User login
The impact of inpatient rehabilitation on outcomes for patients with cancer
The American Cancer Society reports that 1.6 million people are diagnosed with cancer each year, of whom 78% are aged 55 years or older. The 5-year survival rate for cancer is 68%.1 Almost 15.5 million living Americans have been diagnosed with cancer.2 Many patients with cancer have difficulty walking and with activities of daily living. Patients with primary brain tumors or tumors metastatic to the brain may present with focal weakness or cognitive deficits similar to patients with stroke. Patients with tumors metastatic to the spine may have the same deficits as a patient with a traumatic spinal cord injury. Patients with metastasis to bone may have pathologic fractures of the hip or long bones. Patients may develop peripheral neuropathy associated with a paraneoplastic syndrome, chemotherapy, or critical illness neuropathy. Lehmann and colleagues evaluated 805 patients admitted to hospitals affiliated with the University of Washington Medical School with a diagnosis of cancer and found that 15% had difficulty walking and 20% had difficulty with activities of daily living.3
Many patients with cancer can benefit from inpatient rehabilitation.4,5 Study findings have shown that patients with impairments in function related to cancer are often not referred for rehabilitation. Among the reasons mentioned for that are that oncologists are more focused on treating the patients’ cancer than on their functional deficits and that specialists in rehabilitation medicine do not want to be involved with patients with complex medical problems. Rehabilitation facilities may not want to incur the costs associated with caring for patients with cancer.6
The present paper looks at the outcomes of 61 consecutive patients with cancer who were admitted to an inpatient rehabilitation facility (IRF) and received radiation therapy concurrent with rehabilitation. It compares the outcomes of the cancer patients with the outcomes of patients without cancer who were admitted with stroke or spinal cord injury, conditions more commonly treated at an IRF.
Methods
We reviewed electronic medical records of all patients with cancer admitted to the IRF from 2008 through 2013 who received radiation therapy while at the facility. We also reviewed the data of all patients without cancer admitted with a diagnosis of stroke in 2013 and all patients admitted with a diagnosis of traumatic spinal cord injury in 2012 and 2013. No patients were excluded from stroke and traumatic spinal cord injury groups.
We recorded the sex, age, diagnostic group, Functional Independence Measure (FIM) admission score, FIM discharge score, length of stay (LoS) in the IRF, place of discharge of each patient (eg, home, acute care, or subacute care), and calculated the FIM efficiency score (change in FIM/LoS) for each patient. The FIM is an instrument that has 18 items measuring mobility, participation in activities of daily living, ability to communicate, and cognitive function.7 Each item is scored from 1 to 7, with 1 denoting that the patient cannot perform the task and 7 that the activity can be performed independently. The minimum score is 18 (complete dependence), and the maximum score is 126 (independent function). Thirteen items compose the motor FIM score: eating, grooming, bathing, dressing upper body, dressing lower body, toileting, bladder management, management of bowel, transfer to bed or wheelchair, transfer to toilet, tub transfer, walking (or wheelchair use), and climbing stairs. Five items – comprehension, expression, social interaction, problem solving, and memory – compose the cognitive FIM score.
We used a 1-way analysis of variance to evaluate differences between age and cancer type, age and diagnostic group, admission FIM score and cancer type, discharge FIM score and cancer type, change in FIM and cancer type, LoS and cancer type, and LoS and diagnostic group. The Pearson chi-square test was used to test the goodness of fit between the place of disposition and diagnostic group. The paired t test was used to evaluate the improvement in FIM of the patients who were in the cancer groups. The Tukey Simultaneous Tests for Differences of Means was used to compare the FIM efficiency scores of the groups. A 2-sample t test was used to evaluate the factors associated with the need for transfer from the IRF to the acute medical service.
Results
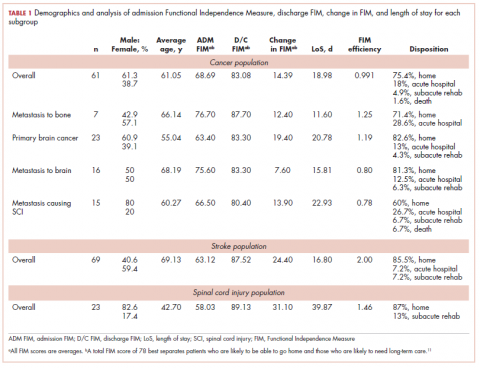
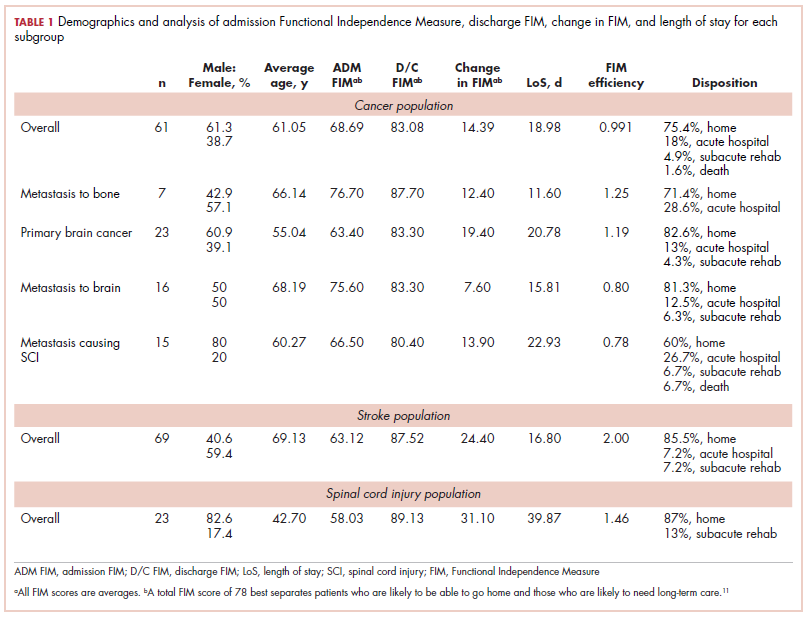
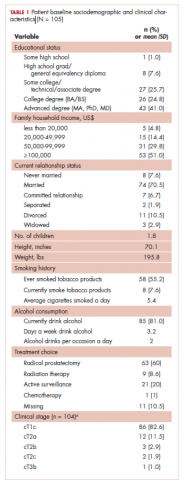
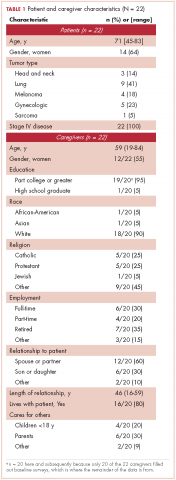
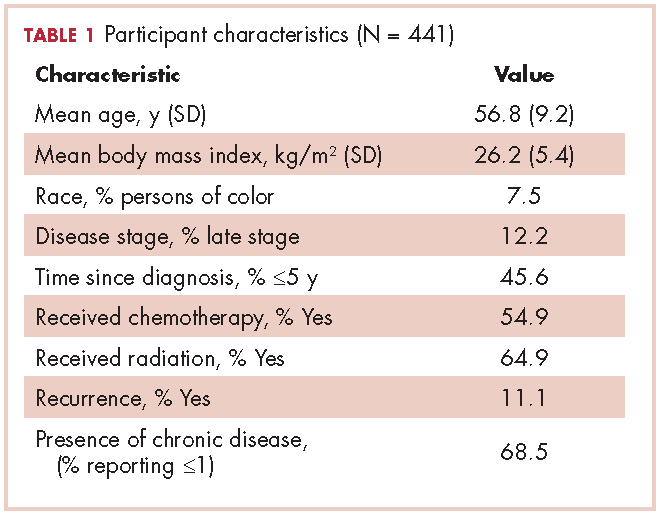
The demographic characteristics of the patients in the study and the admission and discharge FIM scores are reported in Table 1. There were initially 62 cancer patients in the radiation group, which was further divided into 4 subgroups based on the site of the primary tumor or metastasis. In all, 23 had a primary malignant brain tumor and received radiation and temozolomide. Sixteen patients had malignancies metastatic to the brain, 15 patients had tumors metastatic to the spine, and 7 had tumors metastatic to the long bones. One patient had laryngeal cancer and was excluded from the study because we did not think that we could do an analysis of a group with only 1 patient. The final number of patients in the cancer group was therefore 61. There were 69 patients in the stroke group and 23 in the spinal cord injury group.
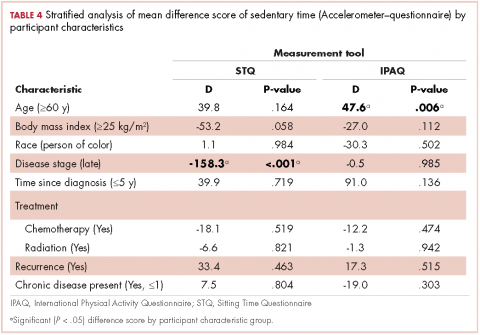
We report improvement in total FIM, motor FIM, and cognitive FIM scores and were able to identify all 18 of the items of the FIM score on 60 of the 61 patients in the cancer group. Improvement in total FIM of the 61 patients in the cancer groups was significant at P P P = .05. Just over 75% of the patients in the cancer group had sufficient enough improvement in their level of function that they were able to return to their homes (Table 1). The average FIM score at the time of discharge was 83.08. This was not significantly different than the level of function of patients discharged after stroke (87.52) or traumatic spinal cord injury (89.13).
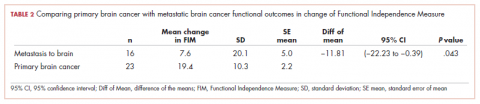
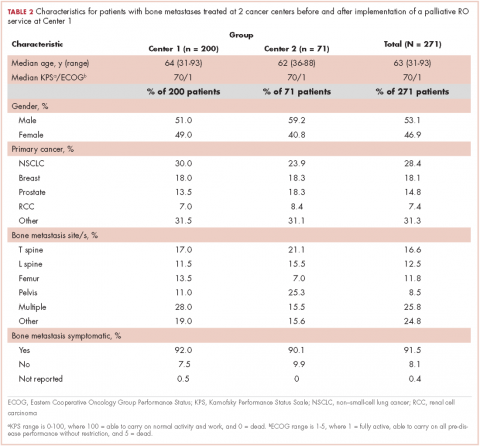
The patients with primary brain tumors were younger than the patients with cancer metastatic to the brain (P = .013). The patients with a primary brain tumor had lower admission FIM scores than patients with tumors metastatic to the brain (P = .027). The patients with a primary brain tumor had a greater increase in FIM score than patients with metastasis to the brain (P = .043; Table 2). There was not a significant difference between these 2 groups in FIM score at discharge or in the likelihood of discharge to home (Table 1). The FIM efficiency score was 1.12 for the patients in the primary brain tumor group and .80 in those with metastasis to the brain. This difference was not significant P = .96.
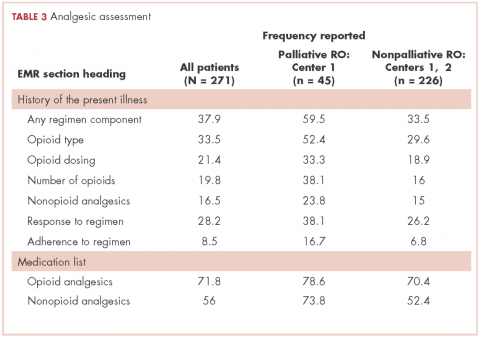
There were 69 patients in the stroke group. We compared the 39 patients with primary or metastatic brain lesion to the stroke group. The patients with primary or metastatic cancer of the brain were younger than the patients with stroke, 60.4 years old versus 69.1 years old (P = .004). The patients in the combined cancer group had a higher admission FIM score compared with the stroke patients (68.4 vs 63.12; P = .05). The discharge FIM scores were 83.3 in the combined cancer group and 87.5 in the stroke group (Table 1). This difference was not significant, but the improvement in the combined cancer group (14.6) was less than the improvement in the stroke group (24.40; P = .002) (Table 3).
The average LoS in the IRF was 18.7 days in the combined cancer group and 16.8 days in the stroke group. This difference was not significant. An average of 82% of the patients in the primary tumor or brain metastasis group and 85.5% of the patients in the stroke group were discharged to home. This difference was not significant. The FIM efficiency score of the patients in the stroke group was 2.0. This was significantly greater than the score for the patients in the metastasis to the brain group (0.80; P = .044) but not significantly greater than the primary brain cancer group (1.19; P = .22).
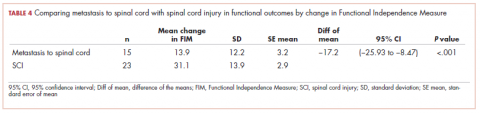
There were 23 patients in the traumatic spinal cord injury group. A comparison of the patients with tumors metastatic to the spine and patients with traumatic spinal cord injury showed that the patients in the cancer group were older (60.27 and 42.70 years, respectively; P = .001). In all, 80% of patients with tumors metastatic to the spine were men. This was not significantly different from the percentage of men in the traumatic spinal cord injury group (82.6%; Table 1). The admission FIM score of the patients with cancer was 66.5 (standard deviation [SD], 13.3) and 58.03 (SD, 15.1) in the patients with a traumatic spinal cord injury (Table 1). The FIM score at discharge was 80.4 (SD, 19.1) in the patients with cancer and 89.1 (SD, 20.3) in the patients with a traumatic spinal cord injury (Table 1). Neither of these were statistically significant. The improvement in patients with cancer was 13.9 (SD, 12.2) and 31.1 (SD, 13.9) in the traumatic spinal cord injured patients. This difference was significant (P
The median LoS was 18.98 days in the cancer metastasis to spine group (interquartile range [IQR] is the 25th-75th percentile, 12-30 days). In the traumatic group the median LoS was 23 days (IQR, 16-50 days). This difference was not significant (P = .14 Mann-Whitney test). The mean FIM efficiency score was 1.46 in the traumatic spinal cord injury group and .78 in the group with cancer metastatic to the spine. This difference was not significant (P = .72). Sixty percent of the patients in the cancer group were discharged to home, and 87% of patients in the traumatic spinal cord group were discharged to home. This difference was not significant (P = .12; Fisher exact test).
As far as we can ascertain, this is the first paper that has looked at the outcomes of patients receiving rehabilitation concurrent with radiation of the long bones. The average improvement in FIM was 12.4 (Table 1). The LoS was 11.6 days, and the FIM efficiency was 1.25. In all, 71.4% made enough progress to go home.
Of the total number of cancer patients, 18% were transferred to the acute medical service of the hospital (Table 1). Neither age, sex, type of cancer, nor admission FIM score were associated with the need for transfer to acute hospital care. Change in FIM score was inversely associated with transfer to acute hospital care (P = .027). Patients whose function did not improve with rehabilitation were most likely to be transferred back to acute hospital care.
Discussion
Radiation therapy is considered a service that is provided to people who come for treatment as an outpatient. Caregivers may have difficulty transporting patients to radiation if the patient has deficits in mobility. This may be particularly true if the patient is heavy, the caregivers are frail, or perhaps if they live in rural settings where there is no wheelchair-accessible public transportation. There are many factors that help determine whether a patient with functional deficits can be discharged to his or her home. These include sex, age, marital status, family and/or community support, income, and insurance.8 The FIM is an instrument that indicates how much help a patient needs with mobility and self-care skills. It also correlates with the amount of time that caregivers must spend helping a patient.9 Study findings have shown that the FIM score is an important determinant of whether a patient can be discharged to home. The total FIM score is as useful as an analysis of the components of the FIM score in predicting whether a patient can return to the community.10,11 Reistetter and colleagues found a total FIM score of 78 to be the score that best separates patients who are likely to be able to go home and patients who are likely to need long-term care.11 Bottemiller and colleagues10 reported that 37% of patients with total discharge FIM scores of less than 40 were discharged to home. They reported that 62% of patients with FIM scores between 40 and 79 were discharged to home, and 88% of patients with scores of 80 or above were discharged to home.10 The goal in bringing patients to the IRF was to accept and treat patients with reasonable community support and potential to achieve a functional level compatible with discharge to the community. Most patients in each of the cancer groups were able to reach an FIM score of 78 to 80 and to be discharged to home.
Most of the patients in the cancer groups had underlying problems that are not considered curable. The primary goal was to enable the patients to have some time at home with their families before requiring readmission to a hospital or hospice care. Reasonable LoS and rate of progress are now expected or required by third-party payors and hospital administrators. Physicians at the Mayo Clinic have indicated that a rehabilitation service should aim for an FIM efficiency score of at least .6 points per day.10 The FIM efficiency of patients in each of the 4 cancer subgroups in this study was higher than this level.
J. Herbert Dietz, Jr was an early advocate of the need to provide comprehensive rehabilitation services for patients with cancer. He first described his work in 1969.12 Since that time, there have been many papers that have documented the benefits of IRF for patients with cancer. O’Toole and Golden have shown outcomes of a large series of patients from an IRF. They reported that at the time of admission, 14% of patients could ambulate, but at discharge, 80% could ambulate without hands-on assistance. They reported significant improvements in continence, FIM score, and score on the Karnofsky Performance Scale.13 Marciniak,14 Hunter,15 Shin,16 and Cole,17 and their respective colleagues have all shown that patients with many different types of cancer benefit from rehabilitation at the IRF level. Gallegos-Kearin and colleagues4 reported on the care of 115,570 patients admitted to IRF with cancer from 2002 to 2014. Patients had significant improvement in function, with more than 70% of patients discharged to home.4 Ng and colleagues studied a group of 200 patients who received IRF care and found there was significant improvement in function. Ninety-four percent of patients rated their stay as either extremely good or very good.5
Metastasis to the spine is a common problem. It is found in 30% of cancer patients at autopsy. The most common sources of metastasis to the spine are breast, lung, prostate, kidney, and thyroid.18 Multiple myeloma and lymphoma may also involve the spine. Several authors have shown that these patients benefit from inpatient rehabilitation. Mckinley and colleagues19 have noted that patients with metastasis to the spine make significant improvement with care at an IRF. Compared with patients with a traumatic spinal cord injury, the cancer patients had shorter LoS, smaller improvement in FIM, equal FIM efficiency (FIM gain/LoS), and equal success in making enough progress to be discharged to home.19 Eriks and colleagues showed that patients at an IRF in Amsterdam made significant improvement in function as measured by the Barthel’s Index.20 Tang .,21 and Parsch22 and their respective colleagues, Murray,23 and New24 and colleagues have published findings confirming that patients with spinal cord injury caused by metastasis to the spine make significant progress with inpatient rehabilitation programs. The present study adds to the literature by showing that patients with metastasis to the spine who are receiving radiation can make progress and be discharged to the community.
There are 24,000 new cases of primary malignant brain tumors in the United States each year.25 The incidence of metastatic cancer to the brain has been estimated to be 100,000 cases per year in the United States. The most common cancer sources are lung, breast, melanoma, kidney, and colon.26,27 The first study of patients admitted to an IRF for treatment of brain tumors was published in 1998 by Huang and colleagues28 who compared the outcomes of 63 patients with brain tumors with the outcomes of 63 patients with stroke. They reported that the patients with the brain tumors made significant improvement in function. There was not a significant difference between the 2 groups of patients in improvement in function, FIM efficiency, or success in discharging the patients to home.28 Greenberg29 and Bartolo30 and their respective colleagues compared the outcomes of patients admitted with brain tumors and patients with stroke and found that improvement in function and discharge to home was similar in the 2 groups. In 2000, Huang and his same colleagues31 compared a group of patients with brain tumors to a group of patients with traumatic brain injury. They found significant improvement in the function of the patients with brain tumors. Patients in the traumatic brain injury group made more progress but had longer LoS. FIM efficiency was not significantly different between the groups.31
Three papers have reported outcomes of patients who received radiation concurrent with inpatient rehabilitation. Tang and colleagues32 reported 63 patients, of whom 48% percent received radiation concurrent with rehabilitation. The patients who received radiation made significant gains in function, and more than 70% were discharged to home. There was no difference in the outcomes of the patients in the radiation and nonradiation groups.32 Marciniak33 and O’Dell34 and their colleagues also reported that patients with brain tumors that required radiation therapy can benefit from inpatient rehabilitation. The present paper is the fourth (with the largest patient group) to show that patients with primary and metastatic tumors to the brain can benefit from a program that provides radiation concurrent with inpatient rehabilitation. We have shown that patients can achieve functional levels and rates of discharge to home that are not significantly different from those of the most commonly admitted group of patients to IRF – patients with stroke.
In the present study, 18% of all of the cancer patients were transferred to medical services and/or acute hospital care (Table 1). This is consistent with a paper by Asher and colleagues35 who reported that 17.4% of patients at an IRF with a diagnosis of cancer required transfer back to medical service, and that low admission motor FIM score correlated with the likelihood of transfer back to medical service. In the present paper, the total admission FIM score was not related to the likelihood of return to medical service, although a lack of improvement in the FIM score did correlate with transfer to medical service.
All of the papers we reviewed found that appropriately selected patients with cancer make significant improvement in function with treatment at an IRF. Tang and colleagues have also shown that for patients with malignant brain tumors and metastasis to the spine, improvement in function correlates with increased survival.32 Our paper confirms that patients with primary malignant brain tumors, malignant tumors metastatic to the brain or spine, and tumors metastatic to long bones may benefit from rehabilitation concurrent with radiation. Rehabilitation units are traditionally associated with treating patients with stroke and spinal cord injury. The patients in our study had cancer and were receiving radiation therapy. They had significant improvement in function and FIM efficiency scores that are not below the threshold set as expected for care at an IRF. Most patients in our study achieved a functional level consistent with what is needed to go home.
There is a prospective payment or reimbursement system for rehabilitation units.36 The payments are based on the admitting diagnosis, the admission FIM score, the age of the patient, and comorbidities. There are 4 tiers for comorbidities with no additional payments for patients in tier 0 but with additional payments for patients with conditions that qualify for tiers 1 through 3. The highest payments are for patients in tier 1. Examples of conditions that can increase payment include morbid obesity, congestive heart failure, vocal cord paralysis, and the need for hemodialysis. There is no increased payment for provision of radiation therapy. There are no reports on the feasibility, in terms of finances, of providing radiation on an IRF. We asked the finance office of the Albany Medical Center to comment on the cost to the hospital of providing radiation therapy to patients on the rehabilitation unit. The hospital’s finance department reviewed available data and reported that the variable cost of providing radiation therapy is about 6.5% of the revenue collected from third-party payors for caring for patients who receive that service (personal communication from the finance office of Albany Medical Center to George Forrest, 2015). Our findings suggest that the Centers for Medicare & Medicaid Services should make an adjustment to the payment system to support the cost of providing radiation to patients at an IRF. Even under the current payment system, for a hospital that has the equipment and personnel to provide radiation treatments, the variable cost of 6.5% of revenue should not be an absolute barrier to providing this service.
Limitations
This study reports on the experience of only 1 facility. The number of patients in the radiation group is greater than the number of patients in any previous report of people receiving radiation at an IRF, but the statistician does not think it is large enough to allow statistical analysis of covariates such as age, sex, and comorbid conditions. In addition, we did not investigate all of the factors that influence the type of care patients are offered and their LoS, such as hospital policy, insurance coverage, income, and family structure.
Conclusions
Acute care medical units are now challenged to both reduce LoS and reduce the number of patients who are readmitted to the hospital. Rehabilitation units are challenged to maintain census, as government and private payors are shifting patients from acute rehabilitation units to subacute rehabilitation units. We found that patients with cancer who need radiation are a population of patients who are seen by payors as needing to be in a facility with excellent nursing, therapy, and comprehensive physician services. A comprehensive cancer care program within a rehabilitation unit can be a great benefit to the acute care services, the IRF, and, most importantly, patients and their families.
1. American Cancer Society. Cancer facts & figures 2016. Atlanta, GA: American Cancer Society; 2016.
2. National Cancer Institute: Office of cancer survivorship: statistics. https://cancercontrol.cancer.gov/ocs/statistics/statistics.html. Updated October 17, 2016. Accessed April 21, 2018.
3. Lehmann JF, DeLisa JA, Warren CG, deLateur BJ, Bryant PL, Nicholson CG. Cancer rehabilitation: assessment of need, development and evaluation of a model of care. Arch Phys Med Rehabil. 1978;59(9):410-419.
4. Gallegos-Kearin V, Knowlton SE, Goldstein R, et al. Outcome trends of adult cancer patients receiving inpatient rehabilitation: a 13-year review [published online Feb 21, 2018]. Am J Phys Med Rehabil. doi:10.1097/PHM.0000000000000911
5. Ng AH, Gupta E, Fontillas RC, et al. Patient-reported usefulness of acute cancer rehabilitation. PM R. 2017;9(11):1135-1143.
6. Cheville AL, Kornblith AB, Basford JR. An examination of the causes for the underutilization of rehabilitation services among people with advanced cancer. Am J Phys Med Rehabil. 2011;90(5 suppl 1):S27-S37.
7. Cohen ME, Marino RJ. The tools of disability outcomes research functional status measures. Arch Phys Med Rehabil. 2000;81(12 suppl 2):S21-S29.
8. Nguyen VQ, PrvuBettger J, Guerrier T, et al. Factors associated with discharge to home versus discharge to institutional care after inpatient stroke rehabilitation. Arch Phys Med Rehabil. 2015;96(7):1297-1303.
9. Forrest G, Schwam A, Cohen E. Time of care required by patients discharged from a rehabilitation unit. Am J Phys Med Rehabil. 2002;81(1):57-62.
10. Bottemiller KL, Bieber PL, Basford JR, Harris M. FIM scores, FIM efficiency and discharge following inpatient stroke rehabilitation. Rehabil Nurs. 2006;31(1):22-25.
11. Reistetter TA, Graham JE, Deutsch A, Granger CV, Markello S, Ottenbacher KJ. Utility of functional status for classifying community versus institutional discharges after inpatient rehabilitation for stroke. Arch Phys Med Rehabil. 2010;91(3):345-350.
12. Dietz JH Jr. Rehabilitation of the cancer patient. Med Clin North Am. 1969;53(3):607-624.
13. O'Toole DM, Golden AM. Evaluating cancer patients for rehabilitation potential. West J Med. 1991;155(4):384-387.
14. Marciniak CM, Sliwa JA, Spill G, Heinemann AW, Semik PE. Functional outcome following rehabilitation of the cancer patient. Arch Phys Med Rehabil. 1996;77(1):54-57.
15. Hunter EG, Baltisberger J. Functional outcomes by age for inpatient cancer rehabilitation: a retrospective chart review. J Appl Gerontol. 2013;32(4):443-456.
16. Shin KY, Guo Y, Konzen B, Fu J, Yadav R, Bruera E. Inpatient cancer rehabilitation: the experience of a national comprehensive cancer center. Am J Phys Med Rehabil. 2011;90(5 suppl 1):S63-S68.
17. Cole RP, Scialla S, Bednarz L. Functional recovery in cancer rehabilitation. Arch Phys Med Rehabil. 2000;81(5):623-627.
18. White AP, Kwon BK, Lindskog DM, Friedlaender GE, Grauer JN. Metastatic disease of the spine. J Am Acad Orthop Surg. 2006;14(11):587-598.
19. McKinley WO, Huang ME, Tewksbury MA. Neoplastic vs traumatic spinal cord injury: an inpatient rehabilitation comparison. Am J Phys Med Rehabil. 2000;79(2):138-144.
20. Eriks IE, Angenot EL, Lankhorst GJ. Epidural metastatic spinal cord compression: functional outcome and survival after inpatient rehabilitation. Spinal Cord. 2004;42(4):235-239.
21. Tang V, Harvey D, Park Dorsay J, Jiang S, Rathbone MP. Prognostic indicators in metastatic spinal cord compression: using functional independence measure and Tokuhashi scale to optimize rehabilitation planning. Spinal Cord. 2007;45(10):671-677.
22. Parsch D, Mikut R, Abel R. Postacute management of patients with spinal cord injury due to metastatic tumor disease: survival and efficacy of rehabilitation. Spinal Cord. 2003;41:205-210.
23. Murray PK. Functional outcome and survival in spinal cord injury secondary to neoplasia. Cancer. 1985;55:197-201.
24. New PW. Functional outcomes and disability after nontraumatic spinal cord injury rehabilitation: results from a retrospective study. Arch Phys Med Rehabil. 2005;86(2):250-261
25. Central Brain Tumor Registry of the United States: 2016 CBTRUS fact sheet. www.cbtrus.org/factsheet/factsheet.html. Updated 2017. Accessed May 28, 2016.
26. Memorial Sloan Kettering Cancer Center: Metastatic brain tumors & secondary brain cancer. https://www.mskcc.org/cancer-care/types/brain-tumors-metastatic. Updated 2018. Accessed April 21, 2018.
27. Bruckner JC, Brown PD, O'Neill BP, Meyer FB, Wetmore CJ, Uhm JH. Central nervous system tumors. Mayo Clin Proc. 2007;82(10):1271-1286.
28. Huang ME, Cifu DX, Keyser-Marcus L. Functional outcome after brain tumor and acute stroke: a comparative analysis. Arch Phys Med Rehabil. 1998;79(11):1386-1390.
29. Greenberg E, Treger I, Ring H. Rehabilitation outcomes in patients with brain tumors and acute stroke: comparative study of inpatient rehabilitation. Am J Phys Med Rehabil. 2006;85(7):568-573.
30. Bartolo M, Zucchella C, Pace A, et al. Early rehabilitation after surgery improves functional outcomes in inpatients with brain tumours. J Neurooncol. 2012;107(3);537-544.
31. Huang ME, Cifu DX, Keyser-Marcus L. Functional outcomes in patients with brain tumor after inpatient rehabilitation: comparison with traumatic brain injury. Am J Phys Med Rehabil. 2000;79(4):327-335.
32. Tang V, Rathbone M, Park Dorsay J, Jiang S, Harvey D. Rehabilitation in primary and metastatic brain tumours: impact of functional outcomes on survival. J Neurol. 2008;255(6):820-827.
33. Marciniak CM, Sliwa JA, Heinemann AW, Semik PE. Functional outcomes of persons with brain tumors after inpatient rehabilitation. Arch Phys Med Rehabil. 2001;82(4):457-463.
34. O'Dell MW, Barr K, Spanier D, Warnick RE. Functional outcome of inpatient rehabilitation in persons with brain tumors. Arch Phys Med Rehabil. 1998;79(12):1530-1534.
35. Asher A, Roberts PS, Bresee C, Zabel G, Riggs RV, Rogatko A. Transferring inpatient rehabilitation facility cancer patients back to acute care (TRIPBAC). PM R. 2014;6(9):808-813.
36. Centers for Medicare and Medicaid Services: Inpatient rehabilitation facilities. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/InpatientRehab.html. Published March 5, 2012. Accessed May 21, 2018.
The American Cancer Society reports that 1.6 million people are diagnosed with cancer each year, of whom 78% are aged 55 years or older. The 5-year survival rate for cancer is 68%.1 Almost 15.5 million living Americans have been diagnosed with cancer.2 Many patients with cancer have difficulty walking and with activities of daily living. Patients with primary brain tumors or tumors metastatic to the brain may present with focal weakness or cognitive deficits similar to patients with stroke. Patients with tumors metastatic to the spine may have the same deficits as a patient with a traumatic spinal cord injury. Patients with metastasis to bone may have pathologic fractures of the hip or long bones. Patients may develop peripheral neuropathy associated with a paraneoplastic syndrome, chemotherapy, or critical illness neuropathy. Lehmann and colleagues evaluated 805 patients admitted to hospitals affiliated with the University of Washington Medical School with a diagnosis of cancer and found that 15% had difficulty walking and 20% had difficulty with activities of daily living.3
Many patients with cancer can benefit from inpatient rehabilitation.4,5 Study findings have shown that patients with impairments in function related to cancer are often not referred for rehabilitation. Among the reasons mentioned for that are that oncologists are more focused on treating the patients’ cancer than on their functional deficits and that specialists in rehabilitation medicine do not want to be involved with patients with complex medical problems. Rehabilitation facilities may not want to incur the costs associated with caring for patients with cancer.6
The present paper looks at the outcomes of 61 consecutive patients with cancer who were admitted to an inpatient rehabilitation facility (IRF) and received radiation therapy concurrent with rehabilitation. It compares the outcomes of the cancer patients with the outcomes of patients without cancer who were admitted with stroke or spinal cord injury, conditions more commonly treated at an IRF.
Methods
We reviewed electronic medical records of all patients with cancer admitted to the IRF from 2008 through 2013 who received radiation therapy while at the facility. We also reviewed the data of all patients without cancer admitted with a diagnosis of stroke in 2013 and all patients admitted with a diagnosis of traumatic spinal cord injury in 2012 and 2013. No patients were excluded from stroke and traumatic spinal cord injury groups.
We recorded the sex, age, diagnostic group, Functional Independence Measure (FIM) admission score, FIM discharge score, length of stay (LoS) in the IRF, place of discharge of each patient (eg, home, acute care, or subacute care), and calculated the FIM efficiency score (change in FIM/LoS) for each patient. The FIM is an instrument that has 18 items measuring mobility, participation in activities of daily living, ability to communicate, and cognitive function.7 Each item is scored from 1 to 7, with 1 denoting that the patient cannot perform the task and 7 that the activity can be performed independently. The minimum score is 18 (complete dependence), and the maximum score is 126 (independent function). Thirteen items compose the motor FIM score: eating, grooming, bathing, dressing upper body, dressing lower body, toileting, bladder management, management of bowel, transfer to bed or wheelchair, transfer to toilet, tub transfer, walking (or wheelchair use), and climbing stairs. Five items – comprehension, expression, social interaction, problem solving, and memory – compose the cognitive FIM score.
We used a 1-way analysis of variance to evaluate differences between age and cancer type, age and diagnostic group, admission FIM score and cancer type, discharge FIM score and cancer type, change in FIM and cancer type, LoS and cancer type, and LoS and diagnostic group. The Pearson chi-square test was used to test the goodness of fit between the place of disposition and diagnostic group. The paired t test was used to evaluate the improvement in FIM of the patients who were in the cancer groups. The Tukey Simultaneous Tests for Differences of Means was used to compare the FIM efficiency scores of the groups. A 2-sample t test was used to evaluate the factors associated with the need for transfer from the IRF to the acute medical service.
Results
The demographic characteristics of the patients in the study and the admission and discharge FIM scores are reported in Table 1. There were initially 62 cancer patients in the radiation group, which was further divided into 4 subgroups based on the site of the primary tumor or metastasis. In all, 23 had a primary malignant brain tumor and received radiation and temozolomide. Sixteen patients had malignancies metastatic to the brain, 15 patients had tumors metastatic to the spine, and 7 had tumors metastatic to the long bones. One patient had laryngeal cancer and was excluded from the study because we did not think that we could do an analysis of a group with only 1 patient. The final number of patients in the cancer group was therefore 61. There were 69 patients in the stroke group and 23 in the spinal cord injury group.
We report improvement in total FIM, motor FIM, and cognitive FIM scores and were able to identify all 18 of the items of the FIM score on 60 of the 61 patients in the cancer group. Improvement in total FIM of the 61 patients in the cancer groups was significant at P P P = .05. Just over 75% of the patients in the cancer group had sufficient enough improvement in their level of function that they were able to return to their homes (Table 1). The average FIM score at the time of discharge was 83.08. This was not significantly different than the level of function of patients discharged after stroke (87.52) or traumatic spinal cord injury (89.13).
The patients with primary brain tumors were younger than the patients with cancer metastatic to the brain (P = .013). The patients with a primary brain tumor had lower admission FIM scores than patients with tumors metastatic to the brain (P = .027). The patients with a primary brain tumor had a greater increase in FIM score than patients with metastasis to the brain (P = .043; Table 2). There was not a significant difference between these 2 groups in FIM score at discharge or in the likelihood of discharge to home (Table 1). The FIM efficiency score was 1.12 for the patients in the primary brain tumor group and .80 in those with metastasis to the brain. This difference was not significant P = .96.
There were 69 patients in the stroke group. We compared the 39 patients with primary or metastatic brain lesion to the stroke group. The patients with primary or metastatic cancer of the brain were younger than the patients with stroke, 60.4 years old versus 69.1 years old (P = .004). The patients in the combined cancer group had a higher admission FIM score compared with the stroke patients (68.4 vs 63.12; P = .05). The discharge FIM scores were 83.3 in the combined cancer group and 87.5 in the stroke group (Table 1). This difference was not significant, but the improvement in the combined cancer group (14.6) was less than the improvement in the stroke group (24.40; P = .002) (Table 3).
The average LoS in the IRF was 18.7 days in the combined cancer group and 16.8 days in the stroke group. This difference was not significant. An average of 82% of the patients in the primary tumor or brain metastasis group and 85.5% of the patients in the stroke group were discharged to home. This difference was not significant. The FIM efficiency score of the patients in the stroke group was 2.0. This was significantly greater than the score for the patients in the metastasis to the brain group (0.80; P = .044) but not significantly greater than the primary brain cancer group (1.19; P = .22).
There were 23 patients in the traumatic spinal cord injury group. A comparison of the patients with tumors metastatic to the spine and patients with traumatic spinal cord injury showed that the patients in the cancer group were older (60.27 and 42.70 years, respectively; P = .001). In all, 80% of patients with tumors metastatic to the spine were men. This was not significantly different from the percentage of men in the traumatic spinal cord injury group (82.6%; Table 1). The admission FIM score of the patients with cancer was 66.5 (standard deviation [SD], 13.3) and 58.03 (SD, 15.1) in the patients with a traumatic spinal cord injury (Table 1). The FIM score at discharge was 80.4 (SD, 19.1) in the patients with cancer and 89.1 (SD, 20.3) in the patients with a traumatic spinal cord injury (Table 1). Neither of these were statistically significant. The improvement in patients with cancer was 13.9 (SD, 12.2) and 31.1 (SD, 13.9) in the traumatic spinal cord injured patients. This difference was significant (P
The median LoS was 18.98 days in the cancer metastasis to spine group (interquartile range [IQR] is the 25th-75th percentile, 12-30 days). In the traumatic group the median LoS was 23 days (IQR, 16-50 days). This difference was not significant (P = .14 Mann-Whitney test). The mean FIM efficiency score was 1.46 in the traumatic spinal cord injury group and .78 in the group with cancer metastatic to the spine. This difference was not significant (P = .72). Sixty percent of the patients in the cancer group were discharged to home, and 87% of patients in the traumatic spinal cord group were discharged to home. This difference was not significant (P = .12; Fisher exact test).
As far as we can ascertain, this is the first paper that has looked at the outcomes of patients receiving rehabilitation concurrent with radiation of the long bones. The average improvement in FIM was 12.4 (Table 1). The LoS was 11.6 days, and the FIM efficiency was 1.25. In all, 71.4% made enough progress to go home.
Of the total number of cancer patients, 18% were transferred to the acute medical service of the hospital (Table 1). Neither age, sex, type of cancer, nor admission FIM score were associated with the need for transfer to acute hospital care. Change in FIM score was inversely associated with transfer to acute hospital care (P = .027). Patients whose function did not improve with rehabilitation were most likely to be transferred back to acute hospital care.
Discussion
Radiation therapy is considered a service that is provided to people who come for treatment as an outpatient. Caregivers may have difficulty transporting patients to radiation if the patient has deficits in mobility. This may be particularly true if the patient is heavy, the caregivers are frail, or perhaps if they live in rural settings where there is no wheelchair-accessible public transportation. There are many factors that help determine whether a patient with functional deficits can be discharged to his or her home. These include sex, age, marital status, family and/or community support, income, and insurance.8 The FIM is an instrument that indicates how much help a patient needs with mobility and self-care skills. It also correlates with the amount of time that caregivers must spend helping a patient.9 Study findings have shown that the FIM score is an important determinant of whether a patient can be discharged to home. The total FIM score is as useful as an analysis of the components of the FIM score in predicting whether a patient can return to the community.10,11 Reistetter and colleagues found a total FIM score of 78 to be the score that best separates patients who are likely to be able to go home and patients who are likely to need long-term care.11 Bottemiller and colleagues10 reported that 37% of patients with total discharge FIM scores of less than 40 were discharged to home. They reported that 62% of patients with FIM scores between 40 and 79 were discharged to home, and 88% of patients with scores of 80 or above were discharged to home.10 The goal in bringing patients to the IRF was to accept and treat patients with reasonable community support and potential to achieve a functional level compatible with discharge to the community. Most patients in each of the cancer groups were able to reach an FIM score of 78 to 80 and to be discharged to home.
Most of the patients in the cancer groups had underlying problems that are not considered curable. The primary goal was to enable the patients to have some time at home with their families before requiring readmission to a hospital or hospice care. Reasonable LoS and rate of progress are now expected or required by third-party payors and hospital administrators. Physicians at the Mayo Clinic have indicated that a rehabilitation service should aim for an FIM efficiency score of at least .6 points per day.10 The FIM efficiency of patients in each of the 4 cancer subgroups in this study was higher than this level.
J. Herbert Dietz, Jr was an early advocate of the need to provide comprehensive rehabilitation services for patients with cancer. He first described his work in 1969.12 Since that time, there have been many papers that have documented the benefits of IRF for patients with cancer. O’Toole and Golden have shown outcomes of a large series of patients from an IRF. They reported that at the time of admission, 14% of patients could ambulate, but at discharge, 80% could ambulate without hands-on assistance. They reported significant improvements in continence, FIM score, and score on the Karnofsky Performance Scale.13 Marciniak,14 Hunter,15 Shin,16 and Cole,17 and their respective colleagues have all shown that patients with many different types of cancer benefit from rehabilitation at the IRF level. Gallegos-Kearin and colleagues4 reported on the care of 115,570 patients admitted to IRF with cancer from 2002 to 2014. Patients had significant improvement in function, with more than 70% of patients discharged to home.4 Ng and colleagues studied a group of 200 patients who received IRF care and found there was significant improvement in function. Ninety-four percent of patients rated their stay as either extremely good or very good.5
Metastasis to the spine is a common problem. It is found in 30% of cancer patients at autopsy. The most common sources of metastasis to the spine are breast, lung, prostate, kidney, and thyroid.18 Multiple myeloma and lymphoma may also involve the spine. Several authors have shown that these patients benefit from inpatient rehabilitation. Mckinley and colleagues19 have noted that patients with metastasis to the spine make significant improvement with care at an IRF. Compared with patients with a traumatic spinal cord injury, the cancer patients had shorter LoS, smaller improvement in FIM, equal FIM efficiency (FIM gain/LoS), and equal success in making enough progress to be discharged to home.19 Eriks and colleagues showed that patients at an IRF in Amsterdam made significant improvement in function as measured by the Barthel’s Index.20 Tang .,21 and Parsch22 and their respective colleagues, Murray,23 and New24 and colleagues have published findings confirming that patients with spinal cord injury caused by metastasis to the spine make significant progress with inpatient rehabilitation programs. The present study adds to the literature by showing that patients with metastasis to the spine who are receiving radiation can make progress and be discharged to the community.
There are 24,000 new cases of primary malignant brain tumors in the United States each year.25 The incidence of metastatic cancer to the brain has been estimated to be 100,000 cases per year in the United States. The most common cancer sources are lung, breast, melanoma, kidney, and colon.26,27 The first study of patients admitted to an IRF for treatment of brain tumors was published in 1998 by Huang and colleagues28 who compared the outcomes of 63 patients with brain tumors with the outcomes of 63 patients with stroke. They reported that the patients with the brain tumors made significant improvement in function. There was not a significant difference between the 2 groups of patients in improvement in function, FIM efficiency, or success in discharging the patients to home.28 Greenberg29 and Bartolo30 and their respective colleagues compared the outcomes of patients admitted with brain tumors and patients with stroke and found that improvement in function and discharge to home was similar in the 2 groups. In 2000, Huang and his same colleagues31 compared a group of patients with brain tumors to a group of patients with traumatic brain injury. They found significant improvement in the function of the patients with brain tumors. Patients in the traumatic brain injury group made more progress but had longer LoS. FIM efficiency was not significantly different between the groups.31
Three papers have reported outcomes of patients who received radiation concurrent with inpatient rehabilitation. Tang and colleagues32 reported 63 patients, of whom 48% percent received radiation concurrent with rehabilitation. The patients who received radiation made significant gains in function, and more than 70% were discharged to home. There was no difference in the outcomes of the patients in the radiation and nonradiation groups.32 Marciniak33 and O’Dell34 and their colleagues also reported that patients with brain tumors that required radiation therapy can benefit from inpatient rehabilitation. The present paper is the fourth (with the largest patient group) to show that patients with primary and metastatic tumors to the brain can benefit from a program that provides radiation concurrent with inpatient rehabilitation. We have shown that patients can achieve functional levels and rates of discharge to home that are not significantly different from those of the most commonly admitted group of patients to IRF – patients with stroke.
In the present study, 18% of all of the cancer patients were transferred to medical services and/or acute hospital care (Table 1). This is consistent with a paper by Asher and colleagues35 who reported that 17.4% of patients at an IRF with a diagnosis of cancer required transfer back to medical service, and that low admission motor FIM score correlated with the likelihood of transfer back to medical service. In the present paper, the total admission FIM score was not related to the likelihood of return to medical service, although a lack of improvement in the FIM score did correlate with transfer to medical service.
All of the papers we reviewed found that appropriately selected patients with cancer make significant improvement in function with treatment at an IRF. Tang and colleagues have also shown that for patients with malignant brain tumors and metastasis to the spine, improvement in function correlates with increased survival.32 Our paper confirms that patients with primary malignant brain tumors, malignant tumors metastatic to the brain or spine, and tumors metastatic to long bones may benefit from rehabilitation concurrent with radiation. Rehabilitation units are traditionally associated with treating patients with stroke and spinal cord injury. The patients in our study had cancer and were receiving radiation therapy. They had significant improvement in function and FIM efficiency scores that are not below the threshold set as expected for care at an IRF. Most patients in our study achieved a functional level consistent with what is needed to go home.
There is a prospective payment or reimbursement system for rehabilitation units.36 The payments are based on the admitting diagnosis, the admission FIM score, the age of the patient, and comorbidities. There are 4 tiers for comorbidities with no additional payments for patients in tier 0 but with additional payments for patients with conditions that qualify for tiers 1 through 3. The highest payments are for patients in tier 1. Examples of conditions that can increase payment include morbid obesity, congestive heart failure, vocal cord paralysis, and the need for hemodialysis. There is no increased payment for provision of radiation therapy. There are no reports on the feasibility, in terms of finances, of providing radiation on an IRF. We asked the finance office of the Albany Medical Center to comment on the cost to the hospital of providing radiation therapy to patients on the rehabilitation unit. The hospital’s finance department reviewed available data and reported that the variable cost of providing radiation therapy is about 6.5% of the revenue collected from third-party payors for caring for patients who receive that service (personal communication from the finance office of Albany Medical Center to George Forrest, 2015). Our findings suggest that the Centers for Medicare & Medicaid Services should make an adjustment to the payment system to support the cost of providing radiation to patients at an IRF. Even under the current payment system, for a hospital that has the equipment and personnel to provide radiation treatments, the variable cost of 6.5% of revenue should not be an absolute barrier to providing this service.
Limitations
This study reports on the experience of only 1 facility. The number of patients in the radiation group is greater than the number of patients in any previous report of people receiving radiation at an IRF, but the statistician does not think it is large enough to allow statistical analysis of covariates such as age, sex, and comorbid conditions. In addition, we did not investigate all of the factors that influence the type of care patients are offered and their LoS, such as hospital policy, insurance coverage, income, and family structure.
Conclusions
Acute care medical units are now challenged to both reduce LoS and reduce the number of patients who are readmitted to the hospital. Rehabilitation units are challenged to maintain census, as government and private payors are shifting patients from acute rehabilitation units to subacute rehabilitation units. We found that patients with cancer who need radiation are a population of patients who are seen by payors as needing to be in a facility with excellent nursing, therapy, and comprehensive physician services. A comprehensive cancer care program within a rehabilitation unit can be a great benefit to the acute care services, the IRF, and, most importantly, patients and their families.
The American Cancer Society reports that 1.6 million people are diagnosed with cancer each year, of whom 78% are aged 55 years or older. The 5-year survival rate for cancer is 68%.1 Almost 15.5 million living Americans have been diagnosed with cancer.2 Many patients with cancer have difficulty walking and with activities of daily living. Patients with primary brain tumors or tumors metastatic to the brain may present with focal weakness or cognitive deficits similar to patients with stroke. Patients with tumors metastatic to the spine may have the same deficits as a patient with a traumatic spinal cord injury. Patients with metastasis to bone may have pathologic fractures of the hip or long bones. Patients may develop peripheral neuropathy associated with a paraneoplastic syndrome, chemotherapy, or critical illness neuropathy. Lehmann and colleagues evaluated 805 patients admitted to hospitals affiliated with the University of Washington Medical School with a diagnosis of cancer and found that 15% had difficulty walking and 20% had difficulty with activities of daily living.3
Many patients with cancer can benefit from inpatient rehabilitation.4,5 Study findings have shown that patients with impairments in function related to cancer are often not referred for rehabilitation. Among the reasons mentioned for that are that oncologists are more focused on treating the patients’ cancer than on their functional deficits and that specialists in rehabilitation medicine do not want to be involved with patients with complex medical problems. Rehabilitation facilities may not want to incur the costs associated with caring for patients with cancer.6
The present paper looks at the outcomes of 61 consecutive patients with cancer who were admitted to an inpatient rehabilitation facility (IRF) and received radiation therapy concurrent with rehabilitation. It compares the outcomes of the cancer patients with the outcomes of patients without cancer who were admitted with stroke or spinal cord injury, conditions more commonly treated at an IRF.
Methods
We reviewed electronic medical records of all patients with cancer admitted to the IRF from 2008 through 2013 who received radiation therapy while at the facility. We also reviewed the data of all patients without cancer admitted with a diagnosis of stroke in 2013 and all patients admitted with a diagnosis of traumatic spinal cord injury in 2012 and 2013. No patients were excluded from stroke and traumatic spinal cord injury groups.
We recorded the sex, age, diagnostic group, Functional Independence Measure (FIM) admission score, FIM discharge score, length of stay (LoS) in the IRF, place of discharge of each patient (eg, home, acute care, or subacute care), and calculated the FIM efficiency score (change in FIM/LoS) for each patient. The FIM is an instrument that has 18 items measuring mobility, participation in activities of daily living, ability to communicate, and cognitive function.7 Each item is scored from 1 to 7, with 1 denoting that the patient cannot perform the task and 7 that the activity can be performed independently. The minimum score is 18 (complete dependence), and the maximum score is 126 (independent function). Thirteen items compose the motor FIM score: eating, grooming, bathing, dressing upper body, dressing lower body, toileting, bladder management, management of bowel, transfer to bed or wheelchair, transfer to toilet, tub transfer, walking (or wheelchair use), and climbing stairs. Five items – comprehension, expression, social interaction, problem solving, and memory – compose the cognitive FIM score.
We used a 1-way analysis of variance to evaluate differences between age and cancer type, age and diagnostic group, admission FIM score and cancer type, discharge FIM score and cancer type, change in FIM and cancer type, LoS and cancer type, and LoS and diagnostic group. The Pearson chi-square test was used to test the goodness of fit between the place of disposition and diagnostic group. The paired t test was used to evaluate the improvement in FIM of the patients who were in the cancer groups. The Tukey Simultaneous Tests for Differences of Means was used to compare the FIM efficiency scores of the groups. A 2-sample t test was used to evaluate the factors associated with the need for transfer from the IRF to the acute medical service.
Results
The demographic characteristics of the patients in the study and the admission and discharge FIM scores are reported in Table 1. There were initially 62 cancer patients in the radiation group, which was further divided into 4 subgroups based on the site of the primary tumor or metastasis. In all, 23 had a primary malignant brain tumor and received radiation and temozolomide. Sixteen patients had malignancies metastatic to the brain, 15 patients had tumors metastatic to the spine, and 7 had tumors metastatic to the long bones. One patient had laryngeal cancer and was excluded from the study because we did not think that we could do an analysis of a group with only 1 patient. The final number of patients in the cancer group was therefore 61. There were 69 patients in the stroke group and 23 in the spinal cord injury group.
We report improvement in total FIM, motor FIM, and cognitive FIM scores and were able to identify all 18 of the items of the FIM score on 60 of the 61 patients in the cancer group. Improvement in total FIM of the 61 patients in the cancer groups was significant at P P P = .05. Just over 75% of the patients in the cancer group had sufficient enough improvement in their level of function that they were able to return to their homes (Table 1). The average FIM score at the time of discharge was 83.08. This was not significantly different than the level of function of patients discharged after stroke (87.52) or traumatic spinal cord injury (89.13).
The patients with primary brain tumors were younger than the patients with cancer metastatic to the brain (P = .013). The patients with a primary brain tumor had lower admission FIM scores than patients with tumors metastatic to the brain (P = .027). The patients with a primary brain tumor had a greater increase in FIM score than patients with metastasis to the brain (P = .043; Table 2). There was not a significant difference between these 2 groups in FIM score at discharge or in the likelihood of discharge to home (Table 1). The FIM efficiency score was 1.12 for the patients in the primary brain tumor group and .80 in those with metastasis to the brain. This difference was not significant P = .96.
There were 69 patients in the stroke group. We compared the 39 patients with primary or metastatic brain lesion to the stroke group. The patients with primary or metastatic cancer of the brain were younger than the patients with stroke, 60.4 years old versus 69.1 years old (P = .004). The patients in the combined cancer group had a higher admission FIM score compared with the stroke patients (68.4 vs 63.12; P = .05). The discharge FIM scores were 83.3 in the combined cancer group and 87.5 in the stroke group (Table 1). This difference was not significant, but the improvement in the combined cancer group (14.6) was less than the improvement in the stroke group (24.40; P = .002) (Table 3).
The average LoS in the IRF was 18.7 days in the combined cancer group and 16.8 days in the stroke group. This difference was not significant. An average of 82% of the patients in the primary tumor or brain metastasis group and 85.5% of the patients in the stroke group were discharged to home. This difference was not significant. The FIM efficiency score of the patients in the stroke group was 2.0. This was significantly greater than the score for the patients in the metastasis to the brain group (0.80; P = .044) but not significantly greater than the primary brain cancer group (1.19; P = .22).
There were 23 patients in the traumatic spinal cord injury group. A comparison of the patients with tumors metastatic to the spine and patients with traumatic spinal cord injury showed that the patients in the cancer group were older (60.27 and 42.70 years, respectively; P = .001). In all, 80% of patients with tumors metastatic to the spine were men. This was not significantly different from the percentage of men in the traumatic spinal cord injury group (82.6%; Table 1). The admission FIM score of the patients with cancer was 66.5 (standard deviation [SD], 13.3) and 58.03 (SD, 15.1) in the patients with a traumatic spinal cord injury (Table 1). The FIM score at discharge was 80.4 (SD, 19.1) in the patients with cancer and 89.1 (SD, 20.3) in the patients with a traumatic spinal cord injury (Table 1). Neither of these were statistically significant. The improvement in patients with cancer was 13.9 (SD, 12.2) and 31.1 (SD, 13.9) in the traumatic spinal cord injured patients. This difference was significant (P
The median LoS was 18.98 days in the cancer metastasis to spine group (interquartile range [IQR] is the 25th-75th percentile, 12-30 days). In the traumatic group the median LoS was 23 days (IQR, 16-50 days). This difference was not significant (P = .14 Mann-Whitney test). The mean FIM efficiency score was 1.46 in the traumatic spinal cord injury group and .78 in the group with cancer metastatic to the spine. This difference was not significant (P = .72). Sixty percent of the patients in the cancer group were discharged to home, and 87% of patients in the traumatic spinal cord group were discharged to home. This difference was not significant (P = .12; Fisher exact test).
As far as we can ascertain, this is the first paper that has looked at the outcomes of patients receiving rehabilitation concurrent with radiation of the long bones. The average improvement in FIM was 12.4 (Table 1). The LoS was 11.6 days, and the FIM efficiency was 1.25. In all, 71.4% made enough progress to go home.
Of the total number of cancer patients, 18% were transferred to the acute medical service of the hospital (Table 1). Neither age, sex, type of cancer, nor admission FIM score were associated with the need for transfer to acute hospital care. Change in FIM score was inversely associated with transfer to acute hospital care (P = .027). Patients whose function did not improve with rehabilitation were most likely to be transferred back to acute hospital care.
Discussion
Radiation therapy is considered a service that is provided to people who come for treatment as an outpatient. Caregivers may have difficulty transporting patients to radiation if the patient has deficits in mobility. This may be particularly true if the patient is heavy, the caregivers are frail, or perhaps if they live in rural settings where there is no wheelchair-accessible public transportation. There are many factors that help determine whether a patient with functional deficits can be discharged to his or her home. These include sex, age, marital status, family and/or community support, income, and insurance.8 The FIM is an instrument that indicates how much help a patient needs with mobility and self-care skills. It also correlates with the amount of time that caregivers must spend helping a patient.9 Study findings have shown that the FIM score is an important determinant of whether a patient can be discharged to home. The total FIM score is as useful as an analysis of the components of the FIM score in predicting whether a patient can return to the community.10,11 Reistetter and colleagues found a total FIM score of 78 to be the score that best separates patients who are likely to be able to go home and patients who are likely to need long-term care.11 Bottemiller and colleagues10 reported that 37% of patients with total discharge FIM scores of less than 40 were discharged to home. They reported that 62% of patients with FIM scores between 40 and 79 were discharged to home, and 88% of patients with scores of 80 or above were discharged to home.10 The goal in bringing patients to the IRF was to accept and treat patients with reasonable community support and potential to achieve a functional level compatible with discharge to the community. Most patients in each of the cancer groups were able to reach an FIM score of 78 to 80 and to be discharged to home.
Most of the patients in the cancer groups had underlying problems that are not considered curable. The primary goal was to enable the patients to have some time at home with their families before requiring readmission to a hospital or hospice care. Reasonable LoS and rate of progress are now expected or required by third-party payors and hospital administrators. Physicians at the Mayo Clinic have indicated that a rehabilitation service should aim for an FIM efficiency score of at least .6 points per day.10 The FIM efficiency of patients in each of the 4 cancer subgroups in this study was higher than this level.
J. Herbert Dietz, Jr was an early advocate of the need to provide comprehensive rehabilitation services for patients with cancer. He first described his work in 1969.12 Since that time, there have been many papers that have documented the benefits of IRF for patients with cancer. O’Toole and Golden have shown outcomes of a large series of patients from an IRF. They reported that at the time of admission, 14% of patients could ambulate, but at discharge, 80% could ambulate without hands-on assistance. They reported significant improvements in continence, FIM score, and score on the Karnofsky Performance Scale.13 Marciniak,14 Hunter,15 Shin,16 and Cole,17 and their respective colleagues have all shown that patients with many different types of cancer benefit from rehabilitation at the IRF level. Gallegos-Kearin and colleagues4 reported on the care of 115,570 patients admitted to IRF with cancer from 2002 to 2014. Patients had significant improvement in function, with more than 70% of patients discharged to home.4 Ng and colleagues studied a group of 200 patients who received IRF care and found there was significant improvement in function. Ninety-four percent of patients rated their stay as either extremely good or very good.5
Metastasis to the spine is a common problem. It is found in 30% of cancer patients at autopsy. The most common sources of metastasis to the spine are breast, lung, prostate, kidney, and thyroid.18 Multiple myeloma and lymphoma may also involve the spine. Several authors have shown that these patients benefit from inpatient rehabilitation. Mckinley and colleagues19 have noted that patients with metastasis to the spine make significant improvement with care at an IRF. Compared with patients with a traumatic spinal cord injury, the cancer patients had shorter LoS, smaller improvement in FIM, equal FIM efficiency (FIM gain/LoS), and equal success in making enough progress to be discharged to home.19 Eriks and colleagues showed that patients at an IRF in Amsterdam made significant improvement in function as measured by the Barthel’s Index.20 Tang .,21 and Parsch22 and their respective colleagues, Murray,23 and New24 and colleagues have published findings confirming that patients with spinal cord injury caused by metastasis to the spine make significant progress with inpatient rehabilitation programs. The present study adds to the literature by showing that patients with metastasis to the spine who are receiving radiation can make progress and be discharged to the community.
There are 24,000 new cases of primary malignant brain tumors in the United States each year.25 The incidence of metastatic cancer to the brain has been estimated to be 100,000 cases per year in the United States. The most common cancer sources are lung, breast, melanoma, kidney, and colon.26,27 The first study of patients admitted to an IRF for treatment of brain tumors was published in 1998 by Huang and colleagues28 who compared the outcomes of 63 patients with brain tumors with the outcomes of 63 patients with stroke. They reported that the patients with the brain tumors made significant improvement in function. There was not a significant difference between the 2 groups of patients in improvement in function, FIM efficiency, or success in discharging the patients to home.28 Greenberg29 and Bartolo30 and their respective colleagues compared the outcomes of patients admitted with brain tumors and patients with stroke and found that improvement in function and discharge to home was similar in the 2 groups. In 2000, Huang and his same colleagues31 compared a group of patients with brain tumors to a group of patients with traumatic brain injury. They found significant improvement in the function of the patients with brain tumors. Patients in the traumatic brain injury group made more progress but had longer LoS. FIM efficiency was not significantly different between the groups.31
Three papers have reported outcomes of patients who received radiation concurrent with inpatient rehabilitation. Tang and colleagues32 reported 63 patients, of whom 48% percent received radiation concurrent with rehabilitation. The patients who received radiation made significant gains in function, and more than 70% were discharged to home. There was no difference in the outcomes of the patients in the radiation and nonradiation groups.32 Marciniak33 and O’Dell34 and their colleagues also reported that patients with brain tumors that required radiation therapy can benefit from inpatient rehabilitation. The present paper is the fourth (with the largest patient group) to show that patients with primary and metastatic tumors to the brain can benefit from a program that provides radiation concurrent with inpatient rehabilitation. We have shown that patients can achieve functional levels and rates of discharge to home that are not significantly different from those of the most commonly admitted group of patients to IRF – patients with stroke.
In the present study, 18% of all of the cancer patients were transferred to medical services and/or acute hospital care (Table 1). This is consistent with a paper by Asher and colleagues35 who reported that 17.4% of patients at an IRF with a diagnosis of cancer required transfer back to medical service, and that low admission motor FIM score correlated with the likelihood of transfer back to medical service. In the present paper, the total admission FIM score was not related to the likelihood of return to medical service, although a lack of improvement in the FIM score did correlate with transfer to medical service.
All of the papers we reviewed found that appropriately selected patients with cancer make significant improvement in function with treatment at an IRF. Tang and colleagues have also shown that for patients with malignant brain tumors and metastasis to the spine, improvement in function correlates with increased survival.32 Our paper confirms that patients with primary malignant brain tumors, malignant tumors metastatic to the brain or spine, and tumors metastatic to long bones may benefit from rehabilitation concurrent with radiation. Rehabilitation units are traditionally associated with treating patients with stroke and spinal cord injury. The patients in our study had cancer and were receiving radiation therapy. They had significant improvement in function and FIM efficiency scores that are not below the threshold set as expected for care at an IRF. Most patients in our study achieved a functional level consistent with what is needed to go home.
