User login
New and Updated FDA Boxed Warnings
The FDA’s MedWatch program safety labeling changes for boxed warnings are compiled quarterly for drugs and therapeutic biologics where important changes have been made to the safety information. These and other label changes are searchable in the Drug Safety Labeling Changes (SLC) database, where data are available to the public in downloadable and searchable formats. Boxed warnings are ordinarily used to highlight either adverse reactions so serious in proportion to the potential bene t from the drug that it is essential that it be considered in assessing the risks and bene ts of using the drug; or serious adverse reactions that can be prevented/reduced in frequency or severity by appropriate use of the drug; or FDA approved the drug with restrictions to ensure safe use because FDA concluded that the drug can be safely used only if distribution or use is restricted.
IMODIUM (LOPERAMIDE HYDROCHLORIDE):
- New warning December 2016
WARNING: TORSADES DE POINTES AND SUDDEN DEATH
Cases of Torsades de Pointes, cardiac arrest, and death have been reported with the use of a higher than recommended dosages of Imodium (see WARNINGS and OVERDOSAGE).
Imodium is contraindicated in pediatric patients less than 2 years of age (see CONTRANIDICATIONS).
Avoid Imodium dosages higher than recommended in adults and pediatric patients 2 years of age and older due to the risk of serious cardiac adverse reactions (see DOSAGE AND ADMINISTRATION).
AUBAGIO (TERIFLUNOMIDE) TABLETS:
- Edited and updated warning December 2016
Risk of Teratogenicity
Aubagio is contraindicated for use in pregnant women and in women of reproductive potential who are not using effective contraception because of the potential for fetal harm. Teratogenicity and embryolethality occurred in animals at plasma teriflunomide exposures lower than that in humans. Exclude pregnancy before the start of treatment with Aubagio in females of reproductive potential. Advise females of reproductive potential to use effective contraception during Aubagio treatment and during an accelerated drug elimination procedure after Aubagio treatment. Stop Aubagio and use an accelerated drug elimination procedure if the patient becomes pregnant.
PROMACTA (ELTROMBOPAG) TABLETS, FOR ORAL USE AND ORAL SUSPENSION:
- Edited and updated warning December 2016
Chronic Hepatitis C
Promacta may increase the risk of severe and potentially lifethreatening hepatotoxicity. Monitor hepatic function and discontinue dosing as recommended.
ICLUSIG (PONATINIB HYDROCHLORIDE):
- Edited and updated warning December 2016
WARNING: ARTERIAL OCCLUSION, VENOUS THROMBOEMBOLISM, HEART FAILURE, and HEPATOTOXICITY
Arterial Occlusion
Arterial occlusions have occurred in at least 35% of Iclusig-treated patients. Some patients experienced more than 1 type of event. Events observed included fatal myocardial infarction, stroke, stenosis of large arterial vessels of the brain, severe peripheral vascular disease, and the need for urgent revascularization procedures. Patients with and without cardiovascular risk factors, including patients age 50 years or younger, experienced these events. Monitor for evidence of arterial occlusion. Interrupt or stop Iclusig immediately for arterial occlusion.
Venous Thromboembolism
Venous occlusive events have occurred in 6% of Iclusig-treated patients. Monitor for evidence of venous thromboembolism. Consider dose modification or discontinuation of Iclusig in patients who develop serious venous thromboembolism.
Heart Failure
Heart failure, including fatalities, occurred in 9% of Iclusig-treated patients.
The FDA’s MedWatch program safety labeling changes for boxed warnings are compiled quarterly for drugs and therapeutic biologics where important changes have been made to the safety information. These and other label changes are searchable in the Drug Safety Labeling Changes (SLC) database, where data are available to the public in downloadable and searchable formats. Boxed warnings are ordinarily used to highlight either adverse reactions so serious in proportion to the potential bene t from the drug that it is essential that it be considered in assessing the risks and bene ts of using the drug; or serious adverse reactions that can be prevented/reduced in frequency or severity by appropriate use of the drug; or FDA approved the drug with restrictions to ensure safe use because FDA concluded that the drug can be safely used only if distribution or use is restricted.
IMODIUM (LOPERAMIDE HYDROCHLORIDE):
- New warning December 2016
WARNING: TORSADES DE POINTES AND SUDDEN DEATH
Cases of Torsades de Pointes, cardiac arrest, and death have been reported with the use of a higher than recommended dosages of Imodium (see WARNINGS and OVERDOSAGE).
Imodium is contraindicated in pediatric patients less than 2 years of age (see CONTRANIDICATIONS).
Avoid Imodium dosages higher than recommended in adults and pediatric patients 2 years of age and older due to the risk of serious cardiac adverse reactions (see DOSAGE AND ADMINISTRATION).
AUBAGIO (TERIFLUNOMIDE) TABLETS:
- Edited and updated warning December 2016
Risk of Teratogenicity
Aubagio is contraindicated for use in pregnant women and in women of reproductive potential who are not using effective contraception because of the potential for fetal harm. Teratogenicity and embryolethality occurred in animals at plasma teriflunomide exposures lower than that in humans. Exclude pregnancy before the start of treatment with Aubagio in females of reproductive potential. Advise females of reproductive potential to use effective contraception during Aubagio treatment and during an accelerated drug elimination procedure after Aubagio treatment. Stop Aubagio and use an accelerated drug elimination procedure if the patient becomes pregnant.
PROMACTA (ELTROMBOPAG) TABLETS, FOR ORAL USE AND ORAL SUSPENSION:
- Edited and updated warning December 2016
Chronic Hepatitis C
Promacta may increase the risk of severe and potentially lifethreatening hepatotoxicity. Monitor hepatic function and discontinue dosing as recommended.
ICLUSIG (PONATINIB HYDROCHLORIDE):
- Edited and updated warning December 2016
WARNING: ARTERIAL OCCLUSION, VENOUS THROMBOEMBOLISM, HEART FAILURE, and HEPATOTOXICITY
Arterial Occlusion
Arterial occlusions have occurred in at least 35% of Iclusig-treated patients. Some patients experienced more than 1 type of event. Events observed included fatal myocardial infarction, stroke, stenosis of large arterial vessels of the brain, severe peripheral vascular disease, and the need for urgent revascularization procedures. Patients with and without cardiovascular risk factors, including patients age 50 years or younger, experienced these events. Monitor for evidence of arterial occlusion. Interrupt or stop Iclusig immediately for arterial occlusion.
Venous Thromboembolism
Venous occlusive events have occurred in 6% of Iclusig-treated patients. Monitor for evidence of venous thromboembolism. Consider dose modification or discontinuation of Iclusig in patients who develop serious venous thromboembolism.
Heart Failure
Heart failure, including fatalities, occurred in 9% of Iclusig-treated patients.
The FDA’s MedWatch program safety labeling changes for boxed warnings are compiled quarterly for drugs and therapeutic biologics where important changes have been made to the safety information. These and other label changes are searchable in the Drug Safety Labeling Changes (SLC) database, where data are available to the public in downloadable and searchable formats. Boxed warnings are ordinarily used to highlight either adverse reactions so serious in proportion to the potential bene t from the drug that it is essential that it be considered in assessing the risks and bene ts of using the drug; or serious adverse reactions that can be prevented/reduced in frequency or severity by appropriate use of the drug; or FDA approved the drug with restrictions to ensure safe use because FDA concluded that the drug can be safely used only if distribution or use is restricted.
IMODIUM (LOPERAMIDE HYDROCHLORIDE):
- New warning December 2016
WARNING: TORSADES DE POINTES AND SUDDEN DEATH
Cases of Torsades de Pointes, cardiac arrest, and death have been reported with the use of a higher than recommended dosages of Imodium (see WARNINGS and OVERDOSAGE).
Imodium is contraindicated in pediatric patients less than 2 years of age (see CONTRANIDICATIONS).
Avoid Imodium dosages higher than recommended in adults and pediatric patients 2 years of age and older due to the risk of serious cardiac adverse reactions (see DOSAGE AND ADMINISTRATION).
AUBAGIO (TERIFLUNOMIDE) TABLETS:
- Edited and updated warning December 2016
Risk of Teratogenicity
Aubagio is contraindicated for use in pregnant women and in women of reproductive potential who are not using effective contraception because of the potential for fetal harm. Teratogenicity and embryolethality occurred in animals at plasma teriflunomide exposures lower than that in humans. Exclude pregnancy before the start of treatment with Aubagio in females of reproductive potential. Advise females of reproductive potential to use effective contraception during Aubagio treatment and during an accelerated drug elimination procedure after Aubagio treatment. Stop Aubagio and use an accelerated drug elimination procedure if the patient becomes pregnant.
PROMACTA (ELTROMBOPAG) TABLETS, FOR ORAL USE AND ORAL SUSPENSION:
- Edited and updated warning December 2016
Chronic Hepatitis C
Promacta may increase the risk of severe and potentially lifethreatening hepatotoxicity. Monitor hepatic function and discontinue dosing as recommended.
ICLUSIG (PONATINIB HYDROCHLORIDE):
- Edited and updated warning December 2016
WARNING: ARTERIAL OCCLUSION, VENOUS THROMBOEMBOLISM, HEART FAILURE, and HEPATOTOXICITY
Arterial Occlusion
Arterial occlusions have occurred in at least 35% of Iclusig-treated patients. Some patients experienced more than 1 type of event. Events observed included fatal myocardial infarction, stroke, stenosis of large arterial vessels of the brain, severe peripheral vascular disease, and the need for urgent revascularization procedures. Patients with and without cardiovascular risk factors, including patients age 50 years or younger, experienced these events. Monitor for evidence of arterial occlusion. Interrupt or stop Iclusig immediately for arterial occlusion.
Venous Thromboembolism
Venous occlusive events have occurred in 6% of Iclusig-treated patients. Monitor for evidence of venous thromboembolism. Consider dose modification or discontinuation of Iclusig in patients who develop serious venous thromboembolism.
Heart Failure
Heart failure, including fatalities, occurred in 9% of Iclusig-treated patients.
IHS Gives Pharmacy Students Hands-On Experience
The IHS has partnered with 3 top American universities to give pharmacy students an opportunity to get real-life work experience and potentially careers at IHS facilities.
Related: Dangerous Staff Shortages in the IHS
In the IHS Advanced Pharmacy Practice Experience Program, PharmD candidates at Howard University, Purdue University, and the University of Southern California will join students from more than 80 universities in 39 states to complete rotations at IHS direct service facilities. “Many return to start their career in providing quality health care to the American Indian and Alaska Native community,” said Mary Smith, IHS principal deputy director.
“My experience with IHS as a student inspired me to apply to work here when I graduated,” said Fengyee Zhou, now a pharmacist at the IHS Whiteriver Indian Hospital in Arizona. “The level of teamwork among all health care disciplines and the extent to which pharmacists engage in patient care activities brought me back to Whiteriver.”
Related: What s the VA? The Largest Educator of Health Care Professionals in the U.S.
The IHS also offers internships, externships, rotations, and residencies to pharmacy, behavioral health, dentistry, optometry, nursing, and medical students.
The IHS has partnered with 3 top American universities to give pharmacy students an opportunity to get real-life work experience and potentially careers at IHS facilities.
Related: Dangerous Staff Shortages in the IHS
In the IHS Advanced Pharmacy Practice Experience Program, PharmD candidates at Howard University, Purdue University, and the University of Southern California will join students from more than 80 universities in 39 states to complete rotations at IHS direct service facilities. “Many return to start their career in providing quality health care to the American Indian and Alaska Native community,” said Mary Smith, IHS principal deputy director.
“My experience with IHS as a student inspired me to apply to work here when I graduated,” said Fengyee Zhou, now a pharmacist at the IHS Whiteriver Indian Hospital in Arizona. “The level of teamwork among all health care disciplines and the extent to which pharmacists engage in patient care activities brought me back to Whiteriver.”
Related: What s the VA? The Largest Educator of Health Care Professionals in the U.S.
The IHS also offers internships, externships, rotations, and residencies to pharmacy, behavioral health, dentistry, optometry, nursing, and medical students.
The IHS has partnered with 3 top American universities to give pharmacy students an opportunity to get real-life work experience and potentially careers at IHS facilities.
Related: Dangerous Staff Shortages in the IHS
In the IHS Advanced Pharmacy Practice Experience Program, PharmD candidates at Howard University, Purdue University, and the University of Southern California will join students from more than 80 universities in 39 states to complete rotations at IHS direct service facilities. “Many return to start their career in providing quality health care to the American Indian and Alaska Native community,” said Mary Smith, IHS principal deputy director.
“My experience with IHS as a student inspired me to apply to work here when I graduated,” said Fengyee Zhou, now a pharmacist at the IHS Whiteriver Indian Hospital in Arizona. “The level of teamwork among all health care disciplines and the extent to which pharmacists engage in patient care activities brought me back to Whiteriver.”
Related: What s the VA? The Largest Educator of Health Care Professionals in the U.S.
The IHS also offers internships, externships, rotations, and residencies to pharmacy, behavioral health, dentistry, optometry, nursing, and medical students.
Recent FDA Boxed Warnings
The FDA’s MedWatch program safety labeling changes for boxed warnings are compiled quarterly for drugs and therapeutic biologics where important changes have been made to the safety information. You can search these and other label changes in the Drug Safety Labeling Changes (SLC) database, where data are available to the public in downloadable and searchable formats. Boxed warnings are ordinarily used to highlight either adverse reactions so serious in proportion to the potential bene t from the drug that it is essential that it be considered in assessing the risks and bene ts of using the drug; or serious adverse reactions that can be prevented/reduced in frequency or severity by appropriate use of the drug; or FDA approved the drug with restrictions to ensure safe use because FDA concluded that the drug can be safely used only if distribution or use is restricted.
QUINOLONE:
- Edited and updated warning September 2016
WARNING: SERIOUS ADVERSE REACTIONS INCLUDING TENDINITIS, TENDON RUPTURE, PERIPHERAL NEUROPATHY, CENTRAL NERVOUS SYSTEM EFFECTS AND EXACERBATION OF MYASTHENIA GRAVIS
Fluoroquinolones have been associated with disabling and potentially irreversible serious adverse reactions that have occurred together including:
- Tendinitis and tendon rupture
- Peripheral neuropathy
- Central nervous system effects
Discontinue immediately and avoid the use of fluoroquinolones in patients who experience any of these serious adverse reactions. Fluoroquinolones may exacerbate muscle weakness in patients with myasthenia gravis. Avoid quinolones in patients with known history of myasthenia gravis. Because fluoroquinolones
have been associated with serious adverse reactions, reserve quinolones for use in patients who have no alternative treatment options for the following
indications:
Avelox (moxifloxacin hydrochloride): Avelox in sodium chloride 0.8% in plastic container; moxifloxacin hydrochloride; Cipro in dextrose 5% in plastic container):
Acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis.
Cipro (ciprofloxacin; ciprofloxacin hydrochloride): Acute exacerbation of chronic bronchitis, acute uncomplicated cystitis, and acute sinusitis.
Cipro XR; Noroxin (norfloxacin): Uncomplicated urinary tract infections.
Factive (gemifloxacin mesylate): Acute bacterial exacerbation of chronic bronchitis.
Levaquin (levofloxacin): Uncomplicated urinary tract infection, acute bacterial exacerbation of chronic bronchitis, and acute bacterial sinusitis.
KRYSTEXXA (PEGLOTICASE):
- Added section to warning September 2016
WARNING: ANAPHYLAXIS AND INFUSION REACTIONS; G6PD DEFICIENCY ASSOCIATED HEMOLYSIS AND METHEMOGLOBINEMIA (Title Updated)
Addition of: Screen patients at risk for G6PD deficiency prior to starting Krystexxa. Hemolysis and methemoglobinemia have been reported with Krystexxa in patients with G6PD deficiency. Do not administer Krystexxa to patients with G6PD deficiency.
PLAVIX (CLOPIDOGREL BISULFATE):
- Edited and updated warning September 2016
WARNING: DIMINISHED ANTIPLATELET EFFECT IN PATIENTS WITH TWO LOSS-OF-FUNCTION ALLELES OF THE CYP2C19 GENE
The effectiveness of Plavix results from its antiplatelet activity, which is dependent on its conversion to an active metabolite by the cytochrome P450 (CYP) system, principally CYP2C19. Plavix at recommended doses forms less of the active metabolite and so has a reduced effect on platelet activity in patients who are homozygous for nonfunctional alleles of the CYP2C19 gene, (termed “CYP2C19 poor metabolizers”). Tests are available to identify patients who are CYP2C19 poor metabolizers. Consider use of another platelet P2Y12 inhibitor in patients identified as CYP2C19 poor metabolizers.
SYNJARDY (EMPAGLIFLOZIN; METFORMIN HYDROCHLORIDE):
- Edited and updated warning September 2016
Postmarketing cases of metformin-associated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. The onset of metformin-associated lactic acidosis is often subtle, accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Metforminassociated lactic acidosis was characterized by elevated blood lactate levels (> 5 mmol/Liter), anion gap acidosis (without evidence of ketonuria or ketonemia), an increased lactate/pyruvate ratio; and metformin plasma levels generally > 5 mcg/mL.
Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (e.g., carbonic anhydrase inhibitors such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (e.g., acute congestive heart failure), excessive alcohol intake, and hepatic impairment.
Steps to reduce the risk of and manage metformin-associated lactic acidosis in these high-risk groups are provided in the full prescribing information.
If metformin-associated lactic acidosis is suspected, immediately discontinue Synjardy and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended.
ZYDELIG (IDELALISIB)
- Edited and updated warning September 2016
WARNING: FATAL AND SERIOUS TOXICITIES: HEPATIC, SEVERE DIARRHEA, COLITIS, PNEUMONITIS, INFECTIONS, AND INTESTINAL PERFORATION
- Fatal and/or serious hepatotoxicity occurred in 11 % to 18% of Zydelig-treated patients. Monitor hepatic function prior to and during treatment. Interrupt and then reduce or discontinue Zydelig as recommended.
- Fatal and/or serious and severe diarrhea or colitis occurred in 14% to 19% of Zydelig-treated patients. Monitor for the development of severe diarrhea or colitis. Interrupt and then reduce or discontinue Zydelig as recommended.
- Fatal and/or serious pneumonitis occurred in 4% of Zydelig-treated patients. Monitor for pulmonary symptoms and bilateral interstitial infiltrates. Interrupt or discontinue Zydelig as recommended.
- Fatal and/or serious infections occurred in 21% to 36% of Zydelig-treated patients. Monitor for signs and symptoms of infection. Interrupt Zydelig if infection is suspected.
- Fatal and serious intestinal perforation can occur in Zydelig-treated patients across clinical trials. Discontinue Zydelig for intestinal perforation.
The FDA’s MedWatch program safety labeling changes for boxed warnings are compiled quarterly for drugs and therapeutic biologics where important changes have been made to the safety information. You can search these and other label changes in the Drug Safety Labeling Changes (SLC) database, where data are available to the public in downloadable and searchable formats. Boxed warnings are ordinarily used to highlight either adverse reactions so serious in proportion to the potential bene t from the drug that it is essential that it be considered in assessing the risks and bene ts of using the drug; or serious adverse reactions that can be prevented/reduced in frequency or severity by appropriate use of the drug; or FDA approved the drug with restrictions to ensure safe use because FDA concluded that the drug can be safely used only if distribution or use is restricted.
QUINOLONE:
- Edited and updated warning September 2016
WARNING: SERIOUS ADVERSE REACTIONS INCLUDING TENDINITIS, TENDON RUPTURE, PERIPHERAL NEUROPATHY, CENTRAL NERVOUS SYSTEM EFFECTS AND EXACERBATION OF MYASTHENIA GRAVIS
Fluoroquinolones have been associated with disabling and potentially irreversible serious adverse reactions that have occurred together including:
- Tendinitis and tendon rupture
- Peripheral neuropathy
- Central nervous system effects
Discontinue immediately and avoid the use of fluoroquinolones in patients who experience any of these serious adverse reactions. Fluoroquinolones may exacerbate muscle weakness in patients with myasthenia gravis. Avoid quinolones in patients with known history of myasthenia gravis. Because fluoroquinolones
have been associated with serious adverse reactions, reserve quinolones for use in patients who have no alternative treatment options for the following
indications:
Avelox (moxifloxacin hydrochloride): Avelox in sodium chloride 0.8% in plastic container; moxifloxacin hydrochloride; Cipro in dextrose 5% in plastic container):
Acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis.
Cipro (ciprofloxacin; ciprofloxacin hydrochloride): Acute exacerbation of chronic bronchitis, acute uncomplicated cystitis, and acute sinusitis.
Cipro XR; Noroxin (norfloxacin): Uncomplicated urinary tract infections.
Factive (gemifloxacin mesylate): Acute bacterial exacerbation of chronic bronchitis.
Levaquin (levofloxacin): Uncomplicated urinary tract infection, acute bacterial exacerbation of chronic bronchitis, and acute bacterial sinusitis.
KRYSTEXXA (PEGLOTICASE):
- Added section to warning September 2016
WARNING: ANAPHYLAXIS AND INFUSION REACTIONS; G6PD DEFICIENCY ASSOCIATED HEMOLYSIS AND METHEMOGLOBINEMIA (Title Updated)
Addition of: Screen patients at risk for G6PD deficiency prior to starting Krystexxa. Hemolysis and methemoglobinemia have been reported with Krystexxa in patients with G6PD deficiency. Do not administer Krystexxa to patients with G6PD deficiency.
PLAVIX (CLOPIDOGREL BISULFATE):
- Edited and updated warning September 2016
WARNING: DIMINISHED ANTIPLATELET EFFECT IN PATIENTS WITH TWO LOSS-OF-FUNCTION ALLELES OF THE CYP2C19 GENE
The effectiveness of Plavix results from its antiplatelet activity, which is dependent on its conversion to an active metabolite by the cytochrome P450 (CYP) system, principally CYP2C19. Plavix at recommended doses forms less of the active metabolite and so has a reduced effect on platelet activity in patients who are homozygous for nonfunctional alleles of the CYP2C19 gene, (termed “CYP2C19 poor metabolizers”). Tests are available to identify patients who are CYP2C19 poor metabolizers. Consider use of another platelet P2Y12 inhibitor in patients identified as CYP2C19 poor metabolizers.
SYNJARDY (EMPAGLIFLOZIN; METFORMIN HYDROCHLORIDE):
- Edited and updated warning September 2016
Postmarketing cases of metformin-associated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. The onset of metformin-associated lactic acidosis is often subtle, accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Metforminassociated lactic acidosis was characterized by elevated blood lactate levels (> 5 mmol/Liter), anion gap acidosis (without evidence of ketonuria or ketonemia), an increased lactate/pyruvate ratio; and metformin plasma levels generally > 5 mcg/mL.
Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (e.g., carbonic anhydrase inhibitors such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (e.g., acute congestive heart failure), excessive alcohol intake, and hepatic impairment.
Steps to reduce the risk of and manage metformin-associated lactic acidosis in these high-risk groups are provided in the full prescribing information.
If metformin-associated lactic acidosis is suspected, immediately discontinue Synjardy and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended.
ZYDELIG (IDELALISIB)
- Edited and updated warning September 2016
WARNING: FATAL AND SERIOUS TOXICITIES: HEPATIC, SEVERE DIARRHEA, COLITIS, PNEUMONITIS, INFECTIONS, AND INTESTINAL PERFORATION
- Fatal and/or serious hepatotoxicity occurred in 11 % to 18% of Zydelig-treated patients. Monitor hepatic function prior to and during treatment. Interrupt and then reduce or discontinue Zydelig as recommended.
- Fatal and/or serious and severe diarrhea or colitis occurred in 14% to 19% of Zydelig-treated patients. Monitor for the development of severe diarrhea or colitis. Interrupt and then reduce or discontinue Zydelig as recommended.
- Fatal and/or serious pneumonitis occurred in 4% of Zydelig-treated patients. Monitor for pulmonary symptoms and bilateral interstitial infiltrates. Interrupt or discontinue Zydelig as recommended.
- Fatal and/or serious infections occurred in 21% to 36% of Zydelig-treated patients. Monitor for signs and symptoms of infection. Interrupt Zydelig if infection is suspected.
- Fatal and serious intestinal perforation can occur in Zydelig-treated patients across clinical trials. Discontinue Zydelig for intestinal perforation.
The FDA’s MedWatch program safety labeling changes for boxed warnings are compiled quarterly for drugs and therapeutic biologics where important changes have been made to the safety information. You can search these and other label changes in the Drug Safety Labeling Changes (SLC) database, where data are available to the public in downloadable and searchable formats. Boxed warnings are ordinarily used to highlight either adverse reactions so serious in proportion to the potential bene t from the drug that it is essential that it be considered in assessing the risks and bene ts of using the drug; or serious adverse reactions that can be prevented/reduced in frequency or severity by appropriate use of the drug; or FDA approved the drug with restrictions to ensure safe use because FDA concluded that the drug can be safely used only if distribution or use is restricted.
QUINOLONE:
- Edited and updated warning September 2016
WARNING: SERIOUS ADVERSE REACTIONS INCLUDING TENDINITIS, TENDON RUPTURE, PERIPHERAL NEUROPATHY, CENTRAL NERVOUS SYSTEM EFFECTS AND EXACERBATION OF MYASTHENIA GRAVIS
Fluoroquinolones have been associated with disabling and potentially irreversible serious adverse reactions that have occurred together including:
- Tendinitis and tendon rupture
- Peripheral neuropathy
- Central nervous system effects
Discontinue immediately and avoid the use of fluoroquinolones in patients who experience any of these serious adverse reactions. Fluoroquinolones may exacerbate muscle weakness in patients with myasthenia gravis. Avoid quinolones in patients with known history of myasthenia gravis. Because fluoroquinolones
have been associated with serious adverse reactions, reserve quinolones for use in patients who have no alternative treatment options for the following
indications:
Avelox (moxifloxacin hydrochloride): Avelox in sodium chloride 0.8% in plastic container; moxifloxacin hydrochloride; Cipro in dextrose 5% in plastic container):
Acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis.
Cipro (ciprofloxacin; ciprofloxacin hydrochloride): Acute exacerbation of chronic bronchitis, acute uncomplicated cystitis, and acute sinusitis.
Cipro XR; Noroxin (norfloxacin): Uncomplicated urinary tract infections.
Factive (gemifloxacin mesylate): Acute bacterial exacerbation of chronic bronchitis.
Levaquin (levofloxacin): Uncomplicated urinary tract infection, acute bacterial exacerbation of chronic bronchitis, and acute bacterial sinusitis.
KRYSTEXXA (PEGLOTICASE):
- Added section to warning September 2016
WARNING: ANAPHYLAXIS AND INFUSION REACTIONS; G6PD DEFICIENCY ASSOCIATED HEMOLYSIS AND METHEMOGLOBINEMIA (Title Updated)
Addition of: Screen patients at risk for G6PD deficiency prior to starting Krystexxa. Hemolysis and methemoglobinemia have been reported with Krystexxa in patients with G6PD deficiency. Do not administer Krystexxa to patients with G6PD deficiency.
PLAVIX (CLOPIDOGREL BISULFATE):
- Edited and updated warning September 2016
WARNING: DIMINISHED ANTIPLATELET EFFECT IN PATIENTS WITH TWO LOSS-OF-FUNCTION ALLELES OF THE CYP2C19 GENE
The effectiveness of Plavix results from its antiplatelet activity, which is dependent on its conversion to an active metabolite by the cytochrome P450 (CYP) system, principally CYP2C19. Plavix at recommended doses forms less of the active metabolite and so has a reduced effect on platelet activity in patients who are homozygous for nonfunctional alleles of the CYP2C19 gene, (termed “CYP2C19 poor metabolizers”). Tests are available to identify patients who are CYP2C19 poor metabolizers. Consider use of another platelet P2Y12 inhibitor in patients identified as CYP2C19 poor metabolizers.
SYNJARDY (EMPAGLIFLOZIN; METFORMIN HYDROCHLORIDE):
- Edited and updated warning September 2016
Postmarketing cases of metformin-associated lactic acidosis have resulted in death, hypothermia, hypotension, and resistant bradyarrhythmias. The onset of metformin-associated lactic acidosis is often subtle, accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, somnolence, and abdominal pain. Metforminassociated lactic acidosis was characterized by elevated blood lactate levels (> 5 mmol/Liter), anion gap acidosis (without evidence of ketonuria or ketonemia), an increased lactate/pyruvate ratio; and metformin plasma levels generally > 5 mcg/mL.
Risk factors for metformin-associated lactic acidosis include renal impairment, concomitant use of certain drugs (e.g., carbonic anhydrase inhibitors such as topiramate), age 65 years old or greater, having a radiological study with contrast, surgery and other procedures, hypoxic states (e.g., acute congestive heart failure), excessive alcohol intake, and hepatic impairment.
Steps to reduce the risk of and manage metformin-associated lactic acidosis in these high-risk groups are provided in the full prescribing information.
If metformin-associated lactic acidosis is suspected, immediately discontinue Synjardy and institute general supportive measures in a hospital setting. Prompt hemodialysis is recommended.
