User login
Why aren’t more psychiatrists prescribing buprenorphine?
After 10 years of heroin dependence, Mr. T, age 36, calls your office and says, “I want to get off heroin.” For 8 months, he’s been using IV heroin 2 to 3 times daily. He says he has tried methadone treatment but found daily dosing cumbersome.
He found your office number on the Substance Abuse and Mental Health Services Administration Web site, which lists physicians qualified to prescribe buprenorphine for opiate detoxification. He has heard about “bup” on the street and wants to know if he is eligible and what he can expect from treatment.
Like Mr. T, 1 million Americans are addicted to opiates.1 As qualified physicians gain experience with using buprenorphine, this agent could revolutionize how opiate-dependent patients are routinely treated. Instead of receiving methadone only in specialized clinics, they can now choose to be treated in physicians’ offices.
Several obstacles, however, are preventing widespread buprenorphine use:
- Too few physicians are trained to offer this office-based treatment.
- Many of the 2,000 doctors who are trained remain uncertain about using buprenorphine.
This article is intended to help overcome obstacles to opiate-dependence treatment by familiarizing psychiatrists—whether trained or not in using buprenorphine—with evidence of this agent’s efficacy and its advantages compared with other treatments.
Efficacy and obstacles
Buprenorphine is a partial opioid agonist that binds to the mu receptor (Box 1).2-5 It is a controlled substance (schedule-III narcotic). Outpatient trials have shown that buprenorphine is more effective than placebo and as effective as methadone for opiate detoxification.6-10
In one of the largest trials, 326 opiate-dependent outpatients were randomly assigned to buprenorphine, buprenorphine/naloxone combination, or placebo for 4 weeks. Both buprenorphine forms were more effective than placebo, as measured by clean urine samples and patient reports of reduced opiate cravings.7
In maintenance treatment, buprenorphine has been shown to be more effective than placebo and as effective as methadone, 60 mg/d, in preventing relapse. In a randomized comparison study, 220 opiate-dependent patients received levomethadyl acetate (LAAM), 75 to 115 mg three times a week; buprenorphine, 16 to 32 mg three times a week; high-dose methadone (60 to 100 mg/d); or low-dose methadone (20 mg/d). Subjects reported using opiates 20 to 30 times in the week before study enrollment. After 17 weeks, treatment retention rates were 58% for buprenorphine, 73% for high-dose methadone, and 20% for low-dose methadone. At the same point, urine samples were negative for opiate use in 40% of patients receiving buprenorphine compared with 39% of those receiving high-dose methadone.10
Buprenorphine is a partial opioid agonist derived from thebaine, an anodyne alkaloid from opium. It binds tightly to the muopiate receptor and produces expected opiate effects such as analgesia and mild euphoria.2 Its partial agonist properties create a ceiling effect and thus a lower likelihood of overdose than with opioid agonists.3
Pharmacokinetics
Buprenorphine has low bioavailability, but its 24- to 60-hour half-life allows once-daily dosing. Because common urine drug screens cannot detect buprenorphine, its use does not cause positive tests for opiates or morphine. Overdose risk is minimal when taken sublingually, with no respiratory depression reported in clinical trials.3,4 The drug is metabolized by the cytochrome P-450 3A4 isoenzyme system and demethylated to norbuprenorphine, which is not significantly bioactive.
Side effects
Nausea, fatigue, constipation, and occasional dysphoria
Abuse potential
Euphoria is less likely with buprenorphine than with opioid agonists because of buprenorphine’s ceiling effects. Theoretically, buprenorphine can be abused by being crushed and injected. The buprenorphine/naloxone combination, if taken parenterally, precipitates opiate withdrawal and thus is preferred for most patients with opioid dependence.
Buprenorphine may be fatal when abused, especially in combination with CNS depressants such as alcohol or high-dose benzodiazepines. However, buprenorphine’s mortality risk remains lower than that of methadone.5
Special precautions
Buprenorphine may precipitate opiate withdrawal during induction when an opioid agonist remains bound to the opiate receptor. Buprenorphine will displace the opiate from the receptor, creating an imbalance in opiate binding that the body interprets as opiate withdrawal.2
To avoid withdrawal, tell the patient not to start buprenorphine until mild withdrawal symptoms occur. In case of withdrawal, tell the patient to continue taking buprenorphine until symptoms are relieved. Adjunctive medications such as benzodiazepines, antiemetics, and antidiarrheals also can be given to control symptoms.
Table
Buprenorphine: A typical dosing strategy
| Phase | Dosage* | Comment |
|---|---|---|
| Induction | Maximum dosage is 32 mg/d; 12 to 24 mg/d typically controls withdrawal symptoms | |
| Day 1 | 4 mg bid (total 8 mg) | |
| Day 2 | 12 mg qd | |
| Day 3 | 16 mg qd | |
| Maintenance | 16 to 24 mg/d is average stabilization dosage | Consider severity of withdrawal symptoms and duration of addiction when deciding when to begin discontinuation |
| Discontinuation | Taper dosage by 2 to 4 mg every 3 to 5 days, then discontinue | Most patients remain on final 2 mg/d at least 1 week; consider alternate-day dosing for patients who experience side effects when attempting to reduce from 2 mg/d to 0 mg/d |
| * Buprenorphine/naloxone is preferred formulation | ||
Slow adoption. Opiate-dependence treatments such as methadone are prescribed in highly regulated environments, which is one reason only 25% of opiate addicts in the United States ever receive treatment.1 Unfortunately, little has changed in the 20 months since the FDA approved buprenorphine for office-based detoxification and maintenance treatment of opiate dependence. More than 2,000 physicians have been trained to use buprenorphine, yet only 20% of them report prescribing it.11
Reasons for this slow introduction include:
- difficulty in obtaining the medication
- lack of appropriate support staff and facilities
- uncertainty about prescribing the medication, despite special training.
Availability. When buprenorphine came to market in late 2003, most commercial pharmacies were not stocking it and it had to be special-ordered. As a result, patients receiving prescriptions had to wait 2 to 3 days for their first dose—a substantial deterrent to prescribing or taking this type of medication. Also, some private physicians and clinics do not keep buprenorphine samples to dispense on-site.
More pharmacies are stocking the medication now, but it remains the physician’s responsibility to ensure that a supply can be dispensed the day it is prescribed.
Support staff and facilities. To prescribe buprenorphine effectively, the physician needs resources for urine testing, physical exams, lab testing, and storing and dispensing buprenorphine. An integrated treatment clinic for opiate-dependent patients, complete with nursing and administrative staff, is ideal. If this support is not available, however, clinicians in private practice can safely prescribe buprenorphine from the office.
Uncertainty. Physicians often adopt new prescription products without hesitation, but buprenorphine’s administration and patient population are unusual. Even some physicians who have taken the special training course remain anxious about using this agent because it may precipitate opiate withdrawal. Also, the training requirement creates a sense that specialist-level knowledge is needed to safely prescribe buprenorphine.
Treatment requirements
For clinicians. The Drug Addiction Treatment Act of 2000 allows physicians to apply for a waiver from the Controlled Substances Act to prescribe buprenorphine for detoxification. A waiver is not required to prescribe buprenorphine for pain.12
To qualify for the waiver, physicians must be board-certified in addiction psychiatry or have completed a buprenorphine training course. Training is offered online and as a 1-day conference by the American Society of Addiction Medicine, American Academy of Addiction Psychiatry, American Medical Association, and American Psychiatric Association.
For patients. Like Mr. T, opiate users who ask about buprenorphine will want to know what to expect from treatment. To be eligible for buprenorphine treatment, a patient must:
- meet criteria for opiate dependence
- commit to keeping regular appointments—at least 3 times a week for the first 2 weeks then usually once weekly until detoxification is complete
- undergo random urine testing
- participate in psychosocial treatments.
So far, patients’ awareness of buprenorphine is highly variable. Asking an opiate user who presents for treatment what he or she knows about buprenorphine can be a useful screening tool. Highly motivated patients will have read about buprenorphine on the Internet, where they probably obtained your office phone number.
When a patient is accepted into treatment, detoxification with buprenorphine includes three phases: induction, stabilization/mainte-nance, and discontinuation.13 After stabilization, some patients remain in maintenance indefinitely and choose not to discontinue buprenorphine. The choice of who to discontinue and who to maintain on buprenorphine is a clinical decision made by the patient and practitioner. Success rates of detoxification with buprenorphine are similar to rates achieved with methadone and clonidine, although most studies have been conducted during buprenorphine maintenance.5
Case continued: Surprised to feel ‘normal’
Mr. T qualified for buprenorphine and came to the office feeling fairly ill. During withdrawal, his usual first symptom is rhinorrhea, followed by malaise, myalgia, restlessness, and intense cravings. His score of 24 on the Clinical Opiate Withdrawal Scale (COWS), indicated moderate withdrawal.
He felt better but not completely well 1 hour after taking buprenorphine/naloxone, 4 mg. He was given a 4-mg tablet to take at home 2 hours later. The next day his COWS score was 8, indicating mild withdrawal. He said he was surprised at how “normal” he was feeling.
Induction: Getting started
Buprenorphine induction is usually done during mild to moderate opiate withdrawal. Starting buprenorphine too soon—while the patient is relatively comfortable—may precipitate withdrawal because the agent will rapidly displace opiate bound to the receptors. In most cases, the first dose is given in the office so that the patient’s response can be monitored.
Two formulations. Buprenorphine comes alone (in 2- or 8-mg tablets) or in combination with naloxone (in 2 mg/0.5 mg and 8 mg/2 mg tablets). Both forms are given sublingually. Contrary to popular belief, IM buprenorphine is not approved for treating opiate addiction.
Naloxone is not absorbed in sublingual form and serves only to deter IV diversion of buprenorphine. Induction with buprenorphine alone is reserved for patients with documented allergy to naloxone or who are being detoxified from long-acting opiates such as methadone.
Dosing strategies are identical for both formulations. The usual starting dosage is 4 mg once daily, with a maximum dosage of 32 mg/d (Table). Withdrawal symptoms are typically controlled with 12 to 24 mg/d.14
If the patient is in active opiate withdrawal, the starting dose usually relieves symptoms in 30 to 45 minutes. If not, a second 4-mg dose can be given. Most patients do not require >8 mg the first day, but some may require 16 to 24 mg to suppress withdrawal symptoms.15
Some clinicians—such as solo practitioners who lack the resources of an outpatient clinic—prefer to have the patient take the first dose at home. Patients are instructed to take the first dose after withdrawal symptoms begin and to repeat the dose in 1 hour if symptoms persist. Thus, patients titrate their own dosages, but the clinician must be immediately available to handle complications. Induction continues until withdrawal symptoms are controlled.
The next day, patients return for evaluation. An objective scale such as the 11-item COWS can quantify withdrawal symptom severity.16 For each symptom—heart rate, nausea, diaphoresis, or restlessness—the COWS assigns a number corresponding to its severity. A total score >25 indicates moderately severe withdrawal.
After withdrawal symptoms are controlled, follow-up visits are scheduled every 2 to 3 days the first week and then weekly. Some physicians maintain daily contact with patients via e-mail or telephone to track symptoms.
Case continued: Steady improvement
By day 3, Mr. T gradually increased his buprenorphine/naloxone dosage to 16 mg once daily. He continued that dosage for 10 days before his next visit. At that point, he was slightly anxious but physically comfortable. He came into the office on days 2, 5, and 10 and his COWS scores decreased each time.
Stabilization and maintenance
When withdrawal symptoms are stabilized, patients begin maintenance therapy at the dosage that stabilized their symptoms. During maintenance therapy, the average buprenorphine dosage is 16 to 24 mg/d. Because of its long half-life, buprenorphine can be taken once daily, though some patients prefer twice-daily dosing for psychological comfort. Several studies comparing buprenorphine with methadone have found that buprenorphine, 8 to 16 mg/d, is similar in effect to approximately 60 mg/d of methadone.5
During the maintenance phase, it is important to have a policy for patients who relapse to substance abuse while taking buprenorphine (Box 2). During buprenorphine maintenance treatment, the estimated relapse rate to opiate use (chance of one positive test for opiates) ranges from 20% to 60%, compared with a relapse rate of 80% to 90% seen with placebo during clinical trials.5
Case continued: Time to taper?
After taking buprenorphine 2 months, Mr. T wants to taper off. He has been seen weekly and receives individual psychotherapy and group counseling. All urine drug screens have been negative for opiates.
With the psychiatrist’s observation, Mr. T. begins to taper his dosage of 16 mg/d by 4 mg a week. He is comfortable when he reaches 4 mg/d, but notices increased anxiety and general achiness when he reduces buprenorphine to 2 mg/d. He elects to remain at 4 mg/d for another 2 months.
Discontinuation
After the patient has reached a stable dose of buprenorphine, the clinician and patient together consider two treatment options:
- sustain the dose as maintenance therapy
- or taper and discontinue buprenorphine.
Screening
Screen patients for alcohol or benzodiazepine use, which may trigger symptoms similar to opiate withdrawal (buprenorphine does not treat withdrawal from these substances)
Induction
Worsening symptoms with buprenorphine indicate that withdrawal was precipitated; repeat buprenorphine dosing until symptoms are relieved (do not exceed 24 mg the first day)
Tell patients:
- to wait as long as possible before taking the first dose to reduce risk of precipitating withdrawal
- not to swallow the sublingual tablet, as this inactivates the medication
- the tablet can take 5 minutes or more to dissolve under the tongue
Maintenance
Set a policy for patients who relapse to substance use while taking buprenorphine. Consequences may include immediate buprenorphine cessation, transfer to methadone treatment, re-induction of buprenorphine, or referral to an inpatient substance abuse treatment center
Tracking
Log how many of your patients are taking buprenorphine; you may not treat more than 30 at a time
Patients who have had multiple relapses and endured severe opiate withdrawal might consider remaining on buprenorphine for several months before tapering. Mild opiate withdrawal may occur if buprenorphine is tapered too rapidly, though this is not as severe or distressing as a full agonist withdrawal.
Tapering recommendations. To taper buprenorphine, reduce by 2 to 4 mg every 3 to 5 days until the patient is taking 2 mg/d. Most patients remain on this dosage at least 1 week and then discontinue. Those who experience side effects when dropping from 2 mg to 0 mg can take 2 mg every other day for 1 week and then discontinue.
After patients are tapered completely off buprenorphine, encourage them to follow up with the treating physician for at least another month. Opiate withdrawal symptoms have been reported to linger 2 to 3 weeks after the last dose of buprenorphine, but these symptoms—usually anxiety or insomnia—tend to be self-limiting.5
Case continued: Time to taper?
Mr. T has been free from heroin use for 4 months and has returned to work. He is rebuilding his life and continues with psychotherapy. He follows up at the buprenorphine clinic monthly for medication management. At each visit, he repeats the COWS test and undergoes a urine drug screen and vital signs check.
Related resources
- Substance Abuse and Mental Health Services Administration. Physician locator for buprenorphine providers.
- American Academy of Addiction Psychiatry. Information about buprenorphine training course. www.aaap.org/buprenorphine/buprenorphine.html
- Buprenorphine manufacturer’s Web site. Answers to frequently-asked questions. www.suboxone.com
Drug brand names
- Buprenorphine • Subutex
- Buprenorphine/naloxone • Suboxone
- Buprenorphine (IM) • Buprenex
- Clonidine • Catapres
1. Vastag B. In-office opiate treatment, “not a panacea:” physicians slow to embrace therapeutic option. JAMA 2003;290:731-5.
2. Marquet P. Pharmacology of high-dose buprenorphine. In: Kintz P, Marquet P (eds). Buprenorphine therapy of opiate addiction. Totowa, NJ: Humana Press, 2002;69-82.
3. Boyd J, Randell T, Luurila H, Kuisma M. Serious overdoses involving buprenorphine in Helsinki. Acta Anaesthesiol Scand 2003;47:1031-3.
4. Johnson RE, Cone EJ, Henningfield JE, Fudala PJ. Use of buprenorphine in the treatment of opiate addiction. Clin Pharmacol Ther 1989;46:335-43.
5. Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend 2003;70:S59-S77.
6. Breen CL, Harris SJ, Lintzeris N, et al. Cessation of methadone maintenance treatment using buprenorphine: transfer from methadone to buprenorphine and subsequent buprenorphine reductions. Drug Alcohol Depend 2003;71:49-55.
7. Fudala PJ, Bridge PT, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med 2003;349:949-58.
8. Ling W, Wesson DR. Clinical efficacy of buprenorphine: comparisons to methadone and placebo. Drug Alcohol Depend 2003;70:S49-S57.
9. Gibson AE, Doran CM, Bell JR, et al. A comparison of buprenorphine treatment in clinic and primary care settings; a randomised trial. Med J Aust 2003;179:38-42.
10. Johnson RE, Chutuape MA, Strain EC, et al. A comparison of levomethadyl acetate, buprenorphine and methadone for opioid dependence. N Engl J Med 2000;343:1290-7.
11. Bivol S. National poll of physicians on barriers to widespread buprenorphine use. Boston University School of Public Health, Join Together 2003;1-7.
12. Clark HW. Office-based practice and opioid-use disorders. N Engl J Med 2003;349:928-30.
13. Ling W, Smith D. Buprenorphine: blending practice and research. J Subst Abuse Treat 2002;23:87-92.
14. Fiellin DA, Pantalon MV, Pakes JP, et al. Treatment of heroin dependence with buprenorphine in primary care. Am J Drug Alcohol Abuse 2002;28:231-41.
15. Greenwald MK, Schuh KJ, Stine SM. Transferring methadone-maintained outpatients to the buprenorphine sublingual tablet: a preliminary study. Am J Addict 2003;12:365-74.
16. Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs 2003;35:253-9.
After 10 years of heroin dependence, Mr. T, age 36, calls your office and says, “I want to get off heroin.” For 8 months, he’s been using IV heroin 2 to 3 times daily. He says he has tried methadone treatment but found daily dosing cumbersome.
He found your office number on the Substance Abuse and Mental Health Services Administration Web site, which lists physicians qualified to prescribe buprenorphine for opiate detoxification. He has heard about “bup” on the street and wants to know if he is eligible and what he can expect from treatment.
Like Mr. T, 1 million Americans are addicted to opiates.1 As qualified physicians gain experience with using buprenorphine, this agent could revolutionize how opiate-dependent patients are routinely treated. Instead of receiving methadone only in specialized clinics, they can now choose to be treated in physicians’ offices.
Several obstacles, however, are preventing widespread buprenorphine use:
- Too few physicians are trained to offer this office-based treatment.
- Many of the 2,000 doctors who are trained remain uncertain about using buprenorphine.
This article is intended to help overcome obstacles to opiate-dependence treatment by familiarizing psychiatrists—whether trained or not in using buprenorphine—with evidence of this agent’s efficacy and its advantages compared with other treatments.
Efficacy and obstacles
Buprenorphine is a partial opioid agonist that binds to the mu receptor (Box 1).2-5 It is a controlled substance (schedule-III narcotic). Outpatient trials have shown that buprenorphine is more effective than placebo and as effective as methadone for opiate detoxification.6-10
In one of the largest trials, 326 opiate-dependent outpatients were randomly assigned to buprenorphine, buprenorphine/naloxone combination, or placebo for 4 weeks. Both buprenorphine forms were more effective than placebo, as measured by clean urine samples and patient reports of reduced opiate cravings.7
In maintenance treatment, buprenorphine has been shown to be more effective than placebo and as effective as methadone, 60 mg/d, in preventing relapse. In a randomized comparison study, 220 opiate-dependent patients received levomethadyl acetate (LAAM), 75 to 115 mg three times a week; buprenorphine, 16 to 32 mg three times a week; high-dose methadone (60 to 100 mg/d); or low-dose methadone (20 mg/d). Subjects reported using opiates 20 to 30 times in the week before study enrollment. After 17 weeks, treatment retention rates were 58% for buprenorphine, 73% for high-dose methadone, and 20% for low-dose methadone. At the same point, urine samples were negative for opiate use in 40% of patients receiving buprenorphine compared with 39% of those receiving high-dose methadone.10
Buprenorphine is a partial opioid agonist derived from thebaine, an anodyne alkaloid from opium. It binds tightly to the muopiate receptor and produces expected opiate effects such as analgesia and mild euphoria.2 Its partial agonist properties create a ceiling effect and thus a lower likelihood of overdose than with opioid agonists.3
Pharmacokinetics
Buprenorphine has low bioavailability, but its 24- to 60-hour half-life allows once-daily dosing. Because common urine drug screens cannot detect buprenorphine, its use does not cause positive tests for opiates or morphine. Overdose risk is minimal when taken sublingually, with no respiratory depression reported in clinical trials.3,4 The drug is metabolized by the cytochrome P-450 3A4 isoenzyme system and demethylated to norbuprenorphine, which is not significantly bioactive.
Side effects
Nausea, fatigue, constipation, and occasional dysphoria
Abuse potential
Euphoria is less likely with buprenorphine than with opioid agonists because of buprenorphine’s ceiling effects. Theoretically, buprenorphine can be abused by being crushed and injected. The buprenorphine/naloxone combination, if taken parenterally, precipitates opiate withdrawal and thus is preferred for most patients with opioid dependence.
Buprenorphine may be fatal when abused, especially in combination with CNS depressants such as alcohol or high-dose benzodiazepines. However, buprenorphine’s mortality risk remains lower than that of methadone.5
Special precautions
Buprenorphine may precipitate opiate withdrawal during induction when an opioid agonist remains bound to the opiate receptor. Buprenorphine will displace the opiate from the receptor, creating an imbalance in opiate binding that the body interprets as opiate withdrawal.2
To avoid withdrawal, tell the patient not to start buprenorphine until mild withdrawal symptoms occur. In case of withdrawal, tell the patient to continue taking buprenorphine until symptoms are relieved. Adjunctive medications such as benzodiazepines, antiemetics, and antidiarrheals also can be given to control symptoms.
Table
Buprenorphine: A typical dosing strategy
| Phase | Dosage* | Comment |
|---|---|---|
| Induction | Maximum dosage is 32 mg/d; 12 to 24 mg/d typically controls withdrawal symptoms | |
| Day 1 | 4 mg bid (total 8 mg) | |
| Day 2 | 12 mg qd | |
| Day 3 | 16 mg qd | |
| Maintenance | 16 to 24 mg/d is average stabilization dosage | Consider severity of withdrawal symptoms and duration of addiction when deciding when to begin discontinuation |
| Discontinuation | Taper dosage by 2 to 4 mg every 3 to 5 days, then discontinue | Most patients remain on final 2 mg/d at least 1 week; consider alternate-day dosing for patients who experience side effects when attempting to reduce from 2 mg/d to 0 mg/d |
| * Buprenorphine/naloxone is preferred formulation | ||
Slow adoption. Opiate-dependence treatments such as methadone are prescribed in highly regulated environments, which is one reason only 25% of opiate addicts in the United States ever receive treatment.1 Unfortunately, little has changed in the 20 months since the FDA approved buprenorphine for office-based detoxification and maintenance treatment of opiate dependence. More than 2,000 physicians have been trained to use buprenorphine, yet only 20% of them report prescribing it.11
Reasons for this slow introduction include:
- difficulty in obtaining the medication
- lack of appropriate support staff and facilities
- uncertainty about prescribing the medication, despite special training.
Availability. When buprenorphine came to market in late 2003, most commercial pharmacies were not stocking it and it had to be special-ordered. As a result, patients receiving prescriptions had to wait 2 to 3 days for their first dose—a substantial deterrent to prescribing or taking this type of medication. Also, some private physicians and clinics do not keep buprenorphine samples to dispense on-site.
More pharmacies are stocking the medication now, but it remains the physician’s responsibility to ensure that a supply can be dispensed the day it is prescribed.
Support staff and facilities. To prescribe buprenorphine effectively, the physician needs resources for urine testing, physical exams, lab testing, and storing and dispensing buprenorphine. An integrated treatment clinic for opiate-dependent patients, complete with nursing and administrative staff, is ideal. If this support is not available, however, clinicians in private practice can safely prescribe buprenorphine from the office.
Uncertainty. Physicians often adopt new prescription products without hesitation, but buprenorphine’s administration and patient population are unusual. Even some physicians who have taken the special training course remain anxious about using this agent because it may precipitate opiate withdrawal. Also, the training requirement creates a sense that specialist-level knowledge is needed to safely prescribe buprenorphine.
Treatment requirements
For clinicians. The Drug Addiction Treatment Act of 2000 allows physicians to apply for a waiver from the Controlled Substances Act to prescribe buprenorphine for detoxification. A waiver is not required to prescribe buprenorphine for pain.12
To qualify for the waiver, physicians must be board-certified in addiction psychiatry or have completed a buprenorphine training course. Training is offered online and as a 1-day conference by the American Society of Addiction Medicine, American Academy of Addiction Psychiatry, American Medical Association, and American Psychiatric Association.
For patients. Like Mr. T, opiate users who ask about buprenorphine will want to know what to expect from treatment. To be eligible for buprenorphine treatment, a patient must:
- meet criteria for opiate dependence
- commit to keeping regular appointments—at least 3 times a week for the first 2 weeks then usually once weekly until detoxification is complete
- undergo random urine testing
- participate in psychosocial treatments.
So far, patients’ awareness of buprenorphine is highly variable. Asking an opiate user who presents for treatment what he or she knows about buprenorphine can be a useful screening tool. Highly motivated patients will have read about buprenorphine on the Internet, where they probably obtained your office phone number.
When a patient is accepted into treatment, detoxification with buprenorphine includes three phases: induction, stabilization/mainte-nance, and discontinuation.13 After stabilization, some patients remain in maintenance indefinitely and choose not to discontinue buprenorphine. The choice of who to discontinue and who to maintain on buprenorphine is a clinical decision made by the patient and practitioner. Success rates of detoxification with buprenorphine are similar to rates achieved with methadone and clonidine, although most studies have been conducted during buprenorphine maintenance.5
Case continued: Surprised to feel ‘normal’
Mr. T qualified for buprenorphine and came to the office feeling fairly ill. During withdrawal, his usual first symptom is rhinorrhea, followed by malaise, myalgia, restlessness, and intense cravings. His score of 24 on the Clinical Opiate Withdrawal Scale (COWS), indicated moderate withdrawal.
He felt better but not completely well 1 hour after taking buprenorphine/naloxone, 4 mg. He was given a 4-mg tablet to take at home 2 hours later. The next day his COWS score was 8, indicating mild withdrawal. He said he was surprised at how “normal” he was feeling.
Induction: Getting started
Buprenorphine induction is usually done during mild to moderate opiate withdrawal. Starting buprenorphine too soon—while the patient is relatively comfortable—may precipitate withdrawal because the agent will rapidly displace opiate bound to the receptors. In most cases, the first dose is given in the office so that the patient’s response can be monitored.
Two formulations. Buprenorphine comes alone (in 2- or 8-mg tablets) or in combination with naloxone (in 2 mg/0.5 mg and 8 mg/2 mg tablets). Both forms are given sublingually. Contrary to popular belief, IM buprenorphine is not approved for treating opiate addiction.
Naloxone is not absorbed in sublingual form and serves only to deter IV diversion of buprenorphine. Induction with buprenorphine alone is reserved for patients with documented allergy to naloxone or who are being detoxified from long-acting opiates such as methadone.
Dosing strategies are identical for both formulations. The usual starting dosage is 4 mg once daily, with a maximum dosage of 32 mg/d (Table). Withdrawal symptoms are typically controlled with 12 to 24 mg/d.14
If the patient is in active opiate withdrawal, the starting dose usually relieves symptoms in 30 to 45 minutes. If not, a second 4-mg dose can be given. Most patients do not require >8 mg the first day, but some may require 16 to 24 mg to suppress withdrawal symptoms.15
Some clinicians—such as solo practitioners who lack the resources of an outpatient clinic—prefer to have the patient take the first dose at home. Patients are instructed to take the first dose after withdrawal symptoms begin and to repeat the dose in 1 hour if symptoms persist. Thus, patients titrate their own dosages, but the clinician must be immediately available to handle complications. Induction continues until withdrawal symptoms are controlled.
The next day, patients return for evaluation. An objective scale such as the 11-item COWS can quantify withdrawal symptom severity.16 For each symptom—heart rate, nausea, diaphoresis, or restlessness—the COWS assigns a number corresponding to its severity. A total score >25 indicates moderately severe withdrawal.
After withdrawal symptoms are controlled, follow-up visits are scheduled every 2 to 3 days the first week and then weekly. Some physicians maintain daily contact with patients via e-mail or telephone to track symptoms.
Case continued: Steady improvement
By day 3, Mr. T gradually increased his buprenorphine/naloxone dosage to 16 mg once daily. He continued that dosage for 10 days before his next visit. At that point, he was slightly anxious but physically comfortable. He came into the office on days 2, 5, and 10 and his COWS scores decreased each time.
Stabilization and maintenance
When withdrawal symptoms are stabilized, patients begin maintenance therapy at the dosage that stabilized their symptoms. During maintenance therapy, the average buprenorphine dosage is 16 to 24 mg/d. Because of its long half-life, buprenorphine can be taken once daily, though some patients prefer twice-daily dosing for psychological comfort. Several studies comparing buprenorphine with methadone have found that buprenorphine, 8 to 16 mg/d, is similar in effect to approximately 60 mg/d of methadone.5
During the maintenance phase, it is important to have a policy for patients who relapse to substance abuse while taking buprenorphine (Box 2). During buprenorphine maintenance treatment, the estimated relapse rate to opiate use (chance of one positive test for opiates) ranges from 20% to 60%, compared with a relapse rate of 80% to 90% seen with placebo during clinical trials.5
Case continued: Time to taper?
After taking buprenorphine 2 months, Mr. T wants to taper off. He has been seen weekly and receives individual psychotherapy and group counseling. All urine drug screens have been negative for opiates.
With the psychiatrist’s observation, Mr. T. begins to taper his dosage of 16 mg/d by 4 mg a week. He is comfortable when he reaches 4 mg/d, but notices increased anxiety and general achiness when he reduces buprenorphine to 2 mg/d. He elects to remain at 4 mg/d for another 2 months.
Discontinuation
After the patient has reached a stable dose of buprenorphine, the clinician and patient together consider two treatment options:
- sustain the dose as maintenance therapy
- or taper and discontinue buprenorphine.
Screening
Screen patients for alcohol or benzodiazepine use, which may trigger symptoms similar to opiate withdrawal (buprenorphine does not treat withdrawal from these substances)
Induction
Worsening symptoms with buprenorphine indicate that withdrawal was precipitated; repeat buprenorphine dosing until symptoms are relieved (do not exceed 24 mg the first day)
Tell patients:
- to wait as long as possible before taking the first dose to reduce risk of precipitating withdrawal
- not to swallow the sublingual tablet, as this inactivates the medication
- the tablet can take 5 minutes or more to dissolve under the tongue
Maintenance
Set a policy for patients who relapse to substance use while taking buprenorphine. Consequences may include immediate buprenorphine cessation, transfer to methadone treatment, re-induction of buprenorphine, or referral to an inpatient substance abuse treatment center
Tracking
Log how many of your patients are taking buprenorphine; you may not treat more than 30 at a time
Patients who have had multiple relapses and endured severe opiate withdrawal might consider remaining on buprenorphine for several months before tapering. Mild opiate withdrawal may occur if buprenorphine is tapered too rapidly, though this is not as severe or distressing as a full agonist withdrawal.
Tapering recommendations. To taper buprenorphine, reduce by 2 to 4 mg every 3 to 5 days until the patient is taking 2 mg/d. Most patients remain on this dosage at least 1 week and then discontinue. Those who experience side effects when dropping from 2 mg to 0 mg can take 2 mg every other day for 1 week and then discontinue.
After patients are tapered completely off buprenorphine, encourage them to follow up with the treating physician for at least another month. Opiate withdrawal symptoms have been reported to linger 2 to 3 weeks after the last dose of buprenorphine, but these symptoms—usually anxiety or insomnia—tend to be self-limiting.5
Case continued: Time to taper?
Mr. T has been free from heroin use for 4 months and has returned to work. He is rebuilding his life and continues with psychotherapy. He follows up at the buprenorphine clinic monthly for medication management. At each visit, he repeats the COWS test and undergoes a urine drug screen and vital signs check.
Related resources
- Substance Abuse and Mental Health Services Administration. Physician locator for buprenorphine providers.
- American Academy of Addiction Psychiatry. Information about buprenorphine training course. www.aaap.org/buprenorphine/buprenorphine.html
- Buprenorphine manufacturer’s Web site. Answers to frequently-asked questions. www.suboxone.com
Drug brand names
- Buprenorphine • Subutex
- Buprenorphine/naloxone • Suboxone
- Buprenorphine (IM) • Buprenex
- Clonidine • Catapres
After 10 years of heroin dependence, Mr. T, age 36, calls your office and says, “I want to get off heroin.” For 8 months, he’s been using IV heroin 2 to 3 times daily. He says he has tried methadone treatment but found daily dosing cumbersome.
He found your office number on the Substance Abuse and Mental Health Services Administration Web site, which lists physicians qualified to prescribe buprenorphine for opiate detoxification. He has heard about “bup” on the street and wants to know if he is eligible and what he can expect from treatment.
Like Mr. T, 1 million Americans are addicted to opiates.1 As qualified physicians gain experience with using buprenorphine, this agent could revolutionize how opiate-dependent patients are routinely treated. Instead of receiving methadone only in specialized clinics, they can now choose to be treated in physicians’ offices.
Several obstacles, however, are preventing widespread buprenorphine use:
- Too few physicians are trained to offer this office-based treatment.
- Many of the 2,000 doctors who are trained remain uncertain about using buprenorphine.
This article is intended to help overcome obstacles to opiate-dependence treatment by familiarizing psychiatrists—whether trained or not in using buprenorphine—with evidence of this agent’s efficacy and its advantages compared with other treatments.
Efficacy and obstacles
Buprenorphine is a partial opioid agonist that binds to the mu receptor (Box 1).2-5 It is a controlled substance (schedule-III narcotic). Outpatient trials have shown that buprenorphine is more effective than placebo and as effective as methadone for opiate detoxification.6-10
In one of the largest trials, 326 opiate-dependent outpatients were randomly assigned to buprenorphine, buprenorphine/naloxone combination, or placebo for 4 weeks. Both buprenorphine forms were more effective than placebo, as measured by clean urine samples and patient reports of reduced opiate cravings.7
In maintenance treatment, buprenorphine has been shown to be more effective than placebo and as effective as methadone, 60 mg/d, in preventing relapse. In a randomized comparison study, 220 opiate-dependent patients received levomethadyl acetate (LAAM), 75 to 115 mg three times a week; buprenorphine, 16 to 32 mg three times a week; high-dose methadone (60 to 100 mg/d); or low-dose methadone (20 mg/d). Subjects reported using opiates 20 to 30 times in the week before study enrollment. After 17 weeks, treatment retention rates were 58% for buprenorphine, 73% for high-dose methadone, and 20% for low-dose methadone. At the same point, urine samples were negative for opiate use in 40% of patients receiving buprenorphine compared with 39% of those receiving high-dose methadone.10
Buprenorphine is a partial opioid agonist derived from thebaine, an anodyne alkaloid from opium. It binds tightly to the muopiate receptor and produces expected opiate effects such as analgesia and mild euphoria.2 Its partial agonist properties create a ceiling effect and thus a lower likelihood of overdose than with opioid agonists.3
Pharmacokinetics
Buprenorphine has low bioavailability, but its 24- to 60-hour half-life allows once-daily dosing. Because common urine drug screens cannot detect buprenorphine, its use does not cause positive tests for opiates or morphine. Overdose risk is minimal when taken sublingually, with no respiratory depression reported in clinical trials.3,4 The drug is metabolized by the cytochrome P-450 3A4 isoenzyme system and demethylated to norbuprenorphine, which is not significantly bioactive.
Side effects
Nausea, fatigue, constipation, and occasional dysphoria
Abuse potential
Euphoria is less likely with buprenorphine than with opioid agonists because of buprenorphine’s ceiling effects. Theoretically, buprenorphine can be abused by being crushed and injected. The buprenorphine/naloxone combination, if taken parenterally, precipitates opiate withdrawal and thus is preferred for most patients with opioid dependence.
Buprenorphine may be fatal when abused, especially in combination with CNS depressants such as alcohol or high-dose benzodiazepines. However, buprenorphine’s mortality risk remains lower than that of methadone.5
Special precautions
Buprenorphine may precipitate opiate withdrawal during induction when an opioid agonist remains bound to the opiate receptor. Buprenorphine will displace the opiate from the receptor, creating an imbalance in opiate binding that the body interprets as opiate withdrawal.2
To avoid withdrawal, tell the patient not to start buprenorphine until mild withdrawal symptoms occur. In case of withdrawal, tell the patient to continue taking buprenorphine until symptoms are relieved. Adjunctive medications such as benzodiazepines, antiemetics, and antidiarrheals also can be given to control symptoms.
Table
Buprenorphine: A typical dosing strategy
| Phase | Dosage* | Comment |
|---|---|---|
| Induction | Maximum dosage is 32 mg/d; 12 to 24 mg/d typically controls withdrawal symptoms | |
| Day 1 | 4 mg bid (total 8 mg) | |
| Day 2 | 12 mg qd | |
| Day 3 | 16 mg qd | |
| Maintenance | 16 to 24 mg/d is average stabilization dosage | Consider severity of withdrawal symptoms and duration of addiction when deciding when to begin discontinuation |
| Discontinuation | Taper dosage by 2 to 4 mg every 3 to 5 days, then discontinue | Most patients remain on final 2 mg/d at least 1 week; consider alternate-day dosing for patients who experience side effects when attempting to reduce from 2 mg/d to 0 mg/d |
| * Buprenorphine/naloxone is preferred formulation | ||
Slow adoption. Opiate-dependence treatments such as methadone are prescribed in highly regulated environments, which is one reason only 25% of opiate addicts in the United States ever receive treatment.1 Unfortunately, little has changed in the 20 months since the FDA approved buprenorphine for office-based detoxification and maintenance treatment of opiate dependence. More than 2,000 physicians have been trained to use buprenorphine, yet only 20% of them report prescribing it.11
Reasons for this slow introduction include:
- difficulty in obtaining the medication
- lack of appropriate support staff and facilities
- uncertainty about prescribing the medication, despite special training.
Availability. When buprenorphine came to market in late 2003, most commercial pharmacies were not stocking it and it had to be special-ordered. As a result, patients receiving prescriptions had to wait 2 to 3 days for their first dose—a substantial deterrent to prescribing or taking this type of medication. Also, some private physicians and clinics do not keep buprenorphine samples to dispense on-site.
More pharmacies are stocking the medication now, but it remains the physician’s responsibility to ensure that a supply can be dispensed the day it is prescribed.
Support staff and facilities. To prescribe buprenorphine effectively, the physician needs resources for urine testing, physical exams, lab testing, and storing and dispensing buprenorphine. An integrated treatment clinic for opiate-dependent patients, complete with nursing and administrative staff, is ideal. If this support is not available, however, clinicians in private practice can safely prescribe buprenorphine from the office.
Uncertainty. Physicians often adopt new prescription products without hesitation, but buprenorphine’s administration and patient population are unusual. Even some physicians who have taken the special training course remain anxious about using this agent because it may precipitate opiate withdrawal. Also, the training requirement creates a sense that specialist-level knowledge is needed to safely prescribe buprenorphine.
Treatment requirements
For clinicians. The Drug Addiction Treatment Act of 2000 allows physicians to apply for a waiver from the Controlled Substances Act to prescribe buprenorphine for detoxification. A waiver is not required to prescribe buprenorphine for pain.12
To qualify for the waiver, physicians must be board-certified in addiction psychiatry or have completed a buprenorphine training course. Training is offered online and as a 1-day conference by the American Society of Addiction Medicine, American Academy of Addiction Psychiatry, American Medical Association, and American Psychiatric Association.
For patients. Like Mr. T, opiate users who ask about buprenorphine will want to know what to expect from treatment. To be eligible for buprenorphine treatment, a patient must:
- meet criteria for opiate dependence
- commit to keeping regular appointments—at least 3 times a week for the first 2 weeks then usually once weekly until detoxification is complete
- undergo random urine testing
- participate in psychosocial treatments.
So far, patients’ awareness of buprenorphine is highly variable. Asking an opiate user who presents for treatment what he or she knows about buprenorphine can be a useful screening tool. Highly motivated patients will have read about buprenorphine on the Internet, where they probably obtained your office phone number.
When a patient is accepted into treatment, detoxification with buprenorphine includes three phases: induction, stabilization/mainte-nance, and discontinuation.13 After stabilization, some patients remain in maintenance indefinitely and choose not to discontinue buprenorphine. The choice of who to discontinue and who to maintain on buprenorphine is a clinical decision made by the patient and practitioner. Success rates of detoxification with buprenorphine are similar to rates achieved with methadone and clonidine, although most studies have been conducted during buprenorphine maintenance.5
Case continued: Surprised to feel ‘normal’
Mr. T qualified for buprenorphine and came to the office feeling fairly ill. During withdrawal, his usual first symptom is rhinorrhea, followed by malaise, myalgia, restlessness, and intense cravings. His score of 24 on the Clinical Opiate Withdrawal Scale (COWS), indicated moderate withdrawal.
He felt better but not completely well 1 hour after taking buprenorphine/naloxone, 4 mg. He was given a 4-mg tablet to take at home 2 hours later. The next day his COWS score was 8, indicating mild withdrawal. He said he was surprised at how “normal” he was feeling.
Induction: Getting started
Buprenorphine induction is usually done during mild to moderate opiate withdrawal. Starting buprenorphine too soon—while the patient is relatively comfortable—may precipitate withdrawal because the agent will rapidly displace opiate bound to the receptors. In most cases, the first dose is given in the office so that the patient’s response can be monitored.
Two formulations. Buprenorphine comes alone (in 2- or 8-mg tablets) or in combination with naloxone (in 2 mg/0.5 mg and 8 mg/2 mg tablets). Both forms are given sublingually. Contrary to popular belief, IM buprenorphine is not approved for treating opiate addiction.
Naloxone is not absorbed in sublingual form and serves only to deter IV diversion of buprenorphine. Induction with buprenorphine alone is reserved for patients with documented allergy to naloxone or who are being detoxified from long-acting opiates such as methadone.
Dosing strategies are identical for both formulations. The usual starting dosage is 4 mg once daily, with a maximum dosage of 32 mg/d (Table). Withdrawal symptoms are typically controlled with 12 to 24 mg/d.14
If the patient is in active opiate withdrawal, the starting dose usually relieves symptoms in 30 to 45 minutes. If not, a second 4-mg dose can be given. Most patients do not require >8 mg the first day, but some may require 16 to 24 mg to suppress withdrawal symptoms.15
Some clinicians—such as solo practitioners who lack the resources of an outpatient clinic—prefer to have the patient take the first dose at home. Patients are instructed to take the first dose after withdrawal symptoms begin and to repeat the dose in 1 hour if symptoms persist. Thus, patients titrate their own dosages, but the clinician must be immediately available to handle complications. Induction continues until withdrawal symptoms are controlled.
The next day, patients return for evaluation. An objective scale such as the 11-item COWS can quantify withdrawal symptom severity.16 For each symptom—heart rate, nausea, diaphoresis, or restlessness—the COWS assigns a number corresponding to its severity. A total score >25 indicates moderately severe withdrawal.
After withdrawal symptoms are controlled, follow-up visits are scheduled every 2 to 3 days the first week and then weekly. Some physicians maintain daily contact with patients via e-mail or telephone to track symptoms.
Case continued: Steady improvement
By day 3, Mr. T gradually increased his buprenorphine/naloxone dosage to 16 mg once daily. He continued that dosage for 10 days before his next visit. At that point, he was slightly anxious but physically comfortable. He came into the office on days 2, 5, and 10 and his COWS scores decreased each time.
Stabilization and maintenance
When withdrawal symptoms are stabilized, patients begin maintenance therapy at the dosage that stabilized their symptoms. During maintenance therapy, the average buprenorphine dosage is 16 to 24 mg/d. Because of its long half-life, buprenorphine can be taken once daily, though some patients prefer twice-daily dosing for psychological comfort. Several studies comparing buprenorphine with methadone have found that buprenorphine, 8 to 16 mg/d, is similar in effect to approximately 60 mg/d of methadone.5
During the maintenance phase, it is important to have a policy for patients who relapse to substance abuse while taking buprenorphine (Box 2). During buprenorphine maintenance treatment, the estimated relapse rate to opiate use (chance of one positive test for opiates) ranges from 20% to 60%, compared with a relapse rate of 80% to 90% seen with placebo during clinical trials.5
Case continued: Time to taper?
After taking buprenorphine 2 months, Mr. T wants to taper off. He has been seen weekly and receives individual psychotherapy and group counseling. All urine drug screens have been negative for opiates.
With the psychiatrist’s observation, Mr. T. begins to taper his dosage of 16 mg/d by 4 mg a week. He is comfortable when he reaches 4 mg/d, but notices increased anxiety and general achiness when he reduces buprenorphine to 2 mg/d. He elects to remain at 4 mg/d for another 2 months.
Discontinuation
After the patient has reached a stable dose of buprenorphine, the clinician and patient together consider two treatment options:
- sustain the dose as maintenance therapy
- or taper and discontinue buprenorphine.
Screening
Screen patients for alcohol or benzodiazepine use, which may trigger symptoms similar to opiate withdrawal (buprenorphine does not treat withdrawal from these substances)
Induction
Worsening symptoms with buprenorphine indicate that withdrawal was precipitated; repeat buprenorphine dosing until symptoms are relieved (do not exceed 24 mg the first day)
Tell patients:
- to wait as long as possible before taking the first dose to reduce risk of precipitating withdrawal
- not to swallow the sublingual tablet, as this inactivates the medication
- the tablet can take 5 minutes or more to dissolve under the tongue
Maintenance
Set a policy for patients who relapse to substance use while taking buprenorphine. Consequences may include immediate buprenorphine cessation, transfer to methadone treatment, re-induction of buprenorphine, or referral to an inpatient substance abuse treatment center
Tracking
Log how many of your patients are taking buprenorphine; you may not treat more than 30 at a time
Patients who have had multiple relapses and endured severe opiate withdrawal might consider remaining on buprenorphine for several months before tapering. Mild opiate withdrawal may occur if buprenorphine is tapered too rapidly, though this is not as severe or distressing as a full agonist withdrawal.
Tapering recommendations. To taper buprenorphine, reduce by 2 to 4 mg every 3 to 5 days until the patient is taking 2 mg/d. Most patients remain on this dosage at least 1 week and then discontinue. Those who experience side effects when dropping from 2 mg to 0 mg can take 2 mg every other day for 1 week and then discontinue.
After patients are tapered completely off buprenorphine, encourage them to follow up with the treating physician for at least another month. Opiate withdrawal symptoms have been reported to linger 2 to 3 weeks after the last dose of buprenorphine, but these symptoms—usually anxiety or insomnia—tend to be self-limiting.5
Case continued: Time to taper?
Mr. T has been free from heroin use for 4 months and has returned to work. He is rebuilding his life and continues with psychotherapy. He follows up at the buprenorphine clinic monthly for medication management. At each visit, he repeats the COWS test and undergoes a urine drug screen and vital signs check.
Related resources
- Substance Abuse and Mental Health Services Administration. Physician locator for buprenorphine providers.
- American Academy of Addiction Psychiatry. Information about buprenorphine training course. www.aaap.org/buprenorphine/buprenorphine.html
- Buprenorphine manufacturer’s Web site. Answers to frequently-asked questions. www.suboxone.com
Drug brand names
- Buprenorphine • Subutex
- Buprenorphine/naloxone • Suboxone
- Buprenorphine (IM) • Buprenex
- Clonidine • Catapres
1. Vastag B. In-office opiate treatment, “not a panacea:” physicians slow to embrace therapeutic option. JAMA 2003;290:731-5.
2. Marquet P. Pharmacology of high-dose buprenorphine. In: Kintz P, Marquet P (eds). Buprenorphine therapy of opiate addiction. Totowa, NJ: Humana Press, 2002;69-82.
3. Boyd J, Randell T, Luurila H, Kuisma M. Serious overdoses involving buprenorphine in Helsinki. Acta Anaesthesiol Scand 2003;47:1031-3.
4. Johnson RE, Cone EJ, Henningfield JE, Fudala PJ. Use of buprenorphine in the treatment of opiate addiction. Clin Pharmacol Ther 1989;46:335-43.
5. Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend 2003;70:S59-S77.
6. Breen CL, Harris SJ, Lintzeris N, et al. Cessation of methadone maintenance treatment using buprenorphine: transfer from methadone to buprenorphine and subsequent buprenorphine reductions. Drug Alcohol Depend 2003;71:49-55.
7. Fudala PJ, Bridge PT, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med 2003;349:949-58.
8. Ling W, Wesson DR. Clinical efficacy of buprenorphine: comparisons to methadone and placebo. Drug Alcohol Depend 2003;70:S49-S57.
9. Gibson AE, Doran CM, Bell JR, et al. A comparison of buprenorphine treatment in clinic and primary care settings; a randomised trial. Med J Aust 2003;179:38-42.
10. Johnson RE, Chutuape MA, Strain EC, et al. A comparison of levomethadyl acetate, buprenorphine and methadone for opioid dependence. N Engl J Med 2000;343:1290-7.
11. Bivol S. National poll of physicians on barriers to widespread buprenorphine use. Boston University School of Public Health, Join Together 2003;1-7.
12. Clark HW. Office-based practice and opioid-use disorders. N Engl J Med 2003;349:928-30.
13. Ling W, Smith D. Buprenorphine: blending practice and research. J Subst Abuse Treat 2002;23:87-92.
14. Fiellin DA, Pantalon MV, Pakes JP, et al. Treatment of heroin dependence with buprenorphine in primary care. Am J Drug Alcohol Abuse 2002;28:231-41.
15. Greenwald MK, Schuh KJ, Stine SM. Transferring methadone-maintained outpatients to the buprenorphine sublingual tablet: a preliminary study. Am J Addict 2003;12:365-74.
16. Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs 2003;35:253-9.
1. Vastag B. In-office opiate treatment, “not a panacea:” physicians slow to embrace therapeutic option. JAMA 2003;290:731-5.
2. Marquet P. Pharmacology of high-dose buprenorphine. In: Kintz P, Marquet P (eds). Buprenorphine therapy of opiate addiction. Totowa, NJ: Humana Press, 2002;69-82.
3. Boyd J, Randell T, Luurila H, Kuisma M. Serious overdoses involving buprenorphine in Helsinki. Acta Anaesthesiol Scand 2003;47:1031-3.
4. Johnson RE, Cone EJ, Henningfield JE, Fudala PJ. Use of buprenorphine in the treatment of opiate addiction. Clin Pharmacol Ther 1989;46:335-43.
5. Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend 2003;70:S59-S77.
6. Breen CL, Harris SJ, Lintzeris N, et al. Cessation of methadone maintenance treatment using buprenorphine: transfer from methadone to buprenorphine and subsequent buprenorphine reductions. Drug Alcohol Depend 2003;71:49-55.
7. Fudala PJ, Bridge PT, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med 2003;349:949-58.
8. Ling W, Wesson DR. Clinical efficacy of buprenorphine: comparisons to methadone and placebo. Drug Alcohol Depend 2003;70:S49-S57.
9. Gibson AE, Doran CM, Bell JR, et al. A comparison of buprenorphine treatment in clinic and primary care settings; a randomised trial. Med J Aust 2003;179:38-42.
10. Johnson RE, Chutuape MA, Strain EC, et al. A comparison of levomethadyl acetate, buprenorphine and methadone for opioid dependence. N Engl J Med 2000;343:1290-7.
11. Bivol S. National poll of physicians on barriers to widespread buprenorphine use. Boston University School of Public Health, Join Together 2003;1-7.
12. Clark HW. Office-based practice and opioid-use disorders. N Engl J Med 2003;349:928-30.
13. Ling W, Smith D. Buprenorphine: blending practice and research. J Subst Abuse Treat 2002;23:87-92.
14. Fiellin DA, Pantalon MV, Pakes JP, et al. Treatment of heroin dependence with buprenorphine in primary care. Am J Drug Alcohol Abuse 2002;28:231-41.
15. Greenwald MK, Schuh KJ, Stine SM. Transferring methadone-maintained outpatients to the buprenorphine sublingual tablet: a preliminary study. Am J Addict 2003;12:365-74.
16. Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs 2003;35:253-9.
Exercise for depression: It really does help—here’s how to get patients moving
Ms. H, age 26, is being evaluated for moderate to severe depressive symptoms, including oversleeping and overeating. She has had difficulty adhering to medication in the past and is ambivalent about taking antidepressants. She takes a passive approach to managing her depression, preferring to “wait for it to pass.”
Her psychiatrist prescribes fluoxetine, 20 mg in the morning, and recommends that Ms. H change her coping strategies from napping and snacking to increased physical activity. She encourages Ms. H to think about what activities interest her and to set exercise goals.
Ms. H says she has considered buying exercise equipment (an elliptical machine) and increasing her walking outside. She sets a goal to walk 20 minutes most days and to spend 10 to 15 minutes using the elliptical machine while watching television.
Physical activity’s mental health benefits are less well-known than its well-documented medical benefits—reduced risk of heart disease, hypertension, and diabetes; weight control; bone mass preservation; better sleep, and improved cholesterol levels.1 By encouraging exercise, you can improve patients’ mood, well-being, and quality of life, independent of medication and psychotherapy. In this article, we:
- explore the relationship between physical activity and mental health
- compare exercise with medication and psychotherapies for easing depression
- discuss counseling strategies shown to be effective in helping sedentary patients become more physically active.
Table 1
Why physical activity may improve mental health
|
| Psychological theories Physical activity:
|
| Source: References 10 and 11 |
Mental benefits of exercise
Adults who exercise regularly report lower levels of depressive and anxiety disorders than the overall U.S. population.2 As a therapeutic intervention, exercise has been studied primarily in depressed individuals, although some data also support its efficacy in:
- reducing anxiety symptoms in panic disorder3
- reducing disruptive behavior in developmentally disabled patients4
- alleviating chronic fatigue symptoms5
- improving body esteem in patients with body image disturbance6
- increasing function in chronic pain7
- reducing urges to smoke and improving smoking abstinence among nicotine-dependent individuals.8
Why exercise helps. Mechanisms that would explain exercise’s positive effect on mood are not well understood.9 Physiologic and psychological hypotheses have been suggested (Table 1),10,11 and researchers are attempting to elucidate them by using animal models.13
Case report: Feeling more energetic
At follow-up 6 weeks later, Ms. H. reported a substantial reduction in depressive symptoms. She noted increased energy, improved sleep, decreased overeating, higher self-esteem, and greater confidence in her ability to manage her depression.
Exercising also helped structure her day. She noticed that on days she did not exercise she was more likely to take a nap, miss her medication, or feel pessimistic about her depression.
Exercise as an antidepressant
Exercise vs psychotherapy. Exercise has been shown to be more effective at reducing depressive symptoms than no treatment, occupational therapy, cognitive therapy, health seminars, routine care, or meditation. Interventions used in these meta-analyses ranged from nonaerobic exercise training several times a week to 1 hour of supervised running 4 times a week.12 Literature reviews also have concluded that exercise training compares favorably with individual or group psychotherapy and with cognitive therapy for treating depression.7
Exercise vs medication. Exercise training has also been compared with drug therapy in treating depression.
In a randomized, controlled trial, 156 men and women over age 50 with major depression received exercise training, sertraline, or exercise plus sertraline. Subjects in the exercise groups completed 40 minutes of aerobic exercise (biking or brisk walking/ jogging) 3 times a week. Subjects treated with sertraline received 50 to 200 mg/d, depending on response.
After 16 weeks, all three groups were significantly improved, with no clinically or statistically significant differences in depressive symptoms, as measured with the Hamilton Rating Scale for Depression (HRSD) and Beck Depression Inventory.13
In a follow-up study 6 months later,14 the exercise group had significantly lower rates of relapse (defined as HRSD scores >15 and meeting diagnostic criteria) than did the medication group. Combining exercise with medication did not provide an added benefit in preventing relapse.
Exercise as monotherapy. Some studies have investigated using exercise instead of medication and psychotherapy. Many of these trials, however, were limited by methodologic weaknesses such as nonrandomized samples or lack of appropriate control groups.12
To address the need for higher-quality evidence, the Depression Outcomes Study of Exercise (DOSE) is investigating the dose-continued from page 12 response effects of exercise as monotherapy for major depressive disorder (MDD).5 The 12-week trial included 80 men and women ages 20 to 45 diagnosed with mild-to-moderate MDD using the Structured Clinical Interview for Depression. They were randomly assigned to one of five supervised exercise regimens:
- 7.0 kcal/kg/week in 3 days/week
- 7.0 kcal/kg/week in 5 days/week
- 17.5 kcal/kg/week in 3 days/week
- 17.5 kcal/kg/week in 5 days/week
- 3 days/week of stretching and flexibility exercises for 15 to 20 min/session.
Table 2
How much physical activity is recommended for adults?
For physical and mental health
|
For weight loss and management
|
Depressive symptoms were measured with the HRSD and Inventory of Depressive Symptoms (clinician and self-report). Other outcome measures included cardiorespiratory fitness, self-efficacy, and quality of life. Results are being prepared for publication and will likely help clarify the role of physical activity in treating patients with MDD.
Table 3
Why patients don’t exercise: Common barriers they perceive
Practical limitations
| |
Medical limitations
| |
Psychological limitations
| |
| Source: References 15 and 16 | |
How much exercise is therapeutic?
In the absence of physical activity guidelines specific to mental health, we suggest using standard public health guidelines (Table 2):
- 30 minutes or more of moderate-intensity physical activity (brisk walking, swimming, dancing, cycling) most days of the week (recommended by the Centers for Disease Control and Prevention and American College of Sports Medicine)1
- 60 minutes of moderate-intensity physical activity daily for weight loss and maintenance (recommended by the Institute of Medicine).16
A recent study investigated the effects of exercise duration and intensity on weight loss in overweight, sedentary women. These researchers recommended setting the initial intervention target at 150 minutes or more of moderate-intensity exercise per week and progressing to 60 minutes per day as appropriate.16
Increasing the number of steps taken per day, as measured by a pedometer, also can be beneficial. Encourage patients to obtain a baseline measure of daily steps and to gradually increase toward a moderate goal of 10,000 steps per day.17
Case report: Accentuating the positive
On follow-up, Ms. H was quick to report the many barriers to exercise she had experienced and the times she did not meet her goal. Rather than dwell on shortcomings, the psychiatrist redirected her to examine the many positive actions she had taken to manage her depression.
As she considered how to overcome barriers to exercise, she reported increased confidence that she could stick with her medication and exercise regimen. She continues to exercise regularly and adheres to her fluoxetine. Her depressive symptoms remain well-controlled.
Overcoming barriers to exercise
Patient obstacles. Many patients acknowledge that regular exercise makes them feel physically and emotionally healthier but have difficulty exercising long term. Less than one-half of those who start an exercise program stick with it beyond 6 months.18 Drop-out reasons include injuries, lack of time, and low motivation (Table 3).19,20
Depressive symptoms—fatigue, loss of interest, low self-esteem, feelings of helplessness, and psychomotor retardation—make exercise adherence even more difficult.
Physician obstacles. The U.S. Preventive Services Task Force recommends that physicians advise all patients to increase physical activity, but the national rate of physician counseling about exercise is low. In a population-based survey of more than 9,000 patients, 34% said their physicians counseled them about exercise at their most recent visit within the past year.21
Physician-reported barriers to exercise counseling include:
- competing demands for limited clinical time
- perceived ineffectiveness of advice to exercise
- lack of training and knowledge about exercise counseling and prescription.22,23
Patients are more likely to become active and continue exercising when their physicians help them set achievable goals.
Project PACE. Physicians can overcome barriers to counseling patients about exercise. Those who participated in Project PACE (Physician-based Assessment and Counseling for Exercise)24 said they felt more confident that they could counsel patients about physical activity in 1 to 5 minutes.
In a controlled study of 212 sedentary adults, patients who received PACE counseling from their physicians significantly increased their minutes of weekly walking compared with a control group. Also, 52% of patients who received PACE counseling adopted some physical activity, compared with 12% of controls.25
Though modest initial goals are not sufficient for achieving the full benefits of exercise, success with a small goal is a powerful motivator. Rather than giving up, patients feel encouraged and are more likely to set a subsequent, more ambitious goal.
Recommendations. To help patients start exercising, determine how motivated and ready they are. Start by asking them to describe their current activities. Ask if they were ever more active and what they liked about it. Did they experience any benefits? Establish which of increased activity’s benefits—improved sleep, reduced depression, increased energy—would most benefit the patient, based on his or her symptoms.
Discuss barriers to physical activity and encourage problem-solving to overcome them and incorporate physical activity into their lives. Encourage patients to seek support from family, friends, coworkers, and exercise groups.
Help them set realistic, achievable goals. Even a modest 10 minutes of activity has been shown to enhance mood,26 and a 10-minute brisk walk is one-third of the day’s public health guideline. Suggest that patients choose a variety of activities they enjoy.
During follow-up visits, reinforce any progress toward change. When patients’ exercise efforts fall short, explain that the process of becoming more active often includes setbacks. Advise them to seek support and to consider adopting more-achievable goals.
Related resources
- Getting started. Resources on nutrition and physical activity from the National Center for Chronic Disease Prevention and Health Promotion. http://www.cdc.gov/nccdphp/dnpa/physical/starting/index.htm
- Marcus B, Forsyth L. Motivating people to be physically active. Champaign, IL: Human Kinetics, 2002.
Drug brand names
- Fluoxetine • Prozac
- Sertraline • Zoloft
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Pate RR, Pratt M, Blair SN, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA 1995;273(5):402-7.
2. Goodwin RD. Association between physical activity and mental disorders among adults in the United States. Prev Med 2003;36:698-703.
3. Broocks A, Bandelow B, Pekrun G, et al. Comparison of aerobic exercise, clomipramine and placebo in the treatment of panic disorder. Am J Psychiatry 1998;155:603-9.
4. Gabler-Halle D, Halle JW, Chung YB. The effects of aerobic exercise on psychological and behavioral variables of individuals with developmental disabilities. A critical review. Res Dev Disabil 1993;14:359-86.
5. Powell P, Bentall RP, Nye FJ, Edwards RH. Patient education to encourage graded exercise in chronic fatigue syndrome. Br J Psychiatry 2004;184:142-6.
6. Pinto BM, Clark MM, Maruyama NC, Feder SI. Psychological and fitness changes associated with exercise participation among women with breast cancer. Psychooncology 2003;12(2):118-26.
7. Tkachuk GA, Martin GL. Exercise therapy for patients with psychiatric disorders: research and clinical implications. Prof Psychol Res Pract 1999;30:275-82
8. Ussher MH, Taylor AH, West R, McEwen A. Does exercise aid smoking cessation? A systematic review. Addiction 2000;95(2):199-208.
9. Van Hoomissen JD, Chambliss HO, Holmes PV, Dishman RK. Effects of chronic exercise and imipramine on mRNA for BDNF after olfactory bulbectomy in rat. Brain Res 2003;974:228-235.
10. Plante TG, Rodin J. Physical fitness and enhanced psychological health. Curr Psychol Res Rev 1990;9:3-24.
11. Weyerer A, Kupfer B. Physical exercise and psychological health. Sports Med 1994;17(2):108-16.
12. Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomized controlled trials. Br Med J 2001;322:1-8.
13. Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older adults with major depression. Arch Intern Med 1999;159:2349-56.
14. Babyak M, Blumenthal JA, Herman S, et al. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med 2000;62:633-8.
15. Dunn AL, Trivedi MH, Kampert JB, et al. The DOSE study: a clinical trial to examine efficacy and dose response of exercise as treatment for depression. Control Clin Trials 2002;23:584-603.
16. Jakicic JM, Marcus BH, Gallagher KI, et al. Effect of exercise duration and intensity on weight loss in overweight, sedentary women. JAMA 2003;290:1323-30.
17. Tudor-Locke C, Bassett DR, Jr. How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med 2004;34(1):1-8.
18. Dishman RK. Compliance/adherence in health-related exercise. Health Psychol 1982;1:237-67.
19. Sallis JF, Hovell MF. Determinants of exercise behavior. Exerc Sport Sci Rev 1990;18:307-30.
20. Heesch KC, Brown DR, Blanton CJ. Perceived barriers to exercise and stage of exercise adoption in older women of different racial/ethnic groups. Women Health 2000;30(4):61-76.
21. Wee CC, McCarthy EP, Davis RB, Phillips RS. Physician counseling about exercise. JAMA 1999;282(16):1583-8.
22. Kennedy MF, Meeuwisse WH. Exercise counseling by family physicians in Canada. Prev Med 2003 Sep;37(3):226-32.
23. Reed BD, Jensen JD, Gorenflo DW. Physicians and exercise promotion. Am J Prev Med 1991;7:410-15.
24. Long BJ, Calfas KJ, Wooten W, et al. A multisite field test of the acceptability of physical activity counseling in primary care: project PACE. Am J Prev Med 1996;12(2):73-81.
25. Calfas KJ, Long BJ, Sallis JF, et al. A controlled trial of physician counseling to promote the adoption of physical activity. Prev Med 1996;25(3):225-33.
