User login
Military sexual trauma: How to identify and treat a unique form of PTSD
Sexual trauma may cause or exacerbate posttraumatic stress disorder (PTSD).See "Traumatized Troops: How to treat combat-related PTSD"). Two recommended questions (Box) for men and women can help you start discussing MST.
Use compassion and sensitivity, recognizing the stigma of sexual assault while fastidiously preserving confidentiality. Establish a comfortable environment for disclosure, be nonjudgmental, and maintain good eye contact as you gradually introduce the questions.
The National Center for Posttraumatic Stress Disorder (Related resources) suggests two screening questions for MST:
- While you were in the military, did you experience any unwanted sexual attention, such as verbal remarks, touching, or pressure for sexual favors?
- Did anyone ever use force or threat of force to have sex with you against your will?
Recommended treatment
Comprehensive MST management includes assessing for PTSD, major depression, and substance abuse. When a veteran screens positive for MST, validation and empathy are first-line treatment. Provide MST education, assess health status, and ask them about their support systems.
Sexual trauma survivors often suffer low selfesteem, self-blame, anger, difficulties with interpersonal relationships, and sexual dysfunction. “PTSD flare-ups” can occur during medical encounters and clinical procedures. Evaluate MST survivors regularly for re-victimization, as they may be at risk to be sexually abused again outside the military.5
Referral. Consider referring veterans to a local VA facility, which all have a “military sexual trauma coordinator” to help veterans obtain treatment. Other VA resources include referrals to the women veterans program manager or mental health clinic.
Veterans living in the community can be referred to readjustment counseling service offices, which have sexual trauma counselors on staff. This may be an option for the veteran who does not want to obtain mental health services from the VA.
- Street A, Stafford J. Military sexual trauma: Issues in caring for veterans. National Center for Posttraumatic Stress Disorder. http://www.ncptsd.org//war/military_sexual_trauma.html.
1. Kang H, Dalager N, Mahan C, et al. The role of sexual assault on the risk of PTSD among Gulf War veterans. Ann Epidemiol 2005;15(3):191-5.
2. Bastian L, Lancaster A, Reyst H. Department of Defense 1995 Sexual Harassment Survey (Report No. 96-014). Arlington, VA: Defense Manpower Data Center; 1996.
3. Department of Veterans Affairs. Report to Congress on the Study of Sexual Trauma among Reservists on Active Duty for Training. According to documents provided March 30, 2006 by the Subcommittee on Health, Committee of Veterans’ Affairs, U.S. House of Representatives.
4. Street A, Stafford J. Military sexual trauma: Issues in caring for veterans. National Center for Posttraumatic Stress Disorder. Available at: http://www.ncptsd.org//war/military_sexual_trauma.html. Accessed March 13, 2006.
5. Carole T, Susane F (eds). Military sexual trauma: Veterans Health Initiative, Department of Veterans Affairs. 2004;77-83.Available at: https://www.ees-learning.net/librix/loginhtml.asp?v=librix. Accessed March 28, 2006.
Sexual trauma may cause or exacerbate posttraumatic stress disorder (PTSD).See "Traumatized Troops: How to treat combat-related PTSD"). Two recommended questions (Box) for men and women can help you start discussing MST.
Use compassion and sensitivity, recognizing the stigma of sexual assault while fastidiously preserving confidentiality. Establish a comfortable environment for disclosure, be nonjudgmental, and maintain good eye contact as you gradually introduce the questions.
The National Center for Posttraumatic Stress Disorder (Related resources) suggests two screening questions for MST:
- While you were in the military, did you experience any unwanted sexual attention, such as verbal remarks, touching, or pressure for sexual favors?
- Did anyone ever use force or threat of force to have sex with you against your will?
Recommended treatment
Comprehensive MST management includes assessing for PTSD, major depression, and substance abuse. When a veteran screens positive for MST, validation and empathy are first-line treatment. Provide MST education, assess health status, and ask them about their support systems.
Sexual trauma survivors often suffer low selfesteem, self-blame, anger, difficulties with interpersonal relationships, and sexual dysfunction. “PTSD flare-ups” can occur during medical encounters and clinical procedures. Evaluate MST survivors regularly for re-victimization, as they may be at risk to be sexually abused again outside the military.5
Referral. Consider referring veterans to a local VA facility, which all have a “military sexual trauma coordinator” to help veterans obtain treatment. Other VA resources include referrals to the women veterans program manager or mental health clinic.
Veterans living in the community can be referred to readjustment counseling service offices, which have sexual trauma counselors on staff. This may be an option for the veteran who does not want to obtain mental health services from the VA.
- Street A, Stafford J. Military sexual trauma: Issues in caring for veterans. National Center for Posttraumatic Stress Disorder. http://www.ncptsd.org//war/military_sexual_trauma.html.
Sexual trauma may cause or exacerbate posttraumatic stress disorder (PTSD).See "Traumatized Troops: How to treat combat-related PTSD"). Two recommended questions (Box) for men and women can help you start discussing MST.
Use compassion and sensitivity, recognizing the stigma of sexual assault while fastidiously preserving confidentiality. Establish a comfortable environment for disclosure, be nonjudgmental, and maintain good eye contact as you gradually introduce the questions.
The National Center for Posttraumatic Stress Disorder (Related resources) suggests two screening questions for MST:
- While you were in the military, did you experience any unwanted sexual attention, such as verbal remarks, touching, or pressure for sexual favors?
- Did anyone ever use force or threat of force to have sex with you against your will?
Recommended treatment
Comprehensive MST management includes assessing for PTSD, major depression, and substance abuse. When a veteran screens positive for MST, validation and empathy are first-line treatment. Provide MST education, assess health status, and ask them about their support systems.
Sexual trauma survivors often suffer low selfesteem, self-blame, anger, difficulties with interpersonal relationships, and sexual dysfunction. “PTSD flare-ups” can occur during medical encounters and clinical procedures. Evaluate MST survivors regularly for re-victimization, as they may be at risk to be sexually abused again outside the military.5
Referral. Consider referring veterans to a local VA facility, which all have a “military sexual trauma coordinator” to help veterans obtain treatment. Other VA resources include referrals to the women veterans program manager or mental health clinic.
Veterans living in the community can be referred to readjustment counseling service offices, which have sexual trauma counselors on staff. This may be an option for the veteran who does not want to obtain mental health services from the VA.
- Street A, Stafford J. Military sexual trauma: Issues in caring for veterans. National Center for Posttraumatic Stress Disorder. http://www.ncptsd.org//war/military_sexual_trauma.html.
1. Kang H, Dalager N, Mahan C, et al. The role of sexual assault on the risk of PTSD among Gulf War veterans. Ann Epidemiol 2005;15(3):191-5.
2. Bastian L, Lancaster A, Reyst H. Department of Defense 1995 Sexual Harassment Survey (Report No. 96-014). Arlington, VA: Defense Manpower Data Center; 1996.
3. Department of Veterans Affairs. Report to Congress on the Study of Sexual Trauma among Reservists on Active Duty for Training. According to documents provided March 30, 2006 by the Subcommittee on Health, Committee of Veterans’ Affairs, U.S. House of Representatives.
4. Street A, Stafford J. Military sexual trauma: Issues in caring for veterans. National Center for Posttraumatic Stress Disorder. Available at: http://www.ncptsd.org//war/military_sexual_trauma.html. Accessed March 13, 2006.
5. Carole T, Susane F (eds). Military sexual trauma: Veterans Health Initiative, Department of Veterans Affairs. 2004;77-83.Available at: https://www.ees-learning.net/librix/loginhtml.asp?v=librix. Accessed March 28, 2006.
1. Kang H, Dalager N, Mahan C, et al. The role of sexual assault on the risk of PTSD among Gulf War veterans. Ann Epidemiol 2005;15(3):191-5.
2. Bastian L, Lancaster A, Reyst H. Department of Defense 1995 Sexual Harassment Survey (Report No. 96-014). Arlington, VA: Defense Manpower Data Center; 1996.
3. Department of Veterans Affairs. Report to Congress on the Study of Sexual Trauma among Reservists on Active Duty for Training. According to documents provided March 30, 2006 by the Subcommittee on Health, Committee of Veterans’ Affairs, U.S. House of Representatives.
4. Street A, Stafford J. Military sexual trauma: Issues in caring for veterans. National Center for Posttraumatic Stress Disorder. Available at: http://www.ncptsd.org//war/military_sexual_trauma.html. Accessed March 13, 2006.
5. Carole T, Susane F (eds). Military sexual trauma: Veterans Health Initiative, Department of Veterans Affairs. 2004;77-83.Available at: https://www.ees-learning.net/librix/loginhtml.asp?v=librix. Accessed March 28, 2006.
TRAUMATIZED TROOPS How to treat combat-related PTSD
Thousands of U.S. troops are seeking mental health care after being deployed in Iraq. Among 222,000 Army and Marine Iraq veterans, 35% sought treatment in the year after returning home—many for posttraumatic stress disorder (PTSD).In a related article, we discuss the diagnosis and treatment of military sexual trauma, a form of PTSD.
Persistent pathology
The greater the intensity of an Iraq/Afghanistan veteran’s combat experiences (“firefights”), the more likely the soldier is to develop PTSD.Traumatic brain injury: Choosing medications for neurobehavioral symptoms”). Sexual trauma also may cause or exacerbate PTSD.Military sexual trauma: How to identify and treat a unique form of PTSD”).
Table 1
3 domains of posttraumatic stress disorder symptoms
| Domain | Symptoms |
|---|---|
| Re-experiencing |
|
| Avoidance and numbing |
|
| Increased arousal |
|
| Source: DSM-IV-TR | |
FigurePTSD screen for war veterans
Source: U.S. Department of Veterans Affairs. Afghan & Iraq Post-Deployment Screen, Attachment B. Screening for risk factors associated with development of post-traumatic stress disorder (PTSD)
Cognitive therapy
Psychotherapy is the cornerstone of PTSD treatment; skilled therapists may achieve greater efficacy and more-durable results than medications do. Evidence strongly supports cognitive behavioral therapy—including exposure therapy, anxiety management, and cognitive therapy.U.S. troops returning home: Are you prepared? Current Psychiatry 2006;5(1):12-22.
- U.S. Department of Veterans Affairs. Seamless Transition Home. http://www.seamlesstransition.va.gov. Accessed March 13, 2006.
- American Psychiatric Association. Practice guideline for the treatment of acute stress disorder and posttraumatic stress disorder. http://www.psych.org/psych_pract/treatg/pg/PTSD-PG-PartsA-B-C-New.pdf.
Drug brand names
- Carbamazepine • Carbatrol
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Lithium • Lithobid, others
- Mirtazapine • Remeron
- Paroxetine • Paxil
- Prazosin • Minipress
- Sertraline • Zoloft
- Trazodone • Desyrel
- Temazepam • Restoril
- Valproic acid • Divalproex, others
- Venlafaxine • Effexor
- Zolpidem • Ambien
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products. Drs. Lineberry, Bostwick, and Rundell served on active duty in the U.S. Air Force. Dr. Ramaswamy is staff psychiatrist, Omaha Veterans Administration. and Director of Psychopharmacology Research, Creighton University, Omaha, NE.
1. Hoge CW, Auchterlonie JL, Milliken CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA 2006;295(9):1023-32.
2. Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med 2004;351:13-22.
3. Schlenger WE, Kulka RA, Fairbank JA, et al. The prevalence of post-traumatic stress disorder in the Vietnam generation: a multimethod, multisource assessment of psychiatric disorder. J Trauma Stress 1992;5:333-63.
4. Prigerson HG, Maciejewski PK, Rosenheck RA. Combat trauma: trauma with highest risk of delayed onset and unresolved posttraumatic stress disorder symptoms, unemployment, and abuse among men. J Nerv Ment Dis 2001;189:99-108.
5. Prigerson HG, Maciejewski PK, Rosenheck RA. Population attributable fractions of psychiatric disorders and behavioral outcomes associated with combat exposures among U.S. men. Am J Public Health 2002;92(1):59-63.
6. Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington, DC: American Psychiatric Association; 2000.
7. Xydakis MS, Fravell MD, Nasser KE, Casler JD. Analysis of battlefield head and neck injuries in Iraq and Afghanistan. Otolaryngol Head Neck Surg 2005;133:497-504.
8. Okie S. Traumatic brain injury in the war zone. N Engl J Med 2005;352(20):2043-7.
9. Kang H, Dalager N, Mahan C, et al. The role of sexual assault on the risk of PTSD among Gulf War veterans. Ann Epidemiol 2005;15(3):191-5.
10. Bradley R, Greene J, Russ E, et al. A multidimensional metaanalysis of psychotherapy for posttraumatic stress disorder. Am J Psychiatry 2005;162:214-27.
11. Foa EB, Hembree EA, Cahill SP, et al. Randomized trial of prolonged exposure for posttraumatic stress disorder with and without cognitive restructuring: outcome at academic and community clinics. J Consult Clin Psychol 2005;73:953-64.
12. Schnurr PP, Lunney CA, Sengupta A. Risk factors for the development versus maintenance of post-traumatic stress disorder. J Trauma Stress 2004;17:85-95.
13. Friedman MJ, Donnelly CL, Mellman TA. Pharmacotherapy for PTSD. Psychiatric Annals 2003;33(8):57-62.
14. Asnis GM, Kohn SR, Henderson M, Brown NL. SSRIs versus non- SSRIs in posttraumatic stress disorder: an update with recommendations. Drugs 2004;6:383-404.
15. Simon GE, Savarino J, Operskalski B, et al. Suicide risk during antidepressant treatment. Am J Psychiatry 2006;163(1):41-7.
16. Oquendo M, Brent DA, Birmaher B, et al. Posttraumatic stress disorder comorbid with major depression: factors mediating the association with suicidal behavior. Am J Psychiatry 2005;162(3):560-6.
17. Schoenfeld FB, Marmar CR, Neylan TC. Current concepts in pharmacotherapy for posttraumatic stress disorder. Psychiatr Serv 2004;55:519-31.
18. Martenyi F, Brown EB, Zhang H, et al. Fluoxetine versus placebo in posttraumatic stress disorder. J Clin Psychiatry 2002;63:199-206.
19. Brady KT, Sonne SC, Roberts JM. Sertraline treatment of comorbid posttraumatic stress disorder and alcohol dependence J Clin Psychiatry 1995;56(11):502-5.
20. Davidson JR, Rothbaum BO, van der Kolk BA, et al. Multicenter, double-blind comparison of sertraline and placebo in the treatment of posttraumatic stress disorder. Arch Gen Psychiatry 2001;58(5):485-92.
21. Zohar J, Amital D, Miodownik C, et al. Double-blind placebocontrolled pilot study of sertraline in military veterans with posttraumatic stress disorder J Clin Psychopharmacol 2002;22(2):190-5.
22. Marshall RD, Beebe KL, Oldham M, et al. Efficacy and safety of paroxetine treatment for chronic PTSD: a fixed-dose, placebocontrolled study. Am J Psychiatr 2001;158(12):1982-8.
23. Tucker P, Zaninelli R, Yehuda R, et al. Paroxetine in the treatment of chronic posttraumatic stress disorder: results of a placebocontrolled, flexible-dosage trial. J Clin Psychiatry 2001;62(11):860-8.
24. Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry 2003;160(2):371-3.
25. Hamner MB, Deitsch SE, Brodrick PS, et al. Quetiapine treatment in patients with posttraumatic stress disorder: an open trial of adjunctive therapy. J Clin Psychopharmacol 2003;23(1):15-20.
26. Villarreal G, Calais L, Pickard J, et al. Open-label aripiprazole monotherapy in the treatment of posttraumatic stress disorder. Poster presented at: annual meeting of the NIMH New Clinical Drug Evaluation Unit; June 6-9, 2005; Boca Raton FL.
27. Vieweg WVR, Julius DA, Fernandez A, et al. Posttraumatic stress disorder in male military veterans with comorbid overweight and obesity: Psychotropic, antihypertensive, and metabolic medications. Prim Care Companion J Clin Psychiatry 2006;8(1):
28. Stein MB, Kline NA, Matloff JL. Adjunctive olanzapine for SSRI-resistant combat-related PTSD: a double-blind, placebo-controlled study. Am J Psychiatry 2002;159(10):1777-9.
29. Kozaric-Kovacic D, Pivac N, Muck-Seler D, et al. Risperidone in psychotic combat-related posttraumatic stress disorder: an open trial. J Clin Psychiatry 2005;66(7):922-7.
30. Ahearn EP, Mussey M, Johnson C, et al. Quetiapine as an adjunctive treatment for post-traumatic stress disorder: an 8-week open-label study. Int Clin Psychopharmacol 2006;21(1):29-33.
31. Lambert MT. Aripiprazole in the management of post-traumatic stress disorder symptoms in returning Global War on Terrorism veterans. Int Clin Psychopharmacol 2006;21(3):185-7.
32. Warner MD, Dorn MR, Peabody CA. Survey on the usefulness of trazodone in patients with PTSD with insomnia or nightmares. Pharmacopsychiatry 2001;34(4):128-31.
33. Ocasio-Tascon ME, Alicea-Colon E, et al., Torres-Palacios A, et al. The veteran population: one at high risk for sleep-disordered breathing. Sleep Breath 2006 Feb 22;[Epub ahead of print].
34. Krakow B, Lowry C, Germain A, et al. A retrospective study on improvements in nightmares and post-traumatic stress disorder following treatment for co-morbid sleep-disordered breathing. J Psychosom Res 2000;49(5):291-8.
35. Forbes D, Creamer M, Hawthorne G, et al. Co-morbidity as a predictor of symptom changes after treatment in combat-related posttraumatic stress disorder. J Nerv Ment Dis 2003;191:93-9.
Thousands of U.S. troops are seeking mental health care after being deployed in Iraq. Among 222,000 Army and Marine Iraq veterans, 35% sought treatment in the year after returning home—many for posttraumatic stress disorder (PTSD).In a related article, we discuss the diagnosis and treatment of military sexual trauma, a form of PTSD.
Persistent pathology
The greater the intensity of an Iraq/Afghanistan veteran’s combat experiences (“firefights”), the more likely the soldier is to develop PTSD.Traumatic brain injury: Choosing medications for neurobehavioral symptoms”). Sexual trauma also may cause or exacerbate PTSD.Military sexual trauma: How to identify and treat a unique form of PTSD”).
Table 1
3 domains of posttraumatic stress disorder symptoms
| Domain | Symptoms |
|---|---|
| Re-experiencing |
|
| Avoidance and numbing |
|
| Increased arousal |
|
| Source: DSM-IV-TR | |
FigurePTSD screen for war veterans
Source: U.S. Department of Veterans Affairs. Afghan & Iraq Post-Deployment Screen, Attachment B. Screening for risk factors associated with development of post-traumatic stress disorder (PTSD)
Cognitive therapy
Psychotherapy is the cornerstone of PTSD treatment; skilled therapists may achieve greater efficacy and more-durable results than medications do. Evidence strongly supports cognitive behavioral therapy—including exposure therapy, anxiety management, and cognitive therapy.U.S. troops returning home: Are you prepared? Current Psychiatry 2006;5(1):12-22.
- U.S. Department of Veterans Affairs. Seamless Transition Home. http://www.seamlesstransition.va.gov. Accessed March 13, 2006.
- American Psychiatric Association. Practice guideline for the treatment of acute stress disorder and posttraumatic stress disorder. http://www.psych.org/psych_pract/treatg/pg/PTSD-PG-PartsA-B-C-New.pdf.
Drug brand names
- Carbamazepine • Carbatrol
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Lithium • Lithobid, others
- Mirtazapine • Remeron
- Paroxetine • Paxil
- Prazosin • Minipress
- Sertraline • Zoloft
- Trazodone • Desyrel
- Temazepam • Restoril
- Valproic acid • Divalproex, others
- Venlafaxine • Effexor
- Zolpidem • Ambien
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products. Drs. Lineberry, Bostwick, and Rundell served on active duty in the U.S. Air Force. Dr. Ramaswamy is staff psychiatrist, Omaha Veterans Administration. and Director of Psychopharmacology Research, Creighton University, Omaha, NE.
Thousands of U.S. troops are seeking mental health care after being deployed in Iraq. Among 222,000 Army and Marine Iraq veterans, 35% sought treatment in the year after returning home—many for posttraumatic stress disorder (PTSD).In a related article, we discuss the diagnosis and treatment of military sexual trauma, a form of PTSD.
Persistent pathology
The greater the intensity of an Iraq/Afghanistan veteran’s combat experiences (“firefights”), the more likely the soldier is to develop PTSD.Traumatic brain injury: Choosing medications for neurobehavioral symptoms”). Sexual trauma also may cause or exacerbate PTSD.Military sexual trauma: How to identify and treat a unique form of PTSD”).
Table 1
3 domains of posttraumatic stress disorder symptoms
| Domain | Symptoms |
|---|---|
| Re-experiencing |
|
| Avoidance and numbing |
|
| Increased arousal |
|
| Source: DSM-IV-TR | |
FigurePTSD screen for war veterans
Source: U.S. Department of Veterans Affairs. Afghan & Iraq Post-Deployment Screen, Attachment B. Screening for risk factors associated with development of post-traumatic stress disorder (PTSD)
Cognitive therapy
Psychotherapy is the cornerstone of PTSD treatment; skilled therapists may achieve greater efficacy and more-durable results than medications do. Evidence strongly supports cognitive behavioral therapy—including exposure therapy, anxiety management, and cognitive therapy.U.S. troops returning home: Are you prepared? Current Psychiatry 2006;5(1):12-22.
- U.S. Department of Veterans Affairs. Seamless Transition Home. http://www.seamlesstransition.va.gov. Accessed March 13, 2006.
- American Psychiatric Association. Practice guideline for the treatment of acute stress disorder and posttraumatic stress disorder. http://www.psych.org/psych_pract/treatg/pg/PTSD-PG-PartsA-B-C-New.pdf.
Drug brand names
- Carbamazepine • Carbatrol
- Fluoxetine • Prozac
- Lamotrigine • Lamictal
- Lithium • Lithobid, others
- Mirtazapine • Remeron
- Paroxetine • Paxil
- Prazosin • Minipress
- Sertraline • Zoloft
- Trazodone • Desyrel
- Temazepam • Restoril
- Valproic acid • Divalproex, others
- Venlafaxine • Effexor
- Zolpidem • Ambien
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products. Drs. Lineberry, Bostwick, and Rundell served on active duty in the U.S. Air Force. Dr. Ramaswamy is staff psychiatrist, Omaha Veterans Administration. and Director of Psychopharmacology Research, Creighton University, Omaha, NE.
1. Hoge CW, Auchterlonie JL, Milliken CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA 2006;295(9):1023-32.
2. Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med 2004;351:13-22.
3. Schlenger WE, Kulka RA, Fairbank JA, et al. The prevalence of post-traumatic stress disorder in the Vietnam generation: a multimethod, multisource assessment of psychiatric disorder. J Trauma Stress 1992;5:333-63.
4. Prigerson HG, Maciejewski PK, Rosenheck RA. Combat trauma: trauma with highest risk of delayed onset and unresolved posttraumatic stress disorder symptoms, unemployment, and abuse among men. J Nerv Ment Dis 2001;189:99-108.
5. Prigerson HG, Maciejewski PK, Rosenheck RA. Population attributable fractions of psychiatric disorders and behavioral outcomes associated with combat exposures among U.S. men. Am J Public Health 2002;92(1):59-63.
6. Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington, DC: American Psychiatric Association; 2000.
7. Xydakis MS, Fravell MD, Nasser KE, Casler JD. Analysis of battlefield head and neck injuries in Iraq and Afghanistan. Otolaryngol Head Neck Surg 2005;133:497-504.
8. Okie S. Traumatic brain injury in the war zone. N Engl J Med 2005;352(20):2043-7.
9. Kang H, Dalager N, Mahan C, et al. The role of sexual assault on the risk of PTSD among Gulf War veterans. Ann Epidemiol 2005;15(3):191-5.
10. Bradley R, Greene J, Russ E, et al. A multidimensional metaanalysis of psychotherapy for posttraumatic stress disorder. Am J Psychiatry 2005;162:214-27.
11. Foa EB, Hembree EA, Cahill SP, et al. Randomized trial of prolonged exposure for posttraumatic stress disorder with and without cognitive restructuring: outcome at academic and community clinics. J Consult Clin Psychol 2005;73:953-64.
12. Schnurr PP, Lunney CA, Sengupta A. Risk factors for the development versus maintenance of post-traumatic stress disorder. J Trauma Stress 2004;17:85-95.
13. Friedman MJ, Donnelly CL, Mellman TA. Pharmacotherapy for PTSD. Psychiatric Annals 2003;33(8):57-62.
14. Asnis GM, Kohn SR, Henderson M, Brown NL. SSRIs versus non- SSRIs in posttraumatic stress disorder: an update with recommendations. Drugs 2004;6:383-404.
15. Simon GE, Savarino J, Operskalski B, et al. Suicide risk during antidepressant treatment. Am J Psychiatry 2006;163(1):41-7.
16. Oquendo M, Brent DA, Birmaher B, et al. Posttraumatic stress disorder comorbid with major depression: factors mediating the association with suicidal behavior. Am J Psychiatry 2005;162(3):560-6.
17. Schoenfeld FB, Marmar CR, Neylan TC. Current concepts in pharmacotherapy for posttraumatic stress disorder. Psychiatr Serv 2004;55:519-31.
18. Martenyi F, Brown EB, Zhang H, et al. Fluoxetine versus placebo in posttraumatic stress disorder. J Clin Psychiatry 2002;63:199-206.
19. Brady KT, Sonne SC, Roberts JM. Sertraline treatment of comorbid posttraumatic stress disorder and alcohol dependence J Clin Psychiatry 1995;56(11):502-5.
20. Davidson JR, Rothbaum BO, van der Kolk BA, et al. Multicenter, double-blind comparison of sertraline and placebo in the treatment of posttraumatic stress disorder. Arch Gen Psychiatry 2001;58(5):485-92.
21. Zohar J, Amital D, Miodownik C, et al. Double-blind placebocontrolled pilot study of sertraline in military veterans with posttraumatic stress disorder J Clin Psychopharmacol 2002;22(2):190-5.
22. Marshall RD, Beebe KL, Oldham M, et al. Efficacy and safety of paroxetine treatment for chronic PTSD: a fixed-dose, placebocontrolled study. Am J Psychiatr 2001;158(12):1982-8.
23. Tucker P, Zaninelli R, Yehuda R, et al. Paroxetine in the treatment of chronic posttraumatic stress disorder: results of a placebocontrolled, flexible-dosage trial. J Clin Psychiatry 2001;62(11):860-8.
24. Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry 2003;160(2):371-3.
25. Hamner MB, Deitsch SE, Brodrick PS, et al. Quetiapine treatment in patients with posttraumatic stress disorder: an open trial of adjunctive therapy. J Clin Psychopharmacol 2003;23(1):15-20.
26. Villarreal G, Calais L, Pickard J, et al. Open-label aripiprazole monotherapy in the treatment of posttraumatic stress disorder. Poster presented at: annual meeting of the NIMH New Clinical Drug Evaluation Unit; June 6-9, 2005; Boca Raton FL.
27. Vieweg WVR, Julius DA, Fernandez A, et al. Posttraumatic stress disorder in male military veterans with comorbid overweight and obesity: Psychotropic, antihypertensive, and metabolic medications. Prim Care Companion J Clin Psychiatry 2006;8(1):
28. Stein MB, Kline NA, Matloff JL. Adjunctive olanzapine for SSRI-resistant combat-related PTSD: a double-blind, placebo-controlled study. Am J Psychiatry 2002;159(10):1777-9.
29. Kozaric-Kovacic D, Pivac N, Muck-Seler D, et al. Risperidone in psychotic combat-related posttraumatic stress disorder: an open trial. J Clin Psychiatry 2005;66(7):922-7.
30. Ahearn EP, Mussey M, Johnson C, et al. Quetiapine as an adjunctive treatment for post-traumatic stress disorder: an 8-week open-label study. Int Clin Psychopharmacol 2006;21(1):29-33.
31. Lambert MT. Aripiprazole in the management of post-traumatic stress disorder symptoms in returning Global War on Terrorism veterans. Int Clin Psychopharmacol 2006;21(3):185-7.
32. Warner MD, Dorn MR, Peabody CA. Survey on the usefulness of trazodone in patients with PTSD with insomnia or nightmares. Pharmacopsychiatry 2001;34(4):128-31.
33. Ocasio-Tascon ME, Alicea-Colon E, et al., Torres-Palacios A, et al. The veteran population: one at high risk for sleep-disordered breathing. Sleep Breath 2006 Feb 22;[Epub ahead of print].
34. Krakow B, Lowry C, Germain A, et al. A retrospective study on improvements in nightmares and post-traumatic stress disorder following treatment for co-morbid sleep-disordered breathing. J Psychosom Res 2000;49(5):291-8.
35. Forbes D, Creamer M, Hawthorne G, et al. Co-morbidity as a predictor of symptom changes after treatment in combat-related posttraumatic stress disorder. J Nerv Ment Dis 2003;191:93-9.
1. Hoge CW, Auchterlonie JL, Milliken CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA 2006;295(9):1023-32.
2. Hoge CW, Castro CA, Messer SC, et al. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med 2004;351:13-22.
3. Schlenger WE, Kulka RA, Fairbank JA, et al. The prevalence of post-traumatic stress disorder in the Vietnam generation: a multimethod, multisource assessment of psychiatric disorder. J Trauma Stress 1992;5:333-63.
4. Prigerson HG, Maciejewski PK, Rosenheck RA. Combat trauma: trauma with highest risk of delayed onset and unresolved posttraumatic stress disorder symptoms, unemployment, and abuse among men. J Nerv Ment Dis 2001;189:99-108.
5. Prigerson HG, Maciejewski PK, Rosenheck RA. Population attributable fractions of psychiatric disorders and behavioral outcomes associated with combat exposures among U.S. men. Am J Public Health 2002;92(1):59-63.
6. Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington, DC: American Psychiatric Association; 2000.
7. Xydakis MS, Fravell MD, Nasser KE, Casler JD. Analysis of battlefield head and neck injuries in Iraq and Afghanistan. Otolaryngol Head Neck Surg 2005;133:497-504.
8. Okie S. Traumatic brain injury in the war zone. N Engl J Med 2005;352(20):2043-7.
9. Kang H, Dalager N, Mahan C, et al. The role of sexual assault on the risk of PTSD among Gulf War veterans. Ann Epidemiol 2005;15(3):191-5.
10. Bradley R, Greene J, Russ E, et al. A multidimensional metaanalysis of psychotherapy for posttraumatic stress disorder. Am J Psychiatry 2005;162:214-27.
11. Foa EB, Hembree EA, Cahill SP, et al. Randomized trial of prolonged exposure for posttraumatic stress disorder with and without cognitive restructuring: outcome at academic and community clinics. J Consult Clin Psychol 2005;73:953-64.
12. Schnurr PP, Lunney CA, Sengupta A. Risk factors for the development versus maintenance of post-traumatic stress disorder. J Trauma Stress 2004;17:85-95.
13. Friedman MJ, Donnelly CL, Mellman TA. Pharmacotherapy for PTSD. Psychiatric Annals 2003;33(8):57-62.
14. Asnis GM, Kohn SR, Henderson M, Brown NL. SSRIs versus non- SSRIs in posttraumatic stress disorder: an update with recommendations. Drugs 2004;6:383-404.
15. Simon GE, Savarino J, Operskalski B, et al. Suicide risk during antidepressant treatment. Am J Psychiatry 2006;163(1):41-7.
16. Oquendo M, Brent DA, Birmaher B, et al. Posttraumatic stress disorder comorbid with major depression: factors mediating the association with suicidal behavior. Am J Psychiatry 2005;162(3):560-6.
17. Schoenfeld FB, Marmar CR, Neylan TC. Current concepts in pharmacotherapy for posttraumatic stress disorder. Psychiatr Serv 2004;55:519-31.
18. Martenyi F, Brown EB, Zhang H, et al. Fluoxetine versus placebo in posttraumatic stress disorder. J Clin Psychiatry 2002;63:199-206.
19. Brady KT, Sonne SC, Roberts JM. Sertraline treatment of comorbid posttraumatic stress disorder and alcohol dependence J Clin Psychiatry 1995;56(11):502-5.
20. Davidson JR, Rothbaum BO, van der Kolk BA, et al. Multicenter, double-blind comparison of sertraline and placebo in the treatment of posttraumatic stress disorder. Arch Gen Psychiatry 2001;58(5):485-92.
21. Zohar J, Amital D, Miodownik C, et al. Double-blind placebocontrolled pilot study of sertraline in military veterans with posttraumatic stress disorder J Clin Psychopharmacol 2002;22(2):190-5.
22. Marshall RD, Beebe KL, Oldham M, et al. Efficacy and safety of paroxetine treatment for chronic PTSD: a fixed-dose, placebocontrolled study. Am J Psychiatr 2001;158(12):1982-8.
23. Tucker P, Zaninelli R, Yehuda R, et al. Paroxetine in the treatment of chronic posttraumatic stress disorder: results of a placebocontrolled, flexible-dosage trial. J Clin Psychiatry 2001;62(11):860-8.
24. Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry 2003;160(2):371-3.
25. Hamner MB, Deitsch SE, Brodrick PS, et al. Quetiapine treatment in patients with posttraumatic stress disorder: an open trial of adjunctive therapy. J Clin Psychopharmacol 2003;23(1):15-20.
26. Villarreal G, Calais L, Pickard J, et al. Open-label aripiprazole monotherapy in the treatment of posttraumatic stress disorder. Poster presented at: annual meeting of the NIMH New Clinical Drug Evaluation Unit; June 6-9, 2005; Boca Raton FL.
27. Vieweg WVR, Julius DA, Fernandez A, et al. Posttraumatic stress disorder in male military veterans with comorbid overweight and obesity: Psychotropic, antihypertensive, and metabolic medications. Prim Care Companion J Clin Psychiatry 2006;8(1):
28. Stein MB, Kline NA, Matloff JL. Adjunctive olanzapine for SSRI-resistant combat-related PTSD: a double-blind, placebo-controlled study. Am J Psychiatry 2002;159(10):1777-9.
29. Kozaric-Kovacic D, Pivac N, Muck-Seler D, et al. Risperidone in psychotic combat-related posttraumatic stress disorder: an open trial. J Clin Psychiatry 2005;66(7):922-7.
30. Ahearn EP, Mussey M, Johnson C, et al. Quetiapine as an adjunctive treatment for post-traumatic stress disorder: an 8-week open-label study. Int Clin Psychopharmacol 2006;21(1):29-33.
31. Lambert MT. Aripiprazole in the management of post-traumatic stress disorder symptoms in returning Global War on Terrorism veterans. Int Clin Psychopharmacol 2006;21(3):185-7.
32. Warner MD, Dorn MR, Peabody CA. Survey on the usefulness of trazodone in patients with PTSD with insomnia or nightmares. Pharmacopsychiatry 2001;34(4):128-31.
33. Ocasio-Tascon ME, Alicea-Colon E, et al., Torres-Palacios A, et al. The veteran population: one at high risk for sleep-disordered breathing. Sleep Breath 2006 Feb 22;[Epub ahead of print].
34. Krakow B, Lowry C, Germain A, et al. A retrospective study on improvements in nightmares and post-traumatic stress disorder following treatment for co-morbid sleep-disordered breathing. J Psychosom Res 2000;49(5):291-8.
35. Forbes D, Creamer M, Hawthorne G, et al. Co-morbidity as a predictor of symptom changes after treatment in combat-related posttraumatic stress disorder. J Nerv Ment Dis 2003;191:93-9.
4 drugs can improve autism’s repetitive behaviors
Autism’s repetitive behaviors and restricted interests interfere with adaptive functioning, social interactions, and learning. No medications are FDA-approved for autistic disorder, but some selective serotonin reuptake inhibitors (SSRIs) and atypical antipsychotics and an anticonvulsant have reduced repetitive behaviors in controlled trials. We discuss how that evidence shapes our approach to patients with or without a comorbid family history of bipolar disorder.
Evidence for SSRIs
Repetitive behaviors and restricted interests are autism’s third core domain, as defined by DSM-IVTR criteria.1 For an autistic disorder diagnosis, a patient must show at least one of these behaviors:
- encompassing preoccupations with stereotyped or restricted patterns of interest
- inflexible routines or rituals
- stereotyped, repetitive motor mannerisms
- or persistent preoccupation with parts of objects.
As in obsessive-compulsive disorder (OCD), rituals and restricted interests are thought to decrease anxiety in autism, whereas self-stimulatory behaviors and stereotypy may regulate arousal. The behaviors persist2,3 but may change across the lifespan.
Because SSRIs improve OCD’s repetitive behaviors, clinicians have also used them to treat autism’s repetitive behaviors, though without supporting data. Recently, however, fluoxetine and fluvoxamine have shown efficacy for autism’s repetitive behaviors in randomized, controlled trials. Results indicate:
- In children, fluoxetine is probably better-tolerated than available dosing forms of fluvoxamine.
- In adults, fluvoxamine is well-tolerated and can improve repetitive behavior.
SSRIs and suicidal ideation in autism. The increased risk of suicidality reported with SSRIs when treating depression and OCD has not been seen in children with autism. But because fewer children with autism have been treated with SSRIs, we recommend that you try to assess suicidal ideation during SSRI treatment in those able to express such concerns (Box). Starting with low SSRI dosages (Table 1) and increasing slowly may help prevent behavioral activation, a possible risk factor for suicidality.
Suicidal ideation has not been reported in studies of selective serotonin reuptake inhibitors (SSRIs) in autism. Even so, children and adolescents with autistic disorder are not excluded from the FDA black-box warning of increased risk of suicidality with SSRIs.
Children with obsessive-compulsive disorder (OCD) treated with SSRIs have shown evidence of suicidal thoughts. Thus, higher-functioning children and adults with autism might think about suicide when they become aware of their deficits.
For lower-functioning patients (generally, those who receive medication), we need markers of possible suicidal ideation other than their reports of symptoms. In clinical trials, investigators measure behavioral activation symptoms as risk factors for suicidality.
Thus, when you start an SSRI in a patient with autistic disorder, educate the caregivers to watch for agitation, increased energy, poor sleep, disinhibition, or new hyperactivity. Encourage them to contact you immediately if these signs of activation occur.
Ask higher-functioning patients taking SSRIs about suicidal thinking in a step-wise fashion: thoughts of death, thoughts of their own death, intent, plan, and finally possible attempts.
Table 1
4 drugs with evidence of benefit for autism’s repetitive behaviors*
| Medication | Suggested target daily dosage |
|---|---|
| Fluoxetine7 | Children: Start at 2.5 mg/d; maximum 20 mg/d |
| Adults: Start at 10 to 20 mg/d; maximum 60 mg/d | |
| Fluvoxamine10 | Children: Not first-line; start at 12.5 mg/d; maximum 150 to200 mg/d |
| Adults: Start at 25 mg/d; maximum 300 mg/d | |
| Risperidone13,16 | Children: Start at 0.25 mg/d; maximum 3 mg/d |
| Adults: Start at 2 mg/d; maximum 4 mg/d | |
| Valproate20 | Children: Start at 125 mg (sprinkles); titrate to clinical effect and blood levels of 50 of 120 mcg/mL |
| Adults: Start at 250 mg; increase by 250mg/week to clinical effect and blood levels of 50 to 120 mcg/mL | |
| *Data from randomized, placebo-controlled trials | |
Fluoxetine. In the first open-label study of fluoxetine in children and adults with autistic disorder, global functioning improved significantly in 15 of 23 patients, as measured by the Clinical Global Impressions (CGI) scale.4 Autism symptoms also improved in follow-up, open-label trials, but these did not target repetitive behaviors specifically.5,6
Our group conducted the first randomized, placebo-controlled study of fluoxetine’s effect on repetitive behaviors in children with autism.7 We measured obsessions and compulsions in 45 children, ages 5 to 16, with the Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS). This 10-item, clinician-rated questionnaire uses a 5-point scale to rate repetitive behaviors by time spent, distress, interference, resistance, and control.
Using a crossover design—two 8-week phases of active or placebo treatment separated by a 4-week washout—we started liquid fluoxetine at 2.5 mg/d and slowly increased the dosage to clinical effect or a maximum of 0.8 mg/kg/day. Mean final dosage was 9.9 (±4.35) mg/d.
Repetitive behaviors improved, even though we used relatively low dosages to avoid side effects. The mean baseline CY-BOCS compulsion score of 13.15 dropped to 11.6 with fluoxetine and to 12.9 with placebo. Fluoxetine’s effect size was moderate to large, and we found no suicidal ideation with this SSRI.
Fluvoxamine. Repetitive behaviors did not change—as measured with the CY-BOCS—when 18 children with autism received fluvoxamine, 1.5 mg/kg/day, in a 10-week prospective, open-label trial.8 Most patients (72%) reported at least one side effect, and 3 discontinued the SSRI because of behavioral activation. Ten completed the trial. Likewise in a randomized trial, children who received fluvoxamine experienced troublesome side effects and limited benefit.9
Compared with outcomes in children, fluvoxamine has shown greater efficacy in adults. In a 12-week, double-blind, placebo-controlled trial of 30 adults with autism, 8 of 15 treated with fluvoxamine (50 mg/d initially and titrated to 300 mg/d) were rated as responders, compared with none of 15 receiving placebo.
Repetitive behaviors and adaptive functioning improved significantly with fluvoxamine, as measured with the Yale-Brown Obsessive Compulsive Scale (YBOCS) and Vineland Adaptive Behavior Scale, respectively.10 The SSRI was well-tolerated, with only mild nausea and sedation reported.
Thus, fluvoxamine may be useful for treating repetitive behaviors but probably is not a first choice for children with autism. Results might be more favorable in children if fluvoxamine were available in doses <12.5 mg.