ZYDELIG (IDELALISIB)
- Edited and updated warning September 2016
WARNING: FATAL AND SERIOUS TOXICITIES: HEPATIC, SEVERE DIARRHEA, COLITIS, PNEUMONITIS, INFECTIONS, AND INTESTINAL PERFORATION
- Fatal and/or serious hepatotoxicity occurred in 11 % to 18% of Zydelig-treated patients. Monitor hepatic function prior to and during treatment. Interrupt and then reduce or discontinue Zydelig as recommended.
- Fatal and/or serious and severe diarrhea or colitis occurred in 14% to 19% of Zydelig-treated patients. Monitor for the development of severe diarrhea or colitis. Interrupt and then reduce or discontinue Zydelig as recommended.
- Fatal and/or serious pneumonitis occurred in 4% of Zydelig-treated patients. Monitor for pulmonary symptoms and bilateral interstitial infiltrates. Interrupt or discontinue Zydelig as recommended.
- Fatal and/or serious infections occurred in 21% to 36% of Zydelig-treated patients. Monitor for signs and symptoms of infection. Interrupt Zydelig if infection is suspected.
- Fatal and serious intestinal perforation can occur in Zydelig-treated patients across clinical trials. Discontinue Zydelig for intestinal perforation.
A Primary Hospital Antimicrobial Stewardship Intervention on Pneumonia Treatment Duration
The safety and the efficacy of shorter durations of antibiotic therapy for uncomplicated pneumonia have been clearly established in the past decade.1,2 Guidelines from the Infectious Diseases Society of America (IDSA) and the American Thoracic Society have been available since 2007. These expert consensus statements recommend that uncomplicated community-acquired pneumonia (CAP) should be treated for 5 to 7 days, as long as the patient exhibits signs and symptoms of clinical stability.3 Similarly, recently updated guidelines for hospital-acquired and ventilator-associated pneumonias call for short-course therapy.4 Despite this guidance, pneumonia treatment duration is often discordant.5 Unnecessary antimicrobial use is associated with greater selection pressure on pathogens, increased risk of adverse events (AEs), and elevated treatment costs.6 The growing burden of antibiotic resistance coupled with limited availability of new antibiotics requires judicious use of these agents.
The IDSA guidelines for Clostridium difficile infection (CDI) note that exposure to antimicrobial agents is the most important modifiable risk factor for the development of CDI.7 Longer durations of antibiotics increase the risk of CDI compared with shorter durations.8,9 Antibiotics are a frequent cause of drug-associated AEs and likely are underestimated.10 To decrease the unwanted effects of excessive therapy, IDSA and CDC suggest that antimicrobial stewardship interventions should be implemented.11-13
Antimicrobial stewardship efforts in small community hospitals (also known as district, rural, general, and primary hospitals) are varied and can be challenging due to limited staff and resources.14,15 The World Health Organization defines a primary care facility as having few specialties, mainly internal medicine and general surgery with limited laboratory services for general (but not specialized) pathologic analysis, and bed size ranging from 30 to 200 beds.16 Although guidance is available for effective intervention strategies in smaller hospitals, there are limited data in the literature regarding successful outcomes.17-22
The purpose of this study was to establish the need and evaluate the impact of a pharmacy-initiated 3-part intervention targeting treatment duration in patients hospitalized with uncomplicated pneumonia in a primary hospital setting. The Veterans Health Care System of the Ozarks (VHSO) in Fayetteville, Arkansas, has 50 acute care beds, including 7 intensive care unit beds and excluding 15 mental health beds. The pharmacy is staffed 24 hours a day. Acute-care providers consist of 7 full-time hospitalists, not including nocturnists and contract physicians. The VHSO does not have an infectious disease physician on staff.
The antimicrobial stewardship committee consists of 3 clinical pharmacists, a pulmonologist, a pathologist, and 2 infection-control nurses. There is 1 full-time equivalent allotted for inpatient clinical pharmacy activities in the acute care areas, including enforcement of all antimicrobial stewardship policies, which are conducted by a single pharmacist.
Methods
This was a retrospective chart review of two 12-month periods using a before and after study design. Medical records were reviewed during October 2012 through September 2013 (before the stewardship implementation) and December 2014 through November 2015 (after implementation). Inclusion criteria consisted of a primary discharge diagnosis of pneumonia as documented by the provider (or secondary diagnosis if sepsis was primary), hospitalization for at least 48 hours, administration of antibiotics for a minimum of 24 hours, and survival to discharge.
Exclusion criteria consisted of direct transfer from another facility, inappropriate empiric therapy as evidenced by culture data (isolated pathogens not covered by prescribed antibiotics), pneumonia that developed 48 hours after admission, extrapulmonary sources of infection, hospitalization > 14 days, discharge without a known duration of outpatient antibiotics, discharge for pneumonia within 28 days prior to admission, documented infection caused by Pseudomonas aeruginosa or other nonlactose fermenting Gram-negative rod, and complicated pneumonias defined as lung abscess, empyema, or severe immunosuppression (eg, cancer with chemotherapy within the previous 30 days, transplant recipients, HIV infection, acquired or congenital immunodeficiency, or absolute neutrophil count 1,500 cell/mm3 within past 28 days).
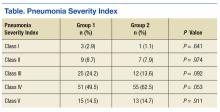
Patients were designated with health care-associated pneumonia (HCAP) if they were hospitalized ≥ 2 days or resided in a skilled nursing or extended-care facility within the previous 90 days; on chronic dialysis; or had wound care, tracheostomy care, or ventilator care from a health care professional within the previous 28 days. Criteria for clinical stability were defined as ≤ 100.4º F temperature, ≤ 100 beats/min heart rate, ≤ 24 breaths/min respiratory rate, ≥ 90 mm Hg systolic blood pressure, ≥ 90% or PaO2 ≥ 60 mm Hg oxygen saturation on room air (or baseline oxygen requirements), and return to baseline mental status. To compare groups, researchers tabulated the pneumonia severity index on hospital day 1.
The intervention consisted of a 3-part process. First, hospitalists were educated on VHSO’s baseline treatment duration data, and these were compared with current IDSA recommendations. The education was followed by an open-discussion component to solicit feedback from providers on perceived barriers to following guidelines. Provider feedback was used to tailor an antimicrobial stewardship intervention to address perceived barriers to optimal antibiotic treatment duration.
After the education component, prospective intervention and feedback were provided for hospitalized patients by a single clinical pharmacist. This pharmacist interacted verbally and in writing with the patients’ providers, discussing antimicrobial appropriateness, de-escalation, duration of therapy, and intravenous to oral switching. Finally, a stewardship note for the Computerized Patient Record System (CPRS) was generated and included a template with reminders of clinical stability, duration of current therapy, and a request to discontinue therapy if the patient met criteria. For patients who remained hospitalized, this note was entered into CPRS on or about day 7 of antibiotic therapy; this required an electronic signature from the provider.
The VHSO Pharmacy and Therapeutics Committee approved both the provider education and the stewardship note in November 2014, and implementation of the stewardship intervention occurred immediately afterward. The pharmacy staff also was educated on the VHSO baseline data and stewardship efforts.
The primary outcome of the study was the change in days of total antibiotic treatment. Secondary outcomes included days of intravenous antibiotic therapy, days of inpatient oral therapy, mean length of stay (LOS), and number of outpatient antibiotic days once discharged. Incidence of CDI and 28-day readmissions were also evaluated. The VHSO Institutional Review Board approved these methods and the procedures that followed were in accord with the ethical standards of the VHSO Committee on Human Experimentation.
Statistical Analysis
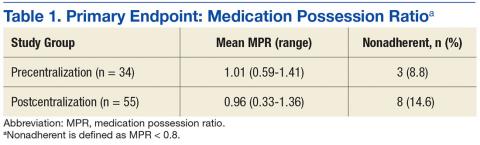
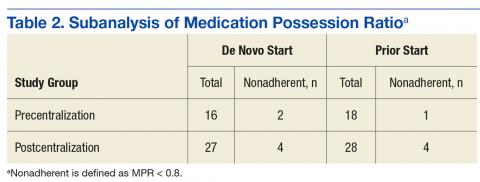
All continuous variables are reported as mean ± standard deviation. Data analysis for significance was performed using a Student t test for continuous variables and a χ2 test (or Fisher exact test) for categorical variables in R Foundation for Statistical Computing version 3.1.0. All samples were 2-tailed. A P value < .05 was considered statistically significant. Using the smaller of the 2 study populations, the investigators calculated that the given sample size of 88 in each group would provide 99% power to detect a 2-day difference in the primary endpoint at a 2-sided significance level of 5%.
Results
During the baseline assessment (group 1), 192 cases were reviewed with 103 meeting the inclusion criteria. Group 1 consisted of 85 cases of CAP and 18 cases of HCAP (mean age, 70.7 years). During the follow-up assessment (group 2), 168 cases were reviewed with 88 meeting the inclusion criteria. Group 2 consisted of 68 cases of CAP and 20 cases of HCAP (mean age, 70.8 years).
There was no difference in inpatient mortality rates between groups (3.1% vs 3.0%, P = .99). This mortality rate is consistent with published reports.23 Empiric antibiotic selection was appropriate because there were no exclusions for drug/pathogen mismatch. Pneumonia severity was similar in both groups (Table).
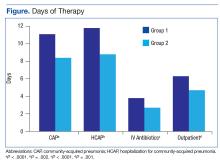
The total duration of antibiotic treatment decreased significantly for CAP and HCAP (Figure). The observed median treatment days for groups 1 and 2 were 11 days and 8 days, respectively. Outpatient antibiotic days also decreased. Mean LOS was shorter in the follow-up group (4.9 ± 2.6 days vs 4.0 ± 2.6 days, P = .02). Length of IV antibiotic duration decreased. Oral antibiotic days while inpatient were not statistically different (1.5 ± 1.8 days vs 1.1 ± 1.5 days, P = .15). During the follow-up period, 26 stewardship notes were entered into CPRS; antibiotics were stopped in 65% of cases.
There were no recorded cases of CDI in either group. There were eleven 28-day readmissions in group 1, only 3 of which were due to infectious causes. One patient had a primary diagnosis of necrotizing pneumonia, 1 had Pseudomonas pneumonia, and 1 patient had a new lung mass and was diagnosed with postobstructive pneumonia. Of eight 28-day readmissions in group 2, only 2 resulted from infectious causes. One readmission primary diagnosis was sinusitis and 1 was recurrent pneumonia (of note, this patient received a 10-day treatment course for pneumonia on initial admission). Two patients died within 28 days of discharge in each group.
Discussion
Other multifaceted single-center interventions have been shown to be effective in large, teaching hospitals,24,25 and it has been suggested that smaller, rural hospitals may be underserved in antimicrobial stewardship activities.26,27 In the global struggle with antimicrobial resistance, McGregor and colleagues highlighted the importance of evaluating successful stewardship methods in an array of clinical settings to help tailor an approach for a specific type of facility.28 To the authors knowledge, this is the first publication showing efficacy of such antimicrobial stewardship interventions specific to pneumonia therapy in a small, primary facility.
The intervention methods used at VHSO are supported by recent IDSA and Society for Healthcare Epidemiology of America guidelines for effective stewardship implementation.29 Prospective audit and feedback is considered a core recommendation, whereas didactic education is recommended only in conjunction with other stewardship activities. Additionally, the guidelines recommend evaluating specific infectious disease syndromes, in this case uncomplicated pneumonia, to focus on specific treatment guidelines. Last, the results of the 3-part intervention can be used to aid in demonstrating facility improvement and encourage continued success.
Of note, VHSO has had established inpatient and outpatient clinical pharmacy roles for several years. Stewardship interventions already in place included an intravenous-to-oral antibiotic switch policy, automatic antibiotic stop dates, as well as pharmacist-driven vancomycin and aminoglycoside dosing. Prior to this multifaceted intervention specific to pneumonia duration, prospective audit and feedback interventions (verbal and written) also were common. The number of interventions specific to this study outside of the stewardship note was not recorded. Using rapid diagnostic testing and biomarkers to aid in stewardship activities at VHSO have been considered, but these tools are not available due to a lab personnel shortage.
Soliciting feedback from providers on their preferred stewardship strategy and perceived barriers was a key component of the educational intervention. Of equal importance was presenting providers with their baseline prescribing data to provide objective evidence of a problem. While all were familiar with existing treatment guidelines, some feedback indicated that it can be difficult to determine accurate antibiotic duration in CPRS. Prescribers reported that identifying antibiotic duration was especially challenging when antibiotics as well as providers change during an admission. Also frequently overlooked were antibiotics given in the emergency department. This could be a key area for clinical pharmacists’ intervention given their familiarity with the CPRS medication sections.
Charani and colleagues suggest that recognizing barriers to implementing best practices and adapting to the local facility culture is paramount for changing prescribing behaviors and developing a successful stewardship intervention.30 At VHSO, the providers were presented with multiple stewardship options but agreed to the new note and template. This process gave providers a voice in selecting their own stewardship intervention. In a culture with no infectious disease physician to champion initiatives, the investigators felt that provider involvement in the intervention selection was unique and may have encouraged provider concurrence.
Although not directly targeted by the intervention strategies, average LOS was shorter in the follow-up group. According to investigators, frequent reminders of clinical stability in the stewardship notes may have influenced this. Even though the note was used only in patients who remained hospitalized for their entire treatment course, investigators felt that it still served as a reminder for prescribing habits as they were also able to show a decrease in outpatient prescription duration.
Limitations
Potential weaknesses of the study include changes in providers. During the transition between group 1 and group 2, 2 hospitalists left and 2 new hospitalists arrived. Given the small size of the staff, this could significantly impact prescribing trends. Another potential weakness is the high exclusion rate, although these rates were similar in both groups (46% group 1, 47% group 2). Furthermore, similar exclusion rates have been reported elsewhere.24,25,31 The most common reasons for exclusion were complicated pneumonias (36%) and immunocompromised patients (18%). These patient populations were not evaluated in the current study, and optimal treatment durations are unknown. Hospital-acquired and ventilator-associated pneumonias also were excluded. Therefore, limitations in applicability of the results should be noted.
The authors acknowledge that, prior to this publication, the IDSA guidelines have removed the designation of HCAP as a separate clinical entity.4 However, this should not affect the significance of the intervention for treatment duration.
The study facility experienced a hiring freeze resulting in a 9.3% decrease in overall admissions from fiscal year 2013 to fiscal year 2015. This is likely why there were fewer admissions for pneumonia in group 2. Regardless, power analysis revealed the study was of adequate sample size to detect its primary outcome. It is possible that patients in either group could have sought health care at other facilities, making the CDI and readmission endpoints less inclusive.
The study was not of a scale to detect changes in antimicrobial resistance pressure or clinical outcomes. Cost savings were not analyzed. However, this study adds to the growing body of evidence that a structured intervention can result in positive outcomes at the facility level. This study shows that interventions targeting pneumonia treatment duration could feasibly be added to the menu of stewardship options available to smaller facilities.
Like other stewardship studies in the literature, the follow-up treatment duration, while improved, still exceeded those recommended in the IDSA guidelines. The investigators noted that not all providers were equal regarding change in prescribing habits, perhaps making the average duration longer. Additionally, the request to discontinue antibiotic therapy through the stewardship note could have been entered earlier (eg, as early as day 5 of therapy) to target the shortest effective date as recommended in the recent stewardship guidelines.29 Future steps include continued feedback to providers on their progress in this area and encouragement to document day of antibiotic treatment in their daily progress notes.
Conclusion
This study showed a significant decrease in antibiotic duration for the treatment of uncomplicated pneumonia using a 3-part pharmacy intervention in a primary hospital setting. The investigators feel that each arm of the strategy was equally important and fewer interventions were not likely to be as effective.32 Although data collection for baseline prescribing and follow-up on outcomes may be a time-consuming task, it can be a valuable component of successful stewardship interventions.
1. Li JZ, Winston LG, Moore DH, Bent S. Efficacy of short-course antibiotic regimens for community-acquired pneumonia: a meta-analysis. Am J Med. 2007;120(9):783-790.
2. Dimopoulos G, Matthaiou DK, Karageorgopoulos DE, Grammatikos AP, Athanassa Z, Falagas ME. Short- versus long-course antibacterial therapy of community-acquired pneumonia: a meta-analysis. Drugs. 2008;68(13):1841-1854.
3. Mandell LA, Wunderink RG, Anzueto A, et al; Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72.
4. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61-e111.
5. Jenkins TC, Stella SA, Cervantes L, et al. Targets for antibiotic and healthcare resource stewardship in inpatient community-acquired pneumonia: a comparison of management practices with National Guideline Recommendations. Infection. 2013; 41(1):135-144.
6. Shlaes DM, Gerding DN, John JF Jr, et al. Society for Healthcare Epidemiology of America, and Infectious Diseases Society of America Joint Committee on the Prevention of Antimicrobial Resistance: guidelines for the prevention of antimicrobial resistance in hospitals. Clin Infect Dis. 1997;25(3):584-599.
7. Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455.
8. Brown E, Talbot GH, Axelrod P, Provencher M, Hoegg C. Risk factors for Clostridium-difficile toxin-associated diarrhea. Infect Control Hosp Epidemiol. 1990;11(6):283-290.
9. McFarland LV, Surawicz CM, Stamm WE. Risk factors for Clostridium-difficile carriage and C. difficile-associated diarrhea in a cohort of hospitalized patients. J Infect Dis. 1990;162(3):678-684.
10. Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47(6):735-743.
11. Dellit TH, Owens RC, McGowan JE Jr, et al; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America Guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159-177.
12. Fridkin S, Baggs J, Fagan R, et al; Centers for Disease Control and Prevention (CDC). Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194-200.
13. Nussenblatt V, Avdic E, Cosgrove S. What is the role of antimicrobial stewardship in improving outcomes of patients with CAP? Infect Dis Clin North Am. 2013;27(1):211-228.
14. Septimus EJ, Owens RC Jr. Need and potential of antimicrobial stewardship in community hospitals. Clin Infect Dis. 2011;53(suppl 1):S8-S14.
15. Hensher M, Price M, Adomakoh S. Referral hospitals. In Jamison DT, Breman JG, Measham AR, eds, et al. Disease Control Priorities in Developing Countries. New York, NY: Oxford University Press; 2006:1230.
16. Mulligan J, Fox-Rushby JA, Adam T, Johns B, Mills A. Unit costs of health care inputs in low and middle income regions. 2003. Working Paper 9, Disease Control Priorities Project. Published September 2003. Revised June 2005.
17. Ohl CA, Dodds Ashley ES. Antimicrobial stewardship programs in community hospitals: the evidence base and case studies. Clin Infect Dis 2011;53(suppl 1):S23-S28.
18. Trevidi KK, Kuper K. Hospital antimicrobial stewardship in the nonuniversity setting. Infect Dis Clin North Am. 2014;28(2):281-289.
19. Yam P, Fales D, Jemison J, Gillum M, Bernstein M. Implementation of an antimicrobial stewardship program in a rural hospital. Am J Health Syst Pharm. 2012;69(13);1142-1148.
20. LaRocco A Jr. Concurrent antibiotic review programs—a role for infectious diseases specialists at small community hospitals. Clin Infect Dis. 2003;37(5):742-743.
21. Bartlett JM, Siola PL. Implementation and first-year results of an antimicrobial stewardship program at a community hospital. Am J Health Syst Pharm. 2014;71(11):943-949.
22. Storey DF, Pate PG, Nguyen AT, Chang F. Implementation of an antimicrobial stewardship program on the medical-surgical service of a 100-bed community hospital. Antimicrob Resist Infect Control. 2012;1(1):32.
23. Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA. 1996;275(2):134-141.
24. Advic E, Cushinotto LA, Hughes AH, et al. Impact of an antimicrobial stewardship intervention on shortening the duration of therapy for community-acquired pneumonia. Clin Infect Dis. 2012;54(11):1581-1587.
25. Carratallà J, Garcia-Vidal C, Ortega L, et al. Effect of a 3-step critical pathway to reduce duration of intravenous antibiotic therapy and length of stay in community-acquired pneumonia: a randomized controlled trial. Arch Intern Med. 2012;172(12):922-928.
26. Stevenson KB, Samore M, Barbera J, et al. Pharmacist involvement in antimicrobial use at rural community hospitals in four Western states. Am J Health Syst Pharm. 2004;61(8):787-792.
27. Reese SM, Gilmartin H, Rich KL, Price CS. Infection prevention needs assessment in Colorado hospitals: rural and urban settings. Am J Infect Control. 2014;42(6):597-601.
28. McGregor JC, Furuno JP. Optimizing research methods used for the evaluation of antimicrobial stewardship programs. Clin Infect Dis. 2014;59(suppl 3):S185-S192.
29. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77.
30. Charani E, Castro-Sánchez E, Holmes A. The role of behavior change in antimicrobial stewardship. Infect Dis Clin N Am. 2014;28(2):169-175.
31. Attridge RT, Frei CR, Restrepo MI, et al. Guideline-concordant therapy and outcomes in healthcare-associated pneumonia. Eur Respir J. 2011;38(4):878-887.
32. MacDougal C, Polk RE. Antimicrobial stewardship programs in health care systems. Clin Microbiol Rev. 2005;18(4):638-656.
The safety and the efficacy of shorter durations of antibiotic therapy for uncomplicated pneumonia have been clearly established in the past decade.1,2 Guidelines from the Infectious Diseases Society of America (IDSA) and the American Thoracic Society have been available since 2007. These expert consensus statements recommend that uncomplicated community-acquired pneumonia (CAP) should be treated for 5 to 7 days, as long as the patient exhibits signs and symptoms of clinical stability.3 Similarly, recently updated guidelines for hospital-acquired and ventilator-associated pneumonias call for short-course therapy.4 Despite this guidance, pneumonia treatment duration is often discordant.5 Unnecessary antimicrobial use is associated with greater selection pressure on pathogens, increased risk of adverse events (AEs), and elevated treatment costs.6 The growing burden of antibiotic resistance coupled with limited availability of new antibiotics requires judicious use of these agents.
The IDSA guidelines for Clostridium difficile infection (CDI) note that exposure to antimicrobial agents is the most important modifiable risk factor for the development of CDI.7 Longer durations of antibiotics increase the risk of CDI compared with shorter durations.8,9 Antibiotics are a frequent cause of drug-associated AEs and likely are underestimated.10 To decrease the unwanted effects of excessive therapy, IDSA and CDC suggest that antimicrobial stewardship interventions should be implemented.11-13
Antimicrobial stewardship efforts in small community hospitals (also known as district, rural, general, and primary hospitals) are varied and can be challenging due to limited staff and resources.14,15 The World Health Organization defines a primary care facility as having few specialties, mainly internal medicine and general surgery with limited laboratory services for general (but not specialized) pathologic analysis, and bed size ranging from 30 to 200 beds.16 Although guidance is available for effective intervention strategies in smaller hospitals, there are limited data in the literature regarding successful outcomes.17-22
The purpose of this study was to establish the need and evaluate the impact of a pharmacy-initiated 3-part intervention targeting treatment duration in patients hospitalized with uncomplicated pneumonia in a primary hospital setting. The Veterans Health Care System of the Ozarks (VHSO) in Fayetteville, Arkansas, has 50 acute care beds, including 7 intensive care unit beds and excluding 15 mental health beds. The pharmacy is staffed 24 hours a day. Acute-care providers consist of 7 full-time hospitalists, not including nocturnists and contract physicians. The VHSO does not have an infectious disease physician on staff.
The antimicrobial stewardship committee consists of 3 clinical pharmacists, a pulmonologist, a pathologist, and 2 infection-control nurses. There is 1 full-time equivalent allotted for inpatient clinical pharmacy activities in the acute care areas, including enforcement of all antimicrobial stewardship policies, which are conducted by a single pharmacist.
Methods
This was a retrospective chart review of two 12-month periods using a before and after study design. Medical records were reviewed during October 2012 through September 2013 (before the stewardship implementation) and December 2014 through November 2015 (after implementation). Inclusion criteria consisted of a primary discharge diagnosis of pneumonia as documented by the provider (or secondary diagnosis if sepsis was primary), hospitalization for at least 48 hours, administration of antibiotics for a minimum of 24 hours, and survival to discharge.
Exclusion criteria consisted of direct transfer from another facility, inappropriate empiric therapy as evidenced by culture data (isolated pathogens not covered by prescribed antibiotics), pneumonia that developed 48 hours after admission, extrapulmonary sources of infection, hospitalization > 14 days, discharge without a known duration of outpatient antibiotics, discharge for pneumonia within 28 days prior to admission, documented infection caused by Pseudomonas aeruginosa or other nonlactose fermenting Gram-negative rod, and complicated pneumonias defined as lung abscess, empyema, or severe immunosuppression (eg, cancer with chemotherapy within the previous 30 days, transplant recipients, HIV infection, acquired or congenital immunodeficiency, or absolute neutrophil count 1,500 cell/mm3 within past 28 days).
Patients were designated with health care-associated pneumonia (HCAP) if they were hospitalized ≥ 2 days or resided in a skilled nursing or extended-care facility within the previous 90 days; on chronic dialysis; or had wound care, tracheostomy care, or ventilator care from a health care professional within the previous 28 days. Criteria for clinical stability were defined as ≤ 100.4º F temperature, ≤ 100 beats/min heart rate, ≤ 24 breaths/min respiratory rate, ≥ 90 mm Hg systolic blood pressure, ≥ 90% or PaO2 ≥ 60 mm Hg oxygen saturation on room air (or baseline oxygen requirements), and return to baseline mental status. To compare groups, researchers tabulated the pneumonia severity index on hospital day 1.
The intervention consisted of a 3-part process. First, hospitalists were educated on VHSO’s baseline treatment duration data, and these were compared with current IDSA recommendations. The education was followed by an open-discussion component to solicit feedback from providers on perceived barriers to following guidelines. Provider feedback was used to tailor an antimicrobial stewardship intervention to address perceived barriers to optimal antibiotic treatment duration.
After the education component, prospective intervention and feedback were provided for hospitalized patients by a single clinical pharmacist. This pharmacist interacted verbally and in writing with the patients’ providers, discussing antimicrobial appropriateness, de-escalation, duration of therapy, and intravenous to oral switching. Finally, a stewardship note for the Computerized Patient Record System (CPRS) was generated and included a template with reminders of clinical stability, duration of current therapy, and a request to discontinue therapy if the patient met criteria. For patients who remained hospitalized, this note was entered into CPRS on or about day 7 of antibiotic therapy; this required an electronic signature from the provider.
The VHSO Pharmacy and Therapeutics Committee approved both the provider education and the stewardship note in November 2014, and implementation of the stewardship intervention occurred immediately afterward. The pharmacy staff also was educated on the VHSO baseline data and stewardship efforts.
The primary outcome of the study was the change in days of total antibiotic treatment. Secondary outcomes included days of intravenous antibiotic therapy, days of inpatient oral therapy, mean length of stay (LOS), and number of outpatient antibiotic days once discharged. Incidence of CDI and 28-day readmissions were also evaluated. The VHSO Institutional Review Board approved these methods and the procedures that followed were in accord with the ethical standards of the VHSO Committee on Human Experimentation.
Statistical Analysis
All continuous variables are reported as mean ± standard deviation. Data analysis for significance was performed using a Student t test for continuous variables and a χ2 test (or Fisher exact test) for categorical variables in R Foundation for Statistical Computing version 3.1.0. All samples were 2-tailed. A P value < .05 was considered statistically significant. Using the smaller of the 2 study populations, the investigators calculated that the given sample size of 88 in each group would provide 99% power to detect a 2-day difference in the primary endpoint at a 2-sided significance level of 5%.
Results
During the baseline assessment (group 1), 192 cases were reviewed with 103 meeting the inclusion criteria. Group 1 consisted of 85 cases of CAP and 18 cases of HCAP (mean age, 70.7 years). During the follow-up assessment (group 2), 168 cases were reviewed with 88 meeting the inclusion criteria. Group 2 consisted of 68 cases of CAP and 20 cases of HCAP (mean age, 70.8 years).
There was no difference in inpatient mortality rates between groups (3.1% vs 3.0%, P = .99). This mortality rate is consistent with published reports.23 Empiric antibiotic selection was appropriate because there were no exclusions for drug/pathogen mismatch. Pneumonia severity was similar in both groups (Table).
The total duration of antibiotic treatment decreased significantly for CAP and HCAP (Figure). The observed median treatment days for groups 1 and 2 were 11 days and 8 days, respectively. Outpatient antibiotic days also decreased. Mean LOS was shorter in the follow-up group (4.9 ± 2.6 days vs 4.0 ± 2.6 days, P = .02). Length of IV antibiotic duration decreased. Oral antibiotic days while inpatient were not statistically different (1.5 ± 1.8 days vs 1.1 ± 1.5 days, P = .15). During the follow-up period, 26 stewardship notes were entered into CPRS; antibiotics were stopped in 65% of cases.
There were no recorded cases of CDI in either group. There were eleven 28-day readmissions in group 1, only 3 of which were due to infectious causes. One patient had a primary diagnosis of necrotizing pneumonia, 1 had Pseudomonas pneumonia, and 1 patient had a new lung mass and was diagnosed with postobstructive pneumonia. Of eight 28-day readmissions in group 2, only 2 resulted from infectious causes. One readmission primary diagnosis was sinusitis and 1 was recurrent pneumonia (of note, this patient received a 10-day treatment course for pneumonia on initial admission). Two patients died within 28 days of discharge in each group.
