User login
February 2020 – ICYMI
Gastroenterology
November 2019
Clip closure after resection of large colorectal lesions with substantial risk of bleeding. Albéniz E et al. 2019 Nov;157(5):1213-21.e4. doi. 10.1053/j.gastro.2019.07.037.
Tumor seeding during colonoscopy as a possible cause for metachronous colorectal cancer. Backes Y et al. 2019 Nov;157(5):1222-32.e4. doi. 10.1053/j.gastro.2019.07.062.
December 2019
How to “DEAL” with disruptive physician behavior. Junga Z et al. 2019 Dec;157(6):1469-72. doi. 10.1053/j.gastro.2019.10.021.
Effect of sex, age, and positivity threshold on fecal immunochemical test accuracy: A systematic review and meta-analysis. Selby K et al. 2019 Dec;(6):1494-505. doi. 10.1053/j.gastro.2019.08.023.
January 2020
How to approach burnout among gastroenterology fellows. DeCross AJ 2020 Jan;158(1):32-5. doi. 10.1053/j.gastro.2019.11.032.
Efficacy and safety of peppermint oil in a randomized, double-blind trial of patients with irritable bowel syndrome. Weerts ZZRM et al. 2020 Jan;158(1):123-36. doi. 10.1053/j.gastro.2019.08.026.
Validation of a machine learning model that outperforms clinical risk scoring systems for upper gastrointestinal bleeding. Shung DL et al. 2020 Jan;158(1):160-7. doi. 10.1053/j.gastro.2019.09.009.
Efficacy and safety of early vs elective colonoscopy for acute lower gastrointestinal bleeding. Niikura R et al. 2020 Jan;158(1):168-75.e6. doi. 10.1053/j.gastro.2019.09.010.
Clinical Gastroenterology and Hepatology
November 2019
Medical professional liability in gastroenterology: Understanding the claims landscape and proposed mechanisms for reform. Adams MA and John I. Allen. 2019 Nov;17(12):2392-6.e1. doi. 10.1016/j.cgh.2019.07.002.
Optimizing use of nonalcoholic fatty liver disease fibrosis score, Fibrosis-4 score, and liver stiffness measurement to identify patients with advanced fibrosis. Chan W-K et al. 2019 Nov;17(12):2570-80.e37. doi. 10.1016/j.cgh.2019.03.006.
December 2019
Clinical and molecular features of post-colonoscopy colorectal cancers. Samadder NJ et al. 2019 Dec;17(12):2731-9.e2. doi. 10.1016/j.cgh.2019.02.040.
Neurologic deficits in patients with newly diagnosed celiac disease are frequent and linked with autoimmunity to transglutaminase 6. Hadjivassiliou M et al. 2019 Dec;17(12):2678-86.e2. doi. 10.1016/j.cgh.2019.03.014.
Increased risk of death in first year after liver transplantation among patients with nonalcoholic steatohepatitis vs liver disease of other etiologies. Nagai S et al. 2019 Dec;17(12):2759-68.e5. doi. 10.1016/j.cgh.2019.04.033.
January 2020
Incorporating high value care into gastroenterology fellowship training. Shah BJ and Janice H. Jou. 2020 Jan;18(1):11-3. doi. 10.1016/j.cgh.2019.10.040.
Association of obesity with colonic diverticulosis in women. Peery AF et al. 2020 Jan;18(1):107-14.e1. doi. 10.1016/j.cgh.2019.04.058.
Endocuff vision reduces inspection time without decreasing lesion detection: A clinical randomized trial. Rex DK et al. 2020 Jan;18(1):158-62.e1 doi. 10.1016/j.cgh.2019.01.015.
Gastroenterology
November 2019
Clip closure after resection of large colorectal lesions with substantial risk of bleeding. Albéniz E et al. 2019 Nov;157(5):1213-21.e4. doi. 10.1053/j.gastro.2019.07.037.
Tumor seeding during colonoscopy as a possible cause for metachronous colorectal cancer. Backes Y et al. 2019 Nov;157(5):1222-32.e4. doi. 10.1053/j.gastro.2019.07.062.
December 2019
How to “DEAL” with disruptive physician behavior. Junga Z et al. 2019 Dec;157(6):1469-72. doi. 10.1053/j.gastro.2019.10.021.
Effect of sex, age, and positivity threshold on fecal immunochemical test accuracy: A systematic review and meta-analysis. Selby K et al. 2019 Dec;(6):1494-505. doi. 10.1053/j.gastro.2019.08.023.
January 2020
How to approach burnout among gastroenterology fellows. DeCross AJ 2020 Jan;158(1):32-5. doi. 10.1053/j.gastro.2019.11.032.
Efficacy and safety of peppermint oil in a randomized, double-blind trial of patients with irritable bowel syndrome. Weerts ZZRM et al. 2020 Jan;158(1):123-36. doi. 10.1053/j.gastro.2019.08.026.
Validation of a machine learning model that outperforms clinical risk scoring systems for upper gastrointestinal bleeding. Shung DL et al. 2020 Jan;158(1):160-7. doi. 10.1053/j.gastro.2019.09.009.
Efficacy and safety of early vs elective colonoscopy for acute lower gastrointestinal bleeding. Niikura R et al. 2020 Jan;158(1):168-75.e6. doi. 10.1053/j.gastro.2019.09.010.
Clinical Gastroenterology and Hepatology
November 2019
Medical professional liability in gastroenterology: Understanding the claims landscape and proposed mechanisms for reform. Adams MA and John I. Allen. 2019 Nov;17(12):2392-6.e1. doi. 10.1016/j.cgh.2019.07.002.
Optimizing use of nonalcoholic fatty liver disease fibrosis score, Fibrosis-4 score, and liver stiffness measurement to identify patients with advanced fibrosis. Chan W-K et al. 2019 Nov;17(12):2570-80.e37. doi. 10.1016/j.cgh.2019.03.006.
December 2019
Clinical and molecular features of post-colonoscopy colorectal cancers. Samadder NJ et al. 2019 Dec;17(12):2731-9.e2. doi. 10.1016/j.cgh.2019.02.040.
Neurologic deficits in patients with newly diagnosed celiac disease are frequent and linked with autoimmunity to transglutaminase 6. Hadjivassiliou M et al. 2019 Dec;17(12):2678-86.e2. doi. 10.1016/j.cgh.2019.03.014.
Increased risk of death in first year after liver transplantation among patients with nonalcoholic steatohepatitis vs liver disease of other etiologies. Nagai S et al. 2019 Dec;17(12):2759-68.e5. doi. 10.1016/j.cgh.2019.04.033.
January 2020
Incorporating high value care into gastroenterology fellowship training. Shah BJ and Janice H. Jou. 2020 Jan;18(1):11-3. doi. 10.1016/j.cgh.2019.10.040.
Association of obesity with colonic diverticulosis in women. Peery AF et al. 2020 Jan;18(1):107-14.e1. doi. 10.1016/j.cgh.2019.04.058.
Endocuff vision reduces inspection time without decreasing lesion detection: A clinical randomized trial. Rex DK et al. 2020 Jan;18(1):158-62.e1 doi. 10.1016/j.cgh.2019.01.015.
Gastroenterology
November 2019
Clip closure after resection of large colorectal lesions with substantial risk of bleeding. Albéniz E et al. 2019 Nov;157(5):1213-21.e4. doi. 10.1053/j.gastro.2019.07.037.
Tumor seeding during colonoscopy as a possible cause for metachronous colorectal cancer. Backes Y et al. 2019 Nov;157(5):1222-32.e4. doi. 10.1053/j.gastro.2019.07.062.
December 2019
How to “DEAL” with disruptive physician behavior. Junga Z et al. 2019 Dec;157(6):1469-72. doi. 10.1053/j.gastro.2019.10.021.
Effect of sex, age, and positivity threshold on fecal immunochemical test accuracy: A systematic review and meta-analysis. Selby K et al. 2019 Dec;(6):1494-505. doi. 10.1053/j.gastro.2019.08.023.
January 2020
How to approach burnout among gastroenterology fellows. DeCross AJ 2020 Jan;158(1):32-5. doi. 10.1053/j.gastro.2019.11.032.
Efficacy and safety of peppermint oil in a randomized, double-blind trial of patients with irritable bowel syndrome. Weerts ZZRM et al. 2020 Jan;158(1):123-36. doi. 10.1053/j.gastro.2019.08.026.
Validation of a machine learning model that outperforms clinical risk scoring systems for upper gastrointestinal bleeding. Shung DL et al. 2020 Jan;158(1):160-7. doi. 10.1053/j.gastro.2019.09.009.
Efficacy and safety of early vs elective colonoscopy for acute lower gastrointestinal bleeding. Niikura R et al. 2020 Jan;158(1):168-75.e6. doi. 10.1053/j.gastro.2019.09.010.
Clinical Gastroenterology and Hepatology
November 2019
Medical professional liability in gastroenterology: Understanding the claims landscape and proposed mechanisms for reform. Adams MA and John I. Allen. 2019 Nov;17(12):2392-6.e1. doi. 10.1016/j.cgh.2019.07.002.
Optimizing use of nonalcoholic fatty liver disease fibrosis score, Fibrosis-4 score, and liver stiffness measurement to identify patients with advanced fibrosis. Chan W-K et al. 2019 Nov;17(12):2570-80.e37. doi. 10.1016/j.cgh.2019.03.006.
December 2019
Clinical and molecular features of post-colonoscopy colorectal cancers. Samadder NJ et al. 2019 Dec;17(12):2731-9.e2. doi. 10.1016/j.cgh.2019.02.040.
Neurologic deficits in patients with newly diagnosed celiac disease are frequent and linked with autoimmunity to transglutaminase 6. Hadjivassiliou M et al. 2019 Dec;17(12):2678-86.e2. doi. 10.1016/j.cgh.2019.03.014.
Increased risk of death in first year after liver transplantation among patients with nonalcoholic steatohepatitis vs liver disease of other etiologies. Nagai S et al. 2019 Dec;17(12):2759-68.e5. doi. 10.1016/j.cgh.2019.04.033.
January 2020
Incorporating high value care into gastroenterology fellowship training. Shah BJ and Janice H. Jou. 2020 Jan;18(1):11-3. doi. 10.1016/j.cgh.2019.10.040.
Association of obesity with colonic diverticulosis in women. Peery AF et al. 2020 Jan;18(1):107-14.e1. doi. 10.1016/j.cgh.2019.04.058.
Endocuff vision reduces inspection time without decreasing lesion detection: A clinical randomized trial. Rex DK et al. 2020 Jan;18(1):158-62.e1 doi. 10.1016/j.cgh.2019.01.015.
AGA News
AGA’s flagship research grant goes to ...
The AGA Research Scholar Award, funded by the AGA Research Foundation, is our premier funding mechanism, providing $100,000 per year for 3 years to early-career faculty working toward independent careers in digestive disease research. Our AGA Research Scholar Award recipients have a proven track record of receiving substantial funding and leadership roles in GI following the receipt of their AGA award. Read about our most recent class of RSA recipients – we’re confident they are future leaders in our field. Learn more about the AGA Research Foundation at www.gastro.org/foundation.
Parambir Dulai, MD
University of California, San Diego
Project title: Development and validation of machine learning optimized predictive models for response to different biologic agents in patients with Crohn’s disease and ulcerative colitis.
Dr. Dulai is using his grant to build and refine a decision-support platform to help providers and patients navigate the complex landscape of choosing between available biologics for the treatment of inflammatory bowel disease (IBD).
Amy Hemperly, DO
University of California, San Diego
AGA-Rady Children’s Institute for Genomic Medicine Research Scholar Award in Pediatric Genomics
Project title: Integration of pharmacogenomics and pharmacometabolomics with pharmacokinetics for biomarker discovery in pediatric inflammatory bowel disease
Dr. Hemperly’s research assesses the influence of genetic variations and metabolic and microbial changes on response to anti–tumor necrosis factor (anti-TNF) therapy in pediatric IBD patients. This work will ultimately elucidate factors that improve a patient’s response to therapy.
Rodney Infante, MD, PhD
University of Texas Southwestern Medical Center, Dallas
Project title: Regulation of gastrointestinal cancer cachexia by a tumor-adipose-hypothalamic axis
Dr. Infante and his lab will use the AGA grant to improve our understanding of the mechanism and clinical relevance of cachexia-associated anorexia and tissue wasting in order to identify effective therapeutic targets.
Suraj Patel, MD, PhD
Massachusetts General Hospital, Boston
Project title: Hepatic IRF3 is a transcriptional regulator of steatosis and insulin resistance in NAFLD
Dr. Patel’s research focuses on the role of innate immunity in cellular metabolism and insulin resistance. Specifically, he’s interested in determining how chronic inflammation fuels the genetic and epigenetic changes we see in overnutritional states such as nonalcoholic fatty liver disease (NAFLD).
Jason Pitarresi, PhD
University of Pennsylvania Health System, Philadelphia
AGA-Bern Schwartz Family Fund Research Scholar Award in Pancreatic Cancer
Project title: PTHLH drives epithelial-to-mesenchymal transition and metastasis in pancreatic cancer
With this funding, Dr. Pitarresi will continue on his quest to identify novel drivers of pancreatic cancer development and metastasis with use of genetically engineered mouse models and patient-derived 3D organoids. Dr. Pitarresi is hoping that anti-PTHLH may fill a treatment void and ultimately increase the quality of life in these patients.
Eric Shah, MD, MBA
Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
AGA-Shire Research Scholar Award in Functional GI and Motility Disorders*
Project title: Office-based anorectal testing to diagnose evacuation disorders and predict outcomes with biofeedback therapy: The rectal expulsion device (RED)
Dr. Shah’s research aims to validate a diagnostic test to triage patients with chronic constipation to the most effective treatment in general GI practice. This work will ultimately help patients with motility and functional bowel conditions and their providers reach a confident diagnosis and understand their treatment options.
*Funded by Shire Plc, now part of Takeda
Shailja Shah, MD, MPH
Vanderbilt University Medical Center, Nashville, Tenn.
Project title: Defining host-specific genetic and non-genetic determinants of Helicobacter pylori eradication failure using a large prospective cohort and genomic biobank
Dr. Shah’s research is focused on personalizing the clinical management of H. pylori such that eradication efforts can be optimized and targeted to the less than 1-3% of the estimated 4.4 billion individuals infected with H. pylori who are most at risk for complications, such as gastric cancer, and avoided in those who are unlikely to benefit and may even experience harm from eradication therapy.
Xiao Tan, MD, PhD
Massachusetts General Hospital, Boston
AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
Project title: Paper-based diagnostics of microbial and host biomarkers to predict responsiveness to IBD therapy
Dr. Tan will develop low-cost, point-of-care microbiome diagnostics to ultimately help physicians’ make diagnoses, monitor, and select treatment for patients with IBD.
Michael Thompson, MD, PhD
Washington University, Saint Louis, Mo.
Project title: Mechanisms of altered bile acid homeostasis and non-alcoholic fatty liver disease in offspring exposed to maternal obesity
Dr. Thompson’s research is focused on how perinatal exposures impact risk for metabolic liver disease in offspring.
My day on Capitol Hill
By Richard K. Sterling, MD, MSC, AGAF
When initially asked to represent AGA on Capitol Hill for the Global Liver Institute (GLI) congressional briefing on liver cancer and the LIVER Act on Oct. 31, I felt both honored and somewhat frightened. Honored that AGA thought enough of me as a hepatologist to represent them and frightened, not because it was Halloween, but because I would be speaking to members of Congress and their staff on issues that may impact policy and thousands if not millions of Americans. Along with myself, were Donna Cryer, founder and CEO of the GLI; John Groopman, PhD, an epidemiologist from Johns Hopkins focusing on liver disease; and two patients with liver disease who had a compelling story to tell. In addition, our briefing and Capitol Hill advocacy day included patients with a history of liver cancer and members of the Hepatitis B Foundation.
In preparation, Andrew Scott from the GLI helped me in identifying the target audience and in developing slides to present to Congress members and their aides that would show those at risk for liver cancer, the increasing incidence of the disease, and the importance of diagnosis at an early stage when curative treatment options are readily available. Travel and hotel logistics were taken care of by Kathleen Teixeira and AGA staff, and it was comforting to see them in the audience.
The briefing took place in the Cannon office building and was standing room only. After a brief introduction by Andrew Scott, I was the first speaker followed by our patient advocates and Dr. Groopman. The LIVER Act (H.R. 3016) is sponsored by Congresswomen Nydia Velazquez (D-NY) and would drive several public health initiatives that would help people of all ages, lifestyles, and ethnic backgrounds to reduce their risk for liver cancer and related illnesses by enhancing the federal government’s prevention, education, and disease surveillance capabilities while empowering local entities to promote treatment and raise awareness. It also supports increased funding to both the Centers for Disease Control and Prevention and the National Institutes of Health for liver disease and liver cancer research.
We had plenty of time for questions from the audience and I saw a lot of nodding from many present acknowledging that they had friends or family who had liver disease. Although our briefing was happening at the same time as the vote on formalizing the impeachment inquiry (you can hear the buzzing going off and the red lights flashing that the vote was about to happen; see Facebook; HepBFoundation video), congressional staff did not leave.
After the meeting, the patient advocates along with members of the GLI, Hepatitis B Foundation, and others met one-on-one with additional members of congress and their staff. While on the train home, I had time to reflect on the day and hoped that our message would be advanced through congress.
AGA, along with our sister societies (American Association for the Study of Liver Diseases and American College of Gastroenterology) are our voice and advocates for advancing legislation through congress. Days like today allow our members to get involved. It is an exciting way to help our congressional representatives take action on what matters most to us: improved patient care, supporting research, promoting education, and reducing the overall burden to accomplish these important goals.
While some say Virginia is for Lovers, I say Virginia is for Livers (#LoveYourLiver). For more on this and the Liver Biliary Council offerings at Digestive Disease Week, follow me on twitter (@RichSterlingMD).
Dr. Sterling is professor of medicine, chief of hepatology, division of gastroenterology, hepatology and nutrition, Virginia Commonwealth University, Richmond; vice-chair, AGA Liver Biliary Section, DDW Council.
New AGA guideline: Management of GIM
AGA released a new clinical practice guideline in Gastroenterology with recommendations for the management of patients with gastric intestinal metaplasia (GIM) detected as part of routine upper endoscopy for reasons including work up of endoscopically identified gastropathy/presumed gastritis, dyspepsia, or exclusion of Helicobacter pylori.
Guideline recommendations
1. In patients with GIM, AGA recommends testing for H. pylori followed by eradication over no testing and eradication. (Strong recommendation: moderate-quality evidence)
2. In patients with GIM, AGA suggests against routine use of endoscopic surveillance. (Conditional recommendation: very-low-quality evidence)
Comment: Patients with GIM at higher risk for gastric cancer who put a high value on potential but uncertain reduction in gastric cancer mortality, and who put a low value on potential risks of surveillance endoscopies, may reasonably elect for surveillance.
Patients with GIM specifically at higher risk of gastric cancer include those with the following:
- Incomplete versus complete GIM.
- Extensive versus limited GIM.
- Family history of gastric cancer.
Patients at overall increased risk for gastric cancer include the following:
- Racial/ethnic minorities.
- Immigrants from high incidence regions.
3. In patients with GIM, AGA suggests against routine repeat short-interval endoscopy with biopsies for the purpose of risk stratification. (Conditional recommendation: very-low-quality evidence)
Comment: Based on shared decision making, patients with GIM and high-risk stigmata, those with concerns about completeness of baseline endoscopy, and/or those who are at overall increased risk for gastric cancer (racial/ethnic minorities, immigrants from regions with high gastric cancer incidence, or individuals with family history of first-degree relative with gastric cancer) may reasonably elect for repeat endoscopy within 1 year for risk stratification.
This guideline will be published in the February print issue of Gastroenterology with additional resources to help you implement in your practice.
A GI society update on MOC reform
Our work was suspended when American Board of Internal Medicine (ABIM) announced the creation of a new longitudinal assessment option for maintenance of certification across all specialties.
GI society leaders are in touch with ABIM. Here’s an update on what we know: The ABIM board of directors committed to evolve its program to provide a longitudinal assessment option for Maintenance of Certification (MOC), offering a self-paced pathway for physicians to acquire and demonstrate ongoing knowledge. The traditional, long-form assessment will also remain an option because some physicians have expressed a preference for a point-in-time exam taken less frequently.
Our next steps include seeking clarity from ABIM including the following:
1. The milestones in the process to create the new pathway.
2. When the new pathway will be available to diplomates.
3. Consideration and integration of the GI societies’ principles in the development of the new pathway for recertification, including these considerations:
- MOC needs to be simpler, less intrusive, and less expensive.
- We continue to support alternatives to the high-stakes, every-10-year recertification exam.
- We do not support single source or time-limited assessments because they do not represent the current realities of medicine in the digital age.
- We support the concept that, for the many diplomates who specialize within certain areas of gastroenterology and hepatology, MOC should not include high-stakes assessments of areas in which the diplomate may not practice.
- We support the principles of lifelong learning, as evidenced by ongoing CME activities, rather than lifelong testing.
4. The role the GI societies, as representatives for thousands of U.S. members who are ABIM diplomates, play in the creation and implementation of the new pathway.
AASLD, ACG, AGA, and American Society for Gastrointestinal Endoscopy want to be fully informed and fully respected partners in an endeavor that touches upon one of the toughest challenges facing our members and the single issue we hear about most often requesting our help.
We will continue to update our members as we learn the answers to these questions from ABIM.
Together, our first priority on the MOC issue remains ensuring that GI diplomates have a pathway for recertification that meets your needs.
Watch your step (therapy) – understanding ‘fail first’
Sometimes known as “fail first,” step therapy is a tool used by insurance companies that requires patients to fail medications before agreeing to cover a health care provider’s initial treatment recommendation.
Often affecting patients with inflammatory bowel disease (IBD), step therapy focuses on the use of insurer-preferred treatments rather than effective, patient-centric therapies. In addition to causing many patient hardships, this protocol allows insurance companies to come between the provider-patient relationship and dictate a patient’s course of treatment.
To help clinicians navigate this challenging landscape, AGA is pleased to offer a new step therapy webpage that details the step therapy protocol and opportunities to advocate for patient protections.
Additional education modules – including videos, podcasts and other resources – are also available for several states that have implemented safe step therapy laws, including Illinois, New York, and Texas.
Visit the Navigating State Step Therapy Laws program page to learn more about the following:
- What is the step therapy protocol?
- How does step therapy impact a health care provider’s ability to provide patient care?
- Which states have implemented step therapy laws?
- How do state step therapy laws provide physician rights and patient protection?
- Tips to share with your patients.
- What are AGA’s advocacy efforts – and how can I help?
Education modules for additional states will be available in early 2020.
AGA’s Navigating State Step Therapy Laws program is funded by an unrestricted educational grant from Takeda and Pfizer.
AGA’s flagship research grant goes to ...
The AGA Research Scholar Award, funded by the AGA Research Foundation, is our premier funding mechanism, providing $100,000 per year for 3 years to early-career faculty working toward independent careers in digestive disease research. Our AGA Research Scholar Award recipients have a proven track record of receiving substantial funding and leadership roles in GI following the receipt of their AGA award. Read about our most recent class of RSA recipients – we’re confident they are future leaders in our field. Learn more about the AGA Research Foundation at www.gastro.org/foundation.
Parambir Dulai, MD
University of California, San Diego
Project title: Development and validation of machine learning optimized predictive models for response to different biologic agents in patients with Crohn’s disease and ulcerative colitis.
Dr. Dulai is using his grant to build and refine a decision-support platform to help providers and patients navigate the complex landscape of choosing between available biologics for the treatment of inflammatory bowel disease (IBD).
Amy Hemperly, DO
University of California, San Diego
AGA-Rady Children’s Institute for Genomic Medicine Research Scholar Award in Pediatric Genomics
Project title: Integration of pharmacogenomics and pharmacometabolomics with pharmacokinetics for biomarker discovery in pediatric inflammatory bowel disease
Dr. Hemperly’s research assesses the influence of genetic variations and metabolic and microbial changes on response to anti–tumor necrosis factor (anti-TNF) therapy in pediatric IBD patients. This work will ultimately elucidate factors that improve a patient’s response to therapy.
Rodney Infante, MD, PhD
University of Texas Southwestern Medical Center, Dallas
Project title: Regulation of gastrointestinal cancer cachexia by a tumor-adipose-hypothalamic axis
Dr. Infante and his lab will use the AGA grant to improve our understanding of the mechanism and clinical relevance of cachexia-associated anorexia and tissue wasting in order to identify effective therapeutic targets.
Suraj Patel, MD, PhD
Massachusetts General Hospital, Boston
Project title: Hepatic IRF3 is a transcriptional regulator of steatosis and insulin resistance in NAFLD
Dr. Patel’s research focuses on the role of innate immunity in cellular metabolism and insulin resistance. Specifically, he’s interested in determining how chronic inflammation fuels the genetic and epigenetic changes we see in overnutritional states such as nonalcoholic fatty liver disease (NAFLD).
Jason Pitarresi, PhD
University of Pennsylvania Health System, Philadelphia
AGA-Bern Schwartz Family Fund Research Scholar Award in Pancreatic Cancer
Project title: PTHLH drives epithelial-to-mesenchymal transition and metastasis in pancreatic cancer
With this funding, Dr. Pitarresi will continue on his quest to identify novel drivers of pancreatic cancer development and metastasis with use of genetically engineered mouse models and patient-derived 3D organoids. Dr. Pitarresi is hoping that anti-PTHLH may fill a treatment void and ultimately increase the quality of life in these patients.
