User login
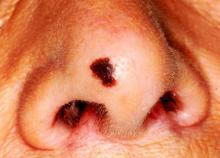
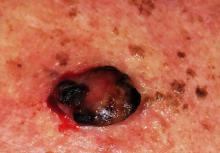
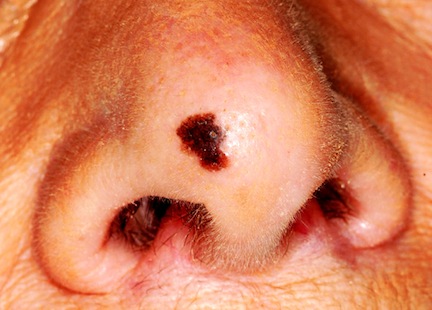
The Truth About Melanoma
Much energy is expended—by patients, parents, publishers, and providers—in obsessing over, looking for, and preventing malignant melanoma. A day rarely goes by without a story in the media purporting to inform the reader about this dreaded cancer.
On the one hand, who can argue with the provision of information that has the potential to save lives and reduce suffering? The answer is: I can.
What I see in the popular media is a veritable cornucopia of misinformation—often rehashed myths, vast generalizations, and the like—that all too often serves to confuse, obfuscate, and generally perpetuate the most dangerous misconceptions. Many of them lead to totally unnecessary deaths.
I know this not only because I read these articles but also because I see the worried patients who’ve read them. They come in to “get their moles checked,” for example, since they’ve been told “that’s where melanomas come from.” As with all myths, there is a grain of truth in that concept, but it is dangerously misleading, as is much of the other information passed on in these pieces.
Unfortunately, many medical providers are as influenced by these reports as the general public. And while I can’t reach the general public, I can reach a few hundred thousand medical professionals with some facts that have the potential to save lives (maybe even their own).
MYTH # 1: MELANOMAS COME FROM MOLES
Easily the most common “melanoma myth,” this is possibly the most dangerous. While patients are busy watching their “moles,” the melanoma is on its way to killing them.
The problem, of course, is the word mole, which means different things to different people. Most patients who use this word mean the raised, fleshy, often hair-bearing papule or nodule on their face, arm, or back, that can be present without changing for years. When that is the case, there is little or no potential for these lesions—usually compound versus intradermal nevi—to undergo malignant transformation. No physical event (trauma, chemicals, or sun exposure) can turn a benign nevus into a cancer.
What we as medical professionals are looking for in any lesion is a history of change: growth, color, elevation, erosion/ulceration. When these are present, our course is clear: We remove the lesion and send it for pathologic evaluation. Just because malignant transformation of a nevus is unlikely doesn’t mean it can’t happen.
MYTH # 2: SUN CAUSES MELANOMA, SO LESIONS ON NON–SUN-EXPOSED SKIN CAN’T BE MELANOMAS
The truth is that about 80% of melanomas are found in areas normally covered by clothing, such as legs, abdomens, thighs, and even the bottoms of the feet, in the scalp, and on genitals. Yes, sun is unquestionably the cause, but the sun does not necessarily have to strike the affected area.
This is only one of a number of seeming contradictions about melanoma. It’s important to understand and accept this fact even if it makes no sense, because lives are in the balance. Many tragic deaths have occurred because of a failure to understand and accept this reality.
MYTH # 3: MELANOMAS MANIFEST AS RAISED LUMPS ON THE SKIN
Dermatology providers see and hear this all day, every day. Seborrheic keratoses typify this misperception. However, they also serve as a terrific example of the benignancy of most epidermal lesions (ie, ones that are “stuck on” the outer layer of skin and thus easily peeled off), which are usually warty, rough and colored brown, tan, gray, or a combination of these colors. They’re usually present in multiples, another good sign (in general), but they are the source of endless worry for patients and providers.
The truth is that at least 80% of melanomas are essentially macular (flat) enough to be difficult to palpate, and they almost never appear in multiples. Having said this, I should note that melanomas can certainly be raised on the skin—but these are in the minority of cases. Again, change is the key thing to watch for/ask about, but not change that occurs overnight or from known trauma.
Much energy is expended—by patients, parents, publishers, and providers—in obsessing over, looking for, and preventing malignant melanoma. A day rarely goes by without a story in the media purporting to inform the reader about this dreaded cancer.
On the one hand, who can argue with the provision of information that has the potential to save lives and reduce suffering? The answer is: I can.
What I see in the popular media is a veritable cornucopia of misinformation—often rehashed myths, vast generalizations, and the like—that all too often serves to confuse, obfuscate, and generally perpetuate the most dangerous misconceptions. Many of them lead to totally unnecessary deaths.
I know this not only because I read these articles but also because I see the worried patients who’ve read them. They come in to “get their moles checked,” for example, since they’ve been told “that’s where melanomas come from.” As with all myths, there is a grain of truth in that concept, but it is dangerously misleading, as is much of the other information passed on in these pieces.
Unfortunately, many medical providers are as influenced by these reports as the general public. And while I can’t reach the general public, I can reach a few hundred thousand medical professionals with some facts that have the potential to save lives (maybe even their own).
MYTH # 1: MELANOMAS COME FROM MOLES
Easily the most common “melanoma myth,” this is possibly the most dangerous. While patients are busy watching their “moles,” the melanoma is on its way to killing them.
The problem, of course, is the word mole, which means different things to different people. Most patients who use this word mean the raised, fleshy, often hair-bearing papule or nodule on their face, arm, or back, that can be present without changing for years. When that is the case, there is little or no potential for these lesions—usually compound versus intradermal nevi—to undergo malignant transformation. No physical event (trauma, chemicals, or sun exposure) can turn a benign nevus into a cancer.
What we as medical professionals are looking for in any lesion is a history of change: growth, color, elevation, erosion/ulceration. When these are present, our course is clear: We remove the lesion and send it for pathologic evaluation. Just because malignant transformation of a nevus is unlikely doesn’t mean it can’t happen.
MYTH # 2: SUN CAUSES MELANOMA, SO LESIONS ON NON–SUN-EXPOSED SKIN CAN’T BE MELANOMAS
The truth is that about 80% of melanomas are found in areas normally covered by clothing, such as legs, abdomens, thighs, and even the bottoms of the feet, in the scalp, and on genitals. Yes, sun is unquestionably the cause, but the sun does not necessarily have to strike the affected area.
This is only one of a number of seeming contradictions about melanoma. It’s important to understand and accept this fact even if it makes no sense, because lives are in the balance. Many tragic deaths have occurred because of a failure to understand and accept this reality.
MYTH # 3: MELANOMAS MANIFEST AS RAISED LUMPS ON THE SKIN
Dermatology providers see and hear this all day, every day. Seborrheic keratoses typify this misperception. However, they also serve as a terrific example of the benignancy of most epidermal lesions (ie, ones that are “stuck on” the outer layer of skin and thus easily peeled off), which are usually warty, rough and colored brown, tan, gray, or a combination of these colors. They’re usually present in multiples, another good sign (in general), but they are the source of endless worry for patients and providers.
The truth is that at least 80% of melanomas are essentially macular (flat) enough to be difficult to palpate, and they almost never appear in multiples. Having said this, I should note that melanomas can certainly be raised on the skin—but these are in the minority of cases. Again, change is the key thing to watch for/ask about, but not change that occurs overnight or from known trauma.
Much energy is expended—by patients, parents, publishers, and providers—in obsessing over, looking for, and preventing malignant melanoma. A day rarely goes by without a story in the media purporting to inform the reader about this dreaded cancer.
On the one hand, who can argue with the provision of information that has the potential to save lives and reduce suffering? The answer is: I can.
What I see in the popular media is a veritable cornucopia of misinformation—often rehashed myths, vast generalizations, and the like—that all too often serves to confuse, obfuscate, and generally perpetuate the most dangerous misconceptions. Many of them lead to totally unnecessary deaths.
I know this not only because I read these articles but also because I see the worried patients who’ve read them. They come in to “get their moles checked,” for example, since they’ve been told “that’s where melanomas come from.” As with all myths, there is a grain of truth in that concept, but it is dangerously misleading, as is much of the other information passed on in these pieces.
Unfortunately, many medical providers are as influenced by these reports as the general public. And while I can’t reach the general public, I can reach a few hundred thousand medical professionals with some facts that have the potential to save lives (maybe even their own).
MYTH # 1: MELANOMAS COME FROM MOLES
Easily the most common “melanoma myth,” this is possibly the most dangerous. While patients are busy watching their “moles,” the melanoma is on its way to killing them.
The problem, of course, is the word mole, which means different things to different people. Most patients who use this word mean the raised, fleshy, often hair-bearing papule or nodule on their face, arm, or back, that can be present without changing for years. When that is the case, there is little or no potential for these lesions—usually compound versus intradermal nevi—to undergo malignant transformation. No physical event (trauma, chemicals, or sun exposure) can turn a benign nevus into a cancer.
What we as medical professionals are looking for in any lesion is a history of change: growth, color, elevation, erosion/ulceration. When these are present, our course is clear: We remove the lesion and send it for pathologic evaluation. Just because malignant transformation of a nevus is unlikely doesn’t mean it can’t happen.
MYTH # 2: SUN CAUSES MELANOMA, SO LESIONS ON NON–SUN-EXPOSED SKIN CAN’T BE MELANOMAS
The truth is that about 80% of melanomas are found in areas normally covered by clothing, such as legs, abdomens, thighs, and even the bottoms of the feet, in the scalp, and on genitals. Yes, sun is unquestionably the cause, but the sun does not necessarily have to strike the affected area.
This is only one of a number of seeming contradictions about melanoma. It’s important to understand and accept this fact even if it makes no sense, because lives are in the balance. Many tragic deaths have occurred because of a failure to understand and accept this reality.
MYTH # 3: MELANOMAS MANIFEST AS RAISED LUMPS ON THE SKIN
Dermatology providers see and hear this all day, every day. Seborrheic keratoses typify this misperception. However, they also serve as a terrific example of the benignancy of most epidermal lesions (ie, ones that are “stuck on” the outer layer of skin and thus easily peeled off), which are usually warty, rough and colored brown, tan, gray, or a combination of these colors. They’re usually present in multiples, another good sign (in general), but they are the source of endless worry for patients and providers.
The truth is that at least 80% of melanomas are essentially macular (flat) enough to be difficult to palpate, and they almost never appear in multiples. Having said this, I should note that melanomas can certainly be raised on the skin—but these are in the minority of cases. Again, change is the key thing to watch for/ask about, but not change that occurs overnight or from known trauma.
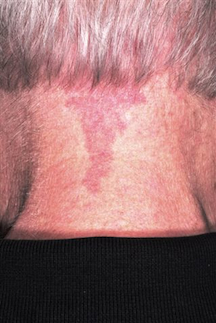
Leg Lesion Represents a Vicious Cycle
CASE
A 38-year-old man is referred to dermatology by his primary care provider (PCP) for evaluation of a lesion on his leg that has been present for more than two years. Concerned friends and family recently urged him to seek medical care.
His PCP thought it probably represented fungal infection, but the nystatin/triamcinolone cream he prescribed was of little or no help. The patient, who is of Indian descent, decided to consult a medical provider during a trip to India, but was dissatisfied with the herbal paste he was advised to obtain and use. When he returned to the United States, he requested referral to dermatology.
The patient denies any other skin problems, now or in the past, but admits to scratching and rubbing the site in question several times a day—partly out of habit, but mostly because it itches. The spot’s progressive darkening has been a major factor in his pursuit of further evaluation.
Examination reveals a single lesion: a uniformly scaly, dark, 8-x-4–cm area of his anterolateral calf. The margins of the lesion are fairly sharply demarcated, but there is no redness, increased warmth, or tenderness associated with it. The patient’s skin overall is quite dark (type V).
DISCUSSION
This presentation is typical of lichen simplex chronicus (LSC; also known as neurodermatitis)—essentially, a reaction to chronic rubbing and scratching. LSC is not a primary diagnosis; it is merely the consequence of mechanical trauma as a reaction to perceived pruritus (with or without actual pathologic cause). Over time, the affected skin tends to thicken in reaction to chronic trauma, which also has the effect of increasing pruritus. Thus, the itch-scratch-itch cycle perpetuates.
Affected skin also tends to darken, especially in darker-skinned patients, and is often the source of considerable consternation. Even when the condition is treated and all rubbing and scratching ceases, it may take months (if not years) for the hyperpigmentation to clear.
The keys to diagnosis include the patient’s admission of regular scratching and his ready access to the area, as well as the lichenification and hyperpigmentation. There are any number of initial triggers, including bug bites, dry skin, eczema, and even psoriasis. However, those conditions take a backseat to the LSC. This exact location (anterior leg) is quite typical in men, but in women, LSC is far more common in the nuchal scalp, where heat and sweat also contribute to the problem.
Many LSC patients have a history of atopic dermatitis that appears to lower their threshold for pruritus. When questioned closely, many if not most will admit to concurrent emotional stress, which is thought to be a contributing factor.
Biopsy is occasionally necessary to distinguish LSC from other items in the differential, including psoriasis, contact dermatitis, and lichen planus. But in most cases, including this one, the twice-daily application of a class 2 or 3 topical steroid cream or ointment for one to two weeks will work wonders. Educating the patient about his own contribution to the problem is essential.
This patient was instructed to return in one month, to ensure that the condition was responding and that he understood the need to gradually decrease the use of this powerful steroid. Unfortunately, his chances for recurrence are quite high, given the habitual and compelling nature of the problem.