There is a prospective payment or reimbursement system for rehabilitation units.36 The payments are based on the admitting diagnosis, the admission FIM score, the age of the patient, and comorbidities. There are 4 tiers for comorbidities with no additional payments for patients in tier 0 but with additional payments for patients with conditions that qualify for tiers 1 through 3. The highest payments are for patients in tier 1. Examples of conditions that can increase payment include morbid obesity, congestive heart failure, vocal cord paralysis, and the need for hemodialysis. There is no increased payment for provision of radiation therapy. There are no reports on the feasibility, in terms of finances, of providing radiation on an IRF. We asked the finance office of the Albany Medical Center to comment on the cost to the hospital of providing radiation therapy to patients on the rehabilitation unit. The hospital’s finance department reviewed available data and reported that the variable cost of providing radiation therapy is about 6.5% of the revenue collected from third-party payors for caring for patients who receive that service (personal communication from the finance office of Albany Medical Center to George Forrest, 2015). Our findings suggest that the Centers for Medicare & Medicaid Services should make an adjustment to the payment system to support the cost of providing radiation to patients at an IRF. Even under the current payment system, for a hospital that has the equipment and personnel to provide radiation treatments, the variable cost of 6.5% of revenue should not be an absolute barrier to providing this service.
Limitations
This study reports on the experience of only 1 facility. The number of patients in the radiation group is greater than the number of patients in any previous report of people receiving radiation at an IRF, but the statistician does not think it is large enough to allow statistical analysis of covariates such as age, sex, and comorbid conditions. In addition, we did not investigate all of the factors that influence the type of care patients are offered and their LoS, such as hospital policy, insurance coverage, income, and family structure.
Conclusions
Acute care medical units are now challenged to both reduce LoS and reduce the number of patients who are readmitted to the hospital. Rehabilitation units are challenged to maintain census, as government and private payors are shifting patients from acute rehabilitation units to subacute rehabilitation units. We found that patients with cancer who need radiation are a population of patients who are seen by payors as needing to be in a facility with excellent nursing, therapy, and comprehensive physician services. A comprehensive cancer care program within a rehabilitation unit can be a great benefit to the acute care services, the IRF, and, most importantly, patients and their families.
1. American Cancer Society. Cancer facts & figures 2016. Atlanta, GA: American Cancer Society; 2016.
2. National Cancer Institute: Office of cancer survivorship: statistics. https://cancercontrol.cancer.gov/ocs/statistics/statistics.html. Updated October 17, 2016. Accessed April 21, 2018.
3. Lehmann JF, DeLisa JA, Warren CG, deLateur BJ, Bryant PL, Nicholson CG. Cancer rehabilitation: assessment of need, development and evaluation of a model of care. Arch Phys Med Rehabil. 1978;59(9):410-419.
4. Gallegos-Kearin V, Knowlton SE, Goldstein R, et al. Outcome trends of adult cancer patients receiving inpatient rehabilitation: a 13-year review [published online Feb 21, 2018]. Am J Phys Med Rehabil. doi:10.1097/PHM.0000000000000911
5. Ng AH, Gupta E, Fontillas RC, et al. Patient-reported usefulness of acute cancer rehabilitation. PM R. 2017;9(11):1135-1143.
6. Cheville AL, Kornblith AB, Basford JR. An examination of the causes for the underutilization of rehabilitation services among people with advanced cancer. Am J Phys Med Rehabil. 2011;90(5 suppl 1):S27-S37.
7. Cohen ME, Marino RJ. The tools of disability outcomes research functional status measures. Arch Phys Med Rehabil. 2000;81(12 suppl 2):S21-S29.
8. Nguyen VQ, PrvuBettger J, Guerrier T, et al. Factors associated with discharge to home versus discharge to institutional care after inpatient stroke rehabilitation. Arch Phys Med Rehabil. 2015;96(7):1297-1303.
9. Forrest G, Schwam A, Cohen E. Time of care required by patients discharged from a rehabilitation unit. Am J Phys Med Rehabil. 2002;81(1):57-62.
10. Bottemiller KL, Bieber PL, Basford JR, Harris M. FIM scores, FIM efficiency and discharge following inpatient stroke rehabilitation. Rehabil Nurs. 2006;31(1):22-25.
11. Reistetter TA, Graham JE, Deutsch A, Granger CV, Markello S, Ottenbacher KJ. Utility of functional status for classifying community versus institutional discharges after inpatient rehabilitation for stroke. Arch Phys Med Rehabil. 2010;91(3):345-350.
12. Dietz JH Jr. Rehabilitation of the cancer patient. Med Clin North Am. 1969;53(3):607-624.
13. O'Toole DM, Golden AM. Evaluating cancer patients for rehabilitation potential. West J Med. 1991;155(4):384-387.
14. Marciniak CM, Sliwa JA, Spill G, Heinemann AW, Semik PE. Functional outcome following rehabilitation of the cancer patient. Arch Phys Med Rehabil. 1996;77(1):54-57.
15. Hunter EG, Baltisberger J. Functional outcomes by age for inpatient cancer rehabilitation: a retrospective chart review. J Appl Gerontol. 2013;32(4):443-456.
16. Shin KY, Guo Y, Konzen B, Fu J, Yadav R, Bruera E. Inpatient cancer rehabilitation: the experience of a national comprehensive cancer center. Am J Phys Med Rehabil. 2011;90(5 suppl 1):S63-S68.
17. Cole RP, Scialla S, Bednarz L. Functional recovery in cancer rehabilitation. Arch Phys Med Rehabil. 2000;81(5):623-627.
18. White AP, Kwon BK, Lindskog DM, Friedlaender GE, Grauer JN. Metastatic disease of the spine. J Am Acad Orthop Surg. 2006;14(11):587-598.
19. McKinley WO, Huang ME, Tewksbury MA. Neoplastic vs traumatic spinal cord injury: an inpatient rehabilitation comparison. Am J Phys Med Rehabil. 2000;79(2):138-144.
20. Eriks IE, Angenot EL, Lankhorst GJ. Epidural metastatic spinal cord compression: functional outcome and survival after inpatient rehabilitation. Spinal Cord. 2004;42(4):235-239.
21. Tang V, Harvey D, Park Dorsay J, Jiang S, Rathbone MP. Prognostic indicators in metastatic spinal cord compression: using functional independence measure and Tokuhashi scale to optimize rehabilitation planning. Spinal Cord. 2007;45(10):671-677.
22. Parsch D, Mikut R, Abel R. Postacute management of patients with spinal cord injury due to metastatic tumor disease: survival and efficacy of rehabilitation. Spinal Cord. 2003;41:205-210.
23. Murray PK. Functional outcome and survival in spinal cord injury secondary to neoplasia. Cancer. 1985;55:197-201.
24. New PW. Functional outcomes and disability after nontraumatic spinal cord injury rehabilitation: results from a retrospective study. Arch Phys Med Rehabil. 2005;86(2):250-261
25. Central Brain Tumor Registry of the United States: 2016 CBTRUS fact sheet. www.cbtrus.org/factsheet/factsheet.html. Updated 2017. Accessed May 28, 2016.
26. Memorial Sloan Kettering Cancer Center: Metastatic brain tumors & secondary brain cancer. https://www.mskcc.org/cancer-care/types/brain-tumors-metastatic. Updated 2018. Accessed April 21, 2018.
27. Bruckner JC, Brown PD, O'Neill BP, Meyer FB, Wetmore CJ, Uhm JH. Central nervous system tumors. Mayo Clin Proc. 2007;82(10):1271-1286.
28. Huang ME, Cifu DX, Keyser-Marcus L. Functional outcome after brain tumor and acute stroke: a comparative analysis. Arch Phys Med Rehabil. 1998;79(11):1386-1390.
29. Greenberg E, Treger I, Ring H. Rehabilitation outcomes in patients with brain tumors and acute stroke: comparative study of inpatient rehabilitation. Am J Phys Med Rehabil. 2006;85(7):568-573.
30. Bartolo M, Zucchella C, Pace A, et al. Early rehabilitation after surgery improves functional outcomes in inpatients with brain tumours. J Neurooncol. 2012;107(3);537-544.
31. Huang ME, Cifu DX, Keyser-Marcus L. Functional outcomes in patients with brain tumor after inpatient rehabilitation: comparison with traumatic brain injury. Am J Phys Med Rehabil. 2000;79(4):327-335.
32. Tang V, Rathbone M, Park Dorsay J, Jiang S, Harvey D. Rehabilitation in primary and metastatic brain tumours: impact of functional outcomes on survival. J Neurol. 2008;255(6):820-827.
33. Marciniak CM, Sliwa JA, Heinemann AW, Semik PE. Functional outcomes of persons with brain tumors after inpatient rehabilitation. Arch Phys Med Rehabil. 2001;82(4):457-463.
34. O'Dell MW, Barr K, Spanier D, Warnick RE. Functional outcome of inpatient rehabilitation in persons with brain tumors. Arch Phys Med Rehabil. 1998;79(12):1530-1534.
35. Asher A, Roberts PS, Bresee C, Zabel G, Riggs RV, Rogatko A. Transferring inpatient rehabilitation facility cancer patients back to acute care (TRIPBAC). PM R. 2014;6(9):808-813.
36. Centers for Medicare and Medicaid Services: Inpatient rehabilitation facilities. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/InpatientRehab.html. Published March 5, 2012. Accessed May 21, 2018.
1. American Cancer Society. Cancer facts & figures 2016. Atlanta, GA: American Cancer Society; 2016.
2. National Cancer Institute: Office of cancer survivorship: statistics. https://cancercontrol.cancer.gov/ocs/statistics/statistics.html. Updated October 17, 2016. Accessed April 21, 2018.
3. Lehmann JF, DeLisa JA, Warren CG, deLateur BJ, Bryant PL, Nicholson CG. Cancer rehabilitation: assessment of need, development and evaluation of a model of care. Arch Phys Med Rehabil. 1978;59(9):410-419.
4. Gallegos-Kearin V, Knowlton SE, Goldstein R, et al. Outcome trends of adult cancer patients receiving inpatient rehabilitation: a 13-year review [published online Feb 21, 2018]. Am J Phys Med Rehabil. doi:10.1097/PHM.0000000000000911
5. Ng AH, Gupta E, Fontillas RC, et al. Patient-reported usefulness of acute cancer rehabilitation. PM R. 2017;9(11):1135-1143.
6. Cheville AL, Kornblith AB, Basford JR. An examination of the causes for the underutilization of rehabilitation services among people with advanced cancer. Am J Phys Med Rehabil. 2011;90(5 suppl 1):S27-S37.
7. Cohen ME, Marino RJ. The tools of disability outcomes research functional status measures. Arch Phys Med Rehabil. 2000;81(12 suppl 2):S21-S29.
8. Nguyen VQ, PrvuBettger J, Guerrier T, et al. Factors associated with discharge to home versus discharge to institutional care after inpatient stroke rehabilitation. Arch Phys Med Rehabil. 2015;96(7):1297-1303.
9. Forrest G, Schwam A, Cohen E. Time of care required by patients discharged from a rehabilitation unit. Am J Phys Med Rehabil. 2002;81(1):57-62.
10. Bottemiller KL, Bieber PL, Basford JR, Harris M. FIM scores, FIM efficiency and discharge following inpatient stroke rehabilitation. Rehabil Nurs. 2006;31(1):22-25.
11. Reistetter TA, Graham JE, Deutsch A, Granger CV, Markello S, Ottenbacher KJ. Utility of functional status for classifying community versus institutional discharges after inpatient rehabilitation for stroke. Arch Phys Med Rehabil. 2010;91(3):345-350.
12. Dietz JH Jr. Rehabilitation of the cancer patient. Med Clin North Am. 1969;53(3):607-624.
13. O'Toole DM, Golden AM. Evaluating cancer patients for rehabilitation potential. West J Med. 1991;155(4):384-387.
14. Marciniak CM, Sliwa JA, Spill G, Heinemann AW, Semik PE. Functional outcome following rehabilitation of the cancer patient. Arch Phys Med Rehabil. 1996;77(1):54-57.
15. Hunter EG, Baltisberger J. Functional outcomes by age for inpatient cancer rehabilitation: a retrospective chart review. J Appl Gerontol. 2013;32(4):443-456.
16. Shin KY, Guo Y, Konzen B, Fu J, Yadav R, Bruera E. Inpatient cancer rehabilitation: the experience of a national comprehensive cancer center. Am J Phys Med Rehabil. 2011;90(5 suppl 1):S63-S68.
17. Cole RP, Scialla S, Bednarz L. Functional recovery in cancer rehabilitation. Arch Phys Med Rehabil. 2000;81(5):623-627.
18. White AP, Kwon BK, Lindskog DM, Friedlaender GE, Grauer JN. Metastatic disease of the spine. J Am Acad Orthop Surg. 2006;14(11):587-598.
19. McKinley WO, Huang ME, Tewksbury MA. Neoplastic vs traumatic spinal cord injury: an inpatient rehabilitation comparison. Am J Phys Med Rehabil. 2000;79(2):138-144.
20. Eriks IE, Angenot EL, Lankhorst GJ. Epidural metastatic spinal cord compression: functional outcome and survival after inpatient rehabilitation. Spinal Cord. 2004;42(4):235-239.
21. Tang V, Harvey D, Park Dorsay J, Jiang S, Rathbone MP. Prognostic indicators in metastatic spinal cord compression: using functional independence measure and Tokuhashi scale to optimize rehabilitation planning. Spinal Cord. 2007;45(10):671-677.
22. Parsch D, Mikut R, Abel R. Postacute management of patients with spinal cord injury due to metastatic tumor disease: survival and efficacy of rehabilitation. Spinal Cord. 2003;41:205-210.
23. Murray PK. Functional outcome and survival in spinal cord injury secondary to neoplasia. Cancer. 1985;55:197-201.
24. New PW. Functional outcomes and disability after nontraumatic spinal cord injury rehabilitation: results from a retrospective study. Arch Phys Med Rehabil. 2005;86(2):250-261
25. Central Brain Tumor Registry of the United States: 2016 CBTRUS fact sheet. www.cbtrus.org/factsheet/factsheet.html. Updated 2017. Accessed May 28, 2016.
26. Memorial Sloan Kettering Cancer Center: Metastatic brain tumors & secondary brain cancer. https://www.mskcc.org/cancer-care/types/brain-tumors-metastatic. Updated 2018. Accessed April 21, 2018.
27. Bruckner JC, Brown PD, O'Neill BP, Meyer FB, Wetmore CJ, Uhm JH. Central nervous system tumors. Mayo Clin Proc. 2007;82(10):1271-1286.
28. Huang ME, Cifu DX, Keyser-Marcus L. Functional outcome after brain tumor and acute stroke: a comparative analysis. Arch Phys Med Rehabil. 1998;79(11):1386-1390.
29. Greenberg E, Treger I, Ring H. Rehabilitation outcomes in patients with brain tumors and acute stroke: comparative study of inpatient rehabilitation. Am J Phys Med Rehabil. 2006;85(7):568-573.
30. Bartolo M, Zucchella C, Pace A, et al. Early rehabilitation after surgery improves functional outcomes in inpatients with brain tumours. J Neurooncol. 2012;107(3);537-544.
31. Huang ME, Cifu DX, Keyser-Marcus L. Functional outcomes in patients with brain tumor after inpatient rehabilitation: comparison with traumatic brain injury. Am J Phys Med Rehabil. 2000;79(4):327-335.
32. Tang V, Rathbone M, Park Dorsay J, Jiang S, Harvey D. Rehabilitation in primary and metastatic brain tumours: impact of functional outcomes on survival. J Neurol. 2008;255(6):820-827.
33. Marciniak CM, Sliwa JA, Heinemann AW, Semik PE. Functional outcomes of persons with brain tumors after inpatient rehabilitation. Arch Phys Med Rehabil. 2001;82(4):457-463.
34. O'Dell MW, Barr K, Spanier D, Warnick RE. Functional outcome of inpatient rehabilitation in persons with brain tumors. Arch Phys Med Rehabil. 1998;79(12):1530-1534.
35. Asher A, Roberts PS, Bresee C, Zabel G, Riggs RV, Rogatko A. Transferring inpatient rehabilitation facility cancer patients back to acute care (TRIPBAC). PM R. 2014;6(9):808-813.
36. Centers for Medicare and Medicaid Services: Inpatient rehabilitation facilities. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/CertificationandComplianc/InpatientRehab.html. Published March 5, 2012. Accessed May 21, 2018.
Psychosocial factors and treatment satisfaction after radical prostatectomy
More than 164,690 men are expected to be diagnosed with prostate cancer in the United States in 2018.1 Men with prostate cancer face not only stress associated with the diagnosis but also decisional conflict regarding different treatment options.2 Most men diagnosed with clinically localized prostate cancer receive 1 or more of the following treatments: radical prostatectomy, external-beam radiation therapy, and/or brachytherapy, all of which are associated with posttreatment urological or sexual side effects including bowel, urinary, or erectile dysfunction.3-5 Men who choose active surveillance may experience increased anxiety associated with the constant vigilance and monitoring of their tumor status along with the uncertainty of not definitively removing or radiating their prostate.6 In addition to direct functional limitations of sexual and urological side effects, treatment can also lead to secondary psychosocial effects, including depression, self-blame, embarrassment, guilt, lower masculine self-esteem, increased reticence to participate socially or engage in sexual activity, and relationship distress.7-9 Therefore, health-related quality of life (HRQoL) and treatment satisfaction are important for this population.
Urological and sexual side effects of prostate cancer treatments are often a primary focus during treatment decision making between patients and providers. However, little prospective empirical data exist regarding the role of HRQoL and other nonurological physical and psychosocial outcomes on overall treatment satisfaction. The purpose of this study was to prospectively evaluate the role of both urological and nonurological outcomes on overall treatment satisfaction in men diagnosed with prostate cancer. We hypothesize that such an understanding can help describe changes in physical and psychosocial factors that are important to men beyond traditional urological outcomes, including their association with overall treatment satisfaction.
Methods
This was a prospective longitudinal assessment of patients from the Department of Urology at Northwestern University’s Feinberg School of Medicine in Chicago. Patients were eligible if they met the following inclusion criteria: they had been diagnosed with clinically localized or locally advanced prostate cancer; they had not yet received a primary treatment (eg, surgery, radiation, active surveillance) before their baseline assessment; they were 18 years or older; and they were able to read, write, speak, and understand English. Patients were excluded if they had a physical debilitation that would make participation not feasible or would create undue hardship, or if they had a history of diagnosed severe mental illness or hospitalization for chronic psychiatric reasons, as identified by referring physicians.
Eligible participants were approached before their treatment decision (if any). Patient enrollment occurred in 2 ways. For patients invited to participate during their clinic visit, the research assistant explained the study and obtained written informed consent for interested patients. A unique user identification and password was created for each patient, and they practiced using the touch screen computer while the research assistant observed and provided guidance as needed. When the patients were ready to start their pretreatment online interview, they completed the questionnaires by themselves. For patients who were invited to participate but were not scheduled to return in the foreseeable future, enrollment was carried out differently. In those cases, participating physicians contacted eligible patients who were not scheduled for a visit and informed them of the study opportunity. Interested patients were contacted by the research assistant who provided them with the study website address, which directed them to the online consent form. After a patient had completed the consent form, he was prompted to self-register. He received a unique user identification and password that could be used to complete the baseline assessment and subsequent assessments. However, for interested patients who did not have access to a computer or Internet connection, the research assistant provided them with paper consent forms and paper versions of all study assessments. After participants had completed the baseline assessment, the research assistant provided them with a written schedule of future assessments, which were expected to occur at 1 month posttreatment, 3 months posttreatment, 6 months posttreatment, and 12 months posttreatment.
For all follow-up appointments, participants could complete assessments either at clinic visits or from home using a secure online assessment platform called Assessment Center.10 The research assistant used a patient log to track participants and their progress in the study, which included study number, patient name (or initials), registration date, date of birth, sex, and timeline of completed or future assessments. The research assistant called or emailed participants (depending on patient preference) about a week before each of their follow-up assessments to facilitate adherence. If the participant did not log into the system by the target day, the research assistant contacted him the following day (target day +1) with a phone or email reminder to log into the system and complete the assessments. If the participant did not log in by midnight 1 day after the target day, the research assistant attempted to contact him one last time (target day +2) with either a reminder to log into the system or to ascertain his status that might be related to his noncompletion. Overall, a participant was called or e-mailed 1 to 3 times to remind him of his assessment. If he was unresponsive after 3 attempts, he was recorded as having withdrawn for an unknown reason.
At baseline and each follow-up time point, study participants completed a battery of patient-reported outcome measures, with most coming from the Patient-Reported Outcomes Measurement Information System (PROMIS)11 and the Surgical Outcomes Measurement System (SOMS).12 PROMIS is a National Institutes of Health (NIH) funded measurement system that has helped standardize and improve self-reported assessment of health status, symptoms, side effects, and different aspects of HRQoL, including physical, emotional, cognitive, and social health. SOMS is a suite of patient-reported outcome measures assessing important aspects of HRQoL after surgery. It was developed with feedback from surgeons, postoperative patients, and surgical nurses. PROMIS items were directly incorporated into numerous SOMS measures to facilitate easier comparisons and score crosswalks across measures and patient populations. In addition to PROMIS and SOMS measures, we also administered several well-known instruments of urological and sexual function, including the International Index of Erectile Function (IIEF) and American Urological Association Symptom Score Index (AUASS).13,14
Outcome measures were compared across sociodemographic and clinical variables at each time point using t tests for numerical variables (age) and with chi-square or Fisher exact tests for categorical variables; those variables with significant differences were used as covariates in statistical models. To examine differences in patient-reported scores over time, we used repeated measures analysis of covariance with general linear modeling methods. We used Pearson correlation coefficients to evaluate for correlations between quality-of-life outcomes and treatment satisfaction.
Not all participants completed each of the follow-up surveys, and reasons for dropout were prospectively documented. Most participants elected surgical resection as their primary treatment compared with the fewer than 10% of patients who chose radiation or chemotherapy as their primary treatment and about 20% of men who chose active surveillance after their initial diagnosis. Therefore, our analysis focused on patients who elected surgical resection. For comparison purposes, we included the HRQoL results from active surveillance patients.
Results
A total of 105 patients diagnosed with prostate cancer were enrolled in the study. Response rates decreased throughout the study (n = 75 at 1 month; n = 71 at 3 months; n = 64 at 6 months; n = 54 at 12 months). Sociodemographic and clinical characteristics of participants are shown in Table 1. The mean change from pretreatment (baseline) scores for each measure in patients treated with surgery is shown in Table 2, and the mean change from pretreatment scores in patients who elected active surveillance is shown in Table 3 (in both tables, a negative score denotes worsened function, and a positive change denotes improvement).
After surgery, patients reported significantly lower erectile function and sexual satisfaction scores. These included statistically significant decreases for IIEF Erectile Function, IIEF Overall Satisfaction, PROMIS Sexual Satisfaction, PROMIS Sexual Interest, and PROMIS Orgasm. In patients treated with surgery, there were significant improvements in anxiety observed for patients at each follow-up time, whereas significantly worse bladder problems were observed on SOMS Bladder at 1 and 3 months but returned to baseline by 12 months after surgery. AUASS was worse at 1 month but significantly improved at 6 and 12 months. Fatigue scores significantly worsened at 1 month but were no longer significant at 6 and 12 months. Physical Function was worsened at 1 month but not throughout the rest of the study. Bowel Problems (SOMS) were significantly worse at 1 month, but changes became nonsignificant on subsequent assessments. The only 2 domains that did not demonstrate any significant changes o
In active surveillance patients, sexual function domains were generally unchanged over the course of the study. However, unlike treated patients, there was no significant improvement in anxiety, depression, pain, fatigue, or sleep. In fact, most of these domains demonstrated worsened functioning, although these were not statistically significant. Urinary domains generally remained unchanged.
Pearson correlation coefficients between HRQoL measures and overall treatment satisfaction (assessed by the question, Are you satisfied with the results of your operation?) at each follow-up time point in patients treated with surgery are shown in Table 4. Relations between treatment satisfaction and sexual outcomes were generally statistically insignificant (r, .08-.56). However, sleep disturbance, depression, pain interference, fatigue, embarrassment, and bladder problems all demonstrated statistically significant positive associations with treatment satisfaction, with coefficients ranging from small to medium in magnitude (r, .32-.61). Other outcomes such as anxiety, physical function, and bowel problems demonstrated small to medium statistically significant associations with treatment satisfaction (r, .04-.60) but not at every time point. We performed t tests to examine treatment satisfaction in patients with detectable initial posttreatment prostate-specific antigen (PSA; >0.01 ng/mL). We found no difference in treatment satisfaction between patients with detectable PSA values and those with undetectable PSA at each time point.
When the patients were asked, Compared with what you expected, how do you rate the results of your operation?, most of those treated with surgery reported that the results of their operation were better than they had expected (Figure 1A; p. e137). More than 75% of the patients had results that were as expected or better than expected. When asked, Compared with what you expected, how do you rate your side effects of the operation?, almost 70% of patients reported side effects no worse than expected (Figure 1B). When asked, Are you satisfied with the results of your operation?, most patients reported that overall, they were satisfied with the results of their operation (Figure 1C).
At 12 months, none of the patients reported overall dissatisfaction with their treatment choice. More than 90% of patients were mostly or completely satisfied with the results of their operation.
Discussion
This prospective study assessed the HRQoL from pretreatment through 12 months posttreatment in men diagnosed with clinically localized prostate cancer that had been treated with surgery. Although the indicators of sexual function significantly decreased over time, they were not meaningfully associated with overall treatment satisfaction. Instead, a host of other factors, including psychosocial (eg, anxiety, depression, body image dissatisfaction, embarrassment), nonurological physical symptoms (pain interference, physical function, sleep disturbance, fatigue), and bladder problems, were significantly related to overall treatment satisfaction. Although this may not be surprising in other clinical oncology paradigms, the sheer surfeit of focus and attention on sexual function has overshadowed aspects of HRQoL that many men report are important to them, despite worsened sexual function outcomes.
Understanding potential treatment-related changes in HRQoL can be challenging for men when choosing providers and different therapeutic options. The increasing complexity of treatment in prostate cancer has created an opportunity to not only understand efficacy on cancer control but also focus on meaningful patient-reported outcomes. Hospitals and medical groups are increasingly aware of the importance of improving the patient care experience. Objective measures of patient satisfaction for health care providers, such as the Press-Ganey and Net Promoter score, exist to measure and improve patient experience. In prostate cancer, clinicians and large groups, including governmental agencies such as the US Preventive Services Task Force, have often focused on declines in urinary and erectile function15 without considering the full impact of prostate cancer treatment on global HRQoL. Our study was a prospective, longitudinal, self-reported examination of the impact, positive and negative, of prostate cancer treatment over a 12-month period.
Numerous studies have documented the treatment-related side effects of erectile, urinary, and bowel dysfunction in patients treated for prostate cancer, which may occur after definitive local therapies.5,16-18 The present study shows a similar impact on urinary, bowel, and erectile domains after treatment. Although erectile function scores remained lower through the course of the 12-month study, bowel and bladder domains returned to baseline by month 12. Unlike other studies, we also examined psychosocial and nonurological aspects of prostate cancer treatment. We found that there was a measurable and significant positive impact on other HRQoL measurements such as decreased anxiety. Despite a variety of declines across HRQoL domains, most patients reported that their results were largely as they had expected, and their side effects were the same or better than they had expected. No patient in the cohort reported being dissatisfied with his overall treatment, and more than 90% of patients were mostly or completely satisfied with their treatment choice. This highlights the point that while sexual and other urological domains of HRQoL are important, impairments in these areas do not necessarily reflect how many patients perceive success or satisfaction with their treatment choice. We also showed correlations between treatment satisfaction and improvement in sleep, anxiety, depression, and fatigue. It is worth noting that although there were decreases in the erectile and sexual function domains after treatment, those factors were not correlated with overall treatment satisfaction. Those factors may not routinely be assessed before, during, and after treatment for prostate cancer in most clinical encounters. However, because they were strongly associated with satisfaction with treatment outcomes in this study, identification in impairments may lead to opportunities to intervene and improve the patient experience. Therefore, important “teachable moments” may be missed (for both patients and providers) during treatment decision-making encounters if other factors beyond sexual and urological outcomes are not adequately considered and addressed. Furthermore, the results of our study may help clinicians counsel patients on their expectations for their recovery after surgery and identify particular issues related to HRQoL to pay close attention to in follow-up visits.
Strengths of our study include its prospective nature, which allowed evaluation of HRQoL outcomes at multiple time points throughout the first year after treatment. In addition, we used existing patient-reported outcome tools validated by the NIH to assess changes in HRQoL. PROMIS is an NIH-supported tool that can be leveraged in the pre- and posttreatment periods to identify patients who have impairments with HRQoL. It can provide clinicians with a unique opportunity to detect and intervene in setbacks and side effects to improve patient satisfaction and HRQoL.
Limitations of the current study include that most patients selected surgery for their treatment choice and that not all patients completed all longitudinal questionnaires, although this is expected in longitudinal studies of this nature. Although all the patients were approached and encouraged to participate, many did not participate and were not captured. In addition, not all patients completed end-of-study surveys. These factors may have biased our results because of unmeasurable factors related to nonparticipation or dropout. Our study encompassed the preoperative period up to 12 months postoperatively, which may fail to identify improvements or declines in HRQoL that may occur more than 12 months postoperatively, particularly related to continence and erectile function. The participants were enrolled by 6 surgeons, and we were not able to standardize the preoperative counseling either preoperatively or postoperatively, which may have biased our results. Finally, our study population consisted of predominantly white, married men of higher socioeconomic status; therefore, our results may not be generalizable to newly diagnosed prostate cancer patients overall.
Conclusions
By using validated self-administered questionnaires, we found that despite decreased sexual and urinary function, patients treated for prostate cancer were satisfied with their treatment choice. Correlates to higher patient satisfaction included decreased anxiety, depression, fatigue, and sleep disturbances.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7‐30.
2. Berry DL, Ellis WJ, Woods NF, Schwien C, Mullen KH, Yang C. Treatment decision-making by men with localized prostate cancer: the influence of personal factors. Urol Oncol. 2003;21(2):93-100.
3. Dubbelman YD, Dohle GR, Schröder FH. Sexual function before and after radical retropubic prostatectomy: a systematic review of prognostic indicators for a successful outcome. Eur Urol. 2006;50(4):711-718; discussion 718-720.
4. McCullough AR. Sexual dysfunction after radical prostatectomy. Rev Urol. 2005;7(2 suppl):S3-S10.
5. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250-1261.
6. Latini DM, Hart SL, Knight SJ, et al. The relationship between anxiety and time to treatment for patients with prostate cancer on surveillance. J Urol. 2007;178(3, pt 1):826-831; discussion 831-832.
7. Meyer JP, Gillatt DA, Lockyer R, Macdonagh R. The effect of erectile dysfunction on the quality of life of men after radical prostatectomy. BJU Int. 2003;92(9):929-931.
8. Casey RG, Corcoran NM, Goldenberg SL. Quality of life issues in men undergoing androgen deprivation therapy: a review. Asian J Androl. 2012;14(2):226-231.
9. Segrin C, Badger TA, Harrington J. Interdependent psychological quality of life in dyads adjusting to prostate cancer. Health Psychol. 2012;31(1):70-79.
10. Gershon RC, Rothrock N, Hanrahan R, Bass M, Cella D. The use of PROMIS and assessment center to deliver patient-reported outcome measures in clinical research. J Appl Meas. 2010;11(3):304-314.
11. Cella D, Yount S, Rothrock N, et al. The patient-reported outcomes measurement information system (PROMIS): progress of an NIH roadmap cooperative group during its first two years. Med Care. 2007;45(5 suppl 1):S3-S11.
12. Zapf M, Denham W, Barrera E, et al. Patient-centered outcomes after laparoscopic cholecystectomy. Surg Endosc. 2013;27(12):4491-4498.
13. Barry MJ, Fowler FJ Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549-1557; discussion 1564.
14. Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822-830.
15. United States Preventive Services Task Force. Final update summary: prostate cancer: screening. http:// www.uspreventiveservicestaskforce.org/Page/ Document/UpdateSummaryFinal/prostate-cancer-screening. Updated July 2015. Accessed April 14, 2017
16. Litwin MS, Gore JL, Kwan L, et al. Quality of life after surgery, external beam irradiation, or brachytherapy for early-stage prostate cancer. Cancer. 2007;109(11):2239-2247.
17. Miwa S, Mizokami A, Konaka H, et al. Prospective longitudinal comparative study of health-related quality of life and treatment satisfaction in patients treated with hormone therapy, radical retropubic prostatectomy, and high or low dose rate brachytherapy for prostate cancer. Prostate Int. 2013;1(3):117-124.
18. Miller DC, Sanda MG, Dunn RL et al. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005;23(12):2772-2780.
More than 164,690 men are expected to be diagnosed with prostate cancer in the United States in 2018.1 Men with prostate cancer face not only stress associated with the diagnosis but also decisional conflict regarding different treatment options.2 Most men diagnosed with clinically localized prostate cancer receive 1 or more of the following treatments: radical prostatectomy, external-beam radiation therapy, and/or brachytherapy, all of which are associated with posttreatment urological or sexual side effects including bowel, urinary, or erectile dysfunction.3-5 Men who choose active surveillance may experience increased anxiety associated with the constant vigilance and monitoring of their tumor status along with the uncertainty of not definitively removing or radiating their prostate.6 In addition to direct functional limitations of sexual and urological side effects, treatment can also lead to secondary psychosocial effects, including depression, self-blame, embarrassment, guilt, lower masculine self-esteem, increased reticence to participate socially or engage in sexual activity, and relationship distress.7-9 Therefore, health-related quality of life (HRQoL) and treatment satisfaction are important for this population.
Urological and sexual side effects of prostate cancer treatments are often a primary focus during treatment decision making between patients and providers. However, little prospective empirical data exist regarding the role of HRQoL and other nonurological physical and psychosocial outcomes on overall treatment satisfaction. The purpose of this study was to prospectively evaluate the role of both urological and nonurological outcomes on overall treatment satisfaction in men diagnosed with prostate cancer. We hypothesize that such an understanding can help describe changes in physical and psychosocial factors that are important to men beyond traditional urological outcomes, including their association with overall treatment satisfaction.
Methods
This was a prospective longitudinal assessment of patients from the Department of Urology at Northwestern University’s Feinberg School of Medicine in Chicago. Patients were eligible if they met the following inclusion criteria: they had been diagnosed with clinically localized or locally advanced prostate cancer; they had not yet received a primary treatment (eg, surgery, radiation, active surveillance) before their baseline assessment; they were 18 years or older; and they were able to read, write, speak, and understand English. Patients were excluded if they had a physical debilitation that would make participation not feasible or would create undue hardship, or if they had a history of diagnosed severe mental illness or hospitalization for chronic psychiatric reasons, as identified by referring physicians.
Eligible participants were approached before their treatment decision (if any). Patient enrollment occurred in 2 ways. For patients invited to participate during their clinic visit, the research assistant explained the study and obtained written informed consent for interested patients. A unique user identification and password was created for each patient, and they practiced using the touch screen computer while the research assistant observed and provided guidance as needed. When the patients were ready to start their pretreatment online interview, they completed the questionnaires by themselves. For patients who were invited to participate but were not scheduled to return in the foreseeable future, enrollment was carried out differently. In those cases, participating physicians contacted eligible patients who were not scheduled for a visit and informed them of the study opportunity. Interested patients were contacted by the research assistant who provided them with the study website address, which directed them to the online consent form. After a patient had completed the consent form, he was prompted to self-register. He received a unique user identification and password that could be used to complete the baseline assessment and subsequent assessments. However, for interested patients who did not have access to a computer or Internet connection, the research assistant provided them with paper consent forms and paper versions of all study assessments. After participants had completed the baseline assessment, the research assistant provided them with a written schedule of future assessments, which were expected to occur at 1 month posttreatment, 3 months posttreatment, 6 months posttreatment, and 12 months posttreatment.
For all follow-up appointments, participants could complete assessments either at clinic visits or from home using a secure online assessment platform called Assessment Center.10 The research assistant used a patient log to track participants and their progress in the study, which included study number, patient name (or initials), registration date, date of birth, sex, and timeline of completed or future assessments. The research assistant called or emailed participants (depending on patient preference) about a week before each of their follow-up assessments to facilitate adherence. If the participant did not log into the system by the target day, the research assistant contacted him the following day (target day +1) with a phone or email reminder to log into the system and complete the assessments. If the participant did not log in by midnight 1 day after the target day, the research assistant attempted to contact him one last time (target day +2) with either a reminder to log into the system or to ascertain his status that might be related to his noncompletion. Overall, a participant was called or e-mailed 1 to 3 times to remind him of his assessment. If he was unresponsive after 3 attempts, he was recorded as having withdrawn for an unknown reason.
At baseline and each follow-up time point, study participants completed a battery of patient-reported outcome measures, with most coming from the Patient-Reported Outcomes Measurement Information System (PROMIS)11 and the Surgical Outcomes Measurement System (SOMS).12 PROMIS is a National Institutes of Health (NIH) funded measurement system that has helped standardize and improve self-reported assessment of health status, symptoms, side effects, and different aspects of HRQoL, including physical, emotional, cognitive, and social health. SOMS is a suite of patient-reported outcome measures assessing important aspects of HRQoL after surgery. It was developed with feedback from surgeons, postoperative patients, and surgical nurses. PROMIS items were directly incorporated into numerous SOMS measures to facilitate easier comparisons and score crosswalks across measures and patient populations. In addition to PROMIS and SOMS measures, we also administered several well-known instruments of urological and sexual function, including the International Index of Erectile Function (IIEF) and American Urological Association Symptom Score Index (AUASS).13,14
Outcome measures were compared across sociodemographic and clinical variables at each time point using t tests for numerical variables (age) and with chi-square or Fisher exact tests for categorical variables; those variables with significant differences were used as covariates in statistical models. To examine differences in patient-reported scores over time, we used repeated measures analysis of covariance with general linear modeling methods. We used Pearson correlation coefficients to evaluate for correlations between quality-of-life outcomes and treatment satisfaction.
Not all participants completed each of the follow-up surveys, and reasons for dropout were prospectively documented. Most participants elected surgical resection as their primary treatment compared with the fewer than 10% of patients who chose radiation or chemotherapy as their primary treatment and about 20% of men who chose active surveillance after their initial diagnosis. Therefore, our analysis focused on patients who elected surgical resection. For comparison purposes, we included the HRQoL results from active surveillance patients.
Results
A total of 105 patients diagnosed with prostate cancer were enrolled in the study. Response rates decreased throughout the study (n = 75 at 1 month; n = 71 at 3 months; n = 64 at 6 months; n = 54 at 12 months). Sociodemographic and clinical characteristics of participants are shown in Table 1. The mean change from pretreatment (baseline) scores for each measure in patients treated with surgery is shown in Table 2, and the mean change from pretreatment scores in patients who elected active surveillance is shown in Table 3 (in both tables, a negative score denotes worsened function, and a positive change denotes improvement).
After surgery, patients reported significantly lower erectile function and sexual satisfaction scores. These included statistically significant decreases for IIEF Erectile Function, IIEF Overall Satisfaction, PROMIS Sexual Satisfaction, PROMIS Sexual Interest, and PROMIS Orgasm. In patients treated with surgery, there were significant improvements in anxiety observed for patients at each follow-up time, whereas significantly worse bladder problems were observed on SOMS Bladder at 1 and 3 months but returned to baseline by 12 months after surgery. AUASS was worse at 1 month but significantly improved at 6 and 12 months. Fatigue scores significantly worsened at 1 month but were no longer significant at 6 and 12 months. Physical Function was worsened at 1 month but not throughout the rest of the study. Bowel Problems (SOMS) were significantly worse at 1 month, but changes became nonsignificant on subsequent assessments. The only 2 domains that did not demonstrate any significant changes o
In active surveillance patients, sexual function domains were generally unchanged over the course of the study. However, unlike treated patients, there was no significant improvement in anxiety, depression, pain, fatigue, or sleep. In fact, most of these domains demonstrated worsened functioning, although these were not statistically significant. Urinary domains generally remained unchanged.
Pearson correlation coefficients between HRQoL measures and overall treatment satisfaction (assessed by the question, Are you satisfied with the results of your operation?) at each follow-up time point in patients treated with surgery are shown in Table 4. Relations between treatment satisfaction and sexual outcomes were generally statistically insignificant (r, .08-.56). However, sleep disturbance, depression, pain interference, fatigue, embarrassment, and bladder problems all demonstrated statistically significant positive associations with treatment satisfaction, with coefficients ranging from small to medium in magnitude (r, .32-.61). Other outcomes such as anxiety, physical function, and bowel problems demonstrated small to medium statistically significant associations with treatment satisfaction (r, .04-.60) but not at every time point. We performed t tests to examine treatment satisfaction in patients with detectable initial posttreatment prostate-specific antigen (PSA; >0.01 ng/mL). We found no difference in treatment satisfaction between patients with detectable PSA values and those with undetectable PSA at each time point.
When the patients were asked, Compared with what you expected, how do you rate the results of your operation?, most of those treated with surgery reported that the results of their operation were better than they had expected (Figure 1A; p. e137). More than 75% of the patients had results that were as expected or better than expected. When asked, Compared with what you expected, how do you rate your side effects of the operation?, almost 70% of patients reported side effects no worse than expected (Figure 1B). When asked, Are you satisfied with the results of your operation?, most patients reported that overall, they were satisfied with the results of their operation (Figure 1C).
At 12 months, none of the patients reported overall dissatisfaction with their treatment choice. More than 90% of patients were mostly or completely satisfied with the results of their operation.
Discussion
This prospective study assessed the HRQoL from pretreatment through 12 months posttreatment in men diagnosed with clinically localized prostate cancer that had been treated with surgery. Although the indicators of sexual function significantly decreased over time, they were not meaningfully associated with overall treatment satisfaction. Instead, a host of other factors, including psychosocial (eg, anxiety, depression, body image dissatisfaction, embarrassment), nonurological physical symptoms (pain interference, physical function, sleep disturbance, fatigue), and bladder problems, were significantly related to overall treatment satisfaction. Although this may not be surprising in other clinical oncology paradigms, the sheer surfeit of focus and attention on sexual function has overshadowed aspects of HRQoL that many men report are important to them, despite worsened sexual function outcomes.
Understanding potential treatment-related changes in HRQoL can be challenging for men when choosing providers and different therapeutic options. The increasing complexity of treatment in prostate cancer has created an opportunity to not only understand efficacy on cancer control but also focus on meaningful patient-reported outcomes. Hospitals and medical groups are increasingly aware of the importance of improving the patient care experience. Objective measures of patient satisfaction for health care providers, such as the Press-Ganey and Net Promoter score, exist to measure and improve patient experience. In prostate cancer, clinicians and large groups, including governmental agencies such as the US Preventive Services Task Force, have often focused on declines in urinary and erectile function15 without considering the full impact of prostate cancer treatment on global HRQoL. Our study was a prospective, longitudinal, self-reported examination of the impact, positive and negative, of prostate cancer treatment over a 12-month period.
Numerous studies have documented the treatment-related side effects of erectile, urinary, and bowel dysfunction in patients treated for prostate cancer, which may occur after definitive local therapies.5,16-18 The present study shows a similar impact on urinary, bowel, and erectile domains after treatment. Although erectile function scores remained lower through the course of the 12-month study, bowel and bladder domains returned to baseline by month 12. Unlike other studies, we also examined psychosocial and nonurological aspects of prostate cancer treatment. We found that there was a measurable and significant positive impact on other HRQoL measurements such as decreased anxiety. Despite a variety of declines across HRQoL domains, most patients reported that their results were largely as they had expected, and their side effects were the same or better than they had expected. No patient in the cohort reported being dissatisfied with his overall treatment, and more than 90% of patients were mostly or completely satisfied with their treatment choice. This highlights the point that while sexual and other urological domains of HRQoL are important, impairments in these areas do not necessarily reflect how many patients perceive success or satisfaction with their treatment choice. We also showed correlations between treatment satisfaction and improvement in sleep, anxiety, depression, and fatigue. It is worth noting that although there were decreases in the erectile and sexual function domains after treatment, those factors were not correlated with overall treatment satisfaction. Those factors may not routinely be assessed before, during, and after treatment for prostate cancer in most clinical encounters. However, because they were strongly associated with satisfaction with treatment outcomes in this study, identification in impairments may lead to opportunities to intervene and improve the patient experience. Therefore, important “teachable moments” may be missed (for both patients and providers) during treatment decision-making encounters if other factors beyond sexual and urological outcomes are not adequately considered and addressed. Furthermore, the results of our study may help clinicians counsel patients on their expectations for their recovery after surgery and identify particular issues related to HRQoL to pay close attention to in follow-up visits.
Strengths of our study include its prospective nature, which allowed evaluation of HRQoL outcomes at multiple time points throughout the first year after treatment. In addition, we used existing patient-reported outcome tools validated by the NIH to assess changes in HRQoL. PROMIS is an NIH-supported tool that can be leveraged in the pre- and posttreatment periods to identify patients who have impairments with HRQoL. It can provide clinicians with a unique opportunity to detect and intervene in setbacks and side effects to improve patient satisfaction and HRQoL.
Limitations of the current study include that most patients selected surgery for their treatment choice and that not all patients completed all longitudinal questionnaires, although this is expected in longitudinal studies of this nature. Although all the patients were approached and encouraged to participate, many did not participate and were not captured. In addition, not all patients completed end-of-study surveys. These factors may have biased our results because of unmeasurable factors related to nonparticipation or dropout. Our study encompassed the preoperative period up to 12 months postoperatively, which may fail to identify improvements or declines in HRQoL that may occur more than 12 months postoperatively, particularly related to continence and erectile function. The participants were enrolled by 6 surgeons, and we were not able to standardize the preoperative counseling either preoperatively or postoperatively, which may have biased our results. Finally, our study population consisted of predominantly white, married men of higher socioeconomic status; therefore, our results may not be generalizable to newly diagnosed prostate cancer patients overall.
Conclusions
By using validated self-administered questionnaires, we found that despite decreased sexual and urinary function, patients treated for prostate cancer were satisfied with their treatment choice. Correlates to higher patient satisfaction included decreased anxiety, depression, fatigue, and sleep disturbances.
More than 164,690 men are expected to be diagnosed with prostate cancer in the United States in 2018.1 Men with prostate cancer face not only stress associated with the diagnosis but also decisional conflict regarding different treatment options.2 Most men diagnosed with clinically localized prostate cancer receive 1 or more of the following treatments: radical prostatectomy, external-beam radiation therapy, and/or brachytherapy, all of which are associated with posttreatment urological or sexual side effects including bowel, urinary, or erectile dysfunction.3-5 Men who choose active surveillance may experience increased anxiety associated with the constant vigilance and monitoring of their tumor status along with the uncertainty of not definitively removing or radiating their prostate.6 In addition to direct functional limitations of sexual and urological side effects, treatment can also lead to secondary psychosocial effects, including depression, self-blame, embarrassment, guilt, lower masculine self-esteem, increased reticence to participate socially or engage in sexual activity, and relationship distress.7-9 Therefore, health-related quality of life (HRQoL) and treatment satisfaction are important for this population.
Urological and sexual side effects of prostate cancer treatments are often a primary focus during treatment decision making between patients and providers. However, little prospective empirical data exist regarding the role of HRQoL and other nonurological physical and psychosocial outcomes on overall treatment satisfaction. The purpose of this study was to prospectively evaluate the role of both urological and nonurological outcomes on overall treatment satisfaction in men diagnosed with prostate cancer. We hypothesize that such an understanding can help describe changes in physical and psychosocial factors that are important to men beyond traditional urological outcomes, including their association with overall treatment satisfaction.
Methods
This was a prospective longitudinal assessment of patients from the Department of Urology at Northwestern University’s Feinberg School of Medicine in Chicago. Patients were eligible if they met the following inclusion criteria: they had been diagnosed with clinically localized or locally advanced prostate cancer; they had not yet received a primary treatment (eg, surgery, radiation, active surveillance) before their baseline assessment; they were 18 years or older; and they were able to read, write, speak, and understand English. Patients were excluded if they had a physical debilitation that would make participation not feasible or would create undue hardship, or if they had a history of diagnosed severe mental illness or hospitalization for chronic psychiatric reasons, as identified by referring physicians.
Eligible participants were approached before their treatment decision (if any). Patient enrollment occurred in 2 ways. For patients invited to participate during their clinic visit, the research assistant explained the study and obtained written informed consent for interested patients. A unique user identification and password was created for each patient, and they practiced using the touch screen computer while the research assistant observed and provided guidance as needed. When the patients were ready to start their pretreatment online interview, they completed the questionnaires by themselves. For patients who were invited to participate but were not scheduled to return in the foreseeable future, enrollment was carried out differently. In those cases, participating physicians contacted eligible patients who were not scheduled for a visit and informed them of the study opportunity. Interested patients were contacted by the research assistant who provided them with the study website address, which directed them to the online consent form. After a patient had completed the consent form, he was prompted to self-register. He received a unique user identification and password that could be used to complete the baseline assessment and subsequent assessments. However, for interested patients who did not have access to a computer or Internet connection, the research assistant provided them with paper consent forms and paper versions of all study assessments. After participants had completed the baseline assessment, the research assistant provided them with a written schedule of future assessments, which were expected to occur at 1 month posttreatment, 3 months posttreatment, 6 months posttreatment, and 12 months posttreatment.
For all follow-up appointments, participants could complete assessments either at clinic visits or from home using a secure online assessment platform called Assessment Center.10 The research assistant used a patient log to track participants and their progress in the study, which included study number, patient name (or initials), registration date, date of birth, sex, and timeline of completed or future assessments. The research assistant called or emailed participants (depending on patient preference) about a week before each of their follow-up assessments to facilitate adherence. If the participant did not log into the system by the target day, the research assistant contacted him the following day (target day +1) with a phone or email reminder to log into the system and complete the assessments. If the participant did not log in by midnight 1 day after the target day, the research assistant attempted to contact him one last time (target day +2) with either a reminder to log into the system or to ascertain his status that might be related to his noncompletion. Overall, a participant was called or e-mailed 1 to 3 times to remind him of his assessment. If he was unresponsive after 3 attempts, he was recorded as having withdrawn for an unknown reason.
At baseline and each follow-up time point, study participants completed a battery of patient-reported outcome measures, with most coming from the Patient-Reported Outcomes Measurement Information System (PROMIS)11 and the Surgical Outcomes Measurement System (SOMS).12 PROMIS is a National Institutes of Health (NIH) funded measurement system that has helped standardize and improve self-reported assessment of health status, symptoms, side effects, and different aspects of HRQoL, including physical, emotional, cognitive, and social health. SOMS is a suite of patient-reported outcome measures assessing important aspects of HRQoL after surgery. It was developed with feedback from surgeons, postoperative patients, and surgical nurses. PROMIS items were directly incorporated into numerous SOMS measures to facilitate easier comparisons and score crosswalks across measures and patient populations. In addition to PROMIS and SOMS measures, we also administered several well-known instruments of urological and sexual function, including the International Index of Erectile Function (IIEF) and American Urological Association Symptom Score Index (AUASS).13,14
Outcome measures were compared across sociodemographic and clinical variables at each time point using t tests for numerical variables (age) and with chi-square or Fisher exact tests for categorical variables; those variables with significant differences were used as covariates in statistical models. To examine differences in patient-reported scores over time, we used repeated measures analysis of covariance with general linear modeling methods. We used Pearson correlation coefficients to evaluate for correlations between quality-of-life outcomes and treatment satisfaction.
Not all participants completed each of the follow-up surveys, and reasons for dropout were prospectively documented. Most participants elected surgical resection as their primary treatment compared with the fewer than 10% of patients who chose radiation or chemotherapy as their primary treatment and about 20% of men who chose active surveillance after their initial diagnosis. Therefore, our analysis focused on patients who elected surgical resection. For comparison purposes, we included the HRQoL results from active surveillance patients.
Results
A total of 105 patients diagnosed with prostate cancer were enrolled in the study. Response rates decreased throughout the study (n = 75 at 1 month; n = 71 at 3 months; n = 64 at 6 months; n = 54 at 12 months). Sociodemographic and clinical characteristics of participants are shown in Table 1. The mean change from pretreatment (baseline) scores for each measure in patients treated with surgery is shown in Table 2, and the mean change from pretreatment scores in patients who elected active surveillance is shown in Table 3 (in both tables, a negative score denotes worsened function, and a positive change denotes improvement).
After surgery, patients reported significantly lower erectile function and sexual satisfaction scores. These included statistically significant decreases for IIEF Erectile Function, IIEF Overall Satisfaction, PROMIS Sexual Satisfaction, PROMIS Sexual Interest, and PROMIS Orgasm. In patients treated with surgery, there were significant improvements in anxiety observed for patients at each follow-up time, whereas significantly worse bladder problems were observed on SOMS Bladder at 1 and 3 months but returned to baseline by 12 months after surgery. AUASS was worse at 1 month but significantly improved at 6 and 12 months. Fatigue scores significantly worsened at 1 month but were no longer significant at 6 and 12 months. Physical Function was worsened at 1 month but not throughout the rest of the study. Bowel Problems (SOMS) were significantly worse at 1 month, but changes became nonsignificant on subsequent assessments. The only 2 domains that did not demonstrate any significant changes o
In active surveillance patients, sexual function domains were generally unchanged over the course of the study. However, unlike treated patients, there was no significant improvement in anxiety, depression, pain, fatigue, or sleep. In fact, most of these domains demonstrated worsened functioning, although these were not statistically significant. Urinary domains generally remained unchanged.
Pearson correlation coefficients between HRQoL measures and overall treatment satisfaction (assessed by the question, Are you satisfied with the results of your operation?) at each follow-up time point in patients treated with surgery are shown in Table 4. Relations between treatment satisfaction and sexual outcomes were generally statistically insignificant (r, .08-.56). However, sleep disturbance, depression, pain interference, fatigue, embarrassment, and bladder problems all demonstrated statistically significant positive associations with treatment satisfaction, with coefficients ranging from small to medium in magnitude (r, .32-.61). Other outcomes such as anxiety, physical function, and bowel problems demonstrated small to medium statistically significant associations with treatment satisfaction (r, .04-.60) but not at every time point. We performed t tests to examine treatment satisfaction in patients with detectable initial posttreatment prostate-specific antigen (PSA; >0.01 ng/mL). We found no difference in treatment satisfaction between patients with detectable PSA values and those with undetectable PSA at each time point.
When the patients were asked, Compared with what you expected, how do you rate the results of your operation?, most of those treated with surgery reported that the results of their operation were better than they had expected (Figure 1A; p. e137). More than 75% of the patients had results that were as expected or better than expected. When asked, Compared with what you expected, how do you rate your side effects of the operation?, almost 70% of patients reported side effects no worse than expected (Figure 1B). When asked, Are you satisfied with the results of your operation?, most patients reported that overall, they were satisfied with the results of their operation (Figure 1C).
At 12 months, none of the patients reported overall dissatisfaction with their treatment choice. More than 90% of patients were mostly or completely satisfied with the results of their operation.
Discussion
This prospective study assessed the HRQoL from pretreatment through 12 months posttreatment in men diagnosed with clinically localized prostate cancer that had been treated with surgery. Although the indicators of sexual function significantly decreased over time, they were not meaningfully associated with overall treatment satisfaction. Instead, a host of other factors, including psychosocial (eg, anxiety, depression, body image dissatisfaction, embarrassment), nonurological physical symptoms (pain interference, physical function, sleep disturbance, fatigue), and bladder problems, were significantly related to overall treatment satisfaction. Although this may not be surprising in other clinical oncology paradigms, the sheer surfeit of focus and attention on sexual function has overshadowed aspects of HRQoL that many men report are important to them, despite worsened sexual function outcomes.
Understanding potential treatment-related changes in HRQoL can be challenging for men when choosing providers and different therapeutic options. The increasing complexity of treatment in prostate cancer has created an opportunity to not only understand efficacy on cancer control but also focus on meaningful patient-reported outcomes. Hospitals and medical groups are increasingly aware of the importance of improving the patient care experience. Objective measures of patient satisfaction for health care providers, such as the Press-Ganey and Net Promoter score, exist to measure and improve patient experience. In prostate cancer, clinicians and large groups, including governmental agencies such as the US Preventive Services Task Force, have often focused on declines in urinary and erectile function15 without considering the full impact of prostate cancer treatment on global HRQoL. Our study was a prospective, longitudinal, self-reported examination of the impact, positive and negative, of prostate cancer treatment over a 12-month period.
Numerous studies have documented the treatment-related side effects of erectile, urinary, and bowel dysfunction in patients treated for prostate cancer, which may occur after definitive local therapies.5,16-18 The present study shows a similar impact on urinary, bowel, and erectile domains after treatment. Although erectile function scores remained lower through the course of the 12-month study, bowel and bladder domains returned to baseline by month 12. Unlike other studies, we also examined psychosocial and nonurological aspects of prostate cancer treatment. We found that there was a measurable and significant positive impact on other HRQoL measurements such as decreased anxiety. Despite a variety of declines across HRQoL domains, most patients reported that their results were largely as they had expected, and their side effects were the same or better than they had expected. No patient in the cohort reported being dissatisfied with his overall treatment, and more than 90% of patients were mostly or completely satisfied with their treatment choice. This highlights the point that while sexual and other urological domains of HRQoL are important, impairments in these areas do not necessarily reflect how many patients perceive success or satisfaction with their treatment choice. We also showed correlations between treatment satisfaction and improvement in sleep, anxiety, depression, and fatigue. It is worth noting that although there were decreases in the erectile and sexual function domains after treatment, those factors were not correlated with overall treatment satisfaction. Those factors may not routinely be assessed before, during, and after treatment for prostate cancer in most clinical encounters. However, because they were strongly associated with satisfaction with treatment outcomes in this study, identification in impairments may lead to opportunities to intervene and improve the patient experience. Therefore, important “teachable moments” may be missed (for both patients and providers) during treatment decision-making encounters if other factors beyond sexual and urological outcomes are not adequately considered and addressed. Furthermore, the results of our study may help clinicians counsel patients on their expectations for their recovery after surgery and identify particular issues related to HRQoL to pay close attention to in follow-up visits.
Strengths of our study include its prospective nature, which allowed evaluation of HRQoL outcomes at multiple time points throughout the first year after treatment. In addition, we used existing patient-reported outcome tools validated by the NIH to assess changes in HRQoL. PROMIS is an NIH-supported tool that can be leveraged in the pre- and posttreatment periods to identify patients who have impairments with HRQoL. It can provide clinicians with a unique opportunity to detect and intervene in setbacks and side effects to improve patient satisfaction and HRQoL.
Limitations of the current study include that most patients selected surgery for their treatment choice and that not all patients completed all longitudinal questionnaires, although this is expected in longitudinal studies of this nature. Although all the patients were approached and encouraged to participate, many did not participate and were not captured. In addition, not all patients completed end-of-study surveys. These factors may have biased our results because of unmeasurable factors related to nonparticipation or dropout. Our study encompassed the preoperative period up to 12 months postoperatively, which may fail to identify improvements or declines in HRQoL that may occur more than 12 months postoperatively, particularly related to continence and erectile function. The participants were enrolled by 6 surgeons, and we were not able to standardize the preoperative counseling either preoperatively or postoperatively, which may have biased our results. Finally, our study population consisted of predominantly white, married men of higher socioeconomic status; therefore, our results may not be generalizable to newly diagnosed prostate cancer patients overall.
Conclusions
By using validated self-administered questionnaires, we found that despite decreased sexual and urinary function, patients treated for prostate cancer were satisfied with their treatment choice. Correlates to higher patient satisfaction included decreased anxiety, depression, fatigue, and sleep disturbances.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7‐30.
2. Berry DL, Ellis WJ, Woods NF, Schwien C, Mullen KH, Yang C. Treatment decision-making by men with localized prostate cancer: the influence of personal factors. Urol Oncol. 2003;21(2):93-100.
3. Dubbelman YD, Dohle GR, Schröder FH. Sexual function before and after radical retropubic prostatectomy: a systematic review of prognostic indicators for a successful outcome. Eur Urol. 2006;50(4):711-718; discussion 718-720.
4. McCullough AR. Sexual dysfunction after radical prostatectomy. Rev Urol. 2005;7(2 suppl):S3-S10.
5. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250-1261.
6. Latini DM, Hart SL, Knight SJ, et al. The relationship between anxiety and time to treatment for patients with prostate cancer on surveillance. J Urol. 2007;178(3, pt 1):826-831; discussion 831-832.
7. Meyer JP, Gillatt DA, Lockyer R, Macdonagh R. The effect of erectile dysfunction on the quality of life of men after radical prostatectomy. BJU Int. 2003;92(9):929-931.
8. Casey RG, Corcoran NM, Goldenberg SL. Quality of life issues in men undergoing androgen deprivation therapy: a review. Asian J Androl. 2012;14(2):226-231.
9. Segrin C, Badger TA, Harrington J. Interdependent psychological quality of life in dyads adjusting to prostate cancer. Health Psychol. 2012;31(1):70-79.
10. Gershon RC, Rothrock N, Hanrahan R, Bass M, Cella D. The use of PROMIS and assessment center to deliver patient-reported outcome measures in clinical research. J Appl Meas. 2010;11(3):304-314.
11. Cella D, Yount S, Rothrock N, et al. The patient-reported outcomes measurement information system (PROMIS): progress of an NIH roadmap cooperative group during its first two years. Med Care. 2007;45(5 suppl 1):S3-S11.
12. Zapf M, Denham W, Barrera E, et al. Patient-centered outcomes after laparoscopic cholecystectomy. Surg Endosc. 2013;27(12):4491-4498.
13. Barry MJ, Fowler FJ Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549-1557; discussion 1564.
14. Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822-830.
15. United States Preventive Services Task Force. Final update summary: prostate cancer: screening. http:// www.uspreventiveservicestaskforce.org/Page/ Document/UpdateSummaryFinal/prostate-cancer-screening. Updated July 2015. Accessed April 14, 2017
16. Litwin MS, Gore JL, Kwan L, et al. Quality of life after surgery, external beam irradiation, or brachytherapy for early-stage prostate cancer. Cancer. 2007;109(11):2239-2247.
17. Miwa S, Mizokami A, Konaka H, et al. Prospective longitudinal comparative study of health-related quality of life and treatment satisfaction in patients treated with hormone therapy, radical retropubic prostatectomy, and high or low dose rate brachytherapy for prostate cancer. Prostate Int. 2013;1(3):117-124.
18. Miller DC, Sanda MG, Dunn RL et al. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005;23(12):2772-2780.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7‐30.