26. Hansen CJ, Stevens LC, Coast JR. Exercise duration and mood state: how much is enough to feel better? Health Psychol 2001;20(4):267-75.
Ms. H, age 26, is being evaluated for moderate to severe depressive symptoms, including oversleeping and overeating. She has had difficulty adhering to medication in the past and is ambivalent about taking antidepressants. She takes a passive approach to managing her depression, preferring to “wait for it to pass.”
Her psychiatrist prescribes fluoxetine, 20 mg in the morning, and recommends that Ms. H change her coping strategies from napping and snacking to increased physical activity. She encourages Ms. H to think about what activities interest her and to set exercise goals.
Ms. H says she has considered buying exercise equipment (an elliptical machine) and increasing her walking outside. She sets a goal to walk 20 minutes most days and to spend 10 to 15 minutes using the elliptical machine while watching television.
Physical activity’s mental health benefits are less well-known than its well-documented medical benefits—reduced risk of heart disease, hypertension, and diabetes; weight control; bone mass preservation; better sleep, and improved cholesterol levels.1 By encouraging exercise, you can improve patients’ mood, well-being, and quality of life, independent of medication and psychotherapy. In this article, we:
- explore the relationship between physical activity and mental health
- compare exercise with medication and psychotherapies for easing depression
- discuss counseling strategies shown to be effective in helping sedentary patients become more physically active.
Table 1
Why physical activity may improve mental health
|
| Psychological theories Physical activity:
|
| Source: References 10 and 11 |
Mental benefits of exercise
Adults who exercise regularly report lower levels of depressive and anxiety disorders than the overall U.S. population.2 As a therapeutic intervention, exercise has been studied primarily in depressed individuals, although some data also support its efficacy in:
- reducing anxiety symptoms in panic disorder3
- reducing disruptive behavior in developmentally disabled patients4
- alleviating chronic fatigue symptoms5
- improving body esteem in patients with body image disturbance6
- increasing function in chronic pain7
- reducing urges to smoke and improving smoking abstinence among nicotine-dependent individuals.8
Why exercise helps. Mechanisms that would explain exercise’s positive effect on mood are not well understood.9 Physiologic and psychological hypotheses have been suggested (Table 1),10,11 and researchers are attempting to elucidate them by using animal models.13
Case report: Feeling more energetic
At follow-up 6 weeks later, Ms. H. reported a substantial reduction in depressive symptoms. She noted increased energy, improved sleep, decreased overeating, higher self-esteem, and greater confidence in her ability to manage her depression.
Exercising also helped structure her day. She noticed that on days she did not exercise she was more likely to take a nap, miss her medication, or feel pessimistic about her depression.
Exercise as an antidepressant
Exercise vs psychotherapy. Exercise has been shown to be more effective at reducing depressive symptoms than no treatment, occupational therapy, cognitive therapy, health seminars, routine care, or meditation. Interventions used in these meta-analyses ranged from nonaerobic exercise training several times a week to 1 hour of supervised running 4 times a week.12 Literature reviews also have concluded that exercise training compares favorably with individual or group psychotherapy and with cognitive therapy for treating depression.7
Exercise vs medication. Exercise training has also been compared with drug therapy in treating depression.
In a randomized, controlled trial, 156 men and women over age 50 with major depression received exercise training, sertraline, or exercise plus sertraline. Subjects in the exercise groups completed 40 minutes of aerobic exercise (biking or brisk walking/ jogging) 3 times a week. Subjects treated with sertraline received 50 to 200 mg/d, depending on response.
After 16 weeks, all three groups were significantly improved, with no clinically or statistically significant differences in depressive symptoms, as measured with the Hamilton Rating Scale for Depression (HRSD) and Beck Depression Inventory.13
In a follow-up study 6 months later,14 the exercise group had significantly lower rates of relapse (defined as HRSD scores >15 and meeting diagnostic criteria) than did the medication group. Combining exercise with medication did not provide an added benefit in preventing relapse.
Exercise as monotherapy. Some studies have investigated using exercise instead of medication and psychotherapy. Many of these trials, however, were limited by methodologic weaknesses such as nonrandomized samples or lack of appropriate control groups.12
To address the need for higher-quality evidence, the Depression Outcomes Study of Exercise (DOSE) is investigating the dose-continued from page 12 response effects of exercise as monotherapy for major depressive disorder (MDD).5 The 12-week trial included 80 men and women ages 20 to 45 diagnosed with mild-to-moderate MDD using the Structured Clinical Interview for Depression. They were randomly assigned to one of five supervised exercise regimens:
- 7.0 kcal/kg/week in 3 days/week
- 7.0 kcal/kg/week in 5 days/week
- 17.5 kcal/kg/week in 3 days/week
- 17.5 kcal/kg/week in 5 days/week
- 3 days/week of stretching and flexibility exercises for 15 to 20 min/session.
Table 2
How much physical activity is recommended for adults?
For physical and mental health
|
For weight loss and management
|
Depressive symptoms were measured with the HRSD and Inventory of Depressive Symptoms (clinician and self-report). Other outcome measures included cardiorespiratory fitness, self-efficacy, and quality of life. Results are being prepared for publication and will likely help clarify the role of physical activity in treating patients with MDD.
Table 3
Why patients don’t exercise: Common barriers they perceive
Practical limitations
| |
Medical limitations
| |
Psychological limitations
| |
| Source: References 15 and 16 | |
How much exercise is therapeutic?
In the absence of physical activity guidelines specific to mental health, we suggest using standard public health guidelines (Table 2):
- 30 minutes or more of moderate-intensity physical activity (brisk walking, swimming, dancing, cycling) most days of the week (recommended by the Centers for Disease Control and Prevention and American College of Sports Medicine)1
- 60 minutes of moderate-intensity physical activity daily for weight loss and maintenance (recommended by the Institute of Medicine).16
A recent study investigated the effects of exercise duration and intensity on weight loss in overweight, sedentary women. These researchers recommended setting the initial intervention target at 150 minutes or more of moderate-intensity exercise per week and progressing to 60 minutes per day as appropriate.16
Increasing the number of steps taken per day, as measured by a pedometer, also can be beneficial. Encourage patients to obtain a baseline measure of daily steps and to gradually increase toward a moderate goal of 10,000 steps per day.17
Case report: Accentuating the positive
On follow-up, Ms. H was quick to report the many barriers to exercise she had experienced and the times she did not meet her goal. Rather than dwell on shortcomings, the psychiatrist redirected her to examine the many positive actions she had taken to manage her depression.
As she considered how to overcome barriers to exercise, she reported increased confidence that she could stick with her medication and exercise regimen. She continues to exercise regularly and adheres to her fluoxetine. Her depressive symptoms remain well-controlled.
Overcoming barriers to exercise
Patient obstacles. Many patients acknowledge that regular exercise makes them feel physically and emotionally healthier but have difficulty exercising long term. Less than one-half of those who start an exercise program stick with it beyond 6 months.18 Drop-out reasons include injuries, lack of time, and low motivation (Table 3).19,20
Depressive symptoms—fatigue, loss of interest, low self-esteem, feelings of helplessness, and psychomotor retardation—make exercise adherence even more difficult.
Physician obstacles. The U.S. Preventive Services Task Force recommends that physicians advise all patients to increase physical activity, but the national rate of physician counseling about exercise is low. In a population-based survey of more than 9,000 patients, 34% said their physicians counseled them about exercise at their most recent visit within the past year.21
Physician-reported barriers to exercise counseling include:
- competing demands for limited clinical time
- perceived ineffectiveness of advice to exercise
- lack of training and knowledge about exercise counseling and prescription.22,23
Patients are more likely to become active and continue exercising when their physicians help them set achievable goals.
Project PACE. Physicians can overcome barriers to counseling patients about exercise. Those who participated in Project PACE (Physician-based Assessment and Counseling for Exercise)24 said they felt more confident that they could counsel patients about physical activity in 1 to 5 minutes.
In a controlled study of 212 sedentary adults, patients who received PACE counseling from their physicians significantly increased their minutes of weekly walking compared with a control group. Also, 52% of patients who received PACE counseling adopted some physical activity, compared with 12% of controls.25
Though modest initial goals are not sufficient for achieving the full benefits of exercise, success with a small goal is a powerful motivator. Rather than giving up, patients feel encouraged and are more likely to set a subsequent, more ambitious goal.
Recommendations. To help patients start exercising, determine how motivated and ready they are. Start by asking them to describe their current activities. Ask if they were ever more active and what they liked about it. Did they experience any benefits? Establish which of increased activity’s benefits—improved sleep, reduced depression, increased energy—would most benefit the patient, based on his or her symptoms.
Discuss barriers to physical activity and encourage problem-solving to overcome them and incorporate physical activity into their lives. Encourage patients to seek support from family, friends, coworkers, and exercise groups.
Help them set realistic, achievable goals. Even a modest 10 minutes of activity has been shown to enhance mood,26 and a 10-minute brisk walk is one-third of the day’s public health guideline. Suggest that patients choose a variety of activities they enjoy.
During follow-up visits, reinforce any progress toward change. When patients’ exercise efforts fall short, explain that the process of becoming more active often includes setbacks. Advise them to seek support and to consider adopting more-achievable goals.
Related resources
- Getting started. Resources on nutrition and physical activity from the National Center for Chronic Disease Prevention and Health Promotion. http://www.cdc.gov/nccdphp/dnpa/physical/starting/index.htm
- Marcus B, Forsyth L. Motivating people to be physically active. Champaign, IL: Human Kinetics, 2002.
Drug brand names
- Fluoxetine • Prozac
- Sertraline • Zoloft
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Ms. H, age 26, is being evaluated for moderate to severe depressive symptoms, including oversleeping and overeating. She has had difficulty adhering to medication in the past and is ambivalent about taking antidepressants. She takes a passive approach to managing her depression, preferring to “wait for it to pass.”
Her psychiatrist prescribes fluoxetine, 20 mg in the morning, and recommends that Ms. H change her coping strategies from napping and snacking to increased physical activity. She encourages Ms. H to think about what activities interest her and to set exercise goals.
Ms. H says she has considered buying exercise equipment (an elliptical machine) and increasing her walking outside. She sets a goal to walk 20 minutes most days and to spend 10 to 15 minutes using the elliptical machine while watching television.
Physical activity’s mental health benefits are less well-known than its well-documented medical benefits—reduced risk of heart disease, hypertension, and diabetes; weight control; bone mass preservation; better sleep, and improved cholesterol levels.1 By encouraging exercise, you can improve patients’ mood, well-being, and quality of life, independent of medication and psychotherapy. In this article, we:
- explore the relationship between physical activity and mental health
- compare exercise with medication and psychotherapies for easing depression
- discuss counseling strategies shown to be effective in helping sedentary patients become more physically active.
Table 1
Why physical activity may improve mental health
|
| Psychological theories Physical activity:
|
| Source: References 10 and 11 |
Mental benefits of exercise
Adults who exercise regularly report lower levels of depressive and anxiety disorders than the overall U.S. population.2 As a therapeutic intervention, exercise has been studied primarily in depressed individuals, although some data also support its efficacy in:
- reducing anxiety symptoms in panic disorder3
- reducing disruptive behavior in developmentally disabled patients4
- alleviating chronic fatigue symptoms5
- improving body esteem in patients with body image disturbance6
- increasing function in chronic pain7
- reducing urges to smoke and improving smoking abstinence among nicotine-dependent individuals.8
Why exercise helps. Mechanisms that would explain exercise’s positive effect on mood are not well understood.9 Physiologic and psychological hypotheses have been suggested (Table 1),10,11 and researchers are attempting to elucidate them by using animal models.13
Case report: Feeling more energetic
At follow-up 6 weeks later, Ms. H. reported a substantial reduction in depressive symptoms. She noted increased energy, improved sleep, decreased overeating, higher self-esteem, and greater confidence in her ability to manage her depression.
Exercising also helped structure her day. She noticed that on days she did not exercise she was more likely to take a nap, miss her medication, or feel pessimistic about her depression.
Exercise as an antidepressant
Exercise vs psychotherapy. Exercise has been shown to be more effective at reducing depressive symptoms than no treatment, occupational therapy, cognitive therapy, health seminars, routine care, or meditation. Interventions used in these meta-analyses ranged from nonaerobic exercise training several times a week to 1 hour of supervised running 4 times a week.12 Literature reviews also have concluded that exercise training compares favorably with individual or group psychotherapy and with cognitive therapy for treating depression.7
Exercise vs medication. Exercise training has also been compared with drug therapy in treating depression.
In a randomized, controlled trial, 156 men and women over age 50 with major depression received exercise training, sertraline, or exercise plus sertraline. Subjects in the exercise groups completed 40 minutes of aerobic exercise (biking or brisk walking/ jogging) 3 times a week. Subjects treated with sertraline received 50 to 200 mg/d, depending on response.
After 16 weeks, all three groups were significantly improved, with no clinically or statistically significant differences in depressive symptoms, as measured with the Hamilton Rating Scale for Depression (HRSD) and Beck Depression Inventory.13
In a follow-up study 6 months later,14 the exercise group had significantly lower rates of relapse (defined as HRSD scores >15 and meeting diagnostic criteria) than did the medication group. Combining exercise with medication did not provide an added benefit in preventing relapse.
Exercise as monotherapy. Some studies have investigated using exercise instead of medication and psychotherapy. Many of these trials, however, were limited by methodologic weaknesses such as nonrandomized samples or lack of appropriate control groups.12
To address the need for higher-quality evidence, the Depression Outcomes Study of Exercise (DOSE) is investigating the dose-continued from page 12 response effects of exercise as monotherapy for major depressive disorder (MDD).5 The 12-week trial included 80 men and women ages 20 to 45 diagnosed with mild-to-moderate MDD using the Structured Clinical Interview for Depression. They were randomly assigned to one of five supervised exercise regimens:
- 7.0 kcal/kg/week in 3 days/week
- 7.0 kcal/kg/week in 5 days/week
- 17.5 kcal/kg/week in 3 days/week
- 17.5 kcal/kg/week in 5 days/week
- 3 days/week of stretching and flexibility exercises for 15 to 20 min/session.
Table 2
How much physical activity is recommended for adults?
For physical and mental health
|
For weight loss and management
|
Depressive symptoms were measured with the HRSD and Inventory of Depressive Symptoms (clinician and self-report). Other outcome measures included cardiorespiratory fitness, self-efficacy, and quality of life. Results are being prepared for publication and will likely help clarify the role of physical activity in treating patients with MDD.
Table 3
Why patients don’t exercise: Common barriers they perceive
Practical limitations
| |
Medical limitations
| |
Psychological limitations
| |
| Source: References 15 and 16 | |
How much exercise is therapeutic?
In the absence of physical activity guidelines specific to mental health, we suggest using standard public health guidelines (Table 2):
- 30 minutes or more of moderate-intensity physical activity (brisk walking, swimming, dancing, cycling) most days of the week (recommended by the Centers for Disease Control and Prevention and American College of Sports Medicine)1
- 60 minutes of moderate-intensity physical activity daily for weight loss and maintenance (recommended by the Institute of Medicine).16
A recent study investigated the effects of exercise duration and intensity on weight loss in overweight, sedentary women. These researchers recommended setting the initial intervention target at 150 minutes or more of moderate-intensity exercise per week and progressing to 60 minutes per day as appropriate.16
Increasing the number of steps taken per day, as measured by a pedometer, also can be beneficial. Encourage patients to obtain a baseline measure of daily steps and to gradually increase toward a moderate goal of 10,000 steps per day.17
Case report: Accentuating the positive
On follow-up, Ms. H was quick to report the many barriers to exercise she had experienced and the times she did not meet her goal. Rather than dwell on shortcomings, the psychiatrist redirected her to examine the many positive actions she had taken to manage her depression.
As she considered how to overcome barriers to exercise, she reported increased confidence that she could stick with her medication and exercise regimen. She continues to exercise regularly and adheres to her fluoxetine. Her depressive symptoms remain well-controlled.
Overcoming barriers to exercise
Patient obstacles. Many patients acknowledge that regular exercise makes them feel physically and emotionally healthier but have difficulty exercising long term. Less than one-half of those who start an exercise program stick with it beyond 6 months.18 Drop-out reasons include injuries, lack of time, and low motivation (Table 3).19,20
Depressive symptoms—fatigue, loss of interest, low self-esteem, feelings of helplessness, and psychomotor retardation—make exercise adherence even more difficult.
Physician obstacles. The U.S. Preventive Services Task Force recommends that physicians advise all patients to increase physical activity, but the national rate of physician counseling about exercise is low. In a population-based survey of more than 9,000 patients, 34% said their physicians counseled them about exercise at their most recent visit within the past year.21
Physician-reported barriers to exercise counseling include:
- competing demands for limited clinical time
- perceived ineffectiveness of advice to exercise
- lack of training and knowledge about exercise counseling and prescription.22,23
Patients are more likely to become active and continue exercising when their physicians help them set achievable goals.
Project PACE. Physicians can overcome barriers to counseling patients about exercise. Those who participated in Project PACE (Physician-based Assessment and Counseling for Exercise)24 said they felt more confident that they could counsel patients about physical activity in 1 to 5 minutes.
In a controlled study of 212 sedentary adults, patients who received PACE counseling from their physicians significantly increased their minutes of weekly walking compared with a control group. Also, 52% of patients who received PACE counseling adopted some physical activity, compared with 12% of controls.25
Though modest initial goals are not sufficient for achieving the full benefits of exercise, success with a small goal is a powerful motivator. Rather than giving up, patients feel encouraged and are more likely to set a subsequent, more ambitious goal.
Recommendations. To help patients start exercising, determine how motivated and ready they are. Start by asking them to describe their current activities. Ask if they were ever more active and what they liked about it. Did they experience any benefits? Establish which of increased activity’s benefits—improved sleep, reduced depression, increased energy—would most benefit the patient, based on his or her symptoms.
Discuss barriers to physical activity and encourage problem-solving to overcome them and incorporate physical activity into their lives. Encourage patients to seek support from family, friends, coworkers, and exercise groups.
Help them set realistic, achievable goals. Even a modest 10 minutes of activity has been shown to enhance mood,26 and a 10-minute brisk walk is one-third of the day’s public health guideline. Suggest that patients choose a variety of activities they enjoy.
During follow-up visits, reinforce any progress toward change. When patients’ exercise efforts fall short, explain that the process of becoming more active often includes setbacks. Advise them to seek support and to consider adopting more-achievable goals.
Related resources
- Getting started. Resources on nutrition and physical activity from the National Center for Chronic Disease Prevention and Health Promotion. http://www.cdc.gov/nccdphp/dnpa/physical/starting/index.htm
- Marcus B, Forsyth L. Motivating people to be physically active. Champaign, IL: Human Kinetics, 2002.
Drug brand names
- Fluoxetine • Prozac
- Sertraline • Zoloft
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Pate RR, Pratt M, Blair SN, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA 1995;273(5):402-7.
2. Goodwin RD. Association between physical activity and mental disorders among adults in the United States. Prev Med 2003;36:698-703.
3. Broocks A, Bandelow B, Pekrun G, et al. Comparison of aerobic exercise, clomipramine and placebo in the treatment of panic disorder. Am J Psychiatry 1998;155:603-9.
4. Gabler-Halle D, Halle JW, Chung YB. The effects of aerobic exercise on psychological and behavioral variables of individuals with developmental disabilities. A critical review. Res Dev Disabil 1993;14:359-86.
5. Powell P, Bentall RP, Nye FJ, Edwards RH. Patient education to encourage graded exercise in chronic fatigue syndrome. Br J Psychiatry 2004;184:142-6.
6. Pinto BM, Clark MM, Maruyama NC, Feder SI. Psychological and fitness changes associated with exercise participation among women with breast cancer. Psychooncology 2003;12(2):118-26.
7. Tkachuk GA, Martin GL. Exercise therapy for patients with psychiatric disorders: research and clinical implications. Prof Psychol Res Pract 1999;30:275-82
8. Ussher MH, Taylor AH, West R, McEwen A. Does exercise aid smoking cessation? A systematic review. Addiction 2000;95(2):199-208.
9. Van Hoomissen JD, Chambliss HO, Holmes PV, Dishman RK. Effects of chronic exercise and imipramine on mRNA for BDNF after olfactory bulbectomy in rat. Brain Res 2003;974:228-235.
10. Plante TG, Rodin J. Physical fitness and enhanced psychological health. Curr Psychol Res Rev 1990;9:3-24.
11. Weyerer A, Kupfer B. Physical exercise and psychological health. Sports Med 1994;17(2):108-16.
12. Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomized controlled trials. Br Med J 2001;322:1-8.
13. Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older adults with major depression. Arch Intern Med 1999;159:2349-56.
14. Babyak M, Blumenthal JA, Herman S, et al. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med 2000;62:633-8.
15. Dunn AL, Trivedi MH, Kampert JB, et al. The DOSE study: a clinical trial to examine efficacy and dose response of exercise as treatment for depression. Control Clin Trials 2002;23:584-603.
16. Jakicic JM, Marcus BH, Gallagher KI, et al. Effect of exercise duration and intensity on weight loss in overweight, sedentary women. JAMA 2003;290:1323-30.
17. Tudor-Locke C, Bassett DR, Jr. How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med 2004;34(1):1-8.
18. Dishman RK. Compliance/adherence in health-related exercise. Health Psychol 1982;1:237-67.
19. Sallis JF, Hovell MF. Determinants of exercise behavior. Exerc Sport Sci Rev 1990;18:307-30.
20. Heesch KC, Brown DR, Blanton CJ. Perceived barriers to exercise and stage of exercise adoption in older women of different racial/ethnic groups. Women Health 2000;30(4):61-76.
21. Wee CC, McCarthy EP, Davis RB, Phillips RS. Physician counseling about exercise. JAMA 1999;282(16):1583-8.
22. Kennedy MF, Meeuwisse WH. Exercise counseling by family physicians in Canada. Prev Med 2003 Sep;37(3):226-32.
23. Reed BD, Jensen JD, Gorenflo DW. Physicians and exercise promotion. Am J Prev Med 1991;7:410-15.
24. Long BJ, Calfas KJ, Wooten W, et al. A multisite field test of the acceptability of physical activity counseling in primary care: project PACE. Am J Prev Med 1996;12(2):73-81.
25. Calfas KJ, Long BJ, Sallis JF, et al. A controlled trial of physician counseling to promote the adoption of physical activity. Prev Med 1996;25(3):225-33.
26. Hansen CJ, Stevens LC, Coast JR. Exercise duration and mood state: how much is enough to feel better? Health Psychol 2001;20(4):267-75.
1. Pate RR, Pratt M, Blair SN, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA 1995;273(5):402-7.
2. Goodwin RD. Association between physical activity and mental disorders among adults in the United States. Prev Med 2003;36:698-703.
3. Broocks A, Bandelow B, Pekrun G, et al. Comparison of aerobic exercise, clomipramine and placebo in the treatment of panic disorder. Am J Psychiatry 1998;155:603-9.
4. Gabler-Halle D, Halle JW, Chung YB. The effects of aerobic exercise on psychological and behavioral variables of individuals with developmental disabilities. A critical review. Res Dev Disabil 1993;14:359-86.
5. Powell P, Bentall RP, Nye FJ, Edwards RH. Patient education to encourage graded exercise in chronic fatigue syndrome. Br J Psychiatry 2004;184:142-6.
6. Pinto BM, Clark MM, Maruyama NC, Feder SI. Psychological and fitness changes associated with exercise participation among women with breast cancer. Psychooncology 2003;12(2):118-26.
7. Tkachuk GA, Martin GL. Exercise therapy for patients with psychiatric disorders: research and clinical implications. Prof Psychol Res Pract 1999;30:275-82
8. Ussher MH, Taylor AH, West R, McEwen A. Does exercise aid smoking cessation? A systematic review. Addiction 2000;95(2):199-208.
9. Van Hoomissen JD, Chambliss HO, Holmes PV, Dishman RK. Effects of chronic exercise and imipramine on mRNA for BDNF after olfactory bulbectomy in rat. Brain Res 2003;974:228-235.
10. Plante TG, Rodin J. Physical fitness and enhanced psychological health. Curr Psychol Res Rev 1990;9:3-24.
11. Weyerer A, Kupfer B. Physical exercise and psychological health. Sports Med 1994;17(2):108-16.
12. Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomized controlled trials. Br Med J 2001;322:1-8.
13. Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older adults with major depression. Arch Intern Med 1999;159:2349-56.
14. Babyak M, Blumenthal JA, Herman S, et al. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med 2000;62:633-8.
15. Dunn AL, Trivedi MH, Kampert JB, et al. The DOSE study: a clinical trial to examine efficacy and dose response of exercise as treatment for depression. Control Clin Trials 2002;23:584-603.
16. Jakicic JM, Marcus BH, Gallagher KI, et al. Effect of exercise duration and intensity on weight loss in overweight, sedentary women. JAMA 2003;290:1323-30.
17. Tudor-Locke C, Bassett DR, Jr. How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med 2004;34(1):1-8.
18. Dishman RK. Compliance/adherence in health-related exercise. Health Psychol 1982;1:237-67.
19. Sallis JF, Hovell MF. Determinants of exercise behavior. Exerc Sport Sci Rev 1990;18:307-30.
20. Heesch KC, Brown DR, Blanton CJ. Perceived barriers to exercise and stage of exercise adoption in older women of different racial/ethnic groups. Women Health 2000;30(4):61-76.
21. Wee CC, McCarthy EP, Davis RB, Phillips RS. Physician counseling about exercise. JAMA 1999;282(16):1583-8.
22. Kennedy MF, Meeuwisse WH. Exercise counseling by family physicians in Canada. Prev Med 2003 Sep;37(3):226-32.
23. Reed BD, Jensen JD, Gorenflo DW. Physicians and exercise promotion. Am J Prev Med 1991;7:410-15.
24. Long BJ, Calfas KJ, Wooten W, et al. A multisite field test of the acceptability of physical activity counseling in primary care: project PACE. Am J Prev Med 1996;12(2):73-81.
25. Calfas KJ, Long BJ, Sallis JF, et al. A controlled trial of physician counseling to promote the adoption of physical activity. Prev Med 1996;25(3):225-33.
26. Hansen CJ, Stevens LC, Coast JR. Exercise duration and mood state: how much is enough to feel better? Health Psychol 2001;20(4):267-75.
8 steps to manage recurrent abdominal pain
Just three words—“My tummy hurts”—can mobilize a child’s parents into a high state of worry, especially on school days. They wonder: Is our child sick? Should he or she stay home? Why is this happening so often?
Although recurrent abdominal pain (RAP) is real, it usually is not caused by tissue damage or serious physical disease. When children with RAP are referred for psychiatric evaluation—often after extensive medical workups—we can help them and their parents manage the problem and function more normally. This article:
- describes physiologic mechanisms that may underlie recurrent GI distress
- discusses the high correlation of psychiatric comorbidities with RAP
- recommends judicious laboratory testing
- reviews evidence on medications and psychotherapies to improve RAP symptoms
- offers advice on building a therapeutic alliance with the patient and family.
Figure Comorbid anxiety and depressive disorders in children with RAP
Children with functional RAP are much more likely to be anxious or depressed than similar pain-free children. A recent blinded study followed 80 children ages 8 to 15 (42 with RAP and 38 controls) identified through screening at primary care pediatric offices. Each was assessed using the Schedule for Affective Disorders and Schizophrenia for School Age Children, Present and Lifetime version (K-SADS-PL). Percentage meeting diagnostic criteria
Source: Reference 5.
RAP: A ‘Functional’ disorder
RAP is a somatoform (or “functional”) disorder, defined as physical symptoms not fully explained by a medical condition, effects of a substance, or another mental disorder. Symptoms cause distress and/or functional impairment and are not intentionally produced.1
A patient with RAP experiences at least three episodes of abdominal pain over 3 months that interfere with daily activities.2 RAP affects 7% to 25% of school-aged children and adolescents,3 most of whom have a functional disorder.4
RAP is equally common among prepubertal boys and girls but more common among girls during adolescence.3 RAP can impair school attendance and performance and stigmatize a child as “sickly.”
Common comorbid symptoms
Physical. Besides stomach pain, children with RAP often experience headaches (including migraines), other GI symptoms, general aches and pains, dizziness, and fatigue.
Patients with RAP who do not experience GI bleeding, anemia, fever, weight loss, growth failure, or persistent vomiting most likely do not have a serious underlying disease.
Psychiatric. Children with RAP have much higher rates of anxiety (80%) and depressive (40%) disorders than do their unaffected peers (Figure).5 We have also seen higher levels of suicidal thinking in children with RAP in primary care settings compared with pain-free controls (14% vs. 4%, P = 0.04; unpublished data).
In most cases, psychiatric comorbidities appear to precede or coincide with RAP onset. Separation fears, generalized anxiety, and social anxiety in particular are common in patients with RAP yet are seldom recognized in medical settings.
Having childhood RAP increases the risk of anxiety, depression, and hypochondriacal fears in adulthood.6 We do not know whether early intervention prevents later disability.
Use of medical services. Abdominal pain accounts for 2% to 4% of all pediatric office visits.7 In one study, 8% of middle school and high school students said they had visited a physician for evaluation of stomach pain during the previous year.8 Children with RAP make more ambulatory health and mental health visits than peers9 and are at risk for unnecessary and potentially dangerous medical tests, procedures, and treatments, including abdominal surgery.10
Four functional GI disorders
To better characterize youths with functional RAP, symptom-based criteria have been developed and applied for functional GI disorders, defined as chronic or recurrent GI symptoms without explanatory structural or biochemical abnormalities.11 Four such disorders are relevant in children with RAP.
Irritable bowel syndrome (IBS): RAP with at least two of the following symptoms: relief with defecation, change in stool frequency, and change in stool form or appearance (occurs in approximately 50% of RAP cases).
Functional dyspepsia: RAP centered in the upper abdomen that is not associated with changes in bowel habits.
Abdominal migraine: Paroxysmal midline abdominal pain lasting 2 hours to several days with symptom-free intervals of weeks to months and at least two of the following: headache during episodes, photophobia during episodes, unilateral headache, aura, and a family history of migraine.
Functional abdominal pain: Continuous or nearly continuous abdominal pain for 6 months or more.
The reliability, validity, and clinical relevance of these criteria have not been demonstrated. Some children with RAP do not meet any criteria for a specific functional GI disorder.
Gut-brain connections. RAP may be associated with a heightened sensitivity to visceral sensations (visceral hyperalgesia) and a low pressure-pain threshold, leading to speculation that these children are hypersensitive to pain.
High rates of anxiety disorders and temperamental harm avoidance also are seen in patients with RAP, along with a tendency to develop pain when faced with unexpected events. Whether these children are more likely than others to perceive novel internal or external perceptions as threatening is open to debate.
Table 1
Recurrent abdominal pain: 8 steps to assessment and diagnosis
|
Serotonin communicates nociceptive information between the gut and brain and may mediate visceral hyperalgesia. Gut enterochromaffin cells contain more than 90% of the body’s total serotonin. They act as sensory transducers, releasing serotonin in response to increased intraluminal pressure or inflammation.
The released serotonin can cause abdominal discomfort by stimulating 5-HT 3 receptors on vagal afferents and can influence gut peristaltic activity by stimulating enteric afferents. The same serotonin transporter responsible for CNS serotonin reuptake is expressed throughout the gut.
A constellation of clues
The ideal RAP evaluation includes information from the child, parents, educators, and other health care professionals (Table 1).
Begin by acknowledging the patient’s suffering and the parent’s concerns; do not challenge the pain’s subjective reality. Rather than prejudging its cause, document the pain’s timing, context, and characteristics, and review the patient’s history. A constellation of clues is most suggestive of RAP (Table 2); single clues are not definitive.12,13
Table 2
Clues that suggest functional pain*
|
| * No single clue is definitive. |
| Source: Adapted from reference 12. |
Diagnostic testing. Be judicious in selecting diagnostic tests and procedures. Continuing to order studies in a haphazard effort to rule out disease can generate concerns that “the doctor doesn’t know what’s wrong” and heighten the family’s fear that a disease has been missed.
The process of “ruling out” physical disease may have no apparent end. Unless you are reasonably comfortable that a serious physical disease has not been missed, it is difficult to explain RAP to the patient and family and lay the foundation for intervention.
On the other hand, you must balance the importance of minimizing your own and the family’s anxiety about unrecognized disease against the physical and psychological risks and costs associated with medical tests and procedures.
Social assessment. Assess social and familial reinforcement (secondary gain) of the pain. Parents sometimes inadvertently encourage their children’s sick-role behaviors by providing excessive attention, rewards, or opportunities to avoid uncomfortable situations. RAP can become an excuse for poor performance (self-handicapping), particularly in children with a learning disorder.
How to deliver the diagnosis
Functional abdominal pain is essentially a clinical diagnosis that relies on presentation, course, and findings. As mentioned, a constellation of “clues” is most supportive, as is having typical IBS symptoms.
Before declaring the diagnosis, discuss with the family the patient’s physical, emotional, and behavioral symptoms and the context in which RAP developed. Doing so can help maintain your credibility and establish a consensus.
Once you declare the diagnosis, discuss it clearly and frankly. Families are not likely to be reassured if you do not offer a plausible explanation for the lack of physical findings.
Precautions. When a definitive diagnosis is not possible, acknowledge that uncertainty. Although you must discuss any recognized psychiatric comorbidity, attempting to “explain” that the disorder is causing the pain is usually impractical and intellectually dishonest.
Also, given the pervasive nature of stigma, do not convey embarrassment or unease about diagnosing functional RAP or any comorbid psychiatric disorder.
Follow-up testing. Once you diagnose functional RAP, further testing is generally not necessary. Tests might be indicated if you:
- receive new information
- observe a change in clinical status
- or are convinced that treatment will not work unless the family is reassured by further investigation.
Collaborative treatment
Reassurance and education. Reassurance that the patient does not have a serious physical disease is necessary but rarely sufficient. Explain that the child’s pain does not appear to reflect tissue damage and is not threatening. On the other hand, avoid giving excessive reassurance, particularly when obsessional illness worry and hypochondriacal fears are prominent. Address illness worry as a problem to be solved together.
Discuss with the patient and family what is known and not known about functional RAP, and encourage them to ask questions. This is an opportunity for you to instill hope and cultivate positive expectations, but avoid promising cure. Discuss the gut-brain connection and relevance of visceral hyperalgesia, including serotonin’s potential roles in RAP pathogenesis.
Partners in care. Collaborative treatment increases the likelihood of success. Discuss the importance of a therapeutic partnership, and clarify any areas of disagreement with the diagnosis or treatment plan.
Clearly delineate your roles and responsibilities and those of the patient, family, and other health care team members. Poor communication is pediatricians’ most common complaint about psychiatrists.14 Good interdisciplinary communication decreases the chance that treatment strategies will be duplicated, diluted, or misinterpreted.
Consolidate medical care with a single clinician—often the primary care physician—based on discussions with the patient, family, and health care team. The coordinating clinician can mediate between the school and family when tensions develop over poor attendance or requests for special treatment. It is often useful for this clinician to spell out:
- what constitutes a legitimate medical excuse for school absence
- who will legitimize excuses.
All parties should understand that the school will view an unexcused absence as truancy and act appropriately.
Diet and lifestyle. Encourage the patient to maintain a regular schedule and a healthy diet. Specific dietary interventions have not been proven effective, despite speculation that lack of dietary fiber or lactose intolerance might cause RAP.15,16 Also encourage adequate sleep and regular exercise.
Medication and psychotherapy
Because no strong evidence-based guidelines address pediatric RAP intervention, family preferences usually guide initial treatment decisions. This highlights the importance of good communication and a therapeutic partnership among the clinician, family, and patient.
Antispasmodics, acid reducers, and antidepressants are commonly prescribed for RAP, though none are well-supported in the literature and no controlled studies have gauged medication’s impact on psychiatric comorbidity.16
Antidepressants. Selective serotonin reuptake inhibitors (SSRIs) might help relieve RAP symptoms, but the evidence is inconclusive. SSRIs are considered potentially beneficial in RAP because they may help communicate nociceptive information between the gut and brain and mediate visceral hyperalgesia.
SSRIs at first may increase serotonin at the synapse, which one might assume would to worsen abdominal symptoms. However, ongoing SSRI use could “down-regulate” postsynaptic 5-HT3 receptors and desensitize postsynaptic cells to the effects of local serotonin.
Our group recently conducted a 12-week open trial of citalopram for functional pediatric RAP.17 The 25 participants received 10 mg/d the first week, then 20 mg/d thereafter if tolerated. At week 4, nonresponders and partial responders who were tolerating the medication began receiving 40 mg/d.
Table 3
SSRI daily dosing for pediatric RAP
| Drug | Starting dosage* | Target dosage† | Maximum dosage‡ |
|---|---|---|---|
| Citalopram | 10 mg | 20 mg | 40 to 60 mg |
| Escitalopram | 5 mg | 10 mg | 20 to 30 mg |
| Fluoxetine | 10 mg | 20 mg | 40 mg |
| Fluvoxamine | 50 mg | 100 mg | 300 mg |
| Sertraline | 25 mg | 50 mg | 200 mg |
| * First 3 to 7 days. | |||
| † If patient tolerates starting dosage, increase to target dosage. | |||
| ‡ If patient does not respond to target dosage in 2 to 3 weeks, consider increasing the dosage. | |||
At trial’s end, more than two-thirds of participants were taking 40 mg/d. We rated 21 of 25 patients (84%) as “much improved” or “very much improved,” using the Clinical Global Impression-Improvement scale. Abdominal pain, anxiety, depression, other somatic symptoms, and functional impairment all improved significantly during treatment. Suicidal thoughts diminished progressively from baseline, and no patient reported suicidal thinking at study’s end. Citalopram was generally well tolerated.
With SSRI treatment, start at a low dosage for 3 to 7 days (Table 3). If tolerated, increase to a typical therapeutic dosage. If symptoms fail to respond after 2 or 3 weeks, consider a higher dosage. A short course of an oral benzodiazepine (such as clonazepam, 0.25 mg bid) during the first weeks of SSRI treatment sometimes helps particularly anxious patients or those whose pain appears closely associated with anxiety or “stress.”
Pediatric gastroenterologists often prescribe a low-dose tricyclic antidepressant as first-line therapy, but we discourage this. TCAs lack efficacy in pediatric depression and pose a greater risk of side effects and safety concerns than SSRIs.18
Other agents have been tried for RAP-associated conditions:
Famotidine, a histamine type 2 receptor blocker, may reduce pain in children with dyspepsia and RAP.19
Peppermint oil reduced abdominal pain in one study of children with IBS but had little effect on other symptoms.20
Medications such as alosetron and tegaserod that interrupt serotonergic neurotransmission in the gut have shown benefit in adults with IBS but have not been studied in children.
Psychotherapy. A few small studies suggest that cognitive-behavioral therapies (CBT) are helpful in RAP, but CBT may be difficult to deliver in medical settings.21,22 A simplified “rehabilitative” approach that incorporates CBT principles involves having the clinician and patient view RAP as a challenge to be overcome, rather than a burden to be endured. Such an approach emphasizes the child’s fundamental strength and adaptability rather than vulnerabilities.
The goal of therapy is redirected from finding a cure to coping with and overcoming the problem. This approach challenges the notion that the child cannot resume normal function until the pain is completely gone. It encourages active, problem-focused coping, and discourages passive acceptance—which is associated with greater symptom burden and functional impairment.
Work with parents to reinforce the child’s health-promoting behaviors and minimize negative and social reinforcements (secondary gain) associated with RAP. Advise parents to:
- encourage and reward full school attendance
- avoid home-bound instruction
- expect the child to function despite physical distress
- insist that the child perform age-appropriate household chores and other responsibilities.
Self-management skills—such as relaxation training, hypnosis, biofeedback, and guided imagery—may help reduce pain and manage physiologic arousal.16 Deception strategies such as placebo or sham interventions are unethical and impractical.
Related resources
- Campo JV. Functional recurrent abdominal pain in children and adolescents. Digestive Health Matters 2003;12(3):15-7.
- International Foundation for Functional Gastrointestinal Disorders. www.iffgd.org
- University of Pittsburgh. Advanced Center for Interventions and Services Research on Early Onset Mood and Anxiety Disorders. www.moodykids.org
Drug brand names
- Alosetron • Lotronex
- Citalopram • Celexa
- Clonazepam • Klonopin
- Escitalopram • Lexapro
- Famotidine • Pepcid
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Sertraline • Zoloft
- Tegaserod • Zelnorm
Disclosure
Dr. Campo’s work has been supported by the National Institute of Mental Health (grant MH01780) and in part by the Advanced Center for Interventions and Services Research on Early Onset Mood and Anxiety Disorders (grant MH66371). He also receives grants from Forest Pharmaceuticals and is a consultant to Eli Lilly and Co.
1. American Psychiatric Association Diagnostic and statistical manual of mental disorders (4th ed, revised). Washington, DC: American Psychiatric Association, 2000.
2. Apley J, Naish N. Recurrent abdominal pains: a field survey of 1,000 school children. Arch Dis Child 1958;33(168):165-70.
3. Scharff L. Recurrent abdominal pain in children: a review of psychological factors and treatment. Clin Psychol Rev 1997;17(2):145-66.
4. Boyle JT. Recurrent abdominal pain: an update. Pediatr Rev 1997;18(9):310-20.
5. Campo JV, Bridge J, Ehmann M, et al. Recurrent abdominal pain, anxiety, and depression in primary care. Pediatrics 2004;113(4):817-24.
6. Campo JV, Di Lorenzo C, Chiappetta L, et al. Adult outcomes of pediatric recurrent abdominal pain: do they just grow out of it? Pediatrics 2001;108(1):E1.-
7. Starfield B, Gross E, Wood M, et al. Psychosocial and psychosomatic diagnoses in primary care of children. Pediatrics 1980;66(2):159-67.
8. Hyams JS, Burke G, Davis PM, et al. Abdominal pain and irritable bowel syndrome in adolescents: a community-based study. J Pediatr 1996;129(2):220-6.
9. Campo JV, Comer DM, Jansen-McWilliams L, et al. Recurrent pain, emotional distress, and health service use in childhood. J Pediatr 2002;141(1):76-83.
10. Stickler GB, Murphy DB. Recurrent abdominal pain. Am J Dis Child 1979;133(5):486-9.
11. Rasquin-Weber A, Hyman PE, Cucchiara S, et al. Childhood functional gastrointestinal disorders. Gut 1999;45(suppl 2):II60-8.
12. Campo JV, Fritz G. A management model for pediatric somatization. Psychosomatics 2001;42(6):467-76.
13. Campo JV, Garber J. Somatization. In: Ammerman RT, Campo JV (eds). Handbook of pediatric psychology and psychiatry. Vol 1 Boston: Allyn and Bacon, 1998;137-61.
14. Fritz GK, Bergman AS. Child psychiatrists seen through pediatricians’ eyes: results of a national survey. J Am Acad Child Psychiatry 1985;24(1):81-6.
15. Huertas-Ceballos A, Macarthur C, Logan S. Dietary interventions for recurrent abdominal pain (RAP) in childhood. Cochrane Database Syst Rev 2002;(2):CD003019.-
16. Weydert JA, Ball TM, Davis MF. Systematic review of treatments for recurrent abdominal pain. Pediatrics 2003;111(1):e1-11.
17. Campo JV, Perel J, Lucas A, et al. Citalopram treatment of pediatric recurrent abdominal pain and comorbid internalizing disorders: An exploratory study (poster). Miami Beach, FL: American Academy of Child and Adolescent Psychiatry annual meeting, October 2003.
18. Geller B, Reising D, Leonard HL, et al. Critical review of tricyclic antidepressant use in children and adolescents. J Am Acad Child Adolesc Psychiatry 1999;38(5):513-6.
19. See MC, Birnbaum AH, Schechter CB, et al. Double-blind, placebo-controlled trial of famotidine in children with abdominal pain and dyspepsia: global and quantitative assessment. Dig Dis Sci 2001;46(5):985-92.
20. Kline RM, Kline JJ, Di Palma J, Barbero GJ. Enteric-coated, pH-dependent peppermint oil capsules for the treatment of irritable bowel syndrome in children. J Pediatr 2001;138(1):125-8.
21. Sanders MR, Rebgetz M, Morrison M, et al. Cognitive-behavioral treatment of recurrent nonspecific abdominal pain in children: an analysis of generalization, maintenance, and side effects. J Consult Clin Psychol 1989;57(2):294-300.
22. Sanders MR, Shepherd RW, Cleghorn G, Woolford H. The treatment of recurrent abdominal pain in children: a controlled comparison of cognitive-behavioral family intervention and standard pediatric care. J Consult Clin Psychol 1994;62(2):306-14.
Just three words—“My tummy hurts”—can mobilize a child’s parents into a high state of worry, especially on school days. They wonder: Is our child sick? Should he or she stay home? Why is this happening so often?
Although recurrent abdominal pain (RAP) is real, it usually is not caused by tissue damage or serious physical disease. When children with RAP are referred for psychiatric evaluation—often after extensive medical workups—we can help them and their parents manage the problem and function more normally. This article:
- describes physiologic mechanisms that may underlie recurrent GI distress
- discusses the high correlation of psychiatric comorbidities with RAP
- recommends judicious laboratory testing
- reviews evidence on medications and psychotherapies to improve RAP symptoms
- offers advice on building a therapeutic alliance with the patient and family.
Figure Comorbid anxiety and depressive disorders in children with RAP
Children with functional RAP are much more likely to be anxious or depressed than similar pain-free children. A recent blinded study followed 80 children ages 8 to 15 (42 with RAP and 38 controls) identified through screening at primary care pediatric offices. Each was assessed using the Schedule for Affective Disorders and Schizophrenia for School Age Children, Present and Lifetime version (K-SADS-PL). Percentage meeting diagnostic criteria
Source: Reference 5.
RAP: A ‘Functional’ disorder
RAP is a somatoform (or “functional”) disorder, defined as physical symptoms not fully explained by a medical condition, effects of a substance, or another mental disorder. Symptoms cause distress and/or functional impairment and are not intentionally produced.1
A patient with RAP experiences at least three episodes of abdominal pain over 3 months that interfere with daily activities.2 RAP affects 7% to 25% of school-aged children and adolescents,3 most of whom have a functional disorder.4
RAP is equally common among prepubertal boys and girls but more common among girls during adolescence.3 RAP can impair school attendance and performance and stigmatize a child as “sickly.”
Common comorbid symptoms
Physical. Besides stomach pain, children with RAP often experience headaches (including migraines), other GI symptoms, general aches and pains, dizziness, and fatigue.
Patients with RAP who do not experience GI bleeding, anemia, fever, weight loss, growth failure, or persistent vomiting most likely do not have a serious underlying disease.
Psychiatric. Children with RAP have much higher rates of anxiety (80%) and depressive (40%) disorders than do their unaffected peers (Figure).5 We have also seen higher levels of suicidal thinking in children with RAP in primary care settings compared with pain-free controls (14% vs. 4%, P = 0.04; unpublished data).
In most cases, psychiatric comorbidities appear to precede or coincide with RAP onset. Separation fears, generalized anxiety, and social anxiety in particular are common in patients with RAP yet are seldom recognized in medical settings.
Having childhood RAP increases the risk of anxiety, depression, and hypochondriacal fears in adulthood.6 We do not know whether early intervention prevents later disability.
Use of medical services. Abdominal pain accounts for 2% to 4% of all pediatric office visits.7 In one study, 8% of middle school and high school students said they had visited a physician for evaluation of stomach pain during the previous year.8 Children with RAP make more ambulatory health and mental health visits than peers9 and are at risk for unnecessary and potentially dangerous medical tests, procedures, and treatments, including abdominal surgery.10
Four functional GI disorders
To better characterize youths with functional RAP, symptom-based criteria have been developed and applied for functional GI disorders, defined as chronic or recurrent GI symptoms without explanatory structural or biochemical abnormalities.11 Four such disorders are relevant in children with RAP.
Irritable bowel syndrome (IBS): RAP with at least two of the following symptoms: relief with defecation, change in stool frequency, and change in stool form or appearance (occurs in approximately 50% of RAP cases).
Functional dyspepsia: RAP centered in the upper abdomen that is not associated with changes in bowel habits.
Abdominal migraine: Paroxysmal midline abdominal pain lasting 2 hours to several days with symptom-free intervals of weeks to months and at least two of the following: headache during episodes, photophobia during episodes, unilateral headache, aura, and a family history of migraine.
Functional abdominal pain: Continuous or nearly continuous abdominal pain for 6 months or more.
The reliability, validity, and clinical relevance of these criteria have not been demonstrated. Some children with RAP do not meet any criteria for a specific functional GI disorder.
Gut-brain connections. RAP may be associated with a heightened sensitivity to visceral sensations (visceral hyperalgesia) and a low pressure-pain threshold, leading to speculation that these children are hypersensitive to pain.
High rates of anxiety disorders and temperamental harm avoidance also are seen in patients with RAP, along with a tendency to develop pain when faced with unexpected events. Whether these children are more likely than others to perceive novel internal or external perceptions as threatening is open to debate.
Table 1
Recurrent abdominal pain: 8 steps to assessment and diagnosis
|
Serotonin communicates nociceptive information between the gut and brain and may mediate visceral hyperalgesia. Gut enterochromaffin cells contain more than 90% of the body’s total serotonin. They act as sensory transducers, releasing serotonin in response to increased intraluminal pressure or inflammation.
The released serotonin can cause abdominal discomfort by stimulating 5-HT 3 receptors on vagal afferents and can influence gut peristaltic activity by stimulating enteric afferents. The same serotonin transporter responsible for CNS serotonin reuptake is expressed throughout the gut.
A constellation of clues
The ideal RAP evaluation includes information from the child, parents, educators, and other health care professionals (Table 1).
Begin by acknowledging the patient’s suffering and the parent’s concerns; do not challenge the pain’s subjective reality. Rather than prejudging its cause, document the pain’s timing, context, and characteristics, and review the patient’s history. A constellation of clues is most suggestive of RAP (Table 2); single clues are not definitive.12,13
Table 2
Clues that suggest functional pain*
|
| * No single clue is definitive. |
| Source: Adapted from reference 12. |
Diagnostic testing. Be judicious in selecting diagnostic tests and procedures. Continuing to order studies in a haphazard effort to rule out disease can generate concerns that “the doctor doesn’t know what’s wrong” and heighten the family’s fear that a disease has been missed.
The process of “ruling out” physical disease may have no apparent end. Unless you are reasonably comfortable that a serious physical disease has not been missed, it is difficult to explain RAP to the patient and family and lay the foundation for intervention.
On the other hand, you must balance the importance of minimizing your own and the family’s anxiety about unrecognized disease against the physical and psychological risks and costs associated with medical tests and procedures.
Social assessment. Assess social and familial reinforcement (secondary gain) of the pain. Parents sometimes inadvertently encourage their children’s sick-role behaviors by providing excessive attention, rewards, or opportunities to avoid uncomfortable situations. RAP can become an excuse for poor performance (self-handicapping), particularly in children with a learning disorder.
How to deliver the diagnosis
Functional abdominal pain is essentially a clinical diagnosis that relies on presentation, course, and findings. As mentioned, a constellation of “clues” is most supportive, as is having typical IBS symptoms.
Before declaring the diagnosis, discuss with the family the patient’s physical, emotional, and behavioral symptoms and the context in which RAP developed. Doing so can help maintain your credibility and establish a consensus.
Once you declare the diagnosis, discuss it clearly and frankly. Families are not likely to be reassured if you do not offer a plausible explanation for the lack of physical findings.
Precautions. When a definitive diagnosis is not possible, acknowledge that uncertainty. Although you must discuss any recognized psychiatric comorbidity, attempting to “explain” that the disorder is causing the pain is usually impractical and intellectually dishonest.
Also, given the pervasive nature of stigma, do not convey embarrassment or unease about diagnosing functional RAP or any comorbid psychiatric disorder.
Follow-up testing. Once you diagnose functional RAP, further testing is generally not necessary. Tests might be indicated if you:
- receive new information
- observe a change in clinical status
- or are convinced that treatment will not work unless the family is reassured by further investigation.
Collaborative treatment
Reassurance and education. Reassurance that the patient does not have a serious physical disease is necessary but rarely sufficient. Explain that the child’s pain does not appear to reflect tissue damage and is not threatening. On the other hand, avoid giving excessive reassurance, particularly when obsessional illness worry and hypochondriacal fears are prominent. Address illness worry as a problem to be solved together.
Discuss with the patient and family what is known and not known about functional RAP, and encourage them to ask questions. This is an opportunity for you to instill hope and cultivate positive expectations, but avoid promising cure. Discuss the gut-brain connection and relevance of visceral hyperalgesia, including serotonin’s potential roles in RAP pathogenesis.
Partners in care. Collaborative treatment increases the likelihood of success. Discuss the importance of a therapeutic partnership, and clarify any areas of disagreement with the diagnosis or treatment plan.
Clearly delineate your roles and responsibilities and those of the patient, family, and other health care team members. Poor communication is pediatricians’ most common complaint about psychiatrists.14 Good interdisciplinary communication decreases the chance that treatment strategies will be duplicated, diluted, or misinterpreted.
Consolidate medical care with a single clinician—often the primary care physician—based on discussions with the patient, family, and health care team. The coordinating clinician can mediate between the school and family when tensions develop over poor attendance or requests for special treatment. It is often useful for this clinician to spell out:
- what constitutes a legitimate medical excuse for school absence
- who will legitimize excuses.
All parties should understand that the school will view an unexcused absence as truancy and act appropriately.
Diet and lifestyle. Encourage the patient to maintain a regular schedule and a healthy diet. Specific dietary interventions have not been proven effective, despite speculation that lack of dietary fiber or lactose intolerance might cause RAP.15,16 Also encourage adequate sleep and regular exercise.
Medication and psychotherapy
Because no strong evidence-based guidelines address pediatric RAP intervention, family preferences usually guide initial treatment decisions. This highlights the importance of good communication and a therapeutic partnership among the clinician, family, and patient.
Antispasmodics, acid reducers, and antidepressants are commonly prescribed for RAP, though none are well-supported in the literature and no controlled studies have gauged medication’s impact on psychiatric comorbidity.16
Antidepressants. Selective serotonin reuptake inhibitors (SSRIs) might help relieve RAP symptoms, but the evidence is inconclusive. SSRIs are considered potentially beneficial in RAP because they may help communicate nociceptive information between the gut and brain and mediate visceral hyperalgesia.
SSRIs at first may increase serotonin at the synapse, which one might assume would to worsen abdominal symptoms. However, ongoing SSRI use could “down-regulate” postsynaptic 5-HT3 receptors and desensitize postsynaptic cells to the effects of local serotonin.
Our group recently conducted a 12-week open trial of citalopram for functional pediatric RAP.17 The 25 participants received 10 mg/d the first week, then 20 mg/d thereafter if tolerated. At week 4, nonresponders and partial responders who were tolerating the medication began receiving 40 mg/d.
Table 3
SSRI daily dosing for pediatric RAP
| Drug | Starting dosage* | Target dosage† | Maximum dosage‡ |
|---|---|---|---|
| Citalopram | 10 mg | 20 mg | 40 to 60 mg |
| Escitalopram | 5 mg | 10 mg | 20 to 30 mg |
| Fluoxetine | 10 mg | 20 mg | 40 mg |
| Fluvoxamine | 50 mg | 100 mg | 300 mg |
| Sertraline | 25 mg | 50 mg | 200 mg |
| * First 3 to 7 days. | |||
| † If patient tolerates starting dosage, increase to target dosage. | |||
| ‡ If patient does not respond to target dosage in 2 to 3 weeks, consider increasing the dosage. | |||
At trial’s end, more than two-thirds of participants were taking 40 mg/d. We rated 21 of 25 patients (84%) as “much improved” or “very much improved,” using the Clinical Global Impression-Improvement scale. Abdominal pain, anxiety, depression, other somatic symptoms, and functional impairment all improved significantly during treatment. Suicidal thoughts diminished progressively from baseline, and no patient reported suicidal thinking at study’s end. Citalopram was generally well tolerated.
With SSRI treatment, start at a low dosage for 3 to 7 days (Table 3). If tolerated, increase to a typical therapeutic dosage. If symptoms fail to respond after 2 or 3 weeks, consider a higher dosage. A short course of an oral benzodiazepine (such as clonazepam, 0.25 mg bid) during the first weeks of SSRI treatment sometimes helps particularly anxious patients or those whose pain appears closely associated with anxiety or “stress.”
Pediatric gastroenterologists often prescribe a low-dose tricyclic antidepressant as first-line therapy, but we discourage this. TCAs lack efficacy in pediatric depression and pose a greater risk of side effects and safety concerns than SSRIs.18
Other agents have been tried for RAP-associated conditions:
Famotidine, a histamine type 2 receptor blocker, may reduce pain in children with dyspepsia and RAP.19
Peppermint oil reduced abdominal pain in one study of children with IBS but had little effect on other symptoms.20
Medications such as alosetron and tegaserod that interrupt serotonergic neurotransmission in the gut have shown benefit in adults with IBS but have not been studied in children.
Psychotherapy. A few small studies suggest that cognitive-behavioral therapies (CBT) are helpful in RAP, but CBT may be difficult to deliver in medical settings.21,22 A simplified “rehabilitative” approach that incorporates CBT principles involves having the clinician and patient view RAP as a challenge to be overcome, rather than a burden to be endured. Such an approach emphasizes the child’s fundamental strength and adaptability rather than vulnerabilities.
The goal of therapy is redirected from finding a cure to coping with and overcoming the problem. This approach challenges the notion that the child cannot resume normal function until the pain is completely gone. It encourages active, problem-focused coping, and discourages passive acceptance—which is associated with greater symptom burden and functional impairment.
Work with parents to reinforce the child’s health-promoting behaviors and minimize negative and social reinforcements (secondary gain) associated with RAP. Advise parents to:
- encourage and reward full school attendance
- avoid home-bound instruction
- expect the child to function despite physical distress
- insist that the child perform age-appropriate household chores and other responsibilities.
Self-management skills—such as relaxation training, hypnosis, biofeedback, and guided imagery—may help reduce pain and manage physiologic arousal.16 Deception strategies such as placebo or sham interventions are unethical and impractical.
Related resources
- Campo JV. Functional recurrent abdominal pain in children and adolescents. Digestive Health Matters 2003;12(3):15-7.
- International Foundation for Functional Gastrointestinal Disorders. www.iffgd.org
- University of Pittsburgh. Advanced Center for Interventions and Services Research on Early Onset Mood and Anxiety Disorders. www.moodykids.org
Drug brand names
- Alosetron • Lotronex
- Citalopram • Celexa
- Clonazepam • Klonopin
- Escitalopram • Lexapro
- Famotidine • Pepcid
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Sertraline • Zoloft
- Tegaserod • Zelnorm
Disclosure
Dr. Campo’s work has been supported by the National Institute of Mental Health (grant MH01780) and in part by the Advanced Center for Interventions and Services Research on Early Onset Mood and Anxiety Disorders (grant MH66371). He also receives grants from Forest Pharmaceuticals and is a consultant to Eli Lilly and Co.
Just three words—“My tummy hurts”—can mobilize a child’s parents into a high state of worry, especially on school days. They wonder: Is our child sick? Should he or she stay home? Why is this happening so often?
Although recurrent abdominal pain (RAP) is real, it usually is not caused by tissue damage or serious physical disease. When children with RAP are referred for psychiatric evaluation—often after extensive medical workups—we can help them and their parents manage the problem and function more normally. This article:
- describes physiologic mechanisms that may underlie recurrent GI distress
- discusses the high correlation of psychiatric comorbidities with RAP
- recommends judicious laboratory testing
- reviews evidence on medications and psychotherapies to improve RAP symptoms
- offers advice on building a therapeutic alliance with the patient and family.
Figure Comorbid anxiety and depressive disorders in children with RAP
Children with functional RAP are much more likely to be anxious or depressed than similar pain-free children. A recent blinded study followed 80 children ages 8 to 15 (42 with RAP and 38 controls) identified through screening at primary care pediatric offices. Each was assessed using the Schedule for Affective Disorders and Schizophrenia for School Age Children, Present and Lifetime version (K-SADS-PL). Percentage meeting diagnostic criteria
Source: Reference 5.
RAP: A ‘Functional’ disorder
RAP is a somatoform (or “functional”) disorder, defined as physical symptoms not fully explained by a medical condition, effects of a substance, or another mental disorder. Symptoms cause distress and/or functional impairment and are not intentionally produced.1
A patient with RAP experiences at least three episodes of abdominal pain over 3 months that interfere with daily activities.2 RAP affects 7% to 25% of school-aged children and adolescents,3 most of whom have a functional disorder.4
RAP is equally common among prepubertal boys and girls but more common among girls during adolescence.3 RAP can impair school attendance and performance and stigmatize a child as “sickly.”
Common comorbid symptoms
Physical. Besides stomach pain, children with RAP often experience headaches (including migraines), other GI symptoms, general aches and pains, dizziness, and fatigue.
Patients with RAP who do not experience GI bleeding, anemia, fever, weight loss, growth failure, or persistent vomiting most likely do not have a serious underlying disease.
Psychiatric. Children with RAP have much higher rates of anxiety (80%) and depressive (40%) disorders than do their unaffected peers (Figure).5 We have also seen higher levels of suicidal thinking in children with RAP in primary care settings compared with pain-free controls (14% vs. 4%, P = 0.04; unpublished data).
In most cases, psychiatric comorbidities appear to precede or coincide with RAP onset. Separation fears, generalized anxiety, and social anxiety in particular are common in patients with RAP yet are seldom recognized in medical settings.
Having childhood RAP increases the risk of anxiety, depression, and hypochondriacal fears in adulthood.6 We do not know whether early intervention prevents later disability.
Use of medical services. Abdominal pain accounts for 2% to 4% of all pediatric office visits.7 In one study, 8% of middle school and high school students said they had visited a physician for evaluation of stomach pain during the previous year.8 Children with RAP make more ambulatory health and mental health visits than peers9 and are at risk for unnecessary and potentially dangerous medical tests, procedures, and treatments, including abdominal surgery.10
Four functional GI disorders
To better characterize youths with functional RAP, symptom-based criteria have been developed and applied for functional GI disorders, defined as chronic or recurrent GI symptoms without explanatory structural or biochemical abnormalities.11 Four such disorders are relevant in children with RAP.
Irritable bowel syndrome (IBS): RAP with at least two of the following symptoms: relief with defecation, change in stool frequency, and change in stool form or appearance (occurs in approximately 50% of RAP cases).
Functional dyspepsia: RAP centered in the upper abdomen that is not associated with changes in bowel habits.
Abdominal migraine: Paroxysmal midline abdominal pain lasting 2 hours to several days with symptom-free intervals of weeks to months and at least two of the following: headache during episodes, photophobia during episodes, unilateral headache, aura, and a family history of migraine.
Functional abdominal pain: Continuous or nearly continuous abdominal pain for 6 months or more.
The reliability, validity, and clinical relevance of these criteria have not been demonstrated. Some children with RAP do not meet any criteria for a specific functional GI disorder.
Gut-brain connections. RAP may be associated with a heightened sensitivity to visceral sensations (visceral hyperalgesia) and a low pressure-pain threshold, leading to speculation that these children are hypersensitive to pain.
High rates of anxiety disorders and temperamental harm avoidance also are seen in patients with RAP, along with a tendency to develop pain when faced with unexpected events. Whether these children are more likely than others to perceive novel internal or external perceptions as threatening is open to debate.
Table 1
Recurrent abdominal pain: 8 steps to assessment and diagnosis
|
Serotonin communicates nociceptive information between the gut and brain and may mediate visceral hyperalgesia. Gut enterochromaffin cells contain more than 90% of the body’s total serotonin. They act as sensory transducers, releasing serotonin in response to increased intraluminal pressure or inflammation.
The released serotonin can cause abdominal discomfort by stimulating 5-HT 3 receptors on vagal afferents and can influence gut peristaltic activity by stimulating enteric afferents. The same serotonin transporter responsible for CNS serotonin reuptake is expressed throughout the gut.
A constellation of clues
The ideal RAP evaluation includes information from the child, parents, educators, and other health care professionals (Table 1).
Begin by acknowledging the patient’s suffering and the parent’s concerns; do not challenge the pain’s subjective reality. Rather than prejudging its cause, document the pain’s timing, context, and characteristics, and review the patient’s history. A constellation of clues is most suggestive of RAP (Table 2); single clues are not definitive.12,13
Table 2
Clues that suggest functional pain*
|
| * No single clue is definitive. |
| Source: Adapted from reference 12. |
Diagnostic testing. Be judicious in selecting diagnostic tests and procedures. Continuing to order studies in a haphazard effort to rule out disease can generate concerns that “the doctor doesn’t know what’s wrong” and heighten the family’s fear that a disease has been missed.
The process of “ruling out” physical disease may have no apparent end. Unless you are reasonably comfortable that a serious physical disease has not been missed, it is difficult to explain RAP to the patient and family and lay the foundation for intervention.
On the other hand, you must balance the importance of minimizing your own and the family’s anxiety about unrecognized disease against the physical and psychological risks and costs associated with medical tests and procedures.
Social assessment. Assess social and familial reinforcement (secondary gain) of the pain. Parents sometimes inadvertently encourage their children’s sick-role behaviors by providing excessive attention, rewards, or opportunities to avoid uncomfortable situations. RAP can become an excuse for poor performance (self-handicapping), particularly in children with a learning disorder.