TAKE-HOME LEARNING POINTS
• Lichen simplex chronicus (LSC) is a very common condition that represents the skin’s reaction to chronic scratching and rubbing.
• LSC is not a primary condition; rather, it is triggered by dry skin, eczema, contact dermatitis, or lichen planus (among others).
• LSC involves thickening of the affected skin and, in darker-skinned patients, a reactive hyperpigmentation.
• LSC commonly manifests on the anterior legs in men and on the nuchal scalp in women.
CASE
A 38-year-old man is referred to dermatology by his primary care provider (PCP) for evaluation of a lesion on his leg that has been present for more than two years. Concerned friends and family recently urged him to seek medical care.
His PCP thought it probably represented fungal infection, but the nystatin/triamcinolone cream he prescribed was of little or no help. The patient, who is of Indian descent, decided to consult a medical provider during a trip to India, but was dissatisfied with the herbal paste he was advised to obtain and use. When he returned to the United States, he requested referral to dermatology.
The patient denies any other skin problems, now or in the past, but admits to scratching and rubbing the site in question several times a day—partly out of habit, but mostly because it itches. The spot’s progressive darkening has been a major factor in his pursuit of further evaluation.
Examination reveals a single lesion: a uniformly scaly, dark, 8-x-4–cm area of his anterolateral calf. The margins of the lesion are fairly sharply demarcated, but there is no redness, increased warmth, or tenderness associated with it. The patient’s skin overall is quite dark (type V).
DISCUSSION
This presentation is typical of lichen simplex chronicus (LSC; also known as neurodermatitis)—essentially, a reaction to chronic rubbing and scratching. LSC is not a primary diagnosis; it is merely the consequence of mechanical trauma as a reaction to perceived pruritus (with or without actual pathologic cause). Over time, the affected skin tends to thicken in reaction to chronic trauma, which also has the effect of increasing pruritus. Thus, the itch-scratch-itch cycle perpetuates.
Affected skin also tends to darken, especially in darker-skinned patients, and is often the source of considerable consternation. Even when the condition is treated and all rubbing and scratching ceases, it may take months (if not years) for the hyperpigmentation to clear.
The keys to diagnosis include the patient’s admission of regular scratching and his ready access to the area, as well as the lichenification and hyperpigmentation. There are any number of initial triggers, including bug bites, dry skin, eczema, and even psoriasis. However, those conditions take a backseat to the LSC. This exact location (anterior leg) is quite typical in men, but in women, LSC is far more common in the nuchal scalp, where heat and sweat also contribute to the problem.
Many LSC patients have a history of atopic dermatitis that appears to lower their threshold for pruritus. When questioned closely, many if not most will admit to concurrent emotional stress, which is thought to be a contributing factor.
Biopsy is occasionally necessary to distinguish LSC from other items in the differential, including psoriasis, contact dermatitis, and lichen planus. But in most cases, including this one, the twice-daily application of a class 2 or 3 topical steroid cream or ointment for one to two weeks will work wonders. Educating the patient about his own contribution to the problem is essential.
This patient was instructed to return in one month, to ensure that the condition was responding and that he understood the need to gradually decrease the use of this powerful steroid. Unfortunately, his chances for recurrence are quite high, given the habitual and compelling nature of the problem.
TAKE-HOME LEARNING POINTS
• Lichen simplex chronicus (LSC) is a very common condition that represents the skin’s reaction to chronic scratching and rubbing.
• LSC is not a primary condition; rather, it is triggered by dry skin, eczema, contact dermatitis, or lichen planus (among others).
• LSC involves thickening of the affected skin and, in darker-skinned patients, a reactive hyperpigmentation.
• LSC commonly manifests on the anterior legs in men and on the nuchal scalp in women.
CASE
A 38-year-old man is referred to dermatology by his primary care provider (PCP) for evaluation of a lesion on his leg that has been present for more than two years. Concerned friends and family recently urged him to seek medical care.
His PCP thought it probably represented fungal infection, but the nystatin/triamcinolone cream he prescribed was of little or no help. The patient, who is of Indian descent, decided to consult a medical provider during a trip to India, but was dissatisfied with the herbal paste he was advised to obtain and use. When he returned to the United States, he requested referral to dermatology.
The patient denies any other skin problems, now or in the past, but admits to scratching and rubbing the site in question several times a day—partly out of habit, but mostly because it itches. The spot’s progressive darkening has been a major factor in his pursuit of further evaluation.
Examination reveals a single lesion: a uniformly scaly, dark, 8-x-4–cm area of his anterolateral calf. The margins of the lesion are fairly sharply demarcated, but there is no redness, increased warmth, or tenderness associated with it. The patient’s skin overall is quite dark (type V).
DISCUSSION
This presentation is typical of lichen simplex chronicus (LSC; also known as neurodermatitis)—essentially, a reaction to chronic rubbing and scratching. LSC is not a primary diagnosis; it is merely the consequence of mechanical trauma as a reaction to perceived pruritus (with or without actual pathologic cause). Over time, the affected skin tends to thicken in reaction to chronic trauma, which also has the effect of increasing pruritus. Thus, the itch-scratch-itch cycle perpetuates.
Affected skin also tends to darken, especially in darker-skinned patients, and is often the source of considerable consternation. Even when the condition is treated and all rubbing and scratching ceases, it may take months (if not years) for the hyperpigmentation to clear.
The keys to diagnosis include the patient’s admission of regular scratching and his ready access to the area, as well as the lichenification and hyperpigmentation. There are any number of initial triggers, including bug bites, dry skin, eczema, and even psoriasis. However, those conditions take a backseat to the LSC. This exact location (anterior leg) is quite typical in men, but in women, LSC is far more common in the nuchal scalp, where heat and sweat also contribute to the problem.
Many LSC patients have a history of atopic dermatitis that appears to lower their threshold for pruritus. When questioned closely, many if not most will admit to concurrent emotional stress, which is thought to be a contributing factor.
Biopsy is occasionally necessary to distinguish LSC from other items in the differential, including psoriasis, contact dermatitis, and lichen planus. But in most cases, including this one, the twice-daily application of a class 2 or 3 topical steroid cream or ointment for one to two weeks will work wonders. Educating the patient about his own contribution to the problem is essential.
This patient was instructed to return in one month, to ensure that the condition was responding and that he understood the need to gradually decrease the use of this powerful steroid. Unfortunately, his chances for recurrence are quite high, given the habitual and compelling nature of the problem.
TAKE-HOME LEARNING POINTS
• Lichen simplex chronicus (LSC) is a very common condition that represents the skin’s reaction to chronic scratching and rubbing.
• LSC is not a primary condition; rather, it is triggered by dry skin, eczema, contact dermatitis, or lichen planus (among others).
• LSC involves thickening of the affected skin and, in darker-skinned patients, a reactive hyperpigmentation.
• LSC commonly manifests on the anterior legs in men and on the nuchal scalp in women.
Baffling reaction to a suspicious lesion
CASE
A 34-year-old woman presents to her primary care provider to “have her moles checked.” She is motivated by a family history of sun-caused skin cancers, as well as her own history of multiple sunburns as a child.
Noting the patient’s fair, freckled skin, red hair, and blue eyes, the primary care provider agrees with the patient’s assessment of her risk. One lesion stands out from the rest: a 1.5-cm dark, irregularly bordered, and pigmented maculopapular lesion on the patient’s right triceps. The primary care provider arranges for a timely referral to dermatology.
The dermatology clinicians know immediately that the triceps lesion is highly suspicious, and tell the patient so while setting up for biopsy. Before that procedure is carried out, a careful examination of all exposed skin is conducted. The patient’s fair, sun-damaged skin is again noted, but no other suspicious lesions are found. There are no palpable nodes detected on her right axilla.
Under local anesthesia (1% lidocaine with epinephrine), the right triceps lesion is removed by deep saucerization (through the deep dermis into the upper adipose layer) using a double-edged razor. Minor bleeding is controlled with light cautery.
The pathology report, received three days later, confirms the malignant nature of the lesion, with a diagnosis of invasive melanoma (nodular, with no horizontal growth phase), measuring 1.7 mm. Fortunately, no other ominous signs—such as a high mitotic rate or vascular invasion—are reported.
The patient is immediately contacted by phone and notified of the results. The potential danger of this diagnosis is reiterated, along with information regarding the next steps in the process. These include consultation with a surgeon for consideration of re-excision and possible lymph node dissection. Evaluation by an oncologist will likely follow.
The patient’s response to this news is puzzling, to say the least. Though she appears to understand what she is being told, she sounds blissfully unconcerned, saying she is “not that worried” and is sure she will “be just fine.”
DISCUSSION
There are patients newly diagnosed with melanoma who overreact. I’ve had patients hop on the next plane to the Mayo Clinic, or, as in one notable case, to Tijuana, which, as we all know, is the home of such questionable practices as coffee-ground enemas and chemically modified amygdalin.
But then there are melanoma patients who go to the other extreme, making us wonder if they really understand the potential seriousness of the situation. It’s not that we want to see any particular “angst” as a reaction, but an appropriate indication or two is reassuring as feedback to the announcement. Questions— “What does this mean?” “What’s going to happen now?” “How serious is this?”— are good to hear in this regard. The answers allow us to convey the sense of where the patient stands, both for the present and in the long term, and help us to get a sense of how well the patient perceives the situation.
This patient had no questions, at all, as if she was totally unconcerned. That concerned me. It left me with a number of questions: “Does she really understand what’s going on?” “Will she follow our instructions and see the specialists we advise her to see?” Over the years, I’ve had several patients like this who went on their merry way, doing nothing we suggested. Some even survived.
This lack of appropriate reaction has been termed la belle indifference. It’s a way of pretending nothing is happening, and represents a way of showing one’s paralysis to others by manipulating their judgment through an attitude of indifference. One doesn’t want to frighten these patients (“Don’t you know this could be fatal?”), so what I do is keep close tabs on them—calling them regularly, making sure they’re following our advice, and documenting our calls and the patient’s responses. When family members can be enlisted to help, so much the better.
So far, this patient is complying with our advice, but it’s early in the process yet. We’ll see. Our job—and her ordeal—is far from done.
Any melanoma over 1 mm in thickness (based on the Breslow scale) is associated with an uncertain prognosis, and nodular melanomas are associated with a relatively poor prognosis. Besides re-excision (probably with 1-cm margins), this patient will probably be a candidate for elective lymph node dissection in the right axilla. PET scans, blood tests, and a visit to the oncologist will most likely follow. The surgeon usually acts as decision-maker in terms of what the patient needs and in what sequence.
Even if she survives all that, this patient will still need to see us every three months or so for a year, then regularly thereafter, to monitor this cancer and watch for new ones.
LEARNING POINTS
• Deep-shave biopsy (sometimes called saucerization) is an appropriate technique for possible melanoma.
• About 75% to 80% of all melanomas are superficial, spreading types, (essentially flat), while 10% or so have only a vertical phase of growth (ie, present as a nodule or mass).
• Survival rates for melanoma are closely tied to tumor thickness, which is most commonly measured (by the pathologist) in millimeters; this staging system is called the Breslow scale. The older system of staging melanoma by the anatomical depth (called the Clark’s level I-V) has fallen into disuse.
• Underreaction to the diagnosis of melanoma (la belle indifference) can be as problematic as overreaction. Consistent monitoring of patients for compliance is often necessary.
CASE
A 34-year-old woman presents to her primary care provider to “have her moles checked.” She is motivated by a family history of sun-caused skin cancers, as well as her own history of multiple sunburns as a child.
Noting the patient’s fair, freckled skin, red hair, and blue eyes, the primary care provider agrees with the patient’s assessment of her risk. One lesion stands out from the rest: a 1.5-cm dark, irregularly bordered, and pigmented maculopapular lesion on the patient’s right triceps. The primary care provider arranges for a timely referral to dermatology.
The dermatology clinicians know immediately that the triceps lesion is highly suspicious, and tell the patient so while setting up for biopsy. Before that procedure is carried out, a careful examination of all exposed skin is conducted. The patient’s fair, sun-damaged skin is again noted, but no other suspicious lesions are found. There are no palpable nodes detected on her right axilla.
Under local anesthesia (1% lidocaine with epinephrine), the right triceps lesion is removed by deep saucerization (through the deep dermis into the upper adipose layer) using a double-edged razor. Minor bleeding is controlled with light cautery.
The pathology report, received three days later, confirms the malignant nature of the lesion, with a diagnosis of invasive melanoma (nodular, with no horizontal growth phase), measuring 1.7 mm. Fortunately, no other ominous signs—such as a high mitotic rate or vascular invasion—are reported.
The patient is immediately contacted by phone and notified of the results. The potential danger of this diagnosis is reiterated, along with information regarding the next steps in the process. These include consultation with a surgeon for consideration of re-excision and possible lymph node dissection. Evaluation by an oncologist will likely follow.
The patient’s response to this news is puzzling, to say the least. Though she appears to understand what she is being told, she sounds blissfully unconcerned, saying she is “not that worried” and is sure she will “be just fine.”
DISCUSSION
There are patients newly diagnosed with melanoma who overreact. I’ve had patients hop on the next plane to the Mayo Clinic, or, as in one notable case, to Tijuana, which, as we all know, is the home of such questionable practices as coffee-ground enemas and chemically modified amygdalin.
But then there are melanoma patients who go to the other extreme, making us wonder if they really understand the potential seriousness of the situation. It’s not that we want to see any particular “angst” as a reaction, but an appropriate indication or two is reassuring as feedback to the announcement. Questions— “What does this mean?” “What’s going to happen now?” “How serious is this?”— are good to hear in this regard. The answers allow us to convey the sense of where the patient stands, both for the present and in the long term, and help us to get a sense of how well the patient perceives the situation.