2. Berry DL, Ellis WJ, Woods NF, Schwien C, Mullen KH, Yang C. Treatment decision-making by men with localized prostate cancer: the influence of personal factors. Urol Oncol. 2003;21(2):93-100.
3. Dubbelman YD, Dohle GR, Schröder FH. Sexual function before and after radical retropubic prostatectomy: a systematic review of prognostic indicators for a successful outcome. Eur Urol. 2006;50(4):711-718; discussion 718-720.
4. McCullough AR. Sexual dysfunction after radical prostatectomy. Rev Urol. 2005;7(2 suppl):S3-S10.
5. Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250-1261.
6. Latini DM, Hart SL, Knight SJ, et al. The relationship between anxiety and time to treatment for patients with prostate cancer on surveillance. J Urol. 2007;178(3, pt 1):826-831; discussion 831-832.
7. Meyer JP, Gillatt DA, Lockyer R, Macdonagh R. The effect of erectile dysfunction on the quality of life of men after radical prostatectomy. BJU Int. 2003;92(9):929-931.
8. Casey RG, Corcoran NM, Goldenberg SL. Quality of life issues in men undergoing androgen deprivation therapy: a review. Asian J Androl. 2012;14(2):226-231.
9. Segrin C, Badger TA, Harrington J. Interdependent psychological quality of life in dyads adjusting to prostate cancer. Health Psychol. 2012;31(1):70-79.
10. Gershon RC, Rothrock N, Hanrahan R, Bass M, Cella D. The use of PROMIS and assessment center to deliver patient-reported outcome measures in clinical research. J Appl Meas. 2010;11(3):304-314.
11. Cella D, Yount S, Rothrock N, et al. The patient-reported outcomes measurement information system (PROMIS): progress of an NIH roadmap cooperative group during its first two years. Med Care. 2007;45(5 suppl 1):S3-S11.
12. Zapf M, Denham W, Barrera E, et al. Patient-centered outcomes after laparoscopic cholecystectomy. Surg Endosc. 2013;27(12):4491-4498.
13. Barry MJ, Fowler FJ Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549-1557; discussion 1564.
14. Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822-830.
15. United States Preventive Services Task Force. Final update summary: prostate cancer: screening. http:// www.uspreventiveservicestaskforce.org/Page/ Document/UpdateSummaryFinal/prostate-cancer-screening. Updated July 2015. Accessed April 14, 2017
16. Litwin MS, Gore JL, Kwan L, et al. Quality of life after surgery, external beam irradiation, or brachytherapy for early-stage prostate cancer. Cancer. 2007;109(11):2239-2247.
17. Miwa S, Mizokami A, Konaka H, et al. Prospective longitudinal comparative study of health-related quality of life and treatment satisfaction in patients treated with hormone therapy, radical retropubic prostatectomy, and high or low dose rate brachytherapy for prostate cancer. Prostate Int. 2013;1(3):117-124.
18. Miller DC, Sanda MG, Dunn RL et al. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005;23(12):2772-2780.
Qualitative assessment of organizational barriers to optimal lung cancer care in a community hospital setting in the United States
Lung cancer is a major public health challenge in the United States. It is the leading cause of cancer death in the United States, accounting for 27% of all cancer deaths, and it has an aggregate 5-year survival rate of 18%.1 Advances in diagnostic and treatment options are rapidly increasing the complexity of lung cancer care delivery, which involves multiple specialty providers and often cuts across health care institutions.2-4 Navigating the process of care while coping with the complexities of the illness can be overwhelming for both the patient and the caregiver.5 With increasing regulations and cost-cutting measures, the health care system in the United States can pose many challenges, especially for those dealing with catastrophic and life-threatening illnesses. Any barrier to accessing care often increases anxiety in patients, who are already trying to cope with the management of their disease.6-8
The concept of barriers to quality care (such as the receipt of timely and appropriate diagnostic and staging work-up and treatment selection according to evidence-based guidelines) is generally used in the context of improving health care management or prevention programs.9-13 Barriers might include high costs, transportation, distance, underinsurance, limited hours for access to care, patient sharing by physicians, and a lack of access to information about physicians’ recommendations.10,14-16 Such barriers have been categorized as organizational (leadership and workforce), structural (process of care), clinical (provider-patient encounter), and macro (policy and population).17,18 Organizational barriers are defined as impediments encountered within the medical system and health care organizations when accessing, receiving, and delivering care.12 Several organizational barriers have been identified in the literature based on characteristics of the targeted population (eg, race, ethnicity, type of illness), key stakeholder views, and aspects of care (eg, screening, preventive practice, care, and treatment).
In a systematic review, Betancourt and colleagues reported provider-patient interactions, processes of care, and language as some of the barriers to receiving quality care.17 Although cancer screening has been shown to reduce mortality in the adult population for several types of cancer,19-21 barriers that impede access to services have been identified as emanating not only from the macro level (eg, age of screening, reimbursement problems, screening guidelines) or inter- and intra-individual levels (eg, awareness of screening, various perspectives on life and cancer, comorbidities, social support), but also from the organization (organizational infrastructure that inhibits screening because of limited participation in research trials) and provider levels (impaired communication regarding screening between patient and physician, low commitment to shared decision-making, provider’s awareness of screening and screening guidelines).18 Other organizational barriers, such as difficulty navigating the health care system, poor interaction between patients and medical staff, and language barriers, have been identified in a systematic review of breast cancer screening in immigrant and minority women.22
Other barriers to quality cancer care reported by patients include knowledge about the disease and treatment, poor communication with providers, lack of coordination and timeliness of care, and lack of attention to care. Providers have identified other barriers to quality care, which include a lack of access to care, reimbursement problems, poor psychosocial support services, accountability of care, provider workload, and inadequate patient education.23 Few qualitative studies have been conducted to understand the organizational barriers that lung cancer patients and their caregivers face within the health care system.
Through the use of focus groups, we sought the perspectives of lung cancer patients and their caregivers on the organizational barriers that they experience while navigating the health care system. Identifying and understanding these barriers can help health care professionals work with patients and their caregivers to alleviate these stressors in an already difficult time.3,24 In addition, a more thorough understanding of patients’ and caregivers’ perspectives on organizational barriers may help improve health care delivery and, thus, patient satisfaction.
Methods
With the approval of the Institutional Review Boards of the University of Memphis and the Baptist Memorial Health Care Corporation, we conducted focus groups with lung cancer patients and their informal caregivers to understand the challenges they encounter while navigating the health care system during their illness. The Baptist Memorial Health Care system is centered in the Mid-South region of the United States, which has some of the highest US lung cancer incidence rates.25
Research staff identified potential participants from a roster of patients provided by treatment clinics within the system. Patients eligible for this study had received care for suspected lung cancer within a community-based health care system within 6 months preceding the date of the focus group. Eligible patients were approached by the research staff by cold calling or in-person contact during clinic visits for their consent to participate in the study. From a compiled list of 219 patients, 89 received initial contact to gauge interest. Of those, 42 patients were formally approached and asked to participate; 22 agreed to participate, and 20 did not participate for reasons including illness, previous participation in other forms of patient feedback, lack of interest, failure to show up to focus group sessions, change of mind, lack of transportation, or other commitments. Patients identified their informal caregivers to form patient-caregiver dyads. All patients and caregivers provided written informed consent before participating in the focus groups.
We conducted 10 focus groups during March 2013 through January 2014 – 5 with 22 patients and 5 with 24 caregivers (Table 1). Eight of the focus groups were conducted in Memphis, Tennessee, and to obtain the perspectives of patients from a rural setting, we conducted 2 focus groups in Grenada, Mississippi. All of the focus groups were facilitated by a medical anthropologist (SK) and a clinical psychologist (KDW), neither of whom was affiliated with the health care system. Each facilitator was accompanied by a note-taker. Patient-caregiver dyads came to the designated location together. Two focus groups (one for patients, the other for caregivers) were then conducted simultaneously in 2 separate rooms. The facilitators used a pilot-tested focus group interview guide during each session. The items in the focus group guide revolved around experience with the health care system in diagnosis and treatment; timeliness with appointments and procedures for diagnosis and subsequent care; physician communication in being informed about the disease, treatment, and getting questions answered; coordination of care; other challenges in receiving quality care; and suggestions for improving the patient and caregiver experience with the health care system.
The focus group sessions lasted 1 to 2 hours and were audiorecorded and transcribed verbatim. The data were analyzed by using Dedoose software version 5.0.11 (Sociocultural Research Consultants, Los Angeles, California). Data collection and analysis were conducted concurrently to achieve theoretical saturation. Creswell’s 7-step analysis framework was used as a guide to code and interpret the data.26 The process involved collecting raw data, preparing and organizing transcripts, reading the transcripts, coding the data with the help of qualitative software, analyzing the data for themes and subthemes, interpreting the themes, and devising the meaning of the themes.26 Initial codes were categorized and compared to determine recurrent themes. Three members of the research team independently reviewed the transcripts, extensively discussed the content, and developed consensus around the identified themes. Critical and rigorous steps were taken throughout data collection and analysis to ensure the credibility, transferability, dependability, and confirmability of the qualitative data.27-29 In addition, elements of the Consolidated Criteria for Reporting Qualitative Research checklist were used to strengthen the data collection, analysis, and reporting process.30
Results
The 10 focus groups included 46 participants: 22 patients and 24 caregivers (some patients brought multiple caregivers). Of the 22 patients, 12 were women and 7 were black. An equal proportion of patients had at least a high school education as had a college or postgraduate degree. Although all of the patients had had a lung lesion suspicious for lung cancer, 19 eventually had a histologic diagnosis of lung cancer. The remaining 3 patients were all evaluated in a thoracic oncology clinic but were eventually found to have metastatic breast cancer, lymphoma, and a granuloma. Nine patients were either currently in the treatment decision-making process or actively receiving treatment, 11 had completed treatment within the preceding 6 months, and 2 had completed treatment more than 6 months previously. Treatment covered the spectrum from curative intent to palliative care. Of the 24 caregivers, 18 were women and 7 were black; 12 caregivers had at least a college education, of whom 2 had postgraduate degrees (Table 2).
Based on participants’ feedback, we identified 4 main levels within the system where barriers to optimal care occurred: policy, institutional, provider, and patient. From our qualitative analyses, we identified a central theme associated with each level, around which the barriers coalesced. The themes were insurance, scheduling, provider communication, and patient knowledge. At the policy level, medical insurance was perceived to affect the timeliness of care and to be a deterrent to timely diagnosis and quality treatment. Lack of insurance was a daunting obstacle for indigent patients. However, even those who were insured felt that dealing with insurance companies was a significant barrier to care. At the institutional level, appointment scheduling caused problems for both patients and their caregivers. At the health care provider level, communication was perceived as a major problem. And finally, at the patient level, both patient and caregiver lack of knowledge of lung cancer and the processes inherent in lung cancer treatment were barriers for optimal diagnosis and treatment (Table 3).
Insurance barriers
At the policy level, health insurance was reported as a significant barrier to accessing health care. Patients and caregivers reported delays in diagnosis and/or treatment because of either lack of insurance or lag time in insurance processing of clinician requests. Insurance restrictions on tests, procedures, and office visits presented difficulties in getting additional opinions from providers regarding diagnosis, prognosis, or treatment plans. Some patients were no longer able to see providers after they had met the test or office visit limit allotted by insurance providers. One patient shared the following experience with office visits leading up to their lung cancer diagnosis:
Patient … with my insurance I just had only 12 office visits … and I had already maxed those out.
In other instances, insurers would not cover hospital or clinic visits if certain logistic protocols were not met. This would sometimes leave patients stranded for a period of time without receiving any care.
Caretaker … went home and went to one of those minor emergency clinics and they sent her to [xx hospital in another city]. There they did nothing. That was then over the weekend, and my son wanted to get her out of there to get her into practice because they weren’t doing anything. They said, ‘No. Your insurance won’t pay for it unless you stay here till we sign the papers to be transferred.’ … It was a bandage. Period.
Some insurers would not cover certain health services outside of routine testing protocols for the patients’ conditions. This lack of coverage caused patients to pay out of pocket for needed care.
Patient Nothing in the lymph nodes, but if it hadn’t been for me going ahead with this [coronary] calcium score, the insurance wasn’t gonna pay anything. If it wouldn’t been for the 79 bucks or the family situation, I wouldn’t be sitting [here] today.
Individuals who had not yet met the age requirement for Medicare reported being without insurance for a period of time, which contributed to delays in accessing care.
Caregiver [xx patient] probably … could’ve been diagnosed maybe even months ago, but she is in that in-between where she gets Social Security but she’s not 65 until November, so she has no insurance.
Scheduling barriers
At the institutional level, patients and caregivers reported problems with appointment scheduling. Logistic problems with adjusting work schedules and arranging for transportation as well as long wait times before evaluation by a provider were recurrent themes expressed by both patients and caregivers. Many had become resigned to the expectation of long wait times during appointments.
Patient I have to call the month before to make the appointment because they don’t take appointments so far—‘Oh, we’re not working on that yet.’ I find that very annoying ....
Caregiver The last time I was there I waited four hours.
Caregiver … your appointment at 9:00 and you get called back at 9:30 or 10:00 and you get to see the doctor by 11:00, but that’s not any different than anywhere, unfortunately ….
Rescheduled appointments also posed a problem for participants. Constant rescheduling was an inconvenience for both patients and caregivers. Many were unhappy with rescheduling because both patients and caregivers had prepared mentally and physically for an appointment, only to be told that they would have to reschedule, which caused delays in the care process.
Patient I think every single visit I had with him gets rescheduled at least twice ....
Caregiver Three times this week we’ve been geared up, ready to have chemo and they keep changing it.
Patient Everything was fine with me, but they keep cancelling my appointments ....
Some participants perceived that the popularity of physicians might explain the difficulty with scheduling. Patients suggested that it is challenging to get appointments with better-known physicians, so they are more accepting of appointments at any time, even if the time is inconvenient for them.
Caregiver Of course, … if you have a popular doctor, sometimes you don’t always get the appointment you want …
Participants also expressed frustration with the way appointments were rescheduled. They felt as though the physicians were not concerned about their lives outside of office visits.
Patient … patients actually have lives. Many of them have jobs or families or responsibilities.
Communication barriers
At the provider level, poor communication between health care providers and patients was perceived as a major impediment to the quality of care patients received. Both patients and caregivers emphasized the importance of open patient-provider interactions and that there was a lack of such open communications in many instances. There was concern regarding the way diagnoses or prognoses were relayed to patients. Many times, physicians were insensitive and disregarded the sentiments of the patients and caregivers when delivering news about the patients’ condition, as one caregiver shared,
Caregiver … the pulmonary man came … in the room and said, ‘Oh, don’t worry about your lungs. Something else will get you first,’ which was a very, very bad thing to say.
Participants also expressed concerns that they were not properly prepared for treatments by their physicians because vital information was not discussed. They felt as if physicians were not realistic about potential outcomes. This resulted in patients and caregivers being too optimistic and later disappointed when the outcome was not what they had originally expected.
Patient Until I got to this office, I was totally oversold on everything. I was told surgery … robotic, not invasive. Day one, surgery. Day two, tubes out. Day three, go home. I expected to be home on Sunday night, stir-frying vegetables, and making dinner, feeding my cat. I was in ICU four days … I went home with oxygen. I mean I thought I was just gonna walk outta there…. You take a little thing out and you put a Band-Aid on, and you go home.
Data also revealed that patients were unsure of their condition, even following treatment. Information was not communicated to patients about the specifics of their disease, either because of miscommunication or minimal patient-provider time spent during office visits. This lack of communication between patients and providers often left patients and caregivers uncertain about exactly what condition they had or what they were being treated for.
Patient I just can’t have the time with Dr. xx, cuz he’s so busy...
Patient … I didn’t understand. Which exactly what type of cancer did I have, cuz I’m—really to tell you the truth—I’m still wondering.
Knowledge barriers
Patients and caregivers also identified a lack of education and knowledge about lung cancer diagnosis and treatment as a barrier to their care. Patients and caregivers were not always fully knowledgeable about lung cancer, treatment options, or the duration of treatments. They relied on the provider to disclose such information or direct them to credible sources. In many instances, patients were misinformed about the causes of lung cancer. There were misconceptions that lung cancer was only caused by a history of smoking or genetic predisposition. Patients who did not smoke or did not have a family history of lung cancer were often confused and dismayed by the diagnosis.
Patient I was trying to figure out, why do I have lung cancer. Never smoked a day in my life.
Patients were often unaware of treatment options or side effects of various treatments. They relied on physicians to relay information and make decisions for them about treatment plans.
Patient I was told chemo would probably be the best thing for me, and I just had faith that Dr. xx knows more about it than me.
Patient I’m doing chemo but it’s — what I’m doing is different. Of course, I don’t know anything about it actually either. It’s what I hear from other people.
Other patients relied on their own sources for information about their condition, either through the Internet, from family members and/or friends, or from preconceived notions.
Patient Well — of course in the meantime, I read — because they said it was a small cell, very aggressive, so I felt like everything I read — that’s the deal. I think we’ve become where we can get on the internet and look up so much, that to me, I was gonna be gone.
Patient When he said, ‘Cancer,’ I said, ‘Well, I thought cancer was a heredity thing? That you have to have somebody in your family that has it…’
Discussion
Organizational barriers are an important consideration in the delivery and receipt of high-quality, patient-centered lung cancer care. This qualitative study of patients being treated for lung cancer and their informal caregivers revealed several common perceived organizational barriers to receiving care, including health insurance coverage restrictions, appointment scheduling difficulties, quality of communication with physicians, and failure to properly educate the patient and family about the disease and what to expect of the treatment process.
The provider communication and patient knowledge barriers seem to reinforce each other and could be improved through focused efforts on the quality of communication between patients and their caregivers and clinical care providers. Patients expect, but are often deprived of, open and active dialogue with their providers. Improved communication can be helpful in educating patients and their caregivers about their disease, prognosis, and treatment goals. Although communication ranks highly as a patient and caregiver priority, there is often a disconnect between patients and caregivers and their physicians.31 Patients and caregivers often want to be more involved in the decision-making process, and effective communication between physicians and patients has been linked to the patient’s ability to understand, and also receive high-quality care.32,33 Failure to communicate effectively and educate patients on key aspects of their condition strips them of their autonomy in decision-making.
The involvement of a navigator for patients being treated for lung cancer could be pivotal in relieving the communication and scheduling barriers. The nurse navigator assists with coordinating effective communication and providing needed information between providers and patients and their caregivers. A navigator also serves as a single point of contact for patients and caregivers to communicate questions outside of physician visits or concerns that may not be urgent enough to warrant immediate physician response.34 The navigator coordinates patients’ appointment schedules and physician referrals and communicates the details of the next steps in the care-delivery process. This helps remove the barriers to care and improve patient outcomes and the quality of health care delivery, especially for patients and caregivers dealing with a life-threatening illness within a complex referral process.35
Multidisciplinary care, a much-recommended alternative care-delivery model, should, in theory, promote connectivity of providers and collaboration between providers, patients, and family members. This model could help reduce barriers for patients and caregivers.3,24,36 A network of connected providers can better coordinate treatment plans, easily share test results, and provide built-in second opinions. Given the increasingly multimodal approach to the diagnosis, staging, and treatment of lung cancer, the multidisciplinary model could allow physicians to consider multiple perspectives and care-delivery options and, ideally, develop consensus around the optimal approach for each individual patient in one setting. This can shorten the length of time before treatment and establish a plan that is tailored to the patient’s needs.24
The National Academy of Medicine (formerly, Institute of Medicine) proposes that modern health care systems have 6 aims for quality improvement: safety, effectiveness, patient-centeredness, timeliness, efficiency, and equity.37 It would take changes in the design and implementation of organizational support systems at the policy, institutional, and provider level for those aims to be achieved. Further investigation of the problems identified by patients and caregivers could lead to innovative solutions to improve lung cancer care. Future work should evaluate the most effective communication styles in patient-provider interactions, particularly in regard to to lung cancer diagnosis and treatment, and investigate how multidisciplinary models influence patient-provider communication and patient care.
Limitations
This study has several limitations. Less than half of those approached for the study participated in the study for various reasons, which may have introduced selection bias in terms of not having the perspectives of patients not willing or able to participate in the study. Though focus groups are known to generate rich in-depth views of certain issues, they have been criticized as potentially lacking rigor and generalizability. To address this concern, we used a standardized script for each focus group and involved multiple members of the research team in data analysis and interpretation. Also, this study enrolled participants from a single health care institution and did not use a comparison group. There might be institutional and geographic differences in the experience of lung cancer care, which might further limit the generalizability of the results of this study.
Conclusions
Despite those limitations, this study offers valuable insight into the barriers that lung cancer patients and caregivers encounter while navigating a community-level health care system. Eliminating or minimizing these barriers will require strategic plans that help mitigate insurance-related, scheduling, provider-patient communication, and patient/caregiver knowledge acquisition problems and translate them into tactical actions for quality improvement. This is one of the first qualitative studies conducted to understand the organizational barriers that lung cancer patients and their caregivers face within a health care system. Additional research is needed to explore these barriers and develop viable solutions.
Disclaimer
All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute or its Board of Governors or Methodology Committee.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30.
2. Riedel RF, Wang X, McCormack M, et al. Impact of a multidisciplinary thoracic oncology clinic on the timeliness of care. J Thorac Oncol. 2006;1(7):692-696.
3. Seek A, Hogle WP. Modeling a better way: navigating the health care system for patients with lung cancer. Clin J Oncol Nurs. 2007;11(1):81-85.
4. Sorensen R, Iedema R. Redefining accountability in health care: managing the plurality of medical interests. Health. 2008;12(1):87-106.
5. Mosher CE, Jaynes HA, Hanna N, Ostroff JS. Distressed family caregivers of lung cancer patients: an examination of psychosocial and practical challenges. Support Care Cancer. 2013;21(2):431-437.
6. Cantril C, Haylock PJ. Patient navigation in the oncology care setting. Semin Oncol Nurs. 2013;29(2):76-90.
7. Freeman HP. Patient navigation: a community based strategy to reduce cancer disparities. J Urban Health. 2006;83(2):139-141.
8. Kwon DH, Tisnado DM, Keating NL, et al. Physician reported barriers to referring cancer patients to specialists: prevalence, factors, and association with career satisfaction. Cancer. 2015;121(1):113-122.
9. Andersen SE. Implementation of a new prescription system. A qualitative survey of organizational barriers. Ugeskr Laeger. 2002;164(38):4449-4453.
10. Rousseau L, Guay M, Archambault D, El m’ala Z, Abdelaziz N. Do organizational barriers to pneumococcal and influenza vaccine access exist? Can J Public Health. 2007;98(2):105-110.
11. Storey J, Buchanan D. Health care governance and organizational barriers to learning from mistakes. J Health Organ Manag. 2008;22(6):642-651.
12. Ziegenfuss JT Jr. Organizational barriers to quality improvement in medical and health care organizations. Qual Assur Util Rev. 1991;6(4):115-122.
13. Rihari-Thomas J, DiGiacomo M, Phillips J, Newton P, Davidson PM. Clinician perspectives of barriers to effective implementation of a rapid response system in an academic health centre: a focus group study. Int J Health Policy Manag. 2017;6(8):447-456.
14. Dutton D. Financial, organizational and professional factors affecting health care utilization. Soc Sci Med. 1986;23(7):721-735.
15. King CJ, Chen J, Dagher RK, Holt CL, Thomas SB. Decomposing differences in medical care access among cancer survivors by race and ethnicity. Am J Med Qual. 2015;30(5):459-469.
16. Renzaho AM, Romios P, Crock C, Sønderlund AL. The effectiveness of cultural competence programs in ethnic minority patient-centered health care—a systematic review of the literature. Int J Qual Health Care. 2013;25(3):261-269.
17. Betancourt JR, Green AR, Carrillo JE, Ananeh-Firempong O. Defining cultural competence: a practical framework for addressing racial/ethnic disparities in health and health care. Public Health Rep. 2003;118(4):293-302.
18. Scheinfeld Gorin S, Gauthier J, Hay J, Miles A, Wardle J. Cancer screening and aging: research barriers and opportunities. Cancer. 2008;113(suppl 12):3493-3504.
19. Humphrey L, Deffebach M, Pappas M, et al. Screening for lung cancer: systematic review to update the US preventive services task force recommendation. Evidence syntheses No. 105. Rockville, MD: Agency for Health Care Research and Quality; 2013.
20. Nelson HD, Fu R, Cantor A, Pappas M, Daeges M, Humphrey L. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force Recommendation. Ann Intern Med. 2016;164(4):244-255.
21. Zauber AG. The impact of screening on colorectal cancer mortality and incidence: has it really made a difference? Dig Dis Sci. 2015;60(3):681-691.
22. Remennick L. The challenge of early breast cancer detection among immigrant and minority women in multicultural societies. Breast J. 2006;12(suppl 1):103-110.
23. Hess LM, Pohl G. Perspectives of quality care in cancer treatment: a review of the literature. Am Health Drug Benefits. 2013;6(6):321-329.
24. Kedia SK, Ward KD, Digney SA, et al. ‘One-stop shop’: lung cancer patients’ and caregivers’ perceptions of multidisciplinary care in a community health care setting. Transl Lung Cancer Res. 2015;4(4):456-464.
25. US Cancer Statistics Working Group. United States cancer statistics: 1999-2014 incidence and mortality web-based report. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute. http://www.cdc.gov/uscs. Published 2017. Accessed November 21, 2017.
26. Creswell JW. Research design: qualitative, quantitative, and mixed methods approaches. 4th ed. Thousand Oaks, CA: SAGE Publications; 2014.
27. Ballinger C. Demonstrating rigour and quality? In Finlay L, Ballinger C, eds. Qualitative research for allied health professionals: challenging choices. Chichester, England: John Wiley & Sons Ltd; 2006:235-246.
28. Finlay L. ‘Rigour’, ‘ethical integrity’ or ‘artistry’? Reflexively reviewing criteria for evaluating qualitative research. Br J Occup Ther. 2006;69(7):319-326.
29. Lincoln YS, Guba EG. Naturalistic inquiry. Newbury Park, CA: SAGE Publications; 1985.
30. Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus group. Int J Qual Health Care. 2007;19(6):349-357.
31. Neeman N, Quinn K, Shoeb M, Mourad M, Sehgal NL, Sliwka D. Postdischarge focus groups to improve the hospital experience. Am J Med Qual. 2013;28(6):536-538.
32. O’Day BL, Killeen M, Iezzoni LI. Improving health care experiences of persons who are blind or have low vision: suggestions from focus groups. Am J Med Qual. 2004;19(5):193-200.
33. Smith B, Lynch WD, Markow C, Lifsey S, Slover M. Consumers’ understanding of and interest in provider- versus practice-level quality characteristics: findings from a focus group study. Am J Med Qual. 2015;30(4):367-373.
34. Islam KM, Opoku ST, Apenteng BA, et al. Coping with an advanced stage lung cancer diagnosis: patient, caregiver, and provider perspectives on the role of the health care system. J Cancer Educ. 2016;31(3):554-558.
35. Pedersen A, Hack TF. Pilots of oncology health care: a concept analysis of the patient navigator role. Oncol Nurs Forum. 2010;37(1):55-60.
36. Gopal R. How to maintain multidisciplinary treatment schedules. J Support Oncol. 2005;3(3):248-256.
37. Committee on Quality of Health Care in America. Improving the 21st century health care system. In: Institute of Medicine, ed. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academies Press; 2001:39-60.
Lung cancer is a major public health challenge in the United States. It is the leading cause of cancer death in the United States, accounting for 27% of all cancer deaths, and it has an aggregate 5-year survival rate of 18%.1 Advances in diagnostic and treatment options are rapidly increasing the complexity of lung cancer care delivery, which involves multiple specialty providers and often cuts across health care institutions.2-4 Navigating the process of care while coping with the complexities of the illness can be overwhelming for both the patient and the caregiver.5 With increasing regulations and cost-cutting measures, the health care system in the United States can pose many challenges, especially for those dealing with catastrophic and life-threatening illnesses. Any barrier to accessing care often increases anxiety in patients, who are already trying to cope with the management of their disease.6-8
The concept of barriers to quality care (such as the receipt of timely and appropriate diagnostic and staging work-up and treatment selection according to evidence-based guidelines) is generally used in the context of improving health care management or prevention programs.9-13 Barriers might include high costs, transportation, distance, underinsurance, limited hours for access to care, patient sharing by physicians, and a lack of access to information about physicians’ recommendations.10,14-16 Such barriers have been categorized as organizational (leadership and workforce), structural (process of care), clinical (provider-patient encounter), and macro (policy and population).17,18 Organizational barriers are defined as impediments encountered within the medical system and health care organizations when accessing, receiving, and delivering care.12 Several organizational barriers have been identified in the literature based on characteristics of the targeted population (eg, race, ethnicity, type of illness), key stakeholder views, and aspects of care (eg, screening, preventive practice, care, and treatment).
In a systematic review, Betancourt and colleagues reported provider-patient interactions, processes of care, and language as some of the barriers to receiving quality care.17 Although cancer screening has been shown to reduce mortality in the adult population for several types of cancer,19-21 barriers that impede access to services have been identified as emanating not only from the macro level (eg, age of screening, reimbursement problems, screening guidelines) or inter- and intra-individual levels (eg, awareness of screening, various perspectives on life and cancer, comorbidities, social support), but also from the organization (organizational infrastructure that inhibits screening because of limited participation in research trials) and provider levels (impaired communication regarding screening between patient and physician, low commitment to shared decision-making, provider’s awareness of screening and screening guidelines).18 Other organizational barriers, such as difficulty navigating the health care system, poor interaction between patients and medical staff, and language barriers, have been identified in a systematic review of breast cancer screening in immigrant and minority women.22
Other barriers to quality cancer care reported by patients include knowledge about the disease and treatment, poor communication with providers, lack of coordination and timeliness of care, and lack of attention to care. Providers have identified other barriers to quality care, which include a lack of access to care, reimbursement problems, poor psychosocial support services, accountability of care, provider workload, and inadequate patient education.23 Few qualitative studies have been conducted to understand the organizational barriers that lung cancer patients and their caregivers face within the health care system.
Through the use of focus groups, we sought the perspectives of lung cancer patients and their caregivers on the organizational barriers that they experience while navigating the health care system. Identifying and understanding these barriers can help health care professionals work with patients and their caregivers to alleviate these stressors in an already difficult time.3,24 In addition, a more thorough understanding of patients’ and caregivers’ perspectives on organizational barriers may help improve health care delivery and, thus, patient satisfaction.
Methods
With the approval of the Institutional Review Boards of the University of Memphis and the Baptist Memorial Health Care Corporation, we conducted focus groups with lung cancer patients and their informal caregivers to understand the challenges they encounter while navigating the health care system during their illness. The Baptist Memorial Health Care system is centered in the Mid-South region of the United States, which has some of the highest US lung cancer incidence rates.25
Research staff identified potential participants from a roster of patients provided by treatment clinics within the system. Patients eligible for this study had received care for suspected lung cancer within a community-based health care system within 6 months preceding the date of the focus group. Eligible patients were approached by the research staff by cold calling or in-person contact during clinic visits for their consent to participate in the study. From a compiled list of 219 patients, 89 received initial contact to gauge interest. Of those, 42 patients were formally approached and asked to participate; 22 agreed to participate, and 20 did not participate for reasons including illness, previous participation in other forms of patient feedback, lack of interest, failure to show up to focus group sessions, change of mind, lack of transportation, or other commitments. Patients identified their informal caregivers to form patient-caregiver dyads. All patients and caregivers provided written informed consent before participating in the focus groups.
We conducted 10 focus groups during March 2013 through January 2014 – 5 with 22 patients and 5 with 24 caregivers (Table 1). Eight of the focus groups were conducted in Memphis, Tennessee, and to obtain the perspectives of patients from a rural setting, we conducted 2 focus groups in Grenada, Mississippi. All of the focus groups were facilitated by a medical anthropologist (SK) and a clinical psychologist (KDW), neither of whom was affiliated with the health care system. Each facilitator was accompanied by a note-taker. Patient-caregiver dyads came to the designated location together. Two focus groups (one for patients, the other for caregivers) were then conducted simultaneously in 2 separate rooms. The facilitators used a pilot-tested focus group interview guide during each session. The items in the focus group guide revolved around experience with the health care system in diagnosis and treatment; timeliness with appointments and procedures for diagnosis and subsequent care; physician communication in being informed about the disease, treatment, and getting questions answered; coordination of care; other challenges in receiving quality care; and suggestions for improving the patient and caregiver experience with the health care system.
The focus group sessions lasted 1 to 2 hours and were audiorecorded and transcribed verbatim. The data were analyzed by using Dedoose software version 5.0.11 (Sociocultural Research Consultants, Los Angeles, California). Data collection and analysis were conducted concurrently to achieve theoretical saturation. Creswell’s 7-step analysis framework was used as a guide to code and interpret the data.26 The process involved collecting raw data, preparing and organizing transcripts, reading the transcripts, coding the data with the help of qualitative software, analyzing the data for themes and subthemes, interpreting the themes, and devising the meaning of the themes.26 Initial codes were categorized and compared to determine recurrent themes. Three members of the research team independently reviewed the transcripts, extensively discussed the content, and developed consensus around the identified themes. Critical and rigorous steps were taken throughout data collection and analysis to ensure the credibility, transferability, dependability, and confirmability of the qualitative data.27-29 In addition, elements of the Consolidated Criteria for Reporting Qualitative Research checklist were used to strengthen the data collection, analysis, and reporting process.30
Results
The 10 focus groups included 46 participants: 22 patients and 24 caregivers (some patients brought multiple caregivers). Of the 22 patients, 12 were women and 7 were black. An equal proportion of patients had at least a high school education as had a college or postgraduate degree. Although all of the patients had had a lung lesion suspicious for lung cancer, 19 eventually had a histologic diagnosis of lung cancer. The remaining 3 patients were all evaluated in a thoracic oncology clinic but were eventually found to have metastatic breast cancer, lymphoma, and a granuloma. Nine patients were either currently in the treatment decision-making process or actively receiving treatment, 11 had completed treatment within the preceding 6 months, and 2 had completed treatment more than 6 months previously. Treatment covered the spectrum from curative intent to palliative care. Of the 24 caregivers, 18 were women and 7 were black; 12 caregivers had at least a college education, of whom 2 had postgraduate degrees (Table 2).
Based on participants’ feedback, we identified 4 main levels within the system where barriers to optimal care occurred: policy, institutional, provider, and patient. From our qualitative analyses, we identified a central theme associated with each level, around which the barriers coalesced. The themes were insurance, scheduling, provider communication, and patient knowledge. At the policy level, medical insurance was perceived to affect the timeliness of care and to be a deterrent to timely diagnosis and quality treatment. Lack of insurance was a daunting obstacle for indigent patients. However, even those who were insured felt that dealing with insurance companies was a significant barrier to care. At the institutional level, appointment scheduling caused problems for both patients and their caregivers. At the health care provider level, communication was perceived as a major problem. And finally, at the patient level, both patient and caregiver lack of knowledge of lung cancer and the processes inherent in lung cancer treatment were barriers for optimal diagnosis and treatment (Table 3).
Insurance barriers
At the policy level, health insurance was reported as a significant barrier to accessing health care. Patients and caregivers reported delays in diagnosis and/or treatment because of either lack of insurance or lag time in insurance processing of clinician requests. Insurance restrictions on tests, procedures, and office visits presented difficulties in getting additional opinions from providers regarding diagnosis, prognosis, or treatment plans. Some patients were no longer able to see providers after they had met the test or office visit limit allotted by insurance providers. One patient shared the following experience with office visits leading up to their lung cancer diagnosis:
Patient … with my insurance I just had only 12 office visits … and I had already maxed those out.
In other instances, insurers would not cover hospital or clinic visits if certain logistic protocols were not met. This would sometimes leave patients stranded for a period of time without receiving any care.
Caretaker … went home and went to one of those minor emergency clinics and they sent her to [xx hospital in another city]. There they did nothing. That was then over the weekend, and my son wanted to get her out of there to get her into practice because they weren’t doing anything. They said, ‘No. Your insurance won’t pay for it unless you stay here till we sign the papers to be transferred.’ … It was a bandage. Period.
Some insurers would not cover certain health services outside of routine testing protocols for the patients’ conditions. This lack of coverage caused patients to pay out of pocket for needed care.
Patient Nothing in the lymph nodes, but if it hadn’t been for me going ahead with this [coronary] calcium score, the insurance wasn’t gonna pay anything. If it wouldn’t been for the 79 bucks or the family situation, I wouldn’t be sitting [here] today.
Individuals who had not yet met the age requirement for Medicare reported being without insurance for a period of time, which contributed to delays in accessing care.
Caregiver [xx patient] probably … could’ve been diagnosed maybe even months ago, but she is in that in-between where she gets Social Security but she’s not 65 until November, so she has no insurance.
Scheduling barriers
At the institutional level, patients and caregivers reported problems with appointment scheduling. Logistic problems with adjusting work schedules and arranging for transportation as well as long wait times before evaluation by a provider were recurrent themes expressed by both patients and caregivers. Many had become resigned to the expectation of long wait times during appointments.
Patient I have to call the month before to make the appointment because they don’t take appointments so far—‘Oh, we’re not working on that yet.’ I find that very annoying ....
Caregiver The last time I was there I waited four hours.
Caregiver … your appointment at 9:00 and you get called back at 9:30 or 10:00 and you get to see the doctor by 11:00, but that’s not any different than anywhere, unfortunately ….
Rescheduled appointments also posed a problem for participants. Constant rescheduling was an inconvenience for both patients and caregivers. Many were unhappy with rescheduling because both patients and caregivers had prepared mentally and physically for an appointment, only to be told that they would have to reschedule, which caused delays in the care process.
Patient I think every single visit I had with him gets rescheduled at least twice ....
Caregiver Three times this week we’ve been geared up, ready to have chemo and they keep changing it.
Patient Everything was fine with me, but they keep cancelling my appointments ....
Some participants perceived that the popularity of physicians might explain the difficulty with scheduling. Patients suggested that it is challenging to get appointments with better-known physicians, so they are more accepting of appointments at any time, even if the time is inconvenient for them.
Caregiver Of course, … if you have a popular doctor, sometimes you don’t always get the appointment you want …
Participants also expressed frustration with the way appointments were rescheduled. They felt as though the physicians were not concerned about their lives outside of office visits.
Patient … patients actually have lives. Many of them have jobs or families or responsibilities.
Communication barriers
At the provider level, poor communication between health care providers and patients was perceived as a major impediment to the quality of care patients received. Both patients and caregivers emphasized the importance of open patient-provider interactions and that there was a lack of such open communications in many instances. There was concern regarding the way diagnoses or prognoses were relayed to patients. Many times, physicians were insensitive and disregarded the sentiments of the patients and caregivers when delivering news about the patients’ condition, as one caregiver shared,
Caregiver … the pulmonary man came … in the room and said, ‘Oh, don’t worry about your lungs. Something else will get you first,’ which was a very, very bad thing to say.
Participants also expressed concerns that they were not properly prepared for treatments by their physicians because vital information was not discussed. They felt as if physicians were not realistic about potential outcomes. This resulted in patients and caregivers being too optimistic and later disappointed when the outcome was not what they had originally expected.
Patient Until I got to this office, I was totally oversold on everything. I was told surgery … robotic, not invasive. Day one, surgery. Day two, tubes out. Day three, go home. I expected to be home on Sunday night, stir-frying vegetables, and making dinner, feeding my cat. I was in ICU four days … I went home with oxygen. I mean I thought I was just gonna walk outta there…. You take a little thing out and you put a Band-Aid on, and you go home.
Data also revealed that patients were unsure of their condition, even following treatment. Information was not communicated to patients about the specifics of their disease, either because of miscommunication or minimal patient-provider time spent during office visits. This lack of communication between patients and providers often left patients and caregivers uncertain about exactly what condition they had or what they were being treated for.
Patient I just can’t have the time with Dr. xx, cuz he’s so busy...
Patient … I didn’t understand. Which exactly what type of cancer did I have, cuz I’m—really to tell you the truth—I’m still wondering.
Knowledge barriers
Patients and caregivers also identified a lack of education and knowledge about lung cancer diagnosis and treatment as a barrier to their care. Patients and caregivers were not always fully knowledgeable about lung cancer, treatment options, or the duration of treatments. They relied on the provider to disclose such information or direct them to credible sources. In many instances, patients were misinformed about the causes of lung cancer. There were misconceptions that lung cancer was only caused by a history of smoking or genetic predisposition. Patients who did not smoke or did not have a family history of lung cancer were often confused and dismayed by the diagnosis.
Patient I was trying to figure out, why do I have lung cancer. Never smoked a day in my life.
Patients were often unaware of treatment options or side effects of various treatments. They relied on physicians to relay information and make decisions for them about treatment plans.
Patient I was told chemo would probably be the best thing for me, and I just had faith that Dr. xx knows more about it than me.
Patient I’m doing chemo but it’s — what I’m doing is different. Of course, I don’t know anything about it actually either. It’s what I hear from other people.
Other patients relied on their own sources for information about their condition, either through the Internet, from family members and/or friends, or from preconceived notions.
Patient Well — of course in the meantime, I read — because they said it was a small cell, very aggressive, so I felt like everything I read — that’s the deal. I think we’ve become where we can get on the internet and look up so much, that to me, I was gonna be gone.
Patient When he said, ‘Cancer,’ I said, ‘Well, I thought cancer was a heredity thing? That you have to have somebody in your family that has it…’
Discussion
Organizational barriers are an important consideration in the delivery and receipt of high-quality, patient-centered lung cancer care. This qualitative study of patients being treated for lung cancer and their informal caregivers revealed several common perceived organizational barriers to receiving care, including health insurance coverage restrictions, appointment scheduling difficulties, quality of communication with physicians, and failure to properly educate the patient and family about the disease and what to expect of the treatment process.
The provider communication and patient knowledge barriers seem to reinforce each other and could be improved through focused efforts on the quality of communication between patients and their caregivers and clinical care providers. Patients expect, but are often deprived of, open and active dialogue with their providers. Improved communication can be helpful in educating patients and their caregivers about their disease, prognosis, and treatment goals. Although communication ranks highly as a patient and caregiver priority, there is often a disconnect between patients and caregivers and their physicians.31 Patients and caregivers often want to be more involved in the decision-making process, and effective communication between physicians and patients has been linked to the patient’s ability to understand, and also receive high-quality care.32,33 Failure to communicate effectively and educate patients on key aspects of their condition strips them of their autonomy in decision-making.
The involvement of a navigator for patients being treated for lung cancer could be pivotal in relieving the communication and scheduling barriers. The nurse navigator assists with coordinating effective communication and providing needed information between providers and patients and their caregivers. A navigator also serves as a single point of contact for patients and caregivers to communicate questions outside of physician visits or concerns that may not be urgent enough to warrant immediate physician response.34 The navigator coordinates patients’ appointment schedules and physician referrals and communicates the details of the next steps in the care-delivery process. This helps remove the barriers to care and improve patient outcomes and the quality of health care delivery, especially for patients and caregivers dealing with a life-threatening illness within a complex referral process.35
Multidisciplinary care, a much-recommended alternative care-delivery model, should, in theory, promote connectivity of providers and collaboration between providers, patients, and family members. This model could help reduce barriers for patients and caregivers.3,24,36 A network of connected providers can better coordinate treatment plans, easily share test results, and provide built-in second opinions. Given the increasingly multimodal approach to the diagnosis, staging, and treatment of lung cancer, the multidisciplinary model could allow physicians to consider multiple perspectives and care-delivery options and, ideally, develop consensus around the optimal approach for each individual patient in one setting. This can shorten the length of time before treatment and establish a plan that is tailored to the patient’s needs.24
The National Academy of Medicine (formerly, Institute of Medicine) proposes that modern health care systems have 6 aims for quality improvement: safety, effectiveness, patient-centeredness, timeliness, efficiency, and equity.37 It would take changes in the design and implementation of organizational support systems at the policy, institutional, and provider level for those aims to be achieved. Further investigation of the problems identified by patients and caregivers could lead to innovative solutions to improve lung cancer care. Future work should evaluate the most effective communication styles in patient-provider interactions, particularly in regard to to lung cancer diagnosis and treatment, and investigate how multidisciplinary models influence patient-provider communication and patient care.
Limitations
This study has several limitations. Less than half of those approached for the study participated in the study for various reasons, which may have introduced selection bias in terms of not having the perspectives of patients not willing or able to participate in the study. Though focus groups are known to generate rich in-depth views of certain issues, they have been criticized as potentially lacking rigor and generalizability. To address this concern, we used a standardized script for each focus group and involved multiple members of the research team in data analysis and interpretation. Also, this study enrolled participants from a single health care institution and did not use a comparison group. There might be institutional and geographic differences in the experience of lung cancer care, which might further limit the generalizability of the results of this study.
Conclusions
Despite those limitations, this study offers valuable insight into the barriers that lung cancer patients and caregivers encounter while navigating a community-level health care system. Eliminating or minimizing these barriers will require strategic plans that help mitigate insurance-related, scheduling, provider-patient communication, and patient/caregiver knowledge acquisition problems and translate them into tactical actions for quality improvement. This is one of the first qualitative studies conducted to understand the organizational barriers that lung cancer patients and their caregivers face within a health care system. Additional research is needed to explore these barriers and develop viable solutions.
Disclaimer
All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute or its Board of Governors or Methodology Committee.
Lung cancer is a major public health challenge in the United States. It is the leading cause of cancer death in the United States, accounting for 27% of all cancer deaths, and it has an aggregate 5-year survival rate of 18%.1 Advances in diagnostic and treatment options are rapidly increasing the complexity of lung cancer care delivery, which involves multiple specialty providers and often cuts across health care institutions.2-4 Navigating the process of care while coping with the complexities of the illness can be overwhelming for both the patient and the caregiver.5 With increasing regulations and cost-cutting measures, the health care system in the United States can pose many challenges, especially for those dealing with catastrophic and life-threatening illnesses. Any barrier to accessing care often increases anxiety in patients, who are already trying to cope with the management of their disease.6-8
The concept of barriers to quality care (such as the receipt of timely and appropriate diagnostic and staging work-up and treatment selection according to evidence-based guidelines) is generally used in the context of improving health care management or prevention programs.9-13 Barriers might include high costs, transportation, distance, underinsurance, limited hours for access to care, patient sharing by physicians, and a lack of access to information about physicians’ recommendations.10,14-16 Such barriers have been categorized as organizational (leadership and workforce), structural (process of care), clinical (provider-patient encounter), and macro (policy and population).17,18 Organizational barriers are defined as impediments encountered within the medical system and health care organizations when accessing, receiving, and delivering care.12 Several organizational barriers have been identified in the literature based on characteristics of the targeted population (eg, race, ethnicity, type of illness), key stakeholder views, and aspects of care (eg, screening, preventive practice, care, and treatment).
In a systematic review, Betancourt and colleagues reported provider-patient interactions, processes of care, and language as some of the barriers to receiving quality care.17 Although cancer screening has been shown to reduce mortality in the adult population for several types of cancer,19-21 barriers that impede access to services have been identified as emanating not only from the macro level (eg, age of screening, reimbursement problems, screening guidelines) or inter- and intra-individual levels (eg, awareness of screening, various perspectives on life and cancer, comorbidities, social support), but also from the organization (organizational infrastructure that inhibits screening because of limited participation in research trials) and provider levels (impaired communication regarding screening between patient and physician, low commitment to shared decision-making, provider’s awareness of screening and screening guidelines).18 Other organizational barriers, such as difficulty navigating the health care system, poor interaction between patients and medical staff, and language barriers, have been identified in a systematic review of breast cancer screening in immigrant and minority women.22
Other barriers to quality cancer care reported by patients include knowledge about the disease and treatment, poor communication with providers, lack of coordination and timeliness of care, and lack of attention to care. Providers have identified other barriers to quality care, which include a lack of access to care, reimbursement problems, poor psychosocial support services, accountability of care, provider workload, and inadequate patient education.23 Few qualitative studies have been conducted to understand the organizational barriers that lung cancer patients and their caregivers face within the health care system.
Through the use of focus groups, we sought the perspectives of lung cancer patients and their caregivers on the organizational barriers that they experience while navigating the health care system. Identifying and understanding these barriers can help health care professionals work with patients and their caregivers to alleviate these stressors in an already difficult time.3,24 In addition, a more thorough understanding of patients’ and caregivers’ perspectives on organizational barriers may help improve health care delivery and, thus, patient satisfaction.
Methods
With the approval of the Institutional Review Boards of the University of Memphis and the Baptist Memorial Health Care Corporation, we conducted focus groups with lung cancer patients and their informal caregivers to understand the challenges they encounter while navigating the health care system during their illness. The Baptist Memorial Health Care system is centered in the Mid-South region of the United States, which has some of the highest US lung cancer incidence rates.25
Research staff identified potential participants from a roster of patients provided by treatment clinics within the system. Patients eligible for this study had received care for suspected lung cancer within a community-based health care system within 6 months preceding the date of the focus group. Eligible patients were approached by the research staff by cold calling or in-person contact during clinic visits for their consent to participate in the study. From a compiled list of 219 patients, 89 received initial contact to gauge interest. Of those, 42 patients were formally approached and asked to participate; 22 agreed to participate, and 20 did not participate for reasons including illness, previous participation in other forms of patient feedback, lack of interest, failure to show up to focus group sessions, change of mind, lack of transportation, or other commitments. Patients identified their informal caregivers to form patient-caregiver dyads. All patients and caregivers provided written informed consent before participating in the focus groups.
We conducted 10 focus groups during March 2013 through January 2014 – 5 with 22 patients and 5 with 24 caregivers (Table 1). Eight of the focus groups were conducted in Memphis, Tennessee, and to obtain the perspectives of patients from a rural setting, we conducted 2 focus groups in Grenada, Mississippi. All of the focus groups were facilitated by a medical anthropologist (SK) and a clinical psychologist (KDW), neither of whom was affiliated with the health care system. Each facilitator was accompanied by a note-taker. Patient-caregiver dyads came to the designated location together. Two focus groups (one for patients, the other for caregivers) were then conducted simultaneously in 2 separate rooms. The facilitators used a pilot-tested focus group interview guide during each session. The items in the focus group guide revolved around experience with the health care system in diagnosis and treatment; timeliness with appointments and procedures for diagnosis and subsequent care; physician communication in being informed about the disease, treatment, and getting questions answered; coordination of care; other challenges in receiving quality care; and suggestions for improving the patient and caregiver experience with the health care system.
The focus group sessions lasted 1 to 2 hours and were audiorecorded and transcribed verbatim. The data were analyzed by using Dedoose software version 5.0.11 (Sociocultural Research Consultants, Los Angeles, California). Data collection and analysis were conducted concurrently to achieve theoretical saturation. Creswell’s 7-step analysis framework was used as a guide to code and interpret the data.26 The process involved collecting raw data, preparing and organizing transcripts, reading the transcripts, coding the data with the help of qualitative software, analyzing the data for themes and subthemes, interpreting the themes, and devising the meaning of the themes.26 Initial codes were categorized and compared to determine recurrent themes. Three members of the research team independently reviewed the transcripts, extensively discussed the content, and developed consensus around the identified themes. Critical and rigorous steps were taken throughout data collection and analysis to ensure the credibility, transferability, dependability, and confirmability of the qualitative data.27-29 In addition, elements of the Consolidated Criteria for Reporting Qualitative Research checklist were used to strengthen the data collection, analysis, and reporting process.30
Results
The 10 focus groups included 46 participants: 22 patients and 24 caregivers (some patients brought multiple caregivers). Of the 22 patients, 12 were women and 7 were black. An equal proportion of patients had at least a high school education as had a college or postgraduate degree. Although all of the patients had had a lung lesion suspicious for lung cancer, 19 eventually had a histologic diagnosis of lung cancer. The remaining 3 patients were all evaluated in a thoracic oncology clinic but were eventually found to have metastatic breast cancer, lymphoma, and a granuloma. Nine patients were either currently in the treatment decision-making process or actively receiving treatment, 11 had completed treatment within the preceding 6 months, and 2 had completed treatment more than 6 months previously. Treatment covered the spectrum from curative intent to palliative care. Of the 24 caregivers, 18 were women and 7 were black; 12 caregivers had at least a college education, of whom 2 had postgraduate degrees (Table 2).
Based on participants’ feedback, we identified 4 main levels within the system where barriers to optimal care occurred: policy, institutional, provider, and patient. From our qualitative analyses, we identified a central theme associated with each level, around which the barriers coalesced. The themes were insurance, scheduling, provider communication, and patient knowledge. At the policy level, medical insurance was perceived to affect the timeliness of care and to be a deterrent to timely diagnosis and quality treatment. Lack of insurance was a daunting obstacle for indigent patients. However, even those who were insured felt that dealing with insurance companies was a significant barrier to care. At the institutional level, appointment scheduling caused problems for both patients and their caregivers. At the health care provider level, communication was perceived as a major problem. And finally, at the patient level, both patient and caregiver lack of knowledge of lung cancer and the processes inherent in lung cancer treatment were barriers for optimal diagnosis and treatment (Table 3).
Insurance barriers
At the policy level, health insurance was reported as a significant barrier to accessing health care. Patients and caregivers reported delays in diagnosis and/or treatment because of either lack of insurance or lag time in insurance processing of clinician requests. Insurance restrictions on tests, procedures, and office visits presented difficulties in getting additional opinions from providers regarding diagnosis, prognosis, or treatment plans. Some patients were no longer able to see providers after they had met the test or office visit limit allotted by insurance providers. One patient shared the following experience with office visits leading up to their lung cancer diagnosis:
Patient … with my insurance I just had only 12 office visits … and I had already maxed those out.
In other instances, insurers would not cover hospital or clinic visits if certain logistic protocols were not met. This would sometimes leave patients stranded for a period of time without receiving any care.
Caretaker … went home and went to one of those minor emergency clinics and they sent her to [xx hospital in another city]. There they did nothing. That was then over the weekend, and my son wanted to get her out of there to get her into practice because they weren’t doing anything. They said, ‘No. Your insurance won’t pay for it unless you stay here till we sign the papers to be transferred.’ … It was a bandage. Period.
Some insurers would not cover certain health services outside of routine testing protocols for the patients’ conditions. This lack of coverage caused patients to pay out of pocket for needed care.
Patient Nothing in the lymph nodes, but if it hadn’t been for me going ahead with this [coronary] calcium score, the insurance wasn’t gonna pay anything. If it wouldn’t been for the 79 bucks or the family situation, I wouldn’t be sitting [here] today.
Individuals who had not yet met the age requirement for Medicare reported being without insurance for a period of time, which contributed to delays in accessing care.
Caregiver [xx patient] probably … could’ve been diagnosed maybe even months ago, but she is in that in-between where she gets Social Security but she’s not 65 until November, so she has no insurance.
Scheduling barriers
At the institutional level, patients and caregivers reported problems with appointment scheduling. Logistic problems with adjusting work schedules and arranging for transportation as well as long wait times before evaluation by a provider were recurrent themes expressed by both patients and caregivers. Many had become resigned to the expectation of long wait times during appointments.
Patient I have to call the month before to make the appointment because they don’t take appointments so far—‘Oh, we’re not working on that yet.’ I find that very annoying ....
Caregiver The last time I was there I waited four hours.
Caregiver … your appointment at 9:00 and you get called back at 9:30 or 10:00 and you get to see the doctor by 11:00, but that’s not any different than anywhere, unfortunately ….
Rescheduled appointments also posed a problem for participants. Constant rescheduling was an inconvenience for both patients and caregivers. Many were unhappy with rescheduling because both patients and caregivers had prepared mentally and physically for an appointment, only to be told that they would have to reschedule, which caused delays in the care process.
Patient I think every single visit I had with him gets rescheduled at least twice ....
Caregiver Three times this week we’ve been geared up, ready to have chemo and they keep changing it.
Patient Everything was fine with me, but they keep cancelling my appointments ....
Some participants perceived that the popularity of physicians might explain the difficulty with scheduling. Patients suggested that it is challenging to get appointments with better-known physicians, so they are more accepting of appointments at any time, even if the time is inconvenient for them.
Caregiver Of course, … if you have a popular doctor, sometimes you don’t always get the appointment you want …
Participants also expressed frustration with the way appointments were rescheduled. They felt as though the physicians were not concerned about their lives outside of office visits.
Patient … patients actually have lives. Many of them have jobs or families or responsibilities.
Communication barriers
At the provider level, poor communication between health care providers and patients was perceived as a major impediment to the quality of care patients received. Both patients and caregivers emphasized the importance of open patient-provider interactions and that there was a lack of such open communications in many instances. There was concern regarding the way diagnoses or prognoses were relayed to patients. Many times, physicians were insensitive and disregarded the sentiments of the patients and caregivers when delivering news about the patients’ condition, as one caregiver shared,
Caregiver … the pulmonary man came … in the room and said, ‘Oh, don’t worry about your lungs. Something else will get you first,’ which was a very, very bad thing to say.
Participants also expressed concerns that they were not properly prepared for treatments by their physicians because vital information was not discussed. They felt as if physicians were not realistic about potential outcomes. This resulted in patients and caregivers being too optimistic and later disappointed when the outcome was not what they had originally expected.
Patient Until I got to this office, I was totally oversold on everything. I was told surgery … robotic, not invasive. Day one, surgery. Day two, tubes out. Day three, go home. I expected to be home on Sunday night, stir-frying vegetables, and making dinner, feeding my cat. I was in ICU four days … I went home with oxygen. I mean I thought I was just gonna walk outta there…. You take a little thing out and you put a Band-Aid on, and you go home.
Data also revealed that patients were unsure of their condition, even following treatment. Information was not communicated to patients about the specifics of their disease, either because of miscommunication or minimal patient-provider time spent during office visits. This lack of communication between patients and providers often left patients and caregivers uncertain about exactly what condition they had or what they were being treated for.
Patient I just can’t have the time with Dr. xx, cuz he’s so busy...
Patient … I didn’t understand. Which exactly what type of cancer did I have, cuz I’m—really to tell you the truth—I’m still wondering.
Knowledge barriers
Patients and caregivers also identified a lack of education and knowledge about lung cancer diagnosis and treatment as a barrier to their care. Patients and caregivers were not always fully knowledgeable about lung cancer, treatment options, or the duration of treatments. They relied on the provider to disclose such information or direct them to credible sources. In many instances, patients were misinformed about the causes of lung cancer. There were misconceptions that lung cancer was only caused by a history of smoking or genetic predisposition. Patients who did not smoke or did not have a family history of lung cancer were often confused and dismayed by the diagnosis.
Patient I was trying to figure out, why do I have lung cancer. Never smoked a day in my life.
Patients were often unaware of treatment options or side effects of various treatments. They relied on physicians to relay information and make decisions for them about treatment plans.
Patient I was told chemo would probably be the best thing for me, and I just had faith that Dr. xx knows more about it than me.
Patient I’m doing chemo but it’s — what I’m doing is different. Of course, I don’t know anything about it actually either. It’s what I hear from other people.
Other patients relied on their own sources for information about their condition, either through the Internet, from family members and/or friends, or from preconceived notions.
Patient Well — of course in the meantime, I read — because they said it was a small cell, very aggressive, so I felt like everything I read — that’s the deal. I think we’ve become where we can get on the internet and look up so much, that to me, I was gonna be gone.
Patient When he said, ‘Cancer,’ I said, ‘Well, I thought cancer was a heredity thing? That you have to have somebody in your family that has it…’
Discussion
Organizational barriers are an important consideration in the delivery and receipt of high-quality, patient-centered lung cancer care. This qualitative study of patients being treated for lung cancer and their informal caregivers revealed several common perceived organizational barriers to receiving care, including health insurance coverage restrictions, appointment scheduling difficulties, quality of communication with physicians, and failure to properly educate the patient and family about the disease and what to expect of the treatment process.
The provider communication and patient knowledge barriers seem to reinforce each other and could be improved through focused efforts on the quality of communication between patients and their caregivers and clinical care providers. Patients expect, but are often deprived of, open and active dialogue with their providers. Improved communication can be helpful in educating patients and their caregivers about their disease, prognosis, and treatment goals. Although communication ranks highly as a patient and caregiver priority, there is often a disconnect between patients and caregivers and their physicians.31 Patients and caregivers often want to be more involved in the decision-making process, and effective communication between physicians and patients has been linked to the patient’s ability to understand, and also receive high-quality care.32,33 Failure to communicate effectively and educate patients on key aspects of their condition strips them of their autonomy in decision-making.
The involvement of a navigator for patients being treated for lung cancer could be pivotal in relieving the communication and scheduling barriers. The nurse navigator assists with coordinating effective communication and providing needed information between providers and patients and their caregivers. A navigator also serves as a single point of contact for patients and caregivers to communicate questions outside of physician visits or concerns that may not be urgent enough to warrant immediate physician response.34 The navigator coordinates patients’ appointment schedules and physician referrals and communicates the details of the next steps in the care-delivery process. This helps remove the barriers to care and improve patient outcomes and the quality of health care delivery, especially for patients and caregivers dealing with a life-threatening illness within a complex referral process.35
Multidisciplinary care, a much-recommended alternative care-delivery model, should, in theory, promote connectivity of providers and collaboration between providers, patients, and family members. This model could help reduce barriers for patients and caregivers.3,24,36 A network of connected providers can better coordinate treatment plans, easily share test results, and provide built-in second opinions. Given the increasingly multimodal approach to the diagnosis, staging, and treatment of lung cancer, the multidisciplinary model could allow physicians to consider multiple perspectives and care-delivery options and, ideally, develop consensus around the optimal approach for each individual patient in one setting. This can shorten the length of time before treatment and establish a plan that is tailored to the patient’s needs.24
The National Academy of Medicine (formerly, Institute of Medicine) proposes that modern health care systems have 6 aims for quality improvement: safety, effectiveness, patient-centeredness, timeliness, efficiency, and equity.37 It would take changes in the design and implementation of organizational support systems at the policy, institutional, and provider level for those aims to be achieved. Further investigation of the problems identified by patients and caregivers could lead to innovative solutions to improve lung cancer care. Future work should evaluate the most effective communication styles in patient-provider interactions, particularly in regard to to lung cancer diagnosis and treatment, and investigate how multidisciplinary models influence patient-provider communication and patient care.
Limitations
This study has several limitations. Less than half of those approached for the study participated in the study for various reasons, which may have introduced selection bias in terms of not having the perspectives of patients not willing or able to participate in the study. Though focus groups are known to generate rich in-depth views of certain issues, they have been criticized as potentially lacking rigor and generalizability. To address this concern, we used a standardized script for each focus group and involved multiple members of the research team in data analysis and interpretation. Also, this study enrolled participants from a single health care institution and did not use a comparison group. There might be institutional and geographic differences in the experience of lung cancer care, which might further limit the generalizability of the results of this study.