Eric Shah, MD, MBA
Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
AGA-Shire Research Scholar Award in Functional GI and Motility Disorders*
Project title: Office-based anorectal testing to diagnose evacuation disorders and predict outcomes with biofeedback therapy: The rectal expulsion device (RED)
Dr. Shah’s research aims to validate a diagnostic test to triage patients with chronic constipation to the most effective treatment in general GI practice. This work will ultimately help patients with motility and functional bowel conditions and their providers reach a confident diagnosis and understand their treatment options.
*Funded by Shire Plc, now part of Takeda
Shailja Shah, MD, MPH
Vanderbilt University Medical Center, Nashville, Tenn.
Project title: Defining host-specific genetic and non-genetic determinants of Helicobacter pylori eradication failure using a large prospective cohort and genomic biobank
Dr. Shah’s research is focused on personalizing the clinical management of H. pylori such that eradication efforts can be optimized and targeted to the less than 1-3% of the estimated 4.4 billion individuals infected with H. pylori who are most at risk for complications, such as gastric cancer, and avoided in those who are unlikely to benefit and may even experience harm from eradication therapy.
Xiao Tan, MD, PhD
Massachusetts General Hospital, Boston
AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
Project title: Paper-based diagnostics of microbial and host biomarkers to predict responsiveness to IBD therapy
Dr. Tan will develop low-cost, point-of-care microbiome diagnostics to ultimately help physicians’ make diagnoses, monitor, and select treatment for patients with IBD.
Michael Thompson, MD, PhD
Washington University, Saint Louis, Mo.
Project title: Mechanisms of altered bile acid homeostasis and non-alcoholic fatty liver disease in offspring exposed to maternal obesity
Dr. Thompson’s research is focused on how perinatal exposures impact risk for metabolic liver disease in offspring.
My day on Capitol Hill
By Richard K. Sterling, MD, MSC, AGAF
When initially asked to represent AGA on Capitol Hill for the Global Liver Institute (GLI) congressional briefing on liver cancer and the LIVER Act on Oct. 31, I felt both honored and somewhat frightened. Honored that AGA thought enough of me as a hepatologist to represent them and frightened, not because it was Halloween, but because I would be speaking to members of Congress and their staff on issues that may impact policy and thousands if not millions of Americans. Along with myself, were Donna Cryer, founder and CEO of the GLI; John Groopman, PhD, an epidemiologist from Johns Hopkins focusing on liver disease; and two patients with liver disease who had a compelling story to tell. In addition, our briefing and Capitol Hill advocacy day included patients with a history of liver cancer and members of the Hepatitis B Foundation.
In preparation, Andrew Scott from the GLI helped me in identifying the target audience and in developing slides to present to Congress members and their aides that would show those at risk for liver cancer, the increasing incidence of the disease, and the importance of diagnosis at an early stage when curative treatment options are readily available. Travel and hotel logistics were taken care of by Kathleen Teixeira and AGA staff, and it was comforting to see them in the audience.
The briefing took place in the Cannon office building and was standing room only. After a brief introduction by Andrew Scott, I was the first speaker followed by our patient advocates and Dr. Groopman. The LIVER Act (H.R. 3016) is sponsored by Congresswomen Nydia Velazquez (D-NY) and would drive several public health initiatives that would help people of all ages, lifestyles, and ethnic backgrounds to reduce their risk for liver cancer and related illnesses by enhancing the federal government’s prevention, education, and disease surveillance capabilities while empowering local entities to promote treatment and raise awareness. It also supports increased funding to both the Centers for Disease Control and Prevention and the National Institutes of Health for liver disease and liver cancer research.
We had plenty of time for questions from the audience and I saw a lot of nodding from many present acknowledging that they had friends or family who had liver disease. Although our briefing was happening at the same time as the vote on formalizing the impeachment inquiry (you can hear the buzzing going off and the red lights flashing that the vote was about to happen; see Facebook; HepBFoundation video), congressional staff did not leave.
After the meeting, the patient advocates along with members of the GLI, Hepatitis B Foundation, and others met one-on-one with additional members of congress and their staff. While on the train home, I had time to reflect on the day and hoped that our message would be advanced through congress.
AGA, along with our sister societies (American Association for the Study of Liver Diseases and American College of Gastroenterology) are our voice and advocates for advancing legislation through congress. Days like today allow our members to get involved. It is an exciting way to help our congressional representatives take action on what matters most to us: improved patient care, supporting research, promoting education, and reducing the overall burden to accomplish these important goals.
While some say Virginia is for Lovers, I say Virginia is for Livers (#LoveYourLiver). For more on this and the Liver Biliary Council offerings at Digestive Disease Week, follow me on twitter (@RichSterlingMD).
Dr. Sterling is professor of medicine, chief of hepatology, division of gastroenterology, hepatology and nutrition, Virginia Commonwealth University, Richmond; vice-chair, AGA Liver Biliary Section, DDW Council.
New AGA guideline: Management of GIM
AGA released a new clinical practice guideline in Gastroenterology with recommendations for the management of patients with gastric intestinal metaplasia (GIM) detected as part of routine upper endoscopy for reasons including work up of endoscopically identified gastropathy/presumed gastritis, dyspepsia, or exclusion of Helicobacter pylori.
Guideline recommendations
1. In patients with GIM, AGA recommends testing for H. pylori followed by eradication over no testing and eradication. (Strong recommendation: moderate-quality evidence)
2. In patients with GIM, AGA suggests against routine use of endoscopic surveillance. (Conditional recommendation: very-low-quality evidence)
Comment: Patients with GIM at higher risk for gastric cancer who put a high value on potential but uncertain reduction in gastric cancer mortality, and who put a low value on potential risks of surveillance endoscopies, may reasonably elect for surveillance.
Patients with GIM specifically at higher risk of gastric cancer include those with the following:
- Incomplete versus complete GIM.
- Extensive versus limited GIM.
- Family history of gastric cancer.
Patients at overall increased risk for gastric cancer include the following:
- Racial/ethnic minorities.
- Immigrants from high incidence regions.
3. In patients with GIM, AGA suggests against routine repeat short-interval endoscopy with biopsies for the purpose of risk stratification. (Conditional recommendation: very-low-quality evidence)
Comment: Based on shared decision making, patients with GIM and high-risk stigmata, those with concerns about completeness of baseline endoscopy, and/or those who are at overall increased risk for gastric cancer (racial/ethnic minorities, immigrants from regions with high gastric cancer incidence, or individuals with family history of first-degree relative with gastric cancer) may reasonably elect for repeat endoscopy within 1 year for risk stratification.
This guideline will be published in the February print issue of Gastroenterology with additional resources to help you implement in your practice.
A GI society update on MOC reform
Our work was suspended when American Board of Internal Medicine (ABIM) announced the creation of a new longitudinal assessment option for maintenance of certification across all specialties.
GI society leaders are in touch with ABIM. Here’s an update on what we know: The ABIM board of directors committed to evolve its program to provide a longitudinal assessment option for Maintenance of Certification (MOC), offering a self-paced pathway for physicians to acquire and demonstrate ongoing knowledge. The traditional, long-form assessment will also remain an option because some physicians have expressed a preference for a point-in-time exam taken less frequently.
Our next steps include seeking clarity from ABIM including the following:
1. The milestones in the process to create the new pathway.
2. When the new pathway will be available to diplomates.
3. Consideration and integration of the GI societies’ principles in the development of the new pathway for recertification, including these considerations:
- MOC needs to be simpler, less intrusive, and less expensive.
- We continue to support alternatives to the high-stakes, every-10-year recertification exam.
- We do not support single source or time-limited assessments because they do not represent the current realities of medicine in the digital age.
- We support the concept that, for the many diplomates who specialize within certain areas of gastroenterology and hepatology, MOC should not include high-stakes assessments of areas in which the diplomate may not practice.
- We support the principles of lifelong learning, as evidenced by ongoing CME activities, rather than lifelong testing.
4. The role the GI societies, as representatives for thousands of U.S. members who are ABIM diplomates, play in the creation and implementation of the new pathway.
AASLD, ACG, AGA, and American Society for Gastrointestinal Endoscopy want to be fully informed and fully respected partners in an endeavor that touches upon one of the toughest challenges facing our members and the single issue we hear about most often requesting our help.
We will continue to update our members as we learn the answers to these questions from ABIM.
Together, our first priority on the MOC issue remains ensuring that GI diplomates have a pathway for recertification that meets your needs.
Watch your step (therapy) – understanding ‘fail first’
Sometimes known as “fail first,” step therapy is a tool used by insurance companies that requires patients to fail medications before agreeing to cover a health care provider’s initial treatment recommendation.
Often affecting patients with inflammatory bowel disease (IBD), step therapy focuses on the use of insurer-preferred treatments rather than effective, patient-centric therapies. In addition to causing many patient hardships, this protocol allows insurance companies to come between the provider-patient relationship and dictate a patient’s course of treatment.
To help clinicians navigate this challenging landscape, AGA is pleased to offer a new step therapy webpage that details the step therapy protocol and opportunities to advocate for patient protections.
Additional education modules – including videos, podcasts and other resources – are also available for several states that have implemented safe step therapy laws, including Illinois, New York, and Texas.
Visit the Navigating State Step Therapy Laws program page to learn more about the following:
- What is the step therapy protocol?
- How does step therapy impact a health care provider’s ability to provide patient care?
- Which states have implemented step therapy laws?
- How do state step therapy laws provide physician rights and patient protection?
- Tips to share with your patients.
- What are AGA’s advocacy efforts – and how can I help?
Education modules for additional states will be available in early 2020.
AGA’s Navigating State Step Therapy Laws program is funded by an unrestricted educational grant from Takeda and Pfizer.
AGA’s flagship research grant goes to ...
The AGA Research Scholar Award, funded by the AGA Research Foundation, is our premier funding mechanism, providing $100,000 per year for 3 years to early-career faculty working toward independent careers in digestive disease research. Our AGA Research Scholar Award recipients have a proven track record of receiving substantial funding and leadership roles in GI following the receipt of their AGA award. Read about our most recent class of RSA recipients – we’re confident they are future leaders in our field. Learn more about the AGA Research Foundation at www.gastro.org/foundation.
Parambir Dulai, MD
University of California, San Diego
Project title: Development and validation of machine learning optimized predictive models for response to different biologic agents in patients with Crohn’s disease and ulcerative colitis.
Dr. Dulai is using his grant to build and refine a decision-support platform to help providers and patients navigate the complex landscape of choosing between available biologics for the treatment of inflammatory bowel disease (IBD).
Amy Hemperly, DO
University of California, San Diego
AGA-Rady Children’s Institute for Genomic Medicine Research Scholar Award in Pediatric Genomics
Project title: Integration of pharmacogenomics and pharmacometabolomics with pharmacokinetics for biomarker discovery in pediatric inflammatory bowel disease
Dr. Hemperly’s research assesses the influence of genetic variations and metabolic and microbial changes on response to anti–tumor necrosis factor (anti-TNF) therapy in pediatric IBD patients. This work will ultimately elucidate factors that improve a patient’s response to therapy.
Rodney Infante, MD, PhD
University of Texas Southwestern Medical Center, Dallas
Project title: Regulation of gastrointestinal cancer cachexia by a tumor-adipose-hypothalamic axis
Dr. Infante and his lab will use the AGA grant to improve our understanding of the mechanism and clinical relevance of cachexia-associated anorexia and tissue wasting in order to identify effective therapeutic targets.
Suraj Patel, MD, PhD
Massachusetts General Hospital, Boston
Project title: Hepatic IRF3 is a transcriptional regulator of steatosis and insulin resistance in NAFLD
Dr. Patel’s research focuses on the role of innate immunity in cellular metabolism and insulin resistance. Specifically, he’s interested in determining how chronic inflammation fuels the genetic and epigenetic changes we see in overnutritional states such as nonalcoholic fatty liver disease (NAFLD).
Jason Pitarresi, PhD
University of Pennsylvania Health System, Philadelphia
AGA-Bern Schwartz Family Fund Research Scholar Award in Pancreatic Cancer
Project title: PTHLH drives epithelial-to-mesenchymal transition and metastasis in pancreatic cancer
With this funding, Dr. Pitarresi will continue on his quest to identify novel drivers of pancreatic cancer development and metastasis with use of genetically engineered mouse models and patient-derived 3D organoids. Dr. Pitarresi is hoping that anti-PTHLH may fill a treatment void and ultimately increase the quality of life in these patients.
Eric Shah, MD, MBA
Dartmouth-Hitchcock Medical Center, Lebanon, N.H.
AGA-Shire Research Scholar Award in Functional GI and Motility Disorders*
Project title: Office-based anorectal testing to diagnose evacuation disorders and predict outcomes with biofeedback therapy: The rectal expulsion device (RED)
Dr. Shah’s research aims to validate a diagnostic test to triage patients with chronic constipation to the most effective treatment in general GI practice. This work will ultimately help patients with motility and functional bowel conditions and their providers reach a confident diagnosis and understand their treatment options.
*Funded by Shire Plc, now part of Takeda
Shailja Shah, MD, MPH
Vanderbilt University Medical Center, Nashville, Tenn.
Project title: Defining host-specific genetic and non-genetic determinants of Helicobacter pylori eradication failure using a large prospective cohort and genomic biobank
Dr. Shah’s research is focused on personalizing the clinical management of H. pylori such that eradication efforts can be optimized and targeted to the less than 1-3% of the estimated 4.4 billion individuals infected with H. pylori who are most at risk for complications, such as gastric cancer, and avoided in those who are unlikely to benefit and may even experience harm from eradication therapy.
Xiao Tan, MD, PhD
Massachusetts General Hospital, Boston
AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
Project title: Paper-based diagnostics of microbial and host biomarkers to predict responsiveness to IBD therapy
Dr. Tan will develop low-cost, point-of-care microbiome diagnostics to ultimately help physicians’ make diagnoses, monitor, and select treatment for patients with IBD.
Michael Thompson, MD, PhD
Washington University, Saint Louis, Mo.
Project title: Mechanisms of altered bile acid homeostasis and non-alcoholic fatty liver disease in offspring exposed to maternal obesity
Dr. Thompson’s research is focused on how perinatal exposures impact risk for metabolic liver disease in offspring.
My day on Capitol Hill
By Richard K. Sterling, MD, MSC, AGAF
When initially asked to represent AGA on Capitol Hill for the Global Liver Institute (GLI) congressional briefing on liver cancer and the LIVER Act on Oct. 31, I felt both honored and somewhat frightened. Honored that AGA thought enough of me as a hepatologist to represent them and frightened, not because it was Halloween, but because I would be speaking to members of Congress and their staff on issues that may impact policy and thousands if not millions of Americans. Along with myself, were Donna Cryer, founder and CEO of the GLI; John Groopman, PhD, an epidemiologist from Johns Hopkins focusing on liver disease; and two patients with liver disease who had a compelling story to tell. In addition, our briefing and Capitol Hill advocacy day included patients with a history of liver cancer and members of the Hepatitis B Foundation.
In preparation, Andrew Scott from the GLI helped me in identifying the target audience and in developing slides to present to Congress members and their aides that would show those at risk for liver cancer, the increasing incidence of the disease, and the importance of diagnosis at an early stage when curative treatment options are readily available. Travel and hotel logistics were taken care of by Kathleen Teixeira and AGA staff, and it was comforting to see them in the audience.
The briefing took place in the Cannon office building and was standing room only. After a brief introduction by Andrew Scott, I was the first speaker followed by our patient advocates and Dr. Groopman. The LIVER Act (H.R. 3016) is sponsored by Congresswomen Nydia Velazquez (D-NY) and would drive several public health initiatives that would help people of all ages, lifestyles, and ethnic backgrounds to reduce their risk for liver cancer and related illnesses by enhancing the federal government’s prevention, education, and disease surveillance capabilities while empowering local entities to promote treatment and raise awareness. It also supports increased funding to both the Centers for Disease Control and Prevention and the National Institutes of Health for liver disease and liver cancer research.
We had plenty of time for questions from the audience and I saw a lot of nodding from many present acknowledging that they had friends or family who had liver disease. Although our briefing was happening at the same time as the vote on formalizing the impeachment inquiry (you can hear the buzzing going off and the red lights flashing that the vote was about to happen; see Facebook; HepBFoundation video), congressional staff did not leave.
After the meeting, the patient advocates along with members of the GLI, Hepatitis B Foundation, and others met one-on-one with additional members of congress and their staff. While on the train home, I had time to reflect on the day and hoped that our message would be advanced through congress.
AGA, along with our sister societies (American Association for the Study of Liver Diseases and American College of Gastroenterology) are our voice and advocates for advancing legislation through congress. Days like today allow our members to get involved. It is an exciting way to help our congressional representatives take action on what matters most to us: improved patient care, supporting research, promoting education, and reducing the overall burden to accomplish these important goals.
While some say Virginia is for Lovers, I say Virginia is for Livers (#LoveYourLiver). For more on this and the Liver Biliary Council offerings at Digestive Disease Week, follow me on twitter (@RichSterlingMD).
Dr. Sterling is professor of medicine, chief of hepatology, division of gastroenterology, hepatology and nutrition, Virginia Commonwealth University, Richmond; vice-chair, AGA Liver Biliary Section, DDW Council.
New AGA guideline: Management of GIM
AGA released a new clinical practice guideline in Gastroenterology with recommendations for the management of patients with gastric intestinal metaplasia (GIM) detected as part of routine upper endoscopy for reasons including work up of endoscopically identified gastropathy/presumed gastritis, dyspepsia, or exclusion of Helicobacter pylori.
Guideline recommendations
1. In patients with GIM, AGA recommends testing for H. pylori followed by eradication over no testing and eradication. (Strong recommendation: moderate-quality evidence)
2. In patients with GIM, AGA suggests against routine use of endoscopic surveillance. (Conditional recommendation: very-low-quality evidence)
Comment: Patients with GIM at higher risk for gastric cancer who put a high value on potential but uncertain reduction in gastric cancer mortality, and who put a low value on potential risks of surveillance endoscopies, may reasonably elect for surveillance.
Patients with GIM specifically at higher risk of gastric cancer include those with the following:
- Incomplete versus complete GIM.
- Extensive versus limited GIM.
- Family history of gastric cancer.
Patients at overall increased risk for gastric cancer include the following:
- Racial/ethnic minorities.
- Immigrants from high incidence regions.
3. In patients with GIM, AGA suggests against routine repeat short-interval endoscopy with biopsies for the purpose of risk stratification. (Conditional recommendation: very-low-quality evidence)
Comment: Based on shared decision making, patients with GIM and high-risk stigmata, those with concerns about completeness of baseline endoscopy, and/or those who are at overall increased risk for gastric cancer (racial/ethnic minorities, immigrants from regions with high gastric cancer incidence, or individuals with family history of first-degree relative with gastric cancer) may reasonably elect for repeat endoscopy within 1 year for risk stratification.
This guideline will be published in the February print issue of Gastroenterology with additional resources to help you implement in your practice.
A GI society update on MOC reform
Our work was suspended when American Board of Internal Medicine (ABIM) announced the creation of a new longitudinal assessment option for maintenance of certification across all specialties.
GI society leaders are in touch with ABIM. Here’s an update on what we know: The ABIM board of directors committed to evolve its program to provide a longitudinal assessment option for Maintenance of Certification (MOC), offering a self-paced pathway for physicians to acquire and demonstrate ongoing knowledge. The traditional, long-form assessment will also remain an option because some physicians have expressed a preference for a point-in-time exam taken less frequently.
Our next steps include seeking clarity from ABIM including the following:
1. The milestones in the process to create the new pathway.
2. When the new pathway will be available to diplomates.
3. Consideration and integration of the GI societies’ principles in the development of the new pathway for recertification, including these considerations:
- MOC needs to be simpler, less intrusive, and less expensive.
- We continue to support alternatives to the high-stakes, every-10-year recertification exam.
- We do not support single source or time-limited assessments because they do not represent the current realities of medicine in the digital age.
- We support the concept that, for the many diplomates who specialize within certain areas of gastroenterology and hepatology, MOC should not include high-stakes assessments of areas in which the diplomate may not practice.
- We support the principles of lifelong learning, as evidenced by ongoing CME activities, rather than lifelong testing.
4. The role the GI societies, as representatives for thousands of U.S. members who are ABIM diplomates, play in the creation and implementation of the new pathway.
AASLD, ACG, AGA, and American Society for Gastrointestinal Endoscopy want to be fully informed and fully respected partners in an endeavor that touches upon one of the toughest challenges facing our members and the single issue we hear about most often requesting our help.
We will continue to update our members as we learn the answers to these questions from ABIM.
Together, our first priority on the MOC issue remains ensuring that GI diplomates have a pathway for recertification that meets your needs.
Watch your step (therapy) – understanding ‘fail first’
Sometimes known as “fail first,” step therapy is a tool used by insurance companies that requires patients to fail medications before agreeing to cover a health care provider’s initial treatment recommendation.
Often affecting patients with inflammatory bowel disease (IBD), step therapy focuses on the use of insurer-preferred treatments rather than effective, patient-centric therapies. In addition to causing many patient hardships, this protocol allows insurance companies to come between the provider-patient relationship and dictate a patient’s course of treatment.
To help clinicians navigate this challenging landscape, AGA is pleased to offer a new step therapy webpage that details the step therapy protocol and opportunities to advocate for patient protections.
Additional education modules – including videos, podcasts and other resources – are also available for several states that have implemented safe step therapy laws, including Illinois, New York, and Texas.
Visit the Navigating State Step Therapy Laws program page to learn more about the following:
- What is the step therapy protocol?
- How does step therapy impact a health care provider’s ability to provide patient care?
- Which states have implemented step therapy laws?
- How do state step therapy laws provide physician rights and patient protection?
- Tips to share with your patients.
- What are AGA’s advocacy efforts – and how can I help?
Education modules for additional states will be available in early 2020.
AGA’s Navigating State Step Therapy Laws program is funded by an unrestricted educational grant from Takeda and Pfizer.
Calendar
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Feb 20; Mar. 24, 2020
Coding and Reimbursement Solutions by McVey Associates, Inc.
Improve the efficiency and performance of your practice by staying current on the latest reimbursement, coding, and compliance changes.
Knoxville, Tenn. (2/20); Birmingham, Ala. (3/24)
Mar. 7-8, 2020
Gut Microbiota for Health World Summit 2020
The focus of the 2020 program will include dietary and nondietary factors shaping the gut microbiome, the microbiome as orchestrator for the immune system, and drug interactions and the microbiome. The summit is sponsored by the European Society for Neurogastroenterology & Motility and the American Gastroenterological Association.
Madrid, Spain
Mar. 10-11; 11-12; 25-26; Apr. 15-16; May 13-14, 2020
Two-Day, In-Depth Coding Seminar by McVey Associates, Inc.
Become a certified GI coder with a two-day, in-depth training course provided by McVey Associates, Inc.
Orlando, Fla. (3/10-11); Novi, Mich. (3/11-12); Charlotte, N.C. (3/25-26); Columbus, Ohio (4/15-16); Chicago, Ill. (5/13-14)
Mar. 21; Apr. 15, 2020
Regional Practice Skills Workshop
AGA Regional Practice Skills workshops are free in-person, half-day courses that provide trainees and early-career gastroenterologists with practical insights about GI business issues that will shape their professional development. Faculty will discuss employment models, reimbursement strategies, health economics and policy, billing issues, contract negotiations, and other subjects to help attendees navigate the rapidly shifting GI business environment. All workshops are open to both members and nonmembers.
Ann Arbor, Mich. (3/21); Philadelphia, Penn. (4/15)
May 2-5, 2020
Digestive Disease Week® (DDW)
Digestive Disease Week® (DDW) is the world’s leading educational forum for academicians, clinicians, researchers, students, and trainees working in gastroenterology, hepatology, GI endoscopy, gastrointestinal surgery, and related fields. Whether you work in patient care, research, education, or administration, the DDW program offers something for you.
Chicago, Ill.
May 2-3, 2020
2020 AGA Postgraduate Course
The AGA Postgraduate Course is a comprehensive 1.5-day program highlighting groundbreaking advances in the delivery of high-quality, patient-centered GI care. Offering general and breakout sessions, learning lunches, and case-based and panel discussions, attendees will gain a deeper understanding of how to diagnose and treat a variety of disease states and digestive disorders.
June 3-6, 2020
2020 AGA Tech Summit
Visit https://techsummit.gastro.org/ for more details.
San Francisco, Calif.
Aug. 14-15, 2020
James W. Freston Single-Topic Conference: Gastrointestinal Organoids and Engineered Organ Systems
The 2020 Freston Conference will focus on GI organoids and engineered organ systems.
Chicago, Ill.
Aug. 14-16, 2020
2020 Principles of GI for the NP and PA
Principles of GI is designed by leading advanced practice providers, GI experts, and physicians to mirror real-life settings for nurse practitioners and physician assistants that lead to GI clinical success.
Denver, Colo.
AWARDS DEADLINES
AGA Fellow Abstract Award
This $500 travel award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Fellow Abstract of the Year and receive a $1,000 award.
Application Deadline: Feb. 26, 2020
AGA Student Abstract Award
This $500 travel award supports recipients who are graduate students, medical students, or medical residents (residents up to postgraduate year three) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW).
Application Deadline: Feb. 26, 2020
AGA-Moti L. & Kamla Rustgi International Travel Awards
This $750 travel award supports recipients who are young (i.e., 35 years of age or younger at the time of DDW) basic, translational, or clinical investigators residing outside North America to support travel and related expenses to attend Digestive Disease Week® (DDW).
Application Deadline: Feb. 26, 2020
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Feb 20; Mar. 24, 2020
Coding and Reimbursement Solutions by McVey Associates, Inc.
Improve the efficiency and performance of your practice by staying current on the latest reimbursement, coding, and compliance changes.
Knoxville, Tenn. (2/20); Birmingham, Ala. (3/24)
Mar. 7-8, 2020
Gut Microbiota for Health World Summit 2020
The focus of the 2020 program will include dietary and nondietary factors shaping the gut microbiome, the microbiome as orchestrator for the immune system, and drug interactions and the microbiome. The summit is sponsored by the European Society for Neurogastroenterology & Motility and the American Gastroenterological Association.
