User login
Development, implementation, and evaluation of a prostate cancer supportive care program
Prostate cancer is the most common malignancy diagnosed in Canadian men. An estimated 21,300 Canadian men were diagnosed with the disease in 2017, representing 21% of all new cancer cases.1 There are about 176,000 men living with prostate cancer in Canada.1 In the United States, there were 2,778,630 survivors of prostate cancer as of 2012 and that population is expected to increase by more than 1 million (40%) to 3,922,600 by 2022.2
Although 96% of men diagnosed with prostate cancer now survive longer than 5 years3, many will suffer from treatment-related sequelae that can have a profound effect on quality of life for themselves and their partners.4,5 Impacts include sexual, urinary, and bowel dysfunctions6 owing to treatment of the primary tumor as well as reduced muscle and bone mass, osteoporosis, fatigue, obesity, and glucose intolerance or diabetes7 owing to androgen-deprivation therapy (ADT). Many men also suffer from psychological issues such as depression, anxiety, anger and irritability, sense of isolation, grief, and loss of masculinity.8,9 The psychological impacts also continue well beyond the completion of treatment and can be significant for both patients and their partners.5,8
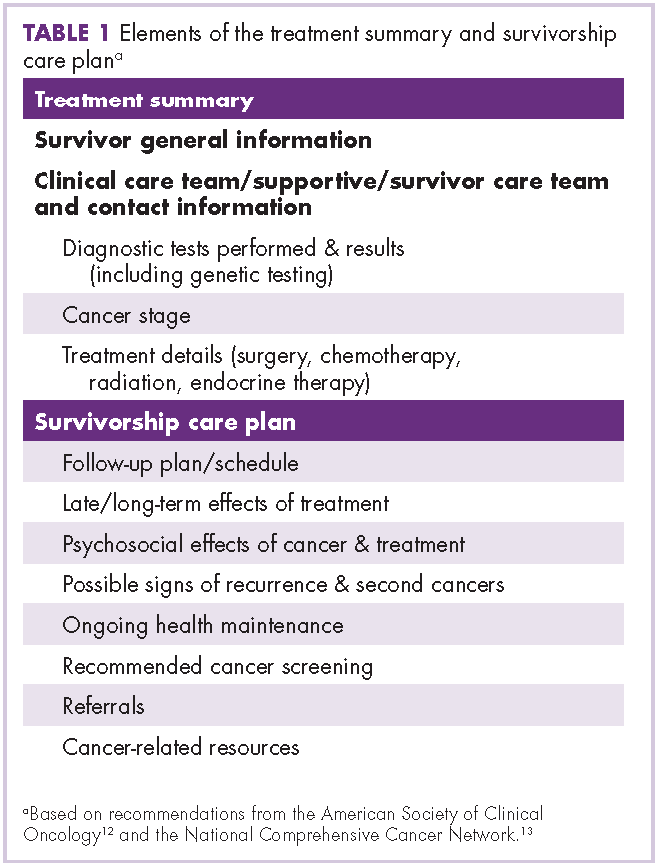
With posttreatment longevity and the associated complex sequelae, prostate cancer is being viewed increasingly as a chronic disease whose effects must be managed for many years after the completion of primary treatment. Supportive care that “[manages] symptoms and side effects, enables adaptation and coping, optimizes understanding and informed decision-making, and minimizes decrements in functioning”10 is becoming recognized as a critical component of direct oncologic care before, during, and after treatment. Health care professionals, scientists, governments, and patient advocates are increasingly calling for the development of comprehensive supportive care programs improve the quality of life for people diagnosed with cancer. A common model for survivorship care is a general program for all cancer survivors that provides disease- and patient-specific care plans. These care plans outline patients’ prior therapies, potential side effects, recommendations for monitoring (for side effects or relapse of cancer), and advice on how patients can maintain a healthy lifestyle.11 However, there are few survivorship programs for men with prostate cancer and their partners, and the evidence base around best practices for these programs is scant.12 Furthermore, up to 87% of men with a prostate cancer diagnosis report specific and significant unmet supportive care needs,10,13 with sexuality-related and psychological issues10,14 being the areas of greatest concern.
To address the complex supportive care needs of men with prostate cancer in British Columbia, Canada, the Vancouver Prostate Centre (VPC) and Department of Urologic Sciences at the University of British Columbia developed the multidisciplinary Prostate Cancer Supportive Care (PCSC) Program. The program aims to address the challenges of decision-making and coping faced by men with prostate cancer and their partners and family members along the entire disease trajectory. Services are provided at no cost to participants. Here, we outline the guiding principles for the PCSC program and its scope, delivery, and evaluation. We provide information on the more than 1,200 patients who have participated in the program since its inception in January of 2013, the rates of participation across the different program modules, and a selection of patient satisfaction measures. We also discuss successes and limitations and ongoing research and evaluation efforts, providing lessons learned to support the development of other supportive care programs in Canada and internationally.
Program description
Guiding principles
The PCSC Program is a clinical, educational, and research-based program, with 4 guiding principles: it is comprehensive, patient- and partner-centered, evidence-based, and supports new research. The program serves patients, partners, and families along the entire disease trajectory, recognizing that cancer is a family disease, affecting both the individual and social network, and that the psychological stress associated with a diagnosis of prostate cancer is borne heavily by partners. It has been designed, implemented, and refined with the best available evidence and with the intention to undergo consistent and repeated evaluation. Finally, it was designed to provide opportunities for targeted research efforts, supporting the growth of the evidence base in this area.
Patient entry and module descriptions
Patients can be referred to the program by a physician or other allied health professional. They may also self-refer, having been made aware of the program through our website, a variety of print materials, or by word of mouth. On referral, the program coordinator collects patients’ basic clinical and demographic data, assesses health literacy and lifestyle factors, and provides them with information on the program modules. As of December 2015, the program consisted of 6 distinct modules, each focusing on different elements of the disease trajectory or on addressing specific physical or mental health concerns. Modules are led by licensed health professionals with experience in oncology. No elements of the program are mandatory, and participants are free to pick and choose the components that are most relevant to them and their partners.
Introduction to prostate cancer and primary treatment options. This is a group-based module that focuses on educating newly diagnosed patients (and those going on or off active surveillance) on the basic biology of prostate cancer, the primary treatment options for localized disease, and the main side effects associated with the treatments. It also includes information about the other services offered by the program and any ongoing research studies. The session is held twice a month in the early evening and is run collectively by a urologist, radiation oncologist, patient representative, and program coordinator. It includes a brief one-on-one discussion between each patient and their partner or family member and the urologist and radiation oncologist to address any remaining questions. A copy of the patient’s biopsy report is on hand for the physician(s). Attendance of this session has been shown to significantly reduce pretreatment distress in both patients and their partners.15
Managing sexual function and intimacy. Sexual intimacy is tied to overall health outcomes, relationship satisfaction, and quality of life.16 Primary therapy for prostate cancer can be associated with substantial side-effects (eg, erectile dysfunction, incontinence, altered libido, penile shortening) that negatively affect sexual intimacy and have an impact on the patient individually as well as the sexual relationship he has with his partner.17
The program’s Sexual Health Service (SHS) provides patients and partners with information on the impact of treatment on sexual health.18 The SHS offers educational sessions led by a sexual rehabilitation nurse and clinical psychologist with a specialization in sexual health. Sessions focus on the impact of prostate cancer treatments on sexual function and therapeutic modalities, promote an understanding of the barriers to sexual adaptation posttreatment, and present options for sexual activity that are not solely dependent on the ability to achieve an erection. Once participants have attended an educational session, they are offered individual consultations with the sexual health nurse every 3 to 6 months for 2 years or longer, depending on the patient’s or couple’s needs. They are referred to the SHS’s sexual medicine physician if further medical intervention is warranted. The sexual health nurse works with the patient and partner to develop an individualized Sexual Health Rehabilitation Action Plan (SHRAP), which assists the couple in sexual adaptation going forward. The SHRAP is a tool devised by the sexual health nurse based on her clinical experience with couples affected by prostate cancer.
Couples who have been evaluated within the SHS are also invited to attend a second workshop on intimacy that is offered quarterly. Workshop participants discuss the impact of sexual changes on relationships, and strategies on how to enhance intimacy and sexual communication are presented. A resource package is provided to each couple to help re-establish and/or strengthen their various dimensions of intimacy.
Lifestyle management. The lifestyle management modules include separate nutrition and physical activity or exercise components. Referral to the smoking cessation program in the Vancouver Coastal Health Authority is made at program registration, if appropriate. The nutrition group-based education session is delivered by a registered dietitian from the British Columbia Cancer Agency who specializes in prostate cancer. The session focuses on evidence-based recommendations on diet after a diagnosis of prostate cancer, the use of dietary supplements, body weight and health, and practical nutrition tips. The exercise session is delivered by an exercise physiologist who specializes in working with cancer patients. It covers the value of exercise in terms of safety, prevention and reduction of treatment side effects (including from ADT), treatment prehabilitation and recovery, advanced cancer management, and long-term survival. A one-on-one exercise counseling clinic is also offered and aims to increase exercise adoption and long-term adherence in line with Canadian Physical Activity guidelines and exercise oncology guidelines,19,20 with follow-up appointments at 3, 6, and 12 months to help patients stay motivated and ensure they are exercising correctly. The individual consultations with the exercise physiologist include physical measures, exercise volume, treatment side effects, and coconstructed goal setting with an individualized formal exercise regimen (exercise prescription).
Adapting to ADT. This is an educational module offered to patients with metastatic prostate cancer who are starting hormone therapy treatments that lower serum testosterone into the castrate range. This program was one of several available through TrueNTH, a portfolio of projects funded by the Movember Foundation, through Prostate Cancer Canada. The session is delivered by a patient facilitator and focuses on strategies to recognize and adapt to the side effects of ADT21 while maintaining a good quality of life and strong intimate relationships with partners.22,23
Pelvic-floor physical therapy for urinary incontinence. This module includes a group-based and individualized education session for patients (either pre- or posttreatment) focused on reducing the effects of surgery and/or radiation therapy on urinary and sexual continence as well as on how to cope with these symptoms and minimize the effect they have on quality of life.24 The session is conducted by a physical therapist who is certified as a pelvic-floor specialist. Supervised pelvic-floor re-education and/or exercise has been shown to successfully reduce the degree of incontinence in this population.25 The module therefore also includes 3 one-on-one clinical appointments for patients who are still experiencing bother from incontinence 12 weeks after a radical prostatectomy or postradiation treatment.
Psycho-oncology. In recognition of the emotional and psychological burden associated with prostate cancer and the important role partners play in facilitating treatment of these psychological and/or psychosocial issues, the program offers appointments with a registered clinical counselor to address acute emotional distress. These are usually 1-hour appointments offered to both patients and partners, either separately or together. Appointments can be attended in person or conducted by telephone. When appropriate, patients are referred for further long-term individual support or couple support with their partners. A group therapy workshop was also initiated in 2016. In this program, men participate in a guided autobiographical life review through a process that focuses on developing a cohesive working group, learning strategic communication skills, and understanding and learning how to manage difficult emotions and transitional life stressors associated with prostate cancer. It also focuses on processing and integrating critical events that contribute to the men’s identity and psychological function and involves the consolidation of the personal learning that occurs. Postgroup referral plans are developed on an individual basis as needed.
Methods
Data
We obtained sociodemographic, diagnostic, and treatment information as well as clinic visit records for all PCSC Program registrants from the electronic medical record held at the VPC. Clinical variables included age at diagnosis, Gleason score, and primary treatment modality (including active surveillance and ADT use). The Gleason score determines the aggressiveness of a patient’s prostate cancer based on biopsy results. A score of 6 or less indicates that the disease is likely to grow slowly. A grade of 7 is considered intermediate risk (with primary score of 3 and secondary 4 being lower risk than those with a primary score of 4 and secondary of 3). A Gleason score of 8 or higher indicates aggressive disease that is poorly differentiated or high grade. Sociodemographic characteristics included age, travel distance to the clinic, and income quintile. We obtained attendance records for the modular education sessions from the program’s database. Patients who did not have any medical visits at the VPC (referred to henceforth as non-VPC patients) did not have a clinic record, so we excluded them from the subset of the analyses that depended on specific clinical variables.
All patients and partners who participate in any PCSC Program education sessions (introduction to prostate cancer, sexual health, nutrition, exercise, ADT, and pelvic-floor physical therapy) are asked to complete voluntary, anonymous feedback forms. These forms assess participant satisfaction using a series of Likert-based and Boolean response items as well as qualitative commentary. They include questions that assess the timing, structure, and content of each session.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Statistical approach
Descriptive statistics were used to analyze participant characteristics, program participation rates, and participant satisfaction. For each module’s education session, we compared the overall satisfaction between patients and partners using t tests. We also compared the level of satisfaction across the different modules using a 1-way analysis of variance. For the sexual health and pelvic-floor physical therapy sessions, we compared satisfaction between participants who attended the education sessions before to those who attended following their primary treatment using t tests. We provide the eta squared (for analyses of variance) and Cohen d (for t tests) to provide an effect size estimate of any significant differences observed.
Results
Participants
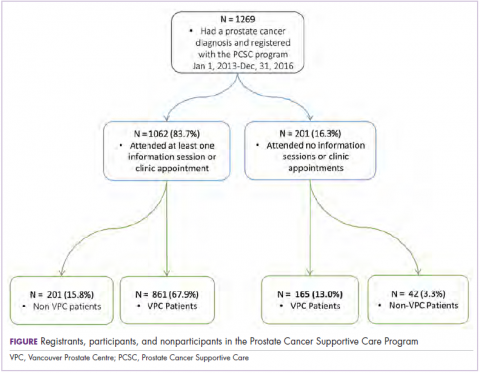
From the program’s founding in January of 2013 to December 31, 2016, a total of 1,269 patients registered (an average of 317 patients a year). Of those, 1,026 (80.9%) had at least 1 prostate cancer–related visit at the VPC. The remaining 243 (19.1%) were non-VPC patients (Figure). Overall, 1,062 men (83.4%) who registered with the program went on to attend at least 1 education session or clinic appointment.
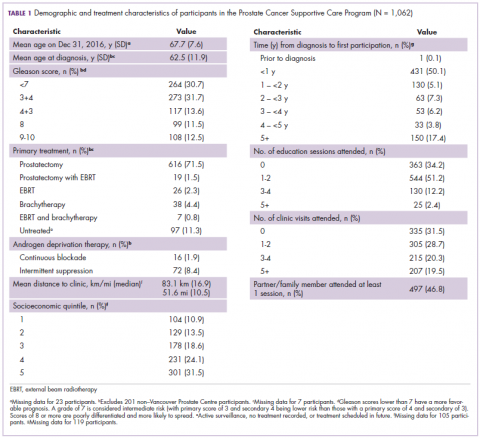
Average age among male program participants was 67.7 years, and age at diagnosis was 62.5 years (Table 1). In all, 273 men (31.7%) had Gleason 3+4, and 117 (13.7%) had Gleason 4+3. Most of the participants (76.9%) elected to undergo radical prostatectomy for primary treatment. Ninety-five men (8.9%) received at least some ADT treatment as an adjunct to radiation or to treat recurrent disease. Participants traveled an average of 83.1 km (51.6 miles; median, 6.9 km and 10.5 miles, respectively) to attend the program; 10% of participants traveled further than 112 km (70 mi) to the clinic. One hundred and four (10.9%) and 301 (31.5%) of registrants were in the lowest and highest income quintiles respectively. Four hundred and ninety-seven (46.8%) attended at lesson 1 session or clinic appointment with a partner or family member.
Program participation
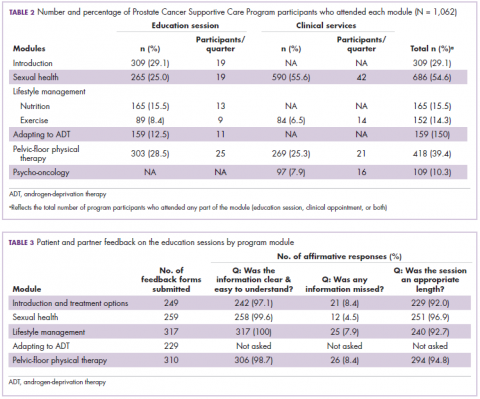
Of the 1,062 men who participated in the program, 867 (80.1%) were patients of the VPC, and 205 (19.1%) were non-VPC patients. The education sessions for the introduction to prostate cancer and sexual health modules had the largest numbers of participants (309 and 265, respectively; Table 2); however, pelvic-floor physical therapy had the highest participation rate per quarter (25 patients). The clinical services offered within the sexual health module had the larger number of participants and highest participation rate per quarter (590 total patients, 42/quarter). Timing of program participation was highly variable, ranging from 6 days to 18.5 years after diagnosis (SD, 1,301 days). More than half of participants attended a session or clinic visit within the first year of their diagnosis. A total of 17% of patients who registered did not attend any part of the program.
Satisfaction
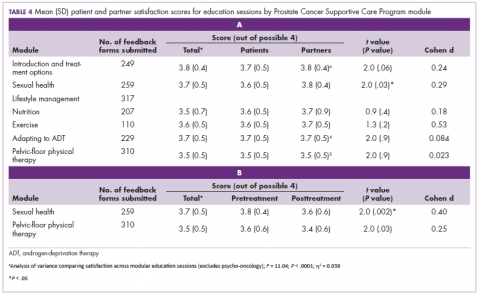
Most patients and partners said that they found the information presented at the modular education sessions comprehensive, clear, and easy to understand (Table 3). Although the overall average satisfaction score varied significantly across sessions, ranging from 3.5 (out of a possible 4) for pelvic-floor physical therapy to 3.8 for introduction to prostate cancer (F = 3.8, P < .001), the effect size of this difference was small (η2 = .039; Table 4A). We found no difference in the level of satisfaction between patients and partners, with the exception of the sexual health module, which was rated better by partners than by patients (patients: 3.6, partners: 3.8; t = 2.0; P = .03); however, the effect size of this difference was again small (Cohen d = .29). A total of 86% of patients found the inclusion of their partners at the sessions useful. For both pelvic-floor physical therapy and sexual health, attendees were more satisfied if they attended before treatment initiation rather than after completion (Table 4B).
Discussion
The purpose of this descriptive analysis was to outline a comprehensive, multidisciplinary supportive care program for men with prostate cancer and to present initial data on the population that has used the program and their satisfaction with the services provided. Within the first 5 years of the PCSC Program, 1,269 patients registered to participate. However, nearly 1 in 6 men who registered for the program did not subsequently attend any education sessions or use any clinical services offered, despite the fact that all services were free of charge. It is possible that nonparticipation may be related to men on active surveillance choosing not to engage with the program until they are faced with making a treatment decision, which may not happen until several years after an initial positive biopsy.26 This and other factors that affect a patient’s decision not to participate will be investigated in a future study. There is existing evidence documenting high levels of distress and anxiety for patients and their partners resulting from decision-making around prostate cancer treatment,27,28 and many face both decisional conflict and subsequent regret.15,29 Further work to help patients access the program could include defining a prehabilitation program for which patients can sign up that automatically selects the education sessions and clinical services most relevant to them.
The number of attendees varied across the 6 education sessions, with introduction to prostate cancer and sexual health being the best attended. This is consistent with the literature concerning the specific unmet supportive care and information needs in this population10,13 and with the value that men have placed on taking an active role in the decisions around their prostate cancer treatment.30 It is also possible that attendance varied because modules were introduced in a stepwise fashion and were offered on different schedules. Patients and partners both reported a high degree of satisfaction with all of the modules’ education sessions, reporting that the length, content, and delivery were appropriate.
Since 2013, a wide research portfolio has grown alongside the program. It has acted as a recruitment site for multicenter national studies and has attracted funding for several in-house research projects and evaluations. In addition, the VPC has implemented clinic-wide electronic collection of several patient-reported outcome measures using iPads. Patients have the option of contributing their data to Canadian (PC360o) and Global (TrueNTH Global Registry – Prostate Cancer Outcomes) registries for prostate cancer. The program has also created educational opportunities by supporting postdoctoral fellows. It has also provided a rich environment for urology and radiation oncology residents and fellows to participate in a multidisciplinary supportive care team, ensuring that the next generation of surgeons and oncologists recognize the importance of this approach to care.
Limitations
This is a brief descriptive study that relies on a mixture of anonymized survey and clinical chart data. Because the program’s patient feedback forms are anonymous, we are not able to link satisfaction scores to differences in sociodemographic, clinical, or prognostic factors. We also have not directly measured clinical, psychological, or quality of life outcomes; however, all 3 will be included in future studies of the program. An additional limitation is that not all program modules were offered for the entirety of the study duration and are offered at different frequencies. Thus, some modules have disproportionally higher participation rates than others. Lastly, we are missing clinical information for 16% of our participants who are not patients at the VPC.
The program is offered within an academic and teaching hospital in a major metropolitan center and depends on the work of a large interdisciplinary team. Cancer programs that are not embedded within a similar environment, such as those located in smaller rural communities, may not have access to the specialized clinical professionals who run our program, affecting its direct generalizability to these locations. Other specialists, such as palliative care teams, could be well positioned to provide support in locations that do not have a similar level of resource available. Furthermore, some program elements will be adapted to be delivered using telemedicine technology, which is an additional approach to improving access for patients who are beyond the reach of a tertiary care facility.
Conclusions
There is a growing need to provide consistent and comprehensive supportive care to patients with prostate cancer and their partners and families throughout the disease and treatment journey. The PCSC Program uses a multidisciplinary, evidenced-based, disease-focused approach to support informed treatment decision-making and address the physical, psychological, and psychosocial effects of prostate cancer diagnosis and treatment. We proactively collect data on disease, personal demographic details, and symptoms or quality of life, supporting opportunities to partner with researchers with the goal of further improving quality of life based on evidenced-based practices. Going forward, we will conduct detailed examinations of the costs and benefits (in terms of symptom management and quality of life) of the PCSC Program, further contributing to the development of evidence-based best practices for supportive care for men with prostate cancer and their families.
Acknowledgments
The authors express their gratitude to the urologists and radiation oncologists who referred their patients to the program and participated in delivering education sessions, including Dr Martin Gleave, Dr Peter Black, Dr Alan So, Dr Scott Tyldesley, and Dr Mira Keyes. They also thank Dr Richard Wassersug for his contributions to the initial program design and implementation. They thank the patients and their families for participating, and all of their current or past staff and collaborators. Lastly, they thank the funders of the program: the Specialist Services Committee, the BC Ministry of Health, the Prostate Cancer Foundation of BC, and philanthropic donors. They acknowledge Vancouver Coastal Health Research Institute and the University of British Columbia for their institutional support.
1. Cancer Research UK. Prostate cancer statistics. http://www.cancer.ca/en/cancer-information/cancer-type/prostate/statistics/?region=sk. Published 2015. Accessed June 22, 2017.
2. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220-241.
3. Canadian Cancer Society. Canadian cancer statistics special topic: predictions of the future burden of cancer in Canada. Ottawa, Canada: Public Health Agency of Canada; 2015.
4. Roth AJ, Weinberger MI, Nelson CJ. Prostate cancer: psychosocial implications and management. Future Oncol. 2008;4(4):561-568.
5. Couper J, Bloch S, Love A, Macvean M, Duchesne GM, Kissane D. Psychosocial adjustment of female partners of men with prostate cancer: a review of the literature. Psychooncology 2006;15(11):937-953.
6. Wilt TJ, MacDonald R, Rutks I, Shamliyan TA, Taylor BC, Kane RL. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med. 2008;148(6):435-448.
7. Galvão DA, Spry NA, Taaffe DR, et al. Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. BJU Int. 2008;102(1):44-47.
8. Watts S, Leydon G, Birch B, et al. Depression and anxiety in prostate cancer: a systematic review and meta-analysis of prevalence rates. BMJ Open. 2014;4(3):e003901.
9. Zaider T, Manne S, Nelson C, Mulhall J, Kissane D. Loss of masculine identity, marital affection, and sexual bother in men with localized prostate cancer. J Sex Med. 2012;9(10):2724-2732.
10. Ream E, Quennell A, Fincham L, et al. Supportive care needs of men living with prostate cancer in England: a survey. Br J Cancer. 2008;98(12):1903-1909.
11. Howell D, Hack TF, Oliver TK, et al. Models of care for post-treatment follow-up of adult cancer survivors: a systematic review and quality appraisal of the evidence. J Cancer Surviv. 2012;6(4):359-371.
12. Halpern MT, Viswanathan M, Evans TS, Birken SA, Basch E, Mayer DK. Models of cancer survivorship care: overview and summary of current evidence. J Oncol Pract. 2015;11(1):e19-e27.
13. Smith DP, Supramaniam R, King MT, Ward J, Berry M, Armstrong BK. Age, health, and education determine supportive care needs of men younger than 70 years with prostate cancer. J Clin Oncol. 2007;25(18):2560-2566.
14. Northouse LL, Mood DW, Montie JE, et al. Living with prostate cancer: patients’ and spouses’ psychosocial status and quality of life. J Clin Oncol. 2007;25(27):4171-4177.
15. Hedden L, Wassersug R, Mahovlich S, et al. Evaluating an educational intervention to alleviate distress amongst men with newly diagnosed prostate cancer and their partners. BJU Int. 2017;120(5B):E21-E29.
16. Bradley EB, Bissonette EA, Theodorescu D. Determinants of long-term quality of life and voiding function of patients treated with radical prostatectomy or permanent brachytherapy for prostate cancer. BJU Int. 2004;94(7):1003-1009.
17. Ramsey SD, Zeliadt SB, Blough DK, et al. Impact of prostate cancer on sexual relationships: a longitudinal perspective on intimate partners’ experiences. J Sex Med. 2013;10(12):3135-3143.
18. Wittmann D, Carolan M, Given B, et al. Exploring the role of the partner in couples’ sexual recovery after surgery for prostate cancer. Support Care Cancer. 2014;22(9):2509-2515.
19. Schmitz KH, Courneya KS, Matthews C, et al. American college of sports medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409-1426.
20. Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243-274.
21. Elliott S, Latini DM, Walker LM, Wassersug R, Robinson JW; ADT Suvivorship Working Group. Androgen deprivation therapy for prostate cancer: recommendations to improve patient and partner quality of life. J Sex Med. 2010;7(9):2996-3010.
22. Wassersug RJ, Walker LM, Robinson JW. Androgen deprivation therapy: an essential guide for prostate cancer patients and their loved ones. New York, NY: Demos Health; 2014.
23. Wibowo E, Walker LM, Wilyman S, et al. Androgen deprivation therapy educational program: a Canadian True NTH initiative. J Clin Oncol. 2016;34(suppl 3):243.
24. Stanford JL, Feng Z, Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer. JAMA. 2000;283(3):354-360.
25. Overgård M, Angelsen A, Lydersen S, Mørkved S. Does physiotherapist-guided pelvic floor muscle training reduce urinary incontinence after radical prostatectomy? A randomised controlled trial. Eur Urol. 2008;54(2):438-448.
26. Godtman RA, Holmberg E, Khatami A, Pihl CG, Stranne J, Hugosson J. Long-term results of active surveillance in the Göteborg randomized, population-based prostate cancer screening trial. Eur Urol. 2016;70(5):760-766.
27. Cohen H, Britten N. Who decides about prostate cancer treatment? A qualitative study. Fam Pract. 2003;20(6):724-729.
28. Denberg TD, Melhado TV, Steiner JF. Patient treatment preferences in localized prostate carcinoma: the influence of emotion, misconception, and anecdote. Cancer. 2006;107(3):620-630.
29. Morris BB, Farnan L, Song L, et al. Treatment decisional regret among men with prostate cancer: racial differences and influential factors in the North Carolina health access and prostate cancer treatment project (HCaP-NC). Cancer. 2015;121(12):2029-2035.
30. Feldman-Stewart D, Capirci C, Brennenstuhl S, et al. Information for decision making by patients with early-stage prostate cancer: a comparison across 9 countries. Med Decis Making. 2011;31(5):754-766.
Prostate cancer is the most common malignancy diagnosed in Canadian men. An estimated 21,300 Canadian men were diagnosed with the disease in 2017, representing 21% of all new cancer cases.1 There are about 176,000 men living with prostate cancer in Canada.1 In the United States, there were 2,778,630 survivors of prostate cancer as of 2012 and that population is expected to increase by more than 1 million (40%) to 3,922,600 by 2022.2
Although 96% of men diagnosed with prostate cancer now survive longer than 5 years3, many will suffer from treatment-related sequelae that can have a profound effect on quality of life for themselves and their partners.4,5 Impacts include sexual, urinary, and bowel dysfunctions6 owing to treatment of the primary tumor as well as reduced muscle and bone mass, osteoporosis, fatigue, obesity, and glucose intolerance or diabetes7 owing to androgen-deprivation therapy (ADT). Many men also suffer from psychological issues such as depression, anxiety, anger and irritability, sense of isolation, grief, and loss of masculinity.8,9 The psychological impacts also continue well beyond the completion of treatment and can be significant for both patients and their partners.5,8
With posttreatment longevity and the associated complex sequelae, prostate cancer is being viewed increasingly as a chronic disease whose effects must be managed for many years after the completion of primary treatment. Supportive care that “[manages] symptoms and side effects, enables adaptation and coping, optimizes understanding and informed decision-making, and minimizes decrements in functioning”10 is becoming recognized as a critical component of direct oncologic care before, during, and after treatment. Health care professionals, scientists, governments, and patient advocates are increasingly calling for the development of comprehensive supportive care programs improve the quality of life for people diagnosed with cancer. A common model for survivorship care is a general program for all cancer survivors that provides disease- and patient-specific care plans. These care plans outline patients’ prior therapies, potential side effects, recommendations for monitoring (for side effects or relapse of cancer), and advice on how patients can maintain a healthy lifestyle.11 However, there are few survivorship programs for men with prostate cancer and their partners, and the evidence base around best practices for these programs is scant.12 Furthermore, up to 87% of men with a prostate cancer diagnosis report specific and significant unmet supportive care needs,10,13 with sexuality-related and psychological issues10,14 being the areas of greatest concern.
To address the complex supportive care needs of men with prostate cancer in British Columbia, Canada, the Vancouver Prostate Centre (VPC) and Department of Urologic Sciences at the University of British Columbia developed the multidisciplinary Prostate Cancer Supportive Care (PCSC) Program. The program aims to address the challenges of decision-making and coping faced by men with prostate cancer and their partners and family members along the entire disease trajectory. Services are provided at no cost to participants. Here, we outline the guiding principles for the PCSC program and its scope, delivery, and evaluation. We provide information on the more than 1,200 patients who have participated in the program since its inception in January of 2013, the rates of participation across the different program modules, and a selection of patient satisfaction measures. We also discuss successes and limitations and ongoing research and evaluation efforts, providing lessons learned to support the development of other supportive care programs in Canada and internationally.
Program description
Guiding principles
The PCSC Program is a clinical, educational, and research-based program, with 4 guiding principles: it is comprehensive, patient- and partner-centered, evidence-based, and supports new research. The program serves patients, partners, and families along the entire disease trajectory, recognizing that cancer is a family disease, affecting both the individual and social network, and that the psychological stress associated with a diagnosis of prostate cancer is borne heavily by partners. It has been designed, implemented, and refined with the best available evidence and with the intention to undergo consistent and repeated evaluation. Finally, it was designed to provide opportunities for targeted research efforts, supporting the growth of the evidence base in this area.
Patient entry and module descriptions
Patients can be referred to the program by a physician or other allied health professional. They may also self-refer, having been made aware of the program through our website, a variety of print materials, or by word of mouth. On referral, the program coordinator collects patients’ basic clinical and demographic data, assesses health literacy and lifestyle factors, and provides them with information on the program modules. As of December 2015, the program consisted of 6 distinct modules, each focusing on different elements of the disease trajectory or on addressing specific physical or mental health concerns. Modules are led by licensed health professionals with experience in oncology. No elements of the program are mandatory, and participants are free to pick and choose the components that are most relevant to them and their partners.
Introduction to prostate cancer and primary treatment options. This is a group-based module that focuses on educating newly diagnosed patients (and those going on or off active surveillance) on the basic biology of prostate cancer, the primary treatment options for localized disease, and the main side effects associated with the treatments. It also includes information about the other services offered by the program and any ongoing research studies. The session is held twice a month in the early evening and is run collectively by a urologist, radiation oncologist, patient representative, and program coordinator. It includes a brief one-on-one discussion between each patient and their partner or family member and the urologist and radiation oncologist to address any remaining questions. A copy of the patient’s biopsy report is on hand for the physician(s). Attendance of this session has been shown to significantly reduce pretreatment distress in both patients and their partners.15
Managing sexual function and intimacy. Sexual intimacy is tied to overall health outcomes, relationship satisfaction, and quality of life.16 Primary therapy for prostate cancer can be associated with substantial side-effects (eg, erectile dysfunction, incontinence, altered libido, penile shortening) that negatively affect sexual intimacy and have an impact on the patient individually as well as the sexual relationship he has with his partner.17
The program’s Sexual Health Service (SHS) provides patients and partners with information on the impact of treatment on sexual health.18 The SHS offers educational sessions led by a sexual rehabilitation nurse and clinical psychologist with a specialization in sexual health. Sessions focus on the impact of prostate cancer treatments on sexual function and therapeutic modalities, promote an understanding of the barriers to sexual adaptation posttreatment, and present options for sexual activity that are not solely dependent on the ability to achieve an erection. Once participants have attended an educational session, they are offered individual consultations with the sexual health nurse every 3 to 6 months for 2 years or longer, depending on the patient’s or couple’s needs. They are referred to the SHS’s sexual medicine physician if further medical intervention is warranted. The sexual health nurse works with the patient and partner to develop an individualized Sexual Health Rehabilitation Action Plan (SHRAP), which assists the couple in sexual adaptation going forward. The SHRAP is a tool devised by the sexual health nurse based on her clinical experience with couples affected by prostate cancer.
Couples who have been evaluated within the SHS are also invited to attend a second workshop on intimacy that is offered quarterly. Workshop participants discuss the impact of sexual changes on relationships, and strategies on how to enhance intimacy and sexual communication are presented. A resource package is provided to each couple to help re-establish and/or strengthen their various dimensions of intimacy.
Lifestyle management. The lifestyle management modules include separate nutrition and physical activity or exercise components. Referral to the smoking cessation program in the Vancouver Coastal Health Authority is made at program registration, if appropriate. The nutrition group-based education session is delivered by a registered dietitian from the British Columbia Cancer Agency who specializes in prostate cancer. The session focuses on evidence-based recommendations on diet after a diagnosis of prostate cancer, the use of dietary supplements, body weight and health, and practical nutrition tips. The exercise session is delivered by an exercise physiologist who specializes in working with cancer patients. It covers the value of exercise in terms of safety, prevention and reduction of treatment side effects (including from ADT), treatment prehabilitation and recovery, advanced cancer management, and long-term survival. A one-on-one exercise counseling clinic is also offered and aims to increase exercise adoption and long-term adherence in line with Canadian Physical Activity guidelines and exercise oncology guidelines,19,20 with follow-up appointments at 3, 6, and 12 months to help patients stay motivated and ensure they are exercising correctly. The individual consultations with the exercise physiologist include physical measures, exercise volume, treatment side effects, and coconstructed goal setting with an individualized formal exercise regimen (exercise prescription).
Adapting to ADT. This is an educational module offered to patients with metastatic prostate cancer who are starting hormone therapy treatments that lower serum testosterone into the castrate range. This program was one of several available through TrueNTH, a portfolio of projects funded by the Movember Foundation, through Prostate Cancer Canada. The session is delivered by a patient facilitator and focuses on strategies to recognize and adapt to the side effects of ADT21 while maintaining a good quality of life and strong intimate relationships with partners.22,23
Pelvic-floor physical therapy for urinary incontinence. This module includes a group-based and individualized education session for patients (either pre- or posttreatment) focused on reducing the effects of surgery and/or radiation therapy on urinary and sexual continence as well as on how to cope with these symptoms and minimize the effect they have on quality of life.24 The session is conducted by a physical therapist who is certified as a pelvic-floor specialist. Supervised pelvic-floor re-education and/or exercise has been shown to successfully reduce the degree of incontinence in this population.25 The module therefore also includes 3 one-on-one clinical appointments for patients who are still experiencing bother from incontinence 12 weeks after a radical prostatectomy or postradiation treatment.
Psycho-oncology. In recognition of the emotional and psychological burden associated with prostate cancer and the important role partners play in facilitating treatment of these psychological and/or psychosocial issues, the program offers appointments with a registered clinical counselor to address acute emotional distress. These are usually 1-hour appointments offered to both patients and partners, either separately or together. Appointments can be attended in person or conducted by telephone. When appropriate, patients are referred for further long-term individual support or couple support with their partners. A group therapy workshop was also initiated in 2016. In this program, men participate in a guided autobiographical life review through a process that focuses on developing a cohesive working group, learning strategic communication skills, and understanding and learning how to manage difficult emotions and transitional life stressors associated with prostate cancer. It also focuses on processing and integrating critical events that contribute to the men’s identity and psychological function and involves the consolidation of the personal learning that occurs. Postgroup referral plans are developed on an individual basis as needed.
Methods
Data
We obtained sociodemographic, diagnostic, and treatment information as well as clinic visit records for all PCSC Program registrants from the electronic medical record held at the VPC. Clinical variables included age at diagnosis, Gleason score, and primary treatment modality (including active surveillance and ADT use). The Gleason score determines the aggressiveness of a patient’s prostate cancer based on biopsy results. A score of 6 or less indicates that the disease is likely to grow slowly. A grade of 7 is considered intermediate risk (with primary score of 3 and secondary 4 being lower risk than those with a primary score of 4 and secondary of 3). A Gleason score of 8 or higher indicates aggressive disease that is poorly differentiated or high grade. Sociodemographic characteristics included age, travel distance to the clinic, and income quintile. We obtained attendance records for the modular education sessions from the program’s database. Patients who did not have any medical visits at the VPC (referred to henceforth as non-VPC patients) did not have a clinic record, so we excluded them from the subset of the analyses that depended on specific clinical variables.
All patients and partners who participate in any PCSC Program education sessions (introduction to prostate cancer, sexual health, nutrition, exercise, ADT, and pelvic-floor physical therapy) are asked to complete voluntary, anonymous feedback forms. These forms assess participant satisfaction using a series of Likert-based and Boolean response items as well as qualitative commentary. They include questions that assess the timing, structure, and content of each session.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Statistical approach
Descriptive statistics were used to analyze participant characteristics, program participation rates, and participant satisfaction. For each module’s education session, we compared the overall satisfaction between patients and partners using t tests. We also compared the level of satisfaction across the different modules using a 1-way analysis of variance. For the sexual health and pelvic-floor physical therapy sessions, we compared satisfaction between participants who attended the education sessions before to those who attended following their primary treatment using t tests. We provide the eta squared (for analyses of variance) and Cohen d (for t tests) to provide an effect size estimate of any significant differences observed.
Results
Participants
From the program’s founding in January of 2013 to December 31, 2016, a total of 1,269 patients registered (an average of 317 patients a year). Of those, 1,026 (80.9%) had at least 1 prostate cancer–related visit at the VPC. The remaining 243 (19.1%) were non-VPC patients (Figure). Overall, 1,062 men (83.4%) who registered with the program went on to attend at least 1 education session or clinic appointment.
Average age among male program participants was 67.7 years, and age at diagnosis was 62.5 years (Table 1). In all, 273 men (31.7%) had Gleason 3+4, and 117 (13.7%) had Gleason 4+3. Most of the participants (76.9%) elected to undergo radical prostatectomy for primary treatment. Ninety-five men (8.9%) received at least some ADT treatment as an adjunct to radiation or to treat recurrent disease. Participants traveled an average of 83.1 km (51.6 miles; median, 6.9 km and 10.5 miles, respectively) to attend the program; 10% of participants traveled further than 112 km (70 mi) to the clinic. One hundred and four (10.9%) and 301 (31.5%) of registrants were in the lowest and highest income quintiles respectively. Four hundred and ninety-seven (46.8%) attended at lesson 1 session or clinic appointment with a partner or family member.
Program participation
Of the 1,062 men who participated in the program, 867 (80.1%) were patients of the VPC, and 205 (19.1%) were non-VPC patients. The education sessions for the introduction to prostate cancer and sexual health modules had the largest numbers of participants (309 and 265, respectively; Table 2); however, pelvic-floor physical therapy had the highest participation rate per quarter (25 patients). The clinical services offered within the sexual health module had the larger number of participants and highest participation rate per quarter (590 total patients, 42/quarter). Timing of program participation was highly variable, ranging from 6 days to 18.5 years after diagnosis (SD, 1,301 days). More than half of participants attended a session or clinic visit within the first year of their diagnosis. A total of 17% of patients who registered did not attend any part of the program.
Satisfaction
Most patients and partners said that they found the information presented at the modular education sessions comprehensive, clear, and easy to understand (Table 3). Although the overall average satisfaction score varied significantly across sessions, ranging from 3.5 (out of a possible 4) for pelvic-floor physical therapy to 3.8 for introduction to prostate cancer (F = 3.8, P < .001), the effect size of this difference was small (η2 = .039; Table 4A). We found no difference in the level of satisfaction between patients and partners, with the exception of the sexual health module, which was rated better by partners than by patients (patients: 3.6, partners: 3.8; t = 2.0; P = .03); however, the effect size of this difference was again small (Cohen d = .29). A total of 86% of patients found the inclusion of their partners at the sessions useful. For both pelvic-floor physical therapy and sexual health, attendees were more satisfied if they attended before treatment initiation rather than after completion (Table 4B).
Discussion
The purpose of this descriptive analysis was to outline a comprehensive, multidisciplinary supportive care program for men with prostate cancer and to present initial data on the population that has used the program and their satisfaction with the services provided. Within the first 5 years of the PCSC Program, 1,269 patients registered to participate. However, nearly 1 in 6 men who registered for the program did not subsequently attend any education sessions or use any clinical services offered, despite the fact that all services were free of charge. It is possible that nonparticipation may be related to men on active surveillance choosing not to engage with the program until they are faced with making a treatment decision, which may not happen until several years after an initial positive biopsy.26 This and other factors that affect a patient’s decision not to participate will be investigated in a future study. There is existing evidence documenting high levels of distress and anxiety for patients and their partners resulting from decision-making around prostate cancer treatment,27,28 and many face both decisional conflict and subsequent regret.15,29 Further work to help patients access the program could include defining a prehabilitation program for which patients can sign up that automatically selects the education sessions and clinical services most relevant to them.
The number of attendees varied across the 6 education sessions, with introduction to prostate cancer and sexual health being the best attended. This is consistent with the literature concerning the specific unmet supportive care and information needs in this population10,13 and with the value that men have placed on taking an active role in the decisions around their prostate cancer treatment.30 It is also possible that attendance varied because modules were introduced in a stepwise fashion and were offered on different schedules. Patients and partners both reported a high degree of satisfaction with all of the modules’ education sessions, reporting that the length, content, and delivery were appropriate.
Since 2013, a wide research portfolio has grown alongside the program. It has acted as a recruitment site for multicenter national studies and has attracted funding for several in-house research projects and evaluations. In addition, the VPC has implemented clinic-wide electronic collection of several patient-reported outcome measures using iPads. Patients have the option of contributing their data to Canadian (PC360o) and Global (TrueNTH Global Registry – Prostate Cancer Outcomes) registries for prostate cancer. The program has also created educational opportunities by supporting postdoctoral fellows. It has also provided a rich environment for urology and radiation oncology residents and fellows to participate in a multidisciplinary supportive care team, ensuring that the next generation of surgeons and oncologists recognize the importance of this approach to care.
Limitations
This is a brief descriptive study that relies on a mixture of anonymized survey and clinical chart data. Because the program’s patient feedback forms are anonymous, we are not able to link satisfaction scores to differences in sociodemographic, clinical, or prognostic factors. We also have not directly measured clinical, psychological, or quality of life outcomes; however, all 3 will be included in future studies of the program. An additional limitation is that not all program modules were offered for the entirety of the study duration and are offered at different frequencies. Thus, some modules have disproportionally higher participation rates than others. Lastly, we are missing clinical information for 16% of our participants who are not patients at the VPC.
The program is offered within an academic and teaching hospital in a major metropolitan center and depends on the work of a large interdisciplinary team. Cancer programs that are not embedded within a similar environment, such as those located in smaller rural communities, may not have access to the specialized clinical professionals who run our program, affecting its direct generalizability to these locations. Other specialists, such as palliative care teams, could be well positioned to provide support in locations that do not have a similar level of resource available. Furthermore, some program elements will be adapted to be delivered using telemedicine technology, which is an additional approach to improving access for patients who are beyond the reach of a tertiary care facility.
Conclusions
There is a growing need to provide consistent and comprehensive supportive care to patients with prostate cancer and their partners and families throughout the disease and treatment journey. The PCSC Program uses a multidisciplinary, evidenced-based, disease-focused approach to support informed treatment decision-making and address the physical, psychological, and psychosocial effects of prostate cancer diagnosis and treatment. We proactively collect data on disease, personal demographic details, and symptoms or quality of life, supporting opportunities to partner with researchers with the goal of further improving quality of life based on evidenced-based practices. Going forward, we will conduct detailed examinations of the costs and benefits (in terms of symptom management and quality of life) of the PCSC Program, further contributing to the development of evidence-based best practices for supportive care for men with prostate cancer and their families.
Acknowledgments
The authors express their gratitude to the urologists and radiation oncologists who referred their patients to the program and participated in delivering education sessions, including Dr Martin Gleave, Dr Peter Black, Dr Alan So, Dr Scott Tyldesley, and Dr Mira Keyes. They also thank Dr Richard Wassersug for his contributions to the initial program design and implementation. They thank the patients and their families for participating, and all of their current or past staff and collaborators. Lastly, they thank the funders of the program: the Specialist Services Committee, the BC Ministry of Health, the Prostate Cancer Foundation of BC, and philanthropic donors. They acknowledge Vancouver Coastal Health Research Institute and the University of British Columbia for their institutional support.
Prostate cancer is the most common malignancy diagnosed in Canadian men. An estimated 21,300 Canadian men were diagnosed with the disease in 2017, representing 21% of all new cancer cases.1 There are about 176,000 men living with prostate cancer in Canada.1 In the United States, there were 2,778,630 survivors of prostate cancer as of 2012 and that population is expected to increase by more than 1 million (40%) to 3,922,600 by 2022.2
Although 96% of men diagnosed with prostate cancer now survive longer than 5 years3, many will suffer from treatment-related sequelae that can have a profound effect on quality of life for themselves and their partners.4,5 Impacts include sexual, urinary, and bowel dysfunctions6 owing to treatment of the primary tumor as well as reduced muscle and bone mass, osteoporosis, fatigue, obesity, and glucose intolerance or diabetes7 owing to androgen-deprivation therapy (ADT). Many men also suffer from psychological issues such as depression, anxiety, anger and irritability, sense of isolation, grief, and loss of masculinity.8,9 The psychological impacts also continue well beyond the completion of treatment and can be significant for both patients and their partners.5,8
With posttreatment longevity and the associated complex sequelae, prostate cancer is being viewed increasingly as a chronic disease whose effects must be managed for many years after the completion of primary treatment. Supportive care that “[manages] symptoms and side effects, enables adaptation and coping, optimizes understanding and informed decision-making, and minimizes decrements in functioning”10 is becoming recognized as a critical component of direct oncologic care before, during, and after treatment. Health care professionals, scientists, governments, and patient advocates are increasingly calling for the development of comprehensive supportive care programs improve the quality of life for people diagnosed with cancer. A common model for survivorship care is a general program for all cancer survivors that provides disease- and patient-specific care plans. These care plans outline patients’ prior therapies, potential side effects, recommendations for monitoring (for side effects or relapse of cancer), and advice on how patients can maintain a healthy lifestyle.11 However, there are few survivorship programs for men with prostate cancer and their partners, and the evidence base around best practices for these programs is scant.12 Furthermore, up to 87% of men with a prostate cancer diagnosis report specific and significant unmet supportive care needs,10,13 with sexuality-related and psychological issues10,14 being the areas of greatest concern.
To address the complex supportive care needs of men with prostate cancer in British Columbia, Canada, the Vancouver Prostate Centre (VPC) and Department of Urologic Sciences at the University of British Columbia developed the multidisciplinary Prostate Cancer Supportive Care (PCSC) Program. The program aims to address the challenges of decision-making and coping faced by men with prostate cancer and their partners and family members along the entire disease trajectory. Services are provided at no cost to participants. Here, we outline the guiding principles for the PCSC program and its scope, delivery, and evaluation. We provide information on the more than 1,200 patients who have participated in the program since its inception in January of 2013, the rates of participation across the different program modules, and a selection of patient satisfaction measures. We also discuss successes and limitations and ongoing research and evaluation efforts, providing lessons learned to support the development of other supportive care programs in Canada and internationally.
Program description
Guiding principles
The PCSC Program is a clinical, educational, and research-based program, with 4 guiding principles: it is comprehensive, patient- and partner-centered, evidence-based, and supports new research. The program serves patients, partners, and families along the entire disease trajectory, recognizing that cancer is a family disease, affecting both the individual and social network, and that the psychological stress associated with a diagnosis of prostate cancer is borne heavily by partners. It has been designed, implemented, and refined with the best available evidence and with the intention to undergo consistent and repeated evaluation. Finally, it was designed to provide opportunities for targeted research efforts, supporting the growth of the evidence base in this area.
