User login
Appreciates treatment options for recurrent BV
“EFFECTIVE TREATMENT OF RECURRENT BACTERIAL VAGINOSIS”
ROBERT L. BARBIERI, MD (EDITORIAL; JULY 2017)
Appreciates treatment options for recurrent BV
I thank Dr. Barbieri for his editorial on effective treatment of recurrent bacterial vaginosis (BV). I practice only outpatient gynecology, and recurrent BV is the most frustrating condition I have to deal with. Now I have 3 treatment options in my armamentarium for taking care of patients. I clipped the article pages from OBG
I have a related question: I see trichomonal vaginitis rarely, maybe 1 to 2 cases in a year. What do you think the reason is?
Vimal Goyle, MD
New York, New York
Beyond BV: Candidiasis and diabetes medications
Thank you for addressing the recurrent BV problem. After many years of throwing antibiotics at this problem I have been underwhelmed. Patients do not want to keep chasing their tails between BV and yeast. I have been suggesting that patients place plain yogurt containing Lactobacillus in a tampon applicator and apply it to the vagina weekly at night, after the original “overgrowth” has been treated, to return the “good bacteria” to the vagina. This avoids overuse of antibiotics (an impending epidemic of resistant organisms), boric acid (a dangerous pill to have around toddlers), and the expense that comes with multiple visits and multiple courses of antibiotics. I believe that in Canada a vaginal ovule with vitamin C and probiotics is available (something to ponder).
Another problem is recurrent yeast infections. We are seeing that many new diabetes medications are increasing the clearance of glucose and are causing severe and intractable Candida vulvovaginitis. In addition, I would like to know the best topical treatments and skin care for yeast in the folds of the panniculus in the morbidly obese. Unfortunately, these patients often have poor or no insurance and therefore cannot afford the cost of many effective remedies.
John Lewis, MD
Bedford, Massachusetts
Another treatment protocol for BV
For recurrent BV, I treat with standard metronidazole 500 mg orally twice daily for 7 days, then immediately start boric acid suppositories for 3 days in a row followed by 1 weekly for 6 weeks, and that usually takes care of it. However, a few caveats: I instruct patients to keep a supply of boric acid suppositories on hand, and if they start to experience symptoms again, to repeat the 3-day, then weekly-for-6 weeks regimen, so essentially they can manage a recurrence themselves.
For patients who come in thinking they have a recurrent yeast infection or BV, which was initially treated elsewhere, I culture for Mycoplasma and Ureaplasma. I often find that one of those organisms is responsible for the infection, requiring completely different treatment.
I also frequently check the vaginal pH, because patients like to see a visual on what I am talking about.
Rebecca Levy-Gantt, DO
Napa, California
Clindamycin appears superior for BV recurrence prevention
In my practice for the past number of years I have been treating BV with clindamycin vaginal cream instead of metronidazole. I have found that the number of women returning with recurrent BV has dropped dramatically. Furthermore, since switching medications, I cannot recall the last time someone required a maintenance dosing regimen. Although anecdotal, the difference between metronidazole and clindamycin treatment seems striking to me.
Daniel N. Sacks, MD
West Palm Beach, Florida
Uses BV regimens in stepwise fashion
To answer Dr. Barbieri’s instant poll question, my preference for treating BV is to start off with Regimen 1 (metronidazole treatment followed by twice weekly vaginal metronidazole for 6 months), as described in his editorial. If problem reports resolve but recur at a later date, then I use Regimen 2 (metronidazole treatment plus 21 days of boric acid vaginal capsules followed by twice weekly vaginal metronidazole for 6 months). I am aware of Regimen 3 (single-dose oral metronidazole plus fluconazole followed by once-monthly metronidazole and fluconazole) but rarely use it.
Carole W. Campbell, DNP, CNM
Gadsden, Alabama
- Gaydos CA, Beqaj S, Schwebke JR, et al. Clinical validation of a test for the diagnosis of vaginitis. Obstet Gynecol. 2017;130(1):181–189.
- Oduyebo OO, Anorlu RI, Ogunsola FL. The effects of antimicrobial therapy on bacterial vaginosis in non-pregnant women. Cochrane Database Syst Rev. 2009;(3):CD006055.
“EFFECTIVE TREATMENT OF RECURRENT BACTERIAL VAGINOSIS”
ROBERT L. BARBIERI, MD (EDITORIAL; JULY 2017)
Appreciates treatment options for recurrent BV
I thank Dr. Barbieri for his editorial on effective treatment of recurrent bacterial vaginosis (BV). I practice only outpatient gynecology, and recurrent BV is the most frustrating condition I have to deal with. Now I have 3 treatment options in my armamentarium for taking care of patients. I clipped the article pages from OBG
I have a related question: I see trichomonal vaginitis rarely, maybe 1 to 2 cases in a year. What do you think the reason is?
Vimal Goyle, MD
New York, New York
Beyond BV: Candidiasis and diabetes medications
Thank you for addressing the recurrent BV problem. After many years of throwing antibiotics at this problem I have been underwhelmed. Patients do not want to keep chasing their tails between BV and yeast. I have been suggesting that patients place plain yogurt containing Lactobacillus in a tampon applicator and apply it to the vagina weekly at night, after the original “overgrowth” has been treated, to return the “good bacteria” to the vagina. This avoids overuse of antibiotics (an impending epidemic of resistant organisms), boric acid (a dangerous pill to have around toddlers), and the expense that comes with multiple visits and multiple courses of antibiotics. I believe that in Canada a vaginal ovule with vitamin C and probiotics is available (something to ponder).
Another problem is recurrent yeast infections. We are seeing that many new diabetes medications are increasing the clearance of glucose and are causing severe and intractable Candida vulvovaginitis. In addition, I would like to know the best topical treatments and skin care for yeast in the folds of the panniculus in the morbidly obese. Unfortunately, these patients often have poor or no insurance and therefore cannot afford the cost of many effective remedies.
John Lewis, MD
Bedford, Massachusetts
Another treatment protocol for BV
For recurrent BV, I treat with standard metronidazole 500 mg orally twice daily for 7 days, then immediately start boric acid suppositories for 3 days in a row followed by 1 weekly for 6 weeks, and that usually takes care of it. However, a few caveats: I instruct patients to keep a supply of boric acid suppositories on hand, and if they start to experience symptoms again, to repeat the 3-day, then weekly-for-6 weeks regimen, so essentially they can manage a recurrence themselves.