Citalopram. Open-label data support using citalopram in autism.11 In a retrospective chart review, 10 of 15 children (73%) were reported “much improved” with citalopram (mean dosage 16.9 mg/d [+/-12.1]), but the review did not specifically address repetitive behaviors. Two patients stopped taking the SSRI because of side effects; agitation, aggressiveness, sedation, and lip dyskinesia were reported.
The National Institutes of Health is sponsoring a multicenter trial of citalopram (starting dosage 2.5 mg/d, up to 20 mg/d) for repetitive behaviors in 144 children with autism, Results are expected in 2007 (see Related resources).
Escitalopram. Some early, open-label evidence suggests that escitalopram may be well-tolerated in autism, but its efficacy for treating repetitive behaviors has not been studied.
Sertraline. One of three reported open-label studies of sertraline in patients with autism measured repetitive behaviors.12 In this study, 42 adults with autism spectrum disorder were treated for 12 weeks with sertraline, 50 to 200 mg/d. One-half were rated “much improved”—mostly in aggressive and repetitive behaviors—with the CGI improvement scale. Sertraline was well-tolerated, although 3 patients dropped out because of persistent agitation.
No randomized trials have examined sertraline in autism.
Clinical recommendations
Family history of bipolar disorder guides our treatment of patients with an autism spectrum disorder and disabling repetitive behaviors (Algorithm).
Algorithm Suggested medications to treat repetitive behaviors in autism
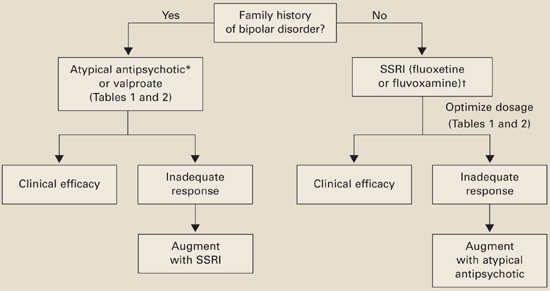
*Risperidone has the most evidence of efficacy, but aripiprazole may be useful for patients with weight-gain problems.
†For children, controlled data support using liquid fluoxetine, starting at 2.5 mg/d.Without bipolar history. SSRIs are usually first-line therapy (although patients with significant irritability and aggression may be an exception and require an atypical antipsychotic first).
If you reach the maximal SSRI dosage without a desired effect, consider adding an atypical (risperidone has the strongest supporting data) or valproate. If behavioral activation symptoms emerge and a lower dosage does not ameliorate them or reduces the clinical effect, consider switching to an atypical or valproate.
With bipolar history. Consider starting with an atypical or valproate; augment with an SSRI if needed.
Monitor for side effects with each medication (Table 2).
Table 2
Medication side effects and recommended monitoring
| Medication | Side effects | Recommended monitoring |
|---|---|---|
| Fluoxetine | Anxiety, insomnia, GI disturbance, appetite and weight changes, mania/hypomania activation, suicidal ideation | Observe closely when starting treatment and while increasing dosage |
| Fluvoxamine | Somnolence, nervousness, insomnia, agitation, GI disturbance, suicidal ideation | Observe closely when starting treatment and while increasing dosage |
| Risperidone | Drowsiness, weight gain, hyperglycemia, GI disturbance, extrapyramidal symptoms, neuroleptic malignant syndrome | Obtain metabolic profile, including serum glucose and lipids |
| Monitor for weight gain and clinical signs of extrapyramidal symptoms | ||
| Valproate | Rash, headaches, weight gain, ataxia, alopecia, GI disturbance, hyperammonemic encephalopathy, sedation, thrombocytopenia, polycystic ovary syndrome, pancreatitis, liver failure, teratogenic effects | CBC with platelets, liver function tests, valproate levels |
| Therapeutic blood levels: 50 to 120 mcg/mL |
Evidence for atypical antipsychotics
Atypicals have been used in autistic disorder to treat irritability and impulsive aggression. Risperidone also has been shown to reduce repetitive behaviors in a controlled trial.13 No evidence or only open-label trials support the use of other atypicals in patients with autism.
Risperidone’s side effects may include metabolic syndrome. No studies have examined lipid profiles and insulin resistance after risperidone treatment in patients with autism, but weight gain has been reported in the Research Units on Pediatric Psychopharmacology (RUPP) trial andothers.13-15 Carefully assess the risk-benefit ratio when you consider using risperidone to treat repetitive behaviors in patients with autism.
Atypical antipsychotics also may increase dyskinesia risk, although extrapyramidal symptoms (EPS) have not been reported in studies of patients with autism and repetitive behaviors. Because EPS could develop after clinical trials are completed, long-term naturalistic studies are needed to address this concern.
Risperidone. The double-blind, placebo-controlled RUPP trial examined risperidone’s efficacy in treating autism’s core symptoms (primarily irritability) in 101 children.13 Mean dosages after 8 weeks and during a 16-week open-label extension for 63 children were 2 and 2.1 mg/d, respectively.
Repetitive behavior—as measured with the CY-BOCS, using RUPP trial data16—improved significantly with risperidone compared with placebo. During the 8-week controlled trial, CY-BOCS scores improved from 15.51 (SD±2.73) to 11.65 (SD±4.02) in the risperidone group, compared with 15.18 (SD±3.88) to 14.21 (SD±4.81) in the placebo group. This response was maintained through the open-label trial.
Side effects included weight gain, fatigue, drowsiness, and drooling. No children receiving risperidone dropped out because of side effects. No EPS were reported, based on weekly Abnormal Involuntary Movement scale and Simpson-Angus scale scores.
Olanzapine. Only open-label studies have examined olanzapine in autism, and one systematically measured repetitive behaviors.17 Eight children with autism or other pervasive developmental disorders were given olanzapine, mean dosage 7.8 (±4.7) mg/d at the end of the 12-week trial.
Repetitive behaviors did not change significantly, as measured with YBOCS. Seven of eight patients completed the trial. Mean weight for the group after 12 weeks was 156±55 lbs, compared with 137±56 lbs at baseline.
Quetiapine. No data support using quetiapine for autism’s repetitive behaviors. Quetiapine, 100 to 350 mg/d (1.6 to 5.2 mg/kg/day) was poorly tolerated in a 16-week open-label safety and efficacy trial among 6 mentally retarded boys with autistic disorder. Side effects included a possible seizure, behavioral activation, increased appetite, and weight gain (0.9 to 8.2 kg). Two patients completed the trial.18
Ziprasidone. Small open-label studies and anecdotal reports of ziprasidone in autism have not examined this drug’s effect on repetitive behaviors.
Aripiprazole. Anecdotal information suggests that clinicians are using this medication to treat patients with autism, but no supporting data exist.
Evidence for valproate
Preliminary trials by our group suggest that valproate may reduce repetitive behaviors in autism. In a retrospective, open-label study, 14 patients (mean age 17) with autism spectrum disorder received divalproex sodium (mean 768±582 mg/d) for a mean 11 months. Ten patients (71%) showed sustained improvement in function, as measured by the CGI-improvement scale, and valproate was generally well-tolerated.19
We then measured valproate’s effect on repetitive behaviors in an 8-week, double-blind, placebo-controlled study of 13 patients (mean age 9) with autism spectrum disorder. Repetitive behaviors improved significantly compared with placebo, as measured by the CY-BOCS, in those who received divalproex (mean 833.93±326.21 mg/d).20
Further studies are needed to replicate this finding. Although it is too early to make general recommendations, valproate may be a reasonable choice for children with autism and epilepsy or affective instability.
- Hollander E, Phillips AT, Yeh CC. Targeted treatments for symptom domains in child and adolescent autism. Lancet 2003;362:732-4.
- Hollander E (ed). Autism spectrum disorders. New York: Marcel Decker; 2003.
- National Institute of Health multicenter study of citalopram for repetitive behaviors in autism. http://www.clinicaltrials.gov/ct/show/nct00086645?order=2
Drug brand names
- Aripiprazole • Abilify
- Citalopram • Celexa
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Valproic acid • Depakote, Depakene
- Ziprasidone • Geodon
Disclosures
Dr. Anagnostou reports no financial interest with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Hollander receives research/grant support from and is a consultant to Abbott Laboratories. He also receives support from the National Institutes of Health (STAART Center) to investigate orphan drug status for fluoxetine in treating autism symptoms.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC; American Psychiatric Association; 2000.
2. Seltzer MM, Shattuck P, Abbeduto L, Greenberg JS. Trajectory of development in adolescents and adults with autism. MRDD Research Reviews 2004;10:234-47.
3. Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. J Child Psychol Psychiatry 2004;45:212-29.
4. Cook EH, Rowlett R, Jaselinkis C, Leventhal BL. Fluoxetine treatment of children and adults with autistic disorder and mental retardation. J Am Acad Child Adolesc Psychiatry 1992;31:739-45.
5. DeLong GR, Teague LA, Kamran MM. Effects of fluoxetine treatment in young children with idiopathic autism. Dev Med Child Neurol 1998;40:551-62.
6. DeLong GR, Ritch CR, Burch S. Fluoxetine response in children with autistic spectrum disorders: correlation with familial major affective disorder and intellectual achievement. Dev Med Child Neurol 2002;44:652-9.
7. Hollander E, Phillips A, Chaplin W, et al. A placebo-controlled cross over trial of liquid fluoxetine on repetitive behaviors in childhood and adolescent autism. Neuropsychopharmacology 2005;30:582-9.
8. Martin A, Koenig K, Anderson GM, Scahill L. Low-dose fluvoxamine treatment of children and adolescents with pervasive developmental disorders: a prospective, open-label study. J Autism Dev Disord 2003;33:77-85.
9. McDougle CJ, Kresch LE, Posey DJ. Repetitive thoughts and behavior in pervasive developmental disorders: treatment with serotonin reuptake inhibitors. J Autism Dev Disord 2000;30:427-35.
10. McDougle CJ, Naylor ST, Cohen DJ, et al. A double-blind, placebo-controlled study of fluvoxamine in adults with autistic disorder. Arch Gen Psychiatry 1996;53:1001-8.
11. Namerow LB, Thomas P, Bostic JQ, et al. Use of citalopram in pervasive developmental disorders. J Dev Behav Pediatr. 2003;24(2):104-8.
12. McDougle CJ, Brodkin ES, Naylor ST, et al. Sertraline in adults with pervasive developmental disorders: a prospective open-label investigation. J Clin Psychopharmacol 1998;18:62-6.
13. McCracken JT, McGough J, Shah B, et al. Research Units on Pediatric Psychopharmacology Autism Network. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347:314-21.
14. Troost PW, Lahuis BE, Steenhuis MP, et al. Long-term effects of risperidone in children with autism spectrum disorders: A placebo discontinuation study. J Am Acad Child Adolesc Psychiatry 2005;44:1137-44.
15. Shea S, Turgay A, Carroll A, et al. Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics 2004;114:e634-e641.
16. McDougle CJ, Scahill L, Aman MG, et al. Risperidone for the core symptom domains of autism: results from the study by the autism network of the research units on pediatric psychopharmacology. Am J Psychiatry 2005;162:1142-8.
17. Potenza MN, Holmes JP, Kanes SJ, McDougle CJ. Olanzapine treatment of children, adolescents and adults with pervasive developmental disorders: an open-label pilot study. J Clin Psychopharmacol 1999;19:37-44.
18. Martin A, Koenig K, Scahill L, Bregman J. Open-label quetiapine in the treatment of children and adolescents with autistic disorder. J Child Adolesc Psychopharmacol 1999;9:99-107.
19. Hollander E, Dolgoff-Kaspar R, Cartwright C, et al. An open trial of divalproex sodium in autism spectrum disorders. J Clin Psychiatry 2001;62:530-4.
20. Hollander E, Soorya LV, Wasserman S, et al. Divalproex sodium vs. placebo in the treatment of repetitive behaviours in autism spectrum disorder. Int J Neuropsychopharmacol 2006;9:209-13.
Autism’s repetitive behaviors and restricted interests interfere with adaptive functioning, social interactions, and learning. No medications are FDA-approved for autistic disorder, but some selective serotonin reuptake inhibitors (SSRIs) and atypical antipsychotics and an anticonvulsant have reduced repetitive behaviors in controlled trials. We discuss how that evidence shapes our approach to patients with or without a comorbid family history of bipolar disorder.
Evidence for SSRIs
Repetitive behaviors and restricted interests are autism’s third core domain, as defined by DSM-IVTR criteria.1 For an autistic disorder diagnosis, a patient must show at least one of these behaviors:
- encompassing preoccupations with stereotyped or restricted patterns of interest
- inflexible routines or rituals
- stereotyped, repetitive motor mannerisms
- or persistent preoccupation with parts of objects.
As in obsessive-compulsive disorder (OCD), rituals and restricted interests are thought to decrease anxiety in autism, whereas self-stimulatory behaviors and stereotypy may regulate arousal. The behaviors persist2,3 but may change across the lifespan.
Because SSRIs improve OCD’s repetitive behaviors, clinicians have also used them to treat autism’s repetitive behaviors, though without supporting data. Recently, however, fluoxetine and fluvoxamine have shown efficacy for autism’s repetitive behaviors in randomized, controlled trials. Results indicate:
- In children, fluoxetine is probably better-tolerated than available dosing forms of fluvoxamine.
- In adults, fluvoxamine is well-tolerated and can improve repetitive behavior.
SSRIs and suicidal ideation in autism. The increased risk of suicidality reported with SSRIs when treating depression and OCD has not been seen in children with autism. But because fewer children with autism have been treated with SSRIs, we recommend that you try to assess suicidal ideation during SSRI treatment in those able to express such concerns (Box). Starting with low SSRI dosages (Table 1) and increasing slowly may help prevent behavioral activation, a possible risk factor for suicidality.
Suicidal ideation has not been reported in studies of selective serotonin reuptake inhibitors (SSRIs) in autism. Even so, children and adolescents with autistic disorder are not excluded from the FDA black-box warning of increased risk of suicidality with SSRIs.
Children with obsessive-compulsive disorder (OCD) treated with SSRIs have shown evidence of suicidal thoughts. Thus, higher-functioning children and adults with autism might think about suicide when they become aware of their deficits.
For lower-functioning patients (generally, those who receive medication), we need markers of possible suicidal ideation other than their reports of symptoms. In clinical trials, investigators measure behavioral activation symptoms as risk factors for suicidality.
Thus, when you start an SSRI in a patient with autistic disorder, educate the caregivers to watch for agitation, increased energy, poor sleep, disinhibition, or new hyperactivity. Encourage them to contact you immediately if these signs of activation occur.
Ask higher-functioning patients taking SSRIs about suicidal thinking in a step-wise fashion: thoughts of death, thoughts of their own death, intent, plan, and finally possible attempts.
Table 1
4 drugs with evidence of benefit for autism’s repetitive behaviors*
| Medication | Suggested target daily dosage |
|---|---|
| Fluoxetine7 | Children: Start at 2.5 mg/d; maximum 20 mg/d |
| Adults: Start at 10 to 20 mg/d; maximum 60 mg/d | |
| Fluvoxamine10 | Children: Not first-line; start at 12.5 mg/d; maximum 150 to200 mg/d |
| Adults: Start at 25 mg/d; maximum 300 mg/d | |
| Risperidone13,16 | Children: Start at 0.25 mg/d; maximum 3 mg/d |
| Adults: Start at 2 mg/d; maximum 4 mg/d | |
| Valproate20 | Children: Start at 125 mg (sprinkles); titrate to clinical effect and blood levels of 50 of 120 mcg/mL |
| Adults: Start at 250 mg; increase by 250mg/week to clinical effect and blood levels of 50 to 120 mcg/mL | |
| *Data from randomized, placebo-controlled trials | |
Fluoxetine. In the first open-label study of fluoxetine in children and adults with autistic disorder, global functioning improved significantly in 15 of 23 patients, as measured by the Clinical Global Impressions (CGI) scale.4 Autism symptoms also improved in follow-up, open-label trials, but these did not target repetitive behaviors specifically.5,6
Our group conducted the first randomized, placebo-controlled study of fluoxetine’s effect on repetitive behaviors in children with autism.7 We measured obsessions and compulsions in 45 children, ages 5 to 16, with the Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS). This 10-item, clinician-rated questionnaire uses a 5-point scale to rate repetitive behaviors by time spent, distress, interference, resistance, and control.
Using a crossover design—two 8-week phases of active or placebo treatment separated by a 4-week washout—we started liquid fluoxetine at 2.5 mg/d and slowly increased the dosage to clinical effect or a maximum of 0.8 mg/kg/day. Mean final dosage was 9.9 (±4.35) mg/d.
Repetitive behaviors improved, even though we used relatively low dosages to avoid side effects. The mean baseline CY-BOCS compulsion score of 13.15 dropped to 11.6 with fluoxetine and to 12.9 with placebo. Fluoxetine’s effect size was moderate to large, and we found no suicidal ideation with this SSRI.
Fluvoxamine. Repetitive behaviors did not change—as measured with the CY-BOCS—when 18 children with autism received fluvoxamine, 1.5 mg/kg/day, in a 10-week prospective, open-label trial.8 Most patients (72%) reported at least one side effect, and 3 discontinued the SSRI because of behavioral activation. Ten completed the trial. Likewise in a randomized trial, children who received fluvoxamine experienced troublesome side effects and limited benefit.9
Compared with outcomes in children, fluvoxamine has shown greater efficacy in adults. In a 12-week, double-blind, placebo-controlled trial of 30 adults with autism, 8 of 15 treated with fluvoxamine (50 mg/d initially and titrated to 300 mg/d) were rated as responders, compared with none of 15 receiving placebo.
Repetitive behaviors and adaptive functioning improved significantly with fluvoxamine, as measured with the Yale-Brown Obsessive Compulsive Scale (YBOCS) and Vineland Adaptive Behavior Scale, respectively.10 The SSRI was well-tolerated, with only mild nausea and sedation reported.
Thus, fluvoxamine may be useful for treating repetitive behaviors but probably is not a first choice for children with autism. Results might be more favorable in children if fluvoxamine were available in doses <12.5 mg.
Citalopram. Open-label data support using citalopram in autism.11 In a retrospective chart review, 10 of 15 children (73%) were reported “much improved” with citalopram (mean dosage 16.9 mg/d [+/-12.1]), but the review did not specifically address repetitive behaviors. Two patients stopped taking the SSRI because of side effects; agitation, aggressiveness, sedation, and lip dyskinesia were reported.
The National Institutes of Health is sponsoring a multicenter trial of citalopram (starting dosage 2.5 mg/d, up to 20 mg/d) for repetitive behaviors in 144 children with autism, Results are expected in 2007 (see Related resources).
Escitalopram. Some early, open-label evidence suggests that escitalopram may be well-tolerated in autism, but its efficacy for treating repetitive behaviors has not been studied.
Sertraline. One of three reported open-label studies of sertraline in patients with autism measured repetitive behaviors.12 In this study, 42 adults with autism spectrum disorder were treated for 12 weeks with sertraline, 50 to 200 mg/d. One-half were rated “much improved”—mostly in aggressive and repetitive behaviors—with the CGI improvement scale. Sertraline was well-tolerated, although 3 patients dropped out because of persistent agitation.
No randomized trials have examined sertraline in autism.
Clinical recommendations
Family history of bipolar disorder guides our treatment of patients with an autism spectrum disorder and disabling repetitive behaviors (Algorithm).
Algorithm Suggested medications to treat repetitive behaviors in autism

*Risperidone has the most evidence of efficacy, but aripiprazole may be useful for patients with weight-gain problems.
†For children, controlled data support using liquid fluoxetine, starting at 2.5 mg/d.Without bipolar history. SSRIs are usually first-line therapy (although patients with significant irritability and aggression may be an exception and require an atypical antipsychotic first).
If you reach the maximal SSRI dosage without a desired effect, consider adding an atypical (risperidone has the strongest supporting data) or valproate. If behavioral activation symptoms emerge and a lower dosage does not ameliorate them or reduces the clinical effect, consider switching to an atypical or valproate.
With bipolar history. Consider starting with an atypical or valproate; augment with an SSRI if needed.
Monitor for side effects with each medication (Table 2).
Table 2
Medication side effects and recommended monitoring
| Medication | Side effects | Recommended monitoring |
|---|---|---|
| Fluoxetine | Anxiety, insomnia, GI disturbance, appetite and weight changes, mania/hypomania activation, suicidal ideation | Observe closely when starting treatment and while increasing dosage |
| Fluvoxamine | Somnolence, nervousness, insomnia, agitation, GI disturbance, suicidal ideation | Observe closely when starting treatment and while increasing dosage |
| Risperidone | Drowsiness, weight gain, hyperglycemia, GI disturbance, extrapyramidal symptoms, neuroleptic malignant syndrome | Obtain metabolic profile, including serum glucose and lipids |
| Monitor for weight gain and clinical signs of extrapyramidal symptoms | ||
| Valproate | Rash, headaches, weight gain, ataxia, alopecia, GI disturbance, hyperammonemic encephalopathy, sedation, thrombocytopenia, polycystic ovary syndrome, pancreatitis, liver failure, teratogenic effects | CBC with platelets, liver function tests, valproate levels |
| Therapeutic blood levels: 50 to 120 mcg/mL |
Evidence for atypical antipsychotics
Atypicals have been used in autistic disorder to treat irritability and impulsive aggression. Risperidone also has been shown to reduce repetitive behaviors in a controlled trial.13 No evidence or only open-label trials support the use of other atypicals in patients with autism.
Risperidone’s side effects may include metabolic syndrome. No studies have examined lipid profiles and insulin resistance after risperidone treatment in patients with autism, but weight gain has been reported in the Research Units on Pediatric Psychopharmacology (RUPP) trial andothers.13-15 Carefully assess the risk-benefit ratio when you consider using risperidone to treat repetitive behaviors in patients with autism.
Atypical antipsychotics also may increase dyskinesia risk, although extrapyramidal symptoms (EPS) have not been reported in studies of patients with autism and repetitive behaviors. Because EPS could develop after clinical trials are completed, long-term naturalistic studies are needed to address this concern.
Risperidone. The double-blind, placebo-controlled RUPP trial examined risperidone’s efficacy in treating autism’s core symptoms (primarily irritability) in 101 children.13 Mean dosages after 8 weeks and during a 16-week open-label extension for 63 children were 2 and 2.1 mg/d, respectively.
Repetitive behavior—as measured with the CY-BOCS, using RUPP trial data16—improved significantly with risperidone compared with placebo. During the 8-week controlled trial, CY-BOCS scores improved from 15.51 (SD±2.73) to 11.65 (SD±4.02) in the risperidone group, compared with 15.18 (SD±3.88) to 14.21 (SD±4.81) in the placebo group. This response was maintained through the open-label trial.
Side effects included weight gain, fatigue, drowsiness, and drooling. No children receiving risperidone dropped out because of side effects. No EPS were reported, based on weekly Abnormal Involuntary Movement scale and Simpson-Angus scale scores.
Olanzapine. Only open-label studies have examined olanzapine in autism, and one systematically measured repetitive behaviors.17 Eight children with autism or other pervasive developmental disorders were given olanzapine, mean dosage 7.8 (±4.7) mg/d at the end of the 12-week trial.
Repetitive behaviors did not change significantly, as measured with YBOCS. Seven of eight patients completed the trial. Mean weight for the group after 12 weeks was 156±55 lbs, compared with 137±56 lbs at baseline.
Quetiapine. No data support using quetiapine for autism’s repetitive behaviors. Quetiapine, 100 to 350 mg/d (1.6 to 5.2 mg/kg/day) was poorly tolerated in a 16-week open-label safety and efficacy trial among 6 mentally retarded boys with autistic disorder. Side effects included a possible seizure, behavioral activation, increased appetite, and weight gain (0.9 to 8.2 kg). Two patients completed the trial.18
Ziprasidone. Small open-label studies and anecdotal reports of ziprasidone in autism have not examined this drug’s effect on repetitive behaviors.
Aripiprazole. Anecdotal information suggests that clinicians are using this medication to treat patients with autism, but no supporting data exist.
Evidence for valproate
Preliminary trials by our group suggest that valproate may reduce repetitive behaviors in autism. In a retrospective, open-label study, 14 patients (mean age 17) with autism spectrum disorder received divalproex sodium (mean 768±582 mg/d) for a mean 11 months. Ten patients (71%) showed sustained improvement in function, as measured by the CGI-improvement scale, and valproate was generally well-tolerated.19
We then measured valproate’s effect on repetitive behaviors in an 8-week, double-blind, placebo-controlled study of 13 patients (mean age 9) with autism spectrum disorder. Repetitive behaviors improved significantly compared with placebo, as measured by the CY-BOCS, in those who received divalproex (mean 833.93±326.21 mg/d).20
Further studies are needed to replicate this finding. Although it is too early to make general recommendations, valproate may be a reasonable choice for children with autism and epilepsy or affective instability.
- Hollander E, Phillips AT, Yeh CC. Targeted treatments for symptom domains in child and adolescent autism. Lancet 2003;362:732-4.
- Hollander E (ed). Autism spectrum disorders. New York: Marcel Decker; 2003.
- National Institute of Health multicenter study of citalopram for repetitive behaviors in autism. http://www.clinicaltrials.gov/ct/show/nct00086645?order=2
Drug brand names
- Aripiprazole • Abilify
- Citalopram • Celexa
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Valproic acid • Depakote, Depakene
- Ziprasidone • Geodon
Disclosures
Dr. Anagnostou reports no financial interest with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Hollander receives research/grant support from and is a consultant to Abbott Laboratories. He also receives support from the National Institutes of Health (STAART Center) to investigate orphan drug status for fluoxetine in treating autism symptoms.
Autism’s repetitive behaviors and restricted interests interfere with adaptive functioning, social interactions, and learning. No medications are FDA-approved for autistic disorder, but some selective serotonin reuptake inhibitors (SSRIs) and atypical antipsychotics and an anticonvulsant have reduced repetitive behaviors in controlled trials. We discuss how that evidence shapes our approach to patients with or without a comorbid family history of bipolar disorder.
Evidence for SSRIs
Repetitive behaviors and restricted interests are autism’s third core domain, as defined by DSM-IVTR criteria.1 For an autistic disorder diagnosis, a patient must show at least one of these behaviors:
- encompassing preoccupations with stereotyped or restricted patterns of interest
- inflexible routines or rituals
- stereotyped, repetitive motor mannerisms
- or persistent preoccupation with parts of objects.
As in obsessive-compulsive disorder (OCD), rituals and restricted interests are thought to decrease anxiety in autism, whereas self-stimulatory behaviors and stereotypy may regulate arousal. The behaviors persist2,3 but may change across the lifespan.
Because SSRIs improve OCD’s repetitive behaviors, clinicians have also used them to treat autism’s repetitive behaviors, though without supporting data. Recently, however, fluoxetine and fluvoxamine have shown efficacy for autism’s repetitive behaviors in randomized, controlled trials. Results indicate:
- In children, fluoxetine is probably better-tolerated than available dosing forms of fluvoxamine.
- In adults, fluvoxamine is well-tolerated and can improve repetitive behavior.
SSRIs and suicidal ideation in autism. The increased risk of suicidality reported with SSRIs when treating depression and OCD has not been seen in children with autism. But because fewer children with autism have been treated with SSRIs, we recommend that you try to assess suicidal ideation during SSRI treatment in those able to express such concerns (Box). Starting with low SSRI dosages (Table 1) and increasing slowly may help prevent behavioral activation, a possible risk factor for suicidality.
Suicidal ideation has not been reported in studies of selective serotonin reuptake inhibitors (SSRIs) in autism. Even so, children and adolescents with autistic disorder are not excluded from the FDA black-box warning of increased risk of suicidality with SSRIs.
Children with obsessive-compulsive disorder (OCD) treated with SSRIs have shown evidence of suicidal thoughts. Thus, higher-functioning children and adults with autism might think about suicide when they become aware of their deficits.
For lower-functioning patients (generally, those who receive medication), we need markers of possible suicidal ideation other than their reports of symptoms. In clinical trials, investigators measure behavioral activation symptoms as risk factors for suicidality.
Thus, when you start an SSRI in a patient with autistic disorder, educate the caregivers to watch for agitation, increased energy, poor sleep, disinhibition, or new hyperactivity. Encourage them to contact you immediately if these signs of activation occur.
Ask higher-functioning patients taking SSRIs about suicidal thinking in a step-wise fashion: thoughts of death, thoughts of their own death, intent, plan, and finally possible attempts.
Table 1
4 drugs with evidence of benefit for autism’s repetitive behaviors*
| Medication | Suggested target daily dosage |
|---|---|
| Fluoxetine7 | Children: Start at 2.5 mg/d; maximum 20 mg/d |
| Adults: Start at 10 to 20 mg/d; maximum 60 mg/d | |
| Fluvoxamine10 | Children: Not first-line; start at 12.5 mg/d; maximum 150 to200 mg/d |
| Adults: Start at 25 mg/d; maximum 300 mg/d | |
| Risperidone13,16 | Children: Start at 0.25 mg/d; maximum 3 mg/d |
| Adults: Start at 2 mg/d; maximum 4 mg/d | |
| Valproate20 | Children: Start at 125 mg (sprinkles); titrate to clinical effect and blood levels of 50 of 120 mcg/mL |
| Adults: Start at 250 mg; increase by 250mg/week to clinical effect and blood levels of 50 to 120 mcg/mL | |
| *Data from randomized, placebo-controlled trials | |
Fluoxetine. In the first open-label study of fluoxetine in children and adults with autistic disorder, global functioning improved significantly in 15 of 23 patients, as measured by the Clinical Global Impressions (CGI) scale.4 Autism symptoms also improved in follow-up, open-label trials, but these did not target repetitive behaviors specifically.5,6
Our group conducted the first randomized, placebo-controlled study of fluoxetine’s effect on repetitive behaviors in children with autism.7 We measured obsessions and compulsions in 45 children, ages 5 to 16, with the Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS). This 10-item, clinician-rated questionnaire uses a 5-point scale to rate repetitive behaviors by time spent, distress, interference, resistance, and control.
Using a crossover design—two 8-week phases of active or placebo treatment separated by a 4-week washout—we started liquid fluoxetine at 2.5 mg/d and slowly increased the dosage to clinical effect or a maximum of 0.8 mg/kg/day. Mean final dosage was 9.9 (±4.35) mg/d.
Repetitive behaviors improved, even though we used relatively low dosages to avoid side effects. The mean baseline CY-BOCS compulsion score of 13.15 dropped to 11.6 with fluoxetine and to 12.9 with placebo. Fluoxetine’s effect size was moderate to large, and we found no suicidal ideation with this SSRI.
Fluvoxamine. Repetitive behaviors did not change—as measured with the CY-BOCS—when 18 children with autism received fluvoxamine, 1.5 mg/kg/day, in a 10-week prospective, open-label trial.8 Most patients (72%) reported at least one side effect, and 3 discontinued the SSRI because of behavioral activation. Ten completed the trial. Likewise in a randomized trial, children who received fluvoxamine experienced troublesome side effects and limited benefit.9
Compared with outcomes in children, fluvoxamine has shown greater efficacy in adults. In a 12-week, double-blind, placebo-controlled trial of 30 adults with autism, 8 of 15 treated with fluvoxamine (50 mg/d initially and titrated to 300 mg/d) were rated as responders, compared with none of 15 receiving placebo.
Repetitive behaviors and adaptive functioning improved significantly with fluvoxamine, as measured with the Yale-Brown Obsessive Compulsive Scale (YBOCS) and Vineland Adaptive Behavior Scale, respectively.10 The SSRI was well-tolerated, with only mild nausea and sedation reported.
Thus, fluvoxamine may be useful for treating repetitive behaviors but probably is not a first choice for children with autism. Results might be more favorable in children if fluvoxamine were available in doses <12.5 mg.
Citalopram. Open-label data support using citalopram in autism.11 In a retrospective chart review, 10 of 15 children (73%) were reported “much improved” with citalopram (mean dosage 16.9 mg/d [+/-12.1]), but the review did not specifically address repetitive behaviors. Two patients stopped taking the SSRI because of side effects; agitation, aggressiveness, sedation, and lip dyskinesia were reported.
The National Institutes of Health is sponsoring a multicenter trial of citalopram (starting dosage 2.5 mg/d, up to 20 mg/d) for repetitive behaviors in 144 children with autism, Results are expected in 2007 (see Related resources).
Escitalopram. Some early, open-label evidence suggests that escitalopram may be well-tolerated in autism, but its efficacy for treating repetitive behaviors has not been studied.
Sertraline. One of three reported open-label studies of sertraline in patients with autism measured repetitive behaviors.12 In this study, 42 adults with autism spectrum disorder were treated for 12 weeks with sertraline, 50 to 200 mg/d. One-half were rated “much improved”—mostly in aggressive and repetitive behaviors—with the CGI improvement scale. Sertraline was well-tolerated, although 3 patients dropped out because of persistent agitation.
No randomized trials have examined sertraline in autism.
Clinical recommendations
Family history of bipolar disorder guides our treatment of patients with an autism spectrum disorder and disabling repetitive behaviors (Algorithm).
Algorithm Suggested medications to treat repetitive behaviors in autism

*Risperidone has the most evidence of efficacy, but aripiprazole may be useful for patients with weight-gain problems.
†For children, controlled data support using liquid fluoxetine, starting at 2.5 mg/d.Without bipolar history. SSRIs are usually first-line therapy (although patients with significant irritability and aggression may be an exception and require an atypical antipsychotic first).
If you reach the maximal SSRI dosage without a desired effect, consider adding an atypical (risperidone has the strongest supporting data) or valproate. If behavioral activation symptoms emerge and a lower dosage does not ameliorate them or reduces the clinical effect, consider switching to an atypical or valproate.
With bipolar history. Consider starting with an atypical or valproate; augment with an SSRI if needed.
Monitor for side effects with each medication (Table 2).
Table 2
Medication side effects and recommended monitoring
| Medication | Side effects | Recommended monitoring |
|---|---|---|
| Fluoxetine | Anxiety, insomnia, GI disturbance, appetite and weight changes, mania/hypomania activation, suicidal ideation | Observe closely when starting treatment and while increasing dosage |
| Fluvoxamine | Somnolence, nervousness, insomnia, agitation, GI disturbance, suicidal ideation | Observe closely when starting treatment and while increasing dosage |
| Risperidone | Drowsiness, weight gain, hyperglycemia, GI disturbance, extrapyramidal symptoms, neuroleptic malignant syndrome | Obtain metabolic profile, including serum glucose and lipids |
| Monitor for weight gain and clinical signs of extrapyramidal symptoms | ||
| Valproate | Rash, headaches, weight gain, ataxia, alopecia, GI disturbance, hyperammonemic encephalopathy, sedation, thrombocytopenia, polycystic ovary syndrome, pancreatitis, liver failure, teratogenic effects | CBC with platelets, liver function tests, valproate levels |
| Therapeutic blood levels: 50 to 120 mcg/mL |
Evidence for atypical antipsychotics
Atypicals have been used in autistic disorder to treat irritability and impulsive aggression. Risperidone also has been shown to reduce repetitive behaviors in a controlled trial.13 No evidence or only open-label trials support the use of other atypicals in patients with autism.
Risperidone’s side effects may include metabolic syndrome. No studies have examined lipid profiles and insulin resistance after risperidone treatment in patients with autism, but weight gain has been reported in the Research Units on Pediatric Psychopharmacology (RUPP) trial andothers.13-15 Carefully assess the risk-benefit ratio when you consider using risperidone to treat repetitive behaviors in patients with autism.
Atypical antipsychotics also may increase dyskinesia risk, although extrapyramidal symptoms (EPS) have not been reported in studies of patients with autism and repetitive behaviors. Because EPS could develop after clinical trials are completed, long-term naturalistic studies are needed to address this concern.
Risperidone. The double-blind, placebo-controlled RUPP trial examined risperidone’s efficacy in treating autism’s core symptoms (primarily irritability) in 101 children.13 Mean dosages after 8 weeks and during a 16-week open-label extension for 63 children were 2 and 2.1 mg/d, respectively.
Repetitive behavior—as measured with the CY-BOCS, using RUPP trial data16—improved significantly with risperidone compared with placebo. During the 8-week controlled trial, CY-BOCS scores improved from 15.51 (SD±2.73) to 11.65 (SD±4.02) in the risperidone group, compared with 15.18 (SD±3.88) to 14.21 (SD±4.81) in the placebo group. This response was maintained through the open-label trial.
Side effects included weight gain, fatigue, drowsiness, and drooling. No children receiving risperidone dropped out because of side effects. No EPS were reported, based on weekly Abnormal Involuntary Movement scale and Simpson-Angus scale scores.
Olanzapine. Only open-label studies have examined olanzapine in autism, and one systematically measured repetitive behaviors.17 Eight children with autism or other pervasive developmental disorders were given olanzapine, mean dosage 7.8 (±4.7) mg/d at the end of the 12-week trial.
Repetitive behaviors did not change significantly, as measured with YBOCS. Seven of eight patients completed the trial. Mean weight for the group after 12 weeks was 156±55 lbs, compared with 137±56 lbs at baseline.
Quetiapine. No data support using quetiapine for autism’s repetitive behaviors. Quetiapine, 100 to 350 mg/d (1.6 to 5.2 mg/kg/day) was poorly tolerated in a 16-week open-label safety and efficacy trial among 6 mentally retarded boys with autistic disorder. Side effects included a possible seizure, behavioral activation, increased appetite, and weight gain (0.9 to 8.2 kg). Two patients completed the trial.18
Ziprasidone. Small open-label studies and anecdotal reports of ziprasidone in autism have not examined this drug’s effect on repetitive behaviors.
Aripiprazole. Anecdotal information suggests that clinicians are using this medication to treat patients with autism, but no supporting data exist.
Evidence for valproate
Preliminary trials by our group suggest that valproate may reduce repetitive behaviors in autism. In a retrospective, open-label study, 14 patients (mean age 17) with autism spectrum disorder received divalproex sodium (mean 768±582 mg/d) for a mean 11 months. Ten patients (71%) showed sustained improvement in function, as measured by the CGI-improvement scale, and valproate was generally well-tolerated.19
We then measured valproate’s effect on repetitive behaviors in an 8-week, double-blind, placebo-controlled study of 13 patients (mean age 9) with autism spectrum disorder. Repetitive behaviors improved significantly compared with placebo, as measured by the CY-BOCS, in those who received divalproex (mean 833.93±326.21 mg/d).20
Further studies are needed to replicate this finding. Although it is too early to make general recommendations, valproate may be a reasonable choice for children with autism and epilepsy or affective instability.
- Hollander E, Phillips AT, Yeh CC. Targeted treatments for symptom domains in child and adolescent autism. Lancet 2003;362:732-4.
- Hollander E (ed). Autism spectrum disorders. New York: Marcel Decker; 2003.
- National Institute of Health multicenter study of citalopram for repetitive behaviors in autism. http://www.clinicaltrials.gov/ct/show/nct00086645?order=2
Drug brand names
- Aripiprazole • Abilify
- Citalopram • Celexa
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Valproic acid • Depakote, Depakene
- Ziprasidone • Geodon
Disclosures
Dr. Anagnostou reports no financial interest with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Hollander receives research/grant support from and is a consultant to Abbott Laboratories. He also receives support from the National Institutes of Health (STAART Center) to investigate orphan drug status for fluoxetine in treating autism symptoms.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC; American Psychiatric Association; 2000.
2. Seltzer MM, Shattuck P, Abbeduto L, Greenberg JS. Trajectory of development in adolescents and adults with autism. MRDD Research Reviews 2004;10:234-47.
3. Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. J Child Psychol Psychiatry 2004;45:212-29.
4. Cook EH, Rowlett R, Jaselinkis C, Leventhal BL. Fluoxetine treatment of children and adults with autistic disorder and mental retardation. J Am Acad Child Adolesc Psychiatry 1992;31:739-45.
5. DeLong GR, Teague LA, Kamran MM. Effects of fluoxetine treatment in young children with idiopathic autism. Dev Med Child Neurol 1998;40:551-62.
6. DeLong GR, Ritch CR, Burch S. Fluoxetine response in children with autistic spectrum disorders: correlation with familial major affective disorder and intellectual achievement. Dev Med Child Neurol 2002;44:652-9.
7. Hollander E, Phillips A, Chaplin W, et al. A placebo-controlled cross over trial of liquid fluoxetine on repetitive behaviors in childhood and adolescent autism. Neuropsychopharmacology 2005;30:582-9.
8. Martin A, Koenig K, Anderson GM, Scahill L. Low-dose fluvoxamine treatment of children and adolescents with pervasive developmental disorders: a prospective, open-label study. J Autism Dev Disord 2003;33:77-85.
9. McDougle CJ, Kresch LE, Posey DJ. Repetitive thoughts and behavior in pervasive developmental disorders: treatment with serotonin reuptake inhibitors. J Autism Dev Disord 2000;30:427-35.
10. McDougle CJ, Naylor ST, Cohen DJ, et al. A double-blind, placebo-controlled study of fluvoxamine in adults with autistic disorder. Arch Gen Psychiatry 1996;53:1001-8.
11. Namerow LB, Thomas P, Bostic JQ, et al. Use of citalopram in pervasive developmental disorders. J Dev Behav Pediatr. 2003;24(2):104-8.
12. McDougle CJ, Brodkin ES, Naylor ST, et al. Sertraline in adults with pervasive developmental disorders: a prospective open-label investigation. J Clin Psychopharmacol 1998;18:62-6.
13. McCracken JT, McGough J, Shah B, et al. Research Units on Pediatric Psychopharmacology Autism Network. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347:314-21.
14. Troost PW, Lahuis BE, Steenhuis MP, et al. Long-term effects of risperidone in children with autism spectrum disorders: A placebo discontinuation study. J Am Acad Child Adolesc Psychiatry 2005;44:1137-44.
