User login
Comparing risk models guiding growth factor use in chemotherapy
Chemotherapy-induced neutropenia (CIN) and its corollary febrile neutropenia (FN) are well recognized, and they are serious consequences of many agents used in the treatment of malignancy. FN in particular has been associated with a considerable risk of morbidity and mortality, namely sepsis with multiorgan failure and eventual death. 1 The mainstay of prophylaxis for patients who are deemed to be at high risk for CIN and FN is colony-stimulating factors (CSF). These agents have been shown to significantly decrease FN-related mortality, and therefore their use is potentially life-saving. 2 However, CSF are not cheap, with the cost of peg-filgrastim as much as US $6195.99 per cycle of chemotherapy. 3 Therefore, not only do FN and CIN pose significant risk to patients, they also carry a high burden of cost to the patient and health care system both in treatment and prophylaxis. 4 As such, it is prudent for oncologists to accurately identify high-risk patients and judiciously use CSF in an evidence-based manner.
However, this has proven to be difficult because of the extent of variability between patients and the heterogeneity of the various risk models in the literature. Currently, there are 2 widely used guidelines, 1 developed by the National Comprehensive Cancer Network (NCCN) and another by the American Society of Clinical Oncology (ASCO). Both guidelines suggest the use of prophylactic CSF if the chemotherapy regimen has an FN risk of more than 20% (high risk). If the chemotherapy is deemed to be of intermediate risk (10%-20% FN risk), then patient-specific factors need to be considered. 5,6
In lung cancer, the NCCN lists only topotecan for small cell carcinomas as being high risk for FN, and therefore it is the only regimen that would warrant definitive use of prophylactic CSF. 5 The most recent ASCO guidelines do not list chemotherapy regimens that are high risk for FN. 6 For intermediate-risk regimens, the NCCN states that CSF prophylaxis should be considered if the patient has had previous chemotherapy or radiation therapy, persistent neutropenia, bone marrow involvement by tumor, recent surgery or open wounds, liver dysfunction (total bilirubin, >2.0 mg/dL), or renal dysfunction (creatinine clearance, <50 mL/min), or is older than 65 years. 5
ASCO guidelines state that in intermediate-risk chemotherapy regimens, the following factors are to be considered: age >65 years, advanced disease, previous chemotherapy or radiation therapy, pre-existing neutropenia or marrow involvement by tumor, infection, open wounds or recent surgery, poor performance status or nutritional status, poor renal function, liver dysfunction (most notably bilirubin elevation), cardiovascular disease, multiple comorbid conditions, and HIV infection. However, in the ASCO guidelines, there is no suggestion as to whether CSF should be administered if patients have one of these risk factors, only to “consider these factors when estimating patients’ overall risk of febrile neutropenia.” 6
There is some uncertainty with the NCCN and ASCO guidelines as to whether prophylactic CSF should be given to these intermediate-risk patients. There are suggestions but no definitive guidelines. In our study, we looked at lung cancer patients treated with intermediate-risk chemotherapy regimens and applied 2 different risk models created by Hosmer 7 and Bozcuk 8 and their respective colleagues (Hosmer and Bozcuk hereinafter). Our goal was to assess the efficacy differences between the 2 risk models and to compare their outcomes and recommendations with the NCCN and ASCO guidelines. This was done to showcase the tools available to a clinical oncologist who must decide whether to prescribe prophylactic CSF in these more challenging clinical situations.
Methods
Study population
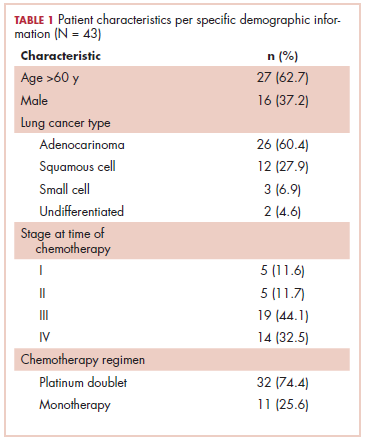
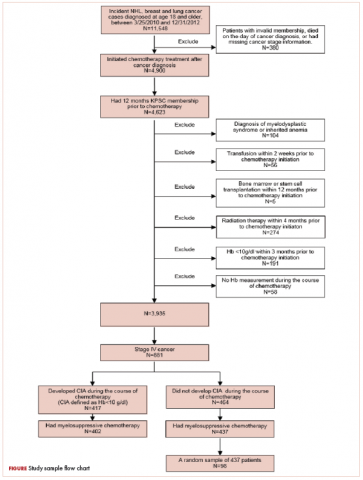
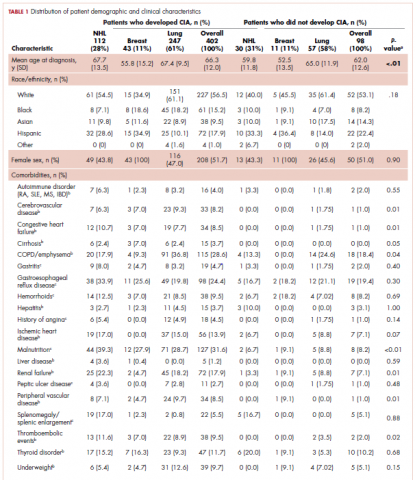
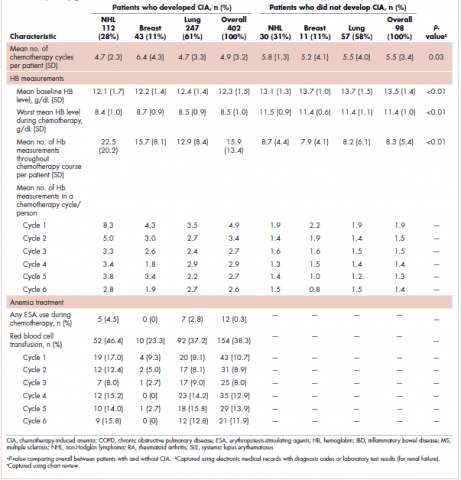
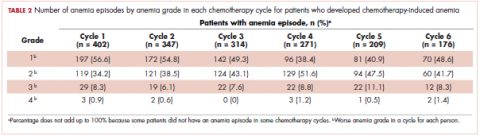
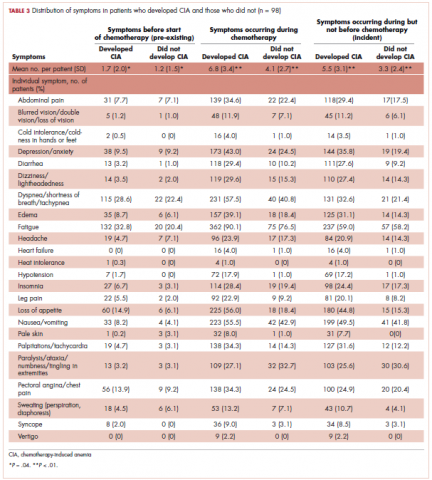
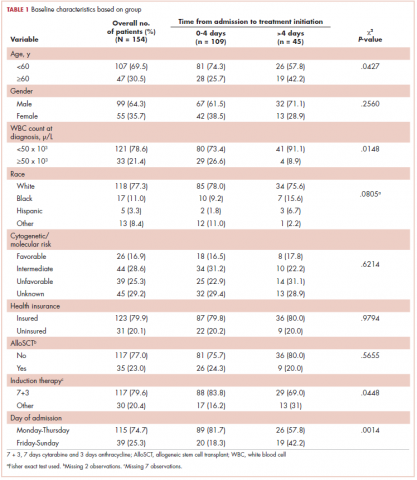
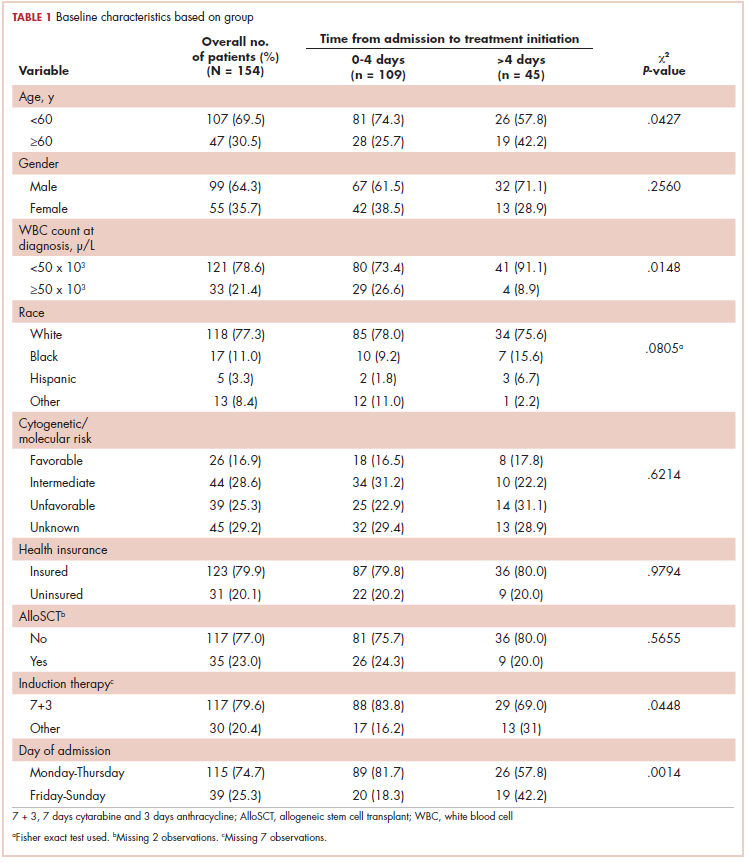
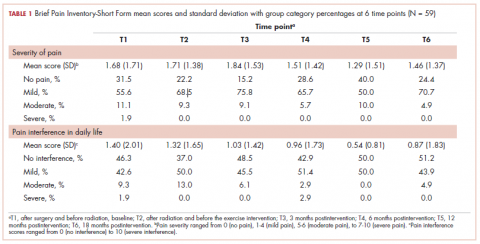
This was a cross-sectional, retrospective study looking at male and female patients aged 18 to 75 years who were treated in the hematology–oncology offices of Drexel University in Philadelphia, Pennsylvania, from 2005 through 2016, who had a diagnosis of lung cancer and were, at some point during their disease, treated with chemotherapy. By using ICD-10 codes for any type of lung cancer, we identified 242 patients. Of those, 106 patients were excluded because they had never received chemotherapy, 16 were excluded either because of miscoding of the type of cancer or because they never actually had cancer, and 61 were excluded either because chemotherapy had not been delivered at our institution or because there were insufficient data to apply the 2 risk models. Of the remaining 59 patients, 16 were excluded because they had received prophylactic CSF with their first cycle of chemotherapy, leaving a total of 43 patients to whom the various risk models and guidelines could be applied (Table 1). If any of the 43 patients were found to be neutropenic, they were given growth factor shortly thereafter.
Chemotherapy for these 43 patients consisted of either a platinum doublet (cisplatin or carboplatin with either etoposide, pemetrexed, gemcitabine, or paclitaxel) or monotherapy with either paclitaxel, abraxane, navelbine, or pemetrexed. Of the 43 patients, 32 had platinum-based doublets, and 11 had monotherapy with one of the listed agents (Table 1).
Formal patient consent was not required because this was a retrospective study.
Defining CIN and FN
Neutropenia was defined as an absolute neutrophil count (ANC) of less than 1500 neutrophils per microliter. The levels of neutropenia were defined as mild (ANC, 1000-1500 neutrophils/μL), moderate (ANC, 500-1000 neutrophils/μL), and severe (ANC, <500 neutrophils/μL). The NCCN guidelines define FN as a single temperature of >38.3°C orally or >38.0°C over 1 hour, with an associated ANC of <500 or <1000 with a predicted decline to <500 over the next 48 hours. 5
Risk models
It should be noted that the Hosmer and Bozcuk calculators were powered to detect occurrence of FN. 7,8 However, we also applied them for the risk of any CIN. In scoring for the Hosmer calculator, points are given to each risk factor and are added together to give a final risk score. This risk score correlates to a percentage of predicted FN. The score for the Hosmer calculator is from minus 18 to plus 19, in which a score of 13 or higher correlates to a 15% predicted risk of FN, and a score of 0 or less correlates to a 1.6% risk of FN. 7 For the Bozcuk calculator, a nomogram is used to calculate risk. Individual points are given to each risk factor and are then summed to give a total that correlates to a risk of FN. The score range for the Bozcuk calculator is 0 to 300, with a score of greater than 190 correlating to a greater than 90% risk of FN, and a score of 0 correlating to a 0% predicted risk of FN. 8
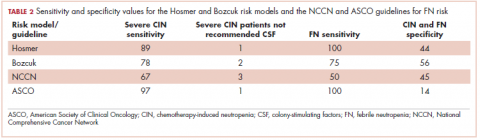
For sensitivity and specificity threshold values, Hosmer reported using a risk score of 10 or above as being a reasonable value for the use of prophylactic CSF. They reported this score would predict an FN risk of about 10%, sensitivity of 24%, and specificity of 93% in detecting FN. 7 Bozcuk reported that using 110 as a cutoff value would correlate to about a 50% FN risk, sensitivity of 100%, and specificity of 49%. However, they did not suggest that value be applied as a threshold for the use of prophylactic CSF as Hosmer did. 8 Despite that, we used the thresholds of 10 and 110 for sensitivity and specificity analyses.
Regarding the current cycle of chemotherapy, the Hosmer calculator looked only at the first cycle, whereas the Bozcuk calculator looked at any cycle of chemotherapy. 7,8 In our study, we used the cycle correlating to the lowest ANC nadir the patient achieved. For example, if a patient achieved a nadir of 1,000 in cycle 1 but 200 in cycle 2, then we used the cycle 2 data to complete the calculators.
With respect to the NCCN and ASCO guidelines, we evaluated our cohort of 43 patients for the risk factors listed in the respective guidelines. If a patient had 1 or more of the risk factors, they were deemed to be high risk and therefore were recommended to receive CSF.
Results
General data
Of the 43 patients studied, 21 developed some level of CIN. Nine patients developed severe CIN, 4 developed moderate CIN, and 8 developed mild CIN. Of the severely neutropenic patients, 4 developed FN. None of the 16 patients who received prophylactic CSF developed FN, although 2 developed severe neutropenia despite CSF administration. Nadirs of ANC were seen on average during cycle 3 of chemotherapy. In all, 15 of the 43 patients achieved lowest ANC nadir during cycle 1.
Risk models
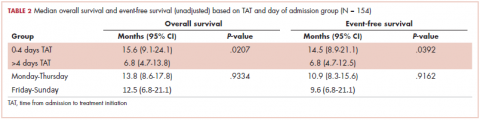
The Bozcuk calculator. A total of 22 patients had risk scores above the calculator’s threshold value of 110. Of those 22 patients, 7 developed severe CIN, 5 developed either mild or moderate CIN, and 3 developed FN. Of the remaining 21 patients who had risk scores of below 110, 2 developed severe CIN, 7 developed mild or moderate CIN, and 1 developed FN. Sensitivity and specificity values are shown in Table 2.
The Hosmer calculator. A total of 26 patients had risk scores above the calculator’s threshold value of 10. Of those 26 patients, 8 developed severe CIN, 4 developed either mild or moderate CIN, and 4 developed FN. Of the remaining 17 patients who had risk scores of less than 10, 1 developed severe CIN, 8 developed mild or moderate CIN, and none developed FN. Sensitivity and specificity values are listed in Table 2.
Current guidelines
NCCN guidelines. If one were to use the NCCN guidelines on our cohort of 43 patients, 25 would have been recommended to receive prophylactic CSF. Of those 25, 6 developed severe CIN (2 with FN), 2 moderate CIN, and 5 mild CIN. Of the 18 patients who would not have been recommended to receive CSF, 3 developed severe CIN (with 2 FN), 2 moderate CIN, and 3 mild CIN. Sensitivity and specificity values are listed in Table 2.
ASCO guidelines. Using the ASCO guidelines on our cohort of 43 patients, 38 had 1 or more of the high-risk features, and, therefore, CSF would have been considered for them. Of those 38 patients, 8 developed severe CIN (4 with FN), 4 developed moderate CIN, and 7 developed mild CIN. Of the 5 patients who would not have received CSF, 1 developed severe CIN and 1 mild CIN. Sensitivity and specificity values are listed in Table 2.
Discussion
In our study, we looked at 2 CIN risk models and compared them with the current NCCN and ASCO guidelines. The models were created to predict risk of FN, but we also looked at their predictive value for any level of CIN. To this end, we found that the Hosmer and Bozcuk calculators both were acceptable for predicting risk of severe CIN and FN. Because of the small number of patients in this study, differences in sensitivities and specificities cannot be quantitatively compared. Nevertheless, qualitatively, it can be said that both calculators were accurate in assigning high-risk scores to patients who developed severe CIN or FN. However, both calculators had many patients with high-risk scores who never developed CIN.
When comparing the 2 risk models with the NCCN and ASCO guidelines, the ASCO guidelines tended to be more liberal in their consideration of CSF use, whereas the NCCN guidelines tended to be more conservative and more similar to the 2 risk models we tested. The NCCN guidelines suggested not giving prophylactic CSF to 2 of our patients who developed FN and to not give CSF to an additional patient who developed severe CIN. The ASCO guidelines suggested considering using CSF for most of our patients, with only 5 patients not to be considered for CSF administration.
The differences in efficacy between the current guidelines and the 2 risk models may be indicative of the fact that the risk models are more accurate in assigning risk in older patients who are clinically more complicated. In our patients, the chemotherapies used were all considered to be intermediate risk, so patient-specific factors were used to guide the administration of CSF. However, because many our patients had at least 1 of the risk factors listed by the NCCN or ASCO, they were automatically deemed to be high risk and to receive prophylactic CSF.
Consequently, the Hosmer and Bozcuk calculators may be of greatest utility in more clinically complicated patients and those who have more comorbidities. The best approach may be a combination of either the NCCN or ASCO guidelines with 1 of the calculators, in our opinion the Hosmer system, for these complicated patients. Likely, the 2 risk models would not be as useful for chemotherapies deemed to have a high risk for FN because, in those situations, the efficacy and benefit of prophylactic CSF are clear. 9 Rather, their use could be beneficial in the grayer areas in which the risk is intermediate and decision-making is more difficult.
Limitations
There were several limitations in our study. First, the size of the cohort was small, and, therefore, the data that we gathered was limited in its scope. However, the goal of this study was to help provide guidance to oncologists in real-world settings about the validity and use of the available risk calculators. A further study should compare the calculators and guidelines in a much larger cohort to see if present results still hold true.
The second possible limitation of the study was our application of the Hosmer calculator because our patient population did not fit the criteria for inclusion in their original study. Hosmer had included only the first cycle of chemotherapy, whereas we included all cycles of chemotherapy. However, despite that, the calculator still performed well and could predict severe CIN and FN even with later cycles of chemotherapy. Therefore, we suggest using this calculator in any cycle of chemotherapy rather than just the first. This would expand its scope and utility in clinical practice.
Conclusions
This article provides oncologists with a comparison of 2 CIN risk models with the currently available NCCN and ASCO guidelines for use in patients with lung cancer. We prefer the Hosmer calculator over the Bozcuk calculator because of its simplicity of use and the accuracy of results. We anticipate that it may be useful and practical as an adjunct tool to the NCCN or ASCO guidelines in patients receiving intermediate-risk chemotherapy regimens. Larger studies combining the calculators and determining accuracy need to be completed to prove this hypothesis.
1. Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258-2266.
2. Kuderer NM, Dale DC, Crawford J, Lyman GH. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol. 2007;25(21):3158-3167.
3. Good Rx, Inc. Peg-filgrastim. https://www.goodrx.com/neulasta. Accessed September 2018.
4. Schilling MB, Parks C, Deeter RG. Costs and outcomes associated with hospitalized cancer patients with neutropenic complications: a retrospective study. Exp Ther Med. 2011;2(5):859-866.
5. National Comprehensive Cancer Network. Myeloid growth factors. In: NCCN Clinical Practice Guidelines in Oncology. Plymouth Meeting, PA: National Comprehensive Cancer Network; 2018.
6. Smith T, Bohlke K, Lyman GH, et al. Recommendations for the use of WBC growth factors: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2015;33(28):3199-3212.
7. Hosmer W, Malin J, Wong M. Development and validation of a prediction model for the risk of developing febrile neutropenia in the first cycle of chemotherapy among elderly patients with breast, lung, colorectal, and prostate cancer. Support Care Cancer. 2011;19(3):333-341.
8. Bozcuk H, Yıldız M, Artaç M, et al. A prospectively validated nomogram for predicting the risk of chemotherapy-induced febrile neutropenia: a multicenter study. Support Care Cancer. 2015;23(6):1759-1767.
9. Vogel CL, Wojtukiewicz MZ, Carroll RR, et al. First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III study. J Clin Oncol. 2005;23(6):1178-1184.
Chemotherapy-induced neutropenia (CIN) and its corollary febrile neutropenia (FN) are well recognized, and they are serious consequences of many agents used in the treatment of malignancy. FN in particular has been associated with a considerable risk of morbidity and mortality, namely sepsis with multiorgan failure and eventual death. 1 The mainstay of prophylaxis for patients who are deemed to be at high risk for CIN and FN is colony-stimulating factors (CSF). These agents have been shown to significantly decrease FN-related mortality, and therefore their use is potentially life-saving. 2 However, CSF are not cheap, with the cost of peg-filgrastim as much as US $6195.99 per cycle of chemotherapy. 3 Therefore, not only do FN and CIN pose significant risk to patients, they also carry a high burden of cost to the patient and health care system both in treatment and prophylaxis. 4 As such, it is prudent for oncologists to accurately identify high-risk patients and judiciously use CSF in an evidence-based manner.
However, this has proven to be difficult because of the extent of variability between patients and the heterogeneity of the various risk models in the literature. Currently, there are 2 widely used guidelines, 1 developed by the National Comprehensive Cancer Network (NCCN) and another by the American Society of Clinical Oncology (ASCO). Both guidelines suggest the use of prophylactic CSF if the chemotherapy regimen has an FN risk of more than 20% (high risk). If the chemotherapy is deemed to be of intermediate risk (10%-20% FN risk), then patient-specific factors need to be considered. 5,6
In lung cancer, the NCCN lists only topotecan for small cell carcinomas as being high risk for FN, and therefore it is the only regimen that would warrant definitive use of prophylactic CSF. 5 The most recent ASCO guidelines do not list chemotherapy regimens that are high risk for FN. 6 For intermediate-risk regimens, the NCCN states that CSF prophylaxis should be considered if the patient has had previous chemotherapy or radiation therapy, persistent neutropenia, bone marrow involvement by tumor, recent surgery or open wounds, liver dysfunction (total bilirubin, >2.0 mg/dL), or renal dysfunction (creatinine clearance, <50 mL/min), or is older than 65 years. 5
ASCO guidelines state that in intermediate-risk chemotherapy regimens, the following factors are to be considered: age >65 years, advanced disease, previous chemotherapy or radiation therapy, pre-existing neutropenia or marrow involvement by tumor, infection, open wounds or recent surgery, poor performance status or nutritional status, poor renal function, liver dysfunction (most notably bilirubin elevation), cardiovascular disease, multiple comorbid conditions, and HIV infection. However, in the ASCO guidelines, there is no suggestion as to whether CSF should be administered if patients have one of these risk factors, only to “consider these factors when estimating patients’ overall risk of febrile neutropenia.” 6
There is some uncertainty with the NCCN and ASCO guidelines as to whether prophylactic CSF should be given to these intermediate-risk patients. There are suggestions but no definitive guidelines. In our study, we looked at lung cancer patients treated with intermediate-risk chemotherapy regimens and applied 2 different risk models created by Hosmer 7 and Bozcuk 8 and their respective colleagues (Hosmer and Bozcuk hereinafter). Our goal was to assess the efficacy differences between the 2 risk models and to compare their outcomes and recommendations with the NCCN and ASCO guidelines. This was done to showcase the tools available to a clinical oncologist who must decide whether to prescribe prophylactic CSF in these more challenging clinical situations.
Methods
Study population
This was a cross-sectional, retrospective study looking at male and female patients aged 18 to 75 years who were treated in the hematology–oncology offices of Drexel University in Philadelphia, Pennsylvania, from 2005 through 2016, who had a diagnosis of lung cancer and were, at some point during their disease, treated with chemotherapy. By using ICD-10 codes for any type of lung cancer, we identified 242 patients. Of those, 106 patients were excluded because they had never received chemotherapy, 16 were excluded either because of miscoding of the type of cancer or because they never actually had cancer, and 61 were excluded either because chemotherapy had not been delivered at our institution or because there were insufficient data to apply the 2 risk models. Of the remaining 59 patients, 16 were excluded because they had received prophylactic CSF with their first cycle of chemotherapy, leaving a total of 43 patients to whom the various risk models and guidelines could be applied (Table 1). If any of the 43 patients were found to be neutropenic, they were given growth factor shortly thereafter.
Chemotherapy for these 43 patients consisted of either a platinum doublet (cisplatin or carboplatin with either etoposide, pemetrexed, gemcitabine, or paclitaxel) or monotherapy with either paclitaxel, abraxane, navelbine, or pemetrexed. Of the 43 patients, 32 had platinum-based doublets, and 11 had monotherapy with one of the listed agents (Table 1).
Formal patient consent was not required because this was a retrospective study.
Defining CIN and FN
Neutropenia was defined as an absolute neutrophil count (ANC) of less than 1500 neutrophils per microliter. The levels of neutropenia were defined as mild (ANC, 1000-1500 neutrophils/μL), moderate (ANC, 500-1000 neutrophils/μL), and severe (ANC, <500 neutrophils/μL). The NCCN guidelines define FN as a single temperature of >38.3°C orally or >38.0°C over 1 hour, with an associated ANC of <500 or <1000 with a predicted decline to <500 over the next 48 hours. 5
Risk models
It should be noted that the Hosmer and Bozcuk calculators were powered to detect occurrence of FN. 7,8 However, we also applied them for the risk of any CIN. In scoring for the Hosmer calculator, points are given to each risk factor and are added together to give a final risk score. This risk score correlates to a percentage of predicted FN. The score for the Hosmer calculator is from minus 18 to plus 19, in which a score of 13 or higher correlates to a 15% predicted risk of FN, and a score of 0 or less correlates to a 1.6% risk of FN. 7 For the Bozcuk calculator, a nomogram is used to calculate risk. Individual points are given to each risk factor and are then summed to give a total that correlates to a risk of FN. The score range for the Bozcuk calculator is 0 to 300, with a score of greater than 190 correlating to a greater than 90% risk of FN, and a score of 0 correlating to a 0% predicted risk of FN. 8
For sensitivity and specificity threshold values, Hosmer reported using a risk score of 10 or above as being a reasonable value for the use of prophylactic CSF. They reported this score would predict an FN risk of about 10%, sensitivity of 24%, and specificity of 93% in detecting FN. 7 Bozcuk reported that using 110 as a cutoff value would correlate to about a 50% FN risk, sensitivity of 100%, and specificity of 49%. However, they did not suggest that value be applied as a threshold for the use of prophylactic CSF as Hosmer did. 8 Despite that, we used the thresholds of 10 and 110 for sensitivity and specificity analyses.
Regarding the current cycle of chemotherapy, the Hosmer calculator looked only at the first cycle, whereas the Bozcuk calculator looked at any cycle of chemotherapy. 7,8 In our study, we used the cycle correlating to the lowest ANC nadir the patient achieved. For example, if a patient achieved a nadir of 1,000 in cycle 1 but 200 in cycle 2, then we used the cycle 2 data to complete the calculators.
With respect to the NCCN and ASCO guidelines, we evaluated our cohort of 43 patients for the risk factors listed in the respective guidelines. If a patient had 1 or more of the risk factors, they were deemed to be high risk and therefore were recommended to receive CSF.
Results
General data
Of the 43 patients studied, 21 developed some level of CIN. Nine patients developed severe CIN, 4 developed moderate CIN, and 8 developed mild CIN. Of the severely neutropenic patients, 4 developed FN. None of the 16 patients who received prophylactic CSF developed FN, although 2 developed severe neutropenia despite CSF administration. Nadirs of ANC were seen on average during cycle 3 of chemotherapy. In all, 15 of the 43 patients achieved lowest ANC nadir during cycle 1.
Risk models
The Bozcuk calculator. A total of 22 patients had risk scores above the calculator’s threshold value of 110. Of those 22 patients, 7 developed severe CIN, 5 developed either mild or moderate CIN, and 3 developed FN. Of the remaining 21 patients who had risk scores of below 110, 2 developed severe CIN, 7 developed mild or moderate CIN, and 1 developed FN. Sensitivity and specificity values are shown in Table 2.
The Hosmer calculator. A total of 26 patients had risk scores above the calculator’s threshold value of 10. Of those 26 patients, 8 developed severe CIN, 4 developed either mild or moderate CIN, and 4 developed FN. Of the remaining 17 patients who had risk scores of less than 10, 1 developed severe CIN, 8 developed mild or moderate CIN, and none developed FN. Sensitivity and specificity values are listed in Table 2.
Current guidelines
NCCN guidelines. If one were to use the NCCN guidelines on our cohort of 43 patients, 25 would have been recommended to receive prophylactic CSF. Of those 25, 6 developed severe CIN (2 with FN), 2 moderate CIN, and 5 mild CIN. Of the 18 patients who would not have been recommended to receive CSF, 3 developed severe CIN (with 2 FN), 2 moderate CIN, and 3 mild CIN. Sensitivity and specificity values are listed in Table 2.
ASCO guidelines. Using the ASCO guidelines on our cohort of 43 patients, 38 had 1 or more of the high-risk features, and, therefore, CSF would have been considered for them. Of those 38 patients, 8 developed severe CIN (4 with FN), 4 developed moderate CIN, and 7 developed mild CIN. Of the 5 patients who would not have received CSF, 1 developed severe CIN and 1 mild CIN. Sensitivity and specificity values are listed in Table 2.
Discussion
In our study, we looked at 2 CIN risk models and compared them with the current NCCN and ASCO guidelines. The models were created to predict risk of FN, but we also looked at their predictive value for any level of CIN. To this end, we found that the Hosmer and Bozcuk calculators both were acceptable for predicting risk of severe CIN and FN. Because of the small number of patients in this study, differences in sensitivities and specificities cannot be quantitatively compared. Nevertheless, qualitatively, it can be said that both calculators were accurate in assigning high-risk scores to patients who developed severe CIN or FN. However, both calculators had many patients with high-risk scores who never developed CIN.
When comparing the 2 risk models with the NCCN and ASCO guidelines, the ASCO guidelines tended to be more liberal in their consideration of CSF use, whereas the NCCN guidelines tended to be more conservative and more similar to the 2 risk models we tested. The NCCN guidelines suggested not giving prophylactic CSF to 2 of our patients who developed FN and to not give CSF to an additional patient who developed severe CIN. The ASCO guidelines suggested considering using CSF for most of our patients, with only 5 patients not to be considered for CSF administration.
The differences in efficacy between the current guidelines and the 2 risk models may be indicative of the fact that the risk models are more accurate in assigning risk in older patients who are clinically more complicated. In our patients, the chemotherapies used were all considered to be intermediate risk, so patient-specific factors were used to guide the administration of CSF. However, because many our patients had at least 1 of the risk factors listed by the NCCN or ASCO, they were automatically deemed to be high risk and to receive prophylactic CSF.
Consequently, the Hosmer and Bozcuk calculators may be of greatest utility in more clinically complicated patients and those who have more comorbidities. The best approach may be a combination of either the NCCN or ASCO guidelines with 1 of the calculators, in our opinion the Hosmer system, for these complicated patients. Likely, the 2 risk models would not be as useful for chemotherapies deemed to have a high risk for FN because, in those situations, the efficacy and benefit of prophylactic CSF are clear. 9 Rather, their use could be beneficial in the grayer areas in which the risk is intermediate and decision-making is more difficult.
Limitations
There were several limitations in our study. First, the size of the cohort was small, and, therefore, the data that we gathered was limited in its scope. However, the goal of this study was to help provide guidance to oncologists in real-world settings about the validity and use of the available risk calculators. A further study should compare the calculators and guidelines in a much larger cohort to see if present results still hold true.
The second possible limitation of the study was our application of the Hosmer calculator because our patient population did not fit the criteria for inclusion in their original study. Hosmer had included only the first cycle of chemotherapy, whereas we included all cycles of chemotherapy. However, despite that, the calculator still performed well and could predict severe CIN and FN even with later cycles of chemotherapy. Therefore, we suggest using this calculator in any cycle of chemotherapy rather than just the first. This would expand its scope and utility in clinical practice.
Conclusions
This article provides oncologists with a comparison of 2 CIN risk models with the currently available NCCN and ASCO guidelines for use in patients with lung cancer. We prefer the Hosmer calculator over the Bozcuk calculator because of its simplicity of use and the accuracy of results. We anticipate that it may be useful and practical as an adjunct tool to the NCCN or ASCO guidelines in patients receiving intermediate-risk chemotherapy regimens. Larger studies combining the calculators and determining accuracy need to be completed to prove this hypothesis.
Chemotherapy-induced neutropenia (CIN) and its corollary febrile neutropenia (FN) are well recognized, and they are serious consequences of many agents used in the treatment of malignancy. FN in particular has been associated with a considerable risk of morbidity and mortality, namely sepsis with multiorgan failure and eventual death. 1 The mainstay of prophylaxis for patients who are deemed to be at high risk for CIN and FN is colony-stimulating factors (CSF). These agents have been shown to significantly decrease FN-related mortality, and therefore their use is potentially life-saving. 2 However, CSF are not cheap, with the cost of peg-filgrastim as much as US $6195.99 per cycle of chemotherapy. 3 Therefore, not only do FN and CIN pose significant risk to patients, they also carry a high burden of cost to the patient and health care system both in treatment and prophylaxis. 4 As such, it is prudent for oncologists to accurately identify high-risk patients and judiciously use CSF in an evidence-based manner.
However, this has proven to be difficult because of the extent of variability between patients and the heterogeneity of the various risk models in the literature. Currently, there are 2 widely used guidelines, 1 developed by the National Comprehensive Cancer Network (NCCN) and another by the American Society of Clinical Oncology (ASCO). Both guidelines suggest the use of prophylactic CSF if the chemotherapy regimen has an FN risk of more than 20% (high risk). If the chemotherapy is deemed to be of intermediate risk (10%-20% FN risk), then patient-specific factors need to be considered. 5,6
In lung cancer, the NCCN lists only topotecan for small cell carcinomas as being high risk for FN, and therefore it is the only regimen that would warrant definitive use of prophylactic CSF. 5 The most recent ASCO guidelines do not list chemotherapy regimens that are high risk for FN. 6 For intermediate-risk regimens, the NCCN states that CSF prophylaxis should be considered if the patient has had previous chemotherapy or radiation therapy, persistent neutropenia, bone marrow involvement by tumor, recent surgery or open wounds, liver dysfunction (total bilirubin, >2.0 mg/dL), or renal dysfunction (creatinine clearance, <50 mL/min), or is older than 65 years. 5
ASCO guidelines state that in intermediate-risk chemotherapy regimens, the following factors are to be considered: age >65 years, advanced disease, previous chemotherapy or radiation therapy, pre-existing neutropenia or marrow involvement by tumor, infection, open wounds or recent surgery, poor performance status or nutritional status, poor renal function, liver dysfunction (most notably bilirubin elevation), cardiovascular disease, multiple comorbid conditions, and HIV infection. However, in the ASCO guidelines, there is no suggestion as to whether CSF should be administered if patients have one of these risk factors, only to “consider these factors when estimating patients’ overall risk of febrile neutropenia.” 6
There is some uncertainty with the NCCN and ASCO guidelines as to whether prophylactic CSF should be given to these intermediate-risk patients. There are suggestions but no definitive guidelines. In our study, we looked at lung cancer patients treated with intermediate-risk chemotherapy regimens and applied 2 different risk models created by Hosmer 7 and Bozcuk 8 and their respective colleagues (Hosmer and Bozcuk hereinafter). Our goal was to assess the efficacy differences between the 2 risk models and to compare their outcomes and recommendations with the NCCN and ASCO guidelines. This was done to showcase the tools available to a clinical oncologist who must decide whether to prescribe prophylactic CSF in these more challenging clinical situations.
Methods
Study population
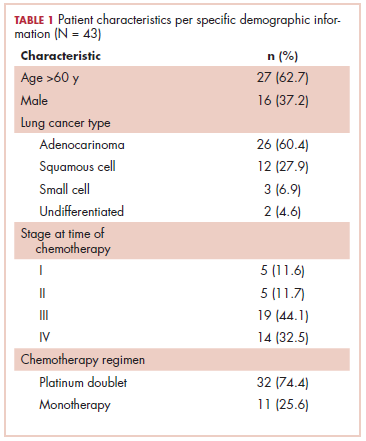
This was a cross-sectional, retrospective study looking at male and female patients aged 18 to 75 years who were treated in the hematology–oncology offices of Drexel University in Philadelphia, Pennsylvania, from 2005 through 2016, who had a diagnosis of lung cancer and were, at some point during their disease, treated with chemotherapy. By using ICD-10 codes for any type of lung cancer, we identified 242 patients. Of those, 106 patients were excluded because they had never received chemotherapy, 16 were excluded either because of miscoding of the type of cancer or because they never actually had cancer, and 61 were excluded either because chemotherapy had not been delivered at our institution or because there were insufficient data to apply the 2 risk models. Of the remaining 59 patients, 16 were excluded because they had received prophylactic CSF with their first cycle of chemotherapy, leaving a total of 43 patients to whom the various risk models and guidelines could be applied (Table 1). If any of the 43 patients were found to be neutropenic, they were given growth factor shortly thereafter.
Chemotherapy for these 43 patients consisted of either a platinum doublet (cisplatin or carboplatin with either etoposide, pemetrexed, gemcitabine, or paclitaxel) or monotherapy with either paclitaxel, abraxane, navelbine, or pemetrexed. Of the 43 patients, 32 had platinum-based doublets, and 11 had monotherapy with one of the listed agents (Table 1).
Formal patient consent was not required because this was a retrospective study.
Defining CIN and FN
Neutropenia was defined as an absolute neutrophil count (ANC) of less than 1500 neutrophils per microliter. The levels of neutropenia were defined as mild (ANC, 1000-1500 neutrophils/μL), moderate (ANC, 500-1000 neutrophils/μL), and severe (ANC, <500 neutrophils/μL). The NCCN guidelines define FN as a single temperature of >38.3°C orally or >38.0°C over 1 hour, with an associated ANC of <500 or <1000 with a predicted decline to <500 over the next 48 hours. 5
Risk models
It should be noted that the Hosmer and Bozcuk calculators were powered to detect occurrence of FN. 7,8 However, we also applied them for the risk of any CIN. In scoring for the Hosmer calculator, points are given to each risk factor and are added together to give a final risk score. This risk score correlates to a percentage of predicted FN. The score for the Hosmer calculator is from minus 18 to plus 19, in which a score of 13 or higher correlates to a 15% predicted risk of FN, and a score of 0 or less correlates to a 1.6% risk of FN. 7 For the Bozcuk calculator, a nomogram is used to calculate risk. Individual points are given to each risk factor and are then summed to give a total that correlates to a risk of FN. The score range for the Bozcuk calculator is 0 to 300, with a score of greater than 190 correlating to a greater than 90% risk of FN, and a score of 0 correlating to a 0% predicted risk of FN. 8
For sensitivity and specificity threshold values, Hosmer reported using a risk score of 10 or above as being a reasonable value for the use of prophylactic CSF. They reported this score would predict an FN risk of about 10%, sensitivity of 24%, and specificity of 93% in detecting FN. 7 Bozcuk reported that using 110 as a cutoff value would correlate to about a 50% FN risk, sensitivity of 100%, and specificity of 49%. However, they did not suggest that value be applied as a threshold for the use of prophylactic CSF as Hosmer did. 8 Despite that, we used the thresholds of 10 and 110 for sensitivity and specificity analyses.
Regarding the current cycle of chemotherapy, the Hosmer calculator looked only at the first cycle, whereas the Bozcuk calculator looked at any cycle of chemotherapy. 7,8 In our study, we used the cycle correlating to the lowest ANC nadir the patient achieved. For example, if a patient achieved a nadir of 1,000 in cycle 1 but 200 in cycle 2, then we used the cycle 2 data to complete the calculators.
With respect to the NCCN and ASCO guidelines, we evaluated our cohort of 43 patients for the risk factors listed in the respective guidelines. If a patient had 1 or more of the risk factors, they were deemed to be high risk and therefore were recommended to receive CSF.
Results
General data
Of the 43 patients studied, 21 developed some level of CIN. Nine patients developed severe CIN, 4 developed moderate CIN, and 8 developed mild CIN. Of the severely neutropenic patients, 4 developed FN. None of the 16 patients who received prophylactic CSF developed FN, although 2 developed severe neutropenia despite CSF administration. Nadirs of ANC were seen on average during cycle 3 of chemotherapy. In all, 15 of the 43 patients achieved lowest ANC nadir during cycle 1.
Risk models
The Bozcuk calculator. A total of 22 patients had risk scores above the calculator’s threshold value of 110. Of those 22 patients, 7 developed severe CIN, 5 developed either mild or moderate CIN, and 3 developed FN. Of the remaining 21 patients who had risk scores of below 110, 2 developed severe CIN, 7 developed mild or moderate CIN, and 1 developed FN. Sensitivity and specificity values are shown in Table 2.
The Hosmer calculator. A total of 26 patients had risk scores above the calculator’s threshold value of 10. Of those 26 patients, 8 developed severe CIN, 4 developed either mild or moderate CIN, and 4 developed FN. Of the remaining 17 patients who had risk scores of less than 10, 1 developed severe CIN, 8 developed mild or moderate CIN, and none developed FN. Sensitivity and specificity values are listed in Table 2.
Current guidelines
NCCN guidelines. If one were to use the NCCN guidelines on our cohort of 43 patients, 25 would have been recommended to receive prophylactic CSF. Of those 25, 6 developed severe CIN (2 with FN), 2 moderate CIN, and 5 mild CIN. Of the 18 patients who would not have been recommended to receive CSF, 3 developed severe CIN (with 2 FN), 2 moderate CIN, and 3 mild CIN. Sensitivity and specificity values are listed in Table 2.
ASCO guidelines. Using the ASCO guidelines on our cohort of 43 patients, 38 had 1 or more of the high-risk features, and, therefore, CSF would have been considered for them. Of those 38 patients, 8 developed severe CIN (4 with FN), 4 developed moderate CIN, and 7 developed mild CIN. Of the 5 patients who would not have received CSF, 1 developed severe CIN and 1 mild CIN. Sensitivity and specificity values are listed in Table 2.
Discussion
In our study, we looked at 2 CIN risk models and compared them with the current NCCN and ASCO guidelines. The models were created to predict risk of FN, but we also looked at their predictive value for any level of CIN. To this end, we found that the Hosmer and Bozcuk calculators both were acceptable for predicting risk of severe CIN and FN. Because of the small number of patients in this study, differences in sensitivities and specificities cannot be quantitatively compared. Nevertheless, qualitatively, it can be said that both calculators were accurate in assigning high-risk scores to patients who developed severe CIN or FN. However, both calculators had many patients with high-risk scores who never developed CIN.
When comparing the 2 risk models with the NCCN and ASCO guidelines, the ASCO guidelines tended to be more liberal in their consideration of CSF use, whereas the NCCN guidelines tended to be more conservative and more similar to the 2 risk models we tested. The NCCN guidelines suggested not giving prophylactic CSF to 2 of our patients who developed FN and to not give CSF to an additional patient who developed severe CIN. The ASCO guidelines suggested considering using CSF for most of our patients, with only 5 patients not to be considered for CSF administration.
The differences in efficacy between the current guidelines and the 2 risk models may be indicative of the fact that the risk models are more accurate in assigning risk in older patients who are clinically more complicated. In our patients, the chemotherapies used were all considered to be intermediate risk, so patient-specific factors were used to guide the administration of CSF. However, because many our patients had at least 1 of the risk factors listed by the NCCN or ASCO, they were automatically deemed to be high risk and to receive prophylactic CSF.
Consequently, the Hosmer and Bozcuk calculators may be of greatest utility in more clinically complicated patients and those who have more comorbidities. The best approach may be a combination of either the NCCN or ASCO guidelines with 1 of the calculators, in our opinion the Hosmer system, for these complicated patients. Likely, the 2 risk models would not be as useful for chemotherapies deemed to have a high risk for FN because, in those situations, the efficacy and benefit of prophylactic CSF are clear. 9 Rather, their use could be beneficial in the grayer areas in which the risk is intermediate and decision-making is more difficult.
Limitations
There were several limitations in our study. First, the size of the cohort was small, and, therefore, the data that we gathered was limited in its scope. However, the goal of this study was to help provide guidance to oncologists in real-world settings about the validity and use of the available risk calculators. A further study should compare the calculators and guidelines in a much larger cohort to see if present results still hold true.
The second possible limitation of the study was our application of the Hosmer calculator because our patient population did not fit the criteria for inclusion in their original study. Hosmer had included only the first cycle of chemotherapy, whereas we included all cycles of chemotherapy. However, despite that, the calculator still performed well and could predict severe CIN and FN even with later cycles of chemotherapy. Therefore, we suggest using this calculator in any cycle of chemotherapy rather than just the first. This would expand its scope and utility in clinical practice.
Conclusions
This article provides oncologists with a comparison of 2 CIN risk models with the currently available NCCN and ASCO guidelines for use in patients with lung cancer. We prefer the Hosmer calculator over the Bozcuk calculator because of its simplicity of use and the accuracy of results. We anticipate that it may be useful and practical as an adjunct tool to the NCCN or ASCO guidelines in patients receiving intermediate-risk chemotherapy regimens. Larger studies combining the calculators and determining accuracy need to be completed to prove this hypothesis.
1. Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258-2266.
2. Kuderer NM, Dale DC, Crawford J, Lyman GH. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol. 2007;25(21):3158-3167.
3. Good Rx, Inc. Peg-filgrastim. https://www.goodrx.com/neulasta. Accessed September 2018.
4. Schilling MB, Parks C, Deeter RG. Costs and outcomes associated with hospitalized cancer patients with neutropenic complications: a retrospective study. Exp Ther Med. 2011;2(5):859-866.
5. National Comprehensive Cancer Network. Myeloid growth factors. In: NCCN Clinical Practice Guidelines in Oncology. Plymouth Meeting, PA: National Comprehensive Cancer Network; 2018.
6. Smith T, Bohlke K, Lyman GH, et al. Recommendations for the use of WBC growth factors: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2015;33(28):3199-3212.
7. Hosmer W, Malin J, Wong M. Development and validation of a prediction model for the risk of developing febrile neutropenia in the first cycle of chemotherapy among elderly patients with breast, lung, colorectal, and prostate cancer. Support Care Cancer. 2011;19(3):333-341.
8. Bozcuk H, Yıldız M, Artaç M, et al. A prospectively validated nomogram for predicting the risk of chemotherapy-induced febrile neutropenia: a multicenter study. Support Care Cancer. 2015;23(6):1759-1767.
9. Vogel CL, Wojtukiewicz MZ, Carroll RR, et al. First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III study. J Clin Oncol. 2005;23(6):1178-1184.
1. Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258-2266.
2. Kuderer NM, Dale DC, Crawford J, Lyman GH. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol. 2007;25(21):3158-3167.
3. Good Rx, Inc. Peg-filgrastim. https://www.goodrx.com/neulasta. Accessed September 2018.
4. Schilling MB, Parks C, Deeter RG. Costs and outcomes associated with hospitalized cancer patients with neutropenic complications: a retrospective study. Exp Ther Med. 2011;2(5):859-866.
5. National Comprehensive Cancer Network. Myeloid growth factors. In: NCCN Clinical Practice Guidelines in Oncology. Plymouth Meeting, PA: National Comprehensive Cancer Network; 2018.
6. Smith T, Bohlke K, Lyman GH, et al. Recommendations for the use of WBC growth factors: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2015;33(28):3199-3212.
7. Hosmer W, Malin J, Wong M. Development and validation of a prediction model for the risk of developing febrile neutropenia in the first cycle of chemotherapy among elderly patients with breast, lung, colorectal, and prostate cancer. Support Care Cancer. 2011;19(3):333-341.
8. Bozcuk H, Yıldız M, Artaç M, et al. A prospectively validated nomogram for predicting the risk of chemotherapy-induced febrile neutropenia: a multicenter study. Support Care Cancer. 2015;23(6):1759-1767.
9. Vogel CL, Wojtukiewicz MZ, Carroll RR, et al. First and subsequent cycle use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III study. J Clin Oncol. 2005;23(6):1178-1184.
Mortality outcomes in hospitalized oncology patients after rapid response team activation
Cancer is the second leading cause of death in the United States, exceeded only by heart disease.1 Despite the overall decline in cancer death rates from 2000 through 2014, physicians struggle to accurately predict disease progression and mortality in patients with cancer who are within 6 months of death.2-8 This prognostic uncertainty makes clinical decision making difficult for patients, families, and health care providers. On a health care system level, an insight into end-of-life prognostication could also have substantial financial implications. In 2013, $74 billion was spent on cancer-related health care in the United States.9 Studies have shown that from 5% to 6% of Medicare beneficiaries with cancer consumed up to 30% of the annual Medicare payments, with a staggering 78% of costs being from acute care in the final 30 days of life.10
Rapid response teams (RRTs) were first introduced in 1995 and are now widely used at many hospitals to identify and provide critical care at the bedside of deteriorating patients outside of the intensive care unit (ICU) to prevent morbidity and mortality.11-15 Although not the original aim, RRTs are commonly activated on patients at the end of life and have therefore come to play an important role in end-of-life care.11,16 RRT activation in the oncology population is of special interest because the activation may predict higher inpatient mortality.17 In addition, RRT activation can serve as a sentinel event that fosters discussion on goals of care, change in code status, and initiation of palliative care or hospice use, particularly when also accompanied by an upgrade in level of care.11,18 As such, the ability to predict mortality after an RRT event, both inpatient and at 100 days after the event, could be of great help in deciding whether to pursue further treatments or, alternatively, palliative or hospice care.
To that end, the purpose of this study was to identify baseline patient characteristics, causes of deterioration leading to the RRT event, and vital signs and laboratory abnormalities in the peri-RRT period –
Methods and materials
A retrospective study was performed at a single, 900+ bed academic center in the northeastern United States during a 2-year study period from October 2014 through November 2016. The Institutional Review Board at Thomas Jefferson University Hospital in Philadelphia, Pennsylvania, reviewed and approved the study.
Through our institution’s RRT database, all consecutive RRT activations during the study period involving hospitalized oncology patients were reviewed. We included patients 18 years or older with a cancer diagnosis, including solid tumor and hematologic malignancy, as well as those who were status post–bone marrow transplantation (BMT), who required rapid response activation while hospitalized at our institution. We excluded patients who activated rapid response while they were in the ICU, including the BMT unit, those on the surgical floors, and those with RRT activation at other hospitals before transfer to our institution. Data for both in-hospital mortality as well as 100-day mortality for all admitted oncology patients was obtained from a separate electronic health record database at our institution from a similar time period.
Our goal was to identify patient characteristics, reasons for the RRT activation, and vital sign and laboratory abnormalities in the peri-RRT period that were associated with increased mortality, both inpatient and at 100 days after RRT activation. Our institution’s RRT database and electronic health records were accessed for data collection. Primary outcome variables for this study were inpatient and 100-day mortality post-RRT activation. We investigated the following predictor variables: age, sex, cancer diagnosis, code status at the time of RRT activation, duration from hospital admission to RRT event, length of hospital stay, time of the day the RRT event occurred (daytime vs nighttime), change in level of care (telemetry upgrade and ICU transfer), previous ICU treatment during the same hospital stay, hospice discharge, reasons cited for the RRT event (increased work of breathing, hypotension, tachyarrhythmia, change in mental status, stroke, gastrointestinal bleed, and seizure), peri-RRT lactate level, international normalized ratio (INR), hemoglobin, positive blood cultures, peri-RRT blood product administration, and scores for systemic inflammatory response syndrome (SIRS) and quick sequential organ failure assessment (qSOFA) in the 24 hours preceding the RRT activation. The SIRS includes abnormal temperature (>38°C or <36°C), heart rate of >90 bpm, increased respiratory rate of >20 times/min, and abnormal white blood cell count (>12,000 cells/mm3, <4,000/mm3, or >10% bands). Its score ranges from 0 to 4, based on the number of SIRS criteria documented. The qSOFA includes hypotension (systolic blood pressure of ≤100 mmHg), increased respiratory rate of ≥22 times/min, and altered mentation and ranges from 0 to 3 based on the number of qSOFA score documented.
