User login
How to address reviewer criticism
Authors of manuscripts typically receive one of three responses from journals: 1. Accepted as submitted; 2. Accepted pending revisions (major or minor); and 3. Rejected. Receiving an unconditional acceptance is an unusual fate worth documenting and celebrating. On the other hand, irreversible rejections are so common that authors need to get accustomed to them. Upon receiving an unqualified editorial rejection without a formal review (usually described as priority-related rejection), send the same manuscript out the next day to another journal (with electronic submissions you can do this on the same day).
If your manuscript is rejected after being reviewed, consider seriously the comments given and try to learn from them. Was your study design flawed? Do you need additional data? Were your analyses incomplete or did you employ suboptimal statistical methods? Was your interpretation of the findings far reaching and out of proportion to the actual data? Use this experience and feedback, revise your manuscript, and submit it to a different journal. It is not uncommon to encounter the same reviewer at the next journal; fixing major issues seems responsive and gets you in the door.
To receive a “conditional acceptance” or “rejection with hope” is the most likely “good” editorial response. Avoid a very quick response, because it may be hasty or create an impression of a hasty response. Because most manuscripts with substantial reviews are sent back to the reviewers, the turnaround time in most journals is several weeks and, therefore, there is little to be gained by sending the revised manuscript in 1 day rather than 1 week. The best course of action is to cool down for 1-2 days and then decide and draft responses in 1 week, including planned additional analyses. In the case of seemingly contradictory or numerous requests from reviewers, it is best to carefully examine clues from the editors or associate editors as to the nature and extent of the revision needed. In most instances, we draft the response letter before revising the manuscript. We use the draft letter to obtain specific input from other authors and ‘brainstorm’ about additional analyses that can best address reviewers concerns.
Do the best that you can to fully address all reviewers’ comments. Adequate time should be spent making real changes, including adding additional data or analyses to the manuscript, and taking utmost care in describing and highlighting these changes. If you believe that the reviewers missed a point that was already included in the paper, then point this out as politely as possible as part of the response letter (see below).
In addition to revising your manuscript, you will be asked to prepare a point-by-point response to each of the reviewers’ comments you receive. Thank the editors and reviewers sincerely for their comments and explain how changes based on the comments have made the paper better; they did spend time reviewing your manuscript, and they have not rejected it yet. Reviewers are usually recognized experts, or their apprentices, in the content or method of research employed in your paper. Reviewers are also likely to be authors on papers cited in your manuscript. Avoid unnecessary arguments when possible, especially about noncore issues or about changes that you already conceded. If you are compelled to contest any of the reviewers’ comments, provide substantial evidence that supports your position and be respectful with your responses. Address each comment separately, beginning with the comments raised by the editors followed by those from reviewer one, two, and so on. After each response, clearly point the reviewers and the editors to the revised sections in the manuscript. In case of similar comments, it is acceptable to direct the second (or the third) reviewer to your previous response. Provide new tables, figures, data elements, and references as part of the response letter to make it a stand-alone document. It can be difficult (and annoying) if the reviewer has to flip back and forth between documents to understand the full story.
Appealing editorial decisions consumes a lot of energy, annoys editors and reviewers, and is generally futile. If it is needed, then write a polite, brief appeal letter that summarizes the reasons for the appeal. The most common editorial response to an appeal, which usually follows a several-week delay, is an equally polite affirmation of the original decision. The second and arguably worse outcome is for the manuscript to be sent to two to three new reviewers with another rejection after a several-month delay.
Have colleagues read and comment on your revised paper and use these comments to improve the draft. There is evidence that writing groups are effective in providing suggestions for improving papers: A writing group also keeps the momentum going during the revision process. Setting realistic time lines with the coauthors of the paper is a useful strategy to maintain momentum during revisions.
Writing (and revising) papers can be a highly rewarding activity. Start early, plan carefully, and do not delay the process. Reviewers’ comments are mostly geared toward enhancing the manuscript. Take them seriously, address them fully, and you will have an improved (and we hope, an accepted) manuscript.
Additional reading
El-Serag HB. Writing and publishing scientific papers. Gastroenterology. 2012 Feb;142(2):197-200. doi: 10.1053/j.gastro.2011.12.021. Epub 2011 Dec 16.
Downey SMet al. Manuscript development and publishing: A 5-step approach. Am J Med Sci. 2017 Feb;353(2):132-6. doi: 10.1016/j.amjms.2016.12.005. Epub 2016 Dec 9.
Sullivan GM. What to do when your paper is rejected. J Grad Med Educ. 2015;7:1-3. doi: 10.4300/JGME-D-14-00686.1
Kotz Det al. Effective writing and publishing scientific papers, part XII: responding to reviewers. J Clin Epidemiol. 2014;67:243. doi: 10.1016/j.jclinepi.2013.10.003. Epub 2014 Jan 9.
Dr. El-Serag is chairman of the Margaret M. and Albert B. Alkek department of medicine, Baylor College of Medicine, Houston; incoming president of the American Gastroenterological Association Institute; and past Editor in Chief, Clinical Gastroenterology and Hepatology. Dr. Kanwal is professor of medicine and chief of the section of gastroenterology and hepatology, department of medicine, Baylor College of Medicine and Michael E. DeBakey Veterans Affairs Medical Center, Houston, and Editor in Chief, Clinical Gastroenterology and Hepatology. This material is based on work supported by Cancer Prevention & Research Institute of Texas grant (RP150587). The work is also supported in part by the Center for Gastrointestinal Development, Infection and Injury (NIDDK P30 DK 56338).
Authors of manuscripts typically receive one of three responses from journals: 1. Accepted as submitted; 2. Accepted pending revisions (major or minor); and 3. Rejected. Receiving an unconditional acceptance is an unusual fate worth documenting and celebrating. On the other hand, irreversible rejections are so common that authors need to get accustomed to them. Upon receiving an unqualified editorial rejection without a formal review (usually described as priority-related rejection), send the same manuscript out the next day to another journal (with electronic submissions you can do this on the same day).
If your manuscript is rejected after being reviewed, consider seriously the comments given and try to learn from them. Was your study design flawed? Do you need additional data? Were your analyses incomplete or did you employ suboptimal statistical methods? Was your interpretation of the findings far reaching and out of proportion to the actual data? Use this experience and feedback, revise your manuscript, and submit it to a different journal. It is not uncommon to encounter the same reviewer at the next journal; fixing major issues seems responsive and gets you in the door.
To receive a “conditional acceptance” or “rejection with hope” is the most likely “good” editorial response. Avoid a very quick response, because it may be hasty or create an impression of a hasty response. Because most manuscripts with substantial reviews are sent back to the reviewers, the turnaround time in most journals is several weeks and, therefore, there is little to be gained by sending the revised manuscript in 1 day rather than 1 week. The best course of action is to cool down for 1-2 days and then decide and draft responses in 1 week, including planned additional analyses. In the case of seemingly contradictory or numerous requests from reviewers, it is best to carefully examine clues from the editors or associate editors as to the nature and extent of the revision needed. In most instances, we draft the response letter before revising the manuscript. We use the draft letter to obtain specific input from other authors and ‘brainstorm’ about additional analyses that can best address reviewers concerns.
Do the best that you can to fully address all reviewers’ comments. Adequate time should be spent making real changes, including adding additional data or analyses to the manuscript, and taking utmost care in describing and highlighting these changes. If you believe that the reviewers missed a point that was already included in the paper, then point this out as politely as possible as part of the response letter (see below).
In addition to revising your manuscript, you will be asked to prepare a point-by-point response to each of the reviewers’ comments you receive. Thank the editors and reviewers sincerely for their comments and explain how changes based on the comments have made the paper better; they did spend time reviewing your manuscript, and they have not rejected it yet. Reviewers are usually recognized experts, or their apprentices, in the content or method of research employed in your paper. Reviewers are also likely to be authors on papers cited in your manuscript. Avoid unnecessary arguments when possible, especially about noncore issues or about changes that you already conceded. If you are compelled to contest any of the reviewers’ comments, provide substantial evidence that supports your position and be respectful with your responses. Address each comment separately, beginning with the comments raised by the editors followed by those from reviewer one, two, and so on. After each response, clearly point the reviewers and the editors to the revised sections in the manuscript. In case of similar comments, it is acceptable to direct the second (or the third) reviewer to your previous response. Provide new tables, figures, data elements, and references as part of the response letter to make it a stand-alone document. It can be difficult (and annoying) if the reviewer has to flip back and forth between documents to understand the full story.
Appealing editorial decisions consumes a lot of energy, annoys editors and reviewers, and is generally futile. If it is needed, then write a polite, brief appeal letter that summarizes the reasons for the appeal. The most common editorial response to an appeal, which usually follows a several-week delay, is an equally polite affirmation of the original decision. The second and arguably worse outcome is for the manuscript to be sent to two to three new reviewers with another rejection after a several-month delay.
Have colleagues read and comment on your revised paper and use these comments to improve the draft. There is evidence that writing groups are effective in providing suggestions for improving papers: A writing group also keeps the momentum going during the revision process. Setting realistic time lines with the coauthors of the paper is a useful strategy to maintain momentum during revisions.
Writing (and revising) papers can be a highly rewarding activity. Start early, plan carefully, and do not delay the process. Reviewers’ comments are mostly geared toward enhancing the manuscript. Take them seriously, address them fully, and you will have an improved (and we hope, an accepted) manuscript.
Additional reading
El-Serag HB. Writing and publishing scientific papers. Gastroenterology. 2012 Feb;142(2):197-200. doi: 10.1053/j.gastro.2011.12.021. Epub 2011 Dec 16.
Downey SMet al. Manuscript development and publishing: A 5-step approach. Am J Med Sci. 2017 Feb;353(2):132-6. doi: 10.1016/j.amjms.2016.12.005. Epub 2016 Dec 9.
Sullivan GM. What to do when your paper is rejected. J Grad Med Educ. 2015;7:1-3. doi: 10.4300/JGME-D-14-00686.1
Kotz Det al. Effective writing and publishing scientific papers, part XII: responding to reviewers. J Clin Epidemiol. 2014;67:243. doi: 10.1016/j.jclinepi.2013.10.003. Epub 2014 Jan 9.
Dr. El-Serag is chairman of the Margaret M. and Albert B. Alkek department of medicine, Baylor College of Medicine, Houston; incoming president of the American Gastroenterological Association Institute; and past Editor in Chief, Clinical Gastroenterology and Hepatology. Dr. Kanwal is professor of medicine and chief of the section of gastroenterology and hepatology, department of medicine, Baylor College of Medicine and Michael E. DeBakey Veterans Affairs Medical Center, Houston, and Editor in Chief, Clinical Gastroenterology and Hepatology. This material is based on work supported by Cancer Prevention & Research Institute of Texas grant (RP150587). The work is also supported in part by the Center for Gastrointestinal Development, Infection and Injury (NIDDK P30 DK 56338).
Authors of manuscripts typically receive one of three responses from journals: 1. Accepted as submitted; 2. Accepted pending revisions (major or minor); and 3. Rejected. Receiving an unconditional acceptance is an unusual fate worth documenting and celebrating. On the other hand, irreversible rejections are so common that authors need to get accustomed to them. Upon receiving an unqualified editorial rejection without a formal review (usually described as priority-related rejection), send the same manuscript out the next day to another journal (with electronic submissions you can do this on the same day).
If your manuscript is rejected after being reviewed, consider seriously the comments given and try to learn from them. Was your study design flawed? Do you need additional data? Were your analyses incomplete or did you employ suboptimal statistical methods? Was your interpretation of the findings far reaching and out of proportion to the actual data? Use this experience and feedback, revise your manuscript, and submit it to a different journal. It is not uncommon to encounter the same reviewer at the next journal; fixing major issues seems responsive and gets you in the door.
To receive a “conditional acceptance” or “rejection with hope” is the most likely “good” editorial response. Avoid a very quick response, because it may be hasty or create an impression of a hasty response. Because most manuscripts with substantial reviews are sent back to the reviewers, the turnaround time in most journals is several weeks and, therefore, there is little to be gained by sending the revised manuscript in 1 day rather than 1 week. The best course of action is to cool down for 1-2 days and then decide and draft responses in 1 week, including planned additional analyses. In the case of seemingly contradictory or numerous requests from reviewers, it is best to carefully examine clues from the editors or associate editors as to the nature and extent of the revision needed. In most instances, we draft the response letter before revising the manuscript. We use the draft letter to obtain specific input from other authors and ‘brainstorm’ about additional analyses that can best address reviewers concerns.
Do the best that you can to fully address all reviewers’ comments. Adequate time should be spent making real changes, including adding additional data or analyses to the manuscript, and taking utmost care in describing and highlighting these changes. If you believe that the reviewers missed a point that was already included in the paper, then point this out as politely as possible as part of the response letter (see below).
In addition to revising your manuscript, you will be asked to prepare a point-by-point response to each of the reviewers’ comments you receive. Thank the editors and reviewers sincerely for their comments and explain how changes based on the comments have made the paper better; they did spend time reviewing your manuscript, and they have not rejected it yet. Reviewers are usually recognized experts, or their apprentices, in the content or method of research employed in your paper. Reviewers are also likely to be authors on papers cited in your manuscript. Avoid unnecessary arguments when possible, especially about noncore issues or about changes that you already conceded. If you are compelled to contest any of the reviewers’ comments, provide substantial evidence that supports your position and be respectful with your responses. Address each comment separately, beginning with the comments raised by the editors followed by those from reviewer one, two, and so on. After each response, clearly point the reviewers and the editors to the revised sections in the manuscript. In case of similar comments, it is acceptable to direct the second (or the third) reviewer to your previous response. Provide new tables, figures, data elements, and references as part of the response letter to make it a stand-alone document. It can be difficult (and annoying) if the reviewer has to flip back and forth between documents to understand the full story.
Appealing editorial decisions consumes a lot of energy, annoys editors and reviewers, and is generally futile. If it is needed, then write a polite, brief appeal letter that summarizes the reasons for the appeal. The most common editorial response to an appeal, which usually follows a several-week delay, is an equally polite affirmation of the original decision. The second and arguably worse outcome is for the manuscript to be sent to two to three new reviewers with another rejection after a several-month delay.
Have colleagues read and comment on your revised paper and use these comments to improve the draft. There is evidence that writing groups are effective in providing suggestions for improving papers: A writing group also keeps the momentum going during the revision process. Setting realistic time lines with the coauthors of the paper is a useful strategy to maintain momentum during revisions.
Writing (and revising) papers can be a highly rewarding activity. Start early, plan carefully, and do not delay the process. Reviewers’ comments are mostly geared toward enhancing the manuscript. Take them seriously, address them fully, and you will have an improved (and we hope, an accepted) manuscript.
Additional reading
El-Serag HB. Writing and publishing scientific papers. Gastroenterology. 2012 Feb;142(2):197-200. doi: 10.1053/j.gastro.2011.12.021. Epub 2011 Dec 16.
Downey SMet al. Manuscript development and publishing: A 5-step approach. Am J Med Sci. 2017 Feb;353(2):132-6. doi: 10.1016/j.amjms.2016.12.005. Epub 2016 Dec 9.
Sullivan GM. What to do when your paper is rejected. J Grad Med Educ. 2015;7:1-3. doi: 10.4300/JGME-D-14-00686.1
Kotz Det al. Effective writing and publishing scientific papers, part XII: responding to reviewers. J Clin Epidemiol. 2014;67:243. doi: 10.1016/j.jclinepi.2013.10.003. Epub 2014 Jan 9.
Dr. El-Serag is chairman of the Margaret M. and Albert B. Alkek department of medicine, Baylor College of Medicine, Houston; incoming president of the American Gastroenterological Association Institute; and past Editor in Chief, Clinical Gastroenterology and Hepatology. Dr. Kanwal is professor of medicine and chief of the section of gastroenterology and hepatology, department of medicine, Baylor College of Medicine and Michael E. DeBakey Veterans Affairs Medical Center, Houston, and Editor in Chief, Clinical Gastroenterology and Hepatology. This material is based on work supported by Cancer Prevention & Research Institute of Texas grant (RP150587). The work is also supported in part by the Center for Gastrointestinal Development, Infection and Injury (NIDDK P30 DK 56338).
Junior faculty guide to preparing a research grant
A wise person once said, “Research is a marathon and not a sprint.” Grant writing is the training for the marathon, and it requires discipline and fortitude to succeed. We are junior faculty members with mentored career development awards who are transitioning to independence. Below, we provide for our junior faculty colleagues some tips that have helped us train for our marathon in research.
Identify great mentors
We all understand that outstanding mentorship is critical to success. With that said, we often struggle to understand what a good mentor is. In regard to grant writing, you need someone who is willing to use red ink. While positive reinforcement may be good for your self-esteem, your mentor needs to be critical so that you can learn how to present the best possible product. In return, you must be an invested mentee who is respectful of the mentor’s time, is prepared for meetings, and responds appropriately to feedback.
Attend workshops
Your home institution and professional societies hold outstanding workshops that provide didactic lessons on both the logistics and mechanics of grant writing and allow you to network with like-minded peers, potential mentors, and staff from the funding organization. One excellent example is the American Gastroenterological Association/American Association for the Study of Liver Diseases (AGA/AASLD) Academic Skills workshop.
Decide on a grant mechanism
There are many different grants available through the government, industry, foundations, and your institution. Each grantor may have a variety of mechanisms from pilot awards to larger multiprovider and institution grants. Deciding which grants to apply for can be more of an art than it is a science. Research the opportunities available to you and develop a long-term plan with your mentors.
Start early and have a plan
An effective grant application is prepared in steps, and every step takes longer than anticipated. About 6 months prior to the deadline, read the instructions and consider using something like a Gantt chart to identify all required sections, special requirements (font, spacing, page limits), and anticipated time of completion. Then, structure a reasonable timeline – and stick to it! Remember to allow ample time for all sections, including the career development plan and research environment. Your institution will probably request the documents early, anywhere from 1 to 2 weeks prior to the deadline so that it can be circulated for institutional signatures. Steady progress wins most races.
Specific aims
There is no grant without a great idea, but not all great ideas are funded. So, the first step is to polish your idea, which must be clearly described on the Specific Aims page, which is a one-page summary that lays the framework for the rest of the grant. For the primary reviewers, it should entice them to read the proposal. For others on the review panel, it may be the only section of your grant that they read. Make sure it is clear and concise. If possible, construct a visually pleasing and easy-to-follow figure that encapsulates your proposal.
Circulate
Ideally, every section of the grant will be circulated but it is critical to have others review the Specific Aims at the very least. Ask not only your mentors and those in the field to critique but also those outside of your area and even your friends and unsuspecting family members; they may not know (or care) about the content but should be able to follow the flow and identify grammatical errors. Remember that everyone is busy, so give ample time for people to review the documents.
Read other proposals
Practice makes perfect. So you can either apply for many grants and make the mistakes yourself or read and review as many proposals as you can to learn from your colleagues’ successes and mistakes. Many institutions, mentors, and colleagues will provide copies of prior applications if you ask. Make sure you know which were successful and try to understand why the others were not successful.
When reading the aims and research strategy, pay close attention to how significance and innovation are detailed. Also, some things like the research environment, which is especially important for career development grants, may be directly applicable to your grant.
Help the reviewer
In general, reviewing grants is a voluntary undertaking. Imagine the reviewer reading your grant at a home filled with screaming children or, alternatively, flying in cramped quarters. Neither situation is stress-free, so put yourself in those positions and decide what you can do to make the reviewer’s job easier.
Use figures and tables to summarize the text, and consider coming back to the figure from your Specific Aims to refer to the specific parts of the proposal. You can decrease reviewer fatigue by using line breaks and fonts to break up sections and highlight important details. This will also be helpful to the reviewers on the panel who were not assigned to your grant and possibly first seeing it during the session.
Learn from rejection
You are either a savant or have not applied for enough grants if you have not received a rejection letter. Often, reviewers provide you with constructive comments, which (after a session of crying in the corner in a fetal position), you can use to improve your grant. Resubmission works!
Apply widely
Identify different possible grants, and work with your mentors on a strategy that allows you to make your idea versatile and package it for various funding mechanisms. Once you have a grant, you can tailor it to other grants as needed. However, remember that quantity does not replace quality, so many poor grants that are not funded will not replace one good one that is funded.
There are multiple approaches to training for the marathon of research, so these tips are not a comprehensive list or mandatory commandments. They have, however, proven invaluable to our mentors and us. Our institutions, societies and government agencies have identified the decline of young scientists and physician-scientists as a major leak in the research pipeline, so there are excellent funding mechanisms that are available to you. Good luck!
We would like to acknowledge Jennifer Weiss, MD, and Sumera Rizvi, MD, for their constructive comments.
Dr. Beyder is with the enteric neuroscience program, a consultant for the department of gastroenterology and hepatology, and an assistant professor of biomedical engineering and physiology at the Mayo Clinic School of Medicine, Rochester, Minn.; Dr. Twyman-Saint Victor is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.
A wise person once said, “Research is a marathon and not a sprint.” Grant writing is the training for the marathon, and it requires discipline and fortitude to succeed. We are junior faculty members with mentored career development awards who are transitioning to independence. Below, we provide for our junior faculty colleagues some tips that have helped us train for our marathon in research.
Identify great mentors
We all understand that outstanding mentorship is critical to success. With that said, we often struggle to understand what a good mentor is. In regard to grant writing, you need someone who is willing to use red ink. While positive reinforcement may be good for your self-esteem, your mentor needs to be critical so that you can learn how to present the best possible product. In return, you must be an invested mentee who is respectful of the mentor’s time, is prepared for meetings, and responds appropriately to feedback.
Attend workshops
Your home institution and professional societies hold outstanding workshops that provide didactic lessons on both the logistics and mechanics of grant writing and allow you to network with like-minded peers, potential mentors, and staff from the funding organization. One excellent example is the American Gastroenterological Association/American Association for the Study of Liver Diseases (AGA/AASLD) Academic Skills workshop.
Decide on a grant mechanism
There are many different grants available through the government, industry, foundations, and your institution. Each grantor may have a variety of mechanisms from pilot awards to larger multiprovider and institution grants. Deciding which grants to apply for can be more of an art than it is a science. Research the opportunities available to you and develop a long-term plan with your mentors.
Start early and have a plan
An effective grant application is prepared in steps, and every step takes longer than anticipated. About 6 months prior to the deadline, read the instructions and consider using something like a Gantt chart to identify all required sections, special requirements (font, spacing, page limits), and anticipated time of completion. Then, structure a reasonable timeline – and stick to it! Remember to allow ample time for all sections, including the career development plan and research environment. Your institution will probably request the documents early, anywhere from 1 to 2 weeks prior to the deadline so that it can be circulated for institutional signatures. Steady progress wins most races.
Specific aims
There is no grant without a great idea, but not all great ideas are funded. So, the first step is to polish your idea, which must be clearly described on the Specific Aims page, which is a one-page summary that lays the framework for the rest of the grant. For the primary reviewers, it should entice them to read the proposal. For others on the review panel, it may be the only section of your grant that they read. Make sure it is clear and concise. If possible, construct a visually pleasing and easy-to-follow figure that encapsulates your proposal.
Circulate
Ideally, every section of the grant will be circulated but it is critical to have others review the Specific Aims at the very least. Ask not only your mentors and those in the field to critique but also those outside of your area and even your friends and unsuspecting family members; they may not know (or care) about the content but should be able to follow the flow and identify grammatical errors. Remember that everyone is busy, so give ample time for people to review the documents.
Read other proposals
Practice makes perfect. So you can either apply for many grants and make the mistakes yourself or read and review as many proposals as you can to learn from your colleagues’ successes and mistakes. Many institutions, mentors, and colleagues will provide copies of prior applications if you ask. Make sure you know which were successful and try to understand why the others were not successful.
When reading the aims and research strategy, pay close attention to how significance and innovation are detailed. Also, some things like the research environment, which is especially important for career development grants, may be directly applicable to your grant.
Help the reviewer
In general, reviewing grants is a voluntary undertaking. Imagine the reviewer reading your grant at a home filled with screaming children or, alternatively, flying in cramped quarters. Neither situation is stress-free, so put yourself in those positions and decide what you can do to make the reviewer’s job easier.
Use figures and tables to summarize the text, and consider coming back to the figure from your Specific Aims to refer to the specific parts of the proposal. You can decrease reviewer fatigue by using line breaks and fonts to break up sections and highlight important details. This will also be helpful to the reviewers on the panel who were not assigned to your grant and possibly first seeing it during the session.
Learn from rejection
You are either a savant or have not applied for enough grants if you have not received a rejection letter. Often, reviewers provide you with constructive comments, which (after a session of crying in the corner in a fetal position), you can use to improve your grant. Resubmission works!
Apply widely
Identify different possible grants, and work with your mentors on a strategy that allows you to make your idea versatile and package it for various funding mechanisms. Once you have a grant, you can tailor it to other grants as needed. However, remember that quantity does not replace quality, so many poor grants that are not funded will not replace one good one that is funded.
There are multiple approaches to training for the marathon of research, so these tips are not a comprehensive list or mandatory commandments. They have, however, proven invaluable to our mentors and us. Our institutions, societies and government agencies have identified the decline of young scientists and physician-scientists as a major leak in the research pipeline, so there are excellent funding mechanisms that are available to you. Good luck!
We would like to acknowledge Jennifer Weiss, MD, and Sumera Rizvi, MD, for their constructive comments.
Dr. Beyder is with the enteric neuroscience program, a consultant for the department of gastroenterology and hepatology, and an assistant professor of biomedical engineering and physiology at the Mayo Clinic School of Medicine, Rochester, Minn.; Dr. Twyman-Saint Victor is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.
A wise person once said, “Research is a marathon and not a sprint.” Grant writing is the training for the marathon, and it requires discipline and fortitude to succeed. We are junior faculty members with mentored career development awards who are transitioning to independence. Below, we provide for our junior faculty colleagues some tips that have helped us train for our marathon in research.
Identify great mentors
We all understand that outstanding mentorship is critical to success. With that said, we often struggle to understand what a good mentor is. In regard to grant writing, you need someone who is willing to use red ink. While positive reinforcement may be good for your self-esteem, your mentor needs to be critical so that you can learn how to present the best possible product. In return, you must be an invested mentee who is respectful of the mentor’s time, is prepared for meetings, and responds appropriately to feedback.
Attend workshops
Your home institution and professional societies hold outstanding workshops that provide didactic lessons on both the logistics and mechanics of grant writing and allow you to network with like-minded peers, potential mentors, and staff from the funding organization. One excellent example is the American Gastroenterological Association/American Association for the Study of Liver Diseases (AGA/AASLD) Academic Skills workshop.
Decide on a grant mechanism
There are many different grants available through the government, industry, foundations, and your institution. Each grantor may have a variety of mechanisms from pilot awards to larger multiprovider and institution grants. Deciding which grants to apply for can be more of an art than it is a science. Research the opportunities available to you and develop a long-term plan with your mentors.
Start early and have a plan
An effective grant application is prepared in steps, and every step takes longer than anticipated. About 6 months prior to the deadline, read the instructions and consider using something like a Gantt chart to identify all required sections, special requirements (font, spacing, page limits), and anticipated time of completion. Then, structure a reasonable timeline – and stick to it! Remember to allow ample time for all sections, including the career development plan and research environment. Your institution will probably request the documents early, anywhere from 1 to 2 weeks prior to the deadline so that it can be circulated for institutional signatures. Steady progress wins most races.
Specific aims
There is no grant without a great idea, but not all great ideas are funded. So, the first step is to polish your idea, which must be clearly described on the Specific Aims page, which is a one-page summary that lays the framework for the rest of the grant. For the primary reviewers, it should entice them to read the proposal. For others on the review panel, it may be the only section of your grant that they read. Make sure it is clear and concise. If possible, construct a visually pleasing and easy-to-follow figure that encapsulates your proposal.
Circulate
Ideally, every section of the grant will be circulated but it is critical to have others review the Specific Aims at the very least. Ask not only your mentors and those in the field to critique but also those outside of your area and even your friends and unsuspecting family members; they may not know (or care) about the content but should be able to follow the flow and identify grammatical errors. Remember that everyone is busy, so give ample time for people to review the documents.
Read other proposals
Practice makes perfect. So you can either apply for many grants and make the mistakes yourself or read and review as many proposals as you can to learn from your colleagues’ successes and mistakes. Many institutions, mentors, and colleagues will provide copies of prior applications if you ask. Make sure you know which were successful and try to understand why the others were not successful.
When reading the aims and research strategy, pay close attention to how significance and innovation are detailed. Also, some things like the research environment, which is especially important for career development grants, may be directly applicable to your grant.
Help the reviewer
In general, reviewing grants is a voluntary undertaking. Imagine the reviewer reading your grant at a home filled with screaming children or, alternatively, flying in cramped quarters. Neither situation is stress-free, so put yourself in those positions and decide what you can do to make the reviewer’s job easier.
Use figures and tables to summarize the text, and consider coming back to the figure from your Specific Aims to refer to the specific parts of the proposal. You can decrease reviewer fatigue by using line breaks and fonts to break up sections and highlight important details. This will also be helpful to the reviewers on the panel who were not assigned to your grant and possibly first seeing it during the session.
Learn from rejection
You are either a savant or have not applied for enough grants if you have not received a rejection letter. Often, reviewers provide you with constructive comments, which (after a session of crying in the corner in a fetal position), you can use to improve your grant. Resubmission works!
Apply widely
Identify different possible grants, and work with your mentors on a strategy that allows you to make your idea versatile and package it for various funding mechanisms. Once you have a grant, you can tailor it to other grants as needed. However, remember that quantity does not replace quality, so many poor grants that are not funded will not replace one good one that is funded.
There are multiple approaches to training for the marathon of research, so these tips are not a comprehensive list or mandatory commandments. They have, however, proven invaluable to our mentors and us. Our institutions, societies and government agencies have identified the decline of young scientists and physician-scientists as a major leak in the research pipeline, so there are excellent funding mechanisms that are available to you. Good luck!
We would like to acknowledge Jennifer Weiss, MD, and Sumera Rizvi, MD, for their constructive comments.
Dr. Beyder is with the enteric neuroscience program, a consultant for the department of gastroenterology and hepatology, and an assistant professor of biomedical engineering and physiology at the Mayo Clinic School of Medicine, Rochester, Minn.; Dr. Twyman-Saint Victor is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.
November 2018
Gastroenterology
How and when to consider genetic testing for colon cancer? Ballester V; Cruz-Correa M. 2018 Oct;155(4):955-9. doi: 10.1053/j.gastro.2018.08.031.
How to approach a patient with eosinophilic esophagitis. Hirano I. 2018 Sept;155(3):601-6. doi: 10.1053/j.gastro.2018.08.001.
How to ensure patient adherence to colorectal cancer screening and surveillance in your practice. Hassan C; Kaminski MF; Repici A. 2018 Aug;155(2):252-7. doi: 10.1053/j.gastro.2018.06.051.
How to approach difficult patient encounters: ROAR. McCarthy JG; Cheatham JG; Singla M. 2018 Aug;155(2):258-61. doi: 10.1053/j.gastro.2018.06.052.
An inside view: AGA advocacy priorities. Jain R. 2018 Aug;155(2):572-3. doi: 10.1053/j.gastro.2018.06.028.
Clin Gastro Hepatol
Adding value to the conversation about colorectal cancer screening: Practical pearls for gastroenterologists. Maratt JK; Naylor K; Saini SD. 2018 Oct;16(10):1545–8. doi: 10.1016/j.cgh.2018.07.002.
Credentialing for endoscopic practice: The Mayo Clinic model. Kane SV; Chandrasekhara V; Sedlack RE; Buttar NS. 2018 Sept;16(9):1370–3.e1 doi: 10.1016/j.cgh.2018.06.020.
Gastroenterology
How and when to consider genetic testing for colon cancer? Ballester V; Cruz-Correa M. 2018 Oct;155(4):955-9. doi: 10.1053/j.gastro.2018.08.031.
How to approach a patient with eosinophilic esophagitis. Hirano I. 2018 Sept;155(3):601-6. doi: 10.1053/j.gastro.2018.08.001.
How to ensure patient adherence to colorectal cancer screening and surveillance in your practice. Hassan C; Kaminski MF; Repici A. 2018 Aug;155(2):252-7. doi: 10.1053/j.gastro.2018.06.051.
How to approach difficult patient encounters: ROAR. McCarthy JG; Cheatham JG; Singla M. 2018 Aug;155(2):258-61. doi: 10.1053/j.gastro.2018.06.052.
An inside view: AGA advocacy priorities. Jain R. 2018 Aug;155(2):572-3. doi: 10.1053/j.gastro.2018.06.028.
Clin Gastro Hepatol
Adding value to the conversation about colorectal cancer screening: Practical pearls for gastroenterologists. Maratt JK; Naylor K; Saini SD. 2018 Oct;16(10):1545–8. doi: 10.1016/j.cgh.2018.07.002.
Credentialing for endoscopic practice: The Mayo Clinic model. Kane SV; Chandrasekhara V; Sedlack RE; Buttar NS. 2018 Sept;16(9):1370–3.e1 doi: 10.1016/j.cgh.2018.06.020.
Gastroenterology
How and when to consider genetic testing for colon cancer? Ballester V; Cruz-Correa M. 2018 Oct;155(4):955-9. doi: 10.1053/j.gastro.2018.08.031.
How to approach a patient with eosinophilic esophagitis. Hirano I. 2018 Sept;155(3):601-6. doi: 10.1053/j.gastro.2018.08.001.
How to ensure patient adherence to colorectal cancer screening and surveillance in your practice. Hassan C; Kaminski MF; Repici A. 2018 Aug;155(2):252-7. doi: 10.1053/j.gastro.2018.06.051.
How to approach difficult patient encounters: ROAR. McCarthy JG; Cheatham JG; Singla M. 2018 Aug;155(2):258-61. doi: 10.1053/j.gastro.2018.06.052.
An inside view: AGA advocacy priorities. Jain R. 2018 Aug;155(2):572-3. doi: 10.1053/j.gastro.2018.06.028.
Clin Gastro Hepatol
Adding value to the conversation about colorectal cancer screening: Practical pearls for gastroenterologists. Maratt JK; Naylor K; Saini SD. 2018 Oct;16(10):1545–8. doi: 10.1016/j.cgh.2018.07.002.
Credentialing for endoscopic practice: The Mayo Clinic model. Kane SV; Chandrasekhara V; Sedlack RE; Buttar NS. 2018 Sept;16(9):1370–3.e1 doi: 10.1016/j.cgh.2018.06.020.
Quality metrics in colonoscopy
Editor's Note:
As quality metrics are becoming increasingly significant throughout all of medicine, our field is no exception. Recent evidence has demonstrated the importance of quality measures in colonoscopy; understanding, reporting, and improving these metrics has become a hot topic of discussion.
In this month’s In Focus article, brought to you by The New Gastroenterologist, Nabiha Shamsi, Ashish Malhotra, and Aasma Shaukat (University of Minnesota/Minneapolis VAMC) provide an outstanding overview of the evidence as well as recommended goals for important quality metrics in colonoscopy. Ultimately, improving colonoscopy quality amongst all gastroenterologists will increase colonoscopy value and lead to further decreases in the incidence and mortality of colorectal cancer.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Introduction
Colonoscopy is a widely used modality to evaluate colorectal cancer because it allows for both identification of early malignancies and removal of precancerous lesions. The increased use of colonoscopy in the last 20 years has been associated with a decline in the incidence and mortality from colorectal cancer.1,2 However, colonoscopy has its limitations. It is an invasive test with inherent risks. Additionally, studies have reported rates of post-colonoscopy cancers, also referred to as interval cancers, of 2%-7%, and miss-rates for adenomas by tandem colonoscopy of 2%-26%.3-5
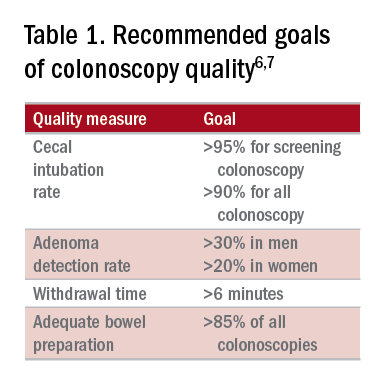
High-quality exams can maximize the value of colonoscopy, and it is important to consider the factors that contribute to high-quality colonoscopies. While there are many metrics proposed,6,7 here we discuss the most evidence-based ones, outlined in Table 1, along with their goal values.
Cecal intubation rate
A high-quality colonoscopy should include a complete examination of the colon. To achieve this, it is necessary to fully intubate the cecum, passing the colonoscope past the ileocecal valve to examine the medial wall of the cecum.8
There are several factors that may contribute to an incomplete colonoscopy, including bowel preparation, anatomy, body habitus, and endoscopist’s skill. To calculate cecal intubation rate as a quality measure, colonoscopies that are incomplete because of poor bowel preparation, severe colitis, or known obstructing lesion are usually excluded.
The U.S. Multi-Society Task Force on Colorectal Cancer recommends a cecal intubation rate of at least 95% for screening colonoscopy and 90% for all colonoscopies.6 There is an expectation of photodocumentation of the ileocecal valve and appendiceal orifice to establish completion of the colonoscopy.6
Some methods used to assist with cecal intubation include changing patient position, applying abdominal pressure, stiffening the colonoscope, and alternating between adult or pediatric colonoscopes.
Adenoma detection rate
Adenoma detection rate (ADR), is defined as the proportion of patients over the age of 50 years undergoing first-time screening colonoscopies in which at least one adenomatous polyp is detected for a given endoscopist in a given time period.
Adenomas are tracked because clearing the colon of neoplasm is the goal of screening colonoscopies; adenomas are tracked instead of more advanced lesions because the higher frequency of adenomas allows for better tracking of variation between endoscopists. Tracking ADR also utilizes the assumption that, if small lesions are identified, larger ones will be as well.
ADR is the only current quality indicator reported to be significantly associated with the risk of interval cancers. In 2010, a study of 45,000 screening colonoscopies by 186 endoscopists validated the use of ADR, finding that patients who underwent colonoscopy by physicians with ADRs below 20% had hazard ratios for development of postcolonoscopy cancer greater than 10 times higher than patients of physicians with ADRs above 20%.9 However, this study had limited power to establish that cancer protection continues to improve when ADRs rise above 20%. Another study, which evaluated the association of ADR in 224,000 patients undergoing colonoscopies by 136 gastroenterologists, showed each 1% increase in ADR is associated with 3% decrease in the risk of interval CRC and 5% decrease in the risk of fatal interval cancers.10
Most recent guidelines propose an adequate ADR for asymptomatic individuals aged 50 years or older undergoing screening colonoscopy should be greater than 30% in men and greater than 20% in women.6 It remains unknown whether there is a threshold for maximum benefit of ADR, in which a very high ADR is not associated with further protective benefit. The answer to this question may depend on why a low ADR is associated with a higher rate of interval cancers and whether every missed polyp, independent of size, is a potential interval cancer or whether hasty, inadequate, or incomplete examinations of the colon are the underlying concern.
Withdrawal time
Optimizing identification of colonic lesions requires a careful and thorough exam of the colon on withdrawal. While this may seem obvious, there is often little focus on the approach to withdrawal. In four chapters on colonoscopy technique from textbooks, the number of pages describing insertion ranged from 20 to 38, while the number of pages focused on withdrawal ranged from 0.5 to 1.5.11-14
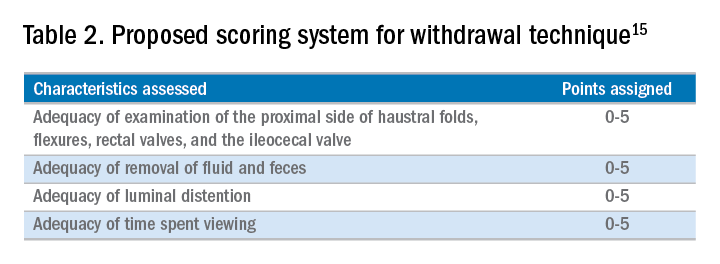
A study examining the difference in withdrawal technique between two endoscopists who were known to differ in adenoma miss rates by tandem colonoscopy proposed the scoring system listed in Table 2 that can assess quality of examination on withdrawal. There was a statistically significant difference in quality scores for the two endoscopists, as assessed by expert review of video recordings of their colonoscopies.15
The endoscopist with the lower adenoma miss rate was also found to have an average withdrawal time of 8 minutes and 55 seconds versus 6 minutes and 41 seconds for the endoscopist with the higher adenoma miss rate. A large, community-based study with over 76,000 colonoscopies found a statistically significant correlation between interval colorectal cancer and withdrawal times shorter than 6 minutes.16 However, there was no association between ADR and colorectal cancer, suggesting that, for practices with optimal ADRs (that is, rates greater than 25%), withdrawal time may be a more sensitive marker of quality of colonoscopy than ADR is.16Intuitively, adequate examination of the colon that includes examining the proximal side of folds, washing and suctioning stool, and even repositioning the patient would likely increase withdrawal time. In a 2008 study examining 2,000 screening colonoscopies of 12 endoscopists, those with withdrawal times greater than 6 minutes had significantly higher rates of detecting adenomas and advanced neoplasia, compared with those with faster withdrawal times.17 The average ADR in this group was 28.3%, compared with 11.8% for physicians who had a withdrawal time less than 6 minutes.17 An evaluation of nearly 11,000 colonoscopies done by 43 endoscopists also identified an increase polyp yield with increased withdrawal time.18 These data drive the recommendation for a minimum withdrawal time of 6 minutes, with 2 minutes spent examining each colonic segment.
Bowel preparation
Diagnosis of colonic lesions is dependent on adequate visualization of the colon. Poor bowel preparation can limit the yield of colonoscopy and lead to missed lesions. It also leads to canceled and rescheduled procedures that reduce efficiency, increase cost, and pose an undue burden on the patient.
The quality of bowel preparation should be assessed after washing and suctioning of colonic mucosa has been completed. Adequate preparation is that which allows identification of lesions greater than 5 mm in size.19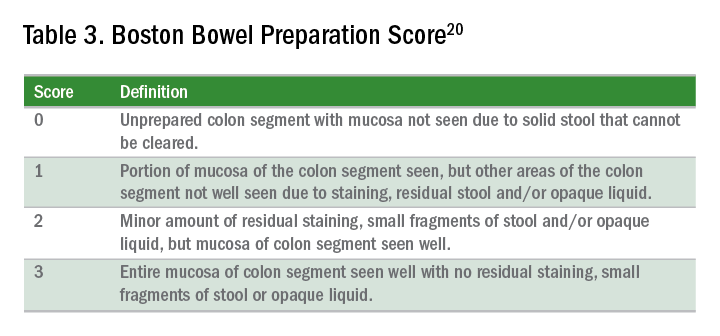
Quality of preparation is assessed subjectively by the endoscopists and often listed as excellent, good, fair, or poor. An alternative method of reporting bowel preparation quality is the Boston Bowel Preparation Score (BBPS) (Table 3).20 This scoring system allows for a more descriptive assessment of each colonic segment by assigning a score from 0 to 3 for the right, transverse, and left colon, leading to a total score between 0 and 9. The BBPS also helps standardize reporting of bowel preparation. The polyp detection rate associated with a BBPS of 5 or greater was 40%, compared with 24% associated with BBPS less than 5.19 A split-dose bowel preparation regimen with at least half of the preparation ingested on the day of the procedure is recommended to optimize quality of bowel preparation.6
The American Society for Gastrointestinal Endoscopy and American College of Gastroenterology task force on quality assurance in endoscopy recommends that bowel preparation should be adequate in 85% of all colonoscopy exams on a per-provider basis.7 One study of completed colonoscopy with inadequate preparation showed an adenoma miss rate of 48%.21 In the setting of inadequate bowel preparation, another study reported 42% of all adenomas detected were only found on repeat colonoscopy. When considering advanced adenomas, there was a 27% miss rate, a relatively high percentage.22
When poor bowel preparation precludes the exam, colonoscopy is appropriately aborted, and the patient asked to return. However, there are situations in which the exam can be completed but the bowel preparation is still inadequate to identify polyps larger than 5 mm. In this setting, the colonoscopy should be repeated with a more aggressive bowel preparation regimen within 1 year.19 Shorter intervals are recommended if advanced neoplasm is detected within an inadequate bowel preparation.19
The appropriate surveillance interval can be unclear when bowel preparation is considered adequate to identify polyps greater than or equal to 5 mm, yet still suboptimal. “Adequate” or “fair” bowel preparation often leads to shorter-than-recommended surveillance intervals because of the concern for small missed lesions. For example, patients with normal colonoscopy results and a fair prep were recommended to undergo a screening colonoscopy in 5 years at 57.4%, while only 23.1% received a 10-year recommendation.23 This increased frequency of colonoscopy leads to increased costs and procedural risks for the patient. Furthermore, a meta-analysis evaluating the effects of bowel preparation reported no significant difference in ADR between adequate and excellent prep.24 These findings suggest that patients with adequate bowel preparation may be followed at guideline-recommended surveillance intervals without significantly affecting colonoscopy quality as measured by ADR.
Endoscopist feedback and report cards
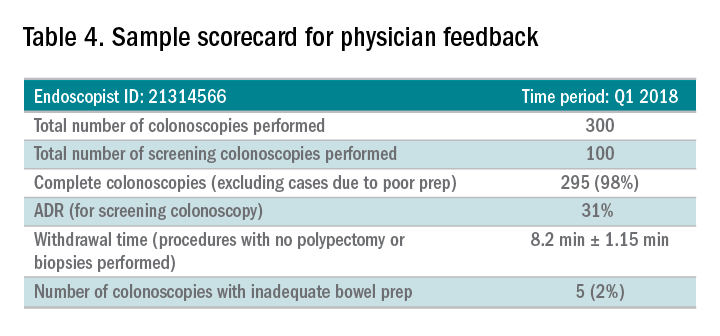
Awareness of quality metrics among individuals and endoscopy practices is crucial to ensuring adequate performance. Several studies have shown improvement with feedback and monitoring of endoscopists.25,26 Some strategies to improve colonoscopy technique and efficiency include having recorded or observed procedures, computer software that measures image resolution/velocity, and scorecards with quality measures. A representation of the scorecards used in our practice is shown in Table 4. Feedback measures both make endoscopists aware of how their performance compares with recommended goals for colonoscopy and help track their improvement. We recommend such feedback should be provided quarterly for most providers and more frequently for providers not meeting benchmarks.
Conclusion
Given we rely on colonoscopy to identify and clear the colon of potential malignancy, it is imperative that we provide high-value exams for our patients. The basis for a quality colonoscopy is complete intubation and careful inspection of the mucosa on withdrawal. Several quality measures are used as surrogates of a good exam such that endoscopists can assess themselves in relation to their peers. These metrics can help us in our goal of remaining mindful during each procedure we are completing and providing the best exam possible.
Dr. Shamsi is a third-year GI fellow. Dr. Malhotra is an assistant professor in the division of gastroenterology at the University of Minnesota, Minneapolis. Dr. Shaukat is a professor of medicine in the division of gastroenterology at the University of Minnesota, Minneapolis, and the GI Section Chief at the Minneapolis VA Medical Center.
References
1. Siegel R et al. CA Cancer J Clin. 2012 Jan-Feb;62(1):10-29.
2. Edwards BK et al. Cancer. 2010 Feb 1;116(3):544-73.
3. Hosokawa O et al. Endoscopy. 2003 Jun;35(6):506-10.
4. Morris EJ et al. Gut. 2015(Aug);64(2):1248-56.
5. Bressler B et al. Gastroenterology. 2004 Aug;127(2):452-6.
6. Rex DK et al. Am J Gastroenterol. 2017 July;12(7):1016-30.
7. Rex DK et al. Gastrointest Endosc. 2015 Jan;81(1):31-53.
8. Anderson J et al. Clin Transl Gastroenterol. 2015 Feb 26;6:e77.
9. Kaminski M et al. N Engl J Med. 2010 May 13;362(19):1795-803.
10. Corley DA et al. N Engl J Med. 2014 Apr 3;370(4):1298-306.
11. Hunt RH. Colonoscopy intubation techniques without fluoroscopy. In: Colonoscopy techniques clinical practice and color atlas. Edited by Hunt RH, Waye JD. London: Chapman and Hall; 1981. p. 109-46.
12. Waye JD. Colonoscopy intubation techniques without fluoroscopy. In: Colonoscopy techniques clinical practice and color atlas. Edited by Hunt RH, Waye JD. London: Chapman and Hall; 1981. p. 147-78.
13. Williams CB et al. In: Colonoscopy principles & techniques. Edited by Raskin J, Juergen NH. New York: Igaku-Shoin Medical Publishers; 1995. p. 121-42.
14. Baillie J. Colonoscopy. In: Gastrointestinal endoscopy basic principles and practice. Oxford (UK): Butterworth-Heinemann; 1992. p. 63-92.
15. Rex DK. Gastrointest Endosc. 2000 Jan;51(1):33-6.
16. Shaukat A et al. Gastroenterol. 2015;149(4):952-7.
17. Barclay R et al. N Engl J Med. 2006 Dec 14;355(24):2533-41.
18. Simmons DT et al. Gastrointest Endosc. 2007;65(5):AB94.
19. Johnson DA et al. Gastrointest Endosc. 2014;80(4):543-62.
20. Calderwood A et al. Gastrointest Endosc. 2010 Oct;72(4):686-92.
21. Chokshi R et al. Gastrointest Endosc. 2012 Jun;75(6):1197-203.
22. Lebwohl B et al. Gastrointest Endosc. 2011 Jun;73(6):1207-14.
23. Menees SB et al. Gastrointest Endosc. 2013 Sep;78(3): 510-6.
24. Clark B et al. Am J Gastroenterol. 2014 Nov;109(11):1714-23.
25. Nielson A et al. BMJ Open Gastro. 2017 Jun. doi: 10.1136/bmjgast-2017-000142.
26. Gurudu S et al. J Gastroenterol Hepatol. 2018 Mar;33(3):645-9.
Editor's Note:
As quality metrics are becoming increasingly significant throughout all of medicine, our field is no exception. Recent evidence has demonstrated the importance of quality measures in colonoscopy; understanding, reporting, and improving these metrics has become a hot topic of discussion.
In this month’s In Focus article, brought to you by The New Gastroenterologist, Nabiha Shamsi, Ashish Malhotra, and Aasma Shaukat (University of Minnesota/Minneapolis VAMC) provide an outstanding overview of the evidence as well as recommended goals for important quality metrics in colonoscopy. Ultimately, improving colonoscopy quality amongst all gastroenterologists will increase colonoscopy value and lead to further decreases in the incidence and mortality of colorectal cancer.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Introduction
Colonoscopy is a widely used modality to evaluate colorectal cancer because it allows for both identification of early malignancies and removal of precancerous lesions. The increased use of colonoscopy in the last 20 years has been associated with a decline in the incidence and mortality from colorectal cancer.1,2 However, colonoscopy has its limitations. It is an invasive test with inherent risks. Additionally, studies have reported rates of post-colonoscopy cancers, also referred to as interval cancers, of 2%-7%, and miss-rates for adenomas by tandem colonoscopy of 2%-26%.3-5

