive coping |
| To connect or reconnect patients to members of their faith community or to help them find a religious community |
Related Resources
- Mohr S, Borras L, Betrisey C, et al. Delusions with religious content in patients with psychosis: how they interact with spiritual coping. Psychiatry. 2010;73(2):158-172.
- Huguelet P, Mohr S, Betrisey C, et al. A randomized trial of spiritual assessment of outpatients with schizophrenia: patients’ and clinicians’ experience. Psychiatr Serv. 2011;62(1):79-86.
Drug Brand Names
- Risperidone • Risperdal
- Zolpidem • Ambien
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgements
The authors acknowledge the support and guidance of Rev. Sean Doll O’Mahoney, Rev. Julie Hanada, Rev. Stephen King, PhD, Rev. George Fitchett, PhD, Patricia Murphy, PhD, LCPC, and Kevin Flannelly, PhD.
1. Siddle R, Haddock G, Tarrier N, et al. Religious delusions in patients admitted to hospital with schizophrenia. Soc Psychiatry Psychiatr Epidemiol. 2002;37(3):130-138.
2. Appelbaum PS, Robbins PC, Roth LH. Dimensional approach to delusions: comparison across types and diagnoses. Am J Psychiatry. 1999;156(12):1938-1943.
3. Field HL, Waldfogel S. Severe ocular self-injury. Gen Hosp Psychiatry. 1995;17(3):224-227.
4. Kushner AW. Two cases of auto-castration due to religious delusions. Br J Med Psychol. 1967;40(3):293-298.
5. Waugh AC. Autocastration and biblical delusions in schizophrenia. Br J Psychiatry. 1986;149:656-658.
6. Silva JA, Leong GB, Weinstock R. Violent behaviors associated with the antichrist delusion. J Forensic Sci. 1997;42(6):1058-1061.
7. Atallah SF, El-Dosoky AR, Coker EM, et al. A 22-year retrospective analysis of the changing frequency and patterns of religious symptoms among inpatients with psychotic illness in Egypt. Soc Psychiatry Psychiatr Epidemiol. 2001;36(8):407-415.
8. Bhavsar V, Bhugra D. Religious delusions: finding meanings in psychosis. Psychopathology. 2008;41(3):165-172.
9. Kim K, Hwu H, Zhang LD, et al. Schizophrenic delusions in Seoul, Shanghai and Taipei: a transcultural study. J Korean Med Sci. 2001;16(1):88-94.
10. Kennedy BL, Feldmann TB. Self-inflicted eye injuries: case presentations and a literature review. Hosp Community Psychiatry. 1994;45(5):470-474.
11. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
12. Sanderson S, Vandenberg B, Paese P. Authentic religious experience or insanity? J Clin Psychol. 1999;55(5):607-616.
13. O’Connor S, Vandenberg B. Psychosis of faith? Clinicians’ assessment of religious beliefs. J Consult Clin Psychol. 2005;73(4):610-616.
14. Spitzer M. On defining delusions. Compr Psychiatry. 1990;31(5):377-397.
15. Pierre JM. Faith or delusion? At the crossroads of religion and psychosis. J Psychiatr Pract. 2001;7(3):163-172.
16. Blass DM. A pragmatic approach to teaching psychiatry residents the assessment and treatment of religious patients. Acad Psychiatry. 2007;31(1):25-31.
17. Westermeyer J. Cultural factors in clinical assessment. J Consult Clin Psychol. 1987;55(4):471-478.
18. Clark RA. Self-mutilation accompanying religious delusions: a case report and review. J Clin Psychiatry. 1981;42(6):243-245.
19. Mohr S, Borras L, Betrisey C, et al. Delusions with religious content in patients with psychosis: how they interact with spiritual coping. Psychiatry. 2010;73(2):158-172.
20. Greenberg D, Witztum E. Problems in the treatment of religious patients. Am J Psychother. 1991;45(4):554-565.
21. Peteet JR. Issues in the treatment of religious patients. Am J Psychother. 1981;35(4):559-564.
22. Borras L, Mohr S, Brandt PY, et al. Religious beliefs in schizophrenia: their relevance for adherence to treatment. Schizophr Bull. 2007;33(5):1238-1246.
23. Ng F. The interface between religion and psychosis. Australas Psychiatry. 2007;15(1):62-66.
24. Sacks JM. Religious issues in psychotherapy. J Relig Health. 1985;24(1):26-30.
25. Shore D, Anderson DJ, Cutler NR. Prediction of self-mutilation in hospitalized schizophrenics. Am J Psychiatry. 1978;135(11):1406-1407.
26. Huguelet P, Mohr S, Betrisey C, et al. A randomized trial of spiritual assessment of outpatients with schizophrenia: patients’ and clinicians’ experience. Psychiatr Serv. 2011;62(1):79-86.
27. Kroll J, Sheehan W. Religious beliefs and practices among 52 psychiatric inpatients in Minnesota. Am J Psychiatry. 1989;146(1):67-72.
28. Tepper L, Rogers SA, Coleman EM, et al. The prevalence of religious coping among persons with persistent mental illness. Psychiatr Serv. 2001;52(5):660-665.
29. Corrigan P, McCorkle B, Schell B, et al. Religion and spirituality in the lives of people with serious mental illness. Community Ment Health J. 2003;39(6):487-499.
30. Koenig HG, McCullough ME, Larson DB. Handbook of religion and health. New York NY: Oxford University Press; 2001.
31. Pargament KI, Koenig HG, Perez LM. The many methods of religious coping: development and initial validation of the RCOPE. J Clin Psychol. 2000;56(4):519-543.
32. Phillips RE, III, Stein CH. God’s will God’s punishment, or God’s limitations? Religious coping strategies reported by young adults living with serious mental illness. J Clin Psychol. 2007;63(6):529-540.
33. Mohr S, Brandt PY, Borras L, et al. Toward an integration of spirituality and religiousness into the psychosocial dimension of schizophrenia. Am J Psychiatry. 2006;163(11):1952-1959.
34. Breier A, Astrachan BM. Characterization of schizophrenic patients who commit suicide. Am J Psychiatry. 1984;141(2):206-209.
35. Jarbin H, Von Knorring AL. Suicide and suicide attempts in adolescent-onset psychotic disorders. Nord J Psychiatry. 2004;58(2):115-123.
36. Brewerton TD. Hyperreligiosity in psychotic disorders. J Nerv Ment Dis. 1994;182(5):302-304.
37. Getz GE, Fleck DE, Strakowski SM. Frequency and severity of religious delusions in Christian patients with psychosis. Psychiatry Res. 2001;103(1):87-91.
1. Siddle R, Haddock G, Tarrier N, et al. Religious delusions in patients admitted to hospital with schizophrenia. Soc Psychiatry Psychiatr Epidemiol. 2002;37(3):130-138.
2. Appelbaum PS, Robbins PC, Roth LH. Dimensional approach to delusions: comparison across types and diagnoses. Am J Psychiatry. 1999;156(12):1938-1943.
3. Field HL, Waldfogel S. Severe ocular self-injury. Gen Hosp Psychiatry. 1995;17(3):224-227.
4. Kushner AW. Two cases of auto-castration due to religious delusions. Br J Med Psychol. 1967;40(3):293-298.
5. Waugh AC. Autocastration and biblical delusions in schizophrenia. Br J Psychiatry. 1986;149:656-658.
6. Silva JA, Leong GB, Weinstock R. Violent behaviors associated with the antichrist delusion. J Forensic Sci. 1997;42(6):1058-1061.
7. Atallah SF, El-Dosoky AR, Coker EM, et al. A 22-year retrospective analysis of the changing frequency and patterns of religious symptoms among inpatients with psychotic illness in Egypt. Soc Psychiatry Psychiatr Epidemiol. 2001;36(8):407-415.
8. Bhavsar V, Bhugra D. Religious delusions: finding meanings in psychosis. Psychopathology. 2008;41(3):165-172.
9. Kim K, Hwu H, Zhang LD, et al. Schizophrenic delusions in Seoul, Shanghai and Taipei: a transcultural study. J Korean Med Sci. 2001;16(1):88-94.
10. Kennedy BL, Feldmann TB. Self-inflicted eye injuries: case presentations and a literature review. Hosp Community Psychiatry. 1994;45(5):470-474.
11. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington DC: American Psychiatric Association; 2000.
12. Sanderson S, Vandenberg B, Paese P. Authentic religious experience or insanity? J Clin Psychol. 1999;55(5):607-616.
13. O’Connor S, Vandenberg B. Psychosis of faith? Clinicians’ assessment of religious beliefs. J Consult Clin Psychol. 2005;73(4):610-616.
14. Spitzer M. On defining delusions. Compr Psychiatry. 1990;31(5):377-397.
15. Pierre JM. Faith or delusion? At the crossroads of religion and psychosis. J Psychiatr Pract. 2001;7(3):163-172.
16. Blass DM. A pragmatic approach to teaching psychiatry residents the assessment and treatment of religious patients. Acad Psychiatry. 2007;31(1):25-31.
17. Westermeyer J. Cultural factors in clinical assessment. J Consult Clin Psychol. 1987;55(4):471-478.
18. Clark RA. Self-mutilation accompanying religious delusions: a case report and review. J Clin Psychiatry. 1981;42(6):243-245.
19. Mohr S, Borras L, Betrisey C, et al. Delusions with religious content in patients with psychosis: how they interact with spiritual coping. Psychiatry. 2010;73(2):158-172.
20. Greenberg D, Witztum E. Problems in the treatment of religious patients. Am J Psychother. 1991;45(4):554-565.
21. Peteet JR. Issues in the treatment of religious patients. Am J Psychother. 1981;35(4):559-564.
22. Borras L, Mohr S, Brandt PY, et al. Religious beliefs in schizophrenia: their relevance for adherence to treatment. Schizophr Bull. 2007;33(5):1238-1246.
23. Ng F. The interface between religion and psychosis. Australas Psychiatry. 2007;15(1):62-66.
24. Sacks JM. Religious issues in psychotherapy. J Relig Health. 1985;24(1):26-30.
25. Shore D, Anderson DJ, Cutler NR. Prediction of self-mutilation in hospitalized schizophrenics. Am J Psychiatry. 1978;135(11):1406-1407.
26. Huguelet P, Mohr S, Betrisey C, et al. A randomized trial of spiritual assessment of outpatients with schizophrenia: patients’ and clinicians’ experience. Psychiatr Serv. 2011;62(1):79-86.
27. Kroll J, Sheehan W. Religious beliefs and practices among 52 psychiatric inpatients in Minnesota. Am J Psychiatry. 1989;146(1):67-72.
28. Tepper L, Rogers SA, Coleman EM, et al. The prevalence of religious coping among persons with persistent mental illness. Psychiatr Serv. 2001;52(5):660-665.
29. Corrigan P, McCorkle B, Schell B, et al. Religion and spirituality in the lives of people with serious mental illness. Community Ment Health J. 2003;39(6):487-499.
30. Koenig HG, McCullough ME, Larson DB. Handbook of religion and health. New York NY: Oxford University Press; 2001.
31. Pargament KI, Koenig HG, Perez LM. The many methods of religious coping: development and initial validation of the RCOPE. J Clin Psychol. 2000;56(4):519-543.
32. Phillips RE, III, Stein CH. God’s will God’s punishment, or God’s limitations? Religious coping strategies reported by young adults living with serious mental illness. J Clin Psychol. 2007;63(6):529-540.
33. Mohr S, Brandt PY, Borras L, et al. Toward an integration of spirituality and religiousness into the psychosocial dimension of schizophrenia. Am J Psychiatry. 2006;163(11):1952-1959.
34. Breier A, Astrachan BM. Characterization of schizophrenic patients who commit suicide. Am J Psychiatry. 1984;141(2):206-209.
35. Jarbin H, Von Knorring AL. Suicide and suicide attempts in adolescent-onset psychotic disorders. Nord J Psychiatry. 2004;58(2):115-123.
36. Brewerton TD. Hyperreligiosity in psychotic disorders. J Nerv Ment Dis. 1994;182(5):302-304.
37. Getz GE, Fleck DE, Strakowski SM. Frequency and severity of religious delusions in Christian patients with psychosis. Psychiatry Res. 2001;103(1):87-91.
Ultra-rapid cycling bipolar disorder: A critical look
Ultra-rapid cycling (URC) entered the psychiatric lexicon in the 1990s as a proposed descriptor for manic/hypomanic, mixed, or depressed episodes of bipolar disorder (BD) that occur every few days or weeks. DSM-IV-TR incorporates rapid cycling (RC)—but not URC—as a course specifier that occurs in 10% to 15% of patients with BD who have ≥4 distinct affective episodes per year, each fulfilling duration criteria and separated by identifiable recovery periods (unless an episode directly changes polarity). Since then, the terms RC and URC have seemingly metamorphosed into imprecise, popular colloquialisms meant to loosely describe frequent mood changes rather than distinct episodes over extended time periods, with little regard for the associated signs that define manic or hypomanic episodes.
This article examines the meaning and validity of URC in BD, its relevance and differentiation from rapid mood shifts in patients without BD, and concepts relevant to treatment extrapolated from studies of RC BD.
Imprecise nomenclature
Post et al1 coined the terms “ultra-rapid cycling” and “ultra-ultra-rapid cycling” (also called “ultradian cycling”) to describe mood episodes that occur monthly (URC) or over the course of as little as 1 day (ultradian cycling). These constructs are controversial because they lack demonstrated content validity and discriminant validity relative to other disorders. (“Content validity” refers to whether the features thought to comprise an entity of interest accurately and meaningfully do so; “discriminant validity” tells researchers and clinicians whether the proposed description of a clinical entity uniquely differentiates it from other disorders—avoiding “false-positive” suspected cases.) Clinicians therefore must pay careful attention to non-bipolar psychiatric problems that can present with rapid mood changes but without the psychomotor and related signs that define bipolar mood episodes. In their looser, nontechnical meanings, “rapid cycling” or “ultra-rapid cycling” may be synonymous with affective lability. RC is neither a diagnosis in itself nor a criterion for diagnosing BD. Rather, it is a course specifier to describe episode frequency in patients with past unambiguous manic or hypomanic episodes.
In children and adolescents, whose presentations often are atypical and can be hard to differentiate from other forms of behavioral or temperamental dysregulation, severe non-episodic mood dysregulation without signs of mania or hypomania may indicate a phenomenon separate from BD.2 Geller and colleagues3 proposed using the term “episodes” to frame the duration of a DSM-IV-defined syndrome of mania/hypomania or depression, while reserving the term “cycling” to connote patterns of mood alternation within a given episode. It is not clear whether this concept of “cycling” differs qualitatively from mood lability that arises during a mood episode in children or adults, and notably, this perspective does not account for changes in psychomotor signs in conjunction with changes in mood.
Clinicians also sometimes blur the concept of “mixed episodes” with RC or URC. DSM-IV-TR defines mixed episodes within bipolar I disorder (BD I) based on criteria for a simultaneous manic and depressive episode, rather than on frequent oscillations between affective poles. These and other differential diagnostic considerations for suspected URC are summarized in Table 1.4
A further concern regarding nomenclature involves the distinction between cyclicity (ie, successive episodes regardless of pole direction) and changes in polarity (ie, switches from depression to mania/hypomania or vice versa). Some mood disorder patients may have rapid oscillations from euthymia to depression while never changing polarity to mania/hypomania and may be best described as having recurrent brief depression.
Table 1
Differential diagnosis in suspected URC
| Phenomenon | Considerations for assessment |
|---|---|
| Mixed episodes in bipolar I disorder, or mixed depressive episodes in bipolar II disorder | DSM-IV-TR mixed episodes entail the co-occurrence of manic and depressive symptoms during the same episode without an intervening period of recovery. ICD-10 includes “rapid alternation of manic, hypomanic or depressive symptoms…from day to day or even hour to hour” in its definition of a mixed episode |
| Distress responses to acute environmental adversities (eg, adjustment disorders with mixed disturbance of emotions and conduct) | One would expect an absence of corresponding sleep-wake cycle changes or speech-language and psychomotor disturbances |
| Intoxication/withdrawal from psychoactive substances or drug-induced mental status changes (eg, corticosteroids, amphetamine, cocaine); a history of substance abuse also may be associated with development of URC in BD patients4 | Substance-induced mood fluctuations caused by intoxication/withdrawal can mimic affective cycling |
| Disinhibition states and frontal lobe syndromes as seen in traumatic brain injury and other CNS disorders, such as multiple sclerosis | Assess for signs of perseveration and history of head trauma or neurologic damage from cumulative toxic-metabolic insults (eg, chronic alcoholism) |
| Autonomic hyperarousal, emotional volatility, and hyperreactivity to environmental stresses, suggestive of PTSD | Determine the presence of a trauma history and review whether DSM-IV-TR symptoms and associated features of PTSD exist, including re-experiencing/reliving and avoidance, as well as paranoid thinking, dissociation, and nightmares |
| Recurrent mood shifts related to premenstrual dysphoric disorder may mimic URC. Other endocrine dysfunctions also may present with URC (eg, thyroid or ovarian malignancies) | Affirm the independent presence of BD before inferring its manifestations solely from premenstrual mood changes |
| Trait affective instability associated with borderline personality disorder | Trait mood instability is more chronic and enduring than episodic, and would not be expected to occur in tandem with signs of psychomotor activation that define mania/hypomania |
| BD: bipolar disorder; ICD-10: International Statistical Classification of Diseases and Related Health Problems, 10th revision; PTSD: posttraumatic stress disorder; URC: ultra-rapid cycling | |
Duration criteria
Clinicians and researchers have debated the minimum duration criteria for identifying manic or hypomanic episodes, and the extent to which suspected hypomanic periods of short duration constitute distinct illness phases. Although DSM-IV-TR designates 4 days as a minimum time for classifying an episode of hypomania, empirical studies suggest that mood symptoms lasting as few as 2 days may comprise a valid and reliably distinct entity relevant to RC.5 More limited data (mainly case observations) identify “affective oscillations” and “mood shifts” occurring faster than once per 24 hours in BD patients without comorbid personality disorders.6 Phenomenologic studies that have focused on 24- to 48-hour switch cycles have described new-onset URC arising spontaneously or following closed head injuries.7 In children and younger adolescents, reports have identified long index manic episodes (mean durations as long as 80 weeks)8 that involve continual (ultradian) mood cycling in as many as 80% of cases.9
Is URC a valid construct?
A central controversy surrounding the validity and meaningfulness of URC as a BD subtype involves its sole focus on mood variation rather than the fuller constellation of associated signs and symptoms that define episodes of mania/hypomania or depression. Abrupt, sudden, drastic, or dramatic mood shifts from one moment to the next are nowhere to be found in the DSM-IV-TR definition of BD, and the construct of mood lability or affective instability is neither a cardinal nor defining element of BD. Although individuals with BD I or bipolar II disorder (BD II) may have periods of affective lability, rapid shifts in mood are neither necessary nor sufficient for a BD diagnosis, and may indicate other types of psychopathology when affective instability occurs in the absence of a history of discernible manic or hypomanic episodes.
Studies by our group10 and others11 have shown that overattention to mood variation without considering associated cognitive, speech-language, chronobiologic, and motor signs of mania/hypomania accounts for substantial overdiagnosis of BD in patients with non-specific mood disturbances, particularly in those with active substance abuse or borderline personality disorder (BPD). Whereas the construct of RC BD attempts to account for changes in energy and psychomotor function as part of recurrent syndromes of mania/hypomania, existing literature on URC does not. Assessing mood changes in <24 hours also precludes assessing associated phenomena that occur over longer periods, such as changes in the sleep-wake cycle.
A rigorous, systematic approach to differential diagnosis for patients with affective instability is essential.
Borderline personality disorder
A common diagnostic debate regarding URC involves how to differentiate it from the chronic mood instability and reactivity inherent to BPD. Although some authors have suggested that RC BD and affective instability in BPD may be the same entity,12 others object to unifying the 2 conditions without considering their phenomenologic and other clinical differences. For example, affective instability arising from borderline character organization is thought to reflect a patient’s impaired capacity to self-regulate his or her internal state and emotional responses to interpersonal and other environmental stresses, or difficulty managing impulses. By contrast, manic or depressive phases of BD tend not to be “triggered” by interpersonal conflicts or frustrations. Furthermore, reframing intense mood reactions to the environment as bipolar variants carries several pitfalls: doing so wrongly accords patients a passive role in their reactions to life events, inaccurately reinforces a sense of victimization in response to stress, and diverts inquiry away from a patient’s active role in life decisions and circumstances that may be unsatisfying, self-defeating, or volatile.
Two key considerations may be helpful in discriminating rapid mood changes in BD vs BPD. First, some longitudinal studies indicate that RC often is a transient, rather than enduring, phenomenon in BD,13 in contrast to the nonvarying, trait feature of affective instability in persons with BPD. It is unknown whether URC is more enduring than transient. Notably, whereas bipolar mood episodes constitute deviations from a baseline state, affective instability in BPD is a baseline characteristic, rather than a deviation from it. Second, by definition, a BPD diagnosis hinges on additional elements unrelated to mood disturbances, such as interpersonal styles or defense mechanisms that involve splitting, projection, and projective identification, feelings of numbness, boredom, or emptiness, identity diffusion, fears of abandonment, and proclivities toward self-mutilation or other self-injurious behaviors as a means to alleviate tension and stress. These characteristics do not overlap with the core elements of BD.
Affective lability in patients with BPD entails prominent oscillations between anger and anxiety, or depression and anxiety, but not depression and elation14; by contrast, affective instability in BD has been linked with greater oscillations between euthymia and depression, and euthymia and elation, but not euthymia and anger.15 Moreover, daily mood fluctuations in patients with BD appear to occur in a relatively random fashion,16 whereas in BPD mood fluctuations are reactions that appear intimately linked to distressing interpersonal experiences.
See the table below, entitled “Rapid cycling and ultra-rapid cycling BD: A comparison,” comparing the phenomenology of RC and URC and a discussion of studies that explored genetic markers or family patterns that may be related to RC or URC.
Treatment considerations
No systematic studies exist for treating URC. Because most clinical trials of BD focus on treatment or prevention of a single episode rather than changes of mood over time, it is difficult to draw inferences about the ability of any treatment to attenuate marked, day-to-day mood variations. Some antimanic drugs, such as carbamazepine, have been suggested to offer better prophylactic efficacy compared with lithium for “non-classical” BD presentations, although the efficacy of carbamazepine has not been studied in URC.
Broadly speaking, treatment for URC, similar to RC, pragmatically involves:
- identifying and eliminating sources of mood destabilization (eg, substance abuse, erratic sleep patterns)
- treating medical comorbidities such as hypothyroidism
- optimizing treatment with mood stabilizing agents
- exercising caution when using antidepressants (see below).
Interestingly, despite frequent allusion to certain medications as “mood stabilizers,” no controlled study has examined mood instability on a day-to-day basis as a primary outcome measure in BD treatment, which limits the ability to surmise that any drug could be expected to diminish mood oscillations that occur over the course of days, or within a single day. However, a post hoc analysis by our group17 compared randomized treatment with lamotrigine or placebo over 6 months in RC BD I or BD II. Using prospective life charting, we found patients who received lamotrigine were almost twice as likely as those receiving placebo to achieve euthymia from one week to the next, which suggests the possibility that lamotrigine may offer benefit for affective instability in BD I or BD II patients, in addition to preventing discrete mood episodes.
Antidepressant controversy. Concerns that antidepressants might acutely induce mania or accelerate cycling frequency over long time periods have led to a contentious, long-standing debate within psychopharmacology. As noted in the box below, several long-term naturalistic follow-up studies have reported RC as a perceived consequence of antidepressants in most RC patients, although efforts to differentiate cycle acceleration caused by antidepressants (or other iatrogenic factors) from the natural course of illness remains exceedingly difficult without prospective randomized trials. (Antidepressants might cause more affective recurrences, but having multiple episodes may also cause more antidepressant prescriptions.) Some researchers (eg, Schneck et al18) have reported more frequent episodes among patients taking antidepressants but did not consider that patients with multiple episodes may be more likely to receive antidepressants, which fail to ameliorate acute or recurrent affective episodes. Importantly, a recent multi-site randomized trial by Ghaemi et al19 found that after a favorable acute response to antidepressants plus mood stabilizers, patients with preexisting RC who were randomized to continue antidepressants for up to 1 year had a 3-fold increased likelihood of developing a new depressive episode, which affirms suggestions that antidepressants do not help—but may exacerbate—cycling in patients with RC. No studies in BD have examined whether URC is more likely to arise as a consequence of antidepressant use.
Mood stabilizers and other biologic therapies. A small body of literature specifically addresses pharmacotherapy of URC in patients with BD (Table 2).19-26 A limitation of most existing literature is its focus on case reports, small open trials, or anecdotal observations rather than large, randomized controlled trials using systematic outcome measures. Extrapolation from reports focusing on patients with DSM-IV-TR RC is limited because it is uncertain whether URC differs fundamentally from RC and studies of DSM-IV-TR RC typically examine acute response during an index episode or time until relapse during maintenance therapy, rather than impact on mood changes over time.