Descriptive statistics were generated, and we then conducted bivariate analysis using chi-square tests or Fisher exact tests for categorical variables and simple logistic regression for continuous variables. Multivariable logistic regression models were performed to identify predictors of inpatient and 100-day mortality. Regression models were fit separately for subsets defined by the type of cancer diagnosis. Variables with P < .2 were included in the models, and backward selection method was performed, keeping variables with P < .2. The results are presented as odds ratios (OR) and 95% confidence intervals (CI). C-statistics were used to measure goodness of fit for the models. A c-statistic value of 0.5 indicates the model is not better than random chance; a value higher than 0.7 indicates moderate accuracy, whereas a value higher than 0.8 indicates strong accuracy. P < .05 was considered significant. All analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
A total of 179 hospitalized oncology patients had an RRT activation during the 2-year study period during October 2014 through November 2016. During that time, 4,654 medical oncology patients were admitted to the hospital, resulting in a rate of RRT activation of 38.4 events per 1,000 admissions. In all, 179 patients were included in the analyses for inpatient mortality, and 175 patients were included for 100-day mortality post-RRT. Patients with unknown mortality status (n = 4) at 100 days after RRT were excluded from the analyses.
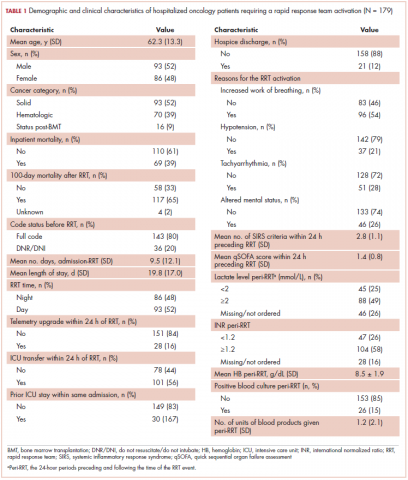
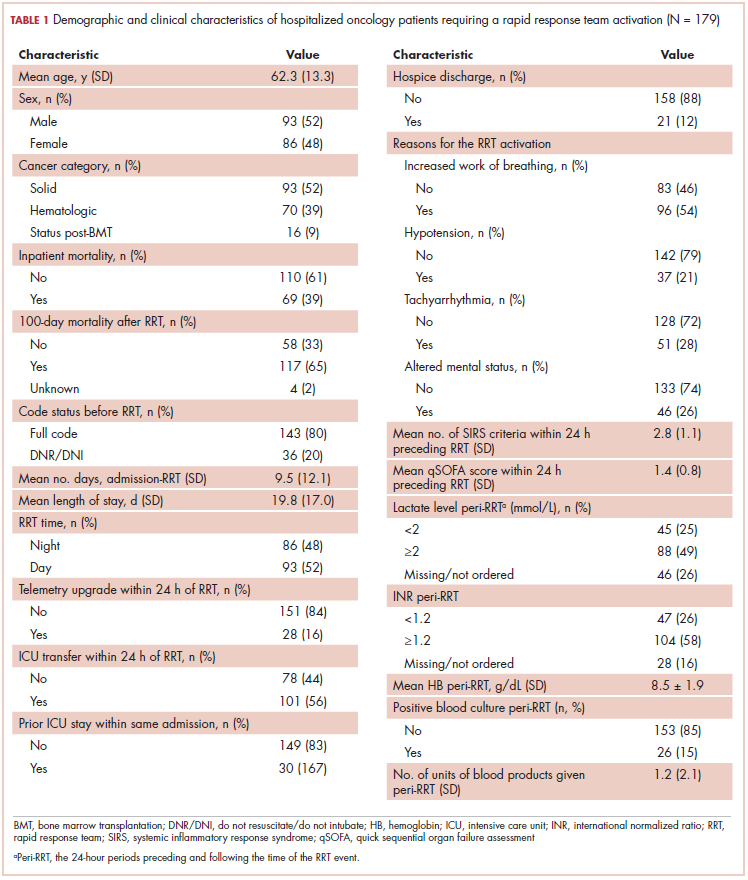
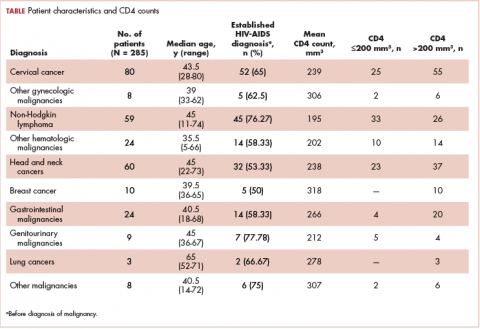
The average age of the study patients was 62.3 years (standard deviation [SD], 13.3; Table 1). They comprised equal proportions of men (52%) and women (48%). Just more than half (52%) of the patients carried a diagnosis of solid malignancy, 39% of hematologic malignancy, and 9% status post-BMT. Most of the patients were full code (80%) at the time of RRT activation. The average number of days from admission to RRT event was 9.5 days (SD, 12.1). Equal proportions of RRT events took place during the daytime (52%) and nighttime (48%), and more than half of the study patients (56%) were transferred to the ICU within 24 hours of the RRT activation. Of all the study patients, 11.7% were discharged to hospice after the RRT event, and 53% required RRT evaluation for increased work of breathing. Forty-nine percent of the total study patients had peri-RRT lactate levels ≥2 mmol/L (reference range, 0.5-2.0 mmol/L), and 58% had peri-RRT INR levels ≥1.2 (reference range, 0.85-1.15). The average SIRS score was 2.8 (SD, 1.1), and the qSOFA score was 1.4 (SD, 0.8) in the 24 hours preceding the RRT activation.
Over the 2-year study period, the inpatient mortality rate for all admitted oncology patients was 2.3% (108 deaths in 4,654 oncology inpatients), according to claims data. By comparison, of the 179 patients who required an RRT activation, 39% did not survive to discharge. When those patients were categorized based on their cancer type, 43% of the solid malignancy patients died within the same hospital stay after an RRT event, 35% of the hematologic malignancy patients died, and 25% of the status post-BMT patients died. Of the 175 patients with known mortality status at 100 days after RRT, 65% of total patients had died within that time compared with only 15.7% (347 deaths in 2,217 patients) of all admitted patients with cancer who did not experience an RRT event. When categorized based on their cancer type, significantly more patients (78%) with solid tumors had died within 100 days after RRT activation, whereas only 55% of those with a hematologic malignancy and 50% of those who were post-BMT died within the same time period.
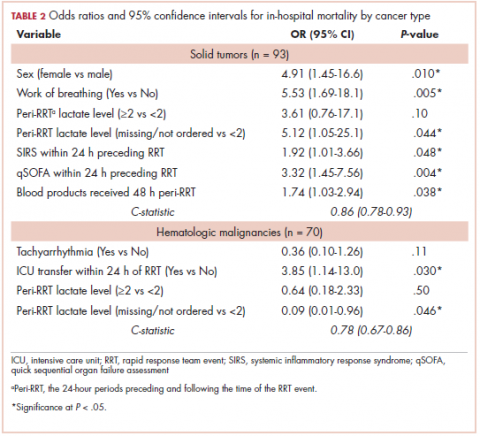
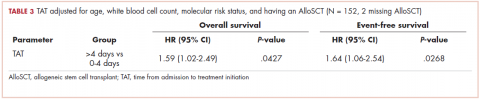
Tables 2 and 3 present major findings from regression models with a moderate to strong level of prediction. The characteristics associated with increased odds of inpatient mortality among solid tumor patients after an RRT event were female sex (OR, 4.91; 95% CI, 1.45-16.6), increased work of breathing as the reason for the RRT activation (OR, 5.53; 95% CI, 1.69-18.1), having no lactate level ordered (OR, 5.12; 95% CI, 1.05-25.1), each unit increase in SIRS score (OR, 1.92; 95% CI, 1.01-3.66), each unit increase in qSOFA score (OR, 3.32; 95% CI, 1.45-7.56), and each unit increase in peri-RRT blood products being given (OR, 1.74; 95% CI, 1.03-2.94). Among hematologic malignancy patients, ICU transfer within 24 hours of the RRT (OR, 3.85; 95% CI, 1.14-13.0) was associated with increased inpatient mortality, whereas having no lactate level ordered (OR, 0.09; 95% CI, 0.01-0.96) was associated with lower odds of inpatient mortality.
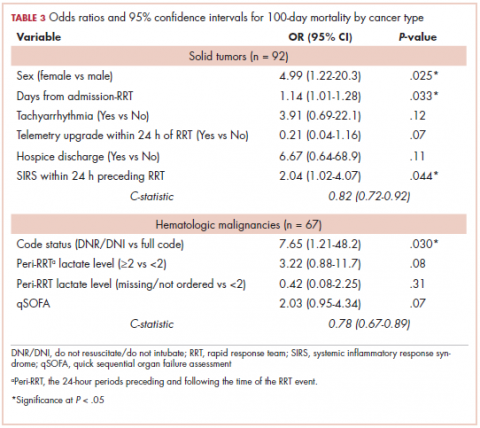
The characteristics associated with increased odds of 100-day mortality in patients with solid tumors were female sex (OR, 4.99; 95% CI, 1.22-20.3), increase in each day from admission to RRT event (OR, 1.14; 95% CI, 1.01-1.18), and each unit increase in SIRS score (OR, 2.04; 95% CI, 1.02-4.07). For hematologic malignancy patients, being do not resuscitate (DNR) or do not intubate (DNI) (OR, 7.65; 95% CI, 1.21-48.2) was associated with increased odds of 100-day mortality.
Discussion
The results of the study highlight the very high mortality rates associated with oncology patients requiring RRT activations, with 39% of patients dying within the same hospital stay and 65% dying within 100 days of the RRT event. These results are particularly notable when contrasted with the 2.3% inpatient and 15.7% 100-day postdischarge mortality rates in the total oncology patient population over a similar time period. The inpatient mortality rate after an RRT activation in our study closely resembled the rate reported by Austin and colleagues, which was 33% (hospital mortality in oncology patients cited during the time was 48.2 deaths per 1,000 patient admissions).17 Of note in our study is that solid tumor patients had higher mortality than the hematologic malignancy patients; 43% died within the same hospital stay and 78% died within 100 days, compared with 35% and 55%, respectively, in patients with hematologic malignancies. The poor prognosis of oncology patients requiring an RRT evaluation must be conveyed to the patients and families and taken into consideration by health care team to determine the most appropriate course of care subsequent to RRT activation.
Our finding that female sex is significantly and strongly associated with increased inpatient and 100-day mortality in patients with solid tumors was unexpected. The cause for this disparity remains elusive. We noted that, in our study, the following types of malignancies were more common in women than men (comparison of women vs men shown in parentheses): lung (53% vs 47%), colon (60% vs 40%), acute lymphoblastic leukemia (83% vs 17%), diffuse large B-cell lymphoma (64% vs 36%), and multiple myeloma (58% vs 42%). Whether these types of cancers are more clinically aggressive and associated with earlier mortality post-RRT could not be ascertained from our data. Gender bias in clinicians’ bedside determination of severity of illness may also play some role in this substantial mortality gap.
Among all the causes for RRT activation, increased work of breathing was the only variable associated with increased inpatient mortality in solid tumor patients. In a study by Austin and colleagues, decreased oxygen saturation was the most common reason for the RRT evaluation, though it did not reach statistical significance as a predictor of inpatient mortality.17 SIRS and qSOFA scores in the 24 hours preceding the RRT event along with peri-RRT blood product administration were all significant predictors of inpatient mortality among patients with solid tumors but were not so for those with hematologic malignancies. It is interesting to note that low hemoglobin was found to be associated with inpatient mortality in a study on 456 hospitalized patients with solid tumors (there was no data on RRT evaluation in their dataset).13 The fact that these well-validated measurements of illness severity correlate positively with RRT activation and increased mortality is intuitive and lends external credibility to other findings in this study.
In patients with hematologic malignancies, ICU transfers within 24 hours of the RRT activation were associated with 4-fold increased odds of inpatient death. This was not shown to be the case in patients with solid tumors. This should be explored in future studies because it could be crucial in conducting goals-of-care discussions in terminally ill cancer patients. The study also showed that patients with hematologic malignancies who were DNR or DNI were associated with almost 8-fold increased odds of 100-day mortality. This argues for a fair predictive ability of the care teams in this particular subgroup. Conversely, hospice referral is underused; of the patients that died at 100 days after the RRT event, only 16.2% were referred to hospice at the time of discharge.
Limitations
Limitations of the study include its retrospective nature at a single medical center on a small group of study participants. Variables such as lactate dehydrogenase level and Eastern Conference Oncology Group Performance Status, which have been found to be predictive of increased mortality in hospitalized oncology patients,19 were not consistently available for analysis in the data set. We had 4 patients whose mortality status was not known at 100 days and were excluded from the study. Because of a lack of documentation, we were also not able to reliably collect the data on patients with multiple RRT events. This presumably would be associated with increased mortality on its own. We only included the data associated with the earliest RRT activation in our electronic health records.
In addition, it is important to note that 26% and 16% of the study patients had missing lactate and INR values, respectively. Given the small size of the study and the unclear significance of the missing lactate and INR, we opted to include the patients with the missing data for final analyses of the regression models. The significance of a care team not ordering a lactate level is perhaps associated with the reason for RRT activation (ie, the patient seemed to be less ill) and perhaps could be associated with non–sepsis-related RRT events.
Conclusions
This study reports on the outcomes of oncology patients admitted to the hospital whose clinical deterioration required activation of a rapid response team. Female sex, increased qSOFA and SIRS scores in the 24 hours preceding the RRT event, and the need for blood product administrations around the time of the RRT event correlated with increased inpatient mortality. Hospitalized oncology patients’ d undestood and response evaluation if perPatientoutcomes, both regarding inpatient and 100-day mortality, demonstrated surprisingly poor survival, with solid malignancy patients bearing significantly higher burden of both inpatient mortality and mortality at 100 days after the RRT event. The findings from the study could help patients, families, and providers make informed decisions regarding advance care and end-of-life planning for terminally ill cancer patients.
The Cancer Center Support Grant 5P30CA056036-17 and the Biostatistics Shared Resource and Thomas Jefferson University Hospital’s Rapid Response Team (RRT) committee.
1. National Center for Health Statistics. Health, United States, 2016: with Chartbook on long-term trends in health. Hyattsville, MD: National Center for Health Statistics; 2017.
2. Lambden J, Zhang B, Friedlander R, Prigerson HG. Accuracy of oncologists’ life-expectancy estimates recalled by their advanced cancer patients: correlates and outcomes. J Palliat Med. 2016;19(12):1296-1303.
3. Maltoni M, Caraceni A, Brunelli C, et al. Prognostic factors in advanced cancer patients: evidence-based clinical recommendations—a study by the Steering Committee of the European Association for Palliative Care. J Clin Oncol. 2005;23(25):6240-6248.
4. Viganó A, Bruera E, Jhangri GS, Newman SC, Fields AL, Suarez-Almazor ME. Clinical survival predictors in patients with advanced cancer. Arch Intern Med. 2000;160(6):861-868.
5. Hauser CA, Stockler MR, Tattersall MH. Prognostic factors in patients with recently diagnosed incurable cancer: a systematic review. Support Care Cancer. 2006;14(10):999-1011.
6. Al-Zahrani AS, El-Kashif AT, Mohammad AA, Elsamany S, Alsirafy SA. Prediction of in-hospital mortality of patients with advanced cancer using the Chuang Prognostic Score. Am J Hosp Palliat Med. 2013;30(7):707-711.
7. Hui D, Kilgore K, Fellman B, et al. Development and cross-validation of the in-hospital mortality prediction in advanced cancer patients score: a preliminary study. J Palliat Med. 2012;15(8):902-909.
8. Shouval R, Labopin M, Bondi O, et al. Prediction of allogeneic hematopoietic stem-cell transplantation mortality 100 days after transplantation using a machine learning algorithm: a European group for blood and marrow transplantation acute leukemia working party retrospective data mining study. J Clin Oncol. 2015;33(28):3144-3151.
9. Agency for Healthcare Research and Quality. Total expenses and percent distribution for selected conditions by type of service: United States, 2013. Medical Expenditure Panel Survey website. https://meps.ahrq.gov/mepsweb/data_stats/tables_compendia_hh_interactive.jsp?_SERVICE=MEPSSocket0&_PROGRAM=MEPSPGM.TC.SAS&File=HCFY2013&Table=HCFY2012_CNDXP_C&_Debug=. Accessed November 10, 2018.
10. McCall N. Utilization and costs of Medicare services by beneficiaries in their last year of life. Med Care. 1984;22(4):329-342.
11. Jones D, Moran J, Winters B, Welch J. The rapid response system and end-of-life care. Curr Opin Crit Care. 2013;19(6):616-623.
12. Solomon RS, Corwin GS, Barclay DC, Quddusi SF, Dannenberg MD. Effectiveness of rapid response teams on rates of in‐hospital cardiopulmonary arrest and mortality: a systematic review and meta‐analysis. J Hosp Med. 2016;11(6):438-445.
13. Jung B, Daurat A, De Jong A, et al. Rapid response team and hospital mortality in hospitalized patients. Intensive Care Med. 2016;42(4):494-504.
14. Sulistio M, Franco M, Vo A, Poon P, William L. Hospital rapid response team and patients with life-limiting illness: a multicentre retrospective cohort study. Palliat Med. 2015;29(4):302-309.
15. Wang J, Hahn SS, Kline M, Cohen RI. Early in-hospital clinical deterioration is not predicted by severity of illness, functional status, or comorbidity. Int J Gen Med. 2017;10:329-334.
16. Dargin JM, Mackey CG, Lei Y, Liesching TN. Resource utilization and end‐of‐life care in a US hospital following medical emergency team‐implemented do not resuscitate orders. J Hosp Med. 2014;9(6):372-378.
17. Austin CA, Hanzaker C, Stafford R, et al. Utilization of rapid response resources and outcomes in a comprehensive cancer center. Crit Care Med. 2014;42(4):905-909.
18. Smith RL, Hayashi VN, Lee YI, Navarro-Mariazeta L, Felner K. The medical emergency team call: a sentinel event that triggers goals of care discussion. Crit Care Med. 2014;42(2):322-327.
19. Bozcuk H, Koyuncu E, Yildiz M, et al. A simple and accurate prediction model to estimate the intrahospital mortality risk of hospitalised cancer patients. Int J Clin Pract. 2004;58(11):1014-1019.
Cancer is the second leading cause of death in the United States, exceeded only by heart disease.1 Despite the overall decline in cancer death rates from 2000 through 2014, physicians struggle to accurately predict disease progression and mortality in patients with cancer who are within 6 months of death.2-8 This prognostic uncertainty makes clinical decision making difficult for patients, families, and health care providers. On a health care system level, an insight into end-of-life prognostication could also have substantial financial implications. In 2013, $74 billion was spent on cancer-related health care in the United States.9 Studies have shown that from 5% to 6% of Medicare beneficiaries with cancer consumed up to 30% of the annual Medicare payments, with a staggering 78% of costs being from acute care in the final 30 days of life.10
Rapid response teams (RRTs) were first introduced in 1995 and are now widely used at many hospitals to identify and provide critical care at the bedside of deteriorating patients outside of the intensive care unit (ICU) to prevent morbidity and mortality.11-15 Although not the original aim, RRTs are commonly activated on patients at the end of life and have therefore come to play an important role in end-of-life care.11,16 RRT activation in the oncology population is of special interest because the activation may predict higher inpatient mortality.17 In addition, RRT activation can serve as a sentinel event that fosters discussion on goals of care, change in code status, and initiation of palliative care or hospice use, particularly when also accompanied by an upgrade in level of care.11,18 As such, the ability to predict mortality after an RRT event, both inpatient and at 100 days after the event, could be of great help in deciding whether to pursue further treatments or, alternatively, palliative or hospice care.
To that end, the purpose of this study was to identify baseline patient characteristics, causes of deterioration leading to the RRT event, and vital signs and laboratory abnormalities in the peri-RRT period –
Methods and materials
A retrospective study was performed at a single, 900+ bed academic center in the northeastern United States during a 2-year study period from October 2014 through November 2016. The Institutional Review Board at Thomas Jefferson University Hospital in Philadelphia, Pennsylvania, reviewed and approved the study.
Through our institution’s RRT database, all consecutive RRT activations during the study period involving hospitalized oncology patients were reviewed. We included patients 18 years or older with a cancer diagnosis, including solid tumor and hematologic malignancy, as well as those who were status post–bone marrow transplantation (BMT), who required rapid response activation while hospitalized at our institution. We excluded patients who activated rapid response while they were in the ICU, including the BMT unit, those on the surgical floors, and those with RRT activation at other hospitals before transfer to our institution. Data for both in-hospital mortality as well as 100-day mortality for all admitted oncology patients was obtained from a separate electronic health record database at our institution from a similar time period.
Our goal was to identify patient characteristics, reasons for the RRT activation, and vital sign and laboratory abnormalities in the peri-RRT period that were associated with increased mortality, both inpatient and at 100 days after RRT activation. Our institution’s RRT database and electronic health records were accessed for data collection. Primary outcome variables for this study were inpatient and 100-day mortality post-RRT activation. We investigated the following predictor variables: age, sex, cancer diagnosis, code status at the time of RRT activation, duration from hospital admission to RRT event, length of hospital stay, time of the day the RRT event occurred (daytime vs nighttime), change in level of care (telemetry upgrade and ICU transfer), previous ICU treatment during the same hospital stay, hospice discharge, reasons cited for the RRT event (increased work of breathing, hypotension, tachyarrhythmia, change in mental status, stroke, gastrointestinal bleed, and seizure), peri-RRT lactate level, international normalized ratio (INR), hemoglobin, positive blood cultures, peri-RRT blood product administration, and scores for systemic inflammatory response syndrome (SIRS) and quick sequential organ failure assessment (qSOFA) in the 24 hours preceding the RRT activation. The SIRS includes abnormal temperature (>38°C or <36°C), heart rate of >90 bpm, increased respiratory rate of >20 times/min, and abnormal white blood cell count (>12,000 cells/mm3, <4,000/mm3, or >10% bands). Its score ranges from 0 to 4, based on the number of SIRS criteria documented. The qSOFA includes hypotension (systolic blood pressure of ≤100 mmHg), increased respiratory rate of ≥22 times/min, and altered mentation and ranges from 0 to 3 based on the number of qSOFA score documented.
Descriptive statistics were generated, and we then conducted bivariate analysis using chi-square tests or Fisher exact tests for categorical variables and simple logistic regression for continuous variables. Multivariable logistic regression models were performed to identify predictors of inpatient and 100-day mortality. Regression models were fit separately for subsets defined by the type of cancer diagnosis. Variables with P < .2 were included in the models, and backward selection method was performed, keeping variables with P < .2. The results are presented as odds ratios (OR) and 95% confidence intervals (CI). C-statistics were used to measure goodness of fit for the models. A c-statistic value of 0.5 indicates the model is not better than random chance; a value higher than 0.7 indicates moderate accuracy, whereas a value higher than 0.8 indicates strong accuracy. P < .05 was considered significant. All analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
A total of 179 hospitalized oncology patients had an RRT activation during the 2-year study period during October 2014 through November 2016. During that time, 4,654 medical oncology patients were admitted to the hospital, resulting in a rate of RRT activation of 38.4 events per 1,000 admissions. In all, 179 patients were included in the analyses for inpatient mortality, and 175 patients were included for 100-day mortality post-RRT. Patients with unknown mortality status (n = 4) at 100 days after RRT were excluded from the analyses.
The average age of the study patients was 62.3 years (standard deviation [SD], 13.3; Table 1). They comprised equal proportions of men (52%) and women (48%). Just more than half (52%) of the patients carried a diagnosis of solid malignancy, 39% of hematologic malignancy, and 9% status post-BMT. Most of the patients were full code (80%) at the time of RRT activation. The average number of days from admission to RRT event was 9.5 days (SD, 12.1). Equal proportions of RRT events took place during the daytime (52%) and nighttime (48%), and more than half of the study patients (56%) were transferred to the ICU within 24 hours of the RRT activation. Of all the study patients, 11.7% were discharged to hospice after the RRT event, and 53% required RRT evaluation for increased work of breathing. Forty-nine percent of the total study patients had peri-RRT lactate levels ≥2 mmol/L (reference range, 0.5-2.0 mmol/L), and 58% had peri-RRT INR levels ≥1.2 (reference range, 0.85-1.15). The average SIRS score was 2.8 (SD, 1.1), and the qSOFA score was 1.4 (SD, 0.8) in the 24 hours preceding the RRT activation.
Over the 2-year study period, the inpatient mortality rate for all admitted oncology patients was 2.3% (108 deaths in 4,654 oncology inpatients), according to claims data. By comparison, of the 179 patients who required an RRT activation, 39% did not survive to discharge. When those patients were categorized based on their cancer type, 43% of the solid malignancy patients died within the same hospital stay after an RRT event, 35% of the hematologic malignancy patients died, and 25% of the status post-BMT patients died. Of the 175 patients with known mortality status at 100 days after RRT, 65% of total patients had died within that time compared with only 15.7% (347 deaths in 2,217 patients) of all admitted patients with cancer who did not experience an RRT event. When categorized based on their cancer type, significantly more patients (78%) with solid tumors had died within 100 days after RRT activation, whereas only 55% of those with a hematologic malignancy and 50% of those who were post-BMT died within the same time period.
Tables 2 and 3 present major findings from regression models with a moderate to strong level of prediction. The characteristics associated with increased odds of inpatient mortality among solid tumor patients after an RRT event were female sex (OR, 4.91; 95% CI, 1.45-16.6), increased work of breathing as the reason for the RRT activation (OR, 5.53; 95% CI, 1.69-18.1), having no lactate level ordered (OR, 5.12; 95% CI, 1.05-25.1), each unit increase in SIRS score (OR, 1.92; 95% CI, 1.01-3.66), each unit increase in qSOFA score (OR, 3.32; 95% CI, 1.45-7.56), and each unit increase in peri-RRT blood products being given (OR, 1.74; 95% CI, 1.03-2.94). Among hematologic malignancy patients, ICU transfer within 24 hours of the RRT (OR, 3.85; 95% CI, 1.14-13.0) was associated with increased inpatient mortality, whereas having no lactate level ordered (OR, 0.09; 95% CI, 0.01-0.96) was associated with lower odds of inpatient mortality.
The characteristics associated with increased odds of 100-day mortality in patients with solid tumors were female sex (OR, 4.99; 95% CI, 1.22-20.3), increase in each day from admission to RRT event (OR, 1.14; 95% CI, 1.01-1.18), and each unit increase in SIRS score (OR, 2.04; 95% CI, 1.02-4.07). For hematologic malignancy patients, being do not resuscitate (DNR) or do not intubate (DNI) (OR, 7.65; 95% CI, 1.21-48.2) was associated with increased odds of 100-day mortality.
Discussion
The results of the study highlight the very high mortality rates associated with oncology patients requiring RRT activations, with 39% of patients dying within the same hospital stay and 65% dying within 100 days of the RRT event. These results are particularly notable when contrasted with the 2.3% inpatient and 15.7% 100-day postdischarge mortality rates in the total oncology patient population over a similar time period. The inpatient mortality rate after an RRT activation in our study closely resembled the rate reported by Austin and colleagues, which was 33% (hospital mortality in oncology patients cited during the time was 48.2 deaths per 1,000 patient admissions).17 Of note in our study is that solid tumor patients had higher mortality than the hematologic malignancy patients; 43% died within the same hospital stay and 78% died within 100 days, compared with 35% and 55%, respectively, in patients with hematologic malignancies. The poor prognosis of oncology patients requiring an RRT evaluation must be conveyed to the patients and families and taken into consideration by health care team to determine the most appropriate course of care subsequent to RRT activation.
Our finding that female sex is significantly and strongly associated with increased inpatient and 100-day mortality in patients with solid tumors was unexpected. The cause for this disparity remains elusive. We noted that, in our study, the following types of malignancies were more common in women than men (comparison of women vs men shown in parentheses): lung (53% vs 47%), colon (60% vs 40%), acute lymphoblastic leukemia (83% vs 17%), diffuse large B-cell lymphoma (64% vs 36%), and multiple myeloma (58% vs 42%). Whether these types of cancers are more clinically aggressive and associated with earlier mortality post-RRT could not be ascertained from our data. Gender bias in clinicians’ bedside determination of severity of illness may also play some role in this substantial mortality gap.
Among all the causes for RRT activation, increased work of breathing was the only variable associated with increased inpatient mortality in solid tumor patients. In a study by Austin and colleagues, decreased oxygen saturation was the most common reason for the RRT evaluation, though it did not reach statistical significance as a predictor of inpatient mortality.17 SIRS and qSOFA scores in the 24 hours preceding the RRT event along with peri-RRT blood product administration were all significant predictors of inpatient mortality among patients with solid tumors but were not so for those with hematologic malignancies. It is interesting to note that low hemoglobin was found to be associated with inpatient mortality in a study on 456 hospitalized patients with solid tumors (there was no data on RRT evaluation in their dataset).13 The fact that these well-validated measurements of illness severity correlate positively with RRT activation and increased mortality is intuitive and lends external credibility to other findings in this study.
In patients with hematologic malignancies, ICU transfers within 24 hours of the RRT activation were associated with 4-fold increased odds of inpatient death. This was not shown to be the case in patients with solid tumors. This should be explored in future studies because it could be crucial in conducting goals-of-care discussions in terminally ill cancer patients. The study also showed that patients with hematologic malignancies who were DNR or DNI were associated with almost 8-fold increased odds of 100-day mortality. This argues for a fair predictive ability of the care teams in this particular subgroup. Conversely, hospice referral is underused; of the patients that died at 100 days after the RRT event, only 16.2% were referred to hospice at the time of discharge.
Limitations
Limitations of the study include its retrospective nature at a single medical center on a small group of study participants. Variables such as lactate dehydrogenase level and Eastern Conference Oncology Group Performance Status, which have been found to be predictive of increased mortality in hospitalized oncology patients,19 were not consistently available for analysis in the data set. We had 4 patients whose mortality status was not known at 100 days and were excluded from the study. Because of a lack of documentation, we were also not able to reliably collect the data on patients with multiple RRT events. This presumably would be associated with increased mortality on its own. We only included the data associated with the earliest RRT activation in our electronic health records.
In addition, it is important to note that 26% and 16% of the study patients had missing lactate and INR values, respectively. Given the small size of the study and the unclear significance of the missing lactate and INR, we opted to include the patients with the missing data for final analyses of the regression models. The significance of a care team not ordering a lactate level is perhaps associated with the reason for RRT activation (ie, the patient seemed to be less ill) and perhaps could be associated with non–sepsis-related RRT events.
Conclusions
This study reports on the outcomes of oncology patients admitted to the hospital whose clinical deterioration required activation of a rapid response team. Female sex, increased qSOFA and SIRS scores in the 24 hours preceding the RRT event, and the need for blood product administrations around the time of the RRT event correlated with increased inpatient mortality. Hospitalized oncology patients’ d undestood and response evaluation if perPatientoutcomes, both regarding inpatient and 100-day mortality, demonstrated surprisingly poor survival, with solid malignancy patients bearing significantly higher burden of both inpatient mortality and mortality at 100 days after the RRT event. The findings from the study could help patients, families, and providers make informed decisions regarding advance care and end-of-life planning for terminally ill cancer patients.
The Cancer Center Support Grant 5P30CA056036-17 and the Biostatistics Shared Resource and Thomas Jefferson University Hospital’s Rapid Response Team (RRT) committee.
Cancer is the second leading cause of death in the United States, exceeded only by heart disease.1 Despite the overall decline in cancer death rates from 2000 through 2014, physicians struggle to accurately predict disease progression and mortality in patients with cancer who are within 6 months of death.2-8 This prognostic uncertainty makes clinical decision making difficult for patients, families, and health care providers. On a health care system level, an insight into end-of-life prognostication could also have substantial financial implications. In 2013, $74 billion was spent on cancer-related health care in the United States.9 Studies have shown that from 5% to 6% of Medicare beneficiaries with cancer consumed up to 30% of the annual Medicare payments, with a staggering 78% of costs being from acute care in the final 30 days of life.10
Rapid response teams (RRTs) were first introduced in 1995 and are now widely used at many hospitals to identify and provide critical care at the bedside of deteriorating patients outside of the intensive care unit (ICU) to prevent morbidity and mortality.11-15 Although not the original aim, RRTs are commonly activated on patients at the end of life and have therefore come to play an important role in end-of-life care.11,16 RRT activation in the oncology population is of special interest because the activation may predict higher inpatient mortality.17 In addition, RRT activation can serve as a sentinel event that fosters discussion on goals of care, change in code status, and initiation of palliative care or hospice use, particularly when also accompanied by an upgrade in level of care.11,18 As such, the ability to predict mortality after an RRT event, both inpatient and at 100 days after the event, could be of great help in deciding whether to pursue further treatments or, alternatively, palliative or hospice care.
To that end, the purpose of this study was to identify baseline patient characteristics, causes of deterioration leading to the RRT event, and vital signs and laboratory abnormalities in the peri-RRT period –
Methods and materials
A retrospective study was performed at a single, 900+ bed academic center in the northeastern United States during a 2-year study period from October 2014 through November 2016. The Institutional Review Board at Thomas Jefferson University Hospital in Philadelphia, Pennsylvania, reviewed and approved the study.
Through our institution’s RRT database, all consecutive RRT activations during the study period involving hospitalized oncology patients were reviewed. We included patients 18 years or older with a cancer diagnosis, including solid tumor and hematologic malignancy, as well as those who were status post–bone marrow transplantation (BMT), who required rapid response activation while hospitalized at our institution. We excluded patients who activated rapid response while they were in the ICU, including the BMT unit, those on the surgical floors, and those with RRT activation at other hospitals before transfer to our institution. Data for both in-hospital mortality as well as 100-day mortality for all admitted oncology patients was obtained from a separate electronic health record database at our institution from a similar time period.
Our goal was to identify patient characteristics, reasons for the RRT activation, and vital sign and laboratory abnormalities in the peri-RRT period that were associated with increased mortality, both inpatient and at 100 days after RRT activation. Our institution’s RRT database and electronic health records were accessed for data collection. Primary outcome variables for this study were inpatient and 100-day mortality post-RRT activation. We investigated the following predictor variables: age, sex, cancer diagnosis, code status at the time of RRT activation, duration from hospital admission to RRT event, length of hospital stay, time of the day the RRT event occurred (daytime vs nighttime), change in level of care (telemetry upgrade and ICU transfer), previous ICU treatment during the same hospital stay, hospice discharge, reasons cited for the RRT event (increased work of breathing, hypotension, tachyarrhythmia, change in mental status, stroke, gastrointestinal bleed, and seizure), peri-RRT lactate level, international normalized ratio (INR), hemoglobin, positive blood cultures, peri-RRT blood product administration, and scores for systemic inflammatory response syndrome (SIRS) and quick sequential organ failure assessment (qSOFA) in the 24 hours preceding the RRT activation. The SIRS includes abnormal temperature (>38°C or <36°C), heart rate of >90 bpm, increased respiratory rate of >20 times/min, and abnormal white blood cell count (>12,000 cells/mm3, <4,000/mm3, or >10% bands). Its score ranges from 0 to 4, based on the number of SIRS criteria documented. The qSOFA includes hypotension (systolic blood pressure of ≤100 mmHg), increased respiratory rate of ≥22 times/min, and altered mentation and ranges from 0 to 3 based on the number of qSOFA score documented.
Descriptive statistics were generated, and we then conducted bivariate analysis using chi-square tests or Fisher exact tests for categorical variables and simple logistic regression for continuous variables. Multivariable logistic regression models were performed to identify predictors of inpatient and 100-day mortality. Regression models were fit separately for subsets defined by the type of cancer diagnosis. Variables with P < .2 were included in the models, and backward selection method was performed, keeping variables with P < .2. The results are presented as odds ratios (OR) and 95% confidence intervals (CI). C-statistics were used to measure goodness of fit for the models. A c-statistic value of 0.5 indicates the model is not better than random chance; a value higher than 0.7 indicates moderate accuracy, whereas a value higher than 0.8 indicates strong accuracy. P < .05 was considered significant. All analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
A total of 179 hospitalized oncology patients had an RRT activation during the 2-year study period during October 2014 through November 2016. During that time, 4,654 medical oncology patients were admitted to the hospital, resulting in a rate of RRT activation of 38.4 events per 1,000 admissions. In all, 179 patients were included in the analyses for inpatient mortality, and 175 patients were included for 100-day mortality post-RRT. Patients with unknown mortality status (n = 4) at 100 days after RRT were excluded from the analyses.
The average age of the study patients was 62.3 years (standard deviation [SD], 13.3; Table 1). They comprised equal proportions of men (52%) and women (48%). Just more than half (52%) of the patients carried a diagnosis of solid malignancy, 39% of hematologic malignancy, and 9% status post-BMT. Most of the patients were full code (80%) at the time of RRT activation. The average number of days from admission to RRT event was 9.5 days (SD, 12.1). Equal proportions of RRT events took place during the daytime (52%) and nighttime (48%), and more than half of the study patients (56%) were transferred to the ICU within 24 hours of the RRT activation. Of all the study patients, 11.7% were discharged to hospice after the RRT event, and 53% required RRT evaluation for increased work of breathing. Forty-nine percent of the total study patients had peri-RRT lactate levels ≥2 mmol/L (reference range, 0.5-2.0 mmol/L), and 58% had peri-RRT INR levels ≥1.2 (reference range, 0.85-1.15). The average SIRS score was 2.8 (SD, 1.1), and the qSOFA score was 1.4 (SD, 0.8) in the 24 hours preceding the RRT activation.
Over the 2-year study period, the inpatient mortality rate for all admitted oncology patients was 2.3% (108 deaths in 4,654 oncology inpatients), according to claims data. By comparison, of the 179 patients who required an RRT activation, 39% did not survive to discharge. When those patients were categorized based on their cancer type, 43% of the solid malignancy patients died within the same hospital stay after an RRT event, 35% of the hematologic malignancy patients died, and 25% of the status post-BMT patients died. Of the 175 patients with known mortality status at 100 days after RRT, 65% of total patients had died within that time compared with only 15.7% (347 deaths in 2,217 patients) of all admitted patients with cancer who did not experience an RRT event. When categorized based on their cancer type, significantly more patients (78%) with solid tumors had died within 100 days after RRT activation, whereas only 55% of those with a hematologic malignancy and 50% of those who were post-BMT died within the same time period.
Tables 2 and 3 present major findings from regression models with a moderate to strong level of prediction. The characteristics associated with increased odds of inpatient mortality among solid tumor patients after an RRT event were female sex (OR, 4.91; 95% CI, 1.45-16.6), increased work of breathing as the reason for the RRT activation (OR, 5.53; 95% CI, 1.69-18.1), having no lactate level ordered (OR, 5.12; 95% CI, 1.05-25.1), each unit increase in SIRS score (OR, 1.92; 95% CI, 1.01-3.66), each unit increase in qSOFA score (OR, 3.32; 95% CI, 1.45-7.56), and each unit increase in peri-RRT blood products being given (OR, 1.74; 95% CI, 1.03-2.94). Among hematologic malignancy patients, ICU transfer within 24 hours of the RRT (OR, 3.85; 95% CI, 1.14-13.0) was associated with increased inpatient mortality, whereas having no lactate level ordered (OR, 0.09; 95% CI, 0.01-0.96) was associated with lower odds of inpatient mortality.
The characteristics associated with increased odds of 100-day mortality in patients with solid tumors were female sex (OR, 4.99; 95% CI, 1.22-20.3), increase in each day from admission to RRT event (OR, 1.14; 95% CI, 1.01-1.18), and each unit increase in SIRS score (OR, 2.04; 95% CI, 1.02-4.07). For hematologic malignancy patients, being do not resuscitate (DNR) or do not intubate (DNI) (OR, 7.65; 95% CI, 1.21-48.2) was associated with increased odds of 100-day mortality.
Discussion
The results of the study highlight the very high mortality rates associated with oncology patients requiring RRT activations, with 39% of patients dying within the same hospital stay and 65% dying within 100 days of the RRT event. These results are particularly notable when contrasted with the 2.3% inpatient and 15.7% 100-day postdischarge mortality rates in the total oncology patient population over a similar time period. The inpatient mortality rate after an RRT activation in our study closely resembled the rate reported by Austin and colleagues, which was 33% (hospital mortality in oncology patients cited during the time was 48.2 deaths per 1,000 patient admissions).17 Of note in our study is that solid tumor patients had higher mortality than the hematologic malignancy patients; 43% died within the same hospital stay and 78% died within 100 days, compared with 35% and 55%, respectively, in patients with hematologic malignancies. The poor prognosis of oncology patients requiring an RRT evaluation must be conveyed to the patients and families and taken into consideration by health care team to determine the most appropriate course of care subsequent to RRT activation.
Our finding that female sex is significantly and strongly associated with increased inpatient and 100-day mortality in patients with solid tumors was unexpected. The cause for this disparity remains elusive. We noted that, in our study, the following types of malignancies were more common in women than men (comparison of women vs men shown in parentheses): lung (53% vs 47%), colon (60% vs 40%), acute lymphoblastic leukemia (83% vs 17%), diffuse large B-cell lymphoma (64% vs 36%), and multiple myeloma (58% vs 42%). Whether these types of cancers are more clinically aggressive and associated with earlier mortality post-RRT could not be ascertained from our data. Gender bias in clinicians’ bedside determination of severity of illness may also play some role in this substantial mortality gap.
Among all the causes for RRT activation, increased work of breathing was the only variable associated with increased inpatient mortality in solid tumor patients. In a study by Austin and colleagues, decreased oxygen saturation was the most common reason for the RRT evaluation, though it did not reach statistical significance as a predictor of inpatient mortality.17 SIRS and qSOFA scores in the 24 hours preceding the RRT event along with peri-RRT blood product administration were all significant predictors of inpatient mortality among patients with solid tumors but were not so for those with hematologic malignancies. It is interesting to note that low hemoglobin was found to be associated with inpatient mortality in a study on 456 hospitalized patients with solid tumors (there was no data on RRT evaluation in their dataset).13 The fact that these well-validated measurements of illness severity correlate positively with RRT activation and increased mortality is intuitive and lends external credibility to other findings in this study.
In patients with hematologic malignancies, ICU transfers within 24 hours of the RRT activation were associated with 4-fold increased odds of inpatient death. This was not shown to be the case in patients with solid tumors. This should be explored in future studies because it could be crucial in conducting goals-of-care discussions in terminally ill cancer patients. The study also showed that patients with hematologic malignancies who were DNR or DNI were associated with almost 8-fold increased odds of 100-day mortality. This argues for a fair predictive ability of the care teams in this particular subgroup. Conversely, hospice referral is underused; of the patients that died at 100 days after the RRT event, only 16.2% were referred to hospice at the time of discharge.
Limitations
Limitations of the study include its retrospective nature at a single medical center on a small group of study participants. Variables such as lactate dehydrogenase level and Eastern Conference Oncology Group Performance Status, which have been found to be predictive of increased mortality in hospitalized oncology patients,19 were not consistently available for analysis in the data set. We had 4 patients whose mortality status was not known at 100 days and were excluded from the study. Because of a lack of documentation, we were also not able to reliably collect the data on patients with multiple RRT events. This presumably would be associated with increased mortality on its own. We only included the data associated with the earliest RRT activation in our electronic health records.
In addition, it is important to note that 26% and 16% of the study patients had missing lactate and INR values, respectively. Given the small size of the study and the unclear significance of the missing lactate and INR, we opted to include the patients with the missing data for final analyses of the regression models. The significance of a care team not ordering a lactate level is perhaps associated with the reason for RRT activation (ie, the patient seemed to be less ill) and perhaps could be associated with non–sepsis-related RRT events.
Conclusions
This study reports on the outcomes of oncology patients admitted to the hospital whose clinical deterioration required activation of a rapid response team. Female sex, increased qSOFA and SIRS scores in the 24 hours preceding the RRT event, and the need for blood product administrations around the time of the RRT event correlated with increased inpatient mortality. Hospitalized oncology patients’ d undestood and response evaluation if perPatientoutcomes, both regarding inpatient and 100-day mortality, demonstrated surprisingly poor survival, with solid malignancy patients bearing significantly higher burden of both inpatient mortality and mortality at 100 days after the RRT event. The findings from the study could help patients, families, and providers make informed decisions regarding advance care and end-of-life planning for terminally ill cancer patients.
The Cancer Center Support Grant 5P30CA056036-17 and the Biostatistics Shared Resource and Thomas Jefferson University Hospital’s Rapid Response Team (RRT) committee.
1. National Center for Health Statistics. Health, United States, 2016: with Chartbook on long-term trends in health. Hyattsville, MD: National Center for Health Statistics; 2017.
2. Lambden J, Zhang B, Friedlander R, Prigerson HG. Accuracy of oncologists’ life-expectancy estimates recalled by their advanced cancer patients: correlates and outcomes. J Palliat Med. 2016;19(12):1296-1303.
3. Maltoni M, Caraceni A, Brunelli C, et al. Prognostic factors in advanced cancer patients: evidence-based clinical recommendations—a study by the Steering Committee of the European Association for Palliative Care. J Clin Oncol. 2005;23(25):6240-6248.
4. Viganó A, Bruera E, Jhangri GS, Newman SC, Fields AL, Suarez-Almazor ME. Clinical survival predictors in patients with advanced cancer. Arch Intern Med. 2000;160(6):861-868.
5. Hauser CA, Stockler MR, Tattersall MH. Prognostic factors in patients with recently diagnosed incurable cancer: a systematic review. Support Care Cancer. 2006;14(10):999-1011.
6. Al-Zahrani AS, El-Kashif AT, Mohammad AA, Elsamany S, Alsirafy SA. Prediction of in-hospital mortality of patients with advanced cancer using the Chuang Prognostic Score. Am J Hosp Palliat Med. 2013;30(7):707-711.
7. Hui D, Kilgore K, Fellman B, et al. Development and cross-validation of the in-hospital mortality prediction in advanced cancer patients score: a preliminary study. J Palliat Med. 2012;15(8):902-909.
8. Shouval R, Labopin M, Bondi O, et al. Prediction of allogeneic hematopoietic stem-cell transplantation mortality 100 days after transplantation using a machine learning algorithm: a European group for blood and marrow transplantation acute leukemia working party retrospective data mining study. J Clin Oncol. 2015;33(28):3144-3151.
9. Agency for Healthcare Research and Quality. Total expenses and percent distribution for selected conditions by type of service: United States, 2013. Medical Expenditure Panel Survey website. https://meps.ahrq.gov/mepsweb/data_stats/tables_compendia_hh_interactive.jsp?_SERVICE=MEPSSocket0&_PROGRAM=MEPSPGM.TC.SAS&File=HCFY2013&Table=HCFY2012_CNDXP_C&_Debug=. Accessed November 10, 2018.
10. McCall N. Utilization and costs of Medicare services by beneficiaries in their last year of life. Med Care. 1984;22(4):329-342.
11. Jones D, Moran J, Winters B, Welch J. The rapid response system and end-of-life care. Curr Opin Crit Care. 2013;19(6):616-623.
12. Solomon RS, Corwin GS, Barclay DC, Quddusi SF, Dannenberg MD. Effectiveness of rapid response teams on rates of in‐hospital cardiopulmonary arrest and mortality: a systematic review and meta‐analysis. J Hosp Med. 2016;11(6):438-445.
13. Jung B, Daurat A, De Jong A, et al. Rapid response team and hospital mortality in hospitalized patients. Intensive Care Med. 2016;42(4):494-504.
14. Sulistio M, Franco M, Vo A, Poon P, William L. Hospital rapid response team and patients with life-limiting illness: a multicentre retrospective cohort study. Palliat Med. 2015;29(4):302-309.
15. Wang J, Hahn SS, Kline M, Cohen RI. Early in-hospital clinical deterioration is not predicted by severity of illness, functional status, or comorbidity. Int J Gen Med. 2017;10:329-334.
16. Dargin JM, Mackey CG, Lei Y, Liesching TN. Resource utilization and end‐of‐life care in a US hospital following medical emergency team‐implemented do not resuscitate orders. J Hosp Med. 2014;9(6):372-378.
17. Austin CA, Hanzaker C, Stafford R, et al. Utilization of rapid response resources and outcomes in a comprehensive cancer center. Crit Care Med. 2014;42(4):905-909.
18. Smith RL, Hayashi VN, Lee YI, Navarro-Mariazeta L, Felner K. The medical emergency team call: a sentinel event that triggers goals of care discussion. Crit Care Med. 2014;42(2):322-327.
19. Bozcuk H, Koyuncu E, Yildiz M, et al. A simple and accurate prediction model to estimate the intrahospital mortality risk of hospitalised cancer patients. Int J Clin Pract. 2004;58(11):1014-1019.
1. National Center for Health Statistics. Health, United States, 2016: with Chartbook on long-term trends in health. Hyattsville, MD: National Center for Health Statistics; 2017.
2. Lambden J, Zhang B, Friedlander R, Prigerson HG. Accuracy of oncologists’ life-expectancy estimates recalled by their advanced cancer patients: correlates and outcomes. J Palliat Med. 2016;19(12):1296-1303.
3. Maltoni M, Caraceni A, Brunelli C, et al. Prognostic factors in advanced cancer patients: evidence-based clinical recommendations—a study by the Steering Committee of the European Association for Palliative Care. J Clin Oncol. 2005;23(25):6240-6248.
4. Viganó A, Bruera E, Jhangri GS, Newman SC, Fields AL, Suarez-Almazor ME. Clinical survival predictors in patients with advanced cancer. Arch Intern Med. 2000;160(6):861-868.
5. Hauser CA, Stockler MR, Tattersall MH. Prognostic factors in patients with recently diagnosed incurable cancer: a systematic review. Support Care Cancer. 2006;14(10):999-1011.
6. Al-Zahrani AS, El-Kashif AT, Mohammad AA, Elsamany S, Alsirafy SA. Prediction of in-hospital mortality of patients with advanced cancer using the Chuang Prognostic Score. Am J Hosp Palliat Med. 2013;30(7):707-711.
7. Hui D, Kilgore K, Fellman B, et al. Development and cross-validation of the in-hospital mortality prediction in advanced cancer patients score: a preliminary study. J Palliat Med. 2012;15(8):902-909.
8. Shouval R, Labopin M, Bondi O, et al. Prediction of allogeneic hematopoietic stem-cell transplantation mortality 100 days after transplantation using a machine learning algorithm: a European group for blood and marrow transplantation acute leukemia working party retrospective data mining study. J Clin Oncol. 2015;33(28):3144-3151.
9. Agency for Healthcare Research and Quality. Total expenses and percent distribution for selected conditions by type of service: United States, 2013. Medical Expenditure Panel Survey website. https://meps.ahrq.gov/mepsweb/data_stats/tables_compendia_hh_interactive.jsp?_SERVICE=MEPSSocket0&_PROGRAM=MEPSPGM.TC.SAS&File=HCFY2013&Table=HCFY2012_CNDXP_C&_Debug=. Accessed November 10, 2018.
10. McCall N. Utilization and costs of Medicare services by beneficiaries in their last year of life. Med Care. 1984;22(4):329-342.
11. Jones D, Moran J, Winters B, Welch J. The rapid response system and end-of-life care. Curr Opin Crit Care. 2013;19(6):616-623.
12. Solomon RS, Corwin GS, Barclay DC, Quddusi SF, Dannenberg MD. Effectiveness of rapid response teams on rates of in‐hospital cardiopulmonary arrest and mortality: a systematic review and meta‐analysis. J Hosp Med. 2016;11(6):438-445.
13. Jung B, Daurat A, De Jong A, et al. Rapid response team and hospital mortality in hospitalized patients. Intensive Care Med. 2016;42(4):494-504.
14. Sulistio M, Franco M, Vo A, Poon P, William L. Hospital rapid response team and patients with life-limiting illness: a multicentre retrospective cohort study. Palliat Med. 2015;29(4):302-309.
15. Wang J, Hahn SS, Kline M, Cohen RI. Early in-hospital clinical deterioration is not predicted by severity of illness, functional status, or comorbidity. Int J Gen Med. 2017;10:329-334.
16. Dargin JM, Mackey CG, Lei Y, Liesching TN. Resource utilization and end‐of‐life care in a US hospital following medical emergency team‐implemented do not resuscitate orders. J Hosp Med. 2014;9(6):372-378.
17. Austin CA, Hanzaker C, Stafford R, et al. Utilization of rapid response resources and outcomes in a comprehensive cancer center. Crit Care Med. 2014;42(4):905-909.
18. Smith RL, Hayashi VN, Lee YI, Navarro-Mariazeta L, Felner K. The medical emergency team call: a sentinel event that triggers goals of care discussion. Crit Care Med. 2014;42(2):322-327.
19. Bozcuk H, Koyuncu E, Yildiz M, et al. A simple and accurate prediction model to estimate the intrahospital mortality risk of hospitalised cancer patients. Int J Clin Pract. 2004;58(11):1014-1019.
Symptom burdens related to chemotherapy-induced anemia in stage IV cancer
Anemia is a common complication of cancer treatment as well as of cancer itself. Most cancer patients undergoing chemotherapy experience anemia sometime during their treatment course.1,2 Moderate to severe anemia is associated with an array of symptoms that are known to compromise the physical functioning and quality of life of cancer patients. Common anemia-related symptoms include fatigue, drowsiness, depression, dyspnea, tachycardia, and dizziness.1,3-7
Symptoms produced by cancer itself or the disease treatment (ie, side effects such as anemia) collectively compose a patient’s symptom burden.8 Although the occurrence of anemia-related fatigue has been described more systematically, other clinical presentations of chemotherapy-induced anemia (CIA) are not well characterized. Furthermore, the overall symptom burdens associated with different ranges of hemoglobin (Hb) concentrations have also not been well reported. Although various tools have been developed to facilitate the reporting of fatigue and other symptoms experienced by patients with CIA, such as the Functional Assessment of Cancer Therapy-Anemia (FACT-An) questionnaire and the MD Anderson Symptom Inventory (MDASI),9-11 these questionnaires have not been extensively used outside of the research context. As such, knowledge on symptom burdens associated with CIA in real-world patient populations remains lacking.