Patient entry and module descriptions
Patients can be referred to the program by a physician or other allied health professional. They may also self-refer, having been made aware of the program through our website, a variety of print materials, or by word of mouth. On referral, the program coordinator collects patients’ basic clinical and demographic data, assesses health literacy and lifestyle factors, and provides them with information on the program modules. As of December 2015, the program consisted of 6 distinct modules, each focusing on different elements of the disease trajectory or on addressing specific physical or mental health concerns. Modules are led by licensed health professionals with experience in oncology. No elements of the program are mandatory, and participants are free to pick and choose the components that are most relevant to them and their partners.
Introduction to prostate cancer and primary treatment options. This is a group-based module that focuses on educating newly diagnosed patients (and those going on or off active surveillance) on the basic biology of prostate cancer, the primary treatment options for localized disease, and the main side effects associated with the treatments. It also includes information about the other services offered by the program and any ongoing research studies. The session is held twice a month in the early evening and is run collectively by a urologist, radiation oncologist, patient representative, and program coordinator. It includes a brief one-on-one discussion between each patient and their partner or family member and the urologist and radiation oncologist to address any remaining questions. A copy of the patient’s biopsy report is on hand for the physician(s). Attendance of this session has been shown to significantly reduce pretreatment distress in both patients and their partners.15
Managing sexual function and intimacy. Sexual intimacy is tied to overall health outcomes, relationship satisfaction, and quality of life.16 Primary therapy for prostate cancer can be associated with substantial side-effects (eg, erectile dysfunction, incontinence, altered libido, penile shortening) that negatively affect sexual intimacy and have an impact on the patient individually as well as the sexual relationship he has with his partner.17
The program’s Sexual Health Service (SHS) provides patients and partners with information on the impact of treatment on sexual health.18 The SHS offers educational sessions led by a sexual rehabilitation nurse and clinical psychologist with a specialization in sexual health. Sessions focus on the impact of prostate cancer treatments on sexual function and therapeutic modalities, promote an understanding of the barriers to sexual adaptation posttreatment, and present options for sexual activity that are not solely dependent on the ability to achieve an erection. Once participants have attended an educational session, they are offered individual consultations with the sexual health nurse every 3 to 6 months for 2 years or longer, depending on the patient’s or couple’s needs. They are referred to the SHS’s sexual medicine physician if further medical intervention is warranted. The sexual health nurse works with the patient and partner to develop an individualized Sexual Health Rehabilitation Action Plan (SHRAP), which assists the couple in sexual adaptation going forward. The SHRAP is a tool devised by the sexual health nurse based on her clinical experience with couples affected by prostate cancer.
Couples who have been evaluated within the SHS are also invited to attend a second workshop on intimacy that is offered quarterly. Workshop participants discuss the impact of sexual changes on relationships, and strategies on how to enhance intimacy and sexual communication are presented. A resource package is provided to each couple to help re-establish and/or strengthen their various dimensions of intimacy.
Lifestyle management. The lifestyle management modules include separate nutrition and physical activity or exercise components. Referral to the smoking cessation program in the Vancouver Coastal Health Authority is made at program registration, if appropriate. The nutrition group-based education session is delivered by a registered dietitian from the British Columbia Cancer Agency who specializes in prostate cancer. The session focuses on evidence-based recommendations on diet after a diagnosis of prostate cancer, the use of dietary supplements, body weight and health, and practical nutrition tips. The exercise session is delivered by an exercise physiologist who specializes in working with cancer patients. It covers the value of exercise in terms of safety, prevention and reduction of treatment side effects (including from ADT), treatment prehabilitation and recovery, advanced cancer management, and long-term survival. A one-on-one exercise counseling clinic is also offered and aims to increase exercise adoption and long-term adherence in line with Canadian Physical Activity guidelines and exercise oncology guidelines,19,20 with follow-up appointments at 3, 6, and 12 months to help patients stay motivated and ensure they are exercising correctly. The individual consultations with the exercise physiologist include physical measures, exercise volume, treatment side effects, and coconstructed goal setting with an individualized formal exercise regimen (exercise prescription).
Adapting to ADT. This is an educational module offered to patients with metastatic prostate cancer who are starting hormone therapy treatments that lower serum testosterone into the castrate range. This program was one of several available through TrueNTH, a portfolio of projects funded by the Movember Foundation, through Prostate Cancer Canada. The session is delivered by a patient facilitator and focuses on strategies to recognize and adapt to the side effects of ADT21 while maintaining a good quality of life and strong intimate relationships with partners.22,23
Pelvic-floor physical therapy for urinary incontinence. This module includes a group-based and individualized education session for patients (either pre- or posttreatment) focused on reducing the effects of surgery and/or radiation therapy on urinary and sexual continence as well as on how to cope with these symptoms and minimize the effect they have on quality of life.24 The session is conducted by a physical therapist who is certified as a pelvic-floor specialist. Supervised pelvic-floor re-education and/or exercise has been shown to successfully reduce the degree of incontinence in this population.25 The module therefore also includes 3 one-on-one clinical appointments for patients who are still experiencing bother from incontinence 12 weeks after a radical prostatectomy or postradiation treatment.
Psycho-oncology. In recognition of the emotional and psychological burden associated with prostate cancer and the important role partners play in facilitating treatment of these psychological and/or psychosocial issues, the program offers appointments with a registered clinical counselor to address acute emotional distress. These are usually 1-hour appointments offered to both patients and partners, either separately or together. Appointments can be attended in person or conducted by telephone. When appropriate, patients are referred for further long-term individual support or couple support with their partners. A group therapy workshop was also initiated in 2016. In this program, men participate in a guided autobiographical life review through a process that focuses on developing a cohesive working group, learning strategic communication skills, and understanding and learning how to manage difficult emotions and transitional life stressors associated with prostate cancer. It also focuses on processing and integrating critical events that contribute to the men’s identity and psychological function and involves the consolidation of the personal learning that occurs. Postgroup referral plans are developed on an individual basis as needed.
Methods
Data
We obtained sociodemographic, diagnostic, and treatment information as well as clinic visit records for all PCSC Program registrants from the electronic medical record held at the VPC. Clinical variables included age at diagnosis, Gleason score, and primary treatment modality (including active surveillance and ADT use). The Gleason score determines the aggressiveness of a patient’s prostate cancer based on biopsy results. A score of 6 or less indicates that the disease is likely to grow slowly. A grade of 7 is considered intermediate risk (with primary score of 3 and secondary 4 being lower risk than those with a primary score of 4 and secondary of 3). A Gleason score of 8 or higher indicates aggressive disease that is poorly differentiated or high grade. Sociodemographic characteristics included age, travel distance to the clinic, and income quintile. We obtained attendance records for the modular education sessions from the program’s database. Patients who did not have any medical visits at the VPC (referred to henceforth as non-VPC patients) did not have a clinic record, so we excluded them from the subset of the analyses that depended on specific clinical variables.
All patients and partners who participate in any PCSC Program education sessions (introduction to prostate cancer, sexual health, nutrition, exercise, ADT, and pelvic-floor physical therapy) are asked to complete voluntary, anonymous feedback forms. These forms assess participant satisfaction using a series of Likert-based and Boolean response items as well as qualitative commentary. They include questions that assess the timing, structure, and content of each session.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Statistical approach
Descriptive statistics were used to analyze participant characteristics, program participation rates, and participant satisfaction. For each module’s education session, we compared the overall satisfaction between patients and partners using t tests. We also compared the level of satisfaction across the different modules using a 1-way analysis of variance. For the sexual health and pelvic-floor physical therapy sessions, we compared satisfaction between participants who attended the education sessions before to those who attended following their primary treatment using t tests. We provide the eta squared (for analyses of variance) and Cohen d (for t tests) to provide an effect size estimate of any significant differences observed.
Results
Participants
From the program’s founding in January of 2013 to December 31, 2016, a total of 1,269 patients registered (an average of 317 patients a year). Of those, 1,026 (80.9%) had at least 1 prostate cancer–related visit at the VPC. The remaining 243 (19.1%) were non-VPC patients (Figure). Overall, 1,062 men (83.4%) who registered with the program went on to attend at least 1 education session or clinic appointment.
Average age among male program participants was 67.7 years, and age at diagnosis was 62.5 years (Table 1). In all, 273 men (31.7%) had Gleason 3+4, and 117 (13.7%) had Gleason 4+3. Most of the participants (76.9%) elected to undergo radical prostatectomy for primary treatment. Ninety-five men (8.9%) received at least some ADT treatment as an adjunct to radiation or to treat recurrent disease. Participants traveled an average of 83.1 km (51.6 miles; median, 6.9 km and 10.5 miles, respectively) to attend the program; 10% of participants traveled further than 112 km (70 mi) to the clinic. One hundred and four (10.9%) and 301 (31.5%) of registrants were in the lowest and highest income quintiles respectively. Four hundred and ninety-seven (46.8%) attended at lesson 1 session or clinic appointment with a partner or family member.
Program participation
Of the 1,062 men who participated in the program, 867 (80.1%) were patients of the VPC, and 205 (19.1%) were non-VPC patients. The education sessions for the introduction to prostate cancer and sexual health modules had the largest numbers of participants (309 and 265, respectively; Table 2); however, pelvic-floor physical therapy had the highest participation rate per quarter (25 patients). The clinical services offered within the sexual health module had the larger number of participants and highest participation rate per quarter (590 total patients, 42/quarter). Timing of program participation was highly variable, ranging from 6 days to 18.5 years after diagnosis (SD, 1,301 days). More than half of participants attended a session or clinic visit within the first year of their diagnosis. A total of 17% of patients who registered did not attend any part of the program.
Satisfaction
Most patients and partners said that they found the information presented at the modular education sessions comprehensive, clear, and easy to understand (Table 3). Although the overall average satisfaction score varied significantly across sessions, ranging from 3.5 (out of a possible 4) for pelvic-floor physical therapy to 3.8 for introduction to prostate cancer (F = 3.8, P < .001), the effect size of this difference was small (η2 = .039; Table 4A). We found no difference in the level of satisfaction between patients and partners, with the exception of the sexual health module, which was rated better by partners than by patients (patients: 3.6, partners: 3.8; t = 2.0; P = .03); however, the effect size of this difference was again small (Cohen d = .29). A total of 86% of patients found the inclusion of their partners at the sessions useful. For both pelvic-floor physical therapy and sexual health, attendees were more satisfied if they attended before treatment initiation rather than after completion (Table 4B).
Discussion
The purpose of this descriptive analysis was to outline a comprehensive, multidisciplinary supportive care program for men with prostate cancer and to present initial data on the population that has used the program and their satisfaction with the services provided. Within the first 5 years of the PCSC Program, 1,269 patients registered to participate. However, nearly 1 in 6 men who registered for the program did not subsequently attend any education sessions or use any clinical services offered, despite the fact that all services were free of charge. It is possible that nonparticipation may be related to men on active surveillance choosing not to engage with the program until they are faced with making a treatment decision, which may not happen until several years after an initial positive biopsy.26 This and other factors that affect a patient’s decision not to participate will be investigated in a future study. There is existing evidence documenting high levels of distress and anxiety for patients and their partners resulting from decision-making around prostate cancer treatment,27,28 and many face both decisional conflict and subsequent regret.15,29 Further work to help patients access the program could include defining a prehabilitation program for which patients can sign up that automatically selects the education sessions and clinical services most relevant to them.
The number of attendees varied across the 6 education sessions, with introduction to prostate cancer and sexual health being the best attended. This is consistent with the literature concerning the specific unmet supportive care and information needs in this population10,13 and with the value that men have placed on taking an active role in the decisions around their prostate cancer treatment.30 It is also possible that attendance varied because modules were introduced in a stepwise fashion and were offered on different schedules. Patients and partners both reported a high degree of satisfaction with all of the modules’ education sessions, reporting that the length, content, and delivery were appropriate.
Since 2013, a wide research portfolio has grown alongside the program. It has acted as a recruitment site for multicenter national studies and has attracted funding for several in-house research projects and evaluations. In addition, the VPC has implemented clinic-wide electronic collection of several patient-reported outcome measures using iPads. Patients have the option of contributing their data to Canadian (PC360o) and Global (TrueNTH Global Registry – Prostate Cancer Outcomes) registries for prostate cancer. The program has also created educational opportunities by supporting postdoctoral fellows. It has also provided a rich environment for urology and radiation oncology residents and fellows to participate in a multidisciplinary supportive care team, ensuring that the next generation of surgeons and oncologists recognize the importance of this approach to care.
Limitations
This is a brief descriptive study that relies on a mixture of anonymized survey and clinical chart data. Because the program’s patient feedback forms are anonymous, we are not able to link satisfaction scores to differences in sociodemographic, clinical, or prognostic factors. We also have not directly measured clinical, psychological, or quality of life outcomes; however, all 3 will be included in future studies of the program. An additional limitation is that not all program modules were offered for the entirety of the study duration and are offered at different frequencies. Thus, some modules have disproportionally higher participation rates than others. Lastly, we are missing clinical information for 16% of our participants who are not patients at the VPC.
The program is offered within an academic and teaching hospital in a major metropolitan center and depends on the work of a large interdisciplinary team. Cancer programs that are not embedded within a similar environment, such as those located in smaller rural communities, may not have access to the specialized clinical professionals who run our program, affecting its direct generalizability to these locations. Other specialists, such as palliative care teams, could be well positioned to provide support in locations that do not have a similar level of resource available. Furthermore, some program elements will be adapted to be delivered using telemedicine technology, which is an additional approach to improving access for patients who are beyond the reach of a tertiary care facility.
Conclusions
There is a growing need to provide consistent and comprehensive supportive care to patients with prostate cancer and their partners and families throughout the disease and treatment journey. The PCSC Program uses a multidisciplinary, evidenced-based, disease-focused approach to support informed treatment decision-making and address the physical, psychological, and psychosocial effects of prostate cancer diagnosis and treatment. We proactively collect data on disease, personal demographic details, and symptoms or quality of life, supporting opportunities to partner with researchers with the goal of further improving quality of life based on evidenced-based practices. Going forward, we will conduct detailed examinations of the costs and benefits (in terms of symptom management and quality of life) of the PCSC Program, further contributing to the development of evidence-based best practices for supportive care for men with prostate cancer and their families.
Acknowledgments
The authors express their gratitude to the urologists and radiation oncologists who referred their patients to the program and participated in delivering education sessions, including Dr Martin Gleave, Dr Peter Black, Dr Alan So, Dr Scott Tyldesley, and Dr Mira Keyes. They also thank Dr Richard Wassersug for his contributions to the initial program design and implementation. They thank the patients and their families for participating, and all of their current or past staff and collaborators. Lastly, they thank the funders of the program: the Specialist Services Committee, the BC Ministry of Health, the Prostate Cancer Foundation of BC, and philanthropic donors. They acknowledge Vancouver Coastal Health Research Institute and the University of British Columbia for their institutional support.
1. Cancer Research UK. Prostate cancer statistics. http://www.cancer.ca/en/cancer-information/cancer-type/prostate/statistics/?region=sk. Published 2015. Accessed June 22, 2017.
2. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220-241.
3. Canadian Cancer Society. Canadian cancer statistics special topic: predictions of the future burden of cancer in Canada. Ottawa, Canada: Public Health Agency of Canada; 2015.
4. Roth AJ, Weinberger MI, Nelson CJ. Prostate cancer: psychosocial implications and management. Future Oncol. 2008;4(4):561-568.
5. Couper J, Bloch S, Love A, Macvean M, Duchesne GM, Kissane D. Psychosocial adjustment of female partners of men with prostate cancer: a review of the literature. Psychooncology 2006;15(11):937-953.
6. Wilt TJ, MacDonald R, Rutks I, Shamliyan TA, Taylor BC, Kane RL. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med. 2008;148(6):435-448.
7. Galvão DA, Spry NA, Taaffe DR, et al. Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. BJU Int. 2008;102(1):44-47.
8. Watts S, Leydon G, Birch B, et al. Depression and anxiety in prostate cancer: a systematic review and meta-analysis of prevalence rates. BMJ Open. 2014;4(3):e003901.
9. Zaider T, Manne S, Nelson C, Mulhall J, Kissane D. Loss of masculine identity, marital affection, and sexual bother in men with localized prostate cancer. J Sex Med. 2012;9(10):2724-2732.
10. Ream E, Quennell A, Fincham L, et al. Supportive care needs of men living with prostate cancer in England: a survey. Br J Cancer. 2008;98(12):1903-1909.
11. Howell D, Hack TF, Oliver TK, et al. Models of care for post-treatment follow-up of adult cancer survivors: a systematic review and quality appraisal of the evidence. J Cancer Surviv. 2012;6(4):359-371.
12. Halpern MT, Viswanathan M, Evans TS, Birken SA, Basch E, Mayer DK. Models of cancer survivorship care: overview and summary of current evidence. J Oncol Pract. 2015;11(1):e19-e27.
13. Smith DP, Supramaniam R, King MT, Ward J, Berry M, Armstrong BK. Age, health, and education determine supportive care needs of men younger than 70 years with prostate cancer. J Clin Oncol. 2007;25(18):2560-2566.
14. Northouse LL, Mood DW, Montie JE, et al. Living with prostate cancer: patients’ and spouses’ psychosocial status and quality of life. J Clin Oncol. 2007;25(27):4171-4177.
15. Hedden L, Wassersug R, Mahovlich S, et al. Evaluating an educational intervention to alleviate distress amongst men with newly diagnosed prostate cancer and their partners. BJU Int. 2017;120(5B):E21-E29.
16. Bradley EB, Bissonette EA, Theodorescu D. Determinants of long-term quality of life and voiding function of patients treated with radical prostatectomy or permanent brachytherapy for prostate cancer. BJU Int. 2004;94(7):1003-1009.
17. Ramsey SD, Zeliadt SB, Blough DK, et al. Impact of prostate cancer on sexual relationships: a longitudinal perspective on intimate partners’ experiences. J Sex Med. 2013;10(12):3135-3143.
18. Wittmann D, Carolan M, Given B, et al. Exploring the role of the partner in couples’ sexual recovery after surgery for prostate cancer. Support Care Cancer. 2014;22(9):2509-2515.
19. Schmitz KH, Courneya KS, Matthews C, et al. American college of sports medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409-1426.
20. Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243-274.
21. Elliott S, Latini DM, Walker LM, Wassersug R, Robinson JW; ADT Suvivorship Working Group. Androgen deprivation therapy for prostate cancer: recommendations to improve patient and partner quality of life. J Sex Med. 2010;7(9):2996-3010.
22. Wassersug RJ, Walker LM, Robinson JW. Androgen deprivation therapy: an essential guide for prostate cancer patients and their loved ones. New York, NY: Demos Health; 2014.
23. Wibowo E, Walker LM, Wilyman S, et al. Androgen deprivation therapy educational program: a Canadian True NTH initiative. J Clin Oncol. 2016;34(suppl 3):243.
24. Stanford JL, Feng Z, Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer. JAMA. 2000;283(3):354-360.
25. Overgård M, Angelsen A, Lydersen S, Mørkved S. Does physiotherapist-guided pelvic floor muscle training reduce urinary incontinence after radical prostatectomy? A randomised controlled trial. Eur Urol. 2008;54(2):438-448.
26. Godtman RA, Holmberg E, Khatami A, Pihl CG, Stranne J, Hugosson J. Long-term results of active surveillance in the Göteborg randomized, population-based prostate cancer screening trial. Eur Urol. 2016;70(5):760-766.
27. Cohen H, Britten N. Who decides about prostate cancer treatment? A qualitative study. Fam Pract. 2003;20(6):724-729.
28. Denberg TD, Melhado TV, Steiner JF. Patient treatment preferences in localized prostate carcinoma: the influence of emotion, misconception, and anecdote. Cancer. 2006;107(3):620-630.
29. Morris BB, Farnan L, Song L, et al. Treatment decisional regret among men with prostate cancer: racial differences and influential factors in the North Carolina health access and prostate cancer treatment project (HCaP-NC). Cancer. 2015;121(12):2029-2035.
30. Feldman-Stewart D, Capirci C, Brennenstuhl S, et al. Information for decision making by patients with early-stage prostate cancer: a comparison across 9 countries. Med Decis Making. 2011;31(5):754-766.
1. Cancer Research UK. Prostate cancer statistics. http://www.cancer.ca/en/cancer-information/cancer-type/prostate/statistics/?region=sk. Published 2015. Accessed June 22, 2017.
2. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220-241.
3. Canadian Cancer Society. Canadian cancer statistics special topic: predictions of the future burden of cancer in Canada. Ottawa, Canada: Public Health Agency of Canada; 2015.
4. Roth AJ, Weinberger MI, Nelson CJ. Prostate cancer: psychosocial implications and management. Future Oncol. 2008;4(4):561-568.
5. Couper J, Bloch S, Love A, Macvean M, Duchesne GM, Kissane D. Psychosocial adjustment of female partners of men with prostate cancer: a review of the literature. Psychooncology 2006;15(11):937-953.
6. Wilt TJ, MacDonald R, Rutks I, Shamliyan TA, Taylor BC, Kane RL. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med. 2008;148(6):435-448.
7. Galvão DA, Spry NA, Taaffe DR, et al. Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. BJU Int. 2008;102(1):44-47.
8. Watts S, Leydon G, Birch B, et al. Depression and anxiety in prostate cancer: a systematic review and meta-analysis of prevalence rates. BMJ Open. 2014;4(3):e003901.
9. Zaider T, Manne S, Nelson C, Mulhall J, Kissane D. Loss of masculine identity, marital affection, and sexual bother in men with localized prostate cancer. J Sex Med. 2012;9(10):2724-2732.
10. Ream E, Quennell A, Fincham L, et al. Supportive care needs of men living with prostate cancer in England: a survey. Br J Cancer. 2008;98(12):1903-1909.
11. Howell D, Hack TF, Oliver TK, et al. Models of care for post-treatment follow-up of adult cancer survivors: a systematic review and quality appraisal of the evidence. J Cancer Surviv. 2012;6(4):359-371.
12. Halpern MT, Viswanathan M, Evans TS, Birken SA, Basch E, Mayer DK. Models of cancer survivorship care: overview and summary of current evidence. J Oncol Pract. 2015;11(1):e19-e27.
13. Smith DP, Supramaniam R, King MT, Ward J, Berry M, Armstrong BK. Age, health, and education determine supportive care needs of men younger than 70 years with prostate cancer. J Clin Oncol. 2007;25(18):2560-2566.
14. Northouse LL, Mood DW, Montie JE, et al. Living with prostate cancer: patients’ and spouses’ psychosocial status and quality of life. J Clin Oncol. 2007;25(27):4171-4177.
15. Hedden L, Wassersug R, Mahovlich S, et al. Evaluating an educational intervention to alleviate distress amongst men with newly diagnosed prostate cancer and their partners. BJU Int. 2017;120(5B):E21-E29.
16. Bradley EB, Bissonette EA, Theodorescu D. Determinants of long-term quality of life and voiding function of patients treated with radical prostatectomy or permanent brachytherapy for prostate cancer. BJU Int. 2004;94(7):1003-1009.
17. Ramsey SD, Zeliadt SB, Blough DK, et al. Impact of prostate cancer on sexual relationships: a longitudinal perspective on intimate partners’ experiences. J Sex Med. 2013;10(12):3135-3143.
18. Wittmann D, Carolan M, Given B, et al. Exploring the role of the partner in couples’ sexual recovery after surgery for prostate cancer. Support Care Cancer. 2014;22(9):2509-2515.
19. Schmitz KH, Courneya KS, Matthews C, et al. American college of sports medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409-1426.
20. Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243-274.
21. Elliott S, Latini DM, Walker LM, Wassersug R, Robinson JW; ADT Suvivorship Working Group. Androgen deprivation therapy for prostate cancer: recommendations to improve patient and partner quality of life. J Sex Med. 2010;7(9):2996-3010.
22. Wassersug RJ, Walker LM, Robinson JW. Androgen deprivation therapy: an essential guide for prostate cancer patients and their loved ones. New York, NY: Demos Health; 2014.
23. Wibowo E, Walker LM, Wilyman S, et al. Androgen deprivation therapy educational program: a Canadian True NTH initiative. J Clin Oncol. 2016;34(suppl 3):243.
24. Stanford JL, Feng Z, Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer. JAMA. 2000;283(3):354-360.
25. Overgård M, Angelsen A, Lydersen S, Mørkved S. Does physiotherapist-guided pelvic floor muscle training reduce urinary incontinence after radical prostatectomy? A randomised controlled trial. Eur Urol. 2008;54(2):438-448.
26. Godtman RA, Holmberg E, Khatami A, Pihl CG, Stranne J, Hugosson J. Long-term results of active surveillance in the Göteborg randomized, population-based prostate cancer screening trial. Eur Urol. 2016;70(5):760-766.
27. Cohen H, Britten N. Who decides about prostate cancer treatment? A qualitative study. Fam Pract. 2003;20(6):724-729.
28. Denberg TD, Melhado TV, Steiner JF. Patient treatment preferences in localized prostate carcinoma: the influence of emotion, misconception, and anecdote. Cancer. 2006;107(3):620-630.
29. Morris BB, Farnan L, Song L, et al. Treatment decisional regret among men with prostate cancer: racial differences and influential factors in the North Carolina health access and prostate cancer treatment project (HCaP-NC). Cancer. 2015;121(12):2029-2035.
30. Feldman-Stewart D, Capirci C, Brennenstuhl S, et al. Information for decision making by patients with early-stage prostate cancer: a comparison across 9 countries. Med Decis Making. 2011;31(5):754-766.
Integrating survivorship care planning in radiation oncology workflow
In January 2016 there were an estimated 15.5 million people in the United States who were living with a cancer diagnosis, representing 4.8% of the population. That number is expected to increase to 20.3 million by 2026.1 The 5-year relative survival rate for all cancers diagnosed during 2005 to 2011 was 69%.2 As more individuals with a cancer diagnosis now live longer, cancer survivorship is receiving increased attention. A report from the Institute of Medicine3 identified the essential components of survivorship care, including the provision of a survivorship care plan (SCP) containing specific diagnosis, treatment, and follow-up information (Table 1). To maintain accreditation in their respective organizations, the American College of Surgeons’ Commission on Cancer and the National Accreditation Program for Breast Centers (NAPBC) have included standards on providing treatment summaries and SCPs in person to those patients who have completed cancer treatments given with curative intent.4,5
SCPs are personalized documents presented to cancer patients at the end of treatment that summarize key aspects of cancer treatment and recommend appropriate ongoing medical care and self-management. The purpose of the SCP is both to educate cancer survivors and to create a portable document that can be shared with primary care providers to facilitate coordinated care.6 There are multiple barriers to SCP implementation, which may include the time required to create an SCP, inadequate reimbursement for the time spent creating and delivering the plan, a lack of risk-stratified guidelines for coordinated care, and the incomplete automation of diagnosis and treatment summarization by the electronic health record (EHR).7
Survivorship care in radiation oncology
The American College of Radiology includes the recommendation for regular, ongoing follow-up in the standards for accreditation for radiation oncology practice.8 Radiation oncology practices often provide the initial follow-up appointment about a month after the prescribed radiation treatment has been completed. The twofold purpose of this appointment is to assess the response to treatment and to evaluate acute treatment-related effects.9 The appointment may include a skin evaluation, assessment for any acute treatment effects, informal counseling on maintaining a healthy lifestyle, and recommendations for posttreatment care and follow-up. The appointment may also be an opportune time for delivering the SCP because radiation therapy is often the final treatment modality in active therapy for breast cancer patients.
A review of the literature yields scant data on the incorporation of SCPs into a radiation oncology practice. A 2014 survey of members of the American Society of Radiation Oncology10for a response percentage of 14.7%. Almost all providers follow their patients after treatment (97% (n = 574 respondents/3987 total membership, 14.4% response rate) showed that although most radiation oncologists provide long-term follow-up care to their patients after treatment completion (97%), fewer than half of those surveyed indicated that they delivered SCPs for curative-intent patients (40%), and even fewer delivered for palliative-intent patients (19%). Standards for the American Society for Radiation Oncology’s Accreditation Program for Excellence11 outline content for end-of-treatment documentation. Typically, the documentation includes a detailed treatment summary prepared by the treating radiation oncologist. This treatment summary includes the patient’s diagnosis, the area treated, radiation doses received, number of fractions delivered, therapy start date, therapy completion date, and overall tolerance of treatment in a clinical summary. The treatment summary is communicated to other providers involved in the patient’s care to promote care coordination, but it is not typically provided to patients.
Development of University of Wisconsin survivorship care planning
As an important component of maintaining NAPBC accreditation, the University of Wisconsin (UW) Health Breast Center began the process of formalizing and optimizing SCPs for breast cancer survivors who are followed at the center. Multidisciplinary input from surgical, medical, and radiation oncology was obtained. Representatives from those disciplines met regularly to reach consensus on the treatment summary and SCP content. The following 3 documents were created for use during a transition visit at the end of treatment: the written individualized SCP to be provided to the survivor and his/her primary care providers, a general survivorship patient education booklet, and a patient questionnaire to identify survivors’ concerns and additional resources that may be beneficial.
Treatment summary
Working in collaboration with IT specialists, we enabled out-of-the-box functionality within our EHR. This cancer-specific functionality provides a central and standard location within each survivor’s problem list to systematically document information regarding cancer diagnosis, stage, and treatment associated with a specific cancer diagnosis. Each treating provider (surgeon, medical oncologist, radiation oncologist, genetic counselor, etc) is responsible for entering and updating the relevant components within the treatment summary (ie, the surgeon enters and maintains the surgical details, the medical oncologist does likewise for chemotherapy and other medical therapies, etc). Information is updated and current, creating a dynamic documentation of diagnosis and treatment that can be used in clinic notes, patient after-visit summaries, and SCPs.
Survivorship care plan
This same EHR functionality is leveraged to generate, populate, and maintain the individualized SCP for each breast cancer survivor. The Treatment Summary section of the SCP can be quickly prepared within the EHR by autopopulating data previously entered by treating providers. Content and language for SCP templates in breast, colorectal, prostate, and gynecologic cancers are in use at the time of publication. The templates are developed as a collaborative effort between oncology subspecialists, with input from the UW Health survivor and family advocacy councils.
Each template contains a Treatment Summary section and an SCP section. The Treatment Summary section includes survivor general information, diagnosis and treatment information, and the clinical and supportive/survivor care team names and contact information. The SCP section includes follow-up recommendations, signs of recurrence and/or symptoms to report, healthy lifestyle and maintenance, chronic or late effects of specific treatment if applicable (eg, surgery, chemotherapy by drug, radiation therapy, and endocrine therapy), and general resources for common psychosocial concerns (Table 1).12,13
Each SCP is visible to the entire health care team, including other specialists and primary care, as long as they have access to UW Health’s EHR.14 The result is a readily accessible, comprehensive document that is individualized for each survivor, residing in a standard location with standardized format and content to facilitate review and use.15
General survivorship patient education booklet
Many cancer survivors request additional information about their posttreatment concerns. The “UW Health Facts for You: Cancer Survivorship, Carbone Cancer Center” booklet was developed by a multidisciplinary team including oncologists, advanced practice providers (APPs), navigators, social workers, program leadership, cancer survivors, and caregivers. The guide includes detailed information for the cancer survivor on topics including nutrition, exercise, sleep, tobacco cessation, sexual health, and spirituality. Common concerns and symptom management are addressed as well as a comprehensive list of community resources. The booklet can be found at http://www.uwhealth.org/healthfacts/cancer/7834.
Survivorship questionnaire
Breast cancer survivors often have multiple concerns as they transition from active treatment to the survivorship phase of their cancer journey. Specific concerns may vary slightly form one survivor to another. Guided by recommendations for the American Society of Clinical Oncology and the National Comprehensive Cancer Network, we developed a 10-question, 2-page questionnaire to identify those concerns with input from members of the Breast Cancer Steering Committee. Members of the committee include surgical, medical, and radiation oncologists, AAPs, radiologists, pathologists, program leadership, and nurses, along with breast cancer survivors. Elements in the questionnaire include nutrition, activity, mood, sleep, sexual health, employment/insurance, pain/swelling, desires regarding pregnancy or prevention, memory/concentration, smoking, alcohol, genetic testing/counseling, and assistance with establishing care with a primary care provider. By completing the questionnaire, breast cancer survivors identify specific concerns within each category and are able to request additional information about those concerns and/or a referral to appropriate resources. They may also select the I need nothing further option if the concern is present but already being addressed.
SCP delivery and the transition visit
The next task in implementation of the care process for survivors encompassed the development of clinical workflows and processes to provide the document to the breast cancer survivor at the completion of treatment. In a study of breast cancer survivors, it was found that the preferred format for survivorship care planning is generally an in-person consultation at completion of treatment with an oncology professional.16 The best time for distribution of the written SCP is, however, unclear. Intuitively, it seems optimal to distribute SCPs around the time of completion of active treatment. However, for SCP delivery to be feasible and sustainable, delivery must be integrated into existing clinical care-delivery processes, and content must be streamlined and focused to meet the needs of their intended recipients without becoming overly burdensome to prepare and deliver.17
Ultimately, and after significant multidisciplinary discussion, it was determined that Stage 0-III breast cancer patients would have a visit focusing on symptoms and transitioning to surveillance follow-up (Transition Visit) as they completed active curative-intent cancer treatment. During this Transition Visit, the SCP document would be provided and reviewed with survivors. The Transition Visit for breast cancer survivors would be conducted by an APP following the completion of their final stage of active, primary treatment (surgery, chemotherapy, and/or radiation therapy). Additional long-term adjuvant therapy for breast cancer survivors (ie, trastuzumab, endocrine therapy) would continue as indicated during and after delivery of the SCP.
The radiation oncology clinic was chosen as a venue for these Transition Visits for breast cancer survivors whose treatment included radiotherapy. Despite little historical experience with delivery of SCPs in radiation oncology clinics, this was a logical choice given that radiotherapy is usually the final phase of active treatment for these breast cancer survivors, and a follow-up visit about a month after completing radiotherapy is already part of standard practice. Collaborating with the multidisciplinary UW Health Breast Center, we therefore integrated the formal breast survivorship care planning process and provision of the SCP into the current radiation oncology workflow. About 40% of the roughly 600 breast cancer patients treated by surgical and/or medical oncology at our institution annually also receive radiation therapy at our site. For the remaining 60% of breast cancer survivors who do not receive radiation therapy or who completed radiotherapy at an outside facility, the SCP is provided by an APP within the UW Health Breast Center.
UW radiation oncology survivorship transition visit
The overall workflow of our Transition Visit is depicted in the Figure. Toward the end of the breast cancer survivor’s radiation treatments, the radiation oncologist instructs the schedulers to arrange the 1-month, post-radiation Transition Visit with the APP and informs the survivor about the nature of the appointment. The Transition Visit is scheduled as a 60-minute appointment. Before the survivor’s arrival, an APP generates the written SCP. The activity includes completing the Treatment Summary, or verifying the accuracy of a prepopulated Treatment Summary, and individualizing the SCP section for the patient based on treatment received and follow-up recommendations using drop-down functionality. As the SCP is printed for review with the survivor, it is simultaneously sent to the survivor’s primary care provider. This is accomplished by using EHR functionality to route the document internally to UW primary care providers or automatically faxing the document to external primary care providers. Each SCP is also marked as complete within the EHR for the purposes of documenting compliance with this activity for later data analysis.
On arrival for the appointment, each breast cancer survivor completes the survivorship questionnaire. During the Transition Visit, the questionnaire is reviewed with the survivor and additional information is provided. Referral options are discussed if indicated with desired referrals made by the APP. The survivor is interviewed and examined for any persistent side effects of treatment. Next, the Treatment Summary and SCP are reviewed with the survivor, emphasizing the follow-up plan, signs or symptoms of breast cancer recurrence, and chronic or late treatment-related toxicities. Ample opportunity is provided for the survivor to ask questions and voice concerns.
Follow-up appointments with members of the patient’s care team (ie, medical, surgical, or radiation oncology) as well as necessary breast imaging (ie, mammogram, MRI) are coordinated and scheduled before the survivor leaves the department. A survey of oncologists (medical, surgical, radiation) identified specific cancer-related components of survivorship care that oncologists felt most responsible for as well as opportunities to improve the quality and efficiency of care provided by oncologists.18 At our institution, the breast surgical, medical, and radiation oncologists all generally participate in follow-up care through at least 1 year following completion of active, primary treatment.
Outcomes, quality improvement opportunities, and continued challenges with the process
There is presently a lack of long-term outcome data about the impact of SCPs. As mandates for the provision of SCPs are made, research focusing on whether SCPs result in improved health behaviors and outcomes, reduced burden in care transitions from the oncology setting, and increased cost-effectiveness will be needed.19 The long-term effects of SCPs on psychological, oncologic, and resource outcomes should be evaluated,20 as well as the impact on health behaviors, such as smoking cessation or participation in rehabilitation programs.21
Following the implementation of our Transition Visits in 2015, we conducted a quality improvement review. This review included summation of 69 recent breast cancer questionnaires from Transition Visits with our APPs (Table 2 and Table 3). The most common concerns raised by our breast cancer survivors include desire for weight loss, improving diet, and increasing physical activity. Of note, concerns did not often translate into a desire for more information or referrals.22 Survivors were generally satisfied with the timing of the Transition Visits and generally indicated that the visits were helpful, with self-reported improvements in their understanding of planned follow-up. A Canadian group evaluating breast and head and neck cancer survivors has suggested that SCPs could produce long-term improvements in healthy lifestyle behaviors; however, further research is needed to determine the extent to which SCPs might improve follow-up care over the long term.23
Finally, although efforts to date have been focused on the breast cancer survivor at the completion of treatment, long-term survivors may also benefit from receiving the SCP. A study by the American Cancer Society found that long-term cancer survivors had unmet informational needs, particularly with regard to screening, long-term cancer and treatment effects, and healthy lifestyle behaviors.24 Identifying and subsequently delivering an SCP to eligible long-term survivors is a challenging prospect, which depends on further refinement of EHR-based tracking of the date of diagnosis, cancer stage, and end-of-treatment date.
Summary and recommendations
Survivorship care has been efficiently integrated into our 1-month post-radiation follow-up appointment for breast cancer survivors. By using current resources in the radiation oncology department, the process has provided an effective way to deliver the SCP to breast cancer survivors. Future plans include implementing the process for all patients receiving curative-intent radiation for additional solid tumor survivors. Quality improvement projects will be developed to assess survivor satisfaction and the impact on health behaviors.
Acknowledgments
The authors thank Amy Heath, MS, RTT, for editorial and manuscript preparation assistance.
1. Statistics. National Cancer Institute, Division of Cancer Control & Population Sciences website. http:///cancercontrol.cancer.gov/ocs/statistics/statistics.html. Updated October 17, 2016. Accessed March 6, 2018.
2. Cancer facts & figures 2016. American Cancer Society website. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2016.html. Published 2016. Accessed February 27, 2018.
3. Hewitt M, Greenfield S, Stovall E, eds. From cancer patient to cancer survivor: lost in transition. Washington, DC: National Academies Press; 2006.
4. Knutson A, McNamara E. Cancer program standards: ensuring patient-centered care. American College of Surgeons website. https://www.facs.org/quality-programs/cancer/coc/standards. Published August 2016. Accessed March 6, 2018.
5. National Accreditation Program for Breast Centers. NAPBC standards manual. American College of Surgeons website. https://www.facs.org/~/media/files/quality%20programs/napbc/2014%20napbc%20standards%20manual.ashx. Published 2014. Accessed March 6, 2018.
6. Salz T, McCabe MS, Onstad EE, et al. Survivorship care plans: is there buy-in from community oncology providers? Cancer. 2014;120(5):722-730.
7. Mayer DK, Nekhlyudov L, Snyder CF, Merrill JK, Wollins DS, Shulman LN. American Society of Clinical Oncology clinical expert statement on cancer survivorship care planning. J Oncol Pract. 2014;10(6):345-351.
8. Dobelbower RR, Cotter G, Schilling PJ, Parsai EI, Carroll JM. Radiation oncology practice accreditation. Rays. 2001;26(3):191-198.
9. Hartford AC, Conway PD, Desai NB, et al. ACR-ASTRO practice parameter for communication: radiation oncology. The American College of Radiology website. http://www.acr.org/-/media/ACR/Files/Practice-Parameters/RadOnc.pdf. Updated 2014. Accessed March 6, 2018.
10. Koontz BF, Benda R, De Los Santos J, et al. US radiation oncology practice patterns for posttreatment survivor care. Pract Radiat Oncol. 2016;6(1):50-56.
11. American Society of Therapeutic Radiation Oncologists. APEx program standards. ASTRO website. http://www.astro.org/uploadedFiles/_MAIN_SITE/Daily_Practice/Accreditation/Content_Pieces/ProgramStandards.pdf. Published February 1, 2016. Accessed March 6, 2018.
12. Clinical practice survivorship guidelines and adaptations. American Society of Clinical Oncology website. http://www.asco.org/practice-guidelines/cancer-care-initiatives/prevention-survivorship. Published 2013. Accessed March 6, 2018.
13. National Comprehensive Cancer Network. Supportive care guidelines. NCNN website. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#supportive. Updated February 16, 2018. Accessed March 6, 2018.
14. Donohue S, Sesto ME, Hahn DL, et al. Evaluating primary care providers’ views on survivorship care plans generated by an electronic health record system. J Oncol Pract. 2015;11(3):e329-e335.
15. Tevaarwerk AJ, Wisinski KB, Buhr KA, et al. Leveraging electronic health record systems to create and provide electronic cancer survivorship care plans: a pilot study. J Oncol Pract. 2014;10(3):e150-e159.
16. Smith SL, Singh-Carlson S, Downie L, Payeur N, Wai ES. Survivors of breast cancer: patient perspectives on survivorship care planning. J Cancer Surviv. 2011;5(4):337-344.
17. Stricker CT, O’Brien M. Implementing the commission on cancer standards for survivorship care plans. Clin J Oncol Nurs. 2014;18(suppl 1):15-22.
18. Neuman HB, Steffens NM, Jacobson N, et al. Oncologists’ perspectives of their roles and responsibilities during multi-disciplinary breast cancer follow-up. Ann Surg Oncol. 2016;23(3):708-714.
19. Palmer SC, Stricker CT, Panzer SL, et al. Outcomes and satisfaction after delivery of a breast cancer survivorship care plan: Results of a multicenter trial. J Oncol Pract. 2015;11(2):e222-e229.
20. Brennan ME, Gormally JF, Butow P, Boyle FM, Spillane AJ. Survivorship care plans in cancer: a systematic review of care plan outcomes. Br J Cancer. 2014;111(10):1899-1908.
21. Chen RC, Hoffman KE, Sher DJ, et al. Development of a standard survivorship care plan template for radiation oncologists. Pract Radiat Oncol. 2016;6(1):57-65.
22. Seaborne LA, Huenerberg KJ, Bohler A, et al. Developing electronic health record based program to deliver survivorship care plans and visits at the UW breast center. Poster presented at American Society of Clinical Oncology Survivorship Symposium; January 15-16, 2016; San Francisco CA.
23. Collie K, McCormick J, Waller A, et al. Qualitative evaluation of care plans for Canadian breast and head-and-neck cancer survivors. Curr Oncol. 2014;21(1):18-28.
24. Playdon M, Ferrucci LM, McCorkle R, et al. Health information needs and preferences in relation to survivorship care plans of long-term cancer survivors in the American Cancer Society’s study of cancer survivors-I. J Cancer Surviv. 2016;10(4):674-685.
In January 2016 there were an estimated 15.5 million people in the United States who were living with a cancer diagnosis, representing 4.8% of the population. That number is expected to increase to 20.3 million by 2026.1 The 5-year relative survival rate for all cancers diagnosed during 2005 to 2011 was 69%.2 As more individuals with a cancer diagnosis now live longer, cancer survivorship is receiving increased attention. A report from the Institute of Medicine3 identified the essential components of survivorship care, including the provision of a survivorship care plan (SCP) containing specific diagnosis, treatment, and follow-up information (Table 1). To maintain accreditation in their respective organizations, the American College of Surgeons’ Commission on Cancer and the National Accreditation Program for Breast Centers (NAPBC) have included standards on providing treatment summaries and SCPs in person to those patients who have completed cancer treatments given with curative intent.4,5
SCPs are personalized documents presented to cancer patients at the end of treatment that summarize key aspects of cancer treatment and recommend appropriate ongoing medical care and self-management. The purpose of the SCP is both to educate cancer survivors and to create a portable document that can be shared with primary care providers to facilitate coordinated care.6 There are multiple barriers to SCP implementation, which may include the time required to create an SCP, inadequate reimbursement for the time spent creating and delivering the plan, a lack of risk-stratified guidelines for coordinated care, and the incomplete automation of diagnosis and treatment summarization by the electronic health record (EHR).7
Survivorship care in radiation oncology
The American College of Radiology includes the recommendation for regular, ongoing follow-up in the standards for accreditation for radiation oncology practice.8 Radiation oncology practices often provide the initial follow-up appointment about a month after the prescribed radiation treatment has been completed. The twofold purpose of this appointment is to assess the response to treatment and to evaluate acute treatment-related effects.9 The appointment may include a skin evaluation, assessment for any acute treatment effects, informal counseling on maintaining a healthy lifestyle, and recommendations for posttreatment care and follow-up. The appointment may also be an opportune time for delivering the SCP because radiation therapy is often the final treatment modality in active therapy for breast cancer patients.
A review of the literature yields scant data on the incorporation of SCPs into a radiation oncology practice. A 2014 survey of members of the American Society of Radiation Oncology10for a response percentage of 14.7%. Almost all providers follow their patients after treatment (97% (n = 574 respondents/3987 total membership, 14.4% response rate) showed that although most radiation oncologists provide long-term follow-up care to their patients after treatment completion (97%), fewer than half of those surveyed indicated that they delivered SCPs for curative-intent patients (40%), and even fewer delivered for palliative-intent patients (19%). Standards for the American Society for Radiation Oncology’s Accreditation Program for Excellence11 outline content for end-of-treatment documentation. Typically, the documentation includes a detailed treatment summary prepared by the treating radiation oncologist. This treatment summary includes the patient’s diagnosis, the area treated, radiation doses received, number of fractions delivered, therapy start date, therapy completion date, and overall tolerance of treatment in a clinical summary. The treatment summary is communicated to other providers involved in the patient’s care to promote care coordination, but it is not typically provided to patients.
Development of University of Wisconsin survivorship care planning
As an important component of maintaining NAPBC accreditation, the University of Wisconsin (UW) Health Breast Center began the process of formalizing and optimizing SCPs for breast cancer survivors who are followed at the center. Multidisciplinary input from surgical, medical, and radiation oncology was obtained. Representatives from those disciplines met regularly to reach consensus on the treatment summary and SCP content. The following 3 documents were created for use during a transition visit at the end of treatment: the written individualized SCP to be provided to the survivor and his/her primary care providers, a general survivorship patient education booklet, and a patient questionnaire to identify survivors’ concerns and additional resources that may be beneficial.
Treatment summary
Working in collaboration with IT specialists, we enabled out-of-the-box functionality within our EHR. This cancer-specific functionality provides a central and standard location within each survivor’s problem list to systematically document information regarding cancer diagnosis, stage, and treatment associated with a specific cancer diagnosis. Each treating provider (surgeon, medical oncologist, radiation oncologist, genetic counselor, etc) is responsible for entering and updating the relevant components within the treatment summary (ie, the surgeon enters and maintains the surgical details, the medical oncologist does likewise for chemotherapy and other medical therapies, etc). Information is updated and current, creating a dynamic documentation of diagnosis and treatment that can be used in clinic notes, patient after-visit summaries, and SCPs.
Survivorship care plan
This same EHR functionality is leveraged to generate, populate, and maintain the individualized SCP for each breast cancer survivor. The Treatment Summary section of the SCP can be quickly prepared within the EHR by autopopulating data previously entered by treating providers. Content and language for SCP templates in breast, colorectal, prostate, and gynecologic cancers are in use at the time of publication. The templates are developed as a collaborative effort between oncology subspecialists, with input from the UW Health survivor and family advocacy councils.
Each template contains a Treatment Summary section and an SCP section. The Treatment Summary section includes survivor general information, diagnosis and treatment information, and the clinical and supportive/survivor care team names and contact information. The SCP section includes follow-up recommendations, signs of recurrence and/or symptoms to report, healthy lifestyle and maintenance, chronic or late effects of specific treatment if applicable (eg, surgery, chemotherapy by drug, radiation therapy, and endocrine therapy), and general resources for common psychosocial concerns (Table 1).12,13
Each SCP is visible to the entire health care team, including other specialists and primary care, as long as they have access to UW Health’s EHR.14 The result is a readily accessible, comprehensive document that is individualized for each survivor, residing in a standard location with standardized format and content to facilitate review and use.15
General survivorship patient education booklet
Many cancer survivors request additional information about their posttreatment concerns. The “UW Health Facts for You: Cancer Survivorship, Carbone Cancer Center” booklet was developed by a multidisciplinary team including oncologists, advanced practice providers (APPs), navigators, social workers, program leadership, cancer survivors, and caregivers. The guide includes detailed information for the cancer survivor on topics including nutrition, exercise, sleep, tobacco cessation, sexual health, and spirituality. Common concerns and symptom management are addressed as well as a comprehensive list of community resources. The booklet can be found at http://www.uwhealth.org/healthfacts/cancer/7834.