This patient had no questions, at all, as if she was totally unconcerned. That concerned me. It left me with a number of questions: “Does she really understand what’s going on?” “Will she follow our instructions and see the specialists we advise her to see?” Over the years, I’ve had several patients like this who went on their merry way, doing nothing we suggested. Some even survived.
This lack of appropriate reaction has been termed la belle indifference. It’s a way of pretending nothing is happening, and represents a way of showing one’s paralysis to others by manipulating their judgment through an attitude of indifference. One doesn’t want to frighten these patients (“Don’t you know this could be fatal?”), so what I do is keep close tabs on them—calling them regularly, making sure they’re following our advice, and documenting our calls and the patient’s responses. When family members can be enlisted to help, so much the better.
So far, this patient is complying with our advice, but it’s early in the process yet. We’ll see. Our job—and her ordeal—is far from done.
Any melanoma over 1 mm in thickness (based on the Breslow scale) is associated with an uncertain prognosis, and nodular melanomas are associated with a relatively poor prognosis. Besides re-excision (probably with 1-cm margins), this patient will probably be a candidate for elective lymph node dissection in the right axilla. PET scans, blood tests, and a visit to the oncologist will most likely follow. The surgeon usually acts as decision-maker in terms of what the patient needs and in what sequence.
Even if she survives all that, this patient will still need to see us every three months or so for a year, then regularly thereafter, to monitor this cancer and watch for new ones.
LEARNING POINTS
• Deep-shave biopsy (sometimes called saucerization) is an appropriate technique for possible melanoma.
• About 75% to 80% of all melanomas are superficial, spreading types, (essentially flat), while 10% or so have only a vertical phase of growth (ie, present as a nodule or mass).
• Survival rates for melanoma are closely tied to tumor thickness, which is most commonly measured (by the pathologist) in millimeters; this staging system is called the Breslow scale. The older system of staging melanoma by the anatomical depth (called the Clark’s level I-V) has fallen into disuse.
• Underreaction to the diagnosis of melanoma (la belle indifference) can be as problematic as overreaction. Consistent monitoring of patients for compliance is often necessary.
CASE
A 34-year-old woman presents to her primary care provider to “have her moles checked.” She is motivated by a family history of sun-caused skin cancers, as well as her own history of multiple sunburns as a child.
Noting the patient’s fair, freckled skin, red hair, and blue eyes, the primary care provider agrees with the patient’s assessment of her risk. One lesion stands out from the rest: a 1.5-cm dark, irregularly bordered, and pigmented maculopapular lesion on the patient’s right triceps. The primary care provider arranges for a timely referral to dermatology.
The dermatology clinicians know immediately that the triceps lesion is highly suspicious, and tell the patient so while setting up for biopsy. Before that procedure is carried out, a careful examination of all exposed skin is conducted. The patient’s fair, sun-damaged skin is again noted, but no other suspicious lesions are found. There are no palpable nodes detected on her right axilla.
Under local anesthesia (1% lidocaine with epinephrine), the right triceps lesion is removed by deep saucerization (through the deep dermis into the upper adipose layer) using a double-edged razor. Minor bleeding is controlled with light cautery.
The pathology report, received three days later, confirms the malignant nature of the lesion, with a diagnosis of invasive melanoma (nodular, with no horizontal growth phase), measuring 1.7 mm. Fortunately, no other ominous signs—such as a high mitotic rate or vascular invasion—are reported.
The patient is immediately contacted by phone and notified of the results. The potential danger of this diagnosis is reiterated, along with information regarding the next steps in the process. These include consultation with a surgeon for consideration of re-excision and possible lymph node dissection. Evaluation by an oncologist will likely follow.
The patient’s response to this news is puzzling, to say the least. Though she appears to understand what she is being told, she sounds blissfully unconcerned, saying she is “not that worried” and is sure she will “be just fine.”
DISCUSSION
There are patients newly diagnosed with melanoma who overreact. I’ve had patients hop on the next plane to the Mayo Clinic, or, as in one notable case, to Tijuana, which, as we all know, is the home of such questionable practices as coffee-ground enemas and chemically modified amygdalin.
But then there are melanoma patients who go to the other extreme, making us wonder if they really understand the potential seriousness of the situation. It’s not that we want to see any particular “angst” as a reaction, but an appropriate indication or two is reassuring as feedback to the announcement. Questions— “What does this mean?” “What’s going to happen now?” “How serious is this?”— are good to hear in this regard. The answers allow us to convey the sense of where the patient stands, both for the present and in the long term, and help us to get a sense of how well the patient perceives the situation.
This patient had no questions, at all, as if she was totally unconcerned. That concerned me. It left me with a number of questions: “Does she really understand what’s going on?” “Will she follow our instructions and see the specialists we advise her to see?” Over the years, I’ve had several patients like this who went on their merry way, doing nothing we suggested. Some even survived.
This lack of appropriate reaction has been termed la belle indifference. It’s a way of pretending nothing is happening, and represents a way of showing one’s paralysis to others by manipulating their judgment through an attitude of indifference. One doesn’t want to frighten these patients (“Don’t you know this could be fatal?”), so what I do is keep close tabs on them—calling them regularly, making sure they’re following our advice, and documenting our calls and the patient’s responses. When family members can be enlisted to help, so much the better.
So far, this patient is complying with our advice, but it’s early in the process yet. We’ll see. Our job—and her ordeal—is far from done.
Any melanoma over 1 mm in thickness (based on the Breslow scale) is associated with an uncertain prognosis, and nodular melanomas are associated with a relatively poor prognosis. Besides re-excision (probably with 1-cm margins), this patient will probably be a candidate for elective lymph node dissection in the right axilla. PET scans, blood tests, and a visit to the oncologist will most likely follow. The surgeon usually acts as decision-maker in terms of what the patient needs and in what sequence.
Even if she survives all that, this patient will still need to see us every three months or so for a year, then regularly thereafter, to monitor this cancer and watch for new ones.
LEARNING POINTS
• Deep-shave biopsy (sometimes called saucerization) is an appropriate technique for possible melanoma.
• About 75% to 80% of all melanomas are superficial, spreading types, (essentially flat), while 10% or so have only a vertical phase of growth (ie, present as a nodule or mass).
• Survival rates for melanoma are closely tied to tumor thickness, which is most commonly measured (by the pathologist) in millimeters; this staging system is called the Breslow scale. The older system of staging melanoma by the anatomical depth (called the Clark’s level I-V) has fallen into disuse.
• Underreaction to the diagnosis of melanoma (la belle indifference) can be as problematic as overreaction. Consistent monitoring of patients for compliance is often necessary.
Skin is skin, no matter the location
HISTORY
A 26-year-old man presents with a lesion on his penis that has existed for many years—at least 10—without significant change and with no attendant symptoms. His new girlfriend, having heard that penile lesions can be dangerous, or even represent contagious disease, insisted that he seek medical evaluation.
He first went to his primary care provider (PCP), who admitted he had no idea what the lesion was; however, he agreed that the longstanding, constant nature of it was reassuring. The PCP advised the patient to consult a urologist. However, the staff at the urology office he contacted advised the patient to consult dermatology instead.
In the dermatology office, a thorough history is taken, including a sexual history that is negative for high-risk factors for HIV or other STDs. Aside from his lesion, the patient has no health-related complaints or causes for concern.
EXAMINATION
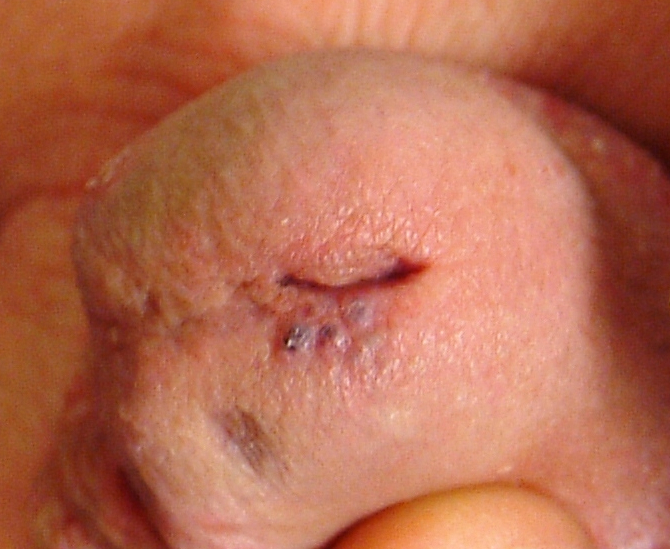
The lesion is actually composed of four grouped purple papules, each about 1 to 1.5 mm in diameter, in aggregate measuring about 8 mm. It is located within 6 mm of the urethral meatus, on the penile glans. The papules are soft and compressible, but barely palpable. Looking elsewhere, no other lesions are seen on the genitals or elsewhere on the body. Notably, the patient is circumcised.
DISCUSSION
I’ve seen these lesions in this exact location several times over a 25-year period. The first was in the early HIV era, and produced enough concern that a biopsy was indicated to rule out things such as Kaposi’s sarcoma or bacillary angiomatosis. What that lesion proved to be is almost certainly what this patient has: angiokeratoma of Fordyce, a totally benign lesion.
These types of angiokeratomas are ectatic, thin-walled vessels in the superficial dermis, with overlying epidermal hyperplasia that forms secondary to normal friction. When they are seen on the scrotum, vulva, or penis, they are usually referred to as angiokeratoma of Fordyce, a type of localized angiokeratoma, other types of which can appear on the legs or hands.
These totally benign lesions must be distinguished from generalized types of angiokeratomata, such as those seen in Fabry disease (angiokeratoma corporis diffusum), an inheritable metabolic disorder. With our patient’s history, his lesion was clearly benign.
Had his lesion been new or changing in any substantive way, additional testing, including a biopsy, might have been necessary to rule out entities such as squamous cell carcinoma (which is almost unknown in circumcised patients), condyloma, melanoma, the aforementioned Kaposi’s sarcoma, or even angiosarcoma.
This case highlights several useful points:
(1) Odd skin lesions need to be sent to the “odd skin lesion” specialist, also known as a dermatology provider. Just because the lesion is on the penis does not mean the patient needs to see a urologist. By the same token, neither does a patient with an odd skin lesion on a foot need to see a podiatrist.
(2) It pays to be a “collector” of anatomic differentials, as in “What lesions might I expect to see on (a given area of the body)?” This concept is valid for any area of the body, and certainly no less true for the penis.
(3) It’s been said that being an effective dermatology provider is as much about “sales” as it is about being an astute diagnostician. In other words, as this case demonstrates, it’s not enough for the provider to be convinced of the identity of the lesion—he must be able to “sell” the concept to a potentially skeptical patient. And in order to do that, the first person he needs to convince is himself.
TAKE-AWAY LEARNING POINTS
• Referral to dermatology is the correct step for any visible lesion or condition, regardless of location—the only exception being internal lesions.
• Just because a lesion is on the penis doesn’t mean it needs to be referred to urology. Skin is skin, irrespective of location.
• It’s not enough to “know” what a given lesion is. Knowing what it isn’t is just as important. And knowing what it means (or doesn’t mean) is often the most important thing of all.
• Anatomic differentials—what types of lesions/conditions are commonly seen in a given anatomic location—are often extremely useful.
HISTORY
A 26-year-old man presents with a lesion on his penis that has existed for many years—at least 10—without significant change and with no attendant symptoms. His new girlfriend, having heard that penile lesions can be dangerous, or even represent contagious disease, insisted that he seek medical evaluation.
He first went to his primary care provider (PCP), who admitted he had no idea what the lesion was; however, he agreed that the longstanding, constant nature of it was reassuring. The PCP advised the patient to consult a urologist. However, the staff at the urology office he contacted advised the patient to consult dermatology instead.
In the dermatology office, a thorough history is taken, including a sexual history that is negative for high-risk factors for HIV or other STDs. Aside from his lesion, the patient has no health-related complaints or causes for concern.
EXAMINATION
The lesion is actually composed of four grouped purple papules, each about 1 to 1.5 mm in diameter, in aggregate measuring about 8 mm. It is located within 6 mm of the urethral meatus, on the penile glans. The papules are soft and compressible, but barely palpable. Looking elsewhere, no other lesions are seen on the genitals or elsewhere on the body. Notably, the patient is circumcised.
DISCUSSION
I’ve seen these lesions in this exact location several times over a 25-year period. The first was in the early HIV era, and produced enough concern that a biopsy was indicated to rule out things such as Kaposi’s sarcoma or bacillary angiomatosis. What that lesion proved to be is almost certainly what this patient has: angiokeratoma of Fordyce, a totally benign lesion.
These types of angiokeratomas are ectatic, thin-walled vessels in the superficial dermis, with overlying epidermal hyperplasia that forms secondary to normal friction. When they are seen on the scrotum, vulva, or penis, they are usually referred to as angiokeratoma of Fordyce, a type of localized angiokeratoma, other types of which can appear on the legs or hands.
These totally benign lesions must be distinguished from generalized types of angiokeratomata, such as those seen in Fabry disease (angiokeratoma corporis diffusum), an inheritable metabolic disorder. With our patient’s history, his lesion was clearly benign.
Had his lesion been new or changing in any substantive way, additional testing, including a biopsy, might have been necessary to rule out entities such as squamous cell carcinoma (which is almost unknown in circumcised patients), condyloma, melanoma, the aforementioned Kaposi’s sarcoma, or even angiosarcoma.