Madrid, Spain
Mar. 10-11; 11-12; 25-26; Apr. 15-16; May 13-14, 2020
Two-Day, In-Depth Coding Seminar by McVey Associates, Inc.
Become a certified GI coder with a two-day, in-depth training course provided by McVey Associates, Inc.
Orlando, Fla. (3/10-11); Novi, Mich. (3/11-12); Charlotte, N.C. (3/25-26); Columbus, Ohio (4/15-16); Chicago, Ill. (5/13-14)
Mar. 21; Apr. 15, 2020
Regional Practice Skills Workshop
AGA Regional Practice Skills workshops are free in-person, half-day courses that provide trainees and early-career gastroenterologists with practical insights about GI business issues that will shape their professional development. Faculty will discuss employment models, reimbursement strategies, health economics and policy, billing issues, contract negotiations, and other subjects to help attendees navigate the rapidly shifting GI business environment. All workshops are open to both members and nonmembers.
Ann Arbor, Mich. (3/21); Philadelphia, Penn. (4/15)
May 2-5, 2020
Digestive Disease Week® (DDW)
Digestive Disease Week® (DDW) is the world’s leading educational forum for academicians, clinicians, researchers, students, and trainees working in gastroenterology, hepatology, GI endoscopy, gastrointestinal surgery, and related fields. Whether you work in patient care, research, education, or administration, the DDW program offers something for you.
Chicago, Ill.
May 2-3, 2020
2020 AGA Postgraduate Course
The AGA Postgraduate Course is a comprehensive 1.5-day program highlighting groundbreaking advances in the delivery of high-quality, patient-centered GI care. Offering general and breakout sessions, learning lunches, and case-based and panel discussions, attendees will gain a deeper understanding of how to diagnose and treat a variety of disease states and digestive disorders.
June 3-6, 2020
2020 AGA Tech Summit
Visit https://techsummit.gastro.org/ for more details.
San Francisco, Calif.
Aug. 14-15, 2020
James W. Freston Single-Topic Conference: Gastrointestinal Organoids and Engineered Organ Systems
The 2020 Freston Conference will focus on GI organoids and engineered organ systems.
Chicago, Ill.
Aug. 14-16, 2020
2020 Principles of GI for the NP and PA
Principles of GI is designed by leading advanced practice providers, GI experts, and physicians to mirror real-life settings for nurse practitioners and physician assistants that lead to GI clinical success.
Denver, Colo.
AWARDS DEADLINES
AGA Fellow Abstract Award
This $500 travel award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Fellow Abstract of the Year and receive a $1,000 award.
Application Deadline: Feb. 26, 2020
AGA Student Abstract Award
This $500 travel award supports recipients who are graduate students, medical students, or medical residents (residents up to postgraduate year three) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW).
Application Deadline: Feb. 26, 2020
AGA-Moti L. & Kamla Rustgi International Travel Awards
This $750 travel award supports recipients who are young (i.e., 35 years of age or younger at the time of DDW) basic, translational, or clinical investigators residing outside North America to support travel and related expenses to attend Digestive Disease Week® (DDW).
Application Deadline: Feb. 26, 2020
For more information about upcoming events and award deadlines, please visit http://agau.gastro.org and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Feb 20; Mar. 24, 2020
Coding and Reimbursement Solutions by McVey Associates, Inc.
Improve the efficiency and performance of your practice by staying current on the latest reimbursement, coding, and compliance changes.
Knoxville, Tenn. (2/20); Birmingham, Ala. (3/24)
Mar. 7-8, 2020
Gut Microbiota for Health World Summit 2020
The focus of the 2020 program will include dietary and nondietary factors shaping the gut microbiome, the microbiome as orchestrator for the immune system, and drug interactions and the microbiome. The summit is sponsored by the European Society for Neurogastroenterology & Motility and the American Gastroenterological Association.
Madrid, Spain
Mar. 10-11; 11-12; 25-26; Apr. 15-16; May 13-14, 2020
Two-Day, In-Depth Coding Seminar by McVey Associates, Inc.
Become a certified GI coder with a two-day, in-depth training course provided by McVey Associates, Inc.
Orlando, Fla. (3/10-11); Novi, Mich. (3/11-12); Charlotte, N.C. (3/25-26); Columbus, Ohio (4/15-16); Chicago, Ill. (5/13-14)
Mar. 21; Apr. 15, 2020
Regional Practice Skills Workshop
AGA Regional Practice Skills workshops are free in-person, half-day courses that provide trainees and early-career gastroenterologists with practical insights about GI business issues that will shape their professional development. Faculty will discuss employment models, reimbursement strategies, health economics and policy, billing issues, contract negotiations, and other subjects to help attendees navigate the rapidly shifting GI business environment. All workshops are open to both members and nonmembers.
Ann Arbor, Mich. (3/21); Philadelphia, Penn. (4/15)
May 2-5, 2020
Digestive Disease Week® (DDW)
Digestive Disease Week® (DDW) is the world’s leading educational forum for academicians, clinicians, researchers, students, and trainees working in gastroenterology, hepatology, GI endoscopy, gastrointestinal surgery, and related fields. Whether you work in patient care, research, education, or administration, the DDW program offers something for you.
Chicago, Ill.
May 2-3, 2020
2020 AGA Postgraduate Course
The AGA Postgraduate Course is a comprehensive 1.5-day program highlighting groundbreaking advances in the delivery of high-quality, patient-centered GI care. Offering general and breakout sessions, learning lunches, and case-based and panel discussions, attendees will gain a deeper understanding of how to diagnose and treat a variety of disease states and digestive disorders.
June 3-6, 2020
2020 AGA Tech Summit
Visit https://techsummit.gastro.org/ for more details.
San Francisco, Calif.
Aug. 14-15, 2020
James W. Freston Single-Topic Conference: Gastrointestinal Organoids and Engineered Organ Systems
The 2020 Freston Conference will focus on GI organoids and engineered organ systems.
Chicago, Ill.
Aug. 14-16, 2020
2020 Principles of GI for the NP and PA
Principles of GI is designed by leading advanced practice providers, GI experts, and physicians to mirror real-life settings for nurse practitioners and physician assistants that lead to GI clinical success.
Denver, Colo.
AWARDS DEADLINES
AGA Fellow Abstract Award
This $500 travel award supports recipients who are MD, PhD, or equivalent fellows giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW). The top-scoring abstract will be designated the Fellow Abstract of the Year and receive a $1,000 award.
Application Deadline: Feb. 26, 2020
AGA Student Abstract Award
This $500 travel award supports recipients who are graduate students, medical students, or medical residents (residents up to postgraduate year three) giving abstract-based oral or poster presentations at Digestive Disease Week® (DDW).
Application Deadline: Feb. 26, 2020
AGA-Moti L. & Kamla Rustgi International Travel Awards
This $750 travel award supports recipients who are young (i.e., 35 years of age or younger at the time of DDW) basic, translational, or clinical investigators residing outside North America to support travel and related expenses to attend Digestive Disease Week® (DDW).
Application Deadline: Feb. 26, 2020
Endoscopy in a do-not-resuscitate patient: Practical and ethical considerations
Editor’s Note: I am very excited to introduce a section to The New Gastroenterologist that will address topics in clinical medical ethics we frequently face as gastroenterologists. There are several inherent ethical issues in gastroenterology that are not often explicitly discussed, such as periprocedural code status, informed consent, transplantation, performance of endoscopy in the critically ill, and nutrition support in the setting of end of life care. Often the most difficult decisions we make as clinicians are fraught with ethical implications which can be daunting and difficult to navigate. The goal of this section is to address these issues in a case-based format to offer some guidance to young gastroenterologists grappling with similar scenarios.
This month’s issue features the inaugural piece for this series, written by Dr. Lauren Feld (University of Washington), which discusses a clinical scenario in which a patient with a preexisting do-not-resuscitate (DNR) order is about to undergo endoscopy. The article provides a systematic approach to periprocedural code status and highlights existing guidelines that are generally not well known among gastroenterologists.
Vijaya L. Rao, MD
Editor in Chief
An 89-year old female with history of heart failure with reduced ejection fraction, chronic obstructive pulmonary disease, and dementia is admitted to the intensive care unit (ICU) with melena and acute post-hemorrhagic anemia. The family member designated as the patient’s power of attorney (POA) agrees that her code status upon admission will be do-not-resuscitate and do-not-intubate (DNR/DNI) without plan for invasive procedures. However, she has continued overt bleeding with concomitant hemodynamic instability. The POA and ICU team are now asking for urgent endoscopic evaluation, but do not agree to temporary code reversal for the duration of the procedure.
This vignette highlights an important distinction between a patient’s goals of care and the code status. While these two terms are often erroneously used interchangeably, “code status” refers to a patient’s wishes in the event of cardiopulmonary arrest, while “goals of care” refers to a more comprehensive understanding of what care fits within a patient’s values. Patients or their families may still desire interventions such as procedures, but not wish to have a resuscitation attempt in the event of cardiopulmonary arrest. This leads to the commonly encountered clinical scenario in which a patient planning to undergo endoscopy has an active DNR order.
Frequently, DNR orders are temporarily rescinded prior to invasive procedures. There are several reasons this occurs. First, patients or decision makers may decide that the improved rates of survival in intraprocedural arrests changes their risk-benefit assessment about resuscitation procedures. Secondly, proceduralists may feel an ethical duty to resuscitate a patient if the cause of the arrest is considered iatrogenic and potentially reversible. In addition, proceduralists may worry about legal or professional risk if a patient suffers cardiopulmonary arrest during a procedure and an attempt at resuscitation does not occur.
While this is a frequently encountered clinical scenario, there is wide variation in clinical practice. This variation led to the creation of guidelines set forth by the American Society of Anesthesiologists in 1993 and subsequently adopted by the American College of Surgeons. These guidelines recommend a discussion between the physician and the patient prior to the procedure, utilizing shared decision-making around three options: 1) a full attempt at resuscitation; 2) a limited attempt at resuscitation defined with regard to specific procedures; and 3) a limited attempt at resuscitation defined with regard to the patient’s goals and values.
However, these guidelines are both not well known and frequently not applied amongst clinicians and ancillary staff. Patients are frequently told that they must reverse their DNR order to full code prior to undergoing endoscopy. Dissemination of a systematic approach to a patient with a DNR order who requires endoscopy is important to ensure patients have autonomy over their medical decision-making, while also ensuring that health care professionals feel comfortable with their decisions.
The first step when encountering this scenario is to ensure that the procedure is indicated in this particular patient. While guidelines and algorithms have a substantial role in deciding the appropriate work-up for a presenting complaint such as a presumed upper gastrointestinal bleed, the art of medicine lies in the role of the physicians to decide if an invasive procedure is indicated in their specific patients. This decision should be based on the patients’ presenting clinical scenario, their overall comorbidities, their values, and their goals of care.
As the medical complexity of the patient increases, the risks of the procedure increase and it is ultimately up to the endoscopist to frame the informed consent conversation such that the patient and family understand the potential risks and benefits in their specific case.
With a patient who has a desire to avoid aggressive resuscitation attempts, the physician, patient, and family should weigh the risks and benefits of the procedure, and carefully examine if the indication is sufficient. For the patient outlined in the case, her dementia limits her decision-making capacity, and the clinical team is working with a surrogate decision-maker, her POA, to understand the patient’s wishes and goals. Her POA reports upon admission that invasive procedures may not be in line with her previously expressed values or in her best interest. However, with the development of an acute decompensation due to a presumed GI bleed, a potentially reversible cause, the POA requests an endoscopy to attempt to intervene. Occasionally, a patient with clear goals of care can have a change in these goals when a decompensation occurs. The gastroenterologist should assess if this represents a true desire for invasive procedures, or if this is a response to pressure from other members of the clinical team or family, or if palliative needs are not being met. In this patient, her POA desires an endoscopy because her likely upper GI bleed may be contributing to an acute decompensation, but does not wish for other aggressive measures if she should suffer cardiopulmonary arrest. Although upper endoscopy is a generally safe and well-tolerated procedure, this patient’s cardiopulmonary comorbidities increase the risk of the procedure; therefore, the gastroenterology team should proceed with a candid, detailed discussion of risks, benefits, and alternatives with the patient’s POA.
If the decision is made to proceed with endoscopy, the next step is to address the patient’s code status surrounding the procedure. This conversation should focus on three key goals: 1) allow the physician to gain understanding of the patient or surrogate’s perspectives on goals of care; 2) provide the patient or surrogate with an understanding of the risks and potential outcomes of the procedure, as well as resuscitation options; and 3) ultimately arrive at a mutual consensus regarding the patient’s periprocedural code status. Plans for postprocedural care should also be discussed.
While gastroenterology societies do not have specific guidelines surrounding this situation, there are several steps clinicians can take to ensure patient safety and autonomy are preserved:
- Physicians should avoid one-size-fits-all policies, such as the expectation that patients routinely return to full code for procedures.
- The patient and/or decision-makers should have a discussion regarding the risks during the procedure and potential reversibility of these risks.
- The patient should be presented with the option to either reverse to full code, refuse specific resuscitative measures such as defibrillation or intubation, or be allowed to explain his or her own views on goals of care and allow the procedural team to use their clinical judgment should an emergency arise.
- Physicians should be specific regarding the duration of the code status change. For example, in a patient who has reversed the code status to allow a full resuscitation attempt, the team and patient should discuss how long the patient will remain intubated after the procedure.
- This discussion should be documented carefully in the chart to assist with dissemination amongst the medical team.
This process will ensure that clear guidelines are defined such that everyone, including the patient’s potential decision makers, understand to what they are agreeing.
While physicians and care teams are primarily concerned with providing high-quality and individualized care to patients, it is true that concerns surrounding medicolegal risk are present. Careful informed consent and informed refusal conversations will reduce risk. Indeed, in a patient who has a DNR order, physicians are more likely to be at risk performing resuscitation efforts than withholding them. Communication between patients, families, and physicians remains the foundation for a trusting relationship and decreased litigation risk.
For this patient, engaging her POA in an honest and thorough discussion about her goals of care, as well as the risks of both performing and not performing the upper endoscopy are critical to her care. If her POA wishes to proceed with the procedure and have her remain DNR during the procedure, this should be documented and adhered to. Ultimately, the best outcome for this patient will occur with an individualized risk-benefit assessment and open, frequent communication among the care team and her POA.
Dr. Feld is a gastroenterology and hepatology fellow in the department of gastroenterology and hepatology, University of Washington, Seattle. She has no conflicts of interest.
Editor’s Note: I am very excited to introduce a section to The New Gastroenterologist that will address topics in clinical medical ethics we frequently face as gastroenterologists. There are several inherent ethical issues in gastroenterology that are not often explicitly discussed, such as periprocedural code status, informed consent, transplantation, performance of endoscopy in the critically ill, and nutrition support in the setting of end of life care. Often the most difficult decisions we make as clinicians are fraught with ethical implications which can be daunting and difficult to navigate. The goal of this section is to address these issues in a case-based format to offer some guidance to young gastroenterologists grappling with similar scenarios.
This month’s issue features the inaugural piece for this series, written by Dr. Lauren Feld (University of Washington), which discusses a clinical scenario in which a patient with a preexisting do-not-resuscitate (DNR) order is about to undergo endoscopy. The article provides a systematic approach to periprocedural code status and highlights existing guidelines that are generally not well known among gastroenterologists.
Vijaya L. Rao, MD
Editor in Chief
An 89-year old female with history of heart failure with reduced ejection fraction, chronic obstructive pulmonary disease, and dementia is admitted to the intensive care unit (ICU) with melena and acute post-hemorrhagic anemia. The family member designated as the patient’s power of attorney (POA) agrees that her code status upon admission will be do-not-resuscitate and do-not-intubate (DNR/DNI) without plan for invasive procedures. However, she has continued overt bleeding with concomitant hemodynamic instability. The POA and ICU team are now asking for urgent endoscopic evaluation, but do not agree to temporary code reversal for the duration of the procedure.
This vignette highlights an important distinction between a patient’s goals of care and the code status. While these two terms are often erroneously used interchangeably, “code status” refers to a patient’s wishes in the event of cardiopulmonary arrest, while “goals of care” refers to a more comprehensive understanding of what care fits within a patient’s values. Patients or their families may still desire interventions such as procedures, but not wish to have a resuscitation attempt in the event of cardiopulmonary arrest. This leads to the commonly encountered clinical scenario in which a patient planning to undergo endoscopy has an active DNR order.
Frequently, DNR orders are temporarily rescinded prior to invasive procedures. There are several reasons this occurs. First, patients or decision makers may decide that the improved rates of survival in intraprocedural arrests changes their risk-benefit assessment about resuscitation procedures. Secondly, proceduralists may feel an ethical duty to resuscitate a patient if the cause of the arrest is considered iatrogenic and potentially reversible. In addition, proceduralists may worry about legal or professional risk if a patient suffers cardiopulmonary arrest during a procedure and an attempt at resuscitation does not occur.
While this is a frequently encountered clinical scenario, there is wide variation in clinical practice. This variation led to the creation of guidelines set forth by the American Society of Anesthesiologists in 1993 and subsequently adopted by the American College of Surgeons. These guidelines recommend a discussion between the physician and the patient prior to the procedure, utilizing shared decision-making around three options: 1) a full attempt at resuscitation; 2) a limited attempt at resuscitation defined with regard to specific procedures; and 3) a limited attempt at resuscitation defined with regard to the patient’s goals and values.
However, these guidelines are both not well known and frequently not applied amongst clinicians and ancillary staff. Patients are frequently told that they must reverse their DNR order to full code prior to undergoing endoscopy. Dissemination of a systematic approach to a patient with a DNR order who requires endoscopy is important to ensure patients have autonomy over their medical decision-making, while also ensuring that health care professionals feel comfortable with their decisions.
The first step when encountering this scenario is to ensure that the procedure is indicated in this particular patient. While guidelines and algorithms have a substantial role in deciding the appropriate work-up for a presenting complaint such as a presumed upper gastrointestinal bleed, the art of medicine lies in the role of the physicians to decide if an invasive procedure is indicated in their specific patients. This decision should be based on the patients’ presenting clinical scenario, their overall comorbidities, their values, and their goals of care.
As the medical complexity of the patient increases, the risks of the procedure increase and it is ultimately up to the endoscopist to frame the informed consent conversation such that the patient and family understand the potential risks and benefits in their specific case.
With a patient who has a desire to avoid aggressive resuscitation attempts, the physician, patient, and family should weigh the risks and benefits of the procedure, and carefully examine if the indication is sufficient. For the patient outlined in the case, her dementia limits her decision-making capacity, and the clinical team is working with a surrogate decision-maker, her POA, to understand the patient’s wishes and goals. Her POA reports upon admission that invasive procedures may not be in line with her previously expressed values or in her best interest. However, with the development of an acute decompensation due to a presumed GI bleed, a potentially reversible cause, the POA requests an endoscopy to attempt to intervene. Occasionally, a patient with clear goals of care can have a change in these goals when a decompensation occurs. The gastroenterologist should assess if this represents a true desire for invasive procedures, or if this is a response to pressure from other members of the clinical team or family, or if palliative needs are not being met. In this patient, her POA desires an endoscopy because her likely upper GI bleed may be contributing to an acute decompensation, but does not wish for other aggressive measures if she should suffer cardiopulmonary arrest. Although upper endoscopy is a generally safe and well-tolerated procedure, this patient’s cardiopulmonary comorbidities increase the risk of the procedure; therefore, the gastroenterology team should proceed with a candid, detailed discussion of risks, benefits, and alternatives with the patient’s POA.
If the decision is made to proceed with endoscopy, the next step is to address the patient’s code status surrounding the procedure. This conversation should focus on three key goals: 1) allow the physician to gain understanding of the patient or surrogate’s perspectives on goals of care; 2) provide the patient or surrogate with an understanding of the risks and potential outcomes of the procedure, as well as resuscitation options; and 3) ultimately arrive at a mutual consensus regarding the patient’s periprocedural code status. Plans for postprocedural care should also be discussed.
While gastroenterology societies do not have specific guidelines surrounding this situation, there are several steps clinicians can take to ensure patient safety and autonomy are preserved:
- Physicians should avoid one-size-fits-all policies, such as the expectation that patients routinely return to full code for procedures.
- The patient and/or decision-makers should have a discussion regarding the risks during the procedure and potential reversibility of these risks.
- The patient should be presented with the option to either reverse to full code, refuse specific resuscitative measures such as defibrillation or intubation, or be allowed to explain his or her own views on goals of care and allow the procedural team to use their clinical judgment should an emergency arise.
- Physicians should be specific regarding the duration of the code status change. For example, in a patient who has reversed the code status to allow a full resuscitation attempt, the team and patient should discuss how long the patient will remain intubated after the procedure.
- This discussion should be documented carefully in the chart to assist with dissemination amongst the medical team.
This process will ensure that clear guidelines are defined such that everyone, including the patient’s potential decision makers, understand to what they are agreeing.
While physicians and care teams are primarily concerned with providing high-quality and individualized care to patients, it is true that concerns surrounding medicolegal risk are present. Careful informed consent and informed refusal conversations will reduce risk. Indeed, in a patient who has a DNR order, physicians are more likely to be at risk performing resuscitation efforts than withholding them. Communication between patients, families, and physicians remains the foundation for a trusting relationship and decreased litigation risk.
For this patient, engaging her POA in an honest and thorough discussion about her goals of care, as well as the risks of both performing and not performing the upper endoscopy are critical to her care. If her POA wishes to proceed with the procedure and have her remain DNR during the procedure, this should be documented and adhered to. Ultimately, the best outcome for this patient will occur with an individualized risk-benefit assessment and open, frequent communication among the care team and her POA.
Dr. Feld is a gastroenterology and hepatology fellow in the department of gastroenterology and hepatology, University of Washington, Seattle. She has no conflicts of interest.
Editor’s Note: I am very excited to introduce a section to The New Gastroenterologist that will address topics in clinical medical ethics we frequently face as gastroenterologists. There are several inherent ethical issues in gastroenterology that are not often explicitly discussed, such as periprocedural code status, informed consent, transplantation, performance of endoscopy in the critically ill, and nutrition support in the setting of end of life care. Often the most difficult decisions we make as clinicians are fraught with ethical implications which can be daunting and difficult to navigate. The goal of this section is to address these issues in a case-based format to offer some guidance to young gastroenterologists grappling with similar scenarios.
This month’s issue features the inaugural piece for this series, written by Dr. Lauren Feld (University of Washington), which discusses a clinical scenario in which a patient with a preexisting do-not-resuscitate (DNR) order is about to undergo endoscopy. The article provides a systematic approach to periprocedural code status and highlights existing guidelines that are generally not well known among gastroenterologists.
Vijaya L. Rao, MD
Editor in Chief
An 89-year old female with history of heart failure with reduced ejection fraction, chronic obstructive pulmonary disease, and dementia is admitted to the intensive care unit (ICU) with melena and acute post-hemorrhagic anemia. The family member designated as the patient’s power of attorney (POA) agrees that her code status upon admission will be do-not-resuscitate and do-not-intubate (DNR/DNI) without plan for invasive procedures. However, she has continued overt bleeding with concomitant hemodynamic instability. The POA and ICU team are now asking for urgent endoscopic evaluation, but do not agree to temporary code reversal for the duration of the procedure.
This vignette highlights an important distinction between a patient’s goals of care and the code status. While these two terms are often erroneously used interchangeably, “code status” refers to a patient’s wishes in the event of cardiopulmonary arrest, while “goals of care” refers to a more comprehensive understanding of what care fits within a patient’s values. Patients or their families may still desire interventions such as procedures, but not wish to have a resuscitation attempt in the event of cardiopulmonary arrest. This leads to the commonly encountered clinical scenario in which a patient planning to undergo endoscopy has an active DNR order.
Frequently, DNR orders are temporarily rescinded prior to invasive procedures. There are several reasons this occurs. First, patients or decision makers may decide that the improved rates of survival in intraprocedural arrests changes their risk-benefit assessment about resuscitation procedures. Secondly, proceduralists may feel an ethical duty to resuscitate a patient if the cause of the arrest is considered iatrogenic and potentially reversible. In addition, proceduralists may worry about legal or professional risk if a patient suffers cardiopulmonary arrest during a procedure and an attempt at resuscitation does not occur.
While this is a frequently encountered clinical scenario, there is wide variation in clinical practice. This variation led to the creation of guidelines set forth by the American Society of Anesthesiologists in 1993 and subsequently adopted by the American College of Surgeons. These guidelines recommend a discussion between the physician and the patient prior to the procedure, utilizing shared decision-making around three options: 1) a full attempt at resuscitation; 2) a limited attempt at resuscitation defined with regard to specific procedures; and 3) a limited attempt at resuscitation defined with regard to the patient’s goals and values.
However, these guidelines are both not well known and frequently not applied amongst clinicians and ancillary staff. Patients are frequently told that they must reverse their DNR order to full code prior to undergoing endoscopy. Dissemination of a systematic approach to a patient with a DNR order who requires endoscopy is important to ensure patients have autonomy over their medical decision-making, while also ensuring that health care professionals feel comfortable with their decisions.
The first step when encountering this scenario is to ensure that the procedure is indicated in this particular patient. While guidelines and algorithms have a substantial role in deciding the appropriate work-up for a presenting complaint such as a presumed upper gastrointestinal bleed, the art of medicine lies in the role of the physicians to decide if an invasive procedure is indicated in their specific patients. This decision should be based on the patients’ presenting clinical scenario, their overall comorbidities, their values, and their goals of care.
As the medical complexity of the patient increases, the risks of the procedure increase and it is ultimately up to the endoscopist to frame the informed consent conversation such that the patient and family understand the potential risks and benefits in their specific case.
