User login
Coaching in medicine: A perspective
Coaching is a new topic in medicine. I first heard about coaching several years ago and met the term with skepticism. I was unsure how coaching was different than mentoring or advising and I wondered about its usefulness. However, the reason that I even started to learn about coaching was because I was struggling. I had finally arrived in my career, I had my dream job with two healthy kids, a perfect house, and good marriage. I kept hearing the refrain in my head: “Is this all there is?” I had this arrival fallacy that after all this striving and straining that I would finally be content. I felt unfulfilled and was dissatisfied with where I was that was affecting all parts of my life.
As I was wrestling with these thoughts, I had an opportunity to become a coach to residents around the country through the Association of Women Surgeons. I discussed with them what fills them up, what gets them down, how to set goals, and what their goals were for the year, as well as imposter syndrome. Impostor syndrome is defined as a pattern in which an individual doubts their accomplishments or talents and has a persistent internalized fear of being exposed as a “fraud.” Despite external evidence of their competence, those experiencing this phenomenon remain convinced that they are fooling everyone around them and do not deserve all they have achieved. Individuals incorrectly attribute their success to luck or interpret it as a result of deceiving others into thinking they are more intelligent than they perceive themselves to be. Imposter syndrome is prevalent and deep in medicine. As perfectionists, we are especially vulnerable to imposter syndrome as we set unrealistic ideals for ourselves. When we fail to reach these ideals, we feel like frauds, setting up this cycle of self-doubt that is toxic. When we feel that we can’t achieve the goals that we are striving for we will always find ourselves lacking. There is a slow, insidious erosion of self over the years. Imposter syndrome is well documented in medicine and is even felt as early as medical school.1,2
When I began coaching these residents the most profound thing that came out of these sessions was that my life was getting better – I knew what filled me up, what got me down, what my goals were for the year, and how I still deal with imposter syndrome. Coaching gave me a framework for helping determine what I wanted for the rest of my life. As I began coaching, I started learning all the ways in which I could figure out my values, my personal and professional goals, and perhaps most importantly, my relationships with myself and others.
Another perspective on coaching is to look at a professional athlete such as Tom Brady, one of the greatest quarterbacks of all time. He has a quarterback coach. No coach is going to be a better quarterback than Tom Brady. A coach for him is to be there as an advocate, break his fundamentals down technically, and help him improve upon what he already knows. A coach also identifies strengths and weaknesses, and helps him capitalize on both by bringing awareness, reflection, accountability, and support. If world-class athletes still want and benefit from coaching in a sport they have already mastered, coaching for physicians is just another tool to help us improve our abilities in and out of medicine.
Coaching is more encompassing than advising or mentoring. It is about examining deeply held beliefs to see if they are really serving us, if they are in line with our values and how we want to live our lives.
Coaching has also been validated in medicine in several papers. In an article by Dyrbye et al. in JAMA Internal Medicine, measures of emotional exhaustion and burnout decreased in physicians who were coached and increased in those who were not.3 In another study from this year by McGonagle et al., a randomized, controlled trial showed that primary care physicians who had sessions (as short as 6 weeks) to address burnout, psychological capital, and job satisfaction experienced an improvement in measures which persisted for 6 months after intervention.4 Numerous other articles in medicine also exist to demonstrate the effect of coaching on mitigating burnout at an institutional level.
Physicians are inherently driven by their love of learning. As physicians, we love getting to the root cause of any problem and coming up with creative solutions. Any challenge we have, or just wanting to improve the quality of our lives, can be addressed with coaching. As perpetual students we can use coaching to truly master ourselves.
Dr. Shah is associate professor of surgery, Rush University Medical Center, Chicago. Instagram: ami.shahmdcoaching.
References
1. Gottlieb M et al. Med Educ. 2020 Feb;54(2):116-24.
2. Villwock JA et al. Int J Med Educ. 2016 Oct 31;7:364-9.
3. Dyrbye LN et al. JAMA Intern Med. 2019 Aug 5;179(10):1406-14.
4. McGonagle AK et al. J Occup Health Psychol. 2020 Apr 16. doi: 10.1037/ocp0000180.
Coaching is a new topic in medicine. I first heard about coaching several years ago and met the term with skepticism. I was unsure how coaching was different than mentoring or advising and I wondered about its usefulness. However, the reason that I even started to learn about coaching was because I was struggling. I had finally arrived in my career, I had my dream job with two healthy kids, a perfect house, and good marriage. I kept hearing the refrain in my head: “Is this all there is?” I had this arrival fallacy that after all this striving and straining that I would finally be content. I felt unfulfilled and was dissatisfied with where I was that was affecting all parts of my life.
As I was wrestling with these thoughts, I had an opportunity to become a coach to residents around the country through the Association of Women Surgeons. I discussed with them what fills them up, what gets them down, how to set goals, and what their goals were for the year, as well as imposter syndrome. Impostor syndrome is defined as a pattern in which an individual doubts their accomplishments or talents and has a persistent internalized fear of being exposed as a “fraud.” Despite external evidence of their competence, those experiencing this phenomenon remain convinced that they are fooling everyone around them and do not deserve all they have achieved. Individuals incorrectly attribute their success to luck or interpret it as a result of deceiving others into thinking they are more intelligent than they perceive themselves to be. Imposter syndrome is prevalent and deep in medicine. As perfectionists, we are especially vulnerable to imposter syndrome as we set unrealistic ideals for ourselves. When we fail to reach these ideals, we feel like frauds, setting up this cycle of self-doubt that is toxic. When we feel that we can’t achieve the goals that we are striving for we will always find ourselves lacking. There is a slow, insidious erosion of self over the years. Imposter syndrome is well documented in medicine and is even felt as early as medical school.1,2
When I began coaching these residents the most profound thing that came out of these sessions was that my life was getting better – I knew what filled me up, what got me down, what my goals were for the year, and how I still deal with imposter syndrome. Coaching gave me a framework for helping determine what I wanted for the rest of my life. As I began coaching, I started learning all the ways in which I could figure out my values, my personal and professional goals, and perhaps most importantly, my relationships with myself and others.
Another perspective on coaching is to look at a professional athlete such as Tom Brady, one of the greatest quarterbacks of all time. He has a quarterback coach. No coach is going to be a better quarterback than Tom Brady. A coach for him is to be there as an advocate, break his fundamentals down technically, and help him improve upon what he already knows. A coach also identifies strengths and weaknesses, and helps him capitalize on both by bringing awareness, reflection, accountability, and support. If world-class athletes still want and benefit from coaching in a sport they have already mastered, coaching for physicians is just another tool to help us improve our abilities in and out of medicine.
Coaching is more encompassing than advising or mentoring. It is about examining deeply held beliefs to see if they are really serving us, if they are in line with our values and how we want to live our lives.
Coaching has also been validated in medicine in several papers. In an article by Dyrbye et al. in JAMA Internal Medicine, measures of emotional exhaustion and burnout decreased in physicians who were coached and increased in those who were not.3 In another study from this year by McGonagle et al., a randomized, controlled trial showed that primary care physicians who had sessions (as short as 6 weeks) to address burnout, psychological capital, and job satisfaction experienced an improvement in measures which persisted for 6 months after intervention.4 Numerous other articles in medicine also exist to demonstrate the effect of coaching on mitigating burnout at an institutional level.
Physicians are inherently driven by their love of learning. As physicians, we love getting to the root cause of any problem and coming up with creative solutions. Any challenge we have, or just wanting to improve the quality of our lives, can be addressed with coaching. As perpetual students we can use coaching to truly master ourselves.
Dr. Shah is associate professor of surgery, Rush University Medical Center, Chicago. Instagram: ami.shahmdcoaching.
References
1. Gottlieb M et al. Med Educ. 2020 Feb;54(2):116-24.
2. Villwock JA et al. Int J Med Educ. 2016 Oct 31;7:364-9.
3. Dyrbye LN et al. JAMA Intern Med. 2019 Aug 5;179(10):1406-14.
4. McGonagle AK et al. J Occup Health Psychol. 2020 Apr 16. doi: 10.1037/ocp0000180.
Coaching is a new topic in medicine. I first heard about coaching several years ago and met the term with skepticism. I was unsure how coaching was different than mentoring or advising and I wondered about its usefulness. However, the reason that I even started to learn about coaching was because I was struggling. I had finally arrived in my career, I had my dream job with two healthy kids, a perfect house, and good marriage. I kept hearing the refrain in my head: “Is this all there is?” I had this arrival fallacy that after all this striving and straining that I would finally be content. I felt unfulfilled and was dissatisfied with where I was that was affecting all parts of my life.
As I was wrestling with these thoughts, I had an opportunity to become a coach to residents around the country through the Association of Women Surgeons. I discussed with them what fills them up, what gets them down, how to set goals, and what their goals were for the year, as well as imposter syndrome. Impostor syndrome is defined as a pattern in which an individual doubts their accomplishments or talents and has a persistent internalized fear of being exposed as a “fraud.” Despite external evidence of their competence, those experiencing this phenomenon remain convinced that they are fooling everyone around them and do not deserve all they have achieved. Individuals incorrectly attribute their success to luck or interpret it as a result of deceiving others into thinking they are more intelligent than they perceive themselves to be. Imposter syndrome is prevalent and deep in medicine. As perfectionists, we are especially vulnerable to imposter syndrome as we set unrealistic ideals for ourselves. When we fail to reach these ideals, we feel like frauds, setting up this cycle of self-doubt that is toxic. When we feel that we can’t achieve the goals that we are striving for we will always find ourselves lacking. There is a slow, insidious erosion of self over the years. Imposter syndrome is well documented in medicine and is even felt as early as medical school.1,2
When I began coaching these residents the most profound thing that came out of these sessions was that my life was getting better – I knew what filled me up, what got me down, what my goals were for the year, and how I still deal with imposter syndrome. Coaching gave me a framework for helping determine what I wanted for the rest of my life. As I began coaching, I started learning all the ways in which I could figure out my values, my personal and professional goals, and perhaps most importantly, my relationships with myself and others.
Another perspective on coaching is to look at a professional athlete such as Tom Brady, one of the greatest quarterbacks of all time. He has a quarterback coach. No coach is going to be a better quarterback than Tom Brady. A coach for him is to be there as an advocate, break his fundamentals down technically, and help him improve upon what he already knows. A coach also identifies strengths and weaknesses, and helps him capitalize on both by bringing awareness, reflection, accountability, and support. If world-class athletes still want and benefit from coaching in a sport they have already mastered, coaching for physicians is just another tool to help us improve our abilities in and out of medicine.
Coaching is more encompassing than advising or mentoring. It is about examining deeply held beliefs to see if they are really serving us, if they are in line with our values and how we want to live our lives.
Coaching has also been validated in medicine in several papers. In an article by Dyrbye et al. in JAMA Internal Medicine, measures of emotional exhaustion and burnout decreased in physicians who were coached and increased in those who were not.3 In another study from this year by McGonagle et al., a randomized, controlled trial showed that primary care physicians who had sessions (as short as 6 weeks) to address burnout, psychological capital, and job satisfaction experienced an improvement in measures which persisted for 6 months after intervention.4 Numerous other articles in medicine also exist to demonstrate the effect of coaching on mitigating burnout at an institutional level.
Physicians are inherently driven by their love of learning. As physicians, we love getting to the root cause of any problem and coming up with creative solutions. Any challenge we have, or just wanting to improve the quality of our lives, can be addressed with coaching. As perpetual students we can use coaching to truly master ourselves.
Dr. Shah is associate professor of surgery, Rush University Medical Center, Chicago. Instagram: ami.shahmdcoaching.
References
1. Gottlieb M et al. Med Educ. 2020 Feb;54(2):116-24.
2. Villwock JA et al. Int J Med Educ. 2016 Oct 31;7:364-9.
3. Dyrbye LN et al. JAMA Intern Med. 2019 Aug 5;179(10):1406-14.
4. McGonagle AK et al. J Occup Health Psychol. 2020 Apr 16. doi: 10.1037/ocp0000180.
Understand the legal implications of telehealth medicine
Telehealth has been steadily gaining mainstream use throughout the last decade, but the practice was recently shoved, almost overnight, into the forefront of the health care profession. Telehealth is now used more frequently by medical groups and physicians than ever before. General reports before the COVID-19 pandemic approximated 90% of health care organizations used or planned to use telehealth in the future. This future may already be a reality, with a McKinsey & Company report estimating that physicians saw 50-175 times more patients over telehealth platforms since the pandemic’s start.1
In general, telehealth includes use of electronic communication and information technologies to deliver long-distance or remote health care. A physician’s use of telemedicine (clinical services) is one of the most common uses, but the industry also includes other professionals, such as pharmacists and nurses.
Telehealth platforms can be used to monitor, diagnose, treat, and counsel patients successfully. It works best for reading images, follow-up care, outpatient care, and long-term care. However, telemedicine is inappropriate for urgent issues, diagnosing underlying health conditions, or any practice where the standard of care would require a physical exam. There is potential liability for decision making without a proper physical exam.
There are many advantages to telehealth over more traditional health care options. Some of these advantages include:
- Increased access to health care.
- Increased access to medical specialists in small and rural communities.
- Improved long-term care from the comfort of patients’ homes.
- Improved platforms to document patient care outside regular business hours.
But along with these benefits, telehealth carries the disadvantage of potential increased liability. This increased liability could stem from:
- Breached standards of care.
- Inadequate or improper licensing.
- Limited care options.
- Decision making without a proper physical exam.
- Increased informed consent requirements.
- Restricted prescription access.
Before expanding any practice into telemedicine, awareness of potential legal issues is crucial.
Standard of care
Currently, telehealth laws and regulations vary significantly from state to state. But one rule is consistent across the board – that the standard of care for practicing medicine through telemedicine is identical to the standard of care required for practicing medicine during physical practice. It still requires the appropriate examination, testing, labs, imaging, and consultations that any in-person diagnosis needs. For physicians, it also includes supervising nonphysician clinicians, where state law requires supervision.
The American Telemedicine Association currently determines the primary governing standards and guidelines for telemedicine. These can help physicians understand best practices in meeting the standard of care through telemedicine. The American Gastroenterological Association provides coding guidelines and other resources to help physicians with telehealth and e-visits. Other professional societies, such as the American College of Radiology and the American Academy of Dermatology, offer guidelines specific to their medical specialties’ standards of care. These standards still vary from state to state, so medical professionals must be aware of any differences before treating patients in multiple states.
Licensing
Licensing is one of telemedicine’s most confusing legal issues. All states require a license to practice medicine (traditional or telehealth) within their borders. Without that license, practicing medicine in the state is a crime. On top of being criminal, unlicensed practice can affect insurance, liability, billing, and malpractice coverage. When in a brick-and-mortar clinic, a physician’s confidence in practicing within the licensed jurisdiction is easy. Now, the distinction is not so clear. Patients and physicians no longer have to be in the same room, city, or even state, meaning there could be unknown conflicting laws between the two locations. With rare exceptions, standards of care are based on the patient’s location, not the physician’s location. This increases the risk of practicing without being correctly licensed to higher than ever.
Because licensing is a significant roadblock in providing telemedicine, efforts are underway to make the process simpler and more streamlined. The Federation of State Medical Boards developed the Interstate Medical Licensure Compact (IMLC).2 This can qualify physicians to practice medicine across state lines within the compact so long as they meet specific eligibility requirements. The IMLC creates a fast-track option for physicians to fill out one application and receive licenses from multiple states at once. Currently, the compact includes 32 states, the District of Columbia, and Guam.3
Informed consent
Telemedicine health care still requires informed consent from patients. In fact, in some states, the requirements for care provided through telehealth are actually stricter than requirements for informed consent obtained in person.
Most informed consent laws require physicians to cover the risks and benefits of a recommended course of treatment and all feasible and reasonable material alternatives. On top of this traditional informed consent, physicians must get additional consent to receive care over a telehealth platform. This unique requirement explains what telehealth is, possible risks and expected benefits, and security measures used to protect patient information. States vary regarding when verbal consent is sufficient, and when written consent is required.
Prescriptions
Telemedicine is still a relatively new industry, and few legal opinions specifically address telemedicine malpractice. However, prescribing medication based on telemedicine information is among the few issues the courts have addressed. A 2008 decision found that a physician review of patient questionnaires submitted over the Internet was insufficient to prescribe medication without a physical examination determining patient health.4 This cautious approach stemmed from telehealth’s early concern about the absence of patient-physician relationships and potential online pharmacy abuse. Since this decision, many states require an “in-person” visit with a patient before prescribing medication. The definition of what qualifies as an in-person visit varies from state to state – some still consider the use of real-time, audiovisual conferencing sufficient.
The law is still evolving for prescriptions. Some states don’t allow any prescriptions, while others allow physicians to prescribe their patients’ medications as part of an appropriate treatment plan according to their professional discretion. Almost every state prohibits the prescription of controlled substances based on telemedicine.
Conclusion
Telemedicine is becoming an increasingly significant part of both physician-patient relationships and the broader health care industry. Used appropriately, it can be an incredibly effective method of care for physicians and patients. Physicians should learn the laws governing telemedicine in every state they want to practice and continue to stay current on any changes. The Center for Connected Health Policy offers a report, updated semiannually, to help physicians stay up to date on their state laws. These efforts will help prevent physicians from exposure to liability and medical malpractice claims.
Mr. Hyde is a partner at Younker Hyde Macfarlane, a law firm that focuses on prosecuting medical malpractice claims on behalf of injured patients. Ms. Johnson is an associate attorney with the firm. You can find them at YHMLaw.com.
References
1. Bestsennyy O, Harris A, Rost J. Telehealth: A quarter-trillion-dollar post-COVID-19 reality? Mckinsey & Company, May 29, 2020.
2. FSMB: Draft Interstate Compact for Physician Licensure Nears Completion, 2014.
3. Interstate Medical Licensure Compact: U.S. State Participation in the Compact.
4. See, Low Cost Pharm., Inc. v. Ariz. State Bd. Of Pharm, 2008 Ariz. App. Unpub. LEXIS 790, referencing conclusion of Arizona Medical Board.
Telehealth has been steadily gaining mainstream use throughout the last decade, but the practice was recently shoved, almost overnight, into the forefront of the health care profession. Telehealth is now used more frequently by medical groups and physicians than ever before. General reports before the COVID-19 pandemic approximated 90% of health care organizations used or planned to use telehealth in the future. This future may already be a reality, with a McKinsey & Company report estimating that physicians saw 50-175 times more patients over telehealth platforms since the pandemic’s start.1
In general, telehealth includes use of electronic communication and information technologies to deliver long-distance or remote health care. A physician’s use of telemedicine (clinical services) is one of the most common uses, but the industry also includes other professionals, such as pharmacists and nurses.
Telehealth platforms can be used to monitor, diagnose, treat, and counsel patients successfully. It works best for reading images, follow-up care, outpatient care, and long-term care. However, telemedicine is inappropriate for urgent issues, diagnosing underlying health conditions, or any practice where the standard of care would require a physical exam. There is potential liability for decision making without a proper physical exam.
There are many advantages to telehealth over more traditional health care options. Some of these advantages include:
- Increased access to health care.
- Increased access to medical specialists in small and rural communities.
- Improved long-term care from the comfort of patients’ homes.
- Improved platforms to document patient care outside regular business hours.
But along with these benefits, telehealth carries the disadvantage of potential increased liability. This increased liability could stem from:
- Breached standards of care.
- Inadequate or improper licensing.
- Limited care options.
- Decision making without a proper physical exam.
- Increased informed consent requirements.
- Restricted prescription access.
Before expanding any practice into telemedicine, awareness of potential legal issues is crucial.
Standard of care
Currently, telehealth laws and regulations vary significantly from state to state. But one rule is consistent across the board – that the standard of care for practicing medicine through telemedicine is identical to the standard of care required for practicing medicine during physical practice. It still requires the appropriate examination, testing, labs, imaging, and consultations that any in-person diagnosis needs. For physicians, it also includes supervising nonphysician clinicians, where state law requires supervision.
The American Telemedicine Association currently determines the primary governing standards and guidelines for telemedicine. These can help physicians understand best practices in meeting the standard of care through telemedicine. The American Gastroenterological Association provides coding guidelines and other resources to help physicians with telehealth and e-visits. Other professional societies, such as the American College of Radiology and the American Academy of Dermatology, offer guidelines specific to their medical specialties’ standards of care. These standards still vary from state to state, so medical professionals must be aware of any differences before treating patients in multiple states.
Licensing
Licensing is one of telemedicine’s most confusing legal issues. All states require a license to practice medicine (traditional or telehealth) within their borders. Without that license, practicing medicine in the state is a crime. On top of being criminal, unlicensed practice can affect insurance, liability, billing, and malpractice coverage. When in a brick-and-mortar clinic, a physician’s confidence in practicing within the licensed jurisdiction is easy. Now, the distinction is not so clear. Patients and physicians no longer have to be in the same room, city, or even state, meaning there could be unknown conflicting laws between the two locations. With rare exceptions, standards of care are based on the patient’s location, not the physician’s location. This increases the risk of practicing without being correctly licensed to higher than ever.
Because licensing is a significant roadblock in providing telemedicine, efforts are underway to make the process simpler and more streamlined. The Federation of State Medical Boards developed the Interstate Medical Licensure Compact (IMLC).2 This can qualify physicians to practice medicine across state lines within the compact so long as they meet specific eligibility requirements. The IMLC creates a fast-track option for physicians to fill out one application and receive licenses from multiple states at once. Currently, the compact includes 32 states, the District of Columbia, and Guam.3
Informed consent
Telemedicine health care still requires informed consent from patients. In fact, in some states, the requirements for care provided through telehealth are actually stricter than requirements for informed consent obtained in person.
Most informed consent laws require physicians to cover the risks and benefits of a recommended course of treatment and all feasible and reasonable material alternatives. On top of this traditional informed consent, physicians must get additional consent to receive care over a telehealth platform. This unique requirement explains what telehealth is, possible risks and expected benefits, and security measures used to protect patient information. States vary regarding when verbal consent is sufficient, and when written consent is required.