Discussion
Other multifaceted single-center interventions have been shown to be effective in large, teaching hospitals,24,25 and it has been suggested that smaller, rural hospitals may be underserved in antimicrobial stewardship activities.26,27 In the global struggle with antimicrobial resistance, McGregor and colleagues highlighted the importance of evaluating successful stewardship methods in an array of clinical settings to help tailor an approach for a specific type of facility.28 To the authors knowledge, this is the first publication showing efficacy of such antimicrobial stewardship interventions specific to pneumonia therapy in a small, primary facility.
The intervention methods used at VHSO are supported by recent IDSA and Society for Healthcare Epidemiology of America guidelines for effective stewardship implementation.29 Prospective audit and feedback is considered a core recommendation, whereas didactic education is recommended only in conjunction with other stewardship activities. Additionally, the guidelines recommend evaluating specific infectious disease syndromes, in this case uncomplicated pneumonia, to focus on specific treatment guidelines. Last, the results of the 3-part intervention can be used to aid in demonstrating facility improvement and encourage continued success.
Of note, VHSO has had established inpatient and outpatient clinical pharmacy roles for several years. Stewardship interventions already in place included an intravenous-to-oral antibiotic switch policy, automatic antibiotic stop dates, as well as pharmacist-driven vancomycin and aminoglycoside dosing. Prior to this multifaceted intervention specific to pneumonia duration, prospective audit and feedback interventions (verbal and written) also were common. The number of interventions specific to this study outside of the stewardship note was not recorded. Using rapid diagnostic testing and biomarkers to aid in stewardship activities at VHSO have been considered, but these tools are not available due to a lab personnel shortage.
Soliciting feedback from providers on their preferred stewardship strategy and perceived barriers was a key component of the educational intervention. Of equal importance was presenting providers with their baseline prescribing data to provide objective evidence of a problem. While all were familiar with existing treatment guidelines, some feedback indicated that it can be difficult to determine accurate antibiotic duration in CPRS. Prescribers reported that identifying antibiotic duration was especially challenging when antibiotics as well as providers change during an admission. Also frequently overlooked were antibiotics given in the emergency department. This could be a key area for clinical pharmacists’ intervention given their familiarity with the CPRS medication sections.
Charani and colleagues suggest that recognizing barriers to implementing best practices and adapting to the local facility culture is paramount for changing prescribing behaviors and developing a successful stewardship intervention.30 At VHSO, the providers were presented with multiple stewardship options but agreed to the new note and template. This process gave providers a voice in selecting their own stewardship intervention. In a culture with no infectious disease physician to champion initiatives, the investigators felt that provider involvement in the intervention selection was unique and may have encouraged provider concurrence.
Although not directly targeted by the intervention strategies, average LOS was shorter in the follow-up group. According to investigators, frequent reminders of clinical stability in the stewardship notes may have influenced this. Even though the note was used only in patients who remained hospitalized for their entire treatment course, investigators felt that it still served as a reminder for prescribing habits as they were also able to show a decrease in outpatient prescription duration.
Limitations
Potential weaknesses of the study include changes in providers. During the transition between group 1 and group 2, 2 hospitalists left and 2 new hospitalists arrived. Given the small size of the staff, this could significantly impact prescribing trends. Another potential weakness is the high exclusion rate, although these rates were similar in both groups (46% group 1, 47% group 2). Furthermore, similar exclusion rates have been reported elsewhere.24,25,31 The most common reasons for exclusion were complicated pneumonias (36%) and immunocompromised patients (18%). These patient populations were not evaluated in the current study, and optimal treatment durations are unknown. Hospital-acquired and ventilator-associated pneumonias also were excluded. Therefore, limitations in applicability of the results should be noted.
The authors acknowledge that, prior to this publication, the IDSA guidelines have removed the designation of HCAP as a separate clinical entity.4 However, this should not affect the significance of the intervention for treatment duration.
The study facility experienced a hiring freeze resulting in a 9.3% decrease in overall admissions from fiscal year 2013 to fiscal year 2015. This is likely why there were fewer admissions for pneumonia in group 2. Regardless, power analysis revealed the study was of adequate sample size to detect its primary outcome. It is possible that patients in either group could have sought health care at other facilities, making the CDI and readmission endpoints less inclusive.
The study was not of a scale to detect changes in antimicrobial resistance pressure or clinical outcomes. Cost savings were not analyzed. However, this study adds to the growing body of evidence that a structured intervention can result in positive outcomes at the facility level. This study shows that interventions targeting pneumonia treatment duration could feasibly be added to the menu of stewardship options available to smaller facilities.
Like other stewardship studies in the literature, the follow-up treatment duration, while improved, still exceeded those recommended in the IDSA guidelines. The investigators noted that not all providers were equal regarding change in prescribing habits, perhaps making the average duration longer. Additionally, the request to discontinue antibiotic therapy through the stewardship note could have been entered earlier (eg, as early as day 5 of therapy) to target the shortest effective date as recommended in the recent stewardship guidelines.29 Future steps include continued feedback to providers on their progress in this area and encouragement to document day of antibiotic treatment in their daily progress notes.
Conclusion
This study showed a significant decrease in antibiotic duration for the treatment of uncomplicated pneumonia using a 3-part pharmacy intervention in a primary hospital setting. The investigators feel that each arm of the strategy was equally important and fewer interventions were not likely to be as effective.32 Although data collection for baseline prescribing and follow-up on outcomes may be a time-consuming task, it can be a valuable component of successful stewardship interventions.
The safety and the efficacy of shorter durations of antibiotic therapy for uncomplicated pneumonia have been clearly established in the past decade.1,2 Guidelines from the Infectious Diseases Society of America (IDSA) and the American Thoracic Society have been available since 2007. These expert consensus statements recommend that uncomplicated community-acquired pneumonia (CAP) should be treated for 5 to 7 days, as long as the patient exhibits signs and symptoms of clinical stability.3 Similarly, recently updated guidelines for hospital-acquired and ventilator-associated pneumonias call for short-course therapy.4 Despite this guidance, pneumonia treatment duration is often discordant.5 Unnecessary antimicrobial use is associated with greater selection pressure on pathogens, increased risk of adverse events (AEs), and elevated treatment costs.6 The growing burden of antibiotic resistance coupled with limited availability of new antibiotics requires judicious use of these agents.
The IDSA guidelines for Clostridium difficile infection (CDI) note that exposure to antimicrobial agents is the most important modifiable risk factor for the development of CDI.7 Longer durations of antibiotics increase the risk of CDI compared with shorter durations.8,9 Antibiotics are a frequent cause of drug-associated AEs and likely are underestimated.10 To decrease the unwanted effects of excessive therapy, IDSA and CDC suggest that antimicrobial stewardship interventions should be implemented.11-13
Antimicrobial stewardship efforts in small community hospitals (also known as district, rural, general, and primary hospitals) are varied and can be challenging due to limited staff and resources.14,15 The World Health Organization defines a primary care facility as having few specialties, mainly internal medicine and general surgery with limited laboratory services for general (but not specialized) pathologic analysis, and bed size ranging from 30 to 200 beds.16 Although guidance is available for effective intervention strategies in smaller hospitals, there are limited data in the literature regarding successful outcomes.17-22
The purpose of this study was to establish the need and evaluate the impact of a pharmacy-initiated 3-part intervention targeting treatment duration in patients hospitalized with uncomplicated pneumonia in a primary hospital setting. The Veterans Health Care System of the Ozarks (VHSO) in Fayetteville, Arkansas, has 50 acute care beds, including 7 intensive care unit beds and excluding 15 mental health beds. The pharmacy is staffed 24 hours a day. Acute-care providers consist of 7 full-time hospitalists, not including nocturnists and contract physicians. The VHSO does not have an infectious disease physician on staff.
The antimicrobial stewardship committee consists of 3 clinical pharmacists, a pulmonologist, a pathologist, and 2 infection-control nurses. There is 1 full-time equivalent allotted for inpatient clinical pharmacy activities in the acute care areas, including enforcement of all antimicrobial stewardship policies, which are conducted by a single pharmacist.
Methods
This was a retrospective chart review of two 12-month periods using a before and after study design. Medical records were reviewed during October 2012 through September 2013 (before the stewardship implementation) and December 2014 through November 2015 (after implementation). Inclusion criteria consisted of a primary discharge diagnosis of pneumonia as documented by the provider (or secondary diagnosis if sepsis was primary), hospitalization for at least 48 hours, administration of antibiotics for a minimum of 24 hours, and survival to discharge.
Exclusion criteria consisted of direct transfer from another facility, inappropriate empiric therapy as evidenced by culture data (isolated pathogens not covered by prescribed antibiotics), pneumonia that developed 48 hours after admission, extrapulmonary sources of infection, hospitalization > 14 days, discharge without a known duration of outpatient antibiotics, discharge for pneumonia within 28 days prior to admission, documented infection caused by Pseudomonas aeruginosa or other nonlactose fermenting Gram-negative rod, and complicated pneumonias defined as lung abscess, empyema, or severe immunosuppression (eg, cancer with chemotherapy within the previous 30 days, transplant recipients, HIV infection, acquired or congenital immunodeficiency, or absolute neutrophil count 1,500 cell/mm3 within past 28 days).
Patients were designated with health care-associated pneumonia (HCAP) if they were hospitalized ≥ 2 days or resided in a skilled nursing or extended-care facility within the previous 90 days; on chronic dialysis; or had wound care, tracheostomy care, or ventilator care from a health care professional within the previous 28 days. Criteria for clinical stability were defined as ≤ 100.4º F temperature, ≤ 100 beats/min heart rate, ≤ 24 breaths/min respiratory rate, ≥ 90 mm Hg systolic blood pressure, ≥ 90% or PaO2 ≥ 60 mm Hg oxygen saturation on room air (or baseline oxygen requirements), and return to baseline mental status. To compare groups, researchers tabulated the pneumonia severity index on hospital day 1.
The intervention consisted of a 3-part process. First, hospitalists were educated on VHSO’s baseline treatment duration data, and these were compared with current IDSA recommendations. The education was followed by an open-discussion component to solicit feedback from providers on perceived barriers to following guidelines. Provider feedback was used to tailor an antimicrobial stewardship intervention to address perceived barriers to optimal antibiotic treatment duration.
After the education component, prospective intervention and feedback were provided for hospitalized patients by a single clinical pharmacist. This pharmacist interacted verbally and in writing with the patients’ providers, discussing antimicrobial appropriateness, de-escalation, duration of therapy, and intravenous to oral switching. Finally, a stewardship note for the Computerized Patient Record System (CPRS) was generated and included a template with reminders of clinical stability, duration of current therapy, and a request to discontinue therapy if the patient met criteria. For patients who remained hospitalized, this note was entered into CPRS on or about day 7 of antibiotic therapy; this required an electronic signature from the provider.
The VHSO Pharmacy and Therapeutics Committee approved both the provider education and the stewardship note in November 2014, and implementation of the stewardship intervention occurred immediately afterward. The pharmacy staff also was educated on the VHSO baseline data and stewardship efforts.
The primary outcome of the study was the change in days of total antibiotic treatment. Secondary outcomes included days of intravenous antibiotic therapy, days of inpatient oral therapy, mean length of stay (LOS), and number of outpatient antibiotic days once discharged. Incidence of CDI and 28-day readmissions were also evaluated. The VHSO Institutional Review Board approved these methods and the procedures that followed were in accord with the ethical standards of the VHSO Committee on Human Experimentation.
Statistical Analysis
All continuous variables are reported as mean ± standard deviation. Data analysis for significance was performed using a Student t test for continuous variables and a χ2 test (or Fisher exact test) for categorical variables in R Foundation for Statistical Computing version 3.1.0. All samples were 2-tailed. A P value < .05 was considered statistically significant. Using the smaller of the 2 study populations, the investigators calculated that the given sample size of 88 in each group would provide 99% power to detect a 2-day difference in the primary endpoint at a 2-sided significance level of 5%.
Results
During the baseline assessment (group 1), 192 cases were reviewed with 103 meeting the inclusion criteria. Group 1 consisted of 85 cases of CAP and 18 cases of HCAP (mean age, 70.7 years). During the follow-up assessment (group 2), 168 cases were reviewed with 88 meeting the inclusion criteria. Group 2 consisted of 68 cases of CAP and 20 cases of HCAP (mean age, 70.8 years).
There was no difference in inpatient mortality rates between groups (3.1% vs 3.0%, P = .99). This mortality rate is consistent with published reports.23 Empiric antibiotic selection was appropriate because there were no exclusions for drug/pathogen mismatch. Pneumonia severity was similar in both groups (Table).
The total duration of antibiotic treatment decreased significantly for CAP and HCAP (Figure). The observed median treatment days for groups 1 and 2 were 11 days and 8 days, respectively. Outpatient antibiotic days also decreased. Mean LOS was shorter in the follow-up group (4.9 ± 2.6 days vs 4.0 ± 2.6 days, P = .02). Length of IV antibiotic duration decreased. Oral antibiotic days while inpatient were not statistically different (1.5 ± 1.8 days vs 1.1 ± 1.5 days, P = .15). During the follow-up period, 26 stewardship notes were entered into CPRS; antibiotics were stopped in 65% of cases.
There were no recorded cases of CDI in either group. There were eleven 28-day readmissions in group 1, only 3 of which were due to infectious causes. One patient had a primary diagnosis of necrotizing pneumonia, 1 had Pseudomonas pneumonia, and 1 patient had a new lung mass and was diagnosed with postobstructive pneumonia. Of eight 28-day readmissions in group 2, only 2 resulted from infectious causes. One readmission primary diagnosis was sinusitis and 1 was recurrent pneumonia (of note, this patient received a 10-day treatment course for pneumonia on initial admission). Two patients died within 28 days of discharge in each group.
Discussion
Other multifaceted single-center interventions have been shown to be effective in large, teaching hospitals,24,25 and it has been suggested that smaller, rural hospitals may be underserved in antimicrobial stewardship activities.26,27 In the global struggle with antimicrobial resistance, McGregor and colleagues highlighted the importance of evaluating successful stewardship methods in an array of clinical settings to help tailor an approach for a specific type of facility.28 To the authors knowledge, this is the first publication showing efficacy of such antimicrobial stewardship interventions specific to pneumonia therapy in a small, primary facility.
The intervention methods used at VHSO are supported by recent IDSA and Society for Healthcare Epidemiology of America guidelines for effective stewardship implementation.29 Prospective audit and feedback is considered a core recommendation, whereas didactic education is recommended only in conjunction with other stewardship activities. Additionally, the guidelines recommend evaluating specific infectious disease syndromes, in this case uncomplicated pneumonia, to focus on specific treatment guidelines. Last, the results of the 3-part intervention can be used to aid in demonstrating facility improvement and encourage continued success.
Of note, VHSO has had established inpatient and outpatient clinical pharmacy roles for several years. Stewardship interventions already in place included an intravenous-to-oral antibiotic switch policy, automatic antibiotic stop dates, as well as pharmacist-driven vancomycin and aminoglycoside dosing. Prior to this multifaceted intervention specific to pneumonia duration, prospective audit and feedback interventions (verbal and written) also were common. The number of interventions specific to this study outside of the stewardship note was not recorded. Using rapid diagnostic testing and biomarkers to aid in stewardship activities at VHSO have been considered, but these tools are not available due to a lab personnel shortage.
Soliciting feedback from providers on their preferred stewardship strategy and perceived barriers was a key component of the educational intervention. Of equal importance was presenting providers with their baseline prescribing data to provide objective evidence of a problem. While all were familiar with existing treatment guidelines, some feedback indicated that it can be difficult to determine accurate antibiotic duration in CPRS. Prescribers reported that identifying antibiotic duration was especially challenging when antibiotics as well as providers change during an admission. Also frequently overlooked were antibiotics given in the emergency department. This could be a key area for clinical pharmacists’ intervention given their familiarity with the CPRS medication sections.
Charani and colleagues suggest that recognizing barriers to implementing best practices and adapting to the local facility culture is paramount for changing prescribing behaviors and developing a successful stewardship intervention.30 At VHSO, the providers were presented with multiple stewardship options but agreed to the new note and template. This process gave providers a voice in selecting their own stewardship intervention. In a culture with no infectious disease physician to champion initiatives, the investigators felt that provider involvement in the intervention selection was unique and may have encouraged provider concurrence.
Although not directly targeted by the intervention strategies, average LOS was shorter in the follow-up group. According to investigators, frequent reminders of clinical stability in the stewardship notes may have influenced this. Even though the note was used only in patients who remained hospitalized for their entire treatment course, investigators felt that it still served as a reminder for prescribing habits as they were also able to show a decrease in outpatient prescription duration.
Limitations
Potential weaknesses of the study include changes in providers. During the transition between group 1 and group 2, 2 hospitalists left and 2 new hospitalists arrived. Given the small size of the staff, this could significantly impact prescribing trends. Another potential weakness is the high exclusion rate, although these rates were similar in both groups (46% group 1, 47% group 2). Furthermore, similar exclusion rates have been reported elsewhere.24,25,31 The most common reasons for exclusion were complicated pneumonias (36%) and immunocompromised patients (18%). These patient populations were not evaluated in the current study, and optimal treatment durations are unknown. Hospital-acquired and ventilator-associated pneumonias also were excluded. Therefore, limitations in applicability of the results should be noted.
The authors acknowledge that, prior to this publication, the IDSA guidelines have removed the designation of HCAP as a separate clinical entity.4 However, this should not affect the significance of the intervention for treatment duration.
The study facility experienced a hiring freeze resulting in a 9.3% decrease in overall admissions from fiscal year 2013 to fiscal year 2015. This is likely why there were fewer admissions for pneumonia in group 2. Regardless, power analysis revealed the study was of adequate sample size to detect its primary outcome. It is possible that patients in either group could have sought health care at other facilities, making the CDI and readmission endpoints less inclusive.
The study was not of a scale to detect changes in antimicrobial resistance pressure or clinical outcomes. Cost savings were not analyzed. However, this study adds to the growing body of evidence that a structured intervention can result in positive outcomes at the facility level. This study shows that interventions targeting pneumonia treatment duration could feasibly be added to the menu of stewardship options available to smaller facilities.
Like other stewardship studies in the literature, the follow-up treatment duration, while improved, still exceeded those recommended in the IDSA guidelines. The investigators noted that not all providers were equal regarding change in prescribing habits, perhaps making the average duration longer. Additionally, the request to discontinue antibiotic therapy through the stewardship note could have been entered earlier (eg, as early as day 5 of therapy) to target the shortest effective date as recommended in the recent stewardship guidelines.29 Future steps include continued feedback to providers on their progress in this area and encouragement to document day of antibiotic treatment in their daily progress notes.
Conclusion
This study showed a significant decrease in antibiotic duration for the treatment of uncomplicated pneumonia using a 3-part pharmacy intervention in a primary hospital setting. The investigators feel that each arm of the strategy was equally important and fewer interventions were not likely to be as effective.32 Although data collection for baseline prescribing and follow-up on outcomes may be a time-consuming task, it can be a valuable component of successful stewardship interventions.
1. Li JZ, Winston LG, Moore DH, Bent S. Efficacy of short-course antibiotic regimens for community-acquired pneumonia: a meta-analysis. Am J Med. 2007;120(9):783-790.
2. Dimopoulos G, Matthaiou DK, Karageorgopoulos DE, Grammatikos AP, Athanassa Z, Falagas ME. Short- versus long-course antibacterial therapy of community-acquired pneumonia: a meta-analysis. Drugs. 2008;68(13):1841-1854.
3. Mandell LA, Wunderink RG, Anzueto A, et al; Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72.
4. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61-e111.
5. Jenkins TC, Stella SA, Cervantes L, et al. Targets for antibiotic and healthcare resource stewardship in inpatient community-acquired pneumonia: a comparison of management practices with National Guideline Recommendations. Infection. 2013; 41(1):135-144.
6. Shlaes DM, Gerding DN, John JF Jr, et al. Society for Healthcare Epidemiology of America, and Infectious Diseases Society of America Joint Committee on the Prevention of Antimicrobial Resistance: guidelines for the prevention of antimicrobial resistance in hospitals. Clin Infect Dis. 1997;25(3):584-599.
7. Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455.
8. Brown E, Talbot GH, Axelrod P, Provencher M, Hoegg C. Risk factors for Clostridium-difficile toxin-associated diarrhea. Infect Control Hosp Epidemiol. 1990;11(6):283-290.
9. McFarland LV, Surawicz CM, Stamm WE. Risk factors for Clostridium-difficile carriage and C. difficile-associated diarrhea in a cohort of hospitalized patients. J Infect Dis. 1990;162(3):678-684.
10. Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47(6):735-743.
11. Dellit TH, Owens RC, McGowan JE Jr, et al; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America Guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159-177.
12. Fridkin S, Baggs J, Fagan R, et al; Centers for Disease Control and Prevention (CDC). Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194-200.
13. Nussenblatt V, Avdic E, Cosgrove S. What is the role of antimicrobial stewardship in improving outcomes of patients with CAP? Infect Dis Clin North Am. 2013;27(1):211-228.
14. Septimus EJ, Owens RC Jr. Need and potential of antimicrobial stewardship in community hospitals. Clin Infect Dis. 2011;53(suppl 1):S8-S14.
15. Hensher M, Price M, Adomakoh S. Referral hospitals. In Jamison DT, Breman JG, Measham AR, eds, et al. Disease Control Priorities in Developing Countries. New York, NY: Oxford University Press; 2006:1230.
16. Mulligan J, Fox-Rushby JA, Adam T, Johns B, Mills A. Unit costs of health care inputs in low and middle income regions. 2003. Working Paper 9, Disease Control Priorities Project. Published September 2003. Revised June 2005.
17. Ohl CA, Dodds Ashley ES. Antimicrobial stewardship programs in community hospitals: the evidence base and case studies. Clin Infect Dis 2011;53(suppl 1):S23-S28.
18. Trevidi KK, Kuper K. Hospital antimicrobial stewardship in the nonuniversity setting. Infect Dis Clin North Am. 2014;28(2):281-289.
19. Yam P, Fales D, Jemison J, Gillum M, Bernstein M. Implementation of an antimicrobial stewardship program in a rural hospital. Am J Health Syst Pharm. 2012;69(13);1142-1148.
20. LaRocco A Jr. Concurrent antibiotic review programs—a role for infectious diseases specialists at small community hospitals. Clin Infect Dis. 2003;37(5):742-743.
21. Bartlett JM, Siola PL. Implementation and first-year results of an antimicrobial stewardship program at a community hospital. Am J Health Syst Pharm. 2014;71(11):943-949.
22. Storey DF, Pate PG, Nguyen AT, Chang F. Implementation of an antimicrobial stewardship program on the medical-surgical service of a 100-bed community hospital. Antimicrob Resist Infect Control. 2012;1(1):32.
23. Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA. 1996;275(2):134-141.
24. Advic E, Cushinotto LA, Hughes AH, et al. Impact of an antimicrobial stewardship intervention on shortening the duration of therapy for community-acquired pneumonia. Clin Infect Dis. 2012;54(11):1581-1587.
25. Carratallà J, Garcia-Vidal C, Ortega L, et al. Effect of a 3-step critical pathway to reduce duration of intravenous antibiotic therapy and length of stay in community-acquired pneumonia: a randomized controlled trial. Arch Intern Med. 2012;172(12):922-928.
26. Stevenson KB, Samore M, Barbera J, et al. Pharmacist involvement in antimicrobial use at rural community hospitals in four Western states. Am J Health Syst Pharm. 2004;61(8):787-792.
27. Reese SM, Gilmartin H, Rich KL, Price CS. Infection prevention needs assessment in Colorado hospitals: rural and urban settings. Am J Infect Control. 2014;42(6):597-601.
28. McGregor JC, Furuno JP. Optimizing research methods used for the evaluation of antimicrobial stewardship programs. Clin Infect Dis. 2014;59(suppl 3):S185-S192.
29. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77.
30. Charani E, Castro-Sánchez E, Holmes A. The role of behavior change in antimicrobial stewardship. Infect Dis Clin N Am. 2014;28(2):169-175.
31. Attridge RT, Frei CR, Restrepo MI, et al. Guideline-concordant therapy and outcomes in healthcare-associated pneumonia. Eur Respir J. 2011;38(4):878-887.
32. MacDougal C, Polk RE. Antimicrobial stewardship programs in health care systems. Clin Microbiol Rev. 2005;18(4):638-656.
1. Li JZ, Winston LG, Moore DH, Bent S. Efficacy of short-course antibiotic regimens for community-acquired pneumonia: a meta-analysis. Am J Med. 2007;120(9):783-790.
2. Dimopoulos G, Matthaiou DK, Karageorgopoulos DE, Grammatikos AP, Athanassa Z, Falagas ME. Short- versus long-course antibacterial therapy of community-acquired pneumonia: a meta-analysis. Drugs. 2008;68(13):1841-1854.
3. Mandell LA, Wunderink RG, Anzueto A, et al; Infectious Diseases Society of America; American Thoracic Society. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27-S72.
4. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61-e111.
5. Jenkins TC, Stella SA, Cervantes L, et al. Targets for antibiotic and healthcare resource stewardship in inpatient community-acquired pneumonia: a comparison of management practices with National Guideline Recommendations. Infection. 2013; 41(1):135-144.
6. Shlaes DM, Gerding DN, John JF Jr, et al. Society for Healthcare Epidemiology of America, and Infectious Diseases Society of America Joint Committee on the Prevention of Antimicrobial Resistance: guidelines for the prevention of antimicrobial resistance in hospitals. Clin Infect Dis. 1997;25(3):584-599.
7. Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455.
8. Brown E, Talbot GH, Axelrod P, Provencher M, Hoegg C. Risk factors for Clostridium-difficile toxin-associated diarrhea. Infect Control Hosp Epidemiol. 1990;11(6):283-290.
9. McFarland LV, Surawicz CM, Stamm WE. Risk factors for Clostridium-difficile carriage and C. difficile-associated diarrhea in a cohort of hospitalized patients. J Infect Dis. 1990;162(3):678-684.
10. Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47(6):735-743.
11. Dellit TH, Owens RC, McGowan JE Jr, et al; Infectious Diseases Society of America; Society for Healthcare Epidemiology of America. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America Guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159-177.
12. Fridkin S, Baggs J, Fagan R, et al; Centers for Disease Control and Prevention (CDC). Vital signs: improving antibiotic use among hospitalized patients. MMWR Morb Mortal Wkly Rep. 2014;63(9):194-200.
13. Nussenblatt V, Avdic E, Cosgrove S. What is the role of antimicrobial stewardship in improving outcomes of patients with CAP? Infect Dis Clin North Am. 2013;27(1):211-228.
14. Septimus EJ, Owens RC Jr. Need and potential of antimicrobial stewardship in community hospitals. Clin Infect Dis. 2011;53(suppl 1):S8-S14.
15. Hensher M, Price M, Adomakoh S. Referral hospitals. In Jamison DT, Breman JG, Measham AR, eds, et al. Disease Control Priorities in Developing Countries. New York, NY: Oxford University Press; 2006:1230.
16. Mulligan J, Fox-Rushby JA, Adam T, Johns B, Mills A. Unit costs of health care inputs in low and middle income regions. 2003. Working Paper 9, Disease Control Priorities Project. Published September 2003. Revised June 2005.
17. Ohl CA, Dodds Ashley ES. Antimicrobial stewardship programs in community hospitals: the evidence base and case studies. Clin Infect Dis 2011;53(suppl 1):S23-S28.
18. Trevidi KK, Kuper K. Hospital antimicrobial stewardship in the nonuniversity setting. Infect Dis Clin North Am. 2014;28(2):281-289.
19. Yam P, Fales D, Jemison J, Gillum M, Bernstein M. Implementation of an antimicrobial stewardship program in a rural hospital. Am J Health Syst Pharm. 2012;69(13);1142-1148.
20. LaRocco A Jr. Concurrent antibiotic review programs—a role for infectious diseases specialists at small community hospitals. Clin Infect Dis. 2003;37(5):742-743.
21. Bartlett JM, Siola PL. Implementation and first-year results of an antimicrobial stewardship program at a community hospital. Am J Health Syst Pharm. 2014;71(11):943-949.
22. Storey DF, Pate PG, Nguyen AT, Chang F. Implementation of an antimicrobial stewardship program on the medical-surgical service of a 100-bed community hospital. Antimicrob Resist Infect Control. 2012;1(1):32.
23. Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA. 1996;275(2):134-141.
24. Advic E, Cushinotto LA, Hughes AH, et al. Impact of an antimicrobial stewardship intervention on shortening the duration of therapy for community-acquired pneumonia. Clin Infect Dis. 2012;54(11):1581-1587.
25. Carratallà J, Garcia-Vidal C, Ortega L, et al. Effect of a 3-step critical pathway to reduce duration of intravenous antibiotic therapy and length of stay in community-acquired pneumonia: a randomized controlled trial. Arch Intern Med. 2012;172(12):922-928.
26. Stevenson KB, Samore M, Barbera J, et al. Pharmacist involvement in antimicrobial use at rural community hospitals in four Western states. Am J Health Syst Pharm. 2004;61(8):787-792.
27. Reese SM, Gilmartin H, Rich KL, Price CS. Infection prevention needs assessment in Colorado hospitals: rural and urban settings. Am J Infect Control. 2014;42(6):597-601.
