User login
Treating persistent catatonia when benzodiazepines fail
Many catatonia cases respond to benzodiazepines—especially lorazepam—but up to 30% do not. Electroconvulsive therapy (ECT) can be effective, but what’s the next step when ECT is unavailable or inappropriate for your patient?
To help you solve this dilemma, we describe our diagnosis and treatment decisions for a patient we call Mr. C. We explain how our process was guided by recent understandings of an abnormal neural circuit that appears to cause catatonia’s complex motor and behavioral symptoms.
This article describes that neurologic pathology and answers common questions about the clinical workup and treatment of catatonia.
CASE: TROUBLE IN TV LAND
Mr. C, age 69, caused a disturbance at a local TV station, demanding that they broadcast a manuscript he had written. Police took him to a local hospital, where he was stabilized and then transferred to a neuropsychiatric hospital for evaluation.
The psychiatric interview revealed that he had developed insomnia, excessive activity, and delusional thinking 2 weeks before admission. His medical history included coronary artery disease (CAD), hypertension, and hypothyroidism. Medications included thyroid hormone replacement therapy, furosemide, potassium, ranitidine, simvastatin, metoprolol, and lisinopril. CAD treatment included stent placement and nitroglycerin as needed.
He had been hospitalized in his 30s and treated with ECT for what he called “bad thoughts.” He said he improved after 1 month and had no subsequent psychiatric history. He denied drug or alcohol abuse.
Shortly after admission, he refused to eat or drink and after 1 week became dehydrated. He also showed mutism, immobility, and stupor. He was transferred to the medical service for IV rehydration.
MANY SCENARIOS AND SIGNS
Mr. C’s symptoms suggest possible catatonia, a neuropsychiatric syndrome of motor dysregulation found in up to 10% of acutely ill psychiatric inpatients.1,2 A movement disorder,1,2 catatonia occurs with general medical conditions and psychiatric disorders (Table 1).
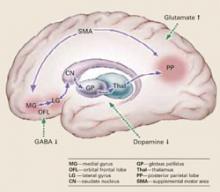
Pathophysiology. Catatonic signs develop when aberrant signals from neurochemical abnormalities trigger a neural circuit that affects the medial gyrus of the orbital frontal lobe, the lateral gyrus, caudate nucleus, globus pallidus, and thalamus (Box).3-5
Presentation. A focused exam is required because patients with catatonia often do not provide a comprehensive or reliable history.2 They show mutism, characteristic postures, rigidity, aberrant speech, negativism, and stereotyped behaviors.1,2 They may present in an excited or retarded state:
- Excited patients may injure themselves or others and develop hyperthermia, tachycardia, and elevated blood pressure from excessive motor activity.
- Patients in a retarded state may present with bradykinesia and poor self-care. They may be unresponsive to external stimuli, develop catatonic stupor, and refuse to eat or drink.
Mr. C’s earlier insomnia, excessive activity, and delusional thinking (such as the TV station incident) may have signaled an excited catatonia. On admission to the medical service, however, he presented in a retarded state.
Signs. Part of the challenge with detecting catatonia’s signs is that there are so many; some rating scales list more than 20. Not all signs need to be present to make the diagnosis, however, and if you find one, others usually turn up in the examination.
A mnemonic from the Bush-Francis Catatonia Screening Instrument (Table 2) represents diagnostic signs in patients with the excited or retarded forms.2 We recommend that you review an authoritative text (see Related resources) to understand catatonia’s psychopathology.2
Table 1
Common diagnoses of patients with catatonia
| Psychiatric |
|
| Organic |
|
Catatonia is caused by neurochemical abnormalities including low GABA activity in the frontal cortex, low dopamine (D2) activity in the basal ganglia, high glutamate—N-methyl-D-aspartate (NMDA)—activity in the parietal cortex, or a combination of these.3-5 Catatonic signs occur when these neurochemical changes cause aberrant signals and trigger a neural circuit affecting the medial gyrus of the orbital frontal lobe, the lateral gyrus, caudate nucleus, globus pallidus, and thalamus (Figure).
Posturing occurs when the aberrant signal reaches the posterior parietal lobe. Patients’ bizarre and mundane postures in catatonia are maintained by “anosognosia of position.” For example, an individual does not know the position of rest for his arm, and it remains in an unusual position as if at rest.3
The PP goes on to influence the supplemental motor area (SMA), causing bradykinesia, rigidity, and other motor phenomena that catatonia shares with Parkinson’s disease. The SMA feeds back to the medial orbital gyrus, completing the neural circuit.3
Regions such as the anterior cingulate area (ACA) and amygdala (1AMG) — also may be recruited into the expanded circuit. ACA recruitment may cause akinetic mutism, and fear is a symptom of AMG recruitment. If the anterior hypothalamus is affected, malignant catatonia or neuroleptic malignant syndrome may occur.3,5
This neural loop demonstrates an integrated model of psychosis. It may help explain why catatonia responds to treatment with lorazepam, ECT, and other agents such as antipsychotics and NMDA antagonists.
Illustration for CURRENT PSYCHIATRY by Marcia Hartsock, CMI
Table 2
WIRED `N MIRED: Mnemonic for detecting catatonia
| Waxy flexibility/catalepsy |
| Immobility/stupor |
| Refusal to eat or drink |
| Excitement |
| Deadpan staring |
| Negativism/negative symptoms |
| Mutism |
| Impulsivity |
| Rigidity |
| Echolalia/echopraxia |
| Direct observation |
CASE CONTINUED: MAKING THE DIAGNOSIS
In the medical unit, Mr. C was found to be in a catatonic stupor, with immobility, mutism (monosyllabic speech), catalepsy, intermittent waxy flexibility, withdrawal (refusal to eat and drink), automatic obedience, and mitgehen (exaggerated movements in response to light finger pressure, despite instructions to stay still). ECT workup was started, along with a trial of lorazepam, 1 mg tid.
Laboratory studies revealed high BUN/creatinine (80/2.0) that returned to normal range (BUN 7 to 21 mg/dL; creatinine 0.5 to 1.2 mg/dL) after 3 days of hydration. Because of Mr. C’s earlier excited symptoms and delusional thinking, we considered a diagnosis of bipolar disorder with catatonia. However, his symptoms did not improve with a trial of valproic acid (serum level 64 mcg/mL).
Head CT showed generalized atrophy and EEG showed delta slowing. Single-photo emission computer tomography (SPECT) showed areas of decreased perfusion in the cortex, with no perfusion in the left posterior parietal area (PP).
Mental status exam found Mr. C disoriented with poor short-term memory and unable to complete the Mini Mental State Examination (MMSE). His Bush-Francis Catatonia Rating Scale score was 28 and included many catatonic signs that would not be seen a patient with simple dehydration.
The workup supported a diagnosis of catatonia due to general medical condition (vascular dementia) and ruled out schizophrenia with catatonic features, bipolar disorder, or major depression with catatonia.
EVALUATION AND DIAGNOSIS
Medical causes. A careful history and thorough physical examination are essential for making an accurate diagnosis and ruling out medical conditions that could present with or mimic catatonia (Table 3). Medications that can induce catatonia include antipsychotics, corticosteroids, and disulfiram at therapeutic doses. Drug abuse (such as with phencyclidine), use of the general anesthetic ketamine, and benzodiazepine withdrawal may also lead to catatonia.
Head CT or MRI is indicated for patients being considered for ECT or for localizing neurologic findings. EEG can be useful when patients present with features of seizure activity—such as tongue biting, incontinence, or stupor—or with catatonia as a manifestation of delirium or dementia.
A history of head injury or neurologic disease warrants further neurologic investigation. Also consider a neurology consult when the patient has prolonged stupor or does not respond to initial drug therapy.
Psychiatric causes. The clinical setting may suggest the most likely primary psychiatric disorders to consider, such as:
- bipolar or major depression in acute inpatient psychiatric units
- autism and pervasive developmental disorders (PDD) in pediatric or PDD units
- catatonic schizophrenia in chronic psychotic patients
- somatoform or factitious disorders in forensic settings.
These generalizations are not clinically exclusive, of course, but may provide a starting point for the treatment team confronted with limited history and exam information.
Table 3
Catatonia workup: Recommended lab tests
| Test | Recommendation |
|---|---|
| Complete blood count with WBC differential | Look for leukocytosis |
| Serum chemistries | Look for electrolyte imbalances |
| Serum iron | May be low in NMS |
| Serum creatine kinase | If NMS is suspected |
| Brain MRI or CT | If structural lesion is suspected |
| Electroencephalography | If seizure disorder or brain abnormality is suspected |
| Lumbar puncture | If encephalitis or meningits is suspected |
| NMS: neuroleptic malignant syndrome | |
Initial treatment. Catatonia related to medical and psychiatric causes has been shown to respond to lorazepam and to ECT.6,7 Lorazepam is preferred because of its specificity for the GABAa receptor and ease of administration (oral, IM, or IV). Other agents that act on GABA—including amobarbital and zolpidem—have also been used. Catatonia’s hallmark features such as mutism and immobility have been shown to respond to lorazepam.8,9
ECT is a first-line treatment for catatonia with life-threatening conditions and should be considered for refractory cases.
Lorazepam. The starting dosage is usually 1 mg tid for healthy adults; 0.5 mg tid can be used for children and the elderly. Observe the patient for improvement in catatonic signs after the first dose and before giving the second. Dosages of up to 16 mg/d have been used.
In many cases, lorazepam can be tapered off after adequate treatment of the primary psychiatric condition. In severe cases, however—such as when patients refuse to eat or drink—lorazepam may be continued for as long as 1 year. Weigh the risk of benzodiazepine tolerance, dependence, and misuse versus the possibility of relapse and rehospitalization.
Medical catatonias and neuroleptic malignant syndrome (NMS) have responded favorably to ECT.8 Addressing the medical cause itself usually does not resolve catatonia, with the possible exception of seizure-induced (“ictal”) catatonia, which may respond to anticonvulsants and lorazepam.6,7
ECT. An ECT workup can begin as soon as a patient presents with catatonia. If lorazepam produces no response within 24 hours, consider ECT.
CASE CONTINUED: PERSISTENT SYMPTOMS
After three 1-mg doses of lorazepam, Mr. C became more alert and oriented but his catatonia symptoms persisted, as indicated by a Bush-Francis score of 23, significant grasp reflex, and gegenhalten (automatic rather than willful resistance to passive limb movement in proportion to the strength of the stimulus). An attempt to gradually increase lorazepam to 2 mg tid produced delirium. He remained confused even when lorazepam was reduced to 0.5 mg tid, so the drug was discontinued.
Mr. C’s neurologist added amantadine, 100 mg tid, and carbidopa/levodopa, 10/100 mg tid, to treat his parkinsonian rigidity.
WHAT NEXT? OTHER OPTIONS
Antipsychotics have been investigated as a possible treatment for catatonia. The literature suggests that conventional antipsychotics may cause catatonia and atypical antipsychotics may improve it. Conventional antipsychotics are best avoided in catatonia because they:
- appear less effective than other treatments in resolving catatonic symptoms8,10
- are associated with catatonic-like side effects, such as rigidity, akinesia, and staring10
- appear to increase NMS risk in patients with catatonic symptoms.11,12
Atypicals appear more effective in treating catatonia and less likely to cause NMS. Case reports13,14 indicate many of these agents can be effective and well tolerated in treating catatonic symptoms, although this was not the case for Mr. C.
Anticonvulsants such as valproate15 and carbamazepine, 600 to 1200 mg/d,16 may take longer to work than lorazepam but may be options for patients who do not respond to benzodiazepines.8,9
Amantadine, an N-methyl-D-aspartate (NMDA) antagonist, has been used with some success for catatonia that does not unrespond to lorazepam.17 However, amantidine’s dopamine agonist activity could worsen underlying psychosis.
Memantine—another NMDA antagonist—differs from amantadine despite having a similar chemical structure. Memantine is a noncompetitive antagonist at the NMDA receptor, without affinity for dopamine, norepinephrine, serotonin, or muscarinic receptors.18
Although no published data support using memantine in patients with catatonia, it might be considered for those who are not candidates for lorazepam or ECT. For instance, a double-blind, placebo-controlled study found that lorazepam was not effective for catatonic schizophrenia.19 We have found memantine to help in some patients with catatonic schizophrenia.
CASE CONTINUED: TRIAL OF MEMANTINE
Mr. C remained in a catatonic stupor, but we decided against ECT because he resumed eating and drinking and was not medically at risk. Quetiapine, 100 to 300 mg/d, was tried to address his dementia symptoms, confusion, and poor mentation. This trial was discontinued after Mr. C fell and was readmitted to the medical unit. We then added memantine, 5 mg bid.
In the first week after beginning memantine, Mr. C’s MMSE score was 21, consistent with vascular dementia, but he remained immobile and staring. Motor signs also persisted, including automatic obedience, ambitendency, and a grasp reflex.
The next week, we increased memantine to 10 mg bid. Mr. C was oriented to person, place, and time, and his affect was blunted. His MMSE score increased to 25, showing improved cognition and memory. His Bush-Francis scale score was 6, showing reduced catatonic signs, with remaining mild immobility, bradykinesia, speech-prompt mutism, staring, and grasp reflex.
He maintained this improvement on carbidopa/levodopa, 10/100 tid; amantadine, 100 mg tid; and memantine, 10 mg bid, and was discharged from the nursing home unit.
IMPROVEMENT WITH MEMANTINE
Memantine may reduce excess glutamate at the NMDA receptor in the parietal-SMA-frontal cortical circuit. It may help to increase GABA and dopamine, which are deficient in catatonia. Our patient with vascular dementia had a severe ischemic deficit in the posterior parietal area, as seen on SPECT.
Amantadine, another NMDA receptor antagonist, acts on dopamine neurons and may have anticholinergic-like side effects, whereas memantine does not. Although both drugs share antagonism at the NMDA glutamate receptor, noncompetitive binding is weak for amantadine and moderate for memantine. Memantine has some serotonin (5-HT3) antagonism, but neither agent has direct GABA activity.
Memantine can improve function in vascular dementia.20 Thus, Mr. C’s improvement may have been caused by the drug’s effect on his vascular dementia, the primary neuropsychiatric illness. However, his catatonic signs improved without antipsychotics, cholinesterase inhibitors, benzodiazepines, or ECT. No anticoagulation treatment or cerebral perfusion procedures account for his improved mental status.
CASE CONCLUSION
Mr. C went to live with his son’s family. Although he has problems with calculation, he shows good selfcare. When asked why he did not respond during his catatonic stupor, Mr. C stated that he believed the physician was an Internal Revenue Service agent asking him about serious tax problems. Upon reflection, he said he no longer believes this.
- Fink M, Taylor MA. Catatonia: a clinician’s guide to diagnosis and treatment. Cambridge, UK: Cambridge University Press, 2003.
- Caroff SN, Mann SC, Francis A, Fricchione GE. Catatonia: from psychopathology to neurobiology. Washington, DC: American Psychiatric Publishing, 2004.
- Mann SC, Caroff SN, Keck PE Jr, Lazarus A. Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Publishing, 2003.
- Neuroleptic Malignant Syndrome Information Service. www.NMSIS.org.
Drug brand names
- Amantadine • Symmetrel
- Amobarbital • Amytal sodium
- Carbamazepine • Carbatrol, Equetro
- Carbidopa/levodopa • Sinemet
- Disulfiram • Antabuse
- Divalproex • Depakote
- Furosemide • Lasix
- Lisinopril • Prinivil, Zestril
- Lorazepam • Ativan
- Memantine • Namenda
- Metoprolol • Lopressor
- Ranitidine • Zantac
- Simvastatin • Zocor
- Valproic acid • Depakene
- Zolpidem • Ambien
Disclosure
Dr. Carroll and Dr. Hawkins are speakers for Forest Laboratories. The other authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The authors thank Dr. Niraj Ahuja, consultant psychiatrist and honorary clinical lecturer (psychiatry), Newcastle, North Tyneside and Northumberland Mental Health Trust, UK, for assistance with the figure.
1. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry 2003;160:1233-41.
2. Bush G, Fink M, Petrides G, et al. Catatonia I: Rating scale and standardized examination. Acta Psychiatr Scand 1996;93:129-36.
3. Northoff G. What catatonia can tell us about “top-down” modulation:” a neuropsychiatric hypothesis. Brain Behav Sci 2002;25:555-604.
4. Carroll BT. The universal field hypothesis of catatonia and neuroleptic malignant syndrome. CNS Spectrums 2000;5(7):26-33.
5. Carroll BT. Catatonia is the rosetta stone of psychosis (poster presentation). New York: American Psychiatric Association annual meeting, 2004.
6. Barnes MP, Saunders M, Walls TJ, et al. The syndrome of Karl Ludwig Kahlbaum. J Neurol Neurosurg Psychiatry 1986;49:991-6.
7. Carroll BT, Anfinson TJ, Kennedy JC, et al. Catatonic disorder due to general medical conditions. J Neuropsychiatry Clin Neurosci 1994;6:122-33.
8. Hawkins JM, Archer KJ, Strakowski SM, Keck PE. Somatic treatments of catatonia. Int J Psychiatry Med 1995;25:345-69.
9. Rosebush PI, Hildebrand AM, Furlong BG, Mazurek MF. Catatonic syndrome in a general psychiatric inpatient population: frequency, clinical presentation, and response to lorazepam. J Clin Psychiatry 1990;51:357-62.
10. Dose M. Neuroleptic-induced pseudo-catatonia. Pharmacopsychiatry 2001;34:262-4.
11. Keck PE, Jr, Pope HG, Jr, Cohen BM, et al. Risk factors for neuroleptic malignant syndrome. Arch Gen Psychiatry 1989;46:914-18.
12. White DAC. 17 catatonic patients diagnosed as neuroleptic malignant syndrome. CNS Spectrums 2000;5:58-65.
13. Levy WO, Nunez CY. Use of ziprasidone to treat bipolar-associated catatonia. Bipolar Disord 2004;6:166-7.
14. Hesslinger B, Walden J, Normann C. Acute and long-term treatment of catatonia with risperidone. Pharmacopsychiatry 2001;34:25-6.
15. Kruger S, Braunig P. Intravenous valproic acid in the treatment of severe catatonia. J Neuropsychiatry Clin Neurosci 2001;13:303-4.
16. Kritzinger PR, Jordaan GP. Catatonia: an open prospective series with carbamazepine. Int J Neuropsychopharmacol 2001;4:251-7.
17. Northoff G, Eckert J, Fritze J. Glutamatergic dysfunction in catatonia? Successful treatment of three acute akinetic catatonic patients with the NMDA antagonist amantadine. J Neurol Neurosurg Psychiatry 1997;62:404-6.
18. Namenda (memantine) Package labeling. Forest Laboratories, 2004.
19. Ungvari GS, Chie HFK, Chow LY, et al. Lorazepam for chronic catatonia: a random, double-blind, placebo-controlled, cross-over study. Psychopharmacol 1999;142:393-8.
20. Mobius HJ. Pharmacologic rationale for memantine in chronic cerebral hypoperfusion, especially vascular dementia. Alz Dis Assoc Disord 1999;13(suppl 3):172-8.
Many catatonia cases respond to benzodiazepines—especially lorazepam—but up to 30% do not. Electroconvulsive therapy (ECT) can be effective, but what’s the next step when ECT is unavailable or inappropriate for your patient?
To help you solve this dilemma, we describe our diagnosis and treatment decisions for a patient we call Mr. C. We explain how our process was guided by recent understandings of an abnormal neural circuit that appears to cause catatonia’s complex motor and behavioral symptoms.
This article describes that neurologic pathology and answers common questions about the clinical workup and treatment of catatonia.
CASE: TROUBLE IN TV LAND
Mr. C, age 69, caused a disturbance at a local TV station, demanding that they broadcast a manuscript he had written. Police took him to a local hospital, where he was stabilized and then transferred to a neuropsychiatric hospital for evaluation.
The psychiatric interview revealed that he had developed insomnia, excessive activity, and delusional thinking 2 weeks before admission. His medical history included coronary artery disease (CAD), hypertension, and hypothyroidism. Medications included thyroid hormone replacement therapy, furosemide, potassium, ranitidine, simvastatin, metoprolol, and lisinopril. CAD treatment included stent placement and nitroglycerin as needed.
He had been hospitalized in his 30s and treated with ECT for what he called “bad thoughts.” He said he improved after 1 month and had no subsequent psychiatric history. He denied drug or alcohol abuse.
Shortly after admission, he refused to eat or drink and after 1 week became dehydrated. He also showed mutism, immobility, and stupor. He was transferred to the medical service for IV rehydration.
MANY SCENARIOS AND SIGNS
Mr. C’s symptoms suggest possible catatonia, a neuropsychiatric syndrome of motor dysregulation found in up to 10% of acutely ill psychiatric inpatients.1,2 A movement disorder,1,2 catatonia occurs with general medical conditions and psychiatric disorders (Table 1).
Pathophysiology. Catatonic signs develop when aberrant signals from neurochemical abnormalities trigger a neural circuit that affects the medial gyrus of the orbital frontal lobe, the lateral gyrus, caudate nucleus, globus pallidus, and thalamus (Box).3-5
Presentation. A focused exam is required because patients with catatonia often do not provide a comprehensive or reliable history.2 They show mutism, characteristic postures, rigidity, aberrant speech, negativism, and stereotyped behaviors.1,2 They may present in an excited or retarded state:
- Excited patients may injure themselves or others and develop hyperthermia, tachycardia, and elevated blood pressure from excessive motor activity.
- Patients in a retarded state may present with bradykinesia and poor self-care. They may be unresponsive to external stimuli, develop catatonic stupor, and refuse to eat or drink.
Mr. C’s earlier insomnia, excessive activity, and delusional thinking (such as the TV station incident) may have signaled an excited catatonia. On admission to the medical service, however, he presented in a retarded state.
Signs. Part of the challenge with detecting catatonia’s signs is that there are so many; some rating scales list more than 20. Not all signs need to be present to make the diagnosis, however, and if you find one, others usually turn up in the examination.
A mnemonic from the Bush-Francis Catatonia Screening Instrument (Table 2) represents diagnostic signs in patients with the excited or retarded forms.2 We recommend that you review an authoritative text (see Related resources) to understand catatonia’s psychopathology.2
Table 1
Common diagnoses of patients with catatonia
| Psychiatric |
|
| Organic |
|
Catatonia is caused by neurochemical abnormalities including low GABA activity in the frontal cortex, low dopamine (D2) activity in the basal ganglia, high glutamate—N-methyl-D-aspartate (NMDA)—activity in the parietal cortex, or a combination of these.3-5 Catatonic signs occur when these neurochemical changes cause aberrant signals and trigger a neural circuit affecting the medial gyrus of the orbital frontal lobe, the lateral gyrus, caudate nucleus, globus pallidus, and thalamus (Figure).
Posturing occurs when the aberrant signal reaches the posterior parietal lobe. Patients’ bizarre and mundane postures in catatonia are maintained by “anosognosia of position.” For example, an individual does not know the position of rest for his arm, and it remains in an unusual position as if at rest.3
The PP goes on to influence the supplemental motor area (SMA), causing bradykinesia, rigidity, and other motor phenomena that catatonia shares with Parkinson’s disease. The SMA feeds back to the medial orbital gyrus, completing the neural circuit.3
Regions such as the anterior cingulate area (ACA) and amygdala (1AMG) — also may be recruited into the expanded circuit. ACA recruitment may cause akinetic mutism, and fear is a symptom of AMG recruitment. If the anterior hypothalamus is affected, malignant catatonia or neuroleptic malignant syndrome may occur.3,5
This neural loop demonstrates an integrated model of psychosis. It may help explain why catatonia responds to treatment with lorazepam, ECT, and other agents such as antipsychotics and NMDA antagonists.
Illustration for CURRENT PSYCHIATRY by Marcia Hartsock, CMI
Table 2
WIRED `N MIRED: Mnemonic for detecting catatonia
| Waxy flexibility/catalepsy |
| Immobility/stupor |
| Refusal to eat or drink |
| Excitement |
| Deadpan staring |
| Negativism/negative symptoms |
| Mutism |
| Impulsivity |
| Rigidity |
| Echolalia/echopraxia |
| Direct observation |
CASE CONTINUED: MAKING THE DIAGNOSIS
In the medical unit, Mr. C was found to be in a catatonic stupor, with immobility, mutism (monosyllabic speech), catalepsy, intermittent waxy flexibility, withdrawal (refusal to eat and drink), automatic obedience, and mitgehen (exaggerated movements in response to light finger pressure, despite instructions to stay still). ECT workup was started, along with a trial of lorazepam, 1 mg tid.
Laboratory studies revealed high BUN/creatinine (80/2.0) that returned to normal range (BUN 7 to 21 mg/dL; creatinine 0.5 to 1.2 mg/dL) after 3 days of hydration. Because of Mr. C’s earlier excited symptoms and delusional thinking, we considered a diagnosis of bipolar disorder with catatonia. However, his symptoms did not improve with a trial of valproic acid (serum level 64 mcg/mL).
Head CT showed generalized atrophy and EEG showed delta slowing. Single-photo emission computer tomography (SPECT) showed areas of decreased perfusion in the cortex, with no perfusion in the left posterior parietal area (PP).
Mental status exam found Mr. C disoriented with poor short-term memory and unable to complete the Mini Mental State Examination (MMSE). His Bush-Francis Catatonia Rating Scale score was 28 and included many catatonic signs that would not be seen a patient with simple dehydration.
The workup supported a diagnosis of catatonia due to general medical condition (vascular dementia) and ruled out schizophrenia with catatonic features, bipolar disorder, or major depression with catatonia.
EVALUATION AND DIAGNOSIS
Medical causes. A careful history and thorough physical examination are essential for making an accurate diagnosis and ruling out medical conditions that could present with or mimic catatonia (Table 3). Medications that can induce catatonia include antipsychotics, corticosteroids, and disulfiram at therapeutic doses. Drug abuse (such as with phencyclidine), use of the general anesthetic ketamine, and benzodiazepine withdrawal may also lead to catatonia.
Head CT or MRI is indicated for patients being considered for ECT or for localizing neurologic findings. EEG can be useful when patients present with features of seizure activity—such as tongue biting, incontinence, or stupor—or with catatonia as a manifestation of delirium or dementia.
A history of head injury or neurologic disease warrants further neurologic investigation. Also consider a neurology consult when the patient has prolonged stupor or does not respond to initial drug therapy.
Psychiatric causes. The clinical setting may suggest the most likely primary psychiatric disorders to consider, such as:
- bipolar or major depression in acute inpatient psychiatric units
- autism and pervasive developmental disorders (PDD) in pediatric or PDD units
- catatonic schizophrenia in chronic psychotic patients
- somatoform or factitious disorders in forensic settings.
These generalizations are not clinically exclusive, of course, but may provide a starting point for the treatment team confronted with limited history and exam information.
Table 3
Catatonia workup: Recommended lab tests
| Test | Recommendation |
|---|---|
| Complete blood count with WBC differential | Look for leukocytosis |
| Serum chemistries | Look for electrolyte imbalances |
| Serum iron | May be low in NMS |
| Serum creatine kinase | If NMS is suspected |
| Brain MRI or CT | If structural lesion is suspected |
| Electroencephalography | If seizure disorder or brain abnormality is suspected |
| Lumbar puncture | If encephalitis or meningits is suspected |
| NMS: neuroleptic malignant syndrome | |
Initial treatment. Catatonia related to medical and psychiatric causes has been shown to respond to lorazepam and to ECT.6,7 Lorazepam is preferred because of its specificity for the GABAa receptor and ease of administration (oral, IM, or IV). Other agents that act on GABA—including amobarbital and zolpidem—have also been used. Catatonia’s hallmark features such as mutism and immobility have been shown to respond to lorazepam.8,9
ECT is a first-line treatment for catatonia with life-threatening conditions and should be considered for refractory cases.
Lorazepam. The starting dosage is usually 1 mg tid for healthy adults; 0.5 mg tid can be used for children and the elderly. Observe the patient for improvement in catatonic signs after the first dose and before giving the second. Dosages of up to 16 mg/d have been used.
In many cases, lorazepam can be tapered off after adequate treatment of the primary psychiatric condition. In severe cases, however—such as when patients refuse to eat or drink—lorazepam may be continued for as long as 1 year. Weigh the risk of benzodiazepine tolerance, dependence, and misuse versus the possibility of relapse and rehospitalization.
Medical catatonias and neuroleptic malignant syndrome (NMS) have responded favorably to ECT.8 Addressing the medical cause itself usually does not resolve catatonia, with the possible exception of seizure-induced (“ictal”) catatonia, which may respond to anticonvulsants and lorazepam.6,7
ECT. An ECT workup can begin as soon as a patient presents with catatonia. If lorazepam produces no response within 24 hours, consider ECT.
CASE CONTINUED: PERSISTENT SYMPTOMS
After three 1-mg doses of lorazepam, Mr. C became more alert and oriented but his catatonia symptoms persisted, as indicated by a Bush-Francis score of 23, significant grasp reflex, and gegenhalten (automatic rather than willful resistance to passive limb movement in proportion to the strength of the stimulus). An attempt to gradually increase lorazepam to 2 mg tid produced delirium. He remained confused even when lorazepam was reduced to 0.5 mg tid, so the drug was discontinued.
Mr. C’s neurologist added amantadine, 100 mg tid, and carbidopa/levodopa, 10/100 mg tid, to treat his parkinsonian rigidity.
WHAT NEXT? OTHER OPTIONS
Antipsychotics have been investigated as a possible treatment for catatonia. The literature suggests that conventional antipsychotics may cause catatonia and atypical antipsychotics may improve it. Conventional antipsychotics are best avoided in catatonia because they:
- appear less effective than other treatments in resolving catatonic symptoms8,10
- are associated with catatonic-like side effects, such as rigidity, akinesia, and staring10
- appear to increase NMS risk in patients with catatonic symptoms.11,12
Atypicals appear more effective in treating catatonia and less likely to cause NMS. Case reports13,14 indicate many of these agents can be effective and well tolerated in treating catatonic symptoms, although this was not the case for Mr. C.
Anticonvulsants such as valproate15 and carbamazepine, 600 to 1200 mg/d,16 may take longer to work than lorazepam but may be options for patients who do not respond to benzodiazepines.8,9
Amantadine, an N-methyl-D-aspartate (NMDA) antagonist, has been used with some success for catatonia that does not unrespond to lorazepam.17 However, amantidine’s dopamine agonist activity could worsen underlying psychosis.
Memantine—another NMDA antagonist—differs from amantadine despite having a similar chemical structure. Memantine is a noncompetitive antagonist at the NMDA receptor, without affinity for dopamine, norepinephrine, serotonin, or muscarinic receptors.18
Although no published data support using memantine in patients with catatonia, it might be considered for those who are not candidates for lorazepam or ECT. For instance, a double-blind, placebo-controlled study found that lorazepam was not effective for catatonic schizophrenia.19 We have found memantine to help in some patients with catatonic schizophrenia.
CASE CONTINUED: TRIAL OF MEMANTINE
Mr. C remained in a catatonic stupor, but we decided against ECT because he resumed eating and drinking and was not medically at risk. Quetiapine, 100 to 300 mg/d, was tried to address his dementia symptoms, confusion, and poor mentation. This trial was discontinued after Mr. C fell and was readmitted to the medical unit. We then added memantine, 5 mg bid.
In the first week after beginning memantine, Mr. C’s MMSE score was 21, consistent with vascular dementia, but he remained immobile and staring. Motor signs also persisted, including automatic obedience, ambitendency, and a grasp reflex.
The next week, we increased memantine to 10 mg bid. Mr. C was oriented to person, place, and time, and his affect was blunted. His MMSE score increased to 25, showing improved cognition and memory. His Bush-Francis scale score was 6, showing reduced catatonic signs, with remaining mild immobility, bradykinesia, speech-prompt mutism, staring, and grasp reflex.
He maintained this improvement on carbidopa/levodopa, 10/100 tid; amantadine, 100 mg tid; and memantine, 10 mg bid, and was discharged from the nursing home unit.
IMPROVEMENT WITH MEMANTINE
Memantine may reduce excess glutamate at the NMDA receptor in the parietal-SMA-frontal cortical circuit. It may help to increase GABA and dopamine, which are deficient in catatonia. Our patient with vascular dementia had a severe ischemic deficit in the posterior parietal area, as seen on SPECT.
Amantadine, another NMDA receptor antagonist, acts on dopamine neurons and may have anticholinergic-like side effects, whereas memantine does not. Although both drugs share antagonism at the NMDA glutamate receptor, noncompetitive binding is weak for amantadine and moderate for memantine. Memantine has some serotonin (5-HT3) antagonism, but neither agent has direct GABA activity.
Memantine can improve function in vascular dementia.20 Thus, Mr. C’s improvement may have been caused by the drug’s effect on his vascular dementia, the primary neuropsychiatric illness. However, his catatonic signs improved without antipsychotics, cholinesterase inhibitors, benzodiazepines, or ECT. No anticoagulation treatment or cerebral perfusion procedures account for his improved mental status.
CASE CONCLUSION
Mr. C went to live with his son’s family. Although he has problems with calculation, he shows good selfcare. When asked why he did not respond during his catatonic stupor, Mr. C stated that he believed the physician was an Internal Revenue Service agent asking him about serious tax problems. Upon reflection, he said he no longer believes this.
- Fink M, Taylor MA. Catatonia: a clinician’s guide to diagnosis and treatment. Cambridge, UK: Cambridge University Press, 2003.
- Caroff SN, Mann SC, Francis A, Fricchione GE. Catatonia: from psychopathology to neurobiology. Washington, DC: American Psychiatric Publishing, 2004.
- Mann SC, Caroff SN, Keck PE Jr, Lazarus A. Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Publishing, 2003.
- Neuroleptic Malignant Syndrome Information Service. www.NMSIS.org.
Drug brand names
- Amantadine • Symmetrel
- Amobarbital • Amytal sodium
- Carbamazepine • Carbatrol, Equetro
- Carbidopa/levodopa • Sinemet
- Disulfiram • Antabuse
- Divalproex • Depakote
- Furosemide • Lasix
- Lisinopril • Prinivil, Zestril
- Lorazepam • Ativan
- Memantine • Namenda
- Metoprolol • Lopressor
- Ranitidine • Zantac
- Simvastatin • Zocor
- Valproic acid • Depakene
- Zolpidem • Ambien
Disclosure
Dr. Carroll and Dr. Hawkins are speakers for Forest Laboratories. The other authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The authors thank Dr. Niraj Ahuja, consultant psychiatrist and honorary clinical lecturer (psychiatry), Newcastle, North Tyneside and Northumberland Mental Health Trust, UK, for assistance with the figure.
Many catatonia cases respond to benzodiazepines—especially lorazepam—but up to 30% do not. Electroconvulsive therapy (ECT) can be effective, but what’s the next step when ECT is unavailable or inappropriate for your patient?
To help you solve this dilemma, we describe our diagnosis and treatment decisions for a patient we call Mr. C. We explain how our process was guided by recent understandings of an abnormal neural circuit that appears to cause catatonia’s complex motor and behavioral symptoms.
This article describes that neurologic pathology and answers common questions about the clinical workup and treatment of catatonia.
CASE: TROUBLE IN TV LAND
Mr. C, age 69, caused a disturbance at a local TV station, demanding that they broadcast a manuscript he had written. Police took him to a local hospital, where he was stabilized and then transferred to a neuropsychiatric hospital for evaluation.
The psychiatric interview revealed that he had developed insomnia, excessive activity, and delusional thinking 2 weeks before admission. His medical history included coronary artery disease (CAD), hypertension, and hypothyroidism. Medications included thyroid hormone replacement therapy, furosemide, potassium, ranitidine, simvastatin, metoprolol, and lisinopril. CAD treatment included stent placement and nitroglycerin as needed.
He had been hospitalized in his 30s and treated with ECT for what he called “bad thoughts.” He said he improved after 1 month and had no subsequent psychiatric history. He denied drug or alcohol abuse.
Shortly after admission, he refused to eat or drink and after 1 week became dehydrated. He also showed mutism, immobility, and stupor. He was transferred to the medical service for IV rehydration.
MANY SCENARIOS AND SIGNS
Mr. C’s symptoms suggest possible catatonia, a neuropsychiatric syndrome of motor dysregulation found in up to 10% of acutely ill psychiatric inpatients.1,2 A movement disorder,1,2 catatonia occurs with general medical conditions and psychiatric disorders (Table 1).
Pathophysiology. Catatonic signs develop when aberrant signals from neurochemical abnormalities trigger a neural circuit that affects the medial gyrus of the orbital frontal lobe, the lateral gyrus, caudate nucleus, globus pallidus, and thalamus (Box).3-5
Presentation. A focused exam is required because patients with catatonia often do not provide a comprehensive or reliable history.2 They show mutism, characteristic postures, rigidity, aberrant speech, negativism, and stereotyped behaviors.1,2 They may present in an excited or retarded state:
- Excited patients may injure themselves or others and develop hyperthermia, tachycardia, and elevated blood pressure from excessive motor activity.
- Patients in a retarded state may present with bradykinesia and poor self-care. They may be unresponsive to external stimuli, develop catatonic stupor, and refuse to eat or drink.
Mr. C’s earlier insomnia, excessive activity, and delusional thinking (such as the TV station incident) may have signaled an excited catatonia. On admission to the medical service, however, he presented in a retarded state.
Signs. Part of the challenge with detecting catatonia’s signs is that there are so many; some rating scales list more than 20. Not all signs need to be present to make the diagnosis, however, and if you find one, others usually turn up in the examination.
A mnemonic from the Bush-Francis Catatonia Screening Instrument (Table 2) represents diagnostic signs in patients with the excited or retarded forms.2 We recommend that you review an authoritative text (see Related resources) to understand catatonia’s psychopathology.2
Table 1
Common diagnoses of patients with catatonia
| Psychiatric |
|
| Organic |
|
Catatonia is caused by neurochemical abnormalities including low GABA activity in the frontal cortex, low dopamine (D2) activity in the basal ganglia, high glutamate—N-methyl-D-aspartate (NMDA)—activity in the parietal cortex, or a combination of these.3-5 Catatonic signs occur when these neurochemical changes cause aberrant signals and trigger a neural circuit affecting the medial gyrus of the orbital frontal lobe, the lateral gyrus, caudate nucleus, globus pallidus, and thalamus (Figure).
Posturing occurs when the aberrant signal reaches the posterior parietal lobe. Patients’ bizarre and mundane postures in catatonia are maintained by “anosognosia of position.” For example, an individual does not know the position of rest for his arm, and it remains in an unusual position as if at rest.3
The PP goes on to influence the supplemental motor area (SMA), causing bradykinesia, rigidity, and other motor phenomena that catatonia shares with Parkinson’s disease. The SMA feeds back to the medial orbital gyrus, completing the neural circuit.3
Regions such as the anterior cingulate area (ACA) and amygdala (1AMG) — also may be recruited into the expanded circuit. ACA recruitment may cause akinetic mutism, and fear is a symptom of AMG recruitment. If the anterior hypothalamus is affected, malignant catatonia or neuroleptic malignant syndrome may occur.3,5
This neural loop demonstrates an integrated model of psychosis. It may help explain why catatonia responds to treatment with lorazepam, ECT, and other agents such as antipsychotics and NMDA antagonists.
Illustration for CURRENT PSYCHIATRY by Marcia Hartsock, CMI
Table 2
WIRED `N MIRED: Mnemonic for detecting catatonia
| Waxy flexibility/catalepsy |
| Immobility/stupor |
| Refusal to eat or drink |
| Excitement |
| Deadpan staring |
| Negativism/negative symptoms |
| Mutism |
| Impulsivity |
| Rigidity |
| Echolalia/echopraxia |
| Direct observation |
CASE CONTINUED: MAKING THE DIAGNOSIS
In the medical unit, Mr. C was found to be in a catatonic stupor, with immobility, mutism (monosyllabic speech), catalepsy, intermittent waxy flexibility, withdrawal (refusal to eat and drink), automatic obedience, and mitgehen (exaggerated movements in response to light finger pressure, despite instructions to stay still). ECT workup was started, along with a trial of lorazepam, 1 mg tid.
Laboratory studies revealed high BUN/creatinine (80/2.0) that returned to normal range (BUN 7 to 21 mg/dL; creatinine 0.5 to 1.2 mg/dL) after 3 days of hydration. Because of Mr. C’s earlier excited symptoms and delusional thinking, we considered a diagnosis of bipolar disorder with catatonia. However, his symptoms did not improve with a trial of valproic acid (serum level 64 mcg/mL).
Head CT showed generalized atrophy and EEG showed delta slowing. Single-photo emission computer tomography (SPECT) showed areas of decreased perfusion in the cortex, with no perfusion in the left posterior parietal area (PP).
Mental status exam found Mr. C disoriented with poor short-term memory and unable to complete the Mini Mental State Examination (MMSE). His Bush-Francis Catatonia Rating Scale score was 28 and included many catatonic signs that would not be seen a patient with simple dehydration.
The workup supported a diagnosis of catatonia due to general medical condition (vascular dementia) and ruled out schizophrenia with catatonic features, bipolar disorder, or major depression with catatonia.
EVALUATION AND DIAGNOSIS
Medical causes. A careful history and thorough physical examination are essential for making an accurate diagnosis and ruling out medical conditions that could present with or mimic catatonia (Table 3). Medications that can induce catatonia include antipsychotics, corticosteroids, and disulfiram at therapeutic doses. Drug abuse (such as with phencyclidine), use of the general anesthetic ketamine, and benzodiazepine withdrawal may also lead to catatonia.
Head CT or MRI is indicated for patients being considered for ECT or for localizing neurologic findings. EEG can be useful when patients present with features of seizure activity—such as tongue biting, incontinence, or stupor—or with catatonia as a manifestation of delirium or dementia.
A history of head injury or neurologic disease warrants further neurologic investigation. Also consider a neurology consult when the patient has prolonged stupor or does not respond to initial drug therapy.
Psychiatric causes. The clinical setting may suggest the most likely primary psychiatric disorders to consider, such as:
- bipolar or major depression in acute inpatient psychiatric units
- autism and pervasive developmental disorders (PDD) in pediatric or PDD units
- catatonic schizophrenia in chronic psychotic patients
- somatoform or factitious disorders in forensic settings.
These generalizations are not clinically exclusive, of course, but may provide a starting point for the treatment team confronted with limited history and exam information.
Table 3
Catatonia workup: Recommended lab tests
| Test | Recommendation |
|---|---|
| Complete blood count with WBC differential | Look for leukocytosis |
| Serum chemistries | Look for electrolyte imbalances |
| Serum iron | May be low in NMS |
| Serum creatine kinase | If NMS is suspected |
| Brain MRI or CT | If structural lesion is suspected |
| Electroencephalography | If seizure disorder or brain abnormality is suspected |
| Lumbar puncture | If encephalitis or meningits is suspected |
| NMS: neuroleptic malignant syndrome | |
Initial treatment. Catatonia related to medical and psychiatric causes has been shown to respond to lorazepam and to ECT.6,7 Lorazepam is preferred because of its specificity for the GABAa receptor and ease of administration (oral, IM, or IV). Other agents that act on GABA—including amobarbital and zolpidem—have also been used. Catatonia’s hallmark features such as mutism and immobility have been shown to respond to lorazepam.8,9
ECT is a first-line treatment for catatonia with life-threatening conditions and should be considered for refractory cases.
Lorazepam. The starting dosage is usually 1 mg tid for healthy adults; 0.5 mg tid can be used for children and the elderly. Observe the patient for improvement in catatonic signs after the first dose and before giving the second. Dosages of up to 16 mg/d have been used.
In many cases, lorazepam can be tapered off after adequate treatment of the primary psychiatric condition. In severe cases, however—such as when patients refuse to eat or drink—lorazepam may be continued for as long as 1 year. Weigh the risk of benzodiazepine tolerance, dependence, and misuse versus the possibility of relapse and rehospitalization.
Medical catatonias and neuroleptic malignant syndrome (NMS) have responded favorably to ECT.8 Addressing the medical cause itself usually does not resolve catatonia, with the possible exception of seizure-induced (“ictal”) catatonia, which may respond to anticonvulsants and lorazepam.6,7
ECT. An ECT workup can begin as soon as a patient presents with catatonia. If lorazepam produces no response within 24 hours, consider ECT.
CASE CONTINUED: PERSISTENT SYMPTOMS
After three 1-mg doses of lorazepam, Mr. C became more alert and oriented but his catatonia symptoms persisted, as indicated by a Bush-Francis score of 23, significant grasp reflex, and gegenhalten (automatic rather than willful resistance to passive limb movement in proportion to the strength of the stimulus). An attempt to gradually increase lorazepam to 2 mg tid produced delirium. He remained confused even when lorazepam was reduced to 0.5 mg tid, so the drug was discontinued.
Mr. C’s neurologist added amantadine, 100 mg tid, and carbidopa/levodopa, 10/100 mg tid, to treat his parkinsonian rigidity.
WHAT NEXT? OTHER OPTIONS
Antipsychotics have been investigated as a possible treatment for catatonia. The literature suggests that conventional antipsychotics may cause catatonia and atypical antipsychotics may improve it. Conventional antipsychotics are best avoided in catatonia because they:
- appear less effective than other treatments in resolving catatonic symptoms8,10
- are associated with catatonic-like side effects, such as rigidity, akinesia, and staring10
- appear to increase NMS risk in patients with catatonic symptoms.11,12
Atypicals appear more effective in treating catatonia and less likely to cause NMS. Case reports13,14 indicate many of these agents can be effective and well tolerated in treating catatonic symptoms, although this was not the case for Mr. C.
Anticonvulsants such as valproate15 and carbamazepine, 600 to 1200 mg/d,16 may take longer to work than lorazepam but may be options for patients who do not respond to benzodiazepines.8,9
Amantadine, an N-methyl-D-aspartate (NMDA) antagonist, has been used with some success for catatonia that does not unrespond to lorazepam.17 However, amantidine’s dopamine agonist activity could worsen underlying psychosis.
Memantine—another NMDA antagonist—differs from amantadine despite having a similar chemical structure. Memantine is a noncompetitive antagonist at the NMDA receptor, without affinity for dopamine, norepinephrine, serotonin, or muscarinic receptors.18
Although no published data support using memantine in patients with catatonia, it might be considered for those who are not candidates for lorazepam or ECT. For instance, a double-blind, placebo-controlled study found that lorazepam was not effective for catatonic schizophrenia.19 We have found memantine to help in some patients with catatonic schizophrenia.
CASE CONTINUED: TRIAL OF MEMANTINE
Mr. C remained in a catatonic stupor, but we decided against ECT because he resumed eating and drinking and was not medically at risk. Quetiapine, 100 to 300 mg/d, was tried to address his dementia symptoms, confusion, and poor mentation. This trial was discontinued after Mr. C fell and was readmitted to the medical unit. We then added memantine, 5 mg bid.
In the first week after beginning memantine, Mr. C’s MMSE score was 21, consistent with vascular dementia, but he remained immobile and staring. Motor signs also persisted, including automatic obedience, ambitendency, and a grasp reflex.
The next week, we increased memantine to 10 mg bid. Mr. C was oriented to person, place, and time, and his affect was blunted. His MMSE score increased to 25, showing improved cognition and memory. His Bush-Francis scale score was 6, showing reduced catatonic signs, with remaining mild immobility, bradykinesia, speech-prompt mutism, staring, and grasp reflex.
He maintained this improvement on carbidopa/levodopa, 10/100 tid; amantadine, 100 mg tid; and memantine, 10 mg bid, and was discharged from the nursing home unit.
IMPROVEMENT WITH MEMANTINE
Memantine may reduce excess glutamate at the NMDA receptor in the parietal-SMA-frontal cortical circuit. It may help to increase GABA and dopamine, which are deficient in catatonia. Our patient with vascular dementia had a severe ischemic deficit in the posterior parietal area, as seen on SPECT.
Amantadine, another NMDA receptor antagonist, acts on dopamine neurons and may have anticholinergic-like side effects, whereas memantine does not. Although both drugs share antagonism at the NMDA glutamate receptor, noncompetitive binding is weak for amantadine and moderate for memantine. Memantine has some serotonin (5-HT3) antagonism, but neither agent has direct GABA activity.
Memantine can improve function in vascular dementia.20 Thus, Mr. C’s improvement may have been caused by the drug’s effect on his vascular dementia, the primary neuropsychiatric illness. However, his catatonic signs improved without antipsychotics, cholinesterase inhibitors, benzodiazepines, or ECT. No anticoagulation treatment or cerebral perfusion procedures account for his improved mental status.
CASE CONCLUSION
Mr. C went to live with his son’s family. Although he has problems with calculation, he shows good selfcare. When asked why he did not respond during his catatonic stupor, Mr. C stated that he believed the physician was an Internal Revenue Service agent asking him about serious tax problems. Upon reflection, he said he no longer believes this.
- Fink M, Taylor MA. Catatonia: a clinician’s guide to diagnosis and treatment. Cambridge, UK: Cambridge University Press, 2003.
- Caroff SN, Mann SC, Francis A, Fricchione GE. Catatonia: from psychopathology to neurobiology. Washington, DC: American Psychiatric Publishing, 2004.
- Mann SC, Caroff SN, Keck PE Jr, Lazarus A. Neuroleptic malignant syndrome and related conditions (2nd ed). Washington, DC: American Psychiatric Publishing, 2003.
- Neuroleptic Malignant Syndrome Information Service. www.NMSIS.org.
Drug brand names
- Amantadine • Symmetrel
- Amobarbital • Amytal sodium
- Carbamazepine • Carbatrol, Equetro
- Carbidopa/levodopa • Sinemet
- Disulfiram • Antabuse
- Divalproex • Depakote
- Furosemide • Lasix
- Lisinopril • Prinivil, Zestril
- Lorazepam • Ativan
- Memantine • Namenda
- Metoprolol • Lopressor
- Ranitidine • Zantac
- Simvastatin • Zocor
- Valproic acid • Depakene
- Zolpidem • Ambien
Disclosure
Dr. Carroll and Dr. Hawkins are speakers for Forest Laboratories. The other authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
The authors thank Dr. Niraj Ahuja, consultant psychiatrist and honorary clinical lecturer (psychiatry), Newcastle, North Tyneside and Northumberland Mental Health Trust, UK, for assistance with the figure.
1. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry 2003;160:1233-41.
2. Bush G, Fink M, Petrides G, et al. Catatonia I: Rating scale and standardized examination. Acta Psychiatr Scand 1996;93:129-36.
3. Northoff G. What catatonia can tell us about “top-down” modulation:” a neuropsychiatric hypothesis. Brain Behav Sci 2002;25:555-604.
4. Carroll BT. The universal field hypothesis of catatonia and neuroleptic malignant syndrome. CNS Spectrums 2000;5(7):26-33.
5. Carroll BT. Catatonia is the rosetta stone of psychosis (poster presentation). New York: American Psychiatric Association annual meeting, 2004.
6. Barnes MP, Saunders M, Walls TJ, et al. The syndrome of Karl Ludwig Kahlbaum. J Neurol Neurosurg Psychiatry 1986;49:991-6.
7. Carroll BT, Anfinson TJ, Kennedy JC, et al. Catatonic disorder due to general medical conditions. J Neuropsychiatry Clin Neurosci 1994;6:122-33.
8. Hawkins JM, Archer KJ, Strakowski SM, Keck PE. Somatic treatments of catatonia. Int J Psychiatry Med 1995;25:345-69.
9. Rosebush PI, Hildebrand AM, Furlong BG, Mazurek MF. Catatonic syndrome in a general psychiatric inpatient population: frequency, clinical presentation, and response to lorazepam. J Clin Psychiatry 1990;51:357-62.
10. Dose M. Neuroleptic-induced pseudo-catatonia. Pharmacopsychiatry 2001;34:262-4.
11. Keck PE, Jr, Pope HG, Jr, Cohen BM, et al. Risk factors for neuroleptic malignant syndrome. Arch Gen Psychiatry 1989;46:914-18.
12. White DAC. 17 catatonic patients diagnosed as neuroleptic malignant syndrome. CNS Spectrums 2000;5:58-65.
13. Levy WO, Nunez CY. Use of ziprasidone to treat bipolar-associated catatonia. Bipolar Disord 2004;6:166-7.
14. Hesslinger B, Walden J, Normann C. Acute and long-term treatment of catatonia with risperidone. Pharmacopsychiatry 2001;34:25-6.
15. Kruger S, Braunig P. Intravenous valproic acid in the treatment of severe catatonia. J Neuropsychiatry Clin Neurosci 2001;13:303-4.
16. Kritzinger PR, Jordaan GP. Catatonia: an open prospective series with carbamazepine. Int J Neuropsychopharmacol 2001;4:251-7.
17. Northoff G, Eckert J, Fritze J. Glutamatergic dysfunction in catatonia? Successful treatment of three acute akinetic catatonic patients with the NMDA antagonist amantadine. J Neurol Neurosurg Psychiatry 1997;62:404-6.
18. Namenda (memantine) Package labeling. Forest Laboratories, 2004.
19. Ungvari GS, Chie HFK, Chow LY, et al. Lorazepam for chronic catatonia: a random, double-blind, placebo-controlled, cross-over study. Psychopharmacol 1999;142:393-8.
20. Mobius HJ. Pharmacologic rationale for memantine in chronic cerebral hypoperfusion, especially vascular dementia. Alz Dis Assoc Disord 1999;13(suppl 3):172-8.
1. Taylor MA, Fink M. Catatonia in psychiatric classification: a home of its own. Am J Psychiatry 2003;160:1233-41.
2. Bush G, Fink M, Petrides G, et al. Catatonia I: Rating scale and standardized examination. Acta Psychiatr Scand 1996;93:129-36.
3. Northoff G. What catatonia can tell us about “top-down” modulation:” a neuropsychiatric hypothesis. Brain Behav Sci 2002;25:555-604.
4. Carroll BT. The universal field hypothesis of catatonia and neuroleptic malignant syndrome. CNS Spectrums 2000;5(7):26-33.
5. Carroll BT. Catatonia is the rosetta stone of psychosis (poster presentation). New York: American Psychiatric Association annual meeting, 2004.
6. Barnes MP, Saunders M, Walls TJ, et al. The syndrome of Karl Ludwig Kahlbaum. J Neurol Neurosurg Psychiatry 1986;49:991-6.
7. Carroll BT, Anfinson TJ, Kennedy JC, et al. Catatonic disorder due to general medical conditions. J Neuropsychiatry Clin Neurosci 1994;6:122-33.
8. Hawkins JM, Archer KJ, Strakowski SM, Keck PE. Somatic treatments of catatonia. Int J Psychiatry Med 1995;25:345-69.
9. Rosebush PI, Hildebrand AM, Furlong BG, Mazurek MF. Catatonic syndrome in a general psychiatric inpatient population: frequency, clinical presentation, and response to lorazepam. J Clin Psychiatry 1990;51:357-62.
10. Dose M. Neuroleptic-induced pseudo-catatonia. Pharmacopsychiatry 2001;34:262-4.
11. Keck PE, Jr, Pope HG, Jr, Cohen BM, et al. Risk factors for neuroleptic malignant syndrome. Arch Gen Psychiatry 1989;46:914-18.
12. White DAC. 17 catatonic patients diagnosed as neuroleptic malignant syndrome. CNS Spectrums 2000;5:58-65.
13. Levy WO, Nunez CY. Use of ziprasidone to treat bipolar-associated catatonia. Bipolar Disord 2004;6:166-7.
14. Hesslinger B, Walden J, Normann C. Acute and long-term treatment of catatonia with risperidone. Pharmacopsychiatry 2001;34:25-6.
15. Kruger S, Braunig P. Intravenous valproic acid in the treatment of severe catatonia. J Neuropsychiatry Clin Neurosci 2001;13:303-4.
16. Kritzinger PR, Jordaan GP. Catatonia: an open prospective series with carbamazepine. Int J Neuropsychopharmacol 2001;4:251-7.
