User login
Medical record documentation: What to do, and what to avoid
Medical record documentation serves as a reminder of previous discussions with patients and what happened during their visits, a reimbursement justification for services, a communication tool to coordinate care with current and future clinicians, and a basis for defense in legal or regulatory matters.1,2 Documentation should be thorough, accurate, timely, and objective, with the ultimate goal of communicating our thoughts in an easily understood manner to other clinicians or attorneys.2 If we fail to achieve this goal, we may inadvertently give the impression that our care was hurried, incomplete, or thoughtless.2
Although not an exhaustive list, this article outlines strategies to employ and practices to avoid in our documentation efforts so we may enhance our defense in case of litigation and ensure the smooth transition of care for our patients.
Strategies to employ
Proper and accurate documentation details the course of patient care, and we should describe our thoughts in a clear and logical manner. Doing so minimizes the risk of misinterpretation by other clinicians or attorneys. Make sure the documentation of each appointment details the reason(s) for the patient’s visit, the effectiveness of treatment, possible treatment nonadherence, our clinical assessment, treatment consent, changes to the patient’s treatment plan, follow-up plans, reasons for not pursuing certain actions (eg, hospitalization), and a suicide risk assessment (and/or a violence risk assessment, if clinically indicated).2 Document missed or rescheduled appointments, and telephone and electronic contact with patients. Also be sure to use only commonly approved abbreviations.2 Document these items sooner rather than later because doing so improves the credibility of your charting.1 If you are handwriting notes, add the date and time to each encounter and make sure your handwriting is legible. Describe the behaviors of patients in objective and nonjudgmental terms.3 Documenting quotes from patients can convey crucial information about what was considered when making clinical decisions.1
Practices to avoid
If there is a need to make changes to previous entries, ensure these corrections are not mistaken for alterations. Each health care institution has its own policy for making corrections and addenda to medical records. Corrections to a patient’s medical record are acceptable, provided they are done appropriately, as I outlined in a previous Pearls article.4 Minimize or eliminate the copying and pasting of information; doing so can improve the efficiency of our documentation, but the practice can undermine the quality of the medical record, increase the risk of outdated and repetitive information being included, lead to clinical errors, and lead to overbilling of services.5 Finally, be sure to avoid speculation, personal commentary about patients and their family members, and language with negative connotations (unless such language is a direct quote from the patient).2,3
1. Mossman D. Tips to make documentation easier, faster, and more satisfying. Current Psychiatry. 2008;7(2):80,84-86.
2. Staus C. Documentation: your very best defense. Psychiatric News. 2022;57(4):7,19.
3. Nelson KJ. How to use patient-centered language in documentation. Current Psychiatry. 2011;10(10):70.
4. Joshi KG. Metadata, malpractice claims, and making changes to the EHR. Current Psychiatry. 2021;20(3):e1-e3. doi:10.12788/cp.0106
5. Neal D. Do’s and don’ts of electronic documentation. Psychiatric News. 2021;56(8):7.
Medical record documentation serves as a reminder of previous discussions with patients and what happened during their visits, a reimbursement justification for services, a communication tool to coordinate care with current and future clinicians, and a basis for defense in legal or regulatory matters.1,2 Documentation should be thorough, accurate, timely, and objective, with the ultimate goal of communicating our thoughts in an easily understood manner to other clinicians or attorneys.2 If we fail to achieve this goal, we may inadvertently give the impression that our care was hurried, incomplete, or thoughtless.2
Although not an exhaustive list, this article outlines strategies to employ and practices to avoid in our documentation efforts so we may enhance our defense in case of litigation and ensure the smooth transition of care for our patients.
Strategies to employ
Proper and accurate documentation details the course of patient care, and we should describe our thoughts in a clear and logical manner. Doing so minimizes the risk of misinterpretation by other clinicians or attorneys. Make sure the documentation of each appointment details the reason(s) for the patient’s visit, the effectiveness of treatment, possible treatment nonadherence, our clinical assessment, treatment consent, changes to the patient’s treatment plan, follow-up plans, reasons for not pursuing certain actions (eg, hospitalization), and a suicide risk assessment (and/or a violence risk assessment, if clinically indicated).2 Document missed or rescheduled appointments, and telephone and electronic contact with patients. Also be sure to use only commonly approved abbreviations.2 Document these items sooner rather than later because doing so improves the credibility of your charting.1 If you are handwriting notes, add the date and time to each encounter and make sure your handwriting is legible. Describe the behaviors of patients in objective and nonjudgmental terms.3 Documenting quotes from patients can convey crucial information about what was considered when making clinical decisions.1
Practices to avoid
If there is a need to make changes to previous entries, ensure these corrections are not mistaken for alterations. Each health care institution has its own policy for making corrections and addenda to medical records. Corrections to a patient’s medical record are acceptable, provided they are done appropriately, as I outlined in a previous Pearls article.4 Minimize or eliminate the copying and pasting of information; doing so can improve the efficiency of our documentation, but the practice can undermine the quality of the medical record, increase the risk of outdated and repetitive information being included, lead to clinical errors, and lead to overbilling of services.5 Finally, be sure to avoid speculation, personal commentary about patients and their family members, and language with negative connotations (unless such language is a direct quote from the patient).2,3
Medical record documentation serves as a reminder of previous discussions with patients and what happened during their visits, a reimbursement justification for services, a communication tool to coordinate care with current and future clinicians, and a basis for defense in legal or regulatory matters.1,2 Documentation should be thorough, accurate, timely, and objective, with the ultimate goal of communicating our thoughts in an easily understood manner to other clinicians or attorneys.2 If we fail to achieve this goal, we may inadvertently give the impression that our care was hurried, incomplete, or thoughtless.2
Although not an exhaustive list, this article outlines strategies to employ and practices to avoid in our documentation efforts so we may enhance our defense in case of litigation and ensure the smooth transition of care for our patients.
Strategies to employ
Proper and accurate documentation details the course of patient care, and we should describe our thoughts in a clear and logical manner. Doing so minimizes the risk of misinterpretation by other clinicians or attorneys. Make sure the documentation of each appointment details the reason(s) for the patient’s visit, the effectiveness of treatment, possible treatment nonadherence, our clinical assessment, treatment consent, changes to the patient’s treatment plan, follow-up plans, reasons for not pursuing certain actions (eg, hospitalization), and a suicide risk assessment (and/or a violence risk assessment, if clinically indicated).2 Document missed or rescheduled appointments, and telephone and electronic contact with patients. Also be sure to use only commonly approved abbreviations.2 Document these items sooner rather than later because doing so improves the credibility of your charting.1 If you are handwriting notes, add the date and time to each encounter and make sure your handwriting is legible. Describe the behaviors of patients in objective and nonjudgmental terms.3 Documenting quotes from patients can convey crucial information about what was considered when making clinical decisions.1
Practices to avoid
If there is a need to make changes to previous entries, ensure these corrections are not mistaken for alterations. Each health care institution has its own policy for making corrections and addenda to medical records. Corrections to a patient’s medical record are acceptable, provided they are done appropriately, as I outlined in a previous Pearls article.4 Minimize or eliminate the copying and pasting of information; doing so can improve the efficiency of our documentation, but the practice can undermine the quality of the medical record, increase the risk of outdated and repetitive information being included, lead to clinical errors, and lead to overbilling of services.5 Finally, be sure to avoid speculation, personal commentary about patients and their family members, and language with negative connotations (unless such language is a direct quote from the patient).2,3
1. Mossman D. Tips to make documentation easier, faster, and more satisfying. Current Psychiatry. 2008;7(2):80,84-86.
2. Staus C. Documentation: your very best defense. Psychiatric News. 2022;57(4):7,19.
3. Nelson KJ. How to use patient-centered language in documentation. Current Psychiatry. 2011;10(10):70.
4. Joshi KG. Metadata, malpractice claims, and making changes to the EHR. Current Psychiatry. 2021;20(3):e1-e3. doi:10.12788/cp.0106
5. Neal D. Do’s and don’ts of electronic documentation. Psychiatric News. 2021;56(8):7.
1. Mossman D. Tips to make documentation easier, faster, and more satisfying. Current Psychiatry. 2008;7(2):80,84-86.
2. Staus C. Documentation: your very best defense. Psychiatric News. 2022;57(4):7,19.
3. Nelson KJ. How to use patient-centered language in documentation. Current Psychiatry. 2011;10(10):70.
4. Joshi KG. Metadata, malpractice claims, and making changes to the EHR. Current Psychiatry. 2021;20(3):e1-e3. doi:10.12788/cp.0106
5. Neal D. Do’s and don’ts of electronic documentation. Psychiatric News. 2021;56(8):7.
Lithium, valproate, and suicide risk: Analysis of 98,831 cases
The current academic psychiatry paradigm reinforces that lithium reduces suicide risk, more so than other medications, including valproate. However, data from multiple sources contradict this “evidence-based” belief.
Data do not support lithium’s supposed advantage
An 8-year prospective study in Sweden by Song et al1 tracked 51,535 patients with bipolar disorder from 2005 to 2013. In their conclusions, the authors of this study omitted some surprising numbers that contradict the dominant paradigm. There were 230 (1.089%) completed suicides in the lithium group (N = 21,129), 99 (1.177%) in the valproate group (N = 8,411), and 308 (1.195%) in the “other medication” group (N = 25,780). This difference of .088% is too small (95% CI, -.180% to .358%) to substantiate the purported advantage of lithium over valproate. More important is that in terms of suicide-related events, the medication group excluding lithium and valproate had 2,018 (7.8%) events vs lithium 2,142 (10.1%) and valproate 1,105 (13.1%). The difference of 2.3% is statistically significant (95% CI, 1.8% to 2.8%). These numbers reflect fewer suicide-related events with psychiatric medications other than lithium and valproate. Compounding the problem is a design flaw in which 3,785 patients were counted twice in the lithium and valproate groups (21,129 + 8,411 + 25,780 = 55,320, which is more than the 51,535 patients in the study). By falsely inflating the denominator (N) for the lithium and valproate groups, the respective published rates are deceptively lower than the actual rates. Song et al1 did not provide an adequate explanation for these findings and omitted them from their conclusions.
