User login
Summertime and Mosquitoes Are Breeding
There are over 3700 types of mosquitoes worldwide and over 200 types in the continental United States, of which only 12 are associated with transmitting diseases to humans. The majority are just a nuisance. Since they cannot readily be distinguished, strategies to prevent any bites are recommended.
West Nile Virus
In the US, West Nile virus (WNV) is the leading cause of neuroinvasive arboviral disease. Just hearing the name took me back to New York in 1999 when sightings of dead birds around the city and boroughs were reported daily. The virus was isolated that same year. The enzootic circle occurs between mosquitoes and birds, which are the primary vertebrate host via the bite of Culex mosquitoes. After a bite from an infected mosquito, humans are usually a dead-end host since the level and duration of viremia needed to infect another mosquito is insufficient.
Human-to-human transmission is documented through blood transfusion and solid organ transplantation. Vertical transmission is rarely described. Initially isolated in New York, WNV quickly spread across North America and has been isolated in every continent except Antarctica. Most cases occur in the summer and autumn.
Most infected individuals are asymptomatic. Those who do develop symptoms have fever, headache, myalgia, arthralgia, nausea, vomiting, and a transient rash. Less than 1% develop meningitis/encephalitis symptoms similar to other causes of aseptic meningitis. Those with encephalitis in addition to fever and headache may have altered mental status and focal neurologic deficits including flaccid paralysis or movement disorders.
Detection of anti-WNV IgM antibodies (AB) in serum or CSF is the most common way to make the diagnosis. IgM AB usually is present within 3-8 days after onset of symptoms and persists up to 90 days. Data from ArboNET, the national arboviral surveillance system managed by Centers for Disease Control and Prevention and state health departments, reveal that from 1999 to 2022 there were 56,575 cases of WNV including 28,684 cases of neuroinvasive disease. In 2023 there were 2,406 and 1,599 cases, respectively. Those historic totals for WNV are 10 times greater than the totals for all the other etiologies of neuroinvasive arboviral diseases in the US combined (Jamestown Canyon, LaCrosse, St. Louis, and Eastern Equine encephalitis n = 1813).
Remember to include WNV in your differential of a febrile patient with neurologic symptoms, mosquito bites, blood transfusions, and organ transplantation. Treatment is supportive care.
The US began screening all blood donations for WNV in 2003. Organ donor screening is not universal.
Dengue
Dengue, another arbovirus, is transmitted by bites of infected Aedes aegypti and Aedes albopictus mosquitoes, which prefer to feed during the daytime. There are four dengue virus serotypes: DENV-1 DENV-2, DENV-3 and DENV-4. In endemic areas, all four serotypes are usually co-circulating and people can be infected by each one.
Long-term immunity is type specific. Heterologous protection lasts only a few months. Dengue is endemic throughout the tropics and subtropics of Asia, Africa, and the Americas. Approximately 53% of the world’s population live in an area where dengue transmission can occur. In the US, most cases are reported from Puerto Rico. Dengue is endemic in the following US territories: Puerto Rico, US Virgin Islands, American Samoa, and free associated states. Most cases reported on the mainland are travel related. However, locally acquired dengue has been reported. From 2010 to 2023 Hawaii reported 250 cases, Florida 438, and Texas 40 locally acquired cases. During that same period, Puerto Rico reported more than 32,000 cases. It is the leading cause of febrile illness for travelers returning from the Caribbean, Latin America, and South Asia. Peru is currently experiencing an outbreak with more than 25,000 cases reported since January 2024. Most cases of dengue occur in adolescents and young adults. Severe disease occurs most often in infants, those with underlying chronic disease, pregnant women, and persons infected with dengue for the second time.
Symptoms range from a mild febrile illness to severe disease associated with hemorrhage and shock. Onset is usually 7-10 days after infection and symptoms include high fever, severe headache, retro-orbital pain, arthralgia and myalgias, nausea, and vomiting; some may develop a generalized rash.
The World Health Organization (WHO) classifies dengue as 1) dengue with or without warning signs for progression of disease and 2) severe dengue. Warning signs for disease progression include abdominal pain or tenderness, persistent vomiting, fluid accumulation (e.g., ascites, pericardial or pleural effusion), mucosal bleeding, restlessness, postural hypotension, liver enlargement greater than 2 cm. Severe dengue is defined as any sign of severe plasma leakage leading to shock, severe bleeding or organ failure, or fluid accumulation with respiratory distress. Management is supportive care.
Prevention: In the US, Dengvaxia, a live attenuated tetravalent vaccine, is approved for use in children aged 9–16 years with laboratory-confirmed previous dengue virus infection and living in areas where dengue is endemic. It is administered at 0, 6, and 12 months. It is not available for purchase on the mainland. Continued control of the vector and personal protection is necessary to prevent recurrent infections.
CHIKV
Chikungunya (CHIKV), which means “that which bends up” in the Mkonde language of Tanzania, refers to the appearance of the person with severe usually symmetric arthralgias characteristic for this infection that otherwise is often clinically confused with dengue and Zika. It too is transmitted by A. aegypti and A. albopictus and is prevalent in tropical Africa, Asia, Central and South America, and the Caribbean. Like dengue it is predominantly an urban disease. The WHO reported the first case in the Western Hemisphere in Saint Martin in December 2013. By August 2014, 31 additional territories and Caribbean or South American countries reported 576,535 suspected cases. Florida first reported locally acquired CHIKV in June 2014. By December an additional 11 cases had been identified. Texas reported one case in 2015. Diagnosis is with IgM ab or PCR. Treatment is supportive with most recovering from acute illness within 2 weeks. Data in adults indicate 40-52% may develop chronic or recurrent joint pain.
Prevention: IXCHIQ, a live attenuated vaccine, was licensed in November 2023 and recommended by the CDC in February 2024 for use in persons at least 18 years of age with travel to destinations where there is a CHIKV outbreak. It may be considered for persons traveling to a country or territory without an outbreak but with evidence of CHIKV transmission among humans within the last 5 years and those staying in endemic areas for a cumulative period of at least 6 months over a 2-year period. Specific recommendations for lab workers and persons older than 65 years were also made. This is good news for your older patients who may be participating in mission trips, volunteering, studying abroad, or just vacationing in an endemic area. Adolescent vaccine trials are ongoing and pediatric trials will soon be initiated. In addition, vector control and use of personal protective measures cannot be emphasized enough.
There are several other mosquito borne diseases, however our discussion here is limited to three. Why these three? WNV as a reminder that it is the most common neuroinvasive agent in the US. Dengue and CHIKV because they are not endemic in the US so they might not routinely be considered in febrile patients; both diseases have been reported and acquired on the mainland and your patients may travel to an endemic area and return home with an unwanted souvenir. You will be ready for them.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Suggested Reading
Chikungunya. Centers for Disease Control and Prevention. 2024. https://www.cdc.gov/vaccines/acip/recommendations.html.
Fagrem AC et al. West Nile and Other Nationally Notifiable Arboviral Diseases–United States, 2021. MMWR Morb Mortal Wkly Rep. 2023 Aug 25;72(34):901-906.
Fever in Returned Travelers, Travel Medicine (Fourth Edition). 2019. doi: 10.1016/B978-0-323-54696-6.00056-2.
Paz-Baily et al. Dengue Vaccine: Recommendations of the Advisory Committee on Immunization Practices, United States, 2021 MMWR Recomm Rep. 2021 Dec 17;70(6):1-16).
Staples JE and Fischer M. Chikungunya virus in the Americas — what a vectorborne pathogen can do. N Engl J Med. 2014 Sep 4;371(10):887-9.
Mosquitoes and Diseases A-Z, Centers for Disease Control and Prevention. https://www.cdc.gov/mosquitoes/about/diseases.html.
There are over 3700 types of mosquitoes worldwide and over 200 types in the continental United States, of which only 12 are associated with transmitting diseases to humans. The majority are just a nuisance. Since they cannot readily be distinguished, strategies to prevent any bites are recommended.
West Nile Virus
In the US, West Nile virus (WNV) is the leading cause of neuroinvasive arboviral disease. Just hearing the name took me back to New York in 1999 when sightings of dead birds around the city and boroughs were reported daily. The virus was isolated that same year. The enzootic circle occurs between mosquitoes and birds, which are the primary vertebrate host via the bite of Culex mosquitoes. After a bite from an infected mosquito, humans are usually a dead-end host since the level and duration of viremia needed to infect another mosquito is insufficient.
Human-to-human transmission is documented through blood transfusion and solid organ transplantation. Vertical transmission is rarely described. Initially isolated in New York, WNV quickly spread across North America and has been isolated in every continent except Antarctica. Most cases occur in the summer and autumn.
Most infected individuals are asymptomatic. Those who do develop symptoms have fever, headache, myalgia, arthralgia, nausea, vomiting, and a transient rash. Less than 1% develop meningitis/encephalitis symptoms similar to other causes of aseptic meningitis. Those with encephalitis in addition to fever and headache may have altered mental status and focal neurologic deficits including flaccid paralysis or movement disorders.
Detection of anti-WNV IgM antibodies (AB) in serum or CSF is the most common way to make the diagnosis. IgM AB usually is present within 3-8 days after onset of symptoms and persists up to 90 days. Data from ArboNET, the national arboviral surveillance system managed by Centers for Disease Control and Prevention and state health departments, reveal that from 1999 to 2022 there were 56,575 cases of WNV including 28,684 cases of neuroinvasive disease. In 2023 there were 2,406 and 1,599 cases, respectively. Those historic totals for WNV are 10 times greater than the totals for all the other etiologies of neuroinvasive arboviral diseases in the US combined (Jamestown Canyon, LaCrosse, St. Louis, and Eastern Equine encephalitis n = 1813).
Remember to include WNV in your differential of a febrile patient with neurologic symptoms, mosquito bites, blood transfusions, and organ transplantation. Treatment is supportive care.
The US began screening all blood donations for WNV in 2003. Organ donor screening is not universal.
Dengue
Dengue, another arbovirus, is transmitted by bites of infected Aedes aegypti and Aedes albopictus mosquitoes, which prefer to feed during the daytime. There are four dengue virus serotypes: DENV-1 DENV-2, DENV-3 and DENV-4. In endemic areas, all four serotypes are usually co-circulating and people can be infected by each one.
Long-term immunity is type specific. Heterologous protection lasts only a few months. Dengue is endemic throughout the tropics and subtropics of Asia, Africa, and the Americas. Approximately 53% of the world’s population live in an area where dengue transmission can occur. In the US, most cases are reported from Puerto Rico. Dengue is endemic in the following US territories: Puerto Rico, US Virgin Islands, American Samoa, and free associated states. Most cases reported on the mainland are travel related. However, locally acquired dengue has been reported. From 2010 to 2023 Hawaii reported 250 cases, Florida 438, and Texas 40 locally acquired cases. During that same period, Puerto Rico reported more than 32,000 cases. It is the leading cause of febrile illness for travelers returning from the Caribbean, Latin America, and South Asia. Peru is currently experiencing an outbreak with more than 25,000 cases reported since January 2024. Most cases of dengue occur in adolescents and young adults. Severe disease occurs most often in infants, those with underlying chronic disease, pregnant women, and persons infected with dengue for the second time.
Symptoms range from a mild febrile illness to severe disease associated with hemorrhage and shock. Onset is usually 7-10 days after infection and symptoms include high fever, severe headache, retro-orbital pain, arthralgia and myalgias, nausea, and vomiting; some may develop a generalized rash.
The World Health Organization (WHO) classifies dengue as 1) dengue with or without warning signs for progression of disease and 2) severe dengue. Warning signs for disease progression include abdominal pain or tenderness, persistent vomiting, fluid accumulation (e.g., ascites, pericardial or pleural effusion), mucosal bleeding, restlessness, postural hypotension, liver enlargement greater than 2 cm. Severe dengue is defined as any sign of severe plasma leakage leading to shock, severe bleeding or organ failure, or fluid accumulation with respiratory distress. Management is supportive care.
Prevention: In the US, Dengvaxia, a live attenuated tetravalent vaccine, is approved for use in children aged 9–16 years with laboratory-confirmed previous dengue virus infection and living in areas where dengue is endemic. It is administered at 0, 6, and 12 months. It is not available for purchase on the mainland. Continued control of the vector and personal protection is necessary to prevent recurrent infections.
CHIKV
Chikungunya (CHIKV), which means “that which bends up” in the Mkonde language of Tanzania, refers to the appearance of the person with severe usually symmetric arthralgias characteristic for this infection that otherwise is often clinically confused with dengue and Zika. It too is transmitted by A. aegypti and A. albopictus and is prevalent in tropical Africa, Asia, Central and South America, and the Caribbean. Like dengue it is predominantly an urban disease. The WHO reported the first case in the Western Hemisphere in Saint Martin in December 2013. By August 2014, 31 additional territories and Caribbean or South American countries reported 576,535 suspected cases. Florida first reported locally acquired CHIKV in June 2014. By December an additional 11 cases had been identified. Texas reported one case in 2015. Diagnosis is with IgM ab or PCR. Treatment is supportive with most recovering from acute illness within 2 weeks. Data in adults indicate 40-52% may develop chronic or recurrent joint pain.
Prevention: IXCHIQ, a live attenuated vaccine, was licensed in November 2023 and recommended by the CDC in February 2024 for use in persons at least 18 years of age with travel to destinations where there is a CHIKV outbreak. It may be considered for persons traveling to a country or territory without an outbreak but with evidence of CHIKV transmission among humans within the last 5 years and those staying in endemic areas for a cumulative period of at least 6 months over a 2-year period. Specific recommendations for lab workers and persons older than 65 years were also made. This is good news for your older patients who may be participating in mission trips, volunteering, studying abroad, or just vacationing in an endemic area. Adolescent vaccine trials are ongoing and pediatric trials will soon be initiated. In addition, vector control and use of personal protective measures cannot be emphasized enough.
There are several other mosquito borne diseases, however our discussion here is limited to three. Why these three? WNV as a reminder that it is the most common neuroinvasive agent in the US. Dengue and CHIKV because they are not endemic in the US so they might not routinely be considered in febrile patients; both diseases have been reported and acquired on the mainland and your patients may travel to an endemic area and return home with an unwanted souvenir. You will be ready for them.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Suggested Reading
Chikungunya. Centers for Disease Control and Prevention. 2024. https://www.cdc.gov/vaccines/acip/recommendations.html.
Fagrem AC et al. West Nile and Other Nationally Notifiable Arboviral Diseases–United States, 2021. MMWR Morb Mortal Wkly Rep. 2023 Aug 25;72(34):901-906.
Fever in Returned Travelers, Travel Medicine (Fourth Edition). 2019. doi: 10.1016/B978-0-323-54696-6.00056-2.
Paz-Baily et al. Dengue Vaccine: Recommendations of the Advisory Committee on Immunization Practices, United States, 2021 MMWR Recomm Rep. 2021 Dec 17;70(6):1-16).
Staples JE and Fischer M. Chikungunya virus in the Americas — what a vectorborne pathogen can do. N Engl J Med. 2014 Sep 4;371(10):887-9.
Mosquitoes and Diseases A-Z, Centers for Disease Control and Prevention. https://www.cdc.gov/mosquitoes/about/diseases.html.
There are over 3700 types of mosquitoes worldwide and over 200 types in the continental United States, of which only 12 are associated with transmitting diseases to humans. The majority are just a nuisance. Since they cannot readily be distinguished, strategies to prevent any bites are recommended.
West Nile Virus
In the US, West Nile virus (WNV) is the leading cause of neuroinvasive arboviral disease. Just hearing the name took me back to New York in 1999 when sightings of dead birds around the city and boroughs were reported daily. The virus was isolated that same year. The enzootic circle occurs between mosquitoes and birds, which are the primary vertebrate host via the bite of Culex mosquitoes. After a bite from an infected mosquito, humans are usually a dead-end host since the level and duration of viremia needed to infect another mosquito is insufficient.
Human-to-human transmission is documented through blood transfusion and solid organ transplantation. Vertical transmission is rarely described. Initially isolated in New York, WNV quickly spread across North America and has been isolated in every continent except Antarctica. Most cases occur in the summer and autumn.
Most infected individuals are asymptomatic. Those who do develop symptoms have fever, headache, myalgia, arthralgia, nausea, vomiting, and a transient rash. Less than 1% develop meningitis/encephalitis symptoms similar to other causes of aseptic meningitis. Those with encephalitis in addition to fever and headache may have altered mental status and focal neurologic deficits including flaccid paralysis or movement disorders.
Detection of anti-WNV IgM antibodies (AB) in serum or CSF is the most common way to make the diagnosis. IgM AB usually is present within 3-8 days after onset of symptoms and persists up to 90 days. Data from ArboNET, the national arboviral surveillance system managed by Centers for Disease Control and Prevention and state health departments, reveal that from 1999 to 2022 there were 56,575 cases of WNV including 28,684 cases of neuroinvasive disease. In 2023 there were 2,406 and 1,599 cases, respectively. Those historic totals for WNV are 10 times greater than the totals for all the other etiologies of neuroinvasive arboviral diseases in the US combined (Jamestown Canyon, LaCrosse, St. Louis, and Eastern Equine encephalitis n = 1813).
Remember to include WNV in your differential of a febrile patient with neurologic symptoms, mosquito bites, blood transfusions, and organ transplantation. Treatment is supportive care.
The US began screening all blood donations for WNV in 2003. Organ donor screening is not universal.
Dengue
Dengue, another arbovirus, is transmitted by bites of infected Aedes aegypti and Aedes albopictus mosquitoes, which prefer to feed during the daytime. There are four dengue virus serotypes: DENV-1 DENV-2, DENV-3 and DENV-4. In endemic areas, all four serotypes are usually co-circulating and people can be infected by each one.
Long-term immunity is type specific. Heterologous protection lasts only a few months. Dengue is endemic throughout the tropics and subtropics of Asia, Africa, and the Americas. Approximately 53% of the world’s population live in an area where dengue transmission can occur. In the US, most cases are reported from Puerto Rico. Dengue is endemic in the following US territories: Puerto Rico, US Virgin Islands, American Samoa, and free associated states. Most cases reported on the mainland are travel related. However, locally acquired dengue has been reported. From 2010 to 2023 Hawaii reported 250 cases, Florida 438, and Texas 40 locally acquired cases. During that same period, Puerto Rico reported more than 32,000 cases. It is the leading cause of febrile illness for travelers returning from the Caribbean, Latin America, and South Asia. Peru is currently experiencing an outbreak with more than 25,000 cases reported since January 2024. Most cases of dengue occur in adolescents and young adults. Severe disease occurs most often in infants, those with underlying chronic disease, pregnant women, and persons infected with dengue for the second time.
Symptoms range from a mild febrile illness to severe disease associated with hemorrhage and shock. Onset is usually 7-10 days after infection and symptoms include high fever, severe headache, retro-orbital pain, arthralgia and myalgias, nausea, and vomiting; some may develop a generalized rash.
The World Health Organization (WHO) classifies dengue as 1) dengue with or without warning signs for progression of disease and 2) severe dengue. Warning signs for disease progression include abdominal pain or tenderness, persistent vomiting, fluid accumulation (e.g., ascites, pericardial or pleural effusion), mucosal bleeding, restlessness, postural hypotension, liver enlargement greater than 2 cm. Severe dengue is defined as any sign of severe plasma leakage leading to shock, severe bleeding or organ failure, or fluid accumulation with respiratory distress. Management is supportive care.
Prevention: In the US, Dengvaxia, a live attenuated tetravalent vaccine, is approved for use in children aged 9–16 years with laboratory-confirmed previous dengue virus infection and living in areas where dengue is endemic. It is administered at 0, 6, and 12 months. It is not available for purchase on the mainland. Continued control of the vector and personal protection is necessary to prevent recurrent infections.
CHIKV
Chikungunya (CHIKV), which means “that which bends up” in the Mkonde language of Tanzania, refers to the appearance of the person with severe usually symmetric arthralgias characteristic for this infection that otherwise is often clinically confused with dengue and Zika. It too is transmitted by A. aegypti and A. albopictus and is prevalent in tropical Africa, Asia, Central and South America, and the Caribbean. Like dengue it is predominantly an urban disease. The WHO reported the first case in the Western Hemisphere in Saint Martin in December 2013. By August 2014, 31 additional territories and Caribbean or South American countries reported 576,535 suspected cases. Florida first reported locally acquired CHIKV in June 2014. By December an additional 11 cases had been identified. Texas reported one case in 2015. Diagnosis is with IgM ab or PCR. Treatment is supportive with most recovering from acute illness within 2 weeks. Data in adults indicate 40-52% may develop chronic or recurrent joint pain.
Prevention: IXCHIQ, a live attenuated vaccine, was licensed in November 2023 and recommended by the CDC in February 2024 for use in persons at least 18 years of age with travel to destinations where there is a CHIKV outbreak. It may be considered for persons traveling to a country or territory without an outbreak but with evidence of CHIKV transmission among humans within the last 5 years and those staying in endemic areas for a cumulative period of at least 6 months over a 2-year period. Specific recommendations for lab workers and persons older than 65 years were also made. This is good news for your older patients who may be participating in mission trips, volunteering, studying abroad, or just vacationing in an endemic area. Adolescent vaccine trials are ongoing and pediatric trials will soon be initiated. In addition, vector control and use of personal protective measures cannot be emphasized enough.
There are several other mosquito borne diseases, however our discussion here is limited to three. Why these three? WNV as a reminder that it is the most common neuroinvasive agent in the US. Dengue and CHIKV because they are not endemic in the US so they might not routinely be considered in febrile patients; both diseases have been reported and acquired on the mainland and your patients may travel to an endemic area and return home with an unwanted souvenir. You will be ready for them.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
Suggested Reading
Chikungunya. Centers for Disease Control and Prevention. 2024. https://www.cdc.gov/vaccines/acip/recommendations.html.
Fagrem AC et al. West Nile and Other Nationally Notifiable Arboviral Diseases–United States, 2021. MMWR Morb Mortal Wkly Rep. 2023 Aug 25;72(34):901-906.
Fever in Returned Travelers, Travel Medicine (Fourth Edition). 2019. doi: 10.1016/B978-0-323-54696-6.00056-2.
Paz-Baily et al. Dengue Vaccine: Recommendations of the Advisory Committee on Immunization Practices, United States, 2021 MMWR Recomm Rep. 2021 Dec 17;70(6):1-16).
