User login
An update on the current standard for ultrasound education in fellowship
Point-of-care ultrasound (POCUS) is an essential part of ICU care. It has been demonstrated to improve patient safety and outcomes through procedural guidance (Brass P, et al. Cochrane Database Syst Rev. 2015 Jan 9;1:CD006962) and aid in accurate and timely diagnosis of cardiopulmonary failure (Lichtenstein DA, Mezière GA. Chest. 2008 Jul;134[1]:117-25). Due in part to increasing affordability and portability of ultrasound technologies, the use of POCUS has become seemingly ubiquitous and will continue to increase in coming years. According to expert groups representing 12 critical care societies worldwide, general critical care ultrasound and basic critical care echocardiography should be mandatory training for ICU physicians (Expert Round Table on Ultrasound in ICU. Intensive Care Med. 2011 Jul;37[7]:1077-83).
Currently, POCUS is not universally taught to pulmonary and critical care fellows (PCCM); and when training does exist, curriculums are not standardized. This is in part due to the broadly worded requirements set forth from the ACGME for pulmonary disease and critical care medicine. The totality of ACGME common program requirements as it regards to ultrasound training are as follows: 1. “Fellows must demonstrate competence in procedural and technical skills, including ... use of ultrasound techniques to perform thoracentesis and place intravascular and intracavitary tubes and catheters”; and 2. “Fellows must demonstrate knowledge of imaging techniques commonly employed in the evaluation of patients with pulmonary disease or critical illness, including the use of ultrasound” (ACGME Program Requirements for Graduate Medical Education in Pulmonary Disease and Critical Care Medicine).
In comparison, recently updated ACGME common program requirements for ultrasound in emergency medicine and anesthesiology residencies are robust and detailed. Requirements for anesthesia residency training include: ” ... competency in using surface ultrasound ... and transthoracic echocardiography to guide the performance of invasive procedures and to evaluate organ function and pathology ... understanding the principles of ultrasound, including the physics of ultrasound transmission, ultrasound transducer construction, and transducer selection for specific applications, to include being able to obtain images with an understanding of limitations and artifacts ... obtaining standard views of the heart and inferior vena cava with transthoracic echocardiography allowing the evaluation of myocardial function, estimation of central venous pressure, and gross pericardial/cardiac pathology (eg, large pericardial effusion) ... using transthoracic ultrasound for the detection of pneumothorax and pleural effusion ... using surface ultrasound to guide vascular access (both central and peripheral) ... describing techniques, views, and findings in standard language” (ACGME Program Requirements for Graduate Medical Education In Anesthesiology).
Herein lies a stark contrast in what is required of programs that train physicians to care for unstable patients and the critically ill. Current requirements leave graduates of PCCM training programs vulnerable to completing ACGME milestones without being adequately prepared to evaluate patients in a modern ICU setting. Increasingly, hospitals credentialing committees expect PCCM graduates to be suitably trained in ultrasound. Regrettably, there is no assurance that is true, or standardized, with current PCCM fellowship training requirements.
There is not a national standard for competency assessment or requirements for credentialing in POCUS for critical care physicians at this time. However, multiple national and international critical care societies, including CHEST, have consensus statements and recommendations outlining the areas of competence expected in critical care ultrasound (Mayo PH, et al. Chest. 2009 Apr;135[4];1050-60, Expert Round Table on Ultrasound in ICU. Intensive Care Med. 2011 Jul;37(7):1077-83). The PCCM ACGME requirements should be updated to reflect such recommendations, thereby placing greater emphasis on ultrasound teaching requirements and standardized curriculums. Despite the current ACGME program requirements, it is incumbent upon critical care training programs to provide competency-based education of this now “standard of care” technology.
Barriers to universal POCUS training exist. Fellowship programs may lack trained, ultrasound confident faculty, time, and funding to successfully develop and sustain an ultrasound curriculum. (Eisen LA, et al. Crit Care Med. 2010;38[10]:1978-83; Patrawalla P, et al. J Intensive Care Med. 2019 Feb 12: [Epub ahead of print].)
Although access to adequate quality and quantity of ultrasound machines is less often a problem than in the past, many institutions lack archival and image review software that allows for quality assurance of image acquisition, and some still may not have a faculty member with expertise and ability to champion the cause.
In attempts to mitigate the local faculty gaps, national and regional solutions have been developed for ultrasonography education. CHEST has educated more than 1,400 learners in the Ultrasound Essentials course since 2013. Also, grassroots efforts have led to the development of courses specifically designed to teach incoming PCCM fellows. Using a collaborative and cost-effective model, these regional programs pool faculty and experts in the field to train multiple fellowship programs simultaneously. The first of these was created over a decade ago in New York City (Patrawalla P, et al. J Intensive Care Med. 2019 Feb 12:[Epub ahead of print].)
Currently, there are at least four regional annual ultrasound courses directed at teaching PCCM fellows. These courses are typically held over multiple days and encompass the basics of critical care ultrasound, including vascular, thoracic, abdominal, cardiac, and procedural imaging. By estimation, these four courses provide a basic ultrasonography education to approximately two-thirds of first year pulmonary and critical care fellows in the United States. In addition to training fellows, these programs also serve as a platform for the development of local faculty experts, so that training can continue at their institutions.
Introductory courses are highly effective (Dinh VA, et al. Crit Care Res Pract. 2015 Aug 5:675041 Patrawalla P, et al. J Intensive Care Med. 2019 Feb 12: [Epub ahead of print]), but ongoing education, assessment, and quality assurance is required to achieve sustained competence. Ideally, training in POCUS should entail a dedicated, intensive introduction to the competencies of critical care ultrasound (such as the above regional courses or CHEST ultrasound courses), followed by a formal curriculum within the PCCM fellowship programs. This curriculum should afford the trainee exposure to critically ill patients in an environment with adequate ultrasound equipment and a method to record studies. The trainee then interprets the acquired studies in clinical context. Preferably, the program will afford the trainee real-time quality assurance for image acquisition and interpretation by a program champion. Quality assurance can be provided on site or remotely using fixed interval review sessions. Lastly, the program should have internal milestones to evaluate when a trainee has reached competency to perform these tasks independently. The completion of training should include a letter to any future employee attesting to the trainee’s acquisition of these skills and ability to apply them safely while caring for the critically ill. This robust education is occurring in many centers across the country. PCCM fellowship programs owe it to their trainees, and patients, that competency-based critical care ultrasound training is robust, standardized, and supported.
Point-of-care ultrasound (POCUS) is an essential part of ICU care. It has been demonstrated to improve patient safety and outcomes through procedural guidance (Brass P, et al. Cochrane Database Syst Rev. 2015 Jan 9;1:CD006962) and aid in accurate and timely diagnosis of cardiopulmonary failure (Lichtenstein DA, Mezière GA. Chest. 2008 Jul;134[1]:117-25). Due in part to increasing affordability and portability of ultrasound technologies, the use of POCUS has become seemingly ubiquitous and will continue to increase in coming years. According to expert groups representing 12 critical care societies worldwide, general critical care ultrasound and basic critical care echocardiography should be mandatory training for ICU physicians (Expert Round Table on Ultrasound in ICU. Intensive Care Med. 2011 Jul;37[7]:1077-83).
Currently, POCUS is not universally taught to pulmonary and critical care fellows (PCCM); and when training does exist, curriculums are not standardized. This is in part due to the broadly worded requirements set forth from the ACGME for pulmonary disease and critical care medicine. The totality of ACGME common program requirements as it regards to ultrasound training are as follows: 1. “Fellows must demonstrate competence in procedural and technical skills, including ... use of ultrasound techniques to perform thoracentesis and place intravascular and intracavitary tubes and catheters”; and 2. “Fellows must demonstrate knowledge of imaging techniques commonly employed in the evaluation of patients with pulmonary disease or critical illness, including the use of ultrasound” (ACGME Program Requirements for Graduate Medical Education in Pulmonary Disease and Critical Care Medicine).
In comparison, recently updated ACGME common program requirements for ultrasound in emergency medicine and anesthesiology residencies are robust and detailed. Requirements for anesthesia residency training include: ” ... competency in using surface ultrasound ... and transthoracic echocardiography to guide the performance of invasive procedures and to evaluate organ function and pathology ... understanding the principles of ultrasound, including the physics of ultrasound transmission, ultrasound transducer construction, and transducer selection for specific applications, to include being able to obtain images with an understanding of limitations and artifacts ... obtaining standard views of the heart and inferior vena cava with transthoracic echocardiography allowing the evaluation of myocardial function, estimation of central venous pressure, and gross pericardial/cardiac pathology (eg, large pericardial effusion) ... using transthoracic ultrasound for the detection of pneumothorax and pleural effusion ... using surface ultrasound to guide vascular access (both central and peripheral) ... describing techniques, views, and findings in standard language” (ACGME Program Requirements for Graduate Medical Education In Anesthesiology).
Herein lies a stark contrast in what is required of programs that train physicians to care for unstable patients and the critically ill. Current requirements leave graduates of PCCM training programs vulnerable to completing ACGME milestones without being adequately prepared to evaluate patients in a modern ICU setting. Increasingly, hospitals credentialing committees expect PCCM graduates to be suitably trained in ultrasound. Regrettably, there is no assurance that is true, or standardized, with current PCCM fellowship training requirements.
There is not a national standard for competency assessment or requirements for credentialing in POCUS for critical care physicians at this time. However, multiple national and international critical care societies, including CHEST, have consensus statements and recommendations outlining the areas of competence expected in critical care ultrasound (Mayo PH, et al. Chest. 2009 Apr;135[4];1050-60, Expert Round Table on Ultrasound in ICU. Intensive Care Med. 2011 Jul;37(7):1077-83). The PCCM ACGME requirements should be updated to reflect such recommendations, thereby placing greater emphasis on ultrasound teaching requirements and standardized curriculums. Despite the current ACGME program requirements, it is incumbent upon critical care training programs to provide competency-based education of this now “standard of care” technology.
Barriers to universal POCUS training exist. Fellowship programs may lack trained, ultrasound confident faculty, time, and funding to successfully develop and sustain an ultrasound curriculum. (Eisen LA, et al. Crit Care Med. 2010;38[10]:1978-83; Patrawalla P, et al. J Intensive Care Med. 2019 Feb 12: [Epub ahead of print].)
Although access to adequate quality and quantity of ultrasound machines is less often a problem than in the past, many institutions lack archival and image review software that allows for quality assurance of image acquisition, and some still may not have a faculty member with expertise and ability to champion the cause.
In attempts to mitigate the local faculty gaps, national and regional solutions have been developed for ultrasonography education. CHEST has educated more than 1,400 learners in the Ultrasound Essentials course since 2013. Also, grassroots efforts have led to the development of courses specifically designed to teach incoming PCCM fellows. Using a collaborative and cost-effective model, these regional programs pool faculty and experts in the field to train multiple fellowship programs simultaneously. The first of these was created over a decade ago in New York City (Patrawalla P, et al. J Intensive Care Med. 2019 Feb 12:[Epub ahead of print].)
Currently, there are at least four regional annual ultrasound courses directed at teaching PCCM fellows. These courses are typically held over multiple days and encompass the basics of critical care ultrasound, including vascular, thoracic, abdominal, cardiac, and procedural imaging. By estimation, these four courses provide a basic ultrasonography education to approximately two-thirds of first year pulmonary and critical care fellows in the United States. In addition to training fellows, these programs also serve as a platform for the development of local faculty experts, so that training can continue at their institutions.
Introductory courses are highly effective (Dinh VA, et al. Crit Care Res Pract. 2015 Aug 5:675041 Patrawalla P, et al. J Intensive Care Med. 2019 Feb 12: [Epub ahead of print]), but ongoing education, assessment, and quality assurance is required to achieve sustained competence. Ideally, training in POCUS should entail a dedicated, intensive introduction to the competencies of critical care ultrasound (such as the above regional courses or CHEST ultrasound courses), followed by a formal curriculum within the PCCM fellowship programs. This curriculum should afford the trainee exposure to critically ill patients in an environment with adequate ultrasound equipment and a method to record studies. The trainee then interprets the acquired studies in clinical context. Preferably, the program will afford the trainee real-time quality assurance for image acquisition and interpretation by a program champion. Quality assurance can be provided on site or remotely using fixed interval review sessions. Lastly, the program should have internal milestones to evaluate when a trainee has reached competency to perform these tasks independently. The completion of training should include a letter to any future employee attesting to the trainee’s acquisition of these skills and ability to apply them safely while caring for the critically ill. This robust education is occurring in many centers across the country. PCCM fellowship programs owe it to their trainees, and patients, that competency-based critical care ultrasound training is robust, standardized, and supported.