Given the common occurrence of CIA, management of CIA and associated symptoms plays an important role to patients’ quality of life during cancer treatment. Symptom control is often the main goal for patients with stage IV cancers, as treatment for disease is most likely palliative or noncurative. To facilitate supportive care planning, it is important to understand patient symptom burdens as chemotherapy progresses over cycles and Hb levels decline. We conducted a comprehensive medical record review study in patients diagnosed with stage IV non-Hodgkin lymphoma (NHL), breast cancer, and lung cancers at Kaiser Permanente Southern California (KPSC), a large community-based health care delivery system. The objective of this study was to report the occurrence of CIA-related symptoms throughout the course of chemotherapy and by Hb levels.
Methods
Study setting and population
KPSC is an integrated managed-care organization that provides comprehensive health services for 4 million racially, ethnically, and socioeconomically diverse members who broadly represent the population in Southern California.12 The organization maintains electronic records of health care received by its members, including physician record notes and clinical databases such as laboratory test results, diagnosis codes, medical procedures, medication dispenses, and disease registries. KPSC’s cancer registry is Surveillance, Epidemiology, and End Results, which is affiliated and routinely collects information on age, sex, race and/or ethnicity, cancer type, histology, and stage at diagnosis.
Patients who met the following inclusion criteria were included in this study: diagnosed with stage IV NHL, breast cancer, or lung cancer at age 18 years or older at KPSC between March 25, 2010 and December 31, 2012; initiated myelosuppressive chemotherapy at KPSC before June 30, 2013 (only the first chemotherapy course was included in this evaluation); and had at least 1 Hb measurement during the course of chemotherapy. Of those who met the inclusion criteria, patients who met the following criteria were excluded if they had less than 12 months KPSC membership before start of chemotherapy, missing information on cancer stage or chemotherapy regimen/agents, a diagnosis of myelodysplastic syndrome before chemotherapy initiation, a diagnosis of inherited anemia, an Hb concentration <10 g/L within 3 months before chemotherapy initiation, a transfusion within 2 weeks before chemotherapy initiation, radiation within 4 months before chemotherapy initiation, or bone marrow transplantation within 12 months before chemotherapy initiation or during the chemotherapy course. These exclusion criteria were applied to evaluate symptom burdens most likely related to CIA as opposed to other cancer treatment or pre-existing anemia.
CIA in this study was defined as moderate to severe anemia with Hb <10 g/dL after chemotherapy initiation. Based on this definition for CIA, all patients who developed CIA between the first chemotherapy administration to 60 days after the last dose of chemotherapy were included for the record review
Data collection
Data on anemia-related symptoms or signs and anemia-related comorbidities (Table 1) were collected by standardized review of physician record notes in the electronic medical records. A set of 24 anemia-related symptoms were identified based on the literature and clinical expertise and included abdominal pain, blurred vision/double vision/loss of vision, cold intolerance/coldness in hands or feet, depression/anxiety, diarrhea, dizziness/lightheadedness, dyspnea/shortness of breath/tachypnea, edema, fatigue, headache, heart failure, heat intolerance, hypotension, insomnia, leg pain, loss of appetite, nausea/vomiting, pale skin, palpitations/tachycardia, paralysis/ataxia/numbness or tingling in extremities, pectoral angina/chest pain, sweating/diaphoresis, syncope, and vertigo. Record review period was defined as 1 month before chemotherapy to 60 days after the last dose of chemotherapy in the first course. To understand the development of new symptoms during chemotherapy treatment, pre-existing symptoms documented within 1 month before chemotherapy initiation were recorded.
The data elements extracted included the date the symptom was documented, date the symptom started, symptom duration (when available), and any relevant comments regarding the symptom (ie, if dyspnea was at rest or on exertion, whether the symptom was a side effect caused by chemotherapy, or change in symptom severity). Ten percent of the records were reviewed independently by 2 abstractors to ensure quality control. Additional quality control measures included SAS algorithms (SAS Institute, Inc., Cary, North Carolina) to check reasonability and logical consistency in the abstracted data.
Patient demographic characteristics, cancer stage, additional selected comorbidities (Table 1), chemotherapy information, Hb test results, and anemia treatment, including erythrocyte stimulating agent (ESA) use and red blood cell transfusion, were collected using KPSC’s cancer registry and clinical databases. Anemia was defined by severity as grade 1 (10 g/dL to lower limit of normal, ie, 14 g/dL for men and 12 g/dL for women), grade 2 (8.0-9.9 g/dL), grade 3 (6.5-7.9 g/dL), and grade 4 (<6.5 g/dL) following the National Cancer Institute’s Common Terminology Criteria for Adverse Events.13
Statistical analysis
Distributions of demographic, cancer, and treatment characteristics were calculated by CIA status, overall and by cancer type. Differences between patients who did and did not develop CIA were assessed using chi-square test and Kruskal-Wallis test. For those who developed CIA, the distribution of the worst anemia grade was also calculated for each cycle of chemotherapy.
Next, the distributions for the following symptom categories were calculated in the 2 study samples defined by CIA status: pre-existing symptoms that occurred before chemotherapy, any symptoms during chemotherapy (ie, whether they started before chemotherapy), and incident symptoms during chemotherapy (ie, new symptoms that only started after chemotherapy). Specifically, the proportion of patients with each individual symptom and the distribution of the number of symptoms per patient were calculated. Differences in symptom distribution by CIA status were assessed using chi-square test.
The distribution of symptoms in each chemotherapy cycle was calculated up to 6 chemotherapy cycles (as >80% of the patients only had treatment up to 6 cycles) in the 2 study samples defined by CIA status. For this analysis, a symptom was “mapped” to a cycle if the date (or date range) of the symptom fell within the date range of that chemotherapy cycle. In patients who developed CIA, the distribution of symptoms was also calculated by anemia grade. This was again done on the chemotherapy cycle level. For each chemotherapy cycle, an anemia grade was assigned (no anemia or anemia grade 1, 2, 3, and 4) using the lowest Hb measurement in that cycle. Symptoms that occurred in a chemotherapy cycle were then “mapped” to the anemia grade of that cycle. Some patients had more than 1 anemia event of the same grade (eg, if a patient’s grade 2 anemia persist across cycles). For these patients, we randomly selected only 1 anemia event of the same grade from each patient to be included in this analysis. Patients could still contribute multiple events of different grades to this analysis. We calculated the mean number of symptoms per patient for each anemia grade (ie, 1-4) separately. Because of the small number of patients who developed grade 4 anemia (n = 11), they were combined with the grade 3 patients when the distributions of individual symptoms were evaluated.
All analyses were repeated stratified by gender. P values for differences between men and women were calculated using chi-square test or t test. All analyses were conducted using SAS version 9.3.
Results
A total of 402 stage IV NHL, breast, and lung cancer patients who developed CIA and 98 patients who did not develop CIA during the first course of chemotherapy were included (Figure 1).
The distribution of cancer types in the study sample were similar across CIA status (Table 1). The mean age at diagnosis was 66 years in patients who developed CIA and 62 years in patients who did not develop CIA. Women accounted for half of the patients in both study samples (52% and 51%, respectively). Most of the study patients were of non-Hispanic white race/ethnicity. Chronic obstructive pulmonary disease/emphysema and gastroesophageal reflux disease were among the most common comorbidities examined in both study samples, while malnutrition and moderate to severe renal disease were also common in patients who developed CIA (Table 1).
The mean Hb level before chemotherapy was lower for patients who developed CIA compared with patients who did not develop CIA (12.3 g/dL and 13.5 g/dL, respectively; Table 1). The mean lowest Hb level during chemotherapy was 8.5 g/dL for patients who developed CIA and 11.4 g/dL for patients without CIA (Table 1). The number of anemia events by grade in each chemotherapy cycle in patients who developed CIA is shown in Table 2.
Table 3 shows the number and proportion of study patients with each of the symptoms documented before and after chemotherapy initiation for the 2 study samples. Patients who developed CIA had statistically significantly more pre-existing symptoms, incident symptoms, or any symptoms that occurred during chemotherapy compared with patients who did not develop CIA.
Table 4 shows the number and proportion of study patients with symptoms that occurred during each chemotherapy cycle. Again, fatigue is the predominant symptom documented throughout cycles for all patients. In patients who developed CIA, the proportion of patients experiencing the following symptoms was relatively stable across chemotherapy cycles: depression/anxiety, dizziness/lightheadedness, fatigue, pale skin, and sweating. The proportion of patients experiencing paralysis/ataxia/numbness/tingling in extremities increased over cycles. For headache, loss of appetite, hypotension, and nausea/vomiting, the proportion of patients with symptom documentation was highest in cycle 1, stabilizing in subsequent cycles (Table 4). In patients without CIA, the cycle-level prevalence of most of the symptoms did not increase over cycles, except for paralysis/ataxia/numbness or tingling in extremities. For insomnia, loss of appetite, and nausea/vomiting, the cycle-level prevalence dropped after the first cycle. There was no clear increasing trend of the mean number of symptoms per patient across chemotherapy cycles in both study samples (Table 4).
Table 5 shows the distribution of symptoms by anemia grade in patients who developed CIA. In general, the prevalence of symptoms increased with higher grades of anemia. The following symptoms especially have a clear increase in prevalence as the severity of anemia progressed: abdominal pain, depression, diarrhea, dizziness/lightheadedness, dyspnea, edema, fatigue, heart failure, headache, hypotension, insomnia, leg pain, loss of appetite, pale skin, palpitations, pectoral angina, and sweating. The mean number of symptoms per patient increased as CIA grade increased, from 3.6 (SD, 2.9) for grade 2 CIA to 5.4 (SD, 3.5) for grades 3 and 4 CIA (specifically, 5.3 [SD, 3.4] for grade 3 CIA and 6.4 [SD, 4.1] for grade 4 CIA; data not shown) (Table 5).
When stratified by gender, there are no material differences between men and women in most analyses. In men, the mean number of pre-existing symptoms was 1.7 (SD, 1.8) and 1.0 (SD, 1.2) for those with and without CIA, respectively (P = .02). The mean number of symptoms that occurred during chemotherapy was 7.0 (SD, 3.4) and 4.2 (SD, 2.4), respectively (P < .01). In women, the mean number of pre-existing symptoms was not statistically different in those with and without CIA (1.6 [SD, 2.2] and 1.3 [SD, 1.8], respectively; P = .46). However, like in men, the mean number of symptoms that occurred during chemotherapy was significantly more in those with CIA (6.5 [SD, 3.3] and 4.0 [SD, 2.9], respectively; P < .01). As in the overall analysis, there was no clear increasing trend of the number of symptoms per patients across chemotherapy cycles in both men and women, but the average number of symptoms increased as the CIA grade increased. For men, the mean number of symptoms per patient increased from 3.7 (SD, 3.0) for grade 2 CIA to 6.0 (SD, 3.5) for grades 3 and 4 CIA (data not shown). For women, the mean number of symptoms per patient increased from 3.6 (SD, 2.9) for grade 2 CIA to 4.7 (SD, 3.3) for grades 3 and 4 CIA (data not shown).
Discussion
In this study, we described the number and type of symptoms documented in the medical record notes among stage IV NHL, breast cancer, and lung cancer patients who did or did not develop CIA during chemotherapy.
Our findings on the prevalence of fatigue are in line with other studies in the literature. Maxwell reported that the prevalence of fatigue was 80% to 96% in cancer patients.17 Cella and colleagues found that using FACT-General questionnaire, 75% of cancer patients reported fatigue.11 The comparability of our estimate and those found in studies based on patient self-report offered some assurance of the validity of assessing symptom prevalence through physician record notes. In addition to fatigue, we described prevalence of 23 additional symptoms, most of which have not been extensively studied in the literature. Gabrilove and colleagues found that a substantial proportion of patients with CIA had moderate to severe score for lack of appetite (36%) and disturbed sleep (41%) using the MDASI.10 The prevalence of loss of appetite and insomnia was around 50% and 25%, respectively, in our study samples. A 2013 systematic review of 21 multinational studies reported the pooled prevalence of several nonfatigue symptoms in cancer patients including headache (23%), sleep disturbance/insomnia (49%), appetite changes (45%), nausea/vomiting (26%), diarrhea (15%), depression (34%), dyspnea (44%), dizziness (26%), numbness/tingling (42%), edema (14%), and sweating (28%).18 Our prevalence estimates in patients with CIA for most of these symptoms were higher, likely because Reilly and colleagues used source studies that included any cancer patients undergoing treatment and not just those with CIA. Our findings on the increased symptom burden in patients who experienced episodes of advanced anemia compared with patients with mild anemia were also consistent with the literature. To this end, several studies using MDASI or the FACT-An reported differential symptom burdens by Hb level based on patient self-report,10,11,19 including data on improvement in symptom burden and quality of life after anemia was amended with the use of ESA.20,21
We found that the number of pre-existing symptoms was significantly higher in patients who went on to develop CIA than in patients who did not develop CIA. Specifically, fatigue, loss of appetite, and pale skin before chemotherapy seemed to be significantly more common in patients who went on to develop CIA. This finding suggested that presentation of these symptoms before chemotherapy initiation may be a predictor for developing moderate or severe anemia during treatment. This is a novel hypothesis, as no studies have evaluated the relationship between pretreatment symptom and risk of CIA. However, our study was not designed to address this specific question. Additional investigation is needed to further shed light on whether the occurrence of anemia-related symptoms in nonanemic patients can be used to effectively risk-stratify patients for subsequent CIA.
Contrary to our expectation, the prevalence of most symptoms did not clearly increase as chemotherapy progressed. There are several possible explanations to this phenomenon, with the most likely being related to reporting of anemia-related symptoms. For example, patients might stop reporting the same symptom repeatedly or become adjusted to the new Hb levels, leading to less symptom manifestation. Clinicians may also be less likely to ask about symptoms in later treatment cycles and/or to document chronic symptoms. Several symptoms were rarely documented altogether, such as cold intolerance, heat intolerance, heart failure, and vertigo. Symptoms reported in earlier cycles could also be managed successfully. Another possible explanation is differential loss of follow-up. Patients who experienced severe adverse events or symptoms may terminate treatment prematurely. Thus, symptom burden found toward later cycles may not represent the true symptom burden should everyone who initiated the chemotherapy treatment complete all planned cycles.
Limitations
In addition to the limitations already discussed, there are several others that should be considered when interpreting our results. We did not have a consistent measure of symptom severity in the medical records. Duration of symptoms was also often poorly documented by physicians. Therefore, our results are not directly comparable with studies such as the MDASI that incorporate severity or duration in their prevalence measure.
Despite the potential limitations, our study has several important strengths.
Conclusions
Our data provide physicians a comprehensive picture of prevalence of various types of symptoms and how symptom burden evolves as chemotherapy cycle and anemia severity progress. High-grade CIA correlates with an increased symptom burden.
1. Barrett-Lee PJ, Ludwig H, Birgegård G, et al. Independent risk factors for anemia in cancer patients receiving chemotherapy: results from the European Cancer Anaemia Survey. Oncology. 2006;70(1):34-48.
2. Kitano T, Tada H, Nishimura T, et al. Prevalence and incidence of anemia in Japanese cancer patients receiving outpatient chemotherapy. Int J Hematol. 2007;86(1):37-41.
3. Birgegård G, Aapro MS, Bokemeyer C, et al. Cancer-related anemia: pathogenesis, prevalence and treatment. Oncology. 2005;68(Suppl 1):3-11.
4. Harper P, Littlewood T. Anaemia of cancer: impact on patient fatigue and long-term outcome. Oncology. 2005;69(Suppl 2):2-7.
5. Nieboer P, Buijs C, Rodenhuis S, et al. Fatigue and relating factors in high-risk breast cancer patients treated with adjuvant standard or high-dose chemotherapy: a longitudinal study. J Clin Oncol. 2005;23(33):8296-8304.
6. Bremberg ER, Brandberg Y, Hising C, Friesland S, Eksborg S. Anemia and quality of life including anemia-related symptoms in patients with solid tumors in clinical practice. Med Oncol. 2007;24(1):95-102.
7. Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl 1):4-10.
8. Cleeland CS. Symptom burden: multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monogr. 2007(37):16-21.
9. Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63-74.
10. Gabrilove JL, Perez EA, Tomita DK, Rossi G, Cleeland CS. Assessing symptom burden using the M. D. Anderson symptom inventory in patients with chemotherapy-induced anemia: results of a multicenter, open-label study (SURPASS) of patients treated with darbepoetin-alpha at a dose of 200 microg every 2 weeks. Cancer. 2007;110(7):1629-1640.
11. Cella D. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34(3 Suppl 2):13-19.
12. Koebnick C, Langer-Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16(3):37-41.
13. Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst. 1999;91(19):1616-1634.
14. Gilreath JA, Stenehjem DD, Rodgers GM. Diagnosis and treatment of cancer-related anemia. Am J Hematol. 2014;89(2):203-212.
15. Rizzo JD, Somerfield MR, Hagerty KL, et al. Use of epoetin and darbepoetin in patients with cancer: 2007 American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update. J Clin Oncol. 2008;26(1):132-149.
16. Bohlius J, Tonia T, Nüesch E, et al. Effects of erythropoiesis-stimulating agents on fatigue- and anaemia-related symptoms in cancer patients: systematic review and meta-analyses of published and unpublished data. Br J Cancer. 2014;111(1):33-45.
17. Maxwell MB. When the cancer patient becomes anemic. Cancer Nurs. 1984;7(4):321-326.
18. Reilly CM, Bruner DW, Mitchell SA, et al. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer. 2013;21(6):1525-1550.
19. Crawford J, Cella D, Cleeland CS, et al. Relationship between changes in hemoglobin level and quality of life during chemotherapy in anemic cancer patients receiving epoetin alfa therapy. Cancer. 2002;95(4):888-895.
20. Mouysset JL, Freier B, van den Bosch J, et al. Hemoglobin levels and quality of life in patients with symptomatic chemotherapy-induced anemia: the eAQUA study. Cancer Manag Res. 2016;8:1-10.
21. Vansteenkiste J, Pirker R, Massuti B, et al. Double-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst. 2002;94(16):1211-1220.
22. Kleinman L, Benjamin K, Viswanathan H, et al. The anemia impact measure (AIM): development and content validation of a patient-reported outcome measure of anemia symptoms and symptom impacts in cancer patients receiving chemotherapy. Qual Life Res. 2012;21(7):1255-1266.
Anemia is a common complication of cancer treatment as well as of cancer itself. Most cancer patients undergoing chemotherapy experience anemia sometime during their treatment course.1,2 Moderate to severe anemia is associated with an array of symptoms that are known to compromise the physical functioning and quality of life of cancer patients. Common anemia-related symptoms include fatigue, drowsiness, depression, dyspnea, tachycardia, and dizziness.1,3-7
Symptoms produced by cancer itself or the disease treatment (ie, side effects such as anemia) collectively compose a patient’s symptom burden.8 Although the occurrence of anemia-related fatigue has been described more systematically, other clinical presentations of chemotherapy-induced anemia (CIA) are not well characterized. Furthermore, the overall symptom burdens associated with different ranges of hemoglobin (Hb) concentrations have also not been well reported. Although various tools have been developed to facilitate the reporting of fatigue and other symptoms experienced by patients with CIA, such as the Functional Assessment of Cancer Therapy-Anemia (FACT-An) questionnaire and the MD Anderson Symptom Inventory (MDASI),9-11 these questionnaires have not been extensively used outside of the research context. As such, knowledge on symptom burdens associated with CIA in real-world patient populations remains lacking.
Given the common occurrence of CIA, management of CIA and associated symptoms plays an important role to patients’ quality of life during cancer treatment. Symptom control is often the main goal for patients with stage IV cancers, as treatment for disease is most likely palliative or noncurative. To facilitate supportive care planning, it is important to understand patient symptom burdens as chemotherapy progresses over cycles and Hb levels decline. We conducted a comprehensive medical record review study in patients diagnosed with stage IV non-Hodgkin lymphoma (NHL), breast cancer, and lung cancers at Kaiser Permanente Southern California (KPSC), a large community-based health care delivery system. The objective of this study was to report the occurrence of CIA-related symptoms throughout the course of chemotherapy and by Hb levels.
Methods
Study setting and population
KPSC is an integrated managed-care organization that provides comprehensive health services for 4 million racially, ethnically, and socioeconomically diverse members who broadly represent the population in Southern California.12 The organization maintains electronic records of health care received by its members, including physician record notes and clinical databases such as laboratory test results, diagnosis codes, medical procedures, medication dispenses, and disease registries. KPSC’s cancer registry is Surveillance, Epidemiology, and End Results, which is affiliated and routinely collects information on age, sex, race and/or ethnicity, cancer type, histology, and stage at diagnosis.
Patients who met the following inclusion criteria were included in this study: diagnosed with stage IV NHL, breast cancer, or lung cancer at age 18 years or older at KPSC between March 25, 2010 and December 31, 2012; initiated myelosuppressive chemotherapy at KPSC before June 30, 2013 (only the first chemotherapy course was included in this evaluation); and had at least 1 Hb measurement during the course of chemotherapy. Of those who met the inclusion criteria, patients who met the following criteria were excluded if they had less than 12 months KPSC membership before start of chemotherapy, missing information on cancer stage or chemotherapy regimen/agents, a diagnosis of myelodysplastic syndrome before chemotherapy initiation, a diagnosis of inherited anemia, an Hb concentration <10 g/L within 3 months before chemotherapy initiation, a transfusion within 2 weeks before chemotherapy initiation, radiation within 4 months before chemotherapy initiation, or bone marrow transplantation within 12 months before chemotherapy initiation or during the chemotherapy course. These exclusion criteria were applied to evaluate symptom burdens most likely related to CIA as opposed to other cancer treatment or pre-existing anemia.
CIA in this study was defined as moderate to severe anemia with Hb <10 g/dL after chemotherapy initiation. Based on this definition for CIA, all patients who developed CIA between the first chemotherapy administration to 60 days after the last dose of chemotherapy were included for the record review
Data collection
Data on anemia-related symptoms or signs and anemia-related comorbidities (Table 1) were collected by standardized review of physician record notes in the electronic medical records. A set of 24 anemia-related symptoms were identified based on the literature and clinical expertise and included abdominal pain, blurred vision/double vision/loss of vision, cold intolerance/coldness in hands or feet, depression/anxiety, diarrhea, dizziness/lightheadedness, dyspnea/shortness of breath/tachypnea, edema, fatigue, headache, heart failure, heat intolerance, hypotension, insomnia, leg pain, loss of appetite, nausea/vomiting, pale skin, palpitations/tachycardia, paralysis/ataxia/numbness or tingling in extremities, pectoral angina/chest pain, sweating/diaphoresis, syncope, and vertigo. Record review period was defined as 1 month before chemotherapy to 60 days after the last dose of chemotherapy in the first course. To understand the development of new symptoms during chemotherapy treatment, pre-existing symptoms documented within 1 month before chemotherapy initiation were recorded.
The data elements extracted included the date the symptom was documented, date the symptom started, symptom duration (when available), and any relevant comments regarding the symptom (ie, if dyspnea was at rest or on exertion, whether the symptom was a side effect caused by chemotherapy, or change in symptom severity). Ten percent of the records were reviewed independently by 2 abstractors to ensure quality control. Additional quality control measures included SAS algorithms (SAS Institute, Inc., Cary, North Carolina) to check reasonability and logical consistency in the abstracted data.
Patient demographic characteristics, cancer stage, additional selected comorbidities (Table 1), chemotherapy information, Hb test results, and anemia treatment, including erythrocyte stimulating agent (ESA) use and red blood cell transfusion, were collected using KPSC’s cancer registry and clinical databases. Anemia was defined by severity as grade 1 (10 g/dL to lower limit of normal, ie, 14 g/dL for men and 12 g/dL for women), grade 2 (8.0-9.9 g/dL), grade 3 (6.5-7.9 g/dL), and grade 4 (<6.5 g/dL) following the National Cancer Institute’s Common Terminology Criteria for Adverse Events.13
Statistical analysis
Distributions of demographic, cancer, and treatment characteristics were calculated by CIA status, overall and by cancer type. Differences between patients who did and did not develop CIA were assessed using chi-square test and Kruskal-Wallis test. For those who developed CIA, the distribution of the worst anemia grade was also calculated for each cycle of chemotherapy.
Next, the distributions for the following symptom categories were calculated in the 2 study samples defined by CIA status: pre-existing symptoms that occurred before chemotherapy, any symptoms during chemotherapy (ie, whether they started before chemotherapy), and incident symptoms during chemotherapy (ie, new symptoms that only started after chemotherapy). Specifically, the proportion of patients with each individual symptom and the distribution of the number of symptoms per patient were calculated. Differences in symptom distribution by CIA status were assessed using chi-square test.
The distribution of symptoms in each chemotherapy cycle was calculated up to 6 chemotherapy cycles (as >80% of the patients only had treatment up to 6 cycles) in the 2 study samples defined by CIA status. For this analysis, a symptom was “mapped” to a cycle if the date (or date range) of the symptom fell within the date range of that chemotherapy cycle. In patients who developed CIA, the distribution of symptoms was also calculated by anemia grade. This was again done on the chemotherapy cycle level. For each chemotherapy cycle, an anemia grade was assigned (no anemia or anemia grade 1, 2, 3, and 4) using the lowest Hb measurement in that cycle. Symptoms that occurred in a chemotherapy cycle were then “mapped” to the anemia grade of that cycle. Some patients had more than 1 anemia event of the same grade (eg, if a patient’s grade 2 anemia persist across cycles). For these patients, we randomly selected only 1 anemia event of the same grade from each patient to be included in this analysis. Patients could still contribute multiple events of different grades to this analysis. We calculated the mean number of symptoms per patient for each anemia grade (ie, 1-4) separately. Because of the small number of patients who developed grade 4 anemia (n = 11), they were combined with the grade 3 patients when the distributions of individual symptoms were evaluated.
All analyses were repeated stratified by gender. P values for differences between men and women were calculated using chi-square test or t test. All analyses were conducted using SAS version 9.3.
Results
A total of 402 stage IV NHL, breast, and lung cancer patients who developed CIA and 98 patients who did not develop CIA during the first course of chemotherapy were included (Figure 1).
The distribution of cancer types in the study sample were similar across CIA status (Table 1). The mean age at diagnosis was 66 years in patients who developed CIA and 62 years in patients who did not develop CIA. Women accounted for half of the patients in both study samples (52% and 51%, respectively). Most of the study patients were of non-Hispanic white race/ethnicity. Chronic obstructive pulmonary disease/emphysema and gastroesophageal reflux disease were among the most common comorbidities examined in both study samples, while malnutrition and moderate to severe renal disease were also common in patients who developed CIA (Table 1).
The mean Hb level before chemotherapy was lower for patients who developed CIA compared with patients who did not develop CIA (12.3 g/dL and 13.5 g/dL, respectively; Table 1). The mean lowest Hb level during chemotherapy was 8.5 g/dL for patients who developed CIA and 11.4 g/dL for patients without CIA (Table 1). The number of anemia events by grade in each chemotherapy cycle in patients who developed CIA is shown in Table 2.
Table 3 shows the number and proportion of study patients with each of the symptoms documented before and after chemotherapy initiation for the 2 study samples. Patients who developed CIA had statistically significantly more pre-existing symptoms, incident symptoms, or any symptoms that occurred during chemotherapy compared with patients who did not develop CIA.
Table 4 shows the number and proportion of study patients with symptoms that occurred during each chemotherapy cycle. Again, fatigue is the predominant symptom documented throughout cycles for all patients. In patients who developed CIA, the proportion of patients experiencing the following symptoms was relatively stable across chemotherapy cycles: depression/anxiety, dizziness/lightheadedness, fatigue, pale skin, and sweating. The proportion of patients experiencing paralysis/ataxia/numbness/tingling in extremities increased over cycles. For headache, loss of appetite, hypotension, and nausea/vomiting, the proportion of patients with symptom documentation was highest in cycle 1, stabilizing in subsequent cycles (Table 4). In patients without CIA, the cycle-level prevalence of most of the symptoms did not increase over cycles, except for paralysis/ataxia/numbness or tingling in extremities. For insomnia, loss of appetite, and nausea/vomiting, the cycle-level prevalence dropped after the first cycle. There was no clear increasing trend of the mean number of symptoms per patient across chemotherapy cycles in both study samples (Table 4).
Table 5 shows the distribution of symptoms by anemia grade in patients who developed CIA. In general, the prevalence of symptoms increased with higher grades of anemia. The following symptoms especially have a clear increase in prevalence as the severity of anemia progressed: abdominal pain, depression, diarrhea, dizziness/lightheadedness, dyspnea, edema, fatigue, heart failure, headache, hypotension, insomnia, leg pain, loss of appetite, pale skin, palpitations, pectoral angina, and sweating. The mean number of symptoms per patient increased as CIA grade increased, from 3.6 (SD, 2.9) for grade 2 CIA to 5.4 (SD, 3.5) for grades 3 and 4 CIA (specifically, 5.3 [SD, 3.4] for grade 3 CIA and 6.4 [SD, 4.1] for grade 4 CIA; data not shown) (Table 5).
When stratified by gender, there are no material differences between men and women in most analyses. In men, the mean number of pre-existing symptoms was 1.7 (SD, 1.8) and 1.0 (SD, 1.2) for those with and without CIA, respectively (P = .02). The mean number of symptoms that occurred during chemotherapy was 7.0 (SD, 3.4) and 4.2 (SD, 2.4), respectively (P < .01). In women, the mean number of pre-existing symptoms was not statistically different in those with and without CIA (1.6 [SD, 2.2] and 1.3 [SD, 1.8], respectively; P = .46). However, like in men, the mean number of symptoms that occurred during chemotherapy was significantly more in those with CIA (6.5 [SD, 3.3] and 4.0 [SD, 2.9], respectively; P < .01). As in the overall analysis, there was no clear increasing trend of the number of symptoms per patients across chemotherapy cycles in both men and women, but the average number of symptoms increased as the CIA grade increased. For men, the mean number of symptoms per patient increased from 3.7 (SD, 3.0) for grade 2 CIA to 6.0 (SD, 3.5) for grades 3 and 4 CIA (data not shown). For women, the mean number of symptoms per patient increased from 3.6 (SD, 2.9) for grade 2 CIA to 4.7 (SD, 3.3) for grades 3 and 4 CIA (data not shown).
Discussion
In this study, we described the number and type of symptoms documented in the medical record notes among stage IV NHL, breast cancer, and lung cancer patients who did or did not develop CIA during chemotherapy.
Our findings on the prevalence of fatigue are in line with other studies in the literature. Maxwell reported that the prevalence of fatigue was 80% to 96% in cancer patients.17 Cella and colleagues found that using FACT-General questionnaire, 75% of cancer patients reported fatigue.11 The comparability of our estimate and those found in studies based on patient self-report offered some assurance of the validity of assessing symptom prevalence through physician record notes. In addition to fatigue, we described prevalence of 23 additional symptoms, most of which have not been extensively studied in the literature. Gabrilove and colleagues found that a substantial proportion of patients with CIA had moderate to severe score for lack of appetite (36%) and disturbed sleep (41%) using the MDASI.10 The prevalence of loss of appetite and insomnia was around 50% and 25%, respectively, in our study samples. A 2013 systematic review of 21 multinational studies reported the pooled prevalence of several nonfatigue symptoms in cancer patients including headache (23%), sleep disturbance/insomnia (49%), appetite changes (45%), nausea/vomiting (26%), diarrhea (15%), depression (34%), dyspnea (44%), dizziness (26%), numbness/tingling (42%), edema (14%), and sweating (28%).18 Our prevalence estimates in patients with CIA for most of these symptoms were higher, likely because Reilly and colleagues used source studies that included any cancer patients undergoing treatment and not just those with CIA. Our findings on the increased symptom burden in patients who experienced episodes of advanced anemia compared with patients with mild anemia were also consistent with the literature. To this end, several studies using MDASI or the FACT-An reported differential symptom burdens by Hb level based on patient self-report,10,11,19 including data on improvement in symptom burden and quality of life after anemia was amended with the use of ESA.20,21
We found that the number of pre-existing symptoms was significantly higher in patients who went on to develop CIA than in patients who did not develop CIA. Specifically, fatigue, loss of appetite, and pale skin before chemotherapy seemed to be significantly more common in patients who went on to develop CIA. This finding suggested that presentation of these symptoms before chemotherapy initiation may be a predictor for developing moderate or severe anemia during treatment. This is a novel hypothesis, as no studies have evaluated the relationship between pretreatment symptom and risk of CIA. However, our study was not designed to address this specific question. Additional investigation is needed to further shed light on whether the occurrence of anemia-related symptoms in nonanemic patients can be used to effectively risk-stratify patients for subsequent CIA.
Contrary to our expectation, the prevalence of most symptoms did not clearly increase as chemotherapy progressed. There are several possible explanations to this phenomenon, with the most likely being related to reporting of anemia-related symptoms. For example, patients might stop reporting the same symptom repeatedly or become adjusted to the new Hb levels, leading to less symptom manifestation. Clinicians may also be less likely to ask about symptoms in later treatment cycles and/or to document chronic symptoms. Several symptoms were rarely documented altogether, such as cold intolerance, heat intolerance, heart failure, and vertigo. Symptoms reported in earlier cycles could also be managed successfully. Another possible explanation is differential loss of follow-up. Patients who experienced severe adverse events or symptoms may terminate treatment prematurely. Thus, symptom burden found toward later cycles may not represent the true symptom burden should everyone who initiated the chemotherapy treatment complete all planned cycles.
Limitations
In addition to the limitations already discussed, there are several others that should be considered when interpreting our results. We did not have a consistent measure of symptom severity in the medical records. Duration of symptoms was also often poorly documented by physicians. Therefore, our results are not directly comparable with studies such as the MDASI that incorporate severity or duration in their prevalence measure.
Despite the potential limitations, our study has several important strengths.
Conclusions
Our data provide physicians a comprehensive picture of prevalence of various types of symptoms and how symptom burden evolves as chemotherapy cycle and anemia severity progress. High-grade CIA correlates with an increased symptom burden.
Anemia is a common complication of cancer treatment as well as of cancer itself. Most cancer patients undergoing chemotherapy experience anemia sometime during their treatment course.1,2 Moderate to severe anemia is associated with an array of symptoms that are known to compromise the physical functioning and quality of life of cancer patients. Common anemia-related symptoms include fatigue, drowsiness, depression, dyspnea, tachycardia, and dizziness.1,3-7
Symptoms produced by cancer itself or the disease treatment (ie, side effects such as anemia) collectively compose a patient’s symptom burden.8 Although the occurrence of anemia-related fatigue has been described more systematically, other clinical presentations of chemotherapy-induced anemia (CIA) are not well characterized. Furthermore, the overall symptom burdens associated with different ranges of hemoglobin (Hb) concentrations have also not been well reported. Although various tools have been developed to facilitate the reporting of fatigue and other symptoms experienced by patients with CIA, such as the Functional Assessment of Cancer Therapy-Anemia (FACT-An) questionnaire and the MD Anderson Symptom Inventory (MDASI),9-11 these questionnaires have not been extensively used outside of the research context. As such, knowledge on symptom burdens associated with CIA in real-world patient populations remains lacking.
Given the common occurrence of CIA, management of CIA and associated symptoms plays an important role to patients’ quality of life during cancer treatment. Symptom control is often the main goal for patients with stage IV cancers, as treatment for disease is most likely palliative or noncurative. To facilitate supportive care planning, it is important to understand patient symptom burdens as chemotherapy progresses over cycles and Hb levels decline. We conducted a comprehensive medical record review study in patients diagnosed with stage IV non-Hodgkin lymphoma (NHL), breast cancer, and lung cancers at Kaiser Permanente Southern California (KPSC), a large community-based health care delivery system. The objective of this study was to report the occurrence of CIA-related symptoms throughout the course of chemotherapy and by Hb levels.
Methods
Study setting and population
KPSC is an integrated managed-care organization that provides comprehensive health services for 4 million racially, ethnically, and socioeconomically diverse members who broadly represent the population in Southern California.12 The organization maintains electronic records of health care received by its members, including physician record notes and clinical databases such as laboratory test results, diagnosis codes, medical procedures, medication dispenses, and disease registries. KPSC’s cancer registry is Surveillance, Epidemiology, and End Results, which is affiliated and routinely collects information on age, sex, race and/or ethnicity, cancer type, histology, and stage at diagnosis.
Patients who met the following inclusion criteria were included in this study: diagnosed with stage IV NHL, breast cancer, or lung cancer at age 18 years or older at KPSC between March 25, 2010 and December 31, 2012; initiated myelosuppressive chemotherapy at KPSC before June 30, 2013 (only the first chemotherapy course was included in this evaluation); and had at least 1 Hb measurement during the course of chemotherapy. Of those who met the inclusion criteria, patients who met the following criteria were excluded if they had less than 12 months KPSC membership before start of chemotherapy, missing information on cancer stage or chemotherapy regimen/agents, a diagnosis of myelodysplastic syndrome before chemotherapy initiation, a diagnosis of inherited anemia, an Hb concentration <10 g/L within 3 months before chemotherapy initiation, a transfusion within 2 weeks before chemotherapy initiation, radiation within 4 months before chemotherapy initiation, or bone marrow transplantation within 12 months before chemotherapy initiation or during the chemotherapy course. These exclusion criteria were applied to evaluate symptom burdens most likely related to CIA as opposed to other cancer treatment or pre-existing anemia.
CIA in this study was defined as moderate to severe anemia with Hb <10 g/dL after chemotherapy initiation. Based on this definition for CIA, all patients who developed CIA between the first chemotherapy administration to 60 days after the last dose of chemotherapy were included for the record review
Data collection
Data on anemia-related symptoms or signs and anemia-related comorbidities (Table 1) were collected by standardized review of physician record notes in the electronic medical records. A set of 24 anemia-related symptoms were identified based on the literature and clinical expertise and included abdominal pain, blurred vision/double vision/loss of vision, cold intolerance/coldness in hands or feet, depression/anxiety, diarrhea, dizziness/lightheadedness, dyspnea/shortness of breath/tachypnea, edema, fatigue, headache, heart failure, heat intolerance, hypotension, insomnia, leg pain, loss of appetite, nausea/vomiting, pale skin, palpitations/tachycardia, paralysis/ataxia/numbness or tingling in extremities, pectoral angina/chest pain, sweating/diaphoresis, syncope, and vertigo. Record review period was defined as 1 month before chemotherapy to 60 days after the last dose of chemotherapy in the first course. To understand the development of new symptoms during chemotherapy treatment, pre-existing symptoms documented within 1 month before chemotherapy initiation were recorded.
The data elements extracted included the date the symptom was documented, date the symptom started, symptom duration (when available), and any relevant comments regarding the symptom (ie, if dyspnea was at rest or on exertion, whether the symptom was a side effect caused by chemotherapy, or change in symptom severity). Ten percent of the records were reviewed independently by 2 abstractors to ensure quality control. Additional quality control measures included SAS algorithms (SAS Institute, Inc., Cary, North Carolina) to check reasonability and logical consistency in the abstracted data.
Patient demographic characteristics, cancer stage, additional selected comorbidities (Table 1), chemotherapy information, Hb test results, and anemia treatment, including erythrocyte stimulating agent (ESA) use and red blood cell transfusion, were collected using KPSC’s cancer registry and clinical databases. Anemia was defined by severity as grade 1 (10 g/dL to lower limit of normal, ie, 14 g/dL for men and 12 g/dL for women), grade 2 (8.0-9.9 g/dL), grade 3 (6.5-7.9 g/dL), and grade 4 (<6.5 g/dL) following the National Cancer Institute’s Common Terminology Criteria for Adverse Events.13
Statistical analysis
Distributions of demographic, cancer, and treatment characteristics were calculated by CIA status, overall and by cancer type. Differences between patients who did and did not develop CIA were assessed using chi-square test and Kruskal-Wallis test. For those who developed CIA, the distribution of the worst anemia grade was also calculated for each cycle of chemotherapy.
Next, the distributions for the following symptom categories were calculated in the 2 study samples defined by CIA status: pre-existing symptoms that occurred before chemotherapy, any symptoms during chemotherapy (ie, whether they started before chemotherapy), and incident symptoms during chemotherapy (ie, new symptoms that only started after chemotherapy). Specifically, the proportion of patients with each individual symptom and the distribution of the number of symptoms per patient were calculated. Differences in symptom distribution by CIA status were assessed using chi-square test.
The distribution of symptoms in each chemotherapy cycle was calculated up to 6 chemotherapy cycles (as >80% of the patients only had treatment up to 6 cycles) in the 2 study samples defined by CIA status. For this analysis, a symptom was “mapped” to a cycle if the date (or date range) of the symptom fell within the date range of that chemotherapy cycle. In patients who developed CIA, the distribution of symptoms was also calculated by anemia grade. This was again done on the chemotherapy cycle level. For each chemotherapy cycle, an anemia grade was assigned (no anemia or anemia grade 1, 2, 3, and 4) using the lowest Hb measurement in that cycle. Symptoms that occurred in a chemotherapy cycle were then “mapped” to the anemia grade of that cycle. Some patients had more than 1 anemia event of the same grade (eg, if a patient’s grade 2 anemia persist across cycles). For these patients, we randomly selected only 1 anemia event of the same grade from each patient to be included in this analysis. Patients could still contribute multiple events of different grades to this analysis. We calculated the mean number of symptoms per patient for each anemia grade (ie, 1-4) separately. Because of the small number of patients who developed grade 4 anemia (n = 11), they were combined with the grade 3 patients when the distributions of individual symptoms were evaluated.
All analyses were repeated stratified by gender. P values for differences between men and women were calculated using chi-square test or t test. All analyses were conducted using SAS version 9.3.
Results
A total of 402 stage IV NHL, breast, and lung cancer patients who developed CIA and 98 patients who did not develop CIA during the first course of chemotherapy were included (Figure 1).
The distribution of cancer types in the study sample were similar across CIA status (Table 1). The mean age at diagnosis was 66 years in patients who developed CIA and 62 years in patients who did not develop CIA. Women accounted for half of the patients in both study samples (52% and 51%, respectively). Most of the study patients were of non-Hispanic white race/ethnicity. Chronic obstructive pulmonary disease/emphysema and gastroesophageal reflux disease were among the most common comorbidities examined in both study samples, while malnutrition and moderate to severe renal disease were also common in patients who developed CIA (Table 1).
The mean Hb level before chemotherapy was lower for patients who developed CIA compared with patients who did not develop CIA (12.3 g/dL and 13.5 g/dL, respectively; Table 1). The mean lowest Hb level during chemotherapy was 8.5 g/dL for patients who developed CIA and 11.4 g/dL for patients without CIA (Table 1). The number of anemia events by grade in each chemotherapy cycle in patients who developed CIA is shown in Table 2.
Table 3 shows the number and proportion of study patients with each of the symptoms documented before and after chemotherapy initiation for the 2 study samples. Patients who developed CIA had statistically significantly more pre-existing symptoms, incident symptoms, or any symptoms that occurred during chemotherapy compared with patients who did not develop CIA.
Table 4 shows the number and proportion of study patients with symptoms that occurred during each chemotherapy cycle. Again, fatigue is the predominant symptom documented throughout cycles for all patients. In patients who developed CIA, the proportion of patients experiencing the following symptoms was relatively stable across chemotherapy cycles: depression/anxiety, dizziness/lightheadedness, fatigue, pale skin, and sweating. The proportion of patients experiencing paralysis/ataxia/numbness/tingling in extremities increased over cycles. For headache, loss of appetite, hypotension, and nausea/vomiting, the proportion of patients with symptom documentation was highest in cycle 1, stabilizing in subsequent cycles (Table 4). In patients without CIA, the cycle-level prevalence of most of the symptoms did not increase over cycles, except for paralysis/ataxia/numbness or tingling in extremities. For insomnia, loss of appetite, and nausea/vomiting, the cycle-level prevalence dropped after the first cycle. There was no clear increasing trend of the mean number of symptoms per patient across chemotherapy cycles in both study samples (Table 4).
Table 5 shows the distribution of symptoms by anemia grade in patients who developed CIA. In general, the prevalence of symptoms increased with higher grades of anemia. The following symptoms especially have a clear increase in prevalence as the severity of anemia progressed: abdominal pain, depression, diarrhea, dizziness/lightheadedness, dyspnea, edema, fatigue, heart failure, headache, hypotension, insomnia, leg pain, loss of appetite, pale skin, palpitations, pectoral angina, and sweating. The mean number of symptoms per patient increased as CIA grade increased, from 3.6 (SD, 2.9) for grade 2 CIA to 5.4 (SD, 3.5) for grades 3 and 4 CIA (specifically, 5.3 [SD, 3.4] for grade 3 CIA and 6.4 [SD, 4.1] for grade 4 CIA; data not shown) (Table 5).
When stratified by gender, there are no material differences between men and women in most analyses. In men, the mean number of pre-existing symptoms was 1.7 (SD, 1.8) and 1.0 (SD, 1.2) for those with and without CIA, respectively (P = .02). The mean number of symptoms that occurred during chemotherapy was 7.0 (SD, 3.4) and 4.2 (SD, 2.4), respectively (P < .01). In women, the mean number of pre-existing symptoms was not statistically different in those with and without CIA (1.6 [SD, 2.2] and 1.3 [SD, 1.8], respectively; P = .46). However, like in men, the mean number of symptoms that occurred during chemotherapy was significantly more in those with CIA (6.5 [SD, 3.3] and 4.0 [SD, 2.9], respectively; P < .01). As in the overall analysis, there was no clear increasing trend of the number of symptoms per patients across chemotherapy cycles in both men and women, but the average number of symptoms increased as the CIA grade increased. For men, the mean number of symptoms per patient increased from 3.7 (SD, 3.0) for grade 2 CIA to 6.0 (SD, 3.5) for grades 3 and 4 CIA (data not shown). For women, the mean number of symptoms per patient increased from 3.6 (SD, 2.9) for grade 2 CIA to 4.7 (SD, 3.3) for grades 3 and 4 CIA (data not shown).
Discussion
In this study, we described the number and type of symptoms documented in the medical record notes among stage IV NHL, breast cancer, and lung cancer patients who did or did not develop CIA during chemotherapy.
Our findings on the prevalence of fatigue are in line with other studies in the literature. Maxwell reported that the prevalence of fatigue was 80% to 96% in cancer patients.17 Cella and colleagues found that using FACT-General questionnaire, 75% of cancer patients reported fatigue.11 The comparability of our estimate and those found in studies based on patient self-report offered some assurance of the validity of assessing symptom prevalence through physician record notes. In addition to fatigue, we described prevalence of 23 additional symptoms, most of which have not been extensively studied in the literature. Gabrilove and colleagues found that a substantial proportion of patients with CIA had moderate to severe score for lack of appetite (36%) and disturbed sleep (41%) using the MDASI.10 The prevalence of loss of appetite and insomnia was around 50% and 25%, respectively, in our study samples. A 2013 systematic review of 21 multinational studies reported the pooled prevalence of several nonfatigue symptoms in cancer patients including headache (23%), sleep disturbance/insomnia (49%), appetite changes (45%), nausea/vomiting (26%), diarrhea (15%), depression (34%), dyspnea (44%), dizziness (26%), numbness/tingling (42%), edema (14%), and sweating (28%).18 Our prevalence estimates in patients with CIA for most of these symptoms were higher, likely because Reilly and colleagues used source studies that included any cancer patients undergoing treatment and not just those with CIA. Our findings on the increased symptom burden in patients who experienced episodes of advanced anemia compared with patients with mild anemia were also consistent with the literature. To this end, several studies using MDASI or the FACT-An reported differential symptom burdens by Hb level based on patient self-report,10,11,19 including data on improvement in symptom burden and quality of life after anemia was amended with the use of ESA.20,21
We found that the number of pre-existing symptoms was significantly higher in patients who went on to develop CIA than in patients who did not develop CIA. Specifically, fatigue, loss of appetite, and pale skin before chemotherapy seemed to be significantly more common in patients who went on to develop CIA. This finding suggested that presentation of these symptoms before chemotherapy initiation may be a predictor for developing moderate or severe anemia during treatment. This is a novel hypothesis, as no studies have evaluated the relationship between pretreatment symptom and risk of CIA. However, our study was not designed to address this specific question. Additional investigation is needed to further shed light on whether the occurrence of anemia-related symptoms in nonanemic patients can be used to effectively risk-stratify patients for subsequent CIA.
Contrary to our expectation, the prevalence of most symptoms did not clearly increase as chemotherapy progressed. There are several possible explanations to this phenomenon, with the most likely being related to reporting of anemia-related symptoms. For example, patients might stop reporting the same symptom repeatedly or become adjusted to the new Hb levels, leading to less symptom manifestation. Clinicians may also be less likely to ask about symptoms in later treatment cycles and/or to document chronic symptoms. Several symptoms were rarely documented altogether, such as cold intolerance, heat intolerance, heart failure, and vertigo. Symptoms reported in earlier cycles could also be managed successfully. Another possible explanation is differential loss of follow-up. Patients who experienced severe adverse events or symptoms may terminate treatment prematurely. Thus, symptom burden found toward later cycles may not represent the true symptom burden should everyone who initiated the chemotherapy treatment complete all planned cycles.
Limitations
In addition to the limitations already discussed, there are several others that should be considered when interpreting our results. We did not have a consistent measure of symptom severity in the medical records. Duration of symptoms was also often poorly documented by physicians. Therefore, our results are not directly comparable with studies such as the MDASI that incorporate severity or duration in their prevalence measure.
Despite the potential limitations, our study has several important strengths.
Conclusions
Our data provide physicians a comprehensive picture of prevalence of various types of symptoms and how symptom burden evolves as chemotherapy cycle and anemia severity progress. High-grade CIA correlates with an increased symptom burden.
1. Barrett-Lee PJ, Ludwig H, Birgegård G, et al. Independent risk factors for anemia in cancer patients receiving chemotherapy: results from the European Cancer Anaemia Survey. Oncology. 2006;70(1):34-48.
2. Kitano T, Tada H, Nishimura T, et al. Prevalence and incidence of anemia in Japanese cancer patients receiving outpatient chemotherapy. Int J Hematol. 2007;86(1):37-41.
3. Birgegård G, Aapro MS, Bokemeyer C, et al. Cancer-related anemia: pathogenesis, prevalence and treatment. Oncology. 2005;68(Suppl 1):3-11.
4. Harper P, Littlewood T. Anaemia of cancer: impact on patient fatigue and long-term outcome. Oncology. 2005;69(Suppl 2):2-7.
5. Nieboer P, Buijs C, Rodenhuis S, et al. Fatigue and relating factors in high-risk breast cancer patients treated with adjuvant standard or high-dose chemotherapy: a longitudinal study. J Clin Oncol. 2005;23(33):8296-8304.
6. Bremberg ER, Brandberg Y, Hising C, Friesland S, Eksborg S. Anemia and quality of life including anemia-related symptoms in patients with solid tumors in clinical practice. Med Oncol. 2007;24(1):95-102.
7. Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl 1):4-10.
8. Cleeland CS. Symptom burden: multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monogr. 2007(37):16-21.
9. Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63-74.
10. Gabrilove JL, Perez EA, Tomita DK, Rossi G, Cleeland CS. Assessing symptom burden using the M. D. Anderson symptom inventory in patients with chemotherapy-induced anemia: results of a multicenter, open-label study (SURPASS) of patients treated with darbepoetin-alpha at a dose of 200 microg every 2 weeks. Cancer. 2007;110(7):1629-1640.
11. Cella D. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34(3 Suppl 2):13-19.
12. Koebnick C, Langer-Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16(3):37-41.
13. Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst. 1999;91(19):1616-1634.
14. Gilreath JA, Stenehjem DD, Rodgers GM. Diagnosis and treatment of cancer-related anemia. Am J Hematol. 2014;89(2):203-212.
15. Rizzo JD, Somerfield MR, Hagerty KL, et al. Use of epoetin and darbepoetin in patients with cancer: 2007 American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update. J Clin Oncol. 2008;26(1):132-149.
16. Bohlius J, Tonia T, Nüesch E, et al. Effects of erythropoiesis-stimulating agents on fatigue- and anaemia-related symptoms in cancer patients: systematic review and meta-analyses of published and unpublished data. Br J Cancer. 2014;111(1):33-45.
17. Maxwell MB. When the cancer patient becomes anemic. Cancer Nurs. 1984;7(4):321-326.
18. Reilly CM, Bruner DW, Mitchell SA, et al. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer. 2013;21(6):1525-1550.
19. Crawford J, Cella D, Cleeland CS, et al. Relationship between changes in hemoglobin level and quality of life during chemotherapy in anemic cancer patients receiving epoetin alfa therapy. Cancer. 2002;95(4):888-895.
20. Mouysset JL, Freier B, van den Bosch J, et al. Hemoglobin levels and quality of life in patients with symptomatic chemotherapy-induced anemia: the eAQUA study. Cancer Manag Res. 2016;8:1-10.