High-quality exams can maximize the value of colonoscopy, and it is important to consider the factors that contribute to high-quality colonoscopies. While there are many metrics proposed,6,7 here we discuss the most evidence-based ones, outlined in Table 1, along with their goal values.
Cecal intubation rate
A high-quality colonoscopy should include a complete examination of the colon. To achieve this, it is necessary to fully intubate the cecum, passing the colonoscope past the ileocecal valve to examine the medial wall of the cecum.8
There are several factors that may contribute to an incomplete colonoscopy, including bowel preparation, anatomy, body habitus, and endoscopist’s skill. To calculate cecal intubation rate as a quality measure, colonoscopies that are incomplete because of poor bowel preparation, severe colitis, or known obstructing lesion are usually excluded.
The U.S. Multi-Society Task Force on Colorectal Cancer recommends a cecal intubation rate of at least 95% for screening colonoscopy and 90% for all colonoscopies.6 There is an expectation of photodocumentation of the ileocecal valve and appendiceal orifice to establish completion of the colonoscopy.6
Some methods used to assist with cecal intubation include changing patient position, applying abdominal pressure, stiffening the colonoscope, and alternating between adult or pediatric colonoscopes.
Adenoma detection rate
Adenoma detection rate (ADR), is defined as the proportion of patients over the age of 50 years undergoing first-time screening colonoscopies in which at least one adenomatous polyp is detected for a given endoscopist in a given time period.
Adenomas are tracked because clearing the colon of neoplasm is the goal of screening colonoscopies; adenomas are tracked instead of more advanced lesions because the higher frequency of adenomas allows for better tracking of variation between endoscopists. Tracking ADR also utilizes the assumption that, if small lesions are identified, larger ones will be as well.
ADR is the only current quality indicator reported to be significantly associated with the risk of interval cancers. In 2010, a study of 45,000 screening colonoscopies by 186 endoscopists validated the use of ADR, finding that patients who underwent colonoscopy by physicians with ADRs below 20% had hazard ratios for development of postcolonoscopy cancer greater than 10 times higher than patients of physicians with ADRs above 20%.9 However, this study had limited power to establish that cancer protection continues to improve when ADRs rise above 20%. Another study, which evaluated the association of ADR in 224,000 patients undergoing colonoscopies by 136 gastroenterologists, showed each 1% increase in ADR is associated with 3% decrease in the risk of interval CRC and 5% decrease in the risk of fatal interval cancers.10
Most recent guidelines propose an adequate ADR for asymptomatic individuals aged 50 years or older undergoing screening colonoscopy should be greater than 30% in men and greater than 20% in women.6 It remains unknown whether there is a threshold for maximum benefit of ADR, in which a very high ADR is not associated with further protective benefit. The answer to this question may depend on why a low ADR is associated with a higher rate of interval cancers and whether every missed polyp, independent of size, is a potential interval cancer or whether hasty, inadequate, or incomplete examinations of the colon are the underlying concern.
Withdrawal time
Optimizing identification of colonic lesions requires a careful and thorough exam of the colon on withdrawal. While this may seem obvious, there is often little focus on the approach to withdrawal. In four chapters on colonoscopy technique from textbooks, the number of pages describing insertion ranged from 20 to 38, while the number of pages focused on withdrawal ranged from 0.5 to 1.5.11-14