Survivorship questionnaire
Breast cancer survivors often have multiple concerns as they transition from active treatment to the survivorship phase of their cancer journey. Specific concerns may vary slightly form one survivor to another. Guided by recommendations for the American Society of Clinical Oncology and the National Comprehensive Cancer Network, we developed a 10-question, 2-page questionnaire to identify those concerns with input from members of the Breast Cancer Steering Committee. Members of the committee include surgical, medical, and radiation oncologists, AAPs, radiologists, pathologists, program leadership, and nurses, along with breast cancer survivors. Elements in the questionnaire include nutrition, activity, mood, sleep, sexual health, employment/insurance, pain/swelling, desires regarding pregnancy or prevention, memory/concentration, smoking, alcohol, genetic testing/counseling, and assistance with establishing care with a primary care provider. By completing the questionnaire, breast cancer survivors identify specific concerns within each category and are able to request additional information about those concerns and/or a referral to appropriate resources. They may also select the I need nothing further option if the concern is present but already being addressed.
SCP delivery and the transition visit
The next task in implementation of the care process for survivors encompassed the development of clinical workflows and processes to provide the document to the breast cancer survivor at the completion of treatment. In a study of breast cancer survivors, it was found that the preferred format for survivorship care planning is generally an in-person consultation at completion of treatment with an oncology professional.16 The best time for distribution of the written SCP is, however, unclear. Intuitively, it seems optimal to distribute SCPs around the time of completion of active treatment. However, for SCP delivery to be feasible and sustainable, delivery must be integrated into existing clinical care-delivery processes, and content must be streamlined and focused to meet the needs of their intended recipients without becoming overly burdensome to prepare and deliver.17
Ultimately, and after significant multidisciplinary discussion, it was determined that Stage 0-III breast cancer patients would have a visit focusing on symptoms and transitioning to surveillance follow-up (Transition Visit) as they completed active curative-intent cancer treatment. During this Transition Visit, the SCP document would be provided and reviewed with survivors. The Transition Visit for breast cancer survivors would be conducted by an APP following the completion of their final stage of active, primary treatment (surgery, chemotherapy, and/or radiation therapy). Additional long-term adjuvant therapy for breast cancer survivors (ie, trastuzumab, endocrine therapy) would continue as indicated during and after delivery of the SCP.
The radiation oncology clinic was chosen as a venue for these Transition Visits for breast cancer survivors whose treatment included radiotherapy. Despite little historical experience with delivery of SCPs in radiation oncology clinics, this was a logical choice given that radiotherapy is usually the final phase of active treatment for these breast cancer survivors, and a follow-up visit about a month after completing radiotherapy is already part of standard practice. Collaborating with the multidisciplinary UW Health Breast Center, we therefore integrated the formal breast survivorship care planning process and provision of the SCP into the current radiation oncology workflow. About 40% of the roughly 600 breast cancer patients treated by surgical and/or medical oncology at our institution annually also receive radiation therapy at our site. For the remaining 60% of breast cancer survivors who do not receive radiation therapy or who completed radiotherapy at an outside facility, the SCP is provided by an APP within the UW Health Breast Center.
UW radiation oncology survivorship transition visit
The overall workflow of our Transition Visit is depicted in the Figure. Toward the end of the breast cancer survivor’s radiation treatments, the radiation oncologist instructs the schedulers to arrange the 1-month, post-radiation Transition Visit with the APP and informs the survivor about the nature of the appointment. The Transition Visit is scheduled as a 60-minute appointment. Before the survivor’s arrival, an APP generates the written SCP. The activity includes completing the Treatment Summary, or verifying the accuracy of a prepopulated Treatment Summary, and individualizing the SCP section for the patient based on treatment received and follow-up recommendations using drop-down functionality. As the SCP is printed for review with the survivor, it is simultaneously sent to the survivor’s primary care provider. This is accomplished by using EHR functionality to route the document internally to UW primary care providers or automatically faxing the document to external primary care providers. Each SCP is also marked as complete within the EHR for the purposes of documenting compliance with this activity for later data analysis.
On arrival for the appointment, each breast cancer survivor completes the survivorship questionnaire. During the Transition Visit, the questionnaire is reviewed with the survivor and additional information is provided. Referral options are discussed if indicated with desired referrals made by the APP. The survivor is interviewed and examined for any persistent side effects of treatment. Next, the Treatment Summary and SCP are reviewed with the survivor, emphasizing the follow-up plan, signs or symptoms of breast cancer recurrence, and chronic or late treatment-related toxicities. Ample opportunity is provided for the survivor to ask questions and voice concerns.
Follow-up appointments with members of the patient’s care team (ie, medical, surgical, or radiation oncology) as well as necessary breast imaging (ie, mammogram, MRI) are coordinated and scheduled before the survivor leaves the department. A survey of oncologists (medical, surgical, radiation) identified specific cancer-related components of survivorship care that oncologists felt most responsible for as well as opportunities to improve the quality and efficiency of care provided by oncologists.18 At our institution, the breast surgical, medical, and radiation oncologists all generally participate in follow-up care through at least 1 year following completion of active, primary treatment.
Outcomes, quality improvement opportunities, and continued challenges with the process
There is presently a lack of long-term outcome data about the impact of SCPs. As mandates for the provision of SCPs are made, research focusing on whether SCPs result in improved health behaviors and outcomes, reduced burden in care transitions from the oncology setting, and increased cost-effectiveness will be needed.19 The long-term effects of SCPs on psychological, oncologic, and resource outcomes should be evaluated,20 as well as the impact on health behaviors, such as smoking cessation or participation in rehabilitation programs.21
Following the implementation of our Transition Visits in 2015, we conducted a quality improvement review. This review included summation of 69 recent breast cancer questionnaires from Transition Visits with our APPs (Table 2 and Table 3). The most common concerns raised by our breast cancer survivors include desire for weight loss, improving diet, and increasing physical activity. Of note, concerns did not often translate into a desire for more information or referrals.22 Survivors were generally satisfied with the timing of the Transition Visits and generally indicated that the visits were helpful, with self-reported improvements in their understanding of planned follow-up. A Canadian group evaluating breast and head and neck cancer survivors has suggested that SCPs could produce long-term improvements in healthy lifestyle behaviors; however, further research is needed to determine the extent to which SCPs might improve follow-up care over the long term.23
Finally, although efforts to date have been focused on the breast cancer survivor at the completion of treatment, long-term survivors may also benefit from receiving the SCP. A study by the American Cancer Society found that long-term cancer survivors had unmet informational needs, particularly with regard to screening, long-term cancer and treatment effects, and healthy lifestyle behaviors.24 Identifying and subsequently delivering an SCP to eligible long-term survivors is a challenging prospect, which depends on further refinement of EHR-based tracking of the date of diagnosis, cancer stage, and end-of-treatment date.
Summary and recommendations
Survivorship care has been efficiently integrated into our 1-month post-radiation follow-up appointment for breast cancer survivors. By using current resources in the radiation oncology department, the process has provided an effective way to deliver the SCP to breast cancer survivors. Future plans include implementing the process for all patients receiving curative-intent radiation for additional solid tumor survivors. Quality improvement projects will be developed to assess survivor satisfaction and the impact on health behaviors.
Acknowledgments
The authors thank Amy Heath, MS, RTT, for editorial and manuscript preparation assistance.
In January 2016 there were an estimated 15.5 million people in the United States who were living with a cancer diagnosis, representing 4.8% of the population. That number is expected to increase to 20.3 million by 2026.1 The 5-year relative survival rate for all cancers diagnosed during 2005 to 2011 was 69%.2 As more individuals with a cancer diagnosis now live longer, cancer survivorship is receiving increased attention. A report from the Institute of Medicine3 identified the essential components of survivorship care, including the provision of a survivorship care plan (SCP) containing specific diagnosis, treatment, and follow-up information (Table 1). To maintain accreditation in their respective organizations, the American College of Surgeons’ Commission on Cancer and the National Accreditation Program for Breast Centers (NAPBC) have included standards on providing treatment summaries and SCPs in person to those patients who have completed cancer treatments given with curative intent.4,5
SCPs are personalized documents presented to cancer patients at the end of treatment that summarize key aspects of cancer treatment and recommend appropriate ongoing medical care and self-management. The purpose of the SCP is both to educate cancer survivors and to create a portable document that can be shared with primary care providers to facilitate coordinated care.6 There are multiple barriers to SCP implementation, which may include the time required to create an SCP, inadequate reimbursement for the time spent creating and delivering the plan, a lack of risk-stratified guidelines for coordinated care, and the incomplete automation of diagnosis and treatment summarization by the electronic health record (EHR).7
Survivorship care in radiation oncology
The American College of Radiology includes the recommendation for regular, ongoing follow-up in the standards for accreditation for radiation oncology practice.8 Radiation oncology practices often provide the initial follow-up appointment about a month after the prescribed radiation treatment has been completed. The twofold purpose of this appointment is to assess the response to treatment and to evaluate acute treatment-related effects.9 The appointment may include a skin evaluation, assessment for any acute treatment effects, informal counseling on maintaining a healthy lifestyle, and recommendations for posttreatment care and follow-up. The appointment may also be an opportune time for delivering the SCP because radiation therapy is often the final treatment modality in active therapy for breast cancer patients.
A review of the literature yields scant data on the incorporation of SCPs into a radiation oncology practice. A 2014 survey of members of the American Society of Radiation Oncology10for a response percentage of 14.7%. Almost all providers follow their patients after treatment (97% (n = 574 respondents/3987 total membership, 14.4% response rate) showed that although most radiation oncologists provide long-term follow-up care to their patients after treatment completion (97%), fewer than half of those surveyed indicated that they delivered SCPs for curative-intent patients (40%), and even fewer delivered for palliative-intent patients (19%). Standards for the American Society for Radiation Oncology’s Accreditation Program for Excellence11 outline content for end-of-treatment documentation. Typically, the documentation includes a detailed treatment summary prepared by the treating radiation oncologist. This treatment summary includes the patient’s diagnosis, the area treated, radiation doses received, number of fractions delivered, therapy start date, therapy completion date, and overall tolerance of treatment in a clinical summary. The treatment summary is communicated to other providers involved in the patient’s care to promote care coordination, but it is not typically provided to patients.
Development of University of Wisconsin survivorship care planning
As an important component of maintaining NAPBC accreditation, the University of Wisconsin (UW) Health Breast Center began the process of formalizing and optimizing SCPs for breast cancer survivors who are followed at the center. Multidisciplinary input from surgical, medical, and radiation oncology was obtained. Representatives from those disciplines met regularly to reach consensus on the treatment summary and SCP content. The following 3 documents were created for use during a transition visit at the end of treatment: the written individualized SCP to be provided to the survivor and his/her primary care providers, a general survivorship patient education booklet, and a patient questionnaire to identify survivors’ concerns and additional resources that may be beneficial.
Treatment summary
Working in collaboration with IT specialists, we enabled out-of-the-box functionality within our EHR. This cancer-specific functionality provides a central and standard location within each survivor’s problem list to systematically document information regarding cancer diagnosis, stage, and treatment associated with a specific cancer diagnosis. Each treating provider (surgeon, medical oncologist, radiation oncologist, genetic counselor, etc) is responsible for entering and updating the relevant components within the treatment summary (ie, the surgeon enters and maintains the surgical details, the medical oncologist does likewise for chemotherapy and other medical therapies, etc). Information is updated and current, creating a dynamic documentation of diagnosis and treatment that can be used in clinic notes, patient after-visit summaries, and SCPs.
Survivorship care plan
This same EHR functionality is leveraged to generate, populate, and maintain the individualized SCP for each breast cancer survivor. The Treatment Summary section of the SCP can be quickly prepared within the EHR by autopopulating data previously entered by treating providers. Content and language for SCP templates in breast, colorectal, prostate, and gynecologic cancers are in use at the time of publication. The templates are developed as a collaborative effort between oncology subspecialists, with input from the UW Health survivor and family advocacy councils.
Each template contains a Treatment Summary section and an SCP section. The Treatment Summary section includes survivor general information, diagnosis and treatment information, and the clinical and supportive/survivor care team names and contact information. The SCP section includes follow-up recommendations, signs of recurrence and/or symptoms to report, healthy lifestyle and maintenance, chronic or late effects of specific treatment if applicable (eg, surgery, chemotherapy by drug, radiation therapy, and endocrine therapy), and general resources for common psychosocial concerns (Table 1).12,13
Each SCP is visible to the entire health care team, including other specialists and primary care, as long as they have access to UW Health’s EHR.14 The result is a readily accessible, comprehensive document that is individualized for each survivor, residing in a standard location with standardized format and content to facilitate review and use.15
General survivorship patient education booklet
Many cancer survivors request additional information about their posttreatment concerns. The “UW Health Facts for You: Cancer Survivorship, Carbone Cancer Center” booklet was developed by a multidisciplinary team including oncologists, advanced practice providers (APPs), navigators, social workers, program leadership, cancer survivors, and caregivers. The guide includes detailed information for the cancer survivor on topics including nutrition, exercise, sleep, tobacco cessation, sexual health, and spirituality. Common concerns and symptom management are addressed as well as a comprehensive list of community resources. The booklet can be found at http://www.uwhealth.org/healthfacts/cancer/7834.
Survivorship questionnaire
Breast cancer survivors often have multiple concerns as they transition from active treatment to the survivorship phase of their cancer journey. Specific concerns may vary slightly form one survivor to another. Guided by recommendations for the American Society of Clinical Oncology and the National Comprehensive Cancer Network, we developed a 10-question, 2-page questionnaire to identify those concerns with input from members of the Breast Cancer Steering Committee. Members of the committee include surgical, medical, and radiation oncologists, AAPs, radiologists, pathologists, program leadership, and nurses, along with breast cancer survivors. Elements in the questionnaire include nutrition, activity, mood, sleep, sexual health, employment/insurance, pain/swelling, desires regarding pregnancy or prevention, memory/concentration, smoking, alcohol, genetic testing/counseling, and assistance with establishing care with a primary care provider. By completing the questionnaire, breast cancer survivors identify specific concerns within each category and are able to request additional information about those concerns and/or a referral to appropriate resources. They may also select the I need nothing further option if the concern is present but already being addressed.
SCP delivery and the transition visit
The next task in implementation of the care process for survivors encompassed the development of clinical workflows and processes to provide the document to the breast cancer survivor at the completion of treatment. In a study of breast cancer survivors, it was found that the preferred format for survivorship care planning is generally an in-person consultation at completion of treatment with an oncology professional.16 The best time for distribution of the written SCP is, however, unclear. Intuitively, it seems optimal to distribute SCPs around the time of completion of active treatment. However, for SCP delivery to be feasible and sustainable, delivery must be integrated into existing clinical care-delivery processes, and content must be streamlined and focused to meet the needs of their intended recipients without becoming overly burdensome to prepare and deliver.17
Ultimately, and after significant multidisciplinary discussion, it was determined that Stage 0-III breast cancer patients would have a visit focusing on symptoms and transitioning to surveillance follow-up (Transition Visit) as they completed active curative-intent cancer treatment. During this Transition Visit, the SCP document would be provided and reviewed with survivors. The Transition Visit for breast cancer survivors would be conducted by an APP following the completion of their final stage of active, primary treatment (surgery, chemotherapy, and/or radiation therapy). Additional long-term adjuvant therapy for breast cancer survivors (ie, trastuzumab, endocrine therapy) would continue as indicated during and after delivery of the SCP.
The radiation oncology clinic was chosen as a venue for these Transition Visits for breast cancer survivors whose treatment included radiotherapy. Despite little historical experience with delivery of SCPs in radiation oncology clinics, this was a logical choice given that radiotherapy is usually the final phase of active treatment for these breast cancer survivors, and a follow-up visit about a month after completing radiotherapy is already part of standard practice. Collaborating with the multidisciplinary UW Health Breast Center, we therefore integrated the formal breast survivorship care planning process and provision of the SCP into the current radiation oncology workflow. About 40% of the roughly 600 breast cancer patients treated by surgical and/or medical oncology at our institution annually also receive radiation therapy at our site. For the remaining 60% of breast cancer survivors who do not receive radiation therapy or who completed radiotherapy at an outside facility, the SCP is provided by an APP within the UW Health Breast Center.
UW radiation oncology survivorship transition visit
The overall workflow of our Transition Visit is depicted in the Figure. Toward the end of the breast cancer survivor’s radiation treatments, the radiation oncologist instructs the schedulers to arrange the 1-month, post-radiation Transition Visit with the APP and informs the survivor about the nature of the appointment. The Transition Visit is scheduled as a 60-minute appointment. Before the survivor’s arrival, an APP generates the written SCP. The activity includes completing the Treatment Summary, or verifying the accuracy of a prepopulated Treatment Summary, and individualizing the SCP section for the patient based on treatment received and follow-up recommendations using drop-down functionality. As the SCP is printed for review with the survivor, it is simultaneously sent to the survivor’s primary care provider. This is accomplished by using EHR functionality to route the document internally to UW primary care providers or automatically faxing the document to external primary care providers. Each SCP is also marked as complete within the EHR for the purposes of documenting compliance with this activity for later data analysis.
On arrival for the appointment, each breast cancer survivor completes the survivorship questionnaire. During the Transition Visit, the questionnaire is reviewed with the survivor and additional information is provided. Referral options are discussed if indicated with desired referrals made by the APP. The survivor is interviewed and examined for any persistent side effects of treatment. Next, the Treatment Summary and SCP are reviewed with the survivor, emphasizing the follow-up plan, signs or symptoms of breast cancer recurrence, and chronic or late treatment-related toxicities. Ample opportunity is provided for the survivor to ask questions and voice concerns.
Follow-up appointments with members of the patient’s care team (ie, medical, surgical, or radiation oncology) as well as necessary breast imaging (ie, mammogram, MRI) are coordinated and scheduled before the survivor leaves the department. A survey of oncologists (medical, surgical, radiation) identified specific cancer-related components of survivorship care that oncologists felt most responsible for as well as opportunities to improve the quality and efficiency of care provided by oncologists.18 At our institution, the breast surgical, medical, and radiation oncologists all generally participate in follow-up care through at least 1 year following completion of active, primary treatment.
Outcomes, quality improvement opportunities, and continued challenges with the process
There is presently a lack of long-term outcome data about the impact of SCPs. As mandates for the provision of SCPs are made, research focusing on whether SCPs result in improved health behaviors and outcomes, reduced burden in care transitions from the oncology setting, and increased cost-effectiveness will be needed.19 The long-term effects of SCPs on psychological, oncologic, and resource outcomes should be evaluated,20 as well as the impact on health behaviors, such as smoking cessation or participation in rehabilitation programs.21
Following the implementation of our Transition Visits in 2015, we conducted a quality improvement review. This review included summation of 69 recent breast cancer questionnaires from Transition Visits with our APPs (Table 2 and Table 3). The most common concerns raised by our breast cancer survivors include desire for weight loss, improving diet, and increasing physical activity. Of note, concerns did not often translate into a desire for more information or referrals.22 Survivors were generally satisfied with the timing of the Transition Visits and generally indicated that the visits were helpful, with self-reported improvements in their understanding of planned follow-up. A Canadian group evaluating breast and head and neck cancer survivors has suggested that SCPs could produce long-term improvements in healthy lifestyle behaviors; however, further research is needed to determine the extent to which SCPs might improve follow-up care over the long term.23
Finally, although efforts to date have been focused on the breast cancer survivor at the completion of treatment, long-term survivors may also benefit from receiving the SCP. A study by the American Cancer Society found that long-term cancer survivors had unmet informational needs, particularly with regard to screening, long-term cancer and treatment effects, and healthy lifestyle behaviors.24 Identifying and subsequently delivering an SCP to eligible long-term survivors is a challenging prospect, which depends on further refinement of EHR-based tracking of the date of diagnosis, cancer stage, and end-of-treatment date.
Summary and recommendations
Survivorship care has been efficiently integrated into our 1-month post-radiation follow-up appointment for breast cancer survivors. By using current resources in the radiation oncology department, the process has provided an effective way to deliver the SCP to breast cancer survivors. Future plans include implementing the process for all patients receiving curative-intent radiation for additional solid tumor survivors. Quality improvement projects will be developed to assess survivor satisfaction and the impact on health behaviors.
Acknowledgments
The authors thank Amy Heath, MS, RTT, for editorial and manuscript preparation assistance.
1. Statistics. National Cancer Institute, Division of Cancer Control & Population Sciences website. http:///cancercontrol.cancer.gov/ocs/statistics/statistics.html. Updated October 17, 2016. Accessed March 6, 2018.
2. Cancer facts & figures 2016. American Cancer Society website. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2016.html. Published 2016. Accessed February 27, 2018.
3. Hewitt M, Greenfield S, Stovall E, eds. From cancer patient to cancer survivor: lost in transition. Washington, DC: National Academies Press; 2006.
4. Knutson A, McNamara E. Cancer program standards: ensuring patient-centered care. American College of Surgeons website. https://www.facs.org/quality-programs/cancer/coc/standards. Published August 2016. Accessed March 6, 2018.
5. National Accreditation Program for Breast Centers. NAPBC standards manual. American College of Surgeons website. https://www.facs.org/~/media/files/quality%20programs/napbc/2014%20napbc%20standards%20manual.ashx. Published 2014. Accessed March 6, 2018.
6. Salz T, McCabe MS, Onstad EE, et al. Survivorship care plans: is there buy-in from community oncology providers? Cancer. 2014;120(5):722-730.
7. Mayer DK, Nekhlyudov L, Snyder CF, Merrill JK, Wollins DS, Shulman LN. American Society of Clinical Oncology clinical expert statement on cancer survivorship care planning. J Oncol Pract. 2014;10(6):345-351.
8. Dobelbower RR, Cotter G, Schilling PJ, Parsai EI, Carroll JM. Radiation oncology practice accreditation. Rays. 2001;26(3):191-198.
9. Hartford AC, Conway PD, Desai NB, et al. ACR-ASTRO practice parameter for communication: radiation oncology. The American College of Radiology website. http://www.acr.org/-/media/ACR/Files/Practice-Parameters/RadOnc.pdf. Updated 2014. Accessed March 6, 2018.
10. Koontz BF, Benda R, De Los Santos J, et al. US radiation oncology practice patterns for posttreatment survivor care. Pract Radiat Oncol. 2016;6(1):50-56.
11. American Society of Therapeutic Radiation Oncologists. APEx program standards. ASTRO website. http://www.astro.org/uploadedFiles/_MAIN_SITE/Daily_Practice/Accreditation/Content_Pieces/ProgramStandards.pdf. Published February 1, 2016. Accessed March 6, 2018.
12. Clinical practice survivorship guidelines and adaptations. American Society of Clinical Oncology website. http://www.asco.org/practice-guidelines/cancer-care-initiatives/prevention-survivorship. Published 2013. Accessed March 6, 2018.
13. National Comprehensive Cancer Network. Supportive care guidelines. NCNN website. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#supportive. Updated February 16, 2018. Accessed March 6, 2018.
14. Donohue S, Sesto ME, Hahn DL, et al. Evaluating primary care providers’ views on survivorship care plans generated by an electronic health record system. J Oncol Pract. 2015;11(3):e329-e335.
15. Tevaarwerk AJ, Wisinski KB, Buhr KA, et al. Leveraging electronic health record systems to create and provide electronic cancer survivorship care plans: a pilot study. J Oncol Pract. 2014;10(3):e150-e159.
16. Smith SL, Singh-Carlson S, Downie L, Payeur N, Wai ES. Survivors of breast cancer: patient perspectives on survivorship care planning. J Cancer Surviv. 2011;5(4):337-344.
17. Stricker CT, O’Brien M. Implementing the commission on cancer standards for survivorship care plans. Clin J Oncol Nurs. 2014;18(suppl 1):15-22.
18. Neuman HB, Steffens NM, Jacobson N, et al. Oncologists’ perspectives of their roles and responsibilities during multi-disciplinary breast cancer follow-up. Ann Surg Oncol. 2016;23(3):708-714.
19. Palmer SC, Stricker CT, Panzer SL, et al. Outcomes and satisfaction after delivery of a breast cancer survivorship care plan: Results of a multicenter trial. J Oncol Pract. 2015;11(2):e222-e229.
20. Brennan ME, Gormally JF, Butow P, Boyle FM, Spillane AJ. Survivorship care plans in cancer: a systematic review of care plan outcomes. Br J Cancer. 2014;111(10):1899-1908.
21. Chen RC, Hoffman KE, Sher DJ, et al. Development of a standard survivorship care plan template for radiation oncologists. Pract Radiat Oncol. 2016;6(1):57-65.
22. Seaborne LA, Huenerberg KJ, Bohler A, et al. Developing electronic health record based program to deliver survivorship care plans and visits at the UW breast center. Poster presented at American Society of Clinical Oncology Survivorship Symposium; January 15-16, 2016; San Francisco CA.
23. Collie K, McCormick J, Waller A, et al. Qualitative evaluation of care plans for Canadian breast and head-and-neck cancer survivors. Curr Oncol. 2014;21(1):18-28.
24. Playdon M, Ferrucci LM, McCorkle R, et al. Health information needs and preferences in relation to survivorship care plans of long-term cancer survivors in the American Cancer Society’s study of cancer survivors-I. J Cancer Surviv. 2016;10(4):674-685.
1. Statistics. National Cancer Institute, Division of Cancer Control & Population Sciences website. http:///cancercontrol.cancer.gov/ocs/statistics/statistics.html. Updated October 17, 2016. Accessed March 6, 2018.
2. Cancer facts & figures 2016. American Cancer Society website. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2016.html. Published 2016. Accessed February 27, 2018.
3. Hewitt M, Greenfield S, Stovall E, eds. From cancer patient to cancer survivor: lost in transition. Washington, DC: National Academies Press; 2006.
4. Knutson A, McNamara E. Cancer program standards: ensuring patient-centered care. American College of Surgeons website. https://www.facs.org/quality-programs/cancer/coc/standards. Published August 2016. Accessed March 6, 2018.
5. National Accreditation Program for Breast Centers. NAPBC standards manual. American College of Surgeons website. https://www.facs.org/~/media/files/quality%20programs/napbc/2014%20napbc%20standards%20manual.ashx. Published 2014. Accessed March 6, 2018.
6. Salz T, McCabe MS, Onstad EE, et al. Survivorship care plans: is there buy-in from community oncology providers? Cancer. 2014;120(5):722-730.
7. Mayer DK, Nekhlyudov L, Snyder CF, Merrill JK, Wollins DS, Shulman LN. American Society of Clinical Oncology clinical expert statement on cancer survivorship care planning. J Oncol Pract. 2014;10(6):345-351.
8. Dobelbower RR, Cotter G, Schilling PJ, Parsai EI, Carroll JM. Radiation oncology practice accreditation. Rays. 2001;26(3):191-198.
9. Hartford AC, Conway PD, Desai NB, et al. ACR-ASTRO practice parameter for communication: radiation oncology. The American College of Radiology website. http://www.acr.org/-/media/ACR/Files/Practice-Parameters/RadOnc.pdf. Updated 2014. Accessed March 6, 2018.
10. Koontz BF, Benda R, De Los Santos J, et al. US radiation oncology practice patterns for posttreatment survivor care. Pract Radiat Oncol. 2016;6(1):50-56.
11. American Society of Therapeutic Radiation Oncologists. APEx program standards. ASTRO website. http://www.astro.org/uploadedFiles/_MAIN_SITE/Daily_Practice/Accreditation/Content_Pieces/ProgramStandards.pdf. Published February 1, 2016. Accessed March 6, 2018.
12. Clinical practice survivorship guidelines and adaptations. American Society of Clinical Oncology website. http://www.asco.org/practice-guidelines/cancer-care-initiatives/prevention-survivorship. Published 2013. Accessed March 6, 2018.
13. National Comprehensive Cancer Network. Supportive care guidelines. NCNN website. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#supportive. Updated February 16, 2018. Accessed March 6, 2018.
14. Donohue S, Sesto ME, Hahn DL, et al. Evaluating primary care providers’ views on survivorship care plans generated by an electronic health record system. J Oncol Pract. 2015;11(3):e329-e335.
15. Tevaarwerk AJ, Wisinski KB, Buhr KA, et al. Leveraging electronic health record systems to create and provide electronic cancer survivorship care plans: a pilot study. J Oncol Pract. 2014;10(3):e150-e159.
16. Smith SL, Singh-Carlson S, Downie L, Payeur N, Wai ES. Survivors of breast cancer: patient perspectives on survivorship care planning. J Cancer Surviv. 2011;5(4):337-344.
17. Stricker CT, O’Brien M. Implementing the commission on cancer standards for survivorship care plans. Clin J Oncol Nurs. 2014;18(suppl 1):15-22.
18. Neuman HB, Steffens NM, Jacobson N, et al. Oncologists’ perspectives of their roles and responsibilities during multi-disciplinary breast cancer follow-up. Ann Surg Oncol. 2016;23(3):708-714.
19. Palmer SC, Stricker CT, Panzer SL, et al. Outcomes and satisfaction after delivery of a breast cancer survivorship care plan: Results of a multicenter trial. J Oncol Pract. 2015;11(2):e222-e229.
20. Brennan ME, Gormally JF, Butow P, Boyle FM, Spillane AJ. Survivorship care plans in cancer: a systematic review of care plan outcomes. Br J Cancer. 2014;111(10):1899-1908.
21. Chen RC, Hoffman KE, Sher DJ, et al. Development of a standard survivorship care plan template for radiation oncologists. Pract Radiat Oncol. 2016;6(1):57-65.
22. Seaborne LA, Huenerberg KJ, Bohler A, et al. Developing electronic health record based program to deliver survivorship care plans and visits at the UW breast center. Poster presented at American Society of Clinical Oncology Survivorship Symposium; January 15-16, 2016; San Francisco CA.
23. Collie K, McCormick J, Waller A, et al. Qualitative evaluation of care plans for Canadian breast and head-and-neck cancer survivors. Curr Oncol. 2014;21(1):18-28.
24. Playdon M, Ferrucci LM, McCorkle R, et al. Health information needs and preferences in relation to survivorship care plans of long-term cancer survivors in the American Cancer Society’s study of cancer survivors-I. J Cancer Surviv. 2016;10(4):674-685.
Myelodysplastic syndromes: etiologies, evaluation, and therapy
In this interview, Dr David Henry, MD, the Editor-in-Chief of JCSO, and David Steensma, MD, of the Dana-Farber Cancer Institute in Boston, talk about myelodysplasic syndromes, from diagnosis, evaluation, and etiologies, to therapy options and molecular sequencing.
Listen to the podcast below, or click on the PDF icon at the top of this introduction to read a transcript of the interview.
In this interview, Dr David Henry, MD, the Editor-in-Chief of JCSO, and David Steensma, MD, of the Dana-Farber Cancer Institute in Boston, talk about myelodysplasic syndromes, from diagnosis, evaluation, and etiologies, to therapy options and molecular sequencing.
Listen to the podcast below, or click on the PDF icon at the top of this introduction to read a transcript of the interview.
In this interview, Dr David Henry, MD, the Editor-in-Chief of JCSO, and David Steensma, MD, of the Dana-Farber Cancer Institute in Boston, talk about myelodysplasic syndromes, from diagnosis, evaluation, and etiologies, to therapy options and molecular sequencing.
Listen to the podcast below, or click on the PDF icon at the top of this introduction to read a transcript of the interview.
From angst to analytics: lessons learned from an oncology care model internal pilot
In March 2016, 13 practices affiliated with the US Oncology Network (USON) were invited to participate in the Oncology Care Model (OCM) proposed by the Center for Medicare and Medicaid Services (CMS) and Center for Medicare and Medicaid Innovation (CMMI). The OCM, a novel value-based care model, was designed to provide higher-quality and better-coordinated oncology care at a lower cost to CMS.1 Of the 13 practices, 12 agreed to participate with a start date for the program of July 1, 2016. At least 40% of the practices’ patients were insured by Medicare, and any eligible patients with active cancer were offered an opportunity to enter the program. USON practices treat more than 25,000 patients with a qualifying episode per year and the overall OCM program sees more than 150,000 beneficiaries per year,2 so we anticipated that the OCM would have a substantial impact on each of the 12 practices on USON.
Faced with the scenario of having only 3 months between notification of approval and launch of the OCM, it was imperative that all the practices be proactive in planning and preparing to launch the OCM. With this goal in mind, representatives from all OCM candidate practices convened to anticipate the needs of the OCM and chart out a program to meet those needs. In this article, we discuss the requirements and scope of the OCM, the development of an internal pilot project, the anticipated gains from the pilot, and the results and findings from the pilot, both expected and unexpected.
The road to the Oncology Care Model
The government and oncology practices have been on separate trajectories to the OCM. In the last 15 years, the major intersections of these trajectories had to do with price and not patient outcomes. In 2003, the Medicare Prescription Drug Improvement and Modernization Act (MMA) focused on drug price reductions from an average wholesale price–based schedule to an average sales price–based schedule.3 There was the sequester in 2013,4 and more recently a proposal to restructure the payment for Part B drugs. In the background, recurrent negotiations to fix the calculation for the sustainable growth rate allowed for periodic draconian cuts to the prices of services. The cumulative effect of these price reductions has been to put economic pressure on community oncologists such that many have moved to a hospital environment.5
This contentious relationship with community oncology began to change with the passage of the Affordable Care Act (ACA) in 2010.6 Section 3022 of the ACA established the Medicare Shared Savings Program (MSSP) with the charge to create a new type of health care entity that was responsible for achieving the triple aim of improving population health, improving individual patient care, and bending the cost curve.7 Additional programs, such as the Pioneer Accountable Care Organization (ACO) program and the Comprehensive Primary Care Initiative were established to test alternative payment models.8-10
The ACA also funded the CMMI with a mandate to “test innovative payment and service delivery models” to achieve the triple aim; US$10 billion were appropriated for the years 2011-2019 for this purpose. The CMMI funded a pilot project for cancer care, the COME HOME [Community Oncology MEdical HOME] initiative, to test whether some aspects of care could be transformed or augmented to reduce overall costs or at least reduce the rate of increase. Findings from COME HOME have helped inform the OCM program.11
Over the same period, practices belonging to the USON were paving a path toward value. An electronic health record (EHR) for the entire network was adopted in 2005. A pathways program in which chemotherapy regimens were assessed on cost as well as benefits and toxicity, was started in 2006. Higher-cost regimens with no additional benefits comparable with other evidence-based regimens were deselected for initial treatment choices at the time of initial decision support. This process was streamlined using web-based technology that improved pathways compliance and tracking of off-pathways exceptions.12 Retrospective studies indicated that pathways had the potential to bend the cost curve by reducing drug spending.13,14 USON and its practices also tested a nurse call system (Innovent Oncology) funded by a monthly management fee. This program guided patients through chemotherapy with regular telephonic symptom assessment and discussion of patient-centered values and advance care planning. Results of these programs indicated relative reductions in both drug and hospital expenses.15
Additional experience has come from participation in the United Healthcare Episodes of Care (EOC) initiative, which eliminated the chemotherapy drug incentives, compensating physicians on a per-episode basis instead. This study showed a significant reduction in the total cost of cancer therapy after modifying the fee-for-service system and incorporating feedback data and financial incentives to reward improved outcomes and cost efficiency.16
The Oncology Care Model represents a convergence of purchaser demand and provider readiness. The purchaser holds providers accountable for cost and quality. The data on outcomes and costs will provide an extensive database that can be analyzed by the participating practices to address variations and reduce unnecessary care and preventable costs. Best practices are rewarded.17
The OCM and practice readiness
As a part of the CMS proposal process, practices were required to submit implementation plans by June 30, 2015. The purpose of the implementation plan was to define how a practice could transform over 6 broad domains: 24/7 coverage; EHR certification; navigation and care coordination; continuous quality improvement; incorporation of the Institute of Medicine’s (IOM’s) Care Management Plan; and adherence to nationally recognized guidelines. The periods of patient eligibility for the program were 6-month treatment episodes triggered by a cancer diagnosis, a provider encounter claim, and a Part B or D drug claim specifically identified as a cancer treatment. The episodes could be repeated if the 3 criteria continued to be met. All charges continued to be billed as fee-for-service as before, but in addition, participating practices could bill a monthly enhanced oncology services (MEOS) payment for the duration of an episode. Reducing the total cost of care while meeting performance metrics thresholds would also qualify a practice for performance-based payments.
Of the primary components, EHR certification and adherence to guidelines had been addressed previously, but the other domains represented significant challenges. Although 24/7 physician coverage with access to an EHR is standard for all practices, most practice sites do not have an insight into the frequency of hospital admissions, the ability to efficiently add sick patients to the daily schedule, or a routine call system to assess chemotherapy toxicity.
The OCM proposes 10 potential navigation/care coordination functions (Table 1) and does not consider those functions to be the role of one person, but rather a team responsibility. Most of our practices perform at least some of these functions, but they are not formally designated, coordinated, or recorded. A similar condition exists for the IOM care plan, which includes recommendations for treatment and adverse event management (Table 2). The prognosis, toxicity, quality-of-life, and goals-of-care requirements are often found in the physician notes, but not systematically documented or searchable.
Similar challenges have been observed for continuous quality improvement programs. Although the data are available, they are often not easy to search and, therefore, are difficult to retrieve and report. The OCM, as with any transformational program, must always weigh the benefit of information with the burden of consumption of physician and staff time to collect and input these data.18
Prepilot project work
In October 2015, lead physicians and managers from the 12 participating practices were brought together with analytic, technical, process management, and business experts from USON and McKesson Specialty Health. The objective of the meeting was to define the areas of greatest need for day 1 of the OCM and to be prepared. The challenges were to identify the changes needed to meet the requirements of the OCM while improving the patient experience, sustaining the viability of the community oncology practices, creating teams to deliver more effective care, and using data to bend the cost curve. Accordingly, 4 work streams were created: Care and Support; Content; Technology; and Communications, Revenue Cycle and Incentives.
Care and Support
The key tasks of the Care and Support team were to define the workflows for navigation and the IOM care plan. As a patient’s journey through the clinic was mapped out, it became clear that although multiple personnel could participate in the navigation and care plans, there was no systematic way to organize and record the components of successful navigation. The goals for the pilot were to test various options for navigation and to identify best practices that could be translated into standard operating procedures.
Content
The Content team was charged with identifying available programs that would fit into the OCM requirements. These included advance care planning, survivorship, chemotherapy teaching, risk assessment, pathways, and symptom assessment. A longer-term goal was the development of care paths, a more comprehensive map of the patient’s journey that would include consultations, coordinated care, imaging, labs, and other services.
Technology
The task for the Technology work stream was to identify processes of care that required documentation and to evaluate current and future technology solutions to improve efficiencies. The electronic medical record satisfied for the input of data with relevant clinical details, demographics, disease types, and staging. A web-based pathways tool supported clinical decision-making, as well as compliance to pathways. The Medicare quality metric programs set the stage for development of capture and reporting tools for data from many sources. The pilot would indicate the adequacy of these tools and the need for expansion or development of new functions or programs. Of particular importance was recording the IOM care plan and navigation functions in a searchable format. As care paths are developed, risk prediction, palliative care, and other services need to be encompassed. Finally, technology will support the identification and enrollment of eligible patients, and billing activities.
Communications, Revenue Cycle, and Incentives
The final work stream was Communications, Revenue Cycle, and Incentives. For the pilot, the focus was on revenue cycle. A new category of patient needed to be identified, enrolled, and billed to CMS for services. At the outset, the technology did not address the identification of patients receiving only oral drugs. The office visit, the diagnosis, and the drug claim all had to be aligned for enrollment and billing. It was critical to understand the workload by patient and total volume to estimate the technology and personnel needs to meet the initial number of new OCM patients. Communication refers to both internal and external parties. Education of the entire practice staff regarding transformation will be critical for success.
Of the 12 participating practices, 3 practice sites were selected for the pilot program. Each had fewer than 10 medical oncologists and at least 1 radiation oncologist. Each site had a physician champion and an administrative lead. All of the sites were part of a larger regional oncology practice. A fourth site had independently started a pilot and that experience was shared with the larger group as well. The sites were distributed across the country in 4 different time zones.
The pilot experience
The pilot experience yielded important findings, some expected and some unexpected. The challenges of navigation, the treatment plan, and team building were anticipated. We were surprised at the sheer number of potential candidates and the difficulty in finding eligible candidates. Not to be overlooked was a need for continued and possibly increased emphasis on adherence to pathways and process changes to reduce hospitalizations and emergency department (ED) visits.
Navigation
At the outset, none of the pilot practices had formal navigation processes as outlined in Table 1. Many of the processes, such as coordinating appointments and facilitating follow-up services and financial support, were provided by the practice, but were not identified or coordinated as navigation. The practices, as a first step, defined who was responsible for those services and identified 1 person who would be responsible for their completion. It was agreed that navigation was a process shared by a team and not an individual responsibility, yet the person who would monitor the completion of the tasks was not identified. It soon became apparent that true navigation included more tasks than initially outlined.
Additional tasks included appropriate patient education regarding treatment toxicities, follow-up after chemotherapy or a hospitalization, and coordination of other aspects of the IOM care plan, such as survivorship and advance care planning. Each of the practices recruited staff internally to assume the navigator role, and standard operating procedures were developed for completing and documenting this expanded responsibility. True navigation, however, depends on building the team character while still having 1 or 2 members of the team identified as being responsible for following and documenting the patient’s journey through an episode. To meet those needs, navigators developed ad hoc methods, such as spreadsheets, to track patients. The technology team developed drop-down check lists within the EHR, but the burden of documentation continued. Lastly, an ongoing challenge is how best to designate responsibility and assess how many additional staffers are needed.
IOM Care Management Plan
Before initiation of the pilot project, no practice was providing patients with a comprehensive, written treatment plan. Considerably more than half of the members of the work-stream teams believed that would be difficult to implement. However, the members of the Care and Support work stream made some fundamental assumptions to make the care plan workable: first, all aspects of the plan did not occur at the same time, and were not completed by the same person; second, and critically, items 2-9 of Table 2 could be completed at one time during the early conversations between the physician and patient about the goals of treatment. Diagnosis, prognosis, treatment intent, response rate, quality of life, and toxicities were included in the treatment plan, and the remaining IOM care plan could be discussed, or at least identified, as issues for further discussion with other team members. These components were incorporated into a 1-page document that was either typed into the record and printed out or handwritten and copied for the patient. This became the treatment plan and was ready for use at the start of the pilot.
The physician response to the treatment plan was mixed. One site adopted it enthusiastically and quickly moved to use the plan for all patients. Other sites had variable uptake. One hurdle was defining response rate to therapy and prognosis. Data were provided but they often did not match the conditions of individual patients. Some physicians were uncomfortable with the process. Documentation was difficult because the plans had to be scanned into the EHR. Patients generally responded favorably to the plans and would bring them to teaching or chemotherapy sessions.
As with navigation, the treatment plan challenges pointed the technology team toward the development and implementation of an electronic version of the plan. The pilot allowed members of the technology team to visit the clinics, to evaluate workflows and make assumptions on how to structure a treatment plan electronically.
Team-based care
None of the pilot sites had a formalized structure for team-based care. Team huddles were developed and weekly and daily huddles were encouraged. The weekly huddles took about 15-30 minutes, during which patients scheduled for the coming week were reviewed. All personnel who saw patients were invited – benefit counselors, advanced practice providers, schedulers, lab technicians, medical assistants, office and infusion nurses, social workers, pharmacists, physicians, and lead administrative staff. The daily huddle was smaller and generally included a nurse, a medical assistant, and a physician, at a minimum, to review the patients in the hospital, those to be seen in the clinic that day, and any follow-up information based on scheduled contact following recent treatments or events. In some sites, these huddles were uniformly endorsed, in others, not at all. Although many physicians felt that the functions were being handled informally and the additional time commitment would not improve the process, once they began to attend the meetings, they appreciated the value of the huddles and continued attending them. As the complexity of delivery and documentation becomes more apparent, these will prove indispensable to coordinated care.
24/7 access
Hospitalization is one of the chief drivers of the total cost of care, so the pilot sites were concerned that more needed to be done to reduce unnecessary hospitalizations. One site surveyed the patients coming into the clinic about their previous ED visits. Many of the visits had been for noncancer-related events and the clinic was not aware of many of the many of the visits. These findings prompted a number of changes. Open slots were created daily for patients who needed to be worked in for any areas of concern. The on-call physician, triage nurse, or navigation lead could fill these slots. All patients discharged from the hospital were called within 48 hours after discharge and scheduled for a clinic visit within 1 week. Night and weekend call logs were scrutinized each morning and patients’ calls were returned for any issues related to symptom or toxicity management. At one site, patients were given wallet cards with the clinic number, the treatment regimen, and, on the reverse, all symptoms that would justify calling the clinic. The patients were encouraged to call the clinic earlier rather than later in the day. On the back end, the clinics were to have processes in place so that patient calls would be answered quickly to facilitate same-day evaluations in the clinic.
Enrollment and revenue cycle
The most intractable problem was the identification and enrollment of OCM patients. As already noted, 3 components were necessary for enrollment: a drug charge for Part B or D Medicare, a provider visit, and an approved cancer diagnosis. To identify those patients, the claims system would churn out a weekly list of all eligible patients. However, the claims system had no mechanism to pick up Part D claims for oral medications. This meant that any patient with a provider visit and an appropriate diagnosis was potentially eligible for enrollment. At one site, the list of potential patients was 2-3 times the number of actual candidates. It took 6 weeks of manual chart review to resolving the list. Collectively, the 12 practices could have as many as 20,000 patients eligible for the July 1 enrollment. The pilot allowed the practices to get an early start on recruitment of business office staff and plans to address the backlog of potentially eligible patients. The process of identifying eligible patients for the OCM still needs a better solution because finding the appropriate patients is a critical first step in this model.
Underlying all of these initiatives is communication, both internal and external. We have to select and celebrate best practices. We have to educate our staffs. We will have to demonstrate that we are giving better care to our patients by using patient and provider testimonials and data.
From angst to analytics
The challenges of practice transformation can be daunting. It will be difficult to formalize processes and document data in ways that were untested before the pilot program was set up. However, the pilot accomplished 2 things: it identified additional areas that needed improvement and it demonstrated that the most challenging aspects of the OCM were feasible. Navigation and the IOM care plan were broken down into parts; each component was separately addressed, and programs were put in place to make the pieces manageable and part of an overall movement toward team-based care. The addition of a technology platform has been a key factor for the success of the value-based care initiative. Additional technology support has been enlisted to facilitate the processes, and an electronic version of the treatment plan is being tested. More difficult will be efforts to address the cultural resistance to change, which we hope to do by using data and outcomes from the CMS claims data files. The OCM represents an unprecedented opportunity for measurement of the quality of care we deliver.
We are now well und
Acknowledgment
The authors thank Supriya Srinivasan, PhD, for editorial support.
1. Oncology Care Model. Centers for Medicare and Medicaid Services 2016. https://innovation.cms.gov/initiatives/oncology-care/. Last updated November 14, 2017. Accessed November 16 2016.
2. Mortimer L, Strawbridge L, Lukens E, et al. CMS’ Oncology Care Model: delivering higher value cancer care. Clin Pharmacol Ther. 2017.
3. Medicare Prescription Drug, Improvement and Modernization Act of 2003, Pub Law No. 108-173; 2003.
4. Mathews D. The sequester: absolutely everything you could possibly need to know, in one FAQ. https://www.washingtonpost.com/news/wonk/wp/2013/02/20/the-sequester-absolutely-everything-you-could-possibly-need-to-know-in-one-faq/?utm_term=.a0f3a768399b. Published February 20, 2013. Accessed December 4, 2017.
5. Community Oncology Alliance. 2016 community oncology practice impact report: tracking the changing landscape of cancer care. https://www.communityoncology.org/wp-content/uploads/2016/09/PracticeImpactReport-2016-Report.pdf. Issued October 4, 2016. Accessed April 10, 2017.
6. The Patient Protection and Affordable Care Act, Pub Law No. 111-148; 2010.
7. Centers for Medicare and Medicaid Services. Shared Savings Program 2016. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/sharedsavingsprogram/index.html?redirect=/sharedsavingsprogram/. Last modified October 12, 2017. Accessed November 16 2016.
8. Dale SB, Ghosh A, Peikes DN, et al. Two-year costs and quality in the comprehensive primary care initiative. N Engl J Med. 2016;374:2345-2356.
9. McWilliams JM, Chernew ME, Landon BE, Schwartz AL. Performance differences in year 1 of pioneer accountable care organizations. N Engl J Med. 2015;372:1927-1936.
10. Rajkumar R, Press MJ, Conway PH. The CMS Innovation Center – a five-year self-assessment. N Engl J Med. 2015;372:1981-1983.
