User login
Open Payments system back online; physician deadline extended
The Open Payments system is up and running again, and federal officials said the technical glitches that caused data to be linked to the wrong physicians have been fixed.
Physicians and teaching hospitals now have until Sept. 8 to review the payment information submitted to the government by drug, device, and biological manufacturers. Despite the delay, the information is still on track to be publicly released on Sept. 30, according to the Centers for Medicare & Medicaid Services.
The Open Payments Program, created by the Affordable Care Act, aims to add transparency to the financial relationships between the health care industry and physicians and teaching hospitals. But the effort to publish data on industry payments was delayed when CMS learned that some information was being incorrectly matched to physicians. The agency took the site offline on Aug. 3 to investigate.
"CMS takes data integrity very seriously and took swift action after a physician reported a problem," Dr. Shantanu Agrawal, deputy administrator and director of the Center for Program Integrity at CMS, said in a statement. "We have identified the root cause of the problem and have instituted a system fix to prevent similar errors."
Dr. Agrawal urged physicians to review their records before the Sept. 8 deadline to identify any potential errors.
The CMS investigation found that manufacturers and group purchasing organizations had submitted intermingled data, adding the wrong state license number or national provider identifier for physicians with the same first and last names. The agency implemented "system fixes," removed the inaccurate data, and revalidated the information.
Since the website used for physicians and teaching hospital to review their data was offline for several days, CMS has extended the deadline for reviewing and disputing data from Aug. 27 to Sept. 8.
On Twitter @maryellenny
The Open Payments system is up and running again, and federal officials said the technical glitches that caused data to be linked to the wrong physicians have been fixed.
Physicians and teaching hospitals now have until Sept. 8 to review the payment information submitted to the government by drug, device, and biological manufacturers. Despite the delay, the information is still on track to be publicly released on Sept. 30, according to the Centers for Medicare & Medicaid Services.
The Open Payments Program, created by the Affordable Care Act, aims to add transparency to the financial relationships between the health care industry and physicians and teaching hospitals. But the effort to publish data on industry payments was delayed when CMS learned that some information was being incorrectly matched to physicians. The agency took the site offline on Aug. 3 to investigate.
"CMS takes data integrity very seriously and took swift action after a physician reported a problem," Dr. Shantanu Agrawal, deputy administrator and director of the Center for Program Integrity at CMS, said in a statement. "We have identified the root cause of the problem and have instituted a system fix to prevent similar errors."
Dr. Agrawal urged physicians to review their records before the Sept. 8 deadline to identify any potential errors.
The CMS investigation found that manufacturers and group purchasing organizations had submitted intermingled data, adding the wrong state license number or national provider identifier for physicians with the same first and last names. The agency implemented "system fixes," removed the inaccurate data, and revalidated the information.
Since the website used for physicians and teaching hospital to review their data was offline for several days, CMS has extended the deadline for reviewing and disputing data from Aug. 27 to Sept. 8.
On Twitter @maryellenny
The Open Payments system is up and running again, and federal officials said the technical glitches that caused data to be linked to the wrong physicians have been fixed.
Physicians and teaching hospitals now have until Sept. 8 to review the payment information submitted to the government by drug, device, and biological manufacturers. Despite the delay, the information is still on track to be publicly released on Sept. 30, according to the Centers for Medicare & Medicaid Services.
The Open Payments Program, created by the Affordable Care Act, aims to add transparency to the financial relationships between the health care industry and physicians and teaching hospitals. But the effort to publish data on industry payments was delayed when CMS learned that some information was being incorrectly matched to physicians. The agency took the site offline on Aug. 3 to investigate.
"CMS takes data integrity very seriously and took swift action after a physician reported a problem," Dr. Shantanu Agrawal, deputy administrator and director of the Center for Program Integrity at CMS, said in a statement. "We have identified the root cause of the problem and have instituted a system fix to prevent similar errors."
Dr. Agrawal urged physicians to review their records before the Sept. 8 deadline to identify any potential errors.
The CMS investigation found that manufacturers and group purchasing organizations had submitted intermingled data, adding the wrong state license number or national provider identifier for physicians with the same first and last names. The agency implemented "system fixes," removed the inaccurate data, and revalidated the information.
Since the website used for physicians and teaching hospital to review their data was offline for several days, CMS has extended the deadline for reviewing and disputing data from Aug. 27 to Sept. 8.
On Twitter @maryellenny
Most Americans satisfied with cost of brand-name drugs
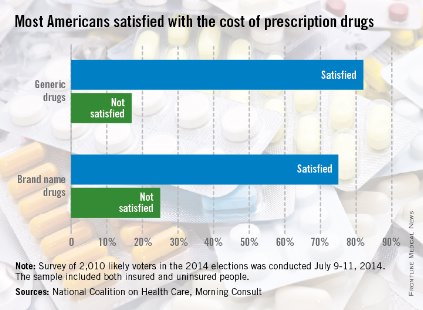
Three-quarters of American adults are satisfied with what they pay for brand-name prescription drugs, according to a survey from the National Coalition on Health Care and digital media company Morning Consult.
Generic drugs are even more popular, getting a satisfaction rate of 82% in the survey, which was conducted July 9-11, among a national sample of 2,010 people likely to vote in the 2014 elections.
When respondents were asked about the transparency of prescription drug pricing, 64% said that there was not enough transparency, 12% said there was enough, and 25% were not sure, the Morning Consult reported.
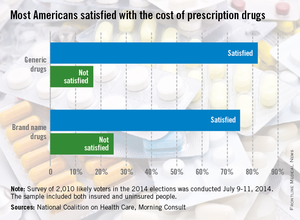
Three-quarters of American adults are satisfied with what they pay for brand-name prescription drugs, according to a survey from the National Coalition on Health Care and digital media company Morning Consult.
Generic drugs are even more popular, getting a satisfaction rate of 82% in the survey, which was conducted July 9-11, among a national sample of 2,010 people likely to vote in the 2014 elections.
When respondents were asked about the transparency of prescription drug pricing, 64% said that there was not enough transparency, 12% said there was enough, and 25% were not sure, the Morning Consult reported.

Three-quarters of American adults are satisfied with what they pay for brand-name prescription drugs, according to a survey from the National Coalition on Health Care and digital media company Morning Consult.
Generic drugs are even more popular, getting a satisfaction rate of 82% in the survey, which was conducted July 9-11, among a national sample of 2,010 people likely to vote in the 2014 elections.
When respondents were asked about the transparency of prescription drug pricing, 64% said that there was not enough transparency, 12% said there was enough, and 25% were not sure, the Morning Consult reported.

Tech problems stall Open Payments Program
The Open Payments system has been temporarily taken offline because of technical glitches that have called into question the accuracy of reported data.
"After an assessment of the data resulting from a complaint, we discovered that a limited number of physician payment records submitted by at least one manufacturer incorrectly contained information about other physicians," officials at the Centers for Medicare & Medicaid Services said in a statement. "CMS takes physician privacy very seriously and we have taken the system offline temporarily and will work with the industry to eliminate incorrect payment records."
The Open Payments Program, created under the Affordable Care Act, aims to increase transparency about financial relationships between the drug, device, and biological industry and physicians and teaching hospitals. Under the program, the industry reports payment data, which can be reviewed and disputed by physicians and teaching hospitals. The goal was for the agency to release the data to the public on Sept. 30.
Agency officials first took the website offline on Aug. 3. On Aug. 7, CMS officials sent an e-mail announcing that physicians and representatives from teaching hospitals would be unable to register or review data while the system was offline. Officials pledged that for each day the system was offline, the agency would accordingly adjust the deadlines for reviews and disputes, and the following 15-day corrections period.
Agency officials found that at least one manufacturer submitted incorrect data by matching the name, address, and National Provider Identifier of a physician with the wrong state medical license. Though the medical license was valid, it belonged to another physician with the same first and last names.
Once that information was entered into the Open Payments website, the system combined the data, allowing one of the physicians to see all the payments, including those made to the other physician.
CMS officials contend the problem is limited to a small number of physicians, but in an effort to keep physicians from viewing another provider’s information, they are temporarily suspending registration in the program and the ability to review data. They have not announced an estimate for when the system will be back online.
On Twitter @maryellenny
The Open Payments system has been temporarily taken offline because of technical glitches that have called into question the accuracy of reported data.
"After an assessment of the data resulting from a complaint, we discovered that a limited number of physician payment records submitted by at least one manufacturer incorrectly contained information about other physicians," officials at the Centers for Medicare & Medicaid Services said in a statement. "CMS takes physician privacy very seriously and we have taken the system offline temporarily and will work with the industry to eliminate incorrect payment records."
The Open Payments Program, created under the Affordable Care Act, aims to increase transparency about financial relationships between the drug, device, and biological industry and physicians and teaching hospitals. Under the program, the industry reports payment data, which can be reviewed and disputed by physicians and teaching hospitals. The goal was for the agency to release the data to the public on Sept. 30.
Agency officials first took the website offline on Aug. 3. On Aug. 7, CMS officials sent an e-mail announcing that physicians and representatives from teaching hospitals would be unable to register or review data while the system was offline. Officials pledged that for each day the system was offline, the agency would accordingly adjust the deadlines for reviews and disputes, and the following 15-day corrections period.
Agency officials found that at least one manufacturer submitted incorrect data by matching the name, address, and National Provider Identifier of a physician with the wrong state medical license. Though the medical license was valid, it belonged to another physician with the same first and last names.
Once that information was entered into the Open Payments website, the system combined the data, allowing one of the physicians to see all the payments, including those made to the other physician.
CMS officials contend the problem is limited to a small number of physicians, but in an effort to keep physicians from viewing another provider’s information, they are temporarily suspending registration in the program and the ability to review data. They have not announced an estimate for when the system will be back online.
On Twitter @maryellenny
The Open Payments system has been temporarily taken offline because of technical glitches that have called into question the accuracy of reported data.
"After an assessment of the data resulting from a complaint, we discovered that a limited number of physician payment records submitted by at least one manufacturer incorrectly contained information about other physicians," officials at the Centers for Medicare & Medicaid Services said in a statement. "CMS takes physician privacy very seriously and we have taken the system offline temporarily and will work with the industry to eliminate incorrect payment records."
The Open Payments Program, created under the Affordable Care Act, aims to increase transparency about financial relationships between the drug, device, and biological industry and physicians and teaching hospitals. Under the program, the industry reports payment data, which can be reviewed and disputed by physicians and teaching hospitals. The goal was for the agency to release the data to the public on Sept. 30.
Agency officials first took the website offline on Aug. 3. On Aug. 7, CMS officials sent an e-mail announcing that physicians and representatives from teaching hospitals would be unable to register or review data while the system was offline. Officials pledged that for each day the system was offline, the agency would accordingly adjust the deadlines for reviews and disputes, and the following 15-day corrections period.
Agency officials found that at least one manufacturer submitted incorrect data by matching the name, address, and National Provider Identifier of a physician with the wrong state medical license. Though the medical license was valid, it belonged to another physician with the same first and last names.
Once that information was entered into the Open Payments website, the system combined the data, allowing one of the physicians to see all the payments, including those made to the other physician.
CMS officials contend the problem is limited to a small number of physicians, but in an effort to keep physicians from viewing another provider’s information, they are temporarily suspending registration in the program and the ability to review data. They have not announced an estimate for when the system will be back online.
On Twitter @maryellenny
Deadline nears to dispute industry payment data
Physicians and teaching hospitals have until Aug. 27 to review and dispute payment data from drug, device, and biological manufacturers under the federal government’s new Open Payments Program. But recent technical problems with the online system could push that deadline back by a few days.
The Centers for Medicare & Medicaid Services had been scheduled to publish information on industry payments to physicians and teaching hospitals on Sept. 30 as part of the new transparency initiative created under the Affordable Care Act.
On Aug. 7, CMS announced that the Open Payments website had been taken offline temporarily and that physicians and other providers would not be able to review data until the system was again operational. For each day that the system is offline, CMS said it will accordingly adjust the review and dispute deadline.