With a patient who has a desire to avoid aggressive resuscitation attempts, the physician, patient, and family should weigh the risks and benefits of the procedure, and carefully examine if the indication is sufficient. For the patient outlined in the case, her dementia limits her decision-making capacity, and the clinical team is working with a surrogate decision-maker, her POA, to understand the patient’s wishes and goals. Her POA reports upon admission that invasive procedures may not be in line with her previously expressed values or in her best interest. However, with the development of an acute decompensation due to a presumed GI bleed, a potentially reversible cause, the POA requests an endoscopy to attempt to intervene. Occasionally, a patient with clear goals of care can have a change in these goals when a decompensation occurs. The gastroenterologist should assess if this represents a true desire for invasive procedures, or if this is a response to pressure from other members of the clinical team or family, or if palliative needs are not being met. In this patient, her POA desires an endoscopy because her likely upper GI bleed may be contributing to an acute decompensation, but does not wish for other aggressive measures if she should suffer cardiopulmonary arrest. Although upper endoscopy is a generally safe and well-tolerated procedure, this patient’s cardiopulmonary comorbidities increase the risk of the procedure; therefore, the gastroenterology team should proceed with a candid, detailed discussion of risks, benefits, and alternatives with the patient’s POA.
If the decision is made to proceed with endoscopy, the next step is to address the patient’s code status surrounding the procedure. This conversation should focus on three key goals: 1) allow the physician to gain understanding of the patient or surrogate’s perspectives on goals of care; 2) provide the patient or surrogate with an understanding of the risks and potential outcomes of the procedure, as well as resuscitation options; and 3) ultimately arrive at a mutual consensus regarding the patient’s periprocedural code status. Plans for postprocedural care should also be discussed.
While gastroenterology societies do not have specific guidelines surrounding this situation, there are several steps clinicians can take to ensure patient safety and autonomy are preserved:
- Physicians should avoid one-size-fits-all policies, such as the expectation that patients routinely return to full code for procedures.
- The patient and/or decision-makers should have a discussion regarding the risks during the procedure and potential reversibility of these risks.
- The patient should be presented with the option to either reverse to full code, refuse specific resuscitative measures such as defibrillation or intubation, or be allowed to explain his or her own views on goals of care and allow the procedural team to use their clinical judgment should an emergency arise.
- Physicians should be specific regarding the duration of the code status change. For example, in a patient who has reversed the code status to allow a full resuscitation attempt, the team and patient should discuss how long the patient will remain intubated after the procedure.
- This discussion should be documented carefully in the chart to assist with dissemination amongst the medical team.
This process will ensure that clear guidelines are defined such that everyone, including the patient’s potential decision makers, understand to what they are agreeing.
While physicians and care teams are primarily concerned with providing high-quality and individualized care to patients, it is true that concerns surrounding medicolegal risk are present. Careful informed consent and informed refusal conversations will reduce risk. Indeed, in a patient who has a DNR order, physicians are more likely to be at risk performing resuscitation efforts than withholding them. Communication between patients, families, and physicians remains the foundation for a trusting relationship and decreased litigation risk.
For this patient, engaging her POA in an honest and thorough discussion about her goals of care, as well as the risks of both performing and not performing the upper endoscopy are critical to her care. If her POA wishes to proceed with the procedure and have her remain DNR during the procedure, this should be documented and adhered to. Ultimately, the best outcome for this patient will occur with an individualized risk-benefit assessment and open, frequent communication among the care team and her POA.
Dr. Feld is a gastroenterology and hepatology fellow in the department of gastroenterology and hepatology, University of Washington, Seattle. She has no conflicts of interest.
Developing a career in nutrition support and small-bowel disorders
The role of diet and nutrition is becoming increasingly recognized in the cause, management, and prevention of disease. Despite the clear importance of the role of nutrition in the field of medicine, among health professionals, formal training in nutrition support is lacking. A lack of nutrition training has been recognized in multiple subspecialty fields1 and is highlighted by a shortage of physicians trained to manage disease-related malnutrition.2 Gastroenterologists, in particular, have a special responsibility related to nutrition in disorders of the gastrointestinal tract and are in a unique position to recognize and manage disorders of maldigestion and malabsorption. Unfortunately, surveys of both U.S. and Canadian fellows have demonstrated deficiencies in the training of nutrition support and management of enteral and parenteral nutrition (PN).3,4
Current status of nutrition training
The impact of diet and nutrition on health and disease is universally recognized but unfortunately lagging with respect to formal training at all levels of medical education. A survey of program directors from primary care, surgery, and anesthesia showed only 26% of respondent programs had a formal curriculum in nutrition education.1 Specific to gastroenterology, a majority of trainees and recent graduates perceived that nutrition education was an important aspect of their training; however, only 50% of respondents had training in nutrition support with 36% reporting mandatory training.3
The Gastroenterology Core Curriculum, most recently updated in 2007 – and sponsored by the American Association for the Study of Liver Diseases, American College of Gastroenterology, the American Society for Gastrointestinal Endoscopy, and the American Gastroenterological Association – includes six domains of nutrition training within the training track: nutrition assessment, basic nutrition requirements, specific gastrointestinal disorders and other allied diseases, enteral nutrition, PN, and diet therapy. Level 1 training is expected for all gastroenterology fellows. Level 2 is comprised, on average, of an additional 12 months with described objectives, either occurring outside of a standard gastroenterology fellowship or coinciding with a dedicated third year of training. Although training durations for level 1 are not defined, level 2 recommends at least 6 months of experience working with an inpatient nutrition support team (NST) and the management of outpatients in nutrition and weight management clinics.5
Role of a nutrition support team
Training in nutrition is a heterogeneous field, with a wide range that covers understanding metabolism in health and disease, micronutrient and macronutrient requirements, nutrient digestion and absorption, and the best route and provision of nutrition support. Therefore, a critical aspect of education includes access to a dedicated NST. Such teams were common and necessary in the late 1900s with the inception of specialized nutrition therapy. However, with an increase in the use of home infusion therapies, NSTs were dismantled in favor of shifting responsibility to decentralized home infusion companies. A dedicated NST often will include some combination of pharmacists with an interest in the safe compounding of parenteral formulas, nurses with experience in the home management of intravenous therapies and catheters, and dietitians with dedicated interests in intestinal failure, recognition of malnutrition, and provision of calories. Collectively, a highly functioning NST also provides dedicated multidisciplinary training to health professionals of varying backgrounds.
My entry into the field of nutrition support
Entering a fellowship in gastroenterology should be pursued with an open mind. We all have varying experiences in the management of patients with gastrointestinal conditions, both in the inpatient and outpatient arenas through residency training. My early experiences in fellowship at the University of Chicago centered on the management of patients with inflammatory bowel disease (IBD) and with research interests related to the clinical course of IBD. I was also fortunate to be part of a fellowship program offering both level 1 and level 2 training with a longstanding track record of graduating fellows responsible for the running of NSTs at their local institutions. Categorical fellows spend 3 months of training on a rotation with combined inpatient and outpatient responsibilities focusing on the management of patients with intestinal failure, inpatient management of complications from PN support, and an outpatient clinic focused on small-bowel disorders (celiac disease, small-bowel bleeding, and intestinal malabsorption). This experience led me to pursue level 2 training at Northwestern University with a combined focus on small-bowel diseases and enteroscopy.
These collective experiences in fellowship and postfellowship training grounded my ideas on the role of nutrition pervading many gastrointestinal conditions from acute and chronic pancreatitis and IBD to rare conditions such as enteropathy associated with immune deficiencies and autoimmune enteropathy. Now, as a junior faculty member with a focus in nutrition support and small-bowel disorders, my clinical responsibilities include a dedicated half-day in the management of outpatients (parenteral and enteral nutrition), inpatient rounding with our dedicated NST focusing on the initiation of PN, management of home PN complications, and dedicated procedural time focusing on enteral access techniques (percutaneous gastrostomy/jejunostomy tubes) and small-bowel enteroscopy. To my surprise, entry into the field of nutrition support and small-bowel disorders has been filled with excitement and a growing list of collaborations and opportunities. While initial work in the management of PN has been in existence since the 1970s and earlier with respect to the development of safe administration techniques, most of my current work transcends specialties as we develop appropriateness criteria related to PN support in collaboration with a wide range of specialties that include surgery, oncology, and palliative care.
Seeking opportunities for additional training
As the field of gastroenterology grows outward in various directions, mastery of subjects has led to subspecialization in specific areas including interventional gastroenterology, pancreatology, IBD, and motility disorders. The field is primed for broader access to specialty training in nutrition support and small-bowel disorders. Exposure to dedicated training in nutrition and nutrition-related disorders is vital as part of a categorical fellowship, but can also be complemented via visiting observerships, access to formal level 2 training programs, and external programs related to promoting nutrition education.
Since 2001, formal nutrition fellowship programs offering level 2 training have been compiled by the National Board of Physician Nutrition Specialists, although attraction of interested fellows has been lacking.2 The Nestlé Nutrition Institute Clinical Nutrition Fellowship, endorsed by the American Society for Parenteral and Enteral Nutrition and the AGA, is an ongoing program that pairs interested trainees with expert program faculty through onsite clinical rotations lasting a total of 4 weeks.2 Attendance at national and international conferences can supplement a fellows training in nutrition, and an increased focus on nutrition lectures should be a priority of meeting education committees to increase the exposure of trainees to leaders in the field.
Conclusion
A career in nutrition support and small-bowel disorders is incredibly rewarding as it incorporates the basic physiologic processes of digestion and absorption with a wide array of pathologic conditions. Incorporation of the basic principles of intestinal absorption allows for a greater understanding of the role of the low–fermentable oligo-, di-, monosaccharides and polyols (FODMAP) diet in the management of irritable bowel syndrome to the varying principles of diets currently under study for the management of IBD. Outside of this spectrum, working with an NST allows for the management of complex cases of malnutrition resulting from disorders ranging from cancer to various postsurgical intestinal alterations. Although observerships and external training programs allow for an introduction into the field, formal level 2 training, combining both work with a NST and small-bowel enteroscopy, allows for exposure to the full range of disorders of the small bowel. As patients continue to seek disease management options rooted in diet, the demand for gastroenterologists with subspecialty training in nutritional disorders will continue to grow and will require further support across training programs to incorporate additional training into categorical fellowships.
References
1. Daley BJ et al. JPEN J Paren Enteral Nutr. 2016;40(1):95-9. doi: 10.1177/0148607115571155.
2. Kiraly LN et al. Nutr Clin Pract. 2014;29(3):332-7. doi: 10.1177/0884533614525212.
3. Hu J et al. Nutr Clin Pract. 2018 Apr;33(2):191-7. doi: 10.1177/0884533617700852.
4. Scolapio JS et al. J Clin Gastroenterol. 2008 Feb;42(2):122-7. doi: 10.1097/MCG.0b013e3181595b6a.
5. American Association for the Study of Liver Diseases et al. The Gastroenterology Core Curriculum, 3rd ed. Gastroenterology. 2007;132(5):2012-8. doi: 10.1053/j.gastro.2007.03.079.
Dr. Micic is assistant professor of medicine, department of internal medicine, section of gastroenterology, hepatology, and nutrition, University of Chicago.
The role of diet and nutrition is becoming increasingly recognized in the cause, management, and prevention of disease. Despite the clear importance of the role of nutrition in the field of medicine, among health professionals, formal training in nutrition support is lacking. A lack of nutrition training has been recognized in multiple subspecialty fields1 and is highlighted by a shortage of physicians trained to manage disease-related malnutrition.2 Gastroenterologists, in particular, have a special responsibility related to nutrition in disorders of the gastrointestinal tract and are in a unique position to recognize and manage disorders of maldigestion and malabsorption. Unfortunately, surveys of both U.S. and Canadian fellows have demonstrated deficiencies in the training of nutrition support and management of enteral and parenteral nutrition (PN).3,4
Current status of nutrition training
The impact of diet and nutrition on health and disease is universally recognized but unfortunately lagging with respect to formal training at all levels of medical education. A survey of program directors from primary care, surgery, and anesthesia showed only 26% of respondent programs had a formal curriculum in nutrition education.1 Specific to gastroenterology, a majority of trainees and recent graduates perceived that nutrition education was an important aspect of their training; however, only 50% of respondents had training in nutrition support with 36% reporting mandatory training.3
The Gastroenterology Core Curriculum, most recently updated in 2007 – and sponsored by the American Association for the Study of Liver Diseases, American College of Gastroenterology, the American Society for Gastrointestinal Endoscopy, and the American Gastroenterological Association – includes six domains of nutrition training within the training track: nutrition assessment, basic nutrition requirements, specific gastrointestinal disorders and other allied diseases, enteral nutrition, PN, and diet therapy. Level 1 training is expected for all gastroenterology fellows. Level 2 is comprised, on average, of an additional 12 months with described objectives, either occurring outside of a standard gastroenterology fellowship or coinciding with a dedicated third year of training. Although training durations for level 1 are not defined, level 2 recommends at least 6 months of experience working with an inpatient nutrition support team (NST) and the management of outpatients in nutrition and weight management clinics.5
Role of a nutrition support team
Training in nutrition is a heterogeneous field, with a wide range that covers understanding metabolism in health and disease, micronutrient and macronutrient requirements, nutrient digestion and absorption, and the best route and provision of nutrition support. Therefore, a critical aspect of education includes access to a dedicated NST. Such teams were common and necessary in the late 1900s with the inception of specialized nutrition therapy. However, with an increase in the use of home infusion therapies, NSTs were dismantled in favor of shifting responsibility to decentralized home infusion companies. A dedicated NST often will include some combination of pharmacists with an interest in the safe compounding of parenteral formulas, nurses with experience in the home management of intravenous therapies and catheters, and dietitians with dedicated interests in intestinal failure, recognition of malnutrition, and provision of calories. Collectively, a highly functioning NST also provides dedicated multidisciplinary training to health professionals of varying backgrounds.
My entry into the field of nutrition support
Entering a fellowship in gastroenterology should be pursued with an open mind. We all have varying experiences in the management of patients with gastrointestinal conditions, both in the inpatient and outpatient arenas through residency training. My early experiences in fellowship at the University of Chicago centered on the management of patients with inflammatory bowel disease (IBD) and with research interests related to the clinical course of IBD. I was also fortunate to be part of a fellowship program offering both level 1 and level 2 training with a longstanding track record of graduating fellows responsible for the running of NSTs at their local institutions. Categorical fellows spend 3 months of training on a rotation with combined inpatient and outpatient responsibilities focusing on the management of patients with intestinal failure, inpatient management of complications from PN support, and an outpatient clinic focused on small-bowel disorders (celiac disease, small-bowel bleeding, and intestinal malabsorption). This experience led me to pursue level 2 training at Northwestern University with a combined focus on small-bowel diseases and enteroscopy.
These collective experiences in fellowship and postfellowship training grounded my ideas on the role of nutrition pervading many gastrointestinal conditions from acute and chronic pancreatitis and IBD to rare conditions such as enteropathy associated with immune deficiencies and autoimmune enteropathy. Now, as a junior faculty member with a focus in nutrition support and small-bowel disorders, my clinical responsibilities include a dedicated half-day in the management of outpatients (parenteral and enteral nutrition), inpatient rounding with our dedicated NST focusing on the initiation of PN, management of home PN complications, and dedicated procedural time focusing on enteral access techniques (percutaneous gastrostomy/jejunostomy tubes) and small-bowel enteroscopy. To my surprise, entry into the field of nutrition support and small-bowel disorders has been filled with excitement and a growing list of collaborations and opportunities. While initial work in the management of PN has been in existence since the 1970s and earlier with respect to the development of safe administration techniques, most of my current work transcends specialties as we develop appropriateness criteria related to PN support in collaboration with a wide range of specialties that include surgery, oncology, and palliative care.
Seeking opportunities for additional training
As the field of gastroenterology grows outward in various directions, mastery of subjects has led to subspecialization in specific areas including interventional gastroenterology, pancreatology, IBD, and motility disorders. The field is primed for broader access to specialty training in nutrition support and small-bowel disorders. Exposure to dedicated training in nutrition and nutrition-related disorders is vital as part of a categorical fellowship, but can also be complemented via visiting observerships, access to formal level 2 training programs, and external programs related to promoting nutrition education.
Since 2001, formal nutrition fellowship programs offering level 2 training have been compiled by the National Board of Physician Nutrition Specialists, although attraction of interested fellows has been lacking.2 The Nestlé Nutrition Institute Clinical Nutrition Fellowship, endorsed by the American Society for Parenteral and Enteral Nutrition and the AGA, is an ongoing program that pairs interested trainees with expert program faculty through onsite clinical rotations lasting a total of 4 weeks.2 Attendance at national and international conferences can supplement a fellows training in nutrition, and an increased focus on nutrition lectures should be a priority of meeting education committees to increase the exposure of trainees to leaders in the field.
Conclusion
A career in nutrition support and small-bowel disorders is incredibly rewarding as it incorporates the basic physiologic processes of digestion and absorption with a wide array of pathologic conditions. Incorporation of the basic principles of intestinal absorption allows for a greater understanding of the role of the low–fermentable oligo-, di-, monosaccharides and polyols (FODMAP) diet in the management of irritable bowel syndrome to the varying principles of diets currently under study for the management of IBD. Outside of this spectrum, working with an NST allows for the management of complex cases of malnutrition resulting from disorders ranging from cancer to various postsurgical intestinal alterations. Although observerships and external training programs allow for an introduction into the field, formal level 2 training, combining both work with a NST and small-bowel enteroscopy, allows for exposure to the full range of disorders of the small bowel. As patients continue to seek disease management options rooted in diet, the demand for gastroenterologists with subspecialty training in nutritional disorders will continue to grow and will require further support across training programs to incorporate additional training into categorical fellowships.
References
1. Daley BJ et al. JPEN J Paren Enteral Nutr. 2016;40(1):95-9. doi: 10.1177/0148607115571155.
2. Kiraly LN et al. Nutr Clin Pract. 2014;29(3):332-7. doi: 10.1177/0884533614525212.
3. Hu J et al. Nutr Clin Pract. 2018 Apr;33(2):191-7. doi: 10.1177/0884533617700852.
4. Scolapio JS et al. J Clin Gastroenterol. 2008 Feb;42(2):122-7. doi: 10.1097/MCG.0b013e3181595b6a.
5. American Association for the Study of Liver Diseases et al. The Gastroenterology Core Curriculum, 3rd ed. Gastroenterology. 2007;132(5):2012-8. doi: 10.1053/j.gastro.2007.03.079.
Dr. Micic is assistant professor of medicine, department of internal medicine, section of gastroenterology, hepatology, and nutrition, University of Chicago.
The role of diet and nutrition is becoming increasingly recognized in the cause, management, and prevention of disease. Despite the clear importance of the role of nutrition in the field of medicine, among health professionals, formal training in nutrition support is lacking. A lack of nutrition training has been recognized in multiple subspecialty fields1 and is highlighted by a shortage of physicians trained to manage disease-related malnutrition.2 Gastroenterologists, in particular, have a special responsibility related to nutrition in disorders of the gastrointestinal tract and are in a unique position to recognize and manage disorders of maldigestion and malabsorption. Unfortunately, surveys of both U.S. and Canadian fellows have demonstrated deficiencies in the training of nutrition support and management of enteral and parenteral nutrition (PN).3,4
Current status of nutrition training
The impact of diet and nutrition on health and disease is universally recognized but unfortunately lagging with respect to formal training at all levels of medical education. A survey of program directors from primary care, surgery, and anesthesia showed only 26% of respondent programs had a formal curriculum in nutrition education.1 Specific to gastroenterology, a majority of trainees and recent graduates perceived that nutrition education was an important aspect of their training; however, only 50% of respondents had training in nutrition support with 36% reporting mandatory training.3
The Gastroenterology Core Curriculum, most recently updated in 2007 – and sponsored by the American Association for the Study of Liver Diseases, American College of Gastroenterology, the American Society for Gastrointestinal Endoscopy, and the American Gastroenterological Association – includes six domains of nutrition training within the training track: nutrition assessment, basic nutrition requirements, specific gastrointestinal disorders and other allied diseases, enteral nutrition, PN, and diet therapy. Level 1 training is expected for all gastroenterology fellows. Level 2 is comprised, on average, of an additional 12 months with described objectives, either occurring outside of a standard gastroenterology fellowship or coinciding with a dedicated third year of training. Although training durations for level 1 are not defined, level 2 recommends at least 6 months of experience working with an inpatient nutrition support team (NST) and the management of outpatients in nutrition and weight management clinics.5
Role of a nutrition support team
Training in nutrition is a heterogeneous field, with a wide range that covers understanding metabolism in health and disease, micronutrient and macronutrient requirements, nutrient digestion and absorption, and the best route and provision of nutrition support. Therefore, a critical aspect of education includes access to a dedicated NST. Such teams were common and necessary in the late 1900s with the inception of specialized nutrition therapy. However, with an increase in the use of home infusion therapies, NSTs were dismantled in favor of shifting responsibility to decentralized home infusion companies. A dedicated NST often will include some combination of pharmacists with an interest in the safe compounding of parenteral formulas, nurses with experience in the home management of intravenous therapies and catheters, and dietitians with dedicated interests in intestinal failure, recognition of malnutrition, and provision of calories. Collectively, a highly functioning NST also provides dedicated multidisciplinary training to health professionals of varying backgrounds.
My entry into the field of nutrition support
Entering a fellowship in gastroenterology should be pursued with an open mind. We all have varying experiences in the management of patients with gastrointestinal conditions, both in the inpatient and outpatient arenas through residency training. My early experiences in fellowship at the University of Chicago centered on the management of patients with inflammatory bowel disease (IBD) and with research interests related to the clinical course of IBD. I was also fortunate to be part of a fellowship program offering both level 1 and level 2 training with a longstanding track record of graduating fellows responsible for the running of NSTs at their local institutions. Categorical fellows spend 3 months of training on a rotation with combined inpatient and outpatient responsibilities focusing on the management of patients with intestinal failure, inpatient management of complications from PN support, and an outpatient clinic focused on small-bowel disorders (celiac disease, small-bowel bleeding, and intestinal malabsorption). This experience led me to pursue level 2 training at Northwestern University with a combined focus on small-bowel diseases and enteroscopy.
These collective experiences in fellowship and postfellowship training grounded my ideas on the role of nutrition pervading many gastrointestinal conditions from acute and chronic pancreatitis and IBD to rare conditions such as enteropathy associated with immune deficiencies and autoimmune enteropathy. Now, as a junior faculty member with a focus in nutrition support and small-bowel disorders, my clinical responsibilities include a dedicated half-day in the management of outpatients (parenteral and enteral nutrition), inpatient rounding with our dedicated NST focusing on the initiation of PN, management of home PN complications, and dedicated procedural time focusing on enteral access techniques (percutaneous gastrostomy/jejunostomy tubes) and small-bowel enteroscopy. To my surprise, entry into the field of nutrition support and small-bowel disorders has been filled with excitement and a growing list of collaborations and opportunities. While initial work in the management of PN has been in existence since the 1970s and earlier with respect to the development of safe administration techniques, most of my current work transcends specialties as we develop appropriateness criteria related to PN support in collaboration with a wide range of specialties that include surgery, oncology, and palliative care.
Seeking opportunities for additional training
As the field of gastroenterology grows outward in various directions, mastery of subjects has led to subspecialization in specific areas including interventional gastroenterology, pancreatology, IBD, and motility disorders. The field is primed for broader access to specialty training in nutrition support and small-bowel disorders. Exposure to dedicated training in nutrition and nutrition-related disorders is vital as part of a categorical fellowship, but can also be complemented via visiting observerships, access to formal level 2 training programs, and external programs related to promoting nutrition education.
Since 2001, formal nutrition fellowship programs offering level 2 training have been compiled by the National Board of Physician Nutrition Specialists, although attraction of interested fellows has been lacking.2 The Nestlé Nutrition Institute Clinical Nutrition Fellowship, endorsed by the American Society for Parenteral and Enteral Nutrition and the AGA, is an ongoing program that pairs interested trainees with expert program faculty through onsite clinical rotations lasting a total of 4 weeks.2 Attendance at national and international conferences can supplement a fellows training in nutrition, and an increased focus on nutrition lectures should be a priority of meeting education committees to increase the exposure of trainees to leaders in the field.
Conclusion
A career in nutrition support and small-bowel disorders is incredibly rewarding as it incorporates the basic physiologic processes of digestion and absorption with a wide array of pathologic conditions. Incorporation of the basic principles of intestinal absorption allows for a greater understanding of the role of the low–fermentable oligo-, di-, monosaccharides and polyols (FODMAP) diet in the management of irritable bowel syndrome to the varying principles of diets currently under study for the management of IBD. Outside of this spectrum, working with an NST allows for the management of complex cases of malnutrition resulting from disorders ranging from cancer to various postsurgical intestinal alterations. Although observerships and external training programs allow for an introduction into the field, formal level 2 training, combining both work with a NST and small-bowel enteroscopy, allows for exposure to the full range of disorders of the small bowel. As patients continue to seek disease management options rooted in diet, the demand for gastroenterologists with subspecialty training in nutritional disorders will continue to grow and will require further support across training programs to incorporate additional training into categorical fellowships.
References
1. Daley BJ et al. JPEN J Paren Enteral Nutr. 2016;40(1):95-9. doi: 10.1177/0148607115571155.
2. Kiraly LN et al. Nutr Clin Pract. 2014;29(3):332-7. doi: 10.1177/0884533614525212.
3. Hu J et al. Nutr Clin Pract. 2018 Apr;33(2):191-7. doi: 10.1177/0884533617700852.
4. Scolapio JS et al. J Clin Gastroenterol. 2008 Feb;42(2):122-7. doi: 10.1097/MCG.0b013e3181595b6a.
5. American Association for the Study of Liver Diseases et al. The Gastroenterology Core Curriculum, 3rd ed. Gastroenterology. 2007;132(5):2012-8. doi: 10.1053/j.gastro.2007.03.079.