Psychotherapy. A limited database on the efficacy of adjunctive cognitive-behavioral therapy (CBT) in RC BD describes improvement in depressive symptoms over short-term follow-up.27 No long-term studies of CBT or other structured psychotherapies have focused on RC or URC. Intuitively, one might expect that psychoeducation targeting sleep hygiene, substance use, stress management and coping skills, medication adherence, and prodrome recognition would be of value to patients with BD who experience frequent mood episodes, especially in those who may be unaware of or unfamiliar with basic concepts related to BD. In addition, relevant concepts from dialectical behavior therapy may be beneficial for BD patients with possible URC, such as skills to enhance emotional regulation, distress tolerance, mindfulness, and interpersonal effectiveness.
Table 2
Evidence-based treatments for ultra-rapid cycling BD
| Intervention | Strength of evidence | Comment |
|---|---|---|
| Antidepressant elimination | Cycling frequency may lengthen during antidepressant-free periods among patients with RC20; long-term (up to 1 year) antidepressant use in RC patients may increase the likelihood of depressive recurrences19 | Findings based mostly on small sample sizes; no controlled trials of antidepressant cessation as an intervention specifically for URC |
| Lithium | Single case report of ECT-induced URC resolved by lithium augmentation during continued ECT22 | No large-scale or randomized trials |
| Carbamazepine | No controlled trials or case reports | Possible anti-cycling benefits relevant for URC could be inferred from post hoc studies among patients with RC |
| Divalproex | Single case report describing resolution of a 48-hour cycle after augmentation of lithium with divalproex23 | No large-scale or randomized trials |
| Lamotrigine | Single case report of 100 mg/d lamotrigine augmentation to divalproex yielded 8 months of remission in a 25-year-old man with BD II and a long-standing pattern of 3 days of hypomania followed by 5 days of depression24 | No large-scale or randomized trials |
| Topiramate | Single case report in URC describing reduction of cycling frequency over 3 years25 | Multiple large scale placebo-controlled studies in bipolar mania have been negative |
| Second-generation antipsychotics | No controlled trials or case reports | Possible anti-cycling benefits relevant for URC could be inferred from post hoc studies in RC |
| Combinations of ≥2 mood stabilizing drugs | No controlled trials or case reports | Combining multiple anti-cycling agents is intuitively logical but largely unstudied |
| Nimodipine | 1 unipolar and 11 BD patients treated in randomized, off-on-off-on fashion (begun at 90 mg/d, increased up to 720 mg/d, mean duration of 12 weeks on active drug)26 | Response in 5 of 9 completers. Findings await replication with larger sample sizes |
| Hypermetabolic thyroid hormone (levothyroxine) | Findings from a small (N = 11) study of adjunctive high-dose levothyroxine (0.15 to 0.4 mg/d, with dosages increased by 0.05 to 0.1 mg/d every 1 to 2 weeks); an unspecified subgroup had “a very rapid cycling pattern” (reviewed by Bauer et al21) | 10 of 11 RC patients had reductions in depressive symptoms, 5 of 7 had improvement from baseline manic symptoms (observation period >60 days) |
| ECT | Case reports of improvement with ECT in refractory RC that was presumed secondary to tricyclic antidepressants | Reports of induction of URC by ECT22; whether or not ECT would more likely improve or exacerbate cyclicity for a given patient may require empirical determination |
| BD: bipolar disorder; BD II: bipolar II disorder; ECT: electroconvulsive therapy; RC: rapid cycling; URC: ultra-rapid cycling | ||
Treatment monitoring. Prospective life charting allows patients to systematically record manic/hypomanic and depressive symptoms day-to-day and week-to-week, thus creating a measure that may be particularly relevant for patients whose moods change rapidly. Simple mood charts (see Related Resources) typically take into account the severity of symptoms of either polarity with ratings of mild, moderate, or severe. Such visual records permit simple calculations over the course of a given interval (eg, week-by-week or across months) of several important parameters, including:
- number of days euthymic
- number of days with depression
- number of days with abnormal mood elevation
- number of occasions in which moods of both polarities occur on the same day.
Tracking these parameters during a treatment allows clinicians to make quantitative comparisons over time as a method of determining whether or not meaningful changes are occurring in cyclicity. See the figure below for an example of a completed mood chart and its interpretation.
Additional recommendations for assessing and managing cyclicity in BD are summarized in Table 3.
Table 3
Tips for managing suspected ultra-rapid cycling BD
| Do’s | Don’ts |
|---|---|
| Ascertain a history of ≥1 lifetime manic or hypomanic episode to diagnose BD | Diagnose BD solely on the presence of rapid mood fluctuations |
| Determine the presence of changes in sleep, energy, speech-language, and related behavior as correlates of mood to differentiate syndromes from isolated variation in mood | Ignore constellations of associated signs and symptoms of mania/hypomania |
| Obtain patient history to assess for head trauma or other medical and neurologic events that could have affective or other psychiatric manifestations | Disregard possible medical etiologies for new-onset affective dysregulation |
| Ascertain the resolution of 1 episode before counting the resurgence of symptoms as constituting a new episode; a waxing and waning course may reflect illness chronicity with incomplete recovery rather than true cyclicity | Misidentify incomplete recovery from an existing episode as the occurrence of new multiple episodes, which would inflate false-positive cases of RC or URC |
| Advise patients to refrain from alcohol or illicit substances that could destabilize mood | Assume that comorbid alcohol or illicit substance abuse will remit only after mood stabilization has been achieved, rather than the reverse |
| Monitor changes in sleep-wake cycles and the effects of erratic sleep or sleep deprivation on mood | Ignore the effects of poor sleep hygiene on mood |
| Minimize antidepressant exposure in patients with RC or URC | Continue long-term antidepressant maintenance therapy in patients with manic or mixed features or ongoing oscillations between mania/hypomania and depression |
| Assure euthyroid status and consider the potential utility of hypermetabolic levothyroxine | Assume that RC or URC will resolve solely by normalizing or optimizing thyroid function |
| Use rational, pharmacodynamically nonredundant anti-cycling drugs | Ignore the cumulative burden of adverse effects of multiple drugs |
| Consider the potential role for ECT as a strategy to arrest URC during any phase of BD | Assume ECT has value only during acute depressive phases of BD |
| Use prospective mood charting to document the evolution of mood changes over time, particularly when gauging treatment efficacy | Rely solely on impressionistic recall of mood states or polarity changes as reflecting distinct phasic changes |
| BD: bipolar disorder; ECT: electroconvulsive therapy; RC: rapid cycling; URC: ultra-rapid cycling | |
Table
Rapid cycling and ultra-rapid cycling BD: A comparison
| Construct | Rapid cycling | Ultra-rapid cycling |
|---|---|---|
| Bipolar I vs II | Predominantly BD IIa | No systematic data |
| Sex | Predominantly women | No systematic data |
| Longitudinal course | May be a transient phenomenon that can occur at any timeb or an enduring phenomenon that may persist for yearsc | Ultradian patterns may be more common across the first several episodes among pediatric BD patientsd |
| Age at onset | Associated with younger age at onsete | May be more evident in prepubescent onset mood disordersd; ultradian cycling more likely when onset occurs before age 13 than in adulthoode |
| Diurnal variation in mood | Morning-to-evening mood switches usually involve depression to mania/hypomania, with the opposite typifying evening-to-morning mood switchesf | Not reported |
| Relationship to environmental stresses | Life stresses may precede initial affective episodes but may be less important as subsequent episodes arise with increasing automaticity | No systematic data |
| Relationship to menstrual cycle | Despite case reports and self-reported links between RC and menstrual mood exacerbations, prospective data do not identify associations between RC and menstrual patternsg,h | No systematic data |
| Subclinical hypothyroidism | Bauer and Whybrow identified hypothyroidism independent of lithium use in 60% of 30 rapidly cycling BD patients, with evidence of improvement in a separate study of 11 RC patients given suprametabolic levothyroxine (reviewed by Bauer et ali) | No systematic data |
| Relationship to psychosis | Nonea | No systematic data |
| Relationship to antidepressant use | Naturalistic observations suggest RC may occur later in the illness course as a result of antidepressant use.c Small open case series data suggest shorter intermorbid intervals on antidepressants with longer intervals off antidepressants.j RC patients often receive antidepressants, but causal relationships are not well-documented.k Some case-control data dispute links between antidepressant use and RCl | No specific published cases |
| Considerations for suicide risk | RC linked with more serious suicide attemptsl | Suicide attempts may be associated with cycling within an episodem or rapid shifting in moodn |
| Time course for judging treatment efficacy | Efforts to diminish acute affective instability may be measured over the course of days to weeks | By definition, treatment of RC involves relapse prevention over the course of 1 year |
| BD: bipolar disorder; RC: rapid cycling | ||
| References a. Schneck CD, Miklowitz DJ, Calabrese JR, et al. Phenomenology of rapid-cycling bipolar disorder: data from the first 500 participants in the Systematic Treatment Enhancement Program. Am J Psychiatry. 2004;161(10):1902-1908. b. Coryell W, Endicott J, Keller M. Rapidly cycling affective disorder. Demographics, diagnosis, family history, and course. Arch Gen Psychiatry. 1992;49(2):126-131. c. Koukopoulos A, Sani G, Koukopoulos AE, et al. Duration and stability of the rapid-cycling course: a long-term personal follow-up of 109 patients. J Affect Disord. 2003;73(1-2):75-85. d. Geller B, Tillman R, Bolhofner K, et al. Child bipolar I disorder: prospective continuity with adult bipolar I disorder; characteristics of second and third episodes; predictors of 8-year outcome. Arch Gen Psychiatry. 2008;65(10):1125-1133. e. Post RM, Leverich GS, Kupka RW, et al. Early-onset bipolar disorder and treatment delay are risk factors for poor outcome in adulthood. J Clin Psychiatry. 2010;71(7):864-872. f. Feldman-Naim S, Turner EH, Leibenluft E. Diurnal variation in the direction of mood switches in patients with rapid-cycling bipolar disorder. J Clin Psychiatry. 1997;58(2):79-84. g. Leibenluft E, Ashman SB, Feldman-Naim S, et al. Lack of relationship between menstrual cycle phase and mood in a sample of women with rapid cycling bipolar disorder. Biol Psychiatry. 1999;46(4):577-580. h. Wehr TA, Sack DA, Rosenthal NE, et al. Rapid cycling affective disorder: contributing factors and treatment responses in 51 patients. Am J Psychiatry. 1988;145(2):179-184. i. Bauer M, Beaulieu S, Dunner DL, et al. Rapid cycling bipolar disorder—diagnostic concepts. Bipolar Disord. 2008;10(1 Pt 2):153-162. j. Wehr TA, Goodwin FK. Can antidepressants cause mania and worsen the course of affective illness? Am J Psychiatry. 1987;144(11):1403-1411. k. Schneck CD, Miklowitz DJ, Miyahara S, et al. The prospective course of rapid-cycling bipolar disorder: findings from the STEP-BD. Am J Psychiatry. 2008;165(3):370-377. l. Coryell W, Solomon D, Turvey C, et al. The long-term course of rapid-cycling bipolar disorder. Arch Gen Psychiatry. 2003;60(9):914-920. m. Fawcett J, Scheftner W, Clark D, et al. Clinical predictors of suicide in patients with major affective disorders: a controlled prospective study. Am J Psychiatry. 1987;144(1):35-40. n. MacKinnon DF, Potash JB, McMahon FJ, et al. Rapid mood switching and suicidality in familial bipolar disorder. Bipolar Disord. 2005;7(5):441-448. | ||
From a biologic perspective, a handful of preliminary studies have examined genetic markers or familial patterns that might be related to rapid cycling (RC) or ultra-rapid cycling (URC). These include a reported link between URC and the low activity variant of the catechol-o-methyltransferase gene polymorphism in a small group of patients with velo-cardio-facial syndrome,a although this finding was not replicated in a larger sample.a Other preliminary reports on RC have implicated both the long (l) and short (s) allelic variants of the serotonin transporter gene (SLC6A4), the val66met variant of the brain-derived neurotrophic factor gene, and the circadian cryptochrome 2 (CRY2) gene (reviewed by Bauer et alb). These candidate loci have been examined in RC but not URC.
URC has not been examined as a familial entity, although in the National Institute of Mental Health Collaborative Depression Study, DSM-IV-TR RC did not occur with elevated frequency in bipolar pedigrees.c Rapid mood switches—abrupt rather than gradual transitions from one affective pole to another—appear to be only slightly, nonsignificantly more common in first-degree bipolar relatives of BD patients who themselves have rapid rather than gradual transitions from one affective pole to the other.d
Neuroimaging studies in BD seldom focus on subpopulations with RC or URC, and have been confined mainly to case reports that have yielded limited, non-generalizable observations, such as state-dependent variations in prefrontal activity during tasks of facial recognition (reviewed by Bauer et alb).
References
a. Papolos DF, Veit S, Faedda GL, et al. Ultra-ultra rapid cycling bipolar disorder is associated with the low activity catecholamine-O-methyltransferase allele. Mol Psychiatry. 1998;3(4):346-349.
b. Bauer M, Beaulieu S, Dunner DL, et al. Rapid cycling bipolar disorder—diagnostic concepts. Bipolar Disord. 2008;10(1 Pt 2):153-162.
c. Coryell W, Endicott J, Keller M. Rapidly cycling affective disorder. Demographics, diagnosis, family history, and course. Arch Gen Psychiatry. 1992;49(2):126-131.
d. MacKinnon DF, Potash JB, McMahon FJ, et al. Rapid mood switching and suicidality in familial bipolar disorder. Bipolar Disord. 2005;7(5):441-448.
Figure: Example of prospective mood charting to document changes in manic/hypomanic and depressive symptoms across time
In the above example, the prevalence and severity of mood symptoms are identified over 21 days. Note the distinctly separate phases of mood elevation followed by depression, whereas euthymia was present only 6 of 21 days (29% of the time). Symptoms of at least moderate severity were more prominently depressive (5 of 21 days, or 24% of the time) than manic/hypomanic (2 of 21 days, or approximately 10% of the time). Mood charting does not capture associate DSM-IV-TR criteria for a mood episode but instead focuses solely on longitudinal changes in mood elevation or depression
Related Resources
- American Psychiatric Association practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(4 suppl):1-50.
- Massachusetts General Hospital Bipolar Clinic and Research Program. Sample mood chart (downloadable). www.manicdepressive.org/moodchart.html.
Drug Brand Names
- Carbamazepine • Equetro, Tegretol
- Divalproex • Depakote
- Lamotrigine • Lamictal
- Levothyroxine • Synthroid
- Lithium • Lithobid
- Nimodipine • Nimotop
- Topiramate • Topamax
Disclosures
Dr. Goldberg is on the speakers’ bureaus for AstraZeneca, Dey Pharmaceuticals, Eli Lilly and Company, Merck, and Sunovion and is a consultant for Axon Advisors, Dey Pharmaceuticals, Eli Lilly and Company, and Grünenthal Group.
Acknowledgment
The author wishes to thank David L. Dunner, MD, for his helpful comments regarding this article.
1. Kramlinger KG, Post RM. Ultra-rapid and ultradian cycling in bipolar affective illness. Br J Psychiatry. 1996;168(3):314-323.
2. Leibenluft E. Severe mood dysregulation irritability, and the diagnostic boundaries of bipolar disorder in youths. Am J Psychiatry. 2011;168(2):129-142.
3. Geller B, Tillman R, Bolhofner K. Proposed definitions of bipolar I disorder episodes and daily rapid cycling phenomena in preschoolers school-aged children, adolescents, and adults. J Child Adolesc Psychopharmacol. 2007;17(2):217-222.
4. Feinman JA, Dunner DL. The effect of alcohol and substance abuse on the course of bipolar affective disorder. J Affect Disord. 1996;37(1):43-49.
5. Maj M, Pirozzi R, Formicola AM, et al. Reliability and validity of four alternative definitions of rapid-cycling bipolar disorder. Am J Psychiatry. 1999;156(9):1421-1424.
6. Jenner FA, Gjessing LR, Cox JR, et al. A manic depressive psychotic with a persistent forty-eight hour cycle. Br J Psychiatry. 1967;113(501):895-910.
7. Zwil AS, McAllister TW, Cohen I, et al. Ultra-rapid cycling bipolar affective disorder following a closed-head injury. Brain Inj. 1993;7(2):147-152.
8. Geller B, Tillman R, Craney JL, et al. Four-year prospective outcome and natural history of mania in children with a prepubertal and early adolescent bipolar disorder phenotype. Arch Gen Psychiatry. 2004;61(5):459-467.
9. Geller B, Sun K, Zimerman B, et al. Complex and rapid-cycling in bipolar children and adolescents: a preliminary study. J Affect Disord. 1995;34(4):259-268.
10. Goldberg JF, Garno JL, Callahan AM, et al. Overdiagnosis of bipolar disorder among substance use disorder inpatients with mood instability. J Clin Psychiatry. 2008;69(11):1751-1757.
11. Zimmerman M, Ruggero CJ, Chelminski I, et al. Psychiatric diagnoses in patients previously overdiagnosed with bipolar disorder. J Clin Psychiatry. 2010;71(1):26-31.
12. MacKinnon DF, Pies R. Affective instability as rapid cycling: theoretical and clinical implications for borderline personality and bipolar spectrum disorders. Bipolar Disord. 2006;8(1):1-14.
13. Coryell W, Endicott J, Keller M. Rapidly cycling affective disorder. Demographics diagnosis, family history, and course. Arch Gen Psychiatry. 1992;49(2):126-131.
14. Koenigsberg HW, Harvey PD, Mitropoulou V, et al. Characterizing affective instability in borderline personality disorder. Am J Psychiatry. 2002;159(5):784-788.
15. Henry C, Mitropoulou V, New AS, et al. Affective instability and impulsivity in borderline personality and bipolar II disorders: similarities and differences. J Psychiatr Res. 2001;35(6):307-312.
16. Gottschalk A, Bauer MS, Whybrow PC. Evidence of chaotic mood variation in bipolar disorder. Arch Gen Psychiatry. 1995;52(11):947-959.
17. Goldberg JF, Bowden CL, Calabrese JR, et al. Six-month prospective life charting of mood symptoms with lamotrigine monotherapy versus placebo in rapid cycling bipolar disorder. Biol Psychiatry. 2008;63(1):125-130.
18. Schneck CD, Miklowitz DJ, Miyahara S, et al. The prospective course of rapid-cycling bipolar disorder: findings from the STEP-BD. Am J Psychiatry. 2008;165(3):370-377.
19. Ghaemi SN, Ostacher MM, El-Mallakh RS, et al. Antidepressant discontinuation in bipolar depression: a Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) randomized clinical trial of long-term effectiveness and safety. J Clin Psychiatry. 2010;71(4):372-380.
20. Wehr TA, Sack DA, Rosenthal NE, et al. Rapid cycling affective disorder: contributing factors and treatment responses in 51 patients. Am J Psychiatry. 1988;145(2):179-184.
21. Bauer M, Beaulieu S, Dunner DL, et al. Rapid cycling bipolar disorder—diagnostic concepts. Bipolar Disord. 2008;10(1 Pt 2):153-162.
22. Zavorotnyy M, Diemer J, Patzelt J, et al. Occurrence of ultra-rapid cycling during electroconvulsive therapy in bipolar depression. World J Biol Psychiatry. 2009;10(4 Pt 3):987-990.
23. Lepkifker E, Iancu I, Dannon P, et al. Valproic acid in ultra-rapid cycling: a case report. Clin Neuropharmacol. 1995;18(1):72-75.
24. Woo YS, Chae JH, Jun TY, et al. Lamotrigine added to valproate successfully treated a case of ultra-rapid cycling bipolar disorder. Psychiatry Clin Neurosci. 2007;61(1):130-131.
25. Karama S, Lal S. Adjunctive topiramate in ultradian cycling bipolar disorder: case report with 3-year follow-up. Eur Psychiatry. 2006;21(4):280-281.
26. Pazzaglia PJ, Post RM, Ketter TA, et al. Preliminary controlled trial of nimodipine in ultra-rapid cycling affective dysregulation. Psychiatry Res. 1993;49(3):257-272.
27. Reilly-Harrington NA, Deckersbach T, Knauz R, et al. Cognitive behavioral therapy for rapid-cycling bipolar disorder: a pilot study. J Psychiatr Pract. 2007;13(5):291-297.
Ultra-rapid cycling (URC) entered the psychiatric lexicon in the 1990s as a proposed descriptor for manic/hypomanic, mixed, or depressed episodes of bipolar disorder (BD) that occur every few days or weeks. DSM-IV-TR incorporates rapid cycling (RC)—but not URC—as a course specifier that occurs in 10% to 15% of patients with BD who have ≥4 distinct affective episodes per year, each fulfilling duration criteria and separated by identifiable recovery periods (unless an episode directly changes polarity). Since then, the terms RC and URC have seemingly metamorphosed into imprecise, popular colloquialisms meant to loosely describe frequent mood changes rather than distinct episodes over extended time periods, with little regard for the associated signs that define manic or hypomanic episodes.
This article examines the meaning and validity of URC in BD, its relevance and differentiation from rapid mood shifts in patients without BD, and concepts relevant to treatment extrapolated from studies of RC BD.
Imprecise nomenclature
Post et al1 coined the terms “ultra-rapid cycling” and “ultra-ultra-rapid cycling” (also called “ultradian cycling”) to describe mood episodes that occur monthly (URC) or over the course of as little as 1 day (ultradian cycling). These constructs are controversial because they lack demonstrated content validity and discriminant validity relative to other disorders. (“Content validity” refers to whether the features thought to comprise an entity of interest accurately and meaningfully do so; “discriminant validity” tells researchers and clinicians whether the proposed description of a clinical entity uniquely differentiates it from other disorders—avoiding “false-positive” suspected cases.) Clinicians therefore must pay careful attention to non-bipolar psychiatric problems that can present with rapid mood changes but without the psychomotor and related signs that define bipolar mood episodes. In their looser, nontechnical meanings, “rapid cycling” or “ultra-rapid cycling” may be synonymous with affective lability. RC is neither a diagnosis in itself nor a criterion for diagnosing BD. Rather, it is a course specifier to describe episode frequency in patients with past unambiguous manic or hypomanic episodes.
In children and adolescents, whose presentations often are atypical and can be hard to differentiate from other forms of behavioral or temperamental dysregulation, severe non-episodic mood dysregulation without signs of mania or hypomania may indicate a phenomenon separate from BD.2 Geller and colleagues3 proposed using the term “episodes” to frame the duration of a DSM-IV-defined syndrome of mania/hypomania or depression, while reserving the term “cycling” to connote patterns of mood alternation within a given episode. It is not clear whether this concept of “cycling” differs qualitatively from mood lability that arises during a mood episode in children or adults, and notably, this perspective does not account for changes in psychomotor signs in conjunction with changes in mood.
Clinicians also sometimes blur the concept of “mixed episodes” with RC or URC. DSM-IV-TR defines mixed episodes within bipolar I disorder (BD I) based on criteria for a simultaneous manic and depressive episode, rather than on frequent oscillations between affective poles. These and other differential diagnostic considerations for suspected URC are summarized in Table 1.4
A further concern regarding nomenclature involves the distinction between cyclicity (ie, successive episodes regardless of pole direction) and changes in polarity (ie, switches from depression to mania/hypomania or vice versa). Some mood disorder patients may have rapid oscillations from euthymia to depression while never changing polarity to mania/hypomania and may be best described as having recurrent brief depression.
