User login
Can a vaccine prevent Alzheimer’s disease?
Deposition of amyloid-β peptide (Aβ) is believed to contribute to Alzheimer’s disease (AD) pathogenesis. Derived from a larger precursor protein, Aβ aggregates into plaques, and may promote neuronal death and, ultimately, dementia.
Current treatments alleviate symptoms without slowing underlying neurodegeneration. The prospect of harnessing the immune system to target the Aβ peptide offers an intriguing option for preventing this devastating, increasingly common disease.
Anti-a BETA antibodies
Transgenic mice bred to overexpress AD genes have responded remarkably in studies using the immune system to target the amyloid-β peptide.3 Several mouse groups have shown plaque reduction (Figure 1) and improved cognitive performance. These findings substantiate the amyloid hypothesis in AD pathogenesis.
A host could acquire anti-Aβ antibodies though two basic approaches (Figure 2):2
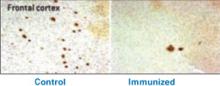
Figure 1 Differences in amyloid deposition between control and immunized mice
Frontal cortex of an unvaccinated mouse (left) shows more amyloid deposits (dark spots) than that of a mouse producing antibodies against the amyloid-β peptide (right).
Source: Image by Cynthia A. Lemere, PhD. Used with permission.Active immunization exposes the subject to the antigen (in this case the Aβ peptide) and allows T cells and B cells to produce anti-Aβ antibodies. This approach has been studied in humans, but adverse effects have stymied its development.
Passive immunization, which involves developing anti-Aβ antibodies in a separate source, aims to clear Aβ peptide without requiring an immunologic response from the host. Large doses of antibodies administered weekly or monthly would be needed to build adequate plasma levels in the CNS, and large quantities of circulating antibodies could cause hemorrhagic stroke.
A troublesome trial
After successful preclinical and phase 1 testing of a vaccine against the Aβ peptide (called AN-1792), a phase 2a placebo-controlled trial in 2001 followed patients with mild to moderate AD. Drug administration was halted after 18 patients (6%) developed meningoencephalitis after several months.4 However, 300 patients with AD and 72 control patients had received at least one injection, and double-blind assessments were maintained for 12 months.
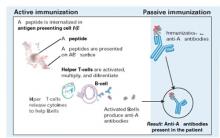
Figure 2 Methods for immunizing against Aβ peptide
Active immunization produces anti-Aβ antibodies via immunologic response to vaccination. With passive immunization, anti-Aβ antibodies are administered directly.
Illustration by Rich LaRocco.All patients with meningoencephalitis had received the vaccine but not all developed an immune response, suggesting that something other than the antibodies—such as T cells—caused the encephalitis. Twelve patients recovered, but six had persistent cognitive and neurologic deficits.
More-optimistic news
Of the 300 patients who received an active vaccine, 20% developed an adequate antibody response.5
The responders showed no significant difference from the placebo group in most outcome measures but showed less worsening in the nine-component Neuropsychological Test Battery (NTB) (P=0.02). Of particular interest, antibody responders showed significant improvement in the NTB—s memory domain (P=0.03). Further, subjects with higher IgG antibody titers showed greater improvement than did other responders.
More work ahead
Although the outcome of this initial AN-1792 trial is disappointing because of its discontinuation and mixed results, T cell infiltration and amyloid depletion were found during postmortem examinations of two vaccine recipients.6
Pharmaceutical companies are testing two compounds for AD immunotherapy:7
- AAB-001, a human monoclonal antibody, targets all 42 Aβ amino acids via passive immunization and has entered phase 2 trials.
- ACC-001, an Aβ immuno-conjugate designed to elicit an active antibody response, began phase 1 testing last fall.
These efforts suggest that an “Alzheimer’s vaccine” could be produced, provided it could attack the Aβ peptide without inducing a significant cellular reaction.
1. Neugroschl JA, Kolevzor A, Samuels SC, Marir DB. Dementia. In: Sadock BJ, Sadock VA (eds). Kaplan & Sadock’s comprehensive text-book of psychiatry (8th ed). Philadelphia: Lippincott Williams & Wilkins; 2005:1068-93.
2. Schenk D. Amyloid-beta immunotherapy for Alzheimer’s disease: the end of the beginning. Nat Rev Neurosci 2002;3:824-8.
3. Schenk D, Hagen M, Seubert P. Current progress in beta-amyloid immunotherapy. Current Opin Immunol 2004;16:599-606.
4. Orgogozo JM, Gilman S, Dartigues JF, et al. Subacute meningoen-cephalitis in a subset of patients with AD after Abeta42 immunization. Neurology 2003;61:46-54.
5. Gilman S, Koller M, Black RS, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology 2005;64:1553-62.
6. Ferrer I, Boada Rovira M, Sanchez Guerra ML, et al. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer’s disease. Brain Pathology 2004;14(1):11-20.
7. Sullivan MG. Immunotherapy studies for AD back on track. Psychiatry News 2005;33(11):69.-
Deposition of amyloid-β peptide (Aβ) is believed to contribute to Alzheimer’s disease (AD) pathogenesis. Derived from a larger precursor protein, Aβ aggregates into plaques, and may promote neuronal death and, ultimately, dementia.
Current treatments alleviate symptoms without slowing underlying neurodegeneration. The prospect of harnessing the immune system to target the Aβ peptide offers an intriguing option for preventing this devastating, increasingly common disease.
Anti-a BETA antibodies
Transgenic mice bred to overexpress AD genes have responded remarkably in studies using the immune system to target the amyloid-β peptide.3 Several mouse groups have shown plaque reduction (Figure 1) and improved cognitive performance. These findings substantiate the amyloid hypothesis in AD pathogenesis.
A host could acquire anti-Aβ antibodies though two basic approaches (Figure 2):2
Figure 1 Differences in amyloid deposition between control and immunized mice
Frontal cortex of an unvaccinated mouse (left) shows more amyloid deposits (dark spots) than that of a mouse producing antibodies against the amyloid-β peptide (right).
Source: Image by Cynthia A. Lemere, PhD. Used with permission.Active immunization exposes the subject to the antigen (in this case the Aβ peptide) and allows T cells and B cells to produce anti-Aβ antibodies. This approach has been studied in humans, but adverse effects have stymied its development.
Passive immunization, which involves developing anti-Aβ antibodies in a separate source, aims to clear Aβ peptide without requiring an immunologic response from the host. Large doses of antibodies administered weekly or monthly would be needed to build adequate plasma levels in the CNS, and large quantities of circulating antibodies could cause hemorrhagic stroke.
A troublesome trial
After successful preclinical and phase 1 testing of a vaccine against the Aβ peptide (called AN-1792), a phase 2a placebo-controlled trial in 2001 followed patients with mild to moderate AD. Drug administration was halted after 18 patients (6%) developed meningoencephalitis after several months.4 However, 300 patients with AD and 72 control patients had received at least one injection, and double-blind assessments were maintained for 12 months.
Figure 2 Methods for immunizing against Aβ peptide
Active immunization produces anti-Aβ antibodies via immunologic response to vaccination. With passive immunization, anti-Aβ antibodies are administered directly.
Illustration by Rich LaRocco.All patients with meningoencephalitis had received the vaccine but not all developed an immune response, suggesting that something other than the antibodies—such as T cells—caused the encephalitis. Twelve patients recovered, but six had persistent cognitive and neurologic deficits.
More-optimistic news
Of the 300 patients who received an active vaccine, 20% developed an adequate antibody response.5
The responders showed no significant difference from the placebo group in most outcome measures but showed less worsening in the nine-component Neuropsychological Test Battery (NTB) (P=0.02). Of particular interest, antibody responders showed significant improvement in the NTB—s memory domain (P=0.03). Further, subjects with higher IgG antibody titers showed greater improvement than did other responders.
More work ahead
Although the outcome of this initial AN-1792 trial is disappointing because of its discontinuation and mixed results, T cell infiltration and amyloid depletion were found during postmortem examinations of two vaccine recipients.6
Pharmaceutical companies are testing two compounds for AD immunotherapy:7
- AAB-001, a human monoclonal antibody, targets all 42 Aβ amino acids via passive immunization and has entered phase 2 trials.
- ACC-001, an Aβ immuno-conjugate designed to elicit an active antibody response, began phase 1 testing last fall.
These efforts suggest that an “Alzheimer’s vaccine” could be produced, provided it could attack the Aβ peptide without inducing a significant cellular reaction.
Deposition of amyloid-β peptide (Aβ) is believed to contribute to Alzheimer’s disease (AD) pathogenesis. Derived from a larger precursor protein, Aβ aggregates into plaques, and may promote neuronal death and, ultimately, dementia.
Current treatments alleviate symptoms without slowing underlying neurodegeneration. The prospect of harnessing the immune system to target the Aβ peptide offers an intriguing option for preventing this devastating, increasingly common disease.
Anti-a BETA antibodies
Transgenic mice bred to overexpress AD genes have responded remarkably in studies using the immune system to target the amyloid-β peptide.3 Several mouse groups have shown plaque reduction (Figure 1) and improved cognitive performance. These findings substantiate the amyloid hypothesis in AD pathogenesis.
A host could acquire anti-Aβ antibodies though two basic approaches (Figure 2):2
Figure 1 Differences in amyloid deposition between control and immunized mice
Frontal cortex of an unvaccinated mouse (left) shows more amyloid deposits (dark spots) than that of a mouse producing antibodies against the amyloid-β peptide (right).
Source: Image by Cynthia A. Lemere, PhD. Used with permission.Active immunization exposes the subject to the antigen (in this case the Aβ peptide) and allows T cells and B cells to produce anti-Aβ antibodies. This approach has been studied in humans, but adverse effects have stymied its development.
Passive immunization, which involves developing anti-Aβ antibodies in a separate source, aims to clear Aβ peptide without requiring an immunologic response from the host. Large doses of antibodies administered weekly or monthly would be needed to build adequate plasma levels in the CNS, and large quantities of circulating antibodies could cause hemorrhagic stroke.
A troublesome trial
After successful preclinical and phase 1 testing of a vaccine against the Aβ peptide (called AN-1792), a phase 2a placebo-controlled trial in 2001 followed patients with mild to moderate AD. Drug administration was halted after 18 patients (6%) developed meningoencephalitis after several months.4 However, 300 patients with AD and 72 control patients had received at least one injection, and double-blind assessments were maintained for 12 months.
Figure 2 Methods for immunizing against Aβ peptide
Active immunization produces anti-Aβ antibodies via immunologic response to vaccination. With passive immunization, anti-Aβ antibodies are administered directly.
Illustration by Rich LaRocco.All patients with meningoencephalitis had received the vaccine but not all developed an immune response, suggesting that something other than the antibodies—such as T cells—caused the encephalitis. Twelve patients recovered, but six had persistent cognitive and neurologic deficits.
More-optimistic news
Of the 300 patients who received an active vaccine, 20% developed an adequate antibody response.5
The responders showed no significant difference from the placebo group in most outcome measures but showed less worsening in the nine-component Neuropsychological Test Battery (NTB) (P=0.02). Of particular interest, antibody responders showed significant improvement in the NTB—s memory domain (P=0.03). Further, subjects with higher IgG antibody titers showed greater improvement than did other responders.
More work ahead
Although the outcome of this initial AN-1792 trial is disappointing because of its discontinuation and mixed results, T cell infiltration and amyloid depletion were found during postmortem examinations of two vaccine recipients.6
Pharmaceutical companies are testing two compounds for AD immunotherapy:7
- AAB-001, a human monoclonal antibody, targets all 42 Aβ amino acids via passive immunization and has entered phase 2 trials.
- ACC-001, an Aβ immuno-conjugate designed to elicit an active antibody response, began phase 1 testing last fall.
These efforts suggest that an “Alzheimer’s vaccine” could be produced, provided it could attack the Aβ peptide without inducing a significant cellular reaction.
1. Neugroschl JA, Kolevzor A, Samuels SC, Marir DB. Dementia. In: Sadock BJ, Sadock VA (eds). Kaplan & Sadock’s comprehensive text-book of psychiatry (8th ed). Philadelphia: Lippincott Williams & Wilkins; 2005:1068-93.
2. Schenk D. Amyloid-beta immunotherapy for Alzheimer’s disease: the end of the beginning. Nat Rev Neurosci 2002;3:824-8.
3. Schenk D, Hagen M, Seubert P. Current progress in beta-amyloid immunotherapy. Current Opin Immunol 2004;16:599-606.
4. Orgogozo JM, Gilman S, Dartigues JF, et al. Subacute meningoen-cephalitis in a subset of patients with AD after Abeta42 immunization. Neurology 2003;61:46-54.
5. Gilman S, Koller M, Black RS, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology 2005;64:1553-62.
6. Ferrer I, Boada Rovira M, Sanchez Guerra ML, et al. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer’s disease. Brain Pathology 2004;14(1):11-20.
7. Sullivan MG. Immunotherapy studies for AD back on track. Psychiatry News 2005;33(11):69.-
1. Neugroschl JA, Kolevzor A, Samuels SC, Marir DB. Dementia. In: Sadock BJ, Sadock VA (eds). Kaplan & Sadock’s comprehensive text-book of psychiatry (8th ed). Philadelphia: Lippincott Williams & Wilkins; 2005:1068-93.
2. Schenk D. Amyloid-beta immunotherapy for Alzheimer’s disease: the end of the beginning. Nat Rev Neurosci 2002;3:824-8.
3. Schenk D, Hagen M, Seubert P. Current progress in beta-amyloid immunotherapy. Current Opin Immunol 2004;16:599-606.
4. Orgogozo JM, Gilman S, Dartigues JF, et al. Subacute meningoen-cephalitis in a subset of patients with AD after Abeta42 immunization. Neurology 2003;61:46-54.
5. Gilman S, Koller M, Black RS, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology 2005;64:1553-62.
6. Ferrer I, Boada Rovira M, Sanchez Guerra ML, et al. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer’s disease. Brain Pathology 2004;14(1):11-20.
7. Sullivan MG. Immunotherapy studies for AD back on track. Psychiatry News 2005;33(11):69.-
CATIE’s surprises: In antipsychotics’ square-off, were there winners or losers?
Investigators faced a dilemma while designing the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE). More than 200 enrollees with chronic schizophrenia had pre-existing tardive dyskinesia (TD). Would it be ethical to give them the antipsychotic most likely to worsen their TD? Would exempting them from taking that drug influence the trial’s outcome?
This issue and others had to be resolved before the largest controlled study of “real world” schizophrenia could begin. Now that data are unfolding, groups with diverse agendas are debating CATIE’s methods and surprising results. This article describes how the trial’s design and findings could transform public policy and clinical practice.
poll here
Efficacy vs Effectiveness
The National Institute of Mental Health funded the prospective CATIE schizophrenia study to compare the effectiveness of atypical antipsychotics versus each other and versus a first-generation (typical) antipsychotic.
All approved atypicals have shown similar efficacy compared with placebo in short-term trials (usually 6 weeks).1 The CATIE trial’s rationale is that short-term efficacy studies required for FDA approval may not necessarily reflect the drugs’ effectiveness in long-term schizophrenia management. Effectiveness measures take into account efficacy as well as safety, tolerability, and unpredictable patient behaviors in the real world.
CATIE’s ‘Real World’ Patients
CATIE investigators enrolled a community sample of chronic schizophrenia patients similar to those many psychiatrists see. Very liberal inclusion and exclusion criteria (Table 1) allowed enrollees to have a history of substance abuse, comorbid psychiatric or medical disorders, be receiving other medications, or show evidence of TD. Their schizophrenia ranged from minimal to severe.2,3
The 1,493 patients who completed the study (Table 2) were enrolled at 57 outpatient treatment settings. One site’s 33 patients were eliminated from analysis because of doubts about the integrity of the data, leaving a total of 1,460 subjects.4
Table 1
Criteria for enrolling patients in the CATIE schizophrenia trial
| Inclusion criteria | Ages 18 to 65 yrs |
| DSM-IV diagnosis of schizophrenia | |
| Able to take oral medication | |
| Able to give informed consent | |
| Exclusion criteria | Diagnosis of schizoaffective disorder, mental retardation, or other cognitive disorders |
| History of serious adverse reactions to one of the study medications | |
| Had only one schizophrenic episode | |
| History of treatment resistance, defined as persistence of severe symptoms despite adequate trials of one of the study antipsychotics or prior treatment with clozapine | |
| Pregnant or breast feeding | |
| Serious and unstable medical conditions |
Table 2
CATIE’s 1,460 ‘real world’ schizophrenia patients at trial entry
| Mean age | 40.6±11.1 yrs |
| Mean age of first treatment | 24.0±8.9 yrs |
| Mean duration of treatment | 14.4±10.7 yrs |
| Gender | 74% male |
| Race | 60% white, 35% black, 5% other |
| Mean education | 12.1±2.3 years |
| Marital status | 59% never married |
| 29% previously married | |
| 11% married | |
| Employment status | 85% unemployed |
| Mean PANSS total score | 75.7±17.6 |
| Mean CGI | 4.0±0.9 |
| Psychiatric comorbidities | 29% drug dependence/abuse |
| 28% depression | |
| 25% alcohol dependence/abuse | |
| 14% anxiety disorder | |
| 5% obsessive-compulsive disorder | |
| Illness severity | 4% severe |
| 20% marked | |
| 47% moderate | |
| 23% mild | |
| 6% minimal | |
| PANSS: Positive and Negative Syndrome Scale | |
| CGI: Clinician-rated Clinical Global Impressions severity score | |
| Source: Reference 5. | |
Medications. Before randomization, 28% of enrollees were not receiving antipsychotics. The remainder were receiving:
- olanzapine (22%)
- risperidone (19%)
- quetiapine (7%)
- ziprasidone (0%; approved after the trial began)
- any combination of olanzapine, risperidone, and quetiapine (7%)
- typical antipsychotics (16%).
Metabolic profile. These outpatients had a high rate of metabolic disorders: 42%—twice the rate in the general population—met criteria for metabolic syndrome,5 putting them at high risk to die of cardiovascular causes within 10 years.6 They had relatively poor physical health self-ratings and increased somatic preoccupation.7 Most worrisome, many were receiving no medications for their metabolic disorders, including 45% of those with diabetes, 89% with hyperlipidemia, and 62% with hypertension.8
Substance abuse. At enrollment, 40% of patients were abstinent from substance use, 22% were using substances without abuse or dependence, and 37% had substance abuse or dependence. Compared with nonusers, substance abusers tended to be male with more childhood problems, higher positive symptoms on the Positive and Negative Syndrome Scale (PANSS), and more likely to have had a recent illness exacerbation.9
Tardive dyskinesia. The 231 subjects who met criteria for probable TD10 were older than the overall sample with more years of antipsychotic treatment, especially with conventional neuroleptics and anticholinergics. Substance abuse was associated with TD, as were severity of psychopathology, extrapyramidal symptoms (EPS), and akathisia.11
Violent behavior. A history of serious violent behavior was reported in:
- 5.4% of patients with high positive and low negative PANSS symptom scores
- 1.7% of patients with low positive and high negative PANSS symptom scores.
Consent. Patients’ capacity to give consent to participate in the study was assessed with the MacArthur Competence Assessment Tool for Clinical Research (MacCAT-CR). Psychosis severity (PANSS positive symptom scale) was not found to affect decision-making capacity, but negative symptoms and diminished working memory did.12
CATIE’s Unique Design
Defining effectiveness. CATIE was designed in three phases (Figure). Phase 1—discussed here—was a blinded, controlled comparison of four atypical antipsychotics and perphenazine. Results of phases 2 and 3 have yet to be published. The primary effectiveness endpoint, “all-cause discontinuation,” was defined as:
- lack of efficacy (patient was switched to another drug assigned at random)
- lack of tolerability (patient requested a drug change)
- safety problem (investigator initiated a switch)
- patient’s decision for any reason (often dropping out of the study).
The longer subjects stayed on the first antipsychotic they received, the more effective that drug was considered to be.
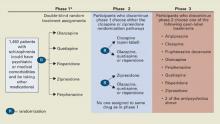
Figure CATIE schizophrenia trial design
* Phase 1A: participants with tardive dyskinesia (N=231) do not get randomized to perphenazine; phase 1B: participants who fail perphenazine will be randomized to an atypical (olanzapine, quetiapine, or risperidone) before eligibility for phase 2.
Source: Reference 2.Medications. Three atypicals—risperidone, olanzapine, and quetiapine—were approved for schizophrenia when the trial began in 1999. Recruitment ended in June 2003, the last subject completed the 18-month trial in December 2004, and data analysis began in January 2005. Ziprasidone was added to phase 1 after 40% of the sample had been enrolled, and aripiprazole was included as an option in the unblinded phase 3.
Perphenazine was chosen to represent typical antipsychotics because it has medium potency and less risk of EPS than high-potency drugs such as haloperidol and is associated with less weight gain than low-potency drugs such as thioridazine.
Dosing. Pharmaceutical manufacturers donated the antipsychotics and were invited to recommend their respective drugs’ starting dosages, dose increments, and maximum dosages. Olanzapine’s maker requested a higher starting dosage (7.5 mg/d instead of 5.0 mg/d) and a maximum dosage 50% higher than the FDA-approved range (30 mg/d instead of 20 mg/d). The others recommended the FDA-approved dosage ranges or less:
- quetiapine, 200 to 800 mg/d
- risperidone, 1.5 to 6 mg/d
- ziprasidone, 40 to 160 mg/d
- perphenazine, 8 to 32 mg/d.
The study team accepted their recommendations.
The medications were packaged in identical capsules. Quetiapine and ziprasidone were given twice daily because of product labeling; risperidone, olanzapine, and perphenazine were given once daily to one-half the patients assigned to them and twice daily to the others to prevent raters from guessing which drug a patient was receiving.
Tardive dyskinesia. For ethical reasons, the 231 patients with TD at enrollment were randomly assigned in phase 1 to atypicals but not to perphenazine because of the well-established link between typical antipsychotics and TD. This exception could have contributed to the closer-than-expected differences in EPS and perhaps in efficacy, given reports that TD patients have more negative symptoms and cognitive dysfunction.13 However, a statistical analysis took that into account.
CATIE’s Key Findings
Discontinuation. A disappointingly high discontinuation rate (74% overall) within a few months was the most important finding (Table 3). A recent effectiveness study with a design similar to the CATIE trial found a similarly high rate of all-cause discontinuation (70%) in patients with first-episode psychosis.14 Thus, patient-initiated drug discontinuation appears to be a core illness behavior from schizophrenia onset to chronic illness.
The high discontinuation rate shows that we need to modify our approach to schizophrenia, emphasizing full adherence to antipsychotic therapy from the onset of the illness.
Table 3
All-cause discontinuation rates in the CATIE trial
| Antipsychotic | Percent discontinued | Duration on antipsychotic (months)* | Dosage (mg/d)* |
| Olanzapine | 64% | 9.2 | 20.1 |
| Perphenazine | 75% | 4.6 | 20.8 |
| Quetiapine | 82% | 4.8 | 543.4 |
| Risperidone | 74% | 5.6 | 3.9 |
| Ziprasidone | 79% | 3.5 | 112.8 |
| Overall | 74% | Median 6.0; mean 8.3 | |
| Notes | |||
| *Mean modal | |||
| Olanzapine’s discontinuation rate was significantly lower than those of perphenazine, quetiapine, and risperidone but not of ziprasidone. | |||
| Olanzapine’s maximum dosage was 30 mg/d (50% higher than FDA-approved 20 mg/d); other agents were dosed within approved ranges. | |||
| Patients reached maximum daily antipsychotic dosages at these rates: 40% with olanzapine, 40% with perphenazine, 44% with quetiapine, 40% with risperidone, and 48% with ziprasidone. | |||
Effectiveness—measured as all-cause discontinuation or switching—was the primary outcome of phase 1. The unexpected finding that perphenazine and the atypicals had similar effectiveness could influence clinical practice. Insurers, for example, might consider promoting cheaper typical antipsychotics for first-line use. CATIE’s cost-effectiveness arm (Rosenheck et al, submitted for publication) will provide additional data on this issue.
Before rushing to use older antipsychotics as first-line treatments for schizophrenia, however, policymakers should consider three factors in the study design that could have enhanced perphenazine’s efficacy and safety profiles.
First, perphenazine was given at lower dosages (up to 32 mg/d) than “real world” clinicians used a decade ago (up to 64 mg/d). Thus, lower rates of serious side effects, especially TD, might have occurred in the study than in past clinical practice. Since atypical antipsychotics were approved, clinicians see far fewer psychiatric patients with pill-rolling tremors, rigid posture, or a shuffling gait, compared with 10 to 15 years ago when typical antipsychotics were widely used.
Second, perphenazine was associated with the highest EPS rate (17%), though its mean modal dosage (20.8 mg/d) is considered moderate. Discontinuation because of EPS was highest with perphenazine and lowest with quetiapine.
Third, excluding enrollees with TD from perphenazine may have increased perphenazine’s effectiveness, whereas including them in the atypicals groups may have reduced the atypicals’ effectiveness. TD patients are at increased risk to develop EPS; they had more-severe illness and a higher substance abuse rate among CATIE patients.11 Even so, investigators did control for TD in the data analysis and found no significant difference between typical and atypical antipsychotics.
No ‘Winners’ or ‘Losers’
Effectiveness, tolerability, and safety findings for each antipsychotic are compared in Tables 4A and 4B. Careful review shows no clear “winners” or “losers;” each agent has weaknesses but also strengths that may benefit individual patients.
Efficacy. Olanzapine showed a relatively higher efficacy and lower discontinuation rate but also had the highest risk of adverse metabolic effects. Some have attributed its greater efficacy to its higher dosing compared with the other antipsychotics. Some also have argued that the antipsychotics that showed lower efficacy, such as quetiapine and ziprasidone, were underdosed in this chronic schizophrenia population with a mean duration of illness of 14 years. Perphenazine, too, was dosed at the lower end of its range (mean modal dose 20.8 mg/d) compared with the old community standard of 36 to 64 mg/d.
Generally, a mean modal dosage of 20.1 mg/d for olanzapine is considered equivalent to ziprasidone, 160 mg; quetiapine, 800 mg; and risperidone, 6 mg. In CATIE phase 1, mean modal dosages were:
- ziprasidone, 112.8 mg/d (30% below 160 mg)
- quetiapine, 543.4 mg/d (32% below 800)
- risperidone, 3.9 mg/d (35% below 6 mg).
Olanzapine’s starting dosage of 7.5 mg/d was relatively higher than those of the other atypicals, which may have produced more-rapid onset of efficacy.
Switching. Another potential “advantage” for olanzapine was that 22% of subjects were taking it when they enrolled. By random assignment, 23% of patients who were taking olanzapine stayed on olanzapine and did not switch. By comparison:
- No patients assigned to ziprasidone were taking it before entering the trial.
- Only 5% of those taking quetiapine stayed on that drug after randomization.
- Few were receiving perphenazine before enrollment.
Switching antipsychotics may increase side effect risk or efficacy problems. For example, a patient switched from olanzapine or quetiapine to ziprasidone or perphenazine may experience insomnia during the transition, which may lead to tolerability complaints.
Metabolic side effects seen in this trial support past observations and reports that olanzapine is associated with higher risk for weight gain, hyperglycemia, and hyperlipidemia than other antipsychotics.15 Data on metabolic changes in CATIE patients taking olanzapine are being analyzed.
Hyperprolactinemia was most common with risperidone and practically nonexistent with other antipsychotics—even perphenazine. On the other hand, risperidone had the most favorable tolerability profile. This implies that elevated prolactin does not necessarily lead to antipsychotic discontinuation because of tolerability among patients with schizophrenia.
QTC interval and cataract data were benign across all antipsychotics. These findings appear to exonerate ziprasidone and quetiapine, respectively, which have been perceived as associated with these side effects.
When data become available, the next article in this series will discuss CATIE phase 2 findings. This phase includes patients who did not improve with the phase 1 regimens because of efficacy or tolerability problems and were switched to other antipsychotic therapies.
Related resources
- Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia study. www.catie.unc.edu/schizophrenia
- Schizophrenia Research Forum. NARSAD, The Mental Health Research Association.www.schizophreniaforum.org
Drug brand names
- Aripiprazole • Abilify
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
Dr Nasrallah receives grants/research support from AstraZeneca, Janssen Pharmaceutica, Eli Lilly & Co., and Pfizer. He is a consultant, advisory board member, and speaker for Abbott Laboratories, AstraZeneca, Janssen Pharmaceutica, Pfizer, and Shire Pharmaceuticals Group.
1. Tandon R, Jibson MD. Efficacy of newer generation antipsychotics in the treatment of schizophrenia. Psychoneuroendocrinol 2003;28(suppl 1):9-26.
2. Stroup TS, McEvoy JP, Swartz MS, et al. The National Institute of Mental Health Clinical Antipsychotic Trial of Intervention Effectiveness (CATIE). Project: schizophrenia trial design and protocol development. Schizophr Bull 2003;29:15-31.
3. Swartz MS, Perkins DO, Stroup TS, et al. Assessing clinical and functional outcomes in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial. Schizophr Bull 2003;29:33-43.
4. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209-23.
5. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the CATIE schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res 2005;80:19-32.
6. Goff D, Sullivan LM, McEvoy JP, et al. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res 2005;80:45-53.
7. Meyer JM, Nasrallah HA, McEvoy JP, et al. The Clinical Antipsychotic Trial of Intervention Effectiveness (CATIE) schizophrenia trial: clinical comparison of subgroups with and without the metabolic syndrome. Schizophr Res 2005;80:9-18.
8. Nasrallah HA, McEvoy JP, Meyer JM, et al. Low rates of treatment for metabolic disorders in the CATIE schizophrenia trial. Neuropsychopharmacol 2005;(suppl 1):204.-
9. Swartz MS, et al. (unpublished data).
10. Schooler NR, Kane JM. Research diagnosis for tardive dyskinesia. Arch Gen Psychiatry 1982;39:486-7.
11. Miller DD, McEvoy JP, Davis SM, et al. Clinical correlates of tardive dyskinesia in schizophrenia: baseline data from the CATIE schizophrenia trial. Schizophr Res 2005;80:33-43.
12. Stroup TS, Applebaum P, Swartz M, et al. Decision-making capacity for research participation among individuals in the CATIE schizophrenia trial. Schizophr Res 2005;80:1-8.
13. Waddington JL, Youssef HA, Dolphin C, et al. Cognitive function, negative symptoms and tardive dyskinesia in schizophrenia. Their association in relation to topography of involuntary movements and criterion of their abnormality. Arch Gen Psychiatry 1987;44:907-12.
14. Keefe R. The CAFÉ effectiveness study. Amsterdam: European College of Neuropsychopharmacology annual meeting, 2005;
15. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, and North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs, obesity, and diabetes. Diabetes Care 2004;27:596-601.
Investigators faced a dilemma while designing the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE). More than 200 enrollees with chronic schizophrenia had pre-existing tardive dyskinesia (TD). Would it be ethical to give them the antipsychotic most likely to worsen their TD? Would exempting them from taking that drug influence the trial’s outcome?
This issue and others had to be resolved before the largest controlled study of “real world” schizophrenia could begin. Now that data are unfolding, groups with diverse agendas are debating CATIE’s methods and surprising results. This article describes how the trial’s design and findings could transform public policy and clinical practice.
poll here
Efficacy vs Effectiveness
The National Institute of Mental Health funded the prospective CATIE schizophrenia study to compare the effectiveness of atypical antipsychotics versus each other and versus a first-generation (typical) antipsychotic.
All approved atypicals have shown similar efficacy compared with placebo in short-term trials (usually 6 weeks).1 The CATIE trial’s rationale is that short-term efficacy studies required for FDA approval may not necessarily reflect the drugs’ effectiveness in long-term schizophrenia management. Effectiveness measures take into account efficacy as well as safety, tolerability, and unpredictable patient behaviors in the real world.
CATIE’s ‘Real World’ Patients
CATIE investigators enrolled a community sample of chronic schizophrenia patients similar to those many psychiatrists see. Very liberal inclusion and exclusion criteria (Table 1) allowed enrollees to have a history of substance abuse, comorbid psychiatric or medical disorders, be receiving other medications, or show evidence of TD. Their schizophrenia ranged from minimal to severe.2,3
The 1,493 patients who completed the study (Table 2) were enrolled at 57 outpatient treatment settings. One site’s 33 patients were eliminated from analysis because of doubts about the integrity of the data, leaving a total of 1,460 subjects.4
Table 1
Criteria for enrolling patients in the CATIE schizophrenia trial
| Inclusion criteria | Ages 18 to 65 yrs |
| DSM-IV diagnosis of schizophrenia | |
| Able to take oral medication | |
| Able to give informed consent | |
| Exclusion criteria | Diagnosis of schizoaffective disorder, mental retardation, or other cognitive disorders |
| History of serious adverse reactions to one of the study medications | |
| Had only one schizophrenic episode | |
| History of treatment resistance, defined as persistence of severe symptoms despite adequate trials of one of the study antipsychotics or prior treatment with clozapine | |
| Pregnant or breast feeding | |
| Serious and unstable medical conditions |
Table 2
CATIE’s 1,460 ‘real world’ schizophrenia patients at trial entry
| Mean age | 40.6±11.1 yrs |
| Mean age of first treatment | 24.0±8.9 yrs |
| Mean duration of treatment | 14.4±10.7 yrs |
| Gender | 74% male |
| Race | 60% white, 35% black, 5% other |
| Mean education | 12.1±2.3 years |
| Marital status | 59% never married |
| 29% previously married | |
| 11% married | |
| Employment status | 85% unemployed |
| Mean PANSS total score | 75.7±17.6 |
| Mean CGI | 4.0±0.9 |
| Psychiatric comorbidities | 29% drug dependence/abuse |
| 28% depression | |
| 25% alcohol dependence/abuse | |
| 14% anxiety disorder | |
| 5% obsessive-compulsive disorder | |
| Illness severity | 4% severe |
| 20% marked | |
| 47% moderate | |
| 23% mild | |
| 6% minimal | |
| PANSS: Positive and Negative Syndrome Scale | |
| CGI: Clinician-rated Clinical Global Impressions severity score | |
| Source: Reference 5. | |
Medications. Before randomization, 28% of enrollees were not receiving antipsychotics. The remainder were receiving:
- olanzapine (22%)
- risperidone (19%)
- quetiapine (7%)
- ziprasidone (0%; approved after the trial began)
- any combination of olanzapine, risperidone, and quetiapine (7%)
- typical antipsychotics (16%).
Metabolic profile. These outpatients had a high rate of metabolic disorders: 42%—twice the rate in the general population—met criteria for metabolic syndrome,5 putting them at high risk to die of cardiovascular causes within 10 years.6 They had relatively poor physical health self-ratings and increased somatic preoccupation.7 Most worrisome, many were receiving no medications for their metabolic disorders, including 45% of those with diabetes, 89% with hyperlipidemia, and 62% with hypertension.8
Substance abuse. At enrollment, 40% of patients were abstinent from substance use, 22% were using substances without abuse or dependence, and 37% had substance abuse or dependence. Compared with nonusers, substance abusers tended to be male with more childhood problems, higher positive symptoms on the Positive and Negative Syndrome Scale (PANSS), and more likely to have had a recent illness exacerbation.9
Tardive dyskinesia. The 231 subjects who met criteria for probable TD10 were older than the overall sample with more years of antipsychotic treatment, especially with conventional neuroleptics and anticholinergics. Substance abuse was associated with TD, as were severity of psychopathology, extrapyramidal symptoms (EPS), and akathisia.11
Violent behavior. A history of serious violent behavior was reported in:
- 5.4% of patients with high positive and low negative PANSS symptom scores
- 1.7% of patients with low positive and high negative PANSS symptom scores.
Consent. Patients’ capacity to give consent to participate in the study was assessed with the MacArthur Competence Assessment Tool for Clinical Research (MacCAT-CR). Psychosis severity (PANSS positive symptom scale) was not found to affect decision-making capacity, but negative symptoms and diminished working memory did.12
CATIE’s Unique Design
Defining effectiveness. CATIE was designed in three phases (Figure). Phase 1—discussed here—was a blinded, controlled comparison of four atypical antipsychotics and perphenazine. Results of phases 2 and 3 have yet to be published. The primary effectiveness endpoint, “all-cause discontinuation,” was defined as:
- lack of efficacy (patient was switched to another drug assigned at random)
- lack of tolerability (patient requested a drug change)
- safety problem (investigator initiated a switch)
- patient’s decision for any reason (often dropping out of the study).
The longer subjects stayed on the first antipsychotic they received, the more effective that drug was considered to be.
Figure CATIE schizophrenia trial design
* Phase 1A: participants with tardive dyskinesia (N=231) do not get randomized to perphenazine; phase 1B: participants who fail perphenazine will be randomized to an atypical (olanzapine, quetiapine, or risperidone) before eligibility for phase 2.
Source: Reference 2.Medications. Three atypicals—risperidone, olanzapine, and quetiapine—were approved for schizophrenia when the trial began in 1999. Recruitment ended in June 2003, the last subject completed the 18-month trial in December 2004, and data analysis began in January 2005. Ziprasidone was added to phase 1 after 40% of the sample had been enrolled, and aripiprazole was included as an option in the unblinded phase 3.
Perphenazine was chosen to represent typical antipsychotics because it has medium potency and less risk of EPS than high-potency drugs such as haloperidol and is associated with less weight gain than low-potency drugs such as thioridazine.
Dosing. Pharmaceutical manufacturers donated the antipsychotics and were invited to recommend their respective drugs’ starting dosages, dose increments, and maximum dosages. Olanzapine’s maker requested a higher starting dosage (7.5 mg/d instead of 5.0 mg/d) and a maximum dosage 50% higher than the FDA-approved range (30 mg/d instead of 20 mg/d). The others recommended the FDA-approved dosage ranges or less:
- quetiapine, 200 to 800 mg/d
- risperidone, 1.5 to 6 mg/d
- ziprasidone, 40 to 160 mg/d
- perphenazine, 8 to 32 mg/d.
The study team accepted their recommendations.
The medications were packaged in identical capsules. Quetiapine and ziprasidone were given twice daily because of product labeling; risperidone, olanzapine, and perphenazine were given once daily to one-half the patients assigned to them and twice daily to the others to prevent raters from guessing which drug a patient was receiving.
Tardive dyskinesia. For ethical reasons, the 231 patients with TD at enrollment were randomly assigned in phase 1 to atypicals but not to perphenazine because of the well-established link between typical antipsychotics and TD. This exception could have contributed to the closer-than-expected differences in EPS and perhaps in efficacy, given reports that TD patients have more negative symptoms and cognitive dysfunction.13 However, a statistical analysis took that into account.
CATIE’s Key Findings
Discontinuation. A disappointingly high discontinuation rate (74% overall) within a few months was the most important finding (Table 3). A recent effectiveness study with a design similar to the CATIE trial found a similarly high rate of all-cause discontinuation (70%) in patients with first-episode psychosis.14 Thus, patient-initiated drug discontinuation appears to be a core illness behavior from schizophrenia onset to chronic illness.
The high discontinuation rate shows that we need to modify our approach to schizophrenia, emphasizing full adherence to antipsychotic therapy from the onset of the illness.
Table 3
All-cause discontinuation rates in the CATIE trial
| Antipsychotic | Percent discontinued | Duration on antipsychotic (months)* | Dosage (mg/d)* |
| Olanzapine | 64% | 9.2 | 20.1 |
| Perphenazine | 75% | 4.6 | 20.8 |
| Quetiapine | 82% | 4.8 | 543.4 |
| Risperidone | 74% | 5.6 | 3.9 |
| Ziprasidone | 79% | 3.5 | 112.8 |
| Overall | 74% | Median 6.0; mean 8.3 | |
| Notes | |||
| *Mean modal | |||
| Olanzapine’s discontinuation rate was significantly lower than those of perphenazine, quetiapine, and risperidone but not of ziprasidone. | |||
| Olanzapine’s maximum dosage was 30 mg/d (50% higher than FDA-approved 20 mg/d); other agents were dosed within approved ranges. | |||
| Patients reached maximum daily antipsychotic dosages at these rates: 40% with olanzapine, 40% with perphenazine, 44% with quetiapine, 40% with risperidone, and 48% with ziprasidone. | |||
Effectiveness—measured as all-cause discontinuation or switching—was the primary outcome of phase 1. The unexpected finding that perphenazine and the atypicals had similar effectiveness could influence clinical practice. Insurers, for example, might consider promoting cheaper typical antipsychotics for first-line use. CATIE’s cost-effectiveness arm (Rosenheck et al, submitted for publication) will provide additional data on this issue.
Before rushing to use older antipsychotics as first-line treatments for schizophrenia, however, policymakers should consider three factors in the study design that could have enhanced perphenazine’s efficacy and safety profiles.
First, perphenazine was given at lower dosages (up to 32 mg/d) than “real world” clinicians used a decade ago (up to 64 mg/d). Thus, lower rates of serious side effects, especially TD, might have occurred in the study than in past clinical practice. Since atypical antipsychotics were approved, clinicians see far fewer psychiatric patients with pill-rolling tremors, rigid posture, or a shuffling gait, compared with 10 to 15 years ago when typical antipsychotics were widely used.
Second, perphenazine was associated with the highest EPS rate (17%), though its mean modal dosage (20.8 mg/d) is considered moderate. Discontinuation because of EPS was highest with perphenazine and lowest with quetiapine.
Third, excluding enrollees with TD from perphenazine may have increased perphenazine’s effectiveness, whereas including them in the atypicals groups may have reduced the atypicals’ effectiveness. TD patients are at increased risk to develop EPS; they had more-severe illness and a higher substance abuse rate among CATIE patients.11 Even so, investigators did control for TD in the data analysis and found no significant difference between typical and atypical antipsychotics.
No ‘Winners’ or ‘Losers’
Effectiveness, tolerability, and safety findings for each antipsychotic are compared in Tables 4A and 4B. Careful review shows no clear “winners” or “losers;” each agent has weaknesses but also strengths that may benefit individual patients.
Efficacy. Olanzapine showed a relatively higher efficacy and lower discontinuation rate but also had the highest risk of adverse metabolic effects. Some have attributed its greater efficacy to its higher dosing compared with the other antipsychotics. Some also have argued that the antipsychotics that showed lower efficacy, such as quetiapine and ziprasidone, were underdosed in this chronic schizophrenia population with a mean duration of illness of 14 years. Perphenazine, too, was dosed at the lower end of its range (mean modal dose 20.8 mg/d) compared with the old community standard of 36 to 64 mg/d.
Generally, a mean modal dosage of 20.1 mg/d for olanzapine is considered equivalent to ziprasidone, 160 mg; quetiapine, 800 mg; and risperidone, 6 mg. In CATIE phase 1, mean modal dosages were:
- ziprasidone, 112.8 mg/d (30% below 160 mg)
- quetiapine, 543.4 mg/d (32% below 800)
- risperidone, 3.9 mg/d (35% below 6 mg).
Olanzapine’s starting dosage of 7.5 mg/d was relatively higher than those of the other atypicals, which may have produced more-rapid onset of efficacy.
Switching. Another potential “advantage” for olanzapine was that 22% of subjects were taking it when they enrolled. By random assignment, 23% of patients who were taking olanzapine stayed on olanzapine and did not switch. By comparison:
- No patients assigned to ziprasidone were taking it before entering the trial.
- Only 5% of those taking quetiapine stayed on that drug after randomization.
- Few were receiving perphenazine before enrollment.
Switching antipsychotics may increase side effect risk or efficacy problems. For example, a patient switched from olanzapine or quetiapine to ziprasidone or perphenazine may experience insomnia during the transition, which may lead to tolerability complaints.
Metabolic side effects seen in this trial support past observations and reports that olanzapine is associated with higher risk for weight gain, hyperglycemia, and hyperlipidemia than other antipsychotics.15 Data on metabolic changes in CATIE patients taking olanzapine are being analyzed.
Hyperprolactinemia was most common with risperidone and practically nonexistent with other antipsychotics—even perphenazine. On the other hand, risperidone had the most favorable tolerability profile. This implies that elevated prolactin does not necessarily lead to antipsychotic discontinuation because of tolerability among patients with schizophrenia.
QTC interval and cataract data were benign across all antipsychotics. These findings appear to exonerate ziprasidone and quetiapine, respectively, which have been perceived as associated with these side effects.
When data become available, the next article in this series will discuss CATIE phase 2 findings. This phase includes patients who did not improve with the phase 1 regimens because of efficacy or tolerability problems and were switched to other antipsychotic therapies.
Related resources
- Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia study. www.catie.unc.edu/schizophrenia
- Schizophrenia Research Forum. NARSAD, The Mental Health Research Association.www.schizophreniaforum.org
Drug brand names
- Aripiprazole • Abilify
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
Dr Nasrallah receives grants/research support from AstraZeneca, Janssen Pharmaceutica, Eli Lilly & Co., and Pfizer. He is a consultant, advisory board member, and speaker for Abbott Laboratories, AstraZeneca, Janssen Pharmaceutica, Pfizer, and Shire Pharmaceuticals Group.
Investigators faced a dilemma while designing the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE). More than 200 enrollees with chronic schizophrenia had pre-existing tardive dyskinesia (TD). Would it be ethical to give them the antipsychotic most likely to worsen their TD? Would exempting them from taking that drug influence the trial’s outcome?
This issue and others had to be resolved before the largest controlled study of “real world” schizophrenia could begin. Now that data are unfolding, groups with diverse agendas are debating CATIE’s methods and surprising results. This article describes how the trial’s design and findings could transform public policy and clinical practice.
poll here
Efficacy vs Effectiveness
The National Institute of Mental Health funded the prospective CATIE schizophrenia study to compare the effectiveness of atypical antipsychotics versus each other and versus a first-generation (typical) antipsychotic.
All approved atypicals have shown similar efficacy compared with placebo in short-term trials (usually 6 weeks).1 The CATIE trial’s rationale is that short-term efficacy studies required for FDA approval may not necessarily reflect the drugs’ effectiveness in long-term schizophrenia management. Effectiveness measures take into account efficacy as well as safety, tolerability, and unpredictable patient behaviors in the real world.
CATIE’s ‘Real World’ Patients
CATIE investigators enrolled a community sample of chronic schizophrenia patients similar to those many psychiatrists see. Very liberal inclusion and exclusion criteria (Table 1) allowed enrollees to have a history of substance abuse, comorbid psychiatric or medical disorders, be receiving other medications, or show evidence of TD. Their schizophrenia ranged from minimal to severe.2,3
The 1,493 patients who completed the study (Table 2) were enrolled at 57 outpatient treatment settings. One site’s 33 patients were eliminated from analysis because of doubts about the integrity of the data, leaving a total of 1,460 subjects.4
Table 1
Criteria for enrolling patients in the CATIE schizophrenia trial
| Inclusion criteria | Ages 18 to 65 yrs |
| DSM-IV diagnosis of schizophrenia | |
| Able to take oral medication | |
| Able to give informed consent | |
| Exclusion criteria | Diagnosis of schizoaffective disorder, mental retardation, or other cognitive disorders |
| History of serious adverse reactions to one of the study medications | |
| Had only one schizophrenic episode | |
| History of treatment resistance, defined as persistence of severe symptoms despite adequate trials of one of the study antipsychotics or prior treatment with clozapine | |
| Pregnant or breast feeding | |
| Serious and unstable medical conditions |
Table 2
CATIE’s 1,460 ‘real world’ schizophrenia patients at trial entry
| Mean age | 40.6±11.1 yrs |
| Mean age of first treatment | 24.0±8.9 yrs |
| Mean duration of treatment | 14.4±10.7 yrs |
| Gender | 74% male |
| Race | 60% white, 35% black, 5% other |
| Mean education | 12.1±2.3 years |
| Marital status | 59% never married |
| 29% previously married | |
| 11% married | |
| Employment status | 85% unemployed |
| Mean PANSS total score | 75.7±17.6 |
| Mean CGI | 4.0±0.9 |
| Psychiatric comorbidities | 29% drug dependence/abuse |
| 28% depression | |
| 25% alcohol dependence/abuse | |
| 14% anxiety disorder | |
| 5% obsessive-compulsive disorder | |
| Illness severity | 4% severe |
| 20% marked | |
| 47% moderate | |
| 23% mild | |
| 6% minimal | |
| PANSS: Positive and Negative Syndrome Scale | |
| CGI: Clinician-rated Clinical Global Impressions severity score | |
| Source: Reference 5. | |
Medications. Before randomization, 28% of enrollees were not receiving antipsychotics. The remainder were receiving:
- olanzapine (22%)
- risperidone (19%)
- quetiapine (7%)
- ziprasidone (0%; approved after the trial began)
- any combination of olanzapine, risperidone, and quetiapine (7%)
- typical antipsychotics (16%).
Metabolic profile. These outpatients had a high rate of metabolic disorders: 42%—twice the rate in the general population—met criteria for metabolic syndrome,5 putting them at high risk to die of cardiovascular causes within 10 years.6 They had relatively poor physical health self-ratings and increased somatic preoccupation.7 Most worrisome, many were receiving no medications for their metabolic disorders, including 45% of those with diabetes, 89% with hyperlipidemia, and 62% with hypertension.8
Substance abuse. At enrollment, 40% of patients were abstinent from substance use, 22% were using substances without abuse or dependence, and 37% had substance abuse or dependence. Compared with nonusers, substance abusers tended to be male with more childhood problems, higher positive symptoms on the Positive and Negative Syndrome Scale (PANSS), and more likely to have had a recent illness exacerbation.9
Tardive dyskinesia. The 231 subjects who met criteria for probable TD10 were older than the overall sample with more years of antipsychotic treatment, especially with conventional neuroleptics and anticholinergics. Substance abuse was associated with TD, as were severity of psychopathology, extrapyramidal symptoms (EPS), and akathisia.11
Violent behavior. A history of serious violent behavior was reported in:
- 5.4% of patients with high positive and low negative PANSS symptom scores
- 1.7% of patients with low positive and high negative PANSS symptom scores.
Consent. Patients’ capacity to give consent to participate in the study was assessed with the MacArthur Competence Assessment Tool for Clinical Research (MacCAT-CR). Psychosis severity (PANSS positive symptom scale) was not found to affect decision-making capacity, but negative symptoms and diminished working memory did.12
CATIE’s Unique Design
Defining effectiveness. CATIE was designed in three phases (Figure). Phase 1—discussed here—was a blinded, controlled comparison of four atypical antipsychotics and perphenazine. Results of phases 2 and 3 have yet to be published. The primary effectiveness endpoint, “all-cause discontinuation,” was defined as:
- lack of efficacy (patient was switched to another drug assigned at random)
- lack of tolerability (patient requested a drug change)
- safety problem (investigator initiated a switch)
- patient’s decision for any reason (often dropping out of the study).
The longer subjects stayed on the first antipsychotic they received, the more effective that drug was considered to be.
Figure CATIE schizophrenia trial design
* Phase 1A: participants with tardive dyskinesia (N=231) do not get randomized to perphenazine; phase 1B: participants who fail perphenazine will be randomized to an atypical (olanzapine, quetiapine, or risperidone) before eligibility for phase 2.
Source: Reference 2.Medications. Three atypicals—risperidone, olanzapine, and quetiapine—were approved for schizophrenia when the trial began in 1999. Recruitment ended in June 2003, the last subject completed the 18-month trial in December 2004, and data analysis began in January 2005. Ziprasidone was added to phase 1 after 40% of the sample had been enrolled, and aripiprazole was included as an option in the unblinded phase 3.
Perphenazine was chosen to represent typical antipsychotics because it has medium potency and less risk of EPS than high-potency drugs such as haloperidol and is associated with less weight gain than low-potency drugs such as thioridazine.
Dosing. Pharmaceutical manufacturers donated the antipsychotics and were invited to recommend their respective drugs’ starting dosages, dose increments, and maximum dosages. Olanzapine’s maker requested a higher starting dosage (7.5 mg/d instead of 5.0 mg/d) and a maximum dosage 50% higher than the FDA-approved range (30 mg/d instead of 20 mg/d). The others recommended the FDA-approved dosage ranges or less:
- quetiapine, 200 to 800 mg/d
- risperidone, 1.5 to 6 mg/d
- ziprasidone, 40 to 160 mg/d
- perphenazine, 8 to 32 mg/d.
The study team accepted their recommendations.
The medications were packaged in identical capsules. Quetiapine and ziprasidone were given twice daily because of product labeling; risperidone, olanzapine, and perphenazine were given once daily to one-half the patients assigned to them and twice daily to the others to prevent raters from guessing which drug a patient was receiving.
Tardive dyskinesia. For ethical reasons, the 231 patients with TD at enrollment were randomly assigned in phase 1 to atypicals but not to perphenazine because of the well-established link between typical antipsychotics and TD. This exception could have contributed to the closer-than-expected differences in EPS and perhaps in efficacy, given reports that TD patients have more negative symptoms and cognitive dysfunction.13 However, a statistical analysis took that into account.
CATIE’s Key Findings
Discontinuation. A disappointingly high discontinuation rate (74% overall) within a few months was the most important finding (Table 3). A recent effectiveness study with a design similar to the CATIE trial found a similarly high rate of all-cause discontinuation (70%) in patients with first-episode psychosis.14 Thus, patient-initiated drug discontinuation appears to be a core illness behavior from schizophrenia onset to chronic illness.
The high discontinuation rate shows that we need to modify our approach to schizophrenia, emphasizing full adherence to antipsychotic therapy from the onset of the illness.
Table 3
All-cause discontinuation rates in the CATIE trial
| Antipsychotic | Percent discontinued | Duration on antipsychotic (months)* | Dosage (mg/d)* |
| Olanzapine | 64% | 9.2 | 20.1 |
| Perphenazine | 75% | 4.6 | 20.8 |
| Quetiapine | 82% | 4.8 | 543.4 |
| Risperidone | 74% | 5.6 | 3.9 |
| Ziprasidone | 79% | 3.5 | 112.8 |
| Overall | 74% | Median 6.0; mean 8.3 | |
| Notes | |||
| *Mean modal | |||
| Olanzapine’s discontinuation rate was significantly lower than those of perphenazine, quetiapine, and risperidone but not of ziprasidone. | |||
| Olanzapine’s maximum dosage was 30 mg/d (50% higher than FDA-approved 20 mg/d); other agents were dosed within approved ranges. | |||
| Patients reached maximum daily antipsychotic dosages at these rates: 40% with olanzapine, 40% with perphenazine, 44% with quetiapine, 40% with risperidone, and 48% with ziprasidone. | |||
Effectiveness—measured as all-cause discontinuation or switching—was the primary outcome of phase 1. The unexpected finding that perphenazine and the atypicals had similar effectiveness could influence clinical practice. Insurers, for example, might consider promoting cheaper typical antipsychotics for first-line use. CATIE’s cost-effectiveness arm (Rosenheck et al, submitted for publication) will provide additional data on this issue.
Before rushing to use older antipsychotics as first-line treatments for schizophrenia, however, policymakers should consider three factors in the study design that could have enhanced perphenazine’s efficacy and safety profiles.
First, perphenazine was given at lower dosages (up to 32 mg/d) than “real world” clinicians used a decade ago (up to 64 mg/d). Thus, lower rates of serious side effects, especially TD, might have occurred in the study than in past clinical practice. Since atypical antipsychotics were approved, clinicians see far fewer psychiatric patients with pill-rolling tremors, rigid posture, or a shuffling gait, compared with 10 to 15 years ago when typical antipsychotics were widely used.
Second, perphenazine was associated with the highest EPS rate (17%), though its mean modal dosage (20.8 mg/d) is considered moderate. Discontinuation because of EPS was highest with perphenazine and lowest with quetiapine.
Third, excluding enrollees with TD from perphenazine may have increased perphenazine’s effectiveness, whereas including them in the atypicals groups may have reduced the atypicals’ effectiveness. TD patients are at increased risk to develop EPS; they had more-severe illness and a higher substance abuse rate among CATIE patients.11 Even so, investigators did control for TD in the data analysis and found no significant difference between typical and atypical antipsychotics.
No ‘Winners’ or ‘Losers’
Effectiveness, tolerability, and safety findings for each antipsychotic are compared in Tables 4A and 4B. Careful review shows no clear “winners” or “losers;” each agent has weaknesses but also strengths that may benefit individual patients.
Efficacy. Olanzapine showed a relatively higher efficacy and lower discontinuation rate but also had the highest risk of adverse metabolic effects. Some have attributed its greater efficacy to its higher dosing compared with the other antipsychotics. Some also have argued that the antipsychotics that showed lower efficacy, such as quetiapine and ziprasidone, were underdosed in this chronic schizophrenia population with a mean duration of illness of 14 years. Perphenazine, too, was dosed at the lower end of its range (mean modal dose 20.8 mg/d) compared with the old community standard of 36 to 64 mg/d.