In Schatzberg’s Manual of Clinical Psychopharmacology, the authors cited Song et al1 but omitted these findings as well, and stated “lithium is clearly effective in preventing suicide attempts and completions in bipolar patients.”2 In Stahl’s Essential Psychopharmacology, the author wrote “lithium actually reduces suicide in patients with bipolar disorder.”3 In a review article,
In an overlapping period, National Poison Data System (NPDS) data of single substance exposures painted a different picture in the United States.6 During 2006-2013, the lithium group (N = 26,144) had 32 deaths (all causes) (.122%), and the valproate group (N = 25,630) had 16 deaths (.062%). During 2006-2020, the lithium group (N = 52,262) had 55 deaths (.105%), and the valproate group (N = 46,569) had 31 deaths (.067%). Clearly there is a major disconnect between lithium’s advertised ability to reduce suicide risk and the actual mortality rate, as evidenced by 98,831 cases reported to NPDS during 2006-2020. One would expect a lower rate in the lithium group, but data show it is higher than in the valproate group. This underscores the common fallacy of most lithium studies: each is based on a very small sample (N < 100), and the statistical inference about the entire population is tenuous. If lithium truly reduces suicide risk 5-fold, it would be seen in a sample of 98,831. The law of large numbers and central limit theorem state that as N increases, the variability of the rate progressively decreases. This can be easily demonstrated with computer simulation models and simple Python code, or on the average fuel economy display of most cars.
What about the relative lethality?
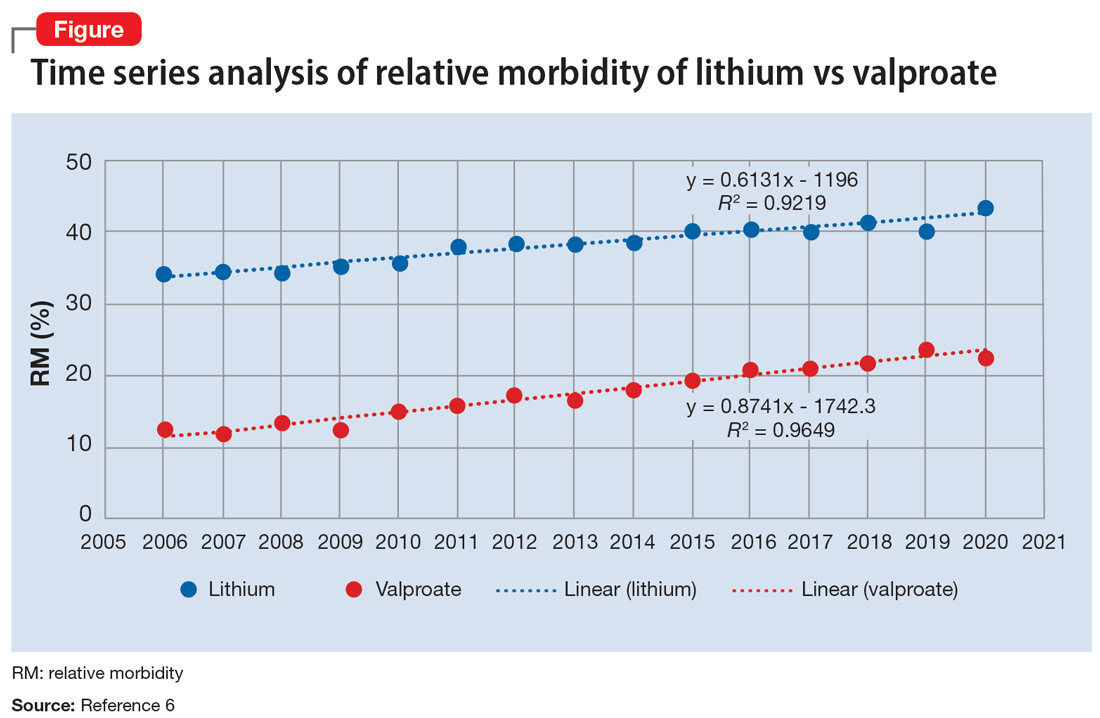
The APA Textbook of Suicide Risk Assessment and Management stated that it is important to consider the relative lethality (RL) of prescription medications.7 The RL equation (RL = 310x / LD50) represents the ratio of a 30-day supply of medication to the human equivalent LD50 for a 60-kg person (x is the daily dose and LD50 is the rat oral lethal dose 50).8 Time series analysis shows that the lithium relative morbidity (RM) is consistently double that of valproate (Figure6). Regression models have shown high correlation and causality between RL and RM.9-11 It is surprising that valproate (RL = 1,666%) has a lower RM than lithium (RL = 1,063%). This paradox can be easily explained with clinical insight. The RL equation compares medications at the maximum daily dose, but in routine practice valproate is commonly prescribed at 1,000 mg/d (28% of the maximum 3,600 mg/d). Lithium is commonly prescribed at 1,200 mg/d (67% of the maximum 1,800 mg/d). Within these dosing parameters, the effective RL is valproate 463% and lithium 709%. The 2020 RM is valproate 22% and lithium 43%.12 The COVID-19 pandemic did not affect the predicted RM. Confirming these numbers, Song et al1 acknowledged “greater safety in case of overdose for valproate in clinical practice.” Baldessarini et al4 asserted “the fatality risk of lithium overdose is only moderate, and very similar to modern antidepressants and second-generation antipsychotics.”4 This claim is contradicted by the RL equation and regression models.7-11 Lithium’s RL is 19 times higher than that of fluoxetine, and 30 times higher than that of olanzapine.8 Lithium’s RM is nearly identical to amitriptyline (42%), vs fluoxetine (12%).12
Data-driven analysis shows that lithium has higher rates of morbidity and mortality than valproate, as evidenced by 98,831 NPDS cases during 2006-2020. These hard numbers speak for themselves and contradict the dominant paradigm, which proclaims lithium’s superiority in reducing suicide risk.
1. Song J, Sjölander A, Joas E, et al. Suicidal behavior during lithium and valproate treatment: a within-individual 8-year prospective study of 50,000 patients with bipolar disorder. Am J Psychiatry. 2017;174(8):795-802.
2. Schatzberg AF, DeBattista C. Schatzberg’s Manual of Clinical Psychopharmacology. 9th ed. American Psychiatric Association Publishing; 2019:335.
3. Stahl SM. Stahl’s Essential Psychopharmacology. 4th ed. Cambridge University Press; 2013:372.
4. Baldessarini RJ, Tondo L, Davis P, et al. Decreased risk of suicides and attempts during long-term lithium treatment: a meta-analytic review. Bipolar Disord. 2006;8(5 Pt 2):625-639.
5. Oquendo MA, Galfalvy HC, Currier D, et al. Treatment of suicide attempters with bipolar disorder: a randomized clinical trial comparing lithium and valproate in the prevention of suicidal behavior. Am J Psychiatry. 2011;168(10):1050-1056.
6. American Association of Poison Control Centers. Annual reports. Accessed August 25, 2022. https://aapcc.org/annual-reports
7. Gold LH, Frierson RL (eds). The American Psychiatric Association Publishing Textbook of Suicide Risk Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020:17-19.
8. Giurca D. Decreasing suicide risk with math. Current Psychiatry. 2018;17(2):57-59,A,B.
9. Giurca D. Data-driven prescribing. Current Psychiatry. 2018;17(10):e6-e8.
10. Giurca D. Time series analysis of poison control data. Current Psychiatry. 2020;19(6):e5-e9.
11. Giurca D, Hodgman MJ. Relative lethality of hypertension drugs. J Med Toxicol. 2022;18(2):81. 2022 American College of Medical Toxicology Annual Scientific Meeting abstract 020.
12. Gummin DD, Mowry JB, Beuhler MD, et al. 2020 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 38th Annual Report. Clin Toxicol (Phila). 2021;59(12):1282-1501.
The current academic psychiatry paradigm reinforces that lithium reduces suicide risk, more so than other medications, including valproate. However, data from multiple sources contradict this “evidence-based” belief.
Data do not support lithium’s supposed advantage
An 8-year prospective study in Sweden by Song et al1 tracked 51,535 patients with bipolar disorder from 2005 to 2013. In their conclusions, the authors of this study omitted some surprising numbers that contradict the dominant paradigm. There were 230 (1.089%) completed suicides in the lithium group (N = 21,129), 99 (1.177%) in the valproate group (N = 8,411), and 308 (1.195%) in the “other medication” group (N = 25,780). This difference of .088% is too small (95% CI, -.180% to .358%) to substantiate the purported advantage of lithium over valproate. More important is that in terms of suicide-related events, the medication group excluding lithium and valproate had 2,018 (7.8%) events vs lithium 2,142 (10.1%) and valproate 1,105 (13.1%). The difference of 2.3% is statistically significant (95% CI, 1.8% to 2.8%). These numbers reflect fewer suicide-related events with psychiatric medications other than lithium and valproate. Compounding the problem is a design flaw in which 3,785 patients were counted twice in the lithium and valproate groups (21,129 + 8,411 + 25,780 = 55,320, which is more than the 51,535 patients in the study). By falsely inflating the denominator (N) for the lithium and valproate groups, the respective published rates are deceptively lower than the actual rates. Song et al1 did not provide an adequate explanation for these findings and omitted them from their conclusions.