A study examining the difference in withdrawal technique between two endoscopists who were known to differ in adenoma miss rates by tandem colonoscopy proposed the scoring system listed in Table 2 that can assess quality of examination on withdrawal. There was a statistically significant difference in quality scores for the two endoscopists, as assessed by expert review of video recordings of their colonoscopies.15
The endoscopist with the lower adenoma miss rate was also found to have an average withdrawal time of 8 minutes and 55 seconds versus 6 minutes and 41 seconds for the endoscopist with the higher adenoma miss rate. A large, community-based study with over 76,000 colonoscopies found a statistically significant correlation between interval colorectal cancer and withdrawal times shorter than 6 minutes.16 However, there was no association between ADR and colorectal cancer, suggesting that, for practices with optimal ADRs (that is, rates greater than 25%), withdrawal time may be a more sensitive marker of quality of colonoscopy than ADR is.16Intuitively, adequate examination of the colon that includes examining the proximal side of folds, washing and suctioning stool, and even repositioning the patient would likely increase withdrawal time. In a 2008 study examining 2,000 screening colonoscopies of 12 endoscopists, those with withdrawal times greater than 6 minutes had significantly higher rates of detecting adenomas and advanced neoplasia, compared with those with faster withdrawal times.17 The average ADR in this group was 28.3%, compared with 11.8% for physicians who had a withdrawal time less than 6 minutes.17 An evaluation of nearly 11,000 colonoscopies done by 43 endoscopists also identified an increase polyp yield with increased withdrawal time.18 These data drive the recommendation for a minimum withdrawal time of 6 minutes, with 2 minutes spent examining each colonic segment.
Bowel preparation
Diagnosis of colonic lesions is dependent on adequate visualization of the colon. Poor bowel preparation can limit the yield of colonoscopy and lead to missed lesions. It also leads to canceled and rescheduled procedures that reduce efficiency, increase cost, and pose an undue burden on the patient.
The quality of bowel preparation should be assessed after washing and suctioning of colonic mucosa has been completed. Adequate preparation is that which allows identification of lesions greater than 5 mm in size.19
Quality of preparation is assessed subjectively by the endoscopists and often listed as excellent, good, fair, or poor. An alternative method of reporting bowel preparation quality is the Boston Bowel Preparation Score (BBPS) (Table 3).20 This scoring system allows for a more descriptive assessment of each colonic segment by assigning a score from 0 to 3 for the right, transverse, and left colon, leading to a total score between 0 and 9. The BBPS also helps standardize reporting of bowel preparation. The polyp detection rate associated with a BBPS of 5 or greater was 40%, compared with 24% associated with BBPS less than 5.19 A split-dose bowel preparation regimen with at least half of the preparation ingested on the day of the procedure is recommended to optimize quality of bowel preparation.6
The American Society for Gastrointestinal Endoscopy and American College of Gastroenterology task force on quality assurance in endoscopy recommends that bowel preparation should be adequate in 85% of all colonoscopy exams on a per-provider basis.7 One study of completed colonoscopy with inadequate preparation showed an adenoma miss rate of 48%.21 In the setting of inadequate bowel preparation, another study reported 42% of all adenomas detected were only found on repeat colonoscopy. When considering advanced adenomas, there was a 27% miss rate, a relatively high percentage.22
When poor bowel preparation precludes the exam, colonoscopy is appropriately aborted, and the patient asked to return. However, there are situations in which the exam can be completed but the bowel preparation is still inadequate to identify polyps larger than 5 mm. In this setting, the colonoscopy should be repeated with a more aggressive bowel preparation regimen within 1 year.19 Shorter intervals are recommended if advanced neoplasm is detected within an inadequate bowel preparation.19
The appropriate surveillance interval can be unclear when bowel preparation is considered adequate to identify polyps greater than or equal to 5 mm, yet still suboptimal. “Adequate” or “fair” bowel preparation often leads to shorter-than-recommended surveillance intervals because of the concern for small missed lesions. For example, patients with normal colonoscopy results and a fair prep were recommended to undergo a screening colonoscopy in 5 years at 57.4%, while only 23.1% received a 10-year recommendation.23 This increased frequency of colonoscopy leads to increased costs and procedural risks for the patient. Furthermore, a meta-analysis evaluating the effects of bowel preparation reported no significant difference in ADR between adequate and excellent prep.24 These findings suggest that patients with adequate bowel preparation may be followed at guideline-recommended surveillance intervals without significantly affecting colonoscopy quality as measured by ADR.
Endoscopist feedback and report cards
Awareness of quality metrics among individuals and endoscopy practices is crucial to ensuring adequate performance. Several studies have shown improvement with feedback and monitoring of endoscopists.25,26 Some strategies to improve colonoscopy technique and efficiency include having recorded or observed procedures, computer software that measures image resolution/velocity, and scorecards with quality measures. A representation of the scorecards used in our practice is shown in Table 4. Feedback measures both make endoscopists aware of how their performance compares with recommended goals for colonoscopy and help track their improvement. We recommend such feedback should be provided quarterly for most providers and more frequently for providers not meeting benchmarks.

