User login
AGA News
Ten tips to help you get a research grant
It’s almost time to submit your application for the American Gastroenterological Association Research Scholar Award, the application deadline is Nov. 13, 2019. Review these tips for writing and preparing your application.
1. Start early. Allow plenty of time to complete your application, give it multiple reviews, and get feedback from others. Most applicants start working on the Specific Aims for the project 6 months in advance of the deadline.
2. Look at examples. Ask your division if there are any templates/prior grant submissions that you can review. There’s no recipe for a successful grant, so the only way to compose one is to have a sense of what has worked in the past. In general, prior awardees are happy to share their applications if you contact them.
3. Request feedback. Ask mentors and colleagues for early feedback on your Specific Aims page. If it makes sense and is interesting to them, reviewers will likely feel the same way.
4. Ask your collaborators for letters of support. In addition to your preceptor, consider including letters of support from prior researchers that you have worked with or any collaborators for the current project, especially if they will help you with a new technique or reagents.
5. Contact the grants staff with questions and concerns early on. If you don’t understand part of the application, aren’t sure if you’re eligible or are having problems with submission, contact the grant staff right away. Don’t wait until the week or day the grant is due when staff may be flooded with calls. They can assist you much better with advance notice, which will allow you to avoid last-minute stress.
6. Each application should be different. Keep in mind the scope of the grant and amount of funding. Don’t just recycle an R01-level application for a 1-year AGA pilot award.
7. More is better than less when it comes to preliminary data. If your expertise in a technique you are proposing is established, you will not need to demonstrate the capability to do the work but will likely need to show preliminary data. If you are looking to build expertise (as a part of your career development), you may need to show that the infrastructure that enables you to do the work is accessible.
8. Don’t take constructive feedback personally. As you share your draft with mentors and colleagues for feedback, you may receive some unanticipated criticism. Try not to take this personally. If you can detach yourself emotionally, you’ll be in a better position to answer critiques and make adjustments.
9. Remember your end goal: To help patients! Even the most basic science proposals are rooted in a clear potential to benefit patients.
10. Stay positive. If you do not succeed on your first application, believe in your work, make it better, and apply again.
Thanks to the following AGA Research Foundation grant recipients for sharing their advice, which resulted in the above 10 tips:
- Arthur Beyder, MD, PhD, 2015 AGA Research Scholar Award
- Barbara Jung, MD, AGAF, 2016 AGA-Elsevier Pilot Research Award
- Benjamin Lebwohl, MD, 2014 AGA Research Scholar Award
- Josephine Ni, MD, 2017 AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
- Sahar Nissim, MD, PhD, 2017 AGA-Caroline Craig Augustyn and Damian Augustyn Award in Digestive Cancer
- Jatin Roper, MD, 2011 AGA Fellowship-to-Faculty Transition Award
- Christina Twyman-Saint Victor, MD, 2015 AGA Research Scholar Award
Visit www.gastro.org/research-funding to review the AGA Research Foundation research grants now open for applications. If you have questions about the AGA awards program, please contact [email protected].
The importance of getting involved for gastroenterology
On Sept. 20, 2019, I had the opportunity to participate in AGA’s Advocacy Day for the second time, joining 40 of our gastroenterology colleagues from across the United States on Capitol Hill to advocate for our profession and our patients.
The evening before Advocacy Day, we discussed strategies for having a successful meeting on Capitol Hill with AGA staff (including Kathleen Teixeira, AGA vice president of government affairs, and Jonathan Sollish, AGA senior coordinator, public policy). We discussed having our “asks” supported with evidence, and “getting personal” about how these policy issues directly affect us and our patients. We also had the chance to hear from Rep. Jim McGovern (D-Mass.) and Sen. Roy Blunt (R-Mo.), both of whom invited our questions. Both congressmen are friends of AGA, with Rep. McGovern serving as chair of the House Rules Committee, and Sen. Blunt serving as chair of the Senate Labor–Health & Human Services Subcommittee on Appropriations.
Advocacy Day began with a group breakfast during which we reviewed some of the policy issues of central importance to gastroenterology:
- Removing Barriers to Colorectal Cancer Screening Act (HR1570/S668), which enjoys strong bipartisan support, would correct the “cost-sharing” problem of screening colonoscopies turning therapeutic (with polypectomy) for our Medicare patients, by waiving the coinsurance for screening colonoscopies — regardless of whether we remove polyps during these colonoscopies.
- Safe Step Act, HR2279, legislation introduced in the House, facilitates a common-sense and timely (72 hours or 24 hours if life threatening) appeals process when our patients are subjected to step therapy (“fail first”) by insurers.
- Improving Seniors’ Timely Access to Care Act of 2019, HR3107, legislation in the House, eases onerous prior authorization burdens by promoting an electronic prior authorization process, ensuring requests are approved by qualified medical professionals who have specialty-specific experience, and mandating that plans report their rates of delays and denials.
- National Institutes of Health research funding facilitates innovative research and supports young investigators in our field.

Full of enthusiasm, our six-strong North Carolina contingent (pictured L-R, Ziad Gellad, MD, MPH, AGAF; David Leiman, MD, MSPH; Animesh Jain, MD; Anne Finefrock Peery, MD; Lisa Gangarosa, MD, AGAF, chair of the AGA Government Affairs Committee; and Amit Patel, MD) met with the offices of Rep. David Price (D-N.C.), and both North Carolina senators, Richard Burr (R) and Thom Tillis (R), on Capitol Hill to convey our “asks.”
At Rep. Price’s office in the stately Rayburn House Office Building, we thanked his team for cosponsorship of H.R. 1570 and H.R. 2279. We also discussed the importance of increasing research funding by the AGA’s goal of $2.5 billion for NIH for fiscal year 2020, noting that a majority of our delegation has received NIH funding for our training and/or research activities. We also encouraged Price’s office to cosponsor H.R. 3107, sharing our personal experiences about the administrative toll of the prior authorization process for obtaining appropriate and recommended medications for our patients – in my case, swallowed topical corticosteroids for patients with eosinophilic esophagitis.
We moved on to Sen. Tillis’s office, where we thanked his office for cosponsorship of S. 668 but encouraged his office to cosponsor upcoming companion Senate legislation for H.R. 2279 and H.R. 3107. Our colleague capably conveyed how an inflammatory bowel disease (IBD) patient he saw recently may require a colectomy because of delays in appropriate treatment stemming from these regulatory processes. We also showed Tillis’s office how NIH funding generates significant economic activity in North Carolina, supporting jobs in our state.
After a quick stop at the U.S. Senate gift shop in the basement to buy souvenirs for our kids, our last meeting was with Sen. Burr’s office. There, we also thanked his office for cosponsorship of S. 668 but encouraged him to sign the “Dear Colleague” letter that Sen. Sherrod Brown (D-Ohio) has circulated asking the Centers for Medicare & Medicaid Services to address the colonoscopy cost-sharing “loophole.” We discussed the importance of cosponsoring upcoming companion Senate legislation for H.R. 2279 and H.R. 3107, sharing stories from our clinical practices about how these regulatory burdens have delayed treatment for our patients.
You can get involved, too.
AGA Advocacy Day was a tremendous experience, but it is not the only way AGA members can get involved and take action. The AGA Advocacy website, gastro.org/advocacy, provides more information on multiple avenues for advocacy. These include an online advocacy tool for sending templated letters on these issues to your elected officials.
Perhaps now more than ever, it is crucial that we get involved to support gastroenterology and advocate for our patients.
Dr. Patel is assistant professor, division of gastroenterology, Duke University, Durham, N.C.; member, AGA Clinical Guidelines Committee.
GI of the week: Arthur Beyder, MD, PhD
Congrats to Arthur Beyder, MD, PhD, who was selected for an NIH Director’s New Innovator Award, part of the NIH director’s high-risk, high-reward research award program. The NIH Director’s New Innovator Award will provide Dr. Beyder with more than $2 million in funding over a 5-year period to continue his project: Does the gut have a sense of touch?
Dr. Beyder’s lab at the Mayo Clinic, Rochester, Minn., recently discovered a novel population of mechanosensitive epithelial sensory cells that are similar to skin’s touch sensors, which prompted a potentially transformative question: “Does the gut have a sense of touch?” We look forward to seeing the results of future research on this topic.
Dr. Beyder – a physician-scientist at the Mayo Clinic – is a 2015 AGA Research Scholar Award recipient and graduate of the 2018 AGA Future Leaders Program. Dr. Beyder currently serves on the AGA Nominating Committee.
Please join us in congratulating Dr. Beyder on Twitter (@BeyderLab) or in the AGA Community.
The NIH director’s high-risk, high-reward research program funds highly innovative, high-impact biomedical research proposed by extraordinarily creative scientists – these awards have one of the lowest funding rates for NIH. Congrats to two additional AGA members who also received a 2019 NIH Director’s New Innovator Award: Maayan Levy, PhD, and Christoph A. Thaiss, PhD, both from the University of Pennsylvania, Philadelphia.
Eight new insights about diet and gut health
During your 4 years of medical school, you likely received only 4 hours of nutrition training. Yet we know diet is so integral to the care of GI patients. That’s why AGA focused the 2019 James W. Freston Conference on the topic: Food at the Intersection of Gut Health and Disease.
Our course directors William Chey, MD, AGAF, Sheila E. Crowe, MD, AGAF, and Gerard E. Mullin, MD, AGAF, share eight points from the meeting that stuck with them and can help all practicing GIs as they consider dietary treatments for their patients.
1. Personalized nutrition is important. Genetic differences lead to differences in health outcomes. One size or recommendation does not fit all. This is why certain diets only work on certain people. There is no one diet for all and for all disease states. Genetic tests can be helpful, but they rely on reporting that isn’t readily available yet.
2. Dietary therapy is key to managing eosinophilic esophagitis (EoE). EoE is becoming more and more prevalent. Genes can’t change that fast, but epigenetic factors can, and the evidence seems to be in food. EoE is not an IgE-mediated disease and therefore most allergy tests will not prove useful; however, food is often the trigger – most common, dairy. Dietary therapy is likely the best way to manage. You want to reduce the number of eliminated foods by way of a reintroduction protocol. The six-food elimination diet is standard, though some are moving to a four-food elimination diet (dairy, wheat, egg, and soy).
3. There has been a reported increase in those with food allergies, sensitivities, celiac disease, and other adverse reactions to food. Many of the food allergy tests available are not helpful. In addition, many afflicted patients are using self-imposed diets rather than working with a GI, allergist, or dietitian. This needs to change.
4. There is currently insufficient evidence to support a gluten-free diet for irritable bowel syndrome (IBS). It is possible that fructans, more than gluten, are causing the GI issues. Typically, the low-FODMAP diet is beneficial to IBS patients if done correctly with the guidance of a dietitian; however, not everyone with IBS improves on it. All the steps are important though, including reintroduction and maintenance.
5. When working with patients on the low-FODMAP or other restrictive diets, it is important to know their food and eating history. Avoidance/restrictive food intake disorder is something we need to be aware of when it comes to patients with a history or likelihood to develop disordered eating/eating disorders. The patient team may need to include an eating disorder therapist.
6. The general population in the United States has increased the adoption of a gluten-free diet although the number of cases of celiac disease has not increased. Many have self-reported gluten sensitivities. Those that have removed gluten, following trends, are more at risk of bowel irregularity (low fiber), weight gain, and disordered eating. Celiac disease is not a do-it-yourself disease, patients will be best served working with a dietitian and GI.
7. Food can induce symptoms in patients with IBD. It can also trigger gut inflammation resulting in incident or relapse. There is experimental plausibility for some factors of the relationship to be causal and we may be able to modify the diet to prevent and manage IBD.
8. The focus on nutrition education must continue! Nutrition should be a required part of continuing medical education for physicians. And physicians should work with dietitians to improve the care of GI patients.
17 fellows advancing GI and patient care
Each year during Digestive Disease Week®, AGA hosts a session titled “Advancing Clinical Practice: GI Fellow-Directed Quality-Improvement Projects.” During the 2019 session, 17 quality improvement initiatives were presented — you can review these abstracts in the July issue of Gastroenterology in the “AGA Section” or review a presenter’s abstract by clicking their name or image. Kudos to the promising fellows featured below, who all served as lead authors for their quality improvement projects.

Manasi Agrawal, MD
Lenox Hill Hospital, New York
@ManasiAgrawalMD
Jessica Breton, MD
Children’s Hospital of Philadelphia
Adam Faye, MD
Columbia University Medical Center, New York
@AdamFaye4
Shelly Gurwara, MD
Wake Forest Baptist Health Medical Center, Winston-Salem, N.C.
Afrin Kamal, MD
Stanford (Calif.) University
Ani Kardashian, MD
University of California, Los Angeles
@AniKardashianMD
Sonali Palchaudhuri, MD
University of Pennsylvania, Philadelphia
@sopalchaudhuri
Nasim Parsa, MD
University of Missouri Health System, Columbia
Sahil Patel, MD
Drexel University, Philadelphia
@sahilr
Vikram Raghu, MD
Children’s Hospital of Pittsburgh
Amit Shah, MD
Children’s Hospital of Philadelphia
Lin Shen, MD
Brigham and Women’s Hospital, Boston
@LinShenMD
Charles Snyder, MD
Icahn School of Medicine at Mount Sinai, New York
Brian Sullivan, MD
Duke University, Durham, N.C.
Ashley Vachon, MD
University of Colorado at Denver, Aurora
Ted Walker, MD
Washington University/Barnes Jewish Hospital, St. Louis
Xiao Jing Wang, MD
Mayo Clinic, Rochester, Minn.
@IrisWangMD
Ten tips to help you get a research grant
It’s almost time to submit your application for the American Gastroenterological Association Research Scholar Award, the application deadline is Nov. 13, 2019. Review these tips for writing and preparing your application.
1. Start early. Allow plenty of time to complete your application, give it multiple reviews, and get feedback from others. Most applicants start working on the Specific Aims for the project 6 months in advance of the deadline.
2. Look at examples. Ask your division if there are any templates/prior grant submissions that you can review. There’s no recipe for a successful grant, so the only way to compose one is to have a sense of what has worked in the past. In general, prior awardees are happy to share their applications if you contact them.
3. Request feedback. Ask mentors and colleagues for early feedback on your Specific Aims page. If it makes sense and is interesting to them, reviewers will likely feel the same way.
4. Ask your collaborators for letters of support. In addition to your preceptor, consider including letters of support from prior researchers that you have worked with or any collaborators for the current project, especially if they will help you with a new technique or reagents.
5. Contact the grants staff with questions and concerns early on. If you don’t understand part of the application, aren’t sure if you’re eligible or are having problems with submission, contact the grant staff right away. Don’t wait until the week or day the grant is due when staff may be flooded with calls. They can assist you much better with advance notice, which will allow you to avoid last-minute stress.
6. Each application should be different. Keep in mind the scope of the grant and amount of funding. Don’t just recycle an R01-level application for a 1-year AGA pilot award.
7. More is better than less when it comes to preliminary data. If your expertise in a technique you are proposing is established, you will not need to demonstrate the capability to do the work but will likely need to show preliminary data. If you are looking to build expertise (as a part of your career development), you may need to show that the infrastructure that enables you to do the work is accessible.
8. Don’t take constructive feedback personally. As you share your draft with mentors and colleagues for feedback, you may receive some unanticipated criticism. Try not to take this personally. If you can detach yourself emotionally, you’ll be in a better position to answer critiques and make adjustments.
9. Remember your end goal: To help patients! Even the most basic science proposals are rooted in a clear potential to benefit patients.
10. Stay positive. If you do not succeed on your first application, believe in your work, make it better, and apply again.
Thanks to the following AGA Research Foundation grant recipients for sharing their advice, which resulted in the above 10 tips:
- Arthur Beyder, MD, PhD, 2015 AGA Research Scholar Award
- Barbara Jung, MD, AGAF, 2016 AGA-Elsevier Pilot Research Award
- Benjamin Lebwohl, MD, 2014 AGA Research Scholar Award
- Josephine Ni, MD, 2017 AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
- Sahar Nissim, MD, PhD, 2017 AGA-Caroline Craig Augustyn and Damian Augustyn Award in Digestive Cancer
- Jatin Roper, MD, 2011 AGA Fellowship-to-Faculty Transition Award
- Christina Twyman-Saint Victor, MD, 2015 AGA Research Scholar Award
Visit www.gastro.org/research-funding to review the AGA Research Foundation research grants now open for applications. If you have questions about the AGA awards program, please contact [email protected].
The importance of getting involved for gastroenterology
On Sept. 20, 2019, I had the opportunity to participate in AGA’s Advocacy Day for the second time, joining 40 of our gastroenterology colleagues from across the United States on Capitol Hill to advocate for our profession and our patients.
The evening before Advocacy Day, we discussed strategies for having a successful meeting on Capitol Hill with AGA staff (including Kathleen Teixeira, AGA vice president of government affairs, and Jonathan Sollish, AGA senior coordinator, public policy). We discussed having our “asks” supported with evidence, and “getting personal” about how these policy issues directly affect us and our patients. We also had the chance to hear from Rep. Jim McGovern (D-Mass.) and Sen. Roy Blunt (R-Mo.), both of whom invited our questions. Both congressmen are friends of AGA, with Rep. McGovern serving as chair of the House Rules Committee, and Sen. Blunt serving as chair of the Senate Labor–Health & Human Services Subcommittee on Appropriations.
Advocacy Day began with a group breakfast during which we reviewed some of the policy issues of central importance to gastroenterology:
- Removing Barriers to Colorectal Cancer Screening Act (HR1570/S668), which enjoys strong bipartisan support, would correct the “cost-sharing” problem of screening colonoscopies turning therapeutic (with polypectomy) for our Medicare patients, by waiving the coinsurance for screening colonoscopies — regardless of whether we remove polyps during these colonoscopies.
- Safe Step Act, HR2279, legislation introduced in the House, facilitates a common-sense and timely (72 hours or 24 hours if life threatening) appeals process when our patients are subjected to step therapy (“fail first”) by insurers.
- Improving Seniors’ Timely Access to Care Act of 2019, HR3107, legislation in the House, eases onerous prior authorization burdens by promoting an electronic prior authorization process, ensuring requests are approved by qualified medical professionals who have specialty-specific experience, and mandating that plans report their rates of delays and denials.
- National Institutes of Health research funding facilitates innovative research and supports young investigators in our field.

Full of enthusiasm, our six-strong North Carolina contingent (pictured L-R, Ziad Gellad, MD, MPH, AGAF; David Leiman, MD, MSPH; Animesh Jain, MD; Anne Finefrock Peery, MD; Lisa Gangarosa, MD, AGAF, chair of the AGA Government Affairs Committee; and Amit Patel, MD) met with the offices of Rep. David Price (D-N.C.), and both North Carolina senators, Richard Burr (R) and Thom Tillis (R), on Capitol Hill to convey our “asks.”
At Rep. Price’s office in the stately Rayburn House Office Building, we thanked his team for cosponsorship of H.R. 1570 and H.R. 2279. We also discussed the importance of increasing research funding by the AGA’s goal of $2.5 billion for NIH for fiscal year 2020, noting that a majority of our delegation has received NIH funding for our training and/or research activities. We also encouraged Price’s office to cosponsor H.R. 3107, sharing our personal experiences about the administrative toll of the prior authorization process for obtaining appropriate and recommended medications for our patients – in my case, swallowed topical corticosteroids for patients with eosinophilic esophagitis.
We moved on to Sen. Tillis’s office, where we thanked his office for cosponsorship of S. 668 but encouraged his office to cosponsor upcoming companion Senate legislation for H.R. 2279 and H.R. 3107. Our colleague capably conveyed how an inflammatory bowel disease (IBD) patient he saw recently may require a colectomy because of delays in appropriate treatment stemming from these regulatory processes. We also showed Tillis’s office how NIH funding generates significant economic activity in North Carolina, supporting jobs in our state.
After a quick stop at the U.S. Senate gift shop in the basement to buy souvenirs for our kids, our last meeting was with Sen. Burr’s office. There, we also thanked his office for cosponsorship of S. 668 but encouraged him to sign the “Dear Colleague” letter that Sen. Sherrod Brown (D-Ohio) has circulated asking the Centers for Medicare & Medicaid Services to address the colonoscopy cost-sharing “loophole.” We discussed the importance of cosponsoring upcoming companion Senate legislation for H.R. 2279 and H.R. 3107, sharing stories from our clinical practices about how these regulatory burdens have delayed treatment for our patients.
You can get involved, too.
AGA Advocacy Day was a tremendous experience, but it is not the only way AGA members can get involved and take action. The AGA Advocacy website, gastro.org/advocacy, provides more information on multiple avenues for advocacy. These include an online advocacy tool for sending templated letters on these issues to your elected officials.
Perhaps now more than ever, it is crucial that we get involved to support gastroenterology and advocate for our patients.
Dr. Patel is assistant professor, division of gastroenterology, Duke University, Durham, N.C.; member, AGA Clinical Guidelines Committee.
GI of the week: Arthur Beyder, MD, PhD
Congrats to Arthur Beyder, MD, PhD, who was selected for an NIH Director’s New Innovator Award, part of the NIH director’s high-risk, high-reward research award program. The NIH Director’s New Innovator Award will provide Dr. Beyder with more than $2 million in funding over a 5-year period to continue his project: Does the gut have a sense of touch?
Dr. Beyder’s lab at the Mayo Clinic, Rochester, Minn., recently discovered a novel population of mechanosensitive epithelial sensory cells that are similar to skin’s touch sensors, which prompted a potentially transformative question: “Does the gut have a sense of touch?” We look forward to seeing the results of future research on this topic.
Dr. Beyder – a physician-scientist at the Mayo Clinic – is a 2015 AGA Research Scholar Award recipient and graduate of the 2018 AGA Future Leaders Program. Dr. Beyder currently serves on the AGA Nominating Committee.
Please join us in congratulating Dr. Beyder on Twitter (@BeyderLab) or in the AGA Community.
The NIH director’s high-risk, high-reward research program funds highly innovative, high-impact biomedical research proposed by extraordinarily creative scientists – these awards have one of the lowest funding rates for NIH. Congrats to two additional AGA members who also received a 2019 NIH Director’s New Innovator Award: Maayan Levy, PhD, and Christoph A. Thaiss, PhD, both from the University of Pennsylvania, Philadelphia.
Eight new insights about diet and gut health
During your 4 years of medical school, you likely received only 4 hours of nutrition training. Yet we know diet is so integral to the care of GI patients. That’s why AGA focused the 2019 James W. Freston Conference on the topic: Food at the Intersection of Gut Health and Disease.
Our course directors William Chey, MD, AGAF, Sheila E. Crowe, MD, AGAF, and Gerard E. Mullin, MD, AGAF, share eight points from the meeting that stuck with them and can help all practicing GIs as they consider dietary treatments for their patients.
1. Personalized nutrition is important. Genetic differences lead to differences in health outcomes. One size or recommendation does not fit all. This is why certain diets only work on certain people. There is no one diet for all and for all disease states. Genetic tests can be helpful, but they rely on reporting that isn’t readily available yet.
2. Dietary therapy is key to managing eosinophilic esophagitis (EoE). EoE is becoming more and more prevalent. Genes can’t change that fast, but epigenetic factors can, and the evidence seems to be in food. EoE is not an IgE-mediated disease and therefore most allergy tests will not prove useful; however, food is often the trigger – most common, dairy. Dietary therapy is likely the best way to manage. You want to reduce the number of eliminated foods by way of a reintroduction protocol. The six-food elimination diet is standard, though some are moving to a four-food elimination diet (dairy, wheat, egg, and soy).
3. There has been a reported increase in those with food allergies, sensitivities, celiac disease, and other adverse reactions to food. Many of the food allergy tests available are not helpful. In addition, many afflicted patients are using self-imposed diets rather than working with a GI, allergist, or dietitian. This needs to change.
4. There is currently insufficient evidence to support a gluten-free diet for irritable bowel syndrome (IBS). It is possible that fructans, more than gluten, are causing the GI issues. Typically, the low-FODMAP diet is beneficial to IBS patients if done correctly with the guidance of a dietitian; however, not everyone with IBS improves on it. All the steps are important though, including reintroduction and maintenance.
5. When working with patients on the low-FODMAP or other restrictive diets, it is important to know their food and eating history. Avoidance/restrictive food intake disorder is something we need to be aware of when it comes to patients with a history or likelihood to develop disordered eating/eating disorders. The patient team may need to include an eating disorder therapist.
6. The general population in the United States has increased the adoption of a gluten-free diet although the number of cases of celiac disease has not increased. Many have self-reported gluten sensitivities. Those that have removed gluten, following trends, are more at risk of bowel irregularity (low fiber), weight gain, and disordered eating. Celiac disease is not a do-it-yourself disease, patients will be best served working with a dietitian and GI.
7. Food can induce symptoms in patients with IBD. It can also trigger gut inflammation resulting in incident or relapse. There is experimental plausibility for some factors of the relationship to be causal and we may be able to modify the diet to prevent and manage IBD.
8. The focus on nutrition education must continue! Nutrition should be a required part of continuing medical education for physicians. And physicians should work with dietitians to improve the care of GI patients.
17 fellows advancing GI and patient care
Each year during Digestive Disease Week®, AGA hosts a session titled “Advancing Clinical Practice: GI Fellow-Directed Quality-Improvement Projects.” During the 2019 session, 17 quality improvement initiatives were presented — you can review these abstracts in the July issue of Gastroenterology in the “AGA Section” or review a presenter’s abstract by clicking their name or image. Kudos to the promising fellows featured below, who all served as lead authors for their quality improvement projects.

Manasi Agrawal, MD
Lenox Hill Hospital, New York
@ManasiAgrawalMD
Jessica Breton, MD
Children’s Hospital of Philadelphia
Adam Faye, MD
Columbia University Medical Center, New York
@AdamFaye4
Shelly Gurwara, MD
Wake Forest Baptist Health Medical Center, Winston-Salem, N.C.