11. Waters TM, Webster JA, Stevens LA, et al. Community oncology medical homes: physician-driven change to improve patient care and reduce costs. J Oncol Pract. 2015;11(6):462-467.
12. Wilfong LS, Hoverman JR, Gosse N, Neubauer MA, Torres V. Changing physician compensation and implementing new technology to enhance pathways compliance. J Clin Oncol. 2016;34(Suppl 7S):Abstr 187.
13. Hoverman JR, Cartwright TH, Patt DA, et al. Pathways, outcomes, and costs in colon cancer: retrospective evaluations in two distinct databases. J Oncol Pract. 2011;7(3 Suppl):52s-59s.
14. Neubauer MA, Hoverman JR, Kolodziej M, et al. Cost effectiveness of evidence-based treatment guidelines for the treatment of non-small-cell lung cancer in the community setting. J Oncol Pract. 2010;6(1):12-18.
15. Hoverman JR, Klein I, Harrison DW, et al. Opening the black box: the impact of an oncology management program consisting of level I pathways and an outbound nurse call system. J Oncol Pract. 2014;10(1):63-67.
16. Newcomer LN, Gould B, Page RD, Donelan SA, Perkins M. Changing physician incentives for affordable, quality cancer care: results of an episode payment model. J Oncol Pract. 2014;10(5):322-326.
17. Meyer JA, Rybowski L, Eichler R. Theory and reality of value-based purchasing: lessons from the pioneers. Rockville, MD.: Agency for Health Care Policy and Research; 1997.
18. Stein CM. Academic clinical research: death by a thousand clicks. Sci Transl Med. 2015;7:318fs349.
19. Churchill W. The end of the beginning. 1st ed. Boston, MA: Little Brown & Co; 1943.
In March 2016, 13 practices affiliated with the US Oncology Network (USON) were invited to participate in the Oncology Care Model (OCM) proposed by the Center for Medicare and Medicaid Services (CMS) and Center for Medicare and Medicaid Innovation (CMMI). The OCM, a novel value-based care model, was designed to provide higher-quality and better-coordinated oncology care at a lower cost to CMS.1 Of the 13 practices, 12 agreed to participate with a start date for the program of July 1, 2016. At least 40% of the practices’ patients were insured by Medicare, and any eligible patients with active cancer were offered an opportunity to enter the program. USON practices treat more than 25,000 patients with a qualifying episode per year and the overall OCM program sees more than 150,000 beneficiaries per year,2 so we anticipated that the OCM would have a substantial impact on each of the 12 practices on USON.
Faced with the scenario of having only 3 months between notification of approval and launch of the OCM, it was imperative that all the practices be proactive in planning and preparing to launch the OCM. With this goal in mind, representatives from all OCM candidate practices convened to anticipate the needs of the OCM and chart out a program to meet those needs. In this article, we discuss the requirements and scope of the OCM, the development of an internal pilot project, the anticipated gains from the pilot, and the results and findings from the pilot, both expected and unexpected.
The road to the Oncology Care Model
The government and oncology practices have been on separate trajectories to the OCM. In the last 15 years, the major intersections of these trajectories had to do with price and not patient outcomes. In 2003, the Medicare Prescription Drug Improvement and Modernization Act (MMA) focused on drug price reductions from an average wholesale price–based schedule to an average sales price–based schedule.3 There was the sequester in 2013,4 and more recently a proposal to restructure the payment for Part B drugs. In the background, recurrent negotiations to fix the calculation for the sustainable growth rate allowed for periodic draconian cuts to the prices of services. The cumulative effect of these price reductions has been to put economic pressure on community oncologists such that many have moved to a hospital environment.5
This contentious relationship with community oncology began to change with the passage of the Affordable Care Act (ACA) in 2010.6 Section 3022 of the ACA established the Medicare Shared Savings Program (MSSP) with the charge to create a new type of health care entity that was responsible for achieving the triple aim of improving population health, improving individual patient care, and bending the cost curve.7 Additional programs, such as the Pioneer Accountable Care Organization (ACO) program and the Comprehensive Primary Care Initiative were established to test alternative payment models.8-10
The ACA also funded the CMMI with a mandate to “test innovative payment and service delivery models” to achieve the triple aim; US$10 billion were appropriated for the years 2011-2019 for this purpose. The CMMI funded a pilot project for cancer care, the COME HOME [Community Oncology MEdical HOME] initiative, to test whether some aspects of care could be transformed or augmented to reduce overall costs or at least reduce the rate of increase. Findings from COME HOME have helped inform the OCM program.11
Over the same period, practices belonging to the USON were paving a path toward value. An electronic health record (EHR) for the entire network was adopted in 2005. A pathways program in which chemotherapy regimens were assessed on cost as well as benefits and toxicity, was started in 2006. Higher-cost regimens with no additional benefits comparable with other evidence-based regimens were deselected for initial treatment choices at the time of initial decision support. This process was streamlined using web-based technology that improved pathways compliance and tracking of off-pathways exceptions.12 Retrospective studies indicated that pathways had the potential to bend the cost curve by reducing drug spending.13,14 USON and its practices also tested a nurse call system (Innovent Oncology) funded by a monthly management fee. This program guided patients through chemotherapy with regular telephonic symptom assessment and discussion of patient-centered values and advance care planning. Results of these programs indicated relative reductions in both drug and hospital expenses.15
Additional experience has come from participation in the United Healthcare Episodes of Care (EOC) initiative, which eliminated the chemotherapy drug incentives, compensating physicians on a per-episode basis instead. This study showed a significant reduction in the total cost of cancer therapy after modifying the fee-for-service system and incorporating feedback data and financial incentives to reward improved outcomes and cost efficiency.16
The Oncology Care Model represents a convergence of purchaser demand and provider readiness. The purchaser holds providers accountable for cost and quality. The data on outcomes and costs will provide an extensive database that can be analyzed by the participating practices to address variations and reduce unnecessary care and preventable costs. Best practices are rewarded.17
The OCM and practice readiness
As a part of the CMS proposal process, practices were required to submit implementation plans by June 30, 2015. The purpose of the implementation plan was to define how a practice could transform over 6 broad domains: 24/7 coverage; EHR certification; navigation and care coordination; continuous quality improvement; incorporation of the Institute of Medicine’s (IOM’s) Care Management Plan; and adherence to nationally recognized guidelines. The periods of patient eligibility for the program were 6-month treatment episodes triggered by a cancer diagnosis, a provider encounter claim, and a Part B or D drug claim specifically identified as a cancer treatment. The episodes could be repeated if the 3 criteria continued to be met. All charges continued to be billed as fee-for-service as before, but in addition, participating practices could bill a monthly enhanced oncology services (MEOS) payment for the duration of an episode. Reducing the total cost of care while meeting performance metrics thresholds would also qualify a practice for performance-based payments.
Of the primary components, EHR certification and adherence to guidelines had been addressed previously, but the other domains represented significant challenges. Although 24/7 physician coverage with access to an EHR is standard for all practices, most practice sites do not have an insight into the frequency of hospital admissions, the ability to efficiently add sick patients to the daily schedule, or a routine call system to assess chemotherapy toxicity.
The OCM proposes 10 potential navigation/care coordination functions (Table 1) and does not consider those functions to be the role of one person, but rather a team responsibility. Most of our practices perform at least some of these functions, but they are not formally designated, coordinated, or recorded. A similar condition exists for the IOM care plan, which includes recommendations for treatment and adverse event management (Table 2). The prognosis, toxicity, quality-of-life, and goals-of-care requirements are often found in the physician notes, but not systematically documented or searchable.
Similar challenges have been observed for continuous quality improvement programs. Although the data are available, they are often not easy to search and, therefore, are difficult to retrieve and report. The OCM, as with any transformational program, must always weigh the benefit of information with the burden of consumption of physician and staff time to collect and input these data.18
Prepilot project work
In October 2015, lead physicians and managers from the 12 participating practices were brought together with analytic, technical, process management, and business experts from USON and McKesson Specialty Health. The objective of the meeting was to define the areas of greatest need for day 1 of the OCM and to be prepared. The challenges were to identify the changes needed to meet the requirements of the OCM while improving the patient experience, sustaining the viability of the community oncology practices, creating teams to deliver more effective care, and using data to bend the cost curve. Accordingly, 4 work streams were created: Care and Support; Content; Technology; and Communications, Revenue Cycle and Incentives.
Care and Support
The key tasks of the Care and Support team were to define the workflows for navigation and the IOM care plan. As a patient’s journey through the clinic was mapped out, it became clear that although multiple personnel could participate in the navigation and care plans, there was no systematic way to organize and record the components of successful navigation. The goals for the pilot were to test various options for navigation and to identify best practices that could be translated into standard operating procedures.
Content
The Content team was charged with identifying available programs that would fit into the OCM requirements. These included advance care planning, survivorship, chemotherapy teaching, risk assessment, pathways, and symptom assessment. A longer-term goal was the development of care paths, a more comprehensive map of the patient’s journey that would include consultations, coordinated care, imaging, labs, and other services.
Technology
The task for the Technology work stream was to identify processes of care that required documentation and to evaluate current and future technology solutions to improve efficiencies. The electronic medical record satisfied for the input of data with relevant clinical details, demographics, disease types, and staging. A web-based pathways tool supported clinical decision-making, as well as compliance to pathways. The Medicare quality metric programs set the stage for development of capture and reporting tools for data from many sources. The pilot would indicate the adequacy of these tools and the need for expansion or development of new functions or programs. Of particular importance was recording the IOM care plan and navigation functions in a searchable format. As care paths are developed, risk prediction, palliative care, and other services need to be encompassed. Finally, technology will support the identification and enrollment of eligible patients, and billing activities.
Communications, Revenue Cycle, and Incentives
The final work stream was Communications, Revenue Cycle, and Incentives. For the pilot, the focus was on revenue cycle. A new category of patient needed to be identified, enrolled, and billed to CMS for services. At the outset, the technology did not address the identification of patients receiving only oral drugs. The office visit, the diagnosis, and the drug claim all had to be aligned for enrollment and billing. It was critical to understand the workload by patient and total volume to estimate the technology and personnel needs to meet the initial number of new OCM patients. Communication refers to both internal and external parties. Education of the entire practice staff regarding transformation will be critical for success.
Of the 12 participating practices, 3 practice sites were selected for the pilot program. Each had fewer than 10 medical oncologists and at least 1 radiation oncologist. Each site had a physician champion and an administrative lead. All of the sites were part of a larger regional oncology practice. A fourth site had independently started a pilot and that experience was shared with the larger group as well. The sites were distributed across the country in 4 different time zones.
The pilot experience
The pilot experience yielded important findings, some expected and some unexpected. The challenges of navigation, the treatment plan, and team building were anticipated. We were surprised at the sheer number of potential candidates and the difficulty in finding eligible candidates. Not to be overlooked was a need for continued and possibly increased emphasis on adherence to pathways and process changes to reduce hospitalizations and emergency department (ED) visits.
Navigation
At the outset, none of the pilot practices had formal navigation processes as outlined in Table 1. Many of the processes, such as coordinating appointments and facilitating follow-up services and financial support, were provided by the practice, but were not identified or coordinated as navigation. The practices, as a first step, defined who was responsible for those services and identified 1 person who would be responsible for their completion. It was agreed that navigation was a process shared by a team and not an individual responsibility, yet the person who would monitor the completion of the tasks was not identified. It soon became apparent that true navigation included more tasks than initially outlined.
Additional tasks included appropriate patient education regarding treatment toxicities, follow-up after chemotherapy or a hospitalization, and coordination of other aspects of the IOM care plan, such as survivorship and advance care planning. Each of the practices recruited staff internally to assume the navigator role, and standard operating procedures were developed for completing and documenting this expanded responsibility. True navigation, however, depends on building the team character while still having 1 or 2 members of the team identified as being responsible for following and documenting the patient’s journey through an episode. To meet those needs, navigators developed ad hoc methods, such as spreadsheets, to track patients. The technology team developed drop-down check lists within the EHR, but the burden of documentation continued. Lastly, an ongoing challenge is how best to designate responsibility and assess how many additional staffers are needed.
IOM Care Management Plan
Before initiation of the pilot project, no practice was providing patients with a comprehensive, written treatment plan. Considerably more than half of the members of the work-stream teams believed that would be difficult to implement. However, the members of the Care and Support work stream made some fundamental assumptions to make the care plan workable: first, all aspects of the plan did not occur at the same time, and were not completed by the same person; second, and critically, items 2-9 of Table 2 could be completed at one time during the early conversations between the physician and patient about the goals of treatment. Diagnosis, prognosis, treatment intent, response rate, quality of life, and toxicities were included in the treatment plan, and the remaining IOM care plan could be discussed, or at least identified, as issues for further discussion with other team members. These components were incorporated into a 1-page document that was either typed into the record and printed out or handwritten and copied for the patient. This became the treatment plan and was ready for use at the start of the pilot.
The physician response to the treatment plan was mixed. One site adopted it enthusiastically and quickly moved to use the plan for all patients. Other sites had variable uptake. One hurdle was defining response rate to therapy and prognosis. Data were provided but they often did not match the conditions of individual patients. Some physicians were uncomfortable with the process. Documentation was difficult because the plans had to be scanned into the EHR. Patients generally responded favorably to the plans and would bring them to teaching or chemotherapy sessions.
As with navigation, the treatment plan challenges pointed the technology team toward the development and implementation of an electronic version of the plan. The pilot allowed members of the technology team to visit the clinics, to evaluate workflows and make assumptions on how to structure a treatment plan electronically.
Team-based care
None of the pilot sites had a formalized structure for team-based care. Team huddles were developed and weekly and daily huddles were encouraged. The weekly huddles took about 15-30 minutes, during which patients scheduled for the coming week were reviewed. All personnel who saw patients were invited – benefit counselors, advanced practice providers, schedulers, lab technicians, medical assistants, office and infusion nurses, social workers, pharmacists, physicians, and lead administrative staff. The daily huddle was smaller and generally included a nurse, a medical assistant, and a physician, at a minimum, to review the patients in the hospital, those to be seen in the clinic that day, and any follow-up information based on scheduled contact following recent treatments or events. In some sites, these huddles were uniformly endorsed, in others, not at all. Although many physicians felt that the functions were being handled informally and the additional time commitment would not improve the process, once they began to attend the meetings, they appreciated the value of the huddles and continued attending them. As the complexity of delivery and documentation becomes more apparent, these will prove indispensable to coordinated care.
24/7 access
Hospitalization is one of the chief drivers of the total cost of care, so the pilot sites were concerned that more needed to be done to reduce unnecessary hospitalizations. One site surveyed the patients coming into the clinic about their previous ED visits. Many of the visits had been for noncancer-related events and the clinic was not aware of many of the many of the visits. These findings prompted a number of changes. Open slots were created daily for patients who needed to be worked in for any areas of concern. The on-call physician, triage nurse, or navigation lead could fill these slots. All patients discharged from the hospital were called within 48 hours after discharge and scheduled for a clinic visit within 1 week. Night and weekend call logs were scrutinized each morning and patients’ calls were returned for any issues related to symptom or toxicity management. At one site, patients were given wallet cards with the clinic number, the treatment regimen, and, on the reverse, all symptoms that would justify calling the clinic. The patients were encouraged to call the clinic earlier rather than later in the day. On the back end, the clinics were to have processes in place so that patient calls would be answered quickly to facilitate same-day evaluations in the clinic.
Enrollment and revenue cycle
The most intractable problem was the identification and enrollment of OCM patients. As already noted, 3 components were necessary for enrollment: a drug charge for Part B or D Medicare, a provider visit, and an approved cancer diagnosis. To identify those patients, the claims system would churn out a weekly list of all eligible patients. However, the claims system had no mechanism to pick up Part D claims for oral medications. This meant that any patient with a provider visit and an appropriate diagnosis was potentially eligible for enrollment. At one site, the list of potential patients was 2-3 times the number of actual candidates. It took 6 weeks of manual chart review to resolving the list. Collectively, the 12 practices could have as many as 20,000 patients eligible for the July 1 enrollment. The pilot allowed the practices to get an early start on recruitment of business office staff and plans to address the backlog of potentially eligible patients. The process of identifying eligible patients for the OCM still needs a better solution because finding the appropriate patients is a critical first step in this model.
Underlying all of these initiatives is communication, both internal and external. We have to select and celebrate best practices. We have to educate our staffs. We will have to demonstrate that we are giving better care to our patients by using patient and provider testimonials and data.
From angst to analytics
The challenges of practice transformation can be daunting. It will be difficult to formalize processes and document data in ways that were untested before the pilot program was set up. However, the pilot accomplished 2 things: it identified additional areas that needed improvement and it demonstrated that the most challenging aspects of the OCM were feasible. Navigation and the IOM care plan were broken down into parts; each component was separately addressed, and programs were put in place to make the pieces manageable and part of an overall movement toward team-based care. The addition of a technology platform has been a key factor for the success of the value-based care initiative. Additional technology support has been enlisted to facilitate the processes, and an electronic version of the treatment plan is being tested. More difficult will be efforts to address the cultural resistance to change, which we hope to do by using data and outcomes from the CMS claims data files. The OCM represents an unprecedented opportunity for measurement of the quality of care we deliver.
We are now well und
Acknowledgment
The authors thank Supriya Srinivasan, PhD, for editorial support.
In March 2016, 13 practices affiliated with the US Oncology Network (USON) were invited to participate in the Oncology Care Model (OCM) proposed by the Center for Medicare and Medicaid Services (CMS) and Center for Medicare and Medicaid Innovation (CMMI). The OCM, a novel value-based care model, was designed to provide higher-quality and better-coordinated oncology care at a lower cost to CMS.1 Of the 13 practices, 12 agreed to participate with a start date for the program of July 1, 2016. At least 40% of the practices’ patients were insured by Medicare, and any eligible patients with active cancer were offered an opportunity to enter the program. USON practices treat more than 25,000 patients with a qualifying episode per year and the overall OCM program sees more than 150,000 beneficiaries per year,2 so we anticipated that the OCM would have a substantial impact on each of the 12 practices on USON.
Faced with the scenario of having only 3 months between notification of approval and launch of the OCM, it was imperative that all the practices be proactive in planning and preparing to launch the OCM. With this goal in mind, representatives from all OCM candidate practices convened to anticipate the needs of the OCM and chart out a program to meet those needs. In this article, we discuss the requirements and scope of the OCM, the development of an internal pilot project, the anticipated gains from the pilot, and the results and findings from the pilot, both expected and unexpected.
The road to the Oncology Care Model
The government and oncology practices have been on separate trajectories to the OCM. In the last 15 years, the major intersections of these trajectories had to do with price and not patient outcomes. In 2003, the Medicare Prescription Drug Improvement and Modernization Act (MMA) focused on drug price reductions from an average wholesale price–based schedule to an average sales price–based schedule.3 There was the sequester in 2013,4 and more recently a proposal to restructure the payment for Part B drugs. In the background, recurrent negotiations to fix the calculation for the sustainable growth rate allowed for periodic draconian cuts to the prices of services. The cumulative effect of these price reductions has been to put economic pressure on community oncologists such that many have moved to a hospital environment.5
This contentious relationship with community oncology began to change with the passage of the Affordable Care Act (ACA) in 2010.6 Section 3022 of the ACA established the Medicare Shared Savings Program (MSSP) with the charge to create a new type of health care entity that was responsible for achieving the triple aim of improving population health, improving individual patient care, and bending the cost curve.7 Additional programs, such as the Pioneer Accountable Care Organization (ACO) program and the Comprehensive Primary Care Initiative were established to test alternative payment models.8-10
The ACA also funded the CMMI with a mandate to “test innovative payment and service delivery models” to achieve the triple aim; US$10 billion were appropriated for the years 2011-2019 for this purpose. The CMMI funded a pilot project for cancer care, the COME HOME [Community Oncology MEdical HOME] initiative, to test whether some aspects of care could be transformed or augmented to reduce overall costs or at least reduce the rate of increase. Findings from COME HOME have helped inform the OCM program.11
Over the same period, practices belonging to the USON were paving a path toward value. An electronic health record (EHR) for the entire network was adopted in 2005. A pathways program in which chemotherapy regimens were assessed on cost as well as benefits and toxicity, was started in 2006. Higher-cost regimens with no additional benefits comparable with other evidence-based regimens were deselected for initial treatment choices at the time of initial decision support. This process was streamlined using web-based technology that improved pathways compliance and tracking of off-pathways exceptions.12 Retrospective studies indicated that pathways had the potential to bend the cost curve by reducing drug spending.13,14 USON and its practices also tested a nurse call system (Innovent Oncology) funded by a monthly management fee. This program guided patients through chemotherapy with regular telephonic symptom assessment and discussion of patient-centered values and advance care planning. Results of these programs indicated relative reductions in both drug and hospital expenses.15
Additional experience has come from participation in the United Healthcare Episodes of Care (EOC) initiative, which eliminated the chemotherapy drug incentives, compensating physicians on a per-episode basis instead. This study showed a significant reduction in the total cost of cancer therapy after modifying the fee-for-service system and incorporating feedback data and financial incentives to reward improved outcomes and cost efficiency.16
The Oncology Care Model represents a convergence of purchaser demand and provider readiness. The purchaser holds providers accountable for cost and quality. The data on outcomes and costs will provide an extensive database that can be analyzed by the participating practices to address variations and reduce unnecessary care and preventable costs. Best practices are rewarded.17
The OCM and practice readiness
As a part of the CMS proposal process, practices were required to submit implementation plans by June 30, 2015. The purpose of the implementation plan was to define how a practice could transform over 6 broad domains: 24/7 coverage; EHR certification; navigation and care coordination; continuous quality improvement; incorporation of the Institute of Medicine’s (IOM’s) Care Management Plan; and adherence to nationally recognized guidelines. The periods of patient eligibility for the program were 6-month treatment episodes triggered by a cancer diagnosis, a provider encounter claim, and a Part B or D drug claim specifically identified as a cancer treatment. The episodes could be repeated if the 3 criteria continued to be met. All charges continued to be billed as fee-for-service as before, but in addition, participating practices could bill a monthly enhanced oncology services (MEOS) payment for the duration of an episode. Reducing the total cost of care while meeting performance metrics thresholds would also qualify a practice for performance-based payments.
Of the primary components, EHR certification and adherence to guidelines had been addressed previously, but the other domains represented significant challenges. Although 24/7 physician coverage with access to an EHR is standard for all practices, most practice sites do not have an insight into the frequency of hospital admissions, the ability to efficiently add sick patients to the daily schedule, or a routine call system to assess chemotherapy toxicity.
The OCM proposes 10 potential navigation/care coordination functions (Table 1) and does not consider those functions to be the role of one person, but rather a team responsibility. Most of our practices perform at least some of these functions, but they are not formally designated, coordinated, or recorded. A similar condition exists for the IOM care plan, which includes recommendations for treatment and adverse event management (Table 2). The prognosis, toxicity, quality-of-life, and goals-of-care requirements are often found in the physician notes, but not systematically documented or searchable.
Similar challenges have been observed for continuous quality improvement programs. Although the data are available, they are often not easy to search and, therefore, are difficult to retrieve and report. The OCM, as with any transformational program, must always weigh the benefit of information with the burden of consumption of physician and staff time to collect and input these data.18
Prepilot project work
In October 2015, lead physicians and managers from the 12 participating practices were brought together with analytic, technical, process management, and business experts from USON and McKesson Specialty Health. The objective of the meeting was to define the areas of greatest need for day 1 of the OCM and to be prepared. The challenges were to identify the changes needed to meet the requirements of the OCM while improving the patient experience, sustaining the viability of the community oncology practices, creating teams to deliver more effective care, and using data to bend the cost curve. Accordingly, 4 work streams were created: Care and Support; Content; Technology; and Communications, Revenue Cycle and Incentives.
Care and Support
The key tasks of the Care and Support team were to define the workflows for navigation and the IOM care plan. As a patient’s journey through the clinic was mapped out, it became clear that although multiple personnel could participate in the navigation and care plans, there was no systematic way to organize and record the components of successful navigation. The goals for the pilot were to test various options for navigation and to identify best practices that could be translated into standard operating procedures.
Content
The Content team was charged with identifying available programs that would fit into the OCM requirements. These included advance care planning, survivorship, chemotherapy teaching, risk assessment, pathways, and symptom assessment. A longer-term goal was the development of care paths, a more comprehensive map of the patient’s journey that would include consultations, coordinated care, imaging, labs, and other services.
Technology
The task for the Technology work stream was to identify processes of care that required documentation and to evaluate current and future technology solutions to improve efficiencies. The electronic medical record satisfied for the input of data with relevant clinical details, demographics, disease types, and staging. A web-based pathways tool supported clinical decision-making, as well as compliance to pathways. The Medicare quality metric programs set the stage for development of capture and reporting tools for data from many sources. The pilot would indicate the adequacy of these tools and the need for expansion or development of new functions or programs. Of particular importance was recording the IOM care plan and navigation functions in a searchable format. As care paths are developed, risk prediction, palliative care, and other services need to be encompassed. Finally, technology will support the identification and enrollment of eligible patients, and billing activities.
Communications, Revenue Cycle, and Incentives
The final work stream was Communications, Revenue Cycle, and Incentives. For the pilot, the focus was on revenue cycle. A new category of patient needed to be identified, enrolled, and billed to CMS for services. At the outset, the technology did not address the identification of patients receiving only oral drugs. The office visit, the diagnosis, and the drug claim all had to be aligned for enrollment and billing. It was critical to understand the workload by patient and total volume to estimate the technology and personnel needs to meet the initial number of new OCM patients. Communication refers to both internal and external parties. Education of the entire practice staff regarding transformation will be critical for success.
Of the 12 participating practices, 3 practice sites were selected for the pilot program. Each had fewer than 10 medical oncologists and at least 1 radiation oncologist. Each site had a physician champion and an administrative lead. All of the sites were part of a larger regional oncology practice. A fourth site had independently started a pilot and that experience was shared with the larger group as well. The sites were distributed across the country in 4 different time zones.
The pilot experience
The pilot experience yielded important findings, some expected and some unexpected. The challenges of navigation, the treatment plan, and team building were anticipated. We were surprised at the sheer number of potential candidates and the difficulty in finding eligible candidates. Not to be overlooked was a need for continued and possibly increased emphasis on adherence to pathways and process changes to reduce hospitalizations and emergency department (ED) visits.
Navigation
At the outset, none of the pilot practices had formal navigation processes as outlined in Table 1. Many of the processes, such as coordinating appointments and facilitating follow-up services and financial support, were provided by the practice, but were not identified or coordinated as navigation. The practices, as a first step, defined who was responsible for those services and identified 1 person who would be responsible for their completion. It was agreed that navigation was a process shared by a team and not an individual responsibility, yet the person who would monitor the completion of the tasks was not identified. It soon became apparent that true navigation included more tasks than initially outlined.
Additional tasks included appropriate patient education regarding treatment toxicities, follow-up after chemotherapy or a hospitalization, and coordination of other aspects of the IOM care plan, such as survivorship and advance care planning. Each of the practices recruited staff internally to assume the navigator role, and standard operating procedures were developed for completing and documenting this expanded responsibility. True navigation, however, depends on building the team character while still having 1 or 2 members of the team identified as being responsible for following and documenting the patient’s journey through an episode. To meet those needs, navigators developed ad hoc methods, such as spreadsheets, to track patients. The technology team developed drop-down check lists within the EHR, but the burden of documentation continued. Lastly, an ongoing challenge is how best to designate responsibility and assess how many additional staffers are needed.
IOM Care Management Plan
Before initiation of the pilot project, no practice was providing patients with a comprehensive, written treatment plan. Considerably more than half of the members of the work-stream teams believed that would be difficult to implement. However, the members of the Care and Support work stream made some fundamental assumptions to make the care plan workable: first, all aspects of the plan did not occur at the same time, and were not completed by the same person; second, and critically, items 2-9 of Table 2 could be completed at one time during the early conversations between the physician and patient about the goals of treatment. Diagnosis, prognosis, treatment intent, response rate, quality of life, and toxicities were included in the treatment plan, and the remaining IOM care plan could be discussed, or at least identified, as issues for further discussion with other team members. These components were incorporated into a 1-page document that was either typed into the record and printed out or handwritten and copied for the patient. This became the treatment plan and was ready for use at the start of the pilot.
The physician response to the treatment plan was mixed. One site adopted it enthusiastically and quickly moved to use the plan for all patients. Other sites had variable uptake. One hurdle was defining response rate to therapy and prognosis. Data were provided but they often did not match the conditions of individual patients. Some physicians were uncomfortable with the process. Documentation was difficult because the plans had to be scanned into the EHR. Patients generally responded favorably to the plans and would bring them to teaching or chemotherapy sessions.
As with navigation, the treatment plan challenges pointed the technology team toward the development and implementation of an electronic version of the plan. The pilot allowed members of the technology team to visit the clinics, to evaluate workflows and make assumptions on how to structure a treatment plan electronically.
Team-based care
None of the pilot sites had a formalized structure for team-based care. Team huddles were developed and weekly and daily huddles were encouraged. The weekly huddles took about 15-30 minutes, during which patients scheduled for the coming week were reviewed. All personnel who saw patients were invited – benefit counselors, advanced practice providers, schedulers, lab technicians, medical assistants, office and infusion nurses, social workers, pharmacists, physicians, and lead administrative staff. The daily huddle was smaller and generally included a nurse, a medical assistant, and a physician, at a minimum, to review the patients in the hospital, those to be seen in the clinic that day, and any follow-up information based on scheduled contact following recent treatments or events. In some sites, these huddles were uniformly endorsed, in others, not at all. Although many physicians felt that the functions were being handled informally and the additional time commitment would not improve the process, once they began to attend the meetings, they appreciated the value of the huddles and continued attending them. As the complexity of delivery and documentation becomes more apparent, these will prove indispensable to coordinated care.
24/7 access
Hospitalization is one of the chief drivers of the total cost of care, so the pilot sites were concerned that more needed to be done to reduce unnecessary hospitalizations. One site surveyed the patients coming into the clinic about their previous ED visits. Many of the visits had been for noncancer-related events and the clinic was not aware of many of the many of the visits. These findings prompted a number of changes. Open slots were created daily for patients who needed to be worked in for any areas of concern. The on-call physician, triage nurse, or navigation lead could fill these slots. All patients discharged from the hospital were called within 48 hours after discharge and scheduled for a clinic visit within 1 week. Night and weekend call logs were scrutinized each morning and patients’ calls were returned for any issues related to symptom or toxicity management. At one site, patients were given wallet cards with the clinic number, the treatment regimen, and, on the reverse, all symptoms that would justify calling the clinic. The patients were encouraged to call the clinic earlier rather than later in the day. On the back end, the clinics were to have processes in place so that patient calls would be answered quickly to facilitate same-day evaluations in the clinic.
Enrollment and revenue cycle
The most intractable problem was the identification and enrollment of OCM patients. As already noted, 3 components were necessary for enrollment: a drug charge for Part B or D Medicare, a provider visit, and an approved cancer diagnosis. To identify those patients, the claims system would churn out a weekly list of all eligible patients. However, the claims system had no mechanism to pick up Part D claims for oral medications. This meant that any patient with a provider visit and an appropriate diagnosis was potentially eligible for enrollment. At one site, the list of potential patients was 2-3 times the number of actual candidates. It took 6 weeks of manual chart review to resolving the list. Collectively, the 12 practices could have as many as 20,000 patients eligible for the July 1 enrollment. The pilot allowed the practices to get an early start on recruitment of business office staff and plans to address the backlog of potentially eligible patients. The process of identifying eligible patients for the OCM still needs a better solution because finding the appropriate patients is a critical first step in this model.
Underlying all of these initiatives is communication, both internal and external. We have to select and celebrate best practices. We have to educate our staffs. We will have to demonstrate that we are giving better care to our patients by using patient and provider testimonials and data.
From angst to analytics
The challenges of practice transformation can be daunting. It will be difficult to formalize processes and document data in ways that were untested before the pilot program was set up. However, the pilot accomplished 2 things: it identified additional areas that needed improvement and it demonstrated that the most challenging aspects of the OCM were feasible. Navigation and the IOM care plan were broken down into parts; each component was separately addressed, and programs were put in place to make the pieces manageable and part of an overall movement toward team-based care. The addition of a technology platform has been a key factor for the success of the value-based care initiative. Additional technology support has been enlisted to facilitate the processes, and an electronic version of the treatment plan is being tested. More difficult will be efforts to address the cultural resistance to change, which we hope to do by using data and outcomes from the CMS claims data files. The OCM represents an unprecedented opportunity for measurement of the quality of care we deliver.
We are now well und
Acknowledgment
The authors thank Supriya Srinivasan, PhD, for editorial support.
1. Oncology Care Model. Centers for Medicare and Medicaid Services 2016. https://innovation.cms.gov/initiatives/oncology-care/. Last updated November 14, 2017. Accessed November 16 2016.
2. Mortimer L, Strawbridge L, Lukens E, et al. CMS’ Oncology Care Model: delivering higher value cancer care. Clin Pharmacol Ther. 2017.
3. Medicare Prescription Drug, Improvement and Modernization Act of 2003, Pub Law No. 108-173; 2003.
4. Mathews D. The sequester: absolutely everything you could possibly need to know, in one FAQ. https://www.washingtonpost.com/news/wonk/wp/2013/02/20/the-sequester-absolutely-everything-you-could-possibly-need-to-know-in-one-faq/?utm_term=.a0f3a768399b. Published February 20, 2013. Accessed December 4, 2017.
5. Community Oncology Alliance. 2016 community oncology practice impact report: tracking the changing landscape of cancer care. https://www.communityoncology.org/wp-content/uploads/2016/09/PracticeImpactReport-2016-Report.pdf. Issued October 4, 2016. Accessed April 10, 2017.
6. The Patient Protection and Affordable Care Act, Pub Law No. 111-148; 2010.
7. Centers for Medicare and Medicaid Services. Shared Savings Program 2016. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/sharedsavingsprogram/index.html?redirect=/sharedsavingsprogram/. Last modified October 12, 2017. Accessed November 16 2016.
8. Dale SB, Ghosh A, Peikes DN, et al. Two-year costs and quality in the comprehensive primary care initiative. N Engl J Med. 2016;374:2345-2356.
9. McWilliams JM, Chernew ME, Landon BE, Schwartz AL. Performance differences in year 1 of pioneer accountable care organizations. N Engl J Med. 2015;372:1927-1936.
10. Rajkumar R, Press MJ, Conway PH. The CMS Innovation Center – a five-year self-assessment. N Engl J Med. 2015;372:1981-1983.
11. Waters TM, Webster JA, Stevens LA, et al. Community oncology medical homes: physician-driven change to improve patient care and reduce costs. J Oncol Pract. 2015;11(6):462-467.
12. Wilfong LS, Hoverman JR, Gosse N, Neubauer MA, Torres V. Changing physician compensation and implementing new technology to enhance pathways compliance. J Clin Oncol. 2016;34(Suppl 7S):Abstr 187.
13. Hoverman JR, Cartwright TH, Patt DA, et al. Pathways, outcomes, and costs in colon cancer: retrospective evaluations in two distinct databases. J Oncol Pract. 2011;7(3 Suppl):52s-59s.
14. Neubauer MA, Hoverman JR, Kolodziej M, et al. Cost effectiveness of evidence-based treatment guidelines for the treatment of non-small-cell lung cancer in the community setting. J Oncol Pract. 2010;6(1):12-18.
15. Hoverman JR, Klein I, Harrison DW, et al. Opening the black box: the impact of an oncology management program consisting of level I pathways and an outbound nurse call system. J Oncol Pract. 2014;10(1):63-67.
16. Newcomer LN, Gould B, Page RD, Donelan SA, Perkins M. Changing physician incentives for affordable, quality cancer care: results of an episode payment model. J Oncol Pract. 2014;10(5):322-326.
17. Meyer JA, Rybowski L, Eichler R. Theory and reality of value-based purchasing: lessons from the pioneers. Rockville, MD.: Agency for Health Care Policy and Research; 1997.
18. Stein CM. Academic clinical research: death by a thousand clicks. Sci Transl Med. 2015;7:318fs349.
19. Churchill W. The end of the beginning. 1st ed. Boston, MA: Little Brown & Co; 1943.
1. Oncology Care Model. Centers for Medicare and Medicaid Services 2016. https://innovation.cms.gov/initiatives/oncology-care/. Last updated November 14, 2017. Accessed November 16 2016.
2. Mortimer L, Strawbridge L, Lukens E, et al. CMS’ Oncology Care Model: delivering higher value cancer care. Clin Pharmacol Ther. 2017.
3. Medicare Prescription Drug, Improvement and Modernization Act of 2003, Pub Law No. 108-173; 2003.
4. Mathews D. The sequester: absolutely everything you could possibly need to know, in one FAQ. https://www.washingtonpost.com/news/wonk/wp/2013/02/20/the-sequester-absolutely-everything-you-could-possibly-need-to-know-in-one-faq/?utm_term=.a0f3a768399b. Published February 20, 2013. Accessed December 4, 2017.
5. Community Oncology Alliance. 2016 community oncology practice impact report: tracking the changing landscape of cancer care. https://www.communityoncology.org/wp-content/uploads/2016/09/PracticeImpactReport-2016-Report.pdf. Issued October 4, 2016. Accessed April 10, 2017.
6. The Patient Protection and Affordable Care Act, Pub Law No. 111-148; 2010.
7. Centers for Medicare and Medicaid Services. Shared Savings Program 2016. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/sharedsavingsprogram/index.html?redirect=/sharedsavingsprogram/. Last modified October 12, 2017. Accessed November 16 2016.
8. Dale SB, Ghosh A, Peikes DN, et al. Two-year costs and quality in the comprehensive primary care initiative. N Engl J Med. 2016;374:2345-2356.
9. McWilliams JM, Chernew ME, Landon BE, Schwartz AL. Performance differences in year 1 of pioneer accountable care organizations. N Engl J Med. 2015;372:1927-1936.
10. Rajkumar R, Press MJ, Conway PH. The CMS Innovation Center – a five-year self-assessment. N Engl J Med. 2015;372:1981-1983.
11. Waters TM, Webster JA, Stevens LA, et al. Community oncology medical homes: physician-driven change to improve patient care and reduce costs. J Oncol Pract. 2015;11(6):462-467.
12. Wilfong LS, Hoverman JR, Gosse N, Neubauer MA, Torres V. Changing physician compensation and implementing new technology to enhance pathways compliance. J Clin Oncol. 2016;34(Suppl 7S):Abstr 187.
13. Hoverman JR, Cartwright TH, Patt DA, et al. Pathways, outcomes, and costs in colon cancer: retrospective evaluations in two distinct databases. J Oncol Pract. 2011;7(3 Suppl):52s-59s.
14. Neubauer MA, Hoverman JR, Kolodziej M, et al. Cost effectiveness of evidence-based treatment guidelines for the treatment of non-small-cell lung cancer in the community setting. J Oncol Pract. 2010;6(1):12-18.
15. Hoverman JR, Klein I, Harrison DW, et al. Opening the black box: the impact of an oncology management program consisting of level I pathways and an outbound nurse call system. J Oncol Pract. 2014;10(1):63-67.
16. Newcomer LN, Gould B, Page RD, Donelan SA, Perkins M. Changing physician incentives for affordable, quality cancer care: results of an episode payment model. J Oncol Pract. 2014;10(5):322-326.
17. Meyer JA, Rybowski L, Eichler R. Theory and reality of value-based purchasing: lessons from the pioneers. Rockville, MD.: Agency for Health Care Policy and Research; 1997.
18. Stein CM. Academic clinical research: death by a thousand clicks. Sci Transl Med. 2015;7:318fs349.
19. Churchill W. The end of the beginning. 1st ed. Boston, MA: Little Brown & Co; 1943.
Goals-of-care discussions
Goals-of-care conversations led by the oncologist are a key opportunity to improve advance care planning and end-of-life care,1 but our patients are not understanding the essentials. Findings from a study of patients’ expectations about chemotherapy showed that more than two-thirds of patients with lung or colorectal cancers thought their palliative chemotherapy,2 radiation, 3 and/or surgery4 could cure them. Failure to effectively educate patients can lead to end-of-life care associated with poor quality of care, including over-aggressive care, poor quality of life with suboptimal symptom management, caregiver distress, and other potentially preventable problems.5
Palliative care routinely includes goals-of-care (GoC) discussions as part of the standard note. At one large urban hospital, the 30-day readmission rate was 10% if palliative care consultation was done, compared with 15% if no consultation was obtained.6 Patients who had consultations that included GoC discussions in addition to a symptom management consultation had a lower hospital readmission rate of 5%, compared with 15% in patients who received symptom management consultations alone (adjusted odds ratio [AOR], 0.36; confidence interval [CI], 0.27-0.48; P < .001). 6 Findings from another study showed that the use of aggressive end-of-life care was reduced by one-third when the patient and provider had a GoC planning session close to the time of diagnosis, instead of at the end of life.7
We prepared a template to be incorporated into the electronic medical record (EMR) to facilitate GoC discussions between the oncologist and patient as part of a randomized phase 3 trial of early versus delayed palliative care for phase 1 clinical trial patients. Documentation is one of the most important elements of efforts to improve end-of-life care, along with advance care planning, GoC discussions, and understanding the system of care.8 Because we have found this approach useful in our everyday oncology practice, we are sharing this simple, doable template in the hope that others will find it effective.
The GoC template
What does it look like?
We developed a document for oncology GoC discussions that was incorporated into the EMR (Table). We use the GoC document in much the same way we’d review a computed-tomography scan with our patients and their families: we bring it up on the computer screen, show them the categories, and type in responses on the allotted spaces on the right-hand side of the document. The input can be done in real time or after the conversation, or even after the patient has left. In our practice, we access the Patient Instructions part of the EMR, use a SmartPhrase to copy the Word template into the chart, and start typing.
The completed GoC sheet can be used in several ways. Most commonly, we print it out for the patient and family, so that everyone has access to the same information. Printing it out as part of the after-visit patient summary also satisfies the meaningful use requirement for EMRs. It is also possible to cut and paste the document into a letter to the care team, or directly into the Progress Note, so that it will be available for members of the care team to see.
The GoC form does take some time to complete. Palliative care teams that have reported on what was done during the palliative care visit have noted that the initial and subsequent visits took about an hour, with about 20 minutes devoted to symptom management, 15 minutes to patient and family coping, and 10 minutes to illness understanding and education, including prognosis.9,10 In our own practice, we do questions 1-9 first (Table, shaded area) because it takes less time than anticipated, just like code status discussions,11 and it is much easier with a script.
We use this template for in- and out-patient consultations and for routine visits when a GoC discussion is warranted. It is important to remember that doing this counts as advance care planning and can be billed using the ACP codes.
How do you use template?
It is critically important to make sure that the patient and family are ready for this discussion. To further facilitate the discussion, we have devised a temporary palliative care tattoo with a script, or prompts, for what questions to ask and in what order they should be asked (Figure). The easy-to-read tattoo is worn on the inner forearm so that it is readily visible to the oncologist or advanced practice nurse.12
We always start by asking, “How do you like to get medical information?” (Table, question 1) and follow up with something like, “Are you the sort of person who wants all the details, or not?” (Table). If the response is yes, they want all the details, then we follow up with another question, “Does that include talking about prognosis and what might happen?” (Table). Most patients will want full disclosure of their circumstances and prognosis, but some will not, and will feel overwhelmed and disempowered if you proceed.
After reviewing the answers to question 1 and any follow-up questions you may have asked, you will be able to gauge whether you should continue with questions 2-4 in the template. Once you know more about the patient and the family’s understanding of the situation (Table, question 2), what is important to them (question 3), what they are hoping for (question 4), and have spoken to them about disease progression, recapped their treatment to date, and checked to see if they might be eligible for any clinical trials (question 5), it will be easier to move on to the next questions, about progressive disease and advance care planning: “You are doing OK now, but have you thought about a time when you could be sicker [and need] a living will or advance directive [question 6, see next section of this article]?” For people unwilling to have this discussion, or have it at that moment, there is an excellent article that outlines the process to help practitioners increase prognostic awareness.14 Patient readiness will change over time as they adjust to the life changes forced by serious illness, and one can put off the discussion until they are more accepting. Just remember that patients are not likely to broach the subject themselves, and part of our job is to offer guidance.
As Singh and colleagues have noted, many patients with incurable disease have poor “prognosis awareness,” 15 so it is important for the oncologist to have a GoC conversation with the patient to be able to guage the patient’s understanding of the prognosis after a scan that shows progressive disease. Singh and his colleagues reported that of 64 taped oncologist-patient conversations about scan results, only 4 included frank discussions about prognosis. The authors suggested asking the question, “Would you like to talk about what this means?” after showing the patient the scan to allow the patient some control and to get permission to disclose crucial information based on the reading of the scan.15
Getting started on the GoC discussions may be the hardest part. Some useful introductory lines might include: “In my experience, it is easier to talk about our goals of care while people are still doing well. I know the future can be more uncertain. That’s why I want us to discuss these things now,” or “I am worried about you, after looking at these scans. I think it is time to have another discussion about what the future holds.”
Starting the hospice information visit
For patients with progressive disease, how do you know when to initiate a discussion about hospice and specifically, the hospice information visit, in a timely manner? It turns out oncologists are fairly adept at sensing when the prognosis has changed, but one useful tip is to routinely ask yourself the “surprise” question: “Would you be surprised if this patient were to die within the next 6 months?”.16 We have developed this hospice information visit practice to ensure that hospice is brought up as part of a natural transition to end-of-life care. We have not formally tested the tool, but every oncologist we know who has adopted the practice has continued it.
If the answer to the “surprise” question is, “No, I would not be surprised if the patient were to die within 6 months,” then we will make a referral to hospice for a hospice information visit. This allows for a timely, carefully planned transition to a known team, working with the oncologist, at some reasonably predictable point on the illness trajectory, usually while the patient is still on treatment. This transition can be difficult for patients and their families, and often they will voice concerns about feeling abandoned by the treating oncologist: “Dr Smith took care of us for seven years, and now – when Mom is the sickest and likely dying – he is sending us to someone whom we have never met before.” The timing of this transition is important, because the tendency is to delay for as long as possible, as demonstrated in a study by O’Connor and colleagues of admissions to hospice, which found that 16% of cancer patients were on hospice for 3 days or less.17
After we have discussed hospice care with the patient, we ask the hospice team to call the patient to set up the hospice information visit. We have been surprised to see that most patients, after the initial shock of talking about hospice care, have indicated that they found this planned transition visit with the hospice team informative and helpful.
One way in which you could broach the topic of hospice care with a patient to avoid the feelings of abandonment, is to say, “I want you to meet the people who will be helping me take care of you if and when we need them.” This emphasizes the continuity of care and the desire for a smooth, planned transition. We try to do this when we think the patient has about 6 months to live. Then, when the patient’s performance status changes or the disease progresses, we will activate the referral and will say, “Remember nurse Bob and the social worker Clare who came out to your house 3 months ago? I think it’s time we touch base with them again. It’s time to change goals from fighting the cancer to maintaining your quality of life. In my experience, hospice support will help us do just that.”
Adelson and colleagues developed standardized criteria (triggers or prompts) for use in palliative care consultation to help improve overall quality of care.18 The criteria included any solid tumor patient with: stage IV solid malignancy or stage III lung or pancreatic cancer; a hospitalization of >7 days; hospitalized in the last 30 days (not including routine chemotherapy); and uncontrolled symptoms (pain, nausea/vomiting, dyspnea, delirium, psychological distress). The investigators showed that use of the standardized criteria for palliative care consultation was associated with a decline in 30-day readmission rates (35% for the intervention group, 13% for the control group), the use of chemotherapy after discharge (18% and 44%, respectively), and use of support services after discharge.
Discussion
We see this approach with GoC discussions as part of our TEAM approach (Time, Education, Assessment, and Management) to care19 that is patient and family centered, education centered, and symptom centered. Other institutions where similar prompt systems have been used have also shown improvements in advance care planning. Temel and colleagues found in a 2010 retrospective review of the EMRs and longitudinal medical records of 2,498 patients with metastatic cancer that only 20% patients had a documented code status, despite their advanced disease state.20 A second study at the same institution, also by Temel and colleagues, showed that during 2009-2011, e-mail prompts encouraging physicians to document their patients’ code status resulted in a doubling of the rate of code status documentation, from 14.5% for historical controls to 33.7% after introducing the prompt system.21
In a study of a disease-management pilot program in Medicare patients with cancer, US Oncology adopted the best practice model of appointing someone in the practice, usually a nurse, to review advance care planning within the first visits of diagnosis of a life-limiting illness, and increased advance care planning to more than 80%.22 In another US Oncology study, Neubauer and colleagues reported that the implementation of an ACP process at 38 member sites resulted in a 15.6% increase in the incidence of code status documentation, and although the incidence of documentation varied considerably, it was as high as 89% at some sites.23
For how long should a terminally ill cancer patient be enrolled in hospice? Von Gunten has suggested increasing length of stay (LoS) in hospice as a quality improvement task. He reported on a study in which oncologists in Ohio were given the LoS recommendations from the state’s Oncology Clinical Guidance Council (LoS, 45-90 days), the national LoS average (43 days), and their peers (19.7 days) at baseline, including a chart showing the median LoS by oncologist. Follow-up with the medical oncologist after a year, showed that there was a doubling of hospice LoS, from the baseline 19.7 days to 39.6 days.24
Patients who have timely end-of-life discussions addressing GoC and understanding of their illness, are more likely to be satisfied with their quality of care, receive care that is closer to their stated preferences, and die at the place of their choosing, and their family members will be less distressed.25 In addition, Enzinger and colleagues have shown that patients who had prognostic discussions with their oncologists revised their self-reported estimates of their survival downward by 17.2 months, which brought them closer to a more realistic expectation of life expectancy, without having a negative impact on their emotional well-being (sadness, anxiety) or relationship with the physician.26 However, it is important to remember, that we, as the oncologist, have to start the GoC, hospice, and EoL conversations, because patients understandably rarely bring it up of their own free will.