CMS did not explain why the system had to be taken offline, but Dr. Robert M. Wah, president of the American Medical Association, said in an interview that CMS officials had discovered earlier in the week a problem in which a manufacturer had attributed payment data to the wrong physician.
While CMS is planning for a small delay because of the technical problems, the AMA and more than 100 specialty and state medical societies are pushing for a 6-month delay on the public release of the payment data, a move that would change the scheduled publication date to March 31, 2015.
In an Aug. 5 letter to CMS, the AMA and the other organizations wrote that while they have "no issue" with efforts to increase transparency in the interactions between physicians and industry, they do have "serious concerns" about the implementation of the program.
A top concern for the groups is the length and complexity of the registration process required for physicians and teaching hospitals to access their data in the Open Payments systems.
CMS officials have told physicians to expect to spend 30-45 minutes to complete a 5-step registration process. But the AMA and the other societies contend that the process is much more involved. They estimate that after the initial preregistration step to verify a physician’s identify, there are another 11 steps in the registration process. And reviewing and disputing data is another 5 steps, they wrote.
"What we’re hearing from physicians is [that] this process is extremely time consuming and difficult," Dr. Wah said. "The user guide to go through this multistep process is 359 pages long. And the time that it’s taking people is in the hours, not minutes, to get through it."
The complexity of the process makes it "effectively impossible" for physicians to review and dispute payment data within the July 14-Aug. 27 window provided by CMS, the organizations wrote in their letter. After Aug. 27, physicians and teaching hospitals can continue to dispute data, but any subsequent corrections would not be reflected in the first publication of data on Sept. 30.
The groups also noted that many physicians are not aware of the program or its deadlines. The medical societies wrote that CMS has not done an adequate job of notifying physicians about the need to review their data. Further, delays by the government in setting a date for physicians to register for the program have made it difficult for medical societies to get information out to their members.
"It takes time to get the word out about these kinds of things, even in our electronically connected world," Dr. Wah said.
On Twitter @maryellenny
Physicians and teaching hospitals have until Aug. 27 to review and dispute payment data from drug, device, and biological manufacturers under the federal government’s new Open Payments Program. But recent technical problems with the online system could push that deadline back by a few days.
The Centers for Medicare & Medicaid Services had been scheduled to publish information on industry payments to physicians and teaching hospitals on Sept. 30 as part of the new transparency initiative created under the Affordable Care Act.
On Aug. 7, CMS announced that the Open Payments website had been taken offline temporarily and that physicians and other providers would not be able to review data until the system was again operational. For each day that the system is offline, CMS said it will accordingly adjust the review and dispute deadline.
CMS did not explain why the system had to be taken offline, but Dr. Robert M. Wah, president of the American Medical Association, said in an interview that CMS officials had discovered earlier in the week a problem in which a manufacturer had attributed payment data to the wrong physician.
While CMS is planning for a small delay because of the technical problems, the AMA and more than 100 specialty and state medical societies are pushing for a 6-month delay on the public release of the payment data, a move that would change the scheduled publication date to March 31, 2015.
In an Aug. 5 letter to CMS, the AMA and the other organizations wrote that while they have "no issue" with efforts to increase transparency in the interactions between physicians and industry, they do have "serious concerns" about the implementation of the program.
A top concern for the groups is the length and complexity of the registration process required for physicians and teaching hospitals to access their data in the Open Payments systems.
CMS officials have told physicians to expect to spend 30-45 minutes to complete a 5-step registration process. But the AMA and the other societies contend that the process is much more involved. They estimate that after the initial preregistration step to verify a physician’s identify, there are another 11 steps in the registration process. And reviewing and disputing data is another 5 steps, they wrote.
"What we’re hearing from physicians is [that] this process is extremely time consuming and difficult," Dr. Wah said. "The user guide to go through this multistep process is 359 pages long. And the time that it’s taking people is in the hours, not minutes, to get through it."
The complexity of the process makes it "effectively impossible" for physicians to review and dispute payment data within the July 14-Aug. 27 window provided by CMS, the organizations wrote in their letter. After Aug. 27, physicians and teaching hospitals can continue to dispute data, but any subsequent corrections would not be reflected in the first publication of data on Sept. 30.
The groups also noted that many physicians are not aware of the program or its deadlines. The medical societies wrote that CMS has not done an adequate job of notifying physicians about the need to review their data. Further, delays by the government in setting a date for physicians to register for the program have made it difficult for medical societies to get information out to their members.
"It takes time to get the word out about these kinds of things, even in our electronically connected world," Dr. Wah said.
On Twitter @maryellenny
Physicians and teaching hospitals have until Aug. 27 to review and dispute payment data from drug, device, and biological manufacturers under the federal government’s new Open Payments Program. But recent technical problems with the online system could push that deadline back by a few days.
The Centers for Medicare & Medicaid Services had been scheduled to publish information on industry payments to physicians and teaching hospitals on Sept. 30 as part of the new transparency initiative created under the Affordable Care Act.
On Aug. 7, CMS announced that the Open Payments website had been taken offline temporarily and that physicians and other providers would not be able to review data until the system was again operational. For each day that the system is offline, CMS said it will accordingly adjust the review and dispute deadline.
CMS did not explain why the system had to be taken offline, but Dr. Robert M. Wah, president of the American Medical Association, said in an interview that CMS officials had discovered earlier in the week a problem in which a manufacturer had attributed payment data to the wrong physician.
While CMS is planning for a small delay because of the technical problems, the AMA and more than 100 specialty and state medical societies are pushing for a 6-month delay on the public release of the payment data, a move that would change the scheduled publication date to March 31, 2015.
In an Aug. 5 letter to CMS, the AMA and the other organizations wrote that while they have "no issue" with efforts to increase transparency in the interactions between physicians and industry, they do have "serious concerns" about the implementation of the program.
A top concern for the groups is the length and complexity of the registration process required for physicians and teaching hospitals to access their data in the Open Payments systems.
CMS officials have told physicians to expect to spend 30-45 minutes to complete a 5-step registration process. But the AMA and the other societies contend that the process is much more involved. They estimate that after the initial preregistration step to verify a physician’s identify, there are another 11 steps in the registration process. And reviewing and disputing data is another 5 steps, they wrote.
"What we’re hearing from physicians is [that] this process is extremely time consuming and difficult," Dr. Wah said. "The user guide to go through this multistep process is 359 pages long. And the time that it’s taking people is in the hours, not minutes, to get through it."
The complexity of the process makes it "effectively impossible" for physicians to review and dispute payment data within the July 14-Aug. 27 window provided by CMS, the organizations wrote in their letter. After Aug. 27, physicians and teaching hospitals can continue to dispute data, but any subsequent corrections would not be reflected in the first publication of data on Sept. 30.
The groups also noted that many physicians are not aware of the program or its deadlines. The medical societies wrote that CMS has not done an adequate job of notifying physicians about the need to review their data. Further, delays by the government in setting a date for physicians to register for the program have made it difficult for medical societies to get information out to their members.
"It takes time to get the word out about these kinds of things, even in our electronically connected world," Dr. Wah said.
On Twitter @maryellenny
VA health care reform signed into law
President Obama has signed a bill that promises to give veterans faster access to medical care, in part by reimbursing services given by physicians and hospitals outside the Veterans Affairs health care system.
The Veterans Access, Choice and Accountability Act of 2014 makes $10 billion available through the "Veterans Choice Fund." The money is available until the funds are spent or until August 2017, whichever comes first.
Under the act, the VA is required to authorize non-VA care for any veteran who is enrolled in the VA system as of Aug. 1 or who is a newly discharged combat veteran if they can’t get an appointment at a VA medical facility within 30 days, or if they live more than 40 miles from the nearest VA medical facility.
The law also will take a closer look at care delivered within the VA, by requiring an independent assessment and establishing a congressional commission that will evaluate access to care throughout the system. It also provides $5 billion to hire new physicians and other medical staff to help increase access to care.
"This will not and cannot be the end of our effort," said President Obama at a signing ceremony Aug. 7 at Ft. Belvoir, Va. He also said that change would not happen overnight. "Implementing this law will take time," said the president.
Physician groups praised the legislation when lawmakers reached a compromise on the act, leading to passage by the House July 30, followed by the Senate July 31.
The American Medical Association supported the bill "because it is an important step to connecting veterans with physicians who can help them right now," AMA President Robert M. Wah said in a statement.
There are a number of reasons to be pleased with the law, added Dr. Reid Blackwelder, president of the American Academy of Family Physicians. Payment will be negotiated at rates not to exceed Medicare, except in very rural areas, he noted in a statement. The law also establishes 1,500 new residency slots in the VA system, with priority given to primary care and mental health.
"While the challenges associated with the current and future physician workforce within the VA will take time to fully resolve, this legislation will allow America’s civilian physicians to fill the void right away, ensuring that those who have served their country will no longer have to wait for primary care," said Dr. Blackwelder.
The American Psychiatric Association also applauded the increase in residency slots, and it welcomed the news that the VA will be able to prioritize hiring each year based on its identification of the five biggest staffing shortage areas. "In 2012, the VA Office of the Inspector General identified recruiting and retaining psychiatrists as the VA’s greatest challenge in the mental health area," said APA President Paul Summergrad in a statement.
"This legislation puts in place several actions to directly address that shortage, and will result in many more veterans having timely access to needed psychiatric services," he said.
The two congressmen who helped shepherd the legislation through the House and Senate also said they were gratified.
"I am pleased President Obama has finally recognized what we have been telling administration officials for years: that VA’s widespread and systemic lack of accountability is jeopardizing the health of veterans and contributing to all of the department’s most pressing problems," said Rep. Jeff Miller (R-Fla.), chairman of the House Committee on Veterans Affairs, in a statement.
"To prevent history from repeating itself, President Obama must become personally involved in solving VA’s many problems," Rep. Miller said. "A good place for him to start would be to meet with family members and veterans who have been struck by the VA scandal, order the department to cooperate with the congressional committees investigating VA, and force [the Department of Defense] and VA to work together to establish a joint electronic health record integrated across all DoD and VA components."
"In a dysfunctional Congress, I’m glad we accomplished something significant for veterans," Sen. Bernie Sanders (I-Vt.) said in a statement. This legislation will go a long way toward ending unacceptably long waiting times for veterans to access health care, and allow the VA the resources to hire the doctors, nurses, and other medical staff it needs to address these problems over the long term."
On Twitter @aliciaault
President Obama has signed a bill that promises to give veterans faster access to medical care, in part by reimbursing services given by physicians and hospitals outside the Veterans Affairs health care system.
The Veterans Access, Choice and Accountability Act of 2014 makes $10 billion available through the "Veterans Choice Fund." The money is available until the funds are spent or until August 2017, whichever comes first.
Under the act, the VA is required to authorize non-VA care for any veteran who is enrolled in the VA system as of Aug. 1 or who is a newly discharged combat veteran if they can’t get an appointment at a VA medical facility within 30 days, or if they live more than 40 miles from the nearest VA medical facility.
The law also will take a closer look at care delivered within the VA, by requiring an independent assessment and establishing a congressional commission that will evaluate access to care throughout the system. It also provides $5 billion to hire new physicians and other medical staff to help increase access to care.
"This will not and cannot be the end of our effort," said President Obama at a signing ceremony Aug. 7 at Ft. Belvoir, Va. He also said that change would not happen overnight. "Implementing this law will take time," said the president.
Physician groups praised the legislation when lawmakers reached a compromise on the act, leading to passage by the House July 30, followed by the Senate July 31.
The American Medical Association supported the bill "because it is an important step to connecting veterans with physicians who can help them right now," AMA President Robert M. Wah said in a statement.
There are a number of reasons to be pleased with the law, added Dr. Reid Blackwelder, president of the American Academy of Family Physicians. Payment will be negotiated at rates not to exceed Medicare, except in very rural areas, he noted in a statement. The law also establishes 1,500 new residency slots in the VA system, with priority given to primary care and mental health.