Table 1
Differential diagnosis in suspected URC
| Phenomenon | Considerations for assessment |
|---|---|
| Mixed episodes in bipolar I disorder, or mixed depressive episodes in bipolar II disorder | DSM-IV-TR mixed episodes entail the co-occurrence of manic and depressive symptoms during the same episode without an intervening period of recovery. ICD-10 includes “rapid alternation of manic, hypomanic or depressive symptoms…from day to day or even hour to hour” in its definition of a mixed episode |
| Distress responses to acute environmental adversities (eg, adjustment disorders with mixed disturbance of emotions and conduct) | One would expect an absence of corresponding sleep-wake cycle changes or speech-language and psychomotor disturbances |
| Intoxication/withdrawal from psychoactive substances or drug-induced mental status changes (eg, corticosteroids, amphetamine, cocaine); a history of substance abuse also may be associated with development of URC in BD patients4 | Substance-induced mood fluctuations caused by intoxication/withdrawal can mimic affective cycling |
| Disinhibition states and frontal lobe syndromes as seen in traumatic brain injury and other CNS disorders, such as multiple sclerosis | Assess for signs of perseveration and history of head trauma or neurologic damage from cumulative toxic-metabolic insults (eg, chronic alcoholism) |
| Autonomic hyperarousal, emotional volatility, and hyperreactivity to environmental stresses, suggestive of PTSD | Determine the presence of a trauma history and review whether DSM-IV-TR symptoms and associated features of PTSD exist, including re-experiencing/reliving and avoidance, as well as paranoid thinking, dissociation, and nightmares |
| Recurrent mood shifts related to premenstrual dysphoric disorder may mimic URC. Other endocrine dysfunctions also may present with URC (eg, thyroid or ovarian malignancies) | Affirm the independent presence of BD before inferring its manifestations solely from premenstrual mood changes |
| Trait affective instability associated with borderline personality disorder | Trait mood instability is more chronic and enduring than episodic, and would not be expected to occur in tandem with signs of psychomotor activation that define mania/hypomania |
| BD: bipolar disorder; ICD-10: International Statistical Classification of Diseases and Related Health Problems, 10th revision; PTSD: posttraumatic stress disorder; URC: ultra-rapid cycling | |
Duration criteria
Clinicians and researchers have debated the minimum duration criteria for identifying manic or hypomanic episodes, and the extent to which suspected hypomanic periods of short duration constitute distinct illness phases. Although DSM-IV-TR designates 4 days as a minimum time for classifying an episode of hypomania, empirical studies suggest that mood symptoms lasting as few as 2 days may comprise a valid and reliably distinct entity relevant to RC.5 More limited data (mainly case observations) identify “affective oscillations” and “mood shifts” occurring faster than once per 24 hours in BD patients without comorbid personality disorders.6 Phenomenologic studies that have focused on 24- to 48-hour switch cycles have described new-onset URC arising spontaneously or following closed head injuries.7 In children and younger adolescents, reports have identified long index manic episodes (mean durations as long as 80 weeks)8 that involve continual (ultradian) mood cycling in as many as 80% of cases.9
Is URC a valid construct?
A central controversy surrounding the validity and meaningfulness of URC as a BD subtype involves its sole focus on mood variation rather than the fuller constellation of associated signs and symptoms that define episodes of mania/hypomania or depression. Abrupt, sudden, drastic, or dramatic mood shifts from one moment to the next are nowhere to be found in the DSM-IV-TR definition of BD, and the construct of mood lability or affective instability is neither a cardinal nor defining element of BD. Although individuals with BD I or bipolar II disorder (BD II) may have periods of affective lability, rapid shifts in mood are neither necessary nor sufficient for a BD diagnosis, and may indicate other types of psychopathology when affective instability occurs in the absence of a history of discernible manic or hypomanic episodes.
Studies by our group10 and others11 have shown that overattention to mood variation without considering associated cognitive, speech-language, chronobiologic, and motor signs of mania/hypomania accounts for substantial overdiagnosis of BD in patients with non-specific mood disturbances, particularly in those with active substance abuse or borderline personality disorder (BPD). Whereas the construct of RC BD attempts to account for changes in energy and psychomotor function as part of recurrent syndromes of mania/hypomania, existing literature on URC does not. Assessing mood changes in <24 hours also precludes assessing associated phenomena that occur over longer periods, such as changes in the sleep-wake cycle.
A rigorous, systematic approach to differential diagnosis for patients with affective instability is essential.
Borderline personality disorder
A common diagnostic debate regarding URC involves how to differentiate it from the chronic mood instability and reactivity inherent to BPD. Although some authors have suggested that RC BD and affective instability in BPD may be the same entity,12 others object to unifying the 2 conditions without considering their phenomenologic and other clinical differences. For example, affective instability arising from borderline character organization is thought to reflect a patient’s impaired capacity to self-regulate his or her internal state and emotional responses to interpersonal and other environmental stresses, or difficulty managing impulses. By contrast, manic or depressive phases of BD tend not to be “triggered” by interpersonal conflicts or frustrations. Furthermore, reframing intense mood reactions to the environment as bipolar variants carries several pitfalls: doing so wrongly accords patients a passive role in their reactions to life events, inaccurately reinforces a sense of victimization in response to stress, and diverts inquiry away from a patient’s active role in life decisions and circumstances that may be unsatisfying, self-defeating, or volatile.
Two key considerations may be helpful in discriminating rapid mood changes in BD vs BPD. First, some longitudinal studies indicate that RC often is a transient, rather than enduring, phenomenon in BD,13 in contrast to the nonvarying, trait feature of affective instability in persons with BPD. It is unknown whether URC is more enduring than transient. Notably, whereas bipolar mood episodes constitute deviations from a baseline state, affective instability in BPD is a baseline characteristic, rather than a deviation from it. Second, by definition, a BPD diagnosis hinges on additional elements unrelated to mood disturbances, such as interpersonal styles or defense mechanisms that involve splitting, projection, and projective identification, feelings of numbness, boredom, or emptiness, identity diffusion, fears of abandonment, and proclivities toward self-mutilation or other self-injurious behaviors as a means to alleviate tension and stress. These characteristics do not overlap with the core elements of BD.
Affective lability in patients with BPD entails prominent oscillations between anger and anxiety, or depression and anxiety, but not depression and elation14; by contrast, affective instability in BD has been linked with greater oscillations between euthymia and depression, and euthymia and elation, but not euthymia and anger.15 Moreover, daily mood fluctuations in patients with BD appear to occur in a relatively random fashion,16 whereas in BPD mood fluctuations are reactions that appear intimately linked to distressing interpersonal experiences.
See the table below, entitled “Rapid cycling and ultra-rapid cycling BD: A comparison,” comparing the phenomenology of RC and URC and a discussion of studies that explored genetic markers or family patterns that may be related to RC or URC.
Treatment considerations
No systematic studies exist for treating URC. Because most clinical trials of BD focus on treatment or prevention of a single episode rather than changes of mood over time, it is difficult to draw inferences about the ability of any treatment to attenuate marked, day-to-day mood variations. Some antimanic drugs, such as carbamazepine, have been suggested to offer better prophylactic efficacy compared with lithium for “non-classical” BD presentations, although the efficacy of carbamazepine has not been studied in URC.
Broadly speaking, treatment for URC, similar to RC, pragmatically involves:
- identifying and eliminating sources of mood destabilization (eg, substance abuse, erratic sleep patterns)
- treating medical comorbidities such as hypothyroidism
- optimizing treatment with mood stabilizing agents
- exercising caution when using antidepressants (see below).
Interestingly, despite frequent allusion to certain medications as “mood stabilizers,” no controlled study has examined mood instability on a day-to-day basis as a primary outcome measure in BD treatment, which limits the ability to surmise that any drug could be expected to diminish mood oscillations that occur over the course of days, or within a single day. However, a post hoc analysis by our group17 compared randomized treatment with lamotrigine or placebo over 6 months in RC BD I or BD II. Using prospective life charting, we found patients who received lamotrigine were almost twice as likely as those receiving placebo to achieve euthymia from one week to the next, which suggests the possibility that lamotrigine may offer benefit for affective instability in BD I or BD II patients, in addition to preventing discrete mood episodes.
Antidepressant controversy. Concerns that antidepressants might acutely induce mania or accelerate cycling frequency over long time periods have led to a contentious, long-standing debate within psychopharmacology. As noted in the box below, several long-term naturalistic follow-up studies have reported RC as a perceived consequence of antidepressants in most RC patients, although efforts to differentiate cycle acceleration caused by antidepressants (or other iatrogenic factors) from the natural course of illness remains exceedingly difficult without prospective randomized trials. (Antidepressants might cause more affective recurrences, but having multiple episodes may also cause more antidepressant prescriptions.) Some researchers (eg, Schneck et al18) have reported more frequent episodes among patients taking antidepressants but did not consider that patients with multiple episodes may be more likely to receive antidepressants, which fail to ameliorate acute or recurrent affective episodes. Importantly, a recent multi-site randomized trial by Ghaemi et al19 found that after a favorable acute response to antidepressants plus mood stabilizers, patients with preexisting RC who were randomized to continue antidepressants for up to 1 year had a 3-fold increased likelihood of developing a new depressive episode, which affirms suggestions that antidepressants do not help—but may exacerbate—cycling in patients with RC. No studies in BD have examined whether URC is more likely to arise as a consequence of antidepressant use.
Mood stabilizers and other biologic therapies. A small body of literature specifically addresses pharmacotherapy of URC in patients with BD (Table 2).19-26 A limitation of most existing literature is its focus on case reports, small open trials, or anecdotal observations rather than large, randomized controlled trials using systematic outcome measures. Extrapolation from reports focusing on patients with DSM-IV-TR RC is limited because it is uncertain whether URC differs fundamentally from RC and studies of DSM-IV-TR RC typically examine acute response during an index episode or time until relapse during maintenance therapy, rather than impact on mood changes over time.
Psychotherapy. A limited database on the efficacy of adjunctive cognitive-behavioral therapy (CBT) in RC BD describes improvement in depressive symptoms over short-term follow-up.27 No long-term studies of CBT or other structured psychotherapies have focused on RC or URC. Intuitively, one might expect that psychoeducation targeting sleep hygiene, substance use, stress management and coping skills, medication adherence, and prodrome recognition would be of value to patients with BD who experience frequent mood episodes, especially in those who may be unaware of or unfamiliar with basic concepts related to BD. In addition, relevant concepts from dialectical behavior therapy may be beneficial for BD patients with possible URC, such as skills to enhance emotional regulation, distress tolerance, mindfulness, and interpersonal effectiveness.
Table 2
Evidence-based treatments for ultra-rapid cycling BD
| Intervention | Strength of evidence | Comment |
|---|---|---|
| Antidepressant elimination | Cycling frequency may lengthen during antidepressant-free periods among patients with RC20; long-term (up to 1 year) antidepressant use in RC patients may increase the likelihood of depressive recurrences19 | Findings based mostly on small sample sizes; no controlled trials of antidepressant cessation as an intervention specifically for URC |
| Lithium | Single case report of ECT-induced URC resolved by lithium augmentation during continued ECT22 | No large-scale or randomized trials |
| Carbamazepine | No controlled trials or case reports | Possible anti-cycling benefits relevant for URC could be inferred from post hoc studies among patients with RC |
| Divalproex | Single case report describing resolution of a 48-hour cycle after augmentation of lithium with divalproex23 | No large-scale or randomized trials |
| Lamotrigine | Single case report of 100 mg/d lamotrigine augmentation to divalproex yielded 8 months of remission in a 25-year-old man with BD II and a long-standing pattern of 3 days of hypomania followed by 5 days of depression24 | No large-scale or randomized trials |
| Topiramate | Single case report in URC describing reduction of cycling frequency over 3 years25 | Multiple large scale placebo-controlled studies in bipolar mania have been negative |
| Second-generation antipsychotics | No controlled trials or case reports | Possible anti-cycling benefits relevant for URC could be inferred from post hoc studies in RC |
| Combinations of ≥2 mood stabilizing drugs | No controlled trials or case reports | Combining multiple anti-cycling agents is intuitively logical but largely unstudied |
| Nimodipine | 1 unipolar and 11 BD patients treated in randomized, off-on-off-on fashion (begun at 90 mg/d, increased up to 720 mg/d, mean duration of 12 weeks on active drug)26 | Response in 5 of 9 completers. Findings await replication with larger sample sizes |
| Hypermetabolic thyroid hormone (levothyroxine) | Findings from a small (N = 11) study of adjunctive high-dose levothyroxine (0.15 to 0.4 mg/d, with dosages increased by 0.05 to 0.1 mg/d every 1 to 2 weeks); an unspecified subgroup had “a very rapid cycling pattern” (reviewed by Bauer et al21) | 10 of 11 RC patients had reductions in depressive symptoms, 5 of 7 had improvement from baseline manic symptoms (observation period >60 days) |
| ECT | Case reports of improvement with ECT in refractory RC that was presumed secondary to tricyclic antidepressants | Reports of induction of URC by ECT22; whether or not ECT would more likely improve or exacerbate cyclicity for a given patient may require empirical determination |
| BD: bipolar disorder; BD II: bipolar II disorder; ECT: electroconvulsive therapy; RC: rapid cycling; URC: ultra-rapid cycling | ||
Treatment monitoring. Prospective life charting allows patients to systematically record manic/hypomanic and depressive symptoms day-to-day and week-to-week, thus creating a measure that may be particularly relevant for patients whose moods change rapidly. Simple mood charts (see Related Resources) typically take into account the severity of symptoms of either polarity with ratings of mild, moderate, or severe. Such visual records permit simple calculations over the course of a given interval (eg, week-by-week or across months) of several important parameters, including:
- number of days euthymic
- number of days with depression
- number of days with abnormal mood elevation
- number of occasions in which moods of both polarities occur on the same day.
Tracking these parameters during a treatment allows clinicians to make quantitative comparisons over time as a method of determining whether or not meaningful changes are occurring in cyclicity. See the figure below for an example of a completed mood chart and its interpretation.
Additional recommendations for assessing and managing cyclicity in BD are summarized in Table 3.
Table 3
Tips for managing suspected ultra-rapid cycling BD
| Do’s | Don’ts |
|---|---|
| Ascertain a history of ≥1 lifetime manic or hypomanic episode to diagnose BD | Diagnose BD solely on the presence of rapid mood fluctuations |
| Determine the presence of changes in sleep, energy, speech-language, and related behavior as correlates of mood to differentiate syndromes from isolated variation in mood | Ignore constellations of associated signs and symptoms of mania/hypomania |
| Obtain patient history to assess for head trauma or other medical and neurologic events that could have affective or other psychiatric manifestations | Disregard possible medical etiologies for new-onset affective dysregulation |
| Ascertain the resolution of 1 episode before counting the resurgence of symptoms as constituting a new episode; a waxing and waning course may reflect illness chronicity with incomplete recovery rather than true cyclicity | Misidentify incomplete recovery from an existing episode as the occurrence of new multiple episodes, which would inflate false-positive cases of RC or URC |
| Advise patients to refrain from alcohol or illicit substances that could destabilize mood | Assume that comorbid alcohol or illicit substance abuse will remit only after mood stabilization has been achieved, rather than the reverse |
| Monitor changes in sleep-wake cycles and the effects of erratic sleep or sleep deprivation on mood | Ignore the effects of poor sleep hygiene on mood |
| Minimize antidepressant exposure in patients with RC or URC | Continue long-term antidepressant maintenance therapy in patients with manic or mixed features or ongoing oscillations between mania/hypomania and depression |
| Assure euthyroid status and consider the potential utility of hypermetabolic levothyroxine | Assume that RC or URC will resolve solely by normalizing or optimizing thyroid function |
| Use rational, pharmacodynamically nonredundant anti-cycling drugs | Ignore the cumulative burden of adverse effects of multiple drugs |
| Consider the potential role for ECT as a strategy to arrest URC during any phase of BD | Assume ECT has value only during acute depressive phases of BD |
| Use prospective mood charting to document the evolution of mood changes over time, particularly when gauging treatment efficacy | Rely solely on impressionistic recall of mood states or polarity changes as reflecting distinct phasic changes |
| BD: bipolar disorder; ECT: electroconvulsive therapy; RC: rapid cycling; URC: ultra-rapid cycling | |
Table
Rapid cycling and ultra-rapid cycling BD: A comparison
| Construct | Rapid cycling | Ultra-rapid cycling |
|---|---|---|
| Bipolar I vs II | Predominantly BD IIa | No systematic data |
| Sex | Predominantly women | No systematic data |
| Longitudinal course | May be a transient phenomenon that can occur at any timeb or an enduring phenomenon that may persist for yearsc | Ultradian patterns may be more common across the first several episodes among pediatric BD patientsd |
| Age at onset | Associated with younger age at onsete | May be more evident in prepubescent onset mood disordersd; ultradian cycling more likely when onset occurs before age 13 than in adulthoode |
| Diurnal variation in mood | Morning-to-evening mood switches usually involve depression to mania/hypomania, with the opposite typifying evening-to-morning mood switchesf | Not reported |
| Relationship to environmental stresses | Life stresses may precede initial affective episodes but may be less important as subsequent episodes arise with increasing automaticity | No systematic data |
| Relationship to menstrual cycle | Despite case reports and self-reported links between RC and menstrual mood exacerbations, prospective data do not identify associations between RC and menstrual patternsg,h | No systematic data |
| Subclinical hypothyroidism | Bauer and Whybrow identified hypothyroidism independent of lithium use in 60% of 30 rapidly cycling BD patients, with evidence of improvement in a separate study of 11 RC patients given suprametabolic levothyroxine (reviewed by Bauer et ali) | No systematic data |
| Relationship to psychosis | Nonea | No systematic data |
| Relationship to antidepressant use | Naturalistic observations suggest RC may occur later in the illness course as a result of antidepressant use.c Small open case series data suggest shorter intermorbid intervals on antidepressants with longer intervals off antidepressants.j RC patients often receive antidepressants, but causal relationships are not well-documented.k Some case-control data dispute links between antidepressant use and RCl | No specific published cases |
| Considerations for suicide risk | RC linked with more serious suicide attemptsl | Suicide attempts may be associated with cycling within an episodem or rapid shifting in moodn |
| Time course for judging treatment efficacy | Efforts to diminish acute affective instability may be measured over the course of days to weeks | By definition, treatment of RC involves relapse prevention over the course of 1 year |
| BD: bipolar disorder; RC: rapid cycling | ||
| References a. Schneck CD, Miklowitz DJ, Calabrese JR, et al. Phenomenology of rapid-cycling bipolar disorder: data from the first 500 participants in the Systematic Treatment Enhancement Program. Am J Psychiatry. 2004;161(10):1902-1908. b. Coryell W, Endicott J, Keller M. Rapidly cycling affective disorder. Demographics, diagnosis, family history, and course. Arch Gen Psychiatry. 1992;49(2):126-131. c. Koukopoulos A, Sani G, Koukopoulos AE, et al. Duration and stability of the rapid-cycling course: a long-term personal follow-up of 109 patients. J Affect Disord. 2003;73(1-2):75-85. d. Geller B, Tillman R, Bolhofner K, et al. Child bipolar I disorder: prospective continuity with adult bipolar I disorder; characteristics of second and third episodes; predictors of 8-year outcome. Arch Gen Psychiatry. 2008;65(10):1125-1133. e. Post RM, Leverich GS, Kupka RW, et al. Early-onset bipolar disorder and treatment delay are risk factors for poor outcome in adulthood. J Clin Psychiatry. 2010;71(7):864-872. f. Feldman-Naim S, Turner EH, Leibenluft E. Diurnal variation in the direction of mood switches in patients with rapid-cycling bipolar disorder. J Clin Psychiatry. 1997;58(2):79-84. g. Leibenluft E, Ashman SB, Feldman-Naim S, et al. Lack of relationship between menstrual cycle phase and mood in a sample of women with rapid cycling bipolar disorder. Biol Psychiatry. 1999;46(4):577-580. h. Wehr TA, Sack DA, Rosenthal NE, et al. Rapid cycling affective disorder: contributing factors and treatment responses in 51 patients. Am J Psychiatry. 1988;145(2):179-184. i. Bauer M, Beaulieu S, Dunner DL, et al. Rapid cycling bipolar disorder—diagnostic concepts. Bipolar Disord. 2008;10(1 Pt 2):153-162. j. Wehr TA, Goodwin FK. Can antidepressants cause mania and worsen the course of affective illness? Am J Psychiatry. 1987;144(11):1403-1411. k. Schneck CD, Miklowitz DJ, Miyahara S, et al. The prospective course of rapid-cycling bipolar disorder: findings from the STEP-BD. Am J Psychiatry. 2008;165(3):370-377. l. Coryell W, Solomon D, Turvey C, et al. The long-term course of rapid-cycling bipolar disorder. Arch Gen Psychiatry. 2003;60(9):914-920. m. Fawcett J, Scheftner W, Clark D, et al. Clinical predictors of suicide in patients with major affective disorders: a controlled prospective study. Am J Psychiatry. 1987;144(1):35-40. n. MacKinnon DF, Potash JB, McMahon FJ, et al. Rapid mood switching and suicidality in familial bipolar disorder. Bipolar Disord. 2005;7(5):441-448. | ||
From a biologic perspective, a handful of preliminary studies have examined genetic markers or familial patterns that might be related to rapid cycling (RC) or ultra-rapid cycling (URC). These include a reported link between URC and the low activity variant of the catechol-o-methyltransferase gene polymorphism in a small group of patients with velo-cardio-facial syndrome,a although this finding was not replicated in a larger sample.a Other preliminary reports on RC have implicated both the long (l) and short (s) allelic variants of the serotonin transporter gene (SLC6A4), the val66met variant of the brain-derived neurotrophic factor gene, and the circadian cryptochrome 2 (CRY2) gene (reviewed by Bauer et alb). These candidate loci have been examined in RC but not URC.
URC has not been examined as a familial entity, although in the National Institute of Mental Health Collaborative Depression Study, DSM-IV-TR RC did not occur with elevated frequency in bipolar pedigrees.c Rapid mood switches—abrupt rather than gradual transitions from one affective pole to another—appear to be only slightly, nonsignificantly more common in first-degree bipolar relatives of BD patients who themselves have rapid rather than gradual transitions from one affective pole to the other.d
Neuroimaging studies in BD seldom focus on subpopulations with RC or URC, and have been confined mainly to case reports that have yielded limited, non-generalizable observations, such as state-dependent variations in prefrontal activity during tasks of facial recognition (reviewed by Bauer et alb).
References
a. Papolos DF, Veit S, Faedda GL, et al. Ultra-ultra rapid cycling bipolar disorder is associated with the low activity catecholamine-O-methyltransferase allele. Mol Psychiatry. 1998;3(4):346-349.
b. Bauer M, Beaulieu S, Dunner DL, et al. Rapid cycling bipolar disorder—diagnostic concepts. Bipolar Disord. 2008;10(1 Pt 2):153-162.
c. Coryell W, Endicott J, Keller M. Rapidly cycling affective disorder. Demographics, diagnosis, family history, and course. Arch Gen Psychiatry. 1992;49(2):126-131.
d. MacKinnon DF, Potash JB, McMahon FJ, et al. Rapid mood switching and suicidality in familial bipolar disorder. Bipolar Disord. 2005;7(5):441-448.
Figure: Example of prospective mood charting to document changes in manic/hypomanic and depressive symptoms across time
In the above example, the prevalence and severity of mood symptoms are identified over 21 days. Note the distinctly separate phases of mood elevation followed by depression, whereas euthymia was present only 6 of 21 days (29% of the time). Symptoms of at least moderate severity were more prominently depressive (5 of 21 days, or 24% of the time) than manic/hypomanic (2 of 21 days, or approximately 10% of the time). Mood charting does not capture associate DSM-IV-TR criteria for a mood episode but instead focuses solely on longitudinal changes in mood elevation or depression
Related Resources
- American Psychiatric Association practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(4 suppl):1-50.
- Massachusetts General Hospital Bipolar Clinic and Research Program. Sample mood chart (downloadable). www.manicdepressive.org/moodchart.html.
Drug Brand Names
- Carbamazepine • Equetro, Tegretol
- Divalproex • Depakote
- Lamotrigine • Lamictal
- Levothyroxine • Synthroid
- Lithium • Lithobid
- Nimodipine • Nimotop
- Topiramate • Topamax
Disclosures
Dr. Goldberg is on the speakers’ bureaus for AstraZeneca, Dey Pharmaceuticals, Eli Lilly and Company, Merck, and Sunovion and is a consultant for Axon Advisors, Dey Pharmaceuticals, Eli Lilly and Company, and Grünenthal Group.
Acknowledgment
The author wishes to thank David L. Dunner, MD, for his helpful comments regarding this article.
Ultra-rapid cycling (URC) entered the psychiatric lexicon in the 1990s as a proposed descriptor for manic/hypomanic, mixed, or depressed episodes of bipolar disorder (BD) that occur every few days or weeks. DSM-IV-TR incorporates rapid cycling (RC)—but not URC—as a course specifier that occurs in 10% to 15% of patients with BD who have ≥4 distinct affective episodes per year, each fulfilling duration criteria and separated by identifiable recovery periods (unless an episode directly changes polarity). Since then, the terms RC and URC have seemingly metamorphosed into imprecise, popular colloquialisms meant to loosely describe frequent mood changes rather than distinct episodes over extended time periods, with little regard for the associated signs that define manic or hypomanic episodes.
This article examines the meaning and validity of URC in BD, its relevance and differentiation from rapid mood shifts in patients without BD, and concepts relevant to treatment extrapolated from studies of RC BD.
Imprecise nomenclature
Post et al1 coined the terms “ultra-rapid cycling” and “ultra-ultra-rapid cycling” (also called “ultradian cycling”) to describe mood episodes that occur monthly (URC) or over the course of as little as 1 day (ultradian cycling). These constructs are controversial because they lack demonstrated content validity and discriminant validity relative to other disorders. (“Content validity” refers to whether the features thought to comprise an entity of interest accurately and meaningfully do so; “discriminant validity” tells researchers and clinicians whether the proposed description of a clinical entity uniquely differentiates it from other disorders—avoiding “false-positive” suspected cases.) Clinicians therefore must pay careful attention to non-bipolar psychiatric problems that can present with rapid mood changes but without the psychomotor and related signs that define bipolar mood episodes. In their looser, nontechnical meanings, “rapid cycling” or “ultra-rapid cycling” may be synonymous with affective lability. RC is neither a diagnosis in itself nor a criterion for diagnosing BD. Rather, it is a course specifier to describe episode frequency in patients with past unambiguous manic or hypomanic episodes.