Point-of-care ultrasound (POCUS) is an essential part of ICU care. It has been demonstrated to improve patient safety and outcomes through procedural guidance (Brass P, et al. Cochrane Database Syst Rev. 2015 Jan 9;1:CD006962) and aid in accurate and timely diagnosis of cardiopulmonary failure (Lichtenstein DA, Mezière GA. Chest. 2008 Jul;134[1]:117-25). Due in part to increasing affordability and portability of ultrasound technologies, the use of POCUS has become seemingly ubiquitous and will continue to increase in coming years. According to expert groups representing 12 critical care societies worldwide, general critical care ultrasound and basic critical care echocardiography should be mandatory training for ICU physicians (Expert Round Table on Ultrasound in ICU. Intensive Care Med. 2011 Jul;37[7]:1077-83).
Currently, POCUS is not universally taught to pulmonary and critical care fellows (PCCM); and when training does exist, curriculums are not standardized. This is in part due to the broadly worded requirements set forth from the ACGME for pulmonary disease and critical care medicine. The totality of ACGME common program requirements as it regards to ultrasound training are as follows: 1. “Fellows must demonstrate competence in procedural and technical skills, including ... use of ultrasound techniques to perform thoracentesis and place intravascular and intracavitary tubes and catheters”; and 2. “Fellows must demonstrate knowledge of imaging techniques commonly employed in the evaluation of patients with pulmonary disease or critical illness, including the use of ultrasound” (ACGME Program Requirements for Graduate Medical Education in Pulmonary Disease and Critical Care Medicine).
In comparison, recently updated ACGME common program requirements for ultrasound in emergency medicine and anesthesiology residencies are robust and detailed. Requirements for anesthesia residency training include: ” ... competency in using surface ultrasound ... and transthoracic echocardiography to guide the performance of invasive procedures and to evaluate organ function and pathology ... understanding the principles of ultrasound, including the physics of ultrasound transmission, ultrasound transducer construction, and transducer selection for specific applications, to include being able to obtain images with an understanding of limitations and artifacts ... obtaining standard views of the heart and inferior vena cava with transthoracic echocardiography allowing the evaluation of myocardial function, estimation of central venous pressure, and gross pericardial/cardiac pathology (eg, large pericardial effusion) ... using transthoracic ultrasound for the detection of pneumothorax and pleural effusion ... using surface ultrasound to guide vascular access (both central and peripheral) ... describing techniques, views, and findings in standard language” (ACGME Program Requirements for Graduate Medical Education In Anesthesiology).
Herein lies a stark contrast in what is required of programs that train physicians to care for unstable patients and the critically ill. Current requirements leave graduates of PCCM training programs vulnerable to completing ACGME milestones without being adequately prepared to evaluate patients in a modern ICU setting. Increasingly, hospitals credentialing committees expect PCCM graduates to be suitably trained in ultrasound. Regrettably, there is no assurance that is true, or standardized, with current PCCM fellowship training requirements.
There is not a national standard for competency assessment or requirements for credentialing in POCUS for critical care physicians at this time. However, multiple national and international critical care societies, including CHEST, have consensus statements and recommendations outlining the areas of competence expected in critical care ultrasound (Mayo PH, et al. Chest. 2009 Apr;135[4];1050-60, Expert Round Table on Ultrasound in ICU. Intensive Care Med. 2011 Jul;37(7):1077-83). The PCCM ACGME requirements should be updated to reflect such recommendations, thereby placing greater emphasis on ultrasound teaching requirements and standardized curriculums. Despite the current ACGME program requirements, it is incumbent upon critical care training programs to provide competency-based education of this now “standard of care” technology.
Barriers to universal POCUS training exist. Fellowship programs may lack trained, ultrasound confident faculty, time, and funding to successfully develop and sustain an ultrasound curriculum. (Eisen LA, et al. Crit Care Med. 2010;38[10]:1978-83; Patrawalla P, et al. J Intensive Care Med. 2019 Feb 12: [Epub ahead of print].)
Although access to adequate quality and quantity of ultrasound machines is less often a problem than in the past, many institutions lack archival and image review software that allows for quality assurance of image acquisition, and some still may not have a faculty member with expertise and ability to champion the cause.
In attempts to mitigate the local faculty gaps, national and regional solutions have been developed for ultrasonography education. CHEST has educated more than 1,400 learners in the Ultrasound Essentials course since 2013. Also, grassroots efforts have led to the development of courses specifically designed to teach incoming PCCM fellows. Using a collaborative and cost-effective model, these regional programs pool faculty and experts in the field to train multiple fellowship programs simultaneously. The first of these was created over a decade ago in New York City (Patrawalla P, et al. J Intensive Care Med. 2019 Feb 12:[Epub ahead of print].)
Currently, there are at least four regional annual ultrasound courses directed at teaching PCCM fellows. These courses are typically held over multiple days and encompass the basics of critical care ultrasound, including vascular, thoracic, abdominal, cardiac, and procedural imaging. By estimation, these four courses provide a basic ultrasonography education to approximately two-thirds of first year pulmonary and critical care fellows in the United States. In addition to training fellows, these programs also serve as a platform for the development of local faculty experts, so that training can continue at their institutions.
Introductory courses are highly effective (Dinh VA, et al. Crit Care Res Pract. 2015 Aug 5:675041 Patrawalla P, et al. J Intensive Care Med. 2019 Feb 12: [Epub ahead of print]), but ongoing education, assessment, and quality assurance is required to achieve sustained competence. Ideally, training in POCUS should entail a dedicated, intensive introduction to the competencies of critical care ultrasound (such as the above regional courses or CHEST ultrasound courses), followed by a formal curriculum within the PCCM fellowship programs. This curriculum should afford the trainee exposure to critically ill patients in an environment with adequate ultrasound equipment and a method to record studies. The trainee then interprets the acquired studies in clinical context. Preferably, the program will afford the trainee real-time quality assurance for image acquisition and interpretation by a program champion. Quality assurance can be provided on site or remotely using fixed interval review sessions. Lastly, the program should have internal milestones to evaluate when a trainee has reached competency to perform these tasks independently. The completion of training should include a letter to any future employee attesting to the trainee’s acquisition of these skills and ability to apply them safely while caring for the critically ill. This robust education is occurring in many centers across the country. PCCM fellowship programs owe it to their trainees, and patients, that competency-based critical care ultrasound training is robust, standardized, and supported.
The emerging role of quantitative CT scans in ILD terms
The role of imaging for interstitial lung disease (ILD) is of paramount importance. With the growth of high resolution chest computed tomography (HRCT) imaging techniques, we are able to visualize nuances between individual ILDs more critically. HRCT is an essential component of an initial ILD evaluation and also has become part of the armamentarium of tools used for routine management of these patients. The technology of HRCT scans has evolved over the years, most recently with the advent of quantitative HRCT (qCT). The technology employs texture-based classification, which identifies and quantifies different radiographic findings. The arrival of qCT scanning has been slowly emerging as a new player in the ILD world. What exactly is qCT, and what role can, and will it serve for our ILD patients?
Quantitative CT scanning has been introduced since the 1980s, but only within the last 15 years has its use for ILD taken form. Human interpretation of CTs is fraught with subjectivity, based on the interpreting radiologist’s training, experience, and individual visual perception of images. This can result in significant variability in radiographic interpretations and, ultimately, affects a patient’s diagnosis, disease monitoring, treatment, and prognosis. Semiquantitative visual scoring by radiologists is highly variable, especially in areas with limited availability of chest radiologists. qCT employs an automated histogram signature technique that utilizes density and texture-based analysis of the lung parenchyma. Utilizing machine learning from pathologically confirmed datasets, computer programs were trained with specialized thoracic radiologists to distinguish some commonly found radiographic abnormalities into four major groups: ground glass, reticular, honeycombing, and emphysema. In addition, these categories are quantified and spatially depicted on an analysis (Bartholmai, et al. J Thorac Imaging. 2013;28[5]:298). Various computer programs have been built to streamline the process and expedite the interpretation of an individual’s HRCT scan. The more commonly familiar program, CALIPER (Computer-Aided Lung Informatics for Pathology Evaluation and Ratings), has been used in multiple research studies of qCT in ILD and IPF. Each patient’s CT scan is uploaded to the program, and a breakdown of the patient’s lungs into each category is presented. Not only is each abnormality quantified and precisely defined, it is also color-coded by segments to help with visual interpretation by the physician.
The benefit of qCT lies not only in the automated, objective evaluation of interstitial lung disease, but also in its possible use in prognostication and mortality prediction. Neither use has been fully validated as of yet. However, growing evidence shows a promising role in both realms. Thus far, there have been some studies correlating PFT data with qCT findings. A follow-up study of the Scleroderma Lung Study II examined qCT changes over 24 months and correlated those findings with PFTs and patient-reported outcomes. Patients in this study were either treated with cyclophosphamide (CYC) for 1 year/placebo 1 year vs mycophenolate mofetil (MMF) for 2 years. A large portion of patients receiving CYC or MMF had a significant correlation between improved or stable qCT scores and their FVC and TLC. Neither CYC nor MMF was superior in qCT scores, aligning with the findings of the study, which showed noninferiority of MMF compared with CYC (Goldin, et al. Ann Am Thorac Soc. 2018 Nov;15[11]:1286). Interestingly, the improvement of ground glass is often viewed by physicians as positive, since this finding is typically thought of as active inflammation. However, if qCT determines that the fibrosis score actually increases over time, despite an improvement in ground glass, this may more accurately reflect the development of subtle fibrosis that is not easily appreciated by the human eye (Goldin, et al. Ann Am Thorac Soc. 2018 Nov;15[11]:1286). In this context, it is feasible that parenchymal changes occur prior to deterioration on PFTs. Diffusing capacity for carbon monoxide (DLCO) correlates largely with the extent of lung involvement on qCT, but DLCO is not a specific biomarker in predicting severity of ILD (ie, because pHTN or anemia can confound DLCO). Forced vital capacity (FVC) in certain diseases may also confound CT correlation (ie, muscle weakness or extrathoracic restriction from skin disease in systemic sclerosis). The usefulness of PFT data as a clinical endpoint in research studies may be replaced by qCTs more consistent and precise detection of disease modification.
IPF has been an interesting area of exploration for the role of qCT in disease monitoring and possible prognostication. It is known that the presence of honeycombing on HRCT is associated with increased mortality. Patients with a progressive fibrotic ILD have similar mortality rates to those with IPF (Adegunsoye, et al. Ann Am Thorac Soc. 2019 May;16[5]:580). The ability to correlate radiographic findings with mortality could potentially become an important marker of clinical deterioration, especially in those patients who are unable to perform PFTs. In addition, it can also be beneficial in those with co-existent emphysema, since PFTs may be confounded by this overlap. Nakagawa and colleagues proposed a computer-aided method for qCT analysis of honeycombing in patients with IPF. The algorithm for the qCT analysis also has specific parameters to exclude emphysematous lesions on imaging. The %honeycomb area (HA) was correlated with a composite physiologic index derived from PFTs (calculated from FEV1, FVC and DLCO). This tool can accurately quantify the percentage of honeycombing and aid in monitoring IPF. Using this protocol, Nakagawa was able to demonstrate a significant correlation with 3-year mortality, with a marked difference found when using a cutoff value of 4.8% (Nakagawa, et al. Plos One. 2019 Mar; 14[3]:e0214278). Furthermore, patient survival in IPF has been compared against the CALIPER program and PFTs. Mortality for patients was significantly associated with pulmonary vessel volume (PVV), an innovative tool that quantified the volume of the pulmonary artery and veins, which may become a new parameter used for disease monitoring. Using qCT in addition to PFTs provides more tangible evidence to help monitor patients with IPF, guide treatment decisions, and plan for transplant or palliative care. The growing use of PVV in qCT has yet to be fully elucidated, but it does have a promising role (Jacob, et al. Eur Respir J. 2017;49[1]. doi: 10.1183/13993003.01011-2016).
Despite the positive outlook for qCT, there are major issues that limit its widespread use. During the image acquisition process, there is a lack of consistency and quality control, stemming from multiple different manufacturers of CT scan machines, reconstitution methods, radiation doses, and noise or inspiratory efforts of patients. The Radiologic Society of North America (RSNA) is attempting to fix this issue by creating a standardized protocol for collecting images used for qCT (Castillo-Saldana, et al. J Thorac Imaging. 2019 Aug 7. doi: 10.1097/RTI.0000000000000440). In order to move forward with adaptation of qCT, a standardized approach and handling of images needs to be created.
Quantitative CT is an exciting new prospect for the care of patients with ILD. As these patients, and their management, becomes more complex, expanding the toolbox for physicians is much needed. It will be fascinating to see how the role of qCT takes shape over the coming years.
Dr. D’Annunzio is with Westmed Medical Group, Rye, N.Y.; Dr. Nayar is a Pulmonary/Critical Care Fellow at NYU School of Medicine; and Dr. Patel is with Columbia University Medical Center.
The role of imaging for interstitial lung disease (ILD) is of paramount importance. With the growth of high resolution chest computed tomography (HRCT) imaging techniques, we are able to visualize nuances between individual ILDs more critically. HRCT is an essential component of an initial ILD evaluation and also has become part of the armamentarium of tools used for routine management of these patients. The technology of HRCT scans has evolved over the years, most recently with the advent of quantitative HRCT (qCT). The technology employs texture-based classification, which identifies and quantifies different radiographic findings. The arrival of qCT scanning has been slowly emerging as a new player in the ILD world. What exactly is qCT, and what role can, and will it serve for our ILD patients?