In Schatzberg’s Manual of Clinical Psychopharmacology, the authors cited Song et al1 but omitted these findings as well, and stated “lithium is clearly effective in preventing suicide attempts and completions in bipolar patients.”2 In Stahl’s Essential Psychopharmacology, the author wrote “lithium actually reduces suicide in patients with bipolar disorder.”3 In a review article,
In an overlapping period, National Poison Data System (NPDS) data of single substance exposures painted a different picture in the United States.6 During 2006-2013, the lithium group (N = 26,144) had 32 deaths (all causes) (.122%), and the valproate group (N = 25,630) had 16 deaths (.062%). During 2006-2020, the lithium group (N = 52,262) had 55 deaths (.105%), and the valproate group (N = 46,569) had 31 deaths (.067%). Clearly there is a major disconnect between lithium’s advertised ability to reduce suicide risk and the actual mortality rate, as evidenced by 98,831 cases reported to NPDS during 2006-2020. One would expect a lower rate in the lithium group, but data show it is higher than in the valproate group. This underscores the common fallacy of most lithium studies: each is based on a very small sample (N < 100), and the statistical inference about the entire population is tenuous. If lithium truly reduces suicide risk 5-fold, it would be seen in a sample of 98,831. The law of large numbers and central limit theorem state that as N increases, the variability of the rate progressively decreases. This can be easily demonstrated with computer simulation models and simple Python code, or on the average fuel economy display of most cars.
What about the relative lethality?
The APA Textbook of Suicide Risk Assessment and Management stated that it is important to consider the relative lethality (RL) of prescription medications.7 The RL equation (RL = 310x / LD50) represents the ratio of a 30-day supply of medication to the human equivalent LD50 for a 60-kg person (x is the daily dose and LD50 is the rat oral lethal dose 50).8 Time series analysis shows that the lithium relative morbidity (RM) is consistently double that of valproate (Figure6). Regression models have shown high correlation and causality between RL and RM.9-11 It is surprising that valproate (RL = 1,666%) has a lower RM than lithium (RL = 1,063%). This paradox can be easily explained with clinical insight. The RL equation compares medications at the maximum daily dose, but in routine practice valproate is commonly prescribed at 1,000 mg/d (28% of the maximum 3,600 mg/d). Lithium is commonly prescribed at 1,200 mg/d (67% of the maximum 1,800 mg/d). Within these dosing parameters, the effective RL is valproate 463% and lithium 709%. The 2020 RM is valproate 22% and lithium 43%.12 The COVID-19 pandemic did not affect the predicted RM. Confirming these numbers, Song et al1 acknowledged “greater safety in case of overdose for valproate in clinical practice.” Baldessarini et al4 asserted “the fatality risk of lithium overdose is only moderate, and very similar to modern antidepressants and second-generation antipsychotics.”4 This claim is contradicted by the RL equation and regression models.7-11 Lithium’s RL is 19 times higher than that of fluoxetine, and 30 times higher than that of olanzapine.8 Lithium’s RM is nearly identical to amitriptyline (42%), vs fluoxetine (12%).12

Data-driven analysis shows that lithium has higher rates of morbidity and mortality than valproate, as evidenced by 98,831 NPDS cases during 2006-2020. These hard numbers speak for themselves and contradict the dominant paradigm, which proclaims lithium’s superiority in reducing suicide risk.
The current academic psychiatry paradigm reinforces that lithium reduces suicide risk, more so than other medications, including valproate. However, data from multiple sources contradict this “evidence-based” belief.
Data do not support lithium’s supposed advantage
An 8-year prospective study in Sweden by Song et al1 tracked 51,535 patients with bipolar disorder from 2005 to 2013. In their conclusions, the authors of this study omitted some surprising numbers that contradict the dominant paradigm. There were 230 (1.089%) completed suicides in the lithium group (N = 21,129), 99 (1.177%) in the valproate group (N = 8,411), and 308 (1.195%) in the “other medication” group (N = 25,780). This difference of .088% is too small (95% CI, -.180% to .358%) to substantiate the purported advantage of lithium over valproate. More important is that in terms of suicide-related events, the medication group excluding lithium and valproate had 2,018 (7.8%) events vs lithium 2,142 (10.1%) and valproate 1,105 (13.1%). The difference of 2.3% is statistically significant (95% CI, 1.8% to 2.8%). These numbers reflect fewer suicide-related events with psychiatric medications other than lithium and valproate. Compounding the problem is a design flaw in which 3,785 patients were counted twice in the lithium and valproate groups (21,129 + 8,411 + 25,780 = 55,320, which is more than the 51,535 patients in the study). By falsely inflating the denominator (N) for the lithium and valproate groups, the respective published rates are deceptively lower than the actual rates. Song et al1 did not provide an adequate explanation for these findings and omitted them from their conclusions.
In Schatzberg’s Manual of Clinical Psychopharmacology, the authors cited Song et al1 but omitted these findings as well, and stated “lithium is clearly effective in preventing suicide attempts and completions in bipolar patients.”2 In Stahl’s Essential Psychopharmacology, the author wrote “lithium actually reduces suicide in patients with bipolar disorder.”3 In a review article,
In an overlapping period, National Poison Data System (NPDS) data of single substance exposures painted a different picture in the United States.6 During 2006-2013, the lithium group (N = 26,144) had 32 deaths (all causes) (.122%), and the valproate group (N = 25,630) had 16 deaths (.062%). During 2006-2020, the lithium group (N = 52,262) had 55 deaths (.105%), and the valproate group (N = 46,569) had 31 deaths (.067%). Clearly there is a major disconnect between lithium’s advertised ability to reduce suicide risk and the actual mortality rate, as evidenced by 98,831 cases reported to NPDS during 2006-2020. One would expect a lower rate in the lithium group, but data show it is higher than in the valproate group. This underscores the common fallacy of most lithium studies: each is based on a very small sample (N < 100), and the statistical inference about the entire population is tenuous. If lithium truly reduces suicide risk 5-fold, it would be seen in a sample of 98,831. The law of large numbers and central limit theorem state that as N increases, the variability of the rate progressively decreases. This can be easily demonstrated with computer simulation models and simple Python code, or on the average fuel economy display of most cars.
What about the relative lethality?
The APA Textbook of Suicide Risk Assessment and Management stated that it is important to consider the relative lethality (RL) of prescription medications.7 The RL equation (RL = 310x / LD50) represents the ratio of a 30-day supply of medication to the human equivalent LD50 for a 60-kg person (x is the daily dose and LD50 is the rat oral lethal dose 50).8 Time series analysis shows that the lithium relative morbidity (RM) is consistently double that of valproate (Figure6). Regression models have shown high correlation and causality between RL and RM.9-11 It is surprising that valproate (RL = 1,666%) has a lower RM than lithium (RL = 1,063%). This paradox can be easily explained with clinical insight. The RL equation compares medications at the maximum daily dose, but in routine practice valproate is commonly prescribed at 1,000 mg/d (28% of the maximum 3,600 mg/d). Lithium is commonly prescribed at 1,200 mg/d (67% of the maximum 1,800 mg/d). Within these dosing parameters, the effective RL is valproate 463% and lithium 709%. The 2020 RM is valproate 22% and lithium 43%.12 The COVID-19 pandemic did not affect the predicted RM. Confirming these numbers, Song et al1 acknowledged “greater safety in case of overdose for valproate in clinical practice.” Baldessarini et al4 asserted “the fatality risk of lithium overdose is only moderate, and very similar to modern antidepressants and second-generation antipsychotics.”4 This claim is contradicted by the RL equation and regression models.7-11 Lithium’s RL is 19 times higher than that of fluoxetine, and 30 times higher than that of olanzapine.8 Lithium’s RM is nearly identical to amitriptyline (42%), vs fluoxetine (12%).12

Data-driven analysis shows that lithium has higher rates of morbidity and mortality than valproate, as evidenced by 98,831 NPDS cases during 2006-2020. These hard numbers speak for themselves and contradict the dominant paradigm, which proclaims lithium’s superiority in reducing suicide risk.
1. Song J, Sjölander A, Joas E, et al. Suicidal behavior during lithium and valproate treatment: a within-individual 8-year prospective study of 50,000 patients with bipolar disorder. Am J Psychiatry. 2017;174(8):795-802.
2. Schatzberg AF, DeBattista C. Schatzberg’s Manual of Clinical Psychopharmacology. 9th ed. American Psychiatric Association Publishing; 2019:335.
3. Stahl SM. Stahl’s Essential Psychopharmacology. 4th ed. Cambridge University Press; 2013:372.
4. Baldessarini RJ, Tondo L, Davis P, et al. Decreased risk of suicides and attempts during long-term lithium treatment: a meta-analytic review. Bipolar Disord. 2006;8(5 Pt 2):625-639.
5. Oquendo MA, Galfalvy HC, Currier D, et al. Treatment of suicide attempters with bipolar disorder: a randomized clinical trial comparing lithium and valproate in the prevention of suicidal behavior. Am J Psychiatry. 2011;168(10):1050-1056.
6. American Association of Poison Control Centers. Annual reports. Accessed August 25, 2022. https://aapcc.org/annual-reports
7. Gold LH, Frierson RL (eds). The American Psychiatric Association Publishing Textbook of Suicide Risk Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020:17-19.
8. Giurca D. Decreasing suicide risk with math. Current Psychiatry. 2018;17(2):57-59,A,B.
9. Giurca D. Data-driven prescribing. Current Psychiatry. 2018;17(10):e6-e8.
10. Giurca D. Time series analysis of poison control data. Current Psychiatry. 2020;19(6):e5-e9.
11. Giurca D, Hodgman MJ. Relative lethality of hypertension drugs. J Med Toxicol. 2022;18(2):81. 2022 American College of Medical Toxicology Annual Scientific Meeting abstract 020.
12. Gummin DD, Mowry JB, Beuhler MD, et al. 2020 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 38th Annual Report. Clin Toxicol (Phila). 2021;59(12):1282-1501.
1. Song J, Sjölander A, Joas E, et al. Suicidal behavior during lithium and valproate treatment: a within-individual 8-year prospective study of 50,000 patients with bipolar disorder. Am J Psychiatry. 2017;174(8):795-802.
2. Schatzberg AF, DeBattista C. Schatzberg’s Manual of Clinical Psychopharmacology. 9th ed. American Psychiatric Association Publishing; 2019:335.