"While the challenges associated with the current and future physician workforce within the VA will take time to fully resolve, this legislation will allow America’s civilian physicians to fill the void right away, ensuring that those who have served their country will no longer have to wait for primary care," said Dr. Blackwelder.
The American Psychiatric Association also applauded the increase in residency slots, and it welcomed the news that the VA will be able to prioritize hiring each year based on its identification of the five biggest staffing shortage areas. "In 2012, the VA Office of the Inspector General identified recruiting and retaining psychiatrists as the VA’s greatest challenge in the mental health area," said APA President Paul Summergrad in a statement.
"This legislation puts in place several actions to directly address that shortage, and will result in many more veterans having timely access to needed psychiatric services," he said.
The two congressmen who helped shepherd the legislation through the House and Senate also said they were gratified.
"I am pleased President Obama has finally recognized what we have been telling administration officials for years: that VA’s widespread and systemic lack of accountability is jeopardizing the health of veterans and contributing to all of the department’s most pressing problems," said Rep. Jeff Miller (R-Fla.), chairman of the House Committee on Veterans Affairs, in a statement.
"To prevent history from repeating itself, President Obama must become personally involved in solving VA’s many problems," Rep. Miller said. "A good place for him to start would be to meet with family members and veterans who have been struck by the VA scandal, order the department to cooperate with the congressional committees investigating VA, and force [the Department of Defense] and VA to work together to establish a joint electronic health record integrated across all DoD and VA components."
"In a dysfunctional Congress, I’m glad we accomplished something significant for veterans," Sen. Bernie Sanders (I-Vt.) said in a statement. This legislation will go a long way toward ending unacceptably long waiting times for veterans to access health care, and allow the VA the resources to hire the doctors, nurses, and other medical staff it needs to address these problems over the long term."
On Twitter @aliciaault
President Obama has signed a bill that promises to give veterans faster access to medical care, in part by reimbursing services given by physicians and hospitals outside the Veterans Affairs health care system.
The Veterans Access, Choice and Accountability Act of 2014 makes $10 billion available through the "Veterans Choice Fund." The money is available until the funds are spent or until August 2017, whichever comes first.
Under the act, the VA is required to authorize non-VA care for any veteran who is enrolled in the VA system as of Aug. 1 or who is a newly discharged combat veteran if they can’t get an appointment at a VA medical facility within 30 days, or if they live more than 40 miles from the nearest VA medical facility.
The law also will take a closer look at care delivered within the VA, by requiring an independent assessment and establishing a congressional commission that will evaluate access to care throughout the system. It also provides $5 billion to hire new physicians and other medical staff to help increase access to care.
"This will not and cannot be the end of our effort," said President Obama at a signing ceremony Aug. 7 at Ft. Belvoir, Va. He also said that change would not happen overnight. "Implementing this law will take time," said the president.
Physician groups praised the legislation when lawmakers reached a compromise on the act, leading to passage by the House July 30, followed by the Senate July 31.
The American Medical Association supported the bill "because it is an important step to connecting veterans with physicians who can help them right now," AMA President Robert M. Wah said in a statement.
There are a number of reasons to be pleased with the law, added Dr. Reid Blackwelder, president of the American Academy of Family Physicians. Payment will be negotiated at rates not to exceed Medicare, except in very rural areas, he noted in a statement. The law also establishes 1,500 new residency slots in the VA system, with priority given to primary care and mental health.
"While the challenges associated with the current and future physician workforce within the VA will take time to fully resolve, this legislation will allow America’s civilian physicians to fill the void right away, ensuring that those who have served their country will no longer have to wait for primary care," said Dr. Blackwelder.
The American Psychiatric Association also applauded the increase in residency slots, and it welcomed the news that the VA will be able to prioritize hiring each year based on its identification of the five biggest staffing shortage areas. "In 2012, the VA Office of the Inspector General identified recruiting and retaining psychiatrists as the VA’s greatest challenge in the mental health area," said APA President Paul Summergrad in a statement.
"This legislation puts in place several actions to directly address that shortage, and will result in many more veterans having timely access to needed psychiatric services," he said.
The two congressmen who helped shepherd the legislation through the House and Senate also said they were gratified.
"I am pleased President Obama has finally recognized what we have been telling administration officials for years: that VA’s widespread and systemic lack of accountability is jeopardizing the health of veterans and contributing to all of the department’s most pressing problems," said Rep. Jeff Miller (R-Fla.), chairman of the House Committee on Veterans Affairs, in a statement.
"To prevent history from repeating itself, President Obama must become personally involved in solving VA’s many problems," Rep. Miller said. "A good place for him to start would be to meet with family members and veterans who have been struck by the VA scandal, order the department to cooperate with the congressional committees investigating VA, and force [the Department of Defense] and VA to work together to establish a joint electronic health record integrated across all DoD and VA components."
"In a dysfunctional Congress, I’m glad we accomplished something significant for veterans," Sen. Bernie Sanders (I-Vt.) said in a statement. This legislation will go a long way toward ending unacceptably long waiting times for veterans to access health care, and allow the VA the resources to hire the doctors, nurses, and other medical staff it needs to address these problems over the long term."
On Twitter @aliciaault
ABIM’s changes to MOC barely quell unrest
The American Board of Internal Medicine is making several changes to its maintenance of certification requirements, but, so far, it is not enough to quell the still-growing tide of anger and resentment against the process.
The ABIM announced the changes just ahead of a July 15 "summit" with 26 specialty societies that receive certification through the organization, and outlined them in a detailed letter that was sent to internal medicine diplomates on July 28. That correspondence also responded in detail to a May 7 letter sent by the American College of Physicians (ACP) on behalf of 14 medical societies, enumerating their concerns about the process.
The Endocrine Society, which attended the July 15 meeting, said that it left with many of the concerns it came in with. In a statement issued shortly thereafter, the Society called on the ABIM to suspend the maintenance of certification (MOC) requirements until it conducted "a formal analysis of all possible unintended consequences of the new MOC requirements."
Among the unintended consequences it says might result: If endocrinologists have to spend more time on MOC, they might spend less time with patients or on research, or they may even leave the field all together. That will put a dent in access to care when there is an increasing need, according to a letter from the Society to the ABIM in early June.
The American Association of Clinical Endocrinologists (AACE) also warned ABIM that by "monopolizing physician time," MOC could end up harming patient care. The group also said that many endocrinologists who previously did not have to recertify (so-called "grandfathers") might leave practice rather than go through the current process. In a letter to ABIM in late June, AACE also called on the Board to suspend the new requirements until there was a better understanding of how it might affect the endocrinology workforce. The ABIM should also "refrain from publicly reporting that a physician does not meet the MOC requirements," said Dr. R. Mack Harrell, AACE’s president, in that letter.
Meanwhile, 3,000 or so physicians have signed on to a "Pledge of Non-Compliance" with the ABIM’s requirements. The pledge was organized by Dr. Paul Teirstein, chief of cardiology and director of interventional cardiology at the Scripps Clinic, La Jolla, Calif., and his colleagues who have formed Physicians for Certification Change, an anti-MOC organization. Dr. Teirstein also launched a petition drive in March to overhaul the MOC process. That petition had about 18,000 signees at press time.
There are several other organizations hoping to derail or significantly change the ABIM’s requirements, including the Association of American Physicians and Surgeons, Change Board Recertification, and Docs4PatientCare.
In addition, many of the 26 professional societies whose members are certified by the ABIM have also been very organized in presenting their discontent.
The ABIM understands the frustration, said Dr. Richard Baron, ABIM president and CEO, in an interview. "One of the things that people have said is that we’re not listening," he said. That is one reason why the ABIM decided to hold what he called a "listening session" on July 15. "We’re a learning organization," he added, noting that the ABIM will "continue to evolve the program," based on the feedback it receives from individuals and from professional societies, among others.
Dr. Baron also acknowledges that by presenting an entirely new MOC process this year, "our timing was not propitious," given the many challenges physicians now face. The ABIM outlined those pressures in the July 28 letter, counting among them "dealing with the Affordable Care Act," fulfilling meaningful use requirements, and responding to changes in payment and practice models, including team-based care.
Dr. Steven E. Weinberger, executive vice president and CEO of the ACP agrees that the ABIM’s timing was pretty poor. He said that there had been a "low to medium level of concern" about MOC for years, but the new requirements announced in January catalyzed the complaints and were "an important tipping point." Added Dr. Weinberger, "To some extent, this was in part the straw that broke the camel’s back."
The ABIM is making a handful of changes to address some of the concerns, including "broadening the kinds of educational activities that can count for self-assessment of knowledge (Part 2)," said Dr. Baron. That is, the ABIM will be broadening the continuing medical education that will count toward the MOC requirements.
There have also been a huge number of complaints about the self-evaluation of practice assessment. There will be less direct data collection by the ABIM and more of a focus "on improvement activities doctors may already be doing in their practice," Dr. Baron said.
Among other things, ABIM is also looking at changes to the secure exam and whether it needs to change how it describes on its website whether physicians are meeting MOC requirements.
Critics are still not satisfied.
"This is nothing more than offering band-aids for gaping wounds," said Dr. Jonathan Weiss, an internist in Middletown, N.Y. "This is the ABIM trying to throw some bones to quiet down the rabble, given the unexpectedly strong pushback they are trying to deal with," he said, adding that he believes that the current process "is too flawed to be fixed."
Dr. Ron Benbassat, an internist in Beverly Hills, Calif., and a founder of Change Board Certification, goes even further. "No one is drinking the ABIM’s Kool-Aid," he said. "We’re on the right side of the truth here, and they know it," he said, adding, "The momentum is increasing and I believe we’re reaching the tipping point. As to what form it will take – widespread noncompliance or political or legal – I don’t have the answer. But we’re getting there."
Dr. Teirstein was a bit more muted in his criticism. "They are certainly taking many good steps. But, the devil will be in the details." He, like many physicians, said that he still sees the ABIM’s fees as a big problem. "To reestablish credibility, ABIM will need to roll back its fee schedule," said Dr. Teirstein.
The ABIM charges $1,940 for a 10-year basic internal medicine certification, which includes a secure examination for each specialty the diplomate chooses to maintain, access to all ABIM self-evaluation products, and any CME credit a physician can claim through completion of an ABIM module. There are basically four requirements: MOC participants take a secure exam; they have to complete a self-assessment of knowledge; they have to assess their practice by applying quality measurement and using the resulting data to improve their practice; and they must assess patient safety and/or include the "patient voice," which could, for instance, consist of a survey of patient satisfaction.
The fee is $2,060 for a focused practice in hospital medicine for 10 years, and $2,560 for any subspecialty for the 10 years.
But it’s more than just a financial cost, say physicians. There’s also an opportunity cost, said Dr. Eric Green, chair of the MOC Task Force at the Society of General Internal Medicine. "When you are doing MOC, what are you not doing?" he asks.
"It’s about a process that seems arbitrary, a little bit burdensome, and perhaps not achieving what we all want, which is the best care for our patients," said Dr. Christopher White, professor and chairman of medicine, the Ochsner Medical Center, New Orleans, in an interview.
Dr. White, who is a founding member of Physicians for Certification Change, has signed the Pledge of Non-Compliance. He is currently certified, and at age 63, wonders if it’s worth the time and effort to recertify in 2019, when he is next due. "It is a good idea that we continually educate physicians and that physicians have continuous improvement," said Dr. White, but he said he questions whether the ABIM process is the best way. "There’s no evidence that this works, or there’s value," he said.
The American College of Cardiology has offered to partner with the ABIM in getting some answers to the evidence question, said ACC President Patrick O’Gara in an interview. "I’m hopeful that our offer to partner to do research will be taken seriously," he said. Although more evidence will "go a long way toward answering this question about relevance," it would be a long-term project, said Dr. O’Gara, who is also director of clinical cardiology at Brigham and Women’s Hospital, Boston.