Afrin Kamal, MD
Stanford (Calif.) University
Ani Kardashian, MD
University of California, Los Angeles
@AniKardashianMD
Sonali Palchaudhuri, MD
University of Pennsylvania, Philadelphia
@sopalchaudhuri
Nasim Parsa, MD
University of Missouri Health System, Columbia
Sahil Patel, MD
Drexel University, Philadelphia
@sahilr
Vikram Raghu, MD
Children’s Hospital of Pittsburgh
Amit Shah, MD
Children’s Hospital of Philadelphia
Lin Shen, MD
Brigham and Women’s Hospital, Boston
@LinShenMD
Charles Snyder, MD
Icahn School of Medicine at Mount Sinai, New York
Brian Sullivan, MD
Duke University, Durham, N.C.
Ashley Vachon, MD
University of Colorado at Denver, Aurora
Ted Walker, MD
Washington University/Barnes Jewish Hospital, St. Louis
Xiao Jing Wang, MD
Mayo Clinic, Rochester, Minn.
@IrisWangMD
Ten tips to help you get a research grant
It’s almost time to submit your application for the American Gastroenterological Association Research Scholar Award, the application deadline is Nov. 13, 2019. Review these tips for writing and preparing your application.
1. Start early. Allow plenty of time to complete your application, give it multiple reviews, and get feedback from others. Most applicants start working on the Specific Aims for the project 6 months in advance of the deadline.
2. Look at examples. Ask your division if there are any templates/prior grant submissions that you can review. There’s no recipe for a successful grant, so the only way to compose one is to have a sense of what has worked in the past. In general, prior awardees are happy to share their applications if you contact them.
3. Request feedback. Ask mentors and colleagues for early feedback on your Specific Aims page. If it makes sense and is interesting to them, reviewers will likely feel the same way.
4. Ask your collaborators for letters of support. In addition to your preceptor, consider including letters of support from prior researchers that you have worked with or any collaborators for the current project, especially if they will help you with a new technique or reagents.
5. Contact the grants staff with questions and concerns early on. If you don’t understand part of the application, aren’t sure if you’re eligible or are having problems with submission, contact the grant staff right away. Don’t wait until the week or day the grant is due when staff may be flooded with calls. They can assist you much better with advance notice, which will allow you to avoid last-minute stress.
6. Each application should be different. Keep in mind the scope of the grant and amount of funding. Don’t just recycle an R01-level application for a 1-year AGA pilot award.
7. More is better than less when it comes to preliminary data. If your expertise in a technique you are proposing is established, you will not need to demonstrate the capability to do the work but will likely need to show preliminary data. If you are looking to build expertise (as a part of your career development), you may need to show that the infrastructure that enables you to do the work is accessible.
8. Don’t take constructive feedback personally. As you share your draft with mentors and colleagues for feedback, you may receive some unanticipated criticism. Try not to take this personally. If you can detach yourself emotionally, you’ll be in a better position to answer critiques and make adjustments.
9. Remember your end goal: To help patients! Even the most basic science proposals are rooted in a clear potential to benefit patients.
10. Stay positive. If you do not succeed on your first application, believe in your work, make it better, and apply again.
Thanks to the following AGA Research Foundation grant recipients for sharing their advice, which resulted in the above 10 tips:
- Arthur Beyder, MD, PhD, 2015 AGA Research Scholar Award
- Barbara Jung, MD, AGAF, 2016 AGA-Elsevier Pilot Research Award
- Benjamin Lebwohl, MD, 2014 AGA Research Scholar Award
- Josephine Ni, MD, 2017 AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
- Sahar Nissim, MD, PhD, 2017 AGA-Caroline Craig Augustyn and Damian Augustyn Award in Digestive Cancer
- Jatin Roper, MD, 2011 AGA Fellowship-to-Faculty Transition Award
- Christina Twyman-Saint Victor, MD, 2015 AGA Research Scholar Award
Visit www.gastro.org/research-funding to review the AGA Research Foundation research grants now open for applications. If you have questions about the AGA awards program, please contact [email protected].
The importance of getting involved for gastroenterology
On Sept. 20, 2019, I had the opportunity to participate in AGA’s Advocacy Day for the second time, joining 40 of our gastroenterology colleagues from across the United States on Capitol Hill to advocate for our profession and our patients.
The evening before Advocacy Day, we discussed strategies for having a successful meeting on Capitol Hill with AGA staff (including Kathleen Teixeira, AGA vice president of government affairs, and Jonathan Sollish, AGA senior coordinator, public policy). We discussed having our “asks” supported with evidence, and “getting personal” about how these policy issues directly affect us and our patients. We also had the chance to hear from Rep. Jim McGovern (D-Mass.) and Sen. Roy Blunt (R-Mo.), both of whom invited our questions. Both congressmen are friends of AGA, with Rep. McGovern serving as chair of the House Rules Committee, and Sen. Blunt serving as chair of the Senate Labor–Health & Human Services Subcommittee on Appropriations.
Advocacy Day began with a group breakfast during which we reviewed some of the policy issues of central importance to gastroenterology:
- Removing Barriers to Colorectal Cancer Screening Act (HR1570/S668), which enjoys strong bipartisan support, would correct the “cost-sharing” problem of screening colonoscopies turning therapeutic (with polypectomy) for our Medicare patients, by waiving the coinsurance for screening colonoscopies — regardless of whether we remove polyps during these colonoscopies.
- Safe Step Act, HR2279, legislation introduced in the House, facilitates a common-sense and timely (72 hours or 24 hours if life threatening) appeals process when our patients are subjected to step therapy (“fail first”) by insurers.
- Improving Seniors’ Timely Access to Care Act of 2019, HR3107, legislation in the House, eases onerous prior authorization burdens by promoting an electronic prior authorization process, ensuring requests are approved by qualified medical professionals who have specialty-specific experience, and mandating that plans report their rates of delays and denials.
- National Institutes of Health research funding facilitates innovative research and supports young investigators in our field.

Full of enthusiasm, our six-strong North Carolina contingent (pictured L-R, Ziad Gellad, MD, MPH, AGAF; David Leiman, MD, MSPH; Animesh Jain, MD; Anne Finefrock Peery, MD; Lisa Gangarosa, MD, AGAF, chair of the AGA Government Affairs Committee; and Amit Patel, MD) met with the offices of Rep. David Price (D-N.C.), and both North Carolina senators, Richard Burr (R) and Thom Tillis (R), on Capitol Hill to convey our “asks.”
At Rep. Price’s office in the stately Rayburn House Office Building, we thanked his team for cosponsorship of H.R. 1570 and H.R. 2279. We also discussed the importance of increasing research funding by the AGA’s goal of $2.5 billion for NIH for fiscal year 2020, noting that a majority of our delegation has received NIH funding for our training and/or research activities. We also encouraged Price’s office to cosponsor H.R. 3107, sharing our personal experiences about the administrative toll of the prior authorization process for obtaining appropriate and recommended medications for our patients – in my case, swallowed topical corticosteroids for patients with eosinophilic esophagitis.
We moved on to Sen. Tillis’s office, where we thanked his office for cosponsorship of S. 668 but encouraged his office to cosponsor upcoming companion Senate legislation for H.R. 2279 and H.R. 3107. Our colleague capably conveyed how an inflammatory bowel disease (IBD) patient he saw recently may require a colectomy because of delays in appropriate treatment stemming from these regulatory processes. We also showed Tillis’s office how NIH funding generates significant economic activity in North Carolina, supporting jobs in our state.
After a quick stop at the U.S. Senate gift shop in the basement to buy souvenirs for our kids, our last meeting was with Sen. Burr’s office. There, we also thanked his office for cosponsorship of S. 668 but encouraged him to sign the “Dear Colleague” letter that Sen. Sherrod Brown (D-Ohio) has circulated asking the Centers for Medicare & Medicaid Services to address the colonoscopy cost-sharing “loophole.” We discussed the importance of cosponsoring upcoming companion Senate legislation for H.R. 2279 and H.R. 3107, sharing stories from our clinical practices about how these regulatory burdens have delayed treatment for our patients.
You can get involved, too.
AGA Advocacy Day was a tremendous experience, but it is not the only way AGA members can get involved and take action. The AGA Advocacy website, gastro.org/advocacy, provides more information on multiple avenues for advocacy. These include an online advocacy tool for sending templated letters on these issues to your elected officials.
Perhaps now more than ever, it is crucial that we get involved to support gastroenterology and advocate for our patients.
Dr. Patel is assistant professor, division of gastroenterology, Duke University, Durham, N.C.; member, AGA Clinical Guidelines Committee.
GI of the week: Arthur Beyder, MD, PhD
Congrats to Arthur Beyder, MD, PhD, who was selected for an NIH Director’s New Innovator Award, part of the NIH director’s high-risk, high-reward research award program. The NIH Director’s New Innovator Award will provide Dr. Beyder with more than $2 million in funding over a 5-year period to continue his project: Does the gut have a sense of touch?
Dr. Beyder’s lab at the Mayo Clinic, Rochester, Minn., recently discovered a novel population of mechanosensitive epithelial sensory cells that are similar to skin’s touch sensors, which prompted a potentially transformative question: “Does the gut have a sense of touch?” We look forward to seeing the results of future research on this topic.
Dr. Beyder – a physician-scientist at the Mayo Clinic – is a 2015 AGA Research Scholar Award recipient and graduate of the 2018 AGA Future Leaders Program. Dr. Beyder currently serves on the AGA Nominating Committee.
Please join us in congratulating Dr. Beyder on Twitter (@BeyderLab) or in the AGA Community.
The NIH director’s high-risk, high-reward research program funds highly innovative, high-impact biomedical research proposed by extraordinarily creative scientists – these awards have one of the lowest funding rates for NIH. Congrats to two additional AGA members who also received a 2019 NIH Director’s New Innovator Award: Maayan Levy, PhD, and Christoph A. Thaiss, PhD, both from the University of Pennsylvania, Philadelphia.
Eight new insights about diet and gut health
During your 4 years of medical school, you likely received only 4 hours of nutrition training. Yet we know diet is so integral to the care of GI patients. That’s why AGA focused the 2019 James W. Freston Conference on the topic: Food at the Intersection of Gut Health and Disease.
Our course directors William Chey, MD, AGAF, Sheila E. Crowe, MD, AGAF, and Gerard E. Mullin, MD, AGAF, share eight points from the meeting that stuck with them and can help all practicing GIs as they consider dietary treatments for their patients.
1. Personalized nutrition is important. Genetic differences lead to differences in health outcomes. One size or recommendation does not fit all. This is why certain diets only work on certain people. There is no one diet for all and for all disease states. Genetic tests can be helpful, but they rely on reporting that isn’t readily available yet.
2. Dietary therapy is key to managing eosinophilic esophagitis (EoE). EoE is becoming more and more prevalent. Genes can’t change that fast, but epigenetic factors can, and the evidence seems to be in food. EoE is not an IgE-mediated disease and therefore most allergy tests will not prove useful; however, food is often the trigger – most common, dairy. Dietary therapy is likely the best way to manage. You want to reduce the number of eliminated foods by way of a reintroduction protocol. The six-food elimination diet is standard, though some are moving to a four-food elimination diet (dairy, wheat, egg, and soy).
3. There has been a reported increase in those with food allergies, sensitivities, celiac disease, and other adverse reactions to food. Many of the food allergy tests available are not helpful. In addition, many afflicted patients are using self-imposed diets rather than working with a GI, allergist, or dietitian. This needs to change.
4. There is currently insufficient evidence to support a gluten-free diet for irritable bowel syndrome (IBS). It is possible that fructans, more than gluten, are causing the GI issues. Typically, the low-FODMAP diet is beneficial to IBS patients if done correctly with the guidance of a dietitian; however, not everyone with IBS improves on it. All the steps are important though, including reintroduction and maintenance.
5. When working with patients on the low-FODMAP or other restrictive diets, it is important to know their food and eating history. Avoidance/restrictive food intake disorder is something we need to be aware of when it comes to patients with a history or likelihood to develop disordered eating/eating disorders. The patient team may need to include an eating disorder therapist.
6. The general population in the United States has increased the adoption of a gluten-free diet although the number of cases of celiac disease has not increased. Many have self-reported gluten sensitivities. Those that have removed gluten, following trends, are more at risk of bowel irregularity (low fiber), weight gain, and disordered eating. Celiac disease is not a do-it-yourself disease, patients will be best served working with a dietitian and GI.
7. Food can induce symptoms in patients with IBD. It can also trigger gut inflammation resulting in incident or relapse. There is experimental plausibility for some factors of the relationship to be causal and we may be able to modify the diet to prevent and manage IBD.
8. The focus on nutrition education must continue! Nutrition should be a required part of continuing medical education for physicians. And physicians should work with dietitians to improve the care of GI patients.
17 fellows advancing GI and patient care
Each year during Digestive Disease Week®, AGA hosts a session titled “Advancing Clinical Practice: GI Fellow-Directed Quality-Improvement Projects.” During the 2019 session, 17 quality improvement initiatives were presented — you can review these abstracts in the July issue of Gastroenterology in the “AGA Section” or review a presenter’s abstract by clicking their name or image. Kudos to the promising fellows featured below, who all served as lead authors for their quality improvement projects.

Manasi Agrawal, MD
Lenox Hill Hospital, New York
@ManasiAgrawalMD
Jessica Breton, MD
Children’s Hospital of Philadelphia
Adam Faye, MD
Columbia University Medical Center, New York
@AdamFaye4
Shelly Gurwara, MD
Wake Forest Baptist Health Medical Center, Winston-Salem, N.C.
Afrin Kamal, MD
Stanford (Calif.) University
Ani Kardashian, MD
University of California, Los Angeles
@AniKardashianMD
Sonali Palchaudhuri, MD
University of Pennsylvania, Philadelphia
@sopalchaudhuri
Nasim Parsa, MD
University of Missouri Health System, Columbia
Sahil Patel, MD
Drexel University, Philadelphia
@sahilr
Vikram Raghu, MD
Children’s Hospital of Pittsburgh
Amit Shah, MD
Children’s Hospital of Philadelphia
Lin Shen, MD
Brigham and Women’s Hospital, Boston
@LinShenMD
Charles Snyder, MD
Icahn School of Medicine at Mount Sinai, New York
Brian Sullivan, MD
Duke University, Durham, N.C.
Ashley Vachon, MD
University of Colorado at Denver, Aurora
Ted Walker, MD
Washington University/Barnes Jewish Hospital, St. Louis
Xiao Jing Wang, MD
Mayo Clinic, Rochester, Minn.
@IrisWangMD
Calendar
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Dec. 9-10, 11-12, 18-19, 2019; Jan. 15-16, 22-23; Feb. 12-13, Mar. 10-11, 11-12, 25-26; Apr. 15-16; May 13-14, 2020
Two-Day, In-Depth Coding Seminar by McVey Associates, Inc.
Become a certified GI coder with a 2-day, in-depth training course provided by McVey Associates, Inc.
Anaheim, Calif. (12/9-10); Houston, Tex. (12/11-12); New Orleans, La. (12/18-19); Phoenix, Ariz. (12/18-19); Pittsburgh, Pa. (1/15-16); Dallas, Tex. (1/22-23); Hartford, Conn. (2/12-13); Orlando, Fla. (3/10-11); Novi, Mich. (3/11-12); Charlotte, N.C. (3/25-26); Columbus, Ohio (4/15-16); Chicago, Ill. (5/13-14)
Jan. 23–25, 2020
2020 Crohn’s & Colitis Congress®
Gain a multidisciplinary perspective on treating inflammatory bowel diseases (IBD). Join health care professionals and researchers at the Crohn’s & Colitis Congress® for the premier conference on IBD. Discover different perspectives, leave with practical information you can immediately implement, and hear about potential treatments on the horizon.
Austin, Tex.
Jan. 23–25, 2020
Gastrointestinal Cancers Symposium
Designed for clinicians, scientists, and all other members of the cancer care and research community, the 2020 Gastrointestinal Cancers Symposium will feature a wide array of multidisciplinary topics and expert faculty will offer insights on the application of gastrointestinal advances in: cancers of the esophagus and stomach, pancreas, small bowel and hepatobiliary tract, and the colon, rectum, and anus.
San Francisco, Calif.
Feb. 8-9, 2020
2020 Academic Skills Workshop
A free biannual meeting for fellows and early-career GIs that is implemented in conjunction with the AASLD. Topics range from leveraging mentor-mentee relationships, promotion strategies, and insights on writing grants. The application deadline is Nov. 18, 2019.
Charlotte, N.C.
Feb. 20; Mar. 24, 2020
Coding and Reimbursement Solutions by McVey Associates, Inc.
Improve the efficiency and performance of your practice by staying current on the latest reimbursement, coding and compliance changes.
Knoxville, Tenn. (2/20); Birmingham, Ala. (3/24)
May 2-5, 2020
Digestive Disease Week® (DDW)
Digestive Disease Week® (DDW) is the world’s leading educational forum for academicians, clinicians, researchers, students, and trainees working in gastroenterology, hepatology, GI endoscopy, gastrointestinal surgery, and related fields. Whether you work in patient care, research, education, or administration, the DDW program offers something for you. Abstract submissions will be due on Dec. 1, and registration will open in January 2020.
Chicago, Ill.
June 3-6, 2020
2020 AGA Tech Summit
Visit https://techsummit.gastro.org/ for more details.
San Francisco, Calif.
AWARDS DEADLINES
AGA Fellow Abstract Award
This $500 travel award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Fellow Abstract of the Year and receive a $1,000 award.
Application Deadline: Feb. 26, 2020
AGA Student Abstract Award
This $500 travel award supports recipients who are graduate students, medical students, or medical residents (residents up to postgraduate year three) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW).
Application Deadline: Feb. 26, 2020
AGA-Moti L. & Kamla Rustgi International Travel Awards
This $750 travel award supports recipients who are young (i.e., 35 years of age or younger at the time of DDW) basic, translational or clinical investigators residing outside North America to support travel and related expenses to attend Digestive Disease Week® (DDW).
Application Deadline: Feb. 26, 2020
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Dec. 9-10, 11-12, 18-19, 2019; Jan. 15-16, 22-23; Feb. 12-13, Mar. 10-11, 11-12, 25-26; Apr. 15-16; May 13-14, 2020
Two-Day, In-Depth Coding Seminar by McVey Associates, Inc.
Become a certified GI coder with a 2-day, in-depth training course provided by McVey Associates, Inc.
Anaheim, Calif. (12/9-10); Houston, Tex. (12/11-12); New Orleans, La. (12/18-19); Phoenix, Ariz. (12/18-19); Pittsburgh, Pa. (1/15-16); Dallas, Tex. (1/22-23); Hartford, Conn. (2/12-13); Orlando, Fla. (3/10-11); Novi, Mich. (3/11-12); Charlotte, N.C. (3/25-26); Columbus, Ohio (4/15-16); Chicago, Ill. (5/13-14)
Jan. 23–25, 2020
2020 Crohn’s & Colitis Congress®
Gain a multidisciplinary perspective on treating inflammatory bowel diseases (IBD). Join health care professionals and researchers at the Crohn’s & Colitis Congress® for the premier conference on IBD. Discover different perspectives, leave with practical information you can immediately implement, and hear about potential treatments on the horizon.
Austin, Tex.
Jan. 23–25, 2020
Gastrointestinal Cancers Symposium
Designed for clinicians, scientists, and all other members of the cancer care and research community, the 2020 Gastrointestinal Cancers Symposium will feature a wide array of multidisciplinary topics and expert faculty will offer insights on the application of gastrointestinal advances in: cancers of the esophagus and stomach, pancreas, small bowel and hepatobiliary tract, and the colon, rectum, and anus.
San Francisco, Calif.
Feb. 8-9, 2020
2020 Academic Skills Workshop
A free biannual meeting for fellows and early-career GIs that is implemented in conjunction with the AASLD. Topics range from leveraging mentor-mentee relationships, promotion strategies, and insights on writing grants. The application deadline is Nov. 18, 2019.
Charlotte, N.C.
Feb. 20; Mar. 24, 2020
Coding and Reimbursement Solutions by McVey Associates, Inc.
Improve the efficiency and performance of your practice by staying current on the latest reimbursement, coding and compliance changes.
Knoxville, Tenn. (2/20); Birmingham, Ala. (3/24)
May 2-5, 2020
Digestive Disease Week® (DDW)
Digestive Disease Week® (DDW) is the world’s leading educational forum for academicians, clinicians, researchers, students, and trainees working in gastroenterology, hepatology, GI endoscopy, gastrointestinal surgery, and related fields. Whether you work in patient care, research, education, or administration, the DDW program offers something for you. Abstract submissions will be due on Dec. 1, and registration will open in January 2020.
Chicago, Ill.
June 3-6, 2020
2020 AGA Tech Summit
Visit https://techsummit.gastro.org/ for more details.
San Francisco, Calif.
AWARDS DEADLINES
AGA Fellow Abstract Award
This $500 travel award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Fellow Abstract of the Year and receive a $1,000 award.
Application Deadline: Feb. 26, 2020
AGA Student Abstract Award
This $500 travel award supports recipients who are graduate students, medical students, or medical residents (residents up to postgraduate year three) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW).
Application Deadline: Feb. 26, 2020
AGA-Moti L. & Kamla Rustgi International Travel Awards
This $750 travel award supports recipients who are young (i.e., 35 years of age or younger at the time of DDW) basic, translational or clinical investigators residing outside North America to support travel and related expenses to attend Digestive Disease Week® (DDW).
Application Deadline: Feb. 26, 2020
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Dec. 9-10, 11-12, 18-19, 2019; Jan. 15-16, 22-23; Feb. 12-13, Mar. 10-11, 11-12, 25-26; Apr. 15-16; May 13-14, 2020
Two-Day, In-Depth Coding Seminar by McVey Associates, Inc.
Become a certified GI coder with a 2-day, in-depth training course provided by McVey Associates, Inc.
Anaheim, Calif. (12/9-10); Houston, Tex. (12/11-12); New Orleans, La. (12/18-19); Phoenix, Ariz. (12/18-19); Pittsburgh, Pa. (1/15-16); Dallas, Tex. (1/22-23); Hartford, Conn. (2/12-13); Orlando, Fla. (3/10-11); Novi, Mich. (3/11-12); Charlotte, N.C. (3/25-26); Columbus, Ohio (4/15-16); Chicago, Ill. (5/13-14)
Jan. 23–25, 2020
2020 Crohn’s & Colitis Congress®
Gain a multidisciplinary perspective on treating inflammatory bowel diseases (IBD). Join health care professionals and researchers at the Crohn’s & Colitis Congress® for the premier conference on IBD. Discover different perspectives, leave with practical information you can immediately implement, and hear about potential treatments on the horizon.
Austin, Tex.
Jan. 23–25, 2020
Gastrointestinal Cancers Symposium
Designed for clinicians, scientists, and all other members of the cancer care and research community, the 2020 Gastrointestinal Cancers Symposium will feature a wide array of multidisciplinary topics and expert faculty will offer insights on the application of gastrointestinal advances in: cancers of the esophagus and stomach, pancreas, small bowel and hepatobiliary tract, and the colon, rectum, and anus.
San Francisco, Calif.
Feb. 8-9, 2020
2020 Academic Skills Workshop
A free biannual meeting for fellows and early-career GIs that is implemented in conjunction with the AASLD. Topics range from leveraging mentor-mentee relationships, promotion strategies, and insights on writing grants. The application deadline is Nov. 18, 2019.
Charlotte, N.C.
Feb. 20; Mar. 24, 2020
Coding and Reimbursement Solutions by McVey Associates, Inc.
Improve the efficiency and performance of your practice by staying current on the latest reimbursement, coding and compliance changes.
Knoxville, Tenn. (2/20); Birmingham, Ala. (3/24)
May 2-5, 2020
Digestive Disease Week® (DDW)
Digestive Disease Week® (DDW) is the world’s leading educational forum for academicians, clinicians, researchers, students, and trainees working in gastroenterology, hepatology, GI endoscopy, gastrointestinal surgery, and related fields. Whether you work in patient care, research, education, or administration, the DDW program offers something for you. Abstract submissions will be due on Dec. 1, and registration will open in January 2020.
Chicago, Ill.
June 3-6, 2020
2020 AGA Tech Summit
Visit https://techsummit.gastro.org/ for more details.
San Francisco, Calif.
AWARDS DEADLINES
AGA Fellow Abstract Award
This $500 travel award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Fellow Abstract of the Year and receive a $1,000 award.
Application Deadline: Feb. 26, 2020
AGA Student Abstract Award
This $500 travel award supports recipients who are graduate students, medical students, or medical residents (residents up to postgraduate year three) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW).
Application Deadline: Feb. 26, 2020
AGA-Moti L. & Kamla Rustgi International Travel Awards
This $750 travel award supports recipients who are young (i.e., 35 years of age or younger at the time of DDW) basic, translational or clinical investigators residing outside North America to support travel and related expenses to attend Digestive Disease Week® (DDW).
Application Deadline: Feb. 26, 2020
An emerging role for physicians in health policy advocacy
The American Board of Internal Medicine has called for “a commitment to the promotion of public health and preventative medicine, as well as public advocacy on the part of each physician.”1 In our responsibility to preserve and promote human life, physicians are not only uniquely positioned for advocacy but also inherently assume the role of becoming health care activists.