3. Stahl SM. Stahl’s Essential Psychopharmacology. 4th ed. Cambridge University Press; 2013:372.
4. Baldessarini RJ, Tondo L, Davis P, et al. Decreased risk of suicides and attempts during long-term lithium treatment: a meta-analytic review. Bipolar Disord. 2006;8(5 Pt 2):625-639.
5. Oquendo MA, Galfalvy HC, Currier D, et al. Treatment of suicide attempters with bipolar disorder: a randomized clinical trial comparing lithium and valproate in the prevention of suicidal behavior. Am J Psychiatry. 2011;168(10):1050-1056.
6. American Association of Poison Control Centers. Annual reports. Accessed August 25, 2022. https://aapcc.org/annual-reports
7. Gold LH, Frierson RL (eds). The American Psychiatric Association Publishing Textbook of Suicide Risk Assessment and Management. 3rd ed. American Psychiatric Association Publishing; 2020:17-19.
8. Giurca D. Decreasing suicide risk with math. Current Psychiatry. 2018;17(2):57-59,A,B.
9. Giurca D. Data-driven prescribing. Current Psychiatry. 2018;17(10):e6-e8.
10. Giurca D. Time series analysis of poison control data. Current Psychiatry. 2020;19(6):e5-e9.
11. Giurca D, Hodgman MJ. Relative lethality of hypertension drugs. J Med Toxicol. 2022;18(2):81. 2022 American College of Medical Toxicology Annual Scientific Meeting abstract 020.
12. Gummin DD, Mowry JB, Beuhler MD, et al. 2020 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 38th Annual Report. Clin Toxicol (Phila). 2021;59(12):1282-1501.
Preparing patients with serious mental illness for extreme HEAT
Climate change is causing intense heat waves that threaten human health across the globe.
A confluence of factors increases risk
Thermoregulatory dysfunction is thought to be intrinsic to patients with schizophrenia partly due to dysregulated dopaminergic neurotransmission.2 This is compounded by these patients’ higher burden of chronic medical comorbidities such as cardiovascular and respiratory illnesses, which together with psychotropic (ie, antipsychotics, antidepressants, lithium, benzodiazepines) and medical medications (ie, certain antihypertensives, diuretics, treatment for urinary incontinence) further disrupt the body’s cooling strategies and increase vulnerability to heat-related illnesses.1,3 Antipsychotics commonly prescribed to patients with SMI increase hyperthermia risk largely by 2 mechanisms: central and peripheral thermal dysregulation, and anticholinergic properties (ie, olanzapine, clozapine, chlorpromazine).2,3 Other anticholinergic medications prescribed to treat extrapyramidal symptoms (ie, diphenhydramine, benztropine, trihexyphenidyl), anxiety, depression, and insomnia (ie, paroxetine, trazodone, doxepin) further add insult to injury because they impair sweating, which decreases the body’s ability to eliminate heat through evaporation.2,3 Additionally, high temperature exacerbates psychiatric symptoms in patients with SMI, resulting in increased hospitalizations and emergency department visits.
How to keep patients safe
The acronym HEAT provides a framework that psychiatrists can use to highlight the importance of planning for heat waves in their institution and guiding discussions with individual patients about heat-related illnesses (Table 1).

Help the health care system where you work plan and prepare for heat waves. In-service training in mental health settings such as outpatient clinics, shelters, group homes, and residential programs can help staff identify patients at particular risk and reinforce key prevention messages.
Educate patients and their caregivers on strategies for preventing heat-related illness. Informational materials can be distributed in clinics, residential settings, and day programs. A 1-page downloadable pamphlet available at https://smiadviser.org/wp-content/uploads/2022/08/SMI-Heat-Stroke-ver1.0-FINAL.pdf summarizes key prevention messages of staying hydrated, staying cool, and staying safe.
Assess personalized heat-related risks. Inquire about patients’ daily activities, access to air conditioning, and water intake. Minimize the use of anticholinergic medications. Identify who patients can turn to for assistance, especially for those who struggle with cognitive impairment and social isolation.
Teach patients, caregivers, and staff the signs and symptoms of heat exhaustion and heat stroke and how to respond in such situations.
HEAT focuses psychiatric clinicians on preparing and protecting patients with SMI against dangerous heat waves. Clinicians can take a proactive leadership role in disseminating basic principles of heat-related illness prevention and heat-wave toolkits by using resources available from organizations such as the Climate Psychiatry Alliance (Table 2). They can also initiate advocacy efforts to raise awareness about the elevated risks of heat-related illnesses in this vulnerable population.

1. Schmeltz MT, Gamble JL. Risk characterization of hospitalizations for mental illness and/or behavioral disorders with concurrent heat-related illness. PLoS One. 2017;12(10):e0186509. doi:10.1371/journal.pone.0186509
2. Lee CP, Chen PJ, Chang CM. Heat stroke during treatment with olanzapine, trihexyphenidyl, and trazodone in a patient with schizophrenia. Acta Neuropsychiatrica. 2015;27(6):380-385.
3. Bongers KS, Salahudeen MS, Peterson GM. Drug-associated non-pyrogenic hyperthermia: a narrative review. Eur J Clin Pharmacol. 2020;76(1):9-16.
Climate change is causing intense heat waves that threaten human health across the globe.
A confluence of factors increases risk
Thermoregulatory dysfunction is thought to be intrinsic to patients with schizophrenia partly due to dysregulated dopaminergic neurotransmission.2 This is compounded by these patients’ higher burden of chronic medical comorbidities such as cardiovascular and respiratory illnesses, which together with psychotropic (ie, antipsychotics, antidepressants, lithium, benzodiazepines) and medical medications (ie, certain antihypertensives, diuretics, treatment for urinary incontinence) further disrupt the body’s cooling strategies and increase vulnerability to heat-related illnesses.1,3 Antipsychotics commonly prescribed to patients with SMI increase hyperthermia risk largely by 2 mechanisms: central and peripheral thermal dysregulation, and anticholinergic properties (ie, olanzapine, clozapine, chlorpromazine).2,3 Other anticholinergic medications prescribed to treat extrapyramidal symptoms (ie, diphenhydramine, benztropine, trihexyphenidyl), anxiety, depression, and insomnia (ie, paroxetine, trazodone, doxepin) further add insult to injury because they impair sweating, which decreases the body’s ability to eliminate heat through evaporation.2,3 Additionally, high temperature exacerbates psychiatric symptoms in patients with SMI, resulting in increased hospitalizations and emergency department visits.
How to keep patients safe
The acronym HEAT provides a framework that psychiatrists can use to highlight the importance of planning for heat waves in their institution and guiding discussions with individual patients about heat-related illnesses (Table 1).

Help the health care system where you work plan and prepare for heat waves. In-service training in mental health settings such as outpatient clinics, shelters, group homes, and residential programs can help staff identify patients at particular risk and reinforce key prevention messages.
Educate patients and their caregivers on strategies for preventing heat-related illness. Informational materials can be distributed in clinics, residential settings, and day programs. A 1-page downloadable pamphlet available at https://smiadviser.org/wp-content/uploads/2022/08/SMI-Heat-Stroke-ver1.0-FINAL.pdf summarizes key prevention messages of staying hydrated, staying cool, and staying safe.
Assess personalized heat-related risks. Inquire about patients’ daily activities, access to air conditioning, and water intake. Minimize the use of anticholinergic medications. Identify who patients can turn to for assistance, especially for those who struggle with cognitive impairment and social isolation.
Teach patients, caregivers, and staff the signs and symptoms of heat exhaustion and heat stroke and how to respond in such situations.
HEAT focuses psychiatric clinicians on preparing and protecting patients with SMI against dangerous heat waves. Clinicians can take a proactive leadership role in disseminating basic principles of heat-related illness prevention and heat-wave toolkits by using resources available from organizations such as the Climate Psychiatry Alliance (Table 2). They can also initiate advocacy efforts to raise awareness about the elevated risks of heat-related illnesses in this vulnerable population.

Climate change is causing intense heat waves that threaten human health across the globe.
A confluence of factors increases risk
Thermoregulatory dysfunction is thought to be intrinsic to patients with schizophrenia partly due to dysregulated dopaminergic neurotransmission.2 This is compounded by these patients’ higher burden of chronic medical comorbidities such as cardiovascular and respiratory illnesses, which together with psychotropic (ie, antipsychotics, antidepressants, lithium, benzodiazepines) and medical medications (ie, certain antihypertensives, diuretics, treatment for urinary incontinence) further disrupt the body’s cooling strategies and increase vulnerability to heat-related illnesses.1,3 Antipsychotics commonly prescribed to patients with SMI increase hyperthermia risk largely by 2 mechanisms: central and peripheral thermal dysregulation, and anticholinergic properties (ie, olanzapine, clozapine, chlorpromazine).2,3 Other anticholinergic medications prescribed to treat extrapyramidal symptoms (ie, diphenhydramine, benztropine, trihexyphenidyl), anxiety, depression, and insomnia (ie, paroxetine, trazodone, doxepin) further add insult to injury because they impair sweating, which decreases the body’s ability to eliminate heat through evaporation.2,3 Additionally, high temperature exacerbates psychiatric symptoms in patients with SMI, resulting in increased hospitalizations and emergency department visits.
How to keep patients safe
The acronym HEAT provides a framework that psychiatrists can use to highlight the importance of planning for heat waves in their institution and guiding discussions with individual patients about heat-related illnesses (Table 1).

Help the health care system where you work plan and prepare for heat waves. In-service training in mental health settings such as outpatient clinics, shelters, group homes, and residential programs can help staff identify patients at particular risk and reinforce key prevention messages.
Educate patients and their caregivers on strategies for preventing heat-related illness. Informational materials can be distributed in clinics, residential settings, and day programs. A 1-page downloadable pamphlet available at https://smiadviser.org/wp-content/uploads/2022/08/SMI-Heat-Stroke-ver1.0-FINAL.pdf summarizes key prevention messages of staying hydrated, staying cool, and staying safe.