28. McGregor JC, Furuno JP. Optimizing research methods used for the evaluation of antimicrobial stewardship programs. Clin Infect Dis. 2014;59(suppl 3):S185-S192.
29. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51-e77.
30. Charani E, Castro-Sánchez E, Holmes A. The role of behavior change in antimicrobial stewardship. Infect Dis Clin N Am. 2014;28(2):169-175.
31. Attridge RT, Frei CR, Restrepo MI, et al. Guideline-concordant therapy and outcomes in healthcare-associated pneumonia. Eur Respir J. 2011;38(4):878-887.
32. MacDougal C, Polk RE. Antimicrobial stewardship programs in health care systems. Clin Microbiol Rev. 2005;18(4):638-656.
Decentralized vs Centralized Pharmacist Treatment of Patients With Atrial Fibrillation Managed With Direct Oral Anticoagulants
In the U.S. about 2.7 to 6.1 million people have atrial fibrillation (AF).1 This condition affects the rhythm of the heart, causes blood in the heart to become stagnant, and puts patients at high risk for developing a systemic embolism, particularly a stroke.1 Recent studies have shown that AF accounts for at least 15% of all strokes in the U.S. and 36% of strokes in people aged > 80 years.2
For patients aged > 60 years, the gold standard of long-term anticoagulation for reducing the risk of stroke has been oral vitamin K antagonist (warfarin) therapy.2 Although overwhelming evidence exists that supports the use of warfarin in these patients, warfarin is a narrow therapeutic index medication that requires frequent laboratory monitoring of international normalized ratio (INR) for dose titration guidance. There is also strong evidence that pharmacist-run anticoagulation clinics have improved patient-centered outcomes in patients prescribed warfarin.3-5
Direct oral anticoagulants (DOACs) are recently approved oral medications used as alternatives to warfarin for anticoagulation in AF. Direct oral anticoagulants do not require INR monitoring or any laboratory test for efficacy. In 2010, the FDA approved the first DOAC, dabigatran, for use in patients with AF. In 2011, rivaroxaban received approval for the same indication. One potential drawback of these new agents relative to warfarin is the lack of availability of a reversal agent that can be used in the event of a life-threatening bleeding event. Dabigatran is the only DOAC with an FDA-approved available reversal agent. In both 2011 and 2012, dabigatran, warfarin, and other anticoagulants topped the Institute for Safe Medicine Practice list of suspect drugs related to adverse events (AEs). These data prompted the Joint Commission to incorporate anticoagulation into the 2017 National Hospital Patient Safety Goals to improve patient outcomes and reduce harm from use of anticoagulants.6
In early 2011, the VHA produced national guidance on the treatment of patients who receive DOACs; this guidance was updated most recently in September 2016.7 Patients who were receiving DOACs at the Ralph H. Johnson VAMC (RHJVAMC) were initially monitored by 12 primary care pharmacists at the main hospital or at community-based outpatient clinics (CBOCs). Ambulatory care pharmacists at RHJVAMC work under a scope of practice to prescribe and adjust certain classes of medications to provide the highest level of care to more than 65,000 veterans in South Carolina and Georgia. Historically at RHJVAMC, warfarin has been the anticoagulant most commonly used for AF, though dabigatran and rivaroxaban have gained in popularity after being added to the national VA formulary.
In November 2012, for better monitoring of patient outcomes, improved efficiency of the primary care pharmacist clinics, and increased access to care in these clinics, treatment of patients prescribed DOACs was shifted to a centralized model that involved 3 anticoagulation clinical pharmacy specialists.
Centralized pharmacy services have a small number of core team members in a specific service for a particular disease, which reduces the number of different pharmacists a patient could talk to for management of a particular condition. Centralized pharmacy services allow for streamlining anticoagulation management to a small group of individual pharmacists considered specialists in anticoagulation. This shift in management to centralized anticoagulation services was supported at RHJVAMC by findings from a study of a pharmacist-run centralized anticoagulation clinic: Patients treated by the centralized clinic were 39% less likely to experience an anticoagulation therapy complication.8
Protocol for dabigatran follow-up and monitoring at RHJVAMC was developed by clinical and supervisory pharmacy staff, to align with national VA guidance. When a provider determines a patient is a candidate for dabigatran, an outpatient consultation is entered for the clinical pharmacy specialist to review the appropriateness of the patient selection for therapy. If the patient is eligible for therapy, the pharmacist contacts the patient to set up an initial visit to confirm selection and to provide the first dabigatran prescription and counseling. For assessments, with specific emphasis on adherence and AE monitoring, the patient is contacted 2 weeks, 1 month, 3 months, and every 6 months after the initial appointment.
Although most of the literature supports pharmacist-managed anticoagulation for patients who receive warfarin, DOACs have become more integrated into practice and more evaluated. Evidence supports pharmacists' interventions on evaluation of patient education and dosing, but there is conflicting evidence regarding pharmacists' impact on adherence after 3 months of therapy.9,10 In a larger VA study of the impact of dabigatran adherence on patient-centered outcomes, patients were mostly nonadherent to prescribed dosing.11 These studies support the need for improved adherence in patients prescribed DOACs and the need for further investigation of pharmacists' roles in improving patient outcomes.
Methods
This single-center, retrospective anticoagulant-use evaluation covered 2 study periods between November 1, 2011 and October 31, 2013. Study approval was obtained from the institutional review board of the Medical University of South Carolina and the research and development committee of RHJVAMC. The study population consisted of veterans who had a diagnosis of AF and received at least 3 outpatient prescription fills of a 30-day supply of dabigatran at RHJVAMC during either or both of the study periods. Patients were excluded if they were pregnant or planning to become pregnant or were incarcerated at any time during the study period. Dabigatran was selected because it was the first DOAC added to the local VA formulary before the start of this study.
Patients who met the inclusion criteria were separated into 2 groups based on the dates of their prescription fills. The precentralization group included patients treated by primary care pharmacists from November 1, 2011 to October 31, 2012; the postcentralization group included patients treated by anticoagulation clinical pharmacy specialists from November 1, 2012 to October 31, 2013. In each group, patients were followed for 1 year during their respective study period. For analysis, patients were included in both study periods if they received at least 3 fills of dabigatran during each period.
Medication possession ratio (MPR), which was used to measure the primary endpoint of adherence, is defined as the proportion of days a patient had dabigatran. The MPR denominator is the total number of days between the first and last prescription refill dates within the 52-week study period; the numerator is calculated by summing the days' supply for all but the last filling of the medication during each respective period. Nonadherence was defined as an MPR < 0.8 (or 80%), which has been used to define poor adherence in the literature.12 The authors calculated all patients' mean MPRs and compared them to determine statistical significance by repeated-measures linear regression. Descriptive statistics on proportion of patients in each study group with MPR < 0.8 were examined. Last, the authors performed a comparative subanalysis of median MPRs to determine whether there was an adherence difference between patients initially started on dabigatran at RHJVAMC and patients who were started on dabigatran before receiving it at RHJVAMC.
The secondary focus of this study was safety outcomes, including any bleeding event or thromboembolism within either study period. A bleeding event was defined as any major or minor bleeding event recognized through ICD-9 codes or any bleeding recorded in the patient's chart and noted during chart review, as well as any serum hemoglobin (Hgb) level decrease of ≥ to 2 g/dL during the study period. Thromboembolism was defined as a thromboembolism recognized through ICD-9 codes or any thromboembolism noted during chart review. Descriptive statistics were reported for this outcome, and a chi-square test was used to compare bleeding events between groups to determine significance.
The tertiary focus of this study was clinical efficiency as determined by number of primary care pharmacist visits during each study period. Primary care pharmacist visits were included for all primary care pharmacists in primary care clinics at the main hospital and in all 6 CBOCs.
For statistical analysis α was set at 0.05, and P < .05 was considered statistically significant. SAS Enterprise Guide software (Cary, North Carolina) was used for all statistical analyses.
Results
An initial data pull was completed from the RHJVAMC prescription records database for patients who had ≥ 3 prescriptions of dabigatran filled for treatment of AF during the study period, which yielded 65 unique patients. There were 34 patients in the precentralization group and 55 patients in the postcentralization group. Twenty-four unique patients were included in both study groups.
Mean MPR was 1.01 (range, 0.59-1.41) for the precentralization study period and 0.96 (range, 0.33-1.36) for the postcentralization period (Table 1). The difference was not statistically significant (P = .91). Number of patients considered nonadherent (MPR < 0.8) was 3 (8.82%) in the precentralization group and 8 (14.6%) in the postcentralization group.
The primary endpoint subanalysis compared the median MPRs for the patients initially started on dabigatran at RHJVAMC (de novo starts) and the patients who were started on dabigatran before receiving it at RHJVAMC (prior starts). In each group, number and percentage of patients determined to be nonadherent by MPR were evaluated as well. De novo patients received initial assessment, counseling, and a dabigatran prescription from RHJVAMC pharmacists before or during the study period, and prior patients were initially prescribed dabigatran at another VA facility or at a non-VA facility (Table 2).
Regarding safety outcomes (secondary endpoint), a bleeding event was identified in 6 (17.7%) of the precentralization patients and 7 (12.7%) of the postcentralization patients. Of the 6 precentralization events, 1 was a case of hemoptysis, 1 was a hematoma on the forehead, 1 was a lower gastrointestinal bleed (unconfirmed), 1 was retinal hemorrhaging (noted by ophthalmologist), and 2 were serum Hgb level decreases of more than 2 g/dL (neither patient required transfusion of packed red blood cells). Of the 7 postcentralization events, 1 was persistent hematochezia caused by hemorrhoids, 1 was hematuria, 1 was a hematoma, 1 was an upper gastrointestinal bleed (required blood transfusion), and 4 were serum Hgb level decreases of more than 2 g/dL (1 of the 4 required transfusion). No precentralization patient had any evidence of thromboembolism during the study period; 1 postcentralization patient had a superficial venous thromboembolism near a hematoma on the elbow.
Discussion
In this single-center, retrospective medication-use evaluation, the authors found a high rate of adherence to dabigatran before and after centralization of outpatient DOAC management by pharmacists. There was no statistically significant difference in bleeding events between the study periods, but primary care pharmacist visits increased by 108% from precentralization to postcentralization. Although the primary outcome findings did not refute the study's null hypothesis, results support implementing centralized pharmacist DOAC management to maintain a high rate of adherence and a low incidence of adverse outcomes and providing more primary care pharmacist services to increase access to care for other chronic diseases.
Although there was no statistically significant difference in adherence rates between study periods, the 2 groups' rates were higher than the national average of 72%, as calculated by the proportion-of-days-covered (PDC) equation (median, 74%) in a 2015 large-scale study of site-level adherence in more than 5,000 VA patients.13 The authors' findings support that study's significant finding of a high rate of adherence to pharmacist-provided dabigatran treatment. This study's adherence rate also was higher than the median PDC rate reported in a 2014 study that focused on dabigatran adherence: 94% (mean, 84%; SD, 22%).11
The RHJVAMC follows national VA guidance on pharmacist follow-up for patients who receive DOACs. This follow-up focuses on frequent counseling over the first 6 months of de novo DOAC treatment and on monitoring and assessing adherence and AEs. Although there is less laboratory monitoring for DOAC treatment than for treatment with vitamin K antagonists (eg, warfarin), telephone monitoring as described in this study has been associated with a high adherence rate and minimization of AEs. The 2014 study with the 94% median PDC rate also showed an association of decreased adherence and increased harm, including combined all-cause mortality and stroke (hazard ratio, 1.13; 95% confidence interval [CI], 1.07-1.19 per 10% decrease in PDC rate).11
This study's subanalysis revealed no difference in adherence between patients initially started on dabigatran at RHJVAMC and patients who were started on dabigatran before receiving it at RHJVAMC. Each group had a high rate of adherence. Shore and colleagues found that most of the VA sites they surveyed (22/41) had anticoagulation clinics monitoring patients who were prescribed dabigatran.13 Pharmacist-led monitoring of adherence and AEs led to increased adherence to dabigatran treatment (relative risk, 1.25; 95% CI, 1.11-1.41), which was the standard of care at RHJVAMC throughout their entire study. Many of these factors may explain the very high rate of adherence found in the present study, specifically in comparison to previously reported national averages.
In addition, the authors found no statistically significant difference in bleeding outcomes between the precentralization and postcentralization groups. Their incidence of bleeding was similar to the 16.6% rate reported in the package insert for dabigatran.14 Furthermore, the safety outcomes were similar for both groups in this study, which may be attributable to the quality of patient care provided by all RHJVAMC pharmacists, particularly in the setting of dabigatran management.
Many studies have found an association between dabigatran use and an increased rate of bleeding, particularly gastrointestinal, as demonstrated in several patients in this study. Evidence of these clinically significant AEs further supports pharmacists' close monitoring to detect these AEs and working with patients' providers to determine whether an alternative anticoagulant should be used.
A significant finding of this study regarding centralization of DOAC management by pharmacists was the increased number of primary care pharmacist visits. By streamlining all anticoagulant services to anticoagulation clinical pharmacy specialists, primary care pharmacists were able to care for more veterans and increase access to care without adding staff. The centralized anticoagulation pharmacists were volunteers who held other positions within the department; they did not have to be replaced when they became anticoagulation providers. This workload reallocation helped the RHJVAMC pharmacy department increase access to care.
Limitations
This study had several potential limitations. First, MPR, a widely studied common tool for assessing adherence, has been criticized for often being imprecise when used with short study periods.12 Another commonly used adherence measure is PDC rate, which has been reported in several large-scale studies of dabigatran therapy. The authors selected MPR for the present study because MPR calculation is more practical in the patient population and because MPR and PDC rate are predicted to yield similar results in assessments of adherence to a single medication.12 It also should be noted that both MPR and PDC rate are surrogate markers for adherence and assume adherence based on the availability of medication to the patient. Assessing adherence in a retrospective study is a challenge, as more reliable adherence assessment--for example, with use of pill counts or blister packs--is not possible. This study's retrospective design was another potential limitation, as an active intervention was not used.
In addition, this study had a small sample, likely attributable to the addition of dabigatran to the VA national formulary just months before the start of the study period. Furthermore, this study was not powered to detect significant differences in safety or efficacy outcomes. Other potential study limitations included having national VA guidance regarding follow-up periods and dabigatran prescription quantity limits during both study periods. Also, there was some potential for pharmacist-initiated refills at follow-up visits, which could falsely increase MPR. Last, the study analyzed only 1 DOAC and not the entire class of medications.
Conclusion
Centralizing DOAC management by clinical pharmacy specialists at a single VA facility helped maintain high rates of dabigatran adherence, above the national average, and low rates of adverse outcomes were maintained in both study groups. In addition, centralization of anticoagulation services improved access to care through an increase in primary care pharmacist visits without the addition of staff. Centralization of DOAC management by pharmacists is a viable option for maintaining high rates of adherence and low rates of adverse outcomes in facilities where the goal is to achieve clinical efficiency.
1. January CT, Wann LS, Alpert JS, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society [published correction appears in J Am Coll Cardiol. 2014;64(21):2305-2307. J Am Coll Cardiol. 2014;64(21):e1-e76.
2. Reiffel JA. New versus traditional approaches to oral anticoagulation in patients with atrial fibrillation. Am J Med. 2014;127(4):e15.
3. Locke C, Ravnan SL, Patel R, Uchizono JA. Reduction in warfarin adverse events requiring patient hospitalization after implementation of pharmacist-managed anticoagulation service. Pharmacotherapy. 2005;25(5):685-689.
4. Poon IO, Lal L, Brown EN, Braun UK. The impact of pharmacist-managed oral anticoagulation therapy in older veterans. J Clin Pharm Ther. 2007;32(1):21-29.
5. Chiquette E, Amato MG, Bussey HI. Comparison of an anticoagulation clinic with usual medical care. Arch Intern Med. 1998;158(15):1641-1647.
6. The Joint Commission. National patient safety goals. https://www.jointcommission.org/as sets/1/6/2017_NPSG_HAP_ER.pdf. Published 2016. Accessed December 6, 2016.
7. Department of Veterans Affairs Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives. Direct oral anticoagulants (DOACs) (formerly called TSOACs) dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis): Criteria for Use for Stroke Prevention in nonvalvular atrial fibrillation (AF) and Edoxaban (SAVAYSA). http://www.pbm.va.gov/PBM/clinicalguidance/criteriaforuse/Anticoagulants_Direct_Oral_DOACs_CFU_and_Algorithm_for_Nonvalvular_Atrial_Fibrillation_Sep_2016.pdf. Updated September 2016. Accessed December 6, 2016.
8. Witt DM, Sadler MA, Shanahan RL, Mazzoli G, Tillman DJ. Effect of a centralized clinical pharmacy anticoagulation service on the outcomes of anticoagulation therapy. Chest. 2005;127(5):1515-1522.
9. Chan LL, Crumpler WL, Jacobson AK. Implementation of pharmacist-managed anticoagulation in patients receiving newer anticoagulants. Am J Health Syst Pharm. 2013;70(15):1285-1286, 1288.
10. Lee PY, Han SY, Miyahara RK. Adherence and outcomes of patients treated with dabigatran: pharmacist-managed anticoagulation clinic versus usual care. Am J Health Syst Pharm. 2013;70(13):1154-1161.
11. Shore S, Carey EP, Turakhia MP, et al. Adherence to dabigatran therapy and longitudinal patient outcomes: insights from the Veterans Health Administration. Am Heart J. 2014;167(6):810-817.
12. Martin BC, Wiley-Exley EK, Richards S, Domino ME, Carey TS, Sleath BL. Contrasting measures of adherence with simple drug use, medication switching and therapeutic duplication. Ann Pharmacother. 2009;43(1):36-44.
13. Shore S, Ho PM, Lambert-Kerzner A, et al. Site-level variation in and practices associated with dabigatran adherence. JAMA. 2015;313(14):1443-1450.
14. Pradaxa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals; 2015.
In the U.S. about 2.7 to 6.1 million people have atrial fibrillation (AF).1 This condition affects the rhythm of the heart, causes blood in the heart to become stagnant, and puts patients at high risk for developing a systemic embolism, particularly a stroke.1 Recent studies have shown that AF accounts for at least 15% of all strokes in the U.S. and 36% of strokes in people aged > 80 years.2
For patients aged > 60 years, the gold standard of long-term anticoagulation for reducing the risk of stroke has been oral vitamin K antagonist (warfarin) therapy.2 Although overwhelming evidence exists that supports the use of warfarin in these patients, warfarin is a narrow therapeutic index medication that requires frequent laboratory monitoring of international normalized ratio (INR) for dose titration guidance. There is also strong evidence that pharmacist-run anticoagulation clinics have improved patient-centered outcomes in patients prescribed warfarin.3-5
Direct oral anticoagulants (DOACs) are recently approved oral medications used as alternatives to warfarin for anticoagulation in AF. Direct oral anticoagulants do not require INR monitoring or any laboratory test for efficacy. In 2010, the FDA approved the first DOAC, dabigatran, for use in patients with AF. In 2011, rivaroxaban received approval for the same indication. One potential drawback of these new agents relative to warfarin is the lack of availability of a reversal agent that can be used in the event of a life-threatening bleeding event. Dabigatran is the only DOAC with an FDA-approved available reversal agent. In both 2011 and 2012, dabigatran, warfarin, and other anticoagulants topped the Institute for Safe Medicine Practice list of suspect drugs related to adverse events (AEs). These data prompted the Joint Commission to incorporate anticoagulation into the 2017 National Hospital Patient Safety Goals to improve patient outcomes and reduce harm from use of anticoagulants.6
In early 2011, the VHA produced national guidance on the treatment of patients who receive DOACs; this guidance was updated most recently in September 2016.7 Patients who were receiving DOACs at the Ralph H. Johnson VAMC (RHJVAMC) were initially monitored by 12 primary care pharmacists at the main hospital or at community-based outpatient clinics (CBOCs). Ambulatory care pharmacists at RHJVAMC work under a scope of practice to prescribe and adjust certain classes of medications to provide the highest level of care to more than 65,000 veterans in South Carolina and Georgia. Historically at RHJVAMC, warfarin has been the anticoagulant most commonly used for AF, though dabigatran and rivaroxaban have gained in popularity after being added to the national VA formulary.
In November 2012, for better monitoring of patient outcomes, improved efficiency of the primary care pharmacist clinics, and increased access to care in these clinics, treatment of patients prescribed DOACs was shifted to a centralized model that involved 3 anticoagulation clinical pharmacy specialists.
Centralized pharmacy services have a small number of core team members in a specific service for a particular disease, which reduces the number of different pharmacists a patient could talk to for management of a particular condition. Centralized pharmacy services allow for streamlining anticoagulation management to a small group of individual pharmacists considered specialists in anticoagulation. This shift in management to centralized anticoagulation services was supported at RHJVAMC by findings from a study of a pharmacist-run centralized anticoagulation clinic: Patients treated by the centralized clinic were 39% less likely to experience an anticoagulation therapy complication.8
Protocol for dabigatran follow-up and monitoring at RHJVAMC was developed by clinical and supervisory pharmacy staff, to align with national VA guidance. When a provider determines a patient is a candidate for dabigatran, an outpatient consultation is entered for the clinical pharmacy specialist to review the appropriateness of the patient selection for therapy. If the patient is eligible for therapy, the pharmacist contacts the patient to set up an initial visit to confirm selection and to provide the first dabigatran prescription and counseling. For assessments, with specific emphasis on adherence and AE monitoring, the patient is contacted 2 weeks, 1 month, 3 months, and every 6 months after the initial appointment.
Although most of the literature supports pharmacist-managed anticoagulation for patients who receive warfarin, DOACs have become more integrated into practice and more evaluated. Evidence supports pharmacists' interventions on evaluation of patient education and dosing, but there is conflicting evidence regarding pharmacists' impact on adherence after 3 months of therapy.9,10 In a larger VA study of the impact of dabigatran adherence on patient-centered outcomes, patients were mostly nonadherent to prescribed dosing.11 These studies support the need for improved adherence in patients prescribed DOACs and the need for further investigation of pharmacists' roles in improving patient outcomes.
Methods
This single-center, retrospective anticoagulant-use evaluation covered 2 study periods between November 1, 2011 and October 31, 2013. Study approval was obtained from the institutional review board of the Medical University of South Carolina and the research and development committee of RHJVAMC. The study population consisted of veterans who had a diagnosis of AF and received at least 3 outpatient prescription fills of a 30-day supply of dabigatran at RHJVAMC during either or both of the study periods. Patients were excluded if they were pregnant or planning to become pregnant or were incarcerated at any time during the study period. Dabigatran was selected because it was the first DOAC added to the local VA formulary before the start of this study.
Patients who met the inclusion criteria were separated into 2 groups based on the dates of their prescription fills. The precentralization group included patients treated by primary care pharmacists from November 1, 2011 to October 31, 2012; the postcentralization group included patients treated by anticoagulation clinical pharmacy specialists from November 1, 2012 to October 31, 2013. In each group, patients were followed for 1 year during their respective study period. For analysis, patients were included in both study periods if they received at least 3 fills of dabigatran during each period.
Medication possession ratio (MPR), which was used to measure the primary endpoint of adherence, is defined as the proportion of days a patient had dabigatran. The MPR denominator is the total number of days between the first and last prescription refill dates within the 52-week study period; the numerator is calculated by summing the days' supply for all but the last filling of the medication during each respective period. Nonadherence was defined as an MPR < 0.8 (or 80%), which has been used to define poor adherence in the literature.12 The authors calculated all patients' mean MPRs and compared them to determine statistical significance by repeated-measures linear regression. Descriptive statistics on proportion of patients in each study group with MPR < 0.8 were examined. Last, the authors performed a comparative subanalysis of median MPRs to determine whether there was an adherence difference between patients initially started on dabigatran at RHJVAMC and patients who were started on dabigatran before receiving it at RHJVAMC.
The secondary focus of this study was safety outcomes, including any bleeding event or thromboembolism within either study period. A bleeding event was defined as any major or minor bleeding event recognized through ICD-9 codes or any bleeding recorded in the patient's chart and noted during chart review, as well as any serum hemoglobin (Hgb) level decrease of ≥ to 2 g/dL during the study period. Thromboembolism was defined as a thromboembolism recognized through ICD-9 codes or any thromboembolism noted during chart review. Descriptive statistics were reported for this outcome, and a chi-square test was used to compare bleeding events between groups to determine significance.
The tertiary focus of this study was clinical efficiency as determined by number of primary care pharmacist visits during each study period. Primary care pharmacist visits were included for all primary care pharmacists in primary care clinics at the main hospital and in all 6 CBOCs.
For statistical analysis α was set at 0.05, and P < .05 was considered statistically significant. SAS Enterprise Guide software (Cary, North Carolina) was used for all statistical analyses.
Results
An initial data pull was completed from the RHJVAMC prescription records database for patients who had ≥ 3 prescriptions of dabigatran filled for treatment of AF during the study period, which yielded 65 unique patients. There were 34 patients in the precentralization group and 55 patients in the postcentralization group. Twenty-four unique patients were included in both study groups.
Mean MPR was 1.01 (range, 0.59-1.41) for the precentralization study period and 0.96 (range, 0.33-1.36) for the postcentralization period (Table 1). The difference was not statistically significant (P = .91). Number of patients considered nonadherent (MPR < 0.8) was 3 (8.82%) in the precentralization group and 8 (14.6%) in the postcentralization group.
The primary endpoint subanalysis compared the median MPRs for the patients initially started on dabigatran at RHJVAMC (de novo starts) and the patients who were started on dabigatran before receiving it at RHJVAMC (prior starts). In each group, number and percentage of patients determined to be nonadherent by MPR were evaluated as well. De novo patients received initial assessment, counseling, and a dabigatran prescription from RHJVAMC pharmacists before or during the study period, and prior patients were initially prescribed dabigatran at another VA facility or at a non-VA facility (Table 2).
Regarding safety outcomes (secondary endpoint), a bleeding event was identified in 6 (17.7%) of the precentralization patients and 7 (12.7%) of the postcentralization patients. Of the 6 precentralization events, 1 was a case of hemoptysis, 1 was a hematoma on the forehead, 1 was a lower gastrointestinal bleed (unconfirmed), 1 was retinal hemorrhaging (noted by ophthalmologist), and 2 were serum Hgb level decreases of more than 2 g/dL (neither patient required transfusion of packed red blood cells). Of the 7 postcentralization events, 1 was persistent hematochezia caused by hemorrhoids, 1 was hematuria, 1 was a hematoma, 1 was an upper gastrointestinal bleed (required blood transfusion), and 4 were serum Hgb level decreases of more than 2 g/dL (1 of the 4 required transfusion). No precentralization patient had any evidence of thromboembolism during the study period; 1 postcentralization patient had a superficial venous thromboembolism near a hematoma on the elbow.
Discussion
In this single-center, retrospective medication-use evaluation, the authors found a high rate of adherence to dabigatran before and after centralization of outpatient DOAC management by pharmacists. There was no statistically significant difference in bleeding events between the study periods, but primary care pharmacist visits increased by 108% from precentralization to postcentralization. Although the primary outcome findings did not refute the study's null hypothesis, results support implementing centralized pharmacist DOAC management to maintain a high rate of adherence and a low incidence of adverse outcomes and providing more primary care pharmacist services to increase access to care for other chronic diseases.
Although there was no statistically significant difference in adherence rates between study periods, the 2 groups' rates were higher than the national average of 72%, as calculated by the proportion-of-days-covered (PDC) equation (median, 74%) in a 2015 large-scale study of site-level adherence in more than 5,000 VA patients.13 The authors' findings support that study's significant finding of a high rate of adherence to pharmacist-provided dabigatran treatment. This study's adherence rate also was higher than the median PDC rate reported in a 2014 study that focused on dabigatran adherence: 94% (mean, 84%; SD, 22%).11
The RHJVAMC follows national VA guidance on pharmacist follow-up for patients who receive DOACs. This follow-up focuses on frequent counseling over the first 6 months of de novo DOAC treatment and on monitoring and assessing adherence and AEs. Although there is less laboratory monitoring for DOAC treatment than for treatment with vitamin K antagonists (eg, warfarin), telephone monitoring as described in this study has been associated with a high adherence rate and minimization of AEs. The 2014 study with the 94% median PDC rate also showed an association of decreased adherence and increased harm, including combined all-cause mortality and stroke (hazard ratio, 1.13; 95% confidence interval [CI], 1.07-1.19 per 10% decrease in PDC rate).11
This study's subanalysis revealed no difference in adherence between patients initially started on dabigatran at RHJVAMC and patients who were started on dabigatran before receiving it at RHJVAMC. Each group had a high rate of adherence. Shore and colleagues found that most of the VA sites they surveyed (22/41) had anticoagulation clinics monitoring patients who were prescribed dabigatran.13 Pharmacist-led monitoring of adherence and AEs led to increased adherence to dabigatran treatment (relative risk, 1.25; 95% CI, 1.11-1.41), which was the standard of care at RHJVAMC throughout their entire study. Many of these factors may explain the very high rate of adherence found in the present study, specifically in comparison to previously reported national averages.
In addition, the authors found no statistically significant difference in bleeding outcomes between the precentralization and postcentralization groups. Their incidence of bleeding was similar to the 16.6% rate reported in the package insert for dabigatran.14 Furthermore, the safety outcomes were similar for both groups in this study, which may be attributable to the quality of patient care provided by all RHJVAMC pharmacists, particularly in the setting of dabigatran management.