Conclusions
Despite those limitations, this study offers valuable insight into the barriers that lung cancer patients and caregivers encounter while navigating a community-level health care system. Eliminating or minimizing these barriers will require strategic plans that help mitigate insurance-related, scheduling, provider-patient communication, and patient/caregiver knowledge acquisition problems and translate them into tactical actions for quality improvement. This is one of the first qualitative studies conducted to understand the organizational barriers that lung cancer patients and their caregivers face within a health care system. Additional research is needed to explore these barriers and develop viable solutions.
Disclaimer
All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute or its Board of Governors or Methodology Committee.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30.
2. Riedel RF, Wang X, McCormack M, et al. Impact of a multidisciplinary thoracic oncology clinic on the timeliness of care. J Thorac Oncol. 2006;1(7):692-696.
3. Seek A, Hogle WP. Modeling a better way: navigating the health care system for patients with lung cancer. Clin J Oncol Nurs. 2007;11(1):81-85.
4. Sorensen R, Iedema R. Redefining accountability in health care: managing the plurality of medical interests. Health. 2008;12(1):87-106.
5. Mosher CE, Jaynes HA, Hanna N, Ostroff JS. Distressed family caregivers of lung cancer patients: an examination of psychosocial and practical challenges. Support Care Cancer. 2013;21(2):431-437.
6. Cantril C, Haylock PJ. Patient navigation in the oncology care setting. Semin Oncol Nurs. 2013;29(2):76-90.
7. Freeman HP. Patient navigation: a community based strategy to reduce cancer disparities. J Urban Health. 2006;83(2):139-141.
8. Kwon DH, Tisnado DM, Keating NL, et al. Physician reported barriers to referring cancer patients to specialists: prevalence, factors, and association with career satisfaction. Cancer. 2015;121(1):113-122.
9. Andersen SE. Implementation of a new prescription system. A qualitative survey of organizational barriers. Ugeskr Laeger. 2002;164(38):4449-4453.
10. Rousseau L, Guay M, Archambault D, El m’ala Z, Abdelaziz N. Do organizational barriers to pneumococcal and influenza vaccine access exist? Can J Public Health. 2007;98(2):105-110.
11. Storey J, Buchanan D. Health care governance and organizational barriers to learning from mistakes. J Health Organ Manag. 2008;22(6):642-651.
12. Ziegenfuss JT Jr. Organizational barriers to quality improvement in medical and health care organizations. Qual Assur Util Rev. 1991;6(4):115-122.
13. Rihari-Thomas J, DiGiacomo M, Phillips J, Newton P, Davidson PM. Clinician perspectives of barriers to effective implementation of a rapid response system in an academic health centre: a focus group study. Int J Health Policy Manag. 2017;6(8):447-456.
14. Dutton D. Financial, organizational and professional factors affecting health care utilization. Soc Sci Med. 1986;23(7):721-735.
15. King CJ, Chen J, Dagher RK, Holt CL, Thomas SB. Decomposing differences in medical care access among cancer survivors by race and ethnicity. Am J Med Qual. 2015;30(5):459-469.
16. Renzaho AM, Romios P, Crock C, Sønderlund AL. The effectiveness of cultural competence programs in ethnic minority patient-centered health care—a systematic review of the literature. Int J Qual Health Care. 2013;25(3):261-269.
17. Betancourt JR, Green AR, Carrillo JE, Ananeh-Firempong O. Defining cultural competence: a practical framework for addressing racial/ethnic disparities in health and health care. Public Health Rep. 2003;118(4):293-302.
18. Scheinfeld Gorin S, Gauthier J, Hay J, Miles A, Wardle J. Cancer screening and aging: research barriers and opportunities. Cancer. 2008;113(suppl 12):3493-3504.
19. Humphrey L, Deffebach M, Pappas M, et al. Screening for lung cancer: systematic review to update the US preventive services task force recommendation. Evidence syntheses No. 105. Rockville, MD: Agency for Health Care Research and Quality; 2013.
20. Nelson HD, Fu R, Cantor A, Pappas M, Daeges M, Humphrey L. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force Recommendation. Ann Intern Med. 2016;164(4):244-255.
21. Zauber AG. The impact of screening on colorectal cancer mortality and incidence: has it really made a difference? Dig Dis Sci. 2015;60(3):681-691.
22. Remennick L. The challenge of early breast cancer detection among immigrant and minority women in multicultural societies. Breast J. 2006;12(suppl 1):103-110.
23. Hess LM, Pohl G. Perspectives of quality care in cancer treatment: a review of the literature. Am Health Drug Benefits. 2013;6(6):321-329.
24. Kedia SK, Ward KD, Digney SA, et al. ‘One-stop shop’: lung cancer patients’ and caregivers’ perceptions of multidisciplinary care in a community health care setting. Transl Lung Cancer Res. 2015;4(4):456-464.
25. US Cancer Statistics Working Group. United States cancer statistics: 1999-2014 incidence and mortality web-based report. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute. http://www.cdc.gov/uscs. Published 2017. Accessed November 21, 2017.
26. Creswell JW. Research design: qualitative, quantitative, and mixed methods approaches. 4th ed. Thousand Oaks, CA: SAGE Publications; 2014.
27. Ballinger C. Demonstrating rigour and quality? In Finlay L, Ballinger C, eds. Qualitative research for allied health professionals: challenging choices. Chichester, England: John Wiley & Sons Ltd; 2006:235-246.
28. Finlay L. ‘Rigour’, ‘ethical integrity’ or ‘artistry’? Reflexively reviewing criteria for evaluating qualitative research. Br J Occup Ther. 2006;69(7):319-326.
29. Lincoln YS, Guba EG. Naturalistic inquiry. Newbury Park, CA: SAGE Publications; 1985.
30. Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus group. Int J Qual Health Care. 2007;19(6):349-357.
31. Neeman N, Quinn K, Shoeb M, Mourad M, Sehgal NL, Sliwka D. Postdischarge focus groups to improve the hospital experience. Am J Med Qual. 2013;28(6):536-538.
32. O’Day BL, Killeen M, Iezzoni LI. Improving health care experiences of persons who are blind or have low vision: suggestions from focus groups. Am J Med Qual. 2004;19(5):193-200.
33. Smith B, Lynch WD, Markow C, Lifsey S, Slover M. Consumers’ understanding of and interest in provider- versus practice-level quality characteristics: findings from a focus group study. Am J Med Qual. 2015;30(4):367-373.
34. Islam KM, Opoku ST, Apenteng BA, et al. Coping with an advanced stage lung cancer diagnosis: patient, caregiver, and provider perspectives on the role of the health care system. J Cancer Educ. 2016;31(3):554-558.
35. Pedersen A, Hack TF. Pilots of oncology health care: a concept analysis of the patient navigator role. Oncol Nurs Forum. 2010;37(1):55-60.
36. Gopal R. How to maintain multidisciplinary treatment schedules. J Support Oncol. 2005;3(3):248-256.
37. Committee on Quality of Health Care in America. Improving the 21st century health care system. In: Institute of Medicine, ed. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academies Press; 2001:39-60.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30.
2. Riedel RF, Wang X, McCormack M, et al. Impact of a multidisciplinary thoracic oncology clinic on the timeliness of care. J Thorac Oncol. 2006;1(7):692-696.
3. Seek A, Hogle WP. Modeling a better way: navigating the health care system for patients with lung cancer. Clin J Oncol Nurs. 2007;11(1):81-85.
4. Sorensen R, Iedema R. Redefining accountability in health care: managing the plurality of medical interests. Health. 2008;12(1):87-106.
5. Mosher CE, Jaynes HA, Hanna N, Ostroff JS. Distressed family caregivers of lung cancer patients: an examination of psychosocial and practical challenges. Support Care Cancer. 2013;21(2):431-437.
6. Cantril C, Haylock PJ. Patient navigation in the oncology care setting. Semin Oncol Nurs. 2013;29(2):76-90.
7. Freeman HP. Patient navigation: a community based strategy to reduce cancer disparities. J Urban Health. 2006;83(2):139-141.
8. Kwon DH, Tisnado DM, Keating NL, et al. Physician reported barriers to referring cancer patients to specialists: prevalence, factors, and association with career satisfaction. Cancer. 2015;121(1):113-122.
9. Andersen SE. Implementation of a new prescription system. A qualitative survey of organizational barriers. Ugeskr Laeger. 2002;164(38):4449-4453.
10. Rousseau L, Guay M, Archambault D, El m’ala Z, Abdelaziz N. Do organizational barriers to pneumococcal and influenza vaccine access exist? Can J Public Health. 2007;98(2):105-110.
11. Storey J, Buchanan D. Health care governance and organizational barriers to learning from mistakes. J Health Organ Manag. 2008;22(6):642-651.
12. Ziegenfuss JT Jr. Organizational barriers to quality improvement in medical and health care organizations. Qual Assur Util Rev. 1991;6(4):115-122.
13. Rihari-Thomas J, DiGiacomo M, Phillips J, Newton P, Davidson PM. Clinician perspectives of barriers to effective implementation of a rapid response system in an academic health centre: a focus group study. Int J Health Policy Manag. 2017;6(8):447-456.
14. Dutton D. Financial, organizational and professional factors affecting health care utilization. Soc Sci Med. 1986;23(7):721-735.
15. King CJ, Chen J, Dagher RK, Holt CL, Thomas SB. Decomposing differences in medical care access among cancer survivors by race and ethnicity. Am J Med Qual. 2015;30(5):459-469.
16. Renzaho AM, Romios P, Crock C, Sønderlund AL. The effectiveness of cultural competence programs in ethnic minority patient-centered health care—a systematic review of the literature. Int J Qual Health Care. 2013;25(3):261-269.
17. Betancourt JR, Green AR, Carrillo JE, Ananeh-Firempong O. Defining cultural competence: a practical framework for addressing racial/ethnic disparities in health and health care. Public Health Rep. 2003;118(4):293-302.
18. Scheinfeld Gorin S, Gauthier J, Hay J, Miles A, Wardle J. Cancer screening and aging: research barriers and opportunities. Cancer. 2008;113(suppl 12):3493-3504.
19. Humphrey L, Deffebach M, Pappas M, et al. Screening for lung cancer: systematic review to update the US preventive services task force recommendation. Evidence syntheses No. 105. Rockville, MD: Agency for Health Care Research and Quality; 2013.
20. Nelson HD, Fu R, Cantor A, Pappas M, Daeges M, Humphrey L. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force Recommendation. Ann Intern Med. 2016;164(4):244-255.
21. Zauber AG. The impact of screening on colorectal cancer mortality and incidence: has it really made a difference? Dig Dis Sci. 2015;60(3):681-691.
22. Remennick L. The challenge of early breast cancer detection among immigrant and minority women in multicultural societies. Breast J. 2006;12(suppl 1):103-110.
23. Hess LM, Pohl G. Perspectives of quality care in cancer treatment: a review of the literature. Am Health Drug Benefits. 2013;6(6):321-329.
24. Kedia SK, Ward KD, Digney SA, et al. ‘One-stop shop’: lung cancer patients’ and caregivers’ perceptions of multidisciplinary care in a community health care setting. Transl Lung Cancer Res. 2015;4(4):456-464.
25. US Cancer Statistics Working Group. United States cancer statistics: 1999-2014 incidence and mortality web-based report. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute. http://www.cdc.gov/uscs. Published 2017. Accessed November 21, 2017.
26. Creswell JW. Research design: qualitative, quantitative, and mixed methods approaches. 4th ed. Thousand Oaks, CA: SAGE Publications; 2014.
27. Ballinger C. Demonstrating rigour and quality? In Finlay L, Ballinger C, eds. Qualitative research for allied health professionals: challenging choices. Chichester, England: John Wiley & Sons Ltd; 2006:235-246.
28. Finlay L. ‘Rigour’, ‘ethical integrity’ or ‘artistry’? Reflexively reviewing criteria for evaluating qualitative research. Br J Occup Ther. 2006;69(7):319-326.
29. Lincoln YS, Guba EG. Naturalistic inquiry. Newbury Park, CA: SAGE Publications; 1985.
30. Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus group. Int J Qual Health Care. 2007;19(6):349-357.
31. Neeman N, Quinn K, Shoeb M, Mourad M, Sehgal NL, Sliwka D. Postdischarge focus groups to improve the hospital experience. Am J Med Qual. 2013;28(6):536-538.
32. O’Day BL, Killeen M, Iezzoni LI. Improving health care experiences of persons who are blind or have low vision: suggestions from focus groups. Am J Med Qual. 2004;19(5):193-200.
33. Smith B, Lynch WD, Markow C, Lifsey S, Slover M. Consumers’ understanding of and interest in provider- versus practice-level quality characteristics: findings from a focus group study. Am J Med Qual. 2015;30(4):367-373.
34. Islam KM, Opoku ST, Apenteng BA, et al. Coping with an advanced stage lung cancer diagnosis: patient, caregiver, and provider perspectives on the role of the health care system. J Cancer Educ. 2016;31(3):554-558.
35. Pedersen A, Hack TF. Pilots of oncology health care: a concept analysis of the patient navigator role. Oncol Nurs Forum. 2010;37(1):55-60.
36. Gopal R. How to maintain multidisciplinary treatment schedules. J Support Oncol. 2005;3(3):248-256.
37. Committee on Quality of Health Care in America. Improving the 21st century health care system. In: Institute of Medicine, ed. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academies Press; 2001:39-60.
The impact of patient education on consideration of enrollment in clinical trials
The low rate of participation in clinical trials is partly owing to the lack of awareness of these trials not only among potential participants but the US population as a whole.1 This lack of awareness, however, can be reversed. For example, findings from a single-institution observational study showed that systematically sending letters about clinical trial participation to all new lung cancer patients was associated with increased trial participation.2 More recently, a large, multicenter, randomized experiment showed that attitudes toward clinical trials were improved through preparatory education about clinical trials before a patient’s first oncologic visit.3
Such clinical trial education can be used before any medical diagnosis to increase clinical trial awareness in the general population. It may be advantageous to do so because people tend to process information more effectively during less stressful times.4 Clinical trial awareness in the US population has increased slightly over time, but in 2012, one study reported that 26% of its participants lacked general awareness about clinical trials.5
Comprehensive educational material, such as a multimedia psychoeducational intervention,6 a 28-video library,3 or a 160-page book,7 which have been proposed for oncology patients, may be too intensive for someone who is not immediately deciding whether to participate in a clinical trial. However, a simple, concise form of education might be preferable and appropriate to increase basic knowledge and awareness among the general population, especially among those who are less educated.8
Our aim in the present study was to evaluate whether providing brief educational material about clinical trials would increase patient willingness to participate in these trials.
Methods
This is a single-group, cross-sectional design study in which all participants were administered the questions and the 240-word educational statement in the same order.
Sample
An electronic survey was conducted by Marketing and Planning Systems (the analytics practice of Kantar Millwardbrown) on behalf of the Memorial Sloan Kettering Cancer Center (MSK). The survey included a national sample of 1011 participants and a local sample of 500 participants from the MSK catchment area (22 counties across the 5 boroughs of New York City, Long Island, southern New York State, northern New Jersey, and southwestern Connecticut).
Survey participants were aged 18 to 69 years in the national sample and 25 to 69 years in the local sample, representing 87% and 75% of the adult populations of those areas, respectively. Respondents who were or who had a family member currently working in the fields of news, advertising/marketing, or medical care were not surveyed. Participants were sourced from an online incentivized panel with millions of potential respondents representative of the US adult population.
Questionnaire
The questionnaire collected data on participant demographics and main medical history (including previous participation in a clinical trial), and asked questions about clinical trials, focusing on:
Analyses
Descriptive and bivariate statistical analyses of participants’ characteristics were weighted to ensure national representativeness for gender, age, ethnicity, and income. Mean standard deviation (SD) was computed for every quantitative variable. Categorical variables were expressed as proportions.
Student t tests and analyses of variance (ANOVAs) were used to compare continuous variables, while chi-square tests were used to compare categorical data. Repeated measures ANOVAs were then used to determine the sociodemographic and medical characteristics associated with the impression of and willingness to enroll in a clinical trial before and after reading the educational material. The interaction between education level and time (pre- or postreading assessment) was tested to determine if the changes after reading the brief statement were different depending on education level.
All statistical analyses were 2-tailed and considered statistically significant at P < .05. Analyses were performed using SPSS PAWS Statistics 24 (IBM Inc, Armonk, New York). Effect sizes (standardized mean differences) and their 95% confidence intervals (CIs) were computed using the compute.es package for R 3.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Participants
From October 23, 2015, through November 12, 2015, 1511 US participants responded to the survey request, including 1507 respondents (99.7%) who reported their education level and are included in the analyses of this report. The mean age of the respondents was 43.5 years (SD, 4.6). More than half of the respondents (57.8%) reported a current medical condition, mainly cardiovascular (20.0%), arthritis (20.0%), or other type or chronic pain (20.0%), and 9.0% reported a cancer diagnosis (current, 2.9%; previous, 6.1%).
Participants who at most had completed high school (18.9%, including 1.4% who had never even attended high school) were more often white women, lived outside urban areas, had lower household income, and were less likely to have health care insurance (Table 2). They also reported a current or previous cancer diagnosis less often than those of similar age who had attended college.
Previous participation in a clinical trial was reported by 9.6% of participants. Most of the clinical trials (75.0%) were testing a new drug. Previous trial participants were more likely to be older than those who had not participated in trials (46.1 years [SD, 14.8] vs 43.3 [SD, 4.6], repectively; P = .033), have a current health condition (86.2% vs 54.8%; P < .001), and know another trial participant (39.9% vs 9.5%; P < .001).
Education level and baseline impression of and
willingness to enroll in a clinical trial
A lower level of education was associated with a decreased likelihood of previous trial participation or of knowing a trial participant, as well as with less awareness and inaccurate perceptions of clinical trials (Table 2).
Participants with a high school degree or less were more likely to have a worse impression of and were less likely to enroll in a future hypothetical clinical trial before reading the educational material (Figure 1). Multivariable analyses confirmed that lower education level was associated with lower baseline overall impression, regardless of other personal characteristics (Table 3). Lowest household income was also associated with a more negative impression of trials, whereas participants with a current medical condition and with previous contact with clinical trials had a more positive impression of them. The same effects were observed with likeliness to enroll in a future hypothetical clinical trial (correlated with the overall impression: r, 0.63; P < .001), except that the negative effect of female gender was statistically significant (Table 3).
Posteducation impression of and willingnes to enroll
willingness to enroll in a clinical trial
The brief educational material was mostly considered believable (86.2%), easy to understand (84.8%), and included information that was new to participants (81.5%; Table 2). Participants with a high school diploma or less more often noted that the material provided them with new information, but they also reported more difficulties in fully understanding and believing the information. Overall, however, few participants found the information difficult to understand (4.6%) or hard to believe (1.8%; Table 2).
Most participants had an improved overall impression of clinical trials (standardized mean difference, 0.42; 95% CI, 0.35-0.50; P < .001) after reading the educational material. This increase was higher among participants with a lower completed level of education (Figure 1; standardized mean difference, 0.62; 95% CI, 0.45-0.79; Pinteraction < .001). The same effects were observed for likeliness to enroll in a future hypothetical clinical trial (P < .001; Figure 1).
After reading the informational statement, education level effect was no longer significantly associated with the overall impression of clinical trials (P = .23) and willingness to enroll in a clinical trial (P = .34), whereas the effects of income, current medical condition, and previous engangement with clinical trials remained statistically significant (Table 3).
Remaining challenges
Regarding hypothetical participation in a future clinical trial after a cancer diagnosis, the most critical concerns were related to side effects and the uncertainty of insurance coverage (Figure 2).10 The lack of understanding of clinical trials was the least critical concern; however, it was significantly higher among participants with a lower completed level of education. These participants also expressed more critical concerns about feeling like a “guinea pig,” inconvenient trial location, and the frequent visits needed.
Discussion
Findings from this survey demonstrated that providing brief educational material about cancer clinical trials was associated with a more favorable impression of clinical trials and higher interest in trial participation. Furthermore, as far as we can ascertain, this is the first report showing how a simple intervention such as this may help close the knowledge gap on clinical trials among people of different educational backgrounds. Although most respondents in this interventional survey noted an increased willingness to consider participation in a clinical trial after reading the educational material, those with a lower level of education and knowledge about clinical trials received the most benefit. Previous participation in a clinical trial was also strongly associated with the impression of and willingness to enroll in a trial, both before and after reading the statement.11
Most of the interventions evaluated to date12 have focused on patients who are faced with having to decide whether to participate in a clinical trial2,3 or on very specific populations, such as select ethnic communities.13,14 However, it may be beneficial to provide simple, concise educational information about clinical trials to the general population, especially to those with minimal education. Although level of education concerns the minority of our sample (19%) sourced from an online panel, in the 2015 Current Population Survey, 41% of US participants aged 25 and older had not reached college education.15 Those with a lower level of education have reported a general lack of familiarity with clinical trials and were more likely to have inaccurate perceptions about trials. This is consistent with previous studies that have shown the lack of awareness and knowledge of clinical trials in this population.5,16,17 Our findings suggest that this knowledge gap can be reversed through a simple educational intervention and result in an increased willingness to participate.
The provision of information on clinical trials was positively associated with the 2 outcomes analyzed – improved impression of clinical trials and increased likelihood to enroll in a hypothetical trial. Such improvement might not translate to improved accrual,18 but it is a step toward closing the overall knowledge gap related to clinical trials and increasing the number of people who would consider trial participation. The lack of awareness of clinical trials has been reported as a legitimate explanation for why participation rates are lower in less-educated patient populations.19,20 This brief educational intervention is a simple, technology-sparing way to increase clinical trial awareness in the general population. In a similar survey, most physicians who reviewed an educational statement noted they were likely to use it with patients.21Less-educated patients, those who lived outside of urban areas, and those with lower household incomes were most concerned about trial location and the frequent visits needed when participating in a trial (Figure 2). Living in a nonurban area was not associated with participant impression of clinical trials or willingness to enroll in a trial. However, rural residency may be a barrier to enrollment depending on distance to the hospital22 and out-of-pocket expenses related to travel.23 Some comprehensive cancer centers, such as MSK, have developed alliances with community centers24 as a means of overcoming geographical barriers and increasing clinical trial participation rates.
Another concern shared by most respondents was the uncertainty in insurance coverage and potential out-of-pocket costs related to care. Lower household income, unlike location of residence and lack of insurance, was significantly associated with negative impressions of clinical trials and lower willingness to enroll in a trial, even after adjusting for education level. Cancer patients with higher financial burden have reported more attitudinal barriers, even after accounting for the negative effect of lower education level.25 Recent studies have also discussed the negative impact of lower income on cancer clinical trial participation,19,20,26,27 and new attention has been paid to the negative financial implications or “financial toxicity” of participating in a trial.23,28
White and older survey participants showed similar interest in clinical trial participation after accounting for other characteristics. There is growing evidence that outcome differences attributed to race may in fact be more dependent on socioeconomic status.8 A recent study among breast cancer patients showed that low socioeconomic status, but not race, was associated with decreased participation in clinical trials.29,30 Previous findings have also indicated that interest in clinical trials and barriers to enrollment among older, less-educated patients31 are often related to ineligibility, comorbidity, or communication difficulties.
Among our participants, the fear of side effects also was a common attitudinal barrier to clinical trial participation, as has been reported in previous studies.3,20 However, contrary to one previous study,20 this fear was not significantly increased among our less-educated participants.
Less-educated participants also reported more difficulties in understanding the information they were provided with, and they remained more concerned about being treated like “guinea pigs.” These concerns are consistent with other results showing that decisional conflict about clinical trial participation among patients with a high school diploma or less remained high even after they had received a National Cancer Institute text as pre-education material.3
Limitations
The lack of randomization makes it difficult to attribute with certainty that the change in acceptability of clinical trial participation is owing to the reading of the educational statement. The survey also sampled only English-speaking and well-educated participants from an online panel (81.1% had at least attended college) despite the use of a weighting procedure to ensure representativeness regarding gender, age, ethnicity, and income. Health literacy level or more specific trial literacy level was not evaluated; however, we were able to show less accurate perceptions of clinical trials among participants with a lower level of education by using agreement toward 8 statements about trials. The responses to hypothetical questions from these participants in the general population may also not be generalizable to a restricted population of patients with cancer. In addition, we measured impression of and willingness to enroll in a clinical trial immediately after providing participants with the educational material. We would have to confirm whether the positive effects of the education persist over time and translate to higher clinical trial participation rates.
Conclusions
Participants were receptive of educational material and expressed greater interest in and likelihood of enrolling in a clinical trial after reading it. The information had a greater effect on those with less education, but increased the willingness of all participants to enroll.
1. Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112(2):228-242.
2. Quinn GP, Bell BA, Bell MY, et al. The guinea pig syndrome: improving clinical trial participation among thoracic patients. J Thorac Oncol. 2007;2(3):191-196.
3. Meropol NJ, Wong Y-N, Albrecht T, et al. Randomized trial of a web-based intervention to address barriers to clinical trials. J Clin Oncol. 2016;34(5):469-478.
4. Goldberg RJ. Disclosure of information to adult cancer patients: issues and update. J Clin Oncol. 1984;2(8):948-955.
5. Leiter A, Diefenbach MA, Doucette J, Oh WK, Galsky MD. Clinical trial awareness: changes over time and sociodemographic disparities. Clin Trials. 2015;12(3):215-223.
6. Jacobsen PB, Wells KJ, Meade CD, et al. Effects of a brief multimedia psychoeducational intervention on the attitudes and interest of patients with cancer regarding clinical trial participation: a multicenter randomized controlled trial. J Clin Oncol. 2012;30(20):2516-2521.
7. Carney PA, Tucker EK, Newby TA, Beer TM. Feasibility, acceptability and findings from a pilot randomized controlled intervention study on the impact of a book designed to inform patients about cancer clinical trials. J Cancer Educ. 2014;29(1):181-187.
8. Sharrocks K, Spicer J, Camidge DR, Papa S. The impact of socioeconomic status on access to cancer clinical trials. Br J Cancer. 2014;111(9):1684-1687.
9. Regan J, Hickey C, Targett C, Masuda S, Sabbatini P. Framing clinical research and the importance of trial participation: patient and physician perspective. Poster presented at: 8th Annual AACI Clinical Research Initiative Meeting; July 20-21 2016; Chicago, IL.
10. Mancini J, Genre D, Dalenc F, et al. Patients’ regrets after participating in a randomized controlled trials depended on their involvement in the decision making. J Clin Epidemiol. 2012;65(6):635-642
11. Murphy ST, Frank LB, Chatterjee JS, et al. Comparing the relative efficacy of narrative vs nonnarrative health messages in reducing health disparities using a randomized trial. Am J Public Health. 2015;105(10):2117-2123.
12. Treweek S, Lockhart P, Pitkethly M, et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open. 2013;3(2):e002360. doi:10.1136/bmjopen-2012-002360.
13. Ma GX, Tan Y, Blakeney NC, et al. The impact of a community-based clinical trial educational intervention among underrepresented Chinese Americans. Cancer Epidemiol Biomarkers Prev. 2014;23(3):424-432.
14. Cupertino AP, Molina CSP, de los Rios JB, et al. Knowledge, awareness, and interest in cancer clinical trials among rural latinos following brief education by promotores de salud. J Community Med Health Educ. 2015;5:2161. doi:10.4172/2161-0711.1000358
15. Ryan CL, Bauman K. Educational attainment in the United States: 2015. https://www.census.gov/content/dam/Census/library/publications/2016/demo/p20-578.pdf. Published March 2016. Accessed October 18, 2016.
16. Lara PN Jr, Paterniti DA, Chiechi C, et al. Evaluation of factors affecting awareness of and willingness to participate in cancer clinical trials. J Clin Oncol. 2005;23(36):9282-9289.
17. Dhai A, Etheredge H, Cleaton-Jones P. A pilot study evaluating an intervention designed to raise awareness of clinical trials among potential participants in the developing world. J Med Ethics. 2010;36(4):238-242.
18. Denicoff AM, McCaskill-Stevens W, Grubbs SS, et al. The National Cancer Institute–American Society of Clinical Oncology Cancer Trial Accrual Symposium: summary and recommendations. J Oncol Pract. 2013;9(6):267-276.
19. Davison BJ, So A, Goldenberg SL, Berkowitz J, Gleave ME. Measurement of factors influencing the participation of patients with prostate cancer in clinical trials: a Canadian perspective. BJU Int. 2008;101(8):982-987.
20. Unger JM, Hershman DL, Albain KS, et al. Patient income level and cancer clinical trial participation. J Clin Oncol. 2013;31(5):536-542.
21. IRB Advisor. Education may overcome reticence to join trials. Relias website. http://www.ahcmedia.com/articles/138088-education-may-overcome-reticence-to-join-trials. Published July 1, 2016. Accessed July 29, 2016.
22. Vanderpool RC, Kornfeld J, Mills L, Byrne MM. Rural-urban differences in discussions of cancer treatment clinical trials. Patient Educ Couns. 2011;85(2):e69-e74.
23. Meropol NJ. Health policy: overcoming cost barriers to clinical trial participation. Nat Rev Clin Oncol. 2016;13(6):333-334.
24. MSK Cancer Alliance. Memorial Sloan Kettering Cancer Center website. https://www.mskcc.org/about/innovative-collaborations/msk-alliance. Updated March 7, 2018. Accessed August 18, 2016.
25. Manne S, Kashy D, Albrecht T, et al. Attitudinal barriers to participation in oncology clinical trials: factor analysis and correlates of barriers. Eur J Cancer Care (Engl). 2015;24(1):28-38.
26. Unger JM, Gralow JR, Albain KS, Ramsey SD, Hershman DL. Patient income level and cancer clinical trial participation: a prospective survey study. JAMA Oncol. 2016;2(1):137-139.
27. Moy B. Clinical trials, disparities, and financial burden: it’s time to intervene. Oncologist. 2015;20(6):571.
28. Bonevski B, Randell M, Paul C, et al. Reaching the hard-to-reach: a systematic review of strategies for improving health and medical research with socially disadvantaged groups. BMC Med Res Methodol. 2014;14:42.
29. Gross CP, Filardo G, Mayne ST, Krumholz HM. The impact of socioeconomic status and race on trial participation for older women with breast cancer. Cancer. 2005;103(3):483-491.
30. Nickell A, Burke NJ, Cohen E, Caprio M, Joseph G. Educating low-SES and LEP survivors about breast cancer research: pilot test of the Health Research Engagement Intervention. J Cancer Educ. 2014;29(4):746-752.
31. Mancini J, Jansen J, Julian-Reynier C, Bechlian D, Vey N, Chabannon C. Preferences of older adults with cancer for involvement in decision-making about research participation. J Am Geriatr Soc. 2014;62(6):1191-1193.
The low rate of participation in clinical trials is partly owing to the lack of awareness of these trials not only among potential participants but the US population as a whole.1 This lack of awareness, however, can be reversed. For example, findings from a single-institution observational study showed that systematically sending letters about clinical trial participation to all new lung cancer patients was associated with increased trial participation.2 More recently, a large, multicenter, randomized experiment showed that attitudes toward clinical trials were improved through preparatory education about clinical trials before a patient’s first oncologic visit.3
Such clinical trial education can be used before any medical diagnosis to increase clinical trial awareness in the general population. It may be advantageous to do so because people tend to process information more effectively during less stressful times.4 Clinical trial awareness in the US population has increased slightly over time, but in 2012, one study reported that 26% of its participants lacked general awareness about clinical trials.5
Comprehensive educational material, such as a multimedia psychoeducational intervention,6 a 28-video library,3 or a 160-page book,7 which have been proposed for oncology patients, may be too intensive for someone who is not immediately deciding whether to participate in a clinical trial. However, a simple, concise form of education might be preferable and appropriate to increase basic knowledge and awareness among the general population, especially among those who are less educated.8
Our aim in the present study was to evaluate whether providing brief educational material about clinical trials would increase patient willingness to participate in these trials.
Methods
This is a single-group, cross-sectional design study in which all participants were administered the questions and the 240-word educational statement in the same order.
Sample
An electronic survey was conducted by Marketing and Planning Systems (the analytics practice of Kantar Millwardbrown) on behalf of the Memorial Sloan Kettering Cancer Center (MSK). The survey included a national sample of 1011 participants and a local sample of 500 participants from the MSK catchment area (22 counties across the 5 boroughs of New York City, Long Island, southern New York State, northern New Jersey, and southwestern Connecticut).
Survey participants were aged 18 to 69 years in the national sample and 25 to 69 years in the local sample, representing 87% and 75% of the adult populations of those areas, respectively. Respondents who were or who had a family member currently working in the fields of news, advertising/marketing, or medical care were not surveyed. Participants were sourced from an online incentivized panel with millions of potential respondents representative of the US adult population.
Questionnaire
The questionnaire collected data on participant demographics and main medical history (including previous participation in a clinical trial), and asked questions about clinical trials, focusing on:
Analyses
Descriptive and bivariate statistical analyses of participants’ characteristics were weighted to ensure national representativeness for gender, age, ethnicity, and income. Mean standard deviation (SD) was computed for every quantitative variable. Categorical variables were expressed as proportions.
Student t tests and analyses of variance (ANOVAs) were used to compare continuous variables, while chi-square tests were used to compare categorical data. Repeated measures ANOVAs were then used to determine the sociodemographic and medical characteristics associated with the impression of and willingness to enroll in a clinical trial before and after reading the educational material. The interaction between education level and time (pre- or postreading assessment) was tested to determine if the changes after reading the brief statement were different depending on education level.
All statistical analyses were 2-tailed and considered statistically significant at P < .05. Analyses were performed using SPSS PAWS Statistics 24 (IBM Inc, Armonk, New York). Effect sizes (standardized mean differences) and their 95% confidence intervals (CIs) were computed using the compute.es package for R 3.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Participants
From October 23, 2015, through November 12, 2015, 1511 US participants responded to the survey request, including 1507 respondents (99.7%) who reported their education level and are included in the analyses of this report. The mean age of the respondents was 43.5 years (SD, 4.6). More than half of the respondents (57.8%) reported a current medical condition, mainly cardiovascular (20.0%), arthritis (20.0%), or other type or chronic pain (20.0%), and 9.0% reported a cancer diagnosis (current, 2.9%; previous, 6.1%).
Participants who at most had completed high school (18.9%, including 1.4% who had never even attended high school) were more often white women, lived outside urban areas, had lower household income, and were less likely to have health care insurance (Table 2). They also reported a current or previous cancer diagnosis less often than those of similar age who had attended college.
Previous participation in a clinical trial was reported by 9.6% of participants. Most of the clinical trials (75.0%) were testing a new drug. Previous trial participants were more likely to be older than those who had not participated in trials (46.1 years [SD, 14.8] vs 43.3 [SD, 4.6], repectively; P = .033), have a current health condition (86.2% vs 54.8%; P < .001), and know another trial participant (39.9% vs 9.5%; P < .001).
Education level and baseline impression of and
willingness to enroll in a clinical trial
A lower level of education was associated with a decreased likelihood of previous trial participation or of knowing a trial participant, as well as with less awareness and inaccurate perceptions of clinical trials (Table 2).
Participants with a high school degree or less were more likely to have a worse impression of and were less likely to enroll in a future hypothetical clinical trial before reading the educational material (Figure 1). Multivariable analyses confirmed that lower education level was associated with lower baseline overall impression, regardless of other personal characteristics (Table 3). Lowest household income was also associated with a more negative impression of trials, whereas participants with a current medical condition and with previous contact with clinical trials had a more positive impression of them. The same effects were observed with likeliness to enroll in a future hypothetical clinical trial (correlated with the overall impression: r, 0.63; P < .001), except that the negative effect of female gender was statistically significant (Table 3).
Posteducation impression of and willingnes to enroll
willingness to enroll in a clinical trial
The brief educational material was mostly considered believable (86.2%), easy to understand (84.8%), and included information that was new to participants (81.5%; Table 2). Participants with a high school diploma or less more often noted that the material provided them with new information, but they also reported more difficulties in fully understanding and believing the information. Overall, however, few participants found the information difficult to understand (4.6%) or hard to believe (1.8%; Table 2).
Most participants had an improved overall impression of clinical trials (standardized mean difference, 0.42; 95% CI, 0.35-0.50; P < .001) after reading the educational material. This increase was higher among participants with a lower completed level of education (Figure 1; standardized mean difference, 0.62; 95% CI, 0.45-0.79; Pinteraction < .001). The same effects were observed for likeliness to enroll in a future hypothetical clinical trial (P < .001; Figure 1).
After reading the informational statement, education level effect was no longer significantly associated with the overall impression of clinical trials (P = .23) and willingness to enroll in a clinical trial (P = .34), whereas the effects of income, current medical condition, and previous engangement with clinical trials remained statistically significant (Table 3).
Remaining challenges
Regarding hypothetical participation in a future clinical trial after a cancer diagnosis, the most critical concerns were related to side effects and the uncertainty of insurance coverage (Figure 2).10 The lack of understanding of clinical trials was the least critical concern; however, it was significantly higher among participants with a lower completed level of education. These participants also expressed more critical concerns about feeling like a “guinea pig,” inconvenient trial location, and the frequent visits needed.
Discussion
Findings from this survey demonstrated that providing brief educational material about cancer clinical trials was associated with a more favorable impression of clinical trials and higher interest in trial participation. Furthermore, as far as we can ascertain, this is the first report showing how a simple intervention such as this may help close the knowledge gap on clinical trials among people of different educational backgrounds. Although most respondents in this interventional survey noted an increased willingness to consider participation in a clinical trial after reading the educational material, those with a lower level of education and knowledge about clinical trials received the most benefit. Previous participation in a clinical trial was also strongly associated with the impression of and willingness to enroll in a trial, both before and after reading the statement.11
Most of the interventions evaluated to date12 have focused on patients who are faced with having to decide whether to participate in a clinical trial2,3 or on very specific populations, such as select ethnic communities.13,14 However, it may be beneficial to provide simple, concise educational information about clinical trials to the general population, especially to those with minimal education. Although level of education concerns the minority of our sample (19%) sourced from an online panel, in the 2015 Current Population Survey, 41% of US participants aged 25 and older had not reached college education.15 Those with a lower level of education have reported a general lack of familiarity with clinical trials and were more likely to have inaccurate perceptions about trials. This is consistent with previous studies that have shown the lack of awareness and knowledge of clinical trials in this population.5,16,17 Our findings suggest that this knowledge gap can be reversed through a simple educational intervention and result in an increased willingness to participate.
The provision of information on clinical trials was positively associated with the 2 outcomes analyzed – improved impression of clinical trials and increased likelihood to enroll in a hypothetical trial. Such improvement might not translate to improved accrual,18 but it is a step toward closing the overall knowledge gap related to clinical trials and increasing the number of people who would consider trial participation. The lack of awareness of clinical trials has been reported as a legitimate explanation for why participation rates are lower in less-educated patient populations.19,20 This brief educational intervention is a simple, technology-sparing way to increase clinical trial awareness in the general population. In a similar survey, most physicians who reviewed an educational statement noted they were likely to use it with patients.21Less-educated patients, those who lived outside of urban areas, and those with lower household incomes were most concerned about trial location and the frequent visits needed when participating in a trial (Figure 2). Living in a nonurban area was not associated with participant impression of clinical trials or willingness to enroll in a trial. However, rural residency may be a barrier to enrollment depending on distance to the hospital22 and out-of-pocket expenses related to travel.23 Some comprehensive cancer centers, such as MSK, have developed alliances with community centers24 as a means of overcoming geographical barriers and increasing clinical trial participation rates.
Another concern shared by most respondents was the uncertainty in insurance coverage and potential out-of-pocket costs related to care. Lower household income, unlike location of residence and lack of insurance, was significantly associated with negative impressions of clinical trials and lower willingness to enroll in a trial, even after adjusting for education level. Cancer patients with higher financial burden have reported more attitudinal barriers, even after accounting for the negative effect of lower education level.25 Recent studies have also discussed the negative impact of lower income on cancer clinical trial participation,19,20,26,27 and new attention has been paid to the negative financial implications or “financial toxicity” of participating in a trial.23,28
White and older survey participants showed similar interest in clinical trial participation after accounting for other characteristics. There is growing evidence that outcome differences attributed to race may in fact be more dependent on socioeconomic status.8 A recent study among breast cancer patients showed that low socioeconomic status, but not race, was associated with decreased participation in clinical trials.29,30 Previous findings have also indicated that interest in clinical trials and barriers to enrollment among older, less-educated patients31 are often related to ineligibility, comorbidity, or communication difficulties.
Among our participants, the fear of side effects also was a common attitudinal barrier to clinical trial participation, as has been reported in previous studies.3,20 However, contrary to one previous study,20 this fear was not significantly increased among our less-educated participants.
Less-educated participants also reported more difficulties in understanding the information they were provided with, and they remained more concerned about being treated like “guinea pigs.” These concerns are consistent with other results showing that decisional conflict about clinical trial participation among patients with a high school diploma or less remained high even after they had received a National Cancer Institute text as pre-education material.3
Limitations
The lack of randomization makes it difficult to attribute with certainty that the change in acceptability of clinical trial participation is owing to the reading of the educational statement. The survey also sampled only English-speaking and well-educated participants from an online panel (81.1% had at least attended college) despite the use of a weighting procedure to ensure representativeness regarding gender, age, ethnicity, and income. Health literacy level or more specific trial literacy level was not evaluated; however, we were able to show less accurate perceptions of clinical trials among participants with a lower level of education by using agreement toward 8 statements about trials. The responses to hypothetical questions from these participants in the general population may also not be generalizable to a restricted population of patients with cancer. In addition, we measured impression of and willingness to enroll in a clinical trial immediately after providing participants with the educational material. We would have to confirm whether the positive effects of the education persist over time and translate to higher clinical trial participation rates.
Conclusions
Participants were receptive of educational material and expressed greater interest in and likelihood of enrolling in a clinical trial after reading it. The information had a greater effect on those with less education, but increased the willingness of all participants to enroll.
The low rate of participation in clinical trials is partly owing to the lack of awareness of these trials not only among potential participants but the US population as a whole.1 This lack of awareness, however, can be reversed. For example, findings from a single-institution observational study showed that systematically sending letters about clinical trial participation to all new lung cancer patients was associated with increased trial participation.2 More recently, a large, multicenter, randomized experiment showed that attitudes toward clinical trials were improved through preparatory education about clinical trials before a patient’s first oncologic visit.3
Such clinical trial education can be used before any medical diagnosis to increase clinical trial awareness in the general population. It may be advantageous to do so because people tend to process information more effectively during less stressful times.4 Clinical trial awareness in the US population has increased slightly over time, but in 2012, one study reported that 26% of its participants lacked general awareness about clinical trials.5
Comprehensive educational material, such as a multimedia psychoeducational intervention,6 a 28-video library,3 or a 160-page book,7 which have been proposed for oncology patients, may be too intensive for someone who is not immediately deciding whether to participate in a clinical trial. However, a simple, concise form of education might be preferable and appropriate to increase basic knowledge and awareness among the general population, especially among those who are less educated.8
Our aim in the present study was to evaluate whether providing brief educational material about clinical trials would increase patient willingness to participate in these trials.
Methods
This is a single-group, cross-sectional design study in which all participants were administered the questions and the 240-word educational statement in the same order.
Sample
An electronic survey was conducted by Marketing and Planning Systems (the analytics practice of Kantar Millwardbrown) on behalf of the Memorial Sloan Kettering Cancer Center (MSK). The survey included a national sample of 1011 participants and a local sample of 500 participants from the MSK catchment area (22 counties across the 5 boroughs of New York City, Long Island, southern New York State, northern New Jersey, and southwestern Connecticut).
Survey participants were aged 18 to 69 years in the national sample and 25 to 69 years in the local sample, representing 87% and 75% of the adult populations of those areas, respectively. Respondents who were or who had a family member currently working in the fields of news, advertising/marketing, or medical care were not surveyed. Participants were sourced from an online incentivized panel with millions of potential respondents representative of the US adult population.
Questionnaire
The questionnaire collected data on participant demographics and main medical history (including previous participation in a clinical trial), and asked questions about clinical trials, focusing on:
Analyses
Descriptive and bivariate statistical analyses of participants’ characteristics were weighted to ensure national representativeness for gender, age, ethnicity, and income. Mean standard deviation (SD) was computed for every quantitative variable. Categorical variables were expressed as proportions.
Student t tests and analyses of variance (ANOVAs) were used to compare continuous variables, while chi-square tests were used to compare categorical data. Repeated measures ANOVAs were then used to determine the sociodemographic and medical characteristics associated with the impression of and willingness to enroll in a clinical trial before and after reading the educational material. The interaction between education level and time (pre- or postreading assessment) was tested to determine if the changes after reading the brief statement were different depending on education level.
All statistical analyses were 2-tailed and considered statistically significant at P < .05. Analyses were performed using SPSS PAWS Statistics 24 (IBM Inc, Armonk, New York). Effect sizes (standardized mean differences) and their 95% confidence intervals (CIs) were computed using the compute.es package for R 3.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Participants
From October 23, 2015, through November 12, 2015, 1511 US participants responded to the survey request, including 1507 respondents (99.7%) who reported their education level and are included in the analyses of this report. The mean age of the respondents was 43.5 years (SD, 4.6). More than half of the respondents (57.8%) reported a current medical condition, mainly cardiovascular (20.0%), arthritis (20.0%), or other type or chronic pain (20.0%), and 9.0% reported a cancer diagnosis (current, 2.9%; previous, 6.1%).
Participants who at most had completed high school (18.9%, including 1.4% who had never even attended high school) were more often white women, lived outside urban areas, had lower household income, and were less likely to have health care insurance (Table 2). They also reported a current or previous cancer diagnosis less often than those of similar age who had attended college.
Previous participation in a clinical trial was reported by 9.6% of participants. Most of the clinical trials (75.0%) were testing a new drug. Previous trial participants were more likely to be older than those who had not participated in trials (46.1 years [SD, 14.8] vs 43.3 [SD, 4.6], repectively; P = .033), have a current health condition (86.2% vs 54.8%; P < .001), and know another trial participant (39.9% vs 9.5%; P < .001).
Education level and baseline impression of and
willingness to enroll in a clinical trial
A lower level of education was associated with a decreased likelihood of previous trial participation or of knowing a trial participant, as well as with less awareness and inaccurate perceptions of clinical trials (Table 2).
Participants with a high school degree or less were more likely to have a worse impression of and were less likely to enroll in a future hypothetical clinical trial before reading the educational material (Figure 1). Multivariable analyses confirmed that lower education level was associated with lower baseline overall impression, regardless of other personal characteristics (Table 3). Lowest household income was also associated with a more negative impression of trials, whereas participants with a current medical condition and with previous contact with clinical trials had a more positive impression of them. The same effects were observed with likeliness to enroll in a future hypothetical clinical trial (correlated with the overall impression: r, 0.63; P < .001), except that the negative effect of female gender was statistically significant (Table 3).
Posteducation impression of and willingnes to enroll
willingness to enroll in a clinical trial
The brief educational material was mostly considered believable (86.2%), easy to understand (84.8%), and included information that was new to participants (81.5%; Table 2). Participants with a high school diploma or less more often noted that the material provided them with new information, but they also reported more difficulties in fully understanding and believing the information. Overall, however, few participants found the information difficult to understand (4.6%) or hard to believe (1.8%; Table 2).
Most participants had an improved overall impression of clinical trials (standardized mean difference, 0.42; 95% CI, 0.35-0.50; P < .001) after reading the educational material. This increase was higher among participants with a lower completed level of education (Figure 1; standardized mean difference, 0.62; 95% CI, 0.45-0.79; Pinteraction < .001). The same effects were observed for likeliness to enroll in a future hypothetical clinical trial (P < .001; Figure 1).
After reading the informational statement, education level effect was no longer significantly associated with the overall impression of clinical trials (P = .23) and willingness to enroll in a clinical trial (P = .34), whereas the effects of income, current medical condition, and previous engangement with clinical trials remained statistically significant (Table 3).
Remaining challenges
Regarding hypothetical participation in a future clinical trial after a cancer diagnosis, the most critical concerns were related to side effects and the uncertainty of insurance coverage (Figure 2).10 The lack of understanding of clinical trials was the least critical concern; however, it was significantly higher among participants with a lower completed level of education. These participants also expressed more critical concerns about feeling like a “guinea pig,” inconvenient trial location, and the frequent visits needed.
Discussion
Findings from this survey demonstrated that providing brief educational material about cancer clinical trials was associated with a more favorable impression of clinical trials and higher interest in trial participation. Furthermore, as far as we can ascertain, this is the first report showing how a simple intervention such as this may help close the knowledge gap on clinical trials among people of different educational backgrounds. Although most respondents in this interventional survey noted an increased willingness to consider participation in a clinical trial after reading the educational material, those with a lower level of education and knowledge about clinical trials received the most benefit. Previous participation in a clinical trial was also strongly associated with the impression of and willingness to enroll in a trial, both before and after reading the statement.11
Most of the interventions evaluated to date12 have focused on patients who are faced with having to decide whether to participate in a clinical trial2,3 or on very specific populations, such as select ethnic communities.13,14 However, it may be beneficial to provide simple, concise educational information about clinical trials to the general population, especially to those with minimal education. Although level of education concerns the minority of our sample (19%) sourced from an online panel, in the 2015 Current Population Survey, 41% of US participants aged 25 and older had not reached college education.15 Those with a lower level of education have reported a general lack of familiarity with clinical trials and were more likely to have inaccurate perceptions about trials. This is consistent with previous studies that have shown the lack of awareness and knowledge of clinical trials in this population.5,16,17 Our findings suggest that this knowledge gap can be reversed through a simple educational intervention and result in an increased willingness to participate.
The provision of information on clinical trials was positively associated with the 2 outcomes analyzed – improved impression of clinical trials and increased likelihood to enroll in a hypothetical trial. Such improvement might not translate to improved accrual,18 but it is a step toward closing the overall knowledge gap related to clinical trials and increasing the number of people who would consider trial participation. The lack of awareness of clinical trials has been reported as a legitimate explanation for why participation rates are lower in less-educated patient populations.19,20 This brief educational intervention is a simple, technology-sparing way to increase clinical trial awareness in the general population. In a similar survey, most physicians who reviewed an educational statement noted they were likely to use it with patients.21Less-educated patients, those who lived outside of urban areas, and those with lower household incomes were most concerned about trial location and the frequent visits needed when participating in a trial (Figure 2). Living in a nonurban area was not associated with participant impression of clinical trials or willingness to enroll in a trial. However, rural residency may be a barrier to enrollment depending on distance to the hospital22 and out-of-pocket expenses related to travel.23 Some comprehensive cancer centers, such as MSK, have developed alliances with community centers24 as a means of overcoming geographical barriers and increasing clinical trial participation rates.
Another concern shared by most respondents was the uncertainty in insurance coverage and potential out-of-pocket costs related to care. Lower household income, unlike location of residence and lack of insurance, was significantly associated with negative impressions of clinical trials and lower willingness to enroll in a trial, even after adjusting for education level. Cancer patients with higher financial burden have reported more attitudinal barriers, even after accounting for the negative effect of lower education level.25 Recent studies have also discussed the negative impact of lower income on cancer clinical trial participation,19,20,26,27 and new attention has been paid to the negative financial implications or “financial toxicity” of participating in a trial.23,28
White and older survey participants showed similar interest in clinical trial participation after accounting for other characteristics. There is growing evidence that outcome differences attributed to race may in fact be more dependent on socioeconomic status.8 A recent study among breast cancer patients showed that low socioeconomic status, but not race, was associated with decreased participation in clinical trials.29,30 Previous findings have also indicated that interest in clinical trials and barriers to enrollment among older, less-educated patients31 are often related to ineligibility, comorbidity, or communication difficulties.
Among our participants, the fear of side effects also was a common attitudinal barrier to clinical trial participation, as has been reported in previous studies.3,20 However, contrary to one previous study,20 this fear was not significantly increased among our less-educated participants.
Less-educated participants also reported more difficulties in understanding the information they were provided with, and they remained more concerned about being treated like “guinea pigs.” These concerns are consistent with other results showing that decisional conflict about clinical trial participation among patients with a high school diploma or less remained high even after they had received a National Cancer Institute text as pre-education material.3
Limitations
The lack of randomization makes it difficult to attribute with certainty that the change in acceptability of clinical trial participation is owing to the reading of the educational statement. The survey also sampled only English-speaking and well-educated participants from an online panel (81.1% had at least attended college) despite the use of a weighting procedure to ensure representativeness regarding gender, age, ethnicity, and income. Health literacy level or more specific trial literacy level was not evaluated; however, we were able to show less accurate perceptions of clinical trials among participants with a lower level of education by using agreement toward 8 statements about trials. The responses to hypothetical questions from these participants in the general population may also not be generalizable to a restricted population of patients with cancer. In addition, we measured impression of and willingness to enroll in a clinical trial immediately after providing participants with the educational material. We would have to confirm whether the positive effects of the education persist over time and translate to higher clinical trial participation rates.
Conclusions
Participants were receptive of educational material and expressed greater interest in and likelihood of enrolling in a clinical trial after reading it. The information had a greater effect on those with less education, but increased the willingness of all participants to enroll.
1. Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112(2):228-242.
2. Quinn GP, Bell BA, Bell MY, et al. The guinea pig syndrome: improving clinical trial participation among thoracic patients. J Thorac Oncol. 2007;2(3):191-196.
3. Meropol NJ, Wong Y-N, Albrecht T, et al. Randomized trial of a web-based intervention to address barriers to clinical trials. J Clin Oncol. 2016;34(5):469-478.
4. Goldberg RJ. Disclosure of information to adult cancer patients: issues and update. J Clin Oncol. 1984;2(8):948-955.
5. Leiter A, Diefenbach MA, Doucette J, Oh WK, Galsky MD. Clinical trial awareness: changes over time and sociodemographic disparities. Clin Trials. 2015;12(3):215-223.
6. Jacobsen PB, Wells KJ, Meade CD, et al. Effects of a brief multimedia psychoeducational intervention on the attitudes and interest of patients with cancer regarding clinical trial participation: a multicenter randomized controlled trial. J Clin Oncol. 2012;30(20):2516-2521.
7. Carney PA, Tucker EK, Newby TA, Beer TM. Feasibility, acceptability and findings from a pilot randomized controlled intervention study on the impact of a book designed to inform patients about cancer clinical trials. J Cancer Educ. 2014;29(1):181-187.
8. Sharrocks K, Spicer J, Camidge DR, Papa S. The impact of socioeconomic status on access to cancer clinical trials. Br J Cancer. 2014;111(9):1684-1687.
9. Regan J, Hickey C, Targett C, Masuda S, Sabbatini P. Framing clinical research and the importance of trial participation: patient and physician perspective. Poster presented at: 8th Annual AACI Clinical Research Initiative Meeting; July 20-21 2016; Chicago, IL.
10. Mancini J, Genre D, Dalenc F, et al. Patients’ regrets after participating in a randomized controlled trials depended on their involvement in the decision making. J Clin Epidemiol. 2012;65(6):635-642
11. Murphy ST, Frank LB, Chatterjee JS, et al. Comparing the relative efficacy of narrative vs nonnarrative health messages in reducing health disparities using a randomized trial. Am J Public Health. 2015;105(10):2117-2123.
12. Treweek S, Lockhart P, Pitkethly M, et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open. 2013;3(2):e002360. doi:10.1136/bmjopen-2012-002360.
13. Ma GX, Tan Y, Blakeney NC, et al. The impact of a community-based clinical trial educational intervention among underrepresented Chinese Americans. Cancer Epidemiol Biomarkers Prev. 2014;23(3):424-432.
14. Cupertino AP, Molina CSP, de los Rios JB, et al. Knowledge, awareness, and interest in cancer clinical trials among rural latinos following brief education by promotores de salud. J Community Med Health Educ. 2015;5:2161. doi:10.4172/2161-0711.1000358
15. Ryan CL, Bauman K. Educational attainment in the United States: 2015. https://www.census.gov/content/dam/Census/library/publications/2016/demo/p20-578.pdf. Published March 2016. Accessed October 18, 2016.
16. Lara PN Jr, Paterniti DA, Chiechi C, et al. Evaluation of factors affecting awareness of and willingness to participate in cancer clinical trials. J Clin Oncol. 2005;23(36):9282-9289.
17. Dhai A, Etheredge H, Cleaton-Jones P. A pilot study evaluating an intervention designed to raise awareness of clinical trials among potential participants in the developing world. J Med Ethics. 2010;36(4):238-242.
18. Denicoff AM, McCaskill-Stevens W, Grubbs SS, et al. The National Cancer Institute–American Society of Clinical Oncology Cancer Trial Accrual Symposium: summary and recommendations. J Oncol Pract. 2013;9(6):267-276.
19. Davison BJ, So A, Goldenberg SL, Berkowitz J, Gleave ME. Measurement of factors influencing the participation of patients with prostate cancer in clinical trials: a Canadian perspective. BJU Int. 2008;101(8):982-987.
20. Unger JM, Hershman DL, Albain KS, et al. Patient income level and cancer clinical trial participation. J Clin Oncol. 2013;31(5):536-542.
21. IRB Advisor. Education may overcome reticence to join trials. Relias website. http://www.ahcmedia.com/articles/138088-education-may-overcome-reticence-to-join-trials. Published July 1, 2016. Accessed July 29, 2016.
22. Vanderpool RC, Kornfeld J, Mills L, Byrne MM. Rural-urban differences in discussions of cancer treatment clinical trials. Patient Educ Couns. 2011;85(2):e69-e74.
23. Meropol NJ. Health policy: overcoming cost barriers to clinical trial participation. Nat Rev Clin Oncol. 2016;13(6):333-334.
24. MSK Cancer Alliance. Memorial Sloan Kettering Cancer Center website. https://www.mskcc.org/about/innovative-collaborations/msk-alliance. Updated March 7, 2018. Accessed August 18, 2016.
25. Manne S, Kashy D, Albrecht T, et al. Attitudinal barriers to participation in oncology clinical trials: factor analysis and correlates of barriers. Eur J Cancer Care (Engl). 2015;24(1):28-38.
26. Unger JM, Gralow JR, Albain KS, Ramsey SD, Hershman DL. Patient income level and cancer clinical trial participation: a prospective survey study. JAMA Oncol. 2016;2(1):137-139.
27. Moy B. Clinical trials, disparities, and financial burden: it’s time to intervene. Oncologist. 2015;20(6):571.
28. Bonevski B, Randell M, Paul C, et al. Reaching the hard-to-reach: a systematic review of strategies for improving health and medical research with socially disadvantaged groups. BMC Med Res Methodol. 2014;14:42.
29. Gross CP, Filardo G, Mayne ST, Krumholz HM. The impact of socioeconomic status and race on trial participation for older women with breast cancer. Cancer. 2005;103(3):483-491.
30. Nickell A, Burke NJ, Cohen E, Caprio M, Joseph G. Educating low-SES and LEP survivors about breast cancer research: pilot test of the Health Research Engagement Intervention. J Cancer Educ. 2014;29(4):746-752.
31. Mancini J, Jansen J, Julian-Reynier C, Bechlian D, Vey N, Chabannon C. Preferences of older adults with cancer for involvement in decision-making about research participation. J Am Geriatr Soc. 2014;62(6):1191-1193.
1. Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112(2):228-242.
2. Quinn GP, Bell BA, Bell MY, et al. The guinea pig syndrome: improving clinical trial participation among thoracic patients. J Thorac Oncol. 2007;2(3):191-196.
3. Meropol NJ, Wong Y-N, Albrecht T, et al. Randomized trial of a web-based intervention to address barriers to clinical trials. J Clin Oncol. 2016;34(5):469-478.
4. Goldberg RJ. Disclosure of information to adult cancer patients: issues and update. J Clin Oncol. 1984;2(8):948-955.
5. Leiter A, Diefenbach MA, Doucette J, Oh WK, Galsky MD. Clinical trial awareness: changes over time and sociodemographic disparities. Clin Trials. 2015;12(3):215-223.
6. Jacobsen PB, Wells KJ, Meade CD, et al. Effects of a brief multimedia psychoeducational intervention on the attitudes and interest of patients with cancer regarding clinical trial participation: a multicenter randomized controlled trial. J Clin Oncol. 2012;30(20):2516-2521.
7. Carney PA, Tucker EK, Newby TA, Beer TM. Feasibility, acceptability and findings from a pilot randomized controlled intervention study on the impact of a book designed to inform patients about cancer clinical trials. J Cancer Educ. 2014;29(1):181-187.
8. Sharrocks K, Spicer J, Camidge DR, Papa S. The impact of socioeconomic status on access to cancer clinical trials. Br J Cancer. 2014;111(9):1684-1687.
9. Regan J, Hickey C, Targett C, Masuda S, Sabbatini P. Framing clinical research and the importance of trial participation: patient and physician perspective. Poster presented at: 8th Annual AACI Clinical Research Initiative Meeting; July 20-21 2016; Chicago, IL.
10. Mancini J, Genre D, Dalenc F, et al. Patients’ regrets after participating in a randomized controlled trials depended on their involvement in the decision making. J Clin Epidemiol. 2012;65(6):635-642
11. Murphy ST, Frank LB, Chatterjee JS, et al. Comparing the relative efficacy of narrative vs nonnarrative health messages in reducing health disparities using a randomized trial. Am J Public Health. 2015;105(10):2117-2123.
12. Treweek S, Lockhart P, Pitkethly M, et al. Methods to improve recruitment to randomised controlled trials: Cochrane systematic review and meta-analysis. BMJ Open. 2013;3(2):e002360. doi:10.1136/bmjopen-2012-002360.
13. Ma GX, Tan Y, Blakeney NC, et al. The impact of a community-based clinical trial educational intervention among underrepresented Chinese Americans. Cancer Epidemiol Biomarkers Prev. 2014;23(3):424-432.
14. Cupertino AP, Molina CSP, de los Rios JB, et al. Knowledge, awareness, and interest in cancer clinical trials among rural latinos following brief education by promotores de salud. J Community Med Health Educ. 2015;5:2161. doi:10.4172/2161-0711.1000358
15. Ryan CL, Bauman K. Educational attainment in the United States: 2015. https://www.census.gov/content/dam/Census/library/publications/2016/demo/p20-578.pdf. Published March 2016. Accessed October 18, 2016.
16. Lara PN Jr, Paterniti DA, Chiechi C, et al. Evaluation of factors affecting awareness of and willingness to participate in cancer clinical trials. J Clin Oncol. 2005;23(36):9282-9289.
17. Dhai A, Etheredge H, Cleaton-Jones P. A pilot study evaluating an intervention designed to raise awareness of clinical trials among potential participants in the developing world. J Med Ethics. 2010;36(4):238-242.
18. Denicoff AM, McCaskill-Stevens W, Grubbs SS, et al. The National Cancer Institute–American Society of Clinical Oncology Cancer Trial Accrual Symposium: summary and recommendations. J Oncol Pract. 2013;9(6):267-276.
19. Davison BJ, So A, Goldenberg SL, Berkowitz J, Gleave ME. Measurement of factors influencing the participation of patients with prostate cancer in clinical trials: a Canadian perspective. BJU Int. 2008;101(8):982-987.
