User login
Leg Lesion Represents a Vicious Cycle
CASE
A 38-year-old man is referred to dermatology by his primary care provider (PCP) for evaluation of a lesion on his leg that has been present for more than two years. Concerned friends and family recently urged him to seek medical care.
His PCP thought it probably represented fungal infection, but the nystatin/triamcinolone cream he prescribed was of little or no help. The patient, who is of Indian descent, decided to consult a medical provider during a trip to India, but was dissatisfied with the herbal paste he was advised to obtain and use. When he returned to the United States, he requested referral to dermatology.
The patient denies any other skin problems, now or in the past, but admits to scratching and rubbing the site in question several times a day—partly out of habit, but mostly because it itches. The spot’s progressive darkening has been a major factor in his pursuit of further evaluation.
Examination reveals a single lesion: a uniformly scaly, dark, 8-x-4–cm area of his anterolateral calf. The margins of the lesion are fairly sharply demarcated, but there is no redness, increased warmth, or tenderness associated with it. The patient’s skin overall is quite dark (type V).
DISCUSSION
This presentation is typical of lichen simplex chronicus (LSC; also known as neurodermatitis)—essentially, a reaction to chronic rubbing and scratching. LSC is not a primary diagnosis; it is merely the consequence of mechanical trauma as a reaction to perceived pruritus (with or without actual pathologic cause). Over time, the affected skin tends to thicken in reaction to chronic trauma, which also has the effect of increasing pruritus. Thus, the itch-scratch-itch cycle perpetuates.
Affected skin also tends to darken, especially in darker-skinned patients, and is often the source of considerable consternation. Even when the condition is treated and all rubbing and scratching ceases, it may take months (if not years) for the hyperpigmentation to clear.
The keys to diagnosis include the patient’s admission of regular scratching and his ready access to the area, as well as the lichenification and hyperpigmentation. There are any number of initial triggers, including bug bites, dry skin, eczema, and even psoriasis. However, those conditions take a backseat to the LSC. This exact location (anterior leg) is quite typical in men, but in women, LSC is far more common in the nuchal scalp, where heat and sweat also contribute to the problem.
Many LSC patients have a history of atopic dermatitis that appears to lower their threshold for pruritus. When questioned closely, many if not most will admit to concurrent emotional stress, which is thought to be a contributing factor.
Biopsy is occasionally necessary to distinguish LSC from other items in the differential, including psoriasis, contact dermatitis, and lichen planus. But in most cases, including this one, the twice-daily application of a class 2 or 3 topical steroid cream or ointment for one to two weeks will work wonders. Educating the patient about his own contribution to the problem is essential.
This patient was instructed to return in one month, to ensure that the condition was responding and that he understood the need to gradually decrease the use of this powerful steroid. Unfortunately, his chances for recurrence are quite high, given the habitual and compelling nature of the problem.
TAKE-HOME LEARNING POINTS
• Lichen simplex chronicus (LSC) is a very common condition that represents the skin’s reaction to chronic scratching and rubbing.
• LSC is not a primary condition; rather, it is triggered by dry skin, eczema, contact dermatitis, or lichen planus (among others).
• LSC involves thickening of the affected skin and, in darker-skinned patients, a reactive hyperpigmentation.
• LSC commonly manifests on the anterior legs in men and on the nuchal scalp in women.
CASE
A 38-year-old man is referred to dermatology by his primary care provider (PCP) for evaluation of a lesion on his leg that has been present for more than two years. Concerned friends and family recently urged him to seek medical care.
His PCP thought it probably represented fungal infection, but the nystatin/triamcinolone cream he prescribed was of little or no help. The patient, who is of Indian descent, decided to consult a medical provider during a trip to India, but was dissatisfied with the herbal paste he was advised to obtain and use. When he returned to the United States, he requested referral to dermatology.
The patient denies any other skin problems, now or in the past, but admits to scratching and rubbing the site in question several times a day—partly out of habit, but mostly because it itches. The spot’s progressive darkening has been a major factor in his pursuit of further evaluation.
Examination reveals a single lesion: a uniformly scaly, dark, 8-x-4–cm area of his anterolateral calf. The margins of the lesion are fairly sharply demarcated, but there is no redness, increased warmth, or tenderness associated with it. The patient’s skin overall is quite dark (type V).
DISCUSSION
This presentation is typical of lichen simplex chronicus (LSC; also known as neurodermatitis)—essentially, a reaction to chronic rubbing and scratching. LSC is not a primary diagnosis; it is merely the consequence of mechanical trauma as a reaction to perceived pruritus (with or without actual pathologic cause). Over time, the affected skin tends to thicken in reaction to chronic trauma, which also has the effect of increasing pruritus. Thus, the itch-scratch-itch cycle perpetuates.
Affected skin also tends to darken, especially in darker-skinned patients, and is often the source of considerable consternation. Even when the condition is treated and all rubbing and scratching ceases, it may take months (if not years) for the hyperpigmentation to clear.
The keys to diagnosis include the patient’s admission of regular scratching and his ready access to the area, as well as the lichenification and hyperpigmentation. There are any number of initial triggers, including bug bites, dry skin, eczema, and even psoriasis. However, those conditions take a backseat to the LSC. This exact location (anterior leg) is quite typical in men, but in women, LSC is far more common in the nuchal scalp, where heat and sweat also contribute to the problem.
Many LSC patients have a history of atopic dermatitis that appears to lower their threshold for pruritus. When questioned closely, many if not most will admit to concurrent emotional stress, which is thought to be a contributing factor.
Biopsy is occasionally necessary to distinguish LSC from other items in the differential, including psoriasis, contact dermatitis, and lichen planus. But in most cases, including this one, the twice-daily application of a class 2 or 3 topical steroid cream or ointment for one to two weeks will work wonders. Educating the patient about his own contribution to the problem is essential.
This patient was instructed to return in one month, to ensure that the condition was responding and that he understood the need to gradually decrease the use of this powerful steroid. Unfortunately, his chances for recurrence are quite high, given the habitual and compelling nature of the problem.
TAKE-HOME LEARNING POINTS
• Lichen simplex chronicus (LSC) is a very common condition that represents the skin’s reaction to chronic scratching and rubbing.
• LSC is not a primary condition; rather, it is triggered by dry skin, eczema, contact dermatitis, or lichen planus (among others).
• LSC involves thickening of the affected skin and, in darker-skinned patients, a reactive hyperpigmentation.
• LSC commonly manifests on the anterior legs in men and on the nuchal scalp in women.
CASE
A 38-year-old man is referred to dermatology by his primary care provider (PCP) for evaluation of a lesion on his leg that has been present for more than two years. Concerned friends and family recently urged him to seek medical care.
His PCP thought it probably represented fungal infection, but the nystatin/triamcinolone cream he prescribed was of little or no help. The patient, who is of Indian descent, decided to consult a medical provider during a trip to India, but was dissatisfied with the herbal paste he was advised to obtain and use. When he returned to the United States, he requested referral to dermatology.
The patient denies any other skin problems, now or in the past, but admits to scratching and rubbing the site in question several times a day—partly out of habit, but mostly because it itches. The spot’s progressive darkening has been a major factor in his pursuit of further evaluation.
Examination reveals a single lesion: a uniformly scaly, dark, 8-x-4–cm area of his anterolateral calf. The margins of the lesion are fairly sharply demarcated, but there is no redness, increased warmth, or tenderness associated with it. The patient’s skin overall is quite dark (type V).
DISCUSSION
This presentation is typical of lichen simplex chronicus (LSC; also known as neurodermatitis)—essentially, a reaction to chronic rubbing and scratching. LSC is not a primary diagnosis; it is merely the consequence of mechanical trauma as a reaction to perceived pruritus (with or without actual pathologic cause). Over time, the affected skin tends to thicken in reaction to chronic trauma, which also has the effect of increasing pruritus. Thus, the itch-scratch-itch cycle perpetuates.
Affected skin also tends to darken, especially in darker-skinned patients, and is often the source of considerable consternation. Even when the condition is treated and all rubbing and scratching ceases, it may take months (if not years) for the hyperpigmentation to clear.
The keys to diagnosis include the patient’s admission of regular scratching and his ready access to the area, as well as the lichenification and hyperpigmentation. There are any number of initial triggers, including bug bites, dry skin, eczema, and even psoriasis. However, those conditions take a backseat to the LSC. This exact location (anterior leg) is quite typical in men, but in women, LSC is far more common in the nuchal scalp, where heat and sweat also contribute to the problem.
Many LSC patients have a history of atopic dermatitis that appears to lower their threshold for pruritus. When questioned closely, many if not most will admit to concurrent emotional stress, which is thought to be a contributing factor.
Biopsy is occasionally necessary to distinguish LSC from other items in the differential, including psoriasis, contact dermatitis, and lichen planus. But in most cases, including this one, the twice-daily application of a class 2 or 3 topical steroid cream or ointment for one to two weeks will work wonders. Educating the patient about his own contribution to the problem is essential.
This patient was instructed to return in one month, to ensure that the condition was responding and that he understood the need to gradually decrease the use of this powerful steroid. Unfortunately, his chances for recurrence are quite high, given the habitual and compelling nature of the problem.
TAKE-HOME LEARNING POINTS
• Lichen simplex chronicus (LSC) is a very common condition that represents the skin’s reaction to chronic scratching and rubbing.
• LSC is not a primary condition; rather, it is triggered by dry skin, eczema, contact dermatitis, or lichen planus (among others).
• LSC involves thickening of the affected skin and, in darker-skinned patients, a reactive hyperpigmentation.
• LSC commonly manifests on the anterior legs in men and on the nuchal scalp in women.
Baffling reaction to a suspicious lesion
CASE
A 34-year-old woman presents to her primary care provider to “have her moles checked.” She is motivated by a family history of sun-caused skin cancers, as well as her own history of multiple sunburns as a child.
Noting the patient’s fair, freckled skin, red hair, and blue eyes, the primary care provider agrees with the patient’s assessment of her risk. One lesion stands out from the rest: a 1.5-cm dark, irregularly bordered, and pigmented maculopapular lesion on the patient’s right triceps. The primary care provider arranges for a timely referral to dermatology.
The dermatology clinicians know immediately that the triceps lesion is highly suspicious, and tell the patient so while setting up for biopsy. Before that procedure is carried out, a careful examination of all exposed skin is conducted. The patient’s fair, sun-damaged skin is again noted, but no other suspicious lesions are found. There are no palpable nodes detected on her right axilla.
Under local anesthesia (1% lidocaine with epinephrine), the right triceps lesion is removed by deep saucerization (through the deep dermis into the upper adipose layer) using a double-edged razor. Minor bleeding is controlled with light cautery.
The pathology report, received three days later, confirms the malignant nature of the lesion, with a diagnosis of invasive melanoma (nodular, with no horizontal growth phase), measuring 1.7 mm. Fortunately, no other ominous signs—such as a high mitotic rate or vascular invasion—are reported.
The patient is immediately contacted by phone and notified of the results. The potential danger of this diagnosis is reiterated, along with information regarding the next steps in the process. These include consultation with a surgeon for consideration of re-excision and possible lymph node dissection. Evaluation by an oncologist will likely follow.
The patient’s response to this news is puzzling, to say the least. Though she appears to understand what she is being told, she sounds blissfully unconcerned, saying she is “not that worried” and is sure she will “be just fine.”
DISCUSSION
There are patients newly diagnosed with melanoma who overreact. I’ve had patients hop on the next plane to the Mayo Clinic, or, as in one notable case, to Tijuana, which, as we all know, is the home of such questionable practices as coffee-ground enemas and chemically modified amygdalin.
But then there are melanoma patients who go to the other extreme, making us wonder if they really understand the potential seriousness of the situation. It’s not that we want to see any particular “angst” as a reaction, but an appropriate indication or two is reassuring as feedback to the announcement. Questions— “What does this mean?” “What’s going to happen now?” “How serious is this?”— are good to hear in this regard. The answers allow us to convey the sense of where the patient stands, both for the present and in the long term, and help us to get a sense of how well the patient perceives the situation.
This patient had no questions, at all, as if she was totally unconcerned. That concerned me. It left me with a number of questions: “Does she really understand what’s going on?” “Will she follow our instructions and see the specialists we advise her to see?” Over the years, I’ve had several patients like this who went on their merry way, doing nothing we suggested. Some even survived.
This lack of appropriate reaction has been termed la belle indifference. It’s a way of pretending nothing is happening, and represents a way of showing one’s paralysis to others by manipulating their judgment through an attitude of indifference. One doesn’t want to frighten these patients (“Don’t you know this could be fatal?”), so what I do is keep close tabs on them—calling them regularly, making sure they’re following our advice, and documenting our calls and the patient’s responses. When family members can be enlisted to help, so much the better.
So far, this patient is complying with our advice, but it’s early in the process yet. We’ll see. Our job—and her ordeal—is far from done.
Any melanoma over 1 mm in thickness (based on the Breslow scale) is associated with an uncertain prognosis, and nodular melanomas are associated with a relatively poor prognosis. Besides re-excision (probably with 1-cm margins), this patient will probably be a candidate for elective lymph node dissection in the right axilla. PET scans, blood tests, and a visit to the oncologist will most likely follow. The surgeon usually acts as decision-maker in terms of what the patient needs and in what sequence.