17. Northoff G, Eckert J, Fritze J. Glutamatergic dysfunction in catatonia? Successful treatment of three acute akinetic catatonic patients with the NMDA antagonist amantadine. J Neurol Neurosurg Psychiatry 1997;62:404-6.
18. Namenda (memantine) Package labeling. Forest Laboratories, 2004.
19. Ungvari GS, Chie HFK, Chow LY, et al. Lorazepam for chronic catatonia: a random, double-blind, placebo-controlled, cross-over study. Psychopharmacol 1999;142:393-8.
20. Mobius HJ. Pharmacologic rationale for memantine in chronic cerebral hypoperfusion, especially vascular dementia. Alz Dis Assoc Disord 1999;13(suppl 3):172-8.
Blame the brain for promiscuity?
Why do some people stay married for decades while others jump from one relationship to the next? If humans are like rodents, recent research suggests that neuropeptides may help forge the ties that bind.
FOLLOWING VOLE BEHAVIOR
The vole, which inhabits many grasslands in the United States, is a rodent resembling a mouse but related to the lemming. Males in some vole species (such as meadow voles) show no partner preference whereas males in other species prefer one partner, share the same nest with her, and help care for their offspring.1
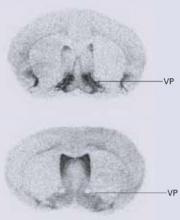
The neuropeptides oxytocin and arginine vasopressin are mediators of pair bonding. The brain of a prairie vole—a species in which males tend to bond with female partners—has more vasopressin receptors than that of the solitary meadow vole (Figure 1).
Figure 1 More vasopressin in bonding voles
Ventral forebrain autoradiograms show greater vasopressin (VP) expression in prairie voles (top) than meadow voles.
Reprinted with permission. © 2004, Nature Publishing Group.Lim et al, however, found that meadow voles were more likely to bond with a single female after the males’ vasopressin receptors were increased.2 The researchers isolated and replicated the gene sequence responsible for the vasopressin receptor in the prairie vole. Then, using a viral vector, they injected the gene into the ventral pallidum of 11 male meadow voles. Eleven other voles received placebo.
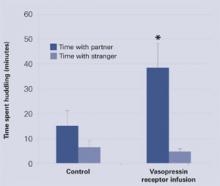
Two weeks later, each sexually naïve male vole was housed for 24 hours with a sexually receptive female vole. Then, each male was placed for 3 hours in a three-chamber apparatus with the partner female in one chamber and a novel female in another. The time spent huddling with each female was recorded.
Across 3 hours, the placebo group voles spent 10 to 15 minutes with either female—normal behavior for this species. By contrast, the voles that received the vasopressin receptors spent approximately 40 minutes with their partners but only about 5 minutes with the novel voles, thus showing more affiliative behavior and a clear preference for their mates (Figure 2). The findings suggest that the researchers may have produced a profound change in social behavior by altering one gene.
IMPLICATIONS FOR HUMANS
How this research relates to humans is unknown, as there is no sound evidence of a link between vole and human pair bonding. Likewise, the influence of the higher cortical areas in orchestrating human behaviors cannot be underestimated.
Neuroimaging, however, has shown that brain regions rich with oxytocin and vasopressin receptors are activated while a person views pictures of loved ones.3 Additionally, mens’ vasopressin levels have been shown to increase when they are sexually aroused.4 Whether these findings one day lead to a medicine that promotes monogamous behavior in men remains to be seen.5
Figure 2 Male meadow voles’ interactions with females after vasopressin or placebo treatment
Source: Adapted from reference 2
1. Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci 2004;7:1048-54.
2. Lim MM, Wang Z, Olazabal DE, et al. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature 2004;429(6993):754-7.
3. Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage 2004;21:1155-66.
4. Murphy MR, Seckl JR, Burton S, et al. Changes in oxytocin and vasopressin secretion during sexual activity in men. J Clin Endocrinol Metab 1987;65:738-41.
5. Konner M. The ties that bind. Nature 2004;429(6993):705.-
Why do some people stay married for decades while others jump from one relationship to the next? If humans are like rodents, recent research suggests that neuropeptides may help forge the ties that bind.
FOLLOWING VOLE BEHAVIOR
The vole, which inhabits many grasslands in the United States, is a rodent resembling a mouse but related to the lemming. Males in some vole species (such as meadow voles) show no partner preference whereas males in other species prefer one partner, share the same nest with her, and help care for their offspring.1
The neuropeptides oxytocin and arginine vasopressin are mediators of pair bonding. The brain of a prairie vole—a species in which males tend to bond with female partners—has more vasopressin receptors than that of the solitary meadow vole (Figure 1).
Figure 1 More vasopressin in bonding voles
Ventral forebrain autoradiograms show greater vasopressin (VP) expression in prairie voles (top) than meadow voles.
Reprinted with permission. © 2004, Nature Publishing Group.Lim et al, however, found that meadow voles were more likely to bond with a single female after the males’ vasopressin receptors were increased.2 The researchers isolated and replicated the gene sequence responsible for the vasopressin receptor in the prairie vole. Then, using a viral vector, they injected the gene into the ventral pallidum of 11 male meadow voles. Eleven other voles received placebo.
Two weeks later, each sexually naïve male vole was housed for 24 hours with a sexually receptive female vole. Then, each male was placed for 3 hours in a three-chamber apparatus with the partner female in one chamber and a novel female in another. The time spent huddling with each female was recorded.
Across 3 hours, the placebo group voles spent 10 to 15 minutes with either female—normal behavior for this species. By contrast, the voles that received the vasopressin receptors spent approximately 40 minutes with their partners but only about 5 minutes with the novel voles, thus showing more affiliative behavior and a clear preference for their mates (Figure 2). The findings suggest that the researchers may have produced a profound change in social behavior by altering one gene.
IMPLICATIONS FOR HUMANS
How this research relates to humans is unknown, as there is no sound evidence of a link between vole and human pair bonding. Likewise, the influence of the higher cortical areas in orchestrating human behaviors cannot be underestimated.
Neuroimaging, however, has shown that brain regions rich with oxytocin and vasopressin receptors are activated while a person views pictures of loved ones.3 Additionally, mens’ vasopressin levels have been shown to increase when they are sexually aroused.4 Whether these findings one day lead to a medicine that promotes monogamous behavior in men remains to be seen.5
Figure 2 Male meadow voles’ interactions with females after vasopressin or placebo treatment
Source: Adapted from reference 2
Why do some people stay married for decades while others jump from one relationship to the next? If humans are like rodents, recent research suggests that neuropeptides may help forge the ties that bind.
FOLLOWING VOLE BEHAVIOR
The vole, which inhabits many grasslands in the United States, is a rodent resembling a mouse but related to the lemming. Males in some vole species (such as meadow voles) show no partner preference whereas males in other species prefer one partner, share the same nest with her, and help care for their offspring.1
The neuropeptides oxytocin and arginine vasopressin are mediators of pair bonding. The brain of a prairie vole—a species in which males tend to bond with female partners—has more vasopressin receptors than that of the solitary meadow vole (Figure 1).
Figure 1 More vasopressin in bonding voles
Ventral forebrain autoradiograms show greater vasopressin (VP) expression in prairie voles (top) than meadow voles.
Reprinted with permission. © 2004, Nature Publishing Group.Lim et al, however, found that meadow voles were more likely to bond with a single female after the males’ vasopressin receptors were increased.2 The researchers isolated and replicated the gene sequence responsible for the vasopressin receptor in the prairie vole. Then, using a viral vector, they injected the gene into the ventral pallidum of 11 male meadow voles. Eleven other voles received placebo.
Two weeks later, each sexually naïve male vole was housed for 24 hours with a sexually receptive female vole. Then, each male was placed for 3 hours in a three-chamber apparatus with the partner female in one chamber and a novel female in another. The time spent huddling with each female was recorded.
Across 3 hours, the placebo group voles spent 10 to 15 minutes with either female—normal behavior for this species. By contrast, the voles that received the vasopressin receptors spent approximately 40 minutes with their partners but only about 5 minutes with the novel voles, thus showing more affiliative behavior and a clear preference for their mates (Figure 2). The findings suggest that the researchers may have produced a profound change in social behavior by altering one gene.
IMPLICATIONS FOR HUMANS
How this research relates to humans is unknown, as there is no sound evidence of a link between vole and human pair bonding. Likewise, the influence of the higher cortical areas in orchestrating human behaviors cannot be underestimated.
Neuroimaging, however, has shown that brain regions rich with oxytocin and vasopressin receptors are activated while a person views pictures of loved ones.3 Additionally, mens’ vasopressin levels have been shown to increase when they are sexually aroused.4 Whether these findings one day lead to a medicine that promotes monogamous behavior in men remains to be seen.5
Figure 2 Male meadow voles’ interactions with females after vasopressin or placebo treatment
Source: Adapted from reference 2
1. Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci 2004;7:1048-54.
2. Lim MM, Wang Z, Olazabal DE, et al. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature 2004;429(6993):754-7.
3. Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage 2004;21:1155-66.
4. Murphy MR, Seckl JR, Burton S, et al. Changes in oxytocin and vasopressin secretion during sexual activity in men. J Clin Endocrinol Metab 1987;65:738-41.
5. Konner M. The ties that bind. Nature 2004;429(6993):705.-
1. Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci 2004;7:1048-54.
2. Lim MM, Wang Z, Olazabal DE, et al. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature 2004;429(6993):754-7.
3. Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage 2004;21:1155-66.
4. Murphy MR, Seckl JR, Burton S, et al. Changes in oxytocin and vasopressin secretion during sexual activity in men. J Clin Endocrinol Metab 1987;65:738-41.
5. Konner M. The ties that bind. Nature 2004;429(6993):705.-
Psychotic prodrome: Are antipsychotics effective? Ethical?
Because 40% of individuals with a psychotic prodrome develop schizophrenia, detecting and preventing this transition could improve many patients’ lives. Unfortunately:
- psychotic prodrome lacks clear-cut symptoms and is difficult to identify
- little evidence exists to help clinicians select psychotropics and decide how long to use them
- treating all prodromal patients would expose those who never develop psychosis to the risk of psychotropics’ side effects.
How, then, can psychiatrists help patients who present with possible prodromal symptoms? Based on research and our experience, this article describes the psychotic prodrome and offers a pragmatic, evidence-based approach to diagnosis and treatment.
WHAT CAUSES PSYCHOTIC CONVERSION?
Reduced gray matter volumes in certain brain regions may be associated with conversion to psychosis (Box 1). Stress also may play a role; elevated stress-reactive cortisol levels are associated with positive symptom severity in the prodrome.1 Other factors being investigated include obstetric complications at birth, maternal age >30, premorbid schizotypal personality disorder, and impaired olfaction.
Symptoms. Nearly 80% of patients with schizophrenia experience a psychotic prodrome that lasts a few months to several years.2 Common features include:
- gradual worsening of perceptual disturbance
- referential thinking
- paranoia
- mild cognitive deficits
- mood lability
- impulsivity
- suicidality
- declining social function and academic performance.3,4
A premorbid phase often precedes the prodrome, with symptoms such as impaired attention, soft neurologic signs, and subtle social deficits. These changes may be harbingers of the prodrome but are too nonspecific to be diagnostic. Other functional impairments—including anxiety, depression, drug abuse, and psychosocial factors such as school stress—may mimic schizophrenic prodrome.
Prognosis. Studies of patients’ first schizophrenia episodes suggest that prodrome duration may predict outcome. A longer prodrome is thought to indicate a poor prognosis,6 such as in patients who wait a year before seeking treatment.7 A review of 22 studies of first-episode psychosis found early psychosocial and pharmacologic interventions improved long-term prognosis, and medication discontinuation predicted more-severe and chronic disease.8
Reduced gray matter volumes in certain brain regions may be associated with conversion to psychosis. Imaging studies have found medial temporal lobe changes—specifically, hippocampal volume alterations—in persons with schizophrenia, genetic high-risk groups, and those thought to be at risk for imminent psychosis.11
MRI imaging of patients with prodromal signs has shown less gray matter in the right medial temporal, lateral temporal, inferior frontal cortex, and bilateral cingulate regions in those who have developed psychosis, compared with those who have not. In the psychotic patients, 12-month longitudinal follow-up has found reduced gray matter in the left hippocampal, fusiform, orbitofrontal, cerebellar cortices, and cingulate gyrus.12
Brain structure is related to genetic liability for schizophrenia in high-risk patients, who seem to have smaller right and left prefrontal lobes and smaller right and left thalami. These findings are consistent with the prodrome’s neurocognitive deficits, which are less than those reported in schizophrenia and greater than those seen in healthy subjects.
Pioneering work by McGorry et al10 identified an “ultra high-risk group” with a psychotic conversion rate of 40% to 60%. These patients present with three symptom patterns:
- attenuated positive symptoms
- brief intermittent psychotic episodes
- genetic risk and recent deterioration syndrome (Table 1).
3 patient groups considered at ‘ultra high risk’ to develop schizophrenia
| Patients with… | Symptoms |
|---|---|
| Attenuated psychotic symptoms | Overvalued ideas, perceptual disorders |
| Present at least 1 week; not >5 years | |
| At least 1 symptom several times a week | |
| Brief intermittent psychotic episodes | Frank psychotic features |
| Resolve spontaneously within 7 days | |
| Can be drug-induced | |
| Genetic risk and recent deterioration syndrome | Psychotic disorder in a first-degree relative |
| Schizotypal personality disorder | |
| Present at least 1 month; not >5 years | |
| Significant functional decline | |
| Source: Adapted from reference 10 | |
The Edinburgh High Risk Study of 162 individuals ages 16 to 25 showed more marked psychopathology in those with at least two close relatives with schizophrenia, compared with control groups. A direct correlation was seen between genetic liability and poor neurocognitive performance.11
PRODROME RATING SCALES
Researchers are using outcome measures to diagnose prodromal symptoms and assess their severity. Operational, validated assessment tools include:
- Bonn Scale for the Assessment of Basic Symptoms (BSABS): captures subtle changes in thinking, feeling, and perception.
- Schizophrenia Prediction Instrument for Adults (SPI-A): defines prepsychotic deviations and rates symptoms that are subjectively experienced by the patient.
- Comprehensive Assessment of At Risk Mental State (CAARMS): defines ultra high-risk criteria and incorporates eight dimensions of psychopathology.
- Scale of Prodromal Symptoms (SOPS): rates psychosis severity. When embedded within the Structured Interview for Prodromal Syndromes (SIPS), the SOPS determines the presence or absence of psychosis and predicts progression to psychopathology.
- Criteria for Prodromal Symptoms (COPS): defines ultra high-risk categories.
- Presence of Psychosis Scale (POPS): rates severity, intensity, and duration of positive prodromal symptoms.12
These instruments may identify prodromal symptoms in psychiatric practice, but further validation of clinical criteria is needed before they could be recommended for routine patient assessment.
PROPHYLACTIC ANTIPSYCHOTICS?
Atypical antipsychotics may be the standard of care for patients with a first psychotic episode, but this intervention is based on few double-blind controlled trials. Not surprisingly, only a handful of studies have examined antipsychotic therapy for the prodrome’s less clear-cut symptoms.
Risperidone. An 8- to 12-week open-label study in adolescents with first- and second-degree relatives with schizophrenia13 included four prodromal and six first-episode psychosis patients who met criteria for a cluster A personality disorder. Risperidone, 1.0 mg/d and 1.8 mg/d, respectively, improved thought disorder and attention symptoms, as measured with the Child Behavior Checklist. Verbal memory improved minimally, and no medication side effects were reported.
An open-label observational study14 identified four middle-aged subjects with a genetic risk of schizophrenia who reported negative symptoms and neurocognitive deficits. Risperidone, started at 0.25 mg/d and gradually increased to a maximum of 2 mg/d, improved negative symptoms, attention, and working memory. Mild side effects including tremors, sedation, dry mouth, and anxiety symptoms were reported.
An open-label, randomized, comparator trial15 examined psychotic transition rates in 59 subjects (mean age, 20) who met ultra high-risk criteria. They received:
- a needs-based intervention (NBI) comprising case management, psychotropics excluding antipsychotics, and supportive psychotherapy
- or a specific preventive intervention (SPI) that included risperidone, 1 to 2 mg/d, and a modified cognitive-behavioral therapy (CBT).
Should you intervene with patients in suspected psychotic prodrome?
| Arguments for: |
|
| Arguments against: |
|
At 12-months’ follow-up, another 3 SPI patients who had been partially adherent or non-adherent to antipsychotic therapy had converted to psychosis. For adherent SPI patients, protection against conversion appeared to persist for 6 months after risperidone therapy ended. All medication side effects were mild and transient.
Table 3
Psychotic prodrome: Unanswered clinical questions
|
In the first year, 11 of 29 placebo-group patients and 5 of 31 receiving olanzapine converted to psychosis. Among patients receiving no treatment in the second year, 2 of 8 former placebo patients and 3 of 9 former olanzapine patients converted to psychosis.
Discontinuation rates were 35% and 28%, respectively. Compared with the placebo group, patients taking olanzapine experienced greater weight gain, suggesting that risks associated with antipsychotic therapy may exceed unproven benefits in this population.
Discussion. Little information exists on using quetiapine, ziprasidone, or aripiprazole in prodromal patients. As cited above, preliminary studies with risperidone and olanzapine suggest that these agents may improve several domains of psychotic prodrome. The evidence does not support firm conclusions, however, given the trials’ small sample sizes and brief duration.
The prevalence of obesity and metabolic syndrome in patients with schizophrenia and the added metabolic risks associated with atypical antipsychotics make their use during the prodrome controversial. Weighing the potential advantages and disadvantages (Table 2), we consider antipsychotics to be the last resort after psychosocial interventions have failed to improve prodromal symptoms.
Low-dose atypical antipsychotics may be warranted for some patients, but their use requires stringent monitoring of:
- weight and waist circumference
- vital signs
- metabolic parameters such as fasting blood glucose and lipid profile
- abnormal involuntary movements
- prolactin elevations.
OTHER THERAPIES
Antidepressants. Researchers are also exploring the efficacy of using antidepressants and anxiolytics in the prodromal phase. The only published naturalistic study of adolescents found antidepressants alone or in combination with mood stabilizers or anxiolytics to be as effective as atypical antipsychotics in treating prodromal symptoms.17 A more substantial study is ongoing.
Psychotherapy. For patients with a suspected psychotic prodrome, nondrug strategies may help minimize functional and cognitive impairments, ease distress, and improve coping skills.
CBT has been shown to reduce psychotic progression over 12 months.18 Use CBT to help patients cope with the illness while focusing on:
- symptom monitoring
- premorbid and present functioning
- establishing a therapeutic alliance
- assessing the patient’s experience of psychosis and any thought distortions.
‘REAL WORLD’ EARLY INTERVENTION
Patients with prodromal symptoms are often referred to psychiatrists by family members, primary care physicians, or other mental health professionals. They tend to be young adults, and a few may present in their teens. Most are experiencing behavioral changes such as social isolation, feeling suspicious, perceptual disturbances, depression, and/or anxiety symptoms that seem abnormal but fall short of DSM-IV criteria for schizophrenia diagnosis.
Many clinical questions about schizophrenia’s prodromal phase remain unanswered (Table 3). Our primary aim is to adequately assess these patients and provide treatment and follow-up, taking into account:
- the individual’s presentation
- risks and benefits of available interventions.
- Provide patients and families information and emotional support; a strong therapeutic alliance may help keep the patient in treatment if schizophrenia develops
- Offer early psychosocial interventions such as vocational training, relapse prevention, substance abuse treatment, family therapy, supportive and CBT
- Explore using low-dose atypical antipsychotics as a last resort for patients with pronounced prodromal symptoms; explain risks of weight gain and other metabolic changes, obtain consent, and document need for such interventions
- Consider referral, if feasible, to a center specializing in psychotic prodrome diagnosis, treatment, and research
Consider atypical antipsychotics for patients with distressing psychotic symptoms, rapidly deteriorating function, increased agitation, and safety risks. Consider antidepressant and/or anxiolytic therapy for depression and anxiety, respectively.
Discuss at length with patients and families the risks and benefits of pharmacologic treatments. When clinically appropriate, cautiously discontinue or taper any medication with patients’ consent, while monitoring for side effects and symptoms.
- Issue devoted to early prodrome research. SchizophrBull 2003;29(4):621-879.
- Diagnostic and therapeutic intervention during psychotic prodrome. CNS Spectrums 2004;9(8):578-606.
- PRIME (Prevention through Risk Identification, Management & Education) Research Clinic. Department of Psychiatry, Yale University. http://info.med.yale.edu/psych/prime/pintro.html.
- Youth Mental Health Update. Schizophrenia: New strategies for early detection and treatment. RAPP Clinic, Zucker Hillside Hospital, Glen Oaks, NY. http://schoolnet.lij.edu/eshare/files/rapp.html
- Aripiprazole • Abilify
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Dr. Narasimhan receives research support from Eli Lilly and Co. and Janssen Pharmaceutica and is a speaker or consultant for Eli Lilly and Co., Pfizer Inc., and Abbott Laboratories.
Dr. Buckley receives research support from AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Co., Eli Lilly and Co., Janssen Pharmaceutica, Novartis Pharmaceuticals Corp., Pfizer Inc., and Solvay Pharmaceuticals. He is a consultant to and/or speaker for Abbott Laboratories, Alamo Pharmaceuticals, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Co., Eli Lilly and Co., Janssen Pharmaceutica, Novartis Pharmaceuticals Corp., Pfizer Inc., and Pharmstar.
1. Corcoran C, Walker E, Huot R, et al. The stress cascade and schizophrenia: etiology and onset. Schizophr Bull 2003;29(4):671-92.
2. Klosterkotter J. Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry 2001;58:158-64.
3. Yung AR. The initial prodrome in psychosis: the prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr Bull 1996;30:587-99.
4. Perkins DO. Evaluating and treating the prodromal stage of schizophrenia. Current Psychiatry Reports 2004;6:289-95.
5. Wood S W, Miller TJ, McGlashan TH. The “prodromal” patient: both symptomatic and at risk. CNS Spectrums 2001;6(3):223-32.
6. Keshavan MS, Haas G, Miewald J, et al. Prolonged untreated illness duration from prodromal onset predicts outcome in first episode psychosis. Schizoph Bull 2003;29(4):757-69.
7. Loebel AD, Lieberman JA, Alvir JM, et al. Duration of psychosis and outcome in first-episode schizophrenia. Am J Psychiatry 1992;149:1183-8.
8. Wyatt RJ, Green MF, Tuma AH. Long-term morbidity associated with delayed treatment of first admission schizophrenic patients: a re-analysis of the Camarillo State Hospital data. Psychol Med 1997;27:261-8.
9. Cornblatt BA, Lencz T, Smith CW, et al. The schizophrenia prodrome revisited: a neuro developmental perspective. Schizophr Bull 2003;29(4):633-51.
10. McGorry PD, Yung AR, Phillips LJ. The “close-in” or ultra high-risk model: a safe and effective strategy for research and clinical intervention in prepsychotic mental disorder. Schizophr Bull 2003;29(4):771-90.
11. Johnstone EC, Lawrie SM, Cosway R. What does the Edinburgh high-risk study tell us about schizophrenia? Am J Med Genet 2002;114(8):906-12.
12. Miller TJ, Clashing TH, Rosen JL, et al. Prodromal assessment with the Structured Interview for Prodromal Syndromes and the Scale of Prodromal Syndromes: predictive validity, interrater reliability, and training to reliability. Schizophr Bull 2003;29(4):703-15.
13. Cannon TD, Huttunen MO, Dahlstrom M, et al. Antipsychotic drug treatment in the prodromal phase in schizophrenia. Am J Psychiatry 2002;159:1230-2.
14. Tsuang MT, Stone WS, Faraone SV. Treatment of nonpsychotic relatives of patients with schizophrenia: four case studies. Biol Psychiatry 1999;45:1412-18.
15. McGorry PD, Yung AR, Phillips LJ, et al. Randomized controlled trial of intervention designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Arch Gen Psychiatry 2002;59:921-8.
16. McGlashan TH, Zipursky R, Perkins DO, et al. The PRIME North America randomized double-blind clinical trial of olanzapine versus placebo in patients at risk of being prodromally symptomatic for psychosis. I. Study rationale and design. Schizophr Res 2003;61:7-18.
17. Cornblatt B, Lencz T, Correll C, et al. Treating the prodrome: naturalistic findings from the RAP program. Acta Psychiatr Scand 2002;106(suppl):44.-
18. Morrison AO, French P, Walford L, et al. Cognitive therapy for the prevention of psychosis in people at ultra-high risk: randomized controlled trial. Br J Psychiatry 2004;185:291-7.
19. Wentzell B. This family experience in a supportive first episode program (abstract S-25-03). Davos, Switzerland: Schizophrenia Research 67(1) 11th Biennial Winter Workshop on Schizophrenia. Feb. 7-13, 2004.
20. Stahl SM. Prophylactic antipsychotics: do they keep you from catching schizophrenia? J Clin Psychiatry 2004;65(11):1445-6.
Because 40% of individuals with a psychotic prodrome develop schizophrenia, detecting and preventing this transition could improve many patients’ lives. Unfortunately:
- psychotic prodrome lacks clear-cut symptoms and is difficult to identify
- little evidence exists to help clinicians select psychotropics and decide how long to use them
- treating all prodromal patients would expose those who never develop psychosis to the risk of psychotropics’ side effects.
How, then, can psychiatrists help patients who present with possible prodromal symptoms? Based on research and our experience, this article describes the psychotic prodrome and offers a pragmatic, evidence-based approach to diagnosis and treatment.
WHAT CAUSES PSYCHOTIC CONVERSION?
Reduced gray matter volumes in certain brain regions may be associated with conversion to psychosis (Box 1). Stress also may play a role; elevated stress-reactive cortisol levels are associated with positive symptom severity in the prodrome.1 Other factors being investigated include obstetric complications at birth, maternal age >30, premorbid schizotypal personality disorder, and impaired olfaction.
Symptoms. Nearly 80% of patients with schizophrenia experience a psychotic prodrome that lasts a few months to several years.2 Common features include:
- gradual worsening of perceptual disturbance
- referential thinking
- paranoia
- mild cognitive deficits
- mood lability
- impulsivity
- suicidality
- declining social function and academic performance.3,4
A premorbid phase often precedes the prodrome, with symptoms such as impaired attention, soft neurologic signs, and subtle social deficits. These changes may be harbingers of the prodrome but are too nonspecific to be diagnostic. Other functional impairments—including anxiety, depression, drug abuse, and psychosocial factors such as school stress—may mimic schizophrenic prodrome.
Prognosis. Studies of patients’ first schizophrenia episodes suggest that prodrome duration may predict outcome. A longer prodrome is thought to indicate a poor prognosis,6 such as in patients who wait a year before seeking treatment.7 A review of 22 studies of first-episode psychosis found early psychosocial and pharmacologic interventions improved long-term prognosis, and medication discontinuation predicted more-severe and chronic disease.8
Reduced gray matter volumes in certain brain regions may be associated with conversion to psychosis. Imaging studies have found medial temporal lobe changes—specifically, hippocampal volume alterations—in persons with schizophrenia, genetic high-risk groups, and those thought to be at risk for imminent psychosis.11
MRI imaging of patients with prodromal signs has shown less gray matter in the right medial temporal, lateral temporal, inferior frontal cortex, and bilateral cingulate regions in those who have developed psychosis, compared with those who have not. In the psychotic patients, 12-month longitudinal follow-up has found reduced gray matter in the left hippocampal, fusiform, orbitofrontal, cerebellar cortices, and cingulate gyrus.12
Brain structure is related to genetic liability for schizophrenia in high-risk patients, who seem to have smaller right and left prefrontal lobes and smaller right and left thalami. These findings are consistent with the prodrome’s neurocognitive deficits, which are less than those reported in schizophrenia and greater than those seen in healthy subjects.
Pioneering work by McGorry et al10 identified an “ultra high-risk group” with a psychotic conversion rate of 40% to 60%. These patients present with three symptom patterns:
- attenuated positive symptoms
- brief intermittent psychotic episodes
- genetic risk and recent deterioration syndrome (Table 1).
3 patient groups considered at ‘ultra high risk’ to develop schizophrenia
| Patients with… | Symptoms |
|---|---|
| Attenuated psychotic symptoms | Overvalued ideas, perceptual disorders |
| Present at least 1 week; not >5 years | |
| At least 1 symptom several times a week | |
| Brief intermittent psychotic episodes | Frank psychotic features |
| Resolve spontaneously within 7 days | |
| Can be drug-induced | |
| Genetic risk and recent deterioration syndrome | Psychotic disorder in a first-degree relative |
| Schizotypal personality disorder | |
| Present at least 1 month; not >5 years | |
| Significant functional decline | |
| Source: Adapted from reference 10 | |
The Edinburgh High Risk Study of 162 individuals ages 16 to 25 showed more marked psychopathology in those with at least two close relatives with schizophrenia, compared with control groups. A direct correlation was seen between genetic liability and poor neurocognitive performance.11
PRODROME RATING SCALES
Researchers are using outcome measures to diagnose prodromal symptoms and assess their severity. Operational, validated assessment tools include:
- Bonn Scale for the Assessment of Basic Symptoms (BSABS): captures subtle changes in thinking, feeling, and perception.
- Schizophrenia Prediction Instrument for Adults (SPI-A): defines prepsychotic deviations and rates symptoms that are subjectively experienced by the patient.
- Comprehensive Assessment of At Risk Mental State (CAARMS): defines ultra high-risk criteria and incorporates eight dimensions of psychopathology.
- Scale of Prodromal Symptoms (SOPS): rates psychosis severity. When embedded within the Structured Interview for Prodromal Syndromes (SIPS), the SOPS determines the presence or absence of psychosis and predicts progression to psychopathology.
- Criteria for Prodromal Symptoms (COPS): defines ultra high-risk categories.
- Presence of Psychosis Scale (POPS): rates severity, intensity, and duration of positive prodromal symptoms.12
These instruments may identify prodromal symptoms in psychiatric practice, but further validation of clinical criteria is needed before they could be recommended for routine patient assessment.
PROPHYLACTIC ANTIPSYCHOTICS?
Atypical antipsychotics may be the standard of care for patients with a first psychotic episode, but this intervention is based on few double-blind controlled trials. Not surprisingly, only a handful of studies have examined antipsychotic therapy for the prodrome’s less clear-cut symptoms.
Risperidone. An 8- to 12-week open-label study in adolescents with first- and second-degree relatives with schizophrenia13 included four prodromal and six first-episode psychosis patients who met criteria for a cluster A personality disorder. Risperidone, 1.0 mg/d and 1.8 mg/d, respectively, improved thought disorder and attention symptoms, as measured with the Child Behavior Checklist. Verbal memory improved minimally, and no medication side effects were reported.
An open-label observational study14 identified four middle-aged subjects with a genetic risk of schizophrenia who reported negative symptoms and neurocognitive deficits. Risperidone, started at 0.25 mg/d and gradually increased to a maximum of 2 mg/d, improved negative symptoms, attention, and working memory. Mild side effects including tremors, sedation, dry mouth, and anxiety symptoms were reported.
An open-label, randomized, comparator trial15 examined psychotic transition rates in 59 subjects (mean age, 20) who met ultra high-risk criteria. They received:
- a needs-based intervention (NBI) comprising case management, psychotropics excluding antipsychotics, and supportive psychotherapy
- or a specific preventive intervention (SPI) that included risperidone, 1 to 2 mg/d, and a modified cognitive-behavioral therapy (CBT).
Should you intervene with patients in suspected psychotic prodrome?
| Arguments for: |
|
| Arguments against: |
|
At 12-months’ follow-up, another 3 SPI patients who had been partially adherent or non-adherent to antipsychotic therapy had converted to psychosis. For adherent SPI patients, protection against conversion appeared to persist for 6 months after risperidone therapy ended. All medication side effects were mild and transient.
Table 3
Psychotic prodrome: Unanswered clinical questions
|
In the first year, 11 of 29 placebo-group patients and 5 of 31 receiving olanzapine converted to psychosis. Among patients receiving no treatment in the second year, 2 of 8 former placebo patients and 3 of 9 former olanzapine patients converted to psychosis.
Discontinuation rates were 35% and 28%, respectively. Compared with the placebo group, patients taking olanzapine experienced greater weight gain, suggesting that risks associated with antipsychotic therapy may exceed unproven benefits in this population.
Discussion. Little information exists on using quetiapine, ziprasidone, or aripiprazole in prodromal patients. As cited above, preliminary studies with risperidone and olanzapine suggest that these agents may improve several domains of psychotic prodrome. The evidence does not support firm conclusions, however, given the trials’ small sample sizes and brief duration.
The prevalence of obesity and metabolic syndrome in patients with schizophrenia and the added metabolic risks associated with atypical antipsychotics make their use during the prodrome controversial. Weighing the potential advantages and disadvantages (Table 2), we consider antipsychotics to be the last resort after psychosocial interventions have failed to improve prodromal symptoms.
Low-dose atypical antipsychotics may be warranted for some patients, but their use requires stringent monitoring of:
- weight and waist circumference
- vital signs
- metabolic parameters such as fasting blood glucose and lipid profile
- abnormal involuntary movements
- prolactin elevations.
OTHER THERAPIES
Antidepressants. Researchers are also exploring the efficacy of using antidepressants and anxiolytics in the prodromal phase. The only published naturalistic study of adolescents found antidepressants alone or in combination with mood stabilizers or anxiolytics to be as effective as atypical antipsychotics in treating prodromal symptoms.17 A more substantial study is ongoing.
Psychotherapy. For patients with a suspected psychotic prodrome, nondrug strategies may help minimize functional and cognitive impairments, ease distress, and improve coping skills.
CBT has been shown to reduce psychotic progression over 12 months.18 Use CBT to help patients cope with the illness while focusing on:
- symptom monitoring
- premorbid and present functioning
- establishing a therapeutic alliance
- assessing the patient’s experience of psychosis and any thought distortions.
‘REAL WORLD’ EARLY INTERVENTION
Patients with prodromal symptoms are often referred to psychiatrists by family members, primary care physicians, or other mental health professionals. They tend to be young adults, and a few may present in their teens. Most are experiencing behavioral changes such as social isolation, feeling suspicious, perceptual disturbances, depression, and/or anxiety symptoms that seem abnormal but fall short of DSM-IV criteria for schizophrenia diagnosis.
Many clinical questions about schizophrenia’s prodromal phase remain unanswered (Table 3). Our primary aim is to adequately assess these patients and provide treatment and follow-up, taking into account:
- the individual’s presentation
- risks and benefits of available interventions.
- Provide patients and families information and emotional support; a strong therapeutic alliance may help keep the patient in treatment if schizophrenia develops
- Offer early psychosocial interventions such as vocational training, relapse prevention, substance abuse treatment, family therapy, supportive and CBT
- Explore using low-dose atypical antipsychotics as a last resort for patients with pronounced prodromal symptoms; explain risks of weight gain and other metabolic changes, obtain consent, and document need for such interventions
- Consider referral, if feasible, to a center specializing in psychotic prodrome diagnosis, treatment, and research
Consider atypical antipsychotics for patients with distressing psychotic symptoms, rapidly deteriorating function, increased agitation, and safety risks. Consider antidepressant and/or anxiolytic therapy for depression and anxiety, respectively.
Discuss at length with patients and families the risks and benefits of pharmacologic treatments. When clinically appropriate, cautiously discontinue or taper any medication with patients’ consent, while monitoring for side effects and symptoms.
- Issue devoted to early prodrome research. SchizophrBull 2003;29(4):621-879.
- Diagnostic and therapeutic intervention during psychotic prodrome. CNS Spectrums 2004;9(8):578-606.
- PRIME (Prevention through Risk Identification, Management & Education) Research Clinic. Department of Psychiatry, Yale University. http://info.med.yale.edu/psych/prime/pintro.html.
- Youth Mental Health Update. Schizophrenia: New strategies for early detection and treatment. RAPP Clinic, Zucker Hillside Hospital, Glen Oaks, NY. http://schoolnet.lij.edu/eshare/files/rapp.html
- Aripiprazole • Abilify
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Dr. Narasimhan receives research support from Eli Lilly and Co. and Janssen Pharmaceutica and is a speaker or consultant for Eli Lilly and Co., Pfizer Inc., and Abbott Laboratories.
Dr. Buckley receives research support from AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Co., Eli Lilly and Co., Janssen Pharmaceutica, Novartis Pharmaceuticals Corp., Pfizer Inc., and Solvay Pharmaceuticals. He is a consultant to and/or speaker for Abbott Laboratories, Alamo Pharmaceuticals, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Co., Eli Lilly and Co., Janssen Pharmaceutica, Novartis Pharmaceuticals Corp., Pfizer Inc., and Pharmstar.
Because 40% of individuals with a psychotic prodrome develop schizophrenia, detecting and preventing this transition could improve many patients’ lives. Unfortunately:
- psychotic prodrome lacks clear-cut symptoms and is difficult to identify
- little evidence exists to help clinicians select psychotropics and decide how long to use them
- treating all prodromal patients would expose those who never develop psychosis to the risk of psychotropics’ side effects.
How, then, can psychiatrists help patients who present with possible prodromal symptoms? Based on research and our experience, this article describes the psychotic prodrome and offers a pragmatic, evidence-based approach to diagnosis and treatment.
WHAT CAUSES PSYCHOTIC CONVERSION?
Reduced gray matter volumes in certain brain regions may be associated with conversion to psychosis (Box 1). Stress also may play a role; elevated stress-reactive cortisol levels are associated with positive symptom severity in the prodrome.1 Other factors being investigated include obstetric complications at birth, maternal age >30, premorbid schizotypal personality disorder, and impaired olfaction.
Symptoms. Nearly 80% of patients with schizophrenia experience a psychotic prodrome that lasts a few months to several years.2 Common features include:
- gradual worsening of perceptual disturbance
- referential thinking
- paranoia
- mild cognitive deficits
- mood lability
- impulsivity
- suicidality
- declining social function and academic performance.3,4
A premorbid phase often precedes the prodrome, with symptoms such as impaired attention, soft neurologic signs, and subtle social deficits. These changes may be harbingers of the prodrome but are too nonspecific to be diagnostic. Other functional impairments—including anxiety, depression, drug abuse, and psychosocial factors such as school stress—may mimic schizophrenic prodrome.
Prognosis. Studies of patients’ first schizophrenia episodes suggest that prodrome duration may predict outcome. A longer prodrome is thought to indicate a poor prognosis,6 such as in patients who wait a year before seeking treatment.7 A review of 22 studies of first-episode psychosis found early psychosocial and pharmacologic interventions improved long-term prognosis, and medication discontinuation predicted more-severe and chronic disease.8
Reduced gray matter volumes in certain brain regions may be associated with conversion to psychosis. Imaging studies have found medial temporal lobe changes—specifically, hippocampal volume alterations—in persons with schizophrenia, genetic high-risk groups, and those thought to be at risk for imminent psychosis.11
MRI imaging of patients with prodromal signs has shown less gray matter in the right medial temporal, lateral temporal, inferior frontal cortex, and bilateral cingulate regions in those who have developed psychosis, compared with those who have not. In the psychotic patients, 12-month longitudinal follow-up has found reduced gray matter in the left hippocampal, fusiform, orbitofrontal, cerebellar cortices, and cingulate gyrus.12
Brain structure is related to genetic liability for schizophrenia in high-risk patients, who seem to have smaller right and left prefrontal lobes and smaller right and left thalami. These findings are consistent with the prodrome’s neurocognitive deficits, which are less than those reported in schizophrenia and greater than those seen in healthy subjects.
Pioneering work by McGorry et al10 identified an “ultra high-risk group” with a psychotic conversion rate of 40% to 60%. These patients present with three symptom patterns:
- attenuated positive symptoms
- brief intermittent psychotic episodes
- genetic risk and recent deterioration syndrome (Table 1).
3 patient groups considered at ‘ultra high risk’ to develop schizophrenia
| Patients with… | Symptoms |
|---|---|
| Attenuated psychotic symptoms | Overvalued ideas, perceptual disorders |
| Present at least 1 week; not >5 years | |
| At least 1 symptom several times a week | |
| Brief intermittent psychotic episodes | Frank psychotic features |
| Resolve spontaneously within 7 days | |
| Can be drug-induced | |
| Genetic risk and recent deterioration syndrome | Psychotic disorder in a first-degree relative |
| Schizotypal personality disorder | |
| Present at least 1 month; not >5 years | |
| Significant functional decline | |
| Source: Adapted from reference 10 | |
The Edinburgh High Risk Study of 162 individuals ages 16 to 25 showed more marked psychopathology in those with at least two close relatives with schizophrenia, compared with control groups. A direct correlation was seen between genetic liability and poor neurocognitive performance.11
PRODROME RATING SCALES
Researchers are using outcome measures to diagnose prodromal symptoms and assess their severity. Operational, validated assessment tools include:
- Bonn Scale for the Assessment of Basic Symptoms (BSABS): captures subtle changes in thinking, feeling, and perception.
- Schizophrenia Prediction Instrument for Adults (SPI-A): defines prepsychotic deviations and rates symptoms that are subjectively experienced by the patient.
- Comprehensive Assessment of At Risk Mental State (CAARMS): defines ultra high-risk criteria and incorporates eight dimensions of psychopathology.
- Scale of Prodromal Symptoms (SOPS): rates psychosis severity. When embedded within the Structured Interview for Prodromal Syndromes (SIPS), the SOPS determines the presence or absence of psychosis and predicts progression to psychopathology.
- Criteria for Prodromal Symptoms (COPS): defines ultra high-risk categories.
- Presence of Psychosis Scale (POPS): rates severity, intensity, and duration of positive prodromal symptoms.12
These instruments may identify prodromal symptoms in psychiatric practice, but further validation of clinical criteria is needed before they could be recommended for routine patient assessment.
PROPHYLACTIC ANTIPSYCHOTICS?
Atypical antipsychotics may be the standard of care for patients with a first psychotic episode, but this intervention is based on few double-blind controlled trials. Not surprisingly, only a handful of studies have examined antipsychotic therapy for the prodrome’s less clear-cut symptoms.
Risperidone. An 8- to 12-week open-label study in adolescents with first- and second-degree relatives with schizophrenia13 included four prodromal and six first-episode psychosis patients who met criteria for a cluster A personality disorder. Risperidone, 1.0 mg/d and 1.8 mg/d, respectively, improved thought disorder and attention symptoms, as measured with the Child Behavior Checklist. Verbal memory improved minimally, and no medication side effects were reported.
An open-label observational study14 identified four middle-aged subjects with a genetic risk of schizophrenia who reported negative symptoms and neurocognitive deficits. Risperidone, started at 0.25 mg/d and gradually increased to a maximum of 2 mg/d, improved negative symptoms, attention, and working memory. Mild side effects including tremors, sedation, dry mouth, and anxiety symptoms were reported.
An open-label, randomized, comparator trial15 examined psychotic transition rates in 59 subjects (mean age, 20) who met ultra high-risk criteria. They received:
- a needs-based intervention (NBI) comprising case management, psychotropics excluding antipsychotics, and supportive psychotherapy
- or a specific preventive intervention (SPI) that included risperidone, 1 to 2 mg/d, and a modified cognitive-behavioral therapy (CBT).
Should you intervene with patients in suspected psychotic prodrome?
| Arguments for: |
|
| Arguments against: |
|
At 12-months’ follow-up, another 3 SPI patients who had been partially adherent or non-adherent to antipsychotic therapy had converted to psychosis. For adherent SPI patients, protection against conversion appeared to persist for 6 months after risperidone therapy ended. All medication side effects were mild and transient.
Table 3
Psychotic prodrome: Unanswered clinical questions
|
In the first year, 11 of 29 placebo-group patients and 5 of 31 receiving olanzapine converted to psychosis. Among patients receiving no treatment in the second year, 2 of 8 former placebo patients and 3 of 9 former olanzapine patients converted to psychosis.
Discontinuation rates were 35% and 28%, respectively. Compared with the placebo group, patients taking olanzapine experienced greater weight gain, suggesting that risks associated with antipsychotic therapy may exceed unproven benefits in this population.
Discussion. Little information exists on using quetiapine, ziprasidone, or aripiprazole in prodromal patients. As cited above, preliminary studies with risperidone and olanzapine suggest that these agents may improve several domains of psychotic prodrome. The evidence does not support firm conclusions, however, given the trials’ small sample sizes and brief duration.
The prevalence of obesity and metabolic syndrome in patients with schizophrenia and the added metabolic risks associated with atypical antipsychotics make their use during the prodrome controversial. Weighing the potential advantages and disadvantages (Table 2), we consider antipsychotics to be the last resort after psychosocial interventions have failed to improve prodromal symptoms.
Low-dose atypical antipsychotics may be warranted for some patients, but their use requires stringent monitoring of:
- weight and waist circumference
- vital signs
- metabolic parameters such as fasting blood glucose and lipid profile
- abnormal involuntary movements
- prolactin elevations.
OTHER THERAPIES
Antidepressants. Researchers are also exploring the efficacy of using antidepressants and anxiolytics in the prodromal phase. The only published naturalistic study of adolescents found antidepressants alone or in combination with mood stabilizers or anxiolytics to be as effective as atypical antipsychotics in treating prodromal symptoms.17 A more substantial study is ongoing.
Psychotherapy. For patients with a suspected psychotic prodrome, nondrug strategies may help minimize functional and cognitive impairments, ease distress, and improve coping skills.
CBT has been shown to reduce psychotic progression over 12 months.18 Use CBT to help patients cope with the illness while focusing on:
- symptom monitoring
- premorbid and present functioning
- establishing a therapeutic alliance
- assessing the patient’s experience of psychosis and any thought distortions.
‘REAL WORLD’ EARLY INTERVENTION
Patients with prodromal symptoms are often referred to psychiatrists by family members, primary care physicians, or other mental health professionals. They tend to be young adults, and a few may present in their teens. Most are experiencing behavioral changes such as social isolation, feeling suspicious, perceptual disturbances, depression, and/or anxiety symptoms that seem abnormal but fall short of DSM-IV criteria for schizophrenia diagnosis.
Many clinical questions about schizophrenia’s prodromal phase remain unanswered (Table 3). Our primary aim is to adequately assess these patients and provide treatment and follow-up, taking into account:
- the individual’s presentation
- risks and benefits of available interventions.
- Provide patients and families information and emotional support; a strong therapeutic alliance may help keep the patient in treatment if schizophrenia develops
- Offer early psychosocial interventions such as vocational training, relapse prevention, substance abuse treatment, family therapy, supportive and CBT
- Explore using low-dose atypical antipsychotics as a last resort for patients with pronounced prodromal symptoms; explain risks of weight gain and other metabolic changes, obtain consent, and document need for such interventions
- Consider referral, if feasible, to a center specializing in psychotic prodrome diagnosis, treatment, and research
Consider atypical antipsychotics for patients with distressing psychotic symptoms, rapidly deteriorating function, increased agitation, and safety risks. Consider antidepressant and/or anxiolytic therapy for depression and anxiety, respectively.
Discuss at length with patients and families the risks and benefits of pharmacologic treatments. When clinically appropriate, cautiously discontinue or taper any medication with patients’ consent, while monitoring for side effects and symptoms.
- Issue devoted to early prodrome research. SchizophrBull 2003;29(4):621-879.
- Diagnostic and therapeutic intervention during psychotic prodrome. CNS Spectrums 2004;9(8):578-606.
- PRIME (Prevention through Risk Identification, Management & Education) Research Clinic. Department of Psychiatry, Yale University. http://info.med.yale.edu/psych/prime/pintro.html.
- Youth Mental Health Update. Schizophrenia: New strategies for early detection and treatment. RAPP Clinic, Zucker Hillside Hospital, Glen Oaks, NY. http://schoolnet.lij.edu/eshare/files/rapp.html
- Aripiprazole • Abilify
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Dr. Narasimhan receives research support from Eli Lilly and Co. and Janssen Pharmaceutica and is a speaker or consultant for Eli Lilly and Co., Pfizer Inc., and Abbott Laboratories.
Dr. Buckley receives research support from AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Co., Eli Lilly and Co., Janssen Pharmaceutica, Novartis Pharmaceuticals Corp., Pfizer Inc., and Solvay Pharmaceuticals. He is a consultant to and/or speaker for Abbott Laboratories, Alamo Pharmaceuticals, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Co., Eli Lilly and Co., Janssen Pharmaceutica, Novartis Pharmaceuticals Corp., Pfizer Inc., and Pharmstar.
1. Corcoran C, Walker E, Huot R, et al. The stress cascade and schizophrenia: etiology and onset. Schizophr Bull 2003;29(4):671-92.
2. Klosterkotter J. Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry 2001;58:158-64.
3. Yung AR. The initial prodrome in psychosis: the prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr Bull 1996;30:587-99.
4. Perkins DO. Evaluating and treating the prodromal stage of schizophrenia. Current Psychiatry Reports 2004;6:289-95.
5. Wood S W, Miller TJ, McGlashan TH. The “prodromal” patient: both symptomatic and at risk. CNS Spectrums 2001;6(3):223-32.
6. Keshavan MS, Haas G, Miewald J, et al. Prolonged untreated illness duration from prodromal onset predicts outcome in first episode psychosis. Schizoph Bull 2003;29(4):757-69.
7. Loebel AD, Lieberman JA, Alvir JM, et al. Duration of psychosis and outcome in first-episode schizophrenia. Am J Psychiatry 1992;149:1183-8.
8. Wyatt RJ, Green MF, Tuma AH. Long-term morbidity associated with delayed treatment of first admission schizophrenic patients: a re-analysis of the Camarillo State Hospital data. Psychol Med 1997;27:261-8.
9. Cornblatt BA, Lencz T, Smith CW, et al. The schizophrenia prodrome revisited: a neuro developmental perspective. Schizophr Bull 2003;29(4):633-51.
10. McGorry PD, Yung AR, Phillips LJ. The “close-in” or ultra high-risk model: a safe and effective strategy for research and clinical intervention in prepsychotic mental disorder. Schizophr Bull 2003;29(4):771-90.
11. Johnstone EC, Lawrie SM, Cosway R. What does the Edinburgh high-risk study tell us about schizophrenia? Am J Med Genet 2002;114(8):906-12.
12. Miller TJ, Clashing TH, Rosen JL, et al. Prodromal assessment with the Structured Interview for Prodromal Syndromes and the Scale of Prodromal Syndromes: predictive validity, interrater reliability, and training to reliability. Schizophr Bull 2003;29(4):703-15.
13. Cannon TD, Huttunen MO, Dahlstrom M, et al. Antipsychotic drug treatment in the prodromal phase in schizophrenia. Am J Psychiatry 2002;159:1230-2.
14. Tsuang MT, Stone WS, Faraone SV. Treatment of nonpsychotic relatives of patients with schizophrenia: four case studies. Biol Psychiatry 1999;45:1412-18.
15. McGorry PD, Yung AR, Phillips LJ, et al. Randomized controlled trial of intervention designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Arch Gen Psychiatry 2002;59:921-8.
16. McGlashan TH, Zipursky R, Perkins DO, et al. The PRIME North America randomized double-blind clinical trial of olanzapine versus placebo in patients at risk of being prodromally symptomatic for psychosis. I. Study rationale and design. Schizophr Res 2003;61:7-18.
17. Cornblatt B, Lencz T, Correll C, et al. Treating the prodrome: naturalistic findings from the RAP program. Acta Psychiatr Scand 2002;106(suppl):44.-
18. Morrison AO, French P, Walford L, et al. Cognitive therapy for the prevention of psychosis in people at ultra-high risk: randomized controlled trial. Br J Psychiatry 2004;185:291-7.
19. Wentzell B. This family experience in a supportive first episode program (abstract S-25-03). Davos, Switzerland: Schizophrenia Research 67(1) 11th Biennial Winter Workshop on Schizophrenia. Feb. 7-13, 2004.
20. Stahl SM. Prophylactic antipsychotics: do they keep you from catching schizophrenia? J Clin Psychiatry 2004;65(11):1445-6.
1. Corcoran C, Walker E, Huot R, et al. The stress cascade and schizophrenia: etiology and onset. Schizophr Bull 2003;29(4):671-92.
2. Klosterkotter J. Diagnosing schizophrenia in the initial prodromal phase. Arch Gen Psychiatry 2001;58:158-64.
3. Yung AR. The initial prodrome in psychosis: the prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr Bull 1996;30:587-99.
4. Perkins DO. Evaluating and treating the prodromal stage of schizophrenia. Current Psychiatry Reports 2004;6:289-95.
5. Wood S W, Miller TJ, McGlashan TH. The “prodromal” patient: both symptomatic and at risk. CNS Spectrums 2001;6(3):223-32.
6. Keshavan MS, Haas G, Miewald J, et al. Prolonged untreated illness duration from prodromal onset predicts outcome in first episode psychosis. Schizoph Bull 2003;29(4):757-69.
7. Loebel AD, Lieberman JA, Alvir JM, et al. Duration of psychosis and outcome in first-episode schizophrenia. Am J Psychiatry 1992;149:1183-8.
8. Wyatt RJ, Green MF, Tuma AH. Long-term morbidity associated with delayed treatment of first admission schizophrenic patients: a re-analysis of the Camarillo State Hospital data. Psychol Med 1997;27:261-8.
9. Cornblatt BA, Lencz T, Smith CW, et al. The schizophrenia prodrome revisited: a neuro developmental perspective. Schizophr Bull 2003;29(4):633-51.
10. McGorry PD, Yung AR, Phillips LJ. The “close-in” or ultra high-risk model: a safe and effective strategy for research and clinical intervention in prepsychotic mental disorder. Schizophr Bull 2003;29(4):771-90.
11. Johnstone EC, Lawrie SM, Cosway R. What does the Edinburgh high-risk study tell us about schizophrenia? Am J Med Genet 2002;114(8):906-12.
12. Miller TJ, Clashing TH, Rosen JL, et al. Prodromal assessment with the Structured Interview for Prodromal Syndromes and the Scale of Prodromal Syndromes: predictive validity, interrater reliability, and training to reliability. Schizophr Bull 2003;29(4):703-15.
13. Cannon TD, Huttunen MO, Dahlstrom M, et al. Antipsychotic drug treatment in the prodromal phase in schizophrenia. Am J Psychiatry 2002;159:1230-2.
14. Tsuang MT, Stone WS, Faraone SV. Treatment of nonpsychotic relatives of patients with schizophrenia: four case studies. Biol Psychiatry 1999;45:1412-18.
15. McGorry PD, Yung AR, Phillips LJ, et al. Randomized controlled trial of intervention designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Arch Gen Psychiatry 2002;59:921-8.
16. McGlashan TH, Zipursky R, Perkins DO, et al. The PRIME North America randomized double-blind clinical trial of olanzapine versus placebo in patients at risk of being prodromally symptomatic for psychosis. I. Study rationale and design. Schizophr Res 2003;61:7-18.
17. Cornblatt B, Lencz T, Correll C, et al. Treating the prodrome: naturalistic findings from the RAP program. Acta Psychiatr Scand 2002;106(suppl):44.-
18. Morrison AO, French P, Walford L, et al. Cognitive therapy for the prevention of psychosis in people at ultra-high risk: randomized controlled trial. Br J Psychiatry 2004;185:291-7.
19. Wentzell B. This family experience in a supportive first episode program (abstract S-25-03). Davos, Switzerland: Schizophrenia Research 67(1) 11th Biennial Winter Workshop on Schizophrenia. Feb. 7-13, 2004.
20. Stahl SM. Prophylactic antipsychotics: do they keep you from catching schizophrenia? J Clin Psychiatry 2004;65(11):1445-6.
Compulsive hoarding: Unclutter lives and homes by breaking anxiety’s grip
Compulsive hoarding behavior is considered notoriously difficult to treat, but targeting its characteristic symptoms with medication and psychotherapy can be successful. This article provides a guide for the psychiatrist—alone or with a cognitive-behavioral therapist—to diagnose compulsive hoarding syndrome and help patients overcome the anxieties that fuel its symptoms.