20. Unger JM, Hershman DL, Albain KS, et al. Patient income level and cancer clinical trial participation. J Clin Oncol. 2013;31(5):536-542.
21. IRB Advisor. Education may overcome reticence to join trials. Relias website. http://www.ahcmedia.com/articles/138088-education-may-overcome-reticence-to-join-trials. Published July 1, 2016. Accessed July 29, 2016.
22. Vanderpool RC, Kornfeld J, Mills L, Byrne MM. Rural-urban differences in discussions of cancer treatment clinical trials. Patient Educ Couns. 2011;85(2):e69-e74.
23. Meropol NJ. Health policy: overcoming cost barriers to clinical trial participation. Nat Rev Clin Oncol. 2016;13(6):333-334.
24. MSK Cancer Alliance. Memorial Sloan Kettering Cancer Center website. https://www.mskcc.org/about/innovative-collaborations/msk-alliance. Updated March 7, 2018. Accessed August 18, 2016.
25. Manne S, Kashy D, Albrecht T, et al. Attitudinal barriers to participation in oncology clinical trials: factor analysis and correlates of barriers. Eur J Cancer Care (Engl). 2015;24(1):28-38.
26. Unger JM, Gralow JR, Albain KS, Ramsey SD, Hershman DL. Patient income level and cancer clinical trial participation: a prospective survey study. JAMA Oncol. 2016;2(1):137-139.
27. Moy B. Clinical trials, disparities, and financial burden: it’s time to intervene. Oncologist. 2015;20(6):571.
28. Bonevski B, Randell M, Paul C, et al. Reaching the hard-to-reach: a systematic review of strategies for improving health and medical research with socially disadvantaged groups. BMC Med Res Methodol. 2014;14:42.
29. Gross CP, Filardo G, Mayne ST, Krumholz HM. The impact of socioeconomic status and race on trial participation for older women with breast cancer. Cancer. 2005;103(3):483-491.
30. Nickell A, Burke NJ, Cohen E, Caprio M, Joseph G. Educating low-SES and LEP survivors about breast cancer research: pilot test of the Health Research Engagement Intervention. J Cancer Educ. 2014;29(4):746-752.
31. Mancini J, Jansen J, Julian-Reynier C, Bechlian D, Vey N, Chabannon C. Preferences of older adults with cancer for involvement in decision-making about research participation. J Am Geriatr Soc. 2014;62(6):1191-1193.
Enhancing communication between oncology care providers and patient caregivers during hospice
Improving the delivery of end-of-life care for patients with advanced cancer has become a priority in the United States.1,2 Quality metrics identifying the components of high-quality end-of-life care have focused on improved symptom management, decreased use of chemotherapy at the end of life, fewer hospitalizations, and increased use of hospice care. Patients and caregivers also consider good communication with the medical team to be a critical component of end-of-life care.3-5 Interventions to improve the quality of end-of-life care are needed.
Caregivers of patients with advanced cancer who receive hospice services report better quality of care and death than those receiving end-of-life care in other settings.6-9 However, the transition for patients from active cancer therapy delivered by their oncologists to end-of-life care delivered by a hospice care team can be abrupt. Patients and their caregivers often feel abandoned by oncology clinicians because of the lack of continuity of care and poor communication.10-13 Caregivers who note continued involvement and communication with their oncology clinicians experience a lower caregiving burden, report higher satisfaction with care, and recount a higher quality of death for their loved one.14-16 Therefore, interventions that prevent abrupt transitions in care from oncology to hospice by ensuring continued communication with oncology clinicians are needed to improve the quality of end-of-life care.17 Recent findings have shown that providing concurrent oncology and palliative care is not only feasible but beneficial for patients with advanced cancer and their caregivers.18-24 However, there is no standard of care for the involvement of oncology clinicians in the care of patients receiving hospice services and their families.
Although interventions may be needed, it could be challenging to deliver them given the multiple demands of caregiving during hospice and the lack of regular contact in clinic. We sought to assess the feasibility of an intervention, Ensuring Communication in Hospice by Oncology (ECHO), to facilitate communication between oncology clinicians and caregivers of patients who enroll in hospice. We also explored caregiver-reported outcomes during hospice care, including satisfaction with care, attitudes toward caregiving, stress, decision regret, and perception of the quality of patients’ end-of-life care.
Methods
Study design
During March 2014-June 2015, caregivers of patients with advanced cancer who enrolled in home hospice services were eligible to participate in the study at Massachusetts General Hospital (MGH) in Boston. The Dana Farber/Harvard Cancer Center Institutional Review Board approved all methods and materials. The study opened with an enrollment goal of 30 participating caregivers. However, due to staff transitions, we closed the study early in June 2015 after 25 caregivers enrolled.
Participants
Caregivers of patients receiving care at the cancer center's thoracic, head and neck, sarcoma, melanoma, and gynecological disease centers were eligible within 10 days after a patient’s enrollment in hospice. Five disease sites were selected to participate in the intervention. We defined caregivers as relatives or friends serving as the primary caregiver of the patient at home during hospice care. Other caregiver eligibility criteria included the ability to read and respond to questions in English or with a translator, access to a telephone and/or computer to communicate with oncology clinicians, and willingness to complete questionnaires. Caregivers were ineligible if the patient was participating in an ongoing palliative care trial.
To identify eligible caregivers, case managers from both the inpatient and outpatient settings, as well as the nurses based in participating disease centers, notified the research team of all patients referred to hospice. If the patient had received oncology care in one of our participating disease centers, the research team contacted their oncology clinician/s (physicians, nurse practitioners [NP], registered nurses [RN], and/or physician assistants [PA]) to inquire if the patient had an involved caregiver and to obtain permission to offer study participation. If the oncology clinician/s did not grant permission, we documented the reason. Otherwise, with permission, research staff contacted the caregiver by telephone to offer study participation and obtain verbal consent. We then sent participating caregivers a copy of the informed consent by mail or e-mail.
Intervention
The ECHO intervention consisted of: supportive phone calls from an oncology clinician to the caregiver; an optional clinic visit with the oncology clinician for the patient to address clinical questions or concerns that was offered during the initial telephone consent; a bereavement call to the participating caregiver (Figure 1). Initially, we designed the intervention to have phone calls occurring twice weekly until the patient died. However, 3 months after starting the study, we received feedback from oncology clinicians and caregivers that calls were too frequent, so we amended the protocol to include phone calls twice weekly for the first 2 weeks of the study and then weekly thereafter. Seven months into the study, we again decreased the number of phone calls to weekly for the first 4 weeks, every other week for 4 weeks, and then monthly until patient death. We informed caregivers of changes by e-mail.
Before we started the study, we conducted training sessions with oncology clinicians from the participating disease centers to review study procedures and expectations of the phone calls. Supportive phone calls during hospice were not a part of standard practice prior to the study. The RN, NP or PA, and/or physician who had an established relationship with the patient and caregiver completed the phone calls. They decided based on their respective relationships with the patients and their workloads who would call each week, though the majority of calls were conducted by the RN or NP. All the clinicians had experience comanaging patients with hospice agencies, and our general practice is for the oncology physician to serve as the hospice attending of record. The calls were intended to offer support and reassurance to caregivers. We did not script the calls so that clinicians could tailor their content to the individual needs of the caregiver, as informed by their established relationship. The calls could include the patient if he/she was able to and interested in speaking to the clinician. There was no standardized communication with hospice as part of the intervention. If a caregiver raised concerns about symptom management during a call, the clinician would advise the caregiver to contact the hospice team directly or the clinician would call the hospice to discuss, depending on the clinical scenario and the clinician’s judgment. Research staff reminded oncology clinicians to call caregivers on the scheduled date and to document the discussion in the electronic medical record. The hospice phone number was included in the e-mail. If the call was not documented, research staff sent a reminder e-mail to the oncology clinicians 24 hours after the call was due.
Caregiver-reported measures
Caregivers completed a demographic questionnaire at baseline in which they reported their age, gender, race, ethnicity, religion, employment status, and relationship to the patient. We collected information about patient characteristics from the electronic medical record, including age, gender, and cancer type. In addition, we administered validated, self-report measures (see below). We limited the number of measures to decrease caregiver burden:
The baseline questionnaire included the FACQ-PC, the FAMCARE scale, the PSS, and the Decision Regret Scale. Initially, the study involved weekly questionnaires after baseline that included the FACQ-PC, the FAMCARE scale, and the PSS. However, after 3 months of study enrollment, we received feedback that the questionnaires were too frequent, so we amended the protocol and changed the frequency to weekly for 2 weeks, then monthly thereafter until the patient died.
Caregiver exit interview
Exit interviews included the toolkit interview, the Quality of End-of-Life Care scale, and the Decision Regret Scale. Caregivers also reported patients’ place and date of death. After the first 6 caregivers enrolled, we amended the exit interview to include open-ended feedback from caregivers. Specifically, we evaluated caregivers’ perceptions of the ECHO intervention by asking them about their perception of and satisfaction with the content and frequency of the oncology clinicians’ phone calls, whether they had an in-person visit with their oncology clinicians after the start of hospice care, whether the clinician/s contacted them after the patient died, and whether there were ways in which the clinician/s could help in the future.
Data collection and storage
Caregivers were given the option of completing study measures by telephone or e-mail so that they could complete them on a computer when it was convenient for them. Caregivers received a link to Research Electronic Data Capture (REDCap), a web-based, HIPAA-compliant application that allows participants to answer questionnaires online. The exit interviews were completed by phone, and research staff entered the data into the REDCap database. In addition, with we obtained caregiver permission to audiorecord the exit interviews, which were then transcribed and de-identified.
Statistical analysis
The primary outcome for the study was feasibility, which we defined as >70% of the caregivers receiving >50% of the phone calls from an oncology clinician, and >70% of the caregivers completing >50% of the questionnaires. All time points for the questionnaires and the exit interview counted toward feasibility. Exploratory endpoints included caregiver-reported satisfaction, stress, quality of end-of-life care, and decision-making regret.
Using STATA (v9.3; StataCorp, College Station, Texas) for all statistical analyses, we summarized participants’ characteristics and outcomes as frequencies and percentages for categorical variables and mean standard deviation for continuous variables. We used the repeated-measures t test to assess changes in caregiver outcomes over time. We used the Fisher exact test to compare clinically meaningful threshold scores of perceived stress between men and women.
We examined caregivers’ open-ended feedback using descriptive analyses to summarize comments about the intervention and to inform possible refinements for a future study.
Results
Baseline characteristics
During March 2014-June 2015, we enrolled caregivers of patients with advanced cancer from 5 participating disease centers: thoracic, head and neck, sarcoma, melanoma, and gynecological malignancy. We screened 123 patients to determine the eligibility of their caregivers (Figure 2). Of 38 eligible caregivers, 7 could not be reached, 6 declined participation, and 25 enrolled in the study (81% enrollment rate). Of the 25 caregivers who enrolled, 3 withdrew – 2 because the patients they were caring for died before the intervention began, and 1 who withdrew from the study because the family wanted less contact with the oncology clinician/s. Thus, we had data for 22 caregivers for our feasibility evaluation. One caregiver stopped study assessments after 3 months because the patient dis-enrolled from hospice. Median time from the patients’ hospice enrollment to caregiver study enrollment was 3 days (range, 1-9). Median time from study enrollment to patient death was 36 days (range, 2-135). Patients were receiving care from 10 different hospice agencies.
All of the patients had metastatic cancer, and 64% were women (Table 1).
Most of the caregivers were white (n = 18, 90%) and women (n = 12, 55%). The majority were the patient’s spouse (n = 12, 60%) or child (n = 6, 30%), and they lived with the patient (n = 16, 80%). Many of the caregivers had other responsibilities in addition to caring for the patients, including part- or full-time work (n = 10, 50%) or caring for others in addition to the patient (n = 10, 50%).
Feasibility
Over the study period, oncology clinicians completed 164 of 180 possible phone calls (91%). All 22 caregivers received >50% of the phone calls. Caregivers completed 78 of 99 possible questionnaires (79%), and 16 of 22 completed >50% of the questionnaires (73%). None of the caregivers/patients wanted to schedule the optional visit with an oncology clinician that was offered as part of the intervention; however, 5 patients had a clinic visit after hospice enrollment. In addition, 2 oncologists visited a patient and caregiver at home. All caregivers received bereavement contact from the oncology team.
Caregiver-reported outcomes
In all, 20 of the 22 enrolled caregivers completed baseline measures (Table 2), and they all chose to complete questionnaires by e-mail. Caregivers’ attitudes toward caregiving and satisfaction with hospice services were overall positive. They reported high mean scores on the 2 domains on the FACQ-PC of positive caregiver appraisal (mean, 4.25; SD, 0.52) and family well-being (mean, 4.09; SD, 0.45). The majority of caregivers (75%-95%) reported they were satisfied or very satisfied with various dimensions of hospice care based on the FAMCARE questionnaire.
Overall, caregivers reported moderate levels of stress (mean, 13.55; SD, 6.08) based on the PSS scores. Of the 20 caregivers who completed the baseline measures, 8 had clinically meaningful stress, and stress was numerically higher in female caregivers than in their male counterparts, but it did not reach statistical significance (55% vs 22%, P = .197). Finally, at baseline, caregivers indicated relatively low levels of decisional regret about enrolling in hospice, although there was considerable variation (mean, 10.25; SD, 14.37).
We conducted exit interviews with 15 caregivers because we were not able to reach 6 of the original 21 (Table 2). Caregivers rated hospice services highly for communication, symptom control, emotional support, and overall care. They also rated quality of death highly (mean, 8.46; SD 1.13). Regret was lower at the end of the study, but this did not reach statistical significance (baseline mean 9.29; end of study mean 3.57; P = .161).
In the recorded exit interviews, all of the caregivers responded they were satisfied with the phone calls. Two caregivers commented that they would have preferred the calls were more scheduled or at more suitable times. Overall, they described the phone calls as excellent, supportive, responsive, comfortable, and appreciated. All caregivers reported contact with the oncology team after the patient died, but one caregiver was disappointed she was only contacted by the nurse practitioner and not the oncologist. Participants did not feel as if there were other ways that the oncology team could have been helpful for them while their loved one was in hospice.
Table 3 highlights other representative comments from the exit interviews.
Discussion
As far as we know, this is the first study to assess the feasibility of an intervention to facilitate communication between oncology clinicians and caregivers of patients with advanced cancer who are receiving hospice care. Despite the challenges of oncology clinicians delivering an intervention to caregivers during hospice, we found this intervention was feasible and acceptable. Although the transition to hospice can be stressful, caregivers reported high satisfaction with hospice care and the quality of the patient’s death. In exit interviews, they also reported high satisfaction with the intervention and appreciation for maintaining their relationship with the oncology team. It is worth noting that no caregiver requested the optional clinic visit after hospice enrollment, and most caregivers were not seen again in clinic after hospice enrollment.
These results suggest that a simple, telephone-based intervention of scheduled calls from the oncology team at prompted intervals is not only feasible, but may also help foster continuity between the patient and caregiver and the oncology team. We received feedback from both oncology clinicians and caregivers that the initial call frequency was too often, suggesting that communication may not need to be very frequent to maintain continuity and provide support. This also suggests that if the calls are too frequent, they may be more intrusive than helpful for both oncologists and caregivers. Alternatively, caregiver suggestion for fewer phone calls may indicate that concerns about abandonment are less prevalent than existing literature has suggested.
Limitations
Our study has several important limitations. The sample size was small as this was a feasibility study conducted at a single tertiary care hospital, and the population was 90% white and 95% college educated, which may limit the generalizability of our results. In addition, the median length of stay in hospice for patients on this study was 36 days, which is long compared with national averages,32,33 and thus the outcomes may not represent the experience of a more heterogeneous population. The longer length of stay in hospice may have contributed to caregivers’ high satisfaction with the quality of end-of-life care.
Oncologists did not grant permission for the study team to approach all eligible caregivers, which may have introduced selection bias. We were also not able to reach 6 participants for exit interviews. People less satisfied with the intervention or with hospice may be more likely to have missing data, which could introduce bias into the satisfaction ratings. Furthermore, we did not explore the oncology clinicians’ perspective of the intervention or assess the time commitment of the calls. Oncology clinicians have many competing responsibilities and have variable experience and comfort with hospice care. Therefore, future studies should explore the perspective of oncology clinicians in regard to the intervention.
Finally, we did not require communication with the hospice agency as part of the intervention as there were ten different hospices involved. Thus, we do not know how the intervention impacted the hospice team’s care of the patient. However, based upon the success of this pilot study, future larger studies should explore the impact of the intervention from the perspective of the hospice care team and include oncology clinician communication with the hospice agency.
Conclusion
These findings demonstrate the feasibility and acceptability of an intervention to enhance communication between oncology clinicians and caregivers of patients with advanced cancer receiving hospice care. Importantly, the high caregiver satisfaction with the intervention in this study suggests that maintaining communication with the primary oncology team during hospice care may be an important component of high quality end-of-life care, though the desire for decreased calls suggests that this communication need not be frequent to maintain the continuity. A randomized study with a larger and more diverse patient/caregiver sample would allow us to explore the impact of the intervention on caregiver feelings of abandonment by the oncology team and short- and long-term caregiver outcomes, as well as to understand the perspective of the oncology and hospice clinicians involved.
1. Institute of Medicine. 2015. Dying in america: improving quality and honoring individual preferences near the end of life. Washington, DC: The National Academies Press. https://doi.org/10.17226/18748.
2. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96-112.
3. Wenrich MD, Curtis JR, Shannon SE, Carline JD, Ambrozy DM, Ramsey PG. Communicating with dying patients within the spectrum of medical care from terminal diagnosis to death. Arch Intern Med. 2001;161(6):868-874.
4. Parker SM, Clayton JM, Hancock K, et al. A systematic review of prognostic/end-of-life communication with adults in the advanced stages of a life-limiting illness: patient/caregiver preferences for the content, style, and timing of information. J Pain Symptom Manage. 2007;34(1):81-93.
5. Steinhauser KE, Clipp EC, McNeilly M, Christakis NA, McIntyre LM, Tulsky JA. In search of a good death: observations of patients, families, and providers. Ann Intern Med. 2000;132(10):825-832.
6. Teno JM, Clarridge BR, Casey V, et al. Family perspectives on end-of-life care at the last place of care. JAMA. 2004;291(1):88-93.
7. Kumar P, Wright AA, Hatfield LA, Temel JS, Keating NL. Family perspectives on hospice care experiences of patients with cancer. J Clin Oncol. 2017;35(4):432-439.
8. Wright AA, Keating NL, Ayanian JZ, et al. Family perspectives on aggressive cancer care near the end of life. JAMA. 2016;315(3):284-292.
9. Duggan KT, Hildebrand Duffus S, D’Agostino RB Jr, Petty WJ, Streer NP, Stephenson RC. The impact of hospice services in the care of patients with advanced stage nonsmall cell lung cancer. J Palliat Med. 2017;20(1):29-34.
10. Curtis JR, Wenrich MD, Carline JD, Shannon SE, Ambrozy DM, Ramsey PG. Understanding physicians’ skills at providing end-of-life care perspectives of patients, families, and health care workers. J Gen Intern Med. 2001;16(1):41-49.
11. Back AL, Young JP, McCown E, et al. Abandonment at the end of life from patient, caregiver, nurse, and physician perspectives: loss of continuity and lack of closure. Arch Intern Med. 2009;169(5):474-479.
12. Vig EK, Starks H, Taylor JS, Hopley EK, Fryer-Edwards K. Why don’t patients enroll in hospice? Can we do anything about it? J Gen Intern Med. 2010;25(10):1009-1019.
13. Waldrop DP, Meeker MA, Kerr C, Skretny J, Tangeman J, Milch R. The nature and timing of family-provider communication in late-stage cancer: a qualitative study of caregivers’ experiences. J Pain Symptom Manage. 2012;43(2):182-194.
14. Emanuel EJ, Fairclough DL, Slutsman J, Emanuel LL. Understanding economic and other burdens of terminal illness: the experience of patients and their caregivers. Ann Intern Med. 2000;132(6):451-459.
15. Curtis JR, Patrick DL, Engelberg RA, Norris K, Asp C, Byock I. A measure of the quality of dying and death. Initial validation using after-death interviews with family members. J Pain Symptom Manage. 2002;24(1):17-31.
16. Fleming DA, Sheppard VB, Mangan PA, et al. Caregiving at the end of life: Perceptions of health care quality and quality of life among patients and caregivers. J Pain Symptom Manage. 2006;31(5):407-420.
17. McMillan SC. Interventions to facilitate family caregiving at the end of life. J Palliat Med. 2005;8(Suppl 1):S132-S139.
18. Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741-749.
19. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742.
20. Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383(9930):1721-1730.
21. Maltoni M, Scarpi E, Dall’Agata M, et al. Systematic versus on-demand early palliative care: a randomised clinical trial assessing quality of care and treatment aggressiveness near the end of life. Eur J Cancer. 2016;69:110-118.
22. El-Jawahri A ea. Early integrated palliative care to improve family caregivers (FC) outcomes for patients with gastrointestinal and lung cancer. J Clin Oncol. 2016;34(suppl; abstr 10131).
23. Temel JS, Greer JA, El-Jawahri A, et al. Effects of early integrated palliative care in patients with lung and gi cancer: a randomized clinical trial. J Clin Oncol. 2017;35(8):834-841.
24. El-Jawahri A, LeBlanc T, VanDusen H, et al. Effect of inpatient palliative care on quality of life 2 weeks after hematopoietic stem cell transplantation: a randomized clinical trial. JAMA. 2016;316(20):2094-2103.
25. Cooper B, Kinsella GJ, Picton C. Development and initial validation of a family appraisal of caregiving questionnaire for palliative care. Psychooncology. 2006;15(7):613-622.
26. Kristjanson LJ. Validity and reliability testing of the FAMCARE Scale: measuring family satisfaction with advanced cancer care. Soc Sci Med. 1993;36(5):693-701.
27. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385-396.
28. Keir ST, Guill AB, Carter KE, Boole LC, Gonzales L, Friedman HS. Differential levels of stress in caregivers of brain tumor patients--observations from a pilot study. Support Care Cancer. 2006;14(12):1258-1261.
29. Brehaut JC, O’Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Decis Making. 2003;23(4):281-292.
30. Teno JM, Clarridge B, Casey V, Edgman-Levitan S, Fowler J. Validation of toolkit after-death bereaved family member interview. J Pain Symptom Manage. 2001;22(3):752-758.
31. Wright AA, Zhang B, Ray A, Mack JW, Trice E, Balboni T, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673.
32. Earle CC, Neville BA, Landrum MB, Ayanian JZ, Block SD, Weeks JC. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22(2):315-321.
33. Obermeyer Z, Makar M, Abujaber S, Dominici F, Block S, Cutler DM. Association between the Medicare hospice benefit and health care utilization and costs for patients with poor-prognosis cancer. JAMA. 2014;312(18):1888-1896.
Improving the delivery of end-of-life care for patients with advanced cancer has become a priority in the United States.1,2 Quality metrics identifying the components of high-quality end-of-life care have focused on improved symptom management, decreased use of chemotherapy at the end of life, fewer hospitalizations, and increased use of hospice care. Patients and caregivers also consider good communication with the medical team to be a critical component of end-of-life care.3-5 Interventions to improve the quality of end-of-life care are needed.
Caregivers of patients with advanced cancer who receive hospice services report better quality of care and death than those receiving end-of-life care in other settings.6-9 However, the transition for patients from active cancer therapy delivered by their oncologists to end-of-life care delivered by a hospice care team can be abrupt. Patients and their caregivers often feel abandoned by oncology clinicians because of the lack of continuity of care and poor communication.10-13 Caregivers who note continued involvement and communication with their oncology clinicians experience a lower caregiving burden, report higher satisfaction with care, and recount a higher quality of death for their loved one.14-16 Therefore, interventions that prevent abrupt transitions in care from oncology to hospice by ensuring continued communication with oncology clinicians are needed to improve the quality of end-of-life care.17 Recent findings have shown that providing concurrent oncology and palliative care is not only feasible but beneficial for patients with advanced cancer and their caregivers.18-24 However, there is no standard of care for the involvement of oncology clinicians in the care of patients receiving hospice services and their families.
Although interventions may be needed, it could be challenging to deliver them given the multiple demands of caregiving during hospice and the lack of regular contact in clinic. We sought to assess the feasibility of an intervention, Ensuring Communication in Hospice by Oncology (ECHO), to facilitate communication between oncology clinicians and caregivers of patients who enroll in hospice. We also explored caregiver-reported outcomes during hospice care, including satisfaction with care, attitudes toward caregiving, stress, decision regret, and perception of the quality of patients’ end-of-life care.
Methods
Study design
During March 2014-June 2015, caregivers of patients with advanced cancer who enrolled in home hospice services were eligible to participate in the study at Massachusetts General Hospital (MGH) in Boston. The Dana Farber/Harvard Cancer Center Institutional Review Board approved all methods and materials. The study opened with an enrollment goal of 30 participating caregivers. However, due to staff transitions, we closed the study early in June 2015 after 25 caregivers enrolled.
Participants
Caregivers of patients receiving care at the cancer center's thoracic, head and neck, sarcoma, melanoma, and gynecological disease centers were eligible within 10 days after a patient’s enrollment in hospice. Five disease sites were selected to participate in the intervention. We defined caregivers as relatives or friends serving as the primary caregiver of the patient at home during hospice care. Other caregiver eligibility criteria included the ability to read and respond to questions in English or with a translator, access to a telephone and/or computer to communicate with oncology clinicians, and willingness to complete questionnaires. Caregivers were ineligible if the patient was participating in an ongoing palliative care trial.
To identify eligible caregivers, case managers from both the inpatient and outpatient settings, as well as the nurses based in participating disease centers, notified the research team of all patients referred to hospice. If the patient had received oncology care in one of our participating disease centers, the research team contacted their oncology clinician/s (physicians, nurse practitioners [NP], registered nurses [RN], and/or physician assistants [PA]) to inquire if the patient had an involved caregiver and to obtain permission to offer study participation. If the oncology clinician/s did not grant permission, we documented the reason. Otherwise, with permission, research staff contacted the caregiver by telephone to offer study participation and obtain verbal consent. We then sent participating caregivers a copy of the informed consent by mail or e-mail.
Intervention
The ECHO intervention consisted of: supportive phone calls from an oncology clinician to the caregiver; an optional clinic visit with the oncology clinician for the patient to address clinical questions or concerns that was offered during the initial telephone consent; a bereavement call to the participating caregiver (Figure 1). Initially, we designed the intervention to have phone calls occurring twice weekly until the patient died. However, 3 months after starting the study, we received feedback from oncology clinicians and caregivers that calls were too frequent, so we amended the protocol to include phone calls twice weekly for the first 2 weeks of the study and then weekly thereafter. Seven months into the study, we again decreased the number of phone calls to weekly for the first 4 weeks, every other week for 4 weeks, and then monthly until patient death. We informed caregivers of changes by e-mail.
Before we started the study, we conducted training sessions with oncology clinicians from the participating disease centers to review study procedures and expectations of the phone calls. Supportive phone calls during hospice were not a part of standard practice prior to the study. The RN, NP or PA, and/or physician who had an established relationship with the patient and caregiver completed the phone calls. They decided based on their respective relationships with the patients and their workloads who would call each week, though the majority of calls were conducted by the RN or NP. All the clinicians had experience comanaging patients with hospice agencies, and our general practice is for the oncology physician to serve as the hospice attending of record. The calls were intended to offer support and reassurance to caregivers. We did not script the calls so that clinicians could tailor their content to the individual needs of the caregiver, as informed by their established relationship. The calls could include the patient if he/she was able to and interested in speaking to the clinician. There was no standardized communication with hospice as part of the intervention. If a caregiver raised concerns about symptom management during a call, the clinician would advise the caregiver to contact the hospice team directly or the clinician would call the hospice to discuss, depending on the clinical scenario and the clinician’s judgment. Research staff reminded oncology clinicians to call caregivers on the scheduled date and to document the discussion in the electronic medical record. The hospice phone number was included in the e-mail. If the call was not documented, research staff sent a reminder e-mail to the oncology clinicians 24 hours after the call was due.
Caregiver-reported measures
Caregivers completed a demographic questionnaire at baseline in which they reported their age, gender, race, ethnicity, religion, employment status, and relationship to the patient. We collected information about patient characteristics from the electronic medical record, including age, gender, and cancer type. In addition, we administered validated, self-report measures (see below). We limited the number of measures to decrease caregiver burden:
The baseline questionnaire included the FACQ-PC, the FAMCARE scale, the PSS, and the Decision Regret Scale. Initially, the study involved weekly questionnaires after baseline that included the FACQ-PC, the FAMCARE scale, and the PSS. However, after 3 months of study enrollment, we received feedback that the questionnaires were too frequent, so we amended the protocol and changed the frequency to weekly for 2 weeks, then monthly thereafter until the patient died.
Caregiver exit interview
Exit interviews included the toolkit interview, the Quality of End-of-Life Care scale, and the Decision Regret Scale. Caregivers also reported patients’ place and date of death. After the first 6 caregivers enrolled, we amended the exit interview to include open-ended feedback from caregivers. Specifically, we evaluated caregivers’ perceptions of the ECHO intervention by asking them about their perception of and satisfaction with the content and frequency of the oncology clinicians’ phone calls, whether they had an in-person visit with their oncology clinicians after the start of hospice care, whether the clinician/s contacted them after the patient died, and whether there were ways in which the clinician/s could help in the future.
Data collection and storage
Caregivers were given the option of completing study measures by telephone or e-mail so that they could complete them on a computer when it was convenient for them. Caregivers received a link to Research Electronic Data Capture (REDCap), a web-based, HIPAA-compliant application that allows participants to answer questionnaires online. The exit interviews were completed by phone, and research staff entered the data into the REDCap database. In addition, with we obtained caregiver permission to audiorecord the exit interviews, which were then transcribed and de-identified.
Statistical analysis
The primary outcome for the study was feasibility, which we defined as >70% of the caregivers receiving >50% of the phone calls from an oncology clinician, and >70% of the caregivers completing >50% of the questionnaires. All time points for the questionnaires and the exit interview counted toward feasibility. Exploratory endpoints included caregiver-reported satisfaction, stress, quality of end-of-life care, and decision-making regret.
Using STATA (v9.3; StataCorp, College Station, Texas) for all statistical analyses, we summarized participants’ characteristics and outcomes as frequencies and percentages for categorical variables and mean standard deviation for continuous variables. We used the repeated-measures t test to assess changes in caregiver outcomes over time. We used the Fisher exact test to compare clinically meaningful threshold scores of perceived stress between men and women.
We examined caregivers’ open-ended feedback using descriptive analyses to summarize comments about the intervention and to inform possible refinements for a future study.
Results
Baseline characteristics
During March 2014-June 2015, we enrolled caregivers of patients with advanced cancer from 5 participating disease centers: thoracic, head and neck, sarcoma, melanoma, and gynecological malignancy. We screened 123 patients to determine the eligibility of their caregivers (Figure 2). Of 38 eligible caregivers, 7 could not be reached, 6 declined participation, and 25 enrolled in the study (81% enrollment rate). Of the 25 caregivers who enrolled, 3 withdrew – 2 because the patients they were caring for died before the intervention began, and 1 who withdrew from the study because the family wanted less contact with the oncology clinician/s. Thus, we had data for 22 caregivers for our feasibility evaluation. One caregiver stopped study assessments after 3 months because the patient dis-enrolled from hospice. Median time from the patients’ hospice enrollment to caregiver study enrollment was 3 days (range, 1-9). Median time from study enrollment to patient death was 36 days (range, 2-135). Patients were receiving care from 10 different hospice agencies.
All of the patients had metastatic cancer, and 64% were women (Table 1).
Most of the caregivers were white (n = 18, 90%) and women (n = 12, 55%). The majority were the patient’s spouse (n = 12, 60%) or child (n = 6, 30%), and they lived with the patient (n = 16, 80%). Many of the caregivers had other responsibilities in addition to caring for the patients, including part- or full-time work (n = 10, 50%) or caring for others in addition to the patient (n = 10, 50%).
Feasibility
Over the study period, oncology clinicians completed 164 of 180 possible phone calls (91%). All 22 caregivers received >50% of the phone calls. Caregivers completed 78 of 99 possible questionnaires (79%), and 16 of 22 completed >50% of the questionnaires (73%). None of the caregivers/patients wanted to schedule the optional visit with an oncology clinician that was offered as part of the intervention; however, 5 patients had a clinic visit after hospice enrollment. In addition, 2 oncologists visited a patient and caregiver at home. All caregivers received bereavement contact from the oncology team.
Caregiver-reported outcomes
In all, 20 of the 22 enrolled caregivers completed baseline measures (Table 2), and they all chose to complete questionnaires by e-mail. Caregivers’ attitudes toward caregiving and satisfaction with hospice services were overall positive. They reported high mean scores on the 2 domains on the FACQ-PC of positive caregiver appraisal (mean, 4.25; SD, 0.52) and family well-being (mean, 4.09; SD, 0.45). The majority of caregivers (75%-95%) reported they were satisfied or very satisfied with various dimensions of hospice care based on the FAMCARE questionnaire.
Overall, caregivers reported moderate levels of stress (mean, 13.55; SD, 6.08) based on the PSS scores. Of the 20 caregivers who completed the baseline measures, 8 had clinically meaningful stress, and stress was numerically higher in female caregivers than in their male counterparts, but it did not reach statistical significance (55% vs 22%, P = .197). Finally, at baseline, caregivers indicated relatively low levels of decisional regret about enrolling in hospice, although there was considerable variation (mean, 10.25; SD, 14.37).
We conducted exit interviews with 15 caregivers because we were not able to reach 6 of the original 21 (Table 2). Caregivers rated hospice services highly for communication, symptom control, emotional support, and overall care. They also rated quality of death highly (mean, 8.46; SD 1.13). Regret was lower at the end of the study, but this did not reach statistical significance (baseline mean 9.29; end of study mean 3.57; P = .161).
In the recorded exit interviews, all of the caregivers responded they were satisfied with the phone calls. Two caregivers commented that they would have preferred the calls were more scheduled or at more suitable times. Overall, they described the phone calls as excellent, supportive, responsive, comfortable, and appreciated. All caregivers reported contact with the oncology team after the patient died, but one caregiver was disappointed she was only contacted by the nurse practitioner and not the oncologist. Participants did not feel as if there were other ways that the oncology team could have been helpful for them while their loved one was in hospice.
Table 3 highlights other representative comments from the exit interviews.
Discussion
As far as we know, this is the first study to assess the feasibility of an intervention to facilitate communication between oncology clinicians and caregivers of patients with advanced cancer who are receiving hospice care. Despite the challenges of oncology clinicians delivering an intervention to caregivers during hospice, we found this intervention was feasible and acceptable. Although the transition to hospice can be stressful, caregivers reported high satisfaction with hospice care and the quality of the patient’s death. In exit interviews, they also reported high satisfaction with the intervention and appreciation for maintaining their relationship with the oncology team. It is worth noting that no caregiver requested the optional clinic visit after hospice enrollment, and most caregivers were not seen again in clinic after hospice enrollment.
These results suggest that a simple, telephone-based intervention of scheduled calls from the oncology team at prompted intervals is not only feasible, but may also help foster continuity between the patient and caregiver and the oncology team. We received feedback from both oncology clinicians and caregivers that the initial call frequency was too often, suggesting that communication may not need to be very frequent to maintain continuity and provide support. This also suggests that if the calls are too frequent, they may be more intrusive than helpful for both oncologists and caregivers. Alternatively, caregiver suggestion for fewer phone calls may indicate that concerns about abandonment are less prevalent than existing literature has suggested.
Limitations
Our study has several important limitations. The sample size was small as this was a feasibility study conducted at a single tertiary care hospital, and the population was 90% white and 95% college educated, which may limit the generalizability of our results. In addition, the median length of stay in hospice for patients on this study was 36 days, which is long compared with national averages,32,33 and thus the outcomes may not represent the experience of a more heterogeneous population. The longer length of stay in hospice may have contributed to caregivers’ high satisfaction with the quality of end-of-life care.
Oncologists did not grant permission for the study team to approach all eligible caregivers, which may have introduced selection bias. We were also not able to reach 6 participants for exit interviews. People less satisfied with the intervention or with hospice may be more likely to have missing data, which could introduce bias into the satisfaction ratings. Furthermore, we did not explore the oncology clinicians’ perspective of the intervention or assess the time commitment of the calls. Oncology clinicians have many competing responsibilities and have variable experience and comfort with hospice care. Therefore, future studies should explore the perspective of oncology clinicians in regard to the intervention.
Finally, we did not require communication with the hospice agency as part of the intervention as there were ten different hospices involved. Thus, we do not know how the intervention impacted the hospice team’s care of the patient. However, based upon the success of this pilot study, future larger studies should explore the impact of the intervention from the perspective of the hospice care team and include oncology clinician communication with the hospice agency.
Conclusion
These findings demonstrate the feasibility and acceptability of an intervention to enhance communication between oncology clinicians and caregivers of patients with advanced cancer receiving hospice care. Importantly, the high caregiver satisfaction with the intervention in this study suggests that maintaining communication with the primary oncology team during hospice care may be an important component of high quality end-of-life care, though the desire for decreased calls suggests that this communication need not be frequent to maintain the continuity. A randomized study with a larger and more diverse patient/caregiver sample would allow us to explore the impact of the intervention on caregiver feelings of abandonment by the oncology team and short- and long-term caregiver outcomes, as well as to understand the perspective of the oncology and hospice clinicians involved.
Improving the delivery of end-of-life care for patients with advanced cancer has become a priority in the United States.1,2 Quality metrics identifying the components of high-quality end-of-life care have focused on improved symptom management, decreased use of chemotherapy at the end of life, fewer hospitalizations, and increased use of hospice care. Patients and caregivers also consider good communication with the medical team to be a critical component of end-of-life care.3-5 Interventions to improve the quality of end-of-life care are needed.
Caregivers of patients with advanced cancer who receive hospice services report better quality of care and death than those receiving end-of-life care in other settings.6-9 However, the transition for patients from active cancer therapy delivered by their oncologists to end-of-life care delivered by a hospice care team can be abrupt. Patients and their caregivers often feel abandoned by oncology clinicians because of the lack of continuity of care and poor communication.10-13 Caregivers who note continued involvement and communication with their oncology clinicians experience a lower caregiving burden, report higher satisfaction with care, and recount a higher quality of death for their loved one.14-16 Therefore, interventions that prevent abrupt transitions in care from oncology to hospice by ensuring continued communication with oncology clinicians are needed to improve the quality of end-of-life care.17 Recent findings have shown that providing concurrent oncology and palliative care is not only feasible but beneficial for patients with advanced cancer and their caregivers.18-24 However, there is no standard of care for the involvement of oncology clinicians in the care of patients receiving hospice services and their families.
Although interventions may be needed, it could be challenging to deliver them given the multiple demands of caregiving during hospice and the lack of regular contact in clinic. We sought to assess the feasibility of an intervention, Ensuring Communication in Hospice by Oncology (ECHO), to facilitate communication between oncology clinicians and caregivers of patients who enroll in hospice. We also explored caregiver-reported outcomes during hospice care, including satisfaction with care, attitudes toward caregiving, stress, decision regret, and perception of the quality of patients’ end-of-life care.
Methods
Study design
During March 2014-June 2015, caregivers of patients with advanced cancer who enrolled in home hospice services were eligible to participate in the study at Massachusetts General Hospital (MGH) in Boston. The Dana Farber/Harvard Cancer Center Institutional Review Board approved all methods and materials. The study opened with an enrollment goal of 30 participating caregivers. However, due to staff transitions, we closed the study early in June 2015 after 25 caregivers enrolled.
Participants
Caregivers of patients receiving care at the cancer center's thoracic, head and neck, sarcoma, melanoma, and gynecological disease centers were eligible within 10 days after a patient’s enrollment in hospice. Five disease sites were selected to participate in the intervention. We defined caregivers as relatives or friends serving as the primary caregiver of the patient at home during hospice care. Other caregiver eligibility criteria included the ability to read and respond to questions in English or with a translator, access to a telephone and/or computer to communicate with oncology clinicians, and willingness to complete questionnaires. Caregivers were ineligible if the patient was participating in an ongoing palliative care trial.
To identify eligible caregivers, case managers from both the inpatient and outpatient settings, as well as the nurses based in participating disease centers, notified the research team of all patients referred to hospice. If the patient had received oncology care in one of our participating disease centers, the research team contacted their oncology clinician/s (physicians, nurse practitioners [NP], registered nurses [RN], and/or physician assistants [PA]) to inquire if the patient had an involved caregiver and to obtain permission to offer study participation. If the oncology clinician/s did not grant permission, we documented the reason. Otherwise, with permission, research staff contacted the caregiver by telephone to offer study participation and obtain verbal consent. We then sent participating caregivers a copy of the informed consent by mail or e-mail.
Intervention
The ECHO intervention consisted of: supportive phone calls from an oncology clinician to the caregiver; an optional clinic visit with the oncology clinician for the patient to address clinical questions or concerns that was offered during the initial telephone consent; a bereavement call to the participating caregiver (Figure 1). Initially, we designed the intervention to have phone calls occurring twice weekly until the patient died. However, 3 months after starting the study, we received feedback from oncology clinicians and caregivers that calls were too frequent, so we amended the protocol to include phone calls twice weekly for the first 2 weeks of the study and then weekly thereafter. Seven months into the study, we again decreased the number of phone calls to weekly for the first 4 weeks, every other week for 4 weeks, and then monthly until patient death. We informed caregivers of changes by e-mail.
Before we started the study, we conducted training sessions with oncology clinicians from the participating disease centers to review study procedures and expectations of the phone calls. Supportive phone calls during hospice were not a part of standard practice prior to the study. The RN, NP or PA, and/or physician who had an established relationship with the patient and caregiver completed the phone calls. They decided based on their respective relationships with the patients and their workloads who would call each week, though the majority of calls were conducted by the RN or NP. All the clinicians had experience comanaging patients with hospice agencies, and our general practice is for the oncology physician to serve as the hospice attending of record. The calls were intended to offer support and reassurance to caregivers. We did not script the calls so that clinicians could tailor their content to the individual needs of the caregiver, as informed by their established relationship. The calls could include the patient if he/she was able to and interested in speaking to the clinician. There was no standardized communication with hospice as part of the intervention. If a caregiver raised concerns about symptom management during a call, the clinician would advise the caregiver to contact the hospice team directly or the clinician would call the hospice to discuss, depending on the clinical scenario and the clinician’s judgment. Research staff reminded oncology clinicians to call caregivers on the scheduled date and to document the discussion in the electronic medical record. The hospice phone number was included in the e-mail. If the call was not documented, research staff sent a reminder e-mail to the oncology clinicians 24 hours after the call was due.
Caregiver-reported measures
Caregivers completed a demographic questionnaire at baseline in which they reported their age, gender, race, ethnicity, religion, employment status, and relationship to the patient. We collected information about patient characteristics from the electronic medical record, including age, gender, and cancer type. In addition, we administered validated, self-report measures (see below). We limited the number of measures to decrease caregiver burden:
The baseline questionnaire included the FACQ-PC, the FAMCARE scale, the PSS, and the Decision Regret Scale. Initially, the study involved weekly questionnaires after baseline that included the FACQ-PC, the FAMCARE scale, and the PSS. However, after 3 months of study enrollment, we received feedback that the questionnaires were too frequent, so we amended the protocol and changed the frequency to weekly for 2 weeks, then monthly thereafter until the patient died.
Caregiver exit interview
Exit interviews included the toolkit interview, the Quality of End-of-Life Care scale, and the Decision Regret Scale. Caregivers also reported patients’ place and date of death. After the first 6 caregivers enrolled, we amended the exit interview to include open-ended feedback from caregivers. Specifically, we evaluated caregivers’ perceptions of the ECHO intervention by asking them about their perception of and satisfaction with the content and frequency of the oncology clinicians’ phone calls, whether they had an in-person visit with their oncology clinicians after the start of hospice care, whether the clinician/s contacted them after the patient died, and whether there were ways in which the clinician/s could help in the future.
Data collection and storage
Caregivers were given the option of completing study measures by telephone or e-mail so that they could complete them on a computer when it was convenient for them. Caregivers received a link to Research Electronic Data Capture (REDCap), a web-based, HIPAA-compliant application that allows participants to answer questionnaires online. The exit interviews were completed by phone, and research staff entered the data into the REDCap database. In addition, with we obtained caregiver permission to audiorecord the exit interviews, which were then transcribed and de-identified.
Statistical analysis
The primary outcome for the study was feasibility, which we defined as >70% of the caregivers receiving >50% of the phone calls from an oncology clinician, and >70% of the caregivers completing >50% of the questionnaires. All time points for the questionnaires and the exit interview counted toward feasibility. Exploratory endpoints included caregiver-reported satisfaction, stress, quality of end-of-life care, and decision-making regret.
Using STATA (v9.3; StataCorp, College Station, Texas) for all statistical analyses, we summarized participants’ characteristics and outcomes as frequencies and percentages for categorical variables and mean standard deviation for continuous variables. We used the repeated-measures t test to assess changes in caregiver outcomes over time. We used the Fisher exact test to compare clinically meaningful threshold scores of perceived stress between men and women.
We examined caregivers’ open-ended feedback using descriptive analyses to summarize comments about the intervention and to inform possible refinements for a future study.
Results
Baseline characteristics
During March 2014-June 2015, we enrolled caregivers of patients with advanced cancer from 5 participating disease centers: thoracic, head and neck, sarcoma, melanoma, and gynecological malignancy. We screened 123 patients to determine the eligibility of their caregivers (Figure 2). Of 38 eligible caregivers, 7 could not be reached, 6 declined participation, and 25 enrolled in the study (81% enrollment rate). Of the 25 caregivers who enrolled, 3 withdrew – 2 because the patients they were caring for died before the intervention began, and 1 who withdrew from the study because the family wanted less contact with the oncology clinician/s. Thus, we had data for 22 caregivers for our feasibility evaluation. One caregiver stopped study assessments after 3 months because the patient dis-enrolled from hospice. Median time from the patients’ hospice enrollment to caregiver study enrollment was 3 days (range, 1-9). Median time from study enrollment to patient death was 36 days (range, 2-135). Patients were receiving care from 10 different hospice agencies.
All of the patients had metastatic cancer, and 64% were women (Table 1).
Most of the caregivers were white (n = 18, 90%) and women (n = 12, 55%). The majority were the patient’s spouse (n = 12, 60%) or child (n = 6, 30%), and they lived with the patient (n = 16, 80%). Many of the caregivers had other responsibilities in addition to caring for the patients, including part- or full-time work (n = 10, 50%) or caring for others in addition to the patient (n = 10, 50%).
Feasibility
Over the study period, oncology clinicians completed 164 of 180 possible phone calls (91%). All 22 caregivers received >50% of the phone calls. Caregivers completed 78 of 99 possible questionnaires (79%), and 16 of 22 completed >50% of the questionnaires (73%). None of the caregivers/patients wanted to schedule the optional visit with an oncology clinician that was offered as part of the intervention; however, 5 patients had a clinic visit after hospice enrollment. In addition, 2 oncologists visited a patient and caregiver at home. All caregivers received bereavement contact from the oncology team.
Caregiver-reported outcomes
In all, 20 of the 22 enrolled caregivers completed baseline measures (Table 2), and they all chose to complete questionnaires by e-mail. Caregivers’ attitudes toward caregiving and satisfaction with hospice services were overall positive. They reported high mean scores on the 2 domains on the FACQ-PC of positive caregiver appraisal (mean, 4.25; SD, 0.52) and family well-being (mean, 4.09; SD, 0.45). The majority of caregivers (75%-95%) reported they were satisfied or very satisfied with various dimensions of hospice care based on the FAMCARE questionnaire.
Overall, caregivers reported moderate levels of stress (mean, 13.55; SD, 6.08) based on the PSS scores. Of the 20 caregivers who completed the baseline measures, 8 had clinically meaningful stress, and stress was numerically higher in female caregivers than in their male counterparts, but it did not reach statistical significance (55% vs 22%, P = .197). Finally, at baseline, caregivers indicated relatively low levels of decisional regret about enrolling in hospice, although there was considerable variation (mean, 10.25; SD, 14.37).
We conducted exit interviews with 15 caregivers because we were not able to reach 6 of the original 21 (Table 2). Caregivers rated hospice services highly for communication, symptom control, emotional support, and overall care. They also rated quality of death highly (mean, 8.46; SD 1.13). Regret was lower at the end of the study, but this did not reach statistical significance (baseline mean 9.29; end of study mean 3.57; P = .161).
In the recorded exit interviews, all of the caregivers responded they were satisfied with the phone calls. Two caregivers commented that they would have preferred the calls were more scheduled or at more suitable times. Overall, they described the phone calls as excellent, supportive, responsive, comfortable, and appreciated. All caregivers reported contact with the oncology team after the patient died, but one caregiver was disappointed she was only contacted by the nurse practitioner and not the oncologist. Participants did not feel as if there were other ways that the oncology team could have been helpful for them while their loved one was in hospice.
Table 3 highlights other representative comments from the exit interviews.
Discussion
As far as we know, this is the first study to assess the feasibility of an intervention to facilitate communication between oncology clinicians and caregivers of patients with advanced cancer who are receiving hospice care. Despite the challenges of oncology clinicians delivering an intervention to caregivers during hospice, we found this intervention was feasible and acceptable. Although the transition to hospice can be stressful, caregivers reported high satisfaction with hospice care and the quality of the patient’s death. In exit interviews, they also reported high satisfaction with the intervention and appreciation for maintaining their relationship with the oncology team. It is worth noting that no caregiver requested the optional clinic visit after hospice enrollment, and most caregivers were not seen again in clinic after hospice enrollment.
These results suggest that a simple, telephone-based intervention of scheduled calls from the oncology team at prompted intervals is not only feasible, but may also help foster continuity between the patient and caregiver and the oncology team. We received feedback from both oncology clinicians and caregivers that the initial call frequency was too often, suggesting that communication may not need to be very frequent to maintain continuity and provide support. This also suggests that if the calls are too frequent, they may be more intrusive than helpful for both oncologists and caregivers. Alternatively, caregiver suggestion for fewer phone calls may indicate that concerns about abandonment are less prevalent than existing literature has suggested.
Limitations
Our study has several important limitations. The sample size was small as this was a feasibility study conducted at a single tertiary care hospital, and the population was 90% white and 95% college educated, which may limit the generalizability of our results. In addition, the median length of stay in hospice for patients on this study was 36 days, which is long compared with national averages,32,33 and thus the outcomes may not represent the experience of a more heterogeneous population. The longer length of stay in hospice may have contributed to caregivers’ high satisfaction with the quality of end-of-life care.
Oncologists did not grant permission for the study team to approach all eligible caregivers, which may have introduced selection bias. We were also not able to reach 6 participants for exit interviews. People less satisfied with the intervention or with hospice may be more likely to have missing data, which could introduce bias into the satisfaction ratings. Furthermore, we did not explore the oncology clinicians’ perspective of the intervention or assess the time commitment of the calls. Oncology clinicians have many competing responsibilities and have variable experience and comfort with hospice care. Therefore, future studies should explore the perspective of oncology clinicians in regard to the intervention.
Finally, we did not require communication with the hospice agency as part of the intervention as there were ten different hospices involved. Thus, we do not know how the intervention impacted the hospice team’s care of the patient. However, based upon the success of this pilot study, future larger studies should explore the impact of the intervention from the perspective of the hospice care team and include oncology clinician communication with the hospice agency.
Conclusion
These findings demonstrate the feasibility and acceptability of an intervention to enhance communication between oncology clinicians and caregivers of patients with advanced cancer receiving hospice care. Importantly, the high caregiver satisfaction with the intervention in this study suggests that maintaining communication with the primary oncology team during hospice care may be an important component of high quality end-of-life care, though the desire for decreased calls suggests that this communication need not be frequent to maintain the continuity. A randomized study with a larger and more diverse patient/caregiver sample would allow us to explore the impact of the intervention on caregiver feelings of abandonment by the oncology team and short- and long-term caregiver outcomes, as well as to understand the perspective of the oncology and hospice clinicians involved.
1. Institute of Medicine. 2015. Dying in america: improving quality and honoring individual preferences near the end of life. Washington, DC: The National Academies Press. https://doi.org/10.17226/18748.
2. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96-112.
3. Wenrich MD, Curtis JR, Shannon SE, Carline JD, Ambrozy DM, Ramsey PG. Communicating with dying patients within the spectrum of medical care from terminal diagnosis to death. Arch Intern Med. 2001;161(6):868-874.
4. Parker SM, Clayton JM, Hancock K, et al. A systematic review of prognostic/end-of-life communication with adults in the advanced stages of a life-limiting illness: patient/caregiver preferences for the content, style, and timing of information. J Pain Symptom Manage. 2007;34(1):81-93.
5. Steinhauser KE, Clipp EC, McNeilly M, Christakis NA, McIntyre LM, Tulsky JA. In search of a good death: observations of patients, families, and providers. Ann Intern Med. 2000;132(10):825-832.
6. Teno JM, Clarridge BR, Casey V, et al. Family perspectives on end-of-life care at the last place of care. JAMA. 2004;291(1):88-93.
7. Kumar P, Wright AA, Hatfield LA, Temel JS, Keating NL. Family perspectives on hospice care experiences of patients with cancer. J Clin Oncol. 2017;35(4):432-439.
8. Wright AA, Keating NL, Ayanian JZ, et al. Family perspectives on aggressive cancer care near the end of life. JAMA. 2016;315(3):284-292.
9. Duggan KT, Hildebrand Duffus S, D’Agostino RB Jr, Petty WJ, Streer NP, Stephenson RC. The impact of hospice services in the care of patients with advanced stage nonsmall cell lung cancer. J Palliat Med. 2017;20(1):29-34.
10. Curtis JR, Wenrich MD, Carline JD, Shannon SE, Ambrozy DM, Ramsey PG. Understanding physicians’ skills at providing end-of-life care perspectives of patients, families, and health care workers. J Gen Intern Med. 2001;16(1):41-49.
11. Back AL, Young JP, McCown E, et al. Abandonment at the end of life from patient, caregiver, nurse, and physician perspectives: loss of continuity and lack of closure. Arch Intern Med. 2009;169(5):474-479.
12. Vig EK, Starks H, Taylor JS, Hopley EK, Fryer-Edwards K. Why don’t patients enroll in hospice? Can we do anything about it? J Gen Intern Med. 2010;25(10):1009-1019.
13. Waldrop DP, Meeker MA, Kerr C, Skretny J, Tangeman J, Milch R. The nature and timing of family-provider communication in late-stage cancer: a qualitative study of caregivers’ experiences. J Pain Symptom Manage. 2012;43(2):182-194.
14. Emanuel EJ, Fairclough DL, Slutsman J, Emanuel LL. Understanding economic and other burdens of terminal illness: the experience of patients and their caregivers. Ann Intern Med. 2000;132(6):451-459.
15. Curtis JR, Patrick DL, Engelberg RA, Norris K, Asp C, Byock I. A measure of the quality of dying and death. Initial validation using after-death interviews with family members. J Pain Symptom Manage. 2002;24(1):17-31.
16. Fleming DA, Sheppard VB, Mangan PA, et al. Caregiving at the end of life: Perceptions of health care quality and quality of life among patients and caregivers. J Pain Symptom Manage. 2006;31(5):407-420.
17. McMillan SC. Interventions to facilitate family caregiving at the end of life. J Palliat Med. 2005;8(Suppl 1):S132-S139.
18. Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741-749.
19. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742.
20. Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383(9930):1721-1730.
21. Maltoni M, Scarpi E, Dall’Agata M, et al. Systematic versus on-demand early palliative care: a randomised clinical trial assessing quality of care and treatment aggressiveness near the end of life. Eur J Cancer. 2016;69:110-118.
22. El-Jawahri A ea. Early integrated palliative care to improve family caregivers (FC) outcomes for patients with gastrointestinal and lung cancer. J Clin Oncol. 2016;34(suppl; abstr 10131).
23. Temel JS, Greer JA, El-Jawahri A, et al. Effects of early integrated palliative care in patients with lung and gi cancer: a randomized clinical trial. J Clin Oncol. 2017;35(8):834-841.
24. El-Jawahri A, LeBlanc T, VanDusen H, et al. Effect of inpatient palliative care on quality of life 2 weeks after hematopoietic stem cell transplantation: a randomized clinical trial. JAMA. 2016;316(20):2094-2103.
25. Cooper B, Kinsella GJ, Picton C. Development and initial validation of a family appraisal of caregiving questionnaire for palliative care. Psychooncology. 2006;15(7):613-622.
26. Kristjanson LJ. Validity and reliability testing of the FAMCARE Scale: measuring family satisfaction with advanced cancer care. Soc Sci Med. 1993;36(5):693-701.
27. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385-396.
28. Keir ST, Guill AB, Carter KE, Boole LC, Gonzales L, Friedman HS. Differential levels of stress in caregivers of brain tumor patients--observations from a pilot study. Support Care Cancer. 2006;14(12):1258-1261.
29. Brehaut JC, O’Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Decis Making. 2003;23(4):281-292.
30. Teno JM, Clarridge B, Casey V, Edgman-Levitan S, Fowler J. Validation of toolkit after-death bereaved family member interview. J Pain Symptom Manage. 2001;22(3):752-758.
31. Wright AA, Zhang B, Ray A, Mack JW, Trice E, Balboni T, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673.
32. Earle CC, Neville BA, Landrum MB, Ayanian JZ, Block SD, Weeks JC. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22(2):315-321.
33. Obermeyer Z, Makar M, Abujaber S, Dominici F, Block S, Cutler DM. Association between the Medicare hospice benefit and health care utilization and costs for patients with poor-prognosis cancer. JAMA. 2014;312(18):1888-1896.
1. Institute of Medicine. 2015. Dying in america: improving quality and honoring individual preferences near the end of life. Washington, DC: The National Academies Press. https://doi.org/10.17226/18748.
2. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96-112.
3. Wenrich MD, Curtis JR, Shannon SE, Carline JD, Ambrozy DM, Ramsey PG. Communicating with dying patients within the spectrum of medical care from terminal diagnosis to death. Arch Intern Med. 2001;161(6):868-874.
4. Parker SM, Clayton JM, Hancock K, et al. A systematic review of prognostic/end-of-life communication with adults in the advanced stages of a life-limiting illness: patient/caregiver preferences for the content, style, and timing of information. J Pain Symptom Manage. 2007;34(1):81-93.
5. Steinhauser KE, Clipp EC, McNeilly M, Christakis NA, McIntyre LM, Tulsky JA. In search of a good death: observations of patients, families, and providers. Ann Intern Med. 2000;132(10):825-832.
6. Teno JM, Clarridge BR, Casey V, et al. Family perspectives on end-of-life care at the last place of care. JAMA. 2004;291(1):88-93.
7. Kumar P, Wright AA, Hatfield LA, Temel JS, Keating NL. Family perspectives on hospice care experiences of patients with cancer. J Clin Oncol. 2017;35(4):432-439.
8. Wright AA, Keating NL, Ayanian JZ, et al. Family perspectives on aggressive cancer care near the end of life. JAMA. 2016;315(3):284-292.
9. Duggan KT, Hildebrand Duffus S, D’Agostino RB Jr, Petty WJ, Streer NP, Stephenson RC. The impact of hospice services in the care of patients with advanced stage nonsmall cell lung cancer. J Palliat Med. 2017;20(1):29-34.
10. Curtis JR, Wenrich MD, Carline JD, Shannon SE, Ambrozy DM, Ramsey PG. Understanding physicians’ skills at providing end-of-life care perspectives of patients, families, and health care workers. J Gen Intern Med. 2001;16(1):41-49.
11. Back AL, Young JP, McCown E, et al. Abandonment at the end of life from patient, caregiver, nurse, and physician perspectives: loss of continuity and lack of closure. Arch Intern Med. 2009;169(5):474-479.
12. Vig EK, Starks H, Taylor JS, Hopley EK, Fryer-Edwards K. Why don’t patients enroll in hospice? Can we do anything about it? J Gen Intern Med. 2010;25(10):1009-1019.
13. Waldrop DP, Meeker MA, Kerr C, Skretny J, Tangeman J, Milch R. The nature and timing of family-provider communication in late-stage cancer: a qualitative study of caregivers’ experiences. J Pain Symptom Manage. 2012;43(2):182-194.
14. Emanuel EJ, Fairclough DL, Slutsman J, Emanuel LL. Understanding economic and other burdens of terminal illness: the experience of patients and their caregivers. Ann Intern Med. 2000;132(6):451-459.
15. Curtis JR, Patrick DL, Engelberg RA, Norris K, Asp C, Byock I. A measure of the quality of dying and death. Initial validation using after-death interviews with family members. J Pain Symptom Manage. 2002;24(1):17-31.
16. Fleming DA, Sheppard VB, Mangan PA, et al. Caregiving at the end of life: Perceptions of health care quality and quality of life among patients and caregivers. J Pain Symptom Manage. 2006;31(5):407-420.
17. McMillan SC. Interventions to facilitate family caregiving at the end of life. J Palliat Med. 2005;8(Suppl 1):S132-S139.
18. Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741-749.
19. Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733-742.
20. Zimmermann C, Swami N, Krzyzanowska M, et al. Early palliative care for patients with advanced cancer: a cluster-randomised controlled trial. Lancet. 2014;383(9930):1721-1730.
21. Maltoni M, Scarpi E, Dall’Agata M, et al. Systematic versus on-demand early palliative care: a randomised clinical trial assessing quality of care and treatment aggressiveness near the end of life. Eur J Cancer. 2016;69:110-118.
22. El-Jawahri A ea. Early integrated palliative care to improve family caregivers (FC) outcomes for patients with gastrointestinal and lung cancer. J Clin Oncol. 2016;34(suppl; abstr 10131).
23. Temel JS, Greer JA, El-Jawahri A, et al. Effects of early integrated palliative care in patients with lung and gi cancer: a randomized clinical trial. J Clin Oncol. 2017;35(8):834-841.
24. El-Jawahri A, LeBlanc T, VanDusen H, et al. Effect of inpatient palliative care on quality of life 2 weeks after hematopoietic stem cell transplantation: a randomized clinical trial. JAMA. 2016;316(20):2094-2103.
25. Cooper B, Kinsella GJ, Picton C. Development and initial validation of a family appraisal of caregiving questionnaire for palliative care. Psychooncology. 2006;15(7):613-622.
26. Kristjanson LJ. Validity and reliability testing of the FAMCARE Scale: measuring family satisfaction with advanced cancer care. Soc Sci Med. 1993;36(5):693-701.
27. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385-396.
28. Keir ST, Guill AB, Carter KE, Boole LC, Gonzales L, Friedman HS. Differential levels of stress in caregivers of brain tumor patients--observations from a pilot study. Support Care Cancer. 2006;14(12):1258-1261.
29. Brehaut JC, O’Connor AM, Wood TJ, et al. Validation of a decision regret scale. Med Decis Making. 2003;23(4):281-292.