Staples JE and Fischer M. Chikungunya virus in the Americas — what a vectorborne pathogen can do. N Engl J Med. 2014 Sep 4;371(10):887-9.
Mosquitoes and Diseases A-Z, Centers for Disease Control and Prevention. https://www.cdc.gov/mosquitoes/about/diseases.html.
Microbiome Impacts Vaccine Responses
When infants are born, they have nearly a clean slate with regard to their immune systems. Virtually all their immune cells are naive. They have no immunity memory. Vaccines at birth, and in the first 2 years of life, elicit variable antibody levels and cellular immune responses. Sometimes, this leaves fully vaccinated children unprotected against vaccine-preventable infectious diseases.
Newborns are bombarded at birth with microbes and other antigenic stimuli from the environment; food in the form of breast milk, formula, water; and vaccines, such as hepatitis B and, in other countries, with BCG. At birth, to avoid immunologically-induced injury, immune responses favor immunologic tolerance. However, adaptation must be rapid to avoid life-threatening infections. To navigate the gauntlet of microbe and environmental exposures and vaccines, the neonatal immune system moves through a gradual maturation process toward immune responsivity. The maturation occurs at different rates in different children.
Reassessing Vaccine Responsiveness
Vaccine responsiveness is usually assessed by measuring antibody levels in blood. Until recently, it was thought to be “bad luck” when a child failed to develop protective immunity following vaccination. The bad luck was suggested to involve illness at the time of vaccination, especially illness occurring with fever, and especially common viral infections. But studies proved that notion incorrect. About 10 years ago I became more interested in variability in vaccine responses in the first 2 years of life. In 2016, my laboratory described a specific population of children with specific cellular immune deficiencies that we classified as low vaccine responders (LVRs).1 To preclude the suggestion that low vaccine responses were to be considered normal biological variation, we chose an a priori definition of LVR as those with sub-protective IgG antibody levels to four (≥ 66 %) of six tested vaccines in DTaP-Hib (diphtheria toxoid, tetanus toxoid, pertussis toxoid, pertactin, and filamentous hemagglutinin [DTaP] and Haemophilus influenzae type b polysaccharide capsule [Hib]). Antibody levels were measured at 1 year of age following primary vaccinations at child age 2, 4, and 6 months old. The remaining 89% of children we termed normal vaccine responders (NVRs). We additionally tested antibody responses to viral protein and pneumococcal polysaccharide conjugated antigens (polio serotypes 1, 2, and 3, hepatitis B, and Streptococcus pneumoniae capsular polysaccharides serotypes 6B, 14, and 23F). Responses to these vaccine antigens were similar to the six vaccines (DTaP/Hib) used to define LVR. We and other groups have used alternative definitions of low vaccine responses that rely on statistics.
I recently reviewed the topic of the determinants of vaccine responses in early life, with a focus on the infant microbiome and metabolome: a.) cesarean section versus vaginal delivery, b.) breast versus formula feeding and c.) antibiotic exposure, that impact the immune response2 (Figure). In the review I also discussed how microbiome may serve as natural adjuvants for vaccine responses, how microbiota-derived metabolites influence vaccine responses, and how low vaccine responses in early life may be linked to increased infection susceptibility (Figure).
Cesarean section births occur in nearly 30% of newborns. Cesarean section birth has been associated with adverse effects on immune development, including predisposing to infections, allergies, and inflammatory disorders. The association of these adverse outcomes has been linked to lower total microbiome diversity. Fecal microbiome seeding from mother to infant in vaginal-delivered infants results in a more favorable and stable microbiome compared with cesarean-delivered infants. Nasopharyngeal microbiome may also be adversely affected by cesarean delivery. In turn, those microbiome differences can be linked to variation in vaccine responsiveness in infants.
Multiple studies strongly support the notion that breastfeeding has a favorable impact on immune development in early life associated with better vaccine responses, mediated by the microbiome. The mechanism of favorable immune responses to vaccines largely relates to the presence of a specific bacteria species, Bifidobacterium infantis. Breast milk contains human milk oligosaccharides that are not digestible by newborns. B. infantis is a strain of bacteria that utilizes these non-digestible oligosaccharides. Thereby, infants fed breast milk provides B. infantis the essential source of nutrition for its growth and predominance in the newborn gut. Studies have shown that Bifidobacterium spp. abundance in early life is correlated with better immune responses to multiple vaccines. Bifidobacterium spp. abundance has been positively correlated with antibody responses measured after 2 years, linking the microbiome composition to the durability of vaccine-induced immune responses.
Antibiotic exposure in early life may disproportionately damage the newborn and infant microbiome compared with later childhood. The average child receives about three antibiotic courses by the age of 2 years. My lab was among the first to describe the adverse effects of antibiotics on vaccine responses in early life.3 We found that broader spectrum antibiotics had a greater adverse effect on vaccine-induced antibody levels than narrower spectrum antibiotics. Ten-day versus five-day treatment courses had a greater negative effect. Multiple antibiotic courses over time (cumulative antibiotic exposure) was negatively associated with vaccine-induced antibody levels.
Over 11 % of live births worldwide occur preterm. Because bacterial infections are frequent complications of preterm birth, 79 % of very low birthweight and 87 % of extremely low birthweight infants in US NICUs receive antibiotics within 3 days of birth. Recently, my group studied full-term infants at birth and found that exposure to parenteral antibiotics at birth or during the first days of life had an adverse effect on vaccine responses.4
Microbiome Impacts Immunity
How does the microbiome affect immunity, and specifically vaccine responses? Microbial-derived metabolites affect host immunity. Gut bacteria produce short chain fatty acids (SCFAs: acetate, propionate, butyrate) [115]. SCFAs positively influence immunity cells. Vitamin D metabolites are generated by intestinal bacteria and those metabolites positively influence immunity. Secondary bile acids produced by Clostridium spp. are involved in favorable immune responses. Increased levels of phenylpyruvic acid produced by gut and/or nasopharyngeal microbiota correlate with reduced vaccine responses and upregulated metabolome genes that encode for oxidative phosphorylation correlate with increased vaccine responses.
In summary, immune development commences at birth. Impairment in responses to vaccination in children have been linked to disturbance in the microbiome. Cesarean section and absence of breastfeeding are associated with adverse microbiota composition. Antibiotics perturb healthy microbiota development. The microbiota affect immunity in several ways, among them are effects by metabolites generated by the commensals that inhabit the child host. A child who responds poorly to vaccines and has specific immune cell dysfunction caused by problems with the microbiome also displays increased infection proneness. But that is a story for another column, later.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Pichichero ME et al. J Infect Dis. 2016 Jun 15;213(12):2014-2019. doi: 10.1093/infdis/jiw053.
2. Pichichero ME. Cell Immunol. 2023 Nov-Dec:393-394:104777. doi: 10.1016/j.cellimm.2023.104777.
3. Chapman TJ et al. Pediatrics. 2022 May 1;149(5):e2021052061. doi: 10.1542/peds.2021-052061.
4. Shaffer M et al. mSystems. 2023 Oct 26;8(5):e0066123. doi: 10.1128/msystems.00661-23.
When infants are born, they have nearly a clean slate with regard to their immune systems. Virtually all their immune cells are naive. They have no immunity memory. Vaccines at birth, and in the first 2 years of life, elicit variable antibody levels and cellular immune responses. Sometimes, this leaves fully vaccinated children unprotected against vaccine-preventable infectious diseases.
Newborns are bombarded at birth with microbes and other antigenic stimuli from the environment; food in the form of breast milk, formula, water; and vaccines, such as hepatitis B and, in other countries, with BCG. At birth, to avoid immunologically-induced injury, immune responses favor immunologic tolerance. However, adaptation must be rapid to avoid life-threatening infections. To navigate the gauntlet of microbe and environmental exposures and vaccines, the neonatal immune system moves through a gradual maturation process toward immune responsivity. The maturation occurs at different rates in different children.
Reassessing Vaccine Responsiveness
Vaccine responsiveness is usually assessed by measuring antibody levels in blood. Until recently, it was thought to be “bad luck” when a child failed to develop protective immunity following vaccination. The bad luck was suggested to involve illness at the time of vaccination, especially illness occurring with fever, and especially common viral infections. But studies proved that notion incorrect. About 10 years ago I became more interested in variability in vaccine responses in the first 2 years of life. In 2016, my laboratory described a specific population of children with specific cellular immune deficiencies that we classified as low vaccine responders (LVRs).1 To preclude the suggestion that low vaccine responses were to be considered normal biological variation, we chose an a priori definition of LVR as those with sub-protective IgG antibody levels to four (≥ 66 %) of six tested vaccines in DTaP-Hib (diphtheria toxoid, tetanus toxoid, pertussis toxoid, pertactin, and filamentous hemagglutinin [DTaP] and Haemophilus influenzae type b polysaccharide capsule [Hib]). Antibody levels were measured at 1 year of age following primary vaccinations at child age 2, 4, and 6 months old. The remaining 89% of children we termed normal vaccine responders (NVRs). We additionally tested antibody responses to viral protein and pneumococcal polysaccharide conjugated antigens (polio serotypes 1, 2, and 3, hepatitis B, and Streptococcus pneumoniae capsular polysaccharides serotypes 6B, 14, and 23F). Responses to these vaccine antigens were similar to the six vaccines (DTaP/Hib) used to define LVR. We and other groups have used alternative definitions of low vaccine responses that rely on statistics.
I recently reviewed the topic of the determinants of vaccine responses in early life, with a focus on the infant microbiome and metabolome: a.) cesarean section versus vaginal delivery, b.) breast versus formula feeding and c.) antibiotic exposure, that impact the immune response2 (Figure). In the review I also discussed how microbiome may serve as natural adjuvants for vaccine responses, how microbiota-derived metabolites influence vaccine responses, and how low vaccine responses in early life may be linked to increased infection susceptibility (Figure).
Cesarean section births occur in nearly 30% of newborns. Cesarean section birth has been associated with adverse effects on immune development, including predisposing to infections, allergies, and inflammatory disorders. The association of these adverse outcomes has been linked to lower total microbiome diversity. Fecal microbiome seeding from mother to infant in vaginal-delivered infants results in a more favorable and stable microbiome compared with cesarean-delivered infants. Nasopharyngeal microbiome may also be adversely affected by cesarean delivery. In turn, those microbiome differences can be linked to variation in vaccine responsiveness in infants.
Multiple studies strongly support the notion that breastfeeding has a favorable impact on immune development in early life associated with better vaccine responses, mediated by the microbiome. The mechanism of favorable immune responses to vaccines largely relates to the presence of a specific bacteria species, Bifidobacterium infantis. Breast milk contains human milk oligosaccharides that are not digestible by newborns. B. infantis is a strain of bacteria that utilizes these non-digestible oligosaccharides. Thereby, infants fed breast milk provides B. infantis the essential source of nutrition for its growth and predominance in the newborn gut. Studies have shown that Bifidobacterium spp. abundance in early life is correlated with better immune responses to multiple vaccines. Bifidobacterium spp. abundance has been positively correlated with antibody responses measured after 2 years, linking the microbiome composition to the durability of vaccine-induced immune responses.
Antibiotic exposure in early life may disproportionately damage the newborn and infant microbiome compared with later childhood. The average child receives about three antibiotic courses by the age of 2 years. My lab was among the first to describe the adverse effects of antibiotics on vaccine responses in early life.3 We found that broader spectrum antibiotics had a greater adverse effect on vaccine-induced antibody levels than narrower spectrum antibiotics. Ten-day versus five-day treatment courses had a greater negative effect. Multiple antibiotic courses over time (cumulative antibiotic exposure) was negatively associated with vaccine-induced antibody levels.
Over 11 % of live births worldwide occur preterm. Because bacterial infections are frequent complications of preterm birth, 79 % of very low birthweight and 87 % of extremely low birthweight infants in US NICUs receive antibiotics within 3 days of birth. Recently, my group studied full-term infants at birth and found that exposure to parenteral antibiotics at birth or during the first days of life had an adverse effect on vaccine responses.4
Microbiome Impacts Immunity
How does the microbiome affect immunity, and specifically vaccine responses? Microbial-derived metabolites affect host immunity. Gut bacteria produce short chain fatty acids (SCFAs: acetate, propionate, butyrate) [115]. SCFAs positively influence immunity cells. Vitamin D metabolites are generated by intestinal bacteria and those metabolites positively influence immunity. Secondary bile acids produced by Clostridium spp. are involved in favorable immune responses. Increased levels of phenylpyruvic acid produced by gut and/or nasopharyngeal microbiota correlate with reduced vaccine responses and upregulated metabolome genes that encode for oxidative phosphorylation correlate with increased vaccine responses.
In summary, immune development commences at birth. Impairment in responses to vaccination in children have been linked to disturbance in the microbiome. Cesarean section and absence of breastfeeding are associated with adverse microbiota composition. Antibiotics perturb healthy microbiota development. The microbiota affect immunity in several ways, among them are effects by metabolites generated by the commensals that inhabit the child host. A child who responds poorly to vaccines and has specific immune cell dysfunction caused by problems with the microbiome also displays increased infection proneness. But that is a story for another column, later.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Pichichero ME et al. J Infect Dis. 2016 Jun 15;213(12):2014-2019. doi: 10.1093/infdis/jiw053.
2. Pichichero ME. Cell Immunol. 2023 Nov-Dec:393-394:104777. doi: 10.1016/j.cellimm.2023.104777.
3. Chapman TJ et al. Pediatrics. 2022 May 1;149(5):e2021052061. doi: 10.1542/peds.2021-052061.
4. Shaffer M et al. mSystems. 2023 Oct 26;8(5):e0066123. doi: 10.1128/msystems.00661-23.
When infants are born, they have nearly a clean slate with regard to their immune systems. Virtually all their immune cells are naive. They have no immunity memory. Vaccines at birth, and in the first 2 years of life, elicit variable antibody levels and cellular immune responses. Sometimes, this leaves fully vaccinated children unprotected against vaccine-preventable infectious diseases.
Newborns are bombarded at birth with microbes and other antigenic stimuli from the environment; food in the form of breast milk, formula, water; and vaccines, such as hepatitis B and, in other countries, with BCG. At birth, to avoid immunologically-induced injury, immune responses favor immunologic tolerance. However, adaptation must be rapid to avoid life-threatening infections. To navigate the gauntlet of microbe and environmental exposures and vaccines, the neonatal immune system moves through a gradual maturation process toward immune responsivity. The maturation occurs at different rates in different children.
Reassessing Vaccine Responsiveness
Vaccine responsiveness is usually assessed by measuring antibody levels in blood. Until recently, it was thought to be “bad luck” when a child failed to develop protective immunity following vaccination. The bad luck was suggested to involve illness at the time of vaccination, especially illness occurring with fever, and especially common viral infections. But studies proved that notion incorrect. About 10 years ago I became more interested in variability in vaccine responses in the first 2 years of life. In 2016, my laboratory described a specific population of children with specific cellular immune deficiencies that we classified as low vaccine responders (LVRs).1 To preclude the suggestion that low vaccine responses were to be considered normal biological variation, we chose an a priori definition of LVR as those with sub-protective IgG antibody levels to four (≥ 66 %) of six tested vaccines in DTaP-Hib (diphtheria toxoid, tetanus toxoid, pertussis toxoid, pertactin, and filamentous hemagglutinin [DTaP] and Haemophilus influenzae type b polysaccharide capsule [Hib]). Antibody levels were measured at 1 year of age following primary vaccinations at child age 2, 4, and 6 months old. The remaining 89% of children we termed normal vaccine responders (NVRs). We additionally tested antibody responses to viral protein and pneumococcal polysaccharide conjugated antigens (polio serotypes 1, 2, and 3, hepatitis B, and Streptococcus pneumoniae capsular polysaccharides serotypes 6B, 14, and 23F). Responses to these vaccine antigens were similar to the six vaccines (DTaP/Hib) used to define LVR. We and other groups have used alternative definitions of low vaccine responses that rely on statistics.
I recently reviewed the topic of the determinants of vaccine responses in early life, with a focus on the infant microbiome and metabolome: a.) cesarean section versus vaginal delivery, b.) breast versus formula feeding and c.) antibiotic exposure, that impact the immune response2 (Figure). In the review I also discussed how microbiome may serve as natural adjuvants for vaccine responses, how microbiota-derived metabolites influence vaccine responses, and how low vaccine responses in early life may be linked to increased infection susceptibility (Figure).
Cesarean section births occur in nearly 30% of newborns. Cesarean section birth has been associated with adverse effects on immune development, including predisposing to infections, allergies, and inflammatory disorders. The association of these adverse outcomes has been linked to lower total microbiome diversity. Fecal microbiome seeding from mother to infant in vaginal-delivered infants results in a more favorable and stable microbiome compared with cesarean-delivered infants. Nasopharyngeal microbiome may also be adversely affected by cesarean delivery. In turn, those microbiome differences can be linked to variation in vaccine responsiveness in infants.
Multiple studies strongly support the notion that breastfeeding has a favorable impact on immune development in early life associated with better vaccine responses, mediated by the microbiome. The mechanism of favorable immune responses to vaccines largely relates to the presence of a specific bacteria species, Bifidobacterium infantis. Breast milk contains human milk oligosaccharides that are not digestible by newborns. B. infantis is a strain of bacteria that utilizes these non-digestible oligosaccharides. Thereby, infants fed breast milk provides B. infantis the essential source of nutrition for its growth and predominance in the newborn gut. Studies have shown that Bifidobacterium spp. abundance in early life is correlated with better immune responses to multiple vaccines. Bifidobacterium spp. abundance has been positively correlated with antibody responses measured after 2 years, linking the microbiome composition to the durability of vaccine-induced immune responses.
Antibiotic exposure in early life may disproportionately damage the newborn and infant microbiome compared with later childhood. The average child receives about three antibiotic courses by the age of 2 years. My lab was among the first to describe the adverse effects of antibiotics on vaccine responses in early life.3 We found that broader spectrum antibiotics had a greater adverse effect on vaccine-induced antibody levels than narrower spectrum antibiotics. Ten-day versus five-day treatment courses had a greater negative effect. Multiple antibiotic courses over time (cumulative antibiotic exposure) was negatively associated with vaccine-induced antibody levels.
Over 11 % of live births worldwide occur preterm. Because bacterial infections are frequent complications of preterm birth, 79 % of very low birthweight and 87 % of extremely low birthweight infants in US NICUs receive antibiotics within 3 days of birth. Recently, my group studied full-term infants at birth and found that exposure to parenteral antibiotics at birth or during the first days of life had an adverse effect on vaccine responses.4
Microbiome Impacts Immunity
How does the microbiome affect immunity, and specifically vaccine responses? Microbial-derived metabolites affect host immunity. Gut bacteria produce short chain fatty acids (SCFAs: acetate, propionate, butyrate) [115]. SCFAs positively influence immunity cells. Vitamin D metabolites are generated by intestinal bacteria and those metabolites positively influence immunity. Secondary bile acids produced by Clostridium spp. are involved in favorable immune responses. Increased levels of phenylpyruvic acid produced by gut and/or nasopharyngeal microbiota correlate with reduced vaccine responses and upregulated metabolome genes that encode for oxidative phosphorylation correlate with increased vaccine responses.
In summary, immune development commences at birth. Impairment in responses to vaccination in children have been linked to disturbance in the microbiome. Cesarean section and absence of breastfeeding are associated with adverse microbiota composition. Antibiotics perturb healthy microbiota development. The microbiota affect immunity in several ways, among them are effects by metabolites generated by the commensals that inhabit the child host. A child who responds poorly to vaccines and has specific immune cell dysfunction caused by problems with the microbiome also displays increased infection proneness. But that is a story for another column, later.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
References
1. Pichichero ME et al. J Infect Dis. 2016 Jun 15;213(12):2014-2019. doi: 10.1093/infdis/jiw053.
2. Pichichero ME. Cell Immunol. 2023 Nov-Dec:393-394:104777. doi: 10.1016/j.cellimm.2023.104777.
3. Chapman TJ et al. Pediatrics. 2022 May 1;149(5):e2021052061. doi: 10.1542/peds.2021-052061.
4. Shaffer M et al. mSystems. 2023 Oct 26;8(5):e0066123. doi: 10.1128/msystems.00661-23.
A Tale of Two Babies and the ‘Family Tragedy’ of Congenital Syphilis
Delivered at 34 weeks’ gestation, Baby “Alex” had an enlarged liver and spleen on his initial newborn exam, poor tone, and a diffuse, peeling rash. Baby “Aaliyah” was born at term and appeared healthy. By 1 month of age, she was gaining weight poorly and developed copious nasal drainage and a salmon-colored rash on the soles of her feet.
The connection? Both babies were ultimately diagnosed with congenital syphilis. Infections in both babies could have been prevented if their mothers had been tested for syphilis and treated during pregnancy. Alex’s mom had no prenatal care. Aaliyah’s mom had tested negative for syphilis during her first trimester but had not been re-tested, despite sharing with her health care provider that she had a new sexual partner.
Alex and Aaliyah are representative of what Centers for Disease Control and Prevention (CDC) Chief Medical Officer Debra Houry, MD, MPH, calls a “family tragedy.” Cases of congenital syphilis are rising rapidly in the United States, reaching a 30-year high in 2021.1 Cases increased by 755% between 2012 and 2021, from 335 in 2012 to 2,865 in 2021. In 2022, cases rose again: 3,761 cases of congenital syphilis were reported, including 231 stillbirths and 51 infant deaths. Infants with congenital syphilis are at risk for lifelong complications, including deafness, blindness, and intellectual disability.
Most of these cases were preventable. Congenital syphilis is rare when pregnant people complete adequate treatment at least 30 days before delivery. In 2022, lack of testing or timely testing contributed to 36.8% of congenital syphilis cases. Nearly 40% of birth parents of infected babies received inadequate treatment during pregnancy, and 11.2% received no treatment or treatment was not documented.
, suggesting ongoing barriers to care related to social determinants of health. In 2021, the highest rates of congenital syphilis were among babies born to individuals who were non-Hispanic American Indian or Alaska Native (384 cases per 100,000 live births), non-Hispanic Native Hawaiian or other Pacific Islander (192 cases per 100,000 live births), and non-Hispanic Black or African American (169 cases per 100,000 live births). Six states had rates of congenital syphilis that exceeded 160 cases per 100,000 population, including Arizona, New Mexico, Louisiana, Mississippi, Texas, and Oklahoma. That is more than twice the national rate of 77.9 cases/100,000.