Many studies have found an association between dabigatran use and an increased rate of bleeding, particularly gastrointestinal, as demonstrated in several patients in this study. Evidence of these clinically significant AEs further supports pharmacists' close monitoring to detect these AEs and working with patients' providers to determine whether an alternative anticoagulant should be used.
A significant finding of this study regarding centralization of DOAC management by pharmacists was the increased number of primary care pharmacist visits. By streamlining all anticoagulant services to anticoagulation clinical pharmacy specialists, primary care pharmacists were able to care for more veterans and increase access to care without adding staff. The centralized anticoagulation pharmacists were volunteers who held other positions within the department; they did not have to be replaced when they became anticoagulation providers. This workload reallocation helped the RHJVAMC pharmacy department increase access to care.
Limitations
This study had several potential limitations. First, MPR, a widely studied common tool for assessing adherence, has been criticized for often being imprecise when used with short study periods.12 Another commonly used adherence measure is PDC rate, which has been reported in several large-scale studies of dabigatran therapy. The authors selected MPR for the present study because MPR calculation is more practical in the patient population and because MPR and PDC rate are predicted to yield similar results in assessments of adherence to a single medication.12 It also should be noted that both MPR and PDC rate are surrogate markers for adherence and assume adherence based on the availability of medication to the patient. Assessing adherence in a retrospective study is a challenge, as more reliable adherence assessment--for example, with use of pill counts or blister packs--is not possible. This study's retrospective design was another potential limitation, as an active intervention was not used.
In addition, this study had a small sample, likely attributable to the addition of dabigatran to the VA national formulary just months before the start of the study period. Furthermore, this study was not powered to detect significant differences in safety or efficacy outcomes. Other potential study limitations included having national VA guidance regarding follow-up periods and dabigatran prescription quantity limits during both study periods. Also, there was some potential for pharmacist-initiated refills at follow-up visits, which could falsely increase MPR. Last, the study analyzed only 1 DOAC and not the entire class of medications.
Conclusion
Centralizing DOAC management by clinical pharmacy specialists at a single VA facility helped maintain high rates of dabigatran adherence, above the national average, and low rates of adverse outcomes were maintained in both study groups. In addition, centralization of anticoagulation services improved access to care through an increase in primary care pharmacist visits without the addition of staff. Centralization of DOAC management by pharmacists is a viable option for maintaining high rates of adherence and low rates of adverse outcomes in facilities where the goal is to achieve clinical efficiency.
In the U.S. about 2.7 to 6.1 million people have atrial fibrillation (AF).1 This condition affects the rhythm of the heart, causes blood in the heart to become stagnant, and puts patients at high risk for developing a systemic embolism, particularly a stroke.1 Recent studies have shown that AF accounts for at least 15% of all strokes in the U.S. and 36% of strokes in people aged > 80 years.2
For patients aged > 60 years, the gold standard of long-term anticoagulation for reducing the risk of stroke has been oral vitamin K antagonist (warfarin) therapy.2 Although overwhelming evidence exists that supports the use of warfarin in these patients, warfarin is a narrow therapeutic index medication that requires frequent laboratory monitoring of international normalized ratio (INR) for dose titration guidance. There is also strong evidence that pharmacist-run anticoagulation clinics have improved patient-centered outcomes in patients prescribed warfarin.3-5
Direct oral anticoagulants (DOACs) are recently approved oral medications used as alternatives to warfarin for anticoagulation in AF. Direct oral anticoagulants do not require INR monitoring or any laboratory test for efficacy. In 2010, the FDA approved the first DOAC, dabigatran, for use in patients with AF. In 2011, rivaroxaban received approval for the same indication. One potential drawback of these new agents relative to warfarin is the lack of availability of a reversal agent that can be used in the event of a life-threatening bleeding event. Dabigatran is the only DOAC with an FDA-approved available reversal agent. In both 2011 and 2012, dabigatran, warfarin, and other anticoagulants topped the Institute for Safe Medicine Practice list of suspect drugs related to adverse events (AEs). These data prompted the Joint Commission to incorporate anticoagulation into the 2017 National Hospital Patient Safety Goals to improve patient outcomes and reduce harm from use of anticoagulants.6
In early 2011, the VHA produced national guidance on the treatment of patients who receive DOACs; this guidance was updated most recently in September 2016.7 Patients who were receiving DOACs at the Ralph H. Johnson VAMC (RHJVAMC) were initially monitored by 12 primary care pharmacists at the main hospital or at community-based outpatient clinics (CBOCs). Ambulatory care pharmacists at RHJVAMC work under a scope of practice to prescribe and adjust certain classes of medications to provide the highest level of care to more than 65,000 veterans in South Carolina and Georgia. Historically at RHJVAMC, warfarin has been the anticoagulant most commonly used for AF, though dabigatran and rivaroxaban have gained in popularity after being added to the national VA formulary.
In November 2012, for better monitoring of patient outcomes, improved efficiency of the primary care pharmacist clinics, and increased access to care in these clinics, treatment of patients prescribed DOACs was shifted to a centralized model that involved 3 anticoagulation clinical pharmacy specialists.
Centralized pharmacy services have a small number of core team members in a specific service for a particular disease, which reduces the number of different pharmacists a patient could talk to for management of a particular condition. Centralized pharmacy services allow for streamlining anticoagulation management to a small group of individual pharmacists considered specialists in anticoagulation. This shift in management to centralized anticoagulation services was supported at RHJVAMC by findings from a study of a pharmacist-run centralized anticoagulation clinic: Patients treated by the centralized clinic were 39% less likely to experience an anticoagulation therapy complication.8
Protocol for dabigatran follow-up and monitoring at RHJVAMC was developed by clinical and supervisory pharmacy staff, to align with national VA guidance. When a provider determines a patient is a candidate for dabigatran, an outpatient consultation is entered for the clinical pharmacy specialist to review the appropriateness of the patient selection for therapy. If the patient is eligible for therapy, the pharmacist contacts the patient to set up an initial visit to confirm selection and to provide the first dabigatran prescription and counseling. For assessments, with specific emphasis on adherence and AE monitoring, the patient is contacted 2 weeks, 1 month, 3 months, and every 6 months after the initial appointment.
Although most of the literature supports pharmacist-managed anticoagulation for patients who receive warfarin, DOACs have become more integrated into practice and more evaluated. Evidence supports pharmacists' interventions on evaluation of patient education and dosing, but there is conflicting evidence regarding pharmacists' impact on adherence after 3 months of therapy.9,10 In a larger VA study of the impact of dabigatran adherence on patient-centered outcomes, patients were mostly nonadherent to prescribed dosing.11 These studies support the need for improved adherence in patients prescribed DOACs and the need for further investigation of pharmacists' roles in improving patient outcomes.
Methods
This single-center, retrospective anticoagulant-use evaluation covered 2 study periods between November 1, 2011 and October 31, 2013. Study approval was obtained from the institutional review board of the Medical University of South Carolina and the research and development committee of RHJVAMC. The study population consisted of veterans who had a diagnosis of AF and received at least 3 outpatient prescription fills of a 30-day supply of dabigatran at RHJVAMC during either or both of the study periods. Patients were excluded if they were pregnant or planning to become pregnant or were incarcerated at any time during the study period. Dabigatran was selected because it was the first DOAC added to the local VA formulary before the start of this study.
Patients who met the inclusion criteria were separated into 2 groups based on the dates of their prescription fills. The precentralization group included patients treated by primary care pharmacists from November 1, 2011 to October 31, 2012; the postcentralization group included patients treated by anticoagulation clinical pharmacy specialists from November 1, 2012 to October 31, 2013. In each group, patients were followed for 1 year during their respective study period. For analysis, patients were included in both study periods if they received at least 3 fills of dabigatran during each period.
Medication possession ratio (MPR), which was used to measure the primary endpoint of adherence, is defined as the proportion of days a patient had dabigatran. The MPR denominator is the total number of days between the first and last prescription refill dates within the 52-week study period; the numerator is calculated by summing the days' supply for all but the last filling of the medication during each respective period. Nonadherence was defined as an MPR < 0.8 (or 80%), which has been used to define poor adherence in the literature.12 The authors calculated all patients' mean MPRs and compared them to determine statistical significance by repeated-measures linear regression. Descriptive statistics on proportion of patients in each study group with MPR < 0.8 were examined. Last, the authors performed a comparative subanalysis of median MPRs to determine whether there was an adherence difference between patients initially started on dabigatran at RHJVAMC and patients who were started on dabigatran before receiving it at RHJVAMC.
The secondary focus of this study was safety outcomes, including any bleeding event or thromboembolism within either study period. A bleeding event was defined as any major or minor bleeding event recognized through ICD-9 codes or any bleeding recorded in the patient's chart and noted during chart review, as well as any serum hemoglobin (Hgb) level decrease of ≥ to 2 g/dL during the study period. Thromboembolism was defined as a thromboembolism recognized through ICD-9 codes or any thromboembolism noted during chart review. Descriptive statistics were reported for this outcome, and a chi-square test was used to compare bleeding events between groups to determine significance.
The tertiary focus of this study was clinical efficiency as determined by number of primary care pharmacist visits during each study period. Primary care pharmacist visits were included for all primary care pharmacists in primary care clinics at the main hospital and in all 6 CBOCs.
For statistical analysis α was set at 0.05, and P < .05 was considered statistically significant. SAS Enterprise Guide software (Cary, North Carolina) was used for all statistical analyses.
Results
An initial data pull was completed from the RHJVAMC prescription records database for patients who had ≥ 3 prescriptions of dabigatran filled for treatment of AF during the study period, which yielded 65 unique patients. There were 34 patients in the precentralization group and 55 patients in the postcentralization group. Twenty-four unique patients were included in both study groups.
Mean MPR was 1.01 (range, 0.59-1.41) for the precentralization study period and 0.96 (range, 0.33-1.36) for the postcentralization period (Table 1). The difference was not statistically significant (P = .91). Number of patients considered nonadherent (MPR < 0.8) was 3 (8.82%) in the precentralization group and 8 (14.6%) in the postcentralization group.
The primary endpoint subanalysis compared the median MPRs for the patients initially started on dabigatran at RHJVAMC (de novo starts) and the patients who were started on dabigatran before receiving it at RHJVAMC (prior starts). In each group, number and percentage of patients determined to be nonadherent by MPR were evaluated as well. De novo patients received initial assessment, counseling, and a dabigatran prescription from RHJVAMC pharmacists before or during the study period, and prior patients were initially prescribed dabigatran at another VA facility or at a non-VA facility (Table 2).
Regarding safety outcomes (secondary endpoint), a bleeding event was identified in 6 (17.7%) of the precentralization patients and 7 (12.7%) of the postcentralization patients. Of the 6 precentralization events, 1 was a case of hemoptysis, 1 was a hematoma on the forehead, 1 was a lower gastrointestinal bleed (unconfirmed), 1 was retinal hemorrhaging (noted by ophthalmologist), and 2 were serum Hgb level decreases of more than 2 g/dL (neither patient required transfusion of packed red blood cells). Of the 7 postcentralization events, 1 was persistent hematochezia caused by hemorrhoids, 1 was hematuria, 1 was a hematoma, 1 was an upper gastrointestinal bleed (required blood transfusion), and 4 were serum Hgb level decreases of more than 2 g/dL (1 of the 4 required transfusion). No precentralization patient had any evidence of thromboembolism during the study period; 1 postcentralization patient had a superficial venous thromboembolism near a hematoma on the elbow.
Discussion
In this single-center, retrospective medication-use evaluation, the authors found a high rate of adherence to dabigatran before and after centralization of outpatient DOAC management by pharmacists. There was no statistically significant difference in bleeding events between the study periods, but primary care pharmacist visits increased by 108% from precentralization to postcentralization. Although the primary outcome findings did not refute the study's null hypothesis, results support implementing centralized pharmacist DOAC management to maintain a high rate of adherence and a low incidence of adverse outcomes and providing more primary care pharmacist services to increase access to care for other chronic diseases.
Although there was no statistically significant difference in adherence rates between study periods, the 2 groups' rates were higher than the national average of 72%, as calculated by the proportion-of-days-covered (PDC) equation (median, 74%) in a 2015 large-scale study of site-level adherence in more than 5,000 VA patients.13 The authors' findings support that study's significant finding of a high rate of adherence to pharmacist-provided dabigatran treatment. This study's adherence rate also was higher than the median PDC rate reported in a 2014 study that focused on dabigatran adherence: 94% (mean, 84%; SD, 22%).11
The RHJVAMC follows national VA guidance on pharmacist follow-up for patients who receive DOACs. This follow-up focuses on frequent counseling over the first 6 months of de novo DOAC treatment and on monitoring and assessing adherence and AEs. Although there is less laboratory monitoring for DOAC treatment than for treatment with vitamin K antagonists (eg, warfarin), telephone monitoring as described in this study has been associated with a high adherence rate and minimization of AEs. The 2014 study with the 94% median PDC rate also showed an association of decreased adherence and increased harm, including combined all-cause mortality and stroke (hazard ratio, 1.13; 95% confidence interval [CI], 1.07-1.19 per 10% decrease in PDC rate).11
This study's subanalysis revealed no difference in adherence between patients initially started on dabigatran at RHJVAMC and patients who were started on dabigatran before receiving it at RHJVAMC. Each group had a high rate of adherence. Shore and colleagues found that most of the VA sites they surveyed (22/41) had anticoagulation clinics monitoring patients who were prescribed dabigatran.13 Pharmacist-led monitoring of adherence and AEs led to increased adherence to dabigatran treatment (relative risk, 1.25; 95% CI, 1.11-1.41), which was the standard of care at RHJVAMC throughout their entire study. Many of these factors may explain the very high rate of adherence found in the present study, specifically in comparison to previously reported national averages.
In addition, the authors found no statistically significant difference in bleeding outcomes between the precentralization and postcentralization groups. Their incidence of bleeding was similar to the 16.6% rate reported in the package insert for dabigatran.14 Furthermore, the safety outcomes were similar for both groups in this study, which may be attributable to the quality of patient care provided by all RHJVAMC pharmacists, particularly in the setting of dabigatran management.
Many studies have found an association between dabigatran use and an increased rate of bleeding, particularly gastrointestinal, as demonstrated in several patients in this study. Evidence of these clinically significant AEs further supports pharmacists' close monitoring to detect these AEs and working with patients' providers to determine whether an alternative anticoagulant should be used.
A significant finding of this study regarding centralization of DOAC management by pharmacists was the increased number of primary care pharmacist visits. By streamlining all anticoagulant services to anticoagulation clinical pharmacy specialists, primary care pharmacists were able to care for more veterans and increase access to care without adding staff. The centralized anticoagulation pharmacists were volunteers who held other positions within the department; they did not have to be replaced when they became anticoagulation providers. This workload reallocation helped the RHJVAMC pharmacy department increase access to care.
Limitations
This study had several potential limitations. First, MPR, a widely studied common tool for assessing adherence, has been criticized for often being imprecise when used with short study periods.12 Another commonly used adherence measure is PDC rate, which has been reported in several large-scale studies of dabigatran therapy. The authors selected MPR for the present study because MPR calculation is more practical in the patient population and because MPR and PDC rate are predicted to yield similar results in assessments of adherence to a single medication.12 It also should be noted that both MPR and PDC rate are surrogate markers for adherence and assume adherence based on the availability of medication to the patient. Assessing adherence in a retrospective study is a challenge, as more reliable adherence assessment--for example, with use of pill counts or blister packs--is not possible. This study's retrospective design was another potential limitation, as an active intervention was not used.
In addition, this study had a small sample, likely attributable to the addition of dabigatran to the VA national formulary just months before the start of the study period. Furthermore, this study was not powered to detect significant differences in safety or efficacy outcomes. Other potential study limitations included having national VA guidance regarding follow-up periods and dabigatran prescription quantity limits during both study periods. Also, there was some potential for pharmacist-initiated refills at follow-up visits, which could falsely increase MPR. Last, the study analyzed only 1 DOAC and not the entire class of medications.
Conclusion
Centralizing DOAC management by clinical pharmacy specialists at a single VA facility helped maintain high rates of dabigatran adherence, above the national average, and low rates of adverse outcomes were maintained in both study groups. In addition, centralization of anticoagulation services improved access to care through an increase in primary care pharmacist visits without the addition of staff. Centralization of DOAC management by pharmacists is a viable option for maintaining high rates of adherence and low rates of adverse outcomes in facilities where the goal is to achieve clinical efficiency.
1. January CT, Wann LS, Alpert JS, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society [published correction appears in J Am Coll Cardiol. 2014;64(21):2305-2307. J Am Coll Cardiol. 2014;64(21):e1-e76.
2. Reiffel JA. New versus traditional approaches to oral anticoagulation in patients with atrial fibrillation. Am J Med. 2014;127(4):e15.
3. Locke C, Ravnan SL, Patel R, Uchizono JA. Reduction in warfarin adverse events requiring patient hospitalization after implementation of pharmacist-managed anticoagulation service. Pharmacotherapy. 2005;25(5):685-689.
4. Poon IO, Lal L, Brown EN, Braun UK. The impact of pharmacist-managed oral anticoagulation therapy in older veterans. J Clin Pharm Ther. 2007;32(1):21-29.
5. Chiquette E, Amato MG, Bussey HI. Comparison of an anticoagulation clinic with usual medical care. Arch Intern Med. 1998;158(15):1641-1647.
6. The Joint Commission. National patient safety goals. https://www.jointcommission.org/as sets/1/6/2017_NPSG_HAP_ER.pdf. Published 2016. Accessed December 6, 2016.
7. Department of Veterans Affairs Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives. Direct oral anticoagulants (DOACs) (formerly called TSOACs) dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis): Criteria for Use for Stroke Prevention in nonvalvular atrial fibrillation (AF) and Edoxaban (SAVAYSA). http://www.pbm.va.gov/PBM/clinicalguidance/criteriaforuse/Anticoagulants_Direct_Oral_DOACs_CFU_and_Algorithm_for_Nonvalvular_Atrial_Fibrillation_Sep_2016.pdf. Updated September 2016. Accessed December 6, 2016.
8. Witt DM, Sadler MA, Shanahan RL, Mazzoli G, Tillman DJ. Effect of a centralized clinical pharmacy anticoagulation service on the outcomes of anticoagulation therapy. Chest. 2005;127(5):1515-1522.
9. Chan LL, Crumpler WL, Jacobson AK. Implementation of pharmacist-managed anticoagulation in patients receiving newer anticoagulants. Am J Health Syst Pharm. 2013;70(15):1285-1286, 1288.
10. Lee PY, Han SY, Miyahara RK. Adherence and outcomes of patients treated with dabigatran: pharmacist-managed anticoagulation clinic versus usual care. Am J Health Syst Pharm. 2013;70(13):1154-1161.
11. Shore S, Carey EP, Turakhia MP, et al. Adherence to dabigatran therapy and longitudinal patient outcomes: insights from the Veterans Health Administration. Am Heart J. 2014;167(6):810-817.
12. Martin BC, Wiley-Exley EK, Richards S, Domino ME, Carey TS, Sleath BL. Contrasting measures of adherence with simple drug use, medication switching and therapeutic duplication. Ann Pharmacother. 2009;43(1):36-44.
13. Shore S, Ho PM, Lambert-Kerzner A, et al. Site-level variation in and practices associated with dabigatran adherence. JAMA. 2015;313(14):1443-1450.
14. Pradaxa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals; 2015.
1. January CT, Wann LS, Alpert JS, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society [published correction appears in J Am Coll Cardiol. 2014;64(21):2305-2307. J Am Coll Cardiol. 2014;64(21):e1-e76.
2. Reiffel JA. New versus traditional approaches to oral anticoagulation in patients with atrial fibrillation. Am J Med. 2014;127(4):e15.
3. Locke C, Ravnan SL, Patel R, Uchizono JA. Reduction in warfarin adverse events requiring patient hospitalization after implementation of pharmacist-managed anticoagulation service. Pharmacotherapy. 2005;25(5):685-689.
4. Poon IO, Lal L, Brown EN, Braun UK. The impact of pharmacist-managed oral anticoagulation therapy in older veterans. J Clin Pharm Ther. 2007;32(1):21-29.
5. Chiquette E, Amato MG, Bussey HI. Comparison of an anticoagulation clinic with usual medical care. Arch Intern Med. 1998;158(15):1641-1647.
6. The Joint Commission. National patient safety goals. https://www.jointcommission.org/as sets/1/6/2017_NPSG_HAP_ER.pdf. Published 2016. Accessed December 6, 2016.
7. Department of Veterans Affairs Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives. Direct oral anticoagulants (DOACs) (formerly called TSOACs) dabigatran (Pradaxa), rivaroxaban (Xarelto), and apixaban (Eliquis): Criteria for Use for Stroke Prevention in nonvalvular atrial fibrillation (AF) and Edoxaban (SAVAYSA). http://www.pbm.va.gov/PBM/clinicalguidance/criteriaforuse/Anticoagulants_Direct_Oral_DOACs_CFU_and_Algorithm_for_Nonvalvular_Atrial_Fibrillation_Sep_2016.pdf. Updated September 2016. Accessed December 6, 2016.
8. Witt DM, Sadler MA, Shanahan RL, Mazzoli G, Tillman DJ. Effect of a centralized clinical pharmacy anticoagulation service on the outcomes of anticoagulation therapy. Chest. 2005;127(5):1515-1522.
9. Chan LL, Crumpler WL, Jacobson AK. Implementation of pharmacist-managed anticoagulation in patients receiving newer anticoagulants. Am J Health Syst Pharm. 2013;70(15):1285-1286, 1288.
10. Lee PY, Han SY, Miyahara RK. Adherence and outcomes of patients treated with dabigatran: pharmacist-managed anticoagulation clinic versus usual care. Am J Health Syst Pharm. 2013;70(13):1154-1161.
11. Shore S, Carey EP, Turakhia MP, et al. Adherence to dabigatran therapy and longitudinal patient outcomes: insights from the Veterans Health Administration. Am Heart J. 2014;167(6):810-817.
12. Martin BC, Wiley-Exley EK, Richards S, Domino ME, Carey TS, Sleath BL. Contrasting measures of adherence with simple drug use, medication switching and therapeutic duplication. Ann Pharmacother. 2009;43(1):36-44.
13. Shore S, Ho PM, Lambert-Kerzner A, et al. Site-level variation in and practices associated with dabigatran adherence. JAMA. 2015;313(14):1443-1450.
14. Pradaxa [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals; 2015.
Novel Screening Test Sparks New Ideas About Old Drugs
Repurposing standard drugs and rethinking drug combinations may lead to more effective ways to combat drug-resistant bacteria, according to findings from an NIH study.
Researchers developed an assay to screen for effectiveness and used it on 5,170 drugs and other biologically active compounds. They identified 25 that suppress the growth of 2 strains of Klebsiella pneumonia (K pneumonia) that are resistant to most antibiotics: 11 FDA-approved drugs and 14 drugs still under investigation, including antibiotics, antifungals, and antiseptics, and an antiviral, antimalarial and anticancer drug/compound.
Related: The Cost of Unused Medications
They also looked for combinations of drugs and paired newly identified drugs from the repurposing screen with a standard-of-care antibiotic that did not work by itself. They found four 2-drug combinations that work against K pneumoniae, meaning the ineffective antibiotics became active again in the presence of the second drug. Combining colistin with doxycycline, for instance, reversed the drug resistance.
They also tested 3-drug combinations against 10 common strains of multidrug-resistant bacteria and found 3 different combinations of broad-acting antibiotics that were effective. For instance, colistin-auranofin-ceftazidime and colistin-auranofin-rifabutin suppressed more than 80% growth of all 10 strains. Rifabutin-colistin-imipenem inhibited more than 75% of the strains, except 2 Acinetobacter baumannii isolates.
Related: DoD Offers ‘Drug Take Back’ Program
Their results demonstrate that their assay has potential as a real-time clinical tool, the researchers say. “The results are very promising,” said one of the investigators. “We think the test can eventually help repurpose approved drugs and other compounds and find clinically relevant drug combinations that can be approved for use in different ways that we have never used before.”
Repurposing standard drugs and rethinking drug combinations may lead to more effective ways to combat drug-resistant bacteria, according to findings from an NIH study.
Researchers developed an assay to screen for effectiveness and used it on 5,170 drugs and other biologically active compounds. They identified 25 that suppress the growth of 2 strains of Klebsiella pneumonia (K pneumonia) that are resistant to most antibiotics: 11 FDA-approved drugs and 14 drugs still under investigation, including antibiotics, antifungals, and antiseptics, and an antiviral, antimalarial and anticancer drug/compound.
Related: The Cost of Unused Medications
They also looked for combinations of drugs and paired newly identified drugs from the repurposing screen with a standard-of-care antibiotic that did not work by itself. They found four 2-drug combinations that work against K pneumoniae, meaning the ineffective antibiotics became active again in the presence of the second drug. Combining colistin with doxycycline, for instance, reversed the drug resistance.
They also tested 3-drug combinations against 10 common strains of multidrug-resistant bacteria and found 3 different combinations of broad-acting antibiotics that were effective. For instance, colistin-auranofin-ceftazidime and colistin-auranofin-rifabutin suppressed more than 80% growth of all 10 strains. Rifabutin-colistin-imipenem inhibited more than 75% of the strains, except 2 Acinetobacter baumannii isolates.
Related: DoD Offers ‘Drug Take Back’ Program
Their results demonstrate that their assay has potential as a real-time clinical tool, the researchers say. “The results are very promising,” said one of the investigators. “We think the test can eventually help repurpose approved drugs and other compounds and find clinically relevant drug combinations that can be approved for use in different ways that we have never used before.”
Repurposing standard drugs and rethinking drug combinations may lead to more effective ways to combat drug-resistant bacteria, according to findings from an NIH study.
Researchers developed an assay to screen for effectiveness and used it on 5,170 drugs and other biologically active compounds. They identified 25 that suppress the growth of 2 strains of Klebsiella pneumonia (K pneumonia) that are resistant to most antibiotics: 11 FDA-approved drugs and 14 drugs still under investigation, including antibiotics, antifungals, and antiseptics, and an antiviral, antimalarial and anticancer drug/compound.
Related: The Cost of Unused Medications
They also looked for combinations of drugs and paired newly identified drugs from the repurposing screen with a standard-of-care antibiotic that did not work by itself. They found four 2-drug combinations that work against K pneumoniae, meaning the ineffective antibiotics became active again in the presence of the second drug. Combining colistin with doxycycline, for instance, reversed the drug resistance.
They also tested 3-drug combinations against 10 common strains of multidrug-resistant bacteria and found 3 different combinations of broad-acting antibiotics that were effective. For instance, colistin-auranofin-ceftazidime and colistin-auranofin-rifabutin suppressed more than 80% growth of all 10 strains. Rifabutin-colistin-imipenem inhibited more than 75% of the strains, except 2 Acinetobacter baumannii isolates.
Related: DoD Offers ‘Drug Take Back’ Program
Their results demonstrate that their assay has potential as a real-time clinical tool, the researchers say. “The results are very promising,” said one of the investigators. “We think the test can eventually help repurpose approved drugs and other compounds and find clinically relevant drug combinations that can be approved for use in different ways that we have never used before.”
Abuse-Deterrent Opioids: What Practitioners Need to Know
Opioid Abuse-Deterrent Formulations
The meaning of the term abuse-deterrent is often misunderstood to mean abuse-proof. The FDA defines abuse-deterrent properties as those properties expected to meaningfully deter abuse even if they do not fully prevent abuse. Abuse-deterrent properties make certain types of abuse, such as crushing in order to snort or dissolving in order to inject, more difficult or less rewarding. However, this does not mean that the product is impossible to abuse or that these properties will necessarily prevent addiction, overdose, or death.
Of note, currently marketed abuse-deterrent formulation technologies do not effectively deter one of the most common forms of opioid abuse—simply swallowing a number of intact tablets or capsules. Abuse-deterrent opioids do not reduce the risk for opioid addiction, and they carry the same warnings about the risk for addiction as do conventional opioids.
Abuse and Misuse Data
The FDA is encouraging pharmaceutical industry efforts to develop pain medicines that are more difficult to abuse and to prioritize the need for data and study methods that will help evaluate the impact of abuse-deterrent opioids on misuse and abuse in the community. To collect this important information, the FDA requires that all companies that have brand-name opioids with labeling describing abuse-deterrent properties conduct postmarketing studies to determine the impact of abuse-deterrent formulation technologies in the real world. Each company is given a time line to which they must adhere. These types of studies take several years to conduct and analyze. Data collected will include the amount prescribed for each product; adverse events related to the use, abuse, and misuse of the products; and epidemiologic data on the rates of abuse and misuse and their consequences (addiction, overdose, and death). These studies should allow the FDA to assess the impact in the community, if any, attributable to the abuse-deterrent properties.