For patients who come in thinking they have a recurrent yeast infection or BV, which was initially treated elsewhere, I culture for Mycoplasma and Ureaplasma. I often find that one of those organisms is responsible for the infection, requiring completely different treatment.
I also frequently check the vaginal pH, because patients like to see a visual on what I am talking about.
Rebecca Levy-Gantt, DO
Napa, California
Clindamycin appears superior for BV recurrence prevention
In my practice for the past number of years I have been treating BV with clindamycin vaginal cream instead of metronidazole. I have found that the number of women returning with recurrent BV has dropped dramatically. Furthermore, since switching medications, I cannot recall the last time someone required a maintenance dosing regimen. Although anecdotal, the difference between metronidazole and clindamycin treatment seems striking to me.
Daniel N. Sacks, MD
West Palm Beach, Florida
Uses BV regimens in stepwise fashion
To answer Dr. Barbieri’s instant poll question, my preference for treating BV is to start off with Regimen 1 (metronidazole treatment followed by twice weekly vaginal metronidazole for 6 months), as described in his editorial. If problem reports resolve but recur at a later date, then I use Regimen 2 (metronidazole treatment plus 21 days of boric acid vaginal capsules followed by twice weekly vaginal metronidazole for 6 months). I am aware of Regimen 3 (single-dose oral metronidazole plus fluconazole followed by once-monthly metronidazole and fluconazole) but rarely use it.
Carole W. Campbell, DNP, CNM
Gadsden, Alabama
“EFFECTIVE TREATMENT OF RECURRENT BACTERIAL VAGINOSIS”
ROBERT L. BARBIERI, MD (EDITORIAL; JULY 2017)
Appreciates treatment options for recurrent BV
I thank Dr. Barbieri for his editorial on effective treatment of recurrent bacterial vaginosis (BV). I practice only outpatient gynecology, and recurrent BV is the most frustrating condition I have to deal with. Now I have 3 treatment options in my armamentarium for taking care of patients. I clipped the article pages from OBG
I have a related question: I see trichomonal vaginitis rarely, maybe 1 to 2 cases in a year. What do you think the reason is?
Vimal Goyle, MD
New York, New York
Beyond BV: Candidiasis and diabetes medications
Thank you for addressing the recurrent BV problem. After many years of throwing antibiotics at this problem I have been underwhelmed. Patients do not want to keep chasing their tails between BV and yeast. I have been suggesting that patients place plain yogurt containing Lactobacillus in a tampon applicator and apply it to the vagina weekly at night, after the original “overgrowth” has been treated, to return the “good bacteria” to the vagina. This avoids overuse of antibiotics (an impending epidemic of resistant organisms), boric acid (a dangerous pill to have around toddlers), and the expense that comes with multiple visits and multiple courses of antibiotics. I believe that in Canada a vaginal ovule with vitamin C and probiotics is available (something to ponder).
Another problem is recurrent yeast infections. We are seeing that many new diabetes medications are increasing the clearance of glucose and are causing severe and intractable Candida vulvovaginitis. In addition, I would like to know the best topical treatments and skin care for yeast in the folds of the panniculus in the morbidly obese. Unfortunately, these patients often have poor or no insurance and therefore cannot afford the cost of many effective remedies.
John Lewis, MD
Bedford, Massachusetts
Another treatment protocol for BV
For recurrent BV, I treat with standard metronidazole 500 mg orally twice daily for 7 days, then immediately start boric acid suppositories for 3 days in a row followed by 1 weekly for 6 weeks, and that usually takes care of it. However, a few caveats: I instruct patients to keep a supply of boric acid suppositories on hand, and if they start to experience symptoms again, to repeat the 3-day, then weekly-for-6 weeks regimen, so essentially they can manage a recurrence themselves.
For patients who come in thinking they have a recurrent yeast infection or BV, which was initially treated elsewhere, I culture for Mycoplasma and Ureaplasma. I often find that one of those organisms is responsible for the infection, requiring completely different treatment.
I also frequently check the vaginal pH, because patients like to see a visual on what I am talking about.
Rebecca Levy-Gantt, DO
Napa, California
Clindamycin appears superior for BV recurrence prevention
In my practice for the past number of years I have been treating BV with clindamycin vaginal cream instead of metronidazole. I have found that the number of women returning with recurrent BV has dropped dramatically. Furthermore, since switching medications, I cannot recall the last time someone required a maintenance dosing regimen. Although anecdotal, the difference between metronidazole and clindamycin treatment seems striking to me.
Daniel N. Sacks, MD
West Palm Beach, Florida
Uses BV regimens in stepwise fashion
To answer Dr. Barbieri’s instant poll question, my preference for treating BV is to start off with Regimen 1 (metronidazole treatment followed by twice weekly vaginal metronidazole for 6 months), as described in his editorial. If problem reports resolve but recur at a later date, then I use Regimen 2 (metronidazole treatment plus 21 days of boric acid vaginal capsules followed by twice weekly vaginal metronidazole for 6 months). I am aware of Regimen 3 (single-dose oral metronidazole plus fluconazole followed by once-monthly metronidazole and fluconazole) but rarely use it.
Carole W. Campbell, DNP, CNM
Gadsden, Alabama
- Gaydos CA, Beqaj S, Schwebke JR, et al. Clinical validation of a test for the diagnosis of vaginitis. Obstet Gynecol. 2017;130(1):181–189.
- Oduyebo OO, Anorlu RI, Ogunsola FL. The effects of antimicrobial therapy on bacterial vaginosis in non-pregnant women. Cochrane Database Syst Rev. 2009;(3):CD006055.
- Gaydos CA, Beqaj S, Schwebke JR, et al. Clinical validation of a test for the diagnosis of vaginitis. Obstet Gynecol. 2017;130(1):181–189.
- Oduyebo OO, Anorlu RI, Ogunsola FL. The effects of antimicrobial therapy on bacterial vaginosis in non-pregnant women. Cochrane Database Syst Rev. 2009;(3):CD006055.
Calls for respect for transgender patients
“CARING FOR THE TRANSGENDER PATIENT: THE ROLE OF THE GYNECOLOGIST”
CECILE A. UNGER, MD, MPH (JUNE 2017)
Calls for respect for transgender patients
We must keep in mind that transgender males are still sexually anatomically female, with all of the medical needs of any other female. Transgender is merely a social construct. We must treat them with kindness and respect.