The ABIM said in its July 28 letter that extensive evidence does exist – with more than half of the studies coming from non-ABIM researchers – but that maybe it hasn’t done the best job of communicating that to diplomates. However, it does say it welcomes partnering on new projects "and a broader discussion" of potential research agendas.
Many say that they are in favor of requiring lifelong learning, but that the ABIM has gone about it the wrong way.
The ACC would like to find a way "to modify the methodology" ABIM is using to achieve that goal, Dr. O’Gara said. Cost is also a big concern for cardiologists, and especially subspecialists, he said. An interventional cardiologist, for instance, not only has to pay for and pass the general cardiology exam, but also a secure board exam in the subspecialty. In an ACC survey this spring, 90% of the 4,400 respondents said they were concerned about the cost of MOC.
The Society for Cardiovascular Angiography and Interventions (SCAI) is concerned about the impact on its members, too, and has formed a work group to "develop an action plan to spur MOC reform," according to a statement. "We believe that maintaining physician competence is of the highest importance," said Dr. Alan Yeung, chair of the MOC Working Group, in the statement. "It is the current process and products for facilitating maintenance of board certification that are not acceptable."
"The problem is not the lofty goals of maintenance of certification, it’s the implementation of it," agreed Dr. Green. The SGIM is also concerned about the one-size-fits-all approach that ABIM seems to be using. The SGIM has a lot of members who are administrators or physician scientists, and the MOC modules as constructed currently aren’t necessarily meaningful to their practice, said Dr. Green, who is also the program director for the internal medicine residency at Mercy Catholic Medical Center, Philadelphia.
Dr. Weinberger of the ACP said that his organization also wants to see more customization of the secure board exam "so that it’s more relevant to a particular physician’s practice."
Many who attended the ABIM’s July 15 meeting said that they felt like the organization had heard their concerns. But they are still waiting to see what will happen next.
The changes already announced "are a very good start," said Dr. Weinberger, who said that the ACP hopes that the ABIM board might make more changes at its meeting in August. "This is clearly a work in evolution," he said, adding that the ACP would continue to work with ABIM.
Likewise, Dr. Green said that the SGIM would continue to work in cooperation with the ABIM. The question is how much the ABIM will use the input from the subspecialty societies, he said.
"I left the [July 15] meeting with the impression that they are generally interested in receiving this information, in processing it, and in determining with their board what’s the best pathway forward," said Dr. O’Gara.
Dr. White, however, is a bit more skeptical. "I think the ABIM is going to have to be a lot less arrogant," he said, calling for more accountability by the organization.
ABIM’s changes to MOC
The new set of changes to the maintenance of certification process announced by the American Board of Internal Medicine represented the first time the organization had responded to the many concerns being voiced by individual physicians and representatives of the 26 societies that receive certification through the ABIM.
The changes were contained in a July 28 letter. Among other things, the ABIM said that it will be more transparent in how it governs, and how it reports its income and expenses, and that it will set up a more formal process for communication among those specialty societies and the ABIM’s boards. The group also said that in August, its board will "discuss website language for ‘meeting MOC requirements’."
The ABIM agreed with a recommendation from the American College of Physicians and 14 other medical societies that it should convene a conference dedicated to addressing the concerns about MOC and that it should focus on working with various societies to identify problems and solutions.
The organization also said it is open to research projects that can help determine whether MOC has any impact on the physician workforce.
Some other changes:
• The ABIM is streamlining the process for validating products from other organizations that can be eligible for credit toward Part 2 (medical knowledge). The ABIM will accept products that are Accreditation Council for Continuing Medical Education–accredited, and it will align its standards with the American Medical Association’s PRA "Category 1 standards, journal-based continuing medical education (CME), test item writing, and Internet point of care learning." The revised program is expected to be available later in 2014.
• If a diplomate takes an exam before his due date and fails, he will have an additional year to pass before being reported as "not certified" or "not meeting MOC requirements."
• Each ABIM specialty board will decide whether underlying certifications are required in each tertiary specialty and conjoint board. A decision is expected by 2015.
• The ABIM acknowledges that the "patient survey" requirement has been confusing. The name will be changed to the "patient voice" and will be designed to ensure that physicians are doing their best to incorporate that voice into their work. There will be four pathways for completing the requirement, including a survey. Specialty boards will determine which options are the most appropriate for each specialty. The deadline for the completion of the patient voice requirement is 2018.
• The organization said it will look into options for discounting its fees for diplomates who want to complete some of their MOC requirements with other groups. However, it said that it might not be able to offer a very deep discount. "We will commit to researching and brainstorming other fee structures," said the ABIM.
On Twitter @aliciaault
The American Board of Internal Medicine is making several changes to its maintenance of certification requirements, but, so far, it is not enough to quell the still-growing tide of anger and resentment against the process.
The ABIM announced the changes just ahead of a July 15 "summit" with 26 specialty societies that receive certification through the organization, and outlined them in a detailed letter that was sent to internal medicine diplomates on July 28. That correspondence also responded in detail to a May 7 letter sent by the American College of Physicians (ACP) on behalf of 14 medical societies, enumerating their concerns about the process.
The Endocrine Society, which attended the July 15 meeting, said that it left with many of the concerns it came in with. In a statement issued shortly thereafter, the Society called on the ABIM to suspend the maintenance of certification (MOC) requirements until it conducted "a formal analysis of all possible unintended consequences of the new MOC requirements."
Among the unintended consequences it says might result: If endocrinologists have to spend more time on MOC, they might spend less time with patients or on research, or they may even leave the field all together. That will put a dent in access to care when there is an increasing need, according to a letter from the Society to the ABIM in early June.
The American Association of Clinical Endocrinologists (AACE) also warned ABIM that by "monopolizing physician time," MOC could end up harming patient care. The group also said that many endocrinologists who previously did not have to recertify (so-called "grandfathers") might leave practice rather than go through the current process. In a letter to ABIM in late June, AACE also called on the Board to suspend the new requirements until there was a better understanding of how it might affect the endocrinology workforce. The ABIM should also "refrain from publicly reporting that a physician does not meet the MOC requirements," said Dr. R. Mack Harrell, AACE’s president, in that letter.
Meanwhile, 3,000 or so physicians have signed on to a "Pledge of Non-Compliance" with the ABIM’s requirements. The pledge was organized by Dr. Paul Teirstein, chief of cardiology and director of interventional cardiology at the Scripps Clinic, La Jolla, Calif., and his colleagues who have formed Physicians for Certification Change, an anti-MOC organization. Dr. Teirstein also launched a petition drive in March to overhaul the MOC process. That petition had about 18,000 signees at press time.
There are several other organizations hoping to derail or significantly change the ABIM’s requirements, including the Association of American Physicians and Surgeons, Change Board Recertification, and Docs4PatientCare.
In addition, many of the 26 professional societies whose members are certified by the ABIM have also been very organized in presenting their discontent.
The ABIM understands the frustration, said Dr. Richard Baron, ABIM president and CEO, in an interview. "One of the things that people have said is that we’re not listening," he said. That is one reason why the ABIM decided to hold what he called a "listening session" on July 15. "We’re a learning organization," he added, noting that the ABIM will "continue to evolve the program," based on the feedback it receives from individuals and from professional societies, among others.
Dr. Baron also acknowledges that by presenting an entirely new MOC process this year, "our timing was not propitious," given the many challenges physicians now face. The ABIM outlined those pressures in the July 28 letter, counting among them "dealing with the Affordable Care Act," fulfilling meaningful use requirements, and responding to changes in payment and practice models, including team-based care.
Dr. Steven E. Weinberger, executive vice president and CEO of the ACP agrees that the ABIM’s timing was pretty poor. He said that there had been a "low to medium level of concern" about MOC for years, but the new requirements announced in January catalyzed the complaints and were "an important tipping point." Added Dr. Weinberger, "To some extent, this was in part the straw that broke the camel’s back."
The ABIM is making a handful of changes to address some of the concerns, including "broadening the kinds of educational activities that can count for self-assessment of knowledge (Part 2)," said Dr. Baron. That is, the ABIM will be broadening the continuing medical education that will count toward the MOC requirements.
There have also been a huge number of complaints about the self-evaluation of practice assessment. There will be less direct data collection by the ABIM and more of a focus "on improvement activities doctors may already be doing in their practice," Dr. Baron said.
Among other things, ABIM is also looking at changes to the secure exam and whether it needs to change how it describes on its website whether physicians are meeting MOC requirements.
Critics are still not satisfied.
"This is nothing more than offering band-aids for gaping wounds," said Dr. Jonathan Weiss, an internist in Middletown, N.Y. "This is the ABIM trying to throw some bones to quiet down the rabble, given the unexpectedly strong pushback they are trying to deal with," he said, adding that he believes that the current process "is too flawed to be fixed."
Dr. Ron Benbassat, an internist in Beverly Hills, Calif., and a founder of Change Board Certification, goes even further. "No one is drinking the ABIM’s Kool-Aid," he said. "We’re on the right side of the truth here, and they know it," he said, adding, "The momentum is increasing and I believe we’re reaching the tipping point. As to what form it will take – widespread noncompliance or political or legal – I don’t have the answer. But we’re getting there."
Dr. Teirstein was a bit more muted in his criticism. "They are certainly taking many good steps. But, the devil will be in the details." He, like many physicians, said that he still sees the ABIM’s fees as a big problem. "To reestablish credibility, ABIM will need to roll back its fee schedule," said Dr. Teirstein.
The ABIM charges $1,940 for a 10-year basic internal medicine certification, which includes a secure examination for each specialty the diplomate chooses to maintain, access to all ABIM self-evaluation products, and any CME credit a physician can claim through completion of an ABIM module. There are basically four requirements: MOC participants take a secure exam; they have to complete a self-assessment of knowledge; they have to assess their practice by applying quality measurement and using the resulting data to improve their practice; and they must assess patient safety and/or include the "patient voice," which could, for instance, consist of a survey of patient satisfaction.
The fee is $2,060 for a focused practice in hospital medicine for 10 years, and $2,560 for any subspecialty for the 10 years.
But it’s more than just a financial cost, say physicians. There’s also an opportunity cost, said Dr. Eric Green, chair of the MOC Task Force at the Society of General Internal Medicine. "When you are doing MOC, what are you not doing?" he asks.
"It’s about a process that seems arbitrary, a little bit burdensome, and perhaps not achieving what we all want, which is the best care for our patients," said Dr. Christopher White, professor and chairman of medicine, the Ochsner Medical Center, New Orleans, in an interview.
Dr. White, who is a founding member of Physicians for Certification Change, has signed the Pledge of Non-Compliance. He is currently certified, and at age 63, wonders if it’s worth the time and effort to recertify in 2019, when he is next due. "It is a good idea that we continually educate physicians and that physicians have continuous improvement," said Dr. White, but he said he questions whether the ABIM process is the best way. "There’s no evidence that this works, or there’s value," he said.
The American College of Cardiology has offered to partner with the ABIM in getting some answers to the evidence question, said ACC President Patrick O’Gara in an interview. "I’m hopeful that our offer to partner to do research will be taken seriously," he said. Although more evidence will "go a long way toward answering this question about relevance," it would be a long-term project, said Dr. O’Gara, who is also director of clinical cardiology at Brigham and Women’s Hospital, Boston.
The ABIM said in its July 28 letter that extensive evidence does exist – with more than half of the studies coming from non-ABIM researchers – but that maybe it hasn’t done the best job of communicating that to diplomates. However, it does say it welcomes partnering on new projects "and a broader discussion" of potential research agendas.
Many say that they are in favor of requiring lifelong learning, but that the ABIM has gone about it the wrong way.
The ACC would like to find a way "to modify the methodology" ABIM is using to achieve that goal, Dr. O’Gara said. Cost is also a big concern for cardiologists, and especially subspecialists, he said. An interventional cardiologist, for instance, not only has to pay for and pass the general cardiology exam, but also a secure board exam in the subspecialty. In an ACC survey this spring, 90% of the 4,400 respondents said they were concerned about the cost of MOC.