Even if she survives all that, this patient will still need to see us every three months or so for a year, then regularly thereafter, to monitor this cancer and watch for new ones.
LEARNING POINTS
• Deep-shave biopsy (sometimes called saucerization) is an appropriate technique for possible melanoma.
• About 75% to 80% of all melanomas are superficial, spreading types, (essentially flat), while 10% or so have only a vertical phase of growth (ie, present as a nodule or mass).
• Survival rates for melanoma are closely tied to tumor thickness, which is most commonly measured (by the pathologist) in millimeters; this staging system is called the Breslow scale. The older system of staging melanoma by the anatomical depth (called the Clark’s level I-V) has fallen into disuse.
• Underreaction to the diagnosis of melanoma (la belle indifference) can be as problematic as overreaction. Consistent monitoring of patients for compliance is often necessary.
CASE
A 34-year-old woman presents to her primary care provider to “have her moles checked.” She is motivated by a family history of sun-caused skin cancers, as well as her own history of multiple sunburns as a child.
Noting the patient’s fair, freckled skin, red hair, and blue eyes, the primary care provider agrees with the patient’s assessment of her risk. One lesion stands out from the rest: a 1.5-cm dark, irregularly bordered, and pigmented maculopapular lesion on the patient’s right triceps. The primary care provider arranges for a timely referral to dermatology.
The dermatology clinicians know immediately that the triceps lesion is highly suspicious, and tell the patient so while setting up for biopsy. Before that procedure is carried out, a careful examination of all exposed skin is conducted. The patient’s fair, sun-damaged skin is again noted, but no other suspicious lesions are found. There are no palpable nodes detected on her right axilla.
Under local anesthesia (1% lidocaine with epinephrine), the right triceps lesion is removed by deep saucerization (through the deep dermis into the upper adipose layer) using a double-edged razor. Minor bleeding is controlled with light cautery.
The pathology report, received three days later, confirms the malignant nature of the lesion, with a diagnosis of invasive melanoma (nodular, with no horizontal growth phase), measuring 1.7 mm. Fortunately, no other ominous signs—such as a high mitotic rate or vascular invasion—are reported.
The patient is immediately contacted by phone and notified of the results. The potential danger of this diagnosis is reiterated, along with information regarding the next steps in the process. These include consultation with a surgeon for consideration of re-excision and possible lymph node dissection. Evaluation by an oncologist will likely follow.
The patient’s response to this news is puzzling, to say the least. Though she appears to understand what she is being told, she sounds blissfully unconcerned, saying she is “not that worried” and is sure she will “be just fine.”
DISCUSSION
There are patients newly diagnosed with melanoma who overreact. I’ve had patients hop on the next plane to the Mayo Clinic, or, as in one notable case, to Tijuana, which, as we all know, is the home of such questionable practices as coffee-ground enemas and chemically modified amygdalin.
But then there are melanoma patients who go to the other extreme, making us wonder if they really understand the potential seriousness of the situation. It’s not that we want to see any particular “angst” as a reaction, but an appropriate indication or two is reassuring as feedback to the announcement. Questions— “What does this mean?” “What’s going to happen now?” “How serious is this?”— are good to hear in this regard. The answers allow us to convey the sense of where the patient stands, both for the present and in the long term, and help us to get a sense of how well the patient perceives the situation.
This patient had no questions, at all, as if she was totally unconcerned. That concerned me. It left me with a number of questions: “Does she really understand what’s going on?” “Will she follow our instructions and see the specialists we advise her to see?” Over the years, I’ve had several patients like this who went on their merry way, doing nothing we suggested. Some even survived.
This lack of appropriate reaction has been termed la belle indifference. It’s a way of pretending nothing is happening, and represents a way of showing one’s paralysis to others by manipulating their judgment through an attitude of indifference. One doesn’t want to frighten these patients (“Don’t you know this could be fatal?”), so what I do is keep close tabs on them—calling them regularly, making sure they’re following our advice, and documenting our calls and the patient’s responses. When family members can be enlisted to help, so much the better.
So far, this patient is complying with our advice, but it’s early in the process yet. We’ll see. Our job—and her ordeal—is far from done.
Any melanoma over 1 mm in thickness (based on the Breslow scale) is associated with an uncertain prognosis, and nodular melanomas are associated with a relatively poor prognosis. Besides re-excision (probably with 1-cm margins), this patient will probably be a candidate for elective lymph node dissection in the right axilla. PET scans, blood tests, and a visit to the oncologist will most likely follow. The surgeon usually acts as decision-maker in terms of what the patient needs and in what sequence.
Even if she survives all that, this patient will still need to see us every three months or so for a year, then regularly thereafter, to monitor this cancer and watch for new ones.
LEARNING POINTS
• Deep-shave biopsy (sometimes called saucerization) is an appropriate technique for possible melanoma.
• About 75% to 80% of all melanomas are superficial, spreading types, (essentially flat), while 10% or so have only a vertical phase of growth (ie, present as a nodule or mass).
• Survival rates for melanoma are closely tied to tumor thickness, which is most commonly measured (by the pathologist) in millimeters; this staging system is called the Breslow scale. The older system of staging melanoma by the anatomical depth (called the Clark’s level I-V) has fallen into disuse.
• Underreaction to the diagnosis of melanoma (la belle indifference) can be as problematic as overreaction. Consistent monitoring of patients for compliance is often necessary.
CASE
A 34-year-old woman presents to her primary care provider to “have her moles checked.” She is motivated by a family history of sun-caused skin cancers, as well as her own history of multiple sunburns as a child.
Noting the patient’s fair, freckled skin, red hair, and blue eyes, the primary care provider agrees with the patient’s assessment of her risk. One lesion stands out from the rest: a 1.5-cm dark, irregularly bordered, and pigmented maculopapular lesion on the patient’s right triceps. The primary care provider arranges for a timely referral to dermatology.
The dermatology clinicians know immediately that the triceps lesion is highly suspicious, and tell the patient so while setting up for biopsy. Before that procedure is carried out, a careful examination of all exposed skin is conducted. The patient’s fair, sun-damaged skin is again noted, but no other suspicious lesions are found. There are no palpable nodes detected on her right axilla.
Under local anesthesia (1% lidocaine with epinephrine), the right triceps lesion is removed by deep saucerization (through the deep dermis into the upper adipose layer) using a double-edged razor. Minor bleeding is controlled with light cautery.
The pathology report, received three days later, confirms the malignant nature of the lesion, with a diagnosis of invasive melanoma (nodular, with no horizontal growth phase), measuring 1.7 mm. Fortunately, no other ominous signs—such as a high mitotic rate or vascular invasion—are reported.
The patient is immediately contacted by phone and notified of the results. The potential danger of this diagnosis is reiterated, along with information regarding the next steps in the process. These include consultation with a surgeon for consideration of re-excision and possible lymph node dissection. Evaluation by an oncologist will likely follow.
The patient’s response to this news is puzzling, to say the least. Though she appears to understand what she is being told, she sounds blissfully unconcerned, saying she is “not that worried” and is sure she will “be just fine.”
DISCUSSION
There are patients newly diagnosed with melanoma who overreact. I’ve had patients hop on the next plane to the Mayo Clinic, or, as in one notable case, to Tijuana, which, as we all know, is the home of such questionable practices as coffee-ground enemas and chemically modified amygdalin.
But then there are melanoma patients who go to the other extreme, making us wonder if they really understand the potential seriousness of the situation. It’s not that we want to see any particular “angst” as a reaction, but an appropriate indication or two is reassuring as feedback to the announcement. Questions— “What does this mean?” “What’s going to happen now?” “How serious is this?”— are good to hear in this regard. The answers allow us to convey the sense of where the patient stands, both for the present and in the long term, and help us to get a sense of how well the patient perceives the situation.
This patient had no questions, at all, as if she was totally unconcerned. That concerned me. It left me with a number of questions: “Does she really understand what’s going on?” “Will she follow our instructions and see the specialists we advise her to see?” Over the years, I’ve had several patients like this who went on their merry way, doing nothing we suggested. Some even survived.
This lack of appropriate reaction has been termed la belle indifference. It’s a way of pretending nothing is happening, and represents a way of showing one’s paralysis to others by manipulating their judgment through an attitude of indifference. One doesn’t want to frighten these patients (“Don’t you know this could be fatal?”), so what I do is keep close tabs on them—calling them regularly, making sure they’re following our advice, and documenting our calls and the patient’s responses. When family members can be enlisted to help, so much the better.
So far, this patient is complying with our advice, but it’s early in the process yet. We’ll see. Our job—and her ordeal—is far from done.
Any melanoma over 1 mm in thickness (based on the Breslow scale) is associated with an uncertain prognosis, and nodular melanomas are associated with a relatively poor prognosis. Besides re-excision (probably with 1-cm margins), this patient will probably be a candidate for elective lymph node dissection in the right axilla. PET scans, blood tests, and a visit to the oncologist will most likely follow. The surgeon usually acts as decision-maker in terms of what the patient needs and in what sequence.
Even if she survives all that, this patient will still need to see us every three months or so for a year, then regularly thereafter, to monitor this cancer and watch for new ones.
LEARNING POINTS
• Deep-shave biopsy (sometimes called saucerization) is an appropriate technique for possible melanoma.
• About 75% to 80% of all melanomas are superficial, spreading types, (essentially flat), while 10% or so have only a vertical phase of growth (ie, present as a nodule or mass).
• Survival rates for melanoma are closely tied to tumor thickness, which is most commonly measured (by the pathologist) in millimeters; this staging system is called the Breslow scale. The older system of staging melanoma by the anatomical depth (called the Clark’s level I-V) has fallen into disuse.
• Underreaction to the diagnosis of melanoma (la belle indifference) can be as problematic as overreaction. Consistent monitoring of patients for compliance is often necessary.
Ongoing agony of the feet
HISTORY
This 66-year-old woman has had a very itchy rash on her left foot for several months. She has tried applying a number of different OTC and prescription medications—including betamethasone dipropionate cream and econazole cream—without successful resolution of the problem.
She denies having any other skin problems, and there is no relevant family history. The patient is retired and lives alone with her cat. Medical history is remarkable for rheumatoid arthritis, for which she takes methotrexate.
EXAMINATION
The dorsum of her left foot is covered with a sharply demarcated, papulosquamous red rash. Interestingly, the interdigital areas are spared, as are the sole and the entire right foot. The patient’s elbows, knees, and scalp exhibit no significant changes. On further examination, a fine, slightly pink, powdery rash is noted on the sides of both feet (including the heels).
A KOH prep of the rim of the feet is positive for fungal elements, as is a similar microscopic examination of scrapings from the dorsum of the left foot. In fact, the fungal elements seen on the latter are so numerous and dense that they are initially difficult to see.
What is the diagnosis?
DISCUSSION
Rashes on the dorsum of the feet are almost never of fungal origin; rather, they usually represent contact (not eczematous) dermatitis. That’s partly because fungi typically require more heat and moisture than the dorsum can provide. Two things are needed to allow it to flourish in this unusual location: immune suppression and a source for the fungi.
Methotrexate and the prolonged use of a potent topical steroid were the likely culprits in terms of immune suppression. Both reduce the chemotactic response that these dermatophytes normally trigger, allowing them to multiply unchecked. But where did the fungi come from in the first place?
Traditionally, three kinds of tinea pedis have been described: the well-known interdigital type, typically affecting the space between the third and fourth or the fourth and fifth toes; the so-called inflammatory type, which presents acutely with highly pruritic vesicles and pustules on the plantar surface (most commonly on the instep); and the most common but least recognized of all, the moccasin type, which causes few if any symptoms and often flies under the patient’s radar. The rash it causes is faint and dry and covers the rim of the foot (sparing the toes).
But, given the right circumstances, it can serve as a reservoir for infection on the foot and leg. The resultant infection can be cured, but moccasin-variety tinea pedis is considered incurable, due to the ubiquitous nature of the organism and patient’s demonstrated susceptibility. The causative organism is almost always Trichophyton rubrum, by far the most common dermatophytic pathogen.
TREATMENT
Fortunately, T. rubrum responds well to oral terbinafine therapy, (250 mg/d). This patient was provided a month’s supply, along with topical miconazole to be applied twice daily until the rash clears. Use of the latter on the sides of the feet is also advised, for purposes of control.
TAKE-HOME-LEARNING-POINTS
• The performance of the KOH prep can be crucial in terms of establishing the correct diagnosis, but it also gives everyone involved confidence in the diagnosis and treatment. The only way to learn how to do KOH preps is to do them.
• Moccasin-variety tinea pedis is the most common but the least recognized of the three major types. Because it is chronic and asymptomatic, patients rarely know what it is, although it can serve as a reservoir for infection elsewhere.
• Fungal infections don’t just happen. Failure to establish a source can doom the patient to repeated episodes.
• Fungal infections (dermatophytosis) in odd places usually involve immune suppression, typically from the use of topical steroids and/or systemic immunosuppressants.