Conclusion
Given we rely on colonoscopy to identify and clear the colon of potential malignancy, it is imperative that we provide high-value exams for our patients. The basis for a quality colonoscopy is complete intubation and careful inspection of the mucosa on withdrawal. Several quality measures are used as surrogates of a good exam such that endoscopists can assess themselves in relation to their peers. These metrics can help us in our goal of remaining mindful during each procedure we are completing and providing the best exam possible.
Dr. Shamsi is a third-year GI fellow. Dr. Malhotra is an assistant professor in the division of gastroenterology at the University of Minnesota, Minneapolis. Dr. Shaukat is a professor of medicine in the division of gastroenterology at the University of Minnesota, Minneapolis, and the GI Section Chief at the Minneapolis VA Medical Center.
References
1. Siegel R et al. CA Cancer J Clin. 2012 Jan-Feb;62(1):10-29.
2. Edwards BK et al. Cancer. 2010 Feb 1;116(3):544-73.
3. Hosokawa O et al. Endoscopy. 2003 Jun;35(6):506-10.
4. Morris EJ et al. Gut. 2015(Aug);64(2):1248-56.
5. Bressler B et al. Gastroenterology. 2004 Aug;127(2):452-6.
6. Rex DK et al. Am J Gastroenterol. 2017 July;12(7):1016-30.
7. Rex DK et al. Gastrointest Endosc. 2015 Jan;81(1):31-53.
8. Anderson J et al. Clin Transl Gastroenterol. 2015 Feb 26;6:e77.
9. Kaminski M et al. N Engl J Med. 2010 May 13;362(19):1795-803.
10. Corley DA et al. N Engl J Med. 2014 Apr 3;370(4):1298-306.
11. Hunt RH. Colonoscopy intubation techniques without fluoroscopy. In: Colonoscopy techniques clinical practice and color atlas. Edited by Hunt RH, Waye JD. London: Chapman and Hall; 1981. p. 109-46.
12. Waye JD. Colonoscopy intubation techniques without fluoroscopy. In: Colonoscopy techniques clinical practice and color atlas. Edited by Hunt RH, Waye JD. London: Chapman and Hall; 1981. p. 147-78.
13. Williams CB et al. In: Colonoscopy principles & techniques. Edited by Raskin J, Juergen NH. New York: Igaku-Shoin Medical Publishers; 1995. p. 121-42.
14. Baillie J. Colonoscopy. In: Gastrointestinal endoscopy basic principles and practice. Oxford (UK): Butterworth-Heinemann; 1992. p. 63-92.
15. Rex DK. Gastrointest Endosc. 2000 Jan;51(1):33-6.
16. Shaukat A et al. Gastroenterol. 2015;149(4):952-7.
17. Barclay R et al. N Engl J Med. 2006 Dec 14;355(24):2533-41.
18. Simmons DT et al. Gastrointest Endosc. 2007;65(5):AB94.
19. Johnson DA et al. Gastrointest Endosc. 2014;80(4):543-62.
20. Calderwood A et al. Gastrointest Endosc. 2010 Oct;72(4):686-92.
21. Chokshi R et al. Gastrointest Endosc. 2012 Jun;75(6):1197-203.
22. Lebwohl B et al. Gastrointest Endosc. 2011 Jun;73(6):1207-14.
23. Menees SB et al. Gastrointest Endosc. 2013 Sep;78(3): 510-6.
24. Clark B et al. Am J Gastroenterol. 2014 Nov;109(11):1714-23.
25. Nielson A et al. BMJ Open Gastro. 2017 Jun. doi: 10.1136/bmjgast-2017-000142.
26. Gurudu S et al. J Gastroenterol Hepatol. 2018 Mar;33(3):645-9.
Editor's Note:
As quality metrics are becoming increasingly significant throughout all of medicine, our field is no exception. Recent evidence has demonstrated the importance of quality measures in colonoscopy; understanding, reporting, and improving these metrics has become a hot topic of discussion.
In this month’s In Focus article, brought to you by The New Gastroenterologist, Nabiha Shamsi, Ashish Malhotra, and Aasma Shaukat (University of Minnesota/Minneapolis VAMC) provide an outstanding overview of the evidence as well as recommended goals for important quality metrics in colonoscopy. Ultimately, improving colonoscopy quality amongst all gastroenterologists will increase colonoscopy value and lead to further decreases in the incidence and mortality of colorectal cancer.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Introduction
Colonoscopy is a widely used modality to evaluate colorectal cancer because it allows for both identification of early malignancies and removal of precancerous lesions. The increased use of colonoscopy in the last 20 years has been associated with a decline in the incidence and mortality from colorectal cancer.1,2 However, colonoscopy has its limitations. It is an invasive test with inherent risks. Additionally, studies have reported rates of post-colonoscopy cancers, also referred to as interval cancers, of 2%-7%, and miss-rates for adenomas by tandem colonoscopy of 2%-26%.3-5

High-quality exams can maximize the value of colonoscopy, and it is important to consider the factors that contribute to high-quality colonoscopies. While there are many metrics proposed,6,7 here we discuss the most evidence-based ones, outlined in Table 1, along with their goal values.
Cecal intubation rate
A high-quality colonoscopy should include a complete examination of the colon. To achieve this, it is necessary to fully intubate the cecum, passing the colonoscope past the ileocecal valve to examine the medial wall of the cecum.8
There are several factors that may contribute to an incomplete colonoscopy, including bowel preparation, anatomy, body habitus, and endoscopist’s skill. To calculate cecal intubation rate as a quality measure, colonoscopies that are incomplete because of poor bowel preparation, severe colitis, or known obstructing lesion are usually excluded.
The U.S. Multi-Society Task Force on Colorectal Cancer recommends a cecal intubation rate of at least 95% for screening colonoscopy and 90% for all colonoscopies.6 There is an expectation of photodocumentation of the ileocecal valve and appendiceal orifice to establish completion of the colonoscopy.6
Some methods used to assist with cecal intubation include changing patient position, applying abdominal pressure, stiffening the colonoscope, and alternating between adult or pediatric colonoscopes.
Adenoma detection rate
Adenoma detection rate (ADR), is defined as the proportion of patients over the age of 50 years undergoing first-time screening colonoscopies in which at least one adenomatous polyp is detected for a given endoscopist in a given time period.
Adenomas are tracked because clearing the colon of neoplasm is the goal of screening colonoscopies; adenomas are tracked instead of more advanced lesions because the higher frequency of adenomas allows for better tracking of variation between endoscopists. Tracking ADR also utilizes the assumption that, if small lesions are identified, larger ones will be as well.
ADR is the only current quality indicator reported to be significantly associated with the risk of interval cancers. In 2010, a study of 45,000 screening colonoscopies by 186 endoscopists validated the use of ADR, finding that patients who underwent colonoscopy by physicians with ADRs below 20% had hazard ratios for development of postcolonoscopy cancer greater than 10 times higher than patients of physicians with ADRs above 20%.9 However, this study had limited power to establish that cancer protection continues to improve when ADRs rise above 20%. Another study, which evaluated the association of ADR in 224,000 patients undergoing colonoscopies by 136 gastroenterologists, showed each 1% increase in ADR is associated with 3% decrease in the risk of interval CRC and 5% decrease in the risk of fatal interval cancers.10
Most recent guidelines propose an adequate ADR for asymptomatic individuals aged 50 years or older undergoing screening colonoscopy should be greater than 30% in men and greater than 20% in women.6 It remains unknown whether there is a threshold for maximum benefit of ADR, in which a very high ADR is not associated with further protective benefit. The answer to this question may depend on why a low ADR is associated with a higher rate of interval cancers and whether every missed polyp, independent of size, is a potential interval cancer or whether hasty, inadequate, or incomplete examinations of the colon are the underlying concern.
Withdrawal time
Optimizing identification of colonic lesions requires a careful and thorough exam of the colon on withdrawal. While this may seem obvious, there is often little focus on the approach to withdrawal. In four chapters on colonoscopy technique from textbooks, the number of pages describing insertion ranged from 20 to 38, while the number of pages focused on withdrawal ranged from 0.5 to 1.5.11-14

A study examining the difference in withdrawal technique between two endoscopists who were known to differ in adenoma miss rates by tandem colonoscopy proposed the scoring system listed in Table 2 that can assess quality of examination on withdrawal. There was a statistically significant difference in quality scores for the two endoscopists, as assessed by expert review of video recordings of their colonoscopies.15
The endoscopist with the lower adenoma miss rate was also found to have an average withdrawal time of 8 minutes and 55 seconds versus 6 minutes and 41 seconds for the endoscopist with the higher adenoma miss rate. A large, community-based study with over 76,000 colonoscopies found a statistically significant correlation between interval colorectal cancer and withdrawal times shorter than 6 minutes.16 However, there was no association between ADR and colorectal cancer, suggesting that, for practices with optimal ADRs (that is, rates greater than 25%), withdrawal time may be a more sensitive marker of quality of colonoscopy than ADR is.16Intuitively, adequate examination of the colon that includes examining the proximal side of folds, washing and suctioning stool, and even repositioning the patient would likely increase withdrawal time. In a 2008 study examining 2,000 screening colonoscopies of 12 endoscopists, those with withdrawal times greater than 6 minutes had significantly higher rates of detecting adenomas and advanced neoplasia, compared with those with faster withdrawal times.17 The average ADR in this group was 28.3%, compared with 11.8% for physicians who had a withdrawal time less than 6 minutes.17 An evaluation of nearly 11,000 colonoscopies done by 43 endoscopists also identified an increase polyp yield with increased withdrawal time.18 These data drive the recommendation for a minimum withdrawal time of 6 minutes, with 2 minutes spent examining each colonic segment.
Bowel preparation
Diagnosis of colonic lesions is dependent on adequate visualization of the colon. Poor bowel preparation can limit the yield of colonoscopy and lead to missed lesions. It also leads to canceled and rescheduled procedures that reduce efficiency, increase cost, and pose an undue burden on the patient.
The quality of bowel preparation should be assessed after washing and suctioning of colonic mucosa has been completed. Adequate preparation is that which allows identification of lesions greater than 5 mm in size.19
Quality of preparation is assessed subjectively by the endoscopists and often listed as excellent, good, fair, or poor. An alternative method of reporting bowel preparation quality is the Boston Bowel Preparation Score (BBPS) (Table 3).20 This scoring system allows for a more descriptive assessment of each colonic segment by assigning a score from 0 to 3 for the right, transverse, and left colon, leading to a total score between 0 and 9. The BBPS also helps standardize reporting of bowel preparation. The polyp detection rate associated with a BBPS of 5 or greater was 40%, compared with 24% associated with BBPS less than 5.19 A split-dose bowel preparation regimen with at least half of the preparation ingested on the day of the procedure is recommended to optimize quality of bowel preparation.6
The American Society for Gastrointestinal Endoscopy and American College of Gastroenterology task force on quality assurance in endoscopy recommends that bowel preparation should be adequate in 85% of all colonoscopy exams on a per-provider basis.7 One study of completed colonoscopy with inadequate preparation showed an adenoma miss rate of 48%.21 In the setting of inadequate bowel preparation, another study reported 42% of all adenomas detected were only found on repeat colonoscopy. When considering advanced adenomas, there was a 27% miss rate, a relatively high percentage.22
When poor bowel preparation precludes the exam, colonoscopy is appropriately aborted, and the patient asked to return. However, there are situations in which the exam can be completed but the bowel preparation is still inadequate to identify polyps larger than 5 mm. In this setting, the colonoscopy should be repeated with a more aggressive bowel preparation regimen within 1 year.19 Shorter intervals are recommended if advanced neoplasm is detected within an inadequate bowel preparation.19
The appropriate surveillance interval can be unclear when bowel preparation is considered adequate to identify polyps greater than or equal to 5 mm, yet still suboptimal. “Adequate” or “fair” bowel preparation often leads to shorter-than-recommended surveillance intervals because of the concern for small missed lesions. For example, patients with normal colonoscopy results and a fair prep were recommended to undergo a screening colonoscopy in 5 years at 57.4%, while only 23.1% received a 10-year recommendation.23 This increased frequency of colonoscopy leads to increased costs and procedural risks for the patient. Furthermore, a meta-analysis evaluating the effects of bowel preparation reported no significant difference in ADR between adequate and excellent prep.24 These findings suggest that patients with adequate bowel preparation may be followed at guideline-recommended surveillance intervals without significantly affecting colonoscopy quality as measured by ADR.
Endoscopist feedback and report cards
Awareness of quality metrics among individuals and endoscopy practices is crucial to ensuring adequate performance. Several studies have shown improvement with feedback and monitoring of endoscopists.25,26 Some strategies to improve colonoscopy technique and efficiency include having recorded or observed procedures, computer software that measures image resolution/velocity, and scorecards with quality measures. A representation of the scorecards used in our practice is shown in Table 4. Feedback measures both make endoscopists aware of how their performance compares with recommended goals for colonoscopy and help track their improvement. We recommend such feedback should be provided quarterly for most providers and more frequently for providers not meeting benchmarks.