Quantitative CT scanning has been introduced since the 1980s, but only within the last 15 years has its use for ILD taken form. Human interpretation of CTs is fraught with subjectivity, based on the interpreting radiologist’s training, experience, and individual visual perception of images. This can result in significant variability in radiographic interpretations and, ultimately, affects a patient’s diagnosis, disease monitoring, treatment, and prognosis. Semiquantitative visual scoring by radiologists is highly variable, especially in areas with limited availability of chest radiologists. qCT employs an automated histogram signature technique that utilizes density and texture-based analysis of the lung parenchyma. Utilizing machine learning from pathologically confirmed datasets, computer programs were trained with specialized thoracic radiologists to distinguish some commonly found radiographic abnormalities into four major groups: ground glass, reticular, honeycombing, and emphysema. In addition, these categories are quantified and spatially depicted on an analysis (Bartholmai, et al. J Thorac Imaging. 2013;28[5]:298). Various computer programs have been built to streamline the process and expedite the interpretation of an individual’s HRCT scan. The more commonly familiar program, CALIPER (Computer-Aided Lung Informatics for Pathology Evaluation and Ratings), has been used in multiple research studies of qCT in ILD and IPF. Each patient’s CT scan is uploaded to the program, and a breakdown of the patient’s lungs into each category is presented. Not only is each abnormality quantified and precisely defined, it is also color-coded by segments to help with visual interpretation by the physician.
The benefit of qCT lies not only in the automated, objective evaluation of interstitial lung disease, but also in its possible use in prognostication and mortality prediction. Neither use has been fully validated as of yet. However, growing evidence shows a promising role in both realms. Thus far, there have been some studies correlating PFT data with qCT findings. A follow-up study of the Scleroderma Lung Study II examined qCT changes over 24 months and correlated those findings with PFTs and patient-reported outcomes. Patients in this study were either treated with cyclophosphamide (CYC) for 1 year/placebo 1 year vs mycophenolate mofetil (MMF) for 2 years. A large portion of patients receiving CYC or MMF had a significant correlation between improved or stable qCT scores and their FVC and TLC. Neither CYC nor MMF was superior in qCT scores, aligning with the findings of the study, which showed noninferiority of MMF compared with CYC (Goldin, et al. Ann Am Thorac Soc. 2018 Nov;15[11]:1286). Interestingly, the improvement of ground glass is often viewed by physicians as positive, since this finding is typically thought of as active inflammation. However, if qCT determines that the fibrosis score actually increases over time, despite an improvement in ground glass, this may more accurately reflect the development of subtle fibrosis that is not easily appreciated by the human eye (Goldin, et al. Ann Am Thorac Soc. 2018 Nov;15[11]:1286). In this context, it is feasible that parenchymal changes occur prior to deterioration on PFTs. Diffusing capacity for carbon monoxide (DLCO) correlates largely with the extent of lung involvement on qCT, but DLCO is not a specific biomarker in predicting severity of ILD (ie, because pHTN or anemia can confound DLCO). Forced vital capacity (FVC) in certain diseases may also confound CT correlation (ie, muscle weakness or extrathoracic restriction from skin disease in systemic sclerosis). The usefulness of PFT data as a clinical endpoint in research studies may be replaced by qCTs more consistent and precise detection of disease modification.
IPF has been an interesting area of exploration for the role of qCT in disease monitoring and possible prognostication. It is known that the presence of honeycombing on HRCT is associated with increased mortality. Patients with a progressive fibrotic ILD have similar mortality rates to those with IPF (Adegunsoye, et al. Ann Am Thorac Soc. 2019 May;16[5]:580). The ability to correlate radiographic findings with mortality could potentially become an important marker of clinical deterioration, especially in those patients who are unable to perform PFTs. In addition, it can also be beneficial in those with co-existent emphysema, since PFTs may be confounded by this overlap. Nakagawa and colleagues proposed a computer-aided method for qCT analysis of honeycombing in patients with IPF. The algorithm for the qCT analysis also has specific parameters to exclude emphysematous lesions on imaging. The %honeycomb area (HA) was correlated with a composite physiologic index derived from PFTs (calculated from FEV1, FVC and DLCO). This tool can accurately quantify the percentage of honeycombing and aid in monitoring IPF. Using this protocol, Nakagawa was able to demonstrate a significant correlation with 3-year mortality, with a marked difference found when using a cutoff value of 4.8% (Nakagawa, et al. Plos One. 2019 Mar; 14[3]:e0214278). Furthermore, patient survival in IPF has been compared against the CALIPER program and PFTs. Mortality for patients was significantly associated with pulmonary vessel volume (PVV), an innovative tool that quantified the volume of the pulmonary artery and veins, which may become a new parameter used for disease monitoring. Using qCT in addition to PFTs provides more tangible evidence to help monitor patients with IPF, guide treatment decisions, and plan for transplant or palliative care. The growing use of PVV in qCT has yet to be fully elucidated, but it does have a promising role (Jacob, et al. Eur Respir J. 2017;49[1]. doi: 10.1183/13993003.01011-2016).
Despite the positive outlook for qCT, there are major issues that limit its widespread use. During the image acquisition process, there is a lack of consistency and quality control, stemming from multiple different manufacturers of CT scan machines, reconstitution methods, radiation doses, and noise or inspiratory efforts of patients. The Radiologic Society of North America (RSNA) is attempting to fix this issue by creating a standardized protocol for collecting images used for qCT (Castillo-Saldana, et al. J Thorac Imaging. 2019 Aug 7. doi: 10.1097/RTI.0000000000000440). In order to move forward with adaptation of qCT, a standardized approach and handling of images needs to be created.
Quantitative CT is an exciting new prospect for the care of patients with ILD. As these patients, and their management, becomes more complex, expanding the toolbox for physicians is much needed. It will be fascinating to see how the role of qCT takes shape over the coming years.
Dr. D’Annunzio is with Westmed Medical Group, Rye, N.Y.; Dr. Nayar is a Pulmonary/Critical Care Fellow at NYU School of Medicine; and Dr. Patel is with Columbia University Medical Center.
The role of imaging for interstitial lung disease (ILD) is of paramount importance. With the growth of high resolution chest computed tomography (HRCT) imaging techniques, we are able to visualize nuances between individual ILDs more critically. HRCT is an essential component of an initial ILD evaluation and also has become part of the armamentarium of tools used for routine management of these patients. The technology of HRCT scans has evolved over the years, most recently with the advent of quantitative HRCT (qCT). The technology employs texture-based classification, which identifies and quantifies different radiographic findings. The arrival of qCT scanning has been slowly emerging as a new player in the ILD world. What exactly is qCT, and what role can, and will it serve for our ILD patients?
Quantitative CT scanning has been introduced since the 1980s, but only within the last 15 years has its use for ILD taken form. Human interpretation of CTs is fraught with subjectivity, based on the interpreting radiologist’s training, experience, and individual visual perception of images. This can result in significant variability in radiographic interpretations and, ultimately, affects a patient’s diagnosis, disease monitoring, treatment, and prognosis. Semiquantitative visual scoring by radiologists is highly variable, especially in areas with limited availability of chest radiologists. qCT employs an automated histogram signature technique that utilizes density and texture-based analysis of the lung parenchyma. Utilizing machine learning from pathologically confirmed datasets, computer programs were trained with specialized thoracic radiologists to distinguish some commonly found radiographic abnormalities into four major groups: ground glass, reticular, honeycombing, and emphysema. In addition, these categories are quantified and spatially depicted on an analysis (Bartholmai, et al. J Thorac Imaging. 2013;28[5]:298). Various computer programs have been built to streamline the process and expedite the interpretation of an individual’s HRCT scan. The more commonly familiar program, CALIPER (Computer-Aided Lung Informatics for Pathology Evaluation and Ratings), has been used in multiple research studies of qCT in ILD and IPF. Each patient’s CT scan is uploaded to the program, and a breakdown of the patient’s lungs into each category is presented. Not only is each abnormality quantified and precisely defined, it is also color-coded by segments to help with visual interpretation by the physician.
The benefit of qCT lies not only in the automated, objective evaluation of interstitial lung disease, but also in its possible use in prognostication and mortality prediction. Neither use has been fully validated as of yet. However, growing evidence shows a promising role in both realms. Thus far, there have been some studies correlating PFT data with qCT findings. A follow-up study of the Scleroderma Lung Study II examined qCT changes over 24 months and correlated those findings with PFTs and patient-reported outcomes. Patients in this study were either treated with cyclophosphamide (CYC) for 1 year/placebo 1 year vs mycophenolate mofetil (MMF) for 2 years. A large portion of patients receiving CYC or MMF had a significant correlation between improved or stable qCT scores and their FVC and TLC. Neither CYC nor MMF was superior in qCT scores, aligning with the findings of the study, which showed noninferiority of MMF compared with CYC (Goldin, et al. Ann Am Thorac Soc. 2018 Nov;15[11]:1286). Interestingly, the improvement of ground glass is often viewed by physicians as positive, since this finding is typically thought of as active inflammation. However, if qCT determines that the fibrosis score actually increases over time, despite an improvement in ground glass, this may more accurately reflect the development of subtle fibrosis that is not easily appreciated by the human eye (Goldin, et al. Ann Am Thorac Soc. 2018 Nov;15[11]:1286). In this context, it is feasible that parenchymal changes occur prior to deterioration on PFTs. Diffusing capacity for carbon monoxide (DLCO) correlates largely with the extent of lung involvement on qCT, but DLCO is not a specific biomarker in predicting severity of ILD (ie, because pHTN or anemia can confound DLCO). Forced vital capacity (FVC) in certain diseases may also confound CT correlation (ie, muscle weakness or extrathoracic restriction from skin disease in systemic sclerosis). The usefulness of PFT data as a clinical endpoint in research studies may be replaced by qCTs more consistent and precise detection of disease modification.
IPF has been an interesting area of exploration for the role of qCT in disease monitoring and possible prognostication. It is known that the presence of honeycombing on HRCT is associated with increased mortality. Patients with a progressive fibrotic ILD have similar mortality rates to those with IPF (Adegunsoye, et al. Ann Am Thorac Soc. 2019 May;16[5]:580). The ability to correlate radiographic findings with mortality could potentially become an important marker of clinical deterioration, especially in those patients who are unable to perform PFTs. In addition, it can also be beneficial in those with co-existent emphysema, since PFTs may be confounded by this overlap. Nakagawa and colleagues proposed a computer-aided method for qCT analysis of honeycombing in patients with IPF. The algorithm for the qCT analysis also has specific parameters to exclude emphysematous lesions on imaging. The %honeycomb area (HA) was correlated with a composite physiologic index derived from PFTs (calculated from FEV1, FVC and DLCO). This tool can accurately quantify the percentage of honeycombing and aid in monitoring IPF. Using this protocol, Nakagawa was able to demonstrate a significant correlation with 3-year mortality, with a marked difference found when using a cutoff value of 4.8% (Nakagawa, et al. Plos One. 2019 Mar; 14[3]:e0214278). Furthermore, patient survival in IPF has been compared against the CALIPER program and PFTs. Mortality for patients was significantly associated with pulmonary vessel volume (PVV), an innovative tool that quantified the volume of the pulmonary artery and veins, which may become a new parameter used for disease monitoring. Using qCT in addition to PFTs provides more tangible evidence to help monitor patients with IPF, guide treatment decisions, and plan for transplant or palliative care. The growing use of PVV in qCT has yet to be fully elucidated, but it does have a promising role (Jacob, et al. Eur Respir J. 2017;49[1]. doi: 10.1183/13993003.01011-2016).
Despite the positive outlook for qCT, there are major issues that limit its widespread use. During the image acquisition process, there is a lack of consistency and quality control, stemming from multiple different manufacturers of CT scan machines, reconstitution methods, radiation doses, and noise or inspiratory efforts of patients. The Radiologic Society of North America (RSNA) is attempting to fix this issue by creating a standardized protocol for collecting images used for qCT (Castillo-Saldana, et al. J Thorac Imaging. 2019 Aug 7. doi: 10.1097/RTI.0000000000000440). In order to move forward with adaptation of qCT, a standardized approach and handling of images needs to be created.
Quantitative CT is an exciting new prospect for the care of patients with ILD. As these patients, and their management, becomes more complex, expanding the toolbox for physicians is much needed. It will be fascinating to see how the role of qCT takes shape over the coming years.
Dr. D’Annunzio is with Westmed Medical Group, Rye, N.Y.; Dr. Nayar is a Pulmonary/Critical Care Fellow at NYU School of Medicine; and Dr. Patel is with Columbia University Medical Center.