15. Shea S, Turgay A, Carroll A, et al. Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics 2004;114:e634-e641.
16. McDougle CJ, Scahill L, Aman MG, et al. Risperidone for the core symptom domains of autism: results from the study by the autism network of the research units on pediatric psychopharmacology. Am J Psychiatry 2005;162:1142-8.
17. Potenza MN, Holmes JP, Kanes SJ, McDougle CJ. Olanzapine treatment of children, adolescents and adults with pervasive developmental disorders: an open-label pilot study. J Clin Psychopharmacol 1999;19:37-44.
18. Martin A, Koenig K, Scahill L, Bregman J. Open-label quetiapine in the treatment of children and adolescents with autistic disorder. J Child Adolesc Psychopharmacol 1999;9:99-107.
19. Hollander E, Dolgoff-Kaspar R, Cartwright C, et al. An open trial of divalproex sodium in autism spectrum disorders. J Clin Psychiatry 2001;62:530-4.
20. Hollander E, Soorya LV, Wasserman S, et al. Divalproex sodium vs. placebo in the treatment of repetitive behaviours in autism spectrum disorder. Int J Neuropsychopharmacol 2006;9:209-13.
1. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC; American Psychiatric Association; 2000.
2. Seltzer MM, Shattuck P, Abbeduto L, Greenberg JS. Trajectory of development in adolescents and adults with autism. MRDD Research Reviews 2004;10:234-47.
3. Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. J Child Psychol Psychiatry 2004;45:212-29.
4. Cook EH, Rowlett R, Jaselinkis C, Leventhal BL. Fluoxetine treatment of children and adults with autistic disorder and mental retardation. J Am Acad Child Adolesc Psychiatry 1992;31:739-45.
5. DeLong GR, Teague LA, Kamran MM. Effects of fluoxetine treatment in young children with idiopathic autism. Dev Med Child Neurol 1998;40:551-62.
6. DeLong GR, Ritch CR, Burch S. Fluoxetine response in children with autistic spectrum disorders: correlation with familial major affective disorder and intellectual achievement. Dev Med Child Neurol 2002;44:652-9.
7. Hollander E, Phillips A, Chaplin W, et al. A placebo-controlled cross over trial of liquid fluoxetine on repetitive behaviors in childhood and adolescent autism. Neuropsychopharmacology 2005;30:582-9.
8. Martin A, Koenig K, Anderson GM, Scahill L. Low-dose fluvoxamine treatment of children and adolescents with pervasive developmental disorders: a prospective, open-label study. J Autism Dev Disord 2003;33:77-85.
9. McDougle CJ, Kresch LE, Posey DJ. Repetitive thoughts and behavior in pervasive developmental disorders: treatment with serotonin reuptake inhibitors. J Autism Dev Disord 2000;30:427-35.
10. McDougle CJ, Naylor ST, Cohen DJ, et al. A double-blind, placebo-controlled study of fluvoxamine in adults with autistic disorder. Arch Gen Psychiatry 1996;53:1001-8.
11. Namerow LB, Thomas P, Bostic JQ, et al. Use of citalopram in pervasive developmental disorders. J Dev Behav Pediatr. 2003;24(2):104-8.
12. McDougle CJ, Brodkin ES, Naylor ST, et al. Sertraline in adults with pervasive developmental disorders: a prospective open-label investigation. J Clin Psychopharmacol 1998;18:62-6.
13. McCracken JT, McGough J, Shah B, et al. Research Units on Pediatric Psychopharmacology Autism Network. Risperidone in children with autism and serious behavioral problems. N Engl J Med 2002;347:314-21.
14. Troost PW, Lahuis BE, Steenhuis MP, et al. Long-term effects of risperidone in children with autism spectrum disorders: A placebo discontinuation study. J Am Acad Child Adolesc Psychiatry 2005;44:1137-44.
15. Shea S, Turgay A, Carroll A, et al. Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics 2004;114:e634-e641.
16. McDougle CJ, Scahill L, Aman MG, et al. Risperidone for the core symptom domains of autism: results from the study by the autism network of the research units on pediatric psychopharmacology. Am J Psychiatry 2005;162:1142-8.
17. Potenza MN, Holmes JP, Kanes SJ, McDougle CJ. Olanzapine treatment of children, adolescents and adults with pervasive developmental disorders: an open-label pilot study. J Clin Psychopharmacol 1999;19:37-44.
18. Martin A, Koenig K, Scahill L, Bregman J. Open-label quetiapine in the treatment of children and adolescents with autistic disorder. J Child Adolesc Psychopharmacol 1999;9:99-107.
19. Hollander E, Dolgoff-Kaspar R, Cartwright C, et al. An open trial of divalproex sodium in autism spectrum disorders. J Clin Psychiatry 2001;62:530-4.
20. Hollander E, Soorya LV, Wasserman S, et al. Divalproex sodium vs. placebo in the treatment of repetitive behaviours in autism spectrum disorder. Int J Neuropsychopharmacol 2006;9:209-13.
SSRI use during pregnancy
Untreated depression can have serious consequences, but many pregnant women resist taking antidepressants because they overestimate the risk of birth defects.Paroxetine in pregnancy”). Further study is needed to define the risks of teratogenesis with paroxetine compared with other antidepressants.
Third-trimester exposure
In a recent meta-analysis, infants exposed to SSRIs in utero showed an increased risk for prematurity (OR; 2.03) and low birth weight (OR; 2.37).15 Other studies, however, showed no differences in these risks in SSRI-exposed infants or attributed the results to untreated maternal depression or smoking.16
A Medline search across the last 20 years17 found 26 case reports, three prospective controlled cohort studies, and other records of >400 women who received fluoxetine, sertraline, or paroxetine in the third trimester. The authors found the evidence “ambiguous” as to the cause of adverse events and concluded that the risk of not treating major depression with adequate SSRI therapy at that stage of pregnancy “most likely” outweighs the risk of harm to infants.
Transient neonatal complications. Thirty percent of neonates exposed to SSRIs in the third trimester experience transient adaptation problems, which peak 48 hours after birth18 (Table 3). Symptoms may include initial lack of crying, increased muscle tonus, flush, irritability, jitteriness, hypothermia, abnormal breathing, and disrupted sleep and motor activity.2,19,20
Transient neonatal symptoms from SSRI exposure are thought to be a serotonin withdrawal syndrome or serotonin overstimulation.21 The syndrome is usually mild, self-limited, and requires only supportive treatments. All antidepressants’ labels warn of these effects.
Table 3
Neonatal SSRI withdrawal: Symptoms, causes, and treatment
| Symptoms | Initial lack of crying |
| Increased muscle tonus | |
| Irritability, jitteriness | |
| Abnormal breathing pattern | |
| Disrupted sleep and motor activity | |
| Hypotheses of cause | Serotonin overstimulation or withdrawal |
| Treatment | Close observation |
| Supportive measures |
Recommendation. Some authors have recommended tapering antidepressants in the third trimester, but the risk of postpartum depression appears to outweigh any potential benefit from discontinuation. Because birth timing is unpredictable, some women whose antidepressants are tapered off could be without medication for a long time.
Thus, we recommend:
- continuing SSRIs during late pregnancy
- monitoring the newborn for 48 hours for transient neonatal adaptation symptoms or PPHN.2,17,18
Long-term effects of SSRI exposure
Do SSRIs during pregnancy have long-term effects on infants’ neurodevelopment? Study results are mixed. For example:
- A prospective, controlled, cohort trial found no adverse effects on IQ, language, or behavioral development in children ages 15 months to 6 years whose mothers took tricyclic antidepressants (N=46) or fluoxetine (N=40) during pregnancy, compared with 36 unexposed controls.23
- Another prospective study showed lower Bayley Psychomotor Developmental Index scores in 31 SSRI-exposed infants compared with 13 infants born to depressed mothers not on antidepressants. Reduced body control, coordination, and fine motor skills might suggest possible subtle effects of SSRIs on motor development in exposed infants, the authors concluded.24
Case continued: A healthy delivery
Ms. P’s depression improves a few weeks after she restarts an SSRI. She delivers a healthy term baby with Apgar score of 7. The baby initially does not cry, awakens easily, and shows mild irritability. His mother’s SSRI use, her severe depression during part of the pregnancy, or some other factor may have caused his mild neonatal complications.
Nursing staff carefully observe the infant for 2 days in the newborn nursery, and his irritability fades away. Ms. P decides to continue taking antidepressants to care for herself and the baby.
Weighing treatment options
For each woman with a history of depression who is pregnant or intends to conceive, we recommend a risk-benefit analysis of her depression severity and need for an antidepressant:
Moderate to severe depression (history of recurrent depressive episodes, hospitalization, or suicidality). Strongly consider medication. If your patient is taking an SSRI, counsel her about:
- the 70% risk of depression relapse if she stops the medication, even for the first trimester
- risks of untreated depression during pregnancy (poor self-care, preterm labor, birth complications, and increased risk for poor stress adaptations in children).
Choosing an SSRI. No one SSRI is the safest choice for all women, especially when data on breast-feeding come into play.
- Fluoxetine has been studied more than other SSRIs during pregnancy; most evidence is reassuring, except for transient neonatal complications. With its long half-life, fluoxetine is not recommended during breastfeeding because it may accumulate in infant sera.
- Sertraline has shown low umbilical cord to maternal serum ratios in small samples and has reassuring breast-feeding data.
- Citalopram, compared with sertraline, has been studied more in pregnancy but has a higher fetal-to-maternal serum ratio (as does escitalopram). These SSRIs are usually second-line for starting a new antidepressant during pregnancy but could be first-line if they have worked well for a patient or she has had adverse effects with fluoxetine or sertraline.
You may need to increase SSRI dosages as pregnancy progresses. Increased metabolism and weight gain during pregnancy can lower SSRI serum levels, allowing depressive symptoms to re-emerge in the third trimester. Counsel the patient to continue taking the antidepressant for at least 12 months postpartum, then re-evaluate the need for medication based on her history.
Paroxetine precautions. If your patient is taking paroxetine and wishes to become pregnant, consider switching to another SSRI (using a slow cross-taper) unless paroxetine has been the only effective medication (Table 4). When discussing risks of any SSRI, explain that the baseline risk for congenital malformations is 3%. Paroxetine might increase this risk by 1% and other SSRIs by less.
If a woman becomes pregnant while taking paroxetine, often the time when cardiac defects occur is passed or will be before you slowly taper the medication to avoid withdrawal. If the patient’s depression has been severe, the risk of shifting her to an untested SSRI is probably higher than the possible 1% increased risk of fetal malformation. If she has taken paroxetine during the first-trimester, refer for ultrasound to monitor for cardiac anomalies.
Table 4
Recommendations for managing paroxetine risk during pregnancy
| Patient status | Recommendation |
|---|---|
| Taking paroxetine and planning pregnancy | Advise of possible 1% increase in risk of fetal malformation |
| Switch to another SSRI unless paroxetine has been the only successful therapy for depression | |
| If stopping paroxetine, slowly taper to avoid withdrawal symptoms | |
| Taking paroxetine and is pregnant | Advise of possible 1% increase in risk of fetal malformation |
| Continue paroxetine; a slow taper probably could not be completed before the first-trimester period associated with increased risk of fetal cardiac defects | |
| If any paroxetine exposure in first trimester, order ultrasound to monitor for fetal malformations |
- California Teratogen Information Service (CTIS). Pediatric department, University of California San Diego Medical Center. www.otispregnancy.org/ctis.html
- MGH Center for Women’s Mental Health, Massachusetts General Hospital. Psychiatric disorders during pregnancy and postpartum. www.womensmentalhealth.com
- MOTHERISK Web site. Teratogen information and updates on reproductive risk research. The Hospital for Sick Children, University of Toronto. www.motherisk.org
- Citalopram • Celexa
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Paroxetine • Paxil
- Sertraline • Zoloft
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bonari L, Koren G, Einarson TR, et al. Use of antidepressants by pregnant women: evaluation of perception of risk, efficacy of evidence-based counseling, and determinants of decision making. Arch Women Ment Health 2005;8:214-20.
2. Hallberg P, Joblom V. The use of selective serotonin reuptake inhibitors during pregnancy and breast-feeding: a review and clinical aspects. J Clin Psychopharmacol 2005;25:59-73.
3. Larsson C, Sydsjo G, Josefsson A. Health, sociodemographic data, and pregnancy outcome in women with antepartum depressive symptoms. Obstet Gynecol 2004;104(3):469-66.
4. Bonari L, Pinto N, Ahn E, et al. Perinatal risks of untreated depression during pregnancy. Can J Psychiatry 2004;49(11):726-35.
5. Cohen L, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA 2006;295(5):499-507.
6. Hendrick V, Altshuler L. Management of major depression during pregnancy. Am J Psychiatry 2002;159:1667-73.
7. Sandman CA, Glynn L, Wadhwa PD, et al. Maternal hypothalamic-pituitary-adrenal dysregulation during the third trimester influences human fetal responses. Dev Neurosci 2003;25(1):41-9.
8. Huot RL, Brennan PA, Stowe ZN, et al. Negative affect in offspring of depressed mothers is predicted by infant cortisol levels at 6 months and maternal depression during pregnancy, but not postpartum. Ann NY Acad Sci 2004;1032:234-6.
9. Gutteling BM, de Weerth C, Buitelaar JK. Prenatal stress and children’s cortisol reaction to the first day of school. Psychoneuroendocrinology 2005;20:541-9.
10. Hendrick V, Stowe ZN, Altshuler LL, et al. Placental passage of antidepressant medications. Am J Psychiatry 2003;5:993-6.
11. GlaxoSmithKline study EPIP083. GSK medicine: buproprion and paroxetine. Epidemiology study: preliminary report on bupropion in pregnancy and the occurrence of cardiovascular and major congenital malformation. Available at: http://ctr.gsk.co.uk/summary/paroxetine/epip083.pdf. Accessed March 13, 2006.
12. Ericson A, Kallen B, Wiholm BE. Delivery outcome after the use of antidepressants in early pregnancy. Eur J Clin Pharmacol 1999;55:503-8.
13. Kulin NA, Pastuszak A, Sage S, et al. Pregnancy outcome following maternal use of the new selective serotonin reuptake inhibitors. A prospective controlled multicenter study. JAMA 1998;279:609-10.
14. Kallen BA, Otterblad Olausson P. Maternal drug use in early pregnancy and infant cardiovascular defect. Reprod Toxicol 2003;17:255-61.
15. Lattimore K, Donn S, Kaciroti N, et al. Selective serotonin reuptake inhibitor use during pregnancy and effects on the fetus and newborn: a meta-analysis. J Perinatol 2005;25:595-604.
16. Levy L, Ragan K, Hower-Hartley A, et al. Psychiatric disorders in pregnancy. Neurol Clin 2004;22:863-93.
17. Nordeng H, Spigset O. Treatment with selective serotonin reuptake inhibitors in the third trimester of pregnancy: effects on the infant. Drug Saf 2005;28(7):565-81.
18. Levinson-Castiel R, Merlob P, Linder N, et al. Neonatal abstinence syndrome after in utero exposure to selective serotonin reuptake inhibitors to term infants. Arch Pediatr Adolesc Med 2006;160:173-6.
19. Oberlander TF, Misri S, Fitzgerald CE, et al. Pharmacologic factors associated with transient neonatal symptoms following prenatal psychotropic medication exposure. J Clin Psychiatry 2004;65(2):230-7.
20. Zeskind PS, Stephens L. Maternal selective serotonin reuptake inhibitor use during pregnancy and newborn neurobehavior. Pediatrics 2004;113:368-75.
21. Moses-Kolko E, Bogen D, Perel J, et al. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors. JAMA 2005;293:2372-83.
22. Chambers CD, Hernandez-Diaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med 2006;354(6):579-87.
23. Nulman I, Rovet J, Stewart DE, et al. Child development following exposure to tricyclic antidepressants or fluoxetine throughout fetal life: a prospective, controlled study. Am J Psychiatry 2002;159:1889-95.
24. Casper RC, Fleisher BE, Lee-Ancajas JC, et al. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr 2003;4(142):402-8.
25. Spinelli M, Endicott J. Controlled clinical trial of interpersonal psychotherapy versus parenting education program for depressed pregnant women. Am J Psychiatry 2003;160:555-62.
26. Oren D, Wisner K, Spinelli M, et al. An open trial of morning light therapy for treatment of antepartum depression. Am J Psychiatry 2002;159:666-9.
Untreated depression can have serious consequences, but many pregnant women resist taking antidepressants because they overestimate the risk of birth defects.Paroxetine in pregnancy”). Further study is needed to define the risks of teratogenesis with paroxetine compared with other antidepressants.
Third-trimester exposure
In a recent meta-analysis, infants exposed to SSRIs in utero showed an increased risk for prematurity (OR; 2.03) and low birth weight (OR; 2.37).15 Other studies, however, showed no differences in these risks in SSRI-exposed infants or attributed the results to untreated maternal depression or smoking.16
A Medline search across the last 20 years17 found 26 case reports, three prospective controlled cohort studies, and other records of >400 women who received fluoxetine, sertraline, or paroxetine in the third trimester. The authors found the evidence “ambiguous” as to the cause of adverse events and concluded that the risk of not treating major depression with adequate SSRI therapy at that stage of pregnancy “most likely” outweighs the risk of harm to infants.
Transient neonatal complications. Thirty percent of neonates exposed to SSRIs in the third trimester experience transient adaptation problems, which peak 48 hours after birth18 (Table 3). Symptoms may include initial lack of crying, increased muscle tonus, flush, irritability, jitteriness, hypothermia, abnormal breathing, and disrupted sleep and motor activity.2,19,20
Transient neonatal symptoms from SSRI exposure are thought to be a serotonin withdrawal syndrome or serotonin overstimulation.21 The syndrome is usually mild, self-limited, and requires only supportive treatments. All antidepressants’ labels warn of these effects.
Table 3
Neonatal SSRI withdrawal: Symptoms, causes, and treatment
| Symptoms | Initial lack of crying |
| Increased muscle tonus | |
| Irritability, jitteriness | |
| Abnormal breathing pattern | |
| Disrupted sleep and motor activity | |
| Hypotheses of cause | Serotonin overstimulation or withdrawal |
| Treatment | Close observation |
| Supportive measures |
Recommendation. Some authors have recommended tapering antidepressants in the third trimester, but the risk of postpartum depression appears to outweigh any potential benefit from discontinuation. Because birth timing is unpredictable, some women whose antidepressants are tapered off could be without medication for a long time.
Thus, we recommend:
- continuing SSRIs during late pregnancy
- monitoring the newborn for 48 hours for transient neonatal adaptation symptoms or PPHN.2,17,18
Long-term effects of SSRI exposure
Do SSRIs during pregnancy have long-term effects on infants’ neurodevelopment? Study results are mixed. For example:
- A prospective, controlled, cohort trial found no adverse effects on IQ, language, or behavioral development in children ages 15 months to 6 years whose mothers took tricyclic antidepressants (N=46) or fluoxetine (N=40) during pregnancy, compared with 36 unexposed controls.23
- Another prospective study showed lower Bayley Psychomotor Developmental Index scores in 31 SSRI-exposed infants compared with 13 infants born to depressed mothers not on antidepressants. Reduced body control, coordination, and fine motor skills might suggest possible subtle effects of SSRIs on motor development in exposed infants, the authors concluded.24
Case continued: A healthy delivery
Ms. P’s depression improves a few weeks after she restarts an SSRI. She delivers a healthy term baby with Apgar score of 7. The baby initially does not cry, awakens easily, and shows mild irritability. His mother’s SSRI use, her severe depression during part of the pregnancy, or some other factor may have caused his mild neonatal complications.
Nursing staff carefully observe the infant for 2 days in the newborn nursery, and his irritability fades away. Ms. P decides to continue taking antidepressants to care for herself and the baby.
Weighing treatment options
For each woman with a history of depression who is pregnant or intends to conceive, we recommend a risk-benefit analysis of her depression severity and need for an antidepressant:
Moderate to severe depression (history of recurrent depressive episodes, hospitalization, or suicidality). Strongly consider medication. If your patient is taking an SSRI, counsel her about:
- the 70% risk of depression relapse if she stops the medication, even for the first trimester
- risks of untreated depression during pregnancy (poor self-care, preterm labor, birth complications, and increased risk for poor stress adaptations in children).
Choosing an SSRI. No one SSRI is the safest choice for all women, especially when data on breast-feeding come into play.
- Fluoxetine has been studied more than other SSRIs during pregnancy; most evidence is reassuring, except for transient neonatal complications. With its long half-life, fluoxetine is not recommended during breastfeeding because it may accumulate in infant sera.
- Sertraline has shown low umbilical cord to maternal serum ratios in small samples and has reassuring breast-feeding data.
- Citalopram, compared with sertraline, has been studied more in pregnancy but has a higher fetal-to-maternal serum ratio (as does escitalopram). These SSRIs are usually second-line for starting a new antidepressant during pregnancy but could be first-line if they have worked well for a patient or she has had adverse effects with fluoxetine or sertraline.
You may need to increase SSRI dosages as pregnancy progresses. Increased metabolism and weight gain during pregnancy can lower SSRI serum levels, allowing depressive symptoms to re-emerge in the third trimester. Counsel the patient to continue taking the antidepressant for at least 12 months postpartum, then re-evaluate the need for medication based on her history.
Paroxetine precautions. If your patient is taking paroxetine and wishes to become pregnant, consider switching to another SSRI (using a slow cross-taper) unless paroxetine has been the only effective medication (Table 4). When discussing risks of any SSRI, explain that the baseline risk for congenital malformations is 3%. Paroxetine might increase this risk by 1% and other SSRIs by less.
If a woman becomes pregnant while taking paroxetine, often the time when cardiac defects occur is passed or will be before you slowly taper the medication to avoid withdrawal. If the patient’s depression has been severe, the risk of shifting her to an untested SSRI is probably higher than the possible 1% increased risk of fetal malformation. If she has taken paroxetine during the first-trimester, refer for ultrasound to monitor for cardiac anomalies.
Table 4
Recommendations for managing paroxetine risk during pregnancy
| Patient status | Recommendation |
|---|---|
| Taking paroxetine and planning pregnancy | Advise of possible 1% increase in risk of fetal malformation |
| Switch to another SSRI unless paroxetine has been the only successful therapy for depression | |
| If stopping paroxetine, slowly taper to avoid withdrawal symptoms | |
| Taking paroxetine and is pregnant | Advise of possible 1% increase in risk of fetal malformation |
| Continue paroxetine; a slow taper probably could not be completed before the first-trimester period associated with increased risk of fetal cardiac defects | |
| If any paroxetine exposure in first trimester, order ultrasound to monitor for fetal malformations |
- California Teratogen Information Service (CTIS). Pediatric department, University of California San Diego Medical Center. www.otispregnancy.org/ctis.html
- MGH Center for Women’s Mental Health, Massachusetts General Hospital. Psychiatric disorders during pregnancy and postpartum. www.womensmentalhealth.com
- MOTHERISK Web site. Teratogen information and updates on reproductive risk research. The Hospital for Sick Children, University of Toronto. www.motherisk.org
- Citalopram • Celexa
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Paroxetine • Paxil
- Sertraline • Zoloft
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Untreated depression can have serious consequences, but many pregnant women resist taking antidepressants because they overestimate the risk of birth defects.Paroxetine in pregnancy”). Further study is needed to define the risks of teratogenesis with paroxetine compared with other antidepressants.
Third-trimester exposure
In a recent meta-analysis, infants exposed to SSRIs in utero showed an increased risk for prematurity (OR; 2.03) and low birth weight (OR; 2.37).15 Other studies, however, showed no differences in these risks in SSRI-exposed infants or attributed the results to untreated maternal depression or smoking.16
A Medline search across the last 20 years17 found 26 case reports, three prospective controlled cohort studies, and other records of >400 women who received fluoxetine, sertraline, or paroxetine in the third trimester. The authors found the evidence “ambiguous” as to the cause of adverse events and concluded that the risk of not treating major depression with adequate SSRI therapy at that stage of pregnancy “most likely” outweighs the risk of harm to infants.
Transient neonatal complications. Thirty percent of neonates exposed to SSRIs in the third trimester experience transient adaptation problems, which peak 48 hours after birth18 (Table 3). Symptoms may include initial lack of crying, increased muscle tonus, flush, irritability, jitteriness, hypothermia, abnormal breathing, and disrupted sleep and motor activity.2,19,20
Transient neonatal symptoms from SSRI exposure are thought to be a serotonin withdrawal syndrome or serotonin overstimulation.21 The syndrome is usually mild, self-limited, and requires only supportive treatments. All antidepressants’ labels warn of these effects.
Table 3
Neonatal SSRI withdrawal: Symptoms, causes, and treatment
| Symptoms | Initial lack of crying |
| Increased muscle tonus | |
| Irritability, jitteriness | |
| Abnormal breathing pattern | |
| Disrupted sleep and motor activity | |
| Hypotheses of cause | Serotonin overstimulation or withdrawal |
| Treatment | Close observation |
| Supportive measures |
Recommendation. Some authors have recommended tapering antidepressants in the third trimester, but the risk of postpartum depression appears to outweigh any potential benefit from discontinuation. Because birth timing is unpredictable, some women whose antidepressants are tapered off could be without medication for a long time.
Thus, we recommend:
- continuing SSRIs during late pregnancy
- monitoring the newborn for 48 hours for transient neonatal adaptation symptoms or PPHN.2,17,18
Long-term effects of SSRI exposure
Do SSRIs during pregnancy have long-term effects on infants’ neurodevelopment? Study results are mixed. For example:
- A prospective, controlled, cohort trial found no adverse effects on IQ, language, or behavioral development in children ages 15 months to 6 years whose mothers took tricyclic antidepressants (N=46) or fluoxetine (N=40) during pregnancy, compared with 36 unexposed controls.23
- Another prospective study showed lower Bayley Psychomotor Developmental Index scores in 31 SSRI-exposed infants compared with 13 infants born to depressed mothers not on antidepressants. Reduced body control, coordination, and fine motor skills might suggest possible subtle effects of SSRIs on motor development in exposed infants, the authors concluded.24
Case continued: A healthy delivery
Ms. P’s depression improves a few weeks after she restarts an SSRI. She delivers a healthy term baby with Apgar score of 7. The baby initially does not cry, awakens easily, and shows mild irritability. His mother’s SSRI use, her severe depression during part of the pregnancy, or some other factor may have caused his mild neonatal complications.
Nursing staff carefully observe the infant for 2 days in the newborn nursery, and his irritability fades away. Ms. P decides to continue taking antidepressants to care for herself and the baby.
Weighing treatment options
For each woman with a history of depression who is pregnant or intends to conceive, we recommend a risk-benefit analysis of her depression severity and need for an antidepressant:
Moderate to severe depression (history of recurrent depressive episodes, hospitalization, or suicidality). Strongly consider medication. If your patient is taking an SSRI, counsel her about:
- the 70% risk of depression relapse if she stops the medication, even for the first trimester
- risks of untreated depression during pregnancy (poor self-care, preterm labor, birth complications, and increased risk for poor stress adaptations in children).
Choosing an SSRI. No one SSRI is the safest choice for all women, especially when data on breast-feeding come into play.
- Fluoxetine has been studied more than other SSRIs during pregnancy; most evidence is reassuring, except for transient neonatal complications. With its long half-life, fluoxetine is not recommended during breastfeeding because it may accumulate in infant sera.
- Sertraline has shown low umbilical cord to maternal serum ratios in small samples and has reassuring breast-feeding data.
- Citalopram, compared with sertraline, has been studied more in pregnancy but has a higher fetal-to-maternal serum ratio (as does escitalopram). These SSRIs are usually second-line for starting a new antidepressant during pregnancy but could be first-line if they have worked well for a patient or she has had adverse effects with fluoxetine or sertraline.
You may need to increase SSRI dosages as pregnancy progresses. Increased metabolism and weight gain during pregnancy can lower SSRI serum levels, allowing depressive symptoms to re-emerge in the third trimester. Counsel the patient to continue taking the antidepressant for at least 12 months postpartum, then re-evaluate the need for medication based on her history.
Paroxetine precautions. If your patient is taking paroxetine and wishes to become pregnant, consider switching to another SSRI (using a slow cross-taper) unless paroxetine has been the only effective medication (Table 4). When discussing risks of any SSRI, explain that the baseline risk for congenital malformations is 3%. Paroxetine might increase this risk by 1% and other SSRIs by less.
If a woman becomes pregnant while taking paroxetine, often the time when cardiac defects occur is passed or will be before you slowly taper the medication to avoid withdrawal. If the patient’s depression has been severe, the risk of shifting her to an untested SSRI is probably higher than the possible 1% increased risk of fetal malformation. If she has taken paroxetine during the first-trimester, refer for ultrasound to monitor for cardiac anomalies.
Table 4
Recommendations for managing paroxetine risk during pregnancy
| Patient status | Recommendation |
|---|---|
| Taking paroxetine and planning pregnancy | Advise of possible 1% increase in risk of fetal malformation |
| Switch to another SSRI unless paroxetine has been the only successful therapy for depression | |
| If stopping paroxetine, slowly taper to avoid withdrawal symptoms | |
| Taking paroxetine and is pregnant | Advise of possible 1% increase in risk of fetal malformation |
| Continue paroxetine; a slow taper probably could not be completed before the first-trimester period associated with increased risk of fetal cardiac defects | |
| If any paroxetine exposure in first trimester, order ultrasound to monitor for fetal malformations |
- California Teratogen Information Service (CTIS). Pediatric department, University of California San Diego Medical Center. www.otispregnancy.org/ctis.html
- MGH Center for Women’s Mental Health, Massachusetts General Hospital. Psychiatric disorders during pregnancy and postpartum. www.womensmentalhealth.com
- MOTHERISK Web site. Teratogen information and updates on reproductive risk research. The Hospital for Sick Children, University of Toronto. www.motherisk.org
- Citalopram • Celexa
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Paroxetine • Paxil
- Sertraline • Zoloft
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Bonari L, Koren G, Einarson TR, et al. Use of antidepressants by pregnant women: evaluation of perception of risk, efficacy of evidence-based counseling, and determinants of decision making. Arch Women Ment Health 2005;8:214-20.
2. Hallberg P, Joblom V. The use of selective serotonin reuptake inhibitors during pregnancy and breast-feeding: a review and clinical aspects. J Clin Psychopharmacol 2005;25:59-73.
3. Larsson C, Sydsjo G, Josefsson A. Health, sociodemographic data, and pregnancy outcome in women with antepartum depressive symptoms. Obstet Gynecol 2004;104(3):469-66.
4. Bonari L, Pinto N, Ahn E, et al. Perinatal risks of untreated depression during pregnancy. Can J Psychiatry 2004;49(11):726-35.
5. Cohen L, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA 2006;295(5):499-507.
6. Hendrick V, Altshuler L. Management of major depression during pregnancy. Am J Psychiatry 2002;159:1667-73.
7. Sandman CA, Glynn L, Wadhwa PD, et al. Maternal hypothalamic-pituitary-adrenal dysregulation during the third trimester influences human fetal responses. Dev Neurosci 2003;25(1):41-9.
8. Huot RL, Brennan PA, Stowe ZN, et al. Negative affect in offspring of depressed mothers is predicted by infant cortisol levels at 6 months and maternal depression during pregnancy, but not postpartum. Ann NY Acad Sci 2004;1032:234-6.
9. Gutteling BM, de Weerth C, Buitelaar JK. Prenatal stress and children’s cortisol reaction to the first day of school. Psychoneuroendocrinology 2005;20:541-9.
10. Hendrick V, Stowe ZN, Altshuler LL, et al. Placental passage of antidepressant medications. Am J Psychiatry 2003;5:993-6.
11. GlaxoSmithKline study EPIP083. GSK medicine: buproprion and paroxetine. Epidemiology study: preliminary report on bupropion in pregnancy and the occurrence of cardiovascular and major congenital malformation. Available at: http://ctr.gsk.co.uk/summary/paroxetine/epip083.pdf. Accessed March 13, 2006.
12. Ericson A, Kallen B, Wiholm BE. Delivery outcome after the use of antidepressants in early pregnancy. Eur J Clin Pharmacol 1999;55:503-8.
13. Kulin NA, Pastuszak A, Sage S, et al. Pregnancy outcome following maternal use of the new selective serotonin reuptake inhibitors. A prospective controlled multicenter study. JAMA 1998;279:609-10.
14. Kallen BA, Otterblad Olausson P. Maternal drug use in early pregnancy and infant cardiovascular defect. Reprod Toxicol 2003;17:255-61.
15. Lattimore K, Donn S, Kaciroti N, et al. Selective serotonin reuptake inhibitor use during pregnancy and effects on the fetus and newborn: a meta-analysis. J Perinatol 2005;25:595-604.
16. Levy L, Ragan K, Hower-Hartley A, et al. Psychiatric disorders in pregnancy. Neurol Clin 2004;22:863-93.
17. Nordeng H, Spigset O. Treatment with selective serotonin reuptake inhibitors in the third trimester of pregnancy: effects on the infant. Drug Saf 2005;28(7):565-81.
18. Levinson-Castiel R, Merlob P, Linder N, et al. Neonatal abstinence syndrome after in utero exposure to selective serotonin reuptake inhibitors to term infants. Arch Pediatr Adolesc Med 2006;160:173-6.
19. Oberlander TF, Misri S, Fitzgerald CE, et al. Pharmacologic factors associated with transient neonatal symptoms following prenatal psychotropic medication exposure. J Clin Psychiatry 2004;65(2):230-7.
20. Zeskind PS, Stephens L. Maternal selective serotonin reuptake inhibitor use during pregnancy and newborn neurobehavior. Pediatrics 2004;113:368-75.
21. Moses-Kolko E, Bogen D, Perel J, et al. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors. JAMA 2005;293:2372-83.
22. Chambers CD, Hernandez-Diaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med 2006;354(6):579-87.
23. Nulman I, Rovet J, Stewart DE, et al. Child development following exposure to tricyclic antidepressants or fluoxetine throughout fetal life: a prospective, controlled study. Am J Psychiatry 2002;159:1889-95.
24. Casper RC, Fleisher BE, Lee-Ancajas JC, et al. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr 2003;4(142):402-8.
25. Spinelli M, Endicott J. Controlled clinical trial of interpersonal psychotherapy versus parenting education program for depressed pregnant women. Am J Psychiatry 2003;160:555-62.
26. Oren D, Wisner K, Spinelli M, et al. An open trial of morning light therapy for treatment of antepartum depression. Am J Psychiatry 2002;159:666-9.
1. Bonari L, Koren G, Einarson TR, et al. Use of antidepressants by pregnant women: evaluation of perception of risk, efficacy of evidence-based counseling, and determinants of decision making. Arch Women Ment Health 2005;8:214-20.
2. Hallberg P, Joblom V. The use of selective serotonin reuptake inhibitors during pregnancy and breast-feeding: a review and clinical aspects. J Clin Psychopharmacol 2005;25:59-73.
3. Larsson C, Sydsjo G, Josefsson A. Health, sociodemographic data, and pregnancy outcome in women with antepartum depressive symptoms. Obstet Gynecol 2004;104(3):469-66.
4. Bonari L, Pinto N, Ahn E, et al. Perinatal risks of untreated depression during pregnancy. Can J Psychiatry 2004;49(11):726-35.
5. Cohen L, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA 2006;295(5):499-507.
6. Hendrick V, Altshuler L. Management of major depression during pregnancy. Am J Psychiatry 2002;159:1667-73.
7. Sandman CA, Glynn L, Wadhwa PD, et al. Maternal hypothalamic-pituitary-adrenal dysregulation during the third trimester influences human fetal responses. Dev Neurosci 2003;25(1):41-9.
8. Huot RL, Brennan PA, Stowe ZN, et al. Negative affect in offspring of depressed mothers is predicted by infant cortisol levels at 6 months and maternal depression during pregnancy, but not postpartum. Ann NY Acad Sci 2004;1032:234-6.
9. Gutteling BM, de Weerth C, Buitelaar JK. Prenatal stress and children’s cortisol reaction to the first day of school. Psychoneuroendocrinology 2005;20:541-9.
10. Hendrick V, Stowe ZN, Altshuler LL, et al. Placental passage of antidepressant medications. Am J Psychiatry 2003;5:993-6.
11. GlaxoSmithKline study EPIP083. GSK medicine: buproprion and paroxetine. Epidemiology study: preliminary report on bupropion in pregnancy and the occurrence of cardiovascular and major congenital malformation. Available at: http://ctr.gsk.co.uk/summary/paroxetine/epip083.pdf. Accessed March 13, 2006.
12. Ericson A, Kallen B, Wiholm BE. Delivery outcome after the use of antidepressants in early pregnancy. Eur J Clin Pharmacol 1999;55:503-8.
13. Kulin NA, Pastuszak A, Sage S, et al. Pregnancy outcome following maternal use of the new selective serotonin reuptake inhibitors. A prospective controlled multicenter study. JAMA 1998;279:609-10.
14. Kallen BA, Otterblad Olausson P. Maternal drug use in early pregnancy and infant cardiovascular defect. Reprod Toxicol 2003;17:255-61.
15. Lattimore K, Donn S, Kaciroti N, et al. Selective serotonin reuptake inhibitor use during pregnancy and effects on the fetus and newborn: a meta-analysis. J Perinatol 2005;25:595-604.
16. Levy L, Ragan K, Hower-Hartley A, et al. Psychiatric disorders in pregnancy. Neurol Clin 2004;22:863-93.
17. Nordeng H, Spigset O. Treatment with selective serotonin reuptake inhibitors in the third trimester of pregnancy: effects on the infant. Drug Saf 2005;28(7):565-81.
18. Levinson-Castiel R, Merlob P, Linder N, et al. Neonatal abstinence syndrome after in utero exposure to selective serotonin reuptake inhibitors to term infants. Arch Pediatr Adolesc Med 2006;160:173-6.
19. Oberlander TF, Misri S, Fitzgerald CE, et al. Pharmacologic factors associated with transient neonatal symptoms following prenatal psychotropic medication exposure. J Clin Psychiatry 2004;65(2):230-7.
20. Zeskind PS, Stephens L. Maternal selective serotonin reuptake inhibitor use during pregnancy and newborn neurobehavior. Pediatrics 2004;113:368-75.
21. Moses-Kolko E, Bogen D, Perel J, et al. Neonatal signs after late in utero exposure to serotonin reuptake inhibitors. JAMA 2005;293:2372-83.
22. Chambers CD, Hernandez-Diaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med 2006;354(6):579-87.
23. Nulman I, Rovet J, Stewart DE, et al. Child development following exposure to tricyclic antidepressants or fluoxetine throughout fetal life: a prospective, controlled study. Am J Psychiatry 2002;159:1889-95.
24. Casper RC, Fleisher BE, Lee-Ancajas JC, et al. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr 2003;4(142):402-8.
25. Spinelli M, Endicott J. Controlled clinical trial of interpersonal psychotherapy versus parenting education program for depressed pregnant women. Am J Psychiatry 2003;160:555-62.
26. Oren D, Wisner K, Spinelli M, et al. An open trial of morning light therapy for treatment of antepartum depression. Am J Psychiatry 2002;159:666-9.
Off-label prescribing: 7 steps for safer, more effective treatment
Have you noticed two curious patterns in off-label prescribing? Psychiatrists avoid agents approved for treating insomnia but prescribe anticonvulsants for a variety of unapproved uses.
Most of us prescribe medications for therapeutic uses not found in FDA-approved labeling. Among 200 psychiatrists surveyed, 65% said they had prescribed off label in the previous month, and only 4% had ever received a patient complaint about the practice.Malpractice Verdicts).
Box
Private insurance. Psychotropic costs are rising 20% a year, contributing to the nation’s annual 13% overall prescription drug cost increase.15 To control rising costs, some medical insurance plans consider off-label use as “unapproved and experimental” and deny coverage. Pharmacy benefit and self-insured employer plans may act similarly, although some states require insurers to cover off-label use of all approved edications.
Government programs. Medicaid does not exclude coverage of off-label prescriptions. How the new Medicare prescription drug plan (Part D) handles off-label prescribing remains unclear.
Why psychiatrists prescribe off-label
| Therapeutic reasons |
| Patient has a disorder for which no drug is labeled |
| Patient falls outside of labeled age or demographic group, such as children, older patients, and pregnant women |
| Patient fails to respond to labeled products |
| Off-label product may potentiate response to a labeled agent or minimize its adverse effects |
| Preferences |
| Manufacturers and respected peers promote use of off-label products as first- or second-line agents18 |
| Practitioner wishes to foster innovative treatments |
| Patients or families request an off-label drug instead of labeled alternatives |
| Practitioner avoids using a particular labeled drug or drug class |
Most state medical practice laws spell out the information required in the patient chart to demonstrate informed consent, defined variously as:
- what a reasonable provider would tell a patient
- what a reasonable patient would expect to hear from the provider
- what a patient would need to hear before deciding on a treatment course.
What the law says
Off-label prescribing is legal, common, necessary, and recognized in some states by statute and by U.S. Supreme Court review.
Court decisions. In a class action suit before the top court (Buckman Company vs. Plaintiff’s Legal Commission, 2001), 5,000 plaintiffs claimed damages from orthopedic screws and plates that were FDA-approved for use in long bones but not for use in the spine. A unanimous court held that such off-label use is an accepted and necessary offshoot of FDA regulatory function and does not interfere with the practice of medicine.
The courts also have determined that off-label use does not mean “experimental” and itself is not a risk. Off-label use may be consistent with the standard of care and does not categorically indicate negligence (though a practitioner who prescribes negligently—such as prescribing a drug to which a patient is known to be allergic—may be found liable).
Drug manufacturers’ risk. The courts recognize that patients receive prescription drugs from doctors, not directly from the manufacturers. The law thus provides some immunity to manufacturers if your patient is injured by a drug you prescribe off-label. The learned-intermediary rule says anufacturers must warn you adequately of a drug’s foreseeable risks, and you then assume the responsibility to warn the patient.