WHAT IS COMPULSIVE HOARDING?
Hoarders acquire and are unable to discard items that others consider of little use or value.1 They most often save newspapers, magazines, old clothing, bags, books, mail, notes, and lists. Hoarding and saving behaviors occur in nonclinical populations and with other neuropsychiatric disorders—schizophrenia, dementia, eating disorders, mental retardation—but are most often found in persons with obsessive-compulsive disorder (OCD).
OCD is a heterogeneous clinical entity with several major symptom domains:2,3
- aggressive, sexual, and religious obsessions with checking compulsions
- symmetry/order obsessions with ordering, arranging, and repeating compulsions
- contamination obsessions with washing and cleaning compulsions
- hoarding and saving symptoms.
Genetics. Compulsive hoarding may have a different pattern of inheritance and comorbidity than other OCD symptom factors. Hoarding/saving symptoms show a recessive inheritance pattern, whereas aggressive/checking and symmetry/order symptoms show a dominant pattern.9 The hoarding phenotype has been significantly associated with genetic markers on chromosomes 4, 5, and 17.14 In other studies:
- Among 20 OCD patients with prominent hoarding, 84% had first-degree relatives with hoarding behaviors and only 37% had first-degree relatives who met DSM-IV criteria for OCD.11
- Among 126 OCD patients, social phobia, personality disorders, and pathologic grooming disorders were more common in hoarders than in nonhoarders. Hoarding and tics were more common in first-degree relatives of hoarders than in those of nonhoarders.12
Neurobiology. Using positron emission tomography (PET) brain imaging, our group13 compared glucose metabolism in patients with compulsive hoarding syndrome with that of nonhoarding OCD patients and normal controls. Compulsive hoarders had unique brain activity, with significantly lower metabolism:
- in the posterior cingulate gyrus and occipital cortex than controls
- in the dorsal anterior cingulate gyrus (AC) and thalamus than nonhoarding OCD patients.
Hoarding severity was significantly correlated with lower activity in the dorsal AC across all OCD patients.
Discussion. Genetic and neurobiologic data suggest that compulsive hoarding syndrome may be a neurobiologically distinct variant of OCD14 and may help explain its clinical symptoms and poor treatment response. Low AC activity may mediate compulsive hoarders’ decision-making and attentional problems, whereas low posterior cingulate activity may be responsible for visuospatial and memory deficits. Moreover:
ASSESSMENT AND TREATMENT PLANNING
To manage compulsive hoarding syndrome, begin with a thorough neuropsychiatric evaluation:
- Rule out primary psychotic disorders, dementia, and other cognitive impairments and neurologic disorders.
- Rule out primary major depression, as clutter and self-neglect may be caused by amotivation, low energy, or hopelessness.
- Determine if the patient has OCD.
Amount of clutter. Living areas may be so cluttered that sleeping in a bed, sitting on chairs, or preparing food on a kitchen counter are impossible. How much of the home is cluttered? How much floor and counter space is usable? Are rooms unusable or inaccessible because of clutter? Can the patient use the laundry, prepare food in the kitchen, use the shower, toilet, etc.?
Health or safety hazards. Huge piles of papers can be a fire hazard. Clutter may be blocking the exits. Collected items may extend beyond patients’ homes to their cars, garages, storage lockers, and even storage areas owned by friends and family.
Beliefs about possessions. Compulsive hoarders often have distorted feelings about their possessions. They may over-buy or impulsively purchase items they feel have emotional or monetary value. They may consider the items extensions of themselves and suffer grief-like loss when discarding things.7
Some collect free items—flyers, coupons, newspapers, discarded goods—hoping to save money or be prepared “just in case” the item is ever needed. This may represent unattainable expectations of perfection, needing to maintain preparedness for every possible contingency. Hoarders often believe they have poor memory and have catastrophic fears of what might happen if they forget something. Thus, their desire to keep their possessions in sight is strong.17
Information processing deficits. Because of anxieties about making mistakes, most hoarders have great difficulty making decisions.18 It is easier to not decide than to suffer the consequences of a “wrong” decision. To gauge this behavior, ask patients how long routine decisions take them and which decisions they procrastinate or avoid.
Compulsive hoarders often have trouble categorizing possessions;6,7 because every item feels unique, they create a special category for each one and resist storing items together.
Table 1
Proposed criteria to diagnose obsessive-compulsive hoarding*
| Patient acquires and fails to discard a large number of possessions that appear useless or of limited value |
| Clutter prevents patient from using living or work spaces for activities for which they were designed |
| Hoarding behavior causes significant distress or functional impairment |
| * Proposed by Frost and Hartl, reference 6. |
Avoidance behaviors are a hallmark of the compulsive hoarding syndrome. To avoid deciding to discard items, they put them in a box, garage, rented storage facility, etc. They may also avoid routine decision-making tasks that could lead to making a mistake.
Daily functioning. Hoarders may take a long time to do even small chores, such as taking an hour to pay one bill. An inordinate amount of time may be spent “churning”—moving items from one pile to another but never discarding any item or establishing a consistent system or organization.
Medication compliance. Compulsive hoarders often forget to take medications or take them at inappropriate times. They may lose their medications in the clutter.
Insight. Hoarders often have little awareness of how their behavior and clutter affect their lives.19 They minimize the clutter in their homes and its health and safety risks. Insight can fluctuate over time and needs to be assessed repeatedly during treatment.
Table 2
Assessing a patient with compulsive hoarding symptoms
| Domain | Useful questions or strategies |
|---|---|
| Amount of clutter | Visit home and/or see pictures |
| Hazards relating to clutter | Ask: What precautions do you take to reduce risk of fire? Have you ever had a problem with rodent or insect infestation as a result of the clutter? Have neighbors complained about the risks of fire or infestation that the clutter might impose on their homes? |
| Beliefs about loss of possessions | Ask: What is the worst thing that would happen if you threw this item away? If you did not have this, what do you think would happen? |
| Information-processing deficits | Ask: How long do routine decisions take you? Which decisions do you procrastinate or avoid? |
| Decision-making and organizational skills | Ask: How do you pay and store your bills? |
| Avoidance behaviors | Ask: Do you avoid other things (sorting mail, returning calls, doing dishes, or paying bills, rent, or taxes)? |
| Daily functioning | Ask: Do you get everything done that you want to do? Are you often late? Do you have difficulty starting or finishing tasks? Describe a typical day. |
| Insight | Ask: Do you think this amount of clutter is normal? Do you think having this clutter has caused problems in your life? |
| Motivation for treatment | Ask: What brings you into therapy now? Do you think you have a problem with excessive hoarding/saving? If it was not for your family, would you come for help? |
| Social and occupational functioning | Ask: How has your clutter affected your personal relationships? When was the last time you had someone come to your home? What prevents you from working right now? Are you working to your full potential? |
| Support from friends and family | Ask: What does your family say about your clutter? Do your friends or family understand what is going on? |
| Treatment compliance | Ask: How long does it typically take before you renew your prescriptions when you run out of medications? |
Cognitive behavioral therapy for compulsive hoarding
| Treatment sequence | Methods and goals |
|---|---|
| Educate patient about hoarding | Help improve insight and motivation |
| Set up treatment | With patient, select target area of clutter |
| Assess items together, creating a hierarchy of least to most difficult areas to sort and items to discard | |
| Create realistic categories and a storage system | |
| Begin discarding | Patient must decide to keep or discard each item and permanently remove it from pile |
| Patient must store saved items appropriately | |
| Continue until area is clear, then move to next area | |
| Plan and implement appropriate use of space | |
| Stop incoming clutter | Cancel subscriptions |
| Address compulsive buying and acquisition | |
| Provide organization training | Organize possessions, time, tasks, etc. |
| Prevent relapse | Replace hoarding with healthier behaviors to prevent clutter from re-accumulating |
| Source: Adapted from reference 23. | |
20
Motivation. Like insight, motivation can fluctuate over time. Patients usually must work tremendously hard to adhere to treatment. To support these efforts, we periodically review with patients compulsive hoarding’s negative effects and the activities they would enjoy—such as improved relationships, greater work capacity, hobbies—if overcoming this behavior allowed them more time and space.
Rating scales. The symptom checklist of the Yale-Brown Obsessive-Compulsive Scale (YBOCS)21 contains two items for hoarding obsessions and compulsions but none for avoidance behaviors, which are prominent with compulsive hoarding. The Saving Inventory-Revised22 is a validated, 23-item self-report measure of clutter, difficulty discarding, and excessive acquisition, which distinguishes compulsive hoarders, nonhoarding OCD patients, and normal controls.
TREATMENT
The compulsive hoarder’s problems will not be solved by someone else throwing away or organizing his or her possessions. These actions often anger patients, who see them as intrusive and a loss of control.
In our experience, family members’ attempts to intervene can disrupt relationships and worsen hoarders’ social withdrawal. “Taking over” also does not help the patient create a sustainable system for keeping clutter-free.
Algorithm
Medication treatment for compulsive hoarding*
| Start with SSRIs, as for nonhoarding OCD (citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline) |
|
| Treat comorbid conditions | Mood disorders, other anxiety disorders, ADHD, psychotic disorders, etc. |
| Use adjunctive medications if SSRIs give only partial response |
|
| * Combine medication treatment with cognitive-behavioral therapy | |
| SSRI: selective serotonin reuptake inhibitor | |
| OCD: obsessive-compulsive disorder | |
| ADHD: attention-deficit/hyperactivity disorder | |
Psychotherapy. Exposure and response prevention (ERP) focuses on preventing incoming clutter, discarding, organizing, and relapse prevention (Table 3).23 Start treatment by explaining compulsive hoarding syndrome to patients as having problems with information processing, obsessional anxiety, and avoiding decisions.
Preventing incoming clutter. Before you focus on discarding, patients must stop incoming clutter; otherwise, it will come in as fast as it goes out. We ask patients to keep a daily log of every item they acquire or buy to build their awareness of what triggers their behavior.
Discarding. To desensitize over time, we repeatedly expose patients to the anxiety, sadness, or anger they feel when discarding items and making decisions. We encourage them to provoke their anxiety by throwing away as many items as possible, keeping only necessary items.
We support ERP with cognitive restructuring, prompting patients to reframe their obsessive fears about losing something necessary or valuable. By thinking through the consequences of discarding their clutter, they challenge their erroneous beliefs that dire consequences will occur.
Organizing. When patients decide they must keep an item, ask them to immediately identify a specific place and deadline to store it. After an area is cleared, patients must keep it clear and use it for its intended purpose. Most patients need training in time management, scheduling, and prioritizing.
Relapse prevention. Replace hoarding behaviors with more-adaptive, healthy behaviors. Teach patients to create a realistic schedule that includes time for chores, eating and sleeping, CBT homework, and recreation. Treatment goals are to:
- extinguish obsessional fears and compulsive saving behaviors
- teach lasting organizational and decision-making skills, thereby reducing relapse risk.
Medications. No controlled studies have examined whether any medications are effective for compulsive hoarding syndrome. The treatment strategies and algorithm described here are based on our clinical experience, controlled trials of OCD patients, and limited OCD studies secondarily examining hoarders’ specific treatment responses.
Selective serotonin reuptake inhibitors (SSRIs) may be less effective for compulsive hoarding than for other compulsive behaviors.14,27 Nevertheless, SSRIs may help alleviate hoarders’ core symptoms, other OCD symptoms, depression, and anxiety. For hoarding treatment to be effective, comorbid disorders must be treated and stabilized.
Several studies of SSRI use in OCD patients have shown modest improvements in compulsive hoarders:
- In a descriptive study of patients with compulsive hoarding, 1 of 18 (6%) patients had a “marked” response to at least one SSRI trial. The others showed a partial response,17 with YBOCS scores decreasing by at least 25% in approximately 50% of this group.
- When 17 OCD patients with hoarding symptoms were treated with paroxetine, CBT, or placebo, 18% responded to active treatment. Response was defined as a 40% reduction on YBOCS scores and “very much” or “much” improved on the Clinical Global Impression Scale (CGI).14
Atypical antipsychotics may be effective for OCD symptoms that do not respond adequately to SSRIs.29 Conventional antipsychotics are also effective adjuncts to SSRIs—particularly for patients with coexisting tic or psychotic disorders30—but consider the potential for extrapyramidal side effects and tardive dyskinesia.
We find that stimulants help some compulsive hoarders, particularly those with comorbid ADHD, other attentional problems, low motivation, or lethargy. Mood stabilizers are necessary to treat comorbid bipolar disorder, cyclothymia, and impulsivity.
Related resources
- Saxena S, Maidment K. Treatment of compulsive hoarding. J Clin Psychol 2004;60(11):1143-54.
- UCLA OCD Intensive Treatment Program. For information on research studies contact Karron Maidment (310) 794-7305 or visit www.mentalhealth.ucla.edu/projects/anxiety/ocdintensivetreatment.htm.
- Compulsive hoarding project. Institute of Living Anxiety Disorders Center, Hartford Hospital. http://instituteofliving.org/adc/compulsive_hoarding.htm
- Obsessive Compulsive Foundation compulsive hoarding Web site. http://www.ocfoundation.org/1005/index.html
- Neziroglu F, Bubrick J, Yaryura-Tobias JA. Overcoming compulsive hoarding. Oakland, CA: New Harbinger Publications, 2004.
- Citalopram • Celexa
- Clomipramine • Anafranil
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Venlafaxine • Effexor
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Frost R, Gross R. The hoarding of possessions. Behav Res Ther 1993;31:367-81.
2. Leckman J, Grice D, Boardman J, et al. Symptoms of obsessive-compulsive disorder. Am J Psychiatry 1997;154:911-17.
3. Leckman J, Zhang H, Alsobrook J, Pauls D. Symptom dimensions in obsessive-compulsive disorder: toward quantitative phenotypes. Am J Med Genet 2001;105(1):28-30.
4. Hanna G. Demographic and clinical features of obsessive-compulsive disorder in children and adolescents. J Am Acad Child Adolesc Psychiatry 1995;34(1):19-27.
5. Rasmussen S, Eisen J. The epidemiology and clinical features of obsessive-compulsive disorder. Psychiatr Clin North Am 1992;15(4):743-58.
6. Frost R, Hartl T. A cognitive-behavioral model of compulsive hoarding. Behav Res Ther 1996;34:341-50.
7. Steketee G, Frost R. Compulsive hoarding: current status of the research. Clin Psychol Rev 2003;23:905-27.
8. Saxena S, Maidment K, Vapnik T, et al. Obsessive-compulsive hoarding: symptom severity and response to multi-modal treatment. J Clin Psychiatry 2002;63:21-7.
9. Leckman JF, Pauls DL, Zhang H, etal. and the Tourette Syndrome Association International Consortium for Genetics. Obsessive-compulsive symptom dimensions in affected sibling pairs diagnosed with Gilles de la Tourette Syndrome. Am J Med Genet 2003;116B:60-8.
10. Zhang H, Leckman JF, Pauls DL, etal. and the Tourette Syndrome Association International Consortium for Genetics. Genome-wide scan of hoarding in sib pairs in which both sibs have Gilles de la Tourette syndrome. Am J Hum Genet 2002;70:896-904.
11. Winsberg M, Cassic K, Koran L. Hoarding in obsessive-compulsive disorder: a report of 20 cases. J Clin Psychiatry 1999;60:591-7.
12. Samuels J, Bienvenu OJ, 3rd, Riddle MA, et al. Hoarding in obsessive compulsive disorder: results from a case-control study. Behav Res Ther 2002;40(5):517-28.
13. Saxena S, Brody A, Maidment K, et al. Cerebral glucose metabolism in obsessive-compulsive hoarding. Am J Psychiatry 2004;161:1038-48.
14. Black D, Monahan P, Gable J, et al. Hoarding and treatment response in non-depressed subjects with obsessive-compulsive disorder. J Clin Psychiatry 1998;59:420-5.
15. Mayberg H, Brannan S, Mahurin R, et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport 1997;8(4):1057-61.
16. Rauch S, Shin L, Dougherty D, et al. Predictors of fluvoxamine response in contamination-related obsessive-compulsive disorder: a PET symptom provocation study. Neuropsychopharmacol 2002;27(5):782-91.
17. Hartl T, Frost R, Allen G, et al. Actual and perceived memory deficits in individuals with compulsive hoarding. Depress Anxiety 2004;20:59-69.
18. Frost R, Krause M, Steketee G. Hoarding and obsessive-compulsive symptoms. Behav Modif 1996;20:116-32.
19. Steketee G, Frost R, Kim H-J. Hoarding by elderly people. Health Soc Work 2001;26:176-84.
20. Frost RO, Steketee G, Williams LF, Warren R. Mood, personality disorder symptoms, and disability in obsessive compulsive hoarders: a comparison with clinical and non-clinical controls. Behav Res Ther 2000;38:1071-81.
21. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry 1989;46(11):1006-11.
22. Frost RO, Steketee G, Grisham J. Measurement of compulsive hoarding: saving inventory-revised. Behav Res Ther 2004;42(10):1163-82.
23. Saxena S, Maidment K. Treatment of compulsive hoarding. J Clin Psychol 2004;60(11):1143-54.
24. Mataix-Cols D, Marks IM, Greist JH, et al. Obsessive-compulsive symptom dimensions as predictors of compliance with and response to behaviour therapy: results from a controlled trial. Psychother Psychosom 2002;71(5):255-62.
25. Abramowitz JS, Franklin ME, Schwartz SA, Furr JM. Symptom presentation and outcome of cognitive-behavioral therapy for obsessive-compulsive disorder. J Consult Clin Psychol 2003;71(6):1049-57.
26. Steketee G, Frost RO, Wincze J, et al. Group and individual treatment of compulsive hoarding: a pilot study. Behav Cognit Psychother 2000;28:259-68.
27. Mataix-Cols D, Rauch S, Manzo P, Jenike M. Use of factor-analyzed symptom dimensions to predict outcome with serotonin reuptake inhibitors and placebo in the treatment of obsessive-compulsive disorder. Am J Psychiatry 1999;156:1409-16.
28. Greist JH, Jefferson JW, Kobak KA, et al. Efficacy and tolerability of serotonin transport inhibitors in obsessive-compulsive disorder. A meta-analysis. Arch Gen Psychiatry 1995;52(1):53-60.
29. Pallanti S, Hollander E, Goodman WK. A qualitative analysis of nonresponse: management of treatment-refractory obsessive-compulsive disorder. J Clin Psychiatry 2004;65(suppl 14):6-10.
30. McDougle C, Goodman W, Leckman J, et al. Haloperidol addition in fluvoxamine-refractory obsessive-compulsive disorder: a double-blind, placebo-controlled study in patients with and without tics. Arch Gen Psychiatry 1994;51:302-8.
Compulsive hoarding behavior is considered notoriously difficult to treat, but targeting its characteristic symptoms with medication and psychotherapy can be successful. This article provides a guide for the psychiatrist—alone or with a cognitive-behavioral therapist—to diagnose compulsive hoarding syndrome and help patients overcome the anxieties that fuel its symptoms.
WHAT IS COMPULSIVE HOARDING?
Hoarders acquire and are unable to discard items that others consider of little use or value.1 They most often save newspapers, magazines, old clothing, bags, books, mail, notes, and lists. Hoarding and saving behaviors occur in nonclinical populations and with other neuropsychiatric disorders—schizophrenia, dementia, eating disorders, mental retardation—but are most often found in persons with obsessive-compulsive disorder (OCD).
OCD is a heterogeneous clinical entity with several major symptom domains:2,3
- aggressive, sexual, and religious obsessions with checking compulsions
- symmetry/order obsessions with ordering, arranging, and repeating compulsions
- contamination obsessions with washing and cleaning compulsions
- hoarding and saving symptoms.
Genetics. Compulsive hoarding may have a different pattern of inheritance and comorbidity than other OCD symptom factors. Hoarding/saving symptoms show a recessive inheritance pattern, whereas aggressive/checking and symmetry/order symptoms show a dominant pattern.9 The hoarding phenotype has been significantly associated with genetic markers on chromosomes 4, 5, and 17.14 In other studies:
- Among 20 OCD patients with prominent hoarding, 84% had first-degree relatives with hoarding behaviors and only 37% had first-degree relatives who met DSM-IV criteria for OCD.11
- Among 126 OCD patients, social phobia, personality disorders, and pathologic grooming disorders were more common in hoarders than in nonhoarders. Hoarding and tics were more common in first-degree relatives of hoarders than in those of nonhoarders.12
Neurobiology. Using positron emission tomography (PET) brain imaging, our group13 compared glucose metabolism in patients with compulsive hoarding syndrome with that of nonhoarding OCD patients and normal controls. Compulsive hoarders had unique brain activity, with significantly lower metabolism:
- in the posterior cingulate gyrus and occipital cortex than controls
- in the dorsal anterior cingulate gyrus (AC) and thalamus than nonhoarding OCD patients.
Hoarding severity was significantly correlated with lower activity in the dorsal AC across all OCD patients.
Discussion. Genetic and neurobiologic data suggest that compulsive hoarding syndrome may be a neurobiologically distinct variant of OCD14 and may help explain its clinical symptoms and poor treatment response. Low AC activity may mediate compulsive hoarders’ decision-making and attentional problems, whereas low posterior cingulate activity may be responsible for visuospatial and memory deficits. Moreover:
ASSESSMENT AND TREATMENT PLANNING
To manage compulsive hoarding syndrome, begin with a thorough neuropsychiatric evaluation:
- Rule out primary psychotic disorders, dementia, and other cognitive impairments and neurologic disorders.
- Rule out primary major depression, as clutter and self-neglect may be caused by amotivation, low energy, or hopelessness.
- Determine if the patient has OCD.
Amount of clutter. Living areas may be so cluttered that sleeping in a bed, sitting on chairs, or preparing food on a kitchen counter are impossible. How much of the home is cluttered? How much floor and counter space is usable? Are rooms unusable or inaccessible because of clutter? Can the patient use the laundry, prepare food in the kitchen, use the shower, toilet, etc.?
Health or safety hazards. Huge piles of papers can be a fire hazard. Clutter may be blocking the exits. Collected items may extend beyond patients’ homes to their cars, garages, storage lockers, and even storage areas owned by friends and family.
Beliefs about possessions. Compulsive hoarders often have distorted feelings about their possessions. They may over-buy or impulsively purchase items they feel have emotional or monetary value. They may consider the items extensions of themselves and suffer grief-like loss when discarding things.7
Some collect free items—flyers, coupons, newspapers, discarded goods—hoping to save money or be prepared “just in case” the item is ever needed. This may represent unattainable expectations of perfection, needing to maintain preparedness for every possible contingency. Hoarders often believe they have poor memory and have catastrophic fears of what might happen if they forget something. Thus, their desire to keep their possessions in sight is strong.17
Information processing deficits. Because of anxieties about making mistakes, most hoarders have great difficulty making decisions.18 It is easier to not decide than to suffer the consequences of a “wrong” decision. To gauge this behavior, ask patients how long routine decisions take them and which decisions they procrastinate or avoid.
Compulsive hoarders often have trouble categorizing possessions;6,7 because every item feels unique, they create a special category for each one and resist storing items together.
Table 1
Proposed criteria to diagnose obsessive-compulsive hoarding*
| Patient acquires and fails to discard a large number of possessions that appear useless or of limited value |
| Clutter prevents patient from using living or work spaces for activities for which they were designed |
| Hoarding behavior causes significant distress or functional impairment |
| * Proposed by Frost and Hartl, reference 6. |
Avoidance behaviors are a hallmark of the compulsive hoarding syndrome. To avoid deciding to discard items, they put them in a box, garage, rented storage facility, etc. They may also avoid routine decision-making tasks that could lead to making a mistake.
Daily functioning. Hoarders may take a long time to do even small chores, such as taking an hour to pay one bill. An inordinate amount of time may be spent “churning”—moving items from one pile to another but never discarding any item or establishing a consistent system or organization.
Medication compliance. Compulsive hoarders often forget to take medications or take them at inappropriate times. They may lose their medications in the clutter.
Insight. Hoarders often have little awareness of how their behavior and clutter affect their lives.19 They minimize the clutter in their homes and its health and safety risks. Insight can fluctuate over time and needs to be assessed repeatedly during treatment.
Table 2
Assessing a patient with compulsive hoarding symptoms
| Domain | Useful questions or strategies |
|---|---|
| Amount of clutter | Visit home and/or see pictures |
| Hazards relating to clutter | Ask: What precautions do you take to reduce risk of fire? Have you ever had a problem with rodent or insect infestation as a result of the clutter? Have neighbors complained about the risks of fire or infestation that the clutter might impose on their homes? |
| Beliefs about loss of possessions | Ask: What is the worst thing that would happen if you threw this item away? If you did not have this, what do you think would happen? |
| Information-processing deficits | Ask: How long do routine decisions take you? Which decisions do you procrastinate or avoid? |
| Decision-making and organizational skills | Ask: How do you pay and store your bills? |
| Avoidance behaviors | Ask: Do you avoid other things (sorting mail, returning calls, doing dishes, or paying bills, rent, or taxes)? |
| Daily functioning | Ask: Do you get everything done that you want to do? Are you often late? Do you have difficulty starting or finishing tasks? Describe a typical day. |
| Insight | Ask: Do you think this amount of clutter is normal? Do you think having this clutter has caused problems in your life? |
| Motivation for treatment | Ask: What brings you into therapy now? Do you think you have a problem with excessive hoarding/saving? If it was not for your family, would you come for help? |
| Social and occupational functioning | Ask: How has your clutter affected your personal relationships? When was the last time you had someone come to your home? What prevents you from working right now? Are you working to your full potential? |
| Support from friends and family | Ask: What does your family say about your clutter? Do your friends or family understand what is going on? |
| Treatment compliance | Ask: How long does it typically take before you renew your prescriptions when you run out of medications? |
Cognitive behavioral therapy for compulsive hoarding
| Treatment sequence | Methods and goals |
|---|---|
| Educate patient about hoarding | Help improve insight and motivation |
| Set up treatment | With patient, select target area of clutter |
| Assess items together, creating a hierarchy of least to most difficult areas to sort and items to discard | |
| Create realistic categories and a storage system | |
| Begin discarding | Patient must decide to keep or discard each item and permanently remove it from pile |
| Patient must store saved items appropriately | |
| Continue until area is clear, then move to next area | |
| Plan and implement appropriate use of space | |
| Stop incoming clutter | Cancel subscriptions |
| Address compulsive buying and acquisition | |
| Provide organization training | Organize possessions, time, tasks, etc. |
| Prevent relapse | Replace hoarding with healthier behaviors to prevent clutter from re-accumulating |
| Source: Adapted from reference 23. | |
20
Motivation. Like insight, motivation can fluctuate over time. Patients usually must work tremendously hard to adhere to treatment. To support these efforts, we periodically review with patients compulsive hoarding’s negative effects and the activities they would enjoy—such as improved relationships, greater work capacity, hobbies—if overcoming this behavior allowed them more time and space.
Rating scales. The symptom checklist of the Yale-Brown Obsessive-Compulsive Scale (YBOCS)21 contains two items for hoarding obsessions and compulsions but none for avoidance behaviors, which are prominent with compulsive hoarding. The Saving Inventory-Revised22 is a validated, 23-item self-report measure of clutter, difficulty discarding, and excessive acquisition, which distinguishes compulsive hoarders, nonhoarding OCD patients, and normal controls.
TREATMENT
The compulsive hoarder’s problems will not be solved by someone else throwing away or organizing his or her possessions. These actions often anger patients, who see them as intrusive and a loss of control.
In our experience, family members’ attempts to intervene can disrupt relationships and worsen hoarders’ social withdrawal. “Taking over” also does not help the patient create a sustainable system for keeping clutter-free.
Algorithm
Medication treatment for compulsive hoarding*
| Start with SSRIs, as for nonhoarding OCD (citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline) |
|
| Treat comorbid conditions | Mood disorders, other anxiety disorders, ADHD, psychotic disorders, etc. |
| Use adjunctive medications if SSRIs give only partial response |
|
| * Combine medication treatment with cognitive-behavioral therapy | |
| SSRI: selective serotonin reuptake inhibitor | |
| OCD: obsessive-compulsive disorder | |
| ADHD: attention-deficit/hyperactivity disorder | |
Psychotherapy. Exposure and response prevention (ERP) focuses on preventing incoming clutter, discarding, organizing, and relapse prevention (Table 3).23 Start treatment by explaining compulsive hoarding syndrome to patients as having problems with information processing, obsessional anxiety, and avoiding decisions.
Preventing incoming clutter. Before you focus on discarding, patients must stop incoming clutter; otherwise, it will come in as fast as it goes out. We ask patients to keep a daily log of every item they acquire or buy to build their awareness of what triggers their behavior.
Discarding. To desensitize over time, we repeatedly expose patients to the anxiety, sadness, or anger they feel when discarding items and making decisions. We encourage them to provoke their anxiety by throwing away as many items as possible, keeping only necessary items.
We support ERP with cognitive restructuring, prompting patients to reframe their obsessive fears about losing something necessary or valuable. By thinking through the consequences of discarding their clutter, they challenge their erroneous beliefs that dire consequences will occur.
Organizing. When patients decide they must keep an item, ask them to immediately identify a specific place and deadline to store it. After an area is cleared, patients must keep it clear and use it for its intended purpose. Most patients need training in time management, scheduling, and prioritizing.
Relapse prevention. Replace hoarding behaviors with more-adaptive, healthy behaviors. Teach patients to create a realistic schedule that includes time for chores, eating and sleeping, CBT homework, and recreation. Treatment goals are to:
- extinguish obsessional fears and compulsive saving behaviors
- teach lasting organizational and decision-making skills, thereby reducing relapse risk.
Medications. No controlled studies have examined whether any medications are effective for compulsive hoarding syndrome. The treatment strategies and algorithm described here are based on our clinical experience, controlled trials of OCD patients, and limited OCD studies secondarily examining hoarders’ specific treatment responses.
Selective serotonin reuptake inhibitors (SSRIs) may be less effective for compulsive hoarding than for other compulsive behaviors.14,27 Nevertheless, SSRIs may help alleviate hoarders’ core symptoms, other OCD symptoms, depression, and anxiety. For hoarding treatment to be effective, comorbid disorders must be treated and stabilized.
Several studies of SSRI use in OCD patients have shown modest improvements in compulsive hoarders:
- In a descriptive study of patients with compulsive hoarding, 1 of 18 (6%) patients had a “marked” response to at least one SSRI trial. The others showed a partial response,17 with YBOCS scores decreasing by at least 25% in approximately 50% of this group.
- When 17 OCD patients with hoarding symptoms were treated with paroxetine, CBT, or placebo, 18% responded to active treatment. Response was defined as a 40% reduction on YBOCS scores and “very much” or “much” improved on the Clinical Global Impression Scale (CGI).14
Atypical antipsychotics may be effective for OCD symptoms that do not respond adequately to SSRIs.29 Conventional antipsychotics are also effective adjuncts to SSRIs—particularly for patients with coexisting tic or psychotic disorders30—but consider the potential for extrapyramidal side effects and tardive dyskinesia.
We find that stimulants help some compulsive hoarders, particularly those with comorbid ADHD, other attentional problems, low motivation, or lethargy. Mood stabilizers are necessary to treat comorbid bipolar disorder, cyclothymia, and impulsivity.
Related resources
- Saxena S, Maidment K. Treatment of compulsive hoarding. J Clin Psychol 2004;60(11):1143-54.
- UCLA OCD Intensive Treatment Program. For information on research studies contact Karron Maidment (310) 794-7305 or visit www.mentalhealth.ucla.edu/projects/anxiety/ocdintensivetreatment.htm.
- Compulsive hoarding project. Institute of Living Anxiety Disorders Center, Hartford Hospital. http://instituteofliving.org/adc/compulsive_hoarding.htm
- Obsessive Compulsive Foundation compulsive hoarding Web site. http://www.ocfoundation.org/1005/index.html
- Neziroglu F, Bubrick J, Yaryura-Tobias JA. Overcoming compulsive hoarding. Oakland, CA: New Harbinger Publications, 2004.
- Citalopram • Celexa
- Clomipramine • Anafranil
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Venlafaxine • Effexor
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Compulsive hoarding behavior is considered notoriously difficult to treat, but targeting its characteristic symptoms with medication and psychotherapy can be successful. This article provides a guide for the psychiatrist—alone or with a cognitive-behavioral therapist—to diagnose compulsive hoarding syndrome and help patients overcome the anxieties that fuel its symptoms.
WHAT IS COMPULSIVE HOARDING?
Hoarders acquire and are unable to discard items that others consider of little use or value.1 They most often save newspapers, magazines, old clothing, bags, books, mail, notes, and lists. Hoarding and saving behaviors occur in nonclinical populations and with other neuropsychiatric disorders—schizophrenia, dementia, eating disorders, mental retardation—but are most often found in persons with obsessive-compulsive disorder (OCD).
OCD is a heterogeneous clinical entity with several major symptom domains:2,3
- aggressive, sexual, and religious obsessions with checking compulsions
- symmetry/order obsessions with ordering, arranging, and repeating compulsions
- contamination obsessions with washing and cleaning compulsions
- hoarding and saving symptoms.
Genetics. Compulsive hoarding may have a different pattern of inheritance and comorbidity than other OCD symptom factors. Hoarding/saving symptoms show a recessive inheritance pattern, whereas aggressive/checking and symmetry/order symptoms show a dominant pattern.9 The hoarding phenotype has been significantly associated with genetic markers on chromosomes 4, 5, and 17.14 In other studies:
- Among 20 OCD patients with prominent hoarding, 84% had first-degree relatives with hoarding behaviors and only 37% had first-degree relatives who met DSM-IV criteria for OCD.11
- Among 126 OCD patients, social phobia, personality disorders, and pathologic grooming disorders were more common in hoarders than in nonhoarders. Hoarding and tics were more common in first-degree relatives of hoarders than in those of nonhoarders.12
Neurobiology. Using positron emission tomography (PET) brain imaging, our group13 compared glucose metabolism in patients with compulsive hoarding syndrome with that of nonhoarding OCD patients and normal controls. Compulsive hoarders had unique brain activity, with significantly lower metabolism:
- in the posterior cingulate gyrus and occipital cortex than controls
- in the dorsal anterior cingulate gyrus (AC) and thalamus than nonhoarding OCD patients.
Hoarding severity was significantly correlated with lower activity in the dorsal AC across all OCD patients.
Discussion. Genetic and neurobiologic data suggest that compulsive hoarding syndrome may be a neurobiologically distinct variant of OCD14 and may help explain its clinical symptoms and poor treatment response. Low AC activity may mediate compulsive hoarders’ decision-making and attentional problems, whereas low posterior cingulate activity may be responsible for visuospatial and memory deficits. Moreover:
ASSESSMENT AND TREATMENT PLANNING
To manage compulsive hoarding syndrome, begin with a thorough neuropsychiatric evaluation:
- Rule out primary psychotic disorders, dementia, and other cognitive impairments and neurologic disorders.
- Rule out primary major depression, as clutter and self-neglect may be caused by amotivation, low energy, or hopelessness.
- Determine if the patient has OCD.
Amount of clutter. Living areas may be so cluttered that sleeping in a bed, sitting on chairs, or preparing food on a kitchen counter are impossible. How much of the home is cluttered? How much floor and counter space is usable? Are rooms unusable or inaccessible because of clutter? Can the patient use the laundry, prepare food in the kitchen, use the shower, toilet, etc.?
Health or safety hazards. Huge piles of papers can be a fire hazard. Clutter may be blocking the exits. Collected items may extend beyond patients’ homes to their cars, garages, storage lockers, and even storage areas owned by friends and family.
Beliefs about possessions. Compulsive hoarders often have distorted feelings about their possessions. They may over-buy or impulsively purchase items they feel have emotional or monetary value. They may consider the items extensions of themselves and suffer grief-like loss when discarding things.7
Some collect free items—flyers, coupons, newspapers, discarded goods—hoping to save money or be prepared “just in case” the item is ever needed. This may represent unattainable expectations of perfection, needing to maintain preparedness for every possible contingency. Hoarders often believe they have poor memory and have catastrophic fears of what might happen if they forget something. Thus, their desire to keep their possessions in sight is strong.17
Information processing deficits. Because of anxieties about making mistakes, most hoarders have great difficulty making decisions.18 It is easier to not decide than to suffer the consequences of a “wrong” decision. To gauge this behavior, ask patients how long routine decisions take them and which decisions they procrastinate or avoid.
Compulsive hoarders often have trouble categorizing possessions;6,7 because every item feels unique, they create a special category for each one and resist storing items together.
Table 1
Proposed criteria to diagnose obsessive-compulsive hoarding*
| Patient acquires and fails to discard a large number of possessions that appear useless or of limited value |
| Clutter prevents patient from using living or work spaces for activities for which they were designed |
| Hoarding behavior causes significant distress or functional impairment |
| * Proposed by Frost and Hartl, reference 6. |
Avoidance behaviors are a hallmark of the compulsive hoarding syndrome. To avoid deciding to discard items, they put them in a box, garage, rented storage facility, etc. They may also avoid routine decision-making tasks that could lead to making a mistake.
Daily functioning. Hoarders may take a long time to do even small chores, such as taking an hour to pay one bill. An inordinate amount of time may be spent “churning”—moving items from one pile to another but never discarding any item or establishing a consistent system or organization.
Medication compliance. Compulsive hoarders often forget to take medications or take them at inappropriate times. They may lose their medications in the clutter.
Insight. Hoarders often have little awareness of how their behavior and clutter affect their lives.19 They minimize the clutter in their homes and its health and safety risks. Insight can fluctuate over time and needs to be assessed repeatedly during treatment.
Table 2
Assessing a patient with compulsive hoarding symptoms
| Domain | Useful questions or strategies |
|---|---|
| Amount of clutter | Visit home and/or see pictures |
| Hazards relating to clutter | Ask: What precautions do you take to reduce risk of fire? Have you ever had a problem with rodent or insect infestation as a result of the clutter? Have neighbors complained about the risks of fire or infestation that the clutter might impose on their homes? |
| Beliefs about loss of possessions | Ask: What is the worst thing that would happen if you threw this item away? If you did not have this, what do you think would happen? |
| Information-processing deficits | Ask: How long do routine decisions take you? Which decisions do you procrastinate or avoid? |
| Decision-making and organizational skills | Ask: How do you pay and store your bills? |
| Avoidance behaviors | Ask: Do you avoid other things (sorting mail, returning calls, doing dishes, or paying bills, rent, or taxes)? |
| Daily functioning | Ask: Do you get everything done that you want to do? Are you often late? Do you have difficulty starting or finishing tasks? Describe a typical day. |
| Insight | Ask: Do you think this amount of clutter is normal? Do you think having this clutter has caused problems in your life? |
| Motivation for treatment | Ask: What brings you into therapy now? Do you think you have a problem with excessive hoarding/saving? If it was not for your family, would you come for help? |
| Social and occupational functioning | Ask: How has your clutter affected your personal relationships? When was the last time you had someone come to your home? What prevents you from working right now? Are you working to your full potential? |
| Support from friends and family | Ask: What does your family say about your clutter? Do your friends or family understand what is going on? |
| Treatment compliance | Ask: How long does it typically take before you renew your prescriptions when you run out of medications? |
Cognitive behavioral therapy for compulsive hoarding
| Treatment sequence | Methods and goals |
|---|---|
| Educate patient about hoarding | Help improve insight and motivation |
| Set up treatment | With patient, select target area of clutter |
| Assess items together, creating a hierarchy of least to most difficult areas to sort and items to discard | |
| Create realistic categories and a storage system | |
| Begin discarding | Patient must decide to keep or discard each item and permanently remove it from pile |
| Patient must store saved items appropriately | |
| Continue until area is clear, then move to next area | |
| Plan and implement appropriate use of space | |
| Stop incoming clutter | Cancel subscriptions |
| Address compulsive buying and acquisition | |
| Provide organization training | Organize possessions, time, tasks, etc. |
| Prevent relapse | Replace hoarding with healthier behaviors to prevent clutter from re-accumulating |
| Source: Adapted from reference 23. | |
20
Motivation. Like insight, motivation can fluctuate over time. Patients usually must work tremendously hard to adhere to treatment. To support these efforts, we periodically review with patients compulsive hoarding’s negative effects and the activities they would enjoy—such as improved relationships, greater work capacity, hobbies—if overcoming this behavior allowed them more time and space.
Rating scales. The symptom checklist of the Yale-Brown Obsessive-Compulsive Scale (YBOCS)21 contains two items for hoarding obsessions and compulsions but none for avoidance behaviors, which are prominent with compulsive hoarding. The Saving Inventory-Revised22 is a validated, 23-item self-report measure of clutter, difficulty discarding, and excessive acquisition, which distinguishes compulsive hoarders, nonhoarding OCD patients, and normal controls.
TREATMENT
The compulsive hoarder’s problems will not be solved by someone else throwing away or organizing his or her possessions. These actions often anger patients, who see them as intrusive and a loss of control.
In our experience, family members’ attempts to intervene can disrupt relationships and worsen hoarders’ social withdrawal. “Taking over” also does not help the patient create a sustainable system for keeping clutter-free.
Algorithm
Medication treatment for compulsive hoarding*
| Start with SSRIs, as for nonhoarding OCD (citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline) |
|
| Treat comorbid conditions | Mood disorders, other anxiety disorders, ADHD, psychotic disorders, etc. |
| Use adjunctive medications if SSRIs give only partial response |
|
| * Combine medication treatment with cognitive-behavioral therapy | |
| SSRI: selective serotonin reuptake inhibitor | |
| OCD: obsessive-compulsive disorder | |
| ADHD: attention-deficit/hyperactivity disorder | |
Psychotherapy. Exposure and response prevention (ERP) focuses on preventing incoming clutter, discarding, organizing, and relapse prevention (Table 3).23 Start treatment by explaining compulsive hoarding syndrome to patients as having problems with information processing, obsessional anxiety, and avoiding decisions.
Preventing incoming clutter. Before you focus on discarding, patients must stop incoming clutter; otherwise, it will come in as fast as it goes out. We ask patients to keep a daily log of every item they acquire or buy to build their awareness of what triggers their behavior.
Discarding. To desensitize over time, we repeatedly expose patients to the anxiety, sadness, or anger they feel when discarding items and making decisions. We encourage them to provoke their anxiety by throwing away as many items as possible, keeping only necessary items.
We support ERP with cognitive restructuring, prompting patients to reframe their obsessive fears about losing something necessary or valuable. By thinking through the consequences of discarding their clutter, they challenge their erroneous beliefs that dire consequences will occur.
Organizing. When patients decide they must keep an item, ask them to immediately identify a specific place and deadline to store it. After an area is cleared, patients must keep it clear and use it for its intended purpose. Most patients need training in time management, scheduling, and prioritizing.
Relapse prevention. Replace hoarding behaviors with more-adaptive, healthy behaviors. Teach patients to create a realistic schedule that includes time for chores, eating and sleeping, CBT homework, and recreation. Treatment goals are to:
- extinguish obsessional fears and compulsive saving behaviors
- teach lasting organizational and decision-making skills, thereby reducing relapse risk.
Medications. No controlled studies have examined whether any medications are effective for compulsive hoarding syndrome. The treatment strategies and algorithm described here are based on our clinical experience, controlled trials of OCD patients, and limited OCD studies secondarily examining hoarders’ specific treatment responses.
Selective serotonin reuptake inhibitors (SSRIs) may be less effective for compulsive hoarding than for other compulsive behaviors.14,27 Nevertheless, SSRIs may help alleviate hoarders’ core symptoms, other OCD symptoms, depression, and anxiety. For hoarding treatment to be effective, comorbid disorders must be treated and stabilized.
Several studies of SSRI use in OCD patients have shown modest improvements in compulsive hoarders:
- In a descriptive study of patients with compulsive hoarding, 1 of 18 (6%) patients had a “marked” response to at least one SSRI trial. The others showed a partial response,17 with YBOCS scores decreasing by at least 25% in approximately 50% of this group.
- When 17 OCD patients with hoarding symptoms were treated with paroxetine, CBT, or placebo, 18% responded to active treatment. Response was defined as a 40% reduction on YBOCS scores and “very much” or “much” improved on the Clinical Global Impression Scale (CGI).14
Atypical antipsychotics may be effective for OCD symptoms that do not respond adequately to SSRIs.29 Conventional antipsychotics are also effective adjuncts to SSRIs—particularly for patients with coexisting tic or psychotic disorders30—but consider the potential for extrapyramidal side effects and tardive dyskinesia.
We find that stimulants help some compulsive hoarders, particularly those with comorbid ADHD, other attentional problems, low motivation, or lethargy. Mood stabilizers are necessary to treat comorbid bipolar disorder, cyclothymia, and impulsivity.
Related resources
- Saxena S, Maidment K. Treatment of compulsive hoarding. J Clin Psychol 2004;60(11):1143-54.
- UCLA OCD Intensive Treatment Program. For information on research studies contact Karron Maidment (310) 794-7305 or visit www.mentalhealth.ucla.edu/projects/anxiety/ocdintensivetreatment.htm.
- Compulsive hoarding project. Institute of Living Anxiety Disorders Center, Hartford Hospital. http://instituteofliving.org/adc/compulsive_hoarding.htm
- Obsessive Compulsive Foundation compulsive hoarding Web site. http://www.ocfoundation.org/1005/index.html
- Neziroglu F, Bubrick J, Yaryura-Tobias JA. Overcoming compulsive hoarding. Oakland, CA: New Harbinger Publications, 2004.
- Citalopram • Celexa
- Clomipramine • Anafranil
- Escitalopram • Lexapro
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Venlafaxine • Effexor
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Frost R, Gross R. The hoarding of possessions. Behav Res Ther 1993;31:367-81.
2. Leckman J, Grice D, Boardman J, et al. Symptoms of obsessive-compulsive disorder. Am J Psychiatry 1997;154:911-17.
3. Leckman J, Zhang H, Alsobrook J, Pauls D. Symptom dimensions in obsessive-compulsive disorder: toward quantitative phenotypes. Am J Med Genet 2001;105(1):28-30.
4. Hanna G. Demographic and clinical features of obsessive-compulsive disorder in children and adolescents. J Am Acad Child Adolesc Psychiatry 1995;34(1):19-27.
5. Rasmussen S, Eisen J. The epidemiology and clinical features of obsessive-compulsive disorder. Psychiatr Clin North Am 1992;15(4):743-58.
6. Frost R, Hartl T. A cognitive-behavioral model of compulsive hoarding. Behav Res Ther 1996;34:341-50.
7. Steketee G, Frost R. Compulsive hoarding: current status of the research. Clin Psychol Rev 2003;23:905-27.
8. Saxena S, Maidment K, Vapnik T, et al. Obsessive-compulsive hoarding: symptom severity and response to multi-modal treatment. J Clin Psychiatry 2002;63:21-7.
9. Leckman JF, Pauls DL, Zhang H, etal. and the Tourette Syndrome Association International Consortium for Genetics. Obsessive-compulsive symptom dimensions in affected sibling pairs diagnosed with Gilles de la Tourette Syndrome. Am J Med Genet 2003;116B:60-8.
10. Zhang H, Leckman JF, Pauls DL, etal. and the Tourette Syndrome Association International Consortium for Genetics. Genome-wide scan of hoarding in sib pairs in which both sibs have Gilles de la Tourette syndrome. Am J Hum Genet 2002;70:896-904.
11. Winsberg M, Cassic K, Koran L. Hoarding in obsessive-compulsive disorder: a report of 20 cases. J Clin Psychiatry 1999;60:591-7.
12. Samuels J, Bienvenu OJ, 3rd, Riddle MA, et al. Hoarding in obsessive compulsive disorder: results from a case-control study. Behav Res Ther 2002;40(5):517-28.
13. Saxena S, Brody A, Maidment K, et al. Cerebral glucose metabolism in obsessive-compulsive hoarding. Am J Psychiatry 2004;161:1038-48.
14. Black D, Monahan P, Gable J, et al. Hoarding and treatment response in non-depressed subjects with obsessive-compulsive disorder. J Clin Psychiatry 1998;59:420-5.
15. Mayberg H, Brannan S, Mahurin R, et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport 1997;8(4):1057-61.
16. Rauch S, Shin L, Dougherty D, et al. Predictors of fluvoxamine response in contamination-related obsessive-compulsive disorder: a PET symptom provocation study. Neuropsychopharmacol 2002;27(5):782-91.
17. Hartl T, Frost R, Allen G, et al. Actual and perceived memory deficits in individuals with compulsive hoarding. Depress Anxiety 2004;20:59-69.
18. Frost R, Krause M, Steketee G. Hoarding and obsessive-compulsive symptoms. Behav Modif 1996;20:116-32.
19. Steketee G, Frost R, Kim H-J. Hoarding by elderly people. Health Soc Work 2001;26:176-84.
20. Frost RO, Steketee G, Williams LF, Warren R. Mood, personality disorder symptoms, and disability in obsessive compulsive hoarders: a comparison with clinical and non-clinical controls. Behav Res Ther 2000;38:1071-81.
21. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry 1989;46(11):1006-11.
22. Frost RO, Steketee G, Grisham J. Measurement of compulsive hoarding: saving inventory-revised. Behav Res Ther 2004;42(10):1163-82.
23. Saxena S, Maidment K. Treatment of compulsive hoarding. J Clin Psychol 2004;60(11):1143-54.
24. Mataix-Cols D, Marks IM, Greist JH, et al. Obsessive-compulsive symptom dimensions as predictors of compliance with and response to behaviour therapy: results from a controlled trial. Psychother Psychosom 2002;71(5):255-62.
25. Abramowitz JS, Franklin ME, Schwartz SA, Furr JM. Symptom presentation and outcome of cognitive-behavioral therapy for obsessive-compulsive disorder. J Consult Clin Psychol 2003;71(6):1049-57.
26. Steketee G, Frost RO, Wincze J, et al. Group and individual treatment of compulsive hoarding: a pilot study. Behav Cognit Psychother 2000;28:259-68.
27. Mataix-Cols D, Rauch S, Manzo P, Jenike M. Use of factor-analyzed symptom dimensions to predict outcome with serotonin reuptake inhibitors and placebo in the treatment of obsessive-compulsive disorder. Am J Psychiatry 1999;156:1409-16.
28. Greist JH, Jefferson JW, Kobak KA, et al. Efficacy and tolerability of serotonin transport inhibitors in obsessive-compulsive disorder. A meta-analysis. Arch Gen Psychiatry 1995;52(1):53-60.
29. Pallanti S, Hollander E, Goodman WK. A qualitative analysis of nonresponse: management of treatment-refractory obsessive-compulsive disorder. J Clin Psychiatry 2004;65(suppl 14):6-10.
30. McDougle C, Goodman W, Leckman J, et al. Haloperidol addition in fluvoxamine-refractory obsessive-compulsive disorder: a double-blind, placebo-controlled study in patients with and without tics. Arch Gen Psychiatry 1994;51:302-8.
1. Frost R, Gross R. The hoarding of possessions. Behav Res Ther 1993;31:367-81.
2. Leckman J, Grice D, Boardman J, et al. Symptoms of obsessive-compulsive disorder. Am J Psychiatry 1997;154:911-17.
3. Leckman J, Zhang H, Alsobrook J, Pauls D. Symptom dimensions in obsessive-compulsive disorder: toward quantitative phenotypes. Am J Med Genet 2001;105(1):28-30.
4. Hanna G. Demographic and clinical features of obsessive-compulsive disorder in children and adolescents. J Am Acad Child Adolesc Psychiatry 1995;34(1):19-27.
5. Rasmussen S, Eisen J. The epidemiology and clinical features of obsessive-compulsive disorder. Psychiatr Clin North Am 1992;15(4):743-58.
6. Frost R, Hartl T. A cognitive-behavioral model of compulsive hoarding. Behav Res Ther 1996;34:341-50.
7. Steketee G, Frost R. Compulsive hoarding: current status of the research. Clin Psychol Rev 2003;23:905-27.
8. Saxena S, Maidment K, Vapnik T, et al. Obsessive-compulsive hoarding: symptom severity and response to multi-modal treatment. J Clin Psychiatry 2002;63:21-7.
9. Leckman JF, Pauls DL, Zhang H, etal. and the Tourette Syndrome Association International Consortium for Genetics. Obsessive-compulsive symptom dimensions in affected sibling pairs diagnosed with Gilles de la Tourette Syndrome. Am J Med Genet 2003;116B:60-8.
10. Zhang H, Leckman JF, Pauls DL, etal. and the Tourette Syndrome Association International Consortium for Genetics. Genome-wide scan of hoarding in sib pairs in which both sibs have Gilles de la Tourette syndrome. Am J Hum Genet 2002;70:896-904.
11. Winsberg M, Cassic K, Koran L. Hoarding in obsessive-compulsive disorder: a report of 20 cases. J Clin Psychiatry 1999;60:591-7.
12. Samuels J, Bienvenu OJ, 3rd, Riddle MA, et al. Hoarding in obsessive compulsive disorder: results from a case-control study. Behav Res Ther 2002;40(5):517-28.
13. Saxena S, Brody A, Maidment K, et al. Cerebral glucose metabolism in obsessive-compulsive hoarding. Am J Psychiatry 2004;161:1038-48.
14. Black D, Monahan P, Gable J, et al. Hoarding and treatment response in non-depressed subjects with obsessive-compulsive disorder. J Clin Psychiatry 1998;59:420-5.
15. Mayberg H, Brannan S, Mahurin R, et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport 1997;8(4):1057-61.
16. Rauch S, Shin L, Dougherty D, et al. Predictors of fluvoxamine response in contamination-related obsessive-compulsive disorder: a PET symptom provocation study. Neuropsychopharmacol 2002;27(5):782-91.
17. Hartl T, Frost R, Allen G, et al. Actual and perceived memory deficits in individuals with compulsive hoarding. Depress Anxiety 2004;20:59-69.
18. Frost R, Krause M, Steketee G. Hoarding and obsessive-compulsive symptoms. Behav Modif 1996;20:116-32.
19. Steketee G, Frost R, Kim H-J. Hoarding by elderly people. Health Soc Work 2001;26:176-84.
20. Frost RO, Steketee G, Williams LF, Warren R. Mood, personality disorder symptoms, and disability in obsessive compulsive hoarders: a comparison with clinical and non-clinical controls. Behav Res Ther 2000;38:1071-81.
21. Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry 1989;46(11):1006-11.
22. Frost RO, Steketee G, Grisham J. Measurement of compulsive hoarding: saving inventory-revised. Behav Res Ther 2004;42(10):1163-82.
23. Saxena S, Maidment K. Treatment of compulsive hoarding. J Clin Psychol 2004;60(11):1143-54.
24. Mataix-Cols D, Marks IM, Greist JH, et al. Obsessive-compulsive symptom dimensions as predictors of compliance with and response to behaviour therapy: results from a controlled trial. Psychother Psychosom 2002;71(5):255-62.
25. Abramowitz JS, Franklin ME, Schwartz SA, Furr JM. Symptom presentation and outcome of cognitive-behavioral therapy for obsessive-compulsive disorder. J Consult Clin Psychol 2003;71(6):1049-57.
26. Steketee G, Frost RO, Wincze J, et al. Group and individual treatment of compulsive hoarding: a pilot study. Behav Cognit Psychother 2000;28:259-68.
27. Mataix-Cols D, Rauch S, Manzo P, Jenike M. Use of factor-analyzed symptom dimensions to predict outcome with serotonin reuptake inhibitors and placebo in the treatment of obsessive-compulsive disorder. Am J Psychiatry 1999;156:1409-16.
28. Greist JH, Jefferson JW, Kobak KA, et al. Efficacy and tolerability of serotonin transport inhibitors in obsessive-compulsive disorder. A meta-analysis. Arch Gen Psychiatry 1995;52(1):53-60.