Prescriptions
Telemedicine is still a relatively new industry, and few legal opinions specifically address telemedicine malpractice. However, prescribing medication based on telemedicine information is among the few issues the courts have addressed. A 2008 decision found that a physician review of patient questionnaires submitted over the Internet was insufficient to prescribe medication without a physical examination determining patient health.4 This cautious approach stemmed from telehealth’s early concern about the absence of patient-physician relationships and potential online pharmacy abuse. Since this decision, many states require an “in-person” visit with a patient before prescribing medication. The definition of what qualifies as an in-person visit varies from state to state – some still consider the use of real-time, audiovisual conferencing sufficient.
The law is still evolving for prescriptions. Some states don’t allow any prescriptions, while others allow physicians to prescribe their patients’ medications as part of an appropriate treatment plan according to their professional discretion. Almost every state prohibits the prescription of controlled substances based on telemedicine.
Conclusion
Telemedicine is becoming an increasingly significant part of both physician-patient relationships and the broader health care industry. Used appropriately, it can be an incredibly effective method of care for physicians and patients. Physicians should learn the laws governing telemedicine in every state they want to practice and continue to stay current on any changes. The Center for Connected Health Policy offers a report, updated semiannually, to help physicians stay up to date on their state laws. These efforts will help prevent physicians from exposure to liability and medical malpractice claims.
Mr. Hyde is a partner at Younker Hyde Macfarlane, a law firm that focuses on prosecuting medical malpractice claims on behalf of injured patients. Ms. Johnson is an associate attorney with the firm. You can find them at YHMLaw.com.
References
1. Bestsennyy O, Harris A, Rost J. Telehealth: A quarter-trillion-dollar post-COVID-19 reality? Mckinsey & Company, May 29, 2020.
2. FSMB: Draft Interstate Compact for Physician Licensure Nears Completion, 2014.
3. Interstate Medical Licensure Compact: U.S. State Participation in the Compact.
4. See, Low Cost Pharm., Inc. v. Ariz. State Bd. Of Pharm, 2008 Ariz. App. Unpub. LEXIS 790, referencing conclusion of Arizona Medical Board.
Telehealth has been steadily gaining mainstream use throughout the last decade, but the practice was recently shoved, almost overnight, into the forefront of the health care profession. Telehealth is now used more frequently by medical groups and physicians than ever before. General reports before the COVID-19 pandemic approximated 90% of health care organizations used or planned to use telehealth in the future. This future may already be a reality, with a McKinsey & Company report estimating that physicians saw 50-175 times more patients over telehealth platforms since the pandemic’s start.1
In general, telehealth includes use of electronic communication and information technologies to deliver long-distance or remote health care. A physician’s use of telemedicine (clinical services) is one of the most common uses, but the industry also includes other professionals, such as pharmacists and nurses.
Telehealth platforms can be used to monitor, diagnose, treat, and counsel patients successfully. It works best for reading images, follow-up care, outpatient care, and long-term care. However, telemedicine is inappropriate for urgent issues, diagnosing underlying health conditions, or any practice where the standard of care would require a physical exam. There is potential liability for decision making without a proper physical exam.
There are many advantages to telehealth over more traditional health care options. Some of these advantages include:
- Increased access to health care.
- Increased access to medical specialists in small and rural communities.
- Improved long-term care from the comfort of patients’ homes.
- Improved platforms to document patient care outside regular business hours.
But along with these benefits, telehealth carries the disadvantage of potential increased liability. This increased liability could stem from:
- Breached standards of care.
- Inadequate or improper licensing.
- Limited care options.
- Decision making without a proper physical exam.
- Increased informed consent requirements.
- Restricted prescription access.
Before expanding any practice into telemedicine, awareness of potential legal issues is crucial.
Standard of care
Currently, telehealth laws and regulations vary significantly from state to state. But one rule is consistent across the board – that the standard of care for practicing medicine through telemedicine is identical to the standard of care required for practicing medicine during physical practice. It still requires the appropriate examination, testing, labs, imaging, and consultations that any in-person diagnosis needs. For physicians, it also includes supervising nonphysician clinicians, where state law requires supervision.
The American Telemedicine Association currently determines the primary governing standards and guidelines for telemedicine. These can help physicians understand best practices in meeting the standard of care through telemedicine. The American Gastroenterological Association provides coding guidelines and other resources to help physicians with telehealth and e-visits. Other professional societies, such as the American College of Radiology and the American Academy of Dermatology, offer guidelines specific to their medical specialties’ standards of care. These standards still vary from state to state, so medical professionals must be aware of any differences before treating patients in multiple states.
Licensing
Licensing is one of telemedicine’s most confusing legal issues. All states require a license to practice medicine (traditional or telehealth) within their borders. Without that license, practicing medicine in the state is a crime. On top of being criminal, unlicensed practice can affect insurance, liability, billing, and malpractice coverage. When in a brick-and-mortar clinic, a physician’s confidence in practicing within the licensed jurisdiction is easy. Now, the distinction is not so clear. Patients and physicians no longer have to be in the same room, city, or even state, meaning there could be unknown conflicting laws between the two locations. With rare exceptions, standards of care are based on the patient’s location, not the physician’s location. This increases the risk of practicing without being correctly licensed to higher than ever.
Because licensing is a significant roadblock in providing telemedicine, efforts are underway to make the process simpler and more streamlined. The Federation of State Medical Boards developed the Interstate Medical Licensure Compact (IMLC).2 This can qualify physicians to practice medicine across state lines within the compact so long as they meet specific eligibility requirements. The IMLC creates a fast-track option for physicians to fill out one application and receive licenses from multiple states at once. Currently, the compact includes 32 states, the District of Columbia, and Guam.3
Informed consent
Telemedicine health care still requires informed consent from patients. In fact, in some states, the requirements for care provided through telehealth are actually stricter than requirements for informed consent obtained in person.
Most informed consent laws require physicians to cover the risks and benefits of a recommended course of treatment and all feasible and reasonable material alternatives. On top of this traditional informed consent, physicians must get additional consent to receive care over a telehealth platform. This unique requirement explains what telehealth is, possible risks and expected benefits, and security measures used to protect patient information. States vary regarding when verbal consent is sufficient, and when written consent is required.
Prescriptions
Telemedicine is still a relatively new industry, and few legal opinions specifically address telemedicine malpractice. However, prescribing medication based on telemedicine information is among the few issues the courts have addressed. A 2008 decision found that a physician review of patient questionnaires submitted over the Internet was insufficient to prescribe medication without a physical examination determining patient health.4 This cautious approach stemmed from telehealth’s early concern about the absence of patient-physician relationships and potential online pharmacy abuse. Since this decision, many states require an “in-person” visit with a patient before prescribing medication. The definition of what qualifies as an in-person visit varies from state to state – some still consider the use of real-time, audiovisual conferencing sufficient.
The law is still evolving for prescriptions. Some states don’t allow any prescriptions, while others allow physicians to prescribe their patients’ medications as part of an appropriate treatment plan according to their professional discretion. Almost every state prohibits the prescription of controlled substances based on telemedicine.
Conclusion
Telemedicine is becoming an increasingly significant part of both physician-patient relationships and the broader health care industry. Used appropriately, it can be an incredibly effective method of care for physicians and patients. Physicians should learn the laws governing telemedicine in every state they want to practice and continue to stay current on any changes. The Center for Connected Health Policy offers a report, updated semiannually, to help physicians stay up to date on their state laws. These efforts will help prevent physicians from exposure to liability and medical malpractice claims.
Mr. Hyde is a partner at Younker Hyde Macfarlane, a law firm that focuses on prosecuting medical malpractice claims on behalf of injured patients. Ms. Johnson is an associate attorney with the firm. You can find them at YHMLaw.com.
References
1. Bestsennyy O, Harris A, Rost J. Telehealth: A quarter-trillion-dollar post-COVID-19 reality? Mckinsey & Company, May 29, 2020.
2. FSMB: Draft Interstate Compact for Physician Licensure Nears Completion, 2014.
3. Interstate Medical Licensure Compact: U.S. State Participation in the Compact.
4. See, Low Cost Pharm., Inc. v. Ariz. State Bd. Of Pharm, 2008 Ariz. App. Unpub. LEXIS 790, referencing conclusion of Arizona Medical Board.
Web-based interviews, financial planning in a pandemic, and more
Dear colleagues,
I’m excited to introduce the November issue of The New Gastroenterologist – the last edition of 2020 features a fantastic line-up of articles! As the year comes to a close, we reflect on what has certainly been an interesting year, defined by a set of unique challenges we have faced as a nation and as a specialty.
The fellowship recruitment season is one that has looked starkly different as interviews have converted to a virtual format. Dr. Wissam Khan, Dr. Nada Al Masalmeh, Dr. Stephanie Judd, and Dr. Diane Levine (Wayne State University) compile a helpful list of tips and tricks on proper interview etiquette in the new era of web-based interviews.
Financial planning in the face of a pandemic is a formidable task – Jonathan Tudor (Fidelity Investments) offers valuable advice for gastroenterologists on how to remain secure in your finances even in uncertain circumstances.
This quarter’s “In Focus” feature, written by Dr. Yutaka Tomizawa (University of Washington), is a comprehensive piece elucidating the role of gastroenterologists in the management of gastric cancer. The article reviews the individual risk factors that exist for gastric cancer and provides guidance on how to stratify patients accordingly, which is critical in the ethnically diverse population of the United States.
Keeping a procedure log during fellowship can seem daunting and cumbersome, but it is important. Dr. Houman Rezaizadeh (University of Connecticut) shares his program’s experience with the AGA Procedure Log, a convenient online tracking tool, which can provide accurate and secure documentation of endoscopic procedures performed throughout fellowship.
Dr. Nazia Hasan (North Bay Health Care) and Dr. Allison Schulman (University of Michigan) broach an incredibly important topic: the paucity of women in interventional endoscopy. Dr. Hasan and Dr. Shulman candidly discuss the barriers women face in pursuing this subspecialty and offer practical solutions on how to approach these challenges – a piece that will surely resonate with many young gastroenterologists.
We wrap up our first year of TNG’s ethics series with two cases discussing the utilization of cannabis therapy in inflammatory bowel disease (IBD). Dr. Jami Kinnucan (University of Michigan) and Dr. Arun Swaminath (Lenox Hill Hospital) systematically review existing data on the efficacy of cannabis use in IBD, the risks associated with therapy, and legal implications for both physicians and patients.
Also in this issue is a high-yield clinical review on the endoscopic drainage of pancreatic fluid collections by Dr. Robert Moran and Dr. Joseph Elmunzer (Medical University of South Carolina). Dr. Manol Jovani (Johns Hopkins) teaches us about confounding – a critical concept to keep in mind when evaluating any manuscript. Lastly, our DHPA Private Practice Perspectives article, written by Dr. Mehul Lalani (US Digestive), reviews how quality measures and initiatives are tracked and implemented in private practice.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Ryan Farrell ([email protected]), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor in Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Dear colleagues,
I’m excited to introduce the November issue of The New Gastroenterologist – the last edition of 2020 features a fantastic line-up of articles! As the year comes to a close, we reflect on what has certainly been an interesting year, defined by a set of unique challenges we have faced as a nation and as a specialty.
The fellowship recruitment season is one that has looked starkly different as interviews have converted to a virtual format. Dr. Wissam Khan, Dr. Nada Al Masalmeh, Dr. Stephanie Judd, and Dr. Diane Levine (Wayne State University) compile a helpful list of tips and tricks on proper interview etiquette in the new era of web-based interviews.
Financial planning in the face of a pandemic is a formidable task – Jonathan Tudor (Fidelity Investments) offers valuable advice for gastroenterologists on how to remain secure in your finances even in uncertain circumstances.
This quarter’s “In Focus” feature, written by Dr. Yutaka Tomizawa (University of Washington), is a comprehensive piece elucidating the role of gastroenterologists in the management of gastric cancer. The article reviews the individual risk factors that exist for gastric cancer and provides guidance on how to stratify patients accordingly, which is critical in the ethnically diverse population of the United States.
Keeping a procedure log during fellowship can seem daunting and cumbersome, but it is important. Dr. Houman Rezaizadeh (University of Connecticut) shares his program’s experience with the AGA Procedure Log, a convenient online tracking tool, which can provide accurate and secure documentation of endoscopic procedures performed throughout fellowship.
Dr. Nazia Hasan (North Bay Health Care) and Dr. Allison Schulman (University of Michigan) broach an incredibly important topic: the paucity of women in interventional endoscopy. Dr. Hasan and Dr. Shulman candidly discuss the barriers women face in pursuing this subspecialty and offer practical solutions on how to approach these challenges – a piece that will surely resonate with many young gastroenterologists.
We wrap up our first year of TNG’s ethics series with two cases discussing the utilization of cannabis therapy in inflammatory bowel disease (IBD). Dr. Jami Kinnucan (University of Michigan) and Dr. Arun Swaminath (Lenox Hill Hospital) systematically review existing data on the efficacy of cannabis use in IBD, the risks associated with therapy, and legal implications for both physicians and patients.
Also in this issue is a high-yield clinical review on the endoscopic drainage of pancreatic fluid collections by Dr. Robert Moran and Dr. Joseph Elmunzer (Medical University of South Carolina). Dr. Manol Jovani (Johns Hopkins) teaches us about confounding – a critical concept to keep in mind when evaluating any manuscript. Lastly, our DHPA Private Practice Perspectives article, written by Dr. Mehul Lalani (US Digestive), reviews how quality measures and initiatives are tracked and implemented in private practice.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Ryan Farrell ([email protected]), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor in Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Dear colleagues,
I’m excited to introduce the November issue of The New Gastroenterologist – the last edition of 2020 features a fantastic line-up of articles! As the year comes to a close, we reflect on what has certainly been an interesting year, defined by a set of unique challenges we have faced as a nation and as a specialty.
The fellowship recruitment season is one that has looked starkly different as interviews have converted to a virtual format. Dr. Wissam Khan, Dr. Nada Al Masalmeh, Dr. Stephanie Judd, and Dr. Diane Levine (Wayne State University) compile a helpful list of tips and tricks on proper interview etiquette in the new era of web-based interviews.
Financial planning in the face of a pandemic is a formidable task – Jonathan Tudor (Fidelity Investments) offers valuable advice for gastroenterologists on how to remain secure in your finances even in uncertain circumstances.
This quarter’s “In Focus” feature, written by Dr. Yutaka Tomizawa (University of Washington), is a comprehensive piece elucidating the role of gastroenterologists in the management of gastric cancer. The article reviews the individual risk factors that exist for gastric cancer and provides guidance on how to stratify patients accordingly, which is critical in the ethnically diverse population of the United States.
Keeping a procedure log during fellowship can seem daunting and cumbersome, but it is important. Dr. Houman Rezaizadeh (University of Connecticut) shares his program’s experience with the AGA Procedure Log, a convenient online tracking tool, which can provide accurate and secure documentation of endoscopic procedures performed throughout fellowship.
Dr. Nazia Hasan (North Bay Health Care) and Dr. Allison Schulman (University of Michigan) broach an incredibly important topic: the paucity of women in interventional endoscopy. Dr. Hasan and Dr. Shulman candidly discuss the barriers women face in pursuing this subspecialty and offer practical solutions on how to approach these challenges – a piece that will surely resonate with many young gastroenterologists.
We wrap up our first year of TNG’s ethics series with two cases discussing the utilization of cannabis therapy in inflammatory bowel disease (IBD). Dr. Jami Kinnucan (University of Michigan) and Dr. Arun Swaminath (Lenox Hill Hospital) systematically review existing data on the efficacy of cannabis use in IBD, the risks associated with therapy, and legal implications for both physicians and patients.
Also in this issue is a high-yield clinical review on the endoscopic drainage of pancreatic fluid collections by Dr. Robert Moran and Dr. Joseph Elmunzer (Medical University of South Carolina). Dr. Manol Jovani (Johns Hopkins) teaches us about confounding – a critical concept to keep in mind when evaluating any manuscript. Lastly, our DHPA Private Practice Perspectives article, written by Dr. Mehul Lalani (US Digestive), reviews how quality measures and initiatives are tracked and implemented in private practice.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Ryan Farrell ([email protected]), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor in Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Role of gastroenterologists in the U.S. in the management of gastric cancer
Introduction
Although gastric cancer is one of the most common causes of cancer death in the world, the burden of gastric cancer in the United States tends to be underestimated relative to that of other cancers of the digestive system. In fact, the 5-year survival rate from gastric cancer remains poor (~32%)1 in the United States, and this is largely because gastric cancers are not diagnosed at an early stage when curative therapeutic options are available. Cumulative epidemiologic data consistently demonstrate that the incidence of gastric cancer in the United States varies according to ethnicity, immigrant status, and country of origin. It is important for practicing gastroenterologists in the United States to recognize individual risk profiles and identify people at higher risk for gastric cancer. Hereditary diffuse gastric cancer is an inherited form of diffuse-type gastric cancer and has pathogenic variants in the E-cadherin gene that are inherited in an autosomal dominant pattern. The lifetime risk of gastric cancer in individuals with HDGC is very high, and prophylactic total gastrectomy is usually advised. This article focuses on intestinal type cancer.
Epidemiology
Gastric cancer (proximal and distal gastric cancer combined) is the fifth most frequently diagnosed cancer and the third most common cause of cancer death worldwide, with 1,033,701 new cases and 782,685 deaths in 2018.2 Gastric cancer is subcategorized based on location (proximal [i.e., esophagogastric junctional, gastric cardia] and distal) and histology (intestinal and diffuse type), and each subtype is considered to have a distinct pathogenesis. Distal intestinal type gastric cancer is most commonly encountered in clinical practice. In this article, gastric cancer will signify distal intestinal type gastric cancer unless it is otherwise noted. In general, incidence rates are about twofold higher in men than in women. There is marked geographic variation in incidence rates, and the age-standardized incidence rates in eastern Asia (32.1 and 13.2, per 100,000) are approximately six times higher than those in northern America (5.6 and 2.8, per 100,000) in both men and women, respectively.2 Recent studies evaluating global trends in the incidence and mortality of gastric cancer have demonstrated decreases worldwide.3-5 However, the degree of decrease in the incidence and mortality of gastric cancer varies substantially across geographic regions, reflecting the heterogeneous distribution of risk profiles. A comprehensive analysis of a U.S. population registry demonstrated a linear decrease in the incidence of gastric cancer in the United States (0.94% decrease per year between 2001 and 2015),6 though the annual percent change in the gastric cancer mortality in the United States was lower (around 2% decrease per year between 1980 and 2011) than in other countries.3Several population-based studies conducted in the United States have demonstrated that the incidence of gastric cancer varied by ethnicity, immigrant status, and country of origin, and the highest incidence was observed among Asian immigrants.7,8 A comprehensive meta-analysis examining the risk of gastric cancer in immigrants from high-incidence regions to low-incidence regions found a persistently higher risk of gastric cancer and related mortality among immigrants.9 These results indicate that there are important risk factors such as environmental and dietary factors in addition to the traditionally considered risk factors including male gender, age, family history, and tobacco use. A survey conducted in an ethnically and culturally diverse U.S. city showed that gastroenterology providers demonstrated knowledge deficiencies in identifying and managing patients with increased risk of gastric cancer.10 Recognizing individualized risk profiles in higher-risk groups (e.g., immigrants from higher-incidence/prevalence regions) is important for optimizing management of gastric cancer in the United States.
Assessment and management of modifiable risk factors
Helicobacter pylori, a group 1 carcinogen, is the most well-recognized risk factor for gastric cancer, particularly noncardia gastric cancer.11 Since a landmark longitudinal follow-up study in Japan demonstrated that people with H. pylori infection are more likely to develop gastric cancer than those without H. pylori infection,12 accumulating evidence largely from Asian countries has shown that eradication of H. pylori is associated with a reduced incidence of gastric cancer regardless of baseline risk.13 There are also data on the protective effect for gastric cancer of H. pylori eradication in asymptomatic individuals. Another meta-analysis of six international randomized control trials demonstrated a 34% relative risk reduction of gastric cancer occurrence in asymptomatic people (relative risk of developing gastric cancer was 0.66 in those who received eradication therapy compared with those with placebo or no treatment, 95% CI, 0.46-0.95).14 A U.S. practice guideline published after these meta-analyses recommends that all patients with a positive test indicating active infection with H. pylori should be offered treatment and testing to prove eradication,15 though the recommendation was not purely intended to reduce the gastric cancer risk in U.S. population. Subsequently, a Department of Veterans Affairs cohort study added valuable insights from a U.S. experience to the body of evidence from other countries with higher prevalence. In this study of more than 370,000 patients with a history of H. pylori infection, the detection and successful eradication of H. pylori was associated with a 76% lower incidence of gastric cancer compared with people without H. pylori treatment.16 This study also provided insight into H. pylori treatment practice patterns. Of patients with a positive H. pylori test result (stool antigen, urea breath test, or pathology), approximately 75% were prescribed an eradication regimen and only 21% of those underwent eradication tests. A low rate (24%) of eradication testing was subsequently reported by the same group among U.S. patients regardless of gastric cancer risk profiles.17 The lesson from the aforementioned study is that treatment and eradication of H. pylori even among asymptomatic U.S. patients reduces the risk of subsequent gastric cancer. However, it may be difficult to generalize the results of this study given the nature of the Veterans Affairs cohort, and more data are required to justify the implementation of nationwide preventive H. pylori screening in the general U.S. population.