Conclusion
Given we rely on colonoscopy to identify and clear the colon of potential malignancy, it is imperative that we provide high-value exams for our patients. The basis for a quality colonoscopy is complete intubation and careful inspection of the mucosa on withdrawal. Several quality measures are used as surrogates of a good exam such that endoscopists can assess themselves in relation to their peers. These metrics can help us in our goal of remaining mindful during each procedure we are completing and providing the best exam possible.
Dr. Shamsi is a third-year GI fellow. Dr. Malhotra is an assistant professor in the division of gastroenterology at the University of Minnesota, Minneapolis. Dr. Shaukat is a professor of medicine in the division of gastroenterology at the University of Minnesota, Minneapolis, and the GI Section Chief at the Minneapolis VA Medical Center.
References
1. Siegel R et al. CA Cancer J Clin. 2012 Jan-Feb;62(1):10-29.
2. Edwards BK et al. Cancer. 2010 Feb 1;116(3):544-73.
3. Hosokawa O et al. Endoscopy. 2003 Jun;35(6):506-10.
4. Morris EJ et al. Gut. 2015(Aug);64(2):1248-56.
5. Bressler B et al. Gastroenterology. 2004 Aug;127(2):452-6.
6. Rex DK et al. Am J Gastroenterol. 2017 July;12(7):1016-30.
7. Rex DK et al. Gastrointest Endosc. 2015 Jan;81(1):31-53.
8. Anderson J et al. Clin Transl Gastroenterol. 2015 Feb 26;6:e77.
9. Kaminski M et al. N Engl J Med. 2010 May 13;362(19):1795-803.
10. Corley DA et al. N Engl J Med. 2014 Apr 3;370(4):1298-306.
11. Hunt RH. Colonoscopy intubation techniques without fluoroscopy. In: Colonoscopy techniques clinical practice and color atlas. Edited by Hunt RH, Waye JD. London: Chapman and Hall; 1981. p. 109-46.
12. Waye JD. Colonoscopy intubation techniques without fluoroscopy. In: Colonoscopy techniques clinical practice and color atlas. Edited by Hunt RH, Waye JD. London: Chapman and Hall; 1981. p. 147-78.
13. Williams CB et al. In: Colonoscopy principles & techniques. Edited by Raskin J, Juergen NH. New York: Igaku-Shoin Medical Publishers; 1995. p. 121-42.
14. Baillie J. Colonoscopy. In: Gastrointestinal endoscopy basic principles and practice. Oxford (UK): Butterworth-Heinemann; 1992. p. 63-92.
15. Rex DK. Gastrointest Endosc. 2000 Jan;51(1):33-6.
16. Shaukat A et al. Gastroenterol. 2015;149(4):952-7.
17. Barclay R et al. N Engl J Med. 2006 Dec 14;355(24):2533-41.
18. Simmons DT et al. Gastrointest Endosc. 2007;65(5):AB94.
19. Johnson DA et al. Gastrointest Endosc. 2014;80(4):543-62.
20. Calderwood A et al. Gastrointest Endosc. 2010 Oct;72(4):686-92.
21. Chokshi R et al. Gastrointest Endosc. 2012 Jun;75(6):1197-203.
22. Lebwohl B et al. Gastrointest Endosc. 2011 Jun;73(6):1207-14.
23. Menees SB et al. Gastrointest Endosc. 2013 Sep;78(3): 510-6.
24. Clark B et al. Am J Gastroenterol. 2014 Nov;109(11):1714-23.
25. Nielson A et al. BMJ Open Gastro. 2017 Jun. doi: 10.1136/bmjgast-2017-000142.
26. Gurudu S et al. J Gastroenterol Hepatol. 2018 Mar;33(3):645-9.
Quality metrics, programs for new GIs, and innovation
Dear Colleagues,
In the August issue of The New Gastroenterologist, we have some fantastic articles that I hope you will find both interesting and useful.
First, as quality metrics are becoming increasingly important in all aspects of health care, it is critical that we have a good understanding of quality metrics within our field. This issue’s “In Focus” article, written by Nabiha Shamsi, Ashish Malhotra, and Aasma Shaukat (University of Minnesota/Minnesota VAMC), provides a helpful overview of the currently accepted quality metrics for colonoscopy as well as the data that support their use. This article can be found online as well as in print in the August issue of GI & Hepatology News.
Additionally, in this issue, we have several articles highlighting important AGA programs that are great opportunities for those of us in our early careers. First, Jennifer Weiss (University of Wisconsin) discusses her experience in the Future Leaders Program, which just graduated its second class at DDW this year. Additionally, Sarah Lieber (UNC Chapel Hill) and Ana Maldonado-Contreras (University of Massachusetts) chronicle their experiences at the AGA Academic Skills Workshop which was held in Charlotte earlier this year. Finally, Eric Shah (University of Michigan), who served as this past year’s Gastroenterology Editorial Fellow, provides insights from his experience in this new position designed specifically for those in their early career.
Also in this issue is an article about pursuing a career in the innovation industry, authored by Chang Hee Kim (GoDx) and Wendy Henderson (NINR/NIH), who were winners of this year’s AGA Shark Tank. Finally, as many will be looking for new jobs in the coming year, one of the most important parts of this process will be the contract. Scott Roman, an attorney with significant expertise in contract law, provides an overview highlighting the important points about contracts that should not be overlooked.
As with prior installments of The New Gastroenterologist e-newsletter, please check out the “In Case You Missed It” section to see recent articles published in the AGA journals that have pertinence to those of us in our early careers. If you have any ideas or are interested in contributing to The New Gastroenterologist, please contact me at [email protected] or the managing editor, Ryan Farrell, at [email protected].
Sincerely,
Bryson W. Katona, MD, PhD
Editor in Chief
Dr. Katona is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.
Dear Colleagues,
In the August issue of The New Gastroenterologist, we have some fantastic articles that I hope you will find both interesting and useful.
First, as quality metrics are becoming increasingly important in all aspects of health care, it is critical that we have a good understanding of quality metrics within our field. This issue’s “In Focus” article, written by Nabiha Shamsi, Ashish Malhotra, and Aasma Shaukat (University of Minnesota/Minnesota VAMC), provides a helpful overview of the currently accepted quality metrics for colonoscopy as well as the data that support their use. This article can be found online as well as in print in the August issue of GI & Hepatology News.
Additionally, in this issue, we have several articles highlighting important AGA programs that are great opportunities for those of us in our early careers. First, Jennifer Weiss (University of Wisconsin) discusses her experience in the Future Leaders Program, which just graduated its second class at DDW this year. Additionally, Sarah Lieber (UNC Chapel Hill) and Ana Maldonado-Contreras (University of Massachusetts) chronicle their experiences at the AGA Academic Skills Workshop which was held in Charlotte earlier this year. Finally, Eric Shah (University of Michigan), who served as this past year’s Gastroenterology Editorial Fellow, provides insights from his experience in this new position designed specifically for those in their early career.
Also in this issue is an article about pursuing a career in the innovation industry, authored by Chang Hee Kim (GoDx) and Wendy Henderson (NINR/NIH), who were winners of this year’s AGA Shark Tank. Finally, as many will be looking for new jobs in the coming year, one of the most important parts of this process will be the contract. Scott Roman, an attorney with significant expertise in contract law, provides an overview highlighting the important points about contracts that should not be overlooked.
As with prior installments of The New Gastroenterologist e-newsletter, please check out the “In Case You Missed It” section to see recent articles published in the AGA journals that have pertinence to those of us in our early careers. If you have any ideas or are interested in contributing to The New Gastroenterologist, please contact me at [email protected] or the managing editor, Ryan Farrell, at [email protected].
Sincerely,
Bryson W. Katona, MD, PhD
Editor in Chief
Dr. Katona is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.
Dear Colleagues,
In the August issue of The New Gastroenterologist, we have some fantastic articles that I hope you will find both interesting and useful.
First, as quality metrics are becoming increasingly important in all aspects of health care, it is critical that we have a good understanding of quality metrics within our field. This issue’s “In Focus” article, written by Nabiha Shamsi, Ashish Malhotra, and Aasma Shaukat (University of Minnesota/Minnesota VAMC), provides a helpful overview of the currently accepted quality metrics for colonoscopy as well as the data that support their use. This article can be found online as well as in print in the August issue of GI & Hepatology News.
Additionally, in this issue, we have several articles highlighting important AGA programs that are great opportunities for those of us in our early careers. First, Jennifer Weiss (University of Wisconsin) discusses her experience in the Future Leaders Program, which just graduated its second class at DDW this year. Additionally, Sarah Lieber (UNC Chapel Hill) and Ana Maldonado-Contreras (University of Massachusetts) chronicle their experiences at the AGA Academic Skills Workshop which was held in Charlotte earlier this year. Finally, Eric Shah (University of Michigan), who served as this past year’s Gastroenterology Editorial Fellow, provides insights from his experience in this new position designed specifically for those in their early career.
Also in this issue is an article about pursuing a career in the innovation industry, authored by Chang Hee Kim (GoDx) and Wendy Henderson (NINR/NIH), who were winners of this year’s AGA Shark Tank. Finally, as many will be looking for new jobs in the coming year, one of the most important parts of this process will be the contract. Scott Roman, an attorney with significant expertise in contract law, provides an overview highlighting the important points about contracts that should not be overlooked.
As with prior installments of The New Gastroenterologist e-newsletter, please check out the “In Case You Missed It” section to see recent articles published in the AGA journals that have pertinence to those of us in our early careers. If you have any ideas or are interested in contributing to The New Gastroenterologist, please contact me at [email protected] or the managing editor, Ryan Farrell, at [email protected].
Sincerely,
Bryson W. Katona, MD, PhD
Editor in Chief
Dr. Katona is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.
Perspectives on the 2018 AGA-AASLD Workshop
In March 2018, the American Gastroenterological Association (AGA) and American Association for the Study of Liver Diseases (AASLD) sponsored the Academic Skills Workshop in Charlotte, N.C. This year’s chairs Barbara Jung, MD, and Michael W. Fried, MD, FAASLD, as well as codirectors Marcia Cruz-Correa, MD, PhD, AGAF, FASGE, and Raymond Chung, MD, FAASLD, led a 2-day workshop featuring educational sessions and opportunities for mentorship and networking in academic gastroenterology and hepatology. The workshop featured sessions on how to navigate the job market, map out a career trajectory, develop fruitful mentoring relationships, apply for grant funding, and showcase research through manuscripts and oral presentations. Fellows and junior faculty from academic institutions across the United States were able to come together. Herein, two participants share their experiences at this event.

Clinical perspective from Sarah R. Lieber, MD
As a second-year gastroenterology fellow and aspiring transplant hepatologist, I found that this workshop provided an excellent framework and foundation for launching a career in academics and clinical research. It was especially effective at providing practical tips and tools for fellows and junior faculty on how to find an academic job, apply for research funding, and conduct written and oral presentations.
I was particularly moved by the personal stories and anecdotes from faculty members – many of whom are renowned leaders in the fields of GI and hepatology – who divulged the challenges and insecurities they had to overcome early in their careers. Sheila E. Crowe, MD, FRCPC, FACP, FACG, AGAF, gave an especially poignant talk on her career path from fellowship to becoming AGA president. She discussed the challenges unique to women in academic GI and hepatology, but left me feeling empowered and inspired by showcasing the many talents and success stories of her female colleagues and herself as well.

On Saturday, the AASLD held a special session that highlighted the personal stories and career trajectories of clinicians and researchers in the forefront of the field of hepatology – including AASLD president-elect Michael W. Fried, MD, FAASLD, and current AASLD President Ana Lok MD, FAASLD – among others. They emphasized the power of collaborative research that included harnessing the tools of molecular biology and Big Data to investigate the role of the microbiome and other novel subjects in liver disease. They advised us to seek out formal training when available, including master degrees or biostatistics training, to help us develop the skill sets necessary as independent researchers. They elaborated about their experiences serving on professional committees and giving oral presentations – essential to their career development – which allowed them to carve out a research niche and gain recognition as experts in their fields.
There were several powerful lessons and important themes that I take away from this workshop. The first is the importance of citizenship: Being a successful academician means not only putting in the clinical hours and being a prolific researcher but also being a good citizen. Supporting your colleagues, teaching mentees, and being a “team player” are all elements crucial to forming meaningful relationships and standing out as a valuable individual in your department. Second, perseverance is equally important; whether you are resubmitting applications for grant funding or reaching out to mentors in your area of interest, perseverance is the key to a successful career in academics. Third, remember that there is the important distinction between mentorship and sponsorship. While it is essential to have a selfless and supportive mentor who helps you cultivate your clinical and research interests, it is equally important to find a sponsor: an influential academician who can help you launch your career by acting as your advocate and opening doors to professional opportunities. Finally,you must always deliver. When mentors and sponsors give you opportunities to showcase your talents, always invest the time and effort to provide a high-quality performance. Be a good citizen who perseveres, seek out influential mentorship and sponsorship, and deliver on important professional tasks which will prime you to succeed as an academic clinical researcher in GI and hepatology.
Basic scientist perspective from Ana Maldonado-Contreras, PhD
The AGA-AASLD Workshop represented an ideal opportunity to regain perspective on my overall career plan. This year, Dr. Jung restructured the format of the program by substituting “lecture-style” sessions with fully interactive discussion panels in which trainees had the opportunity to initiate discussions about various topics of interest. The faculty leading these interactive sessions were committed to providing honest and clear answers to all of our questions. I believe this was a unique opportunity to go beyond PowerPoint presentations to actually gain insights on the dynamics of an academic department. We learned from department chairs what is considered during hiring, promoting, or allocating funds to make their team successful. Among the topics discussed, collegiality and selfless peer support were highlighted among the qualities of an appreciated department member. Panelists insisted that a balancing act between team support and one’s productivity is fundamental to thriving and maintaining focus.

Another topic with personal relevance was securing funding for my newly formed laboratory – and I was not alone! Prior to the meeting, participants were divided into small groups and assigned to a faculty mentor. Each participant was asked to share a research proposal and CV with her respective mentor. Then, each group had the opportunity to meet during the afternoon mentoring sessions. My group was composed of four participants interested in learning more about National Institutes of Health (NIH) funding strategies based on our current situations. Our assigned mentor, John Inadomi, MD – who thoroughly read our proposals and knew who we were before our encounter – provided practical advice about grant mechanisms to pursue given our current positions and provided detailed tips for successful applications. Dr. Inadomi also was greatly insightful about NIH study sessions and the entire review process. This person-to-person interaction was extremely helpful as it opened the possibility of discussing singularities of each participant’s career plans. Similarly, on the next day we had face time with David Saslowsky, PhD, program director of the National Institute of Diabetes and Digestive and Kidney Diseases at NIH. Dr. Saslowsky also reviewed our research proposals and discussed potential venues for funding within the NIDDK based on individual career trajectories.

Most of the second day was dedicated to reviewing grant opportunities and pertinent tips on how to get funded. We discussed the “most common mistakes” made by junior faculty on grant applications and ways to avoid them. All panelists agreed that the most common mistake is overambition. They advised us to critically consider the aims and activities proposed and, more importantly, seek out advice from mentors with more experience in grant writing.
Undoubtedly, networking with other peers represented an essential part of the experience at this academic workshop. As trainees, we were able to connect with not only seasoned colleagues but also with peers facing the same career challenges. Senior faculty were amazingly personable and committed to sharing experiences with the next generation of clinicians and scientists. They shared their failures, frustrations, and fears as well as their successes. Each story and the words of encouragement from this great community of scientists and clinicians helped me realize my hidden strengths and how to build from my past accomplishments to excel on my path toward becoming a fully independent researcher.
Dr. Lieber is a clinical epidemiology fellow, department of medicine, division of gastroenterology and hepatology, University of North Carolina (UNC), Chapel Hill; Dr. Maldonado-Contreras is an instructor in the department of microbiology and physiological systems and the Center for Microbiome Research, University of Massachusetts, Worcester.
In March 2018, the American Gastroenterological Association (AGA) and American Association for the Study of Liver Diseases (AASLD) sponsored the Academic Skills Workshop in Charlotte, N.C. This year’s chairs Barbara Jung, MD, and Michael W. Fried, MD, FAASLD, as well as codirectors Marcia Cruz-Correa, MD, PhD, AGAF, FASGE, and Raymond Chung, MD, FAASLD, led a 2-day workshop featuring educational sessions and opportunities for mentorship and networking in academic gastroenterology and hepatology. The workshop featured sessions on how to navigate the job market, map out a career trajectory, develop fruitful mentoring relationships, apply for grant funding, and showcase research through manuscripts and oral presentations. Fellows and junior faculty from academic institutions across the United States were able to come together. Herein, two participants share their experiences at this event.

Clinical perspective from Sarah R. Lieber, MD
As a second-year gastroenterology fellow and aspiring transplant hepatologist, I found that this workshop provided an excellent framework and foundation for launching a career in academics and clinical research. It was especially effective at providing practical tips and tools for fellows and junior faculty on how to find an academic job, apply for research funding, and conduct written and oral presentations.
I was particularly moved by the personal stories and anecdotes from faculty members – many of whom are renowned leaders in the fields of GI and hepatology – who divulged the challenges and insecurities they had to overcome early in their careers. Sheila E. Crowe, MD, FRCPC, FACP, FACG, AGAF, gave an especially poignant talk on her career path from fellowship to becoming AGA president. She discussed the challenges unique to women in academic GI and hepatology, but left me feeling empowered and inspired by showcasing the many talents and success stories of her female colleagues and herself as well.

On Saturday, the AASLD held a special session that highlighted the personal stories and career trajectories of clinicians and researchers in the forefront of the field of hepatology – including AASLD president-elect Michael W. Fried, MD, FAASLD, and current AASLD President Ana Lok MD, FAASLD – among others. They emphasized the power of collaborative research that included harnessing the tools of molecular biology and Big Data to investigate the role of the microbiome and other novel subjects in liver disease. They advised us to seek out formal training when available, including master degrees or biostatistics training, to help us develop the skill sets necessary as independent researchers. They elaborated about their experiences serving on professional committees and giving oral presentations – essential to their career development – which allowed them to carve out a research niche and gain recognition as experts in their fields.
There were several powerful lessons and important themes that I take away from this workshop. The first is the importance of citizenship: Being a successful academician means not only putting in the clinical hours and being a prolific researcher but also being a good citizen. Supporting your colleagues, teaching mentees, and being a “team player” are all elements crucial to forming meaningful relationships and standing out as a valuable individual in your department. Second, perseverance is equally important; whether you are resubmitting applications for grant funding or reaching out to mentors in your area of interest, perseverance is the key to a successful career in academics. Third, remember that there is the important distinction between mentorship and sponsorship. While it is essential to have a selfless and supportive mentor who helps you cultivate your clinical and research interests, it is equally important to find a sponsor: an influential academician who can help you launch your career by acting as your advocate and opening doors to professional opportunities. Finally,you must always deliver. When mentors and sponsors give you opportunities to showcase your talents, always invest the time and effort to provide a high-quality performance. Be a good citizen who perseveres, seek out influential mentorship and sponsorship, and deliver on important professional tasks which will prime you to succeed as an academic clinical researcher in GI and hepatology.
Basic scientist perspective from Ana Maldonado-Contreras, PhD
The AGA-AASLD Workshop represented an ideal opportunity to regain perspective on my overall career plan. This year, Dr. Jung restructured the format of the program by substituting “lecture-style” sessions with fully interactive discussion panels in which trainees had the opportunity to initiate discussions about various topics of interest. The faculty leading these interactive sessions were committed to providing honest and clear answers to all of our questions. I believe this was a unique opportunity to go beyond PowerPoint presentations to actually gain insights on the dynamics of an academic department. We learned from department chairs what is considered during hiring, promoting, or allocating funds to make their team successful. Among the topics discussed, collegiality and selfless peer support were highlighted among the qualities of an appreciated department member. Panelists insisted that a balancing act between team support and one’s productivity is fundamental to thriving and maintaining focus.

Another topic with personal relevance was securing funding for my newly formed laboratory – and I was not alone! Prior to the meeting, participants were divided into small groups and assigned to a faculty mentor. Each participant was asked to share a research proposal and CV with her respective mentor. Then, each group had the opportunity to meet during the afternoon mentoring sessions. My group was composed of four participants interested in learning more about National Institutes of Health (NIH) funding strategies based on our current situations. Our assigned mentor, John Inadomi, MD – who thoroughly read our proposals and knew who we were before our encounter – provided practical advice about grant mechanisms to pursue given our current positions and provided detailed tips for successful applications. Dr. Inadomi also was greatly insightful about NIH study sessions and the entire review process. This person-to-person interaction was extremely helpful as it opened the possibility of discussing singularities of each participant’s career plans. Similarly, on the next day we had face time with David Saslowsky, PhD, program director of the National Institute of Diabetes and Digestive and Kidney Diseases at NIH. Dr. Saslowsky also reviewed our research proposals and discussed potential venues for funding within the NIDDK based on individual career trajectories.

Most of the second day was dedicated to reviewing grant opportunities and pertinent tips on how to get funded. We discussed the “most common mistakes” made by junior faculty on grant applications and ways to avoid them. All panelists agreed that the most common mistake is overambition. They advised us to critically consider the aims and activities proposed and, more importantly, seek out advice from mentors with more experience in grant writing.
Undoubtedly, networking with other peers represented an essential part of the experience at this academic workshop. As trainees, we were able to connect with not only seasoned colleagues but also with peers facing the same career challenges. Senior faculty were amazingly personable and committed to sharing experiences with the next generation of clinicians and scientists. They shared their failures, frustrations, and fears as well as their successes. Each story and the words of encouragement from this great community of scientists and clinicians helped me realize my hidden strengths and how to build from my past accomplishments to excel on my path toward becoming a fully independent researcher.
Dr. Lieber is a clinical epidemiology fellow, department of medicine, division of gastroenterology and hepatology, University of North Carolina (UNC), Chapel Hill; Dr. Maldonado-Contreras is an instructor in the department of microbiology and physiological systems and the Center for Microbiome Research, University of Massachusetts, Worcester.
In March 2018, the American Gastroenterological Association (AGA) and American Association for the Study of Liver Diseases (AASLD) sponsored the Academic Skills Workshop in Charlotte, N.C. This year’s chairs Barbara Jung, MD, and Michael W. Fried, MD, FAASLD, as well as codirectors Marcia Cruz-Correa, MD, PhD, AGAF, FASGE, and Raymond Chung, MD, FAASLD, led a 2-day workshop featuring educational sessions and opportunities for mentorship and networking in academic gastroenterology and hepatology. The workshop featured sessions on how to navigate the job market, map out a career trajectory, develop fruitful mentoring relationships, apply for grant funding, and showcase research through manuscripts and oral presentations. Fellows and junior faculty from academic institutions across the United States were able to come together. Herein, two participants share their experiences at this event.

Clinical perspective from Sarah R. Lieber, MD
As a second-year gastroenterology fellow and aspiring transplant hepatologist, I found that this workshop provided an excellent framework and foundation for launching a career in academics and clinical research. It was especially effective at providing practical tips and tools for fellows and junior faculty on how to find an academic job, apply for research funding, and conduct written and oral presentations.
I was particularly moved by the personal stories and anecdotes from faculty members – many of whom are renowned leaders in the fields of GI and hepatology – who divulged the challenges and insecurities they had to overcome early in their careers. Sheila E. Crowe, MD, FRCPC, FACP, FACG, AGAF, gave an especially poignant talk on her career path from fellowship to becoming AGA president. She discussed the challenges unique to women in academic GI and hepatology, but left me feeling empowered and inspired by showcasing the many talents and success stories of her female colleagues and herself as well.