29. Pallanti S, Hollander E, Goodman WK. A qualitative analysis of nonresponse: management of treatment-refractory obsessive-compulsive disorder. J Clin Psychiatry 2004;65(suppl 14):6-10.
30. McDougle C, Goodman W, Leckman J, et al. Haloperidol addition in fluvoxamine-refractory obsessive-compulsive disorder: a double-blind, placebo-controlled study in patients with and without tics. Arch Gen Psychiatry 1994;51:302-8.
When not to treat depression in PCOS with antidepressants
Women with depression and polycystic ovary syndrome (PCOS) can be trapped in a vicious cycle of hormonal dysregulation. Treating these patients appropriately—with or without antidepressants—requires an understanding of their underlying metabolic disorder.
This article presents a case1 that exemplifies the association between depression and PCOS (Box 1).2-4 Based on our research and clinical experience, we offer recommendations to help you manage depression in patients with PCOS.
CHRONIC DEPRESSION WITH AMENORRHEA
Ms. K, age 30, presented for psychiatric evaluation of treatment-resistant recurrent major depression. Her symptoms included sad mood, sleep disturbance, decreased energy, anhedonia, poor concentration, and feelings of guilt and worthlessness. Her score of 28 on the Hamilton Rating Scale for Depression (HAM-D-21) indicated severe depression.
Polycystic ovary syndrome (PCOS) is the most common cause of menstrual disturbance in women of reproductive age, affecting approximately 5% of U.S. women.1 Clinically, it is the association of hyperandrogenism with chronic anovulation, without specific underlying adrenal or pituitary gland disease.2
PCOS has been shown to be associated with depression, though little research has been done in this area. We conducted a pilot study (the first, to our knowledge) to examine the rate of depression among women with documented PCOS and to correlate Center for Epidemiological Studies Depression Scale (CES-D) scores indicative of depression with clinical and biochemical markers of PCOS. We found an increased prevalence of depression in women with PCOS and an association between depression, insulin resistance, and body mass index. Specifically, among 32 women with documented PCOS,3 16 (50%) had CES-D scores indicating depression.
This was Ms. K’s third episode of major depression, with the first two occurring at ages 24 and 26. She reported similar symptoms each time. Previous psychiatrists had tried a variety of antidepressants with no therapeutic benefit before she responded to citalopram, 40 mg/d, at age 27. With this selective serotonin reuptake inhibitor, her depressive symptoms resolved for >1 year, except for mild irritability and low mood the week before menses.
Her depressive symptoms returned, however, and her previous psychiatrist treated her for approximately 13 months with extended-release venlafaxine, 225 mg/d. When she remained depressed, she was referred to our clinic for evaluation. Based on her history, we could not determine whether she had become euthymic at age 27 or her symptoms had improved but still met criteria for a major depressive episode.
Ms. K reported having PCOS symptoms from age 19. These included amenorrhea since menarche, hirsutism, hair loss, alopecia, central fat distribution characteristic of PCOS, and male-pattern hair growth on her abdomen and thighs. She was not obese—with a body mass index (BMI) of approximately 25—but had gained 10 to 15 lbs while taking venlafaxine.
Ms. K had never been treated for PCOS but reported that her desire to become pregnant made her more concerned about her symptoms and possible infertility.
Diagnosis. PCOS is usually diagnosed using endocrinologic, clinical, and ultrasonographic criteria (Table 1). Obesity is not a presenting symptom in all women with PCOS. As with Ms. K, about 50% of patients have normal BMI.
Causes of depression in PCOS. Depressed mood in PCOS may be both physiologic and psychological:
- Hypothalamic, pituitary, and other end-organ system dysregulation occurs in both PCOS and affective disorders, which share clinical and biochemical markers including insulin resistance, obesity, and hyperandrogenism.
- PCOS’ clinical sequelae—hirsutism, acne, obesity, hormonal disturbances, fear of infertility, and psychological distress—may damage their self-esteem and female identity.
PCOS’ physical symptoms alone apparently do not account for patients’ worsened mood states. Weiner et al5 found that women with PCOS and free testosterone (FT) of 10 to 26 pg/mL (just above normal range) were more depressed than women without PCOS (FT <10 pg/mL) and women with PCOS and FT >26 pg/mL. Women with PCOS with the lower and higher FT levels had similar demographic profiles, but those with the highest FT levels were not the most depressed.
Similarly, in a study of 32 women with PCOS, we found no association between depression and other possibly distressing PCOS symptoms, including hirsutism, irregular menses, acne, or alopecia.4
Testosterone and mood disorders. Women such as Ms. K with hyperandrogenic syndromes are at increased risk for mood disorders.6 Many metabolic changes associated with PCOS—insulin resistance, obesity, and hyperandrogenism—are also described in patients with affective disorders. Our investigation of reproductive status in women with bipolar disorder found no clinical or biochemical evidence of PCOS with mood-stabilizer treatment. We did find that menstrual disturbances were common, however, and sometimes preceded bipolar disorder onset.7
Insulin resistance and depression. Others have linked insulin resistance and depression;8 depression has been shown to be associated with impaired insulin sensitivity and hyperinsulinemia.9 Depressed persons also tend to eat more sweets, drink more alcohol, exercise less, and sleep fewer hours than the nondepressed—all of which contribute to insulin sensitivity and insulin resistance.10
WHAT LINKS ENDOCRINE, MOOD DISORDERS?
Insulin and serotonin. Insulin affects central serotonin (5-HT) levels.11 Thus, central 5-HT system dysregulation that causes depression might also affect peripheral insulin sensitivity—or vice versa.9
Tryptophan, a serotonin precursor, competes with other large neutral amino acids for access to the transport system that moves them across the blood-brain barrier. Insulin stimulates uptake of the competing amino acids—but not tryptophan—into muscle tissue. The resulting increased tryptophan ratio in plasma affords it greater access to the transport system to contribute toward serotonin synthesis. Insulin also promotes central catecholaminergic activity, perhaps by suppressing norepinephrine reuptake and prolonging its residence in the synaptic cleft.10
Table 1
Diagnostic signs of PCOS
| Endocrine |
| Chronically elevated plasma free and total testosterone levels |
| Increased luteinizing hormone (LH) secretion because of enhanced pituitary sensitivity to GnRH stimulation |
| Low or normal plasma follicle-stimulating hormone (FSH) levels |
| Hyperinsulinemia and peripheral insulin resistance |
| Metabolic abnormalities of hyperinsulinemia and peripheral insulin resistance, including obesity and increased susceptibility to type 2 diabetes and cardiovascular disease |
| Clinical |
| Hirsutism |
| Acne |
| Infertility |
Anovulation or menstrual abnormalities
|
| Morphologic |
| Ovaries may appear to have multiple cysts 8 to 10 mm in diameter |
Several studies have found that insulin resistance and hyperinsulinemia can resolve after depression recovery.9,12 Because insulin resistance is a cardinal feature in PCOS pathophysiology,2 insulin resistance may be a common link between depression and PCOS.
Weight gain and obesity also have been described in patients with affective disorders.13 Not known is whether the weight gain precedes the psychopathology or is caused by long-term exposure to drugs used to treat affective disorders.
CRH antagonists. Corticotropin-releasing hormone (CRH) receptor antagonists have been suggested as possible antidepressants.26 Depression and anxiety scores have declined during treatment with the cortisol synthesis inhibitors metyrapone27 and ketoconazole.28,29 These trials do not reveal whether these agents treat depression symptoms—rather than the underlying pathophysiology—or if the affective disorder will recur after long-term administration.26
GR antagonists. Glucocorticoid receptor (GR) antagonists such as mifepristone (RU-486) have been suggested as antidepressants in depressed patients with elevated basal cortisol levels.30 Mifepristone may be useful for treating psychotic depression, in which the HPA axis is particularly hyperactive.31 Mifepristone is contraindicated in most women with PCOS, however, as its progesterone antagonism would lead to infertility—already a common problem for women with PCOS.
MR antagonists. Spironolactone, a mineralocorticoid receptor (MR) antagonist, decreases insulin resistance and fasting insulin levels in PCOS patients.25 We propose that insulin resistance may provide a common link in the pathophysiology of PCOS and depression. Therefore, treatment with insulin resistance-lowering medications such as spironolactone may induce antidepressant effects in women with depression and PCOS.
We reported a correlation between depression and BMI in women with PCOS.4 Depression might be independently associated with BMI, as weight gain and obesity are distressing symptoms associated with depression.14 However, we found no association between depression and other possibly distressing PCOS symptoms. Thus, the correlation between BMI and depression might more likely reflect the relationship between depression and insulin resistance, as degree of insulin resistance is known to correlate with BMI.
Elevated cortisol. Clearly, other factors—such as hypothalamus-pituitary-adrenal (HPA) axis dysfunction—are known to link affective and endocrine disorders. Hypercortisolemia can lead to both insulin resistance and obesity. Cortisol is one of the glucocorticoids the body secretes in response to stress to mobilize energy by increasing blood glucose levels. Early life stress and chronic emotional stress:
- can impair the negative feedback system that limits cortisol production during stress
- are associated with depression.
Approximately one-half of individuals with depression have elevated serum cortisol.10 Epidemiologic data show a positive correlation between cortisol levels and insulin resistance,15 and an association between HPA dysfunction and obesity has been described.16 Insulin can trigger androgen production by enhancing adrenal sensitivity to adrenocorticotrophic hormone (ACTH).17
CASE: IMPROVING INSULIN RESISTANCE
We referred Ms. K to an endocrinologist for PCOS evaluation and treatment. Her serum glucose and insulin levels were 83 mg/dL (normal range 70 to 125 mg/dL) and 19.0 uIU/mL (normal range <10 uIU/mL), respectively. These values indicated insulin resistance as determined by the homeostasis model assessment (HOMA) ratio (fasting insulin x fasting glucose/22.5). Values >3.2 indicate insulin resistance, and Ms. K had a HOMA ratio of 3.9. The endocrinologist recommended:
- metformin, starting at 850 mg/d and gradually increased to 2,550 mg/d
- spironolactone, 100 mg/d.
Metformin, a biguanide approved for treating for type 2 diabetes, inhibits hepatic glucose production and increases peripheral insulin sensitivity, but it does not modify pancreatic insulin secretion. It may decrease insulin resistance by reducing gut absorption of glucose, improving glucose uptake by tissues, and/or increasing the number of insulin receptors.18 In treating PCOS, metformin can:
- restore ovulation19
- decrease insulin resistance, acne, hirsutism, total and bioavailable testosterone, BMI, and waist-hip ratio.20,21
Although the link between insulin resistance and depression is unclear, insulin is known to contribute to 5-HT synthesis by promoting tryptophan influx into the brain.22 Therefore, drugs used to treat insulin resistance—such as metformin and alpha lipoic acid23—might be useful in treating depression.
Spironolactone, a mineralocorticoid receptor (MR) antagonist, reduces hirsutism in women with PCOS.24 It also can decrease insulin resistance and fasting insulin levels in PCOS patients and reduce serum testosterone.25
Evidence on treating mood disorders with hormonal agents such as spironolactone is scarce, although treatment-resistant depression has been reported to resolve with antiglucocorticoid use (Box 2).25-31 Modulating HPA axis activity to treat affective disorders has been investigated.
CASE: GOING ANTIDEPRESSANT-FREE
At first, Ms. K said she wanted to continue taking venlafaxine with the PCOS treatment. After 2 weeks of combined therapy, however, she chose to stop the antidepressant after her depressive symptoms persisted, and her HAM-D-21 score remained at 28.
During the next 4 weeks, as we tapered off the venlafaxine, Ms. K’s HAM-D-21 score dropped to 7, indicating depressive symptom resolution. Despite slow venlafaxine titration, withdrawal symptoms of excessive crying, not feeling “present,” and tingling sensations occurred. Three days of fluoxetine, 20 mg/d, alleviated these symptoms.
Table 2
Insulin-sensitizing medications used to treat PCOS
| Medication | Normal dosage | Common side effects |
| Metformin | 500 mg tid | Headache, GI effects (nausea, diarrhea, flatulence) at start of therapy, weight loss, taste disturbances |
| Pioglitazone | 15 to 45 mg once daily | Swelling, headache, respiratory infection, abdominal discomfort, muscle soreness |
| Rosiglitazone | 4 to 8 mg once daily | Headache, mild weight gain |
We continued Ms. K’s treatment without antidepressants, and her mood continued to improve with metformin, 2,550 mg/d, and spironolactone, 100 mg/d.
TREATMENT RECOMMENDATIONS
PCOS therapy may take up to 6 months to resolve symptoms such as anovulation or hirsutism, but affective symptoms may improve during the first 6 weeks, as this case shows. Choosing medications to treat depression in patients such as Ms. K depends on whether their PCOS is being treated when they present for psychiatric evaluation.
Patient not being treated for PCOS. Refer her to an endocrinologist for PCOS treatment with an insulin-sensitizing medication, such as metformin (Table 2). Treating the insulin resistance associated with PCOS may also resolve the depression. PCOS drug therapy may also include antiandrogens such as spironolactone to treat hirsutism, male-pattern baldness, and acne. We suggest that spironolactone’s antiandrogen effects may help reduce depressive symptoms.
If your patient is not taking an antidepressant at presentation, try PCOS treatment first. If depressive symptoms persist after 3 months, consider adding an antidepressant. If your patient is taking an antidepressant at presentation but continues to be depressed, offer two options:
- taper off the antidepressant while starting PCOS treatment
- continue the antidepressant while starting PCOS treatment.
The first option allows you to try PCOS treatment alone. If the depression is caused by a common underlying pathophysiology—such as insulin resistance—treating PCOS alone may alleviate her depressive symptoms. Monitor for withdrawal symptoms, which may be minimized with a short-term SSRI, such as fluoxetine.
The second option may help patients who have responded to antidepressants in the past. Adjunctive PCOS treatment may “jump-start” the antidepressant response without withdrawal symptoms, but you will not be sure whether the antidepressant response was caused by PCOS therapy alone or the combination therapy.
Patient being treated for PCOS. Treat her with antidepressants, and consult with her endocrinologist to consider more-aggressive insulin-sensitizing medications, especially if she exhibits high levels of insulin resistance.
Other interventions that increase insulin sensitivity and improve glycemic control—such as improving dietary management and sleep habits, reducing alcohol consumption, and increasing physical activity—might have an antidepressant effect. Therefore, recommend these health practices to patients as adjuncts to drug therapies.
CASE: DEPRESSION IN REMISSION
At the 3-month follow-up visit, Ms. K scored zero on the HAM-D-21 scale. With metformin treatment, she had lost approximately 10 lbs and resumed menstruating approximately every 33 days. She reported experiencing low mood, decreased energy, and irritability during the week before her periods, but these symptoms resolved with menses onset.
Serum glucose was within normal range at 89 mg/dL, and serum insulin was 15.0 uIU/Ml. Her HOMA ratio had dropped to 2.8 (below the 3.2 cut-off for insulin resistance).
Ms. K’s endocrinologist monitored her spironolactone and metformin therapy for approximately 1 year, when she became pregnant.
Discussion. PCOS treatment duration depends on the patient’s response and her goals for therapy. Whether or not she continues PCOS treatment, her primary care physician or endocrinologist should continue to monitor her for insulin resistance’s metabolic consequences, including increased risk of type 2 diabetes and cardiovascular disease.
Related resources
- Legro RS, Winans EA. Overview of polycystic ovary syndrome and its relation to psychiatric illness and treatment. Current Psychiatry 2004;3(Dec[suppl]):12-20.
- The Rotterdam ESHRE/ASRM-sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004 Jan; 81(1):19-25.
- Polycystic ovary syndrome (patient information). The National Women’s Health Information Center. U.S. Department of Health and Human Services, Office on Women’s Health. Available at: http://www.4woman.gov/faq/pcos.htm. Accessed Jan. 13, 2005.
Drug brand names
- Citalopram • Celexa
- Fluoxetine • Prozac
- Ketoconazole • Nizoral
- Metformin • Glucophage
- Metyrapone • Metopirone
- Mifepristone • Mifiprex
- Pioglitazone • Actos
- Rosiglitazone • Avandia
- Spironolactone • Aldactone
- Venlafaxine • Effexor XR
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Rasgon NL, Carter MS, Elman S, et al. Common treatment of polycystic ovarian syndrome and major depressive disorder: case report and review. Curr Drug Targets Immune Endocr Metabol Disord 2002;2(1):97-102.
2. Franks S. Polycystic ovary syndrome. N Engl J Med 1995;333(13):853-61.
3. Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR (eds). Polycystic ovary syndrome. Oxford, UK: Blackwell Scientific, 1992;377-84.
4. Rasgon NL, Rao R, Elman S, et al. Depression in women with polycystic ovary syndrome: clinical and biochemical correlates. J Affect Disord 2003;74(3):299-304.
5. Weiner CL, Primeau M, Ehrmann DA. Androgens and mood dysfunction in women: Comparison of women with polycystic ovarian syndrome to healthy controls. Psychosom Med 2004;66:356-62.
6. Fava GA, Grandi S, Savron G, et al. Psychosomatic assessment of hirsute women. Psychother Psychosom 1989;51:96-100.
7. Rasgon NL, Altshuler LL, Gudeman D, et al. Medication status and PCO syndrome in women with bipolar disorder: a preliminary report. J Clin Psychiatry 2000;61:173-8.
8. Okamura F, Tashiro A, Utsumi A, et al. Insulin resistance in patients with depression and its changes in the clinical course of depression: a report on three cases using the minimal model analysis. Internal Med 1999;38(3):257-60.
9. Okamura F, Tashiro A, Utsumi A, et al. Insulin resistance in patients with depression and its changes during the clinical course of depression: minimal model analysis. Metabolism 2000;49(10):1255-60.
10. Rasgon NL, Altshuler LL, Birtan JA, et al. Menstrual abnormalities in women with bipolar disorder (presentation). San Francisco: American Psychiatric Association annual meeting, May 2003.
11. Figlewicz DP. Endocrine regulation of neurotransmitter transporters. Epilepsy Res 1999;37(3):203-10.
12. Nathan RS, Sachar EJ, Asnis GM, et al. Relative insulin insensitivity and cortisol secretion in depressed patients. Psychiatry Res 1981;4:291.-
13. Elmslie JL, Silverstone TJ, Mann JI, Williams SM. Determinants of overweight and obesity in patients with bipolar disorder. J Clin Psychiatry 2000;61:179.-
14. Istvan J, Zavela K, Weidner G. Body weight and psychological distress in NHANES I. Int J Obes Relat Metab Disord 1992;16(12):999-1003.
15. Andrews RC, Walker BR. Glucocorticoids and insulin resistance: old hormones, new targets. Clin Sci 1999;96(5):513.-
16. Pasquali R, Biscotti D, Spinucci G, et al. Pulsatile secretion of ACTH and cortisol in premenopausal women: effect of obesity and body fat distribution. Clin Endocrinol 1998;48(5):603.-
17. Moghetti P, Castello R, Negri C, et al. Insulin infusion amplifies 17 alpha-hydroxycorticosteroid intermediates response to adrenocorticotropin in hyperandrogenic women: apparent relative impairment of 17,20-lyase activity. J Clin Endocrinol Metabol 1996;81(3):881.-
18. Bailey CJ, Day C, Bray GA, et al. Role of adrenal glands in the development of abnormal glucose and insulin homeostasis in genetically obese (ob/ob) mice. Horm Metab Res 1986;18(6):357.-
19. Sills SE, Perloe M, Palermo GD. Correction of hyperinsulinemia in oligoovulatory women with clomiphene-resistant polycystic ovary syndrome: a review of therapeutic rationale and reproductive outcomes. Eur J Obstet Gynecol Reprod Biol 2000;91(2):135.-
20. Nestler JE, Jakubowicz DJ, Evans WS, Pasquali R. Effects of metformin on spontaneous and clomiphene-induced ovulation in the polycystic ovary syndrome. N Engl J Med 1998;338(26):1876.-
21. Velasquez EM, Mendoza S, Hamer T, et al. Metformin therapy in polycystic ovary syndrome reduces hyperinsulinemia, insulin resistance, hyperandrogenemia, and systolic blood pressure, while facilitating normal menses and pregnancy. Metabolism 1994;43(5):647.-
22. Chaouloff F. Effects of acute physical exercise on central serotonergic systems. Med Sci Sports Exerc 1997;29(1):58.-
23. Salazar M. Alpha lipoic acid: a novel treatment for depression. Medical Hypoth 2000;55:510.-
24. Spritzer PM, Lisboa KO, Mattiello S, Lhullier F. Spironolactone as a single agent for long-term therapy of hirsute patients. Clin Endocrinol 2000;52:587.-
25. Moghetti P, Tosi F, Castello R, et al. The insulin resistance in women with hyperandrogenism is partially reversed by antiandrogen treatment: evidence that androgens impair insulin action in women. J Clin Endocrinol Metabol 1996;81(3):952.-
26. Zobel AW, Nickel T, Kunzel HE. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatric Res 2000;34:171.-
27. Healy DG, Harkin A, Cryan JF, et al. Metyrapone displays antidepressant-like properties in preclinical paradigms. Psychopharmacol 1999;145(3):303.-
28. Bech P, Raabaek Olsen L, Jarløv N, et al. A case of sequential anti-stress medication in a patient with major depression resistant to amine-reuptake inhibitors. Acta Psychiatr Scand 1999;100:76.-
29. Wolkowitz OM, Reus VI, Chan T, et al. Antiglucocorticoid treatment of depression: double-blind ketoconazole. J Biol Psychiatry 1999;45(8):1070.-
30. Murphy BE, Filipini D, Ghadirian AM. Possible use of glucocorticoid receptor antagonists in the treatment of major depression: preliminary results using RU 486. J Psychiatry Neurosci 1993;18(5):209.-
31. Schatzberg AF, Rothschild AJ, Langlais PJ, et al. A corticosteroid/dopamine hypothesis for psychotic depression and related states. J Psychiatr Res 1985;19(1):57.-
Women with depression and polycystic ovary syndrome (PCOS) can be trapped in a vicious cycle of hormonal dysregulation. Treating these patients appropriately—with or without antidepressants—requires an understanding of their underlying metabolic disorder.
This article presents a case1 that exemplifies the association between depression and PCOS (Box 1).2-4 Based on our research and clinical experience, we offer recommendations to help you manage depression in patients with PCOS.
CHRONIC DEPRESSION WITH AMENORRHEA
Ms. K, age 30, presented for psychiatric evaluation of treatment-resistant recurrent major depression. Her symptoms included sad mood, sleep disturbance, decreased energy, anhedonia, poor concentration, and feelings of guilt and worthlessness. Her score of 28 on the Hamilton Rating Scale for Depression (HAM-D-21) indicated severe depression.
Polycystic ovary syndrome (PCOS) is the most common cause of menstrual disturbance in women of reproductive age, affecting approximately 5% of U.S. women.1 Clinically, it is the association of hyperandrogenism with chronic anovulation, without specific underlying adrenal or pituitary gland disease.2
PCOS has been shown to be associated with depression, though little research has been done in this area. We conducted a pilot study (the first, to our knowledge) to examine the rate of depression among women with documented PCOS and to correlate Center for Epidemiological Studies Depression Scale (CES-D) scores indicative of depression with clinical and biochemical markers of PCOS. We found an increased prevalence of depression in women with PCOS and an association between depression, insulin resistance, and body mass index. Specifically, among 32 women with documented PCOS,3 16 (50%) had CES-D scores indicating depression.
This was Ms. K’s third episode of major depression, with the first two occurring at ages 24 and 26. She reported similar symptoms each time. Previous psychiatrists had tried a variety of antidepressants with no therapeutic benefit before she responded to citalopram, 40 mg/d, at age 27. With this selective serotonin reuptake inhibitor, her depressive symptoms resolved for >1 year, except for mild irritability and low mood the week before menses.
Her depressive symptoms returned, however, and her previous psychiatrist treated her for approximately 13 months with extended-release venlafaxine, 225 mg/d. When she remained depressed, she was referred to our clinic for evaluation. Based on her history, we could not determine whether she had become euthymic at age 27 or her symptoms had improved but still met criteria for a major depressive episode.
Ms. K reported having PCOS symptoms from age 19. These included amenorrhea since menarche, hirsutism, hair loss, alopecia, central fat distribution characteristic of PCOS, and male-pattern hair growth on her abdomen and thighs. She was not obese—with a body mass index (BMI) of approximately 25—but had gained 10 to 15 lbs while taking venlafaxine.
Ms. K had never been treated for PCOS but reported that her desire to become pregnant made her more concerned about her symptoms and possible infertility.
Diagnosis. PCOS is usually diagnosed using endocrinologic, clinical, and ultrasonographic criteria (Table 1). Obesity is not a presenting symptom in all women with PCOS. As with Ms. K, about 50% of patients have normal BMI.
Causes of depression in PCOS. Depressed mood in PCOS may be both physiologic and psychological:
- Hypothalamic, pituitary, and other end-organ system dysregulation occurs in both PCOS and affective disorders, which share clinical and biochemical markers including insulin resistance, obesity, and hyperandrogenism.
- PCOS’ clinical sequelae—hirsutism, acne, obesity, hormonal disturbances, fear of infertility, and psychological distress—may damage their self-esteem and female identity.
PCOS’ physical symptoms alone apparently do not account for patients’ worsened mood states. Weiner et al5 found that women with PCOS and free testosterone (FT) of 10 to 26 pg/mL (just above normal range) were more depressed than women without PCOS (FT <10 pg/mL) and women with PCOS and FT >26 pg/mL. Women with PCOS with the lower and higher FT levels had similar demographic profiles, but those with the highest FT levels were not the most depressed.
Similarly, in a study of 32 women with PCOS, we found no association between depression and other possibly distressing PCOS symptoms, including hirsutism, irregular menses, acne, or alopecia.4
Testosterone and mood disorders. Women such as Ms. K with hyperandrogenic syndromes are at increased risk for mood disorders.6 Many metabolic changes associated with PCOS—insulin resistance, obesity, and hyperandrogenism—are also described in patients with affective disorders. Our investigation of reproductive status in women with bipolar disorder found no clinical or biochemical evidence of PCOS with mood-stabilizer treatment. We did find that menstrual disturbances were common, however, and sometimes preceded bipolar disorder onset.7
Insulin resistance and depression. Others have linked insulin resistance and depression;8 depression has been shown to be associated with impaired insulin sensitivity and hyperinsulinemia.9 Depressed persons also tend to eat more sweets, drink more alcohol, exercise less, and sleep fewer hours than the nondepressed—all of which contribute to insulin sensitivity and insulin resistance.10
WHAT LINKS ENDOCRINE, MOOD DISORDERS?
Insulin and serotonin. Insulin affects central serotonin (5-HT) levels.11 Thus, central 5-HT system dysregulation that causes depression might also affect peripheral insulin sensitivity—or vice versa.9
Tryptophan, a serotonin precursor, competes with other large neutral amino acids for access to the transport system that moves them across the blood-brain barrier. Insulin stimulates uptake of the competing amino acids—but not tryptophan—into muscle tissue. The resulting increased tryptophan ratio in plasma affords it greater access to the transport system to contribute toward serotonin synthesis. Insulin also promotes central catecholaminergic activity, perhaps by suppressing norepinephrine reuptake and prolonging its residence in the synaptic cleft.10
Table 1
Diagnostic signs of PCOS
| Endocrine |
| Chronically elevated plasma free and total testosterone levels |
| Increased luteinizing hormone (LH) secretion because of enhanced pituitary sensitivity to GnRH stimulation |
| Low or normal plasma follicle-stimulating hormone (FSH) levels |
| Hyperinsulinemia and peripheral insulin resistance |
| Metabolic abnormalities of hyperinsulinemia and peripheral insulin resistance, including obesity and increased susceptibility to type 2 diabetes and cardiovascular disease |
| Clinical |
| Hirsutism |
| Acne |
| Infertility |
Anovulation or menstrual abnormalities
|
| Morphologic |
| Ovaries may appear to have multiple cysts 8 to 10 mm in diameter |
Several studies have found that insulin resistance and hyperinsulinemia can resolve after depression recovery.9,12 Because insulin resistance is a cardinal feature in PCOS pathophysiology,2 insulin resistance may be a common link between depression and PCOS.
Weight gain and obesity also have been described in patients with affective disorders.13 Not known is whether the weight gain precedes the psychopathology or is caused by long-term exposure to drugs used to treat affective disorders.
CRH antagonists. Corticotropin-releasing hormone (CRH) receptor antagonists have been suggested as possible antidepressants.26 Depression and anxiety scores have declined during treatment with the cortisol synthesis inhibitors metyrapone27 and ketoconazole.28,29 These trials do not reveal whether these agents treat depression symptoms—rather than the underlying pathophysiology—or if the affective disorder will recur after long-term administration.26
GR antagonists. Glucocorticoid receptor (GR) antagonists such as mifepristone (RU-486) have been suggested as antidepressants in depressed patients with elevated basal cortisol levels.30 Mifepristone may be useful for treating psychotic depression, in which the HPA axis is particularly hyperactive.31 Mifepristone is contraindicated in most women with PCOS, however, as its progesterone antagonism would lead to infertility—already a common problem for women with PCOS.
MR antagonists. Spironolactone, a mineralocorticoid receptor (MR) antagonist, decreases insulin resistance and fasting insulin levels in PCOS patients.25 We propose that insulin resistance may provide a common link in the pathophysiology of PCOS and depression. Therefore, treatment with insulin resistance-lowering medications such as spironolactone may induce antidepressant effects in women with depression and PCOS.
We reported a correlation between depression and BMI in women with PCOS.4 Depression might be independently associated with BMI, as weight gain and obesity are distressing symptoms associated with depression.14 However, we found no association between depression and other possibly distressing PCOS symptoms. Thus, the correlation between BMI and depression might more likely reflect the relationship between depression and insulin resistance, as degree of insulin resistance is known to correlate with BMI.
Elevated cortisol. Clearly, other factors—such as hypothalamus-pituitary-adrenal (HPA) axis dysfunction—are known to link affective and endocrine disorders. Hypercortisolemia can lead to both insulin resistance and obesity. Cortisol is one of the glucocorticoids the body secretes in response to stress to mobilize energy by increasing blood glucose levels. Early life stress and chronic emotional stress:
- can impair the negative feedback system that limits cortisol production during stress
- are associated with depression.
Approximately one-half of individuals with depression have elevated serum cortisol.10 Epidemiologic data show a positive correlation between cortisol levels and insulin resistance,15 and an association between HPA dysfunction and obesity has been described.16 Insulin can trigger androgen production by enhancing adrenal sensitivity to adrenocorticotrophic hormone (ACTH).17
CASE: IMPROVING INSULIN RESISTANCE
We referred Ms. K to an endocrinologist for PCOS evaluation and treatment. Her serum glucose and insulin levels were 83 mg/dL (normal range 70 to 125 mg/dL) and 19.0 uIU/mL (normal range <10 uIU/mL), respectively. These values indicated insulin resistance as determined by the homeostasis model assessment (HOMA) ratio (fasting insulin x fasting glucose/22.5). Values >3.2 indicate insulin resistance, and Ms. K had a HOMA ratio of 3.9. The endocrinologist recommended:
- metformin, starting at 850 mg/d and gradually increased to 2,550 mg/d
- spironolactone, 100 mg/d.
Metformin, a biguanide approved for treating for type 2 diabetes, inhibits hepatic glucose production and increases peripheral insulin sensitivity, but it does not modify pancreatic insulin secretion. It may decrease insulin resistance by reducing gut absorption of glucose, improving glucose uptake by tissues, and/or increasing the number of insulin receptors.18 In treating PCOS, metformin can:
- restore ovulation19
- decrease insulin resistance, acne, hirsutism, total and bioavailable testosterone, BMI, and waist-hip ratio.20,21
Although the link between insulin resistance and depression is unclear, insulin is known to contribute to 5-HT synthesis by promoting tryptophan influx into the brain.22 Therefore, drugs used to treat insulin resistance—such as metformin and alpha lipoic acid23—might be useful in treating depression.
Spironolactone, a mineralocorticoid receptor (MR) antagonist, reduces hirsutism in women with PCOS.24 It also can decrease insulin resistance and fasting insulin levels in PCOS patients and reduce serum testosterone.25
Evidence on treating mood disorders with hormonal agents such as spironolactone is scarce, although treatment-resistant depression has been reported to resolve with antiglucocorticoid use (Box 2).25-31 Modulating HPA axis activity to treat affective disorders has been investigated.
CASE: GOING ANTIDEPRESSANT-FREE
At first, Ms. K said she wanted to continue taking venlafaxine with the PCOS treatment. After 2 weeks of combined therapy, however, she chose to stop the antidepressant after her depressive symptoms persisted, and her HAM-D-21 score remained at 28.
During the next 4 weeks, as we tapered off the venlafaxine, Ms. K’s HAM-D-21 score dropped to 7, indicating depressive symptom resolution. Despite slow venlafaxine titration, withdrawal symptoms of excessive crying, not feeling “present,” and tingling sensations occurred. Three days of fluoxetine, 20 mg/d, alleviated these symptoms.
Table 2
Insulin-sensitizing medications used to treat PCOS
| Medication | Normal dosage | Common side effects |
| Metformin | 500 mg tid | Headache, GI effects (nausea, diarrhea, flatulence) at start of therapy, weight loss, taste disturbances |
| Pioglitazone | 15 to 45 mg once daily | Swelling, headache, respiratory infection, abdominal discomfort, muscle soreness |
| Rosiglitazone | 4 to 8 mg once daily | Headache, mild weight gain |
We continued Ms. K’s treatment without antidepressants, and her mood continued to improve with metformin, 2,550 mg/d, and spironolactone, 100 mg/d.
TREATMENT RECOMMENDATIONS
PCOS therapy may take up to 6 months to resolve symptoms such as anovulation or hirsutism, but affective symptoms may improve during the first 6 weeks, as this case shows. Choosing medications to treat depression in patients such as Ms. K depends on whether their PCOS is being treated when they present for psychiatric evaluation.
Patient not being treated for PCOS. Refer her to an endocrinologist for PCOS treatment with an insulin-sensitizing medication, such as metformin (Table 2). Treating the insulin resistance associated with PCOS may also resolve the depression. PCOS drug therapy may also include antiandrogens such as spironolactone to treat hirsutism, male-pattern baldness, and acne. We suggest that spironolactone’s antiandrogen effects may help reduce depressive symptoms.
If your patient is not taking an antidepressant at presentation, try PCOS treatment first. If depressive symptoms persist after 3 months, consider adding an antidepressant. If your patient is taking an antidepressant at presentation but continues to be depressed, offer two options:
- taper off the antidepressant while starting PCOS treatment
- continue the antidepressant while starting PCOS treatment.
The first option allows you to try PCOS treatment alone. If the depression is caused by a common underlying pathophysiology—such as insulin resistance—treating PCOS alone may alleviate her depressive symptoms. Monitor for withdrawal symptoms, which may be minimized with a short-term SSRI, such as fluoxetine.
The second option may help patients who have responded to antidepressants in the past. Adjunctive PCOS treatment may “jump-start” the antidepressant response without withdrawal symptoms, but you will not be sure whether the antidepressant response was caused by PCOS therapy alone or the combination therapy.
Patient being treated for PCOS. Treat her with antidepressants, and consult with her endocrinologist to consider more-aggressive insulin-sensitizing medications, especially if she exhibits high levels of insulin resistance.
Other interventions that increase insulin sensitivity and improve glycemic control—such as improving dietary management and sleep habits, reducing alcohol consumption, and increasing physical activity—might have an antidepressant effect. Therefore, recommend these health practices to patients as adjuncts to drug therapies.
CASE: DEPRESSION IN REMISSION
At the 3-month follow-up visit, Ms. K scored zero on the HAM-D-21 scale. With metformin treatment, she had lost approximately 10 lbs and resumed menstruating approximately every 33 days. She reported experiencing low mood, decreased energy, and irritability during the week before her periods, but these symptoms resolved with menses onset.
Serum glucose was within normal range at 89 mg/dL, and serum insulin was 15.0 uIU/Ml. Her HOMA ratio had dropped to 2.8 (below the 3.2 cut-off for insulin resistance).
Ms. K’s endocrinologist monitored her spironolactone and metformin therapy for approximately 1 year, when she became pregnant.
Discussion. PCOS treatment duration depends on the patient’s response and her goals for therapy. Whether or not she continues PCOS treatment, her primary care physician or endocrinologist should continue to monitor her for insulin resistance’s metabolic consequences, including increased risk of type 2 diabetes and cardiovascular disease.
Related resources
- Legro RS, Winans EA. Overview of polycystic ovary syndrome and its relation to psychiatric illness and treatment. Current Psychiatry 2004;3(Dec[suppl]):12-20.
- The Rotterdam ESHRE/ASRM-sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004 Jan; 81(1):19-25.
- Polycystic ovary syndrome (patient information). The National Women’s Health Information Center. U.S. Department of Health and Human Services, Office on Women’s Health. Available at: http://www.4woman.gov/faq/pcos.htm. Accessed Jan. 13, 2005.
Drug brand names
- Citalopram • Celexa
- Fluoxetine • Prozac
- Ketoconazole • Nizoral
- Metformin • Glucophage
- Metyrapone • Metopirone
- Mifepristone • Mifiprex
- Pioglitazone • Actos
- Rosiglitazone • Avandia
- Spironolactone • Aldactone
- Venlafaxine • Effexor XR
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Women with depression and polycystic ovary syndrome (PCOS) can be trapped in a vicious cycle of hormonal dysregulation. Treating these patients appropriately—with or without antidepressants—requires an understanding of their underlying metabolic disorder.
This article presents a case1 that exemplifies the association between depression and PCOS (Box 1).2-4 Based on our research and clinical experience, we offer recommendations to help you manage depression in patients with PCOS.
CHRONIC DEPRESSION WITH AMENORRHEA
Ms. K, age 30, presented for psychiatric evaluation of treatment-resistant recurrent major depression. Her symptoms included sad mood, sleep disturbance, decreased energy, anhedonia, poor concentration, and feelings of guilt and worthlessness. Her score of 28 on the Hamilton Rating Scale for Depression (HAM-D-21) indicated severe depression.
Polycystic ovary syndrome (PCOS) is the most common cause of menstrual disturbance in women of reproductive age, affecting approximately 5% of U.S. women.1 Clinically, it is the association of hyperandrogenism with chronic anovulation, without specific underlying adrenal or pituitary gland disease.2
PCOS has been shown to be associated with depression, though little research has been done in this area. We conducted a pilot study (the first, to our knowledge) to examine the rate of depression among women with documented PCOS and to correlate Center for Epidemiological Studies Depression Scale (CES-D) scores indicative of depression with clinical and biochemical markers of PCOS. We found an increased prevalence of depression in women with PCOS and an association between depression, insulin resistance, and body mass index. Specifically, among 32 women with documented PCOS,3 16 (50%) had CES-D scores indicating depression.
This was Ms. K’s third episode of major depression, with the first two occurring at ages 24 and 26. She reported similar symptoms each time. Previous psychiatrists had tried a variety of antidepressants with no therapeutic benefit before she responded to citalopram, 40 mg/d, at age 27. With this selective serotonin reuptake inhibitor, her depressive symptoms resolved for >1 year, except for mild irritability and low mood the week before menses.
Her depressive symptoms returned, however, and her previous psychiatrist treated her for approximately 13 months with extended-release venlafaxine, 225 mg/d. When she remained depressed, she was referred to our clinic for evaluation. Based on her history, we could not determine whether she had become euthymic at age 27 or her symptoms had improved but still met criteria for a major depressive episode.
Ms. K reported having PCOS symptoms from age 19. These included amenorrhea since menarche, hirsutism, hair loss, alopecia, central fat distribution characteristic of PCOS, and male-pattern hair growth on her abdomen and thighs. She was not obese—with a body mass index (BMI) of approximately 25—but had gained 10 to 15 lbs while taking venlafaxine.
Ms. K had never been treated for PCOS but reported that her desire to become pregnant made her more concerned about her symptoms and possible infertility.
Diagnosis. PCOS is usually diagnosed using endocrinologic, clinical, and ultrasonographic criteria (Table 1). Obesity is not a presenting symptom in all women with PCOS. As with Ms. K, about 50% of patients have normal BMI.
Causes of depression in PCOS. Depressed mood in PCOS may be both physiologic and psychological:
- Hypothalamic, pituitary, and other end-organ system dysregulation occurs in both PCOS and affective disorders, which share clinical and biochemical markers including insulin resistance, obesity, and hyperandrogenism.
- PCOS’ clinical sequelae—hirsutism, acne, obesity, hormonal disturbances, fear of infertility, and psychological distress—may damage their self-esteem and female identity.
PCOS’ physical symptoms alone apparently do not account for patients’ worsened mood states. Weiner et al5 found that women with PCOS and free testosterone (FT) of 10 to 26 pg/mL (just above normal range) were more depressed than women without PCOS (FT <10 pg/mL) and women with PCOS and FT >26 pg/mL. Women with PCOS with the lower and higher FT levels had similar demographic profiles, but those with the highest FT levels were not the most depressed.
Similarly, in a study of 32 women with PCOS, we found no association between depression and other possibly distressing PCOS symptoms, including hirsutism, irregular menses, acne, or alopecia.4
Testosterone and mood disorders. Women such as Ms. K with hyperandrogenic syndromes are at increased risk for mood disorders.6 Many metabolic changes associated with PCOS—insulin resistance, obesity, and hyperandrogenism—are also described in patients with affective disorders. Our investigation of reproductive status in women with bipolar disorder found no clinical or biochemical evidence of PCOS with mood-stabilizer treatment. We did find that menstrual disturbances were common, however, and sometimes preceded bipolar disorder onset.7
Insulin resistance and depression. Others have linked insulin resistance and depression;8 depression has been shown to be associated with impaired insulin sensitivity and hyperinsulinemia.9 Depressed persons also tend to eat more sweets, drink more alcohol, exercise less, and sleep fewer hours than the nondepressed—all of which contribute to insulin sensitivity and insulin resistance.10
WHAT LINKS ENDOCRINE, MOOD DISORDERS?
Insulin and serotonin. Insulin affects central serotonin (5-HT) levels.11 Thus, central 5-HT system dysregulation that causes depression might also affect peripheral insulin sensitivity—or vice versa.9
Tryptophan, a serotonin precursor, competes with other large neutral amino acids for access to the transport system that moves them across the blood-brain barrier. Insulin stimulates uptake of the competing amino acids—but not tryptophan—into muscle tissue. The resulting increased tryptophan ratio in plasma affords it greater access to the transport system to contribute toward serotonin synthesis. Insulin also promotes central catecholaminergic activity, perhaps by suppressing norepinephrine reuptake and prolonging its residence in the synaptic cleft.10
Table 1
Diagnostic signs of PCOS
| Endocrine |
| Chronically elevated plasma free and total testosterone levels |
| Increased luteinizing hormone (LH) secretion because of enhanced pituitary sensitivity to GnRH stimulation |
| Low or normal plasma follicle-stimulating hormone (FSH) levels |
| Hyperinsulinemia and peripheral insulin resistance |
| Metabolic abnormalities of hyperinsulinemia and peripheral insulin resistance, including obesity and increased susceptibility to type 2 diabetes and cardiovascular disease |
| Clinical |
| Hirsutism |
| Acne |
| Infertility |
Anovulation or menstrual abnormalities
|
| Morphologic |
| Ovaries may appear to have multiple cysts 8 to 10 mm in diameter |
Several studies have found that insulin resistance and hyperinsulinemia can resolve after depression recovery.9,12 Because insulin resistance is a cardinal feature in PCOS pathophysiology,2 insulin resistance may be a common link between depression and PCOS.
Weight gain and obesity also have been described in patients with affective disorders.13 Not known is whether the weight gain precedes the psychopathology or is caused by long-term exposure to drugs used to treat affective disorders.
CRH antagonists. Corticotropin-releasing hormone (CRH) receptor antagonists have been suggested as possible antidepressants.26 Depression and anxiety scores have declined during treatment with the cortisol synthesis inhibitors metyrapone27 and ketoconazole.28,29 These trials do not reveal whether these agents treat depression symptoms—rather than the underlying pathophysiology—or if the affective disorder will recur after long-term administration.26
GR antagonists. Glucocorticoid receptor (GR) antagonists such as mifepristone (RU-486) have been suggested as antidepressants in depressed patients with elevated basal cortisol levels.30 Mifepristone may be useful for treating psychotic depression, in which the HPA axis is particularly hyperactive.31 Mifepristone is contraindicated in most women with PCOS, however, as its progesterone antagonism would lead to infertility—already a common problem for women with PCOS.
MR antagonists. Spironolactone, a mineralocorticoid receptor (MR) antagonist, decreases insulin resistance and fasting insulin levels in PCOS patients.25 We propose that insulin resistance may provide a common link in the pathophysiology of PCOS and depression. Therefore, treatment with insulin resistance-lowering medications such as spironolactone may induce antidepressant effects in women with depression and PCOS.
We reported a correlation between depression and BMI in women with PCOS.4 Depression might be independently associated with BMI, as weight gain and obesity are distressing symptoms associated with depression.14 However, we found no association between depression and other possibly distressing PCOS symptoms. Thus, the correlation between BMI and depression might more likely reflect the relationship between depression and insulin resistance, as degree of insulin resistance is known to correlate with BMI.
Elevated cortisol. Clearly, other factors—such as hypothalamus-pituitary-adrenal (HPA) axis dysfunction—are known to link affective and endocrine disorders. Hypercortisolemia can lead to both insulin resistance and obesity. Cortisol is one of the glucocorticoids the body secretes in response to stress to mobilize energy by increasing blood glucose levels. Early life stress and chronic emotional stress:
- can impair the negative feedback system that limits cortisol production during stress
- are associated with depression.
Approximately one-half of individuals with depression have elevated serum cortisol.10 Epidemiologic data show a positive correlation between cortisol levels and insulin resistance,15 and an association between HPA dysfunction and obesity has been described.16 Insulin can trigger androgen production by enhancing adrenal sensitivity to adrenocorticotrophic hormone (ACTH).17
CASE: IMPROVING INSULIN RESISTANCE
We referred Ms. K to an endocrinologist for PCOS evaluation and treatment. Her serum glucose and insulin levels were 83 mg/dL (normal range 70 to 125 mg/dL) and 19.0 uIU/mL (normal range <10 uIU/mL), respectively. These values indicated insulin resistance as determined by the homeostasis model assessment (HOMA) ratio (fasting insulin x fasting glucose/22.5). Values >3.2 indicate insulin resistance, and Ms. K had a HOMA ratio of 3.9. The endocrinologist recommended:
- metformin, starting at 850 mg/d and gradually increased to 2,550 mg/d
- spironolactone, 100 mg/d.
Metformin, a biguanide approved for treating for type 2 diabetes, inhibits hepatic glucose production and increases peripheral insulin sensitivity, but it does not modify pancreatic insulin secretion. It may decrease insulin resistance by reducing gut absorption of glucose, improving glucose uptake by tissues, and/or increasing the number of insulin receptors.18 In treating PCOS, metformin can:
- restore ovulation19
- decrease insulin resistance, acne, hirsutism, total and bioavailable testosterone, BMI, and waist-hip ratio.20,21
Although the link between insulin resistance and depression is unclear, insulin is known to contribute to 5-HT synthesis by promoting tryptophan influx into the brain.22 Therefore, drugs used to treat insulin resistance—such as metformin and alpha lipoic acid23—might be useful in treating depression.
Spironolactone, a mineralocorticoid receptor (MR) antagonist, reduces hirsutism in women with PCOS.24 It also can decrease insulin resistance and fasting insulin levels in PCOS patients and reduce serum testosterone.25
Evidence on treating mood disorders with hormonal agents such as spironolactone is scarce, although treatment-resistant depression has been reported to resolve with antiglucocorticoid use (Box 2).25-31 Modulating HPA axis activity to treat affective disorders has been investigated.
CASE: GOING ANTIDEPRESSANT-FREE
At first, Ms. K said she wanted to continue taking venlafaxine with the PCOS treatment. After 2 weeks of combined therapy, however, she chose to stop the antidepressant after her depressive symptoms persisted, and her HAM-D-21 score remained at 28.
During the next 4 weeks, as we tapered off the venlafaxine, Ms. K’s HAM-D-21 score dropped to 7, indicating depressive symptom resolution. Despite slow venlafaxine titration, withdrawal symptoms of excessive crying, not feeling “present,” and tingling sensations occurred. Three days of fluoxetine, 20 mg/d, alleviated these symptoms.
Table 2
Insulin-sensitizing medications used to treat PCOS
| Medication | Normal dosage | Common side effects |
| Metformin | 500 mg tid | Headache, GI effects (nausea, diarrhea, flatulence) at start of therapy, weight loss, taste disturbances |
| Pioglitazone | 15 to 45 mg once daily | Swelling, headache, respiratory infection, abdominal discomfort, muscle soreness |
| Rosiglitazone | 4 to 8 mg once daily | Headache, mild weight gain |
We continued Ms. K’s treatment without antidepressants, and her mood continued to improve with metformin, 2,550 mg/d, and spironolactone, 100 mg/d.
TREATMENT RECOMMENDATIONS
PCOS therapy may take up to 6 months to resolve symptoms such as anovulation or hirsutism, but affective symptoms may improve during the first 6 weeks, as this case shows. Choosing medications to treat depression in patients such as Ms. K depends on whether their PCOS is being treated when they present for psychiatric evaluation.
Patient not being treated for PCOS. Refer her to an endocrinologist for PCOS treatment with an insulin-sensitizing medication, such as metformin (Table 2). Treating the insulin resistance associated with PCOS may also resolve the depression. PCOS drug therapy may also include antiandrogens such as spironolactone to treat hirsutism, male-pattern baldness, and acne. We suggest that spironolactone’s antiandrogen effects may help reduce depressive symptoms.
If your patient is not taking an antidepressant at presentation, try PCOS treatment first. If depressive symptoms persist after 3 months, consider adding an antidepressant. If your patient is taking an antidepressant at presentation but continues to be depressed, offer two options:
- taper off the antidepressant while starting PCOS treatment
- continue the antidepressant while starting PCOS treatment.
The first option allows you to try PCOS treatment alone. If the depression is caused by a common underlying pathophysiology—such as insulin resistance—treating PCOS alone may alleviate her depressive symptoms. Monitor for withdrawal symptoms, which may be minimized with a short-term SSRI, such as fluoxetine.
The second option may help patients who have responded to antidepressants in the past. Adjunctive PCOS treatment may “jump-start” the antidepressant response without withdrawal symptoms, but you will not be sure whether the antidepressant response was caused by PCOS therapy alone or the combination therapy.
Patient being treated for PCOS. Treat her with antidepressants, and consult with her endocrinologist to consider more-aggressive insulin-sensitizing medications, especially if she exhibits high levels of insulin resistance.
Other interventions that increase insulin sensitivity and improve glycemic control—such as improving dietary management and sleep habits, reducing alcohol consumption, and increasing physical activity—might have an antidepressant effect. Therefore, recommend these health practices to patients as adjuncts to drug therapies.
CASE: DEPRESSION IN REMISSION
At the 3-month follow-up visit, Ms. K scored zero on the HAM-D-21 scale. With metformin treatment, she had lost approximately 10 lbs and resumed menstruating approximately every 33 days. She reported experiencing low mood, decreased energy, and irritability during the week before her periods, but these symptoms resolved with menses onset.
Serum glucose was within normal range at 89 mg/dL, and serum insulin was 15.0 uIU/Ml. Her HOMA ratio had dropped to 2.8 (below the 3.2 cut-off for insulin resistance).
Ms. K’s endocrinologist monitored her spironolactone and metformin therapy for approximately 1 year, when she became pregnant.
Discussion. PCOS treatment duration depends on the patient’s response and her goals for therapy. Whether or not she continues PCOS treatment, her primary care physician or endocrinologist should continue to monitor her for insulin resistance’s metabolic consequences, including increased risk of type 2 diabetes and cardiovascular disease.
Related resources
- Legro RS, Winans EA. Overview of polycystic ovary syndrome and its relation to psychiatric illness and treatment. Current Psychiatry 2004;3(Dec[suppl]):12-20.
- The Rotterdam ESHRE/ASRM-sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 2004 Jan; 81(1):19-25.
- Polycystic ovary syndrome (patient information). The National Women’s Health Information Center. U.S. Department of Health and Human Services, Office on Women’s Health. Available at: http://www.4woman.gov/faq/pcos.htm. Accessed Jan. 13, 2005.
Drug brand names
- Citalopram • Celexa
- Fluoxetine • Prozac
- Ketoconazole • Nizoral
- Metformin • Glucophage
- Metyrapone • Metopirone
- Mifepristone • Mifiprex
- Pioglitazone • Actos
- Rosiglitazone • Avandia
- Spironolactone • Aldactone
- Venlafaxine • Effexor XR
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Rasgon NL, Carter MS, Elman S, et al. Common treatment of polycystic ovarian syndrome and major depressive disorder: case report and review. Curr Drug Targets Immune Endocr Metabol Disord 2002;2(1):97-102.
2. Franks S. Polycystic ovary syndrome. N Engl J Med 1995;333(13):853-61.
3. Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR (eds). Polycystic ovary syndrome. Oxford, UK: Blackwell Scientific, 1992;377-84.
4. Rasgon NL, Rao R, Elman S, et al. Depression in women with polycystic ovary syndrome: clinical and biochemical correlates. J Affect Disord 2003;74(3):299-304.
5. Weiner CL, Primeau M, Ehrmann DA. Androgens and mood dysfunction in women: Comparison of women with polycystic ovarian syndrome to healthy controls. Psychosom Med 2004;66:356-62.
6. Fava GA, Grandi S, Savron G, et al. Psychosomatic assessment of hirsute women. Psychother Psychosom 1989;51:96-100.
7. Rasgon NL, Altshuler LL, Gudeman D, et al. Medication status and PCO syndrome in women with bipolar disorder: a preliminary report. J Clin Psychiatry 2000;61:173-8.
8. Okamura F, Tashiro A, Utsumi A, et al. Insulin resistance in patients with depression and its changes in the clinical course of depression: a report on three cases using the minimal model analysis. Internal Med 1999;38(3):257-60.
9. Okamura F, Tashiro A, Utsumi A, et al. Insulin resistance in patients with depression and its changes during the clinical course of depression: minimal model analysis. Metabolism 2000;49(10):1255-60.
10. Rasgon NL, Altshuler LL, Birtan JA, et al. Menstrual abnormalities in women with bipolar disorder (presentation). San Francisco: American Psychiatric Association annual meeting, May 2003.
11. Figlewicz DP. Endocrine regulation of neurotransmitter transporters. Epilepsy Res 1999;37(3):203-10.
12. Nathan RS, Sachar EJ, Asnis GM, et al. Relative insulin insensitivity and cortisol secretion in depressed patients. Psychiatry Res 1981;4:291.-
13. Elmslie JL, Silverstone TJ, Mann JI, Williams SM. Determinants of overweight and obesity in patients with bipolar disorder. J Clin Psychiatry 2000;61:179.-
14. Istvan J, Zavela K, Weidner G. Body weight and psychological distress in NHANES I. Int J Obes Relat Metab Disord 1992;16(12):999-1003.