Generally, a mean modal dosage of 20.1 mg/d for olanzapine is considered equivalent to ziprasidone, 160 mg; quetiapine, 800 mg; and risperidone, 6 mg. In CATIE phase 1, mean modal dosages were:
- ziprasidone, 112.8 mg/d (30% below 160 mg)
- quetiapine, 543.4 mg/d (32% below 800)
- risperidone, 3.9 mg/d (35% below 6 mg).
Olanzapine’s starting dosage of 7.5 mg/d was relatively higher than those of the other atypicals, which may have produced more-rapid onset of efficacy.
Switching. Another potential “advantage” for olanzapine was that 22% of subjects were taking it when they enrolled. By random assignment, 23% of patients who were taking olanzapine stayed on olanzapine and did not switch. By comparison:
- No patients assigned to ziprasidone were taking it before entering the trial.
- Only 5% of those taking quetiapine stayed on that drug after randomization.
- Few were receiving perphenazine before enrollment.
Switching antipsychotics may increase side effect risk or efficacy problems. For example, a patient switched from olanzapine or quetiapine to ziprasidone or perphenazine may experience insomnia during the transition, which may lead to tolerability complaints.
Metabolic side effects seen in this trial support past observations and reports that olanzapine is associated with higher risk for weight gain, hyperglycemia, and hyperlipidemia than other antipsychotics.15 Data on metabolic changes in CATIE patients taking olanzapine are being analyzed.
Hyperprolactinemia was most common with risperidone and practically nonexistent with other antipsychotics—even perphenazine. On the other hand, risperidone had the most favorable tolerability profile. This implies that elevated prolactin does not necessarily lead to antipsychotic discontinuation because of tolerability among patients with schizophrenia.
QTC interval and cataract data were benign across all antipsychotics. These findings appear to exonerate ziprasidone and quetiapine, respectively, which have been perceived as associated with these side effects.
When data become available, the next article in this series will discuss CATIE phase 2 findings. This phase includes patients who did not improve with the phase 1 regimens because of efficacy or tolerability problems and were switched to other antipsychotic therapies.
Related resources
- Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia study. www.catie.unc.edu/schizophrenia
- Schizophrenia Research Forum. NARSAD, The Mental Health Research Association.www.schizophreniaforum.org
Drug brand names
- Aripiprazole • Abilify
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Disclosures
Dr Nasrallah receives grants/research support from AstraZeneca, Janssen Pharmaceutica, Eli Lilly & Co., and Pfizer. He is a consultant, advisory board member, and speaker for Abbott Laboratories, AstraZeneca, Janssen Pharmaceutica, Pfizer, and Shire Pharmaceuticals Group.
1. Tandon R, Jibson MD. Efficacy of newer generation antipsychotics in the treatment of schizophrenia. Psychoneuroendocrinol 2003;28(suppl 1):9-26.
2. Stroup TS, McEvoy JP, Swartz MS, et al. The National Institute of Mental Health Clinical Antipsychotic Trial of Intervention Effectiveness (CATIE). Project: schizophrenia trial design and protocol development. Schizophr Bull 2003;29:15-31.
3. Swartz MS, Perkins DO, Stroup TS, et al. Assessing clinical and functional outcomes in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial. Schizophr Bull 2003;29:33-43.
4. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209-23.
5. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the CATIE schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res 2005;80:19-32.
6. Goff D, Sullivan LM, McEvoy JP, et al. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res 2005;80:45-53.
7. Meyer JM, Nasrallah HA, McEvoy JP, et al. The Clinical Antipsychotic Trial of Intervention Effectiveness (CATIE) schizophrenia trial: clinical comparison of subgroups with and without the metabolic syndrome. Schizophr Res 2005;80:9-18.
8. Nasrallah HA, McEvoy JP, Meyer JM, et al. Low rates of treatment for metabolic disorders in the CATIE schizophrenia trial. Neuropsychopharmacol 2005;(suppl 1):204.-
9. Swartz MS, et al. (unpublished data).
10. Schooler NR, Kane JM. Research diagnosis for tardive dyskinesia. Arch Gen Psychiatry 1982;39:486-7.
11. Miller DD, McEvoy JP, Davis SM, et al. Clinical correlates of tardive dyskinesia in schizophrenia: baseline data from the CATIE schizophrenia trial. Schizophr Res 2005;80:33-43.
12. Stroup TS, Applebaum P, Swartz M, et al. Decision-making capacity for research participation among individuals in the CATIE schizophrenia trial. Schizophr Res 2005;80:1-8.
13. Waddington JL, Youssef HA, Dolphin C, et al. Cognitive function, negative symptoms and tardive dyskinesia in schizophrenia. Their association in relation to topography of involuntary movements and criterion of their abnormality. Arch Gen Psychiatry 1987;44:907-12.
14. Keefe R. The CAFÉ effectiveness study. Amsterdam: European College of Neuropsychopharmacology annual meeting, 2005;
15. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, and North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs, obesity, and diabetes. Diabetes Care 2004;27:596-601.
1. Tandon R, Jibson MD. Efficacy of newer generation antipsychotics in the treatment of schizophrenia. Psychoneuroendocrinol 2003;28(suppl 1):9-26.
2. Stroup TS, McEvoy JP, Swartz MS, et al. The National Institute of Mental Health Clinical Antipsychotic Trial of Intervention Effectiveness (CATIE). Project: schizophrenia trial design and protocol development. Schizophr Bull 2003;29:15-31.
3. Swartz MS, Perkins DO, Stroup TS, et al. Assessing clinical and functional outcomes in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial. Schizophr Bull 2003;29:33-43.
4. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209-23.
5. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the CATIE schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res 2005;80:19-32.
6. Goff D, Sullivan LM, McEvoy JP, et al. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res 2005;80:45-53.
7. Meyer JM, Nasrallah HA, McEvoy JP, et al. The Clinical Antipsychotic Trial of Intervention Effectiveness (CATIE) schizophrenia trial: clinical comparison of subgroups with and without the metabolic syndrome. Schizophr Res 2005;80:9-18.
8. Nasrallah HA, McEvoy JP, Meyer JM, et al. Low rates of treatment for metabolic disorders in the CATIE schizophrenia trial. Neuropsychopharmacol 2005;(suppl 1):204.-
9. Swartz MS, et al. (unpublished data).
10. Schooler NR, Kane JM. Research diagnosis for tardive dyskinesia. Arch Gen Psychiatry 1982;39:486-7.
11. Miller DD, McEvoy JP, Davis SM, et al. Clinical correlates of tardive dyskinesia in schizophrenia: baseline data from the CATIE schizophrenia trial. Schizophr Res 2005;80:33-43.
12. Stroup TS, Applebaum P, Swartz M, et al. Decision-making capacity for research participation among individuals in the CATIE schizophrenia trial. Schizophr Res 2005;80:1-8.
13. Waddington JL, Youssef HA, Dolphin C, et al. Cognitive function, negative symptoms and tardive dyskinesia in schizophrenia. Their association in relation to topography of involuntary movements and criterion of their abnormality. Arch Gen Psychiatry 1987;44:907-12.
14. Keefe R. The CAFÉ effectiveness study. Amsterdam: European College of Neuropsychopharmacology annual meeting, 2005;
15. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, and North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs, obesity, and diabetes. Diabetes Care 2004;27:596-601.
Psychosis: 6 steps rule out medical causes in kids
John, age 16, is admitted to our inpatient psychiatric unit, complaining of “a 2-week constant headache” caused by “voices arguing in my head.” He has lived in Mexico with an uncle for 6 months but returned home last week for medical evaluation of his headaches.
His parents report that John developed normally until 3 years ago, when he gradually lost interest in his favorite activities and became socially withdrawn. He has not attended school in 2 years. He has no history of illicit drug use and is not taking prescription or over the-counter medications.
Complete physical examination, neurologic exam, and routine screening lab test results are normal. Thinking that a high lead content of cookware used in Mexico might be causing John’s symptoms, we order a lead level: result-0.2 mg/dL (
We diagnose schizophreniform disorder, but John’s parents refuse to accept this diagnosis. They repeatedly ask if we can do more to identify a medical cause of their son’s psychiatric symptoms.
As in John’s case, young patients or their parents may resist the diagnosis of a chronic mental illness such as schizophrenia. Understandably, they may be invested in trying to identify “medically treatable” causes. You can address their anxieties by showing them that you have systematically evaluated medical causes of psychosis.
We offer such a tool: an algorithm and tables to help you identify common and rare medical conditions that may cause or exacerbate psychotic symptoms in patients ages 3 to 18.
An evidence-based algorithm
Multiple factors—developmental, psychological, family, environmental, or medical—typically cause psychotic symptoms in a child or adolescent. Evaluating all possibilities is essential, but guidelines tend to minimize medical causes. American Academy of Child and Adolescent Psychiatry guidelines, for example, recommend that “all medical disorders (including general medical conditions and substance-induced disorders) are ruled out,”1 but they do not specify which medical conditions to consider.
To supplement existing guidelines, we searched the literature and developed an evidence-based algorithm to help you systematically consider medical causes of pediatric psychotic symptoms. We excluded children age 2
How to use it. The algorithm walks you through a medical systems review. You begin with a complete history, then address six causes of psychotic symptoms: substance abuse, medication reactions, general medical conditions, unexplained somatic symptoms (such as from toxic environmental exposures), developmental and learning disabilities, and atypical presentations.
Don’t stop if you find one possible cause of psychotic symptoms; continue to the end of the algorithm. The more factors you identify, the greater your chance of finding a treatable cause that may ameliorate your patient’s symptoms.
To make the algorithm clinically useful, we listed conditions in order of decreasing probability of causing psychotic symptoms. For example, the first cause listed is substance-induced disorders,3 which are most common among adolescent patients. We also “triaged” medical conditions from common to rare (based on estimated prevalence of association with psychotic symptoms), listing rare causes only in cases of atypical presentation or treatment resistance.
Supporting tables. The following discussion summarizes data that support the algorithm and its tables:
- medications reported to cause psychosis (Table 1)
- medical conditions most likely to cause psychosis (Table 2)
- medical conditions that rarely cause psychosis (Table 3).
Drugs that may cause psychotic symptoms
| Drug class | Psychotic symptoms | |
|---|---|---|
| Bizarre behavior/delusions | Auditory or visual hallucinations | |
| Amphetamine-like drugs | X | X |
| Anabolic steroids | X | |
| Angiotensin-converting enzyme (ACE) inhibitors | X | |
| Anticholinergics and atropine | X | X |
| Antidepressants, tricyclic | X | |
| Antiepileptics | X | |
| Barbiturates | X | X |
| Benzodiazepines | X | X |
| Beta-adrenergic blockers | X | X |
| Calcium channel blockers | X | |
| Cephalosporins | X | X |
| Corticosteroids | X | |
| Dopamine receptor agonists | X | X |
| Fluoroquinolone antibiotics | X | X |
| Histamine H1 receptor blockers | X | |
| Histamine H2 receptor blockers | X | |
| HMG-CoA reductase inhibitors | X | |
| Nonsteroidal anti-inflammatory drugs | X | |
| Opioids | X | X |
| Procaine derivatives (procainamide, procaine penicillin G) | X | X |
| Salicylates | X | X |
| Selective serotonin reuptake inhibitors | X | |
| Sulfonamides | X | |
| Source: Adapted from reference 10. | ||
Common medical conditions that may cause pediatric psychosis symptoms*
| Category | Conditions not to forget | Common symptoms/comments |
|---|---|---|
| Rheumatologic | Lupus erythematosus | Joint pain, fever, facial butterfly rash, prolonged fatigue |
| Infectious | Viral encephalitis | Fever, headache, mental status change; may occur in perinatal period |
| Neurologic | Multiple sclerosis | Varied neurologic deficits, especially ophthalmologic changes and weakness |
| Neurosyphilis | Personality change, ataxia, stroke, ophthalmic symptoms | |
| Seizure (temporal lobe epilepsy, interictal psychosis) | Paroxysmal periods of sudden change in mood, behavior, or motor activity with or without loss of consciousness | |
| Toxicologic | Carbon monoxide poisoning | Shortness of breath, mild nausea, headache, dizziness |
| * Clinically significant symptoms that meet DSM-IV-TR criteria for a primary psychiatric disorder. | ||
| Click here to view citations supporting statements in this table | ||
Medical conditions that rarely cause pediatric psychosis symptoms*
| Category/condition | Symptoms/comments |
|---|---|
| Endocrine | |
| Hyperthyroidism | Tachycardia, weight loss, excessive sweating, tiredness, inability to sleep, diarrhea, shakiness, muscle weakness |
| Thymoma/myasthenia gravis | Shortness of breath, swelling of face, muscle weakness (especially around eyes) |
| Hematologic | |
| Porphyria (acute intermittent porphyria, porphyria variegate) | Intermittent abdominal pain (severe) accompanied by dark urine |
| Genetic | |
| Fabry’s disease | Burning sensations in hands and feet that worsen with exercise and hot weather |
| Niemann-Pick disease, type C | Vertical gaze palsy, hepatosplenomegaly, jaundice, ataxia |
| Prader-Willi syndrome | Obesity, hyperphagia, mild to moderate mental retardation, hypogonadism, tantrums, obsessive-compulsive disorder |
| Infectious | |
| Epstein-Barr virus | Fever, sore throat, adenopathy, fatigue, poor concentration |
| Lyme disease | Target lesion, fever; high-risk geographic area |
| Malaria/typhoid fever | Fever, mental status change; endemic area |
| Mycoplasma pneumonia | Fever, mental status change; may occur in absence of pneumonia |
| Rabies | History of exposure |
| Metabolic | |
| Citrullinemia | Mental status change, high plasma citrulline and ammonia |
| Tay-Sachs disease | Unsteadiness of gait and progressive neurologic deterioration |
| Homocystinuria | Dislocated lenses, blood clots, tall stature, some mental retardation |
| Juvenile metachromatic leukodystrophy | Cognitive decline, ataxia, pyramidal signs, peripheral neuropathy, dystonia; 60% of cases present before age 3 |
| Neurologic | |
| Central pontine myelinolysis | Suspect in patient with pathogenic polydipsia |
| Huntington’s disease | Chorea, myoclonic seizures, poor coordination, emotional lability |
| Moyamoya disease | Paresis, syncopal episodes |
| Narcolepsy | Excessive daytime sleepiness, cataplexy |
| Subacute sclerosing panencephalitis | Visual hallucinations, loss of developmental milestones |
| Traumatic brain injury | Occurring 4 to 5 years after a loss of consciousness >30 minutes |
| Wilson’s disease | Tremors, muscle spasticity, possible liver inflammation |
| Nutritional | |
| Pellagra (vitamin B6 deficiency) | Redness, swelling of mouth and tongue, diarrhea, rash, abnormal mental functioning; seen with isoniazid treatment for tuberculosis |
| Oncologic | |
| Cancers (pancreatic, CNS papilloma, germinoma) | Postural headache, neurologic signs, increased intracranial pressure, early morning nausea, vomiting |
| Toxicologic | |
| Lead intoxication | Headache, fatigue, mental status change |
| Mercury poisoning | Abdominal pain, bleeding gums, metallic taste; history of exposure |
| * Clinically significant symptoms that meet DSM-IV-TR criteria for a primary psychiatric disorder. | |
| Click here to view citations supporting statements in this table | |
Substance abuse
Substance abuse is common among adolescents and adults with psychotic illnesses.4 Drug-induced states can cause delusions, hallucinations, paranoia, and disorganized behavior,5 which are reported most commonly during intoxication and withdrawal.6 Diagnosis is often straightforward because of the temporal association between the substance abuse and onset of psychotic symptoms.
Little evidence supports a causal relationship between drug use and the development of chronic psychotic symptoms, however. Case reports link use of 3,4-methylenedioxymethamphetamine (“Ecstasy”), lysergic acid diethylamide (LSD), and marijuana to chronic schizophrenia-like symptoms.7 The strongest evidence links long-term methamphetamine and cocaine use to chronic psychotic symptoms.8,9
Medications
Side effects of at least 25 drug classes have been reported to mimic psychosis (Table 1),10 but little is known about the incidence and prevalence of this problem. Case reports and chart reviews provide the only data that associate most medications with psychotic symptoms. These disagree on what defines a “psychotic symptom,” and most fail to rule out delirium as a possible cause.
The relationship between glucocorticosteroids and psychotic symptoms has been studied extensively. A clear link has been found between corticosteroids at dosages >40 mg/d and a markedly elevated risk for transient psychotic symptoms.11
Medical conditions
We identified 27 medical conditions that may cause or worsen clinical symptoms of psychosis (Tables 2 and 3) by searching PubMed, psychiatric journals, and neuropsychiatry and consult-liaison textbooks. We included only conditions:
- shown to cause significant morbidity in pediatric populations
- shown to have a statistically significant association with psychotic symptoms, or patients’ symptoms consistently resolved when the condition was treated.
Endocrine disorders. Behavioral disturbances (including psychosis) may be the earliest manifestation of an endocrine disorder.17 Cushing’s syndrome,18 hyperthyroidism,19 and hypothyroidism20—met our inclusion criteria.
Cushing’s syndrome—caused by long-term systemic glucocorticoids and thyroid disorders—is not uncommon in children and adolescents but rarely presents with psychotic behaviors. For each endocrine disorder we included, however, at least one case report described delayed diagnosis because of prominent psychosis. Treating the endocrinopathies resolved the psychotic symptoms.
Genetic disorders. Genetically determined neurodevelopmental disorders usually present in very young children, but some may appear later. Genetic conditions that co-occur with psychotic symptoms at rates significantly greater than the population prevalence include Prader-Willi syndrome,21 metachromatic leukodystrophy,22 Turner’s syndrome,21 velocardiofacial syndrome,23 and Wilson’s disease.15
Acute intermittent porphyria, GM2 gangliosidosis (Tay-Sachs disease), and homocystinuria are rare conditions with unknown prevalence in patients with psychotic disorders. Still, they are important to consider when evaluating youths with psychosis because case reports link their treatment with psychotic symptom resolution.24-26
Infectious disease. An infectious CNS disease does not usually present with psychotic symptoms only. When this does happen, making the correct diagnosis as soon as possible is critical because early treatment is associated with better outcomes.27 Misdiagnosis as a primary psychotic disorder may expose a patient to psychotropics that may adversely affect clinical outcome.
Viruses that affect the CNS (viral encephalopathies) are the infections most likely to cause psychotic symptoms. By decreasing frequency, they are human simian virus, HIV, influenza, measles, Epstein-Barr virus, mumps, and rabies.27,28 Bacterial infections that cause psychosis include mycoplasma pneumonia,29 syphilis,30 typhoid fever,31 and Lyme disease.32
Brain tumor. Childhood brain tumors often present with behavioral symptoms associated with headache, vomiting, visual changes, and motor and cognitive symptoms. A CNS tumor rarely presents with isolated neuropsychiatric symptoms.33 A few case reports describe intracranial tumors initially misdiagnosed as primary psychotic illness because of prominent psychotic symptoms.34,35 In each case, these symptoms resolved with tumor resection.
A temporal relationship does not necessarily equate to a “causal” relationship, however. Tatter et al36 describe a case of “reoccurrence” of manic symptoms initially thought to be caused by an arteriovenous malformation (AVM) 10 years after the AVM was successfully removed. The important point is that, although rarely, pediatric brain tumor can present with prominent psychotic symptoms.
Environmental toxin exposure may cause well-defined psychiatric syndromes,37 although frank psychosis is uncommon at presentation. Most often, environmental toxins produce an encephalopathic process of which psychosis may be one symptom. A few toxic exposures—such as lead,38 carbon monoxide,39 and elemental mercury40 —have presented with prominent psychotic symptoms without other encephalopathic symptoms.
Collagen vascular disease is associated with significantly elevated rates of psychiatric illness, especially depression, but only systemic lupus erythematosus (SLE) is known to be associated with prominent psychosis. Case series report delayed SLE diagnosis in patients with this presentation.41
High-dose pulse corticosteroids have been reported to effectively treat SLE-related psychotic symptoms,42 although high-dose corticosteroids can also cause psychotic symptoms. The timing and character of the symptoms can help you determine whether using corticosteroids is helping or making the patient worse.
Using the algorithm
John’s mother and father fear that the inpatient team’s diagnosis of a primary psychotic disorder means that a medical cause has been permanently “ruled out.” To reassure them, we use the algorithm to explain in concrete terms the thought process that led us to John’s psychiatric diagnosis. We walk them through the algorithm and its tables, explaining how we used evidence to rationally rule out all known medical causes of psychotic symptoms in pediatric patients.
John’s parents are relieved to know that the case is not closed, even though we found no medical cause for their son’s condition. If more clinical data become available, we remain open to considering the possibility that medical conditions could be causing or worsening their son’s symptoms.
Related resources
- American Academy of Child and Adolescent Psychiatry. Practice parameter for the assessment and treatment of children and adolescents with schizophrenia. J Am Acad Child Adolesc Psychiatry 2001;40(7 Suppl):4S-23S.
- Schiffer RB, Klein RF, Sider RC. The medical evaluation of psychiatric patients. New York: Plenum Medical Book Co.; 1998.
- National Organization for Rare Disorders (NORD). www.rarediseases.org.
1. American Academy of Child and Adolescent Psychiatry. Summary of the practice parameters for the assessment and treatment of children and adolescents with schizophrenia. J Am Acad Child Adolesc Psychiatry 2000;39(12):1580-92.
2. Behrman RE (ed). Nelson textbook of pediatrics (17th ed). Philadelphia: WB Saunders; 2003:397-518.
3. Dalmau A, Bergman B, Brismar B. Psychotic disorders among inpatients with abuse of cannabis, amphetamine and opiates. Do dopaminergic stimulants facilitate psychiatric illness? Eur Psychiatry 1999;14(7):366-71.
4. Breslow RE, Klinger BI, Erickson BJ. Acute intoxication and substance abuse among patients presenting to a psychiatric emergency service. Gen Hosp Psychiatry 1996;18(3):183-91.
5. Poole R, Brabbins C. Drug-induced psychosis. Br J Psychiatry 1996;168:137.-
6. DiSclafani A, 2nd, Hall RC, Gardner ER. Drug-induced psychosis: emergency diagnosis and management. Psychosomatics 1981;22(10):845-55.
7. Cohen SI. Substance-induced psychosis. Br J Psychiatry 1996;168(5):651-2.
8. Farrell M, Boys A, Bebbington P, et al. Psychosis and drug dependence: results from a national survey of prisoners. Br J Psychiatry 2002;181:393-8.
9. Ujike H, Sato M. Clinical features of sensitization to methamphetamine observed in patients with methamphetamine dependence and psychosis. Ann NY Acad Sci 2004;1025:279-87.
10. Drugs that may cause psychiatric symptoms. Med Lett Drugs Ther 2002;44:29-62.
11. Lewis DA, Smith RE. Steroid-induced psychiatric symptoms; a report of 14 cases and a review of the literature. J Affect Disord 1983;5(4):319-32.
12. Cummings JL. Organic psychosis. Psychosomatics 1988;29(1):16-26.
13. Roy AK, Rajesh SV, Iby N, et al. A study of epilepsy-related psychosis. Neurol India 2003;51(3):359-60.
14. Mendez MF. Huntington’s disease: update and review of neuropsychiatric aspects. Int J Psychiatry Med 1994;24:189-208.
15. Brewer GJ. Recognition and management of Wilson’s disease. Proc Soc Exp Biol Med 2000;223:39-46.
16. Mendhekar DN, Mehta R, Puri V. Successful steroid therapy in multiple sclerosis presented as acute psychosis. J Assoc Physicians India 2004;52:512-3.
17. Reus VI. Behavioral disturbances associated with endocrine disorders. Ann Rev Med 1986;37:205-14.
18. Hirsch D, Orr G, Kantarovich V, et al. Cushing’s syndrome presenting as a schizophrenia-like psychotic state. Isr J Psychiatry Relat Sci 2000;37(1):46-50.
19. Lu CL, Lee YC, Tsai SJ, et al. Psychiatric disturbances associated with hyperthyroidism: an analysis report of 30 cases. Zhonghua Yi Xue Za Zhi (Taipei) 1995;56(6):393-8.
20. Bhatara V, Alshari MG, Warhol P, et al. Coexistent hypothyroidism, psychosis, and severe obsessions in an adolescent: a 10-year follow-up. J Child Adolesc Psychopharmacol 2004;14(2):315-23.
21. Prior TI, Chue PS, Tibbo P. Investigation of Turner syndrome in schizophrenia. Am J Med Genet 2000;96(3):373-8.
22. Hyde TM, Ziegler JC, Weinberger DR. Psychiatric disturbances in metachromatic leukodystrophy. Insights into the neurobiology of psychosis. Arch Neurol 1992;49(4):401-6.
23. Briegel W, Cohen M. Chromosome 22q11 deletion syndrome and its relevance for child and adolescent psychiatry. An overview of etiology, physical symptoms, aspects of child development and psychiatric disorders. Z Kinder Jugendpsychiatr Psychother 2004;32(2):107-15.
24. Crimlisk HL. The great imitator-porphyria: a neuropsychiatric disorder. J Neurol Neurosurg Psychiatry 2001;62(4):319-28.
25. Ryan MM, Sidhu RK, Alexander J, Megerian JT. Homocystinuria presenting as psychosis in an adolescent. J Child Neurol 2002;17(11):859-60.
26. MacQueen GM, Rosebush PI, Mazurek MF. Neuropsychiatric aspects of the adult variant of Tay-Sachs disease. J Neuropsychiatry Clin Neurosci 1998;10(1):10-9.
27. Caroff SN, Mann SC, Gliatto MF, et al. Psychiatric manifestations of acute viral encephalitis. Psych Annals 2001;31(3):193-204.
28. Caplan R, Tanguay PE, Szekely AG. Subacute sclerosing panencephalitis presenting as childhood psychosis. J Am Acad Child Adolesc Psychiatry 1987;26(3):440-3.
29. Gillberg C. Schizophreniform psychosis in a case of mycoplasma pneumoniae encephalitis. J Autism Dev Disord 1980;10(2):153-8.
30. Gliatto MF, Caroff SN. Neurosyphilis: a history and clinical review. Psych Annals 2001;31(3):153-61.
31. Venkatesh S, Grell GA. Neuropsychiatric manifestations of typhoid fever. West Indian Med J 1989;38(3):137-41.
32. Tager FA, Fallon B. Psychiatric and cognitive features of Lyme disease. Psych Annals 2001;31(3):173-92.
33. Stein MT, Duffner PK, Wery JS, Trauner D. School refusal and emotional liability in a 6 year old boy. J Dev Behav Pediatr 2001;22(suppl):29-32.
34. Carson BS, Weingart JD, Guarnieri M, Fisher PG. Third ventricular choroid plexus papilloma with psychosis. Case report. J Neurosurg 1997;87(1):103-5.
35. Craven C. Pineal germinoma and psychosis. J Am Acad Child Adolesc Psychiatry 2001;40(1):6.-
36. Tatter SB, Ogilvy CS. Recurrent manic episode 10 years after arteriovenous malformation resection. J Clin Psychiatry 1995;56(2):83.-
37. Hartman DE. Missed diagnosis and misdiagnosis of environmental toxicants exposure: the psychiatry of toxic exposure and multiple chemical sensitivity. Psychiatr Clin North Am 1998;21(3):659-70.
38. Bahiga LM, Kotb NA, El-Dessoukey EA. Neurological syndromes produced by some toxic metals encountered industrially or environmentally. Z Ernahrungswiss 1978;17(2):84-8.
39. Olson KR. Carbon monoxide poisoning: mechanisms, presentation, and controversies in management. J Emerg Med 1984;1(3):233-43.
40. Fagala GE, Wigg CL. Psychiatric manifestations of mercury poisoning. J Am Acad Child Adolesc Psychiatry 1992;31(2):306-11.
41. Turkel SB, Miller JH, Reiff A. Case series: neuropsychiatric symptoms with pediatric systemic lupus erythematosus. J Am Acad Child Adolesc Psychiatry 2001;40(4):482-5.
42. Baca V, Lavalle C, Garcia R, et al. Favorable response to intravenous methylprednisolone and cyclophosphamide in children with severe neuropsychiatric lupus. J Rheumatol 1999;26(2):432-9.
John, age 16, is admitted to our inpatient psychiatric unit, complaining of “a 2-week constant headache” caused by “voices arguing in my head.” He has lived in Mexico with an uncle for 6 months but returned home last week for medical evaluation of his headaches.
His parents report that John developed normally until 3 years ago, when he gradually lost interest in his favorite activities and became socially withdrawn. He has not attended school in 2 years. He has no history of illicit drug use and is not taking prescription or over the-counter medications.
Complete physical examination, neurologic exam, and routine screening lab test results are normal. Thinking that a high lead content of cookware used in Mexico might be causing John’s symptoms, we order a lead level: result-0.2 mg/dL (
We diagnose schizophreniform disorder, but John’s parents refuse to accept this diagnosis. They repeatedly ask if we can do more to identify a medical cause of their son’s psychiatric symptoms.
As in John’s case, young patients or their parents may resist the diagnosis of a chronic mental illness such as schizophrenia. Understandably, they may be invested in trying to identify “medically treatable” causes. You can address their anxieties by showing them that you have systematically evaluated medical causes of psychosis.
We offer such a tool: an algorithm and tables to help you identify common and rare medical conditions that may cause or exacerbate psychotic symptoms in patients ages 3 to 18.
An evidence-based algorithm
Multiple factors—developmental, psychological, family, environmental, or medical—typically cause psychotic symptoms in a child or adolescent. Evaluating all possibilities is essential, but guidelines tend to minimize medical causes. American Academy of Child and Adolescent Psychiatry guidelines, for example, recommend that “all medical disorders (including general medical conditions and substance-induced disorders) are ruled out,”1 but they do not specify which medical conditions to consider.
To supplement existing guidelines, we searched the literature and developed an evidence-based algorithm to help you systematically consider medical causes of pediatric psychotic symptoms. We excluded children age 2
How to use it. The algorithm walks you through a medical systems review. You begin with a complete history, then address six causes of psychotic symptoms: substance abuse, medication reactions, general medical conditions, unexplained somatic symptoms (such as from toxic environmental exposures), developmental and learning disabilities, and atypical presentations.
Don’t stop if you find one possible cause of psychotic symptoms; continue to the end of the algorithm. The more factors you identify, the greater your chance of finding a treatable cause that may ameliorate your patient’s symptoms.
To make the algorithm clinically useful, we listed conditions in order of decreasing probability of causing psychotic symptoms. For example, the first cause listed is substance-induced disorders,3 which are most common among adolescent patients. We also “triaged” medical conditions from common to rare (based on estimated prevalence of association with psychotic symptoms), listing rare causes only in cases of atypical presentation or treatment resistance.
Supporting tables. The following discussion summarizes data that support the algorithm and its tables:
- medications reported to cause psychosis (Table 1)
- medical conditions most likely to cause psychosis (Table 2)
- medical conditions that rarely cause psychosis (Table 3).
Drugs that may cause psychotic symptoms
| Drug class | Psychotic symptoms | |
|---|---|---|
| Bizarre behavior/delusions | Auditory or visual hallucinations | |
| Amphetamine-like drugs | X | X |
| Anabolic steroids | X | |
| Angiotensin-converting enzyme (ACE) inhibitors | X | |
| Anticholinergics and atropine | X | X |
| Antidepressants, tricyclic | X | |
| Antiepileptics | X | |
| Barbiturates | X | X |
| Benzodiazepines | X | X |
| Beta-adrenergic blockers | X | X |
| Calcium channel blockers | X | |
| Cephalosporins | X | X |
| Corticosteroids | X | |
| Dopamine receptor agonists | X | X |
| Fluoroquinolone antibiotics | X | X |
| Histamine H1 receptor blockers | X | |
| Histamine H2 receptor blockers | X | |
| HMG-CoA reductase inhibitors | X | |
| Nonsteroidal anti-inflammatory drugs | X | |
| Opioids | X | X |
| Procaine derivatives (procainamide, procaine penicillin G) | X | X |
| Salicylates | X | X |
| Selective serotonin reuptake inhibitors | X | |
| Sulfonamides | X | |
| Source: Adapted from reference 10. | ||
Common medical conditions that may cause pediatric psychosis symptoms*
| Category | Conditions not to forget | Common symptoms/comments |
|---|---|---|
| Rheumatologic | Lupus erythematosus | Joint pain, fever, facial butterfly rash, prolonged fatigue |
| Infectious | Viral encephalitis | Fever, headache, mental status change; may occur in perinatal period |
| Neurologic | Multiple sclerosis | Varied neurologic deficits, especially ophthalmologic changes and weakness |
| Neurosyphilis | Personality change, ataxia, stroke, ophthalmic symptoms | |
| Seizure (temporal lobe epilepsy, interictal psychosis) | Paroxysmal periods of sudden change in mood, behavior, or motor activity with or without loss of consciousness | |
| Toxicologic | Carbon monoxide poisoning | Shortness of breath, mild nausea, headache, dizziness |
| * Clinically significant symptoms that meet DSM-IV-TR criteria for a primary psychiatric disorder. | ||
| Click here to view citations supporting statements in this table | ||
Medical conditions that rarely cause pediatric psychosis symptoms*
| Category/condition | Symptoms/comments |
|---|---|
| Endocrine | |
| Hyperthyroidism | Tachycardia, weight loss, excessive sweating, tiredness, inability to sleep, diarrhea, shakiness, muscle weakness |
| Thymoma/myasthenia gravis | Shortness of breath, swelling of face, muscle weakness (especially around eyes) |
| Hematologic | |
| Porphyria (acute intermittent porphyria, porphyria variegate) | Intermittent abdominal pain (severe) accompanied by dark urine |
| Genetic | |
| Fabry’s disease | Burning sensations in hands and feet that worsen with exercise and hot weather |
| Niemann-Pick disease, type C | Vertical gaze palsy, hepatosplenomegaly, jaundice, ataxia |
| Prader-Willi syndrome | Obesity, hyperphagia, mild to moderate mental retardation, hypogonadism, tantrums, obsessive-compulsive disorder |
| Infectious | |
| Epstein-Barr virus | Fever, sore throat, adenopathy, fatigue, poor concentration |
| Lyme disease | Target lesion, fever; high-risk geographic area |
| Malaria/typhoid fever | Fever, mental status change; endemic area |
| Mycoplasma pneumonia | Fever, mental status change; may occur in absence of pneumonia |
| Rabies | History of exposure |
| Metabolic | |
| Citrullinemia | Mental status change, high plasma citrulline and ammonia |
| Tay-Sachs disease | Unsteadiness of gait and progressive neurologic deterioration |
| Homocystinuria | Dislocated lenses, blood clots, tall stature, some mental retardation |
| Juvenile metachromatic leukodystrophy | Cognitive decline, ataxia, pyramidal signs, peripheral neuropathy, dystonia; 60% of cases present before age 3 |
| Neurologic | |
| Central pontine myelinolysis | Suspect in patient with pathogenic polydipsia |
| Huntington’s disease | Chorea, myoclonic seizures, poor coordination, emotional lability |
| Moyamoya disease | Paresis, syncopal episodes |
| Narcolepsy | Excessive daytime sleepiness, cataplexy |
| Subacute sclerosing panencephalitis | Visual hallucinations, loss of developmental milestones |
| Traumatic brain injury | Occurring 4 to 5 years after a loss of consciousness >30 minutes |
| Wilson’s disease | Tremors, muscle spasticity, possible liver inflammation |
| Nutritional | |
| Pellagra (vitamin B6 deficiency) | Redness, swelling of mouth and tongue, diarrhea, rash, abnormal mental functioning; seen with isoniazid treatment for tuberculosis |
| Oncologic | |
| Cancers (pancreatic, CNS papilloma, germinoma) | Postural headache, neurologic signs, increased intracranial pressure, early morning nausea, vomiting |
| Toxicologic | |
| Lead intoxication | Headache, fatigue, mental status change |
| Mercury poisoning | Abdominal pain, bleeding gums, metallic taste; history of exposure |
| * Clinically significant symptoms that meet DSM-IV-TR criteria for a primary psychiatric disorder. | |
| Click here to view citations supporting statements in this table | |
Substance abuse
Substance abuse is common among adolescents and adults with psychotic illnesses.4 Drug-induced states can cause delusions, hallucinations, paranoia, and disorganized behavior,5 which are reported most commonly during intoxication and withdrawal.6 Diagnosis is often straightforward because of the temporal association between the substance abuse and onset of psychotic symptoms.
Little evidence supports a causal relationship between drug use and the development of chronic psychotic symptoms, however. Case reports link use of 3,4-methylenedioxymethamphetamine (“Ecstasy”), lysergic acid diethylamide (LSD), and marijuana to chronic schizophrenia-like symptoms.7 The strongest evidence links long-term methamphetamine and cocaine use to chronic psychotic symptoms.8,9
Medications
Side effects of at least 25 drug classes have been reported to mimic psychosis (Table 1),10 but little is known about the incidence and prevalence of this problem. Case reports and chart reviews provide the only data that associate most medications with psychotic symptoms. These disagree on what defines a “psychotic symptom,” and most fail to rule out delirium as a possible cause.
The relationship between glucocorticosteroids and psychotic symptoms has been studied extensively. A clear link has been found between corticosteroids at dosages >40 mg/d and a markedly elevated risk for transient psychotic symptoms.11
Medical conditions
We identified 27 medical conditions that may cause or worsen clinical symptoms of psychosis (Tables 2 and 3) by searching PubMed, psychiatric journals, and neuropsychiatry and consult-liaison textbooks. We included only conditions:
- shown to cause significant morbidity in pediatric populations
- shown to have a statistically significant association with psychotic symptoms, or patients’ symptoms consistently resolved when the condition was treated.
Endocrine disorders. Behavioral disturbances (including psychosis) may be the earliest manifestation of an endocrine disorder.17 Cushing’s syndrome,18 hyperthyroidism,19 and hypothyroidism20—met our inclusion criteria.
Cushing’s syndrome—caused by long-term systemic glucocorticoids and thyroid disorders—is not uncommon in children and adolescents but rarely presents with psychotic behaviors. For each endocrine disorder we included, however, at least one case report described delayed diagnosis because of prominent psychosis. Treating the endocrinopathies resolved the psychotic symptoms.
Genetic disorders. Genetically determined neurodevelopmental disorders usually present in very young children, but some may appear later. Genetic conditions that co-occur with psychotic symptoms at rates significantly greater than the population prevalence include Prader-Willi syndrome,21 metachromatic leukodystrophy,22 Turner’s syndrome,21 velocardiofacial syndrome,23 and Wilson’s disease.15
Acute intermittent porphyria, GM2 gangliosidosis (Tay-Sachs disease), and homocystinuria are rare conditions with unknown prevalence in patients with psychotic disorders. Still, they are important to consider when evaluating youths with psychosis because case reports link their treatment with psychotic symptom resolution.24-26
Infectious disease. An infectious CNS disease does not usually present with psychotic symptoms only. When this does happen, making the correct diagnosis as soon as possible is critical because early treatment is associated with better outcomes.27 Misdiagnosis as a primary psychotic disorder may expose a patient to psychotropics that may adversely affect clinical outcome.
Viruses that affect the CNS (viral encephalopathies) are the infections most likely to cause psychotic symptoms. By decreasing frequency, they are human simian virus, HIV, influenza, measles, Epstein-Barr virus, mumps, and rabies.27,28 Bacterial infections that cause psychosis include mycoplasma pneumonia,29 syphilis,30 typhoid fever,31 and Lyme disease.32
Brain tumor. Childhood brain tumors often present with behavioral symptoms associated with headache, vomiting, visual changes, and motor and cognitive symptoms. A CNS tumor rarely presents with isolated neuropsychiatric symptoms.33 A few case reports describe intracranial tumors initially misdiagnosed as primary psychotic illness because of prominent psychotic symptoms.34,35 In each case, these symptoms resolved with tumor resection.
A temporal relationship does not necessarily equate to a “causal” relationship, however. Tatter et al36 describe a case of “reoccurrence” of manic symptoms initially thought to be caused by an arteriovenous malformation (AVM) 10 years after the AVM was successfully removed. The important point is that, although rarely, pediatric brain tumor can present with prominent psychotic symptoms.
Environmental toxin exposure may cause well-defined psychiatric syndromes,37 although frank psychosis is uncommon at presentation. Most often, environmental toxins produce an encephalopathic process of which psychosis may be one symptom. A few toxic exposures—such as lead,38 carbon monoxide,39 and elemental mercury40 —have presented with prominent psychotic symptoms without other encephalopathic symptoms.
Collagen vascular disease is associated with significantly elevated rates of psychiatric illness, especially depression, but only systemic lupus erythematosus (SLE) is known to be associated with prominent psychosis. Case series report delayed SLE diagnosis in patients with this presentation.41
High-dose pulse corticosteroids have been reported to effectively treat SLE-related psychotic symptoms,42 although high-dose corticosteroids can also cause psychotic symptoms. The timing and character of the symptoms can help you determine whether using corticosteroids is helping or making the patient worse.
Using the algorithm
John’s mother and father fear that the inpatient team’s diagnosis of a primary psychotic disorder means that a medical cause has been permanently “ruled out.” To reassure them, we use the algorithm to explain in concrete terms the thought process that led us to John’s psychiatric diagnosis. We walk them through the algorithm and its tables, explaining how we used evidence to rationally rule out all known medical causes of psychotic symptoms in pediatric patients.
John’s parents are relieved to know that the case is not closed, even though we found no medical cause for their son’s condition. If more clinical data become available, we remain open to considering the possibility that medical conditions could be causing or worsening their son’s symptoms.
Related resources
- American Academy of Child and Adolescent Psychiatry. Practice parameter for the assessment and treatment of children and adolescents with schizophrenia. J Am Acad Child Adolesc Psychiatry 2001;40(7 Suppl):4S-23S.
- Schiffer RB, Klein RF, Sider RC. The medical evaluation of psychiatric patients. New York: Plenum Medical Book Co.; 1998.
- National Organization for Rare Disorders (NORD). www.rarediseases.org.
John, age 16, is admitted to our inpatient psychiatric unit, complaining of “a 2-week constant headache” caused by “voices arguing in my head.” He has lived in Mexico with an uncle for 6 months but returned home last week for medical evaluation of his headaches.
His parents report that John developed normally until 3 years ago, when he gradually lost interest in his favorite activities and became socially withdrawn. He has not attended school in 2 years. He has no history of illicit drug use and is not taking prescription or over the-counter medications.
Complete physical examination, neurologic exam, and routine screening lab test results are normal. Thinking that a high lead content of cookware used in Mexico might be causing John’s symptoms, we order a lead level: result-0.2 mg/dL (
We diagnose schizophreniform disorder, but John’s parents refuse to accept this diagnosis. They repeatedly ask if we can do more to identify a medical cause of their son’s psychiatric symptoms.
As in John’s case, young patients or their parents may resist the diagnosis of a chronic mental illness such as schizophrenia. Understandably, they may be invested in trying to identify “medically treatable” causes. You can address their anxieties by showing them that you have systematically evaluated medical causes of psychosis.
We offer such a tool: an algorithm and tables to help you identify common and rare medical conditions that may cause or exacerbate psychotic symptoms in patients ages 3 to 18.
An evidence-based algorithm
Multiple factors—developmental, psychological, family, environmental, or medical—typically cause psychotic symptoms in a child or adolescent. Evaluating all possibilities is essential, but guidelines tend to minimize medical causes. American Academy of Child and Adolescent Psychiatry guidelines, for example, recommend that “all medical disorders (including general medical conditions and substance-induced disorders) are ruled out,”1 but they do not specify which medical conditions to consider.
To supplement existing guidelines, we searched the literature and developed an evidence-based algorithm to help you systematically consider medical causes of pediatric psychotic symptoms. We excluded children age 2
How to use it. The algorithm walks you through a medical systems review. You begin with a complete history, then address six causes of psychotic symptoms: substance abuse, medication reactions, general medical conditions, unexplained somatic symptoms (such as from toxic environmental exposures), developmental and learning disabilities, and atypical presentations.
Don’t stop if you find one possible cause of psychotic symptoms; continue to the end of the algorithm. The more factors you identify, the greater your chance of finding a treatable cause that may ameliorate your patient’s symptoms.
To make the algorithm clinically useful, we listed conditions in order of decreasing probability of causing psychotic symptoms. For example, the first cause listed is substance-induced disorders,3 which are most common among adolescent patients. We also “triaged” medical conditions from common to rare (based on estimated prevalence of association with psychotic symptoms), listing rare causes only in cases of atypical presentation or treatment resistance.
Supporting tables. The following discussion summarizes data that support the algorithm and its tables:
- medications reported to cause psychosis (Table 1)
- medical conditions most likely to cause psychosis (Table 2)
- medical conditions that rarely cause psychosis (Table 3).
Drugs that may cause psychotic symptoms
| Drug class | Psychotic symptoms | |
|---|---|---|
| Bizarre behavior/delusions | Auditory or visual hallucinations | |
| Amphetamine-like drugs | X | X |
| Anabolic steroids | X | |
| Angiotensin-converting enzyme (ACE) inhibitors | X | |
| Anticholinergics and atropine | X | X |
| Antidepressants, tricyclic | X | |
| Antiepileptics | X | |
| Barbiturates | X | X |
| Benzodiazepines | X | X |
| Beta-adrenergic blockers | X | X |
| Calcium channel blockers | X | |
| Cephalosporins | X | X |
| Corticosteroids | X | |
| Dopamine receptor agonists | X | X |
| Fluoroquinolone antibiotics | X | X |
| Histamine H1 receptor blockers | X | |
| Histamine H2 receptor blockers | X | |
| HMG-CoA reductase inhibitors | X | |
| Nonsteroidal anti-inflammatory drugs | X | |
| Opioids | X | X |
| Procaine derivatives (procainamide, procaine penicillin G) | X | X |
| Salicylates | X | X |
| Selective serotonin reuptake inhibitors | X | |
| Sulfonamides | X | |
| Source: Adapted from reference 10. | ||
Common medical conditions that may cause pediatric psychosis symptoms*
| Category | Conditions not to forget | Common symptoms/comments |
|---|---|---|
| Rheumatologic | Lupus erythematosus | Joint pain, fever, facial butterfly rash, prolonged fatigue |
| Infectious | Viral encephalitis | Fever, headache, mental status change; may occur in perinatal period |
| Neurologic | Multiple sclerosis | Varied neurologic deficits, especially ophthalmologic changes and weakness |
| Neurosyphilis | Personality change, ataxia, stroke, ophthalmic symptoms | |
| Seizure (temporal lobe epilepsy, interictal psychosis) | Paroxysmal periods of sudden change in mood, behavior, or motor activity with or without loss of consciousness | |
| Toxicologic | Carbon monoxide poisoning | Shortness of breath, mild nausea, headache, dizziness |
| * Clinically significant symptoms that meet DSM-IV-TR criteria for a primary psychiatric disorder. | ||
| Click here to view citations supporting statements in this table | ||
Medical conditions that rarely cause pediatric psychosis symptoms*
| Category/condition | Symptoms/comments |
|---|---|
| Endocrine | |
| Hyperthyroidism | Tachycardia, weight loss, excessive sweating, tiredness, inability to sleep, diarrhea, shakiness, muscle weakness |
| Thymoma/myasthenia gravis | Shortness of breath, swelling of face, muscle weakness (especially around eyes) |
| Hematologic | |
| Porphyria (acute intermittent porphyria, porphyria variegate) | Intermittent abdominal pain (severe) accompanied by dark urine |
| Genetic | |
| Fabry’s disease | Burning sensations in hands and feet that worsen with exercise and hot weather |
| Niemann-Pick disease, type C | Vertical gaze palsy, hepatosplenomegaly, jaundice, ataxia |
| Prader-Willi syndrome | Obesity, hyperphagia, mild to moderate mental retardation, hypogonadism, tantrums, obsessive-compulsive disorder |
| Infectious | |
| Epstein-Barr virus | Fever, sore throat, adenopathy, fatigue, poor concentration |
| Lyme disease | Target lesion, fever; high-risk geographic area |
| Malaria/typhoid fever | Fever, mental status change; endemic area |
| Mycoplasma pneumonia | Fever, mental status change; may occur in absence of pneumonia |
| Rabies | History of exposure |
| Metabolic | |
| Citrullinemia | Mental status change, high plasma citrulline and ammonia |
| Tay-Sachs disease | Unsteadiness of gait and progressive neurologic deterioration |
| Homocystinuria | Dislocated lenses, blood clots, tall stature, some mental retardation |
| Juvenile metachromatic leukodystrophy | Cognitive decline, ataxia, pyramidal signs, peripheral neuropathy, dystonia; 60% of cases present before age 3 |
| Neurologic | |
| Central pontine myelinolysis | Suspect in patient with pathogenic polydipsia |
| Huntington’s disease | Chorea, myoclonic seizures, poor coordination, emotional lability |
| Moyamoya disease | Paresis, syncopal episodes |
| Narcolepsy | Excessive daytime sleepiness, cataplexy |
| Subacute sclerosing panencephalitis | Visual hallucinations, loss of developmental milestones |
| Traumatic brain injury | Occurring 4 to 5 years after a loss of consciousness >30 minutes |
| Wilson’s disease | Tremors, muscle spasticity, possible liver inflammation |
| Nutritional | |
| Pellagra (vitamin B6 deficiency) | Redness, swelling of mouth and tongue, diarrhea, rash, abnormal mental functioning; seen with isoniazid treatment for tuberculosis |
| Oncologic | |
| Cancers (pancreatic, CNS papilloma, germinoma) | Postural headache, neurologic signs, increased intracranial pressure, early morning nausea, vomiting |
| Toxicologic | |
| Lead intoxication | Headache, fatigue, mental status change |
| Mercury poisoning | Abdominal pain, bleeding gums, metallic taste; history of exposure |
| * Clinically significant symptoms that meet DSM-IV-TR criteria for a primary psychiatric disorder. | |
| Click here to view citations supporting statements in this table | |
Substance abuse
Substance abuse is common among adolescents and adults with psychotic illnesses.4 Drug-induced states can cause delusions, hallucinations, paranoia, and disorganized behavior,5 which are reported most commonly during intoxication and withdrawal.6 Diagnosis is often straightforward because of the temporal association between the substance abuse and onset of psychotic symptoms.
Little evidence supports a causal relationship between drug use and the development of chronic psychotic symptoms, however. Case reports link use of 3,4-methylenedioxymethamphetamine (“Ecstasy”), lysergic acid diethylamide (LSD), and marijuana to chronic schizophrenia-like symptoms.7 The strongest evidence links long-term methamphetamine and cocaine use to chronic psychotic symptoms.8,9
Medications
Side effects of at least 25 drug classes have been reported to mimic psychosis (Table 1),10 but little is known about the incidence and prevalence of this problem. Case reports and chart reviews provide the only data that associate most medications with psychotic symptoms. These disagree on what defines a “psychotic symptom,” and most fail to rule out delirium as a possible cause.
The relationship between glucocorticosteroids and psychotic symptoms has been studied extensively. A clear link has been found between corticosteroids at dosages >40 mg/d and a markedly elevated risk for transient psychotic symptoms.11
Medical conditions
We identified 27 medical conditions that may cause or worsen clinical symptoms of psychosis (Tables 2 and 3) by searching PubMed, psychiatric journals, and neuropsychiatry and consult-liaison textbooks. We included only conditions:
- shown to cause significant morbidity in pediatric populations
- shown to have a statistically significant association with psychotic symptoms, or patients’ symptoms consistently resolved when the condition was treated.
Endocrine disorders. Behavioral disturbances (including psychosis) may be the earliest manifestation of an endocrine disorder.17 Cushing’s syndrome,18 hyperthyroidism,19 and hypothyroidism20—met our inclusion criteria.
Cushing’s syndrome—caused by long-term systemic glucocorticoids and thyroid disorders—is not uncommon in children and adolescents but rarely presents with psychotic behaviors. For each endocrine disorder we included, however, at least one case report described delayed diagnosis because of prominent psychosis. Treating the endocrinopathies resolved the psychotic symptoms.
Genetic disorders. Genetically determined neurodevelopmental disorders usually present in very young children, but some may appear later. Genetic conditions that co-occur with psychotic symptoms at rates significantly greater than the population prevalence include Prader-Willi syndrome,21 metachromatic leukodystrophy,22 Turner’s syndrome,21 velocardiofacial syndrome,23 and Wilson’s disease.15
Acute intermittent porphyria, GM2 gangliosidosis (Tay-Sachs disease), and homocystinuria are rare conditions with unknown prevalence in patients with psychotic disorders. Still, they are important to consider when evaluating youths with psychosis because case reports link their treatment with psychotic symptom resolution.24-26
Infectious disease. An infectious CNS disease does not usually present with psychotic symptoms only. When this does happen, making the correct diagnosis as soon as possible is critical because early treatment is associated with better outcomes.27 Misdiagnosis as a primary psychotic disorder may expose a patient to psychotropics that may adversely affect clinical outcome.
Viruses that affect the CNS (viral encephalopathies) are the infections most likely to cause psychotic symptoms. By decreasing frequency, they are human simian virus, HIV, influenza, measles, Epstein-Barr virus, mumps, and rabies.27,28 Bacterial infections that cause psychosis include mycoplasma pneumonia,29 syphilis,30 typhoid fever,31 and Lyme disease.32
Brain tumor. Childhood brain tumors often present with behavioral symptoms associated with headache, vomiting, visual changes, and motor and cognitive symptoms. A CNS tumor rarely presents with isolated neuropsychiatric symptoms.33 A few case reports describe intracranial tumors initially misdiagnosed as primary psychotic illness because of prominent psychotic symptoms.34,35 In each case, these symptoms resolved with tumor resection.
A temporal relationship does not necessarily equate to a “causal” relationship, however. Tatter et al36 describe a case of “reoccurrence” of manic symptoms initially thought to be caused by an arteriovenous malformation (AVM) 10 years after the AVM was successfully removed. The important point is that, although rarely, pediatric brain tumor can present with prominent psychotic symptoms.
Environmental toxin exposure may cause well-defined psychiatric syndromes,37 although frank psychosis is uncommon at presentation. Most often, environmental toxins produce an encephalopathic process of which psychosis may be one symptom. A few toxic exposures—such as lead,38 carbon monoxide,39 and elemental mercury40 —have presented with prominent psychotic symptoms without other encephalopathic symptoms.
Collagen vascular disease is associated with significantly elevated rates of psychiatric illness, especially depression, but only systemic lupus erythematosus (SLE) is known to be associated with prominent psychosis. Case series report delayed SLE diagnosis in patients with this presentation.41
High-dose pulse corticosteroids have been reported to effectively treat SLE-related psychotic symptoms,42 although high-dose corticosteroids can also cause psychotic symptoms. The timing and character of the symptoms can help you determine whether using corticosteroids is helping or making the patient worse.
Using the algorithm
John’s mother and father fear that the inpatient team’s diagnosis of a primary psychotic disorder means that a medical cause has been permanently “ruled out.” To reassure them, we use the algorithm to explain in concrete terms the thought process that led us to John’s psychiatric diagnosis. We walk them through the algorithm and its tables, explaining how we used evidence to rationally rule out all known medical causes of psychotic symptoms in pediatric patients.
John’s parents are relieved to know that the case is not closed, even though we found no medical cause for their son’s condition. If more clinical data become available, we remain open to considering the possibility that medical conditions could be causing or worsening their son’s symptoms.
Related resources
- American Academy of Child and Adolescent Psychiatry. Practice parameter for the assessment and treatment of children and adolescents with schizophrenia. J Am Acad Child Adolesc Psychiatry 2001;40(7 Suppl):4S-23S.
- Schiffer RB, Klein RF, Sider RC. The medical evaluation of psychiatric patients. New York: Plenum Medical Book Co.; 1998.
- National Organization for Rare Disorders (NORD). www.rarediseases.org.
1. American Academy of Child and Adolescent Psychiatry. Summary of the practice parameters for the assessment and treatment of children and adolescents with schizophrenia. J Am Acad Child Adolesc Psychiatry 2000;39(12):1580-92.
2. Behrman RE (ed). Nelson textbook of pediatrics (17th ed). Philadelphia: WB Saunders; 2003:397-518.
3. Dalmau A, Bergman B, Brismar B. Psychotic disorders among inpatients with abuse of cannabis, amphetamine and opiates. Do dopaminergic stimulants facilitate psychiatric illness? Eur Psychiatry 1999;14(7):366-71.