Reducing the Risk
To reduce rates of congenital syphilis in all people, barriers to testing must be eliminated. The CDC recommends that all pregnant people be tested early in pregnancy, with repeat testing at 28 weeks and at delivery for those at increased risk for infection based on individual risk factors or residence in a high-prevalence community. Rapid syphilis testing and treatment during pregnancy is recommended in settings such as emergency departments, syringe service programs, prisons/jails, and maternal and child health programs to minimize missed opportunities for care.
While pediatric clinicians rarely care for pregnant patients, they also have an essential role to play in reducing the adverse health outcomes associated with congenital syphilis. No infant should be discharged from the newborn nursery without confirming that the birth parent was tested for syphilis at least once and was treated appropriately if positive. Appropriate treatment during pregnancy is a single dose of benzathine penicillin G for primary, secondary, or early latent syphilis. Late-latent syphilis or syphilis of unknown duration is treated with three doses of benzathine penicillin G spaced 7-9 days apart. If the doses are given further than 9 days apart, treatment is considered inadequate, and the series of doses must be restarted. Benzathine penicillin G remains in short supply in the United States, but is the only drug recommended to treat syphilis during pregnancy.
Collaboration between obstetrical and newborn care providers is essential. Those who care for newborns need easy access to birthing parents’ syphilis treatment results. As more health care facilities implement routine syphilis testing at delivery, rapid syphilis testing must be available to avoid prolonging newborn hospital stays.
Pediatricians need to maintain an index of suspicion for congenital syphilis, regardless of maternal history, because symptomatic congenital syphilis can mimic a variety of infectious and noninfectious conditions. Most infected infants look normal at birth. While the majority of cases of congenital syphilis are identified in the newborn period, a 2021 paper published in Pediatrics described 84 infants born between 2014 and 2018 who were diagnosed beyond a month of age.2 These represented 2.2% of all infants born with congenital syphilis. Common symptoms included rash, snuffles, and hepatomegaly. Sixty-nine percent of infants who had long bone radiographs obtained had findings consistent with congenital syphilis. Typical imaging findings include periostitis and demineralization of the metaphysis and diaphysis of long bones, although fractures can also occur. Case reports describe infants who presented with fractures and were initially evaluated for nonaccidental trauma.3
Another critical approach is to treat syphilis in people of childbearing age before pregnancy occurs. The CDC recommends syphilis testing for sexually active females 18-44 years of age and living in communities with high rates of syphilis. County-specific specific rates of syphilis rates are available at https://www.cdc.gov/nchhstp/atlas/syphilis/. Point-of-care tests are now available for syphilis and may facilitate timely treatment.
Additional resources describing syphilis testing and treatment are available from the CDC and the American Academy of Pediatrics.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at [email protected]. (Also [email protected].)
References
1. McDonald R et al. Vital Signs: Missed Opportunities for Preventing Congenital Syphilis — United States, 2022. MMWR Morb Mortal Wkly Rep. 2023 Nov 17;72(46):1269-74. doi: 10.15585/mmwr.mm7246e1.
2. Kimball A et al. Congenital Syphilis Diagnosed Beyond the Neonatal Period in the United States: 2014-2018. Pediatrics. 2021 Sep;148(3):e2020049080. doi: 10.1542/peds.2020-049080.
3. Jacobs K et al. Congenital Syphilis Misdiagnosed as Suspected Nonaccidental Trauma. Pediatrics. 2019 Oct;144(4):e20191564. doi: 10.1542/peds.2019-1564.
Delivered at 34 weeks’ gestation, Baby “Alex” had an enlarged liver and spleen on his initial newborn exam, poor tone, and a diffuse, peeling rash. Baby “Aaliyah” was born at term and appeared healthy. By 1 month of age, she was gaining weight poorly and developed copious nasal drainage and a salmon-colored rash on the soles of her feet.
The connection? Both babies were ultimately diagnosed with congenital syphilis. Infections in both babies could have been prevented if their mothers had been tested for syphilis and treated during pregnancy. Alex’s mom had no prenatal care. Aaliyah’s mom had tested negative for syphilis during her first trimester but had not been re-tested, despite sharing with her health care provider that she had a new sexual partner.
Alex and Aaliyah are representative of what Centers for Disease Control and Prevention (CDC) Chief Medical Officer Debra Houry, MD, MPH, calls a “family tragedy.” Cases of congenital syphilis are rising rapidly in the United States, reaching a 30-year high in 2021.1 Cases increased by 755% between 2012 and 2021, from 335 in 2012 to 2,865 in 2021. In 2022, cases rose again: 3,761 cases of congenital syphilis were reported, including 231 stillbirths and 51 infant deaths. Infants with congenital syphilis are at risk for lifelong complications, including deafness, blindness, and intellectual disability.
Most of these cases were preventable. Congenital syphilis is rare when pregnant people complete adequate treatment at least 30 days before delivery. In 2022, lack of testing or timely testing contributed to 36.8% of congenital syphilis cases. Nearly 40% of birth parents of infected babies received inadequate treatment during pregnancy, and 11.2% received no treatment or treatment was not documented.
, suggesting ongoing barriers to care related to social determinants of health. In 2021, the highest rates of congenital syphilis were among babies born to individuals who were non-Hispanic American Indian or Alaska Native (384 cases per 100,000 live births), non-Hispanic Native Hawaiian or other Pacific Islander (192 cases per 100,000 live births), and non-Hispanic Black or African American (169 cases per 100,000 live births). Six states had rates of congenital syphilis that exceeded 160 cases per 100,000 population, including Arizona, New Mexico, Louisiana, Mississippi, Texas, and Oklahoma. That is more than twice the national rate of 77.9 cases/100,000.
Reducing the Risk
To reduce rates of congenital syphilis in all people, barriers to testing must be eliminated. The CDC recommends that all pregnant people be tested early in pregnancy, with repeat testing at 28 weeks and at delivery for those at increased risk for infection based on individual risk factors or residence in a high-prevalence community. Rapid syphilis testing and treatment during pregnancy is recommended in settings such as emergency departments, syringe service programs, prisons/jails, and maternal and child health programs to minimize missed opportunities for care.
While pediatric clinicians rarely care for pregnant patients, they also have an essential role to play in reducing the adverse health outcomes associated with congenital syphilis. No infant should be discharged from the newborn nursery without confirming that the birth parent was tested for syphilis at least once and was treated appropriately if positive. Appropriate treatment during pregnancy is a single dose of benzathine penicillin G for primary, secondary, or early latent syphilis. Late-latent syphilis or syphilis of unknown duration is treated with three doses of benzathine penicillin G spaced 7-9 days apart. If the doses are given further than 9 days apart, treatment is considered inadequate, and the series of doses must be restarted. Benzathine penicillin G remains in short supply in the United States, but is the only drug recommended to treat syphilis during pregnancy.
Collaboration between obstetrical and newborn care providers is essential. Those who care for newborns need easy access to birthing parents’ syphilis treatment results. As more health care facilities implement routine syphilis testing at delivery, rapid syphilis testing must be available to avoid prolonging newborn hospital stays.
Pediatricians need to maintain an index of suspicion for congenital syphilis, regardless of maternal history, because symptomatic congenital syphilis can mimic a variety of infectious and noninfectious conditions. Most infected infants look normal at birth. While the majority of cases of congenital syphilis are identified in the newborn period, a 2021 paper published in Pediatrics described 84 infants born between 2014 and 2018 who were diagnosed beyond a month of age.2 These represented 2.2% of all infants born with congenital syphilis. Common symptoms included rash, snuffles, and hepatomegaly. Sixty-nine percent of infants who had long bone radiographs obtained had findings consistent with congenital syphilis. Typical imaging findings include periostitis and demineralization of the metaphysis and diaphysis of long bones, although fractures can also occur. Case reports describe infants who presented with fractures and were initially evaluated for nonaccidental trauma.3
Another critical approach is to treat syphilis in people of childbearing age before pregnancy occurs. The CDC recommends syphilis testing for sexually active females 18-44 years of age and living in communities with high rates of syphilis. County-specific specific rates of syphilis rates are available at https://www.cdc.gov/nchhstp/atlas/syphilis/. Point-of-care tests are now available for syphilis and may facilitate timely treatment.
Additional resources describing syphilis testing and treatment are available from the CDC and the American Academy of Pediatrics.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at [email protected]. (Also [email protected].)
References
1. McDonald R et al. Vital Signs: Missed Opportunities for Preventing Congenital Syphilis — United States, 2022. MMWR Morb Mortal Wkly Rep. 2023 Nov 17;72(46):1269-74. doi: 10.15585/mmwr.mm7246e1.
2. Kimball A et al. Congenital Syphilis Diagnosed Beyond the Neonatal Period in the United States: 2014-2018. Pediatrics. 2021 Sep;148(3):e2020049080. doi: 10.1542/peds.2020-049080.
3. Jacobs K et al. Congenital Syphilis Misdiagnosed as Suspected Nonaccidental Trauma. Pediatrics. 2019 Oct;144(4):e20191564. doi: 10.1542/peds.2019-1564.
Delivered at 34 weeks’ gestation, Baby “Alex” had an enlarged liver and spleen on his initial newborn exam, poor tone, and a diffuse, peeling rash. Baby “Aaliyah” was born at term and appeared healthy. By 1 month of age, she was gaining weight poorly and developed copious nasal drainage and a salmon-colored rash on the soles of her feet.
The connection? Both babies were ultimately diagnosed with congenital syphilis. Infections in both babies could have been prevented if their mothers had been tested for syphilis and treated during pregnancy. Alex’s mom had no prenatal care. Aaliyah’s mom had tested negative for syphilis during her first trimester but had not been re-tested, despite sharing with her health care provider that she had a new sexual partner.
Alex and Aaliyah are representative of what Centers for Disease Control and Prevention (CDC) Chief Medical Officer Debra Houry, MD, MPH, calls a “family tragedy.” Cases of congenital syphilis are rising rapidly in the United States, reaching a 30-year high in 2021.1 Cases increased by 755% between 2012 and 2021, from 335 in 2012 to 2,865 in 2021. In 2022, cases rose again: 3,761 cases of congenital syphilis were reported, including 231 stillbirths and 51 infant deaths. Infants with congenital syphilis are at risk for lifelong complications, including deafness, blindness, and intellectual disability.
Most of these cases were preventable. Congenital syphilis is rare when pregnant people complete adequate treatment at least 30 days before delivery. In 2022, lack of testing or timely testing contributed to 36.8% of congenital syphilis cases. Nearly 40% of birth parents of infected babies received inadequate treatment during pregnancy, and 11.2% received no treatment or treatment was not documented.
, suggesting ongoing barriers to care related to social determinants of health. In 2021, the highest rates of congenital syphilis were among babies born to individuals who were non-Hispanic American Indian or Alaska Native (384 cases per 100,000 live births), non-Hispanic Native Hawaiian or other Pacific Islander (192 cases per 100,000 live births), and non-Hispanic Black or African American (169 cases per 100,000 live births). Six states had rates of congenital syphilis that exceeded 160 cases per 100,000 population, including Arizona, New Mexico, Louisiana, Mississippi, Texas, and Oklahoma. That is more than twice the national rate of 77.9 cases/100,000.
Reducing the Risk
To reduce rates of congenital syphilis in all people, barriers to testing must be eliminated. The CDC recommends that all pregnant people be tested early in pregnancy, with repeat testing at 28 weeks and at delivery for those at increased risk for infection based on individual risk factors or residence in a high-prevalence community. Rapid syphilis testing and treatment during pregnancy is recommended in settings such as emergency departments, syringe service programs, prisons/jails, and maternal and child health programs to minimize missed opportunities for care.
While pediatric clinicians rarely care for pregnant patients, they also have an essential role to play in reducing the adverse health outcomes associated with congenital syphilis. No infant should be discharged from the newborn nursery without confirming that the birth parent was tested for syphilis at least once and was treated appropriately if positive. Appropriate treatment during pregnancy is a single dose of benzathine penicillin G for primary, secondary, or early latent syphilis. Late-latent syphilis or syphilis of unknown duration is treated with three doses of benzathine penicillin G spaced 7-9 days apart. If the doses are given further than 9 days apart, treatment is considered inadequate, and the series of doses must be restarted. Benzathine penicillin G remains in short supply in the United States, but is the only drug recommended to treat syphilis during pregnancy.
Collaboration between obstetrical and newborn care providers is essential. Those who care for newborns need easy access to birthing parents’ syphilis treatment results. As more health care facilities implement routine syphilis testing at delivery, rapid syphilis testing must be available to avoid prolonging newborn hospital stays.
Pediatricians need to maintain an index of suspicion for congenital syphilis, regardless of maternal history, because symptomatic congenital syphilis can mimic a variety of infectious and noninfectious conditions. Most infected infants look normal at birth. While the majority of cases of congenital syphilis are identified in the newborn period, a 2021 paper published in Pediatrics described 84 infants born between 2014 and 2018 who were diagnosed beyond a month of age.2 These represented 2.2% of all infants born with congenital syphilis. Common symptoms included rash, snuffles, and hepatomegaly. Sixty-nine percent of infants who had long bone radiographs obtained had findings consistent with congenital syphilis. Typical imaging findings include periostitis and demineralization of the metaphysis and diaphysis of long bones, although fractures can also occur. Case reports describe infants who presented with fractures and were initially evaluated for nonaccidental trauma.3
Another critical approach is to treat syphilis in people of childbearing age before pregnancy occurs. The CDC recommends syphilis testing for sexually active females 18-44 years of age and living in communities with high rates of syphilis. County-specific specific rates of syphilis rates are available at https://www.cdc.gov/nchhstp/atlas/syphilis/. Point-of-care tests are now available for syphilis and may facilitate timely treatment.
Additional resources describing syphilis testing and treatment are available from the CDC and the American Academy of Pediatrics.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta, and Gilead. Email her at [email protected]. (Also [email protected].)
References
1. McDonald R et al. Vital Signs: Missed Opportunities for Preventing Congenital Syphilis — United States, 2022. MMWR Morb Mortal Wkly Rep. 2023 Nov 17;72(46):1269-74. doi: 10.15585/mmwr.mm7246e1.
2. Kimball A et al. Congenital Syphilis Diagnosed Beyond the Neonatal Period in the United States: 2014-2018. Pediatrics. 2021 Sep;148(3):e2020049080. doi: 10.1542/peds.2020-049080.
3. Jacobs K et al. Congenital Syphilis Misdiagnosed as Suspected Nonaccidental Trauma. Pediatrics. 2019 Oct;144(4):e20191564. doi: 10.1542/peds.2019-1564.
Laissez-faire
I read a few articles recently that raised my concern about a laissez faire attitude regarding treatment and prevention of infectious disease and lack of a broader understanding of why we treat our patients.
Strep throat
Let’s start with group A streptococcal pharyngitis – strep throat. There are at least five reasons to treat strep throat with antibiotics.
Lest we forget, there is the prevention of acute rheumatic fever! Of course, acute rheumatic fever is rare in high-income countries like the United States, but we have had outbreaks in the past and we will have outbreaks in the future. All it takes is circulation of rheumatogenic strains and susceptible hosts.
Also, antibiotic treatment may prevent acute post-streptococcal glomerulonephritis, although that benefit is somewhat controversial.
Antibiotic treatment may prevent development of another controversial, nonsuppurative streptococcal complication, namely, pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS).
Second, group A strep causes suppurative complications such as acute otitis media, peritonsillar abscess, mastoiditis, and sepsis, among others, and antibiotic treatment reduces those risks. Group A strep can cause impetigo, cellulitis, necrotizing fasciitis (flesh-eating disease), and toxic shock syndrome; antibiotics reduce those risks.
Third, while strep throat is a self-limited infection in terms of symptoms, it has been clearly shown that antibiotics cause symptoms to resolve more quickly. I must confess that it galls me when pundits suggest that reducing symptoms of any infectious disease by a day or 2 doesn’t matter for children, when adults with even mild symptoms rush to a clinician with hopes of treatment to shorten illness by a day.
Fourth, antibiotics shorten contagion. In fact, treatment in the morning of an office visit can allow a child to return to school the next day.1
Lastly on this topic, if a clinician had a positive strep culture or rapid test on a patient and did not treat with antibiotics, which is not the standard of care, and that patient went on to a nonsuppurative or suppurative complication, then what?
I am not advocating wholesale antibiotic treatment of all sore throats because antibiotics carry risks from use. Most sore throats are not strep throats. The first step is the examination to decide if a strep test is warranted. There are clinical scoring systems available. But the essence of the clinical criteria relies on age of child (strep is mostly seen in 5- to 15-year-olds), season (not summer), known exposure to strep, absence of rhinorrhea, absence of cough, presence of rapid onset of symptoms, usually with fever, and moderate to severe redness, often with exudates. Gratefully, in the United States, we have rapid strep tests that are covered by insurance. This is not the case even in many other high-income countries and certainly, generally, not available at all in moderate to low income countries. With a rapid test, a point-of-care microbiologic diagnosis can be made with reasonable accuracy. Antibiotic treatment should be reserved for patients with positive laboratory confirmation of Group A streptococci, either by rapid test or culture.
Ear infections
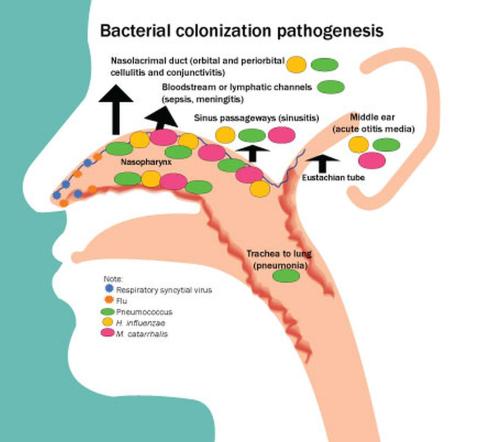
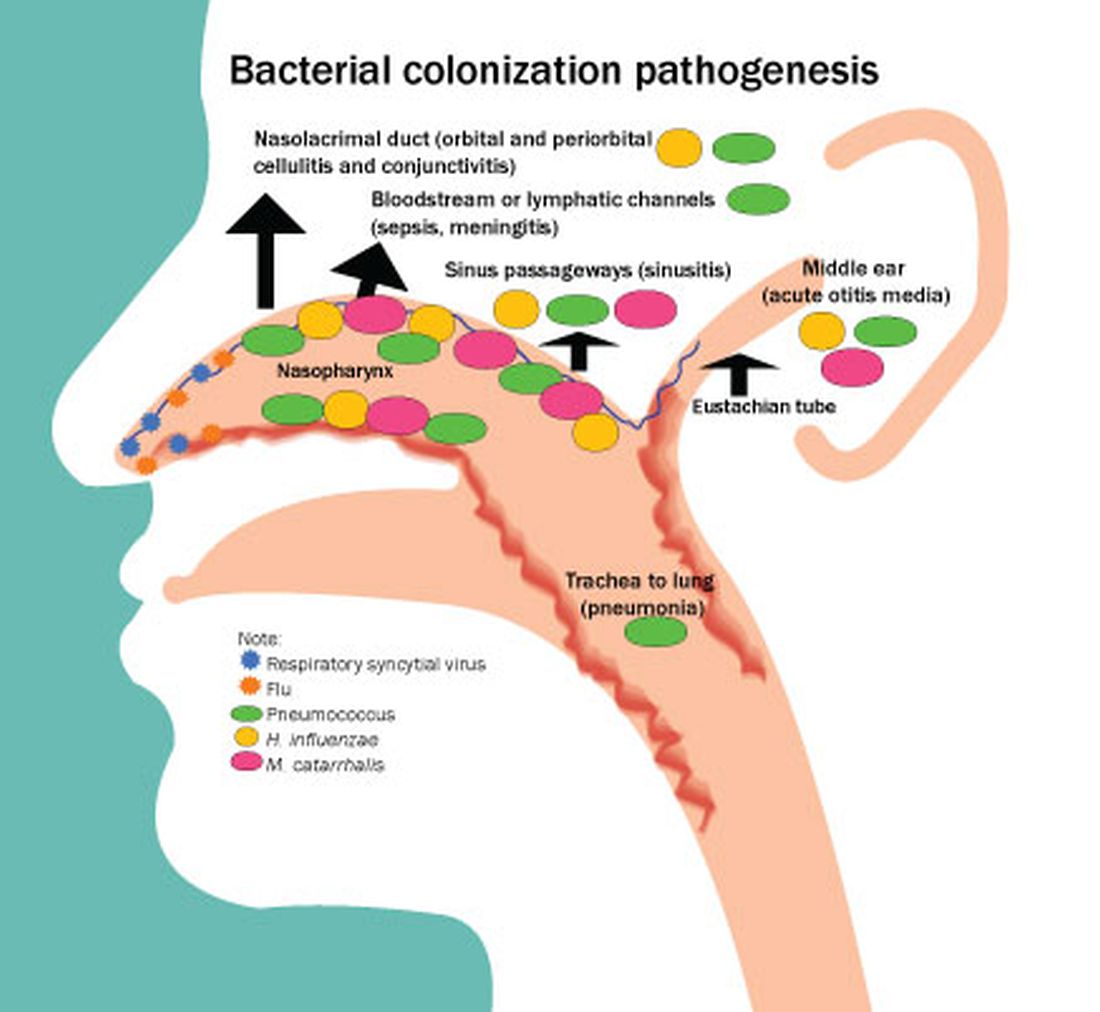
Next, let’s address treatment of acute otitis media – ear infections. There are at least six reasons to treat ear infections with antibiotics. Worldwide, the No. 1 cause of acquired deafness in children today is ear infections. This is rarely seen in the United States because we rarely have patients with chronic suppurative otitis media since antibiotics are typically prescribed.
Second, ear infections have suppurative complications such as mastoiditis, labyrinthitis, malignant otitis, brain abscess, sepsis, and meningitis. The World Health Organization attributes 20,000 deaths per year to complications from ear infections.
Third, ear infections can lead to eardrum rupture and subsequent chronic middle ear drainage.
Fourth, untreated otitis more often progresses to a nonsuppurative complication – a cholesteatoma.
Fifth, while earache is a self-limited illness, antibiotics shorten the acute symptoms by a day or 2 and lessen the duration of middle ear effusion after infection that can cause temporary hearing loss. Once again, as a child advocate, I would point out that pain from an ear infection is often severe and the lingering effects of a middle ear effusion are annoying to say the least.