Smoking has been recognized as the other important risk factor. A study from the European prospective multicenter cohort demonstrated a significant association of cigarette smoking and gastric cancer risk (HR for ever-smokers 1.45 [95% CI, 1.08-1.94], current-smokers in males 1.73 [95% CI, 1.06-2.83], and current smokers in females 1.87 [95% CI, 1.12-3.12], respectively) after adjustment for educational level, dietary consumption profiles, alcohol intake, and body mass index (BMI).18 A subsequent meta-analysis provided solid evidence of smoking as the important behavioral risk factor for gastric cancer.19 Smoking also predisposed to the development of proximal gastric cancer.20 Along with other cancers in the digestive system such as in the esophagus, colon and rectum, liver, gallbladder, and pancreas, a significant association of BMI and the risk of proximal gastric cancer (RR of the highest BMI category compared with normal BMI, 1.8 [95% CI, 1.3-2.5]) was reported, with positive dose-response relationships; however, the association was not sufficient for distal gastric cancer.21 There is also evidence to show a trend of greater alcohol consumption (>45 grams per day [about 3 drinks a day]) associated with the increased risk of gastric cancer.21 It has been thought that salt and salt-preserved food increase the risk of gastric cancer. It should be noted that the observational studies showing the associations were published from Asian countries where such foods were a substantial part of traditional diets (e.g., salted vegetables in Japan) and the incidence of gastric cancer is high. There is also a speculation that preserved foods may have been eaten in more underserved, low socioeconomic regions where refrigeration was not available and prevalence of H. pylori infection was higher. Except for documented inherited form of gastric cancer (e.g., HDGC or hereditary cancer syndromes), most gastric cancers are considered sporadic. A recent randomized study published from South Korea investigated a cohort of higher-risk asymptomatic patients with family history significant for gastric cancer. This study of 1,676 subjects with a median follow-up of 9.2 years showed that successful eradication of H. pylori in the first-degree relatives of those with gastric cancer significantly reduced the risk (HR 0.45 [95% CI, 0.21-0.94]) of developing gastric cancer.22 As previously discussed, in the United States where the prevalence of H. pylori and the incidence of gastric cancer are both lower than in some Asian countries, routine screening of asymptomatic individuals for H. pylori is not justified yet. There may be a role for screening individuals who are first-generation immigrants from areas of high gastric cancer incidence and also have a first-degree relative with gastric cancer.
Who should we consider high risk and offer screening EGD?
With available evidence to date, screening for gastric cancer in a general U.S. population is not recommended. However, it is important to acknowledge the aforementioned varying incidence of gastric cancer in the United States among ethnicity, immigrant status, and country of origin. Immigrants from high-incidence regions maintain a higher risk of gastric cancer and related mortality even after migration to lower-incidence regions. The latter comprehensive study estimated that as many as 12.7 million people (29.4% of total U.S. immigrant population) have emigrated from higher-incidence regions including East Asian and some Central American countries.9 Indeed, an opportunistic nationwide gastric cancer screening program has been implemented in South Korea (beginning at age 40, biannually)23 and Japan (beginning at age 50, biannually).24 Two decision-analytic simulation studies have provided insight into the uncertainty about the cost effectiveness for potential targeted gastric cancer screening in higher-risk populations in the United States. One study demonstrated that esophagogastroduodenoscopy (EGD) screening for otherwise asymptomatic Asian American people (as well as Hispanics and non-Hispanic Blacks) at the time of screening colonoscopy at 50 years of age with continued endoscopic surveillance every 3 years was cost effective, only if gastric intestinal metaplasia (GIM) or more advanced lesions were diagnosed at the index screening EGD.25 Previous studies analyzing the cost effectiveness for gastric cancer screening in the United States had the limitation of not stratifying according to race or ethnicity, or accounting for patients diagnosed with GIM. Subsequently, the same research group extended this model analysis and has published additional findings that this strategy is cost effective for each of the most prevalent Asian American ethnicities (Chinese, Filipino, Southeast Asian, Vietnamese, Korean, and Japanese Americans) in the United States irrespective of sex.26 Although the authors raised a limitation that additional risk factors such as family history, tobacco use, or persistent H. pylori infection were not considered in the model because data regarding differentiated noncardia gastric cancer risk among Asian American ethnicities based on these risk factors are not available.
These two model analytic studies added valuable insights to the body of evidence that subsequent EGDs after the one-time bundled EGD is cost effective for higher-risk asymptomatic people in the United States, if the index screening EGD with gastric mucosal biopsies demonstrates at least GIM. Further population-based research to elucidate risk stratification among higher-risk people will provide a schema that could standardize management and resource allocation as well as increase the cost effectiveness of a gastric cancer screening program in the United States. The degree of risk of developing gastric cancer in autoimmune gastritis varies among the reported studies.27-29 Although the benefit of endoscopic screening in patients with autoimmune gastritis has not been established, a single endoscopic evaluation should be recommended soon after the diagnosis of autoimmune gastritis in order to identify prevalent neoplastic lesions.30
Practical consideration when we perform EGD for early gastric cancer screening
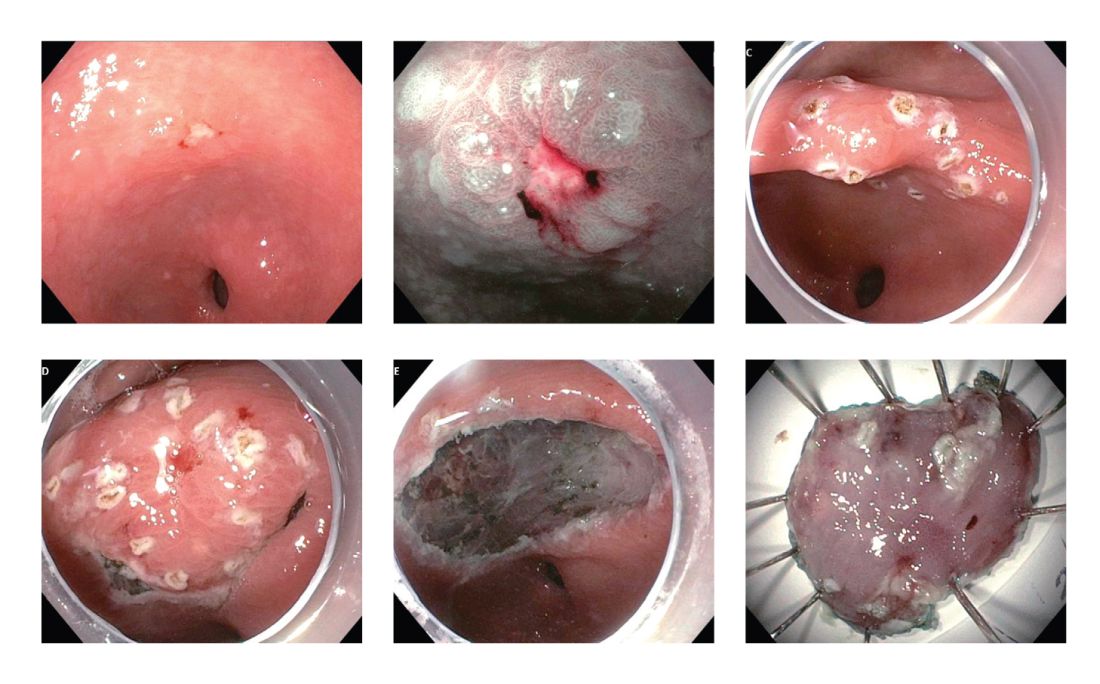
Identification of higher-risk patients should alert an endoscopist to observe mucosa with greater care with a lower threshold to biopsy any suspicious lesions. Preprocedural risk stratification for each individual before performing diagnostic EGD will improve early gastric cancer detection. While we perform EGD, detecting precursor lesions (atrophic gastritis and GIM) is as important as diagnosing an early gastric cancer. Screening and management of patients with precursor lesions (i.e., atrophic gastritis and GIM) is beyond the scope of this article, and this was published in a previous issue of the New Gastroenterologist. It is important to first grossly survey the entire gastric mucosa using high-definition while light (HDWL) endoscopy and screen for any focal irregular (raised or depressed) mucosal lesions. These lesions are often erythematous and should be examined carefully. Use of mucolytic and/or deforming agents (e.g., N-acetylcysteine or simethicone) is recommended for the improvement of visual clarity of gastric mucosa.31 Simethicone is widely used in the United States for colonoscopy and should also be available at the time of EGD for better gastric mucosal visibility. If irregular mucosal lesions are noted, this area should also be examined under narrowband imaging (NBI) in addition to HDWL. According to a simplified classification consisting of mucosal and vascular irregularity, NBI provides better mucosal surface morphology for diagnosis of early gastric cancer compared with HDWL, and a thorough examination of the surface characteristics is a prerequisite.32 This classification was further validated in a randomized control trial, and NBI increased sensitivity for the diagnosis of neoplasia compared with HDWL (92 % vs. 74 %).33 The majority of institutions in the United States have a newer-generation NBI (Olympus America, EVIS EXERA III video system, GIF-HQ190), which provides brighter endoscopic images to better characterize gastric neoplastic lesions. Once we recognize an area suspicious for neoplasia, we should describe the macroscopic features according to a classification system.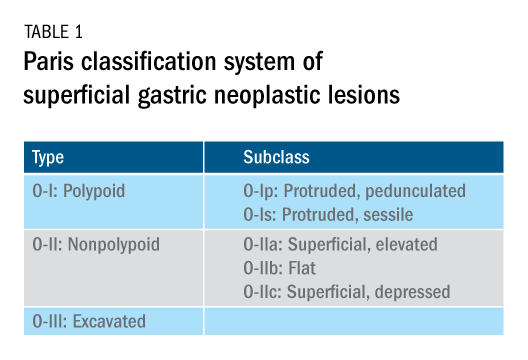
The Paris classification, one of the most widely recognized classification systems among U.S. gastroenterologists, is recommended for gastric neoplastic lesions.34Gastric neoplastic lesions with a “superficial” endoscopic appearance are classified as subtypes of “type 0.” The term “type 0” was chosen to distinguish the classification of “superficial” lesions from the Borrmann classification for “advanced” gastric tumors, which includes types 1 to 4. In the classification, a neoplastic lesion is called “superficial” when its endoscopic appearance suggests that the depth of penetration in the digestive wall is not more than into the submucosa (i.e., there is no infiltration of the muscularis propria). The distinctive characters of polypoid and nonpolypoid lesions are summarized in Table 1. Endoscopic submucosal dissection (ESD) has steadily gained acceptance for the treatment of early gastric cancer in the United States. The American Gastroenterological Association recommended in the 2019 institutional updated clinical practice guideline that ESD should be considered the first-line therapy for visible, endoscopically resectable, superficial gastric neoplasia.35 This recommendation is further supported by the published data on efficacy and safety of ESD for early gastric neoplasia in a large multicenter cohort in the United States.36 For all suspicious lesions, irrespective of pathological neoplastic confirmation, referral to an experienced center for further evaluation and endoscopic management should be considered. Lastly, all patients with early gastric cancer should be evaluated for H. pylori infection and treated if the test is positive. Eradication of H. pylori is associated with a lower rate of metachronous gastric cancer,37 and treatment of H. pylori as secondary prevention is also recommended.
Conclusion
As summarized above, cumulative epidemiologic data consistently demonstrate that the incidence of gastric cancer in the U.S. varies according to ethnicity, immigrant status, and country of origin. New gastroenterologists will need to recognize individual risk profiles and identify people at higher risk for gastric cancer. Risk stratification before performing endoscopic evaluation will improve early gastric cancer detection and make noninvasive, effective therapies an option.
References
1. Surveillance, Epidemiology, and End Results Program cancer statistics. https://seer.cancer.gov/statfacts/html/stomach.html.
2. Bray F et al. Ca Cancer J Clin. 2018;68:394-424.
3. Ferro A et al. Eur J Cancer. 2014;50:1330-44.
4. Luo G et al. Int J Cancer. 2017;141:1333-44.
5. Arnold M et al. Eur J Cancer. 2015;51:1164-87.
6. Thrift AP, El-Serag HB. Clin Gastroenterol Hepatol. 2020;18:534-42.
7. Kim Y et al. Epidemiol Health. 2015;37:e2015066.
8. Kamineni A et al. Cancer Causes Control. 1999;10:77-83.
9. Pabla BS et al. Clin Gastroenterol Hepatol. 2020;18:347-59.
10. Shah SC et al. Knowledge Gaps among Physicians Caring for Multiethnic Populations at Increased Gastric Cancer Risk. Gut Liver. 2018 Jan 15;12(1):38-45.
11. International Agency for Research on Cancer. Monographs on the Identification of Carcinogenic Hazards to Humans. IARC. July 7, 2019. 12. Uemura N et al. N Engl J Med. 2001;345:784-9.
13. Lee YC et al. Gastroenterology. 2016;150:1113-24.
14. Ford AC et al. BMJ. 2014;348:g3174.
15. Chey W et al. Am J Gastroenterol. 2017;112:212-39.
16. Kumar S et al. Gastroenterology. 2020;158:527-36.
17. Kumar S et al. Clin Gastroenterol Hepatol. 2020 Apr 6;S1542-3565(20)30436-5.
18. González CA et al. Int J Cancer. 2003;107:629-34.
19. Ladeiras-Lopes R et al. Cancer Causes Control. 2008;19:689-701.
20. Cavaleiro-Pinto M et al. Cancer Causes Control. 2011;22:375-87.
21. Lauby-Secretan B et al. N Engl J Med. 2016;375:794-8.
22. Choi IJ et al. N Engl J Med. 2020;382:427-36.
23. Kim BJ et al. World J Gastroenterol. 2013;19:736-41.
24. Hamashima C. Jpn J Clin Oncol. 2018;48:278–86.
25. Saumoy M et al. Gastroenterology. 2018;155:648-60.
26. Shah SC et al. Clin Gastroenterol Hepatol. 2020 Jul 21:S1542-3565(20)30993-9. doi: 10.1016/j.cgh.2020.07.031.
27. Brinton LA et al. Br J Cancer. 1989;59:810-3.
28. Hsing AW et al. Cancer. 1993;71:745-50.
29. Schafer LW et al. Mayo Clin Proc. 1985;60:444-8.
30. American Society for Gastrointestinal Endoscopy Standards of Practice Committee. Gastrointest Endosc. 2015;82:1-8.
31. Chiu PWY et al. Gut. 2019;68:186-97.
32. Pimentel-Nunes P et al. Endoscopy. 2012;44:236-46.
33. Pimentel-Nunes P et al. Endoscopy. 2016;48:723-30.
34. Participants in the Paris Workshop. Gastrointest Endosc. 2003;58:S3-43.
35. Draganov PV et al. Clin Gastroenterol Hepatol. 2019;17:16-25.
36. Ngamruengphong S et al. Clin Gastroenterol Hepatol. 2020 Jun 18;S1542-3565(20)30834-X. Online ahead of print.
37. Choi IJ et al. N Engl J Med. 2018;378:1085-95.
Dr. Tomizawa is a clinical assistant professor of medicine in the division of gastroenterology, University of Washington, Seattle.
Introduction
Although gastric cancer is one of the most common causes of cancer death in the world, the burden of gastric cancer in the United States tends to be underestimated relative to that of other cancers of the digestive system. In fact, the 5-year survival rate from gastric cancer remains poor (~32%)1 in the United States, and this is largely because gastric cancers are not diagnosed at an early stage when curative therapeutic options are available. Cumulative epidemiologic data consistently demonstrate that the incidence of gastric cancer in the United States varies according to ethnicity, immigrant status, and country of origin. It is important for practicing gastroenterologists in the United States to recognize individual risk profiles and identify people at higher risk for gastric cancer. Hereditary diffuse gastric cancer is an inherited form of diffuse-type gastric cancer and has pathogenic variants in the E-cadherin gene that are inherited in an autosomal dominant pattern. The lifetime risk of gastric cancer in individuals with HDGC is very high, and prophylactic total gastrectomy is usually advised. This article focuses on intestinal type cancer.
Epidemiology
Gastric cancer (proximal and distal gastric cancer combined) is the fifth most frequently diagnosed cancer and the third most common cause of cancer death worldwide, with 1,033,701 new cases and 782,685 deaths in 2018.2 Gastric cancer is subcategorized based on location (proximal [i.e., esophagogastric junctional, gastric cardia] and distal) and histology (intestinal and diffuse type), and each subtype is considered to have a distinct pathogenesis. Distal intestinal type gastric cancer is most commonly encountered in clinical practice. In this article, gastric cancer will signify distal intestinal type gastric cancer unless it is otherwise noted. In general, incidence rates are about twofold higher in men than in women. There is marked geographic variation in incidence rates, and the age-standardized incidence rates in eastern Asia (32.1 and 13.2, per 100,000) are approximately six times higher than those in northern America (5.6 and 2.8, per 100,000) in both men and women, respectively.2 Recent studies evaluating global trends in the incidence and mortality of gastric cancer have demonstrated decreases worldwide.3-5 However, the degree of decrease in the incidence and mortality of gastric cancer varies substantially across geographic regions, reflecting the heterogeneous distribution of risk profiles. A comprehensive analysis of a U.S. population registry demonstrated a linear decrease in the incidence of gastric cancer in the United States (0.94% decrease per year between 2001 and 2015),6 though the annual percent change in the gastric cancer mortality in the United States was lower (around 2% decrease per year between 1980 and 2011) than in other countries.3Several population-based studies conducted in the United States have demonstrated that the incidence of gastric cancer varied by ethnicity, immigrant status, and country of origin, and the highest incidence was observed among Asian immigrants.7,8 A comprehensive meta-analysis examining the risk of gastric cancer in immigrants from high-incidence regions to low-incidence regions found a persistently higher risk of gastric cancer and related mortality among immigrants.9 These results indicate that there are important risk factors such as environmental and dietary factors in addition to the traditionally considered risk factors including male gender, age, family history, and tobacco use. A survey conducted in an ethnically and culturally diverse U.S. city showed that gastroenterology providers demonstrated knowledge deficiencies in identifying and managing patients with increased risk of gastric cancer.10 Recognizing individualized risk profiles in higher-risk groups (e.g., immigrants from higher-incidence/prevalence regions) is important for optimizing management of gastric cancer in the United States.
Assessment and management of modifiable risk factors
Helicobacter pylori, a group 1 carcinogen, is the most well-recognized risk factor for gastric cancer, particularly noncardia gastric cancer.11 Since a landmark longitudinal follow-up study in Japan demonstrated that people with H. pylori infection are more likely to develop gastric cancer than those without H. pylori infection,12 accumulating evidence largely from Asian countries has shown that eradication of H. pylori is associated with a reduced incidence of gastric cancer regardless of baseline risk.13 There are also data on the protective effect for gastric cancer of H. pylori eradication in asymptomatic individuals. Another meta-analysis of six international randomized control trials demonstrated a 34% relative risk reduction of gastric cancer occurrence in asymptomatic people (relative risk of developing gastric cancer was 0.66 in those who received eradication therapy compared with those with placebo or no treatment, 95% CI, 0.46-0.95).14 A U.S. practice guideline published after these meta-analyses recommends that all patients with a positive test indicating active infection with H. pylori should be offered treatment and testing to prove eradication,15 though the recommendation was not purely intended to reduce the gastric cancer risk in U.S. population. Subsequently, a Department of Veterans Affairs cohort study added valuable insights from a U.S. experience to the body of evidence from other countries with higher prevalence. In this study of more than 370,000 patients with a history of H. pylori infection, the detection and successful eradication of H. pylori was associated with a 76% lower incidence of gastric cancer compared with people without H. pylori treatment.16 This study also provided insight into H. pylori treatment practice patterns. Of patients with a positive H. pylori test result (stool antigen, urea breath test, or pathology), approximately 75% were prescribed an eradication regimen and only 21% of those underwent eradication tests. A low rate (24%) of eradication testing was subsequently reported by the same group among U.S. patients regardless of gastric cancer risk profiles.17 The lesson from the aforementioned study is that treatment and eradication of H. pylori even among asymptomatic U.S. patients reduces the risk of subsequent gastric cancer. However, it may be difficult to generalize the results of this study given the nature of the Veterans Affairs cohort, and more data are required to justify the implementation of nationwide preventive H. pylori screening in the general U.S. population.
Smoking has been recognized as the other important risk factor. A study from the European prospective multicenter cohort demonstrated a significant association of cigarette smoking and gastric cancer risk (HR for ever-smokers 1.45 [95% CI, 1.08-1.94], current-smokers in males 1.73 [95% CI, 1.06-2.83], and current smokers in females 1.87 [95% CI, 1.12-3.12], respectively) after adjustment for educational level, dietary consumption profiles, alcohol intake, and body mass index (BMI).18 A subsequent meta-analysis provided solid evidence of smoking as the important behavioral risk factor for gastric cancer.19 Smoking also predisposed to the development of proximal gastric cancer.20 Along with other cancers in the digestive system such as in the esophagus, colon and rectum, liver, gallbladder, and pancreas, a significant association of BMI and the risk of proximal gastric cancer (RR of the highest BMI category compared with normal BMI, 1.8 [95% CI, 1.3-2.5]) was reported, with positive dose-response relationships; however, the association was not sufficient for distal gastric cancer.21 There is also evidence to show a trend of greater alcohol consumption (>45 grams per day [about 3 drinks a day]) associated with the increased risk of gastric cancer.21 It has been thought that salt and salt-preserved food increase the risk of gastric cancer. It should be noted that the observational studies showing the associations were published from Asian countries where such foods were a substantial part of traditional diets (e.g., salted vegetables in Japan) and the incidence of gastric cancer is high. There is also a speculation that preserved foods may have been eaten in more underserved, low socioeconomic regions where refrigeration was not available and prevalence of H. pylori infection was higher. Except for documented inherited form of gastric cancer (e.g., HDGC or hereditary cancer syndromes), most gastric cancers are considered sporadic. A recent randomized study published from South Korea investigated a cohort of higher-risk asymptomatic patients with family history significant for gastric cancer. This study of 1,676 subjects with a median follow-up of 9.2 years showed that successful eradication of H. pylori in the first-degree relatives of those with gastric cancer significantly reduced the risk (HR 0.45 [95% CI, 0.21-0.94]) of developing gastric cancer.22 As previously discussed, in the United States where the prevalence of H. pylori and the incidence of gastric cancer are both lower than in some Asian countries, routine screening of asymptomatic individuals for H. pylori is not justified yet. There may be a role for screening individuals who are first-generation immigrants from areas of high gastric cancer incidence and also have a first-degree relative with gastric cancer.