In children and adolescents, whose presentations often are atypical and can be hard to differentiate from other forms of behavioral or temperamental dysregulation, severe non-episodic mood dysregulation without signs of mania or hypomania may indicate a phenomenon separate from BD.2 Geller and colleagues3 proposed using the term “episodes” to frame the duration of a DSM-IV-defined syndrome of mania/hypomania or depression, while reserving the term “cycling” to connote patterns of mood alternation within a given episode. It is not clear whether this concept of “cycling” differs qualitatively from mood lability that arises during a mood episode in children or adults, and notably, this perspective does not account for changes in psychomotor signs in conjunction with changes in mood.
Clinicians also sometimes blur the concept of “mixed episodes” with RC or URC. DSM-IV-TR defines mixed episodes within bipolar I disorder (BD I) based on criteria for a simultaneous manic and depressive episode, rather than on frequent oscillations between affective poles. These and other differential diagnostic considerations for suspected URC are summarized in Table 1.4
A further concern regarding nomenclature involves the distinction between cyclicity (ie, successive episodes regardless of pole direction) and changes in polarity (ie, switches from depression to mania/hypomania or vice versa). Some mood disorder patients may have rapid oscillations from euthymia to depression while never changing polarity to mania/hypomania and may be best described as having recurrent brief depression.
Table 1
Differential diagnosis in suspected URC
| Phenomenon | Considerations for assessment |
|---|---|
| Mixed episodes in bipolar I disorder, or mixed depressive episodes in bipolar II disorder | DSM-IV-TR mixed episodes entail the co-occurrence of manic and depressive symptoms during the same episode without an intervening period of recovery. ICD-10 includes “rapid alternation of manic, hypomanic or depressive symptoms…from day to day or even hour to hour” in its definition of a mixed episode |
| Distress responses to acute environmental adversities (eg, adjustment disorders with mixed disturbance of emotions and conduct) | One would expect an absence of corresponding sleep-wake cycle changes or speech-language and psychomotor disturbances |
| Intoxication/withdrawal from psychoactive substances or drug-induced mental status changes (eg, corticosteroids, amphetamine, cocaine); a history of substance abuse also may be associated with development of URC in BD patients4 | Substance-induced mood fluctuations caused by intoxication/withdrawal can mimic affective cycling |
| Disinhibition states and frontal lobe syndromes as seen in traumatic brain injury and other CNS disorders, such as multiple sclerosis | Assess for signs of perseveration and history of head trauma or neurologic damage from cumulative toxic-metabolic insults (eg, chronic alcoholism) |
| Autonomic hyperarousal, emotional volatility, and hyperreactivity to environmental stresses, suggestive of PTSD | Determine the presence of a trauma history and review whether DSM-IV-TR symptoms and associated features of PTSD exist, including re-experiencing/reliving and avoidance, as well as paranoid thinking, dissociation, and nightmares |
| Recurrent mood shifts related to premenstrual dysphoric disorder may mimic URC. Other endocrine dysfunctions also may present with URC (eg, thyroid or ovarian malignancies) | Affirm the independent presence of BD before inferring its manifestations solely from premenstrual mood changes |
| Trait affective instability associated with borderline personality disorder | Trait mood instability is more chronic and enduring than episodic, and would not be expected to occur in tandem with signs of psychomotor activation that define mania/hypomania |
| BD: bipolar disorder; ICD-10: International Statistical Classification of Diseases and Related Health Problems, 10th revision; PTSD: posttraumatic stress disorder; URC: ultra-rapid cycling | |
Duration criteria
Clinicians and researchers have debated the minimum duration criteria for identifying manic or hypomanic episodes, and the extent to which suspected hypomanic periods of short duration constitute distinct illness phases. Although DSM-IV-TR designates 4 days as a minimum time for classifying an episode of hypomania, empirical studies suggest that mood symptoms lasting as few as 2 days may comprise a valid and reliably distinct entity relevant to RC.5 More limited data (mainly case observations) identify “affective oscillations” and “mood shifts” occurring faster than once per 24 hours in BD patients without comorbid personality disorders.6 Phenomenologic studies that have focused on 24- to 48-hour switch cycles have described new-onset URC arising spontaneously or following closed head injuries.7 In children and younger adolescents, reports have identified long index manic episodes (mean durations as long as 80 weeks)8 that involve continual (ultradian) mood cycling in as many as 80% of cases.9
Is URC a valid construct?
A central controversy surrounding the validity and meaningfulness of URC as a BD subtype involves its sole focus on mood variation rather than the fuller constellation of associated signs and symptoms that define episodes of mania/hypomania or depression. Abrupt, sudden, drastic, or dramatic mood shifts from one moment to the next are nowhere to be found in the DSM-IV-TR definition of BD, and the construct of mood lability or affective instability is neither a cardinal nor defining element of BD. Although individuals with BD I or bipolar II disorder (BD II) may have periods of affective lability, rapid shifts in mood are neither necessary nor sufficient for a BD diagnosis, and may indicate other types of psychopathology when affective instability occurs in the absence of a history of discernible manic or hypomanic episodes.
Studies by our group10 and others11 have shown that overattention to mood variation without considering associated cognitive, speech-language, chronobiologic, and motor signs of mania/hypomania accounts for substantial overdiagnosis of BD in patients with non-specific mood disturbances, particularly in those with active substance abuse or borderline personality disorder (BPD). Whereas the construct of RC BD attempts to account for changes in energy and psychomotor function as part of recurrent syndromes of mania/hypomania, existing literature on URC does not. Assessing mood changes in <24 hours also precludes assessing associated phenomena that occur over longer periods, such as changes in the sleep-wake cycle.
A rigorous, systematic approach to differential diagnosis for patients with affective instability is essential.
Borderline personality disorder
A common diagnostic debate regarding URC involves how to differentiate it from the chronic mood instability and reactivity inherent to BPD. Although some authors have suggested that RC BD and affective instability in BPD may be the same entity,12 others object to unifying the 2 conditions without considering their phenomenologic and other clinical differences. For example, affective instability arising from borderline character organization is thought to reflect a patient’s impaired capacity to self-regulate his or her internal state and emotional responses to interpersonal and other environmental stresses, or difficulty managing impulses. By contrast, manic or depressive phases of BD tend not to be “triggered” by interpersonal conflicts or frustrations. Furthermore, reframing intense mood reactions to the environment as bipolar variants carries several pitfalls: doing so wrongly accords patients a passive role in their reactions to life events, inaccurately reinforces a sense of victimization in response to stress, and diverts inquiry away from a patient’s active role in life decisions and circumstances that may be unsatisfying, self-defeating, or volatile.
Two key considerations may be helpful in discriminating rapid mood changes in BD vs BPD. First, some longitudinal studies indicate that RC often is a transient, rather than enduring, phenomenon in BD,13 in contrast to the nonvarying, trait feature of affective instability in persons with BPD. It is unknown whether URC is more enduring than transient. Notably, whereas bipolar mood episodes constitute deviations from a baseline state, affective instability in BPD is a baseline characteristic, rather than a deviation from it. Second, by definition, a BPD diagnosis hinges on additional elements unrelated to mood disturbances, such as interpersonal styles or defense mechanisms that involve splitting, projection, and projective identification, feelings of numbness, boredom, or emptiness, identity diffusion, fears of abandonment, and proclivities toward self-mutilation or other self-injurious behaviors as a means to alleviate tension and stress. These characteristics do not overlap with the core elements of BD.
Affective lability in patients with BPD entails prominent oscillations between anger and anxiety, or depression and anxiety, but not depression and elation14; by contrast, affective instability in BD has been linked with greater oscillations between euthymia and depression, and euthymia and elation, but not euthymia and anger.15 Moreover, daily mood fluctuations in patients with BD appear to occur in a relatively random fashion,16 whereas in BPD mood fluctuations are reactions that appear intimately linked to distressing interpersonal experiences.
See the table below, entitled “Rapid cycling and ultra-rapid cycling BD: A comparison,” comparing the phenomenology of RC and URC and a discussion of studies that explored genetic markers or family patterns that may be related to RC or URC.
Treatment considerations
No systematic studies exist for treating URC. Because most clinical trials of BD focus on treatment or prevention of a single episode rather than changes of mood over time, it is difficult to draw inferences about the ability of any treatment to attenuate marked, day-to-day mood variations. Some antimanic drugs, such as carbamazepine, have been suggested to offer better prophylactic efficacy compared with lithium for “non-classical” BD presentations, although the efficacy of carbamazepine has not been studied in URC.
Broadly speaking, treatment for URC, similar to RC, pragmatically involves:
- identifying and eliminating sources of mood destabilization (eg, substance abuse, erratic sleep patterns)
- treating medical comorbidities such as hypothyroidism
- optimizing treatment with mood stabilizing agents
- exercising caution when using antidepressants (see below).
Interestingly, despite frequent allusion to certain medications as “mood stabilizers,” no controlled study has examined mood instability on a day-to-day basis as a primary outcome measure in BD treatment, which limits the ability to surmise that any drug could be expected to diminish mood oscillations that occur over the course of days, or within a single day. However, a post hoc analysis by our group17 compared randomized treatment with lamotrigine or placebo over 6 months in RC BD I or BD II. Using prospective life charting, we found patients who received lamotrigine were almost twice as likely as those receiving placebo to achieve euthymia from one week to the next, which suggests the possibility that lamotrigine may offer benefit for affective instability in BD I or BD II patients, in addition to preventing discrete mood episodes.
Antidepressant controversy. Concerns that antidepressants might acutely induce mania or accelerate cycling frequency over long time periods have led to a contentious, long-standing debate within psychopharmacology. As noted in the box below, several long-term naturalistic follow-up studies have reported RC as a perceived consequence of antidepressants in most RC patients, although efforts to differentiate cycle acceleration caused by antidepressants (or other iatrogenic factors) from the natural course of illness remains exceedingly difficult without prospective randomized trials. (Antidepressants might cause more affective recurrences, but having multiple episodes may also cause more antidepressant prescriptions.) Some researchers (eg, Schneck et al18) have reported more frequent episodes among patients taking antidepressants but did not consider that patients with multiple episodes may be more likely to receive antidepressants, which fail to ameliorate acute or recurrent affective episodes. Importantly, a recent multi-site randomized trial by Ghaemi et al19 found that after a favorable acute response to antidepressants plus mood stabilizers, patients with preexisting RC who were randomized to continue antidepressants for up to 1 year had a 3-fold increased likelihood of developing a new depressive episode, which affirms suggestions that antidepressants do not help—but may exacerbate—cycling in patients with RC. No studies in BD have examined whether URC is more likely to arise as a consequence of antidepressant use.
Mood stabilizers and other biologic therapies. A small body of literature specifically addresses pharmacotherapy of URC in patients with BD (Table 2).19-26 A limitation of most existing literature is its focus on case reports, small open trials, or anecdotal observations rather than large, randomized controlled trials using systematic outcome measures. Extrapolation from reports focusing on patients with DSM-IV-TR RC is limited because it is uncertain whether URC differs fundamentally from RC and studies of DSM-IV-TR RC typically examine acute response during an index episode or time until relapse during maintenance therapy, rather than impact on mood changes over time.
Psychotherapy. A limited database on the efficacy of adjunctive cognitive-behavioral therapy (CBT) in RC BD describes improvement in depressive symptoms over short-term follow-up.27 No long-term studies of CBT or other structured psychotherapies have focused on RC or URC. Intuitively, one might expect that psychoeducation targeting sleep hygiene, substance use, stress management and coping skills, medication adherence, and prodrome recognition would be of value to patients with BD who experience frequent mood episodes, especially in those who may be unaware of or unfamiliar with basic concepts related to BD. In addition, relevant concepts from dialectical behavior therapy may be beneficial for BD patients with possible URC, such as skills to enhance emotional regulation, distress tolerance, mindfulness, and interpersonal effectiveness.
Table 2
Evidence-based treatments for ultra-rapid cycling BD
| Intervention | Strength of evidence | Comment |
|---|---|---|
| Antidepressant elimination | Cycling frequency may lengthen during antidepressant-free periods among patients with RC20; long-term (up to 1 year) antidepressant use in RC patients may increase the likelihood of depressive recurrences19 | Findings based mostly on small sample sizes; no controlled trials of antidepressant cessation as an intervention specifically for URC |
| Lithium | Single case report of ECT-induced URC resolved by lithium augmentation during continued ECT22 | No large-scale or randomized trials |
| Carbamazepine | No controlled trials or case reports | Possible anti-cycling benefits relevant for URC could be inferred from post hoc studies among patients with RC |
| Divalproex | Single case report describing resolution of a 48-hour cycle after augmentation of lithium with divalproex23 | No large-scale or randomized trials |
| Lamotrigine | Single case report of 100 mg/d lamotrigine augmentation to divalproex yielded 8 months of remission in a 25-year-old man with BD II and a long-standing pattern of 3 days of hypomania followed by 5 days of depression24 | No large-scale or randomized trials |
| Topiramate | Single case report in URC describing reduction of cycling frequency over 3 years25 | Multiple large scale placebo-controlled studies in bipolar mania have been negative |
| Second-generation antipsychotics | No controlled trials or case reports | Possible anti-cycling benefits relevant for URC could be inferred from post hoc studies in RC |
| Combinations of ≥2 mood stabilizing drugs | No controlled trials or case reports | Combining multiple anti-cycling agents is intuitively logical but largely unstudied |
| Nimodipine | 1 unipolar and 11 BD patients treated in randomized, off-on-off-on fashion (begun at 90 mg/d, increased up to 720 mg/d, mean duration of 12 weeks on active drug)26 | Response in 5 of 9 completers. Findings await replication with larger sample sizes |
| Hypermetabolic thyroid hormone (levothyroxine) | Findings from a small (N = 11) study of adjunctive high-dose levothyroxine (0.15 to 0.4 mg/d, with dosages increased by 0.05 to 0.1 mg/d every 1 to 2 weeks); an unspecified subgroup had “a very rapid cycling pattern” (reviewed by Bauer et al21) | 10 of 11 RC patients had reductions in depressive symptoms, 5 of 7 had improvement from baseline manic symptoms (observation period >60 days) |
| ECT | Case reports of improvement with ECT in refractory RC that was presumed secondary to tricyclic antidepressants | Reports of induction of URC by ECT22; whether or not ECT would more likely improve or exacerbate cyclicity for a given patient may require empirical determination |
| BD: bipolar disorder; BD II: bipolar II disorder; ECT: electroconvulsive therapy; RC: rapid cycling; URC: ultra-rapid cycling | ||
Treatment monitoring. Prospective life charting allows patients to systematically record manic/hypomanic and depressive symptoms day-to-day and week-to-week, thus creating a measure that may be particularly relevant for patients whose moods change rapidly. Simple mood charts (see Related Resources) typically take into account the severity of symptoms of either polarity with ratings of mild, moderate, or severe. Such visual records permit simple calculations over the course of a given interval (eg, week-by-week or across months) of several important parameters, including:
- number of days euthymic
- number of days with depression
- number of days with abnormal mood elevation
- number of occasions in which moods of both polarities occur on the same day.
Tracking these parameters during a treatment allows clinicians to make quantitative comparisons over time as a method of determining whether or not meaningful changes are occurring in cyclicity. See the figure below for an example of a completed mood chart and its interpretation.
Additional recommendations for assessing and managing cyclicity in BD are summarized in Table 3.
Table 3
Tips for managing suspected ultra-rapid cycling BD
| Do’s | Don’ts |
|---|---|
| Ascertain a history of ≥1 lifetime manic or hypomanic episode to diagnose BD | Diagnose BD solely on the presence of rapid mood fluctuations |
| Determine the presence of changes in sleep, energy, speech-language, and related behavior as correlates of mood to differentiate syndromes from isolated variation in mood | Ignore constellations of associated signs and symptoms of mania/hypomania |
| Obtain patient history to assess for head trauma or other medical and neurologic events that could have affective or other psychiatric manifestations | Disregard possible medical etiologies for new-onset affective dysregulation |
| Ascertain the resolution of 1 episode before counting the resurgence of symptoms as constituting a new episode; a waxing and waning course may reflect illness chronicity with incomplete recovery rather than true cyclicity | Misidentify incomplete recovery from an existing episode as the occurrence of new multiple episodes, which would inflate false-positive cases of RC or URC |
| Advise patients to refrain from alcohol or illicit substances that could destabilize mood | Assume that comorbid alcohol or illicit substance abuse will remit only after mood stabilization has been achieved, rather than the reverse |
| Monitor changes in sleep-wake cycles and the effects of erratic sleep or sleep deprivation on mood | Ignore the effects of poor sleep hygiene on mood |
| Minimize antidepressant exposure in patients with RC or URC | Continue long-term antidepressant maintenance therapy in patients with manic or mixed features or ongoing oscillations between mania/hypomania and depression |
| Assure euthyroid status and consider the potential utility of hypermetabolic levothyroxine | Assume that RC or URC will resolve solely by normalizing or optimizing thyroid function |
| Use rational, pharmacodynamically nonredundant anti-cycling drugs | Ignore the cumulative burden of adverse effects of multiple drugs |
| Consider the potential role for ECT as a strategy to arrest URC during any phase of BD | Assume ECT has value only during acute depressive phases of BD |
| Use prospective mood charting to document the evolution of mood changes over time, particularly when gauging treatment efficacy | Rely solely on impressionistic recall of mood states or polarity changes as reflecting distinct phasic changes |
| BD: bipolar disorder; ECT: electroconvulsive therapy; RC: rapid cycling; URC: ultra-rapid cycling | |
Table
Rapid cycling and ultra-rapid cycling BD: A comparison
| Construct | Rapid cycling | Ultra-rapid cycling |
|---|---|---|
| Bipolar I vs II | Predominantly BD IIa | No systematic data |
| Sex | Predominantly women | No systematic data |
| Longitudinal course | May be a transient phenomenon that can occur at any timeb or an enduring phenomenon that may persist for yearsc | Ultradian patterns may be more common across the first several episodes among pediatric BD patientsd |
| Age at onset | Associated with younger age at onsete | May be more evident in prepubescent onset mood disordersd; ultradian cycling more likely when onset occurs before age 13 than in adulthoode |
| Diurnal variation in mood | Morning-to-evening mood switches usually involve depression to mania/hypomania, with the opposite typifying evening-to-morning mood switchesf | Not reported |
| Relationship to environmental stresses | Life stresses may precede initial affective episodes but may be less important as subsequent episodes arise with increasing automaticity | No systematic data |
| Relationship to menstrual cycle | Despite case reports and self-reported links between RC and menstrual mood exacerbations, prospective data do not identify associations between RC and menstrual patternsg,h | No systematic data |
| Subclinical hypothyroidism | Bauer and Whybrow identified hypothyroidism independent of lithium use in 60% of 30 rapidly cycling BD patients, with evidence of improvement in a separate study of 11 RC patients given suprametabolic levothyroxine (reviewed by Bauer et ali) | No systematic data |
| Relationship to psychosis | Nonea | No systematic data |
| Relationship to antidepressant use | Naturalistic observations suggest RC may occur later in the illness course as a result of antidepressant use.c Small open case series data suggest shorter intermorbid intervals on antidepressants with longer intervals off antidepressants.j RC patients often receive antidepressants, but causal relationships are not well-documented.k Some case-control data dispute links between antidepressant use and RCl | No specific published cases |
| Considerations for suicide risk | RC linked with more serious suicide attemptsl | Suicide attempts may be associated with cycling within an episodem or rapid shifting in moodn |
| Time course for judging treatment efficacy | Efforts to diminish acute affective instability may be measured over the course of days to weeks | By definition, treatment of RC involves relapse prevention over the course of 1 year |
| BD: bipolar disorder; RC: rapid cycling | ||
| References a. Schneck CD, Miklowitz DJ, Calabrese JR, et al. Phenomenology of rapid-cycling bipolar disorder: data from the first 500 participants in the Systematic Treatment Enhancement Program. Am J Psychiatry. 2004;161(10):1902-1908. b. Coryell W, Endicott J, Keller M. Rapidly cycling affective disorder. Demographics, diagnosis, family history, and course. Arch Gen Psychiatry. 1992;49(2):126-131. c. Koukopoulos A, Sani G, Koukopoulos AE, et al. Duration and stability of the rapid-cycling course: a long-term personal follow-up of 109 patients. J Affect Disord. 2003;73(1-2):75-85. d. Geller B, Tillman R, Bolhofner K, et al. Child bipolar I disorder: prospective continuity with adult bipolar I disorder; characteristics of second and third episodes; predictors of 8-year outcome. Arch Gen Psychiatry. 2008;65(10):1125-1133. e. Post RM, Leverich GS, Kupka RW, et al. Early-onset bipolar disorder and treatment delay are risk factors for poor outcome in adulthood. J Clin Psychiatry. 2010;71(7):864-872. f. Feldman-Naim S, Turner EH, Leibenluft E. Diurnal variation in the direction of mood switches in patients with rapid-cycling bipolar disorder. J Clin Psychiatry. 1997;58(2):79-84. g. Leibenluft E, Ashman SB, Feldman-Naim S, et al. Lack of relationship between menstrual cycle phase and mood in a sample of women with rapid cycling bipolar disorder. Biol Psychiatry. 1999;46(4):577-580. h. Wehr TA, Sack DA, Rosenthal NE, et al. Rapid cycling affective disorder: contributing factors and treatment responses in 51 patients. Am J Psychiatry. 1988;145(2):179-184. i. Bauer M, Beaulieu S, Dunner DL, et al. Rapid cycling bipolar disorder—diagnostic concepts. Bipolar Disord. 2008;10(1 Pt 2):153-162. j. Wehr TA, Goodwin FK. Can antidepressants cause mania and worsen the course of affective illness? Am J Psychiatry. 1987;144(11):1403-1411. k. Schneck CD, Miklowitz DJ, Miyahara S, et al. The prospective course of rapid-cycling bipolar disorder: findings from the STEP-BD. Am J Psychiatry. 2008;165(3):370-377. l. Coryell W, Solomon D, Turvey C, et al. The long-term course of rapid-cycling bipolar disorder. Arch Gen Psychiatry. 2003;60(9):914-920. m. Fawcett J, Scheftner W, Clark D, et al. Clinical predictors of suicide in patients with major affective disorders: a controlled prospective study. Am J Psychiatry. 1987;144(1):35-40. n. MacKinnon DF, Potash JB, McMahon FJ, et al. Rapid mood switching and suicidality in familial bipolar disorder. Bipolar Disord. 2005;7(5):441-448. | ||
From a biologic perspective, a handful of preliminary studies have examined genetic markers or familial patterns that might be related to rapid cycling (RC) or ultra-rapid cycling (URC). These include a reported link between URC and the low activity variant of the catechol-o-methyltransferase gene polymorphism in a small group of patients with velo-cardio-facial syndrome,a although this finding was not replicated in a larger sample.a Other preliminary reports on RC have implicated both the long (l) and short (s) allelic variants of the serotonin transporter gene (SLC6A4), the val66met variant of the brain-derived neurotrophic factor gene, and the circadian cryptochrome 2 (CRY2) gene (reviewed by Bauer et alb). These candidate loci have been examined in RC but not URC.
URC has not been examined as a familial entity, although in the National Institute of Mental Health Collaborative Depression Study, DSM-IV-TR RC did not occur with elevated frequency in bipolar pedigrees.c Rapid mood switches—abrupt rather than gradual transitions from one affective pole to another—appear to be only slightly, nonsignificantly more common in first-degree bipolar relatives of BD patients who themselves have rapid rather than gradual transitions from one affective pole to the other.d
Neuroimaging studies in BD seldom focus on subpopulations with RC or URC, and have been confined mainly to case reports that have yielded limited, non-generalizable observations, such as state-dependent variations in prefrontal activity during tasks of facial recognition (reviewed by Bauer et alb).
References
a. Papolos DF, Veit S, Faedda GL, et al. Ultra-ultra rapid cycling bipolar disorder is associated with the low activity catecholamine-O-methyltransferase allele. Mol Psychiatry. 1998;3(4):346-349.
b. Bauer M, Beaulieu S, Dunner DL, et al. Rapid cycling bipolar disorder—diagnostic concepts. Bipolar Disord. 2008;10(1 Pt 2):153-162.
c. Coryell W, Endicott J, Keller M. Rapidly cycling affective disorder. Demographics, diagnosis, family history, and course. Arch Gen Psychiatry. 1992;49(2):126-131.
d. MacKinnon DF, Potash JB, McMahon FJ, et al. Rapid mood switching and suicidality in familial bipolar disorder. Bipolar Disord. 2005;7(5):441-448.