Assess personalized heat-related risks. Inquire about patients’ daily activities, access to air conditioning, and water intake. Minimize the use of anticholinergic medications. Identify who patients can turn to for assistance, especially for those who struggle with cognitive impairment and social isolation.
Teach patients, caregivers, and staff the signs and symptoms of heat exhaustion and heat stroke and how to respond in such situations.
HEAT focuses psychiatric clinicians on preparing and protecting patients with SMI against dangerous heat waves. Clinicians can take a proactive leadership role in disseminating basic principles of heat-related illness prevention and heat-wave toolkits by using resources available from organizations such as the Climate Psychiatry Alliance (Table 2). They can also initiate advocacy efforts to raise awareness about the elevated risks of heat-related illnesses in this vulnerable population.

1. Schmeltz MT, Gamble JL. Risk characterization of hospitalizations for mental illness and/or behavioral disorders with concurrent heat-related illness. PLoS One. 2017;12(10):e0186509. doi:10.1371/journal.pone.0186509
2. Lee CP, Chen PJ, Chang CM. Heat stroke during treatment with olanzapine, trihexyphenidyl, and trazodone in a patient with schizophrenia. Acta Neuropsychiatrica. 2015;27(6):380-385.
3. Bongers KS, Salahudeen MS, Peterson GM. Drug-associated non-pyrogenic hyperthermia: a narrative review. Eur J Clin Pharmacol. 2020;76(1):9-16.
1. Schmeltz MT, Gamble JL. Risk characterization of hospitalizations for mental illness and/or behavioral disorders with concurrent heat-related illness. PLoS One. 2017;12(10):e0186509. doi:10.1371/journal.pone.0186509
2. Lee CP, Chen PJ, Chang CM. Heat stroke during treatment with olanzapine, trihexyphenidyl, and trazodone in a patient with schizophrenia. Acta Neuropsychiatrica. 2015;27(6):380-385.
3. Bongers KS, Salahudeen MS, Peterson GM. Drug-associated non-pyrogenic hyperthermia: a narrative review. Eur J Clin Pharmacol. 2020;76(1):9-16.
Lithium for bipolar disorder: Which patients will respond?
Though Cade discovered it 70 years ago, lithium is still considered the gold standard treatment for preventing manic and depressive phases of bipolar disorder (BD). In addition to its primary indication as a mood stabilizer, lithium has demonstrated efficacy as an augmenting medication for unipolar major depressive disorder.1 While lithium is a first-line agent for BD, it does not improve symptoms in every patient. In a 2004 meta-analysis of 5 randomized controlled trials of patients with BD, Geddes et al2 found lithium was more effective than placebo in preventing the recurrence of mania, with 60% in the lithium group remaining stable compared to 40% in the placebo group. Being able to predict which patients will respond to lithium is crucial to prevent unnecessary exposure to lithium, which can produce significant adverse effects, including somnolence, nausea, diarrhea, and hypothyroidism.2
Several studies have investigated various clinical factors that might predict which patients with BD will respond to lithium. In a review, Kleindienst et al3 highlighted 3 factors that predicted a positive response to lithium:
- fewer hospitalizations prior to treatment
- an episodic course characterized sequentially by mania, depression, and then euthymia
- a later age (>50) at onset of BD.
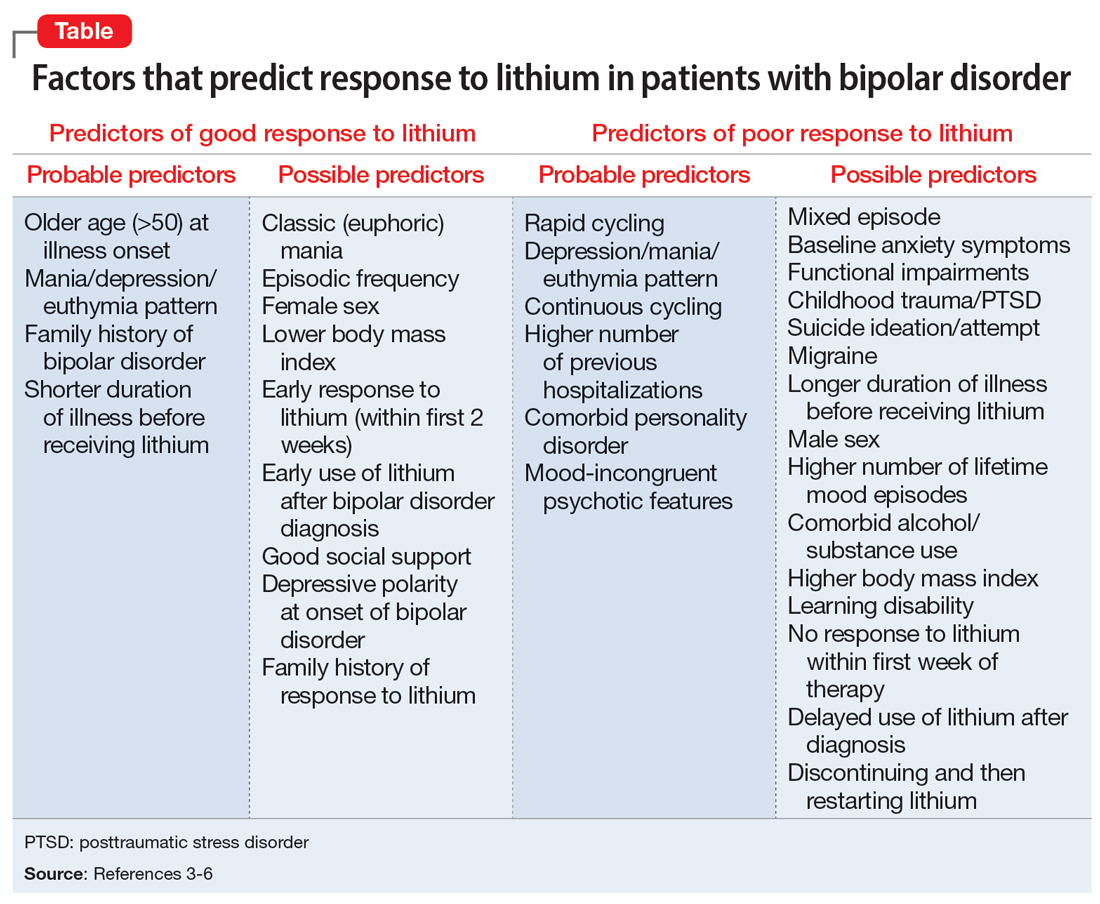
Recent studies and reviews have isolated additional positive predictors, including having a family history of BD and a shorter duration of illness before receiving lithium, as well as negative predictors, such as rapid cycling, a large number of previous hospitalizations, a depression/mania/euthymia pattern, mood-incongruent psychotic features, and the presence of residual symptoms between mood episodes.3,4
The Table provides a list of probable and possible positive and negative predictors for therapeutic response to lithium in patients with BD.3-6 While relevant, the factors listed as possible predictors may not carry as much influence on lithium responsivity as those categorized as probable predictors.
Because of heterogeneity among studies, clinicians should consider their patient’s presentation as a whole, rather than basing medication choice on independent factors. Ultimately, more studies are required to fully determine the most relevant clinical parameters for lithium response. Overall, however, it appears these clinical factors could be extremely useful to guide psychiatrists in the optimal use of lithium while caring for patients with BD.
1. Crossley NA, Bauer M. Acceleration and augmentation of antidepressants with lithium for depressive disorders: two meta-analyses of randomized, placebo-controlled trials. J Clin Psychiatry. 2007;68(6):935-940.
2. Geddes JR, Burgess S, Hawton K, et al. Long-term lithium therapy for bipolar disorder: systematic review and meta-analysis of randomized controlled trials. Am J Psychiatry. 2004;1m61(2):217-222.
3. Kleindienst N, Engel RR, Greil W. Which clinical factors predict response to prophylactic lithium? A systematic review for bipolar disorders. Bipolar Disord. 2005;7(5):404-417.
4. Kleindienst N, Engel RR, Greil W. Psychosocial and demographic factors associated with response to prophylactic lithium: a systematic review for bipolar disorders. Psychol Med. 2005;35(12):1685-1694.
5. Hui TP, Kandola A, Shen L, et al. A systematic review and meta-analysis of clinical predictors of lithium response in bipolar disorder. Acta Psychiatr Scand. 2019;140(2):94-115.
6. Grillault Laroche D, Etain B, Severus E, et al. Socio-demographic and clinical predictors of outcome to long-term treatment with lithium in bipolar disorders: a systematic review of the contemporary literature and recommendations from the ISBD/IGSLI Task Force on treatment with lithium. Int J Bipolar Disord. 2020;8(1):40.
Though Cade discovered it 70 years ago, lithium is still considered the gold standard treatment for preventing manic and depressive phases of bipolar disorder (BD). In addition to its primary indication as a mood stabilizer, lithium has demonstrated efficacy as an augmenting medication for unipolar major depressive disorder.1 While lithium is a first-line agent for BD, it does not improve symptoms in every patient. In a 2004 meta-analysis of 5 randomized controlled trials of patients with BD, Geddes et al2 found lithium was more effective than placebo in preventing the recurrence of mania, with 60% in the lithium group remaining stable compared to 40% in the placebo group. Being able to predict which patients will respond to lithium is crucial to prevent unnecessary exposure to lithium, which can produce significant adverse effects, including somnolence, nausea, diarrhea, and hypothyroidism.2
Several studies have investigated various clinical factors that might predict which patients with BD will respond to lithium. In a review, Kleindienst et al3 highlighted 3 factors that predicted a positive response to lithium:
- fewer hospitalizations prior to treatment
- an episodic course characterized sequentially by mania, depression, and then euthymia
- a later age (>50) at onset of BD.
Recent studies and reviews have isolated additional positive predictors, including having a family history of BD and a shorter duration of illness before receiving lithium, as well as negative predictors, such as rapid cycling, a large number of previous hospitalizations, a depression/mania/euthymia pattern, mood-incongruent psychotic features, and the presence of residual symptoms between mood episodes.3,4
The Table provides a list of probable and possible positive and negative predictors for therapeutic response to lithium in patients with BD.3-6 While relevant, the factors listed as possible predictors may not carry as much influence on lithium responsivity as those categorized as probable predictors.