Laurence Burns, DO
Grand Rapids, Michigan
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“CARING FOR THE TRANSGENDER PATIENT: THE ROLE OF THE GYNECOLOGIST”
CECILE A. UNGER, MD, MPH (JUNE 2017)
Calls for respect for transgender patients
We must keep in mind that transgender males are still sexually anatomically female, with all of the medical needs of any other female. Transgender is merely a social construct. We must treat them with kindness and respect.
Laurence Burns, DO
Grand Rapids, Michigan
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“CARING FOR THE TRANSGENDER PATIENT: THE ROLE OF THE GYNECOLOGIST”
CECILE A. UNGER, MD, MPH (JUNE 2017)
Calls for respect for transgender patients
We must keep in mind that transgender males are still sexually anatomically female, with all of the medical needs of any other female. Transgender is merely a social construct. We must treat them with kindness and respect.
Laurence Burns, DO
Grand Rapids, Michigan
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The Authors Reply, “What Can Be Done to Maintain Positive Patient Experience and Improve Residents’ Satisfaction?” and “Standardized Attending Rounds to Improve the Patient Experience: A Pragmatic Cluster Randomized Controlled Trial”
We thank Talari et al. for their comments in response to our randomized controlled trial evaluating the impact of standardized rounds on patient, attending, and trainee satisfaction. We agree that many factors beyond rounding structure contribute to resident satisfaction, including those highlighted by the authors, and would enthusiastically welcome additional research in this realm.
Because our study intervention addressed rounding structure, we elected to specifically focus on satisfaction with rounds, both from the physician and patient perspectives. We chose to ask about patient satisfaction with attending rounds, as opposed to more generic measures of patient satisfaction, to allow for more direct comparison between attending/resident responses and patient responses. Certainly, there are many other factors that affect overall patient experience. Surveys such as Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) and Press Ganey do not specifically address rounds, are often completed several weeks following hospitalization, and may have low response rates. Relying on such global assessments of patient experience may also reduce the power of the study. Although patient responses to our survey may be higher than scores seen with HCAHPS and Press Ganey, the randomized nature of our study helps control for other differences in the hospitalization experience unrelated to rounding structure. Similarly, because physician teams were randomly assigned, differences in census were not a major factor in the study. Physician blinding was not possible due to the nature of the intervention, which may have affected the satisfaction reports from attendings and residents. For our primary outcome (patient satisfaction with rounds), patients were blinded to the nature of our intervention, and all study team members involved in data collection and statistical analyses were blinded to study arm allocation.
In summary, we feel that evaluating the trade-offs and consequences of interventions should be examined from multiple perspectives, and we welcome additional investigations in this area.
We thank Talari et al. for their comments in response to our randomized controlled trial evaluating the impact of standardized rounds on patient, attending, and trainee satisfaction. We agree that many factors beyond rounding structure contribute to resident satisfaction, including those highlighted by the authors, and would enthusiastically welcome additional research in this realm.
Because our study intervention addressed rounding structure, we elected to specifically focus on satisfaction with rounds, both from the physician and patient perspectives. We chose to ask about patient satisfaction with attending rounds, as opposed to more generic measures of patient satisfaction, to allow for more direct comparison between attending/resident responses and patient responses. Certainly, there are many other factors that affect overall patient experience. Surveys such as Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) and Press Ganey do not specifically address rounds, are often completed several weeks following hospitalization, and may have low response rates. Relying on such global assessments of patient experience may also reduce the power of the study. Although patient responses to our survey may be higher than scores seen with HCAHPS and Press Ganey, the randomized nature of our study helps control for other differences in the hospitalization experience unrelated to rounding structure. Similarly, because physician teams were randomly assigned, differences in census were not a major factor in the study. Physician blinding was not possible due to the nature of the intervention, which may have affected the satisfaction reports from attendings and residents. For our primary outcome (patient satisfaction with rounds), patients were blinded to the nature of our intervention, and all study team members involved in data collection and statistical analyses were blinded to study arm allocation.
In summary, we feel that evaluating the trade-offs and consequences of interventions should be examined from multiple perspectives, and we welcome additional investigations in this area.
We thank Talari et al. for their comments in response to our randomized controlled trial evaluating the impact of standardized rounds on patient, attending, and trainee satisfaction. We agree that many factors beyond rounding structure contribute to resident satisfaction, including those highlighted by the authors, and would enthusiastically welcome additional research in this realm.
Because our study intervention addressed rounding structure, we elected to specifically focus on satisfaction with rounds, both from the physician and patient perspectives. We chose to ask about patient satisfaction with attending rounds, as opposed to more generic measures of patient satisfaction, to allow for more direct comparison between attending/resident responses and patient responses. Certainly, there are many other factors that affect overall patient experience. Surveys such as Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) and Press Ganey do not specifically address rounds, are often completed several weeks following hospitalization, and may have low response rates. Relying on such global assessments of patient experience may also reduce the power of the study. Although patient responses to our survey may be higher than scores seen with HCAHPS and Press Ganey, the randomized nature of our study helps control for other differences in the hospitalization experience unrelated to rounding structure. Similarly, because physician teams were randomly assigned, differences in census were not a major factor in the study. Physician blinding was not possible due to the nature of the intervention, which may have affected the satisfaction reports from attendings and residents. For our primary outcome (patient satisfaction with rounds), patients were blinded to the nature of our intervention, and all study team members involved in data collection and statistical analyses were blinded to study arm allocation.
In summary, we feel that evaluating the trade-offs and consequences of interventions should be examined from multiple perspectives, and we welcome additional investigations in this area.
© 2017 Society of Hospital Medicine
What Can Be Done to Maintain Positive Patient Experience and Improve Residents’ Satisfaction? In Reference to: “Standardized Attending Rounds to Improve the Patient Experience: A Pragmatic Cluster Randomized Controlled Trial”
We read the article by Monash et al.1 published in the March 2017 issue with great interest. This randomized study showed a discrepancy between patients’ and residents’ satisfaction with standardized rounds; for example, residents reported less autonomy, efficiency, teaching, and longer time of rounds.
We agree that letting residents lead the rounds with minimal participation of an attending (only when needed) may improve resident satisfaction. Other factors, such as quality of teaching, positive comments to learners during bedside rounds (whenever appropriate), and a positive attending attitude, might be helpful.2,3 We believe that the adaptation of such a model through the prism of residents’ benefit will lead to better satisfaction among trainees.