Lastly on this topic, if a clinician makes the diagnosis of an ear infection in a patient and does not treat with antibiotics, the decision should be within the guidelines of the standard of care as described by the American Academy of Pediatrics2 with decision-making based on patient age and severity of symptoms.
I am not advocating wholesale antibiotic treatment of all ear pain or presumed ear pain. With this clinical condition we currently do not have a diagnostic test, and therein lies the conundrum. Most acute otitis media occurs among children age 6-24 months old, and this leads most clinicians to overdiagnose the infection. A child in that age group is nonverbal and in the context of a viral upper respiratory illness the symptoms of acute otitis media overlap completely with those of a viral URI. Therefore, an adequate examination is necessary. Confronted with an irritable child who is uncooperative with a challenging otoscopic examination, an ear canal with wax blocking an adequate view of the tympanic membrane, and a parent in a hurry to get back to work or home, the inclination is to observe a “little bit of redness” and prescribe unnecessary antibiotics. Even though redness is not a good diagnostic indicator, whereas a full or bulging eardrum is for the diagnosis of acute otitis media, I shudder at how often I see in a medical record a description of redness of the eardrum and no comment on the fullness that occurs when an authentic infection is most likely.
I could extend this column discussing acute sinusitis and cough illnesses as they are two other conditions associated with infection where antibiotics have their important place and where antibiotics are also overused. Instead, I will end by summarizing my viewpoint that judicious antibiotic use is of high importance for prevention of antibiotic resistance at the individual patient level and the community level. However, we should not become complacent about the risks to untreated children experiencing common respiratory infections because there are many justifiable reasons to treat children as discussed here.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to disclose.
References
1. Schwartz RH et al. A reappraisal of the minimum duration of antibiotic treatment before approval of return to school for children with streptococcal pharyngitis. Pediatr Infect Dis J. 2015 Dec. doi: 10.1097/INF.0000000000000883.
2. Lieberthal AS et al. The diagnosis and management of acute otitis media. Pediatrics. 2013 Mar. doi: 10.1542/peds.2012-3488.
I read a few articles recently that raised my concern about a laissez faire attitude regarding treatment and prevention of infectious disease and lack of a broader understanding of why we treat our patients.
Strep throat
Let’s start with group A streptococcal pharyngitis – strep throat. There are at least five reasons to treat strep throat with antibiotics.
Lest we forget, there is the prevention of acute rheumatic fever! Of course, acute rheumatic fever is rare in high-income countries like the United States, but we have had outbreaks in the past and we will have outbreaks in the future. All it takes is circulation of rheumatogenic strains and susceptible hosts.
Also, antibiotic treatment may prevent acute post-streptococcal glomerulonephritis, although that benefit is somewhat controversial.
Antibiotic treatment may prevent development of another controversial, nonsuppurative streptococcal complication, namely, pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS).
Second, group A strep causes suppurative complications such as acute otitis media, peritonsillar abscess, mastoiditis, and sepsis, among others, and antibiotic treatment reduces those risks. Group A strep can cause impetigo, cellulitis, necrotizing fasciitis (flesh-eating disease), and toxic shock syndrome; antibiotics reduce those risks.
Third, while strep throat is a self-limited infection in terms of symptoms, it has been clearly shown that antibiotics cause symptoms to resolve more quickly. I must confess that it galls me when pundits suggest that reducing symptoms of any infectious disease by a day or 2 doesn’t matter for children, when adults with even mild symptoms rush to a clinician with hopes of treatment to shorten illness by a day.
Fourth, antibiotics shorten contagion. In fact, treatment in the morning of an office visit can allow a child to return to school the next day.1
Lastly on this topic, if a clinician had a positive strep culture or rapid test on a patient and did not treat with antibiotics, which is not the standard of care, and that patient went on to a nonsuppurative or suppurative complication, then what?
I am not advocating wholesale antibiotic treatment of all sore throats because antibiotics carry risks from use. Most sore throats are not strep throats. The first step is the examination to decide if a strep test is warranted. There are clinical scoring systems available. But the essence of the clinical criteria relies on age of child (strep is mostly seen in 5- to 15-year-olds), season (not summer), known exposure to strep, absence of rhinorrhea, absence of cough, presence of rapid onset of symptoms, usually with fever, and moderate to severe redness, often with exudates. Gratefully, in the United States, we have rapid strep tests that are covered by insurance. This is not the case even in many other high-income countries and certainly, generally, not available at all in moderate to low income countries. With a rapid test, a point-of-care microbiologic diagnosis can be made with reasonable accuracy. Antibiotic treatment should be reserved for patients with positive laboratory confirmation of Group A streptococci, either by rapid test or culture.
Ear infections
Next, let’s address treatment of acute otitis media – ear infections. There are at least six reasons to treat ear infections with antibiotics. Worldwide, the No. 1 cause of acquired deafness in children today is ear infections. This is rarely seen in the United States because we rarely have patients with chronic suppurative otitis media since antibiotics are typically prescribed.
Second, ear infections have suppurative complications such as mastoiditis, labyrinthitis, malignant otitis, brain abscess, sepsis, and meningitis. The World Health Organization attributes 20,000 deaths per year to complications from ear infections.
Third, ear infections can lead to eardrum rupture and subsequent chronic middle ear drainage.
Fourth, untreated otitis more often progresses to a nonsuppurative complication – a cholesteatoma.
Fifth, while earache is a self-limited illness, antibiotics shorten the acute symptoms by a day or 2 and lessen the duration of middle ear effusion after infection that can cause temporary hearing loss. Once again, as a child advocate, I would point out that pain from an ear infection is often severe and the lingering effects of a middle ear effusion are annoying to say the least.
Lastly on this topic, if a clinician makes the diagnosis of an ear infection in a patient and does not treat with antibiotics, the decision should be within the guidelines of the standard of care as described by the American Academy of Pediatrics2 with decision-making based on patient age and severity of symptoms.
I am not advocating wholesale antibiotic treatment of all ear pain or presumed ear pain. With this clinical condition we currently do not have a diagnostic test, and therein lies the conundrum. Most acute otitis media occurs among children age 6-24 months old, and this leads most clinicians to overdiagnose the infection. A child in that age group is nonverbal and in the context of a viral upper respiratory illness the symptoms of acute otitis media overlap completely with those of a viral URI. Therefore, an adequate examination is necessary. Confronted with an irritable child who is uncooperative with a challenging otoscopic examination, an ear canal with wax blocking an adequate view of the tympanic membrane, and a parent in a hurry to get back to work or home, the inclination is to observe a “little bit of redness” and prescribe unnecessary antibiotics. Even though redness is not a good diagnostic indicator, whereas a full or bulging eardrum is for the diagnosis of acute otitis media, I shudder at how often I see in a medical record a description of redness of the eardrum and no comment on the fullness that occurs when an authentic infection is most likely.
I could extend this column discussing acute sinusitis and cough illnesses as they are two other conditions associated with infection where antibiotics have their important place and where antibiotics are also overused. Instead, I will end by summarizing my viewpoint that judicious antibiotic use is of high importance for prevention of antibiotic resistance at the individual patient level and the community level. However, we should not become complacent about the risks to untreated children experiencing common respiratory infections because there are many justifiable reasons to treat children as discussed here.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to disclose.
References
1. Schwartz RH et al. A reappraisal of the minimum duration of antibiotic treatment before approval of return to school for children with streptococcal pharyngitis. Pediatr Infect Dis J. 2015 Dec. doi: 10.1097/INF.0000000000000883.
2. Lieberthal AS et al. The diagnosis and management of acute otitis media. Pediatrics. 2013 Mar. doi: 10.1542/peds.2012-3488.
I read a few articles recently that raised my concern about a laissez faire attitude regarding treatment and prevention of infectious disease and lack of a broader understanding of why we treat our patients.
Strep throat
Let’s start with group A streptococcal pharyngitis – strep throat. There are at least five reasons to treat strep throat with antibiotics.
Lest we forget, there is the prevention of acute rheumatic fever! Of course, acute rheumatic fever is rare in high-income countries like the United States, but we have had outbreaks in the past and we will have outbreaks in the future. All it takes is circulation of rheumatogenic strains and susceptible hosts.
Also, antibiotic treatment may prevent acute post-streptococcal glomerulonephritis, although that benefit is somewhat controversial.
Antibiotic treatment may prevent development of another controversial, nonsuppurative streptococcal complication, namely, pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS).
Second, group A strep causes suppurative complications such as acute otitis media, peritonsillar abscess, mastoiditis, and sepsis, among others, and antibiotic treatment reduces those risks. Group A strep can cause impetigo, cellulitis, necrotizing fasciitis (flesh-eating disease), and toxic shock syndrome; antibiotics reduce those risks.
Third, while strep throat is a self-limited infection in terms of symptoms, it has been clearly shown that antibiotics cause symptoms to resolve more quickly. I must confess that it galls me when pundits suggest that reducing symptoms of any infectious disease by a day or 2 doesn’t matter for children, when adults with even mild symptoms rush to a clinician with hopes of treatment to shorten illness by a day.
Fourth, antibiotics shorten contagion. In fact, treatment in the morning of an office visit can allow a child to return to school the next day.1
Lastly on this topic, if a clinician had a positive strep culture or rapid test on a patient and did not treat with antibiotics, which is not the standard of care, and that patient went on to a nonsuppurative or suppurative complication, then what?
I am not advocating wholesale antibiotic treatment of all sore throats because antibiotics carry risks from use. Most sore throats are not strep throats. The first step is the examination to decide if a strep test is warranted. There are clinical scoring systems available. But the essence of the clinical criteria relies on age of child (strep is mostly seen in 5- to 15-year-olds), season (not summer), known exposure to strep, absence of rhinorrhea, absence of cough, presence of rapid onset of symptoms, usually with fever, and moderate to severe redness, often with exudates. Gratefully, in the United States, we have rapid strep tests that are covered by insurance. This is not the case even in many other high-income countries and certainly, generally, not available at all in moderate to low income countries. With a rapid test, a point-of-care microbiologic diagnosis can be made with reasonable accuracy. Antibiotic treatment should be reserved for patients with positive laboratory confirmation of Group A streptococci, either by rapid test or culture.
Ear infections
Next, let’s address treatment of acute otitis media – ear infections. There are at least six reasons to treat ear infections with antibiotics. Worldwide, the No. 1 cause of acquired deafness in children today is ear infections. This is rarely seen in the United States because we rarely have patients with chronic suppurative otitis media since antibiotics are typically prescribed.
Second, ear infections have suppurative complications such as mastoiditis, labyrinthitis, malignant otitis, brain abscess, sepsis, and meningitis. The World Health Organization attributes 20,000 deaths per year to complications from ear infections.
Third, ear infections can lead to eardrum rupture and subsequent chronic middle ear drainage.
Fourth, untreated otitis more often progresses to a nonsuppurative complication – a cholesteatoma.
Fifth, while earache is a self-limited illness, antibiotics shorten the acute symptoms by a day or 2 and lessen the duration of middle ear effusion after infection that can cause temporary hearing loss. Once again, as a child advocate, I would point out that pain from an ear infection is often severe and the lingering effects of a middle ear effusion are annoying to say the least.
Lastly on this topic, if a clinician makes the diagnosis of an ear infection in a patient and does not treat with antibiotics, the decision should be within the guidelines of the standard of care as described by the American Academy of Pediatrics2 with decision-making based on patient age and severity of symptoms.
I am not advocating wholesale antibiotic treatment of all ear pain or presumed ear pain. With this clinical condition we currently do not have a diagnostic test, and therein lies the conundrum. Most acute otitis media occurs among children age 6-24 months old, and this leads most clinicians to overdiagnose the infection. A child in that age group is nonverbal and in the context of a viral upper respiratory illness the symptoms of acute otitis media overlap completely with those of a viral URI. Therefore, an adequate examination is necessary. Confronted with an irritable child who is uncooperative with a challenging otoscopic examination, an ear canal with wax blocking an adequate view of the tympanic membrane, and a parent in a hurry to get back to work or home, the inclination is to observe a “little bit of redness” and prescribe unnecessary antibiotics. Even though redness is not a good diagnostic indicator, whereas a full or bulging eardrum is for the diagnosis of acute otitis media, I shudder at how often I see in a medical record a description of redness of the eardrum and no comment on the fullness that occurs when an authentic infection is most likely.
I could extend this column discussing acute sinusitis and cough illnesses as they are two other conditions associated with infection where antibiotics have their important place and where antibiotics are also overused. Instead, I will end by summarizing my viewpoint that judicious antibiotic use is of high importance for prevention of antibiotic resistance at the individual patient level and the community level. However, we should not become complacent about the risks to untreated children experiencing common respiratory infections because there are many justifiable reasons to treat children as discussed here.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to disclose.
References
1. Schwartz RH et al. A reappraisal of the minimum duration of antibiotic treatment before approval of return to school for children with streptococcal pharyngitis. Pediatr Infect Dis J. 2015 Dec. doi: 10.1097/INF.0000000000000883.
2. Lieberthal AS et al. The diagnosis and management of acute otitis media. Pediatrics. 2013 Mar. doi: 10.1542/peds.2012-3488.
Preparing for the viral trifecta: RSV, influenza, and COVID-19
New armamentaria available to fight an old disease.
In July 2023, nirsevimab (Beyfortus), a monoclonal antibody, was approved by the Food and Drug Administration for the prevention of respiratory syncytial virus (RSV) disease in infants and children younger than 2 years of age. On Aug. 3, 2023, the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention recommended routine use of it for all infants younger than 8 months of age born during or entering their first RSV season. Its use is also recommended for certain children 8-19 months of age who are at increased risk for severe RSV disease at the start of their second RSV season. Hearing the approval, I immediately had a flashback to residency, recalling the multiple infants admitted each fall and winter exhibiting classic symptoms including cough, rhinorrhea, nasal flaring, retractions, and wheezing with many having oxygen requirements and others needing intubation. Only supportive care was available.
RSV is the leading cause of infant hospitalizations. Annually, the CDC estimates there are 50,000-80,000 RSV hospitalizations and 100-300 RSV-related deaths in the United States in persons younger than 5 years of age. While premature infants have the highest rates of hospitalization (three times a term infant) about 79% of hospitalized children younger than 2 years have no underlying medical risks.1 The majority of children will experience RSV as an upper respiratory infection within the first 2 years of life. However, severe disease requiring hospitalization is more likely to occur in premature infants and children younger than 6 months; children younger than 2 with congenital heart disease and/or chronic lung disease; children with severe cystic fibrosis; as well as the immunocompromised child and individuals with neuromuscular disorders that preclude clearing mucous secretions or have difficulty swallowing.
Palivizumab (Synagis), the first monoclonal antibody to prevent RSV in infants was licensed in 1998. Its use was limited to infants meeting specific criteria developed by the American Academy of Pediatrics. Only 5% of infants had access to it. It was a short-acting agent requiring monthly injections, which were very costly ($1,661-$2,584 per dose). Eligible infants could receive up to five injections per season. Several studies proved its use was not cost beneficial.
What are the advantages of nirsevimab? It’s a long-acting monoclonal antibody. Only one dose is required per season. Costs will significantly diminish. It is recommended for all infants younger than 8 months of age born during RSV season. Those children 8-19 months at risk for severe RSV disease can receive it prior to the start of their second RSV season. During RSV season (October 1 to March 31), the initial dose should be administered to newborns just prior to hospital discharge. Older infants and newborns who did not receive it prior to hospital discharge can receive it at their medical home. Newborns should receive it within the first week of life. It is covered by the Vaccine for Children Program. Simultaneous administration with routine childhood immunizations is recommended. Finally, RSV season may vary in tropical areas (Southern Florida, Puerto Rico. etc.) and Alaska. The timing of nirsevimab administration should be based on local RSV activity provided by state and local authorities.
In addition, the FDA approved an RSV vaccine (Abrysvo) for use in adults at least 60 years of age and in pregnant women at 32-36 weeks’ gestation. The latter is administered to prevent lower respiratory tract infection in infants from birth to 6 months. Recommendations have been published for administration in nonpregnant adults. Specific information is forthcoming in terms timing of administration of nirsevimab in infants whose mothers receive Abrysvo.
RSV season is quickly approaching. Detailed recommendations for administration and FAQ questions related to nirsevimab and palivizumab can be found at https://www.aap.org or https://www.cdc.gov/vaccines/hcp/acip-recs/index.html.
Influenza
So, what about influenza? Vaccine composition has been tweaked to match the circulating viruses but the recommended age for annual routine administration remains unchanged. All persons at least 6 months of age should be vaccinated. Children between 6 months and 8 years need two doses at least 4 weeks apart when receiving vaccine for the first time. Immunizing everyone in the household is encouraged especially if there are household contacts at risk for developing severe disease, including infants too young to be vaccinated. Keep in mind children may be coinfected with multiple viruses. Adams and colleagues reviewed the prevalence of coinfection of influenza and Sars-CoV-2 in persons younger than 18 years reported to three CDC surveillance platforms during the 2021-2022 season.2 Thirty-two of 575 hospitalized (6%) coinfections were analyzed and 7 of 44 (16%) deaths. Compared with patients without coinfections, the coinfected patients were more likely to require mechanical ventilation (13% vs. 4%) or CPAP (16% vs. 6%). Only 4 of 23 who were influenza vaccine eligible were vaccinated. Of seven coinfected children who died, none had received influenza vaccine and only one received an antiviral. Only 5 of 31 (16%) infected only with influenza were vaccinated.3
Influenza activity was lower than usual during the 2021-2022 season. However, this report revealed underuse of both influenza vaccine and antiviral therapy, both of which are routinely recommended.
COVID-19
What’s new with COVID-19? On Sept. 12, 2023, ACIP recommended that everyone at least 6 months of age receive the 2023-2024 (monovalent, XBB containing) COVID-19 vaccines. Children at least 5 years of age need one dose and those younger need one or two doses depending on the number of doses previously received. Why the change? Circulating variants continue to change. There is a current uptick in cases including hospitalizations (7.7%) and deaths (4.5%) and it’s just the beginning of the season.4 Symptoms, risk groups and complications have not changed. The primary goal is to prevent infection, hospitalization, long term complications, and death.
We are now armed with the most up-to-date interventions to help prevent the acquisition of these three viruses. Our next step is recommending and delivering them to our patients.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She reported no relevant financial disclosures.
References
1.Suh M et al. J Infect Dis. 2022;226(Suppl 2):S154-36. doi: 10.1093/infdis/jiac120.
2. Adams K et al. MMWR Morb Mortal Wkly Rep. 2022;71:1589-96. doi: http://dx.doi.org/10.15585/mmwr.mm7150a4.
3. Pingali C et al. MMWR Morb Mortal Wkly Rep. 2023 Aug 25;72:912-9. doi: http://dx.doi.org/10.15585/mmwr.mm7234a3.
4. CDC Covid Data Tracker.
New armamentaria available to fight an old disease.
New armamentaria available to fight an old disease.
In July 2023, nirsevimab (Beyfortus), a monoclonal antibody, was approved by the Food and Drug Administration for the prevention of respiratory syncytial virus (RSV) disease in infants and children younger than 2 years of age. On Aug. 3, 2023, the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention recommended routine use of it for all infants younger than 8 months of age born during or entering their first RSV season. Its use is also recommended for certain children 8-19 months of age who are at increased risk for severe RSV disease at the start of their second RSV season. Hearing the approval, I immediately had a flashback to residency, recalling the multiple infants admitted each fall and winter exhibiting classic symptoms including cough, rhinorrhea, nasal flaring, retractions, and wheezing with many having oxygen requirements and others needing intubation. Only supportive care was available.
RSV is the leading cause of infant hospitalizations. Annually, the CDC estimates there are 50,000-80,000 RSV hospitalizations and 100-300 RSV-related deaths in the United States in persons younger than 5 years of age. While premature infants have the highest rates of hospitalization (three times a term infant) about 79% of hospitalized children younger than 2 years have no underlying medical risks.1 The majority of children will experience RSV as an upper respiratory infection within the first 2 years of life. However, severe disease requiring hospitalization is more likely to occur in premature infants and children younger than 6 months; children younger than 2 with congenital heart disease and/or chronic lung disease; children with severe cystic fibrosis; as well as the immunocompromised child and individuals with neuromuscular disorders that preclude clearing mucous secretions or have difficulty swallowing.
Palivizumab (Synagis), the first monoclonal antibody to prevent RSV in infants was licensed in 1998. Its use was limited to infants meeting specific criteria developed by the American Academy of Pediatrics. Only 5% of infants had access to it. It was a short-acting agent requiring monthly injections, which were very costly ($1,661-$2,584 per dose). Eligible infants could receive up to five injections per season. Several studies proved its use was not cost beneficial.
What are the advantages of nirsevimab? It’s a long-acting monoclonal antibody. Only one dose is required per season. Costs will significantly diminish. It is recommended for all infants younger than 8 months of age born during RSV season. Those children 8-19 months at risk for severe RSV disease can receive it prior to the start of their second RSV season. During RSV season (October 1 to March 31), the initial dose should be administered to newborns just prior to hospital discharge. Older infants and newborns who did not receive it prior to hospital discharge can receive it at their medical home. Newborns should receive it within the first week of life. It is covered by the Vaccine for Children Program. Simultaneous administration with routine childhood immunizations is recommended. Finally, RSV season may vary in tropical areas (Southern Florida, Puerto Rico. etc.) and Alaska. The timing of nirsevimab administration should be based on local RSV activity provided by state and local authorities.
In addition, the FDA approved an RSV vaccine (Abrysvo) for use in adults at least 60 years of age and in pregnant women at 32-36 weeks’ gestation. The latter is administered to prevent lower respiratory tract infection in infants from birth to 6 months. Recommendations have been published for administration in nonpregnant adults. Specific information is forthcoming in terms timing of administration of nirsevimab in infants whose mothers receive Abrysvo.
RSV season is quickly approaching. Detailed recommendations for administration and FAQ questions related to nirsevimab and palivizumab can be found at https://www.aap.org or https://www.cdc.gov/vaccines/hcp/acip-recs/index.html.