21. Vansteenkiste J, Pirker R, Massuti B, et al. Double-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst. 2002;94(16):1211-1220.
22. Kleinman L, Benjamin K, Viswanathan H, et al. The anemia impact measure (AIM): development and content validation of a patient-reported outcome measure of anemia symptoms and symptom impacts in cancer patients receiving chemotherapy. Qual Life Res. 2012;21(7):1255-1266.
1. Barrett-Lee PJ, Ludwig H, Birgegård G, et al. Independent risk factors for anemia in cancer patients receiving chemotherapy: results from the European Cancer Anaemia Survey. Oncology. 2006;70(1):34-48.
2. Kitano T, Tada H, Nishimura T, et al. Prevalence and incidence of anemia in Japanese cancer patients receiving outpatient chemotherapy. Int J Hematol. 2007;86(1):37-41.
3. Birgegård G, Aapro MS, Bokemeyer C, et al. Cancer-related anemia: pathogenesis, prevalence and treatment. Oncology. 2005;68(Suppl 1):3-11.
4. Harper P, Littlewood T. Anaemia of cancer: impact on patient fatigue and long-term outcome. Oncology. 2005;69(Suppl 2):2-7.
5. Nieboer P, Buijs C, Rodenhuis S, et al. Fatigue and relating factors in high-risk breast cancer patients treated with adjuvant standard or high-dose chemotherapy: a longitudinal study. J Clin Oncol. 2005;23(33):8296-8304.
6. Bremberg ER, Brandberg Y, Hising C, Friesland S, Eksborg S. Anemia and quality of life including anemia-related symptoms in patients with solid tumors in clinical practice. Med Oncol. 2007;24(1):95-102.
7. Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl 1):4-10.
8. Cleeland CS. Symptom burden: multiple symptoms and their impact as patient-reported outcomes. J Natl Cancer Inst Monogr. 2007(37):16-21.
9. Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63-74.
10. Gabrilove JL, Perez EA, Tomita DK, Rossi G, Cleeland CS. Assessing symptom burden using the M. D. Anderson symptom inventory in patients with chemotherapy-induced anemia: results of a multicenter, open-label study (SURPASS) of patients treated with darbepoetin-alpha at a dose of 200 microg every 2 weeks. Cancer. 2007;110(7):1629-1640.
11. Cella D. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34(3 Suppl 2):13-19.
12. Koebnick C, Langer-Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16(3):37-41.
13. Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst. 1999;91(19):1616-1634.
14. Gilreath JA, Stenehjem DD, Rodgers GM. Diagnosis and treatment of cancer-related anemia. Am J Hematol. 2014;89(2):203-212.
15. Rizzo JD, Somerfield MR, Hagerty KL, et al. Use of epoetin and darbepoetin in patients with cancer: 2007 American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update. J Clin Oncol. 2008;26(1):132-149.
16. Bohlius J, Tonia T, Nüesch E, et al. Effects of erythropoiesis-stimulating agents on fatigue- and anaemia-related symptoms in cancer patients: systematic review and meta-analyses of published and unpublished data. Br J Cancer. 2014;111(1):33-45.
17. Maxwell MB. When the cancer patient becomes anemic. Cancer Nurs. 1984;7(4):321-326.
18. Reilly CM, Bruner DW, Mitchell SA, et al. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer. 2013;21(6):1525-1550.
19. Crawford J, Cella D, Cleeland CS, et al. Relationship between changes in hemoglobin level and quality of life during chemotherapy in anemic cancer patients receiving epoetin alfa therapy. Cancer. 2002;95(4):888-895.
20. Mouysset JL, Freier B, van den Bosch J, et al. Hemoglobin levels and quality of life in patients with symptomatic chemotherapy-induced anemia: the eAQUA study. Cancer Manag Res. 2016;8:1-10.
21. Vansteenkiste J, Pirker R, Massuti B, et al. Double-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. J Natl Cancer Inst. 2002;94(16):1211-1220.
22. Kleinman L, Benjamin K, Viswanathan H, et al. The anemia impact measure (AIM): development and content validation of a patient-reported outcome measure of anemia symptoms and symptom impacts in cancer patients receiving chemotherapy. Qual Life Res. 2012;21(7):1255-1266.
Patterns of malignancies in patients with HIV-AIDS: a single institution observational study
India has the third largest HIV epidemic in the world because of its large population size, with 0.3% of the adult population infected with HIV. That translates to 2.1 million infected people, posing a significant challenge in the management of these individuals.1 In all, 43% of the infected are currently on highly active antiretroviral therapy (HAART).1 There has been a significant decrease in the number of HIV-AIDS–related deaths in recent years because of the remarkable increase in the use of antiretroviral therapy.2 However, the prolonged life expectancy in these patients has resulted in an increase in the risk of various new diseases such as cancers. With the complex interactions between altered immunity and infections, the risk of cancers is markedly increased in patients with HIV-AIDS.3 The spectrum of malignancies in this group of patients differs from that in the general population. In addition, the pattern and the magnitude of malignancies differ in different parts of the world.4 In this study, we have analyzed the pattern of malignancies in patients with HIV-AIDS in a regional cancer center in India. The aim of the study was to analyze the pattern of malignancies in patients with HIV-AIDS based on their age and sex and to document the CD4 counts at the time the malignancy was diagnosed.
Methods
We retrieved data from our institution’s medical records department on all patients who had HIV-AIDS and had been diagnosed with a malignancy. Data of all patients presenting with a malignancy and coexisting HIV-AIDS from January 2013 through December 2016 were analyzed initially. Only patients for whom there was a documented CD4 count were included in the final retrospective analysis. We analyzed the correlation between the patients’ CD4 counts and malignancies subclassified as AIDS-defining malignancies (ADMs; aggressive B-cell non-Hodgkin lymphoma [NHL] and cervical cancer) or non–AIDS-defining malignancies (NADMs; all other malignancies other than aggressive NHL and carcinoma cervix were defined as NADM). We also analyzed the correlation between the CD4 count and NHL and other malignancies. A statistical analysis was performed using SPSS Statistics for Windows, version 23 (IBM Corp, Armonk, NY). The independent sample Mann-Whitney U or Kruskal-Wallis tests were used for comparing the CD4 counts between the various subgroups of malignancies. The study was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Results
A total of 370 patients who were diagnosed with malignancy and have coexisting HIV-AIDS were identified. In all, 85 patients were excluded because there were no CD4 counts available for them, and the remaining 285 patients were included in the final analysis. Of that total, 136 patients (48%) were men, and 149 (52%) were women.
The median age of the population was 44.8 years (5-80 years) at the time of diagnosis with malignancy. The mean CD4 count of the entire population was 235.4 cells/mm3 (50-734 cells/mm3). There were 104 patients with CD4 counts of ≤200 cells/mm3, and 181 patients had CD4 counts of >200 cells/mm3 (Table 1). All patients received the HAART regimen, efavirenz-lamuvidine-tenofovir (600 mg/300 mg/300 mg Telura).
The most common malignancies in this population were gynecologic malignancies, followed by hematologic malignancies. Cervical cancer was the most common malignancy among women as well as in the overall study population. Among men, the most common malignancy was NHL. The second and third most common malignancies in men were carcinoma oral cavity and carcinoma oropharynx, respectively, whereas in women, they were NHL and breast cancer. The distribution of various hematologic, head and neck, and gastrointestinal malignancies in this group of patients is shown in Figures 1, 2, and 3.
The ADMs in the study were NHL, including 2 patients diagnosed with primary central nervous system (CNS) lymphomas, and cervical cancer. No case of Kaposi sarcoma, also considered an ADM, was identified in this study. The common NADMs include head and neck malignancies (Figure 2), gastrointestinal malignancies (Figure 3), gynecological and genitourinary malignancies, and breast cancer. The mean CD4 count in the ADM subgroup was 221 cells/mm3, and in the NADM subgroup, it was 250 cells/mm3. There was a significant difference in the distribution of CD4 counts between the ADM and NADM subgroups (P = .03; Mann-Whitney U test). A statistical difference was also noted when the CD4 counts of the patients with NHL were compared with other malignancies (P = .0001; Mann-Whitney U test) There was no statistically significant difference noted when CD4 counts of patients with cervical cancer were compared with NADMs (P = .914).
Discussion
In 2015, a report from the Indian government estimated the prevalence of HIV in the country as 0.26% (0.22%-0.32%).5 The report also noted a decreasing trend in the number of new cases of HIV diagnosed and a decrease in the number of AIDS-related deaths.5 The decrease in deaths from AIDS is primarily attributed to the widespread use of HAART. With the introduction of HAART therapy, the survival of patients diagnosed with HIV-AIDS has increased markedly.6 However, newer challenges have emerged with improved survival, such as an increasing number of patients being diagnosed with malignancies. In the current HAART era, the pattern of malignancies in people living with HIV-AIDS has changed compared with the pre-HAART era.7 The literature suggests that worldwide, malignancies are encountered in about 30% patients with HIV-AIDS, but that percentage differs sharply from that encountered in India, where it is less than 5%.8 This may partly be explained by opportunistic infections such as tuberculosis in Indian patients, which remains the leading cause of death in the HIV-AIDS population. In our study, we retrospectively analyzed the pattern of malignancies in patients with HIV-AIDS.
Although few studies have quoted NHL as the predominant malignancy in their patients with HIV-AIDS, the predominant malignancy was cervical cancer in our patient population, as seen in few other studies.8-10 Head and neck malignancies also continue to be common malignancies in men with HIV-AIDS.10 Thus, an increase in malignancies induced by the human papillomavirus (HPV) can be seen in this group of patients. Only a few pediatric malignancies were noted in our study, and all of those patients had a vertical transmission of HIV.
Kaposi sarcoma is quite rare in the Indian population, and no case of Kaposi sarcoma was diagnosed in our study population. A similar finding was seen in several earlier publications from India. In the largest published series from India by Dhir and colleagues, evaluating 251 patients with HIV-AIDS and malignancy, no case of Kaposi sarcoma was reported.10 The authors mentioned that this finding might be because of the low seroprevalence of Kaposi sarcoma-associated herpesvirus in the Asian population.10 Three different studies from southern India have also not reported the incidence of Kaposi Sarcoma in their series of HIV-AIDS patients with malignancies,11-13 and similar findings were also reported in a study from northern India.9 The incidence of other immunodeficiency-related malignancies was identical to those reported in other studies in the literature.10,14
As seen in other studies, the CD4 counts in patients with ADM were significantly lower compared with those of patients with NADM, and that difference was not seen when CD4 counts of patients with cervical cancer were compared with patients in the NADM subgroup. The risk of NHL increases proportionally to the degree of immune suppression. The increased susceptibility to various infections in patients with low CD4 counts may also contribute to the occurrence of NHL in patients with low CD4 counts. The occurrence of various other rare cancers in patients with HIV-AIDS may be because of confounding rather than a direct HIV or immunosuppression effect.
An increasing incidence of NADMs has been noted in the Western literature.7,14 ADMs remain the most common malignancies in the HIV-AIDS population, accounting for about 48% of all malignancies.8 This is in concordance with previous publications from India.8,10 With the widespread availability of generic HAART, the incidence of ADMs may decrease even more in the future. In developing countries where the screening procedures for malignancies in both the general population and patients with HIV-AIDS have not yet been implemented at a national level, premalignant lesions of the cervix are not detected.10 Cervical cancer is the most common malignancy in our study population, which underscores the importance of cervical cancer screening in patients with HIV-AIDS.
In the developed countries, following the introduction of HAART in HIV-AIDS management, the incidence of Kaposi sarcoma decreased by 60% to 70%, and the incidence of NHL decreased by 30% to 50%, whereas the rates of cervical cancer remained either stable or declined.15,16 Despite the declining trend, the incidence of these malignancies continues to be high among patients with HIV-AIDS compared with the general population.17 A study from the United States showed increasing trends in various NADMs (such as anal, lung, and liver cancers and Hodgkin lymphoma) from 2006 to 2010.17 In 2003, the number of patients with NADM were higher than the number of patients with ADM in the United States.14 In a population-based study from Brazil, ADMs were the most common malignancies diagnosed in patients with HIV-AIDS. A declining trend was noted in the incidence of ADMs in the population and an increasing trend in the incidence of NADMs. This increase in NADM incidence was contributed by anal and lung cancers.18 Studies from developing countries such as Uganda and Botswana have also shown a decrease in the incidence of Kaposi sarcoma after the introduction of HAART.19-21
Kaposi sarcoma, cervical cancer, NHL (including Burkitt lymphoma, immunoblastic lymphoma, and primary CNS lymphoma [PCNSL]) comprise ADMs. All 3 ADMs have an underlying viral infection as the causative agent.22 Kaposi sarcoma is caused by the Kaposi sarcoma herpes virus, for which seroprevalence varies worldwide.23 As already noted in this article, the incidence of Kaposi sarcoma among the HIV-AIDS population has decreased worldwide since the introduction of HAART. The preinvasive uterine cervix lesions and carcinoma cervix are caused by HPV. NHL in patients with HIV-AIDS is a predominantly aggressive B-cell neoplasm. Epstein-Barr virus is implicated for most of the ADM NHLs.24 PCNSL occurs in patients with low CD4 counts and poses a diagnostic challenge. The treatment outcomes for patients with PCNSL before the HAART era were dismal. With the widespread use of HAART, the treatment outcomes of patients with HIV-AIDS and NHL improved, and, currently, these patients are managed the same way as other patients with NHL.22
The increasing incidence of the NADM is partly attributed to the increasing incidence of these malignancies in the general population. An elevated risk of certain NADMs is also attributable to viral infections. The common NADMs in the United States are lung, anal, oropharyngeal, and hepatocellular cancers and Hodgkin lymphoma.14 The common NADMs in our study population were oral, oropharyngeal, colon, and breast cancers and Hodgkin lymphoma. One-third of head and neck cancers, including most oropharyngeal cancers, and cervical and anal cancers in patients with HIV-AIDS are related to HPV.25 Patients with HIV-AIDS are at increased risk for chronic HPV infection from immunosuppression. Chronic HPV infections and prolonged immunosuppression cause premalignant high-grade squamous intraepithelial lesions and invasive cancers.22 The initiation of and adherence to HAART leads to immune recovery and reduces high-risk HPV-associated morbidity.26 Findings from previous studies have demonstrated the benefits of screening for cervical cancer in patients with HIV-AIDS.27 The HPV vaccine is immunogenic in patients with HIV-AIDS and might help prevent HPV-associated malignancies.28
Conclusions
With the wide use of HAART by patients with HIV-AIDS, we can expect an increase in the survival of that population. The incidence of malignancies may also increase significantly in these patients, and further longitudinal studies are needed, as malignancies may emerge as the most common cause of death in patients with HIV-AIDS. In addition, the extensive use of HAART therapy and implementation of screening programs for cervical cancer in patients with HIV-AIDS could result in a decrease in the incidence of ADMs.
1. UNAIDS. Prevention gap report. http://www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf. Released 2016. Accessed December 27, 2017.
3. Dubrow R, Silverberg MJ, Park LS, Crothers K, Justice AC. HIV infection, aging, and immune function: implications for cancer risk and prevention. Curr Opin Oncol. 2012;24(5):506-516.
4. Biggar RJ, Chaturvedi AK, Bhatia K, Mbulaiteye SM. Cancer risk in persons with HIV-AIDS in India: a review and future directions for research. Infect Agent Cancer. 2009;4:4.
5. National AIDS Control Organisation & National Institute of Medical Statistics, ICMR, Ministry of Health & Family Welfare, Government of India. India HIV estimations 2015, technical report. http://www.naco.gov.in/sites/default/files/India%20HIV%20Estimations%202015.pdf. Published 2015. Accessed December 27, 2017.
6. Bonnet F, Lewden C, May T, et al. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer. 2004;101(2):317-324.
7. Crum-Cianflone N, Hullsiek KH, Marconi V, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS. 2009;23(1):41-50.
8. Sharma S, Soneja M, Ranjan S. Malignancies in human immunodeficiency virus infected patients in India: initial experience in the HAART era. Indian J Med Res. 2015;142(5):563-567.
9. Sachdeva RK, Sharma A, Singh S, Varma S. Spectrum of AIDS defining & non-AIDS defining malignancies in north India. In
10. Dhir AA, Sawant S, Dikshit RP, et al. Spectrum of HIV-AIDS related cancers in India. Cancer Causes Control. 2007;19(2):147-153.
11. Venkatesh KK, Saghayam S, Devaleenal B, et al. Spectrum of malignancies among HIV-infected patients in South India. Indian J Cancer. 2012;49(1):176-180.
12. Shruti P, Narayanan G, Puthuveettil J, Jayasree K, Vijayalakshmi K. Spectrum of HIV/AIDS-associated cancers in south India. J Clin Oncol. 2014;32(suppl):e12534.
13. Paul TR, Uppin MS, Uppin SG, et al. Spectrum of malignancies in human immunodeficiency virus–positive patients at a Tertiary Care Centre in South India. Indian J Cancer. 2014;51(4):459-463.
14. Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103(9):753-762.
15. Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148(10):728-736.
16. Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187-194.
17. Robbins HA, Shiels MS, Pfeiffer RM, Engels EA. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States. AIDS. 2014;28(6):881-890.
18. Tanaka LF, Latorre MDRD, Gutierrez EB, Heumann C, Herbinger KH, Froeschl G. Trends in the incidence of AIDS-defining and non-AIDS-defining cancers in people living with AIDS: a population-based study from São Paulo, Brazil. Int J STD AIDS. 2017;28(12):1190-1198.
19. Mutyaba I, Phipps W, Krantz EM, et al. A population-level evaluation of the effect of antiretroviral therapy on cancer incidence in Kyadondo County, Uganda, 1999–2008. J Acquir Immune Defic Syndr. 2015;69(4):481-486.
20. Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, et al. Cancer incidence following expansion of HIV treatment in Botswana. PLoS ONE. 2015;10(8):e0135602.
21. Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12(1):6-11.
22. Yarchoan R, Uldrick TS. HIV-associated cancers and related diseases. N Engl J Med. 2018;378(11):1029-1041.
23. Gao SJ, Kingsley L, Li M, et al. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med. 1996;2(8):925-928.
24. Epstein-Barr virus and AIDS-associated lymphomas. Lancet. 1991;338(8773):979-981.
25. Picard A, Badoual C, Hourseau M, et al. Human papilloma virus prevalence in HIV patients with head and neck squamous cell carcinoma. AIDS. 2016;30(8):1257-1266.
26. Minkoff H, Zhong Y, Burk RD, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Infect Dis. 2010;201(5):681-690.
27. Ghebre RG, Grover S, Xu MJ, Chuang LT, Simonds H. Cervical cancer control in HIV-infected women: past, present and future. Gynecol Oncol Rep. 2017;21:101-108.
28. Kojic EM, Rana AI, Cu-Uvin S. Human papillomavirus vaccination in HIV-infected women: need for increased coverage. Expert Rev Vaccines. 2016;15(1):105-117.
India has the third largest HIV epidemic in the world because of its large population size, with 0.3% of the adult population infected with HIV. That translates to 2.1 million infected people, posing a significant challenge in the management of these individuals.1 In all, 43% of the infected are currently on highly active antiretroviral therapy (HAART).1 There has been a significant decrease in the number of HIV-AIDS–related deaths in recent years because of the remarkable increase in the use of antiretroviral therapy.2 However, the prolonged life expectancy in these patients has resulted in an increase in the risk of various new diseases such as cancers. With the complex interactions between altered immunity and infections, the risk of cancers is markedly increased in patients with HIV-AIDS.3 The spectrum of malignancies in this group of patients differs from that in the general population. In addition, the pattern and the magnitude of malignancies differ in different parts of the world.4 In this study, we have analyzed the pattern of malignancies in patients with HIV-AIDS in a regional cancer center in India. The aim of the study was to analyze the pattern of malignancies in patients with HIV-AIDS based on their age and sex and to document the CD4 counts at the time the malignancy was diagnosed.
Methods
We retrieved data from our institution’s medical records department on all patients who had HIV-AIDS and had been diagnosed with a malignancy. Data of all patients presenting with a malignancy and coexisting HIV-AIDS from January 2013 through December 2016 were analyzed initially. Only patients for whom there was a documented CD4 count were included in the final retrospective analysis. We analyzed the correlation between the patients’ CD4 counts and malignancies subclassified as AIDS-defining malignancies (ADMs; aggressive B-cell non-Hodgkin lymphoma [NHL] and cervical cancer) or non–AIDS-defining malignancies (NADMs; all other malignancies other than aggressive NHL and carcinoma cervix were defined as NADM). We also analyzed the correlation between the CD4 count and NHL and other malignancies. A statistical analysis was performed using SPSS Statistics for Windows, version 23 (IBM Corp, Armonk, NY). The independent sample Mann-Whitney U or Kruskal-Wallis tests were used for comparing the CD4 counts between the various subgroups of malignancies. The study was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Results
A total of 370 patients who were diagnosed with malignancy and have coexisting HIV-AIDS were identified. In all, 85 patients were excluded because there were no CD4 counts available for them, and the remaining 285 patients were included in the final analysis. Of that total, 136 patients (48%) were men, and 149 (52%) were women.
The median age of the population was 44.8 years (5-80 years) at the time of diagnosis with malignancy. The mean CD4 count of the entire population was 235.4 cells/mm3 (50-734 cells/mm3). There were 104 patients with CD4 counts of ≤200 cells/mm3, and 181 patients had CD4 counts of >200 cells/mm3 (Table 1). All patients received the HAART regimen, efavirenz-lamuvidine-tenofovir (600 mg/300 mg/300 mg Telura).
The most common malignancies in this population were gynecologic malignancies, followed by hematologic malignancies. Cervical cancer was the most common malignancy among women as well as in the overall study population. Among men, the most common malignancy was NHL. The second and third most common malignancies in men were carcinoma oral cavity and carcinoma oropharynx, respectively, whereas in women, they were NHL and breast cancer. The distribution of various hematologic, head and neck, and gastrointestinal malignancies in this group of patients is shown in Figures 1, 2, and 3.
The ADMs in the study were NHL, including 2 patients diagnosed with primary central nervous system (CNS) lymphomas, and cervical cancer. No case of Kaposi sarcoma, also considered an ADM, was identified in this study. The common NADMs include head and neck malignancies (Figure 2), gastrointestinal malignancies (Figure 3), gynecological and genitourinary malignancies, and breast cancer. The mean CD4 count in the ADM subgroup was 221 cells/mm3, and in the NADM subgroup, it was 250 cells/mm3. There was a significant difference in the distribution of CD4 counts between the ADM and NADM subgroups (P = .03; Mann-Whitney U test). A statistical difference was also noted when the CD4 counts of the patients with NHL were compared with other malignancies (P = .0001; Mann-Whitney U test) There was no statistically significant difference noted when CD4 counts of patients with cervical cancer were compared with NADMs (P = .914).
Discussion
In 2015, a report from the Indian government estimated the prevalence of HIV in the country as 0.26% (0.22%-0.32%).5 The report also noted a decreasing trend in the number of new cases of HIV diagnosed and a decrease in the number of AIDS-related deaths.5 The decrease in deaths from AIDS is primarily attributed to the widespread use of HAART. With the introduction of HAART therapy, the survival of patients diagnosed with HIV-AIDS has increased markedly.6 However, newer challenges have emerged with improved survival, such as an increasing number of patients being diagnosed with malignancies. In the current HAART era, the pattern of malignancies in people living with HIV-AIDS has changed compared with the pre-HAART era.7 The literature suggests that worldwide, malignancies are encountered in about 30% patients with HIV-AIDS, but that percentage differs sharply from that encountered in India, where it is less than 5%.8 This may partly be explained by opportunistic infections such as tuberculosis in Indian patients, which remains the leading cause of death in the HIV-AIDS population. In our study, we retrospectively analyzed the pattern of malignancies in patients with HIV-AIDS.
Although few studies have quoted NHL as the predominant malignancy in their patients with HIV-AIDS, the predominant malignancy was cervical cancer in our patient population, as seen in few other studies.8-10 Head and neck malignancies also continue to be common malignancies in men with HIV-AIDS.10 Thus, an increase in malignancies induced by the human papillomavirus (HPV) can be seen in this group of patients. Only a few pediatric malignancies were noted in our study, and all of those patients had a vertical transmission of HIV.
Kaposi sarcoma is quite rare in the Indian population, and no case of Kaposi sarcoma was diagnosed in our study population. A similar finding was seen in several earlier publications from India. In the largest published series from India by Dhir and colleagues, evaluating 251 patients with HIV-AIDS and malignancy, no case of Kaposi sarcoma was reported.10 The authors mentioned that this finding might be because of the low seroprevalence of Kaposi sarcoma-associated herpesvirus in the Asian population.10 Three different studies from southern India have also not reported the incidence of Kaposi Sarcoma in their series of HIV-AIDS patients with malignancies,11-13 and similar findings were also reported in a study from northern India.9 The incidence of other immunodeficiency-related malignancies was identical to those reported in other studies in the literature.10,14
As seen in other studies, the CD4 counts in patients with ADM were significantly lower compared with those of patients with NADM, and that difference was not seen when CD4 counts of patients with cervical cancer were compared with patients in the NADM subgroup. The risk of NHL increases proportionally to the degree of immune suppression. The increased susceptibility to various infections in patients with low CD4 counts may also contribute to the occurrence of NHL in patients with low CD4 counts. The occurrence of various other rare cancers in patients with HIV-AIDS may be because of confounding rather than a direct HIV or immunosuppression effect.
An increasing incidence of NADMs has been noted in the Western literature.7,14 ADMs remain the most common malignancies in the HIV-AIDS population, accounting for about 48% of all malignancies.8 This is in concordance with previous publications from India.8,10 With the widespread availability of generic HAART, the incidence of ADMs may decrease even more in the future. In developing countries where the screening procedures for malignancies in both the general population and patients with HIV-AIDS have not yet been implemented at a national level, premalignant lesions of the cervix are not detected.10 Cervical cancer is the most common malignancy in our study population, which underscores the importance of cervical cancer screening in patients with HIV-AIDS.
In the developed countries, following the introduction of HAART in HIV-AIDS management, the incidence of Kaposi sarcoma decreased by 60% to 70%, and the incidence of NHL decreased by 30% to 50%, whereas the rates of cervical cancer remained either stable or declined.15,16 Despite the declining trend, the incidence of these malignancies continues to be high among patients with HIV-AIDS compared with the general population.17 A study from the United States showed increasing trends in various NADMs (such as anal, lung, and liver cancers and Hodgkin lymphoma) from 2006 to 2010.17 In 2003, the number of patients with NADM were higher than the number of patients with ADM in the United States.14 In a population-based study from Brazil, ADMs were the most common malignancies diagnosed in patients with HIV-AIDS. A declining trend was noted in the incidence of ADMs in the population and an increasing trend in the incidence of NADMs. This increase in NADM incidence was contributed by anal and lung cancers.18 Studies from developing countries such as Uganda and Botswana have also shown a decrease in the incidence of Kaposi sarcoma after the introduction of HAART.19-21
Kaposi sarcoma, cervical cancer, NHL (including Burkitt lymphoma, immunoblastic lymphoma, and primary CNS lymphoma [PCNSL]) comprise ADMs. All 3 ADMs have an underlying viral infection as the causative agent.22 Kaposi sarcoma is caused by the Kaposi sarcoma herpes virus, for which seroprevalence varies worldwide.23 As already noted in this article, the incidence of Kaposi sarcoma among the HIV-AIDS population has decreased worldwide since the introduction of HAART. The preinvasive uterine cervix lesions and carcinoma cervix are caused by HPV. NHL in patients with HIV-AIDS is a predominantly aggressive B-cell neoplasm. Epstein-Barr virus is implicated for most of the ADM NHLs.24 PCNSL occurs in patients with low CD4 counts and poses a diagnostic challenge. The treatment outcomes for patients with PCNSL before the HAART era were dismal. With the widespread use of HAART, the treatment outcomes of patients with HIV-AIDS and NHL improved, and, currently, these patients are managed the same way as other patients with NHL.22
The increasing incidence of the NADM is partly attributed to the increasing incidence of these malignancies in the general population. An elevated risk of certain NADMs is also attributable to viral infections. The common NADMs in the United States are lung, anal, oropharyngeal, and hepatocellular cancers and Hodgkin lymphoma.14 The common NADMs in our study population were oral, oropharyngeal, colon, and breast cancers and Hodgkin lymphoma. One-third of head and neck cancers, including most oropharyngeal cancers, and cervical and anal cancers in patients with HIV-AIDS are related to HPV.25 Patients with HIV-AIDS are at increased risk for chronic HPV infection from immunosuppression. Chronic HPV infections and prolonged immunosuppression cause premalignant high-grade squamous intraepithelial lesions and invasive cancers.22 The initiation of and adherence to HAART leads to immune recovery and reduces high-risk HPV-associated morbidity.26 Findings from previous studies have demonstrated the benefits of screening for cervical cancer in patients with HIV-AIDS.27 The HPV vaccine is immunogenic in patients with HIV-AIDS and might help prevent HPV-associated malignancies.28
Conclusions
With the wide use of HAART by patients with HIV-AIDS, we can expect an increase in the survival of that population. The incidence of malignancies may also increase significantly in these patients, and further longitudinal studies are needed, as malignancies may emerge as the most common cause of death in patients with HIV-AIDS. In addition, the extensive use of HAART therapy and implementation of screening programs for cervical cancer in patients with HIV-AIDS could result in a decrease in the incidence of ADMs.
India has the third largest HIV epidemic in the world because of its large population size, with 0.3% of the adult population infected with HIV. That translates to 2.1 million infected people, posing a significant challenge in the management of these individuals.1 In all, 43% of the infected are currently on highly active antiretroviral therapy (HAART).1 There has been a significant decrease in the number of HIV-AIDS–related deaths in recent years because of the remarkable increase in the use of antiretroviral therapy.2 However, the prolonged life expectancy in these patients has resulted in an increase in the risk of various new diseases such as cancers. With the complex interactions between altered immunity and infections, the risk of cancers is markedly increased in patients with HIV-AIDS.3 The spectrum of malignancies in this group of patients differs from that in the general population. In addition, the pattern and the magnitude of malignancies differ in different parts of the world.4 In this study, we have analyzed the pattern of malignancies in patients with HIV-AIDS in a regional cancer center in India. The aim of the study was to analyze the pattern of malignancies in patients with HIV-AIDS based on their age and sex and to document the CD4 counts at the time the malignancy was diagnosed.
Methods
We retrieved data from our institution’s medical records department on all patients who had HIV-AIDS and had been diagnosed with a malignancy. Data of all patients presenting with a malignancy and coexisting HIV-AIDS from January 2013 through December 2016 were analyzed initially. Only patients for whom there was a documented CD4 count were included in the final retrospective analysis. We analyzed the correlation between the patients’ CD4 counts and malignancies subclassified as AIDS-defining malignancies (ADMs; aggressive B-cell non-Hodgkin lymphoma [NHL] and cervical cancer) or non–AIDS-defining malignancies (NADMs; all other malignancies other than aggressive NHL and carcinoma cervix were defined as NADM). We also analyzed the correlation between the CD4 count and NHL and other malignancies. A statistical analysis was performed using SPSS Statistics for Windows, version 23 (IBM Corp, Armonk, NY). The independent sample Mann-Whitney U or Kruskal-Wallis tests were used for comparing the CD4 counts between the various subgroups of malignancies. The study was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Results
A total of 370 patients who were diagnosed with malignancy and have coexisting HIV-AIDS were identified. In all, 85 patients were excluded because there were no CD4 counts available for them, and the remaining 285 patients were included in the final analysis. Of that total, 136 patients (48%) were men, and 149 (52%) were women.
The median age of the population was 44.8 years (5-80 years) at the time of diagnosis with malignancy. The mean CD4 count of the entire population was 235.4 cells/mm3 (50-734 cells/mm3). There were 104 patients with CD4 counts of ≤200 cells/mm3, and 181 patients had CD4 counts of >200 cells/mm3 (Table 1). All patients received the HAART regimen, efavirenz-lamuvidine-tenofovir (600 mg/300 mg/300 mg Telura).
The most common malignancies in this population were gynecologic malignancies, followed by hematologic malignancies. Cervical cancer was the most common malignancy among women as well as in the overall study population. Among men, the most common malignancy was NHL. The second and third most common malignancies in men were carcinoma oral cavity and carcinoma oropharynx, respectively, whereas in women, they were NHL and breast cancer. The distribution of various hematologic, head and neck, and gastrointestinal malignancies in this group of patients is shown in Figures 1, 2, and 3.
The ADMs in the study were NHL, including 2 patients diagnosed with primary central nervous system (CNS) lymphomas, and cervical cancer. No case of Kaposi sarcoma, also considered an ADM, was identified in this study. The common NADMs include head and neck malignancies (Figure 2), gastrointestinal malignancies (Figure 3), gynecological and genitourinary malignancies, and breast cancer. The mean CD4 count in the ADM subgroup was 221 cells/mm3, and in the NADM subgroup, it was 250 cells/mm3. There was a significant difference in the distribution of CD4 counts between the ADM and NADM subgroups (P = .03; Mann-Whitney U test). A statistical difference was also noted when the CD4 counts of the patients with NHL were compared with other malignancies (P = .0001; Mann-Whitney U test) There was no statistically significant difference noted when CD4 counts of patients with cervical cancer were compared with NADMs (P = .914).
Discussion
In 2015, a report from the Indian government estimated the prevalence of HIV in the country as 0.26% (0.22%-0.32%).5 The report also noted a decreasing trend in the number of new cases of HIV diagnosed and a decrease in the number of AIDS-related deaths.5 The decrease in deaths from AIDS is primarily attributed to the widespread use of HAART. With the introduction of HAART therapy, the survival of patients diagnosed with HIV-AIDS has increased markedly.6 However, newer challenges have emerged with improved survival, such as an increasing number of patients being diagnosed with malignancies. In the current HAART era, the pattern of malignancies in people living with HIV-AIDS has changed compared with the pre-HAART era.7 The literature suggests that worldwide, malignancies are encountered in about 30% patients with HIV-AIDS, but that percentage differs sharply from that encountered in India, where it is less than 5%.8 This may partly be explained by opportunistic infections such as tuberculosis in Indian patients, which remains the leading cause of death in the HIV-AIDS population. In our study, we retrospectively analyzed the pattern of malignancies in patients with HIV-AIDS.
Although few studies have quoted NHL as the predominant malignancy in their patients with HIV-AIDS, the predominant malignancy was cervical cancer in our patient population, as seen in few other studies.8-10 Head and neck malignancies also continue to be common malignancies in men with HIV-AIDS.10 Thus, an increase in malignancies induced by the human papillomavirus (HPV) can be seen in this group of patients. Only a few pediatric malignancies were noted in our study, and all of those patients had a vertical transmission of HIV.
Kaposi sarcoma is quite rare in the Indian population, and no case of Kaposi sarcoma was diagnosed in our study population. A similar finding was seen in several earlier publications from India. In the largest published series from India by Dhir and colleagues, evaluating 251 patients with HIV-AIDS and malignancy, no case of Kaposi sarcoma was reported.10 The authors mentioned that this finding might be because of the low seroprevalence of Kaposi sarcoma-associated herpesvirus in the Asian population.10 Three different studies from southern India have also not reported the incidence of Kaposi Sarcoma in their series of HIV-AIDS patients with malignancies,11-13 and similar findings were also reported in a study from northern India.9 The incidence of other immunodeficiency-related malignancies was identical to those reported in other studies in the literature.10,14
As seen in other studies, the CD4 counts in patients with ADM were significantly lower compared with those of patients with NADM, and that difference was not seen when CD4 counts of patients with cervical cancer were compared with patients in the NADM subgroup. The risk of NHL increases proportionally to the degree of immune suppression. The increased susceptibility to various infections in patients with low CD4 counts may also contribute to the occurrence of NHL in patients with low CD4 counts. The occurrence of various other rare cancers in patients with HIV-AIDS may be because of confounding rather than a direct HIV or immunosuppression effect.
An increasing incidence of NADMs has been noted in the Western literature.7,14 ADMs remain the most common malignancies in the HIV-AIDS population, accounting for about 48% of all malignancies.8 This is in concordance with previous publications from India.8,10 With the widespread availability of generic HAART, the incidence of ADMs may decrease even more in the future. In developing countries where the screening procedures for malignancies in both the general population and patients with HIV-AIDS have not yet been implemented at a national level, premalignant lesions of the cervix are not detected.10 Cervical cancer is the most common malignancy in our study population, which underscores the importance of cervical cancer screening in patients with HIV-AIDS.
In the developed countries, following the introduction of HAART in HIV-AIDS management, the incidence of Kaposi sarcoma decreased by 60% to 70%, and the incidence of NHL decreased by 30% to 50%, whereas the rates of cervical cancer remained either stable or declined.15,16 Despite the declining trend, the incidence of these malignancies continues to be high among patients with HIV-AIDS compared with the general population.17 A study from the United States showed increasing trends in various NADMs (such as anal, lung, and liver cancers and Hodgkin lymphoma) from 2006 to 2010.17 In 2003, the number of patients with NADM were higher than the number of patients with ADM in the United States.14 In a population-based study from Brazil, ADMs were the most common malignancies diagnosed in patients with HIV-AIDS. A declining trend was noted in the incidence of ADMs in the population and an increasing trend in the incidence of NADMs. This increase in NADM incidence was contributed by anal and lung cancers.18 Studies from developing countries such as Uganda and Botswana have also shown a decrease in the incidence of Kaposi sarcoma after the introduction of HAART.19-21
Kaposi sarcoma, cervical cancer, NHL (including Burkitt lymphoma, immunoblastic lymphoma, and primary CNS lymphoma [PCNSL]) comprise ADMs. All 3 ADMs have an underlying viral infection as the causative agent.22 Kaposi sarcoma is caused by the Kaposi sarcoma herpes virus, for which seroprevalence varies worldwide.23 As already noted in this article, the incidence of Kaposi sarcoma among the HIV-AIDS population has decreased worldwide since the introduction of HAART. The preinvasive uterine cervix lesions and carcinoma cervix are caused by HPV. NHL in patients with HIV-AIDS is a predominantly aggressive B-cell neoplasm. Epstein-Barr virus is implicated for most of the ADM NHLs.24 PCNSL occurs in patients with low CD4 counts and poses a diagnostic challenge. The treatment outcomes for patients with PCNSL before the HAART era were dismal. With the widespread use of HAART, the treatment outcomes of patients with HIV-AIDS and NHL improved, and, currently, these patients are managed the same way as other patients with NHL.22
The increasing incidence of the NADM is partly attributed to the increasing incidence of these malignancies in the general population. An elevated risk of certain NADMs is also attributable to viral infections. The common NADMs in the United States are lung, anal, oropharyngeal, and hepatocellular cancers and Hodgkin lymphoma.14 The common NADMs in our study population were oral, oropharyngeal, colon, and breast cancers and Hodgkin lymphoma. One-third of head and neck cancers, including most oropharyngeal cancers, and cervical and anal cancers in patients with HIV-AIDS are related to HPV.25 Patients with HIV-AIDS are at increased risk for chronic HPV infection from immunosuppression. Chronic HPV infections and prolonged immunosuppression cause premalignant high-grade squamous intraepithelial lesions and invasive cancers.22 The initiation of and adherence to HAART leads to immune recovery and reduces high-risk HPV-associated morbidity.26 Findings from previous studies have demonstrated the benefits of screening for cervical cancer in patients with HIV-AIDS.27 The HPV vaccine is immunogenic in patients with HIV-AIDS and might help prevent HPV-associated malignancies.28
Conclusions
With the wide use of HAART by patients with HIV-AIDS, we can expect an increase in the survival of that population. The incidence of malignancies may also increase significantly in these patients, and further longitudinal studies are needed, as malignancies may emerge as the most common cause of death in patients with HIV-AIDS. In addition, the extensive use of HAART therapy and implementation of screening programs for cervical cancer in patients with HIV-AIDS could result in a decrease in the incidence of ADMs.
1. UNAIDS. Prevention gap report. http://www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf. Released 2016. Accessed December 27, 2017.
3. Dubrow R, Silverberg MJ, Park LS, Crothers K, Justice AC. HIV infection, aging, and immune function: implications for cancer risk and prevention. Curr Opin Oncol. 2012;24(5):506-516.
4. Biggar RJ, Chaturvedi AK, Bhatia K, Mbulaiteye SM. Cancer risk in persons with HIV-AIDS in India: a review and future directions for research. Infect Agent Cancer. 2009;4:4.
5. National AIDS Control Organisation & National Institute of Medical Statistics, ICMR, Ministry of Health & Family Welfare, Government of India. India HIV estimations 2015, technical report. http://www.naco.gov.in/sites/default/files/India%20HIV%20Estimations%202015.pdf. Published 2015. Accessed December 27, 2017.
6. Bonnet F, Lewden C, May T, et al. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer. 2004;101(2):317-324.
7. Crum-Cianflone N, Hullsiek KH, Marconi V, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS. 2009;23(1):41-50.
8. Sharma S, Soneja M, Ranjan S. Malignancies in human immunodeficiency virus infected patients in India: initial experience in the HAART era. Indian J Med Res. 2015;142(5):563-567.
9. Sachdeva RK, Sharma A, Singh S, Varma S. Spectrum of AIDS defining & non-AIDS defining malignancies in north India. In
10. Dhir AA, Sawant S, Dikshit RP, et al. Spectrum of HIV-AIDS related cancers in India. Cancer Causes Control. 2007;19(2):147-153.
11. Venkatesh KK, Saghayam S, Devaleenal B, et al. Spectrum of malignancies among HIV-infected patients in South India. Indian J Cancer. 2012;49(1):176-180.
12. Shruti P, Narayanan G, Puthuveettil J, Jayasree K, Vijayalakshmi K. Spectrum of HIV/AIDS-associated cancers in south India. J Clin Oncol. 2014;32(suppl):e12534.
13. Paul TR, Uppin MS, Uppin SG, et al. Spectrum of malignancies in human immunodeficiency virus–positive patients at a Tertiary Care Centre in South India. Indian J Cancer. 2014;51(4):459-463.
14. Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103(9):753-762.
15. Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148(10):728-736.
16. Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187-194.
17. Robbins HA, Shiels MS, Pfeiffer RM, Engels EA. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States. AIDS. 2014;28(6):881-890.
18. Tanaka LF, Latorre MDRD, Gutierrez EB, Heumann C, Herbinger KH, Froeschl G. Trends in the incidence of AIDS-defining and non-AIDS-defining cancers in people living with AIDS: a population-based study from São Paulo, Brazil. Int J STD AIDS. 2017;28(12):1190-1198.
19. Mutyaba I, Phipps W, Krantz EM, et al. A population-level evaluation of the effect of antiretroviral therapy on cancer incidence in Kyadondo County, Uganda, 1999–2008. J Acquir Immune Defic Syndr. 2015;69(4):481-486.
20. Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, et al. Cancer incidence following expansion of HIV treatment in Botswana. PLoS ONE. 2015;10(8):e0135602.
21. Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12(1):6-11.
22. Yarchoan R, Uldrick TS. HIV-associated cancers and related diseases. N Engl J Med. 2018;378(11):1029-1041.
23. Gao SJ, Kingsley L, Li M, et al. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med. 1996;2(8):925-928.
24. Epstein-Barr virus and AIDS-associated lymphomas. Lancet. 1991;338(8773):979-981.
25. Picard A, Badoual C, Hourseau M, et al. Human papilloma virus prevalence in HIV patients with head and neck squamous cell carcinoma. AIDS. 2016;30(8):1257-1266.
26. Minkoff H, Zhong Y, Burk RD, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Infect Dis. 2010;201(5):681-690.
27. Ghebre RG, Grover S, Xu MJ, Chuang LT, Simonds H. Cervical cancer control in HIV-infected women: past, present and future. Gynecol Oncol Rep. 2017;21:101-108.
28. Kojic EM, Rana AI, Cu-Uvin S. Human papillomavirus vaccination in HIV-infected women: need for increased coverage. Expert Rev Vaccines. 2016;15(1):105-117.
1. UNAIDS. Prevention gap report. http://www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf. Released 2016. Accessed December 27, 2017.
3. Dubrow R, Silverberg MJ, Park LS, Crothers K, Justice AC. HIV infection, aging, and immune function: implications for cancer risk and prevention. Curr Opin Oncol. 2012;24(5):506-516.
4. Biggar RJ, Chaturvedi AK, Bhatia K, Mbulaiteye SM. Cancer risk in persons with HIV-AIDS in India: a review and future directions for research. Infect Agent Cancer. 2009;4:4.
5. National AIDS Control Organisation & National Institute of Medical Statistics, ICMR, Ministry of Health & Family Welfare, Government of India. India HIV estimations 2015, technical report. http://www.naco.gov.in/sites/default/files/India%20HIV%20Estimations%202015.pdf. Published 2015. Accessed December 27, 2017.
6. Bonnet F, Lewden C, May T, et al. Malignancy-related causes of death in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Cancer. 2004;101(2):317-324.
7. Crum-Cianflone N, Hullsiek KH, Marconi V, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS. 2009;23(1):41-50.
8. Sharma S, Soneja M, Ranjan S. Malignancies in human immunodeficiency virus infected patients in India: initial experience in the HAART era. Indian J Med Res. 2015;142(5):563-567.
9. Sachdeva RK, Sharma A, Singh S, Varma S. Spectrum of AIDS defining & non-AIDS defining malignancies in north India. In
10. Dhir AA, Sawant S, Dikshit RP, et al. Spectrum of HIV-AIDS related cancers in India. Cancer Causes Control. 2007;19(2):147-153.
11. Venkatesh KK, Saghayam S, Devaleenal B, et al. Spectrum of malignancies among HIV-infected patients in South India. Indian J Cancer. 2012;49(1):176-180.
12. Shruti P, Narayanan G, Puthuveettil J, Jayasree K, Vijayalakshmi K. Spectrum of HIV/AIDS-associated cancers in south India. J Clin Oncol. 2014;32(suppl):e12534.
13. Paul TR, Uppin MS, Uppin SG, et al. Spectrum of malignancies in human immunodeficiency virus–positive patients at a Tertiary Care Centre in South India. Indian J Cancer. 2014;51(4):459-463.
14. Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103(9):753-762.
15. Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148(10):728-736.
16. Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;123(1):187-194.
17. Robbins HA, Shiels MS, Pfeiffer RM, Engels EA. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States. AIDS. 2014;28(6):881-890.
18. Tanaka LF, Latorre MDRD, Gutierrez EB, Heumann C, Herbinger KH, Froeschl G. Trends in the incidence of AIDS-defining and non-AIDS-defining cancers in people living with AIDS: a population-based study from São Paulo, Brazil. Int J STD AIDS. 2017;28(12):1190-1198.
19. Mutyaba I, Phipps W, Krantz EM, et al. A population-level evaluation of the effect of antiretroviral therapy on cancer incidence in Kyadondo County, Uganda, 1999–2008. J Acquir Immune Defic Syndr. 2015;69(4):481-486.
20. Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, et al. Cancer incidence following expansion of HIV treatment in Botswana. PLoS ONE. 2015;10(8):e0135602.
21. Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12(1):6-11.
22. Yarchoan R, Uldrick TS. HIV-associated cancers and related diseases. N Engl J Med. 2018;378(11):1029-1041.
23. Gao SJ, Kingsley L, Li M, et al. KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi’s sarcoma. Nat Med. 1996;2(8):925-928.
24. Epstein-Barr virus and AIDS-associated lymphomas. Lancet. 1991;338(8773):979-981.
25. Picard A, Badoual C, Hourseau M, et al. Human papilloma virus prevalence in HIV patients with head and neck squamous cell carcinoma. AIDS. 2016;30(8):1257-1266.
26. Minkoff H, Zhong Y, Burk RD, et al. Influence of adherent and effective antiretroviral therapy use on human papillomavirus infection and squamous intraepithelial lesions in human immunodeficiency virus-positive women. J Infect Dis. 2010;201(5):681-690.
27. Ghebre RG, Grover S, Xu MJ, Chuang LT, Simonds H. Cervical cancer control in HIV-infected women: past, present and future. Gynecol Oncol Rep. 2017;21:101-108.
28. Kojic EM, Rana AI, Cu-Uvin S. Human papillomavirus vaccination in HIV-infected women: need for increased coverage. Expert Rev Vaccines. 2016;15(1):105-117.
Marriage predicts for survival in patients with stage III non–small-cell lung cancer
Non–small-cell lung cancer (NSCLC) remains the leading cause of cancer death in the United States, where 29% of patients will present with stage III disease.1,2 Ongoing research efforts seek to improve these outcomes using novel systemic therapy options or modern radiation techniques. However, there have also been recent studies showing the importance of marital and/or partner status on clinical outcomes.3-7 For example, in a large Surveillance, Epidemiology, and End Results (SEER) analysis of 734,889 patients diagnosed with several types of cancer (including lung cancer), patients identified as married were less likely to present with metastatic disease, more likely to receive definitive therapy, and had superior cancer-related mortality even after adjusting for other variables such as cancer stage and treatment when compared with single patients.3 Population-based assessments are important in relaying information about trends and general outcomes based on marital status, but because they are large, they often lack patient-specific information such as nutrition, immunologic status, and variability in treatment paradigms, all of which can independently have an impact on overall survival (OS) in stage III NSCLC.8-10 In addition, population analyses have typically included patients of all cancer stages and hence involved a multitude of treatment approaches ranging from curative to palliative. There are limited well-annotated institutional data on the association of marital status on nonmetastatic, locally advanced (LA-NSCLC) in the setting of National Comprehensive Cancer Network-guided, standard-of-care definitive treatment.
The objective of this analysis is to evaluate the effect of marital status on OS and freedom from recurrence (FFR) in patients with stage III NSCLC who were treated at a National Cancer Institute–designated cancer center with curative intent from 2000 through 2013. We performed a detailed multivariate analysis (MVA) of patient-, disease-, and treatment-specific factors, including the interaction with racial, nutritional, and immunologic status, which to our knowledge has not been previously reported, to comprehensively evaluate the benefit of marital status in patients with LA-NSCLC.
Methods
Patient population and treatment
From January 2000 through December 2013, 355 patients diagnosed with clinical stage III NSCLC (American Joint Committee on Cancer 7th edition) were definitively treated at the University of Maryland in Baltimore, Maryland. Their clinical data were retrospectively analyzed under internal review board approval (GCC 1175, Thoracic Oncology Database). All of the patients were evaluated before treatment by a multidisciplinary team consisting of thoracic surgeons and medical and radiation oncologists. Before treatment, the patients underwent standard work-up, which included systemic imaging with positron-emission (PET), computed-tomographic (CT), PET–CT, and/or bone scan, brain imaging consisting of magnetic-resonance imaging or CT with contrast, and routine blood. Patients had documentation of mediastinal disease by either imaging, mediastinoscopy, or endobronchial ultrasound biopsy.
Definitive therapy was administered using the backbone of chemoradiation therapy (CRT) with (trimodality) or without (bimodality) surgical resection. Concurrent CRT was typically administered with weekly carboplatin–paclitaxel (areas under the curve [AUCs], 2 and 50 mg/m2, respectively) and was generally followed with 2 cycles of consolidative treatment with definitive doses of carboplatin–paclitaxel (AUCs, 5-6 and 200-225 mg/m2, respectively) as tolerated. The entire cohort was also assessed for possible trimodality therapy at the time of initial diagnosis, and patients who were potential surgical candidates were reassessed for mediastinal nodal clearance following repeat radiographic staging after full-dose CRT. Patients who experienced pathologic mediastinal clearance of disease underwent resection followed by consolidative chemotherapy. Unless there was evidence of disease progression, patients who did not have mediastinal lymph node clearance or who were found not to be a surgical candidate proceeded directly to consolidative chemotherapy. The details of patient selection for trimodality therapy and the oncological outcomes have been previously reported.10 For follow-up, patients were normally followed with serial CT or PET–CT scans as clinically indicated every 3 months for the first year, 4 to 6 months for the next 2 to 5 years, and then yearly thereafter.
For the analysis, patients were categorized as being either married or single based on self-reporting. As a surrogate for nutrition status, patients were stratified into 4 pretreatment body mass index (BMI) cohorts based on the following World Health Organization criteria: underweight, <18.5 kg/m2; normal weight, 18.5 to <25 kg/m2; overweight, 25 to <30 kg/m2; and obesity, ≥30 kg/m2. Pretreatment albumin was also evaluated as a continuous variable. For assessment of immunological status, neutrophil-to-lymphocyte ratio (NLR) was calculated at the time of diagnosis by dividing the absolute neutrophil count by the absolute lymphocyte count.
Statistics
We used the Pearson chi-square test to compare categorical variables. OS was calculated from the date of diagnosis (by biopsy of either primary tumor or mediastinal nodes) to the time of death or date of last follow-up. Patients were only censored if they were lost to follow-up. FFR was determined by the date of diagnosis to the time of first failure, with either distant or locoregional disease progression. For this analysis, patients were censored at the time of their last follow-up or death. The Kaplan-Meier product limit method was used to estimate OS and FFR, and we applied the log-rank test to compare outcomes between the 2 cohorts.