This case highlights several useful points:
(1) Odd skin lesions need to be sent to the “odd skin lesion” specialist, also known as a dermatology provider. Just because the lesion is on the penis does not mean the patient needs to see a urologist. By the same token, neither does a patient with an odd skin lesion on a foot need to see a podiatrist.
(2) It pays to be a “collector” of anatomic differentials, as in “What lesions might I expect to see on (a given area of the body)?” This concept is valid for any area of the body, and certainly no less true for the penis.
(3) It’s been said that being an effective dermatology provider is as much about “sales” as it is about being an astute diagnostician. In other words, as this case demonstrates, it’s not enough for the provider to be convinced of the identity of the lesion—he must be able to “sell” the concept to a potentially skeptical patient. And in order to do that, the first person he needs to convince is himself.
TAKE-AWAY LEARNING POINTS
• Referral to dermatology is the correct step for any visible lesion or condition, regardless of location—the only exception being internal lesions.
• Just because a lesion is on the penis doesn’t mean it needs to be referred to urology. Skin is skin, irrespective of location.
• It’s not enough to “know” what a given lesion is. Knowing what it isn’t is just as important. And knowing what it means (or doesn’t mean) is often the most important thing of all.
• Anatomic differentials—what types of lesions/conditions are commonly seen in a given anatomic location—are often extremely useful.
HISTORY
A 26-year-old man presents with a lesion on his penis that has existed for many years—at least 10—without significant change and with no attendant symptoms. His new girlfriend, having heard that penile lesions can be dangerous, or even represent contagious disease, insisted that he seek medical evaluation.
He first went to his primary care provider (PCP), who admitted he had no idea what the lesion was; however, he agreed that the longstanding, constant nature of it was reassuring. The PCP advised the patient to consult a urologist. However, the staff at the urology office he contacted advised the patient to consult dermatology instead.
In the dermatology office, a thorough history is taken, including a sexual history that is negative for high-risk factors for HIV or other STDs. Aside from his lesion, the patient has no health-related complaints or causes for concern.
EXAMINATION
The lesion is actually composed of four grouped purple papules, each about 1 to 1.5 mm in diameter, in aggregate measuring about 8 mm. It is located within 6 mm of the urethral meatus, on the penile glans. The papules are soft and compressible, but barely palpable. Looking elsewhere, no other lesions are seen on the genitals or elsewhere on the body. Notably, the patient is circumcised.
DISCUSSION
I’ve seen these lesions in this exact location several times over a 25-year period. The first was in the early HIV era, and produced enough concern that a biopsy was indicated to rule out things such as Kaposi’s sarcoma or bacillary angiomatosis. What that lesion proved to be is almost certainly what this patient has: angiokeratoma of Fordyce, a totally benign lesion.
These types of angiokeratomas are ectatic, thin-walled vessels in the superficial dermis, with overlying epidermal hyperplasia that forms secondary to normal friction. When they are seen on the scrotum, vulva, or penis, they are usually referred to as angiokeratoma of Fordyce, a type of localized angiokeratoma, other types of which can appear on the legs or hands.
These totally benign lesions must be distinguished from generalized types of angiokeratomata, such as those seen in Fabry disease (angiokeratoma corporis diffusum), an inheritable metabolic disorder. With our patient’s history, his lesion was clearly benign.
Had his lesion been new or changing in any substantive way, additional testing, including a biopsy, might have been necessary to rule out entities such as squamous cell carcinoma (which is almost unknown in circumcised patients), condyloma, melanoma, the aforementioned Kaposi’s sarcoma, or even angiosarcoma.
This case highlights several useful points:
(1) Odd skin lesions need to be sent to the “odd skin lesion” specialist, also known as a dermatology provider. Just because the lesion is on the penis does not mean the patient needs to see a urologist. By the same token, neither does a patient with an odd skin lesion on a foot need to see a podiatrist.
(2) It pays to be a “collector” of anatomic differentials, as in “What lesions might I expect to see on (a given area of the body)?” This concept is valid for any area of the body, and certainly no less true for the penis.
(3) It’s been said that being an effective dermatology provider is as much about “sales” as it is about being an astute diagnostician. In other words, as this case demonstrates, it’s not enough for the provider to be convinced of the identity of the lesion—he must be able to “sell” the concept to a potentially skeptical patient. And in order to do that, the first person he needs to convince is himself.
TAKE-AWAY LEARNING POINTS
• Referral to dermatology is the correct step for any visible lesion or condition, regardless of location—the only exception being internal lesions.
• Just because a lesion is on the penis doesn’t mean it needs to be referred to urology. Skin is skin, irrespective of location.
• It’s not enough to “know” what a given lesion is. Knowing what it isn’t is just as important. And knowing what it means (or doesn’t mean) is often the most important thing of all.
• Anatomic differentials—what types of lesions/conditions are commonly seen in a given anatomic location—are often extremely useful.
2013;23(6):W5
A little girl with big skin problems
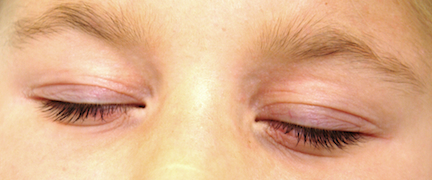
HISTORY
A 6-year-old girl is brought to dermatology for evaluation of several problems: “dry skin” present since birth, as well as new “bumps” that her family has noted recently. There are additional questions about her skin, mostly revolving around its sensitivity (illustrated by overreaction to mosquito bites) and whether there is a connection to food allergy.
She has seen an allergist, who ordered testing that revealed a sensitivity to airborne allergens (eg, dust, pollen, mold). However, the allergist assured the patient and her family that none of these had any relation to her skin complaints.
The child’s two older siblings have similar dermatologic problems, and one also has asthma. All three had terrible diaper rashes as infants.
The parents, who were both allergy-prone as children, wonder if there is a connection between their children’s skin problems and stress. They have noticed a worsening of the itching and scratching with increased levels of anxiety or tension.
EXAMINATION
The child has extremely dry skin all over her body, but especially on her extremities. Her palms exhibit an excessive number of lines. The “bumps” on her skin are widely scattered and firm and average 1 to 1.5 mm in diameter. Several have umbilicated centers.
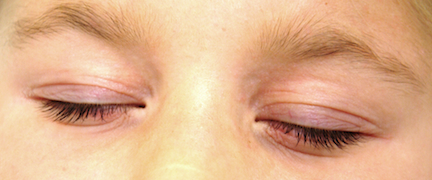
Her periorbital skin is dark, slightly scaly, and edematous, especially beneath the eyes, where extra lines have been created by the edema.
A faint but definite transverse white line is evident on her nose. Transverse ridging is seen on several fingernails.
DISCUSSION
This child and her family could serve as walking textbooks on an extremely common diagnosis: atopic dermatitis (AD), which involves a constellation of skin issues combined with an allergic constitution.
Major diagnostic criteria include a low threshold for pruritus, a personal and/or family history of eczema and atopy, and chronic or chronically relapsing eczematous dermatitis occurring in characteristic distributive patterns (the face and extensor surfaces on children; flexural surfaces on older children and adults).
Minor diagnostic criteria number at least 23, and include several of our patient’s signs: Dennie-Morgan folds, dry skin (xerosis), onset of problems early in life, susceptibility to skin infections such as molluscum, periorbital darkening (so-called allergic shiners), hyperlinearity of the palms, and the transverse nasal crease between the upper two-thirds and lower one-third of her nose, created by years of habitual upward rubbing. The observation by her parents that stress exacerbates the problem is yet another corroborative finding.
It’s important to note that in families like this one, not every child experiences the full measure of allergic and cutaneous problems. It’s not at all unusual, for example, to see one child with dry, sensitive skin and no obvious allergic phenomena, while a sibling might have minimal skin issues but major problems with asthma and seasonal allergies. It’s no wonder families are often quite confused, particularly when they are being told contradictory things by well-meaning medical providers and family members.
It’s critical for us, as medical providers, to have an accurate overview of how utterly common atopic dermatitis is, along with its myriad manifestations. This problem already affects at least 15% of the population to one degree or another, and its incidence is growing rapidly worldwide, primarily among the relatively affluent.
Much research has been done regarding the genetic and physiologic bases of AD, but those issues remain far from settled. What we do know is that AD patients have two basic problems: First, their skin is too thin and dry to serve as an effective barrier. Second, they have a dysfunctional and overreactive immune response to a multitude of allergens that present minor issues to the rest of us. One theory holds that the thin skin is what allows antigens to penetrate and set off the dysfunctional immune response.
In any case, it’s important to be able to recognize this common condition, and to be able to educate patients and parents. The inherited nature of the problem must be stressed, as the manifestations will likely continue (in one form or another) despite any treatment that is attempted. These are key pieces of information, without which patients and parents worry needlessly about the wrong things (often, food is blamed, though it is almost never the cause), to the neglect of potentially effective measures that could be taken.
TAKE-HOME LEARNING POINTS
• Dennie-Morgan folds represent one of many minor diagnostic criteria for atopic dermatitis (AD).
• It is difficult to overstate the pervasive nature of AD, both because it affects so many people (at least 15% of the population) and because it has a variety of manifestations (eg, dry and/or sensitive skin, eczema, hives, asthma).
• The tendency to develop AD is inherited, but it is frequently (and erroneously) blamed on food allergies, too-frequent bathing, or laundry detergent.
• Dry winter weather, long hot showers, and use of colored/perfumed soaps are common triggers for eczema.
• Emotional stress is a major trigger for eczema flares.

HISTORY
A 6-year-old girl is brought to dermatology for evaluation of several problems: “dry skin” present since birth, as well as new “bumps” that her family has noted recently. There are additional questions about her skin, mostly revolving around its sensitivity (illustrated by overreaction to mosquito bites) and whether there is a connection to food allergy.
She has seen an allergist, who ordered testing that revealed a sensitivity to airborne allergens (eg, dust, pollen, mold). However, the allergist assured the patient and her family that none of these had any relation to her skin complaints.
The child’s two older siblings have similar dermatologic problems, and one also has asthma. All three had terrible diaper rashes as infants.
The parents, who were both allergy-prone as children, wonder if there is a connection between their children’s skin problems and stress. They have noticed a worsening of the itching and scratching with increased levels of anxiety or tension.
EXAMINATION
The child has extremely dry skin all over her body, but especially on her extremities. Her palms exhibit an excessive number of lines. The “bumps” on her skin are widely scattered and firm and average 1 to 1.5 mm in diameter. Several have umbilicated centers.
Her periorbital skin is dark, slightly scaly, and edematous, especially beneath the eyes, where extra lines have been created by the edema.
A faint but definite transverse white line is evident on her nose. Transverse ridging is seen on several fingernails.
DISCUSSION
This child and her family could serve as walking textbooks on an extremely common diagnosis: atopic dermatitis (AD), which involves a constellation of skin issues combined with an allergic constitution.
Major diagnostic criteria include a low threshold for pruritus, a personal and/or family history of eczema and atopy, and chronic or chronically relapsing eczematous dermatitis occurring in characteristic distributive patterns (the face and extensor surfaces on children; flexural surfaces on older children and adults).
Minor diagnostic criteria number at least 23, and include several of our patient’s signs: Dennie-Morgan folds, dry skin (xerosis), onset of problems early in life, susceptibility to skin infections such as molluscum, periorbital darkening (so-called allergic shiners), hyperlinearity of the palms, and the transverse nasal crease between the upper two-thirds and lower one-third of her nose, created by years of habitual upward rubbing. The observation by her parents that stress exacerbates the problem is yet another corroborative finding.
It’s important to note that in families like this one, not every child experiences the full measure of allergic and cutaneous problems. It’s not at all unusual, for example, to see one child with dry, sensitive skin and no obvious allergic phenomena, while a sibling might have minimal skin issues but major problems with asthma and seasonal allergies. It’s no wonder families are often quite confused, particularly when they are being told contradictory things by well-meaning medical providers and family members.
It’s critical for us, as medical providers, to have an accurate overview of how utterly common atopic dermatitis is, along with its myriad manifestations. This problem already affects at least 15% of the population to one degree or another, and its incidence is growing rapidly worldwide, primarily among the relatively affluent.
Much research has been done regarding the genetic and physiologic bases of AD, but those issues remain far from settled. What we do know is that AD patients have two basic problems: First, their skin is too thin and dry to serve as an effective barrier. Second, they have a dysfunctional and overreactive immune response to a multitude of allergens that present minor issues to the rest of us. One theory holds that the thin skin is what allows antigens to penetrate and set off the dysfunctional immune response.
In any case, it’s important to be able to recognize this common condition, and to be able to educate patients and parents. The inherited nature of the problem must be stressed, as the manifestations will likely continue (in one form or another) despite any treatment that is attempted. These are key pieces of information, without which patients and parents worry needlessly about the wrong things (often, food is blamed, though it is almost never the cause), to the neglect of potentially effective measures that could be taken.
TAKE-HOME LEARNING POINTS
• Dennie-Morgan folds represent one of many minor diagnostic criteria for atopic dermatitis (AD).
• It is difficult to overstate the pervasive nature of AD, both because it affects so many people (at least 15% of the population) and because it has a variety of manifestations (eg, dry and/or sensitive skin, eczema, hives, asthma).
• The tendency to develop AD is inherited, but it is frequently (and erroneously) blamed on food allergies, too-frequent bathing, or laundry detergent.
• Dry winter weather, long hot showers, and use of colored/perfumed soaps are common triggers for eczema.
• Emotional stress is a major trigger for eczema flares.