The American Medical Association has defined physician advocacy as promoting “social, economic, educational, and political changes that ameliorate suffering and contribute to human well being.”2 For health care professionals, this translates into ensuring the concerns and best interests of patients are at the core of all decisions.3 For generations, physicians have taken extra steps for patient care in daily practice, including submitting prior authorizations, performing peer review, and taking part in family meetings. Many doctors also participate on hospital committees and boards for quality improvement measures and are leaders in designing strategies to improve patient safety and health care experiences. Although these examples may be viewed as a fundamental part of daily practice, in fact, these roles are consistent with advocacy on a local level. A significant number of physicians participate in medical education, research, and societal duties, which include formulating and reviewing guidelines for medical practice. Participation in conference organizing committees and reviewing medical journals are likewise not uncommon roles among medical practitioners. These efforts to provide education to improve patient care are also forms of advocacy on a national or regional level but often viewed as a standard in professionalism.4
It is on the federal and political level in advocacy where physician representation is critical. Health legislation is enacted by Congress and signed into law by the president of the United States.5 These laws can drastically affect clinical practice and patient care, especially in the realm of preventive medicine and pharmaceuticals. Gastroenterology is a unique field in which a large portion of practice is dedicated to cancer prevention, by screening age-appropriate individuals and monitoring high-risk patients. The field is rapidly expanding in the pharmaceutical area with new medications for inflammatory bowel disease and groundbreaking treatments for viral hepatitis. The breadth of practice in gastroenterology calls for antiquated laws to be changed to accommodate the development of patient care guidelines. With physicians representing less than 3% of Congress,6 the rules that govern our practice are largely left to those unfamiliar with the delivery of health care.
Lack of experience, limited time, and a tradition in medicine that prefers physicians to be apolitical are each contributing factors for reduced participation in federal advocacy.7 Professional GI societies, including the American Gastroenterological Association, American College of Gastroenterology, American Society for Gastrointestinal Endoscopy, and American Association for the Study of Liver Disease, have a presence in public policy to educate lawmakers and promote statutes in gastroenterology. The involvement of these organizations in legislation is critical since public policy directly affects the interests and well-being of patients.
The priority public policy issues for GI societies are listed as follows:
- Reducing the administrative burden of prior authorizations.
- Implementing timely appeals for non–first-line therapies as determined by payers (step therapy).
- Eliminating surprise billing and cost-sharing for screening colonoscopy.
- Preserving patient protections, including for preexisting conditions and preventive services.
- Increasing federal funding and research appropriations for gastrointestinal research.
Communication with and development of relationships with legislators are essential to effective advocacy.7,8 Health professionals should be well-informed resources for members of Congress and therefore it is pivotal to provide factual information when presenting topics. There are various ways to reach congressional representatives, including personal visits, writing letters, making phone calls, or attending town halls.
Of the aforementioned, in-person meetings are the best way to directly connect with legislators. These allow for time to discuss a legislative issue, including the background and societal impact, proposed initiative, and personal accounts relating to the topic. Attending town halls also will give face-time with legislators, although the format to ask questions often is abbreviated. GI societies use letter writing as a way to increase support for a proposed bill or measure. The efficacy of letter writing increases with higher involvement. Letters are often generated in an online forum that requires the user’s zip code (so the letter can be routed to the appropriate legislator) and name with electronic signature, which are designed for easy use to boost participation.
Understanding that physicians are advocates in daily practice and that federal initiatives have significant impact on patients and clinical practice is the first step to getting involved. Participation at the local level includes connecting with the district offices of congressional leaders through letter writing, making phone calls, or in-person visits. On regional and national levels, involvement with state legislators, GI societies, or personal like-minded groups are ways to initiate federal advocacy. GI societies have federal policy committees, political action committees, and opportunities for early-career gastroenterologists to become involved in advocacy, including the Congressional Advocates Program from the AGA and the Young Physician Leadership Scholars Program from the ACG. Be sure to visit AGA’s Advocacy & Policy page to keep informed about current and future opportunities.
As the population grows and human life expectancy increases, the practice of medicine is a prime target for legislative changes, which ultimately affect patient care and clinical practice. Physicians are respected members of society, have expansive knowledge in disease processes and the delivery of health care to patients, and are naturally patient advocates. For these reasons, it is imperative for doctors to rise to the calling of federal advocacy, to continue to preserve the best interests and dignity of our patients.
References
1. ABIM Foundation. Ann Intern Med. 2002;136:243-6.
2. Earnest MA et al. Academic Med. 2010;85(1):63-7.
3. Schwartz L. J Med Ethics. 2002;28:37-40.
4. Howell BA et al. J Gen Intern Med. 2019 Aug 5. https://doi.org/10.1007/s11606-019-05184-3. [epub ahead of print]
5. The House of Representatives.
6. AGA News: https://www.gastro.org/news/new-congress-includes-22-health-care-providers
7. Kupfer SS et al. Gastroenterology. 2019;156(4)8:834-7.
8. Grace ND and LB Dennis. Hepatology. 2007;45(6):1337-9.
Dr. Abbasi is a gastroenterologist who works in inflammatory bowel diseases at Cedars-Sinai Medical Center and Santa Monica Gastroenterology, Calif.
The American Board of Internal Medicine has called for “a commitment to the promotion of public health and preventative medicine, as well as public advocacy on the part of each physician.”1 In our responsibility to preserve and promote human life, physicians are not only uniquely positioned for advocacy but also inherently assume the role of becoming health care activists.
The American Medical Association has defined physician advocacy as promoting “social, economic, educational, and political changes that ameliorate suffering and contribute to human well being.”2 For health care professionals, this translates into ensuring the concerns and best interests of patients are at the core of all decisions.3 For generations, physicians have taken extra steps for patient care in daily practice, including submitting prior authorizations, performing peer review, and taking part in family meetings. Many doctors also participate on hospital committees and boards for quality improvement measures and are leaders in designing strategies to improve patient safety and health care experiences. Although these examples may be viewed as a fundamental part of daily practice, in fact, these roles are consistent with advocacy on a local level. A significant number of physicians participate in medical education, research, and societal duties, which include formulating and reviewing guidelines for medical practice. Participation in conference organizing committees and reviewing medical journals are likewise not uncommon roles among medical practitioners. These efforts to provide education to improve patient care are also forms of advocacy on a national or regional level but often viewed as a standard in professionalism.4
It is on the federal and political level in advocacy where physician representation is critical. Health legislation is enacted by Congress and signed into law by the president of the United States.5 These laws can drastically affect clinical practice and patient care, especially in the realm of preventive medicine and pharmaceuticals. Gastroenterology is a unique field in which a large portion of practice is dedicated to cancer prevention, by screening age-appropriate individuals and monitoring high-risk patients. The field is rapidly expanding in the pharmaceutical area with new medications for inflammatory bowel disease and groundbreaking treatments for viral hepatitis. The breadth of practice in gastroenterology calls for antiquated laws to be changed to accommodate the development of patient care guidelines. With physicians representing less than 3% of Congress,6 the rules that govern our practice are largely left to those unfamiliar with the delivery of health care.
Lack of experience, limited time, and a tradition in medicine that prefers physicians to be apolitical are each contributing factors for reduced participation in federal advocacy.7 Professional GI societies, including the American Gastroenterological Association, American College of Gastroenterology, American Society for Gastrointestinal Endoscopy, and American Association for the Study of Liver Disease, have a presence in public policy to educate lawmakers and promote statutes in gastroenterology. The involvement of these organizations in legislation is critical since public policy directly affects the interests and well-being of patients.
The priority public policy issues for GI societies are listed as follows:
- Reducing the administrative burden of prior authorizations.
- Implementing timely appeals for non–first-line therapies as determined by payers (step therapy).
- Eliminating surprise billing and cost-sharing for screening colonoscopy.
- Preserving patient protections, including for preexisting conditions and preventive services.
- Increasing federal funding and research appropriations for gastrointestinal research.
Communication with and development of relationships with legislators are essential to effective advocacy.7,8 Health professionals should be well-informed resources for members of Congress and therefore it is pivotal to provide factual information when presenting topics. There are various ways to reach congressional representatives, including personal visits, writing letters, making phone calls, or attending town halls.
Of the aforementioned, in-person meetings are the best way to directly connect with legislators. These allow for time to discuss a legislative issue, including the background and societal impact, proposed initiative, and personal accounts relating to the topic. Attending town halls also will give face-time with legislators, although the format to ask questions often is abbreviated. GI societies use letter writing as a way to increase support for a proposed bill or measure. The efficacy of letter writing increases with higher involvement. Letters are often generated in an online forum that requires the user’s zip code (so the letter can be routed to the appropriate legislator) and name with electronic signature, which are designed for easy use to boost participation.
Understanding that physicians are advocates in daily practice and that federal initiatives have significant impact on patients and clinical practice is the first step to getting involved. Participation at the local level includes connecting with the district offices of congressional leaders through letter writing, making phone calls, or in-person visits. On regional and national levels, involvement with state legislators, GI societies, or personal like-minded groups are ways to initiate federal advocacy. GI societies have federal policy committees, political action committees, and opportunities for early-career gastroenterologists to become involved in advocacy, including the Congressional Advocates Program from the AGA and the Young Physician Leadership Scholars Program from the ACG. Be sure to visit AGA’s Advocacy & Policy page to keep informed about current and future opportunities.
As the population grows and human life expectancy increases, the practice of medicine is a prime target for legislative changes, which ultimately affect patient care and clinical practice. Physicians are respected members of society, have expansive knowledge in disease processes and the delivery of health care to patients, and are naturally patient advocates. For these reasons, it is imperative for doctors to rise to the calling of federal advocacy, to continue to preserve the best interests and dignity of our patients.
References
1. ABIM Foundation. Ann Intern Med. 2002;136:243-6.
2. Earnest MA et al. Academic Med. 2010;85(1):63-7.
3. Schwartz L. J Med Ethics. 2002;28:37-40.
4. Howell BA et al. J Gen Intern Med. 2019 Aug 5. https://doi.org/10.1007/s11606-019-05184-3. [epub ahead of print]
5. The House of Representatives.
6. AGA News: https://www.gastro.org/news/new-congress-includes-22-health-care-providers
7. Kupfer SS et al. Gastroenterology. 2019;156(4)8:834-7.
8. Grace ND and LB Dennis. Hepatology. 2007;45(6):1337-9.
Dr. Abbasi is a gastroenterologist who works in inflammatory bowel diseases at Cedars-Sinai Medical Center and Santa Monica Gastroenterology, Calif.
The American Board of Internal Medicine has called for “a commitment to the promotion of public health and preventative medicine, as well as public advocacy on the part of each physician.”1 In our responsibility to preserve and promote human life, physicians are not only uniquely positioned for advocacy but also inherently assume the role of becoming health care activists.
The American Medical Association has defined physician advocacy as promoting “social, economic, educational, and political changes that ameliorate suffering and contribute to human well being.”2 For health care professionals, this translates into ensuring the concerns and best interests of patients are at the core of all decisions.3 For generations, physicians have taken extra steps for patient care in daily practice, including submitting prior authorizations, performing peer review, and taking part in family meetings. Many doctors also participate on hospital committees and boards for quality improvement measures and are leaders in designing strategies to improve patient safety and health care experiences. Although these examples may be viewed as a fundamental part of daily practice, in fact, these roles are consistent with advocacy on a local level. A significant number of physicians participate in medical education, research, and societal duties, which include formulating and reviewing guidelines for medical practice. Participation in conference organizing committees and reviewing medical journals are likewise not uncommon roles among medical practitioners. These efforts to provide education to improve patient care are also forms of advocacy on a national or regional level but often viewed as a standard in professionalism.4
It is on the federal and political level in advocacy where physician representation is critical. Health legislation is enacted by Congress and signed into law by the president of the United States.5 These laws can drastically affect clinical practice and patient care, especially in the realm of preventive medicine and pharmaceuticals. Gastroenterology is a unique field in which a large portion of practice is dedicated to cancer prevention, by screening age-appropriate individuals and monitoring high-risk patients. The field is rapidly expanding in the pharmaceutical area with new medications for inflammatory bowel disease and groundbreaking treatments for viral hepatitis. The breadth of practice in gastroenterology calls for antiquated laws to be changed to accommodate the development of patient care guidelines. With physicians representing less than 3% of Congress,6 the rules that govern our practice are largely left to those unfamiliar with the delivery of health care.
Lack of experience, limited time, and a tradition in medicine that prefers physicians to be apolitical are each contributing factors for reduced participation in federal advocacy.7 Professional GI societies, including the American Gastroenterological Association, American College of Gastroenterology, American Society for Gastrointestinal Endoscopy, and American Association for the Study of Liver Disease, have a presence in public policy to educate lawmakers and promote statutes in gastroenterology. The involvement of these organizations in legislation is critical since public policy directly affects the interests and well-being of patients.
The priority public policy issues for GI societies are listed as follows:
- Reducing the administrative burden of prior authorizations.
- Implementing timely appeals for non–first-line therapies as determined by payers (step therapy).
- Eliminating surprise billing and cost-sharing for screening colonoscopy.
- Preserving patient protections, including for preexisting conditions and preventive services.
- Increasing federal funding and research appropriations for gastrointestinal research.
Communication with and development of relationships with legislators are essential to effective advocacy.7,8 Health professionals should be well-informed resources for members of Congress and therefore it is pivotal to provide factual information when presenting topics. There are various ways to reach congressional representatives, including personal visits, writing letters, making phone calls, or attending town halls.
Of the aforementioned, in-person meetings are the best way to directly connect with legislators. These allow for time to discuss a legislative issue, including the background and societal impact, proposed initiative, and personal accounts relating to the topic. Attending town halls also will give face-time with legislators, although the format to ask questions often is abbreviated. GI societies use letter writing as a way to increase support for a proposed bill or measure. The efficacy of letter writing increases with higher involvement. Letters are often generated in an online forum that requires the user’s zip code (so the letter can be routed to the appropriate legislator) and name with electronic signature, which are designed for easy use to boost participation.
Understanding that physicians are advocates in daily practice and that federal initiatives have significant impact on patients and clinical practice is the first step to getting involved. Participation at the local level includes connecting with the district offices of congressional leaders through letter writing, making phone calls, or in-person visits. On regional and national levels, involvement with state legislators, GI societies, or personal like-minded groups are ways to initiate federal advocacy. GI societies have federal policy committees, political action committees, and opportunities for early-career gastroenterologists to become involved in advocacy, including the Congressional Advocates Program from the AGA and the Young Physician Leadership Scholars Program from the ACG. Be sure to visit AGA’s Advocacy & Policy page to keep informed about current and future opportunities.
As the population grows and human life expectancy increases, the practice of medicine is a prime target for legislative changes, which ultimately affect patient care and clinical practice. Physicians are respected members of society, have expansive knowledge in disease processes and the delivery of health care to patients, and are naturally patient advocates. For these reasons, it is imperative for doctors to rise to the calling of federal advocacy, to continue to preserve the best interests and dignity of our patients.
References
1. ABIM Foundation. Ann Intern Med. 2002;136:243-6.
2. Earnest MA et al. Academic Med. 2010;85(1):63-7.
3. Schwartz L. J Med Ethics. 2002;28:37-40.
4. Howell BA et al. J Gen Intern Med. 2019 Aug 5. https://doi.org/10.1007/s11606-019-05184-3. [epub ahead of print]
5. The House of Representatives.
6. AGA News: https://www.gastro.org/news/new-congress-includes-22-health-care-providers
7. Kupfer SS et al. Gastroenterology. 2019;156(4)8:834-7.
8. Grace ND and LB Dennis. Hepatology. 2007;45(6):1337-9.
Dr. Abbasi is a gastroenterologist who works in inflammatory bowel diseases at Cedars-Sinai Medical Center and Santa Monica Gastroenterology, Calif.
AGA promotes workforce diversity in academic gastroenterology: The FORWARD program
“The American Gastroenterological Association (AGA) has worked diligently and effectively to expand the pool of underrepresented in medicine physicians who provide care for patients with digestive diseases,” remarks Byron Cryer, MD, FORWARD principal investigator and associate dean for the office of faculty diversity & development, University of Texas Southwestern Medical Center, Dallas.
This long history of promoting and encouraging diversity in the field is notable; however, the society recognizes that there is still more to be done to prepare and sponsor underrepresented in medicine physician-scientists to assume leadership positions in the field.
“As of 2017, only 3.2% of gastroenterology fellows were African American and 8.5% were Hispanic,” remarked Sandra Quezada, MD, AGA Chair, Diversity Committee. These statistics closely correspond to AGA’s membership demographics, where only 3.36% of AGA members are African American and 5.53% are Hispanic, among those reporting ethnicity.
Under the leadership of principal investigators Byron Cryer, MD, and Sheila Crowe, MD, and coinvestigators Juanita Merchant, MD, and Jesus Rivera-Nieves, MD, AGA developed the FORWARD (Fostering Opportunities Resulting in Workforce and Research Diversity) program.
This program was funded by the National Institute of Diabetes and Digestive and Kidney Diseases through an education project (R25) grant.
The overall objective of the grant is to enhance the diversity of the biomedical science research workforce in gastroenterology and to prepare individuals to assume leadership positions within the AGA and in academic medicine.
The FORWARD program has three aims:
1. Provide skill development in leadership – including general leadership development, executive coaching, and opportunities for leadership experience within the society.
2. Implement a diversity management plan focused on active mentoring approaches, using both one-on-one and networking approaches, to provide opportunities for broad support and sponsorship.
3. Provide training for skill development in research careers including training in research development, management of research groups, scientific manuscript, and grant writing.
The program targets fellows and early-career faculty and was promoted broadly to society membership and to past participants in AGA diversity programs. Through a rigorous selection process, 10 physician-scientists were selected among a qualified pool of applicants. Participants were matched with experienced academic gastroenterologists who committed to serve as their professional mentors throughout the course of their participation in the FORWARD program.
Leadership development
The first FORWARD cohort kicked off in March 2019 and will conclude May 2020. They convened at AGA’s inaugural 2½ day Leadership Development Conference. During the conference, the 10 FORWARD scholars joined with 18 AGA Future Leader program participants and 40 Women’s Leadership Conference attendees to participate in leadership development training that addressed such topics as building resilience, presentation skills, negotiation, career success in an ever-changing scientific environment, emotional intelligence, and other key topics designed to bolster their skills as emerging leaders in gastroenterology. With seven past and current AGA presidents in attendance, the FORWARD participants had the unique opportunity to learn about the career paths and gain advice from key leaders in the field while also networking with fellow emerging leaders in both the Future Leaders program and Women’s Leadership Conference.
To further prepare the FORWARD scholars to assume future leadership positions within the society, they’ve begun “internships” that included shadowing AGA committee meetings during Digestive Disease Week (DDW®) 2019 in San Diego and participating in networking events with AGA leaders at receptions and various events.
Mentoring and coaching
In addition to leadership development training at the conference, each FORWARD scholar identified a research topic that they would work on with their mentor and a grant-writing expert to develop into a full grant proposal by the conclusion of the program. The FORWARD mentors have committed to provide mentoring that addresses scientific research content as well as career guidance. The active mentoring provided to the FORWARD scholars is extensive and includes regular contact with their mentors as well as one-on-one executive coaching by a professional coach committed to learning about their needs and guiding them through an individual development plan.
To facilitate the coaching for their individual development plan, each FORWARD scholar completed a Self-Assessment for Physician Leaders and a 360° assessment, which informed the executive coaching that the scholars receive. For many of the scholars, this is the first time that they have benefited from individual executive coaching.
Academic research skills development
In addition to leadership development training and networking opportunities along with executive coaching and mentoring, the FORWARD scholars benefit from direct evaluation and consultation on grant writing. FORWARD scholars have access to a private learning management system where they participate in online grant-writing skills training designed by a grant-writing expert. Based on their training, they are developing various segments of an actual grant proposal and are receiving both virtual peer critiques as well as individualized critiques and guidance from the grant-writing consultant, their program mentor, and their home institution mentor. Future training modules will include lab management and manuscript writing.
Jesus Rivera-Nieves, MD, FORWARD coinvestigator, stated, “I have had the honor of monitoring the progress of our first class of scholars recently. I am incredibly proud of the caliber of these early-career professionals and have no doubt that they will emerge as leaders in our field, where more diversity is greatly needed.”
The scholars and their mentors will reconvene at DDW® 2020 for an opportunity to give a presentation and participate in a commencement ceremony concluding their involvement in the program. Following the graduation of this first cohort, there will be a period of evaluation prior to recruitment of the second cohort of FORWARD scholars.
The 2018-2020 FORWARD Cohort includes:
Yelina Alvarez, MD, PhD
|
Dominique Bailey, MD
|
|
Oriana M. Damas, MD
|
Patricia D. Jones, MD, MSCR
|
Folasade (Fola) May, MD, PhD, MPhil
|
Antonio Mendoza Ladd, MD, FACG, FASGE
|
Akinbowale Oyalowo, MD
|
|
Eric J. Vargas, MD |
The 2018-2020 – FORWARD Program Mentors
Maria Abreu, MD, AGAF
|
C. Rick Boland, MD, AGAF
|
John Carethers, MD, AGAF
|
Darwin Conwell, MD, MS
|
|
John Inadomi, MD, AGAF
|
|
|
|
Gary Wu, MD |
Dr. Cryer is associate dean for faculty diversity and development, UT Southwestern Medical Center, Dallas; Dr. Rivera-Nieves, is professor of medicine, University of California, San Diego; and Ms. NuQuay, is senior director, member relations and constituency programs, American Gastroenterological Association, Bethesda, Md.
“The American Gastroenterological Association (AGA) has worked diligently and effectively to expand the pool of underrepresented in medicine physicians who provide care for patients with digestive diseases,” remarks Byron Cryer, MD, FORWARD principal investigator and associate dean for the office of faculty diversity & development, University of Texas Southwestern Medical Center, Dallas.
This long history of promoting and encouraging diversity in the field is notable; however, the society recognizes that there is still more to be done to prepare and sponsor underrepresented in medicine physician-scientists to assume leadership positions in the field.
“As of 2017, only 3.2% of gastroenterology fellows were African American and 8.5% were Hispanic,” remarked Sandra Quezada, MD, AGA Chair, Diversity Committee. These statistics closely correspond to AGA’s membership demographics, where only 3.36% of AGA members are African American and 5.53% are Hispanic, among those reporting ethnicity.
Under the leadership of principal investigators Byron Cryer, MD, and Sheila Crowe, MD, and coinvestigators Juanita Merchant, MD, and Jesus Rivera-Nieves, MD, AGA developed the FORWARD (Fostering Opportunities Resulting in Workforce and Research Diversity) program.
This program was funded by the National Institute of Diabetes and Digestive and Kidney Diseases through an education project (R25) grant.
The overall objective of the grant is to enhance the diversity of the biomedical science research workforce in gastroenterology and to prepare individuals to assume leadership positions within the AGA and in academic medicine.
The FORWARD program has three aims:
1. Provide skill development in leadership – including general leadership development, executive coaching, and opportunities for leadership experience within the society.
2. Implement a diversity management plan focused on active mentoring approaches, using both one-on-one and networking approaches, to provide opportunities for broad support and sponsorship.
3. Provide training for skill development in research careers including training in research development, management of research groups, scientific manuscript, and grant writing.
The program targets fellows and early-career faculty and was promoted broadly to society membership and to past participants in AGA diversity programs. Through a rigorous selection process, 10 physician-scientists were selected among a qualified pool of applicants. Participants were matched with experienced academic gastroenterologists who committed to serve as their professional mentors throughout the course of their participation in the FORWARD program.
Leadership development
The first FORWARD cohort kicked off in March 2019 and will conclude May 2020. They convened at AGA’s inaugural 2½ day Leadership Development Conference. During the conference, the 10 FORWARD scholars joined with 18 AGA Future Leader program participants and 40 Women’s Leadership Conference attendees to participate in leadership development training that addressed such topics as building resilience, presentation skills, negotiation, career success in an ever-changing scientific environment, emotional intelligence, and other key topics designed to bolster their skills as emerging leaders in gastroenterology. With seven past and current AGA presidents in attendance, the FORWARD participants had the unique opportunity to learn about the career paths and gain advice from key leaders in the field while also networking with fellow emerging leaders in both the Future Leaders program and Women’s Leadership Conference.
To further prepare the FORWARD scholars to assume future leadership positions within the society, they’ve begun “internships” that included shadowing AGA committee meetings during Digestive Disease Week (DDW®) 2019 in San Diego and participating in networking events with AGA leaders at receptions and various events.
Mentoring and coaching
In addition to leadership development training at the conference, each FORWARD scholar identified a research topic that they would work on with their mentor and a grant-writing expert to develop into a full grant proposal by the conclusion of the program. The FORWARD mentors have committed to provide mentoring that addresses scientific research content as well as career guidance. The active mentoring provided to the FORWARD scholars is extensive and includes regular contact with their mentors as well as one-on-one executive coaching by a professional coach committed to learning about their needs and guiding them through an individual development plan.
To facilitate the coaching for their individual development plan, each FORWARD scholar completed a Self-Assessment for Physician Leaders and a 360° assessment, which informed the executive coaching that the scholars receive. For many of the scholars, this is the first time that they have benefited from individual executive coaching.
Academic research skills development
In addition to leadership development training and networking opportunities along with executive coaching and mentoring, the FORWARD scholars benefit from direct evaluation and consultation on grant writing. FORWARD scholars have access to a private learning management system where they participate in online grant-writing skills training designed by a grant-writing expert. Based on their training, they are developing various segments of an actual grant proposal and are receiving both virtual peer critiques as well as individualized critiques and guidance from the grant-writing consultant, their program mentor, and their home institution mentor. Future training modules will include lab management and manuscript writing.
Jesus Rivera-Nieves, MD, FORWARD coinvestigator, stated, “I have had the honor of monitoring the progress of our first class of scholars recently. I am incredibly proud of the caliber of these early-career professionals and have no doubt that they will emerge as leaders in our field, where more diversity is greatly needed.”
The scholars and their mentors will reconvene at DDW® 2020 for an opportunity to give a presentation and participate in a commencement ceremony concluding their involvement in the program. Following the graduation of this first cohort, there will be a period of evaluation prior to recruitment of the second cohort of FORWARD scholars.