4. Breslow RE, Klinger BI, Erickson BJ. Acute intoxication and substance abuse among patients presenting to a psychiatric emergency service. Gen Hosp Psychiatry 1996;18(3):183-91.
5. Poole R, Brabbins C. Drug-induced psychosis. Br J Psychiatry 1996;168:137.-
6. DiSclafani A, 2nd, Hall RC, Gardner ER. Drug-induced psychosis: emergency diagnosis and management. Psychosomatics 1981;22(10):845-55.
7. Cohen SI. Substance-induced psychosis. Br J Psychiatry 1996;168(5):651-2.
8. Farrell M, Boys A, Bebbington P, et al. Psychosis and drug dependence: results from a national survey of prisoners. Br J Psychiatry 2002;181:393-8.
9. Ujike H, Sato M. Clinical features of sensitization to methamphetamine observed in patients with methamphetamine dependence and psychosis. Ann NY Acad Sci 2004;1025:279-87.
10. Drugs that may cause psychiatric symptoms. Med Lett Drugs Ther 2002;44:29-62.
11. Lewis DA, Smith RE. Steroid-induced psychiatric symptoms; a report of 14 cases and a review of the literature. J Affect Disord 1983;5(4):319-32.
12. Cummings JL. Organic psychosis. Psychosomatics 1988;29(1):16-26.
13. Roy AK, Rajesh SV, Iby N, et al. A study of epilepsy-related psychosis. Neurol India 2003;51(3):359-60.
14. Mendez MF. Huntington’s disease: update and review of neuropsychiatric aspects. Int J Psychiatry Med 1994;24:189-208.
15. Brewer GJ. Recognition and management of Wilson’s disease. Proc Soc Exp Biol Med 2000;223:39-46.
16. Mendhekar DN, Mehta R, Puri V. Successful steroid therapy in multiple sclerosis presented as acute psychosis. J Assoc Physicians India 2004;52:512-3.
17. Reus VI. Behavioral disturbances associated with endocrine disorders. Ann Rev Med 1986;37:205-14.
18. Hirsch D, Orr G, Kantarovich V, et al. Cushing’s syndrome presenting as a schizophrenia-like psychotic state. Isr J Psychiatry Relat Sci 2000;37(1):46-50.
19. Lu CL, Lee YC, Tsai SJ, et al. Psychiatric disturbances associated with hyperthyroidism: an analysis report of 30 cases. Zhonghua Yi Xue Za Zhi (Taipei) 1995;56(6):393-8.
20. Bhatara V, Alshari MG, Warhol P, et al. Coexistent hypothyroidism, psychosis, and severe obsessions in an adolescent: a 10-year follow-up. J Child Adolesc Psychopharmacol 2004;14(2):315-23.
21. Prior TI, Chue PS, Tibbo P. Investigation of Turner syndrome in schizophrenia. Am J Med Genet 2000;96(3):373-8.
22. Hyde TM, Ziegler JC, Weinberger DR. Psychiatric disturbances in metachromatic leukodystrophy. Insights into the neurobiology of psychosis. Arch Neurol 1992;49(4):401-6.
23. Briegel W, Cohen M. Chromosome 22q11 deletion syndrome and its relevance for child and adolescent psychiatry. An overview of etiology, physical symptoms, aspects of child development and psychiatric disorders. Z Kinder Jugendpsychiatr Psychother 2004;32(2):107-15.
24. Crimlisk HL. The great imitator-porphyria: a neuropsychiatric disorder. J Neurol Neurosurg Psychiatry 2001;62(4):319-28.
25. Ryan MM, Sidhu RK, Alexander J, Megerian JT. Homocystinuria presenting as psychosis in an adolescent. J Child Neurol 2002;17(11):859-60.
26. MacQueen GM, Rosebush PI, Mazurek MF. Neuropsychiatric aspects of the adult variant of Tay-Sachs disease. J Neuropsychiatry Clin Neurosci 1998;10(1):10-9.
27. Caroff SN, Mann SC, Gliatto MF, et al. Psychiatric manifestations of acute viral encephalitis. Psych Annals 2001;31(3):193-204.
28. Caplan R, Tanguay PE, Szekely AG. Subacute sclerosing panencephalitis presenting as childhood psychosis. J Am Acad Child Adolesc Psychiatry 1987;26(3):440-3.
29. Gillberg C. Schizophreniform psychosis in a case of mycoplasma pneumoniae encephalitis. J Autism Dev Disord 1980;10(2):153-8.
30. Gliatto MF, Caroff SN. Neurosyphilis: a history and clinical review. Psych Annals 2001;31(3):153-61.
31. Venkatesh S, Grell GA. Neuropsychiatric manifestations of typhoid fever. West Indian Med J 1989;38(3):137-41.
32. Tager FA, Fallon B. Psychiatric and cognitive features of Lyme disease. Psych Annals 2001;31(3):173-92.
33. Stein MT, Duffner PK, Wery JS, Trauner D. School refusal and emotional liability in a 6 year old boy. J Dev Behav Pediatr 2001;22(suppl):29-32.
34. Carson BS, Weingart JD, Guarnieri M, Fisher PG. Third ventricular choroid plexus papilloma with psychosis. Case report. J Neurosurg 1997;87(1):103-5.
35. Craven C. Pineal germinoma and psychosis. J Am Acad Child Adolesc Psychiatry 2001;40(1):6.-
36. Tatter SB, Ogilvy CS. Recurrent manic episode 10 years after arteriovenous malformation resection. J Clin Psychiatry 1995;56(2):83.-
37. Hartman DE. Missed diagnosis and misdiagnosis of environmental toxicants exposure: the psychiatry of toxic exposure and multiple chemical sensitivity. Psychiatr Clin North Am 1998;21(3):659-70.
38. Bahiga LM, Kotb NA, El-Dessoukey EA. Neurological syndromes produced by some toxic metals encountered industrially or environmentally. Z Ernahrungswiss 1978;17(2):84-8.
39. Olson KR. Carbon monoxide poisoning: mechanisms, presentation, and controversies in management. J Emerg Med 1984;1(3):233-43.
40. Fagala GE, Wigg CL. Psychiatric manifestations of mercury poisoning. J Am Acad Child Adolesc Psychiatry 1992;31(2):306-11.
41. Turkel SB, Miller JH, Reiff A. Case series: neuropsychiatric symptoms with pediatric systemic lupus erythematosus. J Am Acad Child Adolesc Psychiatry 2001;40(4):482-5.
42. Baca V, Lavalle C, Garcia R, et al. Favorable response to intravenous methylprednisolone and cyclophosphamide in children with severe neuropsychiatric lupus. J Rheumatol 1999;26(2):432-9.
1. American Academy of Child and Adolescent Psychiatry. Summary of the practice parameters for the assessment and treatment of children and adolescents with schizophrenia. J Am Acad Child Adolesc Psychiatry 2000;39(12):1580-92.
2. Behrman RE (ed). Nelson textbook of pediatrics (17th ed). Philadelphia: WB Saunders; 2003:397-518.
3. Dalmau A, Bergman B, Brismar B. Psychotic disorders among inpatients with abuse of cannabis, amphetamine and opiates. Do dopaminergic stimulants facilitate psychiatric illness? Eur Psychiatry 1999;14(7):366-71.
4. Breslow RE, Klinger BI, Erickson BJ. Acute intoxication and substance abuse among patients presenting to a psychiatric emergency service. Gen Hosp Psychiatry 1996;18(3):183-91.
5. Poole R, Brabbins C. Drug-induced psychosis. Br J Psychiatry 1996;168:137.-
6. DiSclafani A, 2nd, Hall RC, Gardner ER. Drug-induced psychosis: emergency diagnosis and management. Psychosomatics 1981;22(10):845-55.
7. Cohen SI. Substance-induced psychosis. Br J Psychiatry 1996;168(5):651-2.
8. Farrell M, Boys A, Bebbington P, et al. Psychosis and drug dependence: results from a national survey of prisoners. Br J Psychiatry 2002;181:393-8.
9. Ujike H, Sato M. Clinical features of sensitization to methamphetamine observed in patients with methamphetamine dependence and psychosis. Ann NY Acad Sci 2004;1025:279-87.
10. Drugs that may cause psychiatric symptoms. Med Lett Drugs Ther 2002;44:29-62.
11. Lewis DA, Smith RE. Steroid-induced psychiatric symptoms; a report of 14 cases and a review of the literature. J Affect Disord 1983;5(4):319-32.
12. Cummings JL. Organic psychosis. Psychosomatics 1988;29(1):16-26.
13. Roy AK, Rajesh SV, Iby N, et al. A study of epilepsy-related psychosis. Neurol India 2003;51(3):359-60.
14. Mendez MF. Huntington’s disease: update and review of neuropsychiatric aspects. Int J Psychiatry Med 1994;24:189-208.
15. Brewer GJ. Recognition and management of Wilson’s disease. Proc Soc Exp Biol Med 2000;223:39-46.
16. Mendhekar DN, Mehta R, Puri V. Successful steroid therapy in multiple sclerosis presented as acute psychosis. J Assoc Physicians India 2004;52:512-3.
17. Reus VI. Behavioral disturbances associated with endocrine disorders. Ann Rev Med 1986;37:205-14.
18. Hirsch D, Orr G, Kantarovich V, et al. Cushing’s syndrome presenting as a schizophrenia-like psychotic state. Isr J Psychiatry Relat Sci 2000;37(1):46-50.
19. Lu CL, Lee YC, Tsai SJ, et al. Psychiatric disturbances associated with hyperthyroidism: an analysis report of 30 cases. Zhonghua Yi Xue Za Zhi (Taipei) 1995;56(6):393-8.
20. Bhatara V, Alshari MG, Warhol P, et al. Coexistent hypothyroidism, psychosis, and severe obsessions in an adolescent: a 10-year follow-up. J Child Adolesc Psychopharmacol 2004;14(2):315-23.
21. Prior TI, Chue PS, Tibbo P. Investigation of Turner syndrome in schizophrenia. Am J Med Genet 2000;96(3):373-8.
22. Hyde TM, Ziegler JC, Weinberger DR. Psychiatric disturbances in metachromatic leukodystrophy. Insights into the neurobiology of psychosis. Arch Neurol 1992;49(4):401-6.
23. Briegel W, Cohen M. Chromosome 22q11 deletion syndrome and its relevance for child and adolescent psychiatry. An overview of etiology, physical symptoms, aspects of child development and psychiatric disorders. Z Kinder Jugendpsychiatr Psychother 2004;32(2):107-15.
24. Crimlisk HL. The great imitator-porphyria: a neuropsychiatric disorder. J Neurol Neurosurg Psychiatry 2001;62(4):319-28.
25. Ryan MM, Sidhu RK, Alexander J, Megerian JT. Homocystinuria presenting as psychosis in an adolescent. J Child Neurol 2002;17(11):859-60.
26. MacQueen GM, Rosebush PI, Mazurek MF. Neuropsychiatric aspects of the adult variant of Tay-Sachs disease. J Neuropsychiatry Clin Neurosci 1998;10(1):10-9.
27. Caroff SN, Mann SC, Gliatto MF, et al. Psychiatric manifestations of acute viral encephalitis. Psych Annals 2001;31(3):193-204.
28. Caplan R, Tanguay PE, Szekely AG. Subacute sclerosing panencephalitis presenting as childhood psychosis. J Am Acad Child Adolesc Psychiatry 1987;26(3):440-3.
29. Gillberg C. Schizophreniform psychosis in a case of mycoplasma pneumoniae encephalitis. J Autism Dev Disord 1980;10(2):153-8.
30. Gliatto MF, Caroff SN. Neurosyphilis: a history and clinical review. Psych Annals 2001;31(3):153-61.
31. Venkatesh S, Grell GA. Neuropsychiatric manifestations of typhoid fever. West Indian Med J 1989;38(3):137-41.
32. Tager FA, Fallon B. Psychiatric and cognitive features of Lyme disease. Psych Annals 2001;31(3):173-92.
33. Stein MT, Duffner PK, Wery JS, Trauner D. School refusal and emotional liability in a 6 year old boy. J Dev Behav Pediatr 2001;22(suppl):29-32.
34. Carson BS, Weingart JD, Guarnieri M, Fisher PG. Third ventricular choroid plexus papilloma with psychosis. Case report. J Neurosurg 1997;87(1):103-5.
35. Craven C. Pineal germinoma and psychosis. J Am Acad Child Adolesc Psychiatry 2001;40(1):6.-
36. Tatter SB, Ogilvy CS. Recurrent manic episode 10 years after arteriovenous malformation resection. J Clin Psychiatry 1995;56(2):83.-
37. Hartman DE. Missed diagnosis and misdiagnosis of environmental toxicants exposure: the psychiatry of toxic exposure and multiple chemical sensitivity. Psychiatr Clin North Am 1998;21(3):659-70.
38. Bahiga LM, Kotb NA, El-Dessoukey EA. Neurological syndromes produced by some toxic metals encountered industrially or environmentally. Z Ernahrungswiss 1978;17(2):84-8.
39. Olson KR. Carbon monoxide poisoning: mechanisms, presentation, and controversies in management. J Emerg Med 1984;1(3):233-43.
40. Fagala GE, Wigg CL. Psychiatric manifestations of mercury poisoning. J Am Acad Child Adolesc Psychiatry 1992;31(2):306-11.
41. Turkel SB, Miller JH, Reiff A. Case series: neuropsychiatric symptoms with pediatric systemic lupus erythematosus. J Am Acad Child Adolesc Psychiatry 2001;40(4):482-5.
42. Baca V, Lavalle C, Garcia R, et al. Favorable response to intravenous methylprednisolone and cyclophosphamide in children with severe neuropsychiatric lupus. J Rheumatol 1999;26(2):432-9.
Adult with ADHD? Try medication + psychotherapy
Mr. B, age 50, dreams of becoming a computer programmer but fears he will embarrass himself—as he has in many classrooms before. He is seeking evaluation because his teenage son was recently diagnosed with attention-deficit/hyperactivity disorder (ADHD), and he recognizes similar symptoms in himself.
Mr. B received a college degree with great difficulty, putting off assignments until the last minute and “squeaking by.” For years he has changed occupations often, never progressing beyond entry level, and now works as a personal care provider and limousine driver. He reports problems keeping up with work and managing time.
His history includes early childhood hyperactivity, difficulty sitting through classes, sloppy handwriting, disorganization, short attention span, and distractibility. He is restless, fidgety, and has trouble staying on topic. His disorganization has caused marital difficulties, for which he has sought counseling.
After careful evaluation, you determine that Mr. B meets criteria for ADHD, combined type, and for anxiety disorder not otherwise specified. His treatment goals are to increase his ability to focus; procrastinate less; improve his planning, prioritizing, and self-esteem; and to become less sensitive to criticism and less anxious about handling work demands.
Like Mr. B, adults with ADHD need treatment for the disorder’s core symptoms as well as its psychiatric comorbidities and psychosocial consequences. Comprehensive treatment with medications, cognitive-behavioral therapy (CBT), and environmental adaptations is usually recommended.
Comorbidity rules
Core symptoms. ADHD is a lifespan disorder with multiple behavioral, cognitive, and emotional manifestations that impair relationships and academic and vocational functioning. ADHD-like symptoms are seen in other conditions such as mood disorders or substance abuse, but complaints of inattention, distractibility, procrastination, restlessness, and impulsivity—particularly when pervasive and chronic—are highly indicative of ADHD.
In treating adults with ADHD, we have noticed common behavioral patterns that contribute to their psychosocial problems (Table 1). Dysfunctional coping behaviors have short-term advantages, but patients readily admit they would rather accomplish tasks through greater thought and planning.
Chronic frustrations—often associated with deep shame—are typical of adult ADHD. Many patients have maladaptive core beliefs of failure, self-mistrust, and inadequacy (Table 2).
Table 1
Common dysfunctional behavioral patterns in adults with ADHD
| Behavior | Description | Short-term gain/long-term loss |
|---|---|---|
| Anticipatory avoidance | Magnifying the difficulty of a pending task and doubts about being able to complete it; results in rationalizations to justify procrastination | Defers short-term stress, but often creates a self-fulfilling prophecy because the task looms and may seem overwhelming when facing a deadline |
| Brinksmanship | Waiting until the last moment (eg, the night before) to complete a task, often when facing an impending deadline | Deadline-associated stress can be focusing, but this tactic leaves little room for error and may yield a substandard result |
| Pseudoefficiency | Completing several low-priority, manageable tasks (eg, checking e-mail) but avoiding high-priority tasks (eg, a project for work) | Creates sense of productivity by reducing items on to-do list but defers a more difficult project |
| Juggling | Taking on new, exciting projects and feeling ‘busy’ without completing projects already started | It is easier to become motivated to start a novel project than to complete an ongoing one; pattern usually results in several incomplete projects |
Table 2
5 common maladaptive core beliefs of adults with ADHD
| Self-mistrust | ‘I cannot rely on myself to do what I need to do. I let myself down’ |
| Failure | ‘I always have failed and always will fail at what I set out to do.’ |
| Inadequacy | ‘I am basically a bad and defective person.’ |
| Incompetence | ‘I am too inept to handle life’s basic demands.’ |
| Instability | ‘My life will always be chaotic and in turmoil.’ |
Psychiatric comorbidity is the rule in adults with ADHD (Table 3). For example, among 43 patients who received combined medication and CBT at the University of Pennsylvania Adult ADHD Treatment and Research Program, 75% reported at least one comorbid condition, including:
- 27 (63%) with mood disorder
- 23 (54%) with anxiety disorder
- 5 (12%) with substance abuse.1
Other treatment studies have reported similar comorbidity rates in adults with ADHD.2-4
Table 3
Psychiatric comorbidity in adult ADHD
| Disorder | Prevalence |
|---|---|
| Mood disorders | 50% to 65% |
| Recurrent depression | |
| Bipolar disorder | |
| Cyclothymia | |
| Dysthymia | |
| Depressive disorder NOS | |
| Anxiety disorders | 40% to 55% |
| Generalized anxiety disorder | |
| Anxiety disorder NOS | |
| Others | Various |
| Substance use disorder | |
| Learning disabilities | |
| Intermittent explosive disorder | |
| Tourette syndrome | |
| Antisocial personality | |
| Borderline personality disorder | |
| Dependent personality | |
| NOS: Not otherwise specified | |
Making the diagnosis
Diagnosis of adult ADHD is based on a comprehensive assessment, including:
- careful history of presenting complaints
- thorough review of educational, occupational, and family history
- standardized rating scales (such as the Barkley ADHD Behavior Checklists, the Conners’ Adult ADHD Rating Scale, or the Brown Attention Deficit Disorder Scales)
- collateral information
- assessment of mood, anxiety, substance use, and learning/organizational skills. For details, consult references on adult ADHD.5-8
Case continued: Self-fulfilling prophesies
On standardized rating scales, Mr. B meets criteria for combined ADHD for childhood and current symptoms. Information from his wife and brother also confirms the ADHD diagnosis.
He is motivated, resilient, optimistic, and has a good support system. However, his negative automatic thoughts about his ability to succeed in school and to handle increasing time demands suggest deeper beliefs of inadequacy and failure.
Mr. B struggled academically. Without guidance about how to change his approach to difficult situations, he has repeated old thinking and behavior patterns. Believing he will embarrass himself and fail to learn required material, Mr. B procrastinates and avoids doing assignments. In class, his feelings of inadequacy make him self-conscious, which causes him to lose focus and have trouble concentrating.
See the world through the patient’s eyes
Understanding your patient. Before you start treatment, we recommend that you conceptualize how ADHD has influenced your patient’s life, including:
- developmental experiences
- family-of-origin issues, such as conflicts with parents stemming from ADHD symptoms or reciprocal interactions with an ADHD parent
- world view (“schemata”)
- patterns of coping with (or avoiding) stress
- attitudes toward self and important others
- readiness to change.
Developing a working case conceptualization is a dynamic, collaborative process. You talk with patients, and encourage them to reflect on how ADHD affects their view of themselves and their important relationships. The conceptualization takes shape as you:
- observe patients’ behaviors
- elicit how they think and feel
- assess with them the relevance and accuracies of their belief systems and response patterns.
Seeing the world “through their eyes” prepares you to help them accept the diagnosis and learn to manage ADHD symptoms. Then, by providing a blueprint to manage what patients may see as uncontrollable responses, you can help them take charge of their automatic reactions.
Psychoeducation. To set the stage for treatment, encourage patients to learn about ADHD by reading articles and books and consulting Web sites for adults with ADHD (see Related resources). Psychoeducation helps patients:
- review possible treatment approaches, including organizational (environmental) management, medication, and psychotherapy (individual or group)
- become informed participants in setting treatment goals.
Explain the relative contribution of each treatment component. For example, medications can reduce distractibility and improve attention, organizational strategies can reduce disorganization and improve time management, and structured psychotherapy can help the patient develop more effective coping skills.
Case continued: Planning combined treatment
You discuss diagnosis and treatment options with Mr. B, and he agrees to start the methylphenidate compound Concerta, initially at 18 mg/d, and weekly CBT sessions. You recommended a stimulant based on efficacy studies and your clinical experience in treating adults with ADHD. Mr. B wants a medication that will help him focus while working or studying, and he says Concerta has improved his son’s ADHD symptoms.
You instruct Mr. B to increase the dosage by 18 mg each week until he reaches 72 mg/d. You also tell him to keep a medication response log and to note any positive changes and side effects.
If an adult with ADHD expresses preference for a particular medication, we usually prescribe that one first. Most patients to whom we offer both medication and psychotherapy agree to this “top-down” and “bottom-up” approach. “Top down” means giving patients new ways of thinking to help them understand and modify their responses. “Bottom up” refers to the medication reducing their impulsivity, distractibility, and inattentiveness.
CBT for adult ADHD
Medications can ameliorate key symptoms of adult ADHD, but adjunctive interventions are needed to improve functioning and quality of life. Evidence supporting psychosocial treatment for adults with ADHD is limited, but CBT has been studied the most.1,9-13 Safren et al13 found a four-fold greater therapeutic response when patients received adjunctive CBT for residual ADHD symptoms, compared with patients who received medication alone.
We usually provide CBT weekly for 12 weeks and then taper to 8 additional sessions over 3 months (total 20 sessions). We may extend CBT with additional sessions to address complicated issues. CBT helps adults with ADHD to:
- identify dysfunctional thinking, feeling, and behaving patterns
- recognize contexts in which patterns arise
- systematically change these patterns.
CBT can reduce ADHD-associated anxiety and depression and improve coping skills and sense of well-being.1,9,11 Its flexibility allows you to address family issues with patients’ partners, children and other relatives to improve communication, reduce conflict, and develop healthier interactions.
We focus CBT sessions on finding alternate coping strategies. We might try role playing, rehearsing, creating “thought experiments,” and anticipating and preparing to modify typical patterns of avoidance. These approaches have been described elsewhere.10,11,14
We adopt an active stance during therapy to keep ADHD patients’ distractibility from disrupting our conversation. For example, we set the therapeutic agenda, provide feedback about patients’ behaviors, and encourage them to clarify rewards and consequences of using (or avoiding) problem-solving strategies.
Although we typically assign between-session homework, we expect patients to have difficulty completing it. We remain nonjudgmental and collaborative, viewing incomplete assignments as opportunities to learn about patients’ unproductive problem solving and to help them develop more-effective patterns.
Challenging maladaptive beliefs. A strong therapeutic relationship allows adults with ADHD to discuss their chronic frustrations, which often are associated with deep shame. We then shift CBT’s focus to deeper ADHD-related schemata that perpetuate dysfunctional patterns.
We work with patients to elucidate and challenge their maladaptive core beliefs and encourage new ways to view themselves and others. Allowing patients to grieve about the limitations ADHD imposes on their lives also helps to reduce chronic negative self-esteem.
Case continued: ‘less frenetic’
Mr. B achieves good results within 3 weeks of an increasing titration of stimulant medication, reporting significantly less restlessness and greater concentration without significant side effects. His wife confirms that he is less frenetic, can converse without interruptions, and is better at managing his complicated work schedule.
Which medications?
Drug therapy for adult ADHD is not as well-studied as in children and adolescents, but American Academy of Child and Adolescent Psychiatry guidelines and others15-18 recommend stimulant and nonstimulant medications. Your choice depends on the patient’s clinical profile (including risk factors and comorbid conditions), past medication use, treatment goals, preferred medication effects and dosing patterns (once-daily versus multiple times), and potential side effects. Stimulants or atomoxetine are first-line choices for adult ADHD without psychiatric comorbidity.
Stimulants work quickly and are cleared relatively rapidly from the brain without causing euphoria or dependency. They are effective (80% to 90% response rate) and well-tolerated, though long-term effects have not been studied in adults (Table 4).
Stimulants’ effect size of 0.9 is considered substantial. Effect size—a statistical method of reporting an intervention’s effect across different studies—is typically rated as:
- <0.32 very small
- 0.33 to 0.54, moderate
- >0.55, significant or very strong.
When choosing a medication, we usually try methylphenidate and amphetamine first, one after the other. We explain to the patient how stimulants work in the brain and the need for a comparative trial to determine which might work best for him or her. If the patient has tried a stimulant and found it helpful, we start with that class. Similarly, if he/she has not had good results with one type, we start with the other. Approximately one-third of our patients respond equally well to methylphenidate or amphetamine, one-third respond better to methylphenidate, and one-third respond better to amphetamine.
To determine the optimal dosage, we usually titrate up from 10 to 30 mg per dose of an immediate-release preparation. We begin with this form to help patients notice the medication’s onset and duration of action. After we find the optimal dosage, we switch to a longer-acting preparation.
Insomnia, mood instability, and euphoria are unacceptable stimulant side effects, although many patients welcome others such as appetite suppression and weight loss. Closely monitor cardiovascular effects, and review potential interactions with other medications, such as antihypertensives or bronchodilators. Because sudden death has been reported with stimulants in persons with structural cardiac lesions,19 obtain a cardiology consultation for patients with a history of heart disease.
We encourage patients to keep daily medication logs (Box), which we review at each visit and use to make dosing or medication changes. Dosing guidelines resemble those used for children and adolescents, although adults usually tolerate higher maximum dosages (such as methylphenidate, 80 to 100 mg/d).
Because of stimulants’ potential for recreational misuse and abuse, remain wary about choosing stimulants for patients with whom you lack a solid doctor-patient relationship.
Table 4
Stimulant dosages used in treating adult ADHD
| Class (brand name) | Daily dosing | Typical dosing schedule |
|---|---|---|
| Methylphenidate | ||
| Short-acting (Metadate, Ritadex, Ritalin) | Two to four times | 10 to 40 mg bid to qid |
| Intermediate-acting (Metadate SR, Ritalin SR) | Once or twice | 20 to 60 mg qd to bid |
| Extended-release (Concerta, Metadate CD, Ritalin LA) | Once or twice | 18 to 108 mg qd (Concerta) 20 to 40 mg bid (Ritalin LA, Metadate CD) |
| Dextromethylphenidate | ||
| Short-acting (Focalin) | Two to four times | 5 to 20 mg bid to qid |
| Long-acting (Focalin XR) | Once or twice | 10 to 20 mg qd or bid |
| Dextroamphetamine | ||
| Short-acting (Dexedrine) | Twice or three times | 10 to 30 mg bid or tid |
| Intermediate-acting (Dexedrine spansules) | Once or twice | 10 to 30 mg bid |
| Mixed amphetamine salts | ||
| Intermediate-acting (Adderall) | Once or twice | 10 to 30 mg bid or tid |
| Extended-release (Adderall XR) | Once or twice | 10 to 40 mg qd or bid |
Atomoxetine, a nonstimulant, norepinephrine re-uptake inhibitor, is approved for ADHD in adults.20-22 In two double-blind, controlled, randomized trials totalling 536 adults, Michaelson et al20 found significantly reduced ADHD symptoms after 10 weeks of atomoxetine treatment. Effect sizes of 0.35 and 0.40 were reported, with 10% of patients discontinuing because of side effects.
Atomoxetine has a long duration of action (>12 hours) but a more gradual onset (4 to 6 weeks) than that of stimulants. Approximately 60% of patients respond to atomoxetine, though effect sizes are less than those of stimulants. We have found atomoxetine works well for patients who:
- do not tolerate or are uncomfortable with taking stimulants
- are highly anxious
- report emotional dysregulation as a major target symptom.
To reduce risk of common side effects (nausea, GI upset, headache, sedation, reduced sex drive), we start with low dosages (such as 25 mg bid) and increase weekly by 25 mg to a target of 80 to 100 mg/d.
Treating complicated ADHD
Bupropion or tricyclic antidepressants are reasonable options for ADHD with depression. Atomoxetine, a tricyclic, or a stimulant plus a selective serotonin reuptake inhibitor (SSRI) can provide good symptom relief for adults with ADHD and comorbid anxiety and/or depression.
Bupropion. Approximately 50% of adults with ADHD respond to bupropion,23,24 with a treatment effect size of 0.6. Bupropion’s efficacy in smoking cessation adds value for those trying to quit.
We usually start extended-release bupropion at 150 mg/d and increase after 2 weeks to 300 mg/d if response is suboptimal. Headache, dry mouth, insomnia, and nausea are the most common adverse effects. Agitation or irritability is sometimes serious enough to warrant stopping bupropion.
Combining medications. Using SSRIs with stimulants can help adults with ADHD and comorbid anxiety or depression. Any SSRI can be safely combined with stimulants, though we tend to pick:
- more-sedating agents such as paroxetine or sertraline when patients report difficulty with insomnia or overactivation
- less-sedating compounds such as fluoxetine or citalopram when patients complain of being too tired or underactive.
When patients taking SSRIs seek help for ADHD, adding a stimulant usually reduces inattention, distractibility, impulsivity, and/or subjective feelings of restlessness. We prescribe usual dosages because stimulants and SSRIs do not interact. We have not seen serious side effects, but some patients report feeling oversedated.
Tricyclics. We use tricyclics when a stimulant/SSRI combination does not relieve symptoms satisfactorily or a patient complains of side effects. We usually have good results with desipramine or imipramine, 150 to 300 mg/d, or nortriptyline, 50 to 150 mg/d. Spencer et al have reported a response rate of 68% with nortriptyline or desipramine in a retrospective chart review25 and a prospective placebo-controlled trial26 of adults with ADHD.
Case continued: Closer to dream job
After 6 months of combined treatment, Mr. B reports much-improved ADHD symptoms, with minimal stimulant-related side effects. He has made some realistic plans for computer programming school and is taking preliminary courses. Keeping a schedule book has reduced his tardiness and tendency to procrastinate.
He is more comfortable in the classroom and better able to challenge self-critical thinking. When routine difficulties arise, he is using more-adaptive coping strategies. To maintain gains achieved in therapy, he chooses to continue periodic CBT booster sessions.
Long-term treatment
Even with medication and CBT, patients may require referral for organizational coaching, academic counseling, school or workplace accommodations, vocational counseling, cognitive remediation, group therapy, or social skills classes. You can help them obtain quality adjunctive care by collaborating with professionals who offer these services.
No studies have examined long-term care of adults with ADHD. In our experience, ongoing medication and intermittent therapy can sustain symptom control and coping skills for years. Most patients are initially skeptical about staying on medication, but after they experience the benefits most seem willing to continue as long as the medication helps.
Most of our patients sustain changes in thinking, feeling, and behaving that they learn through BT. They may seek additional sessions to meet a challenge, such as a new job or starting a family.
Books
- Kolberg J, Nadeau K. ADD-friendly ways to organize your life. New York: Brunner-Routledge; 2002.
- Hallowell EM, Ratey JJ. Driven to distraction. New York: Touchstone; 1994.
- Hallowell E, Ratey J. Delivered from distraction. New York: Ballantine Books; 2005.
Organizations
- Children and Adults with Attention-Deficit/Hyperactivity Disorder (CHADD). National Resource Center on AD/HD. www.chadd.org.
- Attention Deficit Disorder Association (ADDA). Resources and membership organization for adults with ADHD. www.add.org.
Drug brand names
- Amphetamine • Adderall, Dexedrine
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Desipramine • Norpramin
- Imipramine • Tofranil
- Methylphenidate • Concerta, Focalin, Metadate, Ritalin
- Nortriptyline • Aventyl, Pamelor
Disclosures
Dr. Rostain is a consultant to Shire Pharamaceuticals Group and a speaker for Eli Lilly & Co. and Ortho-McNeil Pharmaceutical
Dr. Ramsay reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Rostain AL, Ramsay JR. A combined treatment approach for adults with attention-deficit/hyperactivity disorder. Results of an open study of 43 patients J Attention Disorders. In press.
2. Shekim WO, Asarnow RF, Hess E, et al. A clinical and demographic profile of a sample of adults with attention deficit hyperactivity disorder, residual state. Comp Psychiatry 1990;31:416-25.
3. Biederman J, Faraone SV, Spencer T, et al. Patterns of psychiatric comorbidity, cognition and psychosocial functioning in adults with attention deficit hyperactivity disorder. Am J Psychiatry 1993;150:1792-8.
4. Wilens TE, Biederman J, Spencer T. Attention-deficit/hyperactivity disorder across the lifespan. Ann Rev Medicine 2002;53:113-31.
5. Barkley RA. Attention deficit hyperactivity disorder: a handbook for diagnosis and treatment. New York: Guilford Press; 1998.
6. Wender PH. ADHD: Attention-deficit hyperactivity disorder in children and adults. New York: Oxford University Press; 2000.
7. Goldstein S, Ellison AT. Clinician’s guide to adult ADHD. San Diego: Academic Press; 2000.
8. Brown TE. Attention-deficit disorder: the unfocused mind in children and adults. New Haven, CT: Yale University Press; 2005.
9. Wilens TE, McDermott SP, Biederman J, et al. Cognitive therapy in the treatment of adults with ADHD: a systematic chart review of 26 cases. J Cogn Ther 1999;13:215-26.
10. Ramsay JR, Rostain AL. A cognitive therapy approach for adult attention-deficit/hyperactivity disorder. J Cogn Psychother 2003;17:319-34.
11. Safren SA, Sprich S, Chulvick S, Otto MW. Psychosocial treatments for adults with attention-deficit/hyperactivity disorder. Psychiatr Clin North Am 2004;27:349-60.
12. Ramsay JR, Rostain AL. Adapting psychotherapy to meet the needs of adults with attention-deficit/hyperactivity disorder. Psychotherapy: Theory, Research, Practice, Training 2005;42:72-84.
13. Safren SA, Otto MW, Sprich S, et al. Cognitive-behavior therapy for ADHD in medication-treated adults with continued symptoms. Behav Res Ther 2005;43:831-42.
14. Ramsay JR, Rostain AL. Girl, repeatedly interrupted: The case of a young adult woman with ADHD. Clinical Case Studies 2005;4:329-46.
15. American Academy of Child and Adolescent Psychiatry. Practice parameters for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;42(suppl 2):26S-49S.
16. Weiss M, Murray C, Weiss G. Adults with attention-deficit/hyperactivity disorder: Current concepts. J Psychiatr Pract 2002;8:99-111.
17. Wilens TE. Drug therapy for adults with attention-deficit hyperactivity disorder. Drugs 2003;63:2395-411.
18. Dodson WW. Pharmacotherapy of adult ADHD. J Clin Psychol 2005;61:589-606.
19. Francis PD. Effects of psychotropic medications on the pediatric electrocardiogram and recommendations for monitoring. Curr Opin Pediatr 2002;14(2):224-30
20. Michaelson D, Adler L, Spencer T. Atomoxetine in adults: Two randomized, placebo-controlled studies. Biol Psychiatry 2003;53:112-20.
21. Simpson D, Plosker GL. Atomoxetine: a review of its use in adults with attention deficit hyperactivity disorder. Drugs 2004;64:205-22.
22. Reimherr FW, Marchant BK, Strong RE, et al. Emotional dysregulation in adult ADHD and response to atomoxetine. Biol Psychiatry 2005;58:125-31.
23. Wilens TE, Spencer T, Biederman J. A controlled clinical trial of bupropion for attention-deficit/hyperactivity disorder in adults. Am J Psychiatry 2001;158:282-8.
24. Wilens TE, Haight BR, Horrigan JP, et al. Bupropion XL in adults with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled study. Biol Psychiatry 2005;57:793-801.
25. Wilens TE, Biederman JB, Mick E, Spencer TJ. A systematic assessment of tricyclic antidepressants in the treatment of adult attention deficit/hyperactivity disorder. J Nerv Ment Dis 1995;183:48-50.
26. Wilens TE, Biederman JB, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention deficit hyperactivity disorder. Am J Psychiatry 1996;153:1147-53.
Mr. B, age 50, dreams of becoming a computer programmer but fears he will embarrass himself—as he has in many classrooms before. He is seeking evaluation because his teenage son was recently diagnosed with attention-deficit/hyperactivity disorder (ADHD), and he recognizes similar symptoms in himself.
Mr. B received a college degree with great difficulty, putting off assignments until the last minute and “squeaking by.” For years he has changed occupations often, never progressing beyond entry level, and now works as a personal care provider and limousine driver. He reports problems keeping up with work and managing time.
His history includes early childhood hyperactivity, difficulty sitting through classes, sloppy handwriting, disorganization, short attention span, and distractibility. He is restless, fidgety, and has trouble staying on topic. His disorganization has caused marital difficulties, for which he has sought counseling.
After careful evaluation, you determine that Mr. B meets criteria for ADHD, combined type, and for anxiety disorder not otherwise specified. His treatment goals are to increase his ability to focus; procrastinate less; improve his planning, prioritizing, and self-esteem; and to become less sensitive to criticism and less anxious about handling work demands.
Like Mr. B, adults with ADHD need treatment for the disorder’s core symptoms as well as its psychiatric comorbidities and psychosocial consequences. Comprehensive treatment with medications, cognitive-behavioral therapy (CBT), and environmental adaptations is usually recommended.
Comorbidity rules
Core symptoms. ADHD is a lifespan disorder with multiple behavioral, cognitive, and emotional manifestations that impair relationships and academic and vocational functioning. ADHD-like symptoms are seen in other conditions such as mood disorders or substance abuse, but complaints of inattention, distractibility, procrastination, restlessness, and impulsivity—particularly when pervasive and chronic—are highly indicative of ADHD.
In treating adults with ADHD, we have noticed common behavioral patterns that contribute to their psychosocial problems (Table 1). Dysfunctional coping behaviors have short-term advantages, but patients readily admit they would rather accomplish tasks through greater thought and planning.
Chronic frustrations—often associated with deep shame—are typical of adult ADHD. Many patients have maladaptive core beliefs of failure, self-mistrust, and inadequacy (Table 2).
Table 1
Common dysfunctional behavioral patterns in adults with ADHD
| Behavior | Description | Short-term gain/long-term loss |
|---|---|---|
| Anticipatory avoidance | Magnifying the difficulty of a pending task and doubts about being able to complete it; results in rationalizations to justify procrastination | Defers short-term stress, but often creates a self-fulfilling prophecy because the task looms and may seem overwhelming when facing a deadline |
| Brinksmanship | Waiting until the last moment (eg, the night before) to complete a task, often when facing an impending deadline | Deadline-associated stress can be focusing, but this tactic leaves little room for error and may yield a substandard result |
| Pseudoefficiency | Completing several low-priority, manageable tasks (eg, checking e-mail) but avoiding high-priority tasks (eg, a project for work) | Creates sense of productivity by reducing items on to-do list but defers a more difficult project |
| Juggling | Taking on new, exciting projects and feeling ‘busy’ without completing projects already started | It is easier to become motivated to start a novel project than to complete an ongoing one; pattern usually results in several incomplete projects |
Table 2
5 common maladaptive core beliefs of adults with ADHD
| Self-mistrust | ‘I cannot rely on myself to do what I need to do. I let myself down’ |
| Failure | ‘I always have failed and always will fail at what I set out to do.’ |
| Inadequacy | ‘I am basically a bad and defective person.’ |
| Incompetence | ‘I am too inept to handle life’s basic demands.’ |
| Instability | ‘My life will always be chaotic and in turmoil.’ |
Psychiatric comorbidity is the rule in adults with ADHD (Table 3). For example, among 43 patients who received combined medication and CBT at the University of Pennsylvania Adult ADHD Treatment and Research Program, 75% reported at least one comorbid condition, including:
- 27 (63%) with mood disorder
- 23 (54%) with anxiety disorder
- 5 (12%) with substance abuse.1
Other treatment studies have reported similar comorbidity rates in adults with ADHD.2-4
Table 3
Psychiatric comorbidity in adult ADHD
| Disorder | Prevalence |
|---|---|
| Mood disorders | 50% to 65% |
| Recurrent depression | |
| Bipolar disorder | |
| Cyclothymia | |
| Dysthymia | |
| Depressive disorder NOS | |
| Anxiety disorders | 40% to 55% |
| Generalized anxiety disorder | |
| Anxiety disorder NOS | |
| Others | Various |
| Substance use disorder | |
| Learning disabilities | |
| Intermittent explosive disorder | |
| Tourette syndrome | |
| Antisocial personality | |
| Borderline personality disorder | |
| Dependent personality | |
| NOS: Not otherwise specified | |
Making the diagnosis
Diagnosis of adult ADHD is based on a comprehensive assessment, including:
- careful history of presenting complaints
- thorough review of educational, occupational, and family history
- standardized rating scales (such as the Barkley ADHD Behavior Checklists, the Conners’ Adult ADHD Rating Scale, or the Brown Attention Deficit Disorder Scales)
- collateral information
- assessment of mood, anxiety, substance use, and learning/organizational skills. For details, consult references on adult ADHD.5-8
Case continued: Self-fulfilling prophesies
On standardized rating scales, Mr. B meets criteria for combined ADHD for childhood and current symptoms. Information from his wife and brother also confirms the ADHD diagnosis.
He is motivated, resilient, optimistic, and has a good support system. However, his negative automatic thoughts about his ability to succeed in school and to handle increasing time demands suggest deeper beliefs of inadequacy and failure.
Mr. B struggled academically. Without guidance about how to change his approach to difficult situations, he has repeated old thinking and behavior patterns. Believing he will embarrass himself and fail to learn required material, Mr. B procrastinates and avoids doing assignments. In class, his feelings of inadequacy make him self-conscious, which causes him to lose focus and have trouble concentrating.
See the world through the patient’s eyes
Understanding your patient. Before you start treatment, we recommend that you conceptualize how ADHD has influenced your patient’s life, including:
- developmental experiences
- family-of-origin issues, such as conflicts with parents stemming from ADHD symptoms or reciprocal interactions with an ADHD parent
- world view (“schemata”)
- patterns of coping with (or avoiding) stress
- attitudes toward self and important others
- readiness to change.
Developing a working case conceptualization is a dynamic, collaborative process. You talk with patients, and encourage them to reflect on how ADHD affects their view of themselves and their important relationships. The conceptualization takes shape as you:
- observe patients’ behaviors
- elicit how they think and feel
- assess with them the relevance and accuracies of their belief systems and response patterns.
Seeing the world “through their eyes” prepares you to help them accept the diagnosis and learn to manage ADHD symptoms. Then, by providing a blueprint to manage what patients may see as uncontrollable responses, you can help them take charge of their automatic reactions.
Psychoeducation. To set the stage for treatment, encourage patients to learn about ADHD by reading articles and books and consulting Web sites for adults with ADHD (see Related resources). Psychoeducation helps patients:
- review possible treatment approaches, including organizational (environmental) management, medication, and psychotherapy (individual or group)
- become informed participants in setting treatment goals.
Explain the relative contribution of each treatment component. For example, medications can reduce distractibility and improve attention, organizational strategies can reduce disorganization and improve time management, and structured psychotherapy can help the patient develop more effective coping skills.
Case continued: Planning combined treatment
You discuss diagnosis and treatment options with Mr. B, and he agrees to start the methylphenidate compound Concerta, initially at 18 mg/d, and weekly CBT sessions. You recommended a stimulant based on efficacy studies and your clinical experience in treating adults with ADHD. Mr. B wants a medication that will help him focus while working or studying, and he says Concerta has improved his son’s ADHD symptoms.
You instruct Mr. B to increase the dosage by 18 mg each week until he reaches 72 mg/d. You also tell him to keep a medication response log and to note any positive changes and side effects.
If an adult with ADHD expresses preference for a particular medication, we usually prescribe that one first. Most patients to whom we offer both medication and psychotherapy agree to this “top-down” and “bottom-up” approach. “Top down” means giving patients new ways of thinking to help them understand and modify their responses. “Bottom up” refers to the medication reducing their impulsivity, distractibility, and inattentiveness.
CBT for adult ADHD
Medications can ameliorate key symptoms of adult ADHD, but adjunctive interventions are needed to improve functioning and quality of life. Evidence supporting psychosocial treatment for adults with ADHD is limited, but CBT has been studied the most.1,9-13 Safren et al13 found a four-fold greater therapeutic response when patients received adjunctive CBT for residual ADHD symptoms, compared with patients who received medication alone.
We usually provide CBT weekly for 12 weeks and then taper to 8 additional sessions over 3 months (total 20 sessions). We may extend CBT with additional sessions to address complicated issues. CBT helps adults with ADHD to:
- identify dysfunctional thinking, feeling, and behaving patterns
- recognize contexts in which patterns arise
- systematically change these patterns.
CBT can reduce ADHD-associated anxiety and depression and improve coping skills and sense of well-being.1,9,11 Its flexibility allows you to address family issues with patients’ partners, children and other relatives to improve communication, reduce conflict, and develop healthier interactions.
We focus CBT sessions on finding alternate coping strategies. We might try role playing, rehearsing, creating “thought experiments,” and anticipating and preparing to modify typical patterns of avoidance. These approaches have been described elsewhere.10,11,14
We adopt an active stance during therapy to keep ADHD patients’ distractibility from disrupting our conversation. For example, we set the therapeutic agenda, provide feedback about patients’ behaviors, and encourage them to clarify rewards and consequences of using (or avoiding) problem-solving strategies.
Although we typically assign between-session homework, we expect patients to have difficulty completing it. We remain nonjudgmental and collaborative, viewing incomplete assignments as opportunities to learn about patients’ unproductive problem solving and to help them develop more-effective patterns.
Challenging maladaptive beliefs. A strong therapeutic relationship allows adults with ADHD to discuss their chronic frustrations, which often are associated with deep shame. We then shift CBT’s focus to deeper ADHD-related schemata that perpetuate dysfunctional patterns.
We work with patients to elucidate and challenge their maladaptive core beliefs and encourage new ways to view themselves and others. Allowing patients to grieve about the limitations ADHD imposes on their lives also helps to reduce chronic negative self-esteem.
Case continued: ‘less frenetic’
Mr. B achieves good results within 3 weeks of an increasing titration of stimulant medication, reporting significantly less restlessness and greater concentration without significant side effects. His wife confirms that he is less frenetic, can converse without interruptions, and is better at managing his complicated work schedule.
Which medications?
Drug therapy for adult ADHD is not as well-studied as in children and adolescents, but American Academy of Child and Adolescent Psychiatry guidelines and others15-18 recommend stimulant and nonstimulant medications. Your choice depends on the patient’s clinical profile (including risk factors and comorbid conditions), past medication use, treatment goals, preferred medication effects and dosing patterns (once-daily versus multiple times), and potential side effects. Stimulants or atomoxetine are first-line choices for adult ADHD without psychiatric comorbidity.
Stimulants work quickly and are cleared relatively rapidly from the brain without causing euphoria or dependency. They are effective (80% to 90% response rate) and well-tolerated, though long-term effects have not been studied in adults (Table 4).
Stimulants’ effect size of 0.9 is considered substantial. Effect size—a statistical method of reporting an intervention’s effect across different studies—is typically rated as:
- <0.32 very small
- 0.33 to 0.54, moderate
- >0.55, significant or very strong.
When choosing a medication, we usually try methylphenidate and amphetamine first, one after the other. We explain to the patient how stimulants work in the brain and the need for a comparative trial to determine which might work best for him or her. If the patient has tried a stimulant and found it helpful, we start with that class. Similarly, if he/she has not had good results with one type, we start with the other. Approximately one-third of our patients respond equally well to methylphenidate or amphetamine, one-third respond better to methylphenidate, and one-third respond better to amphetamine.
To determine the optimal dosage, we usually titrate up from 10 to 30 mg per dose of an immediate-release preparation. We begin with this form to help patients notice the medication’s onset and duration of action. After we find the optimal dosage, we switch to a longer-acting preparation.
Insomnia, mood instability, and euphoria are unacceptable stimulant side effects, although many patients welcome others such as appetite suppression and weight loss. Closely monitor cardiovascular effects, and review potential interactions with other medications, such as antihypertensives or bronchodilators. Because sudden death has been reported with stimulants in persons with structural cardiac lesions,19 obtain a cardiology consultation for patients with a history of heart disease.
We encourage patients to keep daily medication logs (Box), which we review at each visit and use to make dosing or medication changes. Dosing guidelines resemble those used for children and adolescents, although adults usually tolerate higher maximum dosages (such as methylphenidate, 80 to 100 mg/d).
Because of stimulants’ potential for recreational misuse and abuse, remain wary about choosing stimulants for patients with whom you lack a solid doctor-patient relationship.
Table 4
Stimulant dosages used in treating adult ADHD
| Class (brand name) | Daily dosing | Typical dosing schedule |
|---|---|---|
| Methylphenidate | ||
| Short-acting (Metadate, Ritadex, Ritalin) | Two to four times | 10 to 40 mg bid to qid |
| Intermediate-acting (Metadate SR, Ritalin SR) | Once or twice | 20 to 60 mg qd to bid |
| Extended-release (Concerta, Metadate CD, Ritalin LA) | Once or twice | 18 to 108 mg qd (Concerta) 20 to 40 mg bid (Ritalin LA, Metadate CD) |
| Dextromethylphenidate | ||
| Short-acting (Focalin) | Two to four times | 5 to 20 mg bid to qid |
| Long-acting (Focalin XR) | Once or twice | 10 to 20 mg qd or bid |
| Dextroamphetamine | ||
| Short-acting (Dexedrine) | Twice or three times | 10 to 30 mg bid or tid |
| Intermediate-acting (Dexedrine spansules) | Once or twice | 10 to 30 mg bid |
| Mixed amphetamine salts | ||
| Intermediate-acting (Adderall) | Once or twice | 10 to 30 mg bid or tid |
| Extended-release (Adderall XR) | Once or twice | 10 to 40 mg qd or bid |
Atomoxetine, a nonstimulant, norepinephrine re-uptake inhibitor, is approved for ADHD in adults.20-22 In two double-blind, controlled, randomized trials totalling 536 adults, Michaelson et al20 found significantly reduced ADHD symptoms after 10 weeks of atomoxetine treatment. Effect sizes of 0.35 and 0.40 were reported, with 10% of patients discontinuing because of side effects.
Atomoxetine has a long duration of action (>12 hours) but a more gradual onset (4 to 6 weeks) than that of stimulants. Approximately 60% of patients respond to atomoxetine, though effect sizes are less than those of stimulants. We have found atomoxetine works well for patients who:
- do not tolerate or are uncomfortable with taking stimulants
- are highly anxious
- report emotional dysregulation as a major target symptom.
To reduce risk of common side effects (nausea, GI upset, headache, sedation, reduced sex drive), we start with low dosages (such as 25 mg bid) and increase weekly by 25 mg to a target of 80 to 100 mg/d.
Treating complicated ADHD
Bupropion or tricyclic antidepressants are reasonable options for ADHD with depression. Atomoxetine, a tricyclic, or a stimulant plus a selective serotonin reuptake inhibitor (SSRI) can provide good symptom relief for adults with ADHD and comorbid anxiety and/or depression.
Bupropion. Approximately 50% of adults with ADHD respond to bupropion,23,24 with a treatment effect size of 0.6. Bupropion’s efficacy in smoking cessation adds value for those trying to quit.
We usually start extended-release bupropion at 150 mg/d and increase after 2 weeks to 300 mg/d if response is suboptimal. Headache, dry mouth, insomnia, and nausea are the most common adverse effects. Agitation or irritability is sometimes serious enough to warrant stopping bupropion.
Combining medications. Using SSRIs with stimulants can help adults with ADHD and comorbid anxiety or depression. Any SSRI can be safely combined with stimulants, though we tend to pick:
- more-sedating agents such as paroxetine or sertraline when patients report difficulty with insomnia or overactivation
- less-sedating compounds such as fluoxetine or citalopram when patients complain of being too tired or underactive.
When patients taking SSRIs seek help for ADHD, adding a stimulant usually reduces inattention, distractibility, impulsivity, and/or subjective feelings of restlessness. We prescribe usual dosages because stimulants and SSRIs do not interact. We have not seen serious side effects, but some patients report feeling oversedated.
Tricyclics. We use tricyclics when a stimulant/SSRI combination does not relieve symptoms satisfactorily or a patient complains of side effects. We usually have good results with desipramine or imipramine, 150 to 300 mg/d, or nortriptyline, 50 to 150 mg/d. Spencer et al have reported a response rate of 68% with nortriptyline or desipramine in a retrospective chart review25 and a prospective placebo-controlled trial26 of adults with ADHD.
Case continued: Closer to dream job
After 6 months of combined treatment, Mr. B reports much-improved ADHD symptoms, with minimal stimulant-related side effects. He has made some realistic plans for computer programming school and is taking preliminary courses. Keeping a schedule book has reduced his tardiness and tendency to procrastinate.
He is more comfortable in the classroom and better able to challenge self-critical thinking. When routine difficulties arise, he is using more-adaptive coping strategies. To maintain gains achieved in therapy, he chooses to continue periodic CBT booster sessions.
Long-term treatment
Even with medication and CBT, patients may require referral for organizational coaching, academic counseling, school or workplace accommodations, vocational counseling, cognitive remediation, group therapy, or social skills classes. You can help them obtain quality adjunctive care by collaborating with professionals who offer these services.
No studies have examined long-term care of adults with ADHD. In our experience, ongoing medication and intermittent therapy can sustain symptom control and coping skills for years. Most patients are initially skeptical about staying on medication, but after they experience the benefits most seem willing to continue as long as the medication helps.
Most of our patients sustain changes in thinking, feeling, and behaving that they learn through BT. They may seek additional sessions to meet a challenge, such as a new job or starting a family.
Books
- Kolberg J, Nadeau K. ADD-friendly ways to organize your life. New York: Brunner-Routledge; 2002.
- Hallowell EM, Ratey JJ. Driven to distraction. New York: Touchstone; 1994.
- Hallowell E, Ratey J. Delivered from distraction. New York: Ballantine Books; 2005.
Organizations
- Children and Adults with Attention-Deficit/Hyperactivity Disorder (CHADD). National Resource Center on AD/HD. www.chadd.org.
- Attention Deficit Disorder Association (ADDA). Resources and membership organization for adults with ADHD. www.add.org.
Drug brand names
- Amphetamine • Adderall, Dexedrine
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Desipramine • Norpramin
- Imipramine • Tofranil
- Methylphenidate • Concerta, Focalin, Metadate, Ritalin
- Nortriptyline • Aventyl, Pamelor
Disclosures
Dr. Rostain is a consultant to Shire Pharamaceuticals Group and a speaker for Eli Lilly & Co. and Ortho-McNeil Pharmaceutical
Dr. Ramsay reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Mr. B, age 50, dreams of becoming a computer programmer but fears he will embarrass himself—as he has in many classrooms before. He is seeking evaluation because his teenage son was recently diagnosed with attention-deficit/hyperactivity disorder (ADHD), and he recognizes similar symptoms in himself.
Mr. B received a college degree with great difficulty, putting off assignments until the last minute and “squeaking by.” For years he has changed occupations often, never progressing beyond entry level, and now works as a personal care provider and limousine driver. He reports problems keeping up with work and managing time.
His history includes early childhood hyperactivity, difficulty sitting through classes, sloppy handwriting, disorganization, short attention span, and distractibility. He is restless, fidgety, and has trouble staying on topic. His disorganization has caused marital difficulties, for which he has sought counseling.
After careful evaluation, you determine that Mr. B meets criteria for ADHD, combined type, and for anxiety disorder not otherwise specified. His treatment goals are to increase his ability to focus; procrastinate less; improve his planning, prioritizing, and self-esteem; and to become less sensitive to criticism and less anxious about handling work demands.
Like Mr. B, adults with ADHD need treatment for the disorder’s core symptoms as well as its psychiatric comorbidities and psychosocial consequences. Comprehensive treatment with medications, cognitive-behavioral therapy (CBT), and environmental adaptations is usually recommended.