1. Bernacki RE, Block SD; American College of Physicians High Value Care Task Force. Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;174(12):1994-2003.
2. Weeks JC, Catalano PJ, Cronin A, et al. Patients’ expectations about effects of chemotherapy for advanced cancer. N Engl J Med. 2012;367(17):1616-25.
3. Chen AB, Cronin A, Weeks JC, et al. Expectations about the effectiveness of radiation therapy among patients with incurable lung cancer. J Clin Oncol. 2013;31(21):2730-2735.
4. Kim Y, Winner M, Page A, et al. Patient perceptions regarding the likelihood of cure after surgical resection of lung and colorectal cancer. Cancer. 2015;121(20):3564-3573.
5. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673.
6. O’Connor NR, Moyer ME, Behta M, Casarett DJ. The impact of inpatient palliative care consultations on 30-day hospital readmissions. J Palliat Med. 2015;18(11):956-961.
7. Ahluwalia SC, Tisnado DM, Walling AM, et al. Association of early patient-physician care planning discussions and end-of-life care intensity in advanced cancer. J Palliat Med. 2015;18(10):834-841.
8. Sinuff T, Dodek P, You JJ, et al. Improving end-of-life communication and decision making: the development of a conceptual framework and quality indicators. J Pain Symptom Manag. 2015;49:1070-1080.
9. [Behind paywall] Jacobsen J, Jackson V, Dahlin C, et al. Components of early outpatient palliative care consultation in patients with metastatic nonsmall cell lung cancer. J Palliat Med. 2011;14(4):459-464.
10. [Behind paywall] Jorgenson A, Sidebottom AC, Richards H, Kirven J. A description of inpatient palliative care actions for patients with acute heart failure. Am J Hosp Palliat Care. 2016;33(9):863-870.
11. Smith TJ, Desch CE, Hackney MH, Shaw JE. How long does it take to get a ‘do not resuscitate’ order? J Palliat Care. 1997;13(1):5-8. [Available only as PDF on interlibrary loan.]
12. Leong M, Shah M, Smith TJ. How to avoid late chemotherapy. J Oncol Pract. 2016;12(12):1208-1210.
13. [Behind paywall] Spencer JC, Wheeler SB. A systematic review of motivational interviewing interventions in cancer patients and survivors. Patient Educ Couns. 2016;99(7):1099-1105.
14. [Behind paywall] Jackson VA, Jacobsen J, Greer JA, Pirl WF, Temel JS, Back AL. The cultivation of prognostic awareness through the provision of early palliative care in the ambulatory setting: a communication guide. J Palliat Med. 2013;16(8):894-900.
15. [Behind paywall] Singh S, Cortez D, Maynard D, Cleary JF, DuBenske L, Campbell TC. Characterizing the nature of scan results discussions: insights into why patients misunderstand their prognosis. J Oncol Pract. 2017;13(3):e231-e239.
16. [Behind paywall] Gómez-Batiste X, Martínez-Muñoz M, Blay C, et al. Utility of the NECPAL CCOMS-ICO© tool and the Surprise Question as screening tools for early palliative care and to predict mortality in patients with advanced chronic conditions: A cohort study. http://journals.sagepub.com/doi/abs/10.1177/0269216316676647. Published online November 4, 2016. Accessed August 4, 2017.
17. O’Connor NR, Hu R, Harris PS, Ache K, Casarett DJ. Hospice admissions for cancer in the final days of life: independent predictors and implications for quality measures. J Clin Oncol. 2014;32(28):3184-3179.
18. [Behind paywall] Adelson K, Paris J, Horton JR, et al. Standardized criteria for palliative care consultation on a solid tumor oncology service reduces downstream health care use. J Oncol Pract. 2017;13(5):e431-e440.
19. Bakitas MA, El-Jawahri A, Farquhar M, et al. The TEAM approach to improving oncology outcomes by incorporating palliative care in practice. J Onc Pract. In press.
20. Temel JS, Greer JA, Admane S, et al. Code status documentation in the outpatient electronic medical records of patients with metastatic cancer. J Gen Intern Med. 2010;25(2):150-153.
21. Temel JS, Greer JA, Gallagher ER, et al. Electronic prompt to improve outpatient code status documentation for patients with advanced lung cancer. J Clin Oncol. 2013;31(6):710-715.
22. Neubauer MA, Hoverman RA, Jameson M, et al. A disease management pilot program in a Medicare-age population with cancer [abstract 6505]. http://ascopubs.org/doi/abs/10.1200/JCO.2016.34.15_suppl.6505. Published May 2016. Accessed August 4, 2016.
23. Neubauer MA, Taniguchi CB, Hoverman JR. Improving incidence of code status documentation through process and discipline. J Oncol Pract. 2015;11(2):e263-e266.
24. Von Gunten CF. A quality improvement approach to oncologist referrals for hospice care. http://ascopubs.org/doi/abs/10.1200/jco.2016.34.26_suppl.4. Published October 2016. Accessed August 4, 2017.
25. Kumar P, Temel JS. End-of-life care discussions in patients with advanced cancer. J Clin Oncol. 2013;31(27):3315-3319.
26. Enzinger AC, Zhang B, Schrag D, Prigerson HG. Outcomes of prognostic disclosure: associations with prognostic understanding, distress, and relationship with physician among patients with advanced cancer. J Clin Oncol. 2015;33(32):3809-3816.
27. Varon J, Walsh GL, Marik PE, Fromm RE. Should a cancer patient be resuscitated following an in-hospital cardiac arrest? Resuscitation. 1998;36(3):165-168.
28. Wallace S, Ewer MS, Price KJ, Feeley TW. Outcome and cost implications of cardiopulmonary resuscitation in the medical intensive care unit of a comprehensive cancer center. Support Care Cancer. 2002;10(5):425-429.
Goals-of-care conversations led by the oncologist are a key opportunity to improve advance care planning and end-of-life care,1 but our patients are not understanding the essentials. Findings from a study of patients’ expectations about chemotherapy showed that more than two-thirds of patients with lung or colorectal cancers thought their palliative chemotherapy,2 radiation, 3 and/or surgery4 could cure them. Failure to effectively educate patients can lead to end-of-life care associated with poor quality of care, including over-aggressive care, poor quality of life with suboptimal symptom management, caregiver distress, and other potentially preventable problems.5
Palliative care routinely includes goals-of-care (GoC) discussions as part of the standard note. At one large urban hospital, the 30-day readmission rate was 10% if palliative care consultation was done, compared with 15% if no consultation was obtained.6 Patients who had consultations that included GoC discussions in addition to a symptom management consultation had a lower hospital readmission rate of 5%, compared with 15% in patients who received symptom management consultations alone (adjusted odds ratio [AOR], 0.36; confidence interval [CI], 0.27-0.48; P < .001). 6 Findings from another study showed that the use of aggressive end-of-life care was reduced by one-third when the patient and provider had a GoC planning session close to the time of diagnosis, instead of at the end of life.7
We prepared a template to be incorporated into the electronic medical record (EMR) to facilitate GoC discussions between the oncologist and patient as part of a randomized phase 3 trial of early versus delayed palliative care for phase 1 clinical trial patients. Documentation is one of the most important elements of efforts to improve end-of-life care, along with advance care planning, GoC discussions, and understanding the system of care.8 Because we have found this approach useful in our everyday oncology practice, we are sharing this simple, doable template in the hope that others will find it effective.
The GoC template
What does it look like?
We developed a document for oncology GoC discussions that was incorporated into the EMR (Table). We use the GoC document in much the same way we’d review a computed-tomography scan with our patients and their families: we bring it up on the computer screen, show them the categories, and type in responses on the allotted spaces on the right-hand side of the document. The input can be done in real time or after the conversation, or even after the patient has left. In our practice, we access the Patient Instructions part of the EMR, use a SmartPhrase to copy the Word template into the chart, and start typing.
The completed GoC sheet can be used in several ways. Most commonly, we print it out for the patient and family, so that everyone has access to the same information. Printing it out as part of the after-visit patient summary also satisfies the meaningful use requirement for EMRs. It is also possible to cut and paste the document into a letter to the care team, or directly into the Progress Note, so that it will be available for members of the care team to see.
The GoC form does take some time to complete. Palliative care teams that have reported on what was done during the palliative care visit have noted that the initial and subsequent visits took about an hour, with about 20 minutes devoted to symptom management, 15 minutes to patient and family coping, and 10 minutes to illness understanding and education, including prognosis.9,10 In our own practice, we do questions 1-9 first (Table, shaded area) because it takes less time than anticipated, just like code status discussions,11 and it is much easier with a script.
We use this template for in- and out-patient consultations and for routine visits when a GoC discussion is warranted. It is important to remember that doing this counts as advance care planning and can be billed using the ACP codes.
How do you use template?
It is critically important to make sure that the patient and family are ready for this discussion. To further facilitate the discussion, we have devised a temporary palliative care tattoo with a script, or prompts, for what questions to ask and in what order they should be asked (Figure). The easy-to-read tattoo is worn on the inner forearm so that it is readily visible to the oncologist or advanced practice nurse.12
We always start by asking, “How do you like to get medical information?” (Table, question 1) and follow up with something like, “Are you the sort of person who wants all the details, or not?” (Table). If the response is yes, they want all the details, then we follow up with another question, “Does that include talking about prognosis and what might happen?” (Table). Most patients will want full disclosure of their circumstances and prognosis, but some will not, and will feel overwhelmed and disempowered if you proceed.
After reviewing the answers to question 1 and any follow-up questions you may have asked, you will be able to gauge whether you should continue with questions 2-4 in the template. Once you know more about the patient and the family’s understanding of the situation (Table, question 2), what is important to them (question 3), what they are hoping for (question 4), and have spoken to them about disease progression, recapped their treatment to date, and checked to see if they might be eligible for any clinical trials (question 5), it will be easier to move on to the next questions, about progressive disease and advance care planning: “You are doing OK now, but have you thought about a time when you could be sicker [and need] a living will or advance directive [question 6, see next section of this article]?” For people unwilling to have this discussion, or have it at that moment, there is an excellent article that outlines the process to help practitioners increase prognostic awareness.14 Patient readiness will change over time as they adjust to the life changes forced by serious illness, and one can put off the discussion until they are more accepting. Just remember that patients are not likely to broach the subject themselves, and part of our job is to offer guidance.
As Singh and colleagues have noted, many patients with incurable disease have poor “prognosis awareness,” 15 so it is important for the oncologist to have a GoC conversation with the patient to be able to guage the patient’s understanding of the prognosis after a scan that shows progressive disease. Singh and his colleagues reported that of 64 taped oncologist-patient conversations about scan results, only 4 included frank discussions about prognosis. The authors suggested asking the question, “Would you like to talk about what this means?” after showing the patient the scan to allow the patient some control and to get permission to disclose crucial information based on the reading of the scan.15
Getting started on the GoC discussions may be the hardest part. Some useful introductory lines might include: “In my experience, it is easier to talk about our goals of care while people are still doing well. I know the future can be more uncertain. That’s why I want us to discuss these things now,” or “I am worried about you, after looking at these scans. I think it is time to have another discussion about what the future holds.”
Starting the hospice information visit
For patients with progressive disease, how do you know when to initiate a discussion about hospice and specifically, the hospice information visit, in a timely manner? It turns out oncologists are fairly adept at sensing when the prognosis has changed, but one useful tip is to routinely ask yourself the “surprise” question: “Would you be surprised if this patient were to die within the next 6 months?”.16 We have developed this hospice information visit practice to ensure that hospice is brought up as part of a natural transition to end-of-life care. We have not formally tested the tool, but every oncologist we know who has adopted the practice has continued it.
If the answer to the “surprise” question is, “No, I would not be surprised if the patient were to die within 6 months,” then we will make a referral to hospice for a hospice information visit. This allows for a timely, carefully planned transition to a known team, working with the oncologist, at some reasonably predictable point on the illness trajectory, usually while the patient is still on treatment. This transition can be difficult for patients and their families, and often they will voice concerns about feeling abandoned by the treating oncologist: “Dr Smith took care of us for seven years, and now – when Mom is the sickest and likely dying – he is sending us to someone whom we have never met before.” The timing of this transition is important, because the tendency is to delay for as long as possible, as demonstrated in a study by O’Connor and colleagues of admissions to hospice, which found that 16% of cancer patients were on hospice for 3 days or less.17
After we have discussed hospice care with the patient, we ask the hospice team to call the patient to set up the hospice information visit. We have been surprised to see that most patients, after the initial shock of talking about hospice care, have indicated that they found this planned transition visit with the hospice team informative and helpful.
One way in which you could broach the topic of hospice care with a patient to avoid the feelings of abandonment, is to say, “I want you to meet the people who will be helping me take care of you if and when we need them.” This emphasizes the continuity of care and the desire for a smooth, planned transition. We try to do this when we think the patient has about 6 months to live. Then, when the patient’s performance status changes or the disease progresses, we will activate the referral and will say, “Remember nurse Bob and the social worker Clare who came out to your house 3 months ago? I think it’s time we touch base with them again. It’s time to change goals from fighting the cancer to maintaining your quality of life. In my experience, hospice support will help us do just that.”
Adelson and colleagues developed standardized criteria (triggers or prompts) for use in palliative care consultation to help improve overall quality of care.18 The criteria included any solid tumor patient with: stage IV solid malignancy or stage III lung or pancreatic cancer; a hospitalization of >7 days; hospitalized in the last 30 days (not including routine chemotherapy); and uncontrolled symptoms (pain, nausea/vomiting, dyspnea, delirium, psychological distress). The investigators showed that use of the standardized criteria for palliative care consultation was associated with a decline in 30-day readmission rates (35% for the intervention group, 13% for the control group), the use of chemotherapy after discharge (18% and 44%, respectively), and use of support services after discharge.
Discussion
We see this approach with GoC discussions as part of our TEAM approach (Time, Education, Assessment, and Management) to care19 that is patient and family centered, education centered, and symptom centered. Other institutions where similar prompt systems have been used have also shown improvements in advance care planning. Temel and colleagues found in a 2010 retrospective review of the EMRs and longitudinal medical records of 2,498 patients with metastatic cancer that only 20% patients had a documented code status, despite their advanced disease state.20 A second study at the same institution, also by Temel and colleagues, showed that during 2009-2011, e-mail prompts encouraging physicians to document their patients’ code status resulted in a doubling of the rate of code status documentation, from 14.5% for historical controls to 33.7% after introducing the prompt system.21
In a study of a disease-management pilot program in Medicare patients with cancer, US Oncology adopted the best practice model of appointing someone in the practice, usually a nurse, to review advance care planning within the first visits of diagnosis of a life-limiting illness, and increased advance care planning to more than 80%.22 In another US Oncology study, Neubauer and colleagues reported that the implementation of an ACP process at 38 member sites resulted in a 15.6% increase in the incidence of code status documentation, and although the incidence of documentation varied considerably, it was as high as 89% at some sites.23
For how long should a terminally ill cancer patient be enrolled in hospice? Von Gunten has suggested increasing length of stay (LoS) in hospice as a quality improvement task. He reported on a study in which oncologists in Ohio were given the LoS recommendations from the state’s Oncology Clinical Guidance Council (LoS, 45-90 days), the national LoS average (43 days), and their peers (19.7 days) at baseline, including a chart showing the median LoS by oncologist. Follow-up with the medical oncologist after a year, showed that there was a doubling of hospice LoS, from the baseline 19.7 days to 39.6 days.24
Patients who have timely end-of-life discussions addressing GoC and understanding of their illness, are more likely to be satisfied with their quality of care, receive care that is closer to their stated preferences, and die at the place of their choosing, and their family members will be less distressed.25 In addition, Enzinger and colleagues have shown that patients who had prognostic discussions with their oncologists revised their self-reported estimates of their survival downward by 17.2 months, which brought them closer to a more realistic expectation of life expectancy, without having a negative impact on their emotional well-being (sadness, anxiety) or relationship with the physician.26 However, it is important to remember, that we, as the oncologist, have to start the GoC, hospice, and EoL conversations, because patients understandably rarely bring it up of their own free will.
Goals-of-care conversations led by the oncologist are a key opportunity to improve advance care planning and end-of-life care,1 but our patients are not understanding the essentials. Findings from a study of patients’ expectations about chemotherapy showed that more than two-thirds of patients with lung or colorectal cancers thought their palliative chemotherapy,2 radiation, 3 and/or surgery4 could cure them. Failure to effectively educate patients can lead to end-of-life care associated with poor quality of care, including over-aggressive care, poor quality of life with suboptimal symptom management, caregiver distress, and other potentially preventable problems.5
Palliative care routinely includes goals-of-care (GoC) discussions as part of the standard note. At one large urban hospital, the 30-day readmission rate was 10% if palliative care consultation was done, compared with 15% if no consultation was obtained.6 Patients who had consultations that included GoC discussions in addition to a symptom management consultation had a lower hospital readmission rate of 5%, compared with 15% in patients who received symptom management consultations alone (adjusted odds ratio [AOR], 0.36; confidence interval [CI], 0.27-0.48; P < .001). 6 Findings from another study showed that the use of aggressive end-of-life care was reduced by one-third when the patient and provider had a GoC planning session close to the time of diagnosis, instead of at the end of life.7
We prepared a template to be incorporated into the electronic medical record (EMR) to facilitate GoC discussions between the oncologist and patient as part of a randomized phase 3 trial of early versus delayed palliative care for phase 1 clinical trial patients. Documentation is one of the most important elements of efforts to improve end-of-life care, along with advance care planning, GoC discussions, and understanding the system of care.8 Because we have found this approach useful in our everyday oncology practice, we are sharing this simple, doable template in the hope that others will find it effective.
The GoC template
What does it look like?
We developed a document for oncology GoC discussions that was incorporated into the EMR (Table). We use the GoC document in much the same way we’d review a computed-tomography scan with our patients and their families: we bring it up on the computer screen, show them the categories, and type in responses on the allotted spaces on the right-hand side of the document. The input can be done in real time or after the conversation, or even after the patient has left. In our practice, we access the Patient Instructions part of the EMR, use a SmartPhrase to copy the Word template into the chart, and start typing.
The completed GoC sheet can be used in several ways. Most commonly, we print it out for the patient and family, so that everyone has access to the same information. Printing it out as part of the after-visit patient summary also satisfies the meaningful use requirement for EMRs. It is also possible to cut and paste the document into a letter to the care team, or directly into the Progress Note, so that it will be available for members of the care team to see.
The GoC form does take some time to complete. Palliative care teams that have reported on what was done during the palliative care visit have noted that the initial and subsequent visits took about an hour, with about 20 minutes devoted to symptom management, 15 minutes to patient and family coping, and 10 minutes to illness understanding and education, including prognosis.9,10 In our own practice, we do questions 1-9 first (Table, shaded area) because it takes less time than anticipated, just like code status discussions,11 and it is much easier with a script.
We use this template for in- and out-patient consultations and for routine visits when a GoC discussion is warranted. It is important to remember that doing this counts as advance care planning and can be billed using the ACP codes.
How do you use template?
It is critically important to make sure that the patient and family are ready for this discussion. To further facilitate the discussion, we have devised a temporary palliative care tattoo with a script, or prompts, for what questions to ask and in what order they should be asked (Figure). The easy-to-read tattoo is worn on the inner forearm so that it is readily visible to the oncologist or advanced practice nurse.12
We always start by asking, “How do you like to get medical information?” (Table, question 1) and follow up with something like, “Are you the sort of person who wants all the details, or not?” (Table). If the response is yes, they want all the details, then we follow up with another question, “Does that include talking about prognosis and what might happen?” (Table). Most patients will want full disclosure of their circumstances and prognosis, but some will not, and will feel overwhelmed and disempowered if you proceed.
After reviewing the answers to question 1 and any follow-up questions you may have asked, you will be able to gauge whether you should continue with questions 2-4 in the template. Once you know more about the patient and the family’s understanding of the situation (Table, question 2), what is important to them (question 3), what they are hoping for (question 4), and have spoken to them about disease progression, recapped their treatment to date, and checked to see if they might be eligible for any clinical trials (question 5), it will be easier to move on to the next questions, about progressive disease and advance care planning: “You are doing OK now, but have you thought about a time when you could be sicker [and need] a living will or advance directive [question 6, see next section of this article]?” For people unwilling to have this discussion, or have it at that moment, there is an excellent article that outlines the process to help practitioners increase prognostic awareness.14 Patient readiness will change over time as they adjust to the life changes forced by serious illness, and one can put off the discussion until they are more accepting. Just remember that patients are not likely to broach the subject themselves, and part of our job is to offer guidance.
As Singh and colleagues have noted, many patients with incurable disease have poor “prognosis awareness,” 15 so it is important for the oncologist to have a GoC conversation with the patient to be able to guage the patient’s understanding of the prognosis after a scan that shows progressive disease. Singh and his colleagues reported that of 64 taped oncologist-patient conversations about scan results, only 4 included frank discussions about prognosis. The authors suggested asking the question, “Would you like to talk about what this means?” after showing the patient the scan to allow the patient some control and to get permission to disclose crucial information based on the reading of the scan.15
Getting started on the GoC discussions may be the hardest part. Some useful introductory lines might include: “In my experience, it is easier to talk about our goals of care while people are still doing well. I know the future can be more uncertain. That’s why I want us to discuss these things now,” or “I am worried about you, after looking at these scans. I think it is time to have another discussion about what the future holds.”
Starting the hospice information visit
For patients with progressive disease, how do you know when to initiate a discussion about hospice and specifically, the hospice information visit, in a timely manner? It turns out oncologists are fairly adept at sensing when the prognosis has changed, but one useful tip is to routinely ask yourself the “surprise” question: “Would you be surprised if this patient were to die within the next 6 months?”.16 We have developed this hospice information visit practice to ensure that hospice is brought up as part of a natural transition to end-of-life care. We have not formally tested the tool, but every oncologist we know who has adopted the practice has continued it.
If the answer to the “surprise” question is, “No, I would not be surprised if the patient were to die within 6 months,” then we will make a referral to hospice for a hospice information visit. This allows for a timely, carefully planned transition to a known team, working with the oncologist, at some reasonably predictable point on the illness trajectory, usually while the patient is still on treatment. This transition can be difficult for patients and their families, and often they will voice concerns about feeling abandoned by the treating oncologist: “Dr Smith took care of us for seven years, and now – when Mom is the sickest and likely dying – he is sending us to someone whom we have never met before.” The timing of this transition is important, because the tendency is to delay for as long as possible, as demonstrated in a study by O’Connor and colleagues of admissions to hospice, which found that 16% of cancer patients were on hospice for 3 days or less.17
After we have discussed hospice care with the patient, we ask the hospice team to call the patient to set up the hospice information visit. We have been surprised to see that most patients, after the initial shock of talking about hospice care, have indicated that they found this planned transition visit with the hospice team informative and helpful.
One way in which you could broach the topic of hospice care with a patient to avoid the feelings of abandonment, is to say, “I want you to meet the people who will be helping me take care of you if and when we need them.” This emphasizes the continuity of care and the desire for a smooth, planned transition. We try to do this when we think the patient has about 6 months to live. Then, when the patient’s performance status changes or the disease progresses, we will activate the referral and will say, “Remember nurse Bob and the social worker Clare who came out to your house 3 months ago? I think it’s time we touch base with them again. It’s time to change goals from fighting the cancer to maintaining your quality of life. In my experience, hospice support will help us do just that.”
Adelson and colleagues developed standardized criteria (triggers or prompts) for use in palliative care consultation to help improve overall quality of care.18 The criteria included any solid tumor patient with: stage IV solid malignancy or stage III lung or pancreatic cancer; a hospitalization of >7 days; hospitalized in the last 30 days (not including routine chemotherapy); and uncontrolled symptoms (pain, nausea/vomiting, dyspnea, delirium, psychological distress). The investigators showed that use of the standardized criteria for palliative care consultation was associated with a decline in 30-day readmission rates (35% for the intervention group, 13% for the control group), the use of chemotherapy after discharge (18% and 44%, respectively), and use of support services after discharge.
Discussion
We see this approach with GoC discussions as part of our TEAM approach (Time, Education, Assessment, and Management) to care19 that is patient and family centered, education centered, and symptom centered. Other institutions where similar prompt systems have been used have also shown improvements in advance care planning. Temel and colleagues found in a 2010 retrospective review of the EMRs and longitudinal medical records of 2,498 patients with metastatic cancer that only 20% patients had a documented code status, despite their advanced disease state.20 A second study at the same institution, also by Temel and colleagues, showed that during 2009-2011, e-mail prompts encouraging physicians to document their patients’ code status resulted in a doubling of the rate of code status documentation, from 14.5% for historical controls to 33.7% after introducing the prompt system.21
In a study of a disease-management pilot program in Medicare patients with cancer, US Oncology adopted the best practice model of appointing someone in the practice, usually a nurse, to review advance care planning within the first visits of diagnosis of a life-limiting illness, and increased advance care planning to more than 80%.22 In another US Oncology study, Neubauer and colleagues reported that the implementation of an ACP process at 38 member sites resulted in a 15.6% increase in the incidence of code status documentation, and although the incidence of documentation varied considerably, it was as high as 89% at some sites.23
For how long should a terminally ill cancer patient be enrolled in hospice? Von Gunten has suggested increasing length of stay (LoS) in hospice as a quality improvement task. He reported on a study in which oncologists in Ohio were given the LoS recommendations from the state’s Oncology Clinical Guidance Council (LoS, 45-90 days), the national LoS average (43 days), and their peers (19.7 days) at baseline, including a chart showing the median LoS by oncologist. Follow-up with the medical oncologist after a year, showed that there was a doubling of hospice LoS, from the baseline 19.7 days to 39.6 days.24
Patients who have timely end-of-life discussions addressing GoC and understanding of their illness, are more likely to be satisfied with their quality of care, receive care that is closer to their stated preferences, and die at the place of their choosing, and their family members will be less distressed.25 In addition, Enzinger and colleagues have shown that patients who had prognostic discussions with their oncologists revised their self-reported estimates of their survival downward by 17.2 months, which brought them closer to a more realistic expectation of life expectancy, without having a negative impact on their emotional well-being (sadness, anxiety) or relationship with the physician.26 However, it is important to remember, that we, as the oncologist, have to start the GoC, hospice, and EoL conversations, because patients understandably rarely bring it up of their own free will.
1. Bernacki RE, Block SD; American College of Physicians High Value Care Task Force. Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;174(12):1994-2003.
2. Weeks JC, Catalano PJ, Cronin A, et al. Patients’ expectations about effects of chemotherapy for advanced cancer. N Engl J Med. 2012;367(17):1616-25.
3. Chen AB, Cronin A, Weeks JC, et al. Expectations about the effectiveness of radiation therapy among patients with incurable lung cancer. J Clin Oncol. 2013;31(21):2730-2735.
4. Kim Y, Winner M, Page A, et al. Patient perceptions regarding the likelihood of cure after surgical resection of lung and colorectal cancer. Cancer. 2015;121(20):3564-3573.
5. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673.
6. O’Connor NR, Moyer ME, Behta M, Casarett DJ. The impact of inpatient palliative care consultations on 30-day hospital readmissions. J Palliat Med. 2015;18(11):956-961.
7. Ahluwalia SC, Tisnado DM, Walling AM, et al. Association of early patient-physician care planning discussions and end-of-life care intensity in advanced cancer. J Palliat Med. 2015;18(10):834-841.
8. Sinuff T, Dodek P, You JJ, et al. Improving end-of-life communication and decision making: the development of a conceptual framework and quality indicators. J Pain Symptom Manag. 2015;49:1070-1080.
9. [Behind paywall] Jacobsen J, Jackson V, Dahlin C, et al. Components of early outpatient palliative care consultation in patients with metastatic nonsmall cell lung cancer. J Palliat Med. 2011;14(4):459-464.
10. [Behind paywall] Jorgenson A, Sidebottom AC, Richards H, Kirven J. A description of inpatient palliative care actions for patients with acute heart failure. Am J Hosp Palliat Care. 2016;33(9):863-870.
11. Smith TJ, Desch CE, Hackney MH, Shaw JE. How long does it take to get a ‘do not resuscitate’ order? J Palliat Care. 1997;13(1):5-8. [Available only as PDF on interlibrary loan.]
12. Leong M, Shah M, Smith TJ. How to avoid late chemotherapy. J Oncol Pract. 2016;12(12):1208-1210.
13. [Behind paywall] Spencer JC, Wheeler SB. A systematic review of motivational interviewing interventions in cancer patients and survivors. Patient Educ Couns. 2016;99(7):1099-1105.
14. [Behind paywall] Jackson VA, Jacobsen J, Greer JA, Pirl WF, Temel JS, Back AL. The cultivation of prognostic awareness through the provision of early palliative care in the ambulatory setting: a communication guide. J Palliat Med. 2013;16(8):894-900.
15. [Behind paywall] Singh S, Cortez D, Maynard D, Cleary JF, DuBenske L, Campbell TC. Characterizing the nature of scan results discussions: insights into why patients misunderstand their prognosis. J Oncol Pract. 2017;13(3):e231-e239.
16. [Behind paywall] Gómez-Batiste X, Martínez-Muñoz M, Blay C, et al. Utility of the NECPAL CCOMS-ICO© tool and the Surprise Question as screening tools for early palliative care and to predict mortality in patients with advanced chronic conditions: A cohort study. http://journals.sagepub.com/doi/abs/10.1177/0269216316676647. Published online November 4, 2016. Accessed August 4, 2017.
17. O’Connor NR, Hu R, Harris PS, Ache K, Casarett DJ. Hospice admissions for cancer in the final days of life: independent predictors and implications for quality measures. J Clin Oncol. 2014;32(28):3184-3179.
18. [Behind paywall] Adelson K, Paris J, Horton JR, et al. Standardized criteria for palliative care consultation on a solid tumor oncology service reduces downstream health care use. J Oncol Pract. 2017;13(5):e431-e440.
19. Bakitas MA, El-Jawahri A, Farquhar M, et al. The TEAM approach to improving oncology outcomes by incorporating palliative care in practice. J Onc Pract. In press.
20. Temel JS, Greer JA, Admane S, et al. Code status documentation in the outpatient electronic medical records of patients with metastatic cancer. J Gen Intern Med. 2010;25(2):150-153.
21. Temel JS, Greer JA, Gallagher ER, et al. Electronic prompt to improve outpatient code status documentation for patients with advanced lung cancer. J Clin Oncol. 2013;31(6):710-715.
22. Neubauer MA, Hoverman RA, Jameson M, et al. A disease management pilot program in a Medicare-age population with cancer [abstract 6505]. http://ascopubs.org/doi/abs/10.1200/JCO.2016.34.15_suppl.6505. Published May 2016. Accessed August 4, 2016.
23. Neubauer MA, Taniguchi CB, Hoverman JR. Improving incidence of code status documentation through process and discipline. J Oncol Pract. 2015;11(2):e263-e266.
24. Von Gunten CF. A quality improvement approach to oncologist referrals for hospice care. http://ascopubs.org/doi/abs/10.1200/jco.2016.34.26_suppl.4. Published October 2016. Accessed August 4, 2017.
25. Kumar P, Temel JS. End-of-life care discussions in patients with advanced cancer. J Clin Oncol. 2013;31(27):3315-3319.
26. Enzinger AC, Zhang B, Schrag D, Prigerson HG. Outcomes of prognostic disclosure: associations with prognostic understanding, distress, and relationship with physician among patients with advanced cancer. J Clin Oncol. 2015;33(32):3809-3816.
27. Varon J, Walsh GL, Marik PE, Fromm RE. Should a cancer patient be resuscitated following an in-hospital cardiac arrest? Resuscitation. 1998;36(3):165-168.
28. Wallace S, Ewer MS, Price KJ, Feeley TW. Outcome and cost implications of cardiopulmonary resuscitation in the medical intensive care unit of a comprehensive cancer center. Support Care Cancer. 2002;10(5):425-429.
1. Bernacki RE, Block SD; American College of Physicians High Value Care Task Force. Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;174(12):1994-2003.
2. Weeks JC, Catalano PJ, Cronin A, et al. Patients’ expectations about effects of chemotherapy for advanced cancer. N Engl J Med. 2012;367(17):1616-25.
3. Chen AB, Cronin A, Weeks JC, et al. Expectations about the effectiveness of radiation therapy among patients with incurable lung cancer. J Clin Oncol. 2013;31(21):2730-2735.
4. Kim Y, Winner M, Page A, et al. Patient perceptions regarding the likelihood of cure after surgical resection of lung and colorectal cancer. Cancer. 2015;121(20):3564-3573.
5. Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673.
6. O’Connor NR, Moyer ME, Behta M, Casarett DJ. The impact of inpatient palliative care consultations on 30-day hospital readmissions. J Palliat Med. 2015;18(11):956-961.
7. Ahluwalia SC, Tisnado DM, Walling AM, et al. Association of early patient-physician care planning discussions and end-of-life care intensity in advanced cancer. J Palliat Med. 2015;18(10):834-841.
8. Sinuff T, Dodek P, You JJ, et al. Improving end-of-life communication and decision making: the development of a conceptual framework and quality indicators. J Pain Symptom Manag. 2015;49:1070-1080.
9. [Behind paywall] Jacobsen J, Jackson V, Dahlin C, et al. Components of early outpatient palliative care consultation in patients with metastatic nonsmall cell lung cancer. J Palliat Med. 2011;14(4):459-464.
10. [Behind paywall] Jorgenson A, Sidebottom AC, Richards H, Kirven J. A description of inpatient palliative care actions for patients with acute heart failure. Am J Hosp Palliat Care. 2016;33(9):863-870.
11. Smith TJ, Desch CE, Hackney MH, Shaw JE. How long does it take to get a ‘do not resuscitate’ order? J Palliat Care. 1997;13(1):5-8. [Available only as PDF on interlibrary loan.]
12. Leong M, Shah M, Smith TJ. How to avoid late chemotherapy. J Oncol Pract. 2016;12(12):1208-1210.
13. [Behind paywall] Spencer JC, Wheeler SB. A systematic review of motivational interviewing interventions in cancer patients and survivors. Patient Educ Couns. 2016;99(7):1099-1105.
14. [Behind paywall] Jackson VA, Jacobsen J, Greer JA, Pirl WF, Temel JS, Back AL. The cultivation of prognostic awareness through the provision of early palliative care in the ambulatory setting: a communication guide. J Palliat Med. 2013;16(8):894-900.
15. [Behind paywall] Singh S, Cortez D, Maynard D, Cleary JF, DuBenske L, Campbell TC. Characterizing the nature of scan results discussions: insights into why patients misunderstand their prognosis. J Oncol Pract. 2017;13(3):e231-e239.
16. [Behind paywall] Gómez-Batiste X, Martínez-Muñoz M, Blay C, et al. Utility of the NECPAL CCOMS-ICO© tool and the Surprise Question as screening tools for early palliative care and to predict mortality in patients with advanced chronic conditions: A cohort study. http://journals.sagepub.com/doi/abs/10.1177/0269216316676647. Published online November 4, 2016. Accessed August 4, 2017.
17. O’Connor NR, Hu R, Harris PS, Ache K, Casarett DJ. Hospice admissions for cancer in the final days of life: independent predictors and implications for quality measures. J Clin Oncol. 2014;32(28):3184-3179.
18. [Behind paywall] Adelson K, Paris J, Horton JR, et al. Standardized criteria for palliative care consultation on a solid tumor oncology service reduces downstream health care use. J Oncol Pract. 2017;13(5):e431-e440.
19. Bakitas MA, El-Jawahri A, Farquhar M, et al. The TEAM approach to improving oncology outcomes by incorporating palliative care in practice. J Onc Pract. In press.
20. Temel JS, Greer JA, Admane S, et al. Code status documentation in the outpatient electronic medical records of patients with metastatic cancer. J Gen Intern Med. 2010;25(2):150-153.
21. Temel JS, Greer JA, Gallagher ER, et al. Electronic prompt to improve outpatient code status documentation for patients with advanced lung cancer. J Clin Oncol. 2013;31(6):710-715.
22. Neubauer MA, Hoverman RA, Jameson M, et al. A disease management pilot program in a Medicare-age population with cancer [abstract 6505]. http://ascopubs.org/doi/abs/10.1200/JCO.2016.34.15_suppl.6505. Published May 2016. Accessed August 4, 2016.
23. Neubauer MA, Taniguchi CB, Hoverman JR. Improving incidence of code status documentation through process and discipline. J Oncol Pract. 2015;11(2):e263-e266.
24. Von Gunten CF. A quality improvement approach to oncologist referrals for hospice care. http://ascopubs.org/doi/abs/10.1200/jco.2016.34.26_suppl.4. Published October 2016. Accessed August 4, 2017.
25. Kumar P, Temel JS. End-of-life care discussions in patients with advanced cancer. J Clin Oncol. 2013;31(27):3315-3319.
26. Enzinger AC, Zhang B, Schrag D, Prigerson HG. Outcomes of prognostic disclosure: associations with prognostic understanding, distress, and relationship with physician among patients with advanced cancer. J Clin Oncol. 2015;33(32):3809-3816.
27. Varon J, Walsh GL, Marik PE, Fromm RE. Should a cancer patient be resuscitated following an in-hospital cardiac arrest? Resuscitation. 1998;36(3):165-168.
28. Wallace S, Ewer MS, Price KJ, Feeley TW. Outcome and cost implications of cardiopulmonary resuscitation in the medical intensive care unit of a comprehensive cancer center. Support Care Cancer. 2002;10(5):425-429.
Prehabilitation for lymphedema in head and neck cancer patients at a community cancer center
Lymphedema is the swelling of tissue caused by the accumulation of interstitial fluid in any area of the body where lymphatic flow has been compromised.1 Secondary lymphedema is an acquired abnormality in lymph drainage1,2 and is the type commonly seen in cancer patients. Secondary lymphedema can be described as external or internal. Internal lymphedema, swelling of deep structures and tissues, is very difficult to quantify.
Lymphedema in patients with head and neck cancers
Lymphedema is a complicating morbidity frequently seen in head and neck cancer patients who have undergone treatment with surgery, radiation, and chemotherapy. However, although it is one of the most prevalent side effects of treatment, it is both under-recognized and under-treated.3
In head and neck cancer patients, internal swelling may develop in the soft tissues of the upper aero-digestive tract,4 affecting articulation and swallowing. Currently, there does not seem to be an effective practical and reliable tool with which to measure internal lymphedema. In addition, it is generally accepted that there is no effective way to treat internal lymphedema. By contrast, external lymphedema is more readily observed, but both subjective and objective assessments are difficult. External swelling may occur in the face, jaw, and neck. However, the subjective scales currently available are insufficient to capture very important characteristics of external lymphedema.5 The Edge Task Force on Head and Neck Cancer in 2015 was not able to recommend any outcome measures for objectively quantifying external edema.6 Furthermore, objective measurements of head and neck lymphedema can be expensive and time consuming.
Extent and risk
A combination of both internal and external swelling is seen in more than 50% of patients.7 Risk factors include “throat” tumors, multicancer treatment approaches, higher total radiation dose, a greater number of radiation procedures, and radiation at the surgical site.5 More than 500,000 survivors of head and neck cancer in the United States are at risk of lymphedema.5 Although recent advances in treatment have reduced the incidence of other morbidities, 50% of patients who are treated for head and neck cancer may still develop lymphedema.1,8 The reported incidence in some centers may be much higher, with up to 75% of patients developing lymphedema following treatment.9
Measurement modalities for clinical evaluation
There is little current research into lymphedema of the head and neck, despite the high prevalence of the condition.8 According to Deng and colleagues, measurement of head and neck lymphedema is a challenge, which has an impact on clinical assessment, diagnosis, and treatment of this under-recognized, under-reported and under-addressed problem in head and neck cancer patients.10 In a review of the literature, Deng and colleagues identified three measurement modalities available for clinical evaluation: patient-reported outcomes, clinician-reported outcomes, and technology.10 One major factor, though, in detecting lymphedema, is physician awareness: physicians, health care professionals, and even some lymphedema therapists are not well educated about this problem.8
Treatment
The effectiveness of traditional lymphedema treatment is not well defined.8 Currently, complete decongestive therapy (CDT), is considered the standard of care for lymphedema. The National Lymphedema Network has stated that modifications of CDT, especially manual lymphatic drainage and modified compressive garments for external lymphedema, have been shown to be beneficial for the treatment of lymphedema in head and neck cancer patients.11 Most findings in lymphedema research, mainly in breast cancer patients, have shown that early intervention is the best management and yields the best outcomes. As with other chronic conditions, early identification and timely, appropriate treatment of lymphedema is critical to improve clinical outcomes, to decrease symptom burden and functional impairment, and to improve overall quality of life in head and neck cancer patients.10
Improving recognition and treatment
Head and neck oncologic treatment is increasingly offered outside the network of specialist academic hospitals, at hospitals serving more localized communities where the neediest, sickest patient groups may be receiving less than optimal care.3 This challenges community hospitals to provide optimal treatment, similar to that being offered at nationally recognized institutions. In January 2012, we implemented a prehabilitation program in our community hospital cancer center to provide early intervention for our patients based on the understanding that proper and prompt treatment for patients with early signs of lymphedema should be a priority.12 In this article, we outline how we implemented the program and the describe improvements we observed before and after the implementation of the program.
The prehabilitation program
The role of the nurse navigator
Before the introduction of the prehabilitation program, our pattern of practice was to refer patients to oncology rehabilitation for lymphedema management after they had completed their medical treatment with surgery, radiation, and chemotherapy. In 2012, that was changed to a prehabilitation model of care that was overseen by a head and neck nurse navigator. This focus on prehabilitation begins with patients being referred to oncology rehabilitation at the time of cancer diagnosis for baseline assessment of head and neck swelling. In addition, there is assessment of the many possible other side effects associated with head and neck cancer and its treatment, namely loss of range of motion of the neck, jaw (trismus), and/or shoulders, postural deficits, functional loss, pain, balance dysfunction with fall risk, weakness, and fatigue. Therapeutic interventions are initiated as needed and appropriate. This process also raises awareness of a condition that has been described as under-recognized and under-treated.3
The nurse navigator sits in on each radiation oncology consultation and aids in “navigating” patients through their treatment. The nurse ensures that each patient is referred to different ancillary services from the outset, such as seeing a dietician, social worker, physical/occupational therapist and certified lymphedema therapist, speech pathologist, and financial assistance advisor, if necessary (Table 1).
Assessment of lymphedema
Measurement of head and neck lymphedema is a challenge.10 In our program, the physical therapy assessment also includes the evaluation of several other morbidities associated with head and neck cancer and its treatment, such as range of motion, weakness, fatigue, radiation fibrosis, balance dysfunction, and risk of falling (Table 2).
Patient-reported outcomes are essential to fully capture observable and unobservable symptoms (eg, sensations) as well as the functional impacts of lymphedema.10 In addition to lymphedema, there are many other morbidities that may be assessed on the basis of patient-reported outcome tools, such as upper extremity function with QuickDASH.13 At our clinic for head and neck cancer patients we use the Neck Disability Index (NDI)14 and Care Connections (CC)15 survey for the patient-reported outcomes. The Quick DASH, NDI, and CC tools all assess standard functional outcomes that are not specific to lymphedema, but are useful in documenting changes related to lymphedema. We initially used the CC survey and later transitioned to using the NDI. Neck pain is common with lymphedema in the head and neck region, and the NDI is a valid, reliable, responsive and internally consistent clinical tool to measure self-reported disability in patients with neck pain.16 These questionnaires were completed by the patients at their initial assessment, at reassessment, and at time of discharge.
Although objective criteria for external lymphedema have not been established, simple measurements such as using a tape measure to record neck circumference, allow a useful longitudinal assessment. Digital photography may be effective in the documentation and subjective evaluation of changes of external lymphedema.10,17 However, there are some limitations with photography because although external photographs (including digital photography and three-dimensional imaging) can capture some features, such as changes in contours, symmetry, and changes in skin quality and color, they do not detect changes in skin and soft tissue texture and compliance (Table 3).10
Impact on clinical outcomes
We retrospectively reviewed the medical records of 230 head and neck cancer patients who had been treated at our center between June 2008 and June 2015. Complete clinical data were available for 190 patients. The following information was extracted from each patient’s chart: whether they developed lymphedema, tumor stage, had surgery, radiation dose, type of chemotherapy given, their smoking history, if they had had a neck dissection and the primary site of the tumor (Table 3).
Incidence in different time periods. Of the 190 patients with complete records 78 (41%) were found to have lymphedema. These were all patients undergoing treatment for head and neck cancer during June 2008-June 2015. The prehabilitation program was initiated with the hiring of a nurse navigator for head and neck cancer, starting in January 2012. It is interesting to note that the incidence of lymphedema was 27% before the program was started, but after nurse navigator joined the team, the incidence increased significantly to 48% (P = .0002), in line with published expectations. This increase in recorded incidence may be attributable to the greater awareness of lymphedema intentionally fostered by the prehabilitation program.
Smoking history. Patients’ lifetime smoking history was retrieved from their medical records, based on their verbal admission of tobacco use. Most of the patients (n = 110) self-reported a history of smoking. Of those with a history of smoking, 36 (33%) developed external lymphedema after treatment for head and neck cancer, and 74 (67%) did not. However, this difference was not statistically significant. Hence, although smoking is a risk factor for head and neck cancer, it was not associated with the development of external lymphedema in our cohort of patients.
Type of tumor
Most of the patients (n = 156, 82%) had squamous cell carcinomas (SCC). Of those, 45% developed external lymphedema and 55% did not. Therefore, having SCC did not predispose to lymphedema. The other cancers were mixed type, mainly adenocaricoma, but their numbers were too small to draw statistical conclusions.
Stage of the tumor
About two thirds of the patients (n = 121, 64%) had stage 3 or 4 cancer. However, treatment of more advanced cancers was not associated with lymphedema development.
Site of the tumor
The literature suggests that patients with a primary tumor in the throat are at increased risk for lymphedema.5 The American Cancer Society has defined cancers of the oropharynx (throat) as including the base of the tongue (back third of the tongue), the soft palate, the tonsils, and the side and back walls of the throat.18 In our head and neck cancer cohort, patients with primary tumors of the oropharnyx were, perhaps, more susceptible to lymphedema (P = .044, Table 3). By contrast, in our cohort of patients, those with nasopharyngeal, hypopharyngeal, and parotid gland tumors were significantly less likely to develop lymphedema (Ps = .017, .04, .012, respectively).
No surgery
Half of our patients (n = 95) were not treated with surgery. In the patients who did not have surgery, 25 (26%) developed lymphedema, whereas 70 (74%) did not. Hence, although the incidence of lymphedema was significantly lower in patients who did not have surgery (P = .015), lymphedema did develop in patients who did not have a surgical procedure.
Resection of primary tumor without neck dissection
Of the 64 patients who had surgery, but without neck dissection, 35 (55%) developed external lymphedema. Compared with the no-surgery patients, the doubling of the incidence (from 26% to 55%) was highly significant (P = .0004). These findings are compatible with the literature reports that surgery increases the incidence of lymphedema, which is not surprising because surgery and subsequent scarring is known to compromise the lymphatic system.
Resection of primary tumor with neck dissection
The incidence of external lymphedema was increased to 69% when patients were subjected to both surgery and neck dissection. Compared with the June 2008-June 2015 cohort, there was a significant increase in the incidence of lymphedema in the neck dissection group (P = .007). Neck dissection involves the removal of lymph nodes and disruption of the lymphatic vessels, so it is not surprising that there is a higher incidence of external lymphedema. In our practice, neck dissections increased in frequency every year from June 2008 until December 2011, when 8 patients underwent neck dissections, 6 (75%) of whom developed lymphedema. Since January 2012, when the prehabilitation program was implemented, the number of neck dissections have declined, with more patients receiving chemoradiation and surgery being reserved for surgery. Hamoir and colleagues have reported that neck dissection is no longer justified unless there is clinically residual disease in the neck.19
Radiation
Lymphedema occurred in patients regardless of the dose of radiation received. Although the incidence of lymphedema seemed to be higher in patients who received more than 60 cGy, that difference was not statistically significant (Table 3). We had expected a relationship between radiation damage and greater lymphedema, but that was not evident in our patients.