HISTORY
A 6-year-old girl is brought to dermatology for evaluation of several problems: “dry skin” present since birth, as well as new “bumps” that her family has noted recently. There are additional questions about her skin, mostly revolving around its sensitivity (illustrated by overreaction to mosquito bites) and whether there is a connection to food allergy.
She has seen an allergist, who ordered testing that revealed a sensitivity to airborne allergens (eg, dust, pollen, mold). However, the allergist assured the patient and her family that none of these had any relation to her skin complaints.
The child’s two older siblings have similar dermatologic problems, and one also has asthma. All three had terrible diaper rashes as infants.
The parents, who were both allergy-prone as children, wonder if there is a connection between their children’s skin problems and stress. They have noticed a worsening of the itching and scratching with increased levels of anxiety or tension.
EXAMINATION
The child has extremely dry skin all over her body, but especially on her extremities. Her palms exhibit an excessive number of lines. The “bumps” on her skin are widely scattered and firm and average 1 to 1.5 mm in diameter. Several have umbilicated centers.
Her periorbital skin is dark, slightly scaly, and edematous, especially beneath the eyes, where extra lines have been created by the edema.
A faint but definite transverse white line is evident on her nose. Transverse ridging is seen on several fingernails.
DISCUSSION
This child and her family could serve as walking textbooks on an extremely common diagnosis: atopic dermatitis (AD), which involves a constellation of skin issues combined with an allergic constitution.
Major diagnostic criteria include a low threshold for pruritus, a personal and/or family history of eczema and atopy, and chronic or chronically relapsing eczematous dermatitis occurring in characteristic distributive patterns (the face and extensor surfaces on children; flexural surfaces on older children and adults).
Minor diagnostic criteria number at least 23, and include several of our patient’s signs: Dennie-Morgan folds, dry skin (xerosis), onset of problems early in life, susceptibility to skin infections such as molluscum, periorbital darkening (so-called allergic shiners), hyperlinearity of the palms, and the transverse nasal crease between the upper two-thirds and lower one-third of her nose, created by years of habitual upward rubbing. The observation by her parents that stress exacerbates the problem is yet another corroborative finding.
It’s important to note that in families like this one, not every child experiences the full measure of allergic and cutaneous problems. It’s not at all unusual, for example, to see one child with dry, sensitive skin and no obvious allergic phenomena, while a sibling might have minimal skin issues but major problems with asthma and seasonal allergies. It’s no wonder families are often quite confused, particularly when they are being told contradictory things by well-meaning medical providers and family members.
It’s critical for us, as medical providers, to have an accurate overview of how utterly common atopic dermatitis is, along with its myriad manifestations. This problem already affects at least 15% of the population to one degree or another, and its incidence is growing rapidly worldwide, primarily among the relatively affluent.
Much research has been done regarding the genetic and physiologic bases of AD, but those issues remain far from settled. What we do know is that AD patients have two basic problems: First, their skin is too thin and dry to serve as an effective barrier. Second, they have a dysfunctional and overreactive immune response to a multitude of allergens that present minor issues to the rest of us. One theory holds that the thin skin is what allows antigens to penetrate and set off the dysfunctional immune response.
In any case, it’s important to be able to recognize this common condition, and to be able to educate patients and parents. The inherited nature of the problem must be stressed, as the manifestations will likely continue (in one form or another) despite any treatment that is attempted. These are key pieces of information, without which patients and parents worry needlessly about the wrong things (often, food is blamed, though it is almost never the cause), to the neglect of potentially effective measures that could be taken.
TAKE-HOME LEARNING POINTS
• Dennie-Morgan folds represent one of many minor diagnostic criteria for atopic dermatitis (AD).
• It is difficult to overstate the pervasive nature of AD, both because it affects so many people (at least 15% of the population) and because it has a variety of manifestations (eg, dry and/or sensitive skin, eczema, hives, asthma).
• The tendency to develop AD is inherited, but it is frequently (and erroneously) blamed on food allergies, too-frequent bathing, or laundry detergent.
• Dry winter weather, long hot showers, and use of colored/perfumed soaps are common triggers for eczema.
• Emotional stress is a major trigger for eczema flares.
Let’s see you pull that out of my back!

HISTORY
This 50-year-old man first noticed a bulge in his low back several years ago. Every time he brings it to the attention of his medical providers, he is told it is, and will remain, benign and that the best thing he can do is leave it alone.
But it finally grows to a bothersome size, causing pain at times—such as when he leans against hard surfaces—and actually becoming visible under certain clothes. And so he seeks evaluation from a dermatology provider.
His health is otherwise excellent, with no family history of such lesions and no personal history of similar manifestations elsewhere on his body.
EXAMINATION
An ill-defined subcutaneous mass is felt in the L4 area of the patient’s mid-low back; no overlying skin changes can be seen or felt. The mass is rubbery, with a smooth surface, and not at all tender to palpation. The patient’s skin is otherwise free of such lesions.
PROCEDURE
After a thorough discussion of the options available to this patient, the decision is made to excise the lesion. As is customary, this conversation includes disclosure of the anticipated procedure (excision), the indications for the procedure, alternatives, and risks involved in removal.
Under local anesthesia (1% lidocaine with epinephrine) and sterile conditions, a horizontal incision is made. This effectively creates an elliptical window through which the lesion can be visualized, freed by blunt dissection, and then removed in one large piece, which turns out to be in excess of 6 cm—larger than anticipated.
The lesion is consistent in every way with the anticipated diagnosis of lipoma, including the presence of the expected “capsule” (extremely thin web of connective tissue) surrounding the tumor. The defect’s size and depth require three layers to close it, leaving the patient with a 5-cm transverse wound. As is always the case with excised lesions, this one is sent for pathologic examination, which confirms the diagnosis.
DISCUSSION
Obviously, the presentation and history of this lesion were quite consistent with an extremely common diagnosis: lipoma. The other main item in the differential is epidermal cyst, although additional diagnostic possibilities include liposarcoma, glomus tumor, and leiomyoma.
Lipomas are the most common tumor of mesenchymal origin. They are composed of mature fat cells, but differ from normal fat by the demonstration of increased levels of lipoprotein lipase and by the presence of larger numbers of precursor cells.
Lipomas can appear almost anywhere—including internal organs and even the spinal cord—though most commonly, by far, in subcutaneous locations on the trunk and extremities. As in this case, they come to the patient’s attention because of growth or discomfort (or both).
Occasionally, lipomas, even after several years, become painful, and on removal may prove to be a benign innervated variant called angiolipoma. While many other subtypes exist, the vast majority is completely benign. Obviously, rapid growth or other change could signal malignant transformation.
Lipomas are often seen in the context of an inherited condition in which multiple asymptomatic lipomas appear in the third decade of life, usually in forearms and thighs. Termed familial benign lipomatosis, its mode of inheritance is autosomal dominance.
Protease inhibitors given for HIV can cause lipodystrophy of several types, including the appearance of lipomas, as well as the well-known buccal atrophy.
Had this patient’s lipoma undergone rapid growth or had it been accompanied by other significant findings (the so-called fawn’s tail, a tuft of hair, unusual tags, hemangiomas and others in the sacral region), it might well have required pre-operative imaging to rule out spinal dysraphism or other occult embryonic malformation.
This patient made a rapid and uneventful recovery from his surgery. Complications could have included hematoma formation, infection, bleeding, or dysfunctional scarring.
TAKE-HOME LEARNING POINTS
• Lipomas are extremely common and are often seen in the context of an inherited trait called benign familial lipomatosis.
• Epidermal cysts and lipomas can present in similar fashion, though the cysts tend to be more firm and have an overlying punctum. Lipomas tend to be more rubbery and have no overlying punctum.
• Lipomas can be found almost anywhere in the body, including internally.
• Lipomas frequently prove to be larger on removal than would be expected from outward appearance.
• With large lipomas in certain locations—such as in the medial epicondylar area, deep in the forehead, in the scalp, or in the upper trapezius area, for example—consider referral to a surgeon.

HISTORY
This 50-year-old man first noticed a bulge in his low back several years ago. Every time he brings it to the attention of his medical providers, he is told it is, and will remain, benign and that the best thing he can do is leave it alone.
But it finally grows to a bothersome size, causing pain at times—such as when he leans against hard surfaces—and actually becoming visible under certain clothes. And so he seeks evaluation from a dermatology provider.
His health is otherwise excellent, with no family history of such lesions and no personal history of similar manifestations elsewhere on his body.
EXAMINATION
An ill-defined subcutaneous mass is felt in the L4 area of the patient’s mid-low back; no overlying skin changes can be seen or felt. The mass is rubbery, with a smooth surface, and not at all tender to palpation. The patient’s skin is otherwise free of such lesions.
PROCEDURE
After a thorough discussion of the options available to this patient, the decision is made to excise the lesion. As is customary, this conversation includes disclosure of the anticipated procedure (excision), the indications for the procedure, alternatives, and risks involved in removal.
Under local anesthesia (1% lidocaine with epinephrine) and sterile conditions, a horizontal incision is made. This effectively creates an elliptical window through which the lesion can be visualized, freed by blunt dissection, and then removed in one large piece, which turns out to be in excess of 6 cm—larger than anticipated.
The lesion is consistent in every way with the anticipated diagnosis of lipoma, including the presence of the expected “capsule” (extremely thin web of connective tissue) surrounding the tumor. The defect’s size and depth require three layers to close it, leaving the patient with a 5-cm transverse wound. As is always the case with excised lesions, this one is sent for pathologic examination, which confirms the diagnosis.
DISCUSSION
Obviously, the presentation and history of this lesion were quite consistent with an extremely common diagnosis: lipoma. The other main item in the differential is epidermal cyst, although additional diagnostic possibilities include liposarcoma, glomus tumor, and leiomyoma.
Lipomas are the most common tumor of mesenchymal origin. They are composed of mature fat cells, but differ from normal fat by the demonstration of increased levels of lipoprotein lipase and by the presence of larger numbers of precursor cells.
Lipomas can appear almost anywhere—including internal organs and even the spinal cord—though most commonly, by far, in subcutaneous locations on the trunk and extremities. As in this case, they come to the patient’s attention because of growth or discomfort (or both).
Occasionally, lipomas, even after several years, become painful, and on removal may prove to be a benign innervated variant called angiolipoma. While many other subtypes exist, the vast majority is completely benign. Obviously, rapid growth or other change could signal malignant transformation.
Lipomas are often seen in the context of an inherited condition in which multiple asymptomatic lipomas appear in the third decade of life, usually in forearms and thighs. Termed familial benign lipomatosis, its mode of inheritance is autosomal dominance.
Protease inhibitors given for HIV can cause lipodystrophy of several types, including the appearance of lipomas, as well as the well-known buccal atrophy.
Had this patient’s lipoma undergone rapid growth or had it been accompanied by other significant findings (the so-called fawn’s tail, a tuft of hair, unusual tags, hemangiomas and others in the sacral region), it might well have required pre-operative imaging to rule out spinal dysraphism or other occult embryonic malformation.
This patient made a rapid and uneventful recovery from his surgery. Complications could have included hematoma formation, infection, bleeding, or dysfunctional scarring.
TAKE-HOME LEARNING POINTS
• Lipomas are extremely common and are often seen in the context of an inherited trait called benign familial lipomatosis.
• Epidermal cysts and lipomas can present in similar fashion, though the cysts tend to be more firm and have an overlying punctum. Lipomas tend to be more rubbery and have no overlying punctum.
• Lipomas can be found almost anywhere in the body, including internally.
• Lipomas frequently prove to be larger on removal than would be expected from outward appearance.
• With large lipomas in certain locations—such as in the medial epicondylar area, deep in the forehead, in the scalp, or in the upper trapezius area, for example—consider referral to a surgeon.

HISTORY
This 50-year-old man first noticed a bulge in his low back several years ago. Every time he brings it to the attention of his medical providers, he is told it is, and will remain, benign and that the best thing he can do is leave it alone.
But it finally grows to a bothersome size, causing pain at times—such as when he leans against hard surfaces—and actually becoming visible under certain clothes. And so he seeks evaluation from a dermatology provider.
His health is otherwise excellent, with no family history of such lesions and no personal history of similar manifestations elsewhere on his body.
EXAMINATION
An ill-defined subcutaneous mass is felt in the L4 area of the patient’s mid-low back; no overlying skin changes can be seen or felt. The mass is rubbery, with a smooth surface, and not at all tender to palpation. The patient’s skin is otherwise free of such lesions.
PROCEDURE
After a thorough discussion of the options available to this patient, the decision is made to excise the lesion. As is customary, this conversation includes disclosure of the anticipated procedure (excision), the indications for the procedure, alternatives, and risks involved in removal.
Under local anesthesia (1% lidocaine with epinephrine) and sterile conditions, a horizontal incision is made. This effectively creates an elliptical window through which the lesion can be visualized, freed by blunt dissection, and then removed in one large piece, which turns out to be in excess of 6 cm—larger than anticipated.
The lesion is consistent in every way with the anticipated diagnosis of lipoma, including the presence of the expected “capsule” (extremely thin web of connective tissue) surrounding the tumor. The defect’s size and depth require three layers to close it, leaving the patient with a 5-cm transverse wound. As is always the case with excised lesions, this one is sent for pathologic examination, which confirms the diagnosis.
DISCUSSION
Obviously, the presentation and history of this lesion were quite consistent with an extremely common diagnosis: lipoma. The other main item in the differential is epidermal cyst, although additional diagnostic possibilities include liposarcoma, glomus tumor, and leiomyoma.