Figure: Example of prospective mood charting to document changes in manic/hypomanic and depressive symptoms across time
In the above example, the prevalence and severity of mood symptoms are identified over 21 days. Note the distinctly separate phases of mood elevation followed by depression, whereas euthymia was present only 6 of 21 days (29% of the time). Symptoms of at least moderate severity were more prominently depressive (5 of 21 days, or 24% of the time) than manic/hypomanic (2 of 21 days, or approximately 10% of the time). Mood charting does not capture associate DSM-IV-TR criteria for a mood episode but instead focuses solely on longitudinal changes in mood elevation or depression
Related Resources
- American Psychiatric Association practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(4 suppl):1-50.
- Massachusetts General Hospital Bipolar Clinic and Research Program. Sample mood chart (downloadable). www.manicdepressive.org/moodchart.html.
Drug Brand Names
- Carbamazepine • Equetro, Tegretol
- Divalproex • Depakote
- Lamotrigine • Lamictal
- Levothyroxine • Synthroid
- Lithium • Lithobid
- Nimodipine • Nimotop
- Topiramate • Topamax
Disclosures
Dr. Goldberg is on the speakers’ bureaus for AstraZeneca, Dey Pharmaceuticals, Eli Lilly and Company, Merck, and Sunovion and is a consultant for Axon Advisors, Dey Pharmaceuticals, Eli Lilly and Company, and Grünenthal Group.
Acknowledgment
The author wishes to thank David L. Dunner, MD, for his helpful comments regarding this article.
1. Kramlinger KG, Post RM. Ultra-rapid and ultradian cycling in bipolar affective illness. Br J Psychiatry. 1996;168(3):314-323.
2. Leibenluft E. Severe mood dysregulation irritability, and the diagnostic boundaries of bipolar disorder in youths. Am J Psychiatry. 2011;168(2):129-142.
3. Geller B, Tillman R, Bolhofner K. Proposed definitions of bipolar I disorder episodes and daily rapid cycling phenomena in preschoolers school-aged children, adolescents, and adults. J Child Adolesc Psychopharmacol. 2007;17(2):217-222.
4. Feinman JA, Dunner DL. The effect of alcohol and substance abuse on the course of bipolar affective disorder. J Affect Disord. 1996;37(1):43-49.
5. Maj M, Pirozzi R, Formicola AM, et al. Reliability and validity of four alternative definitions of rapid-cycling bipolar disorder. Am J Psychiatry. 1999;156(9):1421-1424.
6. Jenner FA, Gjessing LR, Cox JR, et al. A manic depressive psychotic with a persistent forty-eight hour cycle. Br J Psychiatry. 1967;113(501):895-910.
7. Zwil AS, McAllister TW, Cohen I, et al. Ultra-rapid cycling bipolar affective disorder following a closed-head injury. Brain Inj. 1993;7(2):147-152.
8. Geller B, Tillman R, Craney JL, et al. Four-year prospective outcome and natural history of mania in children with a prepubertal and early adolescent bipolar disorder phenotype. Arch Gen Psychiatry. 2004;61(5):459-467.
9. Geller B, Sun K, Zimerman B, et al. Complex and rapid-cycling in bipolar children and adolescents: a preliminary study. J Affect Disord. 1995;34(4):259-268.
10. Goldberg JF, Garno JL, Callahan AM, et al. Overdiagnosis of bipolar disorder among substance use disorder inpatients with mood instability. J Clin Psychiatry. 2008;69(11):1751-1757.
11. Zimmerman M, Ruggero CJ, Chelminski I, et al. Psychiatric diagnoses in patients previously overdiagnosed with bipolar disorder. J Clin Psychiatry. 2010;71(1):26-31.
12. MacKinnon DF, Pies R. Affective instability as rapid cycling: theoretical and clinical implications for borderline personality and bipolar spectrum disorders. Bipolar Disord. 2006;8(1):1-14.
13. Coryell W, Endicott J, Keller M. Rapidly cycling affective disorder. Demographics diagnosis, family history, and course. Arch Gen Psychiatry. 1992;49(2):126-131.
14. Koenigsberg HW, Harvey PD, Mitropoulou V, et al. Characterizing affective instability in borderline personality disorder. Am J Psychiatry. 2002;159(5):784-788.
15. Henry C, Mitropoulou V, New AS, et al. Affective instability and impulsivity in borderline personality and bipolar II disorders: similarities and differences. J Psychiatr Res. 2001;35(6):307-312.
16. Gottschalk A, Bauer MS, Whybrow PC. Evidence of chaotic mood variation in bipolar disorder. Arch Gen Psychiatry. 1995;52(11):947-959.
17. Goldberg JF, Bowden CL, Calabrese JR, et al. Six-month prospective life charting of mood symptoms with lamotrigine monotherapy versus placebo in rapid cycling bipolar disorder. Biol Psychiatry. 2008;63(1):125-130.
18. Schneck CD, Miklowitz DJ, Miyahara S, et al. The prospective course of rapid-cycling bipolar disorder: findings from the STEP-BD. Am J Psychiatry. 2008;165(3):370-377.
19. Ghaemi SN, Ostacher MM, El-Mallakh RS, et al. Antidepressant discontinuation in bipolar depression: a Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) randomized clinical trial of long-term effectiveness and safety. J Clin Psychiatry. 2010;71(4):372-380.
20. Wehr TA, Sack DA, Rosenthal NE, et al. Rapid cycling affective disorder: contributing factors and treatment responses in 51 patients. Am J Psychiatry. 1988;145(2):179-184.
21. Bauer M, Beaulieu S, Dunner DL, et al. Rapid cycling bipolar disorder—diagnostic concepts. Bipolar Disord. 2008;10(1 Pt 2):153-162.
22. Zavorotnyy M, Diemer J, Patzelt J, et al. Occurrence of ultra-rapid cycling during electroconvulsive therapy in bipolar depression. World J Biol Psychiatry. 2009;10(4 Pt 3):987-990.
23. Lepkifker E, Iancu I, Dannon P, et al. Valproic acid in ultra-rapid cycling: a case report. Clin Neuropharmacol. 1995;18(1):72-75.
24. Woo YS, Chae JH, Jun TY, et al. Lamotrigine added to valproate successfully treated a case of ultra-rapid cycling bipolar disorder. Psychiatry Clin Neurosci. 2007;61(1):130-131.
25. Karama S, Lal S. Adjunctive topiramate in ultradian cycling bipolar disorder: case report with 3-year follow-up. Eur Psychiatry. 2006;21(4):280-281.
26. Pazzaglia PJ, Post RM, Ketter TA, et al. Preliminary controlled trial of nimodipine in ultra-rapid cycling affective dysregulation. Psychiatry Res. 1993;49(3):257-272.
27. Reilly-Harrington NA, Deckersbach T, Knauz R, et al. Cognitive behavioral therapy for rapid-cycling bipolar disorder: a pilot study. J Psychiatr Pract. 2007;13(5):291-297.
1. Kramlinger KG, Post RM. Ultra-rapid and ultradian cycling in bipolar affective illness. Br J Psychiatry. 1996;168(3):314-323.
2. Leibenluft E. Severe mood dysregulation irritability, and the diagnostic boundaries of bipolar disorder in youths. Am J Psychiatry. 2011;168(2):129-142.
3. Geller B, Tillman R, Bolhofner K. Proposed definitions of bipolar I disorder episodes and daily rapid cycling phenomena in preschoolers school-aged children, adolescents, and adults. J Child Adolesc Psychopharmacol. 2007;17(2):217-222.
4. Feinman JA, Dunner DL. The effect of alcohol and substance abuse on the course of bipolar affective disorder. J Affect Disord. 1996;37(1):43-49.
5. Maj M, Pirozzi R, Formicola AM, et al. Reliability and validity of four alternative definitions of rapid-cycling bipolar disorder. Am J Psychiatry. 1999;156(9):1421-1424.
6. Jenner FA, Gjessing LR, Cox JR, et al. A manic depressive psychotic with a persistent forty-eight hour cycle. Br J Psychiatry. 1967;113(501):895-910.
7. Zwil AS, McAllister TW, Cohen I, et al. Ultra-rapid cycling bipolar affective disorder following a closed-head injury. Brain Inj. 1993;7(2):147-152.
8. Geller B, Tillman R, Craney JL, et al. Four-year prospective outcome and natural history of mania in children with a prepubertal and early adolescent bipolar disorder phenotype. Arch Gen Psychiatry. 2004;61(5):459-467.
9. Geller B, Sun K, Zimerman B, et al. Complex and rapid-cycling in bipolar children and adolescents: a preliminary study. J Affect Disord. 1995;34(4):259-268.
10. Goldberg JF, Garno JL, Callahan AM, et al. Overdiagnosis of bipolar disorder among substance use disorder inpatients with mood instability. J Clin Psychiatry. 2008;69(11):1751-1757.
11. Zimmerman M, Ruggero CJ, Chelminski I, et al. Psychiatric diagnoses in patients previously overdiagnosed with bipolar disorder. J Clin Psychiatry. 2010;71(1):26-31.
12. MacKinnon DF, Pies R. Affective instability as rapid cycling: theoretical and clinical implications for borderline personality and bipolar spectrum disorders. Bipolar Disord. 2006;8(1):1-14.
13. Coryell W, Endicott J, Keller M. Rapidly cycling affective disorder. Demographics diagnosis, family history, and course. Arch Gen Psychiatry. 1992;49(2):126-131.
14. Koenigsberg HW, Harvey PD, Mitropoulou V, et al. Characterizing affective instability in borderline personality disorder. Am J Psychiatry. 2002;159(5):784-788.
15. Henry C, Mitropoulou V, New AS, et al. Affective instability and impulsivity in borderline personality and bipolar II disorders: similarities and differences. J Psychiatr Res. 2001;35(6):307-312.
16. Gottschalk A, Bauer MS, Whybrow PC. Evidence of chaotic mood variation in bipolar disorder. Arch Gen Psychiatry. 1995;52(11):947-959.
17. Goldberg JF, Bowden CL, Calabrese JR, et al. Six-month prospective life charting of mood symptoms with lamotrigine monotherapy versus placebo in rapid cycling bipolar disorder. Biol Psychiatry. 2008;63(1):125-130.
18. Schneck CD, Miklowitz DJ, Miyahara S, et al. The prospective course of rapid-cycling bipolar disorder: findings from the STEP-BD. Am J Psychiatry. 2008;165(3):370-377.
19. Ghaemi SN, Ostacher MM, El-Mallakh RS, et al. Antidepressant discontinuation in bipolar depression: a Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) randomized clinical trial of long-term effectiveness and safety. J Clin Psychiatry. 2010;71(4):372-380.
20. Wehr TA, Sack DA, Rosenthal NE, et al. Rapid cycling affective disorder: contributing factors and treatment responses in 51 patients. Am J Psychiatry. 1988;145(2):179-184.
21. Bauer M, Beaulieu S, Dunner DL, et al. Rapid cycling bipolar disorder—diagnostic concepts. Bipolar Disord. 2008;10(1 Pt 2):153-162.
22. Zavorotnyy M, Diemer J, Patzelt J, et al. Occurrence of ultra-rapid cycling during electroconvulsive therapy in bipolar depression. World J Biol Psychiatry. 2009;10(4 Pt 3):987-990.
23. Lepkifker E, Iancu I, Dannon P, et al. Valproic acid in ultra-rapid cycling: a case report. Clin Neuropharmacol. 1995;18(1):72-75.
24. Woo YS, Chae JH, Jun TY, et al. Lamotrigine added to valproate successfully treated a case of ultra-rapid cycling bipolar disorder. Psychiatry Clin Neurosci. 2007;61(1):130-131.
25. Karama S, Lal S. Adjunctive topiramate in ultradian cycling bipolar disorder: case report with 3-year follow-up. Eur Psychiatry. 2006;21(4):280-281.
26. Pazzaglia PJ, Post RM, Ketter TA, et al. Preliminary controlled trial of nimodipine in ultra-rapid cycling affective dysregulation. Psychiatry Res. 1993;49(3):257-272.
27. Reilly-Harrington NA, Deckersbach T, Knauz R, et al. Cognitive behavioral therapy for rapid-cycling bipolar disorder: a pilot study. J Psychiatr Pract. 2007;13(5):291-297.
College mental health: How to provide care for students in need
Each year thousands of college students seek treatment at their school’s mental health service, but few psychiatrists are delivering this care. Most of the 4,500 degree-granting institutions of higher education (IHEs) in the United States1 provide some type of psychological or mental health counseling support to their students, and approximately 10% of the student body seeks care annually.2 In 2010, nearly 24% of students who visited their college counseling service were taking psychiatric medications at the time of their visit, up from 9% in 1994.2
Nevertheless, for various historical and practical reasons, psychiatrists have played—and continue to play—a somewhat peripheral role in college mental health systems. Although interest in psychiatric care at IHEs has been increasing (Box 1),3 until recently, most college counseling services had no direct access to psychiatric services and currently <1% of college services are directed by psychiatrists.2
This article examines some of the unique challenges faced by psychiatrists who work in a college mental health service, including how this setting may affect assessment, medication management, and crisis counseling. I use the terms “counseling services” and “mental health services” interchangeably because schools differ in the name they use for this office.
In recent years, the psychiatric community has begun to take steps to recognize college mental health as a specific practice area.3 In 2004, as president of the American Psychiatric Association (APA), Current Psychiatry Section Editor Michelle B. Riba, MD, MS convened a task force on college mental health. Subsequently, the APA added a section on college mental health to its public information Web site “Healthy Minds. Healthy Lives” (www.healthyminds.org).
The University of Michigan has taken a national leadership role in college psychiatry and college mental health. Since 2003, the University of Michigan Depression Center has hosted a yearly Depression on College Campuses national conference. Content from past conferences is available at www.depressioncenter.org/docc.
Organizations dedicated to college mental health and suicide prevention also have taken a role in disseminating information. Chief among them are the Jed Foundation, Active Minds on Campus, the Suicide Prevention Resource Center, Penn State’s Center for Collegiate Mental Health, and the National Research Consortium of Counseling Centers in Higher Education. More needs to be done to expand efforts related to college mental health and educate the psychiatric community and community at large about these vital concerns.
Managing medications and crises
Most college mental health services are directed by psychologists because of how IHEs historically structured these services (Box 2).4 Although counseling center staffing generally includes relatively few psychiatrists, those who do serve in this setting typically serve 2 primary roles: medication managers and crisis clinicians.
Medication management. Counseling centers are seeing more students who are either already taking psychotropics or need assessment and medication management. In the United States, 14% of students seen at college counseling centers are referred for psychiatric evaluation; however, on average, schools provide 2 hours of psychiatric services per week for every 1,000 students.2
Managing medications for college students poses several challenges. For most students, interaction with their college’s health and/or mental health system may be the first time they receive care not under the direct oversight of their family. Families and their feelings about psychiatric medication can play a major role in planning, executing, and managing psychiatric care, even for students who are legal adults. Family attitudes toward psychiatry and patients’ fears of disappointing parents who may feel distraught because their child has a psychiatric illness may impact a young person’s decision to accept medication or comply with treatment. Students often are insured by their family, and parents might receive an Explanation of Benefits and will learn of the student’s pharmacotherapy even if the student does not want them to know. College psychiatrists and students always need to consider decisions about how and when to include parents in discussions about medications.
College psychiatrists also must be sensitive to the unique vicissitudes of the school calendar and the developmental trajectory of college life. Decisions about when to start a medication or even which medication to prescribe might depend on how close a students is to exams or summer break. For example, a student experiencing severe anxiety a week before exams probably is better treated with a short-term benzodiazepine prescribed on an as-needed basis than a selective serotonin reuptake inhibitor, which in the first few weeks might only disrupt the student’s ability to function academically and not improve symptoms.5
Assessment also must consider the context of the academic year. For example, students may be “homesick” when they first enter college. Although such students may present with prominent and seemingly severe symptoms of anxiety or depression, more often than not the condition is self-limiting and resolves with support and watchful waiting. For example, many years ago a student presented to my institution’s counseling office in severe acute panic at the beginning of his first year at college. He had never been away from home, had not yet received his dorm phone, and did not know how to use a pay phone. His anxiety resolved as soon as I let him use my office phone to call his family. He ultimately made an excellent adjustment to college life.
Because most counseling services are set up primarily to provide talk therapy, most students who receive psychiatric medication also are engaged in psychotherapy. In these situations, psychiatrists must manage the same challenges in communication and coordination of care that occur in any split treatment agreement. These problems may be more easily addressed when the psychotherapist and prescriber both work in the college counseling center. Unfortunately, at some institutions, the psychiatrist or prescribing physician assistant or nurse practitioner may be based at the college’s health service,6 which can make coordination of care more challenging.
Crisis management. College counseling centers often manage students in crisis. Each year, approximately 6% of college students report suicidal ideation and 1% to 2% report suicide attempts.7 In 2010 there were 14 psychiatric hospitalizations for every 10,000 students on college campuses.2 Because psychiatrists are trained to manage patients with severe pathology and have emergency room training, college counseling psychiatrists often are looked to for assessment and consultation for students in crisis. In many cases, a student in crisis also will need psychopharmacologic intervention. In the event of a suicide or death on campus, the college psychiatrist often is called upon to address postvention planning and management of the clinical and community response.
Psychiatrists who manage student crises need to be cognizant of the unique elements of college life: Does the student live in a dormitory or with family? Could a relative who lives nearby help supervise an anxious patient who is cutting herself? Is the student in treatment with a therapist “back home” who could provide history or intervene? A crisis that occurs early in the school year, when a new student is less likely to have a network of friends or other supports, may need to be managed differently from one that occurs later, when the student might have people who could provide some comfort for a short time. The psychiatrist should know what level of support and supervision is available in the residence halls.
Although it is helpful for college counseling centers to maintain ongoing communication and coordination of services with local clinics and/or university medical centers, it is especially important for those who manage crises to have strong communication with local emergency rooms (ERs) and community crisis services. Because these services likely are managed by physicians, the campus psychiatrist is well placed to consult and coordinate care with local ERs because during a crisis, physician-to-physician communication often is more effective than campus counselor-to-ER physician communication. Ideally, college psychiatrists should have regular communication with ER physicians to discuss campus trends—such as particular drugs being used with unusual frequency or suicides on campus that might raise concerns of suicide contagion—and educate ER clinicians about services and programs the college offers.8
Barreira and Snider4 described the early development of college counseling services as flowing from 2 separate streams. Counseling services at colleges began to appear in the middle of the 20th century and grew out of academic and career advising offices. These programs typically had a “developmental”—as opposed to a clinical—orientation. Most of these services were and continue to be directed by counseling psychologists.
At the same time, some larger institutions—particularly the “Ivys”—hired psychiatrists to provide mental health care. Sometimes these clinicians were based at the school health service, while other institutions had parallel systems of counseling and mental health services. Today most colleges have integrated these programs into a single service.
An opportunity for training
Psychiatrists’ training may make them well-suited to address clinical issues that typically arise among college students. For example, students who have difficulty in college often struggle with issues at the border of physical and emotional health. Many students experience significant levels of stress, and many struggle with poor or inconsistent eating and nutrition and inadequate sleep. In fact, severely sleep-deprived students may present with symptoms that mimic depression.5 Psychiatrists have credibility in addressing these issues with individual students and the campus community. Psychiatrists also have training and experience in diagnosing and managing patients with substance abuse and can educate students, parents, faculty, and university administrators about these disorders.
College mental health services can be valuable training venues for senior psychiatric residents and child and adolescent fellows.9 College students can provide exposure to a broad array of problems—such as anxiety disorders, obsessive-compulsive disorder, dysthymic disorder, adjustment disorders, and panic disorder—that psychiatric trainees may not confront in a hospital clinic. Students are particularly responsive to short-term talk therapies with or without medication management, and working with this population can be a strong antidote to the “therapeutic nihilism”—the unfortunate sense that talk therapies are of limited effectiveness and that only pharmacotherapy can help psychiatric problems—often experienced by psychiatric trainees who spend much of their time working with patients with serious, chronic illnesses. Psychiatric residents can be particularly helpful in managing student patients who require combined medication and talk therapy.
College-based psychiatrists are well suited for educating residents about developmental issues of “emerging adulthood,” including:
- exploration of and anxieties about relationships and sexuality
- balancing connectedness to family with increasing sense of autonomy and independence
- establishing personal life goals and values and career choices and goals.10
College counseling services also are an excellent setting for residents to learn principles of community mental health and medico-legal concepts related to confidentiality, duty to warn, and disability law.11
Treatment outside college
Because college students have high rates of substance abuse12 and other psychiatric disorders, it is important for psychiatrists who treat these patients in private practice or community-based clinics to develop a basic awareness of and competency in relevant developmental and clinical issues. Psychiatrists who work in emergency services in areas with high concentrations of college students need to be particularly attuned to issues related to college mental health, substance abuse, and life on campus.8
Related Resources
- The Jed Foundation. www.jedfoundation.org.
- National Research Consortium of Counseling Centers in Higher Education. www.cmhc.utexas.edu/researchconsortium.html.
Disclosure
Dr. Schwartz reports no financial relationship with any companies whose products are mentioned in this article or with manufacturers of competing products.
1. US Department of Education National Center for Education Statistics. Degree-granting institutions by control and type of institution: Selected years, 1949-50 through 2009-10. http://nces.ed.gov/programs/digest/d10/tables/dt10_275.asp. Accessed August 22, 2011.
2. Gallagher RP. National survey of counseling center directors 2010. International Association of Counseling Services. http://www.iacsinc.org. Accessed August 22 2011.
3. Schwartz V, Kay J. The crisis in college and university mental health. Psychiatric Times. July 27 2009.
4. Barreira P, Snider M. History of college counseling and mental health services and role of the community mental health model. In: Kay J Schwartz V, eds. Mental health care in the college community. Hoboken, NJ: Wiley and Sons; 2010:21–31.
5. Schwartz V. Medications. In: Grayson PA Meilman PW, eds. College mental health practice. New York, NY: Routledge; 2006:59–78.
6. Barr V, Rando R, Krylowicz B, et al. The Association for University and College Counseling Centers Directors Annual Survey. 2010. http://aucccd.org/img/pdfs/aucccd_directors_survey_monograph_2010.pdf. Accessed September 1, 2011.
7. American College Health Association. National college health assessment II: reference group executive summary Fall 2010. Linthicum MD: American College Health Association; 2011.
8. Glick RL, Schwartz V. Assessment and management: special considerations for college students in psychiatric crisis. Psychiatric Issues in Emergency Care Settings. 2007;6(4):7-13.
9. Kay J, Schwartz V. Psychiatry residency training in college mental health services. In: Kay J Schwartz V, eds. Mental health care in the college community. Hoboken, NJ: Wiley and Sons; 2010.
10. Arnett JJ. Emerging adulthood: the winding road from the late teens through the twenties. New York NY: Oxford University Press; 2004.
11. Bower K, Schwartz V. Legal and ethical issues in college mental health. In: Kay J Schwartz V, eds. Mental health care in the college community. Hoboken, NJ: Wiley and Sons; 2010.
12. Hingson R, White A. Magnitude and prevention of college alcohol and drug misuse. In: Kay J Schwartz V, eds. Mental health care in the college community. Hoboken, NJ: Wiley and Sons; 2010.
Each year thousands of college students seek treatment at their school’s mental health service, but few psychiatrists are delivering this care. Most of the 4,500 degree-granting institutions of higher education (IHEs) in the United States1 provide some type of psychological or mental health counseling support to their students, and approximately 10% of the student body seeks care annually.2 In 2010, nearly 24% of students who visited their college counseling service were taking psychiatric medications at the time of their visit, up from 9% in 1994.2
Nevertheless, for various historical and practical reasons, psychiatrists have played—and continue to play—a somewhat peripheral role in college mental health systems. Although interest in psychiatric care at IHEs has been increasing (Box 1),3 until recently, most college counseling services had no direct access to psychiatric services and currently <1% of college services are directed by psychiatrists.2
This article examines some of the unique challenges faced by psychiatrists who work in a college mental health service, including how this setting may affect assessment, medication management, and crisis counseling. I use the terms “counseling services” and “mental health services” interchangeably because schools differ in the name they use for this office.
In recent years, the psychiatric community has begun to take steps to recognize college mental health as a specific practice area.3 In 2004, as president of the American Psychiatric Association (APA), Current Psychiatry Section Editor Michelle B. Riba, MD, MS convened a task force on college mental health. Subsequently, the APA added a section on college mental health to its public information Web site “Healthy Minds. Healthy Lives” (www.healthyminds.org).
The University of Michigan has taken a national leadership role in college psychiatry and college mental health. Since 2003, the University of Michigan Depression Center has hosted a yearly Depression on College Campuses national conference. Content from past conferences is available at www.depressioncenter.org/docc.
Organizations dedicated to college mental health and suicide prevention also have taken a role in disseminating information. Chief among them are the Jed Foundation, Active Minds on Campus, the Suicide Prevention Resource Center, Penn State’s Center for Collegiate Mental Health, and the National Research Consortium of Counseling Centers in Higher Education. More needs to be done to expand efforts related to college mental health and educate the psychiatric community and community at large about these vital concerns.