Who should we consider high risk and offer screening EGD?
With available evidence to date, screening for gastric cancer in a general U.S. population is not recommended. However, it is important to acknowledge the aforementioned varying incidence of gastric cancer in the United States among ethnicity, immigrant status, and country of origin. Immigrants from high-incidence regions maintain a higher risk of gastric cancer and related mortality even after migration to lower-incidence regions. The latter comprehensive study estimated that as many as 12.7 million people (29.4% of total U.S. immigrant population) have emigrated from higher-incidence regions including East Asian and some Central American countries.9 Indeed, an opportunistic nationwide gastric cancer screening program has been implemented in South Korea (beginning at age 40, biannually)23 and Japan (beginning at age 50, biannually).24 Two decision-analytic simulation studies have provided insight into the uncertainty about the cost effectiveness for potential targeted gastric cancer screening in higher-risk populations in the United States. One study demonstrated that esophagogastroduodenoscopy (EGD) screening for otherwise asymptomatic Asian American people (as well as Hispanics and non-Hispanic Blacks) at the time of screening colonoscopy at 50 years of age with continued endoscopic surveillance every 3 years was cost effective, only if gastric intestinal metaplasia (GIM) or more advanced lesions were diagnosed at the index screening EGD.25 Previous studies analyzing the cost effectiveness for gastric cancer screening in the United States had the limitation of not stratifying according to race or ethnicity, or accounting for patients diagnosed with GIM. Subsequently, the same research group extended this model analysis and has published additional findings that this strategy is cost effective for each of the most prevalent Asian American ethnicities (Chinese, Filipino, Southeast Asian, Vietnamese, Korean, and Japanese Americans) in the United States irrespective of sex.26 Although the authors raised a limitation that additional risk factors such as family history, tobacco use, or persistent H. pylori infection were not considered in the model because data regarding differentiated noncardia gastric cancer risk among Asian American ethnicities based on these risk factors are not available.

These two model analytic studies added valuable insights to the body of evidence that subsequent EGDs after the one-time bundled EGD is cost effective for higher-risk asymptomatic people in the United States, if the index screening EGD with gastric mucosal biopsies demonstrates at least GIM. Further population-based research to elucidate risk stratification among higher-risk people will provide a schema that could standardize management and resource allocation as well as increase the cost effectiveness of a gastric cancer screening program in the United States. The degree of risk of developing gastric cancer in autoimmune gastritis varies among the reported studies.27-29 Although the benefit of endoscopic screening in patients with autoimmune gastritis has not been established, a single endoscopic evaluation should be recommended soon after the diagnosis of autoimmune gastritis in order to identify prevalent neoplastic lesions.30
Practical consideration when we perform EGD for early gastric cancer screening
Identification of higher-risk patients should alert an endoscopist to observe mucosa with greater care with a lower threshold to biopsy any suspicious lesions. Preprocedural risk stratification for each individual before performing diagnostic EGD will improve early gastric cancer detection. While we perform EGD, detecting precursor lesions (atrophic gastritis and GIM) is as important as diagnosing an early gastric cancer. Screening and management of patients with precursor lesions (i.e., atrophic gastritis and GIM) is beyond the scope of this article, and this was published in a previous issue of the New Gastroenterologist. It is important to first grossly survey the entire gastric mucosa using high-definition while light (HDWL) endoscopy and screen for any focal irregular (raised or depressed) mucosal lesions. These lesions are often erythematous and should be examined carefully. Use of mucolytic and/or deforming agents (e.g., N-acetylcysteine or simethicone) is recommended for the improvement of visual clarity of gastric mucosa.31 Simethicone is widely used in the United States for colonoscopy and should also be available at the time of EGD for better gastric mucosal visibility. If irregular mucosal lesions are noted, this area should also be examined under narrowband imaging (NBI) in addition to HDWL. According to a simplified classification consisting of mucosal and vascular irregularity, NBI provides better mucosal surface morphology for diagnosis of early gastric cancer compared with HDWL, and a thorough examination of the surface characteristics is a prerequisite.32 This classification was further validated in a randomized control trial, and NBI increased sensitivity for the diagnosis of neoplasia compared with HDWL (92 % vs. 74 %).33 The majority of institutions in the United States have a newer-generation NBI (Olympus America, EVIS EXERA III video system, GIF-HQ190), which provides brighter endoscopic images to better characterize gastric neoplastic lesions. Once we recognize an area suspicious for neoplasia, we should describe the macroscopic features according to a classification system.
The Paris classification, one of the most widely recognized classification systems among U.S. gastroenterologists, is recommended for gastric neoplastic lesions.34Gastric neoplastic lesions with a “superficial” endoscopic appearance are classified as subtypes of “type 0.” The term “type 0” was chosen to distinguish the classification of “superficial” lesions from the Borrmann classification for “advanced” gastric tumors, which includes types 1 to 4. In the classification, a neoplastic lesion is called “superficial” when its endoscopic appearance suggests that the depth of penetration in the digestive wall is not more than into the submucosa (i.e., there is no infiltration of the muscularis propria). The distinctive characters of polypoid and nonpolypoid lesions are summarized in Table 1. Endoscopic submucosal dissection (ESD) has steadily gained acceptance for the treatment of early gastric cancer in the United States. The American Gastroenterological Association recommended in the 2019 institutional updated clinical practice guideline that ESD should be considered the first-line therapy for visible, endoscopically resectable, superficial gastric neoplasia.35 This recommendation is further supported by the published data on efficacy and safety of ESD for early gastric neoplasia in a large multicenter cohort in the United States.36 For all suspicious lesions, irrespective of pathological neoplastic confirmation, referral to an experienced center for further evaluation and endoscopic management should be considered. Lastly, all patients with early gastric cancer should be evaluated for H. pylori infection and treated if the test is positive. Eradication of H. pylori is associated with a lower rate of metachronous gastric cancer,37 and treatment of H. pylori as secondary prevention is also recommended.
Conclusion
As summarized above, cumulative epidemiologic data consistently demonstrate that the incidence of gastric cancer in the U.S. varies according to ethnicity, immigrant status, and country of origin. New gastroenterologists will need to recognize individual risk profiles and identify people at higher risk for gastric cancer. Risk stratification before performing endoscopic evaluation will improve early gastric cancer detection and make noninvasive, effective therapies an option.
References
1. Surveillance, Epidemiology, and End Results Program cancer statistics. https://seer.cancer.gov/statfacts/html/stomach.html.
2. Bray F et al. Ca Cancer J Clin. 2018;68:394-424.
3. Ferro A et al. Eur J Cancer. 2014;50:1330-44.
4. Luo G et al. Int J Cancer. 2017;141:1333-44.
5. Arnold M et al. Eur J Cancer. 2015;51:1164-87.
6. Thrift AP, El-Serag HB. Clin Gastroenterol Hepatol. 2020;18:534-42.
7. Kim Y et al. Epidemiol Health. 2015;37:e2015066.
8. Kamineni A et al. Cancer Causes Control. 1999;10:77-83.
9. Pabla BS et al. Clin Gastroenterol Hepatol. 2020;18:347-59.
10. Shah SC et al. Knowledge Gaps among Physicians Caring for Multiethnic Populations at Increased Gastric Cancer Risk. Gut Liver. 2018 Jan 15;12(1):38-45.
11. International Agency for Research on Cancer. Monographs on the Identification of Carcinogenic Hazards to Humans. IARC. July 7, 2019. 12. Uemura N et al. N Engl J Med. 2001;345:784-9.
13. Lee YC et al. Gastroenterology. 2016;150:1113-24.
14. Ford AC et al. BMJ. 2014;348:g3174.
15. Chey W et al. Am J Gastroenterol. 2017;112:212-39.
16. Kumar S et al. Gastroenterology. 2020;158:527-36.
17. Kumar S et al. Clin Gastroenterol Hepatol. 2020 Apr 6;S1542-3565(20)30436-5.
18. González CA et al. Int J Cancer. 2003;107:629-34.
19. Ladeiras-Lopes R et al. Cancer Causes Control. 2008;19:689-701.
20. Cavaleiro-Pinto M et al. Cancer Causes Control. 2011;22:375-87.
21. Lauby-Secretan B et al. N Engl J Med. 2016;375:794-8.
22. Choi IJ et al. N Engl J Med. 2020;382:427-36.
23. Kim BJ et al. World J Gastroenterol. 2013;19:736-41.
24. Hamashima C. Jpn J Clin Oncol. 2018;48:278–86.
25. Saumoy M et al. Gastroenterology. 2018;155:648-60.
26. Shah SC et al. Clin Gastroenterol Hepatol. 2020 Jul 21:S1542-3565(20)30993-9. doi: 10.1016/j.cgh.2020.07.031.
27. Brinton LA et al. Br J Cancer. 1989;59:810-3.
28. Hsing AW et al. Cancer. 1993;71:745-50.
29. Schafer LW et al. Mayo Clin Proc. 1985;60:444-8.
30. American Society for Gastrointestinal Endoscopy Standards of Practice Committee. Gastrointest Endosc. 2015;82:1-8.
31. Chiu PWY et al. Gut. 2019;68:186-97.
32. Pimentel-Nunes P et al. Endoscopy. 2012;44:236-46.
33. Pimentel-Nunes P et al. Endoscopy. 2016;48:723-30.
34. Participants in the Paris Workshop. Gastrointest Endosc. 2003;58:S3-43.
35. Draganov PV et al. Clin Gastroenterol Hepatol. 2019;17:16-25.
36. Ngamruengphong S et al. Clin Gastroenterol Hepatol. 2020 Jun 18;S1542-3565(20)30834-X. Online ahead of print.
37. Choi IJ et al. N Engl J Med. 2018;378:1085-95.
Dr. Tomizawa is a clinical assistant professor of medicine in the division of gastroenterology, University of Washington, Seattle.
Introduction
Although gastric cancer is one of the most common causes of cancer death in the world, the burden of gastric cancer in the United States tends to be underestimated relative to that of other cancers of the digestive system. In fact, the 5-year survival rate from gastric cancer remains poor (~32%)1 in the United States, and this is largely because gastric cancers are not diagnosed at an early stage when curative therapeutic options are available. Cumulative epidemiologic data consistently demonstrate that the incidence of gastric cancer in the United States varies according to ethnicity, immigrant status, and country of origin. It is important for practicing gastroenterologists in the United States to recognize individual risk profiles and identify people at higher risk for gastric cancer. Hereditary diffuse gastric cancer is an inherited form of diffuse-type gastric cancer and has pathogenic variants in the E-cadherin gene that are inherited in an autosomal dominant pattern. The lifetime risk of gastric cancer in individuals with HDGC is very high, and prophylactic total gastrectomy is usually advised. This article focuses on intestinal type cancer.
Epidemiology
Gastric cancer (proximal and distal gastric cancer combined) is the fifth most frequently diagnosed cancer and the third most common cause of cancer death worldwide, with 1,033,701 new cases and 782,685 deaths in 2018.2 Gastric cancer is subcategorized based on location (proximal [i.e., esophagogastric junctional, gastric cardia] and distal) and histology (intestinal and diffuse type), and each subtype is considered to have a distinct pathogenesis. Distal intestinal type gastric cancer is most commonly encountered in clinical practice. In this article, gastric cancer will signify distal intestinal type gastric cancer unless it is otherwise noted. In general, incidence rates are about twofold higher in men than in women. There is marked geographic variation in incidence rates, and the age-standardized incidence rates in eastern Asia (32.1 and 13.2, per 100,000) are approximately six times higher than those in northern America (5.6 and 2.8, per 100,000) in both men and women, respectively.2 Recent studies evaluating global trends in the incidence and mortality of gastric cancer have demonstrated decreases worldwide.3-5 However, the degree of decrease in the incidence and mortality of gastric cancer varies substantially across geographic regions, reflecting the heterogeneous distribution of risk profiles. A comprehensive analysis of a U.S. population registry demonstrated a linear decrease in the incidence of gastric cancer in the United States (0.94% decrease per year between 2001 and 2015),6 though the annual percent change in the gastric cancer mortality in the United States was lower (around 2% decrease per year between 1980 and 2011) than in other countries.3Several population-based studies conducted in the United States have demonstrated that the incidence of gastric cancer varied by ethnicity, immigrant status, and country of origin, and the highest incidence was observed among Asian immigrants.7,8 A comprehensive meta-analysis examining the risk of gastric cancer in immigrants from high-incidence regions to low-incidence regions found a persistently higher risk of gastric cancer and related mortality among immigrants.9 These results indicate that there are important risk factors such as environmental and dietary factors in addition to the traditionally considered risk factors including male gender, age, family history, and tobacco use. A survey conducted in an ethnically and culturally diverse U.S. city showed that gastroenterology providers demonstrated knowledge deficiencies in identifying and managing patients with increased risk of gastric cancer.10 Recognizing individualized risk profiles in higher-risk groups (e.g., immigrants from higher-incidence/prevalence regions) is important for optimizing management of gastric cancer in the United States.
Assessment and management of modifiable risk factors
Helicobacter pylori, a group 1 carcinogen, is the most well-recognized risk factor for gastric cancer, particularly noncardia gastric cancer.11 Since a landmark longitudinal follow-up study in Japan demonstrated that people with H. pylori infection are more likely to develop gastric cancer than those without H. pylori infection,12 accumulating evidence largely from Asian countries has shown that eradication of H. pylori is associated with a reduced incidence of gastric cancer regardless of baseline risk.13 There are also data on the protective effect for gastric cancer of H. pylori eradication in asymptomatic individuals. Another meta-analysis of six international randomized control trials demonstrated a 34% relative risk reduction of gastric cancer occurrence in asymptomatic people (relative risk of developing gastric cancer was 0.66 in those who received eradication therapy compared with those with placebo or no treatment, 95% CI, 0.46-0.95).14 A U.S. practice guideline published after these meta-analyses recommends that all patients with a positive test indicating active infection with H. pylori should be offered treatment and testing to prove eradication,15 though the recommendation was not purely intended to reduce the gastric cancer risk in U.S. population. Subsequently, a Department of Veterans Affairs cohort study added valuable insights from a U.S. experience to the body of evidence from other countries with higher prevalence. In this study of more than 370,000 patients with a history of H. pylori infection, the detection and successful eradication of H. pylori was associated with a 76% lower incidence of gastric cancer compared with people without H. pylori treatment.16 This study also provided insight into H. pylori treatment practice patterns. Of patients with a positive H. pylori test result (stool antigen, urea breath test, or pathology), approximately 75% were prescribed an eradication regimen and only 21% of those underwent eradication tests. A low rate (24%) of eradication testing was subsequently reported by the same group among U.S. patients regardless of gastric cancer risk profiles.17 The lesson from the aforementioned study is that treatment and eradication of H. pylori even among asymptomatic U.S. patients reduces the risk of subsequent gastric cancer. However, it may be difficult to generalize the results of this study given the nature of the Veterans Affairs cohort, and more data are required to justify the implementation of nationwide preventive H. pylori screening in the general U.S. population.
Smoking has been recognized as the other important risk factor. A study from the European prospective multicenter cohort demonstrated a significant association of cigarette smoking and gastric cancer risk (HR for ever-smokers 1.45 [95% CI, 1.08-1.94], current-smokers in males 1.73 [95% CI, 1.06-2.83], and current smokers in females 1.87 [95% CI, 1.12-3.12], respectively) after adjustment for educational level, dietary consumption profiles, alcohol intake, and body mass index (BMI).18 A subsequent meta-analysis provided solid evidence of smoking as the important behavioral risk factor for gastric cancer.19 Smoking also predisposed to the development of proximal gastric cancer.20 Along with other cancers in the digestive system such as in the esophagus, colon and rectum, liver, gallbladder, and pancreas, a significant association of BMI and the risk of proximal gastric cancer (RR of the highest BMI category compared with normal BMI, 1.8 [95% CI, 1.3-2.5]) was reported, with positive dose-response relationships; however, the association was not sufficient for distal gastric cancer.21 There is also evidence to show a trend of greater alcohol consumption (>45 grams per day [about 3 drinks a day]) associated with the increased risk of gastric cancer.21 It has been thought that salt and salt-preserved food increase the risk of gastric cancer. It should be noted that the observational studies showing the associations were published from Asian countries where such foods were a substantial part of traditional diets (e.g., salted vegetables in Japan) and the incidence of gastric cancer is high. There is also a speculation that preserved foods may have been eaten in more underserved, low socioeconomic regions where refrigeration was not available and prevalence of H. pylori infection was higher. Except for documented inherited form of gastric cancer (e.g., HDGC or hereditary cancer syndromes), most gastric cancers are considered sporadic. A recent randomized study published from South Korea investigated a cohort of higher-risk asymptomatic patients with family history significant for gastric cancer. This study of 1,676 subjects with a median follow-up of 9.2 years showed that successful eradication of H. pylori in the first-degree relatives of those with gastric cancer significantly reduced the risk (HR 0.45 [95% CI, 0.21-0.94]) of developing gastric cancer.22 As previously discussed, in the United States where the prevalence of H. pylori and the incidence of gastric cancer are both lower than in some Asian countries, routine screening of asymptomatic individuals for H. pylori is not justified yet. There may be a role for screening individuals who are first-generation immigrants from areas of high gastric cancer incidence and also have a first-degree relative with gastric cancer.
Who should we consider high risk and offer screening EGD?
With available evidence to date, screening for gastric cancer in a general U.S. population is not recommended. However, it is important to acknowledge the aforementioned varying incidence of gastric cancer in the United States among ethnicity, immigrant status, and country of origin. Immigrants from high-incidence regions maintain a higher risk of gastric cancer and related mortality even after migration to lower-incidence regions. The latter comprehensive study estimated that as many as 12.7 million people (29.4% of total U.S. immigrant population) have emigrated from higher-incidence regions including East Asian and some Central American countries.9 Indeed, an opportunistic nationwide gastric cancer screening program has been implemented in South Korea (beginning at age 40, biannually)23 and Japan (beginning at age 50, biannually).24 Two decision-analytic simulation studies have provided insight into the uncertainty about the cost effectiveness for potential targeted gastric cancer screening in higher-risk populations in the United States. One study demonstrated that esophagogastroduodenoscopy (EGD) screening for otherwise asymptomatic Asian American people (as well as Hispanics and non-Hispanic Blacks) at the time of screening colonoscopy at 50 years of age with continued endoscopic surveillance every 3 years was cost effective, only if gastric intestinal metaplasia (GIM) or more advanced lesions were diagnosed at the index screening EGD.25 Previous studies analyzing the cost effectiveness for gastric cancer screening in the United States had the limitation of not stratifying according to race or ethnicity, or accounting for patients diagnosed with GIM. Subsequently, the same research group extended this model analysis and has published additional findings that this strategy is cost effective for each of the most prevalent Asian American ethnicities (Chinese, Filipino, Southeast Asian, Vietnamese, Korean, and Japanese Americans) in the United States irrespective of sex.26 Although the authors raised a limitation that additional risk factors such as family history, tobacco use, or persistent H. pylori infection were not considered in the model because data regarding differentiated noncardia gastric cancer risk among Asian American ethnicities based on these risk factors are not available.

These two model analytic studies added valuable insights to the body of evidence that subsequent EGDs after the one-time bundled EGD is cost effective for higher-risk asymptomatic people in the United States, if the index screening EGD with gastric mucosal biopsies demonstrates at least GIM. Further population-based research to elucidate risk stratification among higher-risk people will provide a schema that could standardize management and resource allocation as well as increase the cost effectiveness of a gastric cancer screening program in the United States. The degree of risk of developing gastric cancer in autoimmune gastritis varies among the reported studies.27-29 Although the benefit of endoscopic screening in patients with autoimmune gastritis has not been established, a single endoscopic evaluation should be recommended soon after the diagnosis of autoimmune gastritis in order to identify prevalent neoplastic lesions.30
Practical consideration when we perform EGD for early gastric cancer screening
Identification of higher-risk patients should alert an endoscopist to observe mucosa with greater care with a lower threshold to biopsy any suspicious lesions. Preprocedural risk stratification for each individual before performing diagnostic EGD will improve early gastric cancer detection. While we perform EGD, detecting precursor lesions (atrophic gastritis and GIM) is as important as diagnosing an early gastric cancer. Screening and management of patients with precursor lesions (i.e., atrophic gastritis and GIM) is beyond the scope of this article, and this was published in a previous issue of the New Gastroenterologist. It is important to first grossly survey the entire gastric mucosa using high-definition while light (HDWL) endoscopy and screen for any focal irregular (raised or depressed) mucosal lesions. These lesions are often erythematous and should be examined carefully. Use of mucolytic and/or deforming agents (e.g., N-acetylcysteine or simethicone) is recommended for the improvement of visual clarity of gastric mucosa.31 Simethicone is widely used in the United States for colonoscopy and should also be available at the time of EGD for better gastric mucosal visibility. If irregular mucosal lesions are noted, this area should also be examined under narrowband imaging (NBI) in addition to HDWL. According to a simplified classification consisting of mucosal and vascular irregularity, NBI provides better mucosal surface morphology for diagnosis of early gastric cancer compared with HDWL, and a thorough examination of the surface characteristics is a prerequisite.32 This classification was further validated in a randomized control trial, and NBI increased sensitivity for the diagnosis of neoplasia compared with HDWL (92 % vs. 74 %).33 The majority of institutions in the United States have a newer-generation NBI (Olympus America, EVIS EXERA III video system, GIF-HQ190), which provides brighter endoscopic images to better characterize gastric neoplastic lesions. Once we recognize an area suspicious for neoplasia, we should describe the macroscopic features according to a classification system.