How to deliver the diagnosis
Functional abdominal pain is essentially a clinical diagnosis that relies on presentation, course, and findings. As mentioned, a constellation of “clues” is most supportive, as is having typical IBS symptoms.
Before declaring the diagnosis, discuss with the family the patient’s physical, emotional, and behavioral symptoms and the context in which RAP developed. Doing so can help maintain your credibility and establish a consensus.
Once you declare the diagnosis, discuss it clearly and frankly. Families are not likely to be reassured if you do not offer a plausible explanation for the lack of physical findings.
Precautions. When a definitive diagnosis is not possible, acknowledge that uncertainty. Although you must discuss any recognized psychiatric comorbidity, attempting to “explain” that the disorder is causing the pain is usually impractical and intellectually dishonest.
Also, given the pervasive nature of stigma, do not convey embarrassment or unease about diagnosing functional RAP or any comorbid psychiatric disorder.
Follow-up testing. Once you diagnose functional RAP, further testing is generally not necessary. Tests might be indicated if you:
- receive new information
- observe a change in clinical status
- or are convinced that treatment will not work unless the family is reassured by further investigation.
Collaborative treatment
Reassurance and education. Reassurance that the patient does not have a serious physical disease is necessary but rarely sufficient. Explain that the child’s pain does not appear to reflect tissue damage and is not threatening. On the other hand, avoid giving excessive reassurance, particularly when obsessional illness worry and hypochondriacal fears are prominent. Address illness worry as a problem to be solved together.
Discuss with the patient and family what is known and not known about functional RAP, and encourage them to ask questions. This is an opportunity for you to instill hope and cultivate positive expectations, but avoid promising cure. Discuss the gut-brain connection and relevance of visceral hyperalgesia, including serotonin’s potential roles in RAP pathogenesis.
Partners in care. Collaborative treatment increases the likelihood of success. Discuss the importance of a therapeutic partnership, and clarify any areas of disagreement with the diagnosis or treatment plan.
Clearly delineate your roles and responsibilities and those of the patient, family, and other health care team members. Poor communication is pediatricians’ most common complaint about psychiatrists.14 Good interdisciplinary communication decreases the chance that treatment strategies will be duplicated, diluted, or misinterpreted.
Consolidate medical care with a single clinician—often the primary care physician—based on discussions with the patient, family, and health care team. The coordinating clinician can mediate between the school and family when tensions develop over poor attendance or requests for special treatment. It is often useful for this clinician to spell out:
- what constitutes a legitimate medical excuse for school absence
- who will legitimize excuses.
All parties should understand that the school will view an unexcused absence as truancy and act appropriately.
Diet and lifestyle. Encourage the patient to maintain a regular schedule and a healthy diet. Specific dietary interventions have not been proven effective, despite speculation that lack of dietary fiber or lactose intolerance might cause RAP.15,16 Also encourage adequate sleep and regular exercise.
Medication and psychotherapy
Because no strong evidence-based guidelines address pediatric RAP intervention, family preferences usually guide initial treatment decisions. This highlights the importance of good communication and a therapeutic partnership among the clinician, family, and patient.
Antispasmodics, acid reducers, and antidepressants are commonly prescribed for RAP, though none are well-supported in the literature and no controlled studies have gauged medication’s impact on psychiatric comorbidity.16
Antidepressants. Selective serotonin reuptake inhibitors (SSRIs) might help relieve RAP symptoms, but the evidence is inconclusive. SSRIs are considered potentially beneficial in RAP because they may help communicate nociceptive information between the gut and brain and mediate visceral hyperalgesia.
SSRIs at first may increase serotonin at the synapse, which one might assume would to worsen abdominal symptoms. However, ongoing SSRI use could “down-regulate” postsynaptic 5-HT3 receptors and desensitize postsynaptic cells to the effects of local serotonin.
Our group recently conducted a 12-week open trial of citalopram for functional pediatric RAP.17 The 25 participants received 10 mg/d the first week, then 20 mg/d thereafter if tolerated. At week 4, nonresponders and partial responders who were tolerating the medication began receiving 40 mg/d.
Table 3
SSRI daily dosing for pediatric RAP
| Drug | Starting dosage* | Target dosage† | Maximum dosage‡ |
|---|---|---|---|
| Citalopram | 10 mg | 20 mg | 40 to 60 mg |
| Escitalopram | 5 mg | 10 mg | 20 to 30 mg |
| Fluoxetine | 10 mg | 20 mg | 40 mg |
| Fluvoxamine | 50 mg | 100 mg | 300 mg |
| Sertraline | 25 mg | 50 mg | 200 mg |
| * First 3 to 7 days. | |||
| † If patient tolerates starting dosage, increase to target dosage. | |||
| ‡ If patient does not respond to target dosage in 2 to 3 weeks, consider increasing the dosage. | |||
At trial’s end, more than two-thirds of participants were taking 40 mg/d. We rated 21 of 25 patients (84%) as “much improved” or “very much improved,” using the Clinical Global Impression-Improvement scale. Abdominal pain, anxiety, depression, other somatic symptoms, and functional impairment all improved significantly during treatment. Suicidal thoughts diminished progressively from baseline, and no patient reported suicidal thinking at study’s end. Citalopram was generally well tolerated.
With SSRI treatment, start at a low dosage for 3 to 7 days (Table 3). If tolerated, increase to a typical therapeutic dosage. If symptoms fail to respond after 2 or 3 weeks, consider a higher dosage. A short course of an oral benzodiazepine (such as clonazepam, 0.25 mg bid) during the first weeks of SSRI treatment sometimes helps particularly anxious patients or those whose pain appears closely associated with anxiety or “stress.”
Pediatric gastroenterologists often prescribe a low-dose tricyclic antidepressant as first-line therapy, but we discourage this. TCAs lack efficacy in pediatric depression and pose a greater risk of side effects and safety concerns than SSRIs.18
Other agents have been tried for RAP-associated conditions:
Famotidine, a histamine type 2 receptor blocker, may reduce pain in children with dyspepsia and RAP.19
Peppermint oil reduced abdominal pain in one study of children with IBS but had little effect on other symptoms.20
Medications such as alosetron and tegaserod that interrupt serotonergic neurotransmission in the gut have shown benefit in adults with IBS but have not been studied in children.
Psychotherapy. A few small studies suggest that cognitive-behavioral therapies (CBT) are helpful in RAP, but CBT may be difficult to deliver in medical settings.21,22 A simplified “rehabilitative” approach that incorporates CBT principles involves having the clinician and patient view RAP as a challenge to be overcome, rather than a burden to be endured. Such an approach emphasizes the child’s fundamental strength and adaptability rather than vulnerabilities.
The goal of therapy is redirected from finding a cure to coping with and overcoming the problem. This approach challenges the notion that the child cannot resume normal function until the pain is completely gone. It encourages active, problem-focused coping, and discourages passive acceptance—which is associated with greater symptom burden and functional impairment.
Work with parents to reinforce the child’s health-promoting behaviors and minimize negative and social reinforcements (secondary gain) associated with RAP. Advise parents to:
- encourage and reward full school attendance
- avoid home-bound instruction
- expect the child to function despite physical distress
- insist that the child perform age-appropriate household chores and other responsibilities.
Self-management skills—such as relaxation training, hypnosis, biofeedback, and guided imagery—may help reduce pain and manage physiologic arousal.16 Deception strategies such as placebo or sham interventions are unethical and impractical.
Related resources
- Campo JV. Functional recurrent abdominal pain in children and adolescents. Digestive Health Matters 2003;12(3):15-7.
- International Foundation for Functional Gastrointestinal Disorders. www.iffgd.org
- University of Pittsburgh. Advanced Center for Interventions and Services Research on Early Onset Mood and Anxiety Disorders. www.moodykids.org
Drug brand names
- Alosetron • Lotronex
- Citalopram • Celexa
- Clonazepam • Klonopin
- Escitalopram • Lexapro
- Famotidine • Pepcid
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Sertraline • Zoloft
- Tegaserod • Zelnorm
Disclosure
Dr. Campo’s work has been supported by the National Institute of Mental Health (grant MH01780) and in part by the Advanced Center for Interventions and Services Research on Early Onset Mood and Anxiety Disorders (grant MH66371). He also receives grants from Forest Pharmaceuticals and is a consultant to Eli Lilly and Co.
1. American Psychiatric Association Diagnostic and statistical manual of mental disorders (4th ed, revised). Washington, DC: American Psychiatric Association, 2000.
2. Apley J, Naish N. Recurrent abdominal pains: a field survey of 1,000 school children. Arch Dis Child 1958;33(168):165-70.
3. Scharff L. Recurrent abdominal pain in children: a review of psychological factors and treatment. Clin Psychol Rev 1997;17(2):145-66.
4. Boyle JT. Recurrent abdominal pain: an update. Pediatr Rev 1997;18(9):310-20.
5. Campo JV, Bridge J, Ehmann M, et al. Recurrent abdominal pain, anxiety, and depression in primary care. Pediatrics 2004;113(4):817-24.
6. Campo JV, Di Lorenzo C, Chiappetta L, et al. Adult outcomes of pediatric recurrent abdominal pain: do they just grow out of it? Pediatrics 2001;108(1):E1.-
7. Starfield B, Gross E, Wood M, et al. Psychosocial and psychosomatic diagnoses in primary care of children. Pediatrics 1980;66(2):159-67.
8. Hyams JS, Burke G, Davis PM, et al. Abdominal pain and irritable bowel syndrome in adolescents: a community-based study. J Pediatr 1996;129(2):220-6.
9. Campo JV, Comer DM, Jansen-McWilliams L, et al. Recurrent pain, emotional distress, and health service use in childhood. J Pediatr 2002;141(1):76-83.
10. Stickler GB, Murphy DB. Recurrent abdominal pain. Am J Dis Child 1979;133(5):486-9.
11. Rasquin-Weber A, Hyman PE, Cucchiara S, et al. Childhood functional gastrointestinal disorders. Gut 1999;45(suppl 2):II60-8.
12. Campo JV, Fritz G. A management model for pediatric somatization. Psychosomatics 2001;42(6):467-76.
13. Campo JV, Garber J. Somatization. In: Ammerman RT, Campo JV (eds). Handbook of pediatric psychology and psychiatry. Vol 1 Boston: Allyn and Bacon, 1998;137-61.
14. Fritz GK, Bergman AS. Child psychiatrists seen through pediatricians’ eyes: results of a national survey. J Am Acad Child Psychiatry 1985;24(1):81-6.
15. Huertas-Ceballos A, Macarthur C, Logan S. Dietary interventions for recurrent abdominal pain (RAP) in childhood. Cochrane Database Syst Rev 2002;(2):CD003019.-
16. Weydert JA, Ball TM, Davis MF. Systematic review of treatments for recurrent abdominal pain. Pediatrics 2003;111(1):e1-11.
17. Campo JV, Perel J, Lucas A, et al. Citalopram treatment of pediatric recurrent abdominal pain and comorbid internalizing disorders: An exploratory study (poster). Miami Beach, FL: American Academy of Child and Adolescent Psychiatry annual meeting, October 2003.
18. Geller B, Reising D, Leonard HL, et al. Critical review of tricyclic antidepressant use in children and adolescents. J Am Acad Child Adolesc Psychiatry 1999;38(5):513-6.
19. See MC, Birnbaum AH, Schechter CB, et al. Double-blind, placebo-controlled trial of famotidine in children with abdominal pain and dyspepsia: global and quantitative assessment. Dig Dis Sci 2001;46(5):985-92.
20. Kline RM, Kline JJ, Di Palma J, Barbero GJ. Enteric-coated, pH-dependent peppermint oil capsules for the treatment of irritable bowel syndrome in children. J Pediatr 2001;138(1):125-8.
21. Sanders MR, Rebgetz M, Morrison M, et al. Cognitive-behavioral treatment of recurrent nonspecific abdominal pain in children: an analysis of generalization, maintenance, and side effects. J Consult Clin Psychol 1989;57(2):294-300.
22. Sanders MR, Shepherd RW, Cleghorn G, Woolford H. The treatment of recurrent abdominal pain in children: a controlled comparison of cognitive-behavioral family intervention and standard pediatric care. J Consult Clin Psychol 1994;62(2):306-14.
1. American Psychiatric Association Diagnostic and statistical manual of mental disorders (4th ed, revised). Washington, DC: American Psychiatric Association, 2000.
2. Apley J, Naish N. Recurrent abdominal pains: a field survey of 1,000 school children. Arch Dis Child 1958;33(168):165-70.
3. Scharff L. Recurrent abdominal pain in children: a review of psychological factors and treatment. Clin Psychol Rev 1997;17(2):145-66.
4. Boyle JT. Recurrent abdominal pain: an update. Pediatr Rev 1997;18(9):310-20.
5. Campo JV, Bridge J, Ehmann M, et al. Recurrent abdominal pain, anxiety, and depression in primary care. Pediatrics 2004;113(4):817-24.
6. Campo JV, Di Lorenzo C, Chiappetta L, et al. Adult outcomes of pediatric recurrent abdominal pain: do they just grow out of it? Pediatrics 2001;108(1):E1.-
7. Starfield B, Gross E, Wood M, et al. Psychosocial and psychosomatic diagnoses in primary care of children. Pediatrics 1980;66(2):159-67.
8. Hyams JS, Burke G, Davis PM, et al. Abdominal pain and irritable bowel syndrome in adolescents: a community-based study. J Pediatr 1996;129(2):220-6.
9. Campo JV, Comer DM, Jansen-McWilliams L, et al. Recurrent pain, emotional distress, and health service use in childhood. J Pediatr 2002;141(1):76-83.
10. Stickler GB, Murphy DB. Recurrent abdominal pain. Am J Dis Child 1979;133(5):486-9.
11. Rasquin-Weber A, Hyman PE, Cucchiara S, et al. Childhood functional gastrointestinal disorders. Gut 1999;45(suppl 2):II60-8.
12. Campo JV, Fritz G. A management model for pediatric somatization. Psychosomatics 2001;42(6):467-76.
13. Campo JV, Garber J. Somatization. In: Ammerman RT, Campo JV (eds). Handbook of pediatric psychology and psychiatry. Vol 1 Boston: Allyn and Bacon, 1998;137-61.
14. Fritz GK, Bergman AS. Child psychiatrists seen through pediatricians’ eyes: results of a national survey. J Am Acad Child Psychiatry 1985;24(1):81-6.
15. Huertas-Ceballos A, Macarthur C, Logan S. Dietary interventions for recurrent abdominal pain (RAP) in childhood. Cochrane Database Syst Rev 2002;(2):CD003019.-
16. Weydert JA, Ball TM, Davis MF. Systematic review of treatments for recurrent abdominal pain. Pediatrics 2003;111(1):e1-11.
17. Campo JV, Perel J, Lucas A, et al. Citalopram treatment of pediatric recurrent abdominal pain and comorbid internalizing disorders: An exploratory study (poster). Miami Beach, FL: American Academy of Child and Adolescent Psychiatry annual meeting, October 2003.
18. Geller B, Reising D, Leonard HL, et al. Critical review of tricyclic antidepressant use in children and adolescents. J Am Acad Child Adolesc Psychiatry 1999;38(5):513-6.
19. See MC, Birnbaum AH, Schechter CB, et al. Double-blind, placebo-controlled trial of famotidine in children with abdominal pain and dyspepsia: global and quantitative assessment. Dig Dis Sci 2001;46(5):985-92.
20. Kline RM, Kline JJ, Di Palma J, Barbero GJ. Enteric-coated, pH-dependent peppermint oil capsules for the treatment of irritable bowel syndrome in children. J Pediatr 2001;138(1):125-8.
21. Sanders MR, Rebgetz M, Morrison M, et al. Cognitive-behavioral treatment of recurrent nonspecific abdominal pain in children: an analysis of generalization, maintenance, and side effects. J Consult Clin Psychol 1989;57(2):294-300.
22. Sanders MR, Shepherd RW, Cleghorn G, Woolford H. The treatment of recurrent abdominal pain in children: a controlled comparison of cognitive-behavioral family intervention and standard pediatric care. J Consult Clin Psychol 1994;62(2):306-14.
Posttraumatic stress disorder: Nature and nurture?
Posttraumatic stress disorder (PTSD) can be one of the most frustrating anxiety disorders for both the patient and clinician. Asymptomatic persons become haunted by an experience they can’t forget. Their resulting anxiety can sour what were once healthy relationships or disable someone who previously was productive.
In some cases, despite aggressive psychopharmacology and psychotherapy, the patient remains incapacitated by inappropriate and unremitting fear. The trauma seems to have broken something—changed something inside the brain—that can’t be fixed.
Brain imaging studies of patients with PTSD—combat veterans and women with histories of childhood sexual abuse—have shown smaller hippocampal volumes compared with patients without PTSD.1,2 This finding has led to speculation that stress hormones (glucocorticoids) adversely affect the hippocampus (Figure 1).
This line of reasoning suggests that prolonged stress causes increased production of glucocorticoids that are neurotoxic to the hippocampus, resulting in hippocampal atrophy.3 Studies of rodents and patients with Cushing’s syndrome support this hypothesis. The hippocampus, therefore, may have been irreversibly damaged in patients with severe PTSD.
Figure 1
The hippocampus, a specialized type of cortex, is key to memory and emotion. As this medial view shows, it extends along the lateral ventricle floor on each side of the brain.
Illustration for Current Psychiatry by Marcia Hartsock, CMI Hippocampus
Intuitively, this theory makes sense, as the hippocampus is crucial for memory and emotion. However, a recent study of identical twins raises doubts.
Surprising evidence
Gilbertson et al recruited 40 pairs of twins, in which one was a Vietnam combat veteran and the other stayed home.4 Using MRI, the researchers measured hippocampal volume in each twin and assessed the presence and severity of PTSD in the combat-exposed twin.
Consistent with earlier reports, the authors found smaller hippocampal volumes in combat-exposed individuals diagnosed with PTSD. However, they found an almost identical correlation between the noncombat-exposed twin’s hippocampal volume and the combat-exposed twin’s PTSD score (Figure 2). In other words, the twin’s hippocampus size was a better predictor of the veteran’s hippocampus size than was the veteran’s trauma exposure or PTSD symptoms.
This finding puts a new spin on the association between small hippocampal volume and PTSD. The authors stated, “these data indicate that smaller hippocampi in PTSD represents a pre-existing, familial vulnerability factor rather than the neurotoxic product of trauma exposure per se.” Put another way, the small hippocampus is not created by stress and trauma but is a preexisting condition. Further, this study suggests that a larger hippocampus may protect a person from developing PTSD.
This study may help explain why different individuals exposed to the same trauma are frequently left with different symptoms.5,6 PTSD would seem to be an excellent example of the combined effects of nature (small hippocampus) and nurture (traumatic experience).
Figure 2 Hippocampal volume correlates with posttraumatic symptoms
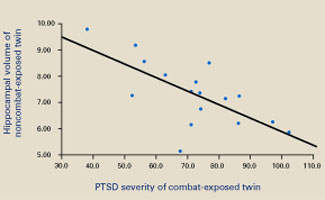
Smaller hippocampal volume in identical twins not exposed to combat was related to more-severe PTSD symptoms in their combat-exposed brothers (P = 0.002). Symptom severity was measured using Clinician-Administered PTSD Scale (CAPS) total scores.
Source: Reprinted with permission from Gilbertson MW, Shenton ME, Ciszewski A, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neurosci 2002;5:1242-7.
1. Bremner JD, Randall P, Scott TM, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry 1995;152:973-81.
2. Bremner JD, Vythilingam M, Vermetten E, et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry 2003;160:924-32.
3. Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry 2000;57:925-35.
4. Gilbertson MW, Shenton ME, Ciszewski A, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci 2002;5:1242-7.
5. Macklin ML, Metzger LJ, Litz BT, et al. Lower precombat intelligence is a risk factor for posttraumatic stress disorder. J Consult Clin Psychol 1998;66:323-6.
6. Schlenger WE, Caddell JM, Ebert L, et al. Psychological reactions to terrorist attacks: findings from the National Study of Americans’ Reactions to September 11. JAMA 2002;288:581-8.
Posttraumatic stress disorder (PTSD) can be one of the most frustrating anxiety disorders for both the patient and clinician. Asymptomatic persons become haunted by an experience they can’t forget. Their resulting anxiety can sour what were once healthy relationships or disable someone who previously was productive.
In some cases, despite aggressive psychopharmacology and psychotherapy, the patient remains incapacitated by inappropriate and unremitting fear. The trauma seems to have broken something—changed something inside the brain—that can’t be fixed.
Brain imaging studies of patients with PTSD—combat veterans and women with histories of childhood sexual abuse—have shown smaller hippocampal volumes compared with patients without PTSD.1,2 This finding has led to speculation that stress hormones (glucocorticoids) adversely affect the hippocampus (Figure 1).
This line of reasoning suggests that prolonged stress causes increased production of glucocorticoids that are neurotoxic to the hippocampus, resulting in hippocampal atrophy.3 Studies of rodents and patients with Cushing’s syndrome support this hypothesis. The hippocampus, therefore, may have been irreversibly damaged in patients with severe PTSD.
Figure 1

The hippocampus, a specialized type of cortex, is key to memory and emotion. As this medial view shows, it extends along the lateral ventricle floor on each side of the brain.
Illustration for Current Psychiatry by Marcia Hartsock, CMI Hippocampus
Intuitively, this theory makes sense, as the hippocampus is crucial for memory and emotion. However, a recent study of identical twins raises doubts.
Surprising evidence
Gilbertson et al recruited 40 pairs of twins, in which one was a Vietnam combat veteran and the other stayed home.4 Using MRI, the researchers measured hippocampal volume in each twin and assessed the presence and severity of PTSD in the combat-exposed twin.
Consistent with earlier reports, the authors found smaller hippocampal volumes in combat-exposed individuals diagnosed with PTSD. However, they found an almost identical correlation between the noncombat-exposed twin’s hippocampal volume and the combat-exposed twin’s PTSD score (Figure 2). In other words, the twin’s hippocampus size was a better predictor of the veteran’s hippocampus size than was the veteran’s trauma exposure or PTSD symptoms.
This finding puts a new spin on the association between small hippocampal volume and PTSD. The authors stated, “these data indicate that smaller hippocampi in PTSD represents a pre-existing, familial vulnerability factor rather than the neurotoxic product of trauma exposure per se.” Put another way, the small hippocampus is not created by stress and trauma but is a preexisting condition. Further, this study suggests that a larger hippocampus may protect a person from developing PTSD.
This study may help explain why different individuals exposed to the same trauma are frequently left with different symptoms.5,6 PTSD would seem to be an excellent example of the combined effects of nature (small hippocampus) and nurture (traumatic experience).
Figure 2 Hippocampal volume correlates with posttraumatic symptoms

Smaller hippocampal volume in identical twins not exposed to combat was related to more-severe PTSD symptoms in their combat-exposed brothers (P = 0.002). Symptom severity was measured using Clinician-Administered PTSD Scale (CAPS) total scores.
Source: Reprinted with permission from Gilbertson MW, Shenton ME, Ciszewski A, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neurosci 2002;5:1242-7.
Posttraumatic stress disorder (PTSD) can be one of the most frustrating anxiety disorders for both the patient and clinician. Asymptomatic persons become haunted by an experience they can’t forget. Their resulting anxiety can sour what were once healthy relationships or disable someone who previously was productive.
In some cases, despite aggressive psychopharmacology and psychotherapy, the patient remains incapacitated by inappropriate and unremitting fear. The trauma seems to have broken something—changed something inside the brain—that can’t be fixed.
Brain imaging studies of patients with PTSD—combat veterans and women with histories of childhood sexual abuse—have shown smaller hippocampal volumes compared with patients without PTSD.1,2 This finding has led to speculation that stress hormones (glucocorticoids) adversely affect the hippocampus (Figure 1).
This line of reasoning suggests that prolonged stress causes increased production of glucocorticoids that are neurotoxic to the hippocampus, resulting in hippocampal atrophy.3 Studies of rodents and patients with Cushing’s syndrome support this hypothesis. The hippocampus, therefore, may have been irreversibly damaged in patients with severe PTSD.
Figure 1

The hippocampus, a specialized type of cortex, is key to memory and emotion. As this medial view shows, it extends along the lateral ventricle floor on each side of the brain.
Illustration for Current Psychiatry by Marcia Hartsock, CMI Hippocampus
Intuitively, this theory makes sense, as the hippocampus is crucial for memory and emotion. However, a recent study of identical twins raises doubts.
Surprising evidence
Gilbertson et al recruited 40 pairs of twins, in which one was a Vietnam combat veteran and the other stayed home.4 Using MRI, the researchers measured hippocampal volume in each twin and assessed the presence and severity of PTSD in the combat-exposed twin.
Consistent with earlier reports, the authors found smaller hippocampal volumes in combat-exposed individuals diagnosed with PTSD. However, they found an almost identical correlation between the noncombat-exposed twin’s hippocampal volume and the combat-exposed twin’s PTSD score (Figure 2). In other words, the twin’s hippocampus size was a better predictor of the veteran’s hippocampus size than was the veteran’s trauma exposure or PTSD symptoms.
This finding puts a new spin on the association between small hippocampal volume and PTSD. The authors stated, “these data indicate that smaller hippocampi in PTSD represents a pre-existing, familial vulnerability factor rather than the neurotoxic product of trauma exposure per se.” Put another way, the small hippocampus is not created by stress and trauma but is a preexisting condition. Further, this study suggests that a larger hippocampus may protect a person from developing PTSD.
This study may help explain why different individuals exposed to the same trauma are frequently left with different symptoms.5,6 PTSD would seem to be an excellent example of the combined effects of nature (small hippocampus) and nurture (traumatic experience).
Figure 2 Hippocampal volume correlates with posttraumatic symptoms

Smaller hippocampal volume in identical twins not exposed to combat was related to more-severe PTSD symptoms in their combat-exposed brothers (P = 0.002). Symptom severity was measured using Clinician-Administered PTSD Scale (CAPS) total scores.
Source: Reprinted with permission from Gilbertson MW, Shenton ME, Ciszewski A, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neurosci 2002;5:1242-7.
1. Bremner JD, Randall P, Scott TM, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry 1995;152:973-81.
2. Bremner JD, Vythilingam M, Vermetten E, et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry 2003;160:924-32.
3. Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry 2000;57:925-35.
4. Gilbertson MW, Shenton ME, Ciszewski A, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci 2002;5:1242-7.
5. Macklin ML, Metzger LJ, Litz BT, et al. Lower precombat intelligence is a risk factor for posttraumatic stress disorder. J Consult Clin Psychol 1998;66:323-6.
6. Schlenger WE, Caddell JM, Ebert L, et al. Psychological reactions to terrorist attacks: findings from the National Study of Americans’ Reactions to September 11. JAMA 2002;288:581-8.
1. Bremner JD, Randall P, Scott TM, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry 1995;152:973-81.
2. Bremner JD, Vythilingam M, Vermetten E, et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry 2003;160:924-32.
3. Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry 2000;57:925-35.
4. Gilbertson MW, Shenton ME, Ciszewski A, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci 2002;5:1242-7.
5. Macklin ML, Metzger LJ, Litz BT, et al. Lower precombat intelligence is a risk factor for posttraumatic stress disorder. J Consult Clin Psychol 1998;66:323-6.
6. Schlenger WE, Caddell JM, Ebert L, et al. Psychological reactions to terrorist attacks: findings from the National Study of Americans’ Reactions to September 11. JAMA 2002;288:581-8.
Treating affective illness in patients with chronic pain
Ms. A, age 44, fell from a 3-foot stool while reaching for a high kitchen shelf and suffered severe neck flexion. Her initial pain persisted for weeks and then months, resulting in chronic neck pain aggravated by movement.
Over the past year, her doctor has prescribed numerous analgesics and muscle relaxants, including tramadol, hydrocodone, oxycodone, tizanidine, and nonsteroidal anti-inflammatory drugs (NSAIDs). Treatments at a pain clinic have included triggerpoint injections, cervical epidural corticosteroid injection, left-sided cervical medial branch blocks, transcutaneous electrical nerve stimulation, and physical therapy. None provided sustained relief.
During a pain clinic visit, Ms. A wept and said she was tired of living with pain. She acknowledged depression and agreed to psychiatric consultation.
As in Ms. A’s case, physicians often refer patients with chronic pain and affective symptoms for psychiatric evaluation. These patients are often fearful, angry, and suspicious of any suggestion that their physical discomfort has a psychiatric component. They typically believe their pain had a clear onset and therefore should have an end point. Many have experienced unproductive specialty evaluations and failed treatments.
To help you overcome these obstacles when treating patients with chronic pain and depression, we discuss:
- strategies to gain patients’ trust and build a therapeutic alliance
- how to assess their pain, depression, and suicide risk
- the role of psychotherapy in treating chronic pain
- and evidence for choosing effective, nonaddicting medications.
Psychiatric evaluation
Depression and pain are linked psychologically and biochemically, sharing neurotransmitters involved in both nociceptive pathways and mood, especially serotonin and norepinephrine.1,2 One-third to one-half of patients with chronic pain report comorbid depression,3 and more than one-half of depressed patients presenting to primary care physicians report only somatic symptoms—various pain complaints among the most common.4,5
Primary care doctors tend to refer chronic pain and depression cases to psychiatrists when:
- patients are preoccupied with medication, have not followed treatment recommendations, or do not respond to treatment as expected
- extensive medical evaluations reveal few or equivocal findings
- somatic complaints are vague and diffuse, or there is marked disparity between pain complaints/disability and objective findings.6,7
Assessing pain. In the initial assessment, validate the patient’s pain experience by asking about the location, quality, and severity of pain. The visual analogue scale (VAS) is commonly used to measure pain severity. The patient marks a spot on a line from “no pain” to “worst possible pain,” or—on a numbered VAS—from 0 (no pain) to 10 (extreme pain). The least and most severe pain over the preceding month can be ranked as baseline values.8
Be sensitive to the patient’s fear that you will attribute the pain to psychosocial issues or imply that “the pain is in your head.” Emphasize that you intend to evaluate the “whole person,” not just the part that hurts. Focus on how the pain affects the patient’s lifestyle—rather than its cause—and explore medication use patterns.
Assessing depression. The word “depression” is emotionally charged for chronic pain patients, who view affective symptoms—if they acknowledge them at all—as secondary to pain. They may strongly resist treatment for anything but pain. One way to defuse this defensiveness is to avoid attributing the pain to stress or depression.
Begin by assessing vegetative symptoms, which overlap in chronic pain and depression. The Beck Depression Inventory-II (Beck-II) may be a useful screening tool in a busy practice; the short form (13 questions) takes about 5 minutes to complete.9
Explore cognitive and behavioral symptoms such as concentration, pleasure and interest level, activity, and self-esteem. Review the chronology of pain onset, mood changes, and stressors (proximate, remote, and cumulative).
Seek clues to endogenous factors by asking about past affective episodes, response to antidepressants, and family history of psychopathology. Substances that may induce depression include reserpine, interferon, and antiparkinsonian agents. Screen for potential organic mood disorders, such as depression secondary to hypothyroidism, corticosteroid use, Parkinson’s disease, lupus, HIV infection, or cerebrovascular disease. Where appropriate, obtain collateral information from family or friends.
Assessing suicide risk. Chronic pain patients may be at greater risk of suicide than the general population. Besides pain, other risk factors for suicide—such as major depression, anxiety disorders, alcohol/substance abuse, sleep disturbances, male gender, diminished social support, and recent loss—are common among these patients.10,11
Screen chronic pain patients with suicidal ideation for these risk factors. Interventions include:
- aggressively treat associated depression, anxiety, or insomnia
- elicit support from family or other caregivers
- pay close attention to talk about suicide
- hospitalize when necessary
- and, of course, treat pain.
Case continued: No stranger to depression
Ms. A’s psychiatric assessments revealed a pain severity ranking of 9 on a 1-to-10 scale, frequent crying, hopelessness, disrupted sleep, low energy, limited ability to concentrate, and fleeting suicidal thoughts. Her history included counseling during her first marriage and severe depression after separation from her second husband 3 years ago. An 8-week trial of fluoxetine, 20 mg/d, did not improve her depression then.
On examination, she displayed obvious pain behavior, constantly shifting her neck position and moving about the room. Her affect was tearful and her mood depressed. She was taking the NSAID celecoxib, 100 mg bid, and the skeletal muscle relaxant tizanidine, 4 mg tid. She was no longer using opioids and had no history of alcohol or illicit drug abuse.
Based on this assessment, the psychiatrist diagnosed Ms. A as having pain disorder with medical and psychological features, including symptom amplification and depression.
Table 1
4 treatment goals for patients with chronic pain and depression
|
Educating the patient
As part of your assessment, explain the reciprocal effects of depression and pain. Acknowledge that:
- chronic pain is different from acute pain, although the patient’s pain experience is the same
- treatment often becomes part of the problem in chronic pain.
Doctors tend to apply acute pain treatments chronically, risking long-term effects of polypharmacy to achieve short-term relief. Depressed patients may be more likely than nondepressed patients to receive opioids for chronic pain,12 and opioids and benzodiazepines may have depressive effects, as reflected by DSM-IV-TR’s inclusion of criteria for “opioid-induced mood disorder” and “sedative-, hypnotic-, or anxiolytic-induced mood disorder.”
To reduce patients’ resistance to antidepressants, reiterate any history of cumulative stressors and affective episodes unrelated to pain. Try using an analogy, such as “stress and pain are like waves on a rock” that eventually damage mood and coping mechanisms, or depression complicating pain is like having “too much on one’s plate.”
Finally, help patients understand that chronic pain is managed, not cured. Encourage them to set treatment goals beyond reducing pain (Table 1) and to make the transition from “patient with pain” to “client managing pain.”
Table 2
Dosing antidepressants and anticonvulsants
for chronic pain and depression
| Drug | Starting (mg/d) | Target (mg/d) | Administration tips |
|---|---|---|---|
| TCAs | Check serum levels for dosages ≥150 mg/d (nortriptyline 100 mg/d) to assess rapid metabolism, adherence, or toxic levels | ||
| Amitriptyline | 10 to 25 | 75 to 300 | |
| Clomipramine | 10 to 25 | 75 to 250 | |
| Desipramine | 10 to 25 | 75 to 200 | |
| Doxepin | 10 to 25 | 75 to 300 | |
| Imipramine | 10 to 25 | 75 to 300 | |
| Nortriptyline | 10 to 25 | 40 to 200 | |
| SNRI | |||
| Venlafaxine | 37.5 to 75 | 75 to 375 | Use XR form to minimize side effects and for once-daily dosing |
| SSRIs | |||
| Citalopram | 10 to 20 | 40 to 60 | |
| Fluoxetine | 10 to 20 | 20 to 80 | May increase carbamazepine, TCA blood levels and inhibit efficacy of codeine, dihydrocodeine, and hydrocodone |
| Paroxetine | 10 to 20 | 20 to 60 | Same as fluoxetine |
| Anticonvulsants | |||
| Carbamazepine | 200 | 800 to 1,200 | Check blood levels; may increase clomipramine levels, reduce acetaminophen, contraceptive levels |
| Clonazepam | 0.5 | 1 to 2 | Habituating potential with chronic use |
| Gabapentin | 300 to 900 | 3,600 to 4,800 | Blood monitoring not necessary |
| Valproate | 250 | 750 to 2,500 (maximum dosage 60 mg/kg/d) | Check blood levels (trough plasma level 50 to 100 μg/mL) |
| TCA: tricyclic antidepressant | |||
| SNRI: serotonin-norepinephrine reuptake inhibitor | |||
| SSRI: selective serotonin reuptake inhibitor | |||
Prescribing principles
Before adding any new pain medications, consider reducing dosages or discontinuing opioids or benzodiazepines and other substances the patient may be taking. Opioid use is associated with risks of dependence, addiction, and side effects including somnolence, cognitive impairment, and reduced activity that amplify depressive symptoms.
Benzodiazepines can generally be tapered by 10% per day, although you may need to extend the final taper over 3 to 4 days or longer, depending upon chronicity of use. Opioids may be tapered by 20% over 5 to 7 days. Breakthrough doses may be needed for marked withdrawal symptoms. Converting to longer half-life agents—such as clonazepam for benzodiazepines or methadone for opioids—often aids tapering, although other agents and strategies exist.13
To gauge patient attempts at self-medication, monitor use of alcohol or illicit drugs with urine screening. For patients with a substantial history of substance abuse or positive toxicology screens, monitor randomly every 2 to 4 weeks.
On the other hand, undertreated pain also may impair mood and function.1 If pain and mood improve and problematic drug-related behaviors resolve with increased opioid analgesia, consider maintaining opioids with regular re-evaluation of mood, coping, and medication adherence.11 Transfer from immediate-release to controlled-release opioids to reduce dosing frequency, clockwatching, and the likelihood of inter-dose pain escalation. In general, maintain and optimize the dosage of nonaddictive analgesics such as NSAIDs, anticonvulsants, or antidepressants.
Case continued: Switching medication
The psychiatrist started Ms. A on nortriptyline, 25 mg at bedtime, to be increased after 3 nights to 50 mg at bedtime. Tizanidine, which had been ineffective, was discontinued to reduce the risk of xerostomia and oversedation in combination with nortriptyline. If tolerated, nortriptyline was to be further increased by 25 mg every 3 days to an initial target dosage of 100 mg at bedtime. The psychiatrist explained to Ms. A that it might take 4 to 6 weeks to gauge the medication’s efficacy.
Psychoeducation addressed the importance of stress reduction, prioritizing commitments, and setting limits on other people’s expectations. The door was left open to future psychotherapeutic exploration of past cumulative stressors.
Because antidepressants may provide an analgesic effect,6,14 they are often used to treat affective symptoms in chronic pain. Headache and neuralgia tend to respond to antidepressants more robustly than do arthritis and low-back pain. Although some patients respond to low-dose antidepressants, a definitive trial requires full doses for 6 to 8 weeks (Table 2).
Matching a patient’s symptoms with medication side effects is useful when choosing antidepressants (Table 3). So-called “adverse” effects may have a corresponding benefit, depending on the clinical presentation. For example, a moreactivating antidepressant—such as the selective serotonin reuptake inhibitor (SSRI) fluoxetine—may help a patient with fatigue, whereas a moresedating agent—such as a tricyclic antidepressant (TCA) or mirtazapine—may improve sleep for a patient with insomnia.
Psychosocial therapies such as cognitive-behavioral therapy (CBT) or relaxation training (Table 4) may help patients with chronic pain to:
- process covert emotions such as fear and anger as well as guilt, loss, and disability
- reduce somatic preoccupation that is aggravating the pain
- adhere to treatment.
Evidence strongly supports using relaxation techniques to reduce chronic pain in many medical conditions and hypnosis to ameliorate cancer pain. CBT and biofeedback appear moderately effective in relieving chronic pain.15 CBT is significantly more effective than waiting list control conditions for relieving chronic nonheadache pain in measures of pain experience, mood/affect, cognitive coping and appraisal, pain behavior and activity level, and social role functioning.16
Pain and opioid medications can impair concentration and affective processing, so initial psychotherapy may need to be supportive while you provide other treatments and simplify medication regimens. Eventually the patient may be ready to address underlying issues that may be contributing to the pain syndrome, such as a history of abuse. However, it is important to address this potentially destabilizing subject only after carefully gauging a patient’s defenses and readiness.
Case continued: A bump in the road
The psychiatrist saw Ms. A 18 months later. Interim history revealed that her pain and mood improved on nortriptyline, 100 mg at bedtime. When she stopped taking nortriptyline 5 months earlier, her neck pain increased and she experienced a “deep blue mood.” Her physician restarted the nortriptyline.
At follow up, Ms. A reported no depressive symptoms and very little neck pain. The psychiatrist discussed with her depression’s relapse rate and the importance of continuing antidepressant therapy. As Ms. A was feeling much better and functioning normally, the psychiatrist decided additional psychotherapeutic intervention was not necessary.
Antidepressant options
TCAs provide analgesia via descending regulatory pathways by inhibiting serotonin and norepinephrine reuptake.17 When using TCAs for chronic pain, start with 10 to 25 mg at bedtime and increase by 10 to 25 mg every 3 to 7 days as tolerated. Increase incrementally until the pain responds or to the full antidepressant dosage (Table 2). Drug levels (when available) can help you provide an appropriate trial and monitor the patient’s adherence.
If the pain does not respond after 6 to 8 weeks, consider switching to another dual-action agent such as venlafaxine or to an SSRI.
SNRIs. Venlafaxine is a serotonin and norepineph-rine reuptake inhibitor (SNRI) with less-troublesome side effects than TCAs. It is structurally similar to tramadol18 and has combined serotonin and norepinephrine inhibition at dosages >75 mg/d. Although venlafaxine is not indicated for chronic pain, some studies have suggested possible benefits, including long-term analgesia, reduced polyneuropathic pain, and migraine prophylaxis.19-21 Venlafaxine may be a reasonable first or second choice for treating depression in patients with chronic pain, especially headache.14
Duloxetine—another SNRI—awaits FDA approval. Some studies have suggested that duloxetine improves painful physical symptoms as well as mood and functioning in major depression.22
SSRIs may be effective for certain types of pain, but the evidence is conflicting. Results of 41controlled trials support TCAs’ analgesic efficacy for neuropathic pain, headache, and central and post-stroke pain, whereas SSRIs’ analgesic efficacy varies from study to study. Comparisons of TCAs and SSRIs as analgesics uniformly show TCAs to be more effective, with the SSRIs often showing no analgesic effect.
Of three controlled trials of SSRIs for diabetic neuropathy, one showed fluoxetine similar to placebo, and two smaller studies showed paroxetine and citalopram more effective than placebo. Fluoxetine has shown analgesic effect for fibromyalgia in one study, but no effect in another. Citalopram showed no analgesic effect for fibromyalgia in another study.23
A prospective, double-blind study comparing fluoxetine, sertraline, paroxetine, and venlafaxine for migraines reported moderate to significant improvement in less than one-half of SSRI-treated patients vs two-thirds of venlafaxine-treated patients.21 SSRIs are no longer recognized by the International Headache Society as primary preventative medications for migraine.
Fluoxetine may help chronic daily headache, and paroxetine and citalopram may be useful for diabetic neuropathy. However, one cannot generalize that all SSRIs are similarly effective as analgesics.14
SSRIs have fewer side effects than TCAs and are less dangerous in overdose. In general, however, SSRIs are a second-line treatment for pain, to be used when dual-action agents pose disadvantageous side effects (Table 3) or have been poorly tolerated or ineffective.
Table 3
Antidepressant side effects:
Limitations and potential benefits in chronic pain
| Side effects/agents | Problems | Conditions potentially benefited | Possible alternatives |
|---|---|---|---|
| Anticholinergic TCAs | Xerostomia, constipation, urinary slowing (esp. when combined with opioids) | Diarrhea-predominant irritable bowel syndrome | SSRIs, nefazodone, venlafaxine |
| Sedation TCAs, mirtazapine, nefazodone, trazodone | Excessive sedation, cognitive impairment, driving risk (esp. when combined with opioids, benzodiazepines) | Pain with insomnia | SSRIs, venlafaxine, bupropion |
| Insomnia SSRIs, venlafaxine | Pain with pre-existing insomnia; equivocal analgesic effects | Excess sedation related to depression, polypharmacy for pain | TCAs, mirtazapine, trazodone, nefazodone |
| Orthostasis TCAs (esp. with methadone), nefazodone | Falls, especially in elderly patients | —— | Nortriptyline, SSRIs bupropion, venlafaxine |
| Weight gain TCAs, mirtazapine | Pain patients are often sedentary, get limited exercise | Pain and depression with weight loss | Bupropion, fluoxetine |
| Hypertension Bupropion, venlafaxine | Pre-existing hypertension | ? Hypotensive state | Citalopram (hypertensive side effects infrequent) |
| Cardiac TCAs | ECG abnormalities, conduction delays, arrhythmias aggravate pre-existing cardiac abnormalities; avoid if recent MI | ——— | SSRIs, bupropion |
| Overdose lethality TCAs | Prominent suicidal ideation | —— | SSRIs, venlafaxine |
| Seizures Esp. maprotiline, clomipramine, bupropion | Lower seizure threshold, aggravation of seizure disorders | ——- | SSRIs |
| Sexual dysfunction SSRIs | Pre-existing sexual dysfunction secondary to pain, medications, stress; equivocal analgesic effects | ——- | Bupropion, nefazodone, mirtazapine |
Table 4
How psychosocial therapies can help treat chronic pain and depression
| Therapies | Purpose/benefits |
|---|---|
| Behavioral therapy | Increase activity and learn to balance activity with limitations Reduce pain behaviors and analgesic use Decrease dependency and secondary gain |
| Cognitive-behavioral therapy | Identify automatic thoughts Challenge negative cognitions, catastrophizing Substitute and rehearse positive thoughts, capabilities Transition from patient role to self-care |
| Couples’ therapy | Assist adaptation to role changes Diminish spousal solicitousness or excessive caretaking Increase communication |
| Biofeedback, relaxation, imagery | Adjunctive role in pain management Reduce tension, comorbid anxiety |
| Hypnosis | Dissociate awareness of pain Substitute, displace, reinterpret pain sensations |
| Vocational rehabilitation | Increase activity, ability to distract Regain sense of control, identity, and productivity Increase socialization |
| Pain management program | Multiple treatment effects Useful for complex pain with affective states |
Monoamine oxidase inhibitors (MAOIs) may have some efficacy for neuropathy and headache, but the need for a tyramine-free diet and potential for drug-drug interactions limit their usefulness. Co-administering an MAOI and meperidine is always contraindicated, as this combination can produce fever, delirium, seizures, circulatory collapse, and death. Similarly, avoid using an MAOI with any other antidepressant.
Others. Evidence is very limited on using other antidepressants such as trazodone, nefazodone, bupropion, and mirtazapine in chronic pain:
- Trazodone may help pediatric migraine, but it is not a consistent analgesic and may not be well-tolerated.
- Case reports suggest bupropion may help with headaches and chronic low-back pain.14
- Mirtazapine and trazodone may be useful adjuncts for treating insomnia in depressed patients with chronic pain.
Other options
Anticonvulsants appear useful for neuropathic pain and are appropriate for chronic pain patients who cannot tolerate TCAs.24 Like TCAs, anticonvulsants are not addictive. Unlike TCAs, anticonvulsants may help stabilize other affective illnesses that may coexist with chronic pain, including bipolar disorder, schizoaffective disorder, and impulsivity/aggression related to dementia or personality disorder.6 If the starting dosage is not effective within 1 week, increase gradually every 2 to 3 days to target dosages comparable to those for anticonvulsant efficacy.
Carbamazepine and gabapentin are recommended first-line medications for neuropathy. Carbamazepine is indicated for treating trigeminal neuralgia, although its cytochrome P-450 3A3/4 isoenzyme induction may reduce serum levels of acetaminophen, opioids, and oral contraceptives. Gabapentin, although not clearly beneficial for bipolar disorder, has anxiolytic effects and a benign side-effect profile, which may help patients with chronic pain.
Valproate can help prevent migraines. Clonazepam can help reduce anxiety and restless legs syndrome but may be habituating. Anticonvulsants’ common adverse effects include sedation, GI upset, dizziness, and fatigue.
Lithium has known efficacy for mood stabilization in bipolar disorder and can ameliorate cluster headaches.
Antipsychotics. Evidence is sparse on whether antipsychotics have analgesic activity. Their side effects generally limit their usefulness to treating pain in patients with psychosis or delirium.6
Stimulants such as dextroamphetamine and methylphenidate can be helpful adjuncts for treating depression, especially for medical inpatients who require a rapid therapeutic response. Stimulants may reduce fatigue or excessive sedation and improve concentration in patients receiving opioids for chronic pain. They also may have analgesic effects when combined with opioids. Potential adverse effects include appetite suppression, anxiety or agitation, confusion, tics, and addiction.6
Precautions. The muscle relaxant carisoprodol is associated with potential dependence and withdrawal. Cyclobenzaprine, another muscle relaxant, has a TCA-like structure and can be lethal in overdose. Baclofen can be useful for chronic pain related to spasticity, although psychotic depression and mania have been reported with abrupt withdrawal.6
Related resources
- American Academy of Pain Medicine. www.painmed.org/
- American Academy of Pain Management. www.aapainmanage.org
- Pain.com: continuing medical education for anesthesiology professionals. www.pain.com/index.cfm
- Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI). Pain 1985;13:345-56
Drug brand names
- Amitriptyline • Elavil
- Baclofen • Lioresal
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Carisoprodol • Soma
- Celecoxib • Celebrex
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Cyclobenzaprine • Flexeril
- Desipramine • Norpramin
- Dihydrocodeine • Synalgos-DC
- Doxepin • Sinequan
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Hydrocodone • Vicodin, Lortab
- Imipramine • Tofranil
- Lithium • Eskalith CR, Lithobid
- Maprotiline • Ludiomil
- Meperidine • Demerol
- Methadone • Dolophine
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Nortriptyline • Pamelor
- Oxycodone • OxyContin
- Paroxetine • Paxil
- Sertraline • Zoloft
- Tizanidine • Zanaflex
- Tramadol • Ultram
- Trazodone • Desyrel
- Valproate • Depakote
- Venlafaxine • Effexor, Effexor XR
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: a biopsychosocial perspective. Biol Psychiatry 2003;54:399-409.
2. Fishbain DA, Cutler R, Rosomoff HL, et al. Chronic painassociated depression: antecedent or consequence of chronic pain? A review. Clin J Pain 1997;13(2):116-37.
3. Banks SM, Kerns RD. Explaining high rates of depression in chronic pain: a diathesis-stress framework. Psychol Bull 1996;119:95-110.
4. Simon GE, VonKorff M, Piccinelli M, et al. An international study of the relation between somatic symptoms and depression. N Engl J Med 1999;341(18):1329-35.
5. Kroenke K, Price RK. Symptoms in the community. Prevalence, classification, and psychiatric comorbidity. Arch Intern Med. 1993;153:2474-80.
6. Leo RJ. Concise guide to pain management for psychiatrists. Arlington, Va: American Psychiatric Publishing, Inc., 2003.
7. Sullivan MD, Turner JA, Romano J. Chronic pain in primary care. Identification and management of psychosocial factors. J Fam Pract 1991;32(2):193-9.
8. Holmgren A, Wise MG, Bouckoms AJ. Pain management. In: Wise MG, Rundell JR (eds). Psychiatry in the medically ill (2nd ed). Washington, DC: American Psychiatric Publishing Inc., 2002;989-1013.
9. Naifeh KH. Psychometric testing in functional GI disorders in: Olden K (ed). Handbook of functional GI disorders. New York: Marcel Dekker, 1996;79-126.
10. Fishbain DA. The association of chronic pain and suicide. Semin Clin Neuropsychiatry 1999;4(3):221-7.
11. Fishbain DA. Medico-legal rounds: medical-legal issues and breaches of “standards of medical care” in opioid tapering for alleged opioid addiction. Pain Med 2002;3(2):135-42.
12. Doan BD, Wadden NP. Relationships between depressive symptoms and descriptions of chronic pain. Pain 1989;36:75-84.
13. Franklin JE, Leamon MH, Frances RJ. Substance-related disorders. In: Wise MG, Rundell JR (eds). Psychiatry in the medically ill (2nd ed). Washington DC: American Psychiatric Publishing, 2002;417-53.
14. Ansari A. The efficacy of newer antidepressants in the treatment of chronic pain: a review of current literature. Harv Rev Psychiatry 2000;7(5):257-77.
15. NIH Technology Assessment Panel. Integration of behavioral relaxation approaches into the treatment of chronic pain and insomnia. JAMA 1996;276(4):313-18.
16. Morley S, Eccleston C, Williams A. Systematic review and metaanalysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain 1999;80:1-13
17. Magni G. On the relationship between chronic pain and depression when there is no organic lesion. Pain 1987;31:1-21.
18. Markowitz JS, Patrick KS. Venlafaxine-tramadol similarities. Med Hypotheses 1998;5:167-8
19. Bradley RH, Barkin RL, Jerome J, et al. Efficacy of venlafaxine for the long-term treatment of chronic pain with associated major depressive disorder. Am J Ther 2003;10(5):318-23.
20. Sindrup SH, Bach FW, Madsen C, et al. Venlafaxine vs. imipramine in painful polyneuropathy—a randomized controlled trial. Neurology 2003;60:1284-9.
21. Kathpal GS. Role of SSRIs in the management of migraine. Headache Quarterly 1998;9:265-6.
22. Mallinckrodt CH, Goldstein DJ, Detke MJ, et al. Duloxetine: a new treatment for the emotional and physical symptoms of depression. Primary Care Companion J Clin Psychiatry 2003;5(1):19-28.
23. Lynch ME. Antidepressants as analgesics: a review of randomized control trials. J Psychiatry Neurosci 2001;26-36.
24. Swerdlow M. Anticonvulsant drugs and chronic pain. Clin Neuropharmacol 1984;7(1):51-82.
Ms. A, age 44, fell from a 3-foot stool while reaching for a high kitchen shelf and suffered severe neck flexion. Her initial pain persisted for weeks and then months, resulting in chronic neck pain aggravated by movement.
Over the past year, her doctor has prescribed numerous analgesics and muscle relaxants, including tramadol, hydrocodone, oxycodone, tizanidine, and nonsteroidal anti-inflammatory drugs (NSAIDs). Treatments at a pain clinic have included triggerpoint injections, cervical epidural corticosteroid injection, left-sided cervical medial branch blocks, transcutaneous electrical nerve stimulation, and physical therapy. None provided sustained relief.
During a pain clinic visit, Ms. A wept and said she was tired of living with pain. She acknowledged depression and agreed to psychiatric consultation.
As in Ms. A’s case, physicians often refer patients with chronic pain and affective symptoms for psychiatric evaluation. These patients are often fearful, angry, and suspicious of any suggestion that their physical discomfort has a psychiatric component. They typically believe their pain had a clear onset and therefore should have an end point. Many have experienced unproductive specialty evaluations and failed treatments.
To help you overcome these obstacles when treating patients with chronic pain and depression, we discuss:
- strategies to gain patients’ trust and build a therapeutic alliance
- how to assess their pain, depression, and suicide risk
- the role of psychotherapy in treating chronic pain
- and evidence for choosing effective, nonaddicting medications.
Psychiatric evaluation
Depression and pain are linked psychologically and biochemically, sharing neurotransmitters involved in both nociceptive pathways and mood, especially serotonin and norepinephrine.1,2 One-third to one-half of patients with chronic pain report comorbid depression,3 and more than one-half of depressed patients presenting to primary care physicians report only somatic symptoms—various pain complaints among the most common.4,5
Primary care doctors tend to refer chronic pain and depression cases to psychiatrists when:
- patients are preoccupied with medication, have not followed treatment recommendations, or do not respond to treatment as expected
- extensive medical evaluations reveal few or equivocal findings
- somatic complaints are vague and diffuse, or there is marked disparity between pain complaints/disability and objective findings.6,7
Assessing pain. In the initial assessment, validate the patient’s pain experience by asking about the location, quality, and severity of pain. The visual analogue scale (VAS) is commonly used to measure pain severity. The patient marks a spot on a line from “no pain” to “worst possible pain,” or—on a numbered VAS—from 0 (no pain) to 10 (extreme pain). The least and most severe pain over the preceding month can be ranked as baseline values.8
Be sensitive to the patient’s fear that you will attribute the pain to psychosocial issues or imply that “the pain is in your head.” Emphasize that you intend to evaluate the “whole person,” not just the part that hurts. Focus on how the pain affects the patient’s lifestyle—rather than its cause—and explore medication use patterns.
Assessing depression. The word “depression” is emotionally charged for chronic pain patients, who view affective symptoms—if they acknowledge them at all—as secondary to pain. They may strongly resist treatment for anything but pain. One way to defuse this defensiveness is to avoid attributing the pain to stress or depression.
Begin by assessing vegetative symptoms, which overlap in chronic pain and depression. The Beck Depression Inventory-II (Beck-II) may be a useful screening tool in a busy practice; the short form (13 questions) takes about 5 minutes to complete.9
Explore cognitive and behavioral symptoms such as concentration, pleasure and interest level, activity, and self-esteem. Review the chronology of pain onset, mood changes, and stressors (proximate, remote, and cumulative).
Seek clues to endogenous factors by asking about past affective episodes, response to antidepressants, and family history of psychopathology. Substances that may induce depression include reserpine, interferon, and antiparkinsonian agents. Screen for potential organic mood disorders, such as depression secondary to hypothyroidism, corticosteroid use, Parkinson’s disease, lupus, HIV infection, or cerebrovascular disease. Where appropriate, obtain collateral information from family or friends.
Assessing suicide risk. Chronic pain patients may be at greater risk of suicide than the general population. Besides pain, other risk factors for suicide—such as major depression, anxiety disorders, alcohol/substance abuse, sleep disturbances, male gender, diminished social support, and recent loss—are common among these patients.10,11
Screen chronic pain patients with suicidal ideation for these risk factors. Interventions include:
- aggressively treat associated depression, anxiety, or insomnia
- elicit support from family or other caregivers
- pay close attention to talk about suicide
- hospitalize when necessary
- and, of course, treat pain.
Case continued: No stranger to depression
Ms. A’s psychiatric assessments revealed a pain severity ranking of 9 on a 1-to-10 scale, frequent crying, hopelessness, disrupted sleep, low energy, limited ability to concentrate, and fleeting suicidal thoughts. Her history included counseling during her first marriage and severe depression after separation from her second husband 3 years ago. An 8-week trial of fluoxetine, 20 mg/d, did not improve her depression then.
On examination, she displayed obvious pain behavior, constantly shifting her neck position and moving about the room. Her affect was tearful and her mood depressed. She was taking the NSAID celecoxib, 100 mg bid, and the skeletal muscle relaxant tizanidine, 4 mg tid. She was no longer using opioids and had no history of alcohol or illicit drug abuse.
Based on this assessment, the psychiatrist diagnosed Ms. A as having pain disorder with medical and psychological features, including symptom amplification and depression.
Table 1
4 treatment goals for patients with chronic pain and depression
|
Educating the patient
As part of your assessment, explain the reciprocal effects of depression and pain. Acknowledge that:
- chronic pain is different from acute pain, although the patient’s pain experience is the same
- treatment often becomes part of the problem in chronic pain.
Doctors tend to apply acute pain treatments chronically, risking long-term effects of polypharmacy to achieve short-term relief. Depressed patients may be more likely than nondepressed patients to receive opioids for chronic pain,12 and opioids and benzodiazepines may have depressive effects, as reflected by DSM-IV-TR’s inclusion of criteria for “opioid-induced mood disorder” and “sedative-, hypnotic-, or anxiolytic-induced mood disorder.”
To reduce patients’ resistance to antidepressants, reiterate any history of cumulative stressors and affective episodes unrelated to pain. Try using an analogy, such as “stress and pain are like waves on a rock” that eventually damage mood and coping mechanisms, or depression complicating pain is like having “too much on one’s plate.”
Finally, help patients understand that chronic pain is managed, not cured. Encourage them to set treatment goals beyond reducing pain (Table 1) and to make the transition from “patient with pain” to “client managing pain.”
Table 2
Dosing antidepressants and anticonvulsants
for chronic pain and depression
| Drug | Starting (mg/d) | Target (mg/d) | Administration tips |
|---|---|---|---|
| TCAs | Check serum levels for dosages ≥150 mg/d (nortriptyline 100 mg/d) to assess rapid metabolism, adherence, or toxic levels | ||
| Amitriptyline | 10 to 25 | 75 to 300 | |
| Clomipramine | 10 to 25 | 75 to 250 | |
| Desipramine | 10 to 25 | 75 to 200 | |
| Doxepin | 10 to 25 | 75 to 300 | |
| Imipramine | 10 to 25 | 75 to 300 | |
| Nortriptyline | 10 to 25 | 40 to 200 | |
| SNRI | |||
| Venlafaxine | 37.5 to 75 | 75 to 375 | Use XR form to minimize side effects and for once-daily dosing |
| SSRIs | |||
| Citalopram | 10 to 20 | 40 to 60 | |
| Fluoxetine | 10 to 20 | 20 to 80 | May increase carbamazepine, TCA blood levels and inhibit efficacy of codeine, dihydrocodeine, and hydrocodone |
| Paroxetine | 10 to 20 | 20 to 60 | Same as fluoxetine |
| Anticonvulsants | |||
| Carbamazepine | 200 | 800 to 1,200 | Check blood levels; may increase clomipramine levels, reduce acetaminophen, contraceptive levels |
| Clonazepam | 0.5 | 1 to 2 | Habituating potential with chronic use |
| Gabapentin | 300 to 900 | 3,600 to 4,800 | Blood monitoring not necessary |
| Valproate | 250 | 750 to 2,500 (maximum dosage 60 mg/kg/d) | Check blood levels (trough plasma level 50 to 100 μg/mL) |
| TCA: tricyclic antidepressant | |||
| SNRI: serotonin-norepinephrine reuptake inhibitor | |||
| SSRI: selective serotonin reuptake inhibitor | |||
Prescribing principles
Before adding any new pain medications, consider reducing dosages or discontinuing opioids or benzodiazepines and other substances the patient may be taking. Opioid use is associated with risks of dependence, addiction, and side effects including somnolence, cognitive impairment, and reduced activity that amplify depressive symptoms.
Benzodiazepines can generally be tapered by 10% per day, although you may need to extend the final taper over 3 to 4 days or longer, depending upon chronicity of use. Opioids may be tapered by 20% over 5 to 7 days. Breakthrough doses may be needed for marked withdrawal symptoms. Converting to longer half-life agents—such as clonazepam for benzodiazepines or methadone for opioids—often aids tapering, although other agents and strategies exist.13
To gauge patient attempts at self-medication, monitor use of alcohol or illicit drugs with urine screening. For patients with a substantial history of substance abuse or positive toxicology screens, monitor randomly every 2 to 4 weeks.
On the other hand, undertreated pain also may impair mood and function.1 If pain and mood improve and problematic drug-related behaviors resolve with increased opioid analgesia, consider maintaining opioids with regular re-evaluation of mood, coping, and medication adherence.11 Transfer from immediate-release to controlled-release opioids to reduce dosing frequency, clockwatching, and the likelihood of inter-dose pain escalation. In general, maintain and optimize the dosage of nonaddictive analgesics such as NSAIDs, anticonvulsants, or antidepressants.
Case continued: Switching medication
The psychiatrist started Ms. A on nortriptyline, 25 mg at bedtime, to be increased after 3 nights to 50 mg at bedtime. Tizanidine, which had been ineffective, was discontinued to reduce the risk of xerostomia and oversedation in combination with nortriptyline. If tolerated, nortriptyline was to be further increased by 25 mg every 3 days to an initial target dosage of 100 mg at bedtime. The psychiatrist explained to Ms. A that it might take 4 to 6 weeks to gauge the medication’s efficacy.
Psychoeducation addressed the importance of stress reduction, prioritizing commitments, and setting limits on other people’s expectations. The door was left open to future psychotherapeutic exploration of past cumulative stressors.
Because antidepressants may provide an analgesic effect,6,14 they are often used to treat affective symptoms in chronic pain. Headache and neuralgia tend to respond to antidepressants more robustly than do arthritis and low-back pain. Although some patients respond to low-dose antidepressants, a definitive trial requires full doses for 6 to 8 weeks (Table 2).
Matching a patient’s symptoms with medication side effects is useful when choosing antidepressants (Table 3). So-called “adverse” effects may have a corresponding benefit, depending on the clinical presentation. For example, a moreactivating antidepressant—such as the selective serotonin reuptake inhibitor (SSRI) fluoxetine—may help a patient with fatigue, whereas a moresedating agent—such as a tricyclic antidepressant (TCA) or mirtazapine—may improve sleep for a patient with insomnia.
Psychosocial therapies such as cognitive-behavioral therapy (CBT) or relaxation training (Table 4) may help patients with chronic pain to:
- process covert emotions such as fear and anger as well as guilt, loss, and disability
- reduce somatic preoccupation that is aggravating the pain
- adhere to treatment.
Evidence strongly supports using relaxation techniques to reduce chronic pain in many medical conditions and hypnosis to ameliorate cancer pain. CBT and biofeedback appear moderately effective in relieving chronic pain.15 CBT is significantly more effective than waiting list control conditions for relieving chronic nonheadache pain in measures of pain experience, mood/affect, cognitive coping and appraisal, pain behavior and activity level, and social role functioning.16
Pain and opioid medications can impair concentration and affective processing, so initial psychotherapy may need to be supportive while you provide other treatments and simplify medication regimens. Eventually the patient may be ready to address underlying issues that may be contributing to the pain syndrome, such as a history of abuse. However, it is important to address this potentially destabilizing subject only after carefully gauging a patient’s defenses and readiness.
Case continued: A bump in the road
The psychiatrist saw Ms. A 18 months later. Interim history revealed that her pain and mood improved on nortriptyline, 100 mg at bedtime. When she stopped taking nortriptyline 5 months earlier, her neck pain increased and she experienced a “deep blue mood.” Her physician restarted the nortriptyline.
At follow up, Ms. A reported no depressive symptoms and very little neck pain. The psychiatrist discussed with her depression’s relapse rate and the importance of continuing antidepressant therapy. As Ms. A was feeling much better and functioning normally, the psychiatrist decided additional psychotherapeutic intervention was not necessary.