Comorbidity rules
Core symptoms. ADHD is a lifespan disorder with multiple behavioral, cognitive, and emotional manifestations that impair relationships and academic and vocational functioning. ADHD-like symptoms are seen in other conditions such as mood disorders or substance abuse, but complaints of inattention, distractibility, procrastination, restlessness, and impulsivity—particularly when pervasive and chronic—are highly indicative of ADHD.
In treating adults with ADHD, we have noticed common behavioral patterns that contribute to their psychosocial problems (Table 1). Dysfunctional coping behaviors have short-term advantages, but patients readily admit they would rather accomplish tasks through greater thought and planning.
Chronic frustrations—often associated with deep shame—are typical of adult ADHD. Many patients have maladaptive core beliefs of failure, self-mistrust, and inadequacy (Table 2).
Table 1
Common dysfunctional behavioral patterns in adults with ADHD
| Behavior | Description | Short-term gain/long-term loss |
|---|---|---|
| Anticipatory avoidance | Magnifying the difficulty of a pending task and doubts about being able to complete it; results in rationalizations to justify procrastination | Defers short-term stress, but often creates a self-fulfilling prophecy because the task looms and may seem overwhelming when facing a deadline |
| Brinksmanship | Waiting until the last moment (eg, the night before) to complete a task, often when facing an impending deadline | Deadline-associated stress can be focusing, but this tactic leaves little room for error and may yield a substandard result |
| Pseudoefficiency | Completing several low-priority, manageable tasks (eg, checking e-mail) but avoiding high-priority tasks (eg, a project for work) | Creates sense of productivity by reducing items on to-do list but defers a more difficult project |
| Juggling | Taking on new, exciting projects and feeling ‘busy’ without completing projects already started | It is easier to become motivated to start a novel project than to complete an ongoing one; pattern usually results in several incomplete projects |
Table 2
5 common maladaptive core beliefs of adults with ADHD
| Self-mistrust | ‘I cannot rely on myself to do what I need to do. I let myself down’ |
| Failure | ‘I always have failed and always will fail at what I set out to do.’ |
| Inadequacy | ‘I am basically a bad and defective person.’ |
| Incompetence | ‘I am too inept to handle life’s basic demands.’ |
| Instability | ‘My life will always be chaotic and in turmoil.’ |
Psychiatric comorbidity is the rule in adults with ADHD (Table 3). For example, among 43 patients who received combined medication and CBT at the University of Pennsylvania Adult ADHD Treatment and Research Program, 75% reported at least one comorbid condition, including:
- 27 (63%) with mood disorder
- 23 (54%) with anxiety disorder
- 5 (12%) with substance abuse.1
Other treatment studies have reported similar comorbidity rates in adults with ADHD.2-4
Table 3
Psychiatric comorbidity in adult ADHD
| Disorder | Prevalence |
|---|---|
| Mood disorders | 50% to 65% |
| Recurrent depression | |
| Bipolar disorder | |
| Cyclothymia | |
| Dysthymia | |
| Depressive disorder NOS | |
| Anxiety disorders | 40% to 55% |
| Generalized anxiety disorder | |
| Anxiety disorder NOS | |
| Others | Various |
| Substance use disorder | |
| Learning disabilities | |
| Intermittent explosive disorder | |
| Tourette syndrome | |
| Antisocial personality | |
| Borderline personality disorder | |
| Dependent personality | |
| NOS: Not otherwise specified | |
Making the diagnosis
Diagnosis of adult ADHD is based on a comprehensive assessment, including:
- careful history of presenting complaints
- thorough review of educational, occupational, and family history
- standardized rating scales (such as the Barkley ADHD Behavior Checklists, the Conners’ Adult ADHD Rating Scale, or the Brown Attention Deficit Disorder Scales)
- collateral information
- assessment of mood, anxiety, substance use, and learning/organizational skills. For details, consult references on adult ADHD.5-8
Case continued: Self-fulfilling prophesies
On standardized rating scales, Mr. B meets criteria for combined ADHD for childhood and current symptoms. Information from his wife and brother also confirms the ADHD diagnosis.
He is motivated, resilient, optimistic, and has a good support system. However, his negative automatic thoughts about his ability to succeed in school and to handle increasing time demands suggest deeper beliefs of inadequacy and failure.
Mr. B struggled academically. Without guidance about how to change his approach to difficult situations, he has repeated old thinking and behavior patterns. Believing he will embarrass himself and fail to learn required material, Mr. B procrastinates and avoids doing assignments. In class, his feelings of inadequacy make him self-conscious, which causes him to lose focus and have trouble concentrating.
See the world through the patient’s eyes
Understanding your patient. Before you start treatment, we recommend that you conceptualize how ADHD has influenced your patient’s life, including:
- developmental experiences
- family-of-origin issues, such as conflicts with parents stemming from ADHD symptoms or reciprocal interactions with an ADHD parent
- world view (“schemata”)
- patterns of coping with (or avoiding) stress
- attitudes toward self and important others
- readiness to change.
Developing a working case conceptualization is a dynamic, collaborative process. You talk with patients, and encourage them to reflect on how ADHD affects their view of themselves and their important relationships. The conceptualization takes shape as you:
- observe patients’ behaviors
- elicit how they think and feel
- assess with them the relevance and accuracies of their belief systems and response patterns.
Seeing the world “through their eyes” prepares you to help them accept the diagnosis and learn to manage ADHD symptoms. Then, by providing a blueprint to manage what patients may see as uncontrollable responses, you can help them take charge of their automatic reactions.
Psychoeducation. To set the stage for treatment, encourage patients to learn about ADHD by reading articles and books and consulting Web sites for adults with ADHD (see Related resources). Psychoeducation helps patients:
- review possible treatment approaches, including organizational (environmental) management, medication, and psychotherapy (individual or group)
- become informed participants in setting treatment goals.
Explain the relative contribution of each treatment component. For example, medications can reduce distractibility and improve attention, organizational strategies can reduce disorganization and improve time management, and structured psychotherapy can help the patient develop more effective coping skills.
Case continued: Planning combined treatment
You discuss diagnosis and treatment options with Mr. B, and he agrees to start the methylphenidate compound Concerta, initially at 18 mg/d, and weekly CBT sessions. You recommended a stimulant based on efficacy studies and your clinical experience in treating adults with ADHD. Mr. B wants a medication that will help him focus while working or studying, and he says Concerta has improved his son’s ADHD symptoms.
You instruct Mr. B to increase the dosage by 18 mg each week until he reaches 72 mg/d. You also tell him to keep a medication response log and to note any positive changes and side effects.
If an adult with ADHD expresses preference for a particular medication, we usually prescribe that one first. Most patients to whom we offer both medication and psychotherapy agree to this “top-down” and “bottom-up” approach. “Top down” means giving patients new ways of thinking to help them understand and modify their responses. “Bottom up” refers to the medication reducing their impulsivity, distractibility, and inattentiveness.
CBT for adult ADHD
Medications can ameliorate key symptoms of adult ADHD, but adjunctive interventions are needed to improve functioning and quality of life. Evidence supporting psychosocial treatment for adults with ADHD is limited, but CBT has been studied the most.1,9-13 Safren et al13 found a four-fold greater therapeutic response when patients received adjunctive CBT for residual ADHD symptoms, compared with patients who received medication alone.
We usually provide CBT weekly for 12 weeks and then taper to 8 additional sessions over 3 months (total 20 sessions). We may extend CBT with additional sessions to address complicated issues. CBT helps adults with ADHD to:
- identify dysfunctional thinking, feeling, and behaving patterns
- recognize contexts in which patterns arise
- systematically change these patterns.
CBT can reduce ADHD-associated anxiety and depression and improve coping skills and sense of well-being.1,9,11 Its flexibility allows you to address family issues with patients’ partners, children and other relatives to improve communication, reduce conflict, and develop healthier interactions.
We focus CBT sessions on finding alternate coping strategies. We might try role playing, rehearsing, creating “thought experiments,” and anticipating and preparing to modify typical patterns of avoidance. These approaches have been described elsewhere.10,11,14
We adopt an active stance during therapy to keep ADHD patients’ distractibility from disrupting our conversation. For example, we set the therapeutic agenda, provide feedback about patients’ behaviors, and encourage them to clarify rewards and consequences of using (or avoiding) problem-solving strategies.
Although we typically assign between-session homework, we expect patients to have difficulty completing it. We remain nonjudgmental and collaborative, viewing incomplete assignments as opportunities to learn about patients’ unproductive problem solving and to help them develop more-effective patterns.
Challenging maladaptive beliefs. A strong therapeutic relationship allows adults with ADHD to discuss their chronic frustrations, which often are associated with deep shame. We then shift CBT’s focus to deeper ADHD-related schemata that perpetuate dysfunctional patterns.
We work with patients to elucidate and challenge their maladaptive core beliefs and encourage new ways to view themselves and others. Allowing patients to grieve about the limitations ADHD imposes on their lives also helps to reduce chronic negative self-esteem.
Case continued: ‘less frenetic’
Mr. B achieves good results within 3 weeks of an increasing titration of stimulant medication, reporting significantly less restlessness and greater concentration without significant side effects. His wife confirms that he is less frenetic, can converse without interruptions, and is better at managing his complicated work schedule.
Which medications?
Drug therapy for adult ADHD is not as well-studied as in children and adolescents, but American Academy of Child and Adolescent Psychiatry guidelines and others15-18 recommend stimulant and nonstimulant medications. Your choice depends on the patient’s clinical profile (including risk factors and comorbid conditions), past medication use, treatment goals, preferred medication effects and dosing patterns (once-daily versus multiple times), and potential side effects. Stimulants or atomoxetine are first-line choices for adult ADHD without psychiatric comorbidity.
Stimulants work quickly and are cleared relatively rapidly from the brain without causing euphoria or dependency. They are effective (80% to 90% response rate) and well-tolerated, though long-term effects have not been studied in adults (Table 4).
Stimulants’ effect size of 0.9 is considered substantial. Effect size—a statistical method of reporting an intervention’s effect across different studies—is typically rated as:
- <0.32 very small
- 0.33 to 0.54, moderate
- >0.55, significant or very strong.
When choosing a medication, we usually try methylphenidate and amphetamine first, one after the other. We explain to the patient how stimulants work in the brain and the need for a comparative trial to determine which might work best for him or her. If the patient has tried a stimulant and found it helpful, we start with that class. Similarly, if he/she has not had good results with one type, we start with the other. Approximately one-third of our patients respond equally well to methylphenidate or amphetamine, one-third respond better to methylphenidate, and one-third respond better to amphetamine.
To determine the optimal dosage, we usually titrate up from 10 to 30 mg per dose of an immediate-release preparation. We begin with this form to help patients notice the medication’s onset and duration of action. After we find the optimal dosage, we switch to a longer-acting preparation.
Insomnia, mood instability, and euphoria are unacceptable stimulant side effects, although many patients welcome others such as appetite suppression and weight loss. Closely monitor cardiovascular effects, and review potential interactions with other medications, such as antihypertensives or bronchodilators. Because sudden death has been reported with stimulants in persons with structural cardiac lesions,19 obtain a cardiology consultation for patients with a history of heart disease.
We encourage patients to keep daily medication logs (Box), which we review at each visit and use to make dosing or medication changes. Dosing guidelines resemble those used for children and adolescents, although adults usually tolerate higher maximum dosages (such as methylphenidate, 80 to 100 mg/d).
Because of stimulants’ potential for recreational misuse and abuse, remain wary about choosing stimulants for patients with whom you lack a solid doctor-patient relationship.
Table 4
Stimulant dosages used in treating adult ADHD
| Class (brand name) | Daily dosing | Typical dosing schedule |
|---|---|---|
| Methylphenidate | ||
| Short-acting (Metadate, Ritadex, Ritalin) | Two to four times | 10 to 40 mg bid to qid |
| Intermediate-acting (Metadate SR, Ritalin SR) | Once or twice | 20 to 60 mg qd to bid |
| Extended-release (Concerta, Metadate CD, Ritalin LA) | Once or twice | 18 to 108 mg qd (Concerta) 20 to 40 mg bid (Ritalin LA, Metadate CD) |
| Dextromethylphenidate | ||
| Short-acting (Focalin) | Two to four times | 5 to 20 mg bid to qid |
| Long-acting (Focalin XR) | Once or twice | 10 to 20 mg qd or bid |
| Dextroamphetamine | ||
| Short-acting (Dexedrine) | Twice or three times | 10 to 30 mg bid or tid |
| Intermediate-acting (Dexedrine spansules) | Once or twice | 10 to 30 mg bid |
| Mixed amphetamine salts | ||
| Intermediate-acting (Adderall) | Once or twice | 10 to 30 mg bid or tid |
| Extended-release (Adderall XR) | Once or twice | 10 to 40 mg qd or bid |
Atomoxetine, a nonstimulant, norepinephrine re-uptake inhibitor, is approved for ADHD in adults.20-22 In two double-blind, controlled, randomized trials totalling 536 adults, Michaelson et al20 found significantly reduced ADHD symptoms after 10 weeks of atomoxetine treatment. Effect sizes of 0.35 and 0.40 were reported, with 10% of patients discontinuing because of side effects.
Atomoxetine has a long duration of action (>12 hours) but a more gradual onset (4 to 6 weeks) than that of stimulants. Approximately 60% of patients respond to atomoxetine, though effect sizes are less than those of stimulants. We have found atomoxetine works well for patients who:
- do not tolerate or are uncomfortable with taking stimulants
- are highly anxious
- report emotional dysregulation as a major target symptom.
To reduce risk of common side effects (nausea, GI upset, headache, sedation, reduced sex drive), we start with low dosages (such as 25 mg bid) and increase weekly by 25 mg to a target of 80 to 100 mg/d.
Treating complicated ADHD
Bupropion or tricyclic antidepressants are reasonable options for ADHD with depression. Atomoxetine, a tricyclic, or a stimulant plus a selective serotonin reuptake inhibitor (SSRI) can provide good symptom relief for adults with ADHD and comorbid anxiety and/or depression.
Bupropion. Approximately 50% of adults with ADHD respond to bupropion,23,24 with a treatment effect size of 0.6. Bupropion’s efficacy in smoking cessation adds value for those trying to quit.
We usually start extended-release bupropion at 150 mg/d and increase after 2 weeks to 300 mg/d if response is suboptimal. Headache, dry mouth, insomnia, and nausea are the most common adverse effects. Agitation or irritability is sometimes serious enough to warrant stopping bupropion.
Combining medications. Using SSRIs with stimulants can help adults with ADHD and comorbid anxiety or depression. Any SSRI can be safely combined with stimulants, though we tend to pick:
- more-sedating agents such as paroxetine or sertraline when patients report difficulty with insomnia or overactivation
- less-sedating compounds such as fluoxetine or citalopram when patients complain of being too tired or underactive.
When patients taking SSRIs seek help for ADHD, adding a stimulant usually reduces inattention, distractibility, impulsivity, and/or subjective feelings of restlessness. We prescribe usual dosages because stimulants and SSRIs do not interact. We have not seen serious side effects, but some patients report feeling oversedated.
Tricyclics. We use tricyclics when a stimulant/SSRI combination does not relieve symptoms satisfactorily or a patient complains of side effects. We usually have good results with desipramine or imipramine, 150 to 300 mg/d, or nortriptyline, 50 to 150 mg/d. Spencer et al have reported a response rate of 68% with nortriptyline or desipramine in a retrospective chart review25 and a prospective placebo-controlled trial26 of adults with ADHD.
Case continued: Closer to dream job
After 6 months of combined treatment, Mr. B reports much-improved ADHD symptoms, with minimal stimulant-related side effects. He has made some realistic plans for computer programming school and is taking preliminary courses. Keeping a schedule book has reduced his tardiness and tendency to procrastinate.
He is more comfortable in the classroom and better able to challenge self-critical thinking. When routine difficulties arise, he is using more-adaptive coping strategies. To maintain gains achieved in therapy, he chooses to continue periodic CBT booster sessions.
Long-term treatment
Even with medication and CBT, patients may require referral for organizational coaching, academic counseling, school or workplace accommodations, vocational counseling, cognitive remediation, group therapy, or social skills classes. You can help them obtain quality adjunctive care by collaborating with professionals who offer these services.
No studies have examined long-term care of adults with ADHD. In our experience, ongoing medication and intermittent therapy can sustain symptom control and coping skills for years. Most patients are initially skeptical about staying on medication, but after they experience the benefits most seem willing to continue as long as the medication helps.
Most of our patients sustain changes in thinking, feeling, and behaving that they learn through BT. They may seek additional sessions to meet a challenge, such as a new job or starting a family.
Books
- Kolberg J, Nadeau K. ADD-friendly ways to organize your life. New York: Brunner-Routledge; 2002.
- Hallowell EM, Ratey JJ. Driven to distraction. New York: Touchstone; 1994.
- Hallowell E, Ratey J. Delivered from distraction. New York: Ballantine Books; 2005.
Organizations
- Children and Adults with Attention-Deficit/Hyperactivity Disorder (CHADD). National Resource Center on AD/HD. www.chadd.org.
- Attention Deficit Disorder Association (ADDA). Resources and membership organization for adults with ADHD. www.add.org.
Drug brand names
- Amphetamine • Adderall, Dexedrine
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Desipramine • Norpramin
- Imipramine • Tofranil
- Methylphenidate • Concerta, Focalin, Metadate, Ritalin
- Nortriptyline • Aventyl, Pamelor
Disclosures
Dr. Rostain is a consultant to Shire Pharamaceuticals Group and a speaker for Eli Lilly & Co. and Ortho-McNeil Pharmaceutical
Dr. Ramsay reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Rostain AL, Ramsay JR. A combined treatment approach for adults with attention-deficit/hyperactivity disorder. Results of an open study of 43 patients J Attention Disorders. In press.
2. Shekim WO, Asarnow RF, Hess E, et al. A clinical and demographic profile of a sample of adults with attention deficit hyperactivity disorder, residual state. Comp Psychiatry 1990;31:416-25.
3. Biederman J, Faraone SV, Spencer T, et al. Patterns of psychiatric comorbidity, cognition and psychosocial functioning in adults with attention deficit hyperactivity disorder. Am J Psychiatry 1993;150:1792-8.
4. Wilens TE, Biederman J, Spencer T. Attention-deficit/hyperactivity disorder across the lifespan. Ann Rev Medicine 2002;53:113-31.
5. Barkley RA. Attention deficit hyperactivity disorder: a handbook for diagnosis and treatment. New York: Guilford Press; 1998.
6. Wender PH. ADHD: Attention-deficit hyperactivity disorder in children and adults. New York: Oxford University Press; 2000.
7. Goldstein S, Ellison AT. Clinician’s guide to adult ADHD. San Diego: Academic Press; 2000.
8. Brown TE. Attention-deficit disorder: the unfocused mind in children and adults. New Haven, CT: Yale University Press; 2005.
9. Wilens TE, McDermott SP, Biederman J, et al. Cognitive therapy in the treatment of adults with ADHD: a systematic chart review of 26 cases. J Cogn Ther 1999;13:215-26.
10. Ramsay JR, Rostain AL. A cognitive therapy approach for adult attention-deficit/hyperactivity disorder. J Cogn Psychother 2003;17:319-34.
11. Safren SA, Sprich S, Chulvick S, Otto MW. Psychosocial treatments for adults with attention-deficit/hyperactivity disorder. Psychiatr Clin North Am 2004;27:349-60.
12. Ramsay JR, Rostain AL. Adapting psychotherapy to meet the needs of adults with attention-deficit/hyperactivity disorder. Psychotherapy: Theory, Research, Practice, Training 2005;42:72-84.
13. Safren SA, Otto MW, Sprich S, et al. Cognitive-behavior therapy for ADHD in medication-treated adults with continued symptoms. Behav Res Ther 2005;43:831-42.
14. Ramsay JR, Rostain AL. Girl, repeatedly interrupted: The case of a young adult woman with ADHD. Clinical Case Studies 2005;4:329-46.
15. American Academy of Child and Adolescent Psychiatry. Practice parameters for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;42(suppl 2):26S-49S.
16. Weiss M, Murray C, Weiss G. Adults with attention-deficit/hyperactivity disorder: Current concepts. J Psychiatr Pract 2002;8:99-111.
17. Wilens TE. Drug therapy for adults with attention-deficit hyperactivity disorder. Drugs 2003;63:2395-411.
18. Dodson WW. Pharmacotherapy of adult ADHD. J Clin Psychol 2005;61:589-606.
19. Francis PD. Effects of psychotropic medications on the pediatric electrocardiogram and recommendations for monitoring. Curr Opin Pediatr 2002;14(2):224-30
20. Michaelson D, Adler L, Spencer T. Atomoxetine in adults: Two randomized, placebo-controlled studies. Biol Psychiatry 2003;53:112-20.
21. Simpson D, Plosker GL. Atomoxetine: a review of its use in adults with attention deficit hyperactivity disorder. Drugs 2004;64:205-22.
22. Reimherr FW, Marchant BK, Strong RE, et al. Emotional dysregulation in adult ADHD and response to atomoxetine. Biol Psychiatry 2005;58:125-31.
23. Wilens TE, Spencer T, Biederman J. A controlled clinical trial of bupropion for attention-deficit/hyperactivity disorder in adults. Am J Psychiatry 2001;158:282-8.
24. Wilens TE, Haight BR, Horrigan JP, et al. Bupropion XL in adults with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled study. Biol Psychiatry 2005;57:793-801.
25. Wilens TE, Biederman JB, Mick E, Spencer TJ. A systematic assessment of tricyclic antidepressants in the treatment of adult attention deficit/hyperactivity disorder. J Nerv Ment Dis 1995;183:48-50.
26. Wilens TE, Biederman JB, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention deficit hyperactivity disorder. Am J Psychiatry 1996;153:1147-53.
1. Rostain AL, Ramsay JR. A combined treatment approach for adults with attention-deficit/hyperactivity disorder. Results of an open study of 43 patients J Attention Disorders. In press.
2. Shekim WO, Asarnow RF, Hess E, et al. A clinical and demographic profile of a sample of adults with attention deficit hyperactivity disorder, residual state. Comp Psychiatry 1990;31:416-25.
3. Biederman J, Faraone SV, Spencer T, et al. Patterns of psychiatric comorbidity, cognition and psychosocial functioning in adults with attention deficit hyperactivity disorder. Am J Psychiatry 1993;150:1792-8.
4. Wilens TE, Biederman J, Spencer T. Attention-deficit/hyperactivity disorder across the lifespan. Ann Rev Medicine 2002;53:113-31.
5. Barkley RA. Attention deficit hyperactivity disorder: a handbook for diagnosis and treatment. New York: Guilford Press; 1998.
6. Wender PH. ADHD: Attention-deficit hyperactivity disorder in children and adults. New York: Oxford University Press; 2000.
7. Goldstein S, Ellison AT. Clinician’s guide to adult ADHD. San Diego: Academic Press; 2000.
8. Brown TE. Attention-deficit disorder: the unfocused mind in children and adults. New Haven, CT: Yale University Press; 2005.
9. Wilens TE, McDermott SP, Biederman J, et al. Cognitive therapy in the treatment of adults with ADHD: a systematic chart review of 26 cases. J Cogn Ther 1999;13:215-26.
10. Ramsay JR, Rostain AL. A cognitive therapy approach for adult attention-deficit/hyperactivity disorder. J Cogn Psychother 2003;17:319-34.
11. Safren SA, Sprich S, Chulvick S, Otto MW. Psychosocial treatments for adults with attention-deficit/hyperactivity disorder. Psychiatr Clin North Am 2004;27:349-60.
12. Ramsay JR, Rostain AL. Adapting psychotherapy to meet the needs of adults with attention-deficit/hyperactivity disorder. Psychotherapy: Theory, Research, Practice, Training 2005;42:72-84.
13. Safren SA, Otto MW, Sprich S, et al. Cognitive-behavior therapy for ADHD in medication-treated adults with continued symptoms. Behav Res Ther 2005;43:831-42.
14. Ramsay JR, Rostain AL. Girl, repeatedly interrupted: The case of a young adult woman with ADHD. Clinical Case Studies 2005;4:329-46.
15. American Academy of Child and Adolescent Psychiatry. Practice parameters for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002;42(suppl 2):26S-49S.
16. Weiss M, Murray C, Weiss G. Adults with attention-deficit/hyperactivity disorder: Current concepts. J Psychiatr Pract 2002;8:99-111.
17. Wilens TE. Drug therapy for adults with attention-deficit hyperactivity disorder. Drugs 2003;63:2395-411.
18. Dodson WW. Pharmacotherapy of adult ADHD. J Clin Psychol 2005;61:589-606.
19. Francis PD. Effects of psychotropic medications on the pediatric electrocardiogram and recommendations for monitoring. Curr Opin Pediatr 2002;14(2):224-30
20. Michaelson D, Adler L, Spencer T. Atomoxetine in adults: Two randomized, placebo-controlled studies. Biol Psychiatry 2003;53:112-20.
21. Simpson D, Plosker GL. Atomoxetine: a review of its use in adults with attention deficit hyperactivity disorder. Drugs 2004;64:205-22.
22. Reimherr FW, Marchant BK, Strong RE, et al. Emotional dysregulation in adult ADHD and response to atomoxetine. Biol Psychiatry 2005;58:125-31.
23. Wilens TE, Spencer T, Biederman J. A controlled clinical trial of bupropion for attention-deficit/hyperactivity disorder in adults. Am J Psychiatry 2001;158:282-8.
24. Wilens TE, Haight BR, Horrigan JP, et al. Bupropion XL in adults with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled study. Biol Psychiatry 2005;57:793-801.
25. Wilens TE, Biederman JB, Mick E, Spencer TJ. A systematic assessment of tricyclic antidepressants in the treatment of adult attention deficit/hyperactivity disorder. J Nerv Ment Dis 1995;183:48-50.
26. Wilens TE, Biederman JB, Prince J, et al. Six-week, double-blind, placebo-controlled study of desipramine for adult attention deficit hyperactivity disorder. Am J Psychiatry 1996;153:1147-53.
Keeping patients physically well: A psychiatrist’s ‘CIVIC’ duty
Many patients with a severe mental disorder go years without preventive medical treatment, leaving them medically ill or at high risk for a medical illness.(See"Acute MI Risk Protecting you patients heart health" September 2005.)
Blood pressure. Check at each visit for patients with a history of hypertension and every 3 to 4 months for nonhypertensive patients. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7)(See"Metabolic syndrome: 5 risk factors guide therapy" April 2005.)
Refer patients with suspected metabolic syndrome to a primary care physician or endocrinologist for management. Refer patients taking anticonvulsants if readings or symptoms suggest hepatitis or dyscrasia. Significant abnormalites include leukocites 9, platelets 14
Table 2
At what point do lipid levels indicate cardiovascular risk?
| Safe | Borderline* | Needs treatment† | Treatment options | |
|---|---|---|---|---|
| Total cholesterol | 200-239 | >240 | See LDL cholesteroltreatment options | |
| LDL cholesterol | 130-159 | >160 | Lifestyle changes | |
| Statins | ||||
| Bile sequestrants | ||||
| Nicotinic acid | ||||
| Fibrate | ||||
| HDL cholesterol | >60¶ | 59-39 | Lifestyle changes | |
| Treat triglycerides | ||||
| Add nicotinic acid or fibrate | ||||
| Triglycerides | 150-199 | >200 | Lifestyle changes | |
| Statins | ||||
| Consider nicotinic acid or fibrate | ||||
| *Treat according to risk factors. See Adult Treatment Panel III guidelines for specific regimens and cautions. | ||||
| †Three- to 6-month trial of lifestyle changes may be warranted in most cases. Urge patients to reduce saturated fat and cholesterol, eat more soluble fiber, and exercise more. | ||||
| ¶Removes one risk factor | ||||
| Source: Adapted from the Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) (www.nhlbi.nih.gov/guidelines/cholesterol) | ||||
VACCINATION HISTORY/INFECTION RISK
Vaccinations. Many psychiatric patients are not up to date with vaccinations against hepatitis, influenza, or pneumonia. Ask the patient to recall his or her vaccination history as accurately as possible. If he or she cannot, contact the primary care physician the patient visited most recently.
If you cannot obtain the history, refer the patient to the municipal health department for influenza vaccine and a blood test to verify hepatitis B immunization. Educate patients on the benefits of vaccination, and coordinate with a primary care doctor or case manager to ensure the patient’s immunization.
Table 3
Who needs which vaccines—and how often
| Vaccine | Targeted group/frequency |
|---|---|
| Tetanus | Two-vaccine series for intravenous drug abusers; vaccine series for adults who did not receive primary series; boosters if ≥10 years since vaccination |
| Hepatitis A | Intravenous drug users, institutionalized persons, homosexual men, and those living or working where hepatitis A is endemic |
| Hepatitis B | Three-vaccine series for health care workers, sexually active heterosexual men and women, homosexual/bisexual men, hemodialysis patients, intravenous drug abusers, institutionalized persons |
| Influenza | Annual vaccination for persons age ≥50; patients with CVD, diabetes, HIV, renal disease, or pulmonary disease; and others who are immunosuppressed, pregnant, or in a nursing home. Check updates from CDC throughout flu season |
| Pneumococcal | Persons age ≥65; institutionalized patients age ≥50; those with alcohol dependence, asplenia, HIV, chronic CVD, chronic lung disease, diabetes, chronic liver disease, renal insufficiency, or who live in settings where pneumococcal disease can spread. Repeat dose on or about 65th birthday if immunized ≥5 years earlier |
| COPD: Chronic obstructive pulmonary disease | |
| STD: Sexually transmitted disease | |
| Source: U.S. Centers for Disease Control and Prevention. Recommended adult immunization schedule, by vaccine and age group (www.cdc.gov/nip/recs/adult-schedule.pdf) | |
Sexually transmitted disease. Neglected general health or malnourishment can weaken the immune system and increase susceptibility to infections. Patients who live in urban areas or public housing—where infections tend to spread—are especially vulnerable.
In addition, mentally ill persons are more likely than the general population to have a sexually transmitted disease (STD)17,18 because:
- mental illness can cloud judgment; for example, patients with bipolar mania are at risk for impulsive, hypersexual behavior
- some mentally ill patients support themselves with prostitution.
Refer sexually active patients to a hospital or private laboratory for an HIV test and an RPR to test for syphilis. Refer sexually active women age ≤25 for DNA cervical probes for gonorrhea and chlamydia. Evidence is equivocal for screening anymptomatic women age >25 for chlamydia or gonorrhea infection. Sexually inactive women or those in monogamous relationships may not need routine screening. For sexually active men, urine testing to screen for chlamydia or gonorrhea is available.19
Consult a local health clinic or gynecologist for the DNA probe, although some clinical laboratories can check urine for signs of cervical problems. Ask sexually active patients if/when they were immunized against hepatitis B. If needed, refer for vaccination.
MANAGING DIETARY INTAKE
Obesity—defined by the National Institutes of Health as BMI ≥30 kg/m2—often precedes preventable chronic diseases and cancer. Persons with chronic severe mental illness tend to be more sedentary than nonmentally ill persons,20 and research suggests that obesity is more common among patients with severe mental illness than among the general population.21 Also, poorer patients have trouble maintaining a balanced diet.
Calculate BMI using the National Heart, Lung and Blood Institute BMI calculator (http://www.nhlbisupport.com/bmi/bmicalc.htm). Encourage patients with BMI >25 kg/m2 to eat more fruits and vegetables, eliminate empty calories (alcohol, soda pop, juices, candy), and decrease fat consumption (especially fast food). Suggest to patients age ≥50 that they incorporate calcium, 1,200 mg/d, and vitamin D, 400 to 800 IU/d, in their diet to prevent osteoporosis.22
Also encourage patients to exercise moderately for a half-hour daily, 5 days a week, to burn calories. Supplement nutritional counseling with behavioral therapy, focusing on changing eating patterns.23
CANCER PREVENTION
Many patients with chronic mental illness are not regularly screened for colon, cervical, breast, or other common early-stage cancers. In addition, their cancer rates are significantly higher than those of the general population.24
Ask men at the initial visit when they were last screened for colon or prostate cancer. Ask women when they were last screened for colon, cervical or breast cancer (Table 4). Ask again once yearly.
Colon cancer. Colonoscopy, done by a gastroenterologist, is indicated for patients age >50 every 5 to 10 years, depending on endoscopic findings. In-office fecal occult blood tests (FOBT), performed annually between colonoscopies, can identify patients who may need closer follow-up. You can do in-office FOBT or refer to a primary care physician.25
Cervical cancer is thought to be caused by human papilloma virus (HPV). Refer women with an intact cervix annually to a gynecologist or hospital clinic for a Pap smear, which usually includes testing for high-risk HPV if atypical cells are discovered. Some guidelines suggest decreasing screening frequency after several negative Pap smears for women in a monogamous sexual relationship.
Breast cancer affects 1 in 8 women, and having a first-degree relative with breast cancer increases the risk. Women should receive annual mammograms starting at age 40. The USPSTF notes that mammography’s benefits improve with increasing age between ages 40 and 70.26
Many hospital radiology departments or community health centers provide mammograms on a rotating schedule. Refer patients with abnormal findings to a general surgeon or breast center.
Table 4
Recommended intervals for cancer screening
| Type of cancer | Recommended test frequency |
|---|---|
| Colon | Asymptomatic persons age >50 should receive colonoscopy every 5 to 10 years, as directed by the gastroenterologist, and annual fecal occult blood tests |
| Cervical | Annual Pap smears for women who have ever been sexually active and still have a cervix |
| Breast | Mammography every 1 to 2 years after age 40 |
| Lung | Evidence does not support routine chest x-rays or sputum cytology in asymptomatic patients |
| Prostate | Refer men age >50 to primary care physician or hospital laboratory for PSA test; counsel patients about the results and treatment |
CASE CONTINUED: TESTING BEGINS
We schedule a battery of laboratory tests for Mrs. J at the local hospital, including a fasting plasma glucose test and lipid profile to gauge her cardiovascular risk and potential effects from olanzapine, and CBC and LFTs to check for adverse effects from oxcarbazepine.
We ask Mrs. J whether she engages in high-risk sexual activity, which she denies. She cannot recall her vaccination history, so we contact the primary care physician she had seen 5 years ago. Depending on her other comorbidities, housing situation, an early pneumococcal vaccine may be indicated.
We also suggest that Mrs. J quit smoking, but she appears to be at a pre-contemplative stage. We hope to promote a change in her attitude by discussing smoking cessation at each visit
To address Mrs. J’s obesity, we briefly review a dietary plan augmented with increased physical activity. She will bring a 3-day food diary to her next visit and promises to walk 30 minutes four to five times weekly. She says she enjoys mall walking with her children.
We strongly urge Mrs. J to schedule a mammogram, as she is past age 50 and says she has never received one. We try to refer her to a primary care physician to arrange a Pap smear and colonoscopy, but she resists, fearing the results. With continued education, exploration, and encouragement, we will briefly follow up with Mrs. J at each visit to ensure that she gets these needed tests (Box 2).
Check with patients at each visit to ensure they are following through on their test referrals. If they are not, find out why.
If the patient is procrastinating, try to uncover an underlying cause. If the patient says he or she is pressed for time, ask: “Are you going through stressful life events? Are you afraid the test will hurt or will reveal a serious disease? Did you have this test before? If so, did it make you uncomfortable?
Tell the patent, “I understand your concerns, but this test is important. You need to make it a higher priority.” To work through the patient’s resistance, start by educating him or her about preventable chronic diseases and screening or treatment resources. Then try problem-solving techniques, motivational interviewing, or dissecting cognitive distortions.
Collaboration with a case manager is key when managing an indigent mentally ill patient. Open communication, setting well-defined goals, and a clear understanding of each other’s treatment roles is crucial. Inform the case manager which target tasks, tests, or appointments the patient has agreed to. The case manager can use this information to help the patient navigate the health care system and encourage full participation in care
Finally, build a referral base for indigent and uninsured patients. Look for a nearby internist, gastroenterologist, and OB/GYN who accept uninsured patients.
- U.S. Preventative Services Task Force. Continually updated, evidence-based screening recommendations for a range of medical problems. www.ahrq.gov/clinic/uspstfix.htm.
- U.S. Public Health Service. Clinical practice guideline: treating tobacco use and dependence. www.surgeongeneral.gov/tobacco/treating_tobacco_use.pdf.
- Olanzapine • Zyprexa
- Oxcarbazepine • Trileptal
Dr. Moss recevies research/grant support from the National Institutes of Health and is a speaker for Janssen Pharmaceutica and Pfizer.
Mr. Brammer and Dr. White report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Carney CP, Allen J, Doebbeling BN. Receipt of clinical preventive medical services among psychiatric patients. Psychiatr Serv 2002;53:1028-30.
2. Joukamaa M, Heliovaara M, Knekt P, et al. Mental disorders and cause-specific mortality. Br J Psychiatry 2001;179:498-502.
3. McCreadie RG. Scottish Schizophrenia Lifestyle Group. Diet, smoking and cardiovascular risk in people with schizophrenia: descriptive study. Br J Psychiatry 2003;183:534-9.
4. Cohn T, Prud’homme D, Streiner D, et al. Characterizing coronary heart disease risk in chronic schizophrenia: high prevalence of the metabolic syndrome. Can J Psychiatry 2004;49:753-60.
5. Hanson D, Gottesman I. Theories of schizophrenia: a geneticinflammatory-vascular synthesis. BMC Med Genet 2005;6:7.-
6. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289(19):2560-72.
7. Berg AO, Atkins D. U.S. Preventive Services Task Force: screening for lipid disorders in adults: recommendations and rationale. Am J Nurs 2002;102(6):p. 91-5.
8. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143-421.
9. Standards of medical care in diabetes. Diabetes Care 2005;28:S4-S36.
10. Clinical practice recommendations 2005. Diabetes Care 2005;28(Suppl 1):S1-S79.
11. Lasser K, Boyd JW, Woolhandler S, et al. Smoking and mental illness: a population-based prevalence study. JAMA 2000;284(20):2606-10.
12. Ockene IS, Miller NH. Cigarette smoking, cardiovascular disease, and stroke: a statement for healthcare professionals from the American Heart Association. American Heart Association Task Force on Risk Reduction. Circulation 1997;96:3243-7.
13. Fiore MC. U.S. Public Health Service clinical practice guideline: treating tobacco use and dependence. Respir Care 2000;45:1200-62.
14. Pellock JM, Willmore LJ. A rational guide to routine blood monitoring in patients receiving antiepileptic drugs. Neurology 1991;41:961-4.
15. Goodwin FK, Goldstein MA. Optimizing lithium treatment in bipolar disorder: a review of the literature and clinical recommendations. J Psychiatr Pract 2003;9:333-43.
16. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004;27:596-601.
17. Erbelding EJ, Hutton HE, Zenilman JM, et al. The prevalence of psychiatric disorders in sexually transmitted disease clinic patients and their association with sexually transmitted risk. Sex Transm Dis 2004;31:8-12.
18. Rein DB, Anderson LA, Irwin KL. Mental health disorders and sexually transmitted diseases in a privately insured population. Am J Manag Care 2004;10:917-24.
19. Screening for sexually transmitted diseases. U.S.Preventive Services Task Force, Washington, DC. Am Fam Physician 1990;42:691-702.
20. Daumit GL, Goldberg RW, Anthony C, et al. Physical activity patterns in adults with severe mental illness. J Nerv Ment Dis 2005;193:641-6.
21. Daumit GL, Clark JM, Steinwachs DM, et al. Prevalence and correlates of obesity in a community sample of individuals with severe and persistent mental illness. J Nerv Ment Dis 2003;191:799-805.
22. Hodgson SF, Watts NB, Bilezikian JP, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the prevention and treatment of postmenopausal osteoporosis: 2001 edition, with selected updates for 2003. Endocr Pract 2003;9:544-64.
23. Jakicic JM, Clark K, Coleman E, et al. American College of Sports Medicine. American College of Sports Medicine position stand. Appropriate intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc 2001;33:2145-56.
24. Lichtermann D, Ekelund J, Pukkala E, et al. Incidence of cancer among persons with schizophrenia and their relatives. Arch Gen Psychiatry 2001;58:573-8.
25. Colon cancer screening (USPSTF recommendation). U.S. Preventive Services Task Force. J Am Geriatr Soc 2000M;48:333-5.
26. Humphrey LL, Helfand M, Chan BK, Woolf SH. Breast cancer screening: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002;137(5 Part 1):347-60.
Many patients with a severe mental disorder go years without preventive medical treatment, leaving them medically ill or at high risk for a medical illness.(See"Acute MI Risk Protecting you patients heart health" September 2005.)
Blood pressure. Check at each visit for patients with a history of hypertension and every 3 to 4 months for nonhypertensive patients. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7)(See"Metabolic syndrome: 5 risk factors guide therapy" April 2005.)
Refer patients with suspected metabolic syndrome to a primary care physician or endocrinologist for management. Refer patients taking anticonvulsants if readings or symptoms suggest hepatitis or dyscrasia. Significant abnormalites include leukocites 9, platelets 14
Table 2
At what point do lipid levels indicate cardiovascular risk?
| Safe | Borderline* | Needs treatment† | Treatment options | |
|---|---|---|---|---|
| Total cholesterol | 200-239 | >240 | See LDL cholesteroltreatment options | |
| LDL cholesterol | 130-159 | >160 | Lifestyle changes | |
| Statins | ||||
| Bile sequestrants | ||||
| Nicotinic acid | ||||
| Fibrate | ||||
| HDL cholesterol | >60¶ | 59-39 | Lifestyle changes | |
| Treat triglycerides | ||||
| Add nicotinic acid or fibrate | ||||
| Triglycerides | 150-199 | >200 | Lifestyle changes | |
| Statins | ||||
| Consider nicotinic acid or fibrate | ||||
| *Treat according to risk factors. See Adult Treatment Panel III guidelines for specific regimens and cautions. | ||||
| †Three- to 6-month trial of lifestyle changes may be warranted in most cases. Urge patients to reduce saturated fat and cholesterol, eat more soluble fiber, and exercise more. | ||||
| ¶Removes one risk factor | ||||
| Source: Adapted from the Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) (www.nhlbi.nih.gov/guidelines/cholesterol) | ||||
VACCINATION HISTORY/INFECTION RISK
Vaccinations. Many psychiatric patients are not up to date with vaccinations against hepatitis, influenza, or pneumonia. Ask the patient to recall his or her vaccination history as accurately as possible. If he or she cannot, contact the primary care physician the patient visited most recently.
If you cannot obtain the history, refer the patient to the municipal health department for influenza vaccine and a blood test to verify hepatitis B immunization. Educate patients on the benefits of vaccination, and coordinate with a primary care doctor or case manager to ensure the patient’s immunization.
Table 3
Who needs which vaccines—and how often
| Vaccine | Targeted group/frequency |
|---|---|
| Tetanus | Two-vaccine series for intravenous drug abusers; vaccine series for adults who did not receive primary series; boosters if ≥10 years since vaccination |
| Hepatitis A | Intravenous drug users, institutionalized persons, homosexual men, and those living or working where hepatitis A is endemic |
| Hepatitis B | Three-vaccine series for health care workers, sexually active heterosexual men and women, homosexual/bisexual men, hemodialysis patients, intravenous drug abusers, institutionalized persons |
| Influenza | Annual vaccination for persons age ≥50; patients with CVD, diabetes, HIV, renal disease, or pulmonary disease; and others who are immunosuppressed, pregnant, or in a nursing home. Check updates from CDC throughout flu season |
| Pneumococcal | Persons age ≥65; institutionalized patients age ≥50; those with alcohol dependence, asplenia, HIV, chronic CVD, chronic lung disease, diabetes, chronic liver disease, renal insufficiency, or who live in settings where pneumococcal disease can spread. Repeat dose on or about 65th birthday if immunized ≥5 years earlier |
| COPD: Chronic obstructive pulmonary disease | |
| STD: Sexually transmitted disease | |
| Source: U.S. Centers for Disease Control and Prevention. Recommended adult immunization schedule, by vaccine and age group (www.cdc.gov/nip/recs/adult-schedule.pdf) | |
Sexually transmitted disease. Neglected general health or malnourishment can weaken the immune system and increase susceptibility to infections. Patients who live in urban areas or public housing—where infections tend to spread—are especially vulnerable.
In addition, mentally ill persons are more likely than the general population to have a sexually transmitted disease (STD)17,18 because:
- mental illness can cloud judgment; for example, patients with bipolar mania are at risk for impulsive, hypersexual behavior
- some mentally ill patients support themselves with prostitution.
Refer sexually active patients to a hospital or private laboratory for an HIV test and an RPR to test for syphilis. Refer sexually active women age ≤25 for DNA cervical probes for gonorrhea and chlamydia. Evidence is equivocal for screening anymptomatic women age >25 for chlamydia or gonorrhea infection. Sexually inactive women or those in monogamous relationships may not need routine screening. For sexually active men, urine testing to screen for chlamydia or gonorrhea is available.19
Consult a local health clinic or gynecologist for the DNA probe, although some clinical laboratories can check urine for signs of cervical problems. Ask sexually active patients if/when they were immunized against hepatitis B. If needed, refer for vaccination.
MANAGING DIETARY INTAKE
Obesity—defined by the National Institutes of Health as BMI ≥30 kg/m2—often precedes preventable chronic diseases and cancer. Persons with chronic severe mental illness tend to be more sedentary than nonmentally ill persons,20 and research suggests that obesity is more common among patients with severe mental illness than among the general population.21 Also, poorer patients have trouble maintaining a balanced diet.
Calculate BMI using the National Heart, Lung and Blood Institute BMI calculator (http://www.nhlbisupport.com/bmi/bmicalc.htm). Encourage patients with BMI >25 kg/m2 to eat more fruits and vegetables, eliminate empty calories (alcohol, soda pop, juices, candy), and decrease fat consumption (especially fast food). Suggest to patients age ≥50 that they incorporate calcium, 1,200 mg/d, and vitamin D, 400 to 800 IU/d, in their diet to prevent osteoporosis.22
Also encourage patients to exercise moderately for a half-hour daily, 5 days a week, to burn calories. Supplement nutritional counseling with behavioral therapy, focusing on changing eating patterns.23
CANCER PREVENTION
Many patients with chronic mental illness are not regularly screened for colon, cervical, breast, or other common early-stage cancers. In addition, their cancer rates are significantly higher than those of the general population.24
Ask men at the initial visit when they were last screened for colon or prostate cancer. Ask women when they were last screened for colon, cervical or breast cancer (Table 4). Ask again once yearly.
Colon cancer. Colonoscopy, done by a gastroenterologist, is indicated for patients age >50 every 5 to 10 years, depending on endoscopic findings. In-office fecal occult blood tests (FOBT), performed annually between colonoscopies, can identify patients who may need closer follow-up. You can do in-office FOBT or refer to a primary care physician.25
Cervical cancer is thought to be caused by human papilloma virus (HPV). Refer women with an intact cervix annually to a gynecologist or hospital clinic for a Pap smear, which usually includes testing for high-risk HPV if atypical cells are discovered. Some guidelines suggest decreasing screening frequency after several negative Pap smears for women in a monogamous sexual relationship.
Breast cancer affects 1 in 8 women, and having a first-degree relative with breast cancer increases the risk. Women should receive annual mammograms starting at age 40. The USPSTF notes that mammography’s benefits improve with increasing age between ages 40 and 70.26
Many hospital radiology departments or community health centers provide mammograms on a rotating schedule. Refer patients with abnormal findings to a general surgeon or breast center.
Table 4
Recommended intervals for cancer screening
| Type of cancer | Recommended test frequency |
|---|---|
| Colon | Asymptomatic persons age >50 should receive colonoscopy every 5 to 10 years, as directed by the gastroenterologist, and annual fecal occult blood tests |
| Cervical | Annual Pap smears for women who have ever been sexually active and still have a cervix |
| Breast | Mammography every 1 to 2 years after age 40 |
| Lung | Evidence does not support routine chest x-rays or sputum cytology in asymptomatic patients |
| Prostate | Refer men age >50 to primary care physician or hospital laboratory for PSA test; counsel patients about the results and treatment |
CASE CONTINUED: TESTING BEGINS
We schedule a battery of laboratory tests for Mrs. J at the local hospital, including a fasting plasma glucose test and lipid profile to gauge her cardiovascular risk and potential effects from olanzapine, and CBC and LFTs to check for adverse effects from oxcarbazepine.
We ask Mrs. J whether she engages in high-risk sexual activity, which she denies. She cannot recall her vaccination history, so we contact the primary care physician she had seen 5 years ago. Depending on her other comorbidities, housing situation, an early pneumococcal vaccine may be indicated.
We also suggest that Mrs. J quit smoking, but she appears to be at a pre-contemplative stage. We hope to promote a change in her attitude by discussing smoking cessation at each visit
To address Mrs. J’s obesity, we briefly review a dietary plan augmented with increased physical activity. She will bring a 3-day food diary to her next visit and promises to walk 30 minutes four to five times weekly. She says she enjoys mall walking with her children.
We strongly urge Mrs. J to schedule a mammogram, as she is past age 50 and says she has never received one. We try to refer her to a primary care physician to arrange a Pap smear and colonoscopy, but she resists, fearing the results. With continued education, exploration, and encouragement, we will briefly follow up with Mrs. J at each visit to ensure that she gets these needed tests (Box 2).
Check with patients at each visit to ensure they are following through on their test referrals. If they are not, find out why.
If the patient is procrastinating, try to uncover an underlying cause. If the patient says he or she is pressed for time, ask: “Are you going through stressful life events? Are you afraid the test will hurt or will reveal a serious disease? Did you have this test before? If so, did it make you uncomfortable?
Tell the patent, “I understand your concerns, but this test is important. You need to make it a higher priority.” To work through the patient’s resistance, start by educating him or her about preventable chronic diseases and screening or treatment resources. Then try problem-solving techniques, motivational interviewing, or dissecting cognitive distortions.
Collaboration with a case manager is key when managing an indigent mentally ill patient. Open communication, setting well-defined goals, and a clear understanding of each other’s treatment roles is crucial. Inform the case manager which target tasks, tests, or appointments the patient has agreed to. The case manager can use this information to help the patient navigate the health care system and encourage full participation in care
Finally, build a referral base for indigent and uninsured patients. Look for a nearby internist, gastroenterologist, and OB/GYN who accept uninsured patients.
- U.S. Preventative Services Task Force. Continually updated, evidence-based screening recommendations for a range of medical problems. www.ahrq.gov/clinic/uspstfix.htm.
- U.S. Public Health Service. Clinical practice guideline: treating tobacco use and dependence. www.surgeongeneral.gov/tobacco/treating_tobacco_use.pdf.
- Olanzapine • Zyprexa
- Oxcarbazepine • Trileptal
Dr. Moss recevies research/grant support from the National Institutes of Health and is a speaker for Janssen Pharmaceutica and Pfizer.
Mr. Brammer and Dr. White report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
Many patients with a severe mental disorder go years without preventive medical treatment, leaving them medically ill or at high risk for a medical illness.(See"Acute MI Risk Protecting you patients heart health" September 2005.)
Blood pressure. Check at each visit for patients with a history of hypertension and every 3 to 4 months for nonhypertensive patients. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7)(See"Metabolic syndrome: 5 risk factors guide therapy" April 2005.)
Refer patients with suspected metabolic syndrome to a primary care physician or endocrinologist for management. Refer patients taking anticonvulsants if readings or symptoms suggest hepatitis or dyscrasia. Significant abnormalites include leukocites 9, platelets 14
Table 2
At what point do lipid levels indicate cardiovascular risk?
| Safe | Borderline* | Needs treatment† | Treatment options | |
|---|---|---|---|---|
| Total cholesterol | 200-239 | >240 | See LDL cholesteroltreatment options | |
| LDL cholesterol | 130-159 | >160 | Lifestyle changes | |
| Statins | ||||
| Bile sequestrants | ||||
| Nicotinic acid | ||||
| Fibrate | ||||
| HDL cholesterol | >60¶ | 59-39 | Lifestyle changes | |
| Treat triglycerides | ||||
| Add nicotinic acid or fibrate | ||||
| Triglycerides | 150-199 | >200 | Lifestyle changes | |
| Statins | ||||
| Consider nicotinic acid or fibrate | ||||
| *Treat according to risk factors. See Adult Treatment Panel III guidelines for specific regimens and cautions. | ||||
| †Three- to 6-month trial of lifestyle changes may be warranted in most cases. Urge patients to reduce saturated fat and cholesterol, eat more soluble fiber, and exercise more. | ||||
| ¶Removes one risk factor | ||||
| Source: Adapted from the Third Report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) (www.nhlbi.nih.gov/guidelines/cholesterol) | ||||
VACCINATION HISTORY/INFECTION RISK
Vaccinations. Many psychiatric patients are not up to date with vaccinations against hepatitis, influenza, or pneumonia. Ask the patient to recall his or her vaccination history as accurately as possible. If he or she cannot, contact the primary care physician the patient visited most recently.
If you cannot obtain the history, refer the patient to the municipal health department for influenza vaccine and a blood test to verify hepatitis B immunization. Educate patients on the benefits of vaccination, and coordinate with a primary care doctor or case manager to ensure the patient’s immunization.
Table 3
Who needs which vaccines—and how often
| Vaccine | Targeted group/frequency |
|---|---|
| Tetanus | Two-vaccine series for intravenous drug abusers; vaccine series for adults who did not receive primary series; boosters if ≥10 years since vaccination |
| Hepatitis A | Intravenous drug users, institutionalized persons, homosexual men, and those living or working where hepatitis A is endemic |
| Hepatitis B | Three-vaccine series for health care workers, sexually active heterosexual men and women, homosexual/bisexual men, hemodialysis patients, intravenous drug abusers, institutionalized persons |
| Influenza | Annual vaccination for persons age ≥50; patients with CVD, diabetes, HIV, renal disease, or pulmonary disease; and others who are immunosuppressed, pregnant, or in a nursing home. Check updates from CDC throughout flu season |
| Pneumococcal | Persons age ≥65; institutionalized patients age ≥50; those with alcohol dependence, asplenia, HIV, chronic CVD, chronic lung disease, diabetes, chronic liver disease, renal insufficiency, or who live in settings where pneumococcal disease can spread. Repeat dose on or about 65th birthday if immunized ≥5 years earlier |
| COPD: Chronic obstructive pulmonary disease | |
| STD: Sexually transmitted disease | |
| Source: U.S. Centers for Disease Control and Prevention. Recommended adult immunization schedule, by vaccine and age group (www.cdc.gov/nip/recs/adult-schedule.pdf) | |
Sexually transmitted disease. Neglected general health or malnourishment can weaken the immune system and increase susceptibility to infections. Patients who live in urban areas or public housing—where infections tend to spread—are especially vulnerable.
In addition, mentally ill persons are more likely than the general population to have a sexually transmitted disease (STD)17,18 because:
- mental illness can cloud judgment; for example, patients with bipolar mania are at risk for impulsive, hypersexual behavior
- some mentally ill patients support themselves with prostitution.
Refer sexually active patients to a hospital or private laboratory for an HIV test and an RPR to test for syphilis. Refer sexually active women age ≤25 for DNA cervical probes for gonorrhea and chlamydia. Evidence is equivocal for screening anymptomatic women age >25 for chlamydia or gonorrhea infection. Sexually inactive women or those in monogamous relationships may not need routine screening. For sexually active men, urine testing to screen for chlamydia or gonorrhea is available.19
Consult a local health clinic or gynecologist for the DNA probe, although some clinical laboratories can check urine for signs of cervical problems. Ask sexually active patients if/when they were immunized against hepatitis B. If needed, refer for vaccination.
MANAGING DIETARY INTAKE
Obesity—defined by the National Institutes of Health as BMI ≥30 kg/m2—often precedes preventable chronic diseases and cancer. Persons with chronic severe mental illness tend to be more sedentary than nonmentally ill persons,20 and research suggests that obesity is more common among patients with severe mental illness than among the general population.21 Also, poorer patients have trouble maintaining a balanced diet.
Calculate BMI using the National Heart, Lung and Blood Institute BMI calculator (http://www.nhlbisupport.com/bmi/bmicalc.htm). Encourage patients with BMI >25 kg/m2 to eat more fruits and vegetables, eliminate empty calories (alcohol, soda pop, juices, candy), and decrease fat consumption (especially fast food). Suggest to patients age ≥50 that they incorporate calcium, 1,200 mg/d, and vitamin D, 400 to 800 IU/d, in their diet to prevent osteoporosis.22
Also encourage patients to exercise moderately for a half-hour daily, 5 days a week, to burn calories. Supplement nutritional counseling with behavioral therapy, focusing on changing eating patterns.23
CANCER PREVENTION
Many patients with chronic mental illness are not regularly screened for colon, cervical, breast, or other common early-stage cancers. In addition, their cancer rates are significantly higher than those of the general population.24
Ask men at the initial visit when they were last screened for colon or prostate cancer. Ask women when they were last screened for colon, cervical or breast cancer (Table 4). Ask again once yearly.