The Society for Cardiovascular Angiography and Interventions (SCAI) is concerned about the impact on its members, too, and has formed a work group to "develop an action plan to spur MOC reform," according to a statement. "We believe that maintaining physician competence is of the highest importance," said Dr. Alan Yeung, chair of the MOC Working Group, in the statement. "It is the current process and products for facilitating maintenance of board certification that are not acceptable."
"The problem is not the lofty goals of maintenance of certification, it’s the implementation of it," agreed Dr. Green. The SGIM is also concerned about the one-size-fits-all approach that ABIM seems to be using. The SGIM has a lot of members who are administrators or physician scientists, and the MOC modules as constructed currently aren’t necessarily meaningful to their practice, said Dr. Green, who is also the program director for the internal medicine residency at Mercy Catholic Medical Center, Philadelphia.
Dr. Weinberger of the ACP said that his organization also wants to see more customization of the secure board exam "so that it’s more relevant to a particular physician’s practice."
Many who attended the ABIM’s July 15 meeting said that they felt like the organization had heard their concerns. But they are still waiting to see what will happen next.
The changes already announced "are a very good start," said Dr. Weinberger, who said that the ACP hopes that the ABIM board might make more changes at its meeting in August. "This is clearly a work in evolution," he said, adding that the ACP would continue to work with ABIM.
Likewise, Dr. Green said that the SGIM would continue to work in cooperation with the ABIM. The question is how much the ABIM will use the input from the subspecialty societies, he said.
"I left the [July 15] meeting with the impression that they are generally interested in receiving this information, in processing it, and in determining with their board what’s the best pathway forward," said Dr. O’Gara.
Dr. White, however, is a bit more skeptical. "I think the ABIM is going to have to be a lot less arrogant," he said, calling for more accountability by the organization.
ABIM’s changes to MOC
The new set of changes to the maintenance of certification process announced by the American Board of Internal Medicine represented the first time the organization had responded to the many concerns being voiced by individual physicians and representatives of the 26 societies that receive certification through the ABIM.
The changes were contained in a July 28 letter. Among other things, the ABIM said that it will be more transparent in how it governs, and how it reports its income and expenses, and that it will set up a more formal process for communication among those specialty societies and the ABIM’s boards. The group also said that in August, its board will "discuss website language for ‘meeting MOC requirements’."
The ABIM agreed with a recommendation from the American College of Physicians and 14 other medical societies that it should convene a conference dedicated to addressing the concerns about MOC and that it should focus on working with various societies to identify problems and solutions.
The organization also said it is open to research projects that can help determine whether MOC has any impact on the physician workforce.
Some other changes:
• The ABIM is streamlining the process for validating products from other organizations that can be eligible for credit toward Part 2 (medical knowledge). The ABIM will accept products that are Accreditation Council for Continuing Medical Education–accredited, and it will align its standards with the American Medical Association’s PRA "Category 1 standards, journal-based continuing medical education (CME), test item writing, and Internet point of care learning." The revised program is expected to be available later in 2014.
• If a diplomate takes an exam before his due date and fails, he will have an additional year to pass before being reported as "not certified" or "not meeting MOC requirements."
• Each ABIM specialty board will decide whether underlying certifications are required in each tertiary specialty and conjoint board. A decision is expected by 2015.
• The ABIM acknowledges that the "patient survey" requirement has been confusing. The name will be changed to the "patient voice" and will be designed to ensure that physicians are doing their best to incorporate that voice into their work. There will be four pathways for completing the requirement, including a survey. Specialty boards will determine which options are the most appropriate for each specialty. The deadline for the completion of the patient voice requirement is 2018.
• The organization said it will look into options for discounting its fees for diplomates who want to complete some of their MOC requirements with other groups. However, it said that it might not be able to offer a very deep discount. "We will commit to researching and brainstorming other fee structures," said the ABIM.
On Twitter @aliciaault
The American Board of Internal Medicine is making several changes to its maintenance of certification requirements, but, so far, it is not enough to quell the still-growing tide of anger and resentment against the process.
The ABIM announced the changes just ahead of a July 15 "summit" with 26 specialty societies that receive certification through the organization, and outlined them in a detailed letter that was sent to internal medicine diplomates on July 28. That correspondence also responded in detail to a May 7 letter sent by the American College of Physicians (ACP) on behalf of 14 medical societies, enumerating their concerns about the process.
The Endocrine Society, which attended the July 15 meeting, said that it left with many of the concerns it came in with. In a statement issued shortly thereafter, the Society called on the ABIM to suspend the maintenance of certification (MOC) requirements until it conducted "a formal analysis of all possible unintended consequences of the new MOC requirements."
Among the unintended consequences it says might result: If endocrinologists have to spend more time on MOC, they might spend less time with patients or on research, or they may even leave the field all together. That will put a dent in access to care when there is an increasing need, according to a letter from the Society to the ABIM in early June.
The American Association of Clinical Endocrinologists (AACE) also warned ABIM that by "monopolizing physician time," MOC could end up harming patient care. The group also said that many endocrinologists who previously did not have to recertify (so-called "grandfathers") might leave practice rather than go through the current process. In a letter to ABIM in late June, AACE also called on the Board to suspend the new requirements until there was a better understanding of how it might affect the endocrinology workforce. The ABIM should also "refrain from publicly reporting that a physician does not meet the MOC requirements," said Dr. R. Mack Harrell, AACE’s president, in that letter.
Meanwhile, 3,000 or so physicians have signed on to a "Pledge of Non-Compliance" with the ABIM’s requirements. The pledge was organized by Dr. Paul Teirstein, chief of cardiology and director of interventional cardiology at the Scripps Clinic, La Jolla, Calif., and his colleagues who have formed Physicians for Certification Change, an anti-MOC organization. Dr. Teirstein also launched a petition drive in March to overhaul the MOC process. That petition had about 18,000 signees at press time.
There are several other organizations hoping to derail or significantly change the ABIM’s requirements, including the Association of American Physicians and Surgeons, Change Board Recertification, and Docs4PatientCare.
In addition, many of the 26 professional societies whose members are certified by the ABIM have also been very organized in presenting their discontent.
The ABIM understands the frustration, said Dr. Richard Baron, ABIM president and CEO, in an interview. "One of the things that people have said is that we’re not listening," he said. That is one reason why the ABIM decided to hold what he called a "listening session" on July 15. "We’re a learning organization," he added, noting that the ABIM will "continue to evolve the program," based on the feedback it receives from individuals and from professional societies, among others.
Dr. Baron also acknowledges that by presenting an entirely new MOC process this year, "our timing was not propitious," given the many challenges physicians now face. The ABIM outlined those pressures in the July 28 letter, counting among them "dealing with the Affordable Care Act," fulfilling meaningful use requirements, and responding to changes in payment and practice models, including team-based care.
Dr. Steven E. Weinberger, executive vice president and CEO of the ACP agrees that the ABIM’s timing was pretty poor. He said that there had been a "low to medium level of concern" about MOC for years, but the new requirements announced in January catalyzed the complaints and were "an important tipping point." Added Dr. Weinberger, "To some extent, this was in part the straw that broke the camel’s back."
The ABIM is making a handful of changes to address some of the concerns, including "broadening the kinds of educational activities that can count for self-assessment of knowledge (Part 2)," said Dr. Baron. That is, the ABIM will be broadening the continuing medical education that will count toward the MOC requirements.
There have also been a huge number of complaints about the self-evaluation of practice assessment. There will be less direct data collection by the ABIM and more of a focus "on improvement activities doctors may already be doing in their practice," Dr. Baron said.
Among other things, ABIM is also looking at changes to the secure exam and whether it needs to change how it describes on its website whether physicians are meeting MOC requirements.
Critics are still not satisfied.
"This is nothing more than offering band-aids for gaping wounds," said Dr. Jonathan Weiss, an internist in Middletown, N.Y. "This is the ABIM trying to throw some bones to quiet down the rabble, given the unexpectedly strong pushback they are trying to deal with," he said, adding that he believes that the current process "is too flawed to be fixed."
Dr. Ron Benbassat, an internist in Beverly Hills, Calif., and a founder of Change Board Certification, goes even further. "No one is drinking the ABIM’s Kool-Aid," he said. "We’re on the right side of the truth here, and they know it," he said, adding, "The momentum is increasing and I believe we’re reaching the tipping point. As to what form it will take – widespread noncompliance or political or legal – I don’t have the answer. But we’re getting there."
Dr. Teirstein was a bit more muted in his criticism. "They are certainly taking many good steps. But, the devil will be in the details." He, like many physicians, said that he still sees the ABIM’s fees as a big problem. "To reestablish credibility, ABIM will need to roll back its fee schedule," said Dr. Teirstein.
The ABIM charges $1,940 for a 10-year basic internal medicine certification, which includes a secure examination for each specialty the diplomate chooses to maintain, access to all ABIM self-evaluation products, and any CME credit a physician can claim through completion of an ABIM module. There are basically four requirements: MOC participants take a secure exam; they have to complete a self-assessment of knowledge; they have to assess their practice by applying quality measurement and using the resulting data to improve their practice; and they must assess patient safety and/or include the "patient voice," which could, for instance, consist of a survey of patient satisfaction.
The fee is $2,060 for a focused practice in hospital medicine for 10 years, and $2,560 for any subspecialty for the 10 years.
But it’s more than just a financial cost, say physicians. There’s also an opportunity cost, said Dr. Eric Green, chair of the MOC Task Force at the Society of General Internal Medicine. "When you are doing MOC, what are you not doing?" he asks.
"It’s about a process that seems arbitrary, a little bit burdensome, and perhaps not achieving what we all want, which is the best care for our patients," said Dr. Christopher White, professor and chairman of medicine, the Ochsner Medical Center, New Orleans, in an interview.
Dr. White, who is a founding member of Physicians for Certification Change, has signed the Pledge of Non-Compliance. He is currently certified, and at age 63, wonders if it’s worth the time and effort to recertify in 2019, when he is next due. "It is a good idea that we continually educate physicians and that physicians have continuous improvement," said Dr. White, but he said he questions whether the ABIM process is the best way. "There’s no evidence that this works, or there’s value," he said.
The American College of Cardiology has offered to partner with the ABIM in getting some answers to the evidence question, said ACC President Patrick O’Gara in an interview. "I’m hopeful that our offer to partner to do research will be taken seriously," he said. Although more evidence will "go a long way toward answering this question about relevance," it would be a long-term project, said Dr. O’Gara, who is also director of clinical cardiology at Brigham and Women’s Hospital, Boston.
The ABIM said in its July 28 letter that extensive evidence does exist – with more than half of the studies coming from non-ABIM researchers – but that maybe it hasn’t done the best job of communicating that to diplomates. However, it does say it welcomes partnering on new projects "and a broader discussion" of potential research agendas.
Many say that they are in favor of requiring lifelong learning, but that the ABIM has gone about it the wrong way.
The ACC would like to find a way "to modify the methodology" ABIM is using to achieve that goal, Dr. O’Gara said. Cost is also a big concern for cardiologists, and especially subspecialists, he said. An interventional cardiologist, for instance, not only has to pay for and pass the general cardiology exam, but also a secure board exam in the subspecialty. In an ACC survey this spring, 90% of the 4,400 respondents said they were concerned about the cost of MOC.
The Society for Cardiovascular Angiography and Interventions (SCAI) is concerned about the impact on its members, too, and has formed a work group to "develop an action plan to spur MOC reform," according to a statement. "We believe that maintaining physician competence is of the highest importance," said Dr. Alan Yeung, chair of the MOC Working Group, in the statement. "It is the current process and products for facilitating maintenance of board certification that are not acceptable."
"The problem is not the lofty goals of maintenance of certification, it’s the implementation of it," agreed Dr. Green. The SGIM is also concerned about the one-size-fits-all approach that ABIM seems to be using. The SGIM has a lot of members who are administrators or physician scientists, and the MOC modules as constructed currently aren’t necessarily meaningful to their practice, said Dr. Green, who is also the program director for the internal medicine residency at Mercy Catholic Medical Center, Philadelphia.