On Saturday, the AASLD held a special session that highlighted the personal stories and career trajectories of clinicians and researchers in the forefront of the field of hepatology – including AASLD president-elect Michael W. Fried, MD, FAASLD, and current AASLD President Ana Lok MD, FAASLD – among others. They emphasized the power of collaborative research that included harnessing the tools of molecular biology and Big Data to investigate the role of the microbiome and other novel subjects in liver disease. They advised us to seek out formal training when available, including master degrees or biostatistics training, to help us develop the skill sets necessary as independent researchers. They elaborated about their experiences serving on professional committees and giving oral presentations – essential to their career development – which allowed them to carve out a research niche and gain recognition as experts in their fields.
There were several powerful lessons and important themes that I take away from this workshop. The first is the importance of citizenship: Being a successful academician means not only putting in the clinical hours and being a prolific researcher but also being a good citizen. Supporting your colleagues, teaching mentees, and being a “team player” are all elements crucial to forming meaningful relationships and standing out as a valuable individual in your department. Second, perseverance is equally important; whether you are resubmitting applications for grant funding or reaching out to mentors in your area of interest, perseverance is the key to a successful career in academics. Third, remember that there is the important distinction between mentorship and sponsorship. While it is essential to have a selfless and supportive mentor who helps you cultivate your clinical and research interests, it is equally important to find a sponsor: an influential academician who can help you launch your career by acting as your advocate and opening doors to professional opportunities. Finally,you must always deliver. When mentors and sponsors give you opportunities to showcase your talents, always invest the time and effort to provide a high-quality performance. Be a good citizen who perseveres, seek out influential mentorship and sponsorship, and deliver on important professional tasks which will prime you to succeed as an academic clinical researcher in GI and hepatology.
Basic scientist perspective from Ana Maldonado-Contreras, PhD
The AGA-AASLD Workshop represented an ideal opportunity to regain perspective on my overall career plan. This year, Dr. Jung restructured the format of the program by substituting “lecture-style” sessions with fully interactive discussion panels in which trainees had the opportunity to initiate discussions about various topics of interest. The faculty leading these interactive sessions were committed to providing honest and clear answers to all of our questions. I believe this was a unique opportunity to go beyond PowerPoint presentations to actually gain insights on the dynamics of an academic department. We learned from department chairs what is considered during hiring, promoting, or allocating funds to make their team successful. Among the topics discussed, collegiality and selfless peer support were highlighted among the qualities of an appreciated department member. Panelists insisted that a balancing act between team support and one’s productivity is fundamental to thriving and maintaining focus.

Another topic with personal relevance was securing funding for my newly formed laboratory – and I was not alone! Prior to the meeting, participants were divided into small groups and assigned to a faculty mentor. Each participant was asked to share a research proposal and CV with her respective mentor. Then, each group had the opportunity to meet during the afternoon mentoring sessions. My group was composed of four participants interested in learning more about National Institutes of Health (NIH) funding strategies based on our current situations. Our assigned mentor, John Inadomi, MD – who thoroughly read our proposals and knew who we were before our encounter – provided practical advice about grant mechanisms to pursue given our current positions and provided detailed tips for successful applications. Dr. Inadomi also was greatly insightful about NIH study sessions and the entire review process. This person-to-person interaction was extremely helpful as it opened the possibility of discussing singularities of each participant’s career plans. Similarly, on the next day we had face time with David Saslowsky, PhD, program director of the National Institute of Diabetes and Digestive and Kidney Diseases at NIH. Dr. Saslowsky also reviewed our research proposals and discussed potential venues for funding within the NIDDK based on individual career trajectories.