Colon cancer. Colonoscopy, done by a gastroenterologist, is indicated for patients age >50 every 5 to 10 years, depending on endoscopic findings. In-office fecal occult blood tests (FOBT), performed annually between colonoscopies, can identify patients who may need closer follow-up. You can do in-office FOBT or refer to a primary care physician.25
Cervical cancer is thought to be caused by human papilloma virus (HPV). Refer women with an intact cervix annually to a gynecologist or hospital clinic for a Pap smear, which usually includes testing for high-risk HPV if atypical cells are discovered. Some guidelines suggest decreasing screening frequency after several negative Pap smears for women in a monogamous sexual relationship.
Breast cancer affects 1 in 8 women, and having a first-degree relative with breast cancer increases the risk. Women should receive annual mammograms starting at age 40. The USPSTF notes that mammography’s benefits improve with increasing age between ages 40 and 70.26
Many hospital radiology departments or community health centers provide mammograms on a rotating schedule. Refer patients with abnormal findings to a general surgeon or breast center.
Table 4
Recommended intervals for cancer screening
| Type of cancer | Recommended test frequency |
|---|---|
| Colon | Asymptomatic persons age >50 should receive colonoscopy every 5 to 10 years, as directed by the gastroenterologist, and annual fecal occult blood tests |
| Cervical | Annual Pap smears for women who have ever been sexually active and still have a cervix |
| Breast | Mammography every 1 to 2 years after age 40 |
| Lung | Evidence does not support routine chest x-rays or sputum cytology in asymptomatic patients |
| Prostate | Refer men age >50 to primary care physician or hospital laboratory for PSA test; counsel patients about the results and treatment |
CASE CONTINUED: TESTING BEGINS
We schedule a battery of laboratory tests for Mrs. J at the local hospital, including a fasting plasma glucose test and lipid profile to gauge her cardiovascular risk and potential effects from olanzapine, and CBC and LFTs to check for adverse effects from oxcarbazepine.
We ask Mrs. J whether she engages in high-risk sexual activity, which she denies. She cannot recall her vaccination history, so we contact the primary care physician she had seen 5 years ago. Depending on her other comorbidities, housing situation, an early pneumococcal vaccine may be indicated.
We also suggest that Mrs. J quit smoking, but she appears to be at a pre-contemplative stage. We hope to promote a change in her attitude by discussing smoking cessation at each visit
To address Mrs. J’s obesity, we briefly review a dietary plan augmented with increased physical activity. She will bring a 3-day food diary to her next visit and promises to walk 30 minutes four to five times weekly. She says she enjoys mall walking with her children.
We strongly urge Mrs. J to schedule a mammogram, as she is past age 50 and says she has never received one. We try to refer her to a primary care physician to arrange a Pap smear and colonoscopy, but she resists, fearing the results. With continued education, exploration, and encouragement, we will briefly follow up with Mrs. J at each visit to ensure that she gets these needed tests (Box 2).
Check with patients at each visit to ensure they are following through on their test referrals. If they are not, find out why.
If the patient is procrastinating, try to uncover an underlying cause. If the patient says he or she is pressed for time, ask: “Are you going through stressful life events? Are you afraid the test will hurt or will reveal a serious disease? Did you have this test before? If so, did it make you uncomfortable?
Tell the patent, “I understand your concerns, but this test is important. You need to make it a higher priority.” To work through the patient’s resistance, start by educating him or her about preventable chronic diseases and screening or treatment resources. Then try problem-solving techniques, motivational interviewing, or dissecting cognitive distortions.
Collaboration with a case manager is key when managing an indigent mentally ill patient. Open communication, setting well-defined goals, and a clear understanding of each other’s treatment roles is crucial. Inform the case manager which target tasks, tests, or appointments the patient has agreed to. The case manager can use this information to help the patient navigate the health care system and encourage full participation in care
Finally, build a referral base for indigent and uninsured patients. Look for a nearby internist, gastroenterologist, and OB/GYN who accept uninsured patients.
- U.S. Preventative Services Task Force. Continually updated, evidence-based screening recommendations for a range of medical problems. www.ahrq.gov/clinic/uspstfix.htm.
- U.S. Public Health Service. Clinical practice guideline: treating tobacco use and dependence. www.surgeongeneral.gov/tobacco/treating_tobacco_use.pdf.
- Olanzapine • Zyprexa
- Oxcarbazepine • Trileptal
Dr. Moss recevies research/grant support from the National Institutes of Health and is a speaker for Janssen Pharmaceutica and Pfizer.
Mr. Brammer and Dr. White report no financial relationship with any company whose products are mentioned in this article, or with manufacturers of competing products.
1. Carney CP, Allen J, Doebbeling BN. Receipt of clinical preventive medical services among psychiatric patients. Psychiatr Serv 2002;53:1028-30.
2. Joukamaa M, Heliovaara M, Knekt P, et al. Mental disorders and cause-specific mortality. Br J Psychiatry 2001;179:498-502.
3. McCreadie RG. Scottish Schizophrenia Lifestyle Group. Diet, smoking and cardiovascular risk in people with schizophrenia: descriptive study. Br J Psychiatry 2003;183:534-9.
4. Cohn T, Prud’homme D, Streiner D, et al. Characterizing coronary heart disease risk in chronic schizophrenia: high prevalence of the metabolic syndrome. Can J Psychiatry 2004;49:753-60.
5. Hanson D, Gottesman I. Theories of schizophrenia: a geneticinflammatory-vascular synthesis. BMC Med Genet 2005;6:7.-
6. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289(19):2560-72.
7. Berg AO, Atkins D. U.S. Preventive Services Task Force: screening for lipid disorders in adults: recommendations and rationale. Am J Nurs 2002;102(6):p. 91-5.
8. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143-421.
9. Standards of medical care in diabetes. Diabetes Care 2005;28:S4-S36.
10. Clinical practice recommendations 2005. Diabetes Care 2005;28(Suppl 1):S1-S79.
11. Lasser K, Boyd JW, Woolhandler S, et al. Smoking and mental illness: a population-based prevalence study. JAMA 2000;284(20):2606-10.
12. Ockene IS, Miller NH. Cigarette smoking, cardiovascular disease, and stroke: a statement for healthcare professionals from the American Heart Association. American Heart Association Task Force on Risk Reduction. Circulation 1997;96:3243-7.
13. Fiore MC. U.S. Public Health Service clinical practice guideline: treating tobacco use and dependence. Respir Care 2000;45:1200-62.
14. Pellock JM, Willmore LJ. A rational guide to routine blood monitoring in patients receiving antiepileptic drugs. Neurology 1991;41:961-4.
15. Goodwin FK, Goldstein MA. Optimizing lithium treatment in bipolar disorder: a review of the literature and clinical recommendations. J Psychiatr Pract 2003;9:333-43.
16. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004;27:596-601.
17. Erbelding EJ, Hutton HE, Zenilman JM, et al. The prevalence of psychiatric disorders in sexually transmitted disease clinic patients and their association with sexually transmitted risk. Sex Transm Dis 2004;31:8-12.
18. Rein DB, Anderson LA, Irwin KL. Mental health disorders and sexually transmitted diseases in a privately insured population. Am J Manag Care 2004;10:917-24.
19. Screening for sexually transmitted diseases. U.S.Preventive Services Task Force, Washington, DC. Am Fam Physician 1990;42:691-702.
20. Daumit GL, Goldberg RW, Anthony C, et al. Physical activity patterns in adults with severe mental illness. J Nerv Ment Dis 2005;193:641-6.
21. Daumit GL, Clark JM, Steinwachs DM, et al. Prevalence and correlates of obesity in a community sample of individuals with severe and persistent mental illness. J Nerv Ment Dis 2003;191:799-805.
22. Hodgson SF, Watts NB, Bilezikian JP, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the prevention and treatment of postmenopausal osteoporosis: 2001 edition, with selected updates for 2003. Endocr Pract 2003;9:544-64.
23. Jakicic JM, Clark K, Coleman E, et al. American College of Sports Medicine. American College of Sports Medicine position stand. Appropriate intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc 2001;33:2145-56.
24. Lichtermann D, Ekelund J, Pukkala E, et al. Incidence of cancer among persons with schizophrenia and their relatives. Arch Gen Psychiatry 2001;58:573-8.
25. Colon cancer screening (USPSTF recommendation). U.S. Preventive Services Task Force. J Am Geriatr Soc 2000M;48:333-5.
26. Humphrey LL, Helfand M, Chan BK, Woolf SH. Breast cancer screening: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002;137(5 Part 1):347-60.
1. Carney CP, Allen J, Doebbeling BN. Receipt of clinical preventive medical services among psychiatric patients. Psychiatr Serv 2002;53:1028-30.
2. Joukamaa M, Heliovaara M, Knekt P, et al. Mental disorders and cause-specific mortality. Br J Psychiatry 2001;179:498-502.
3. McCreadie RG. Scottish Schizophrenia Lifestyle Group. Diet, smoking and cardiovascular risk in people with schizophrenia: descriptive study. Br J Psychiatry 2003;183:534-9.
4. Cohn T, Prud’homme D, Streiner D, et al. Characterizing coronary heart disease risk in chronic schizophrenia: high prevalence of the metabolic syndrome. Can J Psychiatry 2004;49:753-60.
5. Hanson D, Gottesman I. Theories of schizophrenia: a geneticinflammatory-vascular synthesis. BMC Med Genet 2005;6:7.-
6. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289(19):2560-72.
7. Berg AO, Atkins D. U.S. Preventive Services Task Force: screening for lipid disorders in adults: recommendations and rationale. Am J Nurs 2002;102(6):p. 91-5.
8. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143-421.
9. Standards of medical care in diabetes. Diabetes Care 2005;28:S4-S36.
10. Clinical practice recommendations 2005. Diabetes Care 2005;28(Suppl 1):S1-S79.
11. Lasser K, Boyd JW, Woolhandler S, et al. Smoking and mental illness: a population-based prevalence study. JAMA 2000;284(20):2606-10.
12. Ockene IS, Miller NH. Cigarette smoking, cardiovascular disease, and stroke: a statement for healthcare professionals from the American Heart Association. American Heart Association Task Force on Risk Reduction. Circulation 1997;96:3243-7.
13. Fiore MC. U.S. Public Health Service clinical practice guideline: treating tobacco use and dependence. Respir Care 2000;45:1200-62.
14. Pellock JM, Willmore LJ. A rational guide to routine blood monitoring in patients receiving antiepileptic drugs. Neurology 1991;41:961-4.
15. Goodwin FK, Goldstein MA. Optimizing lithium treatment in bipolar disorder: a review of the literature and clinical recommendations. J Psychiatr Pract 2003;9:333-43.
16. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004;27:596-601.
17. Erbelding EJ, Hutton HE, Zenilman JM, et al. The prevalence of psychiatric disorders in sexually transmitted disease clinic patients and their association with sexually transmitted risk. Sex Transm Dis 2004;31:8-12.
18. Rein DB, Anderson LA, Irwin KL. Mental health disorders and sexually transmitted diseases in a privately insured population. Am J Manag Care 2004;10:917-24.
19. Screening for sexually transmitted diseases. U.S.Preventive Services Task Force, Washington, DC. Am Fam Physician 1990;42:691-702.
20. Daumit GL, Goldberg RW, Anthony C, et al. Physical activity patterns in adults with severe mental illness. J Nerv Ment Dis 2005;193:641-6.
21. Daumit GL, Clark JM, Steinwachs DM, et al. Prevalence and correlates of obesity in a community sample of individuals with severe and persistent mental illness. J Nerv Ment Dis 2003;191:799-805.
22. Hodgson SF, Watts NB, Bilezikian JP, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the prevention and treatment of postmenopausal osteoporosis: 2001 edition, with selected updates for 2003. Endocr Pract 2003;9:544-64.
23. Jakicic JM, Clark K, Coleman E, et al. American College of Sports Medicine. American College of Sports Medicine position stand. Appropriate intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc 2001;33:2145-56.
24. Lichtermann D, Ekelund J, Pukkala E, et al. Incidence of cancer among persons with schizophrenia and their relatives. Arch Gen Psychiatry 2001;58:573-8.
25. Colon cancer screening (USPSTF recommendation). U.S. Preventive Services Task Force. J Am Geriatr Soc 2000M;48:333-5.
26. Humphrey LL, Helfand M, Chan BK, Woolf SH. Breast cancer screening: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002;137(5 Part 1):347-60.
When patients can’t sleep
Careful investigation can often reveal insomnia’s cause1—whether a medical or psychiatric condition or poor sleep habits. Understanding why patients can’t sleep is key to effective therapy.
Insomnia is associated with increased risk of accidents, work-related difficulties, and relationship problems.2 Long-term sleeplessness may even increase risk of new psychiatric disorders—most notably major depression.3
Primary Insomnia
DSM-IV-TR criteria for primary insomnia include:4
- For at least 1 month, the patient’s main complaint has been trouble going to sleep, staying asleep, or feeling unrested.
- The insomnia or resulting daytime fatigue causes clinically important distress or impairs work, social, or personal functioning.
- The insomnia does not occur solely in the course of a breathing-related or circadian rhythm sleep disorder, a parasomnia, or as part of another mental disorder such as delirium, generalized anxiety disorder, or major depressive disorder.
Adjustment sleep disorder. Acute emotional stressors—such as bereavement, job loss, or hospitalization—can cause insomnia or daytime sleepiness. Symptoms typically remit soon after the stressors abate, so this insomnia usually lasts a few days (acute) to a few months (short-term). It can also become chronic, lasting3 months or longer.
Psychophysiologic insomnia. Once insomnia begins—regardless of its cause—sleep problems may persist well after precipitating factors resolve. The mechanism may be related to somatized tension and learned sleep-preventing associations (trying too hard to sleep and conditioned arousal to the bedroom). Thus, short-term insomnia may develop into long-term, chronic difficulty with recurring episodes or a constant, daily pattern of insomnia.
Treatment for both adjustment sleep disorder and psychophysiologic insomnia with behavioral therapies and hypnotics6 is warranted if:
- sleepiness and fatigue interfere with daytime function
- the patient is significantly distressed
- a pattern of recurring episodes develops.5
Psychiatric Disorders and Insomnia
Depression. Up to 80% of depressed persons experience insomnia, although no one sleep pattern seems typical.7 Depression may be associated with:
- difficulties in falling asleep
- interrupted nocturnal sleep
- early morning awakening.
Some patients experience panic symptoms while sleeping, possibly in association with mild hypercapnia. Those patients tend to have earlier onset of panic disorder and a higher likelihood of comorbid mood and other anxiety disorders.8
In patients with PTSD, disturbed sleep continuity and increased REM phasic activity—such as eye movements—are directly correlated with PTSD symptom severity. Nightmares and disturbed REM sleep are hallmarks of PTSD.9
Workup of Sleep Complaints
The patient history is an important part of the evaluation and treatment of insomnia and other sleep disturbances (Algorithm).12
Acute. Many short-term insomnias—lasting a few weeks or less—are caused by situational stressors, circadian rhythm changes, or poor sleep hygiene (Table 1).1 A logical approach is to begin sleep hygiene measures and explore the patient’s life situation to uncover what might be causing the insomnia. Hypnotic agents may be considered if insomnia is associated with daytime sleepiness or occupational impairment or if it seems to be escalating and your assessment indicates that it is a primary condition.
Chronic. For longer-term insomnias—lasting more than a few months—consider a more thorough evaluation, including medical and psychiatric history, physical examination, and mental status examination. A differential assessment can be made on the basis of whether a patient has difficulty falling or staying asleep (Table 1). Ask about cardinal symptoms of disorders associated with insomnia, including:
- snoring or breathing pauses during sleep (sleep apnea syndrome)
- restlessness or twitching in the lower extremities (PLMD/RLS).
Carefully review the patient’s weekday and weekend sleep patterns, bedtime habits, sleep hygiene habits, and substance and medication use.
Sleep clinic referrals. Consider an evaluation by a sleep disorders center when the diagnosis remains unclear or treatment of the presumed condition fails after a reasonable time.
Table 1
Possible causes of sleep complaints
| Acute, transient | Recent or recurring stress | |
| Change in sleeping environment | ||
| Acute illness or injury | ||
| New medications | ||
| Jet lag or shift change | ||
| Chronic | Difficulty staying asleep | Difficulty falling asleep |
| Medications | Poor sleep hygiene | |
| Drug or alcohol use | Conditioned insomnia | |
| Psychiatric disorder | Restless legs syndrome | |
| Medical disorder | Circadian rhythm disorder | |
| Sleep-disordered breathing | Advanced sleep-phase syndrome | |
| Periodic limb movement disorder | ||
| Restless legs syndrome | ||
| Source: Adapted and reprinted with permission from reference 13 | ||
Behavioral Treatments
Behavioral treatments—with or without hypnotics—are appropriate for many insomnia complaints, including adjustment sleep disorder and psychophysiologic insomnia. Behavioral measures may work more slowly than drug therapy, but their effects have been shown to last longer in patients with primary insomnia. It may be useful to start with both hypnotic and behavioral treatments and withdraw the hypnotic after behavioral measures take effect.
Sleep hygiene. Many individuals unknowingly engage in habits that impair sleep. Those with insomnia, for example, often try to compensate for lost sleep by staying in bed later in the morning or by napping, which further fragment nocturnal sleep. Advise these patients to adhere to a regular awakening time—regardless of how long they slept the night before—and to avoid naps. Other tips for getting a good night’s sleep are outlined in Table 2.14
Caffeine has a plasma half-life of 3 to 7 hours, although individual sensitivity varies widely and caffeine’s erratic absorption can prolong its effects. Advise patients with insomnia to avoid caffeine-containing beverages—including coffee, tea, and soft drinks—after noon.
Relaxation training. Muscle tension can be reduced through techniques such as electromyography (EMG) biofeedback, abdominal breathing exercises, or progressive muscle relaxation. Relaxation training is usually effective within a few weeks.
Psychological counseling. Counseling can help identify and dispel tension-producing thoughts that may be disrupting sleep, such as preoccupation with unpleasant work experiences or school examinations. Reassurance may help patients overcome fears about sleeplessness; suggest that they deal with anxiety-producing thoughts during counseling sessions and at times other than bedtime.
Table 2
How to get a good night’s sleep
|
Prescribing Hypnotics
Sedative-hypnotics are indicated primarily for short-term insomnia management. Most are used at bedtime until insomnia dissipates or the physician advises the patient to take a break.
Treatment principles. Because many insomnias are recurrent, prolonged hypnotic treatment given in short bouts is often optimal. Longer treatment—months to years—is clearly needed by a few patients with chronic insomnia. In these cases, carefully monitor for tolerance, as manifested by dosage escalation. Hypnotic treatment is generally not suitable for patients with drug abuse or dependence histories.
Although chloral hydrate and barbiturates are effective hypnotics, adverse effects limit their safety and usefulness. Benzodiazepines and more recently introduced agents have milder side-effect profiles (Table 3). Choose agents based on the patient’s situation, preferences, and effects of prior trials with similar agents. Guidelines for hypnotics discourage chronic use to minimize abuse, misuse, and habituation (Table 4).
Elimination half-life is one of the most important pharmacological properties that differentiates the hypnotics from each other:15
- longer half-life: flurazepam, quazepam
- intermediate half-life: estazolam, temazepam, eszopiclone
- short half-life: triazolam, zolpidem, zolpidem ER, zaleplon, ramelteon.
Benzodiazepine receptor agonists. Of the all the drugs in class, zalepon—because of its ultra-short half-life—is least likely to cause residual daytime effects when administered at bedtime. At 10-mg doses, its side effects seem to last no more than 4 hours after administration. Zaleplon can be safely taken after nocturnal awakenings if the patient remains in bed 4 hours or longer after taking it.17
An ultra-short half life is less desirable for patients with difficulty with sleep initiation and discontinuous sleep throughout the night. For them, longer elimination half-life agents—such as zolpidem, zolpidem extended release (ER), and eszopiclone—may be more predictably effective for the entire night.18
Short half-life hypnotics do not offer anxiolysis for patients with daytime anxiety, as the longer half-life agents do.
Zolpidem ER and eszopiclone do not have a limitation imposed on duration of use. Although zolpidem ER has not been investigated in controlled trials greater than 3 weeks, eszopiclone was evaluated during a 6-month study that demonstrated lack of tolerance during the entire period, and lack of rebound after rapid discontinuation.19 Eszopiclone is the only hypnotic indicated for long-term (lasting > 3 weeks) insomnia.
Melatonin receptor agonists. Ramelteon’s activity at MT1 and MT2 receptors is believed to contribute to its sleep-promoting properties. This agent has been found to reduce sleep latency,20,21 and it is indicated to treat insomnia characterized by sleep-onset delays. Although controlled, longterm studies are lacking, ramelteon does not have a limit on duration of use. It demonstrated a lack of abuse liability when compared with triazolam and placebo in subjects with a history of sedative/hypnotic or anxiolytic drug abuse.22
Tolerance and rebound. Tolerance can develop after repeated dosing with benzodiazepines—primarily triazolam—and rebound insomnia can follow abrupt discontinuation. Both can be minimized by using benzodiazepines at the lowest effective dosages and for brief periods. Gradual tapering when discontinuing the drug can help control rebound.
Tolerance and rebound seem to be less of a concern with the newer hypnotics than with benzodiazepines, as shown by controlled studies of eszopiclone19, zolpidem23, and zaleplon.24 However, periodic re-evaluation is still the prudent clinical standard for hypnotics prescribed over long periods of time.
Table 3
Actions and available doses of common hypnotics
| Class/drug | Onset of action | Half-life (hrs) | Active metabolites | Doses (mg) |
|---|---|---|---|---|
| Benzodiazepines | ||||
| Flurazepam | Rapid | 40 to 250 | Yes | 15, 30 |
| Quazepam | Rapid | 40 to 250 | Yes | 7.5, 15 |
| Estazolam | Rapid | 10 to 24 | Yes | 0.5, 1, 2 |
| Temazepam | Intermediate | 8 to 22 | No | 7.5, 15 |
| Triazolam | Rapid | No | 0.125, 0.25, 0.5 | |
| Imidazopyridine | ||||
| Zolpidem | Rapid | 2.5 | No | 5, 10 |
| Zolpidem ER | Rapid | 2.5 | No | 6.25, 12.5 |
| Pyrazolopyrimidines | ||||
| Zaleplon | Rapid | 1 | No | 5, 10, 20 |
| Cyclopyrrolone | ||||
| Eszopiclone | Rapid | 6 | Minor | 2,3 |
| Melatonin receptor agonist | ||||
| Ramelteon | Rapid | 1 to 2.6 | No | 8 |
Guidelines for safe use of hypnotics
|
Nonhypnotic Sleep AIDS
Sedating antidepressants. Some physicians prescribe low doses of sedating antidepressants to control insomnia, a practice supported by controlled clinical trials of some tricyclic antidepressants (TCAs) such as doxepin,25 trazodone,26 and trimipramine.27 Some physicians also advocate using more-sedating antidepressants—at dosages needed to treat depression—to control insomnia in depressed patients.
Evening dosing can minimize daytime sedation. If you choose an activating antidepressant, the potential side effect of insomnia can be managed by judicious use of hypnotic agents. Little is known about antidepressants’ effects on sleep quality after the first 6 to 8 weeks of treatment.28
Although possibly helpful as sleep aids, TCAs are associated with anticholinergic effects such as dry mouth, urinary flow difficulties, and cardiac dysrhythmias.
Alcohol. Patients with insomnia sometimes selfmedicate with alcohol at bedtime because it enhances sleepiness and induces a more rapid sleep onset.29 Drinking a “nightcap” is a poor choice, however, because alcohol can impair sleep quality, resulting in daytime somnolence. Alcohol is also associated with rapid development of tolerance.
Antihistamines and over-the-counter products whose main active ingredients are antihistamines—such as doxylamine and diphenhydramine—are used for insomnia and may help individuals fall asleep and stay asleep. However, antihistamine use is complicated by unpredictable efficacy and side effects such as daytime sedation, confusion, and systemic anticholinergic effects.30
Melatonin is a nonprescription dietary supplement used in dosages of 0.5 to 3,000 mg. Anecdotal reports indicate it may be efficacious in certain subtypes of insomnia—such as shift work, jet lag, blindness, delayed sleep phase syndrome—and in older patients with sleep complaints.
Melatonin’s efficacy has not been established conclusively, however, and concerns have been expressed regarding the purity of over-the-counter preparations and possible coronary artery tissue stimulation, as observed in animal studies.
Related resources
- American Academy of Sleep Medicine. Sleep logs, patient education materials. www.aasmnet.org
- American Sleep Apnea Association. National Sleep Foundation. www.sleepapnea.org
- Doxepin • Inequan
- Estazolam • Prosom
- Eszopiclone • Lunesta
- Flurazepam • Dalmane
- Quazepam • Doral
- Ramelteon • Rozerem
- Temazepam • Restoril
- Trazodone • Desyrel
- Triazolam • Halcion
- Trimipramine • Surmontil
- Zaleplon • Sonata
- Zolpidem • Ambien
Dr. Doghramji receives research grant support from Cephalon, GlaxoSmithKline, Merck & Co., and Sanofi-Synthelabo
1. Sateia MJ, Doghramji K, Hauri PJ, Morin CM. Evaluation of chronic insomnia. Sleep 2000;23:243-81.
2. Zammit GK, Weiner J, Damato N, et al. Quality of life in people with insomnia. Sleep 1999;22(suppl 2):S379-385.
3. Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. JAMA 1989;262:1479-84.
4. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington DC: American Psychiatric Association, 2000.
5. International Classification of Sleep Disorders: Diagnostic and Coding Manual, (2nd ed.) Westchester, IL: The American Academy of Sleep Medicine, 2005.
6. Spielman AJ, Glovinsky P. The varied nature of insomnia. In: Hauri P (ed). Case studies in insomnia. New York: Plenum Press, 1991;1:15.-
7. Reynolds CF, III, Kupfer DJ. Sleep research in affective illness: state of the art circa 1987. Sleep 1987;10:199-215.
8. Labbate LA, Pollack MH, Otto MW, et al. Sleep panic attacks: an association with childhood anxiety and adult psychopathology. Biol Psychiatry 1994;43:840-2.
9. Ross RJ, Ball WA, Sullivan KA, et al. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry 1989;146:697-707.
10. Winokur A, Reynolds CF. The effects of antidepressants on sleep physiology. Primary Psychiatry 1994;6:22-7.
11. Gillin JC, Rapaport M, Erman MK, et al. A comparison of nefazodone and fluoxetine on mood and on objective, subjective, and clinician-rated measures of sleep in depressed patients: a double blind, 8-week clinical trial. J Clin Psychiatry 1997;58(5):185-92.
12. Erman M. Clinical update. Diagnosis of insomnia in the primary care practice. Available at: http://www.medscape.com/viewarticle/478849. Accessed Jan. 18, 2005.
13. Rajput V, Bromley SM. Chronic insomnia: A practical review. Am Fam Physician 1999;60:1431-8.
14. Doghramji K. The evaluation and management of sleep disorders. In: Stoudemire A (ed). Clinical psychiatry for medical students (3rd ed). Philadelphia: JB Lippincott, 1998;783-818.
15. Gillin JC. The long and short of sleeping pills. N Engl J Med 1991;324:1735-7.
16. Lunesta (eszopiclone). Approved labeling text. Marlborough, MA: Sepracor, Inc., 2005.
17. Corser B, Mayleben D, Doghramji K, et al. No next-day residual sedation four hours after middle-of-the-night treatment with zaleplon. Sleep 2000;23(suppl 2):abstract 309.
18. Holm KJ, Goa KL. Zolpidem: an update of its pharmacology, therapeutic efficacy and tolerability in the treatment of insomnia. Drugs 2000;59:865-89.
19. Scarf MB, Roth T, Vogel GW, Walsh JK. A multicenter, placebocontrolled study evaluating zolpidem in the treatment of chronic insomnia. J Clin Psychiatry 1994;55:192-9.
20. Roth T, Stubbs C, Walsh JK. Ramelteon (TAK-375), a selective MT1/MT2-receptor agonist, reduces sleep latency to persistent sleep in a model of transient insomnia related to a novel sleep environment. Sleep 2005;28:303-7.
21. Roth T, Seiden D, Sainati S, et al. Phase III outpatient trial of ramelteon for the treatment of chronic insomniain elderly patients (poster presentation). Orlando, FL: American Geriatric Society annual meeting, 2005.
22. Griffiths R, Seuss P. Ramelteonand triazolam in humans: behavioral effectsand abuse potential (poster presentation). Atlanta, GA: American Psychiatric Association annual meeting, 2005.
23. Elie R, Ruther E, Farr I, et al. Sleep latency is shortened during 4 weeks of treatment with zaleplon, a novel nonbenzodiazepine hypnotic. Zaleplon Clinical Study Group. J Clin Psychiatry 1999;60:536-44.
24. Krystal A, Walsh J, Laska E, et al. Sustained efficacy of eszopiclone over six months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep 2003;26:793-9.
25. Hajak G, Rodenbeck A, Voderholzer U, et al. Doxepin in the treatment of primary insomnia: a placebo-controlled, double-blind, polysomnographic study. J Clin Psychiatry 2001;62:453-63.
26. Walsh JK, Erman M, Erwin CE, et al. Subjective hypnotic efficacy of trazodone and zolpidem in DSM-III-R primary insomnia. Hum Psychopharmacol 1998;13(3):191-8.
27. Hohagen F, Monero RF, Weiss E, et al. Treatment of primary insomnia with trimipramine: an alternative to benzodiazepine hypnotics? Eur Arch Psychiatry Clin Neurosci 1994;244(2):65-72.
28. Thase ME. Antidepressant treatment of the depressed patient with insomnia. J Clin Psychiatry 1999;60(suppl 17):28-31.
29. Johnson EO, Roehrs T, Roth T, Breslau N. Epidemiology of alcohol and medication as aids to sleep in early adulthood. Sleep 1998;21:178-86.
30. Gengo F, Gabos C, Miller JK. The pharmacodynamics of diphenhydramine-induced drowsiness and changes in mental performance. Clin Pharmacol Ther 1989;45:15-21.
Careful investigation can often reveal insomnia’s cause1—whether a medical or psychiatric condition or poor sleep habits. Understanding why patients can’t sleep is key to effective therapy.
Insomnia is associated with increased risk of accidents, work-related difficulties, and relationship problems.2 Long-term sleeplessness may even increase risk of new psychiatric disorders—most notably major depression.3
Primary Insomnia
DSM-IV-TR criteria for primary insomnia include:4
- For at least 1 month, the patient’s main complaint has been trouble going to sleep, staying asleep, or feeling unrested.
- The insomnia or resulting daytime fatigue causes clinically important distress or impairs work, social, or personal functioning.
- The insomnia does not occur solely in the course of a breathing-related or circadian rhythm sleep disorder, a parasomnia, or as part of another mental disorder such as delirium, generalized anxiety disorder, or major depressive disorder.
Adjustment sleep disorder. Acute emotional stressors—such as bereavement, job loss, or hospitalization—can cause insomnia or daytime sleepiness. Symptoms typically remit soon after the stressors abate, so this insomnia usually lasts a few days (acute) to a few months (short-term). It can also become chronic, lasting3 months or longer.
Psychophysiologic insomnia. Once insomnia begins—regardless of its cause—sleep problems may persist well after precipitating factors resolve. The mechanism may be related to somatized tension and learned sleep-preventing associations (trying too hard to sleep and conditioned arousal to the bedroom). Thus, short-term insomnia may develop into long-term, chronic difficulty with recurring episodes or a constant, daily pattern of insomnia.
Treatment for both adjustment sleep disorder and psychophysiologic insomnia with behavioral therapies and hypnotics6 is warranted if:
- sleepiness and fatigue interfere with daytime function
- the patient is significantly distressed
- a pattern of recurring episodes develops.5
Psychiatric Disorders and Insomnia
Depression. Up to 80% of depressed persons experience insomnia, although no one sleep pattern seems typical.7 Depression may be associated with:
- difficulties in falling asleep
- interrupted nocturnal sleep
- early morning awakening.
Some patients experience panic symptoms while sleeping, possibly in association with mild hypercapnia. Those patients tend to have earlier onset of panic disorder and a higher likelihood of comorbid mood and other anxiety disorders.8
In patients with PTSD, disturbed sleep continuity and increased REM phasic activity—such as eye movements—are directly correlated with PTSD symptom severity. Nightmares and disturbed REM sleep are hallmarks of PTSD.9
Workup of Sleep Complaints
The patient history is an important part of the evaluation and treatment of insomnia and other sleep disturbances (Algorithm).12
Acute. Many short-term insomnias—lasting a few weeks or less—are caused by situational stressors, circadian rhythm changes, or poor sleep hygiene (Table 1).1 A logical approach is to begin sleep hygiene measures and explore the patient’s life situation to uncover what might be causing the insomnia. Hypnotic agents may be considered if insomnia is associated with daytime sleepiness or occupational impairment or if it seems to be escalating and your assessment indicates that it is a primary condition.
Chronic. For longer-term insomnias—lasting more than a few months—consider a more thorough evaluation, including medical and psychiatric history, physical examination, and mental status examination. A differential assessment can be made on the basis of whether a patient has difficulty falling or staying asleep (Table 1). Ask about cardinal symptoms of disorders associated with insomnia, including:
- snoring or breathing pauses during sleep (sleep apnea syndrome)
- restlessness or twitching in the lower extremities (PLMD/RLS).
Carefully review the patient’s weekday and weekend sleep patterns, bedtime habits, sleep hygiene habits, and substance and medication use.
Sleep clinic referrals. Consider an evaluation by a sleep disorders center when the diagnosis remains unclear or treatment of the presumed condition fails after a reasonable time.
Table 1
Possible causes of sleep complaints
| Acute, transient | Recent or recurring stress | |
| Change in sleeping environment | ||
| Acute illness or injury | ||
| New medications | ||
| Jet lag or shift change | ||
| Chronic | Difficulty staying asleep | Difficulty falling asleep |
| Medications | Poor sleep hygiene | |
| Drug or alcohol use | Conditioned insomnia | |
| Psychiatric disorder | Restless legs syndrome | |
| Medical disorder | Circadian rhythm disorder | |
| Sleep-disordered breathing | Advanced sleep-phase syndrome | |
| Periodic limb movement disorder | ||
| Restless legs syndrome | ||
| Source: Adapted and reprinted with permission from reference 13 | ||
Behavioral Treatments
Behavioral treatments—with or without hypnotics—are appropriate for many insomnia complaints, including adjustment sleep disorder and psychophysiologic insomnia. Behavioral measures may work more slowly than drug therapy, but their effects have been shown to last longer in patients with primary insomnia. It may be useful to start with both hypnotic and behavioral treatments and withdraw the hypnotic after behavioral measures take effect.
Sleep hygiene. Many individuals unknowingly engage in habits that impair sleep. Those with insomnia, for example, often try to compensate for lost sleep by staying in bed later in the morning or by napping, which further fragment nocturnal sleep. Advise these patients to adhere to a regular awakening time—regardless of how long they slept the night before—and to avoid naps. Other tips for getting a good night’s sleep are outlined in Table 2.14
Caffeine has a plasma half-life of 3 to 7 hours, although individual sensitivity varies widely and caffeine’s erratic absorption can prolong its effects. Advise patients with insomnia to avoid caffeine-containing beverages—including coffee, tea, and soft drinks—after noon.
Relaxation training. Muscle tension can be reduced through techniques such as electromyography (EMG) biofeedback, abdominal breathing exercises, or progressive muscle relaxation. Relaxation training is usually effective within a few weeks.
Psychological counseling. Counseling can help identify and dispel tension-producing thoughts that may be disrupting sleep, such as preoccupation with unpleasant work experiences or school examinations. Reassurance may help patients overcome fears about sleeplessness; suggest that they deal with anxiety-producing thoughts during counseling sessions and at times other than bedtime.
Table 2
How to get a good night’s sleep
|
Prescribing Hypnotics
Sedative-hypnotics are indicated primarily for short-term insomnia management. Most are used at bedtime until insomnia dissipates or the physician advises the patient to take a break.
Treatment principles. Because many insomnias are recurrent, prolonged hypnotic treatment given in short bouts is often optimal. Longer treatment—months to years—is clearly needed by a few patients with chronic insomnia. In these cases, carefully monitor for tolerance, as manifested by dosage escalation. Hypnotic treatment is generally not suitable for patients with drug abuse or dependence histories.
Although chloral hydrate and barbiturates are effective hypnotics, adverse effects limit their safety and usefulness. Benzodiazepines and more recently introduced agents have milder side-effect profiles (Table 3). Choose agents based on the patient’s situation, preferences, and effects of prior trials with similar agents. Guidelines for hypnotics discourage chronic use to minimize abuse, misuse, and habituation (Table 4).
Elimination half-life is one of the most important pharmacological properties that differentiates the hypnotics from each other:15
- longer half-life: flurazepam, quazepam
- intermediate half-life: estazolam, temazepam, eszopiclone
- short half-life: triazolam, zolpidem, zolpidem ER, zaleplon, ramelteon.
Benzodiazepine receptor agonists. Of the all the drugs in class, zalepon—because of its ultra-short half-life—is least likely to cause residual daytime effects when administered at bedtime. At 10-mg doses, its side effects seem to last no more than 4 hours after administration. Zaleplon can be safely taken after nocturnal awakenings if the patient remains in bed 4 hours or longer after taking it.17
An ultra-short half life is less desirable for patients with difficulty with sleep initiation and discontinuous sleep throughout the night. For them, longer elimination half-life agents—such as zolpidem, zolpidem extended release (ER), and eszopiclone—may be more predictably effective for the entire night.18
Short half-life hypnotics do not offer anxiolysis for patients with daytime anxiety, as the longer half-life agents do.
Zolpidem ER and eszopiclone do not have a limitation imposed on duration of use. Although zolpidem ER has not been investigated in controlled trials greater than 3 weeks, eszopiclone was evaluated during a 6-month study that demonstrated lack of tolerance during the entire period, and lack of rebound after rapid discontinuation.19 Eszopiclone is the only hypnotic indicated for long-term (lasting > 3 weeks) insomnia.
Melatonin receptor agonists. Ramelteon’s activity at MT1 and MT2 receptors is believed to contribute to its sleep-promoting properties. This agent has been found to reduce sleep latency,20,21 and it is indicated to treat insomnia characterized by sleep-onset delays. Although controlled, longterm studies are lacking, ramelteon does not have a limit on duration of use. It demonstrated a lack of abuse liability when compared with triazolam and placebo in subjects with a history of sedative/hypnotic or anxiolytic drug abuse.22
Tolerance and rebound. Tolerance can develop after repeated dosing with benzodiazepines—primarily triazolam—and rebound insomnia can follow abrupt discontinuation. Both can be minimized by using benzodiazepines at the lowest effective dosages and for brief periods. Gradual tapering when discontinuing the drug can help control rebound.
Tolerance and rebound seem to be less of a concern with the newer hypnotics than with benzodiazepines, as shown by controlled studies of eszopiclone19, zolpidem23, and zaleplon.24 However, periodic re-evaluation is still the prudent clinical standard for hypnotics prescribed over long periods of time.
Table 3
Actions and available doses of common hypnotics
| Class/drug | Onset of action | Half-life (hrs) | Active metabolites | Doses (mg) |
|---|---|---|---|---|
| Benzodiazepines | ||||
| Flurazepam | Rapid | 40 to 250 | Yes | 15, 30 |
| Quazepam | Rapid | 40 to 250 | Yes | 7.5, 15 |
| Estazolam | Rapid | 10 to 24 | Yes | 0.5, 1, 2 |
| Temazepam | Intermediate | 8 to 22 | No | 7.5, 15 |
| Triazolam | Rapid | No | 0.125, 0.25, 0.5 | |
| Imidazopyridine | ||||
| Zolpidem | Rapid | 2.5 | No | 5, 10 |
| Zolpidem ER | Rapid | 2.5 | No | 6.25, 12.5 |
| Pyrazolopyrimidines | ||||
| Zaleplon | Rapid | 1 | No | 5, 10, 20 |
| Cyclopyrrolone | ||||
| Eszopiclone | Rapid | 6 | Minor | 2,3 |
| Melatonin receptor agonist | ||||
| Ramelteon | Rapid | 1 to 2.6 | No | 8 |
Guidelines for safe use of hypnotics
|
Nonhypnotic Sleep AIDS
Sedating antidepressants. Some physicians prescribe low doses of sedating antidepressants to control insomnia, a practice supported by controlled clinical trials of some tricyclic antidepressants (TCAs) such as doxepin,25 trazodone,26 and trimipramine.27 Some physicians also advocate using more-sedating antidepressants—at dosages needed to treat depression—to control insomnia in depressed patients.
Evening dosing can minimize daytime sedation. If you choose an activating antidepressant, the potential side effect of insomnia can be managed by judicious use of hypnotic agents. Little is known about antidepressants’ effects on sleep quality after the first 6 to 8 weeks of treatment.28
Although possibly helpful as sleep aids, TCAs are associated with anticholinergic effects such as dry mouth, urinary flow difficulties, and cardiac dysrhythmias.
Alcohol. Patients with insomnia sometimes selfmedicate with alcohol at bedtime because it enhances sleepiness and induces a more rapid sleep onset.29 Drinking a “nightcap” is a poor choice, however, because alcohol can impair sleep quality, resulting in daytime somnolence. Alcohol is also associated with rapid development of tolerance.
Antihistamines and over-the-counter products whose main active ingredients are antihistamines—such as doxylamine and diphenhydramine—are used for insomnia and may help individuals fall asleep and stay asleep. However, antihistamine use is complicated by unpredictable efficacy and side effects such as daytime sedation, confusion, and systemic anticholinergic effects.30
Melatonin is a nonprescription dietary supplement used in dosages of 0.5 to 3,000 mg. Anecdotal reports indicate it may be efficacious in certain subtypes of insomnia—such as shift work, jet lag, blindness, delayed sleep phase syndrome—and in older patients with sleep complaints.
Melatonin’s efficacy has not been established conclusively, however, and concerns have been expressed regarding the purity of over-the-counter preparations and possible coronary artery tissue stimulation, as observed in animal studies.
Related resources
- American Academy of Sleep Medicine. Sleep logs, patient education materials. www.aasmnet.org
- American Sleep Apnea Association. National Sleep Foundation. www.sleepapnea.org
- Doxepin • Inequan
- Estazolam • Prosom
- Eszopiclone • Lunesta
- Flurazepam • Dalmane
- Quazepam • Doral
- Ramelteon • Rozerem
- Temazepam • Restoril
- Trazodone • Desyrel
- Triazolam • Halcion
- Trimipramine • Surmontil
- Zaleplon • Sonata
- Zolpidem • Ambien
Dr. Doghramji receives research grant support from Cephalon, GlaxoSmithKline, Merck & Co., and Sanofi-Synthelabo
Careful investigation can often reveal insomnia’s cause1—whether a medical or psychiatric condition or poor sleep habits. Understanding why patients can’t sleep is key to effective therapy.
Insomnia is associated with increased risk of accidents, work-related difficulties, and relationship problems.2 Long-term sleeplessness may even increase risk of new psychiatric disorders—most notably major depression.3
Primary Insomnia
DSM-IV-TR criteria for primary insomnia include:4
- For at least 1 month, the patient’s main complaint has been trouble going to sleep, staying asleep, or feeling unrested.
- The insomnia or resulting daytime fatigue causes clinically important distress or impairs work, social, or personal functioning.
- The insomnia does not occur solely in the course of a breathing-related or circadian rhythm sleep disorder, a parasomnia, or as part of another mental disorder such as delirium, generalized anxiety disorder, or major depressive disorder.
Adjustment sleep disorder. Acute emotional stressors—such as bereavement, job loss, or hospitalization—can cause insomnia or daytime sleepiness. Symptoms typically remit soon after the stressors abate, so this insomnia usually lasts a few days (acute) to a few months (short-term). It can also become chronic, lasting3 months or longer.
Psychophysiologic insomnia. Once insomnia begins—regardless of its cause—sleep problems may persist well after precipitating factors resolve. The mechanism may be related to somatized tension and learned sleep-preventing associations (trying too hard to sleep and conditioned arousal to the bedroom). Thus, short-term insomnia may develop into long-term, chronic difficulty with recurring episodes or a constant, daily pattern of insomnia.
Treatment for both adjustment sleep disorder and psychophysiologic insomnia with behavioral therapies and hypnotics6 is warranted if:
- sleepiness and fatigue interfere with daytime function
- the patient is significantly distressed
- a pattern of recurring episodes develops.5
Psychiatric Disorders and Insomnia
Depression. Up to 80% of depressed persons experience insomnia, although no one sleep pattern seems typical.7 Depression may be associated with:
- difficulties in falling asleep
- interrupted nocturnal sleep
- early morning awakening.
Some patients experience panic symptoms while sleeping, possibly in association with mild hypercapnia. Those patients tend to have earlier onset of panic disorder and a higher likelihood of comorbid mood and other anxiety disorders.8
In patients with PTSD, disturbed sleep continuity and increased REM phasic activity—such as eye movements—are directly correlated with PTSD symptom severity. Nightmares and disturbed REM sleep are hallmarks of PTSD.9
Workup of Sleep Complaints
The patient history is an important part of the evaluation and treatment of insomnia and other sleep disturbances (Algorithm).12
Acute. Many short-term insomnias—lasting a few weeks or less—are caused by situational stressors, circadian rhythm changes, or poor sleep hygiene (Table 1).1 A logical approach is to begin sleep hygiene measures and explore the patient’s life situation to uncover what might be causing the insomnia. Hypnotic agents may be considered if insomnia is associated with daytime sleepiness or occupational impairment or if it seems to be escalating and your assessment indicates that it is a primary condition.
Chronic. For longer-term insomnias—lasting more than a few months—consider a more thorough evaluation, including medical and psychiatric history, physical examination, and mental status examination. A differential assessment can be made on the basis of whether a patient has difficulty falling or staying asleep (Table 1). Ask about cardinal symptoms of disorders associated with insomnia, including:
- snoring or breathing pauses during sleep (sleep apnea syndrome)
- restlessness or twitching in the lower extremities (PLMD/RLS).
Carefully review the patient’s weekday and weekend sleep patterns, bedtime habits, sleep hygiene habits, and substance and medication use.
Sleep clinic referrals. Consider an evaluation by a sleep disorders center when the diagnosis remains unclear or treatment of the presumed condition fails after a reasonable time.
Table 1
Possible causes of sleep complaints
| Acute, transient | Recent or recurring stress | |
| Change in sleeping environment | ||
| Acute illness or injury | ||
| New medications | ||
| Jet lag or shift change | ||
| Chronic | Difficulty staying asleep | Difficulty falling asleep |
| Medications | Poor sleep hygiene | |
| Drug or alcohol use | Conditioned insomnia | |
| Psychiatric disorder | Restless legs syndrome | |
| Medical disorder | Circadian rhythm disorder | |
| Sleep-disordered breathing | Advanced sleep-phase syndrome | |
| Periodic limb movement disorder | ||
| Restless legs syndrome | ||
| Source: Adapted and reprinted with permission from reference 13 | ||
Behavioral Treatments
Behavioral treatments—with or without hypnotics—are appropriate for many insomnia complaints, including adjustment sleep disorder and psychophysiologic insomnia. Behavioral measures may work more slowly than drug therapy, but their effects have been shown to last longer in patients with primary insomnia. It may be useful to start with both hypnotic and behavioral treatments and withdraw the hypnotic after behavioral measures take effect.
Sleep hygiene. Many individuals unknowingly engage in habits that impair sleep. Those with insomnia, for example, often try to compensate for lost sleep by staying in bed later in the morning or by napping, which further fragment nocturnal sleep. Advise these patients to adhere to a regular awakening time—regardless of how long they slept the night before—and to avoid naps. Other tips for getting a good night’s sleep are outlined in Table 2.14
Caffeine has a plasma half-life of 3 to 7 hours, although individual sensitivity varies widely and caffeine’s erratic absorption can prolong its effects. Advise patients with insomnia to avoid caffeine-containing beverages—including coffee, tea, and soft drinks—after noon.
Relaxation training. Muscle tension can be reduced through techniques such as electromyography (EMG) biofeedback, abdominal breathing exercises, or progressive muscle relaxation. Relaxation training is usually effective within a few weeks.
Psychological counseling. Counseling can help identify and dispel tension-producing thoughts that may be disrupting sleep, such as preoccupation with unpleasant work experiences or school examinations. Reassurance may help patients overcome fears about sleeplessness; suggest that they deal with anxiety-producing thoughts during counseling sessions and at times other than bedtime.
Table 2
How to get a good night’s sleep
|
Prescribing Hypnotics
Sedative-hypnotics are indicated primarily for short-term insomnia management. Most are used at bedtime until insomnia dissipates or the physician advises the patient to take a break.
Treatment principles. Because many insomnias are recurrent, prolonged hypnotic treatment given in short bouts is often optimal. Longer treatment—months to years—is clearly needed by a few patients with chronic insomnia. In these cases, carefully monitor for tolerance, as manifested by dosage escalation. Hypnotic treatment is generally not suitable for patients with drug abuse or dependence histories.
Although chloral hydrate and barbiturates are effective hypnotics, adverse effects limit their safety and usefulness. Benzodiazepines and more recently introduced agents have milder side-effect profiles (Table 3). Choose agents based on the patient’s situation, preferences, and effects of prior trials with similar agents. Guidelines for hypnotics discourage chronic use to minimize abuse, misuse, and habituation (Table 4).
Elimination half-life is one of the most important pharmacological properties that differentiates the hypnotics from each other:15
- longer half-life: flurazepam, quazepam
- intermediate half-life: estazolam, temazepam, eszopiclone
- short half-life: triazolam, zolpidem, zolpidem ER, zaleplon, ramelteon.
Benzodiazepine receptor agonists. Of the all the drugs in class, zalepon—because of its ultra-short half-life—is least likely to cause residual daytime effects when administered at bedtime. At 10-mg doses, its side effects seem to last no more than 4 hours after administration. Zaleplon can be safely taken after nocturnal awakenings if the patient remains in bed 4 hours or longer after taking it.17
An ultra-short half life is less desirable for patients with difficulty with sleep initiation and discontinuous sleep throughout the night. For them, longer elimination half-life agents—such as zolpidem, zolpidem extended release (ER), and eszopiclone—may be more predictably effective for the entire night.18
Short half-life hypnotics do not offer anxiolysis for patients with daytime anxiety, as the longer half-life agents do.
Zolpidem ER and eszopiclone do not have a limitation imposed on duration of use. Although zolpidem ER has not been investigated in controlled trials greater than 3 weeks, eszopiclone was evaluated during a 6-month study that demonstrated lack of tolerance during the entire period, and lack of rebound after rapid discontinuation.19 Eszopiclone is the only hypnotic indicated for long-term (lasting > 3 weeks) insomnia.
Melatonin receptor agonists. Ramelteon’s activity at MT1 and MT2 receptors is believed to contribute to its sleep-promoting properties. This agent has been found to reduce sleep latency,20,21 and it is indicated to treat insomnia characterized by sleep-onset delays. Although controlled, longterm studies are lacking, ramelteon does not have a limit on duration of use. It demonstrated a lack of abuse liability when compared with triazolam and placebo in subjects with a history of sedative/hypnotic or anxiolytic drug abuse.22
Tolerance and rebound. Tolerance can develop after repeated dosing with benzodiazepines—primarily triazolam—and rebound insomnia can follow abrupt discontinuation. Both can be minimized by using benzodiazepines at the lowest effective dosages and for brief periods. Gradual tapering when discontinuing the drug can help control rebound.
Tolerance and rebound seem to be less of a concern with the newer hypnotics than with benzodiazepines, as shown by controlled studies of eszopiclone19, zolpidem23, and zaleplon.24 However, periodic re-evaluation is still the prudent clinical standard for hypnotics prescribed over long periods of time.
Table 3
Actions and available doses of common hypnotics
| Class/drug | Onset of action | Half-life (hrs) | Active metabolites | Doses (mg) |
|---|---|---|---|---|
| Benzodiazepines | ||||
| Flurazepam | Rapid | 40 to 250 | Yes | 15, 30 |
| Quazepam | Rapid | 40 to 250 | Yes | 7.5, 15 |
| Estazolam | Rapid | 10 to 24 | Yes | 0.5, 1, 2 |
| Temazepam | Intermediate | 8 to 22 | No | 7.5, 15 |
| Triazolam | Rapid | No | 0.125, 0.25, 0.5 | |
| Imidazopyridine | ||||
| Zolpidem | Rapid | 2.5 | No | 5, 10 |
| Zolpidem ER | Rapid | 2.5 | No | 6.25, 12.5 |
| Pyrazolopyrimidines | ||||
| Zaleplon | Rapid | 1 | No | 5, 10, 20 |
| Cyclopyrrolone | ||||
| Eszopiclone | Rapid | 6 | Minor | 2,3 |
| Melatonin receptor agonist | ||||
| Ramelteon | Rapid | 1 to 2.6 | No | 8 |
Guidelines for safe use of hypnotics
|
Nonhypnotic Sleep AIDS
Sedating antidepressants. Some physicians prescribe low doses of sedating antidepressants to control insomnia, a practice supported by controlled clinical trials of some tricyclic antidepressants (TCAs) such as doxepin,25 trazodone,26 and trimipramine.27 Some physicians also advocate using more-sedating antidepressants—at dosages needed to treat depression—to control insomnia in depressed patients.
Evening dosing can minimize daytime sedation. If you choose an activating antidepressant, the potential side effect of insomnia can be managed by judicious use of hypnotic agents. Little is known about antidepressants’ effects on sleep quality after the first 6 to 8 weeks of treatment.28
Although possibly helpful as sleep aids, TCAs are associated with anticholinergic effects such as dry mouth, urinary flow difficulties, and cardiac dysrhythmias.
Alcohol. Patients with insomnia sometimes selfmedicate with alcohol at bedtime because it enhances sleepiness and induces a more rapid sleep onset.29 Drinking a “nightcap” is a poor choice, however, because alcohol can impair sleep quality, resulting in daytime somnolence. Alcohol is also associated with rapid development of tolerance.
Antihistamines and over-the-counter products whose main active ingredients are antihistamines—such as doxylamine and diphenhydramine—are used for insomnia and may help individuals fall asleep and stay asleep. However, antihistamine use is complicated by unpredictable efficacy and side effects such as daytime sedation, confusion, and systemic anticholinergic effects.30
Melatonin is a nonprescription dietary supplement used in dosages of 0.5 to 3,000 mg. Anecdotal reports indicate it may be efficacious in certain subtypes of insomnia—such as shift work, jet lag, blindness, delayed sleep phase syndrome—and in older patients with sleep complaints.
Melatonin’s efficacy has not been established conclusively, however, and concerns have been expressed regarding the purity of over-the-counter preparations and possible coronary artery tissue stimulation, as observed in animal studies.
Related resources
- American Academy of Sleep Medicine. Sleep logs, patient education materials. www.aasmnet.org
- American Sleep Apnea Association. National Sleep Foundation. www.sleepapnea.org
- Doxepin • Inequan
- Estazolam • Prosom
- Eszopiclone • Lunesta
- Flurazepam • Dalmane
- Quazepam • Doral
- Ramelteon • Rozerem
- Temazepam • Restoril
- Trazodone • Desyrel
- Triazolam • Halcion
- Trimipramine • Surmontil
- Zaleplon • Sonata
- Zolpidem • Ambien
Dr. Doghramji receives research grant support from Cephalon, GlaxoSmithKline, Merck & Co., and Sanofi-Synthelabo
1. Sateia MJ, Doghramji K, Hauri PJ, Morin CM. Evaluation of chronic insomnia. Sleep 2000;23:243-81.
2. Zammit GK, Weiner J, Damato N, et al. Quality of life in people with insomnia. Sleep 1999;22(suppl 2):S379-385.
3. Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. JAMA 1989;262:1479-84.
4. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington DC: American Psychiatric Association, 2000.
5. International Classification of Sleep Disorders: Diagnostic and Coding Manual, (2nd ed.) Westchester, IL: The American Academy of Sleep Medicine, 2005.