Vaping in 2019: Risk vs. reward
The prevalence and popularity of electronic cigarettes or “vaping” have grown dramatically over the last several years in the United States. Although new studies targeting these products are being done at increasing frequency, there remains a relative paucity of data regarding the long-term risks. Proponents argue that they can be used as a cessation tool for smokers, or failing that, a safer replacement for traditional cigarettes. Opponents make the case that the perception of safety could contribute to increased use in people who may have otherwise never smoked, leading to an overall increase in nicotine use and addiction. This is most readily seen in the adolescent population, where use has skyrocketed, leading to concerns about how electronic cigarettes are marketed to youth, as well as the ease of access.
Basics of vaping (devices)
In its most basic form, an electronic cigarette consists of a battery that powers a heating coil. This heating coil applies heat to a wick, which is soaked in liquid, “vape juice,” converting it into a vapor that is then directly inhaled. However, there can be many variations on this simple theme. Early generation products resembled traditional cigarettes in size and shape and were marketed as smoking cessation aids. Newer devices have abandoned this look and strategy. Preloaded cartridges have been replaced by large tanks that the user can fill with the liquid of their choosing. Multiple tanks can be purchased for a single device, enabling the user to have multiple flavors or various levels of nicotine dosing on hand for quick changing, depending on user preference or mood. Additionally, there are variable voltage settings, resulting in different styles of vapor and/or “throat hit” (the description of the desired burning vs smooth effect of the vapor on the oropharynx). This type of device invites experimentation. Multiple flavors can be used in isolation or mixed together at various temperatures. It no longer resembles classic cigarettes, and the flavor and experience are more prominently promoted. One can see that this device has more appeal to a “never smoker” than the original products, and there is concern that it is being marketed as such with some success (Dinakar C, et al. N Engl J Med. 2016;375[14]:1372).
E-liquid
Perhaps more important than the devices themselves is an understanding of the components of the liquid used to generate the inhaled aerosol.
Typically, four components are present:
• Propylene glycol
• Vegetable glycerin
• Flavoring
• Nicotine
The first two components are generally considered nontoxic, based on their use as food additives. However, inhalation is a novel route of entry and the long-term effects on the respiratory tract are unclear.
The third component, “flavorings,” is a catch-all term for the hundreds of different flavors and styles of e-liquids available today, ranging from menthol to fruit or candy and everything in between. It is difficult to account for all the potential effects of the numerous flavorings being used, especially when some are combined by the end user to various degrees.
Nicotine is present, specified in varying doses. However, vaping style, experience, and type of device used can dramatically affect how much is absorbed, making dosages difficult to predict. Additionally, labeled doses are prone to wide ranges of error (Schraufnagel DE, et al. Am J Respir Crit Care Med. 2014;190[6]:611).
What are the risks?
Cancer
A handful of known carcinogens can be found in inhaled vapor, including formaldehyde, acetaldehyde, acrolein, toluene, and nitrosamines. However, they are present in far lower concentrations than in traditional cigarettes (Goniewicz ML, et al. JAMA Netw Open. 2018;1[8]e185937). This leads to the natural assumption that vaping, while not benign, poses a much lower cancer risk when compared with smoking. Whether that is borne out in the long term remains to be seen.
Pulmonary function
The long-term effect on pulmonary function is not known. Small studies have shown no significant changes to spirometry after acute exposure to vapor. More data are needed in this area (Palazzolo DL. Frontiers Public Health. 2013;1[56]1-20).
Wound healing
An animal study has shown evidence of poor wound healing extrapolated from skin flap necrosis in rats. Exposure to vapor vs smoke yielded similar results, and both were worse than the sham arm (Troiano C, et al. JAMA Facial Plast Surg. 2019;21[1]:5). While it is difficult to know how to apply this clinically, it may be prudent to advise patients to abstain while in preparation for elective surgery.
Cardiovascular/stroke
Much of the cardiovascular toxicity from cigarette use is tied to the myriad of complex toxic particles produced in inhaled smoke, the vast majority of which are not present in e-cigarette vapor. While nicotine itself has known acute cardiovascular effects, including tachycardia and vasoconstriction, a tolerance to these effects occurs over time. Previous evaluations of nicotine replacement therapies and smokeless tobacco for their cardiovascular effects have had mixed results. But, there appears to be a trend toward minimal cardiovascular risk when using “cleaner” products, such as nicotine replacement therapy compared with smokeless tobacco (Benowitz NL, et al. Nature Rev Cardiol. 2017;14[8]:447). Whether this can be extrapolated to electronic cigarette use is unknown but is encouraging.
Alternative toxicity
In addition to the above risks that are in comparison to traditional smoking, vaping also introduces novel toxicities. There are case reports of lipoid pneumonia, ARDS, hypersensitivity pneumonitis, eosinophilic pneumonia, and diffuse alveola hemorrhage. Burns from malfunctioning devices must also be considered, as there is a wide array of products available, at differing levels of build quality.
Toxic oral ingestion of nicotine, especially by children, has led to increased calls to poison centers. For a small child, this can be fatal. Regulation of labels and containers could curtail this issue. But, public education regarding the toxicity of these substances when ingested in large quantities is also important. If there is a lack of understanding about this danger, then typical safeguards are easily overlooked by individual users.
Are there benefits?
Smoking cessation
Compared with other products, such as nicotine patches, gum, and pharmaceutical methods, e-cigarettes most closely mimic the actual experience of smoking. For some, the habit and ritual of smoking is as much a part of the addiction as nicotine. Vaping has the potential to help alleviate this difficult aspect of cessation. Data involving early generation products failed to show a significant advantage. Newer devices that are more pleasurable to use and offer more efficient nicotine delivery may be more effective. Indeed, a recent study in the New England Journal of Medicine from this year demonstrated improved smoking cessation compared with traditional methods, using second generation vape devices (Hajek P, et al. N Engl J Med. 2019;380[7]629). It will be interesting to see if this can be repeatable going forward and if protocols can be established to maximize effectiveness.
As outlined above, it is difficult to make definitive conclusions or recommendations regarding electronic cigarette use at the present time. The risk of cancer and cardiopulmonary disease is likely to be significantly lower but not eliminated. Use as a smoking cessation aid is starting to show promise. Even without cessation, ongoing vaping is likely to be safer than ongoing smoking. Two caveats to this remain: some patients, in an effort to quit smoking, may take up vaping but eventually become “dual users.” This scenario has been associated with higher toxic exposure and possibly worse outcomes. The second caveat is that while there is promise to using this as a cessation tool, it should not yet replace other more well-studied, first-line agents in this regard. It should, perhaps, target patients who are motivated to quit but have failed more traditional methods. Finally, there continues to be concern that vaping could appeal to never smokers, given its perceived safety profile and ease of use in public places. This could lead to an overall increase in nicotine addiction, which could be a significant step backwards.
Dr. Clark is Assistant Professor, Pulmonary and Critical Care Medicine, UT Southwestern Medical Center, Dallas, Texas.
The prevalence and popularity of electronic cigarettes or “vaping” have grown dramatically over the last several years in the United States. Although new studies targeting these products are being done at increasing frequency, there remains a relative paucity of data regarding the long-term risks. Proponents argue that they can be used as a cessation tool for smokers, or failing that, a safer replacement for traditional cigarettes. Opponents make the case that the perception of safety could contribute to increased use in people who may have otherwise never smoked, leading to an overall increase in nicotine use and addiction. This is most readily seen in the adolescent population, where use has skyrocketed, leading to concerns about how electronic cigarettes are marketed to youth, as well as the ease of access.
Basics of vaping (devices)
In its most basic form, an electronic cigarette consists of a battery that powers a heating coil. This heating coil applies heat to a wick, which is soaked in liquid, “vape juice,” converting it into a vapor that is then directly inhaled. However, there can be many variations on this simple theme. Early generation products resembled traditional cigarettes in size and shape and were marketed as smoking cessation aids. Newer devices have abandoned this look and strategy. Preloaded cartridges have been replaced by large tanks that the user can fill with the liquid of their choosing. Multiple tanks can be purchased for a single device, enabling the user to have multiple flavors or various levels of nicotine dosing on hand for quick changing, depending on user preference or mood. Additionally, there are variable voltage settings, resulting in different styles of vapor and/or “throat hit” (the description of the desired burning vs smooth effect of the vapor on the oropharynx). This type of device invites experimentation. Multiple flavors can be used in isolation or mixed together at various temperatures. It no longer resembles classic cigarettes, and the flavor and experience are more prominently promoted. One can see that this device has more appeal to a “never smoker” than the original products, and there is concern that it is being marketed as such with some success (Dinakar C, et al. N Engl J Med. 2016;375[14]:1372).
E-liquid
Perhaps more important than the devices themselves is an understanding of the components of the liquid used to generate the inhaled aerosol.
Typically, four components are present:
• Propylene glycol
• Vegetable glycerin
• Flavoring
• Nicotine
The first two components are generally considered nontoxic, based on their use as food additives. However, inhalation is a novel route of entry and the long-term effects on the respiratory tract are unclear.
The third component, “flavorings,” is a catch-all term for the hundreds of different flavors and styles of e-liquids available today, ranging from menthol to fruit or candy and everything in between. It is difficult to account for all the potential effects of the numerous flavorings being used, especially when some are combined by the end user to various degrees.
Nicotine is present, specified in varying doses. However, vaping style, experience, and type of device used can dramatically affect how much is absorbed, making dosages difficult to predict. Additionally, labeled doses are prone to wide ranges of error (Schraufnagel DE, et al. Am J Respir Crit Care Med. 2014;190[6]:611).
What are the risks?
Cancer
A handful of known carcinogens can be found in inhaled vapor, including formaldehyde, acetaldehyde, acrolein, toluene, and nitrosamines. However, they are present in far lower concentrations than in traditional cigarettes (Goniewicz ML, et al. JAMA Netw Open. 2018;1[8]e185937). This leads to the natural assumption that vaping, while not benign, poses a much lower cancer risk when compared with smoking. Whether that is borne out in the long term remains to be seen.
Pulmonary function
The long-term effect on pulmonary function is not known. Small studies have shown no significant changes to spirometry after acute exposure to vapor. More data are needed in this area (Palazzolo DL. Frontiers Public Health. 2013;1[56]1-20).
Wound healing
An animal study has shown evidence of poor wound healing extrapolated from skin flap necrosis in rats. Exposure to vapor vs smoke yielded similar results, and both were worse than the sham arm (Troiano C, et al. JAMA Facial Plast Surg. 2019;21[1]:5). While it is difficult to know how to apply this clinically, it may be prudent to advise patients to abstain while in preparation for elective surgery.
Cardiovascular/stroke
Much of the cardiovascular toxicity from cigarette use is tied to the myriad of complex toxic particles produced in inhaled smoke, the vast majority of which are not present in e-cigarette vapor. While nicotine itself has known acute cardiovascular effects, including tachycardia and vasoconstriction, a tolerance to these effects occurs over time. Previous evaluations of nicotine replacement therapies and smokeless tobacco for their cardiovascular effects have had mixed results. But, there appears to be a trend toward minimal cardiovascular risk when using “cleaner” products, such as nicotine replacement therapy compared with smokeless tobacco (Benowitz NL, et al. Nature Rev Cardiol. 2017;14[8]:447). Whether this can be extrapolated to electronic cigarette use is unknown but is encouraging.
Alternative toxicity
In addition to the above risks that are in comparison to traditional smoking, vaping also introduces novel toxicities. There are case reports of lipoid pneumonia, ARDS, hypersensitivity pneumonitis, eosinophilic pneumonia, and diffuse alveola hemorrhage. Burns from malfunctioning devices must also be considered, as there is a wide array of products available, at differing levels of build quality.
Toxic oral ingestion of nicotine, especially by children, has led to increased calls to poison centers. For a small child, this can be fatal. Regulation of labels and containers could curtail this issue. But, public education regarding the toxicity of these substances when ingested in large quantities is also important. If there is a lack of understanding about this danger, then typical safeguards are easily overlooked by individual users.
Are there benefits?
Smoking cessation
Compared with other products, such as nicotine patches, gum, and pharmaceutical methods, e-cigarettes most closely mimic the actual experience of smoking. For some, the habit and ritual of smoking is as much a part of the addiction as nicotine. Vaping has the potential to help alleviate this difficult aspect of cessation. Data involving early generation products failed to show a significant advantage. Newer devices that are more pleasurable to use and offer more efficient nicotine delivery may be more effective. Indeed, a recent study in the New England Journal of Medicine from this year demonstrated improved smoking cessation compared with traditional methods, using second generation vape devices (Hajek P, et al. N Engl J Med. 2019;380[7]629). It will be interesting to see if this can be repeatable going forward and if protocols can be established to maximize effectiveness.