The 2018-2020 FORWARD Cohort includes:
Yelina Alvarez, MD, PhD
|
Dominique Bailey, MD
|
|
Oriana M. Damas, MD
|
Patricia D. Jones, MD, MSCR
|
Folasade (Fola) May, MD, PhD, MPhil
|
Antonio Mendoza Ladd, MD, FACG, FASGE
|
Akinbowale Oyalowo, MD
|
|
Eric J. Vargas, MD |
The 2018-2020 – FORWARD Program Mentors
Maria Abreu, MD, AGAF
|
C. Rick Boland, MD, AGAF
|
John Carethers, MD, AGAF
|
Darwin Conwell, MD, MS
|
|
John Inadomi, MD, AGAF
|
|
|
|
Gary Wu, MD |
Dr. Cryer is associate dean for faculty diversity and development, UT Southwestern Medical Center, Dallas; Dr. Rivera-Nieves, is professor of medicine, University of California, San Diego; and Ms. NuQuay, is senior director, member relations and constituency programs, American Gastroenterological Association, Bethesda, Md.
“The American Gastroenterological Association (AGA) has worked diligently and effectively to expand the pool of underrepresented in medicine physicians who provide care for patients with digestive diseases,” remarks Byron Cryer, MD, FORWARD principal investigator and associate dean for the office of faculty diversity & development, University of Texas Southwestern Medical Center, Dallas.
This long history of promoting and encouraging diversity in the field is notable; however, the society recognizes that there is still more to be done to prepare and sponsor underrepresented in medicine physician-scientists to assume leadership positions in the field.
“As of 2017, only 3.2% of gastroenterology fellows were African American and 8.5% were Hispanic,” remarked Sandra Quezada, MD, AGA Chair, Diversity Committee. These statistics closely correspond to AGA’s membership demographics, where only 3.36% of AGA members are African American and 5.53% are Hispanic, among those reporting ethnicity.
Under the leadership of principal investigators Byron Cryer, MD, and Sheila Crowe, MD, and coinvestigators Juanita Merchant, MD, and Jesus Rivera-Nieves, MD, AGA developed the FORWARD (Fostering Opportunities Resulting in Workforce and Research Diversity) program.
This program was funded by the National Institute of Diabetes and Digestive and Kidney Diseases through an education project (R25) grant.
The overall objective of the grant is to enhance the diversity of the biomedical science research workforce in gastroenterology and to prepare individuals to assume leadership positions within the AGA and in academic medicine.
The FORWARD program has three aims:
1. Provide skill development in leadership – including general leadership development, executive coaching, and opportunities for leadership experience within the society.
2. Implement a diversity management plan focused on active mentoring approaches, using both one-on-one and networking approaches, to provide opportunities for broad support and sponsorship.
3. Provide training for skill development in research careers including training in research development, management of research groups, scientific manuscript, and grant writing.
The program targets fellows and early-career faculty and was promoted broadly to society membership and to past participants in AGA diversity programs. Through a rigorous selection process, 10 physician-scientists were selected among a qualified pool of applicants. Participants were matched with experienced academic gastroenterologists who committed to serve as their professional mentors throughout the course of their participation in the FORWARD program.
Leadership development
The first FORWARD cohort kicked off in March 2019 and will conclude May 2020. They convened at AGA’s inaugural 2½ day Leadership Development Conference. During the conference, the 10 FORWARD scholars joined with 18 AGA Future Leader program participants and 40 Women’s Leadership Conference attendees to participate in leadership development training that addressed such topics as building resilience, presentation skills, negotiation, career success in an ever-changing scientific environment, emotional intelligence, and other key topics designed to bolster their skills as emerging leaders in gastroenterology. With seven past and current AGA presidents in attendance, the FORWARD participants had the unique opportunity to learn about the career paths and gain advice from key leaders in the field while also networking with fellow emerging leaders in both the Future Leaders program and Women’s Leadership Conference.
To further prepare the FORWARD scholars to assume future leadership positions within the society, they’ve begun “internships” that included shadowing AGA committee meetings during Digestive Disease Week (DDW®) 2019 in San Diego and participating in networking events with AGA leaders at receptions and various events.
Mentoring and coaching
In addition to leadership development training at the conference, each FORWARD scholar identified a research topic that they would work on with their mentor and a grant-writing expert to develop into a full grant proposal by the conclusion of the program. The FORWARD mentors have committed to provide mentoring that addresses scientific research content as well as career guidance. The active mentoring provided to the FORWARD scholars is extensive and includes regular contact with their mentors as well as one-on-one executive coaching by a professional coach committed to learning about their needs and guiding them through an individual development plan.
To facilitate the coaching for their individual development plan, each FORWARD scholar completed a Self-Assessment for Physician Leaders and a 360° assessment, which informed the executive coaching that the scholars receive. For many of the scholars, this is the first time that they have benefited from individual executive coaching.
Academic research skills development
In addition to leadership development training and networking opportunities along with executive coaching and mentoring, the FORWARD scholars benefit from direct evaluation and consultation on grant writing. FORWARD scholars have access to a private learning management system where they participate in online grant-writing skills training designed by a grant-writing expert. Based on their training, they are developing various segments of an actual grant proposal and are receiving both virtual peer critiques as well as individualized critiques and guidance from the grant-writing consultant, their program mentor, and their home institution mentor. Future training modules will include lab management and manuscript writing.
Jesus Rivera-Nieves, MD, FORWARD coinvestigator, stated, “I have had the honor of monitoring the progress of our first class of scholars recently. I am incredibly proud of the caliber of these early-career professionals and have no doubt that they will emerge as leaders in our field, where more diversity is greatly needed.”
The scholars and their mentors will reconvene at DDW® 2020 for an opportunity to give a presentation and participate in a commencement ceremony concluding their involvement in the program. Following the graduation of this first cohort, there will be a period of evaluation prior to recruitment of the second cohort of FORWARD scholars.
The 2018-2020 FORWARD Cohort includes:
Yelina Alvarez, MD, PhD
|
Dominique Bailey, MD
|
|
Oriana M. Damas, MD
|
Patricia D. Jones, MD, MSCR
|
Folasade (Fola) May, MD, PhD, MPhil
|
Antonio Mendoza Ladd, MD, FACG, FASGE
|
Akinbowale Oyalowo, MD
|
|
Eric J. Vargas, MD |
The 2018-2020 – FORWARD Program Mentors
Maria Abreu, MD, AGAF
|
C. Rick Boland, MD, AGAF
|
John Carethers, MD, AGAF
|
Darwin Conwell, MD, MS
|
|
John Inadomi, MD, AGAF
|
|
|
|
Gary Wu, MD |
Dr. Cryer is associate dean for faculty diversity and development, UT Southwestern Medical Center, Dallas; Dr. Rivera-Nieves, is professor of medicine, University of California, San Diego; and Ms. NuQuay, is senior director, member relations and constituency programs, American Gastroenterological Association, Bethesda, Md.
Q&A with DDW 2019 Advancing Clinical Practice: GI Fellow-Directed Quality Improvement Projects session abstract reviewers
The AGA Education & Training Committee sponsors a session during Digestive Disease Week® (DDW) entitled “Advancing Clinical Practice: GI Fellow-Directed Quality Improvement (QI) Projects.” Session participants are selected to give an oral or poster presentation of a quality improvement project they complete during fellowship. The QI project abstracts are peer reviewed and chosen by volunteers from the AGA Young Delegates. We asked several abstract reviewers from the 2019 session for advice on what makes an exceptional QI project and how to make an abstract stand out.
This session will be held again during DDW 2020. Interested participants should submit their abstract to the DDW descriptor GI Fellow-Directed QI Session via the DDW abstract submission site between Oct. 17 and Dec. 1, 2019.
What are the top 3 things that make an exceptional QI project?
Mohammad Bilal, MD, fellow, advanced endoscopy
Beth Israel Deaconess Medical Center Harvard Medical School in Boston
Quality improvement projects are essential to identify areas of improvement in a health care system/institution and to be able to improve them. The best kind of QI projects are those that lead to sustainable positive outcomes. This is not always easy and usually requires a multifocal intervention strategy. In my opinion, the top three things that make an exceptional QI project are:
1. A clear, focused, concise, realistic, and achievable “aim statement,” also known as a “SMART” (Specific, Measurable, Achievable, Relevant/Realistic, Time-Bound) aim statement.
2. A project that directly impacts patient-related outcomes.
3. A project that involves multiple members of the health care team in addition to physicians and patients, such as pharmacists, therapists, schedulers, or IT staff.
Chung Sang Tse, MD, gastroenterology fellow
Brown University Rhode Island Hospital in Providence, R.I.
Some of the most impressive QI abstracts that stood out to my fellow cojudges and I were those that employed novel yet widely applicable solutions to common problems, along with empiric data to assess their effects. For example, one of the most highly rated abstracts was one that used an electronic order set to standardize the acute care of inflammatory bowel disease flare in the emergency department and measured the impact on treatment outcomes. Another memorable abstract tested the use of meditation (via an instructional soundtrack) in the endoscopy suite to assess its effect on sedation use, procedural time, and patient comfort; although the results in the abstract did not reach statistical significance, the reviewers rated this abstract favorably for its attempt to improve the endoscopy experience in a low-cost and replicable manner. For this QI category, the reviewers weighed most heavily on a study’s impact, the rigor of methodology, and novelty of the problem/solution.
What advice would you give to prospective authors to make their abstracts stand out?
Michelle Hughes, MD, assistant professor of internal medicine
Section of digestive diseases, Yale School of Medicine in New Haven, Conn.
Turning your hard work from a QI project into a successful abstract can seem daunting. There are a few things to consider to help your findings reach your target audience. First, an abstract should be clear and concise. Limit background content and focus on highlighting your project’s methodology and results. Remember, readers look for a comprehensive overview so they can easily understand what you did and found without a lot of extra content obscuring the main points. Everything in your abstract should tie back to your stated aim. Does the background build a case for your project? Do the results and conclusion clearly answer the hypothesis and/or clinical question? If a reported result does not help answer your question, then it should probably be left out. Another tip is to always carefully follow the guidelines for abstract submission. Ensure you use correct formatting and follow all outlined rules. Ask a colleague to review and proofread your abstract draft prior to submission. Lastly, before you start writing, decide what take-away message or statement makes your project meaningful. Refer to this as you write and finalize your abstract so that you keep your message clear and remain focused. You did the hard work to execute your project – but without an effective abstract, the impact of your work may get lost.
Manol Jovani, MD, MPH, therapeutic endoscopy fellow
Division of gastroenterology and hepatology, Johns Hopkins Hospital in Baltimore
The abstract is the “elevator pitch” of your year(s)-long work, and as such it must be very carefully crafted. Dedicate specific time to the style and quality of your presentation long before the deadline. As for elevator pitches, first impressions are very important. For an abstract, it begins with the title. Select a declarative title that draws the interest of the reviewers/readers and tells them exactly what to expect, which also accurately represents the main findings. The introduction and aims should be very short and to the point. For example, it is counterproductive to start with “Ulcerative colitis (UC) and Crohn’s disease (CD) are the two major types of inflammatory bowel disease (IBD).” This does not add any information, states what is obvious to any GI reviewer, uses up 92 characters, and possibly gives a subconscious sense of unoriginality. Throughout the description of your methods/results, use clear language. A well-written abstract will explicitly and concisely state the populations included, statistical analyses performed, and specific outcomes. Make it easy for reviewers to follow along by presenting the results in a logical progression and by striving to use as few acronyms as possible. In addition, less is often more. The abstract should have only one main message, enunciated with the title, evident in the results, and interpreted with the conclusion. If you have additional space, use it to make compelling concluding remarks. As in an elevator pitch, the conclusion is as important as the introduction. Conclude by giving a clear message to the audience/reviewers on what the main findings are and suggest specific implications for future research. Do not end with “more studies are needed,” but rather suggest specifically how these results will influence our future understanding. Finally, know your audience and adapt your presentation to their needs, knowledge, and interests. This includes being mindful of the section to which you are submitting your abstract. An excellent abstract that could have been an oral presentation in one section may end up being a poster in another. Something important to remember about abstracts is that, while the content of the research is the most important part, the quality of its presentation serves to alert the reviewers and readers about it.
Ms. Mietzelfeld is a coordinator of data and training initiatives for the American Gastroenterological Association in Bethesda, Md.
The AGA Education & Training Committee sponsors a session during Digestive Disease Week® (DDW) entitled “Advancing Clinical Practice: GI Fellow-Directed Quality Improvement (QI) Projects.” Session participants are selected to give an oral or poster presentation of a quality improvement project they complete during fellowship. The QI project abstracts are peer reviewed and chosen by volunteers from the AGA Young Delegates. We asked several abstract reviewers from the 2019 session for advice on what makes an exceptional QI project and how to make an abstract stand out.
This session will be held again during DDW 2020. Interested participants should submit their abstract to the DDW descriptor GI Fellow-Directed QI Session via the DDW abstract submission site between Oct. 17 and Dec. 1, 2019.
What are the top 3 things that make an exceptional QI project?
Mohammad Bilal, MD, fellow, advanced endoscopy
Beth Israel Deaconess Medical Center Harvard Medical School in Boston
Quality improvement projects are essential to identify areas of improvement in a health care system/institution and to be able to improve them. The best kind of QI projects are those that lead to sustainable positive outcomes. This is not always easy and usually requires a multifocal intervention strategy. In my opinion, the top three things that make an exceptional QI project are:
1. A clear, focused, concise, realistic, and achievable “aim statement,” also known as a “SMART” (Specific, Measurable, Achievable, Relevant/Realistic, Time-Bound) aim statement.
2. A project that directly impacts patient-related outcomes.
3. A project that involves multiple members of the health care team in addition to physicians and patients, such as pharmacists, therapists, schedulers, or IT staff.
Chung Sang Tse, MD, gastroenterology fellow
Brown University Rhode Island Hospital in Providence, R.I.
Some of the most impressive QI abstracts that stood out to my fellow cojudges and I were those that employed novel yet widely applicable solutions to common problems, along with empiric data to assess their effects. For example, one of the most highly rated abstracts was one that used an electronic order set to standardize the acute care of inflammatory bowel disease flare in the emergency department and measured the impact on treatment outcomes. Another memorable abstract tested the use of meditation (via an instructional soundtrack) in the endoscopy suite to assess its effect on sedation use, procedural time, and patient comfort; although the results in the abstract did not reach statistical significance, the reviewers rated this abstract favorably for its attempt to improve the endoscopy experience in a low-cost and replicable manner. For this QI category, the reviewers weighed most heavily on a study’s impact, the rigor of methodology, and novelty of the problem/solution.
What advice would you give to prospective authors to make their abstracts stand out?
Michelle Hughes, MD, assistant professor of internal medicine
Section of digestive diseases, Yale School of Medicine in New Haven, Conn.
Turning your hard work from a QI project into a successful abstract can seem daunting. There are a few things to consider to help your findings reach your target audience. First, an abstract should be clear and concise. Limit background content and focus on highlighting your project’s methodology and results. Remember, readers look for a comprehensive overview so they can easily understand what you did and found without a lot of extra content obscuring the main points. Everything in your abstract should tie back to your stated aim. Does the background build a case for your project? Do the results and conclusion clearly answer the hypothesis and/or clinical question? If a reported result does not help answer your question, then it should probably be left out. Another tip is to always carefully follow the guidelines for abstract submission. Ensure you use correct formatting and follow all outlined rules. Ask a colleague to review and proofread your abstract draft prior to submission. Lastly, before you start writing, decide what take-away message or statement makes your project meaningful. Refer to this as you write and finalize your abstract so that you keep your message clear and remain focused. You did the hard work to execute your project – but without an effective abstract, the impact of your work may get lost.
Manol Jovani, MD, MPH, therapeutic endoscopy fellow
Division of gastroenterology and hepatology, Johns Hopkins Hospital in Baltimore
The abstract is the “elevator pitch” of your year(s)-long work, and as such it must be very carefully crafted. Dedicate specific time to the style and quality of your presentation long before the deadline. As for elevator pitches, first impressions are very important. For an abstract, it begins with the title. Select a declarative title that draws the interest of the reviewers/readers and tells them exactly what to expect, which also accurately represents the main findings. The introduction and aims should be very short and to the point. For example, it is counterproductive to start with “Ulcerative colitis (UC) and Crohn’s disease (CD) are the two major types of inflammatory bowel disease (IBD).” This does not add any information, states what is obvious to any GI reviewer, uses up 92 characters, and possibly gives a subconscious sense of unoriginality. Throughout the description of your methods/results, use clear language. A well-written abstract will explicitly and concisely state the populations included, statistical analyses performed, and specific outcomes. Make it easy for reviewers to follow along by presenting the results in a logical progression and by striving to use as few acronyms as possible. In addition, less is often more. The abstract should have only one main message, enunciated with the title, evident in the results, and interpreted with the conclusion. If you have additional space, use it to make compelling concluding remarks. As in an elevator pitch, the conclusion is as important as the introduction. Conclude by giving a clear message to the audience/reviewers on what the main findings are and suggest specific implications for future research. Do not end with “more studies are needed,” but rather suggest specifically how these results will influence our future understanding. Finally, know your audience and adapt your presentation to their needs, knowledge, and interests. This includes being mindful of the section to which you are submitting your abstract. An excellent abstract that could have been an oral presentation in one section may end up being a poster in another. Something important to remember about abstracts is that, while the content of the research is the most important part, the quality of its presentation serves to alert the reviewers and readers about it.
Ms. Mietzelfeld is a coordinator of data and training initiatives for the American Gastroenterological Association in Bethesda, Md.
The AGA Education & Training Committee sponsors a session during Digestive Disease Week® (DDW) entitled “Advancing Clinical Practice: GI Fellow-Directed Quality Improvement (QI) Projects.” Session participants are selected to give an oral or poster presentation of a quality improvement project they complete during fellowship. The QI project abstracts are peer reviewed and chosen by volunteers from the AGA Young Delegates. We asked several abstract reviewers from the 2019 session for advice on what makes an exceptional QI project and how to make an abstract stand out.
This session will be held again during DDW 2020. Interested participants should submit their abstract to the DDW descriptor GI Fellow-Directed QI Session via the DDW abstract submission site between Oct. 17 and Dec. 1, 2019.
What are the top 3 things that make an exceptional QI project?
Mohammad Bilal, MD, fellow, advanced endoscopy
Beth Israel Deaconess Medical Center Harvard Medical School in Boston
Quality improvement projects are essential to identify areas of improvement in a health care system/institution and to be able to improve them. The best kind of QI projects are those that lead to sustainable positive outcomes. This is not always easy and usually requires a multifocal intervention strategy. In my opinion, the top three things that make an exceptional QI project are:
1. A clear, focused, concise, realistic, and achievable “aim statement,” also known as a “SMART” (Specific, Measurable, Achievable, Relevant/Realistic, Time-Bound) aim statement.
2. A project that directly impacts patient-related outcomes.
3. A project that involves multiple members of the health care team in addition to physicians and patients, such as pharmacists, therapists, schedulers, or IT staff.
Chung Sang Tse, MD, gastroenterology fellow
Brown University Rhode Island Hospital in Providence, R.I.
Some of the most impressive QI abstracts that stood out to my fellow cojudges and I were those that employed novel yet widely applicable solutions to common problems, along with empiric data to assess their effects. For example, one of the most highly rated abstracts was one that used an electronic order set to standardize the acute care of inflammatory bowel disease flare in the emergency department and measured the impact on treatment outcomes. Another memorable abstract tested the use of meditation (via an instructional soundtrack) in the endoscopy suite to assess its effect on sedation use, procedural time, and patient comfort; although the results in the abstract did not reach statistical significance, the reviewers rated this abstract favorably for its attempt to improve the endoscopy experience in a low-cost and replicable manner. For this QI category, the reviewers weighed most heavily on a study’s impact, the rigor of methodology, and novelty of the problem/solution.
What advice would you give to prospective authors to make their abstracts stand out?
Michelle Hughes, MD, assistant professor of internal medicine
Section of digestive diseases, Yale School of Medicine in New Haven, Conn.
Turning your hard work from a QI project into a successful abstract can seem daunting. There are a few things to consider to help your findings reach your target audience. First, an abstract should be clear and concise. Limit background content and focus on highlighting your project’s methodology and results. Remember, readers look for a comprehensive overview so they can easily understand what you did and found without a lot of extra content obscuring the main points. Everything in your abstract should tie back to your stated aim. Does the background build a case for your project? Do the results and conclusion clearly answer the hypothesis and/or clinical question? If a reported result does not help answer your question, then it should probably be left out. Another tip is to always carefully follow the guidelines for abstract submission. Ensure you use correct formatting and follow all outlined rules. Ask a colleague to review and proofread your abstract draft prior to submission. Lastly, before you start writing, decide what take-away message or statement makes your project meaningful. Refer to this as you write and finalize your abstract so that you keep your message clear and remain focused. You did the hard work to execute your project – but without an effective abstract, the impact of your work may get lost.
Manol Jovani, MD, MPH, therapeutic endoscopy fellow
Division of gastroenterology and hepatology, Johns Hopkins Hospital in Baltimore
The abstract is the “elevator pitch” of your year(s)-long work, and as such it must be very carefully crafted. Dedicate specific time to the style and quality of your presentation long before the deadline. As for elevator pitches, first impressions are very important. For an abstract, it begins with the title. Select a declarative title that draws the interest of the reviewers/readers and tells them exactly what to expect, which also accurately represents the main findings. The introduction and aims should be very short and to the point. For example, it is counterproductive to start with “Ulcerative colitis (UC) and Crohn’s disease (CD) are the two major types of inflammatory bowel disease (IBD).” This does not add any information, states what is obvious to any GI reviewer, uses up 92 characters, and possibly gives a subconscious sense of unoriginality. Throughout the description of your methods/results, use clear language. A well-written abstract will explicitly and concisely state the populations included, statistical analyses performed, and specific outcomes. Make it easy for reviewers to follow along by presenting the results in a logical progression and by striving to use as few acronyms as possible. In addition, less is often more. The abstract should have only one main message, enunciated with the title, evident in the results, and interpreted with the conclusion. If you have additional space, use it to make compelling concluding remarks. As in an elevator pitch, the conclusion is as important as the introduction. Conclude by giving a clear message to the audience/reviewers on what the main findings are and suggest specific implications for future research. Do not end with “more studies are needed,” but rather suggest specifically how these results will influence our future understanding. Finally, know your audience and adapt your presentation to their needs, knowledge, and interests. This includes being mindful of the section to which you are submitting your abstract. An excellent abstract that could have been an oral presentation in one section may end up being a poster in another. Something important to remember about abstracts is that, while the content of the research is the most important part, the quality of its presentation serves to alert the reviewers and readers about it.
Ms. Mietzelfeld is a coordinator of data and training initiatives for the American Gastroenterological Association in Bethesda, Md.
The P value: What to make of it? A simple guide for the uninitiated
Introduction
Many clinicians consider the P value as an almost magical number that determines whether treatment effects exist or not. Is that a correct understanding?
In order to grasp the conceptual meaning of the P value, consider comparing two treatments, A and B, and finding that A is twice as effective as B. Does it mean that treatment A is better in reality? We cannot be sure from that information alone. It may be that treatment A is truly better than treatment B (i.e., true positive). However, it may also be that by chance we have collected a sample in which more people respond to treatment A, making it appear as more effective, when in reality it is equally effective as treatment B (i.e., false positive).
An arbitrary definition
If the P value is less than 5% (P less than .05) that means that there is less than a 5% probability that we would observe the above results if in reality treatment A and treatment B were equally effective. Since this probability is very small, the convention is to reject the idea that both treatments are equally effective and declare that treatment A is indeed more effective.
The P value is thus a probability, and “statistical significance” depends simply on 5% being considered the cutoff for sufficiently low enough probability to make chance an unlikely explanation for the observed results. As you can see this is an arbitrary cutoff; it could have been 4% or 6%, and the concept would not have changed.1
Power
Thus, simply looking at the P value itself is insufficient. We need to interpret it in light of other information.2 Before doing that, we need to introduce a new related statistical concept, that of “power.” The power of a study can be conceptually understood as the ability to detect a difference if there truly is one. If there is a difference in reality between treatments A and B, then the power of a study is the ability to detect that difference.
Two factors influence power: the effect size (that is, the difference between A and B) and the sample size. If the effect size is large, then even with small samples we can detect it. For example, if treatment A was effective in 100% of the cases, and treatment B only in 10% of cases, then the difference will be clear even with a small number of patients. Conversely, if the effect size is small, then we would need a very large sample size to detect that difference. For example, if treatment A is effective in 20% of cases, and treatment B is effective in 22% of cases, the difference between them could be observed only if we enrolled a very large number of patients. A large sample size increases the power of a study. This has important implications for the interpretation of the P value.
How (not) to interpret the P value
Many clinicians do not consider other factors when interpreting the P value, and assume that the dichotomization of results as “significant” and “nonsignificant” accurately reflects reality.3
Authors may say something like the following: “Treatment A was effective in 50% of patients, and treatment B was effective in 20% of the patients, but there was no difference between them (P = .059).” The reason why they declare this as “no difference” is because there is no “statistically significant difference” if P = .059. However, this does not mean that there is no difference.
First, if the convention for the cutoff value for significance was another arbitrary value, say 0.06, then this would have been a statistically significant finding.
Second, we should pay attention to the magnitude of the P value when interpreting the results. As per definition above, the P value is simply the probability of a false-positive result. However, these probabilities may be greater than 5% with varying degrees. For example, a probability of false positive of 80% (P = .80) is very different from a probability of 6% (P = .059), even though, technically, both are “nonsignificant.” A P value of .059 can be interpreted to mean that there is possibly some “signal” of real difference in the data. It may be that the study above was not powered enough to see the difference of 30 percentage points between the treatments as statistically significant; had the sample size been larger and thus provided greater power, then the finding could have been significant. Instead of reporting that there is no difference, it would be better to say that these results are suggestive of a difference, but that there was not enough power to detect it. Alternatively, P = .059 can be considered as “marginally nonsignificant” to qualitatively differentiate it from larger values, say P = .80, which are clearly nonsignificant.
Third, a key distinction is that between clinical and statistical significance. In the example above, even though the study was not statistically significant (P = .059), a difference of 30% seems clinically important. The difference between clinical and statistical significance can perhaps be better illustrated with the opposite, and more common, mistake. As mentioned, a large sample size increases power, thus the ability to detect even minor differences. For example, if a study enrolls 100,000 participants in each arm, then even a difference of 0.2% between treatments A and B will be statistically significant. However, this difference is clinically irrelevant. Thus, when researchers report “statistically significant” results, careful attention must be paid to the clinical significance of those results. The purpose of the studies is to uncover reality, not to be technical about conventions.