The courts recognize exceptions, though, and have required manufacturers to warn patients directly about vaccines given in mass immunizations, drugs withdrawn from the market, drugs advertised directly to consumers, and other risks.
- BMJ Publishing Group. Clinical evidence. Summary of what is known—and not known—about more than 200 medical disorders and 2,000 treatments. www.clinicalevidence.com.
- Cochrane Library of evidence-based clinical reviews. www.cochrane.org.
- Agency for Health Care Research and Quality. Draft comparative effectiveness review of off-label use of atypical antipsychotic drugs. http://effectivehealthcare.ahrq.gov/synthesize/reports/draft.cfm.
- Amitriptyline • Elavil, others
- Carbamazepine • Carbetrol; Epitol; Equetro; Tegretol
- Gabapentin • Gabarone; Neurontin
- Lamotrigine • Lamictal
- Trazodone • Desyrel; Trialodine
- Valproate • Depakote; Depakene
Dr. Kramer reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. McCall receives research support from GlaxoSmithKline, Sanofi-Aventis, Sepracor, Takeda, and Wyeth, is an advisor to King Pharmaceuticals and Sepracor, and is a speaker for GlaxoSmtihKline, Sepracor, and Wyeth.
1. Lowe-Ponsford FL, Baldwin DS. Off-label prescribing by psychiatrists. Psychiatr Bull 2000;24(11):415-17.
2. Zito JM, Safer DJ, dosReis S, et al. Trends in the prescribing of psychotropic medications to preschoolers. JAMA 2000;283(8):1025-30.
3. Kelly DL, Love RC, Mackowick M, et al. Atypical antipsychotic use in a state hospital inpatient adolescent population. J Child Adolesc Psychopharmacol 2004;14(1):75-85.
4. Rosenheck R, Leslie D, Sernyak M. From clinical trials to real-world practice: use of atypical antipsychotic medication nationally in the Department of Veterans Affairs. Med Care 2001;39(3):302-8.
5. Fountoulakis KN, Nimatoudis I, Iacovides A, Kaprinis G. Off-label indications for atypical antipsychotics: A systematic review. Ann Gen Hosp Psychiatry 2004;3(1):4.-
6. Pomerantz JM, Finkelstein SN, Berndt ER, et al. Prescriber intent, off-label usage, and early discontinuation of antidepressants: a retrospective physician survey and data analysis. J Clin Psychiatry 2004;65(3):395-404.
1. Beck JM, Azari ED. FDA, off-label use, and informed consent. Food Drug Law J 1998;53:71-104.
8. Hepper F, Fellow-Smith E. Off-label prescribing in a community child and adolescent mental health service: Implications for information giving and informed consent. Clin Manag 2005;13(1):29-33.
9. O’Reilly JD, Dalal A. Off-label or out of bounds? Prescriber and marketer liability for unapproved uses of FDA-approved drugs. Ann Health Law 2003;12:295-324.
10. Ware JC, Pittard JT. Increased deep sleep after trazodone use: a double-blind placebo-controlled study in healthy young adults. J Clin Psychiatry 1990;51:18-22.
11. McCall WV. Use of off-label medications in the treatment of chronic insomnia. J Clin Sleep Med 2005;1(4):e494-5.
12. Chen H, Deshpande AD, Jiang R, Martin BC. An epidemiological investigation of off-label anticonvulsant drug use in the Georgia Medicaid population. Pharmacoepidemiol Drug Saf 2005;14(9):629-38.
13. Mack A. Examination of the evidence for off-label use of gabapentin. J Manag Care Pharm 2003;9(6):559-68.
14. Citrome L, Levine J, Allingham B. Utilization of valproate: extent of inpatient use in the New York State Office of Mental Health. Psychiatr Q 1998;69(4):283-300.
15. De Leon O. Antiepileptic drugs for the acute and maintenance treatment of bipolar disorder. Harv Rev Psychiatry 2001;9(5):209-22.
16. Le Bon O, Murphy JR, Staner L, et al. Double-blind, placebocontrolled study of the efficacy of trazodone in alcohol postwithdrawal syndrome: polysomnographic and clinical evaluations. J Clin Psychopharmacol 2003;23(4):377-83.
17. Zuvekas SH. Prescription drugs and the changing patterns of treatment for mental disorders, 1996-2001. Health Aff (Millwood) 2005;24(1):195-205.
18. Glick ID, Murray SR, Vasudevan P, et al. Treatment with atypical antipsychotics: new indications and new populations. J Psychiatr Res 2001;35(3):187-91.
Have you noticed two curious patterns in off-label prescribing? Psychiatrists avoid agents approved for treating insomnia but prescribe anticonvulsants for a variety of unapproved uses.
Most of us prescribe medications for therapeutic uses not found in FDA-approved labeling. Among 200 psychiatrists surveyed, 65% said they had prescribed off label in the previous month, and only 4% had ever received a patient complaint about the practice.Malpractice Verdicts).
Box
Private insurance. Psychotropic costs are rising 20% a year, contributing to the nation’s annual 13% overall prescription drug cost increase.15 To control rising costs, some medical insurance plans consider off-label use as “unapproved and experimental” and deny coverage. Pharmacy benefit and self-insured employer plans may act similarly, although some states require insurers to cover off-label use of all approved edications.
Government programs. Medicaid does not exclude coverage of off-label prescriptions. How the new Medicare prescription drug plan (Part D) handles off-label prescribing remains unclear.
Why psychiatrists prescribe off-label
| Therapeutic reasons |
| Patient has a disorder for which no drug is labeled |
| Patient falls outside of labeled age or demographic group, such as children, older patients, and pregnant women |
| Patient fails to respond to labeled products |
| Off-label product may potentiate response to a labeled agent or minimize its adverse effects |
| Preferences |
| Manufacturers and respected peers promote use of off-label products as first- or second-line agents18 |
| Practitioner wishes to foster innovative treatments |
| Patients or families request an off-label drug instead of labeled alternatives |
| Practitioner avoids using a particular labeled drug or drug class |
Most state medical practice laws spell out the information required in the patient chart to demonstrate informed consent, defined variously as:
- what a reasonable provider would tell a patient
- what a reasonable patient would expect to hear from the provider
- what a patient would need to hear before deciding on a treatment course.
What the law says
Off-label prescribing is legal, common, necessary, and recognized in some states by statute and by U.S. Supreme Court review.
Court decisions. In a class action suit before the top court (Buckman Company vs. Plaintiff’s Legal Commission, 2001), 5,000 plaintiffs claimed damages from orthopedic screws and plates that were FDA-approved for use in long bones but not for use in the spine. A unanimous court held that such off-label use is an accepted and necessary offshoot of FDA regulatory function and does not interfere with the practice of medicine.
The courts also have determined that off-label use does not mean “experimental” and itself is not a risk. Off-label use may be consistent with the standard of care and does not categorically indicate negligence (though a practitioner who prescribes negligently—such as prescribing a drug to which a patient is known to be allergic—may be found liable).
Drug manufacturers’ risk. The courts recognize that patients receive prescription drugs from doctors, not directly from the manufacturers. The law thus provides some immunity to manufacturers if your patient is injured by a drug you prescribe off-label. The learned-intermediary rule says anufacturers must warn you adequately of a drug’s foreseeable risks, and you then assume the responsibility to warn the patient.
The courts recognize exceptions, though, and have required manufacturers to warn patients directly about vaccines given in mass immunizations, drugs withdrawn from the market, drugs advertised directly to consumers, and other risks.
- BMJ Publishing Group. Clinical evidence. Summary of what is known—and not known—about more than 200 medical disorders and 2,000 treatments. www.clinicalevidence.com.
- Cochrane Library of evidence-based clinical reviews. www.cochrane.org.
- Agency for Health Care Research and Quality. Draft comparative effectiveness review of off-label use of atypical antipsychotic drugs. http://effectivehealthcare.ahrq.gov/synthesize/reports/draft.cfm.
- Amitriptyline • Elavil, others
- Carbamazepine • Carbetrol; Epitol; Equetro; Tegretol
- Gabapentin • Gabarone; Neurontin
- Lamotrigine • Lamictal
- Trazodone • Desyrel; Trialodine
- Valproate • Depakote; Depakene
Dr. Kramer reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. McCall receives research support from GlaxoSmithKline, Sanofi-Aventis, Sepracor, Takeda, and Wyeth, is an advisor to King Pharmaceuticals and Sepracor, and is a speaker for GlaxoSmtihKline, Sepracor, and Wyeth.
Have you noticed two curious patterns in off-label prescribing? Psychiatrists avoid agents approved for treating insomnia but prescribe anticonvulsants for a variety of unapproved uses.
Most of us prescribe medications for therapeutic uses not found in FDA-approved labeling. Among 200 psychiatrists surveyed, 65% said they had prescribed off label in the previous month, and only 4% had ever received a patient complaint about the practice.Malpractice Verdicts).
Box
Private insurance. Psychotropic costs are rising 20% a year, contributing to the nation’s annual 13% overall prescription drug cost increase.15 To control rising costs, some medical insurance plans consider off-label use as “unapproved and experimental” and deny coverage. Pharmacy benefit and self-insured employer plans may act similarly, although some states require insurers to cover off-label use of all approved edications.
Government programs. Medicaid does not exclude coverage of off-label prescriptions. How the new Medicare prescription drug plan (Part D) handles off-label prescribing remains unclear.
Why psychiatrists prescribe off-label
| Therapeutic reasons |
| Patient has a disorder for which no drug is labeled |
| Patient falls outside of labeled age or demographic group, such as children, older patients, and pregnant women |
| Patient fails to respond to labeled products |
| Off-label product may potentiate response to a labeled agent or minimize its adverse effects |
| Preferences |
| Manufacturers and respected peers promote use of off-label products as first- or second-line agents18 |
| Practitioner wishes to foster innovative treatments |
| Patients or families request an off-label drug instead of labeled alternatives |
| Practitioner avoids using a particular labeled drug or drug class |
Most state medical practice laws spell out the information required in the patient chart to demonstrate informed consent, defined variously as:
- what a reasonable provider would tell a patient
- what a reasonable patient would expect to hear from the provider
- what a patient would need to hear before deciding on a treatment course.
What the law says
Off-label prescribing is legal, common, necessary, and recognized in some states by statute and by U.S. Supreme Court review.
Court decisions. In a class action suit before the top court (Buckman Company vs. Plaintiff’s Legal Commission, 2001), 5,000 plaintiffs claimed damages from orthopedic screws and plates that were FDA-approved for use in long bones but not for use in the spine. A unanimous court held that such off-label use is an accepted and necessary offshoot of FDA regulatory function and does not interfere with the practice of medicine.
The courts also have determined that off-label use does not mean “experimental” and itself is not a risk. Off-label use may be consistent with the standard of care and does not categorically indicate negligence (though a practitioner who prescribes negligently—such as prescribing a drug to which a patient is known to be allergic—may be found liable).
Drug manufacturers’ risk. The courts recognize that patients receive prescription drugs from doctors, not directly from the manufacturers. The law thus provides some immunity to manufacturers if your patient is injured by a drug you prescribe off-label. The learned-intermediary rule says anufacturers must warn you adequately of a drug’s foreseeable risks, and you then assume the responsibility to warn the patient.
The courts recognize exceptions, though, and have required manufacturers to warn patients directly about vaccines given in mass immunizations, drugs withdrawn from the market, drugs advertised directly to consumers, and other risks.
- BMJ Publishing Group. Clinical evidence. Summary of what is known—and not known—about more than 200 medical disorders and 2,000 treatments. www.clinicalevidence.com.
- Cochrane Library of evidence-based clinical reviews. www.cochrane.org.
- Agency for Health Care Research and Quality. Draft comparative effectiveness review of off-label use of atypical antipsychotic drugs. http://effectivehealthcare.ahrq.gov/synthesize/reports/draft.cfm.
- Amitriptyline • Elavil, others
- Carbamazepine • Carbetrol; Epitol; Equetro; Tegretol
- Gabapentin • Gabarone; Neurontin
- Lamotrigine • Lamictal
- Trazodone • Desyrel; Trialodine
- Valproate • Depakote; Depakene
Dr. Kramer reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. McCall receives research support from GlaxoSmithKline, Sanofi-Aventis, Sepracor, Takeda, and Wyeth, is an advisor to King Pharmaceuticals and Sepracor, and is a speaker for GlaxoSmtihKline, Sepracor, and Wyeth.
1. Lowe-Ponsford FL, Baldwin DS. Off-label prescribing by psychiatrists. Psychiatr Bull 2000;24(11):415-17.
2. Zito JM, Safer DJ, dosReis S, et al. Trends in the prescribing of psychotropic medications to preschoolers. JAMA 2000;283(8):1025-30.
3. Kelly DL, Love RC, Mackowick M, et al. Atypical antipsychotic use in a state hospital inpatient adolescent population. J Child Adolesc Psychopharmacol 2004;14(1):75-85.
4. Rosenheck R, Leslie D, Sernyak M. From clinical trials to real-world practice: use of atypical antipsychotic medication nationally in the Department of Veterans Affairs. Med Care 2001;39(3):302-8.
5. Fountoulakis KN, Nimatoudis I, Iacovides A, Kaprinis G. Off-label indications for atypical antipsychotics: A systematic review. Ann Gen Hosp Psychiatry 2004;3(1):4.-
6. Pomerantz JM, Finkelstein SN, Berndt ER, et al. Prescriber intent, off-label usage, and early discontinuation of antidepressants: a retrospective physician survey and data analysis. J Clin Psychiatry 2004;65(3):395-404.
1. Beck JM, Azari ED. FDA, off-label use, and informed consent. Food Drug Law J 1998;53:71-104.
8. Hepper F, Fellow-Smith E. Off-label prescribing in a community child and adolescent mental health service: Implications for information giving and informed consent. Clin Manag 2005;13(1):29-33.
9. O’Reilly JD, Dalal A. Off-label or out of bounds? Prescriber and marketer liability for unapproved uses of FDA-approved drugs. Ann Health Law 2003;12:295-324.
10. Ware JC, Pittard JT. Increased deep sleep after trazodone use: a double-blind placebo-controlled study in healthy young adults. J Clin Psychiatry 1990;51:18-22.
11. McCall WV. Use of off-label medications in the treatment of chronic insomnia. J Clin Sleep Med 2005;1(4):e494-5.
12. Chen H, Deshpande AD, Jiang R, Martin BC. An epidemiological investigation of off-label anticonvulsant drug use in the Georgia Medicaid population. Pharmacoepidemiol Drug Saf 2005;14(9):629-38.
13. Mack A. Examination of the evidence for off-label use of gabapentin. J Manag Care Pharm 2003;9(6):559-68.
14. Citrome L, Levine J, Allingham B. Utilization of valproate: extent of inpatient use in the New York State Office of Mental Health. Psychiatr Q 1998;69(4):283-300.
15. De Leon O. Antiepileptic drugs for the acute and maintenance treatment of bipolar disorder. Harv Rev Psychiatry 2001;9(5):209-22.
16. Le Bon O, Murphy JR, Staner L, et al. Double-blind, placebocontrolled study of the efficacy of trazodone in alcohol postwithdrawal syndrome: polysomnographic and clinical evaluations. J Clin Psychopharmacol 2003;23(4):377-83.
17. Zuvekas SH. Prescription drugs and the changing patterns of treatment for mental disorders, 1996-2001. Health Aff (Millwood) 2005;24(1):195-205.
18. Glick ID, Murray SR, Vasudevan P, et al. Treatment with atypical antipsychotics: new indications and new populations. J Psychiatr Res 2001;35(3):187-91.
1. Lowe-Ponsford FL, Baldwin DS. Off-label prescribing by psychiatrists. Psychiatr Bull 2000;24(11):415-17.
2. Zito JM, Safer DJ, dosReis S, et al. Trends in the prescribing of psychotropic medications to preschoolers. JAMA 2000;283(8):1025-30.
3. Kelly DL, Love RC, Mackowick M, et al. Atypical antipsychotic use in a state hospital inpatient adolescent population. J Child Adolesc Psychopharmacol 2004;14(1):75-85.
4. Rosenheck R, Leslie D, Sernyak M. From clinical trials to real-world practice: use of atypical antipsychotic medication nationally in the Department of Veterans Affairs. Med Care 2001;39(3):302-8.
5. Fountoulakis KN, Nimatoudis I, Iacovides A, Kaprinis G. Off-label indications for atypical antipsychotics: A systematic review. Ann Gen Hosp Psychiatry 2004;3(1):4.-
6. Pomerantz JM, Finkelstein SN, Berndt ER, et al. Prescriber intent, off-label usage, and early discontinuation of antidepressants: a retrospective physician survey and data analysis. J Clin Psychiatry 2004;65(3):395-404.
1. Beck JM, Azari ED. FDA, off-label use, and informed consent. Food Drug Law J 1998;53:71-104.
8. Hepper F, Fellow-Smith E. Off-label prescribing in a community child and adolescent mental health service: Implications for information giving and informed consent. Clin Manag 2005;13(1):29-33.
9. O’Reilly JD, Dalal A. Off-label or out of bounds? Prescriber and marketer liability for unapproved uses of FDA-approved drugs. Ann Health Law 2003;12:295-324.
10. Ware JC, Pittard JT. Increased deep sleep after trazodone use: a double-blind placebo-controlled study in healthy young adults. J Clin Psychiatry 1990;51:18-22.
11. McCall WV. Use of off-label medications in the treatment of chronic insomnia. J Clin Sleep Med 2005;1(4):e494-5.
12. Chen H, Deshpande AD, Jiang R, Martin BC. An epidemiological investigation of off-label anticonvulsant drug use in the Georgia Medicaid population. Pharmacoepidemiol Drug Saf 2005;14(9):629-38.
13. Mack A. Examination of the evidence for off-label use of gabapentin. J Manag Care Pharm 2003;9(6):559-68.
14. Citrome L, Levine J, Allingham B. Utilization of valproate: extent of inpatient use in the New York State Office of Mental Health. Psychiatr Q 1998;69(4):283-300.
15. De Leon O. Antiepileptic drugs for the acute and maintenance treatment of bipolar disorder. Harv Rev Psychiatry 2001;9(5):209-22.
16. Le Bon O, Murphy JR, Staner L, et al. Double-blind, placebocontrolled study of the efficacy of trazodone in alcohol postwithdrawal syndrome: polysomnographic and clinical evaluations. J Clin Psychopharmacol 2003;23(4):377-83.
17. Zuvekas SH. Prescription drugs and the changing patterns of treatment for mental disorders, 1996-2001. Health Aff (Millwood) 2005;24(1):195-205.
18. Glick ID, Murray SR, Vasudevan P, et al. Treatment with atypical antipsychotics: new indications and new populations. J Psychiatr Res 2001;35(3):187-91.
Glutamatergic TB drug ‘cools off’ anxiety disorders
Think of anxiety disorders as an overactive brain alarm that psychotropics and exposure therapy quiet via separate mechanisms. Psychotropics “cool off” the alarm by curtailing excitability of the amygdala, brainstem nuclei, and hypothalamus.1 Psychological treatments, particularly exposure therapy, seek to teach the brain not to fear the dreaded object.2
One would assume that combining medication and exposure therapy for anxiety would be beneficial, but results have been disappointing.3 Anxiolytics do not enhance—and many impede—learning that occurs during psychotherapy. When the medications are tapered, patients who receive psychotherapy plus placebo typically experience more-enduring benefit than those receiving psychotherapy plus active medication.4-6
An unlikely candidate—a glutamatergic tuberculosis (TB) drug—may offer a solution. The drug and others in its class may potentiate psychotherapy’s effects by enhancing learning rather than relief.7
Glutamate/learning link
Glutamate neurons have 3 types of glutamatergic receptors, with the NMDA and AMPA types perceived as most important because of their possible role in memory development. Creating new memories may involve strengthening signals between glutamate neurons. The exact cellular mechanism is unknown, but it may involve greater release of neurotransmitters or formation of new synapses.
Stronger signaling—and hence learning—may depend on opening the NMDA receptor to enhance postsynaptic potential.8 Opening both NMDA and AMPA receptors generates a stronger signal and allows calcium influx, compared with opening the AMPA receptor alone (Figure). This combination can activate genes that control protein synthesis and result in structural changes necessary for developing long-term memories.
D-cycloserine—a partial agonist at the NMDA receptor—is usually used as an antibiotic to treat TB. The drug also has been shown to enhance the learning process that underlies fear extinction in rats. A group at Emory University studied the effect of adding the medication to exposure therapy in humans with acrophobia.7
FigureNMDA receptor agonists may enhance learning in psychotherapy
Normally, only glutamate neurons’ AMPA receptors activate in response to glutamate release (A). Both AMPA and NMDA receptors open in response to NMDA receptor agonists like D-cycloserine (B). Consequent stronger signaling may improve memory.
Going ‘up’
The researchers developed a virtual reality exposure system in which participants felt as if they were standing in an elevator, watching the floors recede as they rose 19 stories. Exposure therapy—seven weekly 35- to 45-minute sessions—has been shown to reduce the fear patients with acrophobia experience in virtual elevators.
Of 27 subjects, 10 received placebo and 17 received D-cycloserine, 50 mg or 500 mg. Subjects took their pills 2 to 4 hours before an exposure session. All participants experienced 2 virtual exposure sessions 1 to 2 weeks apart, which is considered suboptimal treatment for acrophobia.
Three months after the study, the D-cycloserine groups showed a markedly reduced fear of heights on the virtual elevator, while the placebo group showed no change from baseline. Fear levels were measured by subjective report of discomfort at each “floor.” D-cycloserine subjects also reported significantly greater reductions in measures of acrophobia in their daily lives.
Interestingly, both the D-cycloserine and placebo groups were equally frightened during virtual reality exposure. Only later did the D-cycloserine groups report less fear when exposed to heights, indicating that D-cycloserine enhanced learning that occurred during exposure therapy.
It’s exciting to think that medications could enhance and accelerate healing by activating the appropriate receptors during psychotherapy and give patients enduring benefits without the need for continued treatment.
If shown to be effective in larger studies, glutamatergic medications plus psychotherapy could provide more effective therapy for anxiety disorder. This approach is reported to be under investigation for treating anorexia nervosa, social phobia, panic disorder, and obsessive-compulsive disorder.9
1. Lydiard RB. Break the ‘fear circuit’ in resistant panic disorder. Current Psychiatry 2003;2(11):12-22.
2. Tynes LL, Tynes SF. Panic attacks: help sufferers recover with cognitive-behavioral therapy. Current Psychiatry 2005;4(11):51-60.
3. Otto MW, Smits JAJ, Reese HE. Combined psychotherapy and pharmacotherapy for mood and anxiety disorders in adults: review and analysis. Clinical Psychology: Science & Practice 2005;12(1):72-86.
4. Barlow DH, Gorman JM, Shear MK, Woods SW. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: A randomized controlled trial. JAMA 2000;283(19):2529-36.
5. Haug TT, Blomhoff S, Hellstrom K, et al. Exposure therapy and sertraline in social phobia: I-year follow-up of a randomised controlled trial. Br J Psychiatry 2003;182:312-8.
6. Marks IM, Swinson RP, Basoglu M, et al. Alprazolam and exposure alone and combined in panic disorder with agoraphobia. A controlled study in London and Toronto. Br J Psychiatry 1993;162:776-87.
7. Ressler KJ, Rothbaum BO, Tannenbaum L, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry 2004;61(11):1136-44.
8. Purves D, Augustine GJ, Fitzpatrick D, et al. Plasticity of mature synapses and circuits. In: Neuroscience (3rd ed). Sunderland, MA: Sinauer; 2004:575-610.
9. O’Connor A. A pill that helps ease grip of irrational fears. New York Times March 22, 2005.
Think of anxiety disorders as an overactive brain alarm that psychotropics and exposure therapy quiet via separate mechanisms. Psychotropics “cool off” the alarm by curtailing excitability of the amygdala, brainstem nuclei, and hypothalamus.1 Psychological treatments, particularly exposure therapy, seek to teach the brain not to fear the dreaded object.2
One would assume that combining medication and exposure therapy for anxiety would be beneficial, but results have been disappointing.3 Anxiolytics do not enhance—and many impede—learning that occurs during psychotherapy. When the medications are tapered, patients who receive psychotherapy plus placebo typically experience more-enduring benefit than those receiving psychotherapy plus active medication.4-6
An unlikely candidate—a glutamatergic tuberculosis (TB) drug—may offer a solution. The drug and others in its class may potentiate psychotherapy’s effects by enhancing learning rather than relief.7
Glutamate/learning link
Glutamate neurons have 3 types of glutamatergic receptors, with the NMDA and AMPA types perceived as most important because of their possible role in memory development. Creating new memories may involve strengthening signals between glutamate neurons. The exact cellular mechanism is unknown, but it may involve greater release of neurotransmitters or formation of new synapses.
Stronger signaling—and hence learning—may depend on opening the NMDA receptor to enhance postsynaptic potential.8 Opening both NMDA and AMPA receptors generates a stronger signal and allows calcium influx, compared with opening the AMPA receptor alone (Figure). This combination can activate genes that control protein synthesis and result in structural changes necessary for developing long-term memories.
D-cycloserine—a partial agonist at the NMDA receptor—is usually used as an antibiotic to treat TB. The drug also has been shown to enhance the learning process that underlies fear extinction in rats. A group at Emory University studied the effect of adding the medication to exposure therapy in humans with acrophobia.7
FigureNMDA receptor agonists may enhance learning in psychotherapy
Normally, only glutamate neurons’ AMPA receptors activate in response to glutamate release (A). Both AMPA and NMDA receptors open in response to NMDA receptor agonists like D-cycloserine (B). Consequent stronger signaling may improve memory.
Going ‘up’
The researchers developed a virtual reality exposure system in which participants felt as if they were standing in an elevator, watching the floors recede as they rose 19 stories. Exposure therapy—seven weekly 35- to 45-minute sessions—has been shown to reduce the fear patients with acrophobia experience in virtual elevators.
Of 27 subjects, 10 received placebo and 17 received D-cycloserine, 50 mg or 500 mg. Subjects took their pills 2 to 4 hours before an exposure session. All participants experienced 2 virtual exposure sessions 1 to 2 weeks apart, which is considered suboptimal treatment for acrophobia.
Three months after the study, the D-cycloserine groups showed a markedly reduced fear of heights on the virtual elevator, while the placebo group showed no change from baseline. Fear levels were measured by subjective report of discomfort at each “floor.” D-cycloserine subjects also reported significantly greater reductions in measures of acrophobia in their daily lives.
Interestingly, both the D-cycloserine and placebo groups were equally frightened during virtual reality exposure. Only later did the D-cycloserine groups report less fear when exposed to heights, indicating that D-cycloserine enhanced learning that occurred during exposure therapy.
It’s exciting to think that medications could enhance and accelerate healing by activating the appropriate receptors during psychotherapy and give patients enduring benefits without the need for continued treatment.
If shown to be effective in larger studies, glutamatergic medications plus psychotherapy could provide more effective therapy for anxiety disorder. This approach is reported to be under investigation for treating anorexia nervosa, social phobia, panic disorder, and obsessive-compulsive disorder.9
Think of anxiety disorders as an overactive brain alarm that psychotropics and exposure therapy quiet via separate mechanisms. Psychotropics “cool off” the alarm by curtailing excitability of the amygdala, brainstem nuclei, and hypothalamus.1 Psychological treatments, particularly exposure therapy, seek to teach the brain not to fear the dreaded object.2
One would assume that combining medication and exposure therapy for anxiety would be beneficial, but results have been disappointing.3 Anxiolytics do not enhance—and many impede—learning that occurs during psychotherapy. When the medications are tapered, patients who receive psychotherapy plus placebo typically experience more-enduring benefit than those receiving psychotherapy plus active medication.4-6
An unlikely candidate—a glutamatergic tuberculosis (TB) drug—may offer a solution. The drug and others in its class may potentiate psychotherapy’s effects by enhancing learning rather than relief.7
Glutamate/learning link
Glutamate neurons have 3 types of glutamatergic receptors, with the NMDA and AMPA types perceived as most important because of their possible role in memory development. Creating new memories may involve strengthening signals between glutamate neurons. The exact cellular mechanism is unknown, but it may involve greater release of neurotransmitters or formation of new synapses.
Stronger signaling—and hence learning—may depend on opening the NMDA receptor to enhance postsynaptic potential.8 Opening both NMDA and AMPA receptors generates a stronger signal and allows calcium influx, compared with opening the AMPA receptor alone (Figure). This combination can activate genes that control protein synthesis and result in structural changes necessary for developing long-term memories.
D-cycloserine—a partial agonist at the NMDA receptor—is usually used as an antibiotic to treat TB. The drug also has been shown to enhance the learning process that underlies fear extinction in rats. A group at Emory University studied the effect of adding the medication to exposure therapy in humans with acrophobia.7
FigureNMDA receptor agonists may enhance learning in psychotherapy
Normally, only glutamate neurons’ AMPA receptors activate in response to glutamate release (A). Both AMPA and NMDA receptors open in response to NMDA receptor agonists like D-cycloserine (B). Consequent stronger signaling may improve memory.
Going ‘up’
The researchers developed a virtual reality exposure system in which participants felt as if they were standing in an elevator, watching the floors recede as they rose 19 stories. Exposure therapy—seven weekly 35- to 45-minute sessions—has been shown to reduce the fear patients with acrophobia experience in virtual elevators.
Of 27 subjects, 10 received placebo and 17 received D-cycloserine, 50 mg or 500 mg. Subjects took their pills 2 to 4 hours before an exposure session. All participants experienced 2 virtual exposure sessions 1 to 2 weeks apart, which is considered suboptimal treatment for acrophobia.
Three months after the study, the D-cycloserine groups showed a markedly reduced fear of heights on the virtual elevator, while the placebo group showed no change from baseline. Fear levels were measured by subjective report of discomfort at each “floor.” D-cycloserine subjects also reported significantly greater reductions in measures of acrophobia in their daily lives.
Interestingly, both the D-cycloserine and placebo groups were equally frightened during virtual reality exposure. Only later did the D-cycloserine groups report less fear when exposed to heights, indicating that D-cycloserine enhanced learning that occurred during exposure therapy.
It’s exciting to think that medications could enhance and accelerate healing by activating the appropriate receptors during psychotherapy and give patients enduring benefits without the need for continued treatment.
If shown to be effective in larger studies, glutamatergic medications plus psychotherapy could provide more effective therapy for anxiety disorder. This approach is reported to be under investigation for treating anorexia nervosa, social phobia, panic disorder, and obsessive-compulsive disorder.9
1. Lydiard RB. Break the ‘fear circuit’ in resistant panic disorder. Current Psychiatry 2003;2(11):12-22.
2. Tynes LL, Tynes SF. Panic attacks: help sufferers recover with cognitive-behavioral therapy. Current Psychiatry 2005;4(11):51-60.
3. Otto MW, Smits JAJ, Reese HE. Combined psychotherapy and pharmacotherapy for mood and anxiety disorders in adults: review and analysis. Clinical Psychology: Science & Practice 2005;12(1):72-86.
4. Barlow DH, Gorman JM, Shear MK, Woods SW. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: A randomized controlled trial. JAMA 2000;283(19):2529-36.
5. Haug TT, Blomhoff S, Hellstrom K, et al. Exposure therapy and sertraline in social phobia: I-year follow-up of a randomised controlled trial. Br J Psychiatry 2003;182:312-8.
6. Marks IM, Swinson RP, Basoglu M, et al. Alprazolam and exposure alone and combined in panic disorder with agoraphobia. A controlled study in London and Toronto. Br J Psychiatry 1993;162:776-87.
7. Ressler KJ, Rothbaum BO, Tannenbaum L, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry 2004;61(11):1136-44.
8. Purves D, Augustine GJ, Fitzpatrick D, et al. Plasticity of mature synapses and circuits. In: Neuroscience (3rd ed). Sunderland, MA: Sinauer; 2004:575-610.
9. O’Connor A. A pill that helps ease grip of irrational fears. New York Times March 22, 2005.
1. Lydiard RB. Break the ‘fear circuit’ in resistant panic disorder. Current Psychiatry 2003;2(11):12-22.
2. Tynes LL, Tynes SF. Panic attacks: help sufferers recover with cognitive-behavioral therapy. Current Psychiatry 2005;4(11):51-60.
3. Otto MW, Smits JAJ, Reese HE. Combined psychotherapy and pharmacotherapy for mood and anxiety disorders in adults: review and analysis. Clinical Psychology: Science & Practice 2005;12(1):72-86.
4. Barlow DH, Gorman JM, Shear MK, Woods SW. Cognitive-behavioral therapy, imipramine, or their combination for panic disorder: A randomized controlled trial. JAMA 2000;283(19):2529-36.
5. Haug TT, Blomhoff S, Hellstrom K, et al. Exposure therapy and sertraline in social phobia: I-year follow-up of a randomised controlled trial. Br J Psychiatry 2003;182:312-8.
6. Marks IM, Swinson RP, Basoglu M, et al. Alprazolam and exposure alone and combined in panic disorder with agoraphobia. A controlled study in London and Toronto. Br J Psychiatry 1993;162:776-87.
7. Ressler KJ, Rothbaum BO, Tannenbaum L, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry 2004;61(11):1136-44.
8. Purves D, Augustine GJ, Fitzpatrick D, et al. Plasticity of mature synapses and circuits. In: Neuroscience (3rd ed). Sunderland, MA: Sinauer; 2004:575-610.
9. O’Connor A. A pill that helps ease grip of irrational fears. New York Times March 22, 2005.
2 therapies lift mood in chronic fatigue syndrome
Chronic fatigue syndrome (CFS) continues to puzzle and provoke. Years of research have failed to find a biomedical cause or to answer fundamental questions, such as “What is it?” and “Does it exist?”
Up to two-thirds of CFS patients have psychiatric disorders,1 but psychiatrists are not the first physicians CFS patients usually see. Primary care physicians may ask you to help confirm the diagnosis, manage patients’ anxiety and depression, differentiate CFS from somatoform disorders, or provide psychotherapy for patients and their families.
Knowing what transpires in the referring physician’s office is key to helping a patient function despite CFS. This article describes:
- CFS clinical features and possible causes
- psychiatric comorbidities and exclusions
- cognitive behavioral therapy (CBT) and graded exercise, the only two therapies shown to improve CFS patients’ daily function.
Case: Anxious, depressed, and tired
Mr. A, age 43, is referred to you with anxiety and depressed mood associated with CFS, diagnosed 2 years ago. Fatigue predated Mr. A’s depression and anxiety, which his primary care physician considers consequences of CFS.
Mr. A is married, has three children, and owns a successful accounting practice. CFS was diagnosed from the classic presentation: abrupt onset of fatigue despite good health and a promising career. Now, overwhelming fatigue reduces his productivity. He makes up for frequent rest breaks by working in snatches of time, even at 4 AM. He is spending little time with his wife and children.
Defining CFS
The Centers for Disease Control and Prevention (CDC) defined CFS in 1988 while investigating infectious causes of fatigue (Table 1).2,3 Epstein-Barr virus was thought to be the cause of CFS.
Most patients who present to their primary care physicians with complaints of fatigue do not meet CDC criteria for CFS, however. These “non-CFS” patients are diagnosed as having “idiopathic chronic fatigue,” a term that is not particularly helpful because CFS remains an idiopathic disorder.
Clinical findings. As with Mr. A, many CFS patients’ fatigue begins suddenly, often with flu-like symptoms. Patients lose tolerance for exercise and alcohol and become unable to work or socialize at pre-illness levels.
Medical symptoms can overlap those of other conditions, such as fibromyalgia, chemical sensitivities, and irritable bowel disorder. These associations make the condition difficult to assess and contribute to some physicians’ difficulty in accepting CFS as a biomedical condition. Mistrust and a poor patient-physician relationship can result when the physician doubts the symptoms’ “medical” nature and the patient resents the implication that the suffering is “all in your head.”
What causes CFS? No consistent factor has been identified that explains the pathophysiology of CFS symptoms. Many possibilities have been examined, but the evidence is confusing and contradictory.
A few preliminary studies suggest possible familial (shared environmental) and genetic components, but data are sparse and no more than suggestive.4 Findings of CNS studies are inconsistent, and the search for a change in immune function or an infectious agent has been fruitless despite some patients’ infection-like symptoms. The early hypothesis that Epstein-Barr virus was responsible has been disproved.
Imaging studies, psychological testing, and neuroendocrine investigations have identified abnormalities in some patients with CFS. The most-promising findings point to abnormalities in the hypothalamic-pituitary-adrenal axis and in serotonergic neurotransmission,5 suggesting an abnormal stress response in some patients.
Table 1
CDC diagnostic criteria for chronic fatigue syndrome
| At least 6 months of fatigue sufficient to “substantially reduce” patient’s level of activity |
4 or more of 8 concurrent symptoms:
|
| No obvious medical or psychiatric causes, such as eating disorders, psychosis, bipolar disorder, melancholia, or substance abuse* |
| CDC: Centers for Disease Control and Prevention |
| * Many nonpsychotic psychiatric disorders (such as atypical depression) do not exclude a CFS diagnosis |
| Source: References 1 and 2 |
Case continued: Test results are normal
Mr. A has undergone extensive medical assessment (complete blood cell count; renal, hepatic, and thyroid function tests; calcium, phosphate, and glucose determinations; and urinalysis), which yielded normal results. Brain MRI findings were also normal; specifically, no evidence of multiple sclerosis.
Even so, he has had nonspecific symptoms of impaired concentration, sore throat, tender cervical nodes, muscle pain, and nonrefreshing sleep. Physical exertion can leave him drained for at least 1 or 2 days.
Psychiatric Disorders
As many as 66% of CFS patients may have one or more psychiatric comorbidities; the most common are generalized anxiety disorder, panic disorder, depression, and somatoform disorder.1 Because CFS symptoms are regarded as being not fully explained by a known medical disorder, patients are often diagnosed as having an undifferentiated somatoform disorder. Either disorder could be diagnosed in some cases, but this differentiation sheds no new light on the condition.
CFS and depression. Could CFS and depression be one and the same? Proponents of that position point out the similarity of symptoms, loss of function, and—in at least some cases—favorable response to antidepressants. Opponents cite other factors such as:
- presence of sore throat, lymphadenopathy, and post-exercise fatigue
- differences in sleep patterns
- frequent absence of psychiatric illness before fatigue onset
- evidence of hypocortisolism (also seen in patients with melancholic depression) in some CFS patients.
Primary Care Workup
Complaints of long-lasting, debilitating fatigue should alert the primary care physician to CFS. Like somatization disorder, CFS requires a physical workup, though as few as 2% of CFS patients are found to have an undiagnosed medical illness that explains the symptoms.9 The evaluation’s goal is not so much to find out what’s causing the fatigue as to reassure the patient that all avenues are considered before the diagnosis is made. When this is accomplished well, the patient is likely to accept psychiatric referral or treatment, if needed.
Two-part initial evaluation. If the initial physical exam and laboratory work find no biomedical cause for the patient’s chronic fatigue symptoms, we recommend a two-part primary care evaluation. This includes a focused discussion with the patient about CFS (Table 2).10 Goals of the first session are to:
- establish a relationship that will survive difficult times
- teach the patient to think of complex medical problems as having psychological and social consequences, if not causes.
Primary care physicians usually request a psychiatric consultation to confirm or rule out psychiatric conditions that exclude a CFS diagnosis (melancholic depression, bipolar disorder, schizophrenia, anorexia nervosa or bulimia, and recent substance abuse). They also may refer in cases of other common disorders with poorly explained symptoms such as fibromyalgia and chemical sensitivity disorder.
Table 2
Two-part initial primary care evaluation of chronic fatigue
First session
|
Case continued: High anxiety
Mr. A describes how fatigue is affecting his work and home life. He is especially worried that he will not be attentive enough to catch accounting errors by his employees.
Interestingly, his anxiety remits but fatigue continues when he goes on vacation. He has no history of melancholic depression, bipolar disorder, psychosis, or substance abuse.
Psychiatric Assessment
The referring physician should provide a full account of the medical workup. This:
- assures you that possible medical causes of fatigue have been excluded
- provides information on psychiatric history and previous treatments
- delineates information on initial treatment efforts.
Realize how defensive a patient may feel about being given a vague and disputed diagnosis such as CFS. Because the diagnosis depends somewhat on examining his or her volitional contribution to the symptoms,6 your listening skills are key to building the patient-physician relationship. Taking the patient’s suffering seriously is essential and may provide great relief.
When you confirm a CFS diagnosis, the next step is to identify any frequently occurring psychiatric comorbidities, such as nonmelancholic depression, anxiety, and somatoform disorders.
Psychiatric Treatment
CBT and exercise. Only CBT and graded exercise therapy yielded “promising results” in a systematic review of all CFS treatments studied in 44 controlled treatment trials.11 By comparison, evidence is inconclusive or insufficient to support the use of:
- immunoglobulins or hydrocortisone
- most psychotropics—including all classes of antidepressants.
- Patients learn about their illness, develop a realistic assessment of their limitations, and come to understand that physical activity will not harm them.
- Patients begin graded exercises designed to slowly extend their exercise tolerance and widen their range of daily activities.
Trained psychologists usually do this work, and your role is to be aware of the key part this approach plays in managing CFS patients and to set up appropriate referrals.
Case continued: Relief and acceptance
Mr. A continues to see you and a therapist for treatment of mild depression and severe anxiety. His behavioral therapy focuses on helping him cope with how his illness limits his relationship to work and family. His therapist also explores with him the personal meanings of his new situation, his feelings about issues such as dependence, and limitations imposed on his life goals.
You start a trial of fluoxetine (up to 40 mg/d for several months) with minimal benefit. You then try nortriptyline, 25 mg nightly, and clonazepam, 0.5 mg bid. Although these drugs can be sedating, Mr. A reports feeling no more fatigued than he was before taking them. He improves slightly after 7 months but not enough that he wants to continue the medication.