Antidepressant options
TCAs provide analgesia via descending regulatory pathways by inhibiting serotonin and norepinephrine reuptake.17 When using TCAs for chronic pain, start with 10 to 25 mg at bedtime and increase by 10 to 25 mg every 3 to 7 days as tolerated. Increase incrementally until the pain responds or to the full antidepressant dosage (Table 2). Drug levels (when available) can help you provide an appropriate trial and monitor the patient’s adherence.
If the pain does not respond after 6 to 8 weeks, consider switching to another dual-action agent such as venlafaxine or to an SSRI.
SNRIs. Venlafaxine is a serotonin and norepineph-rine reuptake inhibitor (SNRI) with less-troublesome side effects than TCAs. It is structurally similar to tramadol18 and has combined serotonin and norepinephrine inhibition at dosages >75 mg/d. Although venlafaxine is not indicated for chronic pain, some studies have suggested possible benefits, including long-term analgesia, reduced polyneuropathic pain, and migraine prophylaxis.19-21 Venlafaxine may be a reasonable first or second choice for treating depression in patients with chronic pain, especially headache.14
Duloxetine—another SNRI—awaits FDA approval. Some studies have suggested that duloxetine improves painful physical symptoms as well as mood and functioning in major depression.22
SSRIs may be effective for certain types of pain, but the evidence is conflicting. Results of 41controlled trials support TCAs’ analgesic efficacy for neuropathic pain, headache, and central and post-stroke pain, whereas SSRIs’ analgesic efficacy varies from study to study. Comparisons of TCAs and SSRIs as analgesics uniformly show TCAs to be more effective, with the SSRIs often showing no analgesic effect.
Of three controlled trials of SSRIs for diabetic neuropathy, one showed fluoxetine similar to placebo, and two smaller studies showed paroxetine and citalopram more effective than placebo. Fluoxetine has shown analgesic effect for fibromyalgia in one study, but no effect in another. Citalopram showed no analgesic effect for fibromyalgia in another study.23
A prospective, double-blind study comparing fluoxetine, sertraline, paroxetine, and venlafaxine for migraines reported moderate to significant improvement in less than one-half of SSRI-treated patients vs two-thirds of venlafaxine-treated patients.21 SSRIs are no longer recognized by the International Headache Society as primary preventative medications for migraine.
Fluoxetine may help chronic daily headache, and paroxetine and citalopram may be useful for diabetic neuropathy. However, one cannot generalize that all SSRIs are similarly effective as analgesics.14
SSRIs have fewer side effects than TCAs and are less dangerous in overdose. In general, however, SSRIs are a second-line treatment for pain, to be used when dual-action agents pose disadvantageous side effects (Table 3) or have been poorly tolerated or ineffective.
Table 3
Antidepressant side effects:
Limitations and potential benefits in chronic pain
| Side effects/agents | Problems | Conditions potentially benefited | Possible alternatives |
|---|---|---|---|
| Anticholinergic TCAs | Xerostomia, constipation, urinary slowing (esp. when combined with opioids) | Diarrhea-predominant irritable bowel syndrome | SSRIs, nefazodone, venlafaxine |
| Sedation TCAs, mirtazapine, nefazodone, trazodone | Excessive sedation, cognitive impairment, driving risk (esp. when combined with opioids, benzodiazepines) | Pain with insomnia | SSRIs, venlafaxine, bupropion |
| Insomnia SSRIs, venlafaxine | Pain with pre-existing insomnia; equivocal analgesic effects | Excess sedation related to depression, polypharmacy for pain | TCAs, mirtazapine, trazodone, nefazodone |
| Orthostasis TCAs (esp. with methadone), nefazodone | Falls, especially in elderly patients | —— | Nortriptyline, SSRIs bupropion, venlafaxine |
| Weight gain TCAs, mirtazapine | Pain patients are often sedentary, get limited exercise | Pain and depression with weight loss | Bupropion, fluoxetine |
| Hypertension Bupropion, venlafaxine | Pre-existing hypertension | ? Hypotensive state | Citalopram (hypertensive side effects infrequent) |
| Cardiac TCAs | ECG abnormalities, conduction delays, arrhythmias aggravate pre-existing cardiac abnormalities; avoid if recent MI | ——— | SSRIs, bupropion |
| Overdose lethality TCAs | Prominent suicidal ideation | —— | SSRIs, venlafaxine |
| Seizures Esp. maprotiline, clomipramine, bupropion | Lower seizure threshold, aggravation of seizure disorders | ——- | SSRIs |
| Sexual dysfunction SSRIs | Pre-existing sexual dysfunction secondary to pain, medications, stress; equivocal analgesic effects | ——- | Bupropion, nefazodone, mirtazapine |
Table 4
How psychosocial therapies can help treat chronic pain and depression
| Therapies | Purpose/benefits |
|---|---|
| Behavioral therapy | Increase activity and learn to balance activity with limitations Reduce pain behaviors and analgesic use Decrease dependency and secondary gain |
| Cognitive-behavioral therapy | Identify automatic thoughts Challenge negative cognitions, catastrophizing Substitute and rehearse positive thoughts, capabilities Transition from patient role to self-care |
| Couples’ therapy | Assist adaptation to role changes Diminish spousal solicitousness or excessive caretaking Increase communication |
| Biofeedback, relaxation, imagery | Adjunctive role in pain management Reduce tension, comorbid anxiety |
| Hypnosis | Dissociate awareness of pain Substitute, displace, reinterpret pain sensations |
| Vocational rehabilitation | Increase activity, ability to distract Regain sense of control, identity, and productivity Increase socialization |
| Pain management program | Multiple treatment effects Useful for complex pain with affective states |
Monoamine oxidase inhibitors (MAOIs) may have some efficacy for neuropathy and headache, but the need for a tyramine-free diet and potential for drug-drug interactions limit their usefulness. Co-administering an MAOI and meperidine is always contraindicated, as this combination can produce fever, delirium, seizures, circulatory collapse, and death. Similarly, avoid using an MAOI with any other antidepressant.
Others. Evidence is very limited on using other antidepressants such as trazodone, nefazodone, bupropion, and mirtazapine in chronic pain:
- Trazodone may help pediatric migraine, but it is not a consistent analgesic and may not be well-tolerated.
- Case reports suggest bupropion may help with headaches and chronic low-back pain.14
- Mirtazapine and trazodone may be useful adjuncts for treating insomnia in depressed patients with chronic pain.
Other options
Anticonvulsants appear useful for neuropathic pain and are appropriate for chronic pain patients who cannot tolerate TCAs.24 Like TCAs, anticonvulsants are not addictive. Unlike TCAs, anticonvulsants may help stabilize other affective illnesses that may coexist with chronic pain, including bipolar disorder, schizoaffective disorder, and impulsivity/aggression related to dementia or personality disorder.6 If the starting dosage is not effective within 1 week, increase gradually every 2 to 3 days to target dosages comparable to those for anticonvulsant efficacy.
Carbamazepine and gabapentin are recommended first-line medications for neuropathy. Carbamazepine is indicated for treating trigeminal neuralgia, although its cytochrome P-450 3A3/4 isoenzyme induction may reduce serum levels of acetaminophen, opioids, and oral contraceptives. Gabapentin, although not clearly beneficial for bipolar disorder, has anxiolytic effects and a benign side-effect profile, which may help patients with chronic pain.
Valproate can help prevent migraines. Clonazepam can help reduce anxiety and restless legs syndrome but may be habituating. Anticonvulsants’ common adverse effects include sedation, GI upset, dizziness, and fatigue.
Lithium has known efficacy for mood stabilization in bipolar disorder and can ameliorate cluster headaches.
Antipsychotics. Evidence is sparse on whether antipsychotics have analgesic activity. Their side effects generally limit their usefulness to treating pain in patients with psychosis or delirium.6
Stimulants such as dextroamphetamine and methylphenidate can be helpful adjuncts for treating depression, especially for medical inpatients who require a rapid therapeutic response. Stimulants may reduce fatigue or excessive sedation and improve concentration in patients receiving opioids for chronic pain. They also may have analgesic effects when combined with opioids. Potential adverse effects include appetite suppression, anxiety or agitation, confusion, tics, and addiction.6
Precautions. The muscle relaxant carisoprodol is associated with potential dependence and withdrawal. Cyclobenzaprine, another muscle relaxant, has a TCA-like structure and can be lethal in overdose. Baclofen can be useful for chronic pain related to spasticity, although psychotic depression and mania have been reported with abrupt withdrawal.6
Related resources
- American Academy of Pain Medicine. www.painmed.org/
- American Academy of Pain Management. www.aapainmanage.org
- Pain.com: continuing medical education for anesthesiology professionals. www.pain.com/index.cfm
- Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI). Pain 1985;13:345-56
Drug brand names
- Amitriptyline • Elavil
- Baclofen • Lioresal
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Carisoprodol • Soma
- Celecoxib • Celebrex
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Cyclobenzaprine • Flexeril
- Desipramine • Norpramin
- Dihydrocodeine • Synalgos-DC
- Doxepin • Sinequan
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Hydrocodone • Vicodin, Lortab
- Imipramine • Tofranil
- Lithium • Eskalith CR, Lithobid
- Maprotiline • Ludiomil
- Meperidine • Demerol
- Methadone • Dolophine
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Nortriptyline • Pamelor
- Oxycodone • OxyContin
- Paroxetine • Paxil
- Sertraline • Zoloft
- Tizanidine • Zanaflex
- Tramadol • Ultram
- Trazodone • Desyrel
- Valproate • Depakote
- Venlafaxine • Effexor, Effexor XR
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Ms. A, age 44, fell from a 3-foot stool while reaching for a high kitchen shelf and suffered severe neck flexion. Her initial pain persisted for weeks and then months, resulting in chronic neck pain aggravated by movement.
Over the past year, her doctor has prescribed numerous analgesics and muscle relaxants, including tramadol, hydrocodone, oxycodone, tizanidine, and nonsteroidal anti-inflammatory drugs (NSAIDs). Treatments at a pain clinic have included triggerpoint injections, cervical epidural corticosteroid injection, left-sided cervical medial branch blocks, transcutaneous electrical nerve stimulation, and physical therapy. None provided sustained relief.
During a pain clinic visit, Ms. A wept and said she was tired of living with pain. She acknowledged depression and agreed to psychiatric consultation.
As in Ms. A’s case, physicians often refer patients with chronic pain and affective symptoms for psychiatric evaluation. These patients are often fearful, angry, and suspicious of any suggestion that their physical discomfort has a psychiatric component. They typically believe their pain had a clear onset and therefore should have an end point. Many have experienced unproductive specialty evaluations and failed treatments.
To help you overcome these obstacles when treating patients with chronic pain and depression, we discuss:
- strategies to gain patients’ trust and build a therapeutic alliance
- how to assess their pain, depression, and suicide risk
- the role of psychotherapy in treating chronic pain
- and evidence for choosing effective, nonaddicting medications.
Psychiatric evaluation
Depression and pain are linked psychologically and biochemically, sharing neurotransmitters involved in both nociceptive pathways and mood, especially serotonin and norepinephrine.1,2 One-third to one-half of patients with chronic pain report comorbid depression,3 and more than one-half of depressed patients presenting to primary care physicians report only somatic symptoms—various pain complaints among the most common.4,5
Primary care doctors tend to refer chronic pain and depression cases to psychiatrists when:
- patients are preoccupied with medication, have not followed treatment recommendations, or do not respond to treatment as expected
- extensive medical evaluations reveal few or equivocal findings
- somatic complaints are vague and diffuse, or there is marked disparity between pain complaints/disability and objective findings.6,7
Assessing pain. In the initial assessment, validate the patient’s pain experience by asking about the location, quality, and severity of pain. The visual analogue scale (VAS) is commonly used to measure pain severity. The patient marks a spot on a line from “no pain” to “worst possible pain,” or—on a numbered VAS—from 0 (no pain) to 10 (extreme pain). The least and most severe pain over the preceding month can be ranked as baseline values.8
Be sensitive to the patient’s fear that you will attribute the pain to psychosocial issues or imply that “the pain is in your head.” Emphasize that you intend to evaluate the “whole person,” not just the part that hurts. Focus on how the pain affects the patient’s lifestyle—rather than its cause—and explore medication use patterns.
Assessing depression. The word “depression” is emotionally charged for chronic pain patients, who view affective symptoms—if they acknowledge them at all—as secondary to pain. They may strongly resist treatment for anything but pain. One way to defuse this defensiveness is to avoid attributing the pain to stress or depression.
Begin by assessing vegetative symptoms, which overlap in chronic pain and depression. The Beck Depression Inventory-II (Beck-II) may be a useful screening tool in a busy practice; the short form (13 questions) takes about 5 minutes to complete.9
Explore cognitive and behavioral symptoms such as concentration, pleasure and interest level, activity, and self-esteem. Review the chronology of pain onset, mood changes, and stressors (proximate, remote, and cumulative).
Seek clues to endogenous factors by asking about past affective episodes, response to antidepressants, and family history of psychopathology. Substances that may induce depression include reserpine, interferon, and antiparkinsonian agents. Screen for potential organic mood disorders, such as depression secondary to hypothyroidism, corticosteroid use, Parkinson’s disease, lupus, HIV infection, or cerebrovascular disease. Where appropriate, obtain collateral information from family or friends.
Assessing suicide risk. Chronic pain patients may be at greater risk of suicide than the general population. Besides pain, other risk factors for suicide—such as major depression, anxiety disorders, alcohol/substance abuse, sleep disturbances, male gender, diminished social support, and recent loss—are common among these patients.10,11
Screen chronic pain patients with suicidal ideation for these risk factors. Interventions include:
- aggressively treat associated depression, anxiety, or insomnia
- elicit support from family or other caregivers
- pay close attention to talk about suicide
- hospitalize when necessary
- and, of course, treat pain.
Case continued: No stranger to depression
Ms. A’s psychiatric assessments revealed a pain severity ranking of 9 on a 1-to-10 scale, frequent crying, hopelessness, disrupted sleep, low energy, limited ability to concentrate, and fleeting suicidal thoughts. Her history included counseling during her first marriage and severe depression after separation from her second husband 3 years ago. An 8-week trial of fluoxetine, 20 mg/d, did not improve her depression then.
On examination, she displayed obvious pain behavior, constantly shifting her neck position and moving about the room. Her affect was tearful and her mood depressed. She was taking the NSAID celecoxib, 100 mg bid, and the skeletal muscle relaxant tizanidine, 4 mg tid. She was no longer using opioids and had no history of alcohol or illicit drug abuse.
Based on this assessment, the psychiatrist diagnosed Ms. A as having pain disorder with medical and psychological features, including symptom amplification and depression.
Table 1
4 treatment goals for patients with chronic pain and depression
|
Educating the patient
As part of your assessment, explain the reciprocal effects of depression and pain. Acknowledge that:
- chronic pain is different from acute pain, although the patient’s pain experience is the same
- treatment often becomes part of the problem in chronic pain.
Doctors tend to apply acute pain treatments chronically, risking long-term effects of polypharmacy to achieve short-term relief. Depressed patients may be more likely than nondepressed patients to receive opioids for chronic pain,12 and opioids and benzodiazepines may have depressive effects, as reflected by DSM-IV-TR’s inclusion of criteria for “opioid-induced mood disorder” and “sedative-, hypnotic-, or anxiolytic-induced mood disorder.”
To reduce patients’ resistance to antidepressants, reiterate any history of cumulative stressors and affective episodes unrelated to pain. Try using an analogy, such as “stress and pain are like waves on a rock” that eventually damage mood and coping mechanisms, or depression complicating pain is like having “too much on one’s plate.”
Finally, help patients understand that chronic pain is managed, not cured. Encourage them to set treatment goals beyond reducing pain (Table 1) and to make the transition from “patient with pain” to “client managing pain.”
Table 2
Dosing antidepressants and anticonvulsants
for chronic pain and depression
| Drug | Starting (mg/d) | Target (mg/d) | Administration tips |
|---|---|---|---|
| TCAs | Check serum levels for dosages ≥150 mg/d (nortriptyline 100 mg/d) to assess rapid metabolism, adherence, or toxic levels | ||
| Amitriptyline | 10 to 25 | 75 to 300 | |
| Clomipramine | 10 to 25 | 75 to 250 | |
| Desipramine | 10 to 25 | 75 to 200 | |
| Doxepin | 10 to 25 | 75 to 300 | |
| Imipramine | 10 to 25 | 75 to 300 | |
| Nortriptyline | 10 to 25 | 40 to 200 | |
| SNRI | |||
| Venlafaxine | 37.5 to 75 | 75 to 375 | Use XR form to minimize side effects and for once-daily dosing |
| SSRIs | |||
| Citalopram | 10 to 20 | 40 to 60 | |
| Fluoxetine | 10 to 20 | 20 to 80 | May increase carbamazepine, TCA blood levels and inhibit efficacy of codeine, dihydrocodeine, and hydrocodone |
| Paroxetine | 10 to 20 | 20 to 60 | Same as fluoxetine |
| Anticonvulsants | |||
| Carbamazepine | 200 | 800 to 1,200 | Check blood levels; may increase clomipramine levels, reduce acetaminophen, contraceptive levels |
| Clonazepam | 0.5 | 1 to 2 | Habituating potential with chronic use |
| Gabapentin | 300 to 900 | 3,600 to 4,800 | Blood monitoring not necessary |
| Valproate | 250 | 750 to 2,500 (maximum dosage 60 mg/kg/d) | Check blood levels (trough plasma level 50 to 100 μg/mL) |
| TCA: tricyclic antidepressant | |||
| SNRI: serotonin-norepinephrine reuptake inhibitor | |||
| SSRI: selective serotonin reuptake inhibitor | |||
Prescribing principles
Before adding any new pain medications, consider reducing dosages or discontinuing opioids or benzodiazepines and other substances the patient may be taking. Opioid use is associated with risks of dependence, addiction, and side effects including somnolence, cognitive impairment, and reduced activity that amplify depressive symptoms.
Benzodiazepines can generally be tapered by 10% per day, although you may need to extend the final taper over 3 to 4 days or longer, depending upon chronicity of use. Opioids may be tapered by 20% over 5 to 7 days. Breakthrough doses may be needed for marked withdrawal symptoms. Converting to longer half-life agents—such as clonazepam for benzodiazepines or methadone for opioids—often aids tapering, although other agents and strategies exist.13
To gauge patient attempts at self-medication, monitor use of alcohol or illicit drugs with urine screening. For patients with a substantial history of substance abuse or positive toxicology screens, monitor randomly every 2 to 4 weeks.
On the other hand, undertreated pain also may impair mood and function.1 If pain and mood improve and problematic drug-related behaviors resolve with increased opioid analgesia, consider maintaining opioids with regular re-evaluation of mood, coping, and medication adherence.11 Transfer from immediate-release to controlled-release opioids to reduce dosing frequency, clockwatching, and the likelihood of inter-dose pain escalation. In general, maintain and optimize the dosage of nonaddictive analgesics such as NSAIDs, anticonvulsants, or antidepressants.
Case continued: Switching medication
The psychiatrist started Ms. A on nortriptyline, 25 mg at bedtime, to be increased after 3 nights to 50 mg at bedtime. Tizanidine, which had been ineffective, was discontinued to reduce the risk of xerostomia and oversedation in combination with nortriptyline. If tolerated, nortriptyline was to be further increased by 25 mg every 3 days to an initial target dosage of 100 mg at bedtime. The psychiatrist explained to Ms. A that it might take 4 to 6 weeks to gauge the medication’s efficacy.
Psychoeducation addressed the importance of stress reduction, prioritizing commitments, and setting limits on other people’s expectations. The door was left open to future psychotherapeutic exploration of past cumulative stressors.
Because antidepressants may provide an analgesic effect,6,14 they are often used to treat affective symptoms in chronic pain. Headache and neuralgia tend to respond to antidepressants more robustly than do arthritis and low-back pain. Although some patients respond to low-dose antidepressants, a definitive trial requires full doses for 6 to 8 weeks (Table 2).
Matching a patient’s symptoms with medication side effects is useful when choosing antidepressants (Table 3). So-called “adverse” effects may have a corresponding benefit, depending on the clinical presentation. For example, a moreactivating antidepressant—such as the selective serotonin reuptake inhibitor (SSRI) fluoxetine—may help a patient with fatigue, whereas a moresedating agent—such as a tricyclic antidepressant (TCA) or mirtazapine—may improve sleep for a patient with insomnia.
Psychosocial therapies such as cognitive-behavioral therapy (CBT) or relaxation training (Table 4) may help patients with chronic pain to:
- process covert emotions such as fear and anger as well as guilt, loss, and disability
- reduce somatic preoccupation that is aggravating the pain
- adhere to treatment.
Evidence strongly supports using relaxation techniques to reduce chronic pain in many medical conditions and hypnosis to ameliorate cancer pain. CBT and biofeedback appear moderately effective in relieving chronic pain.15 CBT is significantly more effective than waiting list control conditions for relieving chronic nonheadache pain in measures of pain experience, mood/affect, cognitive coping and appraisal, pain behavior and activity level, and social role functioning.16
Pain and opioid medications can impair concentration and affective processing, so initial psychotherapy may need to be supportive while you provide other treatments and simplify medication regimens. Eventually the patient may be ready to address underlying issues that may be contributing to the pain syndrome, such as a history of abuse. However, it is important to address this potentially destabilizing subject only after carefully gauging a patient’s defenses and readiness.
Case continued: A bump in the road
The psychiatrist saw Ms. A 18 months later. Interim history revealed that her pain and mood improved on nortriptyline, 100 mg at bedtime. When she stopped taking nortriptyline 5 months earlier, her neck pain increased and she experienced a “deep blue mood.” Her physician restarted the nortriptyline.
At follow up, Ms. A reported no depressive symptoms and very little neck pain. The psychiatrist discussed with her depression’s relapse rate and the importance of continuing antidepressant therapy. As Ms. A was feeling much better and functioning normally, the psychiatrist decided additional psychotherapeutic intervention was not necessary.
Antidepressant options
TCAs provide analgesia via descending regulatory pathways by inhibiting serotonin and norepinephrine reuptake.17 When using TCAs for chronic pain, start with 10 to 25 mg at bedtime and increase by 10 to 25 mg every 3 to 7 days as tolerated. Increase incrementally until the pain responds or to the full antidepressant dosage (Table 2). Drug levels (when available) can help you provide an appropriate trial and monitor the patient’s adherence.
If the pain does not respond after 6 to 8 weeks, consider switching to another dual-action agent such as venlafaxine or to an SSRI.
SNRIs. Venlafaxine is a serotonin and norepineph-rine reuptake inhibitor (SNRI) with less-troublesome side effects than TCAs. It is structurally similar to tramadol18 and has combined serotonin and norepinephrine inhibition at dosages >75 mg/d. Although venlafaxine is not indicated for chronic pain, some studies have suggested possible benefits, including long-term analgesia, reduced polyneuropathic pain, and migraine prophylaxis.19-21 Venlafaxine may be a reasonable first or second choice for treating depression in patients with chronic pain, especially headache.14
Duloxetine—another SNRI—awaits FDA approval. Some studies have suggested that duloxetine improves painful physical symptoms as well as mood and functioning in major depression.22
SSRIs may be effective for certain types of pain, but the evidence is conflicting. Results of 41controlled trials support TCAs’ analgesic efficacy for neuropathic pain, headache, and central and post-stroke pain, whereas SSRIs’ analgesic efficacy varies from study to study. Comparisons of TCAs and SSRIs as analgesics uniformly show TCAs to be more effective, with the SSRIs often showing no analgesic effect.
Of three controlled trials of SSRIs for diabetic neuropathy, one showed fluoxetine similar to placebo, and two smaller studies showed paroxetine and citalopram more effective than placebo. Fluoxetine has shown analgesic effect for fibromyalgia in one study, but no effect in another. Citalopram showed no analgesic effect for fibromyalgia in another study.23
A prospective, double-blind study comparing fluoxetine, sertraline, paroxetine, and venlafaxine for migraines reported moderate to significant improvement in less than one-half of SSRI-treated patients vs two-thirds of venlafaxine-treated patients.21 SSRIs are no longer recognized by the International Headache Society as primary preventative medications for migraine.
Fluoxetine may help chronic daily headache, and paroxetine and citalopram may be useful for diabetic neuropathy. However, one cannot generalize that all SSRIs are similarly effective as analgesics.14
SSRIs have fewer side effects than TCAs and are less dangerous in overdose. In general, however, SSRIs are a second-line treatment for pain, to be used when dual-action agents pose disadvantageous side effects (Table 3) or have been poorly tolerated or ineffective.
Table 3
Antidepressant side effects:
Limitations and potential benefits in chronic pain
| Side effects/agents | Problems | Conditions potentially benefited | Possible alternatives |
|---|---|---|---|
| Anticholinergic TCAs | Xerostomia, constipation, urinary slowing (esp. when combined with opioids) | Diarrhea-predominant irritable bowel syndrome | SSRIs, nefazodone, venlafaxine |
| Sedation TCAs, mirtazapine, nefazodone, trazodone | Excessive sedation, cognitive impairment, driving risk (esp. when combined with opioids, benzodiazepines) | Pain with insomnia | SSRIs, venlafaxine, bupropion |
| Insomnia SSRIs, venlafaxine | Pain with pre-existing insomnia; equivocal analgesic effects | Excess sedation related to depression, polypharmacy for pain | TCAs, mirtazapine, trazodone, nefazodone |
| Orthostasis TCAs (esp. with methadone), nefazodone | Falls, especially in elderly patients | —— | Nortriptyline, SSRIs bupropion, venlafaxine |
| Weight gain TCAs, mirtazapine | Pain patients are often sedentary, get limited exercise | Pain and depression with weight loss | Bupropion, fluoxetine |
| Hypertension Bupropion, venlafaxine | Pre-existing hypertension | ? Hypotensive state | Citalopram (hypertensive side effects infrequent) |
| Cardiac TCAs | ECG abnormalities, conduction delays, arrhythmias aggravate pre-existing cardiac abnormalities; avoid if recent MI | ——— | SSRIs, bupropion |
| Overdose lethality TCAs | Prominent suicidal ideation | —— | SSRIs, venlafaxine |
| Seizures Esp. maprotiline, clomipramine, bupropion | Lower seizure threshold, aggravation of seizure disorders | ——- | SSRIs |
| Sexual dysfunction SSRIs | Pre-existing sexual dysfunction secondary to pain, medications, stress; equivocal analgesic effects | ——- | Bupropion, nefazodone, mirtazapine |
Table 4
How psychosocial therapies can help treat chronic pain and depression
| Therapies | Purpose/benefits |
|---|---|
| Behavioral therapy | Increase activity and learn to balance activity with limitations Reduce pain behaviors and analgesic use Decrease dependency and secondary gain |
| Cognitive-behavioral therapy | Identify automatic thoughts Challenge negative cognitions, catastrophizing Substitute and rehearse positive thoughts, capabilities Transition from patient role to self-care |
| Couples’ therapy | Assist adaptation to role changes Diminish spousal solicitousness or excessive caretaking Increase communication |
| Biofeedback, relaxation, imagery | Adjunctive role in pain management Reduce tension, comorbid anxiety |
| Hypnosis | Dissociate awareness of pain Substitute, displace, reinterpret pain sensations |
| Vocational rehabilitation | Increase activity, ability to distract Regain sense of control, identity, and productivity Increase socialization |
| Pain management program | Multiple treatment effects Useful for complex pain with affective states |
Monoamine oxidase inhibitors (MAOIs) may have some efficacy for neuropathy and headache, but the need for a tyramine-free diet and potential for drug-drug interactions limit their usefulness. Co-administering an MAOI and meperidine is always contraindicated, as this combination can produce fever, delirium, seizures, circulatory collapse, and death. Similarly, avoid using an MAOI with any other antidepressant.
Others. Evidence is very limited on using other antidepressants such as trazodone, nefazodone, bupropion, and mirtazapine in chronic pain:
- Trazodone may help pediatric migraine, but it is not a consistent analgesic and may not be well-tolerated.
- Case reports suggest bupropion may help with headaches and chronic low-back pain.14
- Mirtazapine and trazodone may be useful adjuncts for treating insomnia in depressed patients with chronic pain.
Other options
Anticonvulsants appear useful for neuropathic pain and are appropriate for chronic pain patients who cannot tolerate TCAs.24 Like TCAs, anticonvulsants are not addictive. Unlike TCAs, anticonvulsants may help stabilize other affective illnesses that may coexist with chronic pain, including bipolar disorder, schizoaffective disorder, and impulsivity/aggression related to dementia or personality disorder.6 If the starting dosage is not effective within 1 week, increase gradually every 2 to 3 days to target dosages comparable to those for anticonvulsant efficacy.
Carbamazepine and gabapentin are recommended first-line medications for neuropathy. Carbamazepine is indicated for treating trigeminal neuralgia, although its cytochrome P-450 3A3/4 isoenzyme induction may reduce serum levels of acetaminophen, opioids, and oral contraceptives. Gabapentin, although not clearly beneficial for bipolar disorder, has anxiolytic effects and a benign side-effect profile, which may help patients with chronic pain.
Valproate can help prevent migraines. Clonazepam can help reduce anxiety and restless legs syndrome but may be habituating. Anticonvulsants’ common adverse effects include sedation, GI upset, dizziness, and fatigue.
Lithium has known efficacy for mood stabilization in bipolar disorder and can ameliorate cluster headaches.
Antipsychotics. Evidence is sparse on whether antipsychotics have analgesic activity. Their side effects generally limit their usefulness to treating pain in patients with psychosis or delirium.6
Stimulants such as dextroamphetamine and methylphenidate can be helpful adjuncts for treating depression, especially for medical inpatients who require a rapid therapeutic response. Stimulants may reduce fatigue or excessive sedation and improve concentration in patients receiving opioids for chronic pain. They also may have analgesic effects when combined with opioids. Potential adverse effects include appetite suppression, anxiety or agitation, confusion, tics, and addiction.6
Precautions. The muscle relaxant carisoprodol is associated with potential dependence and withdrawal. Cyclobenzaprine, another muscle relaxant, has a TCA-like structure and can be lethal in overdose. Baclofen can be useful for chronic pain related to spasticity, although psychotic depression and mania have been reported with abrupt withdrawal.6
Related resources
- American Academy of Pain Medicine. www.painmed.org/
- American Academy of Pain Management. www.aapainmanage.org
- Pain.com: continuing medical education for anesthesiology professionals. www.pain.com/index.cfm
- Kerns RD, Turk DC, Rudy TE. The West Haven-Yale Multidimensional Pain Inventory (WHYMPI). Pain 1985;13:345-56
Drug brand names
- Amitriptyline • Elavil
- Baclofen • Lioresal
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Carisoprodol • Soma
- Celecoxib • Celebrex
- Citalopram • Celexa
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Cyclobenzaprine • Flexeril
- Desipramine • Norpramin
- Dihydrocodeine • Synalgos-DC
- Doxepin • Sinequan
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Hydrocodone • Vicodin, Lortab
- Imipramine • Tofranil
- Lithium • Eskalith CR, Lithobid
- Maprotiline • Ludiomil
- Meperidine • Demerol
- Methadone • Dolophine
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Nortriptyline • Pamelor
- Oxycodone • OxyContin
- Paroxetine • Paxil
- Sertraline • Zoloft
- Tizanidine • Zanaflex
- Tramadol • Ultram
- Trazodone • Desyrel
- Valproate • Depakote
- Venlafaxine • Effexor, Effexor XR
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: a biopsychosocial perspective. Biol Psychiatry 2003;54:399-409.
2. Fishbain DA, Cutler R, Rosomoff HL, et al. Chronic painassociated depression: antecedent or consequence of chronic pain? A review. Clin J Pain 1997;13(2):116-37.
3. Banks SM, Kerns RD. Explaining high rates of depression in chronic pain: a diathesis-stress framework. Psychol Bull 1996;119:95-110.
4. Simon GE, VonKorff M, Piccinelli M, et al. An international study of the relation between somatic symptoms and depression. N Engl J Med 1999;341(18):1329-35.
5. Kroenke K, Price RK. Symptoms in the community. Prevalence, classification, and psychiatric comorbidity. Arch Intern Med. 1993;153:2474-80.
6. Leo RJ. Concise guide to pain management for psychiatrists. Arlington, Va: American Psychiatric Publishing, Inc., 2003.
7. Sullivan MD, Turner JA, Romano J. Chronic pain in primary care. Identification and management of psychosocial factors. J Fam Pract 1991;32(2):193-9.
8. Holmgren A, Wise MG, Bouckoms AJ. Pain management. In: Wise MG, Rundell JR (eds). Psychiatry in the medically ill (2nd ed). Washington, DC: American Psychiatric Publishing Inc., 2002;989-1013.
9. Naifeh KH. Psychometric testing in functional GI disorders in: Olden K (ed). Handbook of functional GI disorders. New York: Marcel Dekker, 1996;79-126.
10. Fishbain DA. The association of chronic pain and suicide. Semin Clin Neuropsychiatry 1999;4(3):221-7.
11. Fishbain DA. Medico-legal rounds: medical-legal issues and breaches of “standards of medical care” in opioid tapering for alleged opioid addiction. Pain Med 2002;3(2):135-42.
12. Doan BD, Wadden NP. Relationships between depressive symptoms and descriptions of chronic pain. Pain 1989;36:75-84.
13. Franklin JE, Leamon MH, Frances RJ. Substance-related disorders. In: Wise MG, Rundell JR (eds). Psychiatry in the medically ill (2nd ed). Washington DC: American Psychiatric Publishing, 2002;417-53.
14. Ansari A. The efficacy of newer antidepressants in the treatment of chronic pain: a review of current literature. Harv Rev Psychiatry 2000;7(5):257-77.
15. NIH Technology Assessment Panel. Integration of behavioral relaxation approaches into the treatment of chronic pain and insomnia. JAMA 1996;276(4):313-18.
16. Morley S, Eccleston C, Williams A. Systematic review and metaanalysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain 1999;80:1-13
17. Magni G. On the relationship between chronic pain and depression when there is no organic lesion. Pain 1987;31:1-21.
18. Markowitz JS, Patrick KS. Venlafaxine-tramadol similarities. Med Hypotheses 1998;5:167-8
19. Bradley RH, Barkin RL, Jerome J, et al. Efficacy of venlafaxine for the long-term treatment of chronic pain with associated major depressive disorder. Am J Ther 2003;10(5):318-23.
20. Sindrup SH, Bach FW, Madsen C, et al. Venlafaxine vs. imipramine in painful polyneuropathy—a randomized controlled trial. Neurology 2003;60:1284-9.
21. Kathpal GS. Role of SSRIs in the management of migraine. Headache Quarterly 1998;9:265-6.
22. Mallinckrodt CH, Goldstein DJ, Detke MJ, et al. Duloxetine: a new treatment for the emotional and physical symptoms of depression. Primary Care Companion J Clin Psychiatry 2003;5(1):19-28.
23. Lynch ME. Antidepressants as analgesics: a review of randomized control trials. J Psychiatry Neurosci 2001;26-36.
24. Swerdlow M. Anticonvulsant drugs and chronic pain. Clin Neuropharmacol 1984;7(1):51-82.
1. Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: a biopsychosocial perspective. Biol Psychiatry 2003;54:399-409.
2. Fishbain DA, Cutler R, Rosomoff HL, et al. Chronic painassociated depression: antecedent or consequence of chronic pain? A review. Clin J Pain 1997;13(2):116-37.
3. Banks SM, Kerns RD. Explaining high rates of depression in chronic pain: a diathesis-stress framework. Psychol Bull 1996;119:95-110.
4. Simon GE, VonKorff M, Piccinelli M, et al. An international study of the relation between somatic symptoms and depression. N Engl J Med 1999;341(18):1329-35.
5. Kroenke K, Price RK. Symptoms in the community. Prevalence, classification, and psychiatric comorbidity. Arch Intern Med. 1993;153:2474-80.
6. Leo RJ. Concise guide to pain management for psychiatrists. Arlington, Va: American Psychiatric Publishing, Inc., 2003.
7. Sullivan MD, Turner JA, Romano J. Chronic pain in primary care. Identification and management of psychosocial factors. J Fam Pract 1991;32(2):193-9.
8. Holmgren A, Wise MG, Bouckoms AJ. Pain management. In: Wise MG, Rundell JR (eds). Psychiatry in the medically ill (2nd ed). Washington, DC: American Psychiatric Publishing Inc., 2002;989-1013.
9. Naifeh KH. Psychometric testing in functional GI disorders in: Olden K (ed). Handbook of functional GI disorders. New York: Marcel Dekker, 1996;79-126.
10. Fishbain DA. The association of chronic pain and suicide. Semin Clin Neuropsychiatry 1999;4(3):221-7.
11. Fishbain DA. Medico-legal rounds: medical-legal issues and breaches of “standards of medical care” in opioid tapering for alleged opioid addiction. Pain Med 2002;3(2):135-42.
12. Doan BD, Wadden NP. Relationships between depressive symptoms and descriptions of chronic pain. Pain 1989;36:75-84.
13. Franklin JE, Leamon MH, Frances RJ. Substance-related disorders. In: Wise MG, Rundell JR (eds). Psychiatry in the medically ill (2nd ed). Washington DC: American Psychiatric Publishing, 2002;417-53.
14. Ansari A. The efficacy of newer antidepressants in the treatment of chronic pain: a review of current literature. Harv Rev Psychiatry 2000;7(5):257-77.
15. NIH Technology Assessment Panel. Integration of behavioral relaxation approaches into the treatment of chronic pain and insomnia. JAMA 1996;276(4):313-18.
16. Morley S, Eccleston C, Williams A. Systematic review and metaanalysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain 1999;80:1-13
17. Magni G. On the relationship between chronic pain and depression when there is no organic lesion. Pain 1987;31:1-21.
18. Markowitz JS, Patrick KS. Venlafaxine-tramadol similarities. Med Hypotheses 1998;5:167-8
19. Bradley RH, Barkin RL, Jerome J, et al. Efficacy of venlafaxine for the long-term treatment of chronic pain with associated major depressive disorder. Am J Ther 2003;10(5):318-23.
20. Sindrup SH, Bach FW, Madsen C, et al. Venlafaxine vs. imipramine in painful polyneuropathy—a randomized controlled trial. Neurology 2003;60:1284-9.
21. Kathpal GS. Role of SSRIs in the management of migraine. Headache Quarterly 1998;9:265-6.
22. Mallinckrodt CH, Goldstein DJ, Detke MJ, et al. Duloxetine: a new treatment for the emotional and physical symptoms of depression. Primary Care Companion J Clin Psychiatry 2003;5(1):19-28.
23. Lynch ME. Antidepressants as analgesics: a review of randomized control trials. J Psychiatry Neurosci 2001;26-36.
24. Swerdlow M. Anticonvulsant drugs and chronic pain. Clin Neuropharmacol 1984;7(1):51-82.
Persistent depression? Low libido? Androgen decline may be to blame
When a patient exhibits depressed mood, low energy, anxiety, insomnia, and low libido, do you consider major depression related to testosterone deficiency? Psychiatrists who don’t look for hypogonadism may miss a reversible cause of depression, especially in patients whose affective symptoms don’t respond to antidepressants.
Evidence is revealing how below-normal androgen levels may affect behavior and psychopathology in both men and women. This article describes:
- possible causes and effects of hypogonadism
- how to recognize and treat depression related to testosterone deficiency
- which lab tests provide the most clinically useful measures of testosterone
- potential benefits and adverse effects of testosterone replacement therapy.
Low testosterone and depression
Testosterone deficiency is particularly common in men with treatment-resistant depression. In one study, hypogonadism (total AMtestosterone contrations ≤350 ng/dL) was detected in 24 (43%) of 56 middle-aged men with treatment-resistant depression.1
Table 1
Signs and symptoms of testosterone deficiency
|
|
|
|
Symptoms. Although most depressed patients are not hypogonadal, testosterone deficiency can cause depressed mood, low self-confidence, timidity, fearfulness, irritability, low libido, and impaired sexual function in men1-6 and most likely in women.7
Conversely, robust androgen secretion usually promotes good mood, self-confidence, boldness, dominant behavior, and strong libido. Men’s normally higher testosterone levels may relate to this sex’s lower frequency of depression and generally more violent aggression, compared with women.
Increased male aggression is associated with elevated gonadal steroid levels—from overelaboration of endogenous hormone or, more commonly, use of exogenous anabolic steroids.8 Less well-appreciated is that testosterone deficiency in men is frequently associated with irritability,9 particularly in response to stress. Correcting testosterone deficiency can improve control of hostile feelings and lead to higher self-esteem and less impulsivity.2
In general, correcting hypogonadism improves mood in men,10,11 including those with refractory depression.1,12
Depression in women. Evidence is conflicting and limited on a possible link between testosterone deficiency and depression in women. Psychological well-being in postmenopausal women given exogenous estrogens appears to improve when low-dose testosterone is added. In a recent placebo-controlled trial, testosterone cream, 10 mg/d—sufficient to bring total testosterone to the upper normal range—significantly improved mood in premenopausal women with low libido.13
Diagnosing hypogonadism
Hypogonadism is usually diagnosed by clinical and biochemical findings. Testosterone deficiency’s common signs and symptoms are shown in Table 1. Treated diabetes and obesity are significantly related to testosterone deficiency, as are—to a lesser extent—headaches, age >60, not smoking, treated asthma, low dominance rating, and sleeping <5 hr/night.14
Laboratory evaluation. Measuring total serum testosterone concentrations in blood withdrawn before 9 AMis a useful initial screen for testosterone deficiency. Circulating testosterone concentrations show diurnal variation in both sexes, with higher levels in early morning—typically 7 to 8 AM—and lowest levels in the evening—typically 7 to 8 PM. Morning concentrations of serum and salivary testosterone decline an average 50% to 60% from zenith to nadir.
In men, 90 to 95% of circulating sex hormones originate in the testes; transformation from adrenal-derived DHEA accounts for only about 5 to 10%. In ovulatory women, the ovaries and adrenals (via conversion from DHEA) contribute approximately equally to circulating androgens and estrogens.
Relative concentrations of sex hormones in circulation, CSF, and tissues depend on the concentrations and function of steroidogenic enzymes, whose sexual divergences largely account for differences between men’s and women’s androgen and estrogen levels.
The brain controls sex hormone synthesis and release and is also an important target organ for sex hormone action. Gonadotropin-releasing hormone (GnRH) released from the hypothalamus is the primary brain regulator of gonadal function, via the so-called hypothalamic-pituitary-gonadal (HPG) axis. Pulsatile GnRH stimulates the anterior pituitary to release luteinizing hormone (LH) and follicular-stimulating hormone (FSH). LH and FSH in turn regulate spermatogenesis, ovulation, and synthesis and release of estrogens and androgens.
The brain also regulates adrenal sex hormone synthesis and release but by the hypothalamicpituitary-adrenal (HPA) axis, via pituitary adrenocorticotropic hormone (ACTH). Unlike cortisol, which is also regulated by ACTH, negative feedback suppression of ACTH by DHEA, if it occurs at all, is not significant.
Testosterone begins to decline with age in men after the third decade and in women after menopause. Approximately 90% of men in their 80s have biochemical hypogonadism (testosterone or free testosterone <2.5th percentile for young men), as do 35% of men in their 60s.15 Age-related increases in sex hormone-binding globulin (SHBG) compound the effects of diminishing total testosterone synthesis. Thus, free testosterone decreases with aging proportionately faster than total testosterone.
Total testosterone includes protein-bound and unbound testosterone and is a good measure of testosterone synthesis (Box).15 Normal circulating total testosterone levels are:
- 325 to 1,000 ng/dL in men
- 25 to 90 ng/dL in women (approximately 10% of male levels).
Testosterone assays are usually insensitive in the lower concentration ranges. This makes establishing testosterone deficiency difficult in women.
When total testosterone level is equivocal or low, repeat total testosterone levels once or twice and measure free testosterone, which is the biologically active form. More than 95% of circulating testosterone is bound to plasma proteins, including SHBG and albumin. Also measure free testosterone during the initial screen when you suspect testosterone deficiency.
In cycling women, sex hormone concentrations spike during ovulation and are low when the follicular phase begins. Although longitudinal evaluation is more accurate, the more practical crosssectional screen (AMblood) in the late follicular or late luteal phase is usually adequate.
Evaluating women taking oral contraceptives is biochemically straightforward, as exogenous estrogen suppresses ovarian sex hormone production and induces steady testosterone concentrations.
Postmenopausal women can be screened for sex hormone concentrations on virtually any morning, although perimenopausal women (within 5 years of last menstrual period) are, like premenopausal women, best studied longitudinally. DHEA and DHEA-S concentrations are perhaps more important to measure in women than in men because these sex steroids are responsible for a comparatively much larger component of circulating testosterone in women.
Follow-up tests. If testosterone deficiency is established, measure circulating pituitary hormones LH, FSH, and prolactin to determine if hypogolnadism is primary (gonadal) or secondary to another abnormality (of the brain or pituitary):
- Elevated LH and/or FSH levels are seen in primary hypogonadism, as the pituitary attempts to compensate for poorly functioning or sluggish gonads by increasing their stimulation.
- Diminished or inappropriately normal LH levels during testosterone deficiency (when high levels should be seen) are consistent with central or secondary hypogonadism.
- A combination of primary and secondary hypogonadism is common with advanced age.
Measure serum prolactin concentrations when evaluating hypogonadism because hyperprolactinemia is a common cause.
If the patient is testosterone-deficient, also assess other endocrine systems. If one fails or becomes inflamed, other glands or hormone systems often show insufficiency or inflammation as well, perhaps because of a common pathologic process. Circulating testosterone levels may be normal or elevated in testosterone insensitivity or hyposensitivity syndromes.
Correcting deficiency
Testosterone deficiency can often be corrected without using androgens, such as by changing or supplementing a medication.
Hyperprolactinemia is a common cause of central hypogonadism and testosterone deficiency in psychiatric patients, often as an adverse effect of psychotropics (particularly antipsychotics). Hyperprolactinemia suppresses GnRH and, in turn, LH and gonadal synthesis of testosterone. Hyperprolactinemia depresses libido and causes infertility in both sexes and amenorrhea in women.
Medication changes can usually correct psychotropic-induced hyperprolactinemia. Elevated prolactin levels from other causes (such as a pituitary prolactinoma) usually respond to dopamine agonists such as bromocriptine or cabergoline.
Zinc deficiency can lower testosterone levels. Zinc is highly enriched in the testes and prostate, where it accumulates via a zinc uptake system. The cerebral cortex is also zinc-enriched.
Zinc’s recommended daily allowance (RDA) is 15 mg for men and 12 mg for women. Mild zinc deficiency is common, affecting, for example, about 30% of healthy older men in Detroit16 and many depressed patients.17
Remarkably, dietary zinc restriction (to one-third of the RDA) in healthy young men reduces serum testosterone levels by 75% after 5 to 6 months. Conversely, giving a zinc supplement, 30 mg/d, to marginally zinc-deficient older men nearly doubled their serum testosterone concentrations after 6 months.18
Because serum zinc concentrations do not reliably reflect zinc status, the most expedient clinical approach is to supplement with the RDA—found in widely available multivitamins. Zinc is generally considered low-risk for toxicity, although high doses should be avoided. Much is unknown about zinc’s role in the CNS, where it apparently can be neuroprotective or neurotoxic.
Androgen suppressants. Cholesterol-lowering agents—whether they inhibit cholesterol biosynthesis or absorption—can sometimes lower serum androgen levels. Included are antihyperlipidemic pharmaceuticals and plant sterols that compete with cholesterol for gut absorption. Plant sterols such as beta-sitosterol are marketed as cholesterol-lowering food supplements.
Volatile and fatty oils of the saw palmetto berry (Seranoa repens or Sabal serrulata)—a frequently used over-the-counter phytotherapy for benign prostatic hypertrophy—have antiandrogen properties. They inhibit 5-alpha reductase types I and II, reducing testosterone’s conversion to dihydrotestosterone.19 Flaxseed oil (linseed oil), another over-the-counter herbal supplement, also may alter testosterone levels.
Table 2
Recommended testosterone-replacement preparations
| Preparation | Usual dosage (men) |
|---|---|
| Transdermal patch (2.5 or 5 mg each) | 1 to 2 patch(es) applied daily |
| Gel | 5 to 10 mg/d (in 5 to 10 grams of gel, applied once daily) |
| Oral methyltestosterone | 10 to 200 mg/d |
| Testosterone enanthate IM injection | 50 to 400 mg every 2 weeks |
| Buccal testosterone adhesive | 60 to 90 mg/d |
| Sex hormone precursor | Usual dosage for testosterone replacement (women) |
| Oral DHEA | 25 to 50 mg once daily |
| DHEA: dehydroepiandrosterone | |
Exogenous glucocorticoids suppress DHEA release by negative feedback suppression of adrenocorticotropic hormone (ACTH) at the anterior pituitary. To protect against sex hormone deficiency, give DHEA in replacement doses whenever more than a few glucocorticoid doses are given. This applies particularly to postmenopausal women, in whom DHEA is the major source of circulating androgens.
Testosterone replacement
Preliminary data suggest that correcting testosterone deficiency in depressed men can have an antidepressant effect, especially in men who respond inadequately to standard antidepressants. Moreover, like antidepressants, testosterone replacement therapy can induce hypomania or mania in some individuals.
Depression and/or anxiety associated with sustained, irreversible serum testosterone deficiency—usually with other signs of testosterone deficiency (Table 1)—is the major psychiatric indication for testosterone replacement. Borderline biochemical testosterone deficiency and psychiatric symptoms in a “treatment-resistant” patient—especially one at risk for suicide—may justify an empirical testosterone replacement trial. Do not continue such a trial indefinitely without compelling reasons, however, because gonadal function recovery can be delayed for months after even a 12-week testosterone trial.20
Recommended agents for testosterone replacement are shown in Table 2. In men, testosterone preparations are normally used to increase testosterone levels. In women, I prescribe DHEA (discussed below). In young men and women with secondary hypogonadism, pulsatile use of gonadotropins may be necessary to induce spermatogenesis or ovulation—interventions outside the scope of psychiatric practice.
Contraindications to androgen replacement include hyperandrogenism, prostate cancer, antisocial personality, current mania, pedophilia, hypersexuality, and any psychiatric syndrome characterized by violent or predatory behavior. Pregnant patients (or women without a reliable birth control method) should not receive testosterone. Use caution when replacing androgens in patients with benign prostatic hypertrophy, hypomania, or a history of mania or hypomania.
An antidepressant response to adequate exogenous testosterone (enough to raise free testosterone levels to mid-normal range) is generally seen within 4 weeks. If psychological improvement is not observed, testosterone replacement may still prove beneficial if reversing hypogonadism improves the efficacy of subsequent antidepressants.
Dosage forms for men. Transdermal testosterone patches are normally applied to clean, dry skin on the upper arms, abdomen, thigh, or back and rotated among sites to avoid dermal irritation. When the nonscrotal patch is applied at night, testosterone concentrations mimic the circadian pattern seen in young men without causing supraphysiologic transients.21
Testosterone gel is applied every morning—also in a rotating manner—to clean, dry, intact skin and allowed to dry. Absorption is rapid, with measurable testosterone increases within 30 minutes. Approximately 10% of the testosterone is absorbed, delivering 5 to 10 mg/d into the circulation after 5 to 10 grams of gel (containing 50 to 100 mg of testosterone) is applied. Steady-state concentrations are achieved within 2 to 3 days, so dosages can be adjusted quickly.
Some patients regard 10 grams of gel as too messy to apply comfortably. Testosterone gel residuals can be washed from the skin with soap and water. Prolonged coated-skin contact with another person, such as a sex partner, can increase testosterone concentrations in the untreated individual.
Oral testosterone is absorbed poorly (often requiring high dosages) and cleared rapidly (half-life: 10 to 100 minutes). Only 10-mg capsules of methyltestosterone preparations are readily available—a dose too small for most men and too large for women. Many pharmacists can formulate other dosages for individual patients. Twice-daily doses are often used. Gum irritation and altered taste can occur when using buccal mucoadhesive testosterone.
Oil-based testosterone injections (such as IM testosteroneenanthate) are absorbedslowly and cannot reproduce normal circadian testosterone rhythms and concentrations. In some cases, however, the long-acting effectsof IM testosteroneare beneficial.
DHEA acutely increases testosterone and estrogens in both men and women after a single physiologic dose. During maintenance DHEA replacement, however, clinically significant increases in both sex hormones are seen only in women. DHEA is preferred to increase testosterone levels in women, as it is converted to appropriate proportions of androgens and estrogens by endogenous steroidogenic enzymes.
Table 3
Potential adverse effects of testosterone replacement therapy
|
|
|
|
DHEA, which occurs in yams, is available over-the-counter as a “food supplement” or “nutritional supplement.” However, many of these preparations, which are not regulated by the FDA, are unreliable because of poor quality control.22
Aromatase inhibitors were developed as antibreastcancer agents but also may treat testosterone deficiency. Testosterone administration increases circulating estrogens because testosterone is metabolized by the enzyme aromatase to estradiol. Aromatase inhibitors may prevent excessive estradiol levels—and associated adverse effects, such as gynecomastia—that are sometimes seen during testosterone replacement therapy in men. Available aromatase inhibitors include anastrozole, exemestane, and letrozole.
Potential adverse effects
Short-term testosterone replacement is generally low-risk. Acne is the most common adverse effect (Table 3).
The incidence of adverse events increases as testosterone concentrations are elevated above the normal range. For example, about 5% of men experience a manic or hypomanic arousal within 2 to 6 weeks of induced supraphysiologic testosterone levels.8
Gonadal suppression. Exogenous testosterone (or high-dose DHEA) suppresses endogenous gonadal function in men and premenopausal women. When a sustained course of exogenous androgens is discontinued, gonadal suppression usually does not reverse completely for several months or longer.
Prostatic hypertrophy, commonly considered to be testosterone driven, may be a risk of testosterone replacement therapy. Emergent urinary retention during testosterone replacement therapy has been reported, so use caution when giving testosterone to men with prostatic hypertrophy.
Barring evidence to the contrary, testosterone therapy is contraindicated in patients with prostate cancer. Baseline and post-treatment prostate-specific antigen measures are recommended.
Other risks in men. Men occasionally develop gynecomastia during testosterone replacement, perhaps because of testosterone aromatization to estradiol. Beyond increased hematocrit levels and associated problems, testosterone’s cardiovascular risks are unclear. Testosterone deficiency also has been linked to increased atherosclerosis risk in older men.23
Risks in women. Overtreating women with testosterone (DHEA) can promote hirsutism (including facial hair), loss of hair on scalp, voice lowering, clitoromegaly, breast regression, and muscle hypertrophy.
Related resources
- Daly RC, Su T-P, Schmidt PJ, et al. Cerebrospinal fluid and behavioral changes after methyltestosterone administration. Arch Gen Psychiatry 2001;58:172-7.
- Davis S. Testosterone deficiency in women.J Reprod Med2 001; 46(3 suppl):291-6.
- Rohr UD. The impact of testosterone imbalance on depression and women’s health. Maturitas 2002;41(1 suppl):S25-S46.
- Mantzoros CS, Georgiadis EI. Contribution of dihydrotestosterone to male sexual behaviour. BMJ 1995;310:1289-91.
Drug brand names
- Methyltestosterone (oral) • Android, Methitest, Testred, Virilon
- Testosterone (buccal) • Striant
- Testosterone (gel) • AndroGel, Testim
- Testosterone (transdermal) • Androderm, Testoderm
- Testosterone enanthate (IM injection) • Delatestryl
Disclosure
Dr. Geracioti reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Pope HG, Jr, Cohane GH, Kanayama G, et al. Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. Am J Psychiatry 2003;160:105-11.
2. Ehrenreich H, Halaris A, Ruether E, et al. Psychoendocrine sequelae of chronic testosterone deficiency. J Psychiatric Res 1999;33:379-87.
3. Schweiger U, Deuschle M, Weber B, et al. Testosterone, gonadotropin, and cortisol secretion in male patients with major depression. Psychosom Med 1999;61:292-6.
4. Seidman SN, Walsh BT. Testosterone and depression in aging men. Am J Geriatr Psychiatry 1999;7:18-33.
5. Mulchahey JJ, Ekhator NN, Zhang H, et al. Cerebrospinal fluid and plasma testosterone levels in post-traumatic stress disorder and tobacco dependence. Psychoneuroendocrinology 2001;26:273-85.
6. Shores MM, Sloan KL, Matsumoto AM, et al. Increased incidence of diagnosed depressive illness in hypogonadal older men. Arch Gen Psychiatry 2004;61:162-7.
7. Bachman G, Bancroft J, Braunstein G, et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertil Steril 2002;77:660-5.
8. Pope HG, Jr, Kouri EM, Hudson JI. Effects of supraphysiologic doses of testosterone on mood and aggression in normal men. A randomized controlled trial. Arch Gen Psychiatry 2000;57:133-40.
9. Matsumoto AM. The testis. In: Felig P, Frohman LA (eds). Endocrinology and metabolism (4th ed). New York: McGraw-Hill, 2001;635-705.
10. O’Carroll R, Shapiro C, Bancroft J. Androgens, behavior and nocturnal erection in hypogonadal men: the effects of varying the replacement dose. Clin Endocrinol 1985;23:527-38.
11. Wang C, Swerdloff R, Iranmanesh A, et al. and the Testosterone Gel Study Group. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab 2000;85:2839-53.
12. Seidman SN, Rabkin JG. Testosterone replacement therapy for hypogonadal men with SSRI-refractory depression. J Affect Disord 1998;48(2-3):157-61.
13. Goldstat R, Briganti E, Tran J, et al. Transdermal testosterone therapy improves well-being, mood, and sexual function in premenopausal women. Menopause 2003;10:390-8.
14. Smith KW, Feldman HA, McKinlay JB. Construction and field validation of self-administered screener for testosterone deficiency (hypogonadism) in ageing men. Clin Endocrinol 2000;53:703-11.
15. Harmon SM, Metter EJ, Tobin JD, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab 2001;86:724-31.
16. Prasad AS, Fitzgerald JT, Hess JW, et al. Zinc deficiency in elderly patients. Nutrition 1993;9:218-24.
17. Maes M, D’Haese PC, Scharpe S, et al. Hypozincemia in depression. J Affect Disord 1994;31:135-40.
18. Prasad AS, Mantzoros CS, Beck FW, et al. Zinc status and serum testosterone levels of healthy adults. Nutrition 1996;12:344-8.
19. Weisser H, Tunn S, Behnke B, Krieg M. Effects of the sabal serrulata extract IDS 89 and its subfractions on 5 alpha-reductase activity in human benign prostatic hyperplasia. Prostate 1996;28:300-6.
20. Forbes GB, Porta CR, Herr BE, Griggs RC. Sequence of changes in body composition induced by testosterone and reversal of changes after drug is stopped. JAMA 1992;267(3):397-9.
21. Meikle AW. Transdermal testosterone. Drugs 1998;55:259.-
22. Parasrampuria J, Schwartz K, Petesch R. Quality control of dehydroepiandrosterone dietary supplement products. JAMA 1998;280:1565.-
23. Hak AE, Witteman JC, de Jong FH, et al. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab 2002;87:3632-9.
When a patient exhibits depressed mood, low energy, anxiety, insomnia, and low libido, do you consider major depression related to testosterone deficiency? Psychiatrists who don’t look for hypogonadism may miss a reversible cause of depression, especially in patients whose affective symptoms don’t respond to antidepressants.
Evidence is revealing how below-normal androgen levels may affect behavior and psychopathology in both men and women. This article describes:
- possible causes and effects of hypogonadism
- how to recognize and treat depression related to testosterone deficiency
- which lab tests provide the most clinically useful measures of testosterone
- potential benefits and adverse effects of testosterone replacement therapy.
Low testosterone and depression
Testosterone deficiency is particularly common in men with treatment-resistant depression. In one study, hypogonadism (total AMtestosterone contrations ≤350 ng/dL) was detected in 24 (43%) of 56 middle-aged men with treatment-resistant depression.1
Table 1
Signs and symptoms of testosterone deficiency
|
|
|
|
Symptoms. Although most depressed patients are not hypogonadal, testosterone deficiency can cause depressed mood, low self-confidence, timidity, fearfulness, irritability, low libido, and impaired sexual function in men1-6 and most likely in women.7
Conversely, robust androgen secretion usually promotes good mood, self-confidence, boldness, dominant behavior, and strong libido. Men’s normally higher testosterone levels may relate to this sex’s lower frequency of depression and generally more violent aggression, compared with women.
Increased male aggression is associated with elevated gonadal steroid levels—from overelaboration of endogenous hormone or, more commonly, use of exogenous anabolic steroids.8 Less well-appreciated is that testosterone deficiency in men is frequently associated with irritability,9 particularly in response to stress. Correcting testosterone deficiency can improve control of hostile feelings and lead to higher self-esteem and less impulsivity.2
In general, correcting hypogonadism improves mood in men,10,11 including those with refractory depression.1,12
Depression in women. Evidence is conflicting and limited on a possible link between testosterone deficiency and depression in women. Psychological well-being in postmenopausal women given exogenous estrogens appears to improve when low-dose testosterone is added. In a recent placebo-controlled trial, testosterone cream, 10 mg/d—sufficient to bring total testosterone to the upper normal range—significantly improved mood in premenopausal women with low libido.13
Diagnosing hypogonadism
Hypogonadism is usually diagnosed by clinical and biochemical findings. Testosterone deficiency’s common signs and symptoms are shown in Table 1. Treated diabetes and obesity are significantly related to testosterone deficiency, as are—to a lesser extent—headaches, age >60, not smoking, treated asthma, low dominance rating, and sleeping <5 hr/night.14
Laboratory evaluation. Measuring total serum testosterone concentrations in blood withdrawn before 9 AMis a useful initial screen for testosterone deficiency. Circulating testosterone concentrations show diurnal variation in both sexes, with higher levels in early morning—typically 7 to 8 AM—and lowest levels in the evening—typically 7 to 8 PM. Morning concentrations of serum and salivary testosterone decline an average 50% to 60% from zenith to nadir.
In men, 90 to 95% of circulating sex hormones originate in the testes; transformation from adrenal-derived DHEA accounts for only about 5 to 10%. In ovulatory women, the ovaries and adrenals (via conversion from DHEA) contribute approximately equally to circulating androgens and estrogens.
Relative concentrations of sex hormones in circulation, CSF, and tissues depend on the concentrations and function of steroidogenic enzymes, whose sexual divergences largely account for differences between men’s and women’s androgen and estrogen levels.
The brain controls sex hormone synthesis and release and is also an important target organ for sex hormone action. Gonadotropin-releasing hormone (GnRH) released from the hypothalamus is the primary brain regulator of gonadal function, via the so-called hypothalamic-pituitary-gonadal (HPG) axis. Pulsatile GnRH stimulates the anterior pituitary to release luteinizing hormone (LH) and follicular-stimulating hormone (FSH). LH and FSH in turn regulate spermatogenesis, ovulation, and synthesis and release of estrogens and androgens.
The brain also regulates adrenal sex hormone synthesis and release but by the hypothalamicpituitary-adrenal (HPA) axis, via pituitary adrenocorticotropic hormone (ACTH). Unlike cortisol, which is also regulated by ACTH, negative feedback suppression of ACTH by DHEA, if it occurs at all, is not significant.
Testosterone begins to decline with age in men after the third decade and in women after menopause. Approximately 90% of men in their 80s have biochemical hypogonadism (testosterone or free testosterone <2.5th percentile for young men), as do 35% of men in their 60s.15 Age-related increases in sex hormone-binding globulin (SHBG) compound the effects of diminishing total testosterone synthesis. Thus, free testosterone decreases with aging proportionately faster than total testosterone.
Total testosterone includes protein-bound and unbound testosterone and is a good measure of testosterone synthesis (Box).15 Normal circulating total testosterone levels are:
- 325 to 1,000 ng/dL in men
- 25 to 90 ng/dL in women (approximately 10% of male levels).
Testosterone assays are usually insensitive in the lower concentration ranges. This makes establishing testosterone deficiency difficult in women.
When total testosterone level is equivocal or low, repeat total testosterone levels once or twice and measure free testosterone, which is the biologically active form. More than 95% of circulating testosterone is bound to plasma proteins, including SHBG and albumin. Also measure free testosterone during the initial screen when you suspect testosterone deficiency.
In cycling women, sex hormone concentrations spike during ovulation and are low when the follicular phase begins. Although longitudinal evaluation is more accurate, the more practical crosssectional screen (AMblood) in the late follicular or late luteal phase is usually adequate.
Evaluating women taking oral contraceptives is biochemically straightforward, as exogenous estrogen suppresses ovarian sex hormone production and induces steady testosterone concentrations.
Postmenopausal women can be screened for sex hormone concentrations on virtually any morning, although perimenopausal women (within 5 years of last menstrual period) are, like premenopausal women, best studied longitudinally. DHEA and DHEA-S concentrations are perhaps more important to measure in women than in men because these sex steroids are responsible for a comparatively much larger component of circulating testosterone in women.
Follow-up tests. If testosterone deficiency is established, measure circulating pituitary hormones LH, FSH, and prolactin to determine if hypogolnadism is primary (gonadal) or secondary to another abnormality (of the brain or pituitary):
- Elevated LH and/or FSH levels are seen in primary hypogonadism, as the pituitary attempts to compensate for poorly functioning or sluggish gonads by increasing their stimulation.