Influenza
So, what about influenza? Vaccine composition has been tweaked to match the circulating viruses but the recommended age for annual routine administration remains unchanged. All persons at least 6 months of age should be vaccinated. Children between 6 months and 8 years need two doses at least 4 weeks apart when receiving vaccine for the first time. Immunizing everyone in the household is encouraged especially if there are household contacts at risk for developing severe disease, including infants too young to be vaccinated. Keep in mind children may be coinfected with multiple viruses. Adams and colleagues reviewed the prevalence of coinfection of influenza and Sars-CoV-2 in persons younger than 18 years reported to three CDC surveillance platforms during the 2021-2022 season.2 Thirty-two of 575 hospitalized (6%) coinfections were analyzed and 7 of 44 (16%) deaths. Compared with patients without coinfections, the coinfected patients were more likely to require mechanical ventilation (13% vs. 4%) or CPAP (16% vs. 6%). Only 4 of 23 who were influenza vaccine eligible were vaccinated. Of seven coinfected children who died, none had received influenza vaccine and only one received an antiviral. Only 5 of 31 (16%) infected only with influenza were vaccinated.3
Influenza activity was lower than usual during the 2021-2022 season. However, this report revealed underuse of both influenza vaccine and antiviral therapy, both of which are routinely recommended.
COVID-19
What’s new with COVID-19? On Sept. 12, 2023, ACIP recommended that everyone at least 6 months of age receive the 2023-2024 (monovalent, XBB containing) COVID-19 vaccines. Children at least 5 years of age need one dose and those younger need one or two doses depending on the number of doses previously received. Why the change? Circulating variants continue to change. There is a current uptick in cases including hospitalizations (7.7%) and deaths (4.5%) and it’s just the beginning of the season.4 Symptoms, risk groups and complications have not changed. The primary goal is to prevent infection, hospitalization, long term complications, and death.
We are now armed with the most up-to-date interventions to help prevent the acquisition of these three viruses. Our next step is recommending and delivering them to our patients.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She reported no relevant financial disclosures.
References
1.Suh M et al. J Infect Dis. 2022;226(Suppl 2):S154-36. doi: 10.1093/infdis/jiac120.
2. Adams K et al. MMWR Morb Mortal Wkly Rep. 2022;71:1589-96. doi: http://dx.doi.org/10.15585/mmwr.mm7150a4.
3. Pingali C et al. MMWR Morb Mortal Wkly Rep. 2023 Aug 25;72:912-9. doi: http://dx.doi.org/10.15585/mmwr.mm7234a3.
4. CDC Covid Data Tracker.
In July 2023, nirsevimab (Beyfortus), a monoclonal antibody, was approved by the Food and Drug Administration for the prevention of respiratory syncytial virus (RSV) disease in infants and children younger than 2 years of age. On Aug. 3, 2023, the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention recommended routine use of it for all infants younger than 8 months of age born during or entering their first RSV season. Its use is also recommended for certain children 8-19 months of age who are at increased risk for severe RSV disease at the start of their second RSV season. Hearing the approval, I immediately had a flashback to residency, recalling the multiple infants admitted each fall and winter exhibiting classic symptoms including cough, rhinorrhea, nasal flaring, retractions, and wheezing with many having oxygen requirements and others needing intubation. Only supportive care was available.
RSV is the leading cause of infant hospitalizations. Annually, the CDC estimates there are 50,000-80,000 RSV hospitalizations and 100-300 RSV-related deaths in the United States in persons younger than 5 years of age. While premature infants have the highest rates of hospitalization (three times a term infant) about 79% of hospitalized children younger than 2 years have no underlying medical risks.1 The majority of children will experience RSV as an upper respiratory infection within the first 2 years of life. However, severe disease requiring hospitalization is more likely to occur in premature infants and children younger than 6 months; children younger than 2 with congenital heart disease and/or chronic lung disease; children with severe cystic fibrosis; as well as the immunocompromised child and individuals with neuromuscular disorders that preclude clearing mucous secretions or have difficulty swallowing.
Palivizumab (Synagis), the first monoclonal antibody to prevent RSV in infants was licensed in 1998. Its use was limited to infants meeting specific criteria developed by the American Academy of Pediatrics. Only 5% of infants had access to it. It was a short-acting agent requiring monthly injections, which were very costly ($1,661-$2,584 per dose). Eligible infants could receive up to five injections per season. Several studies proved its use was not cost beneficial.
What are the advantages of nirsevimab? It’s a long-acting monoclonal antibody. Only one dose is required per season. Costs will significantly diminish. It is recommended for all infants younger than 8 months of age born during RSV season. Those children 8-19 months at risk for severe RSV disease can receive it prior to the start of their second RSV season. During RSV season (October 1 to March 31), the initial dose should be administered to newborns just prior to hospital discharge. Older infants and newborns who did not receive it prior to hospital discharge can receive it at their medical home. Newborns should receive it within the first week of life. It is covered by the Vaccine for Children Program. Simultaneous administration with routine childhood immunizations is recommended. Finally, RSV season may vary in tropical areas (Southern Florida, Puerto Rico. etc.) and Alaska. The timing of nirsevimab administration should be based on local RSV activity provided by state and local authorities.
In addition, the FDA approved an RSV vaccine (Abrysvo) for use in adults at least 60 years of age and in pregnant women at 32-36 weeks’ gestation. The latter is administered to prevent lower respiratory tract infection in infants from birth to 6 months. Recommendations have been published for administration in nonpregnant adults. Specific information is forthcoming in terms timing of administration of nirsevimab in infants whose mothers receive Abrysvo.
RSV season is quickly approaching. Detailed recommendations for administration and FAQ questions related to nirsevimab and palivizumab can be found at https://www.aap.org or https://www.cdc.gov/vaccines/hcp/acip-recs/index.html.
Influenza
So, what about influenza? Vaccine composition has been tweaked to match the circulating viruses but the recommended age for annual routine administration remains unchanged. All persons at least 6 months of age should be vaccinated. Children between 6 months and 8 years need two doses at least 4 weeks apart when receiving vaccine for the first time. Immunizing everyone in the household is encouraged especially if there are household contacts at risk for developing severe disease, including infants too young to be vaccinated. Keep in mind children may be coinfected with multiple viruses. Adams and colleagues reviewed the prevalence of coinfection of influenza and Sars-CoV-2 in persons younger than 18 years reported to three CDC surveillance platforms during the 2021-2022 season.2 Thirty-two of 575 hospitalized (6%) coinfections were analyzed and 7 of 44 (16%) deaths. Compared with patients without coinfections, the coinfected patients were more likely to require mechanical ventilation (13% vs. 4%) or CPAP (16% vs. 6%). Only 4 of 23 who were influenza vaccine eligible were vaccinated. Of seven coinfected children who died, none had received influenza vaccine and only one received an antiviral. Only 5 of 31 (16%) infected only with influenza were vaccinated.3
Influenza activity was lower than usual during the 2021-2022 season. However, this report revealed underuse of both influenza vaccine and antiviral therapy, both of which are routinely recommended.
COVID-19
What’s new with COVID-19? On Sept. 12, 2023, ACIP recommended that everyone at least 6 months of age receive the 2023-2024 (monovalent, XBB containing) COVID-19 vaccines. Children at least 5 years of age need one dose and those younger need one or two doses depending on the number of doses previously received. Why the change? Circulating variants continue to change. There is a current uptick in cases including hospitalizations (7.7%) and deaths (4.5%) and it’s just the beginning of the season.4 Symptoms, risk groups and complications have not changed. The primary goal is to prevent infection, hospitalization, long term complications, and death.
We are now armed with the most up-to-date interventions to help prevent the acquisition of these three viruses. Our next step is recommending and delivering them to our patients.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She reported no relevant financial disclosures.
References
1.Suh M et al. J Infect Dis. 2022;226(Suppl 2):S154-36. doi: 10.1093/infdis/jiac120.
2. Adams K et al. MMWR Morb Mortal Wkly Rep. 2022;71:1589-96. doi: http://dx.doi.org/10.15585/mmwr.mm7150a4.
3. Pingali C et al. MMWR Morb Mortal Wkly Rep. 2023 Aug 25;72:912-9. doi: http://dx.doi.org/10.15585/mmwr.mm7234a3.
4. CDC Covid Data Tracker.
Summer diarrhea – Time to think outside the box
It’s “summertime and the livin’ is easy” according to the lyric from an old George Gershwin song. But sometimes, summer activities can lead to illnesses that can disrupt a child’s easy living.
Case: An otherwise healthy 11-year-old presents with four to five loose stools per day, mild nausea, excess flatulence, and cramps for 12 days with a 5-pound weight loss. His loose-to-mushy stools have no blood or mucous but smell worse than usual. He has had no fever, vomiting, rashes, or joint symptoms. A month ago, he went hiking/camping on the Appalachian Trail, drank boiled stream water. and slept in a common-use semi-enclosed shelter. He waded through streams and shared “Trail Magic” (soft drinks being cooled in a fresh mountain stream). Two other campers report similar symptoms.
Differential diagnosis: Broadly, we should consider bacteria, viruses, and parasites. But generally, bacteria are likely to produce more systemic symptoms and usually do not last 12 days. That said, this could be Clostridioides difficile, yet that seems unlikely because he is otherwise healthy and has no apparent risk factors. Salmonella spp., Campylobacter spp. and some Escherichia coli infections may drag on for more than a week but the lack of systemic symptoms or blood/mucous lowers the likelihood. Viral agents (rotavirus, norovirus, adenovirus, astrovirus, calicivirus, or sapovirus) seem unlikely because of the long symptom duration and the child’s preteen age.
The history and presentation seem more likely attributable to a parasite. Uncommonly detected protozoa include Microsporidium (mostly Enterocytozoon bieneusi) and amoeba. Microsporidium is very rare and seen mostly in immune compromised hosts, for example, those living with HIV. Amebiasis occurs mostly after travel to endemic areas, and stools usually contain blood or mucous. Some roundworm or tapeworm infestations cause abdominal pain and abnormal stools, but the usual exposures are absent. Giardia spp., Cryptosporidium spp., Cyclospora cayetanensis, and/or Cystoisospora belli best fit this presentation given his hiking/camping trip.
Workup. Laboratory testing of stool is warranted (because of weight loss and persistent diarrhea) despite a lack of systemic signs. Initially, bacterial culture, C. difficile testing, and viral testing seem unwarranted. The best initial approach, given our most likely suspects, is protozoan/parasite testing.
The Centers for Disease Control and Prevention recommends testing up to three stools collected on separate days.1 Initially, stool testing for giardia and cryptosporidium antigens by EIA assays could be done as a point-of-care test. Such antigen tests are often the first step because of their ease of use, relatively low expense, reasonably high sensitivity and specificity, and rapid turnaround (as little as 1 hour). Alternatively, direct examination of three stools for ova and parasites (O&P) and acid-fast stain or direct fluorescent antibody testing can usually detect our main suspects (giardia, cryptosporidium, cyclospora, and cystoisospora) along with other less likely parasites.
Some laboratories, however, use syndromic stool testing approaches (multiplex nucleic acid panels) that detect over 20 different bacteria, viruses, and select parasites. Multiplex testing has yielded increased detection rates, compared with microscopic examination alone in some settings. Further, they also share ease-of-use and rapid turnaround times with parasite antigen assays while requiring less hands-on time by laboratory personnel, compared with direct microscopic examination. However, multiplex assays are expensive and more readily detect commensal organisms, so they are not necessarily the ideal test in all diarrheal illnesses.
Diagnosis. You decide to first order giardia and cryptosporidium antigen testing because you are highly suspicious that giardia is the cause, based on wild-water exposure, the presentation, and symptom duration. You also order full microscopic O&P examination because you know that parasites can “run in packs.” Results of testing the first stool are positive for giardia. Microscopic examination on each of three stools is negative except for giardia trophozoites (the noninfectious form) in stools two and three.
Giardia overview. Giardia is the most common protozoan causing diarrhea in the United States, is fecal-oral spread, and like Shigella spp., is a low-inoculum infection (ingestion of as few as 10-100 cysts). Acquisition in the United States has been estimated as being 75% from contaminated water (streams are a classic source.2 Other sources are contaminated food (fresh produce is classic) and in some cases sexual encounters (mostly in men who have sex with men). Most detections are sporadic, but outbreaks can occur with case numbers usually below 20; 40% of outbreaks are attributable to contaminated water or food.3 Evaluating symptomatic household members can be important as transmission in families can occur.
After ingestion, the cysts uncoat and form trophozoites, which reside mostly in the small bowel (Figure), causing inflammation and altering gut membrane permeability, thereby reducing nutrient absorption and circulating amino acids. Along with decreased food intake, altered absorption can lead to weight loss and potentially reduce growth in young children. Some trophozoites replicate while others encyst, eventually passing into stool. The cysts can survive for months in water or the environment (lakes, swimming pools, and clear mountain streams). Giardia has been linked to beavers’ feces contaminating wild-water sources, hence the moniker “Beaver fever” and warnings about stream water related to wilderness hiking.4
Management. Supportive therapy as with any diarrheal illness is the cornerstone of management. Specific antiparasitic treatment has traditionally been with metronidazole compounded into a liquid for young children, but the awful taste and frequent dosing often result in poor adherence. Nevertheless, published cure rates range from 80% to 100%. The taste issue, known adverse effects, and lack of FDA approval for giardia, have led to use of other drugs.5 One dose of tinidazole is as effective as metronidazole and can be prescribed for children 3 years old or older. But the drug nitazoxanide is becoming more standard. It is as effective as either alternative, and is FDA approved for children 1 year old and older. Nitazoxanide also is effective against other intestinal parasites (e.g., cryptosporidium). Nitazoxanide’s 3-day course involves every-12-hour dosing with food with each dose being 5 mL (100 mg) for 1- to 3-year-olds, 10 mL (200 mg) for 4- to 11-year-olds, and one tablet (500 mg) or 25 mL (500 mg) for children 12 years old or older.6
Key elements in this subacute nonsystemic diarrheal presentation were primitive camping history, multiple stream water contacts, nearly 2 weeks of symptoms, weight loss, and flatulence/cramping, but no fever or stool blood/mucous. Two friends also appear to be similarly symptomatic, so a common exposure seemed likely This is typical for several summertime activity–related parasites. So,
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at [email protected].
References
1. Diagnosis and Treatment Information for Medical Professionals, Giardia, Parasites. CDC.
2. Krumrie S et al. Curr Res Parasitol Vector Borne Dis. 2022;2:100084. doi: 10.1016/j.crpvbd.2022.100084.
3. Baldursson S and Karanis P. Water Res. 2011 Dec 15. doi: 10.1016/j.watres.2011.10.013.
4. “Water on the Appalachian Trail” AppalachianTrail.com.
5. Giardiasis: Treatment and prevention. UpToDate.
6. Kimberlin D et al. Red Book: 2021-2024 Report of the Committee on Infectious Diseases (Itasca, Ill.: American Academy of Pediatrics, 2021. 32nd ed.) Giardia duodenalis infections. pp. 335-8; and p. 961 (Table 4.11).
It’s “summertime and the livin’ is easy” according to the lyric from an old George Gershwin song. But sometimes, summer activities can lead to illnesses that can disrupt a child’s easy living.
Case: An otherwise healthy 11-year-old presents with four to five loose stools per day, mild nausea, excess flatulence, and cramps for 12 days with a 5-pound weight loss. His loose-to-mushy stools have no blood or mucous but smell worse than usual. He has had no fever, vomiting, rashes, or joint symptoms. A month ago, he went hiking/camping on the Appalachian Trail, drank boiled stream water. and slept in a common-use semi-enclosed shelter. He waded through streams and shared “Trail Magic” (soft drinks being cooled in a fresh mountain stream). Two other campers report similar symptoms.
Differential diagnosis: Broadly, we should consider bacteria, viruses, and parasites. But generally, bacteria are likely to produce more systemic symptoms and usually do not last 12 days. That said, this could be Clostridioides difficile, yet that seems unlikely because he is otherwise healthy and has no apparent risk factors. Salmonella spp., Campylobacter spp. and some Escherichia coli infections may drag on for more than a week but the lack of systemic symptoms or blood/mucous lowers the likelihood. Viral agents (rotavirus, norovirus, adenovirus, astrovirus, calicivirus, or sapovirus) seem unlikely because of the long symptom duration and the child’s preteen age.
The history and presentation seem more likely attributable to a parasite. Uncommonly detected protozoa include Microsporidium (mostly Enterocytozoon bieneusi) and amoeba. Microsporidium is very rare and seen mostly in immune compromised hosts, for example, those living with HIV. Amebiasis occurs mostly after travel to endemic areas, and stools usually contain blood or mucous. Some roundworm or tapeworm infestations cause abdominal pain and abnormal stools, but the usual exposures are absent. Giardia spp., Cryptosporidium spp., Cyclospora cayetanensis, and/or Cystoisospora belli best fit this presentation given his hiking/camping trip.
Workup. Laboratory testing of stool is warranted (because of weight loss and persistent diarrhea) despite a lack of systemic signs. Initially, bacterial culture, C. difficile testing, and viral testing seem unwarranted. The best initial approach, given our most likely suspects, is protozoan/parasite testing.
The Centers for Disease Control and Prevention recommends testing up to three stools collected on separate days.1 Initially, stool testing for giardia and cryptosporidium antigens by EIA assays could be done as a point-of-care test. Such antigen tests are often the first step because of their ease of use, relatively low expense, reasonably high sensitivity and specificity, and rapid turnaround (as little as 1 hour). Alternatively, direct examination of three stools for ova and parasites (O&P) and acid-fast stain or direct fluorescent antibody testing can usually detect our main suspects (giardia, cryptosporidium, cyclospora, and cystoisospora) along with other less likely parasites.
Some laboratories, however, use syndromic stool testing approaches (multiplex nucleic acid panels) that detect over 20 different bacteria, viruses, and select parasites. Multiplex testing has yielded increased detection rates, compared with microscopic examination alone in some settings. Further, they also share ease-of-use and rapid turnaround times with parasite antigen assays while requiring less hands-on time by laboratory personnel, compared with direct microscopic examination. However, multiplex assays are expensive and more readily detect commensal organisms, so they are not necessarily the ideal test in all diarrheal illnesses.
Diagnosis. You decide to first order giardia and cryptosporidium antigen testing because you are highly suspicious that giardia is the cause, based on wild-water exposure, the presentation, and symptom duration. You also order full microscopic O&P examination because you know that parasites can “run in packs.” Results of testing the first stool are positive for giardia. Microscopic examination on each of three stools is negative except for giardia trophozoites (the noninfectious form) in stools two and three.
Giardia overview. Giardia is the most common protozoan causing diarrhea in the United States, is fecal-oral spread, and like Shigella spp., is a low-inoculum infection (ingestion of as few as 10-100 cysts). Acquisition in the United States has been estimated as being 75% from contaminated water (streams are a classic source.2 Other sources are contaminated food (fresh produce is classic) and in some cases sexual encounters (mostly in men who have sex with men). Most detections are sporadic, but outbreaks can occur with case numbers usually below 20; 40% of outbreaks are attributable to contaminated water or food.3 Evaluating symptomatic household members can be important as transmission in families can occur.
After ingestion, the cysts uncoat and form trophozoites, which reside mostly in the small bowel (Figure), causing inflammation and altering gut membrane permeability, thereby reducing nutrient absorption and circulating amino acids. Along with decreased food intake, altered absorption can lead to weight loss and potentially reduce growth in young children. Some trophozoites replicate while others encyst, eventually passing into stool. The cysts can survive for months in water or the environment (lakes, swimming pools, and clear mountain streams). Giardia has been linked to beavers’ feces contaminating wild-water sources, hence the moniker “Beaver fever” and warnings about stream water related to wilderness hiking.4

Management. Supportive therapy as with any diarrheal illness is the cornerstone of management. Specific antiparasitic treatment has traditionally been with metronidazole compounded into a liquid for young children, but the awful taste and frequent dosing often result in poor adherence. Nevertheless, published cure rates range from 80% to 100%. The taste issue, known adverse effects, and lack of FDA approval for giardia, have led to use of other drugs.5 One dose of tinidazole is as effective as metronidazole and can be prescribed for children 3 years old or older. But the drug nitazoxanide is becoming more standard. It is as effective as either alternative, and is FDA approved for children 1 year old and older. Nitazoxanide also is effective against other intestinal parasites (e.g., cryptosporidium). Nitazoxanide’s 3-day course involves every-12-hour dosing with food with each dose being 5 mL (100 mg) for 1- to 3-year-olds, 10 mL (200 mg) for 4- to 11-year-olds, and one tablet (500 mg) or 25 mL (500 mg) for children 12 years old or older.6
Key elements in this subacute nonsystemic diarrheal presentation were primitive camping history, multiple stream water contacts, nearly 2 weeks of symptoms, weight loss, and flatulence/cramping, but no fever or stool blood/mucous. Two friends also appear to be similarly symptomatic, so a common exposure seemed likely This is typical for several summertime activity–related parasites. So,
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at [email protected].
References
1. Diagnosis and Treatment Information for Medical Professionals, Giardia, Parasites. CDC.
2. Krumrie S et al. Curr Res Parasitol Vector Borne Dis. 2022;2:100084. doi: 10.1016/j.crpvbd.2022.100084.
3. Baldursson S and Karanis P. Water Res. 2011 Dec 15. doi: 10.1016/j.watres.2011.10.013.
4. “Water on the Appalachian Trail” AppalachianTrail.com.
5. Giardiasis: Treatment and prevention. UpToDate.
6. Kimberlin D et al. Red Book: 2021-2024 Report of the Committee on Infectious Diseases (Itasca, Ill.: American Academy of Pediatrics, 2021. 32nd ed.) Giardia duodenalis infections. pp. 335-8; and p. 961 (Table 4.11).
It’s “summertime and the livin’ is easy” according to the lyric from an old George Gershwin song. But sometimes, summer activities can lead to illnesses that can disrupt a child’s easy living.
Case: An otherwise healthy 11-year-old presents with four to five loose stools per day, mild nausea, excess flatulence, and cramps for 12 days with a 5-pound weight loss. His loose-to-mushy stools have no blood or mucous but smell worse than usual. He has had no fever, vomiting, rashes, or joint symptoms. A month ago, he went hiking/camping on the Appalachian Trail, drank boiled stream water. and slept in a common-use semi-enclosed shelter. He waded through streams and shared “Trail Magic” (soft drinks being cooled in a fresh mountain stream). Two other campers report similar symptoms.