Lipomas are the most common tumor of mesenchymal origin. They are composed of mature fat cells, but differ from normal fat by the demonstration of increased levels of lipoprotein lipase and by the presence of larger numbers of precursor cells.
Lipomas can appear almost anywhere—including internal organs and even the spinal cord—though most commonly, by far, in subcutaneous locations on the trunk and extremities. As in this case, they come to the patient’s attention because of growth or discomfort (or both).
Occasionally, lipomas, even after several years, become painful, and on removal may prove to be a benign innervated variant called angiolipoma. While many other subtypes exist, the vast majority is completely benign. Obviously, rapid growth or other change could signal malignant transformation.
Lipomas are often seen in the context of an inherited condition in which multiple asymptomatic lipomas appear in the third decade of life, usually in forearms and thighs. Termed familial benign lipomatosis, its mode of inheritance is autosomal dominance.
Protease inhibitors given for HIV can cause lipodystrophy of several types, including the appearance of lipomas, as well as the well-known buccal atrophy.
Had this patient’s lipoma undergone rapid growth or had it been accompanied by other significant findings (the so-called fawn’s tail, a tuft of hair, unusual tags, hemangiomas and others in the sacral region), it might well have required pre-operative imaging to rule out spinal dysraphism or other occult embryonic malformation.
This patient made a rapid and uneventful recovery from his surgery. Complications could have included hematoma formation, infection, bleeding, or dysfunctional scarring.
TAKE-HOME LEARNING POINTS
• Lipomas are extremely common and are often seen in the context of an inherited trait called benign familial lipomatosis.
• Epidermal cysts and lipomas can present in similar fashion, though the cysts tend to be more firm and have an overlying punctum. Lipomas tend to be more rubbery and have no overlying punctum.
• Lipomas can be found almost anywhere in the body, including internally.
• Lipomas frequently prove to be larger on removal than would be expected from outward appearance.
• With large lipomas in certain locations—such as in the medial epicondylar area, deep in the forehead, in the scalp, or in the upper trapezius area, for example—consider referral to a surgeon.
Ongoing agony of the feet
HISTORY
This 66-year-old woman has had a very itchy rash on her left foot for several months. She has tried applying a number of different OTC and prescription medications—including betamethasone dipropionate cream and econazole cream—without successful resolution of the problem.
She denies having any other skin problems, and there is no relevant family history. The patient is retired and lives alone with her cat. Medical history is remarkable for rheumatoid arthritis, for which she takes methotrexate.
EXAMINATION
The dorsum of her left foot is covered with a sharply demarcated, papulosquamous red rash. Interestingly, the interdigital areas are spared, as are the sole and the entire right foot. The patient’s elbows, knees, and scalp exhibit no significant changes. On further examination, a fine, slightly pink, powdery rash is noted on the sides of both feet (including the heels).
A KOH prep of the rim of the feet is positive for fungal elements, as is a similar microscopic examination of scrapings from the dorsum of the left foot. In fact, the fungal elements seen on the latter are so numerous and dense that they are initially difficult to see.
What is the diagnosis?
DISCUSSION
Rashes on the dorsum of the feet are almost never of fungal origin; rather, they usually represent contact (not eczematous) dermatitis. That’s partly because fungi typically require more heat and moisture than the dorsum can provide. Two things are needed to allow it to flourish in this unusual location: immune suppression and a source for the fungi.
Methotrexate and the prolonged use of a potent topical steroid were the likely culprits in terms of immune suppression. Both reduce the chemotactic response that these dermatophytes normally trigger, allowing them to multiply unchecked. But where did the fungi come from in the first place?
Traditionally, three kinds of tinea pedis have been described: the well-known interdigital type, typically affecting the space between the third and fourth or the fourth and fifth toes; the so-called inflammatory type, which presents acutely with highly pruritic vesicles and pustules on the plantar surface (most commonly on the instep); and the most common but least recognized of all, the moccasin type, which causes few if any symptoms and often flies under the patient’s radar. The rash it causes is faint and dry and covers the rim of the foot (sparing the toes).
But, given the right circumstances, it can serve as a reservoir for infection on the foot and leg. The resultant infection can be cured, but moccasin-variety tinea pedis is considered incurable, due to the ubiquitous nature of the organism and patient’s demonstrated susceptibility. The causative organism is almost always Trichophyton rubrum, by far the most common dermatophytic pathogen.
TREATMENT
Fortunately, T. rubrum responds well to oral terbinafine therapy, (250 mg/d). This patient was provided a month’s supply, along with topical miconazole to be applied twice daily until the rash clears. Use of the latter on the sides of the feet is also advised, for purposes of control.
TAKE-HOME-LEARNING-POINTS
• The performance of the KOH prep can be crucial in terms of establishing the correct diagnosis, but it also gives everyone involved confidence in the diagnosis and treatment. The only way to learn how to do KOH preps is to do them.
• Moccasin-variety tinea pedis is the most common but the least recognized of the three major types. Because it is chronic and asymptomatic, patients rarely know what it is, although it can serve as a reservoir for infection elsewhere.
• Fungal infections don’t just happen. Failure to establish a source can doom the patient to repeated episodes.
• Fungal infections (dermatophytosis) in odd places usually involve immune suppression, typically from the use of topical steroids and/or systemic immunosuppressants.
HISTORY
This 66-year-old woman has had a very itchy rash on her left foot for several months. She has tried applying a number of different OTC and prescription medications—including betamethasone dipropionate cream and econazole cream—without successful resolution of the problem.
She denies having any other skin problems, and there is no relevant family history. The patient is retired and lives alone with her cat. Medical history is remarkable for rheumatoid arthritis, for which she takes methotrexate.
EXAMINATION
The dorsum of her left foot is covered with a sharply demarcated, papulosquamous red rash. Interestingly, the interdigital areas are spared, as are the sole and the entire right foot. The patient’s elbows, knees, and scalp exhibit no significant changes. On further examination, a fine, slightly pink, powdery rash is noted on the sides of both feet (including the heels).
A KOH prep of the rim of the feet is positive for fungal elements, as is a similar microscopic examination of scrapings from the dorsum of the left foot. In fact, the fungal elements seen on the latter are so numerous and dense that they are initially difficult to see.
What is the diagnosis?
DISCUSSION
Rashes on the dorsum of the feet are almost never of fungal origin; rather, they usually represent contact (not eczematous) dermatitis. That’s partly because fungi typically require more heat and moisture than the dorsum can provide. Two things are needed to allow it to flourish in this unusual location: immune suppression and a source for the fungi.
Methotrexate and the prolonged use of a potent topical steroid were the likely culprits in terms of immune suppression. Both reduce the chemotactic response that these dermatophytes normally trigger, allowing them to multiply unchecked. But where did the fungi come from in the first place?
Traditionally, three kinds of tinea pedis have been described: the well-known interdigital type, typically affecting the space between the third and fourth or the fourth and fifth toes; the so-called inflammatory type, which presents acutely with highly pruritic vesicles and pustules on the plantar surface (most commonly on the instep); and the most common but least recognized of all, the moccasin type, which causes few if any symptoms and often flies under the patient’s radar. The rash it causes is faint and dry and covers the rim of the foot (sparing the toes).
But, given the right circumstances, it can serve as a reservoir for infection on the foot and leg. The resultant infection can be cured, but moccasin-variety tinea pedis is considered incurable, due to the ubiquitous nature of the organism and patient’s demonstrated susceptibility. The causative organism is almost always Trichophyton rubrum, by far the most common dermatophytic pathogen.
TREATMENT
Fortunately, T. rubrum responds well to oral terbinafine therapy, (250 mg/d). This patient was provided a month’s supply, along with topical miconazole to be applied twice daily until the rash clears. Use of the latter on the sides of the feet is also advised, for purposes of control.
TAKE-HOME-LEARNING-POINTS
• The performance of the KOH prep can be crucial in terms of establishing the correct diagnosis, but it also gives everyone involved confidence in the diagnosis and treatment. The only way to learn how to do KOH preps is to do them.
• Moccasin-variety tinea pedis is the most common but the least recognized of the three major types. Because it is chronic and asymptomatic, patients rarely know what it is, although it can serve as a reservoir for infection elsewhere.
• Fungal infections don’t just happen. Failure to establish a source can doom the patient to repeated episodes.
• Fungal infections (dermatophytosis) in odd places usually involve immune suppression, typically from the use of topical steroids and/or systemic immunosuppressants.
HISTORY
This 66-year-old woman has had a very itchy rash on her left foot for several months. She has tried applying a number of different OTC and prescription medications—including betamethasone dipropionate cream and econazole cream—without successful resolution of the problem.
She denies having any other skin problems, and there is no relevant family history. The patient is retired and lives alone with her cat. Medical history is remarkable for rheumatoid arthritis, for which she takes methotrexate.
EXAMINATION
The dorsum of her left foot is covered with a sharply demarcated, papulosquamous red rash. Interestingly, the interdigital areas are spared, as are the sole and the entire right foot. The patient’s elbows, knees, and scalp exhibit no significant changes. On further examination, a fine, slightly pink, powdery rash is noted on the sides of both feet (including the heels).
A KOH prep of the rim of the feet is positive for fungal elements, as is a similar microscopic examination of scrapings from the dorsum of the left foot. In fact, the fungal elements seen on the latter are so numerous and dense that they are initially difficult to see.
What is the diagnosis?
DISCUSSION
Rashes on the dorsum of the feet are almost never of fungal origin; rather, they usually represent contact (not eczematous) dermatitis. That’s partly because fungi typically require more heat and moisture than the dorsum can provide. Two things are needed to allow it to flourish in this unusual location: immune suppression and a source for the fungi.
Methotrexate and the prolonged use of a potent topical steroid were the likely culprits in terms of immune suppression. Both reduce the chemotactic response that these dermatophytes normally trigger, allowing them to multiply unchecked. But where did the fungi come from in the first place?
Traditionally, three kinds of tinea pedis have been described: the well-known interdigital type, typically affecting the space between the third and fourth or the fourth and fifth toes; the so-called inflammatory type, which presents acutely with highly pruritic vesicles and pustules on the plantar surface (most commonly on the instep); and the most common but least recognized of all, the moccasin type, which causes few if any symptoms and often flies under the patient’s radar. The rash it causes is faint and dry and covers the rim of the foot (sparing the toes).
But, given the right circumstances, it can serve as a reservoir for infection on the foot and leg. The resultant infection can be cured, but moccasin-variety tinea pedis is considered incurable, due to the ubiquitous nature of the organism and patient’s demonstrated susceptibility. The causative organism is almost always Trichophyton rubrum, by far the most common dermatophytic pathogen.
TREATMENT
Fortunately, T. rubrum responds well to oral terbinafine therapy, (250 mg/d). This patient was provided a month’s supply, along with topical miconazole to be applied twice daily until the rash clears. Use of the latter on the sides of the feet is also advised, for purposes of control.
TAKE-HOME-LEARNING-POINTS
• The performance of the KOH prep can be crucial in terms of establishing the correct diagnosis, but it also gives everyone involved confidence in the diagnosis and treatment. The only way to learn how to do KOH preps is to do them.
• Moccasin-variety tinea pedis is the most common but the least recognized of the three major types. Because it is chronic and asymptomatic, patients rarely know what it is, although it can serve as a reservoir for infection elsewhere.
• Fungal infections don’t just happen. Failure to establish a source can doom the patient to repeated episodes.
• Fungal infections (dermatophytosis) in odd places usually involve immune suppression, typically from the use of topical steroids and/or systemic immunosuppressants.
Surgical Removal of Cyst Yields Unsightly Result
HISTORY
This 24-year-old woman presents to dermatology for evaluation of excessive scarring on her chest. It developed slowly in a spot from which a cyst was surgically removed more than two years ago. In addition to being unsightly, the lesion is sometimes symptomatic: It often tingles and feels “tight.” Worst of all, it still seems to be growing.
She has never experienced anything like this—not even following her C-section several years ago. There is no family history of similar problems. The patient says she tans easily, holds a tan well, and rarely burns in the sun.
EXAMINATION
The lesion is clearly cicatricial, quite firm, and slightly pink. It has rounded edges and an exceptionally smooth surface. Located on the left sternal chest wall, the lesion has obliterated any sign of the original surgical scar, except for peripheral scars left by the sutures. The patient’s skin type is a strong IV/VI.
What is the diagnosis?
DISCUSSION
This is a classic case of keloid formation—a real problem since no good, permanent solution exists. In one form or another, this otherwise attractive woman will likely bear this lesion the rest of her life.
Not all excessive scars are keloids. When scarring is excessive, but the outline of the original wound can still be seen, the result is usually termed hypertrophic scarring. By definition, a true keloid, by its thickness, shape, and width, totally obscures the original insult and, unlike a hypertrophic scar, does not spontaneously involute. Viewed as a continuum, there is normal scarring, inappropriate scarring, and severe inappropriate scarring. Unlike the first two, the latter is almost always symptomatic (burning) and does not spontaneously resolve.
Two things could have alerted the surgeon to the possibility that a keloid would form: (1) location, since the chest, shoulders, ear lobes, and neck are especially prone to inappropriate scarring, and (2) skin type, because in general, the darker the skin is, the greater the tendency to form inappropriate scars. While keloids are commonly a postoperative complication, they are also not infrequently triggered by acne, cysts, or even chickenpox. Other high-tension areas prone to keloids are knees and ankles.
The best thing, obviously, would have been for the patient to avoid having the surgery. An alternative to surgical removal of her cyst might have been intralesional injection with triamcinolone solution (5 mg/cc). While this would have been unlikely to produce a cure, it almost certainly would have shrunk the cyst for months at a time.