On the other hand, we note that the nature of the study might have exaggerated patient satisfaction when compared with real-world surveys.4 The survey appears to focus only on attending rounds and did not consider other factors like hospitality, pain control, etc. A low patient census and lack of double blinding are other potential factors.
In conclusion, we want to congratulate the authors for raising this important topic and showing positive patients’ satisfaction with standardized rounds on teaching services. Further research should focus on improving residents’ satisfaction without compromising patients’ experiences.
1. Monash B, Najafi N, Mourad M, et al. Standardized Attending Rounds to Improve the Patient Experience: A Pragmatic Cluster Randomized Controlled Trial. J Hosp Med. 2017;12(3):143-149. PubMed
2. Williams KN, Ramani S, Fraser B, Orlander JD. Improving bedside teaching: findings from a focus group study of learners. Acad Med. 2008;83(3):257-264. PubMed
3. Castiglioni A, Shewchuk RM, Willett LL, Heudebert GR, Centor RM. A pilot study using nominal group technique to assess residents’ perceptions of successful attending rounds. J Gen Intern Med. 2008;23(7):1060-1065. PubMed
4. Siddiqui ZK, Wu AW, Kurbanova N, Qayyum R. Comparison of Hospital Consumer Assessment of Healthcare Providers and Systems patient satisfaction scores for specialty hospitals and general medical hospitals: confounding effect of survey response rate. J Hosp Med. 2014;9(9):590-593. PubMed
We read the article by Monash et al.1 published in the March 2017 issue with great interest. This randomized study showed a discrepancy between patients’ and residents’ satisfaction with standardized rounds; for example, residents reported less autonomy, efficiency, teaching, and longer time of rounds.
We agree that letting residents lead the rounds with minimal participation of an attending (only when needed) may improve resident satisfaction. Other factors, such as quality of teaching, positive comments to learners during bedside rounds (whenever appropriate), and a positive attending attitude, might be helpful.2,3 We believe that the adaptation of such a model through the prism of residents’ benefit will lead to better satisfaction among trainees.
On the other hand, we note that the nature of the study might have exaggerated patient satisfaction when compared with real-world surveys.4 The survey appears to focus only on attending rounds and did not consider other factors like hospitality, pain control, etc. A low patient census and lack of double blinding are other potential factors.
In conclusion, we want to congratulate the authors for raising this important topic and showing positive patients’ satisfaction with standardized rounds on teaching services. Further research should focus on improving residents’ satisfaction without compromising patients’ experiences.
We read the article by Monash et al.1 published in the March 2017 issue with great interest. This randomized study showed a discrepancy between patients’ and residents’ satisfaction with standardized rounds; for example, residents reported less autonomy, efficiency, teaching, and longer time of rounds.
We agree that letting residents lead the rounds with minimal participation of an attending (only when needed) may improve resident satisfaction. Other factors, such as quality of teaching, positive comments to learners during bedside rounds (whenever appropriate), and a positive attending attitude, might be helpful.2,3 We believe that the adaptation of such a model through the prism of residents’ benefit will lead to better satisfaction among trainees.
On the other hand, we note that the nature of the study might have exaggerated patient satisfaction when compared with real-world surveys.4 The survey appears to focus only on attending rounds and did not consider other factors like hospitality, pain control, etc. A low patient census and lack of double blinding are other potential factors.
In conclusion, we want to congratulate the authors for raising this important topic and showing positive patients’ satisfaction with standardized rounds on teaching services. Further research should focus on improving residents’ satisfaction without compromising patients’ experiences.
1. Monash B, Najafi N, Mourad M, et al. Standardized Attending Rounds to Improve the Patient Experience: A Pragmatic Cluster Randomized Controlled Trial. J Hosp Med. 2017;12(3):143-149. PubMed
2. Williams KN, Ramani S, Fraser B, Orlander JD. Improving bedside teaching: findings from a focus group study of learners. Acad Med. 2008;83(3):257-264. PubMed
3. Castiglioni A, Shewchuk RM, Willett LL, Heudebert GR, Centor RM. A pilot study using nominal group technique to assess residents’ perceptions of successful attending rounds. J Gen Intern Med. 2008;23(7):1060-1065. PubMed
4. Siddiqui ZK, Wu AW, Kurbanova N, Qayyum R. Comparison of Hospital Consumer Assessment of Healthcare Providers and Systems patient satisfaction scores for specialty hospitals and general medical hospitals: confounding effect of survey response rate. J Hosp Med. 2014;9(9):590-593. PubMed
1. Monash B, Najafi N, Mourad M, et al. Standardized Attending Rounds to Improve the Patient Experience: A Pragmatic Cluster Randomized Controlled Trial. J Hosp Med. 2017;12(3):143-149. PubMed
2. Williams KN, Ramani S, Fraser B, Orlander JD. Improving bedside teaching: findings from a focus group study of learners. Acad Med. 2008;83(3):257-264. PubMed
3. Castiglioni A, Shewchuk RM, Willett LL, Heudebert GR, Centor RM. A pilot study using nominal group technique to assess residents’ perceptions of successful attending rounds. J Gen Intern Med. 2008;23(7):1060-1065. PubMed
4. Siddiqui ZK, Wu AW, Kurbanova N, Qayyum R. Comparison of Hospital Consumer Assessment of Healthcare Providers and Systems patient satisfaction scores for specialty hospitals and general medical hospitals: confounding effect of survey response rate. J Hosp Med. 2014;9(9):590-593. PubMed
The Authors Reply: “Cost and Utility of Thrombophilia Testing”
We thank Dr. Berse and colleagues for their correspondence about our paper.1,2 We are pleased they agreed with our conclusion: Thrombophilia testing has limited clinical utility in most inpatient settings.
Berse and colleagues critiqued details of our methodology in calculating payer cost, including how we estimated the number of Medicare claims for thrombophilia testing. We estimated that there were at least 280,000 Medicare claims in 2014 using CodeMap® (Wheaton Partners, LLC, Schaumburg, IL), a dataset of utilization data from the Physician Supplier Procedure Summary Master File from all Medicare Part B carriers.3 This estimate was similar to that reported in a previous publication.4
Thus, regardless of the precise estimates, even a conservative estimate of 33 to 80 million dollars of unnecessary spending is far too much. Rather, it is a perfect example of “Things We Do for No Reason.”