As outlined above, it is difficult to make definitive conclusions or recommendations regarding electronic cigarette use at the present time. The risk of cancer and cardiopulmonary disease is likely to be significantly lower but not eliminated. Use as a smoking cessation aid is starting to show promise. Even without cessation, ongoing vaping is likely to be safer than ongoing smoking. Two caveats to this remain: some patients, in an effort to quit smoking, may take up vaping but eventually become “dual users.” This scenario has been associated with higher toxic exposure and possibly worse outcomes. The second caveat is that while there is promise to using this as a cessation tool, it should not yet replace other more well-studied, first-line agents in this regard. It should, perhaps, target patients who are motivated to quit but have failed more traditional methods. Finally, there continues to be concern that vaping could appeal to never smokers, given its perceived safety profile and ease of use in public places. This could lead to an overall increase in nicotine addiction, which could be a significant step backwards.
Dr. Clark is Assistant Professor, Pulmonary and Critical Care Medicine, UT Southwestern Medical Center, Dallas, Texas.
The prevalence and popularity of electronic cigarettes or “vaping” have grown dramatically over the last several years in the United States. Although new studies targeting these products are being done at increasing frequency, there remains a relative paucity of data regarding the long-term risks. Proponents argue that they can be used as a cessation tool for smokers, or failing that, a safer replacement for traditional cigarettes. Opponents make the case that the perception of safety could contribute to increased use in people who may have otherwise never smoked, leading to an overall increase in nicotine use and addiction. This is most readily seen in the adolescent population, where use has skyrocketed, leading to concerns about how electronic cigarettes are marketed to youth, as well as the ease of access.
Basics of vaping (devices)
In its most basic form, an electronic cigarette consists of a battery that powers a heating coil. This heating coil applies heat to a wick, which is soaked in liquid, “vape juice,” converting it into a vapor that is then directly inhaled. However, there can be many variations on this simple theme. Early generation products resembled traditional cigarettes in size and shape and were marketed as smoking cessation aids. Newer devices have abandoned this look and strategy. Preloaded cartridges have been replaced by large tanks that the user can fill with the liquid of their choosing. Multiple tanks can be purchased for a single device, enabling the user to have multiple flavors or various levels of nicotine dosing on hand for quick changing, depending on user preference or mood. Additionally, there are variable voltage settings, resulting in different styles of vapor and/or “throat hit” (the description of the desired burning vs smooth effect of the vapor on the oropharynx). This type of device invites experimentation. Multiple flavors can be used in isolation or mixed together at various temperatures. It no longer resembles classic cigarettes, and the flavor and experience are more prominently promoted. One can see that this device has more appeal to a “never smoker” than the original products, and there is concern that it is being marketed as such with some success (Dinakar C, et al. N Engl J Med. 2016;375[14]:1372).
E-liquid
Perhaps more important than the devices themselves is an understanding of the components of the liquid used to generate the inhaled aerosol.
Typically, four components are present:
• Propylene glycol
• Vegetable glycerin
• Flavoring
• Nicotine
The first two components are generally considered nontoxic, based on their use as food additives. However, inhalation is a novel route of entry and the long-term effects on the respiratory tract are unclear.
The third component, “flavorings,” is a catch-all term for the hundreds of different flavors and styles of e-liquids available today, ranging from menthol to fruit or candy and everything in between. It is difficult to account for all the potential effects of the numerous flavorings being used, especially when some are combined by the end user to various degrees.
Nicotine is present, specified in varying doses. However, vaping style, experience, and type of device used can dramatically affect how much is absorbed, making dosages difficult to predict. Additionally, labeled doses are prone to wide ranges of error (Schraufnagel DE, et al. Am J Respir Crit Care Med. 2014;190[6]:611).
What are the risks?
Cancer
A handful of known carcinogens can be found in inhaled vapor, including formaldehyde, acetaldehyde, acrolein, toluene, and nitrosamines. However, they are present in far lower concentrations than in traditional cigarettes (Goniewicz ML, et al. JAMA Netw Open. 2018;1[8]e185937). This leads to the natural assumption that vaping, while not benign, poses a much lower cancer risk when compared with smoking. Whether that is borne out in the long term remains to be seen.
Pulmonary function
The long-term effect on pulmonary function is not known. Small studies have shown no significant changes to spirometry after acute exposure to vapor. More data are needed in this area (Palazzolo DL. Frontiers Public Health. 2013;1[56]1-20).
Wound healing
An animal study has shown evidence of poor wound healing extrapolated from skin flap necrosis in rats. Exposure to vapor vs smoke yielded similar results, and both were worse than the sham arm (Troiano C, et al. JAMA Facial Plast Surg. 2019;21[1]:5). While it is difficult to know how to apply this clinically, it may be prudent to advise patients to abstain while in preparation for elective surgery.
Cardiovascular/stroke
Much of the cardiovascular toxicity from cigarette use is tied to the myriad of complex toxic particles produced in inhaled smoke, the vast majority of which are not present in e-cigarette vapor. While nicotine itself has known acute cardiovascular effects, including tachycardia and vasoconstriction, a tolerance to these effects occurs over time. Previous evaluations of nicotine replacement therapies and smokeless tobacco for their cardiovascular effects have had mixed results. But, there appears to be a trend toward minimal cardiovascular risk when using “cleaner” products, such as nicotine replacement therapy compared with smokeless tobacco (Benowitz NL, et al. Nature Rev Cardiol. 2017;14[8]:447). Whether this can be extrapolated to electronic cigarette use is unknown but is encouraging.
Alternative toxicity
In addition to the above risks that are in comparison to traditional smoking, vaping also introduces novel toxicities. There are case reports of lipoid pneumonia, ARDS, hypersensitivity pneumonitis, eosinophilic pneumonia, and diffuse alveola hemorrhage. Burns from malfunctioning devices must also be considered, as there is a wide array of products available, at differing levels of build quality.
Toxic oral ingestion of nicotine, especially by children, has led to increased calls to poison centers. For a small child, this can be fatal. Regulation of labels and containers could curtail this issue. But, public education regarding the toxicity of these substances when ingested in large quantities is also important. If there is a lack of understanding about this danger, then typical safeguards are easily overlooked by individual users.
Are there benefits?
Smoking cessation
Compared with other products, such as nicotine patches, gum, and pharmaceutical methods, e-cigarettes most closely mimic the actual experience of smoking. For some, the habit and ritual of smoking is as much a part of the addiction as nicotine. Vaping has the potential to help alleviate this difficult aspect of cessation. Data involving early generation products failed to show a significant advantage. Newer devices that are more pleasurable to use and offer more efficient nicotine delivery may be more effective. Indeed, a recent study in the New England Journal of Medicine from this year demonstrated improved smoking cessation compared with traditional methods, using second generation vape devices (Hajek P, et al. N Engl J Med. 2019;380[7]629). It will be interesting to see if this can be repeatable going forward and if protocols can be established to maximize effectiveness.
As outlined above, it is difficult to make definitive conclusions or recommendations regarding electronic cigarette use at the present time. The risk of cancer and cardiopulmonary disease is likely to be significantly lower but not eliminated. Use as a smoking cessation aid is starting to show promise. Even without cessation, ongoing vaping is likely to be safer than ongoing smoking. Two caveats to this remain: some patients, in an effort to quit smoking, may take up vaping but eventually become “dual users.” This scenario has been associated with higher toxic exposure and possibly worse outcomes. The second caveat is that while there is promise to using this as a cessation tool, it should not yet replace other more well-studied, first-line agents in this regard. It should, perhaps, target patients who are motivated to quit but have failed more traditional methods. Finally, there continues to be concern that vaping could appeal to never smokers, given its perceived safety profile and ease of use in public places. This could lead to an overall increase in nicotine addiction, which could be a significant step backwards.
Dr. Clark is Assistant Professor, Pulmonary and Critical Care Medicine, UT Southwestern Medical Center, Dallas, Texas.
Endobronchial valves for lung volume reduction: What can we offer patients with advanced emphysema?
The global burden COPD is considerable. In the United States, it is the third most common cause of death and is associated with over $50 billion in annual direct and indirect health-care expenditures (Guarascio AJ, et al. Clinicoecon Outcomes Res. 2013;5:235). For patients with severe emphysema with hyperinflation, dyspnea is often a quality of life (QOL)-limiting symptom (O’Donnell DE, et al. Ann Am Thorac Soc. 2017;14:S30). Few proven palliation options exist, particularly for patients with dyspnea refractory to smoking cessation, medical management with bronchodilators, and pulmonary rehabilitation. The recent Food and Drug Administration (FDA) approval of two endobronchial valves for lung volume reduction has established the increasing importance of bronchoscopy as a management tool in advanced COPD.
Why were these valves developed?
For decades, lung volume reduction has been investigated as a mechanical approach to counter-act the physiologic effects of emphysematous hyperinflation. Its goal is to improve lung elastic recoil, respiratory muscle mechanical advantage and efficiency, and ventilation/perfusion matching. The landmark National Emphysema Treatment Trial (NETT), published in 2001 and 2003, demonstrated that in a select patient population (upper lobe-predominant emphysema and low exercise capacity), lung volume reduction surgery (LVRS) lowers mortality and improves QOL and exercise tolerance (Fishman A et al. N Engl J Med. 2003;348:2059). Despite the encouraging results in this study subpopulation, LVRS is per-formed infrequently (Decker MR, et al. J Thorac Cardiovasc Surg. 2014;148:2651). Concern about its morbidity and the specialized nature of the procedure has hindered widespread adoption. Subsequently, endobronchial techniques have been developed as an alternative to surgical lung volume reduction.
How does bronchoscopic lung volume reduction (BLVR) benefit patients with emphysema?
Valves used for ELVR are removable one-way flow devices placed by flexible bronchoscopy into selected airways supplying emphysematous lung. The valves block air entry but allow the exit of secretions and trapped air. This results in atelectasis of the targeted lobe and a decrease in lung volume.
Which endobronchial valves are available in the United States?
In 2018, two valves were approved by the FDA for bronchoscopic lung volume reduction (BLVR) – the Zephyr® EBV (Pulmonx) ( (Fig 1) and the Spiration® Valve System (Olympus) (IBV) (Fig 2). The Zephyr® EBV is a duckbill-shaped silicone valve mounted within a self-expanding nitinol (nickel titanium alloy) stent. It comes in three sizes for airways with a diameter 4 - 8.5 mm. The Spiration® IBV umbrella-shaped valve is com-posed of six nitinol struts surfaced with polyurethane. Its four sizes accommodate airway diameters 5 - 9 mm.
What’s the evidence behind BLVR?
Zephyr® Valves
The Endobronchial Valve for Emphysema Palliation Trial (VENT), the largest valve trial thus far, randomized patients with severe heterogeneous emphysema to receive unilateral Zephyr® valve placement or standard medical care (Sciurba FC, et al. N Engl J Med. 2010;363:1233). Overall improvement in spirometry and dyspnea scores was modest in the valve group. Post-hoc analysis identified an important subgroup of patients with significant clinical benefit, those with a complete fissure. This finding gave guidance to further EBV studies on patients with severe emphysema and absent collateral ventilation (CV).
Identifying a complete fissure on imaging is now used as a surrogate for assessing CV and is an integral part of the initial profiling of patients for EBV therapy (Koster TD, et al. Respiration. 2016;92(3):150).
In the STELVIO trial, 68 patients were randomized to Zephyr ® EBV placement or standard medical care (Klooster K, et al. N Engl J Med. 2015;373:2325). Those with EBV placement had significantly improved lung function and exercise capacity. TRANSFORM, a multicenter trial evaluating Zephyr® EBV placement in heterogeneous emphysema, showed similar results (Kemp SV, et al. Am J Respir Crit Care Med. 2017;196:1535).
The IMPACT trial compared patients with homogenous emphysema without CV to standard medical therapy alone. It showed improvement in FEV1, QOL scores, and exercise tolerance in the EBV group. This study affirmed that the absence of CV, rather than the pattern of emphysema, correlates with the clinical benefit from EBV therapy (Valipour A, et al. Am J Respir Crit Care Med. 2016;194(9):1073). Finally, LIBERATE, a multicenter study on the Zephyr® EBV, examined its placement in patients with heterogenous emphysema. This study demonstrated improvement in spirometry, QOL, and 6-minute walk test (6-MWT) distance (Criner GJ, et al. Am J Respir Crit Care Med. 2018;198:1151) over a longer period, 12 months, bolstering the findings of prior studies. These results prompted the Zephyr® valve’s FDA approval.
Spiration® Valves
Small trials have shown favorable results with the Spiration® IBV for BLVR, including a pilot multicenter cohort study of 30 patients with heterogeneous, upper-lobe emphysema who underwent valve placement (Wood DE, et al. J Thorac Cardiovasc Surg. 2007;133:65). In this trial, investigators found significant improvement in QOL scores, but no change in FEV1 or other physiologic parameters.
The EMPROVE trial is a multicenter, prospective, randomized, controlled study assessing BLVR with the Spiration® IBV. Six- and twelve-month data from the trial were presented in 2018 at the American Thoracic Society Conference and at the European Respiratory Society International Conference.