Chemotherapy
The majority of patients (n = 131, 69%) received chemotherapy. The exposure to chemotherapy was not correlated with the risk of external lymphedema in our cohort of patients, with 58 of the 131 treated patients (44%) developing lymphedema, compared with 73 (56%) of treated patients who did not (Table 3).
Complete decongestive therapy
All patients with documented lymphedema were evaluated for complete decongestive therapy (CDT). Contraindications to CDT included congestive heart failure, renal failure, acute infection, peripheral artery disease, upper-quadrant deep vein thrombosis, and carotid artery stenosis. Eligible patients were referred to a certified lymphedema therapist for CDT. As the program evolved, patients at risk for lymphedema were referred for CDT early on, usually at the time of diagnosis, to improve early identification and surveillance of lymphedema.
CDT included manual lymph drainage,
Patients’ responses to CDT were documented with digital photographs that were taken at each visit and, more recently, use of the NDI.
Communication and education
The head and neck cancer nurse navigator attends the cancer center’s multidisciplinary head and neck tumor board, which has representation from otolaryngology, diagnostic radiology, pathology, radiation oncology, medical oncology, reconstructive surgery, oncology rehabilitation (physical/occupational therapist), dietary services, speech pathology, social services and clinical research. This regular contact allows for earlier awareness about which patients are at greater risk for developing lymphedema, thus enabling early intervention (and patient education) in a timely manner.
Education of the patient, before cancer therapy, of the risks of lymphedema is very important. Before the implementation of the prehabilitation program, some patients did not fully comprehend what a painful and debilitating consequence of cancer treatment lymphedema could be.
Discussion
We introduced a prehabilitation program to detect and treat lymphedema in head and neck cancer patients in January 2012 part way through following an observation cohort from June2008 through June2015. Central to this, in our center, was the appointment of a nurse navigator whose primary focus was on head and neck cancer patients. We placed a high priority on the early detection and treatment of lymphedema because do so has been associated with better outcomes in other centers.
One immediate consequence of the inception of our program was the identification of more patients with external lymphedema. Our detected incidence rose significantly (P = .0002), from 27% in the period June 2008-December 20112010, before the program, to 48% during the January 2012-June 2015 period, after the inception of the program. This later incidence rate is in line with published incidence rates in most centers. However, it is still somewhat short of the 75% suggested in one center,9 which suggests we are either we are underdetecting lymphedema or there are differences in definition criteria or sensitivity levels for defining lymphedema.
There are currently no specific objective measures of lymphedema, so there is bound to be some variation in diagnosis rates. In our program, we rely heavily on the patient-reported outcome measures, the NDI instrument, and digital photography to detect and monitor lymphedema, starting with the pretreatment baseline values that are established for each patient.
The use of digital photography in our community hospital setting, which includes taking photographs before and after treatment and at each visit, motivates and encourages patients and provides a tool for clinical lymphedema therapists to visually document benefits of treatment. Patients’ motivation and compliance with their established home program for head and neck lymphedema self-management are essential. The elements of the home program may include self-manual lymph drainage, home-modified compression bandaging and garment wear, therapeutic exercises, and skin care. Patients with lymphedema who adhered closely with their therapy program were more than 8 times more likely to improve compared with noncompliant patients.17
Some groups of patients have a greater risk of developing lymphedema than others,5 so the development of an algorithm to predict lymphedema seemed possible. However, in our cohort of patients, only neck dissection, with its disruption of the lymphatic system of the neck, was strongly associated with external lymphedema (Table 3). It is important to note that some patients who did not undergo surgery developed lymphedema. In our patients, high doses of radiation alone did not seem to predispose to lymphedema. That suggests that no group of head and neck cancer patients should be ignored, which is why we did routine screening of all patients before, during, and after treatment.
Our protocol falls short in the detection of internal lymphedema. For example, information on swallowing gathered by our speech pathologists (in a different department) has not, so far, been included in our assessment. This is one opportunity to improve on our approach, especially because speech difficulties may be associated with internal lymphedema. In addition, we are not equipped for the requisite internal examinations. Unfortunately, there are no practical and successful treatments for patients suffering from internal swelling. This represents a challenge for the medical community to better meet this need. Therefore, although we are missing some assessments of internal lymphedema, this is of little therapeutic consequence at this time.
The increase in the detected incidence of external lymphedema points to a practice gap that has been resolved by the appointment of a dedicated nurse navigator who attends oncology reviews to share knowledge and information. Another educational effort has been made with the patients themselves to increase compliance and improve continuous care at home.
There is always room for improvement, however, either by feedback acquired from other institutions and hospitals or through the future introduction of more objective assessment techniques.
Conclusions
The introduction of the prehabilitation program at our center has coincided with a significantly improved detection rate for external lymphedema in head and neck cancer patients. It may be because the program emphasizes education about lymphedema that awareness of the condition has increased throughout the center. It is now widely recognized that all patients are at risk of lymphedema regardless of whether they fall into an acknowledged high-risk group. Our experience shows that there is no significant difference between treatment modalities apart from neck dissection. In our population, the use of this procedure is decreasing. External lymphedema can develop even in patients who do not have surgery. Therefore, there is no sound way to predict which patients are most likely to suffer from the accumulation of fluid in their head and neck after treatment for head and neck cancer. Thus, an assessment as described here, during and after treatment for all patients, is warranted. Patients are now being seen earlier as a part of the prehabilitation program, which facilitates access to complete decongestive treatment at an earlier stage, improves patient outcomes, and increases patient satisfaction with their treatment. Our prehabilitation program could serve as a model for other community hospital centers in achieving outcomes that are as good as those in academic centers.
Acknowledgments
The authors thank Irene Kadota and Heather Peters, from the Department of Radiation Oncology, and Julianne Courtenay, from the Department of Physical Therapy at the Disney Family Cancer Center, Burbank, California, for providing the original clinical data for analysis.
1. The National Lymphedema Medical Advisory Committee. The diagnosis and treatment of lymphedema. National Lymphedema Network. http://www.lymphnet.org/pdfDocs/nlntreatment.pdf. Updated February 2011. Accessed April 26, 2017.
2. McGarvey AC, Osmotherly PG, Hoffman GR, Chiarelli PE. Lymphedema following treatment for head and neck cancer: impact on patients, and beliefs of health professionals. Eur J Cancer Care (Engl). 2014;23(3):317-327.
3. Bhattacharyya N, Abemayor E. Patterns of hospital utilization for head and neck cancer care: changing demographics. JAMA Otolaryngol Head Neck Surg. 2015;141(4):307-312.
4. Deng J, Ridner SH, Dietrich MS, et al. Prevalence of secondary lymphedema in patients with head and neck cancer. J Pain Symptom Manage. 2012;43(2):244-252.
5. Deng J, Ridner SH, Dietrich MS, et al. Factors associated with external and internal lymphedema in patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2012;84(3):e319-328.
6. Flores AM, Spinelli BA, Eden MM, Galantino ML. EDGE task force on head and neck cancer outcomes: a systematic review of outcomes measures for quantifying external lymphedema. Rehabil Oncol. 2015;33(2):15-23.
7. Ridner SH, Doersam J, Galford E. An update on lymphedema of the head and neck. http://www.lymphnet.org/pdfDocs/Vol_28-N2_Update_HN.pdf. Published April-June 2015. Accessed April 26, 2017.
8. Smith BG, Hutcheson KA, Little LG, et al. Lymphedema outcomes in patients with head and neck cancer. Otolaryngol Head Neck Surg. 2015;152(2);284-291.
9. Naqvi SHS, Karni RJ, Tan IC, et al. Int J Rad Oncol Biol Phys. 2016;4:927-928.
10. Deng J, Ridner SH, Aulino JM, Murphy BA. Assessment and measurement of head and neck lymphedema: state-of-the-science and future directions. Oral Oncol. 2015; 51(5):431-437.
11. Purcell A. Head and neck lymphedema management practices. J Lymphedema. 2013;8(2):8-15.
12. Paskett ED, Dean JA, Oliveri JM, Harrop JP. Cancer-related lymphedema risk factors, diagnosis treatment and impact: a review. J Clinl Oncol. 2012;30(30):3726-3733.
13. Quick DASH questionnaire. http://www.dash.iwh.on.ca/about-quickdash. [Last update not stated.] Accessed May 18, 2017.
14. Neck Disability Index questionnaire. www.aaos.org/uploadedFiles/NDI.pdf Accessed May 18, 2017.
15. Care Connections questionnaire. http://www.careconnections.com/. Accessed May 18, 2017.
16. Galantino ML, Eden MM, Spinelli BA, Flores AM. EDGE task force on head and neck cancer outcomes a systematic review of outcome measures for temporomandibular-related dysfunction. Rehabil Oncol. 2015;33(1):6-14.
17. Deng J, Ridner SH, Murphy BA. Lymphedema in patients with head and neck cancer. Oncol Nurs Forum. 2011;38(1):e1-e10.
18. What are oral cavity and oropharyngeal cancers? American Cancer Society. http://www.cancer.org/cancer/oralcavityandoropharyngealcancer/detailedguide/oral-cavity-and-oropharyngeal-cancer-what-is-oral-cavity-cancer. Last revised August 8, 2016. Accessed April 26, 2017.
19. Hamoir M, Schmitz S, Gregoire V. The role of neck dissection in squamous cell carcinoma of the head and neck. Curr Treat Options Oncol. 2014;15:611-624.
Lymphedema is the swelling of tissue caused by the accumulation of interstitial fluid in any area of the body where lymphatic flow has been compromised.1 Secondary lymphedema is an acquired abnormality in lymph drainage1,2 and is the type commonly seen in cancer patients. Secondary lymphedema can be described as external or internal. Internal lymphedema, swelling of deep structures and tissues, is very difficult to quantify.
Lymphedema in patients with head and neck cancers
Lymphedema is a complicating morbidity frequently seen in head and neck cancer patients who have undergone treatment with surgery, radiation, and chemotherapy. However, although it is one of the most prevalent side effects of treatment, it is both under-recognized and under-treated.3
In head and neck cancer patients, internal swelling may develop in the soft tissues of the upper aero-digestive tract,4 affecting articulation and swallowing. Currently, there does not seem to be an effective practical and reliable tool with which to measure internal lymphedema. In addition, it is generally accepted that there is no effective way to treat internal lymphedema. By contrast, external lymphedema is more readily observed, but both subjective and objective assessments are difficult. External swelling may occur in the face, jaw, and neck. However, the subjective scales currently available are insufficient to capture very important characteristics of external lymphedema.5 The Edge Task Force on Head and Neck Cancer in 2015 was not able to recommend any outcome measures for objectively quantifying external edema.6 Furthermore, objective measurements of head and neck lymphedema can be expensive and time consuming.
Extent and risk
A combination of both internal and external swelling is seen in more than 50% of patients.7 Risk factors include “throat” tumors, multicancer treatment approaches, higher total radiation dose, a greater number of radiation procedures, and radiation at the surgical site.5 More than 500,000 survivors of head and neck cancer in the United States are at risk of lymphedema.5 Although recent advances in treatment have reduced the incidence of other morbidities, 50% of patients who are treated for head and neck cancer may still develop lymphedema.1,8 The reported incidence in some centers may be much higher, with up to 75% of patients developing lymphedema following treatment.9
Measurement modalities for clinical evaluation
There is little current research into lymphedema of the head and neck, despite the high prevalence of the condition.8 According to Deng and colleagues, measurement of head and neck lymphedema is a challenge, which has an impact on clinical assessment, diagnosis, and treatment of this under-recognized, under-reported and under-addressed problem in head and neck cancer patients.10 In a review of the literature, Deng and colleagues identified three measurement modalities available for clinical evaluation: patient-reported outcomes, clinician-reported outcomes, and technology.10 One major factor, though, in detecting lymphedema, is physician awareness: physicians, health care professionals, and even some lymphedema therapists are not well educated about this problem.8
Treatment
The effectiveness of traditional lymphedema treatment is not well defined.8 Currently, complete decongestive therapy (CDT), is considered the standard of care for lymphedema. The National Lymphedema Network has stated that modifications of CDT, especially manual lymphatic drainage and modified compressive garments for external lymphedema, have been shown to be beneficial for the treatment of lymphedema in head and neck cancer patients.11 Most findings in lymphedema research, mainly in breast cancer patients, have shown that early intervention is the best management and yields the best outcomes. As with other chronic conditions, early identification and timely, appropriate treatment of lymphedema is critical to improve clinical outcomes, to decrease symptom burden and functional impairment, and to improve overall quality of life in head and neck cancer patients.10
Improving recognition and treatment
Head and neck oncologic treatment is increasingly offered outside the network of specialist academic hospitals, at hospitals serving more localized communities where the neediest, sickest patient groups may be receiving less than optimal care.3 This challenges community hospitals to provide optimal treatment, similar to that being offered at nationally recognized institutions. In January 2012, we implemented a prehabilitation program in our community hospital cancer center to provide early intervention for our patients based on the understanding that proper and prompt treatment for patients with early signs of lymphedema should be a priority.12 In this article, we outline how we implemented the program and the describe improvements we observed before and after the implementation of the program.
The prehabilitation program
The role of the nurse navigator
Before the introduction of the prehabilitation program, our pattern of practice was to refer patients to oncology rehabilitation for lymphedema management after they had completed their medical treatment with surgery, radiation, and chemotherapy. In 2012, that was changed to a prehabilitation model of care that was overseen by a head and neck nurse navigator. This focus on prehabilitation begins with patients being referred to oncology rehabilitation at the time of cancer diagnosis for baseline assessment of head and neck swelling. In addition, there is assessment of the many possible other side effects associated with head and neck cancer and its treatment, namely loss of range of motion of the neck, jaw (trismus), and/or shoulders, postural deficits, functional loss, pain, balance dysfunction with fall risk, weakness, and fatigue. Therapeutic interventions are initiated as needed and appropriate. This process also raises awareness of a condition that has been described as under-recognized and under-treated.3
The nurse navigator sits in on each radiation oncology consultation and aids in “navigating” patients through their treatment. The nurse ensures that each patient is referred to different ancillary services from the outset, such as seeing a dietician, social worker, physical/occupational therapist and certified lymphedema therapist, speech pathologist, and financial assistance advisor, if necessary (Table 1).
Assessment of lymphedema
Measurement of head and neck lymphedema is a challenge.10 In our program, the physical therapy assessment also includes the evaluation of several other morbidities associated with head and neck cancer and its treatment, such as range of motion, weakness, fatigue, radiation fibrosis, balance dysfunction, and risk of falling (Table 2).
Patient-reported outcomes are essential to fully capture observable and unobservable symptoms (eg, sensations) as well as the functional impacts of lymphedema.10 In addition to lymphedema, there are many other morbidities that may be assessed on the basis of patient-reported outcome tools, such as upper extremity function with QuickDASH.13 At our clinic for head and neck cancer patients we use the Neck Disability Index (NDI)14 and Care Connections (CC)15 survey for the patient-reported outcomes. The Quick DASH, NDI, and CC tools all assess standard functional outcomes that are not specific to lymphedema, but are useful in documenting changes related to lymphedema. We initially used the CC survey and later transitioned to using the NDI. Neck pain is common with lymphedema in the head and neck region, and the NDI is a valid, reliable, responsive and internally consistent clinical tool to measure self-reported disability in patients with neck pain.16 These questionnaires were completed by the patients at their initial assessment, at reassessment, and at time of discharge.
Although objective criteria for external lymphedema have not been established, simple measurements such as using a tape measure to record neck circumference, allow a useful longitudinal assessment. Digital photography may be effective in the documentation and subjective evaluation of changes of external lymphedema.10,17 However, there are some limitations with photography because although external photographs (including digital photography and three-dimensional imaging) can capture some features, such as changes in contours, symmetry, and changes in skin quality and color, they do not detect changes in skin and soft tissue texture and compliance (Table 3).10
Impact on clinical outcomes
We retrospectively reviewed the medical records of 230 head and neck cancer patients who had been treated at our center between June 2008 and June 2015. Complete clinical data were available for 190 patients. The following information was extracted from each patient’s chart: whether they developed lymphedema, tumor stage, had surgery, radiation dose, type of chemotherapy given, their smoking history, if they had had a neck dissection and the primary site of the tumor (Table 3).
Incidence in different time periods. Of the 190 patients with complete records 78 (41%) were found to have lymphedema. These were all patients undergoing treatment for head and neck cancer during June 2008-June 2015. The prehabilitation program was initiated with the hiring of a nurse navigator for head and neck cancer, starting in January 2012. It is interesting to note that the incidence of lymphedema was 27% before the program was started, but after nurse navigator joined the team, the incidence increased significantly to 48% (P = .0002), in line with published expectations. This increase in recorded incidence may be attributable to the greater awareness of lymphedema intentionally fostered by the prehabilitation program.
Smoking history. Patients’ lifetime smoking history was retrieved from their medical records, based on their verbal admission of tobacco use. Most of the patients (n = 110) self-reported a history of smoking. Of those with a history of smoking, 36 (33%) developed external lymphedema after treatment for head and neck cancer, and 74 (67%) did not. However, this difference was not statistically significant. Hence, although smoking is a risk factor for head and neck cancer, it was not associated with the development of external lymphedema in our cohort of patients.
Type of tumor
Most of the patients (n = 156, 82%) had squamous cell carcinomas (SCC). Of those, 45% developed external lymphedema and 55% did not. Therefore, having SCC did not predispose to lymphedema. The other cancers were mixed type, mainly adenocaricoma, but their numbers were too small to draw statistical conclusions.
Stage of the tumor
About two thirds of the patients (n = 121, 64%) had stage 3 or 4 cancer. However, treatment of more advanced cancers was not associated with lymphedema development.
Site of the tumor
The literature suggests that patients with a primary tumor in the throat are at increased risk for lymphedema.5 The American Cancer Society has defined cancers of the oropharynx (throat) as including the base of the tongue (back third of the tongue), the soft palate, the tonsils, and the side and back walls of the throat.18 In our head and neck cancer cohort, patients with primary tumors of the oropharnyx were, perhaps, more susceptible to lymphedema (P = .044, Table 3). By contrast, in our cohort of patients, those with nasopharyngeal, hypopharyngeal, and parotid gland tumors were significantly less likely to develop lymphedema (Ps = .017, .04, .012, respectively).
No surgery
Half of our patients (n = 95) were not treated with surgery. In the patients who did not have surgery, 25 (26%) developed lymphedema, whereas 70 (74%) did not. Hence, although the incidence of lymphedema was significantly lower in patients who did not have surgery (P = .015), lymphedema did develop in patients who did not have a surgical procedure.
Resection of primary tumor without neck dissection
Of the 64 patients who had surgery, but without neck dissection, 35 (55%) developed external lymphedema. Compared with the no-surgery patients, the doubling of the incidence (from 26% to 55%) was highly significant (P = .0004). These findings are compatible with the literature reports that surgery increases the incidence of lymphedema, which is not surprising because surgery and subsequent scarring is known to compromise the lymphatic system.
Resection of primary tumor with neck dissection
The incidence of external lymphedema was increased to 69% when patients were subjected to both surgery and neck dissection. Compared with the June 2008-June 2015 cohort, there was a significant increase in the incidence of lymphedema in the neck dissection group (P = .007). Neck dissection involves the removal of lymph nodes and disruption of the lymphatic vessels, so it is not surprising that there is a higher incidence of external lymphedema. In our practice, neck dissections increased in frequency every year from June 2008 until December 2011, when 8 patients underwent neck dissections, 6 (75%) of whom developed lymphedema. Since January 2012, when the prehabilitation program was implemented, the number of neck dissections have declined, with more patients receiving chemoradiation and surgery being reserved for surgery. Hamoir and colleagues have reported that neck dissection is no longer justified unless there is clinically residual disease in the neck.19
Radiation
Lymphedema occurred in patients regardless of the dose of radiation received. Although the incidence of lymphedema seemed to be higher in patients who received more than 60 cGy, that difference was not statistically significant (Table 3). We had expected a relationship between radiation damage and greater lymphedema, but that was not evident in our patients.
Chemotherapy
The majority of patients (n = 131, 69%) received chemotherapy. The exposure to chemotherapy was not correlated with the risk of external lymphedema in our cohort of patients, with 58 of the 131 treated patients (44%) developing lymphedema, compared with 73 (56%) of treated patients who did not (Table 3).
Complete decongestive therapy
All patients with documented lymphedema were evaluated for complete decongestive therapy (CDT). Contraindications to CDT included congestive heart failure, renal failure, acute infection, peripheral artery disease, upper-quadrant deep vein thrombosis, and carotid artery stenosis. Eligible patients were referred to a certified lymphedema therapist for CDT. As the program evolved, patients at risk for lymphedema were referred for CDT early on, usually at the time of diagnosis, to improve early identification and surveillance of lymphedema.
CDT included manual lymph drainage,
Patients’ responses to CDT were documented with digital photographs that were taken at each visit and, more recently, use of the NDI.
Communication and education
The head and neck cancer nurse navigator attends the cancer center’s multidisciplinary head and neck tumor board, which has representation from otolaryngology, diagnostic radiology, pathology, radiation oncology, medical oncology, reconstructive surgery, oncology rehabilitation (physical/occupational therapist), dietary services, speech pathology, social services and clinical research. This regular contact allows for earlier awareness about which patients are at greater risk for developing lymphedema, thus enabling early intervention (and patient education) in a timely manner.
Education of the patient, before cancer therapy, of the risks of lymphedema is very important. Before the implementation of the prehabilitation program, some patients did not fully comprehend what a painful and debilitating consequence of cancer treatment lymphedema could be.
Discussion
We introduced a prehabilitation program to detect and treat lymphedema in head and neck cancer patients in January 2012 part way through following an observation cohort from June2008 through June2015. Central to this, in our center, was the appointment of a nurse navigator whose primary focus was on head and neck cancer patients. We placed a high priority on the early detection and treatment of lymphedema because do so has been associated with better outcomes in other centers.
One immediate consequence of the inception of our program was the identification of more patients with external lymphedema. Our detected incidence rose significantly (P = .0002), from 27% in the period June 2008-December 20112010, before the program, to 48% during the January 2012-June 2015 period, after the inception of the program. This later incidence rate is in line with published incidence rates in most centers. However, it is still somewhat short of the 75% suggested in one center,9 which suggests we are either we are underdetecting lymphedema or there are differences in definition criteria or sensitivity levels for defining lymphedema.
There are currently no specific objective measures of lymphedema, so there is bound to be some variation in diagnosis rates. In our program, we rely heavily on the patient-reported outcome measures, the NDI instrument, and digital photography to detect and monitor lymphedema, starting with the pretreatment baseline values that are established for each patient.
The use of digital photography in our community hospital setting, which includes taking photographs before and after treatment and at each visit, motivates and encourages patients and provides a tool for clinical lymphedema therapists to visually document benefits of treatment. Patients’ motivation and compliance with their established home program for head and neck lymphedema self-management are essential. The elements of the home program may include self-manual lymph drainage, home-modified compression bandaging and garment wear, therapeutic exercises, and skin care. Patients with lymphedema who adhered closely with their therapy program were more than 8 times more likely to improve compared with noncompliant patients.17
Some groups of patients have a greater risk of developing lymphedema than others,5 so the development of an algorithm to predict lymphedema seemed possible. However, in our cohort of patients, only neck dissection, with its disruption of the lymphatic system of the neck, was strongly associated with external lymphedema (Table 3). It is important to note that some patients who did not undergo surgery developed lymphedema. In our patients, high doses of radiation alone did not seem to predispose to lymphedema. That suggests that no group of head and neck cancer patients should be ignored, which is why we did routine screening of all patients before, during, and after treatment.
Our protocol falls short in the detection of internal lymphedema. For example, information on swallowing gathered by our speech pathologists (in a different department) has not, so far, been included in our assessment. This is one opportunity to improve on our approach, especially because speech difficulties may be associated with internal lymphedema. In addition, we are not equipped for the requisite internal examinations. Unfortunately, there are no practical and successful treatments for patients suffering from internal swelling. This represents a challenge for the medical community to better meet this need. Therefore, although we are missing some assessments of internal lymphedema, this is of little therapeutic consequence at this time.
The increase in the detected incidence of external lymphedema points to a practice gap that has been resolved by the appointment of a dedicated nurse navigator who attends oncology reviews to share knowledge and information. Another educational effort has been made with the patients themselves to increase compliance and improve continuous care at home.
There is always room for improvement, however, either by feedback acquired from other institutions and hospitals or through the future introduction of more objective assessment techniques.
Conclusions
The introduction of the prehabilitation program at our center has coincided with a significantly improved detection rate for external lymphedema in head and neck cancer patients. It may be because the program emphasizes education about lymphedema that awareness of the condition has increased throughout the center. It is now widely recognized that all patients are at risk of lymphedema regardless of whether they fall into an acknowledged high-risk group. Our experience shows that there is no significant difference between treatment modalities apart from neck dissection. In our population, the use of this procedure is decreasing. External lymphedema can develop even in patients who do not have surgery. Therefore, there is no sound way to predict which patients are most likely to suffer from the accumulation of fluid in their head and neck after treatment for head and neck cancer. Thus, an assessment as described here, during and after treatment for all patients, is warranted. Patients are now being seen earlier as a part of the prehabilitation program, which facilitates access to complete decongestive treatment at an earlier stage, improves patient outcomes, and increases patient satisfaction with their treatment. Our prehabilitation program could serve as a model for other community hospital centers in achieving outcomes that are as good as those in academic centers.
Acknowledgments
The authors thank Irene Kadota and Heather Peters, from the Department of Radiation Oncology, and Julianne Courtenay, from the Department of Physical Therapy at the Disney Family Cancer Center, Burbank, California, for providing the original clinical data for analysis.
Lymphedema is the swelling of tissue caused by the accumulation of interstitial fluid in any area of the body where lymphatic flow has been compromised.1 Secondary lymphedema is an acquired abnormality in lymph drainage1,2 and is the type commonly seen in cancer patients. Secondary lymphedema can be described as external or internal. Internal lymphedema, swelling of deep structures and tissues, is very difficult to quantify.
Lymphedema in patients with head and neck cancers
Lymphedema is a complicating morbidity frequently seen in head and neck cancer patients who have undergone treatment with surgery, radiation, and chemotherapy. However, although it is one of the most prevalent side effects of treatment, it is both under-recognized and under-treated.3
In head and neck cancer patients, internal swelling may develop in the soft tissues of the upper aero-digestive tract,4 affecting articulation and swallowing. Currently, there does not seem to be an effective practical and reliable tool with which to measure internal lymphedema. In addition, it is generally accepted that there is no effective way to treat internal lymphedema. By contrast, external lymphedema is more readily observed, but both subjective and objective assessments are difficult. External swelling may occur in the face, jaw, and neck. However, the subjective scales currently available are insufficient to capture very important characteristics of external lymphedema.5 The Edge Task Force on Head and Neck Cancer in 2015 was not able to recommend any outcome measures for objectively quantifying external edema.6 Furthermore, objective measurements of head and neck lymphedema can be expensive and time consuming.
Extent and risk
A combination of both internal and external swelling is seen in more than 50% of patients.7 Risk factors include “throat” tumors, multicancer treatment approaches, higher total radiation dose, a greater number of radiation procedures, and radiation at the surgical site.5 More than 500,000 survivors of head and neck cancer in the United States are at risk of lymphedema.5 Although recent advances in treatment have reduced the incidence of other morbidities, 50% of patients who are treated for head and neck cancer may still develop lymphedema.1,8 The reported incidence in some centers may be much higher, with up to 75% of patients developing lymphedema following treatment.9
Measurement modalities for clinical evaluation
There is little current research into lymphedema of the head and neck, despite the high prevalence of the condition.8 According to Deng and colleagues, measurement of head and neck lymphedema is a challenge, which has an impact on clinical assessment, diagnosis, and treatment of this under-recognized, under-reported and under-addressed problem in head and neck cancer patients.10 In a review of the literature, Deng and colleagues identified three measurement modalities available for clinical evaluation: patient-reported outcomes, clinician-reported outcomes, and technology.10 One major factor, though, in detecting lymphedema, is physician awareness: physicians, health care professionals, and even some lymphedema therapists are not well educated about this problem.8
Treatment
The effectiveness of traditional lymphedema treatment is not well defined.8 Currently, complete decongestive therapy (CDT), is considered the standard of care for lymphedema. The National Lymphedema Network has stated that modifications of CDT, especially manual lymphatic drainage and modified compressive garments for external lymphedema, have been shown to be beneficial for the treatment of lymphedema in head and neck cancer patients.11 Most findings in lymphedema research, mainly in breast cancer patients, have shown that early intervention is the best management and yields the best outcomes. As with other chronic conditions, early identification and timely, appropriate treatment of lymphedema is critical to improve clinical outcomes, to decrease symptom burden and functional impairment, and to improve overall quality of life in head and neck cancer patients.10
Improving recognition and treatment
Head and neck oncologic treatment is increasingly offered outside the network of specialist academic hospitals, at hospitals serving more localized communities where the neediest, sickest patient groups may be receiving less than optimal care.3 This challenges community hospitals to provide optimal treatment, similar to that being offered at nationally recognized institutions. In January 2012, we implemented a prehabilitation program in our community hospital cancer center to provide early intervention for our patients based on the understanding that proper and prompt treatment for patients with early signs of lymphedema should be a priority.12 In this article, we outline how we implemented the program and the describe improvements we observed before and after the implementation of the program.
The prehabilitation program
The role of the nurse navigator
Before the introduction of the prehabilitation program, our pattern of practice was to refer patients to oncology rehabilitation for lymphedema management after they had completed their medical treatment with surgery, radiation, and chemotherapy. In 2012, that was changed to a prehabilitation model of care that was overseen by a head and neck nurse navigator. This focus on prehabilitation begins with patients being referred to oncology rehabilitation at the time of cancer diagnosis for baseline assessment of head and neck swelling. In addition, there is assessment of the many possible other side effects associated with head and neck cancer and its treatment, namely loss of range of motion of the neck, jaw (trismus), and/or shoulders, postural deficits, functional loss, pain, balance dysfunction with fall risk, weakness, and fatigue. Therapeutic interventions are initiated as needed and appropriate. This process also raises awareness of a condition that has been described as under-recognized and under-treated.3
The nurse navigator sits in on each radiation oncology consultation and aids in “navigating” patients through their treatment. The nurse ensures that each patient is referred to different ancillary services from the outset, such as seeing a dietician, social worker, physical/occupational therapist and certified lymphedema therapist, speech pathologist, and financial assistance advisor, if necessary (Table 1).
Assessment of lymphedema
Measurement of head and neck lymphedema is a challenge.10 In our program, the physical therapy assessment also includes the evaluation of several other morbidities associated with head and neck cancer and its treatment, such as range of motion, weakness, fatigue, radiation fibrosis, balance dysfunction, and risk of falling (Table 2).
Patient-reported outcomes are essential to fully capture observable and unobservable symptoms (eg, sensations) as well as the functional impacts of lymphedema.10 In addition to lymphedema, there are many other morbidities that may be assessed on the basis of patient-reported outcome tools, such as upper extremity function with QuickDASH.13 At our clinic for head and neck cancer patients we use the Neck Disability Index (NDI)14 and Care Connections (CC)15 survey for the patient-reported outcomes. The Quick DASH, NDI, and CC tools all assess standard functional outcomes that are not specific to lymphedema, but are useful in documenting changes related to lymphedema. We initially used the CC survey and later transitioned to using the NDI. Neck pain is common with lymphedema in the head and neck region, and the NDI is a valid, reliable, responsive and internally consistent clinical tool to measure self-reported disability in patients with neck pain.16 These questionnaires were completed by the patients at their initial assessment, at reassessment, and at time of discharge.
Although objective criteria for external lymphedema have not been established, simple measurements such as using a tape measure to record neck circumference, allow a useful longitudinal assessment. Digital photography may be effective in the documentation and subjective evaluation of changes of external lymphedema.10,17 However, there are some limitations with photography because although external photographs (including digital photography and three-dimensional imaging) can capture some features, such as changes in contours, symmetry, and changes in skin quality and color, they do not detect changes in skin and soft tissue texture and compliance (Table 3).10
Impact on clinical outcomes
We retrospectively reviewed the medical records of 230 head and neck cancer patients who had been treated at our center between June 2008 and June 2015. Complete clinical data were available for 190 patients. The following information was extracted from each patient’s chart: whether they developed lymphedema, tumor stage, had surgery, radiation dose, type of chemotherapy given, their smoking history, if they had had a neck dissection and the primary site of the tumor (Table 3).
Incidence in different time periods. Of the 190 patients with complete records 78 (41%) were found to have lymphedema. These were all patients undergoing treatment for head and neck cancer during June 2008-June 2015. The prehabilitation program was initiated with the hiring of a nurse navigator for head and neck cancer, starting in January 2012. It is interesting to note that the incidence of lymphedema was 27% before the program was started, but after nurse navigator joined the team, the incidence increased significantly to 48% (P = .0002), in line with published expectations. This increase in recorded incidence may be attributable to the greater awareness of lymphedema intentionally fostered by the prehabilitation program.
Smoking history. Patients’ lifetime smoking history was retrieved from their medical records, based on their verbal admission of tobacco use. Most of the patients (n = 110) self-reported a history of smoking. Of those with a history of smoking, 36 (33%) developed external lymphedema after treatment for head and neck cancer, and 74 (67%) did not. However, this difference was not statistically significant. Hence, although smoking is a risk factor for head and neck cancer, it was not associated with the development of external lymphedema in our cohort of patients.
Type of tumor
Most of the patients (n = 156, 82%) had squamous cell carcinomas (SCC). Of those, 45% developed external lymphedema and 55% did not. Therefore, having SCC did not predispose to lymphedema. The other cancers were mixed type, mainly adenocaricoma, but their numbers were too small to draw statistical conclusions.
Stage of the tumor
About two thirds of the patients (n = 121, 64%) had stage 3 or 4 cancer. However, treatment of more advanced cancers was not associated with lymphedema development.
Site of the tumor
The literature suggests that patients with a primary tumor in the throat are at increased risk for lymphedema.5 The American Cancer Society has defined cancers of the oropharynx (throat) as including the base of the tongue (back third of the tongue), the soft palate, the tonsils, and the side and back walls of the throat.18 In our head and neck cancer cohort, patients with primary tumors of the oropharnyx were, perhaps, more susceptible to lymphedema (P = .044, Table 3). By contrast, in our cohort of patients, those with nasopharyngeal, hypopharyngeal, and parotid gland tumors were significantly less likely to develop lymphedema (Ps = .017, .04, .012, respectively).
No surgery
Half of our patients (n = 95) were not treated with surgery. In the patients who did not have surgery, 25 (26%) developed lymphedema, whereas 70 (74%) did not. Hence, although the incidence of lymphedema was significantly lower in patients who did not have surgery (P = .015), lymphedema did develop in patients who did not have a surgical procedure.
Resection of primary tumor without neck dissection
Of the 64 patients who had surgery, but without neck dissection, 35 (55%) developed external lymphedema. Compared with the no-surgery patients, the doubling of the incidence (from 26% to 55%) was highly significant (P = .0004). These findings are compatible with the literature reports that surgery increases the incidence of lymphedema, which is not surprising because surgery and subsequent scarring is known to compromise the lymphatic system.
Resection of primary tumor with neck dissection
The incidence of external lymphedema was increased to 69% when patients were subjected to both surgery and neck dissection. Compared with the June 2008-June 2015 cohort, there was a significant increase in the incidence of lymphedema in the neck dissection group (P = .007). Neck dissection involves the removal of lymph nodes and disruption of the lymphatic vessels, so it is not surprising that there is a higher incidence of external lymphedema. In our practice, neck dissections increased in frequency every year from June 2008 until December 2011, when 8 patients underwent neck dissections, 6 (75%) of whom developed lymphedema. Since January 2012, when the prehabilitation program was implemented, the number of neck dissections have declined, with more patients receiving chemoradiation and surgery being reserved for surgery. Hamoir and colleagues have reported that neck dissection is no longer justified unless there is clinically residual disease in the neck.19
Radiation
Lymphedema occurred in patients regardless of the dose of radiation received. Although the incidence of lymphedema seemed to be higher in patients who received more than 60 cGy, that difference was not statistically significant (Table 3). We had expected a relationship between radiation damage and greater lymphedema, but that was not evident in our patients.
Chemotherapy
The majority of patients (n = 131, 69%) received chemotherapy. The exposure to chemotherapy was not correlated with the risk of external lymphedema in our cohort of patients, with 58 of the 131 treated patients (44%) developing lymphedema, compared with 73 (56%) of treated patients who did not (Table 3).
Complete decongestive therapy
All patients with documented lymphedema were evaluated for complete decongestive therapy (CDT). Contraindications to CDT included congestive heart failure, renal failure, acute infection, peripheral artery disease, upper-quadrant deep vein thrombosis, and carotid artery stenosis. Eligible patients were referred to a certified lymphedema therapist for CDT. As the program evolved, patients at risk for lymphedema were referred for CDT early on, usually at the time of diagnosis, to improve early identification and surveillance of lymphedema.
CDT included manual lymph drainage,
Patients’ responses to CDT were documented with digital photographs that were taken at each visit and, more recently, use of the NDI.
Communication and education
The head and neck cancer nurse navigator attends the cancer center’s multidisciplinary head and neck tumor board, which has representation from otolaryngology, diagnostic radiology, pathology, radiation oncology, medical oncology, reconstructive surgery, oncology rehabilitation (physical/occupational therapist), dietary services, speech pathology, social services and clinical research. This regular contact allows for earlier awareness about which patients are at greater risk for developing lymphedema, thus enabling early intervention (and patient education) in a timely manner.
Education of the patient, before cancer therapy, of the risks of lymphedema is very important. Before the implementation of the prehabilitation program, some patients did not fully comprehend what a painful and debilitating consequence of cancer treatment lymphedema could be.
Discussion
We introduced a prehabilitation program to detect and treat lymphedema in head and neck cancer patients in January 2012 part way through following an observation cohort from June2008 through June2015. Central to this, in our center, was the appointment of a nurse navigator whose primary focus was on head and neck cancer patients. We placed a high priority on the early detection and treatment of lymphedema because do so has been associated with better outcomes in other centers.
One immediate consequence of the inception of our program was the identification of more patients with external lymphedema. Our detected incidence rose significantly (P = .0002), from 27% in the period June 2008-December 20112010, before the program, to 48% during the January 2012-June 2015 period, after the inception of the program. This later incidence rate is in line with published incidence rates in most centers. However, it is still somewhat short of the 75% suggested in one center,9 which suggests we are either we are underdetecting lymphedema or there are differences in definition criteria or sensitivity levels for defining lymphedema.
There are currently no specific objective measures of lymphedema, so there is bound to be some variation in diagnosis rates. In our program, we rely heavily on the patient-reported outcome measures, the NDI instrument, and digital photography to detect and monitor lymphedema, starting with the pretreatment baseline values that are established for each patient.
The use of digital photography in our community hospital setting, which includes taking photographs before and after treatment and at each visit, motivates and encourages patients and provides a tool for clinical lymphedema therapists to visually document benefits of treatment. Patients’ motivation and compliance with their established home program for head and neck lymphedema self-management are essential. The elements of the home program may include self-manual lymph drainage, home-modified compression bandaging and garment wear, therapeutic exercises, and skin care. Patients with lymphedema who adhered closely with their therapy program were more than 8 times more likely to improve compared with noncompliant patients.17
Some groups of patients have a greater risk of developing lymphedema than others,5 so the development of an algorithm to predict lymphedema seemed possible. However, in our cohort of patients, only neck dissection, with its disruption of the lymphatic system of the neck, was strongly associated with external lymphedema (Table 3). It is important to note that some patients who did not undergo surgery developed lymphedema. In our patients, high doses of radiation alone did not seem to predispose to lymphedema. That suggests that no group of head and neck cancer patients should be ignored, which is why we did routine screening of all patients before, during, and after treatment.
Our protocol falls short in the detection of internal lymphedema. For example, information on swallowing gathered by our speech pathologists (in a different department) has not, so far, been included in our assessment. This is one opportunity to improve on our approach, especially because speech difficulties may be associated with internal lymphedema. In addition, we are not equipped for the requisite internal examinations. Unfortunately, there are no practical and successful treatments for patients suffering from internal swelling. This represents a challenge for the medical community to better meet this need. Therefore, although we are missing some assessments of internal lymphedema, this is of little therapeutic consequence at this time.
The increase in the detected incidence of external lymphedema points to a practice gap that has been resolved by the appointment of a dedicated nurse navigator who attends oncology reviews to share knowledge and information. Another educational effort has been made with the patients themselves to increase compliance and improve continuous care at home.
There is always room for improvement, however, either by feedback acquired from other institutions and hospitals or through the future introduction of more objective assessment techniques.
Conclusions
The introduction of the prehabilitation program at our center has coincided with a significantly improved detection rate for external lymphedema in head and neck cancer patients. It may be because the program emphasizes education about lymphedema that awareness of the condition has increased throughout the center. It is now widely recognized that all patients are at risk of lymphedema regardless of whether they fall into an acknowledged high-risk group. Our experience shows that there is no significant difference between treatment modalities apart from neck dissection. In our population, the use of this procedure is decreasing. External lymphedema can develop even in patients who do not have surgery. Therefore, there is no sound way to predict which patients are most likely to suffer from the accumulation of fluid in their head and neck after treatment for head and neck cancer. Thus, an assessment as described here, during and after treatment for all patients, is warranted. Patients are now being seen earlier as a part of the prehabilitation program, which facilitates access to complete decongestive treatment at an earlier stage, improves patient outcomes, and increases patient satisfaction with their treatment. Our prehabilitation program could serve as a model for other community hospital centers in achieving outcomes that are as good as those in academic centers.
Acknowledgments
The authors thank Irene Kadota and Heather Peters, from the Department of Radiation Oncology, and Julianne Courtenay, from the Department of Physical Therapy at the Disney Family Cancer Center, Burbank, California, for providing the original clinical data for analysis.
1. The National Lymphedema Medical Advisory Committee. The diagnosis and treatment of lymphedema. National Lymphedema Network. http://www.lymphnet.org/pdfDocs/nlntreatment.pdf. Updated February 2011. Accessed April 26, 2017.
2. McGarvey AC, Osmotherly PG, Hoffman GR, Chiarelli PE. Lymphedema following treatment for head and neck cancer: impact on patients, and beliefs of health professionals. Eur J Cancer Care (Engl). 2014;23(3):317-327.
3. Bhattacharyya N, Abemayor E. Patterns of hospital utilization for head and neck cancer care: changing demographics. JAMA Otolaryngol Head Neck Surg. 2015;141(4):307-312.
4. Deng J, Ridner SH, Dietrich MS, et al. Prevalence of secondary lymphedema in patients with head and neck cancer. J Pain Symptom Manage. 2012;43(2):244-252.
5. Deng J, Ridner SH, Dietrich MS, et al. Factors associated with external and internal lymphedema in patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2012;84(3):e319-328.
6. Flores AM, Spinelli BA, Eden MM, Galantino ML. EDGE task force on head and neck cancer outcomes: a systematic review of outcomes measures for quantifying external lymphedema. Rehabil Oncol. 2015;33(2):15-23.
7. Ridner SH, Doersam J, Galford E. An update on lymphedema of the head and neck. http://www.lymphnet.org/pdfDocs/Vol_28-N2_Update_HN.pdf. Published April-June 2015. Accessed April 26, 2017.
8. Smith BG, Hutcheson KA, Little LG, et al. Lymphedema outcomes in patients with head and neck cancer. Otolaryngol Head Neck Surg. 2015;152(2);284-291.
9. Naqvi SHS, Karni RJ, Tan IC, et al. Int J Rad Oncol Biol Phys. 2016;4:927-928.
10. Deng J, Ridner SH, Aulino JM, Murphy BA. Assessment and measurement of head and neck lymphedema: state-of-the-science and future directions. Oral Oncol. 2015; 51(5):431-437.
11. Purcell A. Head and neck lymphedema management practices. J Lymphedema. 2013;8(2):8-15.
12. Paskett ED, Dean JA, Oliveri JM, Harrop JP. Cancer-related lymphedema risk factors, diagnosis treatment and impact: a review. J Clinl Oncol. 2012;30(30):3726-3733.
13. Quick DASH questionnaire. http://www.dash.iwh.on.ca/about-quickdash. [Last update not stated.] Accessed May 18, 2017.
14. Neck Disability Index questionnaire. www.aaos.org/uploadedFiles/NDI.pdf Accessed May 18, 2017.
15. Care Connections questionnaire. http://www.careconnections.com/. Accessed May 18, 2017.
16. Galantino ML, Eden MM, Spinelli BA, Flores AM. EDGE task force on head and neck cancer outcomes a systematic review of outcome measures for temporomandibular-related dysfunction. Rehabil Oncol. 2015;33(1):6-14.
17. Deng J, Ridner SH, Murphy BA. Lymphedema in patients with head and neck cancer. Oncol Nurs Forum. 2011;38(1):e1-e10.
18. What are oral cavity and oropharyngeal cancers? American Cancer Society. http://www.cancer.org/cancer/oralcavityandoropharyngealcancer/detailedguide/oral-cavity-and-oropharyngeal-cancer-what-is-oral-cavity-cancer. Last revised August 8, 2016. Accessed April 26, 2017.
19. Hamoir M, Schmitz S, Gregoire V. The role of neck dissection in squamous cell carcinoma of the head and neck. Curr Treat Options Oncol. 2014;15:611-624.
1. The National Lymphedema Medical Advisory Committee. The diagnosis and treatment of lymphedema. National Lymphedema Network. http://www.lymphnet.org/pdfDocs/nlntreatment.pdf. Updated February 2011. Accessed April 26, 2017.
2. McGarvey AC, Osmotherly PG, Hoffman GR, Chiarelli PE. Lymphedema following treatment for head and neck cancer: impact on patients, and beliefs of health professionals. Eur J Cancer Care (Engl). 2014;23(3):317-327.
3. Bhattacharyya N, Abemayor E. Patterns of hospital utilization for head and neck cancer care: changing demographics. JAMA Otolaryngol Head Neck Surg. 2015;141(4):307-312.
4. Deng J, Ridner SH, Dietrich MS, et al. Prevalence of secondary lymphedema in patients with head and neck cancer. J Pain Symptom Manage. 2012;43(2):244-252.
5. Deng J, Ridner SH, Dietrich MS, et al. Factors associated with external and internal lymphedema in patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2012;84(3):e319-328.
6. Flores AM, Spinelli BA, Eden MM, Galantino ML. EDGE task force on head and neck cancer outcomes: a systematic review of outcomes measures for quantifying external lymphedema. Rehabil Oncol. 2015;33(2):15-23.
7. Ridner SH, Doersam J, Galford E. An update on lymphedema of the head and neck. http://www.lymphnet.org/pdfDocs/Vol_28-N2_Update_HN.pdf. Published April-June 2015. Accessed April 26, 2017.
8. Smith BG, Hutcheson KA, Little LG, et al. Lymphedema outcomes in patients with head and neck cancer. Otolaryngol Head Neck Surg. 2015;152(2);284-291.
9. Naqvi SHS, Karni RJ, Tan IC, et al. Int J Rad Oncol Biol Phys. 2016;4:927-928.
10. Deng J, Ridner SH, Aulino JM, Murphy BA. Assessment and measurement of head and neck lymphedema: state-of-the-science and future directions. Oral Oncol. 2015; 51(5):431-437.
11. Purcell A. Head and neck lymphedema management practices. J Lymphedema. 2013;8(2):8-15.
12. Paskett ED, Dean JA, Oliveri JM, Harrop JP. Cancer-related lymphedema risk factors, diagnosis treatment and impact: a review. J Clinl Oncol. 2012;30(30):3726-3733.
13. Quick DASH questionnaire. http://www.dash.iwh.on.ca/about-quickdash. [Last update not stated.] Accessed May 18, 2017.
14. Neck Disability Index questionnaire. www.aaos.org/uploadedFiles/NDI.pdf Accessed May 18, 2017.
15. Care Connections questionnaire. http://www.careconnections.com/. Accessed May 18, 2017.
16. Galantino ML, Eden MM, Spinelli BA, Flores AM. EDGE task force on head and neck cancer outcomes a systematic review of outcome measures for temporomandibular-related dysfunction. Rehabil Oncol. 2015;33(1):6-14.
17. Deng J, Ridner SH, Murphy BA. Lymphedema in patients with head and neck cancer. Oncol Nurs Forum. 2011;38(1):e1-e10.
18. What are oral cavity and oropharyngeal cancers? American Cancer Society. http://www.cancer.org/cancer/oralcavityandoropharyngealcancer/detailedguide/oral-cavity-and-oropharyngeal-cancer-what-is-oral-cavity-cancer. Last revised August 8, 2016. Accessed April 26, 2017.
19. Hamoir M, Schmitz S, Gregoire V. The role of neck dissection in squamous cell carcinoma of the head and neck. Curr Treat Options Oncol. 2014;15:611-624.
Distress management in cancer patients in Puerto Rico
A comprehensive, patient-centered approach is required to accomplish cancer best standards of care.1 This approach reflects the holistic conceptualization of health in which the physical, emotional, and social dimensions of the human being are considered when providing medical care. As a result, to look after all patient needs, interdisciplinary and well-coordinated interventions are recommended. Cancer patients should be provided not only with diagnostic, treatment, and follow-up clinical service, but also with the supportive assistance that may positively influence all aspects of their health.