The science of abuse deterrence is relatively new, and both the formulation technologies and the analytical, clinical, and statistical methods for evaluating those technologies ar
Key Points for Practitioners
The FDA’s work to facilitate the safe use of opioids is taking place within a larger policy framework aimed at addressing opioid abuse while ensuring appropriate access to pain treatment. The FDA has undertaken several efforts helpful to clinicians. The FDA’s Extended-Release and Long-Acting Opioid Analgesics Risk Evaluation and Mitigation Strategy (ER/LA REMS) Program is required for all companies who make these products. The program’s goal is to reduce serious adverse outcomes of inappropriate prescribing, misuse, and abuse of ER/LA opioid analgesics while maintaining patient access to pain medications. Adverse outcomes of concern include addiction, unintentional overdose, and death.
As part of the REMS, all ER/LA opioid analgesic pharmaceutical companies must provide education for prescribers of their medications through accredited continuing education activities that are supported by independent educational grants. Companies must also provide information that prescribers can use when counseling patients about the risks and benefits associated with ER/LA opioid analgesic use.
The FDA has developed core messages that are communicated to prescribers in the Blueprint for Prescriber Education. The Blueprint is directed to prescribers of ER/LA opioid analgesics but also may be relevant for other health care professionals (eg, pharmacists). Companies involved in the ER/LA Opioid Analgesics REMS Program have collaborated to implement a single shared REMS. This group provides a list of REMS-compliant continuing education activities, which can be found at http://www.er-la-opioidrems.com.
It is important for practitioners to understand that all currently approved abuse-deterrent opioid products still can be abused, and as scheduled controlled substances, they are addictive. The abuse-deterrent properties are expected to deter but do not wholly prevent abuse. Because in the end opioid medications must be able to deliver the opioid to the patient, there probably always will be potential for abuse of these products. Consequently, practitioners should counsel their patients on the following:
- Keep medicines in a secure location out of the reach and out of sight of children and pets. Put away medicines after every use. Accidental exposure to medicine in the home is a major source of unintentional poisonings in the U.S.
- If medicines are no longer needed, dispose of them properly. Disposing of all unused opioid analgesics reduces access to these medications by family members and household guests seeking opioids for abuse.
- The FDA recommends returning most prescription medications through a local or U.S. Drug Enforcement Administration (DEA)-sponsored take-back program or DEA-authorized collector. For opioid analgesics, the FDA recommends immediate removal from the home by flushing them down the toilet or sink.
Opioids Action Plan
In February 2016, FDA Commissioner Robert Califf (then the deputy commissioner for medical products and tobacco) announced the FDA Opioids Action Plan. The plan focuses on policies aimed at reversing the opioid epidemic while still providing patients in pain access to effective pain relief. The FDA actions include:
- Convening an expert advisory committee before approving any new drug application for an opioid that does not have abuse-deterrent properties;
- Consulting with the Pediatric Advisory Committee about a framework for pediatric opioid labeling before any new labeling is approved;
- Updating the REMS requirements for ER/LA opioid analgesics after considering the advisory committee’s recommendations from a meeting held in May 2016 and reviewing existing requirements;
- Improving access to naloxone (by facilitating the development of an over-the-counter version of naloxone, which is currently available only by prescription, thereby making it more accessible to treat opioid overdose), and medication-assisted treatment options for patients with opioid use disorders; and
- Supporting better pain management options, including alternative, nonaddictive treatments for pain.
The FDA is conducting research on pain measurements for conditions such as chronic low back pain, osteoarthritis, diabetic neuropathy, postherpetic neuralgia, and fibromyalgia. The FDA is also working to support the development of nonopioid options for these patients.
Consistent with the plan, in March 2016, the FDA announced that it was requiring changes to the labeling on immediate-release opioids, including additional warnings and safety information that incorporate elements similar to the ER/LA opioid analgesics labeling. Furthermore, among other steps, the FDA has contracted with the National Academy of Medicine to provide advice on how to incorporate current evidence about the public health impact of opioid use (for patients who are prescribed opioids as well as for nonpatients) into regulatory activities concerning opioids.
The FDA shares the responsibility of keeping patients safe. Working with the health care community and federal and state partners to help reduce opioid misuse and abuse and improve appropriate opioid prescribing while ensuring that patients in pain continue to have appropriate access to opioid analgesics is a top priority for the FDA and part of the targeted approach of the HHS focused on prevention, treatment, and intervention.
Opioid Abuse-Deterrent Formulations
The meaning of the term abuse-deterrent is often misunderstood to mean abuse-proof. The FDA defines abuse-deterrent properties as those properties expected to meaningfully deter abuse even if they do not fully prevent abuse. Abuse-deterrent properties make certain types of abuse, such as crushing in order to snort or dissolving in order to inject, more difficult or less rewarding. However, this does not mean that the product is impossible to abuse or that these properties will necessarily prevent addiction, overdose, or death.
Of note, currently marketed abuse-deterrent formulation technologies do not effectively deter one of the most common forms of opioid abuse—simply swallowing a number of intact tablets or capsules. Abuse-deterrent opioids do not reduce the risk for opioid addiction, and they carry the same warnings about the risk for addiction as do conventional opioids.
Abuse and Misuse Data
The FDA is encouraging pharmaceutical industry efforts to develop pain medicines that are more difficult to abuse and to prioritize the need for data and study methods that will help evaluate the impact of abuse-deterrent opioids on misuse and abuse in the community. To collect this important information, the FDA requires that all companies that have brand-name opioids with labeling describing abuse-deterrent properties conduct postmarketing studies to determine the impact of abuse-deterrent formulation technologies in the real world. Each company is given a time line to which they must adhere. These types of studies take several years to conduct and analyze. Data collected will include the amount prescribed for each product; adverse events related to the use, abuse, and misuse of the products; and epidemiologic data on the rates of abuse and misuse and their consequences (addiction, overdose, and death). These studies should allow the FDA to assess the impact in the community, if any, attributable to the abuse-deterrent properties.
The science of abuse deterrence is relatively new, and both the formulation technologies and the analytical, clinical, and statistical methods for evaluating those technologies ar
Key Points for Practitioners
The FDA’s work to facilitate the safe use of opioids is taking place within a larger policy framework aimed at addressing opioid abuse while ensuring appropriate access to pain treatment. The FDA has undertaken several efforts helpful to clinicians. The FDA’s Extended-Release and Long-Acting Opioid Analgesics Risk Evaluation and Mitigation Strategy (ER/LA REMS) Program is required for all companies who make these products. The program’s goal is to reduce serious adverse outcomes of inappropriate prescribing, misuse, and abuse of ER/LA opioid analgesics while maintaining patient access to pain medications. Adverse outcomes of concern include addiction, unintentional overdose, and death.
As part of the REMS, all ER/LA opioid analgesic pharmaceutical companies must provide education for prescribers of their medications through accredited continuing education activities that are supported by independent educational grants. Companies must also provide information that prescribers can use when counseling patients about the risks and benefits associated with ER/LA opioid analgesic use.
The FDA has developed core messages that are communicated to prescribers in the Blueprint for Prescriber Education. The Blueprint is directed to prescribers of ER/LA opioid analgesics but also may be relevant for other health care professionals (eg, pharmacists). Companies involved in the ER/LA Opioid Analgesics REMS Program have collaborated to implement a single shared REMS. This group provides a list of REMS-compliant continuing education activities, which can be found at http://www.er-la-opioidrems.com.
It is important for practitioners to understand that all currently approved abuse-deterrent opioid products still can be abused, and as scheduled controlled substances, they are addictive. The abuse-deterrent properties are expected to deter but do not wholly prevent abuse. Because in the end opioid medications must be able to deliver the opioid to the patient, there probably always will be potential for abuse of these products. Consequently, practitioners should counsel their patients on the following:
- Keep medicines in a secure location out of the reach and out of sight of children and pets. Put away medicines after every use. Accidental exposure to medicine in the home is a major source of unintentional poisonings in the U.S.
- If medicines are no longer needed, dispose of them properly. Disposing of all unused opioid analgesics reduces access to these medications by family members and household guests seeking opioids for abuse.
- The FDA recommends returning most prescription medications through a local or U.S. Drug Enforcement Administration (DEA)-sponsored take-back program or DEA-authorized collector. For opioid analgesics, the FDA recommends immediate removal from the home by flushing them down the toilet or sink.
Opioids Action Plan
In February 2016, FDA Commissioner Robert Califf (then the deputy commissioner for medical products and tobacco) announced the FDA Opioids Action Plan. The plan focuses on policies aimed at reversing the opioid epidemic while still providing patients in pain access to effective pain relief. The FDA actions include:
- Convening an expert advisory committee before approving any new drug application for an opioid that does not have abuse-deterrent properties;
- Consulting with the Pediatric Advisory Committee about a framework for pediatric opioid labeling before any new labeling is approved;
- Updating the REMS requirements for ER/LA opioid analgesics after considering the advisory committee’s recommendations from a meeting held in May 2016 and reviewing existing requirements;
- Improving access to naloxone (by facilitating the development of an over-the-counter version of naloxone, which is currently available only by prescription, thereby making it more accessible to treat opioid overdose), and medication-assisted treatment options for patients with opioid use disorders; and
- Supporting better pain management options, including alternative, nonaddictive treatments for pain.
The FDA is conducting research on pain measurements for conditions such as chronic low back pain, osteoarthritis, diabetic neuropathy, postherpetic neuralgia, and fibromyalgia. The FDA is also working to support the development of nonopioid options for these patients.
Consistent with the plan, in March 2016, the FDA announced that it was requiring changes to the labeling on immediate-release opioids, including additional warnings and safety information that incorporate elements similar to the ER/LA opioid analgesics labeling. Furthermore, among other steps, the FDA has contracted with the National Academy of Medicine to provide advice on how to incorporate current evidence about the public health impact of opioid use (for patients who are prescribed opioids as well as for nonpatients) into regulatory activities concerning opioids.
The FDA shares the responsibility of keeping patients safe. Working with the health care community and federal and state partners to help reduce opioid misuse and abuse and improve appropriate opioid prescribing while ensuring that patients in pain continue to have appropriate access to opioid analgesics is a top priority for the FDA and part of the targeted approach of the HHS focused on prevention, treatment, and intervention.
Opioid Abuse-Deterrent Formulations
The meaning of the term abuse-deterrent is often misunderstood to mean abuse-proof. The FDA defines abuse-deterrent properties as those properties expected to meaningfully deter abuse even if they do not fully prevent abuse. Abuse-deterrent properties make certain types of abuse, such as crushing in order to snort or dissolving in order to inject, more difficult or less rewarding. However, this does not mean that the product is impossible to abuse or that these properties will necessarily prevent addiction, overdose, or death.
Of note, currently marketed abuse-deterrent formulation technologies do not effectively deter one of the most common forms of opioid abuse—simply swallowing a number of intact tablets or capsules. Abuse-deterrent opioids do not reduce the risk for opioid addiction, and they carry the same warnings about the risk for addiction as do conventional opioids.
Abuse and Misuse Data
The FDA is encouraging pharmaceutical industry efforts to develop pain medicines that are more difficult to abuse and to prioritize the need for data and study methods that will help evaluate the impact of abuse-deterrent opioids on misuse and abuse in the community. To collect this important information, the FDA requires that all companies that have brand-name opioids with labeling describing abuse-deterrent properties conduct postmarketing studies to determine the impact of abuse-deterrent formulation technologies in the real world. Each company is given a time line to which they must adhere. These types of studies take several years to conduct and analyze. Data collected will include the amount prescribed for each product; adverse events related to the use, abuse, and misuse of the products; and epidemiologic data on the rates of abuse and misuse and their consequences (addiction, overdose, and death). These studies should allow the FDA to assess the impact in the community, if any, attributable to the abuse-deterrent properties.
The science of abuse deterrence is relatively new, and both the formulation technologies and the analytical, clinical, and statistical methods for evaluating those technologies ar
Key Points for Practitioners
The FDA’s work to facilitate the safe use of opioids is taking place within a larger policy framework aimed at addressing opioid abuse while ensuring appropriate access to pain treatment. The FDA has undertaken several efforts helpful to clinicians. The FDA’s Extended-Release and Long-Acting Opioid Analgesics Risk Evaluation and Mitigation Strategy (ER/LA REMS) Program is required for all companies who make these products. The program’s goal is to reduce serious adverse outcomes of inappropriate prescribing, misuse, and abuse of ER/LA opioid analgesics while maintaining patient access to pain medications. Adverse outcomes of concern include addiction, unintentional overdose, and death.
As part of the REMS, all ER/LA opioid analgesic pharmaceutical companies must provide education for prescribers of their medications through accredited continuing education activities that are supported by independent educational grants. Companies must also provide information that prescribers can use when counseling patients about the risks and benefits associated with ER/LA opioid analgesic use.
The FDA has developed core messages that are communicated to prescribers in the Blueprint for Prescriber Education. The Blueprint is directed to prescribers of ER/LA opioid analgesics but also may be relevant for other health care professionals (eg, pharmacists). Companies involved in the ER/LA Opioid Analgesics REMS Program have collaborated to implement a single shared REMS. This group provides a list of REMS-compliant continuing education activities, which can be found at http://www.er-la-opioidrems.com.
It is important for practitioners to understand that all currently approved abuse-deterrent opioid products still can be abused, and as scheduled controlled substances, they are addictive. The abuse-deterrent properties are expected to deter but do not wholly prevent abuse. Because in the end opioid medications must be able to deliver the opioid to the patient, there probably always will be potential for abuse of these products. Consequently, practitioners should counsel their patients on the following:
- Keep medicines in a secure location out of the reach and out of sight of children and pets. Put away medicines after every use. Accidental exposure to medicine in the home is a major source of unintentional poisonings in the U.S.
- If medicines are no longer needed, dispose of them properly. Disposing of all unused opioid analgesics reduces access to these medications by family members and household guests seeking opioids for abuse.
- The FDA recommends returning most prescription medications through a local or U.S. Drug Enforcement Administration (DEA)-sponsored take-back program or DEA-authorized collector. For opioid analgesics, the FDA recommends immediate removal from the home by flushing them down the toilet or sink.
Opioids Action Plan
In February 2016, FDA Commissioner Robert Califf (then the deputy commissioner for medical products and tobacco) announced the FDA Opioids Action Plan. The plan focuses on policies aimed at reversing the opioid epidemic while still providing patients in pain access to effective pain relief. The FDA actions include:
- Convening an expert advisory committee before approving any new drug application for an opioid that does not have abuse-deterrent properties;
- Consulting with the Pediatric Advisory Committee about a framework for pediatric opioid labeling before any new labeling is approved;
- Updating the REMS requirements for ER/LA opioid analgesics after considering the advisory committee’s recommendations from a meeting held in May 2016 and reviewing existing requirements;
- Improving access to naloxone (by facilitating the development of an over-the-counter version of naloxone, which is currently available only by prescription, thereby making it more accessible to treat opioid overdose), and medication-assisted treatment options for patients with opioid use disorders; and
- Supporting better pain management options, including alternative, nonaddictive treatments for pain.
The FDA is conducting research on pain measurements for conditions such as chronic low back pain, osteoarthritis, diabetic neuropathy, postherpetic neuralgia, and fibromyalgia. The FDA is also working to support the development of nonopioid options for these patients.
Consistent with the plan, in March 2016, the FDA announced that it was requiring changes to the labeling on immediate-release opioids, including additional warnings and safety information that incorporate elements similar to the ER/LA opioid analgesics labeling. Furthermore, among other steps, the FDA has contracted with the National Academy of Medicine to provide advice on how to incorporate current evidence about the public health impact of opioid use (for patients who are prescribed opioids as well as for nonpatients) into regulatory activities concerning opioids.
The FDA shares the responsibility of keeping patients safe. Working with the health care community and federal and state partners to help reduce opioid misuse and abuse and improve appropriate opioid prescribing while ensuring that patients in pain continue to have appropriate access to opioid analgesics is a top priority for the FDA and part of the targeted approach of the HHS focused on prevention, treatment, and intervention.
Who Overdoses at a VA Emergency Department?
Overdose deaths remain epidemic throughout the U.S. The rates of unintentional overdose deaths, increasing by 137% between 2000 and 2014, have been driven by a 4-fold increase in prescription opioid overdoses during that period.1-3
Veterans died of accidental overdose at a rate of 19.85 deaths/ 100,000 people compared with a rate of 10.49 deaths in the general population, based on 2005 data.4 There is wide state-by-state variation with the lowest age-adjusted opioid overdose death rate of 1.9 deaths/100,000 person-years among veterans in Mississippi and the highest rate in Utah of 33.9 deaths/100,000 person-years, using 2001 to 2009 data.5 These data can be compared with a crude general population overdose death rate of 10.6 deaths per 100,000 person-years in Mississippi and 18.4 deaths per 100,000 person-years in the general Utah population during that same period.6
Overdose deaths in the U.S. occur most often in persons aged 25 to 54 years.7 Older age has been associated with iatrogenic opioid overdose in hospitalized patients.8 Pulmonary, cardiovascular, and psychiatric disorders, including past or present substance use, have been associated with an increased risk of opioid overdose.9 However, veterans with substance use disorders are less likely to be prescribed opioids than are nonveterans with substance use disorders.10 Also, concomitant use of sedating medications, such as benzodiazepines (BZDs), can increase mortality from opioid overdose.11 Patients prescribed opioids for chronic pain conditions often take BZDs for various reasons.12 Veterans seem more likely to receive opioids to treat chronic pain but at lower average daily doses than the doses that nonveterans receive.10
Emergency management of life-threatening opioid overdose includes prompt administration of naloxone.13 Naloxone is FDA approved for complete or partial reversal of opioid-induced clinical effects, most critically respiratory depression.14,15 Naloxone administration in the emergency department (ED) may serve as a surrogate for an overdose event. During the study period, naloxone take-home kits were not available in the VA setting.
A 2010 ED study described demographic information and comorbidities in opioid overdose, but the study did not include veterans.16 The clinical characteristics of veterans treated for opioid overdose have not been published. Because identifying characteristics of veterans who overdose may help tailor overdose prevention efforts, the objective of this study is to describe clinical characteristics of veterans treated for opioid overdose.
Methods
A retrospective chart review and archived data study was approved by the University of Utah and VA institutional review boards, and conducted at the George E. Wahlen VAMC in Salt Lake City, Utah. This chart review included veterans who were admitted to the ED and treated with naloxone between January 1, 2009 and January 1, 2013.
The authors used the Pharmacy Benefits Management Data Manager to extract data from the VA Data Warehouse and verified the data by open chart review (Table). The following data were collected: ED visit date (overdose date); demographic information, including age, gender, and race; evidence of next-of-kin or other contact at the same address as the veteran; diagnoses based on ICD-9 codes, including sleep apnea, obesitycardiac disease, pulmonary disease, mental health diagnoses (ICD-9 codes 290-302 [wild card characters (*) included many subdiagnoses]),
cancer, and substance use disorders and/or dependencies (SUDD); tobacco use; VA-issued prescription opioid and BZD availability, including dose, fill dates, quantities dispensed, and day supplies; specialty of opioid prescriber; urine drug screening (UDS) results; and outcome of the overdose.
No standardized research criteria identify overdose in medical chart review.17 For each identified patient, the authors reviewed provider and nursing notes charted during an ED visit that included naloxone administration. The event was included as an opioid overdose when notes indicated that the veteran was unresponsive and given naloxone, which resulted in increased respirations or increased responsiveness. Cases were excluded if the reason for naloxone administration was anything other than opioid overdose.
Medical, mental health, and SUDD diagnoses were included only if the veteran had more than 3 patient care encounters (PCE) with ICD-9 codes for a specific diagnosis entered by providers. A PCE used in the electronic medical record (EMR) helps collect, manage, and display outpatient encounter data, including providers, procedure codes, and diagnostic codes. Tobacco use was extracted from health factors documented during primary care visit screenings. (Health factors help capture data entered in note templates in the EMR and can be used to query trends.) A diagnosis of obesity was based on a calculated body mass index of > 30 kg/m2 on the day of the ED visit date or the most recently charted height and weight. The type of SUDD was stratified into opioids (ICD-9 codes 304.0*), sedatives (ICD-9 code 304.1*), alcohol (ICD-9 code 303.*), and other (ICD-9 codes 304.2-305.9).
The dosage of opioids and BZDs available to a veteran was determined by using methods similar to those described by Gomes and colleagues: the dose of opioids and BZDs available based on prescriptions dispensed during the 120 days prior to the ED visit date and the dose available on the day of the ED visit date if prescription instructions were being followed.18 Prescription opioids and BZDs were converted to daily morphine equivalent dose (MED) and daily lorazepam-equivalent dose (LED), using established methods.19,20
Veterans were stratified into 4 groups based on prescribed medication availability: opioids only, BZDs only, opioids and BZDs, and neither opioids nor BZDs. The specialty of the opioid prescribers was categorized as primary care, pain specialist, surgeon, emergency specialist, or hospitalist (discharge prescription). Veteran EMRs contain a list of medications obtained outside the VA facility, referred to as non-VA prescriptions. These medications werenot included in the analysis because accuracy could not be verified.
A study author reviewed the results of any UDS performed up to 120 days before the ED visit date to determine whether the result reflected the currently prescribed prescription medications. If the UDS was positive for the prescribed opioids and/or BZDs and for any nonprescribed drug, including alcohol, the UDS was classified as not reflective. If the prescribed BZD was alprazolam, clonazepam, or lorazepam, a BZD-positive UDS was not required for the UDS to be considered reflective because of the sensitivity of the UDS BZD immunoassay
used at the George E. Wahlen VAMC clinical laboratory.21
Outcomes of the overdose were categorized as discharged, hospitalized, or deceased. Descriptive statistical analyses were performed using Microsoft Excel. Group comparisons were performed using Pearson chi-square or Student t test.
Results
The ED at the George E. Wahlen VAMC averages 64 visits per day, almost 94,000 visits within the study period. One hundred seventy ED visits between January 1, 2009 and January 1, 2013, involved naloxone administration. Ninety-two visits met the inclusion criteria of opioid overdose, representing about 0.002% of all ED visits at this facility (Figure 1). Six veterans had multiple ED visits within the study period, including 4 veterans who were in the opioid-only group.
The majority of veterans in this study were non-Hispanic white (n = 83, 90%), male (n = 88, 96%), with a mean age of 63 years. Less than 40% listed a next-of-kin or contact person living at their address.
Based on prescriptions available within 120 days before the overdose, 67 veterans (73%) possessed opioid and/or BZD prescriptions. In this group, the MED available on the day of the ED visit ranged from 7.5 mg to 830 mg. The MED was ≤ 200 mg in 71.6% and ≤ 50 mg in 34.3% of these cases. Veterans prescribed both opioids and BZDs had higher MED (average, 259 mg) available within 120 days of the ED visit than did those prescribed opioids only (average, 118 mg) (P = .015; SD, 132.9). The LED ranged from 1 mg to 12 mg for veterans with available BZDs.
Based on prescriptions available on the day of opioid overdose, 53 veterans (58%) had opioid prescriptions. The ranges of MED and LED available on the day of overdose were the same as the 120-day availability period. The average MED was 183 mg in veterans prescribed both opioids and BZDs and 126 mg in those prescribed opioids only (P = .283; SD, 168.65; Figure 2). The time between the last opioid fill date and the overdose visit date averaged 20 days (range, 0 to 28 days) in veterans prescribed opioids.
All veterans had at least 1 diagnosis that in previous studies was associated with increased risk of overdose.9,15 The most common diagnoses included cardiovascular diseases, mental health disorders, pulmonary diseases, and cancer. Other SUDDs not including tobacco use were documented in at least half the veterans with prescribed opioids and/or BZDs. No veteran in the sample had a documented history of opioid SUDD.
Hydrocodone products were available in > 50% of cases. None of the veterans were prescribed buprenorphine products; other opioids, including tramadol, comprised the remainder (Figure 3). Primary care providers prescribed 72% of opioid prescriptions, with pain specialists, discharge physicians, ED providers, and surgeons prescribing the rest. When both opioids and BZDs were available, combinations of a hydrocodone product plus clonazepam or lorazepam were most common. The time between the last opioid fill date and the overdose visit date averaged 20 days (range, 0 to 28 days) in veterans prescribed opioids.
Overall, 64% of the sample had UDS results prior to the ED visit. Of veterans prescribed opioids and/or BZDs, 53% of UDSs reflected prescribed regimens.
On the day of the ED visit, 1 death occurred. Ninety-one veterans (99%) survived the overdose; 79 veterans (86%) were hospitalized, most for < 24 hours.
Discussion
This retrospective review identified 92 veterans who were treated with naloxone in the ED for opioid overdose during a 4-year period at the George E. Wahlen VAMC. Seventy-eight cases were excluded because the reason entered in charts for naloxone administration was itching, constipation, altered mental status, or unclear documentation.
Veterans in this study were older on average than the overdose fatalities in the U.S. Opioid overdose deaths in the U.S. and in Utah occur most frequently in non-Hispanic white men aged between 35 and 54 years.7,22,23 In the 2010 Nationwide Emergency Department Sample of 136,000 opioid overdoses, of which 98% survived, most were aged 18 to 54 years.16 The older age in this study most likely reflects the age range of veterans served in the VHA; however, as more young veterans enter the VHA, the age range of overdose victims may more closely resemble the age ranges found in previous studies. Post hoc analysis showed 8 veterans (9%) with probable intentional opioid overdose based on chart review, whereas the incidence of intentional prescription drug overdose in the U.S. is 17.1%.24
In Utah, almost 93% of fatal overdoses occur at a residential location.22 Less than half the veterans in this study had a contact or next-of-kin listed as living at the same address. Although veterans may not have identified someone living with them, in many cases, it is likely another person witnessed the overdose. Relying on EMRs to identify who should receive prevention education, in addition to the veteran, may result in missed opportunities to include another person likely to witness an overdose.25 Prescribers should make a conscious effort to ask veterans to identify someone who may be able to assist with rescue efforts in the event of an overdose.
Diagnoses associated with increased risk of opioid overdose death include sleep apnea, morbid obesity, pulmonary or cardiovascular diseases, and/or a history of psychiatric disorders and SUDD.8,9,16 In a large sample of older veterans, only 64% had at least 1 medical or psychiatric diagnosis.26 Less than half the 18,000 VA primary care patients in 5 VA centers had any psychiatric condition, and < 65% had cardiovascular disease, pulmonary disease, or cancer.27 All veterans in this study had medical and psychiatric comorbidity.
In contrast, a large ED sample described by Yokell and colleagues found chronic mental conditions in 33.9%, circulatory disorders in 29.1%, and respiratory conditions in 25.6% of their sample.16 Bohnert and associates found a significantly elevated hazard ratio (HR) for any psychiatric disorder in a sample of nearly 4,500 veterans. There was variation in the HR when individual psychiatric diagnoses were broken out, with bipolar disorder having the largest HR and schizophrenia having the lowest but still elevated HR.9 In this study, individual diagnoses were not broken out because the smaller sample size could diminish the clinical significance of any apparent differences.
Edlund and colleagues found that < 8% of veterans treated with opioids for chronic noncancer pain had nonopioid SUDD.10 Bohnert and colleagues found an HR of 21.95 for overdose death among those with opioid-use disorders.9 The sample in this study had a much higher incidence of nonopioid SUDD compared with that ub the study by Edlund and colleagues, but none of the veterans in this study had a documented history of opioid use disorder. The absence of opioid use disorders in this sample is unexpected and points to a need for providers to screen for opioid use disorder whenever opioids are prescribed or renewed. If prevention practices were directed only to those with opioid SUDDs, none of the veterans in this study would have been included in those efforts. Non-SUDD providers should address the risks of opioid overdose in veterans with sleep apnea, morbid obesity, pulmonary or cardiovascular diseases, and/or a history of psychiatric disorders.
Gomes and colleagues found that > 100 mg MED available on the day of overdose doubled the risk of opioid-related mortality.18 The VA/DoD Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain identifies 200 mg MED as a threshold to define high-dose opioid therapy.28 Fulton-Kehoe and colleagues found that 28% of overdose victims were prescribed < 50 mg MED.29 In this study, the average dose available to veterans was > 100 mg MED; however, one-third of all study veterans had < 50 mg MED available. Using a threshold dose of 50 mg MED to target prevention efforts would capture only two-thirds of those who experienced overdose; a 200 mg MED threshold would exclude the majority, based on the average MED in each group in this study. Overdose education should be provided to veterans with access to any dose of opioids.
Use of BZDs with opioids may result in greater central nervous system (CNS) depression, pharmacokinetic interactions, or pharmacodynamic interactions at the µ opioid receptor.30-32 About one-third of veterans in this study were prescribed opioids and BZDs concurrently, a combination noted in about 33% of opioid overdose deaths reported by the CDC.24 Individuals taking methadone combined with BZDs have been found to have severe medical outcomes.33 If preventive efforts are targeted to those receiving opioids and other CNS depressants, such as BZDs, about half (42%) the veterans in this study would not receive a potentially life-saving message about preventing overdoses. All veterans with opioids should be educated about the additional risk of overdose posed by drug interactions with other CNS depressants.
The time since the last fill of opioid prescription ranged from 0 to 28 days. This time frame indicates that some overdoses may have occurred on the day an opioid was filled but most occurred near the end of the expected days’ supply. Because information about adherence or use of the opioid was not studied, it cannot be assumed that medication misuse is the primary reason for the overdose. Delivering prevention efforts only at the time of medication dispensing would be insufficient. Clinicians should review local and remote prescription data, including via their states’ prescription drug monitoring program when discussing the risk of overdose with veterans.