Managing medications and crises
Most college mental health services are directed by psychologists because of how IHEs historically structured these services (Box 2).4 Although counseling center staffing generally includes relatively few psychiatrists, those who do serve in this setting typically serve 2 primary roles: medication managers and crisis clinicians.
Medication management. Counseling centers are seeing more students who are either already taking psychotropics or need assessment and medication management. In the United States, 14% of students seen at college counseling centers are referred for psychiatric evaluation; however, on average, schools provide 2 hours of psychiatric services per week for every 1,000 students.2
Managing medications for college students poses several challenges. For most students, interaction with their college’s health and/or mental health system may be the first time they receive care not under the direct oversight of their family. Families and their feelings about psychiatric medication can play a major role in planning, executing, and managing psychiatric care, even for students who are legal adults. Family attitudes toward psychiatry and patients’ fears of disappointing parents who may feel distraught because their child has a psychiatric illness may impact a young person’s decision to accept medication or comply with treatment. Students often are insured by their family, and parents might receive an Explanation of Benefits and will learn of the student’s pharmacotherapy even if the student does not want them to know. College psychiatrists and students always need to consider decisions about how and when to include parents in discussions about medications.
College psychiatrists also must be sensitive to the unique vicissitudes of the school calendar and the developmental trajectory of college life. Decisions about when to start a medication or even which medication to prescribe might depend on how close a students is to exams or summer break. For example, a student experiencing severe anxiety a week before exams probably is better treated with a short-term benzodiazepine prescribed on an as-needed basis than a selective serotonin reuptake inhibitor, which in the first few weeks might only disrupt the student’s ability to function academically and not improve symptoms.5
Assessment also must consider the context of the academic year. For example, students may be “homesick” when they first enter college. Although such students may present with prominent and seemingly severe symptoms of anxiety or depression, more often than not the condition is self-limiting and resolves with support and watchful waiting. For example, many years ago a student presented to my institution’s counseling office in severe acute panic at the beginning of his first year at college. He had never been away from home, had not yet received his dorm phone, and did not know how to use a pay phone. His anxiety resolved as soon as I let him use my office phone to call his family. He ultimately made an excellent adjustment to college life.
Because most counseling services are set up primarily to provide talk therapy, most students who receive psychiatric medication also are engaged in psychotherapy. In these situations, psychiatrists must manage the same challenges in communication and coordination of care that occur in any split treatment agreement. These problems may be more easily addressed when the psychotherapist and prescriber both work in the college counseling center. Unfortunately, at some institutions, the psychiatrist or prescribing physician assistant or nurse practitioner may be based at the college’s health service,6 which can make coordination of care more challenging.
Crisis management. College counseling centers often manage students in crisis. Each year, approximately 6% of college students report suicidal ideation and 1% to 2% report suicide attempts.7 In 2010 there were 14 psychiatric hospitalizations for every 10,000 students on college campuses.2 Because psychiatrists are trained to manage patients with severe pathology and have emergency room training, college counseling psychiatrists often are looked to for assessment and consultation for students in crisis. In many cases, a student in crisis also will need psychopharmacologic intervention. In the event of a suicide or death on campus, the college psychiatrist often is called upon to address postvention planning and management of the clinical and community response.
Psychiatrists who manage student crises need to be cognizant of the unique elements of college life: Does the student live in a dormitory or with family? Could a relative who lives nearby help supervise an anxious patient who is cutting herself? Is the student in treatment with a therapist “back home” who could provide history or intervene? A crisis that occurs early in the school year, when a new student is less likely to have a network of friends or other supports, may need to be managed differently from one that occurs later, when the student might have people who could provide some comfort for a short time. The psychiatrist should know what level of support and supervision is available in the residence halls.
Although it is helpful for college counseling centers to maintain ongoing communication and coordination of services with local clinics and/or university medical centers, it is especially important for those who manage crises to have strong communication with local emergency rooms (ERs) and community crisis services. Because these services likely are managed by physicians, the campus psychiatrist is well placed to consult and coordinate care with local ERs because during a crisis, physician-to-physician communication often is more effective than campus counselor-to-ER physician communication. Ideally, college psychiatrists should have regular communication with ER physicians to discuss campus trends—such as particular drugs being used with unusual frequency or suicides on campus that might raise concerns of suicide contagion—and educate ER clinicians about services and programs the college offers.8
Barreira and Snider4 described the early development of college counseling services as flowing from 2 separate streams. Counseling services at colleges began to appear in the middle of the 20th century and grew out of academic and career advising offices. These programs typically had a “developmental”—as opposed to a clinical—orientation. Most of these services were and continue to be directed by counseling psychologists.
At the same time, some larger institutions—particularly the “Ivys”—hired psychiatrists to provide mental health care. Sometimes these clinicians were based at the school health service, while other institutions had parallel systems of counseling and mental health services. Today most colleges have integrated these programs into a single service.
An opportunity for training
Psychiatrists’ training may make them well-suited to address clinical issues that typically arise among college students. For example, students who have difficulty in college often struggle with issues at the border of physical and emotional health. Many students experience significant levels of stress, and many struggle with poor or inconsistent eating and nutrition and inadequate sleep. In fact, severely sleep-deprived students may present with symptoms that mimic depression.5 Psychiatrists have credibility in addressing these issues with individual students and the campus community. Psychiatrists also have training and experience in diagnosing and managing patients with substance abuse and can educate students, parents, faculty, and university administrators about these disorders.
College mental health services can be valuable training venues for senior psychiatric residents and child and adolescent fellows.9 College students can provide exposure to a broad array of problems—such as anxiety disorders, obsessive-compulsive disorder, dysthymic disorder, adjustment disorders, and panic disorder—that psychiatric trainees may not confront in a hospital clinic. Students are particularly responsive to short-term talk therapies with or without medication management, and working with this population can be a strong antidote to the “therapeutic nihilism”—the unfortunate sense that talk therapies are of limited effectiveness and that only pharmacotherapy can help psychiatric problems—often experienced by psychiatric trainees who spend much of their time working with patients with serious, chronic illnesses. Psychiatric residents can be particularly helpful in managing student patients who require combined medication and talk therapy.
College-based psychiatrists are well suited for educating residents about developmental issues of “emerging adulthood,” including:
- exploration of and anxieties about relationships and sexuality
- balancing connectedness to family with increasing sense of autonomy and independence
- establishing personal life goals and values and career choices and goals.10
College counseling services also are an excellent setting for residents to learn principles of community mental health and medico-legal concepts related to confidentiality, duty to warn, and disability law.11
Treatment outside college
Because college students have high rates of substance abuse12 and other psychiatric disorders, it is important for psychiatrists who treat these patients in private practice or community-based clinics to develop a basic awareness of and competency in relevant developmental and clinical issues. Psychiatrists who work in emergency services in areas with high concentrations of college students need to be particularly attuned to issues related to college mental health, substance abuse, and life on campus.8
Related Resources
- The Jed Foundation. www.jedfoundation.org.
- National Research Consortium of Counseling Centers in Higher Education. www.cmhc.utexas.edu/researchconsortium.html.
Disclosure
Dr. Schwartz reports no financial relationship with any companies whose products are mentioned in this article or with manufacturers of competing products.
Each year thousands of college students seek treatment at their school’s mental health service, but few psychiatrists are delivering this care. Most of the 4,500 degree-granting institutions of higher education (IHEs) in the United States1 provide some type of psychological or mental health counseling support to their students, and approximately 10% of the student body seeks care annually.2 In 2010, nearly 24% of students who visited their college counseling service were taking psychiatric medications at the time of their visit, up from 9% in 1994.2
Nevertheless, for various historical and practical reasons, psychiatrists have played—and continue to play—a somewhat peripheral role in college mental health systems. Although interest in psychiatric care at IHEs has been increasing (Box 1),3 until recently, most college counseling services had no direct access to psychiatric services and currently <1% of college services are directed by psychiatrists.2
This article examines some of the unique challenges faced by psychiatrists who work in a college mental health service, including how this setting may affect assessment, medication management, and crisis counseling. I use the terms “counseling services” and “mental health services” interchangeably because schools differ in the name they use for this office.
In recent years, the psychiatric community has begun to take steps to recognize college mental health as a specific practice area.3 In 2004, as president of the American Psychiatric Association (APA), Current Psychiatry Section Editor Michelle B. Riba, MD, MS convened a task force on college mental health. Subsequently, the APA added a section on college mental health to its public information Web site “Healthy Minds. Healthy Lives” (www.healthyminds.org).
The University of Michigan has taken a national leadership role in college psychiatry and college mental health. Since 2003, the University of Michigan Depression Center has hosted a yearly Depression on College Campuses national conference. Content from past conferences is available at www.depressioncenter.org/docc.
Organizations dedicated to college mental health and suicide prevention also have taken a role in disseminating information. Chief among them are the Jed Foundation, Active Minds on Campus, the Suicide Prevention Resource Center, Penn State’s Center for Collegiate Mental Health, and the National Research Consortium of Counseling Centers in Higher Education. More needs to be done to expand efforts related to college mental health and educate the psychiatric community and community at large about these vital concerns.
Managing medications and crises
Most college mental health services are directed by psychologists because of how IHEs historically structured these services (Box 2).4 Although counseling center staffing generally includes relatively few psychiatrists, those who do serve in this setting typically serve 2 primary roles: medication managers and crisis clinicians.
Medication management. Counseling centers are seeing more students who are either already taking psychotropics or need assessment and medication management. In the United States, 14% of students seen at college counseling centers are referred for psychiatric evaluation; however, on average, schools provide 2 hours of psychiatric services per week for every 1,000 students.2
Managing medications for college students poses several challenges. For most students, interaction with their college’s health and/or mental health system may be the first time they receive care not under the direct oversight of their family. Families and their feelings about psychiatric medication can play a major role in planning, executing, and managing psychiatric care, even for students who are legal adults. Family attitudes toward psychiatry and patients’ fears of disappointing parents who may feel distraught because their child has a psychiatric illness may impact a young person’s decision to accept medication or comply with treatment. Students often are insured by their family, and parents might receive an Explanation of Benefits and will learn of the student’s pharmacotherapy even if the student does not want them to know. College psychiatrists and students always need to consider decisions about how and when to include parents in discussions about medications.
College psychiatrists also must be sensitive to the unique vicissitudes of the school calendar and the developmental trajectory of college life. Decisions about when to start a medication or even which medication to prescribe might depend on how close a students is to exams or summer break. For example, a student experiencing severe anxiety a week before exams probably is better treated with a short-term benzodiazepine prescribed on an as-needed basis than a selective serotonin reuptake inhibitor, which in the first few weeks might only disrupt the student’s ability to function academically and not improve symptoms.5
Assessment also must consider the context of the academic year. For example, students may be “homesick” when they first enter college. Although such students may present with prominent and seemingly severe symptoms of anxiety or depression, more often than not the condition is self-limiting and resolves with support and watchful waiting. For example, many years ago a student presented to my institution’s counseling office in severe acute panic at the beginning of his first year at college. He had never been away from home, had not yet received his dorm phone, and did not know how to use a pay phone. His anxiety resolved as soon as I let him use my office phone to call his family. He ultimately made an excellent adjustment to college life.
Because most counseling services are set up primarily to provide talk therapy, most students who receive psychiatric medication also are engaged in psychotherapy. In these situations, psychiatrists must manage the same challenges in communication and coordination of care that occur in any split treatment agreement. These problems may be more easily addressed when the psychotherapist and prescriber both work in the college counseling center. Unfortunately, at some institutions, the psychiatrist or prescribing physician assistant or nurse practitioner may be based at the college’s health service,6 which can make coordination of care more challenging.
Crisis management. College counseling centers often manage students in crisis. Each year, approximately 6% of college students report suicidal ideation and 1% to 2% report suicide attempts.7 In 2010 there were 14 psychiatric hospitalizations for every 10,000 students on college campuses.2 Because psychiatrists are trained to manage patients with severe pathology and have emergency room training, college counseling psychiatrists often are looked to for assessment and consultation for students in crisis. In many cases, a student in crisis also will need psychopharmacologic intervention. In the event of a suicide or death on campus, the college psychiatrist often is called upon to address postvention planning and management of the clinical and community response.
Psychiatrists who manage student crises need to be cognizant of the unique elements of college life: Does the student live in a dormitory or with family? Could a relative who lives nearby help supervise an anxious patient who is cutting herself? Is the student in treatment with a therapist “back home” who could provide history or intervene? A crisis that occurs early in the school year, when a new student is less likely to have a network of friends or other supports, may need to be managed differently from one that occurs later, when the student might have people who could provide some comfort for a short time. The psychiatrist should know what level of support and supervision is available in the residence halls.
Although it is helpful for college counseling centers to maintain ongoing communication and coordination of services with local clinics and/or university medical centers, it is especially important for those who manage crises to have strong communication with local emergency rooms (ERs) and community crisis services. Because these services likely are managed by physicians, the campus psychiatrist is well placed to consult and coordinate care with local ERs because during a crisis, physician-to-physician communication often is more effective than campus counselor-to-ER physician communication. Ideally, college psychiatrists should have regular communication with ER physicians to discuss campus trends—such as particular drugs being used with unusual frequency or suicides on campus that might raise concerns of suicide contagion—and educate ER clinicians about services and programs the college offers.8
Barreira and Snider4 described the early development of college counseling services as flowing from 2 separate streams. Counseling services at colleges began to appear in the middle of the 20th century and grew out of academic and career advising offices. These programs typically had a “developmental”—as opposed to a clinical—orientation. Most of these services were and continue to be directed by counseling psychologists.
At the same time, some larger institutions—particularly the “Ivys”—hired psychiatrists to provide mental health care. Sometimes these clinicians were based at the school health service, while other institutions had parallel systems of counseling and mental health services. Today most colleges have integrated these programs into a single service.
An opportunity for training
Psychiatrists’ training may make them well-suited to address clinical issues that typically arise among college students. For example, students who have difficulty in college often struggle with issues at the border of physical and emotional health. Many students experience significant levels of stress, and many struggle with poor or inconsistent eating and nutrition and inadequate sleep. In fact, severely sleep-deprived students may present with symptoms that mimic depression.5 Psychiatrists have credibility in addressing these issues with individual students and the campus community. Psychiatrists also have training and experience in diagnosing and managing patients with substance abuse and can educate students, parents, faculty, and university administrators about these disorders.
College mental health services can be valuable training venues for senior psychiatric residents and child and adolescent fellows.9 College students can provide exposure to a broad array of problems—such as anxiety disorders, obsessive-compulsive disorder, dysthymic disorder, adjustment disorders, and panic disorder—that psychiatric trainees may not confront in a hospital clinic. Students are particularly responsive to short-term talk therapies with or without medication management, and working with this population can be a strong antidote to the “therapeutic nihilism”—the unfortunate sense that talk therapies are of limited effectiveness and that only pharmacotherapy can help psychiatric problems—often experienced by psychiatric trainees who spend much of their time working with patients with serious, chronic illnesses. Psychiatric residents can be particularly helpful in managing student patients who require combined medication and talk therapy.
College-based psychiatrists are well suited for educating residents about developmental issues of “emerging adulthood,” including:
- exploration of and anxieties about relationships and sexuality
- balancing connectedness to family with increasing sense of autonomy and independence
- establishing personal life goals and values and career choices and goals.10
College counseling services also are an excellent setting for residents to learn principles of community mental health and medico-legal concepts related to confidentiality, duty to warn, and disability law.11
Treatment outside college
Because college students have high rates of substance abuse12 and other psychiatric disorders, it is important for psychiatrists who treat these patients in private practice or community-based clinics to develop a basic awareness of and competency in relevant developmental and clinical issues. Psychiatrists who work in emergency services in areas with high concentrations of college students need to be particularly attuned to issues related to college mental health, substance abuse, and life on campus.8
Related Resources
- The Jed Foundation. www.jedfoundation.org.
- National Research Consortium of Counseling Centers in Higher Education. www.cmhc.utexas.edu/researchconsortium.html.
Disclosure
Dr. Schwartz reports no financial relationship with any companies whose products are mentioned in this article or with manufacturers of competing products.
1. US Department of Education National Center for Education Statistics. Degree-granting institutions by control and type of institution: Selected years, 1949-50 through 2009-10. http://nces.ed.gov/programs/digest/d10/tables/dt10_275.asp. Accessed August 22, 2011.
2. Gallagher RP. National survey of counseling center directors 2010. International Association of Counseling Services. http://www.iacsinc.org. Accessed August 22 2011.
3. Schwartz V, Kay J. The crisis in college and university mental health. Psychiatric Times. July 27 2009.
4. Barreira P, Snider M. History of college counseling and mental health services and role of the community mental health model. In: Kay J Schwartz V, eds. Mental health care in the college community. Hoboken, NJ: Wiley and Sons; 2010:21–31.
5. Schwartz V. Medications. In: Grayson PA Meilman PW, eds. College mental health practice. New York, NY: Routledge; 2006:59–78.
6. Barr V, Rando R, Krylowicz B, et al. The Association for University and College Counseling Centers Directors Annual Survey. 2010. http://aucccd.org/img/pdfs/aucccd_directors_survey_monograph_2010.pdf. Accessed September 1, 2011.
7. American College Health Association. National college health assessment II: reference group executive summary Fall 2010. Linthicum MD: American College Health Association; 2011.
8. Glick RL, Schwartz V. Assessment and management: special considerations for college students in psychiatric crisis. Psychiatric Issues in Emergency Care Settings. 2007;6(4):7-13.
9. Kay J, Schwartz V. Psychiatry residency training in college mental health services. In: Kay J Schwartz V, eds. Mental health care in the college community. Hoboken, NJ: Wiley and Sons; 2010.
10. Arnett JJ. Emerging adulthood: the winding road from the late teens through the twenties. New York NY: Oxford University Press; 2004.
11. Bower K, Schwartz V. Legal and ethical issues in college mental health. In: Kay J Schwartz V, eds. Mental health care in the college community. Hoboken, NJ: Wiley and Sons; 2010.
12. Hingson R, White A. Magnitude and prevention of college alcohol and drug misuse. In: Kay J Schwartz V, eds. Mental health care in the college community. Hoboken, NJ: Wiley and Sons; 2010.
1. US Department of Education National Center for Education Statistics. Degree-granting institutions by control and type of institution: Selected years, 1949-50 through 2009-10. http://nces.ed.gov/programs/digest/d10/tables/dt10_275.asp. Accessed August 22, 2011.
2. Gallagher RP. National survey of counseling center directors 2010. International Association of Counseling Services. http://www.iacsinc.org. Accessed August 22 2011.
3. Schwartz V, Kay J. The crisis in college and university mental health. Psychiatric Times. July 27 2009.
4. Barreira P, Snider M. History of college counseling and mental health services and role of the community mental health model. In: Kay J Schwartz V, eds. Mental health care in the college community. Hoboken, NJ: Wiley and Sons; 2010:21–31.
5. Schwartz V. Medications. In: Grayson PA Meilman PW, eds. College mental health practice. New York, NY: Routledge; 2006:59–78.
6. Barr V, Rando R, Krylowicz B, et al. The Association for University and College Counseling Centers Directors Annual Survey. 2010. http://aucccd.org/img/pdfs/aucccd_directors_survey_monograph_2010.pdf. Accessed September 1, 2011.
7. American College Health Association. National college health assessment II: reference group executive summary Fall 2010. Linthicum MD: American College Health Association; 2011.
8. Glick RL, Schwartz V. Assessment and management: special considerations for college students in psychiatric crisis. Psychiatric Issues in Emergency Care Settings. 2007;6(4):7-13.
9. Kay J, Schwartz V. Psychiatry residency training in college mental health services. In: Kay J Schwartz V, eds. Mental health care in the college community. Hoboken, NJ: Wiley and Sons; 2010.
10. Arnett JJ. Emerging adulthood: the winding road from the late teens through the twenties. New York NY: Oxford University Press; 2004.
11. Bower K, Schwartz V. Legal and ethical issues in college mental health. In: Kay J Schwartz V, eds. Mental health care in the college community. Hoboken, NJ: Wiley and Sons; 2010.
12. Hingson R, White A. Magnitude and prevention of college alcohol and drug misuse. In: Kay J Schwartz V, eds. Mental health care in the college community. Hoboken, NJ: Wiley and Sons; 2010.
Strategies to reduce alcohol use in problem drinkers
Alcohol dependence is not an acute illness. Progression from alcohol use to dependence typically takes years, and alcohol dependence is a chronic illness with symptom severity that increases over time.1,2 As the level of alcohol intake increases, the probability of developing an alcohol use disorder, cirrhosis, seizures, cancer, hypertension, stroke, and injuries significantly increases.3,4 Most alcohol-related harm occurs in high-risk drinkers who do not meet DSM-IV-TR criteria for an alcohol use disorder.5,6
Clinicians therefore have an opportunity to intervene at early stages of alcohol use to help prevent progression to dependence and reduce harm. The concept of a continuum from low-to high-risk alcohol use is replacing the disease-oriented model that focuses on identifying and treating individuals who meet DSM-IV-TR criteria for alcohol use disorders (Figure 1).4,7 Efforts to provide screening and intervention earlier in the disease course have:
- mainly been carried out in the primary care setting
- been aimed at nontreatment-seeking, nondependent individuals
- led to recommendations for safe alcohol consumption limits.8-10
One of the largest outreach efforts is a federally funded screening, brief intervention, referral to treatment (SBIRT) program established in 17 states that targets hospital emergency rooms, community health centers, and trauma centers.11
Although research on the use of brief interventions for problem drinking in psychiatric settings is limited,12-15 psychiatrists can employ these strategies, even during a brief medication management visit.
Figure 1: Recommended treatment based on type of alcohol use
NIAAA: National Institute on Alcohol Abuse and Alcoholism
Source: References 7,16
How much is too much?
The National Institute on Alcohol Abuse and Alcoholism (NIAAA) recommends men age <65 drink no more than 4 drinks/day and no more than 14 drinks/week and women of any age and men age ≥65 drink no more than 3 drinks/day and no more than 7 drinks/week.16 A standard drink is defined as 14 grams of absolute ethanol (12 ounces of beer, 5 ounces of wine, or 1.5 ounces of distilled spirits). Drinking above these levels is likely to result in harm and is defined as hazardous or at-risk use.10,17,18 Harmful use is alcohol consumption that has resulted in adverse mental or physical effects.10,17,18 We use “problem drinking” to describe both at-risk and harmful use as alcohol intake that exceeds NIAAA-recommended limits but does not meet DSM-IV-TR criteria for an alcohol use disorder.
In the United States, approximately 20% of the population exceeds NIAAA recommended alcohol intake guidelines without meeting criteria for an alcohol use disorder.18 Problem drinkers have an increased risk of developing an alcohol use disorder, nicotine dependence, liver disease, financial problems, marital disruptions, injuries, and driver’s license suspensions.4-6,19
Problem drinking is common in psychiatric populations and may lead to difficulties beyond those experienced in the general population.20,21 For example, problem drinking in individuals with bipolar disorder is associated with reduced medication compliance, greater functional impairment, and possibly more suicide attempts.22,23 Even moderate alcohol use below the NIAAA-recommend levels may increase symptom severity in patients with bipolar disorder.22,23
Interviewing techniques
Interventions to identify and treat problem drinkers have focused on the primary care setting because these physicians often are the only medical professionals problem drinkers encounter.8-10 The style of counseling is based on motivational interviewing. Providers use therapeutic empathy, describe risk, deal with ambivalence and resistance, assess motivation to change, emphasize patient responsibility and self-efficacy, and provide a menu of specific strategies to reduce alcohol use. However, unlike standard motivational interviewing, in brief interventions, providers use clear, directive advice to reduce alcohol consumption in the context of the medical provider role. Brief interventions also draw on cognitive-behavioral therapy and general education strategies utilizing contracting, goal setting, and written materials such as self-help manuals.24
Clinicians’ time demands have driven an effort to make interventions for problem drinkers efficient and time-limited. A single short intervention (5 to 15 minutes) can be effective, but 2 to 4 sessions of 5 to 15 minutes each seems to be more effective.8,10 The emphasis is on increasing a patient’s insight and awareness of risk to prompt him or her to establish and manage a goal to reduce alcohol intake. As opposed to abstinence, which is commonly recommended for patients with alcohol use disorders, reducing intake typically is encouraged for problem drinkers.9,10,16,18 For patients who use alcohol over the recommended limits and also meet criteria for an alcohol use disorder, clinicians can deliver a brief intervention, but should recommend abstinence from drinking and provide referral for further treatment.9,10,16,18
Compared with controls, problem drinkers who receive a brief intervention are twice as likely to moderate their drinking at 6 to 12 months25 and significantly reduce alcohol intake by approximately 4 drinks per week at follow-ups of 12 months or more.8,10,26 In addition, providing brief interventions significantly decreases the proportion of individuals whose alcohol intake exceeds recommended levels.10 A meta-analysis and a systemic review indicate brief interventions for problem drinkers reduce morbidity and mortality resulting from motor vehicle crashes, falls, suicide attempts, domestic violence, assaults, and child abuse.5,27
A brief intervention
Screening and brief intervention guidelines suggest a standard approach for assessing and managing problem drinking and alcohol use disorders.7,16 This approach can be described by the “5 As of intervention” (Table 1),10 which is one way to summarize the different brief intervention strategies described in the literature.7,9,10,16-18,28-30
Ask. Asking a question about any alcohol use is a simple way to initiate a conversation about the topic (“Do you sometimes drink beer, wine, or other alcoholic beverages?”). Incorporating questions about alcohol consumption into inquiries about other health habits (eg, smoking, exercise) may decrease patients’ defensiveness. If your patient reports using alcohol, follow up by screening for ≥5 drinks a day for men or ≥4 drinks a day for women (“In the past year, how many times have you had 5 or more drinks a day?”). Use at this level ≥1 times a year indicates a positive screen that provides good sensitivity and specificity for detecting problem drinking and alcohol use disorders.18 Then ask about weekly alcohol intake (“On average, how many days a week do you have an alcoholic drink?” “On a typical drinking day how many drinks do you have?”). Presenting a chart that describes what constitutes a standard drink—available from the NIAAA (see Related Resources)—may be helpful.