Multiple testing and P value
Finally, another almost universally ignored problem in clinical research papers is that of multiple testing. It is not uncommon to read papers in which the authors present results for 20 different and independent hypotheses tests, and when one of them has a P value less than .05 they declare it as a significant finding. However, this is clearly mistaken. The more tests are made, the higher the probability of false positives. Imagine having 20 balls and only one of them is red. If you pick a random ball only once you have a 5% probability of picking the red one. If, however, you try it 10 different times, the probability of picking the red ball is higher (approximately 40%). Similarly, if we perform only one test, then the probability of a false positive is 5%; however, if we perform many tests, then the probability of a false positive is higher than 5%.
There are three main ways to deal with this problem. The first is to have only one main outcome declaring statistical significance for only that outcome and consider the other outcomes as exploratory. The second is to report on multiple findings and correct for multiple testing. The third is to report on multiple findings, but mention explicitly in the paper that they have not corrected for multiple testing and therefore the findings may be significant by chance.
Conclusion
In summary, the P value is the probability of a false-positive finding, and the cutoff of .05 is arbitrary. Instead of dichotomizing results as “significant” and “nonsignificant” purely based on whether the P value is more or less than .05, a more qualitative approach that takes into account the magnitude of the P value and the sample size should be considered, and multiple testing should be taken into account when declaring significant findings.
Dr. Jovani is a therapeutic endoscopy fellow, division of gastroenterology and hepatology, Johns Hopkins Hospital, Baltimore.
References
1. Guyatt G et al. CMAJ. 1995;152:27-32.
2. Guller U and DeLong ER. J Am Coll Surg. 2004;198:441-58.
3. Greenland S et al. Eur J Epidemiol. 2016;31:337-50.
Introduction
Many clinicians consider the P value as an almost magical number that determines whether treatment effects exist or not. Is that a correct understanding?
In order to grasp the conceptual meaning of the P value, consider comparing two treatments, A and B, and finding that A is twice as effective as B. Does it mean that treatment A is better in reality? We cannot be sure from that information alone. It may be that treatment A is truly better than treatment B (i.e., true positive). However, it may also be that by chance we have collected a sample in which more people respond to treatment A, making it appear as more effective, when in reality it is equally effective as treatment B (i.e., false positive).
An arbitrary definition
If the P value is less than 5% (P less than .05) that means that there is less than a 5% probability that we would observe the above results if in reality treatment A and treatment B were equally effective. Since this probability is very small, the convention is to reject the idea that both treatments are equally effective and declare that treatment A is indeed more effective.
The P value is thus a probability, and “statistical significance” depends simply on 5% being considered the cutoff for sufficiently low enough probability to make chance an unlikely explanation for the observed results. As you can see this is an arbitrary cutoff; it could have been 4% or 6%, and the concept would not have changed.1
Power
Thus, simply looking at the P value itself is insufficient. We need to interpret it in light of other information.2 Before doing that, we need to introduce a new related statistical concept, that of “power.” The power of a study can be conceptually understood as the ability to detect a difference if there truly is one. If there is a difference in reality between treatments A and B, then the power of a study is the ability to detect that difference.
Two factors influence power: the effect size (that is, the difference between A and B) and the sample size. If the effect size is large, then even with small samples we can detect it. For example, if treatment A was effective in 100% of the cases, and treatment B only in 10% of cases, then the difference will be clear even with a small number of patients. Conversely, if the effect size is small, then we would need a very large sample size to detect that difference. For example, if treatment A is effective in 20% of cases, and treatment B is effective in 22% of cases, the difference between them could be observed only if we enrolled a very large number of patients. A large sample size increases the power of a study. This has important implications for the interpretation of the P value.
How (not) to interpret the P value
Many clinicians do not consider other factors when interpreting the P value, and assume that the dichotomization of results as “significant” and “nonsignificant” accurately reflects reality.3
Authors may say something like the following: “Treatment A was effective in 50% of patients, and treatment B was effective in 20% of the patients, but there was no difference between them (P = .059).” The reason why they declare this as “no difference” is because there is no “statistically significant difference” if P = .059. However, this does not mean that there is no difference.
First, if the convention for the cutoff value for significance was another arbitrary value, say 0.06, then this would have been a statistically significant finding.
Second, we should pay attention to the magnitude of the P value when interpreting the results. As per definition above, the P value is simply the probability of a false-positive result. However, these probabilities may be greater than 5% with varying degrees. For example, a probability of false positive of 80% (P = .80) is very different from a probability of 6% (P = .059), even though, technically, both are “nonsignificant.” A P value of .059 can be interpreted to mean that there is possibly some “signal” of real difference in the data. It may be that the study above was not powered enough to see the difference of 30 percentage points between the treatments as statistically significant; had the sample size been larger and thus provided greater power, then the finding could have been significant. Instead of reporting that there is no difference, it would be better to say that these results are suggestive of a difference, but that there was not enough power to detect it. Alternatively, P = .059 can be considered as “marginally nonsignificant” to qualitatively differentiate it from larger values, say P = .80, which are clearly nonsignificant.
Third, a key distinction is that between clinical and statistical significance. In the example above, even though the study was not statistically significant (P = .059), a difference of 30% seems clinically important. The difference between clinical and statistical significance can perhaps be better illustrated with the opposite, and more common, mistake. As mentioned, a large sample size increases power, thus the ability to detect even minor differences. For example, if a study enrolls 100,000 participants in each arm, then even a difference of 0.2% between treatments A and B will be statistically significant. However, this difference is clinically irrelevant. Thus, when researchers report “statistically significant” results, careful attention must be paid to the clinical significance of those results. The purpose of the studies is to uncover reality, not to be technical about conventions.
Multiple testing and P value
Finally, another almost universally ignored problem in clinical research papers is that of multiple testing. It is not uncommon to read papers in which the authors present results for 20 different and independent hypotheses tests, and when one of them has a P value less than .05 they declare it as a significant finding. However, this is clearly mistaken. The more tests are made, the higher the probability of false positives. Imagine having 20 balls and only one of them is red. If you pick a random ball only once you have a 5% probability of picking the red one. If, however, you try it 10 different times, the probability of picking the red ball is higher (approximately 40%). Similarly, if we perform only one test, then the probability of a false positive is 5%; however, if we perform many tests, then the probability of a false positive is higher than 5%.
There are three main ways to deal with this problem. The first is to have only one main outcome declaring statistical significance for only that outcome and consider the other outcomes as exploratory. The second is to report on multiple findings and correct for multiple testing. The third is to report on multiple findings, but mention explicitly in the paper that they have not corrected for multiple testing and therefore the findings may be significant by chance.
Conclusion
In summary, the P value is the probability of a false-positive finding, and the cutoff of .05 is arbitrary. Instead of dichotomizing results as “significant” and “nonsignificant” purely based on whether the P value is more or less than .05, a more qualitative approach that takes into account the magnitude of the P value and the sample size should be considered, and multiple testing should be taken into account when declaring significant findings.
Dr. Jovani is a therapeutic endoscopy fellow, division of gastroenterology and hepatology, Johns Hopkins Hospital, Baltimore.
References
1. Guyatt G et al. CMAJ. 1995;152:27-32.
2. Guller U and DeLong ER. J Am Coll Surg. 2004;198:441-58.
3. Greenland S et al. Eur J Epidemiol. 2016;31:337-50.
Introduction
Many clinicians consider the P value as an almost magical number that determines whether treatment effects exist or not. Is that a correct understanding?
In order to grasp the conceptual meaning of the P value, consider comparing two treatments, A and B, and finding that A is twice as effective as B. Does it mean that treatment A is better in reality? We cannot be sure from that information alone. It may be that treatment A is truly better than treatment B (i.e., true positive). However, it may also be that by chance we have collected a sample in which more people respond to treatment A, making it appear as more effective, when in reality it is equally effective as treatment B (i.e., false positive).
An arbitrary definition
If the P value is less than 5% (P less than .05) that means that there is less than a 5% probability that we would observe the above results if in reality treatment A and treatment B were equally effective. Since this probability is very small, the convention is to reject the idea that both treatments are equally effective and declare that treatment A is indeed more effective.
The P value is thus a probability, and “statistical significance” depends simply on 5% being considered the cutoff for sufficiently low enough probability to make chance an unlikely explanation for the observed results. As you can see this is an arbitrary cutoff; it could have been 4% or 6%, and the concept would not have changed.1
Power
Thus, simply looking at the P value itself is insufficient. We need to interpret it in light of other information.2 Before doing that, we need to introduce a new related statistical concept, that of “power.” The power of a study can be conceptually understood as the ability to detect a difference if there truly is one. If there is a difference in reality between treatments A and B, then the power of a study is the ability to detect that difference.
Two factors influence power: the effect size (that is, the difference between A and B) and the sample size. If the effect size is large, then even with small samples we can detect it. For example, if treatment A was effective in 100% of the cases, and treatment B only in 10% of cases, then the difference will be clear even with a small number of patients. Conversely, if the effect size is small, then we would need a very large sample size to detect that difference. For example, if treatment A is effective in 20% of cases, and treatment B is effective in 22% of cases, the difference between them could be observed only if we enrolled a very large number of patients. A large sample size increases the power of a study. This has important implications for the interpretation of the P value.
How (not) to interpret the P value
Many clinicians do not consider other factors when interpreting the P value, and assume that the dichotomization of results as “significant” and “nonsignificant” accurately reflects reality.3
Authors may say something like the following: “Treatment A was effective in 50% of patients, and treatment B was effective in 20% of the patients, but there was no difference between them (P = .059).” The reason why they declare this as “no difference” is because there is no “statistically significant difference” if P = .059. However, this does not mean that there is no difference.
First, if the convention for the cutoff value for significance was another arbitrary value, say 0.06, then this would have been a statistically significant finding.
Second, we should pay attention to the magnitude of the P value when interpreting the results. As per definition above, the P value is simply the probability of a false-positive result. However, these probabilities may be greater than 5% with varying degrees. For example, a probability of false positive of 80% (P = .80) is very different from a probability of 6% (P = .059), even though, technically, both are “nonsignificant.” A P value of .059 can be interpreted to mean that there is possibly some “signal” of real difference in the data. It may be that the study above was not powered enough to see the difference of 30 percentage points between the treatments as statistically significant; had the sample size been larger and thus provided greater power, then the finding could have been significant. Instead of reporting that there is no difference, it would be better to say that these results are suggestive of a difference, but that there was not enough power to detect it. Alternatively, P = .059 can be considered as “marginally nonsignificant” to qualitatively differentiate it from larger values, say P = .80, which are clearly nonsignificant.
Third, a key distinction is that between clinical and statistical significance. In the example above, even though the study was not statistically significant (P = .059), a difference of 30% seems clinically important. The difference between clinical and statistical significance can perhaps be better illustrated with the opposite, and more common, mistake. As mentioned, a large sample size increases power, thus the ability to detect even minor differences. For example, if a study enrolls 100,000 participants in each arm, then even a difference of 0.2% between treatments A and B will be statistically significant. However, this difference is clinically irrelevant. Thus, when researchers report “statistically significant” results, careful attention must be paid to the clinical significance of those results. The purpose of the studies is to uncover reality, not to be technical about conventions.
Multiple testing and P value
Finally, another almost universally ignored problem in clinical research papers is that of multiple testing. It is not uncommon to read papers in which the authors present results for 20 different and independent hypotheses tests, and when one of them has a P value less than .05 they declare it as a significant finding. However, this is clearly mistaken. The more tests are made, the higher the probability of false positives. Imagine having 20 balls and only one of them is red. If you pick a random ball only once you have a 5% probability of picking the red one. If, however, you try it 10 different times, the probability of picking the red ball is higher (approximately 40%). Similarly, if we perform only one test, then the probability of a false positive is 5%; however, if we perform many tests, then the probability of a false positive is higher than 5%.
There are three main ways to deal with this problem. The first is to have only one main outcome declaring statistical significance for only that outcome and consider the other outcomes as exploratory. The second is to report on multiple findings and correct for multiple testing. The third is to report on multiple findings, but mention explicitly in the paper that they have not corrected for multiple testing and therefore the findings may be significant by chance.
Conclusion
In summary, the P value is the probability of a false-positive finding, and the cutoff of .05 is arbitrary. Instead of dichotomizing results as “significant” and “nonsignificant” purely based on whether the P value is more or less than .05, a more qualitative approach that takes into account the magnitude of the P value and the sample size should be considered, and multiple testing should be taken into account when declaring significant findings.
Dr. Jovani is a therapeutic endoscopy fellow, division of gastroenterology and hepatology, Johns Hopkins Hospital, Baltimore.
References
1. Guyatt G et al. CMAJ. 1995;152:27-32.
2. Guller U and DeLong ER. J Am Coll Surg. 2004;198:441-58.
3. Greenland S et al. Eur J Epidemiol. 2016;31:337-50.
Not all labs are created equal: What to look for when searching for the right lab
Introduction
As researchers, we spend a substantial period of our careers as trainees in a lab. As graduate students or postdoctoral fellows, selecting the “right” lab has the potential to make a huge difference for your future career and your mental health. Although much of the decision is based on instinct, there is still some logic and reason that can be applied to ensure the choice is a good fit.
PI/mentor
PI funding. The history of funding and the funding of your specific project are important factors to consider. No matter how significant the scientific question, not having adequate funding can be paralyzing. For both graduate students and postdoctoral fellows, it is in your best interest to proactively inquire about the funding status of the lab. This can be accomplished using online reporters (such as NIH Reporter or Grantome) and asking the PI. It is equally important to identify which grant application/fellowship will fund your stipend and ensure that the funding is secure for 4-6 years.
PI personality. For better or for worse, the PI largely sets the tone for the lab. The personality of the PI can be a key determining factor in finding the right “fit” for a trainee. Ask your peers and current and past lab members about working with the PI. As you speak to lab members and alumni, don’t disregard warning signs of unethical behavior, bullying, or harassment. Even with the rise of movements such as the #MeTooSTEM, academic misconduct is hard to correct. It is far better to avoid these labs. If available, examine the PI’s social networks (LinkedIn, Twitter, Facebook, etc.) or read previously published interviews and look for the PI’s attitude toward its lab members.
Mentoring style. The NIH emphasizes mentorship in numerous funding mechanisms; thus, it isn’t surprising that selecting an appropriate mentor is paramount for a successful training experience. A lot of this decision requires information about you as a trainee. Reflect on what type of scientific mentor will be the best fit for your needs and honestly evaluate your own communication style, expectations, and final career goals. Ask questions of the PI and lab members to identify the mentoring style. Find a mentor who takes his or her responsibility to train you seriously and who genuinely cares about the well-being of the lab personnel. Good mentors advocate for their trainees inside and outside of the lab. A mentor’s vocal support of talented trainees can help propel them toward their career goals.
Career support. One of the most important questions you need to consider is whether your mentor can help you accomplish your career goals. Be prepared to have an honest conversation with the PI about your goals. The majority of PhD scientists will pursue careers outside of academia, so choose a mentor who supports diverse career paths. A mentor who is invested in your success will work with you to finish papers in a timely manner, encourage you to apply for awards and grants, aid in the identification of fruitful collaborations, help develop new skills, and expand your professional network. A good mentor will also ensure that you have freedom in your project to pursue your own scientific interests, and ultimately allow you to carve out a project for you to take with you when your training is complete.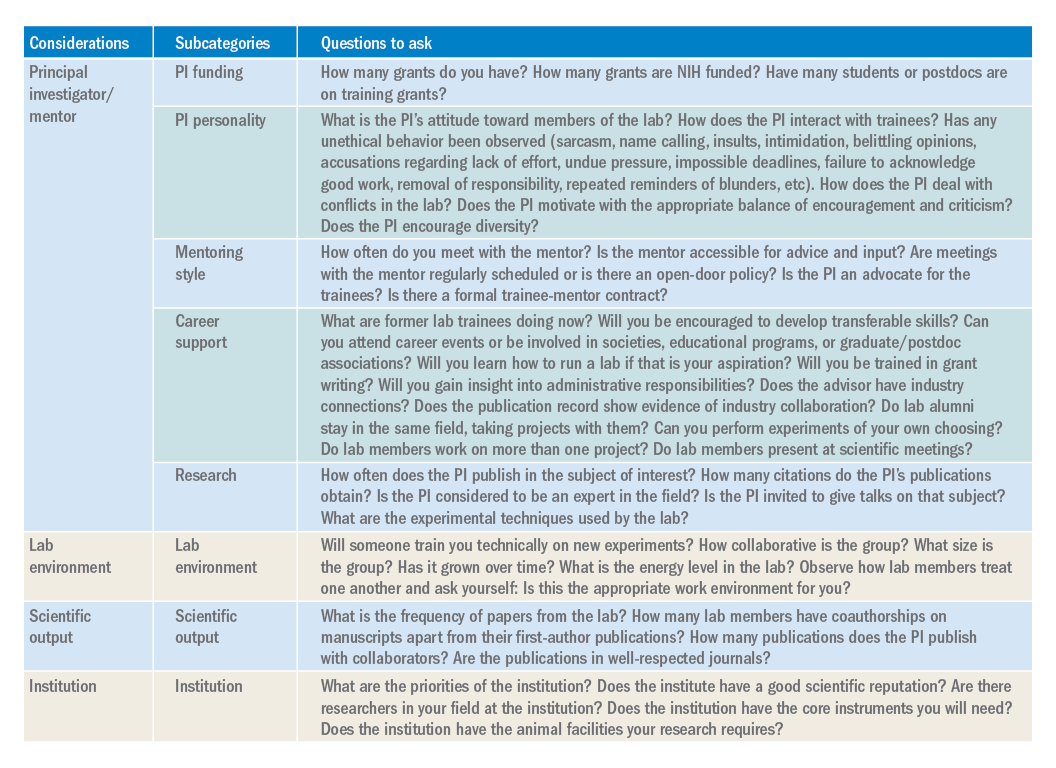
Research. The research done in your lab will also factor into your decision. Especially if you are a postdoctoral fellow, this will be the field/subject/niche in which you will likely establish your career and expertise. If there is a field you want to pursue, identify mentors who have a publication record in that subject. Examine the PIs for publications, citations, and overall contributions to the field. If the PI in question is young, you may not find many citations of papers. However, you can identify the quality of the papers, the lab’s experimental techniques, and how often the PI is invited to give talks on that subject.
Lab environment
The composition of a lab can play a huge role in your overall productivity and mental well-being. Your coworkers will be the ones you interact with on a day-to-day basis. Your lab mates can be a huge asset in terms of mastering new techniques, collaborating on a project, or receiving helpful feedback. Thus, it is to your benefit to get along with your lab mates and to choose a lab that has people who you want to work with or don’t mind working with. The reality of postdoctoral training is that you will spend a large amount of time working in the lab, so choosing a lab that has an environment that fits your personality and desired workload and schedule is important. Considering the valuable contribution of lab members toward your research progression, you should give considerable weight to this factor when choosing a lab.
Scientific output
Although labs with high-impact publications are attractive, it is more important to consider the frequency with which a lab comes out with papers. Search Pubmed to obtain the complete publication roster of the PI. This output will give you an idea about the consistency and regularity of publications and overall lab productivity. When querying the lab’s publication list, check if the lab members have coauthorships on manuscripts apart from their first-author ones. It is desirable to be a coauthor on other manuscripts, as this will help increase your publication record. In addition, look for collaborators of the PI. These collaborations might benefit you by broadening your skill set and experience. Be intentional about reviewing the papers from the lab over the last 5 years. If you want to pursue academic research, you will want to be in a lab that publishes frequently in well-respected journals.
Institution
The institutional environment should also influence your decision. Top universities can attract some of the best researchers. Moreover, funding agencies examine the “Environment and Institutional Commitment” as one of the criteria for awarding grants. When choosing an institution, consider its priorities and ensure that it aligns with your priorities (i.e., research, undergraduate education, etc.). Consider the number of PIs conducting research in your field. These other labs may benefit your career development through collaborations, scientific discussions, letters of recommendation, career support, and feedback.
Conclusion
While there are certain key criteria that should be prioritized when choosing a lab, the path to finding (and joining) the right lab will vary from individual to individual. Gather data, ask questions, research what you can online (lab website, publication records, LinkedIn, ResearchGate, or Twitter), and be ready for the interview with a long list of written questions for the PI and lab members. One of the best ways to begin looking for a new lab is to ask your current mentor, committee members, peers, and others in your professional network for their suggestions. Table 1 has questions to keep in mind as you are searching for a lab. You will spend much of your time in the lab of your choosing, so choose wisely.
Dr. Engevik is an instructor in pathology and immunology, Baylor College of Medicine, Texas Children’s Hospital, Houston.
Introduction
As researchers, we spend a substantial period of our careers as trainees in a lab. As graduate students or postdoctoral fellows, selecting the “right” lab has the potential to make a huge difference for your future career and your mental health. Although much of the decision is based on instinct, there is still some logic and reason that can be applied to ensure the choice is a good fit.
PI/mentor
PI funding. The history of funding and the funding of your specific project are important factors to consider. No matter how significant the scientific question, not having adequate funding can be paralyzing. For both graduate students and postdoctoral fellows, it is in your best interest to proactively inquire about the funding status of the lab. This can be accomplished using online reporters (such as NIH Reporter or Grantome) and asking the PI. It is equally important to identify which grant application/fellowship will fund your stipend and ensure that the funding is secure for 4-6 years.
PI personality. For better or for worse, the PI largely sets the tone for the lab. The personality of the PI can be a key determining factor in finding the right “fit” for a trainee. Ask your peers and current and past lab members about working with the PI. As you speak to lab members and alumni, don’t disregard warning signs of unethical behavior, bullying, or harassment. Even with the rise of movements such as the #MeTooSTEM, academic misconduct is hard to correct. It is far better to avoid these labs. If available, examine the PI’s social networks (LinkedIn, Twitter, Facebook, etc.) or read previously published interviews and look for the PI’s attitude toward its lab members.
Mentoring style. The NIH emphasizes mentorship in numerous funding mechanisms; thus, it isn’t surprising that selecting an appropriate mentor is paramount for a successful training experience. A lot of this decision requires information about you as a trainee. Reflect on what type of scientific mentor will be the best fit for your needs and honestly evaluate your own communication style, expectations, and final career goals. Ask questions of the PI and lab members to identify the mentoring style. Find a mentor who takes his or her responsibility to train you seriously and who genuinely cares about the well-being of the lab personnel. Good mentors advocate for their trainees inside and outside of the lab. A mentor’s vocal support of talented trainees can help propel them toward their career goals.
Career support. One of the most important questions you need to consider is whether your mentor can help you accomplish your career goals. Be prepared to have an honest conversation with the PI about your goals. The majority of PhD scientists will pursue careers outside of academia, so choose a mentor who supports diverse career paths. A mentor who is invested in your success will work with you to finish papers in a timely manner, encourage you to apply for awards and grants, aid in the identification of fruitful collaborations, help develop new skills, and expand your professional network. A good mentor will also ensure that you have freedom in your project to pursue your own scientific interests, and ultimately allow you to carve out a project for you to take with you when your training is complete.
Research. The research done in your lab will also factor into your decision. Especially if you are a postdoctoral fellow, this will be the field/subject/niche in which you will likely establish your career and expertise. If there is a field you want to pursue, identify mentors who have a publication record in that subject. Examine the PIs for publications, citations, and overall contributions to the field. If the PI in question is young, you may not find many citations of papers. However, you can identify the quality of the papers, the lab’s experimental techniques, and how often the PI is invited to give talks on that subject.
Lab environment
The composition of a lab can play a huge role in your overall productivity and mental well-being. Your coworkers will be the ones you interact with on a day-to-day basis. Your lab mates can be a huge asset in terms of mastering new techniques, collaborating on a project, or receiving helpful feedback. Thus, it is to your benefit to get along with your lab mates and to choose a lab that has people who you want to work with or don’t mind working with. The reality of postdoctoral training is that you will spend a large amount of time working in the lab, so choosing a lab that has an environment that fits your personality and desired workload and schedule is important. Considering the valuable contribution of lab members toward your research progression, you should give considerable weight to this factor when choosing a lab.
Scientific output
Although labs with high-impact publications are attractive, it is more important to consider the frequency with which a lab comes out with papers. Search Pubmed to obtain the complete publication roster of the PI. This output will give you an idea about the consistency and regularity of publications and overall lab productivity. When querying the lab’s publication list, check if the lab members have coauthorships on manuscripts apart from their first-author ones. It is desirable to be a coauthor on other manuscripts, as this will help increase your publication record. In addition, look for collaborators of the PI. These collaborations might benefit you by broadening your skill set and experience. Be intentional about reviewing the papers from the lab over the last 5 years. If you want to pursue academic research, you will want to be in a lab that publishes frequently in well-respected journals.
Institution
The institutional environment should also influence your decision. Top universities can attract some of the best researchers. Moreover, funding agencies examine the “Environment and Institutional Commitment” as one of the criteria for awarding grants. When choosing an institution, consider its priorities and ensure that it aligns with your priorities (i.e., research, undergraduate education, etc.). Consider the number of PIs conducting research in your field. These other labs may benefit your career development through collaborations, scientific discussions, letters of recommendation, career support, and feedback.
Conclusion
While there are certain key criteria that should be prioritized when choosing a lab, the path to finding (and joining) the right lab will vary from individual to individual. Gather data, ask questions, research what you can online (lab website, publication records, LinkedIn, ResearchGate, or Twitter), and be ready for the interview with a long list of written questions for the PI and lab members. One of the best ways to begin looking for a new lab is to ask your current mentor, committee members, peers, and others in your professional network for their suggestions. Table 1 has questions to keep in mind as you are searching for a lab. You will spend much of your time in the lab of your choosing, so choose wisely.