Disclosure
Nothing to report.
1. Petrilli CM, Mack M, Petrilli JJ, Hickner A, Saint S, Chopra V. Understanding the role of physician attire on patient perceptions: a systematic review of the literature--targeting attire to improve likelihood of rapport (TAILOR) investigators. BMJ Open. 2015;5(1):e006578. DOI:10.1136/bmjopen-2014-006578. PubMed
2. Berse B, Lynch JA, Bowen S, Grosse SD. In Reference to: “Cost and Utility of Thrombophilia Testing.” J Hosp Med. 2017;12(9):783.
3. CodeMap® https://www.codemap.com/. Accessed March 2, 2017.
4. Somma J, Sussman, II, Rand JH. An evaluation of thrombophilia screening in an urban tertiary care medical center: A “real world” experience. Am J Clin Pathol. 2006;126(1):120-7. DOI:10.1309/KV06-32LJ-8EDM-EWQT. PubMed
We thank Dr. Berse and colleagues for their correspondence about our paper.1,2 We are pleased they agreed with our conclusion: Thrombophilia testing has limited clinical utility in most inpatient settings.
Berse and colleagues critiqued details of our methodology in calculating payer cost, including how we estimated the number of Medicare claims for thrombophilia testing. We estimated that there were at least 280,000 Medicare claims in 2014 using CodeMap® (Wheaton Partners, LLC, Schaumburg, IL), a dataset of utilization data from the Physician Supplier Procedure Summary Master File from all Medicare Part B carriers.3 This estimate was similar to that reported in a previous publication.4
Thus, regardless of the precise estimates, even a conservative estimate of 33 to 80 million dollars of unnecessary spending is far too much. Rather, it is a perfect example of “Things We Do for No Reason.”
Disclosure
Nothing to report.
We thank Dr. Berse and colleagues for their correspondence about our paper.1,2 We are pleased they agreed with our conclusion: Thrombophilia testing has limited clinical utility in most inpatient settings.
Berse and colleagues critiqued details of our methodology in calculating payer cost, including how we estimated the number of Medicare claims for thrombophilia testing. We estimated that there were at least 280,000 Medicare claims in 2014 using CodeMap® (Wheaton Partners, LLC, Schaumburg, IL), a dataset of utilization data from the Physician Supplier Procedure Summary Master File from all Medicare Part B carriers.3 This estimate was similar to that reported in a previous publication.4
Thus, regardless of the precise estimates, even a conservative estimate of 33 to 80 million dollars of unnecessary spending is far too much. Rather, it is a perfect example of “Things We Do for No Reason.”
Disclosure
Nothing to report.
1. Petrilli CM, Mack M, Petrilli JJ, Hickner A, Saint S, Chopra V. Understanding the role of physician attire on patient perceptions: a systematic review of the literature--targeting attire to improve likelihood of rapport (TAILOR) investigators. BMJ Open. 2015;5(1):e006578. DOI:10.1136/bmjopen-2014-006578. PubMed
2. Berse B, Lynch JA, Bowen S, Grosse SD. In Reference to: “Cost and Utility of Thrombophilia Testing.” J Hosp Med. 2017;12(9):783.
3. CodeMap® https://www.codemap.com/. Accessed March 2, 2017.
4. Somma J, Sussman, II, Rand JH. An evaluation of thrombophilia screening in an urban tertiary care medical center: A “real world” experience. Am J Clin Pathol. 2006;126(1):120-7. DOI:10.1309/KV06-32LJ-8EDM-EWQT. PubMed
1. Petrilli CM, Mack M, Petrilli JJ, Hickner A, Saint S, Chopra V. Understanding the role of physician attire on patient perceptions: a systematic review of the literature--targeting attire to improve likelihood of rapport (TAILOR) investigators. BMJ Open. 2015;5(1):e006578. DOI:10.1136/bmjopen-2014-006578. PubMed
2. Berse B, Lynch JA, Bowen S, Grosse SD. In Reference to: “Cost and Utility of Thrombophilia Testing.” J Hosp Med. 2017;12(9):783.
3. CodeMap® https://www.codemap.com/. Accessed March 2, 2017.
4. Somma J, Sussman, II, Rand JH. An evaluation of thrombophilia screening in an urban tertiary care medical center: A “real world” experience. Am J Clin Pathol. 2006;126(1):120-7. DOI:10.1309/KV06-32LJ-8EDM-EWQT. PubMed
© 2017 Society of Hospital Medicine
In Reference to: “Cost and Utility of Thrombophilia Testing”
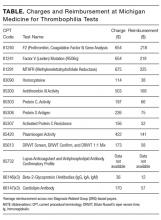
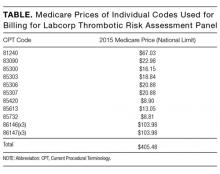
The article by Petrilli et al. points to the important but complicated issue of ordering laboratory testing for thrombophilia despite multiple guidelines that dispute the clinical utility of such testing for many indications.1 We question the basis of these authors’ assertion that Medicare spends $300 to $672 million for thrombophilia testing annually. They arrived at this figure by multiplying the price of a thrombophilia test panel (between $1100 and $2400) by the number of annual Medicare claims for thrombophilia analysis, which they estimated at 280,000. The price of the panel is derived from two papers: (1) a 2001 review2 that lists prices of various thrombophilia-related tests adding up to $1782, and (2) a 2006 evaluation by Somma et al.3 of thrombophilia screening at one hospital in New York in 2005. The latter paper refers to various thrombophilia panels from Quest Diagnostics with list prices ranging from $1311 to $2429. However, the repertoire of available test panels and their prices have changed over the last decade. The cost evaluation of thrombophilia testing should be based on actual current payments for tests, and not on list prices for laboratory offerings from over a decade ago. Several laboratories offer mutational analysis of 3 genes—F5, F2, and MTHFR—as a thrombophilia risk panel. Based on the Current Procedural Terminology (CPT) codes listed by the test suppliers (81240, 81241, and 81291), the average Medicare payment for the combination of these 3 markers in 2013 was $172.4 A broader panel of several biochemical, immunological, and genetic assays had a maximum Medicare payment in 2015 of $405 (Table).5
In conclusion, the cost evaluation of thrombophilia screening is more challenging than the calculation by Petrilli et al. suggests.1 Even if Medicare paid as much as $400 per individual tested and assuming up to 200,000 individuals underwent thrombophilia testing per year, the aggregate Medicare expenditure would have been no more than roughly $80 million. Thus, the estimated range in the article appears to have overstated actual Medicare expenditures by an order of magnitude. This does not take away from their overall conclusion that payers are burdened with significant expenditures for laboratory testing that may not present clinical value for many patients.6 We need research into the patterns of utilization as well as improvements in documentation of expenditures associated with these tests.