To appraise physical, social, emotional and spiritual issues and to develop supportive interventional action plans, the National Comprehensive Cancer Network (NCCN) recommends screening all cancer patients for distress.2 In particular, screening the emotional component of distress occupies a prominent place in this process because it is now recognized as the sixth vital sign in oncology.3 Even though the influence of emotional distress over cancer mortality rates and disease progression is still under scrutiny,4 its plausible implications over treatment compliance have been pointed out. Patients with higher levels of emotional distress show lower adherence to treatment and poorer health outcomes.5 Furthermore, prevalence rates of emotional distress in cancer patients from ambulatory settings6 and oncology surgical units have been studied and have provided justification for distress management.7 Studies have shown low ability among oncologists to identify patients in distress and oncologists’ tendency to judge distress higher than the patients themselves.8 As a consequence, to achieve systematic distress evaluations and appropriate referrals for care, guidelines for distress management should be implemented in clinical settings. It is recommended that tests are conducted to find brief screening instruments and procedures to assure accurate interventions according to patient specific needs.
This article presents the process of implementing a distress management program at HIMA-San Pablo Oncologic Hospital in Caguas, Puerto Rico, with particular emphasis on the management of emotional distress, which has been defined as the feeling of suffering that cancer patients may experience after diagnosis. In addition, we have included data from a pilot study that was completed for content validation of the Patient Health Questionnaire (PHQ-9) to estimate depression levels in Puerto Rican cancer patients.
Methods
HIMA-San Pablo operates a group of privately owned hospitals in Puerto Rico. It established a cancer center in Caguas in 2007, recruiting a multispecialty medical faculty to provide cancer care and bone marrow transplants for adult and pediatric patients. The cancer center, currently named HIMA-San Pablo Oncologic Hospital (HSPOH), is a hospital within a hospital licensed by the Puerto Rico Department of Health. In 2007, a cancer committee was established as the steering committee to ensure the delivery of cancer care according to best standards of care. The committee took responsibility for developing all activities needed to achieve the American College of Surgeons’ Commission on Cancer (CoC) accreditation under the category of Comprehensive Community Cancer Center. The committee established a psychosocial team to develop a protocol for the delivery of distress management for adult patients. (The psychosocial needs of pediatric patients are assessed through other procedures.)
To develop the protocol, principles of input-output model of research and quality analysis in health care were applied.9 The input-output model, with its origin in engineering, helped map systematic activities to transform empirical data on cancer psychosocial care into operational procedures. Focus was given to data gathering (input), information organization and analysis (throughput), and the schematization of emotional distress management care (output).
The input phase
In the input phase, elements of psychosocial care and operational definitions related to distress management in general were identified through literature review (Table 1). Basic parameters for distress management were clarified, resulting in a conceptual framework based in four remarks: First, according to NCCN, distress is a multifactorial unpleasant emotional experience of a psychological, social, and spiritual nature. It may interfere with the ability to cope effectively with cancer, its symptoms and its treatment. Its intensity may fluctuate from feelings of suffering and fear to incapacitating manifestations of anxiety and depression2 and its severity may hamper patient quality of life and treatment compliance.
Second, distress management requires the intervention of an interdisciplinary team with both medical and allied health professionals. This may include mental health specialists and other professionals with training and experience in cancer-related issues, who work with reciprocal channels of communication for the exchange of patient information.
Third, NCCN recommends using the Distress Thermometer for patient initial distress screening.10-12 It consists of a numeric scale ranging from 0 (no distress) to 10 (severe distress) in which patients classify their level of distress. The numeric scale is followed by a section in which patients identify areas of practical, familiar, emotional, spiritual/ religious, and physical concerns. Based on responses, interviews may follow to set distress management interventions.
Fourth, screening and assessment are different but sequential and complementary stages of distress management. Screening is viewed as a rapid strategy to identify cancer patients in distress. Assessment looks out for a broader appraisal and documentation of factors with repercussions over patient distress level and resiliency capability.13 In many instances, the patient’s emotional distress is better understood in the assessment phase.
The throughput phase
Within the throughput phase of information organization and analysis, an inventory of health professionals and other in-house consultants needed for distress management was completed. Roles and procedures for information sharing were determined, and we established collaborative agreements with professionals in the community who could contribute to distress management. Members of the psychosocial team held workshops to discuss elements of NCCN guidelines for distress management and to create an action plan for the implementation of the protocol. Data analyses were performed to create a demographic profile of the oncology population at the hospital and assess patient willingness to receive emotional support services,16 which led to the implementation of support group meetings at which additional substantive information was collected about issues affecting cancer patients’ emotions.
The NCCN Distress Thermometer for measuring distress was translated to Spanish. Its format was adapted, and it was identified as a distress screening tool (DST), which we named Distress Assessment Tool for Oncology Patients (Figure 1). The instrument helps for rapid screening of patient needs and proper determination of initial interventions. In addition, psychometric properties of several instruments were reviewed for instances when patient emotional distress could not be clearly determined. We decided to proceed with the validation of the 9-item Patient Health Questionnaire (PHQ-9) to estimate patient depression level. A proposal for content validation of the PHQ-9 was approved by the University of Puerto Rico institutional review board, and patients were recruited to participate in the pilot study.
The PHQ-9. The PHQ-9 is a self-report version of the PRIME-MD instrument developed to assess mental disorders in clinical settings. It is based on DSM-IV diagnostic criteria.17 The PHQ-9 is the depression module with nine depression symptoms to check off if they become the cause of emotional impairment. Respondents categorized depression symptoms in four frequency degrees representing numeric values: 0 (not at all), 1 (several days), 2 (more than half the days), 3 (nearly every day). Measures of depression severity are subsequently determined in a Likert-type scale according to numeric calculations of responses: 0-4 (none severe depression), 5-9 (mild), 10-14 (moderate), 15-19 (moderately severe), and 20-27 (severe-major depression).
The instrument is widely used because of its validity in small and large populations. It showed adequate reliability and validity in a small sample of head and neck cancer patients, with a Cronbach’s alpha of 0.80 and a correlation coefficient of 0.71.18 Similarly, it showed good performance in identifying major depression in 4264 cancer outpatients, with sensitivity of 93%, specificity of 81%, and a positive predictive value (PPV) of 25% and negative predictive value (NPV) of 99%.19 Even when administered on a touch screen computer, the instrument showed valid data of depression from patients in treatment.20
The Beck Depression Inventory. We used the Beck Depression Inventory (BDI-II) Spanish version as the gold standard measure for the validation study. It is a 22-items inventory that measures attitudes and symptoms of depression.21 It can be administered in 10 minutes and has shown good psychometric measures when administered in Spain and Puerto Rico.22, 23
The pilot study. In all, 44 cancer patients who were receiving outpatient treatment at the radiotherapy unit agreed to participate in the study. The participants signed a consent form after the confidentiality protection measures and the main objectives of the study had been explained to them. Patients were interviewed individually during November and December 2012, with the Spanish versions of the PHQ-9 and BDI-II administered by one of two interviewers. At the beginning of each interview, the patient was asked 10 questions so that we could gather demographic data and confirm participant eligibility: aged 21 years or older, born and raised in Puerto Rico, being a Spanish speaker, and having a primary cancer diagnosis with no previous disease. Three patients were excluded from the sample because they either had cancer previously or had a recurrence or metastasis. The final sample consisted of 41 outpatients (N = 41).
Data analysis for demographics was completed with STATA v.12 software. Measures of central tendency and dispersion as well as PHQ-9 internal consistency analysis were made through Cronbach alpha with SPSS.
From a total of 41 patients surveyed, 22 (54%) were men and 19 (46%) were women, with an overall median age of 61 years. Among the men, 15 (68%) had a prostate cancer diagnosis and among women, 9 (47.4%) had a breast cancer diagnosis. In regard to health insurance, 19 (46%) had Medicare or Veterans/federal insurance coverage, and 13 (32%) had Reforma, the Puerto Rican government health insurance program partially funded by Medicaid funds. In addition, 8 participants (20%) were unemployed or disabled. As previously stated, all of the patients were in ambulatory care. Only 3 (7%) were participating in support groups.
Of all the respondents, 16 (39%) reported some level of depression. In particular, 2 (5%) showed severe-major depression, 4 (10%) moderately severe depression, and 10 (24%) moderate depression. Of those with depression, 8 (50%) were women, 8 (50%) were men. All 6 of the patients with head and neck cancer showed moderate or moderately severe depression (Table 2).
When respondent PHQ-9 scoring reflected moderate to severe depression (>10), a letter was sent to the patient’s radio-oncologist for referral to counseling and clinical psychological evaluation. All participants had access to the support group program, to a radiotherapy education program meeting weekly, and written information about their cancer diagnosis and treatment. They also were interviewed by the psychosocial coordinator or patient navigator for further assessment.
The output phase
In the output phase, a graphic representing the process of emotional assessment at the institution was created and then modified. PHQ-9 was added to the process when it was found suitable to assess level of depression contributing to the identification of patients requiring psychological and psychiatric assistance which by other means would be missed. PHQ-9 was useful in the busy clinical setting as it was completed, scored and interpreted in minutes. It showed the potential for routine evaluations when looking to identify improvement or deterioration in depression levels thus helping to monitor responses to treatment and providing insights for follow up interventions. As stated by NCCN guidelines, distress should be monitored, documented and managed at all stages of the cancer continuum.
Results and discussion
The protocol for distress management at HSPOH is based on the 2013 NCCN guidelines. Cancer patients are screened for levels of distress in all settings (inpatients and outpatients). Screening is held with the DST Spanish translation at the moment of diagnosis or as soon as possible after a diagnosis is made. Screening for distress is also done before or after surgery, in recurrence or progression, and when clinically indicated. Patients are informed that distress management is an essential part of their care and are encouraged to provide information so that we can make a proper need assessment.
Patients are screened by the psychosocial coordinator or patient navigator who administers the DST followed by in-depth interviews for additional appraisal. An action plan is designed based on patient needs, which include their intervention and the intervention of other members of the psychosocial team from the institution and/or from the community. Additional in-house health professionals contributing in distress management include, but are not limited to: physicians; clinical psychologists; health educators; social workers; dietitians; chaplains; and physical, respiratory, speech, and/or swallow therapists. Follow-up and rescreening sessions are scheduled to assure coordination of services between those health professionals as well as to secure continuity of distress management during all stages of the cancer continuum.
The results of the DST are filed in patient medical records. Members of the psychosocial team also document their interventions in the patient medical record, which helps in the exchange of information among the cancer care team. The psychosocial team meets once a month – or as required for extraordinary cases – to review and discuss the cases, determine the best options for distress management, and identify areas for psychosocial care improvement. Those findings and the results of distress management in patient level of satisfaction are then reported and discussed quarterly by the psychosocial coordinator and the cancer committee.
Figure 2 shows in what phase of emotional distress assessment the PHQ-9 was included. Patients reporting four or more of the six areas of concern related to emotional distress in the DST (Figure 1) are automatically referred to a mental health specialist. But when patients report three areas of concern with no clear data on their specific level of depression, PHQ-9 is administered to differentiate those who need a mental health specialist from those who could be adequately supported by health education and support group interventions. In this way detrimental outcomes such as duplicity and over or underuse of services and resources are reduced. In addition, it is recognized that using an interview after the administration of the DST to determine distress management actions does not always provide enough information about a patient’s emotional circumstances and previous comorbidities. Patient responses during interviews may be influenced by the patient’s level of literacy, verbal comprehension, and communication style,24 so emotional distress can go unrecognized during interviews, resulting in delays for treatment and supportive care.
National guidelines in oncology consider such socio-ecological models emphasizing the delivery of patient-centered, interdisciplinary, and evidence-based care. That does not mean that institutions should apply protocols of psychosocial care as previously developed, but that they should test, review, adapt, and improve them during the implementation of the care. In fact, NCCN encourages conducting trials to examine protocols, screening instruments, and models of intervention to determine applicability to particular settings.2
Findings from a study by NCCN member institutions to evaluate progress of implementing distress management guidelines found that 53% (n = 8) of respondent institutions conducted routine distress screening. Of those, 37.5% (3) relied only on interviews. That finding is of concern because if interviews are not standardized and have not been systematically evaluated, then their sensitivity and specificity in identifying distressed patients is unknown.26 Accordingly, the process described in this article and the PHQ-9 validation was an effort to standardize emotional distress management, and was underlined as an achievement during the CoC accreditation visit to the cancer center in December 2013. The hospital was accredited as a comprehensive community cancer center with gold commendations, becoming the first privately owned hospital in Puerto Rico to achieve the accreditation.
1. Commission on Cancer, American College of Surgeons. Cancer Programs Standards 2012: Ensuring Patient-Centered Care. Version 1.2.1. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx. Published 2012. Accessed March 5, 2013.
2. National Comprehensive Cancer Network clinical practice guidelines in oncology (NCCN guidelines): Distress management. Version I. 2012. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#supportive. Accessed March 5, 2013.
3. Bultz BD, Groff SL. Screening for distress, the 6th vital sign in oncology: from theory to practice: http://www.oncologyex.com/issue/2009/vol8_no1/8_comment2_1.html. Published February 2009. Accessed February 16, 2017.
4. Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009;115:5349-5361.
5. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Int Med. 2000;160:2101-2107.
6. Jadoon NA, Munir W, Shahzad MA, Choudhry ZS. Assessment of depression and anxiety in adult cancer outpatients: a cross sectional study. BMC Cancer. 2010;10:594.
7. Fisher D, Wedel B. Anxiety and depression disorders in cancer patients: incidence, diagnosis and therapy. Mag Eur Med Oncol. 2012;5:52-54.
8. Sollner W, DeVries A, Steixner E, et al. How successful are oncologists in identifying patient distress, perceived social support, and in need for psychosocial counselling? Br J Cancer. 2001;84:179-185.
9. Scott RD, Solomon SL, McGowan JE. Applying economic principles to health care: special issue. Emerg Infect Dis. 2001;7:282-285.
10. Adler NE, Page AEK. A model for delivering psychosocial health services. In: Cancer care for the whole patient: meeting psychosocial health needs. Washington, DC: National Academies Press (US); 2008.
11. Holland JC, Alici Y. Management of distress in cancer patients. J Support Oncol. 2010;8:4-12.
12. Jacobsen PB, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005;103:1494-1502.
13. Maihoff SE. Assessment. In Washington CM, Leaver D, eds. Principles and practice of radiation therapy. St Louis, MO: Mosby Elsevier; 2004:243-264.
14. National Academy of Sciences. Adler NE, Page AEK, eds. Cancer care for the whole patient: meeting psychosocial health needs. https://www.ncbi.nlm.nih.gov/books/NBK4015/. Published 2008. Accessed February 22, 2012.
15. Nancarrow SA, Booth A, Ariss
16. Baker-Glenn EA, Park B, Granger L, Symonds P, Mitchell AJ. Desire for psychological support in cancer patients with depression or distress: validation of a simple help question. Psychooncology. 2011;20:525-531.
17. Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
18. Omoro SA, Fann JR, Weymuller EA, Macharia IM, Yueh B. Swahili translation and validation of the Patient Health Questionnaire-9 depression scale in the Kenyan head and neck cancer patient population. Int J Psychiatry Med. 2006;36:367-381.
19. Thekkumpurath P, Walker J, Butcher I, et al. Screening for major depression on cancer outpatients: the diagnostic accuracy of the 9-item Patient Health Questionnaire. Cancer. 2011;117:218-227.
20. Fann JR, Berry DL, Wolpin S, et al. Depression screening using the Patient Health Questionnaire-9 administered on a touch screen computer. Psychooncology. 2009;18:14-22.
21. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:651-571.
22. Sanz J, Perdigón AL, Vázquez C. The Spanish adaptation of Beck’s Depression Inventory–II (BDI-II): psychometric properties in the general population. Clínica y Salud. 2003;14:249-280.
23. Bonilla J, Bernal G, Santos A, Santos D. A revised Spanish version of the Beck Depression Inventory: psychometric properties with a Puerto Rican sample of college students. J Clin Psychol. 2004;60:119-130.
24. Alcántara C, Gone JP. Multicultural issues in the clinical interview and diagnostic process. In Leong FTL, ed. APA handbook of multicultural psychology. Vol 2. Applications and training. Washington, DC: American Psychological Association; 2014:153-163.
25. Sharma M, Romas JA. Theoretical foundations of health education and health promotion. 2nd ed. Burlington, MA: Jones & Barlett Learning; 2012.
26. Jacobsen PB, Ransom S. Implementation of NCCN distress management guidelines by member institutions. J Natl Compr Canc Netw. 2007;5:99-103.
A comprehensive, patient-centered approach is required to accomplish cancer best standards of care.1 This approach reflects the holistic conceptualization of health in which the physical, emotional, and social dimensions of the human being are considered when providing medical care. As a result, to look after all patient needs, interdisciplinary and well-coordinated interventions are recommended. Cancer patients should be provided not only with diagnostic, treatment, and follow-up clinical service, but also with the supportive assistance that may positively influence all aspects of their health.
To appraise physical, social, emotional and spiritual issues and to develop supportive interventional action plans, the National Comprehensive Cancer Network (NCCN) recommends screening all cancer patients for distress.2 In particular, screening the emotional component of distress occupies a prominent place in this process because it is now recognized as the sixth vital sign in oncology.3 Even though the influence of emotional distress over cancer mortality rates and disease progression is still under scrutiny,4 its plausible implications over treatment compliance have been pointed out. Patients with higher levels of emotional distress show lower adherence to treatment and poorer health outcomes.5 Furthermore, prevalence rates of emotional distress in cancer patients from ambulatory settings6 and oncology surgical units have been studied and have provided justification for distress management.7 Studies have shown low ability among oncologists to identify patients in distress and oncologists’ tendency to judge distress higher than the patients themselves.8 As a consequence, to achieve systematic distress evaluations and appropriate referrals for care, guidelines for distress management should be implemented in clinical settings. It is recommended that tests are conducted to find brief screening instruments and procedures to assure accurate interventions according to patient specific needs.
This article presents the process of implementing a distress management program at HIMA-San Pablo Oncologic Hospital in Caguas, Puerto Rico, with particular emphasis on the management of emotional distress, which has been defined as the feeling of suffering that cancer patients may experience after diagnosis. In addition, we have included data from a pilot study that was completed for content validation of the Patient Health Questionnaire (PHQ-9) to estimate depression levels in Puerto Rican cancer patients.
Methods
HIMA-San Pablo operates a group of privately owned hospitals in Puerto Rico. It established a cancer center in Caguas in 2007, recruiting a multispecialty medical faculty to provide cancer care and bone marrow transplants for adult and pediatric patients. The cancer center, currently named HIMA-San Pablo Oncologic Hospital (HSPOH), is a hospital within a hospital licensed by the Puerto Rico Department of Health. In 2007, a cancer committee was established as the steering committee to ensure the delivery of cancer care according to best standards of care. The committee took responsibility for developing all activities needed to achieve the American College of Surgeons’ Commission on Cancer (CoC) accreditation under the category of Comprehensive Community Cancer Center. The committee established a psychosocial team to develop a protocol for the delivery of distress management for adult patients. (The psychosocial needs of pediatric patients are assessed through other procedures.)
To develop the protocol, principles of input-output model of research and quality analysis in health care were applied.9 The input-output model, with its origin in engineering, helped map systematic activities to transform empirical data on cancer psychosocial care into operational procedures. Focus was given to data gathering (input), information organization and analysis (throughput), and the schematization of emotional distress management care (output).
The input phase
In the input phase, elements of psychosocial care and operational definitions related to distress management in general were identified through literature review (Table 1). Basic parameters for distress management were clarified, resulting in a conceptual framework based in four remarks: First, according to NCCN, distress is a multifactorial unpleasant emotional experience of a psychological, social, and spiritual nature. It may interfere with the ability to cope effectively with cancer, its symptoms and its treatment. Its intensity may fluctuate from feelings of suffering and fear to incapacitating manifestations of anxiety and depression2 and its severity may hamper patient quality of life and treatment compliance.
Second, distress management requires the intervention of an interdisciplinary team with both medical and allied health professionals. This may include mental health specialists and other professionals with training and experience in cancer-related issues, who work with reciprocal channels of communication for the exchange of patient information.
Third, NCCN recommends using the Distress Thermometer for patient initial distress screening.10-12 It consists of a numeric scale ranging from 0 (no distress) to 10 (severe distress) in which patients classify their level of distress. The numeric scale is followed by a section in which patients identify areas of practical, familiar, emotional, spiritual/ religious, and physical concerns. Based on responses, interviews may follow to set distress management interventions.
Fourth, screening and assessment are different but sequential and complementary stages of distress management. Screening is viewed as a rapid strategy to identify cancer patients in distress. Assessment looks out for a broader appraisal and documentation of factors with repercussions over patient distress level and resiliency capability.13 In many instances, the patient’s emotional distress is better understood in the assessment phase.
The throughput phase
Within the throughput phase of information organization and analysis, an inventory of health professionals and other in-house consultants needed for distress management was completed. Roles and procedures for information sharing were determined, and we established collaborative agreements with professionals in the community who could contribute to distress management. Members of the psychosocial team held workshops to discuss elements of NCCN guidelines for distress management and to create an action plan for the implementation of the protocol. Data analyses were performed to create a demographic profile of the oncology population at the hospital and assess patient willingness to receive emotional support services,16 which led to the implementation of support group meetings at which additional substantive information was collected about issues affecting cancer patients’ emotions.
The NCCN Distress Thermometer for measuring distress was translated to Spanish. Its format was adapted, and it was identified as a distress screening tool (DST), which we named Distress Assessment Tool for Oncology Patients (Figure 1). The instrument helps for rapid screening of patient needs and proper determination of initial interventions. In addition, psychometric properties of several instruments were reviewed for instances when patient emotional distress could not be clearly determined. We decided to proceed with the validation of the 9-item Patient Health Questionnaire (PHQ-9) to estimate patient depression level. A proposal for content validation of the PHQ-9 was approved by the University of Puerto Rico institutional review board, and patients were recruited to participate in the pilot study.
The PHQ-9. The PHQ-9 is a self-report version of the PRIME-MD instrument developed to assess mental disorders in clinical settings. It is based on DSM-IV diagnostic criteria.17 The PHQ-9 is the depression module with nine depression symptoms to check off if they become the cause of emotional impairment. Respondents categorized depression symptoms in four frequency degrees representing numeric values: 0 (not at all), 1 (several days), 2 (more than half the days), 3 (nearly every day). Measures of depression severity are subsequently determined in a Likert-type scale according to numeric calculations of responses: 0-4 (none severe depression), 5-9 (mild), 10-14 (moderate), 15-19 (moderately severe), and 20-27 (severe-major depression).
The instrument is widely used because of its validity in small and large populations. It showed adequate reliability and validity in a small sample of head and neck cancer patients, with a Cronbach’s alpha of 0.80 and a correlation coefficient of 0.71.18 Similarly, it showed good performance in identifying major depression in 4264 cancer outpatients, with sensitivity of 93%, specificity of 81%, and a positive predictive value (PPV) of 25% and negative predictive value (NPV) of 99%.19 Even when administered on a touch screen computer, the instrument showed valid data of depression from patients in treatment.20
The Beck Depression Inventory. We used the Beck Depression Inventory (BDI-II) Spanish version as the gold standard measure for the validation study. It is a 22-items inventory that measures attitudes and symptoms of depression.21 It can be administered in 10 minutes and has shown good psychometric measures when administered in Spain and Puerto Rico.22, 23
The pilot study. In all, 44 cancer patients who were receiving outpatient treatment at the radiotherapy unit agreed to participate in the study. The participants signed a consent form after the confidentiality protection measures and the main objectives of the study had been explained to them. Patients were interviewed individually during November and December 2012, with the Spanish versions of the PHQ-9 and BDI-II administered by one of two interviewers. At the beginning of each interview, the patient was asked 10 questions so that we could gather demographic data and confirm participant eligibility: aged 21 years or older, born and raised in Puerto Rico, being a Spanish speaker, and having a primary cancer diagnosis with no previous disease. Three patients were excluded from the sample because they either had cancer previously or had a recurrence or metastasis. The final sample consisted of 41 outpatients (N = 41).
Data analysis for demographics was completed with STATA v.12 software. Measures of central tendency and dispersion as well as PHQ-9 internal consistency analysis were made through Cronbach alpha with SPSS.
From a total of 41 patients surveyed, 22 (54%) were men and 19 (46%) were women, with an overall median age of 61 years. Among the men, 15 (68%) had a prostate cancer diagnosis and among women, 9 (47.4%) had a breast cancer diagnosis. In regard to health insurance, 19 (46%) had Medicare or Veterans/federal insurance coverage, and 13 (32%) had Reforma, the Puerto Rican government health insurance program partially funded by Medicaid funds. In addition, 8 participants (20%) were unemployed or disabled. As previously stated, all of the patients were in ambulatory care. Only 3 (7%) were participating in support groups.
Of all the respondents, 16 (39%) reported some level of depression. In particular, 2 (5%) showed severe-major depression, 4 (10%) moderately severe depression, and 10 (24%) moderate depression. Of those with depression, 8 (50%) were women, 8 (50%) were men. All 6 of the patients with head and neck cancer showed moderate or moderately severe depression (Table 2).
When respondent PHQ-9 scoring reflected moderate to severe depression (>10), a letter was sent to the patient’s radio-oncologist for referral to counseling and clinical psychological evaluation. All participants had access to the support group program, to a radiotherapy education program meeting weekly, and written information about their cancer diagnosis and treatment. They also were interviewed by the psychosocial coordinator or patient navigator for further assessment.
The output phase
In the output phase, a graphic representing the process of emotional assessment at the institution was created and then modified. PHQ-9 was added to the process when it was found suitable to assess level of depression contributing to the identification of patients requiring psychological and psychiatric assistance which by other means would be missed. PHQ-9 was useful in the busy clinical setting as it was completed, scored and interpreted in minutes. It showed the potential for routine evaluations when looking to identify improvement or deterioration in depression levels thus helping to monitor responses to treatment and providing insights for follow up interventions. As stated by NCCN guidelines, distress should be monitored, documented and managed at all stages of the cancer continuum.
Results and discussion
The protocol for distress management at HSPOH is based on the 2013 NCCN guidelines. Cancer patients are screened for levels of distress in all settings (inpatients and outpatients). Screening is held with the DST Spanish translation at the moment of diagnosis or as soon as possible after a diagnosis is made. Screening for distress is also done before or after surgery, in recurrence or progression, and when clinically indicated. Patients are informed that distress management is an essential part of their care and are encouraged to provide information so that we can make a proper need assessment.
Patients are screened by the psychosocial coordinator or patient navigator who administers the DST followed by in-depth interviews for additional appraisal. An action plan is designed based on patient needs, which include their intervention and the intervention of other members of the psychosocial team from the institution and/or from the community. Additional in-house health professionals contributing in distress management include, but are not limited to: physicians; clinical psychologists; health educators; social workers; dietitians; chaplains; and physical, respiratory, speech, and/or swallow therapists. Follow-up and rescreening sessions are scheduled to assure coordination of services between those health professionals as well as to secure continuity of distress management during all stages of the cancer continuum.
The results of the DST are filed in patient medical records. Members of the psychosocial team also document their interventions in the patient medical record, which helps in the exchange of information among the cancer care team. The psychosocial team meets once a month – or as required for extraordinary cases – to review and discuss the cases, determine the best options for distress management, and identify areas for psychosocial care improvement. Those findings and the results of distress management in patient level of satisfaction are then reported and discussed quarterly by the psychosocial coordinator and the cancer committee.
Figure 2 shows in what phase of emotional distress assessment the PHQ-9 was included. Patients reporting four or more of the six areas of concern related to emotional distress in the DST (Figure 1) are automatically referred to a mental health specialist. But when patients report three areas of concern with no clear data on their specific level of depression, PHQ-9 is administered to differentiate those who need a mental health specialist from those who could be adequately supported by health education and support group interventions. In this way detrimental outcomes such as duplicity and over or underuse of services and resources are reduced. In addition, it is recognized that using an interview after the administration of the DST to determine distress management actions does not always provide enough information about a patient’s emotional circumstances and previous comorbidities. Patient responses during interviews may be influenced by the patient’s level of literacy, verbal comprehension, and communication style,24 so emotional distress can go unrecognized during interviews, resulting in delays for treatment and supportive care.
National guidelines in oncology consider such socio-ecological models emphasizing the delivery of patient-centered, interdisciplinary, and evidence-based care. That does not mean that institutions should apply protocols of psychosocial care as previously developed, but that they should test, review, adapt, and improve them during the implementation of the care. In fact, NCCN encourages conducting trials to examine protocols, screening instruments, and models of intervention to determine applicability to particular settings.2
Findings from a study by NCCN member institutions to evaluate progress of implementing distress management guidelines found that 53% (n = 8) of respondent institutions conducted routine distress screening. Of those, 37.5% (3) relied only on interviews. That finding is of concern because if interviews are not standardized and have not been systematically evaluated, then their sensitivity and specificity in identifying distressed patients is unknown.26 Accordingly, the process described in this article and the PHQ-9 validation was an effort to standardize emotional distress management, and was underlined as an achievement during the CoC accreditation visit to the cancer center in December 2013. The hospital was accredited as a comprehensive community cancer center with gold commendations, becoming the first privately owned hospital in Puerto Rico to achieve the accreditation.
A comprehensive, patient-centered approach is required to accomplish cancer best standards of care.1 This approach reflects the holistic conceptualization of health in which the physical, emotional, and social dimensions of the human being are considered when providing medical care. As a result, to look after all patient needs, interdisciplinary and well-coordinated interventions are recommended. Cancer patients should be provided not only with diagnostic, treatment, and follow-up clinical service, but also with the supportive assistance that may positively influence all aspects of their health.
To appraise physical, social, emotional and spiritual issues and to develop supportive interventional action plans, the National Comprehensive Cancer Network (NCCN) recommends screening all cancer patients for distress.2 In particular, screening the emotional component of distress occupies a prominent place in this process because it is now recognized as the sixth vital sign in oncology.3 Even though the influence of emotional distress over cancer mortality rates and disease progression is still under scrutiny,4 its plausible implications over treatment compliance have been pointed out. Patients with higher levels of emotional distress show lower adherence to treatment and poorer health outcomes.5 Furthermore, prevalence rates of emotional distress in cancer patients from ambulatory settings6 and oncology surgical units have been studied and have provided justification for distress management.7 Studies have shown low ability among oncologists to identify patients in distress and oncologists’ tendency to judge distress higher than the patients themselves.8 As a consequence, to achieve systematic distress evaluations and appropriate referrals for care, guidelines for distress management should be implemented in clinical settings. It is recommended that tests are conducted to find brief screening instruments and procedures to assure accurate interventions according to patient specific needs.
This article presents the process of implementing a distress management program at HIMA-San Pablo Oncologic Hospital in Caguas, Puerto Rico, with particular emphasis on the management of emotional distress, which has been defined as the feeling of suffering that cancer patients may experience after diagnosis. In addition, we have included data from a pilot study that was completed for content validation of the Patient Health Questionnaire (PHQ-9) to estimate depression levels in Puerto Rican cancer patients.
Methods
HIMA-San Pablo operates a group of privately owned hospitals in Puerto Rico. It established a cancer center in Caguas in 2007, recruiting a multispecialty medical faculty to provide cancer care and bone marrow transplants for adult and pediatric patients. The cancer center, currently named HIMA-San Pablo Oncologic Hospital (HSPOH), is a hospital within a hospital licensed by the Puerto Rico Department of Health. In 2007, a cancer committee was established as the steering committee to ensure the delivery of cancer care according to best standards of care. The committee took responsibility for developing all activities needed to achieve the American College of Surgeons’ Commission on Cancer (CoC) accreditation under the category of Comprehensive Community Cancer Center. The committee established a psychosocial team to develop a protocol for the delivery of distress management for adult patients. (The psychosocial needs of pediatric patients are assessed through other procedures.)
To develop the protocol, principles of input-output model of research and quality analysis in health care were applied.9 The input-output model, with its origin in engineering, helped map systematic activities to transform empirical data on cancer psychosocial care into operational procedures. Focus was given to data gathering (input), information organization and analysis (throughput), and the schematization of emotional distress management care (output).
The input phase
In the input phase, elements of psychosocial care and operational definitions related to distress management in general were identified through literature review (Table 1). Basic parameters for distress management were clarified, resulting in a conceptual framework based in four remarks: First, according to NCCN, distress is a multifactorial unpleasant emotional experience of a psychological, social, and spiritual nature. It may interfere with the ability to cope effectively with cancer, its symptoms and its treatment. Its intensity may fluctuate from feelings of suffering and fear to incapacitating manifestations of anxiety and depression2 and its severity may hamper patient quality of life and treatment compliance.
Second, distress management requires the intervention of an interdisciplinary team with both medical and allied health professionals. This may include mental health specialists and other professionals with training and experience in cancer-related issues, who work with reciprocal channels of communication for the exchange of patient information.
Third, NCCN recommends using the Distress Thermometer for patient initial distress screening.10-12 It consists of a numeric scale ranging from 0 (no distress) to 10 (severe distress) in which patients classify their level of distress. The numeric scale is followed by a section in which patients identify areas of practical, familiar, emotional, spiritual/ religious, and physical concerns. Based on responses, interviews may follow to set distress management interventions.
Fourth, screening and assessment are different but sequential and complementary stages of distress management. Screening is viewed as a rapid strategy to identify cancer patients in distress. Assessment looks out for a broader appraisal and documentation of factors with repercussions over patient distress level and resiliency capability.13 In many instances, the patient’s emotional distress is better understood in the assessment phase.
The throughput phase
Within the throughput phase of information organization and analysis, an inventory of health professionals and other in-house consultants needed for distress management was completed. Roles and procedures for information sharing were determined, and we established collaborative agreements with professionals in the community who could contribute to distress management. Members of the psychosocial team held workshops to discuss elements of NCCN guidelines for distress management and to create an action plan for the implementation of the protocol. Data analyses were performed to create a demographic profile of the oncology population at the hospital and assess patient willingness to receive emotional support services,16 which led to the implementation of support group meetings at which additional substantive information was collected about issues affecting cancer patients’ emotions.
The NCCN Distress Thermometer for measuring distress was translated to Spanish. Its format was adapted, and it was identified as a distress screening tool (DST), which we named Distress Assessment Tool for Oncology Patients (Figure 1). The instrument helps for rapid screening of patient needs and proper determination of initial interventions. In addition, psychometric properties of several instruments were reviewed for instances when patient emotional distress could not be clearly determined. We decided to proceed with the validation of the 9-item Patient Health Questionnaire (PHQ-9) to estimate patient depression level. A proposal for content validation of the PHQ-9 was approved by the University of Puerto Rico institutional review board, and patients were recruited to participate in the pilot study.
The PHQ-9. The PHQ-9 is a self-report version of the PRIME-MD instrument developed to assess mental disorders in clinical settings. It is based on DSM-IV diagnostic criteria.17 The PHQ-9 is the depression module with nine depression symptoms to check off if they become the cause of emotional impairment. Respondents categorized depression symptoms in four frequency degrees representing numeric values: 0 (not at all), 1 (several days), 2 (more than half the days), 3 (nearly every day). Measures of depression severity are subsequently determined in a Likert-type scale according to numeric calculations of responses: 0-4 (none severe depression), 5-9 (mild), 10-14 (moderate), 15-19 (moderately severe), and 20-27 (severe-major depression).
The instrument is widely used because of its validity in small and large populations. It showed adequate reliability and validity in a small sample of head and neck cancer patients, with a Cronbach’s alpha of 0.80 and a correlation coefficient of 0.71.18 Similarly, it showed good performance in identifying major depression in 4264 cancer outpatients, with sensitivity of 93%, specificity of 81%, and a positive predictive value (PPV) of 25% and negative predictive value (NPV) of 99%.19 Even when administered on a touch screen computer, the instrument showed valid data of depression from patients in treatment.20
The Beck Depression Inventory. We used the Beck Depression Inventory (BDI-II) Spanish version as the gold standard measure for the validation study. It is a 22-items inventory that measures attitudes and symptoms of depression.21 It can be administered in 10 minutes and has shown good psychometric measures when administered in Spain and Puerto Rico.22, 23
The pilot study. In all, 44 cancer patients who were receiving outpatient treatment at the radiotherapy unit agreed to participate in the study. The participants signed a consent form after the confidentiality protection measures and the main objectives of the study had been explained to them. Patients were interviewed individually during November and December 2012, with the Spanish versions of the PHQ-9 and BDI-II administered by one of two interviewers. At the beginning of each interview, the patient was asked 10 questions so that we could gather demographic data and confirm participant eligibility: aged 21 years or older, born and raised in Puerto Rico, being a Spanish speaker, and having a primary cancer diagnosis with no previous disease. Three patients were excluded from the sample because they either had cancer previously or had a recurrence or metastasis. The final sample consisted of 41 outpatients (N = 41).
Data analysis for demographics was completed with STATA v.12 software. Measures of central tendency and dispersion as well as PHQ-9 internal consistency analysis were made through Cronbach alpha with SPSS.
From a total of 41 patients surveyed, 22 (54%) were men and 19 (46%) were women, with an overall median age of 61 years. Among the men, 15 (68%) had a prostate cancer diagnosis and among women, 9 (47.4%) had a breast cancer diagnosis. In regard to health insurance, 19 (46%) had Medicare or Veterans/federal insurance coverage, and 13 (32%) had Reforma, the Puerto Rican government health insurance program partially funded by Medicaid funds. In addition, 8 participants (20%) were unemployed or disabled. As previously stated, all of the patients were in ambulatory care. Only 3 (7%) were participating in support groups.
Of all the respondents, 16 (39%) reported some level of depression. In particular, 2 (5%) showed severe-major depression, 4 (10%) moderately severe depression, and 10 (24%) moderate depression. Of those with depression, 8 (50%) were women, 8 (50%) were men. All 6 of the patients with head and neck cancer showed moderate or moderately severe depression (Table 2).
When respondent PHQ-9 scoring reflected moderate to severe depression (>10), a letter was sent to the patient’s radio-oncologist for referral to counseling and clinical psychological evaluation. All participants had access to the support group program, to a radiotherapy education program meeting weekly, and written information about their cancer diagnosis and treatment. They also were interviewed by the psychosocial coordinator or patient navigator for further assessment.
The output phase
In the output phase, a graphic representing the process of emotional assessment at the institution was created and then modified. PHQ-9 was added to the process when it was found suitable to assess level of depression contributing to the identification of patients requiring psychological and psychiatric assistance which by other means would be missed. PHQ-9 was useful in the busy clinical setting as it was completed, scored and interpreted in minutes. It showed the potential for routine evaluations when looking to identify improvement or deterioration in depression levels thus helping to monitor responses to treatment and providing insights for follow up interventions. As stated by NCCN guidelines, distress should be monitored, documented and managed at all stages of the cancer continuum.
Results and discussion
The protocol for distress management at HSPOH is based on the 2013 NCCN guidelines. Cancer patients are screened for levels of distress in all settings (inpatients and outpatients). Screening is held with the DST Spanish translation at the moment of diagnosis or as soon as possible after a diagnosis is made. Screening for distress is also done before or after surgery, in recurrence or progression, and when clinically indicated. Patients are informed that distress management is an essential part of their care and are encouraged to provide information so that we can make a proper need assessment.
Patients are screened by the psychosocial coordinator or patient navigator who administers the DST followed by in-depth interviews for additional appraisal. An action plan is designed based on patient needs, which include their intervention and the intervention of other members of the psychosocial team from the institution and/or from the community. Additional in-house health professionals contributing in distress management include, but are not limited to: physicians; clinical psychologists; health educators; social workers; dietitians; chaplains; and physical, respiratory, speech, and/or swallow therapists. Follow-up and rescreening sessions are scheduled to assure coordination of services between those health professionals as well as to secure continuity of distress management during all stages of the cancer continuum.
The results of the DST are filed in patient medical records. Members of the psychosocial team also document their interventions in the patient medical record, which helps in the exchange of information among the cancer care team. The psychosocial team meets once a month – or as required for extraordinary cases – to review and discuss the cases, determine the best options for distress management, and identify areas for psychosocial care improvement. Those findings and the results of distress management in patient level of satisfaction are then reported and discussed quarterly by the psychosocial coordinator and the cancer committee.
Figure 2 shows in what phase of emotional distress assessment the PHQ-9 was included. Patients reporting four or more of the six areas of concern related to emotional distress in the DST (Figure 1) are automatically referred to a mental health specialist. But when patients report three areas of concern with no clear data on their specific level of depression, PHQ-9 is administered to differentiate those who need a mental health specialist from those who could be adequately supported by health education and support group interventions. In this way detrimental outcomes such as duplicity and over or underuse of services and resources are reduced. In addition, it is recognized that using an interview after the administration of the DST to determine distress management actions does not always provide enough information about a patient’s emotional circumstances and previous comorbidities. Patient responses during interviews may be influenced by the patient’s level of literacy, verbal comprehension, and communication style,24 so emotional distress can go unrecognized during interviews, resulting in delays for treatment and supportive care.
National guidelines in oncology consider such socio-ecological models emphasizing the delivery of patient-centered, interdisciplinary, and evidence-based care. That does not mean that institutions should apply protocols of psychosocial care as previously developed, but that they should test, review, adapt, and improve them during the implementation of the care. In fact, NCCN encourages conducting trials to examine protocols, screening instruments, and models of intervention to determine applicability to particular settings.2
Findings from a study by NCCN member institutions to evaluate progress of implementing distress management guidelines found that 53% (n = 8) of respondent institutions conducted routine distress screening. Of those, 37.5% (3) relied only on interviews. That finding is of concern because if interviews are not standardized and have not been systematically evaluated, then their sensitivity and specificity in identifying distressed patients is unknown.26 Accordingly, the process described in this article and the PHQ-9 validation was an effort to standardize emotional distress management, and was underlined as an achievement during the CoC accreditation visit to the cancer center in December 2013. The hospital was accredited as a comprehensive community cancer center with gold commendations, becoming the first privately owned hospital in Puerto Rico to achieve the accreditation.
1. Commission on Cancer, American College of Surgeons. Cancer Programs Standards 2012: Ensuring Patient-Centered Care. Version 1.2.1. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx. Published 2012. Accessed March 5, 2013.
2. National Comprehensive Cancer Network clinical practice guidelines in oncology (NCCN guidelines): Distress management. Version I. 2012. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#supportive. Accessed March 5, 2013.
3. Bultz BD, Groff SL. Screening for distress, the 6th vital sign in oncology: from theory to practice: http://www.oncologyex.com/issue/2009/vol8_no1/8_comment2_1.html. Published February 2009. Accessed February 16, 2017.
4. Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009;115:5349-5361.
5. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Int Med. 2000;160:2101-2107.
6. Jadoon NA, Munir W, Shahzad MA, Choudhry ZS. Assessment of depression and anxiety in adult cancer outpatients: a cross sectional study. BMC Cancer. 2010;10:594.
7. Fisher D, Wedel B. Anxiety and depression disorders in cancer patients: incidence, diagnosis and therapy. Mag Eur Med Oncol. 2012;5:52-54.
8. Sollner W, DeVries A, Steixner E, et al. How successful are oncologists in identifying patient distress, perceived social support, and in need for psychosocial counselling? Br J Cancer. 2001;84:179-185.
9. Scott RD, Solomon SL, McGowan JE. Applying economic principles to health care: special issue. Emerg Infect Dis. 2001;7:282-285.
10. Adler NE, Page AEK. A model for delivering psychosocial health services. In: Cancer care for the whole patient: meeting psychosocial health needs. Washington, DC: National Academies Press (US); 2008.
11. Holland JC, Alici Y. Management of distress in cancer patients. J Support Oncol. 2010;8:4-12.
12. Jacobsen PB, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005;103:1494-1502.
13. Maihoff SE. Assessment. In Washington CM, Leaver D, eds. Principles and practice of radiation therapy. St Louis, MO: Mosby Elsevier; 2004:243-264.
14. National Academy of Sciences. Adler NE, Page AEK, eds. Cancer care for the whole patient: meeting psychosocial health needs. https://www.ncbi.nlm.nih.gov/books/NBK4015/. Published 2008. Accessed February 22, 2012.
15. Nancarrow SA, Booth A, Ariss
16. Baker-Glenn EA, Park B, Granger L, Symonds P, Mitchell AJ. Desire for psychological support in cancer patients with depression or distress: validation of a simple help question. Psychooncology. 2011;20:525-531.
17. Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
18. Omoro SA, Fann JR, Weymuller EA, Macharia IM, Yueh B. Swahili translation and validation of the Patient Health Questionnaire-9 depression scale in the Kenyan head and neck cancer patient population. Int J Psychiatry Med. 2006;36:367-381.
19. Thekkumpurath P, Walker J, Butcher I, et al. Screening for major depression on cancer outpatients: the diagnostic accuracy of the 9-item Patient Health Questionnaire. Cancer. 2011;117:218-227.
20. Fann JR, Berry DL, Wolpin S, et al. Depression screening using the Patient Health Questionnaire-9 administered on a touch screen computer. Psychooncology. 2009;18:14-22.
21. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:651-571.
22. Sanz J, Perdigón AL, Vázquez C. The Spanish adaptation of Beck’s Depression Inventory–II (BDI-II): psychometric properties in the general population. Clínica y Salud. 2003;14:249-280.
23. Bonilla J, Bernal G, Santos A, Santos D. A revised Spanish version of the Beck Depression Inventory: psychometric properties with a Puerto Rican sample of college students. J Clin Psychol. 2004;60:119-130.
24. Alcántara C, Gone JP. Multicultural issues in the clinical interview and diagnostic process. In Leong FTL, ed. APA handbook of multicultural psychology. Vol 2. Applications and training. Washington, DC: American Psychological Association; 2014:153-163.
25. Sharma M, Romas JA. Theoretical foundations of health education and health promotion. 2nd ed. Burlington, MA: Jones & Barlett Learning; 2012.
26. Jacobsen PB, Ransom S. Implementation of NCCN distress management guidelines by member institutions. J Natl Compr Canc Netw. 2007;5:99-103.
1. Commission on Cancer, American College of Surgeons. Cancer Programs Standards 2012: Ensuring Patient-Centered Care. Version 1.2.1. https://www.facs.org/~/media/files/quality%20programs/cancer/coc/programstandards2012.ashx. Published 2012. Accessed March 5, 2013.
2. National Comprehensive Cancer Network clinical practice guidelines in oncology (NCCN guidelines): Distress management. Version I. 2012. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#supportive. Accessed March 5, 2013.
3. Bultz BD, Groff SL. Screening for distress, the 6th vital sign in oncology: from theory to practice: http://www.oncologyex.com/issue/2009/vol8_no1/8_comment2_1.html. Published February 2009. Accessed February 16, 2017.
4. Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer. 2009;115:5349-5361.
5. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Int Med. 2000;160:2101-2107.
6. Jadoon NA, Munir W, Shahzad MA, Choudhry ZS. Assessment of depression and anxiety in adult cancer outpatients: a cross sectional study. BMC Cancer. 2010;10:594.
7. Fisher D, Wedel B. Anxiety and depression disorders in cancer patients: incidence, diagnosis and therapy. Mag Eur Med Oncol. 2012;5:52-54.
8. Sollner W, DeVries A, Steixner E, et al. How successful are oncologists in identifying patient distress, perceived social support, and in need for psychosocial counselling? Br J Cancer. 2001;84:179-185.
9. Scott RD, Solomon SL, McGowan JE. Applying economic principles to health care: special issue. Emerg Infect Dis. 2001;7:282-285.
10. Adler NE, Page AEK. A model for delivering psychosocial health services. In: Cancer care for the whole patient: meeting psychosocial health needs. Washington, DC: National Academies Press (US); 2008.
11. Holland JC, Alici Y. Management of distress in cancer patients. J Support Oncol. 2010;8:4-12.
12. Jacobsen PB, Donovan KA, Trask PC, et al. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005;103:1494-1502.
13. Maihoff SE. Assessment. In Washington CM, Leaver D, eds. Principles and practice of radiation therapy. St Louis, MO: Mosby Elsevier; 2004:243-264.
14. National Academy of Sciences. Adler NE, Page AEK, eds. Cancer care for the whole patient: meeting psychosocial health needs. https://www.ncbi.nlm.nih.gov/books/NBK4015/. Published 2008. Accessed February 22, 2012.
15. Nancarrow SA, Booth A, Ariss
16. Baker-Glenn EA, Park B, Granger L, Symonds P, Mitchell AJ. Desire for psychological support in cancer patients with depression or distress: validation of a simple help question. Psychooncology. 2011;20:525-531.
17. Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606-613.
18. Omoro SA, Fann JR, Weymuller EA, Macharia IM, Yueh B. Swahili translation and validation of the Patient Health Questionnaire-9 depression scale in the Kenyan head and neck cancer patient population. Int J Psychiatry Med. 2006;36:367-381.
19. Thekkumpurath P, Walker J, Butcher I, et al. Screening for major depression on cancer outpatients: the diagnostic accuracy of the 9-item Patient Health Questionnaire. Cancer. 2011;117:218-227.