When surgery in a high-risk area is necessary (eg, in the case of a skin cancer), the surgical margins can be injected with triamcinolone (2.5 mg/cc) at the time of suture removal and again a month postoperative, which will reduce but not eliminate the risk for keloid formation. Low-tension closure (making generous use of undermining and deep sutures to reduce tension on surface sutures) and proper wound care by the patient can help too.
Surgical removal of a keloid in such a location, in a high-risk patient, is an option. However, such procedures are usually left for the plastic surgeon to deal with. Weapons that can be brought to bear on these difficult lesions include excision, intralesional steroid injection, cryotherapy, ionizing radiation, and laser surgery.
This particular patient chose to have us inject her keloid with 1.5 cc of triamcinolone (20 mg/cc), using a 10-cc syringe and 30-gauge needle. (This was made from stock triamcinolone, which comes in a 40 mg/cc strength, mixed half-and-half with lidocaine 1%.) Had her keloid been so dense as to make injection impossible (which happens fairly often), I would have treated it with liquid nitrogen first (approximately 5 seconds), waited five minutes while the keloid softened, then injected it.
TAKE-HOME-LEARNING-POINTS
• The tendency to form keloids is in large part a function of location and skin type.
• Skin on chests, shoulders, earlobes, and necks is especially prone to keloid formation.
• The darker the patient’s skin, the greater the chance for keloid formation.
• The decision to perform elective surgery should be informed by a full review of the risks involved, including that for keloid formation.
• Alternatives to excision of cysts in high-risk areas include intralesional steroid injection (using their tendency to cause atrophy to advantage), cryotherapy, or benign neglect.
• Once a keloid has formed, no perfect remedy exists. However, several options can be considered: intralesional injection, excision followed by steroid injection of the wound margins, or referral to plastic surgery.
• Viewed as a continuum, scarring can either be normal, excessive (eg, hypertrophic scarring), or lesional (eg keloid). The latter totally obscures the original insult, fails to spontaneously involute, and is often symptomatic (burning).
HISTORY
This 24-year-old woman presents to dermatology for evaluation of excessive scarring on her chest. It developed slowly in a spot from which a cyst was surgically removed more than two years ago. In addition to being unsightly, the lesion is sometimes symptomatic: It often tingles and feels “tight.” Worst of all, it still seems to be growing.
She has never experienced anything like this—not even following her C-section several years ago. There is no family history of similar problems. The patient says she tans easily, holds a tan well, and rarely burns in the sun.
EXAMINATION
The lesion is clearly cicatricial, quite firm, and slightly pink. It has rounded edges and an exceptionally smooth surface. Located on the left sternal chest wall, the lesion has obliterated any sign of the original surgical scar, except for peripheral scars left by the sutures. The patient’s skin type is a strong IV/VI.
What is the diagnosis?
DISCUSSION
This is a classic case of keloid formation—a real problem since no good, permanent solution exists. In one form or another, this otherwise attractive woman will likely bear this lesion the rest of her life.
Not all excessive scars are keloids. When scarring is excessive, but the outline of the original wound can still be seen, the result is usually termed hypertrophic scarring. By definition, a true keloid, by its thickness, shape, and width, totally obscures the original insult and, unlike a hypertrophic scar, does not spontaneously involute. Viewed as a continuum, there is normal scarring, inappropriate scarring, and severe inappropriate scarring. Unlike the first two, the latter is almost always symptomatic (burning) and does not spontaneously resolve.
Two things could have alerted the surgeon to the possibility that a keloid would form: (1) location, since the chest, shoulders, ear lobes, and neck are especially prone to inappropriate scarring, and (2) skin type, because in general, the darker the skin is, the greater the tendency to form inappropriate scars. While keloids are commonly a postoperative complication, they are also not infrequently triggered by acne, cysts, or even chickenpox. Other high-tension areas prone to keloids are knees and ankles.
The best thing, obviously, would have been for the patient to avoid having the surgery. An alternative to surgical removal of her cyst might have been intralesional injection with triamcinolone solution (5 mg/cc). While this would have been unlikely to produce a cure, it almost certainly would have shrunk the cyst for months at a time.
When surgery in a high-risk area is necessary (eg, in the case of a skin cancer), the surgical margins can be injected with triamcinolone (2.5 mg/cc) at the time of suture removal and again a month postoperative, which will reduce but not eliminate the risk for keloid formation. Low-tension closure (making generous use of undermining and deep sutures to reduce tension on surface sutures) and proper wound care by the patient can help too.
Surgical removal of a keloid in such a location, in a high-risk patient, is an option. However, such procedures are usually left for the plastic surgeon to deal with. Weapons that can be brought to bear on these difficult lesions include excision, intralesional steroid injection, cryotherapy, ionizing radiation, and laser surgery.
This particular patient chose to have us inject her keloid with 1.5 cc of triamcinolone (20 mg/cc), using a 10-cc syringe and 30-gauge needle. (This was made from stock triamcinolone, which comes in a 40 mg/cc strength, mixed half-and-half with lidocaine 1%.) Had her keloid been so dense as to make injection impossible (which happens fairly often), I would have treated it with liquid nitrogen first (approximately 5 seconds), waited five minutes while the keloid softened, then injected it.
TAKE-HOME-LEARNING-POINTS
• The tendency to form keloids is in large part a function of location and skin type.
• Skin on chests, shoulders, earlobes, and necks is especially prone to keloid formation.
• The darker the patient’s skin, the greater the chance for keloid formation.
• The decision to perform elective surgery should be informed by a full review of the risks involved, including that for keloid formation.
• Alternatives to excision of cysts in high-risk areas include intralesional steroid injection (using their tendency to cause atrophy to advantage), cryotherapy, or benign neglect.
• Once a keloid has formed, no perfect remedy exists. However, several options can be considered: intralesional injection, excision followed by steroid injection of the wound margins, or referral to plastic surgery.
• Viewed as a continuum, scarring can either be normal, excessive (eg, hypertrophic scarring), or lesional (eg keloid). The latter totally obscures the original insult, fails to spontaneously involute, and is often symptomatic (burning).
HISTORY
This 24-year-old woman presents to dermatology for evaluation of excessive scarring on her chest. It developed slowly in a spot from which a cyst was surgically removed more than two years ago. In addition to being unsightly, the lesion is sometimes symptomatic: It often tingles and feels “tight.” Worst of all, it still seems to be growing.
She has never experienced anything like this—not even following her C-section several years ago. There is no family history of similar problems. The patient says she tans easily, holds a tan well, and rarely burns in the sun.
EXAMINATION
The lesion is clearly cicatricial, quite firm, and slightly pink. It has rounded edges and an exceptionally smooth surface. Located on the left sternal chest wall, the lesion has obliterated any sign of the original surgical scar, except for peripheral scars left by the sutures. The patient’s skin type is a strong IV/VI.
What is the diagnosis?
DISCUSSION
This is a classic case of keloid formation—a real problem since no good, permanent solution exists. In one form or another, this otherwise attractive woman will likely bear this lesion the rest of her life.
Not all excessive scars are keloids. When scarring is excessive, but the outline of the original wound can still be seen, the result is usually termed hypertrophic scarring. By definition, a true keloid, by its thickness, shape, and width, totally obscures the original insult and, unlike a hypertrophic scar, does not spontaneously involute. Viewed as a continuum, there is normal scarring, inappropriate scarring, and severe inappropriate scarring. Unlike the first two, the latter is almost always symptomatic (burning) and does not spontaneously resolve.
Two things could have alerted the surgeon to the possibility that a keloid would form: (1) location, since the chest, shoulders, ear lobes, and neck are especially prone to inappropriate scarring, and (2) skin type, because in general, the darker the skin is, the greater the tendency to form inappropriate scars. While keloids are commonly a postoperative complication, they are also not infrequently triggered by acne, cysts, or even chickenpox. Other high-tension areas prone to keloids are knees and ankles.
The best thing, obviously, would have been for the patient to avoid having the surgery. An alternative to surgical removal of her cyst might have been intralesional injection with triamcinolone solution (5 mg/cc). While this would have been unlikely to produce a cure, it almost certainly would have shrunk the cyst for months at a time.
When surgery in a high-risk area is necessary (eg, in the case of a skin cancer), the surgical margins can be injected with triamcinolone (2.5 mg/cc) at the time of suture removal and again a month postoperative, which will reduce but not eliminate the risk for keloid formation. Low-tension closure (making generous use of undermining and deep sutures to reduce tension on surface sutures) and proper wound care by the patient can help too.
Surgical removal of a keloid in such a location, in a high-risk patient, is an option. However, such procedures are usually left for the plastic surgeon to deal with. Weapons that can be brought to bear on these difficult lesions include excision, intralesional steroid injection, cryotherapy, ionizing radiation, and laser surgery.
This particular patient chose to have us inject her keloid with 1.5 cc of triamcinolone (20 mg/cc), using a 10-cc syringe and 30-gauge needle. (This was made from stock triamcinolone, which comes in a 40 mg/cc strength, mixed half-and-half with lidocaine 1%.) Had her keloid been so dense as to make injection impossible (which happens fairly often), I would have treated it with liquid nitrogen first (approximately 5 seconds), waited five minutes while the keloid softened, then injected it.
TAKE-HOME-LEARNING-POINTS
• The tendency to form keloids is in large part a function of location and skin type.
• Skin on chests, shoulders, earlobes, and necks is especially prone to keloid formation.
• The darker the patient’s skin, the greater the chance for keloid formation.
• The decision to perform elective surgery should be informed by a full review of the risks involved, including that for keloid formation.
• Alternatives to excision of cysts in high-risk areas include intralesional steroid injection (using their tendency to cause atrophy to advantage), cryotherapy, or benign neglect.
• Once a keloid has formed, no perfect remedy exists. However, several options can be considered: intralesional injection, excision followed by steroid injection of the wound margins, or referral to plastic surgery.
• Viewed as a continuum, scarring can either be normal, excessive (eg, hypertrophic scarring), or lesional (eg keloid). The latter totally obscures the original insult, fails to spontaneously involute, and is often symptomatic (burning).
A persistent, nonhealing lesion in a transplant patient
HISTORY
A 64 year-old man is referred to dermatology for investigation of a non-healing asymptomatic lesion present on his left ear for slightly less than a year. His history included his having had a number of basal cell carcinomas removed from his face over the years, and, of course, a history of excessive sun exposure as a young man.
Additional history-taking revealed his having had a kidney transplant 5 years previously, for which he was still under the care of the transplant team, including taking immunosuppressive medications. He had been warned by his transplant providers of the increased risk of skin cancers associated with these medications.
EXAMINATION
A 1 cm friable nodule on the left helical crus could readily be seen, covered by thin eschar, with a faintly red base. Abundant evidence of ancient sun damage could be seen on the ear and face, including telangiectasias and solar lentigines. No nodes or other masses could be detected on palpation of the periauricular and neck regions. A generous shave biopsy of this lesion showed clear evidence of a moderately well-differentiated squamous cell carcinoma.
DISCUSSION
Squamous cell carcinoma (SCC) is the second-most common skin cancer, after basal cell carcinoma (BCC), but unlike the latter, SCC has a very real chance of leading to metastasis and death. 8,000 cases of nodal involvement occur in this country every year, 3,000 of which result in death from metastasis to the brain and/or lung. In general, the more well-differentiated the cancer (similar-appearing cells), the better the prognosis, but the more poorly differentiated the cells are (dissimilar appearance), the greater the chance for invasion and spread.
But several other factors determine the prognosis for any given SCC. Each anatomic site has its own pattern of spread and prognosis. In this particular case, the proximity of the cancer to the external auditory meatus predicts an increased potential for local or nodal spread. Moreover, the patient’s suppressed immune system increases the risk even further.
Approximately 15% of all SCCs occur on upper extremities, but these tend to be relatively safe, especially when sun-caused. Non-solar causation of SCC, such as from HPV, chronic arsenic exposure, or over-exposure to ionizing radiation, tends to have a worse prognosis, especially if it occurs near orifices such as the ear, vagina, mouth or in anogential regions. The size of the tumor matters as well, with tumors greater than 2 cm being increasingly prone to local invasion and metastasis compared to smaller lesions. Beyond these factors, variations in the biologic activity of individual SCCs can be unpredictable, making all SCCs considerably more worrisome than BCCs.
Relatively safe forms of SCC are common, and include intraepidermal SCC in situ, also known as Bowen’s disease, which presents as a slowly growing, fixed papulosquamous plaque on sun-exposed skin. As with all forms of sun-caused SCC, Bowen’s in more common in older, chronically sun-damaged patients.
The lateral and vertical dimensions of this man’s SCC were determined by the Mohs surgeon who excised the lesion, examining the margins of the excised tissue to insure complete removal and to detect any deeper involvement of cartilage or potential perineural involvement. Fortunately, these were not seen in his case, but the operative site was treated with radiation of the site because of the uncertainty caused by his immunosuppressive state.
Obviously, SCCs are not confined to the skin, occurring in a wide variety of areas such as the cervix, lung and oral cavity. The comments made above regarding SCC of the skin have little if any bearing on the diagnosis and treatment of internal squamous cell carcinoma, a subject far beyond the scope of this article.
LEARNING POINTS
1) Any cell in human skin can undergo malignant transformation
2) The tendency to develop SCC is held in check in part by an intact immune system.
3) Non-solar causes of SCC include HPV, chronic exposure to arsenic, and ionizing radiation exposure.
4) SCC, because of its potential for metastasis, is considered more dangerous than BCC.