Medications. Neither psychiatric nor other medication classes have shown efficacy in treating CFS core symptoms.15 One recent study16 found citalopram helped reduce chronic fatigue, but the study was small and uncontrolled. Although the subjects had chronic fatigue, not all met the formal definition of CFS for study inclusion.
Medication does play an important role in treating comorbid anxiety and depression. Usual psychopharmacologic strategies are appropriate. As in Mr. A’s case, most psychiatrists use SSRIs as first-line medications, but side effects are probably the most useful guide to medication choice.
Case continued: Additional treatment
Because medication has had little effect on Mr. A’s anxiety and depressed mood, you suggest adding a graded exercise program to his treatment plan. He improves steadily over time and says he is pleased. Although progress is slow, he finds it reassuring to be accomplishing realistic goals. He realizes that you and the therapist do not have the answer to his illness, but he trusts you and is comforted that you accept his condition and are willing to listen and help.
Related resources
- Afari N, Buchwald D. Chronic fatigue syndrome: a review. Am J Psychiatry 2003;160:221-36.
- Reid S, Chalder T, Cleare A, et al. Chronic fatigue syndrome. Clin Evid 2004;12:1578-93.
- Centers for Disease Control and Prevention. Chronic fatigue syndrome. http://www.cdc.gov/ncidod/diseases/cfs/.
- International Association for Chronic Fatigue Syndrome. http://www.aacfs.org.
- Citalopram • Celexa, others
- Clonazepam • Lorazepam, others
- Fluoxetine • Prozac
- Nortriptyline • Aventyl, others
The authors report no financial relationship with any company whos products are mentioned in this article or with manufacturers of competing products.
1. Kroenke K, Wood DR, Mangelsdorff AD, et al. Chronic fatigue in primary care: prevalence, patient characteristics, and outcome. JAMA 1988;260:929-34.
2. Holmes GP, Kaplan JE, Gantz NM, et al. Chronic fatigue syndrome: a working case definition. Ann Intern Med 1988;108:387-9.
3. Goshorn RK. Chronic fatigue syndrome: a review for clinicians. Semin Neurol 1998;18:237-42.
4. Afari N, Buchwald D. Chronic fatigue syndrome: a review. Am J Psychiatry 2003;160:221-36.
5. Parker AJR, Wessely S, Cleare AJ. The neuroendocrinology of chronic fatigue syndrome and fibromyalgia. Psychol Med 2001;31:1331-45.
6. Clarke JN, James S. The radicalized self: the impact on the self of the contested nature of the diagnosis of chronic fatigue syndrome. Soc Sci Med 2003;57:1387-95.
7. Demitrack MA. The psychobiology of chronic fatigue: the central nervous system as a final common pathway. In: Demitrack MA, Abbey SE (eds). Chronic fatigue syndrome: an integrative approach to evaluation and treatment. New York: Guilford Press; 1996:72-109.
8. Cassem EH. Depression and anxiety secondary to medical illness. Psychiatr Clin North Am 1990;13:597-612.
9. Lane TJ, Matthews DA, Manu P. The low yield of physical examinations and laboratory investigations of patients with chronic fatigue. Am J Med Sci 1990;299:313-18.
10. Ruffin MT, Margo GM, Margo KL. Puzzling physical conditions (monograph, edition No. 209). Home study self-assessment program. Presented at: American Academy of Family Physicians; October 1996; Kansas City, MO.
11. Whiting P, Bagnall A, Sowden A, et al. Interventions for the treatment and management of chronic fatigue syndrome: a systematic review. JAMA 2001;286:1360-8.
12. Deale A, Husain K, Chalder T, Wessely S. Long-term outcome of cognitive behavior therapy versus relaxation therapy for chronic fatigue syndrome: a 5-year follow-up study. Am J Psychiatry 2001;158:2038-42.
13. Sharpe M. Cognitive behavior therapy for chronic fatigue syndrome: efficacy and implications. Am J Med 1998;105:104S-9S.
14. Stulemeijer M, de Jong LW, Fiselier TJ, et al. Cognitive behavior therapy for adolescents with chronic fatigue syndrome: randomized control trial. BMJ 2005;330:1418.-
15. Straus SE. Pharmacotherapy of chronic fatigue syndrome: another gallant attempt. JAMA 2004;292:1234-5.
16. Hartz AJ, Bentler SE, Brake KA, Kelly MW. The effectiveness of citalopram for idiopathic chronic fatigue. J Clin Psychiatry 2003;64:927-35.
Chronic fatigue syndrome (CFS) continues to puzzle and provoke. Years of research have failed to find a biomedical cause or to answer fundamental questions, such as “What is it?” and “Does it exist?”
Up to two-thirds of CFS patients have psychiatric disorders,1 but psychiatrists are not the first physicians CFS patients usually see. Primary care physicians may ask you to help confirm the diagnosis, manage patients’ anxiety and depression, differentiate CFS from somatoform disorders, or provide psychotherapy for patients and their families.
Knowing what transpires in the referring physician’s office is key to helping a patient function despite CFS. This article describes:
- CFS clinical features and possible causes
- psychiatric comorbidities and exclusions
- cognitive behavioral therapy (CBT) and graded exercise, the only two therapies shown to improve CFS patients’ daily function.
Case: Anxious, depressed, and tired
Mr. A, age 43, is referred to you with anxiety and depressed mood associated with CFS, diagnosed 2 years ago. Fatigue predated Mr. A’s depression and anxiety, which his primary care physician considers consequences of CFS.
Mr. A is married, has three children, and owns a successful accounting practice. CFS was diagnosed from the classic presentation: abrupt onset of fatigue despite good health and a promising career. Now, overwhelming fatigue reduces his productivity. He makes up for frequent rest breaks by working in snatches of time, even at 4 AM. He is spending little time with his wife and children.
Defining CFS
The Centers for Disease Control and Prevention (CDC) defined CFS in 1988 while investigating infectious causes of fatigue (Table 1).2,3 Epstein-Barr virus was thought to be the cause of CFS.
Most patients who present to their primary care physicians with complaints of fatigue do not meet CDC criteria for CFS, however. These “non-CFS” patients are diagnosed as having “idiopathic chronic fatigue,” a term that is not particularly helpful because CFS remains an idiopathic disorder.
Clinical findings. As with Mr. A, many CFS patients’ fatigue begins suddenly, often with flu-like symptoms. Patients lose tolerance for exercise and alcohol and become unable to work or socialize at pre-illness levels.
Medical symptoms can overlap those of other conditions, such as fibromyalgia, chemical sensitivities, and irritable bowel disorder. These associations make the condition difficult to assess and contribute to some physicians’ difficulty in accepting CFS as a biomedical condition. Mistrust and a poor patient-physician relationship can result when the physician doubts the symptoms’ “medical” nature and the patient resents the implication that the suffering is “all in your head.”
What causes CFS? No consistent factor has been identified that explains the pathophysiology of CFS symptoms. Many possibilities have been examined, but the evidence is confusing and contradictory.
A few preliminary studies suggest possible familial (shared environmental) and genetic components, but data are sparse and no more than suggestive.4 Findings of CNS studies are inconsistent, and the search for a change in immune function or an infectious agent has been fruitless despite some patients’ infection-like symptoms. The early hypothesis that Epstein-Barr virus was responsible has been disproved.
Imaging studies, psychological testing, and neuroendocrine investigations have identified abnormalities in some patients with CFS. The most-promising findings point to abnormalities in the hypothalamic-pituitary-adrenal axis and in serotonergic neurotransmission,5 suggesting an abnormal stress response in some patients.
Table 1
CDC diagnostic criteria for chronic fatigue syndrome
| At least 6 months of fatigue sufficient to “substantially reduce” patient’s level of activity |
4 or more of 8 concurrent symptoms:
|
| No obvious medical or psychiatric causes, such as eating disorders, psychosis, bipolar disorder, melancholia, or substance abuse* |
| CDC: Centers for Disease Control and Prevention |
| * Many nonpsychotic psychiatric disorders (such as atypical depression) do not exclude a CFS diagnosis |
| Source: References 1 and 2 |
Case continued: Test results are normal
Mr. A has undergone extensive medical assessment (complete blood cell count; renal, hepatic, and thyroid function tests; calcium, phosphate, and glucose determinations; and urinalysis), which yielded normal results. Brain MRI findings were also normal; specifically, no evidence of multiple sclerosis.
Even so, he has had nonspecific symptoms of impaired concentration, sore throat, tender cervical nodes, muscle pain, and nonrefreshing sleep. Physical exertion can leave him drained for at least 1 or 2 days.
Psychiatric Disorders
As many as 66% of CFS patients may have one or more psychiatric comorbidities; the most common are generalized anxiety disorder, panic disorder, depression, and somatoform disorder.1 Because CFS symptoms are regarded as being not fully explained by a known medical disorder, patients are often diagnosed as having an undifferentiated somatoform disorder. Either disorder could be diagnosed in some cases, but this differentiation sheds no new light on the condition.
CFS and depression. Could CFS and depression be one and the same? Proponents of that position point out the similarity of symptoms, loss of function, and—in at least some cases—favorable response to antidepressants. Opponents cite other factors such as:
- presence of sore throat, lymphadenopathy, and post-exercise fatigue
- differences in sleep patterns
- frequent absence of psychiatric illness before fatigue onset
- evidence of hypocortisolism (also seen in patients with melancholic depression) in some CFS patients.
Primary Care Workup
Complaints of long-lasting, debilitating fatigue should alert the primary care physician to CFS. Like somatization disorder, CFS requires a physical workup, though as few as 2% of CFS patients are found to have an undiagnosed medical illness that explains the symptoms.9 The evaluation’s goal is not so much to find out what’s causing the fatigue as to reassure the patient that all avenues are considered before the diagnosis is made. When this is accomplished well, the patient is likely to accept psychiatric referral or treatment, if needed.
Two-part initial evaluation. If the initial physical exam and laboratory work find no biomedical cause for the patient’s chronic fatigue symptoms, we recommend a two-part primary care evaluation. This includes a focused discussion with the patient about CFS (Table 2).10 Goals of the first session are to:
- establish a relationship that will survive difficult times
- teach the patient to think of complex medical problems as having psychological and social consequences, if not causes.
Primary care physicians usually request a psychiatric consultation to confirm or rule out psychiatric conditions that exclude a CFS diagnosis (melancholic depression, bipolar disorder, schizophrenia, anorexia nervosa or bulimia, and recent substance abuse). They also may refer in cases of other common disorders with poorly explained symptoms such as fibromyalgia and chemical sensitivity disorder.
Table 2
Two-part initial primary care evaluation of chronic fatigue
First session
|
Case continued: High anxiety
Mr. A describes how fatigue is affecting his work and home life. He is especially worried that he will not be attentive enough to catch accounting errors by his employees.
Interestingly, his anxiety remits but fatigue continues when he goes on vacation. He has no history of melancholic depression, bipolar disorder, psychosis, or substance abuse.
Psychiatric Assessment
The referring physician should provide a full account of the medical workup. This:
- assures you that possible medical causes of fatigue have been excluded
- provides information on psychiatric history and previous treatments
- delineates information on initial treatment efforts.
Realize how defensive a patient may feel about being given a vague and disputed diagnosis such as CFS. Because the diagnosis depends somewhat on examining his or her volitional contribution to the symptoms,6 your listening skills are key to building the patient-physician relationship. Taking the patient’s suffering seriously is essential and may provide great relief.
When you confirm a CFS diagnosis, the next step is to identify any frequently occurring psychiatric comorbidities, such as nonmelancholic depression, anxiety, and somatoform disorders.
Psychiatric Treatment
CBT and exercise. Only CBT and graded exercise therapy yielded “promising results” in a systematic review of all CFS treatments studied in 44 controlled treatment trials.11 By comparison, evidence is inconclusive or insufficient to support the use of:
- immunoglobulins or hydrocortisone
- most psychotropics—including all classes of antidepressants.
- Patients learn about their illness, develop a realistic assessment of their limitations, and come to understand that physical activity will not harm them.
- Patients begin graded exercises designed to slowly extend their exercise tolerance and widen their range of daily activities.
Trained psychologists usually do this work, and your role is to be aware of the key part this approach plays in managing CFS patients and to set up appropriate referrals.
Case continued: Relief and acceptance
Mr. A continues to see you and a therapist for treatment of mild depression and severe anxiety. His behavioral therapy focuses on helping him cope with how his illness limits his relationship to work and family. His therapist also explores with him the personal meanings of his new situation, his feelings about issues such as dependence, and limitations imposed on his life goals.
You start a trial of fluoxetine (up to 40 mg/d for several months) with minimal benefit. You then try nortriptyline, 25 mg nightly, and clonazepam, 0.5 mg bid. Although these drugs can be sedating, Mr. A reports feeling no more fatigued than he was before taking them. He improves slightly after 7 months but not enough that he wants to continue the medication.
Medications. Neither psychiatric nor other medication classes have shown efficacy in treating CFS core symptoms.15 One recent study16 found citalopram helped reduce chronic fatigue, but the study was small and uncontrolled. Although the subjects had chronic fatigue, not all met the formal definition of CFS for study inclusion.
Medication does play an important role in treating comorbid anxiety and depression. Usual psychopharmacologic strategies are appropriate. As in Mr. A’s case, most psychiatrists use SSRIs as first-line medications, but side effects are probably the most useful guide to medication choice.
Case continued: Additional treatment
Because medication has had little effect on Mr. A’s anxiety and depressed mood, you suggest adding a graded exercise program to his treatment plan. He improves steadily over time and says he is pleased. Although progress is slow, he finds it reassuring to be accomplishing realistic goals. He realizes that you and the therapist do not have the answer to his illness, but he trusts you and is comforted that you accept his condition and are willing to listen and help.
Related resources
- Afari N, Buchwald D. Chronic fatigue syndrome: a review. Am J Psychiatry 2003;160:221-36.
- Reid S, Chalder T, Cleare A, et al. Chronic fatigue syndrome. Clin Evid 2004;12:1578-93.
- Centers for Disease Control and Prevention. Chronic fatigue syndrome. http://www.cdc.gov/ncidod/diseases/cfs/.
- International Association for Chronic Fatigue Syndrome. http://www.aacfs.org.
- Citalopram • Celexa, others
- Clonazepam • Lorazepam, others
- Fluoxetine • Prozac
- Nortriptyline • Aventyl, others
The authors report no financial relationship with any company whos products are mentioned in this article or with manufacturers of competing products.
Chronic fatigue syndrome (CFS) continues to puzzle and provoke. Years of research have failed to find a biomedical cause or to answer fundamental questions, such as “What is it?” and “Does it exist?”
Up to two-thirds of CFS patients have psychiatric disorders,1 but psychiatrists are not the first physicians CFS patients usually see. Primary care physicians may ask you to help confirm the diagnosis, manage patients’ anxiety and depression, differentiate CFS from somatoform disorders, or provide psychotherapy for patients and their families.
Knowing what transpires in the referring physician’s office is key to helping a patient function despite CFS. This article describes:
- CFS clinical features and possible causes
- psychiatric comorbidities and exclusions
- cognitive behavioral therapy (CBT) and graded exercise, the only two therapies shown to improve CFS patients’ daily function.
Case: Anxious, depressed, and tired
Mr. A, age 43, is referred to you with anxiety and depressed mood associated with CFS, diagnosed 2 years ago. Fatigue predated Mr. A’s depression and anxiety, which his primary care physician considers consequences of CFS.
Mr. A is married, has three children, and owns a successful accounting practice. CFS was diagnosed from the classic presentation: abrupt onset of fatigue despite good health and a promising career. Now, overwhelming fatigue reduces his productivity. He makes up for frequent rest breaks by working in snatches of time, even at 4 AM. He is spending little time with his wife and children.
Defining CFS
The Centers for Disease Control and Prevention (CDC) defined CFS in 1988 while investigating infectious causes of fatigue (Table 1).2,3 Epstein-Barr virus was thought to be the cause of CFS.
Most patients who present to their primary care physicians with complaints of fatigue do not meet CDC criteria for CFS, however. These “non-CFS” patients are diagnosed as having “idiopathic chronic fatigue,” a term that is not particularly helpful because CFS remains an idiopathic disorder.
Clinical findings. As with Mr. A, many CFS patients’ fatigue begins suddenly, often with flu-like symptoms. Patients lose tolerance for exercise and alcohol and become unable to work or socialize at pre-illness levels.
Medical symptoms can overlap those of other conditions, such as fibromyalgia, chemical sensitivities, and irritable bowel disorder. These associations make the condition difficult to assess and contribute to some physicians’ difficulty in accepting CFS as a biomedical condition. Mistrust and a poor patient-physician relationship can result when the physician doubts the symptoms’ “medical” nature and the patient resents the implication that the suffering is “all in your head.”
What causes CFS? No consistent factor has been identified that explains the pathophysiology of CFS symptoms. Many possibilities have been examined, but the evidence is confusing and contradictory.
A few preliminary studies suggest possible familial (shared environmental) and genetic components, but data are sparse and no more than suggestive.4 Findings of CNS studies are inconsistent, and the search for a change in immune function or an infectious agent has been fruitless despite some patients’ infection-like symptoms. The early hypothesis that Epstein-Barr virus was responsible has been disproved.
Imaging studies, psychological testing, and neuroendocrine investigations have identified abnormalities in some patients with CFS. The most-promising findings point to abnormalities in the hypothalamic-pituitary-adrenal axis and in serotonergic neurotransmission,5 suggesting an abnormal stress response in some patients.
Table 1
CDC diagnostic criteria for chronic fatigue syndrome
| At least 6 months of fatigue sufficient to “substantially reduce” patient’s level of activity |
4 or more of 8 concurrent symptoms:
|
| No obvious medical or psychiatric causes, such as eating disorders, psychosis, bipolar disorder, melancholia, or substance abuse* |
| CDC: Centers for Disease Control and Prevention |
| * Many nonpsychotic psychiatric disorders (such as atypical depression) do not exclude a CFS diagnosis |
| Source: References 1 and 2 |
Case continued: Test results are normal
Mr. A has undergone extensive medical assessment (complete blood cell count; renal, hepatic, and thyroid function tests; calcium, phosphate, and glucose determinations; and urinalysis), which yielded normal results. Brain MRI findings were also normal; specifically, no evidence of multiple sclerosis.
Even so, he has had nonspecific symptoms of impaired concentration, sore throat, tender cervical nodes, muscle pain, and nonrefreshing sleep. Physical exertion can leave him drained for at least 1 or 2 days.
Psychiatric Disorders
As many as 66% of CFS patients may have one or more psychiatric comorbidities; the most common are generalized anxiety disorder, panic disorder, depression, and somatoform disorder.1 Because CFS symptoms are regarded as being not fully explained by a known medical disorder, patients are often diagnosed as having an undifferentiated somatoform disorder. Either disorder could be diagnosed in some cases, but this differentiation sheds no new light on the condition.
CFS and depression. Could CFS and depression be one and the same? Proponents of that position point out the similarity of symptoms, loss of function, and—in at least some cases—favorable response to antidepressants. Opponents cite other factors such as:
- presence of sore throat, lymphadenopathy, and post-exercise fatigue
- differences in sleep patterns
- frequent absence of psychiatric illness before fatigue onset
- evidence of hypocortisolism (also seen in patients with melancholic depression) in some CFS patients.
Primary Care Workup
Complaints of long-lasting, debilitating fatigue should alert the primary care physician to CFS. Like somatization disorder, CFS requires a physical workup, though as few as 2% of CFS patients are found to have an undiagnosed medical illness that explains the symptoms.9 The evaluation’s goal is not so much to find out what’s causing the fatigue as to reassure the patient that all avenues are considered before the diagnosis is made. When this is accomplished well, the patient is likely to accept psychiatric referral or treatment, if needed.
Two-part initial evaluation. If the initial physical exam and laboratory work find no biomedical cause for the patient’s chronic fatigue symptoms, we recommend a two-part primary care evaluation. This includes a focused discussion with the patient about CFS (Table 2).10 Goals of the first session are to:
- establish a relationship that will survive difficult times
- teach the patient to think of complex medical problems as having psychological and social consequences, if not causes.
Primary care physicians usually request a psychiatric consultation to confirm or rule out psychiatric conditions that exclude a CFS diagnosis (melancholic depression, bipolar disorder, schizophrenia, anorexia nervosa or bulimia, and recent substance abuse). They also may refer in cases of other common disorders with poorly explained symptoms such as fibromyalgia and chemical sensitivity disorder.
Table 2
Two-part initial primary care evaluation of chronic fatigue
First session
|
Case continued: High anxiety
Mr. A describes how fatigue is affecting his work and home life. He is especially worried that he will not be attentive enough to catch accounting errors by his employees.
Interestingly, his anxiety remits but fatigue continues when he goes on vacation. He has no history of melancholic depression, bipolar disorder, psychosis, or substance abuse.
Psychiatric Assessment
The referring physician should provide a full account of the medical workup. This:
- assures you that possible medical causes of fatigue have been excluded
- provides information on psychiatric history and previous treatments
- delineates information on initial treatment efforts.
Realize how defensive a patient may feel about being given a vague and disputed diagnosis such as CFS. Because the diagnosis depends somewhat on examining his or her volitional contribution to the symptoms,6 your listening skills are key to building the patient-physician relationship. Taking the patient’s suffering seriously is essential and may provide great relief.
When you confirm a CFS diagnosis, the next step is to identify any frequently occurring psychiatric comorbidities, such as nonmelancholic depression, anxiety, and somatoform disorders.
Psychiatric Treatment
CBT and exercise. Only CBT and graded exercise therapy yielded “promising results” in a systematic review of all CFS treatments studied in 44 controlled treatment trials.11 By comparison, evidence is inconclusive or insufficient to support the use of:
- immunoglobulins or hydrocortisone
- most psychotropics—including all classes of antidepressants.
- Patients learn about their illness, develop a realistic assessment of their limitations, and come to understand that physical activity will not harm them.
- Patients begin graded exercises designed to slowly extend their exercise tolerance and widen their range of daily activities.
Trained psychologists usually do this work, and your role is to be aware of the key part this approach plays in managing CFS patients and to set up appropriate referrals.
Case continued: Relief and acceptance
Mr. A continues to see you and a therapist for treatment of mild depression and severe anxiety. His behavioral therapy focuses on helping him cope with how his illness limits his relationship to work and family. His therapist also explores with him the personal meanings of his new situation, his feelings about issues such as dependence, and limitations imposed on his life goals.
You start a trial of fluoxetine (up to 40 mg/d for several months) with minimal benefit. You then try nortriptyline, 25 mg nightly, and clonazepam, 0.5 mg bid. Although these drugs can be sedating, Mr. A reports feeling no more fatigued than he was before taking them. He improves slightly after 7 months but not enough that he wants to continue the medication.
Medications. Neither psychiatric nor other medication classes have shown efficacy in treating CFS core symptoms.15 One recent study16 found citalopram helped reduce chronic fatigue, but the study was small and uncontrolled. Although the subjects had chronic fatigue, not all met the formal definition of CFS for study inclusion.
Medication does play an important role in treating comorbid anxiety and depression. Usual psychopharmacologic strategies are appropriate. As in Mr. A’s case, most psychiatrists use SSRIs as first-line medications, but side effects are probably the most useful guide to medication choice.
Case continued: Additional treatment
Because medication has had little effect on Mr. A’s anxiety and depressed mood, you suggest adding a graded exercise program to his treatment plan. He improves steadily over time and says he is pleased. Although progress is slow, he finds it reassuring to be accomplishing realistic goals. He realizes that you and the therapist do not have the answer to his illness, but he trusts you and is comforted that you accept his condition and are willing to listen and help.
Related resources
- Afari N, Buchwald D. Chronic fatigue syndrome: a review. Am J Psychiatry 2003;160:221-36.
- Reid S, Chalder T, Cleare A, et al. Chronic fatigue syndrome. Clin Evid 2004;12:1578-93.
- Centers for Disease Control and Prevention. Chronic fatigue syndrome. http://www.cdc.gov/ncidod/diseases/cfs/.
- International Association for Chronic Fatigue Syndrome. http://www.aacfs.org.
- Citalopram • Celexa, others
- Clonazepam • Lorazepam, others
- Fluoxetine • Prozac
- Nortriptyline • Aventyl, others
The authors report no financial relationship with any company whos products are mentioned in this article or with manufacturers of competing products.
1. Kroenke K, Wood DR, Mangelsdorff AD, et al. Chronic fatigue in primary care: prevalence, patient characteristics, and outcome. JAMA 1988;260:929-34.
2. Holmes GP, Kaplan JE, Gantz NM, et al. Chronic fatigue syndrome: a working case definition. Ann Intern Med 1988;108:387-9.
3. Goshorn RK. Chronic fatigue syndrome: a review for clinicians. Semin Neurol 1998;18:237-42.
4. Afari N, Buchwald D. Chronic fatigue syndrome: a review. Am J Psychiatry 2003;160:221-36.
5. Parker AJR, Wessely S, Cleare AJ. The neuroendocrinology of chronic fatigue syndrome and fibromyalgia. Psychol Med 2001;31:1331-45.
6. Clarke JN, James S. The radicalized self: the impact on the self of the contested nature of the diagnosis of chronic fatigue syndrome. Soc Sci Med 2003;57:1387-95.
7. Demitrack MA. The psychobiology of chronic fatigue: the central nervous system as a final common pathway. In: Demitrack MA, Abbey SE (eds). Chronic fatigue syndrome: an integrative approach to evaluation and treatment. New York: Guilford Press; 1996:72-109.
8. Cassem EH. Depression and anxiety secondary to medical illness. Psychiatr Clin North Am 1990;13:597-612.
9. Lane TJ, Matthews DA, Manu P. The low yield of physical examinations and laboratory investigations of patients with chronic fatigue. Am J Med Sci 1990;299:313-18.
10. Ruffin MT, Margo GM, Margo KL. Puzzling physical conditions (monograph, edition No. 209). Home study self-assessment program. Presented at: American Academy of Family Physicians; October 1996; Kansas City, MO.
11. Whiting P, Bagnall A, Sowden A, et al. Interventions for the treatment and management of chronic fatigue syndrome: a systematic review. JAMA 2001;286:1360-8.
12. Deale A, Husain K, Chalder T, Wessely S. Long-term outcome of cognitive behavior therapy versus relaxation therapy for chronic fatigue syndrome: a 5-year follow-up study. Am J Psychiatry 2001;158:2038-42.
13. Sharpe M. Cognitive behavior therapy for chronic fatigue syndrome: efficacy and implications. Am J Med 1998;105:104S-9S.
14. Stulemeijer M, de Jong LW, Fiselier TJ, et al. Cognitive behavior therapy for adolescents with chronic fatigue syndrome: randomized control trial. BMJ 2005;330:1418.-
15. Straus SE. Pharmacotherapy of chronic fatigue syndrome: another gallant attempt. JAMA 2004;292:1234-5.
16. Hartz AJ, Bentler SE, Brake KA, Kelly MW. The effectiveness of citalopram for idiopathic chronic fatigue. J Clin Psychiatry 2003;64:927-35.
1. Kroenke K, Wood DR, Mangelsdorff AD, et al. Chronic fatigue in primary care: prevalence, patient characteristics, and outcome. JAMA 1988;260:929-34.
2. Holmes GP, Kaplan JE, Gantz NM, et al. Chronic fatigue syndrome: a working case definition. Ann Intern Med 1988;108:387-9.
3. Goshorn RK. Chronic fatigue syndrome: a review for clinicians. Semin Neurol 1998;18:237-42.
4. Afari N, Buchwald D. Chronic fatigue syndrome: a review. Am J Psychiatry 2003;160:221-36.
5. Parker AJR, Wessely S, Cleare AJ. The neuroendocrinology of chronic fatigue syndrome and fibromyalgia. Psychol Med 2001;31:1331-45.
6. Clarke JN, James S. The radicalized self: the impact on the self of the contested nature of the diagnosis of chronic fatigue syndrome. Soc Sci Med 2003;57:1387-95.
7. Demitrack MA. The psychobiology of chronic fatigue: the central nervous system as a final common pathway. In: Demitrack MA, Abbey SE (eds). Chronic fatigue syndrome: an integrative approach to evaluation and treatment. New York: Guilford Press; 1996:72-109.
8. Cassem EH. Depression and anxiety secondary to medical illness. Psychiatr Clin North Am 1990;13:597-612.
9. Lane TJ, Matthews DA, Manu P. The low yield of physical examinations and laboratory investigations of patients with chronic fatigue. Am J Med Sci 1990;299:313-18.
10. Ruffin MT, Margo GM, Margo KL. Puzzling physical conditions (monograph, edition No. 209). Home study self-assessment program. Presented at: American Academy of Family Physicians; October 1996; Kansas City, MO.
11. Whiting P, Bagnall A, Sowden A, et al. Interventions for the treatment and management of chronic fatigue syndrome: a systematic review. JAMA 2001;286:1360-8.
12. Deale A, Husain K, Chalder T, Wessely S. Long-term outcome of cognitive behavior therapy versus relaxation therapy for chronic fatigue syndrome: a 5-year follow-up study. Am J Psychiatry 2001;158:2038-42.
13. Sharpe M. Cognitive behavior therapy for chronic fatigue syndrome: efficacy and implications. Am J Med 1998;105:104S-9S.
14. Stulemeijer M, de Jong LW, Fiselier TJ, et al. Cognitive behavior therapy for adolescents with chronic fatigue syndrome: randomized control trial. BMJ 2005;330:1418.-
15. Straus SE. Pharmacotherapy of chronic fatigue syndrome: another gallant attempt. JAMA 2004;292:1234-5.
16. Hartz AJ, Bentler SE, Brake KA, Kelly MW. The effectiveness of citalopram for idiopathic chronic fatigue. J Clin Psychiatry 2003;64:927-35.
2 NAMES, 1 DISEASE: Does schizophrenia = psychotic bipolar disorder?
When a patient presents with psychotic symptoms, you might not recognize or pursue hints of bipolarity if you assume psychosis means schizophrenia. Yet psychotic bipolar disorder can explain every sign, symptom, course, and other characteristic traditionally assumed to indicate schizophrenia (Table 1). The literature, including recent genetic data,1-6 marshals a persuasive argument that patients diagnosed with schizophrenia usually suffer from a psychotic bipolar disorder.
Consider here how a cascade of changing signs and symptoms, initially unrecognized, caused five sequential re-evaluations of one psychotic patient’s primary Axis I diagnosis. His case highlights why the correct initial diagnosis of the disease causing psychosis is essential to effective treatment.4,7-9
Table 1
DSM-IV-TR criteria for schizophrenia vs. psychotic mood disorder
| Schizophrenia diagnosis6 | Seen in psychotic mood disorders |
|---|---|
| Criterion A | |
| Hallucinations and delusions | 50% to 80% explained by mood16,21 |
| Paranoia | Hides grandiosity4 |
| Catatonia | 75% explained by mood7,8 |
| Disorganized speech and behavior | All patients with moderate to severe mania1-5 |
| Negative symptoms | All patients with moderate to severe depression4 |
| Criterion B | |
| Social and job dysfunction | All patients with moderate to severe bipolar disorder5,13 |
| Criterion C | |
| Chronic continuous symptoms | Patients can have psychotic symptoms continuously for 2 years to life5,6,13 |
Case: Carved in stone
Police officers carry Mr. C, age 30, into the emergency department. He is mentally disorganized and arrives in a rigid, catatonic posture. According to a neighbor, Mr. C was kneeling motionless on his mother’s front lawn, alternating between mutism and inappropriately loud, disorganized religious preaching. When his arm is lifted, it remains as placed. He is admitted to the acute care inpatient unit.
Mr. C’s most striking symptoms are catatonia and psychosis. Postural rigidity, waxy flexibility, and automatic obedience are characteristics of catatonia.6-8 An organic cause is first considered, such as hyperthyroidism, cerebrovascular accident, cerebral neoplasm, head trauma, seizure disorder, dementia, neuroleptic malignant syndrome, pheochromocytoma, or—especially—intoxication from illegal drugs.7
While awaiting results from physical, mental status, and lab exams and imaging studies, staff assign him two admitting diagnoses: catatonic disorder due to a general medical condition and psychotic disorder not otherwise specified.6
Case: Inconclusive workup
Mr. C denies using illegal substances or alcohol, which his mother confirms. He has no history of seizures or other medical conditions. His distractibility prevents him from focusing on a formal mental status exam. Physical exam, urine drug screen, lab results, and imaging studies are unremarkable except for an admitting blood pressure of 145/95 mm Hg and pulse of 115 beats per minute. These readings normalize within 1 hour. IM haloperidol and lorazepam are given as needed for agitation, but physicians withhold scheduled medications to allow staff to observe his symptoms.
Organic causes of catatonia now seem less likely, though past use of drugs such as phencyclidine that can cause chronic psychosis cannot be ruled out. Schizophrenia is considered likely because catatonia is one of schizophrenia’s five core diagnostic symptoms.6 Catatonia can also be a symptom of bipolar disorder.6-9 Staff make a preliminary diagnosis of schizophrenia, catatonic type.
Case: ‘Hit men are after me’
Staff observe Mr. C responding to threatening auditory hallucinations. His affect is “fearful to terrified.” He says he hears the voice of God warning him of danger and continuing a running commentary on his actions. He fears for his life because “hit men have been sent to kill me” and have “infiltrated” the inpatient ward. He does not eat, saying his food is poisoned. He says these beliefs have escalated over the past year.
Mr. C’s catatonic symptoms resolve overnight, but obtaining additional history is difficult because of his paranoia. He denies any history of bizarre behavior or past contact with mental health services. He claims not to be especially religious. He is unmarried and lives with his mother, is college-educated, but has held only menial jobs.
Inpatient staff shifts its diagnostic focus to functional disorders associated with auditory hallucinations, paranoid delusions, and gross disorganization. According to Schneider and the DSM-IV-TR,6,10 hearing a voice “keeping up a running commentary on one’s behavior” is especially diagnostic of schizophrenia.
Because of the rapid resolution of his “catatonic” symptoms and prominence of paranoia, they change his diagnosis on day 2 to schizophrenia, paranoid type. Mr. C meets all diagnostic criteria for schizophrenia except one: the staff has overlooked and has not adequately excluded a psychotic mood disorder.
Case: A turn for the worse
That night, nursing staff find Mr. C naked and cowering in the fetal position in a corner of his room. He has smeared his feces on his face and in his hair and mouth. While being cleaned up, he suddenly begins quoting scripture in a loud, disorganized voice. His expressed thoughts are incomprehensible. He is given haloperidol and lorazepam immediately; oral haloperidol is continued at 10 mg bid.
Both Bleuler and Kraepelin concluded “coprophilia and coprophagia are unique to children and patients with schizophrenia.”11,12 The DSM casebook cites Kraepelin’s description of a catatonic patient who “smeared feces about” as a “classic, textbook case” of schizophrenia.11 The casebook goes on to say: “In the absence of any known general medical condition, the combination of coprophilia, disorganized speech, and catatonic behavior clearly indicates the diagnosis of schizophrenia.”
Mr. C shows each of these. Staff changes his diagnosis again—to schizophrenia, disorganized type, which carries a poor prognosis.11,12
Case: Banking and ray guns
By day 5, Mr. C’s mental status is normalizing and his psychosis improving. He volunteers for a weekly student case conference. There, he reveals additional information that staff could have discovered at admission with more-focused questions.
He reports that 2 years earlier he suffered severe suicidal depression. Six months later, during a hypomanic episode, he began “toying with the idea” that he might become part owner of his local bank. He believes “the Secret Service decided to transfer ownership to me.”
His plans upon acquiring the bank include buying three houses and six cars valued at several million dollars and running for state governor. For weeks before admission, he did not need sleep, experienced an increase in energy and activities, and his mind was racing. His job seemed so “trivial” that he quit. Immediately before his hospital admission, his delusions intensified to include an “evil conspiracy” to murder him for ownership of the bank and he feared his execution was imminent.
He explains his catatonic behavior on the lawn by his belief that “hit men” hiding across the street aimed a “motion-detecting, heat-seeking ray gun” at him so that if he had “moved an inch,” he would die. He says the “feces incident” was an effort to get himself transferred to the state hospital, where he thought he would be safer because his present caretakers were “infiltrated.” He also says his mother received electroconvulsive therapy in her 20s.
These symptoms—especially the striking grandiosity, lack of need for sleep, racing thoughts, hallucinations and delusions—define a manic episode with psychotic features. Only one manic episode as described here is diagnostic of bipolar disorder, type I.2,6,13 Staff changes his diagnosis to schizoaffective disorder, a compromise used to include patients with bipolar and psychotic (schizophrenic) features. Some authors contend schizoaffective disorder is psychotic bipolar disorder and not a separate disease.3,4,9
Case: From SSRI to lithium
After 2 weeks, Mr. C is discharged on haloperidol, 5 mg bid, but no mood stabilizer. He receives follow-up care at a community mental health center. When he develops severe depressive symptoms 6 months after discharge, the attending psychiatrist starts him on a selective serotonin reuptake inhibitor (SSRI). Within 2 weeks, Mr. C switches from depression to a mixed, dysphoric mania. After the SSRI is discontinued and lithium is added to his haloperidol, his mood gradually stabilizes to moderate depression. He develops rigidity, masked faces, and a fine tremor in his hands.
About 10% of bipolar depressed patients given an antidepressant—especially without a mood stabilizer—switch to mania, and their cycle frequency increases.2,13-15 A correct initial diagnosis and treatment with a mood stabilizer might have avoided Mr. C’s switch.
Mixed bipolar disorder with overlapping depressive and manic symptoms is often resistant to monotherapy, requiring two or more mood stabilizers such as lithium and an anticonvulsant.14 Without a mood-stabilizing combination, the mixed, rapid-cycling type of bipolar disorder is likely to progress, with more-rapid and more-severe episodes.2,13-15 Adding lamotrigine, a mood stabilizer with antidepressant effects, can help.2,14
Stopping the SSRI is correct, despite Mr. C’s severe depression, to avoid increasing the cycle frequency.13-15 Some authors recommend tapering the antipsychotic, using it only as needed for psychotic features after psychosis has resolved.14-17 Continuing antipsychotic drugs after psychosis has remitted increases rates of cycling to depression, depressive and extrapyramidal symptoms, and medication discontinuation.17 Lithium may have aggravated Mr. C’s antipsychotic-induced parkinsonism, but discontinuing haloperidol may have been the most therapeutic decision.
The community mental health staff changes his diagnosis again, this time to bipolar disorder, type I, mixed, severe with psychotic features. We concur that this is correct.
Case: A diagnostic step back
Two years later, Mr. C is working and continues to take lithium and haloperidol prescribed at the mental health center. His intermittent depressive episodes persist, but—apparently because he has not had another manic episode—the staff switches his diagnosis back to schizoaffective disorder.
We disagree with this change. A diagnosis of schizoaffective disorder precludes ideal pharmacotherapy for Mr. C’s rapid-cycling bipolar disorder and increases the risk of adverse drug effects and stigma. Persuasive evidence shows that schizoaffective disorder is psychotic bipolar disorder; there is no schizoaffective disorder (Box).3,4,16-18
Three disorders—schizophrenia, schizoaffective disorder, and psychotic bipolar disorder—have been evoked to account for the variance in severity in psychotic patients, but psychotic bipolar disorder expresses the entire spectrum. We concur with others that psychotic bipolar disorder includes patient populations typically diagnosed as having schizophrenia and schizoaffective disorder.3,4,9,16-18 In other words, there is no schizophrenia or schizoaffective disorder.4,19
Based on these data, we advocate re-evaluating all patients diagnosed with schizoaffective disorder and schizophrenia, with detailed inquiry for personal and family histories of mania or hypomania. A mood stabilizer may be warranted in some patients with psychosis but without clear manic symptoms. In such cases, we suggest using a provisional DSM-IV-TR diagnosis of psychotic disorder not otherwise specified while you seek obscure mood and/or organic causes.
Misdiagnosis of psychosis
Bipolar disorder can be missed when patients present with psychotic symptoms, but clinicians could have initially recognized Mr. C’s bipolar disorder. His diagnostic trail illustrates important points about psychotic presentations:
- Predominant psychotic symptoms can obscure mood disturbances.
- Mistakenly believing that psychosis means schizophrenia can jeopardize patient care.
- When paranoia and fear hide grandiosity, then mania—not schizophrenia—is likely.
- Psychotic mood disorders—not schizophrenia—cause functional psychosis; there is no schizophrenia (Box).
- Pursuing mood symptoms in psychotic presentations is critical in an initial diagnostic interview.
Bipolar disorder has a broad spectrum of severity and course; it frequently reaches psychotic levels that can become chronic.2,5,21 Psychotic symptoms of rigorously diagnosed bipolar patients can deteriorate until their overwhelming psychosis obscures bipolar symptoms.5,6,13,21 Like most, if not all, acutely psychotic bipolar patients, Mr. C shows all diagnostic criteria for schizophrenia.1-6,21
Patients with severe, psychotic bipolar disorder can stop responding to medication and suffer chronic deterioration without remission.5,21 They can lose their jobs, families, friends, and health until they are homeless, hungry, sick, and psychotic. A deteriorating course such as this has typically defined the schizophrenic process, but this concept has been reassessed.1-6,13,15
Most, but not all, bipolar type I patients experience psychosis. Mr. C’s bipolar symptoms were not initially obvious because of predominant psychosis and were revealed only with specific, focused questions. Without the student case conference, his diagnosis might have remained schizophrenia. His treatment would have remained substandard because of the conventional belief that schizophrenia requires lifelong antipsychotics, usually without mood stabilizers.
Our patient satisfied all DSM-IV-TR criteria for both schizophrenia and psychotic bipolar. Bleuler and Schneider would have diagnosed him as having schizophrenia because they thought all psychotic disorders were schizophrenic.10,12 They were incorrect, as psychotic symptoms are common in patients with severe bipolar disorder.1-6,13,22
Cinical implications
Our observations about this case suggest four important clinical questions:
- Do data justify diagnosing patients such as Mr. C with bipolar disorder and not schizophrenia?