- Diminished or inappropriately normal LH levels during testosterone deficiency (when high levels should be seen) are consistent with central or secondary hypogonadism.
- A combination of primary and secondary hypogonadism is common with advanced age.
Measure serum prolactin concentrations when evaluating hypogonadism because hyperprolactinemia is a common cause.
If the patient is testosterone-deficient, also assess other endocrine systems. If one fails or becomes inflamed, other glands or hormone systems often show insufficiency or inflammation as well, perhaps because of a common pathologic process. Circulating testosterone levels may be normal or elevated in testosterone insensitivity or hyposensitivity syndromes.
Correcting deficiency
Testosterone deficiency can often be corrected without using androgens, such as by changing or supplementing a medication.
Hyperprolactinemia is a common cause of central hypogonadism and testosterone deficiency in psychiatric patients, often as an adverse effect of psychotropics (particularly antipsychotics). Hyperprolactinemia suppresses GnRH and, in turn, LH and gonadal synthesis of testosterone. Hyperprolactinemia depresses libido and causes infertility in both sexes and amenorrhea in women.
Medication changes can usually correct psychotropic-induced hyperprolactinemia. Elevated prolactin levels from other causes (such as a pituitary prolactinoma) usually respond to dopamine agonists such as bromocriptine or cabergoline.
Zinc deficiency can lower testosterone levels. Zinc is highly enriched in the testes and prostate, where it accumulates via a zinc uptake system. The cerebral cortex is also zinc-enriched.
Zinc’s recommended daily allowance (RDA) is 15 mg for men and 12 mg for women. Mild zinc deficiency is common, affecting, for example, about 30% of healthy older men in Detroit16 and many depressed patients.17
Remarkably, dietary zinc restriction (to one-third of the RDA) in healthy young men reduces serum testosterone levels by 75% after 5 to 6 months. Conversely, giving a zinc supplement, 30 mg/d, to marginally zinc-deficient older men nearly doubled their serum testosterone concentrations after 6 months.18
Because serum zinc concentrations do not reliably reflect zinc status, the most expedient clinical approach is to supplement with the RDA—found in widely available multivitamins. Zinc is generally considered low-risk for toxicity, although high doses should be avoided. Much is unknown about zinc’s role in the CNS, where it apparently can be neuroprotective or neurotoxic.
Androgen suppressants. Cholesterol-lowering agents—whether they inhibit cholesterol biosynthesis or absorption—can sometimes lower serum androgen levels. Included are antihyperlipidemic pharmaceuticals and plant sterols that compete with cholesterol for gut absorption. Plant sterols such as beta-sitosterol are marketed as cholesterol-lowering food supplements.
Volatile and fatty oils of the saw palmetto berry (Seranoa repens or Sabal serrulata)—a frequently used over-the-counter phytotherapy for benign prostatic hypertrophy—have antiandrogen properties. They inhibit 5-alpha reductase types I and II, reducing testosterone’s conversion to dihydrotestosterone.19 Flaxseed oil (linseed oil), another over-the-counter herbal supplement, also may alter testosterone levels.
Table 2
Recommended testosterone-replacement preparations
| Preparation | Usual dosage (men) |
|---|---|
| Transdermal patch (2.5 or 5 mg each) | 1 to 2 patch(es) applied daily |
| Gel | 5 to 10 mg/d (in 5 to 10 grams of gel, applied once daily) |
| Oral methyltestosterone | 10 to 200 mg/d |
| Testosterone enanthate IM injection | 50 to 400 mg every 2 weeks |
| Buccal testosterone adhesive | 60 to 90 mg/d |
| Sex hormone precursor | Usual dosage for testosterone replacement (women) |
| Oral DHEA | 25 to 50 mg once daily |
| DHEA: dehydroepiandrosterone | |
Exogenous glucocorticoids suppress DHEA release by negative feedback suppression of adrenocorticotropic hormone (ACTH) at the anterior pituitary. To protect against sex hormone deficiency, give DHEA in replacement doses whenever more than a few glucocorticoid doses are given. This applies particularly to postmenopausal women, in whom DHEA is the major source of circulating androgens.
Testosterone replacement
Preliminary data suggest that correcting testosterone deficiency in depressed men can have an antidepressant effect, especially in men who respond inadequately to standard antidepressants. Moreover, like antidepressants, testosterone replacement therapy can induce hypomania or mania in some individuals.
Depression and/or anxiety associated with sustained, irreversible serum testosterone deficiency—usually with other signs of testosterone deficiency (Table 1)—is the major psychiatric indication for testosterone replacement. Borderline biochemical testosterone deficiency and psychiatric symptoms in a “treatment-resistant” patient—especially one at risk for suicide—may justify an empirical testosterone replacement trial. Do not continue such a trial indefinitely without compelling reasons, however, because gonadal function recovery can be delayed for months after even a 12-week testosterone trial.20
Recommended agents for testosterone replacement are shown in Table 2. In men, testosterone preparations are normally used to increase testosterone levels. In women, I prescribe DHEA (discussed below). In young men and women with secondary hypogonadism, pulsatile use of gonadotropins may be necessary to induce spermatogenesis or ovulation—interventions outside the scope of psychiatric practice.
Contraindications to androgen replacement include hyperandrogenism, prostate cancer, antisocial personality, current mania, pedophilia, hypersexuality, and any psychiatric syndrome characterized by violent or predatory behavior. Pregnant patients (or women without a reliable birth control method) should not receive testosterone. Use caution when replacing androgens in patients with benign prostatic hypertrophy, hypomania, or a history of mania or hypomania.
An antidepressant response to adequate exogenous testosterone (enough to raise free testosterone levels to mid-normal range) is generally seen within 4 weeks. If psychological improvement is not observed, testosterone replacement may still prove beneficial if reversing hypogonadism improves the efficacy of subsequent antidepressants.
Dosage forms for men. Transdermal testosterone patches are normally applied to clean, dry skin on the upper arms, abdomen, thigh, or back and rotated among sites to avoid dermal irritation. When the nonscrotal patch is applied at night, testosterone concentrations mimic the circadian pattern seen in young men without causing supraphysiologic transients.21
Testosterone gel is applied every morning—also in a rotating manner—to clean, dry, intact skin and allowed to dry. Absorption is rapid, with measurable testosterone increases within 30 minutes. Approximately 10% of the testosterone is absorbed, delivering 5 to 10 mg/d into the circulation after 5 to 10 grams of gel (containing 50 to 100 mg of testosterone) is applied. Steady-state concentrations are achieved within 2 to 3 days, so dosages can be adjusted quickly.
Some patients regard 10 grams of gel as too messy to apply comfortably. Testosterone gel residuals can be washed from the skin with soap and water. Prolonged coated-skin contact with another person, such as a sex partner, can increase testosterone concentrations in the untreated individual.
Oral testosterone is absorbed poorly (often requiring high dosages) and cleared rapidly (half-life: 10 to 100 minutes). Only 10-mg capsules of methyltestosterone preparations are readily available—a dose too small for most men and too large for women. Many pharmacists can formulate other dosages for individual patients. Twice-daily doses are often used. Gum irritation and altered taste can occur when using buccal mucoadhesive testosterone.
Oil-based testosterone injections (such as IM testosteroneenanthate) are absorbedslowly and cannot reproduce normal circadian testosterone rhythms and concentrations. In some cases, however, the long-acting effectsof IM testosteroneare beneficial.
DHEA acutely increases testosterone and estrogens in both men and women after a single physiologic dose. During maintenance DHEA replacement, however, clinically significant increases in both sex hormones are seen only in women. DHEA is preferred to increase testosterone levels in women, as it is converted to appropriate proportions of androgens and estrogens by endogenous steroidogenic enzymes.
Table 3
Potential adverse effects of testosterone replacement therapy
|
|
|
|
DHEA, which occurs in yams, is available over-the-counter as a “food supplement” or “nutritional supplement.” However, many of these preparations, which are not regulated by the FDA, are unreliable because of poor quality control.22
Aromatase inhibitors were developed as antibreastcancer agents but also may treat testosterone deficiency. Testosterone administration increases circulating estrogens because testosterone is metabolized by the enzyme aromatase to estradiol. Aromatase inhibitors may prevent excessive estradiol levels—and associated adverse effects, such as gynecomastia—that are sometimes seen during testosterone replacement therapy in men. Available aromatase inhibitors include anastrozole, exemestane, and letrozole.
Potential adverse effects
Short-term testosterone replacement is generally low-risk. Acne is the most common adverse effect (Table 3).
The incidence of adverse events increases as testosterone concentrations are elevated above the normal range. For example, about 5% of men experience a manic or hypomanic arousal within 2 to 6 weeks of induced supraphysiologic testosterone levels.8
Gonadal suppression. Exogenous testosterone (or high-dose DHEA) suppresses endogenous gonadal function in men and premenopausal women. When a sustained course of exogenous androgens is discontinued, gonadal suppression usually does not reverse completely for several months or longer.
Prostatic hypertrophy, commonly considered to be testosterone driven, may be a risk of testosterone replacement therapy. Emergent urinary retention during testosterone replacement therapy has been reported, so use caution when giving testosterone to men with prostatic hypertrophy.
Barring evidence to the contrary, testosterone therapy is contraindicated in patients with prostate cancer. Baseline and post-treatment prostate-specific antigen measures are recommended.
Other risks in men. Men occasionally develop gynecomastia during testosterone replacement, perhaps because of testosterone aromatization to estradiol. Beyond increased hematocrit levels and associated problems, testosterone’s cardiovascular risks are unclear. Testosterone deficiency also has been linked to increased atherosclerosis risk in older men.23
Risks in women. Overtreating women with testosterone (DHEA) can promote hirsutism (including facial hair), loss of hair on scalp, voice lowering, clitoromegaly, breast regression, and muscle hypertrophy.
Related resources
- Daly RC, Su T-P, Schmidt PJ, et al. Cerebrospinal fluid and behavioral changes after methyltestosterone administration. Arch Gen Psychiatry 2001;58:172-7.
- Davis S. Testosterone deficiency in women.J Reprod Med2 001; 46(3 suppl):291-6.
- Rohr UD. The impact of testosterone imbalance on depression and women’s health. Maturitas 2002;41(1 suppl):S25-S46.
- Mantzoros CS, Georgiadis EI. Contribution of dihydrotestosterone to male sexual behaviour. BMJ 1995;310:1289-91.
Drug brand names
- Methyltestosterone (oral) • Android, Methitest, Testred, Virilon
- Testosterone (buccal) • Striant
- Testosterone (gel) • AndroGel, Testim
- Testosterone (transdermal) • Androderm, Testoderm
- Testosterone enanthate (IM injection) • Delatestryl
Disclosure
Dr. Geracioti reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
When a patient exhibits depressed mood, low energy, anxiety, insomnia, and low libido, do you consider major depression related to testosterone deficiency? Psychiatrists who don’t look for hypogonadism may miss a reversible cause of depression, especially in patients whose affective symptoms don’t respond to antidepressants.
Evidence is revealing how below-normal androgen levels may affect behavior and psychopathology in both men and women. This article describes:
- possible causes and effects of hypogonadism
- how to recognize and treat depression related to testosterone deficiency
- which lab tests provide the most clinically useful measures of testosterone
- potential benefits and adverse effects of testosterone replacement therapy.
Low testosterone and depression
Testosterone deficiency is particularly common in men with treatment-resistant depression. In one study, hypogonadism (total AMtestosterone contrations ≤350 ng/dL) was detected in 24 (43%) of 56 middle-aged men with treatment-resistant depression.1
Table 1
Signs and symptoms of testosterone deficiency
|
|
|
|
Symptoms. Although most depressed patients are not hypogonadal, testosterone deficiency can cause depressed mood, low self-confidence, timidity, fearfulness, irritability, low libido, and impaired sexual function in men1-6 and most likely in women.7
Conversely, robust androgen secretion usually promotes good mood, self-confidence, boldness, dominant behavior, and strong libido. Men’s normally higher testosterone levels may relate to this sex’s lower frequency of depression and generally more violent aggression, compared with women.
Increased male aggression is associated with elevated gonadal steroid levels—from overelaboration of endogenous hormone or, more commonly, use of exogenous anabolic steroids.8 Less well-appreciated is that testosterone deficiency in men is frequently associated with irritability,9 particularly in response to stress. Correcting testosterone deficiency can improve control of hostile feelings and lead to higher self-esteem and less impulsivity.2
In general, correcting hypogonadism improves mood in men,10,11 including those with refractory depression.1,12
Depression in women. Evidence is conflicting and limited on a possible link between testosterone deficiency and depression in women. Psychological well-being in postmenopausal women given exogenous estrogens appears to improve when low-dose testosterone is added. In a recent placebo-controlled trial, testosterone cream, 10 mg/d—sufficient to bring total testosterone to the upper normal range—significantly improved mood in premenopausal women with low libido.13
Diagnosing hypogonadism
Hypogonadism is usually diagnosed by clinical and biochemical findings. Testosterone deficiency’s common signs and symptoms are shown in Table 1. Treated diabetes and obesity are significantly related to testosterone deficiency, as are—to a lesser extent—headaches, age >60, not smoking, treated asthma, low dominance rating, and sleeping <5 hr/night.14
Laboratory evaluation. Measuring total serum testosterone concentrations in blood withdrawn before 9 AMis a useful initial screen for testosterone deficiency. Circulating testosterone concentrations show diurnal variation in both sexes, with higher levels in early morning—typically 7 to 8 AM—and lowest levels in the evening—typically 7 to 8 PM. Morning concentrations of serum and salivary testosterone decline an average 50% to 60% from zenith to nadir.
In men, 90 to 95% of circulating sex hormones originate in the testes; transformation from adrenal-derived DHEA accounts for only about 5 to 10%. In ovulatory women, the ovaries and adrenals (via conversion from DHEA) contribute approximately equally to circulating androgens and estrogens.
Relative concentrations of sex hormones in circulation, CSF, and tissues depend on the concentrations and function of steroidogenic enzymes, whose sexual divergences largely account for differences between men’s and women’s androgen and estrogen levels.
The brain controls sex hormone synthesis and release and is also an important target organ for sex hormone action. Gonadotropin-releasing hormone (GnRH) released from the hypothalamus is the primary brain regulator of gonadal function, via the so-called hypothalamic-pituitary-gonadal (HPG) axis. Pulsatile GnRH stimulates the anterior pituitary to release luteinizing hormone (LH) and follicular-stimulating hormone (FSH). LH and FSH in turn regulate spermatogenesis, ovulation, and synthesis and release of estrogens and androgens.
The brain also regulates adrenal sex hormone synthesis and release but by the hypothalamicpituitary-adrenal (HPA) axis, via pituitary adrenocorticotropic hormone (ACTH). Unlike cortisol, which is also regulated by ACTH, negative feedback suppression of ACTH by DHEA, if it occurs at all, is not significant.
Testosterone begins to decline with age in men after the third decade and in women after menopause. Approximately 90% of men in their 80s have biochemical hypogonadism (testosterone or free testosterone <2.5th percentile for young men), as do 35% of men in their 60s.15 Age-related increases in sex hormone-binding globulin (SHBG) compound the effects of diminishing total testosterone synthesis. Thus, free testosterone decreases with aging proportionately faster than total testosterone.
Total testosterone includes protein-bound and unbound testosterone and is a good measure of testosterone synthesis (Box).15 Normal circulating total testosterone levels are:
- 325 to 1,000 ng/dL in men
- 25 to 90 ng/dL in women (approximately 10% of male levels).
Testosterone assays are usually insensitive in the lower concentration ranges. This makes establishing testosterone deficiency difficult in women.
When total testosterone level is equivocal or low, repeat total testosterone levels once or twice and measure free testosterone, which is the biologically active form. More than 95% of circulating testosterone is bound to plasma proteins, including SHBG and albumin. Also measure free testosterone during the initial screen when you suspect testosterone deficiency.
In cycling women, sex hormone concentrations spike during ovulation and are low when the follicular phase begins. Although longitudinal evaluation is more accurate, the more practical crosssectional screen (AMblood) in the late follicular or late luteal phase is usually adequate.
Evaluating women taking oral contraceptives is biochemically straightforward, as exogenous estrogen suppresses ovarian sex hormone production and induces steady testosterone concentrations.
Postmenopausal women can be screened for sex hormone concentrations on virtually any morning, although perimenopausal women (within 5 years of last menstrual period) are, like premenopausal women, best studied longitudinally. DHEA and DHEA-S concentrations are perhaps more important to measure in women than in men because these sex steroids are responsible for a comparatively much larger component of circulating testosterone in women.
Follow-up tests. If testosterone deficiency is established, measure circulating pituitary hormones LH, FSH, and prolactin to determine if hypogolnadism is primary (gonadal) or secondary to another abnormality (of the brain or pituitary):
- Elevated LH and/or FSH levels are seen in primary hypogonadism, as the pituitary attempts to compensate for poorly functioning or sluggish gonads by increasing their stimulation.
- Diminished or inappropriately normal LH levels during testosterone deficiency (when high levels should be seen) are consistent with central or secondary hypogonadism.
- A combination of primary and secondary hypogonadism is common with advanced age.
Measure serum prolactin concentrations when evaluating hypogonadism because hyperprolactinemia is a common cause.
If the patient is testosterone-deficient, also assess other endocrine systems. If one fails or becomes inflamed, other glands or hormone systems often show insufficiency or inflammation as well, perhaps because of a common pathologic process. Circulating testosterone levels may be normal or elevated in testosterone insensitivity or hyposensitivity syndromes.
Correcting deficiency
Testosterone deficiency can often be corrected without using androgens, such as by changing or supplementing a medication.
Hyperprolactinemia is a common cause of central hypogonadism and testosterone deficiency in psychiatric patients, often as an adverse effect of psychotropics (particularly antipsychotics). Hyperprolactinemia suppresses GnRH and, in turn, LH and gonadal synthesis of testosterone. Hyperprolactinemia depresses libido and causes infertility in both sexes and amenorrhea in women.
Medication changes can usually correct psychotropic-induced hyperprolactinemia. Elevated prolactin levels from other causes (such as a pituitary prolactinoma) usually respond to dopamine agonists such as bromocriptine or cabergoline.
Zinc deficiency can lower testosterone levels. Zinc is highly enriched in the testes and prostate, where it accumulates via a zinc uptake system. The cerebral cortex is also zinc-enriched.
Zinc’s recommended daily allowance (RDA) is 15 mg for men and 12 mg for women. Mild zinc deficiency is common, affecting, for example, about 30% of healthy older men in Detroit16 and many depressed patients.17
Remarkably, dietary zinc restriction (to one-third of the RDA) in healthy young men reduces serum testosterone levels by 75% after 5 to 6 months. Conversely, giving a zinc supplement, 30 mg/d, to marginally zinc-deficient older men nearly doubled their serum testosterone concentrations after 6 months.18
Because serum zinc concentrations do not reliably reflect zinc status, the most expedient clinical approach is to supplement with the RDA—found in widely available multivitamins. Zinc is generally considered low-risk for toxicity, although high doses should be avoided. Much is unknown about zinc’s role in the CNS, where it apparently can be neuroprotective or neurotoxic.
Androgen suppressants. Cholesterol-lowering agents—whether they inhibit cholesterol biosynthesis or absorption—can sometimes lower serum androgen levels. Included are antihyperlipidemic pharmaceuticals and plant sterols that compete with cholesterol for gut absorption. Plant sterols such as beta-sitosterol are marketed as cholesterol-lowering food supplements.
Volatile and fatty oils of the saw palmetto berry (Seranoa repens or Sabal serrulata)—a frequently used over-the-counter phytotherapy for benign prostatic hypertrophy—have antiandrogen properties. They inhibit 5-alpha reductase types I and II, reducing testosterone’s conversion to dihydrotestosterone.19 Flaxseed oil (linseed oil), another over-the-counter herbal supplement, also may alter testosterone levels.
Table 2
Recommended testosterone-replacement preparations
| Preparation | Usual dosage (men) |
|---|---|
| Transdermal patch (2.5 or 5 mg each) | 1 to 2 patch(es) applied daily |
| Gel | 5 to 10 mg/d (in 5 to 10 grams of gel, applied once daily) |
| Oral methyltestosterone | 10 to 200 mg/d |
| Testosterone enanthate IM injection | 50 to 400 mg every 2 weeks |
| Buccal testosterone adhesive | 60 to 90 mg/d |
| Sex hormone precursor | Usual dosage for testosterone replacement (women) |
| Oral DHEA | 25 to 50 mg once daily |
| DHEA: dehydroepiandrosterone | |
Exogenous glucocorticoids suppress DHEA release by negative feedback suppression of adrenocorticotropic hormone (ACTH) at the anterior pituitary. To protect against sex hormone deficiency, give DHEA in replacement doses whenever more than a few glucocorticoid doses are given. This applies particularly to postmenopausal women, in whom DHEA is the major source of circulating androgens.
Testosterone replacement
Preliminary data suggest that correcting testosterone deficiency in depressed men can have an antidepressant effect, especially in men who respond inadequately to standard antidepressants. Moreover, like antidepressants, testosterone replacement therapy can induce hypomania or mania in some individuals.
Depression and/or anxiety associated with sustained, irreversible serum testosterone deficiency—usually with other signs of testosterone deficiency (Table 1)—is the major psychiatric indication for testosterone replacement. Borderline biochemical testosterone deficiency and psychiatric symptoms in a “treatment-resistant” patient—especially one at risk for suicide—may justify an empirical testosterone replacement trial. Do not continue such a trial indefinitely without compelling reasons, however, because gonadal function recovery can be delayed for months after even a 12-week testosterone trial.20
Recommended agents for testosterone replacement are shown in Table 2. In men, testosterone preparations are normally used to increase testosterone levels. In women, I prescribe DHEA (discussed below). In young men and women with secondary hypogonadism, pulsatile use of gonadotropins may be necessary to induce spermatogenesis or ovulation—interventions outside the scope of psychiatric practice.
Contraindications to androgen replacement include hyperandrogenism, prostate cancer, antisocial personality, current mania, pedophilia, hypersexuality, and any psychiatric syndrome characterized by violent or predatory behavior. Pregnant patients (or women without a reliable birth control method) should not receive testosterone. Use caution when replacing androgens in patients with benign prostatic hypertrophy, hypomania, or a history of mania or hypomania.
An antidepressant response to adequate exogenous testosterone (enough to raise free testosterone levels to mid-normal range) is generally seen within 4 weeks. If psychological improvement is not observed, testosterone replacement may still prove beneficial if reversing hypogonadism improves the efficacy of subsequent antidepressants.
Dosage forms for men. Transdermal testosterone patches are normally applied to clean, dry skin on the upper arms, abdomen, thigh, or back and rotated among sites to avoid dermal irritation. When the nonscrotal patch is applied at night, testosterone concentrations mimic the circadian pattern seen in young men without causing supraphysiologic transients.21
Testosterone gel is applied every morning—also in a rotating manner—to clean, dry, intact skin and allowed to dry. Absorption is rapid, with measurable testosterone increases within 30 minutes. Approximately 10% of the testosterone is absorbed, delivering 5 to 10 mg/d into the circulation after 5 to 10 grams of gel (containing 50 to 100 mg of testosterone) is applied. Steady-state concentrations are achieved within 2 to 3 days, so dosages can be adjusted quickly.
Some patients regard 10 grams of gel as too messy to apply comfortably. Testosterone gel residuals can be washed from the skin with soap and water. Prolonged coated-skin contact with another person, such as a sex partner, can increase testosterone concentrations in the untreated individual.
Oral testosterone is absorbed poorly (often requiring high dosages) and cleared rapidly (half-life: 10 to 100 minutes). Only 10-mg capsules of methyltestosterone preparations are readily available—a dose too small for most men and too large for women. Many pharmacists can formulate other dosages for individual patients. Twice-daily doses are often used. Gum irritation and altered taste can occur when using buccal mucoadhesive testosterone.
Oil-based testosterone injections (such as IM testosteroneenanthate) are absorbedslowly and cannot reproduce normal circadian testosterone rhythms and concentrations. In some cases, however, the long-acting effectsof IM testosteroneare beneficial.
DHEA acutely increases testosterone and estrogens in both men and women after a single physiologic dose. During maintenance DHEA replacement, however, clinically significant increases in both sex hormones are seen only in women. DHEA is preferred to increase testosterone levels in women, as it is converted to appropriate proportions of androgens and estrogens by endogenous steroidogenic enzymes.
Table 3
Potential adverse effects of testosterone replacement therapy
|
|
|
|
DHEA, which occurs in yams, is available over-the-counter as a “food supplement” or “nutritional supplement.” However, many of these preparations, which are not regulated by the FDA, are unreliable because of poor quality control.22
Aromatase inhibitors were developed as antibreastcancer agents but also may treat testosterone deficiency. Testosterone administration increases circulating estrogens because testosterone is metabolized by the enzyme aromatase to estradiol. Aromatase inhibitors may prevent excessive estradiol levels—and associated adverse effects, such as gynecomastia—that are sometimes seen during testosterone replacement therapy in men. Available aromatase inhibitors include anastrozole, exemestane, and letrozole.
Potential adverse effects
Short-term testosterone replacement is generally low-risk. Acne is the most common adverse effect (Table 3).
The incidence of adverse events increases as testosterone concentrations are elevated above the normal range. For example, about 5% of men experience a manic or hypomanic arousal within 2 to 6 weeks of induced supraphysiologic testosterone levels.8
Gonadal suppression. Exogenous testosterone (or high-dose DHEA) suppresses endogenous gonadal function in men and premenopausal women. When a sustained course of exogenous androgens is discontinued, gonadal suppression usually does not reverse completely for several months or longer.
Prostatic hypertrophy, commonly considered to be testosterone driven, may be a risk of testosterone replacement therapy. Emergent urinary retention during testosterone replacement therapy has been reported, so use caution when giving testosterone to men with prostatic hypertrophy.
Barring evidence to the contrary, testosterone therapy is contraindicated in patients with prostate cancer. Baseline and post-treatment prostate-specific antigen measures are recommended.
Other risks in men. Men occasionally develop gynecomastia during testosterone replacement, perhaps because of testosterone aromatization to estradiol. Beyond increased hematocrit levels and associated problems, testosterone’s cardiovascular risks are unclear. Testosterone deficiency also has been linked to increased atherosclerosis risk in older men.23
Risks in women. Overtreating women with testosterone (DHEA) can promote hirsutism (including facial hair), loss of hair on scalp, voice lowering, clitoromegaly, breast regression, and muscle hypertrophy.
Related resources
- Daly RC, Su T-P, Schmidt PJ, et al. Cerebrospinal fluid and behavioral changes after methyltestosterone administration. Arch Gen Psychiatry 2001;58:172-7.
- Davis S. Testosterone deficiency in women.J Reprod Med2 001; 46(3 suppl):291-6.
- Rohr UD. The impact of testosterone imbalance on depression and women’s health. Maturitas 2002;41(1 suppl):S25-S46.
- Mantzoros CS, Georgiadis EI. Contribution of dihydrotestosterone to male sexual behaviour. BMJ 1995;310:1289-91.
Drug brand names
- Methyltestosterone (oral) • Android, Methitest, Testred, Virilon
- Testosterone (buccal) • Striant
- Testosterone (gel) • AndroGel, Testim
- Testosterone (transdermal) • Androderm, Testoderm
- Testosterone enanthate (IM injection) • Delatestryl
Disclosure
Dr. Geracioti reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Pope HG, Jr, Cohane GH, Kanayama G, et al. Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. Am J Psychiatry 2003;160:105-11.
2. Ehrenreich H, Halaris A, Ruether E, et al. Psychoendocrine sequelae of chronic testosterone deficiency. J Psychiatric Res 1999;33:379-87.
3. Schweiger U, Deuschle M, Weber B, et al. Testosterone, gonadotropin, and cortisol secretion in male patients with major depression. Psychosom Med 1999;61:292-6.
4. Seidman SN, Walsh BT. Testosterone and depression in aging men. Am J Geriatr Psychiatry 1999;7:18-33.
5. Mulchahey JJ, Ekhator NN, Zhang H, et al. Cerebrospinal fluid and plasma testosterone levels in post-traumatic stress disorder and tobacco dependence. Psychoneuroendocrinology 2001;26:273-85.
6. Shores MM, Sloan KL, Matsumoto AM, et al. Increased incidence of diagnosed depressive illness in hypogonadal older men. Arch Gen Psychiatry 2004;61:162-7.
7. Bachman G, Bancroft J, Braunstein G, et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertil Steril 2002;77:660-5.
8. Pope HG, Jr, Kouri EM, Hudson JI. Effects of supraphysiologic doses of testosterone on mood and aggression in normal men. A randomized controlled trial. Arch Gen Psychiatry 2000;57:133-40.
9. Matsumoto AM. The testis. In: Felig P, Frohman LA (eds). Endocrinology and metabolism (4th ed). New York: McGraw-Hill, 2001;635-705.
10. O’Carroll R, Shapiro C, Bancroft J. Androgens, behavior and nocturnal erection in hypogonadal men: the effects of varying the replacement dose. Clin Endocrinol 1985;23:527-38.
11. Wang C, Swerdloff R, Iranmanesh A, et al. and the Testosterone Gel Study Group. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab 2000;85:2839-53.
12. Seidman SN, Rabkin JG. Testosterone replacement therapy for hypogonadal men with SSRI-refractory depression. J Affect Disord 1998;48(2-3):157-61.
13. Goldstat R, Briganti E, Tran J, et al. Transdermal testosterone therapy improves well-being, mood, and sexual function in premenopausal women. Menopause 2003;10:390-8.
14. Smith KW, Feldman HA, McKinlay JB. Construction and field validation of self-administered screener for testosterone deficiency (hypogonadism) in ageing men. Clin Endocrinol 2000;53:703-11.
15. Harmon SM, Metter EJ, Tobin JD, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab 2001;86:724-31.
16. Prasad AS, Fitzgerald JT, Hess JW, et al. Zinc deficiency in elderly patients. Nutrition 1993;9:218-24.
17. Maes M, D’Haese PC, Scharpe S, et al. Hypozincemia in depression. J Affect Disord 1994;31:135-40.
18. Prasad AS, Mantzoros CS, Beck FW, et al. Zinc status and serum testosterone levels of healthy adults. Nutrition 1996;12:344-8.
19. Weisser H, Tunn S, Behnke B, Krieg M. Effects of the sabal serrulata extract IDS 89 and its subfractions on 5 alpha-reductase activity in human benign prostatic hyperplasia. Prostate 1996;28:300-6.
20. Forbes GB, Porta CR, Herr BE, Griggs RC. Sequence of changes in body composition induced by testosterone and reversal of changes after drug is stopped. JAMA 1992;267(3):397-9.
21. Meikle AW. Transdermal testosterone. Drugs 1998;55:259.-
22. Parasrampuria J, Schwartz K, Petesch R. Quality control of dehydroepiandrosterone dietary supplement products. JAMA 1998;280:1565.-
23. Hak AE, Witteman JC, de Jong FH, et al. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab 2002;87:3632-9.
1. Pope HG, Jr, Cohane GH, Kanayama G, et al. Testosterone gel supplementation for men with refractory depression: a randomized, placebo-controlled trial. Am J Psychiatry 2003;160:105-11.
2. Ehrenreich H, Halaris A, Ruether E, et al. Psychoendocrine sequelae of chronic testosterone deficiency. J Psychiatric Res 1999;33:379-87.
3. Schweiger U, Deuschle M, Weber B, et al. Testosterone, gonadotropin, and cortisol secretion in male patients with major depression. Psychosom Med 1999;61:292-6.
4. Seidman SN, Walsh BT. Testosterone and depression in aging men. Am J Geriatr Psychiatry 1999;7:18-33.
5. Mulchahey JJ, Ekhator NN, Zhang H, et al. Cerebrospinal fluid and plasma testosterone levels in post-traumatic stress disorder and tobacco dependence. Psychoneuroendocrinology 2001;26:273-85.
6. Shores MM, Sloan KL, Matsumoto AM, et al. Increased incidence of diagnosed depressive illness in hypogonadal older men. Arch Gen Psychiatry 2004;61:162-7.
7. Bachman G, Bancroft J, Braunstein G, et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertil Steril 2002;77:660-5.
8. Pope HG, Jr, Kouri EM, Hudson JI. Effects of supraphysiologic doses of testosterone on mood and aggression in normal men. A randomized controlled trial. Arch Gen Psychiatry 2000;57:133-40.
9. Matsumoto AM. The testis. In: Felig P, Frohman LA (eds). Endocrinology and metabolism (4th ed). New York: McGraw-Hill, 2001;635-705.
10. O’Carroll R, Shapiro C, Bancroft J. Androgens, behavior and nocturnal erection in hypogonadal men: the effects of varying the replacement dose. Clin Endocrinol 1985;23:527-38.
11. Wang C, Swerdloff R, Iranmanesh A, et al. and the Testosterone Gel Study Group. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab 2000;85:2839-53.
12. Seidman SN, Rabkin JG. Testosterone replacement therapy for hypogonadal men with SSRI-refractory depression. J Affect Disord 1998;48(2-3):157-61.
13. Goldstat R, Briganti E, Tran J, et al. Transdermal testosterone therapy improves well-being, mood, and sexual function in premenopausal women. Menopause 2003;10:390-8.
14. Smith KW, Feldman HA, McKinlay JB. Construction and field validation of self-administered screener for testosterone deficiency (hypogonadism) in ageing men. Clin Endocrinol 2000;53:703-11.
15. Harmon SM, Metter EJ, Tobin JD, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab 2001;86:724-31.
16. Prasad AS, Fitzgerald JT, Hess JW, et al. Zinc deficiency in elderly patients. Nutrition 1993;9:218-24.
17. Maes M, D’Haese PC, Scharpe S, et al. Hypozincemia in depression. J Affect Disord 1994;31:135-40.
18. Prasad AS, Mantzoros CS, Beck FW, et al. Zinc status and serum testosterone levels of healthy adults. Nutrition 1996;12:344-8.
19. Weisser H, Tunn S, Behnke B, Krieg M. Effects of the sabal serrulata extract IDS 89 and its subfractions on 5 alpha-reductase activity in human benign prostatic hyperplasia. Prostate 1996;28:300-6.
20. Forbes GB, Porta CR, Herr BE, Griggs RC. Sequence of changes in body composition induced by testosterone and reversal of changes after drug is stopped. JAMA 1992;267(3):397-9.
21. Meikle AW. Transdermal testosterone. Drugs 1998;55:259.-
22. Parasrampuria J, Schwartz K, Petesch R. Quality control of dehydroepiandrosterone dietary supplement products. JAMA 1998;280:1565.-
23. Hak AE, Witteman JC, de Jong FH, et al. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab 2002;87:3632-9.
Psychological first aid: Emergency care for terrorism and disaster survivors
The night started like any other for Dr. Z. Kids in bed (too late) by 10:30, dog out by 11, fell asleep reading journal by 11:15. Sirens jolt her out of a solid stage 4. Her eyes widen, pulse quickens, mouth dries as she follows the glow of the TV into the living room. On TV, fire frames shots of tearful faces, body bags, and firefighters in protective suits. She catches the announcer’s voice: “…explosion at City Power and Light nuclear facility at 3:10 AM today. Fifty-five are known dead, and thousands are being told to evacuate. The blast’s cause is unknown, but terrorism is suspected.”
As her numbness slowly ebbs, Dr. Z’s questions rise insistently. How can I help the survivors? My patients? My children? What if the media call me? How could I have been better prepared for this?
Disaster shakes us to our human and biological core. More than any other clinical encounter, it reminds us that psychiatrists share the vulnerabilities of those we seek to help. Yet it also reminds us that even simple concepts and interventions can mobilize the healing process.
Are you ready to provide emergency psychiatric care following a disaster in your community—be it a nuclear accident, tornado, airplane crash, or terrorist act? Here is evidence—some counterintuitive—that can help you prepare.
Table 1
‘Psychological first aid’ for disaster survivors
Re-create sense of safety
|
Encourage social support
|
Re-establish sense of efficacy
|
Disaster’s psychobiology
Human reactions to disaster are often conceptualized as the mammalian survival response: flight, fight, and fright (freezing). In most cases, these reactions are adaptive and dissipate after safety is restored. Posttraumatic stress disorder (PTSD) develops in about 5% of natural disaster victims and 33% of mass shooting victims.1
Responses that do go awry appear to be associated with abnormally low cortisol and persistent adrenergic activation, leading to sensitization of the fear response.2 Reminders of trauma or persistent stressors—such as pain, problems with finances or housing, or bereavement—may exacerbate sensitization. On the other hand, preclinical studies suggest that social support3,4 and active coping5 mitigate physiologic stress responses, confirming numerous clinical observations that associate lack of social support and avoidant coping with eventual PTSD development.
Three basics. Just as our emergency medicine colleagues must often revert to life-support basics, we must remind ourselves of biology’s three basics for psychic resilience:
- safety (including—as much as possible—protection from reminders of trauma and ongoing stress)
- meaningful social connection
- re-establishing a sense of efficacy to overcome helplessness (Table 1).
Like the stress response, these protective factors seem hard-wired into our biological make-up. They form the foundation for all phases of disaster psychiatry interventions, from planning to immediate interventions through longterm follow-up.
Disaster planning
As a mental health professional, plan to operate within established disaster plans and agencies, not only for the sake of efficiency but also because structure and support are paramount in disaster situations. Check with the American Psychiatric Association’s local branch to determine if a disaster mental health plan exists. If not, explore how to work directly with local American Red Cross chapters and hospitals, which recruit personnel for the Disaster Medical Assistance Teams mobilized by the public health service.
Table 2
Normal reactions to disaster for adults and children
| All ages | |
| Emotional | Shock, fear, grief, anger, guilt, shame, helplessness, hopelessness, numbness, emptiness Decreased ability to feel interest, pleasure, love |
| Cognitive | Confusion, disorientation, indecisiveness, worry, shortened attention span, poor concentration, memory difficulties, unwanted memories, self-blame |
| Physical | Tension, fatigue, edginess, insomnia, generalized aches and pains, startling easily, rapid heartbeat, nausea, decreased appetite and sex drive |
| Interpersonal | Difficulties being intimate, being over-controlling, feeling rejected or abandoned |
| Children’s age-specific disaster responses | |
| Preschool | Separation fears, regression, fussiness, temper tantrums, somatization Sleep disturbances including nightmares, somnambulism, night terrors |
| School-age | May still have the above, as well as excessive guilt and worries about others’ safety, poor concentration and loss of school performance, repetitious re-telling or play related to trauma |
| Adolescent | Depression, acting out, wish for revenge, sleeping and eating disturbances, altered view of the future |
Immediate disaster mental health plans vary in detail according to local needs and resources but should at least address:
- providing on-site interventions
- disseminating information about responses to trauma
- identifying and publicizing local mental health resources.
Immediate interventions
Immediately following a disaster, psychiatrists are frequently asked to assist with on-site crisis and medical interventions, evaluate survivors with unusual or intense reactions, and provide public education about psychological reactions to disaster.
On-site response. All responders, regardless of discipline, should provide disaster survivors with “psychological first aid,” which is directed at reestablishing safety, connection, and efficacy. Basic crisis intervention principles are useful when support and reassurance are not enough.6
For example, relaxation exercises can reduce anxiety and improve sleep. Use focused, structured relaxation tools—such as progressive muscle relaxation and breathing training—as unstructured exercises can exacerbate dissociation and re-experiencing. Grounding techniques, by which survivors learn to focus all senses on immediate surroundings, often alleviate dissociation and flashbacks.
Care for children. Because children’s reactions to disaster greatly depend on their caregivers’ responses (social referencing), focus acute interventions for children on:
- re-connecting them with their families
- reducing caregivers’ distress
- educating caregivers about providing age appropriate information and support (see Related resources).
Medical care. As physicians, psychiatrists may be called upon to intervene medically. Although it is generally advisable to stay within our usual practice, medical personnel may be in short supply. Fortunately, Good Samaritan laws exist in every state, and the potential for a successful malpractice suit against a physician responding in a disaster is almost zero, unless the physician’s performance is grossly negligent (such as moving the neck of a patient with obvious head or neck injuries).7
Principles regarding informed consent and right to refuse treatment—along with the usual exceptions—apply during disasters.
Evaluating survivors in shelters and hospitals requires knowing the normal and abnormal responses to disaster, being able to differentially diagnose changes in mental status, and understanding risk factors for trauma’s psychiatric sequelae. Aside from PTSD, trauma’s long-term effects include other anxiety disorders, depression, substance abuse, and eating disorders. In addition to the usual components of a psychiatric evaluation, assessments must address event-related factors such as proximity to the disaster, loss of significant others or property, physical injuries, immediate needs, and social support.8
Normal stress reactions. Frequently described as “a normal response to an abnormal situation,” the normal stress reaction is multidimensional and depends on the person’s developmental level (Table 2). About 10% to 25% of survivors experience intense affect and dissociation, whereas a similar number may appear unusually calm.
Interventions beyond the“first aid” described above are not usually needed unless individuals:
- are a danger to themselves or others
- are psychotic
- have no social supports
- cannot perform tasks necessary for self-care and to begin the recovery process.
Always re-assess when there is any question about a survivor’s immediate reaction to trauma.
In DSM-IV-TR’s trauma-related diagnoses, the symptom clusters often do not capture many disaster survivors’ subjective experience: the shattering of fundamental beliefs regarding themselves (invulnerability), the world (predictability, safety), and others (trust, benevolence).9 By empathizing with these responses, you can help survivors feel less isolated and estranged.
Differential diagnosis. Survivors’ mental status changes may be manifestations of the stress response, but they also may represent:
- exacerbations of pre-existing psychiatric or general medical conditions
- hypoxemia, hypovolemia, or CNS trauma from physical injury
- responses to medications used for resuscitation or pain control, such as atropine, epinephrine, lidocaine, or morphine.
Effects of bioterrorism agents must also be considered. For example, organophosphorus compounds such as the nerve agents sarin and soman can cause impaired concentration, depression, and anxiety. Anthrax can cause rapidly progressing meningitis.10 Delirium must be differentiated from dissociation; patients with dissociation can be re-oriented, and changes will resolve with time rather than fluctuate.11
Psychopathology risk factors. Multiple studies have addressed risk factors for post-disaster psychiatric sequelae (usually PTSD). In general, risk increases with repeated trauma exposure (including TV viewing), prior trauma, lack of social support, injury, pre-existing psychiatric problems, traumatic bereavement (having witnessed the violent death of a loved one), avoidant coping, and having strong negative beliefs about the meanings of normal stress reactions such as tearfulness, anxiety, and insomnia.
Because a recent meta-analysis supports these observations,12 follow-up evaluation for signs of PTSD is recommended for:
- survivors with one or more of the risk factors discussed above
- vulnerable groups such as rescue workers, children, and dependent individuals
- survivors whose symptoms persist after 2 months.13
Decompensation immediately after a disaster seems to be the exception for psychiatric patients, despite their longer-term vulnerability. One psychiatrist who in 1989 survived Hurricane Hugo—the most intense storm to strike the Mid-Atlantic region in 100 years—noted that demand for inpatient psychiatric services did not increase in the storm’s aftermath. The only patient calls she received were inquiries about her own physical safety.14
Caregivers and rescue workers—including psychi-atrists—are also disaster survivors, and you need to tend to your needs for safety and support. Consult frequently with colleagues within and outside the disaster area, as much for support as for information and guidance.15 Remember also that rescue workers are occasionally targets for victims’ rage at their circumstances. Anticipating and explaining this displacement reduces its toxicity.
Using medications
Uses psychotropics judiciously in the first 48 hours of trauma. Medication effects may interfere with neurologic assessment of the injured, and monitoring and follow-up may not be possible.
However, drug therapy should start quickly when survivors are acutely psychotic or their behavior endangers themselves, others, or the milieu. Medications usually include a fast-acting benzodiazepine and/or an antipsychotic, as described in guidelines for managing agitation.16 Always provide structure and supervision for medicated patients.
No guidelines exist for using medications to manage distressing—but less-severe—acute stress-related symptoms. Some experts advocate using adrenergic antagonists such as clonidine, guanfacine, and beta blockers to reduce excessive arousal. These drugs have not been adequately studied in this setting, however, and may harm those with cardiovascular instability from preexisting conditions or injuries.
Table 3
Psychoeducation: Simple messages for workers and trauma survivors
| Get adequate rest, food, sleep |
| Avoid exposure to trauma cues, including TV images |
| Seek support from loved ones and peers |
| Talk about events and feelings only if this feels comfortable and helpful |
| Return to normal routine as much as possible |
| Take action to rebuild, but at a reasonable pace |
| Reach out to others who may need assistance |
Get help:
|
Short-term (<1 week) benzodiazepine use for panic symptoms and severe insomnia is acceptable, but longer-term use may increase PTSD risk.17 A selective serotonin reuptake inhibitor may help individuals with pre-existing PTSD or depression, if you can arrange follow-up.
In the aftermath
‘Debriefing.’ Critical incident stress debriefing (CISD) is a structured, one-session group intervention in which survivors’ experiences and emotional reactions are discussed and education and follow-up recommendations are provided. Developed by Mitchell in 1983,18 CISD was widely used until systematic evaluations revealed that it did not alleviate psychological distress or prevent PTSD.19
Table 4
How to interact with news media during and after a disaster
| For organizations Identify a spokesperson with media experience beforehand Ensure that the spokesperson is well-informed about all aspects of the disaster |
| For spokespersons |
Always
|
Never
|
Thus, although survivor meetings may provide information, education, screening, and support, avoid detailed discussions of events and emotions. Any meetings should be conducted by mental health clinicians and should not be mandatory. Reserve the term “debriefing” for operational reviews by rescue personnel.20
Public education. Educate survivors, rescue workers, health care providers, teachers, and relief agency workers. Provide concise, simple messages as suggested in Table 3. News media provide our most effective means of reaching out to survivors, which is why having a pre-existing relationship is so important. Some guidelines for working with the media are presented in Table 4.
Outreach. Numerous educational resources are available for survivors and their caregivers (see Related resources). Other potentially useful outreach tools include:
- meetings with teachers’ organizations
- continuing medical education activities for primary care providers
- telephone hot lines.
Legal and ethical issues
Disaster scenes are chaotic and informal, and professionals must be flexible, often providing general support and information rather than specific clinical interventions. However, it is important in each encounter to decide whether a patient-physician relationship has begun.
As a general rule, a physician-patient relationship is established whenever diagnosis or treatment is discussed. Once that happens, briefly document:
- signs and symptoms
- working diagnosis
- suicide or violence potential
- treatment and/or follow-up plans.
Confidentiality may be difficult to preserve in chaotic situations involving workers from many agencies. Even in disasters, however, you must obtain permission before sharing information unless the individual’s situation is emergent.
Table 5
Keys to effective disaster psychiatry
Be prepared
|
Care for survivors
|
Care for yourself
|
Long-term interventions
Longer-term disaster interventions include continued outreach and education and needed follow-up services. Existing structures may provide effective follow-up, but additional resources are often needed.
Federal programs. Following a presidentialdeclared disaster, the Federal Emergency Management Agency (FEMA) provides funding for crisis counseling. Programs are typically funded for 9 to 15 months and administered through the emergency services and disaster relief branch of the Substance Abuse and Mental Health Services Administration (SAMHSA) and community mental health organizations. Examples include Project Heartland following the 1995 Oklahoma City federal building bombing and Project COPE following California’s 1989 Loma Prieta earthquake.
Cognitive-behavioral therapy. For adult survivors with acute stress disorder, specific cognitive-behavioral therapy (CBT) provided by trained therapists may prevent PTSD and other trauma sequelae, such as depression.21 CBT interventions may begin as early as 2 weeks after trauma and focus sequentially on anxiety management, cognitive restructuring, imaginal exposure followed by in vivo exposure, and relapse prevention.
Three controlled trials found 6-month PTSD rates of 14% to 20% among acute stress disorder patients treated with CBT, compared with 58% to 67% with supportive counseling.22-24 Although studies of interventions immediately following trauma are lacking, trauma-focused CBT is also recommended for children.25 Evidence-based treatments for PTSD are discussed in detail elsewhere.26
Not unprepared after all
With some reflection, Dr. Z realized she had the tools to help her community. Her feelings of helplessness receded as she envisioned how she could help survivors understand their experiences, re-create a sense of safety, restore important connections to loved ones, and begin to rebuild their lives (Table 5).
For clinicians
- Young BH, Ford JD, Ruzek JI, et al. Disaster mental health: a guidebook for clinicians and administrators. Washington, DC: National Center for Post-Traumatic Stress Disorder, 1998. http://ncptsd.org/publications/disaster/index.html
- Hillman JL. Crisis intervention and trauma: new approaches to evidence-based practice. New York: Kluwer Academic/Plenum Publishers, 2002.
- Office of the Surgeon General Web site on medical aspects of nuclear, biological, and chemical warfare. http://www.nbc-med.org
For survivors and clinicians
- National Center for Post-Traumatic Stress Disorder. Disaster mental health: Dealing with the aftereffects of terrorism. http://www.ncptsd.org/terrorism/index.html
- Substance Abuse and Mental Health Services Administration. Emergency mental health and traumatic stress. http://www.mentalhealth.org/cmhs/EmergencyServices/default.asp
- American Academy of Child and Adolescent Psychiatry. Talking to children about terrorism and war. http://www.aacap.org/publications/factsFam/87.htm
Drug brand names
- Clonidine • Catapres
- Guanfacine • Tenex
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Effects of traumatic stress in a disaster situation. Washington, DC: National Center for Post-Traumatic Stress Disorder. Department of Veterans Affairs, September 12, 2001 (Accessed March 31, 2004 at http://www.ncptsd.org/facts/disasters/fs_effects_disaster.html).
2. Yehuda R, McFarlane AC, Shalev AY. Predicting the development of post-traumatic stress disorder from the acute response to a traumatic event. Biol Psychiatry 1998;44:1305-13.
3. Levine S, Lyons DM, Schatzberg AF. Psychobiological consequences of social relationships. Ann NY Acad Sci 1997;807:210-18.
4. Uchino BN, Garvey TS. The availability of social support reduces cardiovascular reactivity to acute psychological stress. J Behav Med 1997;20:15-27.
5. LeDoux JE, Gorman JM. A call to action: overcoming anxiety through active coping. Am J Psychiatry 2001;158:1953-5.
6. Hillman JL. Crisis intervention and trauma: new approaches to evidence-based practice. New York: Kluwer Academic/Plenum Publishers, 2002.
7. Daniels G. Good Samaritan acts. Emerg Med Clin North Am 1999;17:491-504.
8. Mental-health intervention for disasters. Washington, DC: National Center for Post-Traumatic Stress Disorder, Department of Veterans Affairs, Sept. 12, 2001. (Accessed March 31, 2004 at http://www.ncptsd.org/facts/disasters/fs_treatment_disaster.html.)
9. Difede J, Apfeldorf WJ, Cloitre M, et al. Acute psychiatric responses to the explosion at the World Trade Center: a case series. J Nerv Ment Dis 1997;185:519-22.
10. DiGiovanni C. Domestic terrorism with chemical or biological agents: psychiatric aspects. Am J Psychiatry 1999;156:1500-5.
11. Rundle JR. Psychiatric issues in medical-surgical disaster casualties: consultation-liaison approach. Psychiatr Q 2000;71:245-58.
12. Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for post-traumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol 2000;68:748-66.
13. Mental health and mass violence: evidence-based early psychological intervention for victims/survivors of mass violence. Washington, DC: National Institute of Mental Health, Nov. 1, 2002 (Accessed Oct. 29, 2003 at http://www.nimh.nih.gov/research/massviolence.pdf.)
14. Austin LS. Organizing a disaster response program in one’s home community. In: Austin LS (ed). Responding to disaster: a guide for mental health professionals. Washington, DC: American Psychiatric Publishing, 1992;53-68.
15. Rousseau AW. Notes from Oklahoma City’s recovery. In: Hall RCW, Norwood AE (eds). Disaster psychiatry handbook. Washington, DC: American Psychiatric Association Committee on Psychiatric Dimensions of Disaster, undated:3-11. (Accessed Oct. 29, 2003 at http://www.psych.org/disasterpsych/pdfs/apadisasterhandbk.pdf.)
16. Yildiz A, Sachs GS, Turgay A. Pharmacological management of agitation in emergency settings. Emerg Med J 2003;20:339-46.
17. Gelpin E, Bonne O, Peri T, et al. Treatment of recent trauma survivors with benzodiazepines: a prospective study. J Clin Psychiatry 1996;57:390-4.
18. Mitchell JT. When disaster strikes. J Em Med Serv 1983;8:36-9.
19. Rose S, Bisson J, Wessely S. Psychological debriefing for preventing posttraumatic stress disorder (PTSD). Cochrane Database Syst Rev 2002;(2):CD000560.-
20. Black J. Forming the libidinal cocoon: the Dallas airplane crashes, the Guadalupe river drownings, and Hurricane Hugo in the Virgin Islands. In: Austin LS (ed). Responding to disaster: a guide for mental health professionals. Washington DC: American Psychiatric Publishing, 1992;169:84.-
21. Ehlers A, Clark DM. Early psychological interventions for adult survivors of trauma: a review. Biol Psychiatry 2003;53:817-26.
22. Bryant RA, Moulds M, Guthrie R, Nixon RD. Treating acute stress disorder following mild traumatic brain injury. Am J Psychiatry 2003;160:585-7.
23. Bryant RA, Sackville T, Dang ST, et al. Treating acute stress disorder: an evaluation of cognitive behavior therapy and supportive counseling techniques. Am J Psychiatry 1999;156:1780-6.
24. Bryant RA, Harvey AG, Dang ST, et al. Treatment of acute stress disorder: a comparison of cognitive-behavioral therapy and supportive counseling. J Consult Clin Psychol 1998;66:862-6.
25. Cohen JA. Treating acute posttraumatic reactions in children and adolescents. Biol Psychiatry 2003;53:827-33.
26. Foa EB, Keane TM, Friedman MJ. Effective treatments for PTSD. New York: Guilford Press, 2000.
The night started like any other for Dr. Z. Kids in bed (too late) by 10:30, dog out by 11, fell asleep reading journal by 11:15. Sirens jolt her out of a solid stage 4. Her eyes widen, pulse quickens, mouth dries as she follows the glow of the TV into the living room. On TV, fire frames shots of tearful faces, body bags, and firefighters in protective suits. She catches the announcer’s voice: “…explosion at City Power and Light nuclear facility at 3:10 AM today. Fifty-five are known dead, and thousands are being told to evacuate. The blast’s cause is unknown, but terrorism is suspected.”
As her numbness slowly ebbs, Dr. Z’s questions rise insistently. How can I help the survivors? My patients? My children? What if the media call me? How could I have been better prepared for this?
Disaster shakes us to our human and biological core. More than any other clinical encounter, it reminds us that psychiatrists share the vulnerabilities of those we seek to help. Yet it also reminds us that even simple concepts and interventions can mobilize the healing process.
Are you ready to provide emergency psychiatric care following a disaster in your community—be it a nuclear accident, tornado, airplane crash, or terrorist act? Here is evidence—some counterintuitive—that can help you prepare.
Table 1
‘Psychological first aid’ for disaster survivors
Re-create sense of safety
|
Encourage social support
|
Re-establish sense of efficacy
|
Disaster’s psychobiology
Human reactions to disaster are often conceptualized as the mammalian survival response: flight, fight, and fright (freezing). In most cases, these reactions are adaptive and dissipate after safety is restored. Posttraumatic stress disorder (PTSD) develops in about 5% of natural disaster victims and 33% of mass shooting victims.1
Responses that do go awry appear to be associated with abnormally low cortisol and persistent adrenergic activation, leading to sensitization of the fear response.2 Reminders of trauma or persistent stressors—such as pain, problems with finances or housing, or bereavement—may exacerbate sensitization. On the other hand, preclinical studies suggest that social support3,4 and active coping5 mitigate physiologic stress responses, confirming numerous clinical observations that associate lack of social support and avoidant coping with eventual PTSD development.
Three basics. Just as our emergency medicine colleagues must often revert to life-support basics, we must remind ourselves of biology’s three basics for psychic resilience:
- safety (including—as much as possible—protection from reminders of trauma and ongoing stress)
- meaningful social connection
- re-establishing a sense of efficacy to overcome helplessness (Table 1).
Like the stress response, these protective factors seem hard-wired into our biological make-up. They form the foundation for all phases of disaster psychiatry interventions, from planning to immediate interventions through longterm follow-up.
Disaster planning
As a mental health professional, plan to operate within established disaster plans and agencies, not only for the sake of efficiency but also because structure and support are paramount in disaster situations. Check with the American Psychiatric Association’s local branch to determine if a disaster mental health plan exists. If not, explore how to work directly with local American Red Cross chapters and hospitals, which recruit personnel for the Disaster Medical Assistance Teams mobilized by the public health service.
Table 2
Normal reactions to disaster for adults and children
| All ages | |
| Emotional | Shock, fear, grief, anger, guilt, shame, helplessness, hopelessness, numbness, emptiness Decreased ability to feel interest, pleasure, love |
| Cognitive | Confusion, disorientation, indecisiveness, worry, shortened attention span, poor concentration, memory difficulties, unwanted memories, self-blame |
| Physical | Tension, fatigue, edginess, insomnia, generalized aches and pains, startling easily, rapid heartbeat, nausea, decreased appetite and sex drive |
| Interpersonal | Difficulties being intimate, being over-controlling, feeling rejected or abandoned |
| Children’s age-specific disaster responses | |
| Preschool | Separation fears, regression, fussiness, temper tantrums, somatization Sleep disturbances including nightmares, somnambulism, night terrors |
| School-age | May still have the above, as well as excessive guilt and worries about others’ safety, poor concentration and loss of school performance, repetitious re-telling or play related to trauma |
| Adolescent | Depression, acting out, wish for revenge, sleeping and eating disturbances, altered view of the future |
Immediate disaster mental health plans vary in detail according to local needs and resources but should at least address:
- providing on-site interventions
- disseminating information about responses to trauma
- identifying and publicizing local mental health resources.
Immediate interventions
Immediately following a disaster, psychiatrists are frequently asked to assist with on-site crisis and medical interventions, evaluate survivors with unusual or intense reactions, and provide public education about psychological reactions to disaster.
On-site response. All responders, regardless of discipline, should provide disaster survivors with “psychological first aid,” which is directed at reestablishing safety, connection, and efficacy. Basic crisis intervention principles are useful when support and reassurance are not enough.6
For example, relaxation exercises can reduce anxiety and improve sleep. Use focused, structured relaxation tools—such as progressive muscle relaxation and breathing training—as unstructured exercises can exacerbate dissociation and re-experiencing. Grounding techniques, by which survivors learn to focus all senses on immediate surroundings, often alleviate dissociation and flashbacks.
Care for children. Because children’s reactions to disaster greatly depend on their caregivers’ responses (social referencing), focus acute interventions for children on:
- re-connecting them with their families
- reducing caregivers’ distress
- educating caregivers about providing age appropriate information and support (see Related resources).
Medical care. As physicians, psychiatrists may be called upon to intervene medically. Although it is generally advisable to stay within our usual practice, medical personnel may be in short supply. Fortunately, Good Samaritan laws exist in every state, and the potential for a successful malpractice suit against a physician responding in a disaster is almost zero, unless the physician’s performance is grossly negligent (such as moving the neck of a patient with obvious head or neck injuries).7
Principles regarding informed consent and right to refuse treatment—along with the usual exceptions—apply during disasters.
Evaluating survivors in shelters and hospitals requires knowing the normal and abnormal responses to disaster, being able to differentially diagnose changes in mental status, and understanding risk factors for trauma’s psychiatric sequelae. Aside from PTSD, trauma’s long-term effects include other anxiety disorders, depression, substance abuse, and eating disorders. In addition to the usual components of a psychiatric evaluation, assessments must address event-related factors such as proximity to the disaster, loss of significant others or property, physical injuries, immediate needs, and social support.8
Normal stress reactions. Frequently described as “a normal response to an abnormal situation,” the normal stress reaction is multidimensional and depends on the person’s developmental level (Table 2). About 10% to 25% of survivors experience intense affect and dissociation, whereas a similar number may appear unusually calm.
Interventions beyond the“first aid” described above are not usually needed unless individuals:
- are a danger to themselves or others
- are psychotic
- have no social supports
- cannot perform tasks necessary for self-care and to begin the recovery process.
Always re-assess when there is any question about a survivor’s immediate reaction to trauma.
In DSM-IV-TR’s trauma-related diagnoses, the symptom clusters often do not capture many disaster survivors’ subjective experience: the shattering of fundamental beliefs regarding themselves (invulnerability), the world (predictability, safety), and others (trust, benevolence).9 By empathizing with these responses, you can help survivors feel less isolated and estranged.
Differential diagnosis. Survivors’ mental status changes may be manifestations of the stress response, but they also may represent:
- exacerbations of pre-existing psychiatric or general medical conditions
- hypoxemia, hypovolemia, or CNS trauma from physical injury
- responses to medications used for resuscitation or pain control, such as atropine, epinephrine, lidocaine, or morphine.
Effects of bioterrorism agents must also be considered. For example, organophosphorus compounds such as the nerve agents sarin and soman can cause impaired concentration, depression, and anxiety. Anthrax can cause rapidly progressing meningitis.10 Delirium must be differentiated from dissociation; patients with dissociation can be re-oriented, and changes will resolve with time rather than fluctuate.11
Psychopathology risk factors. Multiple studies have addressed risk factors for post-disaster psychiatric sequelae (usually PTSD). In general, risk increases with repeated trauma exposure (including TV viewing), prior trauma, lack of social support, injury, pre-existing psychiatric problems, traumatic bereavement (having witnessed the violent death of a loved one), avoidant coping, and having strong negative beliefs about the meanings of normal stress reactions such as tearfulness, anxiety, and insomnia.
Because a recent meta-analysis supports these observations,12 follow-up evaluation for signs of PTSD is recommended for:
- survivors with one or more of the risk factors discussed above
- vulnerable groups such as rescue workers, children, and dependent individuals
- survivors whose symptoms persist after 2 months.13
Decompensation immediately after a disaster seems to be the exception for psychiatric patients, despite their longer-term vulnerability. One psychiatrist who in 1989 survived Hurricane Hugo—the most intense storm to strike the Mid-Atlantic region in 100 years—noted that demand for inpatient psychiatric services did not increase in the storm’s aftermath. The only patient calls she received were inquiries about her own physical safety.14
Caregivers and rescue workers—including psychi-atrists—are also disaster survivors, and you need to tend to your needs for safety and support. Consult frequently with colleagues within and outside the disaster area, as much for support as for information and guidance.15 Remember also that rescue workers are occasionally targets for victims’ rage at their circumstances. Anticipating and explaining this displacement reduces its toxicity.
Using medications
Uses psychotropics judiciously in the first 48 hours of trauma. Medication effects may interfere with neurologic assessment of the injured, and monitoring and follow-up may not be possible.
However, drug therapy should start quickly when survivors are acutely psychotic or their behavior endangers themselves, others, or the milieu. Medications usually include a fast-acting benzodiazepine and/or an antipsychotic, as described in guidelines for managing agitation.16 Always provide structure and supervision for medicated patients.
No guidelines exist for using medications to manage distressing—but less-severe—acute stress-related symptoms. Some experts advocate using adrenergic antagonists such as clonidine, guanfacine, and beta blockers to reduce excessive arousal. These drugs have not been adequately studied in this setting, however, and may harm those with cardiovascular instability from preexisting conditions or injuries.
Table 3
Psychoeducation: Simple messages for workers and trauma survivors
| Get adequate rest, food, sleep |
| Avoid exposure to trauma cues, including TV images |
| Seek support from loved ones and peers |
| Talk about events and feelings only if this feels comfortable and helpful |
| Return to normal routine as much as possible |
| Take action to rebuild, but at a reasonable pace |
| Reach out to others who may need assistance |
Get help:
|
Short-term (<1 week) benzodiazepine use for panic symptoms and severe insomnia is acceptable, but longer-term use may increase PTSD risk.17 A selective serotonin reuptake inhibitor may help individuals with pre-existing PTSD or depression, if you can arrange follow-up.
In the aftermath
‘Debriefing.’ Critical incident stress debriefing (CISD) is a structured, one-session group intervention in which survivors’ experiences and emotional reactions are discussed and education and follow-up recommendations are provided. Developed by Mitchell in 1983,18 CISD was widely used until systematic evaluations revealed that it did not alleviate psychological distress or prevent PTSD.19
Table 4
How to interact with news media during and after a disaster
| For organizations Identify a spokesperson with media experience beforehand Ensure that the spokesperson is well-informed about all aspects of the disaster |
| For spokespersons |
Always
|
Never
|
Thus, although survivor meetings may provide information, education, screening, and support, avoid detailed discussions of events and emotions. Any meetings should be conducted by mental health clinicians and should not be mandatory. Reserve the term “debriefing” for operational reviews by rescue personnel.20
Public education. Educate survivors, rescue workers, health care providers, teachers, and relief agency workers. Provide concise, simple messages as suggested in Table 3. News media provide our most effective means of reaching out to survivors, which is why having a pre-existing relationship is so important. Some guidelines for working with the media are presented in Table 4.
Outreach. Numerous educational resources are available for survivors and their caregivers (see Related resources). Other potentially useful outreach tools include:
- meetings with teachers’ organizations
- continuing medical education activities for primary care providers
- telephone hot lines.
Legal and ethical issues
Disaster scenes are chaotic and informal, and professionals must be flexible, often providing general support and information rather than specific clinical interventions. However, it is important in each encounter to decide whether a patient-physician relationship has begun.
As a general rule, a physician-patient relationship is established whenever diagnosis or treatment is discussed. Once that happens, briefly document:
- signs and symptoms
- working diagnosis
- suicide or violence potential
- treatment and/or follow-up plans.
Confidentiality may be difficult to preserve in chaotic situations involving workers from many agencies. Even in disasters, however, you must obtain permission before sharing information unless the individual’s situation is emergent.
Table 5
Keys to effective disaster psychiatry
Be prepared
|
Care for survivors
|
Care for yourself
|
Long-term interventions
Longer-term disaster interventions include continued outreach and education and needed follow-up services. Existing structures may provide effective follow-up, but additional resources are often needed.
Federal programs. Following a presidentialdeclared disaster, the Federal Emergency Management Agency (FEMA) provides funding for crisis counseling. Programs are typically funded for 9 to 15 months and administered through the emergency services and disaster relief branch of the Substance Abuse and Mental Health Services Administration (SAMHSA) and community mental health organizations. Examples include Project Heartland following the 1995 Oklahoma City federal building bombing and Project COPE following California’s 1989 Loma Prieta earthquake.
Cognitive-behavioral therapy. For adult survivors with acute stress disorder, specific cognitive-behavioral therapy (CBT) provided by trained therapists may prevent PTSD and other trauma sequelae, such as depression.21 CBT interventions may begin as early as 2 weeks after trauma and focus sequentially on anxiety management, cognitive restructuring, imaginal exposure followed by in vivo exposure, and relapse prevention.
Three controlled trials found 6-month PTSD rates of 14% to 20% among acute stress disorder patients treated with CBT, compared with 58% to 67% with supportive counseling.22-24 Although studies of interventions immediately following trauma are lacking, trauma-focused CBT is also recommended for children.25 Evidence-based treatments for PTSD are discussed in detail elsewhere.26
Not unprepared after all
With some reflection, Dr. Z realized she had the tools to help her community. Her feelings of helplessness receded as she envisioned how she could help survivors understand their experiences, re-create a sense of safety, restore important connections to loved ones, and begin to rebuild their lives (Table 5).
For clinicians
- Young BH, Ford JD, Ruzek JI, et al. Disaster mental health: a guidebook for clinicians and administrators. Washington, DC: National Center for Post-Traumatic Stress Disorder, 1998. http://ncptsd.org/publications/disaster/index.html
- Hillman JL. Crisis intervention and trauma: new approaches to evidence-based practice. New York: Kluwer Academic/Plenum Publishers, 2002.
- Office of the Surgeon General Web site on medical aspects of nuclear, biological, and chemical warfare. http://www.nbc-med.org
For survivors and clinicians
- National Center for Post-Traumatic Stress Disorder. Disaster mental health: Dealing with the aftereffects of terrorism. http://www.ncptsd.org/terrorism/index.html
- Substance Abuse and Mental Health Services Administration. Emergency mental health and traumatic stress. http://www.mentalhealth.org/cmhs/EmergencyServices/default.asp
- American Academy of Child and Adolescent Psychiatry. Talking to children about terrorism and war. http://www.aacap.org/publications/factsFam/87.htm
Drug brand names
- Clonidine • Catapres
- Guanfacine • Tenex
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
The night started like any other for Dr. Z. Kids in bed (too late) by 10:30, dog out by 11, fell asleep reading journal by 11:15. Sirens jolt her out of a solid stage 4. Her eyes widen, pulse quickens, mouth dries as she follows the glow of the TV into the living room. On TV, fire frames shots of tearful faces, body bags, and firefighters in protective suits. She catches the announcer’s voice: “…explosion at City Power and Light nuclear facility at 3:10 AM today. Fifty-five are known dead, and thousands are being told to evacuate. The blast’s cause is unknown, but terrorism is suspected.”
As her numbness slowly ebbs, Dr. Z’s questions rise insistently. How can I help the survivors? My patients? My children? What if the media call me? How could I have been better prepared for this?
Disaster shakes us to our human and biological core. More than any other clinical encounter, it reminds us that psychiatrists share the vulnerabilities of those we seek to help. Yet it also reminds us that even simple concepts and interventions can mobilize the healing process.
Are you ready to provide emergency psychiatric care following a disaster in your community—be it a nuclear accident, tornado, airplane crash, or terrorist act? Here is evidence—some counterintuitive—that can help you prepare.
Table 1
‘Psychological first aid’ for disaster survivors
Re-create sense of safety
|
Encourage social support
|
Re-establish sense of efficacy
|
Disaster’s psychobiology
Human reactions to disaster are often conceptualized as the mammalian survival response: flight, fight, and fright (freezing). In most cases, these reactions are adaptive and dissipate after safety is restored. Posttraumatic stress disorder (PTSD) develops in about 5% of natural disaster victims and 33% of mass shooting victims.1
Responses that do go awry appear to be associated with abnormally low cortisol and persistent adrenergic activation, leading to sensitization of the fear response.2 Reminders of trauma or persistent stressors—such as pain, problems with finances or housing, or bereavement—may exacerbate sensitization. On the other hand, preclinical studies suggest that social support3,4 and active coping5 mitigate physiologic stress responses, confirming numerous clinical observations that associate lack of social support and avoidant coping with eventual PTSD development.
Three basics. Just as our emergency medicine colleagues must often revert to life-support basics, we must remind ourselves of biology’s three basics for psychic resilience:
- safety (including—as much as possible—protection from reminders of trauma and ongoing stress)
- meaningful social connection
- re-establishing a sense of efficacy to overcome helplessness (Table 1).
Like the stress response, these protective factors seem hard-wired into our biological make-up. They form the foundation for all phases of disaster psychiatry interventions, from planning to immediate interventions through longterm follow-up.
Disaster planning
As a mental health professional, plan to operate within established disaster plans and agencies, not only for the sake of efficiency but also because structure and support are paramount in disaster situations. Check with the American Psychiatric Association’s local branch to determine if a disaster mental health plan exists. If not, explore how to work directly with local American Red Cross chapters and hospitals, which recruit personnel for the Disaster Medical Assistance Teams mobilized by the public health service.
Table 2
Normal reactions to disaster for adults and children
| All ages | |
| Emotional | Shock, fear, grief, anger, guilt, shame, helplessness, hopelessness, numbness, emptiness Decreased ability to feel interest, pleasure, love |
| Cognitive | Confusion, disorientation, indecisiveness, worry, shortened attention span, poor concentration, memory difficulties, unwanted memories, self-blame |
| Physical | Tension, fatigue, edginess, insomnia, generalized aches and pains, startling easily, rapid heartbeat, nausea, decreased appetite and sex drive |
| Interpersonal | Difficulties being intimate, being over-controlling, feeling rejected or abandoned |
| Children’s age-specific disaster responses | |
| Preschool | Separation fears, regression, fussiness, temper tantrums, somatization Sleep disturbances including nightmares, somnambulism, night terrors |
| School-age | May still have the above, as well as excessive guilt and worries about others’ safety, poor concentration and loss of school performance, repetitious re-telling or play related to trauma |
| Adolescent | Depression, acting out, wish for revenge, sleeping and eating disturbances, altered view of the future |
Immediate disaster mental health plans vary in detail according to local needs and resources but should at least address:
- providing on-site interventions
- disseminating information about responses to trauma
- identifying and publicizing local mental health resources.
Immediate interventions
Immediately following a disaster, psychiatrists are frequently asked to assist with on-site crisis and medical interventions, evaluate survivors with unusual or intense reactions, and provide public education about psychological reactions to disaster.
On-site response. All responders, regardless of discipline, should provide disaster survivors with “psychological first aid,” which is directed at reestablishing safety, connection, and efficacy. Basic crisis intervention principles are useful when support and reassurance are not enough.6
For example, relaxation exercises can reduce anxiety and improve sleep. Use focused, structured relaxation tools—such as progressive muscle relaxation and breathing training—as unstructured exercises can exacerbate dissociation and re-experiencing. Grounding techniques, by which survivors learn to focus all senses on immediate surroundings, often alleviate dissociation and flashbacks.
Care for children. Because children’s reactions to disaster greatly depend on their caregivers’ responses (social referencing), focus acute interventions for children on:
- re-connecting them with their families
- reducing caregivers’ distress
- educating caregivers about providing age appropriate information and support (see Related resources).
Medical care. As physicians, psychiatrists may be called upon to intervene medically. Although it is generally advisable to stay within our usual practice, medical personnel may be in short supply. Fortunately, Good Samaritan laws exist in every state, and the potential for a successful malpractice suit against a physician responding in a disaster is almost zero, unless the physician’s performance is grossly negligent (such as moving the neck of a patient with obvious head or neck injuries).7
Principles regarding informed consent and right to refuse treatment—along with the usual exceptions—apply during disasters.
Evaluating survivors in shelters and hospitals requires knowing the normal and abnormal responses to disaster, being able to differentially diagnose changes in mental status, and understanding risk factors for trauma’s psychiatric sequelae. Aside from PTSD, trauma’s long-term effects include other anxiety disorders, depression, substance abuse, and eating disorders. In addition to the usual components of a psychiatric evaluation, assessments must address event-related factors such as proximity to the disaster, loss of significant others or property, physical injuries, immediate needs, and social support.8
Normal stress reactions. Frequently described as “a normal response to an abnormal situation,” the normal stress reaction is multidimensional and depends on the person’s developmental level (Table 2). About 10% to 25% of survivors experience intense affect and dissociation, whereas a similar number may appear unusually calm.
Interventions beyond the“first aid” described above are not usually needed unless individuals:
- are a danger to themselves or others
- are psychotic
- have no social supports
- cannot perform tasks necessary for self-care and to begin the recovery process.
Always re-assess when there is any question about a survivor’s immediate reaction to trauma.
In DSM-IV-TR’s trauma-related diagnoses, the symptom clusters often do not capture many disaster survivors’ subjective experience: the shattering of fundamental beliefs regarding themselves (invulnerability), the world (predictability, safety), and others (trust, benevolence).9 By empathizing with these responses, you can help survivors feel less isolated and estranged.
Differential diagnosis. Survivors’ mental status changes may be manifestations of the stress response, but they also may represent:
- exacerbations of pre-existing psychiatric or general medical conditions
- hypoxemia, hypovolemia, or CNS trauma from physical injury
- responses to medications used for resuscitation or pain control, such as atropine, epinephrine, lidocaine, or morphine.
Effects of bioterrorism agents must also be considered. For example, organophosphorus compounds such as the nerve agents sarin and soman can cause impaired concentration, depression, and anxiety. Anthrax can cause rapidly progressing meningitis.10 Delirium must be differentiated from dissociation; patients with dissociation can be re-oriented, and changes will resolve with time rather than fluctuate.11
Psychopathology risk factors. Multiple studies have addressed risk factors for post-disaster psychiatric sequelae (usually PTSD). In general, risk increases with repeated trauma exposure (including TV viewing), prior trauma, lack of social support, injury, pre-existing psychiatric problems, traumatic bereavement (having witnessed the violent death of a loved one), avoidant coping, and having strong negative beliefs about the meanings of normal stress reactions such as tearfulness, anxiety, and insomnia.
Because a recent meta-analysis supports these observations,12 follow-up evaluation for signs of PTSD is recommended for:
- survivors with one or more of the risk factors discussed above
- vulnerable groups such as rescue workers, children, and dependent individuals
- survivors whose symptoms persist after 2 months.13
Decompensation immediately after a disaster seems to be the exception for psychiatric patients, despite their longer-term vulnerability. One psychiatrist who in 1989 survived Hurricane Hugo—the most intense storm to strike the Mid-Atlantic region in 100 years—noted that demand for inpatient psychiatric services did not increase in the storm’s aftermath. The only patient calls she received were inquiries about her own physical safety.14
Caregivers and rescue workers—including psychi-atrists—are also disaster survivors, and you need to tend to your needs for safety and support. Consult frequently with colleagues within and outside the disaster area, as much for support as for information and guidance.15 Remember also that rescue workers are occasionally targets for victims’ rage at their circumstances. Anticipating and explaining this displacement reduces its toxicity.
Using medications
Uses psychotropics judiciously in the first 48 hours of trauma. Medication effects may interfere with neurologic assessment of the injured, and monitoring and follow-up may not be possible.
However, drug therapy should start quickly when survivors are acutely psychotic or their behavior endangers themselves, others, or the milieu. Medications usually include a fast-acting benzodiazepine and/or an antipsychotic, as described in guidelines for managing agitation.16 Always provide structure and supervision for medicated patients.
No guidelines exist for using medications to manage distressing—but less-severe—acute stress-related symptoms. Some experts advocate using adrenergic antagonists such as clonidine, guanfacine, and beta blockers to reduce excessive arousal. These drugs have not been adequately studied in this setting, however, and may harm those with cardiovascular instability from preexisting conditions or injuries.
Table 3
Psychoeducation: Simple messages for workers and trauma survivors
| Get adequate rest, food, sleep |
| Avoid exposure to trauma cues, including TV images |
| Seek support from loved ones and peers |
| Talk about events and feelings only if this feels comfortable and helpful |
| Return to normal routine as much as possible |
| Take action to rebuild, but at a reasonable pace |
| Reach out to others who may need assistance |
Get help:
|
Short-term (<1 week) benzodiazepine use for panic symptoms and severe insomnia is acceptable, but longer-term use may increase PTSD risk.17 A selective serotonin reuptake inhibitor may help individuals with pre-existing PTSD or depression, if you can arrange follow-up.
In the aftermath
‘Debriefing.’ Critical incident stress debriefing (CISD) is a structured, one-session group intervention in which survivors’ experiences and emotional reactions are discussed and education and follow-up recommendations are provided. Developed by Mitchell in 1983,18 CISD was widely used until systematic evaluations revealed that it did not alleviate psychological distress or prevent PTSD.19
Table 4
How to interact with news media during and after a disaster
| For organizations Identify a spokesperson with media experience beforehand Ensure that the spokesperson is well-informed about all aspects of the disaster |
| For spokespersons |
Always
|
Never
|
Thus, although survivor meetings may provide information, education, screening, and support, avoid detailed discussions of events and emotions. Any meetings should be conducted by mental health clinicians and should not be mandatory. Reserve the term “debriefing” for operational reviews by rescue personnel.20
Public education. Educate survivors, rescue workers, health care providers, teachers, and relief agency workers. Provide concise, simple messages as suggested in Table 3. News media provide our most effective means of reaching out to survivors, which is why having a pre-existing relationship is so important. Some guidelines for working with the media are presented in Table 4.
Outreach. Numerous educational resources are available for survivors and their caregivers (see Related resources). Other potentially useful outreach tools include:
- meetings with teachers’ organizations
- continuing medical education activities for primary care providers
- telephone hot lines.
Legal and ethical issues
Disaster scenes are chaotic and informal, and professionals must be flexible, often providing general support and information rather than specific clinical interventions. However, it is important in each encounter to decide whether a patient-physician relationship has begun.
As a general rule, a physician-patient relationship is established whenever diagnosis or treatment is discussed. Once that happens, briefly document:
- signs and symptoms
- working diagnosis
- suicide or violence potential
- treatment and/or follow-up plans.
Confidentiality may be difficult to preserve in chaotic situations involving workers from many agencies. Even in disasters, however, you must obtain permission before sharing information unless the individual’s situation is emergent.
Table 5
Keys to effective disaster psychiatry
Be prepared
|
Care for survivors
|
Care for yourself
|
Long-term interventions
Longer-term disaster interventions include continued outreach and education and needed follow-up services. Existing structures may provide effective follow-up, but additional resources are often needed.
Federal programs. Following a presidentialdeclared disaster, the Federal Emergency Management Agency (FEMA) provides funding for crisis counseling. Programs are typically funded for 9 to 15 months and administered through the emergency services and disaster relief branch of the Substance Abuse and Mental Health Services Administration (SAMHSA) and community mental health organizations. Examples include Project Heartland following the 1995 Oklahoma City federal building bombing and Project COPE following California’s 1989 Loma Prieta earthquake.
Cognitive-behavioral therapy. For adult survivors with acute stress disorder, specific cognitive-behavioral therapy (CBT) provided by trained therapists may prevent PTSD and other trauma sequelae, such as depression.21 CBT interventions may begin as early as 2 weeks after trauma and focus sequentially on anxiety management, cognitive restructuring, imaginal exposure followed by in vivo exposure, and relapse prevention.
Three controlled trials found 6-month PTSD rates of 14% to 20% among acute stress disorder patients treated with CBT, compared with 58% to 67% with supportive counseling.22-24 Although studies of interventions immediately following trauma are lacking, trauma-focused CBT is also recommended for children.25 Evidence-based treatments for PTSD are discussed in detail elsewhere.26
Not unprepared after all
With some reflection, Dr. Z realized she had the tools to help her community. Her feelings of helplessness receded as she envisioned how she could help survivors understand their experiences, re-create a sense of safety, restore important connections to loved ones, and begin to rebuild their lives (Table 5).
For clinicians
- Young BH, Ford JD, Ruzek JI, et al. Disaster mental health: a guidebook for clinicians and administrators. Washington, DC: National Center for Post-Traumatic Stress Disorder, 1998. http://ncptsd.org/publications/disaster/index.html
- Hillman JL. Crisis intervention and trauma: new approaches to evidence-based practice. New York: Kluwer Academic/Plenum Publishers, 2002.
- Office of the Surgeon General Web site on medical aspects of nuclear, biological, and chemical warfare. http://www.nbc-med.org
For survivors and clinicians
- National Center for Post-Traumatic Stress Disorder. Disaster mental health: Dealing with the aftereffects of terrorism. http://www.ncptsd.org/terrorism/index.html
- Substance Abuse and Mental Health Services Administration. Emergency mental health and traumatic stress. http://www.mentalhealth.org/cmhs/EmergencyServices/default.asp
- American Academy of Child and Adolescent Psychiatry. Talking to children about terrorism and war. http://www.aacap.org/publications/factsFam/87.htm
Drug brand names
- Clonidine • Catapres
- Guanfacine • Tenex
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Effects of traumatic stress in a disaster situation. Washington, DC: National Center for Post-Traumatic Stress Disorder. Department of Veterans Affairs, September 12, 2001 (Accessed March 31, 2004 at http://www.ncptsd.org/facts/disasters/fs_effects_disaster.html).
2. Yehuda R, McFarlane AC, Shalev AY. Predicting the development of post-traumatic stress disorder from the acute response to a traumatic event. Biol Psychiatry 1998;44:1305-13.
3. Levine S, Lyons DM, Schatzberg AF. Psychobiological consequences of social relationships. Ann NY Acad Sci 1997;807:210-18.
4. Uchino BN, Garvey TS. The availability of social support reduces cardiovascular reactivity to acute psychological stress. J Behav Med 1997;20:15-27.
5. LeDoux JE, Gorman JM. A call to action: overcoming anxiety through active coping. Am J Psychiatry 2001;158:1953-5.
6. Hillman JL. Crisis intervention and trauma: new approaches to evidence-based practice. New York: Kluwer Academic/Plenum Publishers, 2002.
7. Daniels G. Good Samaritan acts. Emerg Med Clin North Am 1999;17:491-504.
8. Mental-health intervention for disasters. Washington, DC: National Center for Post-Traumatic Stress Disorder, Department of Veterans Affairs, Sept. 12, 2001. (Accessed March 31, 2004 at http://www.ncptsd.org/facts/disasters/fs_treatment_disaster.html.)
9. Difede J, Apfeldorf WJ, Cloitre M, et al. Acute psychiatric responses to the explosion at the World Trade Center: a case series. J Nerv Ment Dis 1997;185:519-22.
10. DiGiovanni C. Domestic terrorism with chemical or biological agents: psychiatric aspects. Am J Psychiatry 1999;156:1500-5.
11. Rundle JR. Psychiatric issues in medical-surgical disaster casualties: consultation-liaison approach. Psychiatr Q 2000;71:245-58.
12. Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for post-traumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol 2000;68:748-66.
13. Mental health and mass violence: evidence-based early psychological intervention for victims/survivors of mass violence. Washington, DC: National Institute of Mental Health, Nov. 1, 2002 (Accessed Oct. 29, 2003 at http://www.nimh.nih.gov/research/massviolence.pdf.)
14. Austin LS. Organizing a disaster response program in one’s home community. In: Austin LS (ed). Responding to disaster: a guide for mental health professionals. Washington, DC: American Psychiatric Publishing, 1992;53-68.
15. Rousseau AW. Notes from Oklahoma City’s recovery. In: Hall RCW, Norwood AE (eds). Disaster psychiatry handbook. Washington, DC: American Psychiatric Association Committee on Psychiatric Dimensions of Disaster, undated:3-11. (Accessed Oct. 29, 2003 at http://www.psych.org/disasterpsych/pdfs/apadisasterhandbk.pdf.)
16. Yildiz A, Sachs GS, Turgay A. Pharmacological management of agitation in emergency settings. Emerg Med J 2003;20:339-46.
17. Gelpin E, Bonne O, Peri T, et al. Treatment of recent trauma survivors with benzodiazepines: a prospective study. J Clin Psychiatry 1996;57:390-4.
18. Mitchell JT. When disaster strikes. J Em Med Serv 1983;8:36-9.
19. Rose S, Bisson J, Wessely S. Psychological debriefing for preventing posttraumatic stress disorder (PTSD). Cochrane Database Syst Rev 2002;(2):CD000560.-
20. Black J. Forming the libidinal cocoon: the Dallas airplane crashes, the Guadalupe river drownings, and Hurricane Hugo in the Virgin Islands. In: Austin LS (ed). Responding to disaster: a guide for mental health professionals. Washington DC: American Psychiatric Publishing, 1992;169:84.-
21. Ehlers A, Clark DM. Early psychological interventions for adult survivors of trauma: a review. Biol Psychiatry 2003;53:817-26.
22. Bryant RA, Moulds M, Guthrie R, Nixon RD. Treating acute stress disorder following mild traumatic brain injury. Am J Psychiatry 2003;160:585-7.
23. Bryant RA, Sackville T, Dang ST, et al. Treating acute stress disorder: an evaluation of cognitive behavior therapy and supportive counseling techniques. Am J Psychiatry 1999;156:1780-6.
24. Bryant RA, Harvey AG, Dang ST, et al. Treatment of acute stress disorder: a comparison of cognitive-behavioral therapy and supportive counseling. J Consult Clin Psychol 1998;66:862-6.
25. Cohen JA. Treating acute posttraumatic reactions in children and adolescents. Biol Psychiatry 2003;53:827-33.
26. Foa EB, Keane TM, Friedman MJ. Effective treatments for PTSD. New York: Guilford Press, 2000.
1. Effects of traumatic stress in a disaster situation. Washington, DC: National Center for Post-Traumatic Stress Disorder. Department of Veterans Affairs, September 12, 2001 (Accessed March 31, 2004 at http://www.ncptsd.org/facts/disasters/fs_effects_disaster.html).
2. Yehuda R, McFarlane AC, Shalev AY. Predicting the development of post-traumatic stress disorder from the acute response to a traumatic event. Biol Psychiatry 1998;44:1305-13.
3. Levine S, Lyons DM, Schatzberg AF. Psychobiological consequences of social relationships. Ann NY Acad Sci 1997;807:210-18.
4. Uchino BN, Garvey TS. The availability of social support reduces cardiovascular reactivity to acute psychological stress. J Behav Med 1997;20:15-27.
5. LeDoux JE, Gorman JM. A call to action: overcoming anxiety through active coping. Am J Psychiatry 2001;158:1953-5.
6. Hillman JL. Crisis intervention and trauma: new approaches to evidence-based practice. New York: Kluwer Academic/Plenum Publishers, 2002.
7. Daniels G. Good Samaritan acts. Emerg Med Clin North Am 1999;17:491-504.
8. Mental-health intervention for disasters. Washington, DC: National Center for Post-Traumatic Stress Disorder, Department of Veterans Affairs, Sept. 12, 2001. (Accessed March 31, 2004 at http://www.ncptsd.org/facts/disasters/fs_treatment_disaster.html.)
9. Difede J, Apfeldorf WJ, Cloitre M, et al. Acute psychiatric responses to the explosion at the World Trade Center: a case series. J Nerv Ment Dis 1997;185:519-22.
10. DiGiovanni C. Domestic terrorism with chemical or biological agents: psychiatric aspects. Am J Psychiatry 1999;156:1500-5.
11. Rundle JR. Psychiatric issues in medical-surgical disaster casualties: consultation-liaison approach. Psychiatr Q 2000;71:245-58.
12. Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for post-traumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol 2000;68:748-66.
13. Mental health and mass violence: evidence-based early psychological intervention for victims/survivors of mass violence. Washington, DC: National Institute of Mental Health, Nov. 1, 2002 (Accessed Oct. 29, 2003 at http://www.nimh.nih.gov/research/massviolence.pdf.)
14. Austin LS. Organizing a disaster response program in one’s home community. In: Austin LS (ed). Responding to disaster: a guide for mental health professionals. Washington, DC: American Psychiatric Publishing, 1992;53-68.
15. Rousseau AW. Notes from Oklahoma City’s recovery. In: Hall RCW, Norwood AE (eds). Disaster psychiatry handbook. Washington, DC: American Psychiatric Association Committee on Psychiatric Dimensions of Disaster, undated:3-11. (Accessed Oct. 29, 2003 at http://www.psych.org/disasterpsych/pdfs/apadisasterhandbk.pdf.)
16. Yildiz A, Sachs GS, Turgay A. Pharmacological management of agitation in emergency settings. Emerg Med J 2003;20:339-46.
17. Gelpin E, Bonne O, Peri T, et al. Treatment of recent trauma survivors with benzodiazepines: a prospective study. J Clin Psychiatry 1996;57:390-4.
18. Mitchell JT. When disaster strikes. J Em Med Serv 1983;8:36-9.
19. Rose S, Bisson J, Wessely S. Psychological debriefing for preventing posttraumatic stress disorder (PTSD). Cochrane Database Syst Rev 2002;(2):CD000560.-
20. Black J. Forming the libidinal cocoon: the Dallas airplane crashes, the Guadalupe river drownings, and Hurricane Hugo in the Virgin Islands. In: Austin LS (ed). Responding to disaster: a guide for mental health professionals. Washington DC: American Psychiatric Publishing, 1992;169:84.-
21. Ehlers A, Clark DM. Early psychological interventions for adult survivors of trauma: a review. Biol Psychiatry 2003;53:817-26.
22. Bryant RA, Moulds M, Guthrie R, Nixon RD. Treating acute stress disorder following mild traumatic brain injury. Am J Psychiatry 2003;160:585-7.
23. Bryant RA, Sackville T, Dang ST, et al. Treating acute stress disorder: an evaluation of cognitive behavior therapy and supportive counseling techniques. Am J Psychiatry 1999;156:1780-6.
24. Bryant RA, Harvey AG, Dang ST, et al. Treatment of acute stress disorder: a comparison of cognitive-behavioral therapy and supportive counseling. J Consult Clin Psychol 1998;66:862-6.
25. Cohen JA. Treating acute posttraumatic reactions in children and adolescents. Biol Psychiatry 2003;53:827-33.
26. Foa EB, Keane TM, Friedman MJ. Effective treatments for PTSD. New York: Guilford Press, 2000.
Hypnosis: Brief interventions offer key to managing pain and anxiety
Mr. M, a world-class athlete, collapsed suddenly in an alley. He was rushed to a hospital emergency room, where he nearly died of internal bleeding from a grapefruit-sized abdominal lymphoma. He was hospitalized and placed on chemotherapy.
Increasing doses of opiates hardly reduced his pain, and he became extremely anxious. Staff described him as “climbing the walls.” He lay in bed writhing, and his parents feared he was becoming a “drug addict.”
Anxiety about his life-threatening illness was clearly compounding his pain, so his attending physician ordered a psychiatric evaluation. When I interviewed the patient, I felt that hypnosis could help.
Hypnosis—as a state of highly focused attention—can help us treat patients’ anxiety, phobias, pain, posttraumatic stress disorder (PTSD), and dissociative disorders. With training, an experienced psychiatrist can quickly start using hypnosis as an adjunct to other therapies.
This article describes how hypnosis helped Mr. M and a young woman traumatized by a criminal assault. Based on my experience and the literature, I discuss what hypnosis is, what training is required, how to measure hypnotizability, and the value of hypnosis in helping patients control their anxiety, posttraumatic, and dissociative states.
Case continued: ‘Surfing’ in Hawaii
When I met Mr. M in the hospital, I acknowledged his distress and the reasons for it, saying “You don’t really want to be here, do you?”
“How many years of medical training did it take you to figure that out?” he replied.
“Well then,” I said, “let’s go somewhere else. Where would you like to be right now?”
He responded, “I’ve never surfed.”
“Good,” I replied, “let’s go to Hawaii.” In hypnosis, I had him picture himself surfing. He continued to groan, but the pattern changed. “What happened?” I asked. “I fell off the surfboard,” he replied. “OK, get back on, and do it right,” I told him.
He learned to practice self-hypnosis, which markedly reduced his anxiety and pain. Two days later he was off pain medications and joking with the nurses in the hall. The attending physician noted in the patient’s record: “Patient off pain meds. Tumor must be regressing.”
What is hypnosis?
Mr. M’s response, though unusually strong, underscores the fact that hypnosis can rapidly produce analgesia and anxiolysis in the medical setting. Hypnosis—often called “believed-in imagination”—is characterized by an ability to sustain a state of attentive, receptive, intense focal concentration with diminished peripheral awareness. The hypnotized person is awake and alert, not asleep. Hypnosis’ three main components are absorption, dissociation, and suggestibility.
Biological basis. The hypnotic state has no brain “signature” per se, but brain imaging portrays hypnosis as a state of alertness with altered anterior cingulate gyrus activation, which helps to focus attention.1-3 Hypnotized persons can demonstrably alter blood flow in brain regions involved in perceptual processing in response to suggestions of altered perception, whether somatosensory, visual, or olfactory.4,5 Thus, patients report not only reduced pain but changes in how they experience pain with hypnotic analgesia.
The brain’s dopamine neurotransmitter system—especially in the frontal lobes—also may be involved in hypnosis, as highly hypnotizable persons have elevated levels of dopamine metabolites in their cerebrospinal fluid.6
Hypnotic trance. The trance experience is often best explained to patients as similar to being absorbed in a good novel. One loses awareness of one’s surroundings and enters the imagined world. When the novel is finished, the reader requires a moment of reorientation to the surrounding world.
A trance is a state of sustained, attentive-receptive concentration in response to a signal from within or from someone else. The signal activates this shift of awareness and permits more-intensive concentration in a designated direction.
All hypnosis is self-hypnosis. Much of its clinical value is that it can be self-induced throughout the day and whenever symptoms emerge. During the first weeks, patients can be encouraged to practice every 1 or 2 hours.
Applying hypnosis to practice. A well-trained clinician can learn to use hypnosis in classes offered by the two professional hypnosis societies or the American Psychiatric Association (Box 1) Because hypnosis is not something “done to” a patient but rather a capacity to be measured, tapped, and utilized, psychiatrists can integrate hypnosis into clinical practice after some initial training, with ongoing learning and supervision.
Who can be hypnotized?
Not everyone is equally hypnotizable, and hypnotizability is a stable and measurable trait. Approximately one-quarter of adults cannot respond to hypnotic instructions, whereas 10% are extremely hypnotizable.7
Brief, clinically useful tests of hypnotic responsiveness have been developed, such as the Hypnotic Induction Profile (HIP).8 The clinician usually can induce the trance experience and systematically measure the patient’s response within 5 minutes. A HIP score of 5 indicates usable hypnotizability.
The HIP test includes instructions to produce a sense of lightness in the left arm and hand, with tests of response to this instruction. Response is characterized by dissociation, hand elevation after it is lowered, involuntariness, response to the cutoff signal, and altered sensation.
Turning hypnotic induction into a test of hypnotic capacity transforms the initial encounter by:
- removing pressure on the clinician to successfully hypnotize the subject
- reducing patients’ experiences of complying with the clinician’s wishes, rather than exploring and discovering their own hypnotic capacity.
Placing the hypnotic experience in the context of a test also makes it consonant with other medical examinations and procedures.8
Once a patient’s hypnotizability is determined, structured measurement is no longer necessary. The test-retest correlation for hypnotizability scores is 0.7 over 25 years, which is more consistent than IQ testing.7 Subsequent inductions usually can be generated by the patient or signaled by the clinician, and only seconds are required for the shift into trance.
Effective, safe work with hypnosis requires clinical expertise in diagnostic assessment and choosing treatment options. Psychiatrists can learn techniques for inducing, measuring, and using hypnotic responsiveness in introductory and advanced workshops, supplemented by local supervision.
Courses in hypnosis are offered by many medical schools. Postgraduate training is available at annual meetings of the American Psychiatric Association, Society for Clinical and Experimental Hypnosis, and American Society of Clinical Hypnosis. The two hypnosis societies offer intensive workshops for psychiatrists, psychologists, and other health care professionals.
Useful text books also are available:
- Spiegel H, Spiegel D. Trance and treatment: clinical uses of hypnosis. Washington, DC: American Psychiatric Publishing, 2004.
- Zarren JI, Eimer BN. Brief cognitive hypnosis: facilitating the change of dysfunctional behavior. New York: Springer Publishing, 2002.
- Lynn SJ, Kirsch I, Rhue JW. Casebook of clinical hypnosis. Washington, DC: American Psychological Association, 1996.
- Fromm E, Kahn SP. Self-hypnosis: the Chicago paradigm. New York: Guilford Press, 1990.
Reducing anxiety
Anxiety can be understood as a vaguely defined but immobilizing sense of distress. Lack of clarity about the discomfort’s source enhances the patient’s sense of helplessness and avoidance. One therapeutic challenge is to convert anxiety into fear—to give it a focus so that something can be done about it.
Imagine yourself floating in a bath, a lake, a hot tub, or just floating in space. With each breath out, let a little more tension out of your body. Just enjoy this pleasant sense of floating, and notice how you can use your store of memories and fantasies to help yourself and your body feel better.
“While you imagine yourself floating, in your mind’s eye visualize an imaginary screen: a movie, TV, or computer screen, or, if you wish, a piece of clear blue sky. On that screen project your thoughts, fears, worries, ideas, feelings, or memories, while you maintain the pleasant sense of floating in your body. You establish this clear sense of your body floating here, while you relate to your thoughts and ideas out there.
“Once you have established this screen, divide it in half. Use the left side as your ‘worry screen.’ Picture one thing that causes you anxiety on this screen and learn to manage the feelings of discomfort that accompany it. Now use the right side as your ‘problem-solving’ screen. Brainstorm something you can do about the problem on the left, all the while maintaining a sense of floating in your body.
“You may have to ‘freeze’ what is on the ‘worry screen’ and re-establish the floating several times. This allows you to develop new means of coping with the things that are making you anxious, one at a time.”
Anxiety sets up a negative feedback cycle between psychological preoccupation and somatic discomfort, a “snowball effect” in which subjective anxiety and somatic tension reinforce each other. Hypnosis can help reduce anxiety and induce relaxation,9 and its dissociative component can help separate anxiety’s psychological and somatic components.
Hypnosis is as effective at reducing anxiety as 1 mg of alprazolam, at least in a study of college students.10 Student volunteers with high and low hypnotizability were given alprazolam, 1 mg, and a hypnotic suggestion based on their reactions to the drug. Four days later, when students received hypnosis only and hypnosis plus alprazolam:
- combination therapy reduced anxiety more effectively than did hypnosis or alprazolam alone, as measured by the Profile of Mood States tension-anxiety scale
- improvement was comparable with hypnosis or alprazolam alone
- highly hypnotizable students showed significantly greater relaxation than did those with low hypnotizability in all three treatment groups
- EEG data showed similar frontal and occipital changes in the alprazolam and hypnotic suggestion groups.
In randomized trials, simple self-hypnosis training has reduced pain and anxiety during medical procedures, reducing procedure time by an average 17 minutes and resulting in fewer complications.11
A typical hypnotic instruction for managing anxiety is provided in Box 2. This approach teaches patients how to deal with stressors that complicate their anxiety and to control their somatic response. Hypnosis expands patients’ repertoire of responses and enables them to feel less helpless.
Confronting phobias
Phobic symptoms of fear and avoidance or exposure with distress respond especially well to brief hypnosis interventions. Although behavior modification and antidepressants also can treat phobias successfully, one or two hypnosis sessions often can reduce or cure phobic symptoms.
For example, one can help patients with airplane phobia prepare for flight by going into a hypnotic state and learning three concepts:
- Think of the airplane as an extension of the body, such as a bicycle.
- Float with the plane.
- Think about the difference between probability and possibility.
The hypnotic state—with its focused attention and physical relaxation—can amplify this cognitive restructuring technique. Phobic patients can feel more in control of their somatic reactions and, by extrapolation, the flying experience itself. In one study, 52% of patients taught this self-hypnosis exercise remained improved or cured at least 7 years later.12
Treating traumatic reactions
Evidence is growing that trauma elicits dissociation. Thus, hypnosis could help us understand and treat traumatic reactions, including patients with acute and posttraumatic stress disorder (PTSD) and dissociative disorders.
The hypnotic state’s controlled dissociation can be used to model the uncontrolled dissociation represented by posttraumatic phenomena such as flashbacks, numbing, and amnesia.13 This view is supported by evidence that PTSD is associated with high hypnotizability.14,15
Acute stress disorder—as introduced in DSM-IV16—is characterized by prominent dissociative symptoms, with intrusion, avoidance, and hyperarousal. These diagnostic criteria recognize that acute dissociation is a common and predictable reaction to trauma.
Hypnosis involving grief work, exploration of trauma-related transference issues, and emotional expression are effective psychotherapies for persons exposed to trauma. Becoming familiar with hypnotic states can teach patients to recognize, understand, and control their dissociative states.
Evidence suggests that hypnosis’ intense concentration may reverse the dissociative mind fragmentation caused by trauma.17 Traumatic memories may seem less overwhelming and intrusive once patients discover they can:
- exert greater control over memory access and retrieval
- work through and assimilate disturbing thoughts.
The controlled experience of hypnotic abreaction (reliving traumatic and other memories with strong emotion) provides boundaries for psychotherapeutic grief work.18,19 Instead of telling patients not to ruminate over a traumatic event, the clinician instructs the patient how to think about the experience.
The inferred message is that the patient can work on other things—such as relationships and daily living problems—after this therapeutic work is done.
Patients are slowly separated from the victim role. The goal is to help them restructure their memories, both cognitively and emotionally. They bear the memories’ impact, yet come to see the information differently.7 Traumatic input becomes more bearable when linked to a cognitively restructured recognition of an adaptive response.,20 For example, patients may acknowledge what they did during a traumatic event that was self-protective or helped others.
PTSD. Hypnosis shares common elements with other cognitive and behavioral treatments for PTSD, including exposure to traumatic memories for cognitive and emotional processing. Few studies have examined using hypnosis to treat PTSD, but evidence suggests it is at least as effective as other cognitive-behavioral treatments.20,21
Patients can be taught to view PTSD’s intrusive memories and bodily symptoms as re-experiencing painful memories. The memories often intrude less frequently after patients find a controlled method—such as self-hypnosis—to access and work them through.22
Ms. J hoped hypnosis could help her better visualize the face of an assailant who had attacked her as she returned at dusk from the grocery store. She had fought off his attempt to drag her into her apartment and rape her. The police showed little interest in pursuing him, however, because the sexual assault had not been completed. After the police left, she had a grand mal seizure. She had suffered a basalar skull fracture.
Ms. J was highly hypnotizable and learned the split-screen technique. While visualizing the assault on the left screen, she realized something that had not been clear to her before: “From the look on his face, I can see he wants to kill me. If he gets me into my apartment, he will kill me.”
She focused on this realization and the image of his hatred and threat to her. The therapist asked her to picture on the right screen something she had done to protect herself. She said: “He is surprised that I am fighting so hard. He doesn’t expect me to put up such a fight.”
She emerged from hypnosis understanding that she had been in more danger than she realized. Thus, despite the disappointment of having no clearer idea of what he looked like (it was quite dark when he attacked her), she had a restructured perspective about what had occurred.
Before this session, Ms. J had felt guilty that she had gotten herself so seriously injured. Afterward, she could better tolerate the memory of the attack because it was coupled with cognitive awareness that her actions may have saved her life.
Self-blame. Many trauma victims would rather feel guilty than helpless. They blame themselves inappropriately for events over which they had no control, rather than accept their helplessness. They misuse hindsight about the trauma to assume the events were predictable and therefore avoidable. They imagine they can replay the events and change the outcome.
Such an approach to trauma can be profoundly demoralizing, leaving victims burdened by needless guilt and shame. Helping them face and bear the feelings associated with traumatic events can free them from efforts to “undo” or take responsibility for the trauma and accept what happened.
Split-screen technique. Using hypnosis with a “split-screen” technique can help patients restructure the memory of trauma. The left screen symbolizes the trauma in condensed form. The right screen helps patients focus on how they tried to master the situation. This grief work allows patients to acknowledge, bear, and put into perspective the humiliation of the experience and their loss of invulnerability, health, or loved ones (Box 3).18
Dissociation. Dissociating during a threatening situation may enable a person to put aside some awareness of the danger and take self-protective action. Persistent dissociation, however, may make it too easy to avoid working through the traumatic experiences later on.22-24
Dissociation makes subsequent exposure to reminders of the trauma more similar to a reexperiencing rather than a controlled remembering of it. This can trigger physiologic stress reactions and lead to or worsen PTSD.25-27
Dissociative disorders can be understood as chronic and severe PTSDs.28 Many individuals with dissociative disorders have histories of sexual and physical abuse.29-31 Clearly, traumatic experiences sensitize survivors to subsequent trauma through conditioned activation of fear circuitry involving the amygdala, hippocampus, and frontal lobes.32
Hypnosis can be especially helpful—both for diagnosis and therapy.33 It can assist the controlled recovery of memories, while allowing some images to remain dissociated from cognition until the patient is ready to deal with them. The patient can turn memories on and off by entering and exiting the hypnotic state and thereby recover and reprocess memories at a tolerable pace.
Related resources
- Society for Clinical and Experimental Hypnosis. http://ijceh.educ.wsu.edu
- American Society of Clinical Hypnosis. www.asch.net
- American Psychological Association, Division 30. Society of Psychological Hypnosis. http://www.apa.org/about/division/div30.html
1. Spiegel D, Jasiukaitis P. Hypnosis: Brain basis. In: Smith BH (ed). Elsevier’s encyclopedia of neuroscience. The Netherlands: Elsevier Science, 1999.
2. Rainville P, Hofbauer RK, Bushnell MC, et al. Hypnosis modulates activity in brain structures involved in the regulation of consciousness. J Cogn Neurosci 2002;14:887-901.
3. Rainville P, Duncan GH, Price DD, et al. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 1997;277:968-71.
4. Kosslyn SM, Thompson WL, Costantini-Ferrando MF, et al. Hypnotic visual illusion alters color processing in the brain. Am J Psychiatry 2000;157:1279-84.
5. Spiegel D. Negative and positive visual hypnotic hallucinations: attending inside and out. Int J Clin Exp Hypn 2003;51:130-46.
6. Spiegel D, King R. Hypnotizability and CSF HVA levels among psychiatric patients. Biol Psychiatry 1992;31:95-8.
7. Piccione C, Hilgard ER, Zimbardo PG. On the degree of stability of measured hypnotizability over a 25-year period. J Pers Soc Psychol 1989;56:289-95.
8. Spiegel H, Spiegel D. Trance and treatment: Clinical uses of hypnosis. Washington, DC: American Psychiatric Press, 2004.
9. Wertz JM, Sayette MA. Effects of smoking opportunity on attentional bias in smokers. Psychol Addict Behav 2001;15:268-71.
10. Nishith P, Barabasz A, Barabasz M, Warner D. Brief hypnosis substitutes for alprazolam use in college students: transient experiences and quantitative EEG responses. Am J Clin Hypn 1999;41:262-8.
11. Lang EV, Benotsch EG, Fick LJ, et al. Adjunctive nonpharmacological analgesia for invasive medical procedures: a randomised trial. Lancet 2000;355:1486-90.
12. Spiegel D, Frischholz EJ, Maruffi B, Spiegel H. Hypnotic responsitivity and the treatment of flying phobia. Am J Clin Hypn 1981;23:239-47.
13. Butler LD, Duran EFD, Jasiukaitis P, et al. Hypnotizability and traumatic experience: a diathesis-stress model of dissociative symptomatology. Am J Psychiatry 1996;153:42-63.
14. Spiegel D. Dissociation and hypnosis in post-traumatic stress disorder. J Trauma Stress 1988;1:17-33.
15. Stutman RK, Bliss EL. Posttraumatic stress disorder, hypnotizability, and imagery. Am J Psychiatry 1985;142:741-3.
16. Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington, DC: American Psychiatric Association, 2000.
17. Maldonado JR, Spiegel D. Trauma, dissociation and hypnotizability. In: Marmar R, Bremmer D (eds). Trauma, memory and dissociation. Washington, DC: American Psychiatric Press, 1998.
18. Lindemann E. Symptomatology and management of acute grief. Am J Psychiatry 1994;151:155-60.
19. Spiegel D. Vietnam grief work using hypnosis. Am J Clin Hypn 1981;24:33-40.
20. Foa EB, Davidson JRT, Frances A. Treatment of posttraumatic stress disorder. J Clin Psychiatry 1999;50:4-69.
21. Brom D, Kleber RJ, Defare PB. Brief psychotherapy for post-traumatic stress disorder. J Consult Clin Psychol 1989;57:607-12.
22. Spiegel D. Hypnosis and implicit memory: automatic processing of explicit content. Am J Clin Hypn 1998;40:231-40.
23. Spiegel D. Multiple personality as a post-traumatic stress disorder. Psychiatr Clin North Am 1984;7:101-10.
24. Kluft RP. Dissociation as a response to extreme trauma. In: Kluft RP (ed). Childhood antecedents of multiple personality. Washington, DC: American Psychiatric Press, 1985:66-97.
25. Marmar CR, Weiss DS, Metzler T. Peritraumatic dissociation and posttraumatic stress disorder. In: Bremner JD, Marmar C (eds). Trauma, memory, and dissociation. Washington, DC: American Psychiatric Press, 1998;229-52.
26. Birmes P. Peritraumatic dissociation, acute stress, and early posttraumatic stress disorder in victims of general crime. Can J Psychiatry 2001;46:649-51.
27. Spiegel D. Hypnosis, dissociation and trauma. In: Burrows GD, Stanley RO, Bloom PB (eds). Clinical hypnosis. New York: John Wiley & Sons, 2001;143-58.
28. Spiegel D, Cardena E. Disintegrated experience: the dissociative disorders revisited. J Abnorm Psychol 1991;100:366-78.
29. Chu JA, Dill DL. Dissociative symptoms in relation to childhood physical and sexual abuse. Am J Psychiatry 1990;147:887-92.
30. Kluft RP. Childhood antecedents of multiple personality. Washington, DC: American Psychiatric Press, 1985.
31. Spiegel D. Dissociating damage. Am J Clin Hypn 1986;29:123-31.
32. LeDoux J. Synaptic self: How our brains become who we are. New York: Viking Press, 2002.
33. Putnam FW. Using hypnosis for therapeutic abreactions. Psychiatr Med 1992;10:51-65.
Mr. M, a world-class athlete, collapsed suddenly in an alley. He was rushed to a hospital emergency room, where he nearly died of internal bleeding from a grapefruit-sized abdominal lymphoma. He was hospitalized and placed on chemotherapy.
Increasing doses of opiates hardly reduced his pain, and he became extremely anxious. Staff described him as “climbing the walls.” He lay in bed writhing, and his parents feared he was becoming a “drug addict.”
Anxiety about his life-threatening illness was clearly compounding his pain, so his attending physician ordered a psychiatric evaluation. When I interviewed the patient, I felt that hypnosis could help.
Hypnosis—as a state of highly focused attention—can help us treat patients’ anxiety, phobias, pain, posttraumatic stress disorder (PTSD), and dissociative disorders. With training, an experienced psychiatrist can quickly start using hypnosis as an adjunct to other therapies.
This article describes how hypnosis helped Mr. M and a young woman traumatized by a criminal assault. Based on my experience and the literature, I discuss what hypnosis is, what training is required, how to measure hypnotizability, and the value of hypnosis in helping patients control their anxiety, posttraumatic, and dissociative states.
Case continued: ‘Surfing’ in Hawaii
When I met Mr. M in the hospital, I acknowledged his distress and the reasons for it, saying “You don’t really want to be here, do you?”
“How many years of medical training did it take you to figure that out?” he replied.
“Well then,” I said, “let’s go somewhere else. Where would you like to be right now?”
He responded, “I’ve never surfed.”
“Good,” I replied, “let’s go to Hawaii.” In hypnosis, I had him picture himself surfing. He continued to groan, but the pattern changed. “What happened?” I asked. “I fell off the surfboard,” he replied. “OK, get back on, and do it right,” I told him.
He learned to practice self-hypnosis, which markedly reduced his anxiety and pain. Two days later he was off pain medications and joking with the nurses in the hall. The attending physician noted in the patient’s record: “Patient off pain meds. Tumor must be regressing.”
What is hypnosis?
Mr. M’s response, though unusually strong, underscores the fact that hypnosis can rapidly produce analgesia and anxiolysis in the medical setting. Hypnosis—often called “believed-in imagination”—is characterized by an ability to sustain a state of attentive, receptive, intense focal concentration with diminished peripheral awareness. The hypnotized person is awake and alert, not asleep. Hypnosis’ three main components are absorption, dissociation, and suggestibility.
Biological basis. The hypnotic state has no brain “signature” per se, but brain imaging portrays hypnosis as a state of alertness with altered anterior cingulate gyrus activation, which helps to focus attention.1-3 Hypnotized persons can demonstrably alter blood flow in brain regions involved in perceptual processing in response to suggestions of altered perception, whether somatosensory, visual, or olfactory.4,5 Thus, patients report not only reduced pain but changes in how they experience pain with hypnotic analgesia.
The brain’s dopamine neurotransmitter system—especially in the frontal lobes—also may be involved in hypnosis, as highly hypnotizable persons have elevated levels of dopamine metabolites in their cerebrospinal fluid.6
Hypnotic trance. The trance experience is often best explained to patients as similar to being absorbed in a good novel. One loses awareness of one’s surroundings and enters the imagined world. When the novel is finished, the reader requires a moment of reorientation to the surrounding world.
A trance is a state of sustained, attentive-receptive concentration in response to a signal from within or from someone else. The signal activates this shift of awareness and permits more-intensive concentration in a designated direction.
All hypnosis is self-hypnosis. Much of its clinical value is that it can be self-induced throughout the day and whenever symptoms emerge. During the first weeks, patients can be encouraged to practice every 1 or 2 hours.
Applying hypnosis to practice. A well-trained clinician can learn to use hypnosis in classes offered by the two professional hypnosis societies or the American Psychiatric Association (Box 1) Because hypnosis is not something “done to” a patient but rather a capacity to be measured, tapped, and utilized, psychiatrists can integrate hypnosis into clinical practice after some initial training, with ongoing learning and supervision.
Who can be hypnotized?
Not everyone is equally hypnotizable, and hypnotizability is a stable and measurable trait. Approximately one-quarter of adults cannot respond to hypnotic instructions, whereas 10% are extremely hypnotizable.7
Brief, clinically useful tests of hypnotic responsiveness have been developed, such as the Hypnotic Induction Profile (HIP).8 The clinician usually can induce the trance experience and systematically measure the patient’s response within 5 minutes. A HIP score of 5 indicates usable hypnotizability.
The HIP test includes instructions to produce a sense of lightness in the left arm and hand, with tests of response to this instruction. Response is characterized by dissociation, hand elevation after it is lowered, involuntariness, response to the cutoff signal, and altered sensation.
Turning hypnotic induction into a test of hypnotic capacity transforms the initial encounter by:
- removing pressure on the clinician to successfully hypnotize the subject
- reducing patients’ experiences of complying with the clinician’s wishes, rather than exploring and discovering their own hypnotic capacity.
Placing the hypnotic experience in the context of a test also makes it consonant with other medical examinations and procedures.8
Once a patient’s hypnotizability is determined, structured measurement is no longer necessary. The test-retest correlation for hypnotizability scores is 0.7 over 25 years, which is more consistent than IQ testing.7 Subsequent inductions usually can be generated by the patient or signaled by the clinician, and only seconds are required for the shift into trance.
Effective, safe work with hypnosis requires clinical expertise in diagnostic assessment and choosing treatment options. Psychiatrists can learn techniques for inducing, measuring, and using hypnotic responsiveness in introductory and advanced workshops, supplemented by local supervision.
Courses in hypnosis are offered by many medical schools. Postgraduate training is available at annual meetings of the American Psychiatric Association, Society for Clinical and Experimental Hypnosis, and American Society of Clinical Hypnosis. The two hypnosis societies offer intensive workshops for psychiatrists, psychologists, and other health care professionals.
Useful text books also are available:
- Spiegel H, Spiegel D. Trance and treatment: clinical uses of hypnosis. Washington, DC: American Psychiatric Publishing, 2004.
- Zarren JI, Eimer BN. Brief cognitive hypnosis: facilitating the change of dysfunctional behavior. New York: Springer Publishing, 2002.
- Lynn SJ, Kirsch I, Rhue JW. Casebook of clinical hypnosis. Washington, DC: American Psychological Association, 1996.
- Fromm E, Kahn SP. Self-hypnosis: the Chicago paradigm. New York: Guilford Press, 1990.
Reducing anxiety
Anxiety can be understood as a vaguely defined but immobilizing sense of distress. Lack of clarity about the discomfort’s source enhances the patient’s sense of helplessness and avoidance. One therapeutic challenge is to convert anxiety into fear—to give it a focus so that something can be done about it.
Imagine yourself floating in a bath, a lake, a hot tub, or just floating in space. With each breath out, let a little more tension out of your body. Just enjoy this pleasant sense of floating, and notice how you can use your store of memories and fantasies to help yourself and your body feel better.
“While you imagine yourself floating, in your mind’s eye visualize an imaginary screen: a movie, TV, or computer screen, or, if you wish, a piece of clear blue sky. On that screen project your thoughts, fears, worries, ideas, feelings, or memories, while you maintain the pleasant sense of floating in your body. You establish this clear sense of your body floating here, while you relate to your thoughts and ideas out there.
“Once you have established this screen, divide it in half. Use the left side as your ‘worry screen.’ Picture one thing that causes you anxiety on this screen and learn to manage the feelings of discomfort that accompany it. Now use the right side as your ‘problem-solving’ screen. Brainstorm something you can do about the problem on the left, all the while maintaining a sense of floating in your body.
“You may have to ‘freeze’ what is on the ‘worry screen’ and re-establish the floating several times. This allows you to develop new means of coping with the things that are making you anxious, one at a time.”
Anxiety sets up a negative feedback cycle between psychological preoccupation and somatic discomfort, a “snowball effect” in which subjective anxiety and somatic tension reinforce each other. Hypnosis can help reduce anxiety and induce relaxation,9 and its dissociative component can help separate anxiety’s psychological and somatic components.
Hypnosis is as effective at reducing anxiety as 1 mg of alprazolam, at least in a study of college students.10 Student volunteers with high and low hypnotizability were given alprazolam, 1 mg, and a hypnotic suggestion based on their reactions to the drug. Four days later, when students received hypnosis only and hypnosis plus alprazolam:
- combination therapy reduced anxiety more effectively than did hypnosis or alprazolam alone, as measured by the Profile of Mood States tension-anxiety scale
- improvement was comparable with hypnosis or alprazolam alone
- highly hypnotizable students showed significantly greater relaxation than did those with low hypnotizability in all three treatment groups
- EEG data showed similar frontal and occipital changes in the alprazolam and hypnotic suggestion groups.
In randomized trials, simple self-hypnosis training has reduced pain and anxiety during medical procedures, reducing procedure time by an average 17 minutes and resulting in fewer complications.11
A typical hypnotic instruction for managing anxiety is provided in Box 2. This approach teaches patients how to deal with stressors that complicate their anxiety and to control their somatic response. Hypnosis expands patients’ repertoire of responses and enables them to feel less helpless.
Confronting phobias
Phobic symptoms of fear and avoidance or exposure with distress respond especially well to brief hypnosis interventions. Although behavior modification and antidepressants also can treat phobias successfully, one or two hypnosis sessions often can reduce or cure phobic symptoms.
For example, one can help patients with airplane phobia prepare for flight by going into a hypnotic state and learning three concepts:
- Think of the airplane as an extension of the body, such as a bicycle.
- Float with the plane.
- Think about the difference between probability and possibility.
The hypnotic state—with its focused attention and physical relaxation—can amplify this cognitive restructuring technique. Phobic patients can feel more in control of their somatic reactions and, by extrapolation, the flying experience itself. In one study, 52% of patients taught this self-hypnosis exercise remained improved or cured at least 7 years later.12
Treating traumatic reactions
Evidence is growing that trauma elicits dissociation. Thus, hypnosis could help us understand and treat traumatic reactions, including patients with acute and posttraumatic stress disorder (PTSD) and dissociative disorders.
The hypnotic state’s controlled dissociation can be used to model the uncontrolled dissociation represented by posttraumatic phenomena such as flashbacks, numbing, and amnesia.13 This view is supported by evidence that PTSD is associated with high hypnotizability.14,15
Acute stress disorder—as introduced in DSM-IV16—is characterized by prominent dissociative symptoms, with intrusion, avoidance, and hyperarousal. These diagnostic criteria recognize that acute dissociation is a common and predictable reaction to trauma.
Hypnosis involving grief work, exploration of trauma-related transference issues, and emotional expression are effective psychotherapies for persons exposed to trauma. Becoming familiar with hypnotic states can teach patients to recognize, understand, and control their dissociative states.
Evidence suggests that hypnosis’ intense concentration may reverse the dissociative mind fragmentation caused by trauma.17 Traumatic memories may seem less overwhelming and intrusive once patients discover they can:
- exert greater control over memory access and retrieval
- work through and assimilate disturbing thoughts.
The controlled experience of hypnotic abreaction (reliving traumatic and other memories with strong emotion) provides boundaries for psychotherapeutic grief work.18,19 Instead of telling patients not to ruminate over a traumatic event, the clinician instructs the patient how to think about the experience.
The inferred message is that the patient can work on other things—such as relationships and daily living problems—after this therapeutic work is done.
Patients are slowly separated from the victim role. The goal is to help them restructure their memories, both cognitively and emotionally. They bear the memories’ impact, yet come to see the information differently.7 Traumatic input becomes more bearable when linked to a cognitively restructured recognition of an adaptive response.,20 For example, patients may acknowledge what they did during a traumatic event that was self-protective or helped others.
PTSD. Hypnosis shares common elements with other cognitive and behavioral treatments for PTSD, including exposure to traumatic memories for cognitive and emotional processing. Few studies have examined using hypnosis to treat PTSD, but evidence suggests it is at least as effective as other cognitive-behavioral treatments.20,21
Patients can be taught to view PTSD’s intrusive memories and bodily symptoms as re-experiencing painful memories. The memories often intrude less frequently after patients find a controlled method—such as self-hypnosis—to access and work them through.22
Ms. J hoped hypnosis could help her better visualize the face of an assailant who had attacked her as she returned at dusk from the grocery store. She had fought off his attempt to drag her into her apartment and rape her. The police showed little interest in pursuing him, however, because the sexual assault had not been completed. After the police left, she had a grand mal seizure. She had suffered a basalar skull fracture.
Ms. J was highly hypnotizable and learned the split-screen technique. While visualizing the assault on the left screen, she realized something that had not been clear to her before: “From the look on his face, I can see he wants to kill me. If he gets me into my apartment, he will kill me.”
She focused on this realization and the image of his hatred and threat to her. The therapist asked her to picture on the right screen something she had done to protect herself. She said: “He is surprised that I am fighting so hard. He doesn’t expect me to put up such a fight.”
She emerged from hypnosis understanding that she had been in more danger than she realized. Thus, despite the disappointment of having no clearer idea of what he looked like (it was quite dark when he attacked her), she had a restructured perspective about what had occurred.
Before this session, Ms. J had felt guilty that she had gotten herself so seriously injured. Afterward, she could better tolerate the memory of the attack because it was coupled with cognitive awareness that her actions may have saved her life.
Self-blame. Many trauma victims would rather feel guilty than helpless. They blame themselves inappropriately for events over which they had no control, rather than accept their helplessness. They misuse hindsight about the trauma to assume the events were predictable and therefore avoidable. They imagine they can replay the events and change the outcome.
Such an approach to trauma can be profoundly demoralizing, leaving victims burdened by needless guilt and shame. Helping them face and bear the feelings associated with traumatic events can free them from efforts to “undo” or take responsibility for the trauma and accept what happened.
Split-screen technique. Using hypnosis with a “split-screen” technique can help patients restructure the memory of trauma. The left screen symbolizes the trauma in condensed form. The right screen helps patients focus on how they tried to master the situation. This grief work allows patients to acknowledge, bear, and put into perspective the humiliation of the experience and their loss of invulnerability, health, or loved ones (Box 3).18
Dissociation. Dissociating during a threatening situation may enable a person to put aside some awareness of the danger and take self-protective action. Persistent dissociation, however, may make it too easy to avoid working through the traumatic experiences later on.22-24
Dissociation makes subsequent exposure to reminders of the trauma more similar to a reexperiencing rather than a controlled remembering of it. This can trigger physiologic stress reactions and lead to or worsen PTSD.25-27
Dissociative disorders can be understood as chronic and severe PTSDs.28 Many individuals with dissociative disorders have histories of sexual and physical abuse.29-31 Clearly, traumatic experiences sensitize survivors to subsequent trauma through conditioned activation of fear circuitry involving the amygdala, hippocampus, and frontal lobes.32
Hypnosis can be especially helpful—both for diagnosis and therapy.33 It can assist the controlled recovery of memories, while allowing some images to remain dissociated from cognition until the patient is ready to deal with them. The patient can turn memories on and off by entering and exiting the hypnotic state and thereby recover and reprocess memories at a tolerable pace.
Related resources
- Society for Clinical and Experimental Hypnosis. http://ijceh.educ.wsu.edu
- American Society of Clinical Hypnosis. www.asch.net
- American Psychological Association, Division 30. Society of Psychological Hypnosis. http://www.apa.org/about/division/div30.html
Mr. M, a world-class athlete, collapsed suddenly in an alley. He was rushed to a hospital emergency room, where he nearly died of internal bleeding from a grapefruit-sized abdominal lymphoma. He was hospitalized and placed on chemotherapy.
Increasing doses of opiates hardly reduced his pain, and he became extremely anxious. Staff described him as “climbing the walls.” He lay in bed writhing, and his parents feared he was becoming a “drug addict.”
Anxiety about his life-threatening illness was clearly compounding his pain, so his attending physician ordered a psychiatric evaluation. When I interviewed the patient, I felt that hypnosis could help.
Hypnosis—as a state of highly focused attention—can help us treat patients’ anxiety, phobias, pain, posttraumatic stress disorder (PTSD), and dissociative disorders. With training, an experienced psychiatrist can quickly start using hypnosis as an adjunct to other therapies.
This article describes how hypnosis helped Mr. M and a young woman traumatized by a criminal assault. Based on my experience and the literature, I discuss what hypnosis is, what training is required, how to measure hypnotizability, and the value of hypnosis in helping patients control their anxiety, posttraumatic, and dissociative states.
Case continued: ‘Surfing’ in Hawaii
When I met Mr. M in the hospital, I acknowledged his distress and the reasons for it, saying “You don’t really want to be here, do you?”
“How many years of medical training did it take you to figure that out?” he replied.
“Well then,” I said, “let’s go somewhere else. Where would you like to be right now?”
He responded, “I’ve never surfed.”
“Good,” I replied, “let’s go to Hawaii.” In hypnosis, I had him picture himself surfing. He continued to groan, but the pattern changed. “What happened?” I asked. “I fell off the surfboard,” he replied. “OK, get back on, and do it right,” I told him.
He learned to practice self-hypnosis, which markedly reduced his anxiety and pain. Two days later he was off pain medications and joking with the nurses in the hall. The attending physician noted in the patient’s record: “Patient off pain meds. Tumor must be regressing.”
What is hypnosis?
Mr. M’s response, though unusually strong, underscores the fact that hypnosis can rapidly produce analgesia and anxiolysis in the medical setting. Hypnosis—often called “believed-in imagination”—is characterized by an ability to sustain a state of attentive, receptive, intense focal concentration with diminished peripheral awareness. The hypnotized person is awake and alert, not asleep. Hypnosis’ three main components are absorption, dissociation, and suggestibility.
Biological basis. The hypnotic state has no brain “signature” per se, but brain imaging portrays hypnosis as a state of alertness with altered anterior cingulate gyrus activation, which helps to focus attention.1-3 Hypnotized persons can demonstrably alter blood flow in brain regions involved in perceptual processing in response to suggestions of altered perception, whether somatosensory, visual, or olfactory.4,5 Thus, patients report not only reduced pain but changes in how they experience pain with hypnotic analgesia.
The brain’s dopamine neurotransmitter system—especially in the frontal lobes—also may be involved in hypnosis, as highly hypnotizable persons have elevated levels of dopamine metabolites in their cerebrospinal fluid.6
Hypnotic trance. The trance experience is often best explained to patients as similar to being absorbed in a good novel. One loses awareness of one’s surroundings and enters the imagined world. When the novel is finished, the reader requires a moment of reorientation to the surrounding world.
A trance is a state of sustained, attentive-receptive concentration in response to a signal from within or from someone else. The signal activates this shift of awareness and permits more-intensive concentration in a designated direction.
All hypnosis is self-hypnosis. Much of its clinical value is that it can be self-induced throughout the day and whenever symptoms emerge. During the first weeks, patients can be encouraged to practice every 1 or 2 hours.
Applying hypnosis to practice. A well-trained clinician can learn to use hypnosis in classes offered by the two professional hypnosis societies or the American Psychiatric Association (Box 1) Because hypnosis is not something “done to” a patient but rather a capacity to be measured, tapped, and utilized, psychiatrists can integrate hypnosis into clinical practice after some initial training, with ongoing learning and supervision.
Who can be hypnotized?
Not everyone is equally hypnotizable, and hypnotizability is a stable and measurable trait. Approximately one-quarter of adults cannot respond to hypnotic instructions, whereas 10% are extremely hypnotizable.7
Brief, clinically useful tests of hypnotic responsiveness have been developed, such as the Hypnotic Induction Profile (HIP).8 The clinician usually can induce the trance experience and systematically measure the patient’s response within 5 minutes. A HIP score of 5 indicates usable hypnotizability.
The HIP test includes instructions to produce a sense of lightness in the left arm and hand, with tests of response to this instruction. Response is characterized by dissociation, hand elevation after it is lowered, involuntariness, response to the cutoff signal, and altered sensation.
Turning hypnotic induction into a test of hypnotic capacity transforms the initial encounter by:
- removing pressure on the clinician to successfully hypnotize the subject
- reducing patients’ experiences of complying with the clinician’s wishes, rather than exploring and discovering their own hypnotic capacity.
Placing the hypnotic experience in the context of a test also makes it consonant with other medical examinations and procedures.8
Once a patient’s hypnotizability is determined, structured measurement is no longer necessary. The test-retest correlation for hypnotizability scores is 0.7 over 25 years, which is more consistent than IQ testing.7 Subsequent inductions usually can be generated by the patient or signaled by the clinician, and only seconds are required for the shift into trance.
Effective, safe work with hypnosis requires clinical expertise in diagnostic assessment and choosing treatment options. Psychiatrists can learn techniques for inducing, measuring, and using hypnotic responsiveness in introductory and advanced workshops, supplemented by local supervision.
Courses in hypnosis are offered by many medical schools. Postgraduate training is available at annual meetings of the American Psychiatric Association, Society for Clinical and Experimental Hypnosis, and American Society of Clinical Hypnosis. The two hypnosis societies offer intensive workshops for psychiatrists, psychologists, and other health care professionals.
Useful text books also are available:
- Spiegel H, Spiegel D. Trance and treatment: clinical uses of hypnosis. Washington, DC: American Psychiatric Publishing, 2004.
- Zarren JI, Eimer BN. Brief cognitive hypnosis: facilitating the change of dysfunctional behavior. New York: Springer Publishing, 2002.
- Lynn SJ, Kirsch I, Rhue JW. Casebook of clinical hypnosis. Washington, DC: American Psychological Association, 1996.
- Fromm E, Kahn SP. Self-hypnosis: the Chicago paradigm. New York: Guilford Press, 1990.
Reducing anxiety
Anxiety can be understood as a vaguely defined but immobilizing sense of distress. Lack of clarity about the discomfort’s source enhances the patient’s sense of helplessness and avoidance. One therapeutic challenge is to convert anxiety into fear—to give it a focus so that something can be done about it.
Imagine yourself floating in a bath, a lake, a hot tub, or just floating in space. With each breath out, let a little more tension out of your body. Just enjoy this pleasant sense of floating, and notice how you can use your store of memories and fantasies to help yourself and your body feel better.
“While you imagine yourself floating, in your mind’s eye visualize an imaginary screen: a movie, TV, or computer screen, or, if you wish, a piece of clear blue sky. On that screen project your thoughts, fears, worries, ideas, feelings, or memories, while you maintain the pleasant sense of floating in your body. You establish this clear sense of your body floating here, while you relate to your thoughts and ideas out there.
“Once you have established this screen, divide it in half. Use the left side as your ‘worry screen.’ Picture one thing that causes you anxiety on this screen and learn to manage the feelings of discomfort that accompany it. Now use the right side as your ‘problem-solving’ screen. Brainstorm something you can do about the problem on the left, all the while maintaining a sense of floating in your body.
“You may have to ‘freeze’ what is on the ‘worry screen’ and re-establish the floating several times. This allows you to develop new means of coping with the things that are making you anxious, one at a time.”
Anxiety sets up a negative feedback cycle between psychological preoccupation and somatic discomfort, a “snowball effect” in which subjective anxiety and somatic tension reinforce each other. Hypnosis can help reduce anxiety and induce relaxation,9 and its dissociative component can help separate anxiety’s psychological and somatic components.
Hypnosis is as effective at reducing anxiety as 1 mg of alprazolam, at least in a study of college students.10 Student volunteers with high and low hypnotizability were given alprazolam, 1 mg, and a hypnotic suggestion based on their reactions to the drug. Four days later, when students received hypnosis only and hypnosis plus alprazolam:
- combination therapy reduced anxiety more effectively than did hypnosis or alprazolam alone, as measured by the Profile of Mood States tension-anxiety scale
- improvement was comparable with hypnosis or alprazolam alone
- highly hypnotizable students showed significantly greater relaxation than did those with low hypnotizability in all three treatment groups
- EEG data showed similar frontal and occipital changes in the alprazolam and hypnotic suggestion groups.
In randomized trials, simple self-hypnosis training has reduced pain and anxiety during medical procedures, reducing procedure time by an average 17 minutes and resulting in fewer complications.11
A typical hypnotic instruction for managing anxiety is provided in Box 2. This approach teaches patients how to deal with stressors that complicate their anxiety and to control their somatic response. Hypnosis expands patients’ repertoire of responses and enables them to feel less helpless.
Confronting phobias
Phobic symptoms of fear and avoidance or exposure with distress respond especially well to brief hypnosis interventions. Although behavior modification and antidepressants also can treat phobias successfully, one or two hypnosis sessions often can reduce or cure phobic symptoms.
For example, one can help patients with airplane phobia prepare for flight by going into a hypnotic state and learning three concepts:
- Think of the airplane as an extension of the body, such as a bicycle.
- Float with the plane.
- Think about the difference between probability and possibility.
The hypnotic state—with its focused attention and physical relaxation—can amplify this cognitive restructuring technique. Phobic patients can feel more in control of their somatic reactions and, by extrapolation, the flying experience itself. In one study, 52% of patients taught this self-hypnosis exercise remained improved or cured at least 7 years later.12
Treating traumatic reactions
Evidence is growing that trauma elicits dissociation. Thus, hypnosis could help us understand and treat traumatic reactions, including patients with acute and posttraumatic stress disorder (PTSD) and dissociative disorders.
The hypnotic state’s controlled dissociation can be used to model the uncontrolled dissociation represented by posttraumatic phenomena such as flashbacks, numbing, and amnesia.13 This view is supported by evidence that PTSD is associated with high hypnotizability.14,15
Acute stress disorder—as introduced in DSM-IV16—is characterized by prominent dissociative symptoms, with intrusion, avoidance, and hyperarousal. These diagnostic criteria recognize that acute dissociation is a common and predictable reaction to trauma.
Hypnosis involving grief work, exploration of trauma-related transference issues, and emotional expression are effective psychotherapies for persons exposed to trauma. Becoming familiar with hypnotic states can teach patients to recognize, understand, and control their dissociative states.
Evidence suggests that hypnosis’ intense concentration may reverse the dissociative mind fragmentation caused by trauma.17 Traumatic memories may seem less overwhelming and intrusive once patients discover they can:
- exert greater control over memory access and retrieval
- work through and assimilate disturbing thoughts.
The controlled experience of hypnotic abreaction (reliving traumatic and other memories with strong emotion) provides boundaries for psychotherapeutic grief work.18,19 Instead of telling patients not to ruminate over a traumatic event, the clinician instructs the patient how to think about the experience.
The inferred message is that the patient can work on other things—such as relationships and daily living problems—after this therapeutic work is done.
Patients are slowly separated from the victim role. The goal is to help them restructure their memories, both cognitively and emotionally. They bear the memories’ impact, yet come to see the information differently.7 Traumatic input becomes more bearable when linked to a cognitively restructured recognition of an adaptive response.,20 For example, patients may acknowledge what they did during a traumatic event that was self-protective or helped others.
PTSD. Hypnosis shares common elements with other cognitive and behavioral treatments for PTSD, including exposure to traumatic memories for cognitive and emotional processing. Few studies have examined using hypnosis to treat PTSD, but evidence suggests it is at least as effective as other cognitive-behavioral treatments.20,21
Patients can be taught to view PTSD’s intrusive memories and bodily symptoms as re-experiencing painful memories. The memories often intrude less frequently after patients find a controlled method—such as self-hypnosis—to access and work them through.22
Ms. J hoped hypnosis could help her better visualize the face of an assailant who had attacked her as she returned at dusk from the grocery store. She had fought off his attempt to drag her into her apartment and rape her. The police showed little interest in pursuing him, however, because the sexual assault had not been completed. After the police left, she had a grand mal seizure. She had suffered a basalar skull fracture.
Ms. J was highly hypnotizable and learned the split-screen technique. While visualizing the assault on the left screen, she realized something that had not been clear to her before: “From the look on his face, I can see he wants to kill me. If he gets me into my apartment, he will kill me.”
She focused on this realization and the image of his hatred and threat to her. The therapist asked her to picture on the right screen something she had done to protect herself. She said: “He is surprised that I am fighting so hard. He doesn’t expect me to put up such a fight.”
She emerged from hypnosis understanding that she had been in more danger than she realized. Thus, despite the disappointment of having no clearer idea of what he looked like (it was quite dark when he attacked her), she had a restructured perspective about what had occurred.
Before this session, Ms. J had felt guilty that she had gotten herself so seriously injured. Afterward, she could better tolerate the memory of the attack because it was coupled with cognitive awareness that her actions may have saved her life.
Self-blame. Many trauma victims would rather feel guilty than helpless. They blame themselves inappropriately for events over which they had no control, rather than accept their helplessness. They misuse hindsight about the trauma to assume the events were predictable and therefore avoidable. They imagine they can replay the events and change the outcome.
Such an approach to trauma can be profoundly demoralizing, leaving victims burdened by needless guilt and shame. Helping them face and bear the feelings associated with traumatic events can free them from efforts to “undo” or take responsibility for the trauma and accept what happened.
Split-screen technique. Using hypnosis with a “split-screen” technique can help patients restructure the memory of trauma. The left screen symbolizes the trauma in condensed form. The right screen helps patients focus on how they tried to master the situation. This grief work allows patients to acknowledge, bear, and put into perspective the humiliation of the experience and their loss of invulnerability, health, or loved ones (Box 3).18
Dissociation. Dissociating during a threatening situation may enable a person to put aside some awareness of the danger and take self-protective action. Persistent dissociation, however, may make it too easy to avoid working through the traumatic experiences later on.22-24
Dissociation makes subsequent exposure to reminders of the trauma more similar to a reexperiencing rather than a controlled remembering of it. This can trigger physiologic stress reactions and lead to or worsen PTSD.25-27
Dissociative disorders can be understood as chronic and severe PTSDs.28 Many individuals with dissociative disorders have histories of sexual and physical abuse.29-31 Clearly, traumatic experiences sensitize survivors to subsequent trauma through conditioned activation of fear circuitry involving the amygdala, hippocampus, and frontal lobes.32
Hypnosis can be especially helpful—both for diagnosis and therapy.33 It can assist the controlled recovery of memories, while allowing some images to remain dissociated from cognition until the patient is ready to deal with them. The patient can turn memories on and off by entering and exiting the hypnotic state and thereby recover and reprocess memories at a tolerable pace.
Related resources
- Society for Clinical and Experimental Hypnosis. http://ijceh.educ.wsu.edu
- American Society of Clinical Hypnosis. www.asch.net
- American Psychological Association, Division 30. Society of Psychological Hypnosis. http://www.apa.org/about/division/div30.html
1. Spiegel D, Jasiukaitis P. Hypnosis: Brain basis. In: Smith BH (ed). Elsevier’s encyclopedia of neuroscience. The Netherlands: Elsevier Science, 1999.
2. Rainville P, Hofbauer RK, Bushnell MC, et al. Hypnosis modulates activity in brain structures involved in the regulation of consciousness. J Cogn Neurosci 2002;14:887-901.
3. Rainville P, Duncan GH, Price DD, et al. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 1997;277:968-71.
4. Kosslyn SM, Thompson WL, Costantini-Ferrando MF, et al. Hypnotic visual illusion alters color processing in the brain. Am J Psychiatry 2000;157:1279-84.
5. Spiegel D. Negative and positive visual hypnotic hallucinations: attending inside and out. Int J Clin Exp Hypn 2003;51:130-46.
6. Spiegel D, King R. Hypnotizability and CSF HVA levels among psychiatric patients. Biol Psychiatry 1992;31:95-8.
7. Piccione C, Hilgard ER, Zimbardo PG. On the degree of stability of measured hypnotizability over a 25-year period. J Pers Soc Psychol 1989;56:289-95.
8. Spiegel H, Spiegel D. Trance and treatment: Clinical uses of hypnosis. Washington, DC: American Psychiatric Press, 2004.
9. Wertz JM, Sayette MA. Effects of smoking opportunity on attentional bias in smokers. Psychol Addict Behav 2001;15:268-71.
10. Nishith P, Barabasz A, Barabasz M, Warner D. Brief hypnosis substitutes for alprazolam use in college students: transient experiences and quantitative EEG responses. Am J Clin Hypn 1999;41:262-8.
11. Lang EV, Benotsch EG, Fick LJ, et al. Adjunctive nonpharmacological analgesia for invasive medical procedures: a randomised trial. Lancet 2000;355:1486-90.
12. Spiegel D, Frischholz EJ, Maruffi B, Spiegel H. Hypnotic responsitivity and the treatment of flying phobia. Am J Clin Hypn 1981;23:239-47.
13. Butler LD, Duran EFD, Jasiukaitis P, et al. Hypnotizability and traumatic experience: a diathesis-stress model of dissociative symptomatology. Am J Psychiatry 1996;153:42-63.
14. Spiegel D. Dissociation and hypnosis in post-traumatic stress disorder. J Trauma Stress 1988;1:17-33.
15. Stutman RK, Bliss EL. Posttraumatic stress disorder, hypnotizability, and imagery. Am J Psychiatry 1985;142:741-3.
16. Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington, DC: American Psychiatric Association, 2000.
17. Maldonado JR, Spiegel D. Trauma, dissociation and hypnotizability. In: Marmar R, Bremmer D (eds). Trauma, memory and dissociation. Washington, DC: American Psychiatric Press, 1998.
18. Lindemann E. Symptomatology and management of acute grief. Am J Psychiatry 1994;151:155-60.
19. Spiegel D. Vietnam grief work using hypnosis. Am J Clin Hypn 1981;24:33-40.
20. Foa EB, Davidson JRT, Frances A. Treatment of posttraumatic stress disorder. J Clin Psychiatry 1999;50:4-69.
21. Brom D, Kleber RJ, Defare PB. Brief psychotherapy for post-traumatic stress disorder. J Consult Clin Psychol 1989;57:607-12.
22. Spiegel D. Hypnosis and implicit memory: automatic processing of explicit content. Am J Clin Hypn 1998;40:231-40.
23. Spiegel D. Multiple personality as a post-traumatic stress disorder. Psychiatr Clin North Am 1984;7:101-10.
24. Kluft RP. Dissociation as a response to extreme trauma. In: Kluft RP (ed). Childhood antecedents of multiple personality. Washington, DC: American Psychiatric Press, 1985:66-97.
25. Marmar CR, Weiss DS, Metzler T. Peritraumatic dissociation and posttraumatic stress disorder. In: Bremner JD, Marmar C (eds). Trauma, memory, and dissociation. Washington, DC: American Psychiatric Press, 1998;229-52.
26. Birmes P. Peritraumatic dissociation, acute stress, and early posttraumatic stress disorder in victims of general crime. Can J Psychiatry 2001;46:649-51.
27. Spiegel D. Hypnosis, dissociation and trauma. In: Burrows GD, Stanley RO, Bloom PB (eds). Clinical hypnosis. New York: John Wiley & Sons, 2001;143-58.
28. Spiegel D, Cardena E. Disintegrated experience: the dissociative disorders revisited. J Abnorm Psychol 1991;100:366-78.
29. Chu JA, Dill DL. Dissociative symptoms in relation to childhood physical and sexual abuse. Am J Psychiatry 1990;147:887-92.
30. Kluft RP. Childhood antecedents of multiple personality. Washington, DC: American Psychiatric Press, 1985.
31. Spiegel D. Dissociating damage. Am J Clin Hypn 1986;29:123-31.
32. LeDoux J. Synaptic self: How our brains become who we are. New York: Viking Press, 2002.
33. Putnam FW. Using hypnosis for therapeutic abreactions. Psychiatr Med 1992;10:51-65.
1. Spiegel D, Jasiukaitis P. Hypnosis: Brain basis. In: Smith BH (ed). Elsevier’s encyclopedia of neuroscience. The Netherlands: Elsevier Science, 1999.
2. Rainville P, Hofbauer RK, Bushnell MC, et al. Hypnosis modulates activity in brain structures involved in the regulation of consciousness. J Cogn Neurosci 2002;14:887-901.
3. Rainville P, Duncan GH, Price DD, et al. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 1997;277:968-71.
4. Kosslyn SM, Thompson WL, Costantini-Ferrando MF, et al. Hypnotic visual illusion alters color processing in the brain. Am J Psychiatry 2000;157:1279-84.
5. Spiegel D. Negative and positive visual hypnotic hallucinations: attending inside and out. Int J Clin Exp Hypn 2003;51:130-46.
6. Spiegel D, King R. Hypnotizability and CSF HVA levels among psychiatric patients. Biol Psychiatry 1992;31:95-8.
7. Piccione C, Hilgard ER, Zimbardo PG. On the degree of stability of measured hypnotizability over a 25-year period. J Pers Soc Psychol 1989;56:289-95.
8. Spiegel H, Spiegel D. Trance and treatment: Clinical uses of hypnosis. Washington, DC: American Psychiatric Press, 2004.
9. Wertz JM, Sayette MA. Effects of smoking opportunity on attentional bias in smokers. Psychol Addict Behav 2001;15:268-71.
10. Nishith P, Barabasz A, Barabasz M, Warner D. Brief hypnosis substitutes for alprazolam use in college students: transient experiences and quantitative EEG responses. Am J Clin Hypn 1999;41:262-8.
11. Lang EV, Benotsch EG, Fick LJ, et al. Adjunctive nonpharmacological analgesia for invasive medical procedures: a randomised trial. Lancet 2000;355:1486-90.
12. Spiegel D, Frischholz EJ, Maruffi B, Spiegel H. Hypnotic responsitivity and the treatment of flying phobia. Am J Clin Hypn 1981;23:239-47.
13. Butler LD, Duran EFD, Jasiukaitis P, et al. Hypnotizability and traumatic experience: a diathesis-stress model of dissociative symptomatology. Am J Psychiatry 1996;153:42-63.
14. Spiegel D. Dissociation and hypnosis in post-traumatic stress disorder. J Trauma Stress 1988;1:17-33.
15. Stutman RK, Bliss EL. Posttraumatic stress disorder, hypnotizability, and imagery. Am J Psychiatry 1985;142:741-3.
16. Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington, DC: American Psychiatric Association, 2000.
17. Maldonado JR, Spiegel D. Trauma, dissociation and hypnotizability. In: Marmar R, Bremmer D (eds). Trauma, memory and dissociation. Washington, DC: American Psychiatric Press, 1998.
18. Lindemann E. Symptomatology and management of acute grief. Am J Psychiatry 1994;151:155-60.
19. Spiegel D. Vietnam grief work using hypnosis. Am J Clin Hypn 1981;24:33-40.
20. Foa EB, Davidson JRT, Frances A. Treatment of posttraumatic stress disorder. J Clin Psychiatry 1999;50:4-69.
21. Brom D, Kleber RJ, Defare PB. Brief psychotherapy for post-traumatic stress disorder. J Consult Clin Psychol 1989;57:607-12.
22. Spiegel D. Hypnosis and implicit memory: automatic processing of explicit content. Am J Clin Hypn 1998;40:231-40.
23. Spiegel D. Multiple personality as a post-traumatic stress disorder. Psychiatr Clin North Am 1984;7:101-10.
24. Kluft RP. Dissociation as a response to extreme trauma. In: Kluft RP (ed). Childhood antecedents of multiple personality. Washington, DC: American Psychiatric Press, 1985:66-97.
25. Marmar CR, Weiss DS, Metzler T. Peritraumatic dissociation and posttraumatic stress disorder. In: Bremner JD, Marmar C (eds). Trauma, memory, and dissociation. Washington, DC: American Psychiatric Press, 1998;229-52.
26. Birmes P. Peritraumatic dissociation, acute stress, and early posttraumatic stress disorder in victims of general crime. Can J Psychiatry 2001;46:649-51.
27. Spiegel D. Hypnosis, dissociation and trauma. In: Burrows GD, Stanley RO, Bloom PB (eds). Clinical hypnosis. New York: John Wiley & Sons, 2001;143-58.
28. Spiegel D, Cardena E. Disintegrated experience: the dissociative disorders revisited. J Abnorm Psychol 1991;100:366-78.
29. Chu JA, Dill DL. Dissociative symptoms in relation to childhood physical and sexual abuse. Am J Psychiatry 1990;147:887-92.
30. Kluft RP. Childhood antecedents of multiple personality. Washington, DC: American Psychiatric Press, 1985.
31. Spiegel D. Dissociating damage. Am J Clin Hypn 1986;29:123-31.
32. LeDoux J. Synaptic self: How our brains become who we are. New York: Viking Press, 2002.
33. Putnam FW. Using hypnosis for therapeutic abreactions. Psychiatr Med 1992;10:51-65.
Bipolar maintenance: Are atypical antipsychotics really ‘mood stabilizers’?
Maintenance therapy with mood stabilizers is the most critical phase of bipolar disorder treatment but the stage with the least available evidence about medication risks and benefits. The FDA’s recent approval of olanzapine for bipolar maintenance raises the question of whether atypical antipsychotics are really mood stabilizers. This article attempts to answer that question by:
- describing the “ideal” mood stabilizer
- discussing atypicals’ advantages over conventional antipsychotics in bipolar patients
- comparing efficacy data for the six available atypicals
- recommending strategies to prevent and treat atypicals’ potentially serious side effects during long-term therapy.
What is a ‘mood stabilizer’?
Successful mood stabilizer maintenance therapy decreases the time patients are sick and disabled. Although somewhat dated after only 2 years, the most recent American Psychiatric Association (APA) practice guidelines1 support using mood stabilizers for patients with bipolar I and bipolar II disorders.
Table 1
Bipolar maintenance treatment goals
|
| Adapted from American Psychiatric Association practice guidelines for treating patients with bipolar disorder (reference 1) |
The goals of maintenance therapy are listed in Table 1. The ideal mood stabilizer would work in maintenance and all bipolar phases and treatment stages—from treating acute depression, mania, hypomania, and mixed states to preventing abnormal mood elevations and depressions. It would not precipitate depression or mania, rapid cycling, or cycle acceleration.
In other words, the best “mood stabilizer” would work in all four treatment roles of bipolar disorder: treating highs and lows, and preventing highs and lows. No such mood stabilizer exists, although lithium may come closest to the ideal.2
Most U.S. psychiatrists use combination therapies for bipolar disorder, particularly when treating acute manic states. The most common combination is a “known” mood stabilizer—such as lithium or divalproex—plus an antipsychotic to quickly control mania.
After mania remits, clinicians often try to eliminate the antipsychotic in hopes of maintaining mood stability and euthymia with the mood stabilizer alone. This was especially true before atypical antipsychotics were approved, given the risk for tardive dyskinesia (TD) associated with long-term use of conventional antipsychotics.
Unfortunately, patients frequently relapse with this strategy, so psychiatrists may leave their bipolar patients on atypical antipsychotics during long-term maintenance. But how good are atypicals as mood stabilizers? Perhaps more importantly, how safe is long-term use of atypicals in bipolar patients?
Antipsychotics as mood stabilizers
The 2002 APA practice guidelines discuss efficacy data for using lithium, divalproex or valproate, lamotrigine, carbamazepine, and electroconvulsive therapy for bipolar maintenance treatment. Two sentences on antipsychotic drug use note:
- one placebo-controlled study of a conventional antipsychotic showing no efficacy
- some data supporting clozapine as a prophylactic bipolar treatment.1
A 1998 review of five open trials3 touched on conventional depot antipsychotics’ value in reducing manic or affective illness. However, the authors warned:
- no controlled trials existed
- maintenance antipsychotic treatment may be associated with increased risk for tardive movement disorders
- conventional agents can exacerbate depressive symptoms in some patients.
Using conventional antipsychotics long-term in bipolar disorder is not advisable, with the possible exception of depot preparations in nonadhering patients with severe illness. Long-acting injectable atypicals—such as the recently approved IM risperidone—may displace any use of conventional antipsychotics in bipolar patients.
Atypical antipsychotics hold several advantages over conventional agents:
- significantly reduced risk for TD and extrapyramidal symptoms (EPS)
- lack of serum prolactin elevation (except with risperidone)
- improved cognition
- possible decreased suicidality, particularly with clozapine.4
Table 2
Tips for managing atypicals’ potentially serious side-effect risks
| Weight gain/obesity | ||
| Assessment | Prevention | Treatment |
| Evaluate comorbid conditions such as eating disorders or substance abuse Take nutritional and exercise history | Check weight and waist circumference at baseline and every visit Calculate body mass index at every visit Prescribe healthy diet and exercise | Patient education, careful monitoring, and prevention are most-effective treatments Drug therapy for persistent weight gain or early rapid gain (>7% in first 6 months). Agents of potential benefit include topiramate, sibutramine, metformin, zonisamide, and orlistat (see Table 3) |
| Glucose control/type 2 diabetes | ||
| Assessment | Prevention | Treatment |
| Take history of glucose intolerance or diabetes Ask about family history of diabetes, obesity, hypertension, heart disease | Check baseline weight and plasma glucose Obtain fasting plasma glucose every 3 months for first year, then annually Prescribe healthy diet and exercise | Primary prevention through careful monitoring is most effective Discontinue atypical antipsychotic; use other mood stabilizer unless atypical is only effective drug for that patient Oral hypoglycemics (metformin, others) |
| Hyperlipidemia | ||
| Assessment | Prevention | Treatment |
| Take history of hyperlipidemia or cardiovascular disease Ask about family history of hyperlipidemia | Check fasting lipid profile including triglycerides at baseline and every 3 months in first year Prescribe healthy diet and exercise | Monitor diet, exercise, weight, lipids regularly Change atypical antipsychotic or use other mood stabilizer (as described above) Oral antilipemics (simvastatin, others) |
Evidence for atypicals
Olanzapine is the only atypical FDA-approved for relapse prevention in bipolar disorder. This approval is supported by several studies, most notably two 1-year, double-blind trials:
- Mean time to any mood relapse was 174 days in patients taking olanzapine, mean 12.5 mg/d (±5 mg), compared with 22 days in a placebo group (Eli Lilly and Co., data on file).
- Manic relapse rate was 14.3% in patients treated with olanzapine, ~12 mg/d, compared with 28% in patients treated with lithium, ~1,100 mg/d (mean 0.76 mEq/L). The two treatments were similarly effective in preventing depressive relapse.5
As a mood stabilizer, olanzapine was as effective as divalproex in a 47-week randomized, double-blind study of 251 adults with bipolar I disorder.6 Patients treated with olanzapine improved more rapidly and had fewer manic symptoms than those treated with divalproex, but bipolar relapse rates were similar in both treatment groups.
Risperidone appears to have a role as a potential maintenance mood stabilizer in bipolar patients, although double-blind trials are lacking.
In a 6-month, open-label investigation, relapse rates were 16% for depression and 7% for mania in bipolar patients receiving risperidone (average 4 mg/d) combined with mood-stabilizing medications.7 These relapse rates are lower than those typically reported for mood-stabilizing monotherapy.
In another 6-month, open-label study, risperidone monotherapy (average 4 mg/d) was effective for treating mania and maintaining euthymia.8
IM risperidone is a useful option for bipolar patients chronically nonadherent with oral medications; it also substantially reduces the risk of neuroleptic side effects compared with older depot antipsychotics.
Quetiapine was recently approved as an antimanic agent and may possess mood-stabilizing properties. In a preliminary study of 10 patients with bipolar disorder, adding quetiapine (mean 200 mg/d) to existing mood stabilizer therapy for 12 weeks improved psychopathology, mania, and depression rating scale scores.9
More-recent unpublished data suggest dosing quetiapine to approximately 600 mg/d as monotherapy or an adjunct to treat acute mania, though controlled maintenance studies are lacking (AstraZeneca Pharmaceuticals, data on file).
Others. Some early evidence supports using ziprasidone and aripiprazole for bipolar mania:
- Ziprasidone monotherapy, 40 to 80 mg bid, was significantly more effective than placebo in reducing acute mania symptoms in a 3-week, double-blind, randomized trial of 197 patients with bipolar I disorder.10
- Aripiprazole monotherapy, 15 to 30 mg/d, had a significantly greater effect than placebo in a 3-week, double-blind, randomized trial of 262 patients in acute manic or mixed bipolar episodes. Response rates among patients with mania were 40% with aripiprazole and 19% with placebo.11
Both ziprasidone and aripiprazole were well-tolerated in these brief trials, although their efficacy as long-term mood-stabilizers in bipolar disorder is unclear.
Using clozapine raises concerns about potentially serious adverse events, although it remains the only agent with proven efficacy in treatment-refractory mania.12,13 Clozapine also appears to reduce hospitalization and affective relapse rates and improve symptoms and quality of life.14,15
Long-term safety
Compared with conventional antipsychotics, EPS are not a major concern with the atypical agents. Except for risperidone, atypicals’ effect on prolactin levels generally is not clinically meaningful. Atypicals appear to be “mood-friendly,” whereas conventional antipsychotics seem to contribute to dysphoria or cause depression in some patients.
Sedation or other annoying side effects such as dry mouth or dizziness can occur with any atypical. Other more-serious side effects may complicate antipsychotic treatment, as we are coming to understand from using atypicals for long-term schizophrenia management.
Table 3
Weight-loss medications for bipolar patients taking atypical antipsychotics
| Drug | Dosage | Side effects | Recommendations |
|---|---|---|---|
| Metformin | 500 to 1,000 mg bid | Hypoglycemia Diarrhea Nausea/vomiting | First-line in patients with comorbid type 2 diabetes |
| Orlistat | 120 mg tid | GI distress Change in bowel habits | Second-line For patients with BMI >27 Supplement fat-soluble vitamins |
| Sibutramine | 5 to 15 mg/d | Dry mouth Anorexia Insomnia Constipation | Second-line For patients with BMI 27 to 30 Risk of serotonin syndrome if given with serotonergic drugs |
| Topiramate | 50 to 250 mg/d | Somnolence Fatigue Paresthesias | Consider first-line for its potential additive mood-stabilizing effect May help comorbid binge-eating or seizure disorders |
| Zonisamide | 100 to 600 mg/d | Somnolence Dizziness Anorexia | Consider first-line for its potential additive mood-stabilizing effect May help comorbid binge-eating or seizure disorders |
Movement disorders. Antipsychotics appear more likely to cause EPS in patients with mood disorders than with schizophrenia. In one study using conventional antipsychotics, bipolar patients were 4 to 5 times more likely than schizophrenia patients to experience acute dystonia.16
Although atypicals pose some small risk for acute EPS and TD, the risk is near placebo-level with clinically relevant and comparable dosages.17 Even so, it is important to educate patients to watch for emerging signs of TD during long-term treatment with any antipsychotic. EPS risk may be dose-dependent, particularly with risperidone.18
Weight gain and obesity. Patients with bipolar disorder are more likely to be overweight or obese (body mass index [BMI] > 30) than the general population,17,19 though the reasons are unknown. Studies suggest an obesity prevalence of 32% to 35% in bipolar patients, compared with 18% in the general population.20,21
All atypicals can cause weight gain, although olanzapine and clozapine are associated with the greatest mean weight gains. In three long-term trials (47 weeks to 18 months), bipolar patients who received olanzapine gained significantly more weight (mean 2 to 3 kg) than those receiving lithium or divalproex.19
Cases with much greater weight gain—even leading to clinical obesity—have been observed, particularly with olanzapine. Although evidence from registration trials and clinical experience show lesser weight gains with risperidone, quetiapine, ziprasidone, and aripiprazole, some of our patients do gain weight while taking these agents—either alone or in combination with lithium or divalproex.
Weight management. Because patients with bipolar disorder may be at increased risk for weight gain and obesity, weight management techniques may improve their health by:
- decreasing morbidity and mortality tied to weight-related physical illnesses
- enhancing psychological well-being.1
In addition to diet and exercise counseling, some bipolar patients taking long-term atypical antipsychotics may benefit from adjunctive weight-loss medications (Table 3). We generally use such medications for bipolar patients who:
- persistently gain weight despite best dietary practices
- gain substantial weight early in treatment with an atypical antipsychotic that is providing effective symptomatic relief.
Early weight gain—particularly gains of >7% within the first 6 weeks—might predict large weight gain over time.
Diabetes. In September 2003, the FDA requested a class-wide labeling change to warn about a possible link between atypical antipsychotics and diabetes. The FDA recommended blood sugar monitoring of patients taking atypicals, especially those with obesity risk factors or family history of diabetes.
Type 2 diabetes develops in some patients taking atypicals, whether or not they gain substantial weight.22 This suggests that weight gain associated with bipolar disorder and the use of atypical antipsychotics may be independent risk factors for diabetes—a clear concern when treating bipolar patients.
Evidence provides no clear answer as to which atypicals may increase diabetes risk. Cautious use and vigilant monitoring of blood glucose are therefore recommended for every patient taking an atypical for long-term therapy. Also watch for increases in triglycerides and cholesterol17 in patients taking atypicals as bipolar maintenance therapy.
Conclusion
Atypical antipsychotics are valuable therapies in preventing bipolar relapses, although olanzapine is the only atypical with this indication so far. Collective data and clinical experience suggest that atypicals are indeed mood stabilizers, although—like other mood stabilizers such as lithium or divalproex—they have limitations. None achieve ideal efficacy in all four bipolar treatment roles: treating the highs and lows, and preventing the highs and lows. Atypicals seem more effective in treating and preventing the highs than the lows, reminding us that effective depression treatment is the greatest unmet need in bipolar disorder.
More double-blind, randomized, controlled trials are needed to fully understand whether all atypicals are mood stabilizers and to determine their safety and side effects in long-term therapy for patients with bipolar disorder.
Related resources
- Depression and Bipolar Support Alliance. www.dbsalliance.org
- Muzina DJ, Calabrese JR. Guidelines for treatment of bipolar disorder.In: Stein DJ, Kupfer DJ, Schatzberg AF (eds). Textbook of mood disorders Washington, DC: American Psychiatric Publishing, 2004 (in press).
Drug brand names
- Aripiprazole • Abilify
- Carbamazepine • Tegretol
- Clozapine • Clozaril
- Divalproex/valproate • Depakote, Depakene
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid, et al
- Metformin • Glucophage
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal, Risperdal Consta
- Sibutramine • Meridia
- Simvastatin • Zocor
- Topiramate • Topamax
- Ziprasidone • Geodon
- Zonisamide • Zonegran
Disclosure
Dr. Muzina receives research grants from AstraZeneca Pharmaceuticals, Eli Lilly and Co., and Abbott Laboratories, is a consultant to AstraZeneca Pharmaceuticals and Pfizer, Inc., and a speaker for AstraZeneca Pharmaceuticals, Pfizer Inc., Eli Lilly and Co., and GlaxoSmithKline.
1. Hirschfeld RM, Bowden CL, Gitlin MJ, et al. Practice guideline for the treatment of patients with bipolar disorder (rev). Am J Psychiatry 2002;159:1-50.
2. Bauer MS, Mitchner L. What is a “mood stabilizer?” An evidence-based response. Am J Psychiatry 2004;161(1):3-18.
3. Keck PE, Jr, McElroy SL, Strakowski SM. Anticonvulsants and antipsychotics in the treatment of bipolar disorder. J Clin Psychiatry 1998;59(suppl 6):74-81.
4. Sharma V. Atypical antipsychotics and suicide in mood and anxiety disorders. Bipolar Disord 2003;5(suppl 2):48-52.
5. Tohen M, Marneros A, Bowden C, et al. Olanzapine versus lithium in relapse prevention in bipolar disorder: a randomized double-blind controlled 12-month clinical trial (presentation). Freiberg, Germany: Stanley Foundation Bipolar Network, Sept. 11-14, 2002.
6. Tohen M, Ketter TA, Zarate CA, et al. Olanzapine versus divalproex sodium for the treatment of acute mania and maintenance of remission: a 47-week study. Am J Psychiatry 2003;160(7):1263-71.
7. Vieta E, Goikolea JM, Corbella B, et al. Risperidone safety and efficacy in the treatment of bipolar and schizoaffective disorders: results from a 6-month, multicenter, open study. J Clin Psychiatry 2001;62(10):818-25.
8. Vieta E, Brugue E, Goikolea JM, et al. Acute and continuation risperidone monotherapy in mania. Hum Psychopharmacol 2004;19(1):41-5.
9. Sajatovic M, Brescan DW, Perez DE, et al. Quetiapine alone and added to a mood stabilizer for serious mood disorders. J Clin Psychiatry 2001;62(9):728-32.
10. Keck PE, Jr, Versiani M, Potkin S, et al. Ziprasidone in the treatment of acute bipolar mania: a three-week, placebo-controlled, double-blind, randomized trial. Am J Psychiatry 2003;160(4):741-8.
11. Keck PE, Jr, Marcus R, Tourkodimitris S, et al. A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole in patients with acute bipolar mania. Am J Psychiatry 2003;160(9):1651-8.
12. Calabrese JR, Kimmel SE, Woyshville MJ, et al. Clozapine for treatment-refractory mania. Am J Psychiatry 1996;153(6):759-64.
13. Green AI, Tohen M, Patel JK, et al. Clozapine in the treatment of refractory psychotic mania. Am J Psychiatry 2000;157(6):982-6.
14. Zarate CA, Jr, Tohen M, Banov MD, et al. Is clozapine a mood stabilizer? J Clin Psychiatry 1995;56(3):108-12.
15. Suppes T, Webb A, Paul B, et al. Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry 1999;156(8):1164-9.
16. Nasrallah HA, Churchill CM, Hamdan-Allan GA. Higher frequency of neuroleptic-induced dystonia in mania than in schizophrenia. Am J Psychiatry 1988;145(11):1455-6.
17. Chue P, Kovacs CS. Safety and tolerability of atypical antipsychotics in patients with bipolar disorder: prevalence, monitoring and management. Bipolar Disord 2003;5(suppl 2):62-79.
18. Simpson GM, Lindenmayer JP. Extrapyramidal symptoms in patients treated with risperidone. J Clin Psychopharmacol 1997;17(3):194-201.
19. Keck PE, Jr, McElroy SL. Bipolar disorder, obesity, and pharmacotherapy-associated weight gain. J Clin Psychiatry 2003;64(12):1426-35.
20. Fagiolini A, Frank E, Houck PR, et al. Prevalence of obesity and weight change during treatment in patients with bipolar I disorder. J Clin Psychiatry 2002;63(6):528-33.
21. Fagiolini A, Kupfer DJ, Houck PR, et al. Obesity as a correlate of outcome in patients with bipolar I disorder. Am J Psychiatry 2003;160(1):112-17.
22. Haupt DW, Newcomer JW. Abnormalities in glucose regulation associated with mental illness and treatment. J Psychosom Res 2002;53(4):925-33.
Maintenance therapy with mood stabilizers is the most critical phase of bipolar disorder treatment but the stage with the least available evidence about medication risks and benefits. The FDA’s recent approval of olanzapine for bipolar maintenance raises the question of whether atypical antipsychotics are really mood stabilizers. This article attempts to answer that question by:
- describing the “ideal” mood stabilizer
- discussing atypicals’ advantages over conventional antipsychotics in bipolar patients
- comparing efficacy data for the six available atypicals
- recommending strategies to prevent and treat atypicals’ potentially serious side effects during long-term therapy.
What is a ‘mood stabilizer’?
Successful mood stabilizer maintenance therapy decreases the time patients are sick and disabled. Although somewhat dated after only 2 years, the most recent American Psychiatric Association (APA) practice guidelines1 support using mood stabilizers for patients with bipolar I and bipolar II disorders.
Table 1
Bipolar maintenance treatment goals
|
| Adapted from American Psychiatric Association practice guidelines for treating patients with bipolar disorder (reference 1) |
The goals of maintenance therapy are listed in Table 1. The ideal mood stabilizer would work in maintenance and all bipolar phases and treatment stages—from treating acute depression, mania, hypomania, and mixed states to preventing abnormal mood elevations and depressions. It would not precipitate depression or mania, rapid cycling, or cycle acceleration.
In other words, the best “mood stabilizer” would work in all four treatment roles of bipolar disorder: treating highs and lows, and preventing highs and lows. No such mood stabilizer exists, although lithium may come closest to the ideal.2
Most U.S. psychiatrists use combination therapies for bipolar disorder, particularly when treating acute manic states. The most common combination is a “known” mood stabilizer—such as lithium or divalproex—plus an antipsychotic to quickly control mania.
After mania remits, clinicians often try to eliminate the antipsychotic in hopes of maintaining mood stability and euthymia with the mood stabilizer alone. This was especially true before atypical antipsychotics were approved, given the risk for tardive dyskinesia (TD) associated with long-term use of conventional antipsychotics.
Unfortunately, patients frequently relapse with this strategy, so psychiatrists may leave their bipolar patients on atypical antipsychotics during long-term maintenance. But how good are atypicals as mood stabilizers? Perhaps more importantly, how safe is long-term use of atypicals in bipolar patients?
Antipsychotics as mood stabilizers
The 2002 APA practice guidelines discuss efficacy data for using lithium, divalproex or valproate, lamotrigine, carbamazepine, and electroconvulsive therapy for bipolar maintenance treatment. Two sentences on antipsychotic drug use note:
- one placebo-controlled study of a conventional antipsychotic showing no efficacy
- some data supporting clozapine as a prophylactic bipolar treatment.1
A 1998 review of five open trials3 touched on conventional depot antipsychotics’ value in reducing manic or affective illness. However, the authors warned:
- no controlled trials existed
- maintenance antipsychotic treatment may be associated with increased risk for tardive movement disorders
- conventional agents can exacerbate depressive symptoms in some patients.
Using conventional antipsychotics long-term in bipolar disorder is not advisable, with the possible exception of depot preparations in nonadhering patients with severe illness. Long-acting injectable atypicals—such as the recently approved IM risperidone—may displace any use of conventional antipsychotics in bipolar patients.
Atypical antipsychotics hold several advantages over conventional agents:
- significantly reduced risk for TD and extrapyramidal symptoms (EPS)
- lack of serum prolactin elevation (except with risperidone)
- improved cognition
- possible decreased suicidality, particularly with clozapine.4
Table 2
Tips for managing atypicals’ potentially serious side-effect risks
| Weight gain/obesity | ||
| Assessment | Prevention | Treatment |
| Evaluate comorbid conditions such as eating disorders or substance abuse Take nutritional and exercise history | Check weight and waist circumference at baseline and every visit Calculate body mass index at every visit Prescribe healthy diet and exercise | Patient education, careful monitoring, and prevention are most-effective treatments Drug therapy for persistent weight gain or early rapid gain (>7% in first 6 months). Agents of potential benefit include topiramate, sibutramine, metformin, zonisamide, and orlistat (see Table 3) |
| Glucose control/type 2 diabetes | ||
| Assessment | Prevention | Treatment |
| Take history of glucose intolerance or diabetes Ask about family history of diabetes, obesity, hypertension, heart disease | Check baseline weight and plasma glucose Obtain fasting plasma glucose every 3 months for first year, then annually Prescribe healthy diet and exercise | Primary prevention through careful monitoring is most effective Discontinue atypical antipsychotic; use other mood stabilizer unless atypical is only effective drug for that patient Oral hypoglycemics (metformin, others) |
| Hyperlipidemia | ||
| Assessment | Prevention | Treatment |
| Take history of hyperlipidemia or cardiovascular disease Ask about family history of hyperlipidemia | Check fasting lipid profile including triglycerides at baseline and every 3 months in first year Prescribe healthy diet and exercise | Monitor diet, exercise, weight, lipids regularly Change atypical antipsychotic or use other mood stabilizer (as described above) Oral antilipemics (simvastatin, others) |
Evidence for atypicals
Olanzapine is the only atypical FDA-approved for relapse prevention in bipolar disorder. This approval is supported by several studies, most notably two 1-year, double-blind trials:
- Mean time to any mood relapse was 174 days in patients taking olanzapine, mean 12.5 mg/d (±5 mg), compared with 22 days in a placebo group (Eli Lilly and Co., data on file).
- Manic relapse rate was 14.3% in patients treated with olanzapine, ~12 mg/d, compared with 28% in patients treated with lithium, ~1,100 mg/d (mean 0.76 mEq/L). The two treatments were similarly effective in preventing depressive relapse.5
As a mood stabilizer, olanzapine was as effective as divalproex in a 47-week randomized, double-blind study of 251 adults with bipolar I disorder.6 Patients treated with olanzapine improved more rapidly and had fewer manic symptoms than those treated with divalproex, but bipolar relapse rates were similar in both treatment groups.
Risperidone appears to have a role as a potential maintenance mood stabilizer in bipolar patients, although double-blind trials are lacking.
In a 6-month, open-label investigation, relapse rates were 16% for depression and 7% for mania in bipolar patients receiving risperidone (average 4 mg/d) combined with mood-stabilizing medications.7 These relapse rates are lower than those typically reported for mood-stabilizing monotherapy.
In another 6-month, open-label study, risperidone monotherapy (average 4 mg/d) was effective for treating mania and maintaining euthymia.8
IM risperidone is a useful option for bipolar patients chronically nonadherent with oral medications; it also substantially reduces the risk of neuroleptic side effects compared with older depot antipsychotics.
Quetiapine was recently approved as an antimanic agent and may possess mood-stabilizing properties. In a preliminary study of 10 patients with bipolar disorder, adding quetiapine (mean 200 mg/d) to existing mood stabilizer therapy for 12 weeks improved psychopathology, mania, and depression rating scale scores.9
More-recent unpublished data suggest dosing quetiapine to approximately 600 mg/d as monotherapy or an adjunct to treat acute mania, though controlled maintenance studies are lacking (AstraZeneca Pharmaceuticals, data on file).
Others. Some early evidence supports using ziprasidone and aripiprazole for bipolar mania:
- Ziprasidone monotherapy, 40 to 80 mg bid, was significantly more effective than placebo in reducing acute mania symptoms in a 3-week, double-blind, randomized trial of 197 patients with bipolar I disorder.10
- Aripiprazole monotherapy, 15 to 30 mg/d, had a significantly greater effect than placebo in a 3-week, double-blind, randomized trial of 262 patients in acute manic or mixed bipolar episodes. Response rates among patients with mania were 40% with aripiprazole and 19% with placebo.11
Both ziprasidone and aripiprazole were well-tolerated in these brief trials, although their efficacy as long-term mood-stabilizers in bipolar disorder is unclear.
Using clozapine raises concerns about potentially serious adverse events, although it remains the only agent with proven efficacy in treatment-refractory mania.12,13 Clozapine also appears to reduce hospitalization and affective relapse rates and improve symptoms and quality of life.14,15
Long-term safety
Compared with conventional antipsychotics, EPS are not a major concern with the atypical agents. Except for risperidone, atypicals’ effect on prolactin levels generally is not clinically meaningful. Atypicals appear to be “mood-friendly,” whereas conventional antipsychotics seem to contribute to dysphoria or cause depression in some patients.
Sedation or other annoying side effects such as dry mouth or dizziness can occur with any atypical. Other more-serious side effects may complicate antipsychotic treatment, as we are coming to understand from using atypicals for long-term schizophrenia management.
Table 3
Weight-loss medications for bipolar patients taking atypical antipsychotics
| Drug | Dosage | Side effects | Recommendations |
|---|---|---|---|
| Metformin | 500 to 1,000 mg bid | Hypoglycemia Diarrhea Nausea/vomiting | First-line in patients with comorbid type 2 diabetes |
| Orlistat | 120 mg tid | GI distress Change in bowel habits | Second-line For patients with BMI >27 Supplement fat-soluble vitamins |
| Sibutramine | 5 to 15 mg/d | Dry mouth Anorexia Insomnia Constipation | Second-line For patients with BMI 27 to 30 Risk of serotonin syndrome if given with serotonergic drugs |
| Topiramate | 50 to 250 mg/d | Somnolence Fatigue Paresthesias | Consider first-line for its potential additive mood-stabilizing effect May help comorbid binge-eating or seizure disorders |
| Zonisamide | 100 to 600 mg/d | Somnolence Dizziness Anorexia | Consider first-line for its potential additive mood-stabilizing effect May help comorbid binge-eating or seizure disorders |
Movement disorders. Antipsychotics appear more likely to cause EPS in patients with mood disorders than with schizophrenia. In one study using conventional antipsychotics, bipolar patients were 4 to 5 times more likely than schizophrenia patients to experience acute dystonia.16
Although atypicals pose some small risk for acute EPS and TD, the risk is near placebo-level with clinically relevant and comparable dosages.17 Even so, it is important to educate patients to watch for emerging signs of TD during long-term treatment with any antipsychotic. EPS risk may be dose-dependent, particularly with risperidone.18
Weight gain and obesity. Patients with bipolar disorder are more likely to be overweight or obese (body mass index [BMI] > 30) than the general population,17,19 though the reasons are unknown. Studies suggest an obesity prevalence of 32% to 35% in bipolar patients, compared with 18% in the general population.20,21
All atypicals can cause weight gain, although olanzapine and clozapine are associated with the greatest mean weight gains. In three long-term trials (47 weeks to 18 months), bipolar patients who received olanzapine gained significantly more weight (mean 2 to 3 kg) than those receiving lithium or divalproex.19
Cases with much greater weight gain—even leading to clinical obesity—have been observed, particularly with olanzapine. Although evidence from registration trials and clinical experience show lesser weight gains with risperidone, quetiapine, ziprasidone, and aripiprazole, some of our patients do gain weight while taking these agents—either alone or in combination with lithium or divalproex.
Weight management. Because patients with bipolar disorder may be at increased risk for weight gain and obesity, weight management techniques may improve their health by:
- decreasing morbidity and mortality tied to weight-related physical illnesses
- enhancing psychological well-being.1
In addition to diet and exercise counseling, some bipolar patients taking long-term atypical antipsychotics may benefit from adjunctive weight-loss medications (Table 3). We generally use such medications for bipolar patients who:
- persistently gain weight despite best dietary practices
- gain substantial weight early in treatment with an atypical antipsychotic that is providing effective symptomatic relief.
Early weight gain—particularly gains of >7% within the first 6 weeks—might predict large weight gain over time.
Diabetes. In September 2003, the FDA requested a class-wide labeling change to warn about a possible link between atypical antipsychotics and diabetes. The FDA recommended blood sugar monitoring of patients taking atypicals, especially those with obesity risk factors or family history of diabetes.
Type 2 diabetes develops in some patients taking atypicals, whether or not they gain substantial weight.22 This suggests that weight gain associated with bipolar disorder and the use of atypical antipsychotics may be independent risk factors for diabetes—a clear concern when treating bipolar patients.
Evidence provides no clear answer as to which atypicals may increase diabetes risk. Cautious use and vigilant monitoring of blood glucose are therefore recommended for every patient taking an atypical for long-term therapy. Also watch for increases in triglycerides and cholesterol17 in patients taking atypicals as bipolar maintenance therapy.
Conclusion
Atypical antipsychotics are valuable therapies in preventing bipolar relapses, although olanzapine is the only atypical with this indication so far. Collective data and clinical experience suggest that atypicals are indeed mood stabilizers, although—like other mood stabilizers such as lithium or divalproex—they have limitations. None achieve ideal efficacy in all four bipolar treatment roles: treating the highs and lows, and preventing the highs and lows. Atypicals seem more effective in treating and preventing the highs than the lows, reminding us that effective depression treatment is the greatest unmet need in bipolar disorder.
More double-blind, randomized, controlled trials are needed to fully understand whether all atypicals are mood stabilizers and to determine their safety and side effects in long-term therapy for patients with bipolar disorder.
Related resources
- Depression and Bipolar Support Alliance. www.dbsalliance.org
- Muzina DJ, Calabrese JR. Guidelines for treatment of bipolar disorder.In: Stein DJ, Kupfer DJ, Schatzberg AF (eds). Textbook of mood disorders Washington, DC: American Psychiatric Publishing, 2004 (in press).
Drug brand names
- Aripiprazole • Abilify
- Carbamazepine • Tegretol
- Clozapine • Clozaril
- Divalproex/valproate • Depakote, Depakene
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid, et al
- Metformin • Glucophage
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal, Risperdal Consta
- Sibutramine • Meridia
- Simvastatin • Zocor
- Topiramate • Topamax
- Ziprasidone • Geodon
- Zonisamide • Zonegran
Disclosure
Dr. Muzina receives research grants from AstraZeneca Pharmaceuticals, Eli Lilly and Co., and Abbott Laboratories, is a consultant to AstraZeneca Pharmaceuticals and Pfizer, Inc., and a speaker for AstraZeneca Pharmaceuticals, Pfizer Inc., Eli Lilly and Co., and GlaxoSmithKline.
Maintenance therapy with mood stabilizers is the most critical phase of bipolar disorder treatment but the stage with the least available evidence about medication risks and benefits. The FDA’s recent approval of olanzapine for bipolar maintenance raises the question of whether atypical antipsychotics are really mood stabilizers. This article attempts to answer that question by:
- describing the “ideal” mood stabilizer
- discussing atypicals’ advantages over conventional antipsychotics in bipolar patients
- comparing efficacy data for the six available atypicals
- recommending strategies to prevent and treat atypicals’ potentially serious side effects during long-term therapy.
What is a ‘mood stabilizer’?
Successful mood stabilizer maintenance therapy decreases the time patients are sick and disabled. Although somewhat dated after only 2 years, the most recent American Psychiatric Association (APA) practice guidelines1 support using mood stabilizers for patients with bipolar I and bipolar II disorders.
Table 1
Bipolar maintenance treatment goals
|
| Adapted from American Psychiatric Association practice guidelines for treating patients with bipolar disorder (reference 1) |
The goals of maintenance therapy are listed in Table 1. The ideal mood stabilizer would work in maintenance and all bipolar phases and treatment stages—from treating acute depression, mania, hypomania, and mixed states to preventing abnormal mood elevations and depressions. It would not precipitate depression or mania, rapid cycling, or cycle acceleration.
In other words, the best “mood stabilizer” would work in all four treatment roles of bipolar disorder: treating highs and lows, and preventing highs and lows. No such mood stabilizer exists, although lithium may come closest to the ideal.2
Most U.S. psychiatrists use combination therapies for bipolar disorder, particularly when treating acute manic states. The most common combination is a “known” mood stabilizer—such as lithium or divalproex—plus an antipsychotic to quickly control mania.
After mania remits, clinicians often try to eliminate the antipsychotic in hopes of maintaining mood stability and euthymia with the mood stabilizer alone. This was especially true before atypical antipsychotics were approved, given the risk for tardive dyskinesia (TD) associated with long-term use of conventional antipsychotics.
Unfortunately, patients frequently relapse with this strategy, so psychiatrists may leave their bipolar patients on atypical antipsychotics during long-term maintenance. But how good are atypicals as mood stabilizers? Perhaps more importantly, how safe is long-term use of atypicals in bipolar patients?
Antipsychotics as mood stabilizers
The 2002 APA practice guidelines discuss efficacy data for using lithium, divalproex or valproate, lamotrigine, carbamazepine, and electroconvulsive therapy for bipolar maintenance treatment. Two sentences on antipsychotic drug use note:
- one placebo-controlled study of a conventional antipsychotic showing no efficacy
- some data supporting clozapine as a prophylactic bipolar treatment.1
A 1998 review of five open trials3 touched on conventional depot antipsychotics’ value in reducing manic or affective illness. However, the authors warned:
- no controlled trials existed
- maintenance antipsychotic treatment may be associated with increased risk for tardive movement disorders
- conventional agents can exacerbate depressive symptoms in some patients.
Using conventional antipsychotics long-term in bipolar disorder is not advisable, with the possible exception of depot preparations in nonadhering patients with severe illness. Long-acting injectable atypicals—such as the recently approved IM risperidone—may displace any use of conventional antipsychotics in bipolar patients.
Atypical antipsychotics hold several advantages over conventional agents:
- significantly reduced risk for TD and extrapyramidal symptoms (EPS)
- lack of serum prolactin elevation (except with risperidone)
- improved cognition
- possible decreased suicidality, particularly with clozapine.4
Table 2
Tips for managing atypicals’ potentially serious side-effect risks
| Weight gain/obesity | ||
| Assessment | Prevention | Treatment |
| Evaluate comorbid conditions such as eating disorders or substance abuse Take nutritional and exercise history | Check weight and waist circumference at baseline and every visit Calculate body mass index at every visit Prescribe healthy diet and exercise | Patient education, careful monitoring, and prevention are most-effective treatments Drug therapy for persistent weight gain or early rapid gain (>7% in first 6 months). Agents of potential benefit include topiramate, sibutramine, metformin, zonisamide, and orlistat (see Table 3) |
| Glucose control/type 2 diabetes | ||
| Assessment | Prevention | Treatment |
| Take history of glucose intolerance or diabetes Ask about family history of diabetes, obesity, hypertension, heart disease | Check baseline weight and plasma glucose Obtain fasting plasma glucose every 3 months for first year, then annually Prescribe healthy diet and exercise | Primary prevention through careful monitoring is most effective Discontinue atypical antipsychotic; use other mood stabilizer unless atypical is only effective drug for that patient Oral hypoglycemics (metformin, others) |
| Hyperlipidemia | ||
| Assessment | Prevention | Treatment |
| Take history of hyperlipidemia or cardiovascular disease Ask about family history of hyperlipidemia | Check fasting lipid profile including triglycerides at baseline and every 3 months in first year Prescribe healthy diet and exercise | Monitor diet, exercise, weight, lipids regularly Change atypical antipsychotic or use other mood stabilizer (as described above) Oral antilipemics (simvastatin, others) |
Evidence for atypicals
Olanzapine is the only atypical FDA-approved for relapse prevention in bipolar disorder. This approval is supported by several studies, most notably two 1-year, double-blind trials:
- Mean time to any mood relapse was 174 days in patients taking olanzapine, mean 12.5 mg/d (±5 mg), compared with 22 days in a placebo group (Eli Lilly and Co., data on file).
- Manic relapse rate was 14.3% in patients treated with olanzapine, ~12 mg/d, compared with 28% in patients treated with lithium, ~1,100 mg/d (mean 0.76 mEq/L). The two treatments were similarly effective in preventing depressive relapse.5
As a mood stabilizer, olanzapine was as effective as divalproex in a 47-week randomized, double-blind study of 251 adults with bipolar I disorder.6 Patients treated with olanzapine improved more rapidly and had fewer manic symptoms than those treated with divalproex, but bipolar relapse rates were similar in both treatment groups.
Risperidone appears to have a role as a potential maintenance mood stabilizer in bipolar patients, although double-blind trials are lacking.
In a 6-month, open-label investigation, relapse rates were 16% for depression and 7% for mania in bipolar patients receiving risperidone (average 4 mg/d) combined with mood-stabilizing medications.7 These relapse rates are lower than those typically reported for mood-stabilizing monotherapy.
In another 6-month, open-label study, risperidone monotherapy (average 4 mg/d) was effective for treating mania and maintaining euthymia.8
IM risperidone is a useful option for bipolar patients chronically nonadherent with oral medications; it also substantially reduces the risk of neuroleptic side effects compared with older depot antipsychotics.
Quetiapine was recently approved as an antimanic agent and may possess mood-stabilizing properties. In a preliminary study of 10 patients with bipolar disorder, adding quetiapine (mean 200 mg/d) to existing mood stabilizer therapy for 12 weeks improved psychopathology, mania, and depression rating scale scores.9
More-recent unpublished data suggest dosing quetiapine to approximately 600 mg/d as monotherapy or an adjunct to treat acute mania, though controlled maintenance studies are lacking (AstraZeneca Pharmaceuticals, data on file).
Others. Some early evidence supports using ziprasidone and aripiprazole for bipolar mania:
- Ziprasidone monotherapy, 40 to 80 mg bid, was significantly more effective than placebo in reducing acute mania symptoms in a 3-week, double-blind, randomized trial of 197 patients with bipolar I disorder.10
- Aripiprazole monotherapy, 15 to 30 mg/d, had a significantly greater effect than placebo in a 3-week, double-blind, randomized trial of 262 patients in acute manic or mixed bipolar episodes. Response rates among patients with mania were 40% with aripiprazole and 19% with placebo.11
Both ziprasidone and aripiprazole were well-tolerated in these brief trials, although their efficacy as long-term mood-stabilizers in bipolar disorder is unclear.
Using clozapine raises concerns about potentially serious adverse events, although it remains the only agent with proven efficacy in treatment-refractory mania.12,13 Clozapine also appears to reduce hospitalization and affective relapse rates and improve symptoms and quality of life.14,15
Long-term safety
Compared with conventional antipsychotics, EPS are not a major concern with the atypical agents. Except for risperidone, atypicals’ effect on prolactin levels generally is not clinically meaningful. Atypicals appear to be “mood-friendly,” whereas conventional antipsychotics seem to contribute to dysphoria or cause depression in some patients.
Sedation or other annoying side effects such as dry mouth or dizziness can occur with any atypical. Other more-serious side effects may complicate antipsychotic treatment, as we are coming to understand from using atypicals for long-term schizophrenia management.
Table 3
Weight-loss medications for bipolar patients taking atypical antipsychotics
| Drug | Dosage | Side effects | Recommendations |
|---|---|---|---|
| Metformin | 500 to 1,000 mg bid | Hypoglycemia Diarrhea Nausea/vomiting | First-line in patients with comorbid type 2 diabetes |
| Orlistat | 120 mg tid | GI distress Change in bowel habits | Second-line For patients with BMI >27 Supplement fat-soluble vitamins |
| Sibutramine | 5 to 15 mg/d | Dry mouth Anorexia Insomnia Constipation | Second-line For patients with BMI 27 to 30 Risk of serotonin syndrome if given with serotonergic drugs |
| Topiramate | 50 to 250 mg/d | Somnolence Fatigue Paresthesias | Consider first-line for its potential additive mood-stabilizing effect May help comorbid binge-eating or seizure disorders |
| Zonisamide | 100 to 600 mg/d | Somnolence Dizziness Anorexia | Consider first-line for its potential additive mood-stabilizing effect May help comorbid binge-eating or seizure disorders |
Movement disorders. Antipsychotics appear more likely to cause EPS in patients with mood disorders than with schizophrenia. In one study using conventional antipsychotics, bipolar patients were 4 to 5 times more likely than schizophrenia patients to experience acute dystonia.16
Although atypicals pose some small risk for acute EPS and TD, the risk is near placebo-level with clinically relevant and comparable dosages.17 Even so, it is important to educate patients to watch for emerging signs of TD during long-term treatment with any antipsychotic. EPS risk may be dose-dependent, particularly with risperidone.18
Weight gain and obesity. Patients with bipolar disorder are more likely to be overweight or obese (body mass index [BMI] > 30) than the general population,17,19 though the reasons are unknown. Studies suggest an obesity prevalence of 32% to 35% in bipolar patients, compared with 18% in the general population.20,21
All atypicals can cause weight gain, although olanzapine and clozapine are associated with the greatest mean weight gains. In three long-term trials (47 weeks to 18 months), bipolar patients who received olanzapine gained significantly more weight (mean 2 to 3 kg) than those receiving lithium or divalproex.19
Cases with much greater weight gain—even leading to clinical obesity—have been observed, particularly with olanzapine. Although evidence from registration trials and clinical experience show lesser weight gains with risperidone, quetiapine, ziprasidone, and aripiprazole, some of our patients do gain weight while taking these agents—either alone or in combination with lithium or divalproex.
Weight management. Because patients with bipolar disorder may be at increased risk for weight gain and obesity, weight management techniques may improve their health by:
- decreasing morbidity and mortality tied to weight-related physical illnesses
- enhancing psychological well-being.1
In addition to diet and exercise counseling, some bipolar patients taking long-term atypical antipsychotics may benefit from adjunctive weight-loss medications (Table 3). We generally use such medications for bipolar patients who:
- persistently gain weight despite best dietary practices
- gain substantial weight early in treatment with an atypical antipsychotic that is providing effective symptomatic relief.
Early weight gain—particularly gains of >7% within the first 6 weeks—might predict large weight gain over time.
Diabetes. In September 2003, the FDA requested a class-wide labeling change to warn about a possible link between atypical antipsychotics and diabetes. The FDA recommended blood sugar monitoring of patients taking atypicals, especially those with obesity risk factors or family history of diabetes.
Type 2 diabetes develops in some patients taking atypicals, whether or not they gain substantial weight.22 This suggests that weight gain associated with bipolar disorder and the use of atypical antipsychotics may be independent risk factors for diabetes—a clear concern when treating bipolar patients.
Evidence provides no clear answer as to which atypicals may increase diabetes risk. Cautious use and vigilant monitoring of blood glucose are therefore recommended for every patient taking an atypical for long-term therapy. Also watch for increases in triglycerides and cholesterol17 in patients taking atypicals as bipolar maintenance therapy.
Conclusion
Atypical antipsychotics are valuable therapies in preventing bipolar relapses, although olanzapine is the only atypical with this indication so far. Collective data and clinical experience suggest that atypicals are indeed mood stabilizers, although—like other mood stabilizers such as lithium or divalproex—they have limitations. None achieve ideal efficacy in all four bipolar treatment roles: treating the highs and lows, and preventing the highs and lows. Atypicals seem more effective in treating and preventing the highs than the lows, reminding us that effective depression treatment is the greatest unmet need in bipolar disorder.
More double-blind, randomized, controlled trials are needed to fully understand whether all atypicals are mood stabilizers and to determine their safety and side effects in long-term therapy for patients with bipolar disorder.
Related resources
- Depression and Bipolar Support Alliance. www.dbsalliance.org
- Muzina DJ, Calabrese JR. Guidelines for treatment of bipolar disorder.In: Stein DJ, Kupfer DJ, Schatzberg AF (eds). Textbook of mood disorders Washington, DC: American Psychiatric Publishing, 2004 (in press).
Drug brand names
- Aripiprazole • Abilify
- Carbamazepine • Tegretol
- Clozapine • Clozaril
- Divalproex/valproate • Depakote, Depakene
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid, et al
- Metformin • Glucophage
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal, Risperdal Consta
- Sibutramine • Meridia
- Simvastatin • Zocor
- Topiramate • Topamax
- Ziprasidone • Geodon
- Zonisamide • Zonegran
Disclosure
Dr. Muzina receives research grants from AstraZeneca Pharmaceuticals, Eli Lilly and Co., and Abbott Laboratories, is a consultant to AstraZeneca Pharmaceuticals and Pfizer, Inc., and a speaker for AstraZeneca Pharmaceuticals, Pfizer Inc., Eli Lilly and Co., and GlaxoSmithKline.
1. Hirschfeld RM, Bowden CL, Gitlin MJ, et al. Practice guideline for the treatment of patients with bipolar disorder (rev). Am J Psychiatry 2002;159:1-50.
2. Bauer MS, Mitchner L. What is a “mood stabilizer?” An evidence-based response. Am J Psychiatry 2004;161(1):3-18.
3. Keck PE, Jr, McElroy SL, Strakowski SM. Anticonvulsants and antipsychotics in the treatment of bipolar disorder. J Clin Psychiatry 1998;59(suppl 6):74-81.
4. Sharma V. Atypical antipsychotics and suicide in mood and anxiety disorders. Bipolar Disord 2003;5(suppl 2):48-52.
5. Tohen M, Marneros A, Bowden C, et al. Olanzapine versus lithium in relapse prevention in bipolar disorder: a randomized double-blind controlled 12-month clinical trial (presentation). Freiberg, Germany: Stanley Foundation Bipolar Network, Sept. 11-14, 2002.
6. Tohen M, Ketter TA, Zarate CA, et al. Olanzapine versus divalproex sodium for the treatment of acute mania and maintenance of remission: a 47-week study. Am J Psychiatry 2003;160(7):1263-71.
7. Vieta E, Goikolea JM, Corbella B, et al. Risperidone safety and efficacy in the treatment of bipolar and schizoaffective disorders: results from a 6-month, multicenter, open study. J Clin Psychiatry 2001;62(10):818-25.
8. Vieta E, Brugue E, Goikolea JM, et al. Acute and continuation risperidone monotherapy in mania. Hum Psychopharmacol 2004;19(1):41-5.
9. Sajatovic M, Brescan DW, Perez DE, et al. Quetiapine alone and added to a mood stabilizer for serious mood disorders. J Clin Psychiatry 2001;62(9):728-32.
10. Keck PE, Jr, Versiani M, Potkin S, et al. Ziprasidone in the treatment of acute bipolar mania: a three-week, placebo-controlled, double-blind, randomized trial. Am J Psychiatry 2003;160(4):741-8.
11. Keck PE, Jr, Marcus R, Tourkodimitris S, et al. A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole in patients with acute bipolar mania. Am J Psychiatry 2003;160(9):1651-8.
12. Calabrese JR, Kimmel SE, Woyshville MJ, et al. Clozapine for treatment-refractory mania. Am J Psychiatry 1996;153(6):759-64.
13. Green AI, Tohen M, Patel JK, et al. Clozapine in the treatment of refractory psychotic mania. Am J Psychiatry 2000;157(6):982-6.
14. Zarate CA, Jr, Tohen M, Banov MD, et al. Is clozapine a mood stabilizer? J Clin Psychiatry 1995;56(3):108-12.
15. Suppes T, Webb A, Paul B, et al. Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry 1999;156(8):1164-9.
16. Nasrallah HA, Churchill CM, Hamdan-Allan GA. Higher frequency of neuroleptic-induced dystonia in mania than in schizophrenia. Am J Psychiatry 1988;145(11):1455-6.
17. Chue P, Kovacs CS. Safety and tolerability of atypical antipsychotics in patients with bipolar disorder: prevalence, monitoring and management. Bipolar Disord 2003;5(suppl 2):62-79.
18. Simpson GM, Lindenmayer JP. Extrapyramidal symptoms in patients treated with risperidone. J Clin Psychopharmacol 1997;17(3):194-201.
19. Keck PE, Jr, McElroy SL. Bipolar disorder, obesity, and pharmacotherapy-associated weight gain. J Clin Psychiatry 2003;64(12):1426-35.
20. Fagiolini A, Frank E, Houck PR, et al. Prevalence of obesity and weight change during treatment in patients with bipolar I disorder. J Clin Psychiatry 2002;63(6):528-33.
21. Fagiolini A, Kupfer DJ, Houck PR, et al. Obesity as a correlate of outcome in patients with bipolar I disorder. Am J Psychiatry 2003;160(1):112-17.
22. Haupt DW, Newcomer JW. Abnormalities in glucose regulation associated with mental illness and treatment. J Psychosom Res 2002;53(4):925-33.
1. Hirschfeld RM, Bowden CL, Gitlin MJ, et al. Practice guideline for the treatment of patients with bipolar disorder (rev). Am J Psychiatry 2002;159:1-50.
2. Bauer MS, Mitchner L. What is a “mood stabilizer?” An evidence-based response. Am J Psychiatry 2004;161(1):3-18.
3. Keck PE, Jr, McElroy SL, Strakowski SM. Anticonvulsants and antipsychotics in the treatment of bipolar disorder. J Clin Psychiatry 1998;59(suppl 6):74-81.
4. Sharma V. Atypical antipsychotics and suicide in mood and anxiety disorders. Bipolar Disord 2003;5(suppl 2):48-52.
5. Tohen M, Marneros A, Bowden C, et al. Olanzapine versus lithium in relapse prevention in bipolar disorder: a randomized double-blind controlled 12-month clinical trial (presentation). Freiberg, Germany: Stanley Foundation Bipolar Network, Sept. 11-14, 2002.
6. Tohen M, Ketter TA, Zarate CA, et al. Olanzapine versus divalproex sodium for the treatment of acute mania and maintenance of remission: a 47-week study. Am J Psychiatry 2003;160(7):1263-71.
7. Vieta E, Goikolea JM, Corbella B, et al. Risperidone safety and efficacy in the treatment of bipolar and schizoaffective disorders: results from a 6-month, multicenter, open study. J Clin Psychiatry 2001;62(10):818-25.
8. Vieta E, Brugue E, Goikolea JM, et al. Acute and continuation risperidone monotherapy in mania. Hum Psychopharmacol 2004;19(1):41-5.
9. Sajatovic M, Brescan DW, Perez DE, et al. Quetiapine alone and added to a mood stabilizer for serious mood disorders. J Clin Psychiatry 2001;62(9):728-32.
10. Keck PE, Jr, Versiani M, Potkin S, et al. Ziprasidone in the treatment of acute bipolar mania: a three-week, placebo-controlled, double-blind, randomized trial. Am J Psychiatry 2003;160(4):741-8.
11. Keck PE, Jr, Marcus R, Tourkodimitris S, et al. A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole in patients with acute bipolar mania. Am J Psychiatry 2003;160(9):1651-8.
12. Calabrese JR, Kimmel SE, Woyshville MJ, et al. Clozapine for treatment-refractory mania. Am J Psychiatry 1996;153(6):759-64.
13. Green AI, Tohen M, Patel JK, et al. Clozapine in the treatment of refractory psychotic mania. Am J Psychiatry 2000;157(6):982-6.
14. Zarate CA, Jr, Tohen M, Banov MD, et al. Is clozapine a mood stabilizer? J Clin Psychiatry 1995;56(3):108-12.
15. Suppes T, Webb A, Paul B, et al. Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry 1999;156(8):1164-9.
16. Nasrallah HA, Churchill CM, Hamdan-Allan GA. Higher frequency of neuroleptic-induced dystonia in mania than in schizophrenia. Am J Psychiatry 1988;145(11):1455-6.
17. Chue P, Kovacs CS. Safety and tolerability of atypical antipsychotics in patients with bipolar disorder: prevalence, monitoring and management. Bipolar Disord 2003;5(suppl 2):62-79.
18. Simpson GM, Lindenmayer JP. Extrapyramidal symptoms in patients treated with risperidone. J Clin Psychopharmacol 1997;17(3):194-201.
19. Keck PE, Jr, McElroy SL. Bipolar disorder, obesity, and pharmacotherapy-associated weight gain. J Clin Psychiatry 2003;64(12):1426-35.
20. Fagiolini A, Frank E, Houck PR, et al. Prevalence of obesity and weight change during treatment in patients with bipolar I disorder. J Clin Psychiatry 2002;63(6):528-33.
21. Fagiolini A, Kupfer DJ, Houck PR, et al. Obesity as a correlate of outcome in patients with bipolar I disorder. Am J Psychiatry 2003;160(1):112-17.
22. Haupt DW, Newcomer JW. Abnormalities in glucose regulation associated with mental illness and treatment. J Psychosom Res 2002;53(4):925-33.
How to reduce aggression in youths with conduct disorder
Families and schools often pressure clinicians to “do something” when children or adolescents persistently bully, threaten, or injure others. This demand poses a treatment dilemma when psychosocial and educational interventions have failed to manage pediatric aggression.
Aggression is the main reason for drug therapy in youths with conduct disorder, but very little safety and efficacy data exist to help us choose medications. This places young patients at risk for polypharmacy, unmanaged symptoms, short-term side effects, and unknown long-term consequences of exposure to psychotropics.
Table 1
4 precautions when prescribing for pediatric aggression
|
| Source: American Academy of Child and Adolescent Psychiatry1 |
This article reviews the limited data on using medications to reduce aggression in children and adolescents, focusing on double-blind, placebo-controlled trials in conduct disorder. Based on this evidence and our clinical experience, we offer a sample case and treatment recommendations.
Prescribing principles
Precautions. When prescribing drugs to treat aggressive youth, remember the American Academy of Child and Adolescent Psychiatry’s precautions (Table 1)1 Recently published recommendations prepared by expert consensus are also valuable treatment guides.2
Linking treatment to diagnosis. Should we attempt to manage aggression as a manifestation of an underlying psychiatric disorder? Or should we treat it the same across all disorders? The latter approach is akin to the “fever model.”
Fever—regardless of cause—may be treated with a nonsteroidal anti-inflammatory drug. However, evidence from drug studies suggests that underlying psychiatric disorders should help determine the choice of aggression treatment. For example, a recent study in adults found that divalproex was effective for aggressive patients only within a specific diagnostic subgroup (in this case, cluster B personality disorders).3
Clinical experience also links aggression treatment with underlying diagnoses. For example, aggression secondary to agitated depression is treated with an antidepressant, whereas aggression secondary to command hallucinations in schizophrenia is treated with antipsychotics.
In treating aggression in conduct disorder (Table 2), first treat comorbid disorders—such as attention deficit/hyperactivity disorder (ADHD) or bipolar disorder—and address the child’s psychosocial and educational needs. Then if medication is appropriate, consider drugs with evidence of safety and efficacy, such as antipsychotics, lithium, and stimulants.
Antipsychotics
Three conventional antipsychotics—chlorpromazine, haloperidol, and thioridazine—are FDA-approved for controlling disruptive behaviors in children.4 No atypical antipsychotics are so indicated, but atypicals are preferred in children and adolescents because of lower risks for tardive dyskinesia, neuroleptic malignant syndrome, and extrapyramidal symptoms.2
Risperidone is the most-studied atypical antipsychotic for treating pediatric aggression, particularly in patients with low intellectual functioning or mental retardation. In a 6-week, double-blind, placebo-controlled trial, 118 children ages 5 to 12 with severely disruptive behavior and IQs of 36 to 84 were given low-dose risperidone (mean 1.16 mg/d). Risperidone reduced conduct problems significantly more than placebo, although aggression was not measured directly.5 Adverse events included somnolence, headache, vomiting, weight gain, and elevated serum prolactin. Similar results have been reported in other studies.6
Table 2
Diagnostic criteria for conduct disorder
| A. A repetitive and persistent pattern of behavior in which the basic rights of others or major age-appropriate societal norms or rules are violated, as manifested by the persistence of three (or more) of the following criteria in the past 12 months, with at least one criterion present in the past 6 months: | |
| Aggression to people and animals | |
| 1. often bullies, threatens, or intimidates others | 5. has been physically cruel to animals |
| 2. often initiates physical fights | 6. has stolen while confronting a victim (such as mugging, purse snatching, extortion, armed robbery) |
| 3. has used a weapon that can cause serious physical harm to others (such as a bat, brick, broken bottle, knife, gun) | 7. has forced someone into sexual activity |
| 4. has been physically cruel to people | |
| Destruction of property | |
| 8. has deliberately engaged in fire setting with the intention of causing serious damage | 9. has deliberately destroyed others’ property (other than by fire setting) |
| Deceitfulness or theft | |
| 10. has broken into someone else’s house, building, or car | 12. has stolen items of nontrivial value without confronting a victim (such as shoplifting without breaking and entering, or forgery) |
| 11. often lies to obtain goods or favors or to avoid obligations(ie, “cons” others) | |
| Serious violation of rules | |
| 13. often stays out at night despite parental prohibitions, beginning before age 13 | 15. has run away from home overnight at least twice while living in parental or parental surrogate home (or once without returning for a lengthy period) |
| 14. is often truant from school, beginning before age 13 | |
| B. The disturbance in behavior causes clinically significant impairment in social, academic, or occupational functioning | |
| C. If the individual is age 18 or older, criteria are not met for antisocial personality disorder. | |
| Specify severity: | |
| Mild: few if any conduct problems in excess of those required to make the diagnosis and conduct problems cause only minor harm to others (such as lying, truancy, staying out after dark without permission) | |
| Moderate: number of conduct problems and effect on others intermediate between “mild” and severe” (such as stealing without confronting a victim, vandalism) | |
| Severe: many conduct problems in excess of those required to make the diagnosis or conduct problems cause considerable harm to others (such as forced sex, physical cruelty, use of a weapon, stealing while confronting a victim, breaking and entering) | |
| Source: Reprinted with permission from the Diagnostic and statistical manual of mental disorders, 4th ed., text revision. Copyright 2000. American Psychiatric Association. | |
JM, age 12, presented with his mother to address symptoms of hyperactivity and impulsive aggression. The boy also complained that his medications made him fall asleep during the day.
He is receiving five medications: a long-acting stimulant, atypical antipsychotic, anticonvulsant, alpha agonist, and selective serotonin reuptake inhibitor (SSRI). He had received numerous other medications, but prescription records are unavailable or incomplete.
Diagnostic history. Since age 5, JM has been diagnosed as having attention-deficit/hyperactivity disorder (ADHD), oppositional defiant disorder, conduct disorder, bipolar disorder, major depressive disorder, and learning disorders. On examination, the boy met DSM-IV criteria for ADHD, learning disorders, and conduct disorder (Table 2). He has a history of starting fights with peers, bullying, destroying property, lying, and stealing from stores and peers.
His mother stated that her son had always had irritable and labile periods, especially when he did not get his way. She was told during a previous psychiatric evaluation that the boy’s "mood swings" indicated bipolar disorder. On examination, however, he had no other bipolar symptoms, and his condition was chronic, not cyclic.
JM typically cries when he does not get his way, his mother reported, but he has no history of sleep or appetite changes that could suggest depression. He is happy when he can do as he pleases.
Reducing medications. After reviewing JM’s medications and performing the psychiatric assessment, the psychiatrist developed a plan to maximize his psychosocial and educational treatments and alter his medications and dosages. The first step was to increase the stimulant dosage to determine whether JM would be less hyperactive and impulsively aggressive.
The psychiatrist was concerned that the anticonvulsant, alpha agonist, and SSRI were not helping and could cause adverse events. He discussed slowly weaning these drugs one at a time with JM and his mother, and they agreed. The goal was to manage JM over time and to reduce his medications to one (ideally) or two (if necessary), possibly continuing the atypical antipsychotic.
Risperidone also reduced aggression in children with normal intelligence in one small study.7 As a cautionary note, however, long-term risperidone treatment has been associated with withdrawal dyskinesias.8
Olanzapine, quetiapine, ziprasidone, and aripiprazole are less well-studied for treating pediatric aggression but are preferable to conventional agents when antipsychotics are considered.
Recommendation. Expert consensus opinion2 recommends using atypicals when psychosocial treatments and first-line medications for primary conditions have failed. Start with low dosages, and titrate up slowly while monitoring symptoms and side effects. Because no studies have compared any atypical’s efficacy over others for aggressive behavior, base your choices on:
- discussions with the patient and family (Box 1)
- medical comorbidities
- how the patient responded to antipsychotics in the past
- side-effect profile
- long-term treatment planning.2
If the patient cannot tolerate the medication or does not respond after 4 to 6 weeks, try switching atypicals. To improve partial response, consider adding a mood stabilizer such as lithium or divalproex. If aggressive symptoms remit for 6 months or longer, attempt to taper or discontinue the antipsychotic.2
Lithium
In placebo-controlled trials, lithium reduced aggression in:
- male prisoners ages 16 to 24.9
- children ages 7 and 12 with conduct disorder10
- children and adolescents ages 10 to 17 with conduct disorder.11
Among these studies, only ours11 specifically measured aggression. We randomly assigned 40 children to receive 4 weeks of lithium, 900 to 2,100 mg/d (mean 1,425 ± 321 mg/d), or placebo. Serum lithium levels were 0.78 to 1.55 mEq/L (mean 1.07 ± 0.19 mEq/L). We used the Overt Aggression Scale (OAS)12,13 (see Related resources) to track frequency and severity of verbal aggression, aggression against objects, aggression against others, and self-aggression.
Lithium reduced aggression more than did placebo, as measured by the clinician-rated Clinical Global Impressions (CGI) scale and staff-rated Global Clinical Judgments (Consensus) Scale (GCJCS). The CGI showed a 70% response rate with lithium and 20% with placebo. Similarly, the GCJCS scale showed 80% response with lithium and 30% with placebo.
The aggression reduction with lithium was statistically significant and clinically evident. Most subjects (37 of 40) experienced at least one adverse event, however, whether receiving lithium or placebo. Nausea, vomiting, and urinary frequency were significantly more common in the lithium-treated group than with placebo. Fewer adverse events were reported in a similar outpatient study,14 probably because of less-frequent monitoring.
Lithium did not reduce aggression in adolescent girls treated for 2 weeks15 or in an outpatient study of children with ADHD.16
Recommendation. Lithium has shown efficacy for reducing severe aggression in hospitalized children with conduct disorder but not in similar outpatients. Consider this drug to reduce severe aggression in children with conduct disorder, especially if they have failed other treatments.
Anticonvulsants
Anticonvulsants have been used to decrease aggression for more than 50 years, and epidemiologic data show their use is increasing markedly.17 Few controlled studies support this prescribing trend, however.18
Initial reports suggested that anticonvulsants reduce disruptive behaviors, but more-critically designed studies have not supported this finding. For example, phenytoin sodium (diphenylhydantoin) demonstrated efficacy in open trials, but controlled trials found this anticonvulsant no more effective than placebo. In fact, placebo may have reduced aggression more than the active drug. Likewise, earlier controlled trials of carbamazepine indicated efficacy, but more-carefully designed trials using specific measures of aggression did not.
Divalproex is the anticonvulsant most commonly used for aggression in children and adolescents. Only one small, placebo-controlled study has found it effective in reducing aggression in children.19
Twenty children ages 10 to 18 with conduct disorder or oppositional defiant disorder were randomized to divalproex, 750 to 1,500 mg/d, or placebo. Eighteen completed the first phase, and 15 crossed over to the other treatment. Concomitant drug treatment, including stimulants, was allowed. The authors reported that 12 of 15 subjects showed some response to divalproex.
A 7-week study compared divalproex in high dosages (up to 1,500 mg/d) versus low dosages (up to 250 mg/d). This study was not placebo-controlled, but aggression was reduced more in the high-dosage than in the low-dosage group.20
Recommendation. If you use an anticonvulsant, first obtain informed consent from the patient and parent. Divalproex causes weight gain and has been associated with increased risk of polycystic ovary syndrome with masculinizing effects.21
Double-blind, placebo-controlled studies of divalproex and other anticonvulsants in treating aggression are needed, particularly as prescriptions for these agents are rising.
Stimulants
Some small controlled studies suggest that stimulants can reduce aggression in children with ADHD, but their effects on aggression in conduct disorder have not been well studied. Aggression was not measured directly in the National Institute of Mental Health Multimodal Treatment Study of Children with ADHD.21 Most other studies have been small and included children with ADHD but not necessarily conduct disorder.
Recommendation. Stimulants may help reduce aggression in children with ADHD, but studies gauging their effects in conduct disorder are needed.
Alpha agonists
Alpha agonists such as clonidine and guanfacine are increasingly being used to treat children with disruptive disorders, despite limited evidence. The small controlled studies that examined alpha agonists as monotherapy or add-ons in this population did not directly measure aggression.22,23
Recommendation. Little data support alpha agonists for reducing aggression. They should probably be considered second-line treatment.
SSRIs
No double-blind, placebo-controlled studies have tested any selective serotonin reuptake inhibitor (SSRIs) for reducing aggression in conduct disorder. In a 6-week open study, citalopram (mean 27 mg/d) reduced impulsive aggression in 12 children with mixed diagnoses, as measured by the modified OAS,13 Child Behavior Checklist, and CGI.24
Recommendation. Use caution when prescribing SSRIs to aggressive youth, as these drugs may contribute to aggression in some mood-disordered children. More evidence of SSRIs’ safety and efficacy in this population is needed.
- Overt Aggression Scale. In: Coccaro EF, Harvey PD, Kupsaw-Lawrence E, et al. Development of neuropharmacologically-based behavioral assessments of impulsiveaggressive behavior. J Neuropsychiatry 1991;3(2):S44-S51.
Drug brand names
- Aripiprazole • Abilify
- Carbamazepine • Tegretol
- Citalopram • Celexa
- Chlorpromazine • Thorazine
- Clonidine • Catapres
- Phenytoin sodium • Dilantin
- Divalproex • Depakote
- Guanfacine • Tenex
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Ziprasidone • Geodon
Disclosure
Dr. Malone receives research support from Pfizer Inc. and Eli Lilly and Co. and is a consultant to Janssen Pharmaceutica.
Dr. Delaney is a consultant to Shire Pharmaceuticals.
Dr. Sheikh reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Prescribing psychoactive medications for children and adolescents American Academy of Child and Adolescent Psychiatry policy statement, adopted Sept. 20, 2001. Available at:http://www.aacap.org/publications/policy/ps41.htm Accessed Jan. 15, 2004.
2. Pappadopulos E, MacIntyre JC, Crismon ML, et al. Treatment recommendations for the use of antipsychotics for aggressive youth (TRAAY). Part II. J Am Acad Child Adolesc Psychiatry 2003;42(2):145-61.
3. Hollander E, Tracy KA, Swann AC, et al. Divalproex in the treatment of impulsive aggression: efficacy in Cluster B personality disorders. Neuropsychopharmacology 2003;28:1186-97.
4. Physician’s Desk Reference (57th ed). Montvale, NJ: Thomson Healthcare, 2003.
5. Aman MG, DeSmedt G, Derivan A, et al. Double-blind, placebo-controlled study of risperidone for the treatment of disruptive behaviors in children with subaverage intelligence. Am J Psychiatry 2002;159:1337-46.
6. Snyder R, Turgay A, Aman M, et al. Effects of risperidone on conduct and disruptive behavior disorders in children with subaverage IQs. J Am Acad Child Adolesc Psychiatry 2002;41(9):1026-36.
7. Findling RL, McNamara NK, Branicky LA, et al. A double-blind pilot study of risperidone in the treatment of conduct disorder. J Am Acad Child Adolesc Psychiatry 2000;39:509-16.
8. Malone RP, Maislin G, Choudhury MS, et al. Risperidone treatment in children and adolescents with autism: short- and long-term safety and effectiveness. J Am Acad Child Adolesc Psychiatry 2002;41(2):140-7.
9. Sheard MH, Marini JL, Bridges CI, Wagner E. The effect of lithium on impulsive aggressive behavior in man. Am J Psychiatry 1976;133(12):1409-13.
10. Campbell M, Adams PB, Small AM, et al. Lithium in hospitalized aggressive children with conduct disorder: a double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry 1995;34:445-53.
11. Malone RP, Delaney MA, Luebbert JF, et al. A double-blind placebo-controlled study of lithium in hospitalized aggressive children and adolescents with conduct disorder. Arch Gen Psychiatry 2000a;57(7):649-54.
12. Yudofsky SC, Silver JM, Jackson W, et al. The Overt Aggression Scale for the objective rating of verbal and physical aggression. Am J Psychiatry 1986;143:35-9.
13. Coccaro EF, Harvey PD, Kupsaw-Lawrence E, et al. Development of neuropharmacologically-based behavioral assessments of impulsive aggressive behavior. J Neuropsychiatry 1991;3:S44-S5.
14. Malone RP, Delaney MA, Gifford C. Adverse events during lithium treatment in children varies by setting. Miami Beach, FL: American Academy of Child and Adolescent Psychiatry annual meeting, 2003.
15. Rifkin A, Karajgi B, Dicker R, et al. Lithium treatment of conduct disorders in adolescents. Am J Psychiatry 1997;154:554-5.
16. Klein RG. Preliminary results: lithium effects in conduct disorders. New Orleans: American Psychiatric Association annual meeting, 1991.
17. Zito JM, Safer DJ, DosReis S, et al. Psychotropic practice patterns for youth: a 10-year perspective. Arch Pediatr Adolesc Med 2003;157:17-25.
18. Malone RP, Delaney MA. Psychopharmacologic interventions in children with aggression: neuroleptics, lithium, and anticonvulsants. In: Coccaro EF (ed). Aggression: assessment and treatment. New York: Marcel Dekker, 2003b;331-49.
19. Donovan SJ, Stewart JW, Nunes EV, et al. Divalproex treatment for youth with explosive temper and mood lability: a double-blind, placebo-controlled crossover design. Am J Psychiatry 2000;157:818-20.
20. Steiner H. A randomized clinical trial of divalproex sodium in conduct disorders. J Clin Psychiatry (in press).
21. Isojarvi JT, Laatikainen TJ, Knip M, et al. Obesity and endocrine disorders in women taking valproate for epilepsy. Ann Neurol 1996;39:579-84.
22. MTA Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention deficit/hyperactivity disorder. Arch Gen Psychiatry 1999;56:1073-86.
23. Conner DF, Barkley RA, Davis HT. A pilot study of methylphenidate, clonidine, or the combination in ADHD comorbid with aggressive oppositional defiant or conduct disorder. Clin Pediatr 2000;39:15-25.
24. Hazell PL, Stuart JE. A randomized controlled trial of clonidine added to psychostimulant medication for hyperactive and aggressive children. J Am Acad Child Adolesc Psychiatry. 2003;886-94.
25. Armenteros JL, Lewis JE. Citalopram treatment for impulsive aggression in children and adolescents: an open pilot study. J Am Acad Child Adolesc Psychiatry 2002;41:522-9.
Families and schools often pressure clinicians to “do something” when children or adolescents persistently bully, threaten, or injure others. This demand poses a treatment dilemma when psychosocial and educational interventions have failed to manage pediatric aggression.
Aggression is the main reason for drug therapy in youths with conduct disorder, but very little safety and efficacy data exist to help us choose medications. This places young patients at risk for polypharmacy, unmanaged symptoms, short-term side effects, and unknown long-term consequences of exposure to psychotropics.
Table 1
4 precautions when prescribing for pediatric aggression
|
| Source: American Academy of Child and Adolescent Psychiatry1 |
This article reviews the limited data on using medications to reduce aggression in children and adolescents, focusing on double-blind, placebo-controlled trials in conduct disorder. Based on this evidence and our clinical experience, we offer a sample case and treatment recommendations.
Prescribing principles
Precautions. When prescribing drugs to treat aggressive youth, remember the American Academy of Child and Adolescent Psychiatry’s precautions (Table 1)1 Recently published recommendations prepared by expert consensus are also valuable treatment guides.2
Linking treatment to diagnosis. Should we attempt to manage aggression as a manifestation of an underlying psychiatric disorder? Or should we treat it the same across all disorders? The latter approach is akin to the “fever model.”
Fever—regardless of cause—may be treated with a nonsteroidal anti-inflammatory drug. However, evidence from drug studies suggests that underlying psychiatric disorders should help determine the choice of aggression treatment. For example, a recent study in adults found that divalproex was effective for aggressive patients only within a specific diagnostic subgroup (in this case, cluster B personality disorders).3
Clinical experience also links aggression treatment with underlying diagnoses. For example, aggression secondary to agitated depression is treated with an antidepressant, whereas aggression secondary to command hallucinations in schizophrenia is treated with antipsychotics.
In treating aggression in conduct disorder (Table 2), first treat comorbid disorders—such as attention deficit/hyperactivity disorder (ADHD) or bipolar disorder—and address the child’s psychosocial and educational needs. Then if medication is appropriate, consider drugs with evidence of safety and efficacy, such as antipsychotics, lithium, and stimulants.
Antipsychotics
Three conventional antipsychotics—chlorpromazine, haloperidol, and thioridazine—are FDA-approved for controlling disruptive behaviors in children.4 No atypical antipsychotics are so indicated, but atypicals are preferred in children and adolescents because of lower risks for tardive dyskinesia, neuroleptic malignant syndrome, and extrapyramidal symptoms.2
Risperidone is the most-studied atypical antipsychotic for treating pediatric aggression, particularly in patients with low intellectual functioning or mental retardation. In a 6-week, double-blind, placebo-controlled trial, 118 children ages 5 to 12 with severely disruptive behavior and IQs of 36 to 84 were given low-dose risperidone (mean 1.16 mg/d). Risperidone reduced conduct problems significantly more than placebo, although aggression was not measured directly.5 Adverse events included somnolence, headache, vomiting, weight gain, and elevated serum prolactin. Similar results have been reported in other studies.6
Table 2
Diagnostic criteria for conduct disorder
| A. A repetitive and persistent pattern of behavior in which the basic rights of others or major age-appropriate societal norms or rules are violated, as manifested by the persistence of three (or more) of the following criteria in the past 12 months, with at least one criterion present in the past 6 months: | |
| Aggression to people and animals | |
| 1. often bullies, threatens, or intimidates others | 5. has been physically cruel to animals |
| 2. often initiates physical fights | 6. has stolen while confronting a victim (such as mugging, purse snatching, extortion, armed robbery) |
| 3. has used a weapon that can cause serious physical harm to others (such as a bat, brick, broken bottle, knife, gun) | 7. has forced someone into sexual activity |
| 4. has been physically cruel to people | |
| Destruction of property | |
| 8. has deliberately engaged in fire setting with the intention of causing serious damage | 9. has deliberately destroyed others’ property (other than by fire setting) |
| Deceitfulness or theft | |
| 10. has broken into someone else’s house, building, or car | 12. has stolen items of nontrivial value without confronting a victim (such as shoplifting without breaking and entering, or forgery) |
| 11. often lies to obtain goods or favors or to avoid obligations(ie, “cons” others) | |
| Serious violation of rules | |
| 13. often stays out at night despite parental prohibitions, beginning before age 13 | 15. has run away from home overnight at least twice while living in parental or parental surrogate home (or once without returning for a lengthy period) |
| 14. is often truant from school, beginning before age 13 | |
| B. The disturbance in behavior causes clinically significant impairment in social, academic, or occupational functioning | |
| C. If the individual is age 18 or older, criteria are not met for antisocial personality disorder. | |
| Specify severity: | |
| Mild: few if any conduct problems in excess of those required to make the diagnosis and conduct problems cause only minor harm to others (such as lying, truancy, staying out after dark without permission) | |
| Moderate: number of conduct problems and effect on others intermediate between “mild” and severe” (such as stealing without confronting a victim, vandalism) | |
| Severe: many conduct problems in excess of those required to make the diagnosis or conduct problems cause considerable harm to others (such as forced sex, physical cruelty, use of a weapon, stealing while confronting a victim, breaking and entering) | |
| Source: Reprinted with permission from the Diagnostic and statistical manual of mental disorders, 4th ed., text revision. Copyright 2000. American Psychiatric Association. | |
JM, age 12, presented with his mother to address symptoms of hyperactivity and impulsive aggression. The boy also complained that his medications made him fall asleep during the day.
He is receiving five medications: a long-acting stimulant, atypical antipsychotic, anticonvulsant, alpha agonist, and selective serotonin reuptake inhibitor (SSRI). He had received numerous other medications, but prescription records are unavailable or incomplete.
Diagnostic history. Since age 5, JM has been diagnosed as having attention-deficit/hyperactivity disorder (ADHD), oppositional defiant disorder, conduct disorder, bipolar disorder, major depressive disorder, and learning disorders. On examination, the boy met DSM-IV criteria for ADHD, learning disorders, and conduct disorder (Table 2). He has a history of starting fights with peers, bullying, destroying property, lying, and stealing from stores and peers.
His mother stated that her son had always had irritable and labile periods, especially when he did not get his way. She was told during a previous psychiatric evaluation that the boy’s "mood swings" indicated bipolar disorder. On examination, however, he had no other bipolar symptoms, and his condition was chronic, not cyclic.
JM typically cries when he does not get his way, his mother reported, but he has no history of sleep or appetite changes that could suggest depression. He is happy when he can do as he pleases.
Reducing medications. After reviewing JM’s medications and performing the psychiatric assessment, the psychiatrist developed a plan to maximize his psychosocial and educational treatments and alter his medications and dosages. The first step was to increase the stimulant dosage to determine whether JM would be less hyperactive and impulsively aggressive.
The psychiatrist was concerned that the anticonvulsant, alpha agonist, and SSRI were not helping and could cause adverse events. He discussed slowly weaning these drugs one at a time with JM and his mother, and they agreed. The goal was to manage JM over time and to reduce his medications to one (ideally) or two (if necessary), possibly continuing the atypical antipsychotic.
Risperidone also reduced aggression in children with normal intelligence in one small study.7 As a cautionary note, however, long-term risperidone treatment has been associated with withdrawal dyskinesias.8
Olanzapine, quetiapine, ziprasidone, and aripiprazole are less well-studied for treating pediatric aggression but are preferable to conventional agents when antipsychotics are considered.
Recommendation. Expert consensus opinion2 recommends using atypicals when psychosocial treatments and first-line medications for primary conditions have failed. Start with low dosages, and titrate up slowly while monitoring symptoms and side effects. Because no studies have compared any atypical’s efficacy over others for aggressive behavior, base your choices on:
- discussions with the patient and family (Box 1)
- medical comorbidities
- how the patient responded to antipsychotics in the past
- side-effect profile
- long-term treatment planning.2
If the patient cannot tolerate the medication or does not respond after 4 to 6 weeks, try switching atypicals. To improve partial response, consider adding a mood stabilizer such as lithium or divalproex. If aggressive symptoms remit for 6 months or longer, attempt to taper or discontinue the antipsychotic.2
Lithium
In placebo-controlled trials, lithium reduced aggression in:
- male prisoners ages 16 to 24.9
- children ages 7 and 12 with conduct disorder10
- children and adolescents ages 10 to 17 with conduct disorder.11
Among these studies, only ours11 specifically measured aggression. We randomly assigned 40 children to receive 4 weeks of lithium, 900 to 2,100 mg/d (mean 1,425 ± 321 mg/d), or placebo. Serum lithium levels were 0.78 to 1.55 mEq/L (mean 1.07 ± 0.19 mEq/L). We used the Overt Aggression Scale (OAS)12,13 (see Related resources) to track frequency and severity of verbal aggression, aggression against objects, aggression against others, and self-aggression.
Lithium reduced aggression more than did placebo, as measured by the clinician-rated Clinical Global Impressions (CGI) scale and staff-rated Global Clinical Judgments (Consensus) Scale (GCJCS). The CGI showed a 70% response rate with lithium and 20% with placebo. Similarly, the GCJCS scale showed 80% response with lithium and 30% with placebo.
The aggression reduction with lithium was statistically significant and clinically evident. Most subjects (37 of 40) experienced at least one adverse event, however, whether receiving lithium or placebo. Nausea, vomiting, and urinary frequency were significantly more common in the lithium-treated group than with placebo. Fewer adverse events were reported in a similar outpatient study,14 probably because of less-frequent monitoring.
Lithium did not reduce aggression in adolescent girls treated for 2 weeks15 or in an outpatient study of children with ADHD.16
Recommendation. Lithium has shown efficacy for reducing severe aggression in hospitalized children with conduct disorder but not in similar outpatients. Consider this drug to reduce severe aggression in children with conduct disorder, especially if they have failed other treatments.
Anticonvulsants
Anticonvulsants have been used to decrease aggression for more than 50 years, and epidemiologic data show their use is increasing markedly.17 Few controlled studies support this prescribing trend, however.18
Initial reports suggested that anticonvulsants reduce disruptive behaviors, but more-critically designed studies have not supported this finding. For example, phenytoin sodium (diphenylhydantoin) demonstrated efficacy in open trials, but controlled trials found this anticonvulsant no more effective than placebo. In fact, placebo may have reduced aggression more than the active drug. Likewise, earlier controlled trials of carbamazepine indicated efficacy, but more-carefully designed trials using specific measures of aggression did not.
Divalproex is the anticonvulsant most commonly used for aggression in children and adolescents. Only one small, placebo-controlled study has found it effective in reducing aggression in children.19
Twenty children ages 10 to 18 with conduct disorder or oppositional defiant disorder were randomized to divalproex, 750 to 1,500 mg/d, or placebo. Eighteen completed the first phase, and 15 crossed over to the other treatment. Concomitant drug treatment, including stimulants, was allowed. The authors reported that 12 of 15 subjects showed some response to divalproex.
A 7-week study compared divalproex in high dosages (up to 1,500 mg/d) versus low dosages (up to 250 mg/d). This study was not placebo-controlled, but aggression was reduced more in the high-dosage than in the low-dosage group.20
Recommendation. If you use an anticonvulsant, first obtain informed consent from the patient and parent. Divalproex causes weight gain and has been associated with increased risk of polycystic ovary syndrome with masculinizing effects.21
Double-blind, placebo-controlled studies of divalproex and other anticonvulsants in treating aggression are needed, particularly as prescriptions for these agents are rising.
Stimulants
Some small controlled studies suggest that stimulants can reduce aggression in children with ADHD, but their effects on aggression in conduct disorder have not been well studied. Aggression was not measured directly in the National Institute of Mental Health Multimodal Treatment Study of Children with ADHD.21 Most other studies have been small and included children with ADHD but not necessarily conduct disorder.
Recommendation. Stimulants may help reduce aggression in children with ADHD, but studies gauging their effects in conduct disorder are needed.
Alpha agonists
Alpha agonists such as clonidine and guanfacine are increasingly being used to treat children with disruptive disorders, despite limited evidence. The small controlled studies that examined alpha agonists as monotherapy or add-ons in this population did not directly measure aggression.22,23
Recommendation. Little data support alpha agonists for reducing aggression. They should probably be considered second-line treatment.
SSRIs
No double-blind, placebo-controlled studies have tested any selective serotonin reuptake inhibitor (SSRIs) for reducing aggression in conduct disorder. In a 6-week open study, citalopram (mean 27 mg/d) reduced impulsive aggression in 12 children with mixed diagnoses, as measured by the modified OAS,13 Child Behavior Checklist, and CGI.24
Recommendation. Use caution when prescribing SSRIs to aggressive youth, as these drugs may contribute to aggression in some mood-disordered children. More evidence of SSRIs’ safety and efficacy in this population is needed.
- Overt Aggression Scale. In: Coccaro EF, Harvey PD, Kupsaw-Lawrence E, et al. Development of neuropharmacologically-based behavioral assessments of impulsiveaggressive behavior. J Neuropsychiatry 1991;3(2):S44-S51.
Drug brand names
- Aripiprazole • Abilify
- Carbamazepine • Tegretol
- Citalopram • Celexa
- Chlorpromazine • Thorazine
- Clonidine • Catapres
- Phenytoin sodium • Dilantin
- Divalproex • Depakote
- Guanfacine • Tenex
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Ziprasidone • Geodon
Disclosure
Dr. Malone receives research support from Pfizer Inc. and Eli Lilly and Co. and is a consultant to Janssen Pharmaceutica.
Dr. Delaney is a consultant to Shire Pharmaceuticals.
Dr. Sheikh reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Families and schools often pressure clinicians to “do something” when children or adolescents persistently bully, threaten, or injure others. This demand poses a treatment dilemma when psychosocial and educational interventions have failed to manage pediatric aggression.
Aggression is the main reason for drug therapy in youths with conduct disorder, but very little safety and efficacy data exist to help us choose medications. This places young patients at risk for polypharmacy, unmanaged symptoms, short-term side effects, and unknown long-term consequences of exposure to psychotropics.
Table 1
4 precautions when prescribing for pediatric aggression
|
| Source: American Academy of Child and Adolescent Psychiatry1 |
This article reviews the limited data on using medications to reduce aggression in children and adolescents, focusing on double-blind, placebo-controlled trials in conduct disorder. Based on this evidence and our clinical experience, we offer a sample case and treatment recommendations.
Prescribing principles
Precautions. When prescribing drugs to treat aggressive youth, remember the American Academy of Child and Adolescent Psychiatry’s precautions (Table 1)1 Recently published recommendations prepared by expert consensus are also valuable treatment guides.2
Linking treatment to diagnosis. Should we attempt to manage aggression as a manifestation of an underlying psychiatric disorder? Or should we treat it the same across all disorders? The latter approach is akin to the “fever model.”
Fever—regardless of cause—may be treated with a nonsteroidal anti-inflammatory drug. However, evidence from drug studies suggests that underlying psychiatric disorders should help determine the choice of aggression treatment. For example, a recent study in adults found that divalproex was effective for aggressive patients only within a specific diagnostic subgroup (in this case, cluster B personality disorders).3
Clinical experience also links aggression treatment with underlying diagnoses. For example, aggression secondary to agitated depression is treated with an antidepressant, whereas aggression secondary to command hallucinations in schizophrenia is treated with antipsychotics.
In treating aggression in conduct disorder (Table 2), first treat comorbid disorders—such as attention deficit/hyperactivity disorder (ADHD) or bipolar disorder—and address the child’s psychosocial and educational needs. Then if medication is appropriate, consider drugs with evidence of safety and efficacy, such as antipsychotics, lithium, and stimulants.
Antipsychotics
Three conventional antipsychotics—chlorpromazine, haloperidol, and thioridazine—are FDA-approved for controlling disruptive behaviors in children.4 No atypical antipsychotics are so indicated, but atypicals are preferred in children and adolescents because of lower risks for tardive dyskinesia, neuroleptic malignant syndrome, and extrapyramidal symptoms.2
Risperidone is the most-studied atypical antipsychotic for treating pediatric aggression, particularly in patients with low intellectual functioning or mental retardation. In a 6-week, double-blind, placebo-controlled trial, 118 children ages 5 to 12 with severely disruptive behavior and IQs of 36 to 84 were given low-dose risperidone (mean 1.16 mg/d). Risperidone reduced conduct problems significantly more than placebo, although aggression was not measured directly.5 Adverse events included somnolence, headache, vomiting, weight gain, and elevated serum prolactin. Similar results have been reported in other studies.6
Table 2
Diagnostic criteria for conduct disorder
| A. A repetitive and persistent pattern of behavior in which the basic rights of others or major age-appropriate societal norms or rules are violated, as manifested by the persistence of three (or more) of the following criteria in the past 12 months, with at least one criterion present in the past 6 months: | |
| Aggression to people and animals | |
| 1. often bullies, threatens, or intimidates others | 5. has been physically cruel to animals |
| 2. often initiates physical fights | 6. has stolen while confronting a victim (such as mugging, purse snatching, extortion, armed robbery) |
| 3. has used a weapon that can cause serious physical harm to others (such as a bat, brick, broken bottle, knife, gun) | 7. has forced someone into sexual activity |
| 4. has been physically cruel to people | |
| Destruction of property | |
| 8. has deliberately engaged in fire setting with the intention of causing serious damage | 9. has deliberately destroyed others’ property (other than by fire setting) |
| Deceitfulness or theft | |
| 10. has broken into someone else’s house, building, or car | 12. has stolen items of nontrivial value without confronting a victim (such as shoplifting without breaking and entering, or forgery) |
| 11. often lies to obtain goods or favors or to avoid obligations(ie, “cons” others) | |
| Serious violation of rules | |
| 13. often stays out at night despite parental prohibitions, beginning before age 13 | 15. has run away from home overnight at least twice while living in parental or parental surrogate home (or once without returning for a lengthy period) |
| 14. is often truant from school, beginning before age 13 | |
| B. The disturbance in behavior causes clinically significant impairment in social, academic, or occupational functioning | |
| C. If the individual is age 18 or older, criteria are not met for antisocial personality disorder. | |
| Specify severity: | |
| Mild: few if any conduct problems in excess of those required to make the diagnosis and conduct problems cause only minor harm to others (such as lying, truancy, staying out after dark without permission) | |
| Moderate: number of conduct problems and effect on others intermediate between “mild” and severe” (such as stealing without confronting a victim, vandalism) | |
| Severe: many conduct problems in excess of those required to make the diagnosis or conduct problems cause considerable harm to others (such as forced sex, physical cruelty, use of a weapon, stealing while confronting a victim, breaking and entering) | |
| Source: Reprinted with permission from the Diagnostic and statistical manual of mental disorders, 4th ed., text revision. Copyright 2000. American Psychiatric Association. | |
JM, age 12, presented with his mother to address symptoms of hyperactivity and impulsive aggression. The boy also complained that his medications made him fall asleep during the day.
He is receiving five medications: a long-acting stimulant, atypical antipsychotic, anticonvulsant, alpha agonist, and selective serotonin reuptake inhibitor (SSRI). He had received numerous other medications, but prescription records are unavailable or incomplete.
Diagnostic history. Since age 5, JM has been diagnosed as having attention-deficit/hyperactivity disorder (ADHD), oppositional defiant disorder, conduct disorder, bipolar disorder, major depressive disorder, and learning disorders. On examination, the boy met DSM-IV criteria for ADHD, learning disorders, and conduct disorder (Table 2). He has a history of starting fights with peers, bullying, destroying property, lying, and stealing from stores and peers.
His mother stated that her son had always had irritable and labile periods, especially when he did not get his way. She was told during a previous psychiatric evaluation that the boy’s "mood swings" indicated bipolar disorder. On examination, however, he had no other bipolar symptoms, and his condition was chronic, not cyclic.
JM typically cries when he does not get his way, his mother reported, but he has no history of sleep or appetite changes that could suggest depression. He is happy when he can do as he pleases.
Reducing medications. After reviewing JM’s medications and performing the psychiatric assessment, the psychiatrist developed a plan to maximize his psychosocial and educational treatments and alter his medications and dosages. The first step was to increase the stimulant dosage to determine whether JM would be less hyperactive and impulsively aggressive.
The psychiatrist was concerned that the anticonvulsant, alpha agonist, and SSRI were not helping and could cause adverse events. He discussed slowly weaning these drugs one at a time with JM and his mother, and they agreed. The goal was to manage JM over time and to reduce his medications to one (ideally) or two (if necessary), possibly continuing the atypical antipsychotic.
Risperidone also reduced aggression in children with normal intelligence in one small study.7 As a cautionary note, however, long-term risperidone treatment has been associated with withdrawal dyskinesias.8
Olanzapine, quetiapine, ziprasidone, and aripiprazole are less well-studied for treating pediatric aggression but are preferable to conventional agents when antipsychotics are considered.
Recommendation. Expert consensus opinion2 recommends using atypicals when psychosocial treatments and first-line medications for primary conditions have failed. Start with low dosages, and titrate up slowly while monitoring symptoms and side effects. Because no studies have compared any atypical’s efficacy over others for aggressive behavior, base your choices on:
- discussions with the patient and family (Box 1)
- medical comorbidities
- how the patient responded to antipsychotics in the past
- side-effect profile
- long-term treatment planning.2
If the patient cannot tolerate the medication or does not respond after 4 to 6 weeks, try switching atypicals. To improve partial response, consider adding a mood stabilizer such as lithium or divalproex. If aggressive symptoms remit for 6 months or longer, attempt to taper or discontinue the antipsychotic.2
Lithium
In placebo-controlled trials, lithium reduced aggression in:
- male prisoners ages 16 to 24.9
- children ages 7 and 12 with conduct disorder10
- children and adolescents ages 10 to 17 with conduct disorder.11
Among these studies, only ours11 specifically measured aggression. We randomly assigned 40 children to receive 4 weeks of lithium, 900 to 2,100 mg/d (mean 1,425 ± 321 mg/d), or placebo. Serum lithium levels were 0.78 to 1.55 mEq/L (mean 1.07 ± 0.19 mEq/L). We used the Overt Aggression Scale (OAS)12,13 (see Related resources) to track frequency and severity of verbal aggression, aggression against objects, aggression against others, and self-aggression.
Lithium reduced aggression more than did placebo, as measured by the clinician-rated Clinical Global Impressions (CGI) scale and staff-rated Global Clinical Judgments (Consensus) Scale (GCJCS). The CGI showed a 70% response rate with lithium and 20% with placebo. Similarly, the GCJCS scale showed 80% response with lithium and 30% with placebo.
The aggression reduction with lithium was statistically significant and clinically evident. Most subjects (37 of 40) experienced at least one adverse event, however, whether receiving lithium or placebo. Nausea, vomiting, and urinary frequency were significantly more common in the lithium-treated group than with placebo. Fewer adverse events were reported in a similar outpatient study,14 probably because of less-frequent monitoring.
Lithium did not reduce aggression in adolescent girls treated for 2 weeks15 or in an outpatient study of children with ADHD.16
Recommendation. Lithium has shown efficacy for reducing severe aggression in hospitalized children with conduct disorder but not in similar outpatients. Consider this drug to reduce severe aggression in children with conduct disorder, especially if they have failed other treatments.
Anticonvulsants
Anticonvulsants have been used to decrease aggression for more than 50 years, and epidemiologic data show their use is increasing markedly.17 Few controlled studies support this prescribing trend, however.18
Initial reports suggested that anticonvulsants reduce disruptive behaviors, but more-critically designed studies have not supported this finding. For example, phenytoin sodium (diphenylhydantoin) demonstrated efficacy in open trials, but controlled trials found this anticonvulsant no more effective than placebo. In fact, placebo may have reduced aggression more than the active drug. Likewise, earlier controlled trials of carbamazepine indicated efficacy, but more-carefully designed trials using specific measures of aggression did not.
Divalproex is the anticonvulsant most commonly used for aggression in children and adolescents. Only one small, placebo-controlled study has found it effective in reducing aggression in children.19
Twenty children ages 10 to 18 with conduct disorder or oppositional defiant disorder were randomized to divalproex, 750 to 1,500 mg/d, or placebo. Eighteen completed the first phase, and 15 crossed over to the other treatment. Concomitant drug treatment, including stimulants, was allowed. The authors reported that 12 of 15 subjects showed some response to divalproex.
A 7-week study compared divalproex in high dosages (up to 1,500 mg/d) versus low dosages (up to 250 mg/d). This study was not placebo-controlled, but aggression was reduced more in the high-dosage than in the low-dosage group.20
Recommendation. If you use an anticonvulsant, first obtain informed consent from the patient and parent. Divalproex causes weight gain and has been associated with increased risk of polycystic ovary syndrome with masculinizing effects.21
Double-blind, placebo-controlled studies of divalproex and other anticonvulsants in treating aggression are needed, particularly as prescriptions for these agents are rising.
Stimulants
Some small controlled studies suggest that stimulants can reduce aggression in children with ADHD, but their effects on aggression in conduct disorder have not been well studied. Aggression was not measured directly in the National Institute of Mental Health Multimodal Treatment Study of Children with ADHD.21 Most other studies have been small and included children with ADHD but not necessarily conduct disorder.
Recommendation. Stimulants may help reduce aggression in children with ADHD, but studies gauging their effects in conduct disorder are needed.
Alpha agonists
Alpha agonists such as clonidine and guanfacine are increasingly being used to treat children with disruptive disorders, despite limited evidence. The small controlled studies that examined alpha agonists as monotherapy or add-ons in this population did not directly measure aggression.22,23
Recommendation. Little data support alpha agonists for reducing aggression. They should probably be considered second-line treatment.
SSRIs
No double-blind, placebo-controlled studies have tested any selective serotonin reuptake inhibitor (SSRIs) for reducing aggression in conduct disorder. In a 6-week open study, citalopram (mean 27 mg/d) reduced impulsive aggression in 12 children with mixed diagnoses, as measured by the modified OAS,13 Child Behavior Checklist, and CGI.24
Recommendation. Use caution when prescribing SSRIs to aggressive youth, as these drugs may contribute to aggression in some mood-disordered children. More evidence of SSRIs’ safety and efficacy in this population is needed.
- Overt Aggression Scale. In: Coccaro EF, Harvey PD, Kupsaw-Lawrence E, et al. Development of neuropharmacologically-based behavioral assessments of impulsiveaggressive behavior. J Neuropsychiatry 1991;3(2):S44-S51.
Drug brand names
- Aripiprazole • Abilify
- Carbamazepine • Tegretol
- Citalopram • Celexa
- Chlorpromazine • Thorazine
- Clonidine • Catapres
- Phenytoin sodium • Dilantin
- Divalproex • Depakote
- Guanfacine • Tenex
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Ziprasidone • Geodon
Disclosure
Dr. Malone receives research support from Pfizer Inc. and Eli Lilly and Co. and is a consultant to Janssen Pharmaceutica.
Dr. Delaney is a consultant to Shire Pharmaceuticals.
Dr. Sheikh reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Prescribing psychoactive medications for children and adolescents American Academy of Child and Adolescent Psychiatry policy statement, adopted Sept. 20, 2001. Available at:http://www.aacap.org/publications/policy/ps41.htm Accessed Jan. 15, 2004.
2. Pappadopulos E, MacIntyre JC, Crismon ML, et al. Treatment recommendations for the use of antipsychotics for aggressive youth (TRAAY). Part II. J Am Acad Child Adolesc Psychiatry 2003;42(2):145-61.
3. Hollander E, Tracy KA, Swann AC, et al. Divalproex in the treatment of impulsive aggression: efficacy in Cluster B personality disorders. Neuropsychopharmacology 2003;28:1186-97.
4. Physician’s Desk Reference (57th ed). Montvale, NJ: Thomson Healthcare, 2003.
5. Aman MG, DeSmedt G, Derivan A, et al. Double-blind, placebo-controlled study of risperidone for the treatment of disruptive behaviors in children with subaverage intelligence. Am J Psychiatry 2002;159:1337-46.
6. Snyder R, Turgay A, Aman M, et al. Effects of risperidone on conduct and disruptive behavior disorders in children with subaverage IQs. J Am Acad Child Adolesc Psychiatry 2002;41(9):1026-36.
7. Findling RL, McNamara NK, Branicky LA, et al. A double-blind pilot study of risperidone in the treatment of conduct disorder. J Am Acad Child Adolesc Psychiatry 2000;39:509-16.
8. Malone RP, Maislin G, Choudhury MS, et al. Risperidone treatment in children and adolescents with autism: short- and long-term safety and effectiveness. J Am Acad Child Adolesc Psychiatry 2002;41(2):140-7.
9. Sheard MH, Marini JL, Bridges CI, Wagner E. The effect of lithium on impulsive aggressive behavior in man. Am J Psychiatry 1976;133(12):1409-13.
10. Campbell M, Adams PB, Small AM, et al. Lithium in hospitalized aggressive children with conduct disorder: a double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry 1995;34:445-53.
11. Malone RP, Delaney MA, Luebbert JF, et al. A double-blind placebo-controlled study of lithium in hospitalized aggressive children and adolescents with conduct disorder. Arch Gen Psychiatry 2000a;57(7):649-54.
12. Yudofsky SC, Silver JM, Jackson W, et al. The Overt Aggression Scale for the objective rating of verbal and physical aggression. Am J Psychiatry 1986;143:35-9.
13. Coccaro EF, Harvey PD, Kupsaw-Lawrence E, et al. Development of neuropharmacologically-based behavioral assessments of impulsive aggressive behavior. J Neuropsychiatry 1991;3:S44-S5.
14. Malone RP, Delaney MA, Gifford C. Adverse events during lithium treatment in children varies by setting. Miami Beach, FL: American Academy of Child and Adolescent Psychiatry annual meeting, 2003.
15. Rifkin A, Karajgi B, Dicker R, et al. Lithium treatment of conduct disorders in adolescents. Am J Psychiatry 1997;154:554-5.
16. Klein RG. Preliminary results: lithium effects in conduct disorders. New Orleans: American Psychiatric Association annual meeting, 1991.
17. Zito JM, Safer DJ, DosReis S, et al. Psychotropic practice patterns for youth: a 10-year perspective. Arch Pediatr Adolesc Med 2003;157:17-25.
18. Malone RP, Delaney MA. Psychopharmacologic interventions in children with aggression: neuroleptics, lithium, and anticonvulsants. In: Coccaro EF (ed). Aggression: assessment and treatment. New York: Marcel Dekker, 2003b;331-49.
19. Donovan SJ, Stewart JW, Nunes EV, et al. Divalproex treatment for youth with explosive temper and mood lability: a double-blind, placebo-controlled crossover design. Am J Psychiatry 2000;157:818-20.
20. Steiner H. A randomized clinical trial of divalproex sodium in conduct disorders. J Clin Psychiatry (in press).
21. Isojarvi JT, Laatikainen TJ, Knip M, et al. Obesity and endocrine disorders in women taking valproate for epilepsy. Ann Neurol 1996;39:579-84.
22. MTA Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention deficit/hyperactivity disorder. Arch Gen Psychiatry 1999;56:1073-86.
23. Conner DF, Barkley RA, Davis HT. A pilot study of methylphenidate, clonidine, or the combination in ADHD comorbid with aggressive oppositional defiant or conduct disorder. Clin Pediatr 2000;39:15-25.
24. Hazell PL, Stuart JE. A randomized controlled trial of clonidine added to psychostimulant medication for hyperactive and aggressive children. J Am Acad Child Adolesc Psychiatry. 2003;886-94.
25. Armenteros JL, Lewis JE. Citalopram treatment for impulsive aggression in children and adolescents: an open pilot study. J Am Acad Child Adolesc Psychiatry 2002;41:522-9.
1. Prescribing psychoactive medications for children and adolescents American Academy of Child and Adolescent Psychiatry policy statement, adopted Sept. 20, 2001. Available at:http://www.aacap.org/publications/policy/ps41.htm Accessed Jan. 15, 2004.
2. Pappadopulos E, MacIntyre JC, Crismon ML, et al. Treatment recommendations for the use of antipsychotics for aggressive youth (TRAAY). Part II. J Am Acad Child Adolesc Psychiatry 2003;42(2):145-61.
3. Hollander E, Tracy KA, Swann AC, et al. Divalproex in the treatment of impulsive aggression: efficacy in Cluster B personality disorders. Neuropsychopharmacology 2003;28:1186-97.
4. Physician’s Desk Reference (57th ed). Montvale, NJ: Thomson Healthcare, 2003.
5. Aman MG, DeSmedt G, Derivan A, et al. Double-blind, placebo-controlled study of risperidone for the treatment of disruptive behaviors in children with subaverage intelligence. Am J Psychiatry 2002;159:1337-46.
6. Snyder R, Turgay A, Aman M, et al. Effects of risperidone on conduct and disruptive behavior disorders in children with subaverage IQs. J Am Acad Child Adolesc Psychiatry 2002;41(9):1026-36.
7. Findling RL, McNamara NK, Branicky LA, et al. A double-blind pilot study of risperidone in the treatment of conduct disorder. J Am Acad Child Adolesc Psychiatry 2000;39:509-16.
8. Malone RP, Maislin G, Choudhury MS, et al. Risperidone treatment in children and adolescents with autism: short- and long-term safety and effectiveness. J Am Acad Child Adolesc Psychiatry 2002;41(2):140-7.
9. Sheard MH, Marini JL, Bridges CI, Wagner E. The effect of lithium on impulsive aggressive behavior in man. Am J Psychiatry 1976;133(12):1409-13.
10. Campbell M, Adams PB, Small AM, et al. Lithium in hospitalized aggressive children with conduct disorder: a double-blind and placebo-controlled study. J Am Acad Child Adolesc Psychiatry 1995;34:445-53.
11. Malone RP, Delaney MA, Luebbert JF, et al. A double-blind placebo-controlled study of lithium in hospitalized aggressive children and adolescents with conduct disorder. Arch Gen Psychiatry 2000a;57(7):649-54.
12. Yudofsky SC, Silver JM, Jackson W, et al. The Overt Aggression Scale for the objective rating of verbal and physical aggression. Am J Psychiatry 1986;143:35-9.
13. Coccaro EF, Harvey PD, Kupsaw-Lawrence E, et al. Development of neuropharmacologically-based behavioral assessments of impulsive aggressive behavior. J Neuropsychiatry 1991;3:S44-S5.
14. Malone RP, Delaney MA, Gifford C. Adverse events during lithium treatment in children varies by setting. Miami Beach, FL: American Academy of Child and Adolescent Psychiatry annual meeting, 2003.
15. Rifkin A, Karajgi B, Dicker R, et al. Lithium treatment of conduct disorders in adolescents. Am J Psychiatry 1997;154:554-5.
16. Klein RG. Preliminary results: lithium effects in conduct disorders. New Orleans: American Psychiatric Association annual meeting, 1991.
17. Zito JM, Safer DJ, DosReis S, et al. Psychotropic practice patterns for youth: a 10-year perspective. Arch Pediatr Adolesc Med 2003;157:17-25.
18. Malone RP, Delaney MA. Psychopharmacologic interventions in children with aggression: neuroleptics, lithium, and anticonvulsants. In: Coccaro EF (ed). Aggression: assessment and treatment. New York: Marcel Dekker, 2003b;331-49.
19. Donovan SJ, Stewart JW, Nunes EV, et al. Divalproex treatment for youth with explosive temper and mood lability: a double-blind, placebo-controlled crossover design. Am J Psychiatry 2000;157:818-20.
20. Steiner H. A randomized clinical trial of divalproex sodium in conduct disorders. J Clin Psychiatry (in press).
21. Isojarvi JT, Laatikainen TJ, Knip M, et al. Obesity and endocrine disorders in women taking valproate for epilepsy. Ann Neurol 1996;39:579-84.
22. MTA Cooperative Group. A 14-month randomized clinical trial of treatment strategies for attention deficit/hyperactivity disorder. Arch Gen Psychiatry 1999;56:1073-86.
23. Conner DF, Barkley RA, Davis HT. A pilot study of methylphenidate, clonidine, or the combination in ADHD comorbid with aggressive oppositional defiant or conduct disorder. Clin Pediatr 2000;39:15-25.
24. Hazell PL, Stuart JE. A randomized controlled trial of clonidine added to psychostimulant medication for hyperactive and aggressive children. J Am Acad Child Adolesc Psychiatry. 2003;886-94.
25. Armenteros JL, Lewis JE. Citalopram treatment for impulsive aggression in children and adolescents: an open pilot study. J Am Acad Child Adolesc Psychiatry 2002;41:522-9.