HISTORY
This 66-year-old woman has had a very itchy rash on her left foot for several months. She has tried applying a number of different OTC and prescription medications—including betamethasone dipropionate cream and econazole cream—without successful resolution of the problem.
She denies having any other skin problems, and there is no relevant family history. The patient is retired and lives alone with her cat. Medical history is remarkable for rheumatoid arthritis, for which she takes methotrexate.
EXAMINATION
The dorsum of her left foot is covered with a sharply demarcated, papulosquamous red rash. Interestingly, the interdigital areas are spared, as are the sole and the entire right foot. The patient’s elbows, knees, and scalp exhibit no significant changes. On further examination, a fine, slightly pink, powdery rash is noted on the sides of both feet (including the heels).
A KOH prep of the rim of the feet is positive for fungal elements, as is a similar microscopic examination of scrapings from the dorsum of the left foot. In fact, the fungal elements seen on the latter are so numerous and dense that they are initially difficult to see.
What is the diagnosis?
DISCUSSION
Rashes on the dorsum of the feet are almost never of fungal origin; rather, they usually represent contact (not eczematous) dermatitis. That’s partly because fungi typically require more heat and moisture than the dorsum can provide. Two things are needed to allow it to flourish in this unusual location: immune suppression and a source for the fungi.
Methotrexate and the prolonged use of a potent topical steroid were the likely culprits in terms of immune suppression. Both reduce the chemotactic response that these dermatophytes normally trigger, allowing them to multiply unchecked. But where did the fungi come from in the first place?
Traditionally, three kinds of tinea pedis have been described: the well-known interdigital type, typically affecting the space between the third and fourth or the fourth and fifth toes; the so-called inflammatory type, which presents acutely with highly pruritic vesicles and pustules on the plantar surface (most commonly on the instep); and the most common but least recognized of all, the moccasin type, which causes few if any symptoms and often flies under the patient’s radar. The rash it causes is faint and dry and covers the rim of the foot (sparing the toes).
But, given the right circumstances, it can serve as a reservoir for infection on the foot and leg. The resultant infection can be cured, but moccasin-variety tinea pedis is considered incurable, due to the ubiquitous nature of the organism and patient’s demonstrated susceptibility. The causative organism is almost always Trichophyton rubrum, by far the most common dermatophytic pathogen.
TREATMENT
Fortunately, T. rubrum responds well to oral terbinafine therapy, (250 mg/d). This patient was provided a month’s supply, along with topical miconazole to be applied twice daily until the rash clears. Use of the latter on the sides of the feet is also advised, for purposes of control.
TAKE-HOME-LEARNING-POINTS
• The performance of the KOH prep can be crucial in terms of establishing the correct diagnosis, but it also gives everyone involved confidence in the diagnosis and treatment. The only way to learn how to do KOH preps is to do them.
• Moccasin-variety tinea pedis is the most common but the least recognized of the three major types. Because it is chronic and asymptomatic, patients rarely know what it is, although it can serve as a reservoir for infection elsewhere.
• Fungal infections don’t just happen. Failure to establish a source can doom the patient to repeated episodes.
• Fungal infections (dermatophytosis) in odd places usually involve immune suppression, typically from the use of topical steroids and/or systemic immunosuppressants.
HISTORY
This 66-year-old woman has had a very itchy rash on her left foot for several months. She has tried applying a number of different OTC and prescription medications—including betamethasone dipropionate cream and econazole cream—without successful resolution of the problem.
She denies having any other skin problems, and there is no relevant family history. The patient is retired and lives alone with her cat. Medical history is remarkable for rheumatoid arthritis, for which she takes methotrexate.
EXAMINATION
The dorsum of her left foot is covered with a sharply demarcated, papulosquamous red rash. Interestingly, the interdigital areas are spared, as are the sole and the entire right foot. The patient’s elbows, knees, and scalp exhibit no significant changes. On further examination, a fine, slightly pink, powdery rash is noted on the sides of both feet (including the heels).
A KOH prep of the rim of the feet is positive for fungal elements, as is a similar microscopic examination of scrapings from the dorsum of the left foot. In fact, the fungal elements seen on the latter are so numerous and dense that they are initially difficult to see.
What is the diagnosis?
DISCUSSION
Rashes on the dorsum of the feet are almost never of fungal origin; rather, they usually represent contact (not eczematous) dermatitis. That’s partly because fungi typically require more heat and moisture than the dorsum can provide. Two things are needed to allow it to flourish in this unusual location: immune suppression and a source for the fungi.
Methotrexate and the prolonged use of a potent topical steroid were the likely culprits in terms of immune suppression. Both reduce the chemotactic response that these dermatophytes normally trigger, allowing them to multiply unchecked. But where did the fungi come from in the first place?
Traditionally, three kinds of tinea pedis have been described: the well-known interdigital type, typically affecting the space between the third and fourth or the fourth and fifth toes; the so-called inflammatory type, which presents acutely with highly pruritic vesicles and pustules on the plantar surface (most commonly on the instep); and the most common but least recognized of all, the moccasin type, which causes few if any symptoms and often flies under the patient’s radar. The rash it causes is faint and dry and covers the rim of the foot (sparing the toes).
But, given the right circumstances, it can serve as a reservoir for infection on the foot and leg. The resultant infection can be cured, but moccasin-variety tinea pedis is considered incurable, due to the ubiquitous nature of the organism and patient’s demonstrated susceptibility. The causative organism is almost always Trichophyton rubrum, by far the most common dermatophytic pathogen.
TREATMENT
Fortunately, T. rubrum responds well to oral terbinafine therapy, (250 mg/d). This patient was provided a month’s supply, along with topical miconazole to be applied twice daily until the rash clears. Use of the latter on the sides of the feet is also advised, for purposes of control.
TAKE-HOME-LEARNING-POINTS
• The performance of the KOH prep can be crucial in terms of establishing the correct diagnosis, but it also gives everyone involved confidence in the diagnosis and treatment. The only way to learn how to do KOH preps is to do them.
• Moccasin-variety tinea pedis is the most common but the least recognized of the three major types. Because it is chronic and asymptomatic, patients rarely know what it is, although it can serve as a reservoir for infection elsewhere.
• Fungal infections don’t just happen. Failure to establish a source can doom the patient to repeated episodes.
• Fungal infections (dermatophytosis) in odd places usually involve immune suppression, typically from the use of topical steroids and/or systemic immunosuppressants.
Surgical Removal of Cyst Yields Unsightly Result
HISTORY
This 24-year-old woman presents to dermatology for evaluation of excessive scarring on her chest. It developed slowly in a spot from which a cyst was surgically removed more than two years ago. In addition to being unsightly, the lesion is sometimes symptomatic: It often tingles and feels “tight.” Worst of all, it still seems to be growing.
She has never experienced anything like this—not even following her C-section several years ago. There is no family history of similar problems. The patient says she tans easily, holds a tan well, and rarely burns in the sun.
EXAMINATION
The lesion is clearly cicatricial, quite firm, and slightly pink. It has rounded edges and an exceptionally smooth surface. Located on the left sternal chest wall, the lesion has obliterated any sign of the original surgical scar, except for peripheral scars left by the sutures. The patient’s skin type is a strong IV/VI.
What is the diagnosis?
DISCUSSION
This is a classic case of keloid formation—a real problem since no good, permanent solution exists. In one form or another, this otherwise attractive woman will likely bear this lesion the rest of her life.
Not all excessive scars are keloids. When scarring is excessive, but the outline of the original wound can still be seen, the result is usually termed hypertrophic scarring. By definition, a true keloid, by its thickness, shape, and width, totally obscures the original insult and, unlike a hypertrophic scar, does not spontaneously involute. Viewed as a continuum, there is normal scarring, inappropriate scarring, and severe inappropriate scarring. Unlike the first two, the latter is almost always symptomatic (burning) and does not spontaneously resolve.
Two things could have alerted the surgeon to the possibility that a keloid would form: (1) location, since the chest, shoulders, ear lobes, and neck are especially prone to inappropriate scarring, and (2) skin type, because in general, the darker the skin is, the greater the tendency to form inappropriate scars. While keloids are commonly a postoperative complication, they are also not infrequently triggered by acne, cysts, or even chickenpox. Other high-tension areas prone to keloids are knees and ankles.
The best thing, obviously, would have been for the patient to avoid having the surgery. An alternative to surgical removal of her cyst might have been intralesional injection with triamcinolone solution (5 mg/cc). While this would have been unlikely to produce a cure, it almost certainly would have shrunk the cyst for months at a time.
When surgery in a high-risk area is necessary (eg, in the case of a skin cancer), the surgical margins can be injected with triamcinolone (2.5 mg/cc) at the time of suture removal and again a month postoperative, which will reduce but not eliminate the risk for keloid formation. Low-tension closure (making generous use of undermining and deep sutures to reduce tension on surface sutures) and proper wound care by the patient can help too.
Surgical removal of a keloid in such a location, in a high-risk patient, is an option. However, such procedures are usually left for the plastic surgeon to deal with. Weapons that can be brought to bear on these difficult lesions include excision, intralesional steroid injection, cryotherapy, ionizing radiation, and laser surgery.
This particular patient chose to have us inject her keloid with 1.5 cc of triamcinolone (20 mg/cc), using a 10-cc syringe and 30-gauge needle. (This was made from stock triamcinolone, which comes in a 40 mg/cc strength, mixed half-and-half with lidocaine 1%.) Had her keloid been so dense as to make injection impossible (which happens fairly often), I would have treated it with liquid nitrogen first (approximately 5 seconds), waited five minutes while the keloid softened, then injected it.
TAKE-HOME-LEARNING-POINTS
• The tendency to form keloids is in large part a function of location and skin type.
• Skin on chests, shoulders, earlobes, and necks is especially prone to keloid formation.
• The darker the patient’s skin, the greater the chance for keloid formation.
• The decision to perform elective surgery should be informed by a full review of the risks involved, including that for keloid formation.
• Alternatives to excision of cysts in high-risk areas include intralesional steroid injection (using their tendency to cause atrophy to advantage), cryotherapy, or benign neglect.
• Once a keloid has formed, no perfect remedy exists. However, several options can be considered: intralesional injection, excision followed by steroid injection of the wound margins, or referral to plastic surgery.
• Viewed as a continuum, scarring can either be normal, excessive (eg, hypertrophic scarring), or lesional (eg keloid). The latter totally obscures the original insult, fails to spontaneously involute, and is often symptomatic (burning).
HISTORY
This 24-year-old woman presents to dermatology for evaluation of excessive scarring on her chest. It developed slowly in a spot from which a cyst was surgically removed more than two years ago. In addition to being unsightly, the lesion is sometimes symptomatic: It often tingles and feels “tight.” Worst of all, it still seems to be growing.
She has never experienced anything like this—not even following her C-section several years ago. There is no family history of similar problems. The patient says she tans easily, holds a tan well, and rarely burns in the sun.
EXAMINATION
The lesion is clearly cicatricial, quite firm, and slightly pink. It has rounded edges and an exceptionally smooth surface. Located on the left sternal chest wall, the lesion has obliterated any sign of the original surgical scar, except for peripheral scars left by the sutures. The patient’s skin type is a strong IV/VI.
What is the diagnosis?
DISCUSSION
This is a classic case of keloid formation—a real problem since no good, permanent solution exists. In one form or another, this otherwise attractive woman will likely bear this lesion the rest of her life.
Not all excessive scars are keloids. When scarring is excessive, but the outline of the original wound can still be seen, the result is usually termed hypertrophic scarring. By definition, a true keloid, by its thickness, shape, and width, totally obscures the original insult and, unlike a hypertrophic scar, does not spontaneously involute. Viewed as a continuum, there is normal scarring, inappropriate scarring, and severe inappropriate scarring. Unlike the first two, the latter is almost always symptomatic (burning) and does not spontaneously resolve.
Two things could have alerted the surgeon to the possibility that a keloid would form: (1) location, since the chest, shoulders, ear lobes, and neck are especially prone to inappropriate scarring, and (2) skin type, because in general, the darker the skin is, the greater the tendency to form inappropriate scars. While keloids are commonly a postoperative complication, they are also not infrequently triggered by acne, cysts, or even chickenpox. Other high-tension areas prone to keloids are knees and ankles.
The best thing, obviously, would have been for the patient to avoid having the surgery. An alternative to surgical removal of her cyst might have been intralesional injection with triamcinolone solution (5 mg/cc). While this would have been unlikely to produce a cure, it almost certainly would have shrunk the cyst for months at a time.
When surgery in a high-risk area is necessary (eg, in the case of a skin cancer), the surgical margins can be injected with triamcinolone (2.5 mg/cc) at the time of suture removal and again a month postoperative, which will reduce but not eliminate the risk for keloid formation. Low-tension closure (making generous use of undermining and deep sutures to reduce tension on surface sutures) and proper wound care by the patient can help too.
Surgical removal of a keloid in such a location, in a high-risk patient, is an option. However, such procedures are usually left for the plastic surgeon to deal with. Weapons that can be brought to bear on these difficult lesions include excision, intralesional steroid injection, cryotherapy, ionizing radiation, and laser surgery.