Dr. Micic is assistant professor of medicine, department of internal medicine, section of gastroenterology, hepatology, and nutrition, University of Chicago.
Pitfalls in physician-patient communication via patient access support portals
Technology can be used to enhance communication, increase patient safety, and improve overall patient care. For example, many physicians have arranged for remote access to medical records and established a unique system of communication via a patient access support portal. A patient portal is a secure online website that provides patients 24-hour, on-demand access to their health information. Patient portals, while popular and oftentimes quite helpful, are not without drawbacks. Communication by electronic means with your patient can be viewed by some as impersonal and can make patients less tolerant to what they perceive to be a mistake, error, or unwanted outcome. A decrease in face-to-face contact and communication with your patient also gives you less time to resolve any conflict or disagreement. While communication via a patient access support portal has the potential to free up medical staff for direct patient care, such communication also carries liability risk.
Patient access support portal
A physician’s legal responsibility to communicate in a timely and accurate manner does not change, irrespective of the form of communication. However, communication via a patient access portal does have some unique features that must be considered by the practitioner. Practitioners must remember that any communication via the patient portal creates a permanent record, which can and will be used in the event of litigation. For example, when responding to a patient inquiry about a specific complaint, treatment provided, or test result, it will be presumed that the physician had access to the patient’s full medical record and that the full record will be utilized in making a response. Accessing the patient’s chart will leave an audit trail that will provide what is known as metadata, which in the context of electronic medical records, is what allows technicians to verify that the patient record was accessed, and it provides details as to when, and for how long it was accessed. These records are frequently pursued in litigation, so you must understand that parties can often re-create an intricate and accurate timeline of events. While state courts are divided on the issue of whether metadata contained within electronic medical records is discoverable, recent federal court decisions have held that such data is discoverable pursuant to the Federal Rules of Civil Procedure. Thus, once a patient has communicated with you via the portal, you will be responsible for responding in an appropriate and prompt fashion. For these reasons, it is imperative that you create an agreement with your patients as to how the portal will be used and clearly set forth the rules for such use.
Patient portal policies and procedures
In creating patient portal user agreements (See "Sample User Agreement," attached below), it is crucial that an agreement clearly identify the policies and procedures for use. A patient portal user agreement should:
- Set forth the rules and regulations for portal use.
- Include a verification procedure that requires the patients to confirm that they have the legal capacity to consent to the terms of use. This is especially important when treating patients with mental disability, elderly patients with dementia, minors, and any other individuals who may not legally consent.
- Include a verification procedure that requires the patients to confirm that they understand and agree to abide by the user agreement rules.
- Include a detailed list that informs users of the risks and benefits of communicating via the patient portal.
- Stress that communication through the patient portal is for nonemergent matters only.
- Set forth permissible topics for use, such as communicating with the physician or staff, obtaining test results or records, and setting, changing, or canceling appointments.
- Clearly indicate certain topics that should not be discussed via the patient portal, including mental health issues.
- Reiterate that communication via the patient portal is only one option, and that all other standard methods of communication remain available. In doing so, provide office telephone numbers, hotlines, and email addresses for convenience.
- Inform the patients that they should call the office with any questions or concerns regarding use of the patient portal.
- Include a statement that the patient should call 911 or proceed directly to the nearest hospital for any and all urgent or emergent medical matters.
Other considerations
There are, however, equally critical considerations to be made that go beyond the core details of the user agreement. For instance, use of the patient access portal should be limited to only current or active patients, and you should stress to patients the importance of keeping their contact information updated and accurate. This is especially vital in situations in which a patient is unresponsive to communication via the portal, as your staff will need to follow up via other means of communication. It is also imperative to ensure the patient portal is programmed to promptly alert you or your staff following an inquiry from the patient as the patient will likely expect an immediate response.
Notably, communication via the patient portal must still comply with the Health Insurance Portability and Accountability Act (HIPAA). This means that only authorized users are able to access records within the patient portal. To ensure compliance with HIPAA, all users should be instructed in the appropriate practices of maintaining patient privacy. This includes barring the use of shared passwords amongst multiple individuals, requiring that users enable an auto log-off setting, and programming work stations to turn off automatically after brief periods of nonuse. Further, all communications in the patient portal should be encrypted to prevent the patient’s sensitive information from being accessed in the event of an attempted security breach.
Finally, depending upon the practice, there may be instances in which someone other than the patient’s physician would be reading and responding to patient queries. In these situations, the patient should be informed of such potential. This way, if the communication is intended only for the physician, the patient will be afforded the opportunity to call the physician directly rather than communicate via the patient portal.
While the use of patient access portals is becoming far more prevalent, as they offer many practical benefits ranging from increased convenience and efficiency to enhanced patient care, they also carry the potential for increased liability exposure. As such, it is vital that physicians weigh all potential risks and benefits that are inherent in the use of patient access portals prior to making the decision to implement such technology.
Mr. Mills is an equity partner in Cunningham, Meyer & Vedrine, Chicago.
Technology can be used to enhance communication, increase patient safety, and improve overall patient care. For example, many physicians have arranged for remote access to medical records and established a unique system of communication via a patient access support portal. A patient portal is a secure online website that provides patients 24-hour, on-demand access to their health information. Patient portals, while popular and oftentimes quite helpful, are not without drawbacks. Communication by electronic means with your patient can be viewed by some as impersonal and can make patients less tolerant to what they perceive to be a mistake, error, or unwanted outcome. A decrease in face-to-face contact and communication with your patient also gives you less time to resolve any conflict or disagreement. While communication via a patient access support portal has the potential to free up medical staff for direct patient care, such communication also carries liability risk.
Patient access support portal
A physician’s legal responsibility to communicate in a timely and accurate manner does not change, irrespective of the form of communication. However, communication via a patient access portal does have some unique features that must be considered by the practitioner. Practitioners must remember that any communication via the patient portal creates a permanent record, which can and will be used in the event of litigation. For example, when responding to a patient inquiry about a specific complaint, treatment provided, or test result, it will be presumed that the physician had access to the patient’s full medical record and that the full record will be utilized in making a response. Accessing the patient’s chart will leave an audit trail that will provide what is known as metadata, which in the context of electronic medical records, is what allows technicians to verify that the patient record was accessed, and it provides details as to when, and for how long it was accessed. These records are frequently pursued in litigation, so you must understand that parties can often re-create an intricate and accurate timeline of events. While state courts are divided on the issue of whether metadata contained within electronic medical records is discoverable, recent federal court decisions have held that such data is discoverable pursuant to the Federal Rules of Civil Procedure. Thus, once a patient has communicated with you via the portal, you will be responsible for responding in an appropriate and prompt fashion. For these reasons, it is imperative that you create an agreement with your patients as to how the portal will be used and clearly set forth the rules for such use.
Patient portal policies and procedures
In creating patient portal user agreements (See "Sample User Agreement," attached below), it is crucial that an agreement clearly identify the policies and procedures for use. A patient portal user agreement should:
- Set forth the rules and regulations for portal use.
- Include a verification procedure that requires the patients to confirm that they have the legal capacity to consent to the terms of use. This is especially important when treating patients with mental disability, elderly patients with dementia, minors, and any other individuals who may not legally consent.
- Include a verification procedure that requires the patients to confirm that they understand and agree to abide by the user agreement rules.
- Include a detailed list that informs users of the risks and benefits of communicating via the patient portal.
- Stress that communication through the patient portal is for nonemergent matters only.
- Set forth permissible topics for use, such as communicating with the physician or staff, obtaining test results or records, and setting, changing, or canceling appointments.
- Clearly indicate certain topics that should not be discussed via the patient portal, including mental health issues.
- Reiterate that communication via the patient portal is only one option, and that all other standard methods of communication remain available. In doing so, provide office telephone numbers, hotlines, and email addresses for convenience.
- Inform the patients that they should call the office with any questions or concerns regarding use of the patient portal.
- Include a statement that the patient should call 911 or proceed directly to the nearest hospital for any and all urgent or emergent medical matters.
Other considerations
There are, however, equally critical considerations to be made that go beyond the core details of the user agreement. For instance, use of the patient access portal should be limited to only current or active patients, and you should stress to patients the importance of keeping their contact information updated and accurate. This is especially vital in situations in which a patient is unresponsive to communication via the portal, as your staff will need to follow up via other means of communication. It is also imperative to ensure the patient portal is programmed to promptly alert you or your staff following an inquiry from the patient as the patient will likely expect an immediate response.
Notably, communication via the patient portal must still comply with the Health Insurance Portability and Accountability Act (HIPAA). This means that only authorized users are able to access records within the patient portal. To ensure compliance with HIPAA, all users should be instructed in the appropriate practices of maintaining patient privacy. This includes barring the use of shared passwords amongst multiple individuals, requiring that users enable an auto log-off setting, and programming work stations to turn off automatically after brief periods of nonuse. Further, all communications in the patient portal should be encrypted to prevent the patient’s sensitive information from being accessed in the event of an attempted security breach.
Finally, depending upon the practice, there may be instances in which someone other than the patient’s physician would be reading and responding to patient queries. In these situations, the patient should be informed of such potential. This way, if the communication is intended only for the physician, the patient will be afforded the opportunity to call the physician directly rather than communicate via the patient portal.
While the use of patient access portals is becoming far more prevalent, as they offer many practical benefits ranging from increased convenience and efficiency to enhanced patient care, they also carry the potential for increased liability exposure. As such, it is vital that physicians weigh all potential risks and benefits that are inherent in the use of patient access portals prior to making the decision to implement such technology.
Mr. Mills is an equity partner in Cunningham, Meyer & Vedrine, Chicago.
Technology can be used to enhance communication, increase patient safety, and improve overall patient care. For example, many physicians have arranged for remote access to medical records and established a unique system of communication via a patient access support portal. A patient portal is a secure online website that provides patients 24-hour, on-demand access to their health information. Patient portals, while popular and oftentimes quite helpful, are not without drawbacks. Communication by electronic means with your patient can be viewed by some as impersonal and can make patients less tolerant to what they perceive to be a mistake, error, or unwanted outcome. A decrease in face-to-face contact and communication with your patient also gives you less time to resolve any conflict or disagreement. While communication via a patient access support portal has the potential to free up medical staff for direct patient care, such communication also carries liability risk.
Patient access support portal
A physician’s legal responsibility to communicate in a timely and accurate manner does not change, irrespective of the form of communication. However, communication via a patient access portal does have some unique features that must be considered by the practitioner. Practitioners must remember that any communication via the patient portal creates a permanent record, which can and will be used in the event of litigation. For example, when responding to a patient inquiry about a specific complaint, treatment provided, or test result, it will be presumed that the physician had access to the patient’s full medical record and that the full record will be utilized in making a response. Accessing the patient’s chart will leave an audit trail that will provide what is known as metadata, which in the context of electronic medical records, is what allows technicians to verify that the patient record was accessed, and it provides details as to when, and for how long it was accessed. These records are frequently pursued in litigation, so you must understand that parties can often re-create an intricate and accurate timeline of events. While state courts are divided on the issue of whether metadata contained within electronic medical records is discoverable, recent federal court decisions have held that such data is discoverable pursuant to the Federal Rules of Civil Procedure. Thus, once a patient has communicated with you via the portal, you will be responsible for responding in an appropriate and prompt fashion. For these reasons, it is imperative that you create an agreement with your patients as to how the portal will be used and clearly set forth the rules for such use.
Patient portal policies and procedures
In creating patient portal user agreements (See "Sample User Agreement," attached below), it is crucial that an agreement clearly identify the policies and procedures for use. A patient portal user agreement should:
- Set forth the rules and regulations for portal use.
- Include a verification procedure that requires the patients to confirm that they have the legal capacity to consent to the terms of use. This is especially important when treating patients with mental disability, elderly patients with dementia, minors, and any other individuals who may not legally consent.
- Include a verification procedure that requires the patients to confirm that they understand and agree to abide by the user agreement rules.
- Include a detailed list that informs users of the risks and benefits of communicating via the patient portal.
- Stress that communication through the patient portal is for nonemergent matters only.
- Set forth permissible topics for use, such as communicating with the physician or staff, obtaining test results or records, and setting, changing, or canceling appointments.
- Clearly indicate certain topics that should not be discussed via the patient portal, including mental health issues.
- Reiterate that communication via the patient portal is only one option, and that all other standard methods of communication remain available. In doing so, provide office telephone numbers, hotlines, and email addresses for convenience.
- Inform the patients that they should call the office with any questions or concerns regarding use of the patient portal.
- Include a statement that the patient should call 911 or proceed directly to the nearest hospital for any and all urgent or emergent medical matters.
Other considerations
There are, however, equally critical considerations to be made that go beyond the core details of the user agreement. For instance, use of the patient access portal should be limited to only current or active patients, and you should stress to patients the importance of keeping their contact information updated and accurate. This is especially vital in situations in which a patient is unresponsive to communication via the portal, as your staff will need to follow up via other means of communication. It is also imperative to ensure the patient portal is programmed to promptly alert you or your staff following an inquiry from the patient as the patient will likely expect an immediate response.
Notably, communication via the patient portal must still comply with the Health Insurance Portability and Accountability Act (HIPAA). This means that only authorized users are able to access records within the patient portal. To ensure compliance with HIPAA, all users should be instructed in the appropriate practices of maintaining patient privacy. This includes barring the use of shared passwords amongst multiple individuals, requiring that users enable an auto log-off setting, and programming work stations to turn off automatically after brief periods of nonuse. Further, all communications in the patient portal should be encrypted to prevent the patient’s sensitive information from being accessed in the event of an attempted security breach.
Finally, depending upon the practice, there may be instances in which someone other than the patient’s physician would be reading and responding to patient queries. In these situations, the patient should be informed of such potential. This way, if the communication is intended only for the physician, the patient will be afforded the opportunity to call the physician directly rather than communicate via the patient portal.
While the use of patient access portals is becoming far more prevalent, as they offer many practical benefits ranging from increased convenience and efficiency to enhanced patient care, they also carry the potential for increased liability exposure. As such, it is vital that physicians weigh all potential risks and benefits that are inherent in the use of patient access portals prior to making the decision to implement such technology.
Mr. Mills is an equity partner in Cunningham, Meyer & Vedrine, Chicago.
Ambulatory surgery centers 101: What new GIs need to know
Almost 20 years ago, I joined Digestive Disease Specialists in Oklahoma, where I am an owner in two ambulatory endoscopy centers (AECs). Through this experience, I’ve learned a thing or two about the advantages of these centers and what to consider when joining a practice that has ownership in an endoscopy center or an ambulatory surgery center (ASC).
ASCs – or in my case AECs – are highly specialized, modern health care facilities in which physicians provide safe, high-quality procedures to millions of Americans each year, including diagnostic and preventive procedures. ASCs and AECs allow us to provide a more convenient and cost-effective alternative to performing GI procedures in a hospital. As you can imagine, these facilities are a vital part of being an independent gastroenterologist.
Quality care at a lower cost
When looking into practices with ownership in surgery centers, quality of care is one of the most critical considerations. Each center must enter into an agreement with Medicare and meet its certification requirements, which are similar to those required for hospital outpatient departments. We also undergo accreditation by the Accreditation Association for Ambulatory Healthcare. And while not everyone participates in quality registries such as GIquic – which helps us define and refine our endoscopic outcomes – our AEC does because we put patient care first.
This focus on quality leads to better care. A 2016 study of Medicare claims in the Journal of Health Economics shows that ASCs and AECs, on average, provide higher-quality care for outpatient procedures than hospitals.1
We get into medicine because we care about patients and want to provide them with the best and most convenient care possible. Because we don’t have to operate in the context of a large hospital, we can be nimbler when it comes to patient needs. As is true in other areas, the more you specialize, the more effective and efficient you can become performing certain procedures. For instance, our practice is highly specialized in endoscopy. As a result, we can be highly efficient in the turnaround time in between patient procedures. This helps people get back to their daily lives as soon as possible, whereas patients may spend a lot more time waiting to have their procedure in the hospital setting.
Performing procedures in an ASC or AEC also helps us keep costs down, and we spend a lot of time educating policymakers about the role independent physicians play in lowering health care costs. According to a recent study in Health Affairs, hospital-based outpatient care prices grew at four times the rate of physician prices from 2007 to 2014.2 And the National Institute for Health Care Reform found that the average Medicare facility payment for a basic colonoscopy was more than twice as much in a hospital setting.3
The challenges and benefits of running an ASC or AEC
With consolidation of hospitals and insurance companies, operating an independent ASC or AEC has become more challenging. The players are bigger, providing more competition. Where we practice in Oklahoma, we are the only independent gastroenterologists. The way we have kept up is by diversifying the ancillary services we offer. This includes infusion center services, pathology, anesthesia, and research alongside our standard endoscopic and office/hospital-based practice.
As doctors, we are not typically trained on the business side of medicine, but when you own an ASC, you need to learn to be your own boss. You will need to understand the details of how the business operates, from relevant state and federal regulations to the supportive services that make the center run smoothly.
Gastroenterologists who are new to the field can and should ask questions of any practice they are considering joining about the buy-in process for ASCs and the path to becoming a partner. One of the things to consider is what kind of planning has gone into determining how many investors there are in the ASC. It is possible to have too many physician partners, but having too few could also present challenges. If there are too many physician owners, ownership interests could be diluted. On the flip side, if there aren’t many physician owners, it will be more expensive to buy in and there will be greater risk.
Another question to ask is if the practice partners with a hospital for its ASC or AEC. About one in four surgery centers have a hospital partner. This can be helpful with managed care contracting and concerns physicians may have about being excluded from having hospital privileges. Partnering with a hospital is not an indication of a successful center given there are still many surgery centers with hospital partners that are not run well or underperform.
While I have outlined some of the challenges of owning an ASC or AEC, physician ownership can be very attractive under the right circumstances. There are many benefits including having more control over your life. You get to set your own schedules and determine your patient load.
When physicians own the surgery center, we can control the schedule and the environment in the operating room. In a hospital, there is not much we can do if the operating room is not running efficiently. If our cases are bumped, the care of our patients is undermined. In our own surgery center, we can ensure that these things do not happen. We are able to provide our patients the best care possible.
When I look back over my time in our AEC, I wouldn’t change anything. Even though there may be challenges in running an AEC, I would advise young gastroenterologists to consider this path. Nothing worthwhile is easy, but the ability to provide the best care to our patients is its own reward.
References
1. Munnich E et al. J Health Economics, January 2018;57:147-67. https://doi.org/10.1016/j.jhealeco.2017.11.004.
2. Cooper Z et al. https://doi.org/10.1377/hlthaff.2018.05424. Health Affairs 2019;38(2).
3. Reschovsky J et al. NIHCR Research Brief No. 16. http://nihcr.org/wp-content/uploads/2016/07/Research_Brief_No._16.pdf. National Institute for Health Care Reform; June 2014.
Dr. Stokesberry is a practicing gastroenterologist at Digestive Disease Specialists in Oklahoma City and serves as an executive committee member of the Digestive Health Physicians Association.
Almost 20 years ago, I joined Digestive Disease Specialists in Oklahoma, where I am an owner in two ambulatory endoscopy centers (AECs). Through this experience, I’ve learned a thing or two about the advantages of these centers and what to consider when joining a practice that has ownership in an endoscopy center or an ambulatory surgery center (ASC).
ASCs – or in my case AECs – are highly specialized, modern health care facilities in which physicians provide safe, high-quality procedures to millions of Americans each year, including diagnostic and preventive procedures. ASCs and AECs allow us to provide a more convenient and cost-effective alternative to performing GI procedures in a hospital. As you can imagine, these facilities are a vital part of being an independent gastroenterologist.
Quality care at a lower cost
When looking into practices with ownership in surgery centers, quality of care is one of the most critical considerations. Each center must enter into an agreement with Medicare and meet its certification requirements, which are similar to those required for hospital outpatient departments. We also undergo accreditation by the Accreditation Association for Ambulatory Healthcare. And while not everyone participates in quality registries such as GIquic – which helps us define and refine our endoscopic outcomes – our AEC does because we put patient care first.
This focus on quality leads to better care. A 2016 study of Medicare claims in the Journal of Health Economics shows that ASCs and AECs, on average, provide higher-quality care for outpatient procedures than hospitals.1
We get into medicine because we care about patients and want to provide them with the best and most convenient care possible. Because we don’t have to operate in the context of a large hospital, we can be nimbler when it comes to patient needs. As is true in other areas, the more you specialize, the more effective and efficient you can become performing certain procedures. For instance, our practice is highly specialized in endoscopy. As a result, we can be highly efficient in the turnaround time in between patient procedures. This helps people get back to their daily lives as soon as possible, whereas patients may spend a lot more time waiting to have their procedure in the hospital setting.
Performing procedures in an ASC or AEC also helps us keep costs down, and we spend a lot of time educating policymakers about the role independent physicians play in lowering health care costs. According to a recent study in Health Affairs, hospital-based outpatient care prices grew at four times the rate of physician prices from 2007 to 2014.2 And the National Institute for Health Care Reform found that the average Medicare facility payment for a basic colonoscopy was more than twice as much in a hospital setting.3
The challenges and benefits of running an ASC or AEC
With consolidation of hospitals and insurance companies, operating an independent ASC or AEC has become more challenging. The players are bigger, providing more competition. Where we practice in Oklahoma, we are the only independent gastroenterologists. The way we have kept up is by diversifying the ancillary services we offer. This includes infusion center services, pathology, anesthesia, and research alongside our standard endoscopic and office/hospital-based practice.
As doctors, we are not typically trained on the business side of medicine, but when you own an ASC, you need to learn to be your own boss. You will need to understand the details of how the business operates, from relevant state and federal regulations to the supportive services that make the center run smoothly.
Gastroenterologists who are new to the field can and should ask questions of any practice they are considering joining about the buy-in process for ASCs and the path to becoming a partner. One of the things to consider is what kind of planning has gone into determining how many investors there are in the ASC. It is possible to have too many physician partners, but having too few could also present challenges. If there are too many physician owners, ownership interests could be diluted. On the flip side, if there aren’t many physician owners, it will be more expensive to buy in and there will be greater risk.
Another question to ask is if the practice partners with a hospital for its ASC or AEC. About one in four surgery centers have a hospital partner. This can be helpful with managed care contracting and concerns physicians may have about being excluded from having hospital privileges. Partnering with a hospital is not an indication of a successful center given there are still many surgery centers with hospital partners that are not run well or underperform.
While I have outlined some of the challenges of owning an ASC or AEC, physician ownership can be very attractive under the right circumstances. There are many benefits including having more control over your life. You get to set your own schedules and determine your patient load.
When physicians own the surgery center, we can control the schedule and the environment in the operating room. In a hospital, there is not much we can do if the operating room is not running efficiently. If our cases are bumped, the care of our patients is undermined. In our own surgery center, we can ensure that these things do not happen. We are able to provide our patients the best care possible.
When I look back over my time in our AEC, I wouldn’t change anything. Even though there may be challenges in running an AEC, I would advise young gastroenterologists to consider this path. Nothing worthwhile is easy, but the ability to provide the best care to our patients is its own reward.
References
1. Munnich E et al. J Health Economics, January 2018;57:147-67. https://doi.org/10.1016/j.jhealeco.2017.11.004.
2. Cooper Z et al. https://doi.org/10.1377/hlthaff.2018.05424. Health Affairs 2019;38(2).
3. Reschovsky J et al. NIHCR Research Brief No. 16. http://nihcr.org/wp-content/uploads/2016/07/Research_Brief_No._16.pdf. National Institute for Health Care Reform; June 2014.
Dr. Stokesberry is a practicing gastroenterologist at Digestive Disease Specialists in Oklahoma City and serves as an executive committee member of the Digestive Health Physicians Association.
Almost 20 years ago, I joined Digestive Disease Specialists in Oklahoma, where I am an owner in two ambulatory endoscopy centers (AECs). Through this experience, I’ve learned a thing or two about the advantages of these centers and what to consider when joining a practice that has ownership in an endoscopy center or an ambulatory surgery center (ASC).
ASCs – or in my case AECs – are highly specialized, modern health care facilities in which physicians provide safe, high-quality procedures to millions of Americans each year, including diagnostic and preventive procedures. ASCs and AECs allow us to provide a more convenient and cost-effective alternative to performing GI procedures in a hospital. As you can imagine, these facilities are a vital part of being an independent gastroenterologist.
Quality care at a lower cost
When looking into practices with ownership in surgery centers, quality of care is one of the most critical considerations. Each center must enter into an agreement with Medicare and meet its certification requirements, which are similar to those required for hospital outpatient departments. We also undergo accreditation by the Accreditation Association for Ambulatory Healthcare. And while not everyone participates in quality registries such as GIquic – which helps us define and refine our endoscopic outcomes – our AEC does because we put patient care first.
This focus on quality leads to better care. A 2016 study of Medicare claims in the Journal of Health Economics shows that ASCs and AECs, on average, provide higher-quality care for outpatient procedures than hospitals.1
We get into medicine because we care about patients and want to provide them with the best and most convenient care possible. Because we don’t have to operate in the context of a large hospital, we can be nimbler when it comes to patient needs. As is true in other areas, the more you specialize, the more effective and efficient you can become performing certain procedures. For instance, our practice is highly specialized in endoscopy. As a result, we can be highly efficient in the turnaround time in between patient procedures. This helps people get back to their daily lives as soon as possible, whereas patients may spend a lot more time waiting to have their procedure in the hospital setting.