The Paris classification, one of the most widely recognized classification systems among U.S. gastroenterologists, is recommended for gastric neoplastic lesions.34Gastric neoplastic lesions with a “superficial” endoscopic appearance are classified as subtypes of “type 0.” The term “type 0” was chosen to distinguish the classification of “superficial” lesions from the Borrmann classification for “advanced” gastric tumors, which includes types 1 to 4. In the classification, a neoplastic lesion is called “superficial” when its endoscopic appearance suggests that the depth of penetration in the digestive wall is not more than into the submucosa (i.e., there is no infiltration of the muscularis propria). The distinctive characters of polypoid and nonpolypoid lesions are summarized in Table 1. Endoscopic submucosal dissection (ESD) has steadily gained acceptance for the treatment of early gastric cancer in the United States. The American Gastroenterological Association recommended in the 2019 institutional updated clinical practice guideline that ESD should be considered the first-line therapy for visible, endoscopically resectable, superficial gastric neoplasia.35 This recommendation is further supported by the published data on efficacy and safety of ESD for early gastric neoplasia in a large multicenter cohort in the United States.36 For all suspicious lesions, irrespective of pathological neoplastic confirmation, referral to an experienced center for further evaluation and endoscopic management should be considered. Lastly, all patients with early gastric cancer should be evaluated for H. pylori infection and treated if the test is positive. Eradication of H. pylori is associated with a lower rate of metachronous gastric cancer,37 and treatment of H. pylori as secondary prevention is also recommended.
Conclusion
As summarized above, cumulative epidemiologic data consistently demonstrate that the incidence of gastric cancer in the U.S. varies according to ethnicity, immigrant status, and country of origin. New gastroenterologists will need to recognize individual risk profiles and identify people at higher risk for gastric cancer. Risk stratification before performing endoscopic evaluation will improve early gastric cancer detection and make noninvasive, effective therapies an option.
References
1. Surveillance, Epidemiology, and End Results Program cancer statistics. https://seer.cancer.gov/statfacts/html/stomach.html.
2. Bray F et al. Ca Cancer J Clin. 2018;68:394-424.
3. Ferro A et al. Eur J Cancer. 2014;50:1330-44.
4. Luo G et al. Int J Cancer. 2017;141:1333-44.
5. Arnold M et al. Eur J Cancer. 2015;51:1164-87.
6. Thrift AP, El-Serag HB. Clin Gastroenterol Hepatol. 2020;18:534-42.
7. Kim Y et al. Epidemiol Health. 2015;37:e2015066.
8. Kamineni A et al. Cancer Causes Control. 1999;10:77-83.
9. Pabla BS et al. Clin Gastroenterol Hepatol. 2020;18:347-59.
10. Shah SC et al. Knowledge Gaps among Physicians Caring for Multiethnic Populations at Increased Gastric Cancer Risk. Gut Liver. 2018 Jan 15;12(1):38-45.
11. International Agency for Research on Cancer. Monographs on the Identification of Carcinogenic Hazards to Humans. IARC. July 7, 2019. 12. Uemura N et al. N Engl J Med. 2001;345:784-9.
13. Lee YC et al. Gastroenterology. 2016;150:1113-24.
14. Ford AC et al. BMJ. 2014;348:g3174.
15. Chey W et al. Am J Gastroenterol. 2017;112:212-39.
16. Kumar S et al. Gastroenterology. 2020;158:527-36.
17. Kumar S et al. Clin Gastroenterol Hepatol. 2020 Apr 6;S1542-3565(20)30436-5.
18. González CA et al. Int J Cancer. 2003;107:629-34.
19. Ladeiras-Lopes R et al. Cancer Causes Control. 2008;19:689-701.
20. Cavaleiro-Pinto M et al. Cancer Causes Control. 2011;22:375-87.
21. Lauby-Secretan B et al. N Engl J Med. 2016;375:794-8.
22. Choi IJ et al. N Engl J Med. 2020;382:427-36.
23. Kim BJ et al. World J Gastroenterol. 2013;19:736-41.
24. Hamashima C. Jpn J Clin Oncol. 2018;48:278–86.
25. Saumoy M et al. Gastroenterology. 2018;155:648-60.
26. Shah SC et al. Clin Gastroenterol Hepatol. 2020 Jul 21:S1542-3565(20)30993-9. doi: 10.1016/j.cgh.2020.07.031.
27. Brinton LA et al. Br J Cancer. 1989;59:810-3.
28. Hsing AW et al. Cancer. 1993;71:745-50.
29. Schafer LW et al. Mayo Clin Proc. 1985;60:444-8.
30. American Society for Gastrointestinal Endoscopy Standards of Practice Committee. Gastrointest Endosc. 2015;82:1-8.
31. Chiu PWY et al. Gut. 2019;68:186-97.
32. Pimentel-Nunes P et al. Endoscopy. 2012;44:236-46.
33. Pimentel-Nunes P et al. Endoscopy. 2016;48:723-30.
34. Participants in the Paris Workshop. Gastrointest Endosc. 2003;58:S3-43.
35. Draganov PV et al. Clin Gastroenterol Hepatol. 2019;17:16-25.
36. Ngamruengphong S et al. Clin Gastroenterol Hepatol. 2020 Jun 18;S1542-3565(20)30834-X. Online ahead of print.
37. Choi IJ et al. N Engl J Med. 2018;378:1085-95.
Dr. Tomizawa is a clinical assistant professor of medicine in the division of gastroenterology, University of Washington, Seattle.
Calendar
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Jan. 15-17, 2021
Gastrointestinal Cancers Symposium
Through an engaging lineup of novel science, education, and exhibits, the virtual 2021 Gastrointestinal (GI) Cancers Symposium offers new, innovative findings in GI cancer treatment, research, and care.
Early-bird deadline: Dec. 16, 2020.
Jan. 21-24, 2021
Crohn’s & Colitis Congress®Join health care professionals and researchers virtually at the Crohn’s & Colitis Congress® for the premier conference on IBD. Discover different perspectives, practical information you can immediately implement, and potential treatments on the horizon.
Early-bird deadline: Friday, Nov. 6, 2020.
May 21-23, 2021
Digestive Disease Week® (DDW)
Save the date for the world’s leading event in digestive disease. DDW® brings professionals in gastroenterology, hepatology, endoscopy, and GI surgery together. Experience growth when you share your research, converge with trailblazers, and improve the lives of patients suffering from GI and liver diseases.
Abstract submission window Oct. 15 to Dec. 3, 2020.
AWARD DEADLINES
American Gastroenterological Association (AGA) Student Abstract Award
This $500 travel award supports recipients who are graduate students, medical students,or medical residents (residents up to postgraduate year 3) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Student Abstract of the Year and receive a $1,000 award.
Application deadline: Feb. 24, 2021
AGA–Moti L. & Kamla Rustgi International Travel Awards
This $750 travel award provides support to early-career (that is, 35 years of age or younger at the time of DDW) basic, translational or clinical investigators residing outside North America to offset travel and related expenses to attend DDW.
Application deadline: Feb. 24, 2021
AGA Fellow Abstract Award
This $500 travel award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations DDW. The top-scoring abstract will be designated the Fellow Abstract of the Year and receive a $1,000 award.
Application deadline: Feb. 24, 2021
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Jan. 15-17, 2021
Gastrointestinal Cancers Symposium
Through an engaging lineup of novel science, education, and exhibits, the virtual 2021 Gastrointestinal (GI) Cancers Symposium offers new, innovative findings in GI cancer treatment, research, and care.
Early-bird deadline: Dec. 16, 2020.
Jan. 21-24, 2021
Crohn’s & Colitis Congress®Join health care professionals and researchers virtually at the Crohn’s & Colitis Congress® for the premier conference on IBD. Discover different perspectives, practical information you can immediately implement, and potential treatments on the horizon.
Early-bird deadline: Friday, Nov. 6, 2020.
May 21-23, 2021
Digestive Disease Week® (DDW)
Save the date for the world’s leading event in digestive disease. DDW® brings professionals in gastroenterology, hepatology, endoscopy, and GI surgery together. Experience growth when you share your research, converge with trailblazers, and improve the lives of patients suffering from GI and liver diseases.
Abstract submission window Oct. 15 to Dec. 3, 2020.
AWARD DEADLINES
American Gastroenterological Association (AGA) Student Abstract Award
This $500 travel award supports recipients who are graduate students, medical students,or medical residents (residents up to postgraduate year 3) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Student Abstract of the Year and receive a $1,000 award.
Application deadline: Feb. 24, 2021
AGA–Moti L. & Kamla Rustgi International Travel Awards
This $750 travel award provides support to early-career (that is, 35 years of age or younger at the time of DDW) basic, translational or clinical investigators residing outside North America to offset travel and related expenses to attend DDW.
Application deadline: Feb. 24, 2021
AGA Fellow Abstract Award
This $500 travel award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations DDW. The top-scoring abstract will be designated the Fellow Abstract of the Year and receive a $1,000 award.
Application deadline: Feb. 24, 2021
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Jan. 15-17, 2021
Gastrointestinal Cancers Symposium
Through an engaging lineup of novel science, education, and exhibits, the virtual 2021 Gastrointestinal (GI) Cancers Symposium offers new, innovative findings in GI cancer treatment, research, and care.
Early-bird deadline: Dec. 16, 2020.
Jan. 21-24, 2021
Crohn’s & Colitis Congress®Join health care professionals and researchers virtually at the Crohn’s & Colitis Congress® for the premier conference on IBD. Discover different perspectives, practical information you can immediately implement, and potential treatments on the horizon.
Early-bird deadline: Friday, Nov. 6, 2020.
May 21-23, 2021
Digestive Disease Week® (DDW)
Save the date for the world’s leading event in digestive disease. DDW® brings professionals in gastroenterology, hepatology, endoscopy, and GI surgery together. Experience growth when you share your research, converge with trailblazers, and improve the lives of patients suffering from GI and liver diseases.
Abstract submission window Oct. 15 to Dec. 3, 2020.
AWARD DEADLINES
American Gastroenterological Association (AGA) Student Abstract Award
This $500 travel award supports recipients who are graduate students, medical students,or medical residents (residents up to postgraduate year 3) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Student Abstract of the Year and receive a $1,000 award.
Application deadline: Feb. 24, 2021
AGA–Moti L. & Kamla Rustgi International Travel Awards
This $750 travel award provides support to early-career (that is, 35 years of age or younger at the time of DDW) basic, translational or clinical investigators residing outside North America to offset travel and related expenses to attend DDW.
Application deadline: Feb. 24, 2021
AGA Fellow Abstract Award
This $500 travel award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations DDW. The top-scoring abstract will be designated the Fellow Abstract of the Year and receive a $1,000 award.
Application deadline: Feb. 24, 2021
Quality measures and initiatives in private practices
It has been almost 15 years since the American College of Gastroenterology and American Society for Gastrointestinal Endoscopy established the Task Force on Quality Endoscopy and published the first set of quality indicators for GI endoscopic procedures.
This work was motivated by two seminal reports on patient safety that fostered a demand by the public, policy makers, and payers to accurately define and measure the quality of health care services.
While the Centers for Medicare & Medicaid Services initially designated and required reporting on several basic outcome measures, leaders within the field of gastroenterology recognized the importance of developing evidence-based quality measures for our field, and specifically for endoscopic procedures.
Integrating safety measures into our daily operations has always been important, and over the years, policies have been implemented to incentivize health care providers to meet standards in everything from patient safety to patient satisfaction.
Defining quality and how to measure it
The goals of implementing quality measures within private practices include effective patient care and safety, but they also include issues like access and affordability, as well as the professionalism of your physicians and advanced practice providers.
As a larger practice, we have the resources to support a quality coordinator who spends half their time focused on quality measures. Every provider is required to complete annual education on quality parameters.
We have two committees that propose and track quality initiatives in our practice. We have one on the practice side and one for our ambulatory surgery centers (ASCs). The committees are made of physicians who have a particular interest in quality measures. On the ASC side, our ASC center director from our management partner AmSurg is also a member of the committee.
The road to improving quality within a private practice starts by defining the aspects of care that affect the quality of the patient experience.
Tracking quality in the office and in the surgery center
In our practices we have about 60 physicians. Start times and coding accuracy are good examples of what we have tracked in the past as areas of quality improvement. For instance, if only one or two providers get started late, it can cause a domino effect. Schedules get cramped, which can increase stress and possibly cause our team members to rush. Even things that seem like patient satisfaction issues can affect patient care, so it is important to make sure they are being measured.
On the ASC side, we track adenoma detection rates, colonoscopy intervals, complication rates, and many other additional criteria. As an example, when a pathology report is issued, we require our physicians to provide results to our patients within 72 hours.
Data on all providers are tabulated quarterly and then distributed to the providers in the form of a scorecard. The scorecard is then used for constructive feedback on improvements that can be made. A cumulative annual report is given to the providers, which is also incorporated into reviews. Not paying attention to quality measures can potentially have financial ramifications for providers in our group.
Find the right fit from a quality standpoint
In terms of what we are tracking, we are probably not that different from most groups of our size. Standardization will continue to increase, and it is important as an early career physician to familiarize yourself with quality measures in gastroenterology.
I often interview early career physicians who would like to join Regional GI, and the most impressive are the young men and women who ask about our processes for tracking quality measures and implementing programs geared toward improvement. If you are thinking of joining a practice, bring it up. You will be glad you did.
The interest in quality shows that you are invested in providing the best evidence-based patient care. As an independent group, this is critical because so much of what we do depends on having a track record of measurement. For instance, an ASC might not be credentialed if the quality metrics do not meet a certain threshold.
We are looking for potential partners who are seriously interested in joining us on our mission to provide the highest-quality care to our patients. After all, that is why became gastroenterologists in the first place.
Dr. Lalani serves as treasurer on the executive committee of the Digestive Health Physicians Association and is a practicing gastroenterologist at U.S. Digestive Health.
It has been almost 15 years since the American College of Gastroenterology and American Society for Gastrointestinal Endoscopy established the Task Force on Quality Endoscopy and published the first set of quality indicators for GI endoscopic procedures.
This work was motivated by two seminal reports on patient safety that fostered a demand by the public, policy makers, and payers to accurately define and measure the quality of health care services.
While the Centers for Medicare & Medicaid Services initially designated and required reporting on several basic outcome measures, leaders within the field of gastroenterology recognized the importance of developing evidence-based quality measures for our field, and specifically for endoscopic procedures.
Integrating safety measures into our daily operations has always been important, and over the years, policies have been implemented to incentivize health care providers to meet standards in everything from patient safety to patient satisfaction.
Defining quality and how to measure it
The goals of implementing quality measures within private practices include effective patient care and safety, but they also include issues like access and affordability, as well as the professionalism of your physicians and advanced practice providers.
As a larger practice, we have the resources to support a quality coordinator who spends half their time focused on quality measures. Every provider is required to complete annual education on quality parameters.
We have two committees that propose and track quality initiatives in our practice. We have one on the practice side and one for our ambulatory surgery centers (ASCs). The committees are made of physicians who have a particular interest in quality measures. On the ASC side, our ASC center director from our management partner AmSurg is also a member of the committee.
The road to improving quality within a private practice starts by defining the aspects of care that affect the quality of the patient experience.
Tracking quality in the office and in the surgery center
In our practices we have about 60 physicians. Start times and coding accuracy are good examples of what we have tracked in the past as areas of quality improvement. For instance, if only one or two providers get started late, it can cause a domino effect. Schedules get cramped, which can increase stress and possibly cause our team members to rush. Even things that seem like patient satisfaction issues can affect patient care, so it is important to make sure they are being measured.
On the ASC side, we track adenoma detection rates, colonoscopy intervals, complication rates, and many other additional criteria. As an example, when a pathology report is issued, we require our physicians to provide results to our patients within 72 hours.
Data on all providers are tabulated quarterly and then distributed to the providers in the form of a scorecard. The scorecard is then used for constructive feedback on improvements that can be made. A cumulative annual report is given to the providers, which is also incorporated into reviews. Not paying attention to quality measures can potentially have financial ramifications for providers in our group.
Find the right fit from a quality standpoint
In terms of what we are tracking, we are probably not that different from most groups of our size. Standardization will continue to increase, and it is important as an early career physician to familiarize yourself with quality measures in gastroenterology.
I often interview early career physicians who would like to join Regional GI, and the most impressive are the young men and women who ask about our processes for tracking quality measures and implementing programs geared toward improvement. If you are thinking of joining a practice, bring it up. You will be glad you did.
The interest in quality shows that you are invested in providing the best evidence-based patient care. As an independent group, this is critical because so much of what we do depends on having a track record of measurement. For instance, an ASC might not be credentialed if the quality metrics do not meet a certain threshold.
We are looking for potential partners who are seriously interested in joining us on our mission to provide the highest-quality care to our patients. After all, that is why became gastroenterologists in the first place.
Dr. Lalani serves as treasurer on the executive committee of the Digestive Health Physicians Association and is a practicing gastroenterologist at U.S. Digestive Health.
It has been almost 15 years since the American College of Gastroenterology and American Society for Gastrointestinal Endoscopy established the Task Force on Quality Endoscopy and published the first set of quality indicators for GI endoscopic procedures.
This work was motivated by two seminal reports on patient safety that fostered a demand by the public, policy makers, and payers to accurately define and measure the quality of health care services.
While the Centers for Medicare & Medicaid Services initially designated and required reporting on several basic outcome measures, leaders within the field of gastroenterology recognized the importance of developing evidence-based quality measures for our field, and specifically for endoscopic procedures.
Integrating safety measures into our daily operations has always been important, and over the years, policies have been implemented to incentivize health care providers to meet standards in everything from patient safety to patient satisfaction.
Defining quality and how to measure it
The goals of implementing quality measures within private practices include effective patient care and safety, but they also include issues like access and affordability, as well as the professionalism of your physicians and advanced practice providers.
As a larger practice, we have the resources to support a quality coordinator who spends half their time focused on quality measures. Every provider is required to complete annual education on quality parameters.
We have two committees that propose and track quality initiatives in our practice. We have one on the practice side and one for our ambulatory surgery centers (ASCs). The committees are made of physicians who have a particular interest in quality measures. On the ASC side, our ASC center director from our management partner AmSurg is also a member of the committee.
The road to improving quality within a private practice starts by defining the aspects of care that affect the quality of the patient experience.
Tracking quality in the office and in the surgery center
In our practices we have about 60 physicians. Start times and coding accuracy are good examples of what we have tracked in the past as areas of quality improvement. For instance, if only one or two providers get started late, it can cause a domino effect. Schedules get cramped, which can increase stress and possibly cause our team members to rush. Even things that seem like patient satisfaction issues can affect patient care, so it is important to make sure they are being measured.
On the ASC side, we track adenoma detection rates, colonoscopy intervals, complication rates, and many other additional criteria. As an example, when a pathology report is issued, we require our physicians to provide results to our patients within 72 hours.
Data on all providers are tabulated quarterly and then distributed to the providers in the form of a scorecard. The scorecard is then used for constructive feedback on improvements that can be made. A cumulative annual report is given to the providers, which is also incorporated into reviews. Not paying attention to quality measures can potentially have financial ramifications for providers in our group.
Find the right fit from a quality standpoint
In terms of what we are tracking, we are probably not that different from most groups of our size. Standardization will continue to increase, and it is important as an early career physician to familiarize yourself with quality measures in gastroenterology.
I often interview early career physicians who would like to join Regional GI, and the most impressive are the young men and women who ask about our processes for tracking quality measures and implementing programs geared toward improvement. If you are thinking of joining a practice, bring it up. You will be glad you did.
The interest in quality shows that you are invested in providing the best evidence-based patient care. As an independent group, this is critical because so much of what we do depends on having a track record of measurement. For instance, an ASC might not be credentialed if the quality metrics do not meet a certain threshold.
We are looking for potential partners who are seriously interested in joining us on our mission to provide the highest-quality care to our patients. After all, that is why became gastroenterologists in the first place.
Dr. Lalani serves as treasurer on the executive committee of the Digestive Health Physicians Association and is a practicing gastroenterologist at U.S. Digestive Health.
November 2020 – ICYMI
Gastroenterology
July 2020
Role of cannabis and its derivatives in gastrointestinal and hepatic disease. Jonathan Gotfried et al. 2020 July;159(1):62-80. doi: 10.1053/j.gastro.2020.03.087
Effects of blended (yellow) vs forced coagulation (blue) currents on adverse events, complete resection, or polyp recurrence after polypectomy in a large randomized trial. Heiko Pohl et al. 2020 July;159(1):119-28.e2. doi: 10.1053/j.gastro.2020.03.014
Calculating the starting age for screening in relatives of patients with colorectal cancer based on data from large nationwide data sets.