15. Andrews RC, Walker BR. Glucocorticoids and insulin resistance: old hormones, new targets. Clin Sci 1999;96(5):513.-
16. Pasquali R, Biscotti D, Spinucci G, et al. Pulsatile secretion of ACTH and cortisol in premenopausal women: effect of obesity and body fat distribution. Clin Endocrinol 1998;48(5):603.-
17. Moghetti P, Castello R, Negri C, et al. Insulin infusion amplifies 17 alpha-hydroxycorticosteroid intermediates response to adrenocorticotropin in hyperandrogenic women: apparent relative impairment of 17,20-lyase activity. J Clin Endocrinol Metabol 1996;81(3):881.-
18. Bailey CJ, Day C, Bray GA, et al. Role of adrenal glands in the development of abnormal glucose and insulin homeostasis in genetically obese (ob/ob) mice. Horm Metab Res 1986;18(6):357.-
19. Sills SE, Perloe M, Palermo GD. Correction of hyperinsulinemia in oligoovulatory women with clomiphene-resistant polycystic ovary syndrome: a review of therapeutic rationale and reproductive outcomes. Eur J Obstet Gynecol Reprod Biol 2000;91(2):135.-
20. Nestler JE, Jakubowicz DJ, Evans WS, Pasquali R. Effects of metformin on spontaneous and clomiphene-induced ovulation in the polycystic ovary syndrome. N Engl J Med 1998;338(26):1876.-
21. Velasquez EM, Mendoza S, Hamer T, et al. Metformin therapy in polycystic ovary syndrome reduces hyperinsulinemia, insulin resistance, hyperandrogenemia, and systolic blood pressure, while facilitating normal menses and pregnancy. Metabolism 1994;43(5):647.-
22. Chaouloff F. Effects of acute physical exercise on central serotonergic systems. Med Sci Sports Exerc 1997;29(1):58.-
23. Salazar M. Alpha lipoic acid: a novel treatment for depression. Medical Hypoth 2000;55:510.-
24. Spritzer PM, Lisboa KO, Mattiello S, Lhullier F. Spironolactone as a single agent for long-term therapy of hirsute patients. Clin Endocrinol 2000;52:587.-
25. Moghetti P, Tosi F, Castello R, et al. The insulin resistance in women with hyperandrogenism is partially reversed by antiandrogen treatment: evidence that androgens impair insulin action in women. J Clin Endocrinol Metabol 1996;81(3):952.-
26. Zobel AW, Nickel T, Kunzel HE. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatric Res 2000;34:171.-
27. Healy DG, Harkin A, Cryan JF, et al. Metyrapone displays antidepressant-like properties in preclinical paradigms. Psychopharmacol 1999;145(3):303.-
28. Bech P, Raabaek Olsen L, Jarløv N, et al. A case of sequential anti-stress medication in a patient with major depression resistant to amine-reuptake inhibitors. Acta Psychiatr Scand 1999;100:76.-
29. Wolkowitz OM, Reus VI, Chan T, et al. Antiglucocorticoid treatment of depression: double-blind ketoconazole. J Biol Psychiatry 1999;45(8):1070.-
30. Murphy BE, Filipini D, Ghadirian AM. Possible use of glucocorticoid receptor antagonists in the treatment of major depression: preliminary results using RU 486. J Psychiatry Neurosci 1993;18(5):209.-
31. Schatzberg AF, Rothschild AJ, Langlais PJ, et al. A corticosteroid/dopamine hypothesis for psychotic depression and related states. J Psychiatr Res 1985;19(1):57.-
1. Rasgon NL, Carter MS, Elman S, et al. Common treatment of polycystic ovarian syndrome and major depressive disorder: case report and review. Curr Drug Targets Immune Endocr Metabol Disord 2002;2(1):97-102.
2. Franks S. Polycystic ovary syndrome. N Engl J Med 1995;333(13):853-61.
3. Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR (eds). Polycystic ovary syndrome. Oxford, UK: Blackwell Scientific, 1992;377-84.
4. Rasgon NL, Rao R, Elman S, et al. Depression in women with polycystic ovary syndrome: clinical and biochemical correlates. J Affect Disord 2003;74(3):299-304.
5. Weiner CL, Primeau M, Ehrmann DA. Androgens and mood dysfunction in women: Comparison of women with polycystic ovarian syndrome to healthy controls. Psychosom Med 2004;66:356-62.
6. Fava GA, Grandi S, Savron G, et al. Psychosomatic assessment of hirsute women. Psychother Psychosom 1989;51:96-100.
7. Rasgon NL, Altshuler LL, Gudeman D, et al. Medication status and PCO syndrome in women with bipolar disorder: a preliminary report. J Clin Psychiatry 2000;61:173-8.
8. Okamura F, Tashiro A, Utsumi A, et al. Insulin resistance in patients with depression and its changes in the clinical course of depression: a report on three cases using the minimal model analysis. Internal Med 1999;38(3):257-60.
9. Okamura F, Tashiro A, Utsumi A, et al. Insulin resistance in patients with depression and its changes during the clinical course of depression: minimal model analysis. Metabolism 2000;49(10):1255-60.
10. Rasgon NL, Altshuler LL, Birtan JA, et al. Menstrual abnormalities in women with bipolar disorder (presentation). San Francisco: American Psychiatric Association annual meeting, May 2003.
11. Figlewicz DP. Endocrine regulation of neurotransmitter transporters. Epilepsy Res 1999;37(3):203-10.
12. Nathan RS, Sachar EJ, Asnis GM, et al. Relative insulin insensitivity and cortisol secretion in depressed patients. Psychiatry Res 1981;4:291.-
13. Elmslie JL, Silverstone TJ, Mann JI, Williams SM. Determinants of overweight and obesity in patients with bipolar disorder. J Clin Psychiatry 2000;61:179.-
14. Istvan J, Zavela K, Weidner G. Body weight and psychological distress in NHANES I. Int J Obes Relat Metab Disord 1992;16(12):999-1003.
15. Andrews RC, Walker BR. Glucocorticoids and insulin resistance: old hormones, new targets. Clin Sci 1999;96(5):513.-
16. Pasquali R, Biscotti D, Spinucci G, et al. Pulsatile secretion of ACTH and cortisol in premenopausal women: effect of obesity and body fat distribution. Clin Endocrinol 1998;48(5):603.-
17. Moghetti P, Castello R, Negri C, et al. Insulin infusion amplifies 17 alpha-hydroxycorticosteroid intermediates response to adrenocorticotropin in hyperandrogenic women: apparent relative impairment of 17,20-lyase activity. J Clin Endocrinol Metabol 1996;81(3):881.-
18. Bailey CJ, Day C, Bray GA, et al. Role of adrenal glands in the development of abnormal glucose and insulin homeostasis in genetically obese (ob/ob) mice. Horm Metab Res 1986;18(6):357.-
19. Sills SE, Perloe M, Palermo GD. Correction of hyperinsulinemia in oligoovulatory women with clomiphene-resistant polycystic ovary syndrome: a review of therapeutic rationale and reproductive outcomes. Eur J Obstet Gynecol Reprod Biol 2000;91(2):135.-
20. Nestler JE, Jakubowicz DJ, Evans WS, Pasquali R. Effects of metformin on spontaneous and clomiphene-induced ovulation in the polycystic ovary syndrome. N Engl J Med 1998;338(26):1876.-
21. Velasquez EM, Mendoza S, Hamer T, et al. Metformin therapy in polycystic ovary syndrome reduces hyperinsulinemia, insulin resistance, hyperandrogenemia, and systolic blood pressure, while facilitating normal menses and pregnancy. Metabolism 1994;43(5):647.-
22. Chaouloff F. Effects of acute physical exercise on central serotonergic systems. Med Sci Sports Exerc 1997;29(1):58.-
23. Salazar M. Alpha lipoic acid: a novel treatment for depression. Medical Hypoth 2000;55:510.-
24. Spritzer PM, Lisboa KO, Mattiello S, Lhullier F. Spironolactone as a single agent for long-term therapy of hirsute patients. Clin Endocrinol 2000;52:587.-
25. Moghetti P, Tosi F, Castello R, et al. The insulin resistance in women with hyperandrogenism is partially reversed by antiandrogen treatment: evidence that androgens impair insulin action in women. J Clin Endocrinol Metabol 1996;81(3):952.-
26. Zobel AW, Nickel T, Kunzel HE. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatric Res 2000;34:171.-
27. Healy DG, Harkin A, Cryan JF, et al. Metyrapone displays antidepressant-like properties in preclinical paradigms. Psychopharmacol 1999;145(3):303.-
28. Bech P, Raabaek Olsen L, Jarløv N, et al. A case of sequential anti-stress medication in a patient with major depression resistant to amine-reuptake inhibitors. Acta Psychiatr Scand 1999;100:76.-
29. Wolkowitz OM, Reus VI, Chan T, et al. Antiglucocorticoid treatment of depression: double-blind ketoconazole. J Biol Psychiatry 1999;45(8):1070.-
30. Murphy BE, Filipini D, Ghadirian AM. Possible use of glucocorticoid receptor antagonists in the treatment of major depression: preliminary results using RU 486. J Psychiatry Neurosci 1993;18(5):209.-
31. Schatzberg AF, Rothschild AJ, Langlais PJ, et al. A corticosteroid/dopamine hypothesis for psychotic depression and related states. J Psychiatr Res 1985;19(1):57.-
“Diagnostically homeless” Is it ADHD? Mania? Autism? What to do if no diagnosis fits
Children with developmental problems and serious psychopathologies often do not fit neatly into DSM diagnoses.1,2 These “diagnostically homeless” children—handicapped by hyperactivity, volcanic rages, extreme anxieties, and other complex problems—need assessment and treatment that address four domains of dysfunction:
- mood/anxiety problems
- possible psychosis
- language/thought disorder
- relationship/socialization problems.
This article offers snapshots of four children with undetermined diagnoses, explores the dilemma of treating such patients without knowing what they really have, and recommends a treatment approach to help them function better in school and at home.
WHO ARE THE ‘DIAGNOSTICALLY HOMELESS’?
Devon is 5. He is extremely hyperactive and impulsive, with a normal IQ but significant language delay. He exhibits little but not absent interest in peers and rages when changes are imposed on him.
Table 1
Criteria describing impairments in ‘diagnostically homeless’ children
| Domain | Multiple complex developmental disorder (MCDD)* | Multidimensionally impaired (MDI) syndrome† | Schizotypal personality disorder |
|---|---|---|---|
| Anxiety symptoms | Intense generalized anxiety, diffuse tension or irritability; unusual fears and phobias, peculiar in content or intensity; recurrent panic episodes, terror, or flooding with anxiety | Unspecified | Excessive social anxiety associated with paranoid fears |
| Affect regulation | Significant, wide, emotional variability out of proportion to precipitants | Nearly daily periods of emotional lability disproportionate to precipitants | Inappropriate or constricted affect |
| Psychotic-like symptoms | Magical thinking; illogical confusion between reality and fantasy; grandiose fantasies of special powers | Poor ability to separate reality from fantasy | Ideas of reference; unusual perceptual experiences; suspicious; eccentric |
| Thought/language disorder | Thought problems including irrationality, sudden intrusions on normal thought process, neologisms or nonsense words repeated over and over; blatantly illogical, bizarre ideas | Thought disorder specifically excluded | Odd thinking; vague, circumstantial, metaphorical speech, overelaborate or stereotyped |
| Problems with social functioning | Social disinterest, detachment; instrumental relatedness; high degrees of ambivalence to adults, manifested by clinging, overly controlling, needy behavior and/or aggressive, oppositional behavior; limited capacity to empathize | Impaired interpersonal skills despite desire to initiate social interactions with peers | Lack of close friends or confidants other than relatives |
| * PDD NOS (pervasive developmental disorder, not otherwise specified) is the closest DSM-IV-TR designation. | |||
| † Psychosis NOS is the closest DSM-IV-TR designation. | |||
| Source: References 1, 3, 8-13. | |||
Steven is 11, referred “to rule out bipolar disorder” and to evaluate hyperactivity, explosiveness, and nightmares. He didn’t speak until he was 22 months old. He worries that bad people are chasing him, fears skeletons under his bed, has nightmares of vampires, and believes that cartoon characters are real and that Sponge Bob is his protector. He says he sees “scary stuff” out of the corner of his eyes. He does not have a thought disorder; psychotic symptoms are more than an overactive imagination or anxiety.
Lauren, age 12, has been diagnosed with attention-deficit/hyperactivity disorder (ADHD) but now presents with withdrawn, depressed, and defiant behaviors. She is described as a “loner” who has never related well to other children. Lauren speaks about being tortured by her peers to the point of sounding paranoid. Her conversation is extremely circumstantial and rambling.
Richard, age 8, has motor coordination, attachment, and disinhibition problems. He hears voices telling him to do bad things, such as hurt people, steal things, and “break stuff.” He doesn’t mind the voices much, and they don’t pervade his life the way hallucinations do in schizophrenia.
Children such as these are common, and it is unclear whether they have a developmental disorder, the prodrome of a psychotic or mood disorder, or idiosyncratic personalities. They don’t meet criteria for many disorders, including autism, bipolar disorder, schizophrenia, and obsessive-compulsive disorder (OCD). They have more-extensive difficulties than those seen in ADHD, generalized anxiety disorder (GAD), or OCD.
Clinically, they are either forced into a category someone thinks they resemble (such as mania in Devon’s case) or are given a “not otherwise specified” (NOS) label (such as PDD NOS, psychosis NOS, or mood disorder NOS), the severity of which goes unacknowledged.
Problems with ‘NOS.’ Some might consider “NOS” a less-severe problem than a specific diagnosis, but these children are very impaired. They are excluded from treatment studies because they do not meet formal criteria for the designated disorder or they get included erroneously because the structured diagnostic interview doesn’t assess what they really have.
Meaningful psychoeducation for their parents is impossible because no Web site or book exists to help them help their child. Finally, no follow-up studies have been done of this group of children because no one can agree on a diagnosis. Small studies have addressed some of these concerns, but outcomes—not surprisingly—are wide-ranging.3-6
NOS diagnoses also don’t adequately address children with marked anxiety, unusual fears, and perseverative behaviors who are socially clumsy but manage reciprocal social interaction. These children are substantially disabled by:
- attention difficulties
- mood dysregulation (including anxiety and/or manic symptoms)
- trouble with transitions/change
- motor problems (not infrequently)
- pragmatic language/social difficulties.
Diagnostic terms that have tried to classify these children (Table 1) include:
- childhood-onset PDD, described in DSM-III. This category was dropped in DSM III-R to be included in PDD, then largely ignored in DSM-IV when autism criteria were refined.
- multiple complex developmental disorder (MCDD),7-9 which appears to describe children within the autism spectrum (such as PDD NOS)
- multidimensionally-impaired (MDI) syndrome, whose atypical psychosis has been called “psychosis NOS”10-11
- schizotypal personality disorder, which addresses similar symptoms (although mental health professionals are loathe to use a personality disorder diagnosis in a child).12
At this time, however, diagnostic conclusions about this heterogeneous group of children are premature. Our classification system does not do them justice, and we need to study them for what they have, rather than forcing them into our current alternatives.
Prevalence. To find out how many patients in our university-based, tertiary-care clinic do not fit DSM-IV-TR nosology, we examined data from faculty evaluations of 624 children and adolescents.13 These included semi-structured interviews of parent and child, rating scales from parents and teachers, and testing information from the schools in two-thirds of cases.
The result: nearly 25% of our child and adolescent psychiatry outpatients are “diagnostically homeless.” Like the rest of our patient population, these children are:
- 80% male
- 60% under age 12
- 86% Caucasian
- 85% living with their biological mothers.
- ADHD (16%). They have great difficulty with executive functions, such as paying attention, inhibiting impulsive responses, planning and organizing, making transitions from one activity to another, and controlling emotion. Their problems, however, go much beyond ADHD.
- Bipolar disorder (15%) or depression/anxiety (16%). They have catastrophic anxiety and/or frightening rages triggered by apparently trivial circumstances. They balk or “shut down” when people want them to move or act faster than they can move or act.
- To “rule out autism” (19%). More than one-half (56%) of these children have a diagnosable speech or language disorder, compared with 35% among our other child psychiatry outpatients.
- For educational assessment (23%). School systems request guidance for educational interventions because these children are possibly psychotic and disturbing to teachers and children. They may be unable to execute homework assignments and fail their courses but surprisingly do grade-level work on achievement tests.
ASSESSING FOUR DOMAINS
We can consolidate the domains needing assessment into mood/anxiety problems, possible psychosis, language/thought disorder, and relationship/socialization problems. Although evaluating and treating some of these domains may be beyond the psychiatrist’s purview, we must make sure that other professionals attend to them.
Anxiety and mood. Understanding these children’s anxieties is important. A routine fear of bees is a simple phobia, whereas catastrophic anxiety over a highly unlikely impending tornado and perseverative interest in the weather may be more common in a PDD spectrum disorder. Anxiety about going to sleep because a monster is going to suck out one’s brains does not easily fit into the rubric of generalized anxiety.14
Irritability is these youngsters’ most disabling mood symptom. Volcanic anger and rage that prompts referral occurs in numerous conditions, including mania. Many of the children described in Ross Greene’s book, The Explosive Child,15 have conditions other than bipolar disorder. Although parents and teachers often describe these events as occurring without provocation, a good functional behavioral assessment will usually reveal a precipitant.
Table 2
Assessing children’s social and language skills
| Social assessment | Seen in… |
|---|---|
| Are the child’s social abilities delayed? | ADHD |
| Is he uninterested in social situations? | Autism |
| Is he clueless about social interaction? | Autism spectrum disorders including MCDD, MDI, PDD NOS, nonverbal learning disability |
| Are social interactions deviant? | Schizotypal personality disorder/schizophrenia |
| Does child appear shut down/behaviorally inhibited in unfamiliar settings, with greater comfort at home or with familiar people? | Social phobia |
| Language assessment (can be done by psychiatrist) | |
| |
| Useful questions | Seen in… |
| Was communication delayed but then progressed “normally”? | Developmental language disorder |
| Did it begin normally and stop? | Autism |
| Was/is it egocentric and/or unidimensional? | Asperger’s disorder; nonverbal learning disability |
| Was/is it bizarre or paranoid? | Schizotypal personality disorder |
| Pragmatic language problems? | All of the above, MCDD, MDI, ADHD |
| Communication domains (may require speech pathologist assessment) | |
| Expressive and receptive language | |
| Pragmatic language (the child’s ability to communicate in the real world; see Table 3) | |
| Written language | |
| Audiology (hearing and auditory processing) | |
| ADHD: attention-deficit/hyperactivity disorder | |
| MCDD: multiple complex developmental disorder | |
| MDI: multidimensionally impaired syndrome | |
| PDD NOS: pervasive developmental disorder not otherwise specified | |
Possible psychosis. These children may have impaired reality testing that can be difficult to assess; thus, deciding whether the child is experiencing psychotic symptoms can be a challenge. The child may be intensely involved with fantasy characters or imaginary companions to such a degree that he or she insists the character is real.16,17 Developmentally normal fears—as of the dark, monsters, or images from dreams—may preoccupy him or her during the day. Quasi-psychotic symptoms such as these are easily missed if:
- we don’t ask about them
- we assume the child is “just pretending” or has a “great imagination”
- the child does not volunteer the information spontaneously.18
Communication skills children need to learn
|
Language/thought disorder. Parents may not recognize that their child has a thought or language disorder because they have filled in the blanks and interpreted for him or her for so long. Asking the child “yes” and “no” questions will not elucidate these disorders, either. The examiner must talk to the child to determine his or her ability to:
- sustain an extended narration that makes sense
- stay on the topic
- care whether the listener understands what the child is talking about
- make a point.
Nonverbal communication realms include eye contact, appropriate hand gestures and facial expression, tone of voice, and vocal inflection. Other important areas of language to assess are summarized in Table 2.
Relationship/socialization problems. It is important to know whether the child has friends, wants friends, or prefers being with younger children. Peer relationships may be absent, delayed, or deviant.
Other assessments. The diagnostically homeless children we see have complicated family histories of psychopathology. Their first-degree relatives have a higher number of heritable disorders—including bipolar disorder, panic disorder, ADHD, learning disabilities, and “nervous breakdowns”—than do those of children with uncomplicated ADHD, bipolar disorder, or anxiety disorders. Ask about these conditions when taking the family history; if a family member is said to be bipolar, get a description of the person’s symptoms.
Table 4
Targeting drug therapies to treat children’s symptoms
| Drug class | Efficacy by symptom domain |
|---|---|
| Atypical antipsychotics | Psychosis/thought disorder: Can reduce psychotic symptoms |
| Anxiety symptoms: Can reduce extreme anxieties | |
| Affect regulation: Improved by mood-stabilizing effect | |
| Socialization problems: Appear to modify affective aggression, hyperactivity, and impulsivity, which can improve socialization and pragmatic communication | |
| Mood stabilizers | Psychosis/thought disorder: Not primary area of effectiveness |
| Anxiety symptoms: May be helpful; not primary area of effectiveness | |
| Affect regulation: Address mood dysregulation | |
| Socialization problems: May reduce aggressive outbursts and mood, which can improve socialization | |
| Stimulants* | Psychosis/thought disorder: Can produce or intensify psychotic symptoms and agitation |
| Anxiety: Usually do not improve anxiety; can intensify anxiety and agitation | |
| Affect regulation: Not a primary effect in severe cases; address impulsive aggression via mood stabilization | |
| Socialization problems: Can improve functioning via decreased impulsivity, inattention, and aggression | |
| SSRI antidepressants† | Psychosis/thought disorder: Do not directly address |
| Anxiety: Can be effective in decreasing anxiety | |
| Affect regulation: Can improve depressed mood | |
| Socialization problems: Can be improved as a result of improved mood and decreased anxiety | |
| * Stimulants often increase agitation and disinhibition. | |
| † Watch for behavioral disinhibition, possible increase in suicidality, with selective serotonin reuptake inhibitors (SSRIs). | |
A skilled psychologist or speech pathologist can help you determine the presence or absence of cognitive and language dysfunction and learning disabilities. Even before we interview the parents and child, we ask parents and teachers to rate the child’s attention, behavior, mood, PDD-like symptoms, and anxiety, using the Child/Adolescent Symptom Inventory (see Related resources). We use the youth version with children age 10 and older, then review the symptoms with the parents and child to make sure we understand all presenting comorbidities.
TREATMENT
Nonmedical interventions begin with an accurate diagnosis, where possible. Then the four steps of treatment are to:
- address each domain of dysfunction
- translate findings to parent, child, and teachers/school.
- provide settings and resources that allow the child to work most effectively
- develop a behavioral program for the most frequent problems, with consistent response by caretakers and educators.
A communication specialist interested in pragmatics is needed to make sure the child is understood and being understood in the classroom. Table 3, summarizes communications skills the child needs to learn. An educational specialist who serves a resource to other professionals may also help the child. Curriculum should be based on long-term goals rather than on inflexible credit schedules that teach worthless, unlearnable information and demoralize the student.
Finally, the education setting should provide opportunities for structured social interaction and less-structured but supervised—”bully-proofed”—interactions.
Medications. No systematic medical treatment data exist, as there is no way to classify these children. They are usually treated with multiple medications for their specific symptom cluster abnormalities (Table 4). Options include:
- atypical antipsychotics such as risperidone, quetiapine, aripiprazole, ziprasidone, or olanzapine
- mood stabilizers such as valproic acid, lithium, or lamotrigine
- stimulants such as methylphenidate, amphetamine salts, atomoxetine, or bupropion (a mild stimulant and an antidepressant)
- selective serotonin reuptake inhibitors, such as fluoxetine, sertraline, citalopram, paroxetine, or fluvoxamine.
Medication side effects understandably frighten parents—who may be reluctant to have their children use any drug therapies. Counsel the parents in advance that side effects may occur.
- Child/Adolescent Symptom Inventory. http://www.checkmateplus.com. Accessed Jan. 11, 2005.
- Amphetamine • Adderall
- Aripiprazole • Abilify
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Citalopram • Celexa
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Lamotrigine • Lamictal
- Lithium carbonate • Lithobid, others
- Methylphenidate • Concerta, Ritalin
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Valproic acid • Depakote
- Ziprasidone • Geodon
Dr. Weisbrot receives grants from Pfizer Inc.
Dr. Carlson receives grants from or is a speaker for Janssen Pharmaceutica, Eli Lilly and Co., Shire Pharmaceuticals Groups, and Abbott Laboratories; is a consultant to Janssen Pharmaceutica and Eli Lilly and Co.; and is an advisor to Otsuka America Pharmaceutical, Pfizer Inc., and Ortho-McNeil Pharmaceutical.
1. Meijer M, Treffers P. Borderline and schizotypal disorders in children and adolescents. Br J Psychiatry 1991;158:205-12.
2. Petti TA, Vela RM. Borderline disorders of childhood: an overview. J Am Acad Child Adolesc Psychiatry 1990;29:327-37.
3. Wolff S. Loners: the life path of unusual children. London: Routledge, 1995.
4. Kestenbaum C. The borderline child at risk for major psychiatric disorder in adult life: seven case reports with followup. In: Robson KS (ed). The borderline child. New York: McGraw-Hill, 1983;49-82.
5. Lofgren DP, Bemporad J, King J, et al. A prospective follow-up study of so-called borderline children. Am J Psychiatry 1991;148:1541-7.
6. Nicolson R, Lenane M, Brookner F, et al. Children and adolescents with psychotic disorder not otherwise specified: a 2-to-8 year follow-up study. Compr Psychiatry 2001;42:319-25.
7. Towbin KE, Dykens EM, Pearson GS, Cohen DA. Conceptualizing “borderline syndrome of childhood” and “childhood schizophrenia” as a developmental disorder. J Am Acad Child Adolesc Psychiatry 1993;32(4):775-82.
8. Buitelaar JK, van der Gaag RJ. Diagnostic rules for children with PDD-NOS and multiple complex developmental disorder. J Child Psychol Psychiatry 1998;39(6):911-19.
9. Van der Gaag RJ, Buitelaar J, Van den Ban E, et al. A controlled multivariate chart review of multiple complex developmental disorder. J Am Acad Child Adolesc Psychiatry 1995;34(8):1096-106.
10. McKenna K, Gordon C, Lenane M, et al. Looking for childhood-onset schizophrenia: the first 71 cases screened. J Am Acad Child Adolesc Psychiatry 1994;33:636-44.
11. Kumra S, Jacobsen L, Lenane M, et al. “Multidimensionally impaired disorder”: is it a variant of very early-onset schizophrenia? J Am Acad Child Adolesc Psychiatry 1998;37(1):91-99.
12. Nagy J, Satzmari P. A chart review of schizotypal personality disorders in children. J Autism Dev Disord 1986;16(3):351-67.
13. Carlson GA. Unpublished data.
14. Greene R. The explosive child: a new approach for understanding and parenting easily frustrated, chronically inflexible children (2nd ed). New York: Harper Collins, 2001.
15. Weisbrot DM, Gadow KD, DeVincent CJ, et al. The presentation of anxiety in children with pervasive developmental disorders. J Child Adolesc Psychopharmacol 2005 (in press).
16. Garralda ME. Hallucinations in children with conduct and emotional disorders: the clinical phenomena. Psychol Med 1984;14:589-96.
17. Ulloa RE, Birmaher B, Axelson D, et al. Psychosis in a pediatric mood and anxiety disorders clinic: phenomenology and correlates. J Am Acad Child Adolesc Psychiatry 2000;39(3):337-45.
18. Schreier HA. Hallucinations in nonpsychotic children: more common than we think? J Am Acad Child Adolesc Psychiatry 2000;38(5):623-625.
19. Carlson GA, Mick E. Drug-induced disinhibition in psychiatrically hospitalized children. J Child Adolesc Psychopharmacol 2003;13(2):153-63.
Children with developmental problems and serious psychopathologies often do not fit neatly into DSM diagnoses.1,2 These “diagnostically homeless” children—handicapped by hyperactivity, volcanic rages, extreme anxieties, and other complex problems—need assessment and treatment that address four domains of dysfunction:
- mood/anxiety problems
- possible psychosis
- language/thought disorder
- relationship/socialization problems.
This article offers snapshots of four children with undetermined diagnoses, explores the dilemma of treating such patients without knowing what they really have, and recommends a treatment approach to help them function better in school and at home.
WHO ARE THE ‘DIAGNOSTICALLY HOMELESS’?
Devon is 5. He is extremely hyperactive and impulsive, with a normal IQ but significant language delay. He exhibits little but not absent interest in peers and rages when changes are imposed on him.
Table 1
Criteria describing impairments in ‘diagnostically homeless’ children
| Domain | Multiple complex developmental disorder (MCDD)* | Multidimensionally impaired (MDI) syndrome† | Schizotypal personality disorder |
|---|---|---|---|
| Anxiety symptoms | Intense generalized anxiety, diffuse tension or irritability; unusual fears and phobias, peculiar in content or intensity; recurrent panic episodes, terror, or flooding with anxiety | Unspecified | Excessive social anxiety associated with paranoid fears |
| Affect regulation | Significant, wide, emotional variability out of proportion to precipitants | Nearly daily periods of emotional lability disproportionate to precipitants | Inappropriate or constricted affect |
| Psychotic-like symptoms | Magical thinking; illogical confusion between reality and fantasy; grandiose fantasies of special powers | Poor ability to separate reality from fantasy | Ideas of reference; unusual perceptual experiences; suspicious; eccentric |
| Thought/language disorder | Thought problems including irrationality, sudden intrusions on normal thought process, neologisms or nonsense words repeated over and over; blatantly illogical, bizarre ideas | Thought disorder specifically excluded | Odd thinking; vague, circumstantial, metaphorical speech, overelaborate or stereotyped |
| Problems with social functioning | Social disinterest, detachment; instrumental relatedness; high degrees of ambivalence to adults, manifested by clinging, overly controlling, needy behavior and/or aggressive, oppositional behavior; limited capacity to empathize | Impaired interpersonal skills despite desire to initiate social interactions with peers | Lack of close friends or confidants other than relatives |
| * PDD NOS (pervasive developmental disorder, not otherwise specified) is the closest DSM-IV-TR designation. | |||
| † Psychosis NOS is the closest DSM-IV-TR designation. | |||
| Source: References 1, 3, 8-13. | |||
Steven is 11, referred “to rule out bipolar disorder” and to evaluate hyperactivity, explosiveness, and nightmares. He didn’t speak until he was 22 months old. He worries that bad people are chasing him, fears skeletons under his bed, has nightmares of vampires, and believes that cartoon characters are real and that Sponge Bob is his protector. He says he sees “scary stuff” out of the corner of his eyes. He does not have a thought disorder; psychotic symptoms are more than an overactive imagination or anxiety.
Lauren, age 12, has been diagnosed with attention-deficit/hyperactivity disorder (ADHD) but now presents with withdrawn, depressed, and defiant behaviors. She is described as a “loner” who has never related well to other children. Lauren speaks about being tortured by her peers to the point of sounding paranoid. Her conversation is extremely circumstantial and rambling.
Richard, age 8, has motor coordination, attachment, and disinhibition problems. He hears voices telling him to do bad things, such as hurt people, steal things, and “break stuff.” He doesn’t mind the voices much, and they don’t pervade his life the way hallucinations do in schizophrenia.
Children such as these are common, and it is unclear whether they have a developmental disorder, the prodrome of a psychotic or mood disorder, or idiosyncratic personalities. They don’t meet criteria for many disorders, including autism, bipolar disorder, schizophrenia, and obsessive-compulsive disorder (OCD). They have more-extensive difficulties than those seen in ADHD, generalized anxiety disorder (GAD), or OCD.
Clinically, they are either forced into a category someone thinks they resemble (such as mania in Devon’s case) or are given a “not otherwise specified” (NOS) label (such as PDD NOS, psychosis NOS, or mood disorder NOS), the severity of which goes unacknowledged.
Problems with ‘NOS.’ Some might consider “NOS” a less-severe problem than a specific diagnosis, but these children are very impaired. They are excluded from treatment studies because they do not meet formal criteria for the designated disorder or they get included erroneously because the structured diagnostic interview doesn’t assess what they really have.
Meaningful psychoeducation for their parents is impossible because no Web site or book exists to help them help their child. Finally, no follow-up studies have been done of this group of children because no one can agree on a diagnosis. Small studies have addressed some of these concerns, but outcomes—not surprisingly—are wide-ranging.3-6
NOS diagnoses also don’t adequately address children with marked anxiety, unusual fears, and perseverative behaviors who are socially clumsy but manage reciprocal social interaction. These children are substantially disabled by:
- attention difficulties
- mood dysregulation (including anxiety and/or manic symptoms)
- trouble with transitions/change
- motor problems (not infrequently)
- pragmatic language/social difficulties.
Diagnostic terms that have tried to classify these children (Table 1) include:
- childhood-onset PDD, described in DSM-III. This category was dropped in DSM III-R to be included in PDD, then largely ignored in DSM-IV when autism criteria were refined.
- multiple complex developmental disorder (MCDD),7-9 which appears to describe children within the autism spectrum (such as PDD NOS)
- multidimensionally-impaired (MDI) syndrome, whose atypical psychosis has been called “psychosis NOS”10-11
- schizotypal personality disorder, which addresses similar symptoms (although mental health professionals are loathe to use a personality disorder diagnosis in a child).12
At this time, however, diagnostic conclusions about this heterogeneous group of children are premature. Our classification system does not do them justice, and we need to study them for what they have, rather than forcing them into our current alternatives.
Prevalence. To find out how many patients in our university-based, tertiary-care clinic do not fit DSM-IV-TR nosology, we examined data from faculty evaluations of 624 children and adolescents.13 These included semi-structured interviews of parent and child, rating scales from parents and teachers, and testing information from the schools in two-thirds of cases.
The result: nearly 25% of our child and adolescent psychiatry outpatients are “diagnostically homeless.” Like the rest of our patient population, these children are:
- 80% male
- 60% under age 12
- 86% Caucasian
- 85% living with their biological mothers.
- ADHD (16%). They have great difficulty with executive functions, such as paying attention, inhibiting impulsive responses, planning and organizing, making transitions from one activity to another, and controlling emotion. Their problems, however, go much beyond ADHD.
- Bipolar disorder (15%) or depression/anxiety (16%). They have catastrophic anxiety and/or frightening rages triggered by apparently trivial circumstances. They balk or “shut down” when people want them to move or act faster than they can move or act.
- To “rule out autism” (19%). More than one-half (56%) of these children have a diagnosable speech or language disorder, compared with 35% among our other child psychiatry outpatients.
- For educational assessment (23%). School systems request guidance for educational interventions because these children are possibly psychotic and disturbing to teachers and children. They may be unable to execute homework assignments and fail their courses but surprisingly do grade-level work on achievement tests.
ASSESSING FOUR DOMAINS
We can consolidate the domains needing assessment into mood/anxiety problems, possible psychosis, language/thought disorder, and relationship/socialization problems. Although evaluating and treating some of these domains may be beyond the psychiatrist’s purview, we must make sure that other professionals attend to them.
Anxiety and mood. Understanding these children’s anxieties is important. A routine fear of bees is a simple phobia, whereas catastrophic anxiety over a highly unlikely impending tornado and perseverative interest in the weather may be more common in a PDD spectrum disorder. Anxiety about going to sleep because a monster is going to suck out one’s brains does not easily fit into the rubric of generalized anxiety.14
Irritability is these youngsters’ most disabling mood symptom. Volcanic anger and rage that prompts referral occurs in numerous conditions, including mania. Many of the children described in Ross Greene’s book, The Explosive Child,15 have conditions other than bipolar disorder. Although parents and teachers often describe these events as occurring without provocation, a good functional behavioral assessment will usually reveal a precipitant.
Table 2
Assessing children’s social and language skills
| Social assessment | Seen in… |
|---|---|
| Are the child’s social abilities delayed? | ADHD |
| Is he uninterested in social situations? | Autism |
| Is he clueless about social interaction? | Autism spectrum disorders including MCDD, MDI, PDD NOS, nonverbal learning disability |
| Are social interactions deviant? | Schizotypal personality disorder/schizophrenia |
| Does child appear shut down/behaviorally inhibited in unfamiliar settings, with greater comfort at home or with familiar people? | Social phobia |
| Language assessment (can be done by psychiatrist) | |
| |
| Useful questions | Seen in… |
| Was communication delayed but then progressed “normally”? | Developmental language disorder |
| Did it begin normally and stop? | Autism |
| Was/is it egocentric and/or unidimensional? | Asperger’s disorder; nonverbal learning disability |
| Was/is it bizarre or paranoid? | Schizotypal personality disorder |
| Pragmatic language problems? | All of the above, MCDD, MDI, ADHD |
| Communication domains (may require speech pathologist assessment) | |
| Expressive and receptive language | |
| Pragmatic language (the child’s ability to communicate in the real world; see Table 3) | |
| Written language | |
| Audiology (hearing and auditory processing) | |
| ADHD: attention-deficit/hyperactivity disorder | |
| MCDD: multiple complex developmental disorder | |
| MDI: multidimensionally impaired syndrome | |
| PDD NOS: pervasive developmental disorder not otherwise specified | |
Possible psychosis. These children may have impaired reality testing that can be difficult to assess; thus, deciding whether the child is experiencing psychotic symptoms can be a challenge. The child may be intensely involved with fantasy characters or imaginary companions to such a degree that he or she insists the character is real.16,17 Developmentally normal fears—as of the dark, monsters, or images from dreams—may preoccupy him or her during the day. Quasi-psychotic symptoms such as these are easily missed if:
- we don’t ask about them
- we assume the child is “just pretending” or has a “great imagination”
- the child does not volunteer the information spontaneously.18
Communication skills children need to learn
|
Language/thought disorder. Parents may not recognize that their child has a thought or language disorder because they have filled in the blanks and interpreted for him or her for so long. Asking the child “yes” and “no” questions will not elucidate these disorders, either. The examiner must talk to the child to determine his or her ability to:
- sustain an extended narration that makes sense
- stay on the topic
- care whether the listener understands what the child is talking about
- make a point.
Nonverbal communication realms include eye contact, appropriate hand gestures and facial expression, tone of voice, and vocal inflection. Other important areas of language to assess are summarized in Table 2.
Relationship/socialization problems. It is important to know whether the child has friends, wants friends, or prefers being with younger children. Peer relationships may be absent, delayed, or deviant.
Other assessments. The diagnostically homeless children we see have complicated family histories of psychopathology. Their first-degree relatives have a higher number of heritable disorders—including bipolar disorder, panic disorder, ADHD, learning disabilities, and “nervous breakdowns”—than do those of children with uncomplicated ADHD, bipolar disorder, or anxiety disorders. Ask about these conditions when taking the family history; if a family member is said to be bipolar, get a description of the person’s symptoms.
Table 4
Targeting drug therapies to treat children’s symptoms
| Drug class | Efficacy by symptom domain |
|---|---|
| Atypical antipsychotics | Psychosis/thought disorder: Can reduce psychotic symptoms |
| Anxiety symptoms: Can reduce extreme anxieties | |
| Affect regulation: Improved by mood-stabilizing effect | |
| Socialization problems: Appear to modify affective aggression, hyperactivity, and impulsivity, which can improve socialization and pragmatic communication | |
| Mood stabilizers | Psychosis/thought disorder: Not primary area of effectiveness |
| Anxiety symptoms: May be helpful; not primary area of effectiveness | |
| Affect regulation: Address mood dysregulation | |
| Socialization problems: May reduce aggressive outbursts and mood, which can improve socialization | |
| Stimulants* | Psychosis/thought disorder: Can produce or intensify psychotic symptoms and agitation |
| Anxiety: Usually do not improve anxiety; can intensify anxiety and agitation | |
| Affect regulation: Not a primary effect in severe cases; address impulsive aggression via mood stabilization | |
| Socialization problems: Can improve functioning via decreased impulsivity, inattention, and aggression | |
| SSRI antidepressants† | Psychosis/thought disorder: Do not directly address |
| Anxiety: Can be effective in decreasing anxiety | |
| Affect regulation: Can improve depressed mood | |
| Socialization problems: Can be improved as a result of improved mood and decreased anxiety | |
| * Stimulants often increase agitation and disinhibition. | |
| † Watch for behavioral disinhibition, possible increase in suicidality, with selective serotonin reuptake inhibitors (SSRIs). | |
A skilled psychologist or speech pathologist can help you determine the presence or absence of cognitive and language dysfunction and learning disabilities. Even before we interview the parents and child, we ask parents and teachers to rate the child’s attention, behavior, mood, PDD-like symptoms, and anxiety, using the Child/Adolescent Symptom Inventory (see Related resources). We use the youth version with children age 10 and older, then review the symptoms with the parents and child to make sure we understand all presenting comorbidities.
TREATMENT
Nonmedical interventions begin with an accurate diagnosis, where possible. Then the four steps of treatment are to:
- address each domain of dysfunction
- translate findings to parent, child, and teachers/school.
- provide settings and resources that allow the child to work most effectively
- develop a behavioral program for the most frequent problems, with consistent response by caretakers and educators.
A communication specialist interested in pragmatics is needed to make sure the child is understood and being understood in the classroom. Table 3, summarizes communications skills the child needs to learn. An educational specialist who serves a resource to other professionals may also help the child. Curriculum should be based on long-term goals rather than on inflexible credit schedules that teach worthless, unlearnable information and demoralize the student.
Finally, the education setting should provide opportunities for structured social interaction and less-structured but supervised—”bully-proofed”—interactions.
Medications. No systematic medical treatment data exist, as there is no way to classify these children. They are usually treated with multiple medications for their specific symptom cluster abnormalities (Table 4). Options include:
- atypical antipsychotics such as risperidone, quetiapine, aripiprazole, ziprasidone, or olanzapine
- mood stabilizers such as valproic acid, lithium, or lamotrigine
- stimulants such as methylphenidate, amphetamine salts, atomoxetine, or bupropion (a mild stimulant and an antidepressant)
- selective serotonin reuptake inhibitors, such as fluoxetine, sertraline, citalopram, paroxetine, or fluvoxamine.
Medication side effects understandably frighten parents—who may be reluctant to have their children use any drug therapies. Counsel the parents in advance that side effects may occur.
- Child/Adolescent Symptom Inventory. http://www.checkmateplus.com. Accessed Jan. 11, 2005.
- Amphetamine • Adderall
- Aripiprazole • Abilify
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Citalopram • Celexa
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Lamotrigine • Lamictal
- Lithium carbonate • Lithobid, others
- Methylphenidate • Concerta, Ritalin
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Valproic acid • Depakote
- Ziprasidone • Geodon
Dr. Weisbrot receives grants from Pfizer Inc.
Dr. Carlson receives grants from or is a speaker for Janssen Pharmaceutica, Eli Lilly and Co., Shire Pharmaceuticals Groups, and Abbott Laboratories; is a consultant to Janssen Pharmaceutica and Eli Lilly and Co.; and is an advisor to Otsuka America Pharmaceutical, Pfizer Inc., and Ortho-McNeil Pharmaceutical.
Children with developmental problems and serious psychopathologies often do not fit neatly into DSM diagnoses.1,2 These “diagnostically homeless” children—handicapped by hyperactivity, volcanic rages, extreme anxieties, and other complex problems—need assessment and treatment that address four domains of dysfunction:
- mood/anxiety problems
- possible psychosis
- language/thought disorder
- relationship/socialization problems.
This article offers snapshots of four children with undetermined diagnoses, explores the dilemma of treating such patients without knowing what they really have, and recommends a treatment approach to help them function better in school and at home.
WHO ARE THE ‘DIAGNOSTICALLY HOMELESS’?
Devon is 5. He is extremely hyperactive and impulsive, with a normal IQ but significant language delay. He exhibits little but not absent interest in peers and rages when changes are imposed on him.
Table 1
Criteria describing impairments in ‘diagnostically homeless’ children
| Domain | Multiple complex developmental disorder (MCDD)* | Multidimensionally impaired (MDI) syndrome† | Schizotypal personality disorder |
|---|---|---|---|
| Anxiety symptoms | Intense generalized anxiety, diffuse tension or irritability; unusual fears and phobias, peculiar in content or intensity; recurrent panic episodes, terror, or flooding with anxiety | Unspecified | Excessive social anxiety associated with paranoid fears |
| Affect regulation | Significant, wide, emotional variability out of proportion to precipitants | Nearly daily periods of emotional lability disproportionate to precipitants | Inappropriate or constricted affect |
| Psychotic-like symptoms | Magical thinking; illogical confusion between reality and fantasy; grandiose fantasies of special powers | Poor ability to separate reality from fantasy | Ideas of reference; unusual perceptual experiences; suspicious; eccentric |
| Thought/language disorder | Thought problems including irrationality, sudden intrusions on normal thought process, neologisms or nonsense words repeated over and over; blatantly illogical, bizarre ideas | Thought disorder specifically excluded | Odd thinking; vague, circumstantial, metaphorical speech, overelaborate or stereotyped |
| Problems with social functioning | Social disinterest, detachment; instrumental relatedness; high degrees of ambivalence to adults, manifested by clinging, overly controlling, needy behavior and/or aggressive, oppositional behavior; limited capacity to empathize | Impaired interpersonal skills despite desire to initiate social interactions with peers | Lack of close friends or confidants other than relatives |
| * PDD NOS (pervasive developmental disorder, not otherwise specified) is the closest DSM-IV-TR designation. | |||
| † Psychosis NOS is the closest DSM-IV-TR designation. | |||
| Source: References 1, 3, 8-13. | |||
Steven is 11, referred “to rule out bipolar disorder” and to evaluate hyperactivity, explosiveness, and nightmares. He didn’t speak until he was 22 months old. He worries that bad people are chasing him, fears skeletons under his bed, has nightmares of vampires, and believes that cartoon characters are real and that Sponge Bob is his protector. He says he sees “scary stuff” out of the corner of his eyes. He does not have a thought disorder; psychotic symptoms are more than an overactive imagination or anxiety.
Lauren, age 12, has been diagnosed with attention-deficit/hyperactivity disorder (ADHD) but now presents with withdrawn, depressed, and defiant behaviors. She is described as a “loner” who has never related well to other children. Lauren speaks about being tortured by her peers to the point of sounding paranoid. Her conversation is extremely circumstantial and rambling.
Richard, age 8, has motor coordination, attachment, and disinhibition problems. He hears voices telling him to do bad things, such as hurt people, steal things, and “break stuff.” He doesn’t mind the voices much, and they don’t pervade his life the way hallucinations do in schizophrenia.
Children such as these are common, and it is unclear whether they have a developmental disorder, the prodrome of a psychotic or mood disorder, or idiosyncratic personalities. They don’t meet criteria for many disorders, including autism, bipolar disorder, schizophrenia, and obsessive-compulsive disorder (OCD). They have more-extensive difficulties than those seen in ADHD, generalized anxiety disorder (GAD), or OCD.
Clinically, they are either forced into a category someone thinks they resemble (such as mania in Devon’s case) or are given a “not otherwise specified” (NOS) label (such as PDD NOS, psychosis NOS, or mood disorder NOS), the severity of which goes unacknowledged.
Problems with ‘NOS.’ Some might consider “NOS” a less-severe problem than a specific diagnosis, but these children are very impaired. They are excluded from treatment studies because they do not meet formal criteria for the designated disorder or they get included erroneously because the structured diagnostic interview doesn’t assess what they really have.
Meaningful psychoeducation for their parents is impossible because no Web site or book exists to help them help their child. Finally, no follow-up studies have been done of this group of children because no one can agree on a diagnosis. Small studies have addressed some of these concerns, but outcomes—not surprisingly—are wide-ranging.3-6
NOS diagnoses also don’t adequately address children with marked anxiety, unusual fears, and perseverative behaviors who are socially clumsy but manage reciprocal social interaction. These children are substantially disabled by:
- attention difficulties
- mood dysregulation (including anxiety and/or manic symptoms)
- trouble with transitions/change
- motor problems (not infrequently)
- pragmatic language/social difficulties.
Diagnostic terms that have tried to classify these children (Table 1) include:
- childhood-onset PDD, described in DSM-III. This category was dropped in DSM III-R to be included in PDD, then largely ignored in DSM-IV when autism criteria were refined.
- multiple complex developmental disorder (MCDD),7-9 which appears to describe children within the autism spectrum (such as PDD NOS)
- multidimensionally-impaired (MDI) syndrome, whose atypical psychosis has been called “psychosis NOS”10-11
- schizotypal personality disorder, which addresses similar symptoms (although mental health professionals are loathe to use a personality disorder diagnosis in a child).12
At this time, however, diagnostic conclusions about this heterogeneous group of children are premature. Our classification system does not do them justice, and we need to study them for what they have, rather than forcing them into our current alternatives.
Prevalence. To find out how many patients in our university-based, tertiary-care clinic do not fit DSM-IV-TR nosology, we examined data from faculty evaluations of 624 children and adolescents.13 These included semi-structured interviews of parent and child, rating scales from parents and teachers, and testing information from the schools in two-thirds of cases.
The result: nearly 25% of our child and adolescent psychiatry outpatients are “diagnostically homeless.” Like the rest of our patient population, these children are:
- 80% male
- 60% under age 12
- 86% Caucasian
- 85% living with their biological mothers.
- ADHD (16%). They have great difficulty with executive functions, such as paying attention, inhibiting impulsive responses, planning and organizing, making transitions from one activity to another, and controlling emotion. Their problems, however, go much beyond ADHD.
- Bipolar disorder (15%) or depression/anxiety (16%). They have catastrophic anxiety and/or frightening rages triggered by apparently trivial circumstances. They balk or “shut down” when people want them to move or act faster than they can move or act.
- To “rule out autism” (19%). More than one-half (56%) of these children have a diagnosable speech or language disorder, compared with 35% among our other child psychiatry outpatients.
- For educational assessment (23%). School systems request guidance for educational interventions because these children are possibly psychotic and disturbing to teachers and children. They may be unable to execute homework assignments and fail their courses but surprisingly do grade-level work on achievement tests.
ASSESSING FOUR DOMAINS
We can consolidate the domains needing assessment into mood/anxiety problems, possible psychosis, language/thought disorder, and relationship/socialization problems. Although evaluating and treating some of these domains may be beyond the psychiatrist’s purview, we must make sure that other professionals attend to them.
Anxiety and mood. Understanding these children’s anxieties is important. A routine fear of bees is a simple phobia, whereas catastrophic anxiety over a highly unlikely impending tornado and perseverative interest in the weather may be more common in a PDD spectrum disorder. Anxiety about going to sleep because a monster is going to suck out one’s brains does not easily fit into the rubric of generalized anxiety.14
Irritability is these youngsters’ most disabling mood symptom. Volcanic anger and rage that prompts referral occurs in numerous conditions, including mania. Many of the children described in Ross Greene’s book, The Explosive Child,15 have conditions other than bipolar disorder. Although parents and teachers often describe these events as occurring without provocation, a good functional behavioral assessment will usually reveal a precipitant.
Table 2
Assessing children’s social and language skills
| Social assessment | Seen in… |
|---|---|
| Are the child’s social abilities delayed? | ADHD |
| Is he uninterested in social situations? | Autism |
| Is he clueless about social interaction? | Autism spectrum disorders including MCDD, MDI, PDD NOS, nonverbal learning disability |
| Are social interactions deviant? | Schizotypal personality disorder/schizophrenia |
| Does child appear shut down/behaviorally inhibited in unfamiliar settings, with greater comfort at home or with familiar people? | Social phobia |
| Language assessment (can be done by psychiatrist) | |
| |
| Useful questions | Seen in… |
| Was communication delayed but then progressed “normally”? | Developmental language disorder |
| Did it begin normally and stop? | Autism |
| Was/is it egocentric and/or unidimensional? | Asperger’s disorder; nonverbal learning disability |
| Was/is it bizarre or paranoid? | Schizotypal personality disorder |
| Pragmatic language problems? | All of the above, MCDD, MDI, ADHD |
| Communication domains (may require speech pathologist assessment) | |
| Expressive and receptive language | |
| Pragmatic language (the child’s ability to communicate in the real world; see Table 3) | |
| Written language | |
| Audiology (hearing and auditory processing) | |
| ADHD: attention-deficit/hyperactivity disorder | |
| MCDD: multiple complex developmental disorder | |
| MDI: multidimensionally impaired syndrome | |
| PDD NOS: pervasive developmental disorder not otherwise specified | |
Possible psychosis. These children may have impaired reality testing that can be difficult to assess; thus, deciding whether the child is experiencing psychotic symptoms can be a challenge. The child may be intensely involved with fantasy characters or imaginary companions to such a degree that he or she insists the character is real.16,17 Developmentally normal fears—as of the dark, monsters, or images from dreams—may preoccupy him or her during the day. Quasi-psychotic symptoms such as these are easily missed if:
- we don’t ask about them
- we assume the child is “just pretending” or has a “great imagination”
- the child does not volunteer the information spontaneously.18
Communication skills children need to learn
|
Language/thought disorder. Parents may not recognize that their child has a thought or language disorder because they have filled in the blanks and interpreted for him or her for so long. Asking the child “yes” and “no” questions will not elucidate these disorders, either. The examiner must talk to the child to determine his or her ability to:
- sustain an extended narration that makes sense
- stay on the topic
- care whether the listener understands what the child is talking about
- make a point.
Nonverbal communication realms include eye contact, appropriate hand gestures and facial expression, tone of voice, and vocal inflection. Other important areas of language to assess are summarized in Table 2.
Relationship/socialization problems. It is important to know whether the child has friends, wants friends, or prefers being with younger children. Peer relationships may be absent, delayed, or deviant.
Other assessments. The diagnostically homeless children we see have complicated family histories of psychopathology. Their first-degree relatives have a higher number of heritable disorders—including bipolar disorder, panic disorder, ADHD, learning disabilities, and “nervous breakdowns”—than do those of children with uncomplicated ADHD, bipolar disorder, or anxiety disorders. Ask about these conditions when taking the family history; if a family member is said to be bipolar, get a description of the person’s symptoms.
Table 4
Targeting drug therapies to treat children’s symptoms
| Drug class | Efficacy by symptom domain |
|---|---|
| Atypical antipsychotics | Psychosis/thought disorder: Can reduce psychotic symptoms |
| Anxiety symptoms: Can reduce extreme anxieties | |
| Affect regulation: Improved by mood-stabilizing effect | |
| Socialization problems: Appear to modify affective aggression, hyperactivity, and impulsivity, which can improve socialization and pragmatic communication | |
| Mood stabilizers | Psychosis/thought disorder: Not primary area of effectiveness |
| Anxiety symptoms: May be helpful; not primary area of effectiveness | |
| Affect regulation: Address mood dysregulation | |
| Socialization problems: May reduce aggressive outbursts and mood, which can improve socialization | |
| Stimulants* | Psychosis/thought disorder: Can produce or intensify psychotic symptoms and agitation |
| Anxiety: Usually do not improve anxiety; can intensify anxiety and agitation | |
| Affect regulation: Not a primary effect in severe cases; address impulsive aggression via mood stabilization | |
| Socialization problems: Can improve functioning via decreased impulsivity, inattention, and aggression | |
| SSRI antidepressants† | Psychosis/thought disorder: Do not directly address |
| Anxiety: Can be effective in decreasing anxiety | |
| Affect regulation: Can improve depressed mood | |
| Socialization problems: Can be improved as a result of improved mood and decreased anxiety | |
| * Stimulants often increase agitation and disinhibition. | |
| † Watch for behavioral disinhibition, possible increase in suicidality, with selective serotonin reuptake inhibitors (SSRIs). | |
A skilled psychologist or speech pathologist can help you determine the presence or absence of cognitive and language dysfunction and learning disabilities. Even before we interview the parents and child, we ask parents and teachers to rate the child’s attention, behavior, mood, PDD-like symptoms, and anxiety, using the Child/Adolescent Symptom Inventory (see Related resources). We use the youth version with children age 10 and older, then review the symptoms with the parents and child to make sure we understand all presenting comorbidities.
TREATMENT
Nonmedical interventions begin with an accurate diagnosis, where possible. Then the four steps of treatment are to:
- address each domain of dysfunction
- translate findings to parent, child, and teachers/school.
- provide settings and resources that allow the child to work most effectively
- develop a behavioral program for the most frequent problems, with consistent response by caretakers and educators.
A communication specialist interested in pragmatics is needed to make sure the child is understood and being understood in the classroom. Table 3, summarizes communications skills the child needs to learn. An educational specialist who serves a resource to other professionals may also help the child. Curriculum should be based on long-term goals rather than on inflexible credit schedules that teach worthless, unlearnable information and demoralize the student.