Dr. Weinberger of the ACP said that his organization also wants to see more customization of the secure board exam "so that it’s more relevant to a particular physician’s practice."
Many who attended the ABIM’s July 15 meeting said that they felt like the organization had heard their concerns. But they are still waiting to see what will happen next.
The changes already announced "are a very good start," said Dr. Weinberger, who said that the ACP hopes that the ABIM board might make more changes at its meeting in August. "This is clearly a work in evolution," he said, adding that the ACP would continue to work with ABIM.
Likewise, Dr. Green said that the SGIM would continue to work in cooperation with the ABIM. The question is how much the ABIM will use the input from the subspecialty societies, he said.
"I left the [July 15] meeting with the impression that they are generally interested in receiving this information, in processing it, and in determining with their board what’s the best pathway forward," said Dr. O’Gara.
Dr. White, however, is a bit more skeptical. "I think the ABIM is going to have to be a lot less arrogant," he said, calling for more accountability by the organization.
ABIM’s changes to MOC
The new set of changes to the maintenance of certification process announced by the American Board of Internal Medicine represented the first time the organization had responded to the many concerns being voiced by individual physicians and representatives of the 26 societies that receive certification through the ABIM.
The changes were contained in a July 28 letter. Among other things, the ABIM said that it will be more transparent in how it governs, and how it reports its income and expenses, and that it will set up a more formal process for communication among those specialty societies and the ABIM’s boards. The group also said that in August, its board will "discuss website language for ‘meeting MOC requirements’."
The ABIM agreed with a recommendation from the American College of Physicians and 14 other medical societies that it should convene a conference dedicated to addressing the concerns about MOC and that it should focus on working with various societies to identify problems and solutions.
The organization also said it is open to research projects that can help determine whether MOC has any impact on the physician workforce.
Some other changes:
• The ABIM is streamlining the process for validating products from other organizations that can be eligible for credit toward Part 2 (medical knowledge). The ABIM will accept products that are Accreditation Council for Continuing Medical Education–accredited, and it will align its standards with the American Medical Association’s PRA "Category 1 standards, journal-based continuing medical education (CME), test item writing, and Internet point of care learning." The revised program is expected to be available later in 2014.
• If a diplomate takes an exam before his due date and fails, he will have an additional year to pass before being reported as "not certified" or "not meeting MOC requirements."
• Each ABIM specialty board will decide whether underlying certifications are required in each tertiary specialty and conjoint board. A decision is expected by 2015.
• The ABIM acknowledges that the "patient survey" requirement has been confusing. The name will be changed to the "patient voice" and will be designed to ensure that physicians are doing their best to incorporate that voice into their work. There will be four pathways for completing the requirement, including a survey. Specialty boards will determine which options are the most appropriate for each specialty. The deadline for the completion of the patient voice requirement is 2018.
• The organization said it will look into options for discounting its fees for diplomates who want to complete some of their MOC requirements with other groups. However, it said that it might not be able to offer a very deep discount. "We will commit to researching and brainstorming other fee structures," said the ABIM.
On Twitter @aliciaault
Health sector well represented among lobbying top spenders
Four of the 10 largest spenders on lobbying for the first 6 months of 2014 are part of the health sector, the Center for Responsive Politics reported.
Blue Cross/Blue Shield was in third place overall, spending almost $11.3 million through the end of June, while the American Medical Association was fourth at $10.5 million, the American Hospital Association was seventh at $9.7 million, and PhRMA (Pharmaceutical Research and Manufacturers of America) was ninth with spending of just under $8.9 million, according to the center.
The U.S. Chamber of Commerce far outdistanced the health sector, however, putting $54.3 million into its lobbying efforts in the first 6 months of 2014 – about $11 million more than the four health-sector representatives combined. The National Association of Realtors was the second-largest lobbyer, spending $23.6 million, the center’s lobbying data showed.
The data on the center’s OpenSecrets website are compiled using lobbying disclosure reports filed with the Secretary of the Senate’s Office of Public Records.

Four of the 10 largest spenders on lobbying for the first 6 months of 2014 are part of the health sector, the Center for Responsive Politics reported.
Blue Cross/Blue Shield was in third place overall, spending almost $11.3 million through the end of June, while the American Medical Association was fourth at $10.5 million, the American Hospital Association was seventh at $9.7 million, and PhRMA (Pharmaceutical Research and Manufacturers of America) was ninth with spending of just under $8.9 million, according to the center.
The U.S. Chamber of Commerce far outdistanced the health sector, however, putting $54.3 million into its lobbying efforts in the first 6 months of 2014 – about $11 million more than the four health-sector representatives combined. The National Association of Realtors was the second-largest lobbyer, spending $23.6 million, the center’s lobbying data showed.
The data on the center’s OpenSecrets website are compiled using lobbying disclosure reports filed with the Secretary of the Senate’s Office of Public Records.

Four of the 10 largest spenders on lobbying for the first 6 months of 2014 are part of the health sector, the Center for Responsive Politics reported.
Blue Cross/Blue Shield was in third place overall, spending almost $11.3 million through the end of June, while the American Medical Association was fourth at $10.5 million, the American Hospital Association was seventh at $9.7 million, and PhRMA (Pharmaceutical Research and Manufacturers of America) was ninth with spending of just under $8.9 million, according to the center.
The U.S. Chamber of Commerce far outdistanced the health sector, however, putting $54.3 million into its lobbying efforts in the first 6 months of 2014 – about $11 million more than the four health-sector representatives combined. The National Association of Realtors was the second-largest lobbyer, spending $23.6 million, the center’s lobbying data showed.
The data on the center’s OpenSecrets website are compiled using lobbying disclosure reports filed with the Secretary of the Senate’s Office of Public Records.

Big data destined for the bedside within 5 years
Big data shows the promise to transform how health care is delivered, utilizing real-time information to assist doctors and patients in making more informed decisions at the point of care, a transformation that could arrive in the next 5 years.
That time prediction was offered by Dr. Harlan Krumholz, a professor at Yale University, New Haven, Conn.
"We are on the cusp of dramatic change in the way in which we are thinking about leveraging data that’s being generated in everyday clinical practice and in everyday daily life," Dr. Krumholz said in an interview. "I think there’s this recognition that the current clinical scientific enterprise is inadequate to keep up with information needs."
But getting to that point is going to require a shift in the research culture and how that information is brought to doctors as they are treating patients.
"One of the biggest problems with our current research approach is it’s very reductionist," Dr. Krumholz observed. "We try to pursue the scientific method with testing hypotheses. We try to pull out all the complexity around the question we are trying to answer and simplify everything so we can cleanly look at a particular effect of one thing on patients. ... By the time [research results] come out, they are very anachronistic because medicine’s progressed and the patients that they’re studying aren’t exactly the people who are being seen in practice, so the studies aren’t necessarily having the effect they could have."
Dr. Krumholz also is a member of the Patient-Centered Outcomes Research Institute board of governors. The organization is building a research network that will facilitate the gathering and usage of big data to help inform patient decisions.
But it’s more than just health-specific data that need to be incorporated into big data analysis.
"It’s not just the pill, but it’s the pill for a specific patient who’s got a specific profile, who is in a certain social situation, who is taking other medication, maybe has certain access to physicians, and a lot of complicated issues coming together," Dr. Krumholz continued. "We need to be able to learn from everyday experience and we need to embrace the complexity, not reject it, so that our studies are taking into account the complex aspects of medicine, not trying to get rid of them so we have clean studies that may not well-relate back to the real-world situations doctors and patients face everyday."
The next step will be to find something that can truly demonstrate the power of big data.
"I think that once we break the dam open and allow the water to flow and turn the turbines of knowledge generation, people are going to demand better and better information in order to be in a stronger position to make decisions," he predicted. "I think the decision support enterprise, that community of people who are working on decision support tools, is going to start competing to develop and create these things that are going to be at the bedside, that is going to make a whole lot of difference from where we are now."
A number of pilots and areas where big data could have an immediate impact were highlighted in a recent article in the July issue of Health Affairs (doi: 10.1377/hlthaff.2014.0041). For example, Kaiser Permanente Northern California; Harvard University, Boston; and the University of California, San Francisco and Santa Cruz, are pilot testing a two-step protocol aimed at reducing antibiotic prescriptions for newborns by using objective maternal data to determine a preliminary probability for early-onset sepsis. The second step uses a set of clinical findings combined with the estimate based on maternal data to yield a new posterior probability for risk of sepsis following birth. Kaiser anticipates that the combination of these two steps could lead to as many as 240,000 fewer U.S. newborns being treated with systemic antibiotics each year.
Lead author Dr. David Bates, chief of the division of general medicine at Brigham and Women’s Hospital, Boston, and others identified several key areas where big data could have an effect at the point of care, including identifying high-cost patients to more efficiently care for them; helping to reduce readmissions; lowering the risk of complications when a patient is first admitted to a hospital; determining the risk that a patient’s condition will worsen; and understanding the risk of adverse events for patients.
"The better we are at getting to good information about options and consequences [regarding] who’s about to get into trouble, the more people are going to demand [the use of big data,] and the race will be on to create the products that are underneath the hood that are leveraging large data," Dr. Krumholz said.
Big data shows the promise to transform how health care is delivered, utilizing real-time information to assist doctors and patients in making more informed decisions at the point of care, a transformation that could arrive in the next 5 years.
That time prediction was offered by Dr. Harlan Krumholz, a professor at Yale University, New Haven, Conn.
"We are on the cusp of dramatic change in the way in which we are thinking about leveraging data that’s being generated in everyday clinical practice and in everyday daily life," Dr. Krumholz said in an interview. "I think there’s this recognition that the current clinical scientific enterprise is inadequate to keep up with information needs."
But getting to that point is going to require a shift in the research culture and how that information is brought to doctors as they are treating patients.
"One of the biggest problems with our current research approach is it’s very reductionist," Dr. Krumholz observed. "We try to pursue the scientific method with testing hypotheses. We try to pull out all the complexity around the question we are trying to answer and simplify everything so we can cleanly look at a particular effect of one thing on patients. ... By the time [research results] come out, they are very anachronistic because medicine’s progressed and the patients that they’re studying aren’t exactly the people who are being seen in practice, so the studies aren’t necessarily having the effect they could have."
Dr. Krumholz also is a member of the Patient-Centered Outcomes Research Institute board of governors. The organization is building a research network that will facilitate the gathering and usage of big data to help inform patient decisions.
But it’s more than just health-specific data that need to be incorporated into big data analysis.
"It’s not just the pill, but it’s the pill for a specific patient who’s got a specific profile, who is in a certain social situation, who is taking other medication, maybe has certain access to physicians, and a lot of complicated issues coming together," Dr. Krumholz continued. "We need to be able to learn from everyday experience and we need to embrace the complexity, not reject it, so that our studies are taking into account the complex aspects of medicine, not trying to get rid of them so we have clean studies that may not well-relate back to the real-world situations doctors and patients face everyday."
The next step will be to find something that can truly demonstrate the power of big data.
"I think that once we break the dam open and allow the water to flow and turn the turbines of knowledge generation, people are going to demand better and better information in order to be in a stronger position to make decisions," he predicted. "I think the decision support enterprise, that community of people who are working on decision support tools, is going to start competing to develop and create these things that are going to be at the bedside, that is going to make a whole lot of difference from where we are now."
A number of pilots and areas where big data could have an immediate impact were highlighted in a recent article in the July issue of Health Affairs (doi: 10.1377/hlthaff.2014.0041). For example, Kaiser Permanente Northern California; Harvard University, Boston; and the University of California, San Francisco and Santa Cruz, are pilot testing a two-step protocol aimed at reducing antibiotic prescriptions for newborns by using objective maternal data to determine a preliminary probability for early-onset sepsis. The second step uses a set of clinical findings combined with the estimate based on maternal data to yield a new posterior probability for risk of sepsis following birth. Kaiser anticipates that the combination of these two steps could lead to as many as 240,000 fewer U.S. newborns being treated with systemic antibiotics each year.