We conducted the multivariate analyses using Cox regression with forward model selection. Variables analyzed included age (<60 vs ≥60 years), sex, race (black vs nonblack), median household income, insurance status (Yes vs No), Eastern Cooperative Oncology Group Performance Status (ECOG PS) (range: 0-3; 0 = fully active and 3 = capable of limited self-care, confined to bed/chair >50% of day) at time of diagnosis (0 vs ≥1), pre-CRT BMI, smoking (pack-years), chronic obstructive pulmonary disorder (Yes vs No), Charlson Comorbidity Index score (≤6 vs >7; range, 3-15; this score takes into consideration age, cardiovascular disease, malignancy, and other chronic conditions to calculate 1-year mortality), histology, calculated pretreatment NLR (as a continuous variable), pretreatment albumin (as a continuous variable), T stage, N stage, overall stage (IIIA vs IIIB), radiation technique (3D-CRT vs intensity-modulated radiation therapy [IMRT]), date of diagnosis (divided into quartiles based on proportion diagnosed by years: 2000-2002, 2003-2005, 2006-2009, 2010-2013), use of trimodality therapy, and consolidation chemotherapy. SPSS software (version 23.0) was used for statistical analysis (IBM Corp, Armonk, NY).
Results
Treatment cohorts
Table 1 compares and summarizes patient demographics, disease, and treatment characteristics for married (n = 185; 52.1%) and nonmarried (n = 170; 47.9%) patients. Married patients were more likely to self-identify as being white (P < .0001), reside in zip codes with a higher household median income (P < .0001), have an ECOG PS of 0 (P = .001), have a higher distribution of pretreatment albumin levels (P = .009), and undergo trimodality therapy (P = .001), and they were twice as likely to have insurance (P = .029). Both cohorts were evenly distributed in terms of T stage, N stage, and overall staging. There was no difference in pretreatment NLR or pretreatment BMI between married and single patients. Concurrent CRT was used in more than 85% of patients in both groups, with approximately two-thirds also receiving consolidation chemotherapy (Table 1). Median delivered radiation dose was 64.8 Gy (range, 10.8-81.6 Gy). There was no statistically significant difference in radiation dose delivered to either group, with nearly 90% of the cohort receiving ≥60 Gy.
OS and FFR
With a median follow-up of 15 months for all patients and 89 months for surviving patients (range, 1-184 months), married patients had improved OS when compared with the single cohort, with a median survival of 29.6 and 18.4 months, respectively (unadjusted hazard ratio [HR] of married vs nonmarried, .640; 95% confidence interval [CI], 0.502-0.816; P < .0001; Figure 1A). The estimated 2- and 5-year OS for married and single patients were 56% and 31% and 38.6% and 15%, respectively. When stratified by stage, married patients with stage IIIB disease (median survival, 25 months; Figure 1B) had a similar survival to unmarried patients with stage IIIA disease (median survival, 24 months; Figure 1B).
In stage IIIA patients, marital status was associated with an unadjusted HR of .696 (95% CI, 0.497-0.974; P = .035), with a larger OS benefit seen in the IIIB group (unadjusted HR, .601; 95% CI, 0.422-0.856; P = .005).
Survival as it pertains to marital status was further stratified by sex (Figure 2A) and race (Figure 2B). Married men had an improved estimated median survival of 30 months when compared with single men, whose median survival was 16 months (unadjusted HR, .541; 95% CI, 0.392-0.746; P < .0001). On the other hand, marital status had no statistically significant effect on OS when comparing married women with their single counterparts (unadjusted HR, .717; 95% CI, 0.491-1.048; P = .085; Figure 2A), with an overall median survival of approximately 28 months for the entire female cohort. Stratification by race also showed similar results, with married nonblack patients demonstrating better OS when compared with single nonblack patients (HR, .586; 95% CI, 0.420-0.820; P = .002; Figure 2B), with a median survival of 29 and 17 months, respectively. Black patients also had a similar improvement in survival when comparing the married (median survival, 30 months) and nonmarried groups (median survival, 19.6 months; unadjusted HR, .676; 95% CI, 0.457-1.000; P = .050; Figure 2B).
FFR did not differ between the 2 groups, with a median time to failure of 17 and 15 months for married and nonmarried patients, respectively (unadjusted HR, .799; 95% CI, 0.607-1.051; P = .108; Figure 3). Estimated 2- and 5-year FFR for married and nonmarried patients were 39.4% and 27% and 31.5% and 18.5%, respectively (Figure 3).
Clinical predictors of survival
On MVA, factors that were independent predictors for OS are summarized in Table 2. Risk of death was reduced by approximately 65% and 45% in patients who underwent trimodality treatment (P < .0001) or were able to undergo consolidative chemotherapy (P = .004) when compared with those who were treated definitively with bimodality treatment or did not undergo systemic doses of adjuvant chemotherapy, respectively. Having insurance (P = .048) and use of IMRT over 3D-CRT (P = .008) was associated with a reduction of mortality by about half in this cohort. Both gender (improved OS with female sex; P = .004) and marital status (improved OS with marriage; P = .006) were associated with a decreased the risk of death by 40% (Table 2). By contrast, a higher NLR resulted
Discussion
Our study continues to support the notion that marital status is an independent indicator of survival in stage III NSCLC (adjusted HR, .59; 95% CI, 0.404-0.859; P = .006). The benefit of marriage in this population seems to be better than that reported in the SEER analysis for all stages, wherein the HR for death of married patients compared with their single counterparts was .85 (95% CI, 0.83-0.87). In their analysis, the investigators hypothesized that this survival advantage could partially be explained by better access to health care and adherence to therapy, as was supported by the higher likelihood of married patients presenting with localized disease and receiving definitive treatment.3 Another population-based study using the Florida Cancer Data System identified 161,228 lung cancer patients (NSCLC and small-cell lung histology included), and on MVA, marital status remained an important prognostic indicator for OS when compared with never-married patients (HR, .86; P = .001).6 In addition to typically including patients with all stages of diseases, population-based studies often include patients who receive a heterogeneous combination of treatment modalities, possibly confounding the analysis. Furthermore, large population analyses typically do not report on patient-specific variables such as nutrition (ie, BMI and albumin) or immunologic status (ie, NLR), both of which have been shown to be independent predictors of survival in LA-NSCLC.8,9
In contrast, some other studies have failed to demonstrate an OS advantage with marital status in patients with NSCLC. For example, in a meta-analysis that evaluated the influence of race, gender, and marital status on 1,365 nonoperative NSCLC patients who were enrolled in 9 Radiation Therapy Oncology Group (RTOG) trials, the investigators did not find marital status to be independently predictive of survival.11 In addition, for the 5,898 patients who were prospectively enrolled in a Mayo Clinic Lung Cancer Cohort (MCLCC), marital status was also found not to be prognostic for NSCLC outcomes when all stages of the disease were analyzed together.4 There are some possible confounding factors in these studies. Patients recruited for clinical trials tend to be healthier with a better performance status and have a support system (including close monitoring by the study team) when compared with the general population diagnosed with lung cancer. About 70% to 76% of the patients in both the RTOG and MCLCC studies were married, which is significantly higher than both the national average (51%) and our group (52.1%). Like other population-based studies, the MCLCC included patients with all stages getting a variety of treatments. Although no overall impact on survival was noted, the investigators noted that single, divorced, and widowed patients were more likely to not receive cancer therapy(P < .0001). The marital status also influenced the choice of therapy, with subgroup analysis revealing inferior outcomes in widowed and divorced patients with stage IA, IIB, or IIIB disease. The authors also recognized an inherent referral bias from patients, with support system being typically seen at the Mayo clinics, which may have played an additional role. All of the patients in our analysis were appropriately staged and received curative-intent treatment by a team of physicians using essentially identical therapeutic strategies, thus minimizing some of these confounding factors. This allowed us to explore the impact of marital status while a patient was undergoing stage-appropriate treatment. We demonstrated a strong association with marital status and survival that even overcame the effects of stage (IIIA vs IIIB) on clinical outcomes (Figure 1B).
Furthermore, our analysis allowed us to explore the interaction of race and marital status more definitively because the demographics of the patients in the RTOG and MCLCC included 14% and less than 3% of patients identified as being nonwhite, respectively, in contrast to our analysis in which 41% of the patients self-identified as black.12 In our black population, marital status was associated with an observable improvement in OS, similar to our nonblack, predominantly white (97%) cohort (Figure 2B). Also, the results of our analysis may be a more accurate representation of the general population living in large urban or semiurban settings and further implies that an intact social support system could have a greater influence on clinical outcomes.
The current analysis is unique when compared with previous published studies in that beyond conventional demographic and treatment-related factors, we have comprehensively explored potential mechanisms that may explain the survival advantage seen in married patients by evaluating additional factors, such as functional status (ECOG and Charlson’s scores), nutritional status (BMI and albumin), immunologic characteristics (NLR), and other social factors (race, income, insurance status). Although married patients were more likely to have a higher BMI and albumin at diagnosis, when controlling for these factors in the multivariable analysis, marital status remained strongly prognostic (Table 2), suggesting that nutrition alone does not fully account for the observed survival advantage demonstrated. A similar conclusion can be drawn about immunologic status. NLR has previously been shown to be prognostic in a number of cancers,13-16 including in our own cohort.8 Although immune status remains an important predictor for OS in our locally advanced NSCLC population, when we take NLR into consideration in our analysis, marital status continues to be a strong indicator for survival (Table 2). In terms of other variables analyzed, insurance status was a significant predictor of OS in the MVA, though functional status and other social factors including race were not significant.
We also explored cancer control outcomes in the form of FFR. Married patients had an observable, although not statistically significant, improvement in FFR when compared with the single cohort (Figure 2). In our study, married patients were more likely to undergo trimodality therapy (Table 1), which has likely translated to the improvement of FFR seen in our group. In this case, marriage may serve as a surrogate for availability of a support system to undergo aggressive, potentially toxic treatment.3,17,18 Even in the setting of bimodality therapy, the RTOG 0617 study noted about 17.5% treatment interruptions because of adverse effects or illness, with more than 30% of patients experiencing grade 3 or more esophagitis, irrespective of radiation technique.19 In these scenarios, in addition to receiving better attention to nutrition and care, significant others often provide emotional and social support that, in turn, can lead to better compliance. Social supports and socio-demographic factors are especially critical in patient populations in which access to health care is challenging.
Despite the compelling outcomes presented, our study suffers from the common limitations of retrospective analyses. Marital status, in this setting, most likely correlates with improved socioeconomic status and greater support, which have resulted in improved survival. Furthermore, although patients were self-classified as married or single, our data were not able to capture whether patients were single but lived with another adult or had other types of social support. However, even if there was a proportion of the unmarried cohort that had an alternate support system, separating them out is likely to further expand the differences. Quantifying the amount of social, emotional, or even spiritual support was not possible to accomplish in our analysis, though we know that all 3 can play a role in cancer outcomes.20,21 Further prospective studies would have to be done to completely understand how marital status can influence clinical decisions. Understanding whether marital status is a proxy for social provisions may help to identify populations at risk for inferior outcomes. These at-risk patients may benefit from targeted clinical interventions, such as closer physician follow-up, more aggressive supportive care, access to support groups, or nurse navigator visits.
Conclusions
In patients with locally advanced NSCLC treated with curative-intent following uniform treatment algorithms, marital status was linked with improvement in survival even when adjusted for other key variables, with the second highest HR (after insurance status) among pretreatment demographic variables. Although marriage is an unmodifiable factor in itself, it is most likely a surrogate for better psychosocial support. The scale of these positive survival improvements emphasizes the need to institute targeted supportive care strategies to help advance overall outcomes in a tumor for which modern therapeutic approaches (novel systemic therapy and radiation) have yielded only modest improvement in outcomes yet come at the cost of considerable treatment-related toxicity.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
2. Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39-51.
3. Aizer AA, Chen M-H, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31(31):3869-3876.
4. Jatoi A, Novotny P, Cassivi S, et al. Does marital status impact survival and quality of life in patients with non-small cell lung cancer? Observations from the mayo clinic lung cancer cohort. Oncologist. 2007;12(12):1456-1463.
5. Kravdal H, Syse A. Changes over time in the effect of marital status on cancer survival. BMC Public Health. 2011;11:804.
6. Tannenbaum SL, Zhao W, Koru-Sengul T, Miao F, Lee D, Byrne MM. Marital status and its effect on lung cancer survival. Springerplus. 2013;2:504.
7. Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics [published online October 16, 2017]. J Clin Oncol. 2018;36(1):25-33.
8. Scilla KA, Bentzen SM, Lam VK, et al. Neutrophil-lymphocyte ratio is a prognostic marker in patients with locally advanced (stage IIIA and IIIB) non-small cell lung cancer treated with combined modality therapy. Oncologist. 2017;22(6):737-742.
9. Lam VK, Bentzen SM, Mohindra P, et al. Obesity is associated with long-term improved survival in definitively treated locally advanced non-small cell lung cancer (NSCLC). Lung Cancer. 2017;104:52-57.
10. Vyfhuis MAL, Bhooshan N, Burrows WM, et al. Oncological outcomes from trimodality therapy receiving definitive doses of neoadjuvant chemoradiation (≥60 Gy) and factors influencing consideration for surgery in stage III non-small cell lung cancer. Adv Radiat Oncol. 2017;2(3):259-269.
11. Siddiqui F, Bae K, Langer CJ, et al. The influence of gender, race, and marital status on survival in lung cancer patients: analysis of radiation therapy oncology group trials. J Thorac Oncol. 2010;5(5):631-639.
12. Vyfhuis MAL, Bhooshan N, Molitoris J, et al. Clinical outcomes of black vs. non-black patients with locally advanced non–small cell lung cancer. Lung Cancer. 2017;114:44-49.
13. Beltran BE, Castro D, De La Cruz-Vargas JA, et al. The neutrophil-lymphocyte ratio is prognostic in patients with early stage aggressive peripheral T cell lymphoma [published online February 26, 2018]. Br J Haematol. doi:10.1111/bjh.15141.
14. Lee BM, Chung SY, Chang JS, Lee KJ, Seong J. The neutrophil-lymphocyte ratio and platelet-lymphocyte ratio are prognostic factors in patients with locally advanced pancreatic cancer treated with chemoradiotherapy. Gut Liver. 2018;12(3):342-352.
15. Najjar M, Agrawal S, Emond JC, Halazun KJ. Pretreatment neutrophil-lymphocyte ratio: useful prognostic biomarker in hepatocellular carcinoma. J Hepatocell Carcinoma. 2018;5:17-28.
16. Hu W, Yu J, Huang Y, Hu F, Zhang X, Wang Y. Lymphocyte-related inflammation and immune-based scores predict prognosis of chordoma patients after radical resection. Transl Oncol. 2018;11(2):444-449.
17. Mahal BA, Cooperberg MR, Aizer AA, et al. Who bears the greatest burden of aggressive treatment of indolent prostate cancer? Am J Med. 2015;128(6):609-616.
18. Inverso G, Mahal BA, Aizer AA, Donoff RB, Chau NG, Haddad RI. Marital status and head and neck cancer outcomes. Cancer. 2015;121(8):1273-1278.
19. Chun SG, Hu C, Choy H, et al. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: a secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J Clin Oncol. 2017;35(1):56-62.
20. Waite LJ, Lehrer EL. The benefits from marriage and religion in the United States: a comparative analysis. Popul Dev Rev. 2003;29(2):255-276.
21. Osborne C, Ostir GV, Du X, Peek MK, Goodwin JS. The influence of marital status on the stage at diagnosis, treatment, and survival of older women with breast cancer. Breast Cancer Res Treat. 2005;93(1):41-47.
Non–small-cell lung cancer (NSCLC) remains the leading cause of cancer death in the United States, where 29% of patients will present with stage III disease.1,2 Ongoing research efforts seek to improve these outcomes using novel systemic therapy options or modern radiation techniques. However, there have also been recent studies showing the importance of marital and/or partner status on clinical outcomes.3-7 For example, in a large Surveillance, Epidemiology, and End Results (SEER) analysis of 734,889 patients diagnosed with several types of cancer (including lung cancer), patients identified as married were less likely to present with metastatic disease, more likely to receive definitive therapy, and had superior cancer-related mortality even after adjusting for other variables such as cancer stage and treatment when compared with single patients.3 Population-based assessments are important in relaying information about trends and general outcomes based on marital status, but because they are large, they often lack patient-specific information such as nutrition, immunologic status, and variability in treatment paradigms, all of which can independently have an impact on overall survival (OS) in stage III NSCLC.8-10 In addition, population analyses have typically included patients of all cancer stages and hence involved a multitude of treatment approaches ranging from curative to palliative. There are limited well-annotated institutional data on the association of marital status on nonmetastatic, locally advanced (LA-NSCLC) in the setting of National Comprehensive Cancer Network-guided, standard-of-care definitive treatment.
The objective of this analysis is to evaluate the effect of marital status on OS and freedom from recurrence (FFR) in patients with stage III NSCLC who were treated at a National Cancer Institute–designated cancer center with curative intent from 2000 through 2013. We performed a detailed multivariate analysis (MVA) of patient-, disease-, and treatment-specific factors, including the interaction with racial, nutritional, and immunologic status, which to our knowledge has not been previously reported, to comprehensively evaluate the benefit of marital status in patients with LA-NSCLC.
Methods
Patient population and treatment
From January 2000 through December 2013, 355 patients diagnosed with clinical stage III NSCLC (American Joint Committee on Cancer 7th edition) were definitively treated at the University of Maryland in Baltimore, Maryland. Their clinical data were retrospectively analyzed under internal review board approval (GCC 1175, Thoracic Oncology Database). All of the patients were evaluated before treatment by a multidisciplinary team consisting of thoracic surgeons and medical and radiation oncologists. Before treatment, the patients underwent standard work-up, which included systemic imaging with positron-emission (PET), computed-tomographic (CT), PET–CT, and/or bone scan, brain imaging consisting of magnetic-resonance imaging or CT with contrast, and routine blood. Patients had documentation of mediastinal disease by either imaging, mediastinoscopy, or endobronchial ultrasound biopsy.
Definitive therapy was administered using the backbone of chemoradiation therapy (CRT) with (trimodality) or without (bimodality) surgical resection. Concurrent CRT was typically administered with weekly carboplatin–paclitaxel (areas under the curve [AUCs], 2 and 50 mg/m2, respectively) and was generally followed with 2 cycles of consolidative treatment with definitive doses of carboplatin–paclitaxel (AUCs, 5-6 and 200-225 mg/m2, respectively) as tolerated. The entire cohort was also assessed for possible trimodality therapy at the time of initial diagnosis, and patients who were potential surgical candidates were reassessed for mediastinal nodal clearance following repeat radiographic staging after full-dose CRT. Patients who experienced pathologic mediastinal clearance of disease underwent resection followed by consolidative chemotherapy. Unless there was evidence of disease progression, patients who did not have mediastinal lymph node clearance or who were found not to be a surgical candidate proceeded directly to consolidative chemotherapy. The details of patient selection for trimodality therapy and the oncological outcomes have been previously reported.10 For follow-up, patients were normally followed with serial CT or PET–CT scans as clinically indicated every 3 months for the first year, 4 to 6 months for the next 2 to 5 years, and then yearly thereafter.
For the analysis, patients were categorized as being either married or single based on self-reporting. As a surrogate for nutrition status, patients were stratified into 4 pretreatment body mass index (BMI) cohorts based on the following World Health Organization criteria: underweight, <18.5 kg/m2; normal weight, 18.5 to <25 kg/m2; overweight, 25 to <30 kg/m2; and obesity, ≥30 kg/m2. Pretreatment albumin was also evaluated as a continuous variable. For assessment of immunological status, neutrophil-to-lymphocyte ratio (NLR) was calculated at the time of diagnosis by dividing the absolute neutrophil count by the absolute lymphocyte count.
Statistics
We used the Pearson chi-square test to compare categorical variables. OS was calculated from the date of diagnosis (by biopsy of either primary tumor or mediastinal nodes) to the time of death or date of last follow-up. Patients were only censored if they were lost to follow-up. FFR was determined by the date of diagnosis to the time of first failure, with either distant or locoregional disease progression. For this analysis, patients were censored at the time of their last follow-up or death. The Kaplan-Meier product limit method was used to estimate OS and FFR, and we applied the log-rank test to compare outcomes between the 2 cohorts.
We conducted the multivariate analyses using Cox regression with forward model selection. Variables analyzed included age (<60 vs ≥60 years), sex, race (black vs nonblack), median household income, insurance status (Yes vs No), Eastern Cooperative Oncology Group Performance Status (ECOG PS) (range: 0-3; 0 = fully active and 3 = capable of limited self-care, confined to bed/chair >50% of day) at time of diagnosis (0 vs ≥1), pre-CRT BMI, smoking (pack-years), chronic obstructive pulmonary disorder (Yes vs No), Charlson Comorbidity Index score (≤6 vs >7; range, 3-15; this score takes into consideration age, cardiovascular disease, malignancy, and other chronic conditions to calculate 1-year mortality), histology, calculated pretreatment NLR (as a continuous variable), pretreatment albumin (as a continuous variable), T stage, N stage, overall stage (IIIA vs IIIB), radiation technique (3D-CRT vs intensity-modulated radiation therapy [IMRT]), date of diagnosis (divided into quartiles based on proportion diagnosed by years: 2000-2002, 2003-2005, 2006-2009, 2010-2013), use of trimodality therapy, and consolidation chemotherapy. SPSS software (version 23.0) was used for statistical analysis (IBM Corp, Armonk, NY).
Results
Treatment cohorts
Table 1 compares and summarizes patient demographics, disease, and treatment characteristics for married (n = 185; 52.1%) and nonmarried (n = 170; 47.9%) patients. Married patients were more likely to self-identify as being white (P < .0001), reside in zip codes with a higher household median income (P < .0001), have an ECOG PS of 0 (P = .001), have a higher distribution of pretreatment albumin levels (P = .009), and undergo trimodality therapy (P = .001), and they were twice as likely to have insurance (P = .029). Both cohorts were evenly distributed in terms of T stage, N stage, and overall staging. There was no difference in pretreatment NLR or pretreatment BMI between married and single patients. Concurrent CRT was used in more than 85% of patients in both groups, with approximately two-thirds also receiving consolidation chemotherapy (Table 1). Median delivered radiation dose was 64.8 Gy (range, 10.8-81.6 Gy). There was no statistically significant difference in radiation dose delivered to either group, with nearly 90% of the cohort receiving ≥60 Gy.
OS and FFR
With a median follow-up of 15 months for all patients and 89 months for surviving patients (range, 1-184 months), married patients had improved OS when compared with the single cohort, with a median survival of 29.6 and 18.4 months, respectively (unadjusted hazard ratio [HR] of married vs nonmarried, .640; 95% confidence interval [CI], 0.502-0.816; P < .0001; Figure 1A). The estimated 2- and 5-year OS for married and single patients were 56% and 31% and 38.6% and 15%, respectively. When stratified by stage, married patients with stage IIIB disease (median survival, 25 months; Figure 1B) had a similar survival to unmarried patients with stage IIIA disease (median survival, 24 months; Figure 1B).
In stage IIIA patients, marital status was associated with an unadjusted HR of .696 (95% CI, 0.497-0.974; P = .035), with a larger OS benefit seen in the IIIB group (unadjusted HR, .601; 95% CI, 0.422-0.856; P = .005).
Survival as it pertains to marital status was further stratified by sex (Figure 2A) and race (Figure 2B). Married men had an improved estimated median survival of 30 months when compared with single men, whose median survival was 16 months (unadjusted HR, .541; 95% CI, 0.392-0.746; P < .0001). On the other hand, marital status had no statistically significant effect on OS when comparing married women with their single counterparts (unadjusted HR, .717; 95% CI, 0.491-1.048; P = .085; Figure 2A), with an overall median survival of approximately 28 months for the entire female cohort. Stratification by race also showed similar results, with married nonblack patients demonstrating better OS when compared with single nonblack patients (HR, .586; 95% CI, 0.420-0.820; P = .002; Figure 2B), with a median survival of 29 and 17 months, respectively. Black patients also had a similar improvement in survival when comparing the married (median survival, 30 months) and nonmarried groups (median survival, 19.6 months; unadjusted HR, .676; 95% CI, 0.457-1.000; P = .050; Figure 2B).
FFR did not differ between the 2 groups, with a median time to failure of 17 and 15 months for married and nonmarried patients, respectively (unadjusted HR, .799; 95% CI, 0.607-1.051; P = .108; Figure 3). Estimated 2- and 5-year FFR for married and nonmarried patients were 39.4% and 27% and 31.5% and 18.5%, respectively (Figure 3).
Clinical predictors of survival
On MVA, factors that were independent predictors for OS are summarized in Table 2. Risk of death was reduced by approximately 65% and 45% in patients who underwent trimodality treatment (P < .0001) or were able to undergo consolidative chemotherapy (P = .004) when compared with those who were treated definitively with bimodality treatment or did not undergo systemic doses of adjuvant chemotherapy, respectively. Having insurance (P = .048) and use of IMRT over 3D-CRT (P = .008) was associated with a reduction of mortality by about half in this cohort. Both gender (improved OS with female sex; P = .004) and marital status (improved OS with marriage; P = .006) were associated with a decreased the risk of death by 40% (Table 2). By contrast, a higher NLR resulted
Discussion
Our study continues to support the notion that marital status is an independent indicator of survival in stage III NSCLC (adjusted HR, .59; 95% CI, 0.404-0.859; P = .006). The benefit of marriage in this population seems to be better than that reported in the SEER analysis for all stages, wherein the HR for death of married patients compared with their single counterparts was .85 (95% CI, 0.83-0.87). In their analysis, the investigators hypothesized that this survival advantage could partially be explained by better access to health care and adherence to therapy, as was supported by the higher likelihood of married patients presenting with localized disease and receiving definitive treatment.3 Another population-based study using the Florida Cancer Data System identified 161,228 lung cancer patients (NSCLC and small-cell lung histology included), and on MVA, marital status remained an important prognostic indicator for OS when compared with never-married patients (HR, .86; P = .001).6 In addition to typically including patients with all stages of diseases, population-based studies often include patients who receive a heterogeneous combination of treatment modalities, possibly confounding the analysis. Furthermore, large population analyses typically do not report on patient-specific variables such as nutrition (ie, BMI and albumin) or immunologic status (ie, NLR), both of which have been shown to be independent predictors of survival in LA-NSCLC.8,9
In contrast, some other studies have failed to demonstrate an OS advantage with marital status in patients with NSCLC. For example, in a meta-analysis that evaluated the influence of race, gender, and marital status on 1,365 nonoperative NSCLC patients who were enrolled in 9 Radiation Therapy Oncology Group (RTOG) trials, the investigators did not find marital status to be independently predictive of survival.11 In addition, for the 5,898 patients who were prospectively enrolled in a Mayo Clinic Lung Cancer Cohort (MCLCC), marital status was also found not to be prognostic for NSCLC outcomes when all stages of the disease were analyzed together.4 There are some possible confounding factors in these studies. Patients recruited for clinical trials tend to be healthier with a better performance status and have a support system (including close monitoring by the study team) when compared with the general population diagnosed with lung cancer. About 70% to 76% of the patients in both the RTOG and MCLCC studies were married, which is significantly higher than both the national average (51%) and our group (52.1%). Like other population-based studies, the MCLCC included patients with all stages getting a variety of treatments. Although no overall impact on survival was noted, the investigators noted that single, divorced, and widowed patients were more likely to not receive cancer therapy(P < .0001). The marital status also influenced the choice of therapy, with subgroup analysis revealing inferior outcomes in widowed and divorced patients with stage IA, IIB, or IIIB disease. The authors also recognized an inherent referral bias from patients, with support system being typically seen at the Mayo clinics, which may have played an additional role. All of the patients in our analysis were appropriately staged and received curative-intent treatment by a team of physicians using essentially identical therapeutic strategies, thus minimizing some of these confounding factors. This allowed us to explore the impact of marital status while a patient was undergoing stage-appropriate treatment. We demonstrated a strong association with marital status and survival that even overcame the effects of stage (IIIA vs IIIB) on clinical outcomes (Figure 1B).
Furthermore, our analysis allowed us to explore the interaction of race and marital status more definitively because the demographics of the patients in the RTOG and MCLCC included 14% and less than 3% of patients identified as being nonwhite, respectively, in contrast to our analysis in which 41% of the patients self-identified as black.12 In our black population, marital status was associated with an observable improvement in OS, similar to our nonblack, predominantly white (97%) cohort (Figure 2B). Also, the results of our analysis may be a more accurate representation of the general population living in large urban or semiurban settings and further implies that an intact social support system could have a greater influence on clinical outcomes.
The current analysis is unique when compared with previous published studies in that beyond conventional demographic and treatment-related factors, we have comprehensively explored potential mechanisms that may explain the survival advantage seen in married patients by evaluating additional factors, such as functional status (ECOG and Charlson’s scores), nutritional status (BMI and albumin), immunologic characteristics (NLR), and other social factors (race, income, insurance status). Although married patients were more likely to have a higher BMI and albumin at diagnosis, when controlling for these factors in the multivariable analysis, marital status remained strongly prognostic (Table 2), suggesting that nutrition alone does not fully account for the observed survival advantage demonstrated. A similar conclusion can be drawn about immunologic status. NLR has previously been shown to be prognostic in a number of cancers,13-16 including in our own cohort.8 Although immune status remains an important predictor for OS in our locally advanced NSCLC population, when we take NLR into consideration in our analysis, marital status continues to be a strong indicator for survival (Table 2). In terms of other variables analyzed, insurance status was a significant predictor of OS in the MVA, though functional status and other social factors including race were not significant.
We also explored cancer control outcomes in the form of FFR. Married patients had an observable, although not statistically significant, improvement in FFR when compared with the single cohort (Figure 2). In our study, married patients were more likely to undergo trimodality therapy (Table 1), which has likely translated to the improvement of FFR seen in our group. In this case, marriage may serve as a surrogate for availability of a support system to undergo aggressive, potentially toxic treatment.3,17,18 Even in the setting of bimodality therapy, the RTOG 0617 study noted about 17.5% treatment interruptions because of adverse effects or illness, with more than 30% of patients experiencing grade 3 or more esophagitis, irrespective of radiation technique.19 In these scenarios, in addition to receiving better attention to nutrition and care, significant others often provide emotional and social support that, in turn, can lead to better compliance. Social supports and socio-demographic factors are especially critical in patient populations in which access to health care is challenging.
Despite the compelling outcomes presented, our study suffers from the common limitations of retrospective analyses. Marital status, in this setting, most likely correlates with improved socioeconomic status and greater support, which have resulted in improved survival. Furthermore, although patients were self-classified as married or single, our data were not able to capture whether patients were single but lived with another adult or had other types of social support. However, even if there was a proportion of the unmarried cohort that had an alternate support system, separating them out is likely to further expand the differences. Quantifying the amount of social, emotional, or even spiritual support was not possible to accomplish in our analysis, though we know that all 3 can play a role in cancer outcomes.20,21 Further prospective studies would have to be done to completely understand how marital status can influence clinical decisions. Understanding whether marital status is a proxy for social provisions may help to identify populations at risk for inferior outcomes. These at-risk patients may benefit from targeted clinical interventions, such as closer physician follow-up, more aggressive supportive care, access to support groups, or nurse navigator visits.
Conclusions
In patients with locally advanced NSCLC treated with curative-intent following uniform treatment algorithms, marital status was linked with improvement in survival even when adjusted for other key variables, with the second highest HR (after insurance status) among pretreatment demographic variables. Although marriage is an unmodifiable factor in itself, it is most likely a surrogate for better psychosocial support. The scale of these positive survival improvements emphasizes the need to institute targeted supportive care strategies to help advance overall outcomes in a tumor for which modern therapeutic approaches (novel systemic therapy and radiation) have yielded only modest improvement in outcomes yet come at the cost of considerable treatment-related toxicity.
Non–small-cell lung cancer (NSCLC) remains the leading cause of cancer death in the United States, where 29% of patients will present with stage III disease.1,2 Ongoing research efforts seek to improve these outcomes using novel systemic therapy options or modern radiation techniques. However, there have also been recent studies showing the importance of marital and/or partner status on clinical outcomes.3-7 For example, in a large Surveillance, Epidemiology, and End Results (SEER) analysis of 734,889 patients diagnosed with several types of cancer (including lung cancer), patients identified as married were less likely to present with metastatic disease, more likely to receive definitive therapy, and had superior cancer-related mortality even after adjusting for other variables such as cancer stage and treatment when compared with single patients.3 Population-based assessments are important in relaying information about trends and general outcomes based on marital status, but because they are large, they often lack patient-specific information such as nutrition, immunologic status, and variability in treatment paradigms, all of which can independently have an impact on overall survival (OS) in stage III NSCLC.8-10 In addition, population analyses have typically included patients of all cancer stages and hence involved a multitude of treatment approaches ranging from curative to palliative. There are limited well-annotated institutional data on the association of marital status on nonmetastatic, locally advanced (LA-NSCLC) in the setting of National Comprehensive Cancer Network-guided, standard-of-care definitive treatment.
The objective of this analysis is to evaluate the effect of marital status on OS and freedom from recurrence (FFR) in patients with stage III NSCLC who were treated at a National Cancer Institute–designated cancer center with curative intent from 2000 through 2013. We performed a detailed multivariate analysis (MVA) of patient-, disease-, and treatment-specific factors, including the interaction with racial, nutritional, and immunologic status, which to our knowledge has not been previously reported, to comprehensively evaluate the benefit of marital status in patients with LA-NSCLC.
Methods
Patient population and treatment
From January 2000 through December 2013, 355 patients diagnosed with clinical stage III NSCLC (American Joint Committee on Cancer 7th edition) were definitively treated at the University of Maryland in Baltimore, Maryland. Their clinical data were retrospectively analyzed under internal review board approval (GCC 1175, Thoracic Oncology Database). All of the patients were evaluated before treatment by a multidisciplinary team consisting of thoracic surgeons and medical and radiation oncologists. Before treatment, the patients underwent standard work-up, which included systemic imaging with positron-emission (PET), computed-tomographic (CT), PET–CT, and/or bone scan, brain imaging consisting of magnetic-resonance imaging or CT with contrast, and routine blood. Patients had documentation of mediastinal disease by either imaging, mediastinoscopy, or endobronchial ultrasound biopsy.
Definitive therapy was administered using the backbone of chemoradiation therapy (CRT) with (trimodality) or without (bimodality) surgical resection. Concurrent CRT was typically administered with weekly carboplatin–paclitaxel (areas under the curve [AUCs], 2 and 50 mg/m2, respectively) and was generally followed with 2 cycles of consolidative treatment with definitive doses of carboplatin–paclitaxel (AUCs, 5-6 and 200-225 mg/m2, respectively) as tolerated. The entire cohort was also assessed for possible trimodality therapy at the time of initial diagnosis, and patients who were potential surgical candidates were reassessed for mediastinal nodal clearance following repeat radiographic staging after full-dose CRT. Patients who experienced pathologic mediastinal clearance of disease underwent resection followed by consolidative chemotherapy. Unless there was evidence of disease progression, patients who did not have mediastinal lymph node clearance or who were found not to be a surgical candidate proceeded directly to consolidative chemotherapy. The details of patient selection for trimodality therapy and the oncological outcomes have been previously reported.10 For follow-up, patients were normally followed with serial CT or PET–CT scans as clinically indicated every 3 months for the first year, 4 to 6 months for the next 2 to 5 years, and then yearly thereafter.
For the analysis, patients were categorized as being either married or single based on self-reporting. As a surrogate for nutrition status, patients were stratified into 4 pretreatment body mass index (BMI) cohorts based on the following World Health Organization criteria: underweight, <18.5 kg/m2; normal weight, 18.5 to <25 kg/m2; overweight, 25 to <30 kg/m2; and obesity, ≥30 kg/m2. Pretreatment albumin was also evaluated as a continuous variable. For assessment of immunological status, neutrophil-to-lymphocyte ratio (NLR) was calculated at the time of diagnosis by dividing the absolute neutrophil count by the absolute lymphocyte count.
Statistics
We used the Pearson chi-square test to compare categorical variables. OS was calculated from the date of diagnosis (by biopsy of either primary tumor or mediastinal nodes) to the time of death or date of last follow-up. Patients were only censored if they were lost to follow-up. FFR was determined by the date of diagnosis to the time of first failure, with either distant or locoregional disease progression. For this analysis, patients were censored at the time of their last follow-up or death. The Kaplan-Meier product limit method was used to estimate OS and FFR, and we applied the log-rank test to compare outcomes between the 2 cohorts.
We conducted the multivariate analyses using Cox regression with forward model selection. Variables analyzed included age (<60 vs ≥60 years), sex, race (black vs nonblack), median household income, insurance status (Yes vs No), Eastern Cooperative Oncology Group Performance Status (ECOG PS) (range: 0-3; 0 = fully active and 3 = capable of limited self-care, confined to bed/chair >50% of day) at time of diagnosis (0 vs ≥1), pre-CRT BMI, smoking (pack-years), chronic obstructive pulmonary disorder (Yes vs No), Charlson Comorbidity Index score (≤6 vs >7; range, 3-15; this score takes into consideration age, cardiovascular disease, malignancy, and other chronic conditions to calculate 1-year mortality), histology, calculated pretreatment NLR (as a continuous variable), pretreatment albumin (as a continuous variable), T stage, N stage, overall stage (IIIA vs IIIB), radiation technique (3D-CRT vs intensity-modulated radiation therapy [IMRT]), date of diagnosis (divided into quartiles based on proportion diagnosed by years: 2000-2002, 2003-2005, 2006-2009, 2010-2013), use of trimodality therapy, and consolidation chemotherapy. SPSS software (version 23.0) was used for statistical analysis (IBM Corp, Armonk, NY).
Results
Treatment cohorts
Table 1 compares and summarizes patient demographics, disease, and treatment characteristics for married (n = 185; 52.1%) and nonmarried (n = 170; 47.9%) patients. Married patients were more likely to self-identify as being white (P < .0001), reside in zip codes with a higher household median income (P < .0001), have an ECOG PS of 0 (P = .001), have a higher distribution of pretreatment albumin levels (P = .009), and undergo trimodality therapy (P = .001), and they were twice as likely to have insurance (P = .029). Both cohorts were evenly distributed in terms of T stage, N stage, and overall staging. There was no difference in pretreatment NLR or pretreatment BMI between married and single patients. Concurrent CRT was used in more than 85% of patients in both groups, with approximately two-thirds also receiving consolidation chemotherapy (Table 1). Median delivered radiation dose was 64.8 Gy (range, 10.8-81.6 Gy). There was no statistically significant difference in radiation dose delivered to either group, with nearly 90% of the cohort receiving ≥60 Gy.
OS and FFR
With a median follow-up of 15 months for all patients and 89 months for surviving patients (range, 1-184 months), married patients had improved OS when compared with the single cohort, with a median survival of 29.6 and 18.4 months, respectively (unadjusted hazard ratio [HR] of married vs nonmarried, .640; 95% confidence interval [CI], 0.502-0.816; P < .0001; Figure 1A). The estimated 2- and 5-year OS for married and single patients were 56% and 31% and 38.6% and 15%, respectively. When stratified by stage, married patients with stage IIIB disease (median survival, 25 months; Figure 1B) had a similar survival to unmarried patients with stage IIIA disease (median survival, 24 months; Figure 1B).
In stage IIIA patients, marital status was associated with an unadjusted HR of .696 (95% CI, 0.497-0.974; P = .035), with a larger OS benefit seen in the IIIB group (unadjusted HR, .601; 95% CI, 0.422-0.856; P = .005).
Survival as it pertains to marital status was further stratified by sex (Figure 2A) and race (Figure 2B). Married men had an improved estimated median survival of 30 months when compared with single men, whose median survival was 16 months (unadjusted HR, .541; 95% CI, 0.392-0.746; P < .0001). On the other hand, marital status had no statistically significant effect on OS when comparing married women with their single counterparts (unadjusted HR, .717; 95% CI, 0.491-1.048; P = .085; Figure 2A), with an overall median survival of approximately 28 months for the entire female cohort. Stratification by race also showed similar results, with married nonblack patients demonstrating better OS when compared with single nonblack patients (HR, .586; 95% CI, 0.420-0.820; P = .002; Figure 2B), with a median survival of 29 and 17 months, respectively. Black patients also had a similar improvement in survival when comparing the married (median survival, 30 months) and nonmarried groups (median survival, 19.6 months; unadjusted HR, .676; 95% CI, 0.457-1.000; P = .050; Figure 2B).
FFR did not differ between the 2 groups, with a median time to failure of 17 and 15 months for married and nonmarried patients, respectively (unadjusted HR, .799; 95% CI, 0.607-1.051; P = .108; Figure 3). Estimated 2- and 5-year FFR for married and nonmarried patients were 39.4% and 27% and 31.5% and 18.5%, respectively (Figure 3).
Clinical predictors of survival
On MVA, factors that were independent predictors for OS are summarized in Table 2. Risk of death was reduced by approximately 65% and 45% in patients who underwent trimodality treatment (P < .0001) or were able to undergo consolidative chemotherapy (P = .004) when compared with those who were treated definitively with bimodality treatment or did not undergo systemic doses of adjuvant chemotherapy, respectively. Having insurance (P = .048) and use of IMRT over 3D-CRT (P = .008) was associated with a reduction of mortality by about half in this cohort. Both gender (improved OS with female sex; P = .004) and marital status (improved OS with marriage; P = .006) were associated with a decreased the risk of death by 40% (Table 2). By contrast, a higher NLR resulted
Discussion
Our study continues to support the notion that marital status is an independent indicator of survival in stage III NSCLC (adjusted HR, .59; 95% CI, 0.404-0.859; P = .006). The benefit of marriage in this population seems to be better than that reported in the SEER analysis for all stages, wherein the HR for death of married patients compared with their single counterparts was .85 (95% CI, 0.83-0.87). In their analysis, the investigators hypothesized that this survival advantage could partially be explained by better access to health care and adherence to therapy, as was supported by the higher likelihood of married patients presenting with localized disease and receiving definitive treatment.3 Another population-based study using the Florida Cancer Data System identified 161,228 lung cancer patients (NSCLC and small-cell lung histology included), and on MVA, marital status remained an important prognostic indicator for OS when compared with never-married patients (HR, .86; P = .001).6 In addition to typically including patients with all stages of diseases, population-based studies often include patients who receive a heterogeneous combination of treatment modalities, possibly confounding the analysis. Furthermore, large population analyses typically do not report on patient-specific variables such as nutrition (ie, BMI and albumin) or immunologic status (ie, NLR), both of which have been shown to be independent predictors of survival in LA-NSCLC.8,9
In contrast, some other studies have failed to demonstrate an OS advantage with marital status in patients with NSCLC. For example, in a meta-analysis that evaluated the influence of race, gender, and marital status on 1,365 nonoperative NSCLC patients who were enrolled in 9 Radiation Therapy Oncology Group (RTOG) trials, the investigators did not find marital status to be independently predictive of survival.11 In addition, for the 5,898 patients who were prospectively enrolled in a Mayo Clinic Lung Cancer Cohort (MCLCC), marital status was also found not to be prognostic for NSCLC outcomes when all stages of the disease were analyzed together.4 There are some possible confounding factors in these studies. Patients recruited for clinical trials tend to be healthier with a better performance status and have a support system (including close monitoring by the study team) when compared with the general population diagnosed with lung cancer. About 70% to 76% of the patients in both the RTOG and MCLCC studies were married, which is significantly higher than both the national average (51%) and our group (52.1%). Like other population-based studies, the MCLCC included patients with all stages getting a variety of treatments. Although no overall impact on survival was noted, the investigators noted that single, divorced, and widowed patients were more likely to not receive cancer therapy(P < .0001). The marital status also influenced the choice of therapy, with subgroup analysis revealing inferior outcomes in widowed and divorced patients with stage IA, IIB, or IIIB disease. The authors also recognized an inherent referral bias from patients, with support system being typically seen at the Mayo clinics, which may have played an additional role. All of the patients in our analysis were appropriately staged and received curative-intent treatment by a team of physicians using essentially identical therapeutic strategies, thus minimizing some of these confounding factors. This allowed us to explore the impact of marital status while a patient was undergoing stage-appropriate treatment. We demonstrated a strong association with marital status and survival that even overcame the effects of stage (IIIA vs IIIB) on clinical outcomes (Figure 1B).
Furthermore, our analysis allowed us to explore the interaction of race and marital status more definitively because the demographics of the patients in the RTOG and MCLCC included 14% and less than 3% of patients identified as being nonwhite, respectively, in contrast to our analysis in which 41% of the patients self-identified as black.12 In our black population, marital status was associated with an observable improvement in OS, similar to our nonblack, predominantly white (97%) cohort (Figure 2B). Also, the results of our analysis may be a more accurate representation of the general population living in large urban or semiurban settings and further implies that an intact social support system could have a greater influence on clinical outcomes.
The current analysis is unique when compared with previous published studies in that beyond conventional demographic and treatment-related factors, we have comprehensively explored potential mechanisms that may explain the survival advantage seen in married patients by evaluating additional factors, such as functional status (ECOG and Charlson’s scores), nutritional status (BMI and albumin), immunologic characteristics (NLR), and other social factors (race, income, insurance status). Although married patients were more likely to have a higher BMI and albumin at diagnosis, when controlling for these factors in the multivariable analysis, marital status remained strongly prognostic (Table 2), suggesting that nutrition alone does not fully account for the observed survival advantage demonstrated. A similar conclusion can be drawn about immunologic status. NLR has previously been shown to be prognostic in a number of cancers,13-16 including in our own cohort.8 Although immune status remains an important predictor for OS in our locally advanced NSCLC population, when we take NLR into consideration in our analysis, marital status continues to be a strong indicator for survival (Table 2). In terms of other variables analyzed, insurance status was a significant predictor of OS in the MVA, though functional status and other social factors including race were not significant.
We also explored cancer control outcomes in the form of FFR. Married patients had an observable, although not statistically significant, improvement in FFR when compared with the single cohort (Figure 2). In our study, married patients were more likely to undergo trimodality therapy (Table 1), which has likely translated to the improvement of FFR seen in our group. In this case, marriage may serve as a surrogate for availability of a support system to undergo aggressive, potentially toxic treatment.3,17,18 Even in the setting of bimodality therapy, the RTOG 0617 study noted about 17.5% treatment interruptions because of adverse effects or illness, with more than 30% of patients experiencing grade 3 or more esophagitis, irrespective of radiation technique.19 In these scenarios, in addition to receiving better attention to nutrition and care, significant others often provide emotional and social support that, in turn, can lead to better compliance. Social supports and socio-demographic factors are especially critical in patient populations in which access to health care is challenging.
Despite the compelling outcomes presented, our study suffers from the common limitations of retrospective analyses. Marital status, in this setting, most likely correlates with improved socioeconomic status and greater support, which have resulted in improved survival. Furthermore, although patients were self-classified as married or single, our data were not able to capture whether patients were single but lived with another adult or had other types of social support. However, even if there was a proportion of the unmarried cohort that had an alternate support system, separating them out is likely to further expand the differences. Quantifying the amount of social, emotional, or even spiritual support was not possible to accomplish in our analysis, though we know that all 3 can play a role in cancer outcomes.20,21 Further prospective studies would have to be done to completely understand how marital status can influence clinical decisions. Understanding whether marital status is a proxy for social provisions may help to identify populations at risk for inferior outcomes. These at-risk patients may benefit from targeted clinical interventions, such as closer physician follow-up, more aggressive supportive care, access to support groups, or nurse navigator visits.
Conclusions
In patients with locally advanced NSCLC treated with curative-intent following uniform treatment algorithms, marital status was linked with improvement in survival even when adjusted for other key variables, with the second highest HR (after insurance status) among pretreatment demographic variables. Although marriage is an unmodifiable factor in itself, it is most likely a surrogate for better psychosocial support. The scale of these positive survival improvements emphasizes the need to institute targeted supportive care strategies to help advance overall outcomes in a tumor for which modern therapeutic approaches (novel systemic therapy and radiation) have yielded only modest improvement in outcomes yet come at the cost of considerable treatment-related toxicity.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
2. Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39-51.
3. Aizer AA, Chen M-H, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31(31):3869-3876.
4. Jatoi A, Novotny P, Cassivi S, et al. Does marital status impact survival and quality of life in patients with non-small cell lung cancer? Observations from the mayo clinic lung cancer cohort. Oncologist. 2007;12(12):1456-1463.
5. Kravdal H, Syse A. Changes over time in the effect of marital status on cancer survival. BMC Public Health. 2011;11:804.
6. Tannenbaum SL, Zhao W, Koru-Sengul T, Miao F, Lee D, Byrne MM. Marital status and its effect on lung cancer survival. Springerplus. 2013;2:504.
7. Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics [published online October 16, 2017]. J Clin Oncol. 2018;36(1):25-33.
8. Scilla KA, Bentzen SM, Lam VK, et al. Neutrophil-lymphocyte ratio is a prognostic marker in patients with locally advanced (stage IIIA and IIIB) non-small cell lung cancer treated with combined modality therapy. Oncologist. 2017;22(6):737-742.
9. Lam VK, Bentzen SM, Mohindra P, et al. Obesity is associated with long-term improved survival in definitively treated locally advanced non-small cell lung cancer (NSCLC). Lung Cancer. 2017;104:52-57.
10. Vyfhuis MAL, Bhooshan N, Burrows WM, et al. Oncological outcomes from trimodality therapy receiving definitive doses of neoadjuvant chemoradiation (≥60 Gy) and factors influencing consideration for surgery in stage III non-small cell lung cancer. Adv Radiat Oncol. 2017;2(3):259-269.
11. Siddiqui F, Bae K, Langer CJ, et al. The influence of gender, race, and marital status on survival in lung cancer patients: analysis of radiation therapy oncology group trials. J Thorac Oncol. 2010;5(5):631-639.
12. Vyfhuis MAL, Bhooshan N, Molitoris J, et al. Clinical outcomes of black vs. non-black patients with locally advanced non–small cell lung cancer. Lung Cancer. 2017;114:44-49.
13. Beltran BE, Castro D, De La Cruz-Vargas JA, et al. The neutrophil-lymphocyte ratio is prognostic in patients with early stage aggressive peripheral T cell lymphoma [published online February 26, 2018]. Br J Haematol. doi:10.1111/bjh.15141.
14. Lee BM, Chung SY, Chang JS, Lee KJ, Seong J. The neutrophil-lymphocyte ratio and platelet-lymphocyte ratio are prognostic factors in patients with locally advanced pancreatic cancer treated with chemoradiotherapy. Gut Liver. 2018;12(3):342-352.
15. Najjar M, Agrawal S, Emond JC, Halazun KJ. Pretreatment neutrophil-lymphocyte ratio: useful prognostic biomarker in hepatocellular carcinoma. J Hepatocell Carcinoma. 2018;5:17-28.
16. Hu W, Yu J, Huang Y, Hu F, Zhang X, Wang Y. Lymphocyte-related inflammation and immune-based scores predict prognosis of chordoma patients after radical resection. Transl Oncol. 2018;11(2):444-449.
17. Mahal BA, Cooperberg MR, Aizer AA, et al. Who bears the greatest burden of aggressive treatment of indolent prostate cancer? Am J Med. 2015;128(6):609-616.
18. Inverso G, Mahal BA, Aizer AA, Donoff RB, Chau NG, Haddad RI. Marital status and head and neck cancer outcomes. Cancer. 2015;121(8):1273-1278.
19. Chun SG, Hu C, Choy H, et al. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: a secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J Clin Oncol. 2017;35(1):56-62.
20. Waite LJ, Lehrer EL. The benefits from marriage and religion in the United States: a comparative analysis. Popul Dev Rev. 2003;29(2):255-276.
21. Osborne C, Ostir GV, Du X, Peek MK, Goodwin JS. The influence of marital status on the stage at diagnosis, treatment, and survival of older women with breast cancer. Breast Cancer Res Treat. 2005;93(1):41-47.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
2. Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39-51.
3. Aizer AA, Chen M-H, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31(31):3869-3876.
4. Jatoi A, Novotny P, Cassivi S, et al. Does marital status impact survival and quality of life in patients with non-small cell lung cancer? Observations from the mayo clinic lung cancer cohort. Oncologist. 2007;12(12):1456-1463.
5. Kravdal H, Syse A. Changes over time in the effect of marital status on cancer survival. BMC Public Health. 2011;11:804.
6. Tannenbaum SL, Zhao W, Koru-Sengul T, Miao F, Lee D, Byrne MM. Marital status and its effect on lung cancer survival. Springerplus. 2013;2:504.
7. Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics [published online October 16, 2017]. J Clin Oncol. 2018;36(1):25-33.
8. Scilla KA, Bentzen SM, Lam VK, et al. Neutrophil-lymphocyte ratio is a prognostic marker in patients with locally advanced (stage IIIA and IIIB) non-small cell lung cancer treated with combined modality therapy. Oncologist. 2017;22(6):737-742.
9. Lam VK, Bentzen SM, Mohindra P, et al. Obesity is associated with long-term improved survival in definitively treated locally advanced non-small cell lung cancer (NSCLC). Lung Cancer. 2017;104:52-57.
10. Vyfhuis MAL, Bhooshan N, Burrows WM, et al. Oncological outcomes from trimodality therapy receiving definitive doses of neoadjuvant chemoradiation (≥60 Gy) and factors influencing consideration for surgery in stage III non-small cell lung cancer. Adv Radiat Oncol. 2017;2(3):259-269.