Finally, the education setting should provide opportunities for structured social interaction and less-structured but supervised—”bully-proofed”—interactions.
Medications. No systematic medical treatment data exist, as there is no way to classify these children. They are usually treated with multiple medications for their specific symptom cluster abnormalities (Table 4). Options include:
- atypical antipsychotics such as risperidone, quetiapine, aripiprazole, ziprasidone, or olanzapine
- mood stabilizers such as valproic acid, lithium, or lamotrigine
- stimulants such as methylphenidate, amphetamine salts, atomoxetine, or bupropion (a mild stimulant and an antidepressant)
- selective serotonin reuptake inhibitors, such as fluoxetine, sertraline, citalopram, paroxetine, or fluvoxamine.
Medication side effects understandably frighten parents—who may be reluctant to have their children use any drug therapies. Counsel the parents in advance that side effects may occur.
- Child/Adolescent Symptom Inventory. http://www.checkmateplus.com. Accessed Jan. 11, 2005.
- Amphetamine • Adderall
- Aripiprazole • Abilify
- Atomoxetine • Strattera
- Bupropion • Wellbutrin
- Citalopram • Celexa
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Lamotrigine • Lamictal
- Lithium carbonate • Lithobid, others
- Methylphenidate • Concerta, Ritalin
- Olanzapine • Zyprexa
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Valproic acid • Depakote
- Ziprasidone • Geodon
Dr. Weisbrot receives grants from Pfizer Inc.
Dr. Carlson receives grants from or is a speaker for Janssen Pharmaceutica, Eli Lilly and Co., Shire Pharmaceuticals Groups, and Abbott Laboratories; is a consultant to Janssen Pharmaceutica and Eli Lilly and Co.; and is an advisor to Otsuka America Pharmaceutical, Pfizer Inc., and Ortho-McNeil Pharmaceutical.
1. Meijer M, Treffers P. Borderline and schizotypal disorders in children and adolescents. Br J Psychiatry 1991;158:205-12.
2. Petti TA, Vela RM. Borderline disorders of childhood: an overview. J Am Acad Child Adolesc Psychiatry 1990;29:327-37.
3. Wolff S. Loners: the life path of unusual children. London: Routledge, 1995.
4. Kestenbaum C. The borderline child at risk for major psychiatric disorder in adult life: seven case reports with followup. In: Robson KS (ed). The borderline child. New York: McGraw-Hill, 1983;49-82.
5. Lofgren DP, Bemporad J, King J, et al. A prospective follow-up study of so-called borderline children. Am J Psychiatry 1991;148:1541-7.
6. Nicolson R, Lenane M, Brookner F, et al. Children and adolescents with psychotic disorder not otherwise specified: a 2-to-8 year follow-up study. Compr Psychiatry 2001;42:319-25.
7. Towbin KE, Dykens EM, Pearson GS, Cohen DA. Conceptualizing “borderline syndrome of childhood” and “childhood schizophrenia” as a developmental disorder. J Am Acad Child Adolesc Psychiatry 1993;32(4):775-82.
8. Buitelaar JK, van der Gaag RJ. Diagnostic rules for children with PDD-NOS and multiple complex developmental disorder. J Child Psychol Psychiatry 1998;39(6):911-19.
9. Van der Gaag RJ, Buitelaar J, Van den Ban E, et al. A controlled multivariate chart review of multiple complex developmental disorder. J Am Acad Child Adolesc Psychiatry 1995;34(8):1096-106.
10. McKenna K, Gordon C, Lenane M, et al. Looking for childhood-onset schizophrenia: the first 71 cases screened. J Am Acad Child Adolesc Psychiatry 1994;33:636-44.
11. Kumra S, Jacobsen L, Lenane M, et al. “Multidimensionally impaired disorder”: is it a variant of very early-onset schizophrenia? J Am Acad Child Adolesc Psychiatry 1998;37(1):91-99.
12. Nagy J, Satzmari P. A chart review of schizotypal personality disorders in children. J Autism Dev Disord 1986;16(3):351-67.
13. Carlson GA. Unpublished data.
14. Greene R. The explosive child: a new approach for understanding and parenting easily frustrated, chronically inflexible children (2nd ed). New York: Harper Collins, 2001.
15. Weisbrot DM, Gadow KD, DeVincent CJ, et al. The presentation of anxiety in children with pervasive developmental disorders. J Child Adolesc Psychopharmacol 2005 (in press).
16. Garralda ME. Hallucinations in children with conduct and emotional disorders: the clinical phenomena. Psychol Med 1984;14:589-96.
17. Ulloa RE, Birmaher B, Axelson D, et al. Psychosis in a pediatric mood and anxiety disorders clinic: phenomenology and correlates. J Am Acad Child Adolesc Psychiatry 2000;39(3):337-45.
18. Schreier HA. Hallucinations in nonpsychotic children: more common than we think? J Am Acad Child Adolesc Psychiatry 2000;38(5):623-625.
19. Carlson GA, Mick E. Drug-induced disinhibition in psychiatrically hospitalized children. J Child Adolesc Psychopharmacol 2003;13(2):153-63.
1. Meijer M, Treffers P. Borderline and schizotypal disorders in children and adolescents. Br J Psychiatry 1991;158:205-12.
2. Petti TA, Vela RM. Borderline disorders of childhood: an overview. J Am Acad Child Adolesc Psychiatry 1990;29:327-37.
3. Wolff S. Loners: the life path of unusual children. London: Routledge, 1995.
4. Kestenbaum C. The borderline child at risk for major psychiatric disorder in adult life: seven case reports with followup. In: Robson KS (ed). The borderline child. New York: McGraw-Hill, 1983;49-82.
5. Lofgren DP, Bemporad J, King J, et al. A prospective follow-up study of so-called borderline children. Am J Psychiatry 1991;148:1541-7.
6. Nicolson R, Lenane M, Brookner F, et al. Children and adolescents with psychotic disorder not otherwise specified: a 2-to-8 year follow-up study. Compr Psychiatry 2001;42:319-25.
7. Towbin KE, Dykens EM, Pearson GS, Cohen DA. Conceptualizing “borderline syndrome of childhood” and “childhood schizophrenia” as a developmental disorder. J Am Acad Child Adolesc Psychiatry 1993;32(4):775-82.
8. Buitelaar JK, van der Gaag RJ. Diagnostic rules for children with PDD-NOS and multiple complex developmental disorder. J Child Psychol Psychiatry 1998;39(6):911-19.
9. Van der Gaag RJ, Buitelaar J, Van den Ban E, et al. A controlled multivariate chart review of multiple complex developmental disorder. J Am Acad Child Adolesc Psychiatry 1995;34(8):1096-106.
10. McKenna K, Gordon C, Lenane M, et al. Looking for childhood-onset schizophrenia: the first 71 cases screened. J Am Acad Child Adolesc Psychiatry 1994;33:636-44.
11. Kumra S, Jacobsen L, Lenane M, et al. “Multidimensionally impaired disorder”: is it a variant of very early-onset schizophrenia? J Am Acad Child Adolesc Psychiatry 1998;37(1):91-99.
12. Nagy J, Satzmari P. A chart review of schizotypal personality disorders in children. J Autism Dev Disord 1986;16(3):351-67.
13. Carlson GA. Unpublished data.
14. Greene R. The explosive child: a new approach for understanding and parenting easily frustrated, chronically inflexible children (2nd ed). New York: Harper Collins, 2001.
15. Weisbrot DM, Gadow KD, DeVincent CJ, et al. The presentation of anxiety in children with pervasive developmental disorders. J Child Adolesc Psychopharmacol 2005 (in press).
16. Garralda ME. Hallucinations in children with conduct and emotional disorders: the clinical phenomena. Psychol Med 1984;14:589-96.
17. Ulloa RE, Birmaher B, Axelson D, et al. Psychosis in a pediatric mood and anxiety disorders clinic: phenomenology and correlates. J Am Acad Child Adolesc Psychiatry 2000;39(3):337-45.
18. Schreier HA. Hallucinations in nonpsychotic children: more common than we think? J Am Acad Child Adolesc Psychiatry 2000;38(5):623-625.
19. Carlson GA, Mick E. Drug-induced disinhibition in psychiatrically hospitalized children. J Child Adolesc Psychopharmacol 2003;13(2):153-63.
Dependence risk with chronic dextromethorphan abuse
Habitual users of dextromethorphan can develop symptoms that meet DSM-IV criteria for substance dependence. A common ingredient in nonprescription cough syrups, dextromethorphan is considered nonaddictive but is far from benign in excessive dosages.
To illustrate the risks of dextromethorphan abuse, this article:
- presents the case of an adult with apparent dependence
- provides evidence of psychiatric and medical consequences of chronic excessive use of this cough remedy
- offers a glimpse at how dextromethorphan is described on the Internet, where information on its recreational use is readily available.1
Dextromethorphan acts on the brain’s cough center, the medulla oblongata, raising the cough reflex threshold. It is well-absorbed by the GI tract, metabolized in the liver by the cytochrome P-450 2D6 isoenzyme, and excreted in the urine unchanged or as a demethylated metabolite.2,3
Interaction between dextromethorphan and MAOIs resulting in serotonergic syndrome has been well-documented.4
Dextromethorphan has a 15- to 30-minute onset of action and peaks in 2.5 hours. Duration of action is 3 to 6 hours.5 Though dextromethorphan is an opiate analog, it is regarded as having no analgesic or addictive properties.6 When taken in therapeutic dosages—one-sixth to one-third ounce of medication containing 15 to 30 mg dextromethorphan—it is considered highly effective and safe,1 with no analgesic, euphoric, or dependency-producing properties.3
Dextromethorphan has a wide margin of safety. Doses 100 times the recommended amount have not been fatal,1 although overdose deaths have occurred.3
WHY DEXTROMETHORPHAN?
Dextromethorphan is a antitussive (cough suppressant) developed in the 1950s as a nonopioid alternative to codeine. Considered safe and effective at therapeutic dosages (Box 1),1-6 it can cause dissociation and psychotic effects in overdose.
Dextromethorphan is an attractive drug of abuse because it:
- produces the desired intoxicating effect
- is inexpensive—usually less than $5 a bottle
- is easy to purchase without prescription in >120 cough syrup preparations.7
- persons who abuse cough syrup say it tastes terrible
- the hallucinations and dissociation associated with dextromethorphan intoxication can be unpleasant, even frightening
- cough syrup is seen as a drug for “losers.”
CASE: 11 YEARS OF ‘ROBO-ING’
Mr. E, age 26, presented to our clinic for a court-ordered evaluation of substance abuse after his third drunken driving arrest. A college senior and father of three, he denied abusing nonprescription medications but volunteered that his alcohol consumption was “under control.” He said he continued to “drink on occasion,” including “less than three” glasses of wine the night of his arrest.
At the counselor’s recommendation, Mr. E underwent intensive outpatient counseling. He accepted that he had a genetic predisposition to addiction, gained insight into his alcohol abuse, and began a 12-step recovery program. The day he was to be discharged from treatment, however, Mr. E asked for a session with his counselor and revealed that he had been abusing “DXM” (dextromethorphan) in cough syrup for 11 years. He admitted drinking two 6- to 8-oz bottles of Robitussin-DM-brand cough syrup daily for the last 5 years, an activity he called “Robo-ing.”
He claimed to be a “highly revered teacher.” He said he “championed DXM use” and that “everyone looked up to” him because he had introduced “hundreds of people to the high.”
He had taught others to camouflage the cough syrup’s taste by chewing gum or gulping soft drinks. Maintaining a steady DXM level in the body “enhances” any other drug or alcohol use, he said. Mr. E described his DXM use fondly, though now with some fear.
Mr. E begged for help. Because of DXM use, his marriage was failing, he had been fired from his job, he was struggling to pay his legal fines, and he had spent time in jail. He feared he had damaged his brain and worried that his DXM use might have contributed to birth defects in two of his children.
Mr. E continued outpatient psychotherapy for 5 months to address his DXM use triggers—seeing cough syrup in stores, any alcohol use, and stress. He researched DXM addiction and was amazed to find no 12-step programs or information on DXM and birth defects.
We met with him 7 months after discharge. He reported that his marriage “has never been better,” and his children seemed to have no developmental delays. He was graduating from college and returning to his hometown to work.
Two years later, he is back in treatment for dextromethorphan abuse.
DEXTROMETHORPHAN DEPENDENCE
Mr. E believes he is dependent on dextromethorphan, and his behavior meets DSM-IV-TR criteria for dependence (Table):
- His persistent development of a culture of dextromethorphan use consumes much of his time.
- He neglects family and work responsibilities.
- He has tried repeatedly to cut down and stop his cough syrup use.
- His use continues despite marital, work, and legal consequences.
- He can tolerate daily dextromethorphan doses that would not be possible for the naive user.
- He experiences physical and psychological withdrawal when he stops using dextromethorphan.
DSM-IV-TR criteria for substance dependence
| A maladaptive pattern of substance use, leading to clinically significant impairment or distress, as manifested by three (or more) of the following, occurring at any time in the same 12-month period: |
|
| Source: Adapted and reprinted with permission from the Diagnostic and Statistical Manual of Mental Disorders (4th ed, text revision). Copyright 2000. American Psychiatric Association. |
- Cough syrup bottle in home’s medicine cabinet looks more empty than expected
- Child is using the Internet to learn about or attempt to purchase products containing dextromethorphan
- Cough syrups or other products containing dextromethorphan are found in child’s possession
- Child denies using common street drugs or alcohol but displays an unexplained altered state (confusion, ataxia, dizziness, euphoria, or slowed mental processing) or nausea, vomiting, or dizziness (from dextromethorphan withdrawal)
- Child hangs out with peers in drug stores or supermarkets
- Using cough syrup to get high has become a fad in child’s school or peer group
WHO ABUSES ‘DXM’?
The prevalence of dextromethorphan abuse is unclear.8 Abuse has been reported in Sweden, Canada, Australia, Germany, and the United States.1 Sweden has allowed prescription-only sales as a deterrent to abuse since 1986.9
The literature and our experience in treating dextromethorphan abusers suggest that abuse often begins in late childhood or early adolescence but may continue as chronic behavior in adulthood.
Use by adolescents. Case reports note episodic or fad dextromethorphan use—usually by adolescents and young adults—that springs up in a region or within a group and then fades.8 An abuse epidemic by Utah adolescents in the 1980s led drug stores to voluntarily place dextromethorphan-containing products behind the pharmacy counter to monitor and deter purchases.10
In a study of 376 students in grades 4 through 12 in Albuquerque, New Mexico, many knew cough syrup could be used to “get high.” When shown a list of cough syrup brand names, they often could identify those containing dextromethorphan, including NyQuil, Robitussin-DM, and Vicks 44D. The rates at which the students knew these three common cough preparations could be abused were:
- 46%, 25%, and 16%, respectively, for high school students
- 20%, 10%, and 17% for middle schoolers.8
Use by adults. Substance abuse counselors at our agency all have worked with adults who use cough syrup for intoxication. Some adults say they use dextromethorphan to enjoy the high and others as an alternative to alcohol.
A ‘HIGH’ WITH 4 PLATEAUS
Many Internet sites carry information about dextromethorphan.11-13 Its altered state is called a plateau, and four plateaus have been described.
Lower plateaus are considered “recreational.”13 According to the National Institute on Drug Abuse (NIDA), users experience a sense of dissociation and distortion of time and space at doses of about 2 ounces of medication containing 15 to 30 mg of dextromethorphan.14
Users are said to try to attain plateaus 1 or 2 to enhance and enjoy their surroundings. They describe music as being richer, colors more intense, and conversation more meaningful. The experience is said to be similar to a marijuana high or alcohol intoxication.
Objects may appear disproportionately large or small. The normal rhythm of conversation may seem chopped into blocks of words, or words may echo. Users refer to this staccato or strobing quality of sound as “flanging.”13 The bodily experience has been described as dreamlike or as if standing on a wave.
The high at lower plateaus is generally described as a positive experience. However, some describe it as bizarre, weird, and disturbing. It can create panic, nausea, and vomiting.
Upper plateaus. Users consider plateaus 3 and 4 as less recreational and more “spiritual and introspective.”15 Substantial dissociation and hallucinations can occur with “heavy stoning”—which the NIDA defines as using 10 ounces or more of medication containing 15 to 30 mg of dextromethorphan.14 Web sites warn users not to try to attain the upper plateaus unless prepared to “sit it out” or be accompanied by a sober “sitter” to talk the user through a bad trip or get help if needed.13
RISK OF PSYCHOSIS AND DEATH
The upper plateaus of dextromethorphan abuse are described as “intense.” Users report hallucinations, time and space distortions, and out-of-body sensations. Some have reported contacting alien beings or spirits. Although some users report the higher plateaus as pleasant, others report them as “terrifying.”
Upper-level trips can result in panic attacks and psychosis. Case reports have documented dextromethorphan doses that resulted in emergency room visits for psychotic states. For example:
- an adult was treated in the emergency room for psychosis after consuming an estimated 711 mg of dextromethorphan from cough syrup16
- a 23-year-old was treated for agitation and hallucinations in an emergency room after consuming approximately 2,160 mg of dextromethorphan.17
Research is lacking on long-term effects of regular dextromethorphan use. One study reported birth defects in chick embryos exposed to dextromethorphan.18
Fatalities. Dextromethorphan-related deaths have been documented.19 Causes of death include respiratory arrest, seizure, aspiration, and drug-drug interactions.20 Because dextromethorphan is usually not taken in pure form, effects of other drugs in cough syrup—such as bromide or chlorpheniramine—may contribute to the risk of side effects or death.21
Related resources
- National Institute on Drug Abuse. Research Report Series. Hallucinogens and dissociative drugs. Accessed Jan. 4, 2005.
- LSD, PCP, ketamine, and dextromethorphan. Available at: http://www.nida.nih.gov/ResearchReports/hallucinogens/Hallucinogens.html.
- Nature and effects of dextromethorphan. Available at: http://www.drugabuse.gov/ResearchReports/hallucinogens/halluc4.html.
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Cranston JW, Yoast R. Abuse of dextromethorphan (letter). Arch Fam Med 1999;8:99-100.
2. Relling MV, Cherrie J, Schell MJ, et al. Lower prevalence of the debrisoquin oxidative poor metabolizers phenotype in American black and white subjects. Clin Pharmacol Ther 1991;50:308-13.
3. Bem JL, Peck R. Dextromethorphan: an overview of safety issues. Drug Saf 1992;7(3):190-9.
4. Harrison WM, McGarth PJ, Stewart JW, Quitkin F. MAOIs and hypertensive crisis: the role of OTC drugs. J Clin Psychiatry 1989;50:64-5.
5. Albertson TE. Dextromethorphan. In: Obon K (ed). Poisoning and drug overdose (3rd ed). Stanford, CT: Appleton and Lange, 1999;155-6.
6. Gilman AG, Rall TW, Nies AS, et al (eds). Goodman and Gilman’s the pharmacology basics of therapeutics (8th ed). New York: MacMillan, 1990;518.-
7. Helfer J, Oksuk MK. Psychoactive abuse potential of Robitussin-DM (letter). Am J Psychiatry 1990;147(5):672-3.
8. Noonan WC, Miller WR, Feeney DM. Dextromethorphan abuse among youth. Arch Fam Med 2000;9:791-2.
9. Rammer L, Holmgren P, Sandler H. Fatal intoxication by dextromethorphan: a report on two cases. Forensic Sci Int 1988;37:233-6.
10. McElwee NE, Veltri JC. Intentional abuse of dextromethorphan (DM) products: 1985 to 1988 statewide data (abstract). Vet Hum Toxicol 1990;32:355.-
11. White WE. DXM side effects and other things to avoid. Available at: http://www.erowid.org/chemicals/dxm/faq/dxm_side_effects.shtml. Accessed Jan. 4, 2005.
12. Brosien KG. Cough syrup: The over-the-counter underground drug of the 90s. Kent, OH: The Burr, 1997. Available at: http://www.burr.kent.edu/archives/1997/spring/cough/cough.html. Accessed Dec. 16, 2004.
13. White WE. Getting the most out of DXM. Available at: http://www.erowid.org/chemicals/dxm/faq/dxm_most.shtml. Accessed Jan. 4, 2005.
14. Nature and effects of dextromethorphan. National Institute on Drug Abuse. Research Report Series. Hallucinogens and dissociative drugs. Available at http://www.drugabuse.gov/ResearchReports/hallucinogens/halluc4.html. Accessed Jan. 4, 2005.
15. White W. Physiological effects of DXM. Available at: http://www.erowid.org/chemicals/dxm/faq/dxm_physiological.shtml. Accessed Dec. 16, 2004.
16. Price LH, Lebel J. Dextromethorphan-induced psychosis. Am J Psychiatry 2000;157(2):304.-
17. Wolfe TR, Caravati EM. Massive dextromethorphan ingestion and abuse. Am J Emerg Med 1995;13(2):174-6.
18. Andaloro VJ, Monaghan DT, Rosenquist TU. DXM and other methyl-D-aspartate receptor antagonists are teratogenic in the avian embryo model. Pediatr Res 1998;43(1):1-8.
19. Sederstrom J. Friley death attributed to cough medication. Iowa State Daily. Jan. 13, 2003.
20. Iowa State Poison Control Bulletin. Poisindex Management: Dextromethorphan summary of exposure. Sec. 0.2.1. Iowa City, IA: Iowa Statewide Poison Control Center, August 2002.
21. Coricidin (dextromethorphan + chlorpheniramine maleate) harm reduction Web site. Available at: http://www.coricidin.org/. Accessed Dec. 16, 2004.
Habitual users of dextromethorphan can develop symptoms that meet DSM-IV criteria for substance dependence. A common ingredient in nonprescription cough syrups, dextromethorphan is considered nonaddictive but is far from benign in excessive dosages.
To illustrate the risks of dextromethorphan abuse, this article:
- presents the case of an adult with apparent dependence
- provides evidence of psychiatric and medical consequences of chronic excessive use of this cough remedy
- offers a glimpse at how dextromethorphan is described on the Internet, where information on its recreational use is readily available.1
Dextromethorphan acts on the brain’s cough center, the medulla oblongata, raising the cough reflex threshold. It is well-absorbed by the GI tract, metabolized in the liver by the cytochrome P-450 2D6 isoenzyme, and excreted in the urine unchanged or as a demethylated metabolite.2,3
Interaction between dextromethorphan and MAOIs resulting in serotonergic syndrome has been well-documented.4
Dextromethorphan has a 15- to 30-minute onset of action and peaks in 2.5 hours. Duration of action is 3 to 6 hours.5 Though dextromethorphan is an opiate analog, it is regarded as having no analgesic or addictive properties.6 When taken in therapeutic dosages—one-sixth to one-third ounce of medication containing 15 to 30 mg dextromethorphan—it is considered highly effective and safe,1 with no analgesic, euphoric, or dependency-producing properties.3
Dextromethorphan has a wide margin of safety. Doses 100 times the recommended amount have not been fatal,1 although overdose deaths have occurred.3
WHY DEXTROMETHORPHAN?
Dextromethorphan is a antitussive (cough suppressant) developed in the 1950s as a nonopioid alternative to codeine. Considered safe and effective at therapeutic dosages (Box 1),1-6 it can cause dissociation and psychotic effects in overdose.
Dextromethorphan is an attractive drug of abuse because it:
- produces the desired intoxicating effect
- is inexpensive—usually less than $5 a bottle
- is easy to purchase without prescription in >120 cough syrup preparations.7
- persons who abuse cough syrup say it tastes terrible
- the hallucinations and dissociation associated with dextromethorphan intoxication can be unpleasant, even frightening
- cough syrup is seen as a drug for “losers.”
CASE: 11 YEARS OF ‘ROBO-ING’
Mr. E, age 26, presented to our clinic for a court-ordered evaluation of substance abuse after his third drunken driving arrest. A college senior and father of three, he denied abusing nonprescription medications but volunteered that his alcohol consumption was “under control.” He said he continued to “drink on occasion,” including “less than three” glasses of wine the night of his arrest.
At the counselor’s recommendation, Mr. E underwent intensive outpatient counseling. He accepted that he had a genetic predisposition to addiction, gained insight into his alcohol abuse, and began a 12-step recovery program. The day he was to be discharged from treatment, however, Mr. E asked for a session with his counselor and revealed that he had been abusing “DXM” (dextromethorphan) in cough syrup for 11 years. He admitted drinking two 6- to 8-oz bottles of Robitussin-DM-brand cough syrup daily for the last 5 years, an activity he called “Robo-ing.”
He claimed to be a “highly revered teacher.” He said he “championed DXM use” and that “everyone looked up to” him because he had introduced “hundreds of people to the high.”
He had taught others to camouflage the cough syrup’s taste by chewing gum or gulping soft drinks. Maintaining a steady DXM level in the body “enhances” any other drug or alcohol use, he said. Mr. E described his DXM use fondly, though now with some fear.
Mr. E begged for help. Because of DXM use, his marriage was failing, he had been fired from his job, he was struggling to pay his legal fines, and he had spent time in jail. He feared he had damaged his brain and worried that his DXM use might have contributed to birth defects in two of his children.
Mr. E continued outpatient psychotherapy for 5 months to address his DXM use triggers—seeing cough syrup in stores, any alcohol use, and stress. He researched DXM addiction and was amazed to find no 12-step programs or information on DXM and birth defects.
We met with him 7 months after discharge. He reported that his marriage “has never been better,” and his children seemed to have no developmental delays. He was graduating from college and returning to his hometown to work.
Two years later, he is back in treatment for dextromethorphan abuse.
DEXTROMETHORPHAN DEPENDENCE
Mr. E believes he is dependent on dextromethorphan, and his behavior meets DSM-IV-TR criteria for dependence (Table):
- His persistent development of a culture of dextromethorphan use consumes much of his time.
- He neglects family and work responsibilities.
- He has tried repeatedly to cut down and stop his cough syrup use.
- His use continues despite marital, work, and legal consequences.
- He can tolerate daily dextromethorphan doses that would not be possible for the naive user.
- He experiences physical and psychological withdrawal when he stops using dextromethorphan.
DSM-IV-TR criteria for substance dependence
| A maladaptive pattern of substance use, leading to clinically significant impairment or distress, as manifested by three (or more) of the following, occurring at any time in the same 12-month period: |
|
| Source: Adapted and reprinted with permission from the Diagnostic and Statistical Manual of Mental Disorders (4th ed, text revision). Copyright 2000. American Psychiatric Association. |
- Cough syrup bottle in home’s medicine cabinet looks more empty than expected
- Child is using the Internet to learn about or attempt to purchase products containing dextromethorphan
- Cough syrups or other products containing dextromethorphan are found in child’s possession
- Child denies using common street drugs or alcohol but displays an unexplained altered state (confusion, ataxia, dizziness, euphoria, or slowed mental processing) or nausea, vomiting, or dizziness (from dextromethorphan withdrawal)
- Child hangs out with peers in drug stores or supermarkets
- Using cough syrup to get high has become a fad in child’s school or peer group
WHO ABUSES ‘DXM’?
The prevalence of dextromethorphan abuse is unclear.8 Abuse has been reported in Sweden, Canada, Australia, Germany, and the United States.1 Sweden has allowed prescription-only sales as a deterrent to abuse since 1986.9
The literature and our experience in treating dextromethorphan abusers suggest that abuse often begins in late childhood or early adolescence but may continue as chronic behavior in adulthood.
Use by adolescents. Case reports note episodic or fad dextromethorphan use—usually by adolescents and young adults—that springs up in a region or within a group and then fades.8 An abuse epidemic by Utah adolescents in the 1980s led drug stores to voluntarily place dextromethorphan-containing products behind the pharmacy counter to monitor and deter purchases.10
In a study of 376 students in grades 4 through 12 in Albuquerque, New Mexico, many knew cough syrup could be used to “get high.” When shown a list of cough syrup brand names, they often could identify those containing dextromethorphan, including NyQuil, Robitussin-DM, and Vicks 44D. The rates at which the students knew these three common cough preparations could be abused were:
- 46%, 25%, and 16%, respectively, for high school students
- 20%, 10%, and 17% for middle schoolers.8
Use by adults. Substance abuse counselors at our agency all have worked with adults who use cough syrup for intoxication. Some adults say they use dextromethorphan to enjoy the high and others as an alternative to alcohol.
A ‘HIGH’ WITH 4 PLATEAUS
Many Internet sites carry information about dextromethorphan.11-13 Its altered state is called a plateau, and four plateaus have been described.
Lower plateaus are considered “recreational.”13 According to the National Institute on Drug Abuse (NIDA), users experience a sense of dissociation and distortion of time and space at doses of about 2 ounces of medication containing 15 to 30 mg of dextromethorphan.14
Users are said to try to attain plateaus 1 or 2 to enhance and enjoy their surroundings. They describe music as being richer, colors more intense, and conversation more meaningful. The experience is said to be similar to a marijuana high or alcohol intoxication.
Objects may appear disproportionately large or small. The normal rhythm of conversation may seem chopped into blocks of words, or words may echo. Users refer to this staccato or strobing quality of sound as “flanging.”13 The bodily experience has been described as dreamlike or as if standing on a wave.
The high at lower plateaus is generally described as a positive experience. However, some describe it as bizarre, weird, and disturbing. It can create panic, nausea, and vomiting.
Upper plateaus. Users consider plateaus 3 and 4 as less recreational and more “spiritual and introspective.”15 Substantial dissociation and hallucinations can occur with “heavy stoning”—which the NIDA defines as using 10 ounces or more of medication containing 15 to 30 mg of dextromethorphan.14 Web sites warn users not to try to attain the upper plateaus unless prepared to “sit it out” or be accompanied by a sober “sitter” to talk the user through a bad trip or get help if needed.13
RISK OF PSYCHOSIS AND DEATH
The upper plateaus of dextromethorphan abuse are described as “intense.” Users report hallucinations, time and space distortions, and out-of-body sensations. Some have reported contacting alien beings or spirits. Although some users report the higher plateaus as pleasant, others report them as “terrifying.”
Upper-level trips can result in panic attacks and psychosis. Case reports have documented dextromethorphan doses that resulted in emergency room visits for psychotic states. For example:
- an adult was treated in the emergency room for psychosis after consuming an estimated 711 mg of dextromethorphan from cough syrup16
- a 23-year-old was treated for agitation and hallucinations in an emergency room after consuming approximately 2,160 mg of dextromethorphan.17
Research is lacking on long-term effects of regular dextromethorphan use. One study reported birth defects in chick embryos exposed to dextromethorphan.18
Fatalities. Dextromethorphan-related deaths have been documented.19 Causes of death include respiratory arrest, seizure, aspiration, and drug-drug interactions.20 Because dextromethorphan is usually not taken in pure form, effects of other drugs in cough syrup—such as bromide or chlorpheniramine—may contribute to the risk of side effects or death.21
Related resources
- National Institute on Drug Abuse. Research Report Series. Hallucinogens and dissociative drugs. Accessed Jan. 4, 2005.
- LSD, PCP, ketamine, and dextromethorphan. Available at: http://www.nida.nih.gov/ResearchReports/hallucinogens/Hallucinogens.html.
- Nature and effects of dextromethorphan. Available at: http://www.drugabuse.gov/ResearchReports/hallucinogens/halluc4.html.
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Habitual users of dextromethorphan can develop symptoms that meet DSM-IV criteria for substance dependence. A common ingredient in nonprescription cough syrups, dextromethorphan is considered nonaddictive but is far from benign in excessive dosages.
To illustrate the risks of dextromethorphan abuse, this article:
- presents the case of an adult with apparent dependence
- provides evidence of psychiatric and medical consequences of chronic excessive use of this cough remedy
- offers a glimpse at how dextromethorphan is described on the Internet, where information on its recreational use is readily available.1
Dextromethorphan acts on the brain’s cough center, the medulla oblongata, raising the cough reflex threshold. It is well-absorbed by the GI tract, metabolized in the liver by the cytochrome P-450 2D6 isoenzyme, and excreted in the urine unchanged or as a demethylated metabolite.2,3
Interaction between dextromethorphan and MAOIs resulting in serotonergic syndrome has been well-documented.4
Dextromethorphan has a 15- to 30-minute onset of action and peaks in 2.5 hours. Duration of action is 3 to 6 hours.5 Though dextromethorphan is an opiate analog, it is regarded as having no analgesic or addictive properties.6 When taken in therapeutic dosages—one-sixth to one-third ounce of medication containing 15 to 30 mg dextromethorphan—it is considered highly effective and safe,1 with no analgesic, euphoric, or dependency-producing properties.3
Dextromethorphan has a wide margin of safety. Doses 100 times the recommended amount have not been fatal,1 although overdose deaths have occurred.3
WHY DEXTROMETHORPHAN?
Dextromethorphan is a antitussive (cough suppressant) developed in the 1950s as a nonopioid alternative to codeine. Considered safe and effective at therapeutic dosages (Box 1),1-6 it can cause dissociation and psychotic effects in overdose.
Dextromethorphan is an attractive drug of abuse because it:
- produces the desired intoxicating effect
- is inexpensive—usually less than $5 a bottle
- is easy to purchase without prescription in >120 cough syrup preparations.7
- persons who abuse cough syrup say it tastes terrible
- the hallucinations and dissociation associated with dextromethorphan intoxication can be unpleasant, even frightening
- cough syrup is seen as a drug for “losers.”
CASE: 11 YEARS OF ‘ROBO-ING’
Mr. E, age 26, presented to our clinic for a court-ordered evaluation of substance abuse after his third drunken driving arrest. A college senior and father of three, he denied abusing nonprescription medications but volunteered that his alcohol consumption was “under control.” He said he continued to “drink on occasion,” including “less than three” glasses of wine the night of his arrest.
At the counselor’s recommendation, Mr. E underwent intensive outpatient counseling. He accepted that he had a genetic predisposition to addiction, gained insight into his alcohol abuse, and began a 12-step recovery program. The day he was to be discharged from treatment, however, Mr. E asked for a session with his counselor and revealed that he had been abusing “DXM” (dextromethorphan) in cough syrup for 11 years. He admitted drinking two 6- to 8-oz bottles of Robitussin-DM-brand cough syrup daily for the last 5 years, an activity he called “Robo-ing.”
He claimed to be a “highly revered teacher.” He said he “championed DXM use” and that “everyone looked up to” him because he had introduced “hundreds of people to the high.”
He had taught others to camouflage the cough syrup’s taste by chewing gum or gulping soft drinks. Maintaining a steady DXM level in the body “enhances” any other drug or alcohol use, he said. Mr. E described his DXM use fondly, though now with some fear.
Mr. E begged for help. Because of DXM use, his marriage was failing, he had been fired from his job, he was struggling to pay his legal fines, and he had spent time in jail. He feared he had damaged his brain and worried that his DXM use might have contributed to birth defects in two of his children.
Mr. E continued outpatient psychotherapy for 5 months to address his DXM use triggers—seeing cough syrup in stores, any alcohol use, and stress. He researched DXM addiction and was amazed to find no 12-step programs or information on DXM and birth defects.
We met with him 7 months after discharge. He reported that his marriage “has never been better,” and his children seemed to have no developmental delays. He was graduating from college and returning to his hometown to work.
Two years later, he is back in treatment for dextromethorphan abuse.
DEXTROMETHORPHAN DEPENDENCE
Mr. E believes he is dependent on dextromethorphan, and his behavior meets DSM-IV-TR criteria for dependence (Table):
- His persistent development of a culture of dextromethorphan use consumes much of his time.
- He neglects family and work responsibilities.
- He has tried repeatedly to cut down and stop his cough syrup use.
- His use continues despite marital, work, and legal consequences.
- He can tolerate daily dextromethorphan doses that would not be possible for the naive user.
- He experiences physical and psychological withdrawal when he stops using dextromethorphan.
DSM-IV-TR criteria for substance dependence
| A maladaptive pattern of substance use, leading to clinically significant impairment or distress, as manifested by three (or more) of the following, occurring at any time in the same 12-month period: |
|
| Source: Adapted and reprinted with permission from the Diagnostic and Statistical Manual of Mental Disorders (4th ed, text revision). Copyright 2000. American Psychiatric Association. |
- Cough syrup bottle in home’s medicine cabinet looks more empty than expected
- Child is using the Internet to learn about or attempt to purchase products containing dextromethorphan
- Cough syrups or other products containing dextromethorphan are found in child’s possession
- Child denies using common street drugs or alcohol but displays an unexplained altered state (confusion, ataxia, dizziness, euphoria, or slowed mental processing) or nausea, vomiting, or dizziness (from dextromethorphan withdrawal)
- Child hangs out with peers in drug stores or supermarkets
- Using cough syrup to get high has become a fad in child’s school or peer group
WHO ABUSES ‘DXM’?
The prevalence of dextromethorphan abuse is unclear.8 Abuse has been reported in Sweden, Canada, Australia, Germany, and the United States.1 Sweden has allowed prescription-only sales as a deterrent to abuse since 1986.9
The literature and our experience in treating dextromethorphan abusers suggest that abuse often begins in late childhood or early adolescence but may continue as chronic behavior in adulthood.
Use by adolescents. Case reports note episodic or fad dextromethorphan use—usually by adolescents and young adults—that springs up in a region or within a group and then fades.8 An abuse epidemic by Utah adolescents in the 1980s led drug stores to voluntarily place dextromethorphan-containing products behind the pharmacy counter to monitor and deter purchases.10
In a study of 376 students in grades 4 through 12 in Albuquerque, New Mexico, many knew cough syrup could be used to “get high.” When shown a list of cough syrup brand names, they often could identify those containing dextromethorphan, including NyQuil, Robitussin-DM, and Vicks 44D. The rates at which the students knew these three common cough preparations could be abused were:
- 46%, 25%, and 16%, respectively, for high school students
- 20%, 10%, and 17% for middle schoolers.8
Use by adults. Substance abuse counselors at our agency all have worked with adults who use cough syrup for intoxication. Some adults say they use dextromethorphan to enjoy the high and others as an alternative to alcohol.
A ‘HIGH’ WITH 4 PLATEAUS
Many Internet sites carry information about dextromethorphan.11-13 Its altered state is called a plateau, and four plateaus have been described.
Lower plateaus are considered “recreational.”13 According to the National Institute on Drug Abuse (NIDA), users experience a sense of dissociation and distortion of time and space at doses of about 2 ounces of medication containing 15 to 30 mg of dextromethorphan.14
Users are said to try to attain plateaus 1 or 2 to enhance and enjoy their surroundings. They describe music as being richer, colors more intense, and conversation more meaningful. The experience is said to be similar to a marijuana high or alcohol intoxication.
Objects may appear disproportionately large or small. The normal rhythm of conversation may seem chopped into blocks of words, or words may echo. Users refer to this staccato or strobing quality of sound as “flanging.”13 The bodily experience has been described as dreamlike or as if standing on a wave.
The high at lower plateaus is generally described as a positive experience. However, some describe it as bizarre, weird, and disturbing. It can create panic, nausea, and vomiting.
Upper plateaus. Users consider plateaus 3 and 4 as less recreational and more “spiritual and introspective.”15 Substantial dissociation and hallucinations can occur with “heavy stoning”—which the NIDA defines as using 10 ounces or more of medication containing 15 to 30 mg of dextromethorphan.14 Web sites warn users not to try to attain the upper plateaus unless prepared to “sit it out” or be accompanied by a sober “sitter” to talk the user through a bad trip or get help if needed.13
RISK OF PSYCHOSIS AND DEATH
The upper plateaus of dextromethorphan abuse are described as “intense.” Users report hallucinations, time and space distortions, and out-of-body sensations. Some have reported contacting alien beings or spirits. Although some users report the higher plateaus as pleasant, others report them as “terrifying.”
Upper-level trips can result in panic attacks and psychosis. Case reports have documented dextromethorphan doses that resulted in emergency room visits for psychotic states. For example:
- an adult was treated in the emergency room for psychosis after consuming an estimated 711 mg of dextromethorphan from cough syrup16
- a 23-year-old was treated for agitation and hallucinations in an emergency room after consuming approximately 2,160 mg of dextromethorphan.17
Research is lacking on long-term effects of regular dextromethorphan use. One study reported birth defects in chick embryos exposed to dextromethorphan.18
Fatalities. Dextromethorphan-related deaths have been documented.19 Causes of death include respiratory arrest, seizure, aspiration, and drug-drug interactions.20 Because dextromethorphan is usually not taken in pure form, effects of other drugs in cough syrup—such as bromide or chlorpheniramine—may contribute to the risk of side effects or death.21
Related resources
- National Institute on Drug Abuse. Research Report Series. Hallucinogens and dissociative drugs. Accessed Jan. 4, 2005.
- LSD, PCP, ketamine, and dextromethorphan. Available at: http://www.nida.nih.gov/ResearchReports/hallucinogens/Hallucinogens.html.
- Nature and effects of dextromethorphan. Available at: http://www.drugabuse.gov/ResearchReports/hallucinogens/halluc4.html.
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Cranston JW, Yoast R. Abuse of dextromethorphan (letter). Arch Fam Med 1999;8:99-100.
2. Relling MV, Cherrie J, Schell MJ, et al. Lower prevalence of the debrisoquin oxidative poor metabolizers phenotype in American black and white subjects. Clin Pharmacol Ther 1991;50:308-13.
3. Bem JL, Peck R. Dextromethorphan: an overview of safety issues. Drug Saf 1992;7(3):190-9.
4. Harrison WM, McGarth PJ, Stewart JW, Quitkin F. MAOIs and hypertensive crisis: the role of OTC drugs. J Clin Psychiatry 1989;50:64-5.
5. Albertson TE. Dextromethorphan. In: Obon K (ed). Poisoning and drug overdose (3rd ed). Stanford, CT: Appleton and Lange, 1999;155-6.
6. Gilman AG, Rall TW, Nies AS, et al (eds). Goodman and Gilman’s the pharmacology basics of therapeutics (8th ed). New York: MacMillan, 1990;518.-
7. Helfer J, Oksuk MK. Psychoactive abuse potential of Robitussin-DM (letter). Am J Psychiatry 1990;147(5):672-3.
8. Noonan WC, Miller WR, Feeney DM. Dextromethorphan abuse among youth. Arch Fam Med 2000;9:791-2.
9. Rammer L, Holmgren P, Sandler H. Fatal intoxication by dextromethorphan: a report on two cases. Forensic Sci Int 1988;37:233-6.
10. McElwee NE, Veltri JC. Intentional abuse of dextromethorphan (DM) products: 1985 to 1988 statewide data (abstract). Vet Hum Toxicol 1990;32:355.-
11. White WE. DXM side effects and other things to avoid. Available at: http://www.erowid.org/chemicals/dxm/faq/dxm_side_effects.shtml. Accessed Jan. 4, 2005.
12. Brosien KG. Cough syrup: The over-the-counter underground drug of the 90s. Kent, OH: The Burr, 1997. Available at: http://www.burr.kent.edu/archives/1997/spring/cough/cough.html. Accessed Dec. 16, 2004.
13. White WE. Getting the most out of DXM. Available at: http://www.erowid.org/chemicals/dxm/faq/dxm_most.shtml. Accessed Jan. 4, 2005.
14. Nature and effects of dextromethorphan. National Institute on Drug Abuse. Research Report Series. Hallucinogens and dissociative drugs. Available at http://www.drugabuse.gov/ResearchReports/hallucinogens/halluc4.html. Accessed Jan. 4, 2005.
15. White W. Physiological effects of DXM. Available at: http://www.erowid.org/chemicals/dxm/faq/dxm_physiological.shtml. Accessed Dec. 16, 2004.
16. Price LH, Lebel J. Dextromethorphan-induced psychosis. Am J Psychiatry 2000;157(2):304.-
17. Wolfe TR, Caravati EM. Massive dextromethorphan ingestion and abuse. Am J Emerg Med 1995;13(2):174-6.
18. Andaloro VJ, Monaghan DT, Rosenquist TU. DXM and other methyl-D-aspartate receptor antagonists are teratogenic in the avian embryo model. Pediatr Res 1998;43(1):1-8.
19. Sederstrom J. Friley death attributed to cough medication. Iowa State Daily. Jan. 13, 2003.
20. Iowa State Poison Control Bulletin. Poisindex Management: Dextromethorphan summary of exposure. Sec. 0.2.1. Iowa City, IA: Iowa Statewide Poison Control Center, August 2002.
21. Coricidin (dextromethorphan + chlorpheniramine maleate) harm reduction Web site. Available at: http://www.coricidin.org/. Accessed Dec. 16, 2004.
1. Cranston JW, Yoast R. Abuse of dextromethorphan (letter). Arch Fam Med 1999;8:99-100.
2. Relling MV, Cherrie J, Schell MJ, et al. Lower prevalence of the debrisoquin oxidative poor metabolizers phenotype in American black and white subjects. Clin Pharmacol Ther 1991;50:308-13.
3. Bem JL, Peck R. Dextromethorphan: an overview of safety issues. Drug Saf 1992;7(3):190-9.
4. Harrison WM, McGarth PJ, Stewart JW, Quitkin F. MAOIs and hypertensive crisis: the role of OTC drugs. J Clin Psychiatry 1989;50:64-5.
5. Albertson TE. Dextromethorphan. In: Obon K (ed). Poisoning and drug overdose (3rd ed). Stanford, CT: Appleton and Lange, 1999;155-6.
6. Gilman AG, Rall TW, Nies AS, et al (eds). Goodman and Gilman’s the pharmacology basics of therapeutics (8th ed). New York: MacMillan, 1990;518.-
7. Helfer J, Oksuk MK. Psychoactive abuse potential of Robitussin-DM (letter). Am J Psychiatry 1990;147(5):672-3.
8. Noonan WC, Miller WR, Feeney DM. Dextromethorphan abuse among youth. Arch Fam Med 2000;9:791-2.
9. Rammer L, Holmgren P, Sandler H. Fatal intoxication by dextromethorphan: a report on two cases. Forensic Sci Int 1988;37:233-6.
10. McElwee NE, Veltri JC. Intentional abuse of dextromethorphan (DM) products: 1985 to 1988 statewide data (abstract). Vet Hum Toxicol 1990;32:355.-
11. White WE. DXM side effects and other things to avoid. Available at: http://www.erowid.org/chemicals/dxm/faq/dxm_side_effects.shtml. Accessed Jan. 4, 2005.
12. Brosien KG. Cough syrup: The over-the-counter underground drug of the 90s. Kent, OH: The Burr, 1997. Available at: http://www.burr.kent.edu/archives/1997/spring/cough/cough.html. Accessed Dec. 16, 2004.
13. White WE. Getting the most out of DXM. Available at: http://www.erowid.org/chemicals/dxm/faq/dxm_most.shtml. Accessed Jan. 4, 2005.
14. Nature and effects of dextromethorphan. National Institute on Drug Abuse. Research Report Series. Hallucinogens and dissociative drugs. Available at http://www.drugabuse.gov/ResearchReports/hallucinogens/halluc4.html. Accessed Jan. 4, 2005.
15. White W. Physiological effects of DXM. Available at: http://www.erowid.org/chemicals/dxm/faq/dxm_physiological.shtml. Accessed Dec. 16, 2004.
16. Price LH, Lebel J. Dextromethorphan-induced psychosis. Am J Psychiatry 2000;157(2):304.-
17. Wolfe TR, Caravati EM. Massive dextromethorphan ingestion and abuse. Am J Emerg Med 1995;13(2):174-6.
18. Andaloro VJ, Monaghan DT, Rosenquist TU. DXM and other methyl-D-aspartate receptor antagonists are teratogenic in the avian embryo model. Pediatr Res 1998;43(1):1-8.
19. Sederstrom J. Friley death attributed to cough medication. Iowa State Daily. Jan. 13, 2003.
20. Iowa State Poison Control Bulletin. Poisindex Management: Dextromethorphan summary of exposure. Sec. 0.2.1. Iowa City, IA: Iowa Statewide Poison Control Center, August 2002.
21. Coricidin (dextromethorphan + chlorpheniramine maleate) harm reduction Web site. Available at: http://www.coricidin.org/. Accessed Dec. 16, 2004.
Writing progress notes: 10 dos and don’ts
Progress notes must convey that the psychiatrist provided quality care and respected the patient’s condition and wishes. Knowing what information to include—and what to leave out—can help you and your colleagues avoid a malpractice judgment.
Follow these 10 dos and don’ts of writing progress notes:
1. Be concise. Document all necessary information but avoid extraneous details, such as in this example:
“Patient moved to Kansas at age 4. Her parents separated when she was 6 and they moved back to Chicago, then reunited and moved to Indiana, where father took a job as a shoe salesman. When he lost that job, they moved back to Chicago and divorced for good. Mother remarried a fireman, who was an alcoholic; they stayed together for 2 years until …”
Instead, simply write:
“Patient’s childhood was chaotic with many moves; her mother remarried x 3. No physical or sexual abuse …”
2. Include adequate details. Do not exclude information critical to explaining treatment decisions. Describe the symptoms the patient is reporting and the signs you see—or do not see.
This example offers insufficient detail:
“Patient’s parents told her that they just bought a new car. She recalled the first car they had gotten when she was little, and how that made her happy. She talked about the first car she owned. Plan: Add lithium …”
By contrast, the following example explicitly describes signs and symptoms. Also be sure to include a short explanation when changing, discontinuing, or adding a medication:
“Patient reports her mood is much improved. She cannot recall what made her feel so depressed last week. She is hyperverbal, talking rapidly, gesticulating as she talks—much more animated, as compared to psychomotor-retarded presentation of last week, when SSRI was started. Assess: Bipolar switch. Plan: Add lithium, 300 mg bid, and titrate.”
3. Be careful when describing treatment of a patient who is suicidal at presentation. Your notes must contain clear, well-reasoned explanations for:
- discontinuing suicide precautions
- not hospitalizing outpatients who express suicidal ideation.
If the patient attempts or commits suicide shortly after the visit, your progress note may be your best—and only—defense against a malpractice claim. This example offers no convincing argument that the patient will not attempt suicide:
“Patient reports that he feels better. He denies suicidal ideation. He thinks the antidepressant is working. Nursing notes indicate no problems. He would like to get dressed and take a walk outside …”
Instead, verbatim patient statements offer more-concrete proof that the patient wants to live:
“He said he is his family’s sole support and could never abandon them …”
“He said it would kill his mother if he took his own life …”
“She said suicide is against her religion …”
Simply writing “No evidence of suicidal/homicidal ideation” raises the question of whether you asked the patient if he or she has considered suicide or just looked for a sign indicating suicidality. Always ask and record the patient’s exact response.
4. Remember that other clinicians will view the chart to make decisions about your patient’s care. Consider this example:
“Patient just moved to this area and requests amitriptyline and chlorpromazine. The risks of combining these medications were explained to him, but he insisted, so will order.”
If another provider is to grant the patient’s request, more details are needed:
“Patient states that he has been on every antipsychotic and antidepressant on the market—including the newest drugs—over 20 years. He says nothing works for him except this combination. The potential anticholinergic and other severe adverse effects associated with this combination were explained to him, and his responses indicated that he clearly understands the risks. He states, ‘These are the only drugs that have kept me from hearing voices and being depressed and suicidal. I want to stay on this combination.’ ”
5. Write legibly. Many doctors are encouraged to write illegible notes as a defense against legal action. The reasoning: the defendant can testify to anything since no one can read the notes anyway.
Illegible notes annoy and frustrate the people who cannot read them and inspire a lack of trust and confidence in the doctor who wrote them. And they are not likely to fool a jury.
6. Respect patient privacy. Do not name or quote anyone who is not essential to the record. Identifying another patient by name or Social Security number—even the last 4 digits—is a breach of privacy. For example:
“Charlene claimed R2803 followed her into the rest room and raped her…”
Did patient R2803 actually do this? What if Charlene’s psychosis prompted her to make delusional claims about other patients and staff? If her case ends up in court, patient R2803 is named in connection with an unproven allegation. Naming R2803 in Charlene’s chart identifies him as a psychiatric patient at that facility, thus violating his privacy.
If your patient makes accusations toward another patient, describe the alleged encounter this way:
“Charlene was upset over an interaction she described with another patient. Staff allowed her time to ventilate, and (name/dose of sedative) was given. The incident was addressed with the other patient’s treatment team and staff … ”
7. Do not include complaints about other staff members, whether from the patient, staff, or a doctor.
Let’s say a resident pages his backup attending but receives no answer. Entering in the patient’s chart that “Dr. Smith was paged but did not answer” gives the impression that Dr. Smith is ignoring calls, when in fact any of the following may be true:
- the resident does not realize Dr. Smith traded on-call duty with another doctor
- the batteries in Dr. Smith’s pager died
- or Dr. Smith was home, available by telephone, with his pager tucked away in his briefcase.
If the doctor on call cannot be reached, call another doctor—a supervisor or department head—and document your conversation with him or her. Do not identify the doctor who was not available.
Supervisors should address doctor availability issues the following day. Such issues do not belong in a patient’s chart.
8. Document responses to and from other providers. When consulting another doctor for advice, describe the encounter and identify the doctor by name. For example:
“Dr. Mark Jones advised me to accommodate the patient’s request for discharge, because he has known the patient for many years and feels it is safe for the patient to come to see him at the clinic in the morning.”
9. When disregarding a consultant’s advice, clearly explain why. For example:
“Neurology consultant recommended stopping patient’s antipsychotic due to risk of tardive dyskinesia. This patient, however, has been on numerous antipsychotics over the years, and this is the only one that controls his schizophrenia. Patient is aware of the risk of tardive dyskinesia and does not find it problematic. Patient is competent and understands the need to weigh potential side effects against the medication’s benefits, and he prefers to continue the medication.”
10. Never enter derogatory or pejorative statements about a patient. As psychiatrists, we must convey a sense of concern and respect for the patient, regardless of diagnosis and presentation.
Rather than entering, “This patient is obviously lying about his history,” instead write, “This patient’s version of his history is at odds with that in previous hospital records.”
Related resources
- Selden BS, Schnitzer PG, Nolan FX. Medicolegal documentation of prehospital triage. Ann Emerg Med 1990;19:547-51.
- Bjorck JP, Brown J, Goodman M. Casebook for managing managed care: a self-study guide for treatment planning, documentation, and communication (1st ed). Washington, DC: American Psychiatric Association, 2000.
- Wiger DE. The clinical documentation sourcebook: a comprehensive collection of mental health practice forms, handouts, and records (2nd ed). New York: John Wiley &Sons, 1999.
Drug brand names
- Amitriptyline • Elavil
- Chlorpromazine • Thorazine
- Lithium • Eskalith, others
Progress notes must convey that the psychiatrist provided quality care and respected the patient’s condition and wishes. Knowing what information to include—and what to leave out—can help you and your colleagues avoid a malpractice judgment.
Follow these 10 dos and don’ts of writing progress notes:
1. Be concise. Document all necessary information but avoid extraneous details, such as in this example:
“Patient moved to Kansas at age 4. Her parents separated when she was 6 and they moved back to Chicago, then reunited and moved to Indiana, where father took a job as a shoe salesman. When he lost that job, they moved back to Chicago and divorced for good. Mother remarried a fireman, who was an alcoholic; they stayed together for 2 years until …”
Instead, simply write:
“Patient’s childhood was chaotic with many moves; her mother remarried x 3. No physical or sexual abuse …”
2. Include adequate details. Do not exclude information critical to explaining treatment decisions. Describe the symptoms the patient is reporting and the signs you see—or do not see.
This example offers insufficient detail:
“Patient’s parents told her that they just bought a new car. She recalled the first car they had gotten when she was little, and how that made her happy. She talked about the first car she owned. Plan: Add lithium …”
By contrast, the following example explicitly describes signs and symptoms. Also be sure to include a short explanation when changing, discontinuing, or adding a medication:
“Patient reports her mood is much improved. She cannot recall what made her feel so depressed last week. She is hyperverbal, talking rapidly, gesticulating as she talks—much more animated, as compared to psychomotor-retarded presentation of last week, when SSRI was started. Assess: Bipolar switch. Plan: Add lithium, 300 mg bid, and titrate.”