This particular patient chose to have us inject her keloid with 1.5 cc of triamcinolone (20 mg/cc), using a 10-cc syringe and 30-gauge needle. (This was made from stock triamcinolone, which comes in a 40 mg/cc strength, mixed half-and-half with lidocaine 1%.) Had her keloid been so dense as to make injection impossible (which happens fairly often), I would have treated it with liquid nitrogen first (approximately 5 seconds), waited five minutes while the keloid softened, then injected it.
TAKE-HOME-LEARNING-POINTS
• The tendency to form keloids is in large part a function of location and skin type.
• Skin on chests, shoulders, earlobes, and necks is especially prone to keloid formation.
• The darker the patient’s skin, the greater the chance for keloid formation.
• The decision to perform elective surgery should be informed by a full review of the risks involved, including that for keloid formation.
• Alternatives to excision of cysts in high-risk areas include intralesional steroid injection (using their tendency to cause atrophy to advantage), cryotherapy, or benign neglect.
• Once a keloid has formed, no perfect remedy exists. However, several options can be considered: intralesional injection, excision followed by steroid injection of the wound margins, or referral to plastic surgery.
• Viewed as a continuum, scarring can either be normal, excessive (eg, hypertrophic scarring), or lesional (eg keloid). The latter totally obscures the original insult, fails to spontaneously involute, and is often symptomatic (burning).
HISTORY
This 24-year-old woman presents to dermatology for evaluation of excessive scarring on her chest. It developed slowly in a spot from which a cyst was surgically removed more than two years ago. In addition to being unsightly, the lesion is sometimes symptomatic: It often tingles and feels “tight.” Worst of all, it still seems to be growing.
She has never experienced anything like this—not even following her C-section several years ago. There is no family history of similar problems. The patient says she tans easily, holds a tan well, and rarely burns in the sun.
EXAMINATION
The lesion is clearly cicatricial, quite firm, and slightly pink. It has rounded edges and an exceptionally smooth surface. Located on the left sternal chest wall, the lesion has obliterated any sign of the original surgical scar, except for peripheral scars left by the sutures. The patient’s skin type is a strong IV/VI.
What is the diagnosis?
DISCUSSION
This is a classic case of keloid formation—a real problem since no good, permanent solution exists. In one form or another, this otherwise attractive woman will likely bear this lesion the rest of her life.
Not all excessive scars are keloids. When scarring is excessive, but the outline of the original wound can still be seen, the result is usually termed hypertrophic scarring. By definition, a true keloid, by its thickness, shape, and width, totally obscures the original insult and, unlike a hypertrophic scar, does not spontaneously involute. Viewed as a continuum, there is normal scarring, inappropriate scarring, and severe inappropriate scarring. Unlike the first two, the latter is almost always symptomatic (burning) and does not spontaneously resolve.
Two things could have alerted the surgeon to the possibility that a keloid would form: (1) location, since the chest, shoulders, ear lobes, and neck are especially prone to inappropriate scarring, and (2) skin type, because in general, the darker the skin is, the greater the tendency to form inappropriate scars. While keloids are commonly a postoperative complication, they are also not infrequently triggered by acne, cysts, or even chickenpox. Other high-tension areas prone to keloids are knees and ankles.
The best thing, obviously, would have been for the patient to avoid having the surgery. An alternative to surgical removal of her cyst might have been intralesional injection with triamcinolone solution (5 mg/cc). While this would have been unlikely to produce a cure, it almost certainly would have shrunk the cyst for months at a time.
When surgery in a high-risk area is necessary (eg, in the case of a skin cancer), the surgical margins can be injected with triamcinolone (2.5 mg/cc) at the time of suture removal and again a month postoperative, which will reduce but not eliminate the risk for keloid formation. Low-tension closure (making generous use of undermining and deep sutures to reduce tension on surface sutures) and proper wound care by the patient can help too.
Surgical removal of a keloid in such a location, in a high-risk patient, is an option. However, such procedures are usually left for the plastic surgeon to deal with. Weapons that can be brought to bear on these difficult lesions include excision, intralesional steroid injection, cryotherapy, ionizing radiation, and laser surgery.
This particular patient chose to have us inject her keloid with 1.5 cc of triamcinolone (20 mg/cc), using a 10-cc syringe and 30-gauge needle. (This was made from stock triamcinolone, which comes in a 40 mg/cc strength, mixed half-and-half with lidocaine 1%.) Had her keloid been so dense as to make injection impossible (which happens fairly often), I would have treated it with liquid nitrogen first (approximately 5 seconds), waited five minutes while the keloid softened, then injected it.
TAKE-HOME-LEARNING-POINTS
• The tendency to form keloids is in large part a function of location and skin type.
• Skin on chests, shoulders, earlobes, and necks is especially prone to keloid formation.
• The darker the patient’s skin, the greater the chance for keloid formation.
• The decision to perform elective surgery should be informed by a full review of the risks involved, including that for keloid formation.
• Alternatives to excision of cysts in high-risk areas include intralesional steroid injection (using their tendency to cause atrophy to advantage), cryotherapy, or benign neglect.
• Once a keloid has formed, no perfect remedy exists. However, several options can be considered: intralesional injection, excision followed by steroid injection of the wound margins, or referral to plastic surgery.
• Viewed as a continuum, scarring can either be normal, excessive (eg, hypertrophic scarring), or lesional (eg keloid). The latter totally obscures the original insult, fails to spontaneously involute, and is often symptomatic (burning).
Poor prognosis emphasizes need for prevention
A 73-year-old man self-refers to dermatology 18 months after a melanoma was diagnosed and removed from his forearm. Following that discovery, he was referred to a surgeon, who performed a wide excision (the defect from which was closed with a graft) and who went on to do lymph node dissection in the ipsilateral axilla. No positive nodes were found.
The wounds from these procedures are long since healed, and the patient has been doing well. That is, until recently, when he noticed some new lesions developing around the graft site.
EXAMINATION
About 15 to 20 firm, blue-black papules and nodules surround the periphery of the graft site on the patient’s forearm. Some extend out as far as 10 cm, though most are within 3 cm. Obviously intradermal, these lesions display no surface change at all. Punch biopsy confirms the suspicion that these represent satellite metastasis of the patient’s original melanoma, which itself had been more than 3 mm thick.
Fortunately, no nodes are palpable in the axilla, and no evidence of metastasis is found on physical examination, blood work, and PET scan.
DISCUSSION
The image accompanying this case is pregnant with information—some obvious, some less so. For example, the multiple blue-black nodules can easily be seen surrounding the graft site and were just as easily palpated.
Even ignoring those lesions momentarily, a look at the surrounding skin offers a veritable textbook of germane information. The collective term for the skin changes on the patient’s arms is dermatoheliosis, or sun-damaged skin. But that term comprises a number of specific changes, all of which have names and significance.
The casual observer might simply chalk these changes up to age, but for medical providers, more specifics are in order: The sun has thinned the patient’s skin remarkably, hence the term solar atrophy. His dorsal forearms are greatly discolored as well, changes we call poikiloderma. Numerous telangiectasias (also sun-caused) can be seen on his dorsal forearms. These changes are especially appreciated when the dorsal forearm skin is compared to the extensor forearms, which receive relatively little sun exposure.
The point? This patient had every reason to develop a melanoma, making any odd lesion on his skin suspicious. It also means his chances of developing a new primary melanoma are all too real, even if he survives the current one.
As one might imagine, this local recurrence of his melanoma is not a good sign at all. Strictly speaking, it is a form of metastasis—but until it reaches lymph nodes or organs, it only suggests that possibility.
Treatment choices are limited for his melanoma, but include limb perfusion, chemotherapy, and surgery. The truth is, his prognosis is poor. His case emphasizes the need for prevention and early diagnosis, the latter greatly aided by the recognition of patients at risk by virtue of having fair, sun-damaged skin.
As often happens in cases like this, there is a ripple effect as the news of his situation reaches family and friends, whose own skin becomes the subject of attention. In such cases, it’s not unusual for the whole family to then be seen in dermatology over the succeeding months—not only to be examined, but also hopefully educated in terms of prevention and recognition.
TAKE-HOME LEARNING POINTS
• Local recurrence of melanoma is common, especially with primary tumors that exceed 3 mm in thickness.
• UV overexposure has been established as the major contributor to development of melanoma.
• Melanoma is far more common in fair-skinned individuals than in those with darker skin; “fair” is defined as tolerating sun poorly, burning easily, and tanning poorly, if at all.
• Evidence of this excessive sun damage is called dermatoheliosis and consists of specific findings including solar atrophy, telangiectasias, and pigmentary alteration known as poikiloderma.
• The lack of effective treatment for metastatic melanoma underlines the necessity for prevention (protection from the sun) and early detection.
A 73-year-old man self-refers to dermatology 18 months after a melanoma was diagnosed and removed from his forearm. Following that discovery, he was referred to a surgeon, who performed a wide excision (the defect from which was closed with a graft) and who went on to do lymph node dissection in the ipsilateral axilla. No positive nodes were found.
The wounds from these procedures are long since healed, and the patient has been doing well. That is, until recently, when he noticed some new lesions developing around the graft site.
EXAMINATION
About 15 to 20 firm, blue-black papules and nodules surround the periphery of the graft site on the patient’s forearm. Some extend out as far as 10 cm, though most are within 3 cm. Obviously intradermal, these lesions display no surface change at all. Punch biopsy confirms the suspicion that these represent satellite metastasis of the patient’s original melanoma, which itself had been more than 3 mm thick.
Fortunately, no nodes are palpable in the axilla, and no evidence of metastasis is found on physical examination, blood work, and PET scan.
DISCUSSION
The image accompanying this case is pregnant with information—some obvious, some less so. For example, the multiple blue-black nodules can easily be seen surrounding the graft site and were just as easily palpated.
Even ignoring those lesions momentarily, a look at the surrounding skin offers a veritable textbook of germane information. The collective term for the skin changes on the patient’s arms is dermatoheliosis, or sun-damaged skin. But that term comprises a number of specific changes, all of which have names and significance.
The casual observer might simply chalk these changes up to age, but for medical providers, more specifics are in order: The sun has thinned the patient’s skin remarkably, hence the term solar atrophy. His dorsal forearms are greatly discolored as well, changes we call poikiloderma. Numerous telangiectasias (also sun-caused) can be seen on his dorsal forearms. These changes are especially appreciated when the dorsal forearm skin is compared to the extensor forearms, which receive relatively little sun exposure.
The point? This patient had every reason to develop a melanoma, making any odd lesion on his skin suspicious. It also means his chances of developing a new primary melanoma are all too real, even if he survives the current one.
As one might imagine, this local recurrence of his melanoma is not a good sign at all. Strictly speaking, it is a form of metastasis—but until it reaches lymph nodes or organs, it only suggests that possibility.
Treatment choices are limited for his melanoma, but include limb perfusion, chemotherapy, and surgery. The truth is, his prognosis is poor. His case emphasizes the need for prevention and early diagnosis, the latter greatly aided by the recognition of patients at risk by virtue of having fair, sun-damaged skin.
As often happens in cases like this, there is a ripple effect as the news of his situation reaches family and friends, whose own skin becomes the subject of attention. In such cases, it’s not unusual for the whole family to then be seen in dermatology over the succeeding months—not only to be examined, but also hopefully educated in terms of prevention and recognition.
TAKE-HOME LEARNING POINTS
• Local recurrence of melanoma is common, especially with primary tumors that exceed 3 mm in thickness.
• UV overexposure has been established as the major contributor to development of melanoma.
• Melanoma is far more common in fair-skinned individuals than in those with darker skin; “fair” is defined as tolerating sun poorly, burning easily, and tanning poorly, if at all.
• Evidence of this excessive sun damage is called dermatoheliosis and consists of specific findings including solar atrophy, telangiectasias, and pigmentary alteration known as poikiloderma.
• The lack of effective treatment for metastatic melanoma underlines the necessity for prevention (protection from the sun) and early detection.
A 73-year-old man self-refers to dermatology 18 months after a melanoma was diagnosed and removed from his forearm. Following that discovery, he was referred to a surgeon, who performed a wide excision (the defect from which was closed with a graft) and who went on to do lymph node dissection in the ipsilateral axilla. No positive nodes were found.
The wounds from these procedures are long since healed, and the patient has been doing well. That is, until recently, when he noticed some new lesions developing around the graft site.
EXAMINATION
About 15 to 20 firm, blue-black papules and nodules surround the periphery of the graft site on the patient’s forearm. Some extend out as far as 10 cm, though most are within 3 cm. Obviously intradermal, these lesions display no surface change at all. Punch biopsy confirms the suspicion that these represent satellite metastasis of the patient’s original melanoma, which itself had been more than 3 mm thick.
Fortunately, no nodes are palpable in the axilla, and no evidence of metastasis is found on physical examination, blood work, and PET scan.
DISCUSSION
The image accompanying this case is pregnant with information—some obvious, some less so. For example, the multiple blue-black nodules can easily be seen surrounding the graft site and were just as easily palpated.