20. Fann JR, Berry DL, Wolpin S, et al. Depression screening using the Patient Health Questionnaire-9 administered on a touch screen computer. Psychooncology. 2009;18:14-22.
21. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:651-571.
22. Sanz J, Perdigón AL, Vázquez C. The Spanish adaptation of Beck’s Depression Inventory–II (BDI-II): psychometric properties in the general population. Clínica y Salud. 2003;14:249-280.
23. Bonilla J, Bernal G, Santos A, Santos D. A revised Spanish version of the Beck Depression Inventory: psychometric properties with a Puerto Rican sample of college students. J Clin Psychol. 2004;60:119-130.
24. Alcántara C, Gone JP. Multicultural issues in the clinical interview and diagnostic process. In Leong FTL, ed. APA handbook of multicultural psychology. Vol 2. Applications and training. Washington, DC: American Psychological Association; 2014:153-163.
25. Sharma M, Romas JA. Theoretical foundations of health education and health promotion. 2nd ed. Burlington, MA: Jones & Barlett Learning; 2012.
26. Jacobsen PB, Ransom S. Implementation of NCCN distress management guidelines by member institutions. J Natl Compr Canc Netw. 2007;5:99-103.
Demystifying the diagnosis and classification of lymphoma: a guide to the hematopathologist’s galaxy
Lymphomas constitute a very heterogeneous group of neoplasms with diverse clinical presentations, prognoses, and responses to therapy. Approximately 80,500 new cases of lymphoma are expected to be diagnosed in the United States in 2017, of which about one quarter will lead to the death of the patient.1 Perhaps more so than any other group of neoplasms, the diagnosis of lymphoma involves the integration of a multiplicity of clinical, histologic and immunophenotypic findings and, on occasion, cytogenetic and molecular results as well. An accurate diagnosis of lymphoma, usually rendered by hematopathologists, allows hematologists/oncologists to treat patients appropriately. Herein we will describe a simplified approach to the diagnosis and classification of lymphomas (Figure 1).
Lymphoma classification
Lymphomas are clonal neoplasms characterized by the expansion of abnormal lymphoid cells that may develop in any organ but commonly involve lymph nodes. The fourth edition of the World Health Organization (WHO) Classification of Tumours of Haematopoietic and Lymphoid tissues, published in 2008, is the official and most current guideline used for diagnosis of lymphoid neoplasms.2 The WHO scheme classifies lymphomas according to the type of cell from which they are derived (mature and immature B cells, T cells, or natural killer (NK) cells, findings determined by their morphology and immunophenotype) and their clinical, cytogenetic, and/or molecular features. This official classification is currently being updated3 and is expected to be published in full in 2017, at which time it is anticipated to include definitions for more than 70 distinct neoplasms.
Lymphomas are broadly and informally classified as Hodgkin lymphomas (HLs) and non-Hodgkin lymphomas (NHLs), based on the differences these two groups show in their clinical presentation, treatment, prognosis, and proportion of neoplastic cells, among others. NHLs are by far the most common type of lymphomas, accounting for approximately 90% of all new cases of lymphoma in the United States and 70% worldwide.1,2 NHLs are a very heterogeneous group of B-, T-, or NK-cell neoplasms that, in turn, can also be informally subclassified as low-grade (or indolent) or high-grade (or aggressive) according to their predicted clinical behavior. HLs are comparatively rare, less heterogeneous, uniformly of B-cell origin and, in the case of classical Hodgkin lymphoma, highly curable.1,2 It is beyond the scope of this manuscript to outline the features of each of the >70 specific entities, but the reader is referred elsewhere for more detail and encouraged to become familiarized with the complexity, challenges, and beauty of lymphoma diagnosis.2,3
Biopsy procedure
A correct diagnosis begins with an adequate biopsy procedure. It is essential that biopsy specimens for lymphoma evaluation be submitted fresh and unfixed, because some crucial analyses such as flow cytometry or conventional cytogenetics can only be performed on fresh tissue. Indeed, it is important for the hematologist/oncologist and/or surgeon and/or interventional radiologist to converse with the hematopathologist prior to and even during some procedures to ensure the correct processing of the specimen. Also, it is important to limit the compression of the specimen and the excessive use of cauterization during the biopsy procedure, both of which cause artifacts that may render impossible the interpretation of the histopathologic findings.
Given that the diagnosis of lymphoma is based not only on the cytologic details of the lymphoma cells but also on the architectural pattern with which they infiltrate an organ, the larger the biopsy specimen, the easier it will be for a hematopathologist to identify the pattern. In addition, excisional biopsies frequently contain more diagnostic tissue than needle core biopsies and this provides pathologists with the option to submit tissue fragments for ancillary tests that require unfixed tissue as noted above. Needle core biopsies of lymph nodes are increasingly being used because of their association with fewer complications and lower cost than excisional biopsies. However, needle core biopsies provide only a glimpse of the pattern of infiltration and may not be completely representative of the architecture. Therefore, excisional lymph node biopsies of lymph nodes are preferred over needle core biopsies, recognizing that in the setting of deeply seated lymph nodes, needle core biopsies may be the only or the best surgical option.
Clinical presentation
Accurate diagnosis of lymphoma cannot take place in a vacuum. The hematopathologist’s initial approach to the diagnosis of lymphoid processes in tissue biopsies should begin with a thorough review of the clinical history, although some pathology laboratories may not have immediate access to this information. The hematopathologist should evaluate factors such as age, gender, location of the tumor, symptomatology, medications, serology, and prior history of malignancy, immunosuppression or immunodeficiency in every case. Other important but frequently omitted parts of the clinical history are the patient’s occupation, history of exposure to animals, and the presence of tattoos, which may be associated with certain reactive lymphadenopathies.
Histomorphologic evaluation
Despite the plethora of new and increasingly sophisticated tools, histologic and morphologic analysis still remains the cornerstone of diagnosis in hematopathology. However, for the characterization of an increasing number of reactive and neoplastic lymphoid processes, hematopathologists may also require immunophenotypic, molecular, and cytogenetic tests for an accurate diagnosis. Upon review of the clinical information, a microscopic evaluation of the tissue submitted for processing by the histology laboratory will be performed. The results of concurrent flow cytometric evaluation (performed on fresh unfixed material) should also be available in most if not all cases before the H&E-stained slides are available for review. Upon receipt of H&E-stained slides, the hematopathologist will evaluate the quality of the submitted specimen, since many diagnostic difficulties stem from suboptimal techniques related to the biopsy procedure, fixation, processing, cutting, or staining (Figure 1). If deemed suitable for accurate diagnosis, a search for signs of preservation or disruption of the organ that was biopsied will follow. The identification of certain morphologic patterns aids the hematopathologist in answering the first question: “what organ is this and is this consistent with what is indicated on the requisition?” This is usually immediately followed by “is this sufficient and adequate material for a diagnosis?” and “is there any normal architecture?” If the architecture is not normal, “is this alteration due to a reactive or a neoplastic process?” If neoplastic, “is it lymphoma or a non-hematolymphoid neoplasm?”
Both reactive and neoplastic processes have variably unique morphologic features that if properly recognized, guide the subsequent testing. However, some reactive and neoplastic processes can present with overlapping features, and even after extensive immunophenotypic evaluation and the performance of ancillary studies, it may not be possible to conclusively determine its nature. If the lymph node architecture is altered or effaced, the predominant pattern of infiltration (eg, nodular, diffuse, interfollicular, intrasinusoidal) and the degree of alteration of the normal architecture is evaluated, usually at low magnification. When the presence of an infiltrate is recognized, its components must be characterized. If the infiltrate is composed of a homogeneous expansion of lymphoid cells that disrupts or replaces the normal lymphoid architecture, a lymphoma will be suspected or diagnosed. The pattern of distribution of the cells along with their individual morphologic characteristics (ie, size, nuclear shape, chromatin configuration, nucleoli, amount and hue of cytoplasm) are key factors for the diagnosis and classification of the lymphoma that will guide subsequent testing. The immunophenotypic analysis (by immunohistochemistry, flow cytometry or a combination of both) may confirm the reactive or neoplastic nature of the process, and its subclassification. B-cell lymphomas, in particular have variable and distinctive histologic features: as a diffuse infiltrate of large mature lymphoid cells (eg, diffuse large B-cell lymphoma), an expansion of immature lymphoid cells (lymphoblastic lymphoma), and a nodular infiltrate of small, intermediate and/or mature large B cells (eg, follicular lymphoma).
Mature T-cell lymphomas may display similar histologic, features but they can be quite heterogeneous with an infiltrate composed of one predominant cell type or a mixture of small, medium-sized, and large atypical lymphoid cells (on occasion with abundant clear cytoplasm) and a variable number of eosinophils, plasma cells, macrophages (including granulomas), and B cells. HLs most commonly efface the lymph node architecture with a nodular or diffuse infiltrate variably composed of reactive lymphocytes, granulocytes, macrophages, and plasma cells and usually a minority of large neoplastic cells (Hodgkin/Reed-Sternberg cells and/or lymphocyte predominant cells).
Once the H&E-stained slides are evaluated and a diagnosis of lymphoma is suspected or established, the hematopathologist will attempt to determine whether it has mature or immature features, and whether low- or high-grade morphologic characteristics are present. The maturity of lymphoid cells is generally determined by the nature of the chromatin, which if “fine” and homogeneous (with or without a conspicuous nucleolus) will usually, but not always, be considered immature, whereas clumped, vesicular or hyperchromatic chromatin is generally, but not always, associated with maturity. If the chromatin displays immature features, the differential diagnosis will mainly include B- and T-lymphoblastic lymphomas, but also blastoid variants of mature neoplasm such as mantle cell lymphoma, and follicular lymphoma, as well as high-grade B-cell lymphomas. Features associated with low-grade lymphomas (eg, follicular lymphoma, small lymphocytic lymphoma/chronic lymphocytic leukemia, marginal zone lymphoma, lymphoplasmacytic lymphoma) include small cell morphology, mature chromatin, absence of a significant number of mitoses or apoptotic cells, and a low proliferation index as shown by immunohistochemistry for Ki67. High-grade lymphomas, such as lymphoblastic lymphoma, Burkitt lymphoma, or certain large B-cell lymphomas tend to show opposite features, and some of the mature entities are frequently associated with MYC rearrangements. Of note, immature lymphomas tend to be clinically high grade, but not all clinically high-grade lymphomas are immature. Conversely, the majority of low-grade lymphomas are usually mature.
Immunophenotypic evaluation
Immunophenotypic evaluation is essential because the lineage of lymphoma cells cannot be determined by morphology alone. The immunophenotype is the combination of proteins/markers (eg, CD20, CD3, TdT) expressed by cells. Usually, it is evaluated by immunohistochemistry and/or flow cytometry, which help determine the proportion of lymphoid cells that express a certain marker and its location and intensity within the cells. While immunohistochemistry is normally performed on formalin-fixed and paraffin-embedded tissue, flow cytometry can be evaluated only on fresh unfixed tissue. Flow cytometry has the advantage over immunohistochemistry of being faster and better at simultaneously identifying coexpression of multiple markers on multiple cell populations. However, certain markers can only be evaluated by immunohistochemistry.
The immunophenotypic analysis will in most cases reveal whether the lymphomas is of B-, T- or NK-cell origin, and whether a lymphoma subtype associated immunophenotype is present. Typical pan B-cell antigens include PAX5, CD19, and CD79a (CD20 is less broadly expressed throughout B-cell differentiation, although it is usually evident in most mature B-cell lymphomas), and typical pan T-cell antigens include CD2, CD5, and CD7. The immature or mature nature of a lymphoma can also be confirmed by evaluation of the immunophenotype. Immature lymphomas commonly express one or more of TdT, CD10, or CD34; T-lymphoblastic lymphoma cells may also coexpress CD1a. The majority of NHLs and all HLs are derived from (or reflect) B cells at different stages of maturation. Mature B-cell lymphomas are the most common type of lymphoma and typically, but not always, express pan B-cell markers as well as surface membrane immunoglobulin, with the latter also most useful in assessing clonality via a determination of light chain restriction. Some mature B-cell lymphomas tend to acquire markers that are either never physiologically expressed by normal mature B cells (eg, cyclin D1 in mantle cell lymphoma, or BCL2 in germinal center B cells in follicular lymphoma) or only expressed in a minor fraction (eg, CD5 that is characteristically expressed in small lymphocytic and mantle cell lymphoma). The most common mature B-cell lymphomas include diffuse large B-cell lymphoma, follicular lymphoma, small lymphocytic lymphoma, mantle cell lymphoma, marginal zone lymphoma, Burkitt lymphoma, and lymphoplasmacytic lymphoma (Figures 2 and 3). Classical HLs are also lymphomas of B-cell origin that demonstrate diminished preservation of their B-cell immunophenotype (as evidenced by the dim expression of PAX5 but absence of most other pan B-cell antigens), expression of CD30, variable expression of CD15, and loss of CD45 (Figure 1). In contrast, nodular lymphocyte predominant HL shows a preserved B-cell immunophenotypic program and expression of CD45, typically without CD30 and CD15. Of note, the evaluation of the immunophenotype of the neoplastic cells in HL is routinely assessed by immunohistochemistry because most flow cytometry laboratories cannot reliably detect and characterize the low numbers of these cells.
Mature T-cell lymphomas generally express one or more T-cell markers, and tend to display a T-helper (CD4-positive) or cytotoxic (CD8-positive) immunophenotype and may show loss of markers expressed by most normal T-cells (eg, CD5, CD7; Figure 4). However, a subset of them may express markers not commonly detected in normal T cells, such as ALK. NK-cell lymphomas lack surface CD3 (expressing only cytoplasmic CD3) and CD5 but express some pan T-cell antigens (such as CD2 and CD7) as well as CD16 and/or CD56.
Patients with primary or acquired immune dysfunction are at risk for development of lymphoma and other less clearly defined lymphoproliferative disorders, the majority of which are associated with infection of the lymphoid cells with Epstein-Barr virus (EBV). Therefore, evaluation with chromogenic in situ hybridization for an EBV-encoded early RNA (EBER1) is routinely performed in these cases; it is thus essential that the hematopathologist be informed of the altered immune system of the patient. If lymphoma develops, they may be morphologically similar to those that appear in immunocompetent patients, which specifically in the post-transplant setting are known as monomorphic post-transplant lymphoproliferative disorders (PTLD). If the PTLD does not meet the criteria for any of the recognized types of lymphoma, it may be best characterized as a polymorphic PTLD.
Once the lineage (B-, T-, or NK-cell) of the mature lymphoma has been established, the sum (and on occasion the gestalt) of the clinical, morphologic, immunophenotypic and other findings will be considered for the subclassification of the neoplasm.
Cytogenetic and molecular evaluation
If the morphologic and immunophenotypic analysis is inconclusive or nondiagnostic, then molecular and/or cytogenetic testing may further aid in the characterization of the process. Some of available molecular tests include analyses for the rearrangements of the variable region of the immunoglobulin (IG) or T-cell receptor (TCR) genes and for mutations on specific genes. The identification of specific mutations not only confirms the clonal nature of the process but, on occasion, it may also help subclassify the lymphoma, whereas IG or TCR rearrangement studies are used to establish whether a lymphoid expansion is polyclonal or monoclonal. The molecular findings should not be evaluated in isolation, because not all monoclonal rearrangements are diagnostic of lymphoma, and not all lymphomas will show a monoclonal rearrangement. Other methodologies that can aid in the identification of a clonal process or specific genetic abnormalities include metaphase cytogenetics (karyotyping) and fluorescence in situ hybridization (FISH). If any cytogenetic abnormalities are found in sufficient numbers (and constitutional abnormalities are excluded), their identification indicates the presence of a clonal process. Also, some cytogenetic abnormalities are characteristic of certain lymphomas. However, they may be neither 100% diagnostically sensitive nor diagnostically specific, for example, the hallmark t(14;18)/IGH-BCL2 is not present in all follicular lymphomas and not all lymphomas with this translocation are follicular lymphomas. Whereas FISH is generally performed on a minimum of 200 cells, compared with typically 20 metaphase by “conventional” karyotyping, and is therefore considered to have higher analytical sensitivity, it evaluates only for the presence or absence of the abnormality being investigated with a given set of probes, and therefore other abnormalities, if present, will not be identified. The value of FISH cytogenetic studies is perhaps best illustrated in the need to diagnose double hit lymphomas, amongst other scenarios. The detection of certain mutations can aid in the diagnosis of certain lymphomas, such as MYD88 in lymphoplasmacytic lymphoma, prognosis of others, such as in follicular lymphoma and identify pathways that may be precisely therapeutically targeted.
Final remarks
The diagnosis of lymphoma can be complex and usually requires the hematopathologist to integrate multiple parameters. The classification of lymphomas is not static, and new entities or variants are continuously described, and the facets of well-known ones refined. While such changes are often to the chagrin of hematologists/oncologists and hematopathologists alike, we should embrace the incorporation of nascent and typically cool data into our practice, as more therapeutically relevant entities are molded.
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017 ;67(1):7-30.
2. Swerdlow SH, Campo E, Harris NL, et al, eds. WHO classification of tumours of haematopoietic and lymphoid tissues. In: Bosman FT, Jaffe ES, Lakhani SR, Ohgaki H, eds. World Health Organization Classification of Tumours. Lyon, France: IARC; 2008.
3. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016 ;127(20):2375-2390.
Lymphomas constitute a very heterogeneous group of neoplasms with diverse clinical presentations, prognoses, and responses to therapy. Approximately 80,500 new cases of lymphoma are expected to be diagnosed in the United States in 2017, of which about one quarter will lead to the death of the patient.1 Perhaps more so than any other group of neoplasms, the diagnosis of lymphoma involves the integration of a multiplicity of clinical, histologic and immunophenotypic findings and, on occasion, cytogenetic and molecular results as well. An accurate diagnosis of lymphoma, usually rendered by hematopathologists, allows hematologists/oncologists to treat patients appropriately. Herein we will describe a simplified approach to the diagnosis and classification of lymphomas (Figure 1).
Lymphoma classification
Lymphomas are clonal neoplasms characterized by the expansion of abnormal lymphoid cells that may develop in any organ but commonly involve lymph nodes. The fourth edition of the World Health Organization (WHO) Classification of Tumours of Haematopoietic and Lymphoid tissues, published in 2008, is the official and most current guideline used for diagnosis of lymphoid neoplasms.2 The WHO scheme classifies lymphomas according to the type of cell from which they are derived (mature and immature B cells, T cells, or natural killer (NK) cells, findings determined by their morphology and immunophenotype) and their clinical, cytogenetic, and/or molecular features. This official classification is currently being updated3 and is expected to be published in full in 2017, at which time it is anticipated to include definitions for more than 70 distinct neoplasms.
Lymphomas are broadly and informally classified as Hodgkin lymphomas (HLs) and non-Hodgkin lymphomas (NHLs), based on the differences these two groups show in their clinical presentation, treatment, prognosis, and proportion of neoplastic cells, among others. NHLs are by far the most common type of lymphomas, accounting for approximately 90% of all new cases of lymphoma in the United States and 70% worldwide.1,2 NHLs are a very heterogeneous group of B-, T-, or NK-cell neoplasms that, in turn, can also be informally subclassified as low-grade (or indolent) or high-grade (or aggressive) according to their predicted clinical behavior. HLs are comparatively rare, less heterogeneous, uniformly of B-cell origin and, in the case of classical Hodgkin lymphoma, highly curable.1,2 It is beyond the scope of this manuscript to outline the features of each of the >70 specific entities, but the reader is referred elsewhere for more detail and encouraged to become familiarized with the complexity, challenges, and beauty of lymphoma diagnosis.2,3
Biopsy procedure
A correct diagnosis begins with an adequate biopsy procedure. It is essential that biopsy specimens for lymphoma evaluation be submitted fresh and unfixed, because some crucial analyses such as flow cytometry or conventional cytogenetics can only be performed on fresh tissue. Indeed, it is important for the hematologist/oncologist and/or surgeon and/or interventional radiologist to converse with the hematopathologist prior to and even during some procedures to ensure the correct processing of the specimen. Also, it is important to limit the compression of the specimen and the excessive use of cauterization during the biopsy procedure, both of which cause artifacts that may render impossible the interpretation of the histopathologic findings.
Given that the diagnosis of lymphoma is based not only on the cytologic details of the lymphoma cells but also on the architectural pattern with which they infiltrate an organ, the larger the biopsy specimen, the easier it will be for a hematopathologist to identify the pattern. In addition, excisional biopsies frequently contain more diagnostic tissue than needle core biopsies and this provides pathologists with the option to submit tissue fragments for ancillary tests that require unfixed tissue as noted above. Needle core biopsies of lymph nodes are increasingly being used because of their association with fewer complications and lower cost than excisional biopsies. However, needle core biopsies provide only a glimpse of the pattern of infiltration and may not be completely representative of the architecture. Therefore, excisional lymph node biopsies of lymph nodes are preferred over needle core biopsies, recognizing that in the setting of deeply seated lymph nodes, needle core biopsies may be the only or the best surgical option.
Clinical presentation
Accurate diagnosis of lymphoma cannot take place in a vacuum. The hematopathologist’s initial approach to the diagnosis of lymphoid processes in tissue biopsies should begin with a thorough review of the clinical history, although some pathology laboratories may not have immediate access to this information. The hematopathologist should evaluate factors such as age, gender, location of the tumor, symptomatology, medications, serology, and prior history of malignancy, immunosuppression or immunodeficiency in every case. Other important but frequently omitted parts of the clinical history are the patient’s occupation, history of exposure to animals, and the presence of tattoos, which may be associated with certain reactive lymphadenopathies.
Histomorphologic evaluation
Despite the plethora of new and increasingly sophisticated tools, histologic and morphologic analysis still remains the cornerstone of diagnosis in hematopathology. However, for the characterization of an increasing number of reactive and neoplastic lymphoid processes, hematopathologists may also require immunophenotypic, molecular, and cytogenetic tests for an accurate diagnosis. Upon review of the clinical information, a microscopic evaluation of the tissue submitted for processing by the histology laboratory will be performed. The results of concurrent flow cytometric evaluation (performed on fresh unfixed material) should also be available in most if not all cases before the H&E-stained slides are available for review. Upon receipt of H&E-stained slides, the hematopathologist will evaluate the quality of the submitted specimen, since many diagnostic difficulties stem from suboptimal techniques related to the biopsy procedure, fixation, processing, cutting, or staining (Figure 1). If deemed suitable for accurate diagnosis, a search for signs of preservation or disruption of the organ that was biopsied will follow. The identification of certain morphologic patterns aids the hematopathologist in answering the first question: “what organ is this and is this consistent with what is indicated on the requisition?” This is usually immediately followed by “is this sufficient and adequate material for a diagnosis?” and “is there any normal architecture?” If the architecture is not normal, “is this alteration due to a reactive or a neoplastic process?” If neoplastic, “is it lymphoma or a non-hematolymphoid neoplasm?”
Both reactive and neoplastic processes have variably unique morphologic features that if properly recognized, guide the subsequent testing. However, some reactive and neoplastic processes can present with overlapping features, and even after extensive immunophenotypic evaluation and the performance of ancillary studies, it may not be possible to conclusively determine its nature. If the lymph node architecture is altered or effaced, the predominant pattern of infiltration (eg, nodular, diffuse, interfollicular, intrasinusoidal) and the degree of alteration of the normal architecture is evaluated, usually at low magnification. When the presence of an infiltrate is recognized, its components must be characterized. If the infiltrate is composed of a homogeneous expansion of lymphoid cells that disrupts or replaces the normal lymphoid architecture, a lymphoma will be suspected or diagnosed. The pattern of distribution of the cells along with their individual morphologic characteristics (ie, size, nuclear shape, chromatin configuration, nucleoli, amount and hue of cytoplasm) are key factors for the diagnosis and classification of the lymphoma that will guide subsequent testing. The immunophenotypic analysis (by immunohistochemistry, flow cytometry or a combination of both) may confirm the reactive or neoplastic nature of the process, and its subclassification. B-cell lymphomas, in particular have variable and distinctive histologic features: as a diffuse infiltrate of large mature lymphoid cells (eg, diffuse large B-cell lymphoma), an expansion of immature lymphoid cells (lymphoblastic lymphoma), and a nodular infiltrate of small, intermediate and/or mature large B cells (eg, follicular lymphoma).
Mature T-cell lymphomas may display similar histologic, features but they can be quite heterogeneous with an infiltrate composed of one predominant cell type or a mixture of small, medium-sized, and large atypical lymphoid cells (on occasion with abundant clear cytoplasm) and a variable number of eosinophils, plasma cells, macrophages (including granulomas), and B cells. HLs most commonly efface the lymph node architecture with a nodular or diffuse infiltrate variably composed of reactive lymphocytes, granulocytes, macrophages, and plasma cells and usually a minority of large neoplastic cells (Hodgkin/Reed-Sternberg cells and/or lymphocyte predominant cells).
Once the H&E-stained slides are evaluated and a diagnosis of lymphoma is suspected or established, the hematopathologist will attempt to determine whether it has mature or immature features, and whether low- or high-grade morphologic characteristics are present. The maturity of lymphoid cells is generally determined by the nature of the chromatin, which if “fine” and homogeneous (with or without a conspicuous nucleolus) will usually, but not always, be considered immature, whereas clumped, vesicular or hyperchromatic chromatin is generally, but not always, associated with maturity. If the chromatin displays immature features, the differential diagnosis will mainly include B- and T-lymphoblastic lymphomas, but also blastoid variants of mature neoplasm such as mantle cell lymphoma, and follicular lymphoma, as well as high-grade B-cell lymphomas. Features associated with low-grade lymphomas (eg, follicular lymphoma, small lymphocytic lymphoma/chronic lymphocytic leukemia, marginal zone lymphoma, lymphoplasmacytic lymphoma) include small cell morphology, mature chromatin, absence of a significant number of mitoses or apoptotic cells, and a low proliferation index as shown by immunohistochemistry for Ki67. High-grade lymphomas, such as lymphoblastic lymphoma, Burkitt lymphoma, or certain large B-cell lymphomas tend to show opposite features, and some of the mature entities are frequently associated with MYC rearrangements. Of note, immature lymphomas tend to be clinically high grade, but not all clinically high-grade lymphomas are immature. Conversely, the majority of low-grade lymphomas are usually mature.
Immunophenotypic evaluation
Immunophenotypic evaluation is essential because the lineage of lymphoma cells cannot be determined by morphology alone. The immunophenotype is the combination of proteins/markers (eg, CD20, CD3, TdT) expressed by cells. Usually, it is evaluated by immunohistochemistry and/or flow cytometry, which help determine the proportion of lymphoid cells that express a certain marker and its location and intensity within the cells. While immunohistochemistry is normally performed on formalin-fixed and paraffin-embedded tissue, flow cytometry can be evaluated only on fresh unfixed tissue. Flow cytometry has the advantage over immunohistochemistry of being faster and better at simultaneously identifying coexpression of multiple markers on multiple cell populations. However, certain markers can only be evaluated by immunohistochemistry.
The immunophenotypic analysis will in most cases reveal whether the lymphomas is of B-, T- or NK-cell origin, and whether a lymphoma subtype associated immunophenotype is present. Typical pan B-cell antigens include PAX5, CD19, and CD79a (CD20 is less broadly expressed throughout B-cell differentiation, although it is usually evident in most mature B-cell lymphomas), and typical pan T-cell antigens include CD2, CD5, and CD7. The immature or mature nature of a lymphoma can also be confirmed by evaluation of the immunophenotype. Immature lymphomas commonly express one or more of TdT, CD10, or CD34; T-lymphoblastic lymphoma cells may also coexpress CD1a. The majority of NHLs and all HLs are derived from (or reflect) B cells at different stages of maturation. Mature B-cell lymphomas are the most common type of lymphoma and typically, but not always, express pan B-cell markers as well as surface membrane immunoglobulin, with the latter also most useful in assessing clonality via a determination of light chain restriction. Some mature B-cell lymphomas tend to acquire markers that are either never physiologically expressed by normal mature B cells (eg, cyclin D1 in mantle cell lymphoma, or BCL2 in germinal center B cells in follicular lymphoma) or only expressed in a minor fraction (eg, CD5 that is characteristically expressed in small lymphocytic and mantle cell lymphoma). The most common mature B-cell lymphomas include diffuse large B-cell lymphoma, follicular lymphoma, small lymphocytic lymphoma, mantle cell lymphoma, marginal zone lymphoma, Burkitt lymphoma, and lymphoplasmacytic lymphoma (Figures 2 and 3). Classical HLs are also lymphomas of B-cell origin that demonstrate diminished preservation of their B-cell immunophenotype (as evidenced by the dim expression of PAX5 but absence of most other pan B-cell antigens), expression of CD30, variable expression of CD15, and loss of CD45 (Figure 1). In contrast, nodular lymphocyte predominant HL shows a preserved B-cell immunophenotypic program and expression of CD45, typically without CD30 and CD15. Of note, the evaluation of the immunophenotype of the neoplastic cells in HL is routinely assessed by immunohistochemistry because most flow cytometry laboratories cannot reliably detect and characterize the low numbers of these cells.
Mature T-cell lymphomas generally express one or more T-cell markers, and tend to display a T-helper (CD4-positive) or cytotoxic (CD8-positive) immunophenotype and may show loss of markers expressed by most normal T-cells (eg, CD5, CD7; Figure 4). However, a subset of them may express markers not commonly detected in normal T cells, such as ALK. NK-cell lymphomas lack surface CD3 (expressing only cytoplasmic CD3) and CD5 but express some pan T-cell antigens (such as CD2 and CD7) as well as CD16 and/or CD56.
Patients with primary or acquired immune dysfunction are at risk for development of lymphoma and other less clearly defined lymphoproliferative disorders, the majority of which are associated with infection of the lymphoid cells with Epstein-Barr virus (EBV). Therefore, evaluation with chromogenic in situ hybridization for an EBV-encoded early RNA (EBER1) is routinely performed in these cases; it is thus essential that the hematopathologist be informed of the altered immune system of the patient. If lymphoma develops, they may be morphologically similar to those that appear in immunocompetent patients, which specifically in the post-transplant setting are known as monomorphic post-transplant lymphoproliferative disorders (PTLD). If the PTLD does not meet the criteria for any of the recognized types of lymphoma, it may be best characterized as a polymorphic PTLD.
Once the lineage (B-, T-, or NK-cell) of the mature lymphoma has been established, the sum (and on occasion the gestalt) of the clinical, morphologic, immunophenotypic and other findings will be considered for the subclassification of the neoplasm.
Cytogenetic and molecular evaluation
If the morphologic and immunophenotypic analysis is inconclusive or nondiagnostic, then molecular and/or cytogenetic testing may further aid in the characterization of the process. Some of available molecular tests include analyses for the rearrangements of the variable region of the immunoglobulin (IG) or T-cell receptor (TCR) genes and for mutations on specific genes. The identification of specific mutations not only confirms the clonal nature of the process but, on occasion, it may also help subclassify the lymphoma, whereas IG or TCR rearrangement studies are used to establish whether a lymphoid expansion is polyclonal or monoclonal. The molecular findings should not be evaluated in isolation, because not all monoclonal rearrangements are diagnostic of lymphoma, and not all lymphomas will show a monoclonal rearrangement. Other methodologies that can aid in the identification of a clonal process or specific genetic abnormalities include metaphase cytogenetics (karyotyping) and fluorescence in situ hybridization (FISH). If any cytogenetic abnormalities are found in sufficient numbers (and constitutional abnormalities are excluded), their identification indicates the presence of a clonal process. Also, some cytogenetic abnormalities are characteristic of certain lymphomas. However, they may be neither 100% diagnostically sensitive nor diagnostically specific, for example, the hallmark t(14;18)/IGH-BCL2 is not present in all follicular lymphomas and not all lymphomas with this translocation are follicular lymphomas. Whereas FISH is generally performed on a minimum of 200 cells, compared with typically 20 metaphase by “conventional” karyotyping, and is therefore considered to have higher analytical sensitivity, it evaluates only for the presence or absence of the abnormality being investigated with a given set of probes, and therefore other abnormalities, if present, will not be identified. The value of FISH cytogenetic studies is perhaps best illustrated in the need to diagnose double hit lymphomas, amongst other scenarios. The detection of certain mutations can aid in the diagnosis of certain lymphomas, such as MYD88 in lymphoplasmacytic lymphoma, prognosis of others, such as in follicular lymphoma and identify pathways that may be precisely therapeutically targeted.
Final remarks
The diagnosis of lymphoma can be complex and usually requires the hematopathologist to integrate multiple parameters. The classification of lymphomas is not static, and new entities or variants are continuously described, and the facets of well-known ones refined. While such changes are often to the chagrin of hematologists/oncologists and hematopathologists alike, we should embrace the incorporation of nascent and typically cool data into our practice, as more therapeutically relevant entities are molded.
Lymphomas constitute a very heterogeneous group of neoplasms with diverse clinical presentations, prognoses, and responses to therapy. Approximately 80,500 new cases of lymphoma are expected to be diagnosed in the United States in 2017, of which about one quarter will lead to the death of the patient.1 Perhaps more so than any other group of neoplasms, the diagnosis of lymphoma involves the integration of a multiplicity of clinical, histologic and immunophenotypic findings and, on occasion, cytogenetic and molecular results as well. An accurate diagnosis of lymphoma, usually rendered by hematopathologists, allows hematologists/oncologists to treat patients appropriately. Herein we will describe a simplified approach to the diagnosis and classification of lymphomas (Figure 1).
Lymphoma classification
Lymphomas are clonal neoplasms characterized by the expansion of abnormal lymphoid cells that may develop in any organ but commonly involve lymph nodes. The fourth edition of the World Health Organization (WHO) Classification of Tumours of Haematopoietic and Lymphoid tissues, published in 2008, is the official and most current guideline used for diagnosis of lymphoid neoplasms.2 The WHO scheme classifies lymphomas according to the type of cell from which they are derived (mature and immature B cells, T cells, or natural killer (NK) cells, findings determined by their morphology and immunophenotype) and their clinical, cytogenetic, and/or molecular features. This official classification is currently being updated3 and is expected to be published in full in 2017, at which time it is anticipated to include definitions for more than 70 distinct neoplasms.
Lymphomas are broadly and informally classified as Hodgkin lymphomas (HLs) and non-Hodgkin lymphomas (NHLs), based on the differences these two groups show in their clinical presentation, treatment, prognosis, and proportion of neoplastic cells, among others. NHLs are by far the most common type of lymphomas, accounting for approximately 90% of all new cases of lymphoma in the United States and 70% worldwide.1,2 NHLs are a very heterogeneous group of B-, T-, or NK-cell neoplasms that, in turn, can also be informally subclassified as low-grade (or indolent) or high-grade (or aggressive) according to their predicted clinical behavior. HLs are comparatively rare, less heterogeneous, uniformly of B-cell origin and, in the case of classical Hodgkin lymphoma, highly curable.1,2 It is beyond the scope of this manuscript to outline the features of each of the >70 specific entities, but the reader is referred elsewhere for more detail and encouraged to become familiarized with the complexity, challenges, and beauty of lymphoma diagnosis.2,3
Biopsy procedure
A correct diagnosis begins with an adequate biopsy procedure. It is essential that biopsy specimens for lymphoma evaluation be submitted fresh and unfixed, because some crucial analyses such as flow cytometry or conventional cytogenetics can only be performed on fresh tissue. Indeed, it is important for the hematologist/oncologist and/or surgeon and/or interventional radiologist to converse with the hematopathologist prior to and even during some procedures to ensure the correct processing of the specimen. Also, it is important to limit the compression of the specimen and the excessive use of cauterization during the biopsy procedure, both of which cause artifacts that may render impossible the interpretation of the histopathologic findings.
Given that the diagnosis of lymphoma is based not only on the cytologic details of the lymphoma cells but also on the architectural pattern with which they infiltrate an organ, the larger the biopsy specimen, the easier it will be for a hematopathologist to identify the pattern. In addition, excisional biopsies frequently contain more diagnostic tissue than needle core biopsies and this provides pathologists with the option to submit tissue fragments for ancillary tests that require unfixed tissue as noted above. Needle core biopsies of lymph nodes are increasingly being used because of their association with fewer complications and lower cost than excisional biopsies. However, needle core biopsies provide only a glimpse of the pattern of infiltration and may not be completely representative of the architecture. Therefore, excisional lymph node biopsies of lymph nodes are preferred over needle core biopsies, recognizing that in the setting of deeply seated lymph nodes, needle core biopsies may be the only or the best surgical option.
Clinical presentation
Accurate diagnosis of lymphoma cannot take place in a vacuum. The hematopathologist’s initial approach to the diagnosis of lymphoid processes in tissue biopsies should begin with a thorough review of the clinical history, although some pathology laboratories may not have immediate access to this information. The hematopathologist should evaluate factors such as age, gender, location of the tumor, symptomatology, medications, serology, and prior history of malignancy, immunosuppression or immunodeficiency in every case. Other important but frequently omitted parts of the clinical history are the patient’s occupation, history of exposure to animals, and the presence of tattoos, which may be associated with certain reactive lymphadenopathies.
Histomorphologic evaluation
Despite the plethora of new and increasingly sophisticated tools, histologic and morphologic analysis still remains the cornerstone of diagnosis in hematopathology. However, for the characterization of an increasing number of reactive and neoplastic lymphoid processes, hematopathologists may also require immunophenotypic, molecular, and cytogenetic tests for an accurate diagnosis. Upon review of the clinical information, a microscopic evaluation of the tissue submitted for processing by the histology laboratory will be performed. The results of concurrent flow cytometric evaluation (performed on fresh unfixed material) should also be available in most if not all cases before the H&E-stained slides are available for review. Upon receipt of H&E-stained slides, the hematopathologist will evaluate the quality of the submitted specimen, since many diagnostic difficulties stem from suboptimal techniques related to the biopsy procedure, fixation, processing, cutting, or staining (Figure 1). If deemed suitable for accurate diagnosis, a search for signs of preservation or disruption of the organ that was biopsied will follow. The identification of certain morphologic patterns aids the hematopathologist in answering the first question: “what organ is this and is this consistent with what is indicated on the requisition?” This is usually immediately followed by “is this sufficient and adequate material for a diagnosis?” and “is there any normal architecture?” If the architecture is not normal, “is this alteration due to a reactive or a neoplastic process?” If neoplastic, “is it lymphoma or a non-hematolymphoid neoplasm?”
Both reactive and neoplastic processes have variably unique morphologic features that if properly recognized, guide the subsequent testing. However, some reactive and neoplastic processes can present with overlapping features, and even after extensive immunophenotypic evaluation and the performance of ancillary studies, it may not be possible to conclusively determine its nature. If the lymph node architecture is altered or effaced, the predominant pattern of infiltration (eg, nodular, diffuse, interfollicular, intrasinusoidal) and the degree of alteration of the normal architecture is evaluated, usually at low magnification. When the presence of an infiltrate is recognized, its components must be characterized. If the infiltrate is composed of a homogeneous expansion of lymphoid cells that disrupts or replaces the normal lymphoid architecture, a lymphoma will be suspected or diagnosed. The pattern of distribution of the cells along with their individual morphologic characteristics (ie, size, nuclear shape, chromatin configuration, nucleoli, amount and hue of cytoplasm) are key factors for the diagnosis and classification of the lymphoma that will guide subsequent testing. The immunophenotypic analysis (by immunohistochemistry, flow cytometry or a combination of both) may confirm the reactive or neoplastic nature of the process, and its subclassification. B-cell lymphomas, in particular have variable and distinctive histologic features: as a diffuse infiltrate of large mature lymphoid cells (eg, diffuse large B-cell lymphoma), an expansion of immature lymphoid cells (lymphoblastic lymphoma), and a nodular infiltrate of small, intermediate and/or mature large B cells (eg, follicular lymphoma).
Mature T-cell lymphomas may display similar histologic, features but they can be quite heterogeneous with an infiltrate composed of one predominant cell type or a mixture of small, medium-sized, and large atypical lymphoid cells (on occasion with abundant clear cytoplasm) and a variable number of eosinophils, plasma cells, macrophages (including granulomas), and B cells. HLs most commonly efface the lymph node architecture with a nodular or diffuse infiltrate variably composed of reactive lymphocytes, granulocytes, macrophages, and plasma cells and usually a minority of large neoplastic cells (Hodgkin/Reed-Sternberg cells and/or lymphocyte predominant cells).
Once the H&E-stained slides are evaluated and a diagnosis of lymphoma is suspected or established, the hematopathologist will attempt to determine whether it has mature or immature features, and whether low- or high-grade morphologic characteristics are present. The maturity of lymphoid cells is generally determined by the nature of the chromatin, which if “fine” and homogeneous (with or without a conspicuous nucleolus) will usually, but not always, be considered immature, whereas clumped, vesicular or hyperchromatic chromatin is generally, but not always, associated with maturity. If the chromatin displays immature features, the differential diagnosis will mainly include B- and T-lymphoblastic lymphomas, but also blastoid variants of mature neoplasm such as mantle cell lymphoma, and follicular lymphoma, as well as high-grade B-cell lymphomas. Features associated with low-grade lymphomas (eg, follicular lymphoma, small lymphocytic lymphoma/chronic lymphocytic leukemia, marginal zone lymphoma, lymphoplasmacytic lymphoma) include small cell morphology, mature chromatin, absence of a significant number of mitoses or apoptotic cells, and a low proliferation index as shown by immunohistochemistry for Ki67. High-grade lymphomas, such as lymphoblastic lymphoma, Burkitt lymphoma, or certain large B-cell lymphomas tend to show opposite features, and some of the mature entities are frequently associated with MYC rearrangements. Of note, immature lymphomas tend to be clinically high grade, but not all clinically high-grade lymphomas are immature. Conversely, the majority of low-grade lymphomas are usually mature.
Immunophenotypic evaluation
Immunophenotypic evaluation is essential because the lineage of lymphoma cells cannot be determined by morphology alone. The immunophenotype is the combination of proteins/markers (eg, CD20, CD3, TdT) expressed by cells. Usually, it is evaluated by immunohistochemistry and/or flow cytometry, which help determine the proportion of lymphoid cells that express a certain marker and its location and intensity within the cells. While immunohistochemistry is normally performed on formalin-fixed and paraffin-embedded tissue, flow cytometry can be evaluated only on fresh unfixed tissue. Flow cytometry has the advantage over immunohistochemistry of being faster and better at simultaneously identifying coexpression of multiple markers on multiple cell populations. However, certain markers can only be evaluated by immunohistochemistry.
The immunophenotypic analysis will in most cases reveal whether the lymphomas is of B-, T- or NK-cell origin, and whether a lymphoma subtype associated immunophenotype is present. Typical pan B-cell antigens include PAX5, CD19, and CD79a (CD20 is less broadly expressed throughout B-cell differentiation, although it is usually evident in most mature B-cell lymphomas), and typical pan T-cell antigens include CD2, CD5, and CD7. The immature or mature nature of a lymphoma can also be confirmed by evaluation of the immunophenotype. Immature lymphomas commonly express one or more of TdT, CD10, or CD34; T-lymphoblastic lymphoma cells may also coexpress CD1a. The majority of NHLs and all HLs are derived from (or reflect) B cells at different stages of maturation. Mature B-cell lymphomas are the most common type of lymphoma and typically, but not always, express pan B-cell markers as well as surface membrane immunoglobulin, with the latter also most useful in assessing clonality via a determination of light chain restriction. Some mature B-cell lymphomas tend to acquire markers that are either never physiologically expressed by normal mature B cells (eg, cyclin D1 in mantle cell lymphoma, or BCL2 in germinal center B cells in follicular lymphoma) or only expressed in a minor fraction (eg, CD5 that is characteristically expressed in small lymphocytic and mantle cell lymphoma). The most common mature B-cell lymphomas include diffuse large B-cell lymphoma, follicular lymphoma, small lymphocytic lymphoma, mantle cell lymphoma, marginal zone lymphoma, Burkitt lymphoma, and lymphoplasmacytic lymphoma (Figures 2 and 3). Classical HLs are also lymphomas of B-cell origin that demonstrate diminished preservation of their B-cell immunophenotype (as evidenced by the dim expression of PAX5 but absence of most other pan B-cell antigens), expression of CD30, variable expression of CD15, and loss of CD45 (Figure 1). In contrast, nodular lymphocyte predominant HL shows a preserved B-cell immunophenotypic program and expression of CD45, typically without CD30 and CD15. Of note, the evaluation of the immunophenotype of the neoplastic cells in HL is routinely assessed by immunohistochemistry because most flow cytometry laboratories cannot reliably detect and characterize the low numbers of these cells.
Mature T-cell lymphomas generally express one or more T-cell markers, and tend to display a T-helper (CD4-positive) or cytotoxic (CD8-positive) immunophenotype and may show loss of markers expressed by most normal T-cells (eg, CD5, CD7; Figure 4). However, a subset of them may express markers not commonly detected in normal T cells, such as ALK. NK-cell lymphomas lack surface CD3 (expressing only cytoplasmic CD3) and CD5 but express some pan T-cell antigens (such as CD2 and CD7) as well as CD16 and/or CD56.
Patients with primary or acquired immune dysfunction are at risk for development of lymphoma and other less clearly defined lymphoproliferative disorders, the majority of which are associated with infection of the lymphoid cells with Epstein-Barr virus (EBV). Therefore, evaluation with chromogenic in situ hybridization for an EBV-encoded early RNA (EBER1) is routinely performed in these cases; it is thus essential that the hematopathologist be informed of the altered immune system of the patient. If lymphoma develops, they may be morphologically similar to those that appear in immunocompetent patients, which specifically in the post-transplant setting are known as monomorphic post-transplant lymphoproliferative disorders (PTLD). If the PTLD does not meet the criteria for any of the recognized types of lymphoma, it may be best characterized as a polymorphic PTLD.
Once the lineage (B-, T-, or NK-cell) of the mature lymphoma has been established, the sum (and on occasion the gestalt) of the clinical, morphologic, immunophenotypic and other findings will be considered for the subclassification of the neoplasm.
Cytogenetic and molecular evaluation
If the morphologic and immunophenotypic analysis is inconclusive or nondiagnostic, then molecular and/or cytogenetic testing may further aid in the characterization of the process. Some of available molecular tests include analyses for the rearrangements of the variable region of the immunoglobulin (IG) or T-cell receptor (TCR) genes and for mutations on specific genes. The identification of specific mutations not only confirms the clonal nature of the process but, on occasion, it may also help subclassify the lymphoma, whereas IG or TCR rearrangement studies are used to establish whether a lymphoid expansion is polyclonal or monoclonal. The molecular findings should not be evaluated in isolation, because not all monoclonal rearrangements are diagnostic of lymphoma, and not all lymphomas will show a monoclonal rearrangement. Other methodologies that can aid in the identification of a clonal process or specific genetic abnormalities include metaphase cytogenetics (karyotyping) and fluorescence in situ hybridization (FISH). If any cytogenetic abnormalities are found in sufficient numbers (and constitutional abnormalities are excluded), their identification indicates the presence of a clonal process. Also, some cytogenetic abnormalities are characteristic of certain lymphomas. However, they may be neither 100% diagnostically sensitive nor diagnostically specific, for example, the hallmark t(14;18)/IGH-BCL2 is not present in all follicular lymphomas and not all lymphomas with this translocation are follicular lymphomas. Whereas FISH is generally performed on a minimum of 200 cells, compared with typically 20 metaphase by “conventional” karyotyping, and is therefore considered to have higher analytical sensitivity, it evaluates only for the presence or absence of the abnormality being investigated with a given set of probes, and therefore other abnormalities, if present, will not be identified. The value of FISH cytogenetic studies is perhaps best illustrated in the need to diagnose double hit lymphomas, amongst other scenarios. The detection of certain mutations can aid in the diagnosis of certain lymphomas, such as MYD88 in lymphoplasmacytic lymphoma, prognosis of others, such as in follicular lymphoma and identify pathways that may be precisely therapeutically targeted.
Final remarks
The diagnosis of lymphoma can be complex and usually requires the hematopathologist to integrate multiple parameters. The classification of lymphomas is not static, and new entities or variants are continuously described, and the facets of well-known ones refined. While such changes are often to the chagrin of hematologists/oncologists and hematopathologists alike, we should embrace the incorporation of nascent and typically cool data into our practice, as more therapeutically relevant entities are molded.
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017 ;67(1):7-30.
2. Swerdlow SH, Campo E, Harris NL, et al, eds. WHO classification of tumours of haematopoietic and lymphoid tissues. In: Bosman FT, Jaffe ES, Lakhani SR, Ohgaki H, eds. World Health Organization Classification of Tumours. Lyon, France: IARC; 2008.
3. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016 ;127(20):2375-2390.
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017 ;67(1):7-30.
2. Swerdlow SH, Campo E, Harris NL, et al, eds. WHO classification of tumours of haematopoietic and lymphoid tissues. In: Bosman FT, Jaffe ES, Lakhani SR, Ohgaki H, eds. World Health Organization Classification of Tumours. Lyon, France: IARC; 2008.
3. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016 ;127(20):2375-2390.