Differential diagnosis: Broadly, we should consider bacteria, viruses, and parasites. But generally, bacteria are likely to produce more systemic symptoms and usually do not last 12 days. That said, this could be Clostridioides difficile, yet that seems unlikely because he is otherwise healthy and has no apparent risk factors. Salmonella spp., Campylobacter spp. and some Escherichia coli infections may drag on for more than a week but the lack of systemic symptoms or blood/mucous lowers the likelihood. Viral agents (rotavirus, norovirus, adenovirus, astrovirus, calicivirus, or sapovirus) seem unlikely because of the long symptom duration and the child’s preteen age.
The history and presentation seem more likely attributable to a parasite. Uncommonly detected protozoa include Microsporidium (mostly Enterocytozoon bieneusi) and amoeba. Microsporidium is very rare and seen mostly in immune compromised hosts, for example, those living with HIV. Amebiasis occurs mostly after travel to endemic areas, and stools usually contain blood or mucous. Some roundworm or tapeworm infestations cause abdominal pain and abnormal stools, but the usual exposures are absent. Giardia spp., Cryptosporidium spp., Cyclospora cayetanensis, and/or Cystoisospora belli best fit this presentation given his hiking/camping trip.
Workup. Laboratory testing of stool is warranted (because of weight loss and persistent diarrhea) despite a lack of systemic signs. Initially, bacterial culture, C. difficile testing, and viral testing seem unwarranted. The best initial approach, given our most likely suspects, is protozoan/parasite testing.
The Centers for Disease Control and Prevention recommends testing up to three stools collected on separate days.1 Initially, stool testing for giardia and cryptosporidium antigens by EIA assays could be done as a point-of-care test. Such antigen tests are often the first step because of their ease of use, relatively low expense, reasonably high sensitivity and specificity, and rapid turnaround (as little as 1 hour). Alternatively, direct examination of three stools for ova and parasites (O&P) and acid-fast stain or direct fluorescent antibody testing can usually detect our main suspects (giardia, cryptosporidium, cyclospora, and cystoisospora) along with other less likely parasites.
Some laboratories, however, use syndromic stool testing approaches (multiplex nucleic acid panels) that detect over 20 different bacteria, viruses, and select parasites. Multiplex testing has yielded increased detection rates, compared with microscopic examination alone in some settings. Further, they also share ease-of-use and rapid turnaround times with parasite antigen assays while requiring less hands-on time by laboratory personnel, compared with direct microscopic examination. However, multiplex assays are expensive and more readily detect commensal organisms, so they are not necessarily the ideal test in all diarrheal illnesses.
Diagnosis. You decide to first order giardia and cryptosporidium antigen testing because you are highly suspicious that giardia is the cause, based on wild-water exposure, the presentation, and symptom duration. You also order full microscopic O&P examination because you know that parasites can “run in packs.” Results of testing the first stool are positive for giardia. Microscopic examination on each of three stools is negative except for giardia trophozoites (the noninfectious form) in stools two and three.
Giardia overview. Giardia is the most common protozoan causing diarrhea in the United States, is fecal-oral spread, and like Shigella spp., is a low-inoculum infection (ingestion of as few as 10-100 cysts). Acquisition in the United States has been estimated as being 75% from contaminated water (streams are a classic source.2 Other sources are contaminated food (fresh produce is classic) and in some cases sexual encounters (mostly in men who have sex with men). Most detections are sporadic, but outbreaks can occur with case numbers usually below 20; 40% of outbreaks are attributable to contaminated water or food.3 Evaluating symptomatic household members can be important as transmission in families can occur.
After ingestion, the cysts uncoat and form trophozoites, which reside mostly in the small bowel (Figure), causing inflammation and altering gut membrane permeability, thereby reducing nutrient absorption and circulating amino acids. Along with decreased food intake, altered absorption can lead to weight loss and potentially reduce growth in young children. Some trophozoites replicate while others encyst, eventually passing into stool. The cysts can survive for months in water or the environment (lakes, swimming pools, and clear mountain streams). Giardia has been linked to beavers’ feces contaminating wild-water sources, hence the moniker “Beaver fever” and warnings about stream water related to wilderness hiking.4

Management. Supportive therapy as with any diarrheal illness is the cornerstone of management. Specific antiparasitic treatment has traditionally been with metronidazole compounded into a liquid for young children, but the awful taste and frequent dosing often result in poor adherence. Nevertheless, published cure rates range from 80% to 100%. The taste issue, known adverse effects, and lack of FDA approval for giardia, have led to use of other drugs.5 One dose of tinidazole is as effective as metronidazole and can be prescribed for children 3 years old or older. But the drug nitazoxanide is becoming more standard. It is as effective as either alternative, and is FDA approved for children 1 year old and older. Nitazoxanide also is effective against other intestinal parasites (e.g., cryptosporidium). Nitazoxanide’s 3-day course involves every-12-hour dosing with food with each dose being 5 mL (100 mg) for 1- to 3-year-olds, 10 mL (200 mg) for 4- to 11-year-olds, and one tablet (500 mg) or 25 mL (500 mg) for children 12 years old or older.6
Key elements in this subacute nonsystemic diarrheal presentation were primitive camping history, multiple stream water contacts, nearly 2 weeks of symptoms, weight loss, and flatulence/cramping, but no fever or stool blood/mucous. Two friends also appear to be similarly symptomatic, so a common exposure seemed likely This is typical for several summertime activity–related parasites. So,
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at [email protected].
References
1. Diagnosis and Treatment Information for Medical Professionals, Giardia, Parasites. CDC.
2. Krumrie S et al. Curr Res Parasitol Vector Borne Dis. 2022;2:100084. doi: 10.1016/j.crpvbd.2022.100084.
3. Baldursson S and Karanis P. Water Res. 2011 Dec 15. doi: 10.1016/j.watres.2011.10.013.
4. “Water on the Appalachian Trail” AppalachianTrail.com.
5. Giardiasis: Treatment and prevention. UpToDate.
6. Kimberlin D et al. Red Book: 2021-2024 Report of the Committee on Infectious Diseases (Itasca, Ill.: American Academy of Pediatrics, 2021. 32nd ed.) Giardia duodenalis infections. pp. 335-8; and p. 961 (Table 4.11).
Bordetella parapertussis reemerges as a cause of respiratory illness in children
A 4-year-old male presented to an urgent care center with a 2-week history of runny nose and cough. The treating clinician suspected a postviral cough, but the child’s mother was unconvinced. Testing for SARS-CoV-2, influenza, and respiratory syncytial virus performed earlier in the week at the pediatrician’s office was negative. At the mother’s insistence, an expanded respiratory panel was ordered and revealed a surprising result: Bordetella parapertussis.
Just like B. pertussis, B. parapertussis can cause a prolonged cough illness characterized by coughing paroxysms, whoop, and posttussive emesis. Testing is the only way to reliably distinguish between the two infections. In general, disease due to B. parapertussis tends to be milder than typical pertussis and symptoms usually don’t last as long. In one study, 40% of people with B. parapertussis had no symptoms. B. parapertussis does not produce pertussis toxin and this may affect disease severity. Rarely, children can be coinfected with both B. pertussis and B. parapertussis.
The burden of B. parapertussis in the United States is not well described because only pertussis cases caused by B. pertussis are reportable to the Centers for Disease Control and Prevention. Nevertheless, some states include cases in public reporting and outbreaks have been reported. Historically, disease has been cyclical, with peaks in cases every 4 years and no seasonality.
This year, some communities are currently seeing an increase in B. parapertussis cases. Through June 11 of this year, 40 cases of B. parapertussis and no cases of B. pertussis have been identified at Norton Healthcare in Louisville, Ky. For comparison, one case of B. parapertussis was reported in 2022 and no cases were reported in 2021. Chatter on infectious diseases listservs suggests that clinicians in other communities are also seeing an increase in cases.
According to Andi Shane, MD, MPH, chief of the division of pediatric infectious diseases at Emory University and Children’s Healthcare of Atlanta, an unusually high number of children with B. parapertussis were identified in the Atlanta area this spring. “Fortunately, most children had mild illness and of these, only a few required admission to the hospital,” Dr. Shane said.
Back at the urgent care center, the clinician on duty called the patient’s mom to discuss the diagnosis of B. parapertussis. By the time the test result was available, the patient was asymptomatic. The clinician advised that antibiotic therapy was not indicated.
Treatment recommendations diverge for B. pertussis and B. parapertussis and this is a point of emphasis for clinicians. Treatment of B. pertussis during the catarrhal phase may ameliorate disease. Treatment initiated after the catarrhal phase has little impact on symptoms but may reduce spread to others. In most cases, treatment isn’t recommended for B. parapertussis. It is not clear how well antibiotics work against this organism. Macrolides such as erythromycin and azithromycin that are used to treat pertussis may have some activity, along with trimethoprim-sulfamethoxazole and ciprofloxacin. According to the American Academy of Pediatrics, treatment is usually reserved for individuals at risk for more severe disease, including infants, especially those less than 6 months of age, the elderly, and immunocompromised persons. Prophylactic antibiotic therapy is not recommended for most persons exposed to B. parapertussis, although some public health experts also recommend treatment of B. parapertussis-infected people in contact with young infants and others are risk for severe disease.
In recent epidemiologic reports, patients with B. parapertussis infection had received age-appropriate vaccination for pertussis, suggesting that available pertussis vaccines offer little to no protection against this disease. The best prevention strategies are similar to those that are effective against other illness spread by respiratory droplets. Sick people should stay at home and cover their coughs when around others. Everyone should practice good hand hygiene.
Are you seeing increased cases of B. parapertussis in your community? Email me at [email protected].
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at [email protected].
A 4-year-old male presented to an urgent care center with a 2-week history of runny nose and cough. The treating clinician suspected a postviral cough, but the child’s mother was unconvinced. Testing for SARS-CoV-2, influenza, and respiratory syncytial virus performed earlier in the week at the pediatrician’s office was negative. At the mother’s insistence, an expanded respiratory panel was ordered and revealed a surprising result: Bordetella parapertussis.
Just like B. pertussis, B. parapertussis can cause a prolonged cough illness characterized by coughing paroxysms, whoop, and posttussive emesis. Testing is the only way to reliably distinguish between the two infections. In general, disease due to B. parapertussis tends to be milder than typical pertussis and symptoms usually don’t last as long. In one study, 40% of people with B. parapertussis had no symptoms. B. parapertussis does not produce pertussis toxin and this may affect disease severity. Rarely, children can be coinfected with both B. pertussis and B. parapertussis.
The burden of B. parapertussis in the United States is not well described because only pertussis cases caused by B. pertussis are reportable to the Centers for Disease Control and Prevention. Nevertheless, some states include cases in public reporting and outbreaks have been reported. Historically, disease has been cyclical, with peaks in cases every 4 years and no seasonality.
This year, some communities are currently seeing an increase in B. parapertussis cases. Through June 11 of this year, 40 cases of B. parapertussis and no cases of B. pertussis have been identified at Norton Healthcare in Louisville, Ky. For comparison, one case of B. parapertussis was reported in 2022 and no cases were reported in 2021. Chatter on infectious diseases listservs suggests that clinicians in other communities are also seeing an increase in cases.
According to Andi Shane, MD, MPH, chief of the division of pediatric infectious diseases at Emory University and Children’s Healthcare of Atlanta, an unusually high number of children with B. parapertussis were identified in the Atlanta area this spring. “Fortunately, most children had mild illness and of these, only a few required admission to the hospital,” Dr. Shane said.
Back at the urgent care center, the clinician on duty called the patient’s mom to discuss the diagnosis of B. parapertussis. By the time the test result was available, the patient was asymptomatic. The clinician advised that antibiotic therapy was not indicated.
Treatment recommendations diverge for B. pertussis and B. parapertussis and this is a point of emphasis for clinicians. Treatment of B. pertussis during the catarrhal phase may ameliorate disease. Treatment initiated after the catarrhal phase has little impact on symptoms but may reduce spread to others. In most cases, treatment isn’t recommended for B. parapertussis. It is not clear how well antibiotics work against this organism. Macrolides such as erythromycin and azithromycin that are used to treat pertussis may have some activity, along with trimethoprim-sulfamethoxazole and ciprofloxacin. According to the American Academy of Pediatrics, treatment is usually reserved for individuals at risk for more severe disease, including infants, especially those less than 6 months of age, the elderly, and immunocompromised persons. Prophylactic antibiotic therapy is not recommended for most persons exposed to B. parapertussis, although some public health experts also recommend treatment of B. parapertussis-infected people in contact with young infants and others are risk for severe disease.
In recent epidemiologic reports, patients with B. parapertussis infection had received age-appropriate vaccination for pertussis, suggesting that available pertussis vaccines offer little to no protection against this disease. The best prevention strategies are similar to those that are effective against other illness spread by respiratory droplets. Sick people should stay at home and cover their coughs when around others. Everyone should practice good hand hygiene.
Are you seeing increased cases of B. parapertussis in your community? Email me at [email protected].
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at [email protected].
A 4-year-old male presented to an urgent care center with a 2-week history of runny nose and cough. The treating clinician suspected a postviral cough, but the child’s mother was unconvinced. Testing for SARS-CoV-2, influenza, and respiratory syncytial virus performed earlier in the week at the pediatrician’s office was negative. At the mother’s insistence, an expanded respiratory panel was ordered and revealed a surprising result: Bordetella parapertussis.
Just like B. pertussis, B. parapertussis can cause a prolonged cough illness characterized by coughing paroxysms, whoop, and posttussive emesis. Testing is the only way to reliably distinguish between the two infections. In general, disease due to B. parapertussis tends to be milder than typical pertussis and symptoms usually don’t last as long. In one study, 40% of people with B. parapertussis had no symptoms. B. parapertussis does not produce pertussis toxin and this may affect disease severity. Rarely, children can be coinfected with both B. pertussis and B. parapertussis.
The burden of B. parapertussis in the United States is not well described because only pertussis cases caused by B. pertussis are reportable to the Centers for Disease Control and Prevention. Nevertheless, some states include cases in public reporting and outbreaks have been reported. Historically, disease has been cyclical, with peaks in cases every 4 years and no seasonality.
This year, some communities are currently seeing an increase in B. parapertussis cases. Through June 11 of this year, 40 cases of B. parapertussis and no cases of B. pertussis have been identified at Norton Healthcare in Louisville, Ky. For comparison, one case of B. parapertussis was reported in 2022 and no cases were reported in 2021. Chatter on infectious diseases listservs suggests that clinicians in other communities are also seeing an increase in cases.
According to Andi Shane, MD, MPH, chief of the division of pediatric infectious diseases at Emory University and Children’s Healthcare of Atlanta, an unusually high number of children with B. parapertussis were identified in the Atlanta area this spring. “Fortunately, most children had mild illness and of these, only a few required admission to the hospital,” Dr. Shane said.
Back at the urgent care center, the clinician on duty called the patient’s mom to discuss the diagnosis of B. parapertussis. By the time the test result was available, the patient was asymptomatic. The clinician advised that antibiotic therapy was not indicated.
Treatment recommendations diverge for B. pertussis and B. parapertussis and this is a point of emphasis for clinicians. Treatment of B. pertussis during the catarrhal phase may ameliorate disease. Treatment initiated after the catarrhal phase has little impact on symptoms but may reduce spread to others. In most cases, treatment isn’t recommended for B. parapertussis. It is not clear how well antibiotics work against this organism. Macrolides such as erythromycin and azithromycin that are used to treat pertussis may have some activity, along with trimethoprim-sulfamethoxazole and ciprofloxacin. According to the American Academy of Pediatrics, treatment is usually reserved for individuals at risk for more severe disease, including infants, especially those less than 6 months of age, the elderly, and immunocompromised persons. Prophylactic antibiotic therapy is not recommended for most persons exposed to B. parapertussis, although some public health experts also recommend treatment of B. parapertussis-infected people in contact with young infants and others are risk for severe disease.
In recent epidemiologic reports, patients with B. parapertussis infection had received age-appropriate vaccination for pertussis, suggesting that available pertussis vaccines offer little to no protection against this disease. The best prevention strategies are similar to those that are effective against other illness spread by respiratory droplets. Sick people should stay at home and cover their coughs when around others. Everyone should practice good hand hygiene.
Are you seeing increased cases of B. parapertussis in your community? Email me at [email protected].
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She is a member of the AAP’s Committee on Infectious Diseases and one of the lead authors of the AAP’s Recommendations for Prevention and Control of Influenza in Children, 2022-2023. The opinions expressed in this article are her own. Dr. Bryant discloses that she has served as an investigator on clinical trials funded by Pfizer, Enanta and Gilead. Email her at [email protected].
Profile of respiratory bacteria in children younger than 6 months
In this column, I will describe the results of a recently published study from my group.1 We sought to profile Streptococcus pneumoniae (pneumococcus), Haemophilus influenzae (Hflu) and Moraxella catarrhalis (Mcat) in the nasopharynx among 13-valent pneumococcal conjugate vaccine (PCV13)-immunized children, with a focus on the first 6 months of life. The rationale was to provide heretofore unreported contemporary data in a highly PCV13-immunized, community-based child population in the United States. A secondary objective was to assess nasopharyngeal bacterial density because higher density associates with greater likelihood of progression to infection. Thirdly, the serotype distribution and antibiotic susceptibility of pneumococci among children seen in primary care settings in the United States had not been evaluated for strains circulating among infants less than 6 months old and they may differ from strains recovered from older children. Therefore, comparisons were made within the same cohort of children to later child age time points.
Risk factors identified
The study was prospective and collected from a cohort of 101 children in Rochester, N.Y., during 2018-2020. Nasopharyngeal swabs were taken for study at age 1, 2 and 3 weeks, then 1, 2, 4, 6, 9, 12, 15, 18 and 24 months. All children had received PCV13 vaccine according to the Centers for Disease Control and Prevention recommended schedule.
We found two significant risk factors in the first 6 months of life for detection of nasopharyngeal colonization of pneumococcus, Hflu, and Mcat. They were daycare attendance and one or more siblings aged 1-5 years at home.
Colonization by one or more of the three bacteria was detected in only 5% of infants before age 2 months. None of the five children attended daycare but all five had young siblings at home. Pneumococcal colonization was detected in 12%, Hflu in 3%, and Mcat in 21% of nasopharyngeal swabs collected during the first 6 months of life. Nasopharyngeal colonization with the bacteria increased rapidly between age 4 and 6 months of life, coincident with infants going to daycare and other social interaction opportunities. Bacterial density of pneumococcus, Hflu, and Mcat during the first 6 months of life was significantly lower in the nasopharynx compared with bacterial density when samples were collected during child age 7-24 months.
The prevalent pneumococcal serotypes in children up to 6 months old were 23B (17%), 22F (13%), 15B/C (11%), 16F (9%), and 21 (7%), 19F (7%), which differed from those isolated from children age 7-24 months, where serotypes 35B (15%), 21 (10%), 15B (9%), and 23B (7%), 23A (7%) were most commonly observed. Antibiotic resistance among isolates did not significantly differ in comparisons between infants younger than 6 months versus 7- to 24-month-olds.
What is the clinical significance?
Colonization of the nasopharynx is a necessary first step in infection pathogenesis (Figure).
Prevalence of colonization varies among settings and countries, with generally much higher prevalence soon after birth and persisting at high rates in children living in low/middle-income countries versus high-income countries. This is one explanation for higher respiratory infection rates in low/middle-income countries compared with the United States, Europe, and other high-income countries. Environmental risk factors for early life colonization include household crowding, young siblings, no breastfeeding, daycare attendance, antibiotic usage, and passive exposure to smoke.
In a prior study of a different cohort of 358 prospectively-enrolled children, we sought associations between physician-attended illness visits and bacterial colonization in the first 5 years of life.2 We showed that early age of first colonization with pneumococcus, Hflu, and Mcat was associated with respiratory infection proneness and asthma among the children.
Multiple demographic and risk factors may contribute to early life and high-density colonization that in turn may increase risk of infections. High densities and early life pneumococcal colonization in low/middle-income countries might impact PCV responses by induction of immunity tolerance. While it is appealing to study new vaccines in low/middle-income populations with high infection incidence, there are reasons that infection incidence is higher compared with high-income countries like the United States, among them may be early life nasopharyngeal colonization and density of colonization.
Prevalent pneumococcal serotype appear to differ with age. The most common serotypes in the first 6 months of life for the children were 23B> 22F> 16F and 21=19F, but in children 7-24 months, serotypes 35B> 21>15B>23A=23B were most commonly observed. This difference might be due to the impact of antibiotics.3 Pneumococci expressing serotypes 22F and 16F were oxacillin susceptible and antibiotic exposure in the first 6 months of life is very uncommon in our study cohorts. In contrast, all pneumococci expressing 35B capsule were oxacillin resistant and in our cohorts antibiotic exposures are common among 7- to 24-month-olds.
In conclusion, we determined that children in the first 6 months of life seen in pediatric primary care settings in Rochester, N.Y., have very low prevalence and low-density colonization of pneumococcus, Hflu, and Mcat compared with 7- to 24-month olds. Our results may explain the significantly lower rates of infections caused by pneumococci, Hflu, and Mcat in infants younger than 6 months old compared with low/middle-income countries.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to disclose.
References
1. Kaur R and Pichichero M. Colonization, density, and antibiotic resistance of Streptococcus pneumoniae, Haemophilus Influenzae, and Moraxella catarrhalis among PCV13 vaccinated infants in the first six months of life in Rochester, New York. J Pediatric Infect Dis Soc. 2023 Apr 18;12(3):135-42.
2. Chapman T et al. Nasopharyngeal colonization with pathobionts is associated with susceptibility to respiratory illnesses in young children. PLoS One. 2020 Dec 11;15(12):e0243942. doi: 10.1371/journal.pone.0243942.
3. Chapman TJ et al. Antibiotic use and vaccine antibody levels. Pediatrics 2022 May 1;149(5):e2021052061. doi: 10.1542/peds.2021-052061.
In this column, I will describe the results of a recently published study from my group.1 We sought to profile Streptococcus pneumoniae (pneumococcus), Haemophilus influenzae (Hflu) and Moraxella catarrhalis (Mcat) in the nasopharynx among 13-valent pneumococcal conjugate vaccine (PCV13)-immunized children, with a focus on the first 6 months of life. The rationale was to provide heretofore unreported contemporary data in a highly PCV13-immunized, community-based child population in the United States. A secondary objective was to assess nasopharyngeal bacterial density because higher density associates with greater likelihood of progression to infection. Thirdly, the serotype distribution and antibiotic susceptibility of pneumococci among children seen in primary care settings in the United States had not been evaluated for strains circulating among infants less than 6 months old and they may differ from strains recovered from older children. Therefore, comparisons were made within the same cohort of children to later child age time points.