Even ignoring those lesions momentarily, a look at the surrounding skin offers a veritable textbook of germane information. The collective term for the skin changes on the patient’s arms is dermatoheliosis, or sun-damaged skin. But that term comprises a number of specific changes, all of which have names and significance.
The casual observer might simply chalk these changes up to age, but for medical providers, more specifics are in order: The sun has thinned the patient’s skin remarkably, hence the term solar atrophy. His dorsal forearms are greatly discolored as well, changes we call poikiloderma. Numerous telangiectasias (also sun-caused) can be seen on his dorsal forearms. These changes are especially appreciated when the dorsal forearm skin is compared to the extensor forearms, which receive relatively little sun exposure.
The point? This patient had every reason to develop a melanoma, making any odd lesion on his skin suspicious. It also means his chances of developing a new primary melanoma are all too real, even if he survives the current one.
As one might imagine, this local recurrence of his melanoma is not a good sign at all. Strictly speaking, it is a form of metastasis—but until it reaches lymph nodes or organs, it only suggests that possibility.
Treatment choices are limited for his melanoma, but include limb perfusion, chemotherapy, and surgery. The truth is, his prognosis is poor. His case emphasizes the need for prevention and early diagnosis, the latter greatly aided by the recognition of patients at risk by virtue of having fair, sun-damaged skin.
As often happens in cases like this, there is a ripple effect as the news of his situation reaches family and friends, whose own skin becomes the subject of attention. In such cases, it’s not unusual for the whole family to then be seen in dermatology over the succeeding months—not only to be examined, but also hopefully educated in terms of prevention and recognition.
TAKE-HOME LEARNING POINTS
• Local recurrence of melanoma is common, especially with primary tumors that exceed 3 mm in thickness.
• UV overexposure has been established as the major contributor to development of melanoma.
• Melanoma is far more common in fair-skinned individuals than in those with darker skin; “fair” is defined as tolerating sun poorly, burning easily, and tanning poorly, if at all.
• Evidence of this excessive sun damage is called dermatoheliosis and consists of specific findings including solar atrophy, telangiectasias, and pigmentary alteration known as poikiloderma.
• The lack of effective treatment for metastatic melanoma underlines the necessity for prevention (protection from the sun) and early detection.
Reaching Through the Smoke Screen
A 41-year-old woman came in for evaluation of two lesions: one on the dorsum of the left hand, the other on the lateral aspect of the right calf. Both had been present about a year, growing slowly. Neither caused any discomfort, but they were of concern nonetheless. A friend who had seen them insisted that the patient seek evaluation.
On examination, I strongly suspected both lesions were basal cell carcinomas, even though these are unusual on 41-year-old patients. (They are much more common on those 60 or older who usually have had far more time to accumulate the requisite sun exposure). But this patient’s skin, while dark, was extraordinarily sun-damaged, explained by the patient as due to her “love of the great outdoors.”
But there was more going on. For one thing, she had a mask of irregular brownish hyperpigmentation covering her forehead, cheeks, and maxilla. On questioning, this turned out to have been present for at least 10 years, darkening over time. Melasma, also known as the “mask of pregnancy,” was what we were dealing with, even though the patient had never been pregnant or taken birth control (the usual sources of the requisite estrogen); excessive UV exposure and darker skin predispose individuals to this common condition. We talked about her melasma for a few minutes, and I referred her to our cosmetic dermatology section for treatment.
But beyond the sun damage and melasma, there was still a sallow look to her skin. “How’s your health, in general?” I asked, as I often do. Patients tend to assume we know all about their history of hepatitis or liver transplant. But, no, she hadn’t had any of those issues and was otherwise healthy, she said.
Suddenly inspired, I finally asked the right question: “Are you a smoker?”
Tears welled in her eyes. “Yes, I’ve smoked for more than 20 years. How did you know? Do I smell like cigarettes?”
“No,” I responded. “And no offense, but I can see changes in your skin—yellowing—caused by smoking. There’s a way to make that better. Do you have any interest in quitting?”
Though stunned by this information, fortunately she was very much interested in becoming an ex-smoker—arguably, the most significant issue uncovered on this visit. Basal cell carcinomas, serious as they can be, can be removed and hopefully prevented in the future. Melasma is treatable and “merely” a cosmetic issue. But the effects of smoking are protean.
Even though it’s not our primary task to counsel smokers, when I identify one who seems willing to learn about smoking cessation strategies, I take the time to do it. Fortunately, she was the last patient of the morning, so we had an extra five minutes at our disposal.
I could tell my message was well received by the way she listened intently. I think she knew this was potentially the most important day in her life. We’ll see. But studies show that this kind of brief counseling session is amazingly effective in getting patients to stop smoking.
To many providers, such interventions seem like a waste of time, maybe even rude. But I would assert that patients like this one interpret our silence as tacit approval for continuing to smoke. I hear this a lot: “My doctors all tell me I’m healthy. They’ve never even asked me about smoking.”
Is there a more fit topic to discuss with patients than that of smoking, which kills more Americans than murder, suicide, the effects of drug or alcohol abuse, HIV, motor vehicle accidents, drowning, and gunshot wounds combined? And that doesn’t begin to address the millions who don’t die an early death but who have sharply reduced quality of life from the effects of smoking (chronic lung diseases, stroke, heart attack, and bladder cancer, just to name a few).
It is, far and away, the biggest preventable health care problem in this country, the impact of which is almost impossible to overstate. And all this for the only product sold in the US that is not only utterly devoid of an upside, but also certain to create health problems when it is used as intended.
A 41-year-old woman came in for evaluation of two lesions: one on the dorsum of the left hand, the other on the lateral aspect of the right calf. Both had been present about a year, growing slowly. Neither caused any discomfort, but they were of concern nonetheless. A friend who had seen them insisted that the patient seek evaluation.
On examination, I strongly suspected both lesions were basal cell carcinomas, even though these are unusual on 41-year-old patients. (They are much more common on those 60 or older who usually have had far more time to accumulate the requisite sun exposure). But this patient’s skin, while dark, was extraordinarily sun-damaged, explained by the patient as due to her “love of the great outdoors.”
But there was more going on. For one thing, she had a mask of irregular brownish hyperpigmentation covering her forehead, cheeks, and maxilla. On questioning, this turned out to have been present for at least 10 years, darkening over time. Melasma, also known as the “mask of pregnancy,” was what we were dealing with, even though the patient had never been pregnant or taken birth control (the usual sources of the requisite estrogen); excessive UV exposure and darker skin predispose individuals to this common condition. We talked about her melasma for a few minutes, and I referred her to our cosmetic dermatology section for treatment.
But beyond the sun damage and melasma, there was still a sallow look to her skin. “How’s your health, in general?” I asked, as I often do. Patients tend to assume we know all about their history of hepatitis or liver transplant. But, no, she hadn’t had any of those issues and was otherwise healthy, she said.
Suddenly inspired, I finally asked the right question: “Are you a smoker?”
Tears welled in her eyes. “Yes, I’ve smoked for more than 20 years. How did you know? Do I smell like cigarettes?”
“No,” I responded. “And no offense, but I can see changes in your skin—yellowing—caused by smoking. There’s a way to make that better. Do you have any interest in quitting?”
Though stunned by this information, fortunately she was very much interested in becoming an ex-smoker—arguably, the most significant issue uncovered on this visit. Basal cell carcinomas, serious as they can be, can be removed and hopefully prevented in the future. Melasma is treatable and “merely” a cosmetic issue. But the effects of smoking are protean.
Even though it’s not our primary task to counsel smokers, when I identify one who seems willing to learn about smoking cessation strategies, I take the time to do it. Fortunately, she was the last patient of the morning, so we had an extra five minutes at our disposal.
I could tell my message was well received by the way she listened intently. I think she knew this was potentially the most important day in her life. We’ll see. But studies show that this kind of brief counseling session is amazingly effective in getting patients to stop smoking.
To many providers, such interventions seem like a waste of time, maybe even rude. But I would assert that patients like this one interpret our silence as tacit approval for continuing to smoke. I hear this a lot: “My doctors all tell me I’m healthy. They’ve never even asked me about smoking.”
Is there a more fit topic to discuss with patients than that of smoking, which kills more Americans than murder, suicide, the effects of drug or alcohol abuse, HIV, motor vehicle accidents, drowning, and gunshot wounds combined? And that doesn’t begin to address the millions who don’t die an early death but who have sharply reduced quality of life from the effects of smoking (chronic lung diseases, stroke, heart attack, and bladder cancer, just to name a few).
It is, far and away, the biggest preventable health care problem in this country, the impact of which is almost impossible to overstate. And all this for the only product sold in the US that is not only utterly devoid of an upside, but also certain to create health problems when it is used as intended.
A 41-year-old woman came in for evaluation of two lesions: one on the dorsum of the left hand, the other on the lateral aspect of the right calf. Both had been present about a year, growing slowly. Neither caused any discomfort, but they were of concern nonetheless. A friend who had seen them insisted that the patient seek evaluation.
On examination, I strongly suspected both lesions were basal cell carcinomas, even though these are unusual on 41-year-old patients. (They are much more common on those 60 or older who usually have had far more time to accumulate the requisite sun exposure). But this patient’s skin, while dark, was extraordinarily sun-damaged, explained by the patient as due to her “love of the great outdoors.”
But there was more going on. For one thing, she had a mask of irregular brownish hyperpigmentation covering her forehead, cheeks, and maxilla. On questioning, this turned out to have been present for at least 10 years, darkening over time. Melasma, also known as the “mask of pregnancy,” was what we were dealing with, even though the patient had never been pregnant or taken birth control (the usual sources of the requisite estrogen); excessive UV exposure and darker skin predispose individuals to this common condition. We talked about her melasma for a few minutes, and I referred her to our cosmetic dermatology section for treatment.
But beyond the sun damage and melasma, there was still a sallow look to her skin. “How’s your health, in general?” I asked, as I often do. Patients tend to assume we know all about their history of hepatitis or liver transplant. But, no, she hadn’t had any of those issues and was otherwise healthy, she said.
Suddenly inspired, I finally asked the right question: “Are you a smoker?”
Tears welled in her eyes. “Yes, I’ve smoked for more than 20 years. How did you know? Do I smell like cigarettes?”
“No,” I responded. “And no offense, but I can see changes in your skin—yellowing—caused by smoking. There’s a way to make that better. Do you have any interest in quitting?”
Though stunned by this information, fortunately she was very much interested in becoming an ex-smoker—arguably, the most significant issue uncovered on this visit. Basal cell carcinomas, serious as they can be, can be removed and hopefully prevented in the future. Melasma is treatable and “merely” a cosmetic issue. But the effects of smoking are protean.
Even though it’s not our primary task to counsel smokers, when I identify one who seems willing to learn about smoking cessation strategies, I take the time to do it. Fortunately, she was the last patient of the morning, so we had an extra five minutes at our disposal.
I could tell my message was well received by the way she listened intently. I think she knew this was potentially the most important day in her life. We’ll see. But studies show that this kind of brief counseling session is amazingly effective in getting patients to stop smoking.
To many providers, such interventions seem like a waste of time, maybe even rude. But I would assert that patients like this one interpret our silence as tacit approval for continuing to smoke. I hear this a lot: “My doctors all tell me I’m healthy. They’ve never even asked me about smoking.”
Is there a more fit topic to discuss with patients than that of smoking, which kills more Americans than murder, suicide, the effects of drug or alcohol abuse, HIV, motor vehicle accidents, drowning, and gunshot wounds combined? And that doesn’t begin to address the millions who don’t die an early death but who have sharply reduced quality of life from the effects of smoking (chronic lung diseases, stroke, heart attack, and bladder cancer, just to name a few).
It is, far and away, the biggest preventable health care problem in this country, the impact of which is almost impossible to overstate. And all this for the only product sold in the US that is not only utterly devoid of an upside, but also certain to create health problems when it is used as intended.
Is Leprosy the Cause of This Girl's Lesion?
ANSWER
The correct answer is postinflammatory hypopigmentation (choice “c”), in this case secondary to eczema in a classic antecubital location. Leprosy (choice “a”) is more common than one might imagine, but it does not appear overnight and does not involve overt inflammation. Vitiligo (choice “b”) does not appear suddenly and rarely involves the type of inflammation seen in this case. Lichen sclerosis et atrophicus (choice “d”) is an inflammatory condition that presents with hypopigmentation and epidermal atrophy; however, it is gradual in onset and would not exhibit papulosquamous inflammation.
DISCUSSION
The more color in the skin, the more the loss of that color stands out. Patients and families with darker skin are often understandably upset by the contrast. Providers need a differential for pigment loss, including the items mentioned—some of which have the potential to be dreadfully serious.
Two relevant facts stand out in this case: the rapidity of onset and the history of eczema, in which secondary pigment loss can occur. As mentioned, it is especially obvious in those with darker skin. Fortunately, once the eczema calms down, the hypopigmentation resolves and normal color returns.
Paradoxically, it’s not at all unusual to see postinflammatory hyperpigmentation, especially in those with skin of types IV and V (eg, African-Americans, some Hispanics, and those of Indian ancestry). Eczema is a common cause, but the inflammation can be from almost any source, including trauma, burns, or even acne.