Because of heterogeneity among studies, clinicians should consider their patient’s presentation as a whole, rather than basing medication choice on independent factors. Ultimately, more studies are required to fully determine the most relevant clinical parameters for lithium response. Overall, however, it appears these clinical factors could be extremely useful to guide psychiatrists in the optimal use of lithium while caring for patients with BD.
Though Cade discovered it 70 years ago, lithium is still considered the gold standard treatment for preventing manic and depressive phases of bipolar disorder (BD). In addition to its primary indication as a mood stabilizer, lithium has demonstrated efficacy as an augmenting medication for unipolar major depressive disorder.1 While lithium is a first-line agent for BD, it does not improve symptoms in every patient. In a 2004 meta-analysis of 5 randomized controlled trials of patients with BD, Geddes et al2 found lithium was more effective than placebo in preventing the recurrence of mania, with 60% in the lithium group remaining stable compared to 40% in the placebo group. Being able to predict which patients will respond to lithium is crucial to prevent unnecessary exposure to lithium, which can produce significant adverse effects, including somnolence, nausea, diarrhea, and hypothyroidism.2
Several studies have investigated various clinical factors that might predict which patients with BD will respond to lithium. In a review, Kleindienst et al3 highlighted 3 factors that predicted a positive response to lithium:
- fewer hospitalizations prior to treatment
- an episodic course characterized sequentially by mania, depression, and then euthymia
- a later age (>50) at onset of BD.
Recent studies and reviews have isolated additional positive predictors, including having a family history of BD and a shorter duration of illness before receiving lithium, as well as negative predictors, such as rapid cycling, a large number of previous hospitalizations, a depression/mania/euthymia pattern, mood-incongruent psychotic features, and the presence of residual symptoms between mood episodes.3,4
The Table provides a list of probable and possible positive and negative predictors for therapeutic response to lithium in patients with BD.3-6 While relevant, the factors listed as possible predictors may not carry as much influence on lithium responsivity as those categorized as probable predictors.

Because of heterogeneity among studies, clinicians should consider their patient’s presentation as a whole, rather than basing medication choice on independent factors. Ultimately, more studies are required to fully determine the most relevant clinical parameters for lithium response. Overall, however, it appears these clinical factors could be extremely useful to guide psychiatrists in the optimal use of lithium while caring for patients with BD.
1. Crossley NA, Bauer M. Acceleration and augmentation of antidepressants with lithium for depressive disorders: two meta-analyses of randomized, placebo-controlled trials. J Clin Psychiatry. 2007;68(6):935-940.
2. Geddes JR, Burgess S, Hawton K, et al. Long-term lithium therapy for bipolar disorder: systematic review and meta-analysis of randomized controlled trials. Am J Psychiatry. 2004;1m61(2):217-222.
3. Kleindienst N, Engel RR, Greil W. Which clinical factors predict response to prophylactic lithium? A systematic review for bipolar disorders. Bipolar Disord. 2005;7(5):404-417.
4. Kleindienst N, Engel RR, Greil W. Psychosocial and demographic factors associated with response to prophylactic lithium: a systematic review for bipolar disorders. Psychol Med. 2005;35(12):1685-1694.
5. Hui TP, Kandola A, Shen L, et al. A systematic review and meta-analysis of clinical predictors of lithium response in bipolar disorder. Acta Psychiatr Scand. 2019;140(2):94-115.
6. Grillault Laroche D, Etain B, Severus E, et al. Socio-demographic and clinical predictors of outcome to long-term treatment with lithium in bipolar disorders: a systematic review of the contemporary literature and recommendations from the ISBD/IGSLI Task Force on treatment with lithium. Int J Bipolar Disord. 2020;8(1):40.
1. Crossley NA, Bauer M. Acceleration and augmentation of antidepressants with lithium for depressive disorders: two meta-analyses of randomized, placebo-controlled trials. J Clin Psychiatry. 2007;68(6):935-940.
2. Geddes JR, Burgess S, Hawton K, et al. Long-term lithium therapy for bipolar disorder: systematic review and meta-analysis of randomized controlled trials. Am J Psychiatry. 2004;1m61(2):217-222.
3. Kleindienst N, Engel RR, Greil W. Which clinical factors predict response to prophylactic lithium? A systematic review for bipolar disorders. Bipolar Disord. 2005;7(5):404-417.
4. Kleindienst N, Engel RR, Greil W. Psychosocial and demographic factors associated with response to prophylactic lithium: a systematic review for bipolar disorders. Psychol Med. 2005;35(12):1685-1694.
5. Hui TP, Kandola A, Shen L, et al. A systematic review and meta-analysis of clinical predictors of lithium response in bipolar disorder. Acta Psychiatr Scand. 2019;140(2):94-115.
6. Grillault Laroche D, Etain B, Severus E, et al. Socio-demographic and clinical predictors of outcome to long-term treatment with lithium in bipolar disorders: a systematic review of the contemporary literature and recommendations from the ISBD/IGSLI Task Force on treatment with lithium. Int J Bipolar Disord. 2020;8(1):40.
Melatonin as a sleep aid: Are you prescribing it correctly?
Difficulty achieving regular restorative sleep is a common symptom of many psychiatric illnesses and can pose a pharmaceutical challenge, particularly for patients who have contraindications to benzodiazepines or sedative-hypnotics. Melatonin is commonly used to treat insomnia and circadian rhythm disorders in hospitalized patients because it is largely considered safe, nonhabit forming, unlikely to interact with other medications, and possibly protective against delirium.1 We support its short-term use in patients with sleep disruption, even if they do not meet the diagnostic criteria for insomnia or a circadian rhythm sleep-wake disorder. However, this use should be guided by consideration of the known physiological actions of melatonin, and not by an assumption that it acts as a simple sedative-hypnotic.
How melatonin works
Melatonin is an endogenous neurohormone involved in circadian rhythm regulation (sleep/wake regulation), a fundamental process in the functioning of the CNS and in the development of psychiatric disorders.2 Melatonin is commonly described as a sleep-promoting neurotransmitter, but it is more accurately described as a “darkness hormone.”3 With an onset at dusk and offset at sunrise, melatonin is the signal for biological night, not the signal for sleep. Melanopsin-containing retina neurons sensitive to blue light sense the diminishing light of the evening and communicate this cue to the brain’s master clock in the suprachiasmatic nucleus (SCN) of the hypothalamus (via the retinohypothalamic pathway). The SCN then releases its inhibition on the pineal gland, allowing it to release melatonin into the bloodstream and CSF. The timing of this release is known as the dim-light melatonin onset (DLMO).
Selecting the optimal timing and dose
Studies in laboratory and home settings have consistently shown that the DLMO precedes the onset of sleep by approximately 2 to 4 hours.4 Thus, we recommend scheduling melatonin administration for 2 to 4 hours before the intended bedtime.
Lower doses better replicate physiological levels of melatonin. A lower dose is also less likely to lead to a compromise of the entrainment process and the induction of a delayed sleep phase due to the lingering presence of melatonin, or the phase-delaying effects of a strong melatonin signal much later than the ideal DLMO. Giving higher doses at bedtime will induce sleep but may cause a circadian phase delay, effectively “jet lagging” the patient. We recommend prescribing low-dose melatonin (LDM; 0.5 to 1 mg) 2 to 4 hours before the intended bedtime rather than higher doses (≥5 mg) given at bedtime as is common practice and recommended by many melatonin manufacturers. LDM better simulates the natural release and function of melatonin and avoids potential adverse circadian phase delays. T
1. Joseph SG. Melatonin supplementation for the prevention of hospital-associated delirium. Ment Health Clin. 2018;7(4):143-146. doi:10.9740/mhc.2017.07.143
2. Arendt J, Skene DJ. Melatonin as a chronobiotic. Sleep Med Rev. 2005;9(1):25-39. doi:10.1016/j.smrv.2004.05.002
3. Tallavajhula S, Rodgers JJ, Slater JD. Sleep and sleep-wake disorders. In: Arciniengas DB, Yudofsky SC, Hales RE, eds. Textbook of Neuropsychiatry and Clinical Neurosciences. American Psychiatric Association Publishing; 2018:373-393.
4. Sletten TL, Vincenzi S, Redman JR, et al. Timing of sleep and its relationship with the endogenous melatonin rhythm. Front Neurol. 2010;1:137. doi:10.3389/fneur.2010.00137
Difficulty achieving regular restorative sleep is a common symptom of many psychiatric illnesses and can pose a pharmaceutical challenge, particularly for patients who have contraindications to benzodiazepines or sedative-hypnotics. Melatonin is commonly used to treat insomnia and circadian rhythm disorders in hospitalized patients because it is largely considered safe, nonhabit forming, unlikely to interact with other medications, and possibly protective against delirium.1 We support its short-term use in patients with sleep disruption, even if they do not meet the diagnostic criteria for insomnia or a circadian rhythm sleep-wake disorder. However, this use should be guided by consideration of the known physiological actions of melatonin, and not by an assumption that it acts as a simple sedative-hypnotic.
How melatonin works
Melatonin is an endogenous neurohormone involved in circadian rhythm regulation (sleep/wake regulation), a fundamental process in the functioning of the CNS and in the development of psychiatric disorders.2 Melatonin is commonly described as a sleep-promoting neurotransmitter, but it is more accurately described as a “darkness hormone.”3 With an onset at dusk and offset at sunrise, melatonin is the signal for biological night, not the signal for sleep. Melanopsin-containing retina neurons sensitive to blue light sense the diminishing light of the evening and communicate this cue to the brain’s master clock in the suprachiasmatic nucleus (SCN) of the hypothalamus (via the retinohypothalamic pathway). The SCN then releases its inhibition on the pineal gland, allowing it to release melatonin into the bloodstream and CSF. The timing of this release is known as the dim-light melatonin onset (DLMO).