5) Transplant patients are especially prone to SCC.
HISTORY
A 64 year-old man is referred to dermatology for investigation of a non-healing asymptomatic lesion present on his left ear for slightly less than a year. His history included his having had a number of basal cell carcinomas removed from his face over the years, and, of course, a history of excessive sun exposure as a young man.
Additional history-taking revealed his having had a kidney transplant 5 years previously, for which he was still under the care of the transplant team, including taking immunosuppressive medications. He had been warned by his transplant providers of the increased risk of skin cancers associated with these medications.
EXAMINATION
A 1 cm friable nodule on the left helical crus could readily be seen, covered by thin eschar, with a faintly red base. Abundant evidence of ancient sun damage could be seen on the ear and face, including telangiectasias and solar lentigines. No nodes or other masses could be detected on palpation of the periauricular and neck regions. A generous shave biopsy of this lesion showed clear evidence of a moderately well-differentiated squamous cell carcinoma.
DISCUSSION
Squamous cell carcinoma (SCC) is the second-most common skin cancer, after basal cell carcinoma (BCC), but unlike the latter, SCC has a very real chance of leading to metastasis and death. 8,000 cases of nodal involvement occur in this country every year, 3,000 of which result in death from metastasis to the brain and/or lung. In general, the more well-differentiated the cancer (similar-appearing cells), the better the prognosis, but the more poorly differentiated the cells are (dissimilar appearance), the greater the chance for invasion and spread.
But several other factors determine the prognosis for any given SCC. Each anatomic site has its own pattern of spread and prognosis. In this particular case, the proximity of the cancer to the external auditory meatus predicts an increased potential for local or nodal spread. Moreover, the patient’s suppressed immune system increases the risk even further.
Approximately 15% of all SCCs occur on upper extremities, but these tend to be relatively safe, especially when sun-caused. Non-solar causation of SCC, such as from HPV, chronic arsenic exposure, or over-exposure to ionizing radiation, tends to have a worse prognosis, especially if it occurs near orifices such as the ear, vagina, mouth or in anogential regions. The size of the tumor matters as well, with tumors greater than 2 cm being increasingly prone to local invasion and metastasis compared to smaller lesions. Beyond these factors, variations in the biologic activity of individual SCCs can be unpredictable, making all SCCs considerably more worrisome than BCCs.
Relatively safe forms of SCC are common, and include intraepidermal SCC in situ, also known as Bowen’s disease, which presents as a slowly growing, fixed papulosquamous plaque on sun-exposed skin. As with all forms of sun-caused SCC, Bowen’s in more common in older, chronically sun-damaged patients.
The lateral and vertical dimensions of this man’s SCC were determined by the Mohs surgeon who excised the lesion, examining the margins of the excised tissue to insure complete removal and to detect any deeper involvement of cartilage or potential perineural involvement. Fortunately, these were not seen in his case, but the operative site was treated with radiation of the site because of the uncertainty caused by his immunosuppressive state.
Obviously, SCCs are not confined to the skin, occurring in a wide variety of areas such as the cervix, lung and oral cavity. The comments made above regarding SCC of the skin have little if any bearing on the diagnosis and treatment of internal squamous cell carcinoma, a subject far beyond the scope of this article.
LEARNING POINTS
1) Any cell in human skin can undergo malignant transformation
2) The tendency to develop SCC is held in check in part by an intact immune system.
3) Non-solar causes of SCC include HPV, chronic exposure to arsenic, and ionizing radiation exposure.
4) SCC, because of its potential for metastasis, is considered more dangerous than BCC.
5) Transplant patients are especially prone to SCC.
HISTORY
A 64 year-old man is referred to dermatology for investigation of a non-healing asymptomatic lesion present on his left ear for slightly less than a year. His history included his having had a number of basal cell carcinomas removed from his face over the years, and, of course, a history of excessive sun exposure as a young man.
Additional history-taking revealed his having had a kidney transplant 5 years previously, for which he was still under the care of the transplant team, including taking immunosuppressive medications. He had been warned by his transplant providers of the increased risk of skin cancers associated with these medications.
EXAMINATION
A 1 cm friable nodule on the left helical crus could readily be seen, covered by thin eschar, with a faintly red base. Abundant evidence of ancient sun damage could be seen on the ear and face, including telangiectasias and solar lentigines. No nodes or other masses could be detected on palpation of the periauricular and neck regions. A generous shave biopsy of this lesion showed clear evidence of a moderately well-differentiated squamous cell carcinoma.
DISCUSSION
Squamous cell carcinoma (SCC) is the second-most common skin cancer, after basal cell carcinoma (BCC), but unlike the latter, SCC has a very real chance of leading to metastasis and death. 8,000 cases of nodal involvement occur in this country every year, 3,000 of which result in death from metastasis to the brain and/or lung. In general, the more well-differentiated the cancer (similar-appearing cells), the better the prognosis, but the more poorly differentiated the cells are (dissimilar appearance), the greater the chance for invasion and spread.
But several other factors determine the prognosis for any given SCC. Each anatomic site has its own pattern of spread and prognosis. In this particular case, the proximity of the cancer to the external auditory meatus predicts an increased potential for local or nodal spread. Moreover, the patient’s suppressed immune system increases the risk even further.
Approximately 15% of all SCCs occur on upper extremities, but these tend to be relatively safe, especially when sun-caused. Non-solar causation of SCC, such as from HPV, chronic arsenic exposure, or over-exposure to ionizing radiation, tends to have a worse prognosis, especially if it occurs near orifices such as the ear, vagina, mouth or in anogential regions. The size of the tumor matters as well, with tumors greater than 2 cm being increasingly prone to local invasion and metastasis compared to smaller lesions. Beyond these factors, variations in the biologic activity of individual SCCs can be unpredictable, making all SCCs considerably more worrisome than BCCs.
Relatively safe forms of SCC are common, and include intraepidermal SCC in situ, also known as Bowen’s disease, which presents as a slowly growing, fixed papulosquamous plaque on sun-exposed skin. As with all forms of sun-caused SCC, Bowen’s in more common in older, chronically sun-damaged patients.
The lateral and vertical dimensions of this man’s SCC were determined by the Mohs surgeon who excised the lesion, examining the margins of the excised tissue to insure complete removal and to detect any deeper involvement of cartilage or potential perineural involvement. Fortunately, these were not seen in his case, but the operative site was treated with radiation of the site because of the uncertainty caused by his immunosuppressive state.
Obviously, SCCs are not confined to the skin, occurring in a wide variety of areas such as the cervix, lung and oral cavity. The comments made above regarding SCC of the skin have little if any bearing on the diagnosis and treatment of internal squamous cell carcinoma, a subject far beyond the scope of this article.
LEARNING POINTS
1) Any cell in human skin can undergo malignant transformation
2) The tendency to develop SCC is held in check in part by an intact immune system.
3) Non-solar causes of SCC include HPV, chronic exposure to arsenic, and ionizing radiation exposure.
4) SCC, because of its potential for metastasis, is considered more dangerous than BCC.
5) Transplant patients are especially prone to SCC.
A haircut yields memorable findings

HISTORY
In the midst of a hot summer, a 49-year-old man gets a haircut. For the first time in years, his wife can see the back of his neck—and a bright red patch of skin on it that she's never noticed before. The patient, of course, had no idea it was there; nor has he ever experienced any symptoms referable to the lesion. Except for mild hypertension, he is in reasonably good health.
EXAMINATION
The lesion is a bright red, 10 x 4–cm, funnel-shaped patch that runs from just inside the hairline to the C6 area of his midline posterior neck. It lacks any palpable portion and blanches readily on digital palpation.
DISCUSSION
It's a rare medical provider who has never seen one of these extremely common congenital capillary malformations called by various names (including stork bite, salmon patch, and angel kiss) but known officially as nevus flammeus nuchae (NFN). About 25% to 50% of Caucasian newborns are affected by this type of lesion, which can vary a great deal in size.
This particular patient's lesion demonstrated one of the condition's peculiar properties: the tendency to become much more pronounced with extertion, anger, hot weather, or combinations thereof. Many NFNs are effectively concealed by the patient's hair—as in this case, in which the patient had worn his hair long and in a ponytail for many years before getting a significant haircut. On his visit to dermatology, he was shown the photograph above and was so astonished and dismayed that he vowed to grow out his hair again!
NFN is the most common example of congenital vascular malformations; the so-called "port wine stain" is another well-known type. Unlike other lesions in this category, the NFN never resolves on its own, and it grows as the owner grows. Several hypotheses have evolved as to its origin; the leading one asserts that inadequate innervations promote decreased vascular tone.
The diagnosis of NFN is clinical, based on its history, location, and appearance. If performed, a biopsy would show dilated superficial dermal blood vessels, the walls of which can be a bit thinner than normal, but without other significant changes in the endothelial lining.
When desired, the lesion can be covered with makeup. Or ablation can be accomplished by laser treatment.
TAKE-HOME LEARNING POINTS
• Nevus flammeus nuchae (NFN) is extremely common, affecting 25% to 50% of Caucasian newborns.
• NFN is typically permanent and grows as the patient grows.
• NFN is almost always completely flat (macular) and blanches readily with digital pressure.
• NFNs become much more pronounced with exertion, heat, or emotional upset.

HISTORY
In the midst of a hot summer, a 49-year-old man gets a haircut. For the first time in years, his wife can see the back of his neck—and a bright red patch of skin on it that she's never noticed before. The patient, of course, had no idea it was there; nor has he ever experienced any symptoms referable to the lesion. Except for mild hypertension, he is in reasonably good health.
EXAMINATION
The lesion is a bright red, 10 x 4–cm, funnel-shaped patch that runs from just inside the hairline to the C6 area of his midline posterior neck. It lacks any palpable portion and blanches readily on digital palpation.
DISCUSSION
It's a rare medical provider who has never seen one of these extremely common congenital capillary malformations called by various names (including stork bite, salmon patch, and angel kiss) but known officially as nevus flammeus nuchae (NFN). About 25% to 50% of Caucasian newborns are affected by this type of lesion, which can vary a great deal in size.
This particular patient's lesion demonstrated one of the condition's peculiar properties: the tendency to become much more pronounced with extertion, anger, hot weather, or combinations thereof. Many NFNs are effectively concealed by the patient's hair—as in this case, in which the patient had worn his hair long and in a ponytail for many years before getting a significant haircut. On his visit to dermatology, he was shown the photograph above and was so astonished and dismayed that he vowed to grow out his hair again!
NFN is the most common example of congenital vascular malformations; the so-called "port wine stain" is another well-known type. Unlike other lesions in this category, the NFN never resolves on its own, and it grows as the owner grows. Several hypotheses have evolved as to its origin; the leading one asserts that inadequate innervations promote decreased vascular tone.
The diagnosis of NFN is clinical, based on its history, location, and appearance. If performed, a biopsy would show dilated superficial dermal blood vessels, the walls of which can be a bit thinner than normal, but without other significant changes in the endothelial lining.
When desired, the lesion can be covered with makeup. Or ablation can be accomplished by laser treatment.
TAKE-HOME LEARNING POINTS
• Nevus flammeus nuchae (NFN) is extremely common, affecting 25% to 50% of Caucasian newborns.
• NFN is typically permanent and grows as the patient grows.
• NFN is almost always completely flat (macular) and blanches readily with digital pressure.
• NFNs become much more pronounced with exertion, heat, or emotional upset.

HISTORY
In the midst of a hot summer, a 49-year-old man gets a haircut. For the first time in years, his wife can see the back of his neck—and a bright red patch of skin on it that she's never noticed before. The patient, of course, had no idea it was there; nor has he ever experienced any symptoms referable to the lesion. Except for mild hypertension, he is in reasonably good health.
EXAMINATION
The lesion is a bright red, 10 x 4–cm, funnel-shaped patch that runs from just inside the hairline to the C6 area of his midline posterior neck. It lacks any palpable portion and blanches readily on digital palpation.
DISCUSSION
It's a rare medical provider who has never seen one of these extremely common congenital capillary malformations called by various names (including stork bite, salmon patch, and angel kiss) but known officially as nevus flammeus nuchae (NFN). About 25% to 50% of Caucasian newborns are affected by this type of lesion, which can vary a great deal in size.
This particular patient's lesion demonstrated one of the condition's peculiar properties: the tendency to become much more pronounced with extertion, anger, hot weather, or combinations thereof. Many NFNs are effectively concealed by the patient's hair—as in this case, in which the patient had worn his hair long and in a ponytail for many years before getting a significant haircut. On his visit to dermatology, he was shown the photograph above and was so astonished and dismayed that he vowed to grow out his hair again!
NFN is the most common example of congenital vascular malformations; the so-called "port wine stain" is another well-known type. Unlike other lesions in this category, the NFN never resolves on its own, and it grows as the owner grows. Several hypotheses have evolved as to its origin; the leading one asserts that inadequate innervations promote decreased vascular tone.
The diagnosis of NFN is clinical, based on its history, location, and appearance. If performed, a biopsy would show dilated superficial dermal blood vessels, the walls of which can be a bit thinner than normal, but without other significant changes in the endothelial lining.
When desired, the lesion can be covered with makeup. Or ablation can be accomplished by laser treatment.
TAKE-HOME LEARNING POINTS
• Nevus flammeus nuchae (NFN) is extremely common, affecting 25% to 50% of Caucasian newborns.
• NFN is typically permanent and grows as the patient grows.
• NFN is almost always completely flat (macular) and blanches readily with digital pressure.
• NFNs become much more pronounced with exertion, heat, or emotional upset.