Had this patient’s diagnosis not been obvious, a biopsy might have been indicated due to the serious nature of some of the items in the differential. Vitiligo, for example, can be very disfiguring, especially on a dark-skinned individual. It tends to become widespread and permanent, unless it’s caught and treated early on. Other conditions involving hypopigmentation include sarcoidosis, lupus, and morphea.
All of these conditions are unusual, if not rare, compared with atopic dermatitis (AD), which this patient has. AD is so common that almost 20% of newborns develop it. Eczema is one of the more typical manifestations, along with dry, sensitive skin, seasonal allergies, and reactive airway disease. Corroboration of the diagnosis is usually easily accomplished by taking a family history.
TREATMENT
Fortunately, this patient’s hypopigmentation will resolve quickly with treatment of her eczema, using a low-strength steroid cream (eg, hydrocortisone 2.5% cream or ointment). But a good portion of the “treatment” of AD is done by educating the family about the nature of the condition, as well as providing reassurance about the absence of the more serious items in the differential.
ANSWER
The correct answer is postinflammatory hypopigmentation (choice “c”), in this case secondary to eczema in a classic antecubital location. Leprosy (choice “a”) is more common than one might imagine, but it does not appear overnight and does not involve overt inflammation. Vitiligo (choice “b”) does not appear suddenly and rarely involves the type of inflammation seen in this case. Lichen sclerosis et atrophicus (choice “d”) is an inflammatory condition that presents with hypopigmentation and epidermal atrophy; however, it is gradual in onset and would not exhibit papulosquamous inflammation.
DISCUSSION
The more color in the skin, the more the loss of that color stands out. Patients and families with darker skin are often understandably upset by the contrast. Providers need a differential for pigment loss, including the items mentioned—some of which have the potential to be dreadfully serious.
Two relevant facts stand out in this case: the rapidity of onset and the history of eczema, in which secondary pigment loss can occur. As mentioned, it is especially obvious in those with darker skin. Fortunately, once the eczema calms down, the hypopigmentation resolves and normal color returns.
Paradoxically, it’s not at all unusual to see postinflammatory hyperpigmentation, especially in those with skin of types IV and V (eg, African-Americans, some Hispanics, and those of Indian ancestry). Eczema is a common cause, but the inflammation can be from almost any source, including trauma, burns, or even acne.
Had this patient’s diagnosis not been obvious, a biopsy might have been indicated due to the serious nature of some of the items in the differential. Vitiligo, for example, can be very disfiguring, especially on a dark-skinned individual. It tends to become widespread and permanent, unless it’s caught and treated early on. Other conditions involving hypopigmentation include sarcoidosis, lupus, and morphea.
All of these conditions are unusual, if not rare, compared with atopic dermatitis (AD), which this patient has. AD is so common that almost 20% of newborns develop it. Eczema is one of the more typical manifestations, along with dry, sensitive skin, seasonal allergies, and reactive airway disease. Corroboration of the diagnosis is usually easily accomplished by taking a family history.
TREATMENT
Fortunately, this patient’s hypopigmentation will resolve quickly with treatment of her eczema, using a low-strength steroid cream (eg, hydrocortisone 2.5% cream or ointment). But a good portion of the “treatment” of AD is done by educating the family about the nature of the condition, as well as providing reassurance about the absence of the more serious items in the differential.
ANSWER
The correct answer is postinflammatory hypopigmentation (choice “c”), in this case secondary to eczema in a classic antecubital location. Leprosy (choice “a”) is more common than one might imagine, but it does not appear overnight and does not involve overt inflammation. Vitiligo (choice “b”) does not appear suddenly and rarely involves the type of inflammation seen in this case. Lichen sclerosis et atrophicus (choice “d”) is an inflammatory condition that presents with hypopigmentation and epidermal atrophy; however, it is gradual in onset and would not exhibit papulosquamous inflammation.
DISCUSSION
The more color in the skin, the more the loss of that color stands out. Patients and families with darker skin are often understandably upset by the contrast. Providers need a differential for pigment loss, including the items mentioned—some of which have the potential to be dreadfully serious.
Two relevant facts stand out in this case: the rapidity of onset and the history of eczema, in which secondary pigment loss can occur. As mentioned, it is especially obvious in those with darker skin. Fortunately, once the eczema calms down, the hypopigmentation resolves and normal color returns.
Paradoxically, it’s not at all unusual to see postinflammatory hyperpigmentation, especially in those with skin of types IV and V (eg, African-Americans, some Hispanics, and those of Indian ancestry). Eczema is a common cause, but the inflammation can be from almost any source, including trauma, burns, or even acne.
Had this patient’s diagnosis not been obvious, a biopsy might have been indicated due to the serious nature of some of the items in the differential. Vitiligo, for example, can be very disfiguring, especially on a dark-skinned individual. It tends to become widespread and permanent, unless it’s caught and treated early on. Other conditions involving hypopigmentation include sarcoidosis, lupus, and morphea.
All of these conditions are unusual, if not rare, compared with atopic dermatitis (AD), which this patient has. AD is so common that almost 20% of newborns develop it. Eczema is one of the more typical manifestations, along with dry, sensitive skin, seasonal allergies, and reactive airway disease. Corroboration of the diagnosis is usually easily accomplished by taking a family history.
TREATMENT
Fortunately, this patient’s hypopigmentation will resolve quickly with treatment of her eczema, using a low-strength steroid cream (eg, hydrocortisone 2.5% cream or ointment). But a good portion of the “treatment” of AD is done by educating the family about the nature of the condition, as well as providing reassurance about the absence of the more serious items in the differential.
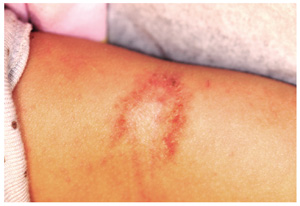
The parents of this 8-month-old infant are alarmed by skin changes that occurred practically overnight on the child’s arm—especially since the child’s grandparents suggested it might represent vitiligo or even leprosy. The child’s pediatrician thought “ringworm” was more likely, but the clotrimazole cream he recommended was no help. The child has an extensive history of atopy, including eczema affecting the trunk and face. The parents have used topical steroid cream on the affected areas with some good effect, but the loss of color in the antecubital site has made them reluctant to use the product on this new site. Examination shows a papulosquamous lesion, 3.5 cm in diameter, on the left lateral antecubital area, with marked central hypopigmentation. The child and her parents are Vietnamese, with type IV skin, making the pigment loss all the more obvious. The periphery of the lesion, in addition to being bumpy and scaly, is moderately inflamed. The rest of the child’s skin is dry but otherwise unremarkable.
Elderly woman baffled by signs of "aging"
HISTORY
A 91-year-old woman is mortified when a friend comments on the “age spots” on the skin of her neck.
“In the first place,” the patient retorts while recounting the story, “I’m not that old. And in the second place, I don’t see anything there—what’s she talking about?”
She is truly upset about what she feels were uncalled-for comments. But more than that, she has no idea what her friend could be referring to. No one else has ever said anything negative about her skin—in fact, everyone who meets her marvels at how young she looks for her age.
On examination, her skin is quite fair and shows extensive signs of sun damage. There is extreme widespread mottling, in colors ranging from yellow to orange, and exceptionally pronounced wrinkling on sun-exposed areas of her face, neck, arms, and chest. Notably, the area of the anterior neck shaded by her chin is pristine and white. Fortunately, no cancerous or other worrisome lesions are seen.
DIAGNOSIS/DISCUSSION
This case illustrates a number of related phenomena. For example, it was shocking that this patient–one of most sun-damaged I’ve ever seen–was unaware of such obvious changes. But these changes had been present for so long and manifested so gradually that they escaped her notice. (Not to mention, the eyesight of a 91-year-old is probably not what it once was.)
Furthermore, when informed that her skin’s condition was a result of sun exposure, she was sure we had lost our minds, because she had not been in the sun “at all” for many, many years. According to her daughter, this was true. But the patient had overlooked the fact that she had grown up on a farm, worked in the fields, played outside, swam and fished, all the while getting a great deal of sun exposure, until she married in her late teens, had children, and moved to town. Being so busy and so fair, she had neither the time nor the inclination to get outdoors much, and that was that—or so she thought. This is a very common set of circumstances for dermatology patients.
Little did she realize that it takes decades (30 to 40 years) for the accumulated effects of sun damage to show up, in the form we see here. This type of “aging” is an example of what we call extrinsic aging. Besides sun, it can be worsened by the effects of wind, low humidity, smoking, alcohol intake, obesity, and some medical conditions. Intrinsic aging, which includes wrinkles, sagging, and general loss of elasticity, is influenced by age, heredity, and ultimately, gravity.
From our standpoint as medical providers, perhaps the most significant issue with this patient is her increased risk for sun-caused skin cancer, specifically basal cell carcinoma (BCC), squamous cell carcinoma (SCC), or melanoma (the three most common forms). Her risk for development of the first two is huge, arguably a certainty given her age, extremely fair skin, and degree of sun damage. Melanomas are different in many ways, actually becoming less likely (statistically) at her age than at age 50. They are also not as much the result of the accumulated effects of sun exposure as are BCC/SCCs.
So, perhaps the most significant outcome of this visit was to get the patient scheduled to see us biannually for skin checks. In our system, that means twice-yearly reminder phone calls to ensure that this actually happens.
TAKE-HOME LEARNING POINTS
• It takes three to five decades for sun damage to eventuate in dermatoheliosis/basal cell carcinoma/squamous cell carcinoma.
• Melanoma is different, since it appears to be related to episodic, poorly tolerated, intense sun damage early in life; the average age of melanoma patients is about 40.
• Aging can be intrinsic (“normal” loss of elasticity, plus the effects of gravity, modified by heredity) in nature.
• Aging can also be extrinsic, caused by UV exposure, smoking, alcohol consumption, wind, and decreased humidity, eventuating in telangiectasias, actinic keratoses, solar elastosis, solar lentigines, atrophy, purpura, poikilodermatous changes, and sun-caused skin cancers.
• Older patients are often incredulous about the role of childhood sun exposure in the eventual development of dermatoheliosis.
HISTORY
A 91-year-old woman is mortified when a friend comments on the “age spots” on the skin of her neck.
“In the first place,” the patient retorts while recounting the story, “I’m not that old. And in the second place, I don’t see anything there—what’s she talking about?”
She is truly upset about what she feels were uncalled-for comments. But more than that, she has no idea what her friend could be referring to. No one else has ever said anything negative about her skin—in fact, everyone who meets her marvels at how young she looks for her age.
On examination, her skin is quite fair and shows extensive signs of sun damage. There is extreme widespread mottling, in colors ranging from yellow to orange, and exceptionally pronounced wrinkling on sun-exposed areas of her face, neck, arms, and chest. Notably, the area of the anterior neck shaded by her chin is pristine and white. Fortunately, no cancerous or other worrisome lesions are seen.
DIAGNOSIS/DISCUSSION
This case illustrates a number of related phenomena. For example, it was shocking that this patient–one of most sun-damaged I’ve ever seen–was unaware of such obvious changes. But these changes had been present for so long and manifested so gradually that they escaped her notice. (Not to mention, the eyesight of a 91-year-old is probably not what it once was.)
Furthermore, when informed that her skin’s condition was a result of sun exposure, she was sure we had lost our minds, because she had not been in the sun “at all” for many, many years. According to her daughter, this was true. But the patient had overlooked the fact that she had grown up on a farm, worked in the fields, played outside, swam and fished, all the while getting a great deal of sun exposure, until she married in her late teens, had children, and moved to town. Being so busy and so fair, she had neither the time nor the inclination to get outdoors much, and that was that—or so she thought. This is a very common set of circumstances for dermatology patients.
Little did she realize that it takes decades (30 to 40 years) for the accumulated effects of sun damage to show up, in the form we see here. This type of “aging” is an example of what we call extrinsic aging. Besides sun, it can be worsened by the effects of wind, low humidity, smoking, alcohol intake, obesity, and some medical conditions. Intrinsic aging, which includes wrinkles, sagging, and general loss of elasticity, is influenced by age, heredity, and ultimately, gravity.
From our standpoint as medical providers, perhaps the most significant issue with this patient is her increased risk for sun-caused skin cancer, specifically basal cell carcinoma (BCC), squamous cell carcinoma (SCC), or melanoma (the three most common forms). Her risk for development of the first two is huge, arguably a certainty given her age, extremely fair skin, and degree of sun damage. Melanomas are different in many ways, actually becoming less likely (statistically) at her age than at age 50. They are also not as much the result of the accumulated effects of sun exposure as are BCC/SCCs.
So, perhaps the most significant outcome of this visit was to get the patient scheduled to see us biannually for skin checks. In our system, that means twice-yearly reminder phone calls to ensure that this actually happens.
TAKE-HOME LEARNING POINTS
• It takes three to five decades for sun damage to eventuate in dermatoheliosis/basal cell carcinoma/squamous cell carcinoma.
• Melanoma is different, since it appears to be related to episodic, poorly tolerated, intense sun damage early in life; the average age of melanoma patients is about 40.
• Aging can be intrinsic (“normal” loss of elasticity, plus the effects of gravity, modified by heredity) in nature.