Selecting the optimal timing and dose
Studies in laboratory and home settings have consistently shown that the DLMO precedes the onset of sleep by approximately 2 to 4 hours.4 Thus, we recommend scheduling melatonin administration for 2 to 4 hours before the intended bedtime.
Lower doses better replicate physiological levels of melatonin. A lower dose is also less likely to lead to a compromise of the entrainment process and the induction of a delayed sleep phase due to the lingering presence of melatonin, or the phase-delaying effects of a strong melatonin signal much later than the ideal DLMO. Giving higher doses at bedtime will induce sleep but may cause a circadian phase delay, effectively “jet lagging” the patient. We recommend prescribing low-dose melatonin (LDM; 0.5 to 1 mg) 2 to 4 hours before the intended bedtime rather than higher doses (≥5 mg) given at bedtime as is common practice and recommended by many melatonin manufacturers. LDM better simulates the natural release and function of melatonin and avoids potential adverse circadian phase delays. T
Difficulty achieving regular restorative sleep is a common symptom of many psychiatric illnesses and can pose a pharmaceutical challenge, particularly for patients who have contraindications to benzodiazepines or sedative-hypnotics. Melatonin is commonly used to treat insomnia and circadian rhythm disorders in hospitalized patients because it is largely considered safe, nonhabit forming, unlikely to interact with other medications, and possibly protective against delirium.1 We support its short-term use in patients with sleep disruption, even if they do not meet the diagnostic criteria for insomnia or a circadian rhythm sleep-wake disorder. However, this use should be guided by consideration of the known physiological actions of melatonin, and not by an assumption that it acts as a simple sedative-hypnotic.
How melatonin works
Melatonin is an endogenous neurohormone involved in circadian rhythm regulation (sleep/wake regulation), a fundamental process in the functioning of the CNS and in the development of psychiatric disorders.2 Melatonin is commonly described as a sleep-promoting neurotransmitter, but it is more accurately described as a “darkness hormone.”3 With an onset at dusk and offset at sunrise, melatonin is the signal for biological night, not the signal for sleep. Melanopsin-containing retina neurons sensitive to blue light sense the diminishing light of the evening and communicate this cue to the brain’s master clock in the suprachiasmatic nucleus (SCN) of the hypothalamus (via the retinohypothalamic pathway). The SCN then releases its inhibition on the pineal gland, allowing it to release melatonin into the bloodstream and CSF. The timing of this release is known as the dim-light melatonin onset (DLMO).
Selecting the optimal timing and dose
Studies in laboratory and home settings have consistently shown that the DLMO precedes the onset of sleep by approximately 2 to 4 hours.4 Thus, we recommend scheduling melatonin administration for 2 to 4 hours before the intended bedtime.
Lower doses better replicate physiological levels of melatonin. A lower dose is also less likely to lead to a compromise of the entrainment process and the induction of a delayed sleep phase due to the lingering presence of melatonin, or the phase-delaying effects of a strong melatonin signal much later than the ideal DLMO. Giving higher doses at bedtime will induce sleep but may cause a circadian phase delay, effectively “jet lagging” the patient. We recommend prescribing low-dose melatonin (LDM; 0.5 to 1 mg) 2 to 4 hours before the intended bedtime rather than higher doses (≥5 mg) given at bedtime as is common practice and recommended by many melatonin manufacturers. LDM better simulates the natural release and function of melatonin and avoids potential adverse circadian phase delays. T
1. Joseph SG. Melatonin supplementation for the prevention of hospital-associated delirium. Ment Health Clin. 2018;7(4):143-146. doi:10.9740/mhc.2017.07.143
2. Arendt J, Skene DJ. Melatonin as a chronobiotic. Sleep Med Rev. 2005;9(1):25-39. doi:10.1016/j.smrv.2004.05.002
3. Tallavajhula S, Rodgers JJ, Slater JD. Sleep and sleep-wake disorders. In: Arciniengas DB, Yudofsky SC, Hales RE, eds. Textbook of Neuropsychiatry and Clinical Neurosciences. American Psychiatric Association Publishing; 2018:373-393.
4. Sletten TL, Vincenzi S, Redman JR, et al. Timing of sleep and its relationship with the endogenous melatonin rhythm. Front Neurol. 2010;1:137. doi:10.3389/fneur.2010.00137
1. Joseph SG. Melatonin supplementation for the prevention of hospital-associated delirium. Ment Health Clin. 2018;7(4):143-146. doi:10.9740/mhc.2017.07.143
2. Arendt J, Skene DJ. Melatonin as a chronobiotic. Sleep Med Rev. 2005;9(1):25-39. doi:10.1016/j.smrv.2004.05.002
3. Tallavajhula S, Rodgers JJ, Slater JD. Sleep and sleep-wake disorders. In: Arciniengas DB, Yudofsky SC, Hales RE, eds. Textbook of Neuropsychiatry and Clinical Neurosciences. American Psychiatric Association Publishing; 2018:373-393.
4. Sletten TL, Vincenzi S, Redman JR, et al. Timing of sleep and its relationship with the endogenous melatonin rhythm. Front Neurol. 2010;1:137. doi:10.3389/fneur.2010.00137
Pregnancy termination: What psychiatrists need to know
Approximately half of pregnancies in the United States are unplanned, and approximately one-fifth of pregnancies end in elective termination.1 Psychiatrists who treat women of childbearing potential should understand critical aspects of abortion that could affect their patients’ mental health.
Discuss the potential for pregnancy with your patients. Individuals with psychiatric illness are less likely to adhere to contraceptive methods and are more likely to have unplanned pregnancies and detect pregnancies late.2 Women receiving psychiatric care could be at risk of not detecting pregnancy early enough to meet state laws that restrict the time frames in which abortions are allowed.
Understand that patients face barriers to abortion. Almost immediately after the Supreme Court overturned Roe v Wade in June 2022, abortion became illegal in several states. Even if abortion remains legal and available in your jurisdiction, patients could face barriers, including strict limits on abortion timing, monetary and travel challenges, preabortion counseling mandates, and timely access to an abortion provider.
Know that most patients can provide informed consent. Most patients with psychiatric illness have capacity to make medical decisions, including whether to consent to an abortion. Pro forma assessment is not necessary. Assessing capacity to consent to abortion should be the same as any other capacity assessment. If a woman lacks medical decision-making capacity, a substitute decision-maker must be used.
Recognize that ambivalence is normal. Even when a woman is certain about her decision to terminate a pregnancy, she might experience ambivalence. Ambivalence about important life decisions is common and should be validated and explored.3
Be aware of bias. As psychiatrists, we must ensure that our personal opinions about abortion do not impact patient care. An impartial and nondirective approach is key, and any effort to persuade or manipulate a woman’s decision is unethical. Because women with mental illness might be vulnerable to coercion, it is important to ensure that the woman’s choice is voluntary.
Accurately communicate information about mental health and abortion to your patients. Abortion does not worsen mental health. Research on abortion and mental health is rife with poorly designed studies that contain methodological flaws, including failure to control for confounding effects, such as pre-existing mental illness, and inadequate control group comparisons.4 For example, the correct comparison group in which to consider mental health outcomes for women who are seeking an abortion is those who sought an abortion but were not able to have one—not women with planned and desired pregnancies. The best predictor of postabortion mental wellness is preabortion mental health.5 Well-designed studies, such as the Turnaway Study, have demonstrated that abortion does not cause a significant increase in mental illness.6 The Turnaway Study was a well-designed, prospective study of thousands of women who obtained a wanted abortion. It compared many outcomes, including mental health, among women who wanted an abortion vs women who could not obtain a wanted abortion.
Continue to: Know that patients might not receive accurate information about the risks and impact of abortion
Know that patients might not receive accurate information about the risks and impact of abortion. A number of states have requirements—known as “informed consent laws”—that mandate physicians to provide state-authored informational packets about the risks and alternatives to abortion to patients seeking abortions. Some of this information is scientifically inaccurate, which poses a significant ethical dilemma for doctors who must choose between legal requirements and an obligation to scientific integrity.7
Recognize that abortion being illegal could negatively impact mental health. The consequences of being forced to carry out an unwanted pregnancy are profound. Women unable to obtain an abortion are more likely to have adverse health and pregnancy outcomes, live in poverty, stay with an abusive partner, and have difficulty bonding with the child.6 Abortion is highly stigmatized in the United States, and belonging to a stigmatized group is a risk factor for adverse mental health sequalae, including anxiety, depression, substance use, and cognitive deficits.4-6
Stay up-to-date on your state’s abortion laws. The legal landscape regarding abortion is changing rapidly, and it is important to stay abreast of these changes.
Restrictions on abortion likely will significantly affect women with psychiatric illness. As psychiatrists, we must be aware of the impact of the country’s changing laws will have on our patients and their mental health.
1. Guttmacher Institute. Accessed July 21, 2022. https://www.guttmacher.org/
2. Miller LJ. Sexuality, reproduction, and family planning in women with schizophrenia. Schizophr Bull. 1997;23(4):623-635. doi:10.1093/schbul/23.4.623
3. Brody BD, Chaudhry SK, Penzner JB, et al. A woman with major depression with psychotic features requesting a termination of pregnancy. Am J Psychiatry. 2016;173(1):12-15. doi:10.1176/appi.ajp.2015.15030380
4. Major B, Appelbaum M, Beckman L, et al. Abortion and mental health: Evaluating the evidence. Am Psychol. 2009;64(9):863-890. doi:10.1037/a0017497
5. Steinberg JR, Tschann JM, Furgerson D, et al. Psychosocial factors and pre-abortion psychological health: the significance of stigma. Soc Sci Med. 2016;150:67-75. doi:10.1016/j.socscimed.2015.12.007
6. ANSIRH. The Turnaway Study. Accessed June 29, 2022. https://www.ansirh.org/research/ongoing/turnaway-study
7. Daniels CR, Ferguson J, Howard G, et al. Informed or misinformed consent? Abortion policy in the United States. J Health Polit Policy Law. 2016;41(2):181-209. doi:10.1215/03616878-3476105
Approximately half of pregnancies in the United States are unplanned, and approximately one-fifth of pregnancies end in elective termination.1 Psychiatrists who treat women of childbearing potential should understand critical aspects of abortion that could affect their patients’ mental health.