30. Teno JM, Clarridge B, Casey V, Edgman-Levitan S, Fowler J. Validation of toolkit after-death bereaved family member interview. J Pain Symptom Manage. 2001;22(3):752-758.
31. Wright AA, Zhang B, Ray A, Mack JW, Trice E, Balboni T, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665-1673.
32. Earle CC, Neville BA, Landrum MB, Ayanian JZ, Block SD, Weeks JC. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22(2):315-321.
33. Obermeyer Z, Makar M, Abujaber S, Dominici F, Block S, Cutler DM. Association between the Medicare hospice benefit and health care utilization and costs for patients with poor-prognosis cancer. JAMA. 2014;312(18):1888-1896.
Analgesic management in radiation oncology for painful bone metastases
Bone metastases are a common cause of pain in patients with advanced cancer, with about three-quarters of patients with bone metastases experiencing pain as the dominant symptom.1 Inadequately treated cancer pain impairs patient quality of life, and is associated with higher rates of depression, anxiety, and fatigue. Palliative radiotherapy (RT) is effective in alleviating pain from bone metastases.4 Local field external beam radiotherapy can provide some pain relief at the site of treated metastasis in 80%-90% of cases, with complete pain relief in 50%-60% of cases.5,6 However, maximal pain relief from RT is delayed, in some cases taking days to up to multiple weeks to attain.7,8 Therefore, optimal management of bone metastases pain may require the use of analgesics until RT takes adequate effect.
National Comprehensive Cancer Network (NCCN) Guidelines for Adult Cancer Pain (v. 2.2015) recommend that pain intensity rating (PIR; range, 0-10, where 0 denotes no pain and 10, worst pain imaginable) be used to quantify pain for all symptomatic patients. These guidelines also recommend the pain medication regimen be assessed for all symptomatic patients. For patients with moderate or severe pain (PIR of ≥4), NCCN guidelines recommend that analgesic regimen be intervened upon by alteration of the analgesic regimen (initiating, rotating, or titrating analgesic) or consideration of referral to pain/symptom management specialty.
Previous findings have demonstrated inadequate analgesic management for cancer pain,2,9 including within the radiation oncology (RO) clinic, suggesting that patients seen in consultation for palliative RT may experience uncontrolled pain for days to weeks before the onset of relief from RT. Possible reasons for inadequate acute pain intervention in the RO clinic may be provider discomfort with analgesic management and infrequent formal integration of palliative care within RO.10
Limited single-institution data from the few institutions with dedicated palliative RO services have suggested that these services improve the quality of palliative care delivery, as demonstrated by providers perceptions’ of the clinical impact of a dedicated service11 and the implementation of expedited palliative RT delivery for acute cancer pain.12,13 To our knowledge, the impact of a dedicated palliative RO service on analgesic management for cancer pain has not been assessed.
Here, we report how often patients with symptomatic bone metastases had assessments of existing analgesic regimens and interventions at RO consultation at 2 cancer centers. Center 1 had implemented a dedicated palliative RO service in 2011, consisting of rotating attending physicians and residents as well as dedicated palliative care trained nurse practitioners and a fellow, with the service structured around daily rounds,11 whereas Center 2 had not yet implemented a dedicated service. Using data from both centers, we assessed the impact of a palliative RO service on analgesic assessment and management in patients with bone metastases.
Methods
We searched our institutional databases for patients seen in RO consultation for bone metastases using ICD-9 code 198.5, and retrospectively reviewed consultation notes for those patients during June-July 2008, January-February 2010, January-February 2013, and June-July 2014. Those time periods were chosen as evenly spaced representative samples before and after implementation of a dedicated palliative RO service in 2011 at Center 1. Center 2 did not implement a dedicated palliative RO service in these time periods.
Within consultation notes, we recorded the following data from the History of the Present Illness section: symptoms from bone metastases (symptomatic was defined as any pain present); PIR (range, 0-10); and whether or not the preconsultation analgesic regimen was reported for symptomatic patients (including analgesic type, dosing, effectiveness, and adherence).
Documentation of the analgesic regimen in the history section of the notes was considered the proxy for analgesic regimen assessment at time of RO consultation. Analgesics within the Medications list, which were autopopulated in the consultation note by the electronic medical record, were recorded.
Whether or not pain was addressed with initiation or titration of analgesics for patients with a PIR of ≥4 was recorded from the Assessment and Plan portion of the notes, and that metric was considered the proxy for pain intervention. In addition, the case was coded as having had pain intervention if there was documentation of the patient declining recommended analgesic intervention, or the patient had been referred to a symptom management service for intervention (eg, referral to a specialty palliative care clinic), or there was recommendation for the patient to discuss uncontrolled pain with the original prescriber. A PIR of 4 was chosen as the threshold for analgesic intervention because at that level, NCCN guidelines for cancer pain state that the analgesic regimen should be titrated, whereas for a PIR of 3 or less, the guidelines recommend only consideration of titrating the analgesic. Only patients with a documented PIR were included in the pain intervention analysis.
Frequencies of analgesic assessment and analgesic intervention were compared using t tests (Wizard Pro, v1.8.5; Evan Miller, Chicago IL).
Results
A total of 271 patients with RO consultation notes were identified at the 2 centers within the 4 time periods (Table 1).
Among symptomatic patients, any component of the preconsultation analgesic regimen (including analgesic type, dosing, pain response, and adherence) was documented for 37.9% of the entire cohort at RO consultation (Table 3). At Centers 1 and 2, the frequencies of analgesic regimen assessment were documented for 41.3% and 28.1%, respectively (P = .06). Among symptomatic patients, 81.5% had an opioid or nonopioid analgesic listed in the Medications section in the electronic medical record at time of consultation.
Patients seen on the dedicated palliative RO service at Center 1 had an analgesic assessment documentation rate of 59.5%, whereas the patients not seen on a palliative RO service (ie, patients seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had an assessment documentation rate of 33.5% (P = .002; Figure 1). There was no significant difference between rates of analgesic regimen assessment between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (28.1% vs 35.9%, respectively; P = .27).
In patients seen at Center 1 only, those seen on the palliative RO service had a higher documentation rate of analgesic assessment compared with those seen by other services after implementation of the dedicated service (59.5% vs 38%, respectively; P = .018). Time period (after versus before 2011) was not significantly associated with the rate of documentation of analgesic assessment at either Center 1 (after vs before 2011: 44.4% vs 31%, P = .23) or Center 2 (31.4% vs 24.1%, P = .60).
Among patients with a PIR of ≥4, analgesic intervention was reported for 17.2% of patients within the entire cohort (20.8% at Center 1 and 0% at Center 2, P = .05). Among those with a PIR of ≥4, documentation of analgesic assessment noted in the History of the Present Illness section was associated with increased documentation of an analgesic intervention in the Assessment and Plan section (25% vs 7.3%; odds ratio [OR], 4.22; 95% confidence interval [CI], 1.1-16.0; P = .03).
Patients seen on the dedicated palliative RO service at Center 1 had a documented analgesic intervention rate of 31.6%, whereas the patients not seen on a palliative RO service (ie, those seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had a documented analgesic intervention rate of 9.2% (P = .01; Figure 2). There was no statistically significant difference between rates of documentation of an analgesic regimen intervention between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (0% vs 17.2%, respectively; P = .07).
Looking at only patients seen at Center 1, patients with a PIR of ≥4 seen on the dedicated palliative RO service had a nearly significant higher rate of documented analgesic interventions in the time period after implementation of the dedicate service (31.6% if seen on the dedicated service vs 12% if seen on a nondedicated service, P = .06).
Discussion
Multiple studies demonstrate the undertreatment of cancer pain in the outpatient setting.2,9,14,15 At 2 cancer centers, we found that about half of patients who present for consideration of palliative RT for bone metastases had a PIR of ≥4, yet only 17% of them had documentation of analgesic intervention as recommended by NCCN guidelines for cancer pain. Underlying this low rate of appropriate intervention may be the assumption of rapid pain relief by RT. However, RT often does not begin at time of consultation,16 and maximal pain relief may take days to weeks after commencement of RT.17 It is estimated that a quarter of all patients with cancer develop bone metastases during the course of their disease,12 and most of those patients suffer from pain. Thus, inherent delay in pain relief before, during, and after RT results in significant morbidity for the cancer patient population if adequate analgesic management is not provided.
The low rate of appropriate analgesic intervention at the time of RO consultation may also be related to the low incidence of proper analgesic assessment. In our cohort, 80% of symptomatic patients had an opioid or nonopioid analgesic listed in their medications within the electronic medical record at time of consultation, but only 38% had the analgesic regimen and/or its effectiveness described in the History of the Present Illness section of the record. Inattentiveness to analgesic type, dosing, and effectiveness during consultation may result in any inadequacies of the analgesic regimen going unnoticed. Consistent with this notion, we found that the rate of appropriate intervention for patients with a PIR of ≥4 was higher among patients who had analgesic regimen reported in the consultation note. Thus, interventions to implement routine review and documentation of the analgesic regimen, for example within the electronic medical record, may be one way to improve pain management.
Another possible reason for low rates of acute pain management within the RO clinic is low provider confidence in regard to analgesic management. In a recent national survey, 96% of radiation oncologists stated they were at least moderately confident with assessment of pain, yet only 77% were at least moderately confident with titrating opioids, and just 56% were at least moderately confident with rotating opioids.10 Educational interventions that improve providers’ facility with analgesic management may increase the frequency of pain management in the RO clinic.
Patients seen on the dedicated palliative RO service had significantly higher rates of documented analgesic regimen assessment and appropriate intervention during RO consultation, compared with patients seen at Center 2 and those not seen on the dedicated palliative RO service at Center 1. The improvements we observed in analgesic assessment and intervention at Center 1 for patients seen on the palliative RO service are likely owing to involvement of palliative RO and not to secular trends, because there were not similar improvements for patients at Center 1 who were not seen by the palliative RO service and those at Center 2, where there was no service.
At Center 1, the dedicated palliative RO service was created to provide specialized care to patients with metastatic disease undergoing palliative radiation. Within its structure, topics within palliative RO, such as technical aspects of palliative RT, symptom management, and communication are taught and reinforced in a case-based approach. Such palliative care awareness, integration, and education within RO achieved by the palliative RO service likely contribute to the improved rates of analgesic management we found in our study. We do note that rate of analgesic intervention in the palliative RO cohort, though higher than in the nonpalliative RO group, was still low, with only a third of patients receiving proper analgesic management. These findings highlight the importance of continued effort in increasing providers’ awareness of the need to assess pain and raise comfort with analgesic initiation and titration and of having dedicated palliative care clinicians embedded within the RO setting.
Since the data for this study was acquired, Center 2 has implemented a short palliative RO didactic course for residents, which improved their comfort levels in assessing analgesic effectiveness and intervening for uncontrolled pain.18 The impact of this intervention on clinical care will need to be evaluated, but the improved provider comfort levels may translate into better-quality care.
Limitations
An important limitation of this retrospective study is the reliance on the documentation provided in the consultation note for determining frequencies of analgesic regimen assessment and intervention. The actual rates of analgesic management that occurred in clinic may have been higher than reported in the documentation. However, such discrepancy in documentation of analgesic management would also be an area for quality improvement. Inadequate documentation limits the ability for proper follow-up of cancer pain as recommended by a joint guidance statement from the American Society of Clinical Oncology and the American Academy of Hospice and Palliative Medicine.19,20 The results of our study may also partly reflect a positive impact in documentation of analgesic management by a dedicated palliative RO service.
Given the multi-institutional nature of this study, it may be that general practice differences confound the impact of the dedicated palliative RO service at Center 1. However, with excluding Center 2, the dedicated service was still strongly associated with a higher rate of analgesic assessment within Center 1 and was almost significantly associated with appropriate analgesic intervention within Center 1.
We used a PIR of ≥4 as a threshold for appropriate analgesic regimen intervention because it is what is recommended by the NCCN guidelines. However, close attention should be paid to the impact that any amount of pain has on an individual patient. The functional, spiritual, and existential impact of pain is unique to each patient’s experience, and optimal symptom management should take those elements into account.
Conclusion
In conclusion, this study indicates that advanced cancer patient pain assessment and intervention according to NCCN cancer pain management guidelines is not common in the RO setting, and it is an area that should be targeted for quality improvement because of the positive implications for patient well-being. Pain assessment and intervention were greater in the setting of a dedicated structure for palliative care within RO, suggesting that the integration of palliative care within RO is a promising means of improving quality of pain management.
This work was presented at the 2016 ASCO Palliative Care in Oncology Symposium (September 9-10, 2016), where this work received a Conquer Cancer Foundation Merit Award.
1. Amichetti M, Orrù P, Madeddu A, et al. Comparative evaluation of two hypofractionated radiotherapy regimens for painful bone metastases. Tumori. 2004;90(1):91-95.
2. Vuong S, Pulenzas N, DeAngelis C, et al. Inadequate pain management in cancer patients attending an outpatient palliative radiotherapy clinic. Support Care Cancer. 2016;24(2):887-892.
3. Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: characteristics and impact in patients with cancer pain. Pain. 1999;81(1-2):129-134.
4. Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy - a systematic review of the randomised trials. Sze WM, ed. Cochrane Database Syst Rev. 2004;(2):CD004721-CD004721.
5. Ratanatharathorn V, Powers WE, Moss WT, Perez CA. Bone metastasis: review and critical analysis of random allocation trials of local field treatment. Int J Radiat Oncol Biol Phys. 1999;44(1):1-18.
6. Kirou-Mauro A, Hird A, Wong J, et al. Is response to radiotherapy in patients related to the severity of pretreatment pain? Int J Radiat Oncol Biol Phys. 2008;71(4):1208-1212.
7. Frassica DA. General principles of external beam radiation therapy for skeletal metastases. Clin Orthop Relat Res. 2003;(415 Suppl):S158-S164.
8. McDonald R, Ding K, Brundage M, et al. Effect of radiotherapy on painful bone metastases: a secondary analysis of the NCIC Clinical Trials Group Symptom Control Trial SC.23. JAMA Oncol. 2017 Jul 1;3(7):953-959.
9. Greco MT, Roberto A, Corli O, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32(36):4149-4154.
10. Wei RL, Mattes MD, Yu J, et al. Attitudes of radiation oncologists toward palliative and supportive care in the united states: report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract Radiat Oncol. 2017;7(2):113-119.
11. Tseng YD, Krishnan MS, Jones JA, et al. Supportive and palliative radiation oncology service: impact of a dedicated service on palliative cancer care. Pract Radiat Oncol. 2014;4(4):247-253.
12. Fairchild A, Pituskin E, Rose B, et al. The rapid access palliative radiotherapy program: blueprint for initiation of a one-stop multidisciplinary bone metastases clinic. Support Care Cancer. 2009;17(2):163-170.
13. de Sa E, Sinclair E, Mitera G, et al. Continued success of the rapid response radiotherapy program: a review of 2004-2008. Support Care Cancer. 2009;17(7):757-762.
14. Deandrea S, Montanari M, Moja L, Apolone G. Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol. 2008;19(12):1985-1991.
15. Mitera G, Zeiadin N, Kirou-Mauro A, et al. Retrospective assessment of cancer pain management in an outpatient palliative radiotherapy clinic using the Pain Management Index. J Pain Symptom Manage. 2010;39(2):259-267.
16. Danjoux C, Chow E, Drossos A, et al. An innovative rapid response radiotherapy program to reduce waiting time for palliative radiotherapy. Support Care Cancer. 2006;14(1):38-43.
17. Feyer PC, Steingraeber M. Radiotherapy of bone metastasis in breast cancer patients – current approaches. Breast Care (Basel). 2012;7(2):108-112.
18. Garcia MA, Braunstein SE, Anderson WG. Palliative Care Didactic Course for Radiation Oncology Residents. Int J Radiat Oncol Biol Phys. 2017;97(5):884-885.
19. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96-112.
20. Bickel KE, McNiff K, Buss MK, et al. Defining high-quality palliative care in oncology practice: an American Society of Clinical Oncology/American Academy of Hospice and Palliative Medicine guidance statement. J Oncol Pract. 2016;12(9):e828-e838.
Bone metastases are a common cause of pain in patients with advanced cancer, with about three-quarters of patients with bone metastases experiencing pain as the dominant symptom.1 Inadequately treated cancer pain impairs patient quality of life, and is associated with higher rates of depression, anxiety, and fatigue. Palliative radiotherapy (RT) is effective in alleviating pain from bone metastases.4 Local field external beam radiotherapy can provide some pain relief at the site of treated metastasis in 80%-90% of cases, with complete pain relief in 50%-60% of cases.5,6 However, maximal pain relief from RT is delayed, in some cases taking days to up to multiple weeks to attain.7,8 Therefore, optimal management of bone metastases pain may require the use of analgesics until RT takes adequate effect.
National Comprehensive Cancer Network (NCCN) Guidelines for Adult Cancer Pain (v. 2.2015) recommend that pain intensity rating (PIR; range, 0-10, where 0 denotes no pain and 10, worst pain imaginable) be used to quantify pain for all symptomatic patients. These guidelines also recommend the pain medication regimen be assessed for all symptomatic patients. For patients with moderate or severe pain (PIR of ≥4), NCCN guidelines recommend that analgesic regimen be intervened upon by alteration of the analgesic regimen (initiating, rotating, or titrating analgesic) or consideration of referral to pain/symptom management specialty.
Previous findings have demonstrated inadequate analgesic management for cancer pain,2,9 including within the radiation oncology (RO) clinic, suggesting that patients seen in consultation for palliative RT may experience uncontrolled pain for days to weeks before the onset of relief from RT. Possible reasons for inadequate acute pain intervention in the RO clinic may be provider discomfort with analgesic management and infrequent formal integration of palliative care within RO.10
Limited single-institution data from the few institutions with dedicated palliative RO services have suggested that these services improve the quality of palliative care delivery, as demonstrated by providers perceptions’ of the clinical impact of a dedicated service11 and the implementation of expedited palliative RT delivery for acute cancer pain.12,13 To our knowledge, the impact of a dedicated palliative RO service on analgesic management for cancer pain has not been assessed.
Here, we report how often patients with symptomatic bone metastases had assessments of existing analgesic regimens and interventions at RO consultation at 2 cancer centers. Center 1 had implemented a dedicated palliative RO service in 2011, consisting of rotating attending physicians and residents as well as dedicated palliative care trained nurse practitioners and a fellow, with the service structured around daily rounds,11 whereas Center 2 had not yet implemented a dedicated service. Using data from both centers, we assessed the impact of a palliative RO service on analgesic assessment and management in patients with bone metastases.
Methods
We searched our institutional databases for patients seen in RO consultation for bone metastases using ICD-9 code 198.5, and retrospectively reviewed consultation notes for those patients during June-July 2008, January-February 2010, January-February 2013, and June-July 2014. Those time periods were chosen as evenly spaced representative samples before and after implementation of a dedicated palliative RO service in 2011 at Center 1. Center 2 did not implement a dedicated palliative RO service in these time periods.
Within consultation notes, we recorded the following data from the History of the Present Illness section: symptoms from bone metastases (symptomatic was defined as any pain present); PIR (range, 0-10); and whether or not the preconsultation analgesic regimen was reported for symptomatic patients (including analgesic type, dosing, effectiveness, and adherence).
Documentation of the analgesic regimen in the history section of the notes was considered the proxy for analgesic regimen assessment at time of RO consultation. Analgesics within the Medications list, which were autopopulated in the consultation note by the electronic medical record, were recorded.
Whether or not pain was addressed with initiation or titration of analgesics for patients with a PIR of ≥4 was recorded from the Assessment and Plan portion of the notes, and that metric was considered the proxy for pain intervention. In addition, the case was coded as having had pain intervention if there was documentation of the patient declining recommended analgesic intervention, or the patient had been referred to a symptom management service for intervention (eg, referral to a specialty palliative care clinic), or there was recommendation for the patient to discuss uncontrolled pain with the original prescriber. A PIR of 4 was chosen as the threshold for analgesic intervention because at that level, NCCN guidelines for cancer pain state that the analgesic regimen should be titrated, whereas for a PIR of 3 or less, the guidelines recommend only consideration of titrating the analgesic. Only patients with a documented PIR were included in the pain intervention analysis.
Frequencies of analgesic assessment and analgesic intervention were compared using t tests (Wizard Pro, v1.8.5; Evan Miller, Chicago IL).
Results
A total of 271 patients with RO consultation notes were identified at the 2 centers within the 4 time periods (Table 1).
Among symptomatic patients, any component of the preconsultation analgesic regimen (including analgesic type, dosing, pain response, and adherence) was documented for 37.9% of the entire cohort at RO consultation (Table 3). At Centers 1 and 2, the frequencies of analgesic regimen assessment were documented for 41.3% and 28.1%, respectively (P = .06). Among symptomatic patients, 81.5% had an opioid or nonopioid analgesic listed in the Medications section in the electronic medical record at time of consultation.
Patients seen on the dedicated palliative RO service at Center 1 had an analgesic assessment documentation rate of 59.5%, whereas the patients not seen on a palliative RO service (ie, patients seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had an assessment documentation rate of 33.5% (P = .002; Figure 1). There was no significant difference between rates of analgesic regimen assessment between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (28.1% vs 35.9%, respectively; P = .27).
In patients seen at Center 1 only, those seen on the palliative RO service had a higher documentation rate of analgesic assessment compared with those seen by other services after implementation of the dedicated service (59.5% vs 38%, respectively; P = .018). Time period (after versus before 2011) was not significantly associated with the rate of documentation of analgesic assessment at either Center 1 (after vs before 2011: 44.4% vs 31%, P = .23) or Center 2 (31.4% vs 24.1%, P = .60).
Among patients with a PIR of ≥4, analgesic intervention was reported for 17.2% of patients within the entire cohort (20.8% at Center 1 and 0% at Center 2, P = .05). Among those with a PIR of ≥4, documentation of analgesic assessment noted in the History of the Present Illness section was associated with increased documentation of an analgesic intervention in the Assessment and Plan section (25% vs 7.3%; odds ratio [OR], 4.22; 95% confidence interval [CI], 1.1-16.0; P = .03).
Patients seen on the dedicated palliative RO service at Center 1 had a documented analgesic intervention rate of 31.6%, whereas the patients not seen on a palliative RO service (ie, those seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had a documented analgesic intervention rate of 9.2% (P = .01; Figure 2). There was no statistically significant difference between rates of documentation of an analgesic regimen intervention between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (0% vs 17.2%, respectively; P = .07).
Looking at only patients seen at Center 1, patients with a PIR of ≥4 seen on the dedicated palliative RO service had a nearly significant higher rate of documented analgesic interventions in the time period after implementation of the dedicate service (31.6% if seen on the dedicated service vs 12% if seen on a nondedicated service, P = .06).
Discussion
Multiple studies demonstrate the undertreatment of cancer pain in the outpatient setting.2,9,14,15 At 2 cancer centers, we found that about half of patients who present for consideration of palliative RT for bone metastases had a PIR of ≥4, yet only 17% of them had documentation of analgesic intervention as recommended by NCCN guidelines for cancer pain. Underlying this low rate of appropriate intervention may be the assumption of rapid pain relief by RT. However, RT often does not begin at time of consultation,16 and maximal pain relief may take days to weeks after commencement of RT.17 It is estimated that a quarter of all patients with cancer develop bone metastases during the course of their disease,12 and most of those patients suffer from pain. Thus, inherent delay in pain relief before, during, and after RT results in significant morbidity for the cancer patient population if adequate analgesic management is not provided.
The low rate of appropriate analgesic intervention at the time of RO consultation may also be related to the low incidence of proper analgesic assessment. In our cohort, 80% of symptomatic patients had an opioid or nonopioid analgesic listed in their medications within the electronic medical record at time of consultation, but only 38% had the analgesic regimen and/or its effectiveness described in the History of the Present Illness section of the record. Inattentiveness to analgesic type, dosing, and effectiveness during consultation may result in any inadequacies of the analgesic regimen going unnoticed. Consistent with this notion, we found that the rate of appropriate intervention for patients with a PIR of ≥4 was higher among patients who had analgesic regimen reported in the consultation note. Thus, interventions to implement routine review and documentation of the analgesic regimen, for example within the electronic medical record, may be one way to improve pain management.
Another possible reason for low rates of acute pain management within the RO clinic is low provider confidence in regard to analgesic management. In a recent national survey, 96% of radiation oncologists stated they were at least moderately confident with assessment of pain, yet only 77% were at least moderately confident with titrating opioids, and just 56% were at least moderately confident with rotating opioids.10 Educational interventions that improve providers’ facility with analgesic management may increase the frequency of pain management in the RO clinic.
Patients seen on the dedicated palliative RO service had significantly higher rates of documented analgesic regimen assessment and appropriate intervention during RO consultation, compared with patients seen at Center 2 and those not seen on the dedicated palliative RO service at Center 1. The improvements we observed in analgesic assessment and intervention at Center 1 for patients seen on the palliative RO service are likely owing to involvement of palliative RO and not to secular trends, because there were not similar improvements for patients at Center 1 who were not seen by the palliative RO service and those at Center 2, where there was no service.
At Center 1, the dedicated palliative RO service was created to provide specialized care to patients with metastatic disease undergoing palliative radiation. Within its structure, topics within palliative RO, such as technical aspects of palliative RT, symptom management, and communication are taught and reinforced in a case-based approach. Such palliative care awareness, integration, and education within RO achieved by the palliative RO service likely contribute to the improved rates of analgesic management we found in our study. We do note that rate of analgesic intervention in the palliative RO cohort, though higher than in the nonpalliative RO group, was still low, with only a third of patients receiving proper analgesic management. These findings highlight the importance of continued effort in increasing providers’ awareness of the need to assess pain and raise comfort with analgesic initiation and titration and of having dedicated palliative care clinicians embedded within the RO setting.
Since the data for this study was acquired, Center 2 has implemented a short palliative RO didactic course for residents, which improved their comfort levels in assessing analgesic effectiveness and intervening for uncontrolled pain.18 The impact of this intervention on clinical care will need to be evaluated, but the improved provider comfort levels may translate into better-quality care.
Limitations
An important limitation of this retrospective study is the reliance on the documentation provided in the consultation note for determining frequencies of analgesic regimen assessment and intervention. The actual rates of analgesic management that occurred in clinic may have been higher than reported in the documentation. However, such discrepancy in documentation of analgesic management would also be an area for quality improvement. Inadequate documentation limits the ability for proper follow-up of cancer pain as recommended by a joint guidance statement from the American Society of Clinical Oncology and the American Academy of Hospice and Palliative Medicine.19,20 The results of our study may also partly reflect a positive impact in documentation of analgesic management by a dedicated palliative RO service.
Given the multi-institutional nature of this study, it may be that general practice differences confound the impact of the dedicated palliative RO service at Center 1. However, with excluding Center 2, the dedicated service was still strongly associated with a higher rate of analgesic assessment within Center 1 and was almost significantly associated with appropriate analgesic intervention within Center 1.
We used a PIR of ≥4 as a threshold for appropriate analgesic regimen intervention because it is what is recommended by the NCCN guidelines. However, close attention should be paid to the impact that any amount of pain has on an individual patient. The functional, spiritual, and existential impact of pain is unique to each patient’s experience, and optimal symptom management should take those elements into account.
Conclusion
In conclusion, this study indicates that advanced cancer patient pain assessment and intervention according to NCCN cancer pain management guidelines is not common in the RO setting, and it is an area that should be targeted for quality improvement because of the positive implications for patient well-being. Pain assessment and intervention were greater in the setting of a dedicated structure for palliative care within RO, suggesting that the integration of palliative care within RO is a promising means of improving quality of pain management.
This work was presented at the 2016 ASCO Palliative Care in Oncology Symposium (September 9-10, 2016), where this work received a Conquer Cancer Foundation Merit Award.
Bone metastases are a common cause of pain in patients with advanced cancer, with about three-quarters of patients with bone metastases experiencing pain as the dominant symptom.1 Inadequately treated cancer pain impairs patient quality of life, and is associated with higher rates of depression, anxiety, and fatigue. Palliative radiotherapy (RT) is effective in alleviating pain from bone metastases.4 Local field external beam radiotherapy can provide some pain relief at the site of treated metastasis in 80%-90% of cases, with complete pain relief in 50%-60% of cases.5,6 However, maximal pain relief from RT is delayed, in some cases taking days to up to multiple weeks to attain.7,8 Therefore, optimal management of bone metastases pain may require the use of analgesics until RT takes adequate effect.
National Comprehensive Cancer Network (NCCN) Guidelines for Adult Cancer Pain (v. 2.2015) recommend that pain intensity rating (PIR; range, 0-10, where 0 denotes no pain and 10, worst pain imaginable) be used to quantify pain for all symptomatic patients. These guidelines also recommend the pain medication regimen be assessed for all symptomatic patients. For patients with moderate or severe pain (PIR of ≥4), NCCN guidelines recommend that analgesic regimen be intervened upon by alteration of the analgesic regimen (initiating, rotating, or titrating analgesic) or consideration of referral to pain/symptom management specialty.
Previous findings have demonstrated inadequate analgesic management for cancer pain,2,9 including within the radiation oncology (RO) clinic, suggesting that patients seen in consultation for palliative RT may experience uncontrolled pain for days to weeks before the onset of relief from RT. Possible reasons for inadequate acute pain intervention in the RO clinic may be provider discomfort with analgesic management and infrequent formal integration of palliative care within RO.10
Limited single-institution data from the few institutions with dedicated palliative RO services have suggested that these services improve the quality of palliative care delivery, as demonstrated by providers perceptions’ of the clinical impact of a dedicated service11 and the implementation of expedited palliative RT delivery for acute cancer pain.12,13 To our knowledge, the impact of a dedicated palliative RO service on analgesic management for cancer pain has not been assessed.
Here, we report how often patients with symptomatic bone metastases had assessments of existing analgesic regimens and interventions at RO consultation at 2 cancer centers. Center 1 had implemented a dedicated palliative RO service in 2011, consisting of rotating attending physicians and residents as well as dedicated palliative care trained nurse practitioners and a fellow, with the service structured around daily rounds,11 whereas Center 2 had not yet implemented a dedicated service. Using data from both centers, we assessed the impact of a palliative RO service on analgesic assessment and management in patients with bone metastases.
Methods
We searched our institutional databases for patients seen in RO consultation for bone metastases using ICD-9 code 198.5, and retrospectively reviewed consultation notes for those patients during June-July 2008, January-February 2010, January-February 2013, and June-July 2014. Those time periods were chosen as evenly spaced representative samples before and after implementation of a dedicated palliative RO service in 2011 at Center 1. Center 2 did not implement a dedicated palliative RO service in these time periods.
Within consultation notes, we recorded the following data from the History of the Present Illness section: symptoms from bone metastases (symptomatic was defined as any pain present); PIR (range, 0-10); and whether or not the preconsultation analgesic regimen was reported for symptomatic patients (including analgesic type, dosing, effectiveness, and adherence).
Documentation of the analgesic regimen in the history section of the notes was considered the proxy for analgesic regimen assessment at time of RO consultation. Analgesics within the Medications list, which were autopopulated in the consultation note by the electronic medical record, were recorded.
Whether or not pain was addressed with initiation or titration of analgesics for patients with a PIR of ≥4 was recorded from the Assessment and Plan portion of the notes, and that metric was considered the proxy for pain intervention. In addition, the case was coded as having had pain intervention if there was documentation of the patient declining recommended analgesic intervention, or the patient had been referred to a symptom management service for intervention (eg, referral to a specialty palliative care clinic), or there was recommendation for the patient to discuss uncontrolled pain with the original prescriber. A PIR of 4 was chosen as the threshold for analgesic intervention because at that level, NCCN guidelines for cancer pain state that the analgesic regimen should be titrated, whereas for a PIR of 3 or less, the guidelines recommend only consideration of titrating the analgesic. Only patients with a documented PIR were included in the pain intervention analysis.
Frequencies of analgesic assessment and analgesic intervention were compared using t tests (Wizard Pro, v1.8.5; Evan Miller, Chicago IL).
Results
A total of 271 patients with RO consultation notes were identified at the 2 centers within the 4 time periods (Table 1).
Among symptomatic patients, any component of the preconsultation analgesic regimen (including analgesic type, dosing, pain response, and adherence) was documented for 37.9% of the entire cohort at RO consultation (Table 3). At Centers 1 and 2, the frequencies of analgesic regimen assessment were documented for 41.3% and 28.1%, respectively (P = .06). Among symptomatic patients, 81.5% had an opioid or nonopioid analgesic listed in the Medications section in the electronic medical record at time of consultation.
Patients seen on the dedicated palliative RO service at Center 1 had an analgesic assessment documentation rate of 59.5%, whereas the patients not seen on a palliative RO service (ie, patients seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had an assessment documentation rate of 33.5% (P = .002; Figure 1). There was no significant difference between rates of analgesic regimen assessment between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (28.1% vs 35.9%, respectively; P = .27).
In patients seen at Center 1 only, those seen on the palliative RO service had a higher documentation rate of analgesic assessment compared with those seen by other services after implementation of the dedicated service (59.5% vs 38%, respectively; P = .018). Time period (after versus before 2011) was not significantly associated with the rate of documentation of analgesic assessment at either Center 1 (after vs before 2011: 44.4% vs 31%, P = .23) or Center 2 (31.4% vs 24.1%, P = .60).
Among patients with a PIR of ≥4, analgesic intervention was reported for 17.2% of patients within the entire cohort (20.8% at Center 1 and 0% at Center 2, P = .05). Among those with a PIR of ≥4, documentation of analgesic assessment noted in the History of the Present Illness section was associated with increased documentation of an analgesic intervention in the Assessment and Plan section (25% vs 7.3%; odds ratio [OR], 4.22; 95% confidence interval [CI], 1.1-16.0; P = .03).
Patients seen on the dedicated palliative RO service at Center 1 had a documented analgesic intervention rate of 31.6%, whereas the patients not seen on a palliative RO service (ie, those seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had a documented analgesic intervention rate of 9.2% (P = .01; Figure 2). There was no statistically significant difference between rates of documentation of an analgesic regimen intervention between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (0% vs 17.2%, respectively; P = .07).
Looking at only patients seen at Center 1, patients with a PIR of ≥4 seen on the dedicated palliative RO service had a nearly significant higher rate of documented analgesic interventions in the time period after implementation of the dedicate service (31.6% if seen on the dedicated service vs 12% if seen on a nondedicated service, P = .06).
Discussion
Multiple studies demonstrate the undertreatment of cancer pain in the outpatient setting.2,9,14,15 At 2 cancer centers, we found that about half of patients who present for consideration of palliative RT for bone metastases had a PIR of ≥4, yet only 17% of them had documentation of analgesic intervention as recommended by NCCN guidelines for cancer pain. Underlying this low rate of appropriate intervention may be the assumption of rapid pain relief by RT. However, RT often does not begin at time of consultation,16 and maximal pain relief may take days to weeks after commencement of RT.17 It is estimated that a quarter of all patients with cancer develop bone metastases during the course of their disease,12 and most of those patients suffer from pain. Thus, inherent delay in pain relief before, during, and after RT results in significant morbidity for the cancer patient population if adequate analgesic management is not provided.
The low rate of appropriate analgesic intervention at the time of RO consultation may also be related to the low incidence of proper analgesic assessment. In our cohort, 80% of symptomatic patients had an opioid or nonopioid analgesic listed in their medications within the electronic medical record at time of consultation, but only 38% had the analgesic regimen and/or its effectiveness described in the History of the Present Illness section of the record. Inattentiveness to analgesic type, dosing, and effectiveness during consultation may result in any inadequacies of the analgesic regimen going unnoticed. Consistent with this notion, we found that the rate of appropriate intervention for patients with a PIR of ≥4 was higher among patients who had analgesic regimen reported in the consultation note. Thus, interventions to implement routine review and documentation of the analgesic regimen, for example within the electronic medical record, may be one way to improve pain management.
Another possible reason for low rates of acute pain management within the RO clinic is low provider confidence in regard to analgesic management. In a recent national survey, 96% of radiation oncologists stated they were at least moderately confident with assessment of pain, yet only 77% were at least moderately confident with titrating opioids, and just 56% were at least moderately confident with rotating opioids.10 Educational interventions that improve providers’ facility with analgesic management may increase the frequency of pain management in the RO clinic.
Patients seen on the dedicated palliative RO service had significantly higher rates of documented analgesic regimen assessment and appropriate intervention during RO consultation, compared with patients seen at Center 2 and those not seen on the dedicated palliative RO service at Center 1. The improvements we observed in analgesic assessment and intervention at Center 1 for patients seen on the palliative RO service are likely owing to involvement of palliative RO and not to secular trends, because there were not similar improvements for patients at Center 1 who were not seen by the palliative RO service and those at Center 2, where there was no service.
At Center 1, the dedicated palliative RO service was created to provide specialized care to patients with metastatic disease undergoing palliative radiation. Within its structure, topics within palliative RO, such as technical aspects of palliative RT, symptom management, and communication are taught and reinforced in a case-based approach. Such palliative care awareness, integration, and education within RO achieved by the palliative RO service likely contribute to the improved rates of analgesic management we found in our study. We do note that rate of analgesic intervention in the palliative RO cohort, though higher than in the nonpalliative RO group, was still low, with only a third of patients receiving proper analgesic management. These findings highlight the importance of continued effort in increasing providers’ awareness of the need to assess pain and raise comfort with analgesic initiation and titration and of having dedicated palliative care clinicians embedded within the RO setting.
Since the data for this study was acquired, Center 2 has implemented a short palliative RO didactic course for residents, which improved their comfort levels in assessing analgesic effectiveness and intervening for uncontrolled pain.18 The impact of this intervention on clinical care will need to be evaluated, but the improved provider comfort levels may translate into better-quality care.
Limitations
An important limitation of this retrospective study is the reliance on the documentation provided in the consultation note for determining frequencies of analgesic regimen assessment and intervention. The actual rates of analgesic management that occurred in clinic may have been higher than reported in the documentation. However, such discrepancy in documentation of analgesic management would also be an area for quality improvement. Inadequate documentation limits the ability for proper follow-up of cancer pain as recommended by a joint guidance statement from the American Society of Clinical Oncology and the American Academy of Hospice and Palliative Medicine.19,20 The results of our study may also partly reflect a positive impact in documentation of analgesic management by a dedicated palliative RO service.
Given the multi-institutional nature of this study, it may be that general practice differences confound the impact of the dedicated palliative RO service at Center 1. However, with excluding Center 2, the dedicated service was still strongly associated with a higher rate of analgesic assessment within Center 1 and was almost significantly associated with appropriate analgesic intervention within Center 1.
We used a PIR of ≥4 as a threshold for appropriate analgesic regimen intervention because it is what is recommended by the NCCN guidelines. However, close attention should be paid to the impact that any amount of pain has on an individual patient. The functional, spiritual, and existential impact of pain is unique to each patient’s experience, and optimal symptom management should take those elements into account.
Conclusion
In conclusion, this study indicates that advanced cancer patient pain assessment and intervention according to NCCN cancer pain management guidelines is not common in the RO setting, and it is an area that should be targeted for quality improvement because of the positive implications for patient well-being. Pain assessment and intervention were greater in the setting of a dedicated structure for palliative care within RO, suggesting that the integration of palliative care within RO is a promising means of improving quality of pain management.
This work was presented at the 2016 ASCO Palliative Care in Oncology Symposium (September 9-10, 2016), where this work received a Conquer Cancer Foundation Merit Award.
1. Amichetti M, Orrù P, Madeddu A, et al. Comparative evaluation of two hypofractionated radiotherapy regimens for painful bone metastases. Tumori. 2004;90(1):91-95.
2. Vuong S, Pulenzas N, DeAngelis C, et al. Inadequate pain management in cancer patients attending an outpatient palliative radiotherapy clinic. Support Care Cancer. 2016;24(2):887-892.
3. Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: characteristics and impact in patients with cancer pain. Pain. 1999;81(1-2):129-134.
4. Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy - a systematic review of the randomised trials. Sze WM, ed. Cochrane Database Syst Rev. 2004;(2):CD004721-CD004721.
5. Ratanatharathorn V, Powers WE, Moss WT, Perez CA. Bone metastasis: review and critical analysis of random allocation trials of local field treatment. Int J Radiat Oncol Biol Phys. 1999;44(1):1-18.
6. Kirou-Mauro A, Hird A, Wong J, et al. Is response to radiotherapy in patients related to the severity of pretreatment pain? Int J Radiat Oncol Biol Phys. 2008;71(4):1208-1212.
7. Frassica DA. General principles of external beam radiation therapy for skeletal metastases. Clin Orthop Relat Res. 2003;(415 Suppl):S158-S164.
8. McDonald R, Ding K, Brundage M, et al. Effect of radiotherapy on painful bone metastases: a secondary analysis of the NCIC Clinical Trials Group Symptom Control Trial SC.23. JAMA Oncol. 2017 Jul 1;3(7):953-959.
9. Greco MT, Roberto A, Corli O, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32(36):4149-4154.
10. Wei RL, Mattes MD, Yu J, et al. Attitudes of radiation oncologists toward palliative and supportive care in the united states: report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract Radiat Oncol. 2017;7(2):113-119.
11. Tseng YD, Krishnan MS, Jones JA, et al. Supportive and palliative radiation oncology service: impact of a dedicated service on palliative cancer care. Pract Radiat Oncol. 2014;4(4):247-253.
12. Fairchild A, Pituskin E, Rose B, et al. The rapid access palliative radiotherapy program: blueprint for initiation of a one-stop multidisciplinary bone metastases clinic. Support Care Cancer. 2009;17(2):163-170.
13. de Sa E, Sinclair E, Mitera G, et al. Continued success of the rapid response radiotherapy program: a review of 2004-2008. Support Care Cancer. 2009;17(7):757-762.
14. Deandrea S, Montanari M, Moja L, Apolone G. Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol. 2008;19(12):1985-1991.
15. Mitera G, Zeiadin N, Kirou-Mauro A, et al. Retrospective assessment of cancer pain management in an outpatient palliative radiotherapy clinic using the Pain Management Index. J Pain Symptom Manage. 2010;39(2):259-267.
16. Danjoux C, Chow E, Drossos A, et al. An innovative rapid response radiotherapy program to reduce waiting time for palliative radiotherapy. Support Care Cancer. 2006;14(1):38-43.
17. Feyer PC, Steingraeber M. Radiotherapy of bone metastasis in breast cancer patients – current approaches. Breast Care (Basel). 2012;7(2):108-112.
18. Garcia MA, Braunstein SE, Anderson WG. Palliative Care Didactic Course for Radiation Oncology Residents. Int J Radiat Oncol Biol Phys. 2017;97(5):884-885.
19. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96-112.
20. Bickel KE, McNiff K, Buss MK, et al. Defining high-quality palliative care in oncology practice: an American Society of Clinical Oncology/American Academy of Hospice and Palliative Medicine guidance statement. J Oncol Pract. 2016;12(9):e828-e838.
1. Amichetti M, Orrù P, Madeddu A, et al. Comparative evaluation of two hypofractionated radiotherapy regimens for painful bone metastases. Tumori. 2004;90(1):91-95.
2. Vuong S, Pulenzas N, DeAngelis C, et al. Inadequate pain management in cancer patients attending an outpatient palliative radiotherapy clinic. Support Care Cancer. 2016;24(2):887-892.
3. Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: characteristics and impact in patients with cancer pain. Pain. 1999;81(1-2):129-134.
4. Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy - a systematic review of the randomised trials. Sze WM, ed. Cochrane Database Syst Rev. 2004;(2):CD004721-CD004721.
5. Ratanatharathorn V, Powers WE, Moss WT, Perez CA. Bone metastasis: review and critical analysis of random allocation trials of local field treatment. Int J Radiat Oncol Biol Phys. 1999;44(1):1-18.
6. Kirou-Mauro A, Hird A, Wong J, et al. Is response to radiotherapy in patients related to the severity of pretreatment pain? Int J Radiat Oncol Biol Phys. 2008;71(4):1208-1212.
7. Frassica DA. General principles of external beam radiation therapy for skeletal metastases. Clin Orthop Relat Res. 2003;(415 Suppl):S158-S164.
8. McDonald R, Ding K, Brundage M, et al. Effect of radiotherapy on painful bone metastases: a secondary analysis of the NCIC Clinical Trials Group Symptom Control Trial SC.23. JAMA Oncol. 2017 Jul 1;3(7):953-959.
9. Greco MT, Roberto A, Corli O, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32(36):4149-4154.
10. Wei RL, Mattes MD, Yu J, et al. Attitudes of radiation oncologists toward palliative and supportive care in the united states: report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract Radiat Oncol. 2017;7(2):113-119.
11. Tseng YD, Krishnan MS, Jones JA, et al. Supportive and palliative radiation oncology service: impact of a dedicated service on palliative cancer care. Pract Radiat Oncol. 2014;4(4):247-253.
12. Fairchild A, Pituskin E, Rose B, et al. The rapid access palliative radiotherapy program: blueprint for initiation of a one-stop multidisciplinary bone metastases clinic. Support Care Cancer. 2009;17(2):163-170.
13. de Sa E, Sinclair E, Mitera G, et al. Continued success of the rapid response radiotherapy program: a review of 2004-2008. Support Care Cancer. 2009;17(7):757-762.
14. Deandrea S, Montanari M, Moja L, Apolone G. Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol. 2008;19(12):1985-1991.
15. Mitera G, Zeiadin N, Kirou-Mauro A, et al. Retrospective assessment of cancer pain management in an outpatient palliative radiotherapy clinic using the Pain Management Index. J Pain Symptom Manage. 2010;39(2):259-267.
16. Danjoux C, Chow E, Drossos A, et al. An innovative rapid response radiotherapy program to reduce waiting time for palliative radiotherapy. Support Care Cancer. 2006;14(1):38-43.
17. Feyer PC, Steingraeber M. Radiotherapy of bone metastasis in breast cancer patients – current approaches. Breast Care (Basel). 2012;7(2):108-112.
18. Garcia MA, Braunstein SE, Anderson WG. Palliative Care Didactic Course for Radiation Oncology Residents. Int J Radiat Oncol Biol Phys. 2017;97(5):884-885.
19. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96-112.
20. Bickel KE, McNiff K, Buss MK, et al. Defining high-quality palliative care in oncology practice: an American Society of Clinical Oncology/American Academy of Hospice and Palliative Medicine guidance statement. J Oncol Pract. 2016;12(9):e828-e838.
Hospitalizations for fracture in patients with metastatic disease: primary source lesions in the United States
It has been well established that metastatic disease to bone has major significance in the morbidity associated with the diagnosis of cancer.1 More than 75% of patients with metastatic cancer will have bone involvement at the time of death.2-4 Moreover, there is a reported 8% incidence of a pathologic fracture in patients who carry the diagnosis of cancer.5 Common sites of involvement include the spine, ribs, pelvis, and long bones such as humerus and femur.6 Pathologic fracture is fracture caused by disease rather than injury or trauma (referred to here as nonpathologic). In any bone, pathologic fracture will be associated with increased morbidity for the patient, but it is the spine and long bones that frequently require surgical intervention and are associated with high mortality and morbidity. Advanced cancer can also increase fracture risk through increasing falls; in one prospective study of patients with advanced cancer, more than half the patients experienced a fall.7
Based on historical studies of patients who have died from common cancers,4,6 it is commonly believed that breast, lung, thyroid, kidney, and prostate cancers are the most common sources of metastasis to bone, and that other common cancers, such as colorectal carcinoma (CRC), have lower rates of metastasis to bone.6,8,9 It has been inferred from this data that cancers such as CRC thereby have lower rates of pathologic fracture.
Presence of bone metastasis at time of death may be less clinically relevant than occurrence of pathologic fracture and, especially, pathologic fracture requiring hospitalization. The authors are aware of no studies that have determined the number of patients hospitalized as a result of pathologic fracture from common tumors. Despite cadaveric findings, clinical experience dictates that colorectal carcinoma is not an uncommon primary tumor in patients presenting with metastatic disease and pathologic fracture, whereas thyroid carcinoma is more rare.
Despite lower rates of metastasis to bone from CRC, progression to advanced disease is common, with projected 50,000 deaths in the United States in 2014, and tumor progression is associated with metastasis to bone.10 Patterns of health care use and costs associated with skeletal-related events in more common metastatic prostate and breast cancer are well documented.11-13 The authors are aware of no population-based studies examining the burden from metastatic fractures or hospitalization incidence attributed to CRC.
Methods
This is a retrospective study of patients hospitalized in the United States with metastatic disease. Data for this study were obtained from the 2003-2010 National (Nationwide) Inpatient Sample (NIS), the Healthcare Cost and Utilization Project (HCUP), and the Agency for Healthcare Research and Quality.14 The NIS is a stratified sample of approximately 20% of inpatient hospitalization discharges in the United States with more than 7 million hospital stays each year. The dataset contains basic patient demographics, dates of admission, discharge, and procedures, as well as diagnosis and procedure codes for unique hospitalizations. The numbers of new cases of each type of cancer diagnosed in the United States during 2003-2010 were determined from fact sheets published by the American Cancer Society.15
In all, 1,008,641 patients with metastatic disease in the NIS database, were identified by the presence of International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM) diagnosis codes 196.0-199.1. Patients were then classified by primary cancer type based on the presence of additional ICD-9-CM codes for a specific cancer type (140.x-189.x) or for a history of a specific cancer type (V10.00 – V10.91). The analysis was limited to the 10 most common types of cancer. Multiple myeloma, leukemia, lymphoma, and primary cancers of bone also cause pathologic fractures, but they were purposefully excluded from the analysis because they do not represent truly metastatic disease. Patients were excluded if they were younger than 18 years (n = 9,425), had been admitted with major significant trauma (Major Diagnostic Category 24; n = 287), or if the cancer type was either not listed in discharge billing data or not one of the 10 most common types (n = 324,249). Therefore, the final study sample consisted of 674,680 hospitalizations.
The primary outcome assessed was pathologic fracture, identified with ICD-9-CM codes 733.10-733,19. Fractures not due to bone metastasis can occur in patients with metastatic disease owing to falls and general debility; therefore, the secondary outcome was nonpathologic fracture, identified with ICD-9-CM codes for fracture (805.0-829.0) in the absence of a code for pathologic fracture. Fractures classified as a “stress fracture” (ICD-9-CM code 733.9x) or where there was a concomitant diagnosis of osteoporosis (ICD-9-CM cod 733.0x) were also considered nonpathologic for the purpose of this study. Thus there were 3 groups of hospitalized patients identified: metastatic disease without fracture (No Fracture); Pathologic Fracture; and Nonpathologic Fracture. The study was limited to the 10 types of cancer with the highest numbers of pathologic fracture, leaving 647,680 hospitalizations for analysis.
Univariate analyses comparing the Pathologic, Nonpathologic, and No Fracture groups were performed with the Student t test for continuous characteristics and chi-square test for categorical characteristics. All analyses were performed with use of Stata 13.1 (StataCorp, College Station, TX).
This study protocol (RSRB00055625) was reviewed by the Office for Human Subject Protection Research Subjects Review Board at the University of Rochester and was determined to meet exemption criteria.
Results
From 2003-2010 there were 674,680 hospitalizations in patients with metastatic cancer that met the inclusion criteria. Hospitalization was most frequent for lung cancer (187,059 admissions), colorectal cancer (172,039), and breast cancer (124,303; Table 1).
There were 17,303 hospitalizations with pathologic fracture and 12,770 hospitalizations with nonpathologic fracture (Figure 1).
Among the most commonly occurring primary cancers in hospitalizations with pathologic fracture were lung, breast, prostate, kidney, and colorectal cancers (Table 1).
Relative to the annual incidence,15 kidney, lung, and breast cancer had the highest rates of hospital admission for pathologic fracture during the study period. Hospital admission with pathologic fracture was more common than nonpathologic fracture for every type of metastatic disease except colorectal and uterine cancer. Pathologic fracture in patients with metastatic disease was most likely to occur in the spine, hip, and femur (Table 2), and ratio of anatomic sites fractured was relatively consistent across each of the 10 primary malignancies (Table 3).
Demographic characteristics of patients in the 3 study groups are shown in Table 4. Patients with pathologic fracture were more likely than those in the no-fracture group to be white (63.0% vs 60.3%, respectively; P < .001) and female (55.5% vs 49.8%; P < .001), but were similar in age (66.4 years; P = 0.7). In-hospital mortality was lower in the pathologic fracture group compared with the no-fracture group (6.4% vs 8.8%; P < .001). People in the pathologic fracture group were more likely than others to be treated at a teaching hospital (P < .001) with ≥450 beds (P < .001), and reside in a zip code with higher income (P < .01).
Pathologic fracture hospitalizations, on average, had higher billed costs and longer length of stay ($62,974, 9.1 days; Table 4), compared with the no-fracture group ($39,576, 6.9 days; both P < .001) and the nonpathologic fracture group ($42,029, 7.2 days; both P < .001). Pathologic fracture in patients with thyroid, liver, and kidney cancer was associated with the highest costs of hospitalization.
In patients with metastatic disease, differences were found between those with pathologic and nonpathologic fractures: those with pathologic fracture were younger (66.4 vs 74.3 years; P < .001), less likely to be white (63.0% vs 69.0%; P < .001), and more commonly treated at a large hospital (68.4% vs 62.1%; P < .001) or a teaching hospital (53.5% vs 41.0%; P < .001).
Discussion
Other investigators have looked at risk factors for pathologic fracture, such as degree of bone involvement, location, and the presence of lytic versus blastic disease, as well as the optimal management of such patients.16-20 In those analyses, there is an emphasis on large, lytic lesions with cortical destruction in weight-bearing long bones, and on functional pain as a key determinant of fracture risk. Although the guidelines outlined by Mirel and others are helpful in predicting fractures, they are not widely applied by practicing oncologists.18 Oncologists and surgeons lack foolproof criteria to predict impending pathologic fracture despite evidence that the pathologic fracture event greatly increases mortality and morbidity.1,4,21,22 As far as we know, this is the first study to determine which types of primary carcinomas were most associated with pathologic fracture requiring hospitalization. This finding will hopefully raise awareness among doctors who care for these patients to be particularly conscientious with patients who present with symptoms of bone pain with activity (functional bone pain) or with lytic disease in the long bones. The results of the present study are similar to those from cadaveric studies, which emphasize the importance of lung, breast, prostate, and kidney cancers as primary tumors that metastasize to bone and lead to pathologic fracture. A novel finding is the nearly 4-fold greater number of pathologic fractures from colorectal carcinoma than thyroid carcinoma.
The importance of detecting patients at risk for pathologic fracture is now more relevant than ever because there are treatment modalities that are readily available to patients with metastatic bone involvement. Two classes of medications, the RANK-ligand inhibitors and bisphosphonates, reduce the number of skeletal events, such as pathologic fracture, in patients with metastatic disease to bone.23-26 However, most of those studies focused on the 3 most common carcinomas (breast, lung, and prostate) to metastasize to bone and cause pathologic fracture. There is greater variability in the prophylactic treatment of other forms of cancer that have metastasized to bone amongst oncologists.
Despite a lower proportion of hospitalizations for fracture in patients with CRC than for thyroid carcinoma (0.5% vs 1.6%, respectively), there were more pathologic fractures from CRC than from thyroid carcinoma because there are far more cases of CRC. SEER data estimate that in 2014 there were 62,000 cases of thyroid cancer and 1,890 deaths, compared with 136,000 cases of CRC and 50,000 deaths.10 Previous findings have shown that bone metastasis from CRC is more common than originally thought, based on autopsies of CRC patients.3 However, the lower rate of bone metastasis in CRC compared with other malignancies has led to a decreased focus on skeletal-related events in CRC. Our results suggest vigilance to bone health is warranted in patients with metastatic CRC. A novel finding is that patients with metastatic CRC also have a high number of hospital admissions for nonpathologic fracture. In establishing that patients with metastatic CRC with bone involvement have a real and significant risk of developing both pathologic and nonpathologic fractures, it may alter the treatment practice for these patients going forward, with greater consideration for an antiresorptive therapy, fall prevention education, or other preventive modalities, such as external-beam radiation therapy after it has been established that patients have metastatic bone disease.
There were some demographic differences between patients with metastatic disease who sustain pathologic fractures and those who do not fracture or sustain nonpathologic fractures. Patients with pathologic fracture were younger than those with nonpathologic fractures, and patients who sustained any fracture were more likely to be white than were patients in the no-fracture group. Known osteoporosis risk factors including older, female, and white with Northern European descent.27 Those findings emphasize the importance of osteoporosis screening and fracture prevention in patients with metastatic disease in general, regardless of the presence of bony metastasis. The present study found that patients who reside in zip codes areas with higher incomes were at slightly increased risk of hospitalization for pathologic fracture. Economic disparities in access to health care and cancer care are well documented,28 and the basis for this finding is a direction for future research.
Both mean billed costs and length of stay were greatest in the pathologic fracture group. The large number of admissions for no-fractured patients may be a final opportunity for intervention and preventative measures in this fragile population. Improved surveillance for bony lesions and attention to pain, especially at night, or unexplained hypercalcemia may help with early diagnosis and prevent some pathologic fractures. Patients with pathologic fracture often undergo additional treatments such as radiation therapy or chemotherapy. These additional treatments may partially explain the higher billed costs associated with inpatient hospitalization; future studies may be able to elucidate treatment differences or other reasons for the increased costs associated with pathologic fractures and identify targets to reduce expenditures.
Limitations
This study is subject to the limitations of a retrospective analysis based on hospital administrative discharge data. It evaluates only billed charges and does not account for costs associated with rehabilitation stays. However, it represents a stratified cross-sample of hospitalizations in the United States, in both teaching and nonteaching hospitals, and is the largest study to date that the authors are aware of looking at the burden of pathologic fractures in patients with metastatic disease.
This study specifically included only patients with metastatic disease, which therefore limits comparisons with the rate of hospitalization for nonpathologic fracture in patients without metastatic disease. Patients with metastatic disease who were not hospitalized during the study period are nevertheless at risk for fracture but would not have been captured in this study. It is also likely that some patients with metastatic disease had multiple hospitalizations, including some that were not for fracture; therefore, this study likely underestimates the percentage of patients with metastatic disease who sustain pathologic and nonpathologic fracture.
Some patients were excluded because we were not able to identify a primary cancer from hospital discharge records. The lack of an included diagnosis may be a result of indeterminate primary during the fracture admission or may represent a failure to accurately code a primary, known cancer. Although the NIS does not permit identification of these patients to determine if a primary cancer was subsequently identified, future studies using other databases may target patients presenting with pathologic fracture and an unknown primary tumor to evaluate subsequent cancer diagnosis.
Summary
The significance of bone metastasis in causing pathologic fractures in lung, breast, prostate, and kidney cancers was confirmed. Colorectal carcinoma has been established as the fifth most common primary cancer in patients with metastatic disease who are hospitalized with pathologic fracture, and a large number of patients with metastatic CRC sustain nonpathologic fractures requiring hospitalization. In patients with metastatic CRC or new skeletal pain, education on fall prevention and increased vigilance should be considered. Further studies are needed to determine the best method for prevention of pathologic fractures in all highly prevalent cancers, with previous hospitalizations without fracture as an appropriate target. Previous paradigms about which cancers metastasize to bone should be reconsidered in the context of which lead to clinically important fractures and hospitalization.
1. Carter JA, Ji X, Botteman MF. Clinical, economic and humanistic burdens of skeletal-related events associated with bone metastases. Expert Rev Pharmacoecon Outcomes Res. 2013;13(4):483-496.
2. Clain A. Secondary malignant disease of bone. Br J Cancer. 1965;19:15-29.
3. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 Pt 2):6243s-6249s.
4. Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1588-1594.
5. Higinbotham NL, Marcove RC. The management of pathological fractures. J Trauma. 1965;5(6):792-798.
6. Hess KR, Varadhachary GR, Taylor SH, et al. Metastatic patterns in adenocarcinoma. Cancer. 2006;106(7):1624-1633.
7. Stone CA, Lawlor PG, Savva GM, Bennett K, Kenny RA. Prospective study of falls and risk factors for falls in adults with advanced cancer. J Clin Oncol. 2012;30(17):2128-2133.
8. Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27(3):165-176.
9. Katoh M, Unakami M, Hara M, Fukuchi S. Bone metastasis from colorectal cancer in autopsy cases. J Gastroenterol. 1995;30(5):615-618.
10. Howlader N NA, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z,Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2011, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission. Posted April 2014. Accessed January 19, 2018.
11. Hagiwara M, Delea TE, Saville MW, Chung K. Healthcare utilization and costs associated with skeletal-related events in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 2013;16(1):23-27.
12. Hagiwara M, Delea TE, Chung K. Healthcare costs associated with skeletal-related events in breast cancer patients with bone metastases. J Med Econ. 2014;17(3):223-230.
13. Yong C, Onukwugha E, Mullins CD. Clinical and economic burden of bone metastasis and skeletal-related events in prostate cancer. Curr Opin Oncol. 2014;26(3):274-283.
14. HCUP Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). 2011. Agency for Healthcare Research and Quality R, MD. http://www.hcup-us.ahrq.gov/nisoverview.jsp. Last modified January 17, 2018. Accessed January 18, 2018.
15. American Cancer Society. Cancer Facts & Figures. Atlanta: American Cancer Society; 2003-2010. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2010.html Published January 2010. Accessed January 17, 2018.
16. Harrington KD. Orthopedic surgical management of skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1614-1627.
17. Harrington KD. Impending pathologic fractures from metastatic malignancy: evaluation and management. Instr Course Lect. 1986;35:357-381.
18. Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989;249:256-264.
19. Weber KL. Evaluation of the adult patient (aged >40 years) with a destructive bone lesion. J Am Acad Orthop Surg. 2010;18(3):169-179.
20. Rougraff BT. Evaluation of the patient with carcinoma of unknown origin metastatic to bone. Clin Orthop Relat Res. 2003(415 Suppl):S105-109.
21. Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987;55(1):61-66.
22. Dijstra S, Wiggers T, van Geel BN, Boxma H. Impending and actual pathological fractures in patients with bone metastases of the long bones. A retrospective study of 233 surgically treated fractures. Eur J Surg. 1994;160(10):535-542.
23. Henry D, Vadhan-Raj S, Hirsh V, et al. Delaying skeletal-related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: an analysis of data from patients with solid tumors. Support Care Cancer. 2014;22(3):679-687.
24. Lorusso V, Duran I, Garzon-Rodriguez C, et al. Health resource utilisation associated with skeletal-related events in European patients with lung cancer: Alpha subgroup analysis from a prospective multinational study. Mol Clin Oncol. 2014;2(5):701-708.
25. Lothgren M, Ribnicsek E, Schmidt L, et al. Cost per patient and potential budget implications of denosumab compared with zoledronic acid in adults with bone metastases from solid tumours who are at risk of skeletal-related events: an analysis for Austria, Sweden and Switzerland. Eu J Hosp Pharm Sci Pract. 2013;20(4):227-231.