6. Spielman AJ, Glovinsky P. The varied nature of insomnia. In: Hauri P (ed). Case studies in insomnia. New York: Plenum Press, 1991;1:15.-
7. Reynolds CF, III, Kupfer DJ. Sleep research in affective illness: state of the art circa 1987. Sleep 1987;10:199-215.
8. Labbate LA, Pollack MH, Otto MW, et al. Sleep panic attacks: an association with childhood anxiety and adult psychopathology. Biol Psychiatry 1994;43:840-2.
9. Ross RJ, Ball WA, Sullivan KA, et al. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry 1989;146:697-707.
10. Winokur A, Reynolds CF. The effects of antidepressants on sleep physiology. Primary Psychiatry 1994;6:22-7.
11. Gillin JC, Rapaport M, Erman MK, et al. A comparison of nefazodone and fluoxetine on mood and on objective, subjective, and clinician-rated measures of sleep in depressed patients: a double blind, 8-week clinical trial. J Clin Psychiatry 1997;58(5):185-92.
12. Erman M. Clinical update. Diagnosis of insomnia in the primary care practice. Available at: http://www.medscape.com/viewarticle/478849. Accessed Jan. 18, 2005.
13. Rajput V, Bromley SM. Chronic insomnia: A practical review. Am Fam Physician 1999;60:1431-8.
14. Doghramji K. The evaluation and management of sleep disorders. In: Stoudemire A (ed). Clinical psychiatry for medical students (3rd ed). Philadelphia: JB Lippincott, 1998;783-818.
15. Gillin JC. The long and short of sleeping pills. N Engl J Med 1991;324:1735-7.
16. Lunesta (eszopiclone). Approved labeling text. Marlborough, MA: Sepracor, Inc., 2005.
17. Corser B, Mayleben D, Doghramji K, et al. No next-day residual sedation four hours after middle-of-the-night treatment with zaleplon. Sleep 2000;23(suppl 2):abstract 309.
18. Holm KJ, Goa KL. Zolpidem: an update of its pharmacology, therapeutic efficacy and tolerability in the treatment of insomnia. Drugs 2000;59:865-89.
19. Scarf MB, Roth T, Vogel GW, Walsh JK. A multicenter, placebocontrolled study evaluating zolpidem in the treatment of chronic insomnia. J Clin Psychiatry 1994;55:192-9.
20. Roth T, Stubbs C, Walsh JK. Ramelteon (TAK-375), a selective MT1/MT2-receptor agonist, reduces sleep latency to persistent sleep in a model of transient insomnia related to a novel sleep environment. Sleep 2005;28:303-7.
21. Roth T, Seiden D, Sainati S, et al. Phase III outpatient trial of ramelteon for the treatment of chronic insomniain elderly patients (poster presentation). Orlando, FL: American Geriatric Society annual meeting, 2005.
22. Griffiths R, Seuss P. Ramelteonand triazolam in humans: behavioral effectsand abuse potential (poster presentation). Atlanta, GA: American Psychiatric Association annual meeting, 2005.
23. Elie R, Ruther E, Farr I, et al. Sleep latency is shortened during 4 weeks of treatment with zaleplon, a novel nonbenzodiazepine hypnotic. Zaleplon Clinical Study Group. J Clin Psychiatry 1999;60:536-44.
24. Krystal A, Walsh J, Laska E, et al. Sustained efficacy of eszopiclone over six months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep 2003;26:793-9.
25. Hajak G, Rodenbeck A, Voderholzer U, et al. Doxepin in the treatment of primary insomnia: a placebo-controlled, double-blind, polysomnographic study. J Clin Psychiatry 2001;62:453-63.
26. Walsh JK, Erman M, Erwin CE, et al. Subjective hypnotic efficacy of trazodone and zolpidem in DSM-III-R primary insomnia. Hum Psychopharmacol 1998;13(3):191-8.
27. Hohagen F, Monero RF, Weiss E, et al. Treatment of primary insomnia with trimipramine: an alternative to benzodiazepine hypnotics? Eur Arch Psychiatry Clin Neurosci 1994;244(2):65-72.
28. Thase ME. Antidepressant treatment of the depressed patient with insomnia. J Clin Psychiatry 1999;60(suppl 17):28-31.
29. Johnson EO, Roehrs T, Roth T, Breslau N. Epidemiology of alcohol and medication as aids to sleep in early adulthood. Sleep 1998;21:178-86.
30. Gengo F, Gabos C, Miller JK. The pharmacodynamics of diphenhydramine-induced drowsiness and changes in mental performance. Clin Pharmacol Ther 1989;45:15-21.
1. Sateia MJ, Doghramji K, Hauri PJ, Morin CM. Evaluation of chronic insomnia. Sleep 2000;23:243-81.
2. Zammit GK, Weiner J, Damato N, et al. Quality of life in people with insomnia. Sleep 1999;22(suppl 2):S379-385.
3. Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. JAMA 1989;262:1479-84.
4. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington DC: American Psychiatric Association, 2000.
5. International Classification of Sleep Disorders: Diagnostic and Coding Manual, (2nd ed.) Westchester, IL: The American Academy of Sleep Medicine, 2005.
6. Spielman AJ, Glovinsky P. The varied nature of insomnia. In: Hauri P (ed). Case studies in insomnia. New York: Plenum Press, 1991;1:15.-
7. Reynolds CF, III, Kupfer DJ. Sleep research in affective illness: state of the art circa 1987. Sleep 1987;10:199-215.
8. Labbate LA, Pollack MH, Otto MW, et al. Sleep panic attacks: an association with childhood anxiety and adult psychopathology. Biol Psychiatry 1994;43:840-2.
9. Ross RJ, Ball WA, Sullivan KA, et al. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry 1989;146:697-707.
10. Winokur A, Reynolds CF. The effects of antidepressants on sleep physiology. Primary Psychiatry 1994;6:22-7.
11. Gillin JC, Rapaport M, Erman MK, et al. A comparison of nefazodone and fluoxetine on mood and on objective, subjective, and clinician-rated measures of sleep in depressed patients: a double blind, 8-week clinical trial. J Clin Psychiatry 1997;58(5):185-92.
12. Erman M. Clinical update. Diagnosis of insomnia in the primary care practice. Available at: http://www.medscape.com/viewarticle/478849. Accessed Jan. 18, 2005.
13. Rajput V, Bromley SM. Chronic insomnia: A practical review. Am Fam Physician 1999;60:1431-8.
14. Doghramji K. The evaluation and management of sleep disorders. In: Stoudemire A (ed). Clinical psychiatry for medical students (3rd ed). Philadelphia: JB Lippincott, 1998;783-818.
15. Gillin JC. The long and short of sleeping pills. N Engl J Med 1991;324:1735-7.
16. Lunesta (eszopiclone). Approved labeling text. Marlborough, MA: Sepracor, Inc., 2005.
17. Corser B, Mayleben D, Doghramji K, et al. No next-day residual sedation four hours after middle-of-the-night treatment with zaleplon. Sleep 2000;23(suppl 2):abstract 309.
18. Holm KJ, Goa KL. Zolpidem: an update of its pharmacology, therapeutic efficacy and tolerability in the treatment of insomnia. Drugs 2000;59:865-89.
19. Scarf MB, Roth T, Vogel GW, Walsh JK. A multicenter, placebocontrolled study evaluating zolpidem in the treatment of chronic insomnia. J Clin Psychiatry 1994;55:192-9.
20. Roth T, Stubbs C, Walsh JK. Ramelteon (TAK-375), a selective MT1/MT2-receptor agonist, reduces sleep latency to persistent sleep in a model of transient insomnia related to a novel sleep environment. Sleep 2005;28:303-7.
21. Roth T, Seiden D, Sainati S, et al. Phase III outpatient trial of ramelteon for the treatment of chronic insomniain elderly patients (poster presentation). Orlando, FL: American Geriatric Society annual meeting, 2005.
22. Griffiths R, Seuss P. Ramelteonand triazolam in humans: behavioral effectsand abuse potential (poster presentation). Atlanta, GA: American Psychiatric Association annual meeting, 2005.
23. Elie R, Ruther E, Farr I, et al. Sleep latency is shortened during 4 weeks of treatment with zaleplon, a novel nonbenzodiazepine hypnotic. Zaleplon Clinical Study Group. J Clin Psychiatry 1999;60:536-44.
24. Krystal A, Walsh J, Laska E, et al. Sustained efficacy of eszopiclone over six months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep 2003;26:793-9.
25. Hajak G, Rodenbeck A, Voderholzer U, et al. Doxepin in the treatment of primary insomnia: a placebo-controlled, double-blind, polysomnographic study. J Clin Psychiatry 2001;62:453-63.
26. Walsh JK, Erman M, Erwin CE, et al. Subjective hypnotic efficacy of trazodone and zolpidem in DSM-III-R primary insomnia. Hum Psychopharmacol 1998;13(3):191-8.
27. Hohagen F, Monero RF, Weiss E, et al. Treatment of primary insomnia with trimipramine: an alternative to benzodiazepine hypnotics? Eur Arch Psychiatry Clin Neurosci 1994;244(2):65-72.
28. Thase ME. Antidepressant treatment of the depressed patient with insomnia. J Clin Psychiatry 1999;60(suppl 17):28-31.
29. Johnson EO, Roehrs T, Roth T, Breslau N. Epidemiology of alcohol and medication as aids to sleep in early adulthood. Sleep 1998;21:178-86.
30. Gengo F, Gabos C, Miller JK. The pharmacodynamics of diphenhydramine-induced drowsiness and changes in mental performance. Clin Pharmacol Ther 1989;45:15-21.
How to avoid burnout and keep your spark
Burnout develops slowly and insidiously; there are no fire alarms, no smoke. It is easy to ignore the warning signs. As psychiatrists, we are at high risk for burnout, and the consequences can be devastating. We have:
- suicide rates 2 to 3 times higher than those of the general population
- higher rates of divorce and substance abuse compared with other physicians and non-physicians (Table 1).1-12
Burnout affects 25% to 57% of our profession at any given time,13 yet we seldom address it. Despite vast literature on burnout in family medicine and other medical specialties, psychiatric burnout is grossly under-recognized. It’s as if we aren’t supposed to burn out; after all, aren’t we the experts others come to when they are burned out?
If you think you may be heading toward burnout, we offer practical, evidence-based information to help you:
- prevent burnout
- diagnose burnout, “brownout,” and “compassion fatigue”
- begin to make immediate changes to over-come burnout and reclaim your life.
Table 1
Relative rates of divorce, suicide, and substance abuse among psychiatrists
| Event | Psychiatrists | Other physicians | General population |
|---|---|---|---|
| Divorce* | 50% (2.7 times risk of other physicians)3 | 22% to 24% in internists and pediatricians3 | 10% to 20% less than among physicians4 |
| Suicide | 28 to 40/100,0001 (2 to 3 times rate in general population) | May be similar to rate among psychiatrists† Equal rates in male and female physicians | 12/100,000 |
| Rate in female physicians is 2 to 4 times that of women in general population1,2,5 | |||
| Substance abuse6-10 | |||
| Benzodiazepine use (past year)6 | 26.3% | 7% to 16% (11.4% across all specialties) | |
| Lifetime abuse/dependence6 | 14.3% | 7.9% | |
| Alcohol only | 7.9% | 4.2% | |
| * Divorce risk across 30 years | |||
| † Some but not all evidence indicates psychiatrists have higher rates of suicide than other physicians1,2,11,12 | |||
Case: ‘Something in me had died’
I (PB) was 50 years old, racing along, seeing patients 45 hours a week, and keeping a full schedule of teaching and writing. Psychotherapy was my primary training and my love, but monitoring medications for other therapists—without getting to know the patients—had become unsatisfying. My practice group had exploded from 5 mental health professionals to more than 20, creating unexpected stresses and conflicts. At the same time, my marriage was failing.
Increasingly overextended, I lost my good humor. I became irritable and short with everyone, and—worse—I felt resentful and burdened by my patients. Once eager for challenges, I avoided new consults and referrals. Every hour was filled with dread, and I struggled to get through the day. Empty, numb, and miserable, I had burned out but did not realize it. I only knew that something in me had died.
I started fantasizing about retiring from clinical work, but what would I do then? What if this was the end of my career?
Burnout is a ‘heart attack’
Most burnout definitions include three features: emotional exhaustion, depersonalization, and diminished feelings of personal accomplishment.14 Some writers describe it as a state of mourning: “A grief syndrome due to loss of our dreams or sense of purpose or mission, leading to the experience of emotional depletion…expectations clash with an imposing reality.”15
Burnout represents a loss of meaning. It resembles a “spiritual heart attack,” with “referred pain” that affects our work, our relationships, and our soul. We become members of the “coronary club” (Box 1).16
- Your job comes first; personal considerations are secondary.
- Go to the office evenings, weekends, and holidays.
- Never say no to a request; always say yes.
- Accept all invitations to meetings, banquets, committees, etc.
- Do not eat a restful, relaxing meal; always plan a meeting for the meal hour.
- Never delegate responsibility to others; carry the entire load by yourself at all times.
Are you getting close to eligibility?
The “15-minute” medication check is probably the most demoralizing hazard. Pressure from managed care to focus on brief contact with patients only for medication management is dispiriting, resulting in:
- little time for empathic connection
- loss of professional autonomy
- fear of greater liability risk than when we handle psychotherapy and medication
- fear of lost income if we opt not to accept medication-only referrals.17-21
Internal causes. Approximately 60% of job satisfaction is related to internal determinants: attitudes, beliefs, lifestyle, and coping techniques. Burnout is not simply the result of overwork, underpay, or increasing demands of a changing medical culture. If all managed care hassles disappeared tomorrow—if paperwork went away and reimbursements flowed freely—burnout would continue because it is the loss of a dream. Freuden-berger23 refers to it as a loss of idealism; a loss of expected goals.
Psychiatry is about intimate human relationships, connectedness, and accompanying our patients over the complex terrain of the human condition. Often, burnout develops when something disrupts the physician-patient bond. As Irvin Yalom reminds us, “It’s the relationship that heals.” That relationship is healing to the physician as well as to the patient.24
Burnout comes from decreased quality of fulfillment we derive from our efforts. It concerns intangible phenomena such as losing our sense of purpose or feeling we are not making a difference. We wonder: Am I doing what I was born to do? Burnout is suffering that goes beyond a worn-down body and approaches “erosion of the soul.”25
Diversify your portfolio
Physician-author Rachel Naomi Remen, MD, clinical professor of family and community medicine at the University of California, San Francisco, reminds us, “Service in medicine is the work of the heart and the soul.”26 To heal ourselves, we must by nurturing and cultivating our inner life. By plumbing these depths, you may rediscover your sense of purpose.
You may need to “diversify your portfolio” with reflective and regenerative activities. These may be as varied as reading poetry, paddling canoes, spiritual practices, gardening, hiking, or visiting art museums.
More importantly, you may need to re-examine and deepen your relationships with:
- your partner (Are you spending enough time together? Is your relationship growing?)
- your patients (Are you getting to know your patients as people?)
- your sense of purpose or spirituality (Do you see a higher or transcendent meaning in your life?)
- the community, the world. (Are you making them better?).
What’s your diagnosis?
How do you know if you have brownout (mild depression; a prodromal phase), classic burnout (severe depression), or compassion fatigue (a form of burnout)?
Brownout vs burnout (Table 2). Look for depressive symptoms: sad mood, lack of pleasure, low energy or motivation, poor concentration or memory, or insomnia. In addition, you may experience a “deadness” at work, as well as “marital deadness.” The “helper’s high” has become the “helper’s low.” You may anger quickly and have tensions with your family or co-workers. Signs of burnout include disorganization and chronic lateness, absenteeism, or “presenteeism” (physically present, spiritually and go beyond the mind and body to address the soul emotionally absent).
Irritability and lack of time for family can cause extensive collateral damage:
- Wife of a burned-out doctor: “My husband wasn’t there for our son’s 6th birthday, and he missed our daughter’s high-school graduation. He’s missed half their childhoods.”
- Husband of a burned-out psychiatrist: “I’m miserable. She’s not the same woman I married. She’s such a workaholic, she’s got nothing left for us.”
- A psychiatrist’s 13-year-old daughter: “He helps his patients have a good life; why can’t he do that with us?”
Are you suffering from brownout or burnout?
| Brownout (mild depression) | Burnout (severe depression) |
|---|---|
| I feel tired | I feel exhausted, listless |
| I’m having less fun and feeling less satisfied | I feel grumpy and joyless |
| I’m drinking more caffeine and eating more junk food | I’m drinking more alcohol, taking more medications, or using illicit drugs |
| I feel less interested and less caring about my patients, residents, and coworkers | I want to leave patient care, and I don’t care about my co-workers |
| I am dissatisfied, troubled | I am impaired |
Table 3
Is it burnout or compassion fatigue?
| Burnout | Compassion fatigue |
|---|---|
| Evolves gradually | Reaction to extreme circumstances or suddenly increased work demands, such as disaster relief, crisis work |
| Loss of meaning, unmet expectations | Vicarious suffering of others’ trauma (“emotional contagion”) |
| Diminished work capacity (depression, withdrawal) | Increased, relentless work effort (ignore physical health, work-‘til-you-drop mentality, obsessive-compulsive behavior) |
Intensive care for burnout
Treating or preventing burnout requires individual solutions, peer strategies, and group/organizational techniques. The first five suggestions below relate to individual steps, and the last two to peer approaches and organizational strategy.
Stop doing what you’re doing. In her book, The Joy of Burnout, Dina Glouberman, PhD, says, “Burn-out is life catching up with us…. Stop doing, and start listening to ourselves in a completely new way, to make space for our true self.”28 Better time management is not the answer; you cannot give what you do not have.
Take time off. Most experts recommend at least 1 month off to rethink things, and 6 months off to renew. I (PB) took 6 months off to recover from my burnout and needed every minute of it.
Take a serious inventory of your life and priorities, and set limits (Box 2). One psychiatrist decided he didn’t want to be on three medical society committees, two hospital committees, and a church task force. His wife had threatened to divorce him, and he was always exhausted.
- Decline (‘Thanks for thinking of me, but I can’t do that right now’)
- Delay (‘Let me think about your request’)
- Delegate
- Dump (‘I thought I could help with this task, but I find it isn’t working for me’)
Get professional counseling. Burnout is moderate or major depression. Practice what you preach.
Join a support group. Let go of, “Real doctors handle things on their own.” Focus on introspection and solutions.
Consider stress management. Options include seminars or retreats and individual, practice-oriented, and organizational consultations (see Related resources).
Burnout as opportunity
Viewing burnout as an opportunity for transformation gives you a chance to:
Re-evaluate your life and priorities. What is most important to you: Money? Family? Making your community a better place? Spiritual growth? If you knew you had only 1 year to live, how would you be living?
Renew/reinvent yourself. One burned-out psychiatrist moved from Denver to San Francisco, where he started over with no expectations or image to uphold. This made it easier to try new professional and personal ventures.
“The geographical solution” is not necessary, however, and can add stress at a vulnerable time. You can “bloom where you’re planted” and renew yourself wherever you are.
Rediscover your passion. Teaching? Art? Part-time practice and run a bed-and-breakfast? Surfing? Guitar lessons? We know physicians who used each of these to help revitalize themselves.
Case continued: recovery
As my life got worse—a drawn-out divorce, two daughters in private universities, and by now a greatly reduced income—I felt trapped and spent. I had to change or die emotionally (possibly even physically).
Not knowing what to do, I took a leap. I cashed in my retirement fund and resigned. I took a 6-month unpaid sabbatical. With no schedule to keep, I had time to read and think. I resumed my own psychotherapy, went through deep reflection, and re-evaluated my priorities and values. I took up acting for fun and started keeping a gratitude journal.
Eventually I remarried. I started changing my workaholic tendencies, limited my practice to 20 hours a week, and established that my priorities were family, friends, and the joy of helping patients and colleagues. I re-discovered my enthusiasm for teaching, including teaching others about preventing burnout.
Preventative medicine
To prevent burnout, we must learn to recognize and address brownout. This is a much better choice than trying to recover from full-fledged burnout: less disruptive, less costly, less damaging interpersonally.
How do we prevent burnout? Several approaches are particularly useful for psychiatrists:
Self-care. Take time off, but beware of “The Vacation Solution”—psychiatrists’ most popular strategy. As one put it: “I work until I’m ready to drop, then I take 2 weeks off.” This is unhealthy:
- physically (gradually wears you down)
- emotionally (we all know the risk of repeated mild depressions, or brownout)
- interpersonally (our family members and colleagues suffer as we get exhausted).
Give and get affirmation and support. Isolating yourself socially is one of the surest roads to burnout. Compared with solo practitioners, psychiatrists working at community mental health centers often report greater career satisfaction. Although they may have difficult case loads and systemic challenges, group practitioners are supported by nurses, social workers, and case managers. The team helps dilute the stress of caring for the most difficult patients.
If you have a solo practice, try to connect and commiserate with other mental health practitioners by joining a professional organization or forming your own process group. If you prefer not to socialize professionally, consider a book club, temple, or church group.
Take time to interact meaningfully: practice appreciating others at least 3 times a day. Saying, “I really admire how you handled that situation,” or “How are you doing?” takes less than 10 seconds. Appreciate your own efforts, too. Write down—now, as you read this—the names of three people you will affirm or offer support to today. Include one person you usually wouldn’t acknowledge.
Find/create meaning in your work life. When you get tired or frustrated, remind yourself that practicing psychiatry is a privilege. We make a difference with people (service) through intimate emotional connection (relationship). Altruism confers benefits to the giver and the recipient. Some psychiatrists derive meaning by seeing our profession as fulfilling a mission or higher purpose, even as a calling.
Be grateful. Gratitude can reduce depression and boost happiness and life satisfaction.29,30 For the next month, try keeping a “gratitude journal,” writing 3 to 5 things you are grateful for each day. It can produce a positive shift in mood, even after a frustrating or demanding workday.31 Start now, by writing what you are grateful for today.
Live fully in this moment. Avoid the “someday” game, waiting to be happy until… you’re caught up with your work, your children are in (or out) of school, you’re wealthy/old enough to retire, etc. Live right now, with this patient, this colleague, this family member. Focus 100% on the person or task of the moment.
Don’t let the task get between us.32 If your spouse or friend wants to visit or share feelings, make the time, even if you’re “busy.” If your patient wants an “extra minute” to thank you for your help or to show you a picture, make time happily.
Play a little every day. Take mini-vacations: walk outside, listen to or make music (a colleague next door lightens my day playing his guitar). Call your spouse or give him/her a funny greeting card, wear a light-hearted tie, bring flowers to your nurses or receptionist. Play with your patients: read poetry; have them bring in pictures of themselves when they were young to help them remember their vitality and spirit. Create your own change of pace (Box 3).
Reading this article is a start, but enduring results will happen only if you commit to follow up. Think about each strategy and decide what appeals to you most. Commit to putting 2 to 4 ideas into practice immediately, and continue them for at least 1 month.
Display the SSPARK mnemonic somewhere to remind you to take care of yourself.
SSPARK =
- Self-care
- Support/affirm
- Purpose/meaning
- Appreciate/gratitude
- Right now
- Kid around/play
- Institute for Healing in Society and Medicine. Martin Sullivan, MD. www.healinginmedicine.org
- Center for Professional Well-Being. John-Henry Pfifferling, PhD. www.cpwb.org
- Renew. Linda Clever, MD. www.renewnow.org
- WorkLife Design. Kernan Manion, MD. www.worklifedesign.org
- Menninger Clinic. Professionals in Crisis Program. www.menningerclinic.com
- Rachel Naomi Remen. Author of Kitchen Table Wisdom: Stories That Heal. www.rachelremen.com.
1. Council on Scientific Affairs: Results and implications of the AMA-APA Physician Mortality Project Stage 2. JAMA 1987;257:2949-53.
2. Hawton K, Clements A, Sakarovitch C, et al. Suicide in doctors: A study of risk according to gender, seniority and specialty in medical practitioners in England and Wales, 1979-1995. J Epidemiol Community Health 2001;55:296-300.
3. Rollman l, Mead LA, Wang N, et al. Medical specialty and the incidence of divorce. N Engl J Med 1997;336(11):800-3.
4. Sotile WM, Sotile MO. The medical marriage: A couple’s survival guide. New York: Carol Publishing; 1996.
5. Pitts FN, Schuller AB, Rich CL, et al. Suicide among U.S. women physicians 1967-1972. Am J Psychiatry 1979;136:694-6.
6. Hughes PH, Storr CL, Brandenburg NA, et al. Physician substance use by medical specialty. J Addict Dis 1999;8(2):23-37.
7. McAuliffe W, Rohman M, Santangelo S, et al. Psychoactive drug use among practicing physicians and medical students. N Engl J Med 1986;315:805-10.
8. Hughes P, Conard S, Baldwin D, et al. Resident physician substance use by specialty. Am J Psychiatry 1992;149:1348-54.
9. Maddux J, Tinnerman I, Costello R. Use of psychoactive substances by residents. J Med Educ 1987;62:852-4.
10. O’Connor PG, Spickard A. Physician impairment by substance abuse. Med Clin North Am 1997;81:1037-52.
11. Craig AG, Pitts FN. Suicide by physicians. Dis Nerv Syst 1968;29:763-72.
12. Rich CL, Pitts FN. Suicide by psychiatrists: a study of medical specialists among 18,730 consecutive physician deaths during a five-year period, 1967-72. J Clin Psychiatry 1980;41:261-3.
13. Montenegro R. A new environmental tragedy: The burn-out of mental health workers. In: Spinetti G, Janiri L (eds). Ecologia e Psichiatria. Rome: CIC Edizioni; 2001.
14. Maslach C. Burnout: The cost of caring. New York: Prentice Hall; 1982.
15. Pfifferling JH, Gilley K. Putting ‘life’ back into your professional life. Fam Pract Manag 1999;6(6):36-42.
16. Adapted from Bits × Pieces January 7 1993. Author unknown.
17. Private Practice Holds Its Own. Psychiatric News April 7, 1995, pp 11, 28.
18. Mizner GL. Current problems in the private practice of psychotherapy. Psychiatric Times August 1994, p 19.
19. Henneberger M. Managed care changing practice of psychotherapy. The New York Times October 9, 1994, pp 1, 50.
20. Pollock EJ. Managed care’s focus on psychiatric drugs alarms many doctors. Wall Street Journal December 1, 1995, pp A1, A11.
21. Hymowitz C. High anxiety: in the name of Freud, why are patients complaining so much? Wall Street Journal December 12, 1995, pp A1, A10.
22. Kalman TP, Goldstein MA. Satisfaction of Manhattan psychiatrists with private practice: assessing the impact of managed care. Medscape Psychiatry & Mental Health eJournal 1998; 3(1).
23. Freudenberger HJ. Staff burn-out. Journal of Social Issues 1974;30:159-65.
24. Yalom ID. Love’s executioner and other tales of psychotherapy. New York: Harper Collins; 1989.
25. Maslach C, Leiter M. The truth about burnout: How organizations cause personal stress and what to do about it. San Francisco, CA: Jossey-Bass Inc.; 1997.
26. Remen RN. Recapturing the soul of medicine: Physicians need to reclaim meaning in their lives. West J Med 2001;174(1):4-5.
27. Figley CR. Compassion fatigue: Coping with secondary post traumatic stress disorder in those who treat the traumatized. New York: Brunner/Mazel; 1995.
28. Glouberman D. The joy of burnout: How the end of the world can be a new beginning. Makawao, HI: Inner Ocean Publishing; 2003.
29. Emmons RA, McCullough ME. Counting blessings versus burdens: Experimental studies of gratitude and subjective well-being in daily life. J Pers Soc Psychol 2003;84(2):377-89.
30. Seligman ME. Positive psychology process: Empirical validation of interventions. Am Psychol 2005;60(5):410-21.
31. Sheldon KM, Lyubomirsky S. How to increase and sustain positive emotion: The benefits of expressing gratitude and visualizing best possible selves. Journal of Positive Psychology, in press.
32. Remen RN. Kitchen table wisdom: Stories that heal. New York: Riverhead Books; 1997.
Burnout develops slowly and insidiously; there are no fire alarms, no smoke. It is easy to ignore the warning signs. As psychiatrists, we are at high risk for burnout, and the consequences can be devastating. We have:
- suicide rates 2 to 3 times higher than those of the general population
- higher rates of divorce and substance abuse compared with other physicians and non-physicians (Table 1).1-12
Burnout affects 25% to 57% of our profession at any given time,13 yet we seldom address it. Despite vast literature on burnout in family medicine and other medical specialties, psychiatric burnout is grossly under-recognized. It’s as if we aren’t supposed to burn out; after all, aren’t we the experts others come to when they are burned out?
If you think you may be heading toward burnout, we offer practical, evidence-based information to help you:
- prevent burnout
- diagnose burnout, “brownout,” and “compassion fatigue”
- begin to make immediate changes to over-come burnout and reclaim your life.
Table 1
Relative rates of divorce, suicide, and substance abuse among psychiatrists
| Event | Psychiatrists | Other physicians | General population |
|---|---|---|---|
| Divorce* | 50% (2.7 times risk of other physicians)3 | 22% to 24% in internists and pediatricians3 | 10% to 20% less than among physicians4 |
| Suicide | 28 to 40/100,0001 (2 to 3 times rate in general population) | May be similar to rate among psychiatrists† Equal rates in male and female physicians | 12/100,000 |
| Rate in female physicians is 2 to 4 times that of women in general population1,2,5 | |||
| Substance abuse6-10 | |||
| Benzodiazepine use (past year)6 | 26.3% | 7% to 16% (11.4% across all specialties) | |
| Lifetime abuse/dependence6 | 14.3% | 7.9% | |
| Alcohol only | 7.9% | 4.2% | |
| * Divorce risk across 30 years | |||
| † Some but not all evidence indicates psychiatrists have higher rates of suicide than other physicians1,2,11,12 | |||
Case: ‘Something in me had died’
I (PB) was 50 years old, racing along, seeing patients 45 hours a week, and keeping a full schedule of teaching and writing. Psychotherapy was my primary training and my love, but monitoring medications for other therapists—without getting to know the patients—had become unsatisfying. My practice group had exploded from 5 mental health professionals to more than 20, creating unexpected stresses and conflicts. At the same time, my marriage was failing.
Increasingly overextended, I lost my good humor. I became irritable and short with everyone, and—worse—I felt resentful and burdened by my patients. Once eager for challenges, I avoided new consults and referrals. Every hour was filled with dread, and I struggled to get through the day. Empty, numb, and miserable, I had burned out but did not realize it. I only knew that something in me had died.
I started fantasizing about retiring from clinical work, but what would I do then? What if this was the end of my career?
Burnout is a ‘heart attack’
Most burnout definitions include three features: emotional exhaustion, depersonalization, and diminished feelings of personal accomplishment.14 Some writers describe it as a state of mourning: “A grief syndrome due to loss of our dreams or sense of purpose or mission, leading to the experience of emotional depletion…expectations clash with an imposing reality.”15
Burnout represents a loss of meaning. It resembles a “spiritual heart attack,” with “referred pain” that affects our work, our relationships, and our soul. We become members of the “coronary club” (Box 1).16
- Your job comes first; personal considerations are secondary.
- Go to the office evenings, weekends, and holidays.
- Never say no to a request; always say yes.
- Accept all invitations to meetings, banquets, committees, etc.
- Do not eat a restful, relaxing meal; always plan a meeting for the meal hour.
- Never delegate responsibility to others; carry the entire load by yourself at all times.
Are you getting close to eligibility?
The “15-minute” medication check is probably the most demoralizing hazard. Pressure from managed care to focus on brief contact with patients only for medication management is dispiriting, resulting in:
- little time for empathic connection
- loss of professional autonomy
- fear of greater liability risk than when we handle psychotherapy and medication
- fear of lost income if we opt not to accept medication-only referrals.17-21
Internal causes. Approximately 60% of job satisfaction is related to internal determinants: attitudes, beliefs, lifestyle, and coping techniques. Burnout is not simply the result of overwork, underpay, or increasing demands of a changing medical culture. If all managed care hassles disappeared tomorrow—if paperwork went away and reimbursements flowed freely—burnout would continue because it is the loss of a dream. Freuden-berger23 refers to it as a loss of idealism; a loss of expected goals.
Psychiatry is about intimate human relationships, connectedness, and accompanying our patients over the complex terrain of the human condition. Often, burnout develops when something disrupts the physician-patient bond. As Irvin Yalom reminds us, “It’s the relationship that heals.” That relationship is healing to the physician as well as to the patient.24
Burnout comes from decreased quality of fulfillment we derive from our efforts. It concerns intangible phenomena such as losing our sense of purpose or feeling we are not making a difference. We wonder: Am I doing what I was born to do? Burnout is suffering that goes beyond a worn-down body and approaches “erosion of the soul.”25
Diversify your portfolio
Physician-author Rachel Naomi Remen, MD, clinical professor of family and community medicine at the University of California, San Francisco, reminds us, “Service in medicine is the work of the heart and the soul.”26 To heal ourselves, we must by nurturing and cultivating our inner life. By plumbing these depths, you may rediscover your sense of purpose.
You may need to “diversify your portfolio” with reflective and regenerative activities. These may be as varied as reading poetry, paddling canoes, spiritual practices, gardening, hiking, or visiting art museums.
More importantly, you may need to re-examine and deepen your relationships with:
- your partner (Are you spending enough time together? Is your relationship growing?)
- your patients (Are you getting to know your patients as people?)
- your sense of purpose or spirituality (Do you see a higher or transcendent meaning in your life?)
- the community, the world. (Are you making them better?).
What’s your diagnosis?
How do you know if you have brownout (mild depression; a prodromal phase), classic burnout (severe depression), or compassion fatigue (a form of burnout)?
Brownout vs burnout (Table 2). Look for depressive symptoms: sad mood, lack of pleasure, low energy or motivation, poor concentration or memory, or insomnia. In addition, you may experience a “deadness” at work, as well as “marital deadness.” The “helper’s high” has become the “helper’s low.” You may anger quickly and have tensions with your family or co-workers. Signs of burnout include disorganization and chronic lateness, absenteeism, or “presenteeism” (physically present, spiritually and go beyond the mind and body to address the soul emotionally absent).
Irritability and lack of time for family can cause extensive collateral damage:
- Wife of a burned-out doctor: “My husband wasn’t there for our son’s 6th birthday, and he missed our daughter’s high-school graduation. He’s missed half their childhoods.”
- Husband of a burned-out psychiatrist: “I’m miserable. She’s not the same woman I married. She’s such a workaholic, she’s got nothing left for us.”
- A psychiatrist’s 13-year-old daughter: “He helps his patients have a good life; why can’t he do that with us?”
Are you suffering from brownout or burnout?
| Brownout (mild depression) | Burnout (severe depression) |
|---|---|
| I feel tired | I feel exhausted, listless |
| I’m having less fun and feeling less satisfied | I feel grumpy and joyless |
| I’m drinking more caffeine and eating more junk food | I’m drinking more alcohol, taking more medications, or using illicit drugs |
| I feel less interested and less caring about my patients, residents, and coworkers | I want to leave patient care, and I don’t care about my co-workers |
| I am dissatisfied, troubled | I am impaired |
Table 3
Is it burnout or compassion fatigue?
| Burnout | Compassion fatigue |
|---|---|
| Evolves gradually | Reaction to extreme circumstances or suddenly increased work demands, such as disaster relief, crisis work |
| Loss of meaning, unmet expectations | Vicarious suffering of others’ trauma (“emotional contagion”) |
| Diminished work capacity (depression, withdrawal) | Increased, relentless work effort (ignore physical health, work-‘til-you-drop mentality, obsessive-compulsive behavior) |
Intensive care for burnout
Treating or preventing burnout requires individual solutions, peer strategies, and group/organizational techniques. The first five suggestions below relate to individual steps, and the last two to peer approaches and organizational strategy.
Stop doing what you’re doing. In her book, The Joy of Burnout, Dina Glouberman, PhD, says, “Burn-out is life catching up with us…. Stop doing, and start listening to ourselves in a completely new way, to make space for our true self.”28 Better time management is not the answer; you cannot give what you do not have.
Take time off. Most experts recommend at least 1 month off to rethink things, and 6 months off to renew. I (PB) took 6 months off to recover from my burnout and needed every minute of it.
Take a serious inventory of your life and priorities, and set limits (Box 2). One psychiatrist decided he didn’t want to be on three medical society committees, two hospital committees, and a church task force. His wife had threatened to divorce him, and he was always exhausted.
- Decline (‘Thanks for thinking of me, but I can’t do that right now’)
- Delay (‘Let me think about your request’)
- Delegate
- Dump (‘I thought I could help with this task, but I find it isn’t working for me’)
Get professional counseling. Burnout is moderate or major depression. Practice what you preach.
Join a support group. Let go of, “Real doctors handle things on their own.” Focus on introspection and solutions.
Consider stress management. Options include seminars or retreats and individual, practice-oriented, and organizational consultations (see Related resources).
Burnout as opportunity
Viewing burnout as an opportunity for transformation gives you a chance to:
Re-evaluate your life and priorities. What is most important to you: Money? Family? Making your community a better place? Spiritual growth? If you knew you had only 1 year to live, how would you be living?
Renew/reinvent yourself. One burned-out psychiatrist moved from Denver to San Francisco, where he started over with no expectations or image to uphold. This made it easier to try new professional and personal ventures.
“The geographical solution” is not necessary, however, and can add stress at a vulnerable time. You can “bloom where you’re planted” and renew yourself wherever you are.
Rediscover your passion. Teaching? Art? Part-time practice and run a bed-and-breakfast? Surfing? Guitar lessons? We know physicians who used each of these to help revitalize themselves.
Case continued: recovery
As my life got worse—a drawn-out divorce, two daughters in private universities, and by now a greatly reduced income—I felt trapped and spent. I had to change or die emotionally (possibly even physically).
Not knowing what to do, I took a leap. I cashed in my retirement fund and resigned. I took a 6-month unpaid sabbatical. With no schedule to keep, I had time to read and think. I resumed my own psychotherapy, went through deep reflection, and re-evaluated my priorities and values. I took up acting for fun and started keeping a gratitude journal.
Eventually I remarried. I started changing my workaholic tendencies, limited my practice to 20 hours a week, and established that my priorities were family, friends, and the joy of helping patients and colleagues. I re-discovered my enthusiasm for teaching, including teaching others about preventing burnout.
Preventative medicine
To prevent burnout, we must learn to recognize and address brownout. This is a much better choice than trying to recover from full-fledged burnout: less disruptive, less costly, less damaging interpersonally.
How do we prevent burnout? Several approaches are particularly useful for psychiatrists:
Self-care. Take time off, but beware of “The Vacation Solution”—psychiatrists’ most popular strategy. As one put it: “I work until I’m ready to drop, then I take 2 weeks off.” This is unhealthy:
- physically (gradually wears you down)
- emotionally (we all know the risk of repeated mild depressions, or brownout)
- interpersonally (our family members and colleagues suffer as we get exhausted).
Give and get affirmation and support. Isolating yourself socially is one of the surest roads to burnout. Compared with solo practitioners, psychiatrists working at community mental health centers often report greater career satisfaction. Although they may have difficult case loads and systemic challenges, group practitioners are supported by nurses, social workers, and case managers. The team helps dilute the stress of caring for the most difficult patients.
If you have a solo practice, try to connect and commiserate with other mental health practitioners by joining a professional organization or forming your own process group. If you prefer not to socialize professionally, consider a book club, temple, or church group.
Take time to interact meaningfully: practice appreciating others at least 3 times a day. Saying, “I really admire how you handled that situation,” or “How are you doing?” takes less than 10 seconds. Appreciate your own efforts, too. Write down—now, as you read this—the names of three people you will affirm or offer support to today. Include one person you usually wouldn’t acknowledge.
Find/create meaning in your work life. When you get tired or frustrated, remind yourself that practicing psychiatry is a privilege. We make a difference with people (service) through intimate emotional connection (relationship). Altruism confers benefits to the giver and the recipient. Some psychiatrists derive meaning by seeing our profession as fulfilling a mission or higher purpose, even as a calling.
Be grateful. Gratitude can reduce depression and boost happiness and life satisfaction.29,30 For the next month, try keeping a “gratitude journal,” writing 3 to 5 things you are grateful for each day. It can produce a positive shift in mood, even after a frustrating or demanding workday.31 Start now, by writing what you are grateful for today.
Live fully in this moment. Avoid the “someday” game, waiting to be happy until… you’re caught up with your work, your children are in (or out) of school, you’re wealthy/old enough to retire, etc. Live right now, with this patient, this colleague, this family member. Focus 100% on the person or task of the moment.
Don’t let the task get between us.32 If your spouse or friend wants to visit or share feelings, make the time, even if you’re “busy.” If your patient wants an “extra minute” to thank you for your help or to show you a picture, make time happily.
Play a little every day. Take mini-vacations: walk outside, listen to or make music (a colleague next door lightens my day playing his guitar). Call your spouse or give him/her a funny greeting card, wear a light-hearted tie, bring flowers to your nurses or receptionist. Play with your patients: read poetry; have them bring in pictures of themselves when they were young to help them remember their vitality and spirit. Create your own change of pace (Box 3).
Reading this article is a start, but enduring results will happen only if you commit to follow up. Think about each strategy and decide what appeals to you most. Commit to putting 2 to 4 ideas into practice immediately, and continue them for at least 1 month.
Display the SSPARK mnemonic somewhere to remind you to take care of yourself.
SSPARK =
- Self-care
- Support/affirm
- Purpose/meaning
- Appreciate/gratitude
- Right now
- Kid around/play
- Institute for Healing in Society and Medicine. Martin Sullivan, MD. www.healinginmedicine.org
- Center for Professional Well-Being. John-Henry Pfifferling, PhD. www.cpwb.org
- Renew. Linda Clever, MD. www.renewnow.org
- WorkLife Design. Kernan Manion, MD. www.worklifedesign.org
- Menninger Clinic. Professionals in Crisis Program. www.menningerclinic.com
- Rachel Naomi Remen. Author of Kitchen Table Wisdom: Stories That Heal. www.rachelremen.com.
Burnout develops slowly and insidiously; there are no fire alarms, no smoke. It is easy to ignore the warning signs. As psychiatrists, we are at high risk for burnout, and the consequences can be devastating. We have:
- suicide rates 2 to 3 times higher than those of the general population
- higher rates of divorce and substance abuse compared with other physicians and non-physicians (Table 1).1-12
Burnout affects 25% to 57% of our profession at any given time,13 yet we seldom address it. Despite vast literature on burnout in family medicine and other medical specialties, psychiatric burnout is grossly under-recognized. It’s as if we aren’t supposed to burn out; after all, aren’t we the experts others come to when they are burned out?
If you think you may be heading toward burnout, we offer practical, evidence-based information to help you:
- prevent burnout
- diagnose burnout, “brownout,” and “compassion fatigue”
- begin to make immediate changes to over-come burnout and reclaim your life.
Table 1
Relative rates of divorce, suicide, and substance abuse among psychiatrists
| Event | Psychiatrists | Other physicians | General population |
|---|---|---|---|
| Divorce* | 50% (2.7 times risk of other physicians)3 | 22% to 24% in internists and pediatricians3 | 10% to 20% less than among physicians4 |
| Suicide | 28 to 40/100,0001 (2 to 3 times rate in general population) | May be similar to rate among psychiatrists† Equal rates in male and female physicians | 12/100,000 |
| Rate in female physicians is 2 to 4 times that of women in general population1,2,5 | |||
| Substance abuse6-10 | |||
| Benzodiazepine use (past year)6 | 26.3% | 7% to 16% (11.4% across all specialties) | |
| Lifetime abuse/dependence6 | 14.3% | 7.9% | |
| Alcohol only | 7.9% | 4.2% | |
| * Divorce risk across 30 years | |||
| † Some but not all evidence indicates psychiatrists have higher rates of suicide than other physicians1,2,11,12 | |||
Case: ‘Something in me had died’
I (PB) was 50 years old, racing along, seeing patients 45 hours a week, and keeping a full schedule of teaching and writing. Psychotherapy was my primary training and my love, but monitoring medications for other therapists—without getting to know the patients—had become unsatisfying. My practice group had exploded from 5 mental health professionals to more than 20, creating unexpected stresses and conflicts. At the same time, my marriage was failing.
Increasingly overextended, I lost my good humor. I became irritable and short with everyone, and—worse—I felt resentful and burdened by my patients. Once eager for challenges, I avoided new consults and referrals. Every hour was filled with dread, and I struggled to get through the day. Empty, numb, and miserable, I had burned out but did not realize it. I only knew that something in me had died.
I started fantasizing about retiring from clinical work, but what would I do then? What if this was the end of my career?
Burnout is a ‘heart attack’
Most burnout definitions include three features: emotional exhaustion, depersonalization, and diminished feelings of personal accomplishment.14 Some writers describe it as a state of mourning: “A grief syndrome due to loss of our dreams or sense of purpose or mission, leading to the experience of emotional depletion…expectations clash with an imposing reality.”15
Burnout represents a loss of meaning. It resembles a “spiritual heart attack,” with “referred pain” that affects our work, our relationships, and our soul. We become members of the “coronary club” (Box 1).16
- Your job comes first; personal considerations are secondary.
- Go to the office evenings, weekends, and holidays.
- Never say no to a request; always say yes.
- Accept all invitations to meetings, banquets, committees, etc.
- Do not eat a restful, relaxing meal; always plan a meeting for the meal hour.
- Never delegate responsibility to others; carry the entire load by yourself at all times.
Are you getting close to eligibility?
The “15-minute” medication check is probably the most demoralizing hazard. Pressure from managed care to focus on brief contact with patients only for medication management is dispiriting, resulting in:
- little time for empathic connection
- loss of professional autonomy
- fear of greater liability risk than when we handle psychotherapy and medication
- fear of lost income if we opt not to accept medication-only referrals.17-21
Internal causes. Approximately 60% of job satisfaction is related to internal determinants: attitudes, beliefs, lifestyle, and coping techniques. Burnout is not simply the result of overwork, underpay, or increasing demands of a changing medical culture. If all managed care hassles disappeared tomorrow—if paperwork went away and reimbursements flowed freely—burnout would continue because it is the loss of a dream. Freuden-berger23 refers to it as a loss of idealism; a loss of expected goals.
Psychiatry is about intimate human relationships, connectedness, and accompanying our patients over the complex terrain of the human condition. Often, burnout develops when something disrupts the physician-patient bond. As Irvin Yalom reminds us, “It’s the relationship that heals.” That relationship is healing to the physician as well as to the patient.24
Burnout comes from decreased quality of fulfillment we derive from our efforts. It concerns intangible phenomena such as losing our sense of purpose or feeling we are not making a difference. We wonder: Am I doing what I was born to do? Burnout is suffering that goes beyond a worn-down body and approaches “erosion of the soul.”25
Diversify your portfolio
Physician-author Rachel Naomi Remen, MD, clinical professor of family and community medicine at the University of California, San Francisco, reminds us, “Service in medicine is the work of the heart and the soul.”26 To heal ourselves, we must by nurturing and cultivating our inner life. By plumbing these depths, you may rediscover your sense of purpose.
You may need to “diversify your portfolio” with reflective and regenerative activities. These may be as varied as reading poetry, paddling canoes, spiritual practices, gardening, hiking, or visiting art museums.
More importantly, you may need to re-examine and deepen your relationships with:
- your partner (Are you spending enough time together? Is your relationship growing?)
- your patients (Are you getting to know your patients as people?)
- your sense of purpose or spirituality (Do you see a higher or transcendent meaning in your life?)
- the community, the world. (Are you making them better?).
What’s your diagnosis?
How do you know if you have brownout (mild depression; a prodromal phase), classic burnout (severe depression), or compassion fatigue (a form of burnout)?
Brownout vs burnout (Table 2). Look for depressive symptoms: sad mood, lack of pleasure, low energy or motivation, poor concentration or memory, or insomnia. In addition, you may experience a “deadness” at work, as well as “marital deadness.” The “helper’s high” has become the “helper’s low.” You may anger quickly and have tensions with your family or co-workers. Signs of burnout include disorganization and chronic lateness, absenteeism, or “presenteeism” (physically present, spiritually and go beyond the mind and body to address the soul emotionally absent).
Irritability and lack of time for family can cause extensive collateral damage:
- Wife of a burned-out doctor: “My husband wasn’t there for our son’s 6th birthday, and he missed our daughter’s high-school graduation. He’s missed half their childhoods.”
- Husband of a burned-out psychiatrist: “I’m miserable. She’s not the same woman I married. She’s such a workaholic, she’s got nothing left for us.”
- A psychiatrist’s 13-year-old daughter: “He helps his patients have a good life; why can’t he do that with us?”
Are you suffering from brownout or burnout?
| Brownout (mild depression) | Burnout (severe depression) |
|---|---|
| I feel tired | I feel exhausted, listless |
| I’m having less fun and feeling less satisfied | I feel grumpy and joyless |
| I’m drinking more caffeine and eating more junk food | I’m drinking more alcohol, taking more medications, or using illicit drugs |
| I feel less interested and less caring about my patients, residents, and coworkers | I want to leave patient care, and I don’t care about my co-workers |
| I am dissatisfied, troubled | I am impaired |
Table 3
Is it burnout or compassion fatigue?
| Burnout | Compassion fatigue |
|---|---|
| Evolves gradually | Reaction to extreme circumstances or suddenly increased work demands, such as disaster relief, crisis work |
| Loss of meaning, unmet expectations | Vicarious suffering of others’ trauma (“emotional contagion”) |
| Diminished work capacity (depression, withdrawal) | Increased, relentless work effort (ignore physical health, work-‘til-you-drop mentality, obsessive-compulsive behavior) |
Intensive care for burnout
Treating or preventing burnout requires individual solutions, peer strategies, and group/organizational techniques. The first five suggestions below relate to individual steps, and the last two to peer approaches and organizational strategy.
Stop doing what you’re doing. In her book, The Joy of Burnout, Dina Glouberman, PhD, says, “Burn-out is life catching up with us…. Stop doing, and start listening to ourselves in a completely new way, to make space for our true self.”28 Better time management is not the answer; you cannot give what you do not have.
Take time off. Most experts recommend at least 1 month off to rethink things, and 6 months off to renew. I (PB) took 6 months off to recover from my burnout and needed every minute of it.
Take a serious inventory of your life and priorities, and set limits (Box 2). One psychiatrist decided he didn’t want to be on three medical society committees, two hospital committees, and a church task force. His wife had threatened to divorce him, and he was always exhausted.
- Decline (‘Thanks for thinking of me, but I can’t do that right now’)
- Delay (‘Let me think about your request’)
- Delegate
- Dump (‘I thought I could help with this task, but I find it isn’t working for me’)
Get professional counseling. Burnout is moderate or major depression. Practice what you preach.
Join a support group. Let go of, “Real doctors handle things on their own.” Focus on introspection and solutions.
Consider stress management. Options include seminars or retreats and individual, practice-oriented, and organizational consultations (see Related resources).
Burnout as opportunity
Viewing burnout as an opportunity for transformation gives you a chance to:
Re-evaluate your life and priorities. What is most important to you: Money? Family? Making your community a better place? Spiritual growth? If you knew you had only 1 year to live, how would you be living?
Renew/reinvent yourself. One burned-out psychiatrist moved from Denver to San Francisco, where he started over with no expectations or image to uphold. This made it easier to try new professional and personal ventures.
“The geographical solution” is not necessary, however, and can add stress at a vulnerable time. You can “bloom where you’re planted” and renew yourself wherever you are.
Rediscover your passion. Teaching? Art? Part-time practice and run a bed-and-breakfast? Surfing? Guitar lessons? We know physicians who used each of these to help revitalize themselves.
Case continued: recovery
As my life got worse—a drawn-out divorce, two daughters in private universities, and by now a greatly reduced income—I felt trapped and spent. I had to change or die emotionally (possibly even physically).
Not knowing what to do, I took a leap. I cashed in my retirement fund and resigned. I took a 6-month unpaid sabbatical. With no schedule to keep, I had time to read and think. I resumed my own psychotherapy, went through deep reflection, and re-evaluated my priorities and values. I took up acting for fun and started keeping a gratitude journal.
Eventually I remarried. I started changing my workaholic tendencies, limited my practice to 20 hours a week, and established that my priorities were family, friends, and the joy of helping patients and colleagues. I re-discovered my enthusiasm for teaching, including teaching others about preventing burnout.
Preventative medicine
To prevent burnout, we must learn to recognize and address brownout. This is a much better choice than trying to recover from full-fledged burnout: less disruptive, less costly, less damaging interpersonally.
How do we prevent burnout? Several approaches are particularly useful for psychiatrists:
Self-care. Take time off, but beware of “The Vacation Solution”—psychiatrists’ most popular strategy. As one put it: “I work until I’m ready to drop, then I take 2 weeks off.” This is unhealthy:
- physically (gradually wears you down)
- emotionally (we all know the risk of repeated mild depressions, or brownout)
- interpersonally (our family members and colleagues suffer as we get exhausted).
Give and get affirmation and support. Isolating yourself socially is one of the surest roads to burnout. Compared with solo practitioners, psychiatrists working at community mental health centers often report greater career satisfaction. Although they may have difficult case loads and systemic challenges, group practitioners are supported by nurses, social workers, and case managers. The team helps dilute the stress of caring for the most difficult patients.
If you have a solo practice, try to connect and commiserate with other mental health practitioners by joining a professional organization or forming your own process group. If you prefer not to socialize professionally, consider a book club, temple, or church group.
Take time to interact meaningfully: practice appreciating others at least 3 times a day. Saying, “I really admire how you handled that situation,” or “How are you doing?” takes less than 10 seconds. Appreciate your own efforts, too. Write down—now, as you read this—the names of three people you will affirm or offer support to today. Include one person you usually wouldn’t acknowledge.
Find/create meaning in your work life. When you get tired or frustrated, remind yourself that practicing psychiatry is a privilege. We make a difference with people (service) through intimate emotional connection (relationship). Altruism confers benefits to the giver and the recipient. Some psychiatrists derive meaning by seeing our profession as fulfilling a mission or higher purpose, even as a calling.
Be grateful. Gratitude can reduce depression and boost happiness and life satisfaction.29,30 For the next month, try keeping a “gratitude journal,” writing 3 to 5 things you are grateful for each day. It can produce a positive shift in mood, even after a frustrating or demanding workday.31 Start now, by writing what you are grateful for today.
Live fully in this moment. Avoid the “someday” game, waiting to be happy until… you’re caught up with your work, your children are in (or out) of school, you’re wealthy/old enough to retire, etc. Live right now, with this patient, this colleague, this family member. Focus 100% on the person or task of the moment.
Don’t let the task get between us.32 If your spouse or friend wants to visit or share feelings, make the time, even if you’re “busy.” If your patient wants an “extra minute” to thank you for your help or to show you a picture, make time happily.
Play a little every day. Take mini-vacations: walk outside, listen to or make music (a colleague next door lightens my day playing his guitar). Call your spouse or give him/her a funny greeting card, wear a light-hearted tie, bring flowers to your nurses or receptionist. Play with your patients: read poetry; have them bring in pictures of themselves when they were young to help them remember their vitality and spirit. Create your own change of pace (Box 3).
Reading this article is a start, but enduring results will happen only if you commit to follow up. Think about each strategy and decide what appeals to you most. Commit to putting 2 to 4 ideas into practice immediately, and continue them for at least 1 month.
Display the SSPARK mnemonic somewhere to remind you to take care of yourself.
SSPARK =
- Self-care
- Support/affirm
- Purpose/meaning
- Appreciate/gratitude
- Right now
- Kid around/play
- Institute for Healing in Society and Medicine. Martin Sullivan, MD. www.healinginmedicine.org
- Center for Professional Well-Being. John-Henry Pfifferling, PhD. www.cpwb.org
- Renew. Linda Clever, MD. www.renewnow.org
- WorkLife Design. Kernan Manion, MD. www.worklifedesign.org
- Menninger Clinic. Professionals in Crisis Program. www.menningerclinic.com
- Rachel Naomi Remen. Author of Kitchen Table Wisdom: Stories That Heal. www.rachelremen.com.
1. Council on Scientific Affairs: Results and implications of the AMA-APA Physician Mortality Project Stage 2. JAMA 1987;257:2949-53.
2. Hawton K, Clements A, Sakarovitch C, et al. Suicide in doctors: A study of risk according to gender, seniority and specialty in medical practitioners in England and Wales, 1979-1995. J Epidemiol Community Health 2001;55:296-300.