Most of the second day was dedicated to reviewing grant opportunities and pertinent tips on how to get funded. We discussed the “most common mistakes” made by junior faculty on grant applications and ways to avoid them. All panelists agreed that the most common mistake is overambition. They advised us to critically consider the aims and activities proposed and, more importantly, seek out advice from mentors with more experience in grant writing.
Undoubtedly, networking with other peers represented an essential part of the experience at this academic workshop. As trainees, we were able to connect with not only seasoned colleagues but also with peers facing the same career challenges. Senior faculty were amazingly personable and committed to sharing experiences with the next generation of clinicians and scientists. They shared their failures, frustrations, and fears as well as their successes. Each story and the words of encouragement from this great community of scientists and clinicians helped me realize my hidden strengths and how to build from my past accomplishments to excel on my path toward becoming a fully independent researcher.
Dr. Lieber is a clinical epidemiology fellow, department of medicine, division of gastroenterology and hepatology, University of North Carolina (UNC), Chapel Hill; Dr. Maldonado-Contreras is an instructor in the department of microbiology and physiological systems and the Center for Microbiome Research, University of Massachusetts, Worcester.
‘Can’t believe we won! (The AGA Shark Tank)’: Building sustainable careers in clinical and translational GI research
Tell us about your recent experience at the AGA Tech Summit.
We attended our first AGA Tech Summit in Boston on March 21-23, flying between New England Nor’easter snowstorms this year. We had been selected as one of the five Shark Tank competition finalists after submitting our application and a video of our technology. We pitched a rapid paper diagnostic that we are developing to detect a multiplex of gastrointestinal pathogens. These pathogens cause infectious diarrhea and are detected from stool in 15 minutes without any instruments or electric power at the point of care (See Figure 1).
The goal is for the test to aid in diagnosis and treatment for patients in real time instead of sending stool samples to the laboratory, which could take days for the return of results. Our idea was the first to be pitched (by Dr. Kim) and it was nerve-racking to be the first presenter and watch others pitch after us. So, we were delightfully surprised that both the “sharks” and the audience picked our technology as the winner!
What led you to go into the innovation industry?
My collaborator, Dr. Henderson, had a dream to create diagnostic products that can be used in real time to diagnose and treat patients with diarrhea during the clinician-patient encounter. The product would be low cost and be run without an electrical power source, making it useful for resource-limited settings. The product would be especially helpful in rural, outbreak, and global settings where mortality from diarrhea is the highest. Approximately 525,000 children a year die of diarrheal diseases, and the elderly and immunocompromised also are significantly affected.
To realize our dream, we invented this technology through a public-private partnership called a Clinical Cooperative Research and Development Agreement between the National Institute of Nursing Research, National Institutes of Health, and GoDx Inc. GoDx, Inc. is a start-up company that Dr. Kim incorporated to develop and commercialize global health technologies into products. Through this partnership, we co-invented the technology, which we patented as a joint invention. We have also obtained IRB approval of a clinical protocol to test our “Stool Tool” on patient samples. Dr. Henderson is the principal investigator of this NIH clinical protocol. Last year, GoDx, Inc. was awarded a Phase 1 Small Business Innovation Research grant by the National Center for Advancing Translational Sciences (NCATS), NIH. They were recently awarded a $1.93 million Phase 2 SBIR grant from the NCATS to further develop the product; we will serve as co-PIs.
What do you enjoy most about the innovation industry?
What we enjoy the most about developing innovative products is the potential to help millions of people. It’s exhilarating to think that the discoveries we make in the lab can turn into innovative and useful new products that help save lives and improve health.
What are important factors for success in the innovation industry?
The first step is having the personal drive and vision toward an innovation. As clinicians and scientists our patients, families, and life experiences give us the drive on a daily basis as we strive to improve patient outcomes through more efficient, patient centered, less costly methods. The next step is having the training to know how to innovate. Dr. Henderson was part of a cohort trained in clinical and translational team science.1 Dr. Kim left the NIH to join his first startup company called Dxterity Diagnostics to learn product development and commercialization firsthand before starting GoDx.
A purposeful long-term commitment to innovation is the cornerstone of success in the implementation science space.2,3 Finding other innovators in your scientific space with similar values and dedication is priceless. An important aspect for someone with an innovative idea for a product is to talk to a patent lawyer or a licensing officer at the technology transfer office to discuss filing a patent. Next steps would be to find or form a company to license the technology, and develop and commercialize the product.
What are the biggest challenges to getting a new product on the market?
One of the biggest challenges for getting a new product to the market is building something that people want to buy. “Technology is the easy part” is a common mantra among bioentrepreneurs. Another mantra is “The market kills innovation.” To address this, GoDx applied for and was awarded a grant supplement to their NCATS Phase 1 SBIR grantto participate in the NIH Innovation Corps (I-Corps) program. As part of the I-Corps program GoDX conducted more than 100 interviews with potential customers and stakeholders for our product. This allowed GoDx to focus their business canvas (an evolving sketch of a business plan) and make key pivots in their customer segments and our technology in order to better achieve a “product-market” fit. While GoDx had thought of the idea from reading journals, when they met real customers and potential strategic partners, GoDx gained a real understanding of who the customers would be and the unmet needs they have. Through the coaching in this I-Corps course and the interviews, GoDx was able to develop a realistic go-to-market strategy. We highly recommend physician entrepreneurs to take part in I-Corps or other Lean Startup courses.
We are so thankful that our innovation was selected as the AGA Shark Tank winner! It garnered us lot of publicity and interest from potential investors and accelerators, and we highly recommend the AGA Tech Summit to all AGA members and GI health professionals who are interested in innovation in the GI space.4 The AGA Tech Summit is an excellent meeting that covers significant practical aspects of innovating technologies in health care including raising capital, patents, commercialization, regulatory approvals, reimbursement, and adoption. The AGA Center for GI Innovation and Technology is an excellent support group that can provide guidance on the different aspects of innovation and commercialization. See you in San Francisco at the 2019 AGA Tech Summit, April 10-12!
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number R44TR001912 and the National Institute of Nursing Research of the National Institutes of Health Intramural Research Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
1. Robinson GF et al. Development, implementation, and evaluation of an interprofessional course in translational research. Clin Transl Sci. 2013;6(1):50-6.
2. Nearing KA et al. Solving the puzzle of recruitment and retention: strategies for building a robust clinical and translational research workforce. Clin Transl Sci. 2015 Oct;8(5):563-7.
3. Manson SM et al. Vision, identity, and career in the clinical and translational sciences: Building upon the formative years. Clin Transl Sci. 2015 Oct;8(5):568-72.
4. Nimgaonkar A, Yock PG, Brinton TJ, et al. Gastroenterology and biodesign: contributing to the future of our specialty. Gastroenterology. 2013 Feb;144(2):258-62.
Dr. Kim is CEO of GoDx. Dr. Henderson is Investigator & Chief, Digestive Disorder Unit, Biobehavioral Branch, National Institute of Nursing Research, National Institutes of Health.
Tell us about your recent experience at the AGA Tech Summit.
We attended our first AGA Tech Summit in Boston on March 21-23, flying between New England Nor’easter snowstorms this year. We had been selected as one of the five Shark Tank competition finalists after submitting our application and a video of our technology. We pitched a rapid paper diagnostic that we are developing to detect a multiplex of gastrointestinal pathogens. These pathogens cause infectious diarrhea and are detected from stool in 15 minutes without any instruments or electric power at the point of care (See Figure 1).
The goal is for the test to aid in diagnosis and treatment for patients in real time instead of sending stool samples to the laboratory, which could take days for the return of results. Our idea was the first to be pitched (by Dr. Kim) and it was nerve-racking to be the first presenter and watch others pitch after us. So, we were delightfully surprised that both the “sharks” and the audience picked our technology as the winner!
What led you to go into the innovation industry?
My collaborator, Dr. Henderson, had a dream to create diagnostic products that can be used in real time to diagnose and treat patients with diarrhea during the clinician-patient encounter. The product would be low cost and be run without an electrical power source, making it useful for resource-limited settings. The product would be especially helpful in rural, outbreak, and global settings where mortality from diarrhea is the highest. Approximately 525,000 children a year die of diarrheal diseases, and the elderly and immunocompromised also are significantly affected.
To realize our dream, we invented this technology through a public-private partnership called a Clinical Cooperative Research and Development Agreement between the National Institute of Nursing Research, National Institutes of Health, and GoDx Inc. GoDx, Inc. is a start-up company that Dr. Kim incorporated to develop and commercialize global health technologies into products. Through this partnership, we co-invented the technology, which we patented as a joint invention. We have also obtained IRB approval of a clinical protocol to test our “Stool Tool” on patient samples. Dr. Henderson is the principal investigator of this NIH clinical protocol. Last year, GoDx, Inc. was awarded a Phase 1 Small Business Innovation Research grant by the National Center for Advancing Translational Sciences (NCATS), NIH. They were recently awarded a $1.93 million Phase 2 SBIR grant from the NCATS to further develop the product; we will serve as co-PIs.
What do you enjoy most about the innovation industry?
What we enjoy the most about developing innovative products is the potential to help millions of people. It’s exhilarating to think that the discoveries we make in the lab can turn into innovative and useful new products that help save lives and improve health.
What are important factors for success in the innovation industry?
The first step is having the personal drive and vision toward an innovation. As clinicians and scientists our patients, families, and life experiences give us the drive on a daily basis as we strive to improve patient outcomes through more efficient, patient centered, less costly methods. The next step is having the training to know how to innovate. Dr. Henderson was part of a cohort trained in clinical and translational team science.1 Dr. Kim left the NIH to join his first startup company called Dxterity Diagnostics to learn product development and commercialization firsthand before starting GoDx.
A purposeful long-term commitment to innovation is the cornerstone of success in the implementation science space.2,3 Finding other innovators in your scientific space with similar values and dedication is priceless. An important aspect for someone with an innovative idea for a product is to talk to a patent lawyer or a licensing officer at the technology transfer office to discuss filing a patent. Next steps would be to find or form a company to license the technology, and develop and commercialize the product.
What are the biggest challenges to getting a new product on the market?
One of the biggest challenges for getting a new product to the market is building something that people want to buy. “Technology is the easy part” is a common mantra among bioentrepreneurs. Another mantra is “The market kills innovation.” To address this, GoDx applied for and was awarded a grant supplement to their NCATS Phase 1 SBIR grantto participate in the NIH Innovation Corps (I-Corps) program. As part of the I-Corps program GoDX conducted more than 100 interviews with potential customers and stakeholders for our product. This allowed GoDx to focus their business canvas (an evolving sketch of a business plan) and make key pivots in their customer segments and our technology in order to better achieve a “product-market” fit. While GoDx had thought of the idea from reading journals, when they met real customers and potential strategic partners, GoDx gained a real understanding of who the customers would be and the unmet needs they have. Through the coaching in this I-Corps course and the interviews, GoDx was able to develop a realistic go-to-market strategy. We highly recommend physician entrepreneurs to take part in I-Corps or other Lean Startup courses.
We are so thankful that our innovation was selected as the AGA Shark Tank winner! It garnered us lot of publicity and interest from potential investors and accelerators, and we highly recommend the AGA Tech Summit to all AGA members and GI health professionals who are interested in innovation in the GI space.4 The AGA Tech Summit is an excellent meeting that covers significant practical aspects of innovating technologies in health care including raising capital, patents, commercialization, regulatory approvals, reimbursement, and adoption. The AGA Center for GI Innovation and Technology is an excellent support group that can provide guidance on the different aspects of innovation and commercialization. See you in San Francisco at the 2019 AGA Tech Summit, April 10-12!
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number R44TR001912 and the National Institute of Nursing Research of the National Institutes of Health Intramural Research Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
1. Robinson GF et al. Development, implementation, and evaluation of an interprofessional course in translational research. Clin Transl Sci. 2013;6(1):50-6.
2. Nearing KA et al. Solving the puzzle of recruitment and retention: strategies for building a robust clinical and translational research workforce. Clin Transl Sci. 2015 Oct;8(5):563-7.
3. Manson SM et al. Vision, identity, and career in the clinical and translational sciences: Building upon the formative years. Clin Transl Sci. 2015 Oct;8(5):568-72.
4. Nimgaonkar A, Yock PG, Brinton TJ, et al. Gastroenterology and biodesign: contributing to the future of our specialty. Gastroenterology. 2013 Feb;144(2):258-62.
Dr. Kim is CEO of GoDx. Dr. Henderson is Investigator & Chief, Digestive Disorder Unit, Biobehavioral Branch, National Institute of Nursing Research, National Institutes of Health.
Tell us about your recent experience at the AGA Tech Summit.
We attended our first AGA Tech Summit in Boston on March 21-23, flying between New England Nor’easter snowstorms this year. We had been selected as one of the five Shark Tank competition finalists after submitting our application and a video of our technology. We pitched a rapid paper diagnostic that we are developing to detect a multiplex of gastrointestinal pathogens. These pathogens cause infectious diarrhea and are detected from stool in 15 minutes without any instruments or electric power at the point of care (See Figure 1).
The goal is for the test to aid in diagnosis and treatment for patients in real time instead of sending stool samples to the laboratory, which could take days for the return of results. Our idea was the first to be pitched (by Dr. Kim) and it was nerve-racking to be the first presenter and watch others pitch after us. So, we were delightfully surprised that both the “sharks” and the audience picked our technology as the winner!
What led you to go into the innovation industry?
My collaborator, Dr. Henderson, had a dream to create diagnostic products that can be used in real time to diagnose and treat patients with diarrhea during the clinician-patient encounter. The product would be low cost and be run without an electrical power source, making it useful for resource-limited settings. The product would be especially helpful in rural, outbreak, and global settings where mortality from diarrhea is the highest. Approximately 525,000 children a year die of diarrheal diseases, and the elderly and immunocompromised also are significantly affected.
To realize our dream, we invented this technology through a public-private partnership called a Clinical Cooperative Research and Development Agreement between the National Institute of Nursing Research, National Institutes of Health, and GoDx Inc. GoDx, Inc. is a start-up company that Dr. Kim incorporated to develop and commercialize global health technologies into products. Through this partnership, we co-invented the technology, which we patented as a joint invention. We have also obtained IRB approval of a clinical protocol to test our “Stool Tool” on patient samples. Dr. Henderson is the principal investigator of this NIH clinical protocol. Last year, GoDx, Inc. was awarded a Phase 1 Small Business Innovation Research grant by the National Center for Advancing Translational Sciences (NCATS), NIH. They were recently awarded a $1.93 million Phase 2 SBIR grant from the NCATS to further develop the product; we will serve as co-PIs.
What do you enjoy most about the innovation industry?
What we enjoy the most about developing innovative products is the potential to help millions of people. It’s exhilarating to think that the discoveries we make in the lab can turn into innovative and useful new products that help save lives and improve health.
What are important factors for success in the innovation industry?
The first step is having the personal drive and vision toward an innovation. As clinicians and scientists our patients, families, and life experiences give us the drive on a daily basis as we strive to improve patient outcomes through more efficient, patient centered, less costly methods. The next step is having the training to know how to innovate. Dr. Henderson was part of a cohort trained in clinical and translational team science.1 Dr. Kim left the NIH to join his first startup company called Dxterity Diagnostics to learn product development and commercialization firsthand before starting GoDx.
A purposeful long-term commitment to innovation is the cornerstone of success in the implementation science space.2,3 Finding other innovators in your scientific space with similar values and dedication is priceless. An important aspect for someone with an innovative idea for a product is to talk to a patent lawyer or a licensing officer at the technology transfer office to discuss filing a patent. Next steps would be to find or form a company to license the technology, and develop and commercialize the product.
What are the biggest challenges to getting a new product on the market?
One of the biggest challenges for getting a new product to the market is building something that people want to buy. “Technology is the easy part” is a common mantra among bioentrepreneurs. Another mantra is “The market kills innovation.” To address this, GoDx applied for and was awarded a grant supplement to their NCATS Phase 1 SBIR grantto participate in the NIH Innovation Corps (I-Corps) program. As part of the I-Corps program GoDX conducted more than 100 interviews with potential customers and stakeholders for our product. This allowed GoDx to focus their business canvas (an evolving sketch of a business plan) and make key pivots in their customer segments and our technology in order to better achieve a “product-market” fit. While GoDx had thought of the idea from reading journals, when they met real customers and potential strategic partners, GoDx gained a real understanding of who the customers would be and the unmet needs they have. Through the coaching in this I-Corps course and the interviews, GoDx was able to develop a realistic go-to-market strategy. We highly recommend physician entrepreneurs to take part in I-Corps or other Lean Startup courses.
We are so thankful that our innovation was selected as the AGA Shark Tank winner! It garnered us lot of publicity and interest from potential investors and accelerators, and we highly recommend the AGA Tech Summit to all AGA members and GI health professionals who are interested in innovation in the GI space.4 The AGA Tech Summit is an excellent meeting that covers significant practical aspects of innovating technologies in health care including raising capital, patents, commercialization, regulatory approvals, reimbursement, and adoption. The AGA Center for GI Innovation and Technology is an excellent support group that can provide guidance on the different aspects of innovation and commercialization. See you in San Francisco at the 2019 AGA Tech Summit, April 10-12!
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number R44TR001912 and the National Institute of Nursing Research of the National Institutes of Health Intramural Research Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
1. Robinson GF et al. Development, implementation, and evaluation of an interprofessional course in translational research. Clin Transl Sci. 2013;6(1):50-6.
2. Nearing KA et al. Solving the puzzle of recruitment and retention: strategies for building a robust clinical and translational research workforce. Clin Transl Sci. 2015 Oct;8(5):563-7.
3. Manson SM et al. Vision, identity, and career in the clinical and translational sciences: Building upon the formative years. Clin Transl Sci. 2015 Oct;8(5):568-72.
4. Nimgaonkar A, Yock PG, Brinton TJ, et al. Gastroenterology and biodesign: contributing to the future of our specialty. Gastroenterology. 2013 Feb;144(2):258-62.
Dr. Kim is CEO of GoDx. Dr. Henderson is Investigator & Chief, Digestive Disorder Unit, Biobehavioral Branch, National Institute of Nursing Research, National Institutes of Health.
Calendar
For more information about upcoming events and award deadlines, please visit http://www.gastro.org/education and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Aug. 15-16; Sept. 19-20; Oct. 10-11, 2018
Two-Day, In-Depth Coding and Billing Seminar
Become a certified GI coder with a two-day, in-depth training course provided by McVey Associates, Inc.
Baltimore, MD (8/15-8/16); Atlanta, GA (9/19-9/20); Las Vegas, NV (10/10-10/11)
Aug. 18-19, 2018
James W. Freston Conference: Obesity and Metabolic Disease — Integrating New Paradigms in Pathophysiology to Advance Treatment
Collaborate with researchers and clinicians to help advance obesity treatment and enhance the continuum of obesity care.
Arlington, VA
Sept. 28, 2018
Partners in Value
Join leaders from AGA, DHPA, and GI trailblazers from across the country for an in-depth look at how your practice can develop and implement strategies to thrive in the changing business of health care, and address the demands of value-based care.
Dallas, TX
Feb. 7–9, 2019
Crohn’s & Colitis Congress™
Expand your knowledge, network with IBD leaders, spark innovative research and get inspired to improve patient care.
Las Vegas, NV
AWARDS APPLICATION DEADLINES
AGA-Allergan Foundation Pilot Research Award in Irritable Bowel Syndrome
This award provides $30,000 for one year to an investigator at any career stage researching the pathophysiology and/or treatment of irritable bowel syndrome (IBS).
Application Deadline: Sept. 7, 2018
AGA-Allergan Foundation Pilot Research Award in Non-Alcoholic Fatty Liver Disease
This award provides $30,000 for one year to an investigator at any career stage researching the pathophysiology and/or treatment of non-alcoholic fatty liver disease (NAFLD).
Application Deadline: Sept. 7, 2018
AGA-Boston Scientific Technology and Innovation Pilot Research Award
This award provides $30,000 for one year to early career and established investigators working in gastroenterology, hepatology or related areas focused on endoscopic technology and innovation.
Application Deadline: Sept. 7, 2018
AGA-Pfizer Young Investigator Pilot Research Award in Inflammatory Bowel Disease
This award provides $30,000 for one year to recipients at any career stage researching new directions focused on improving the diagnosis and treatment of inflammatory bowel disease (IBD).
Application Deadline: Sept. 7, 2018
AGA-Rome Foundation Functional GI and Motility Disorders Pilot Research Award
This one-year, $30,000 research grant is offered to a recipient at any career stage to support pilot research projects pertaining to functional GI and motility disorders. This award is jointly sponsored by AGA and the Rome Foundation.
Application Deadline: Sept. 7, 2018
AGA-Medtronic Research and Development Pilot Award in Technology
This research initiative grant for $30,000 for 1 year is offered to investigators to support the research and development of novel devices or technologies that will potentially impact the diagnosis or treatment of digestive disease.
Application Deadline: Sept. 7, 2018
AGA-Elsevier Pilot Research Award
This award provides $25,000 for one year to a recipient at any career stage performing research in gastroenterology- or hepatology-related areas.
Application Deadline: Sept. 7, 2018
AGA-Rady Children’s Institute for Genomic Medicine Research Scholar Award in Pediatric Genomics
This award provides $90,000 per year for three years (totaling $270,000) to a young investigator, instructor, research associate, or equivalent working toward an independent career in gastroenterology, hepatology, or related areas. The proposed research may be basic, translational, or clinical and must use genomics as an approach to enhance understanding of pediatric digestive diseases toward prevention, treatment, and/or cure of such diseases. The funded research must be conducted full-time at the Rady Children’s Institute for Genomic Medicine in San Diego, Calif., or at Rady Children’s Hospital – San Diego.
Application Deadline: Dec. 14, 2018
AGA Research Scholar Award (RSA)
This award provides $90,000 per year for three years (total $270,000) to a young investigator, instructor, research associate, or equivalent working toward an independent career in gastroenterology, hepatology, or related areas.
Application Deadline: Dec. 14, 2018
AGA-Shire Research Scholar Award in Functional GI and Motility Disorders
This award provides $90,000 per year for 3 years (total $270,000) to a young investigator, instructor, research associate or equivalent working toward an independent career in functional GI and motility disorders research.
Application Deadline: Dec. 14, 2018
AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
This award provides $90,000 per year for 3 years (total $270,000) to a young investigator, instructor, research associate or equivalent working toward an independent career in inflammatory bowel disease research.
Application Deadline: Dec. 14, 2018
For more information about upcoming events and award deadlines, please visit http://www.gastro.org/education and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Aug. 15-16; Sept. 19-20; Oct. 10-11, 2018
Two-Day, In-Depth Coding and Billing Seminar
Become a certified GI coder with a two-day, in-depth training course provided by McVey Associates, Inc.
Baltimore, MD (8/15-8/16); Atlanta, GA (9/19-9/20); Las Vegas, NV (10/10-10/11)
Aug. 18-19, 2018
James W. Freston Conference: Obesity and Metabolic Disease — Integrating New Paradigms in Pathophysiology to Advance Treatment
Collaborate with researchers and clinicians to help advance obesity treatment and enhance the continuum of obesity care.
Arlington, VA
Sept. 28, 2018
Partners in Value
Join leaders from AGA, DHPA, and GI trailblazers from across the country for an in-depth look at how your practice can develop and implement strategies to thrive in the changing business of health care, and address the demands of value-based care.
Dallas, TX
Feb. 7–9, 2019
Crohn’s & Colitis Congress™
Expand your knowledge, network with IBD leaders, spark innovative research and get inspired to improve patient care.
Las Vegas, NV
AWARDS APPLICATION DEADLINES
AGA-Allergan Foundation Pilot Research Award in Irritable Bowel Syndrome
This award provides $30,000 for one year to an investigator at any career stage researching the pathophysiology and/or treatment of irritable bowel syndrome (IBS).
Application Deadline: Sept. 7, 2018
AGA-Allergan Foundation Pilot Research Award in Non-Alcoholic Fatty Liver Disease
This award provides $30,000 for one year to an investigator at any career stage researching the pathophysiology and/or treatment of non-alcoholic fatty liver disease (NAFLD).
Application Deadline: Sept. 7, 2018
AGA-Boston Scientific Technology and Innovation Pilot Research Award
This award provides $30,000 for one year to early career and established investigators working in gastroenterology, hepatology or related areas focused on endoscopic technology and innovation.
Application Deadline: Sept. 7, 2018
AGA-Pfizer Young Investigator Pilot Research Award in Inflammatory Bowel Disease
This award provides $30,000 for one year to recipients at any career stage researching new directions focused on improving the diagnosis and treatment of inflammatory bowel disease (IBD).
Application Deadline: Sept. 7, 2018
AGA-Rome Foundation Functional GI and Motility Disorders Pilot Research Award
This one-year, $30,000 research grant is offered to a recipient at any career stage to support pilot research projects pertaining to functional GI and motility disorders. This award is jointly sponsored by AGA and the Rome Foundation.
Application Deadline: Sept. 7, 2018
AGA-Medtronic Research and Development Pilot Award in Technology
This research initiative grant for $30,000 for 1 year is offered to investigators to support the research and development of novel devices or technologies that will potentially impact the diagnosis or treatment of digestive disease.
Application Deadline: Sept. 7, 2018
AGA-Elsevier Pilot Research Award
This award provides $25,000 for one year to a recipient at any career stage performing research in gastroenterology- or hepatology-related areas.
Application Deadline: Sept. 7, 2018
AGA-Rady Children’s Institute for Genomic Medicine Research Scholar Award in Pediatric Genomics
This award provides $90,000 per year for three years (totaling $270,000) to a young investigator, instructor, research associate, or equivalent working toward an independent career in gastroenterology, hepatology, or related areas. The proposed research may be basic, translational, or clinical and must use genomics as an approach to enhance understanding of pediatric digestive diseases toward prevention, treatment, and/or cure of such diseases. The funded research must be conducted full-time at the Rady Children’s Institute for Genomic Medicine in San Diego, Calif., or at Rady Children’s Hospital – San Diego.
Application Deadline: Dec. 14, 2018
AGA Research Scholar Award (RSA)
This award provides $90,000 per year for three years (total $270,000) to a young investigator, instructor, research associate, or equivalent working toward an independent career in gastroenterology, hepatology, or related areas.
Application Deadline: Dec. 14, 2018
AGA-Shire Research Scholar Award in Functional GI and Motility Disorders
This award provides $90,000 per year for 3 years (total $270,000) to a young investigator, instructor, research associate or equivalent working toward an independent career in functional GI and motility disorders research.
Application Deadline: Dec. 14, 2018
AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
This award provides $90,000 per year for 3 years (total $270,000) to a young investigator, instructor, research associate or equivalent working toward an independent career in inflammatory bowel disease research.
Application Deadline: Dec. 14, 2018
For more information about upcoming events and award deadlines, please visit http://www.gastro.org/education and http://www.gastro.org/research-funding.
UPCOMING EVENTS
Aug. 15-16; Sept. 19-20; Oct. 10-11, 2018
Two-Day, In-Depth Coding and Billing Seminar
Become a certified GI coder with a two-day, in-depth training course provided by McVey Associates, Inc.
Baltimore, MD (8/15-8/16); Atlanta, GA (9/19-9/20); Las Vegas, NV (10/10-10/11)
Aug. 18-19, 2018
James W. Freston Conference: Obesity and Metabolic Disease — Integrating New Paradigms in Pathophysiology to Advance Treatment
Collaborate with researchers and clinicians to help advance obesity treatment and enhance the continuum of obesity care.
Arlington, VA
Sept. 28, 2018
Partners in Value
Join leaders from AGA, DHPA, and GI trailblazers from across the country for an in-depth look at how your practice can develop and implement strategies to thrive in the changing business of health care, and address the demands of value-based care.
Dallas, TX
Feb. 7–9, 2019
Crohn’s & Colitis Congress™
Expand your knowledge, network with IBD leaders, spark innovative research and get inspired to improve patient care.
Las Vegas, NV
AWARDS APPLICATION DEADLINES
AGA-Allergan Foundation Pilot Research Award in Irritable Bowel Syndrome
This award provides $30,000 for one year to an investigator at any career stage researching the pathophysiology and/or treatment of irritable bowel syndrome (IBS).
Application Deadline: Sept. 7, 2018
AGA-Allergan Foundation Pilot Research Award in Non-Alcoholic Fatty Liver Disease
This award provides $30,000 for one year to an investigator at any career stage researching the pathophysiology and/or treatment of non-alcoholic fatty liver disease (NAFLD).
Application Deadline: Sept. 7, 2018
AGA-Boston Scientific Technology and Innovation Pilot Research Award
This award provides $30,000 for one year to early career and established investigators working in gastroenterology, hepatology or related areas focused on endoscopic technology and innovation.
Application Deadline: Sept. 7, 2018
AGA-Pfizer Young Investigator Pilot Research Award in Inflammatory Bowel Disease
This award provides $30,000 for one year to recipients at any career stage researching new directions focused on improving the diagnosis and treatment of inflammatory bowel disease (IBD).
Application Deadline: Sept. 7, 2018
AGA-Rome Foundation Functional GI and Motility Disorders Pilot Research Award
This one-year, $30,000 research grant is offered to a recipient at any career stage to support pilot research projects pertaining to functional GI and motility disorders. This award is jointly sponsored by AGA and the Rome Foundation.
Application Deadline: Sept. 7, 2018
AGA-Medtronic Research and Development Pilot Award in Technology
This research initiative grant for $30,000 for 1 year is offered to investigators to support the research and development of novel devices or technologies that will potentially impact the diagnosis or treatment of digestive disease.
Application Deadline: Sept. 7, 2018
AGA-Elsevier Pilot Research Award
This award provides $25,000 for one year to a recipient at any career stage performing research in gastroenterology- or hepatology-related areas.
Application Deadline: Sept. 7, 2018
AGA-Rady Children’s Institute for Genomic Medicine Research Scholar Award in Pediatric Genomics
This award provides $90,000 per year for three years (totaling $270,000) to a young investigator, instructor, research associate, or equivalent working toward an independent career in gastroenterology, hepatology, or related areas. The proposed research may be basic, translational, or clinical and must use genomics as an approach to enhance understanding of pediatric digestive diseases toward prevention, treatment, and/or cure of such diseases. The funded research must be conducted full-time at the Rady Children’s Institute for Genomic Medicine in San Diego, Calif., or at Rady Children’s Hospital – San Diego.
Application Deadline: Dec. 14, 2018
AGA Research Scholar Award (RSA)
This award provides $90,000 per year for three years (total $270,000) to a young investigator, instructor, research associate, or equivalent working toward an independent career in gastroenterology, hepatology, or related areas.
Application Deadline: Dec. 14, 2018
AGA-Shire Research Scholar Award in Functional GI and Motility Disorders
This award provides $90,000 per year for 3 years (total $270,000) to a young investigator, instructor, research associate or equivalent working toward an independent career in functional GI and motility disorders research.
Application Deadline: Dec. 14, 2018
AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
This award provides $90,000 per year for 3 years (total $270,000) to a young investigator, instructor, research associate or equivalent working toward an independent career in inflammatory bowel disease research.
Application Deadline: Dec. 14, 2018
Lessons learned from the AGA Future Leaders Program
I have been a member of the American Gastroenterological Association since my first year of GI fellowship. Out of all the GI professional societies, I have always considered it to be my home. AGA is the organization I turn to for direction on providing high-value and quality care to my patients, up-to-date information on research and technology innovations in our specialty, education and training programs for the next generation of gastroenterology and hepatology providers, and for my own continued learning. I have received so many benefits from my membership and am always looking to pay back my appreciation. A wonderful way to do this was through my participation in the Future Leaders Program, which “provides a pathway within the organization for selected participants to network, connect with mentors, develop leadership skills, and learn about AGA’s governance and operations while advancing their careers and supporting the profession.” I can honestly say that the program delivers on all of these promises.
The program started with a meeting at AGA headquarters in Bethesda, Md., where we were introduced to key AGA staff, learned about the core mission and goals of AGA, and received training in leadership skills. I was able to gain invaluable insight into the leadership and governance of the AGA and now have a better understanding of the AGA’s commitment to maintaining a vital organization. At this meeting, we were assigned two mentors – one for career coaching and one to guide us through our main project. We worked closely with these mentors throughout the program, and I have no doubt these relationships will continue well into the future. For the main project we were assigned to a team with another participant to research and develop a project that aligned with the overall strategic plan of the AGA and fulfilled a need or gap within the organization. The projects covered topics such as ways to engage early-career professionals in the AGA Community, enhance access to the AGA guidelines, increase membership, highlight top publications from the AGA journals, expand networking among fellows and early career members, develop education metrics, and provide educational opportunities for international members. We worked on our main project remotely with our teams over 6 months, culminating in a final presentation that was shared with all participants in the Future Leaders Program, as well as AGA committees. Some of these projects already are being implemented. It was a truly amazing experience to work closely with the knowledgeable and dedicated AGA staff, gain skills in teamwork and strategic planning, and hear about the innovative ideas from all the participants in the Future Leaders Program.

In addition to the initial meeting in Bethesda, we attended a reception at Digestive Disease Week® with alumni from the prior class of Future Leaders, a face-to-face meeting in Washington held in conjunction with the AGA Joint Committee Meetings, participated in Advocacy Day after the fall meeting, and attended some virtual roundtable discussions. One of the virtual discussions focused on our final project, which allowed us to apply our skills in strategic planning on a smaller scale to proposals for microvolunteerism. These proposals allow for convenient, short-term volunteer activities that move forward the mission of the AGA and cultivate local leaders. The microvolunteerism proposals are a fantastic example of AGA leadership’s understanding that utilizing and engaging members is one of the best ways to ensure a vital and relevant organization. I am excited to see some of the proposals come to fruition over the next few years.
Through the Future Leaders Program, I not only learned a lot about the AGA but also a great deal about myself. I learned about my own leadership style, which for those of you who know me would not be surprised to find out is “harmony.” I see the possibilities in others and try to capitalize on this potential to achieve our mutual goals. I learned how this leadership style can contribute to organizations as a whole, but also the importance of incorporating multiple leadership styles in all projects. This is valuable information that I have no doubt will help throughout my career. This program also provided an opportunity for me to connect and network with colleagues across the country whom I may not have otherwise met. These connections resulted in new research collaborations, as well as new friendships. As the 2018 AGA Future Leaders Program came to a close, I found myself with a deeper understanding of the mission and vision of the AGA, opportunities for more involvement, and a stronger commitment to our organization. I hope to one day participate as a mentor to other future leaders in the program. I encourage everyone interested in gaining leadership skills and more insight into the governance and operations of the AGA to apply.
Since the AGA Future Leaders Program began in the spring of 2015, two classes of 18 participants and nine mentors have participated in the 18-month comprehensive leadership development program. Nearly all of the Future Leaders Program alumni are serving the AGA in a variety of leadership capacities including serving on committees, the editorial boards, or volunteering as speakers and authors for AGA publications and events.
The next Future Leaders Program will begin in the spring of 2019 and the opportunity to apply will launch this fall.
Dr. Weiss is assistant professor, division of gastroenterology and hepatology; director, UW Health Gastrointestinal Genetics Clinic, University of Wisconsin School of Medicine and Public Health, Madison.
I have been a member of the American Gastroenterological Association since my first year of GI fellowship. Out of all the GI professional societies, I have always considered it to be my home. AGA is the organization I turn to for direction on providing high-value and quality care to my patients, up-to-date information on research and technology innovations in our specialty, education and training programs for the next generation of gastroenterology and hepatology providers, and for my own continued learning. I have received so many benefits from my membership and am always looking to pay back my appreciation. A wonderful way to do this was through my participation in the Future Leaders Program, which “provides a pathway within the organization for selected participants to network, connect with mentors, develop leadership skills, and learn about AGA’s governance and operations while advancing their careers and supporting the profession.” I can honestly say that the program delivers on all of these promises.
The program started with a meeting at AGA headquarters in Bethesda, Md., where we were introduced to key AGA staff, learned about the core mission and goals of AGA, and received training in leadership skills. I was able to gain invaluable insight into the leadership and governance of the AGA and now have a better understanding of the AGA’s commitment to maintaining a vital organization. At this meeting, we were assigned two mentors – one for career coaching and one to guide us through our main project. We worked closely with these mentors throughout the program, and I have no doubt these relationships will continue well into the future. For the main project we were assigned to a team with another participant to research and develop a project that aligned with the overall strategic plan of the AGA and fulfilled a need or gap within the organization. The projects covered topics such as ways to engage early-career professionals in the AGA Community, enhance access to the AGA guidelines, increase membership, highlight top publications from the AGA journals, expand networking among fellows and early career members, develop education metrics, and provide educational opportunities for international members. We worked on our main project remotely with our teams over 6 months, culminating in a final presentation that was shared with all participants in the Future Leaders Program, as well as AGA committees. Some of these projects already are being implemented. It was a truly amazing experience to work closely with the knowledgeable and dedicated AGA staff, gain skills in teamwork and strategic planning, and hear about the innovative ideas from all the participants in the Future Leaders Program.