Risk factors identified
The study was prospective and collected from a cohort of 101 children in Rochester, N.Y., during 2018-2020. Nasopharyngeal swabs were taken for study at age 1, 2 and 3 weeks, then 1, 2, 4, 6, 9, 12, 15, 18 and 24 months. All children had received PCV13 vaccine according to the Centers for Disease Control and Prevention recommended schedule.
We found two significant risk factors in the first 6 months of life for detection of nasopharyngeal colonization of pneumococcus, Hflu, and Mcat. They were daycare attendance and one or more siblings aged 1-5 years at home.
Colonization by one or more of the three bacteria was detected in only 5% of infants before age 2 months. None of the five children attended daycare but all five had young siblings at home. Pneumococcal colonization was detected in 12%, Hflu in 3%, and Mcat in 21% of nasopharyngeal swabs collected during the first 6 months of life. Nasopharyngeal colonization with the bacteria increased rapidly between age 4 and 6 months of life, coincident with infants going to daycare and other social interaction opportunities. Bacterial density of pneumococcus, Hflu, and Mcat during the first 6 months of life was significantly lower in the nasopharynx compared with bacterial density when samples were collected during child age 7-24 months.
The prevalent pneumococcal serotypes in children up to 6 months old were 23B (17%), 22F (13%), 15B/C (11%), 16F (9%), and 21 (7%), 19F (7%), which differed from those isolated from children age 7-24 months, where serotypes 35B (15%), 21 (10%), 15B (9%), and 23B (7%), 23A (7%) were most commonly observed. Antibiotic resistance among isolates did not significantly differ in comparisons between infants younger than 6 months versus 7- to 24-month-olds.
What is the clinical significance?
Colonization of the nasopharynx is a necessary first step in infection pathogenesis (Figure).
Prevalence of colonization varies among settings and countries, with generally much higher prevalence soon after birth and persisting at high rates in children living in low/middle-income countries versus high-income countries. This is one explanation for higher respiratory infection rates in low/middle-income countries compared with the United States, Europe, and other high-income countries. Environmental risk factors for early life colonization include household crowding, young siblings, no breastfeeding, daycare attendance, antibiotic usage, and passive exposure to smoke.
In a prior study of a different cohort of 358 prospectively-enrolled children, we sought associations between physician-attended illness visits and bacterial colonization in the first 5 years of life.2 We showed that early age of first colonization with pneumococcus, Hflu, and Mcat was associated with respiratory infection proneness and asthma among the children.
Multiple demographic and risk factors may contribute to early life and high-density colonization that in turn may increase risk of infections. High densities and early life pneumococcal colonization in low/middle-income countries might impact PCV responses by induction of immunity tolerance. While it is appealing to study new vaccines in low/middle-income populations with high infection incidence, there are reasons that infection incidence is higher compared with high-income countries like the United States, among them may be early life nasopharyngeal colonization and density of colonization.
Prevalent pneumococcal serotype appear to differ with age. The most common serotypes in the first 6 months of life for the children were 23B> 22F> 16F and 21=19F, but in children 7-24 months, serotypes 35B> 21>15B>23A=23B were most commonly observed. This difference might be due to the impact of antibiotics.3 Pneumococci expressing serotypes 22F and 16F were oxacillin susceptible and antibiotic exposure in the first 6 months of life is very uncommon in our study cohorts. In contrast, all pneumococci expressing 35B capsule were oxacillin resistant and in our cohorts antibiotic exposures are common among 7- to 24-month-olds.
In conclusion, we determined that children in the first 6 months of life seen in pediatric primary care settings in Rochester, N.Y., have very low prevalence and low-density colonization of pneumococcus, Hflu, and Mcat compared with 7- to 24-month olds. Our results may explain the significantly lower rates of infections caused by pneumococci, Hflu, and Mcat in infants younger than 6 months old compared with low/middle-income countries.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to disclose.
References
1. Kaur R and Pichichero M. Colonization, density, and antibiotic resistance of Streptococcus pneumoniae, Haemophilus Influenzae, and Moraxella catarrhalis among PCV13 vaccinated infants in the first six months of life in Rochester, New York. J Pediatric Infect Dis Soc. 2023 Apr 18;12(3):135-42.
2. Chapman T et al. Nasopharyngeal colonization with pathobionts is associated with susceptibility to respiratory illnesses in young children. PLoS One. 2020 Dec 11;15(12):e0243942. doi: 10.1371/journal.pone.0243942.
3. Chapman TJ et al. Antibiotic use and vaccine antibody levels. Pediatrics 2022 May 1;149(5):e2021052061. doi: 10.1542/peds.2021-052061.
In this column, I will describe the results of a recently published study from my group.1 We sought to profile Streptococcus pneumoniae (pneumococcus), Haemophilus influenzae (Hflu) and Moraxella catarrhalis (Mcat) in the nasopharynx among 13-valent pneumococcal conjugate vaccine (PCV13)-immunized children, with a focus on the first 6 months of life. The rationale was to provide heretofore unreported contemporary data in a highly PCV13-immunized, community-based child population in the United States. A secondary objective was to assess nasopharyngeal bacterial density because higher density associates with greater likelihood of progression to infection. Thirdly, the serotype distribution and antibiotic susceptibility of pneumococci among children seen in primary care settings in the United States had not been evaluated for strains circulating among infants less than 6 months old and they may differ from strains recovered from older children. Therefore, comparisons were made within the same cohort of children to later child age time points.
Risk factors identified
The study was prospective and collected from a cohort of 101 children in Rochester, N.Y., during 2018-2020. Nasopharyngeal swabs were taken for study at age 1, 2 and 3 weeks, then 1, 2, 4, 6, 9, 12, 15, 18 and 24 months. All children had received PCV13 vaccine according to the Centers for Disease Control and Prevention recommended schedule.
We found two significant risk factors in the first 6 months of life for detection of nasopharyngeal colonization of pneumococcus, Hflu, and Mcat. They were daycare attendance and one or more siblings aged 1-5 years at home.
Colonization by one or more of the three bacteria was detected in only 5% of infants before age 2 months. None of the five children attended daycare but all five had young siblings at home. Pneumococcal colonization was detected in 12%, Hflu in 3%, and Mcat in 21% of nasopharyngeal swabs collected during the first 6 months of life. Nasopharyngeal colonization with the bacteria increased rapidly between age 4 and 6 months of life, coincident with infants going to daycare and other social interaction opportunities. Bacterial density of pneumococcus, Hflu, and Mcat during the first 6 months of life was significantly lower in the nasopharynx compared with bacterial density when samples were collected during child age 7-24 months.
The prevalent pneumococcal serotypes in children up to 6 months old were 23B (17%), 22F (13%), 15B/C (11%), 16F (9%), and 21 (7%), 19F (7%), which differed from those isolated from children age 7-24 months, where serotypes 35B (15%), 21 (10%), 15B (9%), and 23B (7%), 23A (7%) were most commonly observed. Antibiotic resistance among isolates did not significantly differ in comparisons between infants younger than 6 months versus 7- to 24-month-olds.
What is the clinical significance?
Colonization of the nasopharynx is a necessary first step in infection pathogenesis (Figure).
Prevalence of colonization varies among settings and countries, with generally much higher prevalence soon after birth and persisting at high rates in children living in low/middle-income countries versus high-income countries. This is one explanation for higher respiratory infection rates in low/middle-income countries compared with the United States, Europe, and other high-income countries. Environmental risk factors for early life colonization include household crowding, young siblings, no breastfeeding, daycare attendance, antibiotic usage, and passive exposure to smoke.
In a prior study of a different cohort of 358 prospectively-enrolled children, we sought associations between physician-attended illness visits and bacterial colonization in the first 5 years of life.2 We showed that early age of first colonization with pneumococcus, Hflu, and Mcat was associated with respiratory infection proneness and asthma among the children.
Multiple demographic and risk factors may contribute to early life and high-density colonization that in turn may increase risk of infections. High densities and early life pneumococcal colonization in low/middle-income countries might impact PCV responses by induction of immunity tolerance. While it is appealing to study new vaccines in low/middle-income populations with high infection incidence, there are reasons that infection incidence is higher compared with high-income countries like the United States, among them may be early life nasopharyngeal colonization and density of colonization.
Prevalent pneumococcal serotype appear to differ with age. The most common serotypes in the first 6 months of life for the children were 23B> 22F> 16F and 21=19F, but in children 7-24 months, serotypes 35B> 21>15B>23A=23B were most commonly observed. This difference might be due to the impact of antibiotics.3 Pneumococci expressing serotypes 22F and 16F were oxacillin susceptible and antibiotic exposure in the first 6 months of life is very uncommon in our study cohorts. In contrast, all pneumococci expressing 35B capsule were oxacillin resistant and in our cohorts antibiotic exposures are common among 7- to 24-month-olds.
In conclusion, we determined that children in the first 6 months of life seen in pediatric primary care settings in Rochester, N.Y., have very low prevalence and low-density colonization of pneumococcus, Hflu, and Mcat compared with 7- to 24-month olds. Our results may explain the significantly lower rates of infections caused by pneumococci, Hflu, and Mcat in infants younger than 6 months old compared with low/middle-income countries.
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute at Rochester (N.Y.) General Hospital. He has no conflicts of interest to disclose.
References
1. Kaur R and Pichichero M. Colonization, density, and antibiotic resistance of Streptococcus pneumoniae, Haemophilus Influenzae, and Moraxella catarrhalis among PCV13 vaccinated infants in the first six months of life in Rochester, New York. J Pediatric Infect Dis Soc. 2023 Apr 18;12(3):135-42.
2. Chapman T et al. Nasopharyngeal colonization with pathobionts is associated with susceptibility to respiratory illnesses in young children. PLoS One. 2020 Dec 11;15(12):e0243942. doi: 10.1371/journal.pone.0243942.
3. Chapman TJ et al. Antibiotic use and vaccine antibody levels. Pediatrics 2022 May 1;149(5):e2021052061. doi: 10.1542/peds.2021-052061.
New outbreaks of Marburg virus disease: What clinicians need to know
What do green monkeys, fruit bats, and python caves all have in common? All have been implicated in outbreaks as transmission sources of the rare but deadly Marburg virus. Marburg virus is in the same Filoviridae family of highly pathogenic RNA viruses as Ebola virus, and similarly can cause a rapidly progressive and fatal viral hemorrhagic fever.
In the first reported Marburg outbreak in 1967, laboratory workers in Marburg and Frankfurt, Germany, and in Belgrade, Yugoslavia, developed severe febrile illnesses with massive hemorrhage and multiorgan system dysfunction after contact with infected African green monkeys imported from Uganda.
The majority of MVD outbreaks have occurred in sub-Saharan Africa, and primarily in three African countries: Angola, the Democratic Republic of Congo, and Uganda. In sub-Saharan Africa, these sporadic outbreaks have had high case fatality rates (up to 80%-90%) and been linked to human exposure to the oral secretions or urinary/fecal droppings of Egyptian fruit bats (Rousettus aegyptiacus), the animal reservoir for Marburg virus. These exposures have primarily occurred among miners or tourists frequenting bat-infested mines or caves, including Uganda’s python cave, where Centers for Disease Control and Prevention investigators have conducted ecological studies on Marburg-infected bats. Person-to-person transmission occurs from direct contact with the blood or bodily fluids of an infected person or contact with a contaminated object (for example, unsterilized needles and syringes in a large nosocomial outbreak in Angola).
On April 6, 2023, the CDC issued a Health Advisory for U.S. clinicians and public health departments regarding two separate MVD outbreaks in Equatorial Guinea and Tanzania. These first-ever MVD outbreaks in both West and East African countries appear to be epidemiologically unrelated. As of March 24, 2023, in Equatorial Guinea, a total of 15 confirmed cases, including 11 deaths, and 23 probable cases, all deceased, have been identified in multiple districts since the outbreak declaration in February 2023. In Tanzania, a total of eight cases, including five deaths, have been reported among villagers in a northwest region since the outbreak declaration in March 2023. While so far cases in the Tanzania MVD outbreak have been epidemiologically linked, in Equatorial Guinea some cases have no identified epidemiological links, raising concern for ongoing community spread.
To date, no cases in these outbreaks have been reported in the United States or outside the affected countries. Overall, the risk of MVD in nonendemic countries, like the United States, is low but there is still a risk of importation. As of May 2, 2023, CDC has issued a Level 2 travel alert (practice enhanced precautions) for Marburg in Equatorial Guinea and a Level 1 travel watch (practice usual precautions) for Marburg in Tanzania. Travelers to these countries are advised to avoid nonessential travel to areas with active outbreaks and practice preventative measures, including avoiding contact with sick people, blood and bodily fluids, dead bodies, fruit bats, and nonhuman primates. International travelers returning to the United States from these countries are advised to self-monitor for Marburg symptoms during travel and for 21 days after country departure. Travelers who develop signs or symptoms of MVD should immediately self-isolate and contact their local health department or clinician.
So, how should clinicians manage such return travelers? In the setting of these new MVD outbreaks in sub-Saharan Africa, what do U.S. clinicians need to know? Clinicians should consider MVD in the differential diagnosis of ill patients with a compatible exposure history and clinical presentation. A detailed exposure history should be obtained to determine if patients have been to an area with an active MVD outbreak during their incubation period (in the past 21 days), had concerning epidemiologic risk factors (for example, presence at funerals, health care facilities, in mines/caves) while in the affected area, and/or had contact with a suspected or confirmed MVD case.
Clinical diagnosis of MVD is challenging as the initial dry symptoms of infection are nonspecific (fever, influenza-like illness, malaise, anorexia, etc.) and can resemble other febrile infectious illnesses. Similarly, presenting alternative or concurrent infections, particularly in febrile return travelers, include malaria, Lassa fever, typhoid, and measles. From these nonspecific symptoms, patients with MVD can then progress to the more severe wet symptoms (for example, vomiting, diarrhea, and bleeding). Common clinical features of MVD have been described based on the clinical presentation and course of cases in MVD outbreaks. Notably, in the original Marburg outbreak, maculopapular rash and conjunctival injection were early patient symptoms and most patient deaths occurred during the second week of illness progression.
Supportive care, including aggressive fluid replacement, is the mainstay of therapy for MVD. Currently, there are no Food and Drug Administration–approved antiviral treatments or vaccines for Marburg virus. Despite their viral similarities, vaccines against Ebola virus have not been shown to be protective against Marburg virus. Marburg virus vaccine development is ongoing, with a few promising candidate vaccines in early phase 1 and 2 clinical trials. In 2022, in response to MVD outbreaks in Ghana and Guinea, the World Health Organization convened an international Marburg virus vaccine consortium which is working to promote global research collaboration for more rapid vaccine development.
In the absence of definitive therapies, early identification of patients with suspected MVD is critical for preventing the spread of infection to close contacts. Like Ebola virus–infected patients, only symptomatic MVD patients are infectious and all patients with suspected MVD should be isolated in a private room and cared for in accordance with infection control procedures. As MVD is a nationally notifiable disease, suspected cases should be reported to local or state health departments as per jurisdictional requirements. Clinicians should also consult with their local or state health department and CDC for guidance on testing patients with suspected MVD and consider prompt evaluation for other infectious etiologies in the patient’s differential diagnosis. Comprehensive guidance for clinicians on screening and diagnosing patients with MVD is available on the CDC website at https://www.cdc.gov/vhf/marburg/index.html.
Dr. Appiah (she/her) is a medical epidemiologist in the division of global migration and quarantine at the CDC. Dr. Appiah holds adjunct faculty appointment in the division of infectious diseases at Emory University, Atlanta. She also holds a commission in the U.S. Public Health Service and is a resident advisor, Uganda, U.S. President’s Malaria Initiative, at the CDC.
What do green monkeys, fruit bats, and python caves all have in common? All have been implicated in outbreaks as transmission sources of the rare but deadly Marburg virus. Marburg virus is in the same Filoviridae family of highly pathogenic RNA viruses as Ebola virus, and similarly can cause a rapidly progressive and fatal viral hemorrhagic fever.
In the first reported Marburg outbreak in 1967, laboratory workers in Marburg and Frankfurt, Germany, and in Belgrade, Yugoslavia, developed severe febrile illnesses with massive hemorrhage and multiorgan system dysfunction after contact with infected African green monkeys imported from Uganda.
The majority of MVD outbreaks have occurred in sub-Saharan Africa, and primarily in three African countries: Angola, the Democratic Republic of Congo, and Uganda. In sub-Saharan Africa, these sporadic outbreaks have had high case fatality rates (up to 80%-90%) and been linked to human exposure to the oral secretions or urinary/fecal droppings of Egyptian fruit bats (Rousettus aegyptiacus), the animal reservoir for Marburg virus. These exposures have primarily occurred among miners or tourists frequenting bat-infested mines or caves, including Uganda’s python cave, where Centers for Disease Control and Prevention investigators have conducted ecological studies on Marburg-infected bats. Person-to-person transmission occurs from direct contact with the blood or bodily fluids of an infected person or contact with a contaminated object (for example, unsterilized needles and syringes in a large nosocomial outbreak in Angola).
On April 6, 2023, the CDC issued a Health Advisory for U.S. clinicians and public health departments regarding two separate MVD outbreaks in Equatorial Guinea and Tanzania. These first-ever MVD outbreaks in both West and East African countries appear to be epidemiologically unrelated. As of March 24, 2023, in Equatorial Guinea, a total of 15 confirmed cases, including 11 deaths, and 23 probable cases, all deceased, have been identified in multiple districts since the outbreak declaration in February 2023. In Tanzania, a total of eight cases, including five deaths, have been reported among villagers in a northwest region since the outbreak declaration in March 2023. While so far cases in the Tanzania MVD outbreak have been epidemiologically linked, in Equatorial Guinea some cases have no identified epidemiological links, raising concern for ongoing community spread.
To date, no cases in these outbreaks have been reported in the United States or outside the affected countries. Overall, the risk of MVD in nonendemic countries, like the United States, is low but there is still a risk of importation. As of May 2, 2023, CDC has issued a Level 2 travel alert (practice enhanced precautions) for Marburg in Equatorial Guinea and a Level 1 travel watch (practice usual precautions) for Marburg in Tanzania. Travelers to these countries are advised to avoid nonessential travel to areas with active outbreaks and practice preventative measures, including avoiding contact with sick people, blood and bodily fluids, dead bodies, fruit bats, and nonhuman primates. International travelers returning to the United States from these countries are advised to self-monitor for Marburg symptoms during travel and for 21 days after country departure. Travelers who develop signs or symptoms of MVD should immediately self-isolate and contact their local health department or clinician.
So, how should clinicians manage such return travelers? In the setting of these new MVD outbreaks in sub-Saharan Africa, what do U.S. clinicians need to know? Clinicians should consider MVD in the differential diagnosis of ill patients with a compatible exposure history and clinical presentation. A detailed exposure history should be obtained to determine if patients have been to an area with an active MVD outbreak during their incubation period (in the past 21 days), had concerning epidemiologic risk factors (for example, presence at funerals, health care facilities, in mines/caves) while in the affected area, and/or had contact with a suspected or confirmed MVD case.
Clinical diagnosis of MVD is challenging as the initial dry symptoms of infection are nonspecific (fever, influenza-like illness, malaise, anorexia, etc.) and can resemble other febrile infectious illnesses. Similarly, presenting alternative or concurrent infections, particularly in febrile return travelers, include malaria, Lassa fever, typhoid, and measles. From these nonspecific symptoms, patients with MVD can then progress to the more severe wet symptoms (for example, vomiting, diarrhea, and bleeding). Common clinical features of MVD have been described based on the clinical presentation and course of cases in MVD outbreaks. Notably, in the original Marburg outbreak, maculopapular rash and conjunctival injection were early patient symptoms and most patient deaths occurred during the second week of illness progression.
Supportive care, including aggressive fluid replacement, is the mainstay of therapy for MVD. Currently, there are no Food and Drug Administration–approved antiviral treatments or vaccines for Marburg virus. Despite their viral similarities, vaccines against Ebola virus have not been shown to be protective against Marburg virus. Marburg virus vaccine development is ongoing, with a few promising candidate vaccines in early phase 1 and 2 clinical trials. In 2022, in response to MVD outbreaks in Ghana and Guinea, the World Health Organization convened an international Marburg virus vaccine consortium which is working to promote global research collaboration for more rapid vaccine development.
In the absence of definitive therapies, early identification of patients with suspected MVD is critical for preventing the spread of infection to close contacts. Like Ebola virus–infected patients, only symptomatic MVD patients are infectious and all patients with suspected MVD should be isolated in a private room and cared for in accordance with infection control procedures. As MVD is a nationally notifiable disease, suspected cases should be reported to local or state health departments as per jurisdictional requirements. Clinicians should also consult with their local or state health department and CDC for guidance on testing patients with suspected MVD and consider prompt evaluation for other infectious etiologies in the patient’s differential diagnosis. Comprehensive guidance for clinicians on screening and diagnosing patients with MVD is available on the CDC website at https://www.cdc.gov/vhf/marburg/index.html.
Dr. Appiah (she/her) is a medical epidemiologist in the division of global migration and quarantine at the CDC. Dr. Appiah holds adjunct faculty appointment in the division of infectious diseases at Emory University, Atlanta. She also holds a commission in the U.S. Public Health Service and is a resident advisor, Uganda, U.S. President’s Malaria Initiative, at the CDC.
What do green monkeys, fruit bats, and python caves all have in common? All have been implicated in outbreaks as transmission sources of the rare but deadly Marburg virus. Marburg virus is in the same Filoviridae family of highly pathogenic RNA viruses as Ebola virus, and similarly can cause a rapidly progressive and fatal viral hemorrhagic fever.
In the first reported Marburg outbreak in 1967, laboratory workers in Marburg and Frankfurt, Germany, and in Belgrade, Yugoslavia, developed severe febrile illnesses with massive hemorrhage and multiorgan system dysfunction after contact with infected African green monkeys imported from Uganda.