Discuss the potential for pregnancy with your patients. Individuals with psychiatric illness are less likely to adhere to contraceptive methods and are more likely to have unplanned pregnancies and detect pregnancies late.2 Women receiving psychiatric care could be at risk of not detecting pregnancy early enough to meet state laws that restrict the time frames in which abortions are allowed.
Understand that patients face barriers to abortion. Almost immediately after the Supreme Court overturned Roe v Wade in June 2022, abortion became illegal in several states. Even if abortion remains legal and available in your jurisdiction, patients could face barriers, including strict limits on abortion timing, monetary and travel challenges, preabortion counseling mandates, and timely access to an abortion provider.
Know that most patients can provide informed consent. Most patients with psychiatric illness have capacity to make medical decisions, including whether to consent to an abortion. Pro forma assessment is not necessary. Assessing capacity to consent to abortion should be the same as any other capacity assessment. If a woman lacks medical decision-making capacity, a substitute decision-maker must be used.
Recognize that ambivalence is normal. Even when a woman is certain about her decision to terminate a pregnancy, she might experience ambivalence. Ambivalence about important life decisions is common and should be validated and explored.3
Be aware of bias. As psychiatrists, we must ensure that our personal opinions about abortion do not impact patient care. An impartial and nondirective approach is key, and any effort to persuade or manipulate a woman’s decision is unethical. Because women with mental illness might be vulnerable to coercion, it is important to ensure that the woman’s choice is voluntary.
Accurately communicate information about mental health and abortion to your patients. Abortion does not worsen mental health. Research on abortion and mental health is rife with poorly designed studies that contain methodological flaws, including failure to control for confounding effects, such as pre-existing mental illness, and inadequate control group comparisons.4 For example, the correct comparison group in which to consider mental health outcomes for women who are seeking an abortion is those who sought an abortion but were not able to have one—not women with planned and desired pregnancies. The best predictor of postabortion mental wellness is preabortion mental health.5 Well-designed studies, such as the Turnaway Study, have demonstrated that abortion does not cause a significant increase in mental illness.6 The Turnaway Study was a well-designed, prospective study of thousands of women who obtained a wanted abortion. It compared many outcomes, including mental health, among women who wanted an abortion vs women who could not obtain a wanted abortion.
Continue to: Know that patients might not receive accurate information about the risks and impact of abortion
Know that patients might not receive accurate information about the risks and impact of abortion. A number of states have requirements—known as “informed consent laws”—that mandate physicians to provide state-authored informational packets about the risks and alternatives to abortion to patients seeking abortions. Some of this information is scientifically inaccurate, which poses a significant ethical dilemma for doctors who must choose between legal requirements and an obligation to scientific integrity.7
Recognize that abortion being illegal could negatively impact mental health. The consequences of being forced to carry out an unwanted pregnancy are profound. Women unable to obtain an abortion are more likely to have adverse health and pregnancy outcomes, live in poverty, stay with an abusive partner, and have difficulty bonding with the child.6 Abortion is highly stigmatized in the United States, and belonging to a stigmatized group is a risk factor for adverse mental health sequalae, including anxiety, depression, substance use, and cognitive deficits.4-6
Stay up-to-date on your state’s abortion laws. The legal landscape regarding abortion is changing rapidly, and it is important to stay abreast of these changes.
Restrictions on abortion likely will significantly affect women with psychiatric illness. As psychiatrists, we must be aware of the impact of the country’s changing laws will have on our patients and their mental health.
Approximately half of pregnancies in the United States are unplanned, and approximately one-fifth of pregnancies end in elective termination.1 Psychiatrists who treat women of childbearing potential should understand critical aspects of abortion that could affect their patients’ mental health.
Discuss the potential for pregnancy with your patients. Individuals with psychiatric illness are less likely to adhere to contraceptive methods and are more likely to have unplanned pregnancies and detect pregnancies late.2 Women receiving psychiatric care could be at risk of not detecting pregnancy early enough to meet state laws that restrict the time frames in which abortions are allowed.
Understand that patients face barriers to abortion. Almost immediately after the Supreme Court overturned Roe v Wade in June 2022, abortion became illegal in several states. Even if abortion remains legal and available in your jurisdiction, patients could face barriers, including strict limits on abortion timing, monetary and travel challenges, preabortion counseling mandates, and timely access to an abortion provider.
Know that most patients can provide informed consent. Most patients with psychiatric illness have capacity to make medical decisions, including whether to consent to an abortion. Pro forma assessment is not necessary. Assessing capacity to consent to abortion should be the same as any other capacity assessment. If a woman lacks medical decision-making capacity, a substitute decision-maker must be used.
Recognize that ambivalence is normal. Even when a woman is certain about her decision to terminate a pregnancy, she might experience ambivalence. Ambivalence about important life decisions is common and should be validated and explored.3
Be aware of bias. As psychiatrists, we must ensure that our personal opinions about abortion do not impact patient care. An impartial and nondirective approach is key, and any effort to persuade or manipulate a woman’s decision is unethical. Because women with mental illness might be vulnerable to coercion, it is important to ensure that the woman’s choice is voluntary.
Accurately communicate information about mental health and abortion to your patients. Abortion does not worsen mental health. Research on abortion and mental health is rife with poorly designed studies that contain methodological flaws, including failure to control for confounding effects, such as pre-existing mental illness, and inadequate control group comparisons.4 For example, the correct comparison group in which to consider mental health outcomes for women who are seeking an abortion is those who sought an abortion but were not able to have one—not women with planned and desired pregnancies. The best predictor of postabortion mental wellness is preabortion mental health.5 Well-designed studies, such as the Turnaway Study, have demonstrated that abortion does not cause a significant increase in mental illness.6 The Turnaway Study was a well-designed, prospective study of thousands of women who obtained a wanted abortion. It compared many outcomes, including mental health, among women who wanted an abortion vs women who could not obtain a wanted abortion.
Continue to: Know that patients might not receive accurate information about the risks and impact of abortion
Know that patients might not receive accurate information about the risks and impact of abortion. A number of states have requirements—known as “informed consent laws”—that mandate physicians to provide state-authored informational packets about the risks and alternatives to abortion to patients seeking abortions. Some of this information is scientifically inaccurate, which poses a significant ethical dilemma for doctors who must choose between legal requirements and an obligation to scientific integrity.7
Recognize that abortion being illegal could negatively impact mental health. The consequences of being forced to carry out an unwanted pregnancy are profound. Women unable to obtain an abortion are more likely to have adverse health and pregnancy outcomes, live in poverty, stay with an abusive partner, and have difficulty bonding with the child.6 Abortion is highly stigmatized in the United States, and belonging to a stigmatized group is a risk factor for adverse mental health sequalae, including anxiety, depression, substance use, and cognitive deficits.4-6
Stay up-to-date on your state’s abortion laws. The legal landscape regarding abortion is changing rapidly, and it is important to stay abreast of these changes.
Restrictions on abortion likely will significantly affect women with psychiatric illness. As psychiatrists, we must be aware of the impact of the country’s changing laws will have on our patients and their mental health.
1. Guttmacher Institute. Accessed July 21, 2022. https://www.guttmacher.org/
2. Miller LJ. Sexuality, reproduction, and family planning in women with schizophrenia. Schizophr Bull. 1997;23(4):623-635. doi:10.1093/schbul/23.4.623
3. Brody BD, Chaudhry SK, Penzner JB, et al. A woman with major depression with psychotic features requesting a termination of pregnancy. Am J Psychiatry. 2016;173(1):12-15. doi:10.1176/appi.ajp.2015.15030380
4. Major B, Appelbaum M, Beckman L, et al. Abortion and mental health: Evaluating the evidence. Am Psychol. 2009;64(9):863-890. doi:10.1037/a0017497
5. Steinberg JR, Tschann JM, Furgerson D, et al. Psychosocial factors and pre-abortion psychological health: the significance of stigma. Soc Sci Med. 2016;150:67-75. doi:10.1016/j.socscimed.2015.12.007
6. ANSIRH. The Turnaway Study. Accessed June 29, 2022. https://www.ansirh.org/research/ongoing/turnaway-study
7. Daniels CR, Ferguson J, Howard G, et al. Informed or misinformed consent? Abortion policy in the United States. J Health Polit Policy Law. 2016;41(2):181-209. doi:10.1215/03616878-3476105
1. Guttmacher Institute. Accessed July 21, 2022. https://www.guttmacher.org/
2. Miller LJ. Sexuality, reproduction, and family planning in women with schizophrenia. Schizophr Bull. 1997;23(4):623-635. doi:10.1093/schbul/23.4.623
3. Brody BD, Chaudhry SK, Penzner JB, et al. A woman with major depression with psychotic features requesting a termination of pregnancy. Am J Psychiatry. 2016;173(1):12-15. doi:10.1176/appi.ajp.2015.15030380
4. Major B, Appelbaum M, Beckman L, et al. Abortion and mental health: Evaluating the evidence. Am Psychol. 2009;64(9):863-890. doi:10.1037/a0017497
5. Steinberg JR, Tschann JM, Furgerson D, et al. Psychosocial factors and pre-abortion psychological health: the significance of stigma. Soc Sci Med. 2016;150:67-75. doi:10.1016/j.socscimed.2015.12.007
6. ANSIRH. The Turnaway Study. Accessed June 29, 2022. https://www.ansirh.org/research/ongoing/turnaway-study
7. Daniels CR, Ferguson J, Howard G, et al. Informed or misinformed consent? Abortion policy in the United States. J Health Polit Policy Law. 2016;41(2):181-209. doi:10.1215/03616878-3476105