Disclosure
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, the Department of Veterans Affairs, or the United States government. The autho
1. Petrilli CM, Heidemann L, Mack M, Durance P, Chopra V. Inpatient inherited thrombophilia testing. J Hosp Med. 2016;11(11):801-804. PubMed
2. Abramson N, Abramson S. Hypercoagulability: clinical assessment and treatment. South Med J. 2001;94(10):1013-1020. PubMed
3. Somma J, Sussman, II, Rand JH. An evaluation of thrombophilia screening in an urban tertiary care medical center: A “real world” experience. Am J Clin Pathol. 2006;126(1):120-127. PubMed
4. Lynch JA, Berse B, Dotson WD, Khoury MJ, Coomer N, Kautter J. Utilization of genetic tests: Analysis of gene-specific billing in Medicare claims data [Published online ahead of print January 26, 2017]. Genet Med. 2017. doi: 10.1038/gim.2016.209. PubMed
5. Centers for Medicare and Medicaid Services. Clinical Laboratory Fee Schedule 2016. https://www.cms.gov/Medicare/Medicare-fee-for-service-Payment/clinicallabfeesched/index.html. Accessed on December 20, 2016.
6. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41(1):154-164. PubMed
The article by Petrilli et al. points to the important but complicated issue of ordering laboratory testing for thrombophilia despite multiple guidelines that dispute the clinical utility of such testing for many indications.1 We question the basis of these authors’ assertion that Medicare spends $300 to $672 million for thrombophilia testing annually. They arrived at this figure by multiplying the price of a thrombophilia test panel (between $1100 and $2400) by the number of annual Medicare claims for thrombophilia analysis, which they estimated at 280,000. The price of the panel is derived from two papers: (1) a 2001 review2 that lists prices of various thrombophilia-related tests adding up to $1782, and (2) a 2006 evaluation by Somma et al.3 of thrombophilia screening at one hospital in New York in 2005. The latter paper refers to various thrombophilia panels from Quest Diagnostics with list prices ranging from $1311 to $2429. However, the repertoire of available test panels and their prices have changed over the last decade. The cost evaluation of thrombophilia testing should be based on actual current payments for tests, and not on list prices for laboratory offerings from over a decade ago. Several laboratories offer mutational analysis of 3 genes—F5, F2, and MTHFR—as a thrombophilia risk panel. Based on the Current Procedural Terminology (CPT) codes listed by the test suppliers (81240, 81241, and 81291), the average Medicare payment for the combination of these 3 markers in 2013 was $172.4 A broader panel of several biochemical, immunological, and genetic assays had a maximum Medicare payment in 2015 of $405 (Table).5
In conclusion, the cost evaluation of thrombophilia screening is more challenging than the calculation by Petrilli et al. suggests.1 Even if Medicare paid as much as $400 per individual tested and assuming up to 200,000 individuals underwent thrombophilia testing per year, the aggregate Medicare expenditure would have been no more than roughly $80 million. Thus, the estimated range in the article appears to have overstated actual Medicare expenditures by an order of magnitude. This does not take away from their overall conclusion that payers are burdened with significant expenditures for laboratory testing that may not present clinical value for many patients.6 We need research into the patterns of utilization as well as improvements in documentation of expenditures associated with these tests.
Disclosure
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, the Department of Veterans Affairs, or the United States government. The autho
The article by Petrilli et al. points to the important but complicated issue of ordering laboratory testing for thrombophilia despite multiple guidelines that dispute the clinical utility of such testing for many indications.1 We question the basis of these authors’ assertion that Medicare spends $300 to $672 million for thrombophilia testing annually. They arrived at this figure by multiplying the price of a thrombophilia test panel (between $1100 and $2400) by the number of annual Medicare claims for thrombophilia analysis, which they estimated at 280,000. The price of the panel is derived from two papers: (1) a 2001 review2 that lists prices of various thrombophilia-related tests adding up to $1782, and (2) a 2006 evaluation by Somma et al.3 of thrombophilia screening at one hospital in New York in 2005. The latter paper refers to various thrombophilia panels from Quest Diagnostics with list prices ranging from $1311 to $2429. However, the repertoire of available test panels and their prices have changed over the last decade. The cost evaluation of thrombophilia testing should be based on actual current payments for tests, and not on list prices for laboratory offerings from over a decade ago. Several laboratories offer mutational analysis of 3 genes—F5, F2, and MTHFR—as a thrombophilia risk panel. Based on the Current Procedural Terminology (CPT) codes listed by the test suppliers (81240, 81241, and 81291), the average Medicare payment for the combination of these 3 markers in 2013 was $172.4 A broader panel of several biochemical, immunological, and genetic assays had a maximum Medicare payment in 2015 of $405 (Table).5
In conclusion, the cost evaluation of thrombophilia screening is more challenging than the calculation by Petrilli et al. suggests.1 Even if Medicare paid as much as $400 per individual tested and assuming up to 200,000 individuals underwent thrombophilia testing per year, the aggregate Medicare expenditure would have been no more than roughly $80 million. Thus, the estimated range in the article appears to have overstated actual Medicare expenditures by an order of magnitude. This does not take away from their overall conclusion that payers are burdened with significant expenditures for laboratory testing that may not present clinical value for many patients.6 We need research into the patterns of utilization as well as improvements in documentation of expenditures associated with these tests.
Disclosure
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, the Department of Veterans Affairs, or the United States government. The autho
1. Petrilli CM, Heidemann L, Mack M, Durance P, Chopra V. Inpatient inherited thrombophilia testing. J Hosp Med. 2016;11(11):801-804. PubMed
2. Abramson N, Abramson S. Hypercoagulability: clinical assessment and treatment. South Med J. 2001;94(10):1013-1020. PubMed
3. Somma J, Sussman, II, Rand JH. An evaluation of thrombophilia screening in an urban tertiary care medical center: A “real world” experience. Am J Clin Pathol. 2006;126(1):120-127. PubMed
4. Lynch JA, Berse B, Dotson WD, Khoury MJ, Coomer N, Kautter J. Utilization of genetic tests: Analysis of gene-specific billing in Medicare claims data [Published online ahead of print January 26, 2017]. Genet Med. 2017. doi: 10.1038/gim.2016.209. PubMed
5. Centers for Medicare and Medicaid Services. Clinical Laboratory Fee Schedule 2016. https://www.cms.gov/Medicare/Medicare-fee-for-service-Payment/clinicallabfeesched/index.html. Accessed on December 20, 2016.
6. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41(1):154-164. PubMed
1. Petrilli CM, Heidemann L, Mack M, Durance P, Chopra V. Inpatient inherited thrombophilia testing. J Hosp Med. 2016;11(11):801-804. PubMed
2. Abramson N, Abramson S. Hypercoagulability: clinical assessment and treatment. South Med J. 2001;94(10):1013-1020. PubMed
3. Somma J, Sussman, II, Rand JH. An evaluation of thrombophilia screening in an urban tertiary care medical center: A “real world” experience. Am J Clin Pathol. 2006;126(1):120-127. PubMed
4. Lynch JA, Berse B, Dotson WD, Khoury MJ, Coomer N, Kautter J. Utilization of genetic tests: Analysis of gene-specific billing in Medicare claims data [Published online ahead of print January 26, 2017]. Genet Med. 2017. doi: 10.1038/gim.2016.209. PubMed
5. Centers for Medicare and Medicaid Services. Clinical Laboratory Fee Schedule 2016. https://www.cms.gov/Medicare/Medicare-fee-for-service-Payment/clinicallabfeesched/index.html. Accessed on December 20, 2016.
6. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41(1):154-164. PubMed
© 2017 Society of Hospital Medicine
Why extended release metformin?
“TREATING POLYCYSTIC OVARY SYNDROME: START USING DUAL MEDICAL THERAPY”
ROBERT L. BARBIERI, MD (EDITORIAL; APRIL 2017)
Why extended release metformin?
I read with interest Dr. Barbieri’s editorial on polycystic ovary syndrome. It left me wondering: Is there a metabolic or pharmacologic reason why you give metformin XR 1,500 mg with dinner instead of 750 mg orally twice per day?
Marcelo Andreoli, MD
Vienna, Virginia
Dr. Barbieri responds
I thank Dr. Andreoli for the important clinical question about one-time or multiple dosing of metformin. To improve patient adherence with metformin treatment, I think once-daily dosing at dinner with an extended-release formulation is more convenient than twice-daily dosing with immediate-release metformin. Following ingestion of immediate- or extended-release metformin, peak metformin blood concentrations are achieved after 2 and 7 hours, respectively.1 There is some evidence that extended-release metformin has fewer gastrointestinal (GI) adverse effects than immediate-release metformin.2 In one study, the reported rates of GI adverse effects were 29% versus 39% with extended-release and immediate-release formulations, respectively.2
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Ali S, Fonseca V. Overview of metformin: special focus on metformin extended release. Expert Opin Pharmacother. 2012;13(12):1797–1805.
- Fujioka K, Pans M, Joyal S. Glycemic control in patients with type 2 diabetes mellitus switched from twice-daily immediate-release metformin to a once-daily extended-release formulation. Clin Ther. 2003;25(2):515–529.
“TREATING POLYCYSTIC OVARY SYNDROME: START USING DUAL MEDICAL THERAPY”
ROBERT L. BARBIERI, MD (EDITORIAL; APRIL 2017)
Why extended release metformin?
I read with interest Dr. Barbieri’s editorial on polycystic ovary syndrome. It left me wondering: Is there a metabolic or pharmacologic reason why you give metformin XR 1,500 mg with dinner instead of 750 mg orally twice per day?
Marcelo Andreoli, MD
Vienna, Virginia
Dr. Barbieri responds
I thank Dr. Andreoli for the important clinical question about one-time or multiple dosing of metformin. To improve patient adherence with metformin treatment, I think once-daily dosing at dinner with an extended-release formulation is more convenient than twice-daily dosing with immediate-release metformin. Following ingestion of immediate- or extended-release metformin, peak metformin blood concentrations are achieved after 2 and 7 hours, respectively.1 There is some evidence that extended-release metformin has fewer gastrointestinal (GI) adverse effects than immediate-release metformin.2 In one study, the reported rates of GI adverse effects were 29% versus 39% with extended-release and immediate-release formulations, respectively.2
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“TREATING POLYCYSTIC OVARY SYNDROME: START USING DUAL MEDICAL THERAPY”
ROBERT L. BARBIERI, MD (EDITORIAL; APRIL 2017)
Why extended release metformin?
I read with interest Dr. Barbieri’s editorial on polycystic ovary syndrome. It left me wondering: Is there a metabolic or pharmacologic reason why you give metformin XR 1,500 mg with dinner instead of 750 mg orally twice per day?
Marcelo Andreoli, MD
Vienna, Virginia
Dr. Barbieri responds
I thank Dr. Andreoli for the important clinical question about one-time or multiple dosing of metformin. To improve patient adherence with metformin treatment, I think once-daily dosing at dinner with an extended-release formulation is more convenient than twice-daily dosing with immediate-release metformin. Following ingestion of immediate- or extended-release metformin, peak metformin blood concentrations are achieved after 2 and 7 hours, respectively.1 There is some evidence that extended-release metformin has fewer gastrointestinal (GI) adverse effects than immediate-release metformin.2 In one study, the reported rates of GI adverse effects were 29% versus 39% with extended-release and immediate-release formulations, respectively.2
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Ali S, Fonseca V. Overview of metformin: special focus on metformin extended release. Expert Opin Pharmacother. 2012;13(12):1797–1805.
- Fujioka K, Pans M, Joyal S. Glycemic control in patients with type 2 diabetes mellitus switched from twice-daily immediate-release metformin to a once-daily extended-release formulation. Clin Ther. 2003;25(2):515–529.
- Ali S, Fonseca V. Overview of metformin: special focus on metformin extended release. Expert Opin Pharmacother. 2012;13(12):1797–1805.
- Fujioka K, Pans M, Joyal S. Glycemic control in patients with type 2 diabetes mellitus switched from twice-daily immediate-release metformin to a once-daily extended-release formulation. Clin Ther. 2003;25(2):515–529.