3. Be careful when describing treatment of a patient who is suicidal at presentation. Your notes must contain clear, well-reasoned explanations for:
- discontinuing suicide precautions
- not hospitalizing outpatients who express suicidal ideation.
If the patient attempts or commits suicide shortly after the visit, your progress note may be your best—and only—defense against a malpractice claim. This example offers no convincing argument that the patient will not attempt suicide:
“Patient reports that he feels better. He denies suicidal ideation. He thinks the antidepressant is working. Nursing notes indicate no problems. He would like to get dressed and take a walk outside …”
Instead, verbatim patient statements offer more-concrete proof that the patient wants to live:
“He said he is his family’s sole support and could never abandon them …”
“He said it would kill his mother if he took his own life …”
“She said suicide is against her religion …”
Simply writing “No evidence of suicidal/homicidal ideation” raises the question of whether you asked the patient if he or she has considered suicide or just looked for a sign indicating suicidality. Always ask and record the patient’s exact response.
4. Remember that other clinicians will view the chart to make decisions about your patient’s care. Consider this example:
“Patient just moved to this area and requests amitriptyline and chlorpromazine. The risks of combining these medications were explained to him, but he insisted, so will order.”
If another provider is to grant the patient’s request, more details are needed:
“Patient states that he has been on every antipsychotic and antidepressant on the market—including the newest drugs—over 20 years. He says nothing works for him except this combination. The potential anticholinergic and other severe adverse effects associated with this combination were explained to him, and his responses indicated that he clearly understands the risks. He states, ‘These are the only drugs that have kept me from hearing voices and being depressed and suicidal. I want to stay on this combination.’ ”
5. Write legibly. Many doctors are encouraged to write illegible notes as a defense against legal action. The reasoning: the defendant can testify to anything since no one can read the notes anyway.
Illegible notes annoy and frustrate the people who cannot read them and inspire a lack of trust and confidence in the doctor who wrote them. And they are not likely to fool a jury.
6. Respect patient privacy. Do not name or quote anyone who is not essential to the record. Identifying another patient by name or Social Security number—even the last 4 digits—is a breach of privacy. For example:
“Charlene claimed R2803 followed her into the rest room and raped her…”
Did patient R2803 actually do this? What if Charlene’s psychosis prompted her to make delusional claims about other patients and staff? If her case ends up in court, patient R2803 is named in connection with an unproven allegation. Naming R2803 in Charlene’s chart identifies him as a psychiatric patient at that facility, thus violating his privacy.
If your patient makes accusations toward another patient, describe the alleged encounter this way:
“Charlene was upset over an interaction she described with another patient. Staff allowed her time to ventilate, and (name/dose of sedative) was given. The incident was addressed with the other patient’s treatment team and staff … ”
7. Do not include complaints about other staff members, whether from the patient, staff, or a doctor.
Let’s say a resident pages his backup attending but receives no answer. Entering in the patient’s chart that “Dr. Smith was paged but did not answer” gives the impression that Dr. Smith is ignoring calls, when in fact any of the following may be true:
- the resident does not realize Dr. Smith traded on-call duty with another doctor
- the batteries in Dr. Smith’s pager died
- or Dr. Smith was home, available by telephone, with his pager tucked away in his briefcase.
If the doctor on call cannot be reached, call another doctor—a supervisor or department head—and document your conversation with him or her. Do not identify the doctor who was not available.
Supervisors should address doctor availability issues the following day. Such issues do not belong in a patient’s chart.
8. Document responses to and from other providers. When consulting another doctor for advice, describe the encounter and identify the doctor by name. For example:
“Dr. Mark Jones advised me to accommodate the patient’s request for discharge, because he has known the patient for many years and feels it is safe for the patient to come to see him at the clinic in the morning.”
9. When disregarding a consultant’s advice, clearly explain why. For example:
“Neurology consultant recommended stopping patient’s antipsychotic due to risk of tardive dyskinesia. This patient, however, has been on numerous antipsychotics over the years, and this is the only one that controls his schizophrenia. Patient is aware of the risk of tardive dyskinesia and does not find it problematic. Patient is competent and understands the need to weigh potential side effects against the medication’s benefits, and he prefers to continue the medication.”
10. Never enter derogatory or pejorative statements about a patient. As psychiatrists, we must convey a sense of concern and respect for the patient, regardless of diagnosis and presentation.
Rather than entering, “This patient is obviously lying about his history,” instead write, “This patient’s version of his history is at odds with that in previous hospital records.”
Related resources
- Selden BS, Schnitzer PG, Nolan FX. Medicolegal documentation of prehospital triage. Ann Emerg Med 1990;19:547-51.
- Bjorck JP, Brown J, Goodman M. Casebook for managing managed care: a self-study guide for treatment planning, documentation, and communication (1st ed). Washington, DC: American Psychiatric Association, 2000.
- Wiger DE. The clinical documentation sourcebook: a comprehensive collection of mental health practice forms, handouts, and records (2nd ed). New York: John Wiley &Sons, 1999.
Drug brand names
- Amitriptyline • Elavil
- Chlorpromazine • Thorazine
- Lithium • Eskalith, others
Progress notes must convey that the psychiatrist provided quality care and respected the patient’s condition and wishes. Knowing what information to include—and what to leave out—can help you and your colleagues avoid a malpractice judgment.
Follow these 10 dos and don’ts of writing progress notes:
1. Be concise. Document all necessary information but avoid extraneous details, such as in this example:
“Patient moved to Kansas at age 4. Her parents separated when she was 6 and they moved back to Chicago, then reunited and moved to Indiana, where father took a job as a shoe salesman. When he lost that job, they moved back to Chicago and divorced for good. Mother remarried a fireman, who was an alcoholic; they stayed together for 2 years until …”
Instead, simply write:
“Patient’s childhood was chaotic with many moves; her mother remarried x 3. No physical or sexual abuse …”
2. Include adequate details. Do not exclude information critical to explaining treatment decisions. Describe the symptoms the patient is reporting and the signs you see—or do not see.
This example offers insufficient detail:
“Patient’s parents told her that they just bought a new car. She recalled the first car they had gotten when she was little, and how that made her happy. She talked about the first car she owned. Plan: Add lithium …”
By contrast, the following example explicitly describes signs and symptoms. Also be sure to include a short explanation when changing, discontinuing, or adding a medication:
“Patient reports her mood is much improved. She cannot recall what made her feel so depressed last week. She is hyperverbal, talking rapidly, gesticulating as she talks—much more animated, as compared to psychomotor-retarded presentation of last week, when SSRI was started. Assess: Bipolar switch. Plan: Add lithium, 300 mg bid, and titrate.”
3. Be careful when describing treatment of a patient who is suicidal at presentation. Your notes must contain clear, well-reasoned explanations for:
- discontinuing suicide precautions
- not hospitalizing outpatients who express suicidal ideation.
If the patient attempts or commits suicide shortly after the visit, your progress note may be your best—and only—defense against a malpractice claim. This example offers no convincing argument that the patient will not attempt suicide:
“Patient reports that he feels better. He denies suicidal ideation. He thinks the antidepressant is working. Nursing notes indicate no problems. He would like to get dressed and take a walk outside …”
Instead, verbatim patient statements offer more-concrete proof that the patient wants to live:
“He said he is his family’s sole support and could never abandon them …”
“He said it would kill his mother if he took his own life …”
“She said suicide is against her religion …”
Simply writing “No evidence of suicidal/homicidal ideation” raises the question of whether you asked the patient if he or she has considered suicide or just looked for a sign indicating suicidality. Always ask and record the patient’s exact response.
4. Remember that other clinicians will view the chart to make decisions about your patient’s care. Consider this example:
“Patient just moved to this area and requests amitriptyline and chlorpromazine. The risks of combining these medications were explained to him, but he insisted, so will order.”
If another provider is to grant the patient’s request, more details are needed:
“Patient states that he has been on every antipsychotic and antidepressant on the market—including the newest drugs—over 20 years. He says nothing works for him except this combination. The potential anticholinergic and other severe adverse effects associated with this combination were explained to him, and his responses indicated that he clearly understands the risks. He states, ‘These are the only drugs that have kept me from hearing voices and being depressed and suicidal. I want to stay on this combination.’ ”
5. Write legibly. Many doctors are encouraged to write illegible notes as a defense against legal action. The reasoning: the defendant can testify to anything since no one can read the notes anyway.
Illegible notes annoy and frustrate the people who cannot read them and inspire a lack of trust and confidence in the doctor who wrote them. And they are not likely to fool a jury.
6. Respect patient privacy. Do not name or quote anyone who is not essential to the record. Identifying another patient by name or Social Security number—even the last 4 digits—is a breach of privacy. For example:
“Charlene claimed R2803 followed her into the rest room and raped her…”
Did patient R2803 actually do this? What if Charlene’s psychosis prompted her to make delusional claims about other patients and staff? If her case ends up in court, patient R2803 is named in connection with an unproven allegation. Naming R2803 in Charlene’s chart identifies him as a psychiatric patient at that facility, thus violating his privacy.
If your patient makes accusations toward another patient, describe the alleged encounter this way:
“Charlene was upset over an interaction she described with another patient. Staff allowed her time to ventilate, and (name/dose of sedative) was given. The incident was addressed with the other patient’s treatment team and staff … ”
7. Do not include complaints about other staff members, whether from the patient, staff, or a doctor.
Let’s say a resident pages his backup attending but receives no answer. Entering in the patient’s chart that “Dr. Smith was paged but did not answer” gives the impression that Dr. Smith is ignoring calls, when in fact any of the following may be true:
- the resident does not realize Dr. Smith traded on-call duty with another doctor
- the batteries in Dr. Smith’s pager died
- or Dr. Smith was home, available by telephone, with his pager tucked away in his briefcase.
If the doctor on call cannot be reached, call another doctor—a supervisor or department head—and document your conversation with him or her. Do not identify the doctor who was not available.
Supervisors should address doctor availability issues the following day. Such issues do not belong in a patient’s chart.
8. Document responses to and from other providers. When consulting another doctor for advice, describe the encounter and identify the doctor by name. For example:
“Dr. Mark Jones advised me to accommodate the patient’s request for discharge, because he has known the patient for many years and feels it is safe for the patient to come to see him at the clinic in the morning.”
9. When disregarding a consultant’s advice, clearly explain why. For example:
“Neurology consultant recommended stopping patient’s antipsychotic due to risk of tardive dyskinesia. This patient, however, has been on numerous antipsychotics over the years, and this is the only one that controls his schizophrenia. Patient is aware of the risk of tardive dyskinesia and does not find it problematic. Patient is competent and understands the need to weigh potential side effects against the medication’s benefits, and he prefers to continue the medication.”
10. Never enter derogatory or pejorative statements about a patient. As psychiatrists, we must convey a sense of concern and respect for the patient, regardless of diagnosis and presentation.
Rather than entering, “This patient is obviously lying about his history,” instead write, “This patient’s version of his history is at odds with that in previous hospital records.”
Related resources
- Selden BS, Schnitzer PG, Nolan FX. Medicolegal documentation of prehospital triage. Ann Emerg Med 1990;19:547-51.
- Bjorck JP, Brown J, Goodman M. Casebook for managing managed care: a self-study guide for treatment planning, documentation, and communication (1st ed). Washington, DC: American Psychiatric Association, 2000.
- Wiger DE. The clinical documentation sourcebook: a comprehensive collection of mental health practice forms, handouts, and records (2nd ed). New York: John Wiley &Sons, 1999.
Drug brand names
- Amitriptyline • Elavil
- Chlorpromazine • Thorazine
- Lithium • Eskalith, others
How—and why—to help psychiatric patients stop smoking
Three myths about cigarette smoking may explain why psychiatrists rarely intervene in their patients’ tobacco dependence:
- Cigarette smoking is an incurable habit in psychiatric patients and thus not worth the effort of intervening.
- Cigarette smoking is an acceptable form of self-medication in persons with psychiatric illness.
- Quitting smoking will worsen psychiatric symptoms.
Smoking by psychiatric patients is treatable, however, and evidence proves that many can quit.1 This article rebuts the “why-bother?” myths and provides practical tips on how to more effectively help psychiatric patients stop smoking.
DEBUNKING THREE MYTHS
Mentally ill women and men consume nearly one-half (44%) of the cigarettes smoked in the United States (Table 1)1-3 and thus are at high risk for tobacco-related premature death, cancer, cardiovascular disease, and respiratory disorders. Although recognized as a leading cause of death, cigarette smoking by psychiatric patients frequently goes unaddressed, contributing to excess mortality in this population.4
Table 1
Cigarette smoking: An epidemic among psychiatric patients
|
| Source: References 1-3 |
- psychiatrists seldom (6,7
- when counseling did occur, nicotine replacement therapy was not prescribed.6
Tobacco dependence is a syndrome with strong genetic and biologic roots. Family, twin, and adoption studies show consistently that tobacco dependence is genetically mediated.8 Genetic polymorphisms are being identified that may modify an individual’s risk for developing nicotine dependence—such as the gene encoding the cytochrome P-450 2A6 isoenzyme (CYP 2A6) that metabolizes nicotine to cotinine.9 Disturbed nicotinic receptor functioning has been shown in persons with schizophrenia, mood disorders, anxiety disorders, and attention-deficit/hyperactivity disorders.3,10,11
Tobacco dependence is a chronic, relapsing condition that usually requires repeated intervention to motivate patients to try to quit and to help those who are willing to quit to succeed. Effective smoking cessation aids include:
- behavioral therapy (brief physician advice, problem-solving skills/skills training)
- pharmacologic therapy (nicotine replacement, sustained-release bupropion).12
Is smoking ‘self-medication’? Compelling evidence indicates that cholinergic mechanisms and nicotinic receptors (nAChRs) are involved in the pathophysiology of schizophrenia and other neuropsychiatric disorders.3,10 Nicotine administration appears to improve sensory-processing and cognitive deficits observed in schizophrenia.2,3 Moreover, the association between depression and smoking13 —and tobacco smoke’s monoamine oxidase-inhibiting and other psychoactive properties14 —have led some to posit that cigarette smoking may have antidepressant actions.10
For all these reasons, some authors have speculated that tobacco use may be a form of self-medication among the psychiatrically ill.3 The problem with this hypothesis, however, is that tobacco smoke is—at best—an untested and potentially lethal cognitive enhancer, antidepressant, or anxiolytic. Animal and human studies may find therapeutic effects of acute nicotine administration, but the cognitive effects of chronic tobacco smoking are not known.
Table 2
5 ‘A’s of brief clinical intervention for tobacco dependence
|
| Source: References 5 and 12 |
Adverse effects from quitting? Smokers with a history of major depressive disorder have been shown to be at risk to:
- develop another depressive episode after they quit smoking15
- experience more severe withdrawal symptoms during abstinence, compared with smokers with no history of depression.13,16
Scant data support the myth that smoking cessation worsens psychiatric symptoms. For example, in a review on tobacco dependence and schizophrenia, George et al2 concluded that the effects of smoking cessation on schizophrenia symptoms are not clear. Two smoking cessation trials in schizophrenic patients treated with nicotine patches found no significant changes in postcessation psychotic symptoms.17,18
Concerns that substance-abusing patients should not attempt to quit smoking during alcohol and other drug dependence treatment are also unsubstantiated. Rather than exacerbating drug addiction, smoking cessation has been found to improve addicts’ abstinence rates.19
USING AVAILABLE THERAPIES
Evidence is insufficient so far to show whether psychiatrically ill smokers would benefit more from specially tailored cessation treatments than from standard treatments, according to the 2000 U.S. Public Health Service clinical practice guide.12 Thus, while researchers try to resolve this issue, psychiatrists are left to use medications found to be effective in smokers overall.
Clinical vignette. Mr. J, age 45, has paranoid-type schizophrenia and has been smoking at least two packs of cigarettes daily for 25 years. He complains of a productive cough and expresses interest in quitting smoking when his psychiatrist raises this topic.
His persecutory delusions are well-controlled on olanzapine, 10 mg/d. He is adhering with his medications and participating in weekly group counseling that provides supportive therapy for patients with serious mental illness.
In this schizophrenic smoker who is willing to try to quit, the psychiatrist performed the first three of “5 ‘A’s” (Table 2) of brief clinical intervention for tobacco dependence.5,12 The next steps are to assist the patient’s effort to quit and arrange follow-up.
When to quit. The best time for a smoker with psychiatric illness to try to quit is when he or she:
- is psychiatrically stable
- is not in crisis
- has no recent or planned psychiatric drug changes.
Smoking cessation may increase blood levels of these psychotropics
| Antipsychotics | Antidepressants | Mood stabilizers | Anxiolytics |
|---|---|---|---|
| Haloperidol | Clomipramine | Carbamazepine | Desmethyldiazepam |
| Chlorpromazine | Desipramine | Oxazepam | |
| Fluphenazine | Doxepin | ||
| Olanzapine | Imipramine | ||
| Clozapine | Nortriptyline | ||
| Source: References 2, 5, and 20 | |||
Olanzapine’s clearance is approximately 40% higher in smokers than in nonsmokers. The psychiatrist discussed this with Mr. J and:
- asked him to call if side effects develop during the quit attempt
- scheduled more-frequent appointments to monitor side effects.
Mr. J’s schizophrenia is stable on maintenance therapy with an atypical antipsychotic. Schizophrenic smokers taking atypicals may be more able to quit smoking with NRT or sustained-release bupropion, compared with those taking conventional antipsychotics.2
The psychiatrist also determined that Mr. J had tried to quit smoking three times. Two of these attempts were done “cold turkey,” without pharmacotherapy, and one involved using nicotine gum. Mr. J said that although the gum “worked well at first,” he stopped using it because it was expensive and made his mouth sore. This information helped the psychiatrist choose medication for this quit attempt.
Most smoking cessation guidelines rely on a stepped-care approach, progressing from minimal to more-intensive interventions as needed.5 Mr. J’s psychiatrist devised an intensive treatment plan because:
- Mr. J has tried to quit before
- schizophrenic patients generally have more difficulty quitting and are more nicotine-dependent than other smokers.
Table 4
Nicotine replacement and other options for smoking cessation
| Drug | Daily dosage | Treatment duration* | Common side effects |
|---|---|---|---|
| Nicotine replacement therapy† | |||
| Transdermal | Skin irritation, insomnia | ||
| 24-hr patch | Starting dose is 21 mg/d; also in 7- and 14-mg patches for tapering dosage | 8 wk | |
| 16-hr patch | 15 mg | 8 wk | |
| Polacrilex (gum) 2- or 4-mg piece | 1 piece/hr ( | 8 to 12 wk | Mouth irritation, sore jaw, dyspepsia, hiccups |
| Vapor inhaler | 6 to 16 cartridges/day (delivers 4/mg/cartridge) | 3 to 6 mo | Mouth and throat irritation, cough |
| Nasal spray | 1 to 2 doses/hr; dose = 1 mg (0.5 mg per nostril); maximum dosage 40 mg/d | 3 to 6 mo | Nasal irritation, sneezing, cough, tearing eyes |
| Lozenge | 2- or 4-mg dose; see dosage formula, titration schedule in over-the-counter package | 12 wk | Hiccups, nausea, heartburn |
| Non-nicotine replacement therapy | |||
| Sustained-release bupropion† | 150 mg/d for 3 days, then 150 mg bid; start 1 week before quit date | 7 to 12 wk; up to 6 mo. to maintain abstinence | Insomnia, dry mouth, agitation |
| Nortriptyline | 75 to 100 mg/d; start 10 to 28 days before quit date at 25 mg/d and increase as tolerated | 12 wk | Dry mouth, sedation, dizziness |
| Clonidine | 0.1 to 0.3 mg bid | 3 to 10 wk | Dry mouth, sedation, dizziness |
| * Treatment duration varies and may be longer in patients with psychiatric disorders. | |||
| † FDA-approved as a smoking cessation aid and recommended as a first-line drug by Public Health Service clinical guidelines. | |||
| Source: Adapted from reference 21. | |||
On the morning of his TQD, Mr. J is to apply the first 21-mg transdermal nicotine patch. He is told not to smoke that day and to apply a new patch daily. The psychiatrist also tells him he will most likely remain on that dosage for 4 weeks. Then the patch strength will be reduced in 7-mg aliquots every 2 to 4 weeks, depending on his progress. The psychiatrist also provides him with educational materials on how to quit successfully.
Follow-up. Recognizing that most relapses occur in the first few days of quitting, the psychiatrist sets Mr. J’s first follow-up appointment for the day after his TQD to assess:
- whether he has smoked and number of cigarettes smoked per day
- presence and severity of withdrawal symptoms
- onset of psychiatric symptoms
- treatment adherence
- how he is handling high-risk situations and urges to smoke
- medication side effects.6
- American Psychiatric Association. Practice guideline for the treatment of patients with nicotine dependence. Am J Psychiatry 1996; 53(153[suppl]): 1-31.
- Fiore MC, Bailey WC, Cohen SJ, et.al. Treating tobacco use and dependence. Clinical practice guideline. Rockville, MD: U.S. Public Health Service, 2000. http://www.ahcpr.gov/path/tobacco.htm. Accessed Dec. 13, 2004.
- Amantadine • Symmetrel
- Bupropion • Wellbutrin SR, Zyban
- Clonidine • Catapres
- Nicotine nasal spray • Nicotrol NS
- Nicotine polacrilex • Nicorette
- Nicotine replacement patch • Nicoderm CQ, Nicotrol, others
- Nicotine vapor inhaler • Nicotrol Inhaler
- Nortriptyline •Aventyl, Pamelor
Dr. Anthenelli receives grant/research support from Sanofi-Aventis and Ortho-McNeil Pharmaceuticals and is a consultant and speaker for Sanofi-Aventis.
Acknowledgments
The author would like to thank Reene Cantwell for technical assistance in preparing this manuscript. This work was supported by grants R01 AA13307 and R01 AA13957 from the National Institute on Alcohol Abuse and Alcoholism and by the Department of Veterans Affairs.
1. Lasser K, Boyd JW, Woolhandler S, et al. Smoking and mental illness: a population-based prevalence study. JAMA 2000;284(20):2606-10.
2. George TP, Vessicchio JC, Termine A. Nicotine and tobacco use in schizophrenia. In: Meyer JM, Nasrallah HR (eds). Medical illness and schizophrenia. Washington, DC: American Psychiatric Publishing, 2003:81-98.
3. Leonard S, Adler LE, Benhammou K, et al. Smoking and mental illness. Pharmacol Biochem Behav 2001;70(4):561-70.
4. Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry 2000;177:212-17.
5. American Psychiatric Association. Practice guideline for the treatment of patients with nicotine dependence. Am J Psychiatry 1996;53[153(suppl)]:1-31.
6. Himelhoch S, Daumit G. To whom do psychiatrists offer smoking-cessation counseling? Am J Psychiatry 2003;160(12):2228-30.
7. Thorndike AN, Stafford RS, Rigotti NA. US physicians’ treatment of smoking in outpatients with psychiatric diagnoses. Nicotine Tob Res 2001;3(1):85-91.
8. Lin SW, Anthenelli RM. Genetic factors in the risk for substance use disorders. In: Lowinson J, Ruiz P, Millman RB, Langrod JC (eds). Substance abuse: a comprehensive textbook (4th ed). Philadelphia: Lippincott Williams and Wilkins, 2004.
9. Tyndale RF, Sellers EM. Genetic variation in CYP2A6-mediated nicotine metabolism alters smoking behavior. Ther Drug Monit 2002;24(1):163-71.
10. Newhouse P, Singh A, Potter A. Nicotine and nicotinic receptor involvement in neuropsychiatric disorders. Curr Top Med Chem 2004;4(3):267-82.
11. McEvoy JP, Allen TB. The importance of nicotinic acetylcholine receptors in schizophrenia, bipolar disorder and Tourette’s syndrome. Curr Drug Target CNS Neurol Disord 2002;1(4):433-42.
12. Fiore MC, Bailey WC, Cohen SJ, et al. Treating tobacco use and dependence. Clinical practice guideline. U.S. Public Health Service. Rockville, MD: Department of Health and Human Services, 2000. Available at http:www.ahcpr.gov/path/tobacco.htm.
13. Covey LS, Glassman AH, Stetner F. Cigarette smoking and major depression. J Addict Disord 1998;17(1):35-46.
14. Berlin I, Anthenelli RM. Monoamine oxidases and tobacco smoking. Intl J Neuropsychopharmacol 2001;4(1):33-42.
15. Killen JD, Fortmann SP, Schatzberg A, et al. Onset of major depression during treatment for nicotine dependence. Addict Behav 2003;28(3):461-70.
16. Breslau N. Psychiatric comorbidity of smoking and nicotine dependence. Behav Genet 1995;25:95-101.
17. Addington J, el Guebaly N, Campbell W, et al. Smoking cessation treatment for patients with schizophrenia. Am J Psychiatry 1998;155(7):974-6.
18. George TP, Ziedonis DM, Feingold A, et al. Nicotine transdermal patch and atypical antipsychotic medications for smoking cessation in schizophrenia. Am J Psychiatry 2000;157(11):1835-42.
19. Lemon SC, Friedmann PD, Stein MD. The impact of smoking cessation on drug abuse treatment outcome. Addict Behav 2003;28(7):1323-31.
20. Prior TI, Baker GB. Interactions between the cytochrome P450 system and the second-generation antipsychotics. J Psychiatry Neurosci 2003;28(2):99-112.
21. Rogotti NA. Clinical practice: treatment of tobacco use and dependence. N Engl J Med 2002;346(7):506-12.
22. Ziedonis D, Williams JM, Smelson D. Serious mental illness and tobacco addiction: a model program to address this common but neglected issue. Am J Med Sci 2003;326(4):223-30.
Three myths about cigarette smoking may explain why psychiatrists rarely intervene in their patients’ tobacco dependence:
- Cigarette smoking is an incurable habit in psychiatric patients and thus not worth the effort of intervening.
- Cigarette smoking is an acceptable form of self-medication in persons with psychiatric illness.
- Quitting smoking will worsen psychiatric symptoms.
Smoking by psychiatric patients is treatable, however, and evidence proves that many can quit.1 This article rebuts the “why-bother?” myths and provides practical tips on how to more effectively help psychiatric patients stop smoking.
DEBUNKING THREE MYTHS
Mentally ill women and men consume nearly one-half (44%) of the cigarettes smoked in the United States (Table 1)1-3 and thus are at high risk for tobacco-related premature death, cancer, cardiovascular disease, and respiratory disorders. Although recognized as a leading cause of death, cigarette smoking by psychiatric patients frequently goes unaddressed, contributing to excess mortality in this population.4
Table 1
Cigarette smoking: An epidemic among psychiatric patients
|
| Source: References 1-3 |
- psychiatrists seldom (6,7
- when counseling did occur, nicotine replacement therapy was not prescribed.6
Tobacco dependence is a syndrome with strong genetic and biologic roots. Family, twin, and adoption studies show consistently that tobacco dependence is genetically mediated.8 Genetic polymorphisms are being identified that may modify an individual’s risk for developing nicotine dependence—such as the gene encoding the cytochrome P-450 2A6 isoenzyme (CYP 2A6) that metabolizes nicotine to cotinine.9 Disturbed nicotinic receptor functioning has been shown in persons with schizophrenia, mood disorders, anxiety disorders, and attention-deficit/hyperactivity disorders.3,10,11
Tobacco dependence is a chronic, relapsing condition that usually requires repeated intervention to motivate patients to try to quit and to help those who are willing to quit to succeed. Effective smoking cessation aids include:
- behavioral therapy (brief physician advice, problem-solving skills/skills training)
- pharmacologic therapy (nicotine replacement, sustained-release bupropion).12
Is smoking ‘self-medication’? Compelling evidence indicates that cholinergic mechanisms and nicotinic receptors (nAChRs) are involved in the pathophysiology of schizophrenia and other neuropsychiatric disorders.3,10 Nicotine administration appears to improve sensory-processing and cognitive deficits observed in schizophrenia.2,3 Moreover, the association between depression and smoking13 —and tobacco smoke’s monoamine oxidase-inhibiting and other psychoactive properties14 —have led some to posit that cigarette smoking may have antidepressant actions.10
For all these reasons, some authors have speculated that tobacco use may be a form of self-medication among the psychiatrically ill.3 The problem with this hypothesis, however, is that tobacco smoke is—at best—an untested and potentially lethal cognitive enhancer, antidepressant, or anxiolytic. Animal and human studies may find therapeutic effects of acute nicotine administration, but the cognitive effects of chronic tobacco smoking are not known.
Table 2
5 ‘A’s of brief clinical intervention for tobacco dependence
|
| Source: References 5 and 12 |
Adverse effects from quitting? Smokers with a history of major depressive disorder have been shown to be at risk to:
- develop another depressive episode after they quit smoking15
- experience more severe withdrawal symptoms during abstinence, compared with smokers with no history of depression.13,16
Scant data support the myth that smoking cessation worsens psychiatric symptoms. For example, in a review on tobacco dependence and schizophrenia, George et al2 concluded that the effects of smoking cessation on schizophrenia symptoms are not clear. Two smoking cessation trials in schizophrenic patients treated with nicotine patches found no significant changes in postcessation psychotic symptoms.17,18
Concerns that substance-abusing patients should not attempt to quit smoking during alcohol and other drug dependence treatment are also unsubstantiated. Rather than exacerbating drug addiction, smoking cessation has been found to improve addicts’ abstinence rates.19
USING AVAILABLE THERAPIES
Evidence is insufficient so far to show whether psychiatrically ill smokers would benefit more from specially tailored cessation treatments than from standard treatments, according to the 2000 U.S. Public Health Service clinical practice guide.12 Thus, while researchers try to resolve this issue, psychiatrists are left to use medications found to be effective in smokers overall.
Clinical vignette. Mr. J, age 45, has paranoid-type schizophrenia and has been smoking at least two packs of cigarettes daily for 25 years. He complains of a productive cough and expresses interest in quitting smoking when his psychiatrist raises this topic.
His persecutory delusions are well-controlled on olanzapine, 10 mg/d. He is adhering with his medications and participating in weekly group counseling that provides supportive therapy for patients with serious mental illness.
In this schizophrenic smoker who is willing to try to quit, the psychiatrist performed the first three of “5 ‘A’s” (Table 2) of brief clinical intervention for tobacco dependence.5,12 The next steps are to assist the patient’s effort to quit and arrange follow-up.
When to quit. The best time for a smoker with psychiatric illness to try to quit is when he or she:
- is psychiatrically stable
- is not in crisis
- has no recent or planned psychiatric drug changes.
Smoking cessation may increase blood levels of these psychotropics
| Antipsychotics | Antidepressants | Mood stabilizers | Anxiolytics |
|---|---|---|---|
| Haloperidol | Clomipramine | Carbamazepine | Desmethyldiazepam |
| Chlorpromazine | Desipramine | Oxazepam | |
| Fluphenazine | Doxepin | ||
| Olanzapine | Imipramine | ||
| Clozapine | Nortriptyline | ||
| Source: References 2, 5, and 20 | |||
Olanzapine’s clearance is approximately 40% higher in smokers than in nonsmokers. The psychiatrist discussed this with Mr. J and:
- asked him to call if side effects develop during the quit attempt
- scheduled more-frequent appointments to monitor side effects.
Mr. J’s schizophrenia is stable on maintenance therapy with an atypical antipsychotic. Schizophrenic smokers taking atypicals may be more able to quit smoking with NRT or sustained-release bupropion, compared with those taking conventional antipsychotics.2
The psychiatrist also determined that Mr. J had tried to quit smoking three times. Two of these attempts were done “cold turkey,” without pharmacotherapy, and one involved using nicotine gum. Mr. J said that although the gum “worked well at first,” he stopped using it because it was expensive and made his mouth sore. This information helped the psychiatrist choose medication for this quit attempt.
Most smoking cessation guidelines rely on a stepped-care approach, progressing from minimal to more-intensive interventions as needed.5 Mr. J’s psychiatrist devised an intensive treatment plan because:
- Mr. J has tried to quit before
- schizophrenic patients generally have more difficulty quitting and are more nicotine-dependent than other smokers.
Table 4
Nicotine replacement and other options for smoking cessation
| Drug | Daily dosage | Treatment duration* | Common side effects |
|---|---|---|---|
| Nicotine replacement therapy† | |||
| Transdermal | Skin irritation, insomnia | ||
| 24-hr patch | Starting dose is 21 mg/d; also in 7- and 14-mg patches for tapering dosage | 8 wk | |
| 16-hr patch | 15 mg | 8 wk | |
| Polacrilex (gum) 2- or 4-mg piece | 1 piece/hr ( | 8 to 12 wk | Mouth irritation, sore jaw, dyspepsia, hiccups |
| Vapor inhaler | 6 to 16 cartridges/day (delivers 4/mg/cartridge) | 3 to 6 mo | Mouth and throat irritation, cough |
| Nasal spray | 1 to 2 doses/hr; dose = 1 mg (0.5 mg per nostril); maximum dosage 40 mg/d | 3 to 6 mo | Nasal irritation, sneezing, cough, tearing eyes |
| Lozenge | 2- or 4-mg dose; see dosage formula, titration schedule in over-the-counter package | 12 wk | Hiccups, nausea, heartburn |
| Non-nicotine replacement therapy | |||
| Sustained-release bupropion† | 150 mg/d for 3 days, then 150 mg bid; start 1 week before quit date | 7 to 12 wk; up to 6 mo. to maintain abstinence | Insomnia, dry mouth, agitation |
| Nortriptyline | 75 to 100 mg/d; start 10 to 28 days before quit date at 25 mg/d and increase as tolerated | 12 wk | Dry mouth, sedation, dizziness |
| Clonidine | 0.1 to 0.3 mg bid | 3 to 10 wk | Dry mouth, sedation, dizziness |
| * Treatment duration varies and may be longer in patients with psychiatric disorders. | |||
| † FDA-approved as a smoking cessation aid and recommended as a first-line drug by Public Health Service clinical guidelines. | |||
| Source: Adapted from reference 21. | |||
On the morning of his TQD, Mr. J is to apply the first 21-mg transdermal nicotine patch. He is told not to smoke that day and to apply a new patch daily. The psychiatrist also tells him he will most likely remain on that dosage for 4 weeks. Then the patch strength will be reduced in 7-mg aliquots every 2 to 4 weeks, depending on his progress. The psychiatrist also provides him with educational materials on how to quit successfully.
Follow-up. Recognizing that most relapses occur in the first few days of quitting, the psychiatrist sets Mr. J’s first follow-up appointment for the day after his TQD to assess:
- whether he has smoked and number of cigarettes smoked per day
- presence and severity of withdrawal symptoms
- onset of psychiatric symptoms
- treatment adherence
- how he is handling high-risk situations and urges to smoke
- medication side effects.6
- American Psychiatric Association. Practice guideline for the treatment of patients with nicotine dependence. Am J Psychiatry 1996; 53(153[suppl]): 1-31.
- Fiore MC, Bailey WC, Cohen SJ, et.al. Treating tobacco use and dependence. Clinical practice guideline. Rockville, MD: U.S. Public Health Service, 2000. http://www.ahcpr.gov/path/tobacco.htm. Accessed Dec. 13, 2004.
- Amantadine • Symmetrel
- Bupropion • Wellbutrin SR, Zyban
- Clonidine • Catapres
- Nicotine nasal spray • Nicotrol NS
- Nicotine polacrilex • Nicorette
- Nicotine replacement patch • Nicoderm CQ, Nicotrol, others
- Nicotine vapor inhaler • Nicotrol Inhaler
- Nortriptyline •Aventyl, Pamelor
Dr. Anthenelli receives grant/research support from Sanofi-Aventis and Ortho-McNeil Pharmaceuticals and is a consultant and speaker for Sanofi-Aventis.
Acknowledgments
The author would like to thank Reene Cantwell for technical assistance in preparing this manuscript. This work was supported by grants R01 AA13307 and R01 AA13957 from the National Institute on Alcohol Abuse and Alcoholism and by the Department of Veterans Affairs.
Three myths about cigarette smoking may explain why psychiatrists rarely intervene in their patients’ tobacco dependence:
- Cigarette smoking is an incurable habit in psychiatric patients and thus not worth the effort of intervening.
- Cigarette smoking is an acceptable form of self-medication in persons with psychiatric illness.
- Quitting smoking will worsen psychiatric symptoms.
Smoking by psychiatric patients is treatable, however, and evidence proves that many can quit.1 This article rebuts the “why-bother?” myths and provides practical tips on how to more effectively help psychiatric patients stop smoking.
DEBUNKING THREE MYTHS
Mentally ill women and men consume nearly one-half (44%) of the cigarettes smoked in the United States (Table 1)1-3 and thus are at high risk for tobacco-related premature death, cancer, cardiovascular disease, and respiratory disorders. Although recognized as a leading cause of death, cigarette smoking by psychiatric patients frequently goes unaddressed, contributing to excess mortality in this population.4
Table 1
Cigarette smoking: An epidemic among psychiatric patients
|
| Source: References 1-3 |
- psychiatrists seldom (6,7
- when counseling did occur, nicotine replacement therapy was not prescribed.6
Tobacco dependence is a syndrome with strong genetic and biologic roots. Family, twin, and adoption studies show consistently that tobacco dependence is genetically mediated.8 Genetic polymorphisms are being identified that may modify an individual’s risk for developing nicotine dependence—such as the gene encoding the cytochrome P-450 2A6 isoenzyme (CYP 2A6) that metabolizes nicotine to cotinine.9 Disturbed nicotinic receptor functioning has been shown in persons with schizophrenia, mood disorders, anxiety disorders, and attention-deficit/hyperactivity disorders.3,10,11
Tobacco dependence is a chronic, relapsing condition that usually requires repeated intervention to motivate patients to try to quit and to help those who are willing to quit to succeed. Effective smoking cessation aids include:
- behavioral therapy (brief physician advice, problem-solving skills/skills training)
- pharmacologic therapy (nicotine replacement, sustained-release bupropion).12
Is smoking ‘self-medication’? Compelling evidence indicates that cholinergic mechanisms and nicotinic receptors (nAChRs) are involved in the pathophysiology of schizophrenia and other neuropsychiatric disorders.3,10 Nicotine administration appears to improve sensory-processing and cognitive deficits observed in schizophrenia.2,3 Moreover, the association between depression and smoking13 —and tobacco smoke’s monoamine oxidase-inhibiting and other psychoactive properties14 —have led some to posit that cigarette smoking may have antidepressant actions.10
For all these reasons, some authors have speculated that tobacco use may be a form of self-medication among the psychiatrically ill.3 The problem with this hypothesis, however, is that tobacco smoke is—at best—an untested and potentially lethal cognitive enhancer, antidepressant, or anxiolytic. Animal and human studies may find therapeutic effects of acute nicotine administration, but the cognitive effects of chronic tobacco smoking are not known.
Table 2
5 ‘A’s of brief clinical intervention for tobacco dependence
|
| Source: References 5 and 12 |
Adverse effects from quitting? Smokers with a history of major depressive disorder have been shown to be at risk to:
- develop another depressive episode after they quit smoking15
- experience more severe withdrawal symptoms during abstinence, compared with smokers with no history of depression.13,16
Scant data support the myth that smoking cessation worsens psychiatric symptoms. For example, in a review on tobacco dependence and schizophrenia, George et al2 concluded that the effects of smoking cessation on schizophrenia symptoms are not clear. Two smoking cessation trials in schizophrenic patients treated with nicotine patches found no significant changes in postcessation psychotic symptoms.17,18
Concerns that substance-abusing patients should not attempt to quit smoking during alcohol and other drug dependence treatment are also unsubstantiated. Rather than exacerbating drug addiction, smoking cessation has been found to improve addicts’ abstinence rates.19
USING AVAILABLE THERAPIES
Evidence is insufficient so far to show whether psychiatrically ill smokers would benefit more from specially tailored cessation treatments than from standard treatments, according to the 2000 U.S. Public Health Service clinical practice guide.12 Thus, while researchers try to resolve this issue, psychiatrists are left to use medications found to be effective in smokers overall.
Clinical vignette. Mr. J, age 45, has paranoid-type schizophrenia and has been smoking at least two packs of cigarettes daily for 25 years. He complains of a productive cough and expresses interest in quitting smoking when his psychiatrist raises this topic.
His persecutory delusions are well-controlled on olanzapine, 10 mg/d. He is adhering with his medications and participating in weekly group counseling that provides supportive therapy for patients with serious mental illness.
In this schizophrenic smoker who is willing to try to quit, the psychiatrist performed the first three of “5 ‘A’s” (Table 2) of brief clinical intervention for tobacco dependence.5,12 The next steps are to assist the patient’s effort to quit and arrange follow-up.
When to quit. The best time for a smoker with psychiatric illness to try to quit is when he or she:
- is psychiatrically stable
- is not in crisis
- has no recent or planned psychiatric drug changes.
Smoking cessation may increase blood levels of these psychotropics
| Antipsychotics | Antidepressants | Mood stabilizers | Anxiolytics |
|---|---|---|---|
| Haloperidol | Clomipramine | Carbamazepine | Desmethyldiazepam |
| Chlorpromazine | Desipramine | Oxazepam | |
| Fluphenazine | Doxepin | ||
| Olanzapine | Imipramine | ||
| Clozapine | Nortriptyline | ||
| Source: References 2, 5, and 20 | |||
Olanzapine’s clearance is approximately 40% higher in smokers than in nonsmokers. The psychiatrist discussed this with Mr. J and:
- asked him to call if side effects develop during the quit attempt
- scheduled more-frequent appointments to monitor side effects.
Mr. J’s schizophrenia is stable on maintenance therapy with an atypical antipsychotic. Schizophrenic smokers taking atypicals may be more able to quit smoking with NRT or sustained-release bupropion, compared with those taking conventional antipsychotics.2
The psychiatrist also determined that Mr. J had tried to quit smoking three times. Two of these attempts were done “cold turkey,” without pharmacotherapy, and one involved using nicotine gum. Mr. J said that although the gum “worked well at first,” he stopped using it because it was expensive and made his mouth sore. This information helped the psychiatrist choose medication for this quit attempt.
Most smoking cessation guidelines rely on a stepped-care approach, progressing from minimal to more-intensive interventions as needed.5 Mr. J’s psychiatrist devised an intensive treatment plan because:
- Mr. J has tried to quit before
- schizophrenic patients generally have more difficulty quitting and are more nicotine-dependent than other smokers.
Table 4
Nicotine replacement and other options for smoking cessation
| Drug | Daily dosage | Treatment duration* | Common side effects |
|---|---|---|---|
| Nicotine replacement therapy† | |||
| Transdermal | Skin irritation, insomnia | ||
| 24-hr patch | Starting dose is 21 mg/d; also in 7- and 14-mg patches for tapering dosage | 8 wk | |
| 16-hr patch | 15 mg | 8 wk | |
| Polacrilex (gum) 2- or 4-mg piece | 1 piece/hr ( | 8 to 12 wk | Mouth irritation, sore jaw, dyspepsia, hiccups |
| Vapor inhaler | 6 to 16 cartridges/day (delivers 4/mg/cartridge) | 3 to 6 mo | Mouth and throat irritation, cough |
| Nasal spray | 1 to 2 doses/hr; dose = 1 mg (0.5 mg per nostril); maximum dosage 40 mg/d | 3 to 6 mo | Nasal irritation, sneezing, cough, tearing eyes |
| Lozenge | 2- or 4-mg dose; see dosage formula, titration schedule in over-the-counter package | 12 wk | Hiccups, nausea, heartburn |
| Non-nicotine replacement therapy | |||
| Sustained-release bupropion† | 150 mg/d for 3 days, then 150 mg bid; start 1 week before quit date | 7 to 12 wk; up to 6 mo. to maintain abstinence | Insomnia, dry mouth, agitation |
| Nortriptyline | 75 to 100 mg/d; start 10 to 28 days before quit date at 25 mg/d and increase as tolerated | 12 wk | Dry mouth, sedation, dizziness |
| Clonidine | 0.1 to 0.3 mg bid | 3 to 10 wk | Dry mouth, sedation, dizziness |
| * Treatment duration varies and may be longer in patients with psychiatric disorders. | |||
| † FDA-approved as a smoking cessation aid and recommended as a first-line drug by Public Health Service clinical guidelines. | |||
| Source: Adapted from reference 21. | |||
On the morning of his TQD, Mr. J is to apply the first 21-mg transdermal nicotine patch. He is told not to smoke that day and to apply a new patch daily. The psychiatrist also tells him he will most likely remain on that dosage for 4 weeks. Then the patch strength will be reduced in 7-mg aliquots every 2 to 4 weeks, depending on his progress. The psychiatrist also provides him with educational materials on how to quit successfully.
Follow-up. Recognizing that most relapses occur in the first few days of quitting, the psychiatrist sets Mr. J’s first follow-up appointment for the day after his TQD to assess:
- whether he has smoked and number of cigarettes smoked per day
- presence and severity of withdrawal symptoms
- onset of psychiatric symptoms
- treatment adherence
- how he is handling high-risk situations and urges to smoke
- medication side effects.6
- American Psychiatric Association. Practice guideline for the treatment of patients with nicotine dependence. Am J Psychiatry 1996; 53(153[suppl]): 1-31.
- Fiore MC, Bailey WC, Cohen SJ, et.al. Treating tobacco use and dependence. Clinical practice guideline. Rockville, MD: U.S. Public Health Service, 2000. http://www.ahcpr.gov/path/tobacco.htm. Accessed Dec. 13, 2004.
- Amantadine • Symmetrel
- Bupropion • Wellbutrin SR, Zyban
- Clonidine • Catapres
- Nicotine nasal spray • Nicotrol NS
- Nicotine polacrilex • Nicorette
- Nicotine replacement patch • Nicoderm CQ, Nicotrol, others
- Nicotine vapor inhaler • Nicotrol Inhaler
- Nortriptyline •Aventyl, Pamelor
Dr. Anthenelli receives grant/research support from Sanofi-Aventis and Ortho-McNeil Pharmaceuticals and is a consultant and speaker for Sanofi-Aventis.
Acknowledgments
The author would like to thank Reene Cantwell for technical assistance in preparing this manuscript. This work was supported by grants R01 AA13307 and R01 AA13957 from the National Institute on Alcohol Abuse and Alcoholism and by the Department of Veterans Affairs.
1. Lasser K, Boyd JW, Woolhandler S, et al. Smoking and mental illness: a population-based prevalence study. JAMA 2000;284(20):2606-10.
2. George TP, Vessicchio JC, Termine A. Nicotine and tobacco use in schizophrenia. In: Meyer JM, Nasrallah HR (eds). Medical illness and schizophrenia. Washington, DC: American Psychiatric Publishing, 2003:81-98.
3. Leonard S, Adler LE, Benhammou K, et al. Smoking and mental illness. Pharmacol Biochem Behav 2001;70(4):561-70.
4. Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry 2000;177:212-17.
5. American Psychiatric Association. Practice guideline for the treatment of patients with nicotine dependence. Am J Psychiatry 1996;53[153(suppl)]:1-31.
6. Himelhoch S, Daumit G. To whom do psychiatrists offer smoking-cessation counseling? Am J Psychiatry 2003;160(12):2228-30.
7. Thorndike AN, Stafford RS, Rigotti NA. US physicians’ treatment of smoking in outpatients with psychiatric diagnoses. Nicotine Tob Res 2001;3(1):85-91.
8. Lin SW, Anthenelli RM. Genetic factors in the risk for substance use disorders. In: Lowinson J, Ruiz P, Millman RB, Langrod JC (eds). Substance abuse: a comprehensive textbook (4th ed). Philadelphia: Lippincott Williams and Wilkins, 2004.
9. Tyndale RF, Sellers EM. Genetic variation in CYP2A6-mediated nicotine metabolism alters smoking behavior. Ther Drug Monit 2002;24(1):163-71.
10. Newhouse P, Singh A, Potter A. Nicotine and nicotinic receptor involvement in neuropsychiatric disorders. Curr Top Med Chem 2004;4(3):267-82.
11. McEvoy JP, Allen TB. The importance of nicotinic acetylcholine receptors in schizophrenia, bipolar disorder and Tourette’s syndrome. Curr Drug Target CNS Neurol Disord 2002;1(4):433-42.
12. Fiore MC, Bailey WC, Cohen SJ, et al. Treating tobacco use and dependence. Clinical practice guideline. U.S. Public Health Service. Rockville, MD: Department of Health and Human Services, 2000. Available at http:www.ahcpr.gov/path/tobacco.htm.
13. Covey LS, Glassman AH, Stetner F. Cigarette smoking and major depression. J Addict Disord 1998;17(1):35-46.
14. Berlin I, Anthenelli RM. Monoamine oxidases and tobacco smoking. Intl J Neuropsychopharmacol 2001;4(1):33-42.
15. Killen JD, Fortmann SP, Schatzberg A, et al. Onset of major depression during treatment for nicotine dependence. Addict Behav 2003;28(3):461-70.
16. Breslau N. Psychiatric comorbidity of smoking and nicotine dependence. Behav Genet 1995;25:95-101.
17. Addington J, el Guebaly N, Campbell W, et al. Smoking cessation treatment for patients with schizophrenia. Am J Psychiatry 1998;155(7):974-6.
18. George TP, Ziedonis DM, Feingold A, et al. Nicotine transdermal patch and atypical antipsychotic medications for smoking cessation in schizophrenia. Am J Psychiatry 2000;157(11):1835-42.
19. Lemon SC, Friedmann PD, Stein MD. The impact of smoking cessation on drug abuse treatment outcome. Addict Behav 2003;28(7):1323-31.
20. Prior TI, Baker GB. Interactions between the cytochrome P450 system and the second-generation antipsychotics. J Psychiatry Neurosci 2003;28(2):99-112.
21. Rogotti NA. Clinical practice: treatment of tobacco use and dependence. N Engl J Med 2002;346(7):506-12.
22. Ziedonis D, Williams JM, Smelson D. Serious mental illness and tobacco addiction: a model program to address this common but neglected issue. Am J Med Sci 2003;326(4):223-30.
1. Lasser K, Boyd JW, Woolhandler S, et al. Smoking and mental illness: a population-based prevalence study. JAMA 2000;284(20):2606-10.
2. George TP, Vessicchio JC, Termine A. Nicotine and tobacco use in schizophrenia. In: Meyer JM, Nasrallah HR (eds). Medical illness and schizophrenia. Washington, DC: American Psychiatric Publishing, 2003:81-98.
3. Leonard S, Adler LE, Benhammou K, et al. Smoking and mental illness. Pharmacol Biochem Behav 2001;70(4):561-70.
4. Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry 2000;177:212-17.
5. American Psychiatric Association. Practice guideline for the treatment of patients with nicotine dependence. Am J Psychiatry 1996;53[153(suppl)]:1-31.
6. Himelhoch S, Daumit G. To whom do psychiatrists offer smoking-cessation counseling? Am J Psychiatry 2003;160(12):2228-30.
7. Thorndike AN, Stafford RS, Rigotti NA. US physicians’ treatment of smoking in outpatients with psychiatric diagnoses. Nicotine Tob Res 2001;3(1):85-91.
8. Lin SW, Anthenelli RM. Genetic factors in the risk for substance use disorders. In: Lowinson J, Ruiz P, Millman RB, Langrod JC (eds). Substance abuse: a comprehensive textbook (4th ed). Philadelphia: Lippincott Williams and Wilkins, 2004.
9. Tyndale RF, Sellers EM. Genetic variation in CYP2A6-mediated nicotine metabolism alters smoking behavior. Ther Drug Monit 2002;24(1):163-71.
10. Newhouse P, Singh A, Potter A. Nicotine and nicotinic receptor involvement in neuropsychiatric disorders. Curr Top Med Chem 2004;4(3):267-82.
11. McEvoy JP, Allen TB. The importance of nicotinic acetylcholine receptors in schizophrenia, bipolar disorder and Tourette’s syndrome. Curr Drug Target CNS Neurol Disord 2002;1(4):433-42.
12. Fiore MC, Bailey WC, Cohen SJ, et al. Treating tobacco use and dependence. Clinical practice guideline. U.S. Public Health Service. Rockville, MD: Department of Health and Human Services, 2000. Available at http:www.ahcpr.gov/path/tobacco.htm.
13. Covey LS, Glassman AH, Stetner F. Cigarette smoking and major depression. J Addict Disord 1998;17(1):35-46.
14. Berlin I, Anthenelli RM. Monoamine oxidases and tobacco smoking. Intl J Neuropsychopharmacol 2001;4(1):33-42.
15. Killen JD, Fortmann SP, Schatzberg A, et al. Onset of major depression during treatment for nicotine dependence. Addict Behav 2003;28(3):461-70.
16. Breslau N. Psychiatric comorbidity of smoking and nicotine dependence. Behav Genet 1995;25:95-101.
17. Addington J, el Guebaly N, Campbell W, et al. Smoking cessation treatment for patients with schizophrenia. Am J Psychiatry 1998;155(7):974-6.
18. George TP, Ziedonis DM, Feingold A, et al. Nicotine transdermal patch and atypical antipsychotic medications for smoking cessation in schizophrenia. Am J Psychiatry 2000;157(11):1835-42.
19. Lemon SC, Friedmann PD, Stein MD. The impact of smoking cessation on drug abuse treatment outcome. Addict Behav 2003;28(7):1323-31.
20. Prior TI, Baker GB. Interactions between the cytochrome P450 system and the second-generation antipsychotics. J Psychiatry Neurosci 2003;28(2):99-112.
21. Rogotti NA. Clinical practice: treatment of tobacco use and dependence. N Engl J Med 2002;346(7):506-12.
22. Ziedonis D, Williams JM, Smelson D. Serious mental illness and tobacco addiction: a model program to address this common but neglected issue. Am J Med Sci 2003;326(4):223-30.
Herbal hazards: Which psychotropics interact with four common supplements
What do you tell your patients who are self-medicating with herbal remedies? Can dietary supplements safely improve mood disorders, insomnia, and other psychiatric complaints?
Evidence is limited on complementary and alternative medicines (CAMs), their active components, pharmacokinetics/dynamics, adverse effects, drug interactions, and therapeutic outcomes. Based on our review of trial data, case reports, and an NIH National Center for Complementary and Alternative Medicine (NCCAM) survey,1 we offer information to help you:
- identify patients using dietary supplements
- avoid serious interactions with common psychotropics
- counsel patients on the efficacy and safety of ginkgo biloba, St. John’s wort, kava kava, and valerian (Table 1).
DON’T BE AFRAID TO ASK
One-third of Americans who took part in the NCCAM survey reported using CAM. After prayer—the number-one CAM—respondents said they most often used natural products such as herbals, botanicals, nutraceuticals, phytomedicinals, and dietary supplements (Figure).1
Table 1
4 herbal supplements with purported psychotropic effects
| Herb | Promoted use | Safety | Recommendation |
|---|---|---|---|
| Ginkgo biloba | Dementia, memory | Bleeding complications, drug interactions a concern | Some data support a trial in dementia; beware of safety concerns |
| St. John’s wort | Depression | Substantial drug interactions | Use not recommended because of wide-ranging drug interactions |
| Kava kava | Anxiety | Hepatotoxicity risk, drug interactions; off market in Europe and Canada | Use not recommended because of safety issues |
| Valerian | Insomnia | Limited data available | Benign (?); monitor for possible adverse events or drug interactions |
Patients tend to use CAM to treat chronic medical conditions such as back pain, depression, and anxiety.1 Although CAM use is common, only 38% of patients say they disclose using CAM to their physicians.2 These use and disclosure patterns are similar in psychiatry.3
Herbal products may produce symptoms that mimic those of mental illnesses, such as psychosis and mania, complicating diagnosis and treatment. Abruptly stopping some herbs can produce withdrawal symptoms similar to those seen with benzodiazepine and antidepressant cessation. Supplements also can inhibit or augment prescribed psychotropics’ effects, and notable consequences include the potential for serotonin syndrome with use of St. John’s wort.