Yu Tian et al. July 2020;159(1):159-168.e3. doi: 10.1053/j.gastro.2020.03.063
August 2020
Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Erica J. Brenner et al. 2020 Aug 159;(2):481-91.e3. doi: 10.1053/j.gastro.2020.05.032
Collagenous colitis is associated with HLA signature and shares genetic risks with other immune-mediated diseases. Eli Stahl et al. 2020 Aug;159(2):549-61.e8. doi: 10.1053/j.gastro.2020.04.063
Efficacy of real-time computer-aided detection of colorectal neoplasia in a randomized trial. Alessandro Repici et al. 2020 Aug;159(2):512-20.e7. doi: 10.1053/j.gastro.2020.04.062
September 2020
Dietary inflammatory potential and risk of Crohn’s disease and ulcerative colitis. Chun-Han Lo et al. 2020 Sept;159(3):p873-83.e1. doi: 10.1053/j.gastro.2020.05.011
Rates of incomplete resection of 1- to 20-mm colorectal polyps: A systematic review and meta-analysis. Roupen Djinbachian et al. 2020 Sept;159(3):904-14.e12. doi: 10.1053/j.gastro.2020.05.018
Clinical Gastroenterology and Hepatology
August 2020
Prevalence and characteristics of avoidant/restrictive food intake disorder in adult neurogastroenterology patients. Helen Burton Murray et al. 2020 Aug;18(9):1995-2002.e1. doi: 10.1016/j.cgh.2019.10.030
Ten things every gastroenterologist should know about antireflux surgery. Steven Park et al. 2020 Aug;18(9):1923-9. doi: 10.1016/j.cgh.2020.02.041
Biopsies from ascending and descending colon are sufficient for diagnosis of microscopic colitis. Boris Virine et al. 2020 Aug;18(9):2003-9. doi: 10.1016/j.cgh.2020.02.036
September 2020
Association between endoscopist annual procedure volume and colonoscopy quality: Systematic review and meta-analysis. Nauzer Forbes et al. 2020 Sept:18(10):2192-208.e12. doi: 10.1016/j.cgh.2020.03.046
Plans to reactivate gastroenterology practices following the COVID-19 pandemic: A survey of North American centers. Vladimir M. Kushnir et al on Behalf of the North American Alliance for the Study of Digestive Manifestations of COVID-19. 2020 Sept;18(10):2287-94.e1. doi: 10.1016/j.cgh.2020.05.030
Cost effectiveness of different strategies for detecting cirrhosis in patients with nonalcoholic fatty liver disease based on United States health care system. Eduardo Vilar-Gomez et al. 2020 Sept;18(10):2305-14.e12. doi: 10.1016/j.cgh.2020.04.017
October 2020
AGA Clinical Practice Update on young adult–onset colorectal cancer diagnosis and management: Expert review. Lisa A. Boardman et al. 2020 Oct:18(11):2415-24. doi: 10.1016/j.cgh.2020.05.058
Frequency of eating disorder pathology among patients with chronic constipation and contribution of gastrointestinal-specific anxiety. Helen Burton Murray et al. 2020 Oct;18(11):2471-8. doi: 10.1016/j.cgh.2019.12.030
Correction of dyssynergic defecation, but not fiber supplementation, reduces symptoms of functional dyspepsia in patients with constipation in a randomized trial. Jose-Walter Huaman et al. 2020 Oct;18(11):2463-70.e1. doi: 10.1016/j.cgh.2019.11.048
Cellular and Molecular Gastroenterology and Hepatology
A new treatment for chronic hepatitis B and D offers novel insights into obesity and hepatic steatosis. Robert Schierwagen et al. 2020;10(3):649-51. doi: 10.1016/j.jcmgh.2020.05.011
Techniques and Innovations in Gastrointestinal Endoscopy
The impact of endoscopic submucosal dissection for gastric adenocarcinomas in the United States. Shria Kumar et al. 2020 July:22(3):93-8. doi: 10.1016/j.tige.2020.03.009
Gastroenterology
July 2020
Role of cannabis and its derivatives in gastrointestinal and hepatic disease. Jonathan Gotfried et al. 2020 July;159(1):62-80. doi: 10.1053/j.gastro.2020.03.087
Effects of blended (yellow) vs forced coagulation (blue) currents on adverse events, complete resection, or polyp recurrence after polypectomy in a large randomized trial. Heiko Pohl et al. 2020 July;159(1):119-28.e2. doi: 10.1053/j.gastro.2020.03.014
Calculating the starting age for screening in relatives of patients with colorectal cancer based on data from large nationwide data sets.
Yu Tian et al. July 2020;159(1):159-168.e3. doi: 10.1053/j.gastro.2020.03.063
August 2020
Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Erica J. Brenner et al. 2020 Aug 159;(2):481-91.e3. doi: 10.1053/j.gastro.2020.05.032
Collagenous colitis is associated with HLA signature and shares genetic risks with other immune-mediated diseases. Eli Stahl et al. 2020 Aug;159(2):549-61.e8. doi: 10.1053/j.gastro.2020.04.063
Efficacy of real-time computer-aided detection of colorectal neoplasia in a randomized trial. Alessandro Repici et al. 2020 Aug;159(2):512-20.e7. doi: 10.1053/j.gastro.2020.04.062
September 2020
Dietary inflammatory potential and risk of Crohn’s disease and ulcerative colitis. Chun-Han Lo et al. 2020 Sept;159(3):p873-83.e1. doi: 10.1053/j.gastro.2020.05.011
Rates of incomplete resection of 1- to 20-mm colorectal polyps: A systematic review and meta-analysis. Roupen Djinbachian et al. 2020 Sept;159(3):904-14.e12. doi: 10.1053/j.gastro.2020.05.018
Clinical Gastroenterology and Hepatology
August 2020
Prevalence and characteristics of avoidant/restrictive food intake disorder in adult neurogastroenterology patients. Helen Burton Murray et al. 2020 Aug;18(9):1995-2002.e1. doi: 10.1016/j.cgh.2019.10.030
Ten things every gastroenterologist should know about antireflux surgery. Steven Park et al. 2020 Aug;18(9):1923-9. doi: 10.1016/j.cgh.2020.02.041
Biopsies from ascending and descending colon are sufficient for diagnosis of microscopic colitis. Boris Virine et al. 2020 Aug;18(9):2003-9. doi: 10.1016/j.cgh.2020.02.036
September 2020
Association between endoscopist annual procedure volume and colonoscopy quality: Systematic review and meta-analysis. Nauzer Forbes et al. 2020 Sept:18(10):2192-208.e12. doi: 10.1016/j.cgh.2020.03.046
Plans to reactivate gastroenterology practices following the COVID-19 pandemic: A survey of North American centers. Vladimir M. Kushnir et al on Behalf of the North American Alliance for the Study of Digestive Manifestations of COVID-19. 2020 Sept;18(10):2287-94.e1. doi: 10.1016/j.cgh.2020.05.030
Cost effectiveness of different strategies for detecting cirrhosis in patients with nonalcoholic fatty liver disease based on United States health care system. Eduardo Vilar-Gomez et al. 2020 Sept;18(10):2305-14.e12. doi: 10.1016/j.cgh.2020.04.017
October 2020
AGA Clinical Practice Update on young adult–onset colorectal cancer diagnosis and management: Expert review. Lisa A. Boardman et al. 2020 Oct:18(11):2415-24. doi: 10.1016/j.cgh.2020.05.058
Frequency of eating disorder pathology among patients with chronic constipation and contribution of gastrointestinal-specific anxiety. Helen Burton Murray et al. 2020 Oct;18(11):2471-8. doi: 10.1016/j.cgh.2019.12.030
Correction of dyssynergic defecation, but not fiber supplementation, reduces symptoms of functional dyspepsia in patients with constipation in a randomized trial. Jose-Walter Huaman et al. 2020 Oct;18(11):2463-70.e1. doi: 10.1016/j.cgh.2019.11.048
Cellular and Molecular Gastroenterology and Hepatology
A new treatment for chronic hepatitis B and D offers novel insights into obesity and hepatic steatosis. Robert Schierwagen et al. 2020;10(3):649-51. doi: 10.1016/j.jcmgh.2020.05.011
Techniques and Innovations in Gastrointestinal Endoscopy
The impact of endoscopic submucosal dissection for gastric adenocarcinomas in the United States. Shria Kumar et al. 2020 July:22(3):93-8. doi: 10.1016/j.tige.2020.03.009
Gastroenterology
July 2020
Role of cannabis and its derivatives in gastrointestinal and hepatic disease. Jonathan Gotfried et al. 2020 July;159(1):62-80. doi: 10.1053/j.gastro.2020.03.087
Effects of blended (yellow) vs forced coagulation (blue) currents on adverse events, complete resection, or polyp recurrence after polypectomy in a large randomized trial. Heiko Pohl et al. 2020 July;159(1):119-28.e2. doi: 10.1053/j.gastro.2020.03.014
Calculating the starting age for screening in relatives of patients with colorectal cancer based on data from large nationwide data sets.
Yu Tian et al. July 2020;159(1):159-168.e3. doi: 10.1053/j.gastro.2020.03.063
August 2020
Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: results from an international registry. Erica J. Brenner et al. 2020 Aug 159;(2):481-91.e3. doi: 10.1053/j.gastro.2020.05.032
Collagenous colitis is associated with HLA signature and shares genetic risks with other immune-mediated diseases. Eli Stahl et al. 2020 Aug;159(2):549-61.e8. doi: 10.1053/j.gastro.2020.04.063
Efficacy of real-time computer-aided detection of colorectal neoplasia in a randomized trial. Alessandro Repici et al. 2020 Aug;159(2):512-20.e7. doi: 10.1053/j.gastro.2020.04.062
September 2020
Dietary inflammatory potential and risk of Crohn’s disease and ulcerative colitis. Chun-Han Lo et al. 2020 Sept;159(3):p873-83.e1. doi: 10.1053/j.gastro.2020.05.011
Rates of incomplete resection of 1- to 20-mm colorectal polyps: A systematic review and meta-analysis. Roupen Djinbachian et al. 2020 Sept;159(3):904-14.e12. doi: 10.1053/j.gastro.2020.05.018
Clinical Gastroenterology and Hepatology
August 2020
Prevalence and characteristics of avoidant/restrictive food intake disorder in adult neurogastroenterology patients. Helen Burton Murray et al. 2020 Aug;18(9):1995-2002.e1. doi: 10.1016/j.cgh.2019.10.030
Ten things every gastroenterologist should know about antireflux surgery. Steven Park et al. 2020 Aug;18(9):1923-9. doi: 10.1016/j.cgh.2020.02.041
Biopsies from ascending and descending colon are sufficient for diagnosis of microscopic colitis. Boris Virine et al. 2020 Aug;18(9):2003-9. doi: 10.1016/j.cgh.2020.02.036
September 2020
Association between endoscopist annual procedure volume and colonoscopy quality: Systematic review and meta-analysis. Nauzer Forbes et al. 2020 Sept:18(10):2192-208.e12. doi: 10.1016/j.cgh.2020.03.046
Plans to reactivate gastroenterology practices following the COVID-19 pandemic: A survey of North American centers. Vladimir M. Kushnir et al on Behalf of the North American Alliance for the Study of Digestive Manifestations of COVID-19. 2020 Sept;18(10):2287-94.e1. doi: 10.1016/j.cgh.2020.05.030
Cost effectiveness of different strategies for detecting cirrhosis in patients with nonalcoholic fatty liver disease based on United States health care system. Eduardo Vilar-Gomez et al. 2020 Sept;18(10):2305-14.e12. doi: 10.1016/j.cgh.2020.04.017
October 2020
AGA Clinical Practice Update on young adult–onset colorectal cancer diagnosis and management: Expert review. Lisa A. Boardman et al. 2020 Oct:18(11):2415-24. doi: 10.1016/j.cgh.2020.05.058
Frequency of eating disorder pathology among patients with chronic constipation and contribution of gastrointestinal-specific anxiety. Helen Burton Murray et al. 2020 Oct;18(11):2471-8. doi: 10.1016/j.cgh.2019.12.030
Correction of dyssynergic defecation, but not fiber supplementation, reduces symptoms of functional dyspepsia in patients with constipation in a randomized trial. Jose-Walter Huaman et al. 2020 Oct;18(11):2463-70.e1. doi: 10.1016/j.cgh.2019.11.048
Cellular and Molecular Gastroenterology and Hepatology
A new treatment for chronic hepatitis B and D offers novel insights into obesity and hepatic steatosis. Robert Schierwagen et al. 2020;10(3):649-51. doi: 10.1016/j.jcmgh.2020.05.011
Techniques and Innovations in Gastrointestinal Endoscopy
The impact of endoscopic submucosal dissection for gastric adenocarcinomas in the United States. Shria Kumar et al. 2020 July:22(3):93-8. doi: 10.1016/j.tige.2020.03.009
AGA News
Receive $300,000 for your research in health disparities
Applications for the research scholar award are due by Nov. 9, 2020.
The American Gastroenterological Association Research Foundation is pleased to announce an important addition to its prestigious awards portfolio. The AGA Research Scholar Award in Digestive Disease Health Disparities supports early-career faculty dedicated to investigating digestive diseases or disorders that disproportionately affect racial or ethnic minority populations in North America.
Applicants must have a full-time faculty (or equivalent) position and may be performing any type of research (clinical, basic, or translational). Awardees will receive a total of $300,000 over 3 years with funding to commence in July 2021. The deadline to apply is Nov. 9, 2020.
This award is just one example of how AGA is helping to improve patient care for those who need it most. Support AGA Giving Day and learn more about the AGA Equity Project – a multiyear effort spanning all aspects of our organization to achieve equity and eradicate disparities in digestive diseases.
Save the date for DDW Virtual™
In 2021, Digestive Disease Week® moves online as a fully virtual meeting with slightly new dates: May 21-23, 2021.
For more than 50 years, the digestive disease community has connected over the best science, education, and networking at DDW, and we’re confident this year will be no exception. In fact, we’re excited by opportunities the new format provides to learn, share, and connect with each other.
Watch the DDW website for more information as it becomes available. In the meantime, check out our FAQs about DDW Virtual™. If you have a question we didn’t answer, please submit a ticket to our help desk.
DDW is jointly sponsored by AGA, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
Virtual 2021 Crohn’s & Colitis Congress® now open for registration
Help forge the roadmap to advance prevention, treatments, and cures for all patients living with inflammatory bowel disease (IBD).
Join the Crohn’s & Colitis Foundation, AGA, and a true community of friends and colleagues at the premier conference on IBD. The fourth annual Crohn’s & Colitis Congress®, taking place virtually Jan. 21-24, 2021, is now open for registration.
The 2021 Crohn’s & Colitis Congress virtual experience will look a little different but will still bring you all the benefits and quality programming you have come to expect. The Congress will offer 4 days of learning, with more than 100 speakers and more than 200 expected abstracts – all from the safety of your home or work. Now at an even more affordable price, access from anywhere, and the ability to hear from the top leaders in the IBD field – this is a unique opportunity to join us as we come together virtually.
By moving our event online, we can now pass on greater savings to you. Registration for the conference provides you with substantial savings over last year and access to all sessions and networking opportunities. This virtual experience will bring our community of IBD professionals together in an engaging, interactive setting which will include breakout rooms, receptions, and much more.
The 2021 congress committee chair David T. Rubin, MD, AGAF, University of Chicago, and cochair Bruce E. Sands, MD, MS, AGAF, Icahn School of Medicine at Mount Sinai, New York, lead a faculty that includes thought leaders in the fields of GI, research investigation, surgery, pediatrics, advanced practice, IBD nursing, diet and nutrition, mental health, radiology, pathology, and more.
Register and get inspired to improve skills and patient outcomes, learn practical information you can immediately implement, hear what’s on the horizon in potential IBD treatments, discover fresh perspectives from multidisciplinary faculty and attendees.
You don’t want to miss the 2021 Crohn’s & Colitis Congress, connecting virtually on Jan. 21-24, 2021.
Register today to save before the early bird deadline of Friday, Nov. 6.
Learn more, submit an abstract, and register by visiting crohnscolitiscongress.org.
AGA releases largest real-world report on safety and effectiveness of fecal microbiota transplantation
About 90% of patients tracked in the AGA FMT National Registry were cured of Clostridioides difficile infection with few serious side effects.
AGA has released the first results from the NIH-funded AGA Fecal Microbiota Transplantation (FMT) National Registry, the largest real-world study on the safety and effectiveness of FMT. Published in Gastroenterology, the registry reported that FMT led to a cure of C. difficile infection in 90% of patients across 20 North American FMT practice sites. Few serious side effects were reported.
“While the value of fecal microbiota transplantation for treating recurrent C. difficile infection is clear from research studies, the potential long-term consequences of altering a patient’s gut microbiota are not fully known,” says Colleen R. Kelly, MD, AGAF, associate professor of medicine at Brown University, Providence, R.I. and coprincipal investigator of the AGA FMT National Registry. “Releasing the initial results of the AGA FMT National Registry is an important step toward understanding the true risks and benefits of microbiota therapeutics in a real-world setting.”
This new report details effectiveness and safety outcomes from the first 259 patients enrolled in the registry between December 2017 and September 2019. Almost all participants received FMT using an unknown donor from stool banks. The most common method of FMT delivery was colonoscopy followed by upper endoscopy. Of the 222 participants who returned for the 1-month follow-up, 200 participants (90%) had their C. difficile infection cured with 197 of those requiring only a single FMT. Infections were reported in 11 participants, but only 2 were thought to be possibly related to the procedure. FMT response was deemed durable, with recurrence of C. difficile infection in the 6 months after successful FMT occurring in only 4% of participants. This data includes patients with comorbidities, such as IBD and immunocompromised status, who are typically excluded from FMT clinical trials.
“These initial results show a high success rate of FMT in the real-world setting. We’ll continue to track these patients for 10 years to assess long-term safety, which will be critical to determining the full safety profile of FMT,” added Dr. Kelly.
AGA raises concerns about recent executive order
We are speaking out to ensure a brighter and more equitable future.
AGA is concerned by the Executive Order on Combating Race and Sex Stereotyping issued on Sept. 22, 2020. This order, while confirming that training of the federal workforce to create an inclusive workspace is beneficial, also leads to a misguided perception of the purpose and outcomes of this type of training. In addition, it may have unintended ramifications for institutions receiving federal research funding.
We believe it is critical and necessary to understand both the positive and negative realities of our nation’s history, so that together we can forge forward into a brighter, and more equitable future.
As highlighted in AGA’s commentary published in Gastroenterology, AGA believes that equity is defined by fair treatment, access, opportunity, and advancement for all, acknowledging that there are historically underserved and underrepresented populations. Equity requires identifying and eliminating barriers that have created unbalanced conditions and prevented the full participation of some groups in order to provide equal opportunity for all groups.
By default, teaching and practicing equity, diversity and inclusion aims not to place any group above or below any other group, or to create division. It rather seeks to achieve fairness and understanding, and fully recognize the dignity of all groups, identities, and individuals.
AGA stands with the Association of American Medical Colleges in our commitment to being a diverse, inclusive, equitable, and antiracist organization.
Our commitment to this issue is manifest in the AGA Equity Project.
Receive $300,000 for your research in health disparities
Applications for the research scholar award are due by Nov. 9, 2020.
The American Gastroenterological Association Research Foundation is pleased to announce an important addition to its prestigious awards portfolio. The AGA Research Scholar Award in Digestive Disease Health Disparities supports early-career faculty dedicated to investigating digestive diseases or disorders that disproportionately affect racial or ethnic minority populations in North America.
Applicants must have a full-time faculty (or equivalent) position and may be performing any type of research (clinical, basic, or translational). Awardees will receive a total of $300,000 over 3 years with funding to commence in July 2021. The deadline to apply is Nov. 9, 2020.
This award is just one example of how AGA is helping to improve patient care for those who need it most. Support AGA Giving Day and learn more about the AGA Equity Project – a multiyear effort spanning all aspects of our organization to achieve equity and eradicate disparities in digestive diseases.
Save the date for DDW Virtual™
In 2021, Digestive Disease Week® moves online as a fully virtual meeting with slightly new dates: May 21-23, 2021.
For more than 50 years, the digestive disease community has connected over the best science, education, and networking at DDW, and we’re confident this year will be no exception. In fact, we’re excited by opportunities the new format provides to learn, share, and connect with each other.
Watch the DDW website for more information as it becomes available. In the meantime, check out our FAQs about DDW Virtual™. If you have a question we didn’t answer, please submit a ticket to our help desk.
DDW is jointly sponsored by AGA, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
Virtual 2021 Crohn’s & Colitis Congress® now open for registration
Help forge the roadmap to advance prevention, treatments, and cures for all patients living with inflammatory bowel disease (IBD).
Join the Crohn’s & Colitis Foundation, AGA, and a true community of friends and colleagues at the premier conference on IBD. The fourth annual Crohn’s & Colitis Congress®, taking place virtually Jan. 21-24, 2021, is now open for registration.
The 2021 Crohn’s & Colitis Congress virtual experience will look a little different but will still bring you all the benefits and quality programming you have come to expect. The Congress will offer 4 days of learning, with more than 100 speakers and more than 200 expected abstracts – all from the safety of your home or work. Now at an even more affordable price, access from anywhere, and the ability to hear from the top leaders in the IBD field – this is a unique opportunity to join us as we come together virtually.
By moving our event online, we can now pass on greater savings to you. Registration for the conference provides you with substantial savings over last year and access to all sessions and networking opportunities. This virtual experience will bring our community of IBD professionals together in an engaging, interactive setting which will include breakout rooms, receptions, and much more.
The 2021 congress committee chair David T. Rubin, MD, AGAF, University of Chicago, and cochair Bruce E. Sands, MD, MS, AGAF, Icahn School of Medicine at Mount Sinai, New York, lead a faculty that includes thought leaders in the fields of GI, research investigation, surgery, pediatrics, advanced practice, IBD nursing, diet and nutrition, mental health, radiology, pathology, and more.
Register and get inspired to improve skills and patient outcomes, learn practical information you can immediately implement, hear what’s on the horizon in potential IBD treatments, discover fresh perspectives from multidisciplinary faculty and attendees.
You don’t want to miss the 2021 Crohn’s & Colitis Congress, connecting virtually on Jan. 21-24, 2021.
Register today to save before the early bird deadline of Friday, Nov. 6.
Learn more, submit an abstract, and register by visiting crohnscolitiscongress.org.
AGA releases largest real-world report on safety and effectiveness of fecal microbiota transplantation
About 90% of patients tracked in the AGA FMT National Registry were cured of Clostridioides difficile infection with few serious side effects.
AGA has released the first results from the NIH-funded AGA Fecal Microbiota Transplantation (FMT) National Registry, the largest real-world study on the safety and effectiveness of FMT. Published in Gastroenterology, the registry reported that FMT led to a cure of C. difficile infection in 90% of patients across 20 North American FMT practice sites. Few serious side effects were reported.
“While the value of fecal microbiota transplantation for treating recurrent C. difficile infection is clear from research studies, the potential long-term consequences of altering a patient’s gut microbiota are not fully known,” says Colleen R. Kelly, MD, AGAF, associate professor of medicine at Brown University, Providence, R.I. and coprincipal investigator of the AGA FMT National Registry. “Releasing the initial results of the AGA FMT National Registry is an important step toward understanding the true risks and benefits of microbiota therapeutics in a real-world setting.”