Collateral Ventilation
Identifying patients in whom there is no CV between lobes is critical to success with BLVR. Collateral ventilation allows air to bypass the valve occlusion distally, thereby negating the desired effect of valve placement, lobar atelectasis. High-resolution computed tomography (HRCT) scanning combined with quantitative software can be used to assess emphysema distribution and fissure integrity. Additionally, a proprietary technology, the Chartis System®, can be employed intra-procedure to estimate CV by measuring airway flow, resistance, and pressure in targeted balloon-occluded segments. Absence of CV based on Chartis evaluation was an inclusion criterion in the aforementioned valve studies.
Which patients with emphysema should be referred for consideration of valve placement?
The following criteria should be used in selecting patients for referral for BLVR:
• FEV1 15% - 45% of predicted value at baseline
• Evidence of hyperinflation: TLC greater than or equal to 100% and RV greater than or equal to 175%
• Baseline postpulmonary rehabilitation 6-MWT distance of 100 - 500 meters
• Clinically stable on < 20 mg prednisone (or equivalent) daily
• Nonsmoking for at least 4 months
• Integrity of one or both major fissures at least 75%
• Ability to provide informed consent and to tolerate bronchoscopy
Complications
The most common complication after valve placement is pneumothorax – a double-edged sword in that it typically indicates the achievement of atelectasis. In published trials, the frequency of pneumothorax varies. Some studies document rates below 10%. Others report rates of nearly 30% (Gompelmann D, et al. Respiration. 2014;87:485). In landmark trials, death related to pneumothorax occurred rarely. Most severe pneumothoraces occur within the first 72 hours after valve placement. This has prompted many centers to observe postprocedure patients in hospital for an extended period. Pneumonia and COPD exacerbations have also been reported after EBV placement. Therefore, in some trials, patients received prophylactic prednisolone and azithromycin. Other less common complications are hemoptysis, granulation tissue formation, and valve migration.
What’s ahead for ELVR?
Overall, valve technology for BLVR is an exciting option in the management of patients with severe emphysema and is now a staple for any advanced emphysema program. Key areas of future interest include management of patients with partial fissures, minimizing adverse procedural effects, and developing programs to optimize and streamline a multidisciplinary approach to timely and efficient referral, assessment, and intervention. As more patients with COPD undergo ELVR, one goal should be to create multi-institution prospective studies as well as registries to delineate further the optimal use of endobronchial valves for lung volume reduction.
Zephyr® Endobronchial Valve (Pulmonx)
Spiration® Valve System (Olympus)
The American College of Chest Physicians (CHEST) does not endorse or supp
The global burden COPD is considerable. In the United States, it is the third most common cause of death and is associated with over $50 billion in annual direct and indirect health-care expenditures (Guarascio AJ, et al. Clinicoecon Outcomes Res. 2013;5:235). For patients with severe emphysema with hyperinflation, dyspnea is often a quality of life (QOL)-limiting symptom (O’Donnell DE, et al. Ann Am Thorac Soc. 2017;14:S30). Few proven palliation options exist, particularly for patients with dyspnea refractory to smoking cessation, medical management with bronchodilators, and pulmonary rehabilitation. The recent Food and Drug Administration (FDA) approval of two endobronchial valves for lung volume reduction has established the increasing importance of bronchoscopy as a management tool in advanced COPD.
Why were these valves developed?
For decades, lung volume reduction has been investigated as a mechanical approach to counter-act the physiologic effects of emphysematous hyperinflation. Its goal is to improve lung elastic recoil, respiratory muscle mechanical advantage and efficiency, and ventilation/perfusion matching. The landmark National Emphysema Treatment Trial (NETT), published in 2001 and 2003, demonstrated that in a select patient population (upper lobe-predominant emphysema and low exercise capacity), lung volume reduction surgery (LVRS) lowers mortality and improves QOL and exercise tolerance (Fishman A et al. N Engl J Med. 2003;348:2059). Despite the encouraging results in this study subpopulation, LVRS is per-formed infrequently (Decker MR, et al. J Thorac Cardiovasc Surg. 2014;148:2651). Concern about its morbidity and the specialized nature of the procedure has hindered widespread adoption. Subsequently, endobronchial techniques have been developed as an alternative to surgical lung volume reduction.
How does bronchoscopic lung volume reduction (BLVR) benefit patients with emphysema?
Valves used for ELVR are removable one-way flow devices placed by flexible bronchoscopy into selected airways supplying emphysematous lung. The valves block air entry but allow the exit of secretions and trapped air. This results in atelectasis of the targeted lobe and a decrease in lung volume.
Which endobronchial valves are available in the United States?
In 2018, two valves were approved by the FDA for bronchoscopic lung volume reduction (BLVR) – the Zephyr® EBV (Pulmonx) ( (Fig 1) and the Spiration® Valve System (Olympus) (IBV) (Fig 2). The Zephyr® EBV is a duckbill-shaped silicone valve mounted within a self-expanding nitinol (nickel titanium alloy) stent. It comes in three sizes for airways with a diameter 4 - 8.5 mm. The Spiration® IBV umbrella-shaped valve is com-posed of six nitinol struts surfaced with polyurethane. Its four sizes accommodate airway diameters 5 - 9 mm.
What’s the evidence behind BLVR?
Zephyr® Valves
The Endobronchial Valve for Emphysema Palliation Trial (VENT), the largest valve trial thus far, randomized patients with severe heterogeneous emphysema to receive unilateral Zephyr® valve placement or standard medical care (Sciurba FC, et al. N Engl J Med. 2010;363:1233). Overall improvement in spirometry and dyspnea scores was modest in the valve group. Post-hoc analysis identified an important subgroup of patients with significant clinical benefit, those with a complete fissure. This finding gave guidance to further EBV studies on patients with severe emphysema and absent collateral ventilation (CV).
Identifying a complete fissure on imaging is now used as a surrogate for assessing CV and is an integral part of the initial profiling of patients for EBV therapy (Koster TD, et al. Respiration. 2016;92(3):150).
In the STELVIO trial, 68 patients were randomized to Zephyr ® EBV placement or standard medical care (Klooster K, et al. N Engl J Med. 2015;373:2325). Those with EBV placement had significantly improved lung function and exercise capacity. TRANSFORM, a multicenter trial evaluating Zephyr® EBV placement in heterogeneous emphysema, showed similar results (Kemp SV, et al. Am J Respir Crit Care Med. 2017;196:1535).
The IMPACT trial compared patients with homogenous emphysema without CV to standard medical therapy alone. It showed improvement in FEV1, QOL scores, and exercise tolerance in the EBV group. This study affirmed that the absence of CV, rather than the pattern of emphysema, correlates with the clinical benefit from EBV therapy (Valipour A, et al. Am J Respir Crit Care Med. 2016;194(9):1073). Finally, LIBERATE, a multicenter study on the Zephyr® EBV, examined its placement in patients with heterogenous emphysema. This study demonstrated improvement in spirometry, QOL, and 6-minute walk test (6-MWT) distance (Criner GJ, et al. Am J Respir Crit Care Med. 2018;198:1151) over a longer period, 12 months, bolstering the findings of prior studies. These results prompted the Zephyr® valve’s FDA approval.
Spiration® Valves
Small trials have shown favorable results with the Spiration® IBV for BLVR, including a pilot multicenter cohort study of 30 patients with heterogeneous, upper-lobe emphysema who underwent valve placement (Wood DE, et al. J Thorac Cardiovasc Surg. 2007;133:65). In this trial, investigators found significant improvement in QOL scores, but no change in FEV1 or other physiologic parameters.
The EMPROVE trial is a multicenter, prospective, randomized, controlled study assessing BLVR with the Spiration® IBV. Six- and twelve-month data from the trial were presented in 2018 at the American Thoracic Society Conference and at the European Respiratory Society International Conference.
Collateral Ventilation
Identifying patients in whom there is no CV between lobes is critical to success with BLVR. Collateral ventilation allows air to bypass the valve occlusion distally, thereby negating the desired effect of valve placement, lobar atelectasis. High-resolution computed tomography (HRCT) scanning combined with quantitative software can be used to assess emphysema distribution and fissure integrity. Additionally, a proprietary technology, the Chartis System®, can be employed intra-procedure to estimate CV by measuring airway flow, resistance, and pressure in targeted balloon-occluded segments. Absence of CV based on Chartis evaluation was an inclusion criterion in the aforementioned valve studies.
Which patients with emphysema should be referred for consideration of valve placement?
The following criteria should be used in selecting patients for referral for BLVR:
• FEV1 15% - 45% of predicted value at baseline
• Evidence of hyperinflation: TLC greater than or equal to 100% and RV greater than or equal to 175%
• Baseline postpulmonary rehabilitation 6-MWT distance of 100 - 500 meters
• Clinically stable on < 20 mg prednisone (or equivalent) daily
• Nonsmoking for at least 4 months
• Integrity of one or both major fissures at least 75%
• Ability to provide informed consent and to tolerate bronchoscopy
Complications
The most common complication after valve placement is pneumothorax – a double-edged sword in that it typically indicates the achievement of atelectasis. In published trials, the frequency of pneumothorax varies. Some studies document rates below 10%. Others report rates of nearly 30% (Gompelmann D, et al. Respiration. 2014;87:485). In landmark trials, death related to pneumothorax occurred rarely. Most severe pneumothoraces occur within the first 72 hours after valve placement. This has prompted many centers to observe postprocedure patients in hospital for an extended period. Pneumonia and COPD exacerbations have also been reported after EBV placement. Therefore, in some trials, patients received prophylactic prednisolone and azithromycin. Other less common complications are hemoptysis, granulation tissue formation, and valve migration.
What’s ahead for ELVR?
Overall, valve technology for BLVR is an exciting option in the management of patients with severe emphysema and is now a staple for any advanced emphysema program. Key areas of future interest include management of patients with partial fissures, minimizing adverse procedural effects, and developing programs to optimize and streamline a multidisciplinary approach to timely and efficient referral, assessment, and intervention. As more patients with COPD undergo ELVR, one goal should be to create multi-institution prospective studies as well as registries to delineate further the optimal use of endobronchial valves for lung volume reduction.
Zephyr® Endobronchial Valve (Pulmonx)
Spiration® Valve System (Olympus)
The American College of Chest Physicians (CHEST) does not endorse or supp
The global burden COPD is considerable. In the United States, it is the third most common cause of death and is associated with over $50 billion in annual direct and indirect health-care expenditures (Guarascio AJ, et al. Clinicoecon Outcomes Res. 2013;5:235). For patients with severe emphysema with hyperinflation, dyspnea is often a quality of life (QOL)-limiting symptom (O’Donnell DE, et al. Ann Am Thorac Soc. 2017;14:S30). Few proven palliation options exist, particularly for patients with dyspnea refractory to smoking cessation, medical management with bronchodilators, and pulmonary rehabilitation. The recent Food and Drug Administration (FDA) approval of two endobronchial valves for lung volume reduction has established the increasing importance of bronchoscopy as a management tool in advanced COPD.
Why were these valves developed?
For decades, lung volume reduction has been investigated as a mechanical approach to counter-act the physiologic effects of emphysematous hyperinflation. Its goal is to improve lung elastic recoil, respiratory muscle mechanical advantage and efficiency, and ventilation/perfusion matching. The landmark National Emphysema Treatment Trial (NETT), published in 2001 and 2003, demonstrated that in a select patient population (upper lobe-predominant emphysema and low exercise capacity), lung volume reduction surgery (LVRS) lowers mortality and improves QOL and exercise tolerance (Fishman A et al. N Engl J Med. 2003;348:2059). Despite the encouraging results in this study subpopulation, LVRS is per-formed infrequently (Decker MR, et al. J Thorac Cardiovasc Surg. 2014;148:2651). Concern about its morbidity and the specialized nature of the procedure has hindered widespread adoption. Subsequently, endobronchial techniques have been developed as an alternative to surgical lung volume reduction.
How does bronchoscopic lung volume reduction (BLVR) benefit patients with emphysema?
Valves used for ELVR are removable one-way flow devices placed by flexible bronchoscopy into selected airways supplying emphysematous lung. The valves block air entry but allow the exit of secretions and trapped air. This results in atelectasis of the targeted lobe and a decrease in lung volume.
Which endobronchial valves are available in the United States?
In 2018, two valves were approved by the FDA for bronchoscopic lung volume reduction (BLVR) – the Zephyr® EBV (Pulmonx) ( (Fig 1) and the Spiration® Valve System (Olympus) (IBV) (Fig 2). The Zephyr® EBV is a duckbill-shaped silicone valve mounted within a self-expanding nitinol (nickel titanium alloy) stent. It comes in three sizes for airways with a diameter 4 - 8.5 mm. The Spiration® IBV umbrella-shaped valve is com-posed of six nitinol struts surfaced with polyurethane. Its four sizes accommodate airway diameters 5 - 9 mm.
What’s the evidence behind BLVR?
Zephyr® Valves
The Endobronchial Valve for Emphysema Palliation Trial (VENT), the largest valve trial thus far, randomized patients with severe heterogeneous emphysema to receive unilateral Zephyr® valve placement or standard medical care (Sciurba FC, et al. N Engl J Med. 2010;363:1233). Overall improvement in spirometry and dyspnea scores was modest in the valve group. Post-hoc analysis identified an important subgroup of patients with significant clinical benefit, those with a complete fissure. This finding gave guidance to further EBV studies on patients with severe emphysema and absent collateral ventilation (CV).