26. Luftner D, Lorusso V, Duran I, et al. Health resource utilization associated with skeletal-related events in patients with advanced breast cancer: results from a prospective, multinational observational study. SpringerPlus. 2014;3:328.
27. Cauley JA. Defining ethnic and racial differences in osteoporosis and fragility fractures. Clin Orthop Relat Res. 2011;469(7):1891-1899.
28. VanEenwyk J, Campo JS, Ossiander EM. Socioeconomic and demographic disparities in treatment for carcinomas of the colon and rectum. Cancer. 2002;95(1):39-46.
It has been well established that metastatic disease to bone has major significance in the morbidity associated with the diagnosis of cancer.1 More than 75% of patients with metastatic cancer will have bone involvement at the time of death.2-4 Moreover, there is a reported 8% incidence of a pathologic fracture in patients who carry the diagnosis of cancer.5 Common sites of involvement include the spine, ribs, pelvis, and long bones such as humerus and femur.6 Pathologic fracture is fracture caused by disease rather than injury or trauma (referred to here as nonpathologic). In any bone, pathologic fracture will be associated with increased morbidity for the patient, but it is the spine and long bones that frequently require surgical intervention and are associated with high mortality and morbidity. Advanced cancer can also increase fracture risk through increasing falls; in one prospective study of patients with advanced cancer, more than half the patients experienced a fall.7
Based on historical studies of patients who have died from common cancers,4,6 it is commonly believed that breast, lung, thyroid, kidney, and prostate cancers are the most common sources of metastasis to bone, and that other common cancers, such as colorectal carcinoma (CRC), have lower rates of metastasis to bone.6,8,9 It has been inferred from this data that cancers such as CRC thereby have lower rates of pathologic fracture.
Presence of bone metastasis at time of death may be less clinically relevant than occurrence of pathologic fracture and, especially, pathologic fracture requiring hospitalization. The authors are aware of no studies that have determined the number of patients hospitalized as a result of pathologic fracture from common tumors. Despite cadaveric findings, clinical experience dictates that colorectal carcinoma is not an uncommon primary tumor in patients presenting with metastatic disease and pathologic fracture, whereas thyroid carcinoma is more rare.
Despite lower rates of metastasis to bone from CRC, progression to advanced disease is common, with projected 50,000 deaths in the United States in 2014, and tumor progression is associated with metastasis to bone.10 Patterns of health care use and costs associated with skeletal-related events in more common metastatic prostate and breast cancer are well documented.11-13 The authors are aware of no population-based studies examining the burden from metastatic fractures or hospitalization incidence attributed to CRC.
Methods
This is a retrospective study of patients hospitalized in the United States with metastatic disease. Data for this study were obtained from the 2003-2010 National (Nationwide) Inpatient Sample (NIS), the Healthcare Cost and Utilization Project (HCUP), and the Agency for Healthcare Research and Quality.14 The NIS is a stratified sample of approximately 20% of inpatient hospitalization discharges in the United States with more than 7 million hospital stays each year. The dataset contains basic patient demographics, dates of admission, discharge, and procedures, as well as diagnosis and procedure codes for unique hospitalizations. The numbers of new cases of each type of cancer diagnosed in the United States during 2003-2010 were determined from fact sheets published by the American Cancer Society.15
In all, 1,008,641 patients with metastatic disease in the NIS database, were identified by the presence of International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM) diagnosis codes 196.0-199.1. Patients were then classified by primary cancer type based on the presence of additional ICD-9-CM codes for a specific cancer type (140.x-189.x) or for a history of a specific cancer type (V10.00 – V10.91). The analysis was limited to the 10 most common types of cancer. Multiple myeloma, leukemia, lymphoma, and primary cancers of bone also cause pathologic fractures, but they were purposefully excluded from the analysis because they do not represent truly metastatic disease. Patients were excluded if they were younger than 18 years (n = 9,425), had been admitted with major significant trauma (Major Diagnostic Category 24; n = 287), or if the cancer type was either not listed in discharge billing data or not one of the 10 most common types (n = 324,249). Therefore, the final study sample consisted of 674,680 hospitalizations.
The primary outcome assessed was pathologic fracture, identified with ICD-9-CM codes 733.10-733,19. Fractures not due to bone metastasis can occur in patients with metastatic disease owing to falls and general debility; therefore, the secondary outcome was nonpathologic fracture, identified with ICD-9-CM codes for fracture (805.0-829.0) in the absence of a code for pathologic fracture. Fractures classified as a “stress fracture” (ICD-9-CM code 733.9x) or where there was a concomitant diagnosis of osteoporosis (ICD-9-CM cod 733.0x) were also considered nonpathologic for the purpose of this study. Thus there were 3 groups of hospitalized patients identified: metastatic disease without fracture (No Fracture); Pathologic Fracture; and Nonpathologic Fracture. The study was limited to the 10 types of cancer with the highest numbers of pathologic fracture, leaving 647,680 hospitalizations for analysis.
Univariate analyses comparing the Pathologic, Nonpathologic, and No Fracture groups were performed with the Student t test for continuous characteristics and chi-square test for categorical characteristics. All analyses were performed with use of Stata 13.1 (StataCorp, College Station, TX).
This study protocol (RSRB00055625) was reviewed by the Office for Human Subject Protection Research Subjects Review Board at the University of Rochester and was determined to meet exemption criteria.
Results
From 2003-2010 there were 674,680 hospitalizations in patients with metastatic cancer that met the inclusion criteria. Hospitalization was most frequent for lung cancer (187,059 admissions), colorectal cancer (172,039), and breast cancer (124,303; Table 1).
There were 17,303 hospitalizations with pathologic fracture and 12,770 hospitalizations with nonpathologic fracture (Figure 1).
Among the most commonly occurring primary cancers in hospitalizations with pathologic fracture were lung, breast, prostate, kidney, and colorectal cancers (Table 1).
Relative to the annual incidence,15 kidney, lung, and breast cancer had the highest rates of hospital admission for pathologic fracture during the study period. Hospital admission with pathologic fracture was more common than nonpathologic fracture for every type of metastatic disease except colorectal and uterine cancer. Pathologic fracture in patients with metastatic disease was most likely to occur in the spine, hip, and femur (Table 2), and ratio of anatomic sites fractured was relatively consistent across each of the 10 primary malignancies (Table 3).
Demographic characteristics of patients in the 3 study groups are shown in Table 4. Patients with pathologic fracture were more likely than those in the no-fracture group to be white (63.0% vs 60.3%, respectively; P < .001) and female (55.5% vs 49.8%; P < .001), but were similar in age (66.4 years; P = 0.7). In-hospital mortality was lower in the pathologic fracture group compared with the no-fracture group (6.4% vs 8.8%; P < .001). People in the pathologic fracture group were more likely than others to be treated at a teaching hospital (P < .001) with ≥450 beds (P < .001), and reside in a zip code with higher income (P < .01).
Pathologic fracture hospitalizations, on average, had higher billed costs and longer length of stay ($62,974, 9.1 days; Table 4), compared with the no-fracture group ($39,576, 6.9 days; both P < .001) and the nonpathologic fracture group ($42,029, 7.2 days; both P < .001). Pathologic fracture in patients with thyroid, liver, and kidney cancer was associated with the highest costs of hospitalization.
In patients with metastatic disease, differences were found between those with pathologic and nonpathologic fractures: those with pathologic fracture were younger (66.4 vs 74.3 years; P < .001), less likely to be white (63.0% vs 69.0%; P < .001), and more commonly treated at a large hospital (68.4% vs 62.1%; P < .001) or a teaching hospital (53.5% vs 41.0%; P < .001).
Discussion
Other investigators have looked at risk factors for pathologic fracture, such as degree of bone involvement, location, and the presence of lytic versus blastic disease, as well as the optimal management of such patients.16-20 In those analyses, there is an emphasis on large, lytic lesions with cortical destruction in weight-bearing long bones, and on functional pain as a key determinant of fracture risk. Although the guidelines outlined by Mirel and others are helpful in predicting fractures, they are not widely applied by practicing oncologists.18 Oncologists and surgeons lack foolproof criteria to predict impending pathologic fracture despite evidence that the pathologic fracture event greatly increases mortality and morbidity.1,4,21,22 As far as we know, this is the first study to determine which types of primary carcinomas were most associated with pathologic fracture requiring hospitalization. This finding will hopefully raise awareness among doctors who care for these patients to be particularly conscientious with patients who present with symptoms of bone pain with activity (functional bone pain) or with lytic disease in the long bones. The results of the present study are similar to those from cadaveric studies, which emphasize the importance of lung, breast, prostate, and kidney cancers as primary tumors that metastasize to bone and lead to pathologic fracture. A novel finding is the nearly 4-fold greater number of pathologic fractures from colorectal carcinoma than thyroid carcinoma.
The importance of detecting patients at risk for pathologic fracture is now more relevant than ever because there are treatment modalities that are readily available to patients with metastatic bone involvement. Two classes of medications, the RANK-ligand inhibitors and bisphosphonates, reduce the number of skeletal events, such as pathologic fracture, in patients with metastatic disease to bone.23-26 However, most of those studies focused on the 3 most common carcinomas (breast, lung, and prostate) to metastasize to bone and cause pathologic fracture. There is greater variability in the prophylactic treatment of other forms of cancer that have metastasized to bone amongst oncologists.
Despite a lower proportion of hospitalizations for fracture in patients with CRC than for thyroid carcinoma (0.5% vs 1.6%, respectively), there were more pathologic fractures from CRC than from thyroid carcinoma because there are far more cases of CRC. SEER data estimate that in 2014 there were 62,000 cases of thyroid cancer and 1,890 deaths, compared with 136,000 cases of CRC and 50,000 deaths.10 Previous findings have shown that bone metastasis from CRC is more common than originally thought, based on autopsies of CRC patients.3 However, the lower rate of bone metastasis in CRC compared with other malignancies has led to a decreased focus on skeletal-related events in CRC. Our results suggest vigilance to bone health is warranted in patients with metastatic CRC. A novel finding is that patients with metastatic CRC also have a high number of hospital admissions for nonpathologic fracture. In establishing that patients with metastatic CRC with bone involvement have a real and significant risk of developing both pathologic and nonpathologic fractures, it may alter the treatment practice for these patients going forward, with greater consideration for an antiresorptive therapy, fall prevention education, or other preventive modalities, such as external-beam radiation therapy after it has been established that patients have metastatic bone disease.
There were some demographic differences between patients with metastatic disease who sustain pathologic fractures and those who do not fracture or sustain nonpathologic fractures. Patients with pathologic fracture were younger than those with nonpathologic fractures, and patients who sustained any fracture were more likely to be white than were patients in the no-fracture group. Known osteoporosis risk factors including older, female, and white with Northern European descent.27 Those findings emphasize the importance of osteoporosis screening and fracture prevention in patients with metastatic disease in general, regardless of the presence of bony metastasis. The present study found that patients who reside in zip codes areas with higher incomes were at slightly increased risk of hospitalization for pathologic fracture. Economic disparities in access to health care and cancer care are well documented,28 and the basis for this finding is a direction for future research.
Both mean billed costs and length of stay were greatest in the pathologic fracture group. The large number of admissions for no-fractured patients may be a final opportunity for intervention and preventative measures in this fragile population. Improved surveillance for bony lesions and attention to pain, especially at night, or unexplained hypercalcemia may help with early diagnosis and prevent some pathologic fractures. Patients with pathologic fracture often undergo additional treatments such as radiation therapy or chemotherapy. These additional treatments may partially explain the higher billed costs associated with inpatient hospitalization; future studies may be able to elucidate treatment differences or other reasons for the increased costs associated with pathologic fractures and identify targets to reduce expenditures.
Limitations
This study is subject to the limitations of a retrospective analysis based on hospital administrative discharge data. It evaluates only billed charges and does not account for costs associated with rehabilitation stays. However, it represents a stratified cross-sample of hospitalizations in the United States, in both teaching and nonteaching hospitals, and is the largest study to date that the authors are aware of looking at the burden of pathologic fractures in patients with metastatic disease.
This study specifically included only patients with metastatic disease, which therefore limits comparisons with the rate of hospitalization for nonpathologic fracture in patients without metastatic disease. Patients with metastatic disease who were not hospitalized during the study period are nevertheless at risk for fracture but would not have been captured in this study. It is also likely that some patients with metastatic disease had multiple hospitalizations, including some that were not for fracture; therefore, this study likely underestimates the percentage of patients with metastatic disease who sustain pathologic and nonpathologic fracture.
Some patients were excluded because we were not able to identify a primary cancer from hospital discharge records. The lack of an included diagnosis may be a result of indeterminate primary during the fracture admission or may represent a failure to accurately code a primary, known cancer. Although the NIS does not permit identification of these patients to determine if a primary cancer was subsequently identified, future studies using other databases may target patients presenting with pathologic fracture and an unknown primary tumor to evaluate subsequent cancer diagnosis.
Summary
The significance of bone metastasis in causing pathologic fractures in lung, breast, prostate, and kidney cancers was confirmed. Colorectal carcinoma has been established as the fifth most common primary cancer in patients with metastatic disease who are hospitalized with pathologic fracture, and a large number of patients with metastatic CRC sustain nonpathologic fractures requiring hospitalization. In patients with metastatic CRC or new skeletal pain, education on fall prevention and increased vigilance should be considered. Further studies are needed to determine the best method for prevention of pathologic fractures in all highly prevalent cancers, with previous hospitalizations without fracture as an appropriate target. Previous paradigms about which cancers metastasize to bone should be reconsidered in the context of which lead to clinically important fractures and hospitalization.
It has been well established that metastatic disease to bone has major significance in the morbidity associated with the diagnosis of cancer.1 More than 75% of patients with metastatic cancer will have bone involvement at the time of death.2-4 Moreover, there is a reported 8% incidence of a pathologic fracture in patients who carry the diagnosis of cancer.5 Common sites of involvement include the spine, ribs, pelvis, and long bones such as humerus and femur.6 Pathologic fracture is fracture caused by disease rather than injury or trauma (referred to here as nonpathologic). In any bone, pathologic fracture will be associated with increased morbidity for the patient, but it is the spine and long bones that frequently require surgical intervention and are associated with high mortality and morbidity. Advanced cancer can also increase fracture risk through increasing falls; in one prospective study of patients with advanced cancer, more than half the patients experienced a fall.7
Based on historical studies of patients who have died from common cancers,4,6 it is commonly believed that breast, lung, thyroid, kidney, and prostate cancers are the most common sources of metastasis to bone, and that other common cancers, such as colorectal carcinoma (CRC), have lower rates of metastasis to bone.6,8,9 It has been inferred from this data that cancers such as CRC thereby have lower rates of pathologic fracture.
Presence of bone metastasis at time of death may be less clinically relevant than occurrence of pathologic fracture and, especially, pathologic fracture requiring hospitalization. The authors are aware of no studies that have determined the number of patients hospitalized as a result of pathologic fracture from common tumors. Despite cadaveric findings, clinical experience dictates that colorectal carcinoma is not an uncommon primary tumor in patients presenting with metastatic disease and pathologic fracture, whereas thyroid carcinoma is more rare.
Despite lower rates of metastasis to bone from CRC, progression to advanced disease is common, with projected 50,000 deaths in the United States in 2014, and tumor progression is associated with metastasis to bone.10 Patterns of health care use and costs associated with skeletal-related events in more common metastatic prostate and breast cancer are well documented.11-13 The authors are aware of no population-based studies examining the burden from metastatic fractures or hospitalization incidence attributed to CRC.
Methods
This is a retrospective study of patients hospitalized in the United States with metastatic disease. Data for this study were obtained from the 2003-2010 National (Nationwide) Inpatient Sample (NIS), the Healthcare Cost and Utilization Project (HCUP), and the Agency for Healthcare Research and Quality.14 The NIS is a stratified sample of approximately 20% of inpatient hospitalization discharges in the United States with more than 7 million hospital stays each year. The dataset contains basic patient demographics, dates of admission, discharge, and procedures, as well as diagnosis and procedure codes for unique hospitalizations. The numbers of new cases of each type of cancer diagnosed in the United States during 2003-2010 were determined from fact sheets published by the American Cancer Society.15
In all, 1,008,641 patients with metastatic disease in the NIS database, were identified by the presence of International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM) diagnosis codes 196.0-199.1. Patients were then classified by primary cancer type based on the presence of additional ICD-9-CM codes for a specific cancer type (140.x-189.x) or for a history of a specific cancer type (V10.00 – V10.91). The analysis was limited to the 10 most common types of cancer. Multiple myeloma, leukemia, lymphoma, and primary cancers of bone also cause pathologic fractures, but they were purposefully excluded from the analysis because they do not represent truly metastatic disease. Patients were excluded if they were younger than 18 years (n = 9,425), had been admitted with major significant trauma (Major Diagnostic Category 24; n = 287), or if the cancer type was either not listed in discharge billing data or not one of the 10 most common types (n = 324,249). Therefore, the final study sample consisted of 674,680 hospitalizations.
The primary outcome assessed was pathologic fracture, identified with ICD-9-CM codes 733.10-733,19. Fractures not due to bone metastasis can occur in patients with metastatic disease owing to falls and general debility; therefore, the secondary outcome was nonpathologic fracture, identified with ICD-9-CM codes for fracture (805.0-829.0) in the absence of a code for pathologic fracture. Fractures classified as a “stress fracture” (ICD-9-CM code 733.9x) or where there was a concomitant diagnosis of osteoporosis (ICD-9-CM cod 733.0x) were also considered nonpathologic for the purpose of this study. Thus there were 3 groups of hospitalized patients identified: metastatic disease without fracture (No Fracture); Pathologic Fracture; and Nonpathologic Fracture. The study was limited to the 10 types of cancer with the highest numbers of pathologic fracture, leaving 647,680 hospitalizations for analysis.
Univariate analyses comparing the Pathologic, Nonpathologic, and No Fracture groups were performed with the Student t test for continuous characteristics and chi-square test for categorical characteristics. All analyses were performed with use of Stata 13.1 (StataCorp, College Station, TX).
This study protocol (RSRB00055625) was reviewed by the Office for Human Subject Protection Research Subjects Review Board at the University of Rochester and was determined to meet exemption criteria.
Results
From 2003-2010 there were 674,680 hospitalizations in patients with metastatic cancer that met the inclusion criteria. Hospitalization was most frequent for lung cancer (187,059 admissions), colorectal cancer (172,039), and breast cancer (124,303; Table 1).
There were 17,303 hospitalizations with pathologic fracture and 12,770 hospitalizations with nonpathologic fracture (Figure 1).
Among the most commonly occurring primary cancers in hospitalizations with pathologic fracture were lung, breast, prostate, kidney, and colorectal cancers (Table 1).
Relative to the annual incidence,15 kidney, lung, and breast cancer had the highest rates of hospital admission for pathologic fracture during the study period. Hospital admission with pathologic fracture was more common than nonpathologic fracture for every type of metastatic disease except colorectal and uterine cancer. Pathologic fracture in patients with metastatic disease was most likely to occur in the spine, hip, and femur (Table 2), and ratio of anatomic sites fractured was relatively consistent across each of the 10 primary malignancies (Table 3).
Demographic characteristics of patients in the 3 study groups are shown in Table 4. Patients with pathologic fracture were more likely than those in the no-fracture group to be white (63.0% vs 60.3%, respectively; P < .001) and female (55.5% vs 49.8%; P < .001), but were similar in age (66.4 years; P = 0.7). In-hospital mortality was lower in the pathologic fracture group compared with the no-fracture group (6.4% vs 8.8%; P < .001). People in the pathologic fracture group were more likely than others to be treated at a teaching hospital (P < .001) with ≥450 beds (P < .001), and reside in a zip code with higher income (P < .01).
Pathologic fracture hospitalizations, on average, had higher billed costs and longer length of stay ($62,974, 9.1 days; Table 4), compared with the no-fracture group ($39,576, 6.9 days; both P < .001) and the nonpathologic fracture group ($42,029, 7.2 days; both P < .001). Pathologic fracture in patients with thyroid, liver, and kidney cancer was associated with the highest costs of hospitalization.
In patients with metastatic disease, differences were found between those with pathologic and nonpathologic fractures: those with pathologic fracture were younger (66.4 vs 74.3 years; P < .001), less likely to be white (63.0% vs 69.0%; P < .001), and more commonly treated at a large hospital (68.4% vs 62.1%; P < .001) or a teaching hospital (53.5% vs 41.0%; P < .001).
Discussion
Other investigators have looked at risk factors for pathologic fracture, such as degree of bone involvement, location, and the presence of lytic versus blastic disease, as well as the optimal management of such patients.16-20 In those analyses, there is an emphasis on large, lytic lesions with cortical destruction in weight-bearing long bones, and on functional pain as a key determinant of fracture risk. Although the guidelines outlined by Mirel and others are helpful in predicting fractures, they are not widely applied by practicing oncologists.18 Oncologists and surgeons lack foolproof criteria to predict impending pathologic fracture despite evidence that the pathologic fracture event greatly increases mortality and morbidity.1,4,21,22 As far as we know, this is the first study to determine which types of primary carcinomas were most associated with pathologic fracture requiring hospitalization. This finding will hopefully raise awareness among doctors who care for these patients to be particularly conscientious with patients who present with symptoms of bone pain with activity (functional bone pain) or with lytic disease in the long bones. The results of the present study are similar to those from cadaveric studies, which emphasize the importance of lung, breast, prostate, and kidney cancers as primary tumors that metastasize to bone and lead to pathologic fracture. A novel finding is the nearly 4-fold greater number of pathologic fractures from colorectal carcinoma than thyroid carcinoma.
The importance of detecting patients at risk for pathologic fracture is now more relevant than ever because there are treatment modalities that are readily available to patients with metastatic bone involvement. Two classes of medications, the RANK-ligand inhibitors and bisphosphonates, reduce the number of skeletal events, such as pathologic fracture, in patients with metastatic disease to bone.23-26 However, most of those studies focused on the 3 most common carcinomas (breast, lung, and prostate) to metastasize to bone and cause pathologic fracture. There is greater variability in the prophylactic treatment of other forms of cancer that have metastasized to bone amongst oncologists.
Despite a lower proportion of hospitalizations for fracture in patients with CRC than for thyroid carcinoma (0.5% vs 1.6%, respectively), there were more pathologic fractures from CRC than from thyroid carcinoma because there are far more cases of CRC. SEER data estimate that in 2014 there were 62,000 cases of thyroid cancer and 1,890 deaths, compared with 136,000 cases of CRC and 50,000 deaths.10 Previous findings have shown that bone metastasis from CRC is more common than originally thought, based on autopsies of CRC patients.3 However, the lower rate of bone metastasis in CRC compared with other malignancies has led to a decreased focus on skeletal-related events in CRC. Our results suggest vigilance to bone health is warranted in patients with metastatic CRC. A novel finding is that patients with metastatic CRC also have a high number of hospital admissions for nonpathologic fracture. In establishing that patients with metastatic CRC with bone involvement have a real and significant risk of developing both pathologic and nonpathologic fractures, it may alter the treatment practice for these patients going forward, with greater consideration for an antiresorptive therapy, fall prevention education, or other preventive modalities, such as external-beam radiation therapy after it has been established that patients have metastatic bone disease.
There were some demographic differences between patients with metastatic disease who sustain pathologic fractures and those who do not fracture or sustain nonpathologic fractures. Patients with pathologic fracture were younger than those with nonpathologic fractures, and patients who sustained any fracture were more likely to be white than were patients in the no-fracture group. Known osteoporosis risk factors including older, female, and white with Northern European descent.27 Those findings emphasize the importance of osteoporosis screening and fracture prevention in patients with metastatic disease in general, regardless of the presence of bony metastasis. The present study found that patients who reside in zip codes areas with higher incomes were at slightly increased risk of hospitalization for pathologic fracture. Economic disparities in access to health care and cancer care are well documented,28 and the basis for this finding is a direction for future research.
Both mean billed costs and length of stay were greatest in the pathologic fracture group. The large number of admissions for no-fractured patients may be a final opportunity for intervention and preventative measures in this fragile population. Improved surveillance for bony lesions and attention to pain, especially at night, or unexplained hypercalcemia may help with early diagnosis and prevent some pathologic fractures. Patients with pathologic fracture often undergo additional treatments such as radiation therapy or chemotherapy. These additional treatments may partially explain the higher billed costs associated with inpatient hospitalization; future studies may be able to elucidate treatment differences or other reasons for the increased costs associated with pathologic fractures and identify targets to reduce expenditures.
Limitations
This study is subject to the limitations of a retrospective analysis based on hospital administrative discharge data. It evaluates only billed charges and does not account for costs associated with rehabilitation stays. However, it represents a stratified cross-sample of hospitalizations in the United States, in both teaching and nonteaching hospitals, and is the largest study to date that the authors are aware of looking at the burden of pathologic fractures in patients with metastatic disease.
This study specifically included only patients with metastatic disease, which therefore limits comparisons with the rate of hospitalization for nonpathologic fracture in patients without metastatic disease. Patients with metastatic disease who were not hospitalized during the study period are nevertheless at risk for fracture but would not have been captured in this study. It is also likely that some patients with metastatic disease had multiple hospitalizations, including some that were not for fracture; therefore, this study likely underestimates the percentage of patients with metastatic disease who sustain pathologic and nonpathologic fracture.
Some patients were excluded because we were not able to identify a primary cancer from hospital discharge records. The lack of an included diagnosis may be a result of indeterminate primary during the fracture admission or may represent a failure to accurately code a primary, known cancer. Although the NIS does not permit identification of these patients to determine if a primary cancer was subsequently identified, future studies using other databases may target patients presenting with pathologic fracture and an unknown primary tumor to evaluate subsequent cancer diagnosis.
Summary
The significance of bone metastasis in causing pathologic fractures in lung, breast, prostate, and kidney cancers was confirmed. Colorectal carcinoma has been established as the fifth most common primary cancer in patients with metastatic disease who are hospitalized with pathologic fracture, and a large number of patients with metastatic CRC sustain nonpathologic fractures requiring hospitalization. In patients with metastatic CRC or new skeletal pain, education on fall prevention and increased vigilance should be considered. Further studies are needed to determine the best method for prevention of pathologic fractures in all highly prevalent cancers, with previous hospitalizations without fracture as an appropriate target. Previous paradigms about which cancers metastasize to bone should be reconsidered in the context of which lead to clinically important fractures and hospitalization.
1. Carter JA, Ji X, Botteman MF. Clinical, economic and humanistic burdens of skeletal-related events associated with bone metastases. Expert Rev Pharmacoecon Outcomes Res. 2013;13(4):483-496.
2. Clain A. Secondary malignant disease of bone. Br J Cancer. 1965;19:15-29.
3. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 Pt 2):6243s-6249s.
4. Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1588-1594.
5. Higinbotham NL, Marcove RC. The management of pathological fractures. J Trauma. 1965;5(6):792-798.
6. Hess KR, Varadhachary GR, Taylor SH, et al. Metastatic patterns in adenocarcinoma. Cancer. 2006;106(7):1624-1633.
7. Stone CA, Lawlor PG, Savva GM, Bennett K, Kenny RA. Prospective study of falls and risk factors for falls in adults with advanced cancer. J Clin Oncol. 2012;30(17):2128-2133.
8. Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27(3):165-176.
9. Katoh M, Unakami M, Hara M, Fukuchi S. Bone metastasis from colorectal cancer in autopsy cases. J Gastroenterol. 1995;30(5):615-618.
10. Howlader N NA, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z,Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2011, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission. Posted April 2014. Accessed January 19, 2018.
11. Hagiwara M, Delea TE, Saville MW, Chung K. Healthcare utilization and costs associated with skeletal-related events in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 2013;16(1):23-27.
12. Hagiwara M, Delea TE, Chung K. Healthcare costs associated with skeletal-related events in breast cancer patients with bone metastases. J Med Econ. 2014;17(3):223-230.
13. Yong C, Onukwugha E, Mullins CD. Clinical and economic burden of bone metastasis and skeletal-related events in prostate cancer. Curr Opin Oncol. 2014;26(3):274-283.
14. HCUP Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). 2011. Agency for Healthcare Research and Quality R, MD. http://www.hcup-us.ahrq.gov/nisoverview.jsp. Last modified January 17, 2018. Accessed January 18, 2018.
15. American Cancer Society. Cancer Facts & Figures. Atlanta: American Cancer Society; 2003-2010. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2010.html Published January 2010. Accessed January 17, 2018.
16. Harrington KD. Orthopedic surgical management of skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1614-1627.
17. Harrington KD. Impending pathologic fractures from metastatic malignancy: evaluation and management. Instr Course Lect. 1986;35:357-381.
18. Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989;249:256-264.
19. Weber KL. Evaluation of the adult patient (aged >40 years) with a destructive bone lesion. J Am Acad Orthop Surg. 2010;18(3):169-179.
20. Rougraff BT. Evaluation of the patient with carcinoma of unknown origin metastatic to bone. Clin Orthop Relat Res. 2003(415 Suppl):S105-109.
21. Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987;55(1):61-66.
22. Dijstra S, Wiggers T, van Geel BN, Boxma H. Impending and actual pathological fractures in patients with bone metastases of the long bones. A retrospective study of 233 surgically treated fractures. Eur J Surg. 1994;160(10):535-542.
23. Henry D, Vadhan-Raj S, Hirsh V, et al. Delaying skeletal-related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: an analysis of data from patients with solid tumors. Support Care Cancer. 2014;22(3):679-687.
24. Lorusso V, Duran I, Garzon-Rodriguez C, et al. Health resource utilisation associated with skeletal-related events in European patients with lung cancer: Alpha subgroup analysis from a prospective multinational study. Mol Clin Oncol. 2014;2(5):701-708.
25. Lothgren M, Ribnicsek E, Schmidt L, et al. Cost per patient and potential budget implications of denosumab compared with zoledronic acid in adults with bone metastases from solid tumours who are at risk of skeletal-related events: an analysis for Austria, Sweden and Switzerland. Eu J Hosp Pharm Sci Pract. 2013;20(4):227-231.
26. Luftner D, Lorusso V, Duran I, et al. Health resource utilization associated with skeletal-related events in patients with advanced breast cancer: results from a prospective, multinational observational study. SpringerPlus. 2014;3:328.
27. Cauley JA. Defining ethnic and racial differences in osteoporosis and fragility fractures. Clin Orthop Relat Res. 2011;469(7):1891-1899.
28. VanEenwyk J, Campo JS, Ossiander EM. Socioeconomic and demographic disparities in treatment for carcinomas of the colon and rectum. Cancer. 2002;95(1):39-46.
1. Carter JA, Ji X, Botteman MF. Clinical, economic and humanistic burdens of skeletal-related events associated with bone metastases. Expert Rev Pharmacoecon Outcomes Res. 2013;13(4):483-496.
2. Clain A. Secondary malignant disease of bone. Br J Cancer. 1965;19:15-29.
3. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 Pt 2):6243s-6249s.
4. Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1588-1594.
5. Higinbotham NL, Marcove RC. The management of pathological fractures. J Trauma. 1965;5(6):792-798.
6. Hess KR, Varadhachary GR, Taylor SH, et al. Metastatic patterns in adenocarcinoma. Cancer. 2006;106(7):1624-1633.
7. Stone CA, Lawlor PG, Savva GM, Bennett K, Kenny RA. Prospective study of falls and risk factors for falls in adults with advanced cancer. J Clin Oncol. 2012;30(17):2128-2133.
8. Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27(3):165-176.
9. Katoh M, Unakami M, Hara M, Fukuchi S. Bone metastasis from colorectal cancer in autopsy cases. J Gastroenterol. 1995;30(5):615-618.
10. Howlader N NA, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z,Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2011, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission. Posted April 2014. Accessed January 19, 2018.
11. Hagiwara M, Delea TE, Saville MW, Chung K. Healthcare utilization and costs associated with skeletal-related events in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 2013;16(1):23-27.
12. Hagiwara M, Delea TE, Chung K. Healthcare costs associated with skeletal-related events in breast cancer patients with bone metastases. J Med Econ. 2014;17(3):223-230.
13. Yong C, Onukwugha E, Mullins CD. Clinical and economic burden of bone metastasis and skeletal-related events in prostate cancer. Curr Opin Oncol. 2014;26(3):274-283.
14. HCUP Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). 2011. Agency for Healthcare Research and Quality R, MD. http://www.hcup-us.ahrq.gov/nisoverview.jsp. Last modified January 17, 2018. Accessed January 18, 2018.
15. American Cancer Society. Cancer Facts & Figures. Atlanta: American Cancer Society; 2003-2010. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2010.html Published January 2010. Accessed January 17, 2018.
16. Harrington KD. Orthopedic surgical management of skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1614-1627.
17. Harrington KD. Impending pathologic fractures from metastatic malignancy: evaluation and management. Instr Course Lect. 1986;35:357-381.
18. Mirels H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin Orthop Relat Res. 1989;249:256-264.
19. Weber KL. Evaluation of the adult patient (aged >40 years) with a destructive bone lesion. J Am Acad Orthop Surg. 2010;18(3):169-179.
20. Rougraff BT. Evaluation of the patient with carcinoma of unknown origin metastatic to bone. Clin Orthop Relat Res. 2003(415 Suppl):S105-109.
21. Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987;55(1):61-66.
22. Dijstra S, Wiggers T, van Geel BN, Boxma H. Impending and actual pathological fractures in patients with bone metastases of the long bones. A retrospective study of 233 surgically treated fractures. Eur J Surg. 1994;160(10):535-542.
23. Henry D, Vadhan-Raj S, Hirsh V, et al. Delaying skeletal-related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: an analysis of data from patients with solid tumors. Support Care Cancer. 2014;22(3):679-687.
24. Lorusso V, Duran I, Garzon-Rodriguez C, et al. Health resource utilisation associated with skeletal-related events in European patients with lung cancer: Alpha subgroup analysis from a prospective multinational study. Mol Clin Oncol. 2014;2(5):701-708.
25. Lothgren M, Ribnicsek E, Schmidt L, et al. Cost per patient and potential budget implications of denosumab compared with zoledronic acid in adults with bone metastases from solid tumours who are at risk of skeletal-related events: an analysis for Austria, Sweden and Switzerland. Eu J Hosp Pharm Sci Pract. 2013;20(4):227-231.
26. Luftner D, Lorusso V, Duran I, et al. Health resource utilization associated with skeletal-related events in patients with advanced breast cancer: results from a prospective, multinational observational study. SpringerPlus. 2014;3:328.
27. Cauley JA. Defining ethnic and racial differences in osteoporosis and fragility fractures. Clin Orthop Relat Res. 2011;469(7):1891-1899.
28. VanEenwyk J, Campo JS, Ossiander EM. Socioeconomic and demographic disparities in treatment for carcinomas of the colon and rectum. Cancer. 2002;95(1):39-46.
Measurement of physical activity and sedentary behavior in breast cancer survivors
Physical activity has numerous physical, mental, and psychosocial benefits for cancer survivors, such as a reduction in the risk of mobility disability, depression, and anxiety, and improved patient quality of life.1,2 In addition, higher levels of physical activity are associated with reduced cancer-specific and all-causes mortality as well as cancer-specific outcomes including reduced risk of cancer progression and recurrence and new primary cancers.3-5 However, fewer than one-third of cancer survivors are meeting government and cancer-specific recommendations of 150 minutes a week of moderate to vigorous physical activity (MPVA; ≥3 metabolic equivalents [METs]).6,7 Growing evidence also demonstrates a significant association between higher levels of sedentary behavior and many deleterious health effects after cancer, including an increased risk for decreased physical functioning and development of other chronic diseases such as cardiovascular disease or diabetes.8 Distinct from physical activity, sedentary behavior is defined as any waking activity resulting in low levels of energy expenditure (≤1.5 METs) while in a seated or reclined position.9 Increased sedentary behavior, even when controlling for moderate and vigorous physical activity (MVPA), is associated with poor quality of life and increased all-cause mortality in cancer survivors.10,11 Given the associations observed between higher levels of physical activity, lower levels of sedentary behavior, and improved health and disease outcomes among the large and increasing number of cancer survivors in the United States, it is important to identify low-cost methods that can be used in a in a variety of settings (ie, research, clinical, community) to accurately and efficiently measure survivors’ lifestyle behaviors to identify high-risk survivors for early intervention, better understand the effects of these behaviors on survivors’ health outcomes and disease trajectories, and ultimately, improve survivors’ health and quality of life.12,13
Two methods commonly used to capture physical activity and sedentary behavior across the lifespan are accelerometry (Actigraph, Pensacola, FL) and self-report questionnaires such as the Godin Leisure-Time Questionnaire (GLTEQ), International Physical Activity Questionnaire (IPAQ), and Sitting Time Questionnaire (STQ).14-17 Each method has unique strengths and weaknesses. Sending accelerometers to multiple individuals at a single time point can be costly, particularly in large-scale epidemiological studies, and the accelerometer’s waist-worn, nonwaterproof design may prevent researchers from capturing certain activities such as swimming and resistance training. However, the accelerometer provides objective, precise assessments of most physical activities and may help remove response bias.18 Conversely, self-report questionnaires rely solely on individuals’ memories and often result in recall bias, inaccurate reporting, and under- or overestimation of physical activity engagement.19,20 Nevertheless, these questionnaires can be widely disseminated at low cost in a variety of settings (eg, clinical, research, community) and are less of a burden to participants.
Recent studies comparing objective (eg, accelerometer) with subjective (eg, self-report) methods of measuring physical activity and sedentary behavior in healthy middle-aged adults and older adults have demonstrated mixed findings with no distinct trends in the degree to which these methods differ.19,21,22 To date, little consideration has been given to the measurement of these lifestyle behaviors in cancer survivors. Boyle and colleagues recently investigated the concurrent validity of an accelerometer to the GLTEQ in colon cancer survivors, finding significant differences in estimated MVPA (~11 minutes). However, no studies, to our knowledge, have compared accelerometer and self-report measures in breast cancer survivors, so it remains unclear how these different measurement tools relate to each another in this population.
It is particularly important to compare these measurement tools among breast cancer survivors because evidence indicates this population’s behavioral habits, self-perceived activity, and sitting time and movement patterns may differ significantly from the general population and other survivor groups across the lifespan.23,24 Further, previous studies examining these behaviors in cancer survivors focused primarily on sitting time and MVPA.15,25,26 Examining other lower-intensity intensities (eg, light activity or lifestyle) in cancer survivors may also be important given that increased levels of activity are associated with health benefits, ranging from reduced disability and fatigue to improved cardiovascular health and quality of life, and that breast cancer survivors engage in fewer of these activities compared with noncancer controls.23 These lower levels of physical activity may be more prevalent among cancer survivors of their high levels of fatigue and propensity toward increased sitting time during the first year of treatment,11 so it is important to be able to accurately assess these activities in this population. The purpose of the present study was to compare estimates of time spent in light physical activity (LPA), MVPA, and sitting time (ST) obtained from an accelerometer and 3 self-report measurement tools (GLTEQ, IPAQ, STQ) in a large, US-based sample of breast cancer survivors. A secondary purpose was to determine whether estimate comparisons among measurements changed by participant characteristics.
Methods
Participants and procedures
This study consisted of a subsample of women who participated in a larger study whose findings have been reported elsewhere by Phillips and McAuley.27 In that study, breast cancer survivors (n = 1,631) were recruited nationally to participate in a 6-month prospective study on quality of life. Eligibility criteria included being aged 18 years or older, having had a diagnosis of breast cancer, being English speaking, and having access to the internet. Once consented to participate in the study, 500 women were randomly selected to wear the accelerometer.
Participants in this group were mailed an accelerometer, an activity log, instructions for use, and a self-addressed stamped envelope to return the monitor. They were asked to wear the accelerometer during all waking hours for 7 consecutive days of usual activity. They were also sent a secure link to complete 3 activity questionnaires online. The questionnaires were to be completed by the end of the 7-day monitoring period. Only women with 3 or more valid days of accelerometer data and complete data on variables of interest (n = 414) were included in the present analyses. All of the participants consented to the study procedures approved by the University of Illinois Institutional Review Board.
Measures
Demographics. The participants self-reported their age, level of education, height, and weight. Their body mass index (BMI; kg/m2) was estimated using the standard equation. They also self-reported their health and cancer history, detailing breast cancer disease stage, time since diagnosis, treatment type, and whether they had had a cancer recurrence. They were also asked to report whether they had ever been diagnosed (Yes/No) with 18 chronic conditions (eg, diabetes, arthritis).
Godin Leisure-Time Exercise Questionnaire.16 The GLTEQ assessed participants’ weekly frequency and mean amount of time performing MVPA (moderate exercise, such as fast walking, combined with vigorous exercise, such as jogging), and LPA (light/mild exercise, eg, easy walking) during the previous 7 days. The mean daily duration (in minutes) for each intensity category (MVPA, LPA) was calculated using activity frequencies and the amount of time spent in each activity presented as minutes/day.
The International Physical Activity Questionnaire.14 The IPAQ evaluated participants’ physical activity of at least moderate intensity in 4 domains of everyday life: job-related physical activity, transportation, housework/caring for family, and leisure-time activity. Within each domain, participants were asked the number of days per week and time per day (hours and minutes) spent performing MVPA. To estimate sitting time, the questionnaire asks participants to report the total amount of time spent sitting per day in 2 conditions, during weekdays and during weekends. The present analysis averaged sitting time for a typical 7-day (5 week days, 2 weekend days) period. We multiplied reported minutes per day and frequency per week of each activity category (MVPA and ST) to calculate the mean number of minutes per day.29,30
Sitting Time Questionnaire.17,28 The STQ estimated the mean time (hours and minutes) participants spent sitting each day on weekdays and at weekends within 5 domains: while traveling to and from places, at work, watching television, using a computer at home, and at leisure, not including watching television (eg, visiting friends, movies, dining out). Mean minutes per day of ST were calculated using all sitting domains.
Actigraph accelerometer (model GT1M, Health One Technology, Fort Walton Beach, FL). The Actigraph GT1M is a reliable and objective measure of physical activity.31-33 Participants wore the monitor on the right hip for 7 consecutive days during all waking hours, except when bathing or swimming. Activity data was analyzed in 1-minute intervals. A valid day of accelerometer wear time was defined as ≥600 minutes with no more than 60 minutes of consecutive zero-values, with allowance of 2 minutes or fewer of observations <100 counts/minute within the nonwear interval.34 Each minute of wear time was classified according to intensity (counts/min) using the following cut-points:34 sedentary, <100 counts/min; LPA, 100-2,019 counts/min; and MVPA, ≥2,020 count/min. Mean daily durations (min/day) spent in each behavior were estimated by dividing the number of minutes in each category by the number of valid days.
Statistical analysis
All statistical analyses were completed in SPSS Statistics 23 (IBM, Chicago, IL). Descriptive statistics were used to define participant characteristics. Rank-order correlation between the methods was assessed using Spearman’s rho (rs) and results were interpreted as follows: rs = 0.10, small; 0.30, moderate; and 0.50, strong.35 Within each activity intensity group, we jointly modeled daily minutes of self-report and accelerometer data using a random-intercept mixed-effects regression model. Differences between measurement tools were assessed based on regression coefficients with accelerometer as the reference category. Finally, we did a post hoc analysis of leisure-time–only MVPA from the IPAQ to compare with other estimates of MVPA.
We calculated the measurement tool difference scores for each estimated intensity category (ST, LPA, MVPA), that is, accelerometer estimated ST minus STQ estimated ST, and GLTEQ estimated MVPA minus IPAQ estimated MVPA. We used these data in an exploratory analysis to examine whether there were statistically significant differences between measurement difference scores by demographic or disease characteristics using linear regression stratified analyses. For example, we were interested in whether there was a significant difference in measurement tool estimates for sitting time in older compared with younger survivors. Analyses were stratified by age (<60/≥60 years), body mass index (<25 kg/m2/≥25 kg/m2), race (white/people of color), disease stage (I and II/III and IV), years since diagnosis (≤5 years/>5 years), recurrence (Yes/No), received chemotherapy (Yes/No ), received radiation (Yes/No ), and the presence of 1 or more chronic diseases (Yes/No ).
Results
Participants
The mean age of the participants was 56.8 years [9.2], they were overweight (BMI, 26.2 kg/m2 [5.4]), and predominantly white (96.7%; Table 1). Table 2 provides a summary of mean daily duration of activity estimates for ST, LPA, and MVPA and the estimate mean difference scores between measurements.
Moderate and vigorous physical activity
Accelerometer−GLTEQ. The mean difference in MVPA estimates between the accelerometer and GLTEQ was less than 5 minutes (Maccelerometer = 20.2 minutes; MGLTEQ = 23.6 minutes), even though the difference was statistically significant (P = .02). Estimates of MVPA from the accelerometer and GLTEQ (rs = 0.564, P < .001) showed a strong relationship. Stratified analyses showed that the difference scores between the GLTEQ and accelerometer were lower for older survivors (≥60 years) compared with younger survivors such that older survivors reported significantly less time in MVPA on the GLTEQ compared with accelerometer estimates (difference score [D] = 6.8 minutes less, P = .001).
Accelerometer−IPAQ. The accelerometer estimated significantly fewer minutes of MVPA per day when compared with the IPAQ (Mdiff = -67.4; 95% confidence interval [CI], -78.6, -55.8; P < .001). Estimates of MVPA from the accelerometer and IPAQ (rs = 0.011, P = .680) were poorly related. Differences between the IPAQ and accelerometer were greater for later-stage breast cancer, compared with early-stage diagnoses such that participants with late-stage disease reported significantly less MVPA on the IPAQ compared with accelerometer estimates (D = 41.8 minutes less than early-stage disease, P = .018). Finally, participants of color reported a greater difference in MVPA between the accelerometer and the IPAQ than did their white counterparts (D = 47.5 minutes, P = .033).
GLTEQ−IPAQ. GLTEQ estimated significantly fewer minutes of MVPA per day compared with the IPAQ (Mdiff = -64.6; 95% CI, -76.6, -52.5; P < .001). The estimates of MVPA from the GLTEQ had a small correlation with IPAQ estimates (rs = 0.128, P = .011).
IPAQ estimates showed almost triple the MVPA minutes per day as were estimated by the accelerometer and GLTEQ. As the MVPA estimate for the IPAQ include nonleisure activities, we conducted a post hoc analyses that only included the leisure-time items from the IPAQ. Leisure-time only IPAQ items, estimates indicated survivors spent a mean 18.5 [SD, 14.2] min/day in MVPA. Although the magnitude of the difference between the accelerometer and GLTEQ estimates (~10 minutes) was much smaller using the leisure-time only IPAQ items, a repeated measures analysis of variance revealed there was still a significant difference between these estimates (P < .05 for both) and negligible correlation.
Light intensity physical activity
Accelerometer−GLTEQ. There was a large and significant difference between LPA estimates from the GLTEQ and accelerometer (Mdiff = 224.5; 95% CI, 218.2, 230.7; P < .001) with estimates from the accelerometer being higher than those for the GLTEQ. Additionally, the measurements showed a negligible correlation (rs = 0.004, P = .94). Difference scores for GLTEQ and accelerometer estimated LPA were significantly different by age, with survivors aged 60 years or older demonstrating a difference that was 18.3 minutes shorter (P = .005) than the difference in younger survivors (<60 years).
Sitting time
Accelerometer−IPAQ. Mean IPAQ estimates were significantly lower (M = 303.8 [63.4]) than accelerometer estimates (M = 603.9 [78.0]). Rank-order correlations between IPAQ and accelerometer estimated ST was small (rs =0.26, P < .001). Difference scores between IPAQ and accelerometer estimates were significantly greater for survivors who were 60 years or older, compared with those younger than 60 years (D = 47.6 minutes, P = .006), indicating that older survivors tended to self-report significantly more ST than estimated by the accelerometer.
Accelerometer−STQ. There was no significant difference in estimated mean ST minutes per day between the STQ and the accelerometer, but the correlation between estimates was low (rs = 0.30, P < .001). Stratified analyses revealed estimates for the difference scores for mean daily ST between the STQ and accelerometer were greater for participants who were diagnosed with later-stage breast cancer (D= -158.3 minutes, P < .001) and those who had received chemotherapy (D= -61.7 minutes, P = .028; Table 2) than for those who were diagnosed with early-stage breast cancer or had not received chemotherapy. Women who had later-stage disease reported significantly less ST than did women diagnosed with early-stage disease, when compared with estimates by the accelerometer.
IPAQ−STQ. The estimated mean ST was significantly lower for IPAQ (M = 303.8 minutes [163.4]) than for the STQ (M = 605.2 minutes [296.2]). There were no significant estimate differences among the stratified groups.
Discussion
The purpose of the present study was to compare 4 measurement tools, an accelerometer-based activity monitor and 3 self-report questionnaires, to estimate ST, LPA, and MVPA in breast cancer survivors. Developing and evaluating accurate and precise measurement tools to assess physical activity and ST in breast cancer survivors remains a critical step toward better understanding the role of physical activity in cancer survivorship. Our results indicate that the congruency of the measurement tools examined was highly dependent on the activity intensity of interest and participants’ demographic or disease characteristics. Overall, the accelerometer estimated a greater amount of time spent sitting and engaging in LPA and less time in MVPA than was estimated on the STQ, GLTEQ, and IPAQ. In addition, our findings suggest significant subgroup differences that will be important in future development and implementation of physical activity measurement for breast cancer survivors.
MVPA has been the most commonly measured activity intensity among cancer survivors to date.15,25,26 The present results indicate mean daily MVPA estimates were significantly higher for the GLTEQ compared with the accelerometer (Mdiff = 2.8 min/d, P = .019), although the magnitude of these differences was relatively small. This difference is lower than in another study that compared these measures in colon cancer survivors and found the GLTEQ over-estimated MVPA by 10.6 min/day compared with the accelerometer (P < .01).15 However, the correlation between the 2 tools in our study was similar to that of Boyle and colleagues (rs = 0.56 and rs = 0.51, respectively). A possible explanation for the equivocal findings across these studies may lie in the difference in study sample demographics; a previous study results finding breast cancer survivors may be better at recalling their physical activities because they may be more attentive to activities they perform daily.26
The IPAQ significantly estimated more than an hour more of MVPA minutes per day compared with the accelerometer and GLTEQ. There are a number of limitations to the reporting of MVPA on the IPAQ. These limitations have been previously reported in the literature and include cross-cultural differences as well as overreporting of nonleisure-time MVPA (eg, occupational or household activities). However, the IPAQ has consistently been shown to be a valid and reliable tool for physical activity surveillance in different populations across the world.29,36,37 This shows that although MVPA was overestimated in our population, we do not mean to undermine the IPAQ value in other populations in which it has shown great utility for overall physical activity surveillance. When we excluded nonleisure-time MVPA, MVPA equated to about 18 min/day, which was closer in magnitude to the GLTEQ and accelerometer. These data highlight the importance of identifying the specific activity parameters of interest when selecting a measurement tool to ensure congruency between the tool and construct of interest.
The differences in MVPA estimation from the 3 tools have significant translational consequences, notably the potential for misclassification of meeting physical activity guidelines. For example, the percentage of women in the present sample that met physical activity guidelines ranged from 0% (using the accelerometer) to 19.5% (using the IPAQ), depending on the measurement tool used. These findings have meaningful implications for future physical activity assessment because multiple measurement tools are cur
For example, scores from the IPAQ may result in a survivor being classified as meeting physical activity guidelines when in fact they are not, and thereby missing the opportunity for intervention; or the accelerometer may classify an active survivor as inactive, which could result in using time and resources for a behavior change intervention that is not necessary. The clinical significance of these findings is to provide providers with data-based information on the strengths and limitations of the measurement tools so that they can accurately estimate physical activity and ST and appropriately optimize resources and treatments.
The degree of measurement tool congruence is likely influenced by a number of factors. First, survivors’ perceptions of the intensity of their activity are relative and subjective to their state of feeling during the activity. For example, breast cancer survivors with lower functional capacity may perceive activities with lower absolute intensity as having a higher relative intensity (ie, they think they are working at a moderate intensity so record an activity as such, but the activity is classified as light by the accelerometer). Second, although our self-report measures asked survivors to record the time they had spent active over the previous 7 days, survivors might report on what they consider a “usual” week, which may reflect the ideal rather than the reality. Third, the accelerometer cut-points used were derived from young, healthy adults on a treadmill. Thus, generalization to an older, sick, less active population that could be experiencing treatment-related side effects could lead to underestimation of time spent in MVPA. To better understand measurement congruency in breast cancer survivors, future research should investigate how functional capacity and activity intensity perceptions are influenced by a breast cancer diagnosis and how those factors may influence subjective and objective physical activity measurement. If those factors were found to have significant influence on activity in breast cancer survivors, it would warrant future development of breast-cancer–specific accelerometer reduction techniques.
The comparison of LPA presented another interesting significant contrast between self-report (GLTEQ) and accelerometry. Results indicated the GLTEQ underestimated LPA by 224.5 [3.2] min/day compared with the accelerometer. This equates to over 3.5 h/day of active time (or about 280 kcal/day) that was potentially unaccounted for by the GLTEQ. The difference between these estimates could be due to the fact that the GLTEQ was designed to measure exercise time and therefore may not be as sensitive as the accelerometer to nonexercise-related LPA. Light intensity activities typically span a large range of domains (ie, occupational, leisure time, household) and tend to occur in higher volumes than MVPA, which may lead to some challenges with recall. Expanding existing LPA questionnaires to encompass these domains would likely provide increased congruency between self-reported and accelerometer-derived estimates for LPA, as it may provide a better trigger for recalling these high volume activities. With increasing literature advocating the important role of LPA in adults’ health in concert with data suggesting survivors may engage in lower levels of LPA than healthy controls,23, accurately accounting for these lower intensity activities to provide a “whole picture” of a survivor’s active day remains an important future research direction. Combining accelerometer and self-report data using ecological momentary assessment to capture these behaviors in real-time in the real world could provide a better understanding of the context in which LPA occurs as well as survivors’ perceptions of intensity to build more accurate and scalable measurement tools for LPA.
Our ST results indicate nonsignificant difference estimates from the accelerometer and the STQ (Mdiff = 1.3 [15.3] min/day) with slightly higher estimates for the STQ versus accelerometer. This finding is consistent with the one other study that has examined these relationships in cancer survivors.15 However, our findings also indicate the IPAQ significantly underestimated ST compared with the accelerometer and the STQ by about half (Table 1). These differences may be because both the STQ and Marshall questionnaire used in the previous study measure multiple domains of sitting (ie, computer, television, travel) on both weekdays and weekends whereas the IPAQ uses only two recall items of overall sitting time (for weekday and weekend separately). The domain-specific, structured approach has been shown to improve recall and may help to prevent underestimation and general underreporting of the high volume, ubiquitous behavior of sitting.17,38 Finally, we would be remiss to not acknowledge the known limitations to estimating ST using the count-based approach on the waist-worn accelerometer. Due to the monitor’s orientation at the hip, the accelerometer may misrepresent total ST by misclassifying standing still as sitting. However, Kozey-Keadle and colleagues have previously examined estimation of ST using waist-worn accelerometers and have shown the 100 count per minute cut off yields ST estimates within 5% range of accuracy for a seated position compared with direct observation.39
Of further interest are our exploratory results indicating that age and disease stage may modify the congruency between activity and ST measures. Specifically, older survivors and those with more advanced disease stage generally reported more PA and less ST than were measured by the accelerometer. These differences raise the question of whether these subgroups are systematically reporting more time physically active, overestimating their intensity, or the accelerometer is misclassifying their activity intensity. These misclassifications could be due to their age, disease stage, fatigue status, functional status, cognitive function, occupational status, etc. and would be important next steps for exploration of measurement of physical activity in breast cancer survivors. Finally, the difference score for MVPA was greater for survivors of color than for white survivors, with survivors of color overreporting MVPA compared with accelerometer-derived estimates. This may be due in part to cultural differences between white survivors and survivors of color. Previous research has suggested that people of color may accumulate a majority of their activity in occupational or household-related domains, thus explaining lower levels of leisure-time MVPA but high levels of reported total MVPA from other nonleisure domains.20 However, given the small number of survivors of color in the present study, these results should be interpreted with caution.
With the multitude of physical activity and ST measurement tools available, many factors including cost, sample size, primary outcome of interest, and activity characteristics of interest (eg, duration, intensity, energy expenditure) need to be considered40 when choosing a tool. Our findings may help inform these decisions for breast cancer survivors. For example, if LPA is of interest, an accelerometer may provide a more comprehensive assessment of these activities than the GLTEQ. In contrast, if MVPA is the activity of interest, our results suggest the GLTEQ and accelerometer were more congruent than the IPAQ was with either measure, therefore, if budgetary constraints are a concern, the more cost-efficient GLTEQ could provide similar results to an accelerometer. In addition to considering measurement congruency, it is also critically important to carefully consider the population (breast cancer survivors) and subsequent burden that accompanies the measurement tool of choice. Overall, our results indicate, when choosing a questionnaire for ST or LPA for breast cancer survivors, the more comprehensive the questions, to encompass multiple domains or time of day, the greater amount of time that will be captured within that activity category. Conversely, since the majority of MVPA is completed in leisure-time, dependent on the age and race of the population, a shorter questionnaire may be sufficient. Additionally, dependent on time since diagnosis and treatment received, activity recall or body movement patterns may be affected which could influence measurement tool selection.23,24 Finally, it is also important to consider the setting in which measurement is taking place. In busy clinical settings, shorter, self-report measures may have a greater chance of being implemented than accelerometers or longer self-report measures and would still provide useful information regarding an overall snapshot of survivors’ MVPA or ST that could be used to initiate a conversation or referral for a program to help survivors positively change one or both of these behaviors.
Limitations
There were a few limitations within the current study that should be taken into account. First, the accelerometer cut-points used were developed with healthy, young adults; therefore using different cut-points may have yielded different results.34 Given the large age range in our participants (23-84 years), we believe the use of these cut-points was justified, in lieu of population-specific (ie, older adults) cut-points. In addition, limitations to estimating activity from an accelerometer include the inability to capture certain activities such as swimming and cycling and the aforementioned inability to distinguish between body postures (ie, sitting vs standing).41 The participants were predominantly white, highly educated, and high earners (85.2% earned ≥$40,000 per year), therefore, the present results may not be generalizable to survivors from more diverse backgrounds. However, as far as we know, this is the first study to report the congruency of estimated ST, LPA, and MVPA across multiple measurement tools in a nationwide sample of breast cancer survivors who were heterogeneous in terms of disease characteristics (ie, stage, treatment, time since diagnosis).
Conclusions
Our findings suggest that physical activity and ST estimates in breast cancer survivors may be dependent on the measurement tool used. In addition, congruency of measurement tools was dependent on activity intensity of interest, and participant age, race, and disease history may also influence these factors. Therefore, researchers should consider the intended outcomes of interest, the context in which the tool is being used (ie, clinical versus research), the available resources, and the participant population before they select a measurement tool for estimating physical activity and sitting time in breast cancer survivors.
Acknowledgment
This work was supported by grant #F31AG034025 from the National Institute on Aging (Dr Phillips); Shahid and Ann Carlson Khan endowed professorship and grant #AG020118 from the National Institute on Aging (Dr McAuley). Dr Phillips is supported by the National Cancer Institute #K07CA196840, and Dr Welch is supported by National Institute of Health/National Cancer Institute training grant CA193193. All data for this study were collected at the University of Illinois Urbana Champaign.
1. Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):87-100.
2. Brenner DR. Cancer incidence due to excess body weight and leisure-time physical inactivity in Canada: implications for prevention. Prev Med. 2014;66:131-139.
3. Courneya KS, Friedenreich CM. Relationship between exercise pattern across the cancer experience and current quality of life in colorectal cancer survivors. J Altern Complement Med. 1997;3(3):215-226.
4. Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol. 2011;28(3):753-765.
5. Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity, risk of death and recurrence in breast cancer survivors: a systematic review and meta-analysis of epidemiological studies. Acta Oncol. 2015;54(5):635-654.
6. Irwin ML, McTiernan A, Bernstein L, et al. Physical activity levels among breast cancer survivors. Med Sci Sports Exerc. 2004;36(9):1484-1491.
7. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409-1426.
8. Lynch BM. Sedentary behavior and cancer: a systematic review of the literature and proposed biological mechanisms. Cancer Epidemiol Biomarkers Prev. 2010;19(11):2691-2709.
9. Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev. 2010;38(3):105-113.
10. Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM. Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. J Clin Oncol. 2013;31(7):876-885.
11. Lynch BM, Dunstan DW, Vallance JK, Owen N. Don’t take cancer sitting down: a new survivorship research agenda. Cancer. 2013;119(11):1928-1935.
12. Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the ‘silver tsunami:’ prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029-1036.
13. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271-289.
14. Booth M. Assessment of physical activity: an international perspective. Res Q Exerc Sport. 2000;71(2 suppl):S114-120.
15. Boyle T, Lynch BM, Courneya KS, Vallance JK. Agreement between accelerometer-assessed and self-reported physical activity and sedentary time in colon cancer survivors. Support Care Cancer. 2015;23(4):1121-1126.
16. Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Canadian journal of applied sport sciences. Can J Appl Sport Sci. 1985;10(3):141-146.
17. Marshall AL, Miller YD, Burton NW, Brown WJ. Measuring total and domain-specific sitting: a study of reliability and validity. Med Sci Sports Exerc. 2010;42(6):1094-1102.
18. Matthews CE, Hagstromer M, Pober DM, Bowles HR. Best practices for using physical activity monitors in population-based research. Med Sci Sports Exerc. 2012;44(1 Suppl 1):S68-76.
19. Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56.
20. Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport. 2000;71(2 Suppl):S1-14.
21. Hart TL, Swartz AM, Cashin SE, Strath SJ. How many days of monitoring predict physical activity and sedentary behaviour in older adults? Int J Behav Nutr Phys Act. 2011;8:62.
22. Hart TL, Ainsworth BE, Tudor-Locke C. Objective and subjective measures of sedentary behavior and physical activity. Med Sci Sports Exerc. 2011;43(3):449-456.
23. Phillips SM, Dodd KW, Steeves J, McClain J, Alfano CM, McAuley E. Physical activity and sedentary behavior in breast cancer survivors: new insight into activity patterns and potential intervention targets. Gynecol Oncol. 2015;138(2):398-404.
24. Boyle T, Vallance JK, Ransom EK, Lynch BM. How sedentary and physically active are breast cancer survivors, and which population subgroups have higher or lower levels of these behaviors? Support Care Cancer. 2016;24(5):2181-2190.
25. Broderick JM, Guinan E, Kennedy MJ, et al. Feasibility and efficacy of a supervised exercise intervention in de-conditioned cancer survivors during the early survivorship phase: the PEACH trial. J Cancer Surviv. 2013;7(4):551-562.
26. Su CC, Lee KD, Yeh CH, Kao CC, Lin CC. Measurement of physical activity in cancer survivors: a validity study. J Cancer Surviv. 2014;8(2):205-212.
27. Phillips SM, McAuley E. Social cognitive influences on physical activity participation in long-term breast cancer survivors. Psychooncology. 2013;22(4):783-791.