- Do data substantiate either diagnosis as valid?
- Does the diagnosis matter?
- What is standard-of-care treatment for these patients?
Evidence for validity? Bipolar disorder’s two extremes in mood and behavior are so different from those in persons without bipolar disorder or with any other condition that homogeneous bipolar populations can be identified and studied with confidence.2,5,13,21 DSM-IV-TR diagnostic symptoms for bipolar disorder are unique (Table 2).
For a psychiatric disorder to be considered valid, patients must share other characteristics. Bipolar disorder has been validated as a specific disease by consistent genetic,1,13,23,24 pharmacologic,2,14,15 and epidemiologic1 data accumulated across 30 years. The concordance for bipolar disorder in monozygotic twins is approximately 75%, and susceptibility loci for bipolar disorder are established.23,24
Table 2
Characteristics indicating a mood disorder, not schizophrenia*
| History | Past diagnosis or symptoms of a mood disorder; family history of mood disorder or alcoholism |
| Past medications | Lithium, valproic acid, or other mood stabilizers |
| Periods of uncharacteristic and excessive goal-directed activities | Political, religious, legal, sexual, business, criminal, medical, physical, spending, calling, writing, preaching, cleaning, planning, exercise |
| Presence of uncharacteristic emotions or conflict | Irritability, anger, violence, conflict with law enforcement, elation, grandiosity (paranoia), sadness, hopelessness, crying, suicidal ideation |
| Periods of appropriate affect | Smiles, laughs, cries, irritable, angry |
| Mood-congruent delusions and/or hallucinations | Consider grandiosity when there is paranoia and fear |
| Episodes of relatively normal function/remission; premorbid personality positive | Friends, dating, team sports, group activities, election to an office/title, club or gang memberships |
| Current social interactions | Enjoys a friendship, active interactions with spouse and own children, regular interactions with others |
| *Absence of any or all does not rule out mood disorder. | |
Does the diagnosis matter? Failing to make an accurate initial diagnosis can worsen the course of patients who present with psychosis (Table 3):
- Bipolar illness not treated with mood stabilizers progresses, with episodes becoming more frequent and severe.2,14,15
- Antipsychotics are given longer and in higher dosages for schizophrenia than for psychotic bipolar disorder and tend to have more common, chronic, and disabling adverse effects than do antidepressants and mood stabilizers.14,16
- Mr. C was given an antidepressant without mood stabilization, which is contraindicated in bipolar I disorder (especially mixed type) because the cycling rate increases.2,14,15
Several initial signs could have raised suspicion that Mr. C had psychotic bipolar disorder (Table 4). Standard-of-care treatment in psychotic patients is predicated on early and correct diagnosis. On the basis of the evidence and our experience, we recommend that you look for bipolar symptoms when a patient:
- presents for the first time with psychosis, and you rule out an organic cause
- is readmitted for treatment of psychotic symptoms after having been diagnosed with schizophrenia.
Consequences of misdiagnosing psychotic mood disorder as schizophrenia
For patient
|
For clinician
|
Mr. C’s symptoms that indicated bipolar disorder
| Religiosity | Loud preaching and no past special interest in religion |
| Catatonia | Most frequently associated with bipolar disorder |
| Paranoia; fear | Usually hides grandiosity, which is diagnostic of mania |
| Distractibility | Could not stay focused in the diagnostic interview; showed ‘flight of ideas’ |
| Pressured speech | Rapid, disorganized thoughts |
| Disorganization | Hallmark of mania; present in all patients with severe mania |
| Functional psychosis | If an organic cause is ruled out, a psychotic mood disorder is the most likely diagnosis |
| Trouble with the law | Police found patient disturbing neighborhood and escorted him to hospital |
| Patient history | Severe depression |
| Family history | Mother was treated for depression with ECT |
| ECT: electroconvulsive therapy | |
- an antipsychotic, with or without a benzodiazepine for sedation, to enhance ward safety and treat acute psychotic symptoms
- and a first-line mood stabilizer such as valproate, carbamazepine, lithium, or lamotrigine, followed by atypical antipsychotics.
The idea that “symptoms should be treated, not the diagnosis” is inaccurate and provides substandard care. When psychotic symptoms overwhelm and obscure bipolar symptoms, giving only antipsychotics is beyond standard of care.
Related resources
- Berrettini WH. Molecular linkage studies of bipolar disorders. Bipolar Disord 2001;3:276-83.
- Lake CR, Hurwitz N. Schizoaffective disorders are psychotic mood disorders; there are no schizoaffective disorders. Psychiatry Res 2006 (in press).
- Pope HG, Lipinski JF. Diagnosis in schizophrenia and manic-depressive illness, a reassessment of the specificity of “schizophrenic” symptoms in the light of current research. Arch Gen Psychiatry 1978;35:811-28.
- Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. Am J Psychiatry 1992;149:999-1010.
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lithium • Lithobid
- Lorazepam • Ativan
- Carbamazepine • Tegretol
- Valproate • Depakote
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgements
The patient described in this case report gave informed, written consent to interviews and to the anonymous publication of his treatment.
The authors thank Anita Swisher for technical assistance.
1. Berrettini WH. Molecular linkage studies of bipolar disorders. Bipolar Disord 2001;3:276-83.
2. Belmaker RH. Bipolar disorder. N Engl J Med 2004;351:476-86.
3. Pope HG, Lipinski JF. Diagnosis in schizophrenia and manic-depressive illness, a reassessment of the specificity of “schizophrenic” symptoms in the light of current research. Arch Gen Psychiatry 1978;35:811-28.
4. Lake CR, Hurwitz N. Schizoaffective disorders are psychotic mood disorders; there are no schizoaffective disorders. Psychiatry Res 2006 (in press).
5. Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. Am J Psychiatry 1992;149:999-1010.
6. Diagnostic and statistical manual of mental disorders 4th ed text rev. Washington, DC: American Psychiatric Association; 2000.
7. Carroll BT, Thomas C, Jayanti K, et al. Treating persistent catatonia when benzodiazepines fail. Current Psychiatry 2005;4:56-64.
8. Abrams R, Taylor MA. Catatonia, a prospective clinical study. Arch Gen Psychiatry 1976;33:579-81.
9. Pope HG. Distinguishing bipolar disorder from schizophrenia in clinical practice: Guidelines and case reports. Hosp Com Psychiatry 1983;34:322-8.
10. Schneider K. Clinical psychopathology. New York: Grune & Stratton; 1959.
11. Kraepelin E. Clinical psychiatry. New York: William Wood Co; 1913.
12. Bleuler E. Dementia praecox or the group of schizophrenias. New York: International Universities Press; 1911/1950.
13. Goodwin FK, Jamison KR. Manic-depressive illness. New York: Oxford University Press; 1990.
14. Calabrese JR, Shelton MD, Bowden CL, et al. Bipolar rapid cycling: Focus on depression as its hallmark. J Clin Psychiatry 2001;62:34-41.
15. Goodwin FK. The biology of recurrence: new directions for the pharmacologic bridge. J Clin Psychiatry 1989;50:40-4.
16. Dieperink ME, Sands JR. Bipolar mania with psychotic features: diagnosis and treatment. Psychiatr Ann 1996;26:633-7.
17. Zarate CA, Tohen M. Double-blind comparison of the continued use of antipsychotic treatment versus its discontinuation in remitted manic patients. Am J Psychiatry 2004;161:169-71.
18. Fowler RC, McCabe MS, Cadoret RJ, Winokur G. The validity of good prognosis schizophrenia. Arch Gen Psychiatry 1972;26:182-5.
19. Harrow M, Grossman LS, Silverstein ML, Meltzer HY. Thought pathology in manic and schizophrenic patients. Its occurrence at hospital admission and 7 weeks later. Arch Gen Psychiatry 1982;39:665-71.
20. Szasz TS. Schizophrenia: the sacred symbol of psychiatry. Br J Psychiatry 1976;129:308-16.
21. Carlson GA, Goodwin FK. The stages of mania. Arch Gen Psychiatry 1973;28:221-8.
22. Pini S, Cassano GB, Dell’Osso L, Amador XF. Insight into illness in schizophrenia, schizoaffective disorder, and mood disorders with psychotic features. Am J Psychiatry 2001;158:122-5.
23. Bertelsen A, Harvald B, Hauge M. A Danish twin study of manic-depressive illness. Br J Psychiatry 1977;130:330-51.
24. Green E, Elvidge G, Jacobson N, et al. Localization of bipolar susceptibility locus by molecular genetic analysis of the chromosome 12q23-q24 region in two pedigrees with bipolar disorder and Darier’s disease. Am J Psychiatry 2005;162:35-42.
When a patient presents with psychotic symptoms, you might not recognize or pursue hints of bipolarity if you assume psychosis means schizophrenia. Yet psychotic bipolar disorder can explain every sign, symptom, course, and other characteristic traditionally assumed to indicate schizophrenia (Table 1). The literature, including recent genetic data,1-6 marshals a persuasive argument that patients diagnosed with schizophrenia usually suffer from a psychotic bipolar disorder.
Consider here how a cascade of changing signs and symptoms, initially unrecognized, caused five sequential re-evaluations of one psychotic patient’s primary Axis I diagnosis. His case highlights why the correct initial diagnosis of the disease causing psychosis is essential to effective treatment.4,7-9
Table 1
DSM-IV-TR criteria for schizophrenia vs. psychotic mood disorder
| Schizophrenia diagnosis6 | Seen in psychotic mood disorders |
|---|---|
| Criterion A | |
| Hallucinations and delusions | 50% to 80% explained by mood16,21 |
| Paranoia | Hides grandiosity4 |
| Catatonia | 75% explained by mood7,8 |
| Disorganized speech and behavior | All patients with moderate to severe mania1-5 |
| Negative symptoms | All patients with moderate to severe depression4 |
| Criterion B | |
| Social and job dysfunction | All patients with moderate to severe bipolar disorder5,13 |
| Criterion C | |
| Chronic continuous symptoms | Patients can have psychotic symptoms continuously for 2 years to life5,6,13 |
Case: Carved in stone
Police officers carry Mr. C, age 30, into the emergency department. He is mentally disorganized and arrives in a rigid, catatonic posture. According to a neighbor, Mr. C was kneeling motionless on his mother’s front lawn, alternating between mutism and inappropriately loud, disorganized religious preaching. When his arm is lifted, it remains as placed. He is admitted to the acute care inpatient unit.
Mr. C’s most striking symptoms are catatonia and psychosis. Postural rigidity, waxy flexibility, and automatic obedience are characteristics of catatonia.6-8 An organic cause is first considered, such as hyperthyroidism, cerebrovascular accident, cerebral neoplasm, head trauma, seizure disorder, dementia, neuroleptic malignant syndrome, pheochromocytoma, or—especially—intoxication from illegal drugs.7
While awaiting results from physical, mental status, and lab exams and imaging studies, staff assign him two admitting diagnoses: catatonic disorder due to a general medical condition and psychotic disorder not otherwise specified.6
Case: Inconclusive workup
Mr. C denies using illegal substances or alcohol, which his mother confirms. He has no history of seizures or other medical conditions. His distractibility prevents him from focusing on a formal mental status exam. Physical exam, urine drug screen, lab results, and imaging studies are unremarkable except for an admitting blood pressure of 145/95 mm Hg and pulse of 115 beats per minute. These readings normalize within 1 hour. IM haloperidol and lorazepam are given as needed for agitation, but physicians withhold scheduled medications to allow staff to observe his symptoms.
Organic causes of catatonia now seem less likely, though past use of drugs such as phencyclidine that can cause chronic psychosis cannot be ruled out. Schizophrenia is considered likely because catatonia is one of schizophrenia’s five core diagnostic symptoms.6 Catatonia can also be a symptom of bipolar disorder.6-9 Staff make a preliminary diagnosis of schizophrenia, catatonic type.
Case: ‘Hit men are after me’
Staff observe Mr. C responding to threatening auditory hallucinations. His affect is “fearful to terrified.” He says he hears the voice of God warning him of danger and continuing a running commentary on his actions. He fears for his life because “hit men have been sent to kill me” and have “infiltrated” the inpatient ward. He does not eat, saying his food is poisoned. He says these beliefs have escalated over the past year.
Mr. C’s catatonic symptoms resolve overnight, but obtaining additional history is difficult because of his paranoia. He denies any history of bizarre behavior or past contact with mental health services. He claims not to be especially religious. He is unmarried and lives with his mother, is college-educated, but has held only menial jobs.
Inpatient staff shifts its diagnostic focus to functional disorders associated with auditory hallucinations, paranoid delusions, and gross disorganization. According to Schneider and the DSM-IV-TR,6,10 hearing a voice “keeping up a running commentary on one’s behavior” is especially diagnostic of schizophrenia.
Because of the rapid resolution of his “catatonic” symptoms and prominence of paranoia, they change his diagnosis on day 2 to schizophrenia, paranoid type. Mr. C meets all diagnostic criteria for schizophrenia except one: the staff has overlooked and has not adequately excluded a psychotic mood disorder.
Case: A turn for the worse
That night, nursing staff find Mr. C naked and cowering in the fetal position in a corner of his room. He has smeared his feces on his face and in his hair and mouth. While being cleaned up, he suddenly begins quoting scripture in a loud, disorganized voice. His expressed thoughts are incomprehensible. He is given haloperidol and lorazepam immediately; oral haloperidol is continued at 10 mg bid.
Both Bleuler and Kraepelin concluded “coprophilia and coprophagia are unique to children and patients with schizophrenia.”11,12 The DSM casebook cites Kraepelin’s description of a catatonic patient who “smeared feces about” as a “classic, textbook case” of schizophrenia.11 The casebook goes on to say: “In the absence of any known general medical condition, the combination of coprophilia, disorganized speech, and catatonic behavior clearly indicates the diagnosis of schizophrenia.”
Mr. C shows each of these. Staff changes his diagnosis again—to schizophrenia, disorganized type, which carries a poor prognosis.11,12
Case: Banking and ray guns
By day 5, Mr. C’s mental status is normalizing and his psychosis improving. He volunteers for a weekly student case conference. There, he reveals additional information that staff could have discovered at admission with more-focused questions.
He reports that 2 years earlier he suffered severe suicidal depression. Six months later, during a hypomanic episode, he began “toying with the idea” that he might become part owner of his local bank. He believes “the Secret Service decided to transfer ownership to me.”
His plans upon acquiring the bank include buying three houses and six cars valued at several million dollars and running for state governor. For weeks before admission, he did not need sleep, experienced an increase in energy and activities, and his mind was racing. His job seemed so “trivial” that he quit. Immediately before his hospital admission, his delusions intensified to include an “evil conspiracy” to murder him for ownership of the bank and he feared his execution was imminent.
He explains his catatonic behavior on the lawn by his belief that “hit men” hiding across the street aimed a “motion-detecting, heat-seeking ray gun” at him so that if he had “moved an inch,” he would die. He says the “feces incident” was an effort to get himself transferred to the state hospital, where he thought he would be safer because his present caretakers were “infiltrated.” He also says his mother received electroconvulsive therapy in her 20s.
These symptoms—especially the striking grandiosity, lack of need for sleep, racing thoughts, hallucinations and delusions—define a manic episode with psychotic features. Only one manic episode as described here is diagnostic of bipolar disorder, type I.2,6,13 Staff changes his diagnosis to schizoaffective disorder, a compromise used to include patients with bipolar and psychotic (schizophrenic) features. Some authors contend schizoaffective disorder is psychotic bipolar disorder and not a separate disease.3,4,9
Case: From SSRI to lithium
After 2 weeks, Mr. C is discharged on haloperidol, 5 mg bid, but no mood stabilizer. He receives follow-up care at a community mental health center. When he develops severe depressive symptoms 6 months after discharge, the attending psychiatrist starts him on a selective serotonin reuptake inhibitor (SSRI). Within 2 weeks, Mr. C switches from depression to a mixed, dysphoric mania. After the SSRI is discontinued and lithium is added to his haloperidol, his mood gradually stabilizes to moderate depression. He develops rigidity, masked faces, and a fine tremor in his hands.
About 10% of bipolar depressed patients given an antidepressant—especially without a mood stabilizer—switch to mania, and their cycle frequency increases.2,13-15 A correct initial diagnosis and treatment with a mood stabilizer might have avoided Mr. C’s switch.
Mixed bipolar disorder with overlapping depressive and manic symptoms is often resistant to monotherapy, requiring two or more mood stabilizers such as lithium and an anticonvulsant.14 Without a mood-stabilizing combination, the mixed, rapid-cycling type of bipolar disorder is likely to progress, with more-rapid and more-severe episodes.2,13-15 Adding lamotrigine, a mood stabilizer with antidepressant effects, can help.2,14
Stopping the SSRI is correct, despite Mr. C’s severe depression, to avoid increasing the cycle frequency.13-15 Some authors recommend tapering the antipsychotic, using it only as needed for psychotic features after psychosis has resolved.14-17 Continuing antipsychotic drugs after psychosis has remitted increases rates of cycling to depression, depressive and extrapyramidal symptoms, and medication discontinuation.17 Lithium may have aggravated Mr. C’s antipsychotic-induced parkinsonism, but discontinuing haloperidol may have been the most therapeutic decision.
The community mental health staff changes his diagnosis again, this time to bipolar disorder, type I, mixed, severe with psychotic features. We concur that this is correct.
Case: A diagnostic step back
Two years later, Mr. C is working and continues to take lithium and haloperidol prescribed at the mental health center. His intermittent depressive episodes persist, but—apparently because he has not had another manic episode—the staff switches his diagnosis back to schizoaffective disorder.
We disagree with this change. A diagnosis of schizoaffective disorder precludes ideal pharmacotherapy for Mr. C’s rapid-cycling bipolar disorder and increases the risk of adverse drug effects and stigma. Persuasive evidence shows that schizoaffective disorder is psychotic bipolar disorder; there is no schizoaffective disorder (Box).3,4,16-18
Three disorders—schizophrenia, schizoaffective disorder, and psychotic bipolar disorder—have been evoked to account for the variance in severity in psychotic patients, but psychotic bipolar disorder expresses the entire spectrum. We concur with others that psychotic bipolar disorder includes patient populations typically diagnosed as having schizophrenia and schizoaffective disorder.3,4,9,16-18 In other words, there is no schizophrenia or schizoaffective disorder.4,19
Based on these data, we advocate re-evaluating all patients diagnosed with schizoaffective disorder and schizophrenia, with detailed inquiry for personal and family histories of mania or hypomania. A mood stabilizer may be warranted in some patients with psychosis but without clear manic symptoms. In such cases, we suggest using a provisional DSM-IV-TR diagnosis of psychotic disorder not otherwise specified while you seek obscure mood and/or organic causes.
Misdiagnosis of psychosis
Bipolar disorder can be missed when patients present with psychotic symptoms, but clinicians could have initially recognized Mr. C’s bipolar disorder. His diagnostic trail illustrates important points about psychotic presentations:
- Predominant psychotic symptoms can obscure mood disturbances.
- Mistakenly believing that psychosis means schizophrenia can jeopardize patient care.
- When paranoia and fear hide grandiosity, then mania—not schizophrenia—is likely.
- Psychotic mood disorders—not schizophrenia—cause functional psychosis; there is no schizophrenia (Box).
- Pursuing mood symptoms in psychotic presentations is critical in an initial diagnostic interview.
Bipolar disorder has a broad spectrum of severity and course; it frequently reaches psychotic levels that can become chronic.2,5,21 Psychotic symptoms of rigorously diagnosed bipolar patients can deteriorate until their overwhelming psychosis obscures bipolar symptoms.5,6,13,21 Like most, if not all, acutely psychotic bipolar patients, Mr. C shows all diagnostic criteria for schizophrenia.1-6,21
Patients with severe, psychotic bipolar disorder can stop responding to medication and suffer chronic deterioration without remission.5,21 They can lose their jobs, families, friends, and health until they are homeless, hungry, sick, and psychotic. A deteriorating course such as this has typically defined the schizophrenic process, but this concept has been reassessed.1-6,13,15
Most, but not all, bipolar type I patients experience psychosis. Mr. C’s bipolar symptoms were not initially obvious because of predominant psychosis and were revealed only with specific, focused questions. Without the student case conference, his diagnosis might have remained schizophrenia. His treatment would have remained substandard because of the conventional belief that schizophrenia requires lifelong antipsychotics, usually without mood stabilizers.
Our patient satisfied all DSM-IV-TR criteria for both schizophrenia and psychotic bipolar. Bleuler and Schneider would have diagnosed him as having schizophrenia because they thought all psychotic disorders were schizophrenic.10,12 They were incorrect, as psychotic symptoms are common in patients with severe bipolar disorder.1-6,13,22
Cinical implications
Our observations about this case suggest four important clinical questions:
- Do data justify diagnosing patients such as Mr. C with bipolar disorder and not schizophrenia?
- Do data substantiate either diagnosis as valid?
- Does the diagnosis matter?
- What is standard-of-care treatment for these patients?
Evidence for validity? Bipolar disorder’s two extremes in mood and behavior are so different from those in persons without bipolar disorder or with any other condition that homogeneous bipolar populations can be identified and studied with confidence.2,5,13,21 DSM-IV-TR diagnostic symptoms for bipolar disorder are unique (Table 2).
For a psychiatric disorder to be considered valid, patients must share other characteristics. Bipolar disorder has been validated as a specific disease by consistent genetic,1,13,23,24 pharmacologic,2,14,15 and epidemiologic1 data accumulated across 30 years. The concordance for bipolar disorder in monozygotic twins is approximately 75%, and susceptibility loci for bipolar disorder are established.23,24
Table 2
Characteristics indicating a mood disorder, not schizophrenia*
| History | Past diagnosis or symptoms of a mood disorder; family history of mood disorder or alcoholism |
| Past medications | Lithium, valproic acid, or other mood stabilizers |
| Periods of uncharacteristic and excessive goal-directed activities | Political, religious, legal, sexual, business, criminal, medical, physical, spending, calling, writing, preaching, cleaning, planning, exercise |
| Presence of uncharacteristic emotions or conflict | Irritability, anger, violence, conflict with law enforcement, elation, grandiosity (paranoia), sadness, hopelessness, crying, suicidal ideation |
| Periods of appropriate affect | Smiles, laughs, cries, irritable, angry |
| Mood-congruent delusions and/or hallucinations | Consider grandiosity when there is paranoia and fear |
| Episodes of relatively normal function/remission; premorbid personality positive | Friends, dating, team sports, group activities, election to an office/title, club or gang memberships |
| Current social interactions | Enjoys a friendship, active interactions with spouse and own children, regular interactions with others |
| *Absence of any or all does not rule out mood disorder. | |
Does the diagnosis matter? Failing to make an accurate initial diagnosis can worsen the course of patients who present with psychosis (Table 3):
- Bipolar illness not treated with mood stabilizers progresses, with episodes becoming more frequent and severe.2,14,15
- Antipsychotics are given longer and in higher dosages for schizophrenia than for psychotic bipolar disorder and tend to have more common, chronic, and disabling adverse effects than do antidepressants and mood stabilizers.14,16
- Mr. C was given an antidepressant without mood stabilization, which is contraindicated in bipolar I disorder (especially mixed type) because the cycling rate increases.2,14,15
Several initial signs could have raised suspicion that Mr. C had psychotic bipolar disorder (Table 4). Standard-of-care treatment in psychotic patients is predicated on early and correct diagnosis. On the basis of the evidence and our experience, we recommend that you look for bipolar symptoms when a patient:
- presents for the first time with psychosis, and you rule out an organic cause
- is readmitted for treatment of psychotic symptoms after having been diagnosed with schizophrenia.
Consequences of misdiagnosing psychotic mood disorder as schizophrenia
For patient
|
For clinician
|
Mr. C’s symptoms that indicated bipolar disorder
| Religiosity | Loud preaching and no past special interest in religion |
| Catatonia | Most frequently associated with bipolar disorder |
| Paranoia; fear | Usually hides grandiosity, which is diagnostic of mania |
| Distractibility | Could not stay focused in the diagnostic interview; showed ‘flight of ideas’ |
| Pressured speech | Rapid, disorganized thoughts |
| Disorganization | Hallmark of mania; present in all patients with severe mania |
| Functional psychosis | If an organic cause is ruled out, a psychotic mood disorder is the most likely diagnosis |
| Trouble with the law | Police found patient disturbing neighborhood and escorted him to hospital |
| Patient history | Severe depression |
| Family history | Mother was treated for depression with ECT |
| ECT: electroconvulsive therapy | |
- an antipsychotic, with or without a benzodiazepine for sedation, to enhance ward safety and treat acute psychotic symptoms
- and a first-line mood stabilizer such as valproate, carbamazepine, lithium, or lamotrigine, followed by atypical antipsychotics.
The idea that “symptoms should be treated, not the diagnosis” is inaccurate and provides substandard care. When psychotic symptoms overwhelm and obscure bipolar symptoms, giving only antipsychotics is beyond standard of care.
Related resources
- Berrettini WH. Molecular linkage studies of bipolar disorders. Bipolar Disord 2001;3:276-83.
- Lake CR, Hurwitz N. Schizoaffective disorders are psychotic mood disorders; there are no schizoaffective disorders. Psychiatry Res 2006 (in press).
- Pope HG, Lipinski JF. Diagnosis in schizophrenia and manic-depressive illness, a reassessment of the specificity of “schizophrenic” symptoms in the light of current research. Arch Gen Psychiatry 1978;35:811-28.
- Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. Am J Psychiatry 1992;149:999-1010.
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lithium • Lithobid
- Lorazepam • Ativan
- Carbamazepine • Tegretol
- Valproate • Depakote
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgements
The patient described in this case report gave informed, written consent to interviews and to the anonymous publication of his treatment.
The authors thank Anita Swisher for technical assistance.
When a patient presents with psychotic symptoms, you might not recognize or pursue hints of bipolarity if you assume psychosis means schizophrenia. Yet psychotic bipolar disorder can explain every sign, symptom, course, and other characteristic traditionally assumed to indicate schizophrenia (Table 1). The literature, including recent genetic data,1-6 marshals a persuasive argument that patients diagnosed with schizophrenia usually suffer from a psychotic bipolar disorder.
Consider here how a cascade of changing signs and symptoms, initially unrecognized, caused five sequential re-evaluations of one psychotic patient’s primary Axis I diagnosis. His case highlights why the correct initial diagnosis of the disease causing psychosis is essential to effective treatment.4,7-9
Table 1
DSM-IV-TR criteria for schizophrenia vs. psychotic mood disorder
| Schizophrenia diagnosis6 | Seen in psychotic mood disorders |
|---|---|
| Criterion A | |
| Hallucinations and delusions | 50% to 80% explained by mood16,21 |
| Paranoia | Hides grandiosity4 |
| Catatonia | 75% explained by mood7,8 |
| Disorganized speech and behavior | All patients with moderate to severe mania1-5 |
| Negative symptoms | All patients with moderate to severe depression4 |
| Criterion B | |
| Social and job dysfunction | All patients with moderate to severe bipolar disorder5,13 |
| Criterion C | |
| Chronic continuous symptoms | Patients can have psychotic symptoms continuously for 2 years to life5,6,13 |
Case: Carved in stone
Police officers carry Mr. C, age 30, into the emergency department. He is mentally disorganized and arrives in a rigid, catatonic posture. According to a neighbor, Mr. C was kneeling motionless on his mother’s front lawn, alternating between mutism and inappropriately loud, disorganized religious preaching. When his arm is lifted, it remains as placed. He is admitted to the acute care inpatient unit.
Mr. C’s most striking symptoms are catatonia and psychosis. Postural rigidity, waxy flexibility, and automatic obedience are characteristics of catatonia.6-8 An organic cause is first considered, such as hyperthyroidism, cerebrovascular accident, cerebral neoplasm, head trauma, seizure disorder, dementia, neuroleptic malignant syndrome, pheochromocytoma, or—especially—intoxication from illegal drugs.7
While awaiting results from physical, mental status, and lab exams and imaging studies, staff assign him two admitting diagnoses: catatonic disorder due to a general medical condition and psychotic disorder not otherwise specified.6
Case: Inconclusive workup
Mr. C denies using illegal substances or alcohol, which his mother confirms. He has no history of seizures or other medical conditions. His distractibility prevents him from focusing on a formal mental status exam. Physical exam, urine drug screen, lab results, and imaging studies are unremarkable except for an admitting blood pressure of 145/95 mm Hg and pulse of 115 beats per minute. These readings normalize within 1 hour. IM haloperidol and lorazepam are given as needed for agitation, but physicians withhold scheduled medications to allow staff to observe his symptoms.
Organic causes of catatonia now seem less likely, though past use of drugs such as phencyclidine that can cause chronic psychosis cannot be ruled out. Schizophrenia is considered likely because catatonia is one of schizophrenia’s five core diagnostic symptoms.6 Catatonia can also be a symptom of bipolar disorder.6-9 Staff make a preliminary diagnosis of schizophrenia, catatonic type.
Case: ‘Hit men are after me’
Staff observe Mr. C responding to threatening auditory hallucinations. His affect is “fearful to terrified.” He says he hears the voice of God warning him of danger and continuing a running commentary on his actions. He fears for his life because “hit men have been sent to kill me” and have “infiltrated” the inpatient ward. He does not eat, saying his food is poisoned. He says these beliefs have escalated over the past year.
Mr. C’s catatonic symptoms resolve overnight, but obtaining additional history is difficult because of his paranoia. He denies any history of bizarre behavior or past contact with mental health services. He claims not to be especially religious. He is unmarried and lives with his mother, is college-educated, but has held only menial jobs.
Inpatient staff shifts its diagnostic focus to functional disorders associated with auditory hallucinations, paranoid delusions, and gross disorganization. According to Schneider and the DSM-IV-TR,6,10 hearing a voice “keeping up a running commentary on one’s behavior” is especially diagnostic of schizophrenia.
Because of the rapid resolution of his “catatonic” symptoms and prominence of paranoia, they change his diagnosis on day 2 to schizophrenia, paranoid type. Mr. C meets all diagnostic criteria for schizophrenia except one: the staff has overlooked and has not adequately excluded a psychotic mood disorder.
Case: A turn for the worse
That night, nursing staff find Mr. C naked and cowering in the fetal position in a corner of his room. He has smeared his feces on his face and in his hair and mouth. While being cleaned up, he suddenly begins quoting scripture in a loud, disorganized voice. His expressed thoughts are incomprehensible. He is given haloperidol and lorazepam immediately; oral haloperidol is continued at 10 mg bid.
Both Bleuler and Kraepelin concluded “coprophilia and coprophagia are unique to children and patients with schizophrenia.”11,12 The DSM casebook cites Kraepelin’s description of a catatonic patient who “smeared feces about” as a “classic, textbook case” of schizophrenia.11 The casebook goes on to say: “In the absence of any known general medical condition, the combination of coprophilia, disorganized speech, and catatonic behavior clearly indicates the diagnosis of schizophrenia.”
Mr. C shows each of these. Staff changes his diagnosis again—to schizophrenia, disorganized type, which carries a poor prognosis.11,12
Case: Banking and ray guns
By day 5, Mr. C’s mental status is normalizing and his psychosis improving. He volunteers for a weekly student case conference. There, he reveals additional information that staff could have discovered at admission with more-focused questions.
He reports that 2 years earlier he suffered severe suicidal depression. Six months later, during a hypomanic episode, he began “toying with the idea” that he might become part owner of his local bank. He believes “the Secret Service decided to transfer ownership to me.”
His plans upon acquiring the bank include buying three houses and six cars valued at several million dollars and running for state governor. For weeks before admission, he did not need sleep, experienced an increase in energy and activities, and his mind was racing. His job seemed so “trivial” that he quit. Immediately before his hospital admission, his delusions intensified to include an “evil conspiracy” to murder him for ownership of the bank and he feared his execution was imminent.
He explains his catatonic behavior on the lawn by his belief that “hit men” hiding across the street aimed a “motion-detecting, heat-seeking ray gun” at him so that if he had “moved an inch,” he would die. He says the “feces incident” was an effort to get himself transferred to the state hospital, where he thought he would be safer because his present caretakers were “infiltrated.” He also says his mother received electroconvulsive therapy in her 20s.
These symptoms—especially the striking grandiosity, lack of need for sleep, racing thoughts, hallucinations and delusions—define a manic episode with psychotic features. Only one manic episode as described here is diagnostic of bipolar disorder, type I.2,6,13 Staff changes his diagnosis to schizoaffective disorder, a compromise used to include patients with bipolar and psychotic (schizophrenic) features. Some authors contend schizoaffective disorder is psychotic bipolar disorder and not a separate disease.3,4,9
Case: From SSRI to lithium
After 2 weeks, Mr. C is discharged on haloperidol, 5 mg bid, but no mood stabilizer. He receives follow-up care at a community mental health center. When he develops severe depressive symptoms 6 months after discharge, the attending psychiatrist starts him on a selective serotonin reuptake inhibitor (SSRI). Within 2 weeks, Mr. C switches from depression to a mixed, dysphoric mania. After the SSRI is discontinued and lithium is added to his haloperidol, his mood gradually stabilizes to moderate depression. He develops rigidity, masked faces, and a fine tremor in his hands.
About 10% of bipolar depressed patients given an antidepressant—especially without a mood stabilizer—switch to mania, and their cycle frequency increases.2,13-15 A correct initial diagnosis and treatment with a mood stabilizer might have avoided Mr. C’s switch.
Mixed bipolar disorder with overlapping depressive and manic symptoms is often resistant to monotherapy, requiring two or more mood stabilizers such as lithium and an anticonvulsant.14 Without a mood-stabilizing combination, the mixed, rapid-cycling type of bipolar disorder is likely to progress, with more-rapid and more-severe episodes.2,13-15 Adding lamotrigine, a mood stabilizer with antidepressant effects, can help.2,14
Stopping the SSRI is correct, despite Mr. C’s severe depression, to avoid increasing the cycle frequency.13-15 Some authors recommend tapering the antipsychotic, using it only as needed for psychotic features after psychosis has resolved.14-17 Continuing antipsychotic drugs after psychosis has remitted increases rates of cycling to depression, depressive and extrapyramidal symptoms, and medication discontinuation.17 Lithium may have aggravated Mr. C’s antipsychotic-induced parkinsonism, but discontinuing haloperidol may have been the most therapeutic decision.
The community mental health staff changes his diagnosis again, this time to bipolar disorder, type I, mixed, severe with psychotic features. We concur that this is correct.
Case: A diagnostic step back
Two years later, Mr. C is working and continues to take lithium and haloperidol prescribed at the mental health center. His intermittent depressive episodes persist, but—apparently because he has not had another manic episode—the staff switches his diagnosis back to schizoaffective disorder.
We disagree with this change. A diagnosis of schizoaffective disorder precludes ideal pharmacotherapy for Mr. C’s rapid-cycling bipolar disorder and increases the risk of adverse drug effects and stigma. Persuasive evidence shows that schizoaffective disorder is psychotic bipolar disorder; there is no schizoaffective disorder (Box).3,4,16-18
Three disorders—schizophrenia, schizoaffective disorder, and psychotic bipolar disorder—have been evoked to account for the variance in severity in psychotic patients, but psychotic bipolar disorder expresses the entire spectrum. We concur with others that psychotic bipolar disorder includes patient populations typically diagnosed as having schizophrenia and schizoaffective disorder.3,4,9,16-18 In other words, there is no schizophrenia or schizoaffective disorder.4,19
Based on these data, we advocate re-evaluating all patients diagnosed with schizoaffective disorder and schizophrenia, with detailed inquiry for personal and family histories of mania or hypomania. A mood stabilizer may be warranted in some patients with psychosis but without clear manic symptoms. In such cases, we suggest using a provisional DSM-IV-TR diagnosis of psychotic disorder not otherwise specified while you seek obscure mood and/or organic causes.
Misdiagnosis of psychosis
Bipolar disorder can be missed when patients present with psychotic symptoms, but clinicians could have initially recognized Mr. C’s bipolar disorder. His diagnostic trail illustrates important points about psychotic presentations:
- Predominant psychotic symptoms can obscure mood disturbances.
- Mistakenly believing that psychosis means schizophrenia can jeopardize patient care.
- When paranoia and fear hide grandiosity, then mania—not schizophrenia—is likely.
- Psychotic mood disorders—not schizophrenia—cause functional psychosis; there is no schizophrenia (Box).
- Pursuing mood symptoms in psychotic presentations is critical in an initial diagnostic interview.
Bipolar disorder has a broad spectrum of severity and course; it frequently reaches psychotic levels that can become chronic.2,5,21 Psychotic symptoms of rigorously diagnosed bipolar patients can deteriorate until their overwhelming psychosis obscures bipolar symptoms.5,6,13,21 Like most, if not all, acutely psychotic bipolar patients, Mr. C shows all diagnostic criteria for schizophrenia.1-6,21
Patients with severe, psychotic bipolar disorder can stop responding to medication and suffer chronic deterioration without remission.5,21 They can lose their jobs, families, friends, and health until they are homeless, hungry, sick, and psychotic. A deteriorating course such as this has typically defined the schizophrenic process, but this concept has been reassessed.1-6,13,15
Most, but not all, bipolar type I patients experience psychosis. Mr. C’s bipolar symptoms were not initially obvious because of predominant psychosis and were revealed only with specific, focused questions. Without the student case conference, his diagnosis might have remained schizophrenia. His treatment would have remained substandard because of the conventional belief that schizophrenia requires lifelong antipsychotics, usually without mood stabilizers.
Our patient satisfied all DSM-IV-TR criteria for both schizophrenia and psychotic bipolar. Bleuler and Schneider would have diagnosed him as having schizophrenia because they thought all psychotic disorders were schizophrenic.10,12 They were incorrect, as psychotic symptoms are common in patients with severe bipolar disorder.1-6,13,22
Cinical implications
Our observations about this case suggest four important clinical questions:
- Do data justify diagnosing patients such as Mr. C with bipolar disorder and not schizophrenia?
- Do data substantiate either diagnosis as valid?
- Does the diagnosis matter?
- What is standard-of-care treatment for these patients?
Evidence for validity? Bipolar disorder’s two extremes in mood and behavior are so different from those in persons without bipolar disorder or with any other condition that homogeneous bipolar populations can be identified and studied with confidence.2,5,13,21 DSM-IV-TR diagnostic symptoms for bipolar disorder are unique (Table 2).
For a psychiatric disorder to be considered valid, patients must share other characteristics. Bipolar disorder has been validated as a specific disease by consistent genetic,1,13,23,24 pharmacologic,2,14,15 and epidemiologic1 data accumulated across 30 years. The concordance for bipolar disorder in monozygotic twins is approximately 75%, and susceptibility loci for bipolar disorder are established.23,24
Table 2
Characteristics indicating a mood disorder, not schizophrenia*
| History | Past diagnosis or symptoms of a mood disorder; family history of mood disorder or alcoholism |
| Past medications | Lithium, valproic acid, or other mood stabilizers |
| Periods of uncharacteristic and excessive goal-directed activities | Political, religious, legal, sexual, business, criminal, medical, physical, spending, calling, writing, preaching, cleaning, planning, exercise |
| Presence of uncharacteristic emotions or conflict | Irritability, anger, violence, conflict with law enforcement, elation, grandiosity (paranoia), sadness, hopelessness, crying, suicidal ideation |
| Periods of appropriate affect | Smiles, laughs, cries, irritable, angry |
| Mood-congruent delusions and/or hallucinations | Consider grandiosity when there is paranoia and fear |
| Episodes of relatively normal function/remission; premorbid personality positive | Friends, dating, team sports, group activities, election to an office/title, club or gang memberships |
| Current social interactions | Enjoys a friendship, active interactions with spouse and own children, regular interactions with others |
| *Absence of any or all does not rule out mood disorder. | |
Does the diagnosis matter? Failing to make an accurate initial diagnosis can worsen the course of patients who present with psychosis (Table 3):
- Bipolar illness not treated with mood stabilizers progresses, with episodes becoming more frequent and severe.2,14,15
- Antipsychotics are given longer and in higher dosages for schizophrenia than for psychotic bipolar disorder and tend to have more common, chronic, and disabling adverse effects than do antidepressants and mood stabilizers.14,16
- Mr. C was given an antidepressant without mood stabilization, which is contraindicated in bipolar I disorder (especially mixed type) because the cycling rate increases.2,14,15
Several initial signs could have raised suspicion that Mr. C had psychotic bipolar disorder (Table 4). Standard-of-care treatment in psychotic patients is predicated on early and correct diagnosis. On the basis of the evidence and our experience, we recommend that you look for bipolar symptoms when a patient:
- presents for the first time with psychosis, and you rule out an organic cause
- is readmitted for treatment of psychotic symptoms after having been diagnosed with schizophrenia.
Consequences of misdiagnosing psychotic mood disorder as schizophrenia
For patient
|
For clinician
|
Mr. C’s symptoms that indicated bipolar disorder
| Religiosity | Loud preaching and no past special interest in religion |
| Catatonia | Most frequently associated with bipolar disorder |
| Paranoia; fear | Usually hides grandiosity, which is diagnostic of mania |
| Distractibility | Could not stay focused in the diagnostic interview; showed ‘flight of ideas’ |
| Pressured speech | Rapid, disorganized thoughts |
| Disorganization | Hallmark of mania; present in all patients with severe mania |
| Functional psychosis | If an organic cause is ruled out, a psychotic mood disorder is the most likely diagnosis |
| Trouble with the law | Police found patient disturbing neighborhood and escorted him to hospital |
| Patient history | Severe depression |
| Family history | Mother was treated for depression with ECT |
| ECT: electroconvulsive therapy | |
- an antipsychotic, with or without a benzodiazepine for sedation, to enhance ward safety and treat acute psychotic symptoms
- and a first-line mood stabilizer such as valproate, carbamazepine, lithium, or lamotrigine, followed by atypical antipsychotics.