Dr. Engevik is an instructor in pathology and immunology, Baylor College of Medicine, Texas Children’s Hospital, Houston.
Introduction
As researchers, we spend a substantial period of our careers as trainees in a lab. As graduate students or postdoctoral fellows, selecting the “right” lab has the potential to make a huge difference for your future career and your mental health. Although much of the decision is based on instinct, there is still some logic and reason that can be applied to ensure the choice is a good fit.
PI/mentor
PI funding. The history of funding and the funding of your specific project are important factors to consider. No matter how significant the scientific question, not having adequate funding can be paralyzing. For both graduate students and postdoctoral fellows, it is in your best interest to proactively inquire about the funding status of the lab. This can be accomplished using online reporters (such as NIH Reporter or Grantome) and asking the PI. It is equally important to identify which grant application/fellowship will fund your stipend and ensure that the funding is secure for 4-6 years.
PI personality. For better or for worse, the PI largely sets the tone for the lab. The personality of the PI can be a key determining factor in finding the right “fit” for a trainee. Ask your peers and current and past lab members about working with the PI. As you speak to lab members and alumni, don’t disregard warning signs of unethical behavior, bullying, or harassment. Even with the rise of movements such as the #MeTooSTEM, academic misconduct is hard to correct. It is far better to avoid these labs. If available, examine the PI’s social networks (LinkedIn, Twitter, Facebook, etc.) or read previously published interviews and look for the PI’s attitude toward its lab members.
Mentoring style. The NIH emphasizes mentorship in numerous funding mechanisms; thus, it isn’t surprising that selecting an appropriate mentor is paramount for a successful training experience. A lot of this decision requires information about you as a trainee. Reflect on what type of scientific mentor will be the best fit for your needs and honestly evaluate your own communication style, expectations, and final career goals. Ask questions of the PI and lab members to identify the mentoring style. Find a mentor who takes his or her responsibility to train you seriously and who genuinely cares about the well-being of the lab personnel. Good mentors advocate for their trainees inside and outside of the lab. A mentor’s vocal support of talented trainees can help propel them toward their career goals.
Career support. One of the most important questions you need to consider is whether your mentor can help you accomplish your career goals. Be prepared to have an honest conversation with the PI about your goals. The majority of PhD scientists will pursue careers outside of academia, so choose a mentor who supports diverse career paths. A mentor who is invested in your success will work with you to finish papers in a timely manner, encourage you to apply for awards and grants, aid in the identification of fruitful collaborations, help develop new skills, and expand your professional network. A good mentor will also ensure that you have freedom in your project to pursue your own scientific interests, and ultimately allow you to carve out a project for you to take with you when your training is complete.
Research. The research done in your lab will also factor into your decision. Especially if you are a postdoctoral fellow, this will be the field/subject/niche in which you will likely establish your career and expertise. If there is a field you want to pursue, identify mentors who have a publication record in that subject. Examine the PIs for publications, citations, and overall contributions to the field. If the PI in question is young, you may not find many citations of papers. However, you can identify the quality of the papers, the lab’s experimental techniques, and how often the PI is invited to give talks on that subject.
Lab environment
The composition of a lab can play a huge role in your overall productivity and mental well-being. Your coworkers will be the ones you interact with on a day-to-day basis. Your lab mates can be a huge asset in terms of mastering new techniques, collaborating on a project, or receiving helpful feedback. Thus, it is to your benefit to get along with your lab mates and to choose a lab that has people who you want to work with or don’t mind working with. The reality of postdoctoral training is that you will spend a large amount of time working in the lab, so choosing a lab that has an environment that fits your personality and desired workload and schedule is important. Considering the valuable contribution of lab members toward your research progression, you should give considerable weight to this factor when choosing a lab.
Scientific output
Although labs with high-impact publications are attractive, it is more important to consider the frequency with which a lab comes out with papers. Search Pubmed to obtain the complete publication roster of the PI. This output will give you an idea about the consistency and regularity of publications and overall lab productivity. When querying the lab’s publication list, check if the lab members have coauthorships on manuscripts apart from their first-author ones. It is desirable to be a coauthor on other manuscripts, as this will help increase your publication record. In addition, look for collaborators of the PI. These collaborations might benefit you by broadening your skill set and experience. Be intentional about reviewing the papers from the lab over the last 5 years. If you want to pursue academic research, you will want to be in a lab that publishes frequently in well-respected journals.
Institution
The institutional environment should also influence your decision. Top universities can attract some of the best researchers. Moreover, funding agencies examine the “Environment and Institutional Commitment” as one of the criteria for awarding grants. When choosing an institution, consider its priorities and ensure that it aligns with your priorities (i.e., research, undergraduate education, etc.). Consider the number of PIs conducting research in your field. These other labs may benefit your career development through collaborations, scientific discussions, letters of recommendation, career support, and feedback.
Conclusion
While there are certain key criteria that should be prioritized when choosing a lab, the path to finding (and joining) the right lab will vary from individual to individual. Gather data, ask questions, research what you can online (lab website, publication records, LinkedIn, ResearchGate, or Twitter), and be ready for the interview with a long list of written questions for the PI and lab members. One of the best ways to begin looking for a new lab is to ask your current mentor, committee members, peers, and others in your professional network for their suggestions. Table 1 has questions to keep in mind as you are searching for a lab. You will spend much of your time in the lab of your choosing, so choose wisely.
Dr. Engevik is an instructor in pathology and immunology, Baylor College of Medicine, Texas Children’s Hospital, Houston.
Clinical research in private practice? It can be done, and here’s how
Not every physician coming out of medical school wants to go down the path of conducting clinical research. But for those who do, the decision is a rewarding one that can make a real difference in patients’ lives.
I took a nontraditional route to get to my current role as the director of clinical research and education at the largest gastroenterology practice in the United States. I had a business degree coming out of college and worked for years in the business world prior to going to medical school. After graduation, I got an offer to join the pharmaceutical industry in medical affairs. There, I focused on inflammatory bowel disease (IBD), where my role was to share high-level scientific data and liaise with IBD disease state experts. Part of the role was working internally with clinical operations and externally with sites conducting research throughout the United States. In the next 6 years, I had the opportunity to observe how research was run in both the academic and community practice settings, noting those characteristics that allowed some to succeed and far more to stagnate or fail.
In 2014, I joined Texas Digestive Disease Consultants with the goal to ramp up the practice’s clinical research arm and create a department expressly for that purpose. To date, the department has become incredibly successful and ultimately sustainable. If you’re considering joining a practice based on its clinical research program or starting one in your current practice, here is what I’ve learned along the way:
Know the benefit to patients
You have to decide to conduct clinical research because of its benefit to the patient, not because you see it as an alternative revenue stream. It will never be sustainable if viewed as a profit center. Clinical research offers therapeutic advancements that will not be available to most for the next 5-10 years. Additionally, patients do not need insurance in order to participate in a study. The sponsor covers all study visits, procedures, and therapeutics.
Know the value to the practice
Having a clinical trials program brings both direct and indirect enterprise value to the practice. It may come in the form of referrals from other practices that don’t have the same capabilities. Patients may view your practice differently, knowing that it has the added value of research. There is a halo effect from having clinical trials capabilities in how you are viewed by patients and other physicians in the community.
Get the right people in place
First, you will need an enthusiastic primary investigator who can take a bit of time from practice to conduct clinical trials. But just as importantly, you need a knowledgeable clinical research coordinator. Without an effective coordinator, the program is doomed. A good coordinator should be well rounded in all aspects of research (e.g., regulatory, patient recruitment, quality assurance, contracts, budgets, running labs, conducting patient visits) and able to deal with the day-to-day intricacies of running clinical trials, which will allow a doctor to take care of the existing practice. They should also be versed in the requisite equipment (e.g., locking refrigerator, freezer, and ambient cabinet, temperature monitoring devices, EKG machine, and centrifuge) necessary to run a clinical study.
Know how to recruit patients
The database at Texas Digestive Disease Consultants/GI Alliance (TDDC/GIA) is vast, which makes identification of patients who may qualify for a trial an easier task. Many practices will have an electronic medical record that will aid in identification, but if that is not the case at your practice, there are a number of ways you can go about this. First, talk to physicians in your practice and in other practices – internists, family physicians, and other gastroenterologists come to mind – about what you can offer. Send a letter or an email out to physicians in the community with the inclusion/exclusion criteria for your study, and always direct them to https://clinicaltrials.gov/ for additional information. Patient advocacy organizations are another good source of referrals for clinical trials. And of course, pharmaceutical companies have recruitment services that they use and can help steer patients your way.
Understand the ethics
There are numerous ethical and legal considerations when it comes to running clinical trials. The principles of Good Clinical Practice (GCP) and Human Subject Protection (HSP) guide the conduct of clinical trials in the United States. Understanding the concepts and regulations around caring for patients in trials is not only “good practice,” it’s a necessity. There are free Good Clinical Practice training courses available, which are required every few years for everyone conducting clinical research. These courses are quite lengthy (3-6 hours), but provide a great overview of the Food and Drug Administration regulations, ethical considerations, and other advice on successfully operating a clinical trials program. Again, a capable clinical research coordinator will help guide you here.
Adopt standard operating procedures
Every clinical trials sponsor will require standard operating procedures for such facets of research as informed consent, reporting requirements, and safety monitoring. Here again, a seasoned clinical trials coordinator can put you on the right path. You may choose to purchase standardized templates or work with another group that already has them and would be willing to share.
Be smart about spending
While this may depend on the scale you want to grow your research, at TDDC/GIA we knew we wanted to build a sustainable program. We invested a lot of time and money into our infrastructure, as well as adopting a robust Clinical Trials Management System (CTMS). A CTMS system will provide the ability to measure productivity, finances, schedule patient visits, and pay patient stipends. Some systems even automate the regulatory process (E-Regulatory) or source process (E-source), which can cut down on the paper burden on a site.
Seek mentors
Look for someone to emulate who is already established in operating a clinical research program in private practice. Reach out to the practices or physicians that are already doing this work and doing it well. For physicians who are just starting out or still in training, look for opportunities to publish or be involved with the clinical trials program at your institution.
Contacts in the pharmaceutical industry are very important to getting a new program off the ground. Ask the pharma sales representatives who visit your clinic to put you in touch with their medical science liaison (MSL). The MSL can get you information about the clinical trials their company is currently running and those they have in the pipeline.
Dr. Chris Fourment, director of clinical research and education, Texas Digestive Disease Consultants/GI Alliance.
Not every physician coming out of medical school wants to go down the path of conducting clinical research. But for those who do, the decision is a rewarding one that can make a real difference in patients’ lives.
I took a nontraditional route to get to my current role as the director of clinical research and education at the largest gastroenterology practice in the United States. I had a business degree coming out of college and worked for years in the business world prior to going to medical school. After graduation, I got an offer to join the pharmaceutical industry in medical affairs. There, I focused on inflammatory bowel disease (IBD), where my role was to share high-level scientific data and liaise with IBD disease state experts. Part of the role was working internally with clinical operations and externally with sites conducting research throughout the United States. In the next 6 years, I had the opportunity to observe how research was run in both the academic and community practice settings, noting those characteristics that allowed some to succeed and far more to stagnate or fail.
In 2014, I joined Texas Digestive Disease Consultants with the goal to ramp up the practice’s clinical research arm and create a department expressly for that purpose. To date, the department has become incredibly successful and ultimately sustainable. If you’re considering joining a practice based on its clinical research program or starting one in your current practice, here is what I’ve learned along the way:
Know the benefit to patients
You have to decide to conduct clinical research because of its benefit to the patient, not because you see it as an alternative revenue stream. It will never be sustainable if viewed as a profit center. Clinical research offers therapeutic advancements that will not be available to most for the next 5-10 years. Additionally, patients do not need insurance in order to participate in a study. The sponsor covers all study visits, procedures, and therapeutics.
Know the value to the practice
Having a clinical trials program brings both direct and indirect enterprise value to the practice. It may come in the form of referrals from other practices that don’t have the same capabilities. Patients may view your practice differently, knowing that it has the added value of research. There is a halo effect from having clinical trials capabilities in how you are viewed by patients and other physicians in the community.
Get the right people in place
First, you will need an enthusiastic primary investigator who can take a bit of time from practice to conduct clinical trials. But just as importantly, you need a knowledgeable clinical research coordinator. Without an effective coordinator, the program is doomed. A good coordinator should be well rounded in all aspects of research (e.g., regulatory, patient recruitment, quality assurance, contracts, budgets, running labs, conducting patient visits) and able to deal with the day-to-day intricacies of running clinical trials, which will allow a doctor to take care of the existing practice. They should also be versed in the requisite equipment (e.g., locking refrigerator, freezer, and ambient cabinet, temperature monitoring devices, EKG machine, and centrifuge) necessary to run a clinical study.
Know how to recruit patients
The database at Texas Digestive Disease Consultants/GI Alliance (TDDC/GIA) is vast, which makes identification of patients who may qualify for a trial an easier task. Many practices will have an electronic medical record that will aid in identification, but if that is not the case at your practice, there are a number of ways you can go about this. First, talk to physicians in your practice and in other practices – internists, family physicians, and other gastroenterologists come to mind – about what you can offer. Send a letter or an email out to physicians in the community with the inclusion/exclusion criteria for your study, and always direct them to https://clinicaltrials.gov/ for additional information. Patient advocacy organizations are another good source of referrals for clinical trials. And of course, pharmaceutical companies have recruitment services that they use and can help steer patients your way.
Understand the ethics
There are numerous ethical and legal considerations when it comes to running clinical trials. The principles of Good Clinical Practice (GCP) and Human Subject Protection (HSP) guide the conduct of clinical trials in the United States. Understanding the concepts and regulations around caring for patients in trials is not only “good practice,” it’s a necessity. There are free Good Clinical Practice training courses available, which are required every few years for everyone conducting clinical research. These courses are quite lengthy (3-6 hours), but provide a great overview of the Food and Drug Administration regulations, ethical considerations, and other advice on successfully operating a clinical trials program. Again, a capable clinical research coordinator will help guide you here.
Adopt standard operating procedures
Every clinical trials sponsor will require standard operating procedures for such facets of research as informed consent, reporting requirements, and safety monitoring. Here again, a seasoned clinical trials coordinator can put you on the right path. You may choose to purchase standardized templates or work with another group that already has them and would be willing to share.
Be smart about spending
While this may depend on the scale you want to grow your research, at TDDC/GIA we knew we wanted to build a sustainable program. We invested a lot of time and money into our infrastructure, as well as adopting a robust Clinical Trials Management System (CTMS). A CTMS system will provide the ability to measure productivity, finances, schedule patient visits, and pay patient stipends. Some systems even automate the regulatory process (E-Regulatory) or source process (E-source), which can cut down on the paper burden on a site.
Seek mentors
Look for someone to emulate who is already established in operating a clinical research program in private practice. Reach out to the practices or physicians that are already doing this work and doing it well. For physicians who are just starting out or still in training, look for opportunities to publish or be involved with the clinical trials program at your institution.
Contacts in the pharmaceutical industry are very important to getting a new program off the ground. Ask the pharma sales representatives who visit your clinic to put you in touch with their medical science liaison (MSL). The MSL can get you information about the clinical trials their company is currently running and those they have in the pipeline.
Dr. Chris Fourment, director of clinical research and education, Texas Digestive Disease Consultants/GI Alliance.
Not every physician coming out of medical school wants to go down the path of conducting clinical research. But for those who do, the decision is a rewarding one that can make a real difference in patients’ lives.
I took a nontraditional route to get to my current role as the director of clinical research and education at the largest gastroenterology practice in the United States. I had a business degree coming out of college and worked for years in the business world prior to going to medical school. After graduation, I got an offer to join the pharmaceutical industry in medical affairs. There, I focused on inflammatory bowel disease (IBD), where my role was to share high-level scientific data and liaise with IBD disease state experts. Part of the role was working internally with clinical operations and externally with sites conducting research throughout the United States. In the next 6 years, I had the opportunity to observe how research was run in both the academic and community practice settings, noting those characteristics that allowed some to succeed and far more to stagnate or fail.
In 2014, I joined Texas Digestive Disease Consultants with the goal to ramp up the practice’s clinical research arm and create a department expressly for that purpose. To date, the department has become incredibly successful and ultimately sustainable. If you’re considering joining a practice based on its clinical research program or starting one in your current practice, here is what I’ve learned along the way:
Know the benefit to patients
You have to decide to conduct clinical research because of its benefit to the patient, not because you see it as an alternative revenue stream. It will never be sustainable if viewed as a profit center. Clinical research offers therapeutic advancements that will not be available to most for the next 5-10 years. Additionally, patients do not need insurance in order to participate in a study. The sponsor covers all study visits, procedures, and therapeutics.
Know the value to the practice
Having a clinical trials program brings both direct and indirect enterprise value to the practice. It may come in the form of referrals from other practices that don’t have the same capabilities. Patients may view your practice differently, knowing that it has the added value of research. There is a halo effect from having clinical trials capabilities in how you are viewed by patients and other physicians in the community.
Get the right people in place
First, you will need an enthusiastic primary investigator who can take a bit of time from practice to conduct clinical trials. But just as importantly, you need a knowledgeable clinical research coordinator. Without an effective coordinator, the program is doomed. A good coordinator should be well rounded in all aspects of research (e.g., regulatory, patient recruitment, quality assurance, contracts, budgets, running labs, conducting patient visits) and able to deal with the day-to-day intricacies of running clinical trials, which will allow a doctor to take care of the existing practice. They should also be versed in the requisite equipment (e.g., locking refrigerator, freezer, and ambient cabinet, temperature monitoring devices, EKG machine, and centrifuge) necessary to run a clinical study.
Know how to recruit patients
The database at Texas Digestive Disease Consultants/GI Alliance (TDDC/GIA) is vast, which makes identification of patients who may qualify for a trial an easier task. Many practices will have an electronic medical record that will aid in identification, but if that is not the case at your practice, there are a number of ways you can go about this. First, talk to physicians in your practice and in other practices – internists, family physicians, and other gastroenterologists come to mind – about what you can offer. Send a letter or an email out to physicians in the community with the inclusion/exclusion criteria for your study, and always direct them to https://clinicaltrials.gov/ for additional information. Patient advocacy organizations are another good source of referrals for clinical trials. And of course, pharmaceutical companies have recruitment services that they use and can help steer patients your way.
Understand the ethics
There are numerous ethical and legal considerations when it comes to running clinical trials. The principles of Good Clinical Practice (GCP) and Human Subject Protection (HSP) guide the conduct of clinical trials in the United States. Understanding the concepts and regulations around caring for patients in trials is not only “good practice,” it’s a necessity. There are free Good Clinical Practice training courses available, which are required every few years for everyone conducting clinical research. These courses are quite lengthy (3-6 hours), but provide a great overview of the Food and Drug Administration regulations, ethical considerations, and other advice on successfully operating a clinical trials program. Again, a capable clinical research coordinator will help guide you here.
Adopt standard operating procedures
Every clinical trials sponsor will require standard operating procedures for such facets of research as informed consent, reporting requirements, and safety monitoring. Here again, a seasoned clinical trials coordinator can put you on the right path. You may choose to purchase standardized templates or work with another group that already has them and would be willing to share.
Be smart about spending
While this may depend on the scale you want to grow your research, at TDDC/GIA we knew we wanted to build a sustainable program. We invested a lot of time and money into our infrastructure, as well as adopting a robust Clinical Trials Management System (CTMS). A CTMS system will provide the ability to measure productivity, finances, schedule patient visits, and pay patient stipends. Some systems even automate the regulatory process (E-Regulatory) or source process (E-source), which can cut down on the paper burden on a site.
Seek mentors
Look for someone to emulate who is already established in operating a clinical research program in private practice. Reach out to the practices or physicians that are already doing this work and doing it well. For physicians who are just starting out or still in training, look for opportunities to publish or be involved with the clinical trials program at your institution.
Contacts in the pharmaceutical industry are very important to getting a new program off the ground. Ask the pharma sales representatives who visit your clinic to put you in touch with their medical science liaison (MSL). The MSL can get you information about the clinical trials their company is currently running and those they have in the pipeline.
Dr. Chris Fourment, director of clinical research and education, Texas Digestive Disease Consultants/GI Alliance.
Diagnosis and management of gastric intestinal metaplasia in the United States
Introduction
Despite a global decline in the incidence of gastric cancer over the past 3 decades, it remains the fifth most commonly diagnosed cancer and the third most common cause of cancer deaths worldwide.1 In the United States it is the fourth most commonly diagnosed GI malignancy, after colorectal, pancreas, and liver cancer. The prevalence remains high in Latin America and Asia, which has implications in the United States because of growing Hispanic and Asian populations.2,3 In recent years, a change in the trend of gastric cancer among non-Hispanic whites has been observed, particularly in women younger than 50 years old.4 5
Etiology
Gastric adenocarcinomas are classified into two subcategories based on location (cardia and noncardia) and histology (intestinal and diffuse types).6,7 Atrophic gastritis and gastric intestinal metaplasia (GIM) are considered precursors of intestinal-type noncardia gastric adenocarcinoma. The Correa cascade is a commonly accepted precancer sequence for noncardia gastric adenocarcinoma that describes mucosal changes from inflammation to atrophy to metaplasia to intraepithelial neoplasia and culminating in carcinoma.8,9 It has been observed that GIM may be the histologic change prior to the development of dysplasia and over 50% of patients with high-grade dysplasia will progress to adenocarcinoma.10-12 In the United States, GIM has the highest prevalence in African Americans, Hispanics, and East Asians, with the overall GIM prevalence regardless of ethnicity reported from 3.05% to 19.2%.5,13
Risk factors and subclassification
Replacement of the foveolar and/or glandular epithelium in the oxyntic and antral mucosa by intestinal epithelium results in GIM. It can be focal when limited to one region of the stomach or extensive when two or more regions are involved.14 The main risk factors for GIM development are Helicobacter pylori infection, tobacco, alcohol consumption, high salt intake, and chronic bile reflux.15,16 Additional risks for developing gastric cancer include older age, certain ethnicities, and male sex.17
CagA strains of H. pylori can promote carcinogenesis by inducing a mitogenic cellular response and downregulating cell adhesion.18,19 Less carcinogenic risk is associated with H. pylori Cag-A negative strains; however, they also have oncogenic potential mediated by expression of babA2 and vacA genes.20 Hence, the combination of multiple virulent factors encoded in babA2, CagA, and vacA genes has been associated with increased risk of GIM, inflammation, and development of gastric cancer.15 The clinical usefulness of genotyping H. pylori strains specifically to survey precancerous gastric lesions remains to be seen because of a lack of sufficient clinical studies. In addition, genotyping H. pylori is not commonly performed as part of clinical practice.
The loss of parietal cells seen in atrophic gastritis due to chronic H. pylori infection has been linked to the development of metaplasia due to possible loss of differentiation-promoting factors. As a result, metaplastic cells emerge that express spasmolytic polypeptide (SP or TFF2); hence, this type of metaplasia is referred to as spasmolytic polypeptide–expressing metaplasia (SPEM). The cellular mechanism that may explain a precursor role of SPEM in the development of GIM remains unknown.14 A second competing theory for the development of GIM is the clonal expansion of stem cells in the gastric isthmus that can lead to dysplasia and cancer development.14
On the basis of histological similarities with small intestinal or colonic epithelium, GIM can be further classified into complete or incomplete intestinal metaplasia.21 Complete intestinal metaplasia most closely resembles small intestinal epithelium with a brush border and goblet cells. Incomplete intestinal metaplasia resembles the colonic epithelium and lacks a brush border. A second classification further classifies GIM into three subtypes: Type I contains nonsecretory absorptive cells and sialomucin secreting goblet cells; type II has few absorptive cells, columnar cells secreting sialomucin, goblet cells secreting mainly sialomucin but some sulphomucin, and presence of Paneth cells; and type III consists of columnar cells secreting predominantly sulphomucin, goblet cells secreting sialomucin or sulphomucin, and absence of Paneth cells.15,22 In this subclassification, type I GIM is known as complete GIM and types II and III as incomplete GIM.23-25
Multiple studies performed outside of the United States have shown a higher progression risk to gastric adenocarcinoma in incomplete intestinal metaplasia, or type III intestinal metaplasia.26-32 Also, the risk of gastric cancer has been demonstrated to be higher among patients with a greater area of metaplasia and extensive intestinal metaplasia, defined as GIM in both the antrum and corpus.33,34 Hence, the extent of the metaplasia determined with mapping biopsies, regardless of the subtype, should also be incorporated into the risk assessment of the patient. Currently, a major limitation in the United States is a standardized method of pathologic reporting including subclassification of incomplete versus complete intestinal metaplasia.
Which patients to screen
Understanding this sequence of carcinogenesis offers a potential window for screening and surveillance. Subsequently, early detection of precancerous mucosal changes would be more amenable for endoscopic submucosal dissection (ESD).35,36 Currently, U.S. society guidelines do not specifically address the management of GIM. The American Society for Gastrointestinal Endoscopy (ASGE) guidelines for management of premalignant and malignant conditions of the stomach recommend surveillance in individuals with a family history of gastric cancer or of high-risk ethnic background but with no specific optimal surveillance interval.37 Also, H. pylori treatment is recommended if identified, but empiric treatment in GIM was felt to be controversial. The AGA recently sought comments on a proposed new guideline for the management of GIM. This guideline should be released after the comment period and help address management of GIM in the United States. In April of 2019, the European Society of Gastrointestinal Endoscopy (ESGE) updated the management of epithelial precancerous conditions and lesions in the stomach (MAPS II) guideline.38 The MAPS II guideline identifies atrophic gastritis and intestinal metaplasia as precancerous lesions. In patients with moderate to marked atrophy or GIM affecting both antral and body mucosa, ESGE recommends endoscopic surveillance with high-definition chromoendoscopy, mapping, and guided biopsies or at least two biopsies taken separately at the lesser and greater curvature of the antrum and body. H. pylori eradication was recommended if the patient tested positive.