The key to learning about a patient’s use of dietary supplements is to ask. Patients commonly say they do not tell their doctors about using dietary supplements because “it wasn’t important for the doctor to know” or “the doctor never asked.”4,5 Ten tips for discussing nutritional supplements with patients are shown in the Box.
Buyer—and psychiatrist—beware. Because of dietary supplements’ regulatory status, physicians and patients need to learn as much as they can about these products’ documented safety and efficacy. The Dietary Supplement Health and Education Act passed by Congress in 1994 does not require manufacturers to prove their products are safe or effective before marketing them. They also can make claims that suggest uses in physical/emotional structure and function, such as “helps maintain a healthy emotional outlook.”
The FDA bears the burden of proof regarding safety and can remove a dietary supplement from the market only after receiving documented adverse event information from the public.6 The FDA has confiscated herbal products containing prescription drugs and misidentified herbal components.
GINKGO BILOBA FOR DEMENTIA
Ginkgo biloba was the third most commonly used herbal product (21%) in the NCCAM survey. Ginkgo is promoted primarily for dementia, cerebrovascular dysfunction, and memory enhancement. The standardized ginkgo extract (EGb 761) contains several components to which its pharmacologic activity has been attributed. Its constituents are thought to act primarily through anticoagulant effects by inhibiting platelet-activating factor and cyclic GMP phosphodiesterase. They also act through membrane stabilization, antioxidant properties, free-radical scavenging, and inhibition of beta-amyloid deposition.7
Efficacy. In patients with dementia, clinical trials using EGb 761 have shown small improvements in or maintenance of cognitive and social functioning, compared with placebo.8,9 The clinical significance of these findings is unclear, however. Ginkgo’s usefulness in enhancing memory is less certain. Controlled clinical trials are evaluating ginkgo’s efficacy in various conditions.10
Adverse effects. Bleeding complications are the primary concern with ginkgo biloba use, and caution is urged when it is taken concomitantly with aspirin or other antithrombotic drugs. Patients receiving warfarin should not take ginkgo because of the combined antiplatelet effects and ginkgo’s inhibition of warfarin metabolism and elimination.
Drug-herb interactions. One report showed that EGb 761 is a strong inhibitor of the cytochrome P-450 2C9 enzyme system (CYP 2C9).11 Other identified inhibitors of CYP 2C9 are fluvoxamine (strong inhibitor), amiodarone, cimetidine, fluoxetine, and omeprazole. Drugs that serve as substrates for that system and can have decreased clearance include phenytoin, warfarin, amitriptyline, and diazepam;12 use caution, therefore, when you co-administer these drugs with ginkgo.
Figure 10 CAM therapies patients report using most often
CAM: Complimentary and alternative medicines
Source: Barnes T, Powell-Griner E, McFann K, Nahin R. Complementary and alternative medicine use among adults: United States, 2002. Atlanta: Centers for Disease Control and Prevention, May 27, 2004: Advance Data Report #343 (available at http://www.cdc.gov/nchs/data/ad/ad343.pdf) Dosage. The usual dosage for standardized ginkgo extract is 120 to 240 mg/d, given in divided doses. Improvements associated with ginkgo use usually are seen within 4 to 12 weeks after starting therapy.7 Consider discontinuing therapy if you see no results after that time. Little is known about ginkgo’s effect after 1 year of use.
Recommendation. Limited data show a slight benefit in treating dementia by slowing cognitive and behavioral decline, but ginkgo biloba cannot be recommended as a first-line treatment until further controlled trials are available.
- Broach the subject without being judgmental; nutritional supplement use is tied to a patient’s health beliefs
- Ask specifically about use of herbs, supplements, teas, elixirs, vitamins, etc., and document in the medical record at each visit
- Include in appointment reminders a request that patients bring all medicines, herbs, and supplements to appointments
- Segue into a discussion of supplements by noting their use by other patients with similar diagnoses
- Learn about and provide objective information on products
- Suggest use of single-ingredient products because:
- Suggest a symptom diary and a plan to discontinue a supplement if desired results are not seen
- Report suspected adverse events or drug interactions to the FDA
- Choose your battles carefully; testimonials and the placebo effect can strongly influence patients’ desire to continue using dietary supplements
- Remember: Patient safety is paramount
ST. JOHN’S WORT FOR DEPRESSION
St. John’s wort (Hypericum perforatum), used to treat mild-to-moderate depression and anxiety,13 is one of the most-recognized herbal remedies. It accounted for 12% of the natural products used in the NCCAM survey. Several clinical trials assessing St. John’s wort are in progress and include placebo-controlled evaluations in obsessive-compulsive disorder and social phobia.14
As with ginkgo, St. John’s wort has many proposed active constituents; most preparations are standardized based on a hypericin content of 0.3%. Its mechanism of action in depression is unclear but laboratory models hint that it may be related to very mild inhibition of:
- monoamine oxidase (MAO)
- catechol-O-methyltransferase(COMT)
- selective serotonin reuptake
- interleukin-6 release (thereby increasing corticotropin-releasing hormone levels)
- norepinephrine uptake.15
Efficacy. St. John’s wort has been studied in mild, moderate, and major depression and compared with placebo and prescription therapies. In treating mild and moderate depression, St. John’s wort has been more effective than placebo and equivalent to tricyclic antidepressants.16,17 Criticisms of these trials include lack of product standardization, lack of comparison with standard antidepressants at appropriate dosages, and small sample sizes. Larger trials comparing St. John’s wort with placebo and sertraline in treating major depression showed no difference in effect among the three.18,19
Adverse effects are generally infrequent and include insomnia, anxiety, GI upset, and photosensitivity reactions.20 St. John’s wort can induce hypomania and mania in patients with bipolar disorder and cause psychosis in schizophrenic patients.13
Drug-herb interactions. Of greatest concern with St. John’s wort use is the remarkable number of drug-herb interactions that have been identified (Table 2 and Table 3).13,21 The primary mechanisms appear to be substantial induction of CYP 3A4, induction of P-glycoprotein mediated drug elimination, and—to a lesser extent—induction of other CYP isoenzymes.22 Interactions resulting in serotonin syndrome have been documented, with restlessness, sweating, and agitation.23
The 3A4 isoenzyme metabolizes most drugs processed via the CYP system.12 Severe interactions seen with St. John’s wort include:
- reduced cyclosporine levels, resulting in heart transplant rejection in two patients
- reduced antiretroviral levels in HIV patients
- pregnancy in women taking oral contraceptives.
Enzyme induction may persist for as long as 14 days after patients stop taking St. John’s wort.
Dosage. The recommended St. John’s wort dosage (using standardized 0.3% hypericin content) is 300 mg 2 or 3 times daily. Dosages of 1,200 to 1,800 mg/d have been used.13,18 Benefits may not be seen for 2 to 3 weeks, and experience with use beyond 8 weeks in mild-to-moderate depression is very limited.
Recommendation. Advise patients to taper off St. John’s wort when stopping therapy to decrease the risk of withdrawal symptoms such as confusion, headache, nausea, insomnia, and fatigue.21 Given the high risk for drug interactions associated with St. John’s wort, we do not recommend its use in patients receiving any other medications.
KAVA KAVA AND LIVER TOXICITY
Kava kava (Piper methysticum) is used by some patients to treat anxiety and insomnia. Compared with other nutritional supplements, kava is less commonly used—by only 6.6% of adults using nutritional supplements,1—and it is not being evaluated in NIH-sponsored trials.
The active-ingredient content varies considerably in kava root, so extracts are standardized to contain 70% kava-lactones (WS 1490). Although its exact mechanism is unclear, kava appears to:
- alter the limbic system
- inhibit monoamine oxidase type B (MAO-B)
- increase the number of gamma-aminobutyric acid (GABA) binding sites
- relax skeletal muscle
- produce anesthesia.24
Table 2
Documented interactions with St. John’s wort
| Alprazolam ↓ | Nevirapine ↓ |
| Amitriptyline ↓ | Oral contraceptives ↓ |
| Buspirone (ss) | Paroxetine (ss) |
| Cyclosporine ↓ | Sertraline (ss, mania) |
| Digoxin ↓ | Simvastatin ↓ |
| Fexofenadine ↓ | Tacrolimus ↓ |
| Indinavir ↓ | Theophylline ↓ |
| Irinotecan ↓ | Tyramine-containing |
| Methadone ↓ | foods (MAO-I reaction) |
| Midazolam ↓ | Venlafaxine (ss) |
| Nefazodone (ss) | Warfarin ↓ |
| ↓= decreased levels/effectiveness | |
| ss = serotonin syndrome | |
| Source: Adapted from reference 21 | |
Table 3
Select potential interactions with St. John’s wort
| Cannabinoids ↓ | Fentanyl ↓ |
| Cocaine ↓ | Temazepam ↓ |
| Diazepam ↓ | Triazolam ↓ |
| Donepezil ↓ | |
| ↓= decreased levels/effectiveness | |
| Source: Adapted from reference 12 | |
Efficacy. A meta-analysis of studies found kava more effective than placebo in treating anxiety,25 although most studies suffer from poor design and/or small sample size.
Adverse effects. Reports have associated kava use with hepatic toxicity, liver failure requiring liver transplantation, and death. The European Union and Canada have banned kava sales, and the FDA issued a consumer advisory noting kava’s risks. Emerging information indicates that kava inhibits virtually all CYP-450 enzymes, which would increase levels of and potential adverse effects from any medications taken with kava.26
Other common adverse effects include GI upset, enlarged pupils, extrapyramidal side effects, and dizziness.24,27
Dosage. Kava is usually given at 100 mg (70 mg of kavalactones) three times daily. Urge patients to avoid driving when taking kava because of its side effects. Anxiety symptoms may improve with 1 to 8 weeks of therapy, but adverse hepatic effects can occur within 3 to 4 weeks of starting kava use.
Recommendation. Avoid kava kava use because of substantial risk of hepatotoxicity and drug interactions.
VALERIAN FOR INSOMNIA, ANXIETY
Valerian (Valeriana officinalis) is promoted as a sedative/hypnotic and anxiolytic. The prevalence of valerian use (5.9%) mirrors that of kava.1 An NCCAM study is enrolling patients to evaluate valerian’s effectiveness in treating Parkinson’s disease-related sleep disturbances.
Efficacy. Information on valerian’s mechanism of action and clinical effectiveness is quite limited. In animal studies, its components produced direct sedative effects and inhibited CNS catabolism of GABA.28 Results are mixed in humans with insomnia; some studies have found reduced sleep latency and improved sleep quality with valerian use, whereas others found no improvements.29 Limited, small evaluations suggest that valerian may be useful in treating anxiety.
Adverse effects. The FDA categorizes valerian as an approved food additive, so it is considered safe in usual amounts found in food. FDA lists no amount that it considers safe in food, however, and the federal code covering valerian states that only enough needed to impart the desired flavor should be used.
When taken in therapeutic amounts, valerian’s most common adverse effects are headache and residual morning drowsiness. Because of the herb’s sedative effects, urge caution if patients drive while using it. On discontinuation, withdrawal symptoms similar to those seen with benzodiazepine withdrawal—anxiety, headache, emotional lability—have been reported.
Dosage. For insomnia, valerian is taken 2 hours to 30 minutes before bedtime; doses start at 300 to 400 mg and increase to 600 to 900 mg. Recommended doses vary, as standardization is less common with valerian than with other herbals. Continuous treatment seems more effective than as-needed dosing, as valerian may take up to 4 weeks to improve insomnia.
Recommendation. Well-controlled trials are lacking, and safety data at therapeutic doses are limited. Monitor patients using valerian for adverse effects and drug interactions.
- Natural Medicines Comprehensive Database.www.naturaldatabase.com
- National Center for Complementary and Alternative Medicine. http://nccam.nih.gov
- FDA MedWatch Adverse Event Reporting Program. www.fda.gov/medwatch/how.htm
- ConsumerLab.com. Independent testing of dietary supplements. www.consumerlab.com
Drug brand names
- Alprazolam • Xanax
- Amiodarone • Cordarone, Pacerone
- Amitriptyline • Elavil
- Buspirone • BuSpar
- Cimetidine • Tagamet
- Cyclosporine • various
- Diazepam • Valium
- Digoxin • Lanoxin
- Donepezil • Aricept
- Fentanyl • Duragesic
- Fexofenadine • Allegra
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Indinavir • Crixivan
- Irinotecan • Camptosar
- Methadone • various
- Midazolam • Versed
- Nefazodone • Serzone
- Nevirapine • Viramune
- Omeprazole • Prilosec
- Paroxetine • Paxil
- Phenytoin • Dilantin
- Sertraline • Zoloft
- Simvastatin • Zocor
- Tacrolimus • Prograf
- Temazepam • Restoril
- Theophylline • various
- Triazolam • Halcion
- Venlafaxine • Effexor
- Warfarin • Coumadin
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. Advance data from vital and health statistics; no 343. Hyattsville, MD: National Center for Health Statistics, 2004.
2. Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997. Results of a follow-up survey. JAMA 1998;280:1569-75.
3. Matthews SC, Camacho A, Lawson K, Dimsdale JE. Use of herbal medications among 200 psychiatric outpatients: prevalence, patterns of use, and potential dangers. Gen Hosp Psychiatry 2003;25:24-6.
4. Eisenberg DM. Advising patients who seek alternative medical therapies. Ann Intern Med 1997;127:61-9.
5. Grant KL. Patient education and herbal dietary supplements. Am J Health-Syst Pharm 2000;57:1997-2003.
6. Harris IM. Regulatory and ethical issues with dietary supplements. Pharmacotherapy 2000;20:1295-1302.
7. Sierpina VS, Wollschlaeger B, Blumenthal M. Ginkgo biloba. Am Fam Physician 2003;68:923-6.
8. LeBars PL, Katz MM, Berman N, et al. A placebo-controlled, double-blind, randomized trial of an extract of ginkgo biloba for dementia. JAMA 1997;278:1327-32.
9. Oken BS, Storzbach DM, Kaye JA. The efficacy of ginkgo biloba on cognitive function in Alzheimer’s disease. Arch Neurol 1998;55:1409-15.
10. Ginkgo biloba clinical trials National Center for Complementary and Alternative Medicine.National Institutes of Health. http://nccam.nih.gov/clinicaltrials/ginkgo.htm. Accessed Dec. 9, 2004.
11. Gaudineau C, Beckerman R, Welbourn S, Auclair K. Inhibition of human P450 enzymes by multiple constituents of the ginkgo biloba extract. Biochem Biophys Res Commun 2004;318:1072-8.
12. Michalets EL. Update: clinically significant cytochrome P-450 drug interactions. Pharmacotherapy 1998;18:84-112.
13. Pepping J. St.John’s wort: hypericum perforatum. Am J HealthSyst Pharm 1999;56:329-30.
14. St John’s wort (hypericum) clinical trials. National Center for Complementary and Alternative Medicine. National Institutes of Health. http://nccam.nih.gov/clinicaltrials/stjohnswort/index.htm. Accessed Dec. 9, 2004.
15. Bennett DA, Phun L, Polk JF, et al. Neuropharmacology of St.John’s wort (hypericum). Ann Pharmacother 1998;32:1201-8.
16. Gaster B, Holroyd J. St.John’s wort for depression: a systematic review. Arch Intern Med 2000;160:152-6.
17. Linde K, Ramirez G, Mulrow CD, et al. St.John’s wort for depression—an overview and meta-analysis of randomized clinical trials. BMJ 1996;313:253-8.
18. Shelton RC, Keller MB, Gelenberg A, et al. Effectiveness of St.John’s wort in major depression: a randomized controlled trial. JAMA 2001;285:1978-86.
19. Hypericum Depression Trial Study Group. Effect of Hypericum perforatum (St.John’s wort) in major depressive disorder: a randomized controlled trial. JAMA 2002;287:1807-14.
20. Beckman SE, Sommi RW, Switzer J. Consumer use of St.John’s wort: a survey on effectiveness, safety, and tolerability. Pharmacotherapy 2000;20:568-74.
21. Izzo AA. Drug interactions with St.John’s wort (hypericum perforatum): a review of the clinical evidence. Int J Clin Pharmacol Ther 2004;42:139-48.
22. Markowitz JS, Donovan JL, DeVane CL, et al. Effect of St.John’s wort on drug metabolism by induction of cytochrome P450 3A4 enzyme. JAMA 2003;290:1500-4.
23. Sternbach H. Serotonin syndrome: how to avoid, identify, and treat dangerous drug reactions. Current Psychiatry 2003;2(5):14-24.
24. Pepping J. Kava: Piper methysticum. Am J Health-Syst Pharm. 11999;56:957-60.
25. Pittler MH, Ernst E. Efficacy of kava extract for treating anxiety: systematic review and meta-analysis. J Clin Psychopharmacol 2000;20:84-9.
26. Matthews JM, Etheridge AS, Black SR. Inhibition of human cytochrome P450 activities by kava extract and kavalactones. Drug Metab Dispos 2002;30:1153-7.
27. Jellin JM, Gregory P, Batz F, et al. Kava Pharmacist’s Letter/Prescriber’s Letter Natural Medicines Comprehensive Database (3rd ed). Stockton, CA: Therapeutic Research Faculty, 2000;625:7.-
28. Hadley S, Petry JJ. Valerian. Am Fam Physician 2003;67:1755-8.
29. Plushner SL. Valerian: Valeriana officinalis. Am J Health-Syst Pharm 2000;57:328-35.
What do you tell your patients who are self-medicating with herbal remedies? Can dietary supplements safely improve mood disorders, insomnia, and other psychiatric complaints?
Evidence is limited on complementary and alternative medicines (CAMs), their active components, pharmacokinetics/dynamics, adverse effects, drug interactions, and therapeutic outcomes. Based on our review of trial data, case reports, and an NIH National Center for Complementary and Alternative Medicine (NCCAM) survey,1 we offer information to help you:
- identify patients using dietary supplements
- avoid serious interactions with common psychotropics
- counsel patients on the efficacy and safety of ginkgo biloba, St. John’s wort, kava kava, and valerian (Table 1).
DON’T BE AFRAID TO ASK
One-third of Americans who took part in the NCCAM survey reported using CAM. After prayer—the number-one CAM—respondents said they most often used natural products such as herbals, botanicals, nutraceuticals, phytomedicinals, and dietary supplements (Figure).1
Table 1
4 herbal supplements with purported psychotropic effects
| Herb | Promoted use | Safety | Recommendation |
|---|---|---|---|
| Ginkgo biloba | Dementia, memory | Bleeding complications, drug interactions a concern | Some data support a trial in dementia; beware of safety concerns |
| St. John’s wort | Depression | Substantial drug interactions | Use not recommended because of wide-ranging drug interactions |
| Kava kava | Anxiety | Hepatotoxicity risk, drug interactions; off market in Europe and Canada | Use not recommended because of safety issues |
| Valerian | Insomnia | Limited data available | Benign (?); monitor for possible adverse events or drug interactions |
Patients tend to use CAM to treat chronic medical conditions such as back pain, depression, and anxiety.1 Although CAM use is common, only 38% of patients say they disclose using CAM to their physicians.2 These use and disclosure patterns are similar in psychiatry.3
Herbal products may produce symptoms that mimic those of mental illnesses, such as psychosis and mania, complicating diagnosis and treatment. Abruptly stopping some herbs can produce withdrawal symptoms similar to those seen with benzodiazepine and antidepressant cessation. Supplements also can inhibit or augment prescribed psychotropics’ effects, and notable consequences include the potential for serotonin syndrome with use of St. John’s wort.
The key to learning about a patient’s use of dietary supplements is to ask. Patients commonly say they do not tell their doctors about using dietary supplements because “it wasn’t important for the doctor to know” or “the doctor never asked.”4,5 Ten tips for discussing nutritional supplements with patients are shown in the Box.
Buyer—and psychiatrist—beware. Because of dietary supplements’ regulatory status, physicians and patients need to learn as much as they can about these products’ documented safety and efficacy. The Dietary Supplement Health and Education Act passed by Congress in 1994 does not require manufacturers to prove their products are safe or effective before marketing them. They also can make claims that suggest uses in physical/emotional structure and function, such as “helps maintain a healthy emotional outlook.”
The FDA bears the burden of proof regarding safety and can remove a dietary supplement from the market only after receiving documented adverse event information from the public.6 The FDA has confiscated herbal products containing prescription drugs and misidentified herbal components.
GINKGO BILOBA FOR DEMENTIA
Ginkgo biloba was the third most commonly used herbal product (21%) in the NCCAM survey. Ginkgo is promoted primarily for dementia, cerebrovascular dysfunction, and memory enhancement. The standardized ginkgo extract (EGb 761) contains several components to which its pharmacologic activity has been attributed. Its constituents are thought to act primarily through anticoagulant effects by inhibiting platelet-activating factor and cyclic GMP phosphodiesterase. They also act through membrane stabilization, antioxidant properties, free-radical scavenging, and inhibition of beta-amyloid deposition.7
Efficacy. In patients with dementia, clinical trials using EGb 761 have shown small improvements in or maintenance of cognitive and social functioning, compared with placebo.8,9 The clinical significance of these findings is unclear, however. Ginkgo’s usefulness in enhancing memory is less certain. Controlled clinical trials are evaluating ginkgo’s efficacy in various conditions.10
Adverse effects. Bleeding complications are the primary concern with ginkgo biloba use, and caution is urged when it is taken concomitantly with aspirin or other antithrombotic drugs. Patients receiving warfarin should not take ginkgo because of the combined antiplatelet effects and ginkgo’s inhibition of warfarin metabolism and elimination.
Drug-herb interactions. One report showed that EGb 761 is a strong inhibitor of the cytochrome P-450 2C9 enzyme system (CYP 2C9).11 Other identified inhibitors of CYP 2C9 are fluvoxamine (strong inhibitor), amiodarone, cimetidine, fluoxetine, and omeprazole. Drugs that serve as substrates for that system and can have decreased clearance include phenytoin, warfarin, amitriptyline, and diazepam;12 use caution, therefore, when you co-administer these drugs with ginkgo.
Figure 10 CAM therapies patients report using most often
CAM: Complimentary and alternative medicines
Source: Barnes T, Powell-Griner E, McFann K, Nahin R. Complementary and alternative medicine use among adults: United States, 2002. Atlanta: Centers for Disease Control and Prevention, May 27, 2004: Advance Data Report #343 (available at http://www.cdc.gov/nchs/data/ad/ad343.pdf) Dosage. The usual dosage for standardized ginkgo extract is 120 to 240 mg/d, given in divided doses. Improvements associated with ginkgo use usually are seen within 4 to 12 weeks after starting therapy.7 Consider discontinuing therapy if you see no results after that time. Little is known about ginkgo’s effect after 1 year of use.
Recommendation. Limited data show a slight benefit in treating dementia by slowing cognitive and behavioral decline, but ginkgo biloba cannot be recommended as a first-line treatment until further controlled trials are available.
- Broach the subject without being judgmental; nutritional supplement use is tied to a patient’s health beliefs
- Ask specifically about use of herbs, supplements, teas, elixirs, vitamins, etc., and document in the medical record at each visit
- Include in appointment reminders a request that patients bring all medicines, herbs, and supplements to appointments
- Segue into a discussion of supplements by noting their use by other patients with similar diagnoses
- Learn about and provide objective information on products
- Suggest use of single-ingredient products because:
- Suggest a symptom diary and a plan to discontinue a supplement if desired results are not seen
- Report suspected adverse events or drug interactions to the FDA
- Choose your battles carefully; testimonials and the placebo effect can strongly influence patients’ desire to continue using dietary supplements
- Remember: Patient safety is paramount
ST. JOHN’S WORT FOR DEPRESSION
St. John’s wort (Hypericum perforatum), used to treat mild-to-moderate depression and anxiety,13 is one of the most-recognized herbal remedies. It accounted for 12% of the natural products used in the NCCAM survey. Several clinical trials assessing St. John’s wort are in progress and include placebo-controlled evaluations in obsessive-compulsive disorder and social phobia.14
As with ginkgo, St. John’s wort has many proposed active constituents; most preparations are standardized based on a hypericin content of 0.3%. Its mechanism of action in depression is unclear but laboratory models hint that it may be related to very mild inhibition of:
- monoamine oxidase (MAO)
- catechol-O-methyltransferase(COMT)
- selective serotonin reuptake
- interleukin-6 release (thereby increasing corticotropin-releasing hormone levels)
- norepinephrine uptake.15
Efficacy. St. John’s wort has been studied in mild, moderate, and major depression and compared with placebo and prescription therapies. In treating mild and moderate depression, St. John’s wort has been more effective than placebo and equivalent to tricyclic antidepressants.16,17 Criticisms of these trials include lack of product standardization, lack of comparison with standard antidepressants at appropriate dosages, and small sample sizes. Larger trials comparing St. John’s wort with placebo and sertraline in treating major depression showed no difference in effect among the three.18,19
Adverse effects are generally infrequent and include insomnia, anxiety, GI upset, and photosensitivity reactions.20 St. John’s wort can induce hypomania and mania in patients with bipolar disorder and cause psychosis in schizophrenic patients.13
Drug-herb interactions. Of greatest concern with St. John’s wort use is the remarkable number of drug-herb interactions that have been identified (Table 2 and Table 3).13,21 The primary mechanisms appear to be substantial induction of CYP 3A4, induction of P-glycoprotein mediated drug elimination, and—to a lesser extent—induction of other CYP isoenzymes.22 Interactions resulting in serotonin syndrome have been documented, with restlessness, sweating, and agitation.23
The 3A4 isoenzyme metabolizes most drugs processed via the CYP system.12 Severe interactions seen with St. John’s wort include:
- reduced cyclosporine levels, resulting in heart transplant rejection in two patients
- reduced antiretroviral levels in HIV patients
- pregnancy in women taking oral contraceptives.
Enzyme induction may persist for as long as 14 days after patients stop taking St. John’s wort.
Dosage. The recommended St. John’s wort dosage (using standardized 0.3% hypericin content) is 300 mg 2 or 3 times daily. Dosages of 1,200 to 1,800 mg/d have been used.13,18 Benefits may not be seen for 2 to 3 weeks, and experience with use beyond 8 weeks in mild-to-moderate depression is very limited.
Recommendation. Advise patients to taper off St. John’s wort when stopping therapy to decrease the risk of withdrawal symptoms such as confusion, headache, nausea, insomnia, and fatigue.21 Given the high risk for drug interactions associated with St. John’s wort, we do not recommend its use in patients receiving any other medications.
KAVA KAVA AND LIVER TOXICITY
Kava kava (Piper methysticum) is used by some patients to treat anxiety and insomnia. Compared with other nutritional supplements, kava is less commonly used—by only 6.6% of adults using nutritional supplements,1—and it is not being evaluated in NIH-sponsored trials.
The active-ingredient content varies considerably in kava root, so extracts are standardized to contain 70% kava-lactones (WS 1490). Although its exact mechanism is unclear, kava appears to:
- alter the limbic system
- inhibit monoamine oxidase type B (MAO-B)
- increase the number of gamma-aminobutyric acid (GABA) binding sites
- relax skeletal muscle
- produce anesthesia.24
Table 2
Documented interactions with St. John’s wort
| Alprazolam ↓ | Nevirapine ↓ |
| Amitriptyline ↓ | Oral contraceptives ↓ |
| Buspirone (ss) | Paroxetine (ss) |
| Cyclosporine ↓ | Sertraline (ss, mania) |
| Digoxin ↓ | Simvastatin ↓ |
| Fexofenadine ↓ | Tacrolimus ↓ |
| Indinavir ↓ | Theophylline ↓ |
| Irinotecan ↓ | Tyramine-containing |
| Methadone ↓ | foods (MAO-I reaction) |
| Midazolam ↓ | Venlafaxine (ss) |
| Nefazodone (ss) | Warfarin ↓ |
| ↓= decreased levels/effectiveness | |
| ss = serotonin syndrome | |
| Source: Adapted from reference 21 | |
Table 3
Select potential interactions with St. John’s wort
| Cannabinoids ↓ | Fentanyl ↓ |
| Cocaine ↓ | Temazepam ↓ |
| Diazepam ↓ | Triazolam ↓ |
| Donepezil ↓ | |
| ↓= decreased levels/effectiveness | |
| Source: Adapted from reference 12 | |
Efficacy. A meta-analysis of studies found kava more effective than placebo in treating anxiety,25 although most studies suffer from poor design and/or small sample size.
Adverse effects. Reports have associated kava use with hepatic toxicity, liver failure requiring liver transplantation, and death. The European Union and Canada have banned kava sales, and the FDA issued a consumer advisory noting kava’s risks. Emerging information indicates that kava inhibits virtually all CYP-450 enzymes, which would increase levels of and potential adverse effects from any medications taken with kava.26
Other common adverse effects include GI upset, enlarged pupils, extrapyramidal side effects, and dizziness.24,27
Dosage. Kava is usually given at 100 mg (70 mg of kavalactones) three times daily. Urge patients to avoid driving when taking kava because of its side effects. Anxiety symptoms may improve with 1 to 8 weeks of therapy, but adverse hepatic effects can occur within 3 to 4 weeks of starting kava use.
Recommendation. Avoid kava kava use because of substantial risk of hepatotoxicity and drug interactions.
VALERIAN FOR INSOMNIA, ANXIETY
Valerian (Valeriana officinalis) is promoted as a sedative/hypnotic and anxiolytic. The prevalence of valerian use (5.9%) mirrors that of kava.1 An NCCAM study is enrolling patients to evaluate valerian’s effectiveness in treating Parkinson’s disease-related sleep disturbances.
Efficacy. Information on valerian’s mechanism of action and clinical effectiveness is quite limited. In animal studies, its components produced direct sedative effects and inhibited CNS catabolism of GABA.28 Results are mixed in humans with insomnia; some studies have found reduced sleep latency and improved sleep quality with valerian use, whereas others found no improvements.29 Limited, small evaluations suggest that valerian may be useful in treating anxiety.
Adverse effects. The FDA categorizes valerian as an approved food additive, so it is considered safe in usual amounts found in food. FDA lists no amount that it considers safe in food, however, and the federal code covering valerian states that only enough needed to impart the desired flavor should be used.
When taken in therapeutic amounts, valerian’s most common adverse effects are headache and residual morning drowsiness. Because of the herb’s sedative effects, urge caution if patients drive while using it. On discontinuation, withdrawal symptoms similar to those seen with benzodiazepine withdrawal—anxiety, headache, emotional lability—have been reported.
Dosage. For insomnia, valerian is taken 2 hours to 30 minutes before bedtime; doses start at 300 to 400 mg and increase to 600 to 900 mg. Recommended doses vary, as standardization is less common with valerian than with other herbals. Continuous treatment seems more effective than as-needed dosing, as valerian may take up to 4 weeks to improve insomnia.
Recommendation. Well-controlled trials are lacking, and safety data at therapeutic doses are limited. Monitor patients using valerian for adverse effects and drug interactions.
- Natural Medicines Comprehensive Database.www.naturaldatabase.com
- National Center for Complementary and Alternative Medicine. http://nccam.nih.gov
- FDA MedWatch Adverse Event Reporting Program. www.fda.gov/medwatch/how.htm
- ConsumerLab.com. Independent testing of dietary supplements. www.consumerlab.com
Drug brand names
- Alprazolam • Xanax
- Amiodarone • Cordarone, Pacerone
- Amitriptyline • Elavil
- Buspirone • BuSpar
- Cimetidine • Tagamet
- Cyclosporine • various
- Diazepam • Valium
- Digoxin • Lanoxin
- Donepezil • Aricept
- Fentanyl • Duragesic
- Fexofenadine • Allegra
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Indinavir • Crixivan
- Irinotecan • Camptosar
- Methadone • various
- Midazolam • Versed
- Nefazodone • Serzone
- Nevirapine • Viramune
- Omeprazole • Prilosec
- Paroxetine • Paxil
- Phenytoin • Dilantin
- Sertraline • Zoloft
- Simvastatin • Zocor
- Tacrolimus • Prograf
- Temazepam • Restoril
- Theophylline • various
- Triazolam • Halcion
- Venlafaxine • Effexor
- Warfarin • Coumadin
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
What do you tell your patients who are self-medicating with herbal remedies? Can dietary supplements safely improve mood disorders, insomnia, and other psychiatric complaints?
Evidence is limited on complementary and alternative medicines (CAMs), their active components, pharmacokinetics/dynamics, adverse effects, drug interactions, and therapeutic outcomes. Based on our review of trial data, case reports, and an NIH National Center for Complementary and Alternative Medicine (NCCAM) survey,1 we offer information to help you:
- identify patients using dietary supplements
- avoid serious interactions with common psychotropics
- counsel patients on the efficacy and safety of ginkgo biloba, St. John’s wort, kava kava, and valerian (Table 1).
DON’T BE AFRAID TO ASK
One-third of Americans who took part in the NCCAM survey reported using CAM. After prayer—the number-one CAM—respondents said they most often used natural products such as herbals, botanicals, nutraceuticals, phytomedicinals, and dietary supplements (Figure).1
Table 1
4 herbal supplements with purported psychotropic effects
| Herb | Promoted use | Safety | Recommendation |
|---|---|---|---|
| Ginkgo biloba | Dementia, memory | Bleeding complications, drug interactions a concern | Some data support a trial in dementia; beware of safety concerns |
| St. John’s wort | Depression | Substantial drug interactions | Use not recommended because of wide-ranging drug interactions |
| Kava kava | Anxiety | Hepatotoxicity risk, drug interactions; off market in Europe and Canada | Use not recommended because of safety issues |
| Valerian | Insomnia | Limited data available | Benign (?); monitor for possible adverse events or drug interactions |
Patients tend to use CAM to treat chronic medical conditions such as back pain, depression, and anxiety.1 Although CAM use is common, only 38% of patients say they disclose using CAM to their physicians.2 These use and disclosure patterns are similar in psychiatry.3
Herbal products may produce symptoms that mimic those of mental illnesses, such as psychosis and mania, complicating diagnosis and treatment. Abruptly stopping some herbs can produce withdrawal symptoms similar to those seen with benzodiazepine and antidepressant cessation. Supplements also can inhibit or augment prescribed psychotropics’ effects, and notable consequences include the potential for serotonin syndrome with use of St. John’s wort.
The key to learning about a patient’s use of dietary supplements is to ask. Patients commonly say they do not tell their doctors about using dietary supplements because “it wasn’t important for the doctor to know” or “the doctor never asked.”4,5 Ten tips for discussing nutritional supplements with patients are shown in the Box.
Buyer—and psychiatrist—beware. Because of dietary supplements’ regulatory status, physicians and patients need to learn as much as they can about these products’ documented safety and efficacy. The Dietary Supplement Health and Education Act passed by Congress in 1994 does not require manufacturers to prove their products are safe or effective before marketing them. They also can make claims that suggest uses in physical/emotional structure and function, such as “helps maintain a healthy emotional outlook.”
The FDA bears the burden of proof regarding safety and can remove a dietary supplement from the market only after receiving documented adverse event information from the public.6 The FDA has confiscated herbal products containing prescription drugs and misidentified herbal components.
GINKGO BILOBA FOR DEMENTIA
Ginkgo biloba was the third most commonly used herbal product (21%) in the NCCAM survey. Ginkgo is promoted primarily for dementia, cerebrovascular dysfunction, and memory enhancement. The standardized ginkgo extract (EGb 761) contains several components to which its pharmacologic activity has been attributed. Its constituents are thought to act primarily through anticoagulant effects by inhibiting platelet-activating factor and cyclic GMP phosphodiesterase. They also act through membrane stabilization, antioxidant properties, free-radical scavenging, and inhibition of beta-amyloid deposition.7
Efficacy. In patients with dementia, clinical trials using EGb 761 have shown small improvements in or maintenance of cognitive and social functioning, compared with placebo.8,9 The clinical significance of these findings is unclear, however. Ginkgo’s usefulness in enhancing memory is less certain. Controlled clinical trials are evaluating ginkgo’s efficacy in various conditions.10
Adverse effects. Bleeding complications are the primary concern with ginkgo biloba use, and caution is urged when it is taken concomitantly with aspirin or other antithrombotic drugs. Patients receiving warfarin should not take ginkgo because of the combined antiplatelet effects and ginkgo’s inhibition of warfarin metabolism and elimination.
Drug-herb interactions. One report showed that EGb 761 is a strong inhibitor of the cytochrome P-450 2C9 enzyme system (CYP 2C9).11 Other identified inhibitors of CYP 2C9 are fluvoxamine (strong inhibitor), amiodarone, cimetidine, fluoxetine, and omeprazole. Drugs that serve as substrates for that system and can have decreased clearance include phenytoin, warfarin, amitriptyline, and diazepam;12 use caution, therefore, when you co-administer these drugs with ginkgo.
Figure 10 CAM therapies patients report using most often
CAM: Complimentary and alternative medicines
Source: Barnes T, Powell-Griner E, McFann K, Nahin R. Complementary and alternative medicine use among adults: United States, 2002. Atlanta: Centers for Disease Control and Prevention, May 27, 2004: Advance Data Report #343 (available at http://www.cdc.gov/nchs/data/ad/ad343.pdf) Dosage. The usual dosage for standardized ginkgo extract is 120 to 240 mg/d, given in divided doses. Improvements associated with ginkgo use usually are seen within 4 to 12 weeks after starting therapy.7 Consider discontinuing therapy if you see no results after that time. Little is known about ginkgo’s effect after 1 year of use.
Recommendation. Limited data show a slight benefit in treating dementia by slowing cognitive and behavioral decline, but ginkgo biloba cannot be recommended as a first-line treatment until further controlled trials are available.
- Broach the subject without being judgmental; nutritional supplement use is tied to a patient’s health beliefs
- Ask specifically about use of herbs, supplements, teas, elixirs, vitamins, etc., and document in the medical record at each visit
- Include in appointment reminders a request that patients bring all medicines, herbs, and supplements to appointments
- Segue into a discussion of supplements by noting their use by other patients with similar diagnoses
- Learn about and provide objective information on products
- Suggest use of single-ingredient products because:
- Suggest a symptom diary and a plan to discontinue a supplement if desired results are not seen
- Report suspected adverse events or drug interactions to the FDA
- Choose your battles carefully; testimonials and the placebo effect can strongly influence patients’ desire to continue using dietary supplements
- Remember: Patient safety is paramount
ST. JOHN’S WORT FOR DEPRESSION
St. John’s wort (Hypericum perforatum), used to treat mild-to-moderate depression and anxiety,13 is one of the most-recognized herbal remedies. It accounted for 12% of the natural products used in the NCCAM survey. Several clinical trials assessing St. John’s wort are in progress and include placebo-controlled evaluations in obsessive-compulsive disorder and social phobia.14
As with ginkgo, St. John’s wort has many proposed active constituents; most preparations are standardized based on a hypericin content of 0.3%. Its mechanism of action in depression is unclear but laboratory models hint that it may be related to very mild inhibition of:
- monoamine oxidase (MAO)
- catechol-O-methyltransferase(COMT)
- selective serotonin reuptake
- interleukin-6 release (thereby increasing corticotropin-releasing hormone levels)
- norepinephrine uptake.15
Efficacy. St. John’s wort has been studied in mild, moderate, and major depression and compared with placebo and prescription therapies. In treating mild and moderate depression, St. John’s wort has been more effective than placebo and equivalent to tricyclic antidepressants.16,17 Criticisms of these trials include lack of product standardization, lack of comparison with standard antidepressants at appropriate dosages, and small sample sizes. Larger trials comparing St. John’s wort with placebo and sertraline in treating major depression showed no difference in effect among the three.18,19
Adverse effects are generally infrequent and include insomnia, anxiety, GI upset, and photosensitivity reactions.20 St. John’s wort can induce hypomania and mania in patients with bipolar disorder and cause psychosis in schizophrenic patients.13
Drug-herb interactions. Of greatest concern with St. John’s wort use is the remarkable number of drug-herb interactions that have been identified (Table 2 and Table 3).13,21 The primary mechanisms appear to be substantial induction of CYP 3A4, induction of P-glycoprotein mediated drug elimination, and—to a lesser extent—induction of other CYP isoenzymes.22 Interactions resulting in serotonin syndrome have been documented, with restlessness, sweating, and agitation.23
The 3A4 isoenzyme metabolizes most drugs processed via the CYP system.12 Severe interactions seen with St. John’s wort include:
- reduced cyclosporine levels, resulting in heart transplant rejection in two patients
- reduced antiretroviral levels in HIV patients
- pregnancy in women taking oral contraceptives.
Enzyme induction may persist for as long as 14 days after patients stop taking St. John’s wort.
Dosage. The recommended St. John’s wort dosage (using standardized 0.3% hypericin content) is 300 mg 2 or 3 times daily. Dosages of 1,200 to 1,800 mg/d have been used.13,18 Benefits may not be seen for 2 to 3 weeks, and experience with use beyond 8 weeks in mild-to-moderate depression is very limited.
Recommendation. Advise patients to taper off St. John’s wort when stopping therapy to decrease the risk of withdrawal symptoms such as confusion, headache, nausea, insomnia, and fatigue.21 Given the high risk for drug interactions associated with St. John’s wort, we do not recommend its use in patients receiving any other medications.
KAVA KAVA AND LIVER TOXICITY
Kava kava (Piper methysticum) is used by some patients to treat anxiety and insomnia. Compared with other nutritional supplements, kava is less commonly used—by only 6.6% of adults using nutritional supplements,1—and it is not being evaluated in NIH-sponsored trials.
The active-ingredient content varies considerably in kava root, so extracts are standardized to contain 70% kava-lactones (WS 1490). Although its exact mechanism is unclear, kava appears to:
- alter the limbic system
- inhibit monoamine oxidase type B (MAO-B)
- increase the number of gamma-aminobutyric acid (GABA) binding sites
- relax skeletal muscle
- produce anesthesia.24
Table 2
Documented interactions with St. John’s wort
| Alprazolam ↓ | Nevirapine ↓ |
| Amitriptyline ↓ | Oral contraceptives ↓ |
| Buspirone (ss) | Paroxetine (ss) |
| Cyclosporine ↓ | Sertraline (ss, mania) |
| Digoxin ↓ | Simvastatin ↓ |
| Fexofenadine ↓ | Tacrolimus ↓ |
| Indinavir ↓ | Theophylline ↓ |
| Irinotecan ↓ | Tyramine-containing |
| Methadone ↓ | foods (MAO-I reaction) |
| Midazolam ↓ | Venlafaxine (ss) |
| Nefazodone (ss) | Warfarin ↓ |
| ↓= decreased levels/effectiveness | |
| ss = serotonin syndrome | |
| Source: Adapted from reference 21 | |
Table 3
Select potential interactions with St. John’s wort
| Cannabinoids ↓ | Fentanyl ↓ |
| Cocaine ↓ | Temazepam ↓ |
| Diazepam ↓ | Triazolam ↓ |
| Donepezil ↓ | |
| ↓= decreased levels/effectiveness | |
| Source: Adapted from reference 12 | |
Efficacy. A meta-analysis of studies found kava more effective than placebo in treating anxiety,25 although most studies suffer from poor design and/or small sample size.
Adverse effects. Reports have associated kava use with hepatic toxicity, liver failure requiring liver transplantation, and death. The European Union and Canada have banned kava sales, and the FDA issued a consumer advisory noting kava’s risks. Emerging information indicates that kava inhibits virtually all CYP-450 enzymes, which would increase levels of and potential adverse effects from any medications taken with kava.26
Other common adverse effects include GI upset, enlarged pupils, extrapyramidal side effects, and dizziness.24,27
Dosage. Kava is usually given at 100 mg (70 mg of kavalactones) three times daily. Urge patients to avoid driving when taking kava because of its side effects. Anxiety symptoms may improve with 1 to 8 weeks of therapy, but adverse hepatic effects can occur within 3 to 4 weeks of starting kava use.
Recommendation. Avoid kava kava use because of substantial risk of hepatotoxicity and drug interactions.
VALERIAN FOR INSOMNIA, ANXIETY
Valerian (Valeriana officinalis) is promoted as a sedative/hypnotic and anxiolytic. The prevalence of valerian use (5.9%) mirrors that of kava.1 An NCCAM study is enrolling patients to evaluate valerian’s effectiveness in treating Parkinson’s disease-related sleep disturbances.
Efficacy. Information on valerian’s mechanism of action and clinical effectiveness is quite limited. In animal studies, its components produced direct sedative effects and inhibited CNS catabolism of GABA.28 Results are mixed in humans with insomnia; some studies have found reduced sleep latency and improved sleep quality with valerian use, whereas others found no improvements.29 Limited, small evaluations suggest that valerian may be useful in treating anxiety.
Adverse effects. The FDA categorizes valerian as an approved food additive, so it is considered safe in usual amounts found in food. FDA lists no amount that it considers safe in food, however, and the federal code covering valerian states that only enough needed to impart the desired flavor should be used.
When taken in therapeutic amounts, valerian’s most common adverse effects are headache and residual morning drowsiness. Because of the herb’s sedative effects, urge caution if patients drive while using it. On discontinuation, withdrawal symptoms similar to those seen with benzodiazepine withdrawal—anxiety, headache, emotional lability—have been reported.
Dosage. For insomnia, valerian is taken 2 hours to 30 minutes before bedtime; doses start at 300 to 400 mg and increase to 600 to 900 mg. Recommended doses vary, as standardization is less common with valerian than with other herbals. Continuous treatment seems more effective than as-needed dosing, as valerian may take up to 4 weeks to improve insomnia.
Recommendation. Well-controlled trials are lacking, and safety data at therapeutic doses are limited. Monitor patients using valerian for adverse effects and drug interactions.
- Natural Medicines Comprehensive Database.www.naturaldatabase.com
- National Center for Complementary and Alternative Medicine. http://nccam.nih.gov
- FDA MedWatch Adverse Event Reporting Program. www.fda.gov/medwatch/how.htm
- ConsumerLab.com. Independent testing of dietary supplements. www.consumerlab.com
Drug brand names
- Alprazolam • Xanax
- Amiodarone • Cordarone, Pacerone
- Amitriptyline • Elavil
- Buspirone • BuSpar
- Cimetidine • Tagamet
- Cyclosporine • various
- Diazepam • Valium
- Digoxin • Lanoxin
- Donepezil • Aricept
- Fentanyl • Duragesic
- Fexofenadine • Allegra
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Indinavir • Crixivan
- Irinotecan • Camptosar
- Methadone • various
- Midazolam • Versed
- Nefazodone • Serzone
- Nevirapine • Viramune
- Omeprazole • Prilosec
- Paroxetine • Paxil
- Phenytoin • Dilantin
- Sertraline • Zoloft
- Simvastatin • Zocor
- Tacrolimus • Prograf
- Temazepam • Restoril
- Theophylline • various
- Triazolam • Halcion
- Venlafaxine • Effexor
- Warfarin • Coumadin
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. Advance data from vital and health statistics; no 343. Hyattsville, MD: National Center for Health Statistics, 2004.
2. Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997. Results of a follow-up survey. JAMA 1998;280:1569-75.
3. Matthews SC, Camacho A, Lawson K, Dimsdale JE. Use of herbal medications among 200 psychiatric outpatients: prevalence, patterns of use, and potential dangers. Gen Hosp Psychiatry 2003;25:24-6.
4. Eisenberg DM. Advising patients who seek alternative medical therapies. Ann Intern Med 1997;127:61-9.
5. Grant KL. Patient education and herbal dietary supplements. Am J Health-Syst Pharm 2000;57:1997-2003.
6. Harris IM. Regulatory and ethical issues with dietary supplements. Pharmacotherapy 2000;20:1295-1302.
7. Sierpina VS, Wollschlaeger B, Blumenthal M. Ginkgo biloba. Am Fam Physician 2003;68:923-6.
8. LeBars PL, Katz MM, Berman N, et al. A placebo-controlled, double-blind, randomized trial of an extract of ginkgo biloba for dementia. JAMA 1997;278:1327-32.
9. Oken BS, Storzbach DM, Kaye JA. The efficacy of ginkgo biloba on cognitive function in Alzheimer’s disease. Arch Neurol 1998;55:1409-15.
10. Ginkgo biloba clinical trials National Center for Complementary and Alternative Medicine.National Institutes of Health. http://nccam.nih.gov/clinicaltrials/ginkgo.htm. Accessed Dec. 9, 2004.
11. Gaudineau C, Beckerman R, Welbourn S, Auclair K. Inhibition of human P450 enzymes by multiple constituents of the ginkgo biloba extract. Biochem Biophys Res Commun 2004;318:1072-8.
12. Michalets EL. Update: clinically significant cytochrome P-450 drug interactions. Pharmacotherapy 1998;18:84-112.
13. Pepping J. St.John’s wort: hypericum perforatum. Am J HealthSyst Pharm 1999;56:329-30.
14. St John’s wort (hypericum) clinical trials. National Center for Complementary and Alternative Medicine. National Institutes of Health. http://nccam.nih.gov/clinicaltrials/stjohnswort/index.htm. Accessed Dec. 9, 2004.
15. Bennett DA, Phun L, Polk JF, et al. Neuropharmacology of St.John’s wort (hypericum). Ann Pharmacother 1998;32:1201-8.
16. Gaster B, Holroyd J. St.John’s wort for depression: a systematic review. Arch Intern Med 2000;160:152-6.
17. Linde K, Ramirez G, Mulrow CD, et al. St.John’s wort for depression—an overview and meta-analysis of randomized clinical trials. BMJ 1996;313:253-8.
18. Shelton RC, Keller MB, Gelenberg A, et al. Effectiveness of St.John’s wort in major depression: a randomized controlled trial. JAMA 2001;285:1978-86.
19. Hypericum Depression Trial Study Group. Effect of Hypericum perforatum (St.John’s wort) in major depressive disorder: a randomized controlled trial. JAMA 2002;287:1807-14.
20. Beckman SE, Sommi RW, Switzer J. Consumer use of St.John’s wort: a survey on effectiveness, safety, and tolerability. Pharmacotherapy 2000;20:568-74.
21. Izzo AA. Drug interactions with St.John’s wort (hypericum perforatum): a review of the clinical evidence. Int J Clin Pharmacol Ther 2004;42:139-48.
22. Markowitz JS, Donovan JL, DeVane CL, et al. Effect of St.John’s wort on drug metabolism by induction of cytochrome P450 3A4 enzyme. JAMA 2003;290:1500-4.
23. Sternbach H. Serotonin syndrome: how to avoid, identify, and treat dangerous drug reactions. Current Psychiatry 2003;2(5):14-24.
24. Pepping J. Kava: Piper methysticum. Am J Health-Syst Pharm. 11999;56:957-60.
25. Pittler MH, Ernst E. Efficacy of kava extract for treating anxiety: systematic review and meta-analysis. J Clin Psychopharmacol 2000;20:84-9.
26. Matthews JM, Etheridge AS, Black SR. Inhibition of human cytochrome P450 activities by kava extract and kavalactones. Drug Metab Dispos 2002;30:1153-7.
27. Jellin JM, Gregory P, Batz F, et al. Kava Pharmacist’s Letter/Prescriber’s Letter Natural Medicines Comprehensive Database (3rd ed). Stockton, CA: Therapeutic Research Faculty, 2000;625:7.-
28. Hadley S, Petry JJ. Valerian. Am Fam Physician 2003;67:1755-8.
29. Plushner SL. Valerian: Valeriana officinalis. Am J Health-Syst Pharm 2000;57:328-35.
1. Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. Advance data from vital and health statistics; no 343. Hyattsville, MD: National Center for Health Statistics, 2004.
2. Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997. Results of a follow-up survey. JAMA 1998;280:1569-75.
3. Matthews SC, Camacho A, Lawson K, Dimsdale JE. Use of herbal medications among 200 psychiatric outpatients: prevalence, patterns of use, and potential dangers. Gen Hosp Psychiatry 2003;25:24-6.
4. Eisenberg DM. Advising patients who seek alternative medical therapies. Ann Intern Med 1997;127:61-9.
5. Grant KL. Patient education and herbal dietary supplements. Am J Health-Syst Pharm 2000;57:1997-2003.
6. Harris IM. Regulatory and ethical issues with dietary supplements. Pharmacotherapy 2000;20:1295-1302.
7. Sierpina VS, Wollschlaeger B, Blumenthal M. Ginkgo biloba. Am Fam Physician 2003;68:923-6.
8. LeBars PL, Katz MM, Berman N, et al. A placebo-controlled, double-blind, randomized trial of an extract of ginkgo biloba for dementia. JAMA 1997;278:1327-32.
9. Oken BS, Storzbach DM, Kaye JA. The efficacy of ginkgo biloba on cognitive function in Alzheimer’s disease. Arch Neurol 1998;55:1409-15.
10. Ginkgo biloba clinical trials National Center for Complementary and Alternative Medicine.National Institutes of Health. http://nccam.nih.gov/clinicaltrials/ginkgo.htm. Accessed Dec. 9, 2004.
11. Gaudineau C, Beckerman R, Welbourn S, Auclair K. Inhibition of human P450 enzymes by multiple constituents of the ginkgo biloba extract. Biochem Biophys Res Commun 2004;318:1072-8.
12. Michalets EL. Update: clinically significant cytochrome P-450 drug interactions. Pharmacotherapy 1998;18:84-112.
13. Pepping J. St.John’s wort: hypericum perforatum. Am J HealthSyst Pharm 1999;56:329-30.
14. St John’s wort (hypericum) clinical trials. National Center for Complementary and Alternative Medicine. National Institutes of Health. http://nccam.nih.gov/clinicaltrials/stjohnswort/index.htm. Accessed Dec. 9, 2004.
15. Bennett DA, Phun L, Polk JF, et al. Neuropharmacology of St.John’s wort (hypericum). Ann Pharmacother 1998;32:1201-8.
16. Gaster B, Holroyd J. St.John’s wort for depression: a systematic review. Arch Intern Med 2000;160:152-6.
17. Linde K, Ramirez G, Mulrow CD, et al. St.John’s wort for depression—an overview and meta-analysis of randomized clinical trials. BMJ 1996;313:253-8.
18. Shelton RC, Keller MB, Gelenberg A, et al. Effectiveness of St.John’s wort in major depression: a randomized controlled trial. JAMA 2001;285:1978-86.
19. Hypericum Depression Trial Study Group. Effect of Hypericum perforatum (St.John’s wort) in major depressive disorder: a randomized controlled trial. JAMA 2002;287:1807-14.
20. Beckman SE, Sommi RW, Switzer J. Consumer use of St.John’s wort: a survey on effectiveness, safety, and tolerability. Pharmacotherapy 2000;20:568-74.
21. Izzo AA. Drug interactions with St.John’s wort (hypericum perforatum): a review of the clinical evidence. Int J Clin Pharmacol Ther 2004;42:139-48.
22. Markowitz JS, Donovan JL, DeVane CL, et al. Effect of St.John’s wort on drug metabolism by induction of cytochrome P450 3A4 enzyme. JAMA 2003;290:1500-4.
23. Sternbach H. Serotonin syndrome: how to avoid, identify, and treat dangerous drug reactions. Current Psychiatry 2003;2(5):14-24.
24. Pepping J. Kava: Piper methysticum. Am J Health-Syst Pharm. 11999;56:957-60.
25. Pittler MH, Ernst E. Efficacy of kava extract for treating anxiety: systematic review and meta-analysis. J Clin Psychopharmacol 2000;20:84-9.
26. Matthews JM, Etheridge AS, Black SR. Inhibition of human cytochrome P450 activities by kava extract and kavalactones. Drug Metab Dispos 2002;30:1153-7.
27. Jellin JM, Gregory P, Batz F, et al. Kava Pharmacist’s Letter/Prescriber’s Letter Natural Medicines Comprehensive Database (3rd ed). Stockton, CA: Therapeutic Research Faculty, 2000;625:7.-
28. Hadley S, Petry JJ. Valerian. Am Fam Physician 2003;67:1755-8.
29. Plushner SL. Valerian: Valeriana officinalis. Am J Health-Syst Pharm 2000;57:328-35.