28. Wojcicki TR, White SM, McAuley E. Assessing outcome expectations in older adults: the multidimensional outcome expectations for exercise scale. J Gerontol B Psychol Sci Soc Sci. 2009;64(1):33-40.
29. Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381-1395.
30. Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498-504.
31. Hacker ED, Ferrans CE. Ecological momentary assessment of fatigue in patients receiving intensive cancer therapy. J Pain Symptom Manage. 2007;33(3):267-275.
32. Swartz AM, Strath SJ, Bassett DR, Jr, O’Brien WL, King GA, Ainsworth BE. Estimation of energy expenditure using CSA accelerometers at hip and wrist sites. Med Sci Sports Exerc. 2000;32(9 Suppl):S450-456.
33. Jim HS, Small B, Faul LA, Franzen J, Apte S, Jacobsen PB. Fatigue, depression, sleep, and activity during chemotherapy: daily and intraday variation and relationships among symptom changes. Ann Behav Med. 2011;42(3):321-333.
34. Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181-188.
35. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: L Erlbaum Associates; 1988.
36. Bauman A, Ainsworth BE, Bull F, et al. Progress and pitfalls in the use of the International Physical Activity Questionnaire (IPAQ) for adult physical activity surveillance. J Phys Act Health. 2009;6 Suppl 1:S5-8.
37. Hagströmer M1, Oja P, Sjöström M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr. 2006;9(6):755-762.
38. Johnson-Kozlow M, Sallis JF, Gilpin EA, Rock CL, Pierce JP. Comparative validation of the IPAQ and the 7-Day PAR among women diagnosed with breast cancer. Int J Behav Nutr Phys Act. 2006;3:7.
39. Kozey-Keadle S, Libertine A, Lyden K, Staudenmayer J, Freedson PS. Validation of wearable monitors for assessing sedentary behavior. Med Sci Sports Exerc. 2011;43(8):1561-1567.
40. Strath SJ, Kaminsky LA, Ainsworth BE, et al. Guide to the assessment of physical activity: clinical and research applications. Circulation. 2013;128(20):2259-2279.
41. Bassett DR. Device-based monitoring in physical activity and public health research. Physiol Meas. 2012;33(11):1769-1783.
Physical activity has numerous physical, mental, and psychosocial benefits for cancer survivors, such as a reduction in the risk of mobility disability, depression, and anxiety, and improved patient quality of life.1,2 In addition, higher levels of physical activity are associated with reduced cancer-specific and all-causes mortality as well as cancer-specific outcomes including reduced risk of cancer progression and recurrence and new primary cancers.3-5 However, fewer than one-third of cancer survivors are meeting government and cancer-specific recommendations of 150 minutes a week of moderate to vigorous physical activity (MPVA; ≥3 metabolic equivalents [METs]).6,7 Growing evidence also demonstrates a significant association between higher levels of sedentary behavior and many deleterious health effects after cancer, including an increased risk for decreased physical functioning and development of other chronic diseases such as cardiovascular disease or diabetes.8 Distinct from physical activity, sedentary behavior is defined as any waking activity resulting in low levels of energy expenditure (≤1.5 METs) while in a seated or reclined position.9 Increased sedentary behavior, even when controlling for moderate and vigorous physical activity (MVPA), is associated with poor quality of life and increased all-cause mortality in cancer survivors.10,11 Given the associations observed between higher levels of physical activity, lower levels of sedentary behavior, and improved health and disease outcomes among the large and increasing number of cancer survivors in the United States, it is important to identify low-cost methods that can be used in a in a variety of settings (ie, research, clinical, community) to accurately and efficiently measure survivors’ lifestyle behaviors to identify high-risk survivors for early intervention, better understand the effects of these behaviors on survivors’ health outcomes and disease trajectories, and ultimately, improve survivors’ health and quality of life.12,13
Two methods commonly used to capture physical activity and sedentary behavior across the lifespan are accelerometry (Actigraph, Pensacola, FL) and self-report questionnaires such as the Godin Leisure-Time Questionnaire (GLTEQ), International Physical Activity Questionnaire (IPAQ), and Sitting Time Questionnaire (STQ).14-17 Each method has unique strengths and weaknesses. Sending accelerometers to multiple individuals at a single time point can be costly, particularly in large-scale epidemiological studies, and the accelerometer’s waist-worn, nonwaterproof design may prevent researchers from capturing certain activities such as swimming and resistance training. However, the accelerometer provides objective, precise assessments of most physical activities and may help remove response bias.18 Conversely, self-report questionnaires rely solely on individuals’ memories and often result in recall bias, inaccurate reporting, and under- or overestimation of physical activity engagement.19,20 Nevertheless, these questionnaires can be widely disseminated at low cost in a variety of settings (eg, clinical, research, community) and are less of a burden to participants.
Recent studies comparing objective (eg, accelerometer) with subjective (eg, self-report) methods of measuring physical activity and sedentary behavior in healthy middle-aged adults and older adults have demonstrated mixed findings with no distinct trends in the degree to which these methods differ.19,21,22 To date, little consideration has been given to the measurement of these lifestyle behaviors in cancer survivors. Boyle and colleagues recently investigated the concurrent validity of an accelerometer to the GLTEQ in colon cancer survivors, finding significant differences in estimated MVPA (~11 minutes). However, no studies, to our knowledge, have compared accelerometer and self-report measures in breast cancer survivors, so it remains unclear how these different measurement tools relate to each another in this population.
It is particularly important to compare these measurement tools among breast cancer survivors because evidence indicates this population’s behavioral habits, self-perceived activity, and sitting time and movement patterns may differ significantly from the general population and other survivor groups across the lifespan.23,24 Further, previous studies examining these behaviors in cancer survivors focused primarily on sitting time and MVPA.15,25,26 Examining other lower-intensity intensities (eg, light activity or lifestyle) in cancer survivors may also be important given that increased levels of activity are associated with health benefits, ranging from reduced disability and fatigue to improved cardiovascular health and quality of life, and that breast cancer survivors engage in fewer of these activities compared with noncancer controls.23 These lower levels of physical activity may be more prevalent among cancer survivors of their high levels of fatigue and propensity toward increased sitting time during the first year of treatment,11 so it is important to be able to accurately assess these activities in this population. The purpose of the present study was to compare estimates of time spent in light physical activity (LPA), MVPA, and sitting time (ST) obtained from an accelerometer and 3 self-report measurement tools (GLTEQ, IPAQ, STQ) in a large, US-based sample of breast cancer survivors. A secondary purpose was to determine whether estimate comparisons among measurements changed by participant characteristics.
Methods
Participants and procedures
This study consisted of a subsample of women who participated in a larger study whose findings have been reported elsewhere by Phillips and McAuley.27 In that study, breast cancer survivors (n = 1,631) were recruited nationally to participate in a 6-month prospective study on quality of life. Eligibility criteria included being aged 18 years or older, having had a diagnosis of breast cancer, being English speaking, and having access to the internet. Once consented to participate in the study, 500 women were randomly selected to wear the accelerometer.
Participants in this group were mailed an accelerometer, an activity log, instructions for use, and a self-addressed stamped envelope to return the monitor. They were asked to wear the accelerometer during all waking hours for 7 consecutive days of usual activity. They were also sent a secure link to complete 3 activity questionnaires online. The questionnaires were to be completed by the end of the 7-day monitoring period. Only women with 3 or more valid days of accelerometer data and complete data on variables of interest (n = 414) were included in the present analyses. All of the participants consented to the study procedures approved by the University of Illinois Institutional Review Board.
Measures
Demographics. The participants self-reported their age, level of education, height, and weight. Their body mass index (BMI; kg/m2) was estimated using the standard equation. They also self-reported their health and cancer history, detailing breast cancer disease stage, time since diagnosis, treatment type, and whether they had had a cancer recurrence. They were also asked to report whether they had ever been diagnosed (Yes/No) with 18 chronic conditions (eg, diabetes, arthritis).
Godin Leisure-Time Exercise Questionnaire.16 The GLTEQ assessed participants’ weekly frequency and mean amount of time performing MVPA (moderate exercise, such as fast walking, combined with vigorous exercise, such as jogging), and LPA (light/mild exercise, eg, easy walking) during the previous 7 days. The mean daily duration (in minutes) for each intensity category (MVPA, LPA) was calculated using activity frequencies and the amount of time spent in each activity presented as minutes/day.
The International Physical Activity Questionnaire.14 The IPAQ evaluated participants’ physical activity of at least moderate intensity in 4 domains of everyday life: job-related physical activity, transportation, housework/caring for family, and leisure-time activity. Within each domain, participants were asked the number of days per week and time per day (hours and minutes) spent performing MVPA. To estimate sitting time, the questionnaire asks participants to report the total amount of time spent sitting per day in 2 conditions, during weekdays and during weekends. The present analysis averaged sitting time for a typical 7-day (5 week days, 2 weekend days) period. We multiplied reported minutes per day and frequency per week of each activity category (MVPA and ST) to calculate the mean number of minutes per day.29,30
Sitting Time Questionnaire.17,28 The STQ estimated the mean time (hours and minutes) participants spent sitting each day on weekdays and at weekends within 5 domains: while traveling to and from places, at work, watching television, using a computer at home, and at leisure, not including watching television (eg, visiting friends, movies, dining out). Mean minutes per day of ST were calculated using all sitting domains.
Actigraph accelerometer (model GT1M, Health One Technology, Fort Walton Beach, FL). The Actigraph GT1M is a reliable and objective measure of physical activity.31-33 Participants wore the monitor on the right hip for 7 consecutive days during all waking hours, except when bathing or swimming. Activity data was analyzed in 1-minute intervals. A valid day of accelerometer wear time was defined as ≥600 minutes with no more than 60 minutes of consecutive zero-values, with allowance of 2 minutes or fewer of observations <100 counts/minute within the nonwear interval.34 Each minute of wear time was classified according to intensity (counts/min) using the following cut-points:34 sedentary, <100 counts/min; LPA, 100-2,019 counts/min; and MVPA, ≥2,020 count/min. Mean daily durations (min/day) spent in each behavior were estimated by dividing the number of minutes in each category by the number of valid days.
Statistical analysis
All statistical analyses were completed in SPSS Statistics 23 (IBM, Chicago, IL). Descriptive statistics were used to define participant characteristics. Rank-order correlation between the methods was assessed using Spearman’s rho (rs) and results were interpreted as follows: rs = 0.10, small; 0.30, moderate; and 0.50, strong.35 Within each activity intensity group, we jointly modeled daily minutes of self-report and accelerometer data using a random-intercept mixed-effects regression model. Differences between measurement tools were assessed based on regression coefficients with accelerometer as the reference category. Finally, we did a post hoc analysis of leisure-time–only MVPA from the IPAQ to compare with other estimates of MVPA.
We calculated the measurement tool difference scores for each estimated intensity category (ST, LPA, MVPA), that is, accelerometer estimated ST minus STQ estimated ST, and GLTEQ estimated MVPA minus IPAQ estimated MVPA. We used these data in an exploratory analysis to examine whether there were statistically significant differences between measurement difference scores by demographic or disease characteristics using linear regression stratified analyses. For example, we were interested in whether there was a significant difference in measurement tool estimates for sitting time in older compared with younger survivors. Analyses were stratified by age (<60/≥60 years), body mass index (<25 kg/m2/≥25 kg/m2), race (white/people of color), disease stage (I and II/III and IV), years since diagnosis (≤5 years/>5 years), recurrence (Yes/No), received chemotherapy (Yes/No ), received radiation (Yes/No ), and the presence of 1 or more chronic diseases (Yes/No ).
Results
Participants
The mean age of the participants was 56.8 years [9.2], they were overweight (BMI, 26.2 kg/m2 [5.4]), and predominantly white (96.7%; Table 1). Table 2 provides a summary of mean daily duration of activity estimates for ST, LPA, and MVPA and the estimate mean difference scores between measurements.
Moderate and vigorous physical activity
Accelerometer−GLTEQ. The mean difference in MVPA estimates between the accelerometer and GLTEQ was less than 5 minutes (Maccelerometer = 20.2 minutes; MGLTEQ = 23.6 minutes), even though the difference was statistically significant (P = .02). Estimates of MVPA from the accelerometer and GLTEQ (rs = 0.564, P < .001) showed a strong relationship. Stratified analyses showed that the difference scores between the GLTEQ and accelerometer were lower for older survivors (≥60 years) compared with younger survivors such that older survivors reported significantly less time in MVPA on the GLTEQ compared with accelerometer estimates (difference score [D] = 6.8 minutes less, P = .001).
Accelerometer−IPAQ. The accelerometer estimated significantly fewer minutes of MVPA per day when compared with the IPAQ (Mdiff = -67.4; 95% confidence interval [CI], -78.6, -55.8; P < .001). Estimates of MVPA from the accelerometer and IPAQ (rs = 0.011, P = .680) were poorly related. Differences between the IPAQ and accelerometer were greater for later-stage breast cancer, compared with early-stage diagnoses such that participants with late-stage disease reported significantly less MVPA on the IPAQ compared with accelerometer estimates (D = 41.8 minutes less than early-stage disease, P = .018). Finally, participants of color reported a greater difference in MVPA between the accelerometer and the IPAQ than did their white counterparts (D = 47.5 minutes, P = .033).
GLTEQ−IPAQ. GLTEQ estimated significantly fewer minutes of MVPA per day compared with the IPAQ (Mdiff = -64.6; 95% CI, -76.6, -52.5; P < .001). The estimates of MVPA from the GLTEQ had a small correlation with IPAQ estimates (rs = 0.128, P = .011).
IPAQ estimates showed almost triple the MVPA minutes per day as were estimated by the accelerometer and GLTEQ. As the MVPA estimate for the IPAQ include nonleisure activities, we conducted a post hoc analyses that only included the leisure-time items from the IPAQ. Leisure-time only IPAQ items, estimates indicated survivors spent a mean 18.5 [SD, 14.2] min/day in MVPA. Although the magnitude of the difference between the accelerometer and GLTEQ estimates (~10 minutes) was much smaller using the leisure-time only IPAQ items, a repeated measures analysis of variance revealed there was still a significant difference between these estimates (P < .05 for both) and negligible correlation.
Light intensity physical activity
Accelerometer−GLTEQ. There was a large and significant difference between LPA estimates from the GLTEQ and accelerometer (Mdiff = 224.5; 95% CI, 218.2, 230.7; P < .001) with estimates from the accelerometer being higher than those for the GLTEQ. Additionally, the measurements showed a negligible correlation (rs = 0.004, P = .94). Difference scores for GLTEQ and accelerometer estimated LPA were significantly different by age, with survivors aged 60 years or older demonstrating a difference that was 18.3 minutes shorter (P = .005) than the difference in younger survivors (<60 years).
Sitting time
Accelerometer−IPAQ. Mean IPAQ estimates were significantly lower (M = 303.8 [63.4]) than accelerometer estimates (M = 603.9 [78.0]). Rank-order correlations between IPAQ and accelerometer estimated ST was small (rs =0.26, P < .001). Difference scores between IPAQ and accelerometer estimates were significantly greater for survivors who were 60 years or older, compared with those younger than 60 years (D = 47.6 minutes, P = .006), indicating that older survivors tended to self-report significantly more ST than estimated by the accelerometer.
Accelerometer−STQ. There was no significant difference in estimated mean ST minutes per day between the STQ and the accelerometer, but the correlation between estimates was low (rs = 0.30, P < .001). Stratified analyses revealed estimates for the difference scores for mean daily ST between the STQ and accelerometer were greater for participants who were diagnosed with later-stage breast cancer (D= -158.3 minutes, P < .001) and those who had received chemotherapy (D= -61.7 minutes, P = .028; Table 2) than for those who were diagnosed with early-stage breast cancer or had not received chemotherapy. Women who had later-stage disease reported significantly less ST than did women diagnosed with early-stage disease, when compared with estimates by the accelerometer.
IPAQ−STQ. The estimated mean ST was significantly lower for IPAQ (M = 303.8 minutes [163.4]) than for the STQ (M = 605.2 minutes [296.2]). There were no significant estimate differences among the stratified groups.
Discussion
The purpose of the present study was to compare 4 measurement tools, an accelerometer-based activity monitor and 3 self-report questionnaires, to estimate ST, LPA, and MVPA in breast cancer survivors. Developing and evaluating accurate and precise measurement tools to assess physical activity and ST in breast cancer survivors remains a critical step toward better understanding the role of physical activity in cancer survivorship. Our results indicate that the congruency of the measurement tools examined was highly dependent on the activity intensity of interest and participants’ demographic or disease characteristics. Overall, the accelerometer estimated a greater amount of time spent sitting and engaging in LPA and less time in MVPA than was estimated on the STQ, GLTEQ, and IPAQ. In addition, our findings suggest significant subgroup differences that will be important in future development and implementation of physical activity measurement for breast cancer survivors.
MVPA has been the most commonly measured activity intensity among cancer survivors to date.15,25,26 The present results indicate mean daily MVPA estimates were significantly higher for the GLTEQ compared with the accelerometer (Mdiff = 2.8 min/d, P = .019), although the magnitude of these differences was relatively small. This difference is lower than in another study that compared these measures in colon cancer survivors and found the GLTEQ over-estimated MVPA by 10.6 min/day compared with the accelerometer (P < .01).15 However, the correlation between the 2 tools in our study was similar to that of Boyle and colleagues (rs = 0.56 and rs = 0.51, respectively). A possible explanation for the equivocal findings across these studies may lie in the difference in study sample demographics; a previous study results finding breast cancer survivors may be better at recalling their physical activities because they may be more attentive to activities they perform daily.26
The IPAQ significantly estimated more than an hour more of MVPA minutes per day compared with the accelerometer and GLTEQ. There are a number of limitations to the reporting of MVPA on the IPAQ. These limitations have been previously reported in the literature and include cross-cultural differences as well as overreporting of nonleisure-time MVPA (eg, occupational or household activities). However, the IPAQ has consistently been shown to be a valid and reliable tool for physical activity surveillance in different populations across the world.29,36,37 This shows that although MVPA was overestimated in our population, we do not mean to undermine the IPAQ value in other populations in which it has shown great utility for overall physical activity surveillance. When we excluded nonleisure-time MVPA, MVPA equated to about 18 min/day, which was closer in magnitude to the GLTEQ and accelerometer. These data highlight the importance of identifying the specific activity parameters of interest when selecting a measurement tool to ensure congruency between the tool and construct of interest.
The differences in MVPA estimation from the 3 tools have significant translational consequences, notably the potential for misclassification of meeting physical activity guidelines. For example, the percentage of women in the present sample that met physical activity guidelines ranged from 0% (using the accelerometer) to 19.5% (using the IPAQ), depending on the measurement tool used. These findings have meaningful implications for future physical activity assessment because multiple measurement tools are cur
For example, scores from the IPAQ may result in a survivor being classified as meeting physical activity guidelines when in fact they are not, and thereby missing the opportunity for intervention; or the accelerometer may classify an active survivor as inactive, which could result in using time and resources for a behavior change intervention that is not necessary. The clinical significance of these findings is to provide providers with data-based information on the strengths and limitations of the measurement tools so that they can accurately estimate physical activity and ST and appropriately optimize resources and treatments.
The degree of measurement tool congruence is likely influenced by a number of factors. First, survivors’ perceptions of the intensity of their activity are relative and subjective to their state of feeling during the activity. For example, breast cancer survivors with lower functional capacity may perceive activities with lower absolute intensity as having a higher relative intensity (ie, they think they are working at a moderate intensity so record an activity as such, but the activity is classified as light by the accelerometer). Second, although our self-report measures asked survivors to record the time they had spent active over the previous 7 days, survivors might report on what they consider a “usual” week, which may reflect the ideal rather than the reality. Third, the accelerometer cut-points used were derived from young, healthy adults on a treadmill. Thus, generalization to an older, sick, less active population that could be experiencing treatment-related side effects could lead to underestimation of time spent in MVPA. To better understand measurement congruency in breast cancer survivors, future research should investigate how functional capacity and activity intensity perceptions are influenced by a breast cancer diagnosis and how those factors may influence subjective and objective physical activity measurement. If those factors were found to have significant influence on activity in breast cancer survivors, it would warrant future development of breast-cancer–specific accelerometer reduction techniques.
The comparison of LPA presented another interesting significant contrast between self-report (GLTEQ) and accelerometry. Results indicated the GLTEQ underestimated LPA by 224.5 [3.2] min/day compared with the accelerometer. This equates to over 3.5 h/day of active time (or about 280 kcal/day) that was potentially unaccounted for by the GLTEQ. The difference between these estimates could be due to the fact that the GLTEQ was designed to measure exercise time and therefore may not be as sensitive as the accelerometer to nonexercise-related LPA. Light intensity activities typically span a large range of domains (ie, occupational, leisure time, household) and tend to occur in higher volumes than MVPA, which may lead to some challenges with recall. Expanding existing LPA questionnaires to encompass these domains would likely provide increased congruency between self-reported and accelerometer-derived estimates for LPA, as it may provide a better trigger for recalling these high volume activities. With increasing literature advocating the important role of LPA in adults’ health in concert with data suggesting survivors may engage in lower levels of LPA than healthy controls,23, accurately accounting for these lower intensity activities to provide a “whole picture” of a survivor’s active day remains an important future research direction. Combining accelerometer and self-report data using ecological momentary assessment to capture these behaviors in real-time in the real world could provide a better understanding of the context in which LPA occurs as well as survivors’ perceptions of intensity to build more accurate and scalable measurement tools for LPA.
Our ST results indicate nonsignificant difference estimates from the accelerometer and the STQ (Mdiff = 1.3 [15.3] min/day) with slightly higher estimates for the STQ versus accelerometer. This finding is consistent with the one other study that has examined these relationships in cancer survivors.15 However, our findings also indicate the IPAQ significantly underestimated ST compared with the accelerometer and the STQ by about half (Table 1). These differences may be because both the STQ and Marshall questionnaire used in the previous study measure multiple domains of sitting (ie, computer, television, travel) on both weekdays and weekends whereas the IPAQ uses only two recall items of overall sitting time (for weekday and weekend separately). The domain-specific, structured approach has been shown to improve recall and may help to prevent underestimation and general underreporting of the high volume, ubiquitous behavior of sitting.17,38 Finally, we would be remiss to not acknowledge the known limitations to estimating ST using the count-based approach on the waist-worn accelerometer. Due to the monitor’s orientation at the hip, the accelerometer may misrepresent total ST by misclassifying standing still as sitting. However, Kozey-Keadle and colleagues have previously examined estimation of ST using waist-worn accelerometers and have shown the 100 count per minute cut off yields ST estimates within 5% range of accuracy for a seated position compared with direct observation.39
Of further interest are our exploratory results indicating that age and disease stage may modify the congruency between activity and ST measures. Specifically, older survivors and those with more advanced disease stage generally reported more PA and less ST than were measured by the accelerometer. These differences raise the question of whether these subgroups are systematically reporting more time physically active, overestimating their intensity, or the accelerometer is misclassifying their activity intensity. These misclassifications could be due to their age, disease stage, fatigue status, functional status, cognitive function, occupational status, etc. and would be important next steps for exploration of measurement of physical activity in breast cancer survivors. Finally, the difference score for MVPA was greater for survivors of color than for white survivors, with survivors of color overreporting MVPA compared with accelerometer-derived estimates. This may be due in part to cultural differences between white survivors and survivors of color. Previous research has suggested that people of color may accumulate a majority of their activity in occupational or household-related domains, thus explaining lower levels of leisure-time MVPA but high levels of reported total MVPA from other nonleisure domains.20 However, given the small number of survivors of color in the present study, these results should be interpreted with caution.
With the multitude of physical activity and ST measurement tools available, many factors including cost, sample size, primary outcome of interest, and activity characteristics of interest (eg, duration, intensity, energy expenditure) need to be considered40 when choosing a tool. Our findings may help inform these decisions for breast cancer survivors. For example, if LPA is of interest, an accelerometer may provide a more comprehensive assessment of these activities than the GLTEQ. In contrast, if MVPA is the activity of interest, our results suggest the GLTEQ and accelerometer were more congruent than the IPAQ was with either measure, therefore, if budgetary constraints are a concern, the more cost-efficient GLTEQ could provide similar results to an accelerometer. In addition to considering measurement congruency, it is also critically important to carefully consider the population (breast cancer survivors) and subsequent burden that accompanies the measurement tool of choice. Overall, our results indicate, when choosing a questionnaire for ST or LPA for breast cancer survivors, the more comprehensive the questions, to encompass multiple domains or time of day, the greater amount of time that will be captured within that activity category. Conversely, since the majority of MVPA is completed in leisure-time, dependent on the age and race of the population, a shorter questionnaire may be sufficient. Additionally, dependent on time since diagnosis and treatment received, activity recall or body movement patterns may be affected which could influence measurement tool selection.23,24 Finally, it is also important to consider the setting in which measurement is taking place. In busy clinical settings, shorter, self-report measures may have a greater chance of being implemented than accelerometers or longer self-report measures and would still provide useful information regarding an overall snapshot of survivors’ MVPA or ST that could be used to initiate a conversation or referral for a program to help survivors positively change one or both of these behaviors.
Limitations
There were a few limitations within the current study that should be taken into account. First, the accelerometer cut-points used were developed with healthy, young adults; therefore using different cut-points may have yielded different results.34 Given the large age range in our participants (23-84 years), we believe the use of these cut-points was justified, in lieu of population-specific (ie, older adults) cut-points. In addition, limitations to estimating activity from an accelerometer include the inability to capture certain activities such as swimming and cycling and the aforementioned inability to distinguish between body postures (ie, sitting vs standing).41 The participants were predominantly white, highly educated, and high earners (85.2% earned ≥$40,000 per year), therefore, the present results may not be generalizable to survivors from more diverse backgrounds. However, as far as we know, this is the first study to report the congruency of estimated ST, LPA, and MVPA across multiple measurement tools in a nationwide sample of breast cancer survivors who were heterogeneous in terms of disease characteristics (ie, stage, treatment, time since diagnosis).
Conclusions
Our findings suggest that physical activity and ST estimates in breast cancer survivors may be dependent on the measurement tool used. In addition, congruency of measurement tools was dependent on activity intensity of interest, and participant age, race, and disease history may also influence these factors. Therefore, researchers should consider the intended outcomes of interest, the context in which the tool is being used (ie, clinical versus research), the available resources, and the participant population before they select a measurement tool for estimating physical activity and sitting time in breast cancer survivors.
Acknowledgment
This work was supported by grant #F31AG034025 from the National Institute on Aging (Dr Phillips); Shahid and Ann Carlson Khan endowed professorship and grant #AG020118 from the National Institute on Aging (Dr McAuley). Dr Phillips is supported by the National Cancer Institute #K07CA196840, and Dr Welch is supported by National Institute of Health/National Cancer Institute training grant CA193193. All data for this study were collected at the University of Illinois Urbana Champaign.
Physical activity has numerous physical, mental, and psychosocial benefits for cancer survivors, such as a reduction in the risk of mobility disability, depression, and anxiety, and improved patient quality of life.1,2 In addition, higher levels of physical activity are associated with reduced cancer-specific and all-causes mortality as well as cancer-specific outcomes including reduced risk of cancer progression and recurrence and new primary cancers.3-5 However, fewer than one-third of cancer survivors are meeting government and cancer-specific recommendations of 150 minutes a week of moderate to vigorous physical activity (MPVA; ≥3 metabolic equivalents [METs]).6,7 Growing evidence also demonstrates a significant association between higher levels of sedentary behavior and many deleterious health effects after cancer, including an increased risk for decreased physical functioning and development of other chronic diseases such as cardiovascular disease or diabetes.8 Distinct from physical activity, sedentary behavior is defined as any waking activity resulting in low levels of energy expenditure (≤1.5 METs) while in a seated or reclined position.9 Increased sedentary behavior, even when controlling for moderate and vigorous physical activity (MVPA), is associated with poor quality of life and increased all-cause mortality in cancer survivors.10,11 Given the associations observed between higher levels of physical activity, lower levels of sedentary behavior, and improved health and disease outcomes among the large and increasing number of cancer survivors in the United States, it is important to identify low-cost methods that can be used in a in a variety of settings (ie, research, clinical, community) to accurately and efficiently measure survivors’ lifestyle behaviors to identify high-risk survivors for early intervention, better understand the effects of these behaviors on survivors’ health outcomes and disease trajectories, and ultimately, improve survivors’ health and quality of life.12,13
Two methods commonly used to capture physical activity and sedentary behavior across the lifespan are accelerometry (Actigraph, Pensacola, FL) and self-report questionnaires such as the Godin Leisure-Time Questionnaire (GLTEQ), International Physical Activity Questionnaire (IPAQ), and Sitting Time Questionnaire (STQ).14-17 Each method has unique strengths and weaknesses. Sending accelerometers to multiple individuals at a single time point can be costly, particularly in large-scale epidemiological studies, and the accelerometer’s waist-worn, nonwaterproof design may prevent researchers from capturing certain activities such as swimming and resistance training. However, the accelerometer provides objective, precise assessments of most physical activities and may help remove response bias.18 Conversely, self-report questionnaires rely solely on individuals’ memories and often result in recall bias, inaccurate reporting, and under- or overestimation of physical activity engagement.19,20 Nevertheless, these questionnaires can be widely disseminated at low cost in a variety of settings (eg, clinical, research, community) and are less of a burden to participants.
Recent studies comparing objective (eg, accelerometer) with subjective (eg, self-report) methods of measuring physical activity and sedentary behavior in healthy middle-aged adults and older adults have demonstrated mixed findings with no distinct trends in the degree to which these methods differ.19,21,22 To date, little consideration has been given to the measurement of these lifestyle behaviors in cancer survivors. Boyle and colleagues recently investigated the concurrent validity of an accelerometer to the GLTEQ in colon cancer survivors, finding significant differences in estimated MVPA (~11 minutes). However, no studies, to our knowledge, have compared accelerometer and self-report measures in breast cancer survivors, so it remains unclear how these different measurement tools relate to each another in this population.
It is particularly important to compare these measurement tools among breast cancer survivors because evidence indicates this population’s behavioral habits, self-perceived activity, and sitting time and movement patterns may differ significantly from the general population and other survivor groups across the lifespan.23,24 Further, previous studies examining these behaviors in cancer survivors focused primarily on sitting time and MVPA.15,25,26 Examining other lower-intensity intensities (eg, light activity or lifestyle) in cancer survivors may also be important given that increased levels of activity are associated with health benefits, ranging from reduced disability and fatigue to improved cardiovascular health and quality of life, and that breast cancer survivors engage in fewer of these activities compared with noncancer controls.23 These lower levels of physical activity may be more prevalent among cancer survivors of their high levels of fatigue and propensity toward increased sitting time during the first year of treatment,11 so it is important to be able to accurately assess these activities in this population. The purpose of the present study was to compare estimates of time spent in light physical activity (LPA), MVPA, and sitting time (ST) obtained from an accelerometer and 3 self-report measurement tools (GLTEQ, IPAQ, STQ) in a large, US-based sample of breast cancer survivors. A secondary purpose was to determine whether estimate comparisons among measurements changed by participant characteristics.
Methods
Participants and procedures
This study consisted of a subsample of women who participated in a larger study whose findings have been reported elsewhere by Phillips and McAuley.27 In that study, breast cancer survivors (n = 1,631) were recruited nationally to participate in a 6-month prospective study on quality of life. Eligibility criteria included being aged 18 years or older, having had a diagnosis of breast cancer, being English speaking, and having access to the internet. Once consented to participate in the study, 500 women were randomly selected to wear the accelerometer.
Participants in this group were mailed an accelerometer, an activity log, instructions for use, and a self-addressed stamped envelope to return the monitor. They were asked to wear the accelerometer during all waking hours for 7 consecutive days of usual activity. They were also sent a secure link to complete 3 activity questionnaires online. The questionnaires were to be completed by the end of the 7-day monitoring period. Only women with 3 or more valid days of accelerometer data and complete data on variables of interest (n = 414) were included in the present analyses. All of the participants consented to the study procedures approved by the University of Illinois Institutional Review Board.
Measures
Demographics. The participants self-reported their age, level of education, height, and weight. Their body mass index (BMI; kg/m2) was estimated using the standard equation. They also self-reported their health and cancer history, detailing breast cancer disease stage, time since diagnosis, treatment type, and whether they had had a cancer recurrence. They were also asked to report whether they had ever been diagnosed (Yes/No) with 18 chronic conditions (eg, diabetes, arthritis).
Godin Leisure-Time Exercise Questionnaire.16 The GLTEQ assessed participants’ weekly frequency and mean amount of time performing MVPA (moderate exercise, such as fast walking, combined with vigorous exercise, such as jogging), and LPA (light/mild exercise, eg, easy walking) during the previous 7 days. The mean daily duration (in minutes) for each intensity category (MVPA, LPA) was calculated using activity frequencies and the amount of time spent in each activity presented as minutes/day.
The International Physical Activity Questionnaire.14 The IPAQ evaluated participants’ physical activity of at least moderate intensity in 4 domains of everyday life: job-related physical activity, transportation, housework/caring for family, and leisure-time activity. Within each domain, participants were asked the number of days per week and time per day (hours and minutes) spent performing MVPA. To estimate sitting time, the questionnaire asks participants to report the total amount of time spent sitting per day in 2 conditions, during weekdays and during weekends. The present analysis averaged sitting time for a typical 7-day (5 week days, 2 weekend days) period. We multiplied reported minutes per day and frequency per week of each activity category (MVPA and ST) to calculate the mean number of minutes per day.29,30
Sitting Time Questionnaire.17,28 The STQ estimated the mean time (hours and minutes) participants spent sitting each day on weekdays and at weekends within 5 domains: while traveling to and from places, at work, watching television, using a computer at home, and at leisure, not including watching television (eg, visiting friends, movies, dining out). Mean minutes per day of ST were calculated using all sitting domains.
Actigraph accelerometer (model GT1M, Health One Technology, Fort Walton Beach, FL). The Actigraph GT1M is a reliable and objective measure of physical activity.31-33 Participants wore the monitor on the right hip for 7 consecutive days during all waking hours, except when bathing or swimming. Activity data was analyzed in 1-minute intervals. A valid day of accelerometer wear time was defined as ≥600 minutes with no more than 60 minutes of consecutive zero-values, with allowance of 2 minutes or fewer of observations <100 counts/minute within the nonwear interval.34 Each minute of wear time was classified according to intensity (counts/min) using the following cut-points:34 sedentary, <100 counts/min; LPA, 100-2,019 counts/min; and MVPA, ≥2,020 count/min. Mean daily durations (min/day) spent in each behavior were estimated by dividing the number of minutes in each category by the number of valid days.
Statistical analysis
All statistical analyses were completed in SPSS Statistics 23 (IBM, Chicago, IL). Descriptive statistics were used to define participant characteristics. Rank-order correlation between the methods was assessed using Spearman’s rho (rs) and results were interpreted as follows: rs = 0.10, small; 0.30, moderate; and 0.50, strong.35 Within each activity intensity group, we jointly modeled daily minutes of self-report and accelerometer data using a random-intercept mixed-effects regression model. Differences between measurement tools were assessed based on regression coefficients with accelerometer as the reference category. Finally, we did a post hoc analysis of leisure-time–only MVPA from the IPAQ to compare with other estimates of MVPA.
We calculated the measurement tool difference scores for each estimated intensity category (ST, LPA, MVPA), that is, accelerometer estimated ST minus STQ estimated ST, and GLTEQ estimated MVPA minus IPAQ estimated MVPA. We used these data in an exploratory analysis to examine whether there were statistically significant differences between measurement difference scores by demographic or disease characteristics using linear regression stratified analyses. For example, we were interested in whether there was a significant difference in measurement tool estimates for sitting time in older compared with younger survivors. Analyses were stratified by age (<60/≥60 years), body mass index (<25 kg/m2/≥25 kg/m2), race (white/people of color), disease stage (I and II/III and IV), years since diagnosis (≤5 years/>5 years), recurrence (Yes/No), received chemotherapy (Yes/No ), received radiation (Yes/No ), and the presence of 1 or more chronic diseases (Yes/No ).
Results
Participants
The mean age of the participants was 56.8 years [9.2], they were overweight (BMI, 26.2 kg/m2 [5.4]), and predominantly white (96.7%; Table 1). Table 2 provides a summary of mean daily duration of activity estimates for ST, LPA, and MVPA and the estimate mean difference scores between measurements.
Moderate and vigorous physical activity
Accelerometer−GLTEQ. The mean difference in MVPA estimates between the accelerometer and GLTEQ was less than 5 minutes (Maccelerometer = 20.2 minutes; MGLTEQ = 23.6 minutes), even though the difference was statistically significant (P = .02). Estimates of MVPA from the accelerometer and GLTEQ (rs = 0.564, P < .001) showed a strong relationship. Stratified analyses showed that the difference scores between the GLTEQ and accelerometer were lower for older survivors (≥60 years) compared with younger survivors such that older survivors reported significantly less time in MVPA on the GLTEQ compared with accelerometer estimates (difference score [D] = 6.8 minutes less, P = .001).
Accelerometer−IPAQ. The accelerometer estimated significantly fewer minutes of MVPA per day when compared with the IPAQ (Mdiff = -67.4; 95% confidence interval [CI], -78.6, -55.8; P < .001). Estimates of MVPA from the accelerometer and IPAQ (rs = 0.011, P = .680) were poorly related. Differences between the IPAQ and accelerometer were greater for later-stage breast cancer, compared with early-stage diagnoses such that participants with late-stage disease reported significantly less MVPA on the IPAQ compared with accelerometer estimates (D = 41.8 minutes less than early-stage disease, P = .018). Finally, participants of color reported a greater difference in MVPA between the accelerometer and the IPAQ than did their white counterparts (D = 47.5 minutes, P = .033).
GLTEQ−IPAQ. GLTEQ estimated significantly fewer minutes of MVPA per day compared with the IPAQ (Mdiff = -64.6; 95% CI, -76.6, -52.5; P < .001). The estimates of MVPA from the GLTEQ had a small correlation with IPAQ estimates (rs = 0.128, P = .011).
IPAQ estimates showed almost triple the MVPA minutes per day as were estimated by the accelerometer and GLTEQ. As the MVPA estimate for the IPAQ include nonleisure activities, we conducted a post hoc analyses that only included the leisure-time items from the IPAQ. Leisure-time only IPAQ items, estimates indicated survivors spent a mean 18.5 [SD, 14.2] min/day in MVPA. Although the magnitude of the difference between the accelerometer and GLTEQ estimates (~10 minutes) was much smaller using the leisure-time only IPAQ items, a repeated measures analysis of variance revealed there was still a significant difference between these estimates (P < .05 for both) and negligible correlation.
Light intensity physical activity
Accelerometer−GLTEQ. There was a large and significant difference between LPA estimates from the GLTEQ and accelerometer (Mdiff = 224.5; 95% CI, 218.2, 230.7; P < .001) with estimates from the accelerometer being higher than those for the GLTEQ. Additionally, the measurements showed a negligible correlation (rs = 0.004, P = .94). Difference scores for GLTEQ and accelerometer estimated LPA were significantly different by age, with survivors aged 60 years or older demonstrating a difference that was 18.3 minutes shorter (P = .005) than the difference in younger survivors (<60 years).
Sitting time
Accelerometer−IPAQ. Mean IPAQ estimates were significantly lower (M = 303.8 [63.4]) than accelerometer estimates (M = 603.9 [78.0]). Rank-order correlations between IPAQ and accelerometer estimated ST was small (rs =0.26, P < .001). Difference scores between IPAQ and accelerometer estimates were significantly greater for survivors who were 60 years or older, compared with those younger than 60 years (D = 47.6 minutes, P = .006), indicating that older survivors tended to self-report significantly more ST than estimated by the accelerometer.
Accelerometer−STQ. There was no significant difference in estimated mean ST minutes per day between the STQ and the accelerometer, but the correlation between estimates was low (rs = 0.30, P < .001). Stratified analyses revealed estimates for the difference scores for mean daily ST between the STQ and accelerometer were greater for participants who were diagnosed with later-stage breast cancer (D= -158.3 minutes, P < .001) and those who had received chemotherapy (D= -61.7 minutes, P = .028; Table 2) than for those who were diagnosed with early-stage breast cancer or had not received chemotherapy. Women who had later-stage disease reported significantly less ST than did women diagnosed with early-stage disease, when compared with estimates by the accelerometer.
IPAQ−STQ. The estimated mean ST was significantly lower for IPAQ (M = 303.8 minutes [163.4]) than for the STQ (M = 605.2 minutes [296.2]). There were no significant estimate differences among the stratified groups.
Discussion
The purpose of the present study was to compare 4 measurement tools, an accelerometer-based activity monitor and 3 self-report questionnaires, to estimate ST, LPA, and MVPA in breast cancer survivors. Developing and evaluating accurate and precise measurement tools to assess physical activity and ST in breast cancer survivors remains a critical step toward better understanding the role of physical activity in cancer survivorship. Our results indicate that the congruency of the measurement tools examined was highly dependent on the activity intensity of interest and participants’ demographic or disease characteristics. Overall, the accelerometer estimated a greater amount of time spent sitting and engaging in LPA and less time in MVPA than was estimated on the STQ, GLTEQ, and IPAQ. In addition, our findings suggest significant subgroup differences that will be important in future development and implementation of physical activity measurement for breast cancer survivors.
MVPA has been the most commonly measured activity intensity among cancer survivors to date.15,25,26 The present results indicate mean daily MVPA estimates were significantly higher for the GLTEQ compared with the accelerometer (Mdiff = 2.8 min/d, P = .019), although the magnitude of these differences was relatively small. This difference is lower than in another study that compared these measures in colon cancer survivors and found the GLTEQ over-estimated MVPA by 10.6 min/day compared with the accelerometer (P < .01).15 However, the correlation between the 2 tools in our study was similar to that of Boyle and colleagues (rs = 0.56 and rs = 0.51, respectively). A possible explanation for the equivocal findings across these studies may lie in the difference in study sample demographics; a previous study results finding breast cancer survivors may be better at recalling their physical activities because they may be more attentive to activities they perform daily.26
The IPAQ significantly estimated more than an hour more of MVPA minutes per day compared with the accelerometer and GLTEQ. There are a number of limitations to the reporting of MVPA on the IPAQ. These limitations have been previously reported in the literature and include cross-cultural differences as well as overreporting of nonleisure-time MVPA (eg, occupational or household activities). However, the IPAQ has consistently been shown to be a valid and reliable tool for physical activity surveillance in different populations across the world.29,36,37 This shows that although MVPA was overestimated in our population, we do not mean to undermine the IPAQ value in other populations in which it has shown great utility for overall physical activity surveillance. When we excluded nonleisure-time MVPA, MVPA equated to about 18 min/day, which was closer in magnitude to the GLTEQ and accelerometer. These data highlight the importance of identifying the specific activity parameters of interest when selecting a measurement tool to ensure congruency between the tool and construct of interest.
The differences in MVPA estimation from the 3 tools have significant translational consequences, notably the potential for misclassification of meeting physical activity guidelines. For example, the percentage of women in the present sample that met physical activity guidelines ranged from 0% (using the accelerometer) to 19.5% (using the IPAQ), depending on the measurement tool used. These findings have meaningful implications for future physical activity assessment because multiple measurement tools are cur
For example, scores from the IPAQ may result in a survivor being classified as meeting physical activity guidelines when in fact they are not, and thereby missing the opportunity for intervention; or the accelerometer may classify an active survivor as inactive, which could result in using time and resources for a behavior change intervention that is not necessary. The clinical significance of these findings is to provide providers with data-based information on the strengths and limitations of the measurement tools so that they can accurately estimate physical activity and ST and appropriately optimize resources and treatments.
The degree of measurement tool congruence is likely influenced by a number of factors. First, survivors’ perceptions of the intensity of their activity are relative and subjective to their state of feeling during the activity. For example, breast cancer survivors with lower functional capacity may perceive activities with lower absolute intensity as having a higher relative intensity (ie, they think they are working at a moderate intensity so record an activity as such, but the activity is classified as light by the accelerometer). Second, although our self-report measures asked survivors to record the time they had spent active over the previous 7 days, survivors might report on what they consider a “usual” week, which may reflect the ideal rather than the reality. Third, the accelerometer cut-points used were derived from young, healthy adults on a treadmill. Thus, generalization to an older, sick, less active population that could be experiencing treatment-related side effects could lead to underestimation of time spent in MVPA. To better understand measurement congruency in breast cancer survivors, future research should investigate how functional capacity and activity intensity perceptions are influenced by a breast cancer diagnosis and how those factors may influence subjective and objective physical activity measurement. If those factors were found to have significant influence on activity in breast cancer survivors, it would warrant future development of breast-cancer–specific accelerometer reduction techniques.
The comparison of LPA presented another interesting significant contrast between self-report (GLTEQ) and accelerometry. Results indicated the GLTEQ underestimated LPA by 224.5 [3.2] min/day compared with the accelerometer. This equates to over 3.5 h/day of active time (or about 280 kcal/day) that was potentially unaccounted for by the GLTEQ. The difference between these estimates could be due to the fact that the GLTEQ was designed to measure exercise time and therefore may not be as sensitive as the accelerometer to nonexercise-related LPA. Light intensity activities typically span a large range of domains (ie, occupational, leisure time, household) and tend to occur in higher volumes than MVPA, which may lead to some challenges with recall. Expanding existing LPA questionnaires to encompass these domains would likely provide increased congruency between self-reported and accelerometer-derived estimates for LPA, as it may provide a better trigger for recalling these high volume activities. With increasing literature advocating the important role of LPA in adults’ health in concert with data suggesting survivors may engage in lower levels of LPA than healthy controls,23, accurately accounting for these lower intensity activities to provide a “whole picture” of a survivor’s active day remains an important future research direction. Combining accelerometer and self-report data using ecological momentary assessment to capture these behaviors in real-time in the real world could provide a better understanding of the context in which LPA occurs as well as survivors’ perceptions of intensity to build more accurate and scalable measurement tools for LPA.
Our ST results indicate nonsignificant difference estimates from the accelerometer and the STQ (Mdiff = 1.3 [15.3] min/day) with slightly higher estimates for the STQ versus accelerometer. This finding is consistent with the one other study that has examined these relationships in cancer survivors.15 However, our findings also indicate the IPAQ significantly underestimated ST compared with the accelerometer and the STQ by about half (Table 1). These differences may be because both the STQ and Marshall questionnaire used in the previous study measure multiple domains of sitting (ie, computer, television, travel) on both weekdays and weekends whereas the IPAQ uses only two recall items of overall sitting time (for weekday and weekend separately). The domain-specific, structured approach has been shown to improve recall and may help to prevent underestimation and general underreporting of the high volume, ubiquitous behavior of sitting.17,38 Finally, we would be remiss to not acknowledge the known limitations to estimating ST using the count-based approach on the waist-worn accelerometer. Due to the monitor’s orientation at the hip, the accelerometer may misrepresent total ST by misclassifying standing still as sitting. However, Kozey-Keadle and colleagues have previously examined estimation of ST using waist-worn accelerometers and have shown the 100 count per minute cut off yields ST estimates within 5% range of accuracy for a seated position compared with direct observation.39
Of further interest are our exploratory results indicating that age and disease stage may modify the congruency between activity and ST measures. Specifically, older survivors and those with more advanced disease stage generally reported more PA and less ST than were measured by the accelerometer. These differences raise the question of whether these subgroups are systematically reporting more time physically active, overestimating their intensity, or the accelerometer is misclassifying their activity intensity. These misclassifications could be due to their age, disease stage, fatigue status, functional status, cognitive function, occupational status, etc. and would be important next steps for exploration of measurement of physical activity in breast cancer survivors. Finally, the difference score for MVPA was greater for survivors of color than for white survivors, with survivors of color overreporting MVPA compared with accelerometer-derived estimates. This may be due in part to cultural differences between white survivors and survivors of color. Previous research has suggested that people of color may accumulate a majority of their activity in occupational or household-related domains, thus explaining lower levels of leisure-time MVPA but high levels of reported total MVPA from other nonleisure domains.20 However, given the small number of survivors of color in the present study, these results should be interpreted with caution.
With the multitude of physical activity and ST measurement tools available, many factors including cost, sample size, primary outcome of interest, and activity characteristics of interest (eg, duration, intensity, energy expenditure) need to be considered40 when choosing a tool. Our findings may help inform these decisions for breast cancer survivors. For example, if LPA is of interest, an accelerometer may provide a more comprehensive assessment of these activities than the GLTEQ. In contrast, if MVPA is the activity of interest, our results suggest the GLTEQ and accelerometer were more congruent than the IPAQ was with either measure, therefore, if budgetary constraints are a concern, the more cost-efficient GLTEQ could provide similar results to an accelerometer. In addition to considering measurement congruency, it is also critically important to carefully consider the population (breast cancer survivors) and subsequent burden that accompanies the measurement tool of choice. Overall, our results indicate, when choosing a questionnaire for ST or LPA for breast cancer survivors, the more comprehensive the questions, to encompass multiple domains or time of day, the greater amount of time that will be captured within that activity category. Conversely, since the majority of MVPA is completed in leisure-time, dependent on the age and race of the population, a shorter questionnaire may be sufficient. Additionally, dependent on time since diagnosis and treatment received, activity recall or body movement patterns may be affected which could influence measurement tool selection.23,24 Finally, it is also important to consider the setting in which measurement is taking place. In busy clinical settings, shorter, self-report measures may have a greater chance of being implemented than accelerometers or longer self-report measures and would still provide useful information regarding an overall snapshot of survivors’ MVPA or ST that could be used to initiate a conversation or referral for a program to help survivors positively change one or both of these behaviors.
Limitations
There were a few limitations within the current study that should be taken into account. First, the accelerometer cut-points used were developed with healthy, young adults; therefore using different cut-points may have yielded different results.34 Given the large age range in our participants (23-84 years), we believe the use of these cut-points was justified, in lieu of population-specific (ie, older adults) cut-points. In addition, limitations to estimating activity from an accelerometer include the inability to capture certain activities such as swimming and cycling and the aforementioned inability to distinguish between body postures (ie, sitting vs standing).41 The participants were predominantly white, highly educated, and high earners (85.2% earned ≥$40,000 per year), therefore, the present results may not be generalizable to survivors from more diverse backgrounds. However, as far as we know, this is the first study to report the congruency of estimated ST, LPA, and MVPA across multiple measurement tools in a nationwide sample of breast cancer survivors who were heterogeneous in terms of disease characteristics (ie, stage, treatment, time since diagnosis).
Conclusions
Our findings suggest that physical activity and ST estimates in breast cancer survivors may be dependent on the measurement tool used. In addition, congruency of measurement tools was dependent on activity intensity of interest, and participant age, race, and disease history may also influence these factors. Therefore, researchers should consider the intended outcomes of interest, the context in which the tool is being used (ie, clinical versus research), the available resources, and the participant population before they select a measurement tool for estimating physical activity and sitting time in breast cancer survivors.
Acknowledgment
This work was supported by grant #F31AG034025 from the National Institute on Aging (Dr Phillips); Shahid and Ann Carlson Khan endowed professorship and grant #AG020118 from the National Institute on Aging (Dr McAuley). Dr Phillips is supported by the National Cancer Institute #K07CA196840, and Dr Welch is supported by National Institute of Health/National Cancer Institute training grant CA193193. All data for this study were collected at the University of Illinois Urbana Champaign.
1. Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):87-100.
2. Brenner DR. Cancer incidence due to excess body weight and leisure-time physical inactivity in Canada: implications for prevention. Prev Med. 2014;66:131-139.
3. Courneya KS, Friedenreich CM. Relationship between exercise pattern across the cancer experience and current quality of life in colorectal cancer survivors. J Altern Complement Med. 1997;3(3):215-226.
4. Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol. 2011;28(3):753-765.
5. Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity, risk of death and recurrence in breast cancer survivors: a systematic review and meta-analysis of epidemiological studies. Acta Oncol. 2015;54(5):635-654.
6. Irwin ML, McTiernan A, Bernstein L, et al. Physical activity levels among breast cancer survivors. Med Sci Sports Exerc. 2004;36(9):1484-1491.
7. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409-1426.
8. Lynch BM. Sedentary behavior and cancer: a systematic review of the literature and proposed biological mechanisms. Cancer Epidemiol Biomarkers Prev. 2010;19(11):2691-2709.
9. Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev. 2010;38(3):105-113.
10. Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM. Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. J Clin Oncol. 2013;31(7):876-885.
11. Lynch BM, Dunstan DW, Vallance JK, Owen N. Don’t take cancer sitting down: a new survivorship research agenda. Cancer. 2013;119(11):1928-1935.
12. Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the ‘silver tsunami:’ prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029-1036.
13. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271-289.
14. Booth M. Assessment of physical activity: an international perspective. Res Q Exerc Sport. 2000;71(2 suppl):S114-120.
15. Boyle T, Lynch BM, Courneya KS, Vallance JK. Agreement between accelerometer-assessed and self-reported physical activity and sedentary time in colon cancer survivors. Support Care Cancer. 2015;23(4):1121-1126.
16. Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Canadian journal of applied sport sciences. Can J Appl Sport Sci. 1985;10(3):141-146.
17. Marshall AL, Miller YD, Burton NW, Brown WJ. Measuring total and domain-specific sitting: a study of reliability and validity. Med Sci Sports Exerc. 2010;42(6):1094-1102.
18. Matthews CE, Hagstromer M, Pober DM, Bowles HR. Best practices for using physical activity monitors in population-based research. Med Sci Sports Exerc. 2012;44(1 Suppl 1):S68-76.
19. Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56.
20. Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport. 2000;71(2 Suppl):S1-14.
21. Hart TL, Swartz AM, Cashin SE, Strath SJ. How many days of monitoring predict physical activity and sedentary behaviour in older adults? Int J Behav Nutr Phys Act. 2011;8:62.
22. Hart TL, Ainsworth BE, Tudor-Locke C. Objective and subjective measures of sedentary behavior and physical activity. Med Sci Sports Exerc. 2011;43(3):449-456.
23. Phillips SM, Dodd KW, Steeves J, McClain J, Alfano CM, McAuley E. Physical activity and sedentary behavior in breast cancer survivors: new insight into activity patterns and potential intervention targets. Gynecol Oncol. 2015;138(2):398-404.
24. Boyle T, Vallance JK, Ransom EK, Lynch BM. How sedentary and physically active are breast cancer survivors, and which population subgroups have higher or lower levels of these behaviors? Support Care Cancer. 2016;24(5):2181-2190.
25. Broderick JM, Guinan E, Kennedy MJ, et al. Feasibility and efficacy of a supervised exercise intervention in de-conditioned cancer survivors during the early survivorship phase: the PEACH trial. J Cancer Surviv. 2013;7(4):551-562.
26. Su CC, Lee KD, Yeh CH, Kao CC, Lin CC. Measurement of physical activity in cancer survivors: a validity study. J Cancer Surviv. 2014;8(2):205-212.
27. Phillips SM, McAuley E. Social cognitive influences on physical activity participation in long-term breast cancer survivors. Psychooncology. 2013;22(4):783-791.
28. Wojcicki TR, White SM, McAuley E. Assessing outcome expectations in older adults: the multidimensional outcome expectations for exercise scale. J Gerontol B Psychol Sci Soc Sci. 2009;64(1):33-40.
29. Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381-1395.
30. Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498-504.
31. Hacker ED, Ferrans CE. Ecological momentary assessment of fatigue in patients receiving intensive cancer therapy. J Pain Symptom Manage. 2007;33(3):267-275.
32. Swartz AM, Strath SJ, Bassett DR, Jr, O’Brien WL, King GA, Ainsworth BE. Estimation of energy expenditure using CSA accelerometers at hip and wrist sites. Med Sci Sports Exerc. 2000;32(9 Suppl):S450-456.
33. Jim HS, Small B, Faul LA, Franzen J, Apte S, Jacobsen PB. Fatigue, depression, sleep, and activity during chemotherapy: daily and intraday variation and relationships among symptom changes. Ann Behav Med. 2011;42(3):321-333.
34. Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181-188.
35. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: L Erlbaum Associates; 1988.
36. Bauman A, Ainsworth BE, Bull F, et al. Progress and pitfalls in the use of the International Physical Activity Questionnaire (IPAQ) for adult physical activity surveillance. J Phys Act Health. 2009;6 Suppl 1:S5-8.
37. Hagströmer M1, Oja P, Sjöström M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr. 2006;9(6):755-762.
38. Johnson-Kozlow M, Sallis JF, Gilpin EA, Rock CL, Pierce JP. Comparative validation of the IPAQ and the 7-Day PAR among women diagnosed with breast cancer. Int J Behav Nutr Phys Act. 2006;3:7.
39. Kozey-Keadle S, Libertine A, Lyden K, Staudenmayer J, Freedson PS. Validation of wearable monitors for assessing sedentary behavior. Med Sci Sports Exerc. 2011;43(8):1561-1567.
40. Strath SJ, Kaminsky LA, Ainsworth BE, et al. Guide to the assessment of physical activity: clinical and research applications. Circulation. 2013;128(20):2259-2279.
41. Bassett DR. Device-based monitoring in physical activity and public health research. Physiol Meas. 2012;33(11):1769-1783.
1. Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4(2):87-100.
2. Brenner DR. Cancer incidence due to excess body weight and leisure-time physical inactivity in Canada: implications for prevention. Prev Med. 2014;66:131-139.
3. Courneya KS, Friedenreich CM. Relationship between exercise pattern across the cancer experience and current quality of life in colorectal cancer survivors. J Altern Complement Med. 1997;3(3):215-226.
4. Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol. 2011;28(3):753-765.
5. Lahart IM, Metsios GS, Nevill AM, Carmichael AR. Physical activity, risk of death and recurrence in breast cancer survivors: a systematic review and meta-analysis of epidemiological studies. Acta Oncol. 2015;54(5):635-654.
6. Irwin ML, McTiernan A, Bernstein L, et al. Physical activity levels among breast cancer survivors. Med Sci Sports Exerc. 2004;36(9):1484-1491.
7. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409-1426.
8. Lynch BM. Sedentary behavior and cancer: a systematic review of the literature and proposed biological mechanisms. Cancer Epidemiol Biomarkers Prev. 2010;19(11):2691-2709.
9. Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev. 2010;38(3):105-113.
10. Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM. Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. J Clin Oncol. 2013;31(7):876-885.
11. Lynch BM, Dunstan DW, Vallance JK, Owen N. Don’t take cancer sitting down: a new survivorship research agenda. Cancer. 2013;119(11):1928-1935.
12. Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the ‘silver tsunami:’ prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029-1036.
13. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271-289.
14. Booth M. Assessment of physical activity: an international perspective. Res Q Exerc Sport. 2000;71(2 suppl):S114-120.
15. Boyle T, Lynch BM, Courneya KS, Vallance JK. Agreement between accelerometer-assessed and self-reported physical activity and sedentary time in colon cancer survivors. Support Care Cancer. 2015;23(4):1121-1126.
16. Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Canadian journal of applied sport sciences. Can J Appl Sport Sci. 1985;10(3):141-146.
17. Marshall AL, Miller YD, Burton NW, Brown WJ. Measuring total and domain-specific sitting: a study of reliability and validity. Med Sci Sports Exerc. 2010;42(6):1094-1102.
18. Matthews CE, Hagstromer M, Pober DM, Bowles HR. Best practices for using physical activity monitors in population-based research. Med Sci Sports Exerc. 2012;44(1 Suppl 1):S68-76.
19. Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56.
20. Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport. 2000;71(2 Suppl):S1-14.
21. Hart TL, Swartz AM, Cashin SE, Strath SJ. How many days of monitoring predict physical activity and sedentary behaviour in older adults? Int J Behav Nutr Phys Act. 2011;8:62.
22. Hart TL, Ainsworth BE, Tudor-Locke C. Objective and subjective measures of sedentary behavior and physical activity. Med Sci Sports Exerc. 2011;43(3):449-456.
23. Phillips SM, Dodd KW, Steeves J, McClain J, Alfano CM, McAuley E. Physical activity and sedentary behavior in breast cancer survivors: new insight into activity patterns and potential intervention targets. Gynecol Oncol. 2015;138(2):398-404.
24. Boyle T, Vallance JK, Ransom EK, Lynch BM. How sedentary and physically active are breast cancer survivors, and which population subgroups have higher or lower levels of these behaviors? Support Care Cancer. 2016;24(5):2181-2190.
25. Broderick JM, Guinan E, Kennedy MJ, et al. Feasibility and efficacy of a supervised exercise intervention in de-conditioned cancer survivors during the early survivorship phase: the PEACH trial. J Cancer Surviv. 2013;7(4):551-562.
26. Su CC, Lee KD, Yeh CH, Kao CC, Lin CC. Measurement of physical activity in cancer survivors: a validity study. J Cancer Surviv. 2014;8(2):205-212.
27. Phillips SM, McAuley E. Social cognitive influences on physical activity participation in long-term breast cancer survivors. Psychooncology. 2013;22(4):783-791.
28. Wojcicki TR, White SM, McAuley E. Assessing outcome expectations in older adults: the multidimensional outcome expectations for exercise scale. J Gerontol B Psychol Sci Soc Sci. 2009;64(1):33-40.
29. Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381-1395.
30. Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498-504.
31. Hacker ED, Ferrans CE. Ecological momentary assessment of fatigue in patients receiving intensive cancer therapy. J Pain Symptom Manage. 2007;33(3):267-275.
32. Swartz AM, Strath SJ, Bassett DR, Jr, O’Brien WL, King GA, Ainsworth BE. Estimation of energy expenditure using CSA accelerometers at hip and wrist sites. Med Sci Sports Exerc. 2000;32(9 Suppl):S450-456.
33. Jim HS, Small B, Faul LA, Franzen J, Apte S, Jacobsen PB. Fatigue, depression, sleep, and activity during chemotherapy: daily and intraday variation and relationships among symptom changes. Ann Behav Med. 2011;42(3):321-333.
34. Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181-188.
35. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: L Erlbaum Associates; 1988.
36. Bauman A, Ainsworth BE, Bull F, et al. Progress and pitfalls in the use of the International Physical Activity Questionnaire (IPAQ) for adult physical activity surveillance. J Phys Act Health. 2009;6 Suppl 1:S5-8.
37. Hagströmer M1, Oja P, Sjöström M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr. 2006;9(6):755-762.
38. Johnson-Kozlow M, Sallis JF, Gilpin EA, Rock CL, Pierce JP. Comparative validation of the IPAQ and the 7-Day PAR among women diagnosed with breast cancer. Int J Behav Nutr Phys Act. 2006;3:7.
39. Kozey-Keadle S, Libertine A, Lyden K, Staudenmayer J, Freedson PS. Validation of wearable monitors for assessing sedentary behavior. Med Sci Sports Exerc. 2011;43(8):1561-1567.
40. Strath SJ, Kaminsky LA, Ainsworth BE, et al. Guide to the assessment of physical activity: clinical and research applications. Circulation. 2013;128(20):2259-2279.
41. Bassett DR. Device-based monitoring in physical activity and public health research. Physiol Meas. 2012;33(11):1769-1783.