11. Siddiqui F, Bae K, Langer CJ, et al. The influence of gender, race, and marital status on survival in lung cancer patients: analysis of radiation therapy oncology group trials. J Thorac Oncol. 2010;5(5):631-639.
12. Vyfhuis MAL, Bhooshan N, Molitoris J, et al. Clinical outcomes of black vs. non-black patients with locally advanced non–small cell lung cancer. Lung Cancer. 2017;114:44-49.
13. Beltran BE, Castro D, De La Cruz-Vargas JA, et al. The neutrophil-lymphocyte ratio is prognostic in patients with early stage aggressive peripheral T cell lymphoma [published online February 26, 2018]. Br J Haematol. doi:10.1111/bjh.15141.
14. Lee BM, Chung SY, Chang JS, Lee KJ, Seong J. The neutrophil-lymphocyte ratio and platelet-lymphocyte ratio are prognostic factors in patients with locally advanced pancreatic cancer treated with chemoradiotherapy. Gut Liver. 2018;12(3):342-352.
15. Najjar M, Agrawal S, Emond JC, Halazun KJ. Pretreatment neutrophil-lymphocyte ratio: useful prognostic biomarker in hepatocellular carcinoma. J Hepatocell Carcinoma. 2018;5:17-28.
16. Hu W, Yu J, Huang Y, Hu F, Zhang X, Wang Y. Lymphocyte-related inflammation and immune-based scores predict prognosis of chordoma patients after radical resection. Transl Oncol. 2018;11(2):444-449.
17. Mahal BA, Cooperberg MR, Aizer AA, et al. Who bears the greatest burden of aggressive treatment of indolent prostate cancer? Am J Med. 2015;128(6):609-616.
18. Inverso G, Mahal BA, Aizer AA, Donoff RB, Chau NG, Haddad RI. Marital status and head and neck cancer outcomes. Cancer. 2015;121(8):1273-1278.
19. Chun SG, Hu C, Choy H, et al. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: a secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J Clin Oncol. 2017;35(1):56-62.
20. Waite LJ, Lehrer EL. The benefits from marriage and religion in the United States: a comparative analysis. Popul Dev Rev. 2003;29(2):255-276.
21. Osborne C, Ostir GV, Du X, Peek MK, Goodwin JS. The influence of marital status on the stage at diagnosis, treatment, and survival of older women with breast cancer. Breast Cancer Res Treat. 2005;93(1):41-47.
Effect of time of admission to treatment initiation on outcomes of patients with acute myeloid leukemia: a tertiary care referral center experience
Acute myeloid leukemia (AML) is the most common acute leukemia in adults in the United States.1 In 2018, the estimated annual incidence of AML is 19,520 (32.4% of all new leukemia cases), with 10,670 projected deaths (43.8% of all leukemia deaths).1 New molecularly targeted treatments are increasingly being used in treating AML, and some of them have shown improved health outcomes. In general, age, white blood cell (WBC) count at presentation, cytogenetics, and molecular characteristics are the major determinants of prognosis and treatment outcome. Studies analyzing the Surveillance Epidemiology and End Results database have also shown racial differences in outcomes.2 It is well known to the oncology community that patients with similar characteristics may respond differently to treatment and that outcome is not uniformly related to the well-defined clinical and laboratory characteristics. Issues related to health care disparities and access to health care are also known to affect the outcome in patients with cancer.3-9
AML is generally considered by the medical community as a time-sensitive condition. Treatment of patients with AML usually consists of induction chemotherapy followed by consolidation treatment with consideration for stem cell transplant. The duration of time from admission to treatment (TAT) of AML with induction chemotherapy is dependent on multiple factors. These may include the assessment of comorbid conditions and the availability of molecular studies at the time of treatment, which can be time consuming. The effect of treatment delays after AML diagnosis has been investigated, but with conflicting results. One study showed that time from diagnosis to treatment initiation affects survival in younger patients, and another showed it has no effect on survival regardless of patient age.10,11 We describe here the results of a retrospective analysis evaluating the impact of TAT and day of admission on outcomes of patients with AML who received treatment at a tertiary care referral center.
Methods and materials
We did a retrospective medical record review of all newly diagnosed AML patients at the Oklahoma University Health Sciences Center (OUHSC). Our sample was composed of 154 adult patients. Our inclusion criteria were an age of 18 years or older with complete insurance data, a diagnosis of AML, and having received treatment at our institution from January 2000 through June 2015. Data were obtained on laboratory values at diagnosis, pathology data including cytogenetics, molecular data, and bone marrow biopsies. Data on patient characteristics such as age, race and/or ethnicity, and comorbidities were obtained from the electronic medical records. Treatment data on type and dose of chemotherapy during induction, subsequent treatment phases, and number of treatments to achieve complete response (CR) as well as response data of CR achievement, relapse, date of CR, date of relapse, stem cell transplantation data, date of death, and date of last follow-up visit were recorded retrospectively from the electronic medical record. The study was approved by the OUHSC Institutional Review Board.
Statistical analysis
TAT was analyzed categorically (0-4 days vs >4 days), and day of admission was analyzed categorically (Monday to Thursday vs Friday to Sunday). Descriptive statistics were calculated overall and by TAT group. The chi-square test was used to compare the association between our covariates and TAT. Kaplan-Meier estimates (with a log-rank test) were used to assess the unadjusted effect of TAT with overall survival (OS) and event-free survival (EFS). Median OS and EFS and 95% confidence intervals (CIs) were also calculated. We used the Cox proportional hazards regression modeling to evaluate the relationship between OS and TAT. The initial model was built by including covariates, with P < .25 for the association between the covariates with OS. TAT was maintained in the final model because it was the primary variable of interest, whereas age and risk group were also included in the final model because those covariates are known prognostic risk factors in AML. Among the set of variables screened in, all 2-way interactions were assessed using P < .05. No significant interactions were found. Backward elimination was then performed. During the backward elimination, confounding was deemed to have been present if the measure of association of significant variables in the model changed by more than 20% and the P-value of the confounding variable was less than .30. Variables with P-values of less than .05 or deemed a confounder would then be retained. A similar modeling approach was used to examine EFS and CR. To evaluate the association between CR with potential predictors, binary logistic regression was used, whereby day of admission and time to treatment were explored unadjusted and then adjusted for age, WBC count, risk group, and undergoing allogeneic stem cell transplant (AlloSCT). SAS version 9.4 (SAS Institute Inc, Cary, North Carolina) was used for all analyses. A final alpha of 0.05 was used unless otherwise noted.
Results
Baseline characteristics are presented in Table 1. Treatment was initiated within 4 days for 71% (109/154) of patients. Most patients in our study were younger than 60 years (70%), male (64%), and white (77%). Most patients were admitted to the hospital for treatment between Monday and Thursday (75%). A higher proportion of patients in the 0-4 days TAT group were <60 years of age compared with patients in the >4 days TAT group (P = .0427). A higher proportion of patients in the 0-4 days TAT group had a WBC count of ≥50 x 103 μ/L compared with patients in the >4 days TAT group (27% vs 9%, respectively; P = .0148). A higher proportion of patients were admitted Friday to Sunday in the TAT >4 days group. Insured and uninsured patients were equally distributed between the 2 groups (P = .0014). Cytogenetic and/or molecular risk was not statistically different between the 0-4 days and >4 days TAT groups (unfavorable risk, 25% vs 23%, respectively; P = .6214). A higher proportion of patients received 7 + 3 induction chemotherapy (7 days cytarabine and 3 days anthracycline) in the TAT 0-4 days group compared with the >4 days TAT group (84% vs 69%, respectively; P = .0448). The most common intensive chemotherapy regimen used was 7 + 3 (80%). The rest of the patients (20%) received high-dose cytarabine clofarabine-based chemotherapy, hypomethylating agents, or other treatments. The proportion of patients who received an AlloSCT did not differ between the 0-4 days and >4 days TAT groups (24% vs 20%, respectively; P = .5655).
The median OS for all patients was 10.9 months (95% CI, 8.3-15.1), and the median EFS was 9.1 months (95% CI, 7.4-13.8). Median follow-up time was 8.6 months (95% CI, 6.7-11). We found a significant association between TAT and both OS and EFS without any adjustment (Table 2).
The median OS for the TAT 0-4 days group was 15.6 months, and for the TAT >4 days group, it was 6.8 months (P = .0207; Figure 1). The median EFS for the TAT 0-4 days group was 14.5 months, and for the TAT >4 days group, it was 6.8 months (P = .0240; Figure 2).
We found no association between the day of admission to hospital (Monday-Thursday vs Friday-Sunday) and either OS or EFS. After adjusting for age, WBC count, molecular risk status, and undergoing AlloSCT, the OS was shorter for those who received treatment >4 days after admission compared with those who received treatment within 0 to 4 days, with a hazard ratio (HR) of 1.59 (95% CI, 1.02-2.49; P = .0427; Table 3).
There was no association between day of admission with OS in the multivariable analysis. Similarly, after adjusting for age, WBC count, molecular risk status, and undergoing AlloSCT, EFS was shorter in patients who received treatment >4 days after admission compared with those who received treatment within 0 to 4 days (HR, 1.64; 95% CI, 1.06-2.54; P = .0268). There was no association between day of admission with EFS in the multivariable model. Although there was a trend for a higher CR rate with earlier treatment, this was not statistically significant (Table 4).
Discussion
Treatment outcomes for patients with AML are known to be affected by several patient- and disease-related factors. Patient-related factors can include age, performance status, comorbidities, and availability of a stem cell donor. Examples of disease-related factors include molecular alterations and site of disease involvement. Little is known about whether the timing of treatment initiation affects patient outcomes. Short-term treatment delays after the diagnosis of leukemia are not uncommon. Generally, patients are treated with anthracycline-based induction chemotherapy, but the response rate and survival are particularly poor in the older age group.12 Moreover, increasing comorbidities with aging are expected to lead to lower treatment tolerability.13 Therefore, elderly patients are particularly prone to treatment delays while providers await the results of the molecular studies to guide the use of less intensive targeted therapies.10 Other reasons for treatment delays may also include transfers between hospitals, suspected or documented infections, and evaluation of chronic illnesses. Our analysis also indicates that admission to the hospital on the weekend contributes to a delay in therapy compared with admission on a weekday.
We found a decreased OS and EFS in patients who received treatment >4 days after admission to the hospital compared with patients who received treatment within 0 to 4 days of admission. This association was statistically significant in a bivariate analysis as well as in a multivariable analysis with adjustment for age, WBC count on presentation, molecular risk group, and undergoing AlloSCT. A previous large retrospective study showed that the time from diagnosis to treatment initiation predicts survival in younger, but not older, patients with AML.10 This remained true after adjusting for age, performance status, WBC count, and the type of AML in a multivariable analysis. In our study, the declines in overall survival and event-free survival were evident after a delay of more than 4 days.
Another retrospective study that included 599 newly diagnosed AML patients, with a median time from diagnosis to treatment of 8 days, did not show any impact of treatment delay on overall survival, early death, or response rate.11 These differences in the effect of treatment delay on outcomes could be related to the differences in baseline characteristics of patients in these studies. Our study had a higher proportion of patients younger than 60 years, for example. We hypothesize that treatment delays, especially in patients with a high WBC count on presentation, might lead to further organ compromise and poorer outcomes with chemotherapy.
In our study, a higher proportion of patients were admitted over the weekend in the >4 days TAT group, but when we analyzed the day of admission to hospital separately, it was not associated with OS or EFS. Admission over the weekend was also not associated with clinical outcomes including 30-day mortality in a larger study that included 422 patients treated at a large teaching referral hospital.14
Limitations of our study include a small sample size and a short median follow-up time. Most of our patients were young and white, which may not be representative of the general population.
In conclusion, we found that treatment delays are associated with inferior outcomes in AML patients. It remains to be elucidated whether the benefit gained from using targeted and less-intensive chemotherapy, especially in elderly patients, outweighs the potential harm from delaying treatment. Additional studies are needed to confirm our findings in different settings and patient populations.
Acknowledgment
Statistical support was provided by the Stephenson Cancer Center Biostatistics and Research Design Shared Resource.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
2. Patel MI, Ma Y, Mitchell B, Rhoads KF. How do differences in treatment impact racial and ethnic disparities in acute myeloid leukemia? Cancer Epidemiol Biomarkers Prev. 2015;24(2):344-349.
3. Weber JJ, Kachare SD, Vohra NA, Fitzgerald TF, Wong JH. Regional disparities in breast cancer outcomes and the process of care. Am Surg. 2014;80(7):669-674.
4. Shippee TP, Kozhimannil KB, Rowan K, Virnig BA. Health insurance coverage and racial disparities in breast reconstruction after mastectomy. Womens Health Issues. 2014;24(3):e261-e269.
5. Dickens C, Joffe M, Jacobson J, et al. Stage at breast cancer diagnosis and distance from diagnostic hospital in a periurban setting: a South African public hospital case series of over 1,000 women. Int J Cancer. 2014;135(9):2173-2182.
6. Nguyen-Pham S, Leung J, McLaughlin D. Disparities in breast cancer stage at diagnosis in urban and rural adult women: a systematic review and meta-analysis. Ann Epidemiol. 2014;24(3):228-235.
7. Gong G, Belasco E, Hargrave KA, Lyford CP, Philips BU Jr. Determinants of delayed detection of cancers in Texas counties in the United States of America. Int J Equity Health. 2012;11:29.
8. Erhunmwunsee L, Joshi MB, Conlon DH, Harpole DH Jr. Neighborhood‐level socioeconomic determinants impact outcomes in nonsmall cell lung cancer patients in the Southeastern United States. Cancer. 2012;118(20):5117-5123.
9. Steele CB, Pisu M, Richardson LC. Urban/rural patterns in receipt of treatment for non–small cell lung cancer among black and white Medicare beneficiaries, 2000-2003. J Natl Med Assoc. 2011;103(8):711-718.
10. Sekeres MA, Elson P, Kalaycio ME, et al. Time from diagnosis to treatment initiation predicts survival in younger, but not older, acute myeloid leukemia patients. Blood. 2009;113(1):28-36.
11. Bertoli S, Bérard E, Huguet F, et al. Time from diagnosis to intensive chemotherapy initiation does not adversely impact the outcome of patients with acute myeloid leukemia. Blood. 2013:121(14):2618-2626.
12. Shah A, Andersson TM, Rachet B, Björkholm M, Lambert PC. Survival and cure of acute myeloid leukaemia in England, 1971-2006: a population-based study. Br J Haematol. 2013;162(4):509-516.
13. Mohammadi M, Cao Y, Glimelius I, Bottai M, Eloranta S, Smedby KE. The impact of comorbid disease history on all-cause and cancer-specific mortality in myeloid leukemia and myeloma – a Swedish population-based study. BMC Cancer. 2015;15:850.
14. Bejanyan N, Fu AZ, Lazaryan A, et al. Impact of weekend admissions on quality of care and outcomes in patients with acute myeloid leukemia. Cancer. 2010;116(15):3614-3620.
Acute myeloid leukemia (AML) is the most common acute leukemia in adults in the United States.1 In 2018, the estimated annual incidence of AML is 19,520 (32.4% of all new leukemia cases), with 10,670 projected deaths (43.8% of all leukemia deaths).1 New molecularly targeted treatments are increasingly being used in treating AML, and some of them have shown improved health outcomes. In general, age, white blood cell (WBC) count at presentation, cytogenetics, and molecular characteristics are the major determinants of prognosis and treatment outcome. Studies analyzing the Surveillance Epidemiology and End Results database have also shown racial differences in outcomes.2 It is well known to the oncology community that patients with similar characteristics may respond differently to treatment and that outcome is not uniformly related to the well-defined clinical and laboratory characteristics. Issues related to health care disparities and access to health care are also known to affect the outcome in patients with cancer.3-9
AML is generally considered by the medical community as a time-sensitive condition. Treatment of patients with AML usually consists of induction chemotherapy followed by consolidation treatment with consideration for stem cell transplant. The duration of time from admission to treatment (TAT) of AML with induction chemotherapy is dependent on multiple factors. These may include the assessment of comorbid conditions and the availability of molecular studies at the time of treatment, which can be time consuming. The effect of treatment delays after AML diagnosis has been investigated, but with conflicting results. One study showed that time from diagnosis to treatment initiation affects survival in younger patients, and another showed it has no effect on survival regardless of patient age.10,11 We describe here the results of a retrospective analysis evaluating the impact of TAT and day of admission on outcomes of patients with AML who received treatment at a tertiary care referral center.
Methods and materials
We did a retrospective medical record review of all newly diagnosed AML patients at the Oklahoma University Health Sciences Center (OUHSC). Our sample was composed of 154 adult patients. Our inclusion criteria were an age of 18 years or older with complete insurance data, a diagnosis of AML, and having received treatment at our institution from January 2000 through June 2015. Data were obtained on laboratory values at diagnosis, pathology data including cytogenetics, molecular data, and bone marrow biopsies. Data on patient characteristics such as age, race and/or ethnicity, and comorbidities were obtained from the electronic medical records. Treatment data on type and dose of chemotherapy during induction, subsequent treatment phases, and number of treatments to achieve complete response (CR) as well as response data of CR achievement, relapse, date of CR, date of relapse, stem cell transplantation data, date of death, and date of last follow-up visit were recorded retrospectively from the electronic medical record. The study was approved by the OUHSC Institutional Review Board.
Statistical analysis
TAT was analyzed categorically (0-4 days vs >4 days), and day of admission was analyzed categorically (Monday to Thursday vs Friday to Sunday). Descriptive statistics were calculated overall and by TAT group. The chi-square test was used to compare the association between our covariates and TAT. Kaplan-Meier estimates (with a log-rank test) were used to assess the unadjusted effect of TAT with overall survival (OS) and event-free survival (EFS). Median OS and EFS and 95% confidence intervals (CIs) were also calculated. We used the Cox proportional hazards regression modeling to evaluate the relationship between OS and TAT. The initial model was built by including covariates, with P < .25 for the association between the covariates with OS. TAT was maintained in the final model because it was the primary variable of interest, whereas age and risk group were also included in the final model because those covariates are known prognostic risk factors in AML. Among the set of variables screened in, all 2-way interactions were assessed using P < .05. No significant interactions were found. Backward elimination was then performed. During the backward elimination, confounding was deemed to have been present if the measure of association of significant variables in the model changed by more than 20% and the P-value of the confounding variable was less than .30. Variables with P-values of less than .05 or deemed a confounder would then be retained. A similar modeling approach was used to examine EFS and CR. To evaluate the association between CR with potential predictors, binary logistic regression was used, whereby day of admission and time to treatment were explored unadjusted and then adjusted for age, WBC count, risk group, and undergoing allogeneic stem cell transplant (AlloSCT). SAS version 9.4 (SAS Institute Inc, Cary, North Carolina) was used for all analyses. A final alpha of 0.05 was used unless otherwise noted.
Results
Baseline characteristics are presented in Table 1. Treatment was initiated within 4 days for 71% (109/154) of patients. Most patients in our study were younger than 60 years (70%), male (64%), and white (77%). Most patients were admitted to the hospital for treatment between Monday and Thursday (75%). A higher proportion of patients in the 0-4 days TAT group were <60 years of age compared with patients in the >4 days TAT group (P = .0427). A higher proportion of patients in the 0-4 days TAT group had a WBC count of ≥50 x 103 μ/L compared with patients in the >4 days TAT group (27% vs 9%, respectively; P = .0148). A higher proportion of patients were admitted Friday to Sunday in the TAT >4 days group. Insured and uninsured patients were equally distributed between the 2 groups (P = .0014). Cytogenetic and/or molecular risk was not statistically different between the 0-4 days and >4 days TAT groups (unfavorable risk, 25% vs 23%, respectively; P = .6214). A higher proportion of patients received 7 + 3 induction chemotherapy (7 days cytarabine and 3 days anthracycline) in the TAT 0-4 days group compared with the >4 days TAT group (84% vs 69%, respectively; P = .0448). The most common intensive chemotherapy regimen used was 7 + 3 (80%). The rest of the patients (20%) received high-dose cytarabine clofarabine-based chemotherapy, hypomethylating agents, or other treatments. The proportion of patients who received an AlloSCT did not differ between the 0-4 days and >4 days TAT groups (24% vs 20%, respectively; P = .5655).
The median OS for all patients was 10.9 months (95% CI, 8.3-15.1), and the median EFS was 9.1 months (95% CI, 7.4-13.8). Median follow-up time was 8.6 months (95% CI, 6.7-11). We found a significant association between TAT and both OS and EFS without any adjustment (Table 2).
The median OS for the TAT 0-4 days group was 15.6 months, and for the TAT >4 days group, it was 6.8 months (P = .0207; Figure 1). The median EFS for the TAT 0-4 days group was 14.5 months, and for the TAT >4 days group, it was 6.8 months (P = .0240; Figure 2).
We found no association between the day of admission to hospital (Monday-Thursday vs Friday-Sunday) and either OS or EFS. After adjusting for age, WBC count, molecular risk status, and undergoing AlloSCT, the OS was shorter for those who received treatment >4 days after admission compared with those who received treatment within 0 to 4 days, with a hazard ratio (HR) of 1.59 (95% CI, 1.02-2.49; P = .0427; Table 3).
There was no association between day of admission with OS in the multivariable analysis. Similarly, after adjusting for age, WBC count, molecular risk status, and undergoing AlloSCT, EFS was shorter in patients who received treatment >4 days after admission compared with those who received treatment within 0 to 4 days (HR, 1.64; 95% CI, 1.06-2.54; P = .0268). There was no association between day of admission with EFS in the multivariable model. Although there was a trend for a higher CR rate with earlier treatment, this was not statistically significant (Table 4).
Discussion
Treatment outcomes for patients with AML are known to be affected by several patient- and disease-related factors. Patient-related factors can include age, performance status, comorbidities, and availability of a stem cell donor. Examples of disease-related factors include molecular alterations and site of disease involvement. Little is known about whether the timing of treatment initiation affects patient outcomes. Short-term treatment delays after the diagnosis of leukemia are not uncommon. Generally, patients are treated with anthracycline-based induction chemotherapy, but the response rate and survival are particularly poor in the older age group.12 Moreover, increasing comorbidities with aging are expected to lead to lower treatment tolerability.13 Therefore, elderly patients are particularly prone to treatment delays while providers await the results of the molecular studies to guide the use of less intensive targeted therapies.10 Other reasons for treatment delays may also include transfers between hospitals, suspected or documented infections, and evaluation of chronic illnesses. Our analysis also indicates that admission to the hospital on the weekend contributes to a delay in therapy compared with admission on a weekday.
We found a decreased OS and EFS in patients who received treatment >4 days after admission to the hospital compared with patients who received treatment within 0 to 4 days of admission. This association was statistically significant in a bivariate analysis as well as in a multivariable analysis with adjustment for age, WBC count on presentation, molecular risk group, and undergoing AlloSCT. A previous large retrospective study showed that the time from diagnosis to treatment initiation predicts survival in younger, but not older, patients with AML.10 This remained true after adjusting for age, performance status, WBC count, and the type of AML in a multivariable analysis. In our study, the declines in overall survival and event-free survival were evident after a delay of more than 4 days.
Another retrospective study that included 599 newly diagnosed AML patients, with a median time from diagnosis to treatment of 8 days, did not show any impact of treatment delay on overall survival, early death, or response rate.11 These differences in the effect of treatment delay on outcomes could be related to the differences in baseline characteristics of patients in these studies. Our study had a higher proportion of patients younger than 60 years, for example. We hypothesize that treatment delays, especially in patients with a high WBC count on presentation, might lead to further organ compromise and poorer outcomes with chemotherapy.
In our study, a higher proportion of patients were admitted over the weekend in the >4 days TAT group, but when we analyzed the day of admission to hospital separately, it was not associated with OS or EFS. Admission over the weekend was also not associated with clinical outcomes including 30-day mortality in a larger study that included 422 patients treated at a large teaching referral hospital.14
Limitations of our study include a small sample size and a short median follow-up time. Most of our patients were young and white, which may not be representative of the general population.
In conclusion, we found that treatment delays are associated with inferior outcomes in AML patients. It remains to be elucidated whether the benefit gained from using targeted and less-intensive chemotherapy, especially in elderly patients, outweighs the potential harm from delaying treatment. Additional studies are needed to confirm our findings in different settings and patient populations.
Acknowledgment
Statistical support was provided by the Stephenson Cancer Center Biostatistics and Research Design Shared Resource.
Acute myeloid leukemia (AML) is the most common acute leukemia in adults in the United States.1 In 2018, the estimated annual incidence of AML is 19,520 (32.4% of all new leukemia cases), with 10,670 projected deaths (43.8% of all leukemia deaths).1 New molecularly targeted treatments are increasingly being used in treating AML, and some of them have shown improved health outcomes. In general, age, white blood cell (WBC) count at presentation, cytogenetics, and molecular characteristics are the major determinants of prognosis and treatment outcome. Studies analyzing the Surveillance Epidemiology and End Results database have also shown racial differences in outcomes.2 It is well known to the oncology community that patients with similar characteristics may respond differently to treatment and that outcome is not uniformly related to the well-defined clinical and laboratory characteristics. Issues related to health care disparities and access to health care are also known to affect the outcome in patients with cancer.3-9
AML is generally considered by the medical community as a time-sensitive condition. Treatment of patients with AML usually consists of induction chemotherapy followed by consolidation treatment with consideration for stem cell transplant. The duration of time from admission to treatment (TAT) of AML with induction chemotherapy is dependent on multiple factors. These may include the assessment of comorbid conditions and the availability of molecular studies at the time of treatment, which can be time consuming. The effect of treatment delays after AML diagnosis has been investigated, but with conflicting results. One study showed that time from diagnosis to treatment initiation affects survival in younger patients, and another showed it has no effect on survival regardless of patient age.10,11 We describe here the results of a retrospective analysis evaluating the impact of TAT and day of admission on outcomes of patients with AML who received treatment at a tertiary care referral center.
Methods and materials
We did a retrospective medical record review of all newly diagnosed AML patients at the Oklahoma University Health Sciences Center (OUHSC). Our sample was composed of 154 adult patients. Our inclusion criteria were an age of 18 years or older with complete insurance data, a diagnosis of AML, and having received treatment at our institution from January 2000 through June 2015. Data were obtained on laboratory values at diagnosis, pathology data including cytogenetics, molecular data, and bone marrow biopsies. Data on patient characteristics such as age, race and/or ethnicity, and comorbidities were obtained from the electronic medical records. Treatment data on type and dose of chemotherapy during induction, subsequent treatment phases, and number of treatments to achieve complete response (CR) as well as response data of CR achievement, relapse, date of CR, date of relapse, stem cell transplantation data, date of death, and date of last follow-up visit were recorded retrospectively from the electronic medical record. The study was approved by the OUHSC Institutional Review Board.
Statistical analysis
TAT was analyzed categorically (0-4 days vs >4 days), and day of admission was analyzed categorically (Monday to Thursday vs Friday to Sunday). Descriptive statistics were calculated overall and by TAT group. The chi-square test was used to compare the association between our covariates and TAT. Kaplan-Meier estimates (with a log-rank test) were used to assess the unadjusted effect of TAT with overall survival (OS) and event-free survival (EFS). Median OS and EFS and 95% confidence intervals (CIs) were also calculated. We used the Cox proportional hazards regression modeling to evaluate the relationship between OS and TAT. The initial model was built by including covariates, with P < .25 for the association between the covariates with OS. TAT was maintained in the final model because it was the primary variable of interest, whereas age and risk group were also included in the final model because those covariates are known prognostic risk factors in AML. Among the set of variables screened in, all 2-way interactions were assessed using P < .05. No significant interactions were found. Backward elimination was then performed. During the backward elimination, confounding was deemed to have been present if the measure of association of significant variables in the model changed by more than 20% and the P-value of the confounding variable was less than .30. Variables with P-values of less than .05 or deemed a confounder would then be retained. A similar modeling approach was used to examine EFS and CR. To evaluate the association between CR with potential predictors, binary logistic regression was used, whereby day of admission and time to treatment were explored unadjusted and then adjusted for age, WBC count, risk group, and undergoing allogeneic stem cell transplant (AlloSCT). SAS version 9.4 (SAS Institute Inc, Cary, North Carolina) was used for all analyses. A final alpha of 0.05 was used unless otherwise noted.
Results
Baseline characteristics are presented in Table 1. Treatment was initiated within 4 days for 71% (109/154) of patients. Most patients in our study were younger than 60 years (70%), male (64%), and white (77%). Most patients were admitted to the hospital for treatment between Monday and Thursday (75%). A higher proportion of patients in the 0-4 days TAT group were <60 years of age compared with patients in the >4 days TAT group (P = .0427). A higher proportion of patients in the 0-4 days TAT group had a WBC count of ≥50 x 103 μ/L compared with patients in the >4 days TAT group (27% vs 9%, respectively; P = .0148). A higher proportion of patients were admitted Friday to Sunday in the TAT >4 days group. Insured and uninsured patients were equally distributed between the 2 groups (P = .0014). Cytogenetic and/or molecular risk was not statistically different between the 0-4 days and >4 days TAT groups (unfavorable risk, 25% vs 23%, respectively; P = .6214). A higher proportion of patients received 7 + 3 induction chemotherapy (7 days cytarabine and 3 days anthracycline) in the TAT 0-4 days group compared with the >4 days TAT group (84% vs 69%, respectively; P = .0448). The most common intensive chemotherapy regimen used was 7 + 3 (80%). The rest of the patients (20%) received high-dose cytarabine clofarabine-based chemotherapy, hypomethylating agents, or other treatments. The proportion of patients who received an AlloSCT did not differ between the 0-4 days and >4 days TAT groups (24% vs 20%, respectively; P = .5655).
The median OS for all patients was 10.9 months (95% CI, 8.3-15.1), and the median EFS was 9.1 months (95% CI, 7.4-13.8). Median follow-up time was 8.6 months (95% CI, 6.7-11). We found a significant association between TAT and both OS and EFS without any adjustment (Table 2).
The median OS for the TAT 0-4 days group was 15.6 months, and for the TAT >4 days group, it was 6.8 months (P = .0207; Figure 1). The median EFS for the TAT 0-4 days group was 14.5 months, and for the TAT >4 days group, it was 6.8 months (P = .0240; Figure 2).
We found no association between the day of admission to hospital (Monday-Thursday vs Friday-Sunday) and either OS or EFS. After adjusting for age, WBC count, molecular risk status, and undergoing AlloSCT, the OS was shorter for those who received treatment >4 days after admission compared with those who received treatment within 0 to 4 days, with a hazard ratio (HR) of 1.59 (95% CI, 1.02-2.49; P = .0427; Table 3).
There was no association between day of admission with OS in the multivariable analysis. Similarly, after adjusting for age, WBC count, molecular risk status, and undergoing AlloSCT, EFS was shorter in patients who received treatment >4 days after admission compared with those who received treatment within 0 to 4 days (HR, 1.64; 95% CI, 1.06-2.54; P = .0268). There was no association between day of admission with EFS in the multivariable model. Although there was a trend for a higher CR rate with earlier treatment, this was not statistically significant (Table 4).
Discussion
Treatment outcomes for patients with AML are known to be affected by several patient- and disease-related factors. Patient-related factors can include age, performance status, comorbidities, and availability of a stem cell donor. Examples of disease-related factors include molecular alterations and site of disease involvement. Little is known about whether the timing of treatment initiation affects patient outcomes. Short-term treatment delays after the diagnosis of leukemia are not uncommon. Generally, patients are treated with anthracycline-based induction chemotherapy, but the response rate and survival are particularly poor in the older age group.12 Moreover, increasing comorbidities with aging are expected to lead to lower treatment tolerability.13 Therefore, elderly patients are particularly prone to treatment delays while providers await the results of the molecular studies to guide the use of less intensive targeted therapies.10 Other reasons for treatment delays may also include transfers between hospitals, suspected or documented infections, and evaluation of chronic illnesses. Our analysis also indicates that admission to the hospital on the weekend contributes to a delay in therapy compared with admission on a weekday.
We found a decreased OS and EFS in patients who received treatment >4 days after admission to the hospital compared with patients who received treatment within 0 to 4 days of admission. This association was statistically significant in a bivariate analysis as well as in a multivariable analysis with adjustment for age, WBC count on presentation, molecular risk group, and undergoing AlloSCT. A previous large retrospective study showed that the time from diagnosis to treatment initiation predicts survival in younger, but not older, patients with AML.10 This remained true after adjusting for age, performance status, WBC count, and the type of AML in a multivariable analysis. In our study, the declines in overall survival and event-free survival were evident after a delay of more than 4 days.
Another retrospective study that included 599 newly diagnosed AML patients, with a median time from diagnosis to treatment of 8 days, did not show any impact of treatment delay on overall survival, early death, or response rate.11 These differences in the effect of treatment delay on outcomes could be related to the differences in baseline characteristics of patients in these studies. Our study had a higher proportion of patients younger than 60 years, for example. We hypothesize that treatment delays, especially in patients with a high WBC count on presentation, might lead to further organ compromise and poorer outcomes with chemotherapy.
In our study, a higher proportion of patients were admitted over the weekend in the >4 days TAT group, but when we analyzed the day of admission to hospital separately, it was not associated with OS or EFS. Admission over the weekend was also not associated with clinical outcomes including 30-day mortality in a larger study that included 422 patients treated at a large teaching referral hospital.14
Limitations of our study include a small sample size and a short median follow-up time. Most of our patients were young and white, which may not be representative of the general population.
In conclusion, we found that treatment delays are associated with inferior outcomes in AML patients. It remains to be elucidated whether the benefit gained from using targeted and less-intensive chemotherapy, especially in elderly patients, outweighs the potential harm from delaying treatment. Additional studies are needed to confirm our findings in different settings and patient populations.
Acknowledgment
Statistical support was provided by the Stephenson Cancer Center Biostatistics and Research Design Shared Resource.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
2. Patel MI, Ma Y, Mitchell B, Rhoads KF. How do differences in treatment impact racial and ethnic disparities in acute myeloid leukemia? Cancer Epidemiol Biomarkers Prev. 2015;24(2):344-349.
3. Weber JJ, Kachare SD, Vohra NA, Fitzgerald TF, Wong JH. Regional disparities in breast cancer outcomes and the process of care. Am Surg. 2014;80(7):669-674.
4. Shippee TP, Kozhimannil KB, Rowan K, Virnig BA. Health insurance coverage and racial disparities in breast reconstruction after mastectomy. Womens Health Issues. 2014;24(3):e261-e269.
5. Dickens C, Joffe M, Jacobson J, et al. Stage at breast cancer diagnosis and distance from diagnostic hospital in a periurban setting: a South African public hospital case series of over 1,000 women. Int J Cancer. 2014;135(9):2173-2182.
6. Nguyen-Pham S, Leung J, McLaughlin D. Disparities in breast cancer stage at diagnosis in urban and rural adult women: a systematic review and meta-analysis. Ann Epidemiol. 2014;24(3):228-235.
7. Gong G, Belasco E, Hargrave KA, Lyford CP, Philips BU Jr. Determinants of delayed detection of cancers in Texas counties in the United States of America. Int J Equity Health. 2012;11:29.
8. Erhunmwunsee L, Joshi MB, Conlon DH, Harpole DH Jr. Neighborhood‐level socioeconomic determinants impact outcomes in nonsmall cell lung cancer patients in the Southeastern United States. Cancer. 2012;118(20):5117-5123.
9. Steele CB, Pisu M, Richardson LC. Urban/rural patterns in receipt of treatment for non–small cell lung cancer among black and white Medicare beneficiaries, 2000-2003. J Natl Med Assoc. 2011;103(8):711-718.
10. Sekeres MA, Elson P, Kalaycio ME, et al. Time from diagnosis to treatment initiation predicts survival in younger, but not older, acute myeloid leukemia patients. Blood. 2009;113(1):28-36.
11. Bertoli S, Bérard E, Huguet F, et al. Time from diagnosis to intensive chemotherapy initiation does not adversely impact the outcome of patients with acute myeloid leukemia. Blood. 2013:121(14):2618-2626.
12. Shah A, Andersson TM, Rachet B, Björkholm M, Lambert PC. Survival and cure of acute myeloid leukaemia in England, 1971-2006: a population-based study. Br J Haematol. 2013;162(4):509-516.
13. Mohammadi M, Cao Y, Glimelius I, Bottai M, Eloranta S, Smedby KE. The impact of comorbid disease history on all-cause and cancer-specific mortality in myeloid leukemia and myeloma – a Swedish population-based study. BMC Cancer. 2015;15:850.
14. Bejanyan N, Fu AZ, Lazaryan A, et al. Impact of weekend admissions on quality of care and outcomes in patients with acute myeloid leukemia. Cancer. 2010;116(15):3614-3620.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
2. Patel MI, Ma Y, Mitchell B, Rhoads KF. How do differences in treatment impact racial and ethnic disparities in acute myeloid leukemia? Cancer Epidemiol Biomarkers Prev. 2015;24(2):344-349.
3. Weber JJ, Kachare SD, Vohra NA, Fitzgerald TF, Wong JH. Regional disparities in breast cancer outcomes and the process of care. Am Surg. 2014;80(7):669-674.
4. Shippee TP, Kozhimannil KB, Rowan K, Virnig BA. Health insurance coverage and racial disparities in breast reconstruction after mastectomy. Womens Health Issues. 2014;24(3):e261-e269.
5. Dickens C, Joffe M, Jacobson J, et al. Stage at breast cancer diagnosis and distance from diagnostic hospital in a periurban setting: a South African public hospital case series of over 1,000 women. Int J Cancer. 2014;135(9):2173-2182.
6. Nguyen-Pham S, Leung J, McLaughlin D. Disparities in breast cancer stage at diagnosis in urban and rural adult women: a systematic review and meta-analysis. Ann Epidemiol. 2014;24(3):228-235.
7. Gong G, Belasco E, Hargrave KA, Lyford CP, Philips BU Jr. Determinants of delayed detection of cancers in Texas counties in the United States of America. Int J Equity Health. 2012;11:29.
8. Erhunmwunsee L, Joshi MB, Conlon DH, Harpole DH Jr. Neighborhood‐level socioeconomic determinants impact outcomes in nonsmall cell lung cancer patients in the Southeastern United States. Cancer. 2012;118(20):5117-5123.
9. Steele CB, Pisu M, Richardson LC. Urban/rural patterns in receipt of treatment for non–small cell lung cancer among black and white Medicare beneficiaries, 2000-2003. J Natl Med Assoc. 2011;103(8):711-718.
10. Sekeres MA, Elson P, Kalaycio ME, et al. Time from diagnosis to treatment initiation predicts survival in younger, but not older, acute myeloid leukemia patients. Blood. 2009;113(1):28-36.
11. Bertoli S, Bérard E, Huguet F, et al. Time from diagnosis to intensive chemotherapy initiation does not adversely impact the outcome of patients with acute myeloid leukemia. Blood. 2013:121(14):2618-2626.
12. Shah A, Andersson TM, Rachet B, Björkholm M, Lambert PC. Survival and cure of acute myeloid leukaemia in England, 1971-2006: a population-based study. Br J Haematol. 2013;162(4):509-516.
13. Mohammadi M, Cao Y, Glimelius I, Bottai M, Eloranta S, Smedby KE. The impact of comorbid disease history on all-cause and cancer-specific mortality in myeloid leukemia and myeloma – a Swedish population-based study. BMC Cancer. 2015;15:850.
14. Bejanyan N, Fu AZ, Lazaryan A, et al. Impact of weekend admissions on quality of care and outcomes in patients with acute myeloid leukemia. Cancer. 2010;116(15):3614-3620.
Characteristics of urgent palliative cancer care consultations encountered by radiation oncologists
Palliative radiation therapy (PRT) plays a major role in the management of incurable cancers. Study findings have demonstrated the efficacy of using PRT in treating tumor-related bone pain,1 brain metastases and related symptoms,2 thoracic disease-causing hemoptysis or obstruction,3 gastrointestinal involvement causing bleeding and/or obstruction,4 and genitourinary and/or gynecologic involvement causing bleeding.5,6
PRT accounts for between 30% and 50% of courses of radiotherapy delivered.3 These courses of RT typically require urgent evaluation since patients are seen because of new and/or progressive symptoms that give cause for concern. The urgency of presentation requires radiation oncologists and the departments receiving these patients to be equipped to manage these cases efficiently and effectively. Furthermore, the types of cases seen, including PRT indications and related symptoms requiring management, inform the training of radiation oncology physicians as well as nursing and other clinical staff. Finally, characterizing the types of urgent PRT cases that are seen can also guide research and quality improvement endeavors for advanced cancer care in radiation oncology settings.
There is currently a paucity of data characterizing the types and frequencies of urgent PRT indications in patients who present to radiation oncology departments, as well as a lack of data detailing the related symptoms radiation oncology clinicians are managing. The aim of this study was to characterize the types and frequencies of urgent PRT consultations and the related symptoms that radiation oncologists are managing as part of patient care.
Methods
Based on national palliative care practice and national oncology care practice guidelines,7,8 we designed a survey to categorize the cancer-related palliative care issues seen by radiation oncologists. Physical symptoms, psychosocial issues, cultural consideration, spiritual needs, care coordination, advanced-care planning, goals of care, and ethical and legal issues comprised the 8 palliative care domains that we evaluated. A survey was developed and critically evaluated by 3 investigators (MK, VL, TB). Each palliative care domain was ranked by clinicians by its relevance (5-point Likert scale [range, 1-5]: 1, Not Relevant, to 5, Extremely Relevant) to the patient’s care point in radiation oncology. In addition, 31 palliative care subissues related to the primary domains were identified by clinicians based on their presence (Yes, No, Not Assessed). Clinicians were also asked whether the consulted patient’s metastatic cancer diagnosis was established (longer than 1 month) or new (within the last 1 month). In addition, clinicians noted whether the patient was returning to active oncologic care (eg, chemotherapy) or to no further anticancer therapies (eg, hospice care) after RT consultation and intervention (if deemed necessary).
The survey’s face and content validity, ease of completion, and time of completion was assessed by a panel of 7 clinicians with expertise in medical oncology, radiation oncology, palliative care, and/or survey construction. The survey was then sent in a sequential manner to 1 member of the panel at a time after incorporating each panel member’s initial comments. After each panel member’s review, the survey was edited until 2 consecutive panel members had no further suggestions for improvement.
After receiving approval from the institutional review boards of participating radiation oncology centers, we electronically surveyed radiation oncology clinicians who were conducting PRT consultations. From May 19 to September 26, 2014, all consultations were evaluated prospectively for consideration of PRT performed by a dedicated PRT service at 3 centers (a large academic cancer center and 2 participating clinicians at affiliated regional hospitals). The consultations for patients aged 18 years or older with incurable, metastatic cancers were considered eligible. The consulting clinician was e-mailed a survey for completion within 5 business days immediately after each PRT consult. Three reminders to complete the survey were sent during the 5–business-day interval. Over the entire study period, 162 consecutive patients were identified, resulting in 162 surveys being sent to 15 radiation oncology clinicians, including nurse practitioners, resident physicians, and attending physicians. Each clinician received a $25 gift card for participating, regardless of the number of surveys completed. In total, 140 of the 162 surveys were returned, resulting in a response rate of 86%.
The investigators then collected patient demographics (age, gender, race, marital status) and disease characteristics (primary cancer type, Eastern Cooperative Oncology Group Performance Status, reasons for urgent RT consult, physical symptoms requiring management at presentation, patient’s place in illness trajectory, and RT recommendation) pertaining to each completed survey from the electronic medical record. Urgent consultations were defined as any patients who needed to be seen on the same day or within a few days of the consult request.
The descriptive statistics of all these data were calculated in terms of frequencies and percentage of categorical variables. Chi-squared statistics, Fisher exact test, and nonparametric rank sum tests were applied to various categories to determine any statistically significant differences between groups.
Results
In total, 162 patients were seen in consultation for PRT during the 19-week enrollment period, or an average of 8.7 consults a week. Of that total, surveys for 140 patients were returned (Table).
The median patient age was 63 years (range, 29-89 years). A sizeable minority (20%) was 50 years or younger. The most common cancer diagnosis was lung cancer (28%), followed by breast (13%) and prostate (10%) cancers, melanoma (10%), and sarcoma (7%). Other diagnoses accounted for the remaining 32%.
Timing of PRT consult in cancer trajectory
The points in the advanced cancer illness trajectory at which patients were seen for PRT evaluation are shown in Figure 1. Most patients (63%) were seen for a PRT evaluation at the time of an established diagnosis (>1 month after diagnosis of metastatic cancer) and were continuing to further cancer therapies. An additional 19% of patients with an established diagnosis proceeded to hospice or end-of-life care after the PRT evaluation. A notable minority of patients (18%) were seen for a PRT evaluation at the time of a new diagnosis (<1 month of diagnosis of metastatic cancer), and of those, 17% went on to receive anticancer therapy after the PRT evaluation and 1% proceeded to hospice or end-of-life care.
Characteristics of PRT consults and symptoms at presentation
The primary reasons for urgent consultation for PRT are shown in Figure 2. Cancer-related pain (57%), brain metastases (29%), and malignant spinal cord or cauda equina compression (13%) were the predominant reasons for consults. Notable minorities were seen for tumor-related dyspnea (10%), bleeding (8%), and bone fractures (4%).
PRT recommendations and targets
Recommendations regarding PRT are shown in Figure 4A. Of the total 140 patients, 18 (13%) were not recommended for RT. Of the 122 patients for whom PRT was recommended, 11 (9%) received RT at more than 1 site.
Discussion and conclusions
The present study provides a descriptive overview of urgent metastatic cancer patient presentations to radiation oncology clinicians through a comprehensive evaluation of 140 consults for PRT. The most common reasons for urgent evaluation were cancer-related pain (57%), but brain metastases (29%), spinal cord compression (13%), and respiratory symptoms (10%) were also common. Other less-common indications included cancer-related dysphagia, bleeding, and poststabilization management of bone fractures. The most common symptoms requiring management by radiation oncology clinicians were pain (69%), neurologic symptoms (51%), and fatigue (49%). The study also provides a comprehensive characterization of the timeframe of PRT consultation and the treatment recommendations in this cohort. Though most PRT consults occurred at the time of an established metastatic cancer diagnosis and before further anticancer therapies, sizeable minorities occurred at the time of a new diagnosis of metastatic cancer (18%) and before comfort-focused, end-of-life care and no further anticancer therapies (20%). Most patients (87%) were recommended PRT, and of those recommended RT, 11% received RT to more than 1 site. The most common PRT sites were to bone (46%), followed by brain (29%), nonlung soft-tissue sites (17%), and lung (8%). This comprehensive description of the day-to-day urgent, advanced cancer care issues seen and managed in radiation oncology practice can help guide PRT clinical structures, education, research, and quality improvement measures in clinical practice.
Our study provides an insight into urgent symptoms encountered by radiation oncology practitioners during their routine practice. Cancer-related pain remains the most common symptom requiring management. Given the frequency with which pain management is needed among PRT patients, this study highlights the need for radiation oncologists to be well trained in symptom management, particularly as the pain response to RT can often take several days. However, studies suggest that cancer-related pain is not frequently managed by radiation oncologists.9 For example, findings from an Italian study showed that the involvement of radiation oncologists in cancer pain management remains minimal compared with other medical professionals; during the treatment course, only half of the radiation oncologists implemented specific treatment for breakthrough pain.10 A nationwide survey in the United States implicated a number of barriers to adequate pain management, including poor assessment by the physician, reluctance in prescribing opioid analgesics, perceived excessive regulation, and patient reluctance to report pain.11 Notably, in a survey of the Radiation Therapy Oncology Group study physicians, 83% believed cancer patients with pain were undermedicated, and 40% reported that pain relief in their own practice setting was suboptimal. Furthermore, in the treatment plan, adjuvants and prophylactic side effect management were frequently not used properly.12 Education of radiation oncologists in pain assessment and management is key to overcome these barriers and to ensure adequate pain management and quality of life for patients in radiation oncology.
The next most common reason for which patients presented for palliative radiation oncology consultation was for central nervous system (CNS) metastatic disease, including brain metastases and spinal cord compression. Correspondingly, the next most common issue requiring management was neurologic symptoms. Management of CNS disease is becoming increasingly complex, and it benefits from multidisciplinary evaluation to guide optimal and personalized care for each patient, including medical oncology, radiation oncology, neurosurgery and/or orthopedic spine surgery, and palliative medicine. Treatment options include supportive care or corticosteroids alone, surgical resection, whole-brain RT, and/or radiosurgery or stereotactic RT alone. These treatment options are considered on the basis of global patient factors, such as prognosis, together with metastatic-site–specific factors, such as site-related symptoms and the number of metastatic diseases or the burden of the disease.13 For example, the use of the diagnosis-specific Graded Prognostic Assessment index to predict life expectancy can help tailor management of brain metastases based on performance status, age, number of brain metastases, extracranial metastases, and cancer type. Highlighting the complexity of this common PRT presentation, Tsao and colleagues showed that there was a lack of uniform agreement among radiation oncologists for common management issues in patients with brain metastatic disease.14
For metastatic spinal cord or cauda equina compression and the associated neurologic symptoms, initiation of immediate corticosteroids and implementation of local therapy within 24 hours of presentation is paramount,15 highlighting the need for rapid, comprehensive care decision-making for these patients. Treatment options that must be weighed include the potential benefit of upfront decompressive surgery, as supported by a randomized controlled trial by Patchell and colleagues16 for patients who are surgical candidates with true cord or cauda compression and have at least 1 neurologic symptom, a prognosis of ≥3 months, paraplegia of no longer than 48 hours, and no previous RT to the site or brain metastases. Compared with the RT alone, patients receiving surgery before RT had improved ambulatory status and overall survival. Hence, neurosurgical or orthopedic consultation should be standard in the evaluation of metastatic spinal cord or cauda equina compression patients. However, patients frequently do not meet these criteria, and corticosteroids and RT alone are considered. In addition to playing a role in surgical decision-making, prognosis also has a key role in decision-making about the RT fractionation. Short-course RT (8 Gy × 1) is as effectual as longer-course regimens (3 Gy × 10) in terms of motor function.17,18 However, more dose-intense or longer-course regimens, such as 3 Gy × 10, have been shown to have more durability beyond about 6 months and are therefore considered for intermediate to good prognosis.18 The common urgent presentation of CNS metastatic disease to radiation oncology clinics together with the complexity of management and urgency of care decision-making point to the need for dedicated structures of care for these patients in radiation oncology settings. For example, dedicated PRT programs, such as the Rapid Response Radiotherapy Program in Toronto and the Supportive and Palliative Radiation Oncology service in Boston, have demonstrated improved quality of care for patients being urgently evaluated for PRT.19
Following management of pain and neurologic symptoms, clinicians were faced with managing fatigue in nearly half of the patients (49%). The prevalence of fatigue among cancer patients and its impact on quality of life20 highlight the need for this key symptom to be addressed throughout the continuum of cancer care. National Comprehensive Cancer Network guidelines provide a comprehensive framework for addressing cancer-related fatigue.7 However, cancer-related fatigue is a largely underreported, underestimated, and thus undertreated problem.20 In a nationwide survey of members of the American Society for Radiation Oncology, radiation oncologists reported being significantly less confident in managing fatigue compared with managing other common symptoms.21 Furthermore, in a national survey of radiation oncology trainees, 67% of respondents indicated that they were not at all minimally or somewhat confident in their ability to manage fatigue symptoms. The frequency of this symptom together with the demonstrated need for improved education in fatigue management point to a need for radiation oncology palliative educational structures to include dedicated emphasis on managing fatigue in addition to other commonly encountered symptoms, such as pain.
Patients evaluated for PRT are seen across the trajectory of their metastatic cancer diagnosis. In our study, patients presented at all stages in their advanced cancers. These include patients seen at the time of initial diagnosis of cancer as well as those seen near the end of life when end-of-life care planning was underway. The broad spectrum of timing of PRT care underscores that radiation oncologists must be prepared to handle generalist palliative care issues encountered throughout the trajectory of advanced cancer care and hence need comprehensive education in generalist palliative care competencies. These include symptom management, end-of-life care coordination, and communication or goals-of-care discussions. Notably, a recent national survey of radiation oncology residents indicated that most residents, 77% on average, perceived their educational training as suboptimal across domains of generalist palliative care competencies needed in oncology practice.22 Furthermore, a majority (81%) desired greater palliative care education within training.
The most common sites treated in this study were bone, brain, and lung sites. These data provide guidance to both education and research initiatives aiming to advance PRT curriculum and care structures within departments. For example, a same-day simulation and radiation treatment program developed at Princess Margaret Hospital Palliative Radiation Oncology Program (Ontario, Canada) aids in providing streamlined care for patients with bone metastases, the most common presentation for PRT.23 Furthermore, education and research in the application of PRT techniques to bone, brain, and thoracic disease cover the majority of PRT presentations. It is notable, however, that 17% were other soft tissue body sites.
Limitations
There are a few limitations to this study. First, this is a survey-based study conducted at a single academic center within an urban setting and surrounding community regions, which affects its generalizability. Second, this study presents perspectives of radiation oncology practitioners evaluating patients and does not directly reflect patient perceptions or report of symptoms. Third, the data provided by this study are solely descriptive in nature. However, this can guide hypothesis-driven research regarding the evaluation and management of urgent palliative care issues encountered by radiation oncology clinicians and suggest educational objectives to address the needs of these patients.