Lead author Dr. David Bates, chief of the division of general medicine at Brigham and Women’s Hospital, Boston, and others identified several key areas where big data could have an effect at the point of care, including identifying high-cost patients to more efficiently care for them; helping to reduce readmissions; lowering the risk of complications when a patient is first admitted to a hospital; determining the risk that a patient’s condition will worsen; and understanding the risk of adverse events for patients.
"The better we are at getting to good information about options and consequences [regarding] who’s about to get into trouble, the more people are going to demand [the use of big data,] and the race will be on to create the products that are underneath the hood that are leveraging large data," Dr. Krumholz said.
Big data shows the promise to transform how health care is delivered, utilizing real-time information to assist doctors and patients in making more informed decisions at the point of care, a transformation that could arrive in the next 5 years.
That time prediction was offered by Dr. Harlan Krumholz, a professor at Yale University, New Haven, Conn.
"We are on the cusp of dramatic change in the way in which we are thinking about leveraging data that’s being generated in everyday clinical practice and in everyday daily life," Dr. Krumholz said in an interview. "I think there’s this recognition that the current clinical scientific enterprise is inadequate to keep up with information needs."
But getting to that point is going to require a shift in the research culture and how that information is brought to doctors as they are treating patients.
"One of the biggest problems with our current research approach is it’s very reductionist," Dr. Krumholz observed. "We try to pursue the scientific method with testing hypotheses. We try to pull out all the complexity around the question we are trying to answer and simplify everything so we can cleanly look at a particular effect of one thing on patients. ... By the time [research results] come out, they are very anachronistic because medicine’s progressed and the patients that they’re studying aren’t exactly the people who are being seen in practice, so the studies aren’t necessarily having the effect they could have."
Dr. Krumholz also is a member of the Patient-Centered Outcomes Research Institute board of governors. The organization is building a research network that will facilitate the gathering and usage of big data to help inform patient decisions.
But it’s more than just health-specific data that need to be incorporated into big data analysis.
"It’s not just the pill, but it’s the pill for a specific patient who’s got a specific profile, who is in a certain social situation, who is taking other medication, maybe has certain access to physicians, and a lot of complicated issues coming together," Dr. Krumholz continued. "We need to be able to learn from everyday experience and we need to embrace the complexity, not reject it, so that our studies are taking into account the complex aspects of medicine, not trying to get rid of them so we have clean studies that may not well-relate back to the real-world situations doctors and patients face everyday."
The next step will be to find something that can truly demonstrate the power of big data.
"I think that once we break the dam open and allow the water to flow and turn the turbines of knowledge generation, people are going to demand better and better information in order to be in a stronger position to make decisions," he predicted. "I think the decision support enterprise, that community of people who are working on decision support tools, is going to start competing to develop and create these things that are going to be at the bedside, that is going to make a whole lot of difference from where we are now."
A number of pilots and areas where big data could have an immediate impact were highlighted in a recent article in the July issue of Health Affairs (doi: 10.1377/hlthaff.2014.0041). For example, Kaiser Permanente Northern California; Harvard University, Boston; and the University of California, San Francisco and Santa Cruz, are pilot testing a two-step protocol aimed at reducing antibiotic prescriptions for newborns by using objective maternal data to determine a preliminary probability for early-onset sepsis. The second step uses a set of clinical findings combined with the estimate based on maternal data to yield a new posterior probability for risk of sepsis following birth. Kaiser anticipates that the combination of these two steps could lead to as many as 240,000 fewer U.S. newborns being treated with systemic antibiotics each year.
Lead author Dr. David Bates, chief of the division of general medicine at Brigham and Women’s Hospital, Boston, and others identified several key areas where big data could have an effect at the point of care, including identifying high-cost patients to more efficiently care for them; helping to reduce readmissions; lowering the risk of complications when a patient is first admitted to a hospital; determining the risk that a patient’s condition will worsen; and understanding the risk of adverse events for patients.
"The better we are at getting to good information about options and consequences [regarding] who’s about to get into trouble, the more people are going to demand [the use of big data,] and the race will be on to create the products that are underneath the hood that are leveraging large data," Dr. Krumholz said.
Questions surround EHR security
Despite government efforts to certify otherwise, questions remain as to whether the Dept. of Health & Human Services Office of the National Coordinator for Health Information Technology is doing enough to ensure that commercially available electronic health record software programs are doing enough to secure patient information.
Concerns were raised by HHS Office of Inspector General (OIG) in a report released Aug. 4. The agency watchdog examined certification work conducted by authorized testing and certification bodies (ATCBs), which early on in the meaningful use program certified that electronic health records (EHRs) met established criteria that would allow doctors and hospitals to obtain Medicare or Medicaid incentive payments.
According to the report, as of Aug. 30, 2013, a total of 3,590 certified EHRs were available to health care providers, 95% of which were certified by ATCBs under a temporary certification program.
In examining the work done by ATCBs, the OIG found that oversight by the HHS Office of the National Coordinator for Health Information Technology (ONC) "did not fully ensure that test procedures and standards could adequately secure and protect patient information contained in EHRs," the report states. OIG claimed that the health IT office did not ensure that ATCBs "developed procedures to periodically evaluate whether certified EHRs continued to meet Federal standards and developed a training program to ensure that their personnel were competent to test and certify EHRs and to secure proprietary or sensitive information."
OIG notes that the ATCB standards and procedures met all National Institute of Standards and Technology (NIST) test procedure requirements that the ONC approved, but those procedures "were not sufficient to ensure that EHRs would adequately secure and protect patient health information; in particular, the procedures allowed ATCBs to certify EHRs that demonstrated the use of a single-character password during testing." NIST procedures also did not address common security issues, including password complexity and logging emergency access or user privilege changes.
In response to the draft, included as part of the report, ONC noted that ATCBs are no longer active in the certification. New certification criteria approved earlier this year have "strengthened test procedures for common security and privacy features for inclusion in EHRs." Additionally, ONC has "substantially revised the ‘auditable events and tamper resistance’ certification criterion, and we adopted a new ‘end-user device encryption’ criterion," as well as other security capabilities, according to a spokesperson. ONC will review the OIG’s comments before determining the appropriate next steps, the spokesperson added.
However, the OIG does not agree that the current certification regulations "sufficiently address our security concerns regarding the Temporary Program," such as multifactor authentication.
OIG also criticized the health IT office for not addressing the authority to remove EHRs from the market that are shown to have privacy and security flaws.
If an EHR "is exploited and used to conduct malicious activities, ONC is not able to remove the EHR, even temporarily, from the product list to prevent further purchases of it."
Despite government efforts to certify otherwise, questions remain as to whether the Dept. of Health & Human Services Office of the National Coordinator for Health Information Technology is doing enough to ensure that commercially available electronic health record software programs are doing enough to secure patient information.
Concerns were raised by HHS Office of Inspector General (OIG) in a report released Aug. 4. The agency watchdog examined certification work conducted by authorized testing and certification bodies (ATCBs), which early on in the meaningful use program certified that electronic health records (EHRs) met established criteria that would allow doctors and hospitals to obtain Medicare or Medicaid incentive payments.
According to the report, as of Aug. 30, 2013, a total of 3,590 certified EHRs were available to health care providers, 95% of which were certified by ATCBs under a temporary certification program.
In examining the work done by ATCBs, the OIG found that oversight by the HHS Office of the National Coordinator for Health Information Technology (ONC) "did not fully ensure that test procedures and standards could adequately secure and protect patient information contained in EHRs," the report states. OIG claimed that the health IT office did not ensure that ATCBs "developed procedures to periodically evaluate whether certified EHRs continued to meet Federal standards and developed a training program to ensure that their personnel were competent to test and certify EHRs and to secure proprietary or sensitive information."
OIG notes that the ATCB standards and procedures met all National Institute of Standards and Technology (NIST) test procedure requirements that the ONC approved, but those procedures "were not sufficient to ensure that EHRs would adequately secure and protect patient health information; in particular, the procedures allowed ATCBs to certify EHRs that demonstrated the use of a single-character password during testing." NIST procedures also did not address common security issues, including password complexity and logging emergency access or user privilege changes.
In response to the draft, included as part of the report, ONC noted that ATCBs are no longer active in the certification. New certification criteria approved earlier this year have "strengthened test procedures for common security and privacy features for inclusion in EHRs." Additionally, ONC has "substantially revised the ‘auditable events and tamper resistance’ certification criterion, and we adopted a new ‘end-user device encryption’ criterion," as well as other security capabilities, according to a spokesperson. ONC will review the OIG’s comments before determining the appropriate next steps, the spokesperson added.
However, the OIG does not agree that the current certification regulations "sufficiently address our security concerns regarding the Temporary Program," such as multifactor authentication.
OIG also criticized the health IT office for not addressing the authority to remove EHRs from the market that are shown to have privacy and security flaws.
If an EHR "is exploited and used to conduct malicious activities, ONC is not able to remove the EHR, even temporarily, from the product list to prevent further purchases of it."
Despite government efforts to certify otherwise, questions remain as to whether the Dept. of Health & Human Services Office of the National Coordinator for Health Information Technology is doing enough to ensure that commercially available electronic health record software programs are doing enough to secure patient information.
Concerns were raised by HHS Office of Inspector General (OIG) in a report released Aug. 4. The agency watchdog examined certification work conducted by authorized testing and certification bodies (ATCBs), which early on in the meaningful use program certified that electronic health records (EHRs) met established criteria that would allow doctors and hospitals to obtain Medicare or Medicaid incentive payments.
According to the report, as of Aug. 30, 2013, a total of 3,590 certified EHRs were available to health care providers, 95% of which were certified by ATCBs under a temporary certification program.
In examining the work done by ATCBs, the OIG found that oversight by the HHS Office of the National Coordinator for Health Information Technology (ONC) "did not fully ensure that test procedures and standards could adequately secure and protect patient information contained in EHRs," the report states. OIG claimed that the health IT office did not ensure that ATCBs "developed procedures to periodically evaluate whether certified EHRs continued to meet Federal standards and developed a training program to ensure that their personnel were competent to test and certify EHRs and to secure proprietary or sensitive information."
OIG notes that the ATCB standards and procedures met all National Institute of Standards and Technology (NIST) test procedure requirements that the ONC approved, but those procedures "were not sufficient to ensure that EHRs would adequately secure and protect patient health information; in particular, the procedures allowed ATCBs to certify EHRs that demonstrated the use of a single-character password during testing." NIST procedures also did not address common security issues, including password complexity and logging emergency access or user privilege changes.
In response to the draft, included as part of the report, ONC noted that ATCBs are no longer active in the certification. New certification criteria approved earlier this year have "strengthened test procedures for common security and privacy features for inclusion in EHRs." Additionally, ONC has "substantially revised the ‘auditable events and tamper resistance’ certification criterion, and we adopted a new ‘end-user device encryption’ criterion," as well as other security capabilities, according to a spokesperson. ONC will review the OIG’s comments before determining the appropriate next steps, the spokesperson added.
However, the OIG does not agree that the current certification regulations "sufficiently address our security concerns regarding the Temporary Program," such as multifactor authentication.
OIG also criticized the health IT office for not addressing the authority to remove EHRs from the market that are shown to have privacy and security flaws.
If an EHR "is exploited and used to conduct malicious activities, ONC is not able to remove the EHR, even temporarily, from the product list to prevent further purchases of it."
CMS finalizes plan to cut hospital payments, move ahead with two-midnight policy
Medicare payments to general acute care hospitals will drop by more than $750 million over the next year, and the controversial two-midnight policy will move forward as scheduled, according to a new federal regulation.
On Aug. 4, the Centers for Medicare & Medicaid Services released the final rule governing payment policies for general acute care hospitals and long term care hospitals for fiscal year 2015, which begins on Oct. 1, 2014.