This new report details effectiveness and safety outcomes from the first 259 patients enrolled in the registry between December 2017 and September 2019. Almost all participants received FMT using an unknown donor from stool banks. The most common method of FMT delivery was colonoscopy followed by upper endoscopy. Of the 222 participants who returned for the 1-month follow-up, 200 participants (90%) had their C. difficile infection cured with 197 of those requiring only a single FMT. Infections were reported in 11 participants, but only 2 were thought to be possibly related to the procedure. FMT response was deemed durable, with recurrence of C. difficile infection in the 6 months after successful FMT occurring in only 4% of participants. This data includes patients with comorbidities, such as IBD and immunocompromised status, who are typically excluded from FMT clinical trials.
“These initial results show a high success rate of FMT in the real-world setting. We’ll continue to track these patients for 10 years to assess long-term safety, which will be critical to determining the full safety profile of FMT,” added Dr. Kelly.
AGA raises concerns about recent executive order
We are speaking out to ensure a brighter and more equitable future.
AGA is concerned by the Executive Order on Combating Race and Sex Stereotyping issued on Sept. 22, 2020. This order, while confirming that training of the federal workforce to create an inclusive workspace is beneficial, also leads to a misguided perception of the purpose and outcomes of this type of training. In addition, it may have unintended ramifications for institutions receiving federal research funding.
We believe it is critical and necessary to understand both the positive and negative realities of our nation’s history, so that together we can forge forward into a brighter, and more equitable future.
As highlighted in AGA’s commentary published in Gastroenterology, AGA believes that equity is defined by fair treatment, access, opportunity, and advancement for all, acknowledging that there are historically underserved and underrepresented populations. Equity requires identifying and eliminating barriers that have created unbalanced conditions and prevented the full participation of some groups in order to provide equal opportunity for all groups.
By default, teaching and practicing equity, diversity and inclusion aims not to place any group above or below any other group, or to create division. It rather seeks to achieve fairness and understanding, and fully recognize the dignity of all groups, identities, and individuals.
AGA stands with the Association of American Medical Colleges in our commitment to being a diverse, inclusive, equitable, and antiracist organization.
Our commitment to this issue is manifest in the AGA Equity Project.
Receive $300,000 for your research in health disparities
Applications for the research scholar award are due by Nov. 9, 2020.
The American Gastroenterological Association Research Foundation is pleased to announce an important addition to its prestigious awards portfolio. The AGA Research Scholar Award in Digestive Disease Health Disparities supports early-career faculty dedicated to investigating digestive diseases or disorders that disproportionately affect racial or ethnic minority populations in North America.
Applicants must have a full-time faculty (or equivalent) position and may be performing any type of research (clinical, basic, or translational). Awardees will receive a total of $300,000 over 3 years with funding to commence in July 2021. The deadline to apply is Nov. 9, 2020.
This award is just one example of how AGA is helping to improve patient care for those who need it most. Support AGA Giving Day and learn more about the AGA Equity Project – a multiyear effort spanning all aspects of our organization to achieve equity and eradicate disparities in digestive diseases.
Save the date for DDW Virtual™
In 2021, Digestive Disease Week® moves online as a fully virtual meeting with slightly new dates: May 21-23, 2021.
For more than 50 years, the digestive disease community has connected over the best science, education, and networking at DDW, and we’re confident this year will be no exception. In fact, we’re excited by opportunities the new format provides to learn, share, and connect with each other.
Watch the DDW website for more information as it becomes available. In the meantime, check out our FAQs about DDW Virtual™. If you have a question we didn’t answer, please submit a ticket to our help desk.
DDW is jointly sponsored by AGA, the American Association for the Study of Liver Diseases, the American Society for Gastrointestinal Endoscopy, and the Society for Surgery of the Alimentary Tract.
Virtual 2021 Crohn’s & Colitis Congress® now open for registration
Help forge the roadmap to advance prevention, treatments, and cures for all patients living with inflammatory bowel disease (IBD).
Join the Crohn’s & Colitis Foundation, AGA, and a true community of friends and colleagues at the premier conference on IBD. The fourth annual Crohn’s & Colitis Congress®, taking place virtually Jan. 21-24, 2021, is now open for registration.
The 2021 Crohn’s & Colitis Congress virtual experience will look a little different but will still bring you all the benefits and quality programming you have come to expect. The Congress will offer 4 days of learning, with more than 100 speakers and more than 200 expected abstracts – all from the safety of your home or work. Now at an even more affordable price, access from anywhere, and the ability to hear from the top leaders in the IBD field – this is a unique opportunity to join us as we come together virtually.
By moving our event online, we can now pass on greater savings to you. Registration for the conference provides you with substantial savings over last year and access to all sessions and networking opportunities. This virtual experience will bring our community of IBD professionals together in an engaging, interactive setting which will include breakout rooms, receptions, and much more.
The 2021 congress committee chair David T. Rubin, MD, AGAF, University of Chicago, and cochair Bruce E. Sands, MD, MS, AGAF, Icahn School of Medicine at Mount Sinai, New York, lead a faculty that includes thought leaders in the fields of GI, research investigation, surgery, pediatrics, advanced practice, IBD nursing, diet and nutrition, mental health, radiology, pathology, and more.
Register and get inspired to improve skills and patient outcomes, learn practical information you can immediately implement, hear what’s on the horizon in potential IBD treatments, discover fresh perspectives from multidisciplinary faculty and attendees.
You don’t want to miss the 2021 Crohn’s & Colitis Congress, connecting virtually on Jan. 21-24, 2021.
Register today to save before the early bird deadline of Friday, Nov. 6.
Learn more, submit an abstract, and register by visiting crohnscolitiscongress.org.
AGA releases largest real-world report on safety and effectiveness of fecal microbiota transplantation
About 90% of patients tracked in the AGA FMT National Registry were cured of Clostridioides difficile infection with few serious side effects.
AGA has released the first results from the NIH-funded AGA Fecal Microbiota Transplantation (FMT) National Registry, the largest real-world study on the safety and effectiveness of FMT. Published in Gastroenterology, the registry reported that FMT led to a cure of C. difficile infection in 90% of patients across 20 North American FMT practice sites. Few serious side effects were reported.
“While the value of fecal microbiota transplantation for treating recurrent C. difficile infection is clear from research studies, the potential long-term consequences of altering a patient’s gut microbiota are not fully known,” says Colleen R. Kelly, MD, AGAF, associate professor of medicine at Brown University, Providence, R.I. and coprincipal investigator of the AGA FMT National Registry. “Releasing the initial results of the AGA FMT National Registry is an important step toward understanding the true risks and benefits of microbiota therapeutics in a real-world setting.”
This new report details effectiveness and safety outcomes from the first 259 patients enrolled in the registry between December 2017 and September 2019. Almost all participants received FMT using an unknown donor from stool banks. The most common method of FMT delivery was colonoscopy followed by upper endoscopy. Of the 222 participants who returned for the 1-month follow-up, 200 participants (90%) had their C. difficile infection cured with 197 of those requiring only a single FMT. Infections were reported in 11 participants, but only 2 were thought to be possibly related to the procedure. FMT response was deemed durable, with recurrence of C. difficile infection in the 6 months after successful FMT occurring in only 4% of participants. This data includes patients with comorbidities, such as IBD and immunocompromised status, who are typically excluded from FMT clinical trials.
“These initial results show a high success rate of FMT in the real-world setting. We’ll continue to track these patients for 10 years to assess long-term safety, which will be critical to determining the full safety profile of FMT,” added Dr. Kelly.
AGA raises concerns about recent executive order
We are speaking out to ensure a brighter and more equitable future.
AGA is concerned by the Executive Order on Combating Race and Sex Stereotyping issued on Sept. 22, 2020. This order, while confirming that training of the federal workforce to create an inclusive workspace is beneficial, also leads to a misguided perception of the purpose and outcomes of this type of training. In addition, it may have unintended ramifications for institutions receiving federal research funding.
We believe it is critical and necessary to understand both the positive and negative realities of our nation’s history, so that together we can forge forward into a brighter, and more equitable future.
As highlighted in AGA’s commentary published in Gastroenterology, AGA believes that equity is defined by fair treatment, access, opportunity, and advancement for all, acknowledging that there are historically underserved and underrepresented populations. Equity requires identifying and eliminating barriers that have created unbalanced conditions and prevented the full participation of some groups in order to provide equal opportunity for all groups.
By default, teaching and practicing equity, diversity and inclusion aims not to place any group above or below any other group, or to create division. It rather seeks to achieve fairness and understanding, and fully recognize the dignity of all groups, identities, and individuals.
AGA stands with the Association of American Medical Colleges in our commitment to being a diverse, inclusive, equitable, and antiracist organization.
Our commitment to this issue is manifest in the AGA Equity Project.
Endoscopic drainage of pancreatic fluid collections
Pancreatic fluid collections (PFCs) are common after acute pancreatitis but almost always resolve spontaneously. Persistent collections that cause symptoms, become infected, and/or compress vital structures require treatment. Open surgery had traditionally been considered the standard method for this indication;1 Given its inherently less invasive nature, endoscopic transmural drainage (ETMD) has become a mainstay of this step-up philosophy – it is now the dominant strategy for pseudocyst drainage and, on the basis of emerging randomized trial data, compares very favorably with surgery for the treatment of walled-off necrosis (WON).
According to the step-up approach, the initial treatment of symptomatic and/or infected collections that are within 4 weeks of an attack of pancreatitis involves conservative management because the wall of the collection is typically immature; the systemic inflammation may be significantly exacerbated by definitive drainage, particularly surgery. In this early phase, failure of conservative management is addressed by percutaneous catheter placement, stepping up to a minimally invasive operation if the response to percutaneous drainage and antibiotics is insufficient.
Collections that are at least 4 weeks from the onset of acute pancreatitis are considered mature and termed pseudocysts or WONs depending on whether they contain pure fluid or necrotic tissue. In this phase, endoscopic treatment plays a primary management role because these collections are generally adherent to the stomach or duodenal wall and their capsule is organized enough to withstand endoscopic intervention. If treatment can be held off until this phase, then percutaneous and surgical drainage can often be avoided.
In practice, the 4-week rule holds true for most, but not all, PFCs. ETMD can be performed in some particularly mature collections prior to 4 weeks if the indication is strong and the collection appears to have a mature wall. However, the potential for cyst wall perforation is higher and should be considered in the risk-benefit discussion. Conversely, some collections beyond 4 weeks lack an adequately organized wall and require additional time for maturation.
While endoscopic drainage of pseudocysts has essentially supplanted surgery, the management of WON is more complex and remains multidisciplinary. Two recent randomized trials demonstrated no difference in major complications and/or death between a surgical and endoscopic step-up strategy for WON.2,3 Rates of pancreatic fistulae, hospital stay, and overall treatment costs, however, favored endoscopy. Nevertheless, defining the ideal strategy for many of these patients with complexity requires multidisciplinary discussion. Surgery continues to play a primary role in several scenarios, including collections that are not close to the upper GI tract, those that are particularly complex and extend caudally, and situations in which the endoscopic progress is too slow.
The three most important questions when deciding to embark on ETMD are: (1) whether drainage is indicated (that is, is the patient symptomatic or is there evidence that the PFC is infected?), (2) whether the wall of the collection is adequately mature and apposed to the GI tract wall; and (3) whether the collection contains necrosis? This last question has critical implications in the technical approach to drainage. While CT scan with IV contrast is accurate for assessing wall maturity, it is inadequate to evaluate the presence or quantity of necrosum. Transabdominal ultrasound, endoscopic ultrasound, and MRI (on a T2 sequence) are all superior for this purpose. MRI has the additional benefit of assessing the pancreatic duct integrity, which may influence subsequent management.
Pseudocysts can be managed by cyst-gastrostomy or cyst-duodenostomy alone, whereas most WONs require the additional step of endoscopic necrosectomy – the process of entering the cyst cavity to mechanically debride necrotic tissue. Because of a higher rate of technical success, endoscopic ultrasound–directed creation of the transmural drainage pathway has become standard practice. In addition, it is likely safer, allowing for the identification and avoidance of interceding vessels and other vital structures. The role of endoscopic retrograde cholangiopancreatography with pancreatic stent placement as primary therapy for PFCs is limited to the drainage of small collections (<5 cm), for which it is the preferred treatment strategy. It is as effective as ETMD, which may not be feasible or safe for small PFCs.
Plastic double-pigtail stents have traditionally been used to maintain the transmural tract for both pseudocyst and WON. Recently, however, metallic stents have become more popular. Fully covered biliary self-expanding metallic stents (SEMS) are easier to place, have a larger lumen, and are associated with improved outcomes, compared with plastic stents in observational studies of pseudocyst drainage. Lumen-apposing metallic stents (LAMS) have become the preferred prosthesis for WON drainage given the ability to near-simultaneously establish access and deploy the stent, as well as their much larger caliber lumen which permits seamless entry into the cavity with an endoscope. Based on ease and efficiency of use, LAMS are also commonly employed for pseudocyst drainage, although entry into the cavity is unnecessary.
Plastic stents have been shown to be more cost effective than LAMS for pseudocyst drainage, although the economics around biliary SEMS in this context have not been explored. Robust comparative effectiveness data defining the optimal prostheses for pseudocysts are needed. The literature comparing LAMS to plastic stents for the management of WON is mixed. Studies have shown LAMS to be more cost effective, but a small randomized trial demonstrated no difference in clinical success or in the number of procedures to achieve WON resolution.4 We generally favor LAMS for WON since large-caliber balloon dilation of the tract seems safer within the lumen of the LAMS (which could seal small perforations and tamponade bleeding vessels) than within a freshly created tract.
Secondary infection of the cavity, usually because of stent occlusion, and bleeding are the most common complications of ETMD. Even in the absence of stent occlusion, contamination of the collection after ETMD is ubiquitous and, as such, we prescribe prophylactic antibiotics for 1-2 weeks after the procedure, although this practice is not evidence based. Hemorrhage appears to be increasing in frequency with the diffusion of LAMS; this has been postulated to be due to particularly rapid cyst cavity collapse resulting in erosion of the stent into contralateral cyst wall vessels. CT angiography followed by an embolization procedure for a possible pseudoaneurysm is the mainstay of treatment. Serious venous bleeding is more challenging to address because angiographic options are limited.
Despite tremendous recent advances, several important controversies in the endoscopic management of PFCs persist. The optimal prosthesis, the importance of first-session endoscopic necroscopy (compared with stepping up to endoscopic necroscopy only if necessary), the roles of adjunctive drain placement and chemical debridement (such as hydrogen peroxide), the need for concomitant pancreatic stent placement, and the preferred long-term management of a disconnected pancreatic duct are areas for which additional research is sorely needed. We further discuss these questions and many additional technical considerations pertaining to endoscopic drainage in a recent review.5
In summary, endoscopic transmural drainage of mature PFCs is effective and safe. Existing evidence supports its use as the favored treatment modality in appropriate candidates and has rendered it a mainstay of the therapeutic armamentarium for this disease. Further studies are needed to address critical unanswered questions and to develop a uniform endoscopic management paradigm.
References
1. van Santvoort HC et al. N Engl J Med. 2010;362(16):1491-502.
2. van Brunschot S et al. Lancet. 2018;391(10115):51-8.
3. Bang JY et al. Gastroenterology. 2019;156(4):1027-40.
4. Bang JY et al. Gut. 2019;68(7):1200-9.
5. Elmunzer BJ. Clin Gastroenterol Hepatol. 2018;16(12):1851-63.
Dr. Moran is assistant professor of medicine, division of gastroenterology and hepatology, Medical University of South Carolina, Charleston; Dr. Elmunzer is the Peter Cotton Professor of Medicine and Endoscopic Innovation, division of gastroenterology and hepatology, Medical University of South Carolina. The authors have no conflicts of interest pertaining to this review.
Pancreatic fluid collections (PFCs) are common after acute pancreatitis but almost always resolve spontaneously. Persistent collections that cause symptoms, become infected, and/or compress vital structures require treatment. Open surgery had traditionally been considered the standard method for this indication;1 Given its inherently less invasive nature, endoscopic transmural drainage (ETMD) has become a mainstay of this step-up philosophy – it is now the dominant strategy for pseudocyst drainage and, on the basis of emerging randomized trial data, compares very favorably with surgery for the treatment of walled-off necrosis (WON).
According to the step-up approach, the initial treatment of symptomatic and/or infected collections that are within 4 weeks of an attack of pancreatitis involves conservative management because the wall of the collection is typically immature; the systemic inflammation may be significantly exacerbated by definitive drainage, particularly surgery. In this early phase, failure of conservative management is addressed by percutaneous catheter placement, stepping up to a minimally invasive operation if the response to percutaneous drainage and antibiotics is insufficient.
Collections that are at least 4 weeks from the onset of acute pancreatitis are considered mature and termed pseudocysts or WONs depending on whether they contain pure fluid or necrotic tissue. In this phase, endoscopic treatment plays a primary management role because these collections are generally adherent to the stomach or duodenal wall and their capsule is organized enough to withstand endoscopic intervention. If treatment can be held off until this phase, then percutaneous and surgical drainage can often be avoided.
In practice, the 4-week rule holds true for most, but not all, PFCs. ETMD can be performed in some particularly mature collections prior to 4 weeks if the indication is strong and the collection appears to have a mature wall. However, the potential for cyst wall perforation is higher and should be considered in the risk-benefit discussion. Conversely, some collections beyond 4 weeks lack an adequately organized wall and require additional time for maturation.
While endoscopic drainage of pseudocysts has essentially supplanted surgery, the management of WON is more complex and remains multidisciplinary. Two recent randomized trials demonstrated no difference in major complications and/or death between a surgical and endoscopic step-up strategy for WON.2,3 Rates of pancreatic fistulae, hospital stay, and overall treatment costs, however, favored endoscopy. Nevertheless, defining the ideal strategy for many of these patients with complexity requires multidisciplinary discussion. Surgery continues to play a primary role in several scenarios, including collections that are not close to the upper GI tract, those that are particularly complex and extend caudally, and situations in which the endoscopic progress is too slow.
The three most important questions when deciding to embark on ETMD are: (1) whether drainage is indicated (that is, is the patient symptomatic or is there evidence that the PFC is infected?), (2) whether the wall of the collection is adequately mature and apposed to the GI tract wall; and (3) whether the collection contains necrosis? This last question has critical implications in the technical approach to drainage. While CT scan with IV contrast is accurate for assessing wall maturity, it is inadequate to evaluate the presence or quantity of necrosum. Transabdominal ultrasound, endoscopic ultrasound, and MRI (on a T2 sequence) are all superior for this purpose. MRI has the additional benefit of assessing the pancreatic duct integrity, which may influence subsequent management.
Pseudocysts can be managed by cyst-gastrostomy or cyst-duodenostomy alone, whereas most WONs require the additional step of endoscopic necrosectomy – the process of entering the cyst cavity to mechanically debride necrotic tissue. Because of a higher rate of technical success, endoscopic ultrasound–directed creation of the transmural drainage pathway has become standard practice. In addition, it is likely safer, allowing for the identification and avoidance of interceding vessels and other vital structures. The role of endoscopic retrograde cholangiopancreatography with pancreatic stent placement as primary therapy for PFCs is limited to the drainage of small collections (<5 cm), for which it is the preferred treatment strategy. It is as effective as ETMD, which may not be feasible or safe for small PFCs.
Plastic double-pigtail stents have traditionally been used to maintain the transmural tract for both pseudocyst and WON. Recently, however, metallic stents have become more popular. Fully covered biliary self-expanding metallic stents (SEMS) are easier to place, have a larger lumen, and are associated with improved outcomes, compared with plastic stents in observational studies of pseudocyst drainage. Lumen-apposing metallic stents (LAMS) have become the preferred prosthesis for WON drainage given the ability to near-simultaneously establish access and deploy the stent, as well as their much larger caliber lumen which permits seamless entry into the cavity with an endoscope. Based on ease and efficiency of use, LAMS are also commonly employed for pseudocyst drainage, although entry into the cavity is unnecessary.
Plastic stents have been shown to be more cost effective than LAMS for pseudocyst drainage, although the economics around biliary SEMS in this context have not been explored. Robust comparative effectiveness data defining the optimal prostheses for pseudocysts are needed. The literature comparing LAMS to plastic stents for the management of WON is mixed. Studies have shown LAMS to be more cost effective, but a small randomized trial demonstrated no difference in clinical success or in the number of procedures to achieve WON resolution.4 We generally favor LAMS for WON since large-caliber balloon dilation of the tract seems safer within the lumen of the LAMS (which could seal small perforations and tamponade bleeding vessels) than within a freshly created tract.
Secondary infection of the cavity, usually because of stent occlusion, and bleeding are the most common complications of ETMD. Even in the absence of stent occlusion, contamination of the collection after ETMD is ubiquitous and, as such, we prescribe prophylactic antibiotics for 1-2 weeks after the procedure, although this practice is not evidence based. Hemorrhage appears to be increasing in frequency with the diffusion of LAMS; this has been postulated to be due to particularly rapid cyst cavity collapse resulting in erosion of the stent into contralateral cyst wall vessels. CT angiography followed by an embolization procedure for a possible pseudoaneurysm is the mainstay of treatment. Serious venous bleeding is more challenging to address because angiographic options are limited.
Despite tremendous recent advances, several important controversies in the endoscopic management of PFCs persist. The optimal prosthesis, the importance of first-session endoscopic necroscopy (compared with stepping up to endoscopic necroscopy only if necessary), the roles of adjunctive drain placement and chemical debridement (such as hydrogen peroxide), the need for concomitant pancreatic stent placement, and the preferred long-term management of a disconnected pancreatic duct are areas for which additional research is sorely needed. We further discuss these questions and many additional technical considerations pertaining to endoscopic drainage in a recent review.5
In summary, endoscopic transmural drainage of mature PFCs is effective and safe. Existing evidence supports its use as the favored treatment modality in appropriate candidates and has rendered it a mainstay of the therapeutic armamentarium for this disease. Further studies are needed to address critical unanswered questions and to develop a uniform endoscopic management paradigm.