Identifying a complete fissure on imaging is now used as a surrogate for assessing CV and is an integral part of the initial profiling of patients for EBV therapy (Koster TD, et al. Respiration. 2016;92(3):150).
In the STELVIO trial, 68 patients were randomized to Zephyr ® EBV placement or standard medical care (Klooster K, et al. N Engl J Med. 2015;373:2325). Those with EBV placement had significantly improved lung function and exercise capacity. TRANSFORM, a multicenter trial evaluating Zephyr® EBV placement in heterogeneous emphysema, showed similar results (Kemp SV, et al. Am J Respir Crit Care Med. 2017;196:1535).
The IMPACT trial compared patients with homogenous emphysema without CV to standard medical therapy alone. It showed improvement in FEV1, QOL scores, and exercise tolerance in the EBV group. This study affirmed that the absence of CV, rather than the pattern of emphysema, correlates with the clinical benefit from EBV therapy (Valipour A, et al. Am J Respir Crit Care Med. 2016;194(9):1073). Finally, LIBERATE, a multicenter study on the Zephyr® EBV, examined its placement in patients with heterogenous emphysema. This study demonstrated improvement in spirometry, QOL, and 6-minute walk test (6-MWT) distance (Criner GJ, et al. Am J Respir Crit Care Med. 2018;198:1151) over a longer period, 12 months, bolstering the findings of prior studies. These results prompted the Zephyr® valve’s FDA approval.
Spiration® Valves
Small trials have shown favorable results with the Spiration® IBV for BLVR, including a pilot multicenter cohort study of 30 patients with heterogeneous, upper-lobe emphysema who underwent valve placement (Wood DE, et al. J Thorac Cardiovasc Surg. 2007;133:65). In this trial, investigators found significant improvement in QOL scores, but no change in FEV1 or other physiologic parameters.
The EMPROVE trial is a multicenter, prospective, randomized, controlled study assessing BLVR with the Spiration® IBV. Six- and twelve-month data from the trial were presented in 2018 at the American Thoracic Society Conference and at the European Respiratory Society International Conference.
Collateral Ventilation
Identifying patients in whom there is no CV between lobes is critical to success with BLVR. Collateral ventilation allows air to bypass the valve occlusion distally, thereby negating the desired effect of valve placement, lobar atelectasis. High-resolution computed tomography (HRCT) scanning combined with quantitative software can be used to assess emphysema distribution and fissure integrity. Additionally, a proprietary technology, the Chartis System®, can be employed intra-procedure to estimate CV by measuring airway flow, resistance, and pressure in targeted balloon-occluded segments. Absence of CV based on Chartis evaluation was an inclusion criterion in the aforementioned valve studies.
Which patients with emphysema should be referred for consideration of valve placement?
The following criteria should be used in selecting patients for referral for BLVR:
• FEV1 15% - 45% of predicted value at baseline
• Evidence of hyperinflation: TLC greater than or equal to 100% and RV greater than or equal to 175%
• Baseline postpulmonary rehabilitation 6-MWT distance of 100 - 500 meters
• Clinically stable on < 20 mg prednisone (or equivalent) daily
• Nonsmoking for at least 4 months
• Integrity of one or both major fissures at least 75%
• Ability to provide informed consent and to tolerate bronchoscopy
Complications
The most common complication after valve placement is pneumothorax – a double-edged sword in that it typically indicates the achievement of atelectasis. In published trials, the frequency of pneumothorax varies. Some studies document rates below 10%. Others report rates of nearly 30% (Gompelmann D, et al. Respiration. 2014;87:485). In landmark trials, death related to pneumothorax occurred rarely. Most severe pneumothoraces occur within the first 72 hours after valve placement. This has prompted many centers to observe postprocedure patients in hospital for an extended period. Pneumonia and COPD exacerbations have also been reported after EBV placement. Therefore, in some trials, patients received prophylactic prednisolone and azithromycin. Other less common complications are hemoptysis, granulation tissue formation, and valve migration.
What’s ahead for ELVR?
Overall, valve technology for BLVR is an exciting option in the management of patients with severe emphysema and is now a staple for any advanced emphysema program. Key areas of future interest include management of patients with partial fissures, minimizing adverse procedural effects, and developing programs to optimize and streamline a multidisciplinary approach to timely and efficient referral, assessment, and intervention. As more patients with COPD undergo ELVR, one goal should be to create multi-institution prospective studies as well as registries to delineate further the optimal use of endobronchial valves for lung volume reduction.
Zephyr® Endobronchial Valve (Pulmonx)
Spiration® Valve System (Olympus)
The American College of Chest Physicians (CHEST) does not endorse or supp
Social media for physicians: Strong medicine or snake oil?
For most of us, social media is a daunting new reality that we are pressured to be part of but that we struggle to fit into our increasingly demanding schedules. My first social media foray as a physician was a Facebook fan page as a hobby rather than a professional presence. Years later, I have learned the incredible benefit that being on social media in other platforms brought to my profession.
What’s social media going to bring to my medical practice?
The days where physicians retreat to the safety of our offices to deliver our care, or to issue carefully structured opinions, or interactions with patients have made way for more direct interaction. Social media has, indeed, allowed us to share more personal glimpses of our daily struggle to save lives, behind-the-scenes snapshot of ethical struggles in decision making, our difficulties qualifying patients for therapies due to insurance complications, or real-time addressing medical news and combating misinformation. Moreover, when patients self-refer, or are referred to my practice, they look me up online before coming to my office. Online profiles are the new “first impression” of the bedside manner of a physician.
Other personal examples of social media benefits include being informed of new publications, since many journals now have an online presence; being able to interact in real-time with authors; learning from physicians in other countries how they handled issues, such as shortage of critical medications; or earning CME, such as the Twitter chats hosted by CHEST (eg, new biologic agents in difficult to treat asthma, or patient selection in triple therapy for COPD).
Why should I pay attention to social media presence?
The pace by which social media changed the landscape took the medical community by surprise. Patients, third-party websites, and online review agencies (official or not) adopted it well before physicians became comfortable with it. As such, when I decided to google myself online, I was shocked at the level of misinformation about me (as a pulmonologist, I didn’t know I had performed sigmoidoscopies, yet that’s what my patients learned before they met me). That was an important lesson: If I don’t control the narrative, someone else will. Consequently, I dedicated a few hours to establish an online presence in order to introduce myself accurately and to be accessible to my patients and colleagues online.
Who decides what’s ethical and what’s not?
As the lines blurred, our community struggled to define what was appropriate and what was not. Finally, we welcomed with relief the issuance of a Code of Ethics, regarding social media use by physicians, from several societies, including the American Medical Association (https://www.ama-assn.org/delivering-care/ethics/professionalism-use-social-media). The principles guiding physicians use of social media include respect for human dignity and rights, honesty and upholding the standards of professionalism, and the duty to safeguard patient confidences and privacy.
Which platform should I use? There are so many.
While any content can be shared on any platform, social media sites have organically differentiated into being more amenable to one content vs the other. Some accounts tend to be more for professional use (ie, Twitter and LinkedIn), and other accounts for personal use (ie, Facebook, Instagram, Snapchat, and Pinterest). CHEST has selected Twitter to host its CME chats regarding preselected topics, post information about an upcoming lecture during the CHEST meeting, etc. New social media sites are now “physician only,” such as Sermo, Doximity, QuantiMD, and Doc2Doc. Many of these sites require doctors to submit their credentials to a site gatekeeper, recreating the intimacy of a “physicians’ lounge” in an online environment (J Med Internet Res. 2014:Feb 11;16[2]:e13). Lastly, Figure1 is a media sharing app between physicians allowing discussions of de-identified images or cases, recreating the “curbside” consult concept online.
I heard about hashtags. What are they?
Hashtags are simply clickable topic titles (#COPD #Sepsis # Education, etc.) that can be added to a post, in order to widen its reach. For instance, if I am interested in sepsis, I can click on the hashtag #Sepsis, and it would bring up all the posts on any Twitter account that added that hashtag. It’s a filter that takes me to that topic of interest. I can then click on the button “Like” on the message or the account itself where the post was found. The “Like” is similar to a bookmark for that account on my own Twitter. In the future, all the posts from that account would be available to me.
What are influencers or thought leaders?
Anyone who “liked” my account is now “following” me. The number of followers has become a measure of the popularity of anyone on social media. If it reaches a high level, then the person with the account is dubbed an “influencer.” Social media “influencers” are individuals whose opinion is followed by hundreds of thousands. Influencers may even be rewarded for harnessing their reach to make money off advertising. One can easily see how it is powerful for a physician to become an influencer or a “thought leader,” not to make money but to expand their reach on social media to spread the correct information about diets, drugs, e-cigarettes, and vaccinations, to name a few.
Can social media get me in trouble?
In 2012, a survey of the state medical boards published by JAMA (2012;307[11]:1141) revealed that approximately 30% of state medical boards reported complaints of “online violations of patient confidentiality.” More than 10% stated they had encountered a case of an “online depiction of intoxication.”
Another study a year earlier revealed that 13% of physicians reported they have discussed individual, though anonymized, cases with other physicians in public online forums (http://www.quantiamd.com/qqcp/DoctorsPatientSocialMedia.pdf).
Even if posted anonymously, or on a “personal” rather than professional social media site, various investigative methods may potentially be used to directly link information to a specific person or incident. The most current case law dictates that such information is “discoverable.” In fact, Facebook’s policy for the use of data informs users that, “we may access, preserve, and share your information in response to a legal request” both within and outside of U.S. jurisdiction”.
What kind of trouble could I be exposed to?
Poor quality of information, damage to our professional image, breaches of patient’s privacy, violation of patient-physician boundary, license revoking by state boards, and erroneous medical advice given in the absence of examining a patient, are all potential pitfalls for physicians in the careless use of social media.
How can I minimize my legal risk when interacting online?
It has been suggested that a legally sound approach in response to requests for online medical advice would be to send a standard response form that:
• informs the inquirer that the health-care provider does not answer online questions;
• supplies offline contact information so that an appointment can be made, if desired; and
• identifies a source for emergency services if the inquirer cannot wait for an appointment.
In circumstances where a patient–physician relationship already exists, informed consent should be obtained, which should include a careful explanation regarding the risks of online communication, expected response times, and the handling of emergencies, then documented in the patient’s chart (PT. 2014 Jul;39[7]:491,520).
In Summary
Social media, much like any area of medicine one is interested in, can be daunting and exciting but fraught with potential difficulties. I liken its adoption in our daily practice to any other decision or interest, including being in a private or academic setting, adopting procedural medicine or sticking to diagnostic consultations, or participating in research. In the end, it’s an individual expression of our desire to practice medicine. However, verifying information already existing online about us is of paramount importance. If I don’t tell my story, someone else will, and they may not be as truthful.
Dr. Bencheqroun is Assistant Professor, University of California Riverside School of Medicine, Pulmonary/Critical Care Faculty Program Coordinator & Research Mentor - Internal Medicine Residency Program Desert Regional Medical Center, Palm Springs CA; and Immediate Past Chair of the CHEST Council of Networks.
For most of us, social media is a daunting new reality that we are pressured to be part of but that we struggle to fit into our increasingly demanding schedules. My first social media foray as a physician was a Facebook fan page as a hobby rather than a professional presence. Years later, I have learned the incredible benefit that being on social media in other platforms brought to my profession.
What’s social media going to bring to my medical practice?
The days where physicians retreat to the safety of our offices to deliver our care, or to issue carefully structured opinions, or interactions with patients have made way for more direct interaction. Social media has, indeed, allowed us to share more personal glimpses of our daily struggle to save lives, behind-the-scenes snapshot of ethical struggles in decision making, our difficulties qualifying patients for therapies due to insurance complications, or real-time addressing medical news and combating misinformation. Moreover, when patients self-refer, or are referred to my practice, they look me up online before coming to my office. Online profiles are the new “first impression” of the bedside manner of a physician.
Other personal examples of social media benefits include being informed of new publications, since many journals now have an online presence; being able to interact in real-time with authors; learning from physicians in other countries how they handled issues, such as shortage of critical medications; or earning CME, such as the Twitter chats hosted by CHEST (eg, new biologic agents in difficult to treat asthma, or patient selection in triple therapy for COPD).
Why should I pay attention to social media presence?
The pace by which social media changed the landscape took the medical community by surprise. Patients, third-party websites, and online review agencies (official or not) adopted it well before physicians became comfortable with it. As such, when I decided to google myself online, I was shocked at the level of misinformation about me (as a pulmonologist, I didn’t know I had performed sigmoidoscopies, yet that’s what my patients learned before they met me). That was an important lesson: If I don’t control the narrative, someone else will. Consequently, I dedicated a few hours to establish an online presence in order to introduce myself accurately and to be accessible to my patients and colleagues online.