The majority of MVD outbreaks have occurred in sub-Saharan Africa, and primarily in three African countries: Angola, the Democratic Republic of Congo, and Uganda. In sub-Saharan Africa, these sporadic outbreaks have had high case fatality rates (up to 80%-90%) and been linked to human exposure to the oral secretions or urinary/fecal droppings of Egyptian fruit bats (Rousettus aegyptiacus), the animal reservoir for Marburg virus. These exposures have primarily occurred among miners or tourists frequenting bat-infested mines or caves, including Uganda’s python cave, where Centers for Disease Control and Prevention investigators have conducted ecological studies on Marburg-infected bats. Person-to-person transmission occurs from direct contact with the blood or bodily fluids of an infected person or contact with a contaminated object (for example, unsterilized needles and syringes in a large nosocomial outbreak in Angola).
On April 6, 2023, the CDC issued a Health Advisory for U.S. clinicians and public health departments regarding two separate MVD outbreaks in Equatorial Guinea and Tanzania. These first-ever MVD outbreaks in both West and East African countries appear to be epidemiologically unrelated. As of March 24, 2023, in Equatorial Guinea, a total of 15 confirmed cases, including 11 deaths, and 23 probable cases, all deceased, have been identified in multiple districts since the outbreak declaration in February 2023. In Tanzania, a total of eight cases, including five deaths, have been reported among villagers in a northwest region since the outbreak declaration in March 2023. While so far cases in the Tanzania MVD outbreak have been epidemiologically linked, in Equatorial Guinea some cases have no identified epidemiological links, raising concern for ongoing community spread.
To date, no cases in these outbreaks have been reported in the United States or outside the affected countries. Overall, the risk of MVD in nonendemic countries, like the United States, is low but there is still a risk of importation. As of May 2, 2023, CDC has issued a Level 2 travel alert (practice enhanced precautions) for Marburg in Equatorial Guinea and a Level 1 travel watch (practice usual precautions) for Marburg in Tanzania. Travelers to these countries are advised to avoid nonessential travel to areas with active outbreaks and practice preventative measures, including avoiding contact with sick people, blood and bodily fluids, dead bodies, fruit bats, and nonhuman primates. International travelers returning to the United States from these countries are advised to self-monitor for Marburg symptoms during travel and for 21 days after country departure. Travelers who develop signs or symptoms of MVD should immediately self-isolate and contact their local health department or clinician.
So, how should clinicians manage such return travelers? In the setting of these new MVD outbreaks in sub-Saharan Africa, what do U.S. clinicians need to know? Clinicians should consider MVD in the differential diagnosis of ill patients with a compatible exposure history and clinical presentation. A detailed exposure history should be obtained to determine if patients have been to an area with an active MVD outbreak during their incubation period (in the past 21 days), had concerning epidemiologic risk factors (for example, presence at funerals, health care facilities, in mines/caves) while in the affected area, and/or had contact with a suspected or confirmed MVD case.
Clinical diagnosis of MVD is challenging as the initial dry symptoms of infection are nonspecific (fever, influenza-like illness, malaise, anorexia, etc.) and can resemble other febrile infectious illnesses. Similarly, presenting alternative or concurrent infections, particularly in febrile return travelers, include malaria, Lassa fever, typhoid, and measles. From these nonspecific symptoms, patients with MVD can then progress to the more severe wet symptoms (for example, vomiting, diarrhea, and bleeding). Common clinical features of MVD have been described based on the clinical presentation and course of cases in MVD outbreaks. Notably, in the original Marburg outbreak, maculopapular rash and conjunctival injection were early patient symptoms and most patient deaths occurred during the second week of illness progression.
Supportive care, including aggressive fluid replacement, is the mainstay of therapy for MVD. Currently, there are no Food and Drug Administration–approved antiviral treatments or vaccines for Marburg virus. Despite their viral similarities, vaccines against Ebola virus have not been shown to be protective against Marburg virus. Marburg virus vaccine development is ongoing, with a few promising candidate vaccines in early phase 1 and 2 clinical trials. In 2022, in response to MVD outbreaks in Ghana and Guinea, the World Health Organization convened an international Marburg virus vaccine consortium which is working to promote global research collaboration for more rapid vaccine development.
In the absence of definitive therapies, early identification of patients with suspected MVD is critical for preventing the spread of infection to close contacts. Like Ebola virus–infected patients, only symptomatic MVD patients are infectious and all patients with suspected MVD should be isolated in a private room and cared for in accordance with infection control procedures. As MVD is a nationally notifiable disease, suspected cases should be reported to local or state health departments as per jurisdictional requirements. Clinicians should also consult with their local or state health department and CDC for guidance on testing patients with suspected MVD and consider prompt evaluation for other infectious etiologies in the patient’s differential diagnosis. Comprehensive guidance for clinicians on screening and diagnosing patients with MVD is available on the CDC website at https://www.cdc.gov/vhf/marburg/index.html.
Dr. Appiah (she/her) is a medical epidemiologist in the division of global migration and quarantine at the CDC. Dr. Appiah holds adjunct faculty appointment in the division of infectious diseases at Emory University, Atlanta. She also holds a commission in the U.S. Public Health Service and is a resident advisor, Uganda, U.S. President’s Malaria Initiative, at the CDC.
Rabies: How to respond to parents’ questions
When most families hear the word rabies, they envision a dog foaming at the mouth and think about receiving multiple painful, often intra-abdominal injections. However, the epidemiology of rabies has changed in the United States. Postexposure prophylaxis (PEP) may not always be indicated and for certain persons preexposure prophylaxis (PrEP) is available and recommended.
Rabies is a Lyssavirus that is transmitted through saliva most often from the bite or scratch of an infected animal. Sometimes it’s via direct contact with mucous membranes. Although rare, cases have been described in which an undiagnosed donor passed the virus via transplant to recipients and four cases of aerosolized transmission were documented in two spelunkers and two laboratory technicians working with the virus. Worldwide it’s estimated that rabies causes 59,000 deaths annually.
Most cases (98%) are secondary to canine rabies. Prior to 1960, dogs were the major reservoir in the United States; however, after introduction of leash laws and animal vaccination in 1947, there was a drastic decline in cases caused by the canine rabies virus variant (CRVV). By 2004, CRVV was eliminated in the United States.
However, the proportion of strains associated with wildlife including raccoons, skunks, foxes, bats, coyotes, and mongoose now account for most of the cases in humans. Wildlife rabies is found in all states except Hawaii. Between 1960 and 2018, 89 cases were acquired in the United States and 62 (70%) were from bat exposure. Dog bites acquired during international travel were the cause of 36 cases.
Once signs and symptoms of disease develop there is no treatment. Regardless of the species variant, rabies virus infection is fatal in over 99% of cases. However, disease can be prevented with prompt initiation of PEP, which includes administration of rabies immune globulin (RIG) and rabies vaccine. Let’s look at a few different scenarios.
1. A delivery person is bitten by your neighbor’s dog while making a delivery. He was told to get rabies vaccine. What should we advise?
Canine rabies has been eliminated in the United States. However, unvaccinated canines can acquire rabies from wildlife. In this situation, you can determine the immunization status of the dog. Contact your local/state health department to assist with enforcement and management. Bites by cats and ferrets should be managed similarly.
Healthy dog:
1. Observe for 10 days.
2. PEP is not indicated unless the animal develops signs/symptoms of rabies. Then euthanize and begin PEP.
Dog appears rabid or suspected to be rabid:
1. Begin PEP.
2. Animal should be euthanized. If immunofluorescent test is negative discontinue PEP.
Dog unavailable:
Contact local/state health department. They are more familiar with rabies surveillance data.
2. Patient relocating to Malaysia for 3-4 years. Rabies PrEP was recommended but the family wants your opinion before receiving the vaccine. What would you advise?
Canine rabies is felt to be the primary cause of rabies outside of the United States. Canines are not routinely vaccinated in many foreign destinations, and the availability of RIG and rabies vaccine is not guaranteed in developing countries. As noted above, dog bites during international travel accounted for 28% of U.S. cases between 1960 and 2018.
In May 2022 recommendations for a modified two-dose PrEP schedule was published that identifies five risk groups and includes specific timing for checking rabies titers. The third rabies dose can now be administered up until year 3 (Morb Mortal Wkly Rep. 2022 May 6;71[18]:619-27). For individuals relocating to countries where CRVV is present, I prefer the traditional three-dose PrEP schedule administered between 21 and 28 days. However, we now have options. If exposure occurs any time after completion of a three-dose PrEP series or within 3 years after completion of a two-dose PrEP series, RIG would not be required. All patients would receive two doses of rabies vaccine (days 0, 3). If exposure occurs after 3 years in a person who received two doses of PrEP who did not have documentation of a protective rabies titer (> 5 IU/mL), treatment will include RIG plus four doses of vaccine (days 0, 3, 7, 14).
For this relocating patient, supporting PrEP would be strongly recommended.
3. A mother tells you she sees bats flying around her home at night and a few have even gotten into the home. This morning she saw one in her child’s room. He was still sleeping. Is there anything she needs to do?
Bats have become the predominant source of rabies in the United States. In addition to the cases noted above, three fatal cases occurred between Sept. 28 and Nov. 10, 2021, after bat exposures in August 2021 (MMWR Morb Mortal Wkly Rep. 2022 Jan 7;71:31-2). All had recognized contact with a bat 3-7 weeks prior to onset of symptoms and died 2-3 weeks after symptom onset. One declined PEP and the other two did not realize the risk for rabies from their exposure or did not notice a scratch or bite. Bites from bats may be small and unnoticed. Exposure to a bat in a closed room while sleeping is considered an exposure. Hawaii is the only state not reporting rabid bats.
PEP is recommended for her child. She should identify potential areas bats may enter the home and seal them in addition to removal of any bat roosts.
4. A parent realizes a house guest has been feeding raccoons in the backyard. What’s your response?
While bat rabies is the predominant variant associated with disease in the United States, as illustrated in Figure 1, other species of wildlife including raccoons are a major source of rabies. The geographic spread of the raccoon variant of rabies has been limited by oral vaccination via bait. In the situation noted here, the raccoons have returned because food was being offered thus increasing the families chance of a potential rabies exposure. Wildlife including skunks, raccoons, coyotes, foxes, and mongooses are always considered rabid until proven negative by laboratory testing.
You recommend to stop feeding wildlife and never to approach them. Have them contact the local rabies control unit and/or state wildlife services to assist with removal of the raccoons. Depending on the locale, pest control may be required at the owners expense. Inform the family to seek PEP if anyone is bitten or scratched by the raccoons.
As per the Centers for Disease Control and Prevention, about 55,000 residents receive PEP annually with health-associated expenditures including diagnostics, prevention, and control estimated between $245 and $510 million annually. Rabies is one of the most fatal diseases that can be prevented by avoiding contact with wild animals, maintenance of high immunization rates in pets, and keeping people informed of potential sources including bats. One can’t determine if an animal has rabies by looking at it. Rabies remains an urgent disease that we have to remember to address with our patients and their families. For additional information go to www.CDC.gov/rabies.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She has no relevant financial disclosures.
When most families hear the word rabies, they envision a dog foaming at the mouth and think about receiving multiple painful, often intra-abdominal injections. However, the epidemiology of rabies has changed in the United States. Postexposure prophylaxis (PEP) may not always be indicated and for certain persons preexposure prophylaxis (PrEP) is available and recommended.
Rabies is a Lyssavirus that is transmitted through saliva most often from the bite or scratch of an infected animal. Sometimes it’s via direct contact with mucous membranes. Although rare, cases have been described in which an undiagnosed donor passed the virus via transplant to recipients and four cases of aerosolized transmission were documented in two spelunkers and two laboratory technicians working with the virus. Worldwide it’s estimated that rabies causes 59,000 deaths annually.

Most cases (98%) are secondary to canine rabies. Prior to 1960, dogs were the major reservoir in the United States; however, after introduction of leash laws and animal vaccination in 1947, there was a drastic decline in cases caused by the canine rabies virus variant (CRVV). By 2004, CRVV was eliminated in the United States.
However, the proportion of strains associated with wildlife including raccoons, skunks, foxes, bats, coyotes, and mongoose now account for most of the cases in humans. Wildlife rabies is found in all states except Hawaii. Between 1960 and 2018, 89 cases were acquired in the United States and 62 (70%) were from bat exposure. Dog bites acquired during international travel were the cause of 36 cases.
Once signs and symptoms of disease develop there is no treatment. Regardless of the species variant, rabies virus infection is fatal in over 99% of cases. However, disease can be prevented with prompt initiation of PEP, which includes administration of rabies immune globulin (RIG) and rabies vaccine. Let’s look at a few different scenarios.
1. A delivery person is bitten by your neighbor’s dog while making a delivery. He was told to get rabies vaccine. What should we advise?
Canine rabies has been eliminated in the United States. However, unvaccinated canines can acquire rabies from wildlife. In this situation, you can determine the immunization status of the dog. Contact your local/state health department to assist with enforcement and management. Bites by cats and ferrets should be managed similarly.
Healthy dog:
1. Observe for 10 days.
2. PEP is not indicated unless the animal develops signs/symptoms of rabies. Then euthanize and begin PEP.
Dog appears rabid or suspected to be rabid:
1. Begin PEP.
2. Animal should be euthanized. If immunofluorescent test is negative discontinue PEP.
Dog unavailable:
Contact local/state health department. They are more familiar with rabies surveillance data.
2. Patient relocating to Malaysia for 3-4 years. Rabies PrEP was recommended but the family wants your opinion before receiving the vaccine. What would you advise?
Canine rabies is felt to be the primary cause of rabies outside of the United States. Canines are not routinely vaccinated in many foreign destinations, and the availability of RIG and rabies vaccine is not guaranteed in developing countries. As noted above, dog bites during international travel accounted for 28% of U.S. cases between 1960 and 2018.
In May 2022 recommendations for a modified two-dose PrEP schedule was published that identifies five risk groups and includes specific timing for checking rabies titers. The third rabies dose can now be administered up until year 3 (Morb Mortal Wkly Rep. 2022 May 6;71[18]:619-27). For individuals relocating to countries where CRVV is present, I prefer the traditional three-dose PrEP schedule administered between 21 and 28 days. However, we now have options. If exposure occurs any time after completion of a three-dose PrEP series or within 3 years after completion of a two-dose PrEP series, RIG would not be required. All patients would receive two doses of rabies vaccine (days 0, 3). If exposure occurs after 3 years in a person who received two doses of PrEP who did not have documentation of a protective rabies titer (> 5 IU/mL), treatment will include RIG plus four doses of vaccine (days 0, 3, 7, 14).
For this relocating patient, supporting PrEP would be strongly recommended.
3. A mother tells you she sees bats flying around her home at night and a few have even gotten into the home. This morning she saw one in her child’s room. He was still sleeping. Is there anything she needs to do?
Bats have become the predominant source of rabies in the United States. In addition to the cases noted above, three fatal cases occurred between Sept. 28 and Nov. 10, 2021, after bat exposures in August 2021 (MMWR Morb Mortal Wkly Rep. 2022 Jan 7;71:31-2). All had recognized contact with a bat 3-7 weeks prior to onset of symptoms and died 2-3 weeks after symptom onset. One declined PEP and the other two did not realize the risk for rabies from their exposure or did not notice a scratch or bite. Bites from bats may be small and unnoticed. Exposure to a bat in a closed room while sleeping is considered an exposure. Hawaii is the only state not reporting rabid bats.
PEP is recommended for her child. She should identify potential areas bats may enter the home and seal them in addition to removal of any bat roosts.
4. A parent realizes a house guest has been feeding raccoons in the backyard. What’s your response?
While bat rabies is the predominant variant associated with disease in the United States, as illustrated in Figure 1, other species of wildlife including raccoons are a major source of rabies. The geographic spread of the raccoon variant of rabies has been limited by oral vaccination via bait. In the situation noted here, the raccoons have returned because food was being offered thus increasing the families chance of a potential rabies exposure. Wildlife including skunks, raccoons, coyotes, foxes, and mongooses are always considered rabid until proven negative by laboratory testing.

You recommend to stop feeding wildlife and never to approach them. Have them contact the local rabies control unit and/or state wildlife services to assist with removal of the raccoons. Depending on the locale, pest control may be required at the owners expense. Inform the family to seek PEP if anyone is bitten or scratched by the raccoons.
As per the Centers for Disease Control and Prevention, about 55,000 residents receive PEP annually with health-associated expenditures including diagnostics, prevention, and control estimated between $245 and $510 million annually. Rabies is one of the most fatal diseases that can be prevented by avoiding contact with wild animals, maintenance of high immunization rates in pets, and keeping people informed of potential sources including bats. One can’t determine if an animal has rabies by looking at it. Rabies remains an urgent disease that we have to remember to address with our patients and their families. For additional information go to www.CDC.gov/rabies.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She has no relevant financial disclosures.
When most families hear the word rabies, they envision a dog foaming at the mouth and think about receiving multiple painful, often intra-abdominal injections. However, the epidemiology of rabies has changed in the United States. Postexposure prophylaxis (PEP) may not always be indicated and for certain persons preexposure prophylaxis (PrEP) is available and recommended.
Rabies is a Lyssavirus that is transmitted through saliva most often from the bite or scratch of an infected animal. Sometimes it’s via direct contact with mucous membranes. Although rare, cases have been described in which an undiagnosed donor passed the virus via transplant to recipients and four cases of aerosolized transmission were documented in two spelunkers and two laboratory technicians working with the virus. Worldwide it’s estimated that rabies causes 59,000 deaths annually.

Most cases (98%) are secondary to canine rabies. Prior to 1960, dogs were the major reservoir in the United States; however, after introduction of leash laws and animal vaccination in 1947, there was a drastic decline in cases caused by the canine rabies virus variant (CRVV). By 2004, CRVV was eliminated in the United States.
However, the proportion of strains associated with wildlife including raccoons, skunks, foxes, bats, coyotes, and mongoose now account for most of the cases in humans. Wildlife rabies is found in all states except Hawaii. Between 1960 and 2018, 89 cases were acquired in the United States and 62 (70%) were from bat exposure. Dog bites acquired during international travel were the cause of 36 cases.
Once signs and symptoms of disease develop there is no treatment. Regardless of the species variant, rabies virus infection is fatal in over 99% of cases. However, disease can be prevented with prompt initiation of PEP, which includes administration of rabies immune globulin (RIG) and rabies vaccine. Let’s look at a few different scenarios.
1. A delivery person is bitten by your neighbor’s dog while making a delivery. He was told to get rabies vaccine. What should we advise?
Canine rabies has been eliminated in the United States. However, unvaccinated canines can acquire rabies from wildlife. In this situation, you can determine the immunization status of the dog. Contact your local/state health department to assist with enforcement and management. Bites by cats and ferrets should be managed similarly.
Healthy dog:
1. Observe for 10 days.
2. PEP is not indicated unless the animal develops signs/symptoms of rabies. Then euthanize and begin PEP.
Dog appears rabid or suspected to be rabid:
1. Begin PEP.
2. Animal should be euthanized. If immunofluorescent test is negative discontinue PEP.
Dog unavailable:
Contact local/state health department. They are more familiar with rabies surveillance data.
2. Patient relocating to Malaysia for 3-4 years. Rabies PrEP was recommended but the family wants your opinion before receiving the vaccine. What would you advise?
Canine rabies is felt to be the primary cause of rabies outside of the United States. Canines are not routinely vaccinated in many foreign destinations, and the availability of RIG and rabies vaccine is not guaranteed in developing countries. As noted above, dog bites during international travel accounted for 28% of U.S. cases between 1960 and 2018.
In May 2022 recommendations for a modified two-dose PrEP schedule was published that identifies five risk groups and includes specific timing for checking rabies titers. The third rabies dose can now be administered up until year 3 (Morb Mortal Wkly Rep. 2022 May 6;71[18]:619-27). For individuals relocating to countries where CRVV is present, I prefer the traditional three-dose PrEP schedule administered between 21 and 28 days. However, we now have options. If exposure occurs any time after completion of a three-dose PrEP series or within 3 years after completion of a two-dose PrEP series, RIG would not be required. All patients would receive two doses of rabies vaccine (days 0, 3). If exposure occurs after 3 years in a person who received two doses of PrEP who did not have documentation of a protective rabies titer (> 5 IU/mL), treatment will include RIG plus four doses of vaccine (days 0, 3, 7, 14).
For this relocating patient, supporting PrEP would be strongly recommended.
3. A mother tells you she sees bats flying around her home at night and a few have even gotten into the home. This morning she saw one in her child’s room. He was still sleeping. Is there anything she needs to do?
Bats have become the predominant source of rabies in the United States. In addition to the cases noted above, three fatal cases occurred between Sept. 28 and Nov. 10, 2021, after bat exposures in August 2021 (MMWR Morb Mortal Wkly Rep. 2022 Jan 7;71:31-2). All had recognized contact with a bat 3-7 weeks prior to onset of symptoms and died 2-3 weeks after symptom onset. One declined PEP and the other two did not realize the risk for rabies from their exposure or did not notice a scratch or bite. Bites from bats may be small and unnoticed. Exposure to a bat in a closed room while sleeping is considered an exposure. Hawaii is the only state not reporting rabid bats.
PEP is recommended for her child. She should identify potential areas bats may enter the home and seal them in addition to removal of any bat roosts.
4. A parent realizes a house guest has been feeding raccoons in the backyard. What’s your response?
While bat rabies is the predominant variant associated with disease in the United States, as illustrated in Figure 1, other species of wildlife including raccoons are a major source of rabies. The geographic spread of the raccoon variant of rabies has been limited by oral vaccination via bait. In the situation noted here, the raccoons have returned because food was being offered thus increasing the families chance of a potential rabies exposure. Wildlife including skunks, raccoons, coyotes, foxes, and mongooses are always considered rabid until proven negative by laboratory testing.

You recommend to stop feeding wildlife and never to approach them. Have them contact the local rabies control unit and/or state wildlife services to assist with removal of the raccoons. Depending on the locale, pest control may be required at the owners expense. Inform the family to seek PEP if anyone is bitten or scratched by the raccoons.
As per the Centers for Disease Control and Prevention, about 55,000 residents receive PEP annually with health-associated expenditures including diagnostics, prevention, and control estimated between $245 and $510 million annually. Rabies is one of the most fatal diseases that can be prevented by avoiding contact with wild animals, maintenance of high immunization rates in pets, and keeping people informed of potential sources including bats. One can’t determine if an animal has rabies by looking at it. Rabies remains an urgent disease that we have to remember to address with our patients and their families. For additional information go to www.CDC.gov/rabies.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She has no relevant financial disclosures.