In addition to the initial meeting in Bethesda, we attended a reception at Digestive Disease Week® with alumni from the prior class of Future Leaders, a face-to-face meeting in Washington held in conjunction with the AGA Joint Committee Meetings, participated in Advocacy Day after the fall meeting, and attended some virtual roundtable discussions. One of the virtual discussions focused on our final project, which allowed us to apply our skills in strategic planning on a smaller scale to proposals for microvolunteerism. These proposals allow for convenient, short-term volunteer activities that move forward the mission of the AGA and cultivate local leaders. The microvolunteerism proposals are a fantastic example of AGA leadership’s understanding that utilizing and engaging members is one of the best ways to ensure a vital and relevant organization. I am excited to see some of the proposals come to fruition over the next few years.
Through the Future Leaders Program, I not only learned a lot about the AGA but also a great deal about myself. I learned about my own leadership style, which for those of you who know me would not be surprised to find out is “harmony.” I see the possibilities in others and try to capitalize on this potential to achieve our mutual goals. I learned how this leadership style can contribute to organizations as a whole, but also the importance of incorporating multiple leadership styles in all projects. This is valuable information that I have no doubt will help throughout my career. This program also provided an opportunity for me to connect and network with colleagues across the country whom I may not have otherwise met. These connections resulted in new research collaborations, as well as new friendships. As the 2018 AGA Future Leaders Program came to a close, I found myself with a deeper understanding of the mission and vision of the AGA, opportunities for more involvement, and a stronger commitment to our organization. I hope to one day participate as a mentor to other future leaders in the program. I encourage everyone interested in gaining leadership skills and more insight into the governance and operations of the AGA to apply.
Since the AGA Future Leaders Program began in the spring of 2015, two classes of 18 participants and nine mentors have participated in the 18-month comprehensive leadership development program. Nearly all of the Future Leaders Program alumni are serving the AGA in a variety of leadership capacities including serving on committees, the editorial boards, or volunteering as speakers and authors for AGA publications and events.
The next Future Leaders Program will begin in the spring of 2019 and the opportunity to apply will launch this fall.
Dr. Weiss is assistant professor, division of gastroenterology and hepatology; director, UW Health Gastrointestinal Genetics Clinic, University of Wisconsin School of Medicine and Public Health, Madison.
I have been a member of the American Gastroenterological Association since my first year of GI fellowship. Out of all the GI professional societies, I have always considered it to be my home. AGA is the organization I turn to for direction on providing high-value and quality care to my patients, up-to-date information on research and technology innovations in our specialty, education and training programs for the next generation of gastroenterology and hepatology providers, and for my own continued learning. I have received so many benefits from my membership and am always looking to pay back my appreciation. A wonderful way to do this was through my participation in the Future Leaders Program, which “provides a pathway within the organization for selected participants to network, connect with mentors, develop leadership skills, and learn about AGA’s governance and operations while advancing their careers and supporting the profession.” I can honestly say that the program delivers on all of these promises.
The program started with a meeting at AGA headquarters in Bethesda, Md., where we were introduced to key AGA staff, learned about the core mission and goals of AGA, and received training in leadership skills. I was able to gain invaluable insight into the leadership and governance of the AGA and now have a better understanding of the AGA’s commitment to maintaining a vital organization. At this meeting, we were assigned two mentors – one for career coaching and one to guide us through our main project. We worked closely with these mentors throughout the program, and I have no doubt these relationships will continue well into the future. For the main project we were assigned to a team with another participant to research and develop a project that aligned with the overall strategic plan of the AGA and fulfilled a need or gap within the organization. The projects covered topics such as ways to engage early-career professionals in the AGA Community, enhance access to the AGA guidelines, increase membership, highlight top publications from the AGA journals, expand networking among fellows and early career members, develop education metrics, and provide educational opportunities for international members. We worked on our main project remotely with our teams over 6 months, culminating in a final presentation that was shared with all participants in the Future Leaders Program, as well as AGA committees. Some of these projects already are being implemented. It was a truly amazing experience to work closely with the knowledgeable and dedicated AGA staff, gain skills in teamwork and strategic planning, and hear about the innovative ideas from all the participants in the Future Leaders Program.

In addition to the initial meeting in Bethesda, we attended a reception at Digestive Disease Week® with alumni from the prior class of Future Leaders, a face-to-face meeting in Washington held in conjunction with the AGA Joint Committee Meetings, participated in Advocacy Day after the fall meeting, and attended some virtual roundtable discussions. One of the virtual discussions focused on our final project, which allowed us to apply our skills in strategic planning on a smaller scale to proposals for microvolunteerism. These proposals allow for convenient, short-term volunteer activities that move forward the mission of the AGA and cultivate local leaders. The microvolunteerism proposals are a fantastic example of AGA leadership’s understanding that utilizing and engaging members is one of the best ways to ensure a vital and relevant organization. I am excited to see some of the proposals come to fruition over the next few years.
Through the Future Leaders Program, I not only learned a lot about the AGA but also a great deal about myself. I learned about my own leadership style, which for those of you who know me would not be surprised to find out is “harmony.” I see the possibilities in others and try to capitalize on this potential to achieve our mutual goals. I learned how this leadership style can contribute to organizations as a whole, but also the importance of incorporating multiple leadership styles in all projects. This is valuable information that I have no doubt will help throughout my career. This program also provided an opportunity for me to connect and network with colleagues across the country whom I may not have otherwise met. These connections resulted in new research collaborations, as well as new friendships. As the 2018 AGA Future Leaders Program came to a close, I found myself with a deeper understanding of the mission and vision of the AGA, opportunities for more involvement, and a stronger commitment to our organization. I hope to one day participate as a mentor to other future leaders in the program. I encourage everyone interested in gaining leadership skills and more insight into the governance and operations of the AGA to apply.
Since the AGA Future Leaders Program began in the spring of 2015, two classes of 18 participants and nine mentors have participated in the 18-month comprehensive leadership development program. Nearly all of the Future Leaders Program alumni are serving the AGA in a variety of leadership capacities including serving on committees, the editorial boards, or volunteering as speakers and authors for AGA publications and events.
The next Future Leaders Program will begin in the spring of 2019 and the opportunity to apply will launch this fall.
Dr. Weiss is assistant professor, division of gastroenterology and hepatology; director, UW Health Gastrointestinal Genetics Clinic, University of Wisconsin School of Medicine and Public Health, Madison.
Meet our 2018 AGA Research Scholar Award Recipients
In 2018, the AGA Research Foundation was proud to provide more than $2 million in research funding to 41 investigators.
AGA’s flagship award, the AGA Research Scholar Award, was given to five exceptional early-career investigators who represent the future of GI research. In addition, one researcher was awarded the AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease. Read about the 2018 awardees’ research projects below.
Sarah Andres, PhD
University of Pennsylvania, Philadelphia
Project title: The mRNA-binding protein IMP1 regulates intestinal epithelial exosome biology during homeostasis and metastasis
Dr. Andres will use this award to delve more deeply into understanding how RNA-binding proteins regulate exosomes within the intestinal and colonic epithelium and how this plays a part in health and disease. RNA-binding proteins provide an exquisite layer of biological regulation to gene expression and downstream cellular processes, which is only beginning to be appreciated. Dr. Andres’ long-term hope is that her work will improve the diagnosis, treatment and ultimately survival of patients with colon cancer.
Swathi Eluri, MD, MSCR
University North Carolina at Chapel Hill
Project title: Improving Barrett’s esophagus screening practices in primary care
Dr. Eluri’s AGA-funded project will gather data to develop and test a multilevel screening intervention for Barrett’s esophagus to be implemented in primary care. The ultimate goal of her work is to improve esophageal adenocarcinoma detection. Given our highly effective endoscopic therapies for early neoplasia in Barrett’s esophagus, early detection has the potential to yield substantial benefits for patients.
Jill Hoffman, PhD
University of California, Los Angeles
AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
Project title: Characterization of CRHR2-mediated enteric glial cell function during colitis
Dr. Hoffman will use her AGA-Takeda funding to define a role for corticotropin-releasing hormone (CRH) signaling in enteric glial cell function and determine CRHR2-dependent crosstalk between enteric glial cells and the intestinal epithelium during inflammation. Through research aimed at understanding the basic mechanisms of cell-to-cell signaling during intestinal inflammation, Dr. Hoffman hopes to determine how to harness these pathways to limit inflammation and promote repair in patients with IBD.
Elizabeth Jensen, MPH, PhD
Wake Forest University, Winston-Salem, N.C.
Project title: Early-life factors, gene-environment interaction and eosinophilic esophagitis (EoE)
With this funding, Dr. Jensen will conduct the largest study to date on early-life factors and EoE, using data that have been collected prospectively through population-based registries in Denmark. Ultimately, Dr. Jensen hopes her research will lead to advancements in our understanding of etiologic factors for development of immune-mediated GI diseases, such as EoE, and will lead to the identification of modifiable factors for disease prevention.
Sumera Rizvi, MD
Mayo Clinic, Rochester, Minn.
Project title: Necrosis enhances tumor immunogenicity and augments cholangiocarcinoma tumor suppression in combination with PD-L1 blockade
Dr. Rizvi’s research is focused on elucidating immunogenic cell death mechanisms and exploring novel, immune-mediated therapeutic approaches in cholangiocarcinoma. This work has the potential to open novel therapeutic avenues for treatment of cholangiocarcinoma, which will ultimately improve the outcomes of patients with this devastating malignancy.
Niels Vande Casteele, PhD
University of California, San Diego
Project title: Identifying optimal thresholds & personalized dosing regimens of infliximab to maximize endoscopic remission rates in patients with ulcerative colitis
Dr. Vande Casteele’s research project is all about determining the right drug for the right patient at the right time using the right dose. By studying optimal thresholds and personalized dosing regimens of infliximab, Dr. Vande Casteele will build the basis for exposure-based dosing regimens that can be applied to other anti-TNF antibodies and antibodies with other targets used in the treatment of patients with IBD, as well as other chronic inflammatory diseases and/or oncology. Dr. Vande Casteele’s goal is for his work to have a direct impact on patients by allowing us to achieve better treatment outcomes with minimal side effects.
View the 2019 AGA research funding opportunities. Please review the deadlines as application deadlines have shifted. Research Scholar Award applications open Sept. 7, 2018.
In 2018, the AGA Research Foundation was proud to provide more than $2 million in research funding to 41 investigators.
AGA’s flagship award, the AGA Research Scholar Award, was given to five exceptional early-career investigators who represent the future of GI research. In addition, one researcher was awarded the AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease. Read about the 2018 awardees’ research projects below.
Sarah Andres, PhD
University of Pennsylvania, Philadelphia
Project title: The mRNA-binding protein IMP1 regulates intestinal epithelial exosome biology during homeostasis and metastasis
Dr. Andres will use this award to delve more deeply into understanding how RNA-binding proteins regulate exosomes within the intestinal and colonic epithelium and how this plays a part in health and disease. RNA-binding proteins provide an exquisite layer of biological regulation to gene expression and downstream cellular processes, which is only beginning to be appreciated. Dr. Andres’ long-term hope is that her work will improve the diagnosis, treatment and ultimately survival of patients with colon cancer.
Swathi Eluri, MD, MSCR
University North Carolina at Chapel Hill
Project title: Improving Barrett’s esophagus screening practices in primary care
Dr. Eluri’s AGA-funded project will gather data to develop and test a multilevel screening intervention for Barrett’s esophagus to be implemented in primary care. The ultimate goal of her work is to improve esophageal adenocarcinoma detection. Given our highly effective endoscopic therapies for early neoplasia in Barrett’s esophagus, early detection has the potential to yield substantial benefits for patients.
Jill Hoffman, PhD
University of California, Los Angeles
AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
Project title: Characterization of CRHR2-mediated enteric glial cell function during colitis
Dr. Hoffman will use her AGA-Takeda funding to define a role for corticotropin-releasing hormone (CRH) signaling in enteric glial cell function and determine CRHR2-dependent crosstalk between enteric glial cells and the intestinal epithelium during inflammation. Through research aimed at understanding the basic mechanisms of cell-to-cell signaling during intestinal inflammation, Dr. Hoffman hopes to determine how to harness these pathways to limit inflammation and promote repair in patients with IBD.
Elizabeth Jensen, MPH, PhD
Wake Forest University, Winston-Salem, N.C.
Project title: Early-life factors, gene-environment interaction and eosinophilic esophagitis (EoE)
With this funding, Dr. Jensen will conduct the largest study to date on early-life factors and EoE, using data that have been collected prospectively through population-based registries in Denmark. Ultimately, Dr. Jensen hopes her research will lead to advancements in our understanding of etiologic factors for development of immune-mediated GI diseases, such as EoE, and will lead to the identification of modifiable factors for disease prevention.
Sumera Rizvi, MD
Mayo Clinic, Rochester, Minn.
Project title: Necrosis enhances tumor immunogenicity and augments cholangiocarcinoma tumor suppression in combination with PD-L1 blockade
Dr. Rizvi’s research is focused on elucidating immunogenic cell death mechanisms and exploring novel, immune-mediated therapeutic approaches in cholangiocarcinoma. This work has the potential to open novel therapeutic avenues for treatment of cholangiocarcinoma, which will ultimately improve the outcomes of patients with this devastating malignancy.
Niels Vande Casteele, PhD
University of California, San Diego
Project title: Identifying optimal thresholds & personalized dosing regimens of infliximab to maximize endoscopic remission rates in patients with ulcerative colitis
Dr. Vande Casteele’s research project is all about determining the right drug for the right patient at the right time using the right dose. By studying optimal thresholds and personalized dosing regimens of infliximab, Dr. Vande Casteele will build the basis for exposure-based dosing regimens that can be applied to other anti-TNF antibodies and antibodies with other targets used in the treatment of patients with IBD, as well as other chronic inflammatory diseases and/or oncology. Dr. Vande Casteele’s goal is for his work to have a direct impact on patients by allowing us to achieve better treatment outcomes with minimal side effects.
View the 2019 AGA research funding opportunities. Please review the deadlines as application deadlines have shifted. Research Scholar Award applications open Sept. 7, 2018.
In 2018, the AGA Research Foundation was proud to provide more than $2 million in research funding to 41 investigators.
AGA’s flagship award, the AGA Research Scholar Award, was given to five exceptional early-career investigators who represent the future of GI research. In addition, one researcher was awarded the AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease. Read about the 2018 awardees’ research projects below.
Sarah Andres, PhD
University of Pennsylvania, Philadelphia
Project title: The mRNA-binding protein IMP1 regulates intestinal epithelial exosome biology during homeostasis and metastasis
Dr. Andres will use this award to delve more deeply into understanding how RNA-binding proteins regulate exosomes within the intestinal and colonic epithelium and how this plays a part in health and disease. RNA-binding proteins provide an exquisite layer of biological regulation to gene expression and downstream cellular processes, which is only beginning to be appreciated. Dr. Andres’ long-term hope is that her work will improve the diagnosis, treatment and ultimately survival of patients with colon cancer.
Swathi Eluri, MD, MSCR
University North Carolina at Chapel Hill
Project title: Improving Barrett’s esophagus screening practices in primary care
Dr. Eluri’s AGA-funded project will gather data to develop and test a multilevel screening intervention for Barrett’s esophagus to be implemented in primary care. The ultimate goal of her work is to improve esophageal adenocarcinoma detection. Given our highly effective endoscopic therapies for early neoplasia in Barrett’s esophagus, early detection has the potential to yield substantial benefits for patients.
Jill Hoffman, PhD
University of California, Los Angeles
AGA-Takeda Pharmaceuticals Research Scholar Award in Inflammatory Bowel Disease
Project title: Characterization of CRHR2-mediated enteric glial cell function during colitis
Dr. Hoffman will use her AGA-Takeda funding to define a role for corticotropin-releasing hormone (CRH) signaling in enteric glial cell function and determine CRHR2-dependent crosstalk between enteric glial cells and the intestinal epithelium during inflammation. Through research aimed at understanding the basic mechanisms of cell-to-cell signaling during intestinal inflammation, Dr. Hoffman hopes to determine how to harness these pathways to limit inflammation and promote repair in patients with IBD.
Elizabeth Jensen, MPH, PhD
Wake Forest University, Winston-Salem, N.C.
Project title: Early-life factors, gene-environment interaction and eosinophilic esophagitis (EoE)
With this funding, Dr. Jensen will conduct the largest study to date on early-life factors and EoE, using data that have been collected prospectively through population-based registries in Denmark. Ultimately, Dr. Jensen hopes her research will lead to advancements in our understanding of etiologic factors for development of immune-mediated GI diseases, such as EoE, and will lead to the identification of modifiable factors for disease prevention.
Sumera Rizvi, MD
Mayo Clinic, Rochester, Minn.
Project title: Necrosis enhances tumor immunogenicity and augments cholangiocarcinoma tumor suppression in combination with PD-L1 blockade
Dr. Rizvi’s research is focused on elucidating immunogenic cell death mechanisms and exploring novel, immune-mediated therapeutic approaches in cholangiocarcinoma. This work has the potential to open novel therapeutic avenues for treatment of cholangiocarcinoma, which will ultimately improve the outcomes of patients with this devastating malignancy.
Niels Vande Casteele, PhD
University of California, San Diego
Project title: Identifying optimal thresholds & personalized dosing regimens of infliximab to maximize endoscopic remission rates in patients with ulcerative colitis
Dr. Vande Casteele’s research project is all about determining the right drug for the right patient at the right time using the right dose. By studying optimal thresholds and personalized dosing regimens of infliximab, Dr. Vande Casteele will build the basis for exposure-based dosing regimens that can be applied to other anti-TNF antibodies and antibodies with other targets used in the treatment of patients with IBD, as well as other chronic inflammatory diseases and/or oncology. Dr. Vande Casteele’s goal is for his work to have a direct impact on patients by allowing us to achieve better treatment outcomes with minimal side effects.
View the 2019 AGA research funding opportunities. Please review the deadlines as application deadlines have shifted. Research Scholar Award applications open Sept. 7, 2018.