Furthermore, MAPS II proposed replacing atrophic gastritis (AG) in the Operative Link on Gastritis Assessment (OLGA) staging by GIM (OLGIM) as it is considered a more reliable predictor of an individual’s gastric neoplasia risk, based on the interobserver agreement kappa value 0.6 for AG versus 0.9 for GIM.39 Five biopsies (two from the antrum, two from the corpus, and one from the incisura angularis) are needed for the OLGA/OLGIM score system to be considered an accurate predictor of this risk.39 This is supported by the early findings of gastric atrophy and GIM in the incisura angularis.23 In addition, for patients with GIM only in either the antrum or the body, a family history of gastric cancer, incomplete GIM, autoimmune gastritis, or persistent H. pylori infection was felt to increase the risk to warrant surveillance every 3 years. In those patients with atrophy or GIM in both the antrum and body with a first-degree relative with gastric cancer, surveillance was recommended every 1-2 years. Patients with any dysplasia and a visible lesion should have staging and resection. With no visible lesion, a follow-up endoscopy should be performed in 6 months with high-grade dysplasia and with low-grade dysplasia a repeat in 12 months. Patients with mild to moderate atrophy in the antrum and no intestinal metaplasia were not felt to warrant any further surveillance. (See Figure 1.)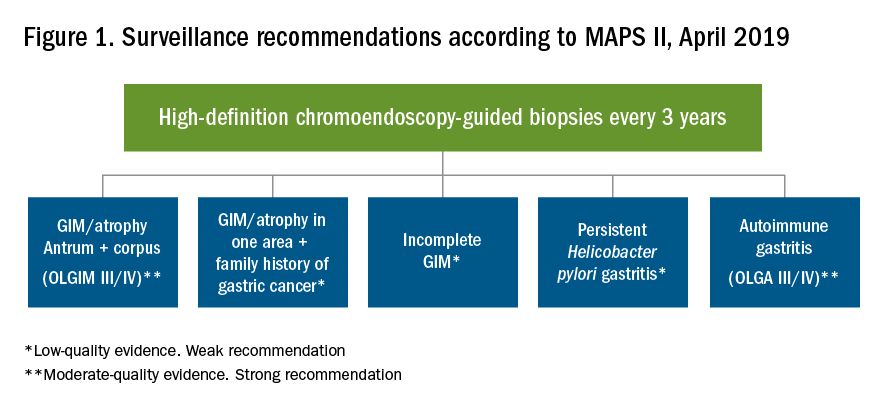
How to screen
Previous studies have found a poor correlation between the endoscopic determination of gastric atrophy and the histologic diagnosis.42 Several studies also found that gastric cancer was missed on initial endoscopic examinations. Sensitivity of endoscopy to detect gastric cancer has ranged from 77% to 93%.43,44 In the United States, there is a lack of standardized quality indicators for upper endoscopy exams. The ESGE has suggested several performance measures to ensure a quality endoscopy exam, including accurate photo documentation, sufficient procedure time of at least 7 minutes, adherence to biopsy protocols, and low complication rates.45 In Asia, a systematic screening protocol is used for photo documentation, and simple techniques such as adequate air insufflation and irrigation to remove mucus are routinely used to improve the endoscopy exam.46,47 The mean time of an endoscopy exam has also been found to increase the detection of neoplastic lesions, as slow endoscopists – with a mean exam duration of 8.6 ± 4.2 min during upper endoscopy – detected threefold more neoplastic lesions than did fast endoscopists.48
A standardized biopsy approach is also important when screening patients. The updated Sydney protocol has been suggested for mapping the stomach to screen for atrophy and GIM. This protocol recommends two biopsies from the antrum (at the lesser and greater curvature), two from the body (at the lesser and greater curvature), and one from the incisura.23 This biopsy protocol was also suggested in the recent MAPS II update, with the biopsy of the incisura felt to be an additional biopsy left to the discretion of the endoscopist. Notably, abnormal appearing mucosal areas should be biopsied separately from the mapping biopsies.
High-definition endoscopy with virtual chromoendoscopy is felt to be better than white-light endoscopy alone at detecting precancerous gastric lesions.38 (See Figure 2.)
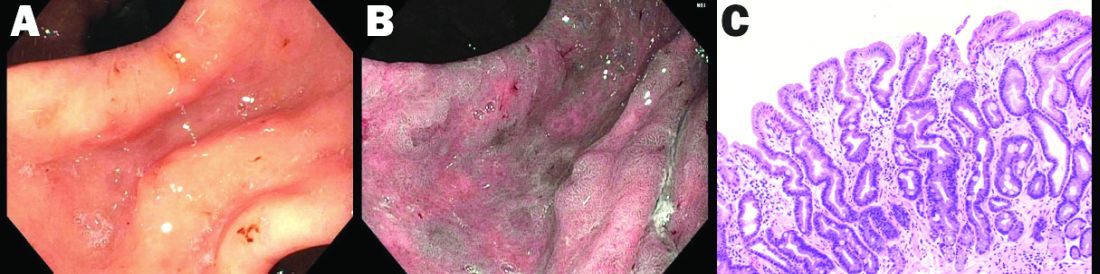
In particular, narrow-band imaging (NBI) has been studied and found to increase the diagnostic yield of GIM and dysplasia compared with white light alone.49 Several studies have shown an increased accuracy for the detection of GIM with magnification NBI.50-52 An unfortunate limitation is the geographic availability of magnification NBI: It is not available in the United States. A multicenter study in Portugal developed a new classification system for the appearance of precancerous lesions with NBI and tested its accuracy in endoscopists with a wide range of NBI experience. An abnormal mucosal pattern that showed light blue crests/regular ridge or a tubulovillous appearance and a regular mucosal pattern was found with GIM. An irregular vascular pattern with a white opaque substance and an absent or irregular mucosal pattern was most often found with dysplasia. Furthermore, the reproducibility of these patterns was high between endoscopists.53 Multiple studies have been performed on additional imaging technologies to enhance the detection of gastric neoplasia; however, these technologies are still investigational and currently not recommended for screening.54-57
Serum pepsinogens have been studied in Europe and Asia as noninvasive indicators of gastric atrophy to determine who should be screened with endoscopy.58 A low serum pepsinogen I level below 70 ng/mL and pepsinogen I/II ratio below 3 has generally been used to detect atrophic gastritis and at-risk populations. However, the studies performed in Europe and Asia used different methods for quantifying pepsinogen levels. Therefore, cutoff values cannot be generalized for all assays and should be validated for the specific tests used.38
Summary
Gastric atrophy and gastric intestinal metaplasia are considered precancerous lesions with an increased risk of development of gastric cancer. H. pylori is a major risk factor for the development of GIM. The extent of GIM as well as the presence of incomplete intestinal metaplasia, or type III intestinal metaplasia has been found to have the highest gastric cancer risk. Currently, in the United States, specific guidelines on endoscopic screening and surveillance for noncardia gastric adenocarcinoma based on histological subtype of GIM, location, and extension are lacking. The ESGE recently updated guidelines that recommend surveillance of patients with extensive atrophy and intestinal metaplasia or with a significant family history. Location and extension of intestinal metaplasia plays a role in increased risk. Screening should include a standardized upper endoscopy approach with high-definition white- light endoscopy and NBI, at least a 7-minute examination, adequate insufflation and cleaning, adequate photo documentation, and a standardized biopsy protocol. Further studies are needed to determine an appropriate surveillance interval and standardized pathology reporting approach as well.
Diana Curras-Martin MD, is an internal medicine resident at Hackensack Meridian Jersey Shore University Medical Center. Susana Gonzalez, MD, is assistant professor of medicine in the division of gastroenterology and hepatology (@WCM_GI), Weill Cornell Medicine, New York Presbyterian Hospital–Cornell.
References
1. Bray F et al. CA Cancer J Clin. 2018;68(6):394-424.
2. Global Burden of Disease Cancer Collaboration et al. JAMA Oncol. 2018;4(11):1553-68.
3. Balakrishnan M et al. Curr Gastroenterol Rep. 2017;19(8):36.
4. Anderson WF et al. J Natl Cancer Inst. 2018;110(6):608-15.
5. Trieu JA et al. Dig Dis Sci. 2019;64(5):1079-88.
6. Lauren P. Acta Pathol Microbiol Scand. 1965;64:31-49.
7. Correa P, Schneider BG. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1865-8.
8. Correa P. Cancer Res. 1992;52(24):6735-40.
9. Correa P, Piazuelo MB. J Dig Dis. 2012;13(1):2-9.
10. Correa P et al. J Natl Cancer Inst. 1970;44(2):297-306.
11. Correa P. Semin Oncol. 1985;12(1):2-10.
12. Rugge M et al. Hum Pathol. 1991;22(10):1002-8.
13. Simko V et al. Bratisl Lek Listy. 2015;116(1):3-8.
14. Giroux V, Rustgi AK. Nat Rev Cancer. 2017;17(10):594-604.
15. Jencks DS et al. Gastroenterol Hepatol (N Y). 2018;14(2):92-101.
16. Amieva M, Peek RM Jr. Gastroenterology. 2016;150(1):64-78.
17. Karimi P et al. Cancer Epidemiol Biomarkers Prev. 2014;23(5):700-13.
18. Hatakeyama M. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(4):196-219.
19. Tsutsumi R et al. Mol Cell Biol. 2006;26(1):261-76.
20. Kikuchi S et al. Am J Gastroenterol. 1999;94(12):3455-9.
21. Jass JR, Filipe MI. Histopathology. 1980;4(3):271-9.
22. Jass JR, Filipe MI. Histochem J. 1981;13(6):931-9.
23. Dixon MF et al. Am J Surg Pathol. 1996;20(10):1161-81.
24. Kang KP et al. J Gastroenterol Hepatol. 2009;24(1):140-8.
25. Gonzalez CA et al. Int J Cancer. 2010;127(11):2654-60.
26. Filipe MI et al. Gut. 1985;26(12):1319-26.
27. Filipe MI et al. Int J Cancer. 1994;57(3):324-9.
28. Gonzalez CA et al. J Gastroenterol Hepatol. 2016;31(5):953-8.
29. Cassaro M et al. Am J Gastroenterol. 2000;95(6):1431-8.
30. Shao L et al. Int J Cancer. Apr 29. 2018.
31. Stemmermann GN. Cancer. 1994;74(2):556-64.
32. Gonzalez CA et al. Int J Cancer. 2013;133(5):1023-32.
33. Reddy KM et al. Clin Gastroenterol Hepatol. 2016;14(10):1420-5.
34. Tava F et al. Hum Pathol. 2006;37(11):1489-97.
35. Fernandez-Esparrach G et al. Rev Esp Enferm Dig. 2014;106(2):120-32.
36. Ono H et al. Dig Endosc. 2016;28(1):3-15.
37. Evans JA, DeWitt JM. Gastrointest Endosc. 2016;83(1):274.
38. Pimentel-Nunes P et al. Endoscopy. 2019;51(4):365-88.
39. Capelle LG et al. Gastrointest Endosc. 2010;71(7):1150-8.
40. Saumoy M et al. Gastroenterology. 2018;155(3):648-60.
41. Gupta N et al. Gastrointest Endosc. 2011;74(3):610-24 e612.
42. Eshmuratov A et al. Dig Dis Sci. 2010;55(5):1364-75.
43. Nam JH et al. Cancer. 2012;118(20):4953-60.
44. Amin A et al. J R Coll Surg Edinb. 2002;47(5):681-4.
45. Bisschops R et al. United European Gastroenterol J. 2016;4(5):629-56.
46. Uedo N et al. Gastroenterol Clin North Am. 2013;42(2):317-35.
47. Yao K. Ann Gastroenterol. 2013;26(1):11-22.
48. Teh JL et al. Clin Gastroenterol Hepatol. 2015;13(3):480-7 e482.
49. Capelle LG et al. Dig Dis Sci. 2010;55(12):3442-8.
50. Bansal A et al. Gastrointest Endosc. 2008;67(2):210-6.
51. Tahara T et al. Gastrointest Endosc. 2009;70(2):246-53.
52. Uedo N et al. Endoscopy. 2006;38(8):819-24.
53. Pimentel-Nunes P et al. Endoscopy. 2012;44(3):236-46.
54. Kato M et al. Gastrointest Endosc. 2009;70(5):899-906.
55. Nishimura J et al. Gastroenterol Res Pract. 2014;2014:819395.
56. Dohi O et al. Gastrointest Endosc. 2019;89(1):47-57.
57. Osawa H et al. World J Gastrointest Endosc. 2012;4(8):356-61.
58. Pasechnikov V et al. World J Gastroenterol. 2014;20(38):13842-62.
Introduction
Despite a global decline in the incidence of gastric cancer over the past 3 decades, it remains the fifth most commonly diagnosed cancer and the third most common cause of cancer deaths worldwide.1 In the United States it is the fourth most commonly diagnosed GI malignancy, after colorectal, pancreas, and liver cancer. The prevalence remains high in Latin America and Asia, which has implications in the United States because of growing Hispanic and Asian populations.2,3 In recent years, a change in the trend of gastric cancer among non-Hispanic whites has been observed, particularly in women younger than 50 years old.4 5
Etiology
Gastric adenocarcinomas are classified into two subcategories based on location (cardia and noncardia) and histology (intestinal and diffuse types).6,7 Atrophic gastritis and gastric intestinal metaplasia (GIM) are considered precursors of intestinal-type noncardia gastric adenocarcinoma. The Correa cascade is a commonly accepted precancer sequence for noncardia gastric adenocarcinoma that describes mucosal changes from inflammation to atrophy to metaplasia to intraepithelial neoplasia and culminating in carcinoma.8,9 It has been observed that GIM may be the histologic change prior to the development of dysplasia and over 50% of patients with high-grade dysplasia will progress to adenocarcinoma.10-12 In the United States, GIM has the highest prevalence in African Americans, Hispanics, and East Asians, with the overall GIM prevalence regardless of ethnicity reported from 3.05% to 19.2%.5,13
Risk factors and subclassification
Replacement of the foveolar and/or glandular epithelium in the oxyntic and antral mucosa by intestinal epithelium results in GIM. It can be focal when limited to one region of the stomach or extensive when two or more regions are involved.14 The main risk factors for GIM development are Helicobacter pylori infection, tobacco, alcohol consumption, high salt intake, and chronic bile reflux.15,16 Additional risks for developing gastric cancer include older age, certain ethnicities, and male sex.17
CagA strains of H. pylori can promote carcinogenesis by inducing a mitogenic cellular response and downregulating cell adhesion.18,19 Less carcinogenic risk is associated with H. pylori Cag-A negative strains; however, they also have oncogenic potential mediated by expression of babA2 and vacA genes.20 Hence, the combination of multiple virulent factors encoded in babA2, CagA, and vacA genes has been associated with increased risk of GIM, inflammation, and development of gastric cancer.15 The clinical usefulness of genotyping H. pylori strains specifically to survey precancerous gastric lesions remains to be seen because of a lack of sufficient clinical studies. In addition, genotyping H. pylori is not commonly performed as part of clinical practice.
The loss of parietal cells seen in atrophic gastritis due to chronic H. pylori infection has been linked to the development of metaplasia due to possible loss of differentiation-promoting factors. As a result, metaplastic cells emerge that express spasmolytic polypeptide (SP or TFF2); hence, this type of metaplasia is referred to as spasmolytic polypeptide–expressing metaplasia (SPEM). The cellular mechanism that may explain a precursor role of SPEM in the development of GIM remains unknown.14 A second competing theory for the development of GIM is the clonal expansion of stem cells in the gastric isthmus that can lead to dysplasia and cancer development.14
On the basis of histological similarities with small intestinal or colonic epithelium, GIM can be further classified into complete or incomplete intestinal metaplasia.21 Complete intestinal metaplasia most closely resembles small intestinal epithelium with a brush border and goblet cells. Incomplete intestinal metaplasia resembles the colonic epithelium and lacks a brush border. A second classification further classifies GIM into three subtypes: Type I contains nonsecretory absorptive cells and sialomucin secreting goblet cells; type II has few absorptive cells, columnar cells secreting sialomucin, goblet cells secreting mainly sialomucin but some sulphomucin, and presence of Paneth cells; and type III consists of columnar cells secreting predominantly sulphomucin, goblet cells secreting sialomucin or sulphomucin, and absence of Paneth cells.15,22 In this subclassification, type I GIM is known as complete GIM and types II and III as incomplete GIM.23-25
Multiple studies performed outside of the United States have shown a higher progression risk to gastric adenocarcinoma in incomplete intestinal metaplasia, or type III intestinal metaplasia.26-32 Also, the risk of gastric cancer has been demonstrated to be higher among patients with a greater area of metaplasia and extensive intestinal metaplasia, defined as GIM in both the antrum and corpus.33,34 Hence, the extent of the metaplasia determined with mapping biopsies, regardless of the subtype, should also be incorporated into the risk assessment of the patient. Currently, a major limitation in the United States is a standardized method of pathologic reporting including subclassification of incomplete versus complete intestinal metaplasia.
Which patients to screen
Understanding this sequence of carcinogenesis offers a potential window for screening and surveillance. Subsequently, early detection of precancerous mucosal changes would be more amenable for endoscopic submucosal dissection (ESD).35,36 Currently, U.S. society guidelines do not specifically address the management of GIM. The American Society for Gastrointestinal Endoscopy (ASGE) guidelines for management of premalignant and malignant conditions of the stomach recommend surveillance in individuals with a family history of gastric cancer or of high-risk ethnic background but with no specific optimal surveillance interval.37 Also, H. pylori treatment is recommended if identified, but empiric treatment in GIM was felt to be controversial. The AGA recently sought comments on a proposed new guideline for the management of GIM. This guideline should be released after the comment period and help address management of GIM in the United States. In April of 2019, the European Society of Gastrointestinal Endoscopy (ESGE) updated the management of epithelial precancerous conditions and lesions in the stomach (MAPS II) guideline.38 The MAPS II guideline identifies atrophic gastritis and intestinal metaplasia as precancerous lesions. In patients with moderate to marked atrophy or GIM affecting both antral and body mucosa, ESGE recommends endoscopic surveillance with high-definition chromoendoscopy, mapping, and guided biopsies or at least two biopsies taken separately at the lesser and greater curvature of the antrum and body. H. pylori eradication was recommended if the patient tested positive.
Furthermore, MAPS II proposed replacing atrophic gastritis (AG) in the Operative Link on Gastritis Assessment (OLGA) staging by GIM (OLGIM) as it is considered a more reliable predictor of an individual’s gastric neoplasia risk, based on the interobserver agreement kappa value 0.6 for AG versus 0.9 for GIM.39 Five biopsies (two from the antrum, two from the corpus, and one from the incisura angularis) are needed for the OLGA/OLGIM score system to be considered an accurate predictor of this risk.39 This is supported by the early findings of gastric atrophy and GIM in the incisura angularis.23 In addition, for patients with GIM only in either the antrum or the body, a family history of gastric cancer, incomplete GIM, autoimmune gastritis, or persistent H. pylori infection was felt to increase the risk to warrant surveillance every 3 years. In those patients with atrophy or GIM in both the antrum and body with a first-degree relative with gastric cancer, surveillance was recommended every 1-2 years. Patients with any dysplasia and a visible lesion should have staging and resection. With no visible lesion, a follow-up endoscopy should be performed in 6 months with high-grade dysplasia and with low-grade dysplasia a repeat in 12 months. Patients with mild to moderate atrophy in the antrum and no intestinal metaplasia were not felt to warrant any further surveillance. (See Figure 1.)
How to screen
Previous studies have found a poor correlation between the endoscopic determination of gastric atrophy and the histologic diagnosis.42 Several studies also found that gastric cancer was missed on initial endoscopic examinations. Sensitivity of endoscopy to detect gastric cancer has ranged from 77% to 93%.43,44 In the United States, there is a lack of standardized quality indicators for upper endoscopy exams. The ESGE has suggested several performance measures to ensure a quality endoscopy exam, including accurate photo documentation, sufficient procedure time of at least 7 minutes, adherence to biopsy protocols, and low complication rates.45 In Asia, a systematic screening protocol is used for photo documentation, and simple techniques such as adequate air insufflation and irrigation to remove mucus are routinely used to improve the endoscopy exam.46,47 The mean time of an endoscopy exam has also been found to increase the detection of neoplastic lesions, as slow endoscopists – with a mean exam duration of 8.6 ± 4.2 min during upper endoscopy – detected threefold more neoplastic lesions than did fast endoscopists.48
A standardized biopsy approach is also important when screening patients. The updated Sydney protocol has been suggested for mapping the stomach to screen for atrophy and GIM. This protocol recommends two biopsies from the antrum (at the lesser and greater curvature), two from the body (at the lesser and greater curvature), and one from the incisura.23 This biopsy protocol was also suggested in the recent MAPS II update, with the biopsy of the incisura felt to be an additional biopsy left to the discretion of the endoscopist. Notably, abnormal appearing mucosal areas should be biopsied separately from the mapping biopsies.
High-definition endoscopy with virtual chromoendoscopy is felt to be better than white-light endoscopy alone at detecting precancerous gastric lesions.38 (See Figure 2.)

In particular, narrow-band imaging (NBI) has been studied and found to increase the diagnostic yield of GIM and dysplasia compared with white light alone.49 Several studies have shown an increased accuracy for the detection of GIM with magnification NBI.50-52 An unfortunate limitation is the geographic availability of magnification NBI: It is not available in the United States. A multicenter study in Portugal developed a new classification system for the appearance of precancerous lesions with NBI and tested its accuracy in endoscopists with a wide range of NBI experience. An abnormal mucosal pattern that showed light blue crests/regular ridge or a tubulovillous appearance and a regular mucosal pattern was found with GIM. An irregular vascular pattern with a white opaque substance and an absent or irregular mucosal pattern was most often found with dysplasia. Furthermore, the reproducibility of these patterns was high between endoscopists.53 Multiple studies have been performed on additional imaging technologies to enhance the detection of gastric neoplasia; however, these technologies are still investigational and currently not recommended for screening.54-57
Serum pepsinogens have been studied in Europe and Asia as noninvasive indicators of gastric atrophy to determine who should be screened with endoscopy.58 A low serum pepsinogen I level below 70 ng/mL and pepsinogen I/II ratio below 3 has generally been used to detect atrophic gastritis and at-risk populations. However, the studies performed in Europe and Asia used different methods for quantifying pepsinogen levels. Therefore, cutoff values cannot be generalized for all assays and should be validated for the specific tests used.38
Summary
Gastric atrophy and gastric intestinal metaplasia are considered precancerous lesions with an increased risk of development of gastric cancer. H. pylori is a major risk factor for the development of GIM. The extent of GIM as well as the presence of incomplete intestinal metaplasia, or type III intestinal metaplasia has been found to have the highest gastric cancer risk. Currently, in the United States, specific guidelines on endoscopic screening and surveillance for noncardia gastric adenocarcinoma based on histological subtype of GIM, location, and extension are lacking. The ESGE recently updated guidelines that recommend surveillance of patients with extensive atrophy and intestinal metaplasia or with a significant family history. Location and extension of intestinal metaplasia plays a role in increased risk. Screening should include a standardized upper endoscopy approach with high-definition white- light endoscopy and NBI, at least a 7-minute examination, adequate insufflation and cleaning, adequate photo documentation, and a standardized biopsy protocol. Further studies are needed to determine an appropriate surveillance interval and standardized pathology reporting approach as well.
Diana Curras-Martin MD, is an internal medicine resident at Hackensack Meridian Jersey Shore University Medical Center. Susana Gonzalez, MD, is assistant professor of medicine in the division of gastroenterology and hepatology (@WCM_GI), Weill Cornell Medicine, New York Presbyterian Hospital–Cornell.