• Aging can also be extrinsic, caused by UV exposure, smoking, alcohol consumption, wind, and decreased humidity, eventuating in telangiectasias, actinic keratoses, solar elastosis, solar lentigines, atrophy, purpura, poikilodermatous changes, and sun-caused skin cancers.
• Older patients are often incredulous about the role of childhood sun exposure in the eventual development of dermatoheliosis.
HISTORY
A 91-year-old woman is mortified when a friend comments on the “age spots” on the skin of her neck.
“In the first place,” the patient retorts while recounting the story, “I’m not that old. And in the second place, I don’t see anything there—what’s she talking about?”
She is truly upset about what she feels were uncalled-for comments. But more than that, she has no idea what her friend could be referring to. No one else has ever said anything negative about her skin—in fact, everyone who meets her marvels at how young she looks for her age.
On examination, her skin is quite fair and shows extensive signs of sun damage. There is extreme widespread mottling, in colors ranging from yellow to orange, and exceptionally pronounced wrinkling on sun-exposed areas of her face, neck, arms, and chest. Notably, the area of the anterior neck shaded by her chin is pristine and white. Fortunately, no cancerous or other worrisome lesions are seen.
DIAGNOSIS/DISCUSSION
This case illustrates a number of related phenomena. For example, it was shocking that this patient–one of most sun-damaged I’ve ever seen–was unaware of such obvious changes. But these changes had been present for so long and manifested so gradually that they escaped her notice. (Not to mention, the eyesight of a 91-year-old is probably not what it once was.)
Furthermore, when informed that her skin’s condition was a result of sun exposure, she was sure we had lost our minds, because she had not been in the sun “at all” for many, many years. According to her daughter, this was true. But the patient had overlooked the fact that she had grown up on a farm, worked in the fields, played outside, swam and fished, all the while getting a great deal of sun exposure, until she married in her late teens, had children, and moved to town. Being so busy and so fair, she had neither the time nor the inclination to get outdoors much, and that was that—or so she thought. This is a very common set of circumstances for dermatology patients.
Little did she realize that it takes decades (30 to 40 years) for the accumulated effects of sun damage to show up, in the form we see here. This type of “aging” is an example of what we call extrinsic aging. Besides sun, it can be worsened by the effects of wind, low humidity, smoking, alcohol intake, obesity, and some medical conditions. Intrinsic aging, which includes wrinkles, sagging, and general loss of elasticity, is influenced by age, heredity, and ultimately, gravity.
From our standpoint as medical providers, perhaps the most significant issue with this patient is her increased risk for sun-caused skin cancer, specifically basal cell carcinoma (BCC), squamous cell carcinoma (SCC), or melanoma (the three most common forms). Her risk for development of the first two is huge, arguably a certainty given her age, extremely fair skin, and degree of sun damage. Melanomas are different in many ways, actually becoming less likely (statistically) at her age than at age 50. They are also not as much the result of the accumulated effects of sun exposure as are BCC/SCCs.
So, perhaps the most significant outcome of this visit was to get the patient scheduled to see us biannually for skin checks. In our system, that means twice-yearly reminder phone calls to ensure that this actually happens.
TAKE-HOME LEARNING POINTS
• It takes three to five decades for sun damage to eventuate in dermatoheliosis/basal cell carcinoma/squamous cell carcinoma.
• Melanoma is different, since it appears to be related to episodic, poorly tolerated, intense sun damage early in life; the average age of melanoma patients is about 40.
• Aging can be intrinsic (“normal” loss of elasticity, plus the effects of gravity, modified by heredity) in nature.
• Aging can also be extrinsic, caused by UV exposure, smoking, alcohol consumption, wind, and decreased humidity, eventuating in telangiectasias, actinic keratoses, solar elastosis, solar lentigines, atrophy, purpura, poikilodermatous changes, and sun-caused skin cancers.
• Older patients are often incredulous about the role of childhood sun exposure in the eventual development of dermatoheliosis.
Wife is Worried That Her Husband's Condition is Contagious
ANSWER
The correct answer is petaloid seborrheic dermatitis (choice “d”), named for the flowerlike appearance of its polycyclic borders. Psoriasis (choice “a”) can present in this area, but tends to be scalier and usually involves multiple areas (eg, elbows, knees, and nails).
Rashes like this patient’s are often termed yeast infection (choice “b”). However, while a commensal yeast (Pityrosporum) can play a role in its formation, it appears that seborrhea represents an idiosyncratic reaction to increased numbers of this organism, rather than an actual infection.
Bowen’s disease (choice “c”) is a superficial squamous cell carcinoma, usually caused by overexposure to sunlight. Its lesions will be fixed, slowly growing larger with time, while seborrheic dermatitis will typically come and go. Biopsy is sometimes necessary to distinguish one from the other.
DISCUSSION
Seborrheic dermatitis (SD, aka seborrhea) is common, affecting up to 5% of the population. Dandruff is its usual manifestation, but it affects numerous other areas (as in this case), including the axillae, groin, beard, and genitals.
Presenting with scaling on an erythematous base, SD often flares and remits with the season (especially winter), with stress, and with increases in alcohol intake. Although it is usually mild, some cases can be severe. SD is associated with or accentuated by several other conditions, including Parkinson’s, stroke, and HIV. Severe SD in infants raises the possibility of Langerhans cell histiocytosis, especially when the presentation is atypical.
The diagnosis of SD can be difficult when it appears elsewhere than the scalp and face (eg, as an axillary or genital rash). Likewise, sternal petaloid SD is mystifying, unless other corroboratory manifestations are sought and found.
A few patients show signs of SD and psoriasis such that a definitive diagnosis cannot be made. Such overlap cases are sometimes termed sebopsoriasis. But psoriasis will usually exhibit signs not seen with SD, such as pitting of the nails, involvement of extensor surfaces of elbows and knees, and characteristic signs of psoriatic arthropathy in about 20% of cases. Pinpoint bleeding caused by peeling away scale, called the Auspitz sign, is seen with psoriasis and not with SD.
TREATMENT
This patient’s chest involvement responded rapidly to topical betamethasone foam, quickly tapered to avoid thinning the skin. Less powerful steroid creams, lotions, or gels (eg, triamcinolone 0.025%) can be used on other areas, such as ears and face. The daily use of an OTC dandruff shampoo (containing selenium sulfide, zinc pyrithione, tar, or ketoconazole) is an effective approach to controlling scalp involvement, but the product should be changed weekly.
Once the initial inflammation is controlled, topical antiyeast/antifungal preparations (eg, ketoconazole cream or any of the imidazoles, such as clotrimazole or oxiconazole) can be useful.
Finally, emphasis must be placed on educating the patient to expect control of the condition but not a cure.
ANSWER
The correct answer is petaloid seborrheic dermatitis (choice “d”), named for the flowerlike appearance of its polycyclic borders. Psoriasis (choice “a”) can present in this area, but tends to be scalier and usually involves multiple areas (eg, elbows, knees, and nails).
Rashes like this patient’s are often termed yeast infection (choice “b”). However, while a commensal yeast (Pityrosporum) can play a role in its formation, it appears that seborrhea represents an idiosyncratic reaction to increased numbers of this organism, rather than an actual infection.
Bowen’s disease (choice “c”) is a superficial squamous cell carcinoma, usually caused by overexposure to sunlight. Its lesions will be fixed, slowly growing larger with time, while seborrheic dermatitis will typically come and go. Biopsy is sometimes necessary to distinguish one from the other.
DISCUSSION
Seborrheic dermatitis (SD, aka seborrhea) is common, affecting up to 5% of the population. Dandruff is its usual manifestation, but it affects numerous other areas (as in this case), including the axillae, groin, beard, and genitals.
Presenting with scaling on an erythematous base, SD often flares and remits with the season (especially winter), with stress, and with increases in alcohol intake. Although it is usually mild, some cases can be severe. SD is associated with or accentuated by several other conditions, including Parkinson’s, stroke, and HIV. Severe SD in infants raises the possibility of Langerhans cell histiocytosis, especially when the presentation is atypical.
The diagnosis of SD can be difficult when it appears elsewhere than the scalp and face (eg, as an axillary or genital rash). Likewise, sternal petaloid SD is mystifying, unless other corroboratory manifestations are sought and found.
A few patients show signs of SD and psoriasis such that a definitive diagnosis cannot be made. Such overlap cases are sometimes termed sebopsoriasis. But psoriasis will usually exhibit signs not seen with SD, such as pitting of the nails, involvement of extensor surfaces of elbows and knees, and characteristic signs of psoriatic arthropathy in about 20% of cases. Pinpoint bleeding caused by peeling away scale, called the Auspitz sign, is seen with psoriasis and not with SD.
TREATMENT
This patient’s chest involvement responded rapidly to topical betamethasone foam, quickly tapered to avoid thinning the skin. Less powerful steroid creams, lotions, or gels (eg, triamcinolone 0.025%) can be used on other areas, such as ears and face. The daily use of an OTC dandruff shampoo (containing selenium sulfide, zinc pyrithione, tar, or ketoconazole) is an effective approach to controlling scalp involvement, but the product should be changed weekly.
Once the initial inflammation is controlled, topical antiyeast/antifungal preparations (eg, ketoconazole cream or any of the imidazoles, such as clotrimazole or oxiconazole) can be useful.
Finally, emphasis must be placed on educating the patient to expect control of the condition but not a cure.
ANSWER
The correct answer is petaloid seborrheic dermatitis (choice “d”), named for the flowerlike appearance of its polycyclic borders. Psoriasis (choice “a”) can present in this area, but tends to be scalier and usually involves multiple areas (eg, elbows, knees, and nails).
Rashes like this patient’s are often termed yeast infection (choice “b”). However, while a commensal yeast (Pityrosporum) can play a role in its formation, it appears that seborrhea represents an idiosyncratic reaction to increased numbers of this organism, rather than an actual infection.
Bowen’s disease (choice “c”) is a superficial squamous cell carcinoma, usually caused by overexposure to sunlight. Its lesions will be fixed, slowly growing larger with time, while seborrheic dermatitis will typically come and go. Biopsy is sometimes necessary to distinguish one from the other.
DISCUSSION
Seborrheic dermatitis (SD, aka seborrhea) is common, affecting up to 5% of the population. Dandruff is its usual manifestation, but it affects numerous other areas (as in this case), including the axillae, groin, beard, and genitals.
Presenting with scaling on an erythematous base, SD often flares and remits with the season (especially winter), with stress, and with increases in alcohol intake. Although it is usually mild, some cases can be severe. SD is associated with or accentuated by several other conditions, including Parkinson’s, stroke, and HIV. Severe SD in infants raises the possibility of Langerhans cell histiocytosis, especially when the presentation is atypical.
The diagnosis of SD can be difficult when it appears elsewhere than the scalp and face (eg, as an axillary or genital rash). Likewise, sternal petaloid SD is mystifying, unless other corroboratory manifestations are sought and found.
A few patients show signs of SD and psoriasis such that a definitive diagnosis cannot be made. Such overlap cases are sometimes termed sebopsoriasis. But psoriasis will usually exhibit signs not seen with SD, such as pitting of the nails, involvement of extensor surfaces of elbows and knees, and characteristic signs of psoriatic arthropathy in about 20% of cases. Pinpoint bleeding caused by peeling away scale, called the Auspitz sign, is seen with psoriasis and not with SD.
TREATMENT
This patient’s chest involvement responded rapidly to topical betamethasone foam, quickly tapered to avoid thinning the skin. Less powerful steroid creams, lotions, or gels (eg, triamcinolone 0.025%) can be used on other areas, such as ears and face. The daily use of an OTC dandruff shampoo (containing selenium sulfide, zinc pyrithione, tar, or ketoconazole) is an effective approach to controlling scalp involvement, but the product should be changed weekly.
Once the initial inflammation is controlled, topical antiyeast/antifungal preparations (eg, ketoconazole cream or any of the imidazoles, such as clotrimazole or oxiconazole) can be useful.
Finally, emphasis must be placed on educating the patient to expect control of the condition but not a cure.
A 70-year-old man presents with a slightly itchy rash on his sternum that has appeared intermittently for years. Told it is “ringworm” by his primary care provider, the patient tried tolnaftate cream, to no avail. He is seeking additional consultation primarily because his wife is concerned she will catch the “infection.” The patient denies other skin problems, but then remembers that he has dandruff that flares from time to time, as well as a curious scaly red rash that “comes and goes” between his eyes, in his nasolabial folds, and behind his ears, especially in the winter. His father had similar problems. The patient is otherwise healthy, except for mild hypertension. The rash, located on the lower right sternum, measures about 6 cm at its largest dimension. Faintly pink, it has a papulosquamous surface, especially on its pol-ycyclic borders. Results of a KOH prep are negative for fungal elements. Elsewhere, a faintly scaly, orange-red rash is seen in the glabellar area and behind both ears. The man’s knees, elbows, and nails are free of any changes.
A dermatologic problem is "all in the family"
HISTORY
A 48-year-old woman presents to dermatology for evaluation of “ringworm” that has been unresponsive to antifungal creams and a subsequent course of terbinafine (250 mg/d for 10 days).