References
1. van Santvoort HC et al. N Engl J Med. 2010;362(16):1491-502.
2. van Brunschot S et al. Lancet. 2018;391(10115):51-8.
3. Bang JY et al. Gastroenterology. 2019;156(4):1027-40.
4. Bang JY et al. Gut. 2019;68(7):1200-9.
5. Elmunzer BJ. Clin Gastroenterol Hepatol. 2018;16(12):1851-63.
Dr. Moran is assistant professor of medicine, division of gastroenterology and hepatology, Medical University of South Carolina, Charleston; Dr. Elmunzer is the Peter Cotton Professor of Medicine and Endoscopic Innovation, division of gastroenterology and hepatology, Medical University of South Carolina. The authors have no conflicts of interest pertaining to this review.
Pancreatic fluid collections (PFCs) are common after acute pancreatitis but almost always resolve spontaneously. Persistent collections that cause symptoms, become infected, and/or compress vital structures require treatment. Open surgery had traditionally been considered the standard method for this indication;1 Given its inherently less invasive nature, endoscopic transmural drainage (ETMD) has become a mainstay of this step-up philosophy – it is now the dominant strategy for pseudocyst drainage and, on the basis of emerging randomized trial data, compares very favorably with surgery for the treatment of walled-off necrosis (WON).
According to the step-up approach, the initial treatment of symptomatic and/or infected collections that are within 4 weeks of an attack of pancreatitis involves conservative management because the wall of the collection is typically immature; the systemic inflammation may be significantly exacerbated by definitive drainage, particularly surgery. In this early phase, failure of conservative management is addressed by percutaneous catheter placement, stepping up to a minimally invasive operation if the response to percutaneous drainage and antibiotics is insufficient.
Collections that are at least 4 weeks from the onset of acute pancreatitis are considered mature and termed pseudocysts or WONs depending on whether they contain pure fluid or necrotic tissue. In this phase, endoscopic treatment plays a primary management role because these collections are generally adherent to the stomach or duodenal wall and their capsule is organized enough to withstand endoscopic intervention. If treatment can be held off until this phase, then percutaneous and surgical drainage can often be avoided.
In practice, the 4-week rule holds true for most, but not all, PFCs. ETMD can be performed in some particularly mature collections prior to 4 weeks if the indication is strong and the collection appears to have a mature wall. However, the potential for cyst wall perforation is higher and should be considered in the risk-benefit discussion. Conversely, some collections beyond 4 weeks lack an adequately organized wall and require additional time for maturation.
While endoscopic drainage of pseudocysts has essentially supplanted surgery, the management of WON is more complex and remains multidisciplinary. Two recent randomized trials demonstrated no difference in major complications and/or death between a surgical and endoscopic step-up strategy for WON.2,3 Rates of pancreatic fistulae, hospital stay, and overall treatment costs, however, favored endoscopy. Nevertheless, defining the ideal strategy for many of these patients with complexity requires multidisciplinary discussion. Surgery continues to play a primary role in several scenarios, including collections that are not close to the upper GI tract, those that are particularly complex and extend caudally, and situations in which the endoscopic progress is too slow.
The three most important questions when deciding to embark on ETMD are: (1) whether drainage is indicated (that is, is the patient symptomatic or is there evidence that the PFC is infected?), (2) whether the wall of the collection is adequately mature and apposed to the GI tract wall; and (3) whether the collection contains necrosis? This last question has critical implications in the technical approach to drainage. While CT scan with IV contrast is accurate for assessing wall maturity, it is inadequate to evaluate the presence or quantity of necrosum. Transabdominal ultrasound, endoscopic ultrasound, and MRI (on a T2 sequence) are all superior for this purpose. MRI has the additional benefit of assessing the pancreatic duct integrity, which may influence subsequent management.
Pseudocysts can be managed by cyst-gastrostomy or cyst-duodenostomy alone, whereas most WONs require the additional step of endoscopic necrosectomy – the process of entering the cyst cavity to mechanically debride necrotic tissue. Because of a higher rate of technical success, endoscopic ultrasound–directed creation of the transmural drainage pathway has become standard practice. In addition, it is likely safer, allowing for the identification and avoidance of interceding vessels and other vital structures. The role of endoscopic retrograde cholangiopancreatography with pancreatic stent placement as primary therapy for PFCs is limited to the drainage of small collections (<5 cm), for which it is the preferred treatment strategy. It is as effective as ETMD, which may not be feasible or safe for small PFCs.
Plastic double-pigtail stents have traditionally been used to maintain the transmural tract for both pseudocyst and WON. Recently, however, metallic stents have become more popular. Fully covered biliary self-expanding metallic stents (SEMS) are easier to place, have a larger lumen, and are associated with improved outcomes, compared with plastic stents in observational studies of pseudocyst drainage. Lumen-apposing metallic stents (LAMS) have become the preferred prosthesis for WON drainage given the ability to near-simultaneously establish access and deploy the stent, as well as their much larger caliber lumen which permits seamless entry into the cavity with an endoscope. Based on ease and efficiency of use, LAMS are also commonly employed for pseudocyst drainage, although entry into the cavity is unnecessary.
Plastic stents have been shown to be more cost effective than LAMS for pseudocyst drainage, although the economics around biliary SEMS in this context have not been explored. Robust comparative effectiveness data defining the optimal prostheses for pseudocysts are needed. The literature comparing LAMS to plastic stents for the management of WON is mixed. Studies have shown LAMS to be more cost effective, but a small randomized trial demonstrated no difference in clinical success or in the number of procedures to achieve WON resolution.4 We generally favor LAMS for WON since large-caliber balloon dilation of the tract seems safer within the lumen of the LAMS (which could seal small perforations and tamponade bleeding vessels) than within a freshly created tract.
Secondary infection of the cavity, usually because of stent occlusion, and bleeding are the most common complications of ETMD. Even in the absence of stent occlusion, contamination of the collection after ETMD is ubiquitous and, as such, we prescribe prophylactic antibiotics for 1-2 weeks after the procedure, although this practice is not evidence based. Hemorrhage appears to be increasing in frequency with the diffusion of LAMS; this has been postulated to be due to particularly rapid cyst cavity collapse resulting in erosion of the stent into contralateral cyst wall vessels. CT angiography followed by an embolization procedure for a possible pseudoaneurysm is the mainstay of treatment. Serious venous bleeding is more challenging to address because angiographic options are limited.
Despite tremendous recent advances, several important controversies in the endoscopic management of PFCs persist. The optimal prosthesis, the importance of first-session endoscopic necroscopy (compared with stepping up to endoscopic necroscopy only if necessary), the roles of adjunctive drain placement and chemical debridement (such as hydrogen peroxide), the need for concomitant pancreatic stent placement, and the preferred long-term management of a disconnected pancreatic duct are areas for which additional research is sorely needed. We further discuss these questions and many additional technical considerations pertaining to endoscopic drainage in a recent review.5
In summary, endoscopic transmural drainage of mature PFCs is effective and safe. Existing evidence supports its use as the favored treatment modality in appropriate candidates and has rendered it a mainstay of the therapeutic armamentarium for this disease. Further studies are needed to address critical unanswered questions and to develop a uniform endoscopic management paradigm.
References
1. van Santvoort HC et al. N Engl J Med. 2010;362(16):1491-502.
2. van Brunschot S et al. Lancet. 2018;391(10115):51-8.
3. Bang JY et al. Gastroenterology. 2019;156(4):1027-40.
4. Bang JY et al. Gut. 2019;68(7):1200-9.
5. Elmunzer BJ. Clin Gastroenterol Hepatol. 2018;16(12):1851-63.
Dr. Moran is assistant professor of medicine, division of gastroenterology and hepatology, Medical University of South Carolina, Charleston; Dr. Elmunzer is the Peter Cotton Professor of Medicine and Endoscopic Innovation, division of gastroenterology and hepatology, Medical University of South Carolina. The authors have no conflicts of interest pertaining to this review.
Fellowship procedure logs: A word of advice for fellows and a call to action for fellowship programs
As a GI fellow, I never would have imagined I would be writing an article on GI fellowship procedure logs. At the time, in my naiveté, I looked at the procedure log as a necessary evil and part of the “red tape” imposed on fellowship programs by the Accreditation Council for Graduate Medical Education (ACGME). While the importance of keeping a log was highlighted and enforced by my program, the large majority of the recommended numbers were easily achievable. As a result, even my sporadic tracking of completed procedures was sufficient to meet the requirements. My poor compliance wasn’t because I was lazy or careless, but rather because of the absence of a formal system, which resulted in homegrown methods that were highly inaccurate. I wasn’t alone in my follies. As I discussed this issue with fellows across the nation, I learned that these sentiments were universally shared. It seemed that everyone had come up with their own unique way of keeping a log – from Word and Excel documents, to a binder of patient stickers, to a daily folded sheet of paper with scribbled technical notes – all of which were an inconvenience to trainees already stretched thin. However, when the time came for employee credentialing, I came to realize the importance of keeping an accurate record. This once-neglected document would become the ultimate record of my capabilities for independent practice. The pitfalls and shortcomings of how we currently log procedures is why it was the first thing I worked on improving once I was an academic faculty member. There had to be a better way!
I started by reviewing what ACGME actually mandates trainees in GI to track, and to my surprise, they no longer set minimum procedure requirements, but rather competencies. The current requirements state that “Fellows must demonstrate competence in performance of ... procedures”1 and specifically state that competence should “not be based solely on a minimum number of procedures performed.” So, where does the need for a procedure log and minimum numbers come from? Your fellowship programs’ review committee. Programs recognize that, in order to approve requests for independent practice privileges, they need to substantiate the competency of the fellow, which ultimately is best evidenced through procedure logs. Therefore, the committee sets the minimum number of cases they believe is necessary for trainees to practice safely and independently.2 Our program leadership at UConn Health in Farmington, Conn., annually assesses our procedure activity and, over the years, has settled on the procedure guideline numbers provided to fellows at orientation and reviewed with them semiannually.
Once I understood exactly why we need procedure logs, I started looking at how other specialties handle them, particularly surgical programs in which accurate procedure logs are vitally important. It turns out that they universally use, and look favorably on, the ACGME Case Log System - an online, all encompassing, tracking software. This system is provided to surgical programs despite ACGME’s focus on competencies rather than numbers. Why this system is not offered for GI programs is unclear. However, in my endeavor, I was able to find the American Gastroenterological Association (AGA) Procedure Log system. When we reviewed the system in 2015 for use in our program, it was more of a concept than an all-encompassing tool. Fortunately, the AGA Information Technology (IT) and Training departments were kind enough to work with us to develop a complete online tracking tool that could be used nationally by all trainees in GI. Finally, we had a system to keep an accurate, secure log online and in real time.
A plea to fellows
With this, understand that in today’s document driven and litigious world, your procedure log is as vital to endoscopy as the scope itself. Without it, you may not be granted permission to do x, y, or z procedure. Indirectly, it can lead to delays in patient care and may prevent you from performing certain tasks and ultimately lead to repetitive training. Treat it as an official legal document of what you’ve done and what you are capable of doing. Recognize that it will be used by your mentors as supporting evidence regarding your competency for independent practice. Ask your training program to provide a clear list of expectations and requirements for graduation and a method for you to accurately track them, such as the AGA Procedure Log. An online, mobile system will allow you to document cases immediately after you finish while the procedure is fresh in your mind. Taking an extra minute after each case will prevent headaches down the road. The faculty and your cofellows all know of the end of the year “procedure scavenger” (i.e., the fellow who searches for procedures and takes them from others to make sure they meet their numbers for graduation). Please don’t be that person.
A request for program directors
As GI educators, we all know the mention of procedure logs to fellows is typically accompanied by eye rolls. It doesn’t have to be that way. Provide your fellows with clear expectations and a quick, easy, and accurate way to track their accomplishments. Help them recognize the importance of an accurate and complete procedure log. Consider an online tracking system such as the AGA Procedure Log. Studies have demonstrated that a computer-based system increases compliance and accuracy.3 Not providing one will surely lead to difficulties in the long run and is a disservice to those we work to empower, educate, and prepare for success.
References
1. ACGME Program Requirements for Graduate Medical Education in Gastroenterology. Accreditation Council for Graduate Medical Education. 2020 Jul 1. pp 21, 28. Accessed Sept. 13, 2020. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/144_Gastroenterology_2020.pdf.
2. Steven J et al. J Grad Med Educat. 2012;4(2):257-60.
3. Rowe BH et al. Can Fam Physician. 1995;41:2113–20.
Dr. Rezaizadeh is an assistant professor of medicine, associate program director, gastroenterology fellowship program, UConn Health, Farmington, Conn.
As a GI fellow, I never would have imagined I would be writing an article on GI fellowship procedure logs. At the time, in my naiveté, I looked at the procedure log as a necessary evil and part of the “red tape” imposed on fellowship programs by the Accreditation Council for Graduate Medical Education (ACGME). While the importance of keeping a log was highlighted and enforced by my program, the large majority of the recommended numbers were easily achievable. As a result, even my sporadic tracking of completed procedures was sufficient to meet the requirements. My poor compliance wasn’t because I was lazy or careless, but rather because of the absence of a formal system, which resulted in homegrown methods that were highly inaccurate. I wasn’t alone in my follies. As I discussed this issue with fellows across the nation, I learned that these sentiments were universally shared. It seemed that everyone had come up with their own unique way of keeping a log – from Word and Excel documents, to a binder of patient stickers, to a daily folded sheet of paper with scribbled technical notes – all of which were an inconvenience to trainees already stretched thin. However, when the time came for employee credentialing, I came to realize the importance of keeping an accurate record. This once-neglected document would become the ultimate record of my capabilities for independent practice. The pitfalls and shortcomings of how we currently log procedures is why it was the first thing I worked on improving once I was an academic faculty member. There had to be a better way!
I started by reviewing what ACGME actually mandates trainees in GI to track, and to my surprise, they no longer set minimum procedure requirements, but rather competencies. The current requirements state that “Fellows must demonstrate competence in performance of ... procedures”1 and specifically state that competence should “not be based solely on a minimum number of procedures performed.” So, where does the need for a procedure log and minimum numbers come from? Your fellowship programs’ review committee. Programs recognize that, in order to approve requests for independent practice privileges, they need to substantiate the competency of the fellow, which ultimately is best evidenced through procedure logs. Therefore, the committee sets the minimum number of cases they believe is necessary for trainees to practice safely and independently.2 Our program leadership at UConn Health in Farmington, Conn., annually assesses our procedure activity and, over the years, has settled on the procedure guideline numbers provided to fellows at orientation and reviewed with them semiannually.
Once I understood exactly why we need procedure logs, I started looking at how other specialties handle them, particularly surgical programs in which accurate procedure logs are vitally important. It turns out that they universally use, and look favorably on, the ACGME Case Log System - an online, all encompassing, tracking software. This system is provided to surgical programs despite ACGME’s focus on competencies rather than numbers. Why this system is not offered for GI programs is unclear. However, in my endeavor, I was able to find the American Gastroenterological Association (AGA) Procedure Log system. When we reviewed the system in 2015 for use in our program, it was more of a concept than an all-encompassing tool. Fortunately, the AGA Information Technology (IT) and Training departments were kind enough to work with us to develop a complete online tracking tool that could be used nationally by all trainees in GI. Finally, we had a system to keep an accurate, secure log online and in real time.
A plea to fellows
With this, understand that in today’s document driven and litigious world, your procedure log is as vital to endoscopy as the scope itself. Without it, you may not be granted permission to do x, y, or z procedure. Indirectly, it can lead to delays in patient care and may prevent you from performing certain tasks and ultimately lead to repetitive training. Treat it as an official legal document of what you’ve done and what you are capable of doing. Recognize that it will be used by your mentors as supporting evidence regarding your competency for independent practice. Ask your training program to provide a clear list of expectations and requirements for graduation and a method for you to accurately track them, such as the AGA Procedure Log. An online, mobile system will allow you to document cases immediately after you finish while the procedure is fresh in your mind. Taking an extra minute after each case will prevent headaches down the road. The faculty and your cofellows all know of the end of the year “procedure scavenger” (i.e., the fellow who searches for procedures and takes them from others to make sure they meet their numbers for graduation). Please don’t be that person.
A request for program directors
As GI educators, we all know the mention of procedure logs to fellows is typically accompanied by eye rolls. It doesn’t have to be that way. Provide your fellows with clear expectations and a quick, easy, and accurate way to track their accomplishments. Help them recognize the importance of an accurate and complete procedure log. Consider an online tracking system such as the AGA Procedure Log. Studies have demonstrated that a computer-based system increases compliance and accuracy.3 Not providing one will surely lead to difficulties in the long run and is a disservice to those we work to empower, educate, and prepare for success.
References
1. ACGME Program Requirements for Graduate Medical Education in Gastroenterology. Accreditation Council for Graduate Medical Education. 2020 Jul 1. pp 21, 28. Accessed Sept. 13, 2020. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/144_Gastroenterology_2020.pdf.
2. Steven J et al. J Grad Med Educat. 2012;4(2):257-60.
3. Rowe BH et al. Can Fam Physician. 1995;41:2113–20.
Dr. Rezaizadeh is an assistant professor of medicine, associate program director, gastroenterology fellowship program, UConn Health, Farmington, Conn.
As a GI fellow, I never would have imagined I would be writing an article on GI fellowship procedure logs. At the time, in my naiveté, I looked at the procedure log as a necessary evil and part of the “red tape” imposed on fellowship programs by the Accreditation Council for Graduate Medical Education (ACGME). While the importance of keeping a log was highlighted and enforced by my program, the large majority of the recommended numbers were easily achievable. As a result, even my sporadic tracking of completed procedures was sufficient to meet the requirements. My poor compliance wasn’t because I was lazy or careless, but rather because of the absence of a formal system, which resulted in homegrown methods that were highly inaccurate. I wasn’t alone in my follies. As I discussed this issue with fellows across the nation, I learned that these sentiments were universally shared. It seemed that everyone had come up with their own unique way of keeping a log – from Word and Excel documents, to a binder of patient stickers, to a daily folded sheet of paper with scribbled technical notes – all of which were an inconvenience to trainees already stretched thin. However, when the time came for employee credentialing, I came to realize the importance of keeping an accurate record. This once-neglected document would become the ultimate record of my capabilities for independent practice. The pitfalls and shortcomings of how we currently log procedures is why it was the first thing I worked on improving once I was an academic faculty member. There had to be a better way!
I started by reviewing what ACGME actually mandates trainees in GI to track, and to my surprise, they no longer set minimum procedure requirements, but rather competencies. The current requirements state that “Fellows must demonstrate competence in performance of ... procedures”1 and specifically state that competence should “not be based solely on a minimum number of procedures performed.” So, where does the need for a procedure log and minimum numbers come from? Your fellowship programs’ review committee. Programs recognize that, in order to approve requests for independent practice privileges, they need to substantiate the competency of the fellow, which ultimately is best evidenced through procedure logs. Therefore, the committee sets the minimum number of cases they believe is necessary for trainees to practice safely and independently.2 Our program leadership at UConn Health in Farmington, Conn., annually assesses our procedure activity and, over the years, has settled on the procedure guideline numbers provided to fellows at orientation and reviewed with them semiannually.
Once I understood exactly why we need procedure logs, I started looking at how other specialties handle them, particularly surgical programs in which accurate procedure logs are vitally important. It turns out that they universally use, and look favorably on, the ACGME Case Log System - an online, all encompassing, tracking software. This system is provided to surgical programs despite ACGME’s focus on competencies rather than numbers. Why this system is not offered for GI programs is unclear. However, in my endeavor, I was able to find the American Gastroenterological Association (AGA) Procedure Log system. When we reviewed the system in 2015 for use in our program, it was more of a concept than an all-encompassing tool. Fortunately, the AGA Information Technology (IT) and Training departments were kind enough to work with us to develop a complete online tracking tool that could be used nationally by all trainees in GI. Finally, we had a system to keep an accurate, secure log online and in real time.
A plea to fellows
With this, understand that in today’s document driven and litigious world, your procedure log is as vital to endoscopy as the scope itself. Without it, you may not be granted permission to do x, y, or z procedure. Indirectly, it can lead to delays in patient care and may prevent you from performing certain tasks and ultimately lead to repetitive training. Treat it as an official legal document of what you’ve done and what you are capable of doing. Recognize that it will be used by your mentors as supporting evidence regarding your competency for independent practice. Ask your training program to provide a clear list of expectations and requirements for graduation and a method for you to accurately track them, such as the AGA Procedure Log. An online, mobile system will allow you to document cases immediately after you finish while the procedure is fresh in your mind. Taking an extra minute after each case will prevent headaches down the road. The faculty and your cofellows all know of the end of the year “procedure scavenger” (i.e., the fellow who searches for procedures and takes them from others to make sure they meet their numbers for graduation). Please don’t be that person.
A request for program directors
As GI educators, we all know the mention of procedure logs to fellows is typically accompanied by eye rolls. It doesn’t have to be that way. Provide your fellows with clear expectations and a quick, easy, and accurate way to track their accomplishments. Help them recognize the importance of an accurate and complete procedure log. Consider an online tracking system such as the AGA Procedure Log. Studies have demonstrated that a computer-based system increases compliance and accuracy.3 Not providing one will surely lead to difficulties in the long run and is a disservice to those we work to empower, educate, and prepare for success.
References
1. ACGME Program Requirements for Graduate Medical Education in Gastroenterology. Accreditation Council for Graduate Medical Education. 2020 Jul 1. pp 21, 28. Accessed Sept. 13, 2020. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/144_Gastroenterology_2020.pdf.
2. Steven J et al. J Grad Med Educat. 2012;4(2):257-60.
3. Rowe BH et al. Can Fam Physician. 1995;41:2113–20.
Dr. Rezaizadeh is an assistant professor of medicine, associate program director, gastroenterology fellowship program, UConn Health, Farmington, Conn.