Most veterans had at least 1 UDS result in the chart. Although half the UDSs obtained reflected prescribed medications, the possibility of aberrant behaviors, which increases the risk of overdose, cannot be ruled out with the methods used in this study.34 Medication management agreements that require UDSs for veterans with chronic pain were not mandatory at the George E. Wahlen VAMC during the study period, and those used did not mandate discontinuation of opioid therapy if suspected aberrant behaviors were present.
A Utah study based on interviews of overdose victims’ next-of-kin found that 76% were concerned about victims’ aberrant behaviors, such as medication misuse, prior to the death.22 In contrast, a study of commercial and Medicaid recipients estimated medication misuse rates in
≤ 30% of the sample.35 Urine drug screening results not reflective of the prescribed regimens have been found in up to 50% of patients receiving chronic opioid therapy.
The UDS findings in this study were determined by the authors and did not capture clinical decisions or interpretations made after results were available or whether these decisions resulted in overdose prevention strategies, such as targeted education or changes in prescription availability. Targeting preventive efforts toward veterans only with UDS results suggesting medication misuse would have missed more than half the veterans in this study. Urine drug screening should be used as a clinical monitoring tool whenever opioids, BZDs, or other substances are used or prescribed.
The VA now has a nationwide program, Opioid Overdose Education and Naloxone Distribution (OEND) promoting overdose education and take-home naloxone distribution for providers and patients to prevent opioid-related overdose deaths. A national SharePoint site has been created within the VA that lists resources to support this effort.
Almost all veterans in this review survived the overdose and were hospitalized following the ED visit. Other investigators also have found that the majority (51% to 98%) of overdose victims reaching the ED survived, but fewer patients (3% to 51%) in those studies were hospitalized.16,36 It is unknown whether there are differences in risk factors associated with survived or fatal overdoses.
Limitations
Although Utah ranked third for drug overdose death rates in 2008 and had the highest death rate among veterans from 2001 to 2009, this review captured only overdoses among veterans treated during the study period at the George E. Wahlen VAMC ED.5,6 The number and characteristics of veterans during this same period who were treated for overdose in other community EDs or urgent care centers throughout Utah is unknown.
The definition of opioid and BZD dose available in this study may not represent actual use of opioids or BZDs because it was based on chart review of prescription dispensing information and UDS procedures at the George E. Wahlen VAMC, and medication misuse cannot be ruled out. This study did not evaluate specific aberrant behaviors.
Conclusion
Current overdose prevention screening efforts primarily identify patients on high-dose opioids and those with SUDD. Many veterans in this study were older than the average U.S. victims’ age, did not have SUDD, were prescribed opioid doses not considered high risk by current guidelines, were nearer the end of their medication supply, and had UDS reflective of prescribed medications. This study suggests that any veteran with access to opioids, whether prescribed or not, is at risk for an opioid overdose. Established risk factors may aid in developing overdose prevention programs, but prevention should not be limited to veterans with prescribed opioids and known risk factors. Prescribers should screen for opioid use disorder whenever opioids are prescribed and continue to screen throughout therapy. Broader screening for overdose risk is needed to avoid missing important opportunities for overdose prevention.
Acknowledgments
Gale Anderson, VISN 19 PBM Data Manager, performed initial data query for the study.
References
1. Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR. 2015;64(50):1-5.
2. Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374(2):154-163.
3. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981-1985.
4. Bohnert AS, Ilgen MA, Galea S, McCarthy JF, Blow FC. Accidental poisoning mortality among patients in the Department of Veterans Affairs Health System. Med Care. 2011;49(4):393-396.
5. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends and regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-612.
6. Centers for Disease Control and Prevention. Policy impact: prescription, painkiller, overdoses. http://www.cdc.gov/drugoverdose/pdf/policyimpact-prescriptionpainkillerod-a.pdf. Published November 2011. Accessed August 25, 2016.
7. Xu J, Murphy SL, Kochanek KD, Bastian BA; Division of Vital Statistics. Deaths: final data for 2013. http://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_02.pdf. Published February 16, 2016. Accessed August 25, 2016.
8. The Joint Commission. Sentinel event alert issue 49: safe use of opioids in the hospital. https://www.jointcommission.org/assets/1/18/SEA_49_opioids_8_2_12_final.pdf. Published August 8, 2012. Accessed April 25, 2015.
9. Bohnert AS, Ilgen MA, Ignacio RV, McCarthy JF, Valenstein M, Blow FC. Risk of death from accidental overdose associated with psychiatric and substance use disorders. Am J Psychiatry. 2012;169(1):64-70.
10. Edlund MJ, Austen MA, Sullivan MD, et al. Patterns of opioid use for chronic noncancer pain in the Veterans Health Administration from 2009 to 2011. Pain. 2014;155:2337-2343.
11. Jann M, Kennedy WK, Lopez G. Benzodiazepines: a major component in unintentional prescription drug overdoses with opioid analgesics. J Pharm Pract. 2014;27(1):5-16.
12. McMillin G, Kusukawa N, Nelson G. Benzodiazepines.Salt Lake City, UT: ARUP Laboratories; 2012.
13. Naloxone hydrochloride [package insert].Lake Forest, IL: Hospira Inc; 2007.
14. Boyer EW. Management of opioid analgesic overdose. N Engl J Med. 2012;367(2):146-155.
15. Hoffman JR, Schriger DL, Luo JS. The empiric use of naloxone in patients with altered mental status: a reappraisal. Ann Emerg Med. 1991;20(3):246-252.
16. Yokell MA, Delgado MK, Zaller ND, Wang NE, McGowan SK, Green TC. Presentation of prescription and nonprescription opioid overdoses to US emergency departments. JAMA Intern Med. 2014;174(12):2034-2037.
17. Binswanger I, Gardner E, Gabella B, Broderick K, Glanz K. Development of case criteria to define pharmaceutical opioid and heroin overdoses in clinical records. Platform presented at: Association for Medical Education and Research in Substance Abuse 38th Annual National Conference; November 7, 2014; San Francisco, CA.
18. Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171(7):686-691.
19. Jaeger TM, Lohr RH, Pankratz VS. Symptom-triggered therapy for alcohol withdrawal syndrome in medical inpatients. Mayo Clin Proc. 2001;76(7):695-701.20. Washington State Agency Medical Directors’ Group. Opioid dose clculator. http://www
.agencymeddirectors.wa.gov/Calculator/DoseCalcula tor.htm. Accessed October 10, 2016.
21. EMIT II Plus Benzodiazepine Assay [package insert]. Brea, CA: Beckman Coulter, Inc; 2010.
22. Johnson EM, Lanier WA, Merrill RM, et al. Unintentional prescription opioid-related overdose deaths: description of decedents by next of kin or best contact, Utah, 2008-2009. J Gen Intern Med. 2013;28(4):522-529.
23. Utah Department of Health. Fact sheet: prescription pain medication deaths in Utah, 2012. https://www.health.utah.gov/vipp/pdf/FactSheets/2012RxOpioidDeaths.pdf. Updated October 2013. Accessed October 10, 2016.
24. Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309(7):657-659.
25. Bohnert AS, Tracy M, Galea S. Characteristics of drug users who witness many overdoses: implications for overdose prevention. Drug Alcohol Depend. 2012;120(1-3):168-173.
26. Yoon J, Zulman D, Scott JY, Maciejewski ML. Costs associated with multimorbidity among VA patients. Med Care. 2014;52(suppl 3):S31-S36.
27. Yoon J, Yano EM, Altman L, et al. Reducing costs of acute care for ambulatory care-sensitive medical conditions: the central roles of comorbid mental illness. Med Care. 2012;50(8):705-713.
28. Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain. Guideline summary. http://www.va.gov/painmanagement/docs/cpg_opioidtherapy_summary.pdf. Published May 2010. Accessed August 25, 2016.
29. Fulton-Kehoe D, Sullivan MD, Turner JA, et al. Opioid poisonings in Washington state Medicaid: trends, dosing, and guidelines. Med Care. 2015;53(8):679-685.
30. Gudin JA, Mogali S, Jones JD, Comer SD. Risks, management, and monitoring of combination opioid, benzodiazepines, and/or alcohol use. Postgrad Med. 2013;125(4):115-130.
31. Poisnel G, Dhilly M, Le Boisselier R, Barre L, Debruyne D. Comparison of five benzodiazepine-receptor agonists on buprenorphine-induced mu-opioid receptor regulation. J Pharmacol Sci. 2009;110(1):36-46.
32. Webster LR, Cochella S, Dasgupta N, et al. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med. 2011;12(suppl 2):S26-S35.
33. Lee SC, Klein-Schwartz W, Doyon S, Welsh C. Comparison of toxicity associated with nonmedical use of benzodiazepines with buprenorphine or methadone. Drug Alcohol Depend. 2014;138:118-123.
34. Owen GT, Burton AW, Schade CM, Passik S. Urine drug testing: current recommendations and best practices. Pain Physician. 2012;15(suppl 3):ES119–ES133.
35. Sullivan MD, Edlund MJ, Fan MY, Devries A, Brennan Braden J, Martin BC. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: the TROUP study. Pain. 2010;150(2):332-339.
36. Sporer KA, Firestone J, Isaacs SM. Out-of-hospital treatment of opioid overdoses in an urban setting. Acad Emerg Med. 1996;3(7):660-667.
Overdose deaths remain epidemic throughout the U.S. The rates of unintentional overdose deaths, increasing by 137% between 2000 and 2014, have been driven by a 4-fold increase in prescription opioid overdoses during that period.1-3
Veterans died of accidental overdose at a rate of 19.85 deaths/ 100,000 people compared with a rate of 10.49 deaths in the general population, based on 2005 data.4 There is wide state-by-state variation with the lowest age-adjusted opioid overdose death rate of 1.9 deaths/100,000 person-years among veterans in Mississippi and the highest rate in Utah of 33.9 deaths/100,000 person-years, using 2001 to 2009 data.5 These data can be compared with a crude general population overdose death rate of 10.6 deaths per 100,000 person-years in Mississippi and 18.4 deaths per 100,000 person-years in the general Utah population during that same period.6
Overdose deaths in the U.S. occur most often in persons aged 25 to 54 years.7 Older age has been associated with iatrogenic opioid overdose in hospitalized patients.8 Pulmonary, cardiovascular, and psychiatric disorders, including past or present substance use, have been associated with an increased risk of opioid overdose.9 However, veterans with substance use disorders are less likely to be prescribed opioids than are nonveterans with substance use disorders.10 Also, concomitant use of sedating medications, such as benzodiazepines (BZDs), can increase mortality from opioid overdose.11 Patients prescribed opioids for chronic pain conditions often take BZDs for various reasons.12 Veterans seem more likely to receive opioids to treat chronic pain but at lower average daily doses than the doses that nonveterans receive.10
Emergency management of life-threatening opioid overdose includes prompt administration of naloxone.13 Naloxone is FDA approved for complete or partial reversal of opioid-induced clinical effects, most critically respiratory depression.14,15 Naloxone administration in the emergency department (ED) may serve as a surrogate for an overdose event. During the study period, naloxone take-home kits were not available in the VA setting.
A 2010 ED study described demographic information and comorbidities in opioid overdose, but the study did not include veterans.16 The clinical characteristics of veterans treated for opioid overdose have not been published. Because identifying characteristics of veterans who overdose may help tailor overdose prevention efforts, the objective of this study is to describe clinical characteristics of veterans treated for opioid overdose.
Methods
A retrospective chart review and archived data study was approved by the University of Utah and VA institutional review boards, and conducted at the George E. Wahlen VAMC in Salt Lake City, Utah. This chart review included veterans who were admitted to the ED and treated with naloxone between January 1, 2009 and January 1, 2013.
The authors used the Pharmacy Benefits Management Data Manager to extract data from the VA Data Warehouse and verified the data by open chart review (Table). The following data were collected: ED visit date (overdose date); demographic information, including age, gender, and race; evidence of next-of-kin or other contact at the same address as the veteran; diagnoses based on ICD-9 codes, including sleep apnea, obesitycardiac disease, pulmonary disease, mental health diagnoses (ICD-9 codes 290-302 [wild card characters (*) included many subdiagnoses]),
cancer, and substance use disorders and/or dependencies (SUDD); tobacco use; VA-issued prescription opioid and BZD availability, including dose, fill dates, quantities dispensed, and day supplies; specialty of opioid prescriber; urine drug screening (UDS) results; and outcome of the overdose.
No standardized research criteria identify overdose in medical chart review.17 For each identified patient, the authors reviewed provider and nursing notes charted during an ED visit that included naloxone administration. The event was included as an opioid overdose when notes indicated that the veteran was unresponsive and given naloxone, which resulted in increased respirations or increased responsiveness. Cases were excluded if the reason for naloxone administration was anything other than opioid overdose.
Medical, mental health, and SUDD diagnoses were included only if the veteran had more than 3 patient care encounters (PCE) with ICD-9 codes for a specific diagnosis entered by providers. A PCE used in the electronic medical record (EMR) helps collect, manage, and display outpatient encounter data, including providers, procedure codes, and diagnostic codes. Tobacco use was extracted from health factors documented during primary care visit screenings. (Health factors help capture data entered in note templates in the EMR and can be used to query trends.) A diagnosis of obesity was based on a calculated body mass index of > 30 kg/m2 on the day of the ED visit date or the most recently charted height and weight. The type of SUDD was stratified into opioids (ICD-9 codes 304.0*), sedatives (ICD-9 code 304.1*), alcohol (ICD-9 code 303.*), and other (ICD-9 codes 304.2-305.9).
The dosage of opioids and BZDs available to a veteran was determined by using methods similar to those described by Gomes and colleagues: the dose of opioids and BZDs available based on prescriptions dispensed during the 120 days prior to the ED visit date and the dose available on the day of the ED visit date if prescription instructions were being followed.18 Prescription opioids and BZDs were converted to daily morphine equivalent dose (MED) and daily lorazepam-equivalent dose (LED), using established methods.19,20
Veterans were stratified into 4 groups based on prescribed medication availability: opioids only, BZDs only, opioids and BZDs, and neither opioids nor BZDs. The specialty of the opioid prescribers was categorized as primary care, pain specialist, surgeon, emergency specialist, or hospitalist (discharge prescription). Veteran EMRs contain a list of medications obtained outside the VA facility, referred to as non-VA prescriptions. These medications werenot included in the analysis because accuracy could not be verified.
A study author reviewed the results of any UDS performed up to 120 days before the ED visit date to determine whether the result reflected the currently prescribed prescription medications. If the UDS was positive for the prescribed opioids and/or BZDs and for any nonprescribed drug, including alcohol, the UDS was classified as not reflective. If the prescribed BZD was alprazolam, clonazepam, or lorazepam, a BZD-positive UDS was not required for the UDS to be considered reflective because of the sensitivity of the UDS BZD immunoassay
used at the George E. Wahlen VAMC clinical laboratory.21
Outcomes of the overdose were categorized as discharged, hospitalized, or deceased. Descriptive statistical analyses were performed using Microsoft Excel. Group comparisons were performed using Pearson chi-square or Student t test.
Results
The ED at the George E. Wahlen VAMC averages 64 visits per day, almost 94,000 visits within the study period. One hundred seventy ED visits between January 1, 2009 and January 1, 2013, involved naloxone administration. Ninety-two visits met the inclusion criteria of opioid overdose, representing about 0.002% of all ED visits at this facility (Figure 1). Six veterans had multiple ED visits within the study period, including 4 veterans who were in the opioid-only group.
The majority of veterans in this study were non-Hispanic white (n = 83, 90%), male (n = 88, 96%), with a mean age of 63 years. Less than 40% listed a next-of-kin or contact person living at their address.
Based on prescriptions available within 120 days before the overdose, 67 veterans (73%) possessed opioid and/or BZD prescriptions. In this group, the MED available on the day of the ED visit ranged from 7.5 mg to 830 mg. The MED was ≤ 200 mg in 71.6% and ≤ 50 mg in 34.3% of these cases. Veterans prescribed both opioids and BZDs had higher MED (average, 259 mg) available within 120 days of the ED visit than did those prescribed opioids only (average, 118 mg) (P = .015; SD, 132.9). The LED ranged from 1 mg to 12 mg for veterans with available BZDs.
Based on prescriptions available on the day of opioid overdose, 53 veterans (58%) had opioid prescriptions. The ranges of MED and LED available on the day of overdose were the same as the 120-day availability period. The average MED was 183 mg in veterans prescribed both opioids and BZDs and 126 mg in those prescribed opioids only (P = .283; SD, 168.65; Figure 2). The time between the last opioid fill date and the overdose visit date averaged 20 days (range, 0 to 28 days) in veterans prescribed opioids.
All veterans had at least 1 diagnosis that in previous studies was associated with increased risk of overdose.9,15 The most common diagnoses included cardiovascular diseases, mental health disorders, pulmonary diseases, and cancer. Other SUDDs not including tobacco use were documented in at least half the veterans with prescribed opioids and/or BZDs. No veteran in the sample had a documented history of opioid SUDD.
Hydrocodone products were available in > 50% of cases. None of the veterans were prescribed buprenorphine products; other opioids, including tramadol, comprised the remainder (Figure 3). Primary care providers prescribed 72% of opioid prescriptions, with pain specialists, discharge physicians, ED providers, and surgeons prescribing the rest. When both opioids and BZDs were available, combinations of a hydrocodone product plus clonazepam or lorazepam were most common. The time between the last opioid fill date and the overdose visit date averaged 20 days (range, 0 to 28 days) in veterans prescribed opioids.
Overall, 64% of the sample had UDS results prior to the ED visit. Of veterans prescribed opioids and/or BZDs, 53% of UDSs reflected prescribed regimens.
On the day of the ED visit, 1 death occurred. Ninety-one veterans (99%) survived the overdose; 79 veterans (86%) were hospitalized, most for < 24 hours.
Discussion
This retrospective review identified 92 veterans who were treated with naloxone in the ED for opioid overdose during a 4-year period at the George E. Wahlen VAMC. Seventy-eight cases were excluded because the reason entered in charts for naloxone administration was itching, constipation, altered mental status, or unclear documentation.
Veterans in this study were older on average than the overdose fatalities in the U.S. Opioid overdose deaths in the U.S. and in Utah occur most frequently in non-Hispanic white men aged between 35 and 54 years.7,22,23 In the 2010 Nationwide Emergency Department Sample of 136,000 opioid overdoses, of which 98% survived, most were aged 18 to 54 years.16 The older age in this study most likely reflects the age range of veterans served in the VHA; however, as more young veterans enter the VHA, the age range of overdose victims may more closely resemble the age ranges found in previous studies. Post hoc analysis showed 8 veterans (9%) with probable intentional opioid overdose based on chart review, whereas the incidence of intentional prescription drug overdose in the U.S. is 17.1%.24
In Utah, almost 93% of fatal overdoses occur at a residential location.22 Less than half the veterans in this study had a contact or next-of-kin listed as living at the same address. Although veterans may not have identified someone living with them, in many cases, it is likely another person witnessed the overdose. Relying on EMRs to identify who should receive prevention education, in addition to the veteran, may result in missed opportunities to include another person likely to witness an overdose.25 Prescribers should make a conscious effort to ask veterans to identify someone who may be able to assist with rescue efforts in the event of an overdose.
Diagnoses associated with increased risk of opioid overdose death include sleep apnea, morbid obesity, pulmonary or cardiovascular diseases, and/or a history of psychiatric disorders and SUDD.8,9,16 In a large sample of older veterans, only 64% had at least 1 medical or psychiatric diagnosis.26 Less than half the 18,000 VA primary care patients in 5 VA centers had any psychiatric condition, and < 65% had cardiovascular disease, pulmonary disease, or cancer.27 All veterans in this study had medical and psychiatric comorbidity.
In contrast, a large ED sample described by Yokell and colleagues found chronic mental conditions in 33.9%, circulatory disorders in 29.1%, and respiratory conditions in 25.6% of their sample.16 Bohnert and associates found a significantly elevated hazard ratio (HR) for any psychiatric disorder in a sample of nearly 4,500 veterans. There was variation in the HR when individual psychiatric diagnoses were broken out, with bipolar disorder having the largest HR and schizophrenia having the lowest but still elevated HR.9 In this study, individual diagnoses were not broken out because the smaller sample size could diminish the clinical significance of any apparent differences.
Edlund and colleagues found that < 8% of veterans treated with opioids for chronic noncancer pain had nonopioid SUDD.10 Bohnert and colleagues found an HR of 21.95 for overdose death among those with opioid-use disorders.9 The sample in this study had a much higher incidence of nonopioid SUDD compared with that ub the study by Edlund and colleagues, but none of the veterans in this study had a documented history of opioid use disorder. The absence of opioid use disorders in this sample is unexpected and points to a need for providers to screen for opioid use disorder whenever opioids are prescribed or renewed. If prevention practices were directed only to those with opioid SUDDs, none of the veterans in this study would have been included in those efforts. Non-SUDD providers should address the risks of opioid overdose in veterans with sleep apnea, morbid obesity, pulmonary or cardiovascular diseases, and/or a history of psychiatric disorders.
Gomes and colleagues found that > 100 mg MED available on the day of overdose doubled the risk of opioid-related mortality.18 The VA/DoD Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain identifies 200 mg MED as a threshold to define high-dose opioid therapy.28 Fulton-Kehoe and colleagues found that 28% of overdose victims were prescribed < 50 mg MED.29 In this study, the average dose available to veterans was > 100 mg MED; however, one-third of all study veterans had < 50 mg MED available. Using a threshold dose of 50 mg MED to target prevention efforts would capture only two-thirds of those who experienced overdose; a 200 mg MED threshold would exclude the majority, based on the average MED in each group in this study. Overdose education should be provided to veterans with access to any dose of opioids.
Use of BZDs with opioids may result in greater central nervous system (CNS) depression, pharmacokinetic interactions, or pharmacodynamic interactions at the µ opioid receptor.30-32 About one-third of veterans in this study were prescribed opioids and BZDs concurrently, a combination noted in about 33% of opioid overdose deaths reported by the CDC.24 Individuals taking methadone combined with BZDs have been found to have severe medical outcomes.33 If preventive efforts are targeted to those receiving opioids and other CNS depressants, such as BZDs, about half (42%) the veterans in this study would not receive a potentially life-saving message about preventing overdoses. All veterans with opioids should be educated about the additional risk of overdose posed by drug interactions with other CNS depressants.
The time since the last fill of opioid prescription ranged from 0 to 28 days. This time frame indicates that some overdoses may have occurred on the day an opioid was filled but most occurred near the end of the expected days’ supply. Because information about adherence or use of the opioid was not studied, it cannot be assumed that medication misuse is the primary reason for the overdose. Delivering prevention efforts only at the time of medication dispensing would be insufficient. Clinicians should review local and remote prescription data, including via their states’ prescription drug monitoring program when discussing the risk of overdose with veterans.
Most veterans had at least 1 UDS result in the chart. Although half the UDSs obtained reflected prescribed medications, the possibility of aberrant behaviors, which increases the risk of overdose, cannot be ruled out with the methods used in this study.34 Medication management agreements that require UDSs for veterans with chronic pain were not mandatory at the George E. Wahlen VAMC during the study period, and those used did not mandate discontinuation of opioid therapy if suspected aberrant behaviors were present.
A Utah study based on interviews of overdose victims’ next-of-kin found that 76% were concerned about victims’ aberrant behaviors, such as medication misuse, prior to the death.22 In contrast, a study of commercial and Medicaid recipients estimated medication misuse rates in
≤ 30% of the sample.35 Urine drug screening results not reflective of the prescribed regimens have been found in up to 50% of patients receiving chronic opioid therapy.
The UDS findings in this study were determined by the authors and did not capture clinical decisions or interpretations made after results were available or whether these decisions resulted in overdose prevention strategies, such as targeted education or changes in prescription availability. Targeting preventive efforts toward veterans only with UDS results suggesting medication misuse would have missed more than half the veterans in this study. Urine drug screening should be used as a clinical monitoring tool whenever opioids, BZDs, or other substances are used or prescribed.
The VA now has a nationwide program, Opioid Overdose Education and Naloxone Distribution (OEND) promoting overdose education and take-home naloxone distribution for providers and patients to prevent opioid-related overdose deaths. A national SharePoint site has been created within the VA that lists resources to support this effort.
Almost all veterans in this review survived the overdose and were hospitalized following the ED visit. Other investigators also have found that the majority (51% to 98%) of overdose victims reaching the ED survived, but fewer patients (3% to 51%) in those studies were hospitalized.16,36 It is unknown whether there are differences in risk factors associated with survived or fatal overdoses.
Limitations
Although Utah ranked third for drug overdose death rates in 2008 and had the highest death rate among veterans from 2001 to 2009, this review captured only overdoses among veterans treated during the study period at the George E. Wahlen VAMC ED.5,6 The number and characteristics of veterans during this same period who were treated for overdose in other community EDs or urgent care centers throughout Utah is unknown.
The definition of opioid and BZD dose available in this study may not represent actual use of opioids or BZDs because it was based on chart review of prescription dispensing information and UDS procedures at the George E. Wahlen VAMC, and medication misuse cannot be ruled out. This study did not evaluate specific aberrant behaviors.
Conclusion
Current overdose prevention screening efforts primarily identify patients on high-dose opioids and those with SUDD. Many veterans in this study were older than the average U.S. victims’ age, did not have SUDD, were prescribed opioid doses not considered high risk by current guidelines, were nearer the end of their medication supply, and had UDS reflective of prescribed medications. This study suggests that any veteran with access to opioids, whether prescribed or not, is at risk for an opioid overdose. Established risk factors may aid in developing overdose prevention programs, but prevention should not be limited to veterans with prescribed opioids and known risk factors. Prescribers should screen for opioid use disorder whenever opioids are prescribed and continue to screen throughout therapy. Broader screening for overdose risk is needed to avoid missing important opportunities for overdose prevention.
Acknowledgments
Gale Anderson, VISN 19 PBM Data Manager, performed initial data query for the study.
Overdose deaths remain epidemic throughout the U.S. The rates of unintentional overdose deaths, increasing by 137% between 2000 and 2014, have been driven by a 4-fold increase in prescription opioid overdoses during that period.1-3
Veterans died of accidental overdose at a rate of 19.85 deaths/ 100,000 people compared with a rate of 10.49 deaths in the general population, based on 2005 data.4 There is wide state-by-state variation with the lowest age-adjusted opioid overdose death rate of 1.9 deaths/100,000 person-years among veterans in Mississippi and the highest rate in Utah of 33.9 deaths/100,000 person-years, using 2001 to 2009 data.5 These data can be compared with a crude general population overdose death rate of 10.6 deaths per 100,000 person-years in Mississippi and 18.4 deaths per 100,000 person-years in the general Utah population during that same period.6
Overdose deaths in the U.S. occur most often in persons aged 25 to 54 years.7 Older age has been associated with iatrogenic opioid overdose in hospitalized patients.8 Pulmonary, cardiovascular, and psychiatric disorders, including past or present substance use, have been associated with an increased risk of opioid overdose.9 However, veterans with substance use disorders are less likely to be prescribed opioids than are nonveterans with substance use disorders.10 Also, concomitant use of sedating medications, such as benzodiazepines (BZDs), can increase mortality from opioid overdose.11 Patients prescribed opioids for chronic pain conditions often take BZDs for various reasons.12 Veterans seem more likely to receive opioids to treat chronic pain but at lower average daily doses than the doses that nonveterans receive.10
Emergency management of life-threatening opioid overdose includes prompt administration of naloxone.13 Naloxone is FDA approved for complete or partial reversal of opioid-induced clinical effects, most critically respiratory depression.14,15 Naloxone administration in the emergency department (ED) may serve as a surrogate for an overdose event. During the study period, naloxone take-home kits were not available in the VA setting.
A 2010 ED study described demographic information and comorbidities in opioid overdose, but the study did not include veterans.16 The clinical characteristics of veterans treated for opioid overdose have not been published. Because identifying characteristics of veterans who overdose may help tailor overdose prevention efforts, the objective of this study is to describe clinical characteristics of veterans treated for opioid overdose.
Methods
A retrospective chart review and archived data study was approved by the University of Utah and VA institutional review boards, and conducted at the George E. Wahlen VAMC in Salt Lake City, Utah. This chart review included veterans who were admitted to the ED and treated with naloxone between January 1, 2009 and January 1, 2013.
The authors used the Pharmacy Benefits Management Data Manager to extract data from the VA Data Warehouse and verified the data by open chart review (Table). The following data were collected: ED visit date (overdose date); demographic information, including age, gender, and race; evidence of next-of-kin or other contact at the same address as the veteran; diagnoses based on ICD-9 codes, including sleep apnea, obesitycardiac disease, pulmonary disease, mental health diagnoses (ICD-9 codes 290-302 [wild card characters (*) included many subdiagnoses]),
cancer, and substance use disorders and/or dependencies (SUDD); tobacco use; VA-issued prescription opioid and BZD availability, including dose, fill dates, quantities dispensed, and day supplies; specialty of opioid prescriber; urine drug screening (UDS) results; and outcome of the overdose.
No standardized research criteria identify overdose in medical chart review.17 For each identified patient, the authors reviewed provider and nursing notes charted during an ED visit that included naloxone administration. The event was included as an opioid overdose when notes indicated that the veteran was unresponsive and given naloxone, which resulted in increased respirations or increased responsiveness. Cases were excluded if the reason for naloxone administration was anything other than opioid overdose.