3. Rollman l, Mead LA, Wang N, et al. Medical specialty and the incidence of divorce. N Engl J Med 1997;336(11):800-3.
4. Sotile WM, Sotile MO. The medical marriage: A couple’s survival guide. New York: Carol Publishing; 1996.
5. Pitts FN, Schuller AB, Rich CL, et al. Suicide among U.S. women physicians 1967-1972. Am J Psychiatry 1979;136:694-6.
6. Hughes PH, Storr CL, Brandenburg NA, et al. Physician substance use by medical specialty. J Addict Dis 1999;8(2):23-37.
7. McAuliffe W, Rohman M, Santangelo S, et al. Psychoactive drug use among practicing physicians and medical students. N Engl J Med 1986;315:805-10.
8. Hughes P, Conard S, Baldwin D, et al. Resident physician substance use by specialty. Am J Psychiatry 1992;149:1348-54.
9. Maddux J, Tinnerman I, Costello R. Use of psychoactive substances by residents. J Med Educ 1987;62:852-4.
10. O’Connor PG, Spickard A. Physician impairment by substance abuse. Med Clin North Am 1997;81:1037-52.
11. Craig AG, Pitts FN. Suicide by physicians. Dis Nerv Syst 1968;29:763-72.
12. Rich CL, Pitts FN. Suicide by psychiatrists: a study of medical specialists among 18,730 consecutive physician deaths during a five-year period, 1967-72. J Clin Psychiatry 1980;41:261-3.
13. Montenegro R. A new environmental tragedy: The burn-out of mental health workers. In: Spinetti G, Janiri L (eds). Ecologia e Psichiatria. Rome: CIC Edizioni; 2001.
14. Maslach C. Burnout: The cost of caring. New York: Prentice Hall; 1982.
15. Pfifferling JH, Gilley K. Putting ‘life’ back into your professional life. Fam Pract Manag 1999;6(6):36-42.
16. Adapted from Bits × Pieces January 7 1993. Author unknown.
17. Private Practice Holds Its Own. Psychiatric News April 7, 1995, pp 11, 28.
18. Mizner GL. Current problems in the private practice of psychotherapy. Psychiatric Times August 1994, p 19.
19. Henneberger M. Managed care changing practice of psychotherapy. The New York Times October 9, 1994, pp 1, 50.
20. Pollock EJ. Managed care’s focus on psychiatric drugs alarms many doctors. Wall Street Journal December 1, 1995, pp A1, A11.
21. Hymowitz C. High anxiety: in the name of Freud, why are patients complaining so much? Wall Street Journal December 12, 1995, pp A1, A10.
22. Kalman TP, Goldstein MA. Satisfaction of Manhattan psychiatrists with private practice: assessing the impact of managed care. Medscape Psychiatry & Mental Health eJournal 1998; 3(1).
23. Freudenberger HJ. Staff burn-out. Journal of Social Issues 1974;30:159-65.
24. Yalom ID. Love’s executioner and other tales of psychotherapy. New York: Harper Collins; 1989.
25. Maslach C, Leiter M. The truth about burnout: How organizations cause personal stress and what to do about it. San Francisco, CA: Jossey-Bass Inc.; 1997.
26. Remen RN. Recapturing the soul of medicine: Physicians need to reclaim meaning in their lives. West J Med 2001;174(1):4-5.
27. Figley CR. Compassion fatigue: Coping with secondary post traumatic stress disorder in those who treat the traumatized. New York: Brunner/Mazel; 1995.
28. Glouberman D. The joy of burnout: How the end of the world can be a new beginning. Makawao, HI: Inner Ocean Publishing; 2003.
29. Emmons RA, McCullough ME. Counting blessings versus burdens: Experimental studies of gratitude and subjective well-being in daily life. J Pers Soc Psychol 2003;84(2):377-89.
30. Seligman ME. Positive psychology process: Empirical validation of interventions. Am Psychol 2005;60(5):410-21.
31. Sheldon KM, Lyubomirsky S. How to increase and sustain positive emotion: The benefits of expressing gratitude and visualizing best possible selves. Journal of Positive Psychology, in press.
32. Remen RN. Kitchen table wisdom: Stories that heal. New York: Riverhead Books; 1997.
1. Council on Scientific Affairs: Results and implications of the AMA-APA Physician Mortality Project Stage 2. JAMA 1987;257:2949-53.
2. Hawton K, Clements A, Sakarovitch C, et al. Suicide in doctors: A study of risk according to gender, seniority and specialty in medical practitioners in England and Wales, 1979-1995. J Epidemiol Community Health 2001;55:296-300.
3. Rollman l, Mead LA, Wang N, et al. Medical specialty and the incidence of divorce. N Engl J Med 1997;336(11):800-3.
4. Sotile WM, Sotile MO. The medical marriage: A couple’s survival guide. New York: Carol Publishing; 1996.
5. Pitts FN, Schuller AB, Rich CL, et al. Suicide among U.S. women physicians 1967-1972. Am J Psychiatry 1979;136:694-6.
6. Hughes PH, Storr CL, Brandenburg NA, et al. Physician substance use by medical specialty. J Addict Dis 1999;8(2):23-37.
7. McAuliffe W, Rohman M, Santangelo S, et al. Psychoactive drug use among practicing physicians and medical students. N Engl J Med 1986;315:805-10.
8. Hughes P, Conard S, Baldwin D, et al. Resident physician substance use by specialty. Am J Psychiatry 1992;149:1348-54.
9. Maddux J, Tinnerman I, Costello R. Use of psychoactive substances by residents. J Med Educ 1987;62:852-4.
10. O’Connor PG, Spickard A. Physician impairment by substance abuse. Med Clin North Am 1997;81:1037-52.
11. Craig AG, Pitts FN. Suicide by physicians. Dis Nerv Syst 1968;29:763-72.
12. Rich CL, Pitts FN. Suicide by psychiatrists: a study of medical specialists among 18,730 consecutive physician deaths during a five-year period, 1967-72. J Clin Psychiatry 1980;41:261-3.
13. Montenegro R. A new environmental tragedy: The burn-out of mental health workers. In: Spinetti G, Janiri L (eds). Ecologia e Psichiatria. Rome: CIC Edizioni; 2001.
14. Maslach C. Burnout: The cost of caring. New York: Prentice Hall; 1982.
15. Pfifferling JH, Gilley K. Putting ‘life’ back into your professional life. Fam Pract Manag 1999;6(6):36-42.
16. Adapted from Bits × Pieces January 7 1993. Author unknown.
17. Private Practice Holds Its Own. Psychiatric News April 7, 1995, pp 11, 28.
18. Mizner GL. Current problems in the private practice of psychotherapy. Psychiatric Times August 1994, p 19.
19. Henneberger M. Managed care changing practice of psychotherapy. The New York Times October 9, 1994, pp 1, 50.
20. Pollock EJ. Managed care’s focus on psychiatric drugs alarms many doctors. Wall Street Journal December 1, 1995, pp A1, A11.
21. Hymowitz C. High anxiety: in the name of Freud, why are patients complaining so much? Wall Street Journal December 12, 1995, pp A1, A10.
22. Kalman TP, Goldstein MA. Satisfaction of Manhattan psychiatrists with private practice: assessing the impact of managed care. Medscape Psychiatry & Mental Health eJournal 1998; 3(1).
23. Freudenberger HJ. Staff burn-out. Journal of Social Issues 1974;30:159-65.
24. Yalom ID. Love’s executioner and other tales of psychotherapy. New York: Harper Collins; 1989.
25. Maslach C, Leiter M. The truth about burnout: How organizations cause personal stress and what to do about it. San Francisco, CA: Jossey-Bass Inc.; 1997.
26. Remen RN. Recapturing the soul of medicine: Physicians need to reclaim meaning in their lives. West J Med 2001;174(1):4-5.
27. Figley CR. Compassion fatigue: Coping with secondary post traumatic stress disorder in those who treat the traumatized. New York: Brunner/Mazel; 1995.
28. Glouberman D. The joy of burnout: How the end of the world can be a new beginning. Makawao, HI: Inner Ocean Publishing; 2003.
29. Emmons RA, McCullough ME. Counting blessings versus burdens: Experimental studies of gratitude and subjective well-being in daily life. J Pers Soc Psychol 2003;84(2):377-89.
30. Seligman ME. Positive psychology process: Empirical validation of interventions. Am Psychol 2005;60(5):410-21.
31. Sheldon KM, Lyubomirsky S. How to increase and sustain positive emotion: The benefits of expressing gratitude and visualizing best possible selves. Journal of Positive Psychology, in press.
32. Remen RN. Kitchen table wisdom: Stories that heal. New York: Riverhead Books; 1997.
U.S. troops returning home: Are you prepared?
National Guard and Army Reserve troops constitute an estimated 30% to 40% of the 1 million-plus U.S. military personnel deployed in Iraq and Afghanistan.1-3 Many of these civilian soldiers—once considered “weekend warriors”—are serving a first combat tour, returning home, and being redeployed for additional tours of duty.
Because of these unprecedented deployment policies, civilian psychiatrists will likely play a greater role in treating combat-related mental health problems than in any previous U.S. war. You may need to provide initial and long-term psychiatric care for reservists and Guard members returning to your community during 2006 and beyond.
To help you prepare, we discuss the combat situations these soldiers are experiencing, types of psychiatric problems they are reporting in anonymous surveys, and their attitudes about seeking psychiatric care. We also offer practical resources on combat-related posttraumatic stress disorder (PTSD) for nonmilitary or Veterans Administration clinicians.
A soldier’s story: ‘He’s always jumpy’
Mr. L, age 39, is supervisor for a local construction company and a sergeant first class with 18 years of Army Reserve service who returned from Iraq 7 months ago. He tells you, “My wife made me come see you—I didn’t want to.”
Though he does not think he needs a psychiatrist, his irritability and poor sleep worry his wife. “He isn’t the same anymore,” she says. “He’s always jumpy.”
Reported psychiatric problems
Stress-related symptoms. Within 4 months of returning home from Iraq or Afghanistan, 3 in 10 soldiers have developed “stress-related mental health problems” such as anxiety, depression, nightmares, anger, and concentration difficulties, reports Army Surgeon General Lt. Gen. Kevin Kiley.4 An unknown smaller percentage were reportedly diagnosed with PTSD.
Strained marriages, suicidal thoughts/feelings, nightmares or flashbacks, and fear of losing control or injuring someone else were among problems soldiers acknowledged during post-deployment health assessments between January and August 2005. In these surveys, 28% of 193,000 returnees endorsed mental health problems, according to the Army Center for Health Promotion and Preventive Medicine (Table 1).5
Table 1
Mental health problems reported by troops returning from combat in Iraq*
| Problem | Number among 193,000 U.S. soldiers |
|---|---|
| Nightmares or unwanted war recollections | 20,000 |
| Might “hurt or lose control” with someone else | 3,700 |
| Suicidal thoughts/feeling better off dead | 1,700 |
| * 28% of returnees reported mental health problems in post-deployment surveys between January and August 2005. | |
| Source: Army Center for Health Promotion and Preventive Medicine, reference 5. | |
Unfortunately, this new information may underestimate the number of returnees with psychiatric problems and the severity of those problems. In an anonymous survey of returning Army and Marine soldiers, Hoge et al7 found that those who met criteria for psychiatric diagnoses were less likely to seek assistance because of perceived stigma and concerns about their military careers than those without a psychiatric diagnosis (Table 2).
Table 2
Perceived barriers to seeking mental health services cited by U.S. soldiers after combat duty in Iraq and Afghanistan*
| Perceived barrier | Met screening criteria for a mental disorder? | |
|---|---|---|
| Yes (N=731) | No (N=5,422) | |
| I would be seen as weak | 65% | 31% |
| My unit leadership might treat me differently | 63% | 33% |
| Members of my unit might have less confidence in me | 59% | 31% |
| I would have difficulty getting time off work for treatment | 55% | 22% |
| My leaders would blame me for the problem | 51% | 20% |
| It would harm my career | 50% | 24% |
| It is difficult to schedule an appointment | 45% | 17% |
| It would be too embarrassing | 41% | 18% |
| I don’t trust mental health professionals | 38% | 17% |
| Mental health care costs too much money | 25% | 10% |
| Mental health care doesn’t work | 25% | 9% |
| I don’t know where to get help | 22% | 6% |
| I don’t have adequate transportation | 18% | 6% |
| * Anonymous survey. Those who met criteria for psychiatric diagnoses were less likely to seek assistance because of perceived stigma and concerns about their military careers than those without a psychiatric diagnosis. | ||
| Source: Adapted and reprinted with permission from Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med 2004;351:13-22. | ||
Gender per se may not be the most important variable; age, number of years in the military, type of military unit, and ethnic group are also risk factors for developing a war-related psychiatric disorder. Further studies of OIF- and OEF-related psychiatric disorders are needed to determine whether female veterans’ clinical needs differ in important ways from those of male veterans.
PTSD in combat veterans
Every war has seen new names and descriptions for combinations of psychological and somatic symptoms resulting from war experiences (Box).9,11 Compared with persons with PTSD from other types of trauma, combat veterans appear to have the highest rate of delayed-onset PTSD and are less responsive to treatment.9
Initial PTSD rates for soldiers returning from Iraq ranged from 12.2% (Marines) to 12.9% (Army), using diagnostic criteria requiring functional impairment.7 These rates are 2.5 times the rate observed before combat (5%) and 3 to 4 times that of the general population (3.6%), using the same methodology.10
If 12.5% of 1 million combat-exposed service members develop PTSD, 125,000 service members may be affected. This rough estimate—7 times the number of personnel officially reported as “wounded”—does not take into account the wide variability of combat exposure among deployed troops or the effects of combat stress interventions (which might decrease the rate). Nor does it consider the impact of multiple rotations and possible decreased combat simulation training in reserve troops (which might increase the rate).
During the Civil War, soldiers with pathologic reactions to combat were described as having “irritable heart” or “soldier’s heart.”9 Since then, every war has seen new names and descriptions for combinations of psychological and somatic symptoms resulting from war experiences.
Affected troops in World War I were said to have “shell shock,” whereas those in World War II and the Korean War had “combat fatigue.” Those fighting in the jungles of Vietnam had posttraumatic stress disorder (PTSD).
Along with evolving psychiatric nomenclature and diagnostic schema, each war—including those in Iraq and Afghanistan—has had unique symptom constellations.11 These differences relate to the contemporary state of scientific and medical knowledge, sociocultural factors, and popular press concerns. Some differences stem from actual or perceived weapon effects (such as chemical warfare or depleted uranium).
For example, World War I physicians at first considered “shell shock” to result from traumatic effects of high-explosive shells on the brain. This explanation proved inadequate when soldiers without direct concussive exposure expressed trauma-related symptoms.12
To develop, PTSD requires synergy between a severe stressor and a neurobiologic response. Because of genetic endowment or experience, not all persons are susceptible to the high levels of stress and associated hypothalamus-pituitary-adrenal axis activation required for the disorder to occur. Specific individual differences in coping, trauma history, and biology may predispose some individuals to PTSD.11
Mr. L’s story: Detached and irritable
As a combat infantryman, Mr. L was in seven fire fights, in which three of his buddies died. In responding to your questions, he admits feeling disconnected from his children and from his old friends who did not go to Iraq. He describes frequent arguments with his wife, though they had rarely argued previously. He denies psychiatric problems before his 12-month rotation in Iraq.
Being wounded in combat, surviving multiple life-threatening events, and experiencing combat of greater intensity and duration all increase the risk of developing PTSD. Mr. L’s multiple fire fights, loss of three friends, and other combat experiences place him at high risk for developing PTSD.
Typical combat experiences in Iraq and Afghanistan reported by Army and Marine troops are outlined in Table 3.7 Familiarizing yourself with these experiences can help you interview combat-exposed patients after you develop trust and rapport with them.
Table 3
Combat experiences reported by U.S. troops
after deployment in Iraq or Afghanistan
| Experience | Army groups | Marine group | |
|---|---|---|---|
| Afghanistan | Iraq | ||
| Being attacked or ambushed | 58% | 89% | 95% |
| Receiving incoming artillery, rocket or mortar fire | 84% | 86% | 92% |
| Being shot at or receiving small-arms fire | 66% | 93% | 97% |
| Shooting or directing fire at the enemy | 27% | 77% | 87% |
| Being responsible for death of an enemy combatant | 12% | 48% | 65% |
| Being responsible for death of a noncombatant | 1% | 14% | 28% |
| Seeing dead bodies or human remains | 39% | 95% | 94% |
| Seeing dead or seriously injured Americans | 30% | 65% | 75% |
| Knowing someone seriously injured or killed | 43% | 86% | 87% |
| Participating in demining operations | 16% | 38% | 34% |
| Seeing ill or injured women or children whom you were unable to help | 46% | 69% | 83% |
| Being wounded or injured | 5% | 14% | 9% |
| Being shot or hit, but protective gear saved you | * | 8% | 10% |
| Having a buddy who was near you shot or hit | * | 22% | 26% |
| Clearing or searching homes or buildings | 57% | 80% | 86% |
| Engaging in hand-to-hand combat | 3% | 22% | 9% |
| Saved the life of a soldier or civilian | 6% | 21% | 19% |
| * Question not included in this survey | |||
| Source: Adapted and reprinted with permission from Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med 2004; 351:13-22. | |||
Workup of combat veterans
Military psychiatrists provide support and treatment during and immediately after combat, but they do not associate acute reactions with specific psychiatric diagnoses to avoid “pathologizing” brief reactions to combat. To provide appropriate treatment for returning troops, however, you will need to characterize clinically significant psychopathology by using DSM IV-TR criteria for acute stress disorder and PTSD.12
We recommend that you manage a returning service member according to usual clinical practice. This includes a thorough history and appropriate medical and laboratory workup. Because soldiers commonly minimize their symptoms and concerns—particularly if they fear full disclosure could jeopardize their military careers—consider including collateral history from the patient’s family and friends in your assessment.
Differential diagnosis of mental disorders in combat troops is broad. You will need to obtain a thorough substance abuse history, with particular attention to use of alcohol to self-medicate symptoms. It will be important to assess safety issues, including potential for suicide, homicide, and domestic violence.
Many soldiers report difficulties with re-entering family life. Marital and sexual problems may develop because of role changes that occurred during a long separation. Pre-existing marital problems may be exacerbated, and both military members and spouses may express concerns about infidelity. Separation and divorce rates may be high.
Mr. L’s story: Alcohol ‘helps me sleep’
When you ask Mr. L about his use of alcohol, he notes that he was cited for driving while intoxicated at age 28. “I used to have a problem with drinking, but after my ticket I didn’t drink ‘til I came back from Iraq,” he says. “Now it’s the only thing that calms me down and helps me sleep.”
Comorbid diagnoses associated with PTSD are the rule. Mr. L’s drinking to self-medicate his PTSD symptoms puts him at risk of redeveloping alcohol problems. Use current best practices for managing depression, anxiety disorders, and substance abuse (if present) to guide treatment.
Suicidal behavior has also been strongly associated with PTSD.13 Thus, address Mr. L’s access to firearms, and include suicide assessment and regular followup in any treatment plan.
Head injuries in iraq
The use of effective body armor has dramatically changed the types of wounds and injuries sustained in combat. Kevlar body armor has decreased the frequency of mortal chest and abdominal wounds, leading to an unprecedented proportion of head and neck wounds, including eye injuries. In the war in Iraq and Afghanistan, 22% of evacuated casualties have injuries to the head, neck, and face.14
At the same time, rapid treatment of open and closed head injuries—often fatal in past wars—has improved survival. As a result, the prevalence of traumatic brain injury in veteran populations is believed to be substantially higher now than in previous conflicts.15
Mr. L’s story: ‘I forget everything’
Mr. L reports that after he served 8 months in Iraq, his vehicle was destroyed by a roadside bomb. He lost consciousness and was hospitalized briefly before returning to duty and completing his tour.
“I’m having trouble concentrating at work, and it seems like I forget everything,” he says. “My boss has complained about mistakes I make when planning our construction jobs. Could that explosion be causing my problems?”
Mr. L’s loss of consciousness associated with a blast injury and his cognitive complaints suggest possible mild traumatic brain injury. Consider neuropsychological testing and brain imaging studies, along with possible referral to appropriate rehabilitation programs if needed.
Treatment resources
The Iraq War Clinician Guide16 delineates military approaches to prevention, as well as acute intervention and initial treatment after evacuation from a war zone. This guide also:
- outlines rationales for removing affected service members from combat and eventually returning them to duty or medically retiring them if severe symptoms continue to interfere with ability to function.
- describes the biopsychosocial approach used by the Walter Reed Army Medical Center Psychiatric Consultation Service to address the multifactorial needs of the traumatized amputee.
- National Center for PTSD: The War in Iraq. www.ncptsd.va.gov/topics/war.html
Comprehensive Web site designated by Congress to provide information for military veterans with PTSD. Clinician’s guide available, plus fact sheets for family and patients. - National Center for PTSD. Iraq War Clinician Guide (2nd ed). www.ncptsd.va.gov/war/iraq_clinician_guide_v2/iraq_clinician_guide_v2.pdf
Detailed guide for treating the soldier in combat. Includes treatment options for PTSD and the veteran with amputation. - U.S. Army Center for Health Promotion and Preventive Medicine. Supporting Guidelines. www.pdhealth.mil/clinicians/support.asp
Collection of guidelines for PTSD, major depression, and medically unexplained symptoms following combat. - Military One Source. www.militaryonesource.com
Resource for active duty and reserve soldiers and family members. Portal for support services, policies, and education. Brief confidential counseling support for soldiers and family members. - Veterans Administration (VA)/Department of Defense (DOD) Clinical Practice Guideline for Management of PTSD, January 2004. www.oqp.med.va.gov/cpg/PTSD/PTSD_Base.htm
Includes list of clinical trials, medication dosing, and evidence basis for treatment with pharmacotherapy and psychotherapy. - American Psychiatric Association. Practice Guideline for the Treatment of Patients With Acute Stress Disorder and Posttraumatic Stress Disorder. http://www.psych.org/psych_pract/treatg/pg/PTSD-PG-PartsA-B-C-New.pdf
Background and guidelines for managing PTSD, including treatment recommendations, evidence basis, background, and areas for future research.
Disclosure
Drs. Lineberry, Bostwick, and Rundell previously served on active duty in the U.S. Air Force for 12, 5, and 23 years, respectively.
1. Benjamin M. How many have gone to war? Salon.com April 12, 2005. Available at: http://www.salon.com/news/feature/2005/04/12/troops_numbers/index_np.html. Accessed Dec. 13, 2005.
2. United States Government Accountability Office. Testimony before the Committee on Government Reform, House of Representatives. Reserve forces. Army National Guard’s role, organization, and equipment need to be reexamined. Oct. 20, 2005. Available at: http://www.gao.gov/new.items/d06170t.pdf. Accessed Oct. 26, 2005.
3. Wetzel K. Senators told of Guard struggles. Seattle Times Oct. 20, 2005. Available at: http://seattletimes.nwsource.com/html/local-news/2002571967_soldiers20m.html. Accessed Oct. 26, 2005.
4. Associated Press. Survey: 30% of returning Iraq vets suffer mental ills. USA Today July 28, 2005. Available at: http://www.usatoday.com/news/health/2005-07-28-iraq-vets-health_x.htm?csp=34. Accessed Oct. 26, 2005.
5. Zoroya G. One in four Iraq vets ailing on return. USA Today Oct. 18, 2005. Available at: http://www.usatoday.com/news/world/iraq/2005-10-18-troops-side_x.htm. Accessed Oct. 26, 2005.
6. Zoroya G. Troops screened as never before. USA Today Oct. 18, 2005. Available at: http://www.usatoday.com/news/world/iraq/2005-10-18-troops-stress-side_x.htm. Accessed Oct. 26, 2005.
7. Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med 2004;351:13-22.
8. Prigerson HG, Maciejewski PK, Rosenheck RA. Combat trauma: trauma with highest risk of delayed onset and unresolved posttraumatic stress disorder symptoms, unemployment, and abuse among men. J Nerv Ment Dis 2001;189:99-108.
9. Narrow WE, Rae DS, Robins LN, Regier DA. Revised prevalence estimates of mental disorders in the United States: using a clinical significance criterion to reconcile 2 surveys’ estimates. Arch Gen Psychiatry 2002;59:115-23.
10. Lieberman HR, Bathalon GP, Falco CN, et al. Severe decrements in cognition, function and mood induced by sleep loss, heat, dehydration, and under-nutrition during simulated combat. Biol Psychiatry 2005;57:422-9.
11. Nemeroff CB, Bremner JD, Foa EB, et al. Posttraumatic stress disorder: a state-of-the-science review. J Psychiatr Res (In press; Oct 2005 epub ahead of publication).
12. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington, DC: American Psychiatric Association; 2000.
13. Sareen J, Houlahan T, Cox BJ, Asmundson GJ. Anxiety disorders associated with suicidal ideation and suicide attempts in the national comorbidity survey. J Nerv Ment Dis 2005;193:450-4.
14. Xydakis MS, Fravell MD, Nasser KE, Casler JD. Analysis of battlefield head and neck injuries in Iraq and Afghanistan. Otolarnygol Head Neck Surg 2005;133:497-504.
15. Okie S. Traumatic brain injury in the war zone. N Engl J Med 2005;352(20):2043-7.
16. Cozza SJ, Benedek DM, Bradley JC, et al. Topics specific to the psychiatric treatment of military personnel. In: Schnurr PP, Cozza SJ (eds). Iraq War clinician guide (2nd ed). Washington, DC: National Center for PTSD. Walter Reed Army Medical Center; 2004:4-20. Available at: http://www.ncptsd.va.gov/war/iraq_clinician_guide_v2/iraq_clinician_guide_v2.pdf Accessed Oct. 26, 2005.
National Guard and Army Reserve troops constitute an estimated 30% to 40% of the 1 million-plus U.S. military personnel deployed in Iraq and Afghanistan.1-3 Many of these civilian soldiers—once considered “weekend warriors”—are serving a first combat tour, returning home, and being redeployed for additional tours of duty.
Because of these unprecedented deployment policies, civilian psychiatrists will likely play a greater role in treating combat-related mental health problems than in any previous U.S. war. You may need to provide initial and long-term psychiatric care for reservists and Guard members returning to your community during 2006 and beyond.
To help you prepare, we discuss the combat situations these soldiers are experiencing, types of psychiatric problems they are reporting in anonymous surveys, and their attitudes about seeking psychiatric care. We also offer practical resources on combat-related posttraumatic stress disorder (PTSD) for nonmilitary or Veterans Administration clinicians.
A soldier’s story: ‘He’s always jumpy’
Mr. L, age 39, is supervisor for a local construction company and a sergeant first class with 18 years of Army Reserve service who returned from Iraq 7 months ago. He tells you, “My wife made me come see you—I didn’t want to.”
Though he does not think he needs a psychiatrist, his irritability and poor sleep worry his wife. “He isn’t the same anymore,” she says. “He’s always jumpy.”
Reported psychiatric problems
Stress-related symptoms. Within 4 months of returning home from Iraq or Afghanistan, 3 in 10 soldiers have developed “stress-related mental health problems” such as anxiety, depression, nightmares, anger, and concentration difficulties, reports Army Surgeon General Lt. Gen. Kevin Kiley.4 An unknown smaller percentage were reportedly diagnosed with PTSD.
Strained marriages, suicidal thoughts/feelings, nightmares or flashbacks, and fear of losing control or injuring someone else were among problems soldiers acknowledged during post-deployment health assessments between January and August 2005. In these surveys, 28% of 193,000 returnees endorsed mental health problems, according to the Army Center for Health Promotion and Preventive Medicine (Table 1).5
Table 1
Mental health problems reported by troops returning from combat in Iraq*
| Problem | Number among 193,000 U.S. soldiers |
|---|---|
| Nightmares or unwanted war recollections | 20,000 |
| Might “hurt or lose control” with someone else | 3,700 |
| Suicidal thoughts/feeling better off dead | 1,700 |
| * 28% of returnees reported mental health problems in post-deployment surveys between January and August 2005. | |
| Source: Army Center for Health Promotion and Preventive Medicine, reference 5. | |
Unfortunately, this new information may underestimate the number of returnees with psychiatric problems and the severity of those problems. In an anonymous survey of returning Army and Marine soldiers, Hoge et al7 found that those who met criteria for psychiatric diagnoses were less likely to seek assistance because of perceived stigma and concerns about their military careers than those without a psychiatric diagnosis (Table 2).
Table 2
Perceived barriers to seeking mental health services cited by U.S. soldiers after combat duty in Iraq and Afghanistan*
| Perceived barrier | Met screening criteria for a mental disorder? | |
|---|---|---|
| Yes (N=731) | No (N=5,422) | |
| I would be seen as weak | 65% | 31% |
| My unit leadership might treat me differently | 63% | 33% |
| Members of my unit might have less confidence in me | 59% | 31% |
| I would have difficulty getting time off work for treatment | 55% | 22% |
| My leaders would blame me for the problem | 51% | 20% |
| It would harm my career | 50% | 24% |
| It is difficult to schedule an appointment | 45% | 17% |
| It would be too embarrassing | 41% | 18% |
| I don’t trust mental health professionals | 38% | 17% |
| Mental health care costs too much money | 25% | 10% |
| Mental health care doesn’t work | 25% | 9% |
| I don’t know where to get help | 22% | 6% |
| I don’t have adequate transportation | 18% | 6% |
| * Anonymous survey. Those who met criteria for psychiatric diagnoses were less likely to seek assistance because of perceived stigma and concerns about their military careers than those without a psychiatric diagnosis. | ||
| Source: Adapted and reprinted with permission from Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med 2004;351:13-22. | ||
Gender per se may not be the most important variable; age, number of years in the military, type of military unit, and ethnic group are also risk factors for developing a war-related psychiatric disorder. Further studies of OIF- and OEF-related psychiatric disorders are needed to determine whether female veterans’ clinical needs differ in important ways from those of male veterans.
PTSD in combat veterans
Every war has seen new names and descriptions for combinations of psychological and somatic symptoms resulting from war experiences (Box).9,11 Compared with persons with PTSD from other types of trauma, combat veterans appear to have the highest rate of delayed-onset PTSD and are less responsive to treatment.9
Initial PTSD rates for soldiers returning from Iraq ranged from 12.2% (Marines) to 12.9% (Army), using diagnostic criteria requiring functional impairment.7 These rates are 2.5 times the rate observed before combat (5%) and 3 to 4 times that of the general population (3.6%), using the same methodology.10
If 12.5% of 1 million combat-exposed service members develop PTSD, 125,000 service members may be affected. This rough estimate—7 times the number of personnel officially reported as “wounded”—does not take into account the wide variability of combat exposure among deployed troops or the effects of combat stress interventions (which might decrease the rate). Nor does it consider the impact of multiple rotations and possible decreased combat simulation training in reserve troops (which might increase the rate).
During the Civil War, soldiers with pathologic reactions to combat were described as having “irritable heart” or “soldier’s heart.”9 Since then, every war has seen new names and descriptions for combinations of psychological and somatic symptoms resulting from war experiences.
Affected troops in World War I were said to have “shell shock,” whereas those in World War II and the Korean War had “combat fatigue.” Those fighting in the jungles of Vietnam had posttraumatic stress disorder (PTSD).
Along with evolving psychiatric nomenclature and diagnostic schema, each war—including those in Iraq and Afghanistan—has had unique symptom constellations.11 These differences relate to the contemporary state of scientific and medical knowledge, sociocultural factors, and popular press concerns. Some differences stem from actual or perceived weapon effects (such as chemical warfare or depleted uranium).
For example, World War I physicians at first considered “shell shock” to result from traumatic effects of high-explosive shells on the brain. This explanation proved inadequate when soldiers without direct concussive exposure expressed trauma-related symptoms.12
To develop, PTSD requires synergy between a severe stressor and a neurobiologic response. Because of genetic endowment or experience, not all persons are susceptible to the high levels of stress and associated hypothalamus-pituitary-adrenal axis activation required for the disorder to occur. Specific individual differences in coping, trauma history, and biology may predispose some individuals to PTSD.11
Mr. L’s story: Detached and irritable
As a combat infantryman, Mr. L was in seven fire fights, in which three of his buddies died. In responding to your questions, he admits feeling disconnected from his children and from his old friends who did not go to Iraq. He describes frequent arguments with his wife, though they had rarely argued previously. He denies psychiatric problems before his 12-month rotation in Iraq.
Being wounded in combat, surviving multiple life-threatening events, and experiencing combat of greater intensity and duration all increase the risk of developing PTSD. Mr. L’s multiple fire fights, loss of three friends, and other combat experiences place him at high risk for developing PTSD.
Typical combat experiences in Iraq and Afghanistan reported by Army and Marine troops are outlined in Table 3.7 Familiarizing yourself with these experiences can help you interview combat-exposed patients after you develop trust and rapport with them.
Table 3
Combat experiences reported by U.S. troops
after deployment in Iraq or Afghanistan
| Experience | Army groups | Marine group | |
|---|---|---|---|
| Afghanistan | Iraq | ||
| Being attacked or ambushed | 58% | 89% | 95% |
| Receiving incoming artillery, rocket or mortar fire | 84% | 86% | 92% |
| Being shot at or receiving small-arms fire | 66% | 93% | 97% |
| Shooting or directing fire at the enemy | 27% | 77% | 87% |
| Being responsible for death of an enemy combatant | 12% | 48% | 65% |
| Being responsible for death of a noncombatant | 1% | 14% | 28% |
| Seeing dead bodies or human remains | 39% | 95% | 94% |
| Seeing dead or seriously injured Americans | 30% | 65% | 75% |
| Knowing someone seriously injured or killed | 43% | 86% | 87% |
| Participating in demining operations | 16% | 38% | 34% |
| Seeing ill or injured women or children whom you were unable to help | 46% | 69% | 83% |
| Being wounded or injured | 5% | 14% | 9% |
| Being shot or hit, but protective gear saved you | * | 8% | 10% |
| Having a buddy who was near you shot or hit | * | 22% | 26% |
| Clearing or searching homes or buildings | 57% | 80% | 86% |
| Engaging in hand-to-hand combat | 3% | 22% | 9% |
| Saved the life of a soldier or civilian | 6% | 21% | 19% |
| * Question not included in this survey | |||
| Source: Adapted and reprinted with permission from Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med 2004; 351:13-22. | |||
Workup of combat veterans
Military psychiatrists provide support and treatment during and immediately after combat, but they do not associate acute reactions with specific psychiatric diagnoses to avoid “pathologizing” brief reactions to combat. To provide appropriate treatment for returning troops, however, you will need to characterize clinically significant psychopathology by using DSM IV-TR criteria for acute stress disorder and PTSD.12
We recommend that you manage a returning service member according to usual clinical practice. This includes a thorough history and appropriate medical and laboratory workup. Because soldiers commonly minimize their symptoms and concerns—particularly if they fear full disclosure could jeopardize their military careers—consider including collateral history from the patient’s family and friends in your assessment.
Differential diagnosis of mental disorders in combat troops is broad. You will need to obtain a thorough substance abuse history, with particular attention to use of alcohol to self-medicate symptoms. It will be important to assess safety issues, including potential for suicide, homicide, and domestic violence.
Many soldiers report difficulties with re-entering family life. Marital and sexual problems may develop because of role changes that occurred during a long separation. Pre-existing marital problems may be exacerbated, and both military members and spouses may express concerns about infidelity. Separation and divorce rates may be high.
Mr. L’s story: Alcohol ‘helps me sleep’
When you ask Mr. L about his use of alcohol, he notes that he was cited for driving while intoxicated at age 28. “I used to have a problem with drinking, but after my ticket I didn’t drink ‘til I came back from Iraq,” he says. “Now it’s the only thing that calms me down and helps me sleep.”
Comorbid diagnoses associated with PTSD are the rule. Mr. L’s drinking to self-medicate his PTSD symptoms puts him at risk of redeveloping alcohol problems. Use current best practices for managing depression, anxiety disorders, and substance abuse (if present) to guide treatment.
Suicidal behavior has also been strongly associated with PTSD.13 Thus, address Mr. L’s access to firearms, and include suicide assessment and regular followup in any treatment plan.
Head injuries in iraq
The use of effective body armor has dramatically changed the types of wounds and injuries sustained in combat. Kevlar body armor has decreased the frequency of mortal chest and abdominal wounds, leading to an unprecedented proportion of head and neck wounds, including eye injuries. In the war in Iraq and Afghanistan, 22% of evacuated casualties have injuries to the head, neck, and face.14
At the same time, rapid treatment of open and closed head injuries—often fatal in past wars—has improved survival. As a result, the prevalence of traumatic brain injury in veteran populations is believed to be substantially higher now than in previous conflicts.15
Mr. L’s story: ‘I forget everything’
Mr. L reports that after he served 8 months in Iraq, his vehicle was destroyed by a roadside bomb. He lost consciousness and was hospitalized briefly before returning to duty and completing his tour.
“I’m having trouble concentrating at work, and it seems like I forget everything,” he says. “My boss has complained about mistakes I make when planning our construction jobs. Could that explosion be causing my problems?”
Mr. L’s loss of consciousness associated with a blast injury and his cognitive complaints suggest possible mild traumatic brain injury. Consider neuropsychological testing and brain imaging studies, along with possible referral to appropriate rehabilitation programs if needed.
Treatment resources
The Iraq War Clinician Guide16 delineates military approaches to prevention, as well as acute intervention and initial treatment after evacuation from a war zone. This guide also:
- outlines rationales for removing affected service members from combat and eventually returning them to duty or medically retiring them if severe symptoms continue to interfere with ability to function.
- describes the biopsychosocial approach used by the Walter Reed Army Medical Center Psychiatric Consultation Service to address the multifactorial needs of the traumatized amputee.
- National Center for PTSD: The War in Iraq. www.ncptsd.va.gov/topics/war.html
Comprehensive Web site designated by Congress to provide information for military veterans with PTSD. Clinician’s guide available, plus fact sheets for family and patients. - National Center for PTSD. Iraq War Clinician Guide (2nd ed). www.ncptsd.va.gov/war/iraq_clinician_guide_v2/iraq_clinician_guide_v2.pdf
Detailed guide for treating the soldier in combat. Includes treatment options for PTSD and the veteran with amputation. - U.S. Army Center for Health Promotion and Preventive Medicine. Supporting Guidelines. www.pdhealth.mil/clinicians/support.asp
Collection of guidelines for PTSD, major depression, and medically unexplained symptoms following combat. - Military One Source. www.militaryonesource.com
Resource for active duty and reserve soldiers and family members. Portal for support services, policies, and education. Brief confidential counseling support for soldiers and family members. - Veterans Administration (VA)/Department of Defense (DOD) Clinical Practice Guideline for Management of PTSD, January 2004. www.oqp.med.va.gov/cpg/PTSD/PTSD_Base.htm
Includes list of clinical trials, medication dosing, and evidence basis for treatment with pharmacotherapy and psychotherapy. - American Psychiatric Association. Practice Guideline for the Treatment of Patients With Acute Stress Disorder and Posttraumatic Stress Disorder. http://www.psych.org/psych_pract/treatg/pg/PTSD-PG-PartsA-B-C-New.pdf
Background and guidelines for managing PTSD, including treatment recommendations, evidence basis, background, and areas for future research.
Disclosure
Drs. Lineberry, Bostwick, and Rundell previously served on active duty in the U.S. Air Force for 12, 5, and 23 years, respectively.
National Guard and Army Reserve troops constitute an estimated 30% to 40% of the 1 million-plus U.S. military personnel deployed in Iraq and Afghanistan.1-3 Many of these civilian soldiers—once considered “weekend warriors”—are serving a first combat tour, returning home, and being redeployed for additional tours of duty.
Because of these unprecedented deployment policies, civilian psychiatrists will likely play a greater role in treating combat-related mental health problems than in any previous U.S. war. You may need to provide initial and long-term psychiatric care for reservists and Guard members returning to your community during 2006 and beyond.
To help you prepare, we discuss the combat situations these soldiers are experiencing, types of psychiatric problems they are reporting in anonymous surveys, and their attitudes about seeking psychiatric care. We also offer practical resources on combat-related posttraumatic stress disorder (PTSD) for nonmilitary or Veterans Administration clinicians.
A soldier’s story: ‘He’s always jumpy’
Mr. L, age 39, is supervisor for a local construction company and a sergeant first class with 18 years of Army Reserve service who returned from Iraq 7 months ago. He tells you, “My wife made me come see you—I didn’t want to.”
Though he does not think he needs a psychiatrist, his irritability and poor sleep worry his wife. “He isn’t the same anymore,” she says. “He’s always jumpy.”
Reported psychiatric problems
Stress-related symptoms. Within 4 months of returning home from Iraq or Afghanistan, 3 in 10 soldiers have developed “stress-related mental health problems” such as anxiety, depression, nightmares, anger, and concentration difficulties, reports Army Surgeon General Lt. Gen. Kevin Kiley.4 An unknown smaller percentage were reportedly diagnosed with PTSD.
Strained marriages, suicidal thoughts/feelings, nightmares or flashbacks, and fear of losing control or injuring someone else were among problems soldiers acknowledged during post-deployment health assessments between January and August 2005. In these surveys, 28% of 193,000 returnees endorsed mental health problems, according to the Army Center for Health Promotion and Preventive Medicine (Table 1).5
Table 1
Mental health problems reported by troops returning from combat in Iraq*
| Problem | Number among 193,000 U.S. soldiers |
|---|---|
| Nightmares or unwanted war recollections | 20,000 |
| Might “hurt or lose control” with someone else | 3,700 |
| Suicidal thoughts/feeling better off dead | 1,700 |
| * 28% of returnees reported mental health problems in post-deployment surveys between January and August 2005. | |
| Source: Army Center for Health Promotion and Preventive Medicine, reference 5. | |
Unfortunately, this new information may underestimate the number of returnees with psychiatric problems and the severity of those problems. In an anonymous survey of returning Army and Marine soldiers, Hoge et al7 found that those who met criteria for psychiatric diagnoses were less likely to seek assistance because of perceived stigma and concerns about their military careers than those without a psychiatric diagnosis (Table 2).
Table 2
Perceived barriers to seeking mental health services cited by U.S. soldiers after combat duty in Iraq and Afghanistan*
| Perceived barrier | Met screening criteria for a mental disorder? | |
|---|---|---|
| Yes (N=731) | No (N=5,422) | |
| I would be seen as weak | 65% | 31% |
| My unit leadership might treat me differently | 63% | 33% |
| Members of my unit might have less confidence in me | 59% | 31% |
| I would have difficulty getting time off work for treatment | 55% | 22% |
| My leaders would blame me for the problem | 51% | 20% |
| It would harm my career | 50% | 24% |
| It is difficult to schedule an appointment | 45% | 17% |
| It would be too embarrassing | 41% | 18% |
| I don’t trust mental health professionals | 38% | 17% |
| Mental health care costs too much money | 25% | 10% |
| Mental health care doesn’t work | 25% | 9% |
| I don’t know where to get help | 22% | 6% |
| I don’t have adequate transportation | 18% | 6% |
| * Anonymous survey. Those who met criteria for psychiatric diagnoses were less likely to seek assistance because of perceived stigma and concerns about their military careers than those without a psychiatric diagnosis. | ||
| Source: Adapted and reprinted with permission from Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med 2004;351:13-22. | ||
Gender per se may not be the most important variable; age, number of years in the military, type of military unit, and ethnic group are also risk factors for developing a war-related psychiatric disorder. Further studies of OIF- and OEF-related psychiatric disorders are needed to determine whether female veterans’ clinical needs differ in important ways from those of male veterans.
PTSD in combat veterans
Every war has seen new names and descriptions for combinations of psychological and somatic symptoms resulting from war experiences (Box).9,11 Compared with persons with PTSD from other types of trauma, combat veterans appear to have the highest rate of delayed-onset PTSD and are less responsive to treatment.9
Initial PTSD rates for soldiers returning from Iraq ranged from 12.2% (Marines) to 12.9% (Army), using diagnostic criteria requiring functional impairment.7 These rates are 2.5 times the rate observed before combat (5%) and 3 to 4 times that of the general population (3.6%), using the same methodology.10
If 12.5% of 1 million combat-exposed service members develop PTSD, 125,000 service members may be affected. This rough estimate—7 times the number of personnel officially reported as “wounded”—does not take into account the wide variability of combat exposure among deployed troops or the effects of combat stress interventions (which might decrease the rate). Nor does it consider the impact of multiple rotations and possible decreased combat simulation training in reserve troops (which might increase the rate).
During the Civil War, soldiers with pathologic reactions to combat were described as having “irritable heart” or “soldier’s heart.”9 Since then, every war has seen new names and descriptions for combinations of psychological and somatic symptoms resulting from war experiences.
Affected troops in World War I were said to have “shell shock,” whereas those in World War II and the Korean War had “combat fatigue.” Those fighting in the jungles of Vietnam had posttraumatic stress disorder (PTSD).
Along with evolving psychiatric nomenclature and diagnostic schema, each war—including those in Iraq and Afghanistan—has had unique symptom constellations.11 These differences relate to the contemporary state of scientific and medical knowledge, sociocultural factors, and popular press concerns. Some differences stem from actual or perceived weapon effects (such as chemical warfare or depleted uranium).
For example, World War I physicians at first considered “shell shock” to result from traumatic effects of high-explosive shells on the brain. This explanation proved inadequate when soldiers without direct concussive exposure expressed trauma-related symptoms.12
To develop, PTSD requires synergy between a severe stressor and a neurobiologic response. Because of genetic endowment or experience, not all persons are susceptible to the high levels of stress and associated hypothalamus-pituitary-adrenal axis activation required for the disorder to occur. Specific individual differences in coping, trauma history, and biology may predispose some individuals to PTSD.11
Mr. L’s story: Detached and irritable
As a combat infantryman, Mr. L was in seven fire fights, in which three of his buddies died. In responding to your questions, he admits feeling disconnected from his children and from his old friends who did not go to Iraq. He describes frequent arguments with his wife, though they had rarely argued previously. He denies psychiatric problems before his 12-month rotation in Iraq.
Being wounded in combat, surviving multiple life-threatening events, and experiencing combat of greater intensity and duration all increase the risk of developing PTSD. Mr. L’s multiple fire fights, loss of three friends, and other combat experiences place him at high risk for developing PTSD.
Typical combat experiences in Iraq and Afghanistan reported by Army and Marine troops are outlined in Table 3.7 Familiarizing yourself with these experiences can help you interview combat-exposed patients after you develop trust and rapport with them.
Table 3
Combat experiences reported by U.S. troops
after deployment in Iraq or Afghanistan
| Experience | Army groups | Marine group | |
|---|---|---|---|
| Afghanistan | Iraq | ||
| Being attacked or ambushed | 58% | 89% | 95% |
| Receiving incoming artillery, rocket or mortar fire | 84% | 86% | 92% |
| Being shot at or receiving small-arms fire | 66% | 93% | 97% |
| Shooting or directing fire at the enemy | 27% | 77% | 87% |
| Being responsible for death of an enemy combatant | 12% | 48% | 65% |
| Being responsible for death of a noncombatant | 1% | 14% | 28% |
| Seeing dead bodies or human remains | 39% | 95% | 94% |
| Seeing dead or seriously injured Americans | 30% | 65% | 75% |
| Knowing someone seriously injured or killed | 43% | 86% | 87% |
| Participating in demining operations | 16% | 38% | 34% |
| Seeing ill or injured women or children whom you were unable to help | 46% | 69% | 83% |
| Being wounded or injured | 5% | 14% | 9% |
| Being shot or hit, but protective gear saved you | * | 8% | 10% |
| Having a buddy who was near you shot or hit | * | 22% | 26% |
| Clearing or searching homes or buildings | 57% | 80% | 86% |
| Engaging in hand-to-hand combat | 3% | 22% | 9% |
| Saved the life of a soldier or civilian | 6% | 21% | 19% |
| * Question not included in this survey | |||
| Source: Adapted and reprinted with permission from Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med 2004; 351:13-22. | |||
Workup of combat veterans
Military psychiatrists provide support and treatment during and immediately after combat, but they do not associate acute reactions with specific psychiatric diagnoses to avoid “pathologizing” brief reactions to combat. To provide appropriate treatment for returning troops, however, you will need to characterize clinically significant psychopathology by using DSM IV-TR criteria for acute stress disorder and PTSD.12
We recommend that you manage a returning service member according to usual clinical practice. This includes a thorough history and appropriate medical and laboratory workup. Because soldiers commonly minimize their symptoms and concerns—particularly if they fear full disclosure could jeopardize their military careers—consider including collateral history from the patient’s family and friends in your assessment.
Differential diagnosis of mental disorders in combat troops is broad. You will need to obtain a thorough substance abuse history, with particular attention to use of alcohol to self-medicate symptoms. It will be important to assess safety issues, including potential for suicide, homicide, and domestic violence.
Many soldiers report difficulties with re-entering family life. Marital and sexual problems may develop because of role changes that occurred during a long separation. Pre-existing marital problems may be exacerbated, and both military members and spouses may express concerns about infidelity. Separation and divorce rates may be high.
Mr. L’s story: Alcohol ‘helps me sleep’
When you ask Mr. L about his use of alcohol, he notes that he was cited for driving while intoxicated at age 28. “I used to have a problem with drinking, but after my ticket I didn’t drink ‘til I came back from Iraq,” he says. “Now it’s the only thing that calms me down and helps me sleep.”
Comorbid diagnoses associated with PTSD are the rule. Mr. L’s drinking to self-medicate his PTSD symptoms puts him at risk of redeveloping alcohol problems. Use current best practices for managing depression, anxiety disorders, and substance abuse (if present) to guide treatment.
Suicidal behavior has also been strongly associated with PTSD.13 Thus, address Mr. L’s access to firearms, and include suicide assessment and regular followup in any treatment plan.
Head injuries in iraq
The use of effective body armor has dramatically changed the types of wounds and injuries sustained in combat. Kevlar body armor has decreased the frequency of mortal chest and abdominal wounds, leading to an unprecedented proportion of head and neck wounds, including eye injuries. In the war in Iraq and Afghanistan, 22% of evacuated casualties have injuries to the head, neck, and face.14
At the same time, rapid treatment of open and closed head injuries—often fatal in past wars—has improved survival. As a result, the prevalence of traumatic brain injury in veteran populations is believed to be substantially higher now than in previous conflicts.15
Mr. L’s story: ‘I forget everything’
Mr. L reports that after he served 8 months in Iraq, his vehicle was destroyed by a roadside bomb. He lost consciousness and was hospitalized briefly before returning to duty and completing his tour.
“I’m having trouble concentrating at work, and it seems like I forget everything,” he says. “My boss has complained about mistakes I make when planning our construction jobs. Could that explosion be causing my problems?”
Mr. L’s loss of consciousness associated with a blast injury and his cognitive complaints suggest possible mild traumatic brain injury. Consider neuropsychological testing and brain imaging studies, along with possible referral to appropriate rehabilitation programs if needed.
Treatment resources
The Iraq War Clinician Guide16 delineates military approaches to prevention, as well as acute intervention and initial treatment after evacuation from a war zone. This guide also:
- outlines rationales for removing affected service members from combat and eventually returning them to duty or medically retiring them if severe symptoms continue to interfere with ability to function.
- describes the biopsychosocial approach used by the Walter Reed Army Medical Center Psychiatric Consultation Service to address the multifactorial needs of the traumatized amputee.
- National Center for PTSD: The War in Iraq. www.ncptsd.va.gov/topics/war.html
Comprehensive Web site designated by Congress to provide information for military veterans with PTSD. Clinician’s guide available, plus fact sheets for family and patients. - National Center for PTSD. Iraq War Clinician Guide (2nd ed). www.ncptsd.va.gov/war/iraq_clinician_guide_v2/iraq_clinician_guide_v2.pdf
Detailed guide for treating the soldier in combat. Includes treatment options for PTSD and the veteran with amputation. - U.S. Army Center for Health Promotion and Preventive Medicine. Supporting Guidelines. www.pdhealth.mil/clinicians/support.asp
Collection of guidelines for PTSD, major depression, and medically unexplained symptoms following combat. - Military One Source. www.militaryonesource.com
Resource for active duty and reserve soldiers and family members. Portal for support services, policies, and education. Brief confidential counseling support for soldiers and family members. - Veterans Administration (VA)/Department of Defense (DOD) Clinical Practice Guideline for Management of PTSD, January 2004. www.oqp.med.va.gov/cpg/PTSD/PTSD_Base.htm
Includes list of clinical trials, medication dosing, and evidence basis for treatment with pharmacotherapy and psychotherapy. - American Psychiatric Association. Practice Guideline for the Treatment of Patients With Acute Stress Disorder and Posttraumatic Stress Disorder. http://www.psych.org/psych_pract/treatg/pg/PTSD-PG-PartsA-B-C-New.pdf
Background and guidelines for managing PTSD, including treatment recommendations, evidence basis, background, and areas for future research.
Disclosure
Drs. Lineberry, Bostwick, and Rundell previously served on active duty in the U.S. Air Force for 12, 5, and 23 years, respectively.
1. Benjamin M. How many have gone to war? Salon.com April 12, 2005. Available at: http://www.salon.com/news/feature/2005/04/12/troops_numbers/index_np.html. Accessed Dec. 13, 2005.
2. United States Government Accountability Office. Testimony before the Committee on Government Reform, House of Representatives. Reserve forces. Army National Guard’s role, organization, and equipment need to be reexamined. Oct. 20, 2005. Available at: http://www.gao.gov/new.items/d06170t.pdf. Accessed Oct. 26, 2005.
3. Wetzel K. Senators told of Guard struggles. Seattle Times Oct. 20, 2005. Available at: http://seattletimes.nwsource.com/html/local-news/2002571967_soldiers20m.html. Accessed Oct. 26, 2005.
4. Associated Press. Survey: 30% of returning Iraq vets suffer mental ills. USA Today July 28, 2005. Available at: http://www.usatoday.com/news/health/2005-07-28-iraq-vets-health_x.htm?csp=34. Accessed Oct. 26, 2005.
5. Zoroya G. One in four Iraq vets ailing on return. USA Today Oct. 18, 2005. Available at: http://www.usatoday.com/news/world/iraq/2005-10-18-troops-side_x.htm. Accessed Oct. 26, 2005.
6. Zoroya G. Troops screened as never before. USA Today Oct. 18, 2005. Available at: http://www.usatoday.com/news/world/iraq/2005-10-18-troops-stress-side_x.htm. Accessed Oct. 26, 2005.
7. Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med 2004;351:13-22.
8. Prigerson HG, Maciejewski PK, Rosenheck RA. Combat trauma: trauma with highest risk of delayed onset and unresolved posttraumatic stress disorder symptoms, unemployment, and abuse among men. J Nerv Ment Dis 2001;189:99-108.
9. Narrow WE, Rae DS, Robins LN, Regier DA. Revised prevalence estimates of mental disorders in the United States: using a clinical significance criterion to reconcile 2 surveys’ estimates. Arch Gen Psychiatry 2002;59:115-23.
10. Lieberman HR, Bathalon GP, Falco CN, et al. Severe decrements in cognition, function and mood induced by sleep loss, heat, dehydration, and under-nutrition during simulated combat. Biol Psychiatry 2005;57:422-9.
11. Nemeroff CB, Bremner JD, Foa EB, et al. Posttraumatic stress disorder: a state-of-the-science review. J Psychiatr Res (In press; Oct 2005 epub ahead of publication).
12. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington, DC: American Psychiatric Association; 2000.
13. Sareen J, Houlahan T, Cox BJ, Asmundson GJ. Anxiety disorders associated with suicidal ideation and suicide attempts in the national comorbidity survey. J Nerv Ment Dis 2005;193:450-4.
14. Xydakis MS, Fravell MD, Nasser KE, Casler JD. Analysis of battlefield head and neck injuries in Iraq and Afghanistan. Otolarnygol Head Neck Surg 2005;133:497-504.
15. Okie S. Traumatic brain injury in the war zone. N Engl J Med 2005;352(20):2043-7.
16. Cozza SJ, Benedek DM, Bradley JC, et al. Topics specific to the psychiatric treatment of military personnel. In: Schnurr PP, Cozza SJ (eds). Iraq War clinician guide (2nd ed). Washington, DC: National Center for PTSD. Walter Reed Army Medical Center; 2004:4-20. Available at: http://www.ncptsd.va.gov/war/iraq_clinician_guide_v2/iraq_clinician_guide_v2.pdf Accessed Oct. 26, 2005.