Performing procedures in an ASC or AEC also helps us keep costs down, and we spend a lot of time educating policymakers about the role independent physicians play in lowering health care costs. According to a recent study in Health Affairs, hospital-based outpatient care prices grew at four times the rate of physician prices from 2007 to 2014.2 And the National Institute for Health Care Reform found that the average Medicare facility payment for a basic colonoscopy was more than twice as much in a hospital setting.3
The challenges and benefits of running an ASC or AEC
With consolidation of hospitals and insurance companies, operating an independent ASC or AEC has become more challenging. The players are bigger, providing more competition. Where we practice in Oklahoma, we are the only independent gastroenterologists. The way we have kept up is by diversifying the ancillary services we offer. This includes infusion center services, pathology, anesthesia, and research alongside our standard endoscopic and office/hospital-based practice.
As doctors, we are not typically trained on the business side of medicine, but when you own an ASC, you need to learn to be your own boss. You will need to understand the details of how the business operates, from relevant state and federal regulations to the supportive services that make the center run smoothly.
Gastroenterologists who are new to the field can and should ask questions of any practice they are considering joining about the buy-in process for ASCs and the path to becoming a partner. One of the things to consider is what kind of planning has gone into determining how many investors there are in the ASC. It is possible to have too many physician partners, but having too few could also present challenges. If there are too many physician owners, ownership interests could be diluted. On the flip side, if there aren’t many physician owners, it will be more expensive to buy in and there will be greater risk.
Another question to ask is if the practice partners with a hospital for its ASC or AEC. About one in four surgery centers have a hospital partner. This can be helpful with managed care contracting and concerns physicians may have about being excluded from having hospital privileges. Partnering with a hospital is not an indication of a successful center given there are still many surgery centers with hospital partners that are not run well or underperform.
While I have outlined some of the challenges of owning an ASC or AEC, physician ownership can be very attractive under the right circumstances. There are many benefits including having more control over your life. You get to set your own schedules and determine your patient load.
When physicians own the surgery center, we can control the schedule and the environment in the operating room. In a hospital, there is not much we can do if the operating room is not running efficiently. If our cases are bumped, the care of our patients is undermined. In our own surgery center, we can ensure that these things do not happen. We are able to provide our patients the best care possible.
When I look back over my time in our AEC, I wouldn’t change anything. Even though there may be challenges in running an AEC, I would advise young gastroenterologists to consider this path. Nothing worthwhile is easy, but the ability to provide the best care to our patients is its own reward.
References
1. Munnich E et al. J Health Economics, January 2018;57:147-67. https://doi.org/10.1016/j.jhealeco.2017.11.004.
2. Cooper Z et al. https://doi.org/10.1377/hlthaff.2018.05424. Health Affairs 2019;38(2).
3. Reschovsky J et al. NIHCR Research Brief No. 16. http://nihcr.org/wp-content/uploads/2016/07/Research_Brief_No._16.pdf. National Institute for Health Care Reform; June 2014.
Dr. Stokesberry is a practicing gastroenterologist at Digestive Disease Specialists in Oklahoma City and serves as an executive committee member of the Digestive Health Physicians Association.
Gastroenterology practice evaluations: Can patients get satisfaction?
Although largely untouched by the first and second industrial revolutions in the 18th and 20th centuries, the practice of medicine in the 21st century is increasingly susceptible to the vast transformative power of the third – and rapidly approaching fourth – industrial revolutions. New technological advances and their associated distribution of knowledge and connectedness have allowed patients unprecedented access to health care information. The salutary effects of this change is manifest in a diversity of areas, including registries that facilitate participation in state of the art research such as ClinicalTrials.gov and the ability to track nascent trends in infectious diseases with Google searches.1
Although the stakes may seem lower when patients go online to choose a practitioner, the reality demonstrates just how important those search results can be. With parallels of similar trends in other sectors, there is an increasing emphasis on ranking health care facilities, practitioners, and medical experiences. This phenomenon extends beyond private Internet sites into government scorecards, which has significant implications. But even with widespread access to information, there is frequently a lack of context for interpreting these data. Consequently, it is worth exploring why measuring satisfaction can be important, how patients can rate practitioners, and what to do with the available information to improve care delivery.
The idea to measure patient satisfaction of delivered health care began in earnest during the 1980s with Irwin Press and Rodney Ganey collaborating to create formal processes for collecting data on the “salient aspects of ... health care experience, [involving] the interaction of expectations, preferences, and satisfaction with medical care.”2,3 The enthusiasm for collecting these data has grown greatly since that time. More recently, the federal government began obtaining data in 2002 when the Centers for Medicaid & Medicare Services and the Agency for Healthcare Research and Quality (AHRQ) collaborated to develop a standardized questionnaire for hospitalized patients known as the Hospital Consumer Assessment of Healthcare Providers and Systems, or HCAHPS.4 Subsequently, standardized survey instruments have been developed for nearly every phase of care, including outpatient care (CG-CAHPS), emergency care (ED-CAHPS), and ambulatory surgery care (OAS-CAHPS). These instruments are particularly relevant to gastroenterologists, with questions querying patients about preprocedure instructions, surgery center check-in processes, comfort of procedure and waiting rooms, friendliness of providers, and quality of postprocedure information.
The focus on rating satisfaction intensified in 2010 after the passage of the Affordable Care Act (ACA). Around this time, patient satisfaction and health outcomes became more deeply integrated concepts in health care quality. As part of a broader emphasis in this area, CMS initiated the hospital value-based purchasing (VBP) program, which tied incentive payments for Medicare beneficiaries to hospital-based health care quality and patient satisfaction. Within this schema, 25% of performance, and its associated economic stakes, is measured by HCAHPS scores.5 Other value programs such as the Merit-Based Incentive Payment Program (MIPS) include CAHPS instruments as optional assessments of quality.
Given the financial risks linked to satisfaction rankings and their online visibility, many argue that patient satisfaction is prioritized in organizations above more clinically meaningful metrics. Studies have shown, however, that high levels of patient satisfaction can lead to increased patient loyalty, treatment adherence, patient retention, staff morale, and personal and professional satisfaction.6,7 In fact, not surprisingly, there is an inverse correlation between patient satisfaction and the rates of malpractice lawsuits.7-10
Despite the growing relevance of patient perceptions to clinical practice, measuring satisfaction remains a challenge. While current metrics are particular to an individual patient’s experiences, underlying health conditions influence opinions of these episodes of care. Specifically, patients with depression and anxiety are, in general, less satisfied with the care they receive.11,12 Similarly, patients with chronic diseases on multiple medications and those with more severe symptoms are commonly less satisfied with their care than are patients with acute issues2 and with milder symptoms.3 As gastroenterologists, seeing sicker patients with chronic conditions is not uncommon, and this could serve as a disadvantage when compared with peers in other specialties because scores are not typically adjusted.
Since patient-centered metrics are likely to remain relevant in the future, and with the unique challenges this can present to practicing gastroenterologists, achieving higher degrees of patient satisfaction remains both aspirational and difficult. We will be asked to reconcile and manage not only clinical conundrums but also seemingly conflicting realities of patient preferences. For example, it has been shown that, among patients with irritable bowel syndrome (IBS), more testing led to higher satisfaction only until that testing was performed within the context of a gastroenterologist’s care.13 In contrast, within the endoscopy setting, a preprocedure diagnosis of IBS did not increase the risk for procedure-related dissatisfaction, provided patients were not prescribed chronic psychotropic medication, nervous prior to the procedure, distressed or in pain during the procedure, or had unmet physical or emotional needs during the procedure.14 Furthermore, there is poor correlation between endoscopic quality measures with strong evidence – such as adenoma detection rate, withdrawal time, and cecal intubation rate – and patient satisfaction.15
So, when considering these conflicting findings and evidence that patients’ global rating of their health care is not reliably associated with the quality of the care they receive,16 should we emphasize experience over outcome? As clinicians practicing in an increasingly transparent and value-based health care environment, we are subject to many priorities contending for our attention. We strive to provide care that is at once patient centric, evidence based, and low cost; however, achieving these goals often requires different strategies. At the end of the day, our primary aim is to provide consistently excellent patient care. We believe that quality and experience are not competing principles. Patient satisfaction is relevant and important, but it should not preclude adherence to our primary responsibility of providing high-quality care.
When trying to make clinical decisions that may compromise one of these goals for another, it can be helpful to recall the “me and my family” rule: What kind of care would I want for myself or my loved ones in this situation?
Acknowledgement
We thank Dr. Ziad Gellad (Duke University, Durham, N.C.) for his assistance in reviewing and providing feedback on this manuscript.
1. Proc Natl Acad Sci U S A. 2015;112(47):14473-8. 2. Am J Manag Care. 1997;3(4):579-94.
3. Gut. 2004;53(SUPPL. 4):40-4.
4. Virtual Mentor. 2013;15(11):982-7.
5. J Hosp Med. 2013;8(5):271-7.
6. Int J Health Care Qual Assur. 2011;24(4):266-73.
7. J Cutan Aesthet Surg. 2010;3(3):151-5.
8. Am J Med. 2005;118(10):1126-33.
9. JAMA. 2002;287(22):2951-7. 10. JAMA. 1994;272(20):1583-7.
11. J Diabetes Metab. 2012;3(7):1000210.
12. Am Heart J. 2000;140(1):105-10.
13. J Clin Gastroenterol. 2018;52(7):614-21.
14. Dig Dis Sci. 2005;50(10):1860-71.15. Am J Gastroenterol. 2014;109(7):1089-91.
16. Ann Intern Med. 2006;144(9):665-72.
Dr. Finn is a gastroenterologist with the Palo Alto Medical Foundation, Mountain View, Calif.; Dr. Leiman is assistant professor of medicine, director of esophageal research and quality in the division of gastroenterology, Duke University, Duke Clinical Research Institute, and chair-elect of the AGA Quality Committee.
Although largely untouched by the first and second industrial revolutions in the 18th and 20th centuries, the practice of medicine in the 21st century is increasingly susceptible to the vast transformative power of the third – and rapidly approaching fourth – industrial revolutions. New technological advances and their associated distribution of knowledge and connectedness have allowed patients unprecedented access to health care information. The salutary effects of this change is manifest in a diversity of areas, including registries that facilitate participation in state of the art research such as ClinicalTrials.gov and the ability to track nascent trends in infectious diseases with Google searches.1
Although the stakes may seem lower when patients go online to choose a practitioner, the reality demonstrates just how important those search results can be. With parallels of similar trends in other sectors, there is an increasing emphasis on ranking health care facilities, practitioners, and medical experiences. This phenomenon extends beyond private Internet sites into government scorecards, which has significant implications. But even with widespread access to information, there is frequently a lack of context for interpreting these data. Consequently, it is worth exploring why measuring satisfaction can be important, how patients can rate practitioners, and what to do with the available information to improve care delivery.
The idea to measure patient satisfaction of delivered health care began in earnest during the 1980s with Irwin Press and Rodney Ganey collaborating to create formal processes for collecting data on the “salient aspects of ... health care experience, [involving] the interaction of expectations, preferences, and satisfaction with medical care.”2,3 The enthusiasm for collecting these data has grown greatly since that time. More recently, the federal government began obtaining data in 2002 when the Centers for Medicaid & Medicare Services and the Agency for Healthcare Research and Quality (AHRQ) collaborated to develop a standardized questionnaire for hospitalized patients known as the Hospital Consumer Assessment of Healthcare Providers and Systems, or HCAHPS.4 Subsequently, standardized survey instruments have been developed for nearly every phase of care, including outpatient care (CG-CAHPS), emergency care (ED-CAHPS), and ambulatory surgery care (OAS-CAHPS). These instruments are particularly relevant to gastroenterologists, with questions querying patients about preprocedure instructions, surgery center check-in processes, comfort of procedure and waiting rooms, friendliness of providers, and quality of postprocedure information.
The focus on rating satisfaction intensified in 2010 after the passage of the Affordable Care Act (ACA). Around this time, patient satisfaction and health outcomes became more deeply integrated concepts in health care quality. As part of a broader emphasis in this area, CMS initiated the hospital value-based purchasing (VBP) program, which tied incentive payments for Medicare beneficiaries to hospital-based health care quality and patient satisfaction. Within this schema, 25% of performance, and its associated economic stakes, is measured by HCAHPS scores.5 Other value programs such as the Merit-Based Incentive Payment Program (MIPS) include CAHPS instruments as optional assessments of quality.
Given the financial risks linked to satisfaction rankings and their online visibility, many argue that patient satisfaction is prioritized in organizations above more clinically meaningful metrics. Studies have shown, however, that high levels of patient satisfaction can lead to increased patient loyalty, treatment adherence, patient retention, staff morale, and personal and professional satisfaction.6,7 In fact, not surprisingly, there is an inverse correlation between patient satisfaction and the rates of malpractice lawsuits.7-10
Despite the growing relevance of patient perceptions to clinical practice, measuring satisfaction remains a challenge. While current metrics are particular to an individual patient’s experiences, underlying health conditions influence opinions of these episodes of care. Specifically, patients with depression and anxiety are, in general, less satisfied with the care they receive.11,12 Similarly, patients with chronic diseases on multiple medications and those with more severe symptoms are commonly less satisfied with their care than are patients with acute issues2 and with milder symptoms.3 As gastroenterologists, seeing sicker patients with chronic conditions is not uncommon, and this could serve as a disadvantage when compared with peers in other specialties because scores are not typically adjusted.
Since patient-centered metrics are likely to remain relevant in the future, and with the unique challenges this can present to practicing gastroenterologists, achieving higher degrees of patient satisfaction remains both aspirational and difficult. We will be asked to reconcile and manage not only clinical conundrums but also seemingly conflicting realities of patient preferences. For example, it has been shown that, among patients with irritable bowel syndrome (IBS), more testing led to higher satisfaction only until that testing was performed within the context of a gastroenterologist’s care.13 In contrast, within the endoscopy setting, a preprocedure diagnosis of IBS did not increase the risk for procedure-related dissatisfaction, provided patients were not prescribed chronic psychotropic medication, nervous prior to the procedure, distressed or in pain during the procedure, or had unmet physical or emotional needs during the procedure.14 Furthermore, there is poor correlation between endoscopic quality measures with strong evidence – such as adenoma detection rate, withdrawal time, and cecal intubation rate – and patient satisfaction.15
So, when considering these conflicting findings and evidence that patients’ global rating of their health care is not reliably associated with the quality of the care they receive,16 should we emphasize experience over outcome? As clinicians practicing in an increasingly transparent and value-based health care environment, we are subject to many priorities contending for our attention. We strive to provide care that is at once patient centric, evidence based, and low cost; however, achieving these goals often requires different strategies. At the end of the day, our primary aim is to provide consistently excellent patient care. We believe that quality and experience are not competing principles. Patient satisfaction is relevant and important, but it should not preclude adherence to our primary responsibility of providing high-quality care.
When trying to make clinical decisions that may compromise one of these goals for another, it can be helpful to recall the “me and my family” rule: What kind of care would I want for myself or my loved ones in this situation?
Acknowledgement
We thank Dr. Ziad Gellad (Duke University, Durham, N.C.) for his assistance in reviewing and providing feedback on this manuscript.
1. Proc Natl Acad Sci U S A. 2015;112(47):14473-8. 2. Am J Manag Care. 1997;3(4):579-94.
3. Gut. 2004;53(SUPPL. 4):40-4.
4. Virtual Mentor. 2013;15(11):982-7.
5. J Hosp Med. 2013;8(5):271-7.
6. Int J Health Care Qual Assur. 2011;24(4):266-73.
7. J Cutan Aesthet Surg. 2010;3(3):151-5.
8. Am J Med. 2005;118(10):1126-33.
9. JAMA. 2002;287(22):2951-7. 10. JAMA. 1994;272(20):1583-7.
11. J Diabetes Metab. 2012;3(7):1000210.
12. Am Heart J. 2000;140(1):105-10.
13. J Clin Gastroenterol. 2018;52(7):614-21.
14. Dig Dis Sci. 2005;50(10):1860-71.15. Am J Gastroenterol. 2014;109(7):1089-91.
16. Ann Intern Med. 2006;144(9):665-72.
Dr. Finn is a gastroenterologist with the Palo Alto Medical Foundation, Mountain View, Calif.; Dr. Leiman is assistant professor of medicine, director of esophageal research and quality in the division of gastroenterology, Duke University, Duke Clinical Research Institute, and chair-elect of the AGA Quality Committee.
Although largely untouched by the first and second industrial revolutions in the 18th and 20th centuries, the practice of medicine in the 21st century is increasingly susceptible to the vast transformative power of the third – and rapidly approaching fourth – industrial revolutions. New technological advances and their associated distribution of knowledge and connectedness have allowed patients unprecedented access to health care information. The salutary effects of this change is manifest in a diversity of areas, including registries that facilitate participation in state of the art research such as ClinicalTrials.gov and the ability to track nascent trends in infectious diseases with Google searches.1
Although the stakes may seem lower when patients go online to choose a practitioner, the reality demonstrates just how important those search results can be. With parallels of similar trends in other sectors, there is an increasing emphasis on ranking health care facilities, practitioners, and medical experiences. This phenomenon extends beyond private Internet sites into government scorecards, which has significant implications. But even with widespread access to information, there is frequently a lack of context for interpreting these data. Consequently, it is worth exploring why measuring satisfaction can be important, how patients can rate practitioners, and what to do with the available information to improve care delivery.
The idea to measure patient satisfaction of delivered health care began in earnest during the 1980s with Irwin Press and Rodney Ganey collaborating to create formal processes for collecting data on the “salient aspects of ... health care experience, [involving] the interaction of expectations, preferences, and satisfaction with medical care.”2,3 The enthusiasm for collecting these data has grown greatly since that time. More recently, the federal government began obtaining data in 2002 when the Centers for Medicaid & Medicare Services and the Agency for Healthcare Research and Quality (AHRQ) collaborated to develop a standardized questionnaire for hospitalized patients known as the Hospital Consumer Assessment of Healthcare Providers and Systems, or HCAHPS.4 Subsequently, standardized survey instruments have been developed for nearly every phase of care, including outpatient care (CG-CAHPS), emergency care (ED-CAHPS), and ambulatory surgery care (OAS-CAHPS). These instruments are particularly relevant to gastroenterologists, with questions querying patients about preprocedure instructions, surgery center check-in processes, comfort of procedure and waiting rooms, friendliness of providers, and quality of postprocedure information.
The focus on rating satisfaction intensified in 2010 after the passage of the Affordable Care Act (ACA). Around this time, patient satisfaction and health outcomes became more deeply integrated concepts in health care quality. As part of a broader emphasis in this area, CMS initiated the hospital value-based purchasing (VBP) program, which tied incentive payments for Medicare beneficiaries to hospital-based health care quality and patient satisfaction. Within this schema, 25% of performance, and its associated economic stakes, is measured by HCAHPS scores.5 Other value programs such as the Merit-Based Incentive Payment Program (MIPS) include CAHPS instruments as optional assessments of quality.
Given the financial risks linked to satisfaction rankings and their online visibility, many argue that patient satisfaction is prioritized in organizations above more clinically meaningful metrics. Studies have shown, however, that high levels of patient satisfaction can lead to increased patient loyalty, treatment adherence, patient retention, staff morale, and personal and professional satisfaction.6,7 In fact, not surprisingly, there is an inverse correlation between patient satisfaction and the rates of malpractice lawsuits.7-10
Despite the growing relevance of patient perceptions to clinical practice, measuring satisfaction remains a challenge. While current metrics are particular to an individual patient’s experiences, underlying health conditions influence opinions of these episodes of care. Specifically, patients with depression and anxiety are, in general, less satisfied with the care they receive.11,12 Similarly, patients with chronic diseases on multiple medications and those with more severe symptoms are commonly less satisfied with their care than are patients with acute issues2 and with milder symptoms.3 As gastroenterologists, seeing sicker patients with chronic conditions is not uncommon, and this could serve as a disadvantage when compared with peers in other specialties because scores are not typically adjusted.
Since patient-centered metrics are likely to remain relevant in the future, and with the unique challenges this can present to practicing gastroenterologists, achieving higher degrees of patient satisfaction remains both aspirational and difficult. We will be asked to reconcile and manage not only clinical conundrums but also seemingly conflicting realities of patient preferences. For example, it has been shown that, among patients with irritable bowel syndrome (IBS), more testing led to higher satisfaction only until that testing was performed within the context of a gastroenterologist’s care.13 In contrast, within the endoscopy setting, a preprocedure diagnosis of IBS did not increase the risk for procedure-related dissatisfaction, provided patients were not prescribed chronic psychotropic medication, nervous prior to the procedure, distressed or in pain during the procedure, or had unmet physical or emotional needs during the procedure.14 Furthermore, there is poor correlation between endoscopic quality measures with strong evidence – such as adenoma detection rate, withdrawal time, and cecal intubation rate – and patient satisfaction.15
So, when considering these conflicting findings and evidence that patients’ global rating of their health care is not reliably associated with the quality of the care they receive,16 should we emphasize experience over outcome? As clinicians practicing in an increasingly transparent and value-based health care environment, we are subject to many priorities contending for our attention. We strive to provide care that is at once patient centric, evidence based, and low cost; however, achieving these goals often requires different strategies. At the end of the day, our primary aim is to provide consistently excellent patient care. We believe that quality and experience are not competing principles. Patient satisfaction is relevant and important, but it should not preclude adherence to our primary responsibility of providing high-quality care.
When trying to make clinical decisions that may compromise one of these goals for another, it can be helpful to recall the “me and my family” rule: What kind of care would I want for myself or my loved ones in this situation?
Acknowledgement
We thank Dr. Ziad Gellad (Duke University, Durham, N.C.) for his assistance in reviewing and providing feedback on this manuscript.
1. Proc Natl Acad Sci U S A. 2015;112(47):14473-8. 2. Am J Manag Care. 1997;3(4):579-94.
3. Gut. 2004;53(SUPPL. 4):40-4.
4. Virtual Mentor. 2013;15(11):982-7.
5. J Hosp Med. 2013;8(5):271-7.
6. Int J Health Care Qual Assur. 2011;24(4):266-73.
7. J Cutan Aesthet Surg. 2010;3(3):151-5.
8. Am J Med. 2005;118(10):1126-33.
9. JAMA. 2002;287(22):2951-7. 10. JAMA. 1994;272(20):1583-7.
11. J Diabetes Metab. 2012;3(7):1000210.
12. Am Heart J. 2000;140(1):105-10.
13. J Clin Gastroenterol. 2018;52(7):614-21.
14. Dig Dis Sci. 2005;50(10):1860-71.15. Am J Gastroenterol. 2014;109(7):1089-91.
16. Ann Intern Med. 2006;144(9):665-72.
Dr. Finn is a gastroenterologist with the Palo Alto Medical Foundation, Mountain View, Calif.; Dr. Leiman is assistant professor of medicine, director of esophageal research and quality in the division of gastroenterology, Duke University, Duke Clinical Research Institute, and chair-elect of the AGA Quality Committee.
Taking the editorial torch
Dear colleagues,
I am excited to introduce the November issue of The New Gastroenterologist – which is also my first issue as the new Editor in Chief! First, I am incredibly grateful for this opportunity to be a part of the only existing publication tailored toward trainees and early-career gastroenterologists. Bryson Katona has done a remarkable job for the last 5 years as the publication’s inaugural EIC, as he has laid a great deal of groundwork and really set the standard going forward. Each issue has been a multifaceted compilation of salient clinical topics paired with brief but high-yield articles to help guide personal and professional growth; I hope to continue to do the same and maintain a high level of interest in our newsletter.
In this issue, the In Focus article, brought to you by Adeeti Chiplunker and Christina Ha (Cedars Sinai), discusses inpatient management of acute severe ulcerative colitis. It is an excellent review of the diagnostic workup and therapeutic options, and an important one, as therapies are quickly evolving in inflammatory bowel disease. We also have Manol Jovani (Johns Hopkins) help us navigate the daunting world of statistics, specifically focusing on the interpretation of the P value.
For those interested in or already pursuing careers in private practice but would not like to relinquish their research interests, Chris Fourment (Texas Digestive Disease Consultants) provides a series of helpful tips on how to be effective in conducting clinical research endeavors. In the realm of basic science, Melinda Engevik (Baylor College of Medicine) gives an informative breakdown on how to choose a lab that is the right fit for you.
Also in this issue, Sadeea Abbasi (Cedars Sinai) provides an array of tangible ways for gastroenterologists to become involved in health policy advocacy. Byron Cryer (UT Southwestern), Jesus Rivera-Nieves (UCSD), and Celena NuQuay (AGA) describe how the AGA has been promoting workforce diversity in academic gastroenterology via the FORWARD (Fostering Opportunities Resulting in Workforce and Research Diversity) program.
Finally, as the submission deadline for DDW® 2020 approaches, abstract reviewers for the fellow-directed quality improvement (QI) projects from this past year share helpful tips on crafting memorable QI abstracts (Mohammad Bilal, UT-Galveston; Chung Sang Tse, Brown University; Manol Jovani, Johns Hopkins; and Mer Mietzelfeld, AGA).
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Ryan Farrell ([email protected]), managing editor of TNG.
Sincerely,
Vijaya L. Rao, MD
Editor in Chief
Dr. Rao is assistant professor of medicine, University of Chicago, section of gastroenterology, hepatology & nutrition.
Dear colleagues,
I am excited to introduce the November issue of The New Gastroenterologist – which is also my first issue as the new Editor in Chief! First, I am incredibly grateful for this opportunity to be a part of the only existing publication tailored toward trainees and early-career gastroenterologists. Bryson Katona has done a remarkable job for the last 5 years as the publication’s inaugural EIC, as he has laid a great deal of groundwork and really set the standard going forward. Each issue has been a multifaceted compilation of salient clinical topics paired with brief but high-yield articles to help guide personal and professional growth; I hope to continue to do the same and maintain a high level of interest in our newsletter.