Conclusions
Radiation oncologists are involved throughout the trajectory of care for advanced cancer patients. Furthermore, they manage a variety of urgent oncologic issues, most commonly metastases causing pain, brain metastases, and spinal cord or cauda equina compression. Radiation oncologists also manage many cancer-related symptoms, mostly pain, neurologic symptoms, fatigue, and gastrointestinal symptoms. These findings point toward the need for palliative care to be well integrated into radiation oncology training curricula and the need for dedicated care structures that enable rapid and multidisciplinary palliative oncology care within radiation oncology departments.
1. Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25(11):1423-1436.
2. van Oorschot B, Rades D, Schulze W, Beckmann G, Feyer P. Palliative radiotherapy--new approaches. Semin Oncol. 2011;38(3):443-449.
3. Simone CB II, Jones JA. Palliative care for patients with locally advanced and metastatic non-small cell lung cancer. Ann Palliat Med. 2013;2(4):178-188.
4. Cihoric N, Crowe S, Eychmüller S, Aebersold DM, Ghadjar P. Clinically significant bleeding in incurable cancer patients: effectiveness of hemostatic radiotherapy. Radiat Oncol. 2012;7:132.
5. Duchesne GM, Bolger JJ, Griffiths GO, et al. A randomized trial of hypofractionated schedules of palliative radiotherapy in the management of bladder carcinoma: results of medical research council trial BA09. Int J Radiat Oncol Biol Phys. 2000;47(2):379-388.
6. Onsrud M, Hagen B, Strickert T. 10-Gy single-fraction pelvic irradiation for palliation and life prolongation in patients with cancer of the cervix and corpus uteri. Gynecol Oncol. 2001;82(1):167-171.
7. NCCN Guidelines(R) Updates. J Natl Compr Canc Netw. 2013;11(9):xxxii-xxxvi.
8. Colby WH, Dahlin C, Lantos J, Carney J, Christopher M. The National Consensus Project for Quality Palliative Care Clinical Practice Guidelines Domain 8: ethical and legal aspects of care. HEC Forum. 2010;22(2):117-131.
9. Stockler MR, Wilcken NR. Why is management of cancer pain still a problem? J Clin Oncol. 2012;30(16):1907-1908.
10. Caravatta L, Ramella S, Melano A, et al. Breakthrough pain management in patients undergoing radiotherapy: a national survey on behalf of the Palliative and Supportive Care Study Group. Tumori. 2015;101(6):603-608.
11. Breuer B, Fleishman SB, Cruciani RA, Portenoy RK. Medical oncologists’ attitudes and practice in cancer pain management: a national survey. J Clin Oncol. 2011;29(36):4769-4775.
12. Cleeland CS, Janjan NA, Scott CB, Seiferheld WF, Curran WJ. Cancer pain management by radiotherapists: a survey of radiation therapy oncology group physicians. Int J Radiat Oncol Biol Phys. 2000;47(1):203-208.
13. Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2(3):210-225.
14. Tsao MN, Rades D, Wirth A, et al. International practice survey on the management of brain metastases: third international consensus workshop on palliative radiotherapy and symptom control. Clin Oncol (R Coll Radiol). 2012;24(6):e81-e92.
15. Tang V, Harvey D, Park Dorsay J, Jiang S, Rathbone MP. Prognostic indicators in metastatic spinal cord compression: using functional independence measure and Tokuhashi scale to optimize rehabilitation planning. Spinal Cord. 2007;45(10):671-677.
16. Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643-648.
17. Rades D, Stalpers LJ, Veninga T, et al. Evaluation of five radiation schedules and prognostic factors for metastatic spinal cord compression. J Clin Oncol. 2005;23(15):3366-3375.
18. Rades D, Stalpers LJ, Hulshof MC, et al. Comparison of 1 x 8 Gy and 10 x 3 Gy for functional outcome in patients with metastatic spinal cord compression. Int J Radiat Oncol Biol Phys. 2005;62(2):514-518.
19. Dennis K, Linden K, Balboni T, Chow E. Rapid access palliative radiation therapy programs: an efficient model of care. Future Oncol. 2015;11(17):2417-2426.
20. Kapoor A, Singhal MK, Bagri PK, Narayan S, Beniwal S, Kumar HS. Cancer related fatigue: a ubiquitous problem yet so under reported, under recognized and under treated. South Asian J Cancer. 2015;4(1):21-23.
21. Wei RL, Mattes MD, Yu J, et al. Attitudes of radiation oncologists toward palliative and supportive care in the United States: report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract Radiat Oncol. 2017;7(2):113-119.
22. Krishnan M, Racsa M, Jones J, et al. Radiation oncology resident palliative education. Pract Radiat Oncol. 2017;7(6):e439-e448.
23. McDonald R, Chow E, Lam H, Rowbottom L, Soliman H. International patterns of practice in radiotherapy for bone metastases: a review of the literature. J Bone Oncol. 2014;3(3-4):96-102.
Palliative radiation therapy (PRT) plays a major role in the management of incurable cancers. Study findings have demonstrated the efficacy of using PRT in treating tumor-related bone pain,1 brain metastases and related symptoms,2 thoracic disease-causing hemoptysis or obstruction,3 gastrointestinal involvement causing bleeding and/or obstruction,4 and genitourinary and/or gynecologic involvement causing bleeding.5,6
PRT accounts for between 30% and 50% of courses of radiotherapy delivered.3 These courses of RT typically require urgent evaluation since patients are seen because of new and/or progressive symptoms that give cause for concern. The urgency of presentation requires radiation oncologists and the departments receiving these patients to be equipped to manage these cases efficiently and effectively. Furthermore, the types of cases seen, including PRT indications and related symptoms requiring management, inform the training of radiation oncology physicians as well as nursing and other clinical staff. Finally, characterizing the types of urgent PRT cases that are seen can also guide research and quality improvement endeavors for advanced cancer care in radiation oncology settings.
There is currently a paucity of data characterizing the types and frequencies of urgent PRT indications in patients who present to radiation oncology departments, as well as a lack of data detailing the related symptoms radiation oncology clinicians are managing. The aim of this study was to characterize the types and frequencies of urgent PRT consultations and the related symptoms that radiation oncologists are managing as part of patient care.
Methods
Based on national palliative care practice and national oncology care practice guidelines,7,8 we designed a survey to categorize the cancer-related palliative care issues seen by radiation oncologists. Physical symptoms, psychosocial issues, cultural consideration, spiritual needs, care coordination, advanced-care planning, goals of care, and ethical and legal issues comprised the 8 palliative care domains that we evaluated. A survey was developed and critically evaluated by 3 investigators (MK, VL, TB). Each palliative care domain was ranked by clinicians by its relevance (5-point Likert scale [range, 1-5]: 1, Not Relevant, to 5, Extremely Relevant) to the patient’s care point in radiation oncology. In addition, 31 palliative care subissues related to the primary domains were identified by clinicians based on their presence (Yes, No, Not Assessed). Clinicians were also asked whether the consulted patient’s metastatic cancer diagnosis was established (longer than 1 month) or new (within the last 1 month). In addition, clinicians noted whether the patient was returning to active oncologic care (eg, chemotherapy) or to no further anticancer therapies (eg, hospice care) after RT consultation and intervention (if deemed necessary).
The survey’s face and content validity, ease of completion, and time of completion was assessed by a panel of 7 clinicians with expertise in medical oncology, radiation oncology, palliative care, and/or survey construction. The survey was then sent in a sequential manner to 1 member of the panel at a time after incorporating each panel member’s initial comments. After each panel member’s review, the survey was edited until 2 consecutive panel members had no further suggestions for improvement.
After receiving approval from the institutional review boards of participating radiation oncology centers, we electronically surveyed radiation oncology clinicians who were conducting PRT consultations. From May 19 to September 26, 2014, all consultations were evaluated prospectively for consideration of PRT performed by a dedicated PRT service at 3 centers (a large academic cancer center and 2 participating clinicians at affiliated regional hospitals). The consultations for patients aged 18 years or older with incurable, metastatic cancers were considered eligible. The consulting clinician was e-mailed a survey for completion within 5 business days immediately after each PRT consult. Three reminders to complete the survey were sent during the 5–business-day interval. Over the entire study period, 162 consecutive patients were identified, resulting in 162 surveys being sent to 15 radiation oncology clinicians, including nurse practitioners, resident physicians, and attending physicians. Each clinician received a $25 gift card for participating, regardless of the number of surveys completed. In total, 140 of the 162 surveys were returned, resulting in a response rate of 86%.
The investigators then collected patient demographics (age, gender, race, marital status) and disease characteristics (primary cancer type, Eastern Cooperative Oncology Group Performance Status, reasons for urgent RT consult, physical symptoms requiring management at presentation, patient’s place in illness trajectory, and RT recommendation) pertaining to each completed survey from the electronic medical record. Urgent consultations were defined as any patients who needed to be seen on the same day or within a few days of the consult request.
The descriptive statistics of all these data were calculated in terms of frequencies and percentage of categorical variables. Chi-squared statistics, Fisher exact test, and nonparametric rank sum tests were applied to various categories to determine any statistically significant differences between groups.
Results
In total, 162 patients were seen in consultation for PRT during the 19-week enrollment period, or an average of 8.7 consults a week. Of that total, surveys for 140 patients were returned (Table).
The median patient age was 63 years (range, 29-89 years). A sizeable minority (20%) was 50 years or younger. The most common cancer diagnosis was lung cancer (28%), followed by breast (13%) and prostate (10%) cancers, melanoma (10%), and sarcoma (7%). Other diagnoses accounted for the remaining 32%.
Timing of PRT consult in cancer trajectory
The points in the advanced cancer illness trajectory at which patients were seen for PRT evaluation are shown in Figure 1. Most patients (63%) were seen for a PRT evaluation at the time of an established diagnosis (>1 month after diagnosis of metastatic cancer) and were continuing to further cancer therapies. An additional 19% of patients with an established diagnosis proceeded to hospice or end-of-life care after the PRT evaluation. A notable minority of patients (18%) were seen for a PRT evaluation at the time of a new diagnosis (<1 month of diagnosis of metastatic cancer), and of those, 17% went on to receive anticancer therapy after the PRT evaluation and 1% proceeded to hospice or end-of-life care.
Characteristics of PRT consults and symptoms at presentation
The primary reasons for urgent consultation for PRT are shown in Figure 2. Cancer-related pain (57%), brain metastases (29%), and malignant spinal cord or cauda equina compression (13%) were the predominant reasons for consults. Notable minorities were seen for tumor-related dyspnea (10%), bleeding (8%), and bone fractures (4%).
PRT recommendations and targets
Recommendations regarding PRT are shown in Figure 4A. Of the total 140 patients, 18 (13%) were not recommended for RT. Of the 122 patients for whom PRT was recommended, 11 (9%) received RT at more than 1 site.
Discussion and conclusions
The present study provides a descriptive overview of urgent metastatic cancer patient presentations to radiation oncology clinicians through a comprehensive evaluation of 140 consults for PRT. The most common reasons for urgent evaluation were cancer-related pain (57%), but brain metastases (29%), spinal cord compression (13%), and respiratory symptoms (10%) were also common. Other less-common indications included cancer-related dysphagia, bleeding, and poststabilization management of bone fractures. The most common symptoms requiring management by radiation oncology clinicians were pain (69%), neurologic symptoms (51%), and fatigue (49%). The study also provides a comprehensive characterization of the timeframe of PRT consultation and the treatment recommendations in this cohort. Though most PRT consults occurred at the time of an established metastatic cancer diagnosis and before further anticancer therapies, sizeable minorities occurred at the time of a new diagnosis of metastatic cancer (18%) and before comfort-focused, end-of-life care and no further anticancer therapies (20%). Most patients (87%) were recommended PRT, and of those recommended RT, 11% received RT to more than 1 site. The most common PRT sites were to bone (46%), followed by brain (29%), nonlung soft-tissue sites (17%), and lung (8%). This comprehensive description of the day-to-day urgent, advanced cancer care issues seen and managed in radiation oncology practice can help guide PRT clinical structures, education, research, and quality improvement measures in clinical practice.
Our study provides an insight into urgent symptoms encountered by radiation oncology practitioners during their routine practice. Cancer-related pain remains the most common symptom requiring management. Given the frequency with which pain management is needed among PRT patients, this study highlights the need for radiation oncologists to be well trained in symptom management, particularly as the pain response to RT can often take several days. However, studies suggest that cancer-related pain is not frequently managed by radiation oncologists.9 For example, findings from an Italian study showed that the involvement of radiation oncologists in cancer pain management remains minimal compared with other medical professionals; during the treatment course, only half of the radiation oncologists implemented specific treatment for breakthrough pain.10 A nationwide survey in the United States implicated a number of barriers to adequate pain management, including poor assessment by the physician, reluctance in prescribing opioid analgesics, perceived excessive regulation, and patient reluctance to report pain.11 Notably, in a survey of the Radiation Therapy Oncology Group study physicians, 83% believed cancer patients with pain were undermedicated, and 40% reported that pain relief in their own practice setting was suboptimal. Furthermore, in the treatment plan, adjuvants and prophylactic side effect management were frequently not used properly.12 Education of radiation oncologists in pain assessment and management is key to overcome these barriers and to ensure adequate pain management and quality of life for patients in radiation oncology.
The next most common reason for which patients presented for palliative radiation oncology consultation was for central nervous system (CNS) metastatic disease, including brain metastases and spinal cord compression. Correspondingly, the next most common issue requiring management was neurologic symptoms. Management of CNS disease is becoming increasingly complex, and it benefits from multidisciplinary evaluation to guide optimal and personalized care for each patient, including medical oncology, radiation oncology, neurosurgery and/or orthopedic spine surgery, and palliative medicine. Treatment options include supportive care or corticosteroids alone, surgical resection, whole-brain RT, and/or radiosurgery or stereotactic RT alone. These treatment options are considered on the basis of global patient factors, such as prognosis, together with metastatic-site–specific factors, such as site-related symptoms and the number of metastatic diseases or the burden of the disease.13 For example, the use of the diagnosis-specific Graded Prognostic Assessment index to predict life expectancy can help tailor management of brain metastases based on performance status, age, number of brain metastases, extracranial metastases, and cancer type. Highlighting the complexity of this common PRT presentation, Tsao and colleagues showed that there was a lack of uniform agreement among radiation oncologists for common management issues in patients with brain metastatic disease.14
For metastatic spinal cord or cauda equina compression and the associated neurologic symptoms, initiation of immediate corticosteroids and implementation of local therapy within 24 hours of presentation is paramount,15 highlighting the need for rapid, comprehensive care decision-making for these patients. Treatment options that must be weighed include the potential benefit of upfront decompressive surgery, as supported by a randomized controlled trial by Patchell and colleagues16 for patients who are surgical candidates with true cord or cauda compression and have at least 1 neurologic symptom, a prognosis of ≥3 months, paraplegia of no longer than 48 hours, and no previous RT to the site or brain metastases. Compared with the RT alone, patients receiving surgery before RT had improved ambulatory status and overall survival. Hence, neurosurgical or orthopedic consultation should be standard in the evaluation of metastatic spinal cord or cauda equina compression patients. However, patients frequently do not meet these criteria, and corticosteroids and RT alone are considered. In addition to playing a role in surgical decision-making, prognosis also has a key role in decision-making about the RT fractionation. Short-course RT (8 Gy × 1) is as effectual as longer-course regimens (3 Gy × 10) in terms of motor function.17,18 However, more dose-intense or longer-course regimens, such as 3 Gy × 10, have been shown to have more durability beyond about 6 months and are therefore considered for intermediate to good prognosis.18 The common urgent presentation of CNS metastatic disease to radiation oncology clinics together with the complexity of management and urgency of care decision-making point to the need for dedicated structures of care for these patients in radiation oncology settings. For example, dedicated PRT programs, such as the Rapid Response Radiotherapy Program in Toronto and the Supportive and Palliative Radiation Oncology service in Boston, have demonstrated improved quality of care for patients being urgently evaluated for PRT.19
Following management of pain and neurologic symptoms, clinicians were faced with managing fatigue in nearly half of the patients (49%). The prevalence of fatigue among cancer patients and its impact on quality of life20 highlight the need for this key symptom to be addressed throughout the continuum of cancer care. National Comprehensive Cancer Network guidelines provide a comprehensive framework for addressing cancer-related fatigue.7 However, cancer-related fatigue is a largely underreported, underestimated, and thus undertreated problem.20 In a nationwide survey of members of the American Society for Radiation Oncology, radiation oncologists reported being significantly less confident in managing fatigue compared with managing other common symptoms.21 Furthermore, in a national survey of radiation oncology trainees, 67% of respondents indicated that they were not at all minimally or somewhat confident in their ability to manage fatigue symptoms. The frequency of this symptom together with the demonstrated need for improved education in fatigue management point to a need for radiation oncology palliative educational structures to include dedicated emphasis on managing fatigue in addition to other commonly encountered symptoms, such as pain.
Patients evaluated for PRT are seen across the trajectory of their metastatic cancer diagnosis. In our study, patients presented at all stages in their advanced cancers. These include patients seen at the time of initial diagnosis of cancer as well as those seen near the end of life when end-of-life care planning was underway. The broad spectrum of timing of PRT care underscores that radiation oncologists must be prepared to handle generalist palliative care issues encountered throughout the trajectory of advanced cancer care and hence need comprehensive education in generalist palliative care competencies. These include symptom management, end-of-life care coordination, and communication or goals-of-care discussions. Notably, a recent national survey of radiation oncology residents indicated that most residents, 77% on average, perceived their educational training as suboptimal across domains of generalist palliative care competencies needed in oncology practice.22 Furthermore, a majority (81%) desired greater palliative care education within training.
The most common sites treated in this study were bone, brain, and lung sites. These data provide guidance to both education and research initiatives aiming to advance PRT curriculum and care structures within departments. For example, a same-day simulation and radiation treatment program developed at Princess Margaret Hospital Palliative Radiation Oncology Program (Ontario, Canada) aids in providing streamlined care for patients with bone metastases, the most common presentation for PRT.23 Furthermore, education and research in the application of PRT techniques to bone, brain, and thoracic disease cover the majority of PRT presentations. It is notable, however, that 17% were other soft tissue body sites.
Limitations
There are a few limitations to this study. First, this is a survey-based study conducted at a single academic center within an urban setting and surrounding community regions, which affects its generalizability. Second, this study presents perspectives of radiation oncology practitioners evaluating patients and does not directly reflect patient perceptions or report of symptoms. Third, the data provided by this study are solely descriptive in nature. However, this can guide hypothesis-driven research regarding the evaluation and management of urgent palliative care issues encountered by radiation oncology clinicians and suggest educational objectives to address the needs of these patients.
Conclusions
Radiation oncologists are involved throughout the trajectory of care for advanced cancer patients. Furthermore, they manage a variety of urgent oncologic issues, most commonly metastases causing pain, brain metastases, and spinal cord or cauda equina compression. Radiation oncologists also manage many cancer-related symptoms, mostly pain, neurologic symptoms, fatigue, and gastrointestinal symptoms. These findings point toward the need for palliative care to be well integrated into radiation oncology training curricula and the need for dedicated care structures that enable rapid and multidisciplinary palliative oncology care within radiation oncology departments.
Palliative radiation therapy (PRT) plays a major role in the management of incurable cancers. Study findings have demonstrated the efficacy of using PRT in treating tumor-related bone pain,1 brain metastases and related symptoms,2 thoracic disease-causing hemoptysis or obstruction,3 gastrointestinal involvement causing bleeding and/or obstruction,4 and genitourinary and/or gynecologic involvement causing bleeding.5,6
PRT accounts for between 30% and 50% of courses of radiotherapy delivered.3 These courses of RT typically require urgent evaluation since patients are seen because of new and/or progressive symptoms that give cause for concern. The urgency of presentation requires radiation oncologists and the departments receiving these patients to be equipped to manage these cases efficiently and effectively. Furthermore, the types of cases seen, including PRT indications and related symptoms requiring management, inform the training of radiation oncology physicians as well as nursing and other clinical staff. Finally, characterizing the types of urgent PRT cases that are seen can also guide research and quality improvement endeavors for advanced cancer care in radiation oncology settings.
There is currently a paucity of data characterizing the types and frequencies of urgent PRT indications in patients who present to radiation oncology departments, as well as a lack of data detailing the related symptoms radiation oncology clinicians are managing. The aim of this study was to characterize the types and frequencies of urgent PRT consultations and the related symptoms that radiation oncologists are managing as part of patient care.
Methods
Based on national palliative care practice and national oncology care practice guidelines,7,8 we designed a survey to categorize the cancer-related palliative care issues seen by radiation oncologists. Physical symptoms, psychosocial issues, cultural consideration, spiritual needs, care coordination, advanced-care planning, goals of care, and ethical and legal issues comprised the 8 palliative care domains that we evaluated. A survey was developed and critically evaluated by 3 investigators (MK, VL, TB). Each palliative care domain was ranked by clinicians by its relevance (5-point Likert scale [range, 1-5]: 1, Not Relevant, to 5, Extremely Relevant) to the patient’s care point in radiation oncology. In addition, 31 palliative care subissues related to the primary domains were identified by clinicians based on their presence (Yes, No, Not Assessed). Clinicians were also asked whether the consulted patient’s metastatic cancer diagnosis was established (longer than 1 month) or new (within the last 1 month). In addition, clinicians noted whether the patient was returning to active oncologic care (eg, chemotherapy) or to no further anticancer therapies (eg, hospice care) after RT consultation and intervention (if deemed necessary).
The survey’s face and content validity, ease of completion, and time of completion was assessed by a panel of 7 clinicians with expertise in medical oncology, radiation oncology, palliative care, and/or survey construction. The survey was then sent in a sequential manner to 1 member of the panel at a time after incorporating each panel member’s initial comments. After each panel member’s review, the survey was edited until 2 consecutive panel members had no further suggestions for improvement.
After receiving approval from the institutional review boards of participating radiation oncology centers, we electronically surveyed radiation oncology clinicians who were conducting PRT consultations. From May 19 to September 26, 2014, all consultations were evaluated prospectively for consideration of PRT performed by a dedicated PRT service at 3 centers (a large academic cancer center and 2 participating clinicians at affiliated regional hospitals). The consultations for patients aged 18 years or older with incurable, metastatic cancers were considered eligible. The consulting clinician was e-mailed a survey for completion within 5 business days immediately after each PRT consult. Three reminders to complete the survey were sent during the 5–business-day interval. Over the entire study period, 162 consecutive patients were identified, resulting in 162 surveys being sent to 15 radiation oncology clinicians, including nurse practitioners, resident physicians, and attending physicians. Each clinician received a $25 gift card for participating, regardless of the number of surveys completed. In total, 140 of the 162 surveys were returned, resulting in a response rate of 86%.
The investigators then collected patient demographics (age, gender, race, marital status) and disease characteristics (primary cancer type, Eastern Cooperative Oncology Group Performance Status, reasons for urgent RT consult, physical symptoms requiring management at presentation, patient’s place in illness trajectory, and RT recommendation) pertaining to each completed survey from the electronic medical record. Urgent consultations were defined as any patients who needed to be seen on the same day or within a few days of the consult request.
The descriptive statistics of all these data were calculated in terms of frequencies and percentage of categorical variables. Chi-squared statistics, Fisher exact test, and nonparametric rank sum tests were applied to various categories to determine any statistically significant differences between groups.
Results
In total, 162 patients were seen in consultation for PRT during the 19-week enrollment period, or an average of 8.7 consults a week. Of that total, surveys for 140 patients were returned (Table).
The median patient age was 63 years (range, 29-89 years). A sizeable minority (20%) was 50 years or younger. The most common cancer diagnosis was lung cancer (28%), followed by breast (13%) and prostate (10%) cancers, melanoma (10%), and sarcoma (7%). Other diagnoses accounted for the remaining 32%.
Timing of PRT consult in cancer trajectory
The points in the advanced cancer illness trajectory at which patients were seen for PRT evaluation are shown in Figure 1. Most patients (63%) were seen for a PRT evaluation at the time of an established diagnosis (>1 month after diagnosis of metastatic cancer) and were continuing to further cancer therapies. An additional 19% of patients with an established diagnosis proceeded to hospice or end-of-life care after the PRT evaluation. A notable minority of patients (18%) were seen for a PRT evaluation at the time of a new diagnosis (<1 month of diagnosis of metastatic cancer), and of those, 17% went on to receive anticancer therapy after the PRT evaluation and 1% proceeded to hospice or end-of-life care.
Characteristics of PRT consults and symptoms at presentation
The primary reasons for urgent consultation for PRT are shown in Figure 2. Cancer-related pain (57%), brain metastases (29%), and malignant spinal cord or cauda equina compression (13%) were the predominant reasons for consults. Notable minorities were seen for tumor-related dyspnea (10%), bleeding (8%), and bone fractures (4%).
PRT recommendations and targets
Recommendations regarding PRT are shown in Figure 4A. Of the total 140 patients, 18 (13%) were not recommended for RT. Of the 122 patients for whom PRT was recommended, 11 (9%) received RT at more than 1 site.
Discussion and conclusions
The present study provides a descriptive overview of urgent metastatic cancer patient presentations to radiation oncology clinicians through a comprehensive evaluation of 140 consults for PRT. The most common reasons for urgent evaluation were cancer-related pain (57%), but brain metastases (29%), spinal cord compression (13%), and respiratory symptoms (10%) were also common. Other less-common indications included cancer-related dysphagia, bleeding, and poststabilization management of bone fractures. The most common symptoms requiring management by radiation oncology clinicians were pain (69%), neurologic symptoms (51%), and fatigue (49%). The study also provides a comprehensive characterization of the timeframe of PRT consultation and the treatment recommendations in this cohort. Though most PRT consults occurred at the time of an established metastatic cancer diagnosis and before further anticancer therapies, sizeable minorities occurred at the time of a new diagnosis of metastatic cancer (18%) and before comfort-focused, end-of-life care and no further anticancer therapies (20%). Most patients (87%) were recommended PRT, and of those recommended RT, 11% received RT to more than 1 site. The most common PRT sites were to bone (46%), followed by brain (29%), nonlung soft-tissue sites (17%), and lung (8%). This comprehensive description of the day-to-day urgent, advanced cancer care issues seen and managed in radiation oncology practice can help guide PRT clinical structures, education, research, and quality improvement measures in clinical practice.
Our study provides an insight into urgent symptoms encountered by radiation oncology practitioners during their routine practice. Cancer-related pain remains the most common symptom requiring management. Given the frequency with which pain management is needed among PRT patients, this study highlights the need for radiation oncologists to be well trained in symptom management, particularly as the pain response to RT can often take several days. However, studies suggest that cancer-related pain is not frequently managed by radiation oncologists.9 For example, findings from an Italian study showed that the involvement of radiation oncologists in cancer pain management remains minimal compared with other medical professionals; during the treatment course, only half of the radiation oncologists implemented specific treatment for breakthrough pain.10 A nationwide survey in the United States implicated a number of barriers to adequate pain management, including poor assessment by the physician, reluctance in prescribing opioid analgesics, perceived excessive regulation, and patient reluctance to report pain.11 Notably, in a survey of the Radiation Therapy Oncology Group study physicians, 83% believed cancer patients with pain were undermedicated, and 40% reported that pain relief in their own practice setting was suboptimal. Furthermore, in the treatment plan, adjuvants and prophylactic side effect management were frequently not used properly.12 Education of radiation oncologists in pain assessment and management is key to overcome these barriers and to ensure adequate pain management and quality of life for patients in radiation oncology.
The next most common reason for which patients presented for palliative radiation oncology consultation was for central nervous system (CNS) metastatic disease, including brain metastases and spinal cord compression. Correspondingly, the next most common issue requiring management was neurologic symptoms. Management of CNS disease is becoming increasingly complex, and it benefits from multidisciplinary evaluation to guide optimal and personalized care for each patient, including medical oncology, radiation oncology, neurosurgery and/or orthopedic spine surgery, and palliative medicine. Treatment options include supportive care or corticosteroids alone, surgical resection, whole-brain RT, and/or radiosurgery or stereotactic RT alone. These treatment options are considered on the basis of global patient factors, such as prognosis, together with metastatic-site–specific factors, such as site-related symptoms and the number of metastatic diseases or the burden of the disease.13 For example, the use of the diagnosis-specific Graded Prognostic Assessment index to predict life expectancy can help tailor management of brain metastases based on performance status, age, number of brain metastases, extracranial metastases, and cancer type. Highlighting the complexity of this common PRT presentation, Tsao and colleagues showed that there was a lack of uniform agreement among radiation oncologists for common management issues in patients with brain metastatic disease.14
For metastatic spinal cord or cauda equina compression and the associated neurologic symptoms, initiation of immediate corticosteroids and implementation of local therapy within 24 hours of presentation is paramount,15 highlighting the need for rapid, comprehensive care decision-making for these patients. Treatment options that must be weighed include the potential benefit of upfront decompressive surgery, as supported by a randomized controlled trial by Patchell and colleagues16 for patients who are surgical candidates with true cord or cauda compression and have at least 1 neurologic symptom, a prognosis of ≥3 months, paraplegia of no longer than 48 hours, and no previous RT to the site or brain metastases. Compared with the RT alone, patients receiving surgery before RT had improved ambulatory status and overall survival. Hence, neurosurgical or orthopedic consultation should be standard in the evaluation of metastatic spinal cord or cauda equina compression patients. However, patients frequently do not meet these criteria, and corticosteroids and RT alone are considered. In addition to playing a role in surgical decision-making, prognosis also has a key role in decision-making about the RT fractionation. Short-course RT (8 Gy × 1) is as effectual as longer-course regimens (3 Gy × 10) in terms of motor function.17,18 However, more dose-intense or longer-course regimens, such as 3 Gy × 10, have been shown to have more durability beyond about 6 months and are therefore considered for intermediate to good prognosis.18 The common urgent presentation of CNS metastatic disease to radiation oncology clinics together with the complexity of management and urgency of care decision-making point to the need for dedicated structures of care for these patients in radiation oncology settings. For example, dedicated PRT programs, such as the Rapid Response Radiotherapy Program in Toronto and the Supportive and Palliative Radiation Oncology service in Boston, have demonstrated improved quality of care for patients being urgently evaluated for PRT.19
Following management of pain and neurologic symptoms, clinicians were faced with managing fatigue in nearly half of the patients (49%). The prevalence of fatigue among cancer patients and its impact on quality of life20 highlight the need for this key symptom to be addressed throughout the continuum of cancer care. National Comprehensive Cancer Network guidelines provide a comprehensive framework for addressing cancer-related fatigue.7 However, cancer-related fatigue is a largely underreported, underestimated, and thus undertreated problem.20 In a nationwide survey of members of the American Society for Radiation Oncology, radiation oncologists reported being significantly less confident in managing fatigue compared with managing other common symptoms.21 Furthermore, in a national survey of radiation oncology trainees, 67% of respondents indicated that they were not at all minimally or somewhat confident in their ability to manage fatigue symptoms. The frequency of this symptom together with the demonstrated need for improved education in fatigue management point to a need for radiation oncology palliative educational structures to include dedicated emphasis on managing fatigue in addition to other commonly encountered symptoms, such as pain.
Patients evaluated for PRT are seen across the trajectory of their metastatic cancer diagnosis. In our study, patients presented at all stages in their advanced cancers. These include patients seen at the time of initial diagnosis of cancer as well as those seen near the end of life when end-of-life care planning was underway. The broad spectrum of timing of PRT care underscores that radiation oncologists must be prepared to handle generalist palliative care issues encountered throughout the trajectory of advanced cancer care and hence need comprehensive education in generalist palliative care competencies. These include symptom management, end-of-life care coordination, and communication or goals-of-care discussions. Notably, a recent national survey of radiation oncology residents indicated that most residents, 77% on average, perceived their educational training as suboptimal across domains of generalist palliative care competencies needed in oncology practice.22 Furthermore, a majority (81%) desired greater palliative care education within training.
The most common sites treated in this study were bone, brain, and lung sites. These data provide guidance to both education and research initiatives aiming to advance PRT curriculum and care structures within departments. For example, a same-day simulation and radiation treatment program developed at Princess Margaret Hospital Palliative Radiation Oncology Program (Ontario, Canada) aids in providing streamlined care for patients with bone metastases, the most common presentation for PRT.23 Furthermore, education and research in the application of PRT techniques to bone, brain, and thoracic disease cover the majority of PRT presentations. It is notable, however, that 17% were other soft tissue body sites.
Limitations
There are a few limitations to this study. First, this is a survey-based study conducted at a single academic center within an urban setting and surrounding community regions, which affects its generalizability. Second, this study presents perspectives of radiation oncology practitioners evaluating patients and does not directly reflect patient perceptions or report of symptoms. Third, the data provided by this study are solely descriptive in nature. However, this can guide hypothesis-driven research regarding the evaluation and management of urgent palliative care issues encountered by radiation oncology clinicians and suggest educational objectives to address the needs of these patients.
Conclusions
Radiation oncologists are involved throughout the trajectory of care for advanced cancer patients. Furthermore, they manage a variety of urgent oncologic issues, most commonly metastases causing pain, brain metastases, and spinal cord or cauda equina compression. Radiation oncologists also manage many cancer-related symptoms, mostly pain, neurologic symptoms, fatigue, and gastrointestinal symptoms. These findings point toward the need for palliative care to be well integrated into radiation oncology training curricula and the need for dedicated care structures that enable rapid and multidisciplinary palliative oncology care within radiation oncology departments.
1. Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25(11):1423-1436.
2. van Oorschot B, Rades D, Schulze W, Beckmann G, Feyer P. Palliative radiotherapy--new approaches. Semin Oncol. 2011;38(3):443-449.
3. Simone CB II, Jones JA. Palliative care for patients with locally advanced and metastatic non-small cell lung cancer. Ann Palliat Med. 2013;2(4):178-188.
4. Cihoric N, Crowe S, Eychmüller S, Aebersold DM, Ghadjar P. Clinically significant bleeding in incurable cancer patients: effectiveness of hemostatic radiotherapy. Radiat Oncol. 2012;7:132.
5. Duchesne GM, Bolger JJ, Griffiths GO, et al. A randomized trial of hypofractionated schedules of palliative radiotherapy in the management of bladder carcinoma: results of medical research council trial BA09. Int J Radiat Oncol Biol Phys. 2000;47(2):379-388.
6. Onsrud M, Hagen B, Strickert T. 10-Gy single-fraction pelvic irradiation for palliation and life prolongation in patients with cancer of the cervix and corpus uteri. Gynecol Oncol. 2001;82(1):167-171.
7. NCCN Guidelines(R) Updates. J Natl Compr Canc Netw. 2013;11(9):xxxii-xxxvi.
8. Colby WH, Dahlin C, Lantos J, Carney J, Christopher M. The National Consensus Project for Quality Palliative Care Clinical Practice Guidelines Domain 8: ethical and legal aspects of care. HEC Forum. 2010;22(2):117-131.
9. Stockler MR, Wilcken NR. Why is management of cancer pain still a problem? J Clin Oncol. 2012;30(16):1907-1908.
10. Caravatta L, Ramella S, Melano A, et al. Breakthrough pain management in patients undergoing radiotherapy: a national survey on behalf of the Palliative and Supportive Care Study Group. Tumori. 2015;101(6):603-608.
11. Breuer B, Fleishman SB, Cruciani RA, Portenoy RK. Medical oncologists’ attitudes and practice in cancer pain management: a national survey. J Clin Oncol. 2011;29(36):4769-4775.
12. Cleeland CS, Janjan NA, Scott CB, Seiferheld WF, Curran WJ. Cancer pain management by radiotherapists: a survey of radiation therapy oncology group physicians. Int J Radiat Oncol Biol Phys. 2000;47(1):203-208.
13. Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2(3):210-225.
14. Tsao MN, Rades D, Wirth A, et al. International practice survey on the management of brain metastases: third international consensus workshop on palliative radiotherapy and symptom control. Clin Oncol (R Coll Radiol). 2012;24(6):e81-e92.
15. Tang V, Harvey D, Park Dorsay J, Jiang S, Rathbone MP. Prognostic indicators in metastatic spinal cord compression: using functional independence measure and Tokuhashi scale to optimize rehabilitation planning. Spinal Cord. 2007;45(10):671-677.
16. Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643-648.
17. Rades D, Stalpers LJ, Veninga T, et al. Evaluation of five radiation schedules and prognostic factors for metastatic spinal cord compression. J Clin Oncol. 2005;23(15):3366-3375.
18. Rades D, Stalpers LJ, Hulshof MC, et al. Comparison of 1 x 8 Gy and 10 x 3 Gy for functional outcome in patients with metastatic spinal cord compression. Int J Radiat Oncol Biol Phys. 2005;62(2):514-518.
19. Dennis K, Linden K, Balboni T, Chow E. Rapid access palliative radiation therapy programs: an efficient model of care. Future Oncol. 2015;11(17):2417-2426.
20. Kapoor A, Singhal MK, Bagri PK, Narayan S, Beniwal S, Kumar HS. Cancer related fatigue: a ubiquitous problem yet so under reported, under recognized and under treated. South Asian J Cancer. 2015;4(1):21-23.
21. Wei RL, Mattes MD, Yu J, et al. Attitudes of radiation oncologists toward palliative and supportive care in the United States: report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract Radiat Oncol. 2017;7(2):113-119.
22. Krishnan M, Racsa M, Jones J, et al. Radiation oncology resident palliative education. Pract Radiat Oncol. 2017;7(6):e439-e448.
23. McDonald R, Chow E, Lam H, Rowbottom L, Soliman H. International patterns of practice in radiotherapy for bone metastases: a review of the literature. J Bone Oncol. 2014;3(3-4):96-102.
1. Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25(11):1423-1436.
2. van Oorschot B, Rades D, Schulze W, Beckmann G, Feyer P. Palliative radiotherapy--new approaches. Semin Oncol. 2011;38(3):443-449.
3. Simone CB II, Jones JA. Palliative care for patients with locally advanced and metastatic non-small cell lung cancer. Ann Palliat Med. 2013;2(4):178-188.
4. Cihoric N, Crowe S, Eychmüller S, Aebersold DM, Ghadjar P. Clinically significant bleeding in incurable cancer patients: effectiveness of hemostatic radiotherapy. Radiat Oncol. 2012;7:132.
5. Duchesne GM, Bolger JJ, Griffiths GO, et al. A randomized trial of hypofractionated schedules of palliative radiotherapy in the management of bladder carcinoma: results of medical research council trial BA09. Int J Radiat Oncol Biol Phys. 2000;47(2):379-388.
6. Onsrud M, Hagen B, Strickert T. 10-Gy single-fraction pelvic irradiation for palliation and life prolongation in patients with cancer of the cervix and corpus uteri. Gynecol Oncol. 2001;82(1):167-171.
7. NCCN Guidelines(R) Updates. J Natl Compr Canc Netw. 2013;11(9):xxxii-xxxvi.
8. Colby WH, Dahlin C, Lantos J, Carney J, Christopher M. The National Consensus Project for Quality Palliative Care Clinical Practice Guidelines Domain 8: ethical and legal aspects of care. HEC Forum. 2010;22(2):117-131.
9. Stockler MR, Wilcken NR. Why is management of cancer pain still a problem? J Clin Oncol. 2012;30(16):1907-1908.
10. Caravatta L, Ramella S, Melano A, et al. Breakthrough pain management in patients undergoing radiotherapy: a national survey on behalf of the Palliative and Supportive Care Study Group. Tumori. 2015;101(6):603-608.
11. Breuer B, Fleishman SB, Cruciani RA, Portenoy RK. Medical oncologists’ attitudes and practice in cancer pain management: a national survey. J Clin Oncol. 2011;29(36):4769-4775.
12. Cleeland CS, Janjan NA, Scott CB, Seiferheld WF, Curran WJ. Cancer pain management by radiotherapists: a survey of radiation therapy oncology group physicians. Int J Radiat Oncol Biol Phys. 2000;47(1):203-208.
13. Tsao MN, Rades D, Wirth A, et al. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2(3):210-225.
14. Tsao MN, Rades D, Wirth A, et al. International practice survey on the management of brain metastases: third international consensus workshop on palliative radiotherapy and symptom control. Clin Oncol (R Coll Radiol). 2012;24(6):e81-e92.
15. Tang V, Harvey D, Park Dorsay J, Jiang S, Rathbone MP. Prognostic indicators in metastatic spinal cord compression: using functional independence measure and Tokuhashi scale to optimize rehabilitation planning. Spinal Cord. 2007;45(10):671-677.
16. Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643-648.
17. Rades D, Stalpers LJ, Veninga T, et al. Evaluation of five radiation schedules and prognostic factors for metastatic spinal cord compression. J Clin Oncol. 2005;23(15):3366-3375.
18. Rades D, Stalpers LJ, Hulshof MC, et al. Comparison of 1 x 8 Gy and 10 x 3 Gy for functional outcome in patients with metastatic spinal cord compression. Int J Radiat Oncol Biol Phys. 2005;62(2):514-518.
19. Dennis K, Linden K, Balboni T, Chow E. Rapid access palliative radiation therapy programs: an efficient model of care. Future Oncol. 2015;11(17):2417-2426.
20. Kapoor A, Singhal MK, Bagri PK, Narayan S, Beniwal S, Kumar HS. Cancer related fatigue: a ubiquitous problem yet so under reported, under recognized and under treated. South Asian J Cancer. 2015;4(1):21-23.
21. Wei RL, Mattes MD, Yu J, et al. Attitudes of radiation oncologists toward palliative and supportive care in the United States: report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract Radiat Oncol. 2017;7(2):113-119.
22. Krishnan M, Racsa M, Jones J, et al. Radiation oncology resident palliative education. Pract Radiat Oncol. 2017;7(6):e439-e448.
23. McDonald R, Chow E, Lam H, Rowbottom L, Soliman H. International patterns of practice in radiotherapy for bone metastases: a review of the literature. J Bone Oncol. 2014;3(3-4):96-102.
The long-term effects of posttreatment exercise on pain in young women with breast cancer
Breast cancer is one of the most prevalent cancers in women worldwide, with more than 1 million new cases diagnosed annually.1 Prognosis for the disease has improved significantly, but 25% to 60% of women living with breast cancer experience some level of pain ranging from mild to severe, the nature of which can evolve from acute to chronic.2 Pre-, intra-, and post-treatment risk factors have been found to correlate with the development of acute and chronic pain and include young age, type of breast surgery (lumpectomy or total mastectomy), axillary node dissection, radiation therapy, and hormonal therapy.3-5 Chemotherapy, particularly anthracycline- and taxane-based regimens, has also been shown to induce pain, arthralgia, myalgia, and peripheral neuropathy during treatment.6 In particular, postradiation pain may result from subcutaneous fibrosis with fixation to underlying musculature and the development of fibrous flaps in the internal axilla.7 These tissue changes are commonly subclinical, occurring 4 to 12 months postradiation,8 and can progress undetected until pain and upper-limb disability develop.
The presence of persistent pain has a considerable impact on the quality of life in survivors of breast cancer: psychological distress is prevalent (anxiety, depression, worry, fear), the performance of daily activities is diminished (eg, bathing, dressing, preparing meals, shopping), and economic independence is compromised by the inability to work or reduced employment and income. These factors directly and indirectly contribute to an increase in the use of health care services.9,10
The management of pain is often characterized by pharmacologic-related treatment, such as the use of opioids and nonsteroidal anti-inflammatory medications, and nonpharmacologic-related treatment, such as exercise. Empirical evidence has shown that rehabilitative exercise programs, which commonly include a combination of resistance training and aerobic exercises, can effectively reduce pain in breast cancer survivors.10-12 Women living with breast cancer who are directed to rehabilitative exercise programs experience an improvement not only in pain levels but also in their ability to engage in activities of daily living, in their psychological health, and in their overall quality of life.13-15 However, despite evidence to support exercise programs to reduce pain related to breast cancer treatment, residual pain and upper-limb discomfort are common complaints in breast cancer survivors, and there is little focus on the duration of effectiveness of such programs for reducing pain after treatment for breast cancer. The objective of this study was to determine if an exercise program initiated postradiation would improve long-term pain levels in a carefully selected population of young women who were living with breast cancer and had no history of shoulder pathology or significant treatment complications.
Methods
Design
We used a pilot randomized control trial to compare the long-term effectiveness of a 12-week postradiation exercise program versus standard care on residual pain levels in young women (aged 18-45 years) living with breast cancer. The program was initiated 3 to 4 weeks postradiation to allow for acute inflammatory reactions to subside. Pain severity and interference were assessed using the Brief Pain Inventory-Short Form (BPI-SF), a tool for assessing cancer pain.16,17 Pain levels for isolated shoulder movements were also recorded on examination by a physical therapist. All measures were collected at 6 time points (T1-T6): postsurgery and preradiation (T1, baseline), postradiation and preintervention (T2), and 4 points during an 18-month period postradiation (T3-T6 at 3, 6, 12, and 18 months postradiation).
Sample
Young women living with breast cancer who met our eligibility criteria were identified from 2 clinics at the Jewish General Hospital – the Segal Cancer Center and the Department of Radiation Oncology in Montréal, Québec, Canada. Inclusion criteria included women with a diagnosis of stage I to stage III breast cancer, who were 18 to 45 years old, were scheduled for postoperative adjuvant radiation therapy, had an Eastern Cooperative Oncology Group Performance Status of 0 or 1 (normal ambulatory function, minimal symptoms), and who consented to participate in the study. Exclusion criteria included women with a metastatic (stage IV) diagnosis; significant musculoskeletal, cardiac, pulmonary, or metabolic comorbidities that would not allow for participation in physical activity; a previous breast cancer diagnosis with treatment to the ipsilateral or contralateral sides; postsurgical lymphedema; postsurgical capsulitis, tendonitis, or other shoulder inflammatory complications; and any contraindication to exercise. The recruitment goal was outlined as 50 patients per group; however, a protracted accrual time because of the stringent study criteria yielded a sample of 29 and 30 patients for the intervention and control groups, respectively, which was sufficient for significant testing of differences between the 2 study groups.18
Variables and measures
Clinical characteristics. We used standardized questions and chart review to document the participants’ clinical characteristics and to capture information on the following: the stage and subtype of breast cancer, hormonal and human epidermal growth factor receptors (HER2) (estrogen receptor, progesterone receptor, and HER2 status), extent of surgery (lumpectomy or total mastectomy), and other modalities of treatment (eg, chemotherapy, radiation therapy).
Pain assessment. The BPI-SF was used to assess participants’ cancer-related pain. Pain severity ranged from 0 (no pain), 1 to 4 (mild pain), 5 to 6 (moderate pain), to 7 to 10 (severe pain).18,19 The questionnaire also identifies the pain interference in daily activities using a Likert scale ranging from 0 (Does not interfere) to 10 (Completely interferes) in the following 7 domains or subscales: General Activity, Walking, Mood, Sleep, Work, Relations with Others, and Enjoyment of Life.16 For the purpose of this study, mean scores were tabulated using both pain intensity and interference scales.
Another important component of the BPI-SF instructs participants to localize pain by means of a body diagram. For purpose of analysis, 3 pain regions were established: shoulder girdle/chest wall on the affected side; neck and other upper extremity, including hand(s), forearm(s), wrist(s), and finger(s); and other regions, including abdominal discomfort, leg(s), hip(s), knee(s), ankle(s), lower back, and feet. In addition, pain levels on movement (Yes/No) were recorded for isolated shoulder flexion, abduction, and horizontal abduction (sitting and standing). The measurements were completed by a single physical therapist throughout the course of the study to minimize variance.
Procedure
The study protocol was approved by the Research Ethics Board at the Jewish General Hospital. Recruitment occurred from 2011 through 2015. The research was in accordance with the ethical standards of the responsible committee on human experimentation. Eligible women were recruited by the research coordinator who described the purpose, risks, and benefits of the study; advised on confidentiality, data collection, and intervention allocation procedures; and highlighted voluntary participation. The research coordinator addressed any concerns on the part of the participants before obtaining their written informed consent. Random allocation to the intervention and control groups was established using a web-based randomization plan generator (www.randomization.com). A single individual was responsible for the randomization process, and treatment assignments were revealed after each participant’s name had been entered. A physical therapist performed 6 sequential evaluations (T1-T6) at the time of participants’ medical follow-up appointments.
Intervention
The 12-week exercise intervention started 3 weeks postradiation and was composed of an initial 6-week program of low-level cardiovascular and resistance exercises that progressed to a set of more advanced exercises for the remaining 6 weeks. Participants were instructed to warm up for at least 10 minutes with a cardiovascular exercise of their choice (eg, a recumbent cross trainer, walking, or stairs) before doing a combined strength, endurance, and stretching exercise program for the upper body.20 The final portion of the exercise intervention included a period of light cool-down. Weight training resistance levels were based on a maximum 8 to 10 repetitions for strength and a maximum of 20 repetitions for endurance training exercises, which progressed gradually over the course of the 12-week exercise program to ensure participant safety.21,22 Participants in the intervention group were supervised at least once a week by an exercise physiologist at a center for oncology patients (Hope & Cope Wellness Centre), and patients were encouraged to perform the program at home 2 to 3 times a week. Those who were not able to exercise consistently at the center were provided with equipment and instructed on how to do the program safely at home.
By comparison, the control group received standard care, which included advice on the benefits of an active lifestyle, including exercise, but without a specific intervention. Participants were not restricted in their physical activity and/or sport participation levels, and their weekly activity levels were calculated using the Metabolic Equivalent of Task and recorded at each of the 6 time points.
Statistical analysis
Descriptive statistics were used to examine participant characteristics. The quantitative data collected through the BPI-SF measures were analyzed with JMP software (version 11.2; SAS Institute, Cary, NC). Continuous variables were tested for statistical significance (P ≤ .05) through the chi-square (categorical), analysis of variance, and nonparametric Wilcoxon tests. The analyses did not include missing data.
Results
A total of 59 young women were randomized into the intervention (n = 29) and control (n = 30) groups. Of those, 2 participants dropped out of the study because of family and time constraints, and 3 participants died, 2 from the control and 1 from the intervention group, after subsequently developing metastatic disease. Baseline data including comparative tumor characteristics, surgical interventions, and treatment interventions have been published in relation to other elements of this study.23,24 The participants had a mean age of 39.2 years (standard deviation [SD], 5.0). More than half of them had an invasive ductal carcinoma (69.5%) and were estrogen positive (78.0%), progesterone positive (74.6%), or HER2 positive (20.3%), whereas 10.2% were triple negative. Most of the participants had undergone breast-sparing procedures (86.4% lumpectomy), and 18.6% had a total mastectomy. By random chance, the intervention group had higher rates of total mastectomy (24.4% and 13.3%, respectively) and surgical reconstruction (12.2% and 6.7%, respectively) compared with the control group. Most of the women (71.2%) received chemotherapy, and all received radiation therapy. In the intervention group, 37.2% received radiation therapy localized to the axilla, and 88% received a boost of radiation to the surgical bed. Self-reported exercise diaries were returned by 15 of the 29 intervention participants, and training frequencies among them varied significantly (1-6 times a week).
The findings showed that there was little variance between the intervention and control groups in BPI-SF severity scores from T1 to T6, so the means and SDs of the BPI-SF scores were grouped at 6 time points (Table 1). There was no statistically significant difference between baseline measures at T1 (1.68; SD, 1.17) and measures at 18 months postintervention (T6: 1.46; SD, 1.37). At baseline, 87.7% of the women reported no pain (31.5%) or mild levels of pain (55.6%), and 13% reported moderate or severe pain. Over the duration of the study from T1 to T6, these primarily low levels of pain (BPI-SF, 0-4) remained consistent with a favorable shift toward having no pain (T1: 31.5%; T6: 24.4%). By 18 months postintervention, 95.7% of women reported no or mild pain, with 4.9% reporting moderate pain.
Similarly, there was little variance over time (T1-T6) and no statistically significant differences between the 2 groups in BPI-SF–measured levels of pain interference in daily activities (Table 2). Moreover, a domain analysis showed that there were no statistically significant differences in pain interference scores when comparing the type and extent of surgery (total mastectomy: 0.59 [1.17]; lumpectomy: 0.94 [1.96]). By chance – and not related directly to the objectives of this study – there was a statistically significant difference between the intervention and control groups in the interference of pain on the Enjoyment of Life domain in favor of the control group.
The sites of pain captured by the BPI-SF shed light on the preceding findings (Figure 1). At baseline (T1, postsurgery and preradiation), 37.0% of participants reported pain in the shoulder girdle–chest wall region, whereas 20.4% reported pain in the general neck–upper extremity region and 50% in other regions. Postradiation, shoulder girdle–chest wall pain was identified as the highest reported site of pain (49.1%; T2, postradiation and preintervention) and remained elevated at 3 months (T3) and 6 months (T4) postradiation (46.9% and 45.5%, respectively). At 12 and 18 months postradiation (T5 and T6), the principal focus of pain shifted once again to “other” regions at 30% and 32.5%, respectively, and the neck–upper extremity region at 10% and 15%, respectively. Shoulder girdle–chest wall pain concomitantly improved at those time points (15% and 25% respectively) but was not eliminated.