The problem started several weeks ago, when she experienced mild itching in the upper intergluteal area. Her husband examined the area and noted several patches of a round, scaly rash that they assumed must be ringworm. The patient tried applying OTC clotrimazole cream twice a day, with no good effect.
She next visited a local urgent care center, where the provider agreed with the self-diagnosis. Nystatin cream was prescribed for twice-daily use, but this treatment was also unsuccessful.
Next up was the patient’s primary care provider, who reiterated the diagnosis of ringworm and prescribed the aforementioned course of terbinafine. When it too failed, the patient, still worried, requested a referral to dermatology.
During the history taking, it is revealed that the patient has hypertension, is 50 lb overweight, and has been smoking for more than 40 years. A few weeks prior to the appearance of the rash, she lost her job when the company she had been employed by for more than 20 years closed. At that point, her consumption of alcohol began to increase markedly.
Questions about family history of skin disease reveal several first- and second-degree relatives with psoriasis. In addition, the patient purposely brought her 17-year-old son with her, in order to show his “ringworm,” which she is convinced he passed on to her.
The son’s rash (pictured) has been present for several years, always confined to his axillae and only slightly itchy. It is composed of almost perfectly round pinkish brown patches covering the roofs of both axillae. It bears a striking resemblance to his mother’s intergluteal rash, including the color, and displays annular margins as well.
Additional questioning confirms the fact of his long-standing dandruff and the presence of a chronic scaly rash in and around the ears. At least two of his siblings have similar problems. On hearing this, the patient admits she has suffered with dandruff for years as well.
DISCUSSION
In all likelihood, both mother and son have a papulosquamous condition called seborrheic dermatitis (SD). SD most commonly manifests as dandruff, but it can present in a number of other, less well-known forms.
Up to 20% of the population at large have one or more types of SD, which tends to favor fair-skinned individuals of Northern European ancestry. It appears to be related to a commensal yeast organism, Malassezia furfur, which is markedly lipophilic and needs copious sebum (ubiquitously present on all humans) to flourish. The needed quantity of sebum rarely develops before puberty, due to hormonal influence. As these organisms feed on sebum, the resulting metabolic byproducts trigger an inflammatory response in the susceptible individual.
The resulting characteristic papulosquamous rash can manifest behind the ears, in the external auditory meati, in the glabellar area of the mid-forehead, into the brows, focally in the beard, and in the nasolabial folds. Less commonly, SD appears on the mid-sternum, in the periumbilical area, on genitals, in the intergluteal area, and in the axillae (as in the case of the patient’s son).
Connecting all these dots is an impossible task for most patients, whose only explanation for round and scaly is “ringworm” (tinea corporis). Ringworm, however, requires a source—such as a new cat, guinea pig, dog, livestock, or child with active disease, none of which were present in this case. Little did the patient know that her son had “caught” SD from her!
What we did have in this case was a sharp increase in the patient’s stress level, as well as an increase in her alcohol intake. These factors are known to exacerbate SD, and may form part of the answer to the (reasonable) question: Why this patient, why now?
TREATMENT
In addition to providing patient education about the diagnosis, I treated both mother and son with topical 2.5% hydrocortisone and arranged for follow-up.
Since a cure is not possible, prevention is the real objective. With that in mind, they were advised to use OTC dandruff shampoos (containing zinc pyrithione, ketoconazole, or selenium sulfide) as body wash, rotating the choice of product on a weekly basis to avoid tachyphylaxis.
Other products that have been useful in treating SD include sodium sulfacetamide cleansers and lotions, imidazole creams and foams, and the calcineurin inhibitors, such as tacrolimus and pimecrolimus.
TAKE-HOME LEARNING POINTS
• Seborrheic dermatitis (SD) is an extremely common papulosquamous condition, affecting up to 20% of the population.
• It tends to run in families of Northern European ancestry.
• SD can manifest in numerous locations, including the scalp, in and behind the ears, or on the face, chest, genitals, axillae, and intergluteal areas.
• Stress is a common trigger for exacerbations of SD.
• Often displaying an annular configuration, SD is frequently mistaken for fungal infection (“ringworm”).
• A flare of SD can be an early sign of Parkinson’s disease or undiagnosed HIV.
• A significant part of the treatment of SD is patient education, not only about the diagnosis and treatment, but also regarding prognosis.
HISTORY
A 48-year-old woman presents to dermatology for evaluation of “ringworm” that has been unresponsive to antifungal creams and a subsequent course of terbinafine (250 mg/d for 10 days).
The problem started several weeks ago, when she experienced mild itching in the upper intergluteal area. Her husband examined the area and noted several patches of a round, scaly rash that they assumed must be ringworm. The patient tried applying OTC clotrimazole cream twice a day, with no good effect.
She next visited a local urgent care center, where the provider agreed with the self-diagnosis. Nystatin cream was prescribed for twice-daily use, but this treatment was also unsuccessful.
Next up was the patient’s primary care provider, who reiterated the diagnosis of ringworm and prescribed the aforementioned course of terbinafine. When it too failed, the patient, still worried, requested a referral to dermatology.
During the history taking, it is revealed that the patient has hypertension, is 50 lb overweight, and has been smoking for more than 40 years. A few weeks prior to the appearance of the rash, she lost her job when the company she had been employed by for more than 20 years closed. At that point, her consumption of alcohol began to increase markedly.
Questions about family history of skin disease reveal several first- and second-degree relatives with psoriasis. In addition, the patient purposely brought her 17-year-old son with her, in order to show his “ringworm,” which she is convinced he passed on to her.
The son’s rash (pictured) has been present for several years, always confined to his axillae and only slightly itchy. It is composed of almost perfectly round pinkish brown patches covering the roofs of both axillae. It bears a striking resemblance to his mother’s intergluteal rash, including the color, and displays annular margins as well.
Additional questioning confirms the fact of his long-standing dandruff and the presence of a chronic scaly rash in and around the ears. At least two of his siblings have similar problems. On hearing this, the patient admits she has suffered with dandruff for years as well.
DISCUSSION
In all likelihood, both mother and son have a papulosquamous condition called seborrheic dermatitis (SD). SD most commonly manifests as dandruff, but it can present in a number of other, less well-known forms.
Up to 20% of the population at large have one or more types of SD, which tends to favor fair-skinned individuals of Northern European ancestry. It appears to be related to a commensal yeast organism, Malassezia furfur, which is markedly lipophilic and needs copious sebum (ubiquitously present on all humans) to flourish. The needed quantity of sebum rarely develops before puberty, due to hormonal influence. As these organisms feed on sebum, the resulting metabolic byproducts trigger an inflammatory response in the susceptible individual.
The resulting characteristic papulosquamous rash can manifest behind the ears, in the external auditory meati, in the glabellar area of the mid-forehead, into the brows, focally in the beard, and in the nasolabial folds. Less commonly, SD appears on the mid-sternum, in the periumbilical area, on genitals, in the intergluteal area, and in the axillae (as in the case of the patient’s son).
Connecting all these dots is an impossible task for most patients, whose only explanation for round and scaly is “ringworm” (tinea corporis). Ringworm, however, requires a source—such as a new cat, guinea pig, dog, livestock, or child with active disease, none of which were present in this case. Little did the patient know that her son had “caught” SD from her!
What we did have in this case was a sharp increase in the patient’s stress level, as well as an increase in her alcohol intake. These factors are known to exacerbate SD, and may form part of the answer to the (reasonable) question: Why this patient, why now?
TREATMENT
In addition to providing patient education about the diagnosis, I treated both mother and son with topical 2.5% hydrocortisone and arranged for follow-up.
Since a cure is not possible, prevention is the real objective. With that in mind, they were advised to use OTC dandruff shampoos (containing zinc pyrithione, ketoconazole, or selenium sulfide) as body wash, rotating the choice of product on a weekly basis to avoid tachyphylaxis.
Other products that have been useful in treating SD include sodium sulfacetamide cleansers and lotions, imidazole creams and foams, and the calcineurin inhibitors, such as tacrolimus and pimecrolimus.
TAKE-HOME LEARNING POINTS
• Seborrheic dermatitis (SD) is an extremely common papulosquamous condition, affecting up to 20% of the population.
• It tends to run in families of Northern European ancestry.
• SD can manifest in numerous locations, including the scalp, in and behind the ears, or on the face, chest, genitals, axillae, and intergluteal areas.
• Stress is a common trigger for exacerbations of SD.
• Often displaying an annular configuration, SD is frequently mistaken for fungal infection (“ringworm”).
• A flare of SD can be an early sign of Parkinson’s disease or undiagnosed HIV.
• A significant part of the treatment of SD is patient education, not only about the diagnosis and treatment, but also regarding prognosis.
HISTORY
A 48-year-old woman presents to dermatology for evaluation of “ringworm” that has been unresponsive to antifungal creams and a subsequent course of terbinafine (250 mg/d for 10 days).
The problem started several weeks ago, when she experienced mild itching in the upper intergluteal area. Her husband examined the area and noted several patches of a round, scaly rash that they assumed must be ringworm. The patient tried applying OTC clotrimazole cream twice a day, with no good effect.
She next visited a local urgent care center, where the provider agreed with the self-diagnosis. Nystatin cream was prescribed for twice-daily use, but this treatment was also unsuccessful.
Next up was the patient’s primary care provider, who reiterated the diagnosis of ringworm and prescribed the aforementioned course of terbinafine. When it too failed, the patient, still worried, requested a referral to dermatology.
During the history taking, it is revealed that the patient has hypertension, is 50 lb overweight, and has been smoking for more than 40 years. A few weeks prior to the appearance of the rash, she lost her job when the company she had been employed by for more than 20 years closed. At that point, her consumption of alcohol began to increase markedly.
Questions about family history of skin disease reveal several first- and second-degree relatives with psoriasis. In addition, the patient purposely brought her 17-year-old son with her, in order to show his “ringworm,” which she is convinced he passed on to her.
The son’s rash (pictured) has been present for several years, always confined to his axillae and only slightly itchy. It is composed of almost perfectly round pinkish brown patches covering the roofs of both axillae. It bears a striking resemblance to his mother’s intergluteal rash, including the color, and displays annular margins as well.
Additional questioning confirms the fact of his long-standing dandruff and the presence of a chronic scaly rash in and around the ears. At least two of his siblings have similar problems. On hearing this, the patient admits she has suffered with dandruff for years as well.
DISCUSSION
In all likelihood, both mother and son have a papulosquamous condition called seborrheic dermatitis (SD). SD most commonly manifests as dandruff, but it can present in a number of other, less well-known forms.
Up to 20% of the population at large have one or more types of SD, which tends to favor fair-skinned individuals of Northern European ancestry. It appears to be related to a commensal yeast organism, Malassezia furfur, which is markedly lipophilic and needs copious sebum (ubiquitously present on all humans) to flourish. The needed quantity of sebum rarely develops before puberty, due to hormonal influence. As these organisms feed on sebum, the resulting metabolic byproducts trigger an inflammatory response in the susceptible individual.
The resulting characteristic papulosquamous rash can manifest behind the ears, in the external auditory meati, in the glabellar area of the mid-forehead, into the brows, focally in the beard, and in the nasolabial folds. Less commonly, SD appears on the mid-sternum, in the periumbilical area, on genitals, in the intergluteal area, and in the axillae (as in the case of the patient’s son).
Connecting all these dots is an impossible task for most patients, whose only explanation for round and scaly is “ringworm” (tinea corporis). Ringworm, however, requires a source—such as a new cat, guinea pig, dog, livestock, or child with active disease, none of which were present in this case. Little did the patient know that her son had “caught” SD from her!
What we did have in this case was a sharp increase in the patient’s stress level, as well as an increase in her alcohol intake. These factors are known to exacerbate SD, and may form part of the answer to the (reasonable) question: Why this patient, why now?
TREATMENT
In addition to providing patient education about the diagnosis, I treated both mother and son with topical 2.5% hydrocortisone and arranged for follow-up.
Since a cure is not possible, prevention is the real objective. With that in mind, they were advised to use OTC dandruff shampoos (containing zinc pyrithione, ketoconazole, or selenium sulfide) as body wash, rotating the choice of product on a weekly basis to avoid tachyphylaxis.
Other products that have been useful in treating SD include sodium sulfacetamide cleansers and lotions, imidazole creams and foams, and the calcineurin inhibitors, such as tacrolimus and pimecrolimus.
TAKE-HOME LEARNING POINTS
• Seborrheic dermatitis (SD) is an extremely common papulosquamous condition, affecting up to 20% of the population.
• It tends to run in families of Northern European ancestry.
• SD can manifest in numerous locations, including the scalp, in and behind the ears, or on the face, chest, genitals, axillae, and intergluteal areas.
• Stress is a common trigger for exacerbations of SD.
• Often displaying an annular configuration, SD is frequently mistaken for fungal infection (“ringworm”).
• A flare of SD can be an early sign of Parkinson’s disease or undiagnosed HIV.
• A significant part of the treatment of SD is patient education, not only about the diagnosis and treatment, but also regarding prognosis.