Medical, mental health, and SUDD diagnoses were included only if the veteran had more than 3 patient care encounters (PCE) with ICD-9 codes for a specific diagnosis entered by providers. A PCE used in the electronic medical record (EMR) helps collect, manage, and display outpatient encounter data, including providers, procedure codes, and diagnostic codes. Tobacco use was extracted from health factors documented during primary care visit screenings. (Health factors help capture data entered in note templates in the EMR and can be used to query trends.) A diagnosis of obesity was based on a calculated body mass index of > 30 kg/m2 on the day of the ED visit date or the most recently charted height and weight. The type of SUDD was stratified into opioids (ICD-9 codes 304.0*), sedatives (ICD-9 code 304.1*), alcohol (ICD-9 code 303.*), and other (ICD-9 codes 304.2-305.9).
The dosage of opioids and BZDs available to a veteran was determined by using methods similar to those described by Gomes and colleagues: the dose of opioids and BZDs available based on prescriptions dispensed during the 120 days prior to the ED visit date and the dose available on the day of the ED visit date if prescription instructions were being followed.18 Prescription opioids and BZDs were converted to daily morphine equivalent dose (MED) and daily lorazepam-equivalent dose (LED), using established methods.19,20
Veterans were stratified into 4 groups based on prescribed medication availability: opioids only, BZDs only, opioids and BZDs, and neither opioids nor BZDs. The specialty of the opioid prescribers was categorized as primary care, pain specialist, surgeon, emergency specialist, or hospitalist (discharge prescription). Veteran EMRs contain a list of medications obtained outside the VA facility, referred to as non-VA prescriptions. These medications werenot included in the analysis because accuracy could not be verified.
A study author reviewed the results of any UDS performed up to 120 days before the ED visit date to determine whether the result reflected the currently prescribed prescription medications. If the UDS was positive for the prescribed opioids and/or BZDs and for any nonprescribed drug, including alcohol, the UDS was classified as not reflective. If the prescribed BZD was alprazolam, clonazepam, or lorazepam, a BZD-positive UDS was not required for the UDS to be considered reflective because of the sensitivity of the UDS BZD immunoassay
used at the George E. Wahlen VAMC clinical laboratory.21
Outcomes of the overdose were categorized as discharged, hospitalized, or deceased. Descriptive statistical analyses were performed using Microsoft Excel. Group comparisons were performed using Pearson chi-square or Student t test.
Results
The ED at the George E. Wahlen VAMC averages 64 visits per day, almost 94,000 visits within the study period. One hundred seventy ED visits between January 1, 2009 and January 1, 2013, involved naloxone administration. Ninety-two visits met the inclusion criteria of opioid overdose, representing about 0.002% of all ED visits at this facility (Figure 1). Six veterans had multiple ED visits within the study period, including 4 veterans who were in the opioid-only group.
The majority of veterans in this study were non-Hispanic white (n = 83, 90%), male (n = 88, 96%), with a mean age of 63 years. Less than 40% listed a next-of-kin or contact person living at their address.
Based on prescriptions available within 120 days before the overdose, 67 veterans (73%) possessed opioid and/or BZD prescriptions. In this group, the MED available on the day of the ED visit ranged from 7.5 mg to 830 mg. The MED was ≤ 200 mg in 71.6% and ≤ 50 mg in 34.3% of these cases. Veterans prescribed both opioids and BZDs had higher MED (average, 259 mg) available within 120 days of the ED visit than did those prescribed opioids only (average, 118 mg) (P = .015; SD, 132.9). The LED ranged from 1 mg to 12 mg for veterans with available BZDs.
Based on prescriptions available on the day of opioid overdose, 53 veterans (58%) had opioid prescriptions. The ranges of MED and LED available on the day of overdose were the same as the 120-day availability period. The average MED was 183 mg in veterans prescribed both opioids and BZDs and 126 mg in those prescribed opioids only (P = .283; SD, 168.65; Figure 2). The time between the last opioid fill date and the overdose visit date averaged 20 days (range, 0 to 28 days) in veterans prescribed opioids.
All veterans had at least 1 diagnosis that in previous studies was associated with increased risk of overdose.9,15 The most common diagnoses included cardiovascular diseases, mental health disorders, pulmonary diseases, and cancer. Other SUDDs not including tobacco use were documented in at least half the veterans with prescribed opioids and/or BZDs. No veteran in the sample had a documented history of opioid SUDD.
Hydrocodone products were available in > 50% of cases. None of the veterans were prescribed buprenorphine products; other opioids, including tramadol, comprised the remainder (Figure 3). Primary care providers prescribed 72% of opioid prescriptions, with pain specialists, discharge physicians, ED providers, and surgeons prescribing the rest. When both opioids and BZDs were available, combinations of a hydrocodone product plus clonazepam or lorazepam were most common. The time between the last opioid fill date and the overdose visit date averaged 20 days (range, 0 to 28 days) in veterans prescribed opioids.
Overall, 64% of the sample had UDS results prior to the ED visit. Of veterans prescribed opioids and/or BZDs, 53% of UDSs reflected prescribed regimens.
On the day of the ED visit, 1 death occurred. Ninety-one veterans (99%) survived the overdose; 79 veterans (86%) were hospitalized, most for < 24 hours.
Discussion
This retrospective review identified 92 veterans who were treated with naloxone in the ED for opioid overdose during a 4-year period at the George E. Wahlen VAMC. Seventy-eight cases were excluded because the reason entered in charts for naloxone administration was itching, constipation, altered mental status, or unclear documentation.
Veterans in this study were older on average than the overdose fatalities in the U.S. Opioid overdose deaths in the U.S. and in Utah occur most frequently in non-Hispanic white men aged between 35 and 54 years.7,22,23 In the 2010 Nationwide Emergency Department Sample of 136,000 opioid overdoses, of which 98% survived, most were aged 18 to 54 years.16 The older age in this study most likely reflects the age range of veterans served in the VHA; however, as more young veterans enter the VHA, the age range of overdose victims may more closely resemble the age ranges found in previous studies. Post hoc analysis showed 8 veterans (9%) with probable intentional opioid overdose based on chart review, whereas the incidence of intentional prescription drug overdose in the U.S. is 17.1%.24
In Utah, almost 93% of fatal overdoses occur at a residential location.22 Less than half the veterans in this study had a contact or next-of-kin listed as living at the same address. Although veterans may not have identified someone living with them, in many cases, it is likely another person witnessed the overdose. Relying on EMRs to identify who should receive prevention education, in addition to the veteran, may result in missed opportunities to include another person likely to witness an overdose.25 Prescribers should make a conscious effort to ask veterans to identify someone who may be able to assist with rescue efforts in the event of an overdose.
Diagnoses associated with increased risk of opioid overdose death include sleep apnea, morbid obesity, pulmonary or cardiovascular diseases, and/or a history of psychiatric disorders and SUDD.8,9,16 In a large sample of older veterans, only 64% had at least 1 medical or psychiatric diagnosis.26 Less than half the 18,000 VA primary care patients in 5 VA centers had any psychiatric condition, and < 65% had cardiovascular disease, pulmonary disease, or cancer.27 All veterans in this study had medical and psychiatric comorbidity.
In contrast, a large ED sample described by Yokell and colleagues found chronic mental conditions in 33.9%, circulatory disorders in 29.1%, and respiratory conditions in 25.6% of their sample.16 Bohnert and associates found a significantly elevated hazard ratio (HR) for any psychiatric disorder in a sample of nearly 4,500 veterans. There was variation in the HR when individual psychiatric diagnoses were broken out, with bipolar disorder having the largest HR and schizophrenia having the lowest but still elevated HR.9 In this study, individual diagnoses were not broken out because the smaller sample size could diminish the clinical significance of any apparent differences.
Edlund and colleagues found that < 8% of veterans treated with opioids for chronic noncancer pain had nonopioid SUDD.10 Bohnert and colleagues found an HR of 21.95 for overdose death among those with opioid-use disorders.9 The sample in this study had a much higher incidence of nonopioid SUDD compared with that ub the study by Edlund and colleagues, but none of the veterans in this study had a documented history of opioid use disorder. The absence of opioid use disorders in this sample is unexpected and points to a need for providers to screen for opioid use disorder whenever opioids are prescribed or renewed. If prevention practices were directed only to those with opioid SUDDs, none of the veterans in this study would have been included in those efforts. Non-SUDD providers should address the risks of opioid overdose in veterans with sleep apnea, morbid obesity, pulmonary or cardiovascular diseases, and/or a history of psychiatric disorders.
Gomes and colleagues found that > 100 mg MED available on the day of overdose doubled the risk of opioid-related mortality.18 The VA/DoD Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain identifies 200 mg MED as a threshold to define high-dose opioid therapy.28 Fulton-Kehoe and colleagues found that 28% of overdose victims were prescribed < 50 mg MED.29 In this study, the average dose available to veterans was > 100 mg MED; however, one-third of all study veterans had < 50 mg MED available. Using a threshold dose of 50 mg MED to target prevention efforts would capture only two-thirds of those who experienced overdose; a 200 mg MED threshold would exclude the majority, based on the average MED in each group in this study. Overdose education should be provided to veterans with access to any dose of opioids.
Use of BZDs with opioids may result in greater central nervous system (CNS) depression, pharmacokinetic interactions, or pharmacodynamic interactions at the µ opioid receptor.30-32 About one-third of veterans in this study were prescribed opioids and BZDs concurrently, a combination noted in about 33% of opioid overdose deaths reported by the CDC.24 Individuals taking methadone combined with BZDs have been found to have severe medical outcomes.33 If preventive efforts are targeted to those receiving opioids and other CNS depressants, such as BZDs, about half (42%) the veterans in this study would not receive a potentially life-saving message about preventing overdoses. All veterans with opioids should be educated about the additional risk of overdose posed by drug interactions with other CNS depressants.
The time since the last fill of opioid prescription ranged from 0 to 28 days. This time frame indicates that some overdoses may have occurred on the day an opioid was filled but most occurred near the end of the expected days’ supply. Because information about adherence or use of the opioid was not studied, it cannot be assumed that medication misuse is the primary reason for the overdose. Delivering prevention efforts only at the time of medication dispensing would be insufficient. Clinicians should review local and remote prescription data, including via their states’ prescription drug monitoring program when discussing the risk of overdose with veterans.
Most veterans had at least 1 UDS result in the chart. Although half the UDSs obtained reflected prescribed medications, the possibility of aberrant behaviors, which increases the risk of overdose, cannot be ruled out with the methods used in this study.34 Medication management agreements that require UDSs for veterans with chronic pain were not mandatory at the George E. Wahlen VAMC during the study period, and those used did not mandate discontinuation of opioid therapy if suspected aberrant behaviors were present.
A Utah study based on interviews of overdose victims’ next-of-kin found that 76% were concerned about victims’ aberrant behaviors, such as medication misuse, prior to the death.22 In contrast, a study of commercial and Medicaid recipients estimated medication misuse rates in
≤ 30% of the sample.35 Urine drug screening results not reflective of the prescribed regimens have been found in up to 50% of patients receiving chronic opioid therapy.
The UDS findings in this study were determined by the authors and did not capture clinical decisions or interpretations made after results were available or whether these decisions resulted in overdose prevention strategies, such as targeted education or changes in prescription availability. Targeting preventive efforts toward veterans only with UDS results suggesting medication misuse would have missed more than half the veterans in this study. Urine drug screening should be used as a clinical monitoring tool whenever opioids, BZDs, or other substances are used or prescribed.
The VA now has a nationwide program, Opioid Overdose Education and Naloxone Distribution (OEND) promoting overdose education and take-home naloxone distribution for providers and patients to prevent opioid-related overdose deaths. A national SharePoint site has been created within the VA that lists resources to support this effort.
Almost all veterans in this review survived the overdose and were hospitalized following the ED visit. Other investigators also have found that the majority (51% to 98%) of overdose victims reaching the ED survived, but fewer patients (3% to 51%) in those studies were hospitalized.16,36 It is unknown whether there are differences in risk factors associated with survived or fatal overdoses.
Limitations
Although Utah ranked third for drug overdose death rates in 2008 and had the highest death rate among veterans from 2001 to 2009, this review captured only overdoses among veterans treated during the study period at the George E. Wahlen VAMC ED.5,6 The number and characteristics of veterans during this same period who were treated for overdose in other community EDs or urgent care centers throughout Utah is unknown.
The definition of opioid and BZD dose available in this study may not represent actual use of opioids or BZDs because it was based on chart review of prescription dispensing information and UDS procedures at the George E. Wahlen VAMC, and medication misuse cannot be ruled out. This study did not evaluate specific aberrant behaviors.
Conclusion
Current overdose prevention screening efforts primarily identify patients on high-dose opioids and those with SUDD. Many veterans in this study were older than the average U.S. victims’ age, did not have SUDD, were prescribed opioid doses not considered high risk by current guidelines, were nearer the end of their medication supply, and had UDS reflective of prescribed medications. This study suggests that any veteran with access to opioids, whether prescribed or not, is at risk for an opioid overdose. Established risk factors may aid in developing overdose prevention programs, but prevention should not be limited to veterans with prescribed opioids and known risk factors. Prescribers should screen for opioid use disorder whenever opioids are prescribed and continue to screen throughout therapy. Broader screening for overdose risk is needed to avoid missing important opportunities for overdose prevention.
Acknowledgments
Gale Anderson, VISN 19 PBM Data Manager, performed initial data query for the study.
References
1. Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR. 2015;64(50):1-5.
2. Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374(2):154-163.
3. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981-1985.
4. Bohnert AS, Ilgen MA, Galea S, McCarthy JF, Blow FC. Accidental poisoning mortality among patients in the Department of Veterans Affairs Health System. Med Care. 2011;49(4):393-396.
5. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends and regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-612.
6. Centers for Disease Control and Prevention. Policy impact: prescription, painkiller, overdoses. http://www.cdc.gov/drugoverdose/pdf/policyimpact-prescriptionpainkillerod-a.pdf. Published November 2011. Accessed August 25, 2016.
7. Xu J, Murphy SL, Kochanek KD, Bastian BA; Division of Vital Statistics. Deaths: final data for 2013. http://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_02.pdf. Published February 16, 2016. Accessed August 25, 2016.
8. The Joint Commission. Sentinel event alert issue 49: safe use of opioids in the hospital. https://www.jointcommission.org/assets/1/18/SEA_49_opioids_8_2_12_final.pdf. Published August 8, 2012. Accessed April 25, 2015.
9. Bohnert AS, Ilgen MA, Ignacio RV, McCarthy JF, Valenstein M, Blow FC. Risk of death from accidental overdose associated with psychiatric and substance use disorders. Am J Psychiatry. 2012;169(1):64-70.
10. Edlund MJ, Austen MA, Sullivan MD, et al. Patterns of opioid use for chronic noncancer pain in the Veterans Health Administration from 2009 to 2011. Pain. 2014;155:2337-2343.
11. Jann M, Kennedy WK, Lopez G. Benzodiazepines: a major component in unintentional prescription drug overdoses with opioid analgesics. J Pharm Pract. 2014;27(1):5-16.
12. McMillin G, Kusukawa N, Nelson G. Benzodiazepines.Salt Lake City, UT: ARUP Laboratories; 2012.
13. Naloxone hydrochloride [package insert].Lake Forest, IL: Hospira Inc; 2007.
14. Boyer EW. Management of opioid analgesic overdose. N Engl J Med. 2012;367(2):146-155.
15. Hoffman JR, Schriger DL, Luo JS. The empiric use of naloxone in patients with altered mental status: a reappraisal. Ann Emerg Med. 1991;20(3):246-252.
16. Yokell MA, Delgado MK, Zaller ND, Wang NE, McGowan SK, Green TC. Presentation of prescription and nonprescription opioid overdoses to US emergency departments. JAMA Intern Med. 2014;174(12):2034-2037.
17. Binswanger I, Gardner E, Gabella B, Broderick K, Glanz K. Development of case criteria to define pharmaceutical opioid and heroin overdoses in clinical records. Platform presented at: Association for Medical Education and Research in Substance Abuse 38th Annual National Conference; November 7, 2014; San Francisco, CA.
18. Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171(7):686-691.
19. Jaeger TM, Lohr RH, Pankratz VS. Symptom-triggered therapy for alcohol withdrawal syndrome in medical inpatients. Mayo Clin Proc. 2001;76(7):695-701.20. Washington State Agency Medical Directors’ Group. Opioid dose clculator. http://www
.agencymeddirectors.wa.gov/Calculator/DoseCalcula tor.htm. Accessed October 10, 2016.
21. EMIT II Plus Benzodiazepine Assay [package insert]. Brea, CA: Beckman Coulter, Inc; 2010.
22. Johnson EM, Lanier WA, Merrill RM, et al. Unintentional prescription opioid-related overdose deaths: description of decedents by next of kin or best contact, Utah, 2008-2009. J Gen Intern Med. 2013;28(4):522-529.
23. Utah Department of Health. Fact sheet: prescription pain medication deaths in Utah, 2012. https://www.health.utah.gov/vipp/pdf/FactSheets/2012RxOpioidDeaths.pdf. Updated October 2013. Accessed October 10, 2016.
24. Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309(7):657-659.
25. Bohnert AS, Tracy M, Galea S. Characteristics of drug users who witness many overdoses: implications for overdose prevention. Drug Alcohol Depend. 2012;120(1-3):168-173.
26. Yoon J, Zulman D, Scott JY, Maciejewski ML. Costs associated with multimorbidity among VA patients. Med Care. 2014;52(suppl 3):S31-S36.
27. Yoon J, Yano EM, Altman L, et al. Reducing costs of acute care for ambulatory care-sensitive medical conditions: the central roles of comorbid mental illness. Med Care. 2012;50(8):705-713.
28. Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain. Guideline summary. http://www.va.gov/painmanagement/docs/cpg_opioidtherapy_summary.pdf. Published May 2010. Accessed August 25, 2016.
29. Fulton-Kehoe D, Sullivan MD, Turner JA, et al. Opioid poisonings in Washington state Medicaid: trends, dosing, and guidelines. Med Care. 2015;53(8):679-685.
30. Gudin JA, Mogali S, Jones JD, Comer SD. Risks, management, and monitoring of combination opioid, benzodiazepines, and/or alcohol use. Postgrad Med. 2013;125(4):115-130.
31. Poisnel G, Dhilly M, Le Boisselier R, Barre L, Debruyne D. Comparison of five benzodiazepine-receptor agonists on buprenorphine-induced mu-opioid receptor regulation. J Pharmacol Sci. 2009;110(1):36-46.
32. Webster LR, Cochella S, Dasgupta N, et al. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med. 2011;12(suppl 2):S26-S35.
33. Lee SC, Klein-Schwartz W, Doyon S, Welsh C. Comparison of toxicity associated with nonmedical use of benzodiazepines with buprenorphine or methadone. Drug Alcohol Depend. 2014;138:118-123.
34. Owen GT, Burton AW, Schade CM, Passik S. Urine drug testing: current recommendations and best practices. Pain Physician. 2012;15(suppl 3):ES119–ES133.
35. Sullivan MD, Edlund MJ, Fan MY, Devries A, Brennan Braden J, Martin BC. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: the TROUP study. Pain. 2010;150(2):332-339.
36. Sporer KA, Firestone J, Isaacs SM. Out-of-hospital treatment of opioid overdoses in an urban setting. Acad Emerg Med. 1996;3(7):660-667.
References
1. Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR. 2015;64(50):1-5.
2. Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374(2):154-163.
3. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363(21):1981-1985.
4. Bohnert AS, Ilgen MA, Galea S, McCarthy JF, Blow FC. Accidental poisoning mortality among patients in the Department of Veterans Affairs Health System. Med Care. 2011;49(4):393-396.
5. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends and regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-612.
6. Centers for Disease Control and Prevention. Policy impact: prescription, painkiller, overdoses. http://www.cdc.gov/drugoverdose/pdf/policyimpact-prescriptionpainkillerod-a.pdf. Published November 2011. Accessed August 25, 2016.
7. Xu J, Murphy SL, Kochanek KD, Bastian BA; Division of Vital Statistics. Deaths: final data for 2013. http://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_02.pdf. Published February 16, 2016. Accessed August 25, 2016.
8. The Joint Commission. Sentinel event alert issue 49: safe use of opioids in the hospital. https://www.jointcommission.org/assets/1/18/SEA_49_opioids_8_2_12_final.pdf. Published August 8, 2012. Accessed April 25, 2015.
9. Bohnert AS, Ilgen MA, Ignacio RV, McCarthy JF, Valenstein M, Blow FC. Risk of death from accidental overdose associated with psychiatric and substance use disorders. Am J Psychiatry. 2012;169(1):64-70.
10. Edlund MJ, Austen MA, Sullivan MD, et al. Patterns of opioid use for chronic noncancer pain in the Veterans Health Administration from 2009 to 2011. Pain. 2014;155:2337-2343.
11. Jann M, Kennedy WK, Lopez G. Benzodiazepines: a major component in unintentional prescription drug overdoses with opioid analgesics. J Pharm Pract. 2014;27(1):5-16.
12. McMillin G, Kusukawa N, Nelson G. Benzodiazepines.Salt Lake City, UT: ARUP Laboratories; 2012.
13. Naloxone hydrochloride [package insert].Lake Forest, IL: Hospira Inc; 2007.
14. Boyer EW. Management of opioid analgesic overdose. N Engl J Med. 2012;367(2):146-155.
15. Hoffman JR, Schriger DL, Luo JS. The empiric use of naloxone in patients with altered mental status: a reappraisal. Ann Emerg Med. 1991;20(3):246-252.
16. Yokell MA, Delgado MK, Zaller ND, Wang NE, McGowan SK, Green TC. Presentation of prescription and nonprescription opioid overdoses to US emergency departments. JAMA Intern Med. 2014;174(12):2034-2037.
17. Binswanger I, Gardner E, Gabella B, Broderick K, Glanz K. Development of case criteria to define pharmaceutical opioid and heroin overdoses in clinical records. Platform presented at: Association for Medical Education and Research in Substance Abuse 38th Annual National Conference; November 7, 2014; San Francisco, CA.
18. Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171(7):686-691.
19. Jaeger TM, Lohr RH, Pankratz VS. Symptom-triggered therapy for alcohol withdrawal syndrome in medical inpatients. Mayo Clin Proc. 2001;76(7):695-701.20. Washington State Agency Medical Directors’ Group. Opioid dose clculator. http://www
.agencymeddirectors.wa.gov/Calculator/DoseCalcula tor.htm. Accessed October 10, 2016.
21. EMIT II Plus Benzodiazepine Assay [package insert]. Brea, CA: Beckman Coulter, Inc; 2010.
22. Johnson EM, Lanier WA, Merrill RM, et al. Unintentional prescription opioid-related overdose deaths: description of decedents by next of kin or best contact, Utah, 2008-2009. J Gen Intern Med. 2013;28(4):522-529.
23. Utah Department of Health. Fact sheet: prescription pain medication deaths in Utah, 2012. https://www.health.utah.gov/vipp/pdf/FactSheets/2012RxOpioidDeaths.pdf. Updated October 2013. Accessed October 10, 2016.
24. Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309(7):657-659.
25. Bohnert AS, Tracy M, Galea S. Characteristics of drug users who witness many overdoses: implications for overdose prevention. Drug Alcohol Depend. 2012;120(1-3):168-173.
26. Yoon J, Zulman D, Scott JY, Maciejewski ML. Costs associated with multimorbidity among VA patients. Med Care. 2014;52(suppl 3):S31-S36.
27. Yoon J, Yano EM, Altman L, et al. Reducing costs of acute care for ambulatory care-sensitive medical conditions: the central roles of comorbid mental illness. Med Care. 2012;50(8):705-713.
28. Department of Veterans Affairs, Department of Defense. VA/DoD Clinical Practice Guideline for Management of Opioid Therapy for Chronic Pain. Guideline summary. http://www.va.gov/painmanagement/docs/cpg_opioidtherapy_summary.pdf. Published May 2010. Accessed August 25, 2016.
29. Fulton-Kehoe D, Sullivan MD, Turner JA, et al. Opioid poisonings in Washington state Medicaid: trends, dosing, and guidelines. Med Care. 2015;53(8):679-685.
30. Gudin JA, Mogali S, Jones JD, Comer SD. Risks, management, and monitoring of combination opioid, benzodiazepines, and/or alcohol use. Postgrad Med. 2013;125(4):115-130.
31. Poisnel G, Dhilly M, Le Boisselier R, Barre L, Debruyne D. Comparison of five benzodiazepine-receptor agonists on buprenorphine-induced mu-opioid receptor regulation. J Pharmacol Sci. 2009;110(1):36-46.
32. Webster LR, Cochella S, Dasgupta N, et al. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med. 2011;12(suppl 2):S26-S35.
33. Lee SC, Klein-Schwartz W, Doyon S, Welsh C. Comparison of toxicity associated with nonmedical use of benzodiazepines with buprenorphine or methadone. Drug Alcohol Depend. 2014;138:118-123.
34. Owen GT, Burton AW, Schade CM, Passik S. Urine drug testing: current recommendations and best practices. Pain Physician. 2012;15(suppl 3):ES119–ES133.
35. Sullivan MD, Edlund MJ, Fan MY, Devries A, Brennan Braden J, Martin BC. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: the TROUP study. Pain. 2010;150(2):332-339.
36. Sporer KA, Firestone J, Isaacs SM. Out-of-hospital treatment of opioid overdoses in an urban setting. Acad Emerg Med. 1996;3(7):660-667.
Ebola Treatment Is Promising—But Not Definitively Better
The experimental Ebola treatment ZMapp, which is composed of 3 different monoclonal antibodies, prevents progression of Ebola virus disease by targeting the main surface protein of the virus. According to findings from the clinical trial PREVAIL II, ZMapp is safe and well tolerated. But because the Ebola epidemic is “waning,” NIH says, the study enrolled too few people to determine definitively whether it is a better treatment than the best available standard of care.
Related: Novel Treatment for Ebola Virus
The study involved 72 men and women with confirmed infection. However, the researchers closed the study early because they could not enroll the target number of 200 participants due to the decline in cases. All patients received the optimized standard of care—IV fluids, electrolyte balance, maintaining oxygen and blood pressure levels—and half also received 3 IV infusions of ZMapp 3 days apart.
At 28 days, 13 of the 35 patients (37%) in the standard care group had died, compared with 8 of 36 (22%) in the ZMapp group. That difference, a 40% lower risk of death with ZMapp, still did not reach statistical significance.
Related: Ebola Virus Persists in Semen Long Term
The findings are “promising and provide valuable scientific data,” says Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases. Moreover, he adds, “Importantly, the study establishes that it is feasible to conduct a randomized, controlled trial during a major public health emergency in a scientifically and ethically sound manner.”
The experimental Ebola treatment ZMapp, which is composed of 3 different monoclonal antibodies, prevents progression of Ebola virus disease by targeting the main surface protein of the virus. According to findings from the clinical trial PREVAIL II, ZMapp is safe and well tolerated. But because the Ebola epidemic is “waning,” NIH says, the study enrolled too few people to determine definitively whether it is a better treatment than the best available standard of care.
Related: Novel Treatment for Ebola Virus
The study involved 72 men and women with confirmed infection. However, the researchers closed the study early because they could not enroll the target number of 200 participants due to the decline in cases. All patients received the optimized standard of care—IV fluids, electrolyte balance, maintaining oxygen and blood pressure levels—and half also received 3 IV infusions of ZMapp 3 days apart.
At 28 days, 13 of the 35 patients (37%) in the standard care group had died, compared with 8 of 36 (22%) in the ZMapp group. That difference, a 40% lower risk of death with ZMapp, still did not reach statistical significance.
Related: Ebola Virus Persists in Semen Long Term
The findings are “promising and provide valuable scientific data,” says Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases. Moreover, he adds, “Importantly, the study establishes that it is feasible to conduct a randomized, controlled trial during a major public health emergency in a scientifically and ethically sound manner.”
The experimental Ebola treatment ZMapp, which is composed of 3 different monoclonal antibodies, prevents progression of Ebola virus disease by targeting the main surface protein of the virus. According to findings from the clinical trial PREVAIL II, ZMapp is safe and well tolerated. But because the Ebola epidemic is “waning,” NIH says, the study enrolled too few people to determine definitively whether it is a better treatment than the best available standard of care.
Related: Novel Treatment for Ebola Virus
The study involved 72 men and women with confirmed infection. However, the researchers closed the study early because they could not enroll the target number of 200 participants due to the decline in cases. All patients received the optimized standard of care—IV fluids, electrolyte balance, maintaining oxygen and blood pressure levels—and half also received 3 IV infusions of ZMapp 3 days apart.
At 28 days, 13 of the 35 patients (37%) in the standard care group had died, compared with 8 of 36 (22%) in the ZMapp group. That difference, a 40% lower risk of death with ZMapp, still did not reach statistical significance.
Related: Ebola Virus Persists in Semen Long Term
The findings are “promising and provide valuable scientific data,” says Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases. Moreover, he adds, “Importantly, the study establishes that it is feasible to conduct a randomized, controlled trial during a major public health emergency in a scientifically and ethically sound manner.”