In this issue, the In Focus article, brought to you by Adeeti Chiplunker and Christina Ha (Cedars Sinai), discusses inpatient management of acute severe ulcerative colitis. It is an excellent review of the diagnostic workup and therapeutic options, and an important one, as therapies are quickly evolving in inflammatory bowel disease. We also have Manol Jovani (Johns Hopkins) help us navigate the daunting world of statistics, specifically focusing on the interpretation of the P value.
For those interested in or already pursuing careers in private practice but would not like to relinquish their research interests, Chris Fourment (Texas Digestive Disease Consultants) provides a series of helpful tips on how to be effective in conducting clinical research endeavors. In the realm of basic science, Melinda Engevik (Baylor College of Medicine) gives an informative breakdown on how to choose a lab that is the right fit for you.
Also in this issue, Sadeea Abbasi (Cedars Sinai) provides an array of tangible ways for gastroenterologists to become involved in health policy advocacy. Byron Cryer (UT Southwestern), Jesus Rivera-Nieves (UCSD), and Celena NuQuay (AGA) describe how the AGA has been promoting workforce diversity in academic gastroenterology via the FORWARD (Fostering Opportunities Resulting in Workforce and Research Diversity) program.
Finally, as the submission deadline for DDW® 2020 approaches, abstract reviewers for the fellow-directed quality improvement (QI) projects from this past year share helpful tips on crafting memorable QI abstracts (Mohammad Bilal, UT-Galveston; Chung Sang Tse, Brown University; Manol Jovani, Johns Hopkins; and Mer Mietzelfeld, AGA).
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Ryan Farrell ([email protected]), managing editor of TNG.
Sincerely,
Vijaya L. Rao, MD
Editor in Chief
Dr. Rao is assistant professor of medicine, University of Chicago, section of gastroenterology, hepatology & nutrition.
Dear colleagues,
I am excited to introduce the November issue of The New Gastroenterologist – which is also my first issue as the new Editor in Chief! First, I am incredibly grateful for this opportunity to be a part of the only existing publication tailored toward trainees and early-career gastroenterologists. Bryson Katona has done a remarkable job for the last 5 years as the publication’s inaugural EIC, as he has laid a great deal of groundwork and really set the standard going forward. Each issue has been a multifaceted compilation of salient clinical topics paired with brief but high-yield articles to help guide personal and professional growth; I hope to continue to do the same and maintain a high level of interest in our newsletter.
In this issue, the In Focus article, brought to you by Adeeti Chiplunker and Christina Ha (Cedars Sinai), discusses inpatient management of acute severe ulcerative colitis. It is an excellent review of the diagnostic workup and therapeutic options, and an important one, as therapies are quickly evolving in inflammatory bowel disease. We also have Manol Jovani (Johns Hopkins) help us navigate the daunting world of statistics, specifically focusing on the interpretation of the P value.
For those interested in or already pursuing careers in private practice but would not like to relinquish their research interests, Chris Fourment (Texas Digestive Disease Consultants) provides a series of helpful tips on how to be effective in conducting clinical research endeavors. In the realm of basic science, Melinda Engevik (Baylor College of Medicine) gives an informative breakdown on how to choose a lab that is the right fit for you.
Also in this issue, Sadeea Abbasi (Cedars Sinai) provides an array of tangible ways for gastroenterologists to become involved in health policy advocacy. Byron Cryer (UT Southwestern), Jesus Rivera-Nieves (UCSD), and Celena NuQuay (AGA) describe how the AGA has been promoting workforce diversity in academic gastroenterology via the FORWARD (Fostering Opportunities Resulting in Workforce and Research Diversity) program.
Finally, as the submission deadline for DDW® 2020 approaches, abstract reviewers for the fellow-directed quality improvement (QI) projects from this past year share helpful tips on crafting memorable QI abstracts (Mohammad Bilal, UT-Galveston; Chung Sang Tse, Brown University; Manol Jovani, Johns Hopkins; and Mer Mietzelfeld, AGA).
If you are interested in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Ryan Farrell ([email protected]), managing editor of TNG.
Sincerely,
Vijaya L. Rao, MD
Editor in Chief
Dr. Rao is assistant professor of medicine, University of Chicago, section of gastroenterology, hepatology & nutrition.
Management of the hospitalized ulcerative colitis patient: A primer for the initial approach to care for the practicing gastroenterologist
Introduction
Inpatient management of acute ulcerative colitis (UC) flares can be challenging because of the multiple patient and disease-related factors influencing therapeutic decision making. The clinical course during the first 24-72 hours of the hospitalization will likely guide the decision between rescue medical and surgical therapy. Using available evidence from clinical practice guidelines, we present a day-by-day guide to managing most hospitalized UC patients.
Day 0 – The emergency department (ED)
When an UC patient presents to the ED for evaluation, the initial assessments should focus on the acuity and severity of the flare. Key clinical features of disease severity include the presence of fever, tachycardia, hypotension, or weight loss in addition to worsened gastrointestinal symptoms of stool frequency relative to baseline, rectal bleeding, and abdominal pain. Acute severe ulcerative colitis (ASUC) is often defined using the modified Truelove and Witts criteria.1 A patient meets criteria for ASUC if they have at least six bloody stools per day and at least one sign of systemic toxicity, such as heart rate greater than 90 bpm, temperature at or above 37.8° C, hemoglobin level below 10.5 g/dL, or elevated inflammatory markers.
Initial laboratory assessments should include complete blood counts to identify anemia, potential superimposed infection, or toxicity and a comprehensive metabolic profile to evaluate for dehydration, electrolyte abnormalities, hepatic injury or hypoalbuminemia (an important predictor of surgery), as well as assessment of response to treatment and readmission.2,3 An evaluation at admission of C-reactive protein (CRP) is crucial because changes from the initial value will determine steroid response and predict need for surgical intervention or rescue therapy. A baseline fecal calprotectin can serve as a noninvasive marker that can be followed after discharge to monitor response to therapy.
Clostridioides difficile infection (CDI) must be ruled out in all patients presenting with ASUC regardless of history of antibiotic use or prior negative testing. Concomitant UC and CDI are associated with a four- to sixfold increased risk of in-hospital mortality and a two- to sixfold increased risk of bowel surgery.4-6 Immunoassay testing is inexpensive and fast with a high specificity but has low sensitivity; nucleic acid amplification testing with polymerase chain reaction has a high sensitivity and specificity.7 Knowing which testing algorithm the hospital lab uses helps guide interpretation of results.
For patients meeting criteria for ASUC, obtaining at least an abdominal x-ray is important to assess for colonic dilation to further stratify the patient by risk. Colonic dilation, defined as a transverse colon diameter greater than 5.5 cm, places the patient in the category of fulminant colitis and colorectal surgical consultation should be obtained.8 A CT scan is often ordered first because it can provide a rapid assessment of intra-abdominal processes but is not routinely needed unless hemodynamic instability, an acute abdomen, or markedly abnormal laboratory testing (specifically white blood cell count with bandemia) is present as these can be indicators of toxic megacolon or perforation.8-10
Day 1 – Assess disease severity and assemble the team
Obtaining a thorough clinical history is essential to classify disease severity and identify potential triggers for the acute exacerbation. Potential triggers may include infections, new medications, recent antibiotic use, recent travel, sick contacts, or cessation of treatments. Standard questions include asking about the timing of onset of symptoms, bowel movements during a 24-hour period, and particularly the presence of nocturnal bowel movements. If patients report bloody stools, inquire how often they see blood relative to the total number of bowel movements. The presence and nature of abdominal pain should be elicited, particularly changes in abdominal pain and comparison with previous disease flares. These clinical parameters are used to assess response to treatment; therefore, ask patients to keep a log of their stool frequency, consistency, rectal urgency, and bleeding each day to report to the team during daily rounds.
For patients with ASUC, a full colonoscopy is rarely indicated in the inpatient setting because it is unlikely to change management and poses a risk of perforation.11 However, a sigmoidoscopy within the first 24 hours of admission will provide useful information about the endoscopic disease activity, particularly if features such as deep or well-like ulcers, large mucosal abrasions, or extensive loss of the mucosal layer are present because these are predictors of colectomy.8 Tissue biopsies can exclude cytomegalovirus (CMV) infection, an important consideration for patients on immunosuppression including corticosteroids.12-16
Venous thromboembolism (VTE) prophylaxis is extremely important for hospitalized inflammatory bowel disease (IBD) patients. At baseline, IBD patients have a threefold higher risk of VTE than do non-IBD patients, which increases to approximately sixfold during flares.17 Pharmacologic VTE prophylaxis is recommended for all hospitalized IBD patients, even those with rectal bleeding. This may seem counterintuitive in the setting of “GI bleeding,” so it is important to counsel both patients and team members regarding VTE risks and the role of the prophylactic regimen to ensure adherence. Mechanical VTE prophylaxis can be used in patients with severe bleeding and hemodynamic instability until pharmacologic VTE prophylaxis can be safely initiated.17
Narcotics should be used sparingly for hospitalized IBD patients. Narcotic use is associated with greater likelihood of subsequent IBD hospitalizations, ED visits, and higher costs of health care for patients with IBD.18 Heavy use of opiates, defined as continuous use for more than 30 days at a dose exceeding 50 mg morphine per day or equivalent, was strongly associated with an increased overall mortality in IBD patients.19 Opiates also slow bowel motility and precipitate toxic megacolon, along with any other agent that slows bowel motility, such as anticholinergic medications.8 These agents may also mask bowel frequency symptoms that would otherwise indicate a failure of medical therapy. Similarly, use of NSAIDS should also be avoided because these have been associated with disease relapse and escalating intestinal inflammation.20
Once disease severity has been determined, intravenous corticosteroid therapy may be initiated, ideally once CDI and CMV have been excluded. The recommended dosing of intravenous corticosteroids is methylprednisolone 20 mg IV every 8 hours or equivalent. There is no evidence to support additional benefit for doses exceeding these amounts.8 Prior to starting parenteral corticosteroids, it is important to keep in mind the possible need for rescue therapy during the admission. Recommended testing includes hepatitis B surface antigen and antibody, hepatitis B core antibody and tuberculosis testing if there is no documented negative testing within the past 6-12 months. These labs should be drawn prior to steroid treatment to avoid delays in care and indeterminate results. Finally, a lipid profile is recommended for patients who may be cyclosporine candidates pending response to intravenous corticosteroids. Unless the patient has been admitted with a bowel obstruction, which should raise the suspicion that the diagnosis is actually Crohn’s disease, enteral feeding is preferred for UC patients even if they may have significant food aversion. The early involvement of a registered dietitian is valuable to guide dietary choices and recommend appropriate enteral nutrition supplements. During acute flares, patients may find a low-residue diet to be less stimulating to their gut while their acute flare is being treated. Electrolyte abnormalities should be repleted and consistently monitored during the hospitalization. Providing parenteral intravenous iron for anemic patients will expedite correction of the anemia alongside treatment of the underlying UC.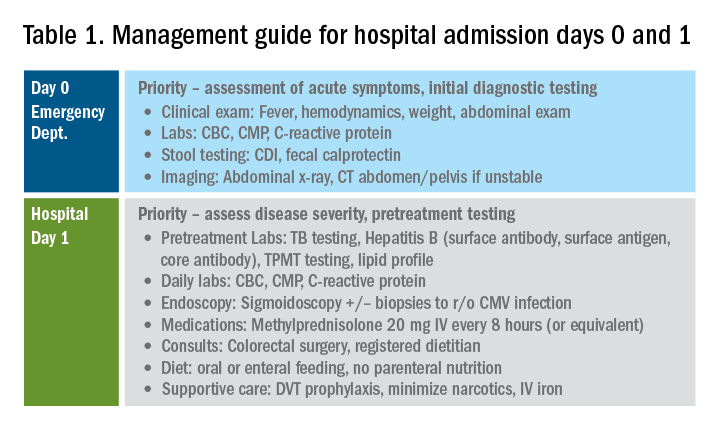
Most UC patients admitted to the hospital will require a multidisciplinary approach with gastroenterologists, surgeons, radiologists, dietitians, and case coordinators/social workers, among others. It is essential to assemble the team, especially the surgeons, earlier during the hospitalization rather than later. It is especially important to discuss the role of the surgeon in the management of UC and explain why the surgeon is being consulted in the context of the patient’s acute presentation. Being transparent about the parameters the GI team are monitoring to determine if and when surgery is the most appropriate and safe approach will improve patients’ acceptance of the surgical team’s role in their care. Specific indications for surgery in ASUC include toxic megacolon, colonic perforation, severe refractory hemorrhage, and failure to respond to medical therapy (Table 1).8
Day 3 – Assessing response to corticosteroids
In addition to daily symptom assessments, a careful abdominal exam should be performed every day with the understanding that steroids (and also narcotics) may mask perforation or pain. Any abrupt decrease or cessation of bowel movements, increasing abdominal distention, or a sudden increase in abdominal pain or tenderness may require abdominal imaging to ensure no interim perforation or severe colonic dilation has occurred while receiving steroid therapy. In these circumstances, the addition of broad spectrum intravenous antibiotics should be considered, particularly if hemodynamic instability (such as tachycardia) is present.
Patients should be assessed for response to intravenous steroid therapy after 3 days of treatment. A meaningful response to corticosteroids is present if the patient has had more than 50% improvement in symptoms, particularly rectal bleeding and stool frequency. A more than 75% improvement in CRP should also be noted from admission to day 3 with an overall trend of improvement.2,21 Additionally, patients should be afebrile, require minimal to no narcotic usage, tolerating oral intake, and be ambulatory. If the patient has met all these parameters, it is reasonable to transition to oral corticosteroids, such as prednisone 40-60 mg daily after a course of 3-5 days of intravenous corticosteroids. Ideally, patients should be observed for 24-48 hours in the hospital after transitioning to oral corticosteroids to make sure that symptoms do not worsen with the switch.
Patients with more than eight bowel movements per day, CRP greater than 4.5 g/dL, deep ulcers on endoscopy, or albumin less than 3.0 g/dL have a higher likelihood of failing intravenous corticosteroid therapy, and these patients should be prepared for rescue therapy.2,21 A patient has failed intravenous corticosteroids by day 3 if they have sustained fever in the absence of an infection, continued CRP elevation or lack of CRP decrease, or ongoing high stool frequency, bleeding, and pain with less than 50% improvement from baseline on admission.8 In the setting of nonresponse to intravenous corticosteroids, it is prudent to involve colorectal surgery to discuss colectomy as an option of equal merit to medical salvage therapies such as infliximab or cyclosporine.
Infliximab is the most readily available rescue therapy for steroid-refractory patients and has been shown to increase colectomy-free survival in patients with ASUC.8 However, patients with the same predictors for intravenous steroid failures (low albumin, high CRP, and/or deep ulcers on endoscopy) are also at the highest risk for infliximab nonresponse. These factors are important to discuss with the patients and colorectal surgery teams when providing the options of treatment strategy, particularly with medication dosing. ASUC with more severe disease biochemically (low albumin, elevated CRP, possibly bandemia) benefit from a higher dose of infliximab at 10 mg/kg, given the likelihood of increased drug clearance in this situation.22,23
From a practical standpoint, it is important to confirm the patient’s insurance status prior to medication administration to make sure therapy can be continued after hospital discharge. Early involvement of the social workers and case coordinators is key to ensuring timely administration of the next dose of treatment. Patients who receive infliximab rescue therapy should be monitored for an additional 1-2 days after administration to ensure they are responding to this therapy with continued monitoring of CRP and symptoms during this period. If there is no response at this point, an additional dose of infliximab may be considered but surgery should not be delayed if there is no meaningful response after the first dose.
Another option for intravenous corticosteroid nonresponders is intravenous cyclosporine because treatment failure rates for cyclosporine and infliximab were similar in head-to-head studies.24 However, patient selection is key to successful utilization of this agent. Unlike infliximab, cyclosporine is primarily an induction agent for steroid nonresponders rather than a maintenance strategy. Therefore, in patients in whom cyclosporine is being considered, thiopurines or vedolizumab are potential options for maintenance therapy. If the patient has poor renal function, low cholesterol, advanced age, significant comorbidities, or a history of nonadherence to therapy, cyclosporine should not be given. Additionally, clinical experience with intravenous cyclosporine administration and monitoring both during inpatient and outpatient care settings should be factored into the decision making for infliximab versus cyclosporine.8
Day 5 and beyond – Discharge planning
Patients who have responded to the initial intravenous steroid course by hospital day 5 should have successfully transitioned to oral steroids with plans to start an appropriate steroid-sparing therapy shortly after discharge. Treatment planning should commence prior to discharge and should be communicated with the outpatient GI team to ensure a smooth transition to the ambulatory care setting, primarily to begin insurance authorizations as soon as possible. If the patient has had a meaningful response to infliximab rescue therapy (improvement by more than 50% in bowel frequency, amount of blood, abdominal pain), discharge planning needs to prioritize obtaining authorization for the second dose within 2 weeks of the initial infusion. These patients are high risk for readmission, and close outpatient follow-up by the ambulatory GI care team is necessary to help direct the tapering of steroids and monitor response to treatment.
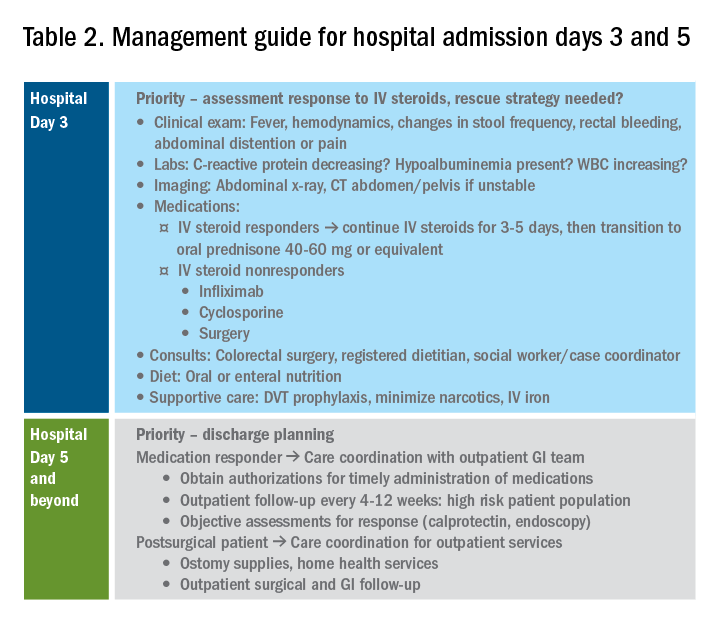
If the patient has not responded to intravenous steroid therapy, infliximab, or cyclosporine by day 5-7, then surgery should be strongly considered. Delaying surgery may worsen outcomes as patients become more malnourished, anemic, and continue to receive intravenous steroids. Additional preoperative optimization may be required depending on the patient’s course up to this point (Table 2).
Summary
The cornerstones of inpatient UC management center on a thorough initial evaluation including imaging and endoscopy as appropriate, establishment of baseline parameters, and daily assessment of response to therapy through a combination of patient-reported outcomes and biomarkers of inflammation. With this strategy in mind, practitioners and care teams can manage these complex patients using a consistent strategy focusing on multidisciplinary, evidence-based care.
References
1. Truelove SC et al. Br Med J. 1955 Oct 23;2(4947):1041-8.
2. Ho GT et al. Aliment Pharmacol Ther. 2004 May 15;19(10):1079-87.
3. Tinsley A et al. Scand J Gastroenterol. 2015;50(9):1103-9.
4. Issa M et al. Clin Gastroenterol Hepatol. 2007 Mar;5(3):345-51.
5. Ananthakrishnan AN et al. Gut. 2008 Feb;57(2):205-10.
6. Negron ME et al. Am J Gastroenterol. 2016 May;111(5):691-704.
7. Taylor KN et al. Gynecol Oncol. 2017 Feb;144(2):428-37.
8. Rubin DT et al. Am J Gastroenterol. 2019 Mar;114(3):384-413.
9. Jalan KN et al. Gastroenterology. 1969 Jul;57(1):68-82.
10. Gan SI et al. Am J Gastroenterol. 2003 Nov;98(11):2363-71.
11. Makkar R et al. Gastroenterol Hepatol (N Y). 2013 Sep;9(9):573-83.
12. Hindryckx P et al. Nat Rev Gastroenterol Hepatol. 2016 Nov;13(11):654-64.
13. Yerushalmy-Feler A et al. Curr Infect Dis Rep. 2019 Feb 15;21(2):5.
14. Shukla T et al. J Clin Gastroenterol. 2017 May/Jun;51(5):394-401.
15. McCurdy JD et al. Clin Gastroenterol Hepatol. 2015 Jan;13(1):131-7; quiz e7.
16. Cottone M et al. Am J Gastroenterol. 2001 Mar;96(3):773-5.
17. Nguyen GC et al. Gastroenterology. 2014 Mar;146(3):835-48 e6.
18. Limsrivilai J et al. Clin Gastroenterol Hepatol. 2017 Mar;15(3):385-92 e2.
19. Targownik LE et al. Am J Gastroenterol. 2014 Oct;109(10):1613-20.
20. Takeuchi K et al. Clin Gastroenterol Hepatol. 2006 Feb;4(2):196-202.
21. Travis SP et al. Gut. 1996 Jun;38(6):905-10.
22. Syal G et al. Mo1891 - Gastroenterology. 2018;154:S841.
23. Ungar B et al. Aliment Pharmacol Ther. 2016 Jun;43(12):1293-9.
24. Laharie D et al. Lancet 2012 Dec 1;380(9857):1909-15.
Dr. Chiplunker is an advanced inflammatory bowel disease fellow; Dr. Ha is associate professor of medicine at the Inflammatory Bowel Disease Center at Cedars-Sinai Medical Center, Los Angeles.
Introduction
Inpatient management of acute ulcerative colitis (UC) flares can be challenging because of the multiple patient and disease-related factors influencing therapeutic decision making. The clinical course during the first 24-72 hours of the hospitalization will likely guide the decision between rescue medical and surgical therapy. Using available evidence from clinical practice guidelines, we present a day-by-day guide to managing most hospitalized UC patients.
Day 0 – The emergency department (ED)
When an UC patient presents to the ED for evaluation, the initial assessments should focus on the acuity and severity of the flare. Key clinical features of disease severity include the presence of fever, tachycardia, hypotension, or weight loss in addition to worsened gastrointestinal symptoms of stool frequency relative to baseline, rectal bleeding, and abdominal pain. Acute severe ulcerative colitis (ASUC) is often defined using the modified Truelove and Witts criteria.1 A patient meets criteria for ASUC if they have at least six bloody stools per day and at least one sign of systemic toxicity, such as heart rate greater than 90 bpm, temperature at or above 37.8° C, hemoglobin level below 10.5 g/dL, or elevated inflammatory markers.
Initial laboratory assessments should include complete blood counts to identify anemia, potential superimposed infection, or toxicity and a comprehensive metabolic profile to evaluate for dehydration, electrolyte abnormalities, hepatic injury or hypoalbuminemia (an important predictor of surgery), as well as assessment of response to treatment and readmission.2,3 An evaluation at admission of C-reactive protein (CRP) is crucial because changes from the initial value will determine steroid response and predict need for surgical intervention or rescue therapy. A baseline fecal calprotectin can serve as a noninvasive marker that can be followed after discharge to monitor response to therapy.
Clostridioides difficile infection (CDI) must be ruled out in all patients presenting with ASUC regardless of history of antibiotic use or prior negative testing. Concomitant UC and CDI are associated with a four- to sixfold increased risk of in-hospital mortality and a two- to sixfold increased risk of bowel surgery.4-6 Immunoassay testing is inexpensive and fast with a high specificity but has low sensitivity; nucleic acid amplification testing with polymerase chain reaction has a high sensitivity and specificity.7 Knowing which testing algorithm the hospital lab uses helps guide interpretation of results.
For patients meeting criteria for ASUC, obtaining at least an abdominal x-ray is important to assess for colonic dilation to further stratify the patient by risk. Colonic dilation, defined as a transverse colon diameter greater than 5.5 cm, places the patient in the category of fulminant colitis and colorectal surgical consultation should be obtained.8 A CT scan is often ordered first because it can provide a rapid assessment of intra-abdominal processes but is not routinely needed unless hemodynamic instability, an acute abdomen, or markedly abnormal laboratory testing (specifically white blood cell count with bandemia) is present as these can be indicators of toxic megacolon or perforation.8-10
Day 1 – Assess disease severity and assemble the team
Obtaining a thorough clinical history is essential to classify disease severity and identify potential triggers for the acute exacerbation. Potential triggers may include infections, new medications, recent antibiotic use, recent travel, sick contacts, or cessation of treatments. Standard questions include asking about the timing of onset of symptoms, bowel movements during a 24-hour period, and particularly the presence of nocturnal bowel movements. If patients report bloody stools, inquire how often they see blood relative to the total number of bowel movements. The presence and nature of abdominal pain should be elicited, particularly changes in abdominal pain and comparison with previous disease flares. These clinical parameters are used to assess response to treatment; therefore, ask patients to keep a log of their stool frequency, consistency, rectal urgency, and bleeding each day to report to the team during daily rounds.