While Medicare will increase the payment rate for general acute care hospitals by 1.4% in fiscal year 2015, a combination of policy changes and penalties under quality programs will actually decrease payments overall by 0.6%, or about a $756 million drop in Medicare spending on inpatient hospital services overall, according to CMS. In contrast, CMS projects that payments to long-term-care hospitals will rise by about 1.1% in fiscal year 2015.
"Today’s policies further support our efforts to continue improving the care our Medicare beneficiaries receive while also cutting the growth of Medicare costs," Marilyn Tavenner, CMS administrator, said in a statement. "This final rule builds on our recent efforts to improve hospital performance while giving hospitals the clarity and resources they need to deliver the best possible patient care."
The final inpatient hospital payment rule does not make changes to the controversial two-midnight policy released by Medicare officials last year. Under the rule, the decision to admit a Medicare patient as an inpatient depends on two factors: whether the condition meets medical necessity requiring a patient to be in a hospital setting and the expectation that their time in the hospital will surpass two midnights.
The two-midnight policy technically went into effect on Oct. 1, 2013, but enforcement through postpayment claims audits by Recovery Audit Contractors has been delayed until March 31, 2015. In the meantime, Medicare Administrative Contractors have been performing prepayment reviews on samples of short-stay inpatient claims to determine hospital compliance with the new policy.
In the final inpatient hospital rule, CMS officials said that they will spend the summer and fall of 2014 to evaluate the results of the prepayment reviews and issue additional guidance on the application of the policy. The agency is also accepting suggestions for exceptions to the two-midnight policy at [email protected].
The Association of American Medical Colleges criticized CMS for failing to either revise or replace the two-midnight policy in this most recent round of rulemaking.
The current policy "continues to ignore physicians’ medical judgment, results in inadequate reimbursement to hospitals for medically necessary care, and creates confusion and new financial liabilities for Medicare beneficiaries," Dr. Darrell G. Kirch, AAMC president and CEO, said in a statement.
Dr. Kirch called on CMS to issue supplemental guidance that would allow hospitals to bill certain short stays as inpatient, under Medicare Part A, when the physician determines that the stay is medically necessary. He also urged the agency to suspend the prepayment reviews being conducted by Medicare Administrative Contractors until the policy is either revised or replaced.
Dr. Bradley Flansbaum, a hospitalist at Lenox Hill Hospital in New York and a member of the Society of Hospital Medicine’s public policy committee, said he thinks CMS could still make changes to the two-midnight policy, despite the lack of action in the recently released inpatient payment rule.
"CMS sees the delay as a cooling off and retrenchment period. They know the two-midnight rule and auditing practice have raised the hackles of many folks," he said. "Business as usual won’t return, and I am pretty certain we will see changes, both in how we make observation determinations and in the payment and surveillance process."
In a proposed rule issued earlier this year, CMS raised the possibility of creating a new payment method under Medicare for short, but intensive inpatient hospital stays. The final rule summarized some of the public comments received so far, but did not propose any new policies.
The inpatient hospital payment rule also contains updates for several of Medicare’s quality improvement programs, including increasing the penalties for hospitals that perform poorly on the measures.
Starting Oct. 1, 2014, hospitals will be subject to a 3% penalty on their Medicare payments if they have high readmission rates for acute myocardial infarction, heart failure, pneumonia, chronic obstructive pulmonary disease, and hip/knee arthroplasty. Starting in October 2016, CMS will add another measure to the Hospital Readmissions Reduction Program: coronary artery bypass graft surgical procedures.
The inpatient payment rule also makes changes to the Hospital Value-Based Purchasing Program. For fiscal year 2015, the penalty will rise to 1.5% of base operating DRG payment amounts to all participating hospitals. CMS estimates that there will be about $1.4 billion available for value-based incentive payments in fiscal year 2015.
On Twitter @maryellenny
Medicare payments to general acute care hospitals will drop by more than $750 million over the next year, and the controversial two-midnight policy will move forward as scheduled, according to a new federal regulation.
On Aug. 4, the Centers for Medicare & Medicaid Services released the final rule governing payment policies for general acute care hospitals and long term care hospitals for fiscal year 2015, which begins on Oct. 1, 2014.
While Medicare will increase the payment rate for general acute care hospitals by 1.4% in fiscal year 2015, a combination of policy changes and penalties under quality programs will actually decrease payments overall by 0.6%, or about a $756 million drop in Medicare spending on inpatient hospital services overall, according to CMS. In contrast, CMS projects that payments to long-term-care hospitals will rise by about 1.1% in fiscal year 2015.
"Today’s policies further support our efforts to continue improving the care our Medicare beneficiaries receive while also cutting the growth of Medicare costs," Marilyn Tavenner, CMS administrator, said in a statement. "This final rule builds on our recent efforts to improve hospital performance while giving hospitals the clarity and resources they need to deliver the best possible patient care."
The final inpatient hospital payment rule does not make changes to the controversial two-midnight policy released by Medicare officials last year. Under the rule, the decision to admit a Medicare patient as an inpatient depends on two factors: whether the condition meets medical necessity requiring a patient to be in a hospital setting and the expectation that their time in the hospital will surpass two midnights.
The two-midnight policy technically went into effect on Oct. 1, 2013, but enforcement through postpayment claims audits by Recovery Audit Contractors has been delayed until March 31, 2015. In the meantime, Medicare Administrative Contractors have been performing prepayment reviews on samples of short-stay inpatient claims to determine hospital compliance with the new policy.
In the final inpatient hospital rule, CMS officials said that they will spend the summer and fall of 2014 to evaluate the results of the prepayment reviews and issue additional guidance on the application of the policy. The agency is also accepting suggestions for exceptions to the two-midnight policy at [email protected].
The Association of American Medical Colleges criticized CMS for failing to either revise or replace the two-midnight policy in this most recent round of rulemaking.
The current policy "continues to ignore physicians’ medical judgment, results in inadequate reimbursement to hospitals for medically necessary care, and creates confusion and new financial liabilities for Medicare beneficiaries," Dr. Darrell G. Kirch, AAMC president and CEO, said in a statement.
Dr. Kirch called on CMS to issue supplemental guidance that would allow hospitals to bill certain short stays as inpatient, under Medicare Part A, when the physician determines that the stay is medically necessary. He also urged the agency to suspend the prepayment reviews being conducted by Medicare Administrative Contractors until the policy is either revised or replaced.
Dr. Bradley Flansbaum, a hospitalist at Lenox Hill Hospital in New York and a member of the Society of Hospital Medicine’s public policy committee, said he thinks CMS could still make changes to the two-midnight policy, despite the lack of action in the recently released inpatient payment rule.
"CMS sees the delay as a cooling off and retrenchment period. They know the two-midnight rule and auditing practice have raised the hackles of many folks," he said. "Business as usual won’t return, and I am pretty certain we will see changes, both in how we make observation determinations and in the payment and surveillance process."
In a proposed rule issued earlier this year, CMS raised the possibility of creating a new payment method under Medicare for short, but intensive inpatient hospital stays. The final rule summarized some of the public comments received so far, but did not propose any new policies.
The inpatient hospital payment rule also contains updates for several of Medicare’s quality improvement programs, including increasing the penalties for hospitals that perform poorly on the measures.
Starting Oct. 1, 2014, hospitals will be subject to a 3% penalty on their Medicare payments if they have high readmission rates for acute myocardial infarction, heart failure, pneumonia, chronic obstructive pulmonary disease, and hip/knee arthroplasty. Starting in October 2016, CMS will add another measure to the Hospital Readmissions Reduction Program: coronary artery bypass graft surgical procedures.
The inpatient payment rule also makes changes to the Hospital Value-Based Purchasing Program. For fiscal year 2015, the penalty will rise to 1.5% of base operating DRG payment amounts to all participating hospitals. CMS estimates that there will be about $1.4 billion available for value-based incentive payments in fiscal year 2015.
On Twitter @maryellenny
Medicare payments to general acute care hospitals will drop by more than $750 million over the next year, and the controversial two-midnight policy will move forward as scheduled, according to a new federal regulation.
On Aug. 4, the Centers for Medicare & Medicaid Services released the final rule governing payment policies for general acute care hospitals and long term care hospitals for fiscal year 2015, which begins on Oct. 1, 2014.
While Medicare will increase the payment rate for general acute care hospitals by 1.4% in fiscal year 2015, a combination of policy changes and penalties under quality programs will actually decrease payments overall by 0.6%, or about a $756 million drop in Medicare spending on inpatient hospital services overall, according to CMS. In contrast, CMS projects that payments to long-term-care hospitals will rise by about 1.1% in fiscal year 2015.
"Today’s policies further support our efforts to continue improving the care our Medicare beneficiaries receive while also cutting the growth of Medicare costs," Marilyn Tavenner, CMS administrator, said in a statement. "This final rule builds on our recent efforts to improve hospital performance while giving hospitals the clarity and resources they need to deliver the best possible patient care."
The final inpatient hospital payment rule does not make changes to the controversial two-midnight policy released by Medicare officials last year. Under the rule, the decision to admit a Medicare patient as an inpatient depends on two factors: whether the condition meets medical necessity requiring a patient to be in a hospital setting and the expectation that their time in the hospital will surpass two midnights.
The two-midnight policy technically went into effect on Oct. 1, 2013, but enforcement through postpayment claims audits by Recovery Audit Contractors has been delayed until March 31, 2015. In the meantime, Medicare Administrative Contractors have been performing prepayment reviews on samples of short-stay inpatient claims to determine hospital compliance with the new policy.
In the final inpatient hospital rule, CMS officials said that they will spend the summer and fall of 2014 to evaluate the results of the prepayment reviews and issue additional guidance on the application of the policy. The agency is also accepting suggestions for exceptions to the two-midnight policy at [email protected].
The Association of American Medical Colleges criticized CMS for failing to either revise or replace the two-midnight policy in this most recent round of rulemaking.
The current policy "continues to ignore physicians’ medical judgment, results in inadequate reimbursement to hospitals for medically necessary care, and creates confusion and new financial liabilities for Medicare beneficiaries," Dr. Darrell G. Kirch, AAMC president and CEO, said in a statement.
Dr. Kirch called on CMS to issue supplemental guidance that would allow hospitals to bill certain short stays as inpatient, under Medicare Part A, when the physician determines that the stay is medically necessary. He also urged the agency to suspend the prepayment reviews being conducted by Medicare Administrative Contractors until the policy is either revised or replaced.
Dr. Bradley Flansbaum, a hospitalist at Lenox Hill Hospital in New York and a member of the Society of Hospital Medicine’s public policy committee, said he thinks CMS could still make changes to the two-midnight policy, despite the lack of action in the recently released inpatient payment rule.
"CMS sees the delay as a cooling off and retrenchment period. They know the two-midnight rule and auditing practice have raised the hackles of many folks," he said. "Business as usual won’t return, and I am pretty certain we will see changes, both in how we make observation determinations and in the payment and surveillance process."
In a proposed rule issued earlier this year, CMS raised the possibility of creating a new payment method under Medicare for short, but intensive inpatient hospital stays. The final rule summarized some of the public comments received so far, but did not propose any new policies.
The inpatient hospital payment rule also contains updates for several of Medicare’s quality improvement programs, including increasing the penalties for hospitals that perform poorly on the measures.
Starting Oct. 1, 2014, hospitals will be subject to a 3% penalty on their Medicare payments if they have high readmission rates for acute myocardial infarction, heart failure, pneumonia, chronic obstructive pulmonary disease, and hip/knee arthroplasty. Starting in October 2016, CMS will add another measure to the Hospital Readmissions Reduction Program: coronary artery bypass graft surgical procedures.
The inpatient payment rule also makes changes to the Hospital Value-Based Purchasing Program. For fiscal year 2015, the penalty will rise to 1.5% of base operating DRG payment amounts to all participating hospitals. CMS estimates that there will be about $1.4 billion available for value-based incentive payments in fiscal year 2015.
On Twitter @maryellenny