Who decides what’s ethical and what’s not?
As the lines blurred, our community struggled to define what was appropriate and what was not. Finally, we welcomed with relief the issuance of a Code of Ethics, regarding social media use by physicians, from several societies, including the American Medical Association (https://www.ama-assn.org/delivering-care/ethics/professionalism-use-social-media). The principles guiding physicians use of social media include respect for human dignity and rights, honesty and upholding the standards of professionalism, and the duty to safeguard patient confidences and privacy.
Which platform should I use? There are so many.
While any content can be shared on any platform, social media sites have organically differentiated into being more amenable to one content vs the other. Some accounts tend to be more for professional use (ie, Twitter and LinkedIn), and other accounts for personal use (ie, Facebook, Instagram, Snapchat, and Pinterest). CHEST has selected Twitter to host its CME chats regarding preselected topics, post information about an upcoming lecture during the CHEST meeting, etc. New social media sites are now “physician only,” such as Sermo, Doximity, QuantiMD, and Doc2Doc. Many of these sites require doctors to submit their credentials to a site gatekeeper, recreating the intimacy of a “physicians’ lounge” in an online environment (J Med Internet Res. 2014:Feb 11;16[2]:e13). Lastly, Figure1 is a media sharing app between physicians allowing discussions of de-identified images or cases, recreating the “curbside” consult concept online.
I heard about hashtags. What are they?
Hashtags are simply clickable topic titles (#COPD #Sepsis # Education, etc.) that can be added to a post, in order to widen its reach. For instance, if I am interested in sepsis, I can click on the hashtag #Sepsis, and it would bring up all the posts on any Twitter account that added that hashtag. It’s a filter that takes me to that topic of interest. I can then click on the button “Like” on the message or the account itself where the post was found. The “Like” is similar to a bookmark for that account on my own Twitter. In the future, all the posts from that account would be available to me.
What are influencers or thought leaders?
Anyone who “liked” my account is now “following” me. The number of followers has become a measure of the popularity of anyone on social media. If it reaches a high level, then the person with the account is dubbed an “influencer.” Social media “influencers” are individuals whose opinion is followed by hundreds of thousands. Influencers may even be rewarded for harnessing their reach to make money off advertising. One can easily see how it is powerful for a physician to become an influencer or a “thought leader,” not to make money but to expand their reach on social media to spread the correct information about diets, drugs, e-cigarettes, and vaccinations, to name a few.
Can social media get me in trouble?
In 2012, a survey of the state medical boards published by JAMA (2012;307[11]:1141) revealed that approximately 30% of state medical boards reported complaints of “online violations of patient confidentiality.” More than 10% stated they had encountered a case of an “online depiction of intoxication.”
Another study a year earlier revealed that 13% of physicians reported they have discussed individual, though anonymized, cases with other physicians in public online forums (http://www.quantiamd.com/qqcp/DoctorsPatientSocialMedia.pdf).
Even if posted anonymously, or on a “personal” rather than professional social media site, various investigative methods may potentially be used to directly link information to a specific person or incident. The most current case law dictates that such information is “discoverable.” In fact, Facebook’s policy for the use of data informs users that, “we may access, preserve, and share your information in response to a legal request” both within and outside of U.S. jurisdiction”.
What kind of trouble could I be exposed to?
Poor quality of information, damage to our professional image, breaches of patient’s privacy, violation of patient-physician boundary, license revoking by state boards, and erroneous medical advice given in the absence of examining a patient, are all potential pitfalls for physicians in the careless use of social media.
How can I minimize my legal risk when interacting online?
It has been suggested that a legally sound approach in response to requests for online medical advice would be to send a standard response form that:
• informs the inquirer that the health-care provider does not answer online questions;
• supplies offline contact information so that an appointment can be made, if desired; and
• identifies a source for emergency services if the inquirer cannot wait for an appointment.
In circumstances where a patient–physician relationship already exists, informed consent should be obtained, which should include a careful explanation regarding the risks of online communication, expected response times, and the handling of emergencies, then documented in the patient’s chart (PT. 2014 Jul;39[7]:491,520).
In Summary
Social media, much like any area of medicine one is interested in, can be daunting and exciting but fraught with potential difficulties. I liken its adoption in our daily practice to any other decision or interest, including being in a private or academic setting, adopting procedural medicine or sticking to diagnostic consultations, or participating in research. In the end, it’s an individual expression of our desire to practice medicine. However, verifying information already existing online about us is of paramount importance. If I don’t tell my story, someone else will, and they may not be as truthful.
Dr. Bencheqroun is Assistant Professor, University of California Riverside School of Medicine, Pulmonary/Critical Care Faculty Program Coordinator & Research Mentor - Internal Medicine Residency Program Desert Regional Medical Center, Palm Springs CA; and Immediate Past Chair of the CHEST Council of Networks.
For most of us, social media is a daunting new reality that we are pressured to be part of but that we struggle to fit into our increasingly demanding schedules. My first social media foray as a physician was a Facebook fan page as a hobby rather than a professional presence. Years later, I have learned the incredible benefit that being on social media in other platforms brought to my profession.
What’s social media going to bring to my medical practice?
The days where physicians retreat to the safety of our offices to deliver our care, or to issue carefully structured opinions, or interactions with patients have made way for more direct interaction. Social media has, indeed, allowed us to share more personal glimpses of our daily struggle to save lives, behind-the-scenes snapshot of ethical struggles in decision making, our difficulties qualifying patients for therapies due to insurance complications, or real-time addressing medical news and combating misinformation. Moreover, when patients self-refer, or are referred to my practice, they look me up online before coming to my office. Online profiles are the new “first impression” of the bedside manner of a physician.
Other personal examples of social media benefits include being informed of new publications, since many journals now have an online presence; being able to interact in real-time with authors; learning from physicians in other countries how they handled issues, such as shortage of critical medications; or earning CME, such as the Twitter chats hosted by CHEST (eg, new biologic agents in difficult to treat asthma, or patient selection in triple therapy for COPD).
Why should I pay attention to social media presence?
The pace by which social media changed the landscape took the medical community by surprise. Patients, third-party websites, and online review agencies (official or not) adopted it well before physicians became comfortable with it. As such, when I decided to google myself online, I was shocked at the level of misinformation about me (as a pulmonologist, I didn’t know I had performed sigmoidoscopies, yet that’s what my patients learned before they met me). That was an important lesson: If I don’t control the narrative, someone else will. Consequently, I dedicated a few hours to establish an online presence in order to introduce myself accurately and to be accessible to my patients and colleagues online.
Who decides what’s ethical and what’s not?
As the lines blurred, our community struggled to define what was appropriate and what was not. Finally, we welcomed with relief the issuance of a Code of Ethics, regarding social media use by physicians, from several societies, including the American Medical Association (https://www.ama-assn.org/delivering-care/ethics/professionalism-use-social-media). The principles guiding physicians use of social media include respect for human dignity and rights, honesty and upholding the standards of professionalism, and the duty to safeguard patient confidences and privacy.
Which platform should I use? There are so many.
While any content can be shared on any platform, social media sites have organically differentiated into being more amenable to one content vs the other. Some accounts tend to be more for professional use (ie, Twitter and LinkedIn), and other accounts for personal use (ie, Facebook, Instagram, Snapchat, and Pinterest). CHEST has selected Twitter to host its CME chats regarding preselected topics, post information about an upcoming lecture during the CHEST meeting, etc. New social media sites are now “physician only,” such as Sermo, Doximity, QuantiMD, and Doc2Doc. Many of these sites require doctors to submit their credentials to a site gatekeeper, recreating the intimacy of a “physicians’ lounge” in an online environment (J Med Internet Res. 2014:Feb 11;16[2]:e13). Lastly, Figure1 is a media sharing app between physicians allowing discussions of de-identified images or cases, recreating the “curbside” consult concept online.
I heard about hashtags. What are they?
Hashtags are simply clickable topic titles (#COPD #Sepsis # Education, etc.) that can be added to a post, in order to widen its reach. For instance, if I am interested in sepsis, I can click on the hashtag #Sepsis, and it would bring up all the posts on any Twitter account that added that hashtag. It’s a filter that takes me to that topic of interest. I can then click on the button “Like” on the message or the account itself where the post was found. The “Like” is similar to a bookmark for that account on my own Twitter. In the future, all the posts from that account would be available to me.
What are influencers or thought leaders?
Anyone who “liked” my account is now “following” me. The number of followers has become a measure of the popularity of anyone on social media. If it reaches a high level, then the person with the account is dubbed an “influencer.” Social media “influencers” are individuals whose opinion is followed by hundreds of thousands. Influencers may even be rewarded for harnessing their reach to make money off advertising. One can easily see how it is powerful for a physician to become an influencer or a “thought leader,” not to make money but to expand their reach on social media to spread the correct information about diets, drugs, e-cigarettes, and vaccinations, to name a few.
Can social media get me in trouble?
In 2012, a survey of the state medical boards published by JAMA (2012;307[11]:1141) revealed that approximately 30% of state medical boards reported complaints of “online violations of patient confidentiality.” More than 10% stated they had encountered a case of an “online depiction of intoxication.”
Another study a year earlier revealed that 13% of physicians reported they have discussed individual, though anonymized, cases with other physicians in public online forums (http://www.quantiamd.com/qqcp/DoctorsPatientSocialMedia.pdf).
Even if posted anonymously, or on a “personal” rather than professional social media site, various investigative methods may potentially be used to directly link information to a specific person or incident. The most current case law dictates that such information is “discoverable.” In fact, Facebook’s policy for the use of data informs users that, “we may access, preserve, and share your information in response to a legal request” both within and outside of U.S. jurisdiction”.
What kind of trouble could I be exposed to?
Poor quality of information, damage to our professional image, breaches of patient’s privacy, violation of patient-physician boundary, license revoking by state boards, and erroneous medical advice given in the absence of examining a patient, are all potential pitfalls for physicians in the careless use of social media.
How can I minimize my legal risk when interacting online?
It has been suggested that a legally sound approach in response to requests for online medical advice would be to send a standard response form that:
• informs the inquirer that the health-care provider does not answer online questions;
• supplies offline contact information so that an appointment can be made, if desired; and
• identifies a source for emergency services if the inquirer cannot wait for an appointment.
In circumstances where a patient–physician relationship already exists, informed consent should be obtained, which should include a careful explanation regarding the risks of online communication, expected response times, and the handling of emergencies, then documented in the patient’s chart (PT. 2014 Jul;39[7]:491,520).
In Summary
Social media, much like any area of medicine one is interested in, can be daunting and exciting but fraught with potential difficulties. I liken its adoption in our daily practice to any other decision or interest, including being in a private or academic setting, adopting procedural medicine or sticking to diagnostic consultations, or participating in research. In the end, it’s an individual expression of our desire to practice medicine. However, verifying information already existing online about us is of paramount importance. If I don’t tell my story, someone else will, and they may not be as truthful.
Dr. Bencheqroun is Assistant Professor, University of California Riverside School of Medicine, Pulmonary/Critical Care Faculty Program Coordinator & Research Mentor - Internal Medicine Residency Program Desert Regional Medical Center, Palm Springs CA; and Immediate Past Chair of the CHEST Council of Networks.
An update on chronic thromboembolic pulmonary hypertension
The “fixable” form of PH that you don’t want to miss
Chronic thromboembolic pulmonary hypertension (CTEPH) is an elevation in pulmonary vascular resistance (PVR) resulting from chronic, “scarred-in” thromboembolic material partially occluding the pulmonary arteries. This vascular obstruction, over time, results in failure of the right ventricle and early mortality.
CTEPH was first characterized in an autopsy series from the Massachusetts General Hospital in 1931. On these postmortem examinations, it was noted that the affected patients had large pulmonary artery vascular obstruction, but also normal pulmonary parenchyma distal to this vascular obstruction and extensive bronchial collateral blood flow (Means J. Ann Intern Med. 1931;5:417). Although this observation set the groundwork for the theory that surgically removing the vascular obstruction to this preserved lung tissue could improve the condition of these patients, it would take until the mid-20th century until imaging and cardiac catheterization techniques allowed the recognition of the disease in real time.
CTEPH is thought to begin with an acute pulmonary embolus, but in approximately 3.4% of patients, rather than resolving over time, the thrombus will organize and incorporate into the pulmonary artery intimal layer (Simonneau G, et al. Eur Respir Rev. 2017;26:160112) A history of venous thromboembolism in a patient with persistent dyspnea should spur a screening evaluation for CTEPH; 75% of patients with CTEPH have a history of prior known acute pulmonary embolus and 56% of patients report a prior diagnosis of deep venous thrombosis. An acute pulmonary embolus will fibrinolyse early with the vast majority of the vascular obstruction resolving by the third month. Therefore, if the patient continues to report a significant exercise limitation after 3 months of therapeutic anticoagulation therapy, or has concerning physical exam signs, a workup should be pursued. The initial evaluation for CTEPH begins with a transthoracic echocardiogram (TTE) and ventilation/perfusion (V/Q