The idea that “symptoms should be treated, not the diagnosis” is inaccurate and provides substandard care. When psychotic symptoms overwhelm and obscure bipolar symptoms, giving only antipsychotics is beyond standard of care.
Related resources
- Berrettini WH. Molecular linkage studies of bipolar disorders. Bipolar Disord 2001;3:276-83.
- Lake CR, Hurwitz N. Schizoaffective disorders are psychotic mood disorders; there are no schizoaffective disorders. Psychiatry Res 2006 (in press).
- Pope HG, Lipinski JF. Diagnosis in schizophrenia and manic-depressive illness, a reassessment of the specificity of “schizophrenic” symptoms in the light of current research. Arch Gen Psychiatry 1978;35:811-28.
- Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. Am J Psychiatry 1992;149:999-1010.
- Haloperidol • Haldol
- Lamotrigine • Lamictal
- Lithium • Lithobid
- Lorazepam • Ativan
- Carbamazepine • Tegretol
- Valproate • Depakote
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgements
The patient described in this case report gave informed, written consent to interviews and to the anonymous publication of his treatment.
The authors thank Anita Swisher for technical assistance.
1. Berrettini WH. Molecular linkage studies of bipolar disorders. Bipolar Disord 2001;3:276-83.
2. Belmaker RH. Bipolar disorder. N Engl J Med 2004;351:476-86.
3. Pope HG, Lipinski JF. Diagnosis in schizophrenia and manic-depressive illness, a reassessment of the specificity of “schizophrenic” symptoms in the light of current research. Arch Gen Psychiatry 1978;35:811-28.
4. Lake CR, Hurwitz N. Schizoaffective disorders are psychotic mood disorders; there are no schizoaffective disorders. Psychiatry Res 2006 (in press).
5. Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. Am J Psychiatry 1992;149:999-1010.
6. Diagnostic and statistical manual of mental disorders 4th ed text rev. Washington, DC: American Psychiatric Association; 2000.
7. Carroll BT, Thomas C, Jayanti K, et al. Treating persistent catatonia when benzodiazepines fail. Current Psychiatry 2005;4:56-64.
8. Abrams R, Taylor MA. Catatonia, a prospective clinical study. Arch Gen Psychiatry 1976;33:579-81.
9. Pope HG. Distinguishing bipolar disorder from schizophrenia in clinical practice: Guidelines and case reports. Hosp Com Psychiatry 1983;34:322-8.
10. Schneider K. Clinical psychopathology. New York: Grune & Stratton; 1959.
11. Kraepelin E. Clinical psychiatry. New York: William Wood Co; 1913.
12. Bleuler E. Dementia praecox or the group of schizophrenias. New York: International Universities Press; 1911/1950.
13. Goodwin FK, Jamison KR. Manic-depressive illness. New York: Oxford University Press; 1990.
14. Calabrese JR, Shelton MD, Bowden CL, et al. Bipolar rapid cycling: Focus on depression as its hallmark. J Clin Psychiatry 2001;62:34-41.
15. Goodwin FK. The biology of recurrence: new directions for the pharmacologic bridge. J Clin Psychiatry 1989;50:40-4.
16. Dieperink ME, Sands JR. Bipolar mania with psychotic features: diagnosis and treatment. Psychiatr Ann 1996;26:633-7.
17. Zarate CA, Tohen M. Double-blind comparison of the continued use of antipsychotic treatment versus its discontinuation in remitted manic patients. Am J Psychiatry 2004;161:169-71.
18. Fowler RC, McCabe MS, Cadoret RJ, Winokur G. The validity of good prognosis schizophrenia. Arch Gen Psychiatry 1972;26:182-5.
19. Harrow M, Grossman LS, Silverstein ML, Meltzer HY. Thought pathology in manic and schizophrenic patients. Its occurrence at hospital admission and 7 weeks later. Arch Gen Psychiatry 1982;39:665-71.
20. Szasz TS. Schizophrenia: the sacred symbol of psychiatry. Br J Psychiatry 1976;129:308-16.
21. Carlson GA, Goodwin FK. The stages of mania. Arch Gen Psychiatry 1973;28:221-8.
22. Pini S, Cassano GB, Dell’Osso L, Amador XF. Insight into illness in schizophrenia, schizoaffective disorder, and mood disorders with psychotic features. Am J Psychiatry 2001;158:122-5.
23. Bertelsen A, Harvald B, Hauge M. A Danish twin study of manic-depressive illness. Br J Psychiatry 1977;130:330-51.
24. Green E, Elvidge G, Jacobson N, et al. Localization of bipolar susceptibility locus by molecular genetic analysis of the chromosome 12q23-q24 region in two pedigrees with bipolar disorder and Darier’s disease. Am J Psychiatry 2005;162:35-42.
1. Berrettini WH. Molecular linkage studies of bipolar disorders. Bipolar Disord 2001;3:276-83.
2. Belmaker RH. Bipolar disorder. N Engl J Med 2004;351:476-86.
3. Pope HG, Lipinski JF. Diagnosis in schizophrenia and manic-depressive illness, a reassessment of the specificity of “schizophrenic” symptoms in the light of current research. Arch Gen Psychiatry 1978;35:811-28.
4. Lake CR, Hurwitz N. Schizoaffective disorders are psychotic mood disorders; there are no schizoaffective disorders. Psychiatry Res 2006 (in press).
5. Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. Am J Psychiatry 1992;149:999-1010.
6. Diagnostic and statistical manual of mental disorders 4th ed text rev. Washington, DC: American Psychiatric Association; 2000.
7. Carroll BT, Thomas C, Jayanti K, et al. Treating persistent catatonia when benzodiazepines fail. Current Psychiatry 2005;4:56-64.
8. Abrams R, Taylor MA. Catatonia, a prospective clinical study. Arch Gen Psychiatry 1976;33:579-81.
9. Pope HG. Distinguishing bipolar disorder from schizophrenia in clinical practice: Guidelines and case reports. Hosp Com Psychiatry 1983;34:322-8.
10. Schneider K. Clinical psychopathology. New York: Grune & Stratton; 1959.
11. Kraepelin E. Clinical psychiatry. New York: William Wood Co; 1913.
12. Bleuler E. Dementia praecox or the group of schizophrenias. New York: International Universities Press; 1911/1950.
13. Goodwin FK, Jamison KR. Manic-depressive illness. New York: Oxford University Press; 1990.
14. Calabrese JR, Shelton MD, Bowden CL, et al. Bipolar rapid cycling: Focus on depression as its hallmark. J Clin Psychiatry 2001;62:34-41.
15. Goodwin FK. The biology of recurrence: new directions for the pharmacologic bridge. J Clin Psychiatry 1989;50:40-4.
16. Dieperink ME, Sands JR. Bipolar mania with psychotic features: diagnosis and treatment. Psychiatr Ann 1996;26:633-7.
17. Zarate CA, Tohen M. Double-blind comparison of the continued use of antipsychotic treatment versus its discontinuation in remitted manic patients. Am J Psychiatry 2004;161:169-71.
18. Fowler RC, McCabe MS, Cadoret RJ, Winokur G. The validity of good prognosis schizophrenia. Arch Gen Psychiatry 1972;26:182-5.
19. Harrow M, Grossman LS, Silverstein ML, Meltzer HY. Thought pathology in manic and schizophrenic patients. Its occurrence at hospital admission and 7 weeks later. Arch Gen Psychiatry 1982;39:665-71.
20. Szasz TS. Schizophrenia: the sacred symbol of psychiatry. Br J Psychiatry 1976;129:308-16.
21. Carlson GA, Goodwin FK. The stages of mania. Arch Gen Psychiatry 1973;28:221-8.
22. Pini S, Cassano GB, Dell’Osso L, Amador XF. Insight into illness in schizophrenia, schizoaffective disorder, and mood disorders with psychotic features. Am J Psychiatry 2001;158:122-5.
23. Bertelsen A, Harvald B, Hauge M. A Danish twin study of manic-depressive illness. Br J Psychiatry 1977;130:330-51.
24. Green E, Elvidge G, Jacobson N, et al. Localization of bipolar susceptibility locus by molecular genetic analysis of the chromosome 12q23-q24 region in two pedigrees with bipolar disorder and Darier’s disease. Am J Psychiatry 2005;162:35-42.
When a child can’t sleep, start by treating the parents
Sleep problems are very common in children but more complicated to manage than in adults. That’s because you usually must consider the parents’ opinions in making the child’s diagnosis and change the parents’ behavior for the treatment to succeed.
This article describes sleep disorders of children and adolescents, the most effective behavioral therapies, and the limited situations when hypnotic therapy may be appropriate.
A Symptom, Not a Diagnosis
Pediatric insomnia is significant difficulty in initiating and/or maintaining sleep that impairs a child’s or caregiver’s daytime function (Table 1).1-4 Childhood sleep disorders may manifest primarily as daytime sleepiness and neurobehavioral symptoms or occur with comorbid psychiatric diagnoses such as depression, anxiety, or attention-deficit/hyperactivity disorder (ADHD).
It is important to view insomnia as a symptom—not a diagnosis. Causes of insomnia in children may be medical (drug-related, pain-induced, or obstructive sleep apnea syndrome), behavioral (poor sleep hygiene or negative sleep-onset associations), or multiple factors (Table 2).
Sleep hygiene. Before starting therapy, educate parents and children about normal sleep development and sleep hygiene, which includes:
- environmental factors (temperature, noise, ambient light)
- scheduling (regular sleep-wake schedule)
- sleep practice (bedtime routine)
- physiologic factors (exercise, timing of meals, caffeine intake).
- insufficient sleep for individual physiologic needs (“lifestyle” sleep restriction, delayed sleep onset related to behavioral insomnia)
- adequate sleep but fragmented or disrupted by conditions such as obstructive sleep apnea or periodic limb movement disorder that cause frequent or prolonged arousals
- primary disorders of excessive daytime sleepiness such as narcolepsy (less common than in adults but under-recognized in children and adolescents)
- circadian rhythm disorders in which sleep is usually normal in structure and duration but occurs at an undesired time (delayed sleep phase syndrome).
Table 1
Insomnia’s negative effects on children and adolescents
| Problem | Manifestations |
|---|---|
| Daytime sleepiness | Yawning, rubbing eyes, resting head on desk |
| Neurocognitive dysfunction | Decreased cognitive flexibility and verbal creativity |
| Poor abstract reasoning | |
| Impaired motor skills | |
| Decreased attention and vigilance | |
| Memory impairment | |
| Externalizing behaviors | Increased impulsivity, hyperactivity, and aggressiveness |
| Mood dysregulation | Increased irritability |
| Decreased positive mood | |
| Poor affect modulation | |
| Source: References 1-4 | |
Diagnostic types of pediatric insomnia
| Diagnosis | Characteristics |
|---|---|
| Behavioral insomnia of childhood | Learned behaviors that interfere with sleep onset or maintenance |
| Sleep-onset association | Prolonged nighttime arousals because child can fall asleep only with certain sleep associations, such as being soothed by parent |
| Limit-setting subtype | Active resistance, verbal protests, and repeated demands by child at bedtime |
| Psychophysiologic insomnia | Conditioned anxiety about sleep difficulty heightens physiologic and emotional arousal, further compromising ability to sleep |
| Delayed sleep phase disorder | Common in adolescents; persistent phase shift in sleep-wake schedule (later bedtime and wake time) that conflicts with school and lifestyle demands |
| Secondary insomnia | Not primary; related to other diagnoses or factors |
| Psychiatric disorders | Depression, anxiety, posttraumatic stress disorder, attention-deficit/hyperactivity disorder |
| Medical disorders | Obstructive sleep apnea syndrome, pain |
| Medication | Psychostimulants used to treat ADHD and antidepressants used for major depression may cause sleep-onset delay |
With Psychiatric Disorders
Sleep disturbances can profoundly affect the clinical presentation, severity, and management of psychiatric disorders in children and adolescents.5-7 Up to 75% of children with a major depressive disorder have insomnia (severe in 30%), and one-third of depressed adolescents have delayed sleep-onset. Sleep complaints—especially bedtime resistance, refusal to sleep alone, increased nighttime fears, and nightmares—are also common in anxious children and those who have experienced severe trauma (including physical and sexual abuse).
Growing evidence suggests that pediatric “primary” insomnia with no concurrent psychiatric disorder is a risk factor for developing psychiatric conditions later in life—particularly depressive and anxiety disorders. Psychotropics such as psychostimulants and antidepressants also may interfere with sleep.
ADHD. Parents often report that children with ADHD have sleep disturbances, especially difficulty initiating sleep, poor sleep quality, restless sleep, frequent nighttime arousals, and shortened sleep duration.8 Parental observations notwithstanding, most objective methods of examining sleep and sleep architecture (polysomnography, actigraphy) have shown few or inconsistent differences between children with ADHD and controls.
Sleep problems in children with ADHD are often multifactorial. Potential causes include:
- psychostimulant-mediated sleep-onset delay
- bedtime resistance related to comorbid anxiety, oppositional defiant disorder, or circadian phase delay
- settling difficulties related to deficits in sensory integration associated with ADHD.
When managing a child with ADHD, evaluate comorbid sleep problems and provide diagnostically driven behavioral and/or drug therapy.
Behavioral Insomnia of Childhood
Behavioral insomnia of childhood may manifest as sleep-onset association and limit-setting types.9 The two often coexist, and many children present with both bedtime delays and nighttime arousals.
Sleep-onset association type. The presenting problem is usually prolonged nighttime arousals resulting in insufficient sleep. The child has learned to fall asleep only with sleep associations, such as being soothed by a parent, that usually are available at bedtime.
During the night, when the child experiences the type of brief arousal that normally occurs at the end of each sleep cycle (every 60 to 90 minutes) or awakens for other reasons, he is unable to get back to sleep (“self-soothe”) unless those same conditions are available to him. The child then “signals” the caregiver by crying (or coming into the parents’ bedroom) until the necessary associations are provided.
Limit-setting type is characterized by active resistance, verbal protests, and repeated demands at bedtime (“curtain calls”) rather than nighttime arousals. If sufficiently prolonged, the sleep-onset delay may result in inadequate sleep duration.
Sometimes bedtime resistance is related to:
- an underlying problem (a medical condition such as asthma or medication use, a sleep disorder such as restless legs, or anxiety)
- a mismatch between the child’s intrinsic circadian preferences (“night owl”) and parental expectations.
Behavioral therapy can alleviate bedtime resistance and nighttime arousals in young children.10 Controlled group studies strongly support three techniques: unmodified extinction, graduated extinction, and preventive parental education (Table 3).
To use graduated extinction, tell parents to ignore bedtime crying and tantrums for specified periods before checking. Tailor the duration or interval between check-ins to the child’s age and temperament; the limiting factor is how much crying the parents can tolerate, as checking is often more to reassure them than the child.
For younger children, parents might check every 2 minutes initially, then gradually lengthen to 5-, 10-, and 15-minute intervals. A common scenario is to double the time between each successive check-in (2 minutes, 4 minutes, 8 minutes, etc.). For older children, checking could start at 5- or 10-minute intervals.
During check-ins, the parents briefly comfort the child (usually 15 seconds to 1 minute). Advise parents to minimize interactions that may reinforce the child’s attention-seeking behavior.
To treat limit-setting sleep problems, recommend a combination of:
- decreased parental attention to bedtime-delaying behavior
- establishing a consistent bedtime routine that does not include stimulating activities such as television viewing
- bedtime “fading” (temporarily setting bedtime to the current sleep-onset time and then gradually advancing bedtime)
- positive reinforcement (sticker charts) for appropriate behavior at bedtime.
Behavioral treatment strategies require parental consistency to avoid inadvertently reinforcing nighttime arousals. Warn parents that children’s protests frequently escalate temporarily as treatment begins (“postextinction burst”).
How parents define a sleep “problem” and how well they accept your treatment recommendations can depend on their cultural values and beliefs about sleep’s meaning, importance, and role in daily life. Family attitudes vary about solitary sleep versus co-sleeping and about offering children transitional objects such as a blanket or toy to help them sleep.
Parents who repeatedly fail to start or enforce behavioral management may have other issues to address, such as depression or marital conflict.
Table 3
3 treatments for behavioral insomnia of childhood
| Treatment | Definition/examples |
|---|---|
| Extinction | Withdrawing parental assistance at sleep onset and during the night (‘systematic ignoring’) |
| Graduated extinction | Gradual rather than abrupt extinction treatment |
| For toddlers, parents check child briefly at successively longer intervals during wake-sleep transition | |
| For older children, parents introduce transitional sleep association objects (a blanket or toy) and use positive reinforcement (stickers for remaining in bed) | |
| Preventive parental education | Parents must consistently use behavioral treatment strategies to avoid reinforcing the child’s nighttime arousals |
Psychophysiologic Insomnia
Psychophysiologic insomnia (sleep onset and/or maintenance) occurs primarily in older children and adolescents and results from:
- predisposing factors (genetic vulnerability, underlying medical or psychiatric conditions)
- precipitating factors (acute stress)
- perpetuating factors (poor sleep habits, caffeine use, maladaptive thoughts about sleep).
- using the bed only for sleep
- getting out of bed if unable to fall asleep (stimulus control)
- restricting time in bed to actual time asleep (sleep restriction)
- learning relaxation techniques to reduce anxiety.
12 The problem is the timing rather than quality of sleep.
Sleep quantity may be compromised if the individual must arise before obtaining adequate sleep. Sleep-onset delays resolve, however, when the patient is allowed to follow his or her preferred later bedtime and wake time.
The typical DSPS sleep-wake pattern is a consistently preferred bedtime/sleep-onset time after midnight and wake time after 10 AM on weekdays and weekends. Adolescents with DSPS often complain of sleep-onset insomnia, extreme difficulty waking in the morning, and profound daytime sleepiness.
A 1- to 2-hour phase shift to a later bedtime and wake time is part of normal pubertal development and has been cited as a rationale for delaying high school start times. The phase shift in DSPS is typically much more dramatic and intractable than the norm.
Treatment options for DSPS include:
- strict sleep-wake schedule (such as 9:30 or 10 PM to 6:30 AM on school nights, with no more than a 1-hour discrepancy on non-school nights)
- melatonin, 3 to 5 mg, given 3 to 4 hours before the desired bedtime, if sleep schedule strategies are unsuccessful
- bright-light therapy in the morning to suppress melatonin secretion and “reset” the body clock, especially if morning waking is particularly difficult.13
If the adolescent also has school avoidance or a mood disorder—which is often the case—noncompliance with treatment is common. More-intensive behavioral and medication approaches may be needed.
Use Hypnotics?
Most insomnia in children and adolescents can be managed from infancy on with behavior therapy alone. If not, combined behavioral and drug interventions may be appropriate, such as when:
- the family is overwhelmed by the sleep problem and cannot execute behavioral strategies
- the child’s safety is at risk (engaging in dangerous activities during night awakenings, for example)
- treating specific populations (such as children with ADHD or autistic disorders).
- antihistamines such as diphenhydramine
- tricyclic antidepressants (amitriptyline, trazodone, and others)
- benzodiazepines (clonazepam)
- nonbenzodiazepine hypnotics (zolpidem, zaleplon)
- alpha-agonists (clonidine).14,15
Use these medications with caution in children, as safety and tolerability are unknown. Prescribe the lowest dosage for the briefest time possible, and use in combination with behavioral management strategies. Choose the shortest-acting agents to avoid morning grogginess. Chloral hydrate and barbiturates are rarely indicated in children because of side effects.
Over-the-counter products. Parents often use nonprescription products such as diphenhydramine, melatonin, and herbal preparations to treat children’s sleep problems, with or without a clinician’s recommendation. Most herbal preparations are generally safe but remain untested in pediatric patients.
Antihistamines such as diphenhydramine are generally well-tolerated, but they may have a paradoxical agitating effect. Tolerance also tends to develop, leading to increasing doses. Parents may inadvertently overdose a child by giving multiple nonprescription products with diphenhydramine as the active ingredient (such as combining Benadryl with Tylenol PM).
Related resources
- National Sleep Foundation. Information for patients and clinicians. www.sleepfoundation.org.
- American Academy of Sleep Medicine. Professional and patient resources and links. www.aasmnet.org.
- Mindell J, Owens J. A clinical guide to pediatric sleep: diagnosis and management of sleep problems in children and adolescents. Philadelphia: Lippincott Williams and Wilkins; 2003.
- Owens J, Mindell J. Take charge of your child’s sleep: the all-in-one resource for solving sleep problems in kids and teens. New York: Marlowe & Co.; 2005.
- Amitriptyline • Elavil
- Clonazepam • Klonopin
- Clonidine • Catapres
- Diphenhydramine • Benadryl and others (nonprescription)
- Divalproex sodium • Depakote
- Mirtazapine • Remeron
- Risperidone • Risperdal
- Trazodone • Desyrel
- Zaleplon • Sonata
- Zolpidem • Ambien
Dr. Owens receives research support from Sepracor, Eli Lilly & Co., and Cephalon; is a consultant to Eli Lilly & Co., Cephalon, and Shire; and is a speaker for Eli Lilly & Co., Cephalon, and Johnson & Johnson.
1. Fallone G, Owens J, Deane J. Sleepiness in children and adolescents: clinical implications. Sleep Med Rev 2002;6(2):287-306.
2. Smedje H, Broman JE, Hetta J. Associations between disturbed sleep and behavioural difficulties in 635 children aged 6-8 years: a study based on parents’ perceptions. Eur Child Adolesc Psychiatry 2001;10(1):1-9.
3. Dahl RE. The regulation of sleep and arousal: development and psychopathology. Dev Psychopathol 1996;8:3-27.
4. Randazzo AC, Muehlbach MJ, Schweitzer PK, Walsh JK. Cognitive function following acute sleep restriction in children ages 10-14. Sleep 1998;21:861-8.
5. Sadeh A, McGuire JP, Sachs H. Sleep and psychological characteristics of children on a psychiatric inpatient unit. J Am Acad Child Adolesc Psychiatry 1995;33:1303-46.
6. Sachs H, McGuire J, Sadeh A, et al. Cognitive and behavioural correlates of mother-reported sleep problems in psychiatrically hospitalized children. Sleep Res 1994;23:207-13.
7. Dahl RE, Ryan ND, Matty MK, et al. Sleep onset abnormalities in depressed adolescents. Biol Psychiatry 1996;39:400-10.
8. Owens J. The ADHD and sleep conundrum: A review. J Develop Behav Pediatr 2005;26(4):312-22.
9. The International Classification of Sleep Disorders. Diagnosis and Coding Manual (ICSD-2) (2nd ed). Westchester, IL: American Academy of Sleep Medicine; 2005.
10. Mindell J, Kuhn B, Lewin D, et al. Behavioral treatment of bedtime problems and night wakings in infants and young children. An American Academy of Sleep Medicine Review. Sleep. In press.
11. Hohagen F. Nonpharmacologic treatment of insomnia. Sleep 1996;19(8):S50-51.
12. Garcia J, Rosen G, Mahowald M. Circadian rhythms and circadian rhythm disturbances in children and adolescents. Semin Pediatr Neurol 2001;8:229-40.
13. Sack RL, Lewy AJ, Hughes RJ. Use of melatonin for sleep and circadian rhythm disorders. Ann Med 1998;30:115-21.
14. Owens J, Rosen C, Mindell J. Medication use in the treatment of pediatric insomnia: results of a survey of community-based pediatricians. Pediatrics 2003;111(5):e628-35.
15. Owens J, Babcock D, Blumer J, et al. The use of pharmacotherapy in the treatment of pediatric insomnia in primary care: rational approaches. A consensus meeting summary. J Clin Sleep Med 2005;1(1):49-59.
Sleep problems are very common in children but more complicated to manage than in adults. That’s because you usually must consider the parents’ opinions in making the child’s diagnosis and change the parents’ behavior for the treatment to succeed.
This article describes sleep disorders of children and adolescents, the most effective behavioral therapies, and the limited situations when hypnotic therapy may be appropriate.
A Symptom, Not a Diagnosis
Pediatric insomnia is significant difficulty in initiating and/or maintaining sleep that impairs a child’s or caregiver’s daytime function (Table 1).1-4 Childhood sleep disorders may manifest primarily as daytime sleepiness and neurobehavioral symptoms or occur with comorbid psychiatric diagnoses such as depression, anxiety, or attention-deficit/hyperactivity disorder (ADHD).
It is important to view insomnia as a symptom—not a diagnosis. Causes of insomnia in children may be medical (drug-related, pain-induced, or obstructive sleep apnea syndrome), behavioral (poor sleep hygiene or negative sleep-onset associations), or multiple factors (Table 2).
Sleep hygiene. Before starting therapy, educate parents and children about normal sleep development and sleep hygiene, which includes:
- environmental factors (temperature, noise, ambient light)
- scheduling (regular sleep-wake schedule)
- sleep practice (bedtime routine)
- physiologic factors (exercise, timing of meals, caffeine intake).
- insufficient sleep for individual physiologic needs (“lifestyle” sleep restriction, delayed sleep onset related to behavioral insomnia)
- adequate sleep but fragmented or disrupted by conditions such as obstructive sleep apnea or periodic limb movement disorder that cause frequent or prolonged arousals
- primary disorders of excessive daytime sleepiness such as narcolepsy (less common than in adults but under-recognized in children and adolescents)
- circadian rhythm disorders in which sleep is usually normal in structure and duration but occurs at an undesired time (delayed sleep phase syndrome).
Table 1
Insomnia’s negative effects on children and adolescents
| Problem | Manifestations |
|---|---|
| Daytime sleepiness | Yawning, rubbing eyes, resting head on desk |
| Neurocognitive dysfunction | Decreased cognitive flexibility and verbal creativity |
| Poor abstract reasoning | |
| Impaired motor skills | |
| Decreased attention and vigilance | |
| Memory impairment | |
| Externalizing behaviors | Increased impulsivity, hyperactivity, and aggressiveness |
| Mood dysregulation | Increased irritability |
| Decreased positive mood | |
| Poor affect modulation | |
| Source: References 1-4 | |
Diagnostic types of pediatric insomnia
| Diagnosis | Characteristics |
|---|---|
| Behavioral insomnia of childhood | Learned behaviors that interfere with sleep onset or maintenance |
| Sleep-onset association | Prolonged nighttime arousals because child can fall asleep only with certain sleep associations, such as being soothed by parent |
| Limit-setting subtype | Active resistance, verbal protests, and repeated demands by child at bedtime |
| Psychophysiologic insomnia | Conditioned anxiety about sleep difficulty heightens physiologic and emotional arousal, further compromising ability to sleep |
| Delayed sleep phase disorder | Common in adolescents; persistent phase shift in sleep-wake schedule (later bedtime and wake time) that conflicts with school and lifestyle demands |
| Secondary insomnia | Not primary; related to other diagnoses or factors |
| Psychiatric disorders | Depression, anxiety, posttraumatic stress disorder, attention-deficit/hyperactivity disorder |
| Medical disorders | Obstructive sleep apnea syndrome, pain |
| Medication | Psychostimulants used to treat ADHD and antidepressants used for major depression may cause sleep-onset delay |
With Psychiatric Disorders
Sleep disturbances can profoundly affect the clinical presentation, severity, and management of psychiatric disorders in children and adolescents.5-7 Up to 75% of children with a major depressive disorder have insomnia (severe in 30%), and one-third of depressed adolescents have delayed sleep-onset. Sleep complaints—especially bedtime resistance, refusal to sleep alone, increased nighttime fears, and nightmares—are also common in anxious children and those who have experienced severe trauma (including physical and sexual abuse).
Growing evidence suggests that pediatric “primary” insomnia with no concurrent psychiatric disorder is a risk factor for developing psychiatric conditions later in life—particularly depressive and anxiety disorders. Psychotropics such as psychostimulants and antidepressants also may interfere with sleep.
ADHD. Parents often report that children with ADHD have sleep disturbances, especially difficulty initiating sleep, poor sleep quality, restless sleep, frequent nighttime arousals, and shortened sleep duration.8 Parental observations notwithstanding, most objective methods of examining sleep and sleep architecture (polysomnography, actigraphy) have shown few or inconsistent differences between children with ADHD and controls.
Sleep problems in children with ADHD are often multifactorial. Potential causes include:
- psychostimulant-mediated sleep-onset delay
- bedtime resistance related to comorbid anxiety, oppositional defiant disorder, or circadian phase delay
- settling difficulties related to deficits in sensory integration associated with ADHD.
When managing a child with ADHD, evaluate comorbid sleep problems and provide diagnostically driven behavioral and/or drug therapy.
Behavioral Insomnia of Childhood
Behavioral insomnia of childhood may manifest as sleep-onset association and limit-setting types.9 The two often coexist, and many children present with both bedtime delays and nighttime arousals.
Sleep-onset association type. The presenting problem is usually prolonged nighttime arousals resulting in insufficient sleep. The child has learned to fall asleep only with sleep associations, such as being soothed by a parent, that usually are available at bedtime.
During the night, when the child experiences the type of brief arousal that normally occurs at the end of each sleep cycle (every 60 to 90 minutes) or awakens for other reasons, he is unable to get back to sleep (“self-soothe”) unless those same conditions are available to him. The child then “signals” the caregiver by crying (or coming into the parents’ bedroom) until the necessary associations are provided.
Limit-setting type is characterized by active resistance, verbal protests, and repeated demands at bedtime (“curtain calls”) rather than nighttime arousals. If sufficiently prolonged, the sleep-onset delay may result in inadequate sleep duration.
Sometimes bedtime resistance is related to:
- an underlying problem (a medical condition such as asthma or medication use, a sleep disorder such as restless legs, or anxiety)
- a mismatch between the child’s intrinsic circadian preferences (“night owl”) and parental expectations.
Behavioral therapy can alleviate bedtime resistance and nighttime arousals in young children.10 Controlled group studies strongly support three techniques: unmodified extinction, graduated extinction, and preventive parental education (Table 3).
To use graduated extinction, tell parents to ignore bedtime crying and tantrums for specified periods before checking. Tailor the duration or interval between check-ins to the child’s age and temperament; the limiting factor is how much crying the parents can tolerate, as checking is often more to reassure them than the child.
For younger children, parents might check every 2 minutes initially, then gradually lengthen to 5-, 10-, and 15-minute intervals. A common scenario is to double the time between each successive check-in (2 minutes, 4 minutes, 8 minutes, etc.). For older children, checking could start at 5- or 10-minute intervals.
During check-ins, the parents briefly comfort the child (usually 15 seconds to 1 minute). Advise parents to minimize interactions that may reinforce the child’s attention-seeking behavior.
To treat limit-setting sleep problems, recommend a combination of:
- decreased parental attention to bedtime-delaying behavior
- establishing a consistent bedtime routine that does not include stimulating activities such as television viewing
- bedtime “fading” (temporarily setting bedtime to the current sleep-onset time and then gradually advancing bedtime)
- positive reinforcement (sticker charts) for appropriate behavior at bedtime.
Behavioral treatment strategies require parental consistency to avoid inadvertently reinforcing nighttime arousals. Warn parents that children’s protests frequently escalate temporarily as treatment begins (“postextinction burst”).
How parents define a sleep “problem” and how well they accept your treatment recommendations can depend on their cultural values and beliefs about sleep’s meaning, importance, and role in daily life. Family attitudes vary about solitary sleep versus co-sleeping and about offering children transitional objects such as a blanket or toy to help them sleep.
Parents who repeatedly fail to start or enforce behavioral management may have other issues to address, such as depression or marital conflict.
Table 3
3 treatments for behavioral insomnia of childhood
| Treatment | Definition/examples |
|---|---|
| Extinction | Withdrawing parental assistance at sleep onset and during the night (‘systematic ignoring’) |
| Graduated extinction | Gradual rather than abrupt extinction treatment |
| For toddlers, parents check child briefly at successively longer intervals during wake-sleep transition | |
| For older children, parents introduce transitional sleep association objects (a blanket or toy) and use positive reinforcement (stickers for remaining in bed) | |
| Preventive parental education | Parents must consistently use behavioral treatment strategies to avoid reinforcing the child’s nighttime arousals |
Psychophysiologic Insomnia
Psychophysiologic insomnia (sleep onset and/or maintenance) occurs primarily in older children and adolescents and results from:
- predisposing factors (genetic vulnerability, underlying medical or psychiatric conditions)
- precipitating factors (acute stress)
- perpetuating factors (poor sleep habits, caffeine use, maladaptive thoughts about sleep).
- using the bed only for sleep
- getting out of bed if unable to fall asleep (stimulus control)
- restricting time in bed to actual time asleep (sleep restriction)
- learning relaxation techniques to reduce anxiety.
12 The problem is the timing rather than quality of sleep.
Sleep quantity may be compromised if the individual must arise before obtaining adequate sleep. Sleep-onset delays resolve, however, when the patient is allowed to follow his or her preferred later bedtime and wake time.
The typical DSPS sleep-wake pattern is a consistently preferred bedtime/sleep-onset time after midnight and wake time after 10 AM on weekdays and weekends. Adolescents with DSPS often complain of sleep-onset insomnia, extreme difficulty waking in the morning, and profound daytime sleepiness.
A 1- to 2-hour phase shift to a later bedtime and wake time is part of normal pubertal development and has been cited as a rationale for delaying high school start times. The phase shift in DSPS is typically much more dramatic and intractable than the norm.
Treatment options for DSPS include:
- strict sleep-wake schedule (such as 9:30 or 10 PM to 6:30 AM on school nights, with no more than a 1-hour discrepancy on non-school nights)
- melatonin, 3 to 5 mg, given 3 to 4 hours before the desired bedtime, if sleep schedule strategies are unsuccessful
- bright-light therapy in the morning to suppress melatonin secretion and “reset” the body clock, especially if morning waking is particularly difficult.13
If the adolescent also has school avoidance or a mood disorder—which is often the case—noncompliance with treatment is common. More-intensive behavioral and medication approaches may be needed.
Use Hypnotics?
Most insomnia in children and adolescents can be managed from infancy on with behavior therapy alone. If not, combined behavioral and drug interventions may be appropriate, such as when:
- the family is overwhelmed by the sleep problem and cannot execute behavioral strategies
- the child’s safety is at risk (engaging in dangerous activities during night awakenings, for example)
- treating specific populations (such as children with ADHD or autistic disorders).
- antihistamines such as diphenhydramine
- tricyclic antidepressants (amitriptyline, trazodone, and others)
- benzodiazepines (clonazepam)
- nonbenzodiazepine hypnotics (zolpidem, zaleplon)
- alpha-agonists (clonidine).14,15
Use these medications with caution in children, as safety and tolerability are unknown. Prescribe the lowest dosage for the briefest time possible, and use in combination with behavioral management strategies. Choose the shortest-acting agents to avoid morning grogginess. Chloral hydrate and barbiturates are rarely indicated in children because of side effects.
Over-the-counter products. Parents often use nonprescription products such as diphenhydramine, melatonin, and herbal preparations to treat children’s sleep problems, with or without a clinician’s recommendation. Most herbal preparations are generally safe but remain untested in pediatric patients.
Antihistamines such as diphenhydramine are generally well-tolerated, but they may have a paradoxical agitating effect. Tolerance also tends to develop, leading to increasing doses. Parents may inadvertently overdose a child by giving multiple nonprescription products with diphenhydramine as the active ingredient (such as combining Benadryl with Tylenol PM).
Related resources
- National Sleep Foundation. Information for patients and clinicians. www.sleepfoundation.org.
- American Academy of Sleep Medicine. Professional and patient resources and links. www.aasmnet.org.
- Mindell J, Owens J. A clinical guide to pediatric sleep: diagnosis and management of sleep problems in children and adolescents. Philadelphia: Lippincott Williams and Wilkins; 2003.
- Owens J, Mindell J. Take charge of your child’s sleep: the all-in-one resource for solving sleep problems in kids and teens. New York: Marlowe & Co.; 2005.
- Amitriptyline • Elavil
- Clonazepam • Klonopin
- Clonidine • Catapres
- Diphenhydramine • Benadryl and others (nonprescription)
- Divalproex sodium • Depakote
- Mirtazapine • Remeron
- Risperidone • Risperdal
- Trazodone • Desyrel
- Zaleplon • Sonata
- Zolpidem • Ambien
Dr. Owens receives research support from Sepracor, Eli Lilly & Co., and Cephalon; is a consultant to Eli Lilly & Co., Cephalon, and Shire; and is a speaker for Eli Lilly & Co., Cephalon, and Johnson & Johnson.
Sleep problems are very common in children but more complicated to manage than in adults. That’s because you usually must consider the parents’ opinions in making the child’s diagnosis and change the parents’ behavior for the treatment to succeed.
This article describes sleep disorders of children and adolescents, the most effective behavioral therapies, and the limited situations when hypnotic therapy may be appropriate.
A Symptom, Not a Diagnosis
Pediatric insomnia is significant difficulty in initiating and/or maintaining sleep that impairs a child’s or caregiver’s daytime function (Table 1).1-4 Childhood sleep disorders may manifest primarily as daytime sleepiness and neurobehavioral symptoms or occur with comorbid psychiatric diagnoses such as depression, anxiety, or attention-deficit/hyperactivity disorder (ADHD).
It is important to view insomnia as a symptom—not a diagnosis. Causes of insomnia in children may be medical (drug-related, pain-induced, or obstructive sleep apnea syndrome), behavioral (poor sleep hygiene or negative sleep-onset associations), or multiple factors (Table 2).
Sleep hygiene. Before starting therapy, educate parents and children about normal sleep development and sleep hygiene, which includes:
- environmental factors (temperature, noise, ambient light)
- scheduling (regular sleep-wake schedule)
- sleep practice (bedtime routine)
- physiologic factors (exercise, timing of meals, caffeine intake).
- insufficient sleep for individual physiologic needs (“lifestyle” sleep restriction, delayed sleep onset related to behavioral insomnia)
- adequate sleep but fragmented or disrupted by conditions such as obstructive sleep apnea or periodic limb movement disorder that cause frequent or prolonged arousals
- primary disorders of excessive daytime sleepiness such as narcolepsy (less common than in adults but under-recognized in children and adolescents)
- circadian rhythm disorders in which sleep is usually normal in structure and duration but occurs at an undesired time (delayed sleep phase syndrome).
Table 1
Insomnia’s negative effects on children and adolescents
| Problem | Manifestations |
|---|---|
| Daytime sleepiness | Yawning, rubbing eyes, resting head on desk |
| Neurocognitive dysfunction | Decreased cognitive flexibility and verbal creativity |
| Poor abstract reasoning | |
| Impaired motor skills | |
| Decreased attention and vigilance | |
| Memory impairment | |
| Externalizing behaviors | Increased impulsivity, hyperactivity, and aggressiveness |
| Mood dysregulation | Increased irritability |
| Decreased positive mood | |
| Poor affect modulation | |
| Source: References 1-4 | |
Diagnostic types of pediatric insomnia
| Diagnosis | Characteristics |
|---|---|
| Behavioral insomnia of childhood | Learned behaviors that interfere with sleep onset or maintenance |
| Sleep-onset association | Prolonged nighttime arousals because child can fall asleep only with certain sleep associations, such as being soothed by parent |
| Limit-setting subtype | Active resistance, verbal protests, and repeated demands by child at bedtime |
| Psychophysiologic insomnia | Conditioned anxiety about sleep difficulty heightens physiologic and emotional arousal, further compromising ability to sleep |
| Delayed sleep phase disorder | Common in adolescents; persistent phase shift in sleep-wake schedule (later bedtime and wake time) that conflicts with school and lifestyle demands |
| Secondary insomnia | Not primary; related to other diagnoses or factors |
| Psychiatric disorders | Depression, anxiety, posttraumatic stress disorder, attention-deficit/hyperactivity disorder |
| Medical disorders | Obstructive sleep apnea syndrome, pain |
| Medication | Psychostimulants used to treat ADHD and antidepressants used for major depression may cause sleep-onset delay |
With Psychiatric Disorders
Sleep disturbances can profoundly affect the clinical presentation, severity, and management of psychiatric disorders in children and adolescents.5-7 Up to 75% of children with a major depressive disorder have insomnia (severe in 30%), and one-third of depressed adolescents have delayed sleep-onset. Sleep complaints—especially bedtime resistance, refusal to sleep alone, increased nighttime fears, and nightmares—are also common in anxious children and those who have experienced severe trauma (including physical and sexual abuse).
Growing evidence suggests that pediatric “primary” insomnia with no concurrent psychiatric disorder is a risk factor for developing psychiatric conditions later in life—particularly depressive and anxiety disorders. Psychotropics such as psychostimulants and antidepressants also may interfere with sleep.
ADHD. Parents often report that children with ADHD have sleep disturbances, especially difficulty initiating sleep, poor sleep quality, restless sleep, frequent nighttime arousals, and shortened sleep duration.8 Parental observations notwithstanding, most objective methods of examining sleep and sleep architecture (polysomnography, actigraphy) have shown few or inconsistent differences between children with ADHD and controls.
Sleep problems in children with ADHD are often multifactorial. Potential causes include:
- psychostimulant-mediated sleep-onset delay
- bedtime resistance related to comorbid anxiety, oppositional defiant disorder, or circadian phase delay
- settling difficulties related to deficits in sensory integration associated with ADHD.
When managing a child with ADHD, evaluate comorbid sleep problems and provide diagnostically driven behavioral and/or drug therapy.
Behavioral Insomnia of Childhood
Behavioral insomnia of childhood may manifest as sleep-onset association and limit-setting types.9 The two often coexist, and many children present with both bedtime delays and nighttime arousals.
Sleep-onset association type. The presenting problem is usually prolonged nighttime arousals resulting in insufficient sleep. The child has learned to fall asleep only with sleep associations, such as being soothed by a parent, that usually are available at bedtime.
During the night, when the child experiences the type of brief arousal that normally occurs at the end of each sleep cycle (every 60 to 90 minutes) or awakens for other reasons, he is unable to get back to sleep (“self-soothe”) unless those same conditions are available to him. The child then “signals” the caregiver by crying (or coming into the parents’ bedroom) until the necessary associations are provided.