References
1. Bray F et al. CA Cancer J Clin. 2018;68(6):394-424.
2. Global Burden of Disease Cancer Collaboration et al. JAMA Oncol. 2018;4(11):1553-68.
3. Balakrishnan M et al. Curr Gastroenterol Rep. 2017;19(8):36.
4. Anderson WF et al. J Natl Cancer Inst. 2018;110(6):608-15.
5. Trieu JA et al. Dig Dis Sci. 2019;64(5):1079-88.
6. Lauren P. Acta Pathol Microbiol Scand. 1965;64:31-49.
7. Correa P, Schneider BG. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1865-8.
8. Correa P. Cancer Res. 1992;52(24):6735-40.
9. Correa P, Piazuelo MB. J Dig Dis. 2012;13(1):2-9.
10. Correa P et al. J Natl Cancer Inst. 1970;44(2):297-306.
11. Correa P. Semin Oncol. 1985;12(1):2-10.
12. Rugge M et al. Hum Pathol. 1991;22(10):1002-8.
13. Simko V et al. Bratisl Lek Listy. 2015;116(1):3-8.
14. Giroux V, Rustgi AK. Nat Rev Cancer. 2017;17(10):594-604.
15. Jencks DS et al. Gastroenterol Hepatol (N Y). 2018;14(2):92-101.
16. Amieva M, Peek RM Jr. Gastroenterology. 2016;150(1):64-78.
17. Karimi P et al. Cancer Epidemiol Biomarkers Prev. 2014;23(5):700-13.
18. Hatakeyama M. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(4):196-219.
19. Tsutsumi R et al. Mol Cell Biol. 2006;26(1):261-76.
20. Kikuchi S et al. Am J Gastroenterol. 1999;94(12):3455-9.
21. Jass JR, Filipe MI. Histopathology. 1980;4(3):271-9.
22. Jass JR, Filipe MI. Histochem J. 1981;13(6):931-9.
23. Dixon MF et al. Am J Surg Pathol. 1996;20(10):1161-81.
24. Kang KP et al. J Gastroenterol Hepatol. 2009;24(1):140-8.
25. Gonzalez CA et al. Int J Cancer. 2010;127(11):2654-60.
26. Filipe MI et al. Gut. 1985;26(12):1319-26.
27. Filipe MI et al. Int J Cancer. 1994;57(3):324-9.
28. Gonzalez CA et al. J Gastroenterol Hepatol. 2016;31(5):953-8.
29. Cassaro M et al. Am J Gastroenterol. 2000;95(6):1431-8.
30. Shao L et al. Int J Cancer. Apr 29. 2018.
31. Stemmermann GN. Cancer. 1994;74(2):556-64.
32. Gonzalez CA et al. Int J Cancer. 2013;133(5):1023-32.
33. Reddy KM et al. Clin Gastroenterol Hepatol. 2016;14(10):1420-5.
34. Tava F et al. Hum Pathol. 2006;37(11):1489-97.
35. Fernandez-Esparrach G et al. Rev Esp Enferm Dig. 2014;106(2):120-32.
36. Ono H et al. Dig Endosc. 2016;28(1):3-15.
37. Evans JA, DeWitt JM. Gastrointest Endosc. 2016;83(1):274.
38. Pimentel-Nunes P et al. Endoscopy. 2019;51(4):365-88.
39. Capelle LG et al. Gastrointest Endosc. 2010;71(7):1150-8.
40. Saumoy M et al. Gastroenterology. 2018;155(3):648-60.
41. Gupta N et al. Gastrointest Endosc. 2011;74(3):610-24 e612.
42. Eshmuratov A et al. Dig Dis Sci. 2010;55(5):1364-75.
43. Nam JH et al. Cancer. 2012;118(20):4953-60.
44. Amin A et al. J R Coll Surg Edinb. 2002;47(5):681-4.
45. Bisschops R et al. United European Gastroenterol J. 2016;4(5):629-56.
46. Uedo N et al. Gastroenterol Clin North Am. 2013;42(2):317-35.
47. Yao K. Ann Gastroenterol. 2013;26(1):11-22.
48. Teh JL et al. Clin Gastroenterol Hepatol. 2015;13(3):480-7 e482.
49. Capelle LG et al. Dig Dis Sci. 2010;55(12):3442-8.
50. Bansal A et al. Gastrointest Endosc. 2008;67(2):210-6.
51. Tahara T et al. Gastrointest Endosc. 2009;70(2):246-53.
52. Uedo N et al. Endoscopy. 2006;38(8):819-24.
53. Pimentel-Nunes P et al. Endoscopy. 2012;44(3):236-46.
54. Kato M et al. Gastrointest Endosc. 2009;70(5):899-906.
55. Nishimura J et al. Gastroenterol Res Pract. 2014;2014:819395.
56. Dohi O et al. Gastrointest Endosc. 2019;89(1):47-57.
57. Osawa H et al. World J Gastrointest Endosc. 2012;4(8):356-61.
58. Pasechnikov V et al. World J Gastroenterol. 2014;20(38):13842-62.
Introduction
Despite a global decline in the incidence of gastric cancer over the past 3 decades, it remains the fifth most commonly diagnosed cancer and the third most common cause of cancer deaths worldwide.1 In the United States it is the fourth most commonly diagnosed GI malignancy, after colorectal, pancreas, and liver cancer. The prevalence remains high in Latin America and Asia, which has implications in the United States because of growing Hispanic and Asian populations.2,3 In recent years, a change in the trend of gastric cancer among non-Hispanic whites has been observed, particularly in women younger than 50 years old.4 5
Etiology
Gastric adenocarcinomas are classified into two subcategories based on location (cardia and noncardia) and histology (intestinal and diffuse types).6,7 Atrophic gastritis and gastric intestinal metaplasia (GIM) are considered precursors of intestinal-type noncardia gastric adenocarcinoma. The Correa cascade is a commonly accepted precancer sequence for noncardia gastric adenocarcinoma that describes mucosal changes from inflammation to atrophy to metaplasia to intraepithelial neoplasia and culminating in carcinoma.8,9 It has been observed that GIM may be the histologic change prior to the development of dysplasia and over 50% of patients with high-grade dysplasia will progress to adenocarcinoma.10-12 In the United States, GIM has the highest prevalence in African Americans, Hispanics, and East Asians, with the overall GIM prevalence regardless of ethnicity reported from 3.05% to 19.2%.5,13
Risk factors and subclassification
Replacement of the foveolar and/or glandular epithelium in the oxyntic and antral mucosa by intestinal epithelium results in GIM. It can be focal when limited to one region of the stomach or extensive when two or more regions are involved.14 The main risk factors for GIM development are Helicobacter pylori infection, tobacco, alcohol consumption, high salt intake, and chronic bile reflux.15,16 Additional risks for developing gastric cancer include older age, certain ethnicities, and male sex.17
CagA strains of H. pylori can promote carcinogenesis by inducing a mitogenic cellular response and downregulating cell adhesion.18,19 Less carcinogenic risk is associated with H. pylori Cag-A negative strains; however, they also have oncogenic potential mediated by expression of babA2 and vacA genes.20 Hence, the combination of multiple virulent factors encoded in babA2, CagA, and vacA genes has been associated with increased risk of GIM, inflammation, and development of gastric cancer.15 The clinical usefulness of genotyping H. pylori strains specifically to survey precancerous gastric lesions remains to be seen because of a lack of sufficient clinical studies. In addition, genotyping H. pylori is not commonly performed as part of clinical practice.
The loss of parietal cells seen in atrophic gastritis due to chronic H. pylori infection has been linked to the development of metaplasia due to possible loss of differentiation-promoting factors. As a result, metaplastic cells emerge that express spasmolytic polypeptide (SP or TFF2); hence, this type of metaplasia is referred to as spasmolytic polypeptide–expressing metaplasia (SPEM). The cellular mechanism that may explain a precursor role of SPEM in the development of GIM remains unknown.14 A second competing theory for the development of GIM is the clonal expansion of stem cells in the gastric isthmus that can lead to dysplasia and cancer development.14
On the basis of histological similarities with small intestinal or colonic epithelium, GIM can be further classified into complete or incomplete intestinal metaplasia.21 Complete intestinal metaplasia most closely resembles small intestinal epithelium with a brush border and goblet cells. Incomplete intestinal metaplasia resembles the colonic epithelium and lacks a brush border. A second classification further classifies GIM into three subtypes: Type I contains nonsecretory absorptive cells and sialomucin secreting goblet cells; type II has few absorptive cells, columnar cells secreting sialomucin, goblet cells secreting mainly sialomucin but some sulphomucin, and presence of Paneth cells; and type III consists of columnar cells secreting predominantly sulphomucin, goblet cells secreting sialomucin or sulphomucin, and absence of Paneth cells.15,22 In this subclassification, type I GIM is known as complete GIM and types II and III as incomplete GIM.23-25
Multiple studies performed outside of the United States have shown a higher progression risk to gastric adenocarcinoma in incomplete intestinal metaplasia, or type III intestinal metaplasia.26-32 Also, the risk of gastric cancer has been demonstrated to be higher among patients with a greater area of metaplasia and extensive intestinal metaplasia, defined as GIM in both the antrum and corpus.33,34 Hence, the extent of the metaplasia determined with mapping biopsies, regardless of the subtype, should also be incorporated into the risk assessment of the patient. Currently, a major limitation in the United States is a standardized method of pathologic reporting including subclassification of incomplete versus complete intestinal metaplasia.
Which patients to screen
Understanding this sequence of carcinogenesis offers a potential window for screening and surveillance. Subsequently, early detection of precancerous mucosal changes would be more amenable for endoscopic submucosal dissection (ESD).35,36 Currently, U.S. society guidelines do not specifically address the management of GIM. The American Society for Gastrointestinal Endoscopy (ASGE) guidelines for management of premalignant and malignant conditions of the stomach recommend surveillance in individuals with a family history of gastric cancer or of high-risk ethnic background but with no specific optimal surveillance interval.37 Also, H. pylori treatment is recommended if identified, but empiric treatment in GIM was felt to be controversial. The AGA recently sought comments on a proposed new guideline for the management of GIM. This guideline should be released after the comment period and help address management of GIM in the United States. In April of 2019, the European Society of Gastrointestinal Endoscopy (ESGE) updated the management of epithelial precancerous conditions and lesions in the stomach (MAPS II) guideline.38 The MAPS II guideline identifies atrophic gastritis and intestinal metaplasia as precancerous lesions. In patients with moderate to marked atrophy or GIM affecting both antral and body mucosa, ESGE recommends endoscopic surveillance with high-definition chromoendoscopy, mapping, and guided biopsies or at least two biopsies taken separately at the lesser and greater curvature of the antrum and body. H. pylori eradication was recommended if the patient tested positive.
Furthermore, MAPS II proposed replacing atrophic gastritis (AG) in the Operative Link on Gastritis Assessment (OLGA) staging by GIM (OLGIM) as it is considered a more reliable predictor of an individual’s gastric neoplasia risk, based on the interobserver agreement kappa value 0.6 for AG versus 0.9 for GIM.39 Five biopsies (two from the antrum, two from the corpus, and one from the incisura angularis) are needed for the OLGA/OLGIM score system to be considered an accurate predictor of this risk.39 This is supported by the early findings of gastric atrophy and GIM in the incisura angularis.23 In addition, for patients with GIM only in either the antrum or the body, a family history of gastric cancer, incomplete GIM, autoimmune gastritis, or persistent H. pylori infection was felt to increase the risk to warrant surveillance every 3 years. In those patients with atrophy or GIM in both the antrum and body with a first-degree relative with gastric cancer, surveillance was recommended every 1-2 years. Patients with any dysplasia and a visible lesion should have staging and resection. With no visible lesion, a follow-up endoscopy should be performed in 6 months with high-grade dysplasia and with low-grade dysplasia a repeat in 12 months. Patients with mild to moderate atrophy in the antrum and no intestinal metaplasia were not felt to warrant any further surveillance. (See Figure 1.)
How to screen
Previous studies have found a poor correlation between the endoscopic determination of gastric atrophy and the histologic diagnosis.42 Several studies also found that gastric cancer was missed on initial endoscopic examinations. Sensitivity of endoscopy to detect gastric cancer has ranged from 77% to 93%.43,44 In the United States, there is a lack of standardized quality indicators for upper endoscopy exams. The ESGE has suggested several performance measures to ensure a quality endoscopy exam, including accurate photo documentation, sufficient procedure time of at least 7 minutes, adherence to biopsy protocols, and low complication rates.45 In Asia, a systematic screening protocol is used for photo documentation, and simple techniques such as adequate air insufflation and irrigation to remove mucus are routinely used to improve the endoscopy exam.46,47 The mean time of an endoscopy exam has also been found to increase the detection of neoplastic lesions, as slow endoscopists – with a mean exam duration of 8.6 ± 4.2 min during upper endoscopy – detected threefold more neoplastic lesions than did fast endoscopists.48
A standardized biopsy approach is also important when screening patients. The updated Sydney protocol has been suggested for mapping the stomach to screen for atrophy and GIM. This protocol recommends two biopsies from the antrum (at the lesser and greater curvature), two from the body (at the lesser and greater curvature), and one from the incisura.23 This biopsy protocol was also suggested in the recent MAPS II update, with the biopsy of the incisura felt to be an additional biopsy left to the discretion of the endoscopist. Notably, abnormal appearing mucosal areas should be biopsied separately from the mapping biopsies.
High-definition endoscopy with virtual chromoendoscopy is felt to be better than white-light endoscopy alone at detecting precancerous gastric lesions.38 (See Figure 2.)

In particular, narrow-band imaging (NBI) has been studied and found to increase the diagnostic yield of GIM and dysplasia compared with white light alone.49 Several studies have shown an increased accuracy for the detection of GIM with magnification NBI.50-52 An unfortunate limitation is the geographic availability of magnification NBI: It is not available in the United States. A multicenter study in Portugal developed a new classification system for the appearance of precancerous lesions with NBI and tested its accuracy in endoscopists with a wide range of NBI experience. An abnormal mucosal pattern that showed light blue crests/regular ridge or a tubulovillous appearance and a regular mucosal pattern was found with GIM. An irregular vascular pattern with a white opaque substance and an absent or irregular mucosal pattern was most often found with dysplasia. Furthermore, the reproducibility of these patterns was high between endoscopists.53 Multiple studies have been performed on additional imaging technologies to enhance the detection of gastric neoplasia; however, these technologies are still investigational and currently not recommended for screening.54-57
Serum pepsinogens have been studied in Europe and Asia as noninvasive indicators of gastric atrophy to determine who should be screened with endoscopy.58 A low serum pepsinogen I level below 70 ng/mL and pepsinogen I/II ratio below 3 has generally been used to detect atrophic gastritis and at-risk populations. However, the studies performed in Europe and Asia used different methods for quantifying pepsinogen levels. Therefore, cutoff values cannot be generalized for all assays and should be validated for the specific tests used.38
Summary
Gastric atrophy and gastric intestinal metaplasia are considered precancerous lesions with an increased risk of development of gastric cancer. H. pylori is a major risk factor for the development of GIM. The extent of GIM as well as the presence of incomplete intestinal metaplasia, or type III intestinal metaplasia has been found to have the highest gastric cancer risk. Currently, in the United States, specific guidelines on endoscopic screening and surveillance for noncardia gastric adenocarcinoma based on histological subtype of GIM, location, and extension are lacking. The ESGE recently updated guidelines that recommend surveillance of patients with extensive atrophy and intestinal metaplasia or with a significant family history. Location and extension of intestinal metaplasia plays a role in increased risk. Screening should include a standardized upper endoscopy approach with high-definition white- light endoscopy and NBI, at least a 7-minute examination, adequate insufflation and cleaning, adequate photo documentation, and a standardized biopsy protocol. Further studies are needed to determine an appropriate surveillance interval and standardized pathology reporting approach as well.
Diana Curras-Martin MD, is an internal medicine resident at Hackensack Meridian Jersey Shore University Medical Center. Susana Gonzalez, MD, is assistant professor of medicine in the division of gastroenterology and hepatology (@WCM_GI), Weill Cornell Medicine, New York Presbyterian Hospital–Cornell.
References
1. Bray F et al. CA Cancer J Clin. 2018;68(6):394-424.
2. Global Burden of Disease Cancer Collaboration et al. JAMA Oncol. 2018;4(11):1553-68.
3. Balakrishnan M et al. Curr Gastroenterol Rep. 2017;19(8):36.
4. Anderson WF et al. J Natl Cancer Inst. 2018;110(6):608-15.
5. Trieu JA et al. Dig Dis Sci. 2019;64(5):1079-88.
6. Lauren P. Acta Pathol Microbiol Scand. 1965;64:31-49.
7. Correa P, Schneider BG. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1865-8.
8. Correa P. Cancer Res. 1992;52(24):6735-40.
9. Correa P, Piazuelo MB. J Dig Dis. 2012;13(1):2-9.
10. Correa P et al. J Natl Cancer Inst. 1970;44(2):297-306.
11. Correa P. Semin Oncol. 1985;12(1):2-10.
12. Rugge M et al. Hum Pathol. 1991;22(10):1002-8.
13. Simko V et al. Bratisl Lek Listy. 2015;116(1):3-8.
14. Giroux V, Rustgi AK. Nat Rev Cancer. 2017;17(10):594-604.
15. Jencks DS et al. Gastroenterol Hepatol (N Y). 2018;14(2):92-101.
16. Amieva M, Peek RM Jr. Gastroenterology. 2016;150(1):64-78.
17. Karimi P et al. Cancer Epidemiol Biomarkers Prev. 2014;23(5):700-13.
18. Hatakeyama M. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93(4):196-219.
19. Tsutsumi R et al. Mol Cell Biol. 2006;26(1):261-76.
20. Kikuchi S et al. Am J Gastroenterol. 1999;94(12):3455-9.
21. Jass JR, Filipe MI. Histopathology. 1980;4(3):271-9.
22. Jass JR, Filipe MI. Histochem J. 1981;13(6):931-9.
23. Dixon MF et al. Am J Surg Pathol. 1996;20(10):1161-81.
24. Kang KP et al. J Gastroenterol Hepatol. 2009;24(1):140-8.
25. Gonzalez CA et al. Int J Cancer. 2010;127(11):2654-60.
26. Filipe MI et al. Gut. 1985;26(12):1319-26.
27. Filipe MI et al. Int J Cancer. 1994;57(3):324-9.
28. Gonzalez CA et al. J Gastroenterol Hepatol. 2016;31(5):953-8.
29. Cassaro M et al. Am J Gastroenterol. 2000;95(6):1431-8.
30. Shao L et al. Int J Cancer. Apr 29. 2018.
31. Stemmermann GN. Cancer. 1994;74(2):556-64.
32. Gonzalez CA et al. Int J Cancer. 2013;133(5):1023-32.
33. Reddy KM et al. Clin Gastroenterol Hepatol. 2016;14(10):1420-5.
34. Tava F et al. Hum Pathol. 2006;37(11):1489-97.
35. Fernandez-Esparrach G et al. Rev Esp Enferm Dig. 2014;106(2):120-32.
36. Ono H et al. Dig Endosc. 2016;28(1):3-15.
37. Evans JA, DeWitt JM. Gastrointest Endosc. 2016;83(1):274.
38. Pimentel-Nunes P et al. Endoscopy. 2019;51(4):365-88.
39. Capelle LG et al. Gastrointest Endosc. 2010;71(7):1150-8.
40. Saumoy M et al. Gastroenterology. 2018;155(3):648-60.
41. Gupta N et al. Gastrointest Endosc. 2011;74(3):610-24 e612.
42. Eshmuratov A et al. Dig Dis Sci. 2010;55(5):1364-75.
43. Nam JH et al. Cancer. 2012;118(20):4953-60.
44. Amin A et al. J R Coll Surg Edinb. 2002;47(5):681-4.
45. Bisschops R et al. United European Gastroenterol J. 2016;4(5):629-56.
46. Uedo N et al. Gastroenterol Clin North Am. 2013;42(2):317-35.
47. Yao K. Ann Gastroenterol. 2013;26(1):11-22.
48. Teh JL et al. Clin Gastroenterol Hepatol. 2015;13(3):480-7 e482.
49. Capelle LG et al. Dig Dis Sci. 2010;55(12):3442-8.
50. Bansal A et al. Gastrointest Endosc. 2008;67(2):210-6.
51. Tahara T et al. Gastrointest Endosc. 2009;70(2):246-53.
52. Uedo N et al. Endoscopy. 2006;38(8):819-24.
53. Pimentel-Nunes P et al. Endoscopy. 2012;44(3):236-46.
54. Kato M et al. Gastrointest Endosc. 2009;70(5):899-906.
55. Nishimura J et al. Gastroenterol Res Pract. 2014;2014:819395.
56. Dohi O et al. Gastrointest Endosc. 2019;89(1):47-57.
57. Osawa H et al. World J Gastrointest Endosc. 2012;4(8):356-61.
58. Pasechnikov V et al. World J Gastroenterol. 2014;20(38):13842-62.
Passing the torch
Dear Colleagues,
It’s hard to believe that The New Gastroenterologist (TNG) is now in its 5th year of publication! Since the inception of TNG, it has been a true honor and pleasure to serve as the inaugural editor in chief (EIC), and it has been an experience that I will never forget. When the idea of TNG was first conceived nearly 5 years ago, the goal of the publication was to provide a dedicated home for content for early-career GIs and trainees, an area that was a clear void in the GI community. Over 4 years later, TNG remains a one-of-a-kind resource for our field, and I hope that you have enjoyed the content published.
As my term is ending soon, it is my pleasure to turn TNG over to the next EIC, Vijaya Rao from the University of Chicago. I have no doubt that Vijaya will do a fantastic job continuing TNG, and I am excited to see how she applies many of her innovative ideas to grow the publication and make it even more valuable to the early-career and trainee GI community. Finally, I would just like to thank all of the people who have made invaluable contributions to make TNG a success including Erin Landis and Ryan Farrell from the AGA; the staff of our publisher Frontline Medical Communications, especially Lora McGlade; and current editor in chief of GI & Hepatology News, John Allen.
As for this issue of TNG, my last issue as EIC, there is a fantastic line-up of content. The “In Focus” article, by Diana Curras-Martin and Susana Gonzalez (Cornell), addresses the controversial topic of gastric intestinal metaplasia, and will no doubt be very helpful for dealing with this condition when it’s encountered in clinical practice. Additionally, Edward Barnes (UNC Chapel Hill) covers the importance of mentoring during the early-career stage, while Josh Sloan (Hopkins) provides an overview of options for extra training in motility, including motility fellowships.
Also in this issue of TNG, Rishi Naik (Vanderbilt) outlines some of the important lessons he learned during his 1-year term as the Gastroenterology editorial fellow, and Latha Alaparthi (Gastroenterology Center of Connecticut) discusses tips for building an effective community practice as part of our “Private Practice Perspectives” section cosponsored by the Digestive Health Physicians Association. Finally, lawyers Matthew D’Emilio and Jeremy Riley cover estate planning, which is a topic that is important for all to be familiar with, regardless of age or current health status.
If you’re interested in contributing or have ideas for TNG, please contact me ([email protected]), incoming editor in chief Vijaya Rao ([email protected]), or Ryan Farrell (rfarrell@gastro.org), managing editor of TNG.
Thank you, this has been a true pleasure.
Sincerely,
Bryson W. Katona, MD, PhD
(outgoing) Editor in Chief
Dr. Katona is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.
Dear Colleagues,
It’s hard to believe that The New Gastroenterologist (TNG) is now in its 5th year of publication! Since the inception of TNG, it has been a true honor and pleasure to serve as the inaugural editor in chief (EIC), and it has been an experience that I will never forget. When the idea of TNG was first conceived nearly 5 years ago, the goal of the publication was to provide a dedicated home for content for early-career GIs and trainees, an area that was a clear void in the GI community. Over 4 years later, TNG remains a one-of-a-kind resource for our field, and I hope that you have enjoyed the content published.
As my term is ending soon, it is my pleasure to turn TNG over to the next EIC, Vijaya Rao from the University of Chicago. I have no doubt that Vijaya will do a fantastic job continuing TNG, and I am excited to see how she applies many of her innovative ideas to grow the publication and make it even more valuable to the early-career and trainee GI community. Finally, I would just like to thank all of the people who have made invaluable contributions to make TNG a success including Erin Landis and Ryan Farrell from the AGA; the staff of our publisher Frontline Medical Communications, especially Lora McGlade; and current editor in chief of GI & Hepatology News, John Allen.
As for this issue of TNG, my last issue as EIC, there is a fantastic line-up of content. The “In Focus” article, by Diana Curras-Martin and Susana Gonzalez (Cornell), addresses the controversial topic of gastric intestinal metaplasia, and will no doubt be very helpful for dealing with this condition when it’s encountered in clinical practice. Additionally, Edward Barnes (UNC Chapel Hill) covers the importance of mentoring during the early-career stage, while Josh Sloan (Hopkins) provides an overview of options for extra training in motility, including motility fellowships.
Also in this issue of TNG, Rishi Naik (Vanderbilt) outlines some of the important lessons he learned during his 1-year term as the Gastroenterology editorial fellow, and Latha Alaparthi (Gastroenterology Center of Connecticut) discusses tips for building an effective community practice as part of our “Private Practice Perspectives” section cosponsored by the Digestive Health Physicians Association. Finally, lawyers Matthew D’Emilio and Jeremy Riley cover estate planning, which is a topic that is important for all to be familiar with, regardless of age or current health status.
If you’re interested in contributing or have ideas for TNG, please contact me ([email protected]), incoming editor in chief Vijaya Rao ([email protected]), or Ryan Farrell (rfarrell@gastro.org), managing editor of TNG.
Thank you, this has been a true pleasure.
Sincerely,
Bryson W. Katona, MD, PhD
(outgoing) Editor in Chief
Dr. Katona is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.
Dear Colleagues,
It’s hard to believe that The New Gastroenterologist (TNG) is now in its 5th year of publication! Since the inception of TNG, it has been a true honor and pleasure to serve as the inaugural editor in chief (EIC), and it has been an experience that I will never forget. When the idea of TNG was first conceived nearly 5 years ago, the goal of the publication was to provide a dedicated home for content for early-career GIs and trainees, an area that was a clear void in the GI community. Over 4 years later, TNG remains a one-of-a-kind resource for our field, and I hope that you have enjoyed the content published.
As my term is ending soon, it is my pleasure to turn TNG over to the next EIC, Vijaya Rao from the University of Chicago. I have no doubt that Vijaya will do a fantastic job continuing TNG, and I am excited to see how she applies many of her innovative ideas to grow the publication and make it even more valuable to the early-career and trainee GI community. Finally, I would just like to thank all of the people who have made invaluable contributions to make TNG a success including Erin Landis and Ryan Farrell from the AGA; the staff of our publisher Frontline Medical Communications, especially Lora McGlade; and current editor in chief of GI & Hepatology News, John Allen.
As for this issue of TNG, my last issue as EIC, there is a fantastic line-up of content. The “In Focus” article, by Diana Curras-Martin and Susana Gonzalez (Cornell), addresses the controversial topic of gastric intestinal metaplasia, and will no doubt be very helpful for dealing with this condition when it’s encountered in clinical practice. Additionally, Edward Barnes (UNC Chapel Hill) covers the importance of mentoring during the early-career stage, while Josh Sloan (Hopkins) provides an overview of options for extra training in motility, including motility fellowships.
Also in this issue of TNG, Rishi Naik (Vanderbilt) outlines some of the important lessons he learned during his 1-year term as the Gastroenterology editorial fellow, and Latha Alaparthi (Gastroenterology Center of Connecticut) discusses tips for building an effective community practice as part of our “Private Practice Perspectives” section cosponsored by the Digestive Health Physicians Association. Finally, lawyers Matthew D’Emilio and Jeremy Riley cover estate planning, which is a topic that is important for all to be familiar with, regardless of age or current health status.
If you’re interested in contributing or have ideas for TNG, please contact me ([email protected]), incoming editor in chief Vijaya Rao ([email protected]), or Ryan Farrell (rfarrell@gastro.org), managing editor of TNG.
Thank you, this has been a true pleasure.
Sincerely,
Bryson W. Katona, MD, PhD
(outgoing) Editor in Chief
Dr. Katona is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.