Look for symptoms of IBS, PCOS, and PMS
“WHY ARE THERE DELAYS IN THE DIAGNOSIS OF ENDOMETRIOSIS?”
ROBERT L. BARBIERI, MD (EDITORIAL; MARCH 2017)
Look for symptoms of IBS, PCOS, and PMS
I practiced reproductive endocrinology for 40 years and saw too many patients whose endometriosis had been ignored or undertreated. I found that the initial suspicion for the disease could be discovered by looking for symptoms of 3 comorbidities: irritable bowel syndrome, polycystic ovary syndrome, and premenstrual syndrome.
Wilbur (Dub) Howard, MD
Dallas, Texas
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“WHY ARE THERE DELAYS IN THE DIAGNOSIS OF ENDOMETRIOSIS?”
ROBERT L. BARBIERI, MD (EDITORIAL; MARCH 2017)
Look for symptoms of IBS, PCOS, and PMS
I practiced reproductive endocrinology for 40 years and saw too many patients whose endometriosis had been ignored or undertreated. I found that the initial suspicion for the disease could be discovered by looking for symptoms of 3 comorbidities: irritable bowel syndrome, polycystic ovary syndrome, and premenstrual syndrome.
Wilbur (Dub) Howard, MD
Dallas, Texas
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“WHY ARE THERE DELAYS IN THE DIAGNOSIS OF ENDOMETRIOSIS?”
ROBERT L. BARBIERI, MD (EDITORIAL; MARCH 2017)
Look for symptoms of IBS, PCOS, and PMS
I practiced reproductive endocrinology for 40 years and saw too many patients whose endometriosis had been ignored or undertreated. I found that the initial suspicion for the disease could be discovered by looking for symptoms of 3 comorbidities: irritable bowel syndrome, polycystic ovary syndrome, and premenstrual syndrome.
Wilbur (Dub) Howard, MD
Dallas, Texas
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
The fallopian tube should have been removed
OBIANUJU SANDRA MADUEKE-LAVEAUX, MD, MPH; BETH W. RACKOW, MD; AND ARNOLD P. ADVINCULA, MD (VIDEO; JANUARY 2017)
The fallopian tube should have been removed
I watched the video by Dr. Advincula and colleagues and as always was impressed with the surgical skills demonstrated. While the robot-assisted approach is quite nice, this case could have been accomplished with only three 5-mm lower abdominal port sites and traditional straight-stick laparoscopic methods. The cosmetic benefit to a 15-year-old patient of this alternative should have been considered.
More importantly, the fallopian tube separated from the rudimentary horn should have been removed. Leaving the right tube in situ exposes the patient to the possibility of a future ectopic pregnancy in that tube and provides no benefit to the patient.
David L. Zisow, MD
Baltimore, Maryland
Dr. Advincula and team respond
We appreciate Dr. Zisow’s perspective. As is known, tool selection is based on surgeon preference. Inherent to this point, a discussion about route of surgery, and any implications it would have, such as cosmesis, was had. Cosmesis was not an issue with this patient, and she was quite pleased with her cosmetic outcome.
We also discussed preoperatively, among our team and with the patient, the right fallopian tube. Although removal would have been optimal, there was concern intraoperatively of possible compromise to the ovary. Hence, a decision was made to forego removal particularly in light of the extremely rare risk of transperitoneal migration of spermatozoa weighed against the risk of compromising a perfectly healthy ovary in a 15-year-old woman.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
OBIANUJU SANDRA MADUEKE-LAVEAUX, MD, MPH; BETH W. RACKOW, MD; AND ARNOLD P. ADVINCULA, MD (VIDEO; JANUARY 2017)
The fallopian tube should have been removed
I watched the video by Dr. Advincula and colleagues and as always was impressed with the surgical skills demonstrated. While the robot-assisted approach is quite nice, this case could have been accomplished with only three 5-mm lower abdominal port sites and traditional straight-stick laparoscopic methods. The cosmetic benefit to a 15-year-old patient of this alternative should have been considered.
More importantly, the fallopian tube separated from the rudimentary horn should have been removed. Leaving the right tube in situ exposes the patient to the possibility of a future ectopic pregnancy in that tube and provides no benefit to the patient.
David L. Zisow, MD
Baltimore, Maryland
Dr. Advincula and team respond
We appreciate Dr. Zisow’s perspective. As is known, tool selection is based on surgeon preference. Inherent to this point, a discussion about route of surgery, and any implications it would have, such as cosmesis, was had. Cosmesis was not an issue with this patient, and she was quite pleased with her cosmetic outcome.
We also discussed preoperatively, among our team and with the patient, the right fallopian tube. Although removal would have been optimal, there was concern intraoperatively of possible compromise to the ovary. Hence, a decision was made to forego removal particularly in light of the extremely rare risk of transperitoneal migration of spermatozoa weighed against the risk of compromising a perfectly healthy ovary in a 15-year-old woman.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
OBIANUJU SANDRA MADUEKE-LAVEAUX, MD, MPH; BETH W. RACKOW, MD; AND ARNOLD P. ADVINCULA, MD (VIDEO; JANUARY 2017)
The fallopian tube should have been removed
I watched the video by Dr. Advincula and colleagues and as always was impressed with the surgical skills demonstrated. While the robot-assisted approach is quite nice, this case could have been accomplished with only three 5-mm lower abdominal port sites and traditional straight-stick laparoscopic methods. The cosmetic benefit to a 15-year-old patient of this alternative should have been considered.
More importantly, the fallopian tube separated from the rudimentary horn should have been removed. Leaving the right tube in situ exposes the patient to the possibility of a future ectopic pregnancy in that tube and provides no benefit to the patient.
David L. Zisow, MD
Baltimore, Maryland
Dr. Advincula and team respond
We appreciate Dr. Zisow’s perspective. As is known, tool selection is based on surgeon preference. Inherent to this point, a discussion about route of surgery, and any implications it would have, such as cosmesis, was had. Cosmesis was not an issue with this patient, and she was quite pleased with her cosmetic outcome.
We also discussed preoperatively, among our team and with the patient, the right fallopian tube. Although removal would have been optimal, there was concern intraoperatively of possible compromise to the ovary. Hence, a decision was made to forego removal particularly in light of the extremely rare risk of transperitoneal migration of spermatozoa weighed against the risk of compromising a perfectly healthy ovary in a 15-year-old woman.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.