You also can use additional questions from the CAGE screening test (Table 2).31 In clinics with a more formal screening protocol, patients may be asked to complete a written self-report such as the Alcohol Use Disorders Identification Test (AUDIT). This 10-question survey covers domains of alcohol consumption, drinking behavior, and alcohol-related problems.17,18 If your patient is a problem drinker, further assessment could determine if he or she has an alcohol use disorder.
Advise. Provide feedback about your patient’s drinking and its consequences along with clear recommendations in an empathic, nonconfrontational manner (“You’re drinking more than is medically safe; I strongly recommend you cut down”). Comparing your patient’s drinking pattern to population norms may be helpful (“Less than 20% of people drink a much as you”).7,16 When possible, tie the consequences of the patient’s drinking to his or her current physical, mental, family, social, and legal concerns. Convey your concerns empathically, as a medical provider providing health recommendations (“As your doctor, I am concerned about how much you drink and how it is affecting your health”). However, to respect patient autonomy, give a medical recommendation, rather than a directive (“As your doctor, I feel I should tell you” rather than “You should”). Finally, express a clear message of your willingness to help.
Assess your patient’s readiness to change (“Given what we’ve talked about, are you willing to consider making changes in your drinking?”). Showing the links between alcohol use and personal consequences may improve patient engagement (“If you keep drinking at your current levels, do you think your goal of improving your grades will be easier, harder, or no different?”).28 If your patient is willing to make a change, negotiate a patient-specific goal, such as reducing drinking to within the recommended limits, using alcohol only a few days a week instead of every day, or abstaining for a defined period. If the patient is not willing to change, restate your concern regarding his or her health-related consequences and your willingness to help.
Assist. After you’ve negotiated a goal with your patient, discuss a treatment plan to help the patient achieve the goal. This should include steps the patient will take to reduce or quit drinking. Consider offering handouts on standard drink sizes, alcohol-associated harms, and strategies for cutting down or abstaining (eg, pacing use, spacing drinks by including nonalcoholic beverages, plans to handle urges, using alcohol money for other items), and calendars for tracking drinking (ie, a drinking diary). Help your patient identify situations where he or she is likely to have difficulty achieving the goal and strategies for avoiding or managing such situations. Ask patients to identify a family member or friend who can help them. Refer patients who have an alcohol use disorder to addiction treatment.
For patients who are not willing to change, discuss the perceived benefits of continued drinking vs reducing or stopping drinking to encourage them to reflect on their alcohol use patterns. Discuss any potential barriers to change. Keep in mind ambivalence and reluctance to change drinking patterns are common and many patients are unaware of the risks of their alcohol use. This discussion may lead patients to contemplate change later.
Arrange. Schedule a follow-up appointment to reinforce the treatment plan with further support, feedback, and assistance in setting, achieving, and maintaining realistic goals. Consider a follow-up phone call 2 weeks after the brief intervention to check on progress and a follow-up appointment in 1 month. Clinical staff may have the opportunity to e-mail or text message patients to check on progress between face-to-face visits. At the follow-up appointment, ask if your patient was able to meet and sustain the drinking goal. If so, support continued adherence, renegotiate drinking goals if indicated, and encourage follow-up with at least annual rescreening. When patients are unable to meet their treatment goals, acknowledge that change is difficult, encourage any positive changes, and address barriers to reaching the goal. Reemphasize your willingness to help, reevaluate the diagnosis, treatment plan, and goals, and schedule close follow-up. Consider engaging significant others in the treatment process.
Table 1
Brief interventions: ‘5As’ to address alcohol use
Ask: Screen for use
|
Advise: Provide strong direct personal advice to change
|
Assess: Determine willingness to change
|
Assist: Help the patient make a change if he or she is ready
|
Arrange: Reinforce change effort with follow-up
|
| Source: Reference 10 |
Table 2
CAGE questionnaire to detect alcohol use disorders
| Cut down | ‘Have you ever felt you ought to cut down on your drinking?’ |
| Annoyed | ‘Have people annoyed you by criticizing your drinking?’ |
| Guilt | ‘Have you ever felt bad or guilty about your drinking?’ |
| Eye-opener | ‘Have you ever had a drink first thing in the morning to steady your nerves or get rid of a hangover?’ |
| Source: Reference 31 | |
Use in psychiatric practice
Problem drinking may not be adequately addressed in psychiatric settings. In a survey of problem drinkers identified in the general population, only 64% discussed their drinking during a mental health visit and only 40% received counseling about their alcohol use from their mental health provider.32 In a study of 200 psychiatric inpatients, 49% exhibited problem drinking as measured by AUDIT, but only 27% of patients had alcohol use recorded in their medical record.33 In addition, routine use of screening tools such as CAGE or AUDIT appears to be low in many psychiatric settings even though research has shown that tools such as AUDIT or computerized screening may be effective for identifying problem drinking in psychiatric outpatient settings.20,21,34
Several small studies support the efficacy of brief interventions for problem drinking in psychiatric settings. A study in a psychiatric emergency service found patients with either schizophrenia/bipolar disorder or depression/anxiety decreased their drinking by about 7 drinks a week over 6 months after a brief intervention.15 This study was small and the decrease in alcohol intake was not significant within the 2 population groups (P = .10 for schizophrenia/bipolar disorder, n = 34, P = .05 for depression/anxiety, n = 53); however, there was a significant decrease for all patients with follow-up (P = .0096, N = 55).15 In another study, psychiatric inpatients with problem drinking who received a brief motivational intervention demonstrated a statistically significant reduction in alcohol consumption at 6 months compared with patients who received only an information packet,13 but health-related outcomes at 5 years did not differ between the 2 groups.14 Finally, in a study of 344 nonpsychotic psychiatric outpatients with problem drinking, one-half of those who received a brief telephone intervention reduced their drinking to non-hazardous levels at a 6-month follow-up (intervention 43.8%, control 27.7%).12
- National Institute on Alcohol Abuse and Alcoholism. www.niaaa.nih.gov.
- Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption—II. Addiction. 1993;88(6):791-804.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Schuckit MA, Anthenelli RM, Bucholz KK, et al. The time course of development of alcohol-related problems in men and women. J Stud Alcohol. 1995;56(2):218-225.
2. Wagner FA, Anthony JC. From first drug use to drug dependence; developmental periods of risk for dependence upon marijuana cocaine, and alcohol. Neuropsychopharmacology. 2002;26(4):479-488.
3. Rehm J, Room R, Graham K, et al. The relationship of average volume of alcohol consumption and patterns of drinking to burden of disease: an overview. Addiction. 2003;98(9):1209-1228.
4. Dawson DA, Li TK, Grant BF. A prospective study of risk drinking: at risk for what? Drug Alcohol Depend. 2008;95(1-2):62-72.
5. Dinh-Zarr T, Goss C, Heitman E, et al. Interventions for preventing injuries in problem drinkers. Cochrane Database Syst Rev. 2004;(3):CD001857.-
6. Bradley KA, Donovan DM, Larson EB. How much is too much? Advising patients about safe levels of alcohol consumption. Arch Intern Med. 1993;153(24):2734-2740.
7. Babor TF, Higgins-Biddle JC. Brief intervention for hazardous and harmful drinking: a manual for use in primary care. World Health Organization. http://whqlibdoc.who.int/hq/2001/WHO_MSD_MSB_01.6b.pdf. Accessed August 18 2011.
8. Kaner EF, Dickinson HO, Beyer F, et al. The effectiveness of brief alcohol interventions in primary care settings: a systematic review. Drug Alcohol Rev. 2009;28(3):301-323.
9. Fleming M, Manwell LB. Brief intervention in primary care settings. A primary treatment method for at-risk problem, and dependent drinkers. Alcohol Res Health. 1999;23(2):128-137.
10. Whitlock EP, Polen MR, Green CA, et al. Behavioral counseling interventions in primary care to reduce risky/harmful alcohol use by adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2004;140(7):557-568.
11. Madras BK, Compton WM, Avula D, et al. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99(1-3):280-295.
12. Eberhard S, Nordström G, Höglund P, et al. Secondary prevention of hazardous alcohol consumption in psychiatric out-patients: a randomised controlled study. Soc Psychiatry Psychiatr Epidemiol. 2009;44(12):1013-1021.
13. Hulse GK, Tait RJ. Six-month outcomes associated with a brief alcohol intervention for adult in-patients with psychiatric disorders. Drug Alcohol Rev. 2002;21(2):105-112.
14. Hulse GK, Tait RJ. Five-year outcomes of a brief alcohol intervention for adult in-patients with psychiatric disorders. Addiction. 2003;98(8):1061-1068.
15. Milner KK, Barry KL, Blow FC, et al. Brief interventions for patients presenting to the Psychiatric Emergency Service (PES) with major mental illnesses and at-risk drinking. Community Ment Health J. 2010;46(2):149-155.
16. U.S. Department of Health and Human Services. Helping patients who drink too much: a clinician’s guide. http://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/guide.pdf. Updated 2005. Accessed August 18, 2011.
17. Fiellin DA, Reid MC, O’Connor PG. Outpatient management of patients with alcohol problems. Ann Intern Med. 2000;133(10):815-827.
18. Willenbring ML, Massey SH, Gardner MB. Helping patients who drink too much: an evidence-based guide for primary care clinicians. Am Fam Physician. 2009;80(1):44-50.
19. Batty GD, Lewars H, Emslie C, et al. Internationally recognized guidelines for ‘sensible’ alcohol consumption: is exceeding them actually detrimental to health and social circumstances? Evidence from a population-based cohort study. J Public Health (Oxf). 2009;31(3):360-365.
20. Barry KL, Milner K, Blow FC, et al. Screening psychiatric emergency department patients with major mental illnesses for at-risk drinking. Psychiatr Serv. 2006;57(7):1039-1042.
21. Satre D, Wolfe W, Eisendrath S, et al. Computerized screening for alcohol and drug use among adults seeking outpatient psychiatric services. Psychiatr Serv. 2008;59(4):441-444.
22. Goldstein BI, Velyvis VP, Parikh SV. The association between moderate alcohol use and illness severity in bipolar disorder: a preliminary report. J Clin Psychiatry. 2006;67(1):102-106.
23. Lagerberg TV, Andreassen OA, Ringen PA, et al. Excessive substance use in bipolar disorder is associated with impaired functioning rather than clinical characteristics, a descriptive study. BMC Psychiatry. 2010;10:9.-
24. Fleming MF, Balousek SL, Grossberg PM, et al. Brief physician advice for heavy drinking college students: a randomized controlled trial in college health clinics. J Stud Alcohol Drugs. 2010;71(1):23-31.
25. Wilk AI, Jensen NM, Havighurst TC. Meta-analysis of randomized control trials addressing brief interventions in heavy alcohol drinkers. J Gen Intern Med. 1997;12(5):274-283.
26. Bertholet N, Daeppen JB, Wietlisbach V, et al. Reduction of alcohol consumption by brief alcohol intervention in primary care: systematic review and meta-analysis. Arch Intern Med. 2005;165(9):986-995.
27. Cuijpers P, Riper H, Lemmers L. The effects on mortality of brief interventions for problem drinking: a meta-analysis. Addiction. 2004;99(7):839-845.
28. Grossberg P, Halperin A, Mackenzie S, et al. Inside the physician’s black bag: critical ingredients of brief alcohol interventions. Subst Abus. 2010;31(4):240-250.
29. D’Onofrio G, Pantalon MV, Degutis LC, et al. Development and implementation of an emergency practitioner-performed brief intervention for hazardous and harmful drinkers in the emergency department. Acad Emerg Med. 2005;12(3):249-256.
30. Moyer A, Finney JW, Swearingen CE, et al. Brief interventions for alcohol problems: a meta-analytic review of controlled investigations in treatment-seeking and non-treatment-seeking populations. Addiction. 2002;97(3):279-292.
31. Ewing JA. Detecting alcoholism: the CAGE questionnaire. JAMA. 1984;252(14):1905-1907.
32. Weisner C, Matzger H. Missed opportunities in addressing drinking behavior in medical and mental health services. Alcohol Clin Exp Res. 2003;27(7):1132-1141.
33. Barnaby B, Drummond C, McCloud A, et al. Substance misuse in psychiatric inpatients: comparison of a screening questionnaire survey with case notes. BMJ. 2003;327(7418):783-784.
34. Freimuth M. Another missed opportunity? Recognition of alcohol use problems by mental health providers. Psychotherapy: Theory Research, Practice, Training. 2008;45(3):405-409.
Alcohol dependence is not an acute illness. Progression from alcohol use to dependence typically takes years, and alcohol dependence is a chronic illness with symptom severity that increases over time.1,2 As the level of alcohol intake increases, the probability of developing an alcohol use disorder, cirrhosis, seizures, cancer, hypertension, stroke, and injuries significantly increases.3,4 Most alcohol-related harm occurs in high-risk drinkers who do not meet DSM-IV-TR criteria for an alcohol use disorder.5,6
Clinicians therefore have an opportunity to intervene at early stages of alcohol use to help prevent progression to dependence and reduce harm. The concept of a continuum from low-to high-risk alcohol use is replacing the disease-oriented model that focuses on identifying and treating individuals who meet DSM-IV-TR criteria for alcohol use disorders (Figure 1).4,7 Efforts to provide screening and intervention earlier in the disease course have:
- mainly been carried out in the primary care setting
- been aimed at nontreatment-seeking, nondependent individuals
- led to recommendations for safe alcohol consumption limits.8-10
One of the largest outreach efforts is a federally funded screening, brief intervention, referral to treatment (SBIRT) program established in 17 states that targets hospital emergency rooms, community health centers, and trauma centers.11
Although research on the use of brief interventions for problem drinking in psychiatric settings is limited,12-15 psychiatrists can employ these strategies, even during a brief medication management visit.
Figure 1: Recommended treatment based on type of alcohol use
NIAAA: National Institute on Alcohol Abuse and Alcoholism
Source: References 7,16
How much is too much?
The National Institute on Alcohol Abuse and Alcoholism (NIAAA) recommends men age <65 drink no more than 4 drinks/day and no more than 14 drinks/week and women of any age and men age ≥65 drink no more than 3 drinks/day and no more than 7 drinks/week.16 A standard drink is defined as 14 grams of absolute ethanol (12 ounces of beer, 5 ounces of wine, or 1.5 ounces of distilled spirits). Drinking above these levels is likely to result in harm and is defined as hazardous or at-risk use.10,17,18 Harmful use is alcohol consumption that has resulted in adverse mental or physical effects.10,17,18 We use “problem drinking” to describe both at-risk and harmful use as alcohol intake that exceeds NIAAA-recommended limits but does not meet DSM-IV-TR criteria for an alcohol use disorder.
In the United States, approximately 20% of the population exceeds NIAAA recommended alcohol intake guidelines without meeting criteria for an alcohol use disorder.18 Problem drinkers have an increased risk of developing an alcohol use disorder, nicotine dependence, liver disease, financial problems, marital disruptions, injuries, and driver’s license suspensions.4-6,19
Problem drinking is common in psychiatric populations and may lead to difficulties beyond those experienced in the general population.20,21 For example, problem drinking in individuals with bipolar disorder is associated with reduced medication compliance, greater functional impairment, and possibly more suicide attempts.22,23 Even moderate alcohol use below the NIAAA-recommend levels may increase symptom severity in patients with bipolar disorder.22,23
Interviewing techniques
Interventions to identify and treat problem drinkers have focused on the primary care setting because these physicians often are the only medical professionals problem drinkers encounter.8-10 The style of counseling is based on motivational interviewing. Providers use therapeutic empathy, describe risk, deal with ambivalence and resistance, assess motivation to change, emphasize patient responsibility and self-efficacy, and provide a menu of specific strategies to reduce alcohol use. However, unlike standard motivational interviewing, in brief interventions, providers use clear, directive advice to reduce alcohol consumption in the context of the medical provider role. Brief interventions also draw on cognitive-behavioral therapy and general education strategies utilizing contracting, goal setting, and written materials such as self-help manuals.24
Clinicians’ time demands have driven an effort to make interventions for problem drinkers efficient and time-limited. A single short intervention (5 to 15 minutes) can be effective, but 2 to 4 sessions of 5 to 15 minutes each seems to be more effective.8,10 The emphasis is on increasing a patient’s insight and awareness of risk to prompt him or her to establish and manage a goal to reduce alcohol intake. As opposed to abstinence, which is commonly recommended for patients with alcohol use disorders, reducing intake typically is encouraged for problem drinkers.9,10,16,18 For patients who use alcohol over the recommended limits and also meet criteria for an alcohol use disorder, clinicians can deliver a brief intervention, but should recommend abstinence from drinking and provide referral for further treatment.9,10,16,18
Compared with controls, problem drinkers who receive a brief intervention are twice as likely to moderate their drinking at 6 to 12 months25 and significantly reduce alcohol intake by approximately 4 drinks per week at follow-ups of 12 months or more.8,10,26 In addition, providing brief interventions significantly decreases the proportion of individuals whose alcohol intake exceeds recommended levels.10 A meta-analysis and a systemic review indicate brief interventions for problem drinkers reduce morbidity and mortality resulting from motor vehicle crashes, falls, suicide attempts, domestic violence, assaults, and child abuse.5,27
A brief intervention
Screening and brief intervention guidelines suggest a standard approach for assessing and managing problem drinking and alcohol use disorders.7,16 This approach can be described by the “5 As of intervention” (Table 1),10 which is one way to summarize the different brief intervention strategies described in the literature.7,9,10,16-18,28-30
Ask. Asking a question about any alcohol use is a simple way to initiate a conversation about the topic (“Do you sometimes drink beer, wine, or other alcoholic beverages?”). Incorporating questions about alcohol consumption into inquiries about other health habits (eg, smoking, exercise) may decrease patients’ defensiveness. If your patient reports using alcohol, follow up by screening for ≥5 drinks a day for men or ≥4 drinks a day for women (“In the past year, how many times have you had 5 or more drinks a day?”). Use at this level ≥1 times a year indicates a positive screen that provides good sensitivity and specificity for detecting problem drinking and alcohol use disorders.18 Then ask about weekly alcohol intake (“On average, how many days a week do you have an alcoholic drink?” “On a typical drinking day how many drinks do you have?”). Presenting a chart that describes what constitutes a standard drink—available from the NIAAA (see Related Resources)—may be helpful.
You also can use additional questions from the CAGE screening test (Table 2).31 In clinics with a more formal screening protocol, patients may be asked to complete a written self-report such as the Alcohol Use Disorders Identification Test (AUDIT). This 10-question survey covers domains of alcohol consumption, drinking behavior, and alcohol-related problems.17,18 If your patient is a problem drinker, further assessment could determine if he or she has an alcohol use disorder.
Advise. Provide feedback about your patient’s drinking and its consequences along with clear recommendations in an empathic, nonconfrontational manner (“You’re drinking more than is medically safe; I strongly recommend you cut down”). Comparing your patient’s drinking pattern to population norms may be helpful (“Less than 20% of people drink a much as you”).7,16 When possible, tie the consequences of the patient’s drinking to his or her current physical, mental, family, social, and legal concerns. Convey your concerns empathically, as a medical provider providing health recommendations (“As your doctor, I am concerned about how much you drink and how it is affecting your health”). However, to respect patient autonomy, give a medical recommendation, rather than a directive (“As your doctor, I feel I should tell you” rather than “You should”). Finally, express a clear message of your willingness to help.
Assess your patient’s readiness to change (“Given what we’ve talked about, are you willing to consider making changes in your drinking?”). Showing the links between alcohol use and personal consequences may improve patient engagement (“If you keep drinking at your current levels, do you think your goal of improving your grades will be easier, harder, or no different?”).28 If your patient is willing to make a change, negotiate a patient-specific goal, such as reducing drinking to within the recommended limits, using alcohol only a few days a week instead of every day, or abstaining for a defined period. If the patient is not willing to change, restate your concern regarding his or her health-related consequences and your willingness to help.
Assist. After you’ve negotiated a goal with your patient, discuss a treatment plan to help the patient achieve the goal. This should include steps the patient will take to reduce or quit drinking. Consider offering handouts on standard drink sizes, alcohol-associated harms, and strategies for cutting down or abstaining (eg, pacing use, spacing drinks by including nonalcoholic beverages, plans to handle urges, using alcohol money for other items), and calendars for tracking drinking (ie, a drinking diary). Help your patient identify situations where he or she is likely to have difficulty achieving the goal and strategies for avoiding or managing such situations. Ask patients to identify a family member or friend who can help them. Refer patients who have an alcohol use disorder to addiction treatment.
For patients who are not willing to change, discuss the perceived benefits of continued drinking vs reducing or stopping drinking to encourage them to reflect on their alcohol use patterns. Discuss any potential barriers to change. Keep in mind ambivalence and reluctance to change drinking patterns are common and many patients are unaware of the risks of their alcohol use. This discussion may lead patients to contemplate change later.
Arrange. Schedule a follow-up appointment to reinforce the treatment plan with further support, feedback, and assistance in setting, achieving, and maintaining realistic goals. Consider a follow-up phone call 2 weeks after the brief intervention to check on progress and a follow-up appointment in 1 month. Clinical staff may have the opportunity to e-mail or text message patients to check on progress between face-to-face visits. At the follow-up appointment, ask if your patient was able to meet and sustain the drinking goal. If so, support continued adherence, renegotiate drinking goals if indicated, and encourage follow-up with at least annual rescreening. When patients are unable to meet their treatment goals, acknowledge that change is difficult, encourage any positive changes, and address barriers to reaching the goal. Reemphasize your willingness to help, reevaluate the diagnosis, treatment plan, and goals, and schedule close follow-up. Consider engaging significant others in the treatment process.
Table 1
Brief interventions: ‘5As’ to address alcohol use
Ask: Screen for use
|
Advise: Provide strong direct personal advice to change
|
Assess: Determine willingness to change
|
Assist: Help the patient make a change if he or she is ready
|
Arrange: Reinforce change effort with follow-up
|
| Source: Reference 10 |
Table 2
CAGE questionnaire to detect alcohol use disorders
| Cut down | ‘Have you ever felt you ought to cut down on your drinking?’ |
| Annoyed | ‘Have people annoyed you by criticizing your drinking?’ |
| Guilt | ‘Have you ever felt bad or guilty about your drinking?’ |
| Eye-opener | ‘Have you ever had a drink first thing in the morning to steady your nerves or get rid of a hangover?’ |
| Source: Reference 31 | |
Use in psychiatric practice
Problem drinking may not be adequately addressed in psychiatric settings. In a survey of problem drinkers identified in the general population, only 64% discussed their drinking during a mental health visit and only 40% received counseling about their alcohol use from their mental health provider.32 In a study of 200 psychiatric inpatients, 49% exhibited problem drinking as measured by AUDIT, but only 27% of patients had alcohol use recorded in their medical record.33 In addition, routine use of screening tools such as CAGE or AUDIT appears to be low in many psychiatric settings even though research has shown that tools such as AUDIT or computerized screening may be effective for identifying problem drinking in psychiatric outpatient settings.20,21,34
Several small studies support the efficacy of brief interventions for problem drinking in psychiatric settings. A study in a psychiatric emergency service found patients with either schizophrenia/bipolar disorder or depression/anxiety decreased their drinking by about 7 drinks a week over 6 months after a brief intervention.15 This study was small and the decrease in alcohol intake was not significant within the 2 population groups (P = .10 for schizophrenia/bipolar disorder, n = 34, P = .05 for depression/anxiety, n = 53); however, there was a significant decrease for all patients with follow-up (P = .0096, N = 55).15 In another study, psychiatric inpatients with problem drinking who received a brief motivational intervention demonstrated a statistically significant reduction in alcohol consumption at 6 months compared with patients who received only an information packet,13 but health-related outcomes at 5 years did not differ between the 2 groups.14 Finally, in a study of 344 nonpsychotic psychiatric outpatients with problem drinking, one-half of those who received a brief telephone intervention reduced their drinking to non-hazardous levels at a 6-month follow-up (intervention 43.8%, control 27.7%).12
- National Institute on Alcohol Abuse and Alcoholism. www.niaaa.nih.gov.
- Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption—II. Addiction. 1993;88(6):791-804.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Alcohol dependence is not an acute illness. Progression from alcohol use to dependence typically takes years, and alcohol dependence is a chronic illness with symptom severity that increases over time.1,2 As the level of alcohol intake increases, the probability of developing an alcohol use disorder, cirrhosis, seizures, cancer, hypertension, stroke, and injuries significantly increases.3,4 Most alcohol-related harm occurs in high-risk drinkers who do not meet DSM-IV-TR criteria for an alcohol use disorder.5,6
Clinicians therefore have an opportunity to intervene at early stages of alcohol use to help prevent progression to dependence and reduce harm. The concept of a continuum from low-to high-risk alcohol use is replacing the disease-oriented model that focuses on identifying and treating individuals who meet DSM-IV-TR criteria for alcohol use disorders (Figure 1).4,7 Efforts to provide screening and intervention earlier in the disease course have:
- mainly been carried out in the primary care setting
- been aimed at nontreatment-seeking, nondependent individuals
- led to recommendations for safe alcohol consumption limits.8-10
One of the largest outreach efforts is a federally funded screening, brief intervention, referral to treatment (SBIRT) program established in 17 states that targets hospital emergency rooms, community health centers, and trauma centers.11
Although research on the use of brief interventions for problem drinking in psychiatric settings is limited,12-15 psychiatrists can employ these strategies, even during a brief medication management visit.
Figure 1: Recommended treatment based on type of alcohol use
NIAAA: National Institute on Alcohol Abuse and Alcoholism
Source: References 7,16
How much is too much?
The National Institute on Alcohol Abuse and Alcoholism (NIAAA) recommends men age <65 drink no more than 4 drinks/day and no more than 14 drinks/week and women of any age and men age ≥65 drink no more than 3 drinks/day and no more than 7 drinks/week.16 A standard drink is defined as 14 grams of absolute ethanol (12 ounces of beer, 5 ounces of wine, or 1.5 ounces of distilled spirits). Drinking above these levels is likely to result in harm and is defined as hazardous or at-risk use.10,17,18 Harmful use is alcohol consumption that has resulted in adverse mental or physical effects.10,17,18 We use “problem drinking” to describe both at-risk and harmful use as alcohol intake that exceeds NIAAA-recommended limits but does not meet DSM-IV-TR criteria for an alcohol use disorder.
In the United States, approximately 20% of the population exceeds NIAAA recommended alcohol intake guidelines without meeting criteria for an alcohol use disorder.18 Problem drinkers have an increased risk of developing an alcohol use disorder, nicotine dependence, liver disease, financial problems, marital disruptions, injuries, and driver’s license suspensions.4-6,19
Problem drinking is common in psychiatric populations and may lead to difficulties beyond those experienced in the general population.20,21 For example, problem drinking in individuals with bipolar disorder is associated with reduced medication compliance, greater functional impairment, and possibly more suicide attempts.22,23 Even moderate alcohol use below the NIAAA-recommend levels may increase symptom severity in patients with bipolar disorder.22,23
Interviewing techniques
Interventions to identify and treat problem drinkers have focused on the primary care setting because these physicians often are the only medical professionals problem drinkers encounter.8-10 The style of counseling is based on motivational interviewing. Providers use therapeutic empathy, describe risk, deal with ambivalence and resistance, assess motivation to change, emphasize patient responsibility and self-efficacy, and provide a menu of specific strategies to reduce alcohol use. However, unlike standard motivational interviewing, in brief interventions, providers use clear, directive advice to reduce alcohol consumption in the context of the medical provider role. Brief interventions also draw on cognitive-behavioral therapy and general education strategies utilizing contracting, goal setting, and written materials such as self-help manuals.24
Clinicians’ time demands have driven an effort to make interventions for problem drinkers efficient and time-limited. A single short intervention (5 to 15 minutes) can be effective, but 2 to 4 sessions of 5 to 15 minutes each seems to be more effective.8,10 The emphasis is on increasing a patient’s insight and awareness of risk to prompt him or her to establish and manage a goal to reduce alcohol intake. As opposed to abstinence, which is commonly recommended for patients with alcohol use disorders, reducing intake typically is encouraged for problem drinkers.9,10,16,18 For patients who use alcohol over the recommended limits and also meet criteria for an alcohol use disorder, clinicians can deliver a brief intervention, but should recommend abstinence from drinking and provide referral for further treatment.9,10,16,18
Compared with controls, problem drinkers who receive a brief intervention are twice as likely to moderate their drinking at 6 to 12 months25 and significantly reduce alcohol intake by approximately 4 drinks per week at follow-ups of 12 months or more.8,10,26 In addition, providing brief interventions significantly decreases the proportion of individuals whose alcohol intake exceeds recommended levels.10 A meta-analysis and a systemic review indicate brief interventions for problem drinkers reduce morbidity and mortality resulting from motor vehicle crashes, falls, suicide attempts, domestic violence, assaults, and child abuse.5,27
A brief intervention
Screening and brief intervention guidelines suggest a standard approach for assessing and managing problem drinking and alcohol use disorders.7,16 This approach can be described by the “5 As of intervention” (Table 1),10 which is one way to summarize the different brief intervention strategies described in the literature.7,9,10,16-18,28-30
Ask. Asking a question about any alcohol use is a simple way to initiate a conversation about the topic (“Do you sometimes drink beer, wine, or other alcoholic beverages?”). Incorporating questions about alcohol consumption into inquiries about other health habits (eg, smoking, exercise) may decrease patients’ defensiveness. If your patient reports using alcohol, follow up by screening for ≥5 drinks a day for men or ≥4 drinks a day for women (“In the past year, how many times have you had 5 or more drinks a day?”). Use at this level ≥1 times a year indicates a positive screen that provides good sensitivity and specificity for detecting problem drinking and alcohol use disorders.18 Then ask about weekly alcohol intake (“On average, how many days a week do you have an alcoholic drink?” “On a typical drinking day how many drinks do you have?”). Presenting a chart that describes what constitutes a standard drink—available from the NIAAA (see Related Resources)—may be helpful.
You also can use additional questions from the CAGE screening test (Table 2).31 In clinics with a more formal screening protocol, patients may be asked to complete a written self-report such as the Alcohol Use Disorders Identification Test (AUDIT). This 10-question survey covers domains of alcohol consumption, drinking behavior, and alcohol-related problems.17,18 If your patient is a problem drinker, further assessment could determine if he or she has an alcohol use disorder.
Advise. Provide feedback about your patient’s drinking and its consequences along with clear recommendations in an empathic, nonconfrontational manner (“You’re drinking more than is medically safe; I strongly recommend you cut down”). Comparing your patient’s drinking pattern to population norms may be helpful (“Less than 20% of people drink a much as you”).7,16 When possible, tie the consequences of the patient’s drinking to his or her current physical, mental, family, social, and legal concerns. Convey your concerns empathically, as a medical provider providing health recommendations (“As your doctor, I am concerned about how much you drink and how it is affecting your health”). However, to respect patient autonomy, give a medical recommendation, rather than a directive (“As your doctor, I feel I should tell you” rather than “You should”). Finally, express a clear message of your willingness to help.
Assess your patient’s readiness to change (“Given what we’ve talked about, are you willing to consider making changes in your drinking?”). Showing the links between alcohol use and personal consequences may improve patient engagement (“If you keep drinking at your current levels, do you think your goal of improving your grades will be easier, harder, or no different?”).28 If your patient is willing to make a change, negotiate a patient-specific goal, such as reducing drinking to within the recommended limits, using alcohol only a few days a week instead of every day, or abstaining for a defined period. If the patient is not willing to change, restate your concern regarding his or her health-related consequences and your willingness to help.
Assist. After you’ve negotiated a goal with your patient, discuss a treatment plan to help the patient achieve the goal. This should include steps the patient will take to reduce or quit drinking. Consider offering handouts on standard drink sizes, alcohol-associated harms, and strategies for cutting down or abstaining (eg, pacing use, spacing drinks by including nonalcoholic beverages, plans to handle urges, using alcohol money for other items), and calendars for tracking drinking (ie, a drinking diary). Help your patient identify situations where he or she is likely to have difficulty achieving the goal and strategies for avoiding or managing such situations. Ask patients to identify a family member or friend who can help them. Refer patients who have an alcohol use disorder to addiction treatment.
For patients who are not willing to change, discuss the perceived benefits of continued drinking vs reducing or stopping drinking to encourage them to reflect on their alcohol use patterns. Discuss any potential barriers to change. Keep in mind ambivalence and reluctance to change drinking patterns are common and many patients are unaware of the risks of their alcohol use. This discussion may lead patients to contemplate change later.
Arrange. Schedule a follow-up appointment to reinforce the treatment plan with further support, feedback, and assistance in setting, achieving, and maintaining realistic goals. Consider a follow-up phone call 2 weeks after the brief intervention to check on progress and a follow-up appointment in 1 month. Clinical staff may have the opportunity to e-mail or text message patients to check on progress between face-to-face visits. At the follow-up appointment, ask if your patient was able to meet and sustain the drinking goal. If so, support continued adherence, renegotiate drinking goals if indicated, and encourage follow-up with at least annual rescreening. When patients are unable to meet their treatment goals, acknowledge that change is difficult, encourage any positive changes, and address barriers to reaching the goal. Reemphasize your willingness to help, reevaluate the diagnosis, treatment plan, and goals, and schedule close follow-up. Consider engaging significant others in the treatment process.
Table 1
Brief interventions: ‘5As’ to address alcohol use
Ask: Screen for use
|
Advise: Provide strong direct personal advice to change
|
Assess: Determine willingness to change
|
Assist: Help the patient make a change if he or she is ready
|
Arrange: Reinforce change effort with follow-up
|
| Source: Reference 10 |
Table 2
CAGE questionnaire to detect alcohol use disorders
| Cut down | ‘Have you ever felt you ought to cut down on your drinking?’ |
| Annoyed | ‘Have people annoyed you by criticizing your drinking?’ |
| Guilt | ‘Have you ever felt bad or guilty about your drinking?’ |
| Eye-opener | ‘Have you ever had a drink first thing in the morning to steady your nerves or get rid of a hangover?’ |
| Source: Reference 31 | |
Use in psychiatric practice
Problem drinking may not be adequately addressed in psychiatric settings. In a survey of problem drinkers identified in the general population, only 64% discussed their drinking during a mental health visit and only 40% received counseling about their alcohol use from their mental health provider.32 In a study of 200 psychiatric inpatients, 49% exhibited problem drinking as measured by AUDIT, but only 27% of patients had alcohol use recorded in their medical record.33 In addition, routine use of screening tools such as CAGE or AUDIT appears to be low in many psychiatric settings even though research has shown that tools such as AUDIT or computerized screening may be effective for identifying problem drinking in psychiatric outpatient settings.20,21,34
Several small studies support the efficacy of brief interventions for problem drinking in psychiatric settings. A study in a psychiatric emergency service found patients with either schizophrenia/bipolar disorder or depression/anxiety decreased their drinking by about 7 drinks a week over 6 months after a brief intervention.15 This study was small and the decrease in alcohol intake was not significant within the 2 population groups (P = .10 for schizophrenia/bipolar disorder, n = 34, P = .05 for depression/anxiety, n = 53); however, there was a significant decrease for all patients with follow-up (P = .0096, N = 55).15 In another study, psychiatric inpatients with problem drinking who received a brief motivational intervention demonstrated a statistically significant reduction in alcohol consumption at 6 months compared with patients who received only an information packet,13 but health-related outcomes at 5 years did not differ between the 2 groups.14 Finally, in a study of 344 nonpsychotic psychiatric outpatients with problem drinking, one-half of those who received a brief telephone intervention reduced their drinking to non-hazardous levels at a 6-month follow-up (intervention 43.8%, control 27.7%).12
- National Institute on Alcohol Abuse and Alcoholism. www.niaaa.nih.gov.
- Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption—II. Addiction. 1993;88(6):791-804.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Schuckit MA, Anthenelli RM, Bucholz KK, et al. The time course of development of alcohol-related problems in men and women. J Stud Alcohol. 1995;56(2):218-225.
2. Wagner FA, Anthony JC. From first drug use to drug dependence; developmental periods of risk for dependence upon marijuana cocaine, and alcohol. Neuropsychopharmacology. 2002;26(4):479-488.
3. Rehm J, Room R, Graham K, et al. The relationship of average volume of alcohol consumption and patterns of drinking to burden of disease: an overview. Addiction. 2003;98(9):1209-1228.
4. Dawson DA, Li TK, Grant BF. A prospective study of risk drinking: at risk for what? Drug Alcohol Depend. 2008;95(1-2):62-72.
5. Dinh-Zarr T, Goss C, Heitman E, et al. Interventions for preventing injuries in problem drinkers. Cochrane Database Syst Rev. 2004;(3):CD001857.-
6. Bradley KA, Donovan DM, Larson EB. How much is too much? Advising patients about safe levels of alcohol consumption. Arch Intern Med. 1993;153(24):2734-2740.
7. Babor TF, Higgins-Biddle JC. Brief intervention for hazardous and harmful drinking: a manual for use in primary care. World Health Organization. http://whqlibdoc.who.int/hq/2001/WHO_MSD_MSB_01.6b.pdf. Accessed August 18 2011.
8. Kaner EF, Dickinson HO, Beyer F, et al. The effectiveness of brief alcohol interventions in primary care settings: a systematic review. Drug Alcohol Rev. 2009;28(3):301-323.
9. Fleming M, Manwell LB. Brief intervention in primary care settings. A primary treatment method for at-risk problem, and dependent drinkers. Alcohol Res Health. 1999;23(2):128-137.
10. Whitlock EP, Polen MR, Green CA, et al. Behavioral counseling interventions in primary care to reduce risky/harmful alcohol use by adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2004;140(7):557-568.
11. Madras BK, Compton WM, Avula D, et al. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99(1-3):280-295.
12. Eberhard S, Nordström G, Höglund P, et al. Secondary prevention of hazardous alcohol consumption in psychiatric out-patients: a randomised controlled study. Soc Psychiatry Psychiatr Epidemiol. 2009;44(12):1013-1021.
13. Hulse GK, Tait RJ. Six-month outcomes associated with a brief alcohol intervention for adult in-patients with psychiatric disorders. Drug Alcohol Rev. 2002;21(2):105-112.
14. Hulse GK, Tait RJ. Five-year outcomes of a brief alcohol intervention for adult in-patients with psychiatric disorders. Addiction. 2003;98(8):1061-1068.
15. Milner KK, Barry KL, Blow FC, et al. Brief interventions for patients presenting to the Psychiatric Emergency Service (PES) with major mental illnesses and at-risk drinking. Community Ment Health J. 2010;46(2):149-155.
16. U.S. Department of Health and Human Services. Helping patients who drink too much: a clinician’s guide. http://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/guide.pdf. Updated 2005. Accessed August 18, 2011.
17. Fiellin DA, Reid MC, O’Connor PG. Outpatient management of patients with alcohol problems. Ann Intern Med. 2000;133(10):815-827.
18. Willenbring ML, Massey SH, Gardner MB. Helping patients who drink too much: an evidence-based guide for primary care clinicians. Am Fam Physician. 2009;80(1):44-50.
19. Batty GD, Lewars H, Emslie C, et al. Internationally recognized guidelines for ‘sensible’ alcohol consumption: is exceeding them actually detrimental to health and social circumstances? Evidence from a population-based cohort study. J Public Health (Oxf). 2009;31(3):360-365.
20. Barry KL, Milner K, Blow FC, et al. Screening psychiatric emergency department patients with major mental illnesses for at-risk drinking. Psychiatr Serv. 2006;57(7):1039-1042.
21. Satre D, Wolfe W, Eisendrath S, et al. Computerized screening for alcohol and drug use among adults seeking outpatient psychiatric services. Psychiatr Serv. 2008;59(4):441-444.
22. Goldstein BI, Velyvis VP, Parikh SV. The association between moderate alcohol use and illness severity in bipolar disorder: a preliminary report. J Clin Psychiatry. 2006;67(1):102-106.
23. Lagerberg TV, Andreassen OA, Ringen PA, et al. Excessive substance use in bipolar disorder is associated with impaired functioning rather than clinical characteristics, a descriptive study. BMC Psychiatry. 2010;10:9.-
24. Fleming MF, Balousek SL, Grossberg PM, et al. Brief physician advice for heavy drinking college students: a randomized controlled trial in college health clinics. J Stud Alcohol Drugs. 2010;71(1):23-31.
25. Wilk AI, Jensen NM, Havighurst TC. Meta-analysis of randomized control trials addressing brief interventions in heavy alcohol drinkers. J Gen Intern Med. 1997;12(5):274-283.
26. Bertholet N, Daeppen JB, Wietlisbach V, et al. Reduction of alcohol consumption by brief alcohol intervention in primary care: systematic review and meta-analysis. Arch Intern Med. 2005;165(9):986-995.
27. Cuijpers P, Riper H, Lemmers L. The effects on mortality of brief interventions for problem drinking: a meta-analysis. Addiction. 2004;99(7):839-845.
28. Grossberg P, Halperin A, Mackenzie S, et al. Inside the physician’s black bag: critical ingredients of brief alcohol interventions. Subst Abus. 2010;31(4):240-250.
29. D’Onofrio G, Pantalon MV, Degutis LC, et al. Development and implementation of an emergency practitioner-performed brief intervention for hazardous and harmful drinkers in the emergency department. Acad Emerg Med. 2005;12(3):249-256.
30. Moyer A, Finney JW, Swearingen CE, et al. Brief interventions for alcohol problems: a meta-analytic review of controlled investigations in treatment-seeking and non-treatment-seeking populations. Addiction. 2002;97(3):279-292.
31. Ewing JA. Detecting alcoholism: the CAGE questionnaire. JAMA. 1984;252(14):1905-1907.
32. Weisner C, Matzger H. Missed opportunities in addressing drinking behavior in medical and mental health services. Alcohol Clin Exp Res. 2003;27(7):1132-1141.
33. Barnaby B, Drummond C, McCloud A, et al. Substance misuse in psychiatric inpatients: comparison of a screening questionnaire survey with case notes. BMJ. 2003;327(7418):783-784.
34. Freimuth M. Another missed opportunity? Recognition of alcohol use problems by mental health providers. Psychotherapy: Theory Research, Practice, Training. 2008;45(3):405-409.
1. Schuckit MA, Anthenelli RM, Bucholz KK, et al. The time course of development of alcohol-related problems in men and women. J Stud Alcohol. 1995;56(2):218-225.
2. Wagner FA, Anthony JC. From first drug use to drug dependence; developmental periods of risk for dependence upon marijuana cocaine, and alcohol. Neuropsychopharmacology. 2002;26(4):479-488.
3. Rehm J, Room R, Graham K, et al. The relationship of average volume of alcohol consumption and patterns of drinking to burden of disease: an overview. Addiction. 2003;98(9):1209-1228.
4. Dawson DA, Li TK, Grant BF. A prospective study of risk drinking: at risk for what? Drug Alcohol Depend. 2008;95(1-2):62-72.
5. Dinh-Zarr T, Goss C, Heitman E, et al. Interventions for preventing injuries in problem drinkers. Cochrane Database Syst Rev. 2004;(3):CD001857.-
6. Bradley KA, Donovan DM, Larson EB. How much is too much? Advising patients about safe levels of alcohol consumption. Arch Intern Med. 1993;153(24):2734-2740.
7. Babor TF, Higgins-Biddle JC. Brief intervention for hazardous and harmful drinking: a manual for use in primary care. World Health Organization. http://whqlibdoc.who.int/hq/2001/WHO_MSD_MSB_01.6b.pdf. Accessed August 18 2011.
8. Kaner EF, Dickinson HO, Beyer F, et al. The effectiveness of brief alcohol interventions in primary care settings: a systematic review. Drug Alcohol Rev. 2009;28(3):301-323.
9. Fleming M, Manwell LB. Brief intervention in primary care settings. A primary treatment method for at-risk problem, and dependent drinkers. Alcohol Res Health. 1999;23(2):128-137.
10. Whitlock EP, Polen MR, Green CA, et al. Behavioral counseling interventions in primary care to reduce risky/harmful alcohol use by adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2004;140(7):557-568.
11. Madras BK, Compton WM, Avula D, et al. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99(1-3):280-295.
12. Eberhard S, Nordström G, Höglund P, et al. Secondary prevention of hazardous alcohol consumption in psychiatric out-patients: a randomised controlled study. Soc Psychiatry Psychiatr Epidemiol. 2009;44(12):1013-1021.
13. Hulse GK, Tait RJ. Six-month outcomes associated with a brief alcohol intervention for adult in-patients with psychiatric disorders. Drug Alcohol Rev. 2002;21(2):105-112.
14. Hulse GK, Tait RJ. Five-year outcomes of a brief alcohol intervention for adult in-patients with psychiatric disorders. Addiction. 2003;98(8):1061-1068.
15. Milner KK, Barry KL, Blow FC, et al. Brief interventions for patients presenting to the Psychiatric Emergency Service (PES) with major mental illnesses and at-risk drinking. Community Ment Health J. 2010;46(2):149-155.
16. U.S. Department of Health and Human Services. Helping patients who drink too much: a clinician’s guide. http://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/guide.pdf. Updated 2005. Accessed August 18, 2011.
17. Fiellin DA, Reid MC, O’Connor PG. Outpatient management of patients with alcohol problems. Ann Intern Med. 2000;133(10):815-827.
18. Willenbring ML, Massey SH, Gardner MB. Helping patients who drink too much: an evidence-based guide for primary care clinicians. Am Fam Physician. 2009;80(1):44-50.
19. Batty GD, Lewars H, Emslie C, et al. Internationally recognized guidelines for ‘sensible’ alcohol consumption: is exceeding them actually detrimental to health and social circumstances? Evidence from a population-based cohort study. J Public Health (Oxf). 2009;31(3):360-365.
20. Barry KL, Milner K, Blow FC, et al. Screening psychiatric emergency department patients with major mental illnesses for at-risk drinking. Psychiatr Serv. 2006;57(7):1039-1042.
21. Satre D, Wolfe W, Eisendrath S, et al. Computerized screening for alcohol and drug use among adults seeking outpatient psychiatric services. Psychiatr Serv. 2008;59(4):441-444.
22. Goldstein BI, Velyvis VP, Parikh SV. The association between moderate alcohol use and illness severity in bipolar disorder: a preliminary report. J Clin Psychiatry. 2006;67(1):102-106.
23. Lagerberg TV, Andreassen OA, Ringen PA, et al. Excessive substance use in bipolar disorder is associated with impaired functioning rather than clinical characteristics, a descriptive study. BMC Psychiatry. 2010;10:9.-
24. Fleming MF, Balousek SL, Grossberg PM, et al. Brief physician advice for heavy drinking college students: a randomized controlled trial in college health clinics. J Stud Alcohol Drugs. 2010;71(1):23-31.
25. Wilk AI, Jensen NM, Havighurst TC. Meta-analysis of randomized control trials addressing brief interventions in heavy alcohol drinkers. J Gen Intern Med. 1997;12(5):274-283.
26. Bertholet N, Daeppen JB, Wietlisbach V, et al. Reduction of alcohol consumption by brief alcohol intervention in primary care: systematic review and meta-analysis. Arch Intern Med. 2005;165(9):986-995.
27. Cuijpers P, Riper H, Lemmers L. The effects on mortality of brief interventions for problem drinking: a meta-analysis. Addiction. 2004;99(7):839-845.
28. Grossberg P, Halperin A, Mackenzie S, et al. Inside the physician’s black bag: critical ingredients of brief alcohol interventions. Subst Abus. 2010;31(4):240-250.
29. D’Onofrio G, Pantalon MV, Degutis LC, et al. Development and implementation of an emergency practitioner-performed brief intervention for hazardous and harmful drinkers in the emergency department. Acad Emerg Med. 2005;12(3):249-256.
30. Moyer A, Finney JW, Swearingen CE, et al. Brief interventions for alcohol problems: a meta-analytic review of controlled investigations in treatment-seeking and non-treatment-seeking populations. Addiction. 2002;97(3):279-292.
31. Ewing JA. Detecting alcoholism: the CAGE questionnaire. JAMA. 1984;252(14):1905-1907.
32. Weisner C, Matzger H. Missed opportunities in addressing drinking behavior in medical and mental health services. Alcohol Clin Exp Res. 2003;27(7):1132-1141.
33. Barnaby B, Drummond C, McCloud A, et al. Substance misuse in psychiatric inpatients: comparison of a screening questionnaire survey with case notes. BMJ. 2003;327(7418):783-784.
34. Freimuth M. Another missed opportunity? Recognition of alcohol use problems by mental health providers. Psychotherapy: Theory Research, Practice, Training. 2008;45(3):405-409.