For patients with ASUC, a full colonoscopy is rarely indicated in the inpatient setting because it is unlikely to change management and poses a risk of perforation.11 However, a sigmoidoscopy within the first 24 hours of admission will provide useful information about the endoscopic disease activity, particularly if features such as deep or well-like ulcers, large mucosal abrasions, or extensive loss of the mucosal layer are present because these are predictors of colectomy.8 Tissue biopsies can exclude cytomegalovirus (CMV) infection, an important consideration for patients on immunosuppression including corticosteroids.12-16
Venous thromboembolism (VTE) prophylaxis is extremely important for hospitalized inflammatory bowel disease (IBD) patients. At baseline, IBD patients have a threefold higher risk of VTE than do non-IBD patients, which increases to approximately sixfold during flares.17 Pharmacologic VTE prophylaxis is recommended for all hospitalized IBD patients, even those with rectal bleeding. This may seem counterintuitive in the setting of “GI bleeding,” so it is important to counsel both patients and team members regarding VTE risks and the role of the prophylactic regimen to ensure adherence. Mechanical VTE prophylaxis can be used in patients with severe bleeding and hemodynamic instability until pharmacologic VTE prophylaxis can be safely initiated.17
Narcotics should be used sparingly for hospitalized IBD patients. Narcotic use is associated with greater likelihood of subsequent IBD hospitalizations, ED visits, and higher costs of health care for patients with IBD.18 Heavy use of opiates, defined as continuous use for more than 30 days at a dose exceeding 50 mg morphine per day or equivalent, was strongly associated with an increased overall mortality in IBD patients.19 Opiates also slow bowel motility and precipitate toxic megacolon, along with any other agent that slows bowel motility, such as anticholinergic medications.8 These agents may also mask bowel frequency symptoms that would otherwise indicate a failure of medical therapy. Similarly, use of NSAIDS should also be avoided because these have been associated with disease relapse and escalating intestinal inflammation.20
Once disease severity has been determined, intravenous corticosteroid therapy may be initiated, ideally once CDI and CMV have been excluded. The recommended dosing of intravenous corticosteroids is methylprednisolone 20 mg IV every 8 hours or equivalent. There is no evidence to support additional benefit for doses exceeding these amounts.8 Prior to starting parenteral corticosteroids, it is important to keep in mind the possible need for rescue therapy during the admission. Recommended testing includes hepatitis B surface antigen and antibody, hepatitis B core antibody and tuberculosis testing if there is no documented negative testing within the past 6-12 months. These labs should be drawn prior to steroid treatment to avoid delays in care and indeterminate results. Finally, a lipid profile is recommended for patients who may be cyclosporine candidates pending response to intravenous corticosteroids. Unless the patient has been admitted with a bowel obstruction, which should raise the suspicion that the diagnosis is actually Crohn’s disease, enteral feeding is preferred for UC patients even if they may have significant food aversion. The early involvement of a registered dietitian is valuable to guide dietary choices and recommend appropriate enteral nutrition supplements. During acute flares, patients may find a low-residue diet to be less stimulating to their gut while their acute flare is being treated. Electrolyte abnormalities should be repleted and consistently monitored during the hospitalization. Providing parenteral intravenous iron for anemic patients will expedite correction of the anemia alongside treatment of the underlying UC.
Most UC patients admitted to the hospital will require a multidisciplinary approach with gastroenterologists, surgeons, radiologists, dietitians, and case coordinators/social workers, among others. It is essential to assemble the team, especially the surgeons, earlier during the hospitalization rather than later. It is especially important to discuss the role of the surgeon in the management of UC and explain why the surgeon is being consulted in the context of the patient’s acute presentation. Being transparent about the parameters the GI team are monitoring to determine if and when surgery is the most appropriate and safe approach will improve patients’ acceptance of the surgical team’s role in their care. Specific indications for surgery in ASUC include toxic megacolon, colonic perforation, severe refractory hemorrhage, and failure to respond to medical therapy (Table 1).8
Day 3 – Assessing response to corticosteroids
In addition to daily symptom assessments, a careful abdominal exam should be performed every day with the understanding that steroids (and also narcotics) may mask perforation or pain. Any abrupt decrease or cessation of bowel movements, increasing abdominal distention, or a sudden increase in abdominal pain or tenderness may require abdominal imaging to ensure no interim perforation or severe colonic dilation has occurred while receiving steroid therapy. In these circumstances, the addition of broad spectrum intravenous antibiotics should be considered, particularly if hemodynamic instability (such as tachycardia) is present.
Patients should be assessed for response to intravenous steroid therapy after 3 days of treatment. A meaningful response to corticosteroids is present if the patient has had more than 50% improvement in symptoms, particularly rectal bleeding and stool frequency. A more than 75% improvement in CRP should also be noted from admission to day 3 with an overall trend of improvement.2,21 Additionally, patients should be afebrile, require minimal to no narcotic usage, tolerating oral intake, and be ambulatory. If the patient has met all these parameters, it is reasonable to transition to oral corticosteroids, such as prednisone 40-60 mg daily after a course of 3-5 days of intravenous corticosteroids. Ideally, patients should be observed for 24-48 hours in the hospital after transitioning to oral corticosteroids to make sure that symptoms do not worsen with the switch.
Patients with more than eight bowel movements per day, CRP greater than 4.5 g/dL, deep ulcers on endoscopy, or albumin less than 3.0 g/dL have a higher likelihood of failing intravenous corticosteroid therapy, and these patients should be prepared for rescue therapy.2,21 A patient has failed intravenous corticosteroids by day 3 if they have sustained fever in the absence of an infection, continued CRP elevation or lack of CRP decrease, or ongoing high stool frequency, bleeding, and pain with less than 50% improvement from baseline on admission.8 In the setting of nonresponse to intravenous corticosteroids, it is prudent to involve colorectal surgery to discuss colectomy as an option of equal merit to medical salvage therapies such as infliximab or cyclosporine.
Infliximab is the most readily available rescue therapy for steroid-refractory patients and has been shown to increase colectomy-free survival in patients with ASUC.8 However, patients with the same predictors for intravenous steroid failures (low albumin, high CRP, and/or deep ulcers on endoscopy) are also at the highest risk for infliximab nonresponse. These factors are important to discuss with the patients and colorectal surgery teams when providing the options of treatment strategy, particularly with medication dosing. ASUC with more severe disease biochemically (low albumin, elevated CRP, possibly bandemia) benefit from a higher dose of infliximab at 10 mg/kg, given the likelihood of increased drug clearance in this situation.22,23
From a practical standpoint, it is important to confirm the patient’s insurance status prior to medication administration to make sure therapy can be continued after hospital discharge. Early involvement of the social workers and case coordinators is key to ensuring timely administration of the next dose of treatment. Patients who receive infliximab rescue therapy should be monitored for an additional 1-2 days after administration to ensure they are responding to this therapy with continued monitoring of CRP and symptoms during this period. If there is no response at this point, an additional dose of infliximab may be considered but surgery should not be delayed if there is no meaningful response after the first dose.
Another option for intravenous corticosteroid nonresponders is intravenous cyclosporine because treatment failure rates for cyclosporine and infliximab were similar in head-to-head studies.24 However, patient selection is key to successful utilization of this agent. Unlike infliximab, cyclosporine is primarily an induction agent for steroid nonresponders rather than a maintenance strategy. Therefore, in patients in whom cyclosporine is being considered, thiopurines or vedolizumab are potential options for maintenance therapy. If the patient has poor renal function, low cholesterol, advanced age, significant comorbidities, or a history of nonadherence to therapy, cyclosporine should not be given. Additionally, clinical experience with intravenous cyclosporine administration and monitoring both during inpatient and outpatient care settings should be factored into the decision making for infliximab versus cyclosporine.8
Day 5 and beyond – Discharge planning
Patients who have responded to the initial intravenous steroid course by hospital day 5 should have successfully transitioned to oral steroids with plans to start an appropriate steroid-sparing therapy shortly after discharge. Treatment planning should commence prior to discharge and should be communicated with the outpatient GI team to ensure a smooth transition to the ambulatory care setting, primarily to begin insurance authorizations as soon as possible. If the patient has had a meaningful response to infliximab rescue therapy (improvement by more than 50% in bowel frequency, amount of blood, abdominal pain), discharge planning needs to prioritize obtaining authorization for the second dose within 2 weeks of the initial infusion. These patients are high risk for readmission, and close outpatient follow-up by the ambulatory GI care team is necessary to help direct the tapering of steroids and monitor response to treatment.

If the patient has not responded to intravenous steroid therapy, infliximab, or cyclosporine by day 5-7, then surgery should be strongly considered. Delaying surgery may worsen outcomes as patients become more malnourished, anemic, and continue to receive intravenous steroids. Additional preoperative optimization may be required depending on the patient’s course up to this point (Table 2).
Summary
The cornerstones of inpatient UC management center on a thorough initial evaluation including imaging and endoscopy as appropriate, establishment of baseline parameters, and daily assessment of response to therapy through a combination of patient-reported outcomes and biomarkers of inflammation. With this strategy in mind, practitioners and care teams can manage these complex patients using a consistent strategy focusing on multidisciplinary, evidence-based care.
References
1. Truelove SC et al. Br Med J. 1955 Oct 23;2(4947):1041-8.
2. Ho GT et al. Aliment Pharmacol Ther. 2004 May 15;19(10):1079-87.
3. Tinsley A et al. Scand J Gastroenterol. 2015;50(9):1103-9.
4. Issa M et al. Clin Gastroenterol Hepatol. 2007 Mar;5(3):345-51.
5. Ananthakrishnan AN et al. Gut. 2008 Feb;57(2):205-10.
6. Negron ME et al. Am J Gastroenterol. 2016 May;111(5):691-704.
7. Taylor KN et al. Gynecol Oncol. 2017 Feb;144(2):428-37.
8. Rubin DT et al. Am J Gastroenterol. 2019 Mar;114(3):384-413.
9. Jalan KN et al. Gastroenterology. 1969 Jul;57(1):68-82.
10. Gan SI et al. Am J Gastroenterol. 2003 Nov;98(11):2363-71.
11. Makkar R et al. Gastroenterol Hepatol (N Y). 2013 Sep;9(9):573-83.
12. Hindryckx P et al. Nat Rev Gastroenterol Hepatol. 2016 Nov;13(11):654-64.
13. Yerushalmy-Feler A et al. Curr Infect Dis Rep. 2019 Feb 15;21(2):5.
14. Shukla T et al. J Clin Gastroenterol. 2017 May/Jun;51(5):394-401.
15. McCurdy JD et al. Clin Gastroenterol Hepatol. 2015 Jan;13(1):131-7; quiz e7.
16. Cottone M et al. Am J Gastroenterol. 2001 Mar;96(3):773-5.
17. Nguyen GC et al. Gastroenterology. 2014 Mar;146(3):835-48 e6.
18. Limsrivilai J et al. Clin Gastroenterol Hepatol. 2017 Mar;15(3):385-92 e2.
19. Targownik LE et al. Am J Gastroenterol. 2014 Oct;109(10):1613-20.
20. Takeuchi K et al. Clin Gastroenterol Hepatol. 2006 Feb;4(2):196-202.
21. Travis SP et al. Gut. 1996 Jun;38(6):905-10.
22. Syal G et al. Mo1891 - Gastroenterology. 2018;154:S841.
23. Ungar B et al. Aliment Pharmacol Ther. 2016 Jun;43(12):1293-9.
24. Laharie D et al. Lancet 2012 Dec 1;380(9857):1909-15.
Dr. Chiplunker is an advanced inflammatory bowel disease fellow; Dr. Ha is associate professor of medicine at the Inflammatory Bowel Disease Center at Cedars-Sinai Medical Center, Los Angeles.
Introduction
Inpatient management of acute ulcerative colitis (UC) flares can be challenging because of the multiple patient and disease-related factors influencing therapeutic decision making. The clinical course during the first 24-72 hours of the hospitalization will likely guide the decision between rescue medical and surgical therapy. Using available evidence from clinical practice guidelines, we present a day-by-day guide to managing most hospitalized UC patients.
Day 0 – The emergency department (ED)
When an UC patient presents to the ED for evaluation, the initial assessments should focus on the acuity and severity of the flare. Key clinical features of disease severity include the presence of fever, tachycardia, hypotension, or weight loss in addition to worsened gastrointestinal symptoms of stool frequency relative to baseline, rectal bleeding, and abdominal pain. Acute severe ulcerative colitis (ASUC) is often defined using the modified Truelove and Witts criteria.1 A patient meets criteria for ASUC if they have at least six bloody stools per day and at least one sign of systemic toxicity, such as heart rate greater than 90 bpm, temperature at or above 37.8° C, hemoglobin level below 10.5 g/dL, or elevated inflammatory markers.
Initial laboratory assessments should include complete blood counts to identify anemia, potential superimposed infection, or toxicity and a comprehensive metabolic profile to evaluate for dehydration, electrolyte abnormalities, hepatic injury or hypoalbuminemia (an important predictor of surgery), as well as assessment of response to treatment and readmission.2,3 An evaluation at admission of C-reactive protein (CRP) is crucial because changes from the initial value will determine steroid response and predict need for surgical intervention or rescue therapy. A baseline fecal calprotectin can serve as a noninvasive marker that can be followed after discharge to monitor response to therapy.
Clostridioides difficile infection (CDI) must be ruled out in all patients presenting with ASUC regardless of history of antibiotic use or prior negative testing. Concomitant UC and CDI are associated with a four- to sixfold increased risk of in-hospital mortality and a two- to sixfold increased risk of bowel surgery.4-6 Immunoassay testing is inexpensive and fast with a high specificity but has low sensitivity; nucleic acid amplification testing with polymerase chain reaction has a high sensitivity and specificity.7 Knowing which testing algorithm the hospital lab uses helps guide interpretation of results.
For patients meeting criteria for ASUC, obtaining at least an abdominal x-ray is important to assess for colonic dilation to further stratify the patient by risk. Colonic dilation, defined as a transverse colon diameter greater than 5.5 cm, places the patient in the category of fulminant colitis and colorectal surgical consultation should be obtained.8 A CT scan is often ordered first because it can provide a rapid assessment of intra-abdominal processes but is not routinely needed unless hemodynamic instability, an acute abdomen, or markedly abnormal laboratory testing (specifically white blood cell count with bandemia) is present as these can be indicators of toxic megacolon or perforation.8-10
Day 1 – Assess disease severity and assemble the team
Obtaining a thorough clinical history is essential to classify disease severity and identify potential triggers for the acute exacerbation. Potential triggers may include infections, new medications, recent antibiotic use, recent travel, sick contacts, or cessation of treatments. Standard questions include asking about the timing of onset of symptoms, bowel movements during a 24-hour period, and particularly the presence of nocturnal bowel movements. If patients report bloody stools, inquire how often they see blood relative to the total number of bowel movements. The presence and nature of abdominal pain should be elicited, particularly changes in abdominal pain and comparison with previous disease flares. These clinical parameters are used to assess response to treatment; therefore, ask patients to keep a log of their stool frequency, consistency, rectal urgency, and bleeding each day to report to the team during daily rounds.
For patients with ASUC, a full colonoscopy is rarely indicated in the inpatient setting because it is unlikely to change management and poses a risk of perforation.11 However, a sigmoidoscopy within the first 24 hours of admission will provide useful information about the endoscopic disease activity, particularly if features such as deep or well-like ulcers, large mucosal abrasions, or extensive loss of the mucosal layer are present because these are predictors of colectomy.8 Tissue biopsies can exclude cytomegalovirus (CMV) infection, an important consideration for patients on immunosuppression including corticosteroids.12-16
Venous thromboembolism (VTE) prophylaxis is extremely important for hospitalized inflammatory bowel disease (IBD) patients. At baseline, IBD patients have a threefold higher risk of VTE than do non-IBD patients, which increases to approximately sixfold during flares.17 Pharmacologic VTE prophylaxis is recommended for all hospitalized IBD patients, even those with rectal bleeding. This may seem counterintuitive in the setting of “GI bleeding,” so it is important to counsel both patients and team members regarding VTE risks and the role of the prophylactic regimen to ensure adherence. Mechanical VTE prophylaxis can be used in patients with severe bleeding and hemodynamic instability until pharmacologic VTE prophylaxis can be safely initiated.17
Narcotics should be used sparingly for hospitalized IBD patients. Narcotic use is associated with greater likelihood of subsequent IBD hospitalizations, ED visits, and higher costs of health care for patients with IBD.18 Heavy use of opiates, defined as continuous use for more than 30 days at a dose exceeding 50 mg morphine per day or equivalent, was strongly associated with an increased overall mortality in IBD patients.19 Opiates also slow bowel motility and precipitate toxic megacolon, along with any other agent that slows bowel motility, such as anticholinergic medications.8 These agents may also mask bowel frequency symptoms that would otherwise indicate a failure of medical therapy. Similarly, use of NSAIDS should also be avoided because these have been associated with disease relapse and escalating intestinal inflammation.20
Once disease severity has been determined, intravenous corticosteroid therapy may be initiated, ideally once CDI and CMV have been excluded. The recommended dosing of intravenous corticosteroids is methylprednisolone 20 mg IV every 8 hours or equivalent. There is no evidence to support additional benefit for doses exceeding these amounts.8 Prior to starting parenteral corticosteroids, it is important to keep in mind the possible need for rescue therapy during the admission. Recommended testing includes hepatitis B surface antigen and antibody, hepatitis B core antibody and tuberculosis testing if there is no documented negative testing within the past 6-12 months. These labs should be drawn prior to steroid treatment to avoid delays in care and indeterminate results. Finally, a lipid profile is recommended for patients who may be cyclosporine candidates pending response to intravenous corticosteroids. Unless the patient has been admitted with a bowel obstruction, which should raise the suspicion that the diagnosis is actually Crohn’s disease, enteral feeding is preferred for UC patients even if they may have significant food aversion. The early involvement of a registered dietitian is valuable to guide dietary choices and recommend appropriate enteral nutrition supplements. During acute flares, patients may find a low-residue diet to be less stimulating to their gut while their acute flare is being treated. Electrolyte abnormalities should be repleted and consistently monitored during the hospitalization. Providing parenteral intravenous iron for anemic patients will expedite correction of the anemia alongside treatment of the underlying UC.
Most UC patients admitted to the hospital will require a multidisciplinary approach with gastroenterologists, surgeons, radiologists, dietitians, and case coordinators/social workers, among others. It is essential to assemble the team, especially the surgeons, earlier during the hospitalization rather than later. It is especially important to discuss the role of the surgeon in the management of UC and explain why the surgeon is being consulted in the context of the patient’s acute presentation. Being transparent about the parameters the GI team are monitoring to determine if and when surgery is the most appropriate and safe approach will improve patients’ acceptance of the surgical team’s role in their care. Specific indications for surgery in ASUC include toxic megacolon, colonic perforation, severe refractory hemorrhage, and failure to respond to medical therapy (Table 1).8
Day 3 – Assessing response to corticosteroids
In addition to daily symptom assessments, a careful abdominal exam should be performed every day with the understanding that steroids (and also narcotics) may mask perforation or pain. Any abrupt decrease or cessation of bowel movements, increasing abdominal distention, or a sudden increase in abdominal pain or tenderness may require abdominal imaging to ensure no interim perforation or severe colonic dilation has occurred while receiving steroid therapy. In these circumstances, the addition of broad spectrum intravenous antibiotics should be considered, particularly if hemodynamic instability (such as tachycardia) is present.
Patients should be assessed for response to intravenous steroid therapy after 3 days of treatment. A meaningful response to corticosteroids is present if the patient has had more than 50% improvement in symptoms, particularly rectal bleeding and stool frequency. A more than 75% improvement in CRP should also be noted from admission to day 3 with an overall trend of improvement.2,21 Additionally, patients should be afebrile, require minimal to no narcotic usage, tolerating oral intake, and be ambulatory. If the patient has met all these parameters, it is reasonable to transition to oral corticosteroids, such as prednisone 40-60 mg daily after a course of 3-5 days of intravenous corticosteroids. Ideally, patients should be observed for 24-48 hours in the hospital after transitioning to oral corticosteroids to make sure that symptoms do not worsen with the switch.
Patients with more than eight bowel movements per day, CRP greater than 4.5 g/dL, deep ulcers on endoscopy, or albumin less than 3.0 g/dL have a higher likelihood of failing intravenous corticosteroid therapy, and these patients should be prepared for rescue therapy.2,21 A patient has failed intravenous corticosteroids by day 3 if they have sustained fever in the absence of an infection, continued CRP elevation or lack of CRP decrease, or ongoing high stool frequency, bleeding, and pain with less than 50% improvement from baseline on admission.8 In the setting of nonresponse to intravenous corticosteroids, it is prudent to involve colorectal surgery to discuss colectomy as an option of equal merit to medical salvage therapies such as infliximab or cyclosporine.
Infliximab is the most readily available rescue therapy for steroid-refractory patients and has been shown to increase colectomy-free survival in patients with ASUC.8 However, patients with the same predictors for intravenous steroid failures (low albumin, high CRP, and/or deep ulcers on endoscopy) are also at the highest risk for infliximab nonresponse. These factors are important to discuss with the patients and colorectal surgery teams when providing the options of treatment strategy, particularly with medication dosing. ASUC with more severe disease biochemically (low albumin, elevated CRP, possibly bandemia) benefit from a higher dose of infliximab at 10 mg/kg, given the likelihood of increased drug clearance in this situation.22,23
From a practical standpoint, it is important to confirm the patient’s insurance status prior to medication administration to make sure therapy can be continued after hospital discharge. Early involvement of the social workers and case coordinators is key to ensuring timely administration of the next dose of treatment. Patients who receive infliximab rescue therapy should be monitored for an additional 1-2 days after administration to ensure they are responding to this therapy with continued monitoring of CRP and symptoms during this period. If there is no response at this point, an additional dose of infliximab may be considered but surgery should not be delayed if there is no meaningful response after the first dose.
Another option for intravenous corticosteroid nonresponders is intravenous cyclosporine because treatment failure rates for cyclosporine and infliximab were similar in head-to-head studies.24 However, patient selection is key to successful utilization of this agent. Unlike infliximab, cyclosporine is primarily an induction agent for steroid nonresponders rather than a maintenance strategy. Therefore, in patients in whom cyclosporine is being considered, thiopurines or vedolizumab are potential options for maintenance therapy. If the patient has poor renal function, low cholesterol, advanced age, significant comorbidities, or a history of nonadherence to therapy, cyclosporine should not be given. Additionally, clinical experience with intravenous cyclosporine administration and monitoring both during inpatient and outpatient care settings should be factored into the decision making for infliximab versus cyclosporine.8
Day 5 and beyond – Discharge planning
Patients who have responded to the initial intravenous steroid course by hospital day 5 should have successfully transitioned to oral steroids with plans to start an appropriate steroid-sparing therapy shortly after discharge. Treatment planning should commence prior to discharge and should be communicated with the outpatient GI team to ensure a smooth transition to the ambulatory care setting, primarily to begin insurance authorizations as soon as possible. If the patient has had a meaningful response to infliximab rescue therapy (improvement by more than 50% in bowel frequency, amount of blood, abdominal pain), discharge planning needs to prioritize obtaining authorization for the second dose within 2 weeks of the initial infusion. These patients are high risk for readmission, and close outpatient follow-up by the ambulatory GI care team is necessary to help direct the tapering of steroids and monitor response to treatment.

If the patient has not responded to intravenous steroid therapy, infliximab, or cyclosporine by day 5-7, then surgery should be strongly considered. Delaying surgery may worsen outcomes as patients become more malnourished, anemic, and continue to receive intravenous steroids. Additional preoperative optimization may be required depending on the patient’s course up to this point (Table 2).
Summary
The cornerstones of inpatient UC management center on a thorough initial evaluation including imaging and endoscopy as appropriate, establishment of baseline parameters, and daily assessment of response to therapy through a combination of patient-reported outcomes and biomarkers of inflammation. With this strategy in mind, practitioners and care teams can manage these complex patients using a consistent strategy focusing on multidisciplinary, evidence-based care.
References
1. Truelove SC et al. Br Med J. 1955 Oct 23;2(4947):1041-8.
2. Ho GT et al. Aliment Pharmacol Ther. 2004 May 15;19(10):1079-87.
3. Tinsley A et al. Scand J Gastroenterol. 2015;50(9):1103-9.
4. Issa M et al. Clin Gastroenterol Hepatol. 2007 Mar;5(3):345-51.
5. Ananthakrishnan AN et al. Gut. 2008 Feb;57(2):205-10.
6. Negron ME et al. Am J Gastroenterol. 2016 May;111(5):691-704.
7. Taylor KN et al. Gynecol Oncol. 2017 Feb;144(2):428-37.
8. Rubin DT et al. Am J Gastroenterol. 2019 Mar;114(3):384-413.
9. Jalan KN et al. Gastroenterology. 1969 Jul;57(1):68-82.
10. Gan SI et al. Am J Gastroenterol. 2003 Nov;98(11):2363-71.
11. Makkar R et al. Gastroenterol Hepatol (N Y). 2013 Sep;9(9):573-83.
12. Hindryckx P et al. Nat Rev Gastroenterol Hepatol. 2016 Nov;13(11):654-64.
13. Yerushalmy-Feler A et al. Curr Infect Dis Rep. 2019 Feb 15;21(2):5.
14. Shukla T et al. J Clin Gastroenterol. 2017 May/Jun;51(5):394-401.
15. McCurdy JD et al. Clin Gastroenterol Hepatol. 2015 Jan;13(1):131-7; quiz e7.
16. Cottone M et al. Am J Gastroenterol. 2001 Mar;96(3):773-5.
17. Nguyen GC et al. Gastroenterology. 2014 Mar;146(3):835-48 e6.
18. Limsrivilai J et al. Clin Gastroenterol Hepatol. 2017 Mar;15(3):385-92 e2.
19. Targownik LE et al. Am J Gastroenterol. 2014 Oct;109(10):1613-20.
20. Takeuchi K et al. Clin Gastroenterol Hepatol. 2006 Feb;4(2):196-202.
21. Travis SP et al. Gut. 1996 Jun;38(6):905-10.
22. Syal G et al. Mo1891 - Gastroenterology. 2018;154:S841.
23. Ungar B et al. Aliment Pharmacol Ther. 2016 Jun;43(12):1293-9.
24. Laharie D et al. Lancet 2012 Dec 1;380(9857):1909-15.
Dr. Chiplunker is an advanced inflammatory bowel disease fellow; Dr. Ha is associate professor of medicine at the Inflammatory Bowel Disease Center at Cedars-Sinai Medical Center, Los Angeles.