Pain levels recorded on physical examination for isolated shoulder range of movements were recently published,24 and they have been abbreviated and reproduced in this paper (Figure 2) to allow for a comparison of findings between the exercise intervention group and the control group to help determine the sensitivity of these tools for use in breast cancer patients. At baseline, pain levels with active movement were noted to be slightly greater in the intervention group for flexion and abduction.
Following the intervention, at 3 and 6 months postradiation (T3 and T4), the intervention group showed a steady decrease in pain levels in flexion and abduction, whereas the control group showed a 5-fold increase in pain with horizontal abduction. Furthermore, participants in the intervention group reported having no pain on movement 12 months postradiation (T5); however, recurrence of pain was apparent with all shoulder movements by 18 months postradiation (T6) in both the intervention and control groups.
Discussion
Previous studies have hypothesized that younger age (18-39 years), adjuvant radiotherapy, and axillary node dissection are risk factors for chronic pain in breast cancer survivors.22,25 Persistent pain is prevalent in 12% to 51% of breast cancer survivors, with up to one-third experiencing some pain more than 5 years after treatment,26,27 and our study outcomes concur with those findings. In our study, pain, as measured by the BPI-SF, was found to persist for most participants (75.6%) after the 18-month follow-up. The results of our trial showed that a 12-week exercise intervention administered postsurgery and postradiation had no statistically significant effect on long-term (18 months) pain severity and its interference in daily life. It is worth noting that body regions that had not been directly related to either surgical or radiation treatment for breast cancer were commonly identified as areas of pain but were not specifically targeted by our intervention. However, focusing on pain severity (BPI-SF), our findings suggest that the benefits of targeted upper-extremity exercise on pain in the intermediate time course of follow-up (T3, T4, and T5) was notable compared with the control group, which received standard care. The apparent recurrence of pain at 18 months in both groups was not anticipated and needs to be further investigated.
More specific objective assessments of pain on active shoulder movement identified distinct patterns of pain that could not be isolated using the BPI-SF alone. The incidence and localization of pain on movement differed between the population of women who received a specific exercise intervention and those who received standard care (Figure 2). Patterns of pain over time fluctuated in the control group, whereas the intervention group reported a linear decrease in pain. Residual pain on shoulder movement remained apparent in both groups at 18-months postradiation, but that finding was not reflected in the BPI-SF results. The literature supports our findings on persistent pain among breast cancer survivors,3,7,8,28-30 and in our study of young women carefully screened and excluded for pre-existent shoulder conditions or comorbid medical conditions, recurrent articular pain was nonetheless prevalent. It seems that unidentified or multiple factors may be part of the etiology of pain in this young adult cohort.
Although the BPI-SF is a generic measurement tool commonly used to assess and measure cancer patients’ pain levels, the lack of variance in our BPI-SF severity and interference outcomes over time (T1-T6) (Table 1, Table 2), the variety of “other” unrelated regions (Figure 1) identified by the BPI-SF, and the contrast in our findings on specific physical examination emphasize the potential limitations of this clinical tool.
Moreover, the BPI-SF has not been validated specifically for breast cancer. Harrington and colleagues have recommended using the BPI-SF to assess pain in women with breast cancer,31 but the use of a more multidimensional measurement tool that evaluates axillary, chest, trunk, and upper-limb pain may prove to be more valuable in this population.
Limitations
Recruitment of young adult women was difficult because of our stringent inclusion criteria, the long-term follow-up, and the relatively small population of breast cancer patients in this age demographic. Therefore, the duration of the recruitment phase, despite our having access to a specialized young adult and adolescent clinic in our institute, greatly surpassed the expectations we had when we designed the study. In addition, there remains an inherent bias in participants who accept participation in a study that includes exercise interventions. Potential participants who exercise regularly or have a positive inclination toward doing exercise are more likely to participate. Despite the prescription of a targeted 12-week upper-limb intervention in this study, the general activity levels of both groups may have had an impact on the significance of this study. In addition, the low adherence to the use of self-reported logs failed to capture the true compliance rates of our participants because their lack of tracking does not indicate failure to comply with the program. The use of weekly or biweekly telephone calls to monitor compliance rates of activity more vigilantly may be used in future studies.
Conclusions
Advances in clinical management of breast cancer have improved survival outcomes, and morbidity over recent years, yet symptoms such as pain remain prevalent in this population. The results of this study showed that a targeted, 12-week upper-limb exercise intervention postradiation transiently improved levels of shoulder pain without a concomitant impact on chronic pain or any positive influence on activities of daily living 18 months posttreatment. Furthermore, future studies should use a variety of measurement tools to evaluate trunk and upper-limb pain in women with breast cancer and investigate the optimal timing of postradiation exercise interventions.
Acknowledgments
The authors thank Hope & Cope, the CURE foundation, and the Jewish General Hospital Foundation/Weekend to End Breast Cancer for providing the financial resources needed to sustain this research study. They also thank the McGill Adolescent and Young Adult program for its continued support. Previous oral presentations of research Muanza TM, et al. Randomized clinical trial of a progressive exercise program for young women with breast cancer undergoing radiation therapy. Int J Radiat Oncol Biol Phys. 2015;93(3):s35-s36.
1. World Health Organization. Breast cancer: prevention and control. www.who.int/cancer/detection/breastcancer/en/. Updated 2017. Accessed September 16, 2016.
2. Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain. 2011;12(7):725-746.
3. Ernst MF, Voogd AC, Balder W, Klinkenbijl JH, Roukema JA. Early and late morbidity associated with axillary levels I-III dissection in breast cancer. J Surg Oncol. 2002;79(3):151-155; discussion 156.
4. Gulluoglu BM, Cingi A, Cakir T, Gercek A, Barlas A, Eti Z. Factors related to post-treatment chronic pain in breast cancer survivors: the interference of pain with life functions. Int J Fertil Womens Med. 2006;51(2):75-82.
5. Jung BF, Ahrendt GM, Oaklander AL, Dworkin RH. Neuropathic pain following breast cancer surgery: proposed classification and research update. Pain. 2003;104(1-2):1-13.
6. Saibil S, Fitzgerald B, Freedman OC, et al. Incidence of taxane-induced pain and distress in patients receiving chemotherapy for early-stage breast cancer: a retrospective, outcomes-based survey. Curr Oncol. 2010;17(4):42-47.
7. Tengrup I, Tennvall-Nittby L, Christiansson I, Laurin M. Arm morbidity after breast-conserving therapy for breast cancer. Acta Oncol. 2000;39(3):393-397.
8. Johansen J, Overgaard J, Blichert-Toft M, Overgaard M. Treatment of morbidity associated with the management of the axilla in breast-conserving therapy. Acta Oncol. 2000;39(3):349-354.
9. Mittmann N, Porter JM, Rangrej J, et al. Health system costs for stage-specific breast cancer: a population-based approach. Curr Oncol. 2014;21(6):281-293.
10. Page A. Keeping patients safe: transforming the work environment of nurses. Washington, DC: National Academies Press; 2004.
11. McNeely ML, Campbell K, Ospina M, et al. Exercise interventions for upper-limb dysfunction due to breast cancer treatment. Cochrane Database Syst Rev. 2010;(6):CD005211. doi:10.1002/14651858.CD005211.pub2
12. Wong P, Muanza T, Hijal T, et al. Effect of exercise in reducing breast and chest-wall pain in patients with breast cancer: a pilot study. Curr Oncol. 2012;19(3):e129-e135.
13. Fernández-Lao C, Cantarero-Villanueva I, Fernández-de-Las-Peñas C, del Moral-Ávila R, Castro-Sánchez AM, Arroyo-Morales M. Effectiveness of a multidimensional physical therapy program on pain, pressure hypersensitivity, and trigger points in breast cancer survivors: a randomized controlled clinical trial. Clin J Pain. 2012;28(2):113-121.
14. Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21(9):1660-1668.
15. Segal R, Evans W, Johnson D, et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. J Clin Oncol. 2001;19(3):657-665.
16. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129-138.
17. Kumar SP. Utilization of Brief Pain Inventory as an assessment tool for pain in patients with cancer: a focused review. Indian J Palliat Care. 2011;17(2):108-115.
18. Van Voorhis CRW, Morgan BL. Understanding power and rules of thumb for determining sample sizes. Tutor Quant Methods Psychol. 2007;3(2):43-50.
19. Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61(2):277-284.
20. Lee TS, Kilbreath SL, Refshauge KM, Pendlebury SC, Beith JM, Lee MJ. Pectoral stretching program for women undergoing radiotherapy for breast cancer. Breast Cancer Res Treat. 2007;102(3):313-321.
21. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409-1426.
22. Pollock ML, Gaesser GA, Butcher JD, et al. ACSM position stand: the recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30(6):975-991.
23. Ibrahim M, Muanza T, Smirnow N, et al. Time course of upper limb function and return-to-work post-radiotherapy in young adults with breast cancer: a pilot randomized control trial on effects of targeted exercise program. J Cancer Surviv. 2017;11(6):791-799.
24. Ibrahim M, Muanza T, Smirnow N, et al. A pilot randomized controlled trial on the effects of a progressive exercise program on the range of motion and upper extremity grip strength in young adults with breast cancer. Clin Breast Cancer. 2018;18(1):e55-e64.
25. Gärtner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. 2009;302(18):1985-1992.
26. Hayes SC, Johansson K, Stout NL, et al. Upper-body morbidity after breast cancer: incidence and evidence for evaluation, prevention, and management within a prospective surveillance model of care. Cancer. 2012;118(suppl 8):2237-2249.
27. Kärki A, Simonen R, Mälkiä E, Selfe J. Impairments, activity limitations and participation restrictions 6 and 12 months after breast cancer operation. J Rehabil Med. 2005;37(3):180-188.
28. Katz J, Poleshuck EL, Andrus CH, et al. Risk factors for acute pain and its persistence following breast cancer surgery. Pain. 2005;119(1-3):16-25.
29. Tasmuth T, von Smitten K, Hietanen P, Kataja M, Kalso E. Pain and other symptoms after different treatment modalities of breast cancer. Ann Oncol. 1995;6(5):453-459.
30. Whelan TJ, Levine M, Julian J, Kirkbride P, Skingley P. The effects of radiation therapy on quality of life of women with breast carcinoma: results of a randomized trial. Ontario Clinical Oncology Group. Cancer. 2000;88(10):2260-2266.
31. Harrington S, Gilchrist L, Sander A. Breast cancer EDGE task force outcomes: clinical measures of pain. Rehabil Oncol. 2014;32(1):13-21.
Breast cancer is one of the most prevalent cancers in women worldwide, with more than 1 million new cases diagnosed annually.1 Prognosis for the disease has improved significantly, but 25% to 60% of women living with breast cancer experience some level of pain ranging from mild to severe, the nature of which can evolve from acute to chronic.2 Pre-, intra-, and post-treatment risk factors have been found to correlate with the development of acute and chronic pain and include young age, type of breast surgery (lumpectomy or total mastectomy), axillary node dissection, radiation therapy, and hormonal therapy.3-5 Chemotherapy, particularly anthracycline- and taxane-based regimens, has also been shown to induce pain, arthralgia, myalgia, and peripheral neuropathy during treatment.6 In particular, postradiation pain may result from subcutaneous fibrosis with fixation to underlying musculature and the development of fibrous flaps in the internal axilla.7 These tissue changes are commonly subclinical, occurring 4 to 12 months postradiation,8 and can progress undetected until pain and upper-limb disability develop.
The presence of persistent pain has a considerable impact on the quality of life in survivors of breast cancer: psychological distress is prevalent (anxiety, depression, worry, fear), the performance of daily activities is diminished (eg, bathing, dressing, preparing meals, shopping), and economic independence is compromised by the inability to work or reduced employment and income. These factors directly and indirectly contribute to an increase in the use of health care services.9,10
The management of pain is often characterized by pharmacologic-related treatment, such as the use of opioids and nonsteroidal anti-inflammatory medications, and nonpharmacologic-related treatment, such as exercise. Empirical evidence has shown that rehabilitative exercise programs, which commonly include a combination of resistance training and aerobic exercises, can effectively reduce pain in breast cancer survivors.10-12 Women living with breast cancer who are directed to rehabilitative exercise programs experience an improvement not only in pain levels but also in their ability to engage in activities of daily living, in their psychological health, and in their overall quality of life.13-15 However, despite evidence to support exercise programs to reduce pain related to breast cancer treatment, residual pain and upper-limb discomfort are common complaints in breast cancer survivors, and there is little focus on the duration of effectiveness of such programs for reducing pain after treatment for breast cancer. The objective of this study was to determine if an exercise program initiated postradiation would improve long-term pain levels in a carefully selected population of young women who were living with breast cancer and had no history of shoulder pathology or significant treatment complications.
Methods
Design
We used a pilot randomized control trial to compare the long-term effectiveness of a 12-week postradiation exercise program versus standard care on residual pain levels in young women (aged 18-45 years) living with breast cancer. The program was initiated 3 to 4 weeks postradiation to allow for acute inflammatory reactions to subside. Pain severity and interference were assessed using the Brief Pain Inventory-Short Form (BPI-SF), a tool for assessing cancer pain.16,17 Pain levels for isolated shoulder movements were also recorded on examination by a physical therapist. All measures were collected at 6 time points (T1-T6): postsurgery and preradiation (T1, baseline), postradiation and preintervention (T2), and 4 points during an 18-month period postradiation (T3-T6 at 3, 6, 12, and 18 months postradiation).
Sample
Young women living with breast cancer who met our eligibility criteria were identified from 2 clinics at the Jewish General Hospital – the Segal Cancer Center and the Department of Radiation Oncology in Montréal, Québec, Canada. Inclusion criteria included women with a diagnosis of stage I to stage III breast cancer, who were 18 to 45 years old, were scheduled for postoperative adjuvant radiation therapy, had an Eastern Cooperative Oncology Group Performance Status of 0 or 1 (normal ambulatory function, minimal symptoms), and who consented to participate in the study. Exclusion criteria included women with a metastatic (stage IV) diagnosis; significant musculoskeletal, cardiac, pulmonary, or metabolic comorbidities that would not allow for participation in physical activity; a previous breast cancer diagnosis with treatment to the ipsilateral or contralateral sides; postsurgical lymphedema; postsurgical capsulitis, tendonitis, or other shoulder inflammatory complications; and any contraindication to exercise. The recruitment goal was outlined as 50 patients per group; however, a protracted accrual time because of the stringent study criteria yielded a sample of 29 and 30 patients for the intervention and control groups, respectively, which was sufficient for significant testing of differences between the 2 study groups.18
Variables and measures
Clinical characteristics. We used standardized questions and chart review to document the participants’ clinical characteristics and to capture information on the following: the stage and subtype of breast cancer, hormonal and human epidermal growth factor receptors (HER2) (estrogen receptor, progesterone receptor, and HER2 status), extent of surgery (lumpectomy or total mastectomy), and other modalities of treatment (eg, chemotherapy, radiation therapy).
Pain assessment. The BPI-SF was used to assess participants’ cancer-related pain. Pain severity ranged from 0 (no pain), 1 to 4 (mild pain), 5 to 6 (moderate pain), to 7 to 10 (severe pain).18,19 The questionnaire also identifies the pain interference in daily activities using a Likert scale ranging from 0 (Does not interfere) to 10 (Completely interferes) in the following 7 domains or subscales: General Activity, Walking, Mood, Sleep, Work, Relations with Others, and Enjoyment of Life.16 For the purpose of this study, mean scores were tabulated using both pain intensity and interference scales.
Another important component of the BPI-SF instructs participants to localize pain by means of a body diagram. For purpose of analysis, 3 pain regions were established: shoulder girdle/chest wall on the affected side; neck and other upper extremity, including hand(s), forearm(s), wrist(s), and finger(s); and other regions, including abdominal discomfort, leg(s), hip(s), knee(s), ankle(s), lower back, and feet. In addition, pain levels on movement (Yes/No) were recorded for isolated shoulder flexion, abduction, and horizontal abduction (sitting and standing). The measurements were completed by a single physical therapist throughout the course of the study to minimize variance.
Procedure
The study protocol was approved by the Research Ethics Board at the Jewish General Hospital. Recruitment occurred from 2011 through 2015. The research was in accordance with the ethical standards of the responsible committee on human experimentation. Eligible women were recruited by the research coordinator who described the purpose, risks, and benefits of the study; advised on confidentiality, data collection, and intervention allocation procedures; and highlighted voluntary participation. The research coordinator addressed any concerns on the part of the participants before obtaining their written informed consent. Random allocation to the intervention and control groups was established using a web-based randomization plan generator (www.randomization.com). A single individual was responsible for the randomization process, and treatment assignments were revealed after each participant’s name had been entered. A physical therapist performed 6 sequential evaluations (T1-T6) at the time of participants’ medical follow-up appointments.
Intervention
The 12-week exercise intervention started 3 weeks postradiation and was composed of an initial 6-week program of low-level cardiovascular and resistance exercises that progressed to a set of more advanced exercises for the remaining 6 weeks. Participants were instructed to warm up for at least 10 minutes with a cardiovascular exercise of their choice (eg, a recumbent cross trainer, walking, or stairs) before doing a combined strength, endurance, and stretching exercise program for the upper body.20 The final portion of the exercise intervention included a period of light cool-down. Weight training resistance levels were based on a maximum 8 to 10 repetitions for strength and a maximum of 20 repetitions for endurance training exercises, which progressed gradually over the course of the 12-week exercise program to ensure participant safety.21,22 Participants in the intervention group were supervised at least once a week by an exercise physiologist at a center for oncology patients (Hope & Cope Wellness Centre), and patients were encouraged to perform the program at home 2 to 3 times a week. Those who were not able to exercise consistently at the center were provided with equipment and instructed on how to do the program safely at home.
By comparison, the control group received standard care, which included advice on the benefits of an active lifestyle, including exercise, but without a specific intervention. Participants were not restricted in their physical activity and/or sport participation levels, and their weekly activity levels were calculated using the Metabolic Equivalent of Task and recorded at each of the 6 time points.
Statistical analysis
Descriptive statistics were used to examine participant characteristics. The quantitative data collected through the BPI-SF measures were analyzed with JMP software (version 11.2; SAS Institute, Cary, NC). Continuous variables were tested for statistical significance (P ≤ .05) through the chi-square (categorical), analysis of variance, and nonparametric Wilcoxon tests. The analyses did not include missing data.
Results
A total of 59 young women were randomized into the intervention (n = 29) and control (n = 30) groups. Of those, 2 participants dropped out of the study because of family and time constraints, and 3 participants died, 2 from the control and 1 from the intervention group, after subsequently developing metastatic disease. Baseline data including comparative tumor characteristics, surgical interventions, and treatment interventions have been published in relation to other elements of this study.23,24 The participants had a mean age of 39.2 years (standard deviation [SD], 5.0). More than half of them had an invasive ductal carcinoma (69.5%) and were estrogen positive (78.0%), progesterone positive (74.6%), or HER2 positive (20.3%), whereas 10.2% were triple negative. Most of the participants had undergone breast-sparing procedures (86.4% lumpectomy), and 18.6% had a total mastectomy. By random chance, the intervention group had higher rates of total mastectomy (24.4% and 13.3%, respectively) and surgical reconstruction (12.2% and 6.7%, respectively) compared with the control group. Most of the women (71.2%) received chemotherapy, and all received radiation therapy. In the intervention group, 37.2% received radiation therapy localized to the axilla, and 88% received a boost of radiation to the surgical bed. Self-reported exercise diaries were returned by 15 of the 29 intervention participants, and training frequencies among them varied significantly (1-6 times a week).
The findings showed that there was little variance between the intervention and control groups in BPI-SF severity scores from T1 to T6, so the means and SDs of the BPI-SF scores were grouped at 6 time points (Table 1). There was no statistically significant difference between baseline measures at T1 (1.68; SD, 1.17) and measures at 18 months postintervention (T6: 1.46; SD, 1.37). At baseline, 87.7% of the women reported no pain (31.5%) or mild levels of pain (55.6%), and 13% reported moderate or severe pain. Over the duration of the study from T1 to T6, these primarily low levels of pain (BPI-SF, 0-4) remained consistent with a favorable shift toward having no pain (T1: 31.5%; T6: 24.4%). By 18 months postintervention, 95.7% of women reported no or mild pain, with 4.9% reporting moderate pain.
Similarly, there was little variance over time (T1-T6) and no statistically significant differences between the 2 groups in BPI-SF–measured levels of pain interference in daily activities (Table 2). Moreover, a domain analysis showed that there were no statistically significant differences in pain interference scores when comparing the type and extent of surgery (total mastectomy: 0.59 [1.17]; lumpectomy: 0.94 [1.96]). By chance – and not related directly to the objectives of this study – there was a statistically significant difference between the intervention and control groups in the interference of pain on the Enjoyment of Life domain in favor of the control group.
The sites of pain captured by the BPI-SF shed light on the preceding findings (Figure 1). At baseline (T1, postsurgery and preradiation), 37.0% of participants reported pain in the shoulder girdle–chest wall region, whereas 20.4% reported pain in the general neck–upper extremity region and 50% in other regions. Postradiation, shoulder girdle–chest wall pain was identified as the highest reported site of pain (49.1%; T2, postradiation and preintervention) and remained elevated at 3 months (T3) and 6 months (T4) postradiation (46.9% and 45.5%, respectively). At 12 and 18 months postradiation (T5 and T6), the principal focus of pain shifted once again to “other” regions at 30% and 32.5%, respectively, and the neck–upper extremity region at 10% and 15%, respectively. Shoulder girdle–chest wall pain concomitantly improved at those time points (15% and 25% respectively) but was not eliminated.
Pain levels recorded on physical examination for isolated shoulder range of movements were recently published,24 and they have been abbreviated and reproduced in this paper (Figure 2) to allow for a comparison of findings between the exercise intervention group and the control group to help determine the sensitivity of these tools for use in breast cancer patients. At baseline, pain levels with active movement were noted to be slightly greater in the intervention group for flexion and abduction.
Following the intervention, at 3 and 6 months postradiation (T3 and T4), the intervention group showed a steady decrease in pain levels in flexion and abduction, whereas the control group showed a 5-fold increase in pain with horizontal abduction. Furthermore, participants in the intervention group reported having no pain on movement 12 months postradiation (T5); however, recurrence of pain was apparent with all shoulder movements by 18 months postradiation (T6) in both the intervention and control groups.
Discussion
Previous studies have hypothesized that younger age (18-39 years), adjuvant radiotherapy, and axillary node dissection are risk factors for chronic pain in breast cancer survivors.22,25 Persistent pain is prevalent in 12% to 51% of breast cancer survivors, with up to one-third experiencing some pain more than 5 years after treatment,26,27 and our study outcomes concur with those findings. In our study, pain, as measured by the BPI-SF, was found to persist for most participants (75.6%) after the 18-month follow-up. The results of our trial showed that a 12-week exercise intervention administered postsurgery and postradiation had no statistically significant effect on long-term (18 months) pain severity and its interference in daily life. It is worth noting that body regions that had not been directly related to either surgical or radiation treatment for breast cancer were commonly identified as areas of pain but were not specifically targeted by our intervention. However, focusing on pain severity (BPI-SF), our findings suggest that the benefits of targeted upper-extremity exercise on pain in the intermediate time course of follow-up (T3, T4, and T5) was notable compared with the control group, which received standard care. The apparent recurrence of pain at 18 months in both groups was not anticipated and needs to be further investigated.
More specific objective assessments of pain on active shoulder movement identified distinct patterns of pain that could not be isolated using the BPI-SF alone. The incidence and localization of pain on movement differed between the population of women who received a specific exercise intervention and those who received standard care (Figure 2). Patterns of pain over time fluctuated in the control group, whereas the intervention group reported a linear decrease in pain. Residual pain on shoulder movement remained apparent in both groups at 18-months postradiation, but that finding was not reflected in the BPI-SF results. The literature supports our findings on persistent pain among breast cancer survivors,3,7,8,28-30 and in our study of young women carefully screened and excluded for pre-existent shoulder conditions or comorbid medical conditions, recurrent articular pain was nonetheless prevalent. It seems that unidentified or multiple factors may be part of the etiology of pain in this young adult cohort.
Although the BPI-SF is a generic measurement tool commonly used to assess and measure cancer patients’ pain levels, the lack of variance in our BPI-SF severity and interference outcomes over time (T1-T6) (Table 1, Table 2), the variety of “other” unrelated regions (Figure 1) identified by the BPI-SF, and the contrast in our findings on specific physical examination emphasize the potential limitations of this clinical tool.
Moreover, the BPI-SF has not been validated specifically for breast cancer. Harrington and colleagues have recommended using the BPI-SF to assess pain in women with breast cancer,31 but the use of a more multidimensional measurement tool that evaluates axillary, chest, trunk, and upper-limb pain may prove to be more valuable in this population.
Limitations
Recruitment of young adult women was difficult because of our stringent inclusion criteria, the long-term follow-up, and the relatively small population of breast cancer patients in this age demographic. Therefore, the duration of the recruitment phase, despite our having access to a specialized young adult and adolescent clinic in our institute, greatly surpassed the expectations we had when we designed the study. In addition, there remains an inherent bias in participants who accept participation in a study that includes exercise interventions. Potential participants who exercise regularly or have a positive inclination toward doing exercise are more likely to participate. Despite the prescription of a targeted 12-week upper-limb intervention in this study, the general activity levels of both groups may have had an impact on the significance of this study. In addition, the low adherence to the use of self-reported logs failed to capture the true compliance rates of our participants because their lack of tracking does not indicate failure to comply with the program. The use of weekly or biweekly telephone calls to monitor compliance rates of activity more vigilantly may be used in future studies.
Conclusions
Advances in clinical management of breast cancer have improved survival outcomes, and morbidity over recent years, yet symptoms such as pain remain prevalent in this population. The results of this study showed that a targeted, 12-week upper-limb exercise intervention postradiation transiently improved levels of shoulder pain without a concomitant impact on chronic pain or any positive influence on activities of daily living 18 months posttreatment. Furthermore, future studies should use a variety of measurement tools to evaluate trunk and upper-limb pain in women with breast cancer and investigate the optimal timing of postradiation exercise interventions.
Acknowledgments
The authors thank Hope & Cope, the CURE foundation, and the Jewish General Hospital Foundation/Weekend to End Breast Cancer for providing the financial resources needed to sustain this research study. They also thank the McGill Adolescent and Young Adult program for its continued support. Previous oral presentations of research Muanza TM, et al. Randomized clinical trial of a progressive exercise program for young women with breast cancer undergoing radiation therapy. Int J Radiat Oncol Biol Phys. 2015;93(3):s35-s36.
Breast cancer is one of the most prevalent cancers in women worldwide, with more than 1 million new cases diagnosed annually.1 Prognosis for the disease has improved significantly, but 25% to 60% of women living with breast cancer experience some level of pain ranging from mild to severe, the nature of which can evolve from acute to chronic.2 Pre-, intra-, and post-treatment risk factors have been found to correlate with the development of acute and chronic pain and include young age, type of breast surgery (lumpectomy or total mastectomy), axillary node dissection, radiation therapy, and hormonal therapy.3-5 Chemotherapy, particularly anthracycline- and taxane-based regimens, has also been shown to induce pain, arthralgia, myalgia, and peripheral neuropathy during treatment.6 In particular, postradiation pain may result from subcutaneous fibrosis with fixation to underlying musculature and the development of fibrous flaps in the internal axilla.7 These tissue changes are commonly subclinical, occurring 4 to 12 months postradiation,8 and can progress undetected until pain and upper-limb disability develop.
The presence of persistent pain has a considerable impact on the quality of life in survivors of breast cancer: psychological distress is prevalent (anxiety, depression, worry, fear), the performance of daily activities is diminished (eg, bathing, dressing, preparing meals, shopping), and economic independence is compromised by the inability to work or reduced employment and income. These factors directly and indirectly contribute to an increase in the use of health care services.9,10
The management of pain is often characterized by pharmacologic-related treatment, such as the use of opioids and nonsteroidal anti-inflammatory medications, and nonpharmacologic-related treatment, such as exercise. Empirical evidence has shown that rehabilitative exercise programs, which commonly include a combination of resistance training and aerobic exercises, can effectively reduce pain in breast cancer survivors.10-12 Women living with breast cancer who are directed to rehabilitative exercise programs experience an improvement not only in pain levels but also in their ability to engage in activities of daily living, in their psychological health, and in their overall quality of life.13-15 However, despite evidence to support exercise programs to reduce pain related to breast cancer treatment, residual pain and upper-limb discomfort are common complaints in breast cancer survivors, and there is little focus on the duration of effectiveness of such programs for reducing pain after treatment for breast cancer. The objective of this study was to determine if an exercise program initiated postradiation would improve long-term pain levels in a carefully selected population of young women who were living with breast cancer and had no history of shoulder pathology or significant treatment complications.
Methods
Design
We used a pilot randomized control trial to compare the long-term effectiveness of a 12-week postradiation exercise program versus standard care on residual pain levels in young women (aged 18-45 years) living with breast cancer. The program was initiated 3 to 4 weeks postradiation to allow for acute inflammatory reactions to subside. Pain severity and interference were assessed using the Brief Pain Inventory-Short Form (BPI-SF), a tool for assessing cancer pain.16,17 Pain levels for isolated shoulder movements were also recorded on examination by a physical therapist. All measures were collected at 6 time points (T1-T6): postsurgery and preradiation (T1, baseline), postradiation and preintervention (T2), and 4 points during an 18-month period postradiation (T3-T6 at 3, 6, 12, and 18 months postradiation).
Sample
Young women living with breast cancer who met our eligibility criteria were identified from 2 clinics at the Jewish General Hospital – the Segal Cancer Center and the Department of Radiation Oncology in Montréal, Québec, Canada. Inclusion criteria included women with a diagnosis of stage I to stage III breast cancer, who were 18 to 45 years old, were scheduled for postoperative adjuvant radiation therapy, had an Eastern Cooperative Oncology Group Performance Status of 0 or 1 (normal ambulatory function, minimal symptoms), and who consented to participate in the study. Exclusion criteria included women with a metastatic (stage IV) diagnosis; significant musculoskeletal, cardiac, pulmonary, or metabolic comorbidities that would not allow for participation in physical activity; a previous breast cancer diagnosis with treatment to the ipsilateral or contralateral sides; postsurgical lymphedema; postsurgical capsulitis, tendonitis, or other shoulder inflammatory complications; and any contraindication to exercise. The recruitment goal was outlined as 50 patients per group; however, a protracted accrual time because of the stringent study criteria yielded a sample of 29 and 30 patients for the intervention and control groups, respectively, which was sufficient for significant testing of differences between the 2 study groups.18
Variables and measures
Clinical characteristics. We used standardized questions and chart review to document the participants’ clinical characteristics and to capture information on the following: the stage and subtype of breast cancer, hormonal and human epidermal growth factor receptors (HER2) (estrogen receptor, progesterone receptor, and HER2 status), extent of surgery (lumpectomy or total mastectomy), and other modalities of treatment (eg, chemotherapy, radiation therapy).
Pain assessment. The BPI-SF was used to assess participants’ cancer-related pain. Pain severity ranged from 0 (no pain), 1 to 4 (mild pain), 5 to 6 (moderate pain), to 7 to 10 (severe pain).18,19 The questionnaire also identifies the pain interference in daily activities using a Likert scale ranging from 0 (Does not interfere) to 10 (Completely interferes) in the following 7 domains or subscales: General Activity, Walking, Mood, Sleep, Work, Relations with Others, and Enjoyment of Life.16 For the purpose of this study, mean scores were tabulated using both pain intensity and interference scales.
Another important component of the BPI-SF instructs participants to localize pain by means of a body diagram. For purpose of analysis, 3 pain regions were established: shoulder girdle/chest wall on the affected side; neck and other upper extremity, including hand(s), forearm(s), wrist(s), and finger(s); and other regions, including abdominal discomfort, leg(s), hip(s), knee(s), ankle(s), lower back, and feet. In addition, pain levels on movement (Yes/No) were recorded for isolated shoulder flexion, abduction, and horizontal abduction (sitting and standing). The measurements were completed by a single physical therapist throughout the course of the study to minimize variance.
Procedure
The study protocol was approved by the Research Ethics Board at the Jewish General Hospital. Recruitment occurred from 2011 through 2015. The research was in accordance with the ethical standards of the responsible committee on human experimentation. Eligible women were recruited by the research coordinator who described the purpose, risks, and benefits of the study; advised on confidentiality, data collection, and intervention allocation procedures; and highlighted voluntary participation. The research coordinator addressed any concerns on the part of the participants before obtaining their written informed consent. Random allocation to the intervention and control groups was established using a web-based randomization plan generator (www.randomization.com). A single individual was responsible for the randomization process, and treatment assignments were revealed after each participant’s name had been entered. A physical therapist performed 6 sequential evaluations (T1-T6) at the time of participants’ medical follow-up appointments.
Intervention
The 12-week exercise intervention started 3 weeks postradiation and was composed of an initial 6-week program of low-level cardiovascular and resistance exercises that progressed to a set of more advanced exercises for the remaining 6 weeks. Participants were instructed to warm up for at least 10 minutes with a cardiovascular exercise of their choice (eg, a recumbent cross trainer, walking, or stairs) before doing a combined strength, endurance, and stretching exercise program for the upper body.20 The final portion of the exercise intervention included a period of light cool-down. Weight training resistance levels were based on a maximum 8 to 10 repetitions for strength and a maximum of 20 repetitions for endurance training exercises, which progressed gradually over the course of the 12-week exercise program to ensure participant safety.21,22 Participants in the intervention group were supervised at least once a week by an exercise physiologist at a center for oncology patients (Hope & Cope Wellness Centre), and patients were encouraged to perform the program at home 2 to 3 times a week. Those who were not able to exercise consistently at the center were provided with equipment and instructed on how to do the program safely at home.
By comparison, the control group received standard care, which included advice on the benefits of an active lifestyle, including exercise, but without a specific intervention. Participants were not restricted in their physical activity and/or sport participation levels, and their weekly activity levels were calculated using the Metabolic Equivalent of Task and recorded at each of the 6 time points.
Statistical analysis
Descriptive statistics were used to examine participant characteristics. The quantitative data collected through the BPI-SF measures were analyzed with JMP software (version 11.2; SAS Institute, Cary, NC). Continuous variables were tested for statistical significance (P ≤ .05) through the chi-square (categorical), analysis of variance, and nonparametric Wilcoxon tests. The analyses did not include missing data.
Results
A total of 59 young women were randomized into the intervention (n = 29) and control (n = 30) groups. Of those, 2 participants dropped out of the study because of family and time constraints, and 3 participants died, 2 from the control and 1 from the intervention group, after subsequently developing metastatic disease. Baseline data including comparative tumor characteristics, surgical interventions, and treatment interventions have been published in relation to other elements of this study.23,24 The participants had a mean age of 39.2 years (standard deviation [SD], 5.0). More than half of them had an invasive ductal carcinoma (69.5%) and were estrogen positive (78.0%), progesterone positive (74.6%), or HER2 positive (20.3%), whereas 10.2% were triple negative. Most of the participants had undergone breast-sparing procedures (86.4% lumpectomy), and 18.6% had a total mastectomy. By random chance, the intervention group had higher rates of total mastectomy (24.4% and 13.3%, respectively) and surgical reconstruction (12.2% and 6.7%, respectively) compared with the control group. Most of the women (71.2%) received chemotherapy, and all received radiation therapy. In the intervention group, 37.2% received radiation therapy localized to the axilla, and 88% received a boost of radiation to the surgical bed. Self-reported exercise diaries were returned by 15 of the 29 intervention participants, and training frequencies among them varied significantly (1-6 times a week).
The findings showed that there was little variance between the intervention and control groups in BPI-SF severity scores from T1 to T6, so the means and SDs of the BPI-SF scores were grouped at 6 time points (Table 1). There was no statistically significant difference between baseline measures at T1 (1.68; SD, 1.17) and measures at 18 months postintervention (T6: 1.46; SD, 1.37). At baseline, 87.7% of the women reported no pain (31.5%) or mild levels of pain (55.6%), and 13% reported moderate or severe pain. Over the duration of the study from T1 to T6, these primarily low levels of pain (BPI-SF, 0-4) remained consistent with a favorable shift toward having no pain (T1: 31.5%; T6: 24.4%). By 18 months postintervention, 95.7% of women reported no or mild pain, with 4.9% reporting moderate pain.
Similarly, there was little variance over time (T1-T6) and no statistically significant differences between the 2 groups in BPI-SF–measured levels of pain interference in daily activities (Table 2). Moreover, a domain analysis showed that there were no statistically significant differences in pain interference scores when comparing the type and extent of surgery (total mastectomy: 0.59 [1.17]; lumpectomy: 0.94 [1.96]). By chance – and not related directly to the objectives of this study – there was a statistically significant difference between the intervention and control groups in the interference of pain on the Enjoyment of Life domain in favor of the control group.
The sites of pain captured by the BPI-SF shed light on the preceding findings (Figure 1). At baseline (T1, postsurgery and preradiation), 37.0% of participants reported pain in the shoulder girdle–chest wall region, whereas 20.4% reported pain in the general neck–upper extremity region and 50% in other regions. Postradiation, shoulder girdle–chest wall pain was identified as the highest reported site of pain (49.1%; T2, postradiation and preintervention) and remained elevated at 3 months (T3) and 6 months (T4) postradiation (46.9% and 45.5%, respectively). At 12 and 18 months postradiation (T5 and T6), the principal focus of pain shifted once again to “other” regions at 30% and 32.5%, respectively, and the neck–upper extremity region at 10% and 15%, respectively. Shoulder girdle–chest wall pain concomitantly improved at those time points (15% and 25% respectively) but was not eliminated.
Pain levels recorded on physical examination for isolated shoulder range of movements were recently published,24 and they have been abbreviated and reproduced in this paper (Figure 2) to allow for a comparison of findings between the exercise intervention group and the control group to help determine the sensitivity of these tools for use in breast cancer patients. At baseline, pain levels with active movement were noted to be slightly greater in the intervention group for flexion and abduction.
Following the intervention, at 3 and 6 months postradiation (T3 and T4), the intervention group showed a steady decrease in pain levels in flexion and abduction, whereas the control group showed a 5-fold increase in pain with horizontal abduction. Furthermore, participants in the intervention group reported having no pain on movement 12 months postradiation (T5); however, recurrence of pain was apparent with all shoulder movements by 18 months postradiation (T6) in both the intervention and control groups.
Discussion
Previous studies have hypothesized that younger age (18-39 years), adjuvant radiotherapy, and axillary node dissection are risk factors for chronic pain in breast cancer survivors.22,25 Persistent pain is prevalent in 12% to 51% of breast cancer survivors, with up to one-third experiencing some pain more than 5 years after treatment,26,27 and our study outcomes concur with those findings. In our study, pain, as measured by the BPI-SF, was found to persist for most participants (75.6%) after the 18-month follow-up. The results of our trial showed that a 12-week exercise intervention administered postsurgery and postradiation had no statistically significant effect on long-term (18 months) pain severity and its interference in daily life. It is worth noting that body regions that had not been directly related to either surgical or radiation treatment for breast cancer were commonly identified as areas of pain but were not specifically targeted by our intervention. However, focusing on pain severity (BPI-SF), our findings suggest that the benefits of targeted upper-extremity exercise on pain in the intermediate time course of follow-up (T3, T4, and T5) was notable compared with the control group, which received standard care. The apparent recurrence of pain at 18 months in both groups was not anticipated and needs to be further investigated.
More specific objective assessments of pain on active shoulder movement identified distinct patterns of pain that could not be isolated using the BPI-SF alone. The incidence and localization of pain on movement differed between the population of women who received a specific exercise intervention and those who received standard care (Figure 2). Patterns of pain over time fluctuated in the control group, whereas the intervention group reported a linear decrease in pain. Residual pain on shoulder movement remained apparent in both groups at 18-months postradiation, but that finding was not reflected in the BPI-SF results. The literature supports our findings on persistent pain among breast cancer survivors,3,7,8,28-30 and in our study of young women carefully screened and excluded for pre-existent shoulder conditions or comorbid medical conditions, recurrent articular pain was nonetheless prevalent. It seems that unidentified or multiple factors may be part of the etiology of pain in this young adult cohort.
Although the BPI-SF is a generic measurement tool commonly used to assess and measure cancer patients’ pain levels, the lack of variance in our BPI-SF severity and interference outcomes over time (T1-T6) (Table 1, Table 2), the variety of “other” unrelated regions (Figure 1) identified by the BPI-SF, and the contrast in our findings on specific physical examination emphasize the potential limitations of this clinical tool.
Moreover, the BPI-SF has not been validated specifically for breast cancer. Harrington and colleagues have recommended using the BPI-SF to assess pain in women with breast cancer,31 but the use of a more multidimensional measurement tool that evaluates axillary, chest, trunk, and upper-limb pain may prove to be more valuable in this population.
Limitations
Recruitment of young adult women was difficult because of our stringent inclusion criteria, the long-term follow-up, and the relatively small population of breast cancer patients in this age demographic. Therefore, the duration of the recruitment phase, despite our having access to a specialized young adult and adolescent clinic in our institute, greatly surpassed the expectations we had when we designed the study. In addition, there remains an inherent bias in participants who accept participation in a study that includes exercise interventions. Potential participants who exercise regularly or have a positive inclination toward doing exercise are more likely to participate. Despite the prescription of a targeted 12-week upper-limb intervention in this study, the general activity levels of both groups may have had an impact on the significance of this study. In addition, the low adherence to the use of self-reported logs failed to capture the true compliance rates of our participants because their lack of tracking does not indicate failure to comply with the program. The use of weekly or biweekly telephone calls to monitor compliance rates of activity more vigilantly may be used in future studies.
Conclusions
Advances in clinical management of breast cancer have improved survival outcomes, and morbidity over recent years, yet symptoms such as pain remain prevalent in this population. The results of this study showed that a targeted, 12-week upper-limb exercise intervention postradiation transiently improved levels of shoulder pain without a concomitant impact on chronic pain or any positive influence on activities of daily living 18 months posttreatment. Furthermore, future studies should use a variety of measurement tools to evaluate trunk and upper-limb pain in women with breast cancer and investigate the optimal timing of postradiation exercise interventions.
Acknowledgments
The authors thank Hope & Cope, the CURE foundation, and the Jewish General Hospital Foundation/Weekend to End Breast Cancer for providing the financial resources needed to sustain this research study. They also thank the McGill Adolescent and Young Adult program for its continued support. Previous oral presentations of research Muanza TM, et al. Randomized clinical trial of a progressive exercise program for young women with breast cancer undergoing radiation therapy. Int J Radiat Oncol Biol Phys. 2015;93(3):s35-s36.
1. World Health Organization. Breast cancer: prevention and control. www.who.int/cancer/detection/breastcancer/en/. Updated 2017. Accessed September 16, 2016.
2. Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain. 2011;12(7):725-746.
3. Ernst MF, Voogd AC, Balder W, Klinkenbijl JH, Roukema JA. Early and late morbidity associated with axillary levels I-III dissection in breast cancer. J Surg Oncol. 2002;79(3):151-155; discussion 156.
4. Gulluoglu BM, Cingi A, Cakir T, Gercek A, Barlas A, Eti Z. Factors related to post-treatment chronic pain in breast cancer survivors: the interference of pain with life functions. Int J Fertil Womens Med. 2006;51(2):75-82.
5. Jung BF, Ahrendt GM, Oaklander AL, Dworkin RH. Neuropathic pain following breast cancer surgery: proposed classification and research update. Pain. 2003;104(1-2):1-13.
6. Saibil S, Fitzgerald B, Freedman OC, et al. Incidence of taxane-induced pain and distress in patients receiving chemotherapy for early-stage breast cancer: a retrospective, outcomes-based survey. Curr Oncol. 2010;17(4):42-47.
7. Tengrup I, Tennvall-Nittby L, Christiansson I, Laurin M. Arm morbidity after breast-conserving therapy for breast cancer. Acta Oncol. 2000;39(3):393-397.
8. Johansen J, Overgaard J, Blichert-Toft M, Overgaard M. Treatment of morbidity associated with the management of the axilla in breast-conserving therapy. Acta Oncol. 2000;39(3):349-354.
9. Mittmann N, Porter JM, Rangrej J, et al. Health system costs for stage-specific breast cancer: a population-based approach. Curr Oncol. 2014;21(6):281-293.
10. Page A. Keeping patients safe: transforming the work environment of nurses. Washington, DC: National Academies Press; 2004.
11. McNeely ML, Campbell K, Ospina M, et al. Exercise interventions for upper-limb dysfunction due to breast cancer treatment. Cochrane Database Syst Rev. 2010;(6):CD005211. doi:10.1002/14651858.CD005211.pub2
12. Wong P, Muanza T, Hijal T, et al. Effect of exercise in reducing breast and chest-wall pain in patients with breast cancer: a pilot study. Curr Oncol. 2012;19(3):e129-e135.
13. Fernández-Lao C, Cantarero-Villanueva I, Fernández-de-Las-Peñas C, del Moral-Ávila R, Castro-Sánchez AM, Arroyo-Morales M. Effectiveness of a multidimensional physical therapy program on pain, pressure hypersensitivity, and trigger points in breast cancer survivors: a randomized controlled clinical trial. Clin J Pain. 2012;28(2):113-121.
14. Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21(9):1660-1668.
15. Segal R, Evans W, Johnson D, et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. J Clin Oncol. 2001;19(3):657-665.
16. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129-138.
17. Kumar SP. Utilization of Brief Pain Inventory as an assessment tool for pain in patients with cancer: a focused review. Indian J Palliat Care. 2011;17(2):108-115.
18. Van Voorhis CRW, Morgan BL. Understanding power and rules of thumb for determining sample sizes. Tutor Quant Methods Psychol. 2007;3(2):43-50.
19. Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61(2):277-284.
20. Lee TS, Kilbreath SL, Refshauge KM, Pendlebury SC, Beith JM, Lee MJ. Pectoral stretching program for women undergoing radiotherapy for breast cancer. Breast Cancer Res Treat. 2007;102(3):313-321.
21. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409-1426.
22. Pollock ML, Gaesser GA, Butcher JD, et al. ACSM position stand: the recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30(6):975-991.
23. Ibrahim M, Muanza T, Smirnow N, et al. Time course of upper limb function and return-to-work post-radiotherapy in young adults with breast cancer: a pilot randomized control trial on effects of targeted exercise program. J Cancer Surviv. 2017;11(6):791-799.
24. Ibrahim M, Muanza T, Smirnow N, et al. A pilot randomized controlled trial on the effects of a progressive exercise program on the range of motion and upper extremity grip strength in young adults with breast cancer. Clin Breast Cancer. 2018;18(1):e55-e64.
25. Gärtner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. 2009;302(18):1985-1992.
26. Hayes SC, Johansson K, Stout NL, et al. Upper-body morbidity after breast cancer: incidence and evidence for evaluation, prevention, and management within a prospective surveillance model of care. Cancer. 2012;118(suppl 8):2237-2249.
27. Kärki A, Simonen R, Mälkiä E, Selfe J. Impairments, activity limitations and participation restrictions 6 and 12 months after breast cancer operation. J Rehabil Med. 2005;37(3):180-188.
28. Katz J, Poleshuck EL, Andrus CH, et al. Risk factors for acute pain and its persistence following breast cancer surgery. Pain. 2005;119(1-3):16-25.
29. Tasmuth T, von Smitten K, Hietanen P, Kataja M, Kalso E. Pain and other symptoms after different treatment modalities of breast cancer. Ann Oncol. 1995;6(5):453-459.
30. Whelan TJ, Levine M, Julian J, Kirkbride P, Skingley P. The effects of radiation therapy on quality of life of women with breast carcinoma: results of a randomized trial. Ontario Clinical Oncology Group. Cancer. 2000;88(10):2260-2266.
31. Harrington S, Gilchrist L, Sander A. Breast cancer EDGE task force outcomes: clinical measures of pain. Rehabil Oncol. 2014;32(1):13-21.
1. World Health Organization. Breast cancer: prevention and control. www.who.int/cancer/detection/breastcancer/en/. Updated 2017. Accessed September 16, 2016.
2. Andersen KG, Kehlet H. Persistent pain after breast cancer treatment: a critical review of risk factors and strategies for prevention. J Pain. 2011;12(7):725-746.
3. Ernst MF, Voogd AC, Balder W, Klinkenbijl JH, Roukema JA. Early and late morbidity associated with axillary levels I-III dissection in breast cancer. J Surg Oncol. 2002;79(3):151-155; discussion 156.
4. Gulluoglu BM, Cingi A, Cakir T, Gercek A, Barlas A, Eti Z. Factors related to post-treatment chronic pain in breast cancer survivors: the interference of pain with life functions. Int J Fertil Womens Med. 2006;51(2):75-82.
5. Jung BF, Ahrendt GM, Oaklander AL, Dworkin RH. Neuropathic pain following breast cancer surgery: proposed classification and research update. Pain. 2003;104(1-2):1-13.
6. Saibil S, Fitzgerald B, Freedman OC, et al. Incidence of taxane-induced pain and distress in patients receiving chemotherapy for early-stage breast cancer: a retrospective, outcomes-based survey. Curr Oncol. 2010;17(4):42-47.
7. Tengrup I, Tennvall-Nittby L, Christiansson I, Laurin M. Arm morbidity after breast-conserving therapy for breast cancer. Acta Oncol. 2000;39(3):393-397.
8. Johansen J, Overgaard J, Blichert-Toft M, Overgaard M. Treatment of morbidity associated with the management of the axilla in breast-conserving therapy. Acta Oncol. 2000;39(3):349-354.
9. Mittmann N, Porter JM, Rangrej J, et al. Health system costs for stage-specific breast cancer: a population-based approach. Curr Oncol. 2014;21(6):281-293.
10. Page A. Keeping patients safe: transforming the work environment of nurses. Washington, DC: National Academies Press; 2004.
11. McNeely ML, Campbell K, Ospina M, et al. Exercise interventions for upper-limb dysfunction due to breast cancer treatment. Cochrane Database Syst Rev. 2010;(6):CD005211. doi:10.1002/14651858.CD005211.pub2
12. Wong P, Muanza T, Hijal T, et al. Effect of exercise in reducing breast and chest-wall pain in patients with breast cancer: a pilot study. Curr Oncol. 2012;19(3):e129-e135.
13. Fernández-Lao C, Cantarero-Villanueva I, Fernández-de-Las-Peñas C, del Moral-Ávila R, Castro-Sánchez AM, Arroyo-Morales M. Effectiveness of a multidimensional physical therapy program on pain, pressure hypersensitivity, and trigger points in breast cancer survivors: a randomized controlled clinical trial. Clin J Pain. 2012;28(2):113-121.
14. Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21(9):1660-1668.
15. Segal R, Evans W, Johnson D, et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. J Clin Oncol. 2001;19(3):657-665.
16. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129-138.
17. Kumar SP. Utilization of Brief Pain Inventory as an assessment tool for pain in patients with cancer: a focused review. Indian J Palliat Care. 2011;17(2):108-115.
18. Van Voorhis CRW, Morgan BL. Understanding power and rules of thumb for determining sample sizes. Tutor Quant Methods Psychol. 2007;3(2):43-50.
19. Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61(2):277-284.
20. Lee TS, Kilbreath SL, Refshauge KM, Pendlebury SC, Beith JM, Lee MJ. Pectoral stretching program for women undergoing radiotherapy for breast cancer. Breast Cancer Res Treat. 2007;102(3):313-321.
21. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409-1426.
22. Pollock ML, Gaesser GA, Butcher JD, et al. ACSM position stand: the recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30(6):975-991.
23. Ibrahim M, Muanza T, Smirnow N, et al. Time course of upper limb function and return-to-work post-radiotherapy in young adults with breast cancer: a pilot randomized control trial on effects of targeted exercise program. J Cancer Surviv. 2017;11(6):791-799.
24. Ibrahim M, Muanza T, Smirnow N, et al. A pilot randomized controlled trial on the effects of a progressive exercise program on the range of motion and upper extremity grip strength in young adults with breast cancer. Clin Breast Cancer. 2018;18(1):e55-e64.
25. Gärtner R, Jensen MB, Nielsen J, Ewertz M, Kroman N, Kehlet H. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA. 2009;302(18):1985-1992.
26. Hayes SC, Johansson K, Stout NL, et al. Upper-body morbidity after breast cancer: incidence and evidence for evaluation, prevention, and management within a prospective surveillance model of care. Cancer. 2012;118(suppl 8):2237-2249.
27. Kärki A, Simonen R, Mälkiä E, Selfe J. Impairments, activity limitations and participation restrictions 6 and 12 months after breast cancer operation. J Rehabil Med. 2005;37(3):180-188.
28. Katz J, Poleshuck EL, Andrus CH, et al. Risk factors for acute pain and its persistence following breast cancer surgery. Pain. 2005;119(1-3):16-25.
29. Tasmuth T, von Smitten K, Hietanen P, Kataja M, Kalso E. Pain and other symptoms after different treatment modalities of breast cancer. Ann Oncol. 1995;6(5):453-459.
30. Whelan TJ, Levine M, Julian J, Kirkbride P, Skingley P. The effects of radiation therapy on quality of life of women with breast carcinoma: results of a randomized trial. Ontario Clinical Oncology Group. Cancer. 2000;88(10):2260-2266.
31. Harrington S, Gilchrist L, Sander A. Breast cancer EDGE task force outcomes: clinical measures of pain. Rehabil Oncol. 2014;32(1):13-21.