Limit-setting type is characterized by active resistance, verbal protests, and repeated demands at bedtime (“curtain calls”) rather than nighttime arousals. If sufficiently prolonged, the sleep-onset delay may result in inadequate sleep duration.
Sometimes bedtime resistance is related to:
- an underlying problem (a medical condition such as asthma or medication use, a sleep disorder such as restless legs, or anxiety)
- a mismatch between the child’s intrinsic circadian preferences (“night owl”) and parental expectations.
Behavioral therapy can alleviate bedtime resistance and nighttime arousals in young children.10 Controlled group studies strongly support three techniques: unmodified extinction, graduated extinction, and preventive parental education (Table 3).
To use graduated extinction, tell parents to ignore bedtime crying and tantrums for specified periods before checking. Tailor the duration or interval between check-ins to the child’s age and temperament; the limiting factor is how much crying the parents can tolerate, as checking is often more to reassure them than the child.
For younger children, parents might check every 2 minutes initially, then gradually lengthen to 5-, 10-, and 15-minute intervals. A common scenario is to double the time between each successive check-in (2 minutes, 4 minutes, 8 minutes, etc.). For older children, checking could start at 5- or 10-minute intervals.
During check-ins, the parents briefly comfort the child (usually 15 seconds to 1 minute). Advise parents to minimize interactions that may reinforce the child’s attention-seeking behavior.
To treat limit-setting sleep problems, recommend a combination of:
- decreased parental attention to bedtime-delaying behavior
- establishing a consistent bedtime routine that does not include stimulating activities such as television viewing
- bedtime “fading” (temporarily setting bedtime to the current sleep-onset time and then gradually advancing bedtime)
- positive reinforcement (sticker charts) for appropriate behavior at bedtime.
Behavioral treatment strategies require parental consistency to avoid inadvertently reinforcing nighttime arousals. Warn parents that children’s protests frequently escalate temporarily as treatment begins (“postextinction burst”).
How parents define a sleep “problem” and how well they accept your treatment recommendations can depend on their cultural values and beliefs about sleep’s meaning, importance, and role in daily life. Family attitudes vary about solitary sleep versus co-sleeping and about offering children transitional objects such as a blanket or toy to help them sleep.
Parents who repeatedly fail to start or enforce behavioral management may have other issues to address, such as depression or marital conflict.
Table 3
3 treatments for behavioral insomnia of childhood
| Treatment | Definition/examples |
|---|---|
| Extinction | Withdrawing parental assistance at sleep onset and during the night (‘systematic ignoring’) |
| Graduated extinction | Gradual rather than abrupt extinction treatment |
| For toddlers, parents check child briefly at successively longer intervals during wake-sleep transition | |
| For older children, parents introduce transitional sleep association objects (a blanket or toy) and use positive reinforcement (stickers for remaining in bed) | |
| Preventive parental education | Parents must consistently use behavioral treatment strategies to avoid reinforcing the child’s nighttime arousals |
Psychophysiologic Insomnia
Psychophysiologic insomnia (sleep onset and/or maintenance) occurs primarily in older children and adolescents and results from:
- predisposing factors (genetic vulnerability, underlying medical or psychiatric conditions)
- precipitating factors (acute stress)
- perpetuating factors (poor sleep habits, caffeine use, maladaptive thoughts about sleep).
- using the bed only for sleep
- getting out of bed if unable to fall asleep (stimulus control)
- restricting time in bed to actual time asleep (sleep restriction)
- learning relaxation techniques to reduce anxiety.
12 The problem is the timing rather than quality of sleep.
Sleep quantity may be compromised if the individual must arise before obtaining adequate sleep. Sleep-onset delays resolve, however, when the patient is allowed to follow his or her preferred later bedtime and wake time.
The typical DSPS sleep-wake pattern is a consistently preferred bedtime/sleep-onset time after midnight and wake time after 10 AM on weekdays and weekends. Adolescents with DSPS often complain of sleep-onset insomnia, extreme difficulty waking in the morning, and profound daytime sleepiness.
A 1- to 2-hour phase shift to a later bedtime and wake time is part of normal pubertal development and has been cited as a rationale for delaying high school start times. The phase shift in DSPS is typically much more dramatic and intractable than the norm.
Treatment options for DSPS include:
- strict sleep-wake schedule (such as 9:30 or 10 PM to 6:30 AM on school nights, with no more than a 1-hour discrepancy on non-school nights)
- melatonin, 3 to 5 mg, given 3 to 4 hours before the desired bedtime, if sleep schedule strategies are unsuccessful
- bright-light therapy in the morning to suppress melatonin secretion and “reset” the body clock, especially if morning waking is particularly difficult.13
If the adolescent also has school avoidance or a mood disorder—which is often the case—noncompliance with treatment is common. More-intensive behavioral and medication approaches may be needed.
Use Hypnotics?
Most insomnia in children and adolescents can be managed from infancy on with behavior therapy alone. If not, combined behavioral and drug interventions may be appropriate, such as when:
- the family is overwhelmed by the sleep problem and cannot execute behavioral strategies
- the child’s safety is at risk (engaging in dangerous activities during night awakenings, for example)
- treating specific populations (such as children with ADHD or autistic disorders).
- antihistamines such as diphenhydramine
- tricyclic antidepressants (amitriptyline, trazodone, and others)
- benzodiazepines (clonazepam)
- nonbenzodiazepine hypnotics (zolpidem, zaleplon)
- alpha-agonists (clonidine).14,15
Use these medications with caution in children, as safety and tolerability are unknown. Prescribe the lowest dosage for the briefest time possible, and use in combination with behavioral management strategies. Choose the shortest-acting agents to avoid morning grogginess. Chloral hydrate and barbiturates are rarely indicated in children because of side effects.
Over-the-counter products. Parents often use nonprescription products such as diphenhydramine, melatonin, and herbal preparations to treat children’s sleep problems, with or without a clinician’s recommendation. Most herbal preparations are generally safe but remain untested in pediatric patients.
Antihistamines such as diphenhydramine are generally well-tolerated, but they may have a paradoxical agitating effect. Tolerance also tends to develop, leading to increasing doses. Parents may inadvertently overdose a child by giving multiple nonprescription products with diphenhydramine as the active ingredient (such as combining Benadryl with Tylenol PM).
Related resources
- National Sleep Foundation. Information for patients and clinicians. www.sleepfoundation.org.
- American Academy of Sleep Medicine. Professional and patient resources and links. www.aasmnet.org.
- Mindell J, Owens J. A clinical guide to pediatric sleep: diagnosis and management of sleep problems in children and adolescents. Philadelphia: Lippincott Williams and Wilkins; 2003.
- Owens J, Mindell J. Take charge of your child’s sleep: the all-in-one resource for solving sleep problems in kids and teens. New York: Marlowe & Co.; 2005.
- Amitriptyline • Elavil
- Clonazepam • Klonopin
- Clonidine • Catapres
- Diphenhydramine • Benadryl and others (nonprescription)
- Divalproex sodium • Depakote
- Mirtazapine • Remeron
- Risperidone • Risperdal
- Trazodone • Desyrel
- Zaleplon • Sonata
- Zolpidem • Ambien
Dr. Owens receives research support from Sepracor, Eli Lilly & Co., and Cephalon; is a consultant to Eli Lilly & Co., Cephalon, and Shire; and is a speaker for Eli Lilly & Co., Cephalon, and Johnson & Johnson.
1. Fallone G, Owens J, Deane J. Sleepiness in children and adolescents: clinical implications. Sleep Med Rev 2002;6(2):287-306.
2. Smedje H, Broman JE, Hetta J. Associations between disturbed sleep and behavioural difficulties in 635 children aged 6-8 years: a study based on parents’ perceptions. Eur Child Adolesc Psychiatry 2001;10(1):1-9.
3. Dahl RE. The regulation of sleep and arousal: development and psychopathology. Dev Psychopathol 1996;8:3-27.
4. Randazzo AC, Muehlbach MJ, Schweitzer PK, Walsh JK. Cognitive function following acute sleep restriction in children ages 10-14. Sleep 1998;21:861-8.
5. Sadeh A, McGuire JP, Sachs H. Sleep and psychological characteristics of children on a psychiatric inpatient unit. J Am Acad Child Adolesc Psychiatry 1995;33:1303-46.
6. Sachs H, McGuire J, Sadeh A, et al. Cognitive and behavioural correlates of mother-reported sleep problems in psychiatrically hospitalized children. Sleep Res 1994;23:207-13.
7. Dahl RE, Ryan ND, Matty MK, et al. Sleep onset abnormalities in depressed adolescents. Biol Psychiatry 1996;39:400-10.
8. Owens J. The ADHD and sleep conundrum: A review. J Develop Behav Pediatr 2005;26(4):312-22.
9. The International Classification of Sleep Disorders. Diagnosis and Coding Manual (ICSD-2) (2nd ed). Westchester, IL: American Academy of Sleep Medicine; 2005.
10. Mindell J, Kuhn B, Lewin D, et al. Behavioral treatment of bedtime problems and night wakings in infants and young children. An American Academy of Sleep Medicine Review. Sleep. In press.
11. Hohagen F. Nonpharmacologic treatment of insomnia. Sleep 1996;19(8):S50-51.
12. Garcia J, Rosen G, Mahowald M. Circadian rhythms and circadian rhythm disturbances in children and adolescents. Semin Pediatr Neurol 2001;8:229-40.
13. Sack RL, Lewy AJ, Hughes RJ. Use of melatonin for sleep and circadian rhythm disorders. Ann Med 1998;30:115-21.
14. Owens J, Rosen C, Mindell J. Medication use in the treatment of pediatric insomnia: results of a survey of community-based pediatricians. Pediatrics 2003;111(5):e628-35.
15. Owens J, Babcock D, Blumer J, et al. The use of pharmacotherapy in the treatment of pediatric insomnia in primary care: rational approaches. A consensus meeting summary. J Clin Sleep Med 2005;1(1):49-59.
1. Fallone G, Owens J, Deane J. Sleepiness in children and adolescents: clinical implications. Sleep Med Rev 2002;6(2):287-306.
2. Smedje H, Broman JE, Hetta J. Associations between disturbed sleep and behavioural difficulties in 635 children aged 6-8 years: a study based on parents’ perceptions. Eur Child Adolesc Psychiatry 2001;10(1):1-9.
3. Dahl RE. The regulation of sleep and arousal: development and psychopathology. Dev Psychopathol 1996;8:3-27.
4. Randazzo AC, Muehlbach MJ, Schweitzer PK, Walsh JK. Cognitive function following acute sleep restriction in children ages 10-14. Sleep 1998;21:861-8.
5. Sadeh A, McGuire JP, Sachs H. Sleep and psychological characteristics of children on a psychiatric inpatient unit. J Am Acad Child Adolesc Psychiatry 1995;33:1303-46.
6. Sachs H, McGuire J, Sadeh A, et al. Cognitive and behavioural correlates of mother-reported sleep problems in psychiatrically hospitalized children. Sleep Res 1994;23:207-13.
7. Dahl RE, Ryan ND, Matty MK, et al. Sleep onset abnormalities in depressed adolescents. Biol Psychiatry 1996;39:400-10.
8. Owens J. The ADHD and sleep conundrum: A review. J Develop Behav Pediatr 2005;26(4):312-22.
9. The International Classification of Sleep Disorders. Diagnosis and Coding Manual (ICSD-2) (2nd ed). Westchester, IL: American Academy of Sleep Medicine; 2005.
10. Mindell J, Kuhn B, Lewin D, et al. Behavioral treatment of bedtime problems and night wakings in infants and young children. An American Academy of Sleep Medicine Review. Sleep. In press.
11. Hohagen F. Nonpharmacologic treatment of insomnia. Sleep 1996;19(8):S50-51.
12. Garcia J, Rosen G, Mahowald M. Circadian rhythms and circadian rhythm disturbances in children and adolescents. Semin Pediatr Neurol 2001;8:229-40.
13. Sack RL, Lewy AJ, Hughes RJ. Use of melatonin for sleep and circadian rhythm disorders. Ann Med 1998;30:115-21.
14. Owens J, Rosen C, Mindell J. Medication use in the treatment of pediatric insomnia: results of a survey of community-based pediatricians. Pediatrics 2003;111(5):e628-35.
15. Owens J, Babcock D, Blumer J, et al. The use of pharmacotherapy in the treatment of pediatric insomnia in primary care: rational approaches. A consensus meeting summary. J Clin Sleep Med 2005;1(1):49-59.
Is your patient making the ‘wrong’ treatment choice?
Consultation/liaison (C/L) psychiatrists assess capacity in 1 of 6 consults,1 and these evaluations must be quick but systematic. Hospital time is precious, and asking for a psychiatry consult inevitably slows down the medical team’s efforts to care for sick or injured patients.
We suggest an approach our C/L service developed to rapidly weigh capacity’s three dimensions—risks, benefits, and patient decisions—to formulate appropriate opinions for the medical team.
A standard for capacity
In most cases, capacity must be assessed and considered adequate before a patient can provide informed consent for a medical intervention. Because a patient might be capable of making some decisions but not others, the standard for determining capacity is not black and white but a sliding scale that depends on the magnitude of the decision being made.
As physicians, psychiatrists understand doctors’ frustrations when they believe a patient is making the wrong treatment choice. When the primary team turns to us, they want us to help determine the most appropriate course of action.
Capacity is determined by weighing whether the patient is competent to exercise his or her autonomy in making a decision about medical treatment. We do not assess global capacity; the goal is to provide an unbiased opinion about specific capacity for a given situation–“Does Mr. X have the ability to accept/refuse this treatment option presented to him?”
Capacity’s three dimensions. The means to achieve this goal are often complex. Roth et al2 proposed that capacity could be measured on a sliding scale. Wise and Rundell3 agreed and developed a two-dimension table to show that capacity can be evaluated at different thresholds, depending on the patient’s clinical situation. We expanded this model (Figure) to include three dimensions to consider when you evaluate capacity:
- risk of the proposed treatment (high vs. low)
- benefit of the treatment (high vs. low)
- the patient’s decision about the treatment (accept vs. refuse).
If a treatment’s benefits far outweigh the risks and the patient accepts that treatment, he is probably capable of making that decision and a lenient (low) threshold to establish capacity applies. If the same patient refuses the high-benefit, low-risk treatment, then he might be incapable of making that decision and a stringent (high) threshold to establish capacity comes into play. Our C/L service often uses this model when discussing capacity evaluations with the primary team. It explains why some capacity evaluations—when a patient agrees to a low-risk, high-benefit procedure—might take minutes, whereas others—those that fall into the medium threshold for capacity—take hours. Consider the following cases.
Figure 3-dimension model for evaluating capacity
Three cases: Is capacity evaluation needed?
Mr. X, age 25, was in a motor vehicle accident that caused trauma to his esophagus. He requires a feeding tube because he will be unable to eat for several weeks. The risk of the procedure (feeding tube placement) is low, and the benefit (getting possibly life-saving nutrition) is high.
If Mr. X refuses the feeding tube, he may be incapable of making this decision and would require a rigorous capacity evaluation (high-threshold capacity). If he consents, he is making a choice with which most reasonable people would agree, and establishing capacity would be less important (low-threshold capacity).
Mr. J, age 95, has congestive heart failure, diabetes, and liver disease. If he consents to a liver transplant—a treatment likely to be low-benefit and high-risk—he would require a rigorous capacity evaluation. If he refuses this surgical intervention, then more-lenient capacity criteria would apply.
Mrs. F, age 59, has breast cancer with metastases. Her oncologist is recommending bilateral mastectomy, radiation, chemotherapy, and an experimental treatment that has shown favorable results. The risk of treatment is high, and the benefit is unknown but most likely high. Since this is a high-risk, high-benefit intervention, the capacity threshold is medium. Whether she consents to or refuses treatment, you must weigh risks and benefits very carefully with her.
The primary team’s role
A common myth holds that only psychiatrists can determine capacity, but any physician can.4 The primary team may feel comfortable deciding a patient’s capacity without seeking consultation after asking the screening questions in Table 1.5,6 A patient who gives consistent and appropriate answers to these screening questions usually also can answer the more detailed questions psychiatrists would ask and thus has sufficient capacity.
When uncertainties remain after screening, we recommend that the primary team ask psychiatry for an opinion. Knowing what the primary team is thinking about a difficult case often helps the psychiatric consultant. So when consulting with psychiatry, we suggest that the primary team:
- clarify the question (such as, “Does Mrs. Z have the capacity to refuse dialysis?”)
- give an opinion about whether the patient does or does not have capacity and why.
When sharing of opinions was studied at institutions trying this idea, C/L teams agreed with the primary teams’ initial impression of patients’ capacity 80% of the time.4 Most consults occurred because the patient was refusing an intervention the primary team felt was “essential,” or the patient and primary team disagreed on code status. At our institution, anecdotal evidence shows that if the primary team spends a few minutes asking screening questions, the C/L service and primary team agree on the patient’s capacity >90% of the time.
Table 1
Primary team capacity evaluation: 5 W’s
Explain to the patient the treatment you recommend. Review risks and benefits of accepting and of refusing the treatment. Describe alternatives. Then ask these screening questions to assess capacity:
|
| Source: References 5,6 |
Tips for the psychiatrist
C/L psychiatrists are usually asked to evaluate capacity in complicated cases, such as when the:
- family disagrees with the patient’s decision
- patient changes his mind several times
- patient has a formidable psychiatric history.
Determining capacity requires that you assess the patient’s ability to communicate choices, understand and retain information about his condition and proposed treatment, appreciate likely consequences, and rationally manipulate information (Table 2).7
You can often gauge a patient’s attitude the moment you walk into his or her room. Those who feel insulted or defensive about being evaluated by a psychiatrist say things like:
- “I’m not crazy; I don’t need to talk to you.”
- “I think you need to evaluate my doctors, not me.”
- “Why is it so hard to believe that I’m ready to die? You can’t change my mind. Get out!”
To put the patient at ease, consider an inoffensive introduction such as: “My name is Dr. Y and I’m one of the psychiatrists who work here. I’m often called by the primary team to help explain the pros and cons of the various treatments we can provide to you. I’m not here to change your mind; I just want to make sure you are aware of all your options.”
Table 2
Psychiatry C/L service capacity evaluation
Ability to communicate choices
|
Ability to understand information about a treatment
|
Appreciation of likely consequences
|
Rational manipulation of information
|
| Source: References 5,6 |
Is it ever ok not to assess capacity?
In rare situations, informed consent does not need to be pursued and neither does capacity. Informed consent occurs when a capable patient receives adequate information to make a decision and voluntarily consents to the proposed intervention.8 Informed consent is not required in emergency, patient waiver, or therapeutic privilege situations.8,9
Emergency exception is permitted if the patient lacks the capacity to consent and the harm of postponing therapy is imminent and outweighs the proposed intervention’s risks. These cases are usually life-threatening situations in the emergency department, such as when a patient suffers severe physical trauma in a motor vehicle accident and is unable to communicate. Although capacity cannot be established, patients are taken immediately to the operating room.
If a patient with capacity refuses emergent treatment, however, the treating physician cannot override the patient’s wishes simply because it is an emergency. For example:
Mrs. L, age 32, lost several liters of blood during a complicated vaginal delivery. Her obstetrician felt she needed an emergent blood transfusion to avoid further medical complications. Mrs. L—a Jehovah’s Witness—refused the transfusion because of her religious beliefs. She was deemed capable of making this decision, and the transfusion was deferred.8-10
Patient waiver applies when a patient does not want to know all the relevant information about a procedure; he or she may wish for the physician (or another person) to make decisions.
Therapeutic privilege, a controversial idea, allows the physician to make decisions for the patient without informed consent when the physician believes the risk of giving pertinent information poses a serious detriment to the patient. In the rare cases when this is invoked, obtain family input if possible. For example:
Mrs. J, age 70, has severe health anxiety. When the primary care physician she has seen for 30 years tries to discuss treatments with her, Mrs. J fixates on potential harms and refuses treatments with even minimal risk. Her doctor tells her that it may be in her best interest to not hear the risks of treatment. Mrs. J agrees and gives her doctor permission to discusses treatment risks and benefits with her daughter, who is intricately involved in her mother’s health care.
Related resources
- Harvard Medical School department of psychiatry. Web site on forensic psychiatry and medicine. www.forensic-psych.com.
- Stern TA, Fricchione GL, Cassem, NH, et al (eds). Massachusetts General Hospital handbook of general hospital psychiatry (5th ed). Philadelphia: CV Mosby; 2004:355-9.
1. Viswanathan R, Schindler B, Brendel RW, et al. Should APM develop practice guidelines for decisional capacity assessments in the medical setting? Presented at: Annual Meeting of the Academy of Psychosomatic Medicine; November 16-20, 2005; Santa Ana Pueblo, NM.
2. Roth LH, Meisel A, Lidz CW. Tests of competency to consent to treatment. Am J Psychiatry 1977;124:279-84.
3. Wise MG, Rundell JR. Medicolegal issues in consultation. In: Clinical manual of psychosomatic medicine: a guide to consultation-liaison psychiatry. Arlington, VA: American Psychiatric Publishing; 2005:254-67.
4. Muskin PR, Kornfeld DS, Aladjem A, Tahil F. Determining capacity: Is it just capacity? Plenary workshop at: Annual Meeting of the Academy of Psychosomatic Medicine; November 16-20, 2005; Santa Ana Pueblo, NM.
5. Malin PJ. Creighton University. Educational handout (adapted with permission).
6. Poole K, Singh M, Murphy J. Law and medicine: the dilemmas of capacity, consultation/liaison psychiatry. Presented at: The Mayo Clinic; February 15, 2001; Rochester, MN.
7. Grisso T, Appelbaum PS. Assessing competence to consent to treatment: A guide for physicians and other health professionals. New York: Oxford University Press; 1998.
8. Nora LM, Benvenuti RJ. Iatrogenic disorders: medicolegal aspects of informed consent. Neurol Clin 1998;16:207-16.
9. Coulson KM, Glasser BL, Liang BA. Informed consent: issues for providers. Hematol Oncol Clin North Am 2002;16:1365-80.
10. Magid M, Reichenberg JS, Philbrick KL. To cut or not to cut: that was the question. Poster presented at: Meeting of the American Academy of Psychiatry and the Law; October 2005; Montreal, Canada.
Consultation/liaison (C/L) psychiatrists assess capacity in 1 of 6 consults,1 and these evaluations must be quick but systematic. Hospital time is precious, and asking for a psychiatry consult inevitably slows down the medical team’s efforts to care for sick or injured patients.
We suggest an approach our C/L service developed to rapidly weigh capacity’s three dimensions—risks, benefits, and patient decisions—to formulate appropriate opinions for the medical team.
A standard for capacity
In most cases, capacity must be assessed and considered adequate before a patient can provide informed consent for a medical intervention. Because a patient might be capable of making some decisions but not others, the standard for determining capacity is not black and white but a sliding scale that depends on the magnitude of the decision being made.
As physicians, psychiatrists understand doctors’ frustrations when they believe a patient is making the wrong treatment choice. When the primary team turns to us, they want us to help determine the most appropriate course of action.
Capacity is determined by weighing whether the patient is competent to exercise his or her autonomy in making a decision about medical treatment. We do not assess global capacity; the goal is to provide an unbiased opinion about specific capacity for a given situation–“Does Mr. X have the ability to accept/refuse this treatment option presented to him?”
Capacity’s three dimensions. The means to achieve this goal are often complex. Roth et al2 proposed that capacity could be measured on a sliding scale. Wise and Rundell3 agreed and developed a two-dimension table to show that capacity can be evaluated at different thresholds, depending on the patient’s clinical situation. We expanded this model (Figure) to include three dimensions to consider when you evaluate capacity:
- risk of the proposed treatment (high vs. low)
- benefit of the treatment (high vs. low)
- the patient’s decision about the treatment (accept vs. refuse).
If a treatment’s benefits far outweigh the risks and the patient accepts that treatment, he is probably capable of making that decision and a lenient (low) threshold to establish capacity applies. If the same patient refuses the high-benefit, low-risk treatment, then he might be incapable of making that decision and a stringent (high) threshold to establish capacity comes into play. Our C/L service often uses this model when discussing capacity evaluations with the primary team. It explains why some capacity evaluations—when a patient agrees to a low-risk, high-benefit procedure—might take minutes, whereas others—those that fall into the medium threshold for capacity—take hours. Consider the following cases.
Figure 3-dimension model for evaluating capacity
Three cases: Is capacity evaluation needed?
Mr. X, age 25, was in a motor vehicle accident that caused trauma to his esophagus. He requires a feeding tube because he will be unable to eat for several weeks. The risk of the procedure (feeding tube placement) is low, and the benefit (getting possibly life-saving nutrition) is high.
If Mr. X refuses the feeding tube, he may be incapable of making this decision and would require a rigorous capacity evaluation (high-threshold capacity). If he consents, he is making a choice with which most reasonable people would agree, and establishing capacity would be less important (low-threshold capacity).
Mr. J, age 95, has congestive heart failure, diabetes, and liver disease. If he consents to a liver transplant—a treatment likely to be low-benefit and high-risk—he would require a rigorous capacity evaluation. If he refuses this surgical intervention, then more-lenient capacity criteria would apply.
Mrs. F, age 59, has breast cancer with metastases. Her oncologist is recommending bilateral mastectomy, radiation, chemotherapy, and an experimental treatment that has shown favorable results. The risk of treatment is high, and the benefit is unknown but most likely high. Since this is a high-risk, high-benefit intervention, the capacity threshold is medium. Whether she consents to or refuses treatment, you must weigh risks and benefits very carefully with her.
The primary team’s role
A common myth holds that only psychiatrists can determine capacity, but any physician can.4 The primary team may feel comfortable deciding a patient’s capacity without seeking consultation after asking the screening questions in Table 1.5,6 A patient who gives consistent and appropriate answers to these screening questions usually also can answer the more detailed questions psychiatrists would ask and thus has sufficient capacity.
When uncertainties remain after screening, we recommend that the primary team ask psychiatry for an opinion. Knowing what the primary team is thinking about a difficult case often helps the psychiatric consultant. So when consulting with psychiatry, we suggest that the primary team:
- clarify the question (such as, “Does Mrs. Z have the capacity to refuse dialysis?”)
- give an opinion about whether the patient does or does not have capacity and why.
When sharing of opinions was studied at institutions trying this idea, C/L teams agreed with the primary teams’ initial impression of patients’ capacity 80% of the time.4 Most consults occurred because the patient was refusing an intervention the primary team felt was “essential,” or the patient and primary team disagreed on code status. At our institution, anecdotal evidence shows that if the primary team spends a few minutes asking screening questions, the C/L service and primary team agree on the patient’s capacity >90% of the time.
Table 1
Primary team capacity evaluation: 5 W’s
Explain to the patient the treatment you recommend. Review risks and benefits of accepting and of refusing the treatment. Describe alternatives. Then ask these screening questions to assess capacity:
|
| Source: References 5,6 |
Tips for the psychiatrist
C/L psychiatrists are usually asked to evaluate capacity in complicated cases, such as when the:
- family disagrees with the patient’s decision
- patient changes his mind several times
- patient has a formidable psychiatric history.
Determining capacity requires that you assess the patient’s ability to communicate choices, understand and retain information about his condition and proposed treatment, appreciate likely consequences, and rationally manipulate information (Table 2).7
You can often gauge a patient’s attitude the moment you walk into his or her room. Those who feel insulted or defensive about being evaluated by a psychiatrist say things like:
- “I’m not crazy; I don’t need to talk to you.”
- “I think you need to evaluate my doctors, not me.”
- “Why is it so hard to believe that I’m ready to die? You can’t change my mind. Get out!”
To put the patient at ease, consider an inoffensive introduction such as: “My name is Dr. Y and I’m one of the psychiatrists who work here. I’m often called by the primary team to help explain the pros and cons of the various treatments we can provide to you. I’m not here to change your mind; I just want to make sure you are aware of all your options.”
Table 2
Psychiatry C/L service capacity evaluation
Ability to communicate choices
|
Ability to understand information about a treatment
|
Appreciation of likely consequences
|
Rational manipulation of information
|
| Source: References 5,6 |
Is it ever ok not to assess capacity?
In rare situations, informed consent does not need to be pursued and neither does capacity. Informed consent occurs when a capable patient receives adequate information to make a decision and voluntarily consents to the proposed intervention.8 Informed consent is not required in emergency, patient waiver, or therapeutic privilege situations.8,9
Emergency exception is permitted if the patient lacks the capacity to consent and the harm of postponing therapy is imminent and outweighs the proposed intervention’s risks. These cases are usually life-threatening situations in the emergency department, such as when a patient suffers severe physical trauma in a motor vehicle accident and is unable to communicate. Although capacity cannot be established, patients are taken immediately to the operating room.
If a patient with capacity refuses emergent treatment, however, the treating physician cannot override the patient’s wishes simply because it is an emergency. For example:
Mrs. L, age 32, lost several liters of blood during a complicated vaginal delivery. Her obstetrician felt she needed an emergent blood transfusion to avoid further medical complications. Mrs. L—a Jehovah’s Witness—refused the transfusion because of her religious beliefs. She was deemed capable of making this decision, and the transfusion was deferred.8-10
Patient waiver applies when a patient does not want to know all the relevant information about a procedure; he or she may wish for the physician (or another person) to make decisions.
Therapeutic privilege, a controversial idea, allows the physician to make decisions for the patient without informed consent when the physician believes the risk of giving pertinent information poses a serious detriment to the patient. In the rare cases when this is invoked, obtain family input if possible. For example:
Mrs. J, age 70, has severe health anxiety. When the primary care physician she has seen for 30 years tries to discuss treatments with her, Mrs. J fixates on potential harms and refuses treatments with even minimal risk. Her doctor tells her that it may be in her best interest to not hear the risks of treatment. Mrs. J agrees and gives her doctor permission to discusses treatment risks and benefits with her daughter, who is intricately involved in her mother’s health care.
Related resources
- Harvard Medical School department of psychiatry. Web site on forensic psychiatry and medicine. www.forensic-psych.com.
- Stern TA, Fricchione GL, Cassem, NH, et al (eds). Massachusetts General Hospital handbook of general hospital psychiatry (5th ed). Philadelphia: CV Mosby; 2004:355-9.
Consultation/liaison (C/L) psychiatrists assess capacity in 1 of 6 consults,1 and these evaluations must be quick but systematic. Hospital time is precious, and asking for a psychiatry consult inevitably slows down the medical team’s efforts to care for sick or injured patients.
We suggest an approach our C/L service developed to rapidly weigh capacity’s three dimensions—risks, benefits, and patient decisions—to formulate appropriate opinions for the medical team.
A standard for capacity
In most cases, capacity must be assessed and considered adequate before a patient can provide informed consent for a medical intervention. Because a patient might be capable of making some decisions but not others, the standard for determining capacity is not black and white but a sliding scale that depends on the magnitude of the decision being made.
As physicians, psychiatrists understand doctors’ frustrations when they believe a patient is making the wrong treatment choice. When the primary team turns to us, they want us to help determine the most appropriate course of action.
Capacity is determined by weighing whether the patient is competent to exercise his or her autonomy in making a decision about medical treatment. We do not assess global capacity; the goal is to provide an unbiased opinion about specific capacity for a given situation–“Does Mr. X have the ability to accept/refuse this treatment option presented to him?”
Capacity’s three dimensions. The means to achieve this goal are often complex. Roth et al2 proposed that capacity could be measured on a sliding scale. Wise and Rundell3 agreed and developed a two-dimension table to show that capacity can be evaluated at different thresholds, depending on the patient’s clinical situation. We expanded this model (Figure) to include three dimensions to consider when you evaluate capacity:
- risk of the proposed treatment (high vs. low)
- benefit of the treatment (high vs. low)
- the patient’s decision about the treatment (accept vs. refuse).
If a treatment’s benefits far outweigh the risks and the patient accepts that treatment, he is probably capable of making that decision and a lenient (low) threshold to establish capacity applies. If the same patient refuses the high-benefit, low-risk treatment, then he might be incapable of making that decision and a stringent (high) threshold to establish capacity comes into play. Our C/L service often uses this model when discussing capacity evaluations with the primary team. It explains why some capacity evaluations—when a patient agrees to a low-risk, high-benefit procedure—might take minutes, whereas others—those that fall into the medium threshold for capacity—take hours. Consider the following cases.
Figure 3-dimension model for evaluating capacity
Three cases: Is capacity evaluation needed?
Mr. X, age 25, was in a motor vehicle accident that caused trauma to his esophagus. He requires a feeding tube because he will be unable to eat for several weeks. The risk of the procedure (feeding tube placement) is low, and the benefit (getting possibly life-saving nutrition) is high.
If Mr. X refuses the feeding tube, he may be incapable of making this decision and would require a rigorous capacity evaluation (high-threshold capacity). If he consents, he is making a choice with which most reasonable people would agree, and establishing capacity would be less important (low-threshold capacity).
Mr. J, age 95, has congestive heart failure, diabetes, and liver disease. If he consents to a liver transplant—a treatment likely to be low-benefit and high-risk—he would require a rigorous capacity evaluation. If he refuses this surgical intervention, then more-lenient capacity criteria would apply.
Mrs. F, age 59, has breast cancer with metastases. Her oncologist is recommending bilateral mastectomy, radiation, chemotherapy, and an experimental treatment that has shown favorable results. The risk of treatment is high, and the benefit is unknown but most likely high. Since this is a high-risk, high-benefit intervention, the capacity threshold is medium. Whether she consents to or refuses treatment, you must weigh risks and benefits very carefully with her.
The primary team’s role
A common myth holds that only psychiatrists can determine capacity, but any physician can.4 The primary team may feel comfortable deciding a patient’s capacity without seeking consultation after asking the screening questions in Table 1.5,6 A patient who gives consistent and appropriate answers to these screening questions usually also can answer the more detailed questions psychiatrists would ask and thus has sufficient capacity.
When uncertainties remain after screening, we recommend that the primary team ask psychiatry for an opinion. Knowing what the primary team is thinking about a difficult case often helps the psychiatric consultant. So when consulting with psychiatry, we suggest that the primary team:
- clarify the question (such as, “Does Mrs. Z have the capacity to refuse dialysis?”)
- give an opinion about whether the patient does or does not have capacity and why.
When sharing of opinions was studied at institutions trying this idea, C/L teams agreed with the primary teams’ initial impression of patients’ capacity 80% of the time.4 Most consults occurred because the patient was refusing an intervention the primary team felt was “essential,” or the patient and primary team disagreed on code status. At our institution, anecdotal evidence shows that if the primary team spends a few minutes asking screening questions, the C/L service and primary team agree on the patient’s capacity >90% of the time.
Table 1
Primary team capacity evaluation: 5 W’s
Explain to the patient the treatment you recommend. Review risks and benefits of accepting and of refusing the treatment. Describe alternatives. Then ask these screening questions to assess capacity:
|
| Source: References 5,6 |
Tips for the psychiatrist
C/L psychiatrists are usually asked to evaluate capacity in complicated cases, such as when the:
- family disagrees with the patient’s decision
- patient changes his mind several times
- patient has a formidable psychiatric history.
Determining capacity requires that you assess the patient’s ability to communicate choices, understand and retain information about his condition and proposed treatment, appreciate likely consequences, and rationally manipulate information (Table 2).7
You can often gauge a patient’s attitude the moment you walk into his or her room. Those who feel insulted or defensive about being evaluated by a psychiatrist say things like:
- “I’m not crazy; I don’t need to talk to you.”
- “I think you need to evaluate my doctors, not me.”
- “Why is it so hard to believe that I’m ready to die? You can’t change my mind. Get out!”
To put the patient at ease, consider an inoffensive introduction such as: “My name is Dr. Y and I’m one of the psychiatrists who work here. I’m often called by the primary team to help explain the pros and cons of the various treatments we can provide to you. I’m not here to change your mind; I just want to make sure you are aware of all your options.”
Table 2
Psychiatry C/L service capacity evaluation
Ability to communicate choices
|
Ability to understand information about a treatment
|
Appreciation of likely consequences
|
Rational manipulation of information
|
| Source: References 5,6 |
Is it ever ok not to assess capacity?
In rare situations, informed consent does not need to be pursued and neither does capacity. Informed consent occurs when a capable patient receives adequate information to make a decision and voluntarily consents to the proposed intervention.8 Informed consent is not required in emergency, patient waiver, or therapeutic privilege situations.8,9
Emergency exception is permitted if the patient lacks the capacity to consent and the harm of postponing therapy is imminent and outweighs the proposed intervention’s risks. These cases are usually life-threatening situations in the emergency department, such as when a patient suffers severe physical trauma in a motor vehicle accident and is unable to communicate. Although capacity cannot be established, patients are taken immediately to the operating room.
If a patient with capacity refuses emergent treatment, however, the treating physician cannot override the patient’s wishes simply because it is an emergency. For example:
Mrs. L, age 32, lost several liters of blood during a complicated vaginal delivery. Her obstetrician felt she needed an emergent blood transfusion to avoid further medical complications. Mrs. L—a Jehovah’s Witness—refused the transfusion because of her religious beliefs. She was deemed capable of making this decision, and the transfusion was deferred.8-10
Patient waiver applies when a patient does not want to know all the relevant information about a procedure; he or she may wish for the physician (or another person) to make decisions.
Therapeutic privilege, a controversial idea, allows the physician to make decisions for the patient without informed consent when the physician believes the risk of giving pertinent information poses a serious detriment to the patient. In the rare cases when this is invoked, obtain family input if possible. For example:
Mrs. J, age 70, has severe health anxiety. When the primary care physician she has seen for 30 years tries to discuss treatments with her, Mrs. J fixates on potential harms and refuses treatments with even minimal risk. Her doctor tells her that it may be in her best interest to not hear the risks of treatment. Mrs. J agrees and gives her doctor permission to discusses treatment risks and benefits with her daughter, who is intricately involved in her mother’s health care.
Related resources
- Harvard Medical School department of psychiatry. Web site on forensic psychiatry and medicine. www.forensic-psych.com.
- Stern TA, Fricchione GL, Cassem, NH, et al (eds). Massachusetts General Hospital handbook of general hospital psychiatry (5th ed). Philadelphia: CV Mosby; 2004:355-9.
1. Viswanathan R, Schindler B, Brendel RW, et al. Should APM develop practice guidelines for decisional capacity assessments in the medical setting? Presented at: Annual Meeting of the Academy of Psychosomatic Medicine; November 16-20, 2005; Santa Ana Pueblo, NM.
2. Roth LH, Meisel A, Lidz CW. Tests of competency to consent to treatment. Am J Psychiatry 1977;124:279-84.
3. Wise MG, Rundell JR. Medicolegal issues in consultation. In: Clinical manual of psychosomatic medicine: a guide to consultation-liaison psychiatry. Arlington, VA: American Psychiatric Publishing; 2005:254-67.
4. Muskin PR, Kornfeld DS, Aladjem A, Tahil F. Determining capacity: Is it just capacity? Plenary workshop at: Annual Meeting of the Academy of Psychosomatic Medicine; November 16-20, 2005; Santa Ana Pueblo, NM.
5. Malin PJ. Creighton University. Educational handout (adapted with permission).
6. Poole K, Singh M, Murphy J. Law and medicine: the dilemmas of capacity, consultation/liaison psychiatry. Presented at: The Mayo Clinic; February 15, 2001; Rochester, MN.
7. Grisso T, Appelbaum PS. Assessing competence to consent to treatment: A guide for physicians and other health professionals. New York: Oxford University Press; 1998.
8. Nora LM, Benvenuti RJ. Iatrogenic disorders: medicolegal aspects of informed consent. Neurol Clin 1998;16:207-16.
9. Coulson KM, Glasser BL, Liang BA. Informed consent: issues for providers. Hematol Oncol Clin North Am 2002;16:1365-80.
10. Magid M, Reichenberg JS, Philbrick KL. To cut or not to cut: that was the question. Poster presented at: Meeting of the American Academy of Psychiatry and the Law; October 2005; Montreal, Canada.
1. Viswanathan R, Schindler B, Brendel RW, et al. Should APM develop practice guidelines for decisional capacity assessments in the medical setting? Presented at: Annual Meeting of the Academy of Psychosomatic Medicine; November 16-20, 2005; Santa Ana Pueblo, NM.
2. Roth LH, Meisel A, Lidz CW. Tests of competency to consent to treatment. Am J Psychiatry 1977;124:279-84.
3. Wise MG, Rundell JR. Medicolegal issues in consultation. In: Clinical manual of psychosomatic medicine: a guide to consultation-liaison psychiatry. Arlington, VA: American Psychiatric Publishing; 2005:254-67.
4. Muskin PR, Kornfeld DS, Aladjem A, Tahil F. Determining capacity: Is it just capacity? Plenary workshop at: Annual Meeting of the Academy of Psychosomatic Medicine; November 16-20, 2005; Santa Ana Pueblo, NM.
5. Malin PJ. Creighton University. Educational handout (adapted with permission).
6. Poole K, Singh M, Murphy J. Law and medicine: the dilemmas of capacity, consultation/liaison psychiatry. Presented at: The Mayo Clinic; February 15, 2001; Rochester, MN.
7. Grisso T, Appelbaum PS. Assessing competence to consent to treatment: A guide for physicians and other health professionals. New York: Oxford University Press; 1998.
8. Nora LM, Benvenuti RJ. Iatrogenic disorders: medicolegal aspects of informed consent. Neurol Clin 1998;16:207-16.
9. Coulson KM, Glasser BL, Liang BA. Informed consent: issues for providers. Hematol Oncol Clin North Am 2002;16:1365-80.
10. Magid M, Reichenberg JS, Philbrick KL. To cut or not to cut: that was the question. Poster presented at: Meeting of the American Academy of Psychiatry and the Law; October 2005; Montreal, Canada.