1. Benjamin M. How many have gone to war? Salon.com April 12, 2005. Available at: http://www.salon.com/news/feature/2005/04/12/troops_numbers/index_np.html. Accessed Dec. 13, 2005.
2. United States Government Accountability Office. Testimony before the Committee on Government Reform, House of Representatives. Reserve forces. Army National Guard’s role, organization, and equipment need to be reexamined. Oct. 20, 2005. Available at: http://www.gao.gov/new.items/d06170t.pdf. Accessed Oct. 26, 2005.
3. Wetzel K. Senators told of Guard struggles. Seattle Times Oct. 20, 2005. Available at: http://seattletimes.nwsource.com/html/local-news/2002571967_soldiers20m.html. Accessed Oct. 26, 2005.
4. Associated Press. Survey: 30% of returning Iraq vets suffer mental ills. USA Today July 28, 2005. Available at: http://www.usatoday.com/news/health/2005-07-28-iraq-vets-health_x.htm?csp=34. Accessed Oct. 26, 2005.
5. Zoroya G. One in four Iraq vets ailing on return. USA Today Oct. 18, 2005. Available at: http://www.usatoday.com/news/world/iraq/2005-10-18-troops-side_x.htm. Accessed Oct. 26, 2005.
6. Zoroya G. Troops screened as never before. USA Today Oct. 18, 2005. Available at: http://www.usatoday.com/news/world/iraq/2005-10-18-troops-stress-side_x.htm. Accessed Oct. 26, 2005.
7. Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med 2004;351:13-22.
8. Prigerson HG, Maciejewski PK, Rosenheck RA. Combat trauma: trauma with highest risk of delayed onset and unresolved posttraumatic stress disorder symptoms, unemployment, and abuse among men. J Nerv Ment Dis 2001;189:99-108.
9. Narrow WE, Rae DS, Robins LN, Regier DA. Revised prevalence estimates of mental disorders in the United States: using a clinical significance criterion to reconcile 2 surveys’ estimates. Arch Gen Psychiatry 2002;59:115-23.
10. Lieberman HR, Bathalon GP, Falco CN, et al. Severe decrements in cognition, function and mood induced by sleep loss, heat, dehydration, and under-nutrition during simulated combat. Biol Psychiatry 2005;57:422-9.
11. Nemeroff CB, Bremner JD, Foa EB, et al. Posttraumatic stress disorder: a state-of-the-science review. J Psychiatr Res (In press; Oct 2005 epub ahead of publication).
12. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington, DC: American Psychiatric Association; 2000.
13. Sareen J, Houlahan T, Cox BJ, Asmundson GJ. Anxiety disorders associated with suicidal ideation and suicide attempts in the national comorbidity survey. J Nerv Ment Dis 2005;193:450-4.
14. Xydakis MS, Fravell MD, Nasser KE, Casler JD. Analysis of battlefield head and neck injuries in Iraq and Afghanistan. Otolarnygol Head Neck Surg 2005;133:497-504.
15. Okie S. Traumatic brain injury in the war zone. N Engl J Med 2005;352(20):2043-7.
16. Cozza SJ, Benedek DM, Bradley JC, et al. Topics specific to the psychiatric treatment of military personnel. In: Schnurr PP, Cozza SJ (eds). Iraq War clinician guide (2nd ed). Washington, DC: National Center for PTSD. Walter Reed Army Medical Center; 2004:4-20. Available at: http://www.ncptsd.va.gov/war/iraq_clinician_guide_v2/iraq_clinician_guide_v2.pdf Accessed Oct. 26, 2005.
Does chronic pain shrink the brain?
Chronic pain—particularly lower back pain—is frustrating to both patient and clinician. Because most cases lack an obvious physical explanation, the doctor may wonder if the patient is faking or exaggerating—that the pain is “in the patient’s head.” Studies suggest this cerebral component may exist—but not in ways you might expect.
How the brain processes pain
According to traditional belief, the brain passively receives noxious signals from injured tissue (nociceptive) or damaged nerve (neuropathic). Extensive—some would say excessive—tests are often conducted in search of a bone or muscle injury that might explain the pain.
Functional imaging studies across 15 years have shown activity in various brain regions when subjects feel pain. In addition to the somatosensory cortex, pain also activates brain areas involved with mood, attention, and anxiety. More important, the brain does not passively receive signals from the periphery but can inhibit ascending signals with endogenous opioids, such as endorphins and enkephalins.
Apkarian et alPosttraumatic stress disorder: Nature and nurture?, May 2004.)
Drugs. Medications and other substances taken to alleviate pain might also reduce gray matter. Excessive alcohol and opioid use have long-term adverse effects on the CNS.3 Is treatment or self-medication mildly toxic to the brain?
Overuse atrophy. Apkarian et al1 propose that cortical loss may be secondary to overuse. They suggest that persistent pain perception— and the resultant negative affect and stress—causes an excitotoxic and inflammatory state that wears out portions of the brain circuitry. If this is true, then chronic pain itself causes cerebral atrophy.
Whatever the explanation, this study indicates that chronic lower back pain pathology extends beyond the lower back.
Related resources
- Deyo RA, Weinstein JN. Low back pain. N Engl J Med 2001;344:363-70.
- International Association for the Study of Pain. www.iasp-pain.org.
1. Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci 2004;24:10410-5.
2. Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain 2003;126:1079-91.
3. Goldman D, Barr CS. Restoring the addicted brain. N Engl J Med 2002;347:843-5.
Dr. Higgins is clinical associate professor of family medicine and psychiatry, Medical University of South Carolina, Charleston ([email protected]).
Chronic pain—particularly lower back pain—is frustrating to both patient and clinician. Because most cases lack an obvious physical explanation, the doctor may wonder if the patient is faking or exaggerating—that the pain is “in the patient’s head.” Studies suggest this cerebral component may exist—but not in ways you might expect.
How the brain processes pain
According to traditional belief, the brain passively receives noxious signals from injured tissue (nociceptive) or damaged nerve (neuropathic). Extensive—some would say excessive—tests are often conducted in search of a bone or muscle injury that might explain the pain.
Functional imaging studies across 15 years have shown activity in various brain regions when subjects feel pain. In addition to the somatosensory cortex, pain also activates brain areas involved with mood, attention, and anxiety. More important, the brain does not passively receive signals from the periphery but can inhibit ascending signals with endogenous opioids, such as endorphins and enkephalins.
Apkarian et alPosttraumatic stress disorder: Nature and nurture?, May 2004.)
Drugs. Medications and other substances taken to alleviate pain might also reduce gray matter. Excessive alcohol and opioid use have long-term adverse effects on the CNS.3 Is treatment or self-medication mildly toxic to the brain?
Overuse atrophy. Apkarian et al1 propose that cortical loss may be secondary to overuse. They suggest that persistent pain perception— and the resultant negative affect and stress—causes an excitotoxic and inflammatory state that wears out portions of the brain circuitry. If this is true, then chronic pain itself causes cerebral atrophy.
Whatever the explanation, this study indicates that chronic lower back pain pathology extends beyond the lower back.
Related resources
- Deyo RA, Weinstein JN. Low back pain. N Engl J Med 2001;344:363-70.
- International Association for the Study of Pain. www.iasp-pain.org.
Chronic pain—particularly lower back pain—is frustrating to both patient and clinician. Because most cases lack an obvious physical explanation, the doctor may wonder if the patient is faking or exaggerating—that the pain is “in the patient’s head.” Studies suggest this cerebral component may exist—but not in ways you might expect.
How the brain processes pain
According to traditional belief, the brain passively receives noxious signals from injured tissue (nociceptive) or damaged nerve (neuropathic). Extensive—some would say excessive—tests are often conducted in search of a bone or muscle injury that might explain the pain.
Functional imaging studies across 15 years have shown activity in various brain regions when subjects feel pain. In addition to the somatosensory cortex, pain also activates brain areas involved with mood, attention, and anxiety. More important, the brain does not passively receive signals from the periphery but can inhibit ascending signals with endogenous opioids, such as endorphins and enkephalins.
Apkarian et alPosttraumatic stress disorder: Nature and nurture?, May 2004.)
Drugs. Medications and other substances taken to alleviate pain might also reduce gray matter. Excessive alcohol and opioid use have long-term adverse effects on the CNS.3 Is treatment or self-medication mildly toxic to the brain?
Overuse atrophy. Apkarian et al1 propose that cortical loss may be secondary to overuse. They suggest that persistent pain perception— and the resultant negative affect and stress—causes an excitotoxic and inflammatory state that wears out portions of the brain circuitry. If this is true, then chronic pain itself causes cerebral atrophy.
Whatever the explanation, this study indicates that chronic lower back pain pathology extends beyond the lower back.
Related resources
- Deyo RA, Weinstein JN. Low back pain. N Engl J Med 2001;344:363-70.
- International Association for the Study of Pain. www.iasp-pain.org.
1. Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci 2004;24:10410-5.
2. Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain 2003;126:1079-91.
3. Goldman D, Barr CS. Restoring the addicted brain. N Engl J Med 2002;347:843-5.
Dr. Higgins is clinical associate professor of family medicine and psychiatry, Medical University of South Carolina, Charleston ([email protected]).
1. Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci 2004;24:10410-5.
2. Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain 2003;126:1079-91.
3. Goldman D, Barr CS. Restoring the addicted brain. N Engl J Med 2002;347:843-5.
Dr. Higgins is clinical associate professor of family medicine and psychiatry, Medical University of South Carolina, Charleston ([email protected]).
Are undiagnosed eating disorders keeping your patients sick?
The internist next door asks you about his patient with bulimia, who routinely has potassium levels of 2.0 mEq/L. “She admits it’s a problem but thinks she’ll get fat if she stops purging. What can I tell her to get her into treatment?”
That afternoon, your longtime patient Mr. J—age 56 with depression, obesity, and hypertension—arrives for his appointment. With the day’s earlier conversation in mind, you ask him if he has an eating problem. Staring at the floor, he describes a lifelong battle with nighttime eating binges, which he has never mentioned to you before.
Mr. J may have concealed his binge eating because of shame or ambivalence about stopping a psychologically protective behavior. And his eating disorder may be complicating his depression treatment.
But outpatient psychiatrists can often manage patients like Mr. J in consultation with a nutritionist and primary care physician. Eating disorders are treatable,1,2 and many patients can recover. This article describes how to identify eating disorders so that treatment can begin.
Psychiatric comorbidity
Eating disorders are common in outpatient practice (Box)3,4 and coexist with a variety of psychiatric diagnoses (Table 1). For example, in 248 women with anorexia, bulimia, or unspecified eating disorders, 74% had another Axis I disorder, including:
- anxiety disorders (54%)
- affective disorders (52%)
- substance-related disorders (25%).
Eating disorders also are much more common in persons who present with psychiatric problems than in the general population. For example:
- Among 62 patients with a primary diagnosis of obsessive-compulsive disorder, 13% had anorexia or bulimia nervosa and another 18% met subthreshold criteria.6
- In 257 female patients with anxiety disorders, nearly 12% also met criteria for a possible eating disorder.7
Some 40% of persons with eating disorders meet DSM-IV-TR criteria for anorexia or bulimia nervosa. The other 60%—with eating disorder, not otherwise specified (ED-NOS)—are divided nearly evenly between binge eating disorder and subsyndromal anorexia or bulimia. Outpatient psychiatrists see these eating disorders most often.
Anorexia and bulimia nervosa prevalence rates are estimated to be 0.3% and 1%, respectively.3 But including ED-NOS patients increases the overall eating disorder prevalence to 2% to 3%—equal to or greater than the combined rates of schizophrenia and bipolar I disorder.
Although considered “subsyndromal,” ED-NOS patients suffer psychopathology, impairment, and medical comorbidity similar to those of persons who meet DSM-IV-TR criteria for anorexia or bulimia nervosa.4
Common psychiatric comorbidities in patients with eating disorders
| Disorder | Comorbidities |
|---|---|
| Anorexia and bulimia nervosa | Anxiety disorders (social phobia, PTSD, OCD) |
| Mood disorders (major depressive disorder, dysthymia, bipolar disorder) | |
| Substance use disorders (more common in patients who binge and/or purge) | |
| Personality disorders (cluster C more common in restricting anorexia, cluster B more common in patients who binge and purge) | |
| Binge eating disorder | Anxiety disorders (PTSD) |
| Mood disorders (major depressive disorder, bipolar disorder) | |
| PTSD: posttraumatic stress disorder | |
| OCD: obsessive-compulsive disorder | |
Who has eating disorders?
Most eating disorder patients are adolescent girls or young women with pronounced body image dissatisfaction. Other patients include:
Atypical young women. Some young women—usually Asian—meet most criteria for anorexia nervosa but lack the characteristic drive for thinness. They tend to have less psychopathology and better prognosis than typical female patients.9
Boys and men. Female-to-male ratios are approximately 11:1 for anorexia, 5:1 for bulimia, and 3:1 for binge eating disorder. Men and boys with eating disorders are similar to their female counterparts but are more likely to report:
- comorbid substance abuse
- having begun weight loss and purging in response to teasing or concerns about health, sports performance, or gay relationships, rather than appearance.10
Middle-aged to late-life. Midlife onset of eating disorders may be precipitated by losses or concerns about aging. In the elderly, eating disorders may be manifestations of complicated bereavement, and ruling out medical causes of weight loss is crucial in this age group.
Night-eating syndrome. Some patients eat at least 25% of daily calories after the evening meal. They experience insomnia, morning anorexia, and sometimes amnesia for the nocturnal eating episodes. Anxiety, depression, or sleep disorders may be contributing factors.12
Identifying eating disorders
Screening. No formal guidelines recommend which psychiatric patients to screen for eating disorders. We suggest screening any patients who are over- or underweight or have eating disorder risk factors, such as:
- young women (teens and early 20s)
- athletes in certain sports (gymnastics, ballet, figure skating, running, body building, wrestling)
- history of childhood sexual abuse.13
Interviewing. Evaluate all 4 illness domains—nutritional, medical, psychological, and social. Because patients often do not volunteer information, ask about:
- symptoms and complications
- onset and development of eating and weight problems
- history of being teased or criticized about weight
- weight history (premorbid, lowest, highest, and preferred weights).
DSM-IV-TR defines binging as consuming a large quantity of food in a discrete time and feeling out of control of eating. Ask specifically how the patient defines “binge,” and seek details of a typical binge. Also ask about compensatory behaviors (purging by vomiting or using laxatives or diuretics). Is the patient abusing ipecac, diet pills, or thyroid hormone? Does he or she fast or exercise compulsively (such as even while ill)?
Eating and exercise patterns. Ask the patient to recall everything eaten in the past 24 hours. This history can help estimate caloric intake and may reveal problematic eating patterns. For example, does the patient:
- avoid certain foods, consider others to be “safe,” or use diet products, gum, or mints?
- engage in food rituals, such as slow eating, hoarding food, or eating odd combinations?
- steal food, weigh him/herself frequently, or visit pro-anorexia/pro-bulimia Web sites?
Table 2
Potential medical complications of anorexia and bulimia nervosa
| Organ system | Symptoms | Signs, syndromes, laboratory abnormalities |
|---|---|---|
| Cardiovascular | Palpitations, dyspnea, chest pain, dizziness | Bradycardia, orthostasis, acrocyanosis |
| Prolonged PR and QTc intervals on ECG, mitral valve prolapse, cardiomyopathy in ipecac abusers | ||
| CNS | Anxious, depressed, or irritable mood; obsessiveness; cognitive deficits; seizures (rare) | Enlarged ventricles on CT or MRI, deficits on neuropsychological testing, abnormal EEG, signs of peripheral neuropathy |
| Dermatologic | Hair loss, dry skin | Xerosis, carotenoderma, cheilitis, lanugo, brittle hair and nails, Russell’s sign (callus on dorsum of hand used to induce vomiting) |
| Endocrine | Fatigue, cold intolerance | Hypothermia, hypoglycemia, hypercortisolemia, ↓ T3 and T4 |
| Gastrointestinal | Bloating, constipation, spontaneous vomiting, reflux, abdominal pain, heartburn, hematemesis | Abnormal bowel sounds, delayed gastric emptying, superior mesenteric artery syndrome, pancreatitis |
| In patients who vomit: Mallory-Weiss tears, Barrett’s esophagus, occult blood in stool, ↑ amylase, gingivitis, dental caries, sialadenosis, perimolysis | ||
| Genitourinary | Polyuria, oliguria | ↑ BUN, nephrolithiasis, hypokalemic nephropathy, renal failure (rare). |
| Hematologic | Fatigue, bruising | Anemia; ↓ numbers of WBCs, RBCs and platelets; ↓ ferritin, B12, folate |
| Metabolic | Weakness, cardiac or CNS manifestations | ↓ K, Na, Mg, phosphate; ↑ cholesterol; metabolic alkalosis (from vomiting), or acidosis (from laxatives); thiamin and niacin deficiencies (rare). |
| Musculoskeletal | Weakness, cramps, bone pain | Wasting, ↑ CK (rare), decreased bone mineral density, pathologic fractures |
| Reproductive | Amenorrhea, ↓ libido, infertility, ↑ pregnancy, neonatal complications | Arrested sexual development; ↓ estrogen or testosterone; prepubertal levels of LH and FSH |
Interview adjuncts
Assessment tools. In addition to patient interviews, some clinicians use self-report scales to screen for eating disorders or to monitor treatment. Reliable and valid self-report questionnaires include the Eating Disorder Examination-Q (36 items),14 Eating Disorder Inventory (91 items),15 and Eating Attitudes Test (26 items).16
The Eating Attitudes Test takes 10 minutes to complete and is widely used for screening. A cut-off score of 20 indicates a potential eating disorder and the need for a follow-up interview.
Self-report diaries can help identify binge eating triggers—usually dietary restriction combined with interpersonal stressors. Ask the patient to record all meals, snacks, binges, purges, and exercise activities, plus time of day and associated feelings, thoughts, and situations. Diaries can also reveal maladaptive thoughts, such as body image distortion, and problematic coping strategies, such as purging or excessive exercising.
Medical workup
Measure height and weight, calculate body mass index, and check vital signs (including supine and standing blood pressure and pulse) and hydration status. Perform a neurologic exam, particularly for peripheral neuropathy, and check for cardiac, dermatologic, and GI complications (Tables 2 and 3). Include a dental examination if the patient admits or you suspect self-induced vomiting.
If treating eating disorders’ medical consequences is beyond the scope of your practice, refer the patient for evaluation by a physician with this experience.
Table 3
Common medical complications of binge eating disorder
| Obesity (body mass index>30) and related comorbidities: |
| Hypertension |
| Diabetes mellitus |
| Hyperlipidemia |
| Increased cardiovascular mortality |
| Obstructive sleep apnea |
| Degenerative arthritis |
| Gastroesophageal reflux symptoms and complications |
- Weight for height in women: 100 lbs for the first 5 feet, +5 lbs/inch over 5 feet
- Weight for height in men: 106 lbs for the first 5 feet, +6 lbs/inch over 5 feet.
For adolescents with suspected anorexia nervosa, estimate expected body weight from individual growth curves or standard growth charts posted on the Centers for Disease Control and Prevention Web site (see Related resources).
Note that the DSM-IV-TR weight criterion for anorexia of “less than 85% of expected” is an example, not an absolute cutoff. Anorexia nervosa would be an appropriate diagnosis for a patient who weighs more than 85% of expected weight but has lost substantial weight and meets the other diagnostic criteria.
BMI
Laboratory tests vary, depending on patients’ suspected eating disorders (Table 4). In 214 outpatient women with anorexia, the most common abnormalities were anemia (38.6%), leukocytopenia (34.4%), hyponatremia (19.7%) and hypokalemia (19.7%).17 With few exceptions, abnormal values are not predicted by the apparent degree of undernutrition.
Table 4
Laboratory studies for patients with suspected eating disorders
| For whom | Recommended tests |
|---|---|
| All eating disorder patients | Comprehensive metabolic panel (electrolytes, glucose, albumin, measures of hepatic and renal function), complete blood count, urinalysis, ECG, TSH |
| Add for patients with anorexia | Serum magnesium, phosphate, calcium; creatinine clearance; chest radiography; estrogen in women, testosterone in men; DEXA bone density scan; consider echocardiography, brain MRI; screen urine for unreported substances of abuse |
| Add for patients with bulimia and purging type anorexia | Serum magnesium, phosphate, calcium; DEXA scan if patient is amenorrheic or has history of anorexia; amylase (fractionated, if possible); consider fecal occult blood, urine for electrolytes and laxatives, urine drug screen |
| Add for patients with binge eating disorder | Fasting blood glucose, fasting lipid profile |
From diagnosis to treatment
Talking with patients. Discussing abnormal lab results with patients can be therapeutic. In our experience, recovered patients often report that worry about medical complications was their primary reason to seek treatment for eating disorders.
Relate the patient’s cognitive, mood, and physical symptoms to abnormal eating behavior, then present the eating disorder diagnosis as the beginning of treatment. For example, you could praise Mr. J for his courage in revealing his binge eating and tell him that identifying this problem is the first step toward solving it. Not only can he overcome binge eating, but treatment will also likely improve his mood, weight, and blood pressure.
Eating disorder patients who are medically stable, motivated for treatment, have good support, and are able and willing to come for frequent appointments are good candidates for outpatient eating disorder treatment.
For clinicians
- Standard growth charts. National Center for Health Statistics. Centers for Disease Control and Prevention. www.cdc.gov/growthcharts.
- Brewerton TD. Clinical handbook of eating disorders: an integrated approach. New York: Marcel Dekker; 2004.
- Work group on eating disorders. Practice guideline for the treatment of patients with eating disorders (2nd ed.). Washington, DC: American Psychiatric Publishing; 2000. Available at: http://www.psych.org/psych_pract/treatg/pg/eating_revisebook_index.cfm.
- Zerbe KJ. The body betrayed: a deeper understanding of women, eating disorders, and treatment. Carlsbad, CA: Gürze Books; 1995.
- National Eating Disorders Association. www.nationaleatingdisorders.org.
- National Association of Anorexia Nervosa and Associated Disorders. www.anad.org.
1. Reas Dl, Williamson DA, Martin CK, Zucker NL. Duration of illness predicts outcome for bulimia nervosa: a long-term outcome study. Int J Eat Disord 2000;27:428-34.
2. Nielsen S, Moller-Madsen S, Isager T, et al. Standardized mortality in eating disorders—a quantitative summary of previously published and new evidence. J Psychosom Res 1998;44:413-34.
3. Hoek HW, van Hoeken D. Review of the prevalence and incidence of eating disorders. Int J Eat Disord 2003;34(4):383-96.
4. Watson TL, Andersen AE. A critical examination of the amenorrhea and weight criteria for diagnosing anorexia nervosa. Acta Psychiatr Scand 2003;108:175-82.
5. Milos G, Spindler A, Schnyder U. Psychiatric comorbidity and eating disorder inventory (EDI) profiles in eating disorder patients. Can J Psychiatry 2004;49:179-84.
6. Rubenstein CS, Pigott TA, L’ Heureux F, et al. A preliminary investigation of the lifetime prevalence of anorexia and bulimia nervosa in patients with obsessive compulsive disorder. J Clin Psychiatry 1992;53(9):309-14.
7. Becker CB, DeViva JC, Zayfert C. Eating disorder symptoms among female anxiety disorder patients in clinical practice: the importance of anxiety comorbidity assessment. J Anxiety Disord 2004;18(3):255-74.
8. Keys A, Brozek J, Henschel A, et al. The biology of human starvation. Minneapolis: University of Minnesota Press; 1950.
9. Ramacciotti CE, Dell’Osso L, Paoli RA, et al. Characteristics of eating disorder patients without a drive for thinness. Int J Eat Disord 2002;32:206-12.
10. Andersen AE. Males with eating disorders: medical considerations. In: Mehler PS, Andersen AE (eds). Eating disorders: a guide to medical care and complications. Baltimore: The Johns Hopkins University Press; 1999;214-26.
11. Lantzouni E, Frank GR, Golden NH, Shenker RI. Reversibility of growth stunting in early onset anorexia nervosa: a prospective study. J Adolesc Health 2002;31(2):162-5.
12. Napolitano MA, Head S, Babyak MA, Blumenthal JA. Binge eating disorder and night eating syndrome: psychological and behavioral characteristics. Int J Eating Disord 2001;30:193-203.
13. Jacobi C, Morris L, de Zwaan M. Overview of risk factors for anorexia nervosa, bulimia nervosa, and binge eating disorder. In: Brewerton, TD (ed). Clinical handbook of eating disorders: An integrated approach. New York: Marcel Dekker; 2004;183-208.
14. Fairburn CG, Beglin SJ. Assessment of eating disorders: interview or self-report questionnaire. Int J Eating Disord 1994;16:363-70.
15. Garner DM. Eating Disorder Inventory-2 professional manual. Odessa, FL: Psychological Assessment Resources; 1991.
16. Garner DM. Psychoeducational principles in treatment. In: Garner DM, Garfinkel PE (eds). Handbook of treatment for eating disorders (2nd ed). New York: Guilford Press; 1997;145-77.
17. Miller KK, Grinspoon SK, Ciampa J, et al. Medical findings in outpatients with anorexia nervosa. Arch Intern Med 2005;165(5):561-6.
The internist next door asks you about his patient with bulimia, who routinely has potassium levels of 2.0 mEq/L. “She admits it’s a problem but thinks she’ll get fat if she stops purging. What can I tell her to get her into treatment?”
That afternoon, your longtime patient Mr. J—age 56 with depression, obesity, and hypertension—arrives for his appointment. With the day’s earlier conversation in mind, you ask him if he has an eating problem. Staring at the floor, he describes a lifelong battle with nighttime eating binges, which he has never mentioned to you before.
Mr. J may have concealed his binge eating because of shame or ambivalence about stopping a psychologically protective behavior. And his eating disorder may be complicating his depression treatment.
But outpatient psychiatrists can often manage patients like Mr. J in consultation with a nutritionist and primary care physician. Eating disorders are treatable,1,2 and many patients can recover. This article describes how to identify eating disorders so that treatment can begin.
Psychiatric comorbidity
Eating disorders are common in outpatient practice (Box)3,4 and coexist with a variety of psychiatric diagnoses (Table 1). For example, in 248 women with anorexia, bulimia, or unspecified eating disorders, 74% had another Axis I disorder, including:
- anxiety disorders (54%)
- affective disorders (52%)
- substance-related disorders (25%).
Eating disorders also are much more common in persons who present with psychiatric problems than in the general population. For example:
- Among 62 patients with a primary diagnosis of obsessive-compulsive disorder, 13% had anorexia or bulimia nervosa and another 18% met subthreshold criteria.6
- In 257 female patients with anxiety disorders, nearly 12% also met criteria for a possible eating disorder.7
Some 40% of persons with eating disorders meet DSM-IV-TR criteria for anorexia or bulimia nervosa. The other 60%—with eating disorder, not otherwise specified (ED-NOS)—are divided nearly evenly between binge eating disorder and subsyndromal anorexia or bulimia. Outpatient psychiatrists see these eating disorders most often.
Anorexia and bulimia nervosa prevalence rates are estimated to be 0.3% and 1%, respectively.3 But including ED-NOS patients increases the overall eating disorder prevalence to 2% to 3%—equal to or greater than the combined rates of schizophrenia and bipolar I disorder.
Although considered “subsyndromal,” ED-NOS patients suffer psychopathology, impairment, and medical comorbidity similar to those of persons who meet DSM-IV-TR criteria for anorexia or bulimia nervosa.4
Common psychiatric comorbidities in patients with eating disorders
| Disorder | Comorbidities |
|---|---|
| Anorexia and bulimia nervosa | Anxiety disorders (social phobia, PTSD, OCD) |
| Mood disorders (major depressive disorder, dysthymia, bipolar disorder) | |
| Substance use disorders (more common in patients who binge and/or purge) | |
| Personality disorders (cluster C more common in restricting anorexia, cluster B more common in patients who binge and purge) | |
| Binge eating disorder | Anxiety disorders (PTSD) |
| Mood disorders (major depressive disorder, bipolar disorder) | |
| PTSD: posttraumatic stress disorder | |
| OCD: obsessive-compulsive disorder | |
Who has eating disorders?
Most eating disorder patients are adolescent girls or young women with pronounced body image dissatisfaction. Other patients include:
Atypical young women. Some young women—usually Asian—meet most criteria for anorexia nervosa but lack the characteristic drive for thinness. They tend to have less psychopathology and better prognosis than typical female patients.9
Boys and men. Female-to-male ratios are approximately 11:1 for anorexia, 5:1 for bulimia, and 3:1 for binge eating disorder. Men and boys with eating disorders are similar to their female counterparts but are more likely to report:
- comorbid substance abuse
- having begun weight loss and purging in response to teasing or concerns about health, sports performance, or gay relationships, rather than appearance.10
Middle-aged to late-life. Midlife onset of eating disorders may be precipitated by losses or concerns about aging. In the elderly, eating disorders may be manifestations of complicated bereavement, and ruling out medical causes of weight loss is crucial in this age group.
Night-eating syndrome. Some patients eat at least 25% of daily calories after the evening meal. They experience insomnia, morning anorexia, and sometimes amnesia for the nocturnal eating episodes. Anxiety, depression, or sleep disorders may be contributing factors.12
Identifying eating disorders
Screening. No formal guidelines recommend which psychiatric patients to screen for eating disorders. We suggest screening any patients who are over- or underweight or have eating disorder risk factors, such as:
- young women (teens and early 20s)
- athletes in certain sports (gymnastics, ballet, figure skating, running, body building, wrestling)
- history of childhood sexual abuse.13
Interviewing. Evaluate all 4 illness domains—nutritional, medical, psychological, and social. Because patients often do not volunteer information, ask about:
- symptoms and complications
- onset and development of eating and weight problems
- history of being teased or criticized about weight
- weight history (premorbid, lowest, highest, and preferred weights).
DSM-IV-TR defines binging as consuming a large quantity of food in a discrete time and feeling out of control of eating. Ask specifically how the patient defines “binge,” and seek details of a typical binge. Also ask about compensatory behaviors (purging by vomiting or using laxatives or diuretics). Is the patient abusing ipecac, diet pills, or thyroid hormone? Does he or she fast or exercise compulsively (such as even while ill)?
Eating and exercise patterns. Ask the patient to recall everything eaten in the past 24 hours. This history can help estimate caloric intake and may reveal problematic eating patterns. For example, does the patient:
- avoid certain foods, consider others to be “safe,” or use diet products, gum, or mints?
- engage in food rituals, such as slow eating, hoarding food, or eating odd combinations?
- steal food, weigh him/herself frequently, or visit pro-anorexia/pro-bulimia Web sites?
Table 2
Potential medical complications of anorexia and bulimia nervosa
| Organ system | Symptoms | Signs, syndromes, laboratory abnormalities |
|---|---|---|
| Cardiovascular | Palpitations, dyspnea, chest pain, dizziness | Bradycardia, orthostasis, acrocyanosis |
| Prolonged PR and QTc intervals on ECG, mitral valve prolapse, cardiomyopathy in ipecac abusers | ||
| CNS | Anxious, depressed, or irritable mood; obsessiveness; cognitive deficits; seizures (rare) | Enlarged ventricles on CT or MRI, deficits on neuropsychological testing, abnormal EEG, signs of peripheral neuropathy |
| Dermatologic | Hair loss, dry skin | Xerosis, carotenoderma, cheilitis, lanugo, brittle hair and nails, Russell’s sign (callus on dorsum of hand used to induce vomiting) |
| Endocrine | Fatigue, cold intolerance | Hypothermia, hypoglycemia, hypercortisolemia, ↓ T3 and T4 |
| Gastrointestinal | Bloating, constipation, spontaneous vomiting, reflux, abdominal pain, heartburn, hematemesis | Abnormal bowel sounds, delayed gastric emptying, superior mesenteric artery syndrome, pancreatitis |
| In patients who vomit: Mallory-Weiss tears, Barrett’s esophagus, occult blood in stool, ↑ amylase, gingivitis, dental caries, sialadenosis, perimolysis | ||
| Genitourinary | Polyuria, oliguria | ↑ BUN, nephrolithiasis, hypokalemic nephropathy, renal failure (rare). |
| Hematologic | Fatigue, bruising | Anemia; ↓ numbers of WBCs, RBCs and platelets; ↓ ferritin, B12, folate |
| Metabolic | Weakness, cardiac or CNS manifestations | ↓ K, Na, Mg, phosphate; ↑ cholesterol; metabolic alkalosis (from vomiting), or acidosis (from laxatives); thiamin and niacin deficiencies (rare). |
| Musculoskeletal | Weakness, cramps, bone pain | Wasting, ↑ CK (rare), decreased bone mineral density, pathologic fractures |
| Reproductive | Amenorrhea, ↓ libido, infertility, ↑ pregnancy, neonatal complications | Arrested sexual development; ↓ estrogen or testosterone; prepubertal levels of LH and FSH |
Interview adjuncts
Assessment tools. In addition to patient interviews, some clinicians use self-report scales to screen for eating disorders or to monitor treatment. Reliable and valid self-report questionnaires include the Eating Disorder Examination-Q (36 items),14 Eating Disorder Inventory (91 items),15 and Eating Attitudes Test (26 items).16
The Eating Attitudes Test takes 10 minutes to complete and is widely used for screening. A cut-off score of 20 indicates a potential eating disorder and the need for a follow-up interview.
Self-report diaries can help identify binge eating triggers—usually dietary restriction combined with interpersonal stressors. Ask the patient to record all meals, snacks, binges, purges, and exercise activities, plus time of day and associated feelings, thoughts, and situations. Diaries can also reveal maladaptive thoughts, such as body image distortion, and problematic coping strategies, such as purging or excessive exercising.
Medical workup
Measure height and weight, calculate body mass index, and check vital signs (including supine and standing blood pressure and pulse) and hydration status. Perform a neurologic exam, particularly for peripheral neuropathy, and check for cardiac, dermatologic, and GI complications (Tables 2 and 3). Include a dental examination if the patient admits or you suspect self-induced vomiting.
If treating eating disorders’ medical consequences is beyond the scope of your practice, refer the patient for evaluation by a physician with this experience.
Table 3
Common medical complications of binge eating disorder
| Obesity (body mass index>30) and related comorbidities: |
| Hypertension |
| Diabetes mellitus |
| Hyperlipidemia |
| Increased cardiovascular mortality |
| Obstructive sleep apnea |
| Degenerative arthritis |
| Gastroesophageal reflux symptoms and complications |
- Weight for height in women: 100 lbs for the first 5 feet, +5 lbs/inch over 5 feet
- Weight for height in men: 106 lbs for the first 5 feet, +6 lbs/inch over 5 feet.
For adolescents with suspected anorexia nervosa, estimate expected body weight from individual growth curves or standard growth charts posted on the Centers for Disease Control and Prevention Web site (see Related resources).
Note that the DSM-IV-TR weight criterion for anorexia of “less than 85% of expected” is an example, not an absolute cutoff. Anorexia nervosa would be an appropriate diagnosis for a patient who weighs more than 85% of expected weight but has lost substantial weight and meets the other diagnostic criteria.
BMI
Laboratory tests vary, depending on patients’ suspected eating disorders (Table 4). In 214 outpatient women with anorexia, the most common abnormalities were anemia (38.6%), leukocytopenia (34.4%), hyponatremia (19.7%) and hypokalemia (19.7%).17 With few exceptions, abnormal values are not predicted by the apparent degree of undernutrition.
Table 4
Laboratory studies for patients with suspected eating disorders
| For whom | Recommended tests |
|---|---|
| All eating disorder patients | Comprehensive metabolic panel (electrolytes, glucose, albumin, measures of hepatic and renal function), complete blood count, urinalysis, ECG, TSH |
| Add for patients with anorexia | Serum magnesium, phosphate, calcium; creatinine clearance; chest radiography; estrogen in women, testosterone in men; DEXA bone density scan; consider echocardiography, brain MRI; screen urine for unreported substances of abuse |
| Add for patients with bulimia and purging type anorexia | Serum magnesium, phosphate, calcium; DEXA scan if patient is amenorrheic or has history of anorexia; amylase (fractionated, if possible); consider fecal occult blood, urine for electrolytes and laxatives, urine drug screen |
| Add for patients with binge eating disorder | Fasting blood glucose, fasting lipid profile |
From diagnosis to treatment
Talking with patients. Discussing abnormal lab results with patients can be therapeutic. In our experience, recovered patients often report that worry about medical complications was their primary reason to seek treatment for eating disorders.
Relate the patient’s cognitive, mood, and physical symptoms to abnormal eating behavior, then present the eating disorder diagnosis as the beginning of treatment. For example, you could praise Mr. J for his courage in revealing his binge eating and tell him that identifying this problem is the first step toward solving it. Not only can he overcome binge eating, but treatment will also likely improve his mood, weight, and blood pressure.
Eating disorder patients who are medically stable, motivated for treatment, have good support, and are able and willing to come for frequent appointments are good candidates for outpatient eating disorder treatment.
For clinicians
- Standard growth charts. National Center for Health Statistics. Centers for Disease Control and Prevention. www.cdc.gov/growthcharts.
- Brewerton TD. Clinical handbook of eating disorders: an integrated approach. New York: Marcel Dekker; 2004.
- Work group on eating disorders. Practice guideline for the treatment of patients with eating disorders (2nd ed.). Washington, DC: American Psychiatric Publishing; 2000. Available at: http://www.psych.org/psych_pract/treatg/pg/eating_revisebook_index.cfm.
- Zerbe KJ. The body betrayed: a deeper understanding of women, eating disorders, and treatment. Carlsbad, CA: Gürze Books; 1995.
- National Eating Disorders Association. www.nationaleatingdisorders.org.
- National Association of Anorexia Nervosa and Associated Disorders. www.anad.org.
The internist next door asks you about his patient with bulimia, who routinely has potassium levels of 2.0 mEq/L. “She admits it’s a problem but thinks she’ll get fat if she stops purging. What can I tell her to get her into treatment?”
That afternoon, your longtime patient Mr. J—age 56 with depression, obesity, and hypertension—arrives for his appointment. With the day’s earlier conversation in mind, you ask him if he has an eating problem. Staring at the floor, he describes a lifelong battle with nighttime eating binges, which he has never mentioned to you before.
Mr. J may have concealed his binge eating because of shame or ambivalence about stopping a psychologically protective behavior. And his eating disorder may be complicating his depression treatment.
But outpatient psychiatrists can often manage patients like Mr. J in consultation with a nutritionist and primary care physician. Eating disorders are treatable,1,2 and many patients can recover. This article describes how to identify eating disorders so that treatment can begin.
Psychiatric comorbidity
Eating disorders are common in outpatient practice (Box)3,4 and coexist with a variety of psychiatric diagnoses (Table 1). For example, in 248 women with anorexia, bulimia, or unspecified eating disorders, 74% had another Axis I disorder, including:
- anxiety disorders (54%)
- affective disorders (52%)
- substance-related disorders (25%).
Eating disorders also are much more common in persons who present with psychiatric problems than in the general population. For example:
- Among 62 patients with a primary diagnosis of obsessive-compulsive disorder, 13% had anorexia or bulimia nervosa and another 18% met subthreshold criteria.6
- In 257 female patients with anxiety disorders, nearly 12% also met criteria for a possible eating disorder.7
Some 40% of persons with eating disorders meet DSM-IV-TR criteria for anorexia or bulimia nervosa. The other 60%—with eating disorder, not otherwise specified (ED-NOS)—are divided nearly evenly between binge eating disorder and subsyndromal anorexia or bulimia. Outpatient psychiatrists see these eating disorders most often.
Anorexia and bulimia nervosa prevalence rates are estimated to be 0.3% and 1%, respectively.3 But including ED-NOS patients increases the overall eating disorder prevalence to 2% to 3%—equal to or greater than the combined rates of schizophrenia and bipolar I disorder.
Although considered “subsyndromal,” ED-NOS patients suffer psychopathology, impairment, and medical comorbidity similar to those of persons who meet DSM-IV-TR criteria for anorexia or bulimia nervosa.4
Common psychiatric comorbidities in patients with eating disorders
| Disorder | Comorbidities |
|---|---|
| Anorexia and bulimia nervosa | Anxiety disorders (social phobia, PTSD, OCD) |
| Mood disorders (major depressive disorder, dysthymia, bipolar disorder) | |
| Substance use disorders (more common in patients who binge and/or purge) | |
| Personality disorders (cluster C more common in restricting anorexia, cluster B more common in patients who binge and purge) | |
| Binge eating disorder | Anxiety disorders (PTSD) |
| Mood disorders (major depressive disorder, bipolar disorder) | |
| PTSD: posttraumatic stress disorder | |
| OCD: obsessive-compulsive disorder | |
Who has eating disorders?
Most eating disorder patients are adolescent girls or young women with pronounced body image dissatisfaction. Other patients include:
Atypical young women. Some young women—usually Asian—meet most criteria for anorexia nervosa but lack the characteristic drive for thinness. They tend to have less psychopathology and better prognosis than typical female patients.9
Boys and men. Female-to-male ratios are approximately 11:1 for anorexia, 5:1 for bulimia, and 3:1 for binge eating disorder. Men and boys with eating disorders are similar to their female counterparts but are more likely to report:
- comorbid substance abuse
- having begun weight loss and purging in response to teasing or concerns about health, sports performance, or gay relationships, rather than appearance.10
Middle-aged to late-life. Midlife onset of eating disorders may be precipitated by losses or concerns about aging. In the elderly, eating disorders may be manifestations of complicated bereavement, and ruling out medical causes of weight loss is crucial in this age group.
Night-eating syndrome. Some patients eat at least 25% of daily calories after the evening meal. They experience insomnia, morning anorexia, and sometimes amnesia for the nocturnal eating episodes. Anxiety, depression, or sleep disorders may be contributing factors.12
Identifying eating disorders
Screening. No formal guidelines recommend which psychiatric patients to screen for eating disorders. We suggest screening any patients who are over- or underweight or have eating disorder risk factors, such as:
- young women (teens and early 20s)
- athletes in certain sports (gymnastics, ballet, figure skating, running, body building, wrestling)
- history of childhood sexual abuse.13
Interviewing. Evaluate all 4 illness domains—nutritional, medical, psychological, and social. Because patients often do not volunteer information, ask about:
- symptoms and complications
- onset and development of eating and weight problems
- history of being teased or criticized about weight
- weight history (premorbid, lowest, highest, and preferred weights).
DSM-IV-TR defines binging as consuming a large quantity of food in a discrete time and feeling out of control of eating. Ask specifically how the patient defines “binge,” and seek details of a typical binge. Also ask about compensatory behaviors (purging by vomiting or using laxatives or diuretics). Is the patient abusing ipecac, diet pills, or thyroid hormone? Does he or she fast or exercise compulsively (such as even while ill)?
Eating and exercise patterns. Ask the patient to recall everything eaten in the past 24 hours. This history can help estimate caloric intake and may reveal problematic eating patterns. For example, does the patient:
- avoid certain foods, consider others to be “safe,” or use diet products, gum, or mints?
- engage in food rituals, such as slow eating, hoarding food, or eating odd combinations?
- steal food, weigh him/herself frequently, or visit pro-anorexia/pro-bulimia Web sites?
Table 2
Potential medical complications of anorexia and bulimia nervosa
| Organ system | Symptoms | Signs, syndromes, laboratory abnormalities |
|---|---|---|
| Cardiovascular | Palpitations, dyspnea, chest pain, dizziness | Bradycardia, orthostasis, acrocyanosis |
| Prolonged PR and QTc intervals on ECG, mitral valve prolapse, cardiomyopathy in ipecac abusers | ||
| CNS | Anxious, depressed, or irritable mood; obsessiveness; cognitive deficits; seizures (rare) | Enlarged ventricles on CT or MRI, deficits on neuropsychological testing, abnormal EEG, signs of peripheral neuropathy |
| Dermatologic | Hair loss, dry skin | Xerosis, carotenoderma, cheilitis, lanugo, brittle hair and nails, Russell’s sign (callus on dorsum of hand used to induce vomiting) |
| Endocrine | Fatigue, cold intolerance | Hypothermia, hypoglycemia, hypercortisolemia, ↓ T3 and T4 |
| Gastrointestinal | Bloating, constipation, spontaneous vomiting, reflux, abdominal pain, heartburn, hematemesis | Abnormal bowel sounds, delayed gastric emptying, superior mesenteric artery syndrome, pancreatitis |
| In patients who vomit: Mallory-Weiss tears, Barrett’s esophagus, occult blood in stool, ↑ amylase, gingivitis, dental caries, sialadenosis, perimolysis | ||
| Genitourinary | Polyuria, oliguria | ↑ BUN, nephrolithiasis, hypokalemic nephropathy, renal failure (rare). |
| Hematologic | Fatigue, bruising | Anemia; ↓ numbers of WBCs, RBCs and platelets; ↓ ferritin, B12, folate |
| Metabolic | Weakness, cardiac or CNS manifestations | ↓ K, Na, Mg, phosphate; ↑ cholesterol; metabolic alkalosis (from vomiting), or acidosis (from laxatives); thiamin and niacin deficiencies (rare). |
| Musculoskeletal | Weakness, cramps, bone pain | Wasting, ↑ CK (rare), decreased bone mineral density, pathologic fractures |
| Reproductive | Amenorrhea, ↓ libido, infertility, ↑ pregnancy, neonatal complications | Arrested sexual development; ↓ estrogen or testosterone; prepubertal levels of LH and FSH |
Interview adjuncts
Assessment tools. In addition to patient interviews, some clinicians use self-report scales to screen for eating disorders or to monitor treatment. Reliable and valid self-report questionnaires include the Eating Disorder Examination-Q (36 items),14 Eating Disorder Inventory (91 items),15 and Eating Attitudes Test (26 items).16
The Eating Attitudes Test takes 10 minutes to complete and is widely used for screening. A cut-off score of 20 indicates a potential eating disorder and the need for a follow-up interview.
Self-report diaries can help identify binge eating triggers—usually dietary restriction combined with interpersonal stressors. Ask the patient to record all meals, snacks, binges, purges, and exercise activities, plus time of day and associated feelings, thoughts, and situations. Diaries can also reveal maladaptive thoughts, such as body image distortion, and problematic coping strategies, such as purging or excessive exercising.
Medical workup
Measure height and weight, calculate body mass index, and check vital signs (including supine and standing blood pressure and pulse) and hydration status. Perform a neurologic exam, particularly for peripheral neuropathy, and check for cardiac, dermatologic, and GI complications (Tables 2 and 3). Include a dental examination if the patient admits or you suspect self-induced vomiting.
If treating eating disorders’ medical consequences is beyond the scope of your practice, refer the patient for evaluation by a physician with this experience.
Table 3
Common medical complications of binge eating disorder
| Obesity (body mass index>30) and related comorbidities: |
| Hypertension |
| Diabetes mellitus |
| Hyperlipidemia |
| Increased cardiovascular mortality |
| Obstructive sleep apnea |
| Degenerative arthritis |
| Gastroesophageal reflux symptoms and complications |
- Weight for height in women: 100 lbs for the first 5 feet, +5 lbs/inch over 5 feet
- Weight for height in men: 106 lbs for the first 5 feet, +6 lbs/inch over 5 feet.
For adolescents with suspected anorexia nervosa, estimate expected body weight from individual growth curves or standard growth charts posted on the Centers for Disease Control and Prevention Web site (see Related resources).
Note that the DSM-IV-TR weight criterion for anorexia of “less than 85% of expected” is an example, not an absolute cutoff. Anorexia nervosa would be an appropriate diagnosis for a patient who weighs more than 85% of expected weight but has lost substantial weight and meets the other diagnostic criteria.
BMI
Laboratory tests vary, depending on patients’ suspected eating disorders (Table 4). In 214 outpatient women with anorexia, the most common abnormalities were anemia (38.6%), leukocytopenia (34.4%), hyponatremia (19.7%) and hypokalemia (19.7%).17 With few exceptions, abnormal values are not predicted by the apparent degree of undernutrition.
Table 4
Laboratory studies for patients with suspected eating disorders
| For whom | Recommended tests |
|---|---|
| All eating disorder patients | Comprehensive metabolic panel (electrolytes, glucose, albumin, measures of hepatic and renal function), complete blood count, urinalysis, ECG, TSH |
| Add for patients with anorexia | Serum magnesium, phosphate, calcium; creatinine clearance; chest radiography; estrogen in women, testosterone in men; DEXA bone density scan; consider echocardiography, brain MRI; screen urine for unreported substances of abuse |
| Add for patients with bulimia and purging type anorexia | Serum magnesium, phosphate, calcium; DEXA scan if patient is amenorrheic or has history of anorexia; amylase (fractionated, if possible); consider fecal occult blood, urine for electrolytes and laxatives, urine drug screen |
| Add for patients with binge eating disorder | Fasting blood glucose, fasting lipid profile |
From diagnosis to treatment
Talking with patients. Discussing abnormal lab results with patients can be therapeutic. In our experience, recovered patients often report that worry about medical complications was their primary reason to seek treatment for eating disorders.
Relate the patient’s cognitive, mood, and physical symptoms to abnormal eating behavior, then present the eating disorder diagnosis as the beginning of treatment. For example, you could praise Mr. J for his courage in revealing his binge eating and tell him that identifying this problem is the first step toward solving it. Not only can he overcome binge eating, but treatment will also likely improve his mood, weight, and blood pressure.
Eating disorder patients who are medically stable, motivated for treatment, have good support, and are able and willing to come for frequent appointments are good candidates for outpatient eating disorder treatment.
For clinicians
- Standard growth charts. National Center for Health Statistics. Centers for Disease Control and Prevention. www.cdc.gov/growthcharts.
- Brewerton TD. Clinical handbook of eating disorders: an integrated approach. New York: Marcel Dekker; 2004.
- Work group on eating disorders. Practice guideline for the treatment of patients with eating disorders (2nd ed.). Washington, DC: American Psychiatric Publishing; 2000. Available at: http://www.psych.org/psych_pract/treatg/pg/eating_revisebook_index.cfm.
- Zerbe KJ. The body betrayed: a deeper understanding of women, eating disorders, and treatment. Carlsbad, CA: Gürze Books; 1995.
- National Eating Disorders Association. www.nationaleatingdisorders.org.
- National Association of Anorexia Nervosa and Associated Disorders. www.anad.org.
1. Reas Dl, Williamson DA, Martin CK, Zucker NL. Duration of illness predicts outcome for bulimia nervosa: a long-term outcome study. Int J Eat Disord 2000;27:428-34.
2. Nielsen S, Moller-Madsen S, Isager T, et al. Standardized mortality in eating disorders—a quantitative summary of previously published and new evidence. J Psychosom Res 1998;44:413-34.
3. Hoek HW, van Hoeken D. Review of the prevalence and incidence of eating disorders. Int J Eat Disord 2003;34(4):383-96.
4. Watson TL, Andersen AE. A critical examination of the amenorrhea and weight criteria for diagnosing anorexia nervosa. Acta Psychiatr Scand 2003;108:175-82.
5. Milos G, Spindler A, Schnyder U. Psychiatric comorbidity and eating disorder inventory (EDI) profiles in eating disorder patients. Can J Psychiatry 2004;49:179-84.
6. Rubenstein CS, Pigott TA, L’ Heureux F, et al. A preliminary investigation of the lifetime prevalence of anorexia and bulimia nervosa in patients with obsessive compulsive disorder. J Clin Psychiatry 1992;53(9):309-14.
7. Becker CB, DeViva JC, Zayfert C. Eating disorder symptoms among female anxiety disorder patients in clinical practice: the importance of anxiety comorbidity assessment. J Anxiety Disord 2004;18(3):255-74.
8. Keys A, Brozek J, Henschel A, et al. The biology of human starvation. Minneapolis: University of Minnesota Press; 1950.
9. Ramacciotti CE, Dell’Osso L, Paoli RA, et al. Characteristics of eating disorder patients without a drive for thinness. Int J Eat Disord 2002;32:206-12.
10. Andersen AE. Males with eating disorders: medical considerations. In: Mehler PS, Andersen AE (eds). Eating disorders: a guide to medical care and complications. Baltimore: The Johns Hopkins University Press; 1999;214-26.
11. Lantzouni E, Frank GR, Golden NH, Shenker RI. Reversibility of growth stunting in early onset anorexia nervosa: a prospective study. J Adolesc Health 2002;31(2):162-5.
12. Napolitano MA, Head S, Babyak MA, Blumenthal JA. Binge eating disorder and night eating syndrome: psychological and behavioral characteristics. Int J Eating Disord 2001;30:193-203.
13. Jacobi C, Morris L, de Zwaan M. Overview of risk factors for anorexia nervosa, bulimia nervosa, and binge eating disorder. In: Brewerton, TD (ed). Clinical handbook of eating disorders: An integrated approach. New York: Marcel Dekker; 2004;183-208.
14. Fairburn CG, Beglin SJ. Assessment of eating disorders: interview or self-report questionnaire. Int J Eating Disord 1994;16:363-70.
15. Garner DM. Eating Disorder Inventory-2 professional manual. Odessa, FL: Psychological Assessment Resources; 1991.
16. Garner DM. Psychoeducational principles in treatment. In: Garner DM, Garfinkel PE (eds). Handbook of treatment for eating disorders (2nd ed). New York: Guilford Press; 1997;145-77.
17. Miller KK, Grinspoon SK, Ciampa J, et al. Medical findings in outpatients with anorexia nervosa. Arch Intern Med 2005;165(5):561-6.
1. Reas Dl, Williamson DA, Martin CK, Zucker NL. Duration of illness predicts outcome for bulimia nervosa: a long-term outcome study. Int J Eat Disord 2000;27:428-34.
2. Nielsen S, Moller-Madsen S, Isager T, et al. Standardized mortality in eating disorders—a quantitative summary of previously published and new evidence. J Psychosom Res 1998;44:413-34.
3. Hoek HW, van Hoeken D. Review of the prevalence and incidence of eating disorders. Int J Eat Disord 2003;34(4):383-96.
4. Watson TL, Andersen AE. A critical examination of the amenorrhea and weight criteria for diagnosing anorexia nervosa. Acta Psychiatr Scand 2003;108:175-82.
5. Milos G, Spindler A, Schnyder U. Psychiatric comorbidity and eating disorder inventory (EDI) profiles in eating disorder patients. Can J Psychiatry 2004;49:179-84.
6. Rubenstein CS, Pigott TA, L’ Heureux F, et al. A preliminary investigation of the lifetime prevalence of anorexia and bulimia nervosa in patients with obsessive compulsive disorder. J Clin Psychiatry 1992;53(9):309-14.
7. Becker CB, DeViva JC, Zayfert C. Eating disorder symptoms among female anxiety disorder patients in clinical practice: the importance of anxiety comorbidity assessment. J Anxiety Disord 2004;18(3):255-74.
8. Keys A, Brozek J, Henschel A, et al. The biology of human starvation. Minneapolis: University of Minnesota Press; 1950.
9. Ramacciotti CE, Dell’Osso L, Paoli RA, et al. Characteristics of eating disorder patients without a drive for thinness. Int J Eat Disord 2002;32:206-12.
10. Andersen AE. Males with eating disorders: medical considerations. In: Mehler PS, Andersen AE (eds). Eating disorders: a guide to medical care and complications. Baltimore: The Johns Hopkins University Press; 1999;214-26.
11. Lantzouni E, Frank GR, Golden NH, Shenker RI. Reversibility of growth stunting in early onset anorexia nervosa: a prospective study. J Adolesc Health 2002;31(2):162-5.
12. Napolitano MA, Head S, Babyak MA, Blumenthal JA. Binge eating disorder and night eating syndrome: psychological and behavioral characteristics. Int J Eating Disord 2001;30:193-203.
13. Jacobi C, Morris L, de Zwaan M. Overview of risk factors for anorexia nervosa, bulimia nervosa, and binge eating disorder. In: Brewerton, TD (ed). Clinical handbook of eating disorders: An integrated approach. New York: Marcel Dekker; 2004;183-208.
14. Fairburn CG, Beglin SJ. Assessment of eating disorders: interview or self-report questionnaire. Int J Eating Disord 1994;16:363-70.
15. Garner DM. Eating Disorder Inventory-2 professional manual. Odessa, FL: Psychological Assessment Resources; 1991.
16. Garner DM. Psychoeducational principles in treatment. In: Garner DM, Garfinkel PE (eds). Handbook of treatment for eating disorders (2nd ed). New York: Guilford Press; 1997;145-77.
17. Miller KK, Grinspoon SK, Ciampa J, et al. Medical findings in outpatients with anorexia nervosa. Arch Intern Med 2005;165(5):561-6.