User login
A physical deformity no clinician has asked about

HISTORY
A 37-year-old man presents to dermatology for refill of his rosacea medication, at which time the clinician notices an entirely incidental deformity of the patient's fingers. The patient says his "crooked little fingers" have been present since birth, and he claims to be otherwise healthy. There is no family history of serious medical problems, and the patient denies any issues with the function of his fingers or hands.
EXAMINATION
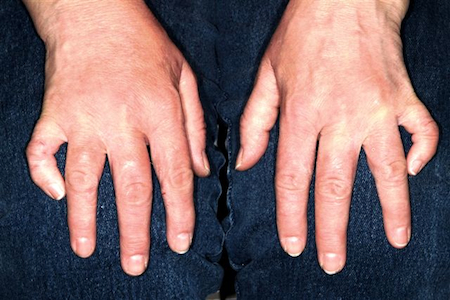
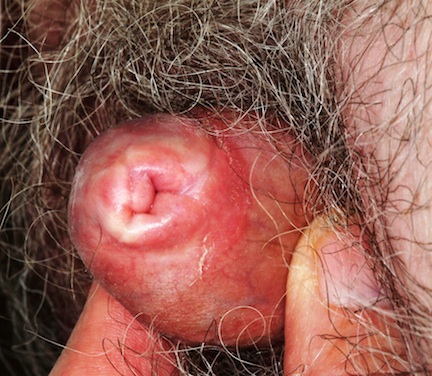
The fifth fingers of both hands are markedly curved inward, effectively shortening the fingers, but without any rotational curvature. No other defect can be seen in the fingers. The patient's appearance is otherwise unremarkable.
DISCUSSION
Physical diagnosis is a skill unto its own in medicine, spilling over into every specialty. Many findings are subtle, but some are so obvious as to escape detection—as in the case of this patient, who said no medical provider he'd ever seen had mentioned his fingers.
I was ignorant of what this defect might represent, or even what it was called. But I'm aware of a good "rule of thumb," which is: If it looks like something, it probably is. In other words, if it looks like it ought to have a name, it probably does—and it probably has implications that are potentially meaningful.
I promised the patient I'd find out what his deformity was called, and even though it took a while, I was able to do just that. (In retrospect, I should have simply asked an orthopedic PA, but instead I did an Internet search for "deformities of the fingers.") Here is what I found:
This patient's diagnosis was clinodactyly, a radial curving of the fingers. The fifth is most likely to be affected, though other fingers may be involved, and the condition is usually bilateral, as in this case. Although I wasn't familiar with it, clinodactyly is quite common: It affects 1% to 20% of children, appearing either at birth or shortly thereafter. Most commonly, it is insignificant and does not affect normal function.
However, up to 80% of Down syndrome patients have clinodactyly, and it has been associated with many other syndromes, usually as an incidental finding. Its presence in an infant should prompt a search for any other possibly related phenomena. Likewise, when seen on a prenatal ultrasound, chromosomal analysis is indicated.
Classified as to degree of involvement, clinodactyly presents in several variations. The basic defect is in the wedge shape of the distal phalanx, which should be rectangular. Extreme cases can require surgery to restore normal function and appearance.
As one would expect, there are a huge number of deformities of the fingers, with infinite variations. For example, children can be born with fingers fused together, a condition called syndactyly. Camptodactyly is a type of deformity in which there is a fixed-flexion deformity of a finger. All of these can be part of an inherited condition, usually in an autosomal dominant mode of transmission.
Obviously, this patient had suffered little if at all with his condition, though he recalled being ashamed as a child because his fingers were so different from his peers'.
TAKE-HOME LEARNING POINTS
• Clinodactyly is common, affecting 1% to 20% of all newborns, and is usually a normal variant.
• However, it has been associated with many other diseases and syndromes, most notably Down syndrome.
• It is therefore reasonable to search for other related abnormalities.
• Clinodactyly is a good example of the principle: If it looks like it's "something," it probably has a name and implications for the patient's health.

HISTORY
A 37-year-old man presents to dermatology for refill of his rosacea medication, at which time the clinician notices an entirely incidental deformity of the patient's fingers. The patient says his "crooked little fingers" have been present since birth, and he claims to be otherwise healthy. There is no family history of serious medical problems, and the patient denies any issues with the function of his fingers or hands.
EXAMINATION
The fifth fingers of both hands are markedly curved inward, effectively shortening the fingers, but without any rotational curvature. No other defect can be seen in the fingers. The patient's appearance is otherwise unremarkable.
DISCUSSION
Physical diagnosis is a skill unto its own in medicine, spilling over into every specialty. Many findings are subtle, but some are so obvious as to escape detection—as in the case of this patient, who said no medical provider he'd ever seen had mentioned his fingers.
I was ignorant of what this defect might represent, or even what it was called. But I'm aware of a good "rule of thumb," which is: If it looks like something, it probably is. In other words, if it looks like it ought to have a name, it probably does—and it probably has implications that are potentially meaningful.
I promised the patient I'd find out what his deformity was called, and even though it took a while, I was able to do just that. (In retrospect, I should have simply asked an orthopedic PA, but instead I did an Internet search for "deformities of the fingers.") Here is what I found:
This patient's diagnosis was clinodactyly, a radial curving of the fingers. The fifth is most likely to be affected, though other fingers may be involved, and the condition is usually bilateral, as in this case. Although I wasn't familiar with it, clinodactyly is quite common: It affects 1% to 20% of children, appearing either at birth or shortly thereafter. Most commonly, it is insignificant and does not affect normal function.
However, up to 80% of Down syndrome patients have clinodactyly, and it has been associated with many other syndromes, usually as an incidental finding. Its presence in an infant should prompt a search for any other possibly related phenomena. Likewise, when seen on a prenatal ultrasound, chromosomal analysis is indicated.
Classified as to degree of involvement, clinodactyly presents in several variations. The basic defect is in the wedge shape of the distal phalanx, which should be rectangular. Extreme cases can require surgery to restore normal function and appearance.
As one would expect, there are a huge number of deformities of the fingers, with infinite variations. For example, children can be born with fingers fused together, a condition called syndactyly. Camptodactyly is a type of deformity in which there is a fixed-flexion deformity of a finger. All of these can be part of an inherited condition, usually in an autosomal dominant mode of transmission.
Obviously, this patient had suffered little if at all with his condition, though he recalled being ashamed as a child because his fingers were so different from his peers'.
TAKE-HOME LEARNING POINTS
• Clinodactyly is common, affecting 1% to 20% of all newborns, and is usually a normal variant.
• However, it has been associated with many other diseases and syndromes, most notably Down syndrome.
• It is therefore reasonable to search for other related abnormalities.
• Clinodactyly is a good example of the principle: If it looks like it's "something," it probably has a name and implications for the patient's health.

HISTORY
A 37-year-old man presents to dermatology for refill of his rosacea medication, at which time the clinician notices an entirely incidental deformity of the patient's fingers. The patient says his "crooked little fingers" have been present since birth, and he claims to be otherwise healthy. There is no family history of serious medical problems, and the patient denies any issues with the function of his fingers or hands.
EXAMINATION
The fifth fingers of both hands are markedly curved inward, effectively shortening the fingers, but without any rotational curvature. No other defect can be seen in the fingers. The patient's appearance is otherwise unremarkable.
DISCUSSION
Physical diagnosis is a skill unto its own in medicine, spilling over into every specialty. Many findings are subtle, but some are so obvious as to escape detection—as in the case of this patient, who said no medical provider he'd ever seen had mentioned his fingers.
I was ignorant of what this defect might represent, or even what it was called. But I'm aware of a good "rule of thumb," which is: If it looks like something, it probably is. In other words, if it looks like it ought to have a name, it probably does—and it probably has implications that are potentially meaningful.
I promised the patient I'd find out what his deformity was called, and even though it took a while, I was able to do just that. (In retrospect, I should have simply asked an orthopedic PA, but instead I did an Internet search for "deformities of the fingers.") Here is what I found:
This patient's diagnosis was clinodactyly, a radial curving of the fingers. The fifth is most likely to be affected, though other fingers may be involved, and the condition is usually bilateral, as in this case. Although I wasn't familiar with it, clinodactyly is quite common: It affects 1% to 20% of children, appearing either at birth or shortly thereafter. Most commonly, it is insignificant and does not affect normal function.
However, up to 80% of Down syndrome patients have clinodactyly, and it has been associated with many other syndromes, usually as an incidental finding. Its presence in an infant should prompt a search for any other possibly related phenomena. Likewise, when seen on a prenatal ultrasound, chromosomal analysis is indicated.
Classified as to degree of involvement, clinodactyly presents in several variations. The basic defect is in the wedge shape of the distal phalanx, which should be rectangular. Extreme cases can require surgery to restore normal function and appearance.
As one would expect, there are a huge number of deformities of the fingers, with infinite variations. For example, children can be born with fingers fused together, a condition called syndactyly. Camptodactyly is a type of deformity in which there is a fixed-flexion deformity of a finger. All of these can be part of an inherited condition, usually in an autosomal dominant mode of transmission.
Obviously, this patient had suffered little if at all with his condition, though he recalled being ashamed as a child because his fingers were so different from his peers'.
TAKE-HOME LEARNING POINTS
• Clinodactyly is common, affecting 1% to 20% of all newborns, and is usually a normal variant.
• However, it has been associated with many other diseases and syndromes, most notably Down syndrome.
• It is therefore reasonable to search for other related abnormalities.
• Clinodactyly is a good example of the principle: If it looks like it's "something," it probably has a name and implications for the patient's health.
A fingernail "infection" present since birth
HISTORY
A 25-year-old man presents to dermatology with what he describes as a "fungal infection" of the fingernails that he's had since birth. His family physician made the diagnosis, noting similar changes in the nails of the patient's mother and several siblings.
The patient's family is part of a close-knit religious community of farmers whose northern European ancestors immigrated to this country several generations ago. For the most part, they keep to themselves, eschewing modern technology and marrying almost exclusively within the community.
EXAMINATION
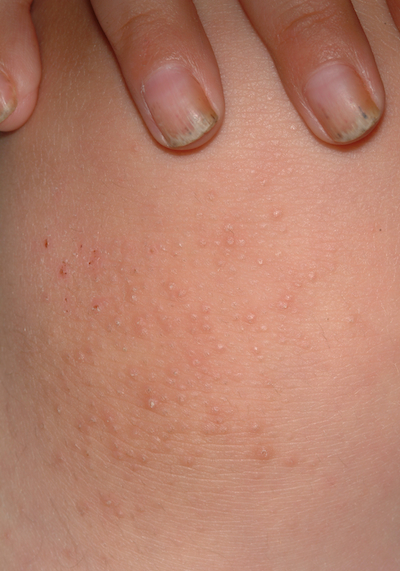
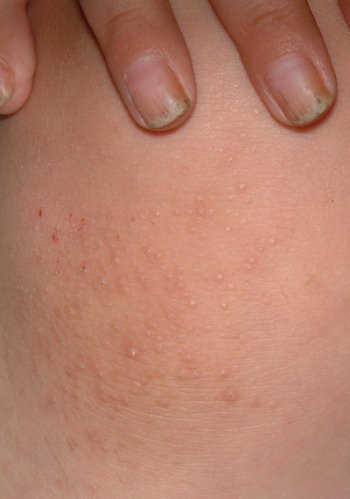
The patient's fingernails are uniformly thickened and dystrophic, but without significant discoloration. All 10 toenails are similarly, though not as severely, affected. The patient's palms and soles are hyperkeratotic, and the upper anterior legs are covered by a folliculocentric papular hyperkeratosis reminiscent of a coarse keratosis pilaris.
DIAGNOSIS/DISCUSSION
This case of pachyonychia congenita (PC) is but one example of a large category of inheritable conditions involving skin, hair, and nails. These are sometimes referred to collectively as the "genodermatoses," a group that includes better-known entities such as neurofibromatosis, tuberous sclerosis, and Ehlers-Danlos syndrome.
PC is a rare condition that represents a mutation of keratin genes and is usually of autosomal dominant inheritance. First described by Muller in 1904, it was eventually categorized into one of two types: type I, MIM 167200, also known as Jadassohn-Lewandowsky, the most common type, and type II, MIM 167210, also known as Jackson-Lawler, with slightly different features. Today, a more common view is that no such divisions exist—only variations of PC that exhibit overlapping features.
PC appears to affect both genders equally and does not seem to have any effect on lifespan. However, when PC affects fingernails (87% of cases), there can be significant loss of function. In addition, the hyperkeratosis of palms and soles can be bothersome, even painful. In extreme cases, blisters can develop at the periphery of the hyperkeratotic areas. As these break down, the patient is subject to even more pain and the risk for secondary infection.
Cases such as this one mimic a significant item in the differential: epidermolysis bullosa. Patient education and reassurance serve well to assuage the patient's fears of fungal infection. A useful pearl for the clinician in that regard is that fungal infections of the fingernails are to be doubted, since they are quite uncommon.
A curious feature of this condition is that many children with PC are born with natal teeth, causing significant pain for the breastfeeding mother. These teeth are lost as permanent teeth grow in.
TREATMENT
The only treatment for PC is surgical ablation of the fingernails, a singularly impractical and unpredictable procedure. It is best reserved for the highly motivated patient.
TAKE-HOME LEARNING POINTS
• Pachyonychia congenita (PC) is a rare inherited mutation of keratin genes manifesting with thickened, dystrophic nails and hyperkeratosis of palms and soles.
• PC is only one of dozens of so-called "genodermatoses," inherited conditions involving skin, hair, nails, and mucous membranes; other examples include neurofibromatosis, tuberous sclerosis, and Ehlers-Danlos syndrome.
• A family history of similar problems, along with that of natal teeth, help to establish the diagnosis.
• Fungal infections of fingernails occur approximately 1/20 as often as onychomycosis of toenails.

HISTORY
A 25-year-old man presents to dermatology with what he describes as a "fungal infection" of the fingernails that he's had since birth. His family physician made the diagnosis, noting similar changes in the nails of the patient's mother and several siblings.
The patient's family is part of a close-knit religious community of farmers whose northern European ancestors immigrated to this country several generations ago. For the most part, they keep to themselves, eschewing modern technology and marrying almost exclusively within the community.
EXAMINATION
The patient's fingernails are uniformly thickened and dystrophic, but without significant discoloration. All 10 toenails are similarly, though not as severely, affected. The patient's palms and soles are hyperkeratotic, and the upper anterior legs are covered by a folliculocentric papular hyperkeratosis reminiscent of a coarse keratosis pilaris.
DIAGNOSIS/DISCUSSION
This case of pachyonychia congenita (PC) is but one example of a large category of inheritable conditions involving skin, hair, and nails. These are sometimes referred to collectively as the "genodermatoses," a group that includes better-known entities such as neurofibromatosis, tuberous sclerosis, and Ehlers-Danlos syndrome.
PC is a rare condition that represents a mutation of keratin genes and is usually of autosomal dominant inheritance. First described by Muller in 1904, it was eventually categorized into one of two types: type I, MIM 167200, also known as Jadassohn-Lewandowsky, the most common type, and type II, MIM 167210, also known as Jackson-Lawler, with slightly different features. Today, a more common view is that no such divisions exist—only variations of PC that exhibit overlapping features.
PC appears to affect both genders equally and does not seem to have any effect on lifespan. However, when PC affects fingernails (87% of cases), there can be significant loss of function. In addition, the hyperkeratosis of palms and soles can be bothersome, even painful. In extreme cases, blisters can develop at the periphery of the hyperkeratotic areas. As these break down, the patient is subject to even more pain and the risk for secondary infection.
Cases such as this one mimic a significant item in the differential: epidermolysis bullosa. Patient education and reassurance serve well to assuage the patient's fears of fungal infection. A useful pearl for the clinician in that regard is that fungal infections of the fingernails are to be doubted, since they are quite uncommon.
A curious feature of this condition is that many children with PC are born with natal teeth, causing significant pain for the breastfeeding mother. These teeth are lost as permanent teeth grow in.
TREATMENT
The only treatment for PC is surgical ablation of the fingernails, a singularly impractical and unpredictable procedure. It is best reserved for the highly motivated patient.
TAKE-HOME LEARNING POINTS
• Pachyonychia congenita (PC) is a rare inherited mutation of keratin genes manifesting with thickened, dystrophic nails and hyperkeratosis of palms and soles.
• PC is only one of dozens of so-called "genodermatoses," inherited conditions involving skin, hair, nails, and mucous membranes; other examples include neurofibromatosis, tuberous sclerosis, and Ehlers-Danlos syndrome.
• A family history of similar problems, along with that of natal teeth, help to establish the diagnosis.
• Fungal infections of fingernails occur approximately 1/20 as often as onychomycosis of toenails.

HISTORY
A 25-year-old man presents to dermatology with what he describes as a "fungal infection" of the fingernails that he's had since birth. His family physician made the diagnosis, noting similar changes in the nails of the patient's mother and several siblings.
The patient's family is part of a close-knit religious community of farmers whose northern European ancestors immigrated to this country several generations ago. For the most part, they keep to themselves, eschewing modern technology and marrying almost exclusively within the community.
EXAMINATION
The patient's fingernails are uniformly thickened and dystrophic, but without significant discoloration. All 10 toenails are similarly, though not as severely, affected. The patient's palms and soles are hyperkeratotic, and the upper anterior legs are covered by a folliculocentric papular hyperkeratosis reminiscent of a coarse keratosis pilaris.
DIAGNOSIS/DISCUSSION
This case of pachyonychia congenita (PC) is but one example of a large category of inheritable conditions involving skin, hair, and nails. These are sometimes referred to collectively as the "genodermatoses," a group that includes better-known entities such as neurofibromatosis, tuberous sclerosis, and Ehlers-Danlos syndrome.
PC is a rare condition that represents a mutation of keratin genes and is usually of autosomal dominant inheritance. First described by Muller in 1904, it was eventually categorized into one of two types: type I, MIM 167200, also known as Jadassohn-Lewandowsky, the most common type, and type II, MIM 167210, also known as Jackson-Lawler, with slightly different features. Today, a more common view is that no such divisions exist—only variations of PC that exhibit overlapping features.
PC appears to affect both genders equally and does not seem to have any effect on lifespan. However, when PC affects fingernails (87% of cases), there can be significant loss of function. In addition, the hyperkeratosis of palms and soles can be bothersome, even painful. In extreme cases, blisters can develop at the periphery of the hyperkeratotic areas. As these break down, the patient is subject to even more pain and the risk for secondary infection.
Cases such as this one mimic a significant item in the differential: epidermolysis bullosa. Patient education and reassurance serve well to assuage the patient's fears of fungal infection. A useful pearl for the clinician in that regard is that fungal infections of the fingernails are to be doubted, since they are quite uncommon.
A curious feature of this condition is that many children with PC are born with natal teeth, causing significant pain for the breastfeeding mother. These teeth are lost as permanent teeth grow in.
TREATMENT
The only treatment for PC is surgical ablation of the fingernails, a singularly impractical and unpredictable procedure. It is best reserved for the highly motivated patient.
TAKE-HOME LEARNING POINTS
• Pachyonychia congenita (PC) is a rare inherited mutation of keratin genes manifesting with thickened, dystrophic nails and hyperkeratosis of palms and soles.
• PC is only one of dozens of so-called "genodermatoses," inherited conditions involving skin, hair, nails, and mucous membranes; other examples include neurofibromatosis, tuberous sclerosis, and Ehlers-Danlos syndrome.
• A family history of similar problems, along with that of natal teeth, help to establish the diagnosis.
• Fungal infections of fingernails occur approximately 1/20 as often as onychomycosis of toenails.
Hair loss comes and goes, always causing distress
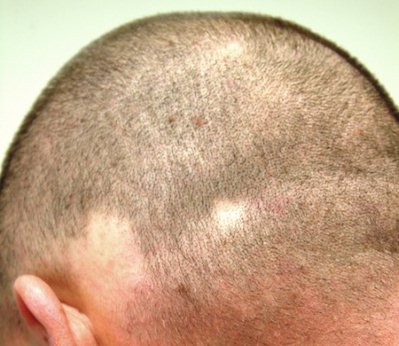
HISTORY
This 38-year-old man has had recurrent episodes of focal hair loss. His scalp has been the most affected area, but he has also noticed hair loss in his beard and in the suprapubic area. Although the problem resolves in weeks to months, it is very distressing for him.
Early in each episode, he experiences a slight tingling in the area, followed by noticeable hair loss—usually in a round pattern. He has consulted a number of providers, but no one in dermatology (until now, that is).
Additional history taking reveals a strong connection between stress and these episodes of hair loss. Moreover, there is a family history of similar hair loss, as well as of thyroid disease.
EXAMINATION
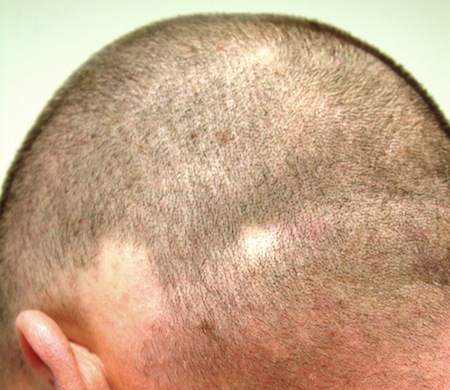
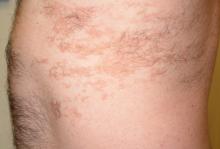
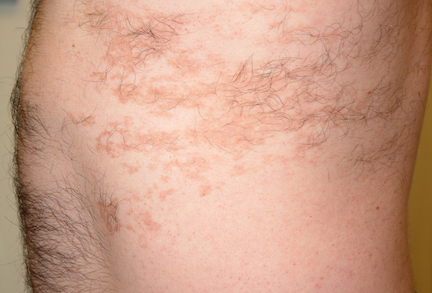
Several areas of complete hair loss, in annular configuration, are noted in the patient's scalp. No epidermal changes (eg, scaling, redness, edema) are present. Two of the sites are slightly larger than 5 cm in diameter.
DISCUSSION
Hair loss (alopecia) is an exceedingly common complaint, but alopecia areata (AA) is one of the more prolific types. Stress appears to trigger the episodes. Ironically, many patients find the hair loss itself to be extremely stressful, which of course compounds the problem.
The most widely accepted theory is that AA is an autoimmune phenomenon, mediated by T-cells and occurring in genetically predisposed individuals. Support for this theory is abundant: increased levels of antibodies directed to various hair follicle structures and a perifollicular lymphocytic infiltrate seen histologically.
Likewise, the genetic basis for predisposition to AA appears valid. For example, 10% to 20% of AA patients report a positive family history. (The more severe the AA, the more likely the patient is to have that family history.) When one twin has AA, the other is quite likely to develop it during his/her lifetime. The high association of Down's syndrome with AA suggests the involvement of a gene located on chromosome 21, but other genes have also been implicated.
PROGNOSIS AND TREATMENT
In the majority of cases, AA resolves, with or without treatment, within weeks to months. As this particular case illustrates, recurrences are quite common. In a study of more than 700 patients, 90% experienced a recurrence of AA within five years.
A tiny percentage of AA patients will progress to the permanent loss of all scalp hair (termed alopecia totalis), and a small percentage of those patients will go on to lose every hair on their body (alopecia universalis). In addition to a family history of such problems, other factors that predict this outcome include youth, atopy, and the extent of involvement of the peripheral scalp (ophiasis).
Local intralesional steroid injection (triamcinolone 5 mg/cc) usually stimulates modest hair regrowth, but must be continued at regular intervals for maintenance. Many other systemic and topically applied medications have been tried, but none appear to have a curative effect.
DIFFERENTIAL DIAGNOSIS
Aside from androgenetic alopecia (the so-called male pattern baldness seen in both men and women), the next most common type of hair loss is telogen effluvium. Seen almost exclusively in women, TE involves uniform hair loss from all over the scalp; the lost hair can be found in the comb, brush, or sink.
Occasionally, AA can be so atypical as to require biopsy to distinguish it from another major item in the differential: trichotillomania. The latter condition is characterized by focal hair loss caused by obsessive twirling or other digital manipulation by the patient, who often has an obsessive-compulsive disorder. This process usually leaves hairs of unequal lengths in the affected location (whereas in AA, total hair loss is typical).
The process of evaluating patients for hair loss is often complicated by the presence of more than one diagnosis. For example, it's quite common for a woman to have longstanding, mild androgenetic alopecia, with thinning mostly confined to the crown of the scalp, but then to experience the onset of AA or TE superimposed on the chronic hair loss. This can make for a confusing clinical picture.
The potential for hair loss due to other conditions—such as connective tissue diseases (lupus is a prime example), secondary syphilis, thyroid disease, or any number of inflammatory conditions, including lichen planopilaris—further complicates the process. And as if all this were not enough, alopecia patients are usually, and understandably, anxious about their problem. Prompt referral of these patients to dermatology is often advisable.
TAKE-HOME LEARNING POINTS
• Localized, complete hair loss in a well-defined annular pattern is probably alopecia areata (AA).
• One of the more common types of hair loss, AA is polygenic in origin, with an autoimmune basis, and manifests in a genetically predisposed patient.
• No treatment has been shown to influence the long-term outcome of AA, which is usually self-limiting.
• Recurrences of AA are quite common; stress appears to be the triggering factor in many cases.
• The fact that AA is common in patients with Down's syndrome suggests the possible involvement of a gene located on chromosome 21.
• Alopecia patients are often quite anxious and therefore may benefit from referral to dermatology for evaluation of what can be a complex problem.

HISTORY
This 38-year-old man has had recurrent episodes of focal hair loss. His scalp has been the most affected area, but he has also noticed hair loss in his beard and in the suprapubic area. Although the problem resolves in weeks to months, it is very distressing for him.
Early in each episode, he experiences a slight tingling in the area, followed by noticeable hair loss—usually in a round pattern. He has consulted a number of providers, but no one in dermatology (until now, that is).
Additional history taking reveals a strong connection between stress and these episodes of hair loss. Moreover, there is a family history of similar hair loss, as well as of thyroid disease.
EXAMINATION
Several areas of complete hair loss, in annular configuration, are noted in the patient's scalp. No epidermal changes (eg, scaling, redness, edema) are present. Two of the sites are slightly larger than 5 cm in diameter.
DISCUSSION
Hair loss (alopecia) is an exceedingly common complaint, but alopecia areata (AA) is one of the more prolific types. Stress appears to trigger the episodes. Ironically, many patients find the hair loss itself to be extremely stressful, which of course compounds the problem.
The most widely accepted theory is that AA is an autoimmune phenomenon, mediated by T-cells and occurring in genetically predisposed individuals. Support for this theory is abundant: increased levels of antibodies directed to various hair follicle structures and a perifollicular lymphocytic infiltrate seen histologically.
Likewise, the genetic basis for predisposition to AA appears valid. For example, 10% to 20% of AA patients report a positive family history. (The more severe the AA, the more likely the patient is to have that family history.) When one twin has AA, the other is quite likely to develop it during his/her lifetime. The high association of Down's syndrome with AA suggests the involvement of a gene located on chromosome 21, but other genes have also been implicated.
PROGNOSIS AND TREATMENT
In the majority of cases, AA resolves, with or without treatment, within weeks to months. As this particular case illustrates, recurrences are quite common. In a study of more than 700 patients, 90% experienced a recurrence of AA within five years.
A tiny percentage of AA patients will progress to the permanent loss of all scalp hair (termed alopecia totalis), and a small percentage of those patients will go on to lose every hair on their body (alopecia universalis). In addition to a family history of such problems, other factors that predict this outcome include youth, atopy, and the extent of involvement of the peripheral scalp (ophiasis).
Local intralesional steroid injection (triamcinolone 5 mg/cc) usually stimulates modest hair regrowth, but must be continued at regular intervals for maintenance. Many other systemic and topically applied medications have been tried, but none appear to have a curative effect.
DIFFERENTIAL DIAGNOSIS
Aside from androgenetic alopecia (the so-called male pattern baldness seen in both men and women), the next most common type of hair loss is telogen effluvium. Seen almost exclusively in women, TE involves uniform hair loss from all over the scalp; the lost hair can be found in the comb, brush, or sink.
Occasionally, AA can be so atypical as to require biopsy to distinguish it from another major item in the differential: trichotillomania. The latter condition is characterized by focal hair loss caused by obsessive twirling or other digital manipulation by the patient, who often has an obsessive-compulsive disorder. This process usually leaves hairs of unequal lengths in the affected location (whereas in AA, total hair loss is typical).
The process of evaluating patients for hair loss is often complicated by the presence of more than one diagnosis. For example, it's quite common for a woman to have longstanding, mild androgenetic alopecia, with thinning mostly confined to the crown of the scalp, but then to experience the onset of AA or TE superimposed on the chronic hair loss. This can make for a confusing clinical picture.
The potential for hair loss due to other conditions—such as connective tissue diseases (lupus is a prime example), secondary syphilis, thyroid disease, or any number of inflammatory conditions, including lichen planopilaris—further complicates the process. And as if all this were not enough, alopecia patients are usually, and understandably, anxious about their problem. Prompt referral of these patients to dermatology is often advisable.
TAKE-HOME LEARNING POINTS
• Localized, complete hair loss in a well-defined annular pattern is probably alopecia areata (AA).
• One of the more common types of hair loss, AA is polygenic in origin, with an autoimmune basis, and manifests in a genetically predisposed patient.
• No treatment has been shown to influence the long-term outcome of AA, which is usually self-limiting.
• Recurrences of AA are quite common; stress appears to be the triggering factor in many cases.
• The fact that AA is common in patients with Down's syndrome suggests the possible involvement of a gene located on chromosome 21.
• Alopecia patients are often quite anxious and therefore may benefit from referral to dermatology for evaluation of what can be a complex problem.

HISTORY
This 38-year-old man has had recurrent episodes of focal hair loss. His scalp has been the most affected area, but he has also noticed hair loss in his beard and in the suprapubic area. Although the problem resolves in weeks to months, it is very distressing for him.
Early in each episode, he experiences a slight tingling in the area, followed by noticeable hair loss—usually in a round pattern. He has consulted a number of providers, but no one in dermatology (until now, that is).
Additional history taking reveals a strong connection between stress and these episodes of hair loss. Moreover, there is a family history of similar hair loss, as well as of thyroid disease.
EXAMINATION
Several areas of complete hair loss, in annular configuration, are noted in the patient's scalp. No epidermal changes (eg, scaling, redness, edema) are present. Two of the sites are slightly larger than 5 cm in diameter.
DISCUSSION
Hair loss (alopecia) is an exceedingly common complaint, but alopecia areata (AA) is one of the more prolific types. Stress appears to trigger the episodes. Ironically, many patients find the hair loss itself to be extremely stressful, which of course compounds the problem.
The most widely accepted theory is that AA is an autoimmune phenomenon, mediated by T-cells and occurring in genetically predisposed individuals. Support for this theory is abundant: increased levels of antibodies directed to various hair follicle structures and a perifollicular lymphocytic infiltrate seen histologically.
Likewise, the genetic basis for predisposition to AA appears valid. For example, 10% to 20% of AA patients report a positive family history. (The more severe the AA, the more likely the patient is to have that family history.) When one twin has AA, the other is quite likely to develop it during his/her lifetime. The high association of Down's syndrome with AA suggests the involvement of a gene located on chromosome 21, but other genes have also been implicated.
PROGNOSIS AND TREATMENT
In the majority of cases, AA resolves, with or without treatment, within weeks to months. As this particular case illustrates, recurrences are quite common. In a study of more than 700 patients, 90% experienced a recurrence of AA within five years.
A tiny percentage of AA patients will progress to the permanent loss of all scalp hair (termed alopecia totalis), and a small percentage of those patients will go on to lose every hair on their body (alopecia universalis). In addition to a family history of such problems, other factors that predict this outcome include youth, atopy, and the extent of involvement of the peripheral scalp (ophiasis).
Local intralesional steroid injection (triamcinolone 5 mg/cc) usually stimulates modest hair regrowth, but must be continued at regular intervals for maintenance. Many other systemic and topically applied medications have been tried, but none appear to have a curative effect.
DIFFERENTIAL DIAGNOSIS
Aside from androgenetic alopecia (the so-called male pattern baldness seen in both men and women), the next most common type of hair loss is telogen effluvium. Seen almost exclusively in women, TE involves uniform hair loss from all over the scalp; the lost hair can be found in the comb, brush, or sink.
Occasionally, AA can be so atypical as to require biopsy to distinguish it from another major item in the differential: trichotillomania. The latter condition is characterized by focal hair loss caused by obsessive twirling or other digital manipulation by the patient, who often has an obsessive-compulsive disorder. This process usually leaves hairs of unequal lengths in the affected location (whereas in AA, total hair loss is typical).
The process of evaluating patients for hair loss is often complicated by the presence of more than one diagnosis. For example, it's quite common for a woman to have longstanding, mild androgenetic alopecia, with thinning mostly confined to the crown of the scalp, but then to experience the onset of AA or TE superimposed on the chronic hair loss. This can make for a confusing clinical picture.
The potential for hair loss due to other conditions—such as connective tissue diseases (lupus is a prime example), secondary syphilis, thyroid disease, or any number of inflammatory conditions, including lichen planopilaris—further complicates the process. And as if all this were not enough, alopecia patients are usually, and understandably, anxious about their problem. Prompt referral of these patients to dermatology is often advisable.
TAKE-HOME LEARNING POINTS
• Localized, complete hair loss in a well-defined annular pattern is probably alopecia areata (AA).
• One of the more common types of hair loss, AA is polygenic in origin, with an autoimmune basis, and manifests in a genetically predisposed patient.
• No treatment has been shown to influence the long-term outcome of AA, which is usually self-limiting.
• Recurrences of AA are quite common; stress appears to be the triggering factor in many cases.
• The fact that AA is common in patients with Down's syndrome suggests the possible involvement of a gene located on chromosome 21.
• Alopecia patients are often quite anxious and therefore may benefit from referral to dermatology for evaluation of what can be a complex problem.
Giving providers a (diagnostic) hand with rash
HISTORY
Rashes of the hand are commonly seen in both primary care and dermatology practices. Their location makes them problematic, in terms of interference with normal activities and difficulty with treatment.
This 51-year-old man’s rash appeared about a month ago, with no premonitory signs. He has consulted numerous providers about it, including his primary care clinician. That practitioner diagnosed a probable “fungal infection” and prescribed a combination clotrimazole/betamethasone dipropionate cream. The rash subsequently improved, though not substantially.
The patient recalls developing a similar rash several other times during adulthood, but says it was never this severe. His mother has had similar eruptions, as well as sensitive skin in general. Both the patient and his mother are plagued by seasonal allergies, asthma, and sweating of the palms.
EXAMINATION
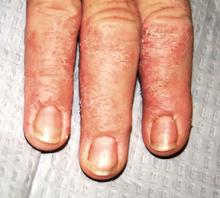
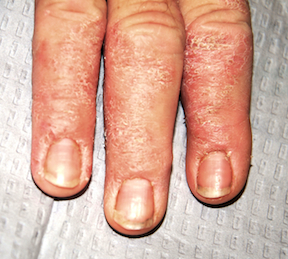
The distal portions of the second through fourth fingers of the right hand are affected. This is typical, according to the patient. The cuticles of all four affected nails are detached from the nail plates, with two of the three nail plates showing mild transverse ridging.
There is circumferential involvement of the distal half of all four fingers, with a well-defined margin and a blistery look to the papulosquamous process. Neither the left hand nor the feet are impacted.
DISCUSSION
This is a classic picture of a condition that goes by several names, most commonly pompholyx, also known as dishidrotic eczema. Despite the efforts of many investigators, with much information gleaned, it remains quite mysterious. It can be chronic or acute and can closely resemble what is known simply as hand dermatitis.
Pompholyx is associated with atopy in at least 50% of cases (such as this one). Hyperhidrosis of the palms is also common, and resolution of difficult cases has been achieved with injection of botulinum toxin A. This hypothesis is bolstered by the fact that many patients report onset in summer months; however, there are many whose history fails to follow this pattern.
Research has revealed that more than a few patients with pompholyx are allergic to one of several ingested metals, such as nickel and cobalt. But challenge tests with those substances have failed to consistently replicate the eruption.
Stress is another reported factor in the genesis of this condition. It is well known to exacerbate related conditions (eg, atopic dermatitis), but again, many patients deny any such connection.
When confronted with this clinical picture, dermatology providers are trained to look at the patient’s feet, where a flare of tinea pedis can sometimes be found. This common foot infection can, under certain circumstances, trigger a clinically indistinguishable pompholyx-like eruption on the hands, called an id reaction. In these cases, the tinea pedis always precedes the hand rash. Both resolve with adequate treatment (ie, oral antifungals).
The differential also includes irritant or contact dermatitis. However, patients are likely to report a contributing factor early on, lessening the clinical mystery.
A major diagnostic clue in this and similar cases is the effect on the cuticles and nails. Both are good indicators of the chronicity and nature of the problem.
TREATMENT
Treatment of pompholyx is notoriously difficult. Potent topical corticosteroids (eg, clobetasol cream), applied under occlusion to dampened skin at bedtime, are the cornerstone. In this and many such cases, while not curative by themselves, oral antibiotics can help to reduce colonization by staphylococcus.
A two-week course of prednisone (eg, 20 mg bid for a week, then 20 mg/d for another week) can be extremely helpful. Alternatively, an intramuscular injection of triamcinolone (40 to 60 mg) can be used, especially if the patient has a history of reflux or peptic ulcer. Relative contraindications to systemic steroid use include diabetes, poorly controlled hypertension, congestive heart failure, and dementia.
Phototherapy has also been used, but botulinum injection is becoming common in cases in which hyperhidrosis is the major culprit.
TAKE-HOME LEARNING POINTS
• Pompholyx manifests as a papulosquamous, well-defined rash, often composed of tiny fluid-filled blisters, on the fingers and hand.
• The cause of this condition is unknown, but potential triggers include atopy, stress, hyperhidrosis, and ingestion of metals (eg, nickel or cobalt).
• Since acute tinea pedis can trigger a similar eruption on the hands (and responds to oral antifungals, as does pompholyx), checking the patient’s feet can provide a diagnostic clue.
• Patients may find it helpful to apply a potent topical corticosteroid cream (eg, clobetasol) to dampened hands (which allows for increased penetration of the medication) and cover with cotton gloves overnight.
HISTORY
Rashes of the hand are commonly seen in both primary care and dermatology practices. Their location makes them problematic, in terms of interference with normal activities and difficulty with treatment.
This 51-year-old man’s rash appeared about a month ago, with no premonitory signs. He has consulted numerous providers about it, including his primary care clinician. That practitioner diagnosed a probable “fungal infection” and prescribed a combination clotrimazole/betamethasone dipropionate cream. The rash subsequently improved, though not substantially.
The patient recalls developing a similar rash several other times during adulthood, but says it was never this severe. His mother has had similar eruptions, as well as sensitive skin in general. Both the patient and his mother are plagued by seasonal allergies, asthma, and sweating of the palms.
EXAMINATION
The distal portions of the second through fourth fingers of the right hand are affected. This is typical, according to the patient. The cuticles of all four affected nails are detached from the nail plates, with two of the three nail plates showing mild transverse ridging.
There is circumferential involvement of the distal half of all four fingers, with a well-defined margin and a blistery look to the papulosquamous process. Neither the left hand nor the feet are impacted.
DISCUSSION
This is a classic picture of a condition that goes by several names, most commonly pompholyx, also known as dishidrotic eczema. Despite the efforts of many investigators, with much information gleaned, it remains quite mysterious. It can be chronic or acute and can closely resemble what is known simply as hand dermatitis.
Pompholyx is associated with atopy in at least 50% of cases (such as this one). Hyperhidrosis of the palms is also common, and resolution of difficult cases has been achieved with injection of botulinum toxin A. This hypothesis is bolstered by the fact that many patients report onset in summer months; however, there are many whose history fails to follow this pattern.
Research has revealed that more than a few patients with pompholyx are allergic to one of several ingested metals, such as nickel and cobalt. But challenge tests with those substances have failed to consistently replicate the eruption.
Stress is another reported factor in the genesis of this condition. It is well known to exacerbate related conditions (eg, atopic dermatitis), but again, many patients deny any such connection.
When confronted with this clinical picture, dermatology providers are trained to look at the patient’s feet, where a flare of tinea pedis can sometimes be found. This common foot infection can, under certain circumstances, trigger a clinically indistinguishable pompholyx-like eruption on the hands, called an id reaction. In these cases, the tinea pedis always precedes the hand rash. Both resolve with adequate treatment (ie, oral antifungals).
The differential also includes irritant or contact dermatitis. However, patients are likely to report a contributing factor early on, lessening the clinical mystery.
A major diagnostic clue in this and similar cases is the effect on the cuticles and nails. Both are good indicators of the chronicity and nature of the problem.
TREATMENT
Treatment of pompholyx is notoriously difficult. Potent topical corticosteroids (eg, clobetasol cream), applied under occlusion to dampened skin at bedtime, are the cornerstone. In this and many such cases, while not curative by themselves, oral antibiotics can help to reduce colonization by staphylococcus.
A two-week course of prednisone (eg, 20 mg bid for a week, then 20 mg/d for another week) can be extremely helpful. Alternatively, an intramuscular injection of triamcinolone (40 to 60 mg) can be used, especially if the patient has a history of reflux or peptic ulcer. Relative contraindications to systemic steroid use include diabetes, poorly controlled hypertension, congestive heart failure, and dementia.
Phototherapy has also been used, but botulinum injection is becoming common in cases in which hyperhidrosis is the major culprit.
TAKE-HOME LEARNING POINTS
• Pompholyx manifests as a papulosquamous, well-defined rash, often composed of tiny fluid-filled blisters, on the fingers and hand.
• The cause of this condition is unknown, but potential triggers include atopy, stress, hyperhidrosis, and ingestion of metals (eg, nickel or cobalt).
• Since acute tinea pedis can trigger a similar eruption on the hands (and responds to oral antifungals, as does pompholyx), checking the patient’s feet can provide a diagnostic clue.
• Patients may find it helpful to apply a potent topical corticosteroid cream (eg, clobetasol) to dampened hands (which allows for increased penetration of the medication) and cover with cotton gloves overnight.
HISTORY
Rashes of the hand are commonly seen in both primary care and dermatology practices. Their location makes them problematic, in terms of interference with normal activities and difficulty with treatment.
This 51-year-old man’s rash appeared about a month ago, with no premonitory signs. He has consulted numerous providers about it, including his primary care clinician. That practitioner diagnosed a probable “fungal infection” and prescribed a combination clotrimazole/betamethasone dipropionate cream. The rash subsequently improved, though not substantially.
The patient recalls developing a similar rash several other times during adulthood, but says it was never this severe. His mother has had similar eruptions, as well as sensitive skin in general. Both the patient and his mother are plagued by seasonal allergies, asthma, and sweating of the palms.
EXAMINATION
The distal portions of the second through fourth fingers of the right hand are affected. This is typical, according to the patient. The cuticles of all four affected nails are detached from the nail plates, with two of the three nail plates showing mild transverse ridging.
There is circumferential involvement of the distal half of all four fingers, with a well-defined margin and a blistery look to the papulosquamous process. Neither the left hand nor the feet are impacted.
DISCUSSION
This is a classic picture of a condition that goes by several names, most commonly pompholyx, also known as dishidrotic eczema. Despite the efforts of many investigators, with much information gleaned, it remains quite mysterious. It can be chronic or acute and can closely resemble what is known simply as hand dermatitis.
Pompholyx is associated with atopy in at least 50% of cases (such as this one). Hyperhidrosis of the palms is also common, and resolution of difficult cases has been achieved with injection of botulinum toxin A. This hypothesis is bolstered by the fact that many patients report onset in summer months; however, there are many whose history fails to follow this pattern.
Research has revealed that more than a few patients with pompholyx are allergic to one of several ingested metals, such as nickel and cobalt. But challenge tests with those substances have failed to consistently replicate the eruption.
Stress is another reported factor in the genesis of this condition. It is well known to exacerbate related conditions (eg, atopic dermatitis), but again, many patients deny any such connection.
When confronted with this clinical picture, dermatology providers are trained to look at the patient’s feet, where a flare of tinea pedis can sometimes be found. This common foot infection can, under certain circumstances, trigger a clinically indistinguishable pompholyx-like eruption on the hands, called an id reaction. In these cases, the tinea pedis always precedes the hand rash. Both resolve with adequate treatment (ie, oral antifungals).
The differential also includes irritant or contact dermatitis. However, patients are likely to report a contributing factor early on, lessening the clinical mystery.
A major diagnostic clue in this and similar cases is the effect on the cuticles and nails. Both are good indicators of the chronicity and nature of the problem.
TREATMENT
Treatment of pompholyx is notoriously difficult. Potent topical corticosteroids (eg, clobetasol cream), applied under occlusion to dampened skin at bedtime, are the cornerstone. In this and many such cases, while not curative by themselves, oral antibiotics can help to reduce colonization by staphylococcus.
A two-week course of prednisone (eg, 20 mg bid for a week, then 20 mg/d for another week) can be extremely helpful. Alternatively, an intramuscular injection of triamcinolone (40 to 60 mg) can be used, especially if the patient has a history of reflux or peptic ulcer. Relative contraindications to systemic steroid use include diabetes, poorly controlled hypertension, congestive heart failure, and dementia.
Phototherapy has also been used, but botulinum injection is becoming common in cases in which hyperhidrosis is the major culprit.
TAKE-HOME LEARNING POINTS
• Pompholyx manifests as a papulosquamous, well-defined rash, often composed of tiny fluid-filled blisters, on the fingers and hand.
• The cause of this condition is unknown, but potential triggers include atopy, stress, hyperhidrosis, and ingestion of metals (eg, nickel or cobalt).
• Since acute tinea pedis can trigger a similar eruption on the hands (and responds to oral antifungals, as does pompholyx), checking the patient’s feet can provide a diagnostic clue.
• Patients may find it helpful to apply a potent topical corticosteroid cream (eg, clobetasol) to dampened hands (which allows for increased penetration of the medication) and cover with cotton gloves overnight.
After 28 years, a diagnosis is sought
HISTORY
This 40-year-old man was urged by his wife to consult dermatology about a lesion he first noted on his left flank when he was 12. Since then, it has grown darker and more hairy but otherwise has not changed. It has never been symptomatic, and he has no other lesions. The patient claims to be in good health.
The lesion, which covers a good portion of the patient’s left lateral chest wall, is uniformly light brown. The surface is slightly rough and decidedly hypertrichotic, with markedly irregular margins. No other significant lesions are seen elsewhere on the patient’s skin.
DISCUSSION
The shoulder and pectoral areas are far more common locations than the chest wall for Becker’s nevus. S. William Becker first described this curious condition in 1948, in two young men who had acquired melanosis and hypertrichosis in unilateral distribution. Originally called Becker’s melanosis, it has long been known by its present name and has now been extensively investigated.
Though the exact pathogenesis of Becker’s nevus remains uncertain, much has been learned. For example, it appears that androgens play a role in its acquisition. Supporting evidence includes the history of peripubertal development, male preponderance, presence of hypertrichosis, and even the occasional development of acneiform eruptions within the patch. Special studies have also reported a significant increase in the number of androgen receptors in these patches.
The typical natural history of this lesion is as follows: First, smaller, widely scattered tan-to-orange macules appear, increase in number, and then coalesce to form a larger patch. Over the next few months to years, darker hairs begin to appear within the lesion, as well as on its periphery (though a small percentage of BN lesions never develop hair), while the skin in the middle of the lesion may become slightly thicker. The configuration of the lesion’s borders is said to be geographic (ie, indented and irregular).
Histologically, Becker’s is distinguished by mild acanthosis and hyperkeratosis, with regular elongation of rete ridges. There is also hyperpigmentation of the epidermis, an increase in smooth muscle, and increased numbers of hair follicles.
The main item in the differential is McCune-Albright syndrome, a rare genetic disorder involving defects of bones, skin pigmentation, and hormonal abnormalities such as premature puberty. Highly variable in its presentation, it often presents with unilateral café-au-lait macules at birth, but these have no hypertrichosis.
Far more men than women develop Becker’s nevus. Rarely, unilateral breast hypoplasia has been reported.
TREATMENT
Primarily sought for cosmetic reasons, treatment entails the use of lasers, with variable results. Elimination of hairs is relatively easy to achieve, but resolution of the hyperpigmentation is less predictable.
This particular patient was satisfied just to know the correct diagnosis and benign prognosis, though he was instructed to watch the lesion for signs of significant change.
TAKE-HOME LEARNING POINTS
• Becker’s nevus (BN) is usually found on the shoulder, but can develop anywhere on the trunk and is occasionally seen on the extremities.
• The chances of malignant transformation within a BN are extremely low.
• There is abundant evidence of the influence of androgens on the development of BNs, including hypertrichosis, prevalence in males, and peripubertal onset.
• Ipsilateral hypoplasia of the breast has been reported in conjunction with BN.
• Multiple-laser treatment of BN can lighten the hyperpigmentation but may result in unacceptable loss of normal pigment in treated areas.
HISTORY
This 40-year-old man was urged by his wife to consult dermatology about a lesion he first noted on his left flank when he was 12. Since then, it has grown darker and more hairy but otherwise has not changed. It has never been symptomatic, and he has no other lesions. The patient claims to be in good health.
The lesion, which covers a good portion of the patient’s left lateral chest wall, is uniformly light brown. The surface is slightly rough and decidedly hypertrichotic, with markedly irregular margins. No other significant lesions are seen elsewhere on the patient’s skin.
DISCUSSION
The shoulder and pectoral areas are far more common locations than the chest wall for Becker’s nevus. S. William Becker first described this curious condition in 1948, in two young men who had acquired melanosis and hypertrichosis in unilateral distribution. Originally called Becker’s melanosis, it has long been known by its present name and has now been extensively investigated.
Though the exact pathogenesis of Becker’s nevus remains uncertain, much has been learned. For example, it appears that androgens play a role in its acquisition. Supporting evidence includes the history of peripubertal development, male preponderance, presence of hypertrichosis, and even the occasional development of acneiform eruptions within the patch. Special studies have also reported a significant increase in the number of androgen receptors in these patches.
The typical natural history of this lesion is as follows: First, smaller, widely scattered tan-to-orange macules appear, increase in number, and then coalesce to form a larger patch. Over the next few months to years, darker hairs begin to appear within the lesion, as well as on its periphery (though a small percentage of BN lesions never develop hair), while the skin in the middle of the lesion may become slightly thicker. The configuration of the lesion’s borders is said to be geographic (ie, indented and irregular).
Histologically, Becker’s is distinguished by mild acanthosis and hyperkeratosis, with regular elongation of rete ridges. There is also hyperpigmentation of the epidermis, an increase in smooth muscle, and increased numbers of hair follicles.
The main item in the differential is McCune-Albright syndrome, a rare genetic disorder involving defects of bones, skin pigmentation, and hormonal abnormalities such as premature puberty. Highly variable in its presentation, it often presents with unilateral café-au-lait macules at birth, but these have no hypertrichosis.
Far more men than women develop Becker’s nevus. Rarely, unilateral breast hypoplasia has been reported.
TREATMENT
Primarily sought for cosmetic reasons, treatment entails the use of lasers, with variable results. Elimination of hairs is relatively easy to achieve, but resolution of the hyperpigmentation is less predictable.
This particular patient was satisfied just to know the correct diagnosis and benign prognosis, though he was instructed to watch the lesion for signs of significant change.
TAKE-HOME LEARNING POINTS
• Becker’s nevus (BN) is usually found on the shoulder, but can develop anywhere on the trunk and is occasionally seen on the extremities.
• The chances of malignant transformation within a BN are extremely low.
• There is abundant evidence of the influence of androgens on the development of BNs, including hypertrichosis, prevalence in males, and peripubertal onset.
• Ipsilateral hypoplasia of the breast has been reported in conjunction with BN.
• Multiple-laser treatment of BN can lighten the hyperpigmentation but may result in unacceptable loss of normal pigment in treated areas.
HISTORY
This 40-year-old man was urged by his wife to consult dermatology about a lesion he first noted on his left flank when he was 12. Since then, it has grown darker and more hairy but otherwise has not changed. It has never been symptomatic, and he has no other lesions. The patient claims to be in good health.
The lesion, which covers a good portion of the patient’s left lateral chest wall, is uniformly light brown. The surface is slightly rough and decidedly hypertrichotic, with markedly irregular margins. No other significant lesions are seen elsewhere on the patient’s skin.
DISCUSSION
The shoulder and pectoral areas are far more common locations than the chest wall for Becker’s nevus. S. William Becker first described this curious condition in 1948, in two young men who had acquired melanosis and hypertrichosis in unilateral distribution. Originally called Becker’s melanosis, it has long been known by its present name and has now been extensively investigated.
Though the exact pathogenesis of Becker’s nevus remains uncertain, much has been learned. For example, it appears that androgens play a role in its acquisition. Supporting evidence includes the history of peripubertal development, male preponderance, presence of hypertrichosis, and even the occasional development of acneiform eruptions within the patch. Special studies have also reported a significant increase in the number of androgen receptors in these patches.
The typical natural history of this lesion is as follows: First, smaller, widely scattered tan-to-orange macules appear, increase in number, and then coalesce to form a larger patch. Over the next few months to years, darker hairs begin to appear within the lesion, as well as on its periphery (though a small percentage of BN lesions never develop hair), while the skin in the middle of the lesion may become slightly thicker. The configuration of the lesion’s borders is said to be geographic (ie, indented and irregular).
Histologically, Becker’s is distinguished by mild acanthosis and hyperkeratosis, with regular elongation of rete ridges. There is also hyperpigmentation of the epidermis, an increase in smooth muscle, and increased numbers of hair follicles.
The main item in the differential is McCune-Albright syndrome, a rare genetic disorder involving defects of bones, skin pigmentation, and hormonal abnormalities such as premature puberty. Highly variable in its presentation, it often presents with unilateral café-au-lait macules at birth, but these have no hypertrichosis.
Far more men than women develop Becker’s nevus. Rarely, unilateral breast hypoplasia has been reported.
TREATMENT
Primarily sought for cosmetic reasons, treatment entails the use of lasers, with variable results. Elimination of hairs is relatively easy to achieve, but resolution of the hyperpigmentation is less predictable.
This particular patient was satisfied just to know the correct diagnosis and benign prognosis, though he was instructed to watch the lesion for signs of significant change.
TAKE-HOME LEARNING POINTS
• Becker’s nevus (BN) is usually found on the shoulder, but can develop anywhere on the trunk and is occasionally seen on the extremities.
• The chances of malignant transformation within a BN are extremely low.
• There is abundant evidence of the influence of androgens on the development of BNs, including hypertrichosis, prevalence in males, and peripubertal onset.
• Ipsilateral hypoplasia of the breast has been reported in conjunction with BN.
• Multiple-laser treatment of BN can lighten the hyperpigmentation but may result in unacceptable loss of normal pigment in treated areas.
Poor prognosis emphasizes need for prevention
A 73-year-old man self-refers to dermatology 18 months after a melanoma was diagnosed and removed from his forearm. Following that discovery, he was referred to a surgeon, who performed a wide excision (the defect from which was closed with a graft) and who went on to do lymph node dissection in the ipsilateral axilla. No positive nodes were found.
The wounds from these procedures are long since healed, and the patient has been doing well. That is, until recently, when he noticed some new lesions developing around the graft site.
EXAMINATION
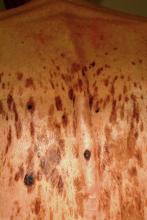
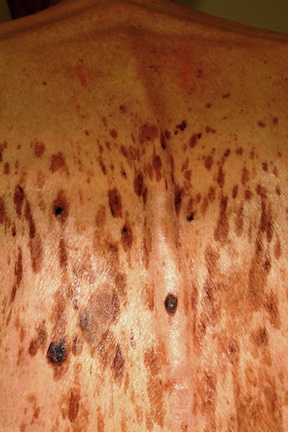
About 15 to 20 firm, blue-black papules and nodules surround the periphery of the graft site on the patient’s forearm. Some extend out as far as 10 cm, though most are within 3 cm. Obviously intradermal, these lesions display no surface change at all. Punch biopsy confirms the suspicion that these represent satellite metastasis of the patient’s original melanoma, which itself had been more than 3 mm thick.
Fortunately, no nodes are palpable in the axilla, and no evidence of metastasis is found on physical examination, blood work, and PET scan.
DISCUSSION
The image accompanying this case is pregnant with information—some obvious, some less so. For example, the multiple blue-black nodules can easily be seen surrounding the graft site and were just as easily palpated.
Even ignoring those lesions momentarily, a look at the surrounding skin offers a veritable textbook of germane information. The collective term for the skin changes on the patient’s arms is dermatoheliosis, or sun-damaged skin. But that term comprises a number of specific changes, all of which have names and significance.
The casual observer might simply chalk these changes up to age, but for medical providers, more specifics are in order: The sun has thinned the patient’s skin remarkably, hence the term solar atrophy. His dorsal forearms are greatly discolored as well, changes we call poikiloderma. Numerous telangiectasias (also sun-caused) can be seen on his dorsal forearms. These changes are especially appreciated when the dorsal forearm skin is compared to the extensor forearms, which receive relatively little sun exposure.
The point? This patient had every reason to develop a melanoma, making any odd lesion on his skin suspicious. It also means his chances of developing a new primary melanoma are all too real, even if he survives the current one.
As one might imagine, this local recurrence of his melanoma is not a good sign at all. Strictly speaking, it is a form of metastasis—but until it reaches lymph nodes or organs, it only suggests that possibility.
Treatment choices are limited for his melanoma, but include limb perfusion, chemotherapy, and surgery. The truth is, his prognosis is poor. His case emphasizes the need for prevention and early diagnosis, the latter greatly aided by the recognition of patients at risk by virtue of having fair, sun-damaged skin.
As often happens in cases like this, there is a ripple effect as the news of his situation reaches family and friends, whose own skin becomes the subject of attention. In such cases, it’s not unusual for the whole family to then be seen in dermatology over the succeeding months—not only to be examined, but also hopefully educated in terms of prevention and recognition.
TAKE-HOME LEARNING POINTS
• Local recurrence of melanoma is common, especially with primary tumors that exceed 3 mm in thickness.
• UV overexposure has been established as the major contributor to development of melanoma.
• Melanoma is far more common in fair-skinned individuals than in those with darker skin; “fair” is defined as tolerating sun poorly, burning easily, and tanning poorly, if at all.
• Evidence of this excessive sun damage is called dermatoheliosis and consists of specific findings including solar atrophy, telangiectasias, and pigmentary alteration known as poikiloderma.
• The lack of effective treatment for metastatic melanoma underlines the necessity for prevention (protection from the sun) and early detection.
A 73-year-old man self-refers to dermatology 18 months after a melanoma was diagnosed and removed from his forearm. Following that discovery, he was referred to a surgeon, who performed a wide excision (the defect from which was closed with a graft) and who went on to do lymph node dissection in the ipsilateral axilla. No positive nodes were found.
The wounds from these procedures are long since healed, and the patient has been doing well. That is, until recently, when he noticed some new lesions developing around the graft site.
EXAMINATION
About 15 to 20 firm, blue-black papules and nodules surround the periphery of the graft site on the patient’s forearm. Some extend out as far as 10 cm, though most are within 3 cm. Obviously intradermal, these lesions display no surface change at all. Punch biopsy confirms the suspicion that these represent satellite metastasis of the patient’s original melanoma, which itself had been more than 3 mm thick.
Fortunately, no nodes are palpable in the axilla, and no evidence of metastasis is found on physical examination, blood work, and PET scan.
DISCUSSION
The image accompanying this case is pregnant with information—some obvious, some less so. For example, the multiple blue-black nodules can easily be seen surrounding the graft site and were just as easily palpated.
Even ignoring those lesions momentarily, a look at the surrounding skin offers a veritable textbook of germane information. The collective term for the skin changes on the patient’s arms is dermatoheliosis, or sun-damaged skin. But that term comprises a number of specific changes, all of which have names and significance.
The casual observer might simply chalk these changes up to age, but for medical providers, more specifics are in order: The sun has thinned the patient’s skin remarkably, hence the term solar atrophy. His dorsal forearms are greatly discolored as well, changes we call poikiloderma. Numerous telangiectasias (also sun-caused) can be seen on his dorsal forearms. These changes are especially appreciated when the dorsal forearm skin is compared to the extensor forearms, which receive relatively little sun exposure.
The point? This patient had every reason to develop a melanoma, making any odd lesion on his skin suspicious. It also means his chances of developing a new primary melanoma are all too real, even if he survives the current one.
As one might imagine, this local recurrence of his melanoma is not a good sign at all. Strictly speaking, it is a form of metastasis—but until it reaches lymph nodes or organs, it only suggests that possibility.
Treatment choices are limited for his melanoma, but include limb perfusion, chemotherapy, and surgery. The truth is, his prognosis is poor. His case emphasizes the need for prevention and early diagnosis, the latter greatly aided by the recognition of patients at risk by virtue of having fair, sun-damaged skin.
As often happens in cases like this, there is a ripple effect as the news of his situation reaches family and friends, whose own skin becomes the subject of attention. In such cases, it’s not unusual for the whole family to then be seen in dermatology over the succeeding months—not only to be examined, but also hopefully educated in terms of prevention and recognition.
TAKE-HOME LEARNING POINTS
• Local recurrence of melanoma is common, especially with primary tumors that exceed 3 mm in thickness.
• UV overexposure has been established as the major contributor to development of melanoma.
• Melanoma is far more common in fair-skinned individuals than in those with darker skin; “fair” is defined as tolerating sun poorly, burning easily, and tanning poorly, if at all.
• Evidence of this excessive sun damage is called dermatoheliosis and consists of specific findings including solar atrophy, telangiectasias, and pigmentary alteration known as poikiloderma.
• The lack of effective treatment for metastatic melanoma underlines the necessity for prevention (protection from the sun) and early detection.
A 73-year-old man self-refers to dermatology 18 months after a melanoma was diagnosed and removed from his forearm. Following that discovery, he was referred to a surgeon, who performed a wide excision (the defect from which was closed with a graft) and who went on to do lymph node dissection in the ipsilateral axilla. No positive nodes were found.
The wounds from these procedures are long since healed, and the patient has been doing well. That is, until recently, when he noticed some new lesions developing around the graft site.
EXAMINATION
About 15 to 20 firm, blue-black papules and nodules surround the periphery of the graft site on the patient’s forearm. Some extend out as far as 10 cm, though most are within 3 cm. Obviously intradermal, these lesions display no surface change at all. Punch biopsy confirms the suspicion that these represent satellite metastasis of the patient’s original melanoma, which itself had been more than 3 mm thick.
Fortunately, no nodes are palpable in the axilla, and no evidence of metastasis is found on physical examination, blood work, and PET scan.
DISCUSSION
The image accompanying this case is pregnant with information—some obvious, some less so. For example, the multiple blue-black nodules can easily be seen surrounding the graft site and were just as easily palpated.
Even ignoring those lesions momentarily, a look at the surrounding skin offers a veritable textbook of germane information. The collective term for the skin changes on the patient’s arms is dermatoheliosis, or sun-damaged skin. But that term comprises a number of specific changes, all of which have names and significance.
The casual observer might simply chalk these changes up to age, but for medical providers, more specifics are in order: The sun has thinned the patient’s skin remarkably, hence the term solar atrophy. His dorsal forearms are greatly discolored as well, changes we call poikiloderma. Numerous telangiectasias (also sun-caused) can be seen on his dorsal forearms. These changes are especially appreciated when the dorsal forearm skin is compared to the extensor forearms, which receive relatively little sun exposure.
The point? This patient had every reason to develop a melanoma, making any odd lesion on his skin suspicious. It also means his chances of developing a new primary melanoma are all too real, even if he survives the current one.
As one might imagine, this local recurrence of his melanoma is not a good sign at all. Strictly speaking, it is a form of metastasis—but until it reaches lymph nodes or organs, it only suggests that possibility.
Treatment choices are limited for his melanoma, but include limb perfusion, chemotherapy, and surgery. The truth is, his prognosis is poor. His case emphasizes the need for prevention and early diagnosis, the latter greatly aided by the recognition of patients at risk by virtue of having fair, sun-damaged skin.
As often happens in cases like this, there is a ripple effect as the news of his situation reaches family and friends, whose own skin becomes the subject of attention. In such cases, it’s not unusual for the whole family to then be seen in dermatology over the succeeding months—not only to be examined, but also hopefully educated in terms of prevention and recognition.
TAKE-HOME LEARNING POINTS
• Local recurrence of melanoma is common, especially with primary tumors that exceed 3 mm in thickness.
• UV overexposure has been established as the major contributor to development of melanoma.
• Melanoma is far more common in fair-skinned individuals than in those with darker skin; “fair” is defined as tolerating sun poorly, burning easily, and tanning poorly, if at all.
• Evidence of this excessive sun damage is called dermatoheliosis and consists of specific findings including solar atrophy, telangiectasias, and pigmentary alteration known as poikiloderma.
• The lack of effective treatment for metastatic melanoma underlines the necessity for prevention (protection from the sun) and early detection.
Elderly woman baffled by signs of "aging"
HISTORY
A 91-year-old woman is mortified when a friend comments on the “age spots” on the skin of her neck.
“In the first place,” the patient retorts while recounting the story, “I’m not that old. And in the second place, I don’t see anything there—what’s she talking about?”
She is truly upset about what she feels were uncalled-for comments. But more than that, she has no idea what her friend could be referring to. No one else has ever said anything negative about her skin—in fact, everyone who meets her marvels at how young she looks for her age.
On examination, her skin is quite fair and shows extensive signs of sun damage. There is extreme widespread mottling, in colors ranging from yellow to orange, and exceptionally pronounced wrinkling on sun-exposed areas of her face, neck, arms, and chest. Notably, the area of the anterior neck shaded by her chin is pristine and white. Fortunately, no cancerous or other worrisome lesions are seen.
DIAGNOSIS/DISCUSSION
This case illustrates a number of related phenomena. For example, it was shocking that this patient–one of most sun-damaged I’ve ever seen–was unaware of such obvious changes. But these changes had been present for so long and manifested so gradually that they escaped her notice. (Not to mention, the eyesight of a 91-year-old is probably not what it once was.)
Furthermore, when informed that her skin’s condition was a result of sun exposure, she was sure we had lost our minds, because she had not been in the sun “at all” for many, many years. According to her daughter, this was true. But the patient had overlooked the fact that she had grown up on a farm, worked in the fields, played outside, swam and fished, all the while getting a great deal of sun exposure, until she married in her late teens, had children, and moved to town. Being so busy and so fair, she had neither the time nor the inclination to get outdoors much, and that was that—or so she thought. This is a very common set of circumstances for dermatology patients.
Little did she realize that it takes decades (30 to 40 years) for the accumulated effects of sun damage to show up, in the form we see here. This type of “aging” is an example of what we call extrinsic aging. Besides sun, it can be worsened by the effects of wind, low humidity, smoking, alcohol intake, obesity, and some medical conditions. Intrinsic aging, which includes wrinkles, sagging, and general loss of elasticity, is influenced by age, heredity, and ultimately, gravity.
From our standpoint as medical providers, perhaps the most significant issue with this patient is her increased risk for sun-caused skin cancer, specifically basal cell carcinoma (BCC), squamous cell carcinoma (SCC), or melanoma (the three most common forms). Her risk for development of the first two is huge, arguably a certainty given her age, extremely fair skin, and degree of sun damage. Melanomas are different in many ways, actually becoming less likely (statistically) at her age than at age 50. They are also not as much the result of the accumulated effects of sun exposure as are BCC/SCCs.
So, perhaps the most significant outcome of this visit was to get the patient scheduled to see us biannually for skin checks. In our system, that means twice-yearly reminder phone calls to ensure that this actually happens.
TAKE-HOME LEARNING POINTS
• It takes three to five decades for sun damage to eventuate in dermatoheliosis/basal cell carcinoma/squamous cell carcinoma.
• Melanoma is different, since it appears to be related to episodic, poorly tolerated, intense sun damage early in life; the average age of melanoma patients is about 40.
• Aging can be intrinsic (“normal” loss of elasticity, plus the effects of gravity, modified by heredity) in nature.
• Aging can also be extrinsic, caused by UV exposure, smoking, alcohol consumption, wind, and decreased humidity, eventuating in telangiectasias, actinic keratoses, solar elastosis, solar lentigines, atrophy, purpura, poikilodermatous changes, and sun-caused skin cancers.
• Older patients are often incredulous about the role of childhood sun exposure in the eventual development of dermatoheliosis.
HISTORY
A 91-year-old woman is mortified when a friend comments on the “age spots” on the skin of her neck.
“In the first place,” the patient retorts while recounting the story, “I’m not that old. And in the second place, I don’t see anything there—what’s she talking about?”
She is truly upset about what she feels were uncalled-for comments. But more than that, she has no idea what her friend could be referring to. No one else has ever said anything negative about her skin—in fact, everyone who meets her marvels at how young she looks for her age.
On examination, her skin is quite fair and shows extensive signs of sun damage. There is extreme widespread mottling, in colors ranging from yellow to orange, and exceptionally pronounced wrinkling on sun-exposed areas of her face, neck, arms, and chest. Notably, the area of the anterior neck shaded by her chin is pristine and white. Fortunately, no cancerous or other worrisome lesions are seen.
DIAGNOSIS/DISCUSSION
This case illustrates a number of related phenomena. For example, it was shocking that this patient–one of most sun-damaged I’ve ever seen–was unaware of such obvious changes. But these changes had been present for so long and manifested so gradually that they escaped her notice. (Not to mention, the eyesight of a 91-year-old is probably not what it once was.)
Furthermore, when informed that her skin’s condition was a result of sun exposure, she was sure we had lost our minds, because she had not been in the sun “at all” for many, many years. According to her daughter, this was true. But the patient had overlooked the fact that she had grown up on a farm, worked in the fields, played outside, swam and fished, all the while getting a great deal of sun exposure, until she married in her late teens, had children, and moved to town. Being so busy and so fair, she had neither the time nor the inclination to get outdoors much, and that was that—or so she thought. This is a very common set of circumstances for dermatology patients.
Little did she realize that it takes decades (30 to 40 years) for the accumulated effects of sun damage to show up, in the form we see here. This type of “aging” is an example of what we call extrinsic aging. Besides sun, it can be worsened by the effects of wind, low humidity, smoking, alcohol intake, obesity, and some medical conditions. Intrinsic aging, which includes wrinkles, sagging, and general loss of elasticity, is influenced by age, heredity, and ultimately, gravity.
From our standpoint as medical providers, perhaps the most significant issue with this patient is her increased risk for sun-caused skin cancer, specifically basal cell carcinoma (BCC), squamous cell carcinoma (SCC), or melanoma (the three most common forms). Her risk for development of the first two is huge, arguably a certainty given her age, extremely fair skin, and degree of sun damage. Melanomas are different in many ways, actually becoming less likely (statistically) at her age than at age 50. They are also not as much the result of the accumulated effects of sun exposure as are BCC/SCCs.
So, perhaps the most significant outcome of this visit was to get the patient scheduled to see us biannually for skin checks. In our system, that means twice-yearly reminder phone calls to ensure that this actually happens.
TAKE-HOME LEARNING POINTS
• It takes three to five decades for sun damage to eventuate in dermatoheliosis/basal cell carcinoma/squamous cell carcinoma.
• Melanoma is different, since it appears to be related to episodic, poorly tolerated, intense sun damage early in life; the average age of melanoma patients is about 40.
• Aging can be intrinsic (“normal” loss of elasticity, plus the effects of gravity, modified by heredity) in nature.
• Aging can also be extrinsic, caused by UV exposure, smoking, alcohol consumption, wind, and decreased humidity, eventuating in telangiectasias, actinic keratoses, solar elastosis, solar lentigines, atrophy, purpura, poikilodermatous changes, and sun-caused skin cancers.
• Older patients are often incredulous about the role of childhood sun exposure in the eventual development of dermatoheliosis.
HISTORY
A 91-year-old woman is mortified when a friend comments on the “age spots” on the skin of her neck.
“In the first place,” the patient retorts while recounting the story, “I’m not that old. And in the second place, I don’t see anything there—what’s she talking about?”
She is truly upset about what she feels were uncalled-for comments. But more than that, she has no idea what her friend could be referring to. No one else has ever said anything negative about her skin—in fact, everyone who meets her marvels at how young she looks for her age.
On examination, her skin is quite fair and shows extensive signs of sun damage. There is extreme widespread mottling, in colors ranging from yellow to orange, and exceptionally pronounced wrinkling on sun-exposed areas of her face, neck, arms, and chest. Notably, the area of the anterior neck shaded by her chin is pristine and white. Fortunately, no cancerous or other worrisome lesions are seen.
DIAGNOSIS/DISCUSSION
This case illustrates a number of related phenomena. For example, it was shocking that this patient–one of most sun-damaged I’ve ever seen–was unaware of such obvious changes. But these changes had been present for so long and manifested so gradually that they escaped her notice. (Not to mention, the eyesight of a 91-year-old is probably not what it once was.)
Furthermore, when informed that her skin’s condition was a result of sun exposure, she was sure we had lost our minds, because she had not been in the sun “at all” for many, many years. According to her daughter, this was true. But the patient had overlooked the fact that she had grown up on a farm, worked in the fields, played outside, swam and fished, all the while getting a great deal of sun exposure, until she married in her late teens, had children, and moved to town. Being so busy and so fair, she had neither the time nor the inclination to get outdoors much, and that was that—or so she thought. This is a very common set of circumstances for dermatology patients.
Little did she realize that it takes decades (30 to 40 years) for the accumulated effects of sun damage to show up, in the form we see here. This type of “aging” is an example of what we call extrinsic aging. Besides sun, it can be worsened by the effects of wind, low humidity, smoking, alcohol intake, obesity, and some medical conditions. Intrinsic aging, which includes wrinkles, sagging, and general loss of elasticity, is influenced by age, heredity, and ultimately, gravity.
From our standpoint as medical providers, perhaps the most significant issue with this patient is her increased risk for sun-caused skin cancer, specifically basal cell carcinoma (BCC), squamous cell carcinoma (SCC), or melanoma (the three most common forms). Her risk for development of the first two is huge, arguably a certainty given her age, extremely fair skin, and degree of sun damage. Melanomas are different in many ways, actually becoming less likely (statistically) at her age than at age 50. They are also not as much the result of the accumulated effects of sun exposure as are BCC/SCCs.
So, perhaps the most significant outcome of this visit was to get the patient scheduled to see us biannually for skin checks. In our system, that means twice-yearly reminder phone calls to ensure that this actually happens.
TAKE-HOME LEARNING POINTS
• It takes three to five decades for sun damage to eventuate in dermatoheliosis/basal cell carcinoma/squamous cell carcinoma.
• Melanoma is different, since it appears to be related to episodic, poorly tolerated, intense sun damage early in life; the average age of melanoma patients is about 40.
• Aging can be intrinsic (“normal” loss of elasticity, plus the effects of gravity, modified by heredity) in nature.
• Aging can also be extrinsic, caused by UV exposure, smoking, alcohol consumption, wind, and decreased humidity, eventuating in telangiectasias, actinic keratoses, solar elastosis, solar lentigines, atrophy, purpura, poikilodermatous changes, and sun-caused skin cancers.
• Older patients are often incredulous about the role of childhood sun exposure in the eventual development of dermatoheliosis.
A dermatologic problem is "all in the family"
HISTORY
A 48-year-old woman presents to dermatology for evaluation of “ringworm” that has been unresponsive to antifungal creams and a subsequent course of terbinafine (250 mg/d for 10 days).
The problem started several weeks ago, when she experienced mild itching in the upper intergluteal area. Her husband examined the area and noted several patches of a round, scaly rash that they assumed must be ringworm. The patient tried applying OTC clotrimazole cream twice a day, with no good effect.
She next visited a local urgent care center, where the provider agreed with the self-diagnosis. Nystatin cream was prescribed for twice-daily use, but this treatment was also unsuccessful.
Next up was the patient’s primary care provider, who reiterated the diagnosis of ringworm and prescribed the aforementioned course of terbinafine. When it too failed, the patient, still worried, requested a referral to dermatology.
During the history taking, it is revealed that the patient has hypertension, is 50 lb overweight, and has been smoking for more than 40 years. A few weeks prior to the appearance of the rash, she lost her job when the company she had been employed by for more than 20 years closed. At that point, her consumption of alcohol began to increase markedly.
Questions about family history of skin disease reveal several first- and second-degree relatives with psoriasis. In addition, the patient purposely brought her 17-year-old son with her, in order to show his “ringworm,” which she is convinced he passed on to her.
The son’s rash (pictured) has been present for several years, always confined to his axillae and only slightly itchy. It is composed of almost perfectly round pinkish brown patches covering the roofs of both axillae. It bears a striking resemblance to his mother’s intergluteal rash, including the color, and displays annular margins as well.
Additional questioning confirms the fact of his long-standing dandruff and the presence of a chronic scaly rash in and around the ears. At least two of his siblings have similar problems. On hearing this, the patient admits she has suffered with dandruff for years as well.
DISCUSSION
In all likelihood, both mother and son have a papulosquamous condition called seborrheic dermatitis (SD). SD most commonly manifests as dandruff, but it can present in a number of other, less well-known forms.
Up to 20% of the population at large have one or more types of SD, which tends to favor fair-skinned individuals of Northern European ancestry. It appears to be related to a commensal yeast organism, Malassezia furfur, which is markedly lipophilic and needs copious sebum (ubiquitously present on all humans) to flourish. The needed quantity of sebum rarely develops before puberty, due to hormonal influence. As these organisms feed on sebum, the resulting metabolic byproducts trigger an inflammatory response in the susceptible individual.
The resulting characteristic papulosquamous rash can manifest behind the ears, in the external auditory meati, in the glabellar area of the mid-forehead, into the brows, focally in the beard, and in the nasolabial folds. Less commonly, SD appears on the mid-sternum, in the periumbilical area, on genitals, in the intergluteal area, and in the axillae (as in the case of the patient’s son).
Connecting all these dots is an impossible task for most patients, whose only explanation for round and scaly is “ringworm” (tinea corporis). Ringworm, however, requires a source—such as a new cat, guinea pig, dog, livestock, or child with active disease, none of which were present in this case. Little did the patient know that her son had “caught” SD from her!
What we did have in this case was a sharp increase in the patient’s stress level, as well as an increase in her alcohol intake. These factors are known to exacerbate SD, and may form part of the answer to the (reasonable) question: Why this patient, why now?
TREATMENT
In addition to providing patient education about the diagnosis, I treated both mother and son with topical 2.5% hydrocortisone and arranged for follow-up.
Since a cure is not possible, prevention is the real objective. With that in mind, they were advised to use OTC dandruff shampoos (containing zinc pyrithione, ketoconazole, or selenium sulfide) as body wash, rotating the choice of product on a weekly basis to avoid tachyphylaxis.
Other products that have been useful in treating SD include sodium sulfacetamide cleansers and lotions, imidazole creams and foams, and the calcineurin inhibitors, such as tacrolimus and pimecrolimus.
TAKE-HOME LEARNING POINTS
• Seborrheic dermatitis (SD) is an extremely common papulosquamous condition, affecting up to 20% of the population.
• It tends to run in families of Northern European ancestry.
• SD can manifest in numerous locations, including the scalp, in and behind the ears, or on the face, chest, genitals, axillae, and intergluteal areas.
• Stress is a common trigger for exacerbations of SD.
• Often displaying an annular configuration, SD is frequently mistaken for fungal infection (“ringworm”).
• A flare of SD can be an early sign of Parkinson’s disease or undiagnosed HIV.
• A significant part of the treatment of SD is patient education, not only about the diagnosis and treatment, but also regarding prognosis.
HISTORY
A 48-year-old woman presents to dermatology for evaluation of “ringworm” that has been unresponsive to antifungal creams and a subsequent course of terbinafine (250 mg/d for 10 days).
The problem started several weeks ago, when she experienced mild itching in the upper intergluteal area. Her husband examined the area and noted several patches of a round, scaly rash that they assumed must be ringworm. The patient tried applying OTC clotrimazole cream twice a day, with no good effect.
She next visited a local urgent care center, where the provider agreed with the self-diagnosis. Nystatin cream was prescribed for twice-daily use, but this treatment was also unsuccessful.
Next up was the patient’s primary care provider, who reiterated the diagnosis of ringworm and prescribed the aforementioned course of terbinafine. When it too failed, the patient, still worried, requested a referral to dermatology.
During the history taking, it is revealed that the patient has hypertension, is 50 lb overweight, and has been smoking for more than 40 years. A few weeks prior to the appearance of the rash, she lost her job when the company she had been employed by for more than 20 years closed. At that point, her consumption of alcohol began to increase markedly.
Questions about family history of skin disease reveal several first- and second-degree relatives with psoriasis. In addition, the patient purposely brought her 17-year-old son with her, in order to show his “ringworm,” which she is convinced he passed on to her.
The son’s rash (pictured) has been present for several years, always confined to his axillae and only slightly itchy. It is composed of almost perfectly round pinkish brown patches covering the roofs of both axillae. It bears a striking resemblance to his mother’s intergluteal rash, including the color, and displays annular margins as well.
Additional questioning confirms the fact of his long-standing dandruff and the presence of a chronic scaly rash in and around the ears. At least two of his siblings have similar problems. On hearing this, the patient admits she has suffered with dandruff for years as well.
DISCUSSION
In all likelihood, both mother and son have a papulosquamous condition called seborrheic dermatitis (SD). SD most commonly manifests as dandruff, but it can present in a number of other, less well-known forms.
Up to 20% of the population at large have one or more types of SD, which tends to favor fair-skinned individuals of Northern European ancestry. It appears to be related to a commensal yeast organism, Malassezia furfur, which is markedly lipophilic and needs copious sebum (ubiquitously present on all humans) to flourish. The needed quantity of sebum rarely develops before puberty, due to hormonal influence. As these organisms feed on sebum, the resulting metabolic byproducts trigger an inflammatory response in the susceptible individual.
The resulting characteristic papulosquamous rash can manifest behind the ears, in the external auditory meati, in the glabellar area of the mid-forehead, into the brows, focally in the beard, and in the nasolabial folds. Less commonly, SD appears on the mid-sternum, in the periumbilical area, on genitals, in the intergluteal area, and in the axillae (as in the case of the patient’s son).
Connecting all these dots is an impossible task for most patients, whose only explanation for round and scaly is “ringworm” (tinea corporis). Ringworm, however, requires a source—such as a new cat, guinea pig, dog, livestock, or child with active disease, none of which were present in this case. Little did the patient know that her son had “caught” SD from her!
What we did have in this case was a sharp increase in the patient’s stress level, as well as an increase in her alcohol intake. These factors are known to exacerbate SD, and may form part of the answer to the (reasonable) question: Why this patient, why now?
TREATMENT
In addition to providing patient education about the diagnosis, I treated both mother and son with topical 2.5% hydrocortisone and arranged for follow-up.
Since a cure is not possible, prevention is the real objective. With that in mind, they were advised to use OTC dandruff shampoos (containing zinc pyrithione, ketoconazole, or selenium sulfide) as body wash, rotating the choice of product on a weekly basis to avoid tachyphylaxis.
Other products that have been useful in treating SD include sodium sulfacetamide cleansers and lotions, imidazole creams and foams, and the calcineurin inhibitors, such as tacrolimus and pimecrolimus.
TAKE-HOME LEARNING POINTS
• Seborrheic dermatitis (SD) is an extremely common papulosquamous condition, affecting up to 20% of the population.
• It tends to run in families of Northern European ancestry.
• SD can manifest in numerous locations, including the scalp, in and behind the ears, or on the face, chest, genitals, axillae, and intergluteal areas.
• Stress is a common trigger for exacerbations of SD.
• Often displaying an annular configuration, SD is frequently mistaken for fungal infection (“ringworm”).
• A flare of SD can be an early sign of Parkinson’s disease or undiagnosed HIV.
• A significant part of the treatment of SD is patient education, not only about the diagnosis and treatment, but also regarding prognosis.
HISTORY
A 48-year-old woman presents to dermatology for evaluation of “ringworm” that has been unresponsive to antifungal creams and a subsequent course of terbinafine (250 mg/d for 10 days).
The problem started several weeks ago, when she experienced mild itching in the upper intergluteal area. Her husband examined the area and noted several patches of a round, scaly rash that they assumed must be ringworm. The patient tried applying OTC clotrimazole cream twice a day, with no good effect.
She next visited a local urgent care center, where the provider agreed with the self-diagnosis. Nystatin cream was prescribed for twice-daily use, but this treatment was also unsuccessful.
Next up was the patient’s primary care provider, who reiterated the diagnosis of ringworm and prescribed the aforementioned course of terbinafine. When it too failed, the patient, still worried, requested a referral to dermatology.
During the history taking, it is revealed that the patient has hypertension, is 50 lb overweight, and has been smoking for more than 40 years. A few weeks prior to the appearance of the rash, she lost her job when the company she had been employed by for more than 20 years closed. At that point, her consumption of alcohol began to increase markedly.
Questions about family history of skin disease reveal several first- and second-degree relatives with psoriasis. In addition, the patient purposely brought her 17-year-old son with her, in order to show his “ringworm,” which she is convinced he passed on to her.
The son’s rash (pictured) has been present for several years, always confined to his axillae and only slightly itchy. It is composed of almost perfectly round pinkish brown patches covering the roofs of both axillae. It bears a striking resemblance to his mother’s intergluteal rash, including the color, and displays annular margins as well.
Additional questioning confirms the fact of his long-standing dandruff and the presence of a chronic scaly rash in and around the ears. At least two of his siblings have similar problems. On hearing this, the patient admits she has suffered with dandruff for years as well.
DISCUSSION
In all likelihood, both mother and son have a papulosquamous condition called seborrheic dermatitis (SD). SD most commonly manifests as dandruff, but it can present in a number of other, less well-known forms.
Up to 20% of the population at large have one or more types of SD, which tends to favor fair-skinned individuals of Northern European ancestry. It appears to be related to a commensal yeast organism, Malassezia furfur, which is markedly lipophilic and needs copious sebum (ubiquitously present on all humans) to flourish. The needed quantity of sebum rarely develops before puberty, due to hormonal influence. As these organisms feed on sebum, the resulting metabolic byproducts trigger an inflammatory response in the susceptible individual.
The resulting characteristic papulosquamous rash can manifest behind the ears, in the external auditory meati, in the glabellar area of the mid-forehead, into the brows, focally in the beard, and in the nasolabial folds. Less commonly, SD appears on the mid-sternum, in the periumbilical area, on genitals, in the intergluteal area, and in the axillae (as in the case of the patient’s son).
Connecting all these dots is an impossible task for most patients, whose only explanation for round and scaly is “ringworm” (tinea corporis). Ringworm, however, requires a source—such as a new cat, guinea pig, dog, livestock, or child with active disease, none of which were present in this case. Little did the patient know that her son had “caught” SD from her!
What we did have in this case was a sharp increase in the patient’s stress level, as well as an increase in her alcohol intake. These factors are known to exacerbate SD, and may form part of the answer to the (reasonable) question: Why this patient, why now?
TREATMENT
In addition to providing patient education about the diagnosis, I treated both mother and son with topical 2.5% hydrocortisone and arranged for follow-up.
Since a cure is not possible, prevention is the real objective. With that in mind, they were advised to use OTC dandruff shampoos (containing zinc pyrithione, ketoconazole, or selenium sulfide) as body wash, rotating the choice of product on a weekly basis to avoid tachyphylaxis.
Other products that have been useful in treating SD include sodium sulfacetamide cleansers and lotions, imidazole creams and foams, and the calcineurin inhibitors, such as tacrolimus and pimecrolimus.
TAKE-HOME LEARNING POINTS
• Seborrheic dermatitis (SD) is an extremely common papulosquamous condition, affecting up to 20% of the population.
• It tends to run in families of Northern European ancestry.
• SD can manifest in numerous locations, including the scalp, in and behind the ears, or on the face, chest, genitals, axillae, and intergluteal areas.
• Stress is a common trigger for exacerbations of SD.
• Often displaying an annular configuration, SD is frequently mistaken for fungal infection (“ringworm”).
• A flare of SD can be an early sign of Parkinson’s disease or undiagnosed HIV.
• A significant part of the treatment of SD is patient education, not only about the diagnosis and treatment, but also regarding prognosis.
Man has very uncomfortable problem
HISTORY
A 59-year-old uncircumcised man is referred by his primary care provider for evaluation of what he deems a “yeast infection” of the distal penis and foreskin. Numerous OTC creams (eg, tolnaftate, clotrimazole, hydrocortisone cream) have been tried, over a period of months, with no improvement in the condition. Now, the patient is experiencing increasing discomfort, not only with the rash itself, but also with actual urinary obstruction. Urination, he reports, has been “difficult” and “messy.”
EXAMINATION
The patient’s foreskin is bound down so tightly that it cannot be retracted without pain. Only a tiny opening remains through which the patient can urinate (with difficulty). The surface of the foreskin is atrophic, dry, and shiny. There is little, if any, redness or swelling, but focal areas of purpura are noted over the area.
DISCUSSION
Penile conditions are problematic for several reasons, not the least of which is the location. Many clinicians are not inclined to even look at the area, pleading ignorance of what they might see and preferring to stay in the diagnostic dark. It is certainly true that a provider would do well to have some idea of what he/she might see when examining a particular area of the body—a principle that applies just as much to elbows and fingernails as to penises.
This patient’s condition is called balanitis xerotica obliterans (BXO), a diagnosis of sufficient obscurity to almost guarantee initial misdiagnosis as “yeast infection” or “herpes.” One treatment failure after another eventually leads to referral to a provider familiar with BXO, which is the male version of lichen sclerosus et atrophicus (LS&A) and usually affects the glans, foreskin, and distal shaft.
The causes of these conditions are as yet unknown. However, much is known about how they present, how they look under a microscope, and how to treat them.
LS&A in women itches terribly and presents with whitish atrophic skin changes that affect the perivaginal and perianal areas, sharply sparing the perineum, which gives it a figure-eight or butterfly look. Localized trauma can produce areas of purpura or even bullae. LS&A is not uncommon in children (females > males), in whom the associated focal purpura can be mistaken for a sign of sexual abuse.
Treatment entails use of the most powerful topical steroid ointments, which are so effective that they have completely replaced previous treatment options (eg, testosterone ointment). While a cure is unlikely, control is a realistic goal. If it is left untreated, not only are affected women miserable, but also the condition can lead to stenosis of the introitus and/or meatal stenosis and eventual urinary blockage.
BXO, as in this case, can also cause urinary obstruction, both from the overlying foreskin and from actual meatal stenosis. This is why advanced cases need referral to urology for possible circumcision. As with many penile diagnoses (eg, squamous cell carcinoma or condyloma), BXO is far more common in the uncircumcised.
Other conditions that belong in the differential include seborrhea, psoriasis, dermatophytosis, candidiasis, Bowen’s disease, and irritant/contact dermatitis. Biopsy is typically needed to confirm the diagnosis.
A more common presentation of BXO is a focally atrophic finish to the glans penis, with whitish highlights and a notably dry feel to the affected skin. But, as in this case, it can also affect the foreskin, leading to inflammation and adhesion of the foreskin to the shaft and glans.
In relatively uncomplicated cases, class 1 steroid ointments, such as clobetasol, are prescribed for twice-daily use in the beginning, with a reduction to once-daily use within about two weeks, and then finally to occasional use as the situation demands. Normally, a class 1 steroid would not be used on a thin-skinned area, but BXO responds quite well to it (and lower-strength steroids usually fail). Once control is achieved, the frequency of application is, of course, limited to as-needed use.
TAKE-HOME LEARNING POINTS
1. There is a differential for penile conditions that includes a number of items beyond “yeast infection”: squamous cell carcinoma, psoriasis, seborrheic dermatitis, contact dermatitis, lichen planus, and balanitis xerotica obliterans (BXO).
2. Dermatology is the relevant initial specialty for these patients, not urology, although the ultimate resolution for balanitis is often surgical.
3. The female counterpart to BXO is called lichen sclerosus et atrophicus (LS&A); it is far more common but equally mysterious to providers unacquainted with it.
4. Biopsy—safe and easy to perform—is often necessary to establish the correct diagnosis. For example, a 3-mm punch biopsy, performed under local anesthesia and closed with a single polyglactin stitch, would provide an adequate specimen. Other things being equal, the use of lidocaine-containing epinephrine is perfectly acceptable (and often necessary!) in this area.
5. BXO/LS&A are now routinely treated with class 1 steroid ointments, such as clobetasol, which has almost totally supplanted older choices (eg, testosterone ointment).
HISTORY
A 59-year-old uncircumcised man is referred by his primary care provider for evaluation of what he deems a “yeast infection” of the distal penis and foreskin. Numerous OTC creams (eg, tolnaftate, clotrimazole, hydrocortisone cream) have been tried, over a period of months, with no improvement in the condition. Now, the patient is experiencing increasing discomfort, not only with the rash itself, but also with actual urinary obstruction. Urination, he reports, has been “difficult” and “messy.”
EXAMINATION
The patient’s foreskin is bound down so tightly that it cannot be retracted without pain. Only a tiny opening remains through which the patient can urinate (with difficulty). The surface of the foreskin is atrophic, dry, and shiny. There is little, if any, redness or swelling, but focal areas of purpura are noted over the area.
DISCUSSION
Penile conditions are problematic for several reasons, not the least of which is the location. Many clinicians are not inclined to even look at the area, pleading ignorance of what they might see and preferring to stay in the diagnostic dark. It is certainly true that a provider would do well to have some idea of what he/she might see when examining a particular area of the body—a principle that applies just as much to elbows and fingernails as to penises.
This patient’s condition is called balanitis xerotica obliterans (BXO), a diagnosis of sufficient obscurity to almost guarantee initial misdiagnosis as “yeast infection” or “herpes.” One treatment failure after another eventually leads to referral to a provider familiar with BXO, which is the male version of lichen sclerosus et atrophicus (LS&A) and usually affects the glans, foreskin, and distal shaft.
The causes of these conditions are as yet unknown. However, much is known about how they present, how they look under a microscope, and how to treat them.
LS&A in women itches terribly and presents with whitish atrophic skin changes that affect the perivaginal and perianal areas, sharply sparing the perineum, which gives it a figure-eight or butterfly look. Localized trauma can produce areas of purpura or even bullae. LS&A is not uncommon in children (females > males), in whom the associated focal purpura can be mistaken for a sign of sexual abuse.
Treatment entails use of the most powerful topical steroid ointments, which are so effective that they have completely replaced previous treatment options (eg, testosterone ointment). While a cure is unlikely, control is a realistic goal. If it is left untreated, not only are affected women miserable, but also the condition can lead to stenosis of the introitus and/or meatal stenosis and eventual urinary blockage.
BXO, as in this case, can also cause urinary obstruction, both from the overlying foreskin and from actual meatal stenosis. This is why advanced cases need referral to urology for possible circumcision. As with many penile diagnoses (eg, squamous cell carcinoma or condyloma), BXO is far more common in the uncircumcised.
Other conditions that belong in the differential include seborrhea, psoriasis, dermatophytosis, candidiasis, Bowen’s disease, and irritant/contact dermatitis. Biopsy is typically needed to confirm the diagnosis.
A more common presentation of BXO is a focally atrophic finish to the glans penis, with whitish highlights and a notably dry feel to the affected skin. But, as in this case, it can also affect the foreskin, leading to inflammation and adhesion of the foreskin to the shaft and glans.
In relatively uncomplicated cases, class 1 steroid ointments, such as clobetasol, are prescribed for twice-daily use in the beginning, with a reduction to once-daily use within about two weeks, and then finally to occasional use as the situation demands. Normally, a class 1 steroid would not be used on a thin-skinned area, but BXO responds quite well to it (and lower-strength steroids usually fail). Once control is achieved, the frequency of application is, of course, limited to as-needed use.
TAKE-HOME LEARNING POINTS
1. There is a differential for penile conditions that includes a number of items beyond “yeast infection”: squamous cell carcinoma, psoriasis, seborrheic dermatitis, contact dermatitis, lichen planus, and balanitis xerotica obliterans (BXO).
2. Dermatology is the relevant initial specialty for these patients, not urology, although the ultimate resolution for balanitis is often surgical.
3. The female counterpart to BXO is called lichen sclerosus et atrophicus (LS&A); it is far more common but equally mysterious to providers unacquainted with it.
4. Biopsy—safe and easy to perform—is often necessary to establish the correct diagnosis. For example, a 3-mm punch biopsy, performed under local anesthesia and closed with a single polyglactin stitch, would provide an adequate specimen. Other things being equal, the use of lidocaine-containing epinephrine is perfectly acceptable (and often necessary!) in this area.
5. BXO/LS&A are now routinely treated with class 1 steroid ointments, such as clobetasol, which has almost totally supplanted older choices (eg, testosterone ointment).
HISTORY
A 59-year-old uncircumcised man is referred by his primary care provider for evaluation of what he deems a “yeast infection” of the distal penis and foreskin. Numerous OTC creams (eg, tolnaftate, clotrimazole, hydrocortisone cream) have been tried, over a period of months, with no improvement in the condition. Now, the patient is experiencing increasing discomfort, not only with the rash itself, but also with actual urinary obstruction. Urination, he reports, has been “difficult” and “messy.”
EXAMINATION
The patient’s foreskin is bound down so tightly that it cannot be retracted without pain. Only a tiny opening remains through which the patient can urinate (with difficulty). The surface of the foreskin is atrophic, dry, and shiny. There is little, if any, redness or swelling, but focal areas of purpura are noted over the area.
DISCUSSION
Penile conditions are problematic for several reasons, not the least of which is the location. Many clinicians are not inclined to even look at the area, pleading ignorance of what they might see and preferring to stay in the diagnostic dark. It is certainly true that a provider would do well to have some idea of what he/she might see when examining a particular area of the body—a principle that applies just as much to elbows and fingernails as to penises.
This patient’s condition is called balanitis xerotica obliterans (BXO), a diagnosis of sufficient obscurity to almost guarantee initial misdiagnosis as “yeast infection” or “herpes.” One treatment failure after another eventually leads to referral to a provider familiar with BXO, which is the male version of lichen sclerosus et atrophicus (LS&A) and usually affects the glans, foreskin, and distal shaft.
The causes of these conditions are as yet unknown. However, much is known about how they present, how they look under a microscope, and how to treat them.
LS&A in women itches terribly and presents with whitish atrophic skin changes that affect the perivaginal and perianal areas, sharply sparing the perineum, which gives it a figure-eight or butterfly look. Localized trauma can produce areas of purpura or even bullae. LS&A is not uncommon in children (females > males), in whom the associated focal purpura can be mistaken for a sign of sexual abuse.
Treatment entails use of the most powerful topical steroid ointments, which are so effective that they have completely replaced previous treatment options (eg, testosterone ointment). While a cure is unlikely, control is a realistic goal. If it is left untreated, not only are affected women miserable, but also the condition can lead to stenosis of the introitus and/or meatal stenosis and eventual urinary blockage.
BXO, as in this case, can also cause urinary obstruction, both from the overlying foreskin and from actual meatal stenosis. This is why advanced cases need referral to urology for possible circumcision. As with many penile diagnoses (eg, squamous cell carcinoma or condyloma), BXO is far more common in the uncircumcised.
Other conditions that belong in the differential include seborrhea, psoriasis, dermatophytosis, candidiasis, Bowen’s disease, and irritant/contact dermatitis. Biopsy is typically needed to confirm the diagnosis.
A more common presentation of BXO is a focally atrophic finish to the glans penis, with whitish highlights and a notably dry feel to the affected skin. But, as in this case, it can also affect the foreskin, leading to inflammation and adhesion of the foreskin to the shaft and glans.
In relatively uncomplicated cases, class 1 steroid ointments, such as clobetasol, are prescribed for twice-daily use in the beginning, with a reduction to once-daily use within about two weeks, and then finally to occasional use as the situation demands. Normally, a class 1 steroid would not be used on a thin-skinned area, but BXO responds quite well to it (and lower-strength steroids usually fail). Once control is achieved, the frequency of application is, of course, limited to as-needed use.
TAKE-HOME LEARNING POINTS
1. There is a differential for penile conditions that includes a number of items beyond “yeast infection”: squamous cell carcinoma, psoriasis, seborrheic dermatitis, contact dermatitis, lichen planus, and balanitis xerotica obliterans (BXO).
2. Dermatology is the relevant initial specialty for these patients, not urology, although the ultimate resolution for balanitis is often surgical.
3. The female counterpart to BXO is called lichen sclerosus et atrophicus (LS&A); it is far more common but equally mysterious to providers unacquainted with it.
4. Biopsy—safe and easy to perform—is often necessary to establish the correct diagnosis. For example, a 3-mm punch biopsy, performed under local anesthesia and closed with a single polyglactin stitch, would provide an adequate specimen. Other things being equal, the use of lidocaine-containing epinephrine is perfectly acceptable (and often necessary!) in this area.
5. BXO/LS&A are now routinely treated with class 1 steroid ointments, such as clobetasol, which has almost totally supplanted older choices (eg, testosterone ointment).
Is common problem of aging benign or malignant?
HISTORY
A 61-year-old man is urgently referred to dermatology by his primary care provider, at the request of the patient’s family. During a recent visit, family members happened to see his exposed back, which is covered in lesions. The patient claims the lesions have “been there for years,” growing in size and number. However, his family was greatly alarmed and insisted he seek immediate evaluation.
The patient denies any symptoms associated with the lesions, but does volunteer that they are very much like those his father had as an older man.
DISCUSSION
These are, of course, benign seborrheic keratoses—arguably the single most common problem seen in dermatology offices and the source of much consternation, often to everyone except the patient. Dark, sometimes large, often numerous, they look worrisome. But what are they, exactly?
Seborrheic keratoses (SKs) are benign epidermal excrescences that exhibit a variety of colors and finishes. Varying greatly in size, the typical SK is about a centimeter in diameter, papular to nodular, and tan to grayish brown, with a rough, friable surface. Though little (if any) evidence proves that sun causes them, they tend to appear on the sun-exposed skin of patients ages 50 and older. However, I’ve seen them on patients in their 30s. SKs larger than 10 cm are not unusual, especially on the mid-trunk.
Not everyone develops as many lesions as this patient, but multiple SKs are the rule rather than the exception. Many patients mislabel them as “age spots,” “liver spots,” or “barnacles.” But just as many know that development of SKs is probably hereditary in nature; patients often recall seeing them on their parents or grandparents. Hardly anyone is pleased when they appear, though they are an inevitable consequence of aging for most of us.
The provider’s role, of course, is to distinguish benign from malignant, and that task isn’t always easy. But there are ways to make sure an SK is just that, and not something dangerous.
First of all, anytime you see a multitude of similar lesions that have been present, unchanged, for extended periods on older patients, it’s likely they’re benign.
Next, the “stuck-on” (epidermal) nature of SKs is a very reassuring sign of benignancy. SKs are rough, warty, and dry and clearly develop on the surface of the skin. Their appearance is in sharp contrast to the intradermal nature of, say, melanoma, which may have a raised portion (though at least 80% of melanomas are essentially flat) but cannot be scraped off. Patients will often comment that they’re able to peel SKs off with minor trauma, something one cannot do to a malignant lesion.
Third, on close inspection of SKs, tiny comedone-like pores can be seen scattered over their surface, a feature accentuated by application of liquid nitrogen (which is thus diagnostic as well as therapeutic).
Finally, the application of liquid nitrogen also highlights another indication of the benignancy of SKs: Since SKs have almost no superficial vasculature, they are relatively cool, and when treated with liquid nitrogen, the white color that results persists for several seconds longer than in normal well-vascularized skin or in most melanomas.
This may sound like trivia to the unwary. Unfortunately, there is no rule that says a melanoma cannot develop in the midst of numerous SKs, or even concurrent with an SK. So it does pay to “stay awake” while freezing SKs.
SKs are common on the trunk, arms (especially the triceps), face, scalp, and legs. On the latter, which have fewer oil glands, SKs tend to be drier and whiter. On the face, where sebum is relatively abundant, SKs can be quite dark and shiny. SKs are also relatively common on areas that never see the sun, such as genitals, but they spare the palms and soles, preferring the company of hair follicles.
Biopsy is often required to rule out the other items in the differential, such as melanoma, pigmented basal cell carcinoma, Bowen’s disease, angiokeratoma, and warts.
TAKE-HOME LEARNING POINTS
1. Seborrheic keratoses (SKs) are, by far, the most common benign skin lesions seen in dermatology.
2. The fact that they appear in large numbers speaks loudly to their benignancy, other things being equal (older patient, sun-exposed skin, unchanging nature).
3. The color of SKs can vary a great deal—from orange to brown, gray, or tan to black—in part related to their location.
4. Liquid nitrogen treatment is not only highly effective in treating SKs, but serves as a useful diagnostic tool in two ways: First, it highlights the surface pore-like structures, called horn pseudocysts, which are pathognomic for SKs. Second, the frozen SK stays white considerably longer than relatively well-vascularized normal skin or melanoma, which thaw almost instantly.
5. When in doubt, a deep shave biopsy (“saucerization”) is indicated.
HISTORY
A 61-year-old man is urgently referred to dermatology by his primary care provider, at the request of the patient’s family. During a recent visit, family members happened to see his exposed back, which is covered in lesions. The patient claims the lesions have “been there for years,” growing in size and number. However, his family was greatly alarmed and insisted he seek immediate evaluation.
The patient denies any symptoms associated with the lesions, but does volunteer that they are very much like those his father had as an older man.
DISCUSSION
These are, of course, benign seborrheic keratoses—arguably the single most common problem seen in dermatology offices and the source of much consternation, often to everyone except the patient. Dark, sometimes large, often numerous, they look worrisome. But what are they, exactly?
Seborrheic keratoses (SKs) are benign epidermal excrescences that exhibit a variety of colors and finishes. Varying greatly in size, the typical SK is about a centimeter in diameter, papular to nodular, and tan to grayish brown, with a rough, friable surface. Though little (if any) evidence proves that sun causes them, they tend to appear on the sun-exposed skin of patients ages 50 and older. However, I’ve seen them on patients in their 30s. SKs larger than 10 cm are not unusual, especially on the mid-trunk.
Not everyone develops as many lesions as this patient, but multiple SKs are the rule rather than the exception. Many patients mislabel them as “age spots,” “liver spots,” or “barnacles.” But just as many know that development of SKs is probably hereditary in nature; patients often recall seeing them on their parents or grandparents. Hardly anyone is pleased when they appear, though they are an inevitable consequence of aging for most of us.
The provider’s role, of course, is to distinguish benign from malignant, and that task isn’t always easy. But there are ways to make sure an SK is just that, and not something dangerous.
First of all, anytime you see a multitude of similar lesions that have been present, unchanged, for extended periods on older patients, it’s likely they’re benign.
Next, the “stuck-on” (epidermal) nature of SKs is a very reassuring sign of benignancy. SKs are rough, warty, and dry and clearly develop on the surface of the skin. Their appearance is in sharp contrast to the intradermal nature of, say, melanoma, which may have a raised portion (though at least 80% of melanomas are essentially flat) but cannot be scraped off. Patients will often comment that they’re able to peel SKs off with minor trauma, something one cannot do to a malignant lesion.
Third, on close inspection of SKs, tiny comedone-like pores can be seen scattered over their surface, a feature accentuated by application of liquid nitrogen (which is thus diagnostic as well as therapeutic).
Finally, the application of liquid nitrogen also highlights another indication of the benignancy of SKs: Since SKs have almost no superficial vasculature, they are relatively cool, and when treated with liquid nitrogen, the white color that results persists for several seconds longer than in normal well-vascularized skin or in most melanomas.
This may sound like trivia to the unwary. Unfortunately, there is no rule that says a melanoma cannot develop in the midst of numerous SKs, or even concurrent with an SK. So it does pay to “stay awake” while freezing SKs.
SKs are common on the trunk, arms (especially the triceps), face, scalp, and legs. On the latter, which have fewer oil glands, SKs tend to be drier and whiter. On the face, where sebum is relatively abundant, SKs can be quite dark and shiny. SKs are also relatively common on areas that never see the sun, such as genitals, but they spare the palms and soles, preferring the company of hair follicles.
Biopsy is often required to rule out the other items in the differential, such as melanoma, pigmented basal cell carcinoma, Bowen’s disease, angiokeratoma, and warts.
TAKE-HOME LEARNING POINTS
1. Seborrheic keratoses (SKs) are, by far, the most common benign skin lesions seen in dermatology.
2. The fact that they appear in large numbers speaks loudly to their benignancy, other things being equal (older patient, sun-exposed skin, unchanging nature).
3. The color of SKs can vary a great deal—from orange to brown, gray, or tan to black—in part related to their location.
4. Liquid nitrogen treatment is not only highly effective in treating SKs, but serves as a useful diagnostic tool in two ways: First, it highlights the surface pore-like structures, called horn pseudocysts, which are pathognomic for SKs. Second, the frozen SK stays white considerably longer than relatively well-vascularized normal skin or melanoma, which thaw almost instantly.
5. When in doubt, a deep shave biopsy (“saucerization”) is indicated.
HISTORY
A 61-year-old man is urgently referred to dermatology by his primary care provider, at the request of the patient’s family. During a recent visit, family members happened to see his exposed back, which is covered in lesions. The patient claims the lesions have “been there for years,” growing in size and number. However, his family was greatly alarmed and insisted he seek immediate evaluation.
The patient denies any symptoms associated with the lesions, but does volunteer that they are very much like those his father had as an older man.
DISCUSSION
These are, of course, benign seborrheic keratoses—arguably the single most common problem seen in dermatology offices and the source of much consternation, often to everyone except the patient. Dark, sometimes large, often numerous, they look worrisome. But what are they, exactly?
Seborrheic keratoses (SKs) are benign epidermal excrescences that exhibit a variety of colors and finishes. Varying greatly in size, the typical SK is about a centimeter in diameter, papular to nodular, and tan to grayish brown, with a rough, friable surface. Though little (if any) evidence proves that sun causes them, they tend to appear on the sun-exposed skin of patients ages 50 and older. However, I’ve seen them on patients in their 30s. SKs larger than 10 cm are not unusual, especially on the mid-trunk.
Not everyone develops as many lesions as this patient, but multiple SKs are the rule rather than the exception. Many patients mislabel them as “age spots,” “liver spots,” or “barnacles.” But just as many know that development of SKs is probably hereditary in nature; patients often recall seeing them on their parents or grandparents. Hardly anyone is pleased when they appear, though they are an inevitable consequence of aging for most of us.
The provider’s role, of course, is to distinguish benign from malignant, and that task isn’t always easy. But there are ways to make sure an SK is just that, and not something dangerous.
First of all, anytime you see a multitude of similar lesions that have been present, unchanged, for extended periods on older patients, it’s likely they’re benign.
Next, the “stuck-on” (epidermal) nature of SKs is a very reassuring sign of benignancy. SKs are rough, warty, and dry and clearly develop on the surface of the skin. Their appearance is in sharp contrast to the intradermal nature of, say, melanoma, which may have a raised portion (though at least 80% of melanomas are essentially flat) but cannot be scraped off. Patients will often comment that they’re able to peel SKs off with minor trauma, something one cannot do to a malignant lesion.
Third, on close inspection of SKs, tiny comedone-like pores can be seen scattered over their surface, a feature accentuated by application of liquid nitrogen (which is thus diagnostic as well as therapeutic).
Finally, the application of liquid nitrogen also highlights another indication of the benignancy of SKs: Since SKs have almost no superficial vasculature, they are relatively cool, and when treated with liquid nitrogen, the white color that results persists for several seconds longer than in normal well-vascularized skin or in most melanomas.
This may sound like trivia to the unwary. Unfortunately, there is no rule that says a melanoma cannot develop in the midst of numerous SKs, or even concurrent with an SK. So it does pay to “stay awake” while freezing SKs.
SKs are common on the trunk, arms (especially the triceps), face, scalp, and legs. On the latter, which have fewer oil glands, SKs tend to be drier and whiter. On the face, where sebum is relatively abundant, SKs can be quite dark and shiny. SKs are also relatively common on areas that never see the sun, such as genitals, but they spare the palms and soles, preferring the company of hair follicles.
Biopsy is often required to rule out the other items in the differential, such as melanoma, pigmented basal cell carcinoma, Bowen’s disease, angiokeratoma, and warts.
TAKE-HOME LEARNING POINTS
1. Seborrheic keratoses (SKs) are, by far, the most common benign skin lesions seen in dermatology.
2. The fact that they appear in large numbers speaks loudly to their benignancy, other things being equal (older patient, sun-exposed skin, unchanging nature).
3. The color of SKs can vary a great deal—from orange to brown, gray, or tan to black—in part related to their location.
4. Liquid nitrogen treatment is not only highly effective in treating SKs, but serves as a useful diagnostic tool in two ways: First, it highlights the surface pore-like structures, called horn pseudocysts, which are pathognomic for SKs. Second, the frozen SK stays white considerably longer than relatively well-vascularized normal skin or melanoma, which thaw almost instantly.
5. When in doubt, a deep shave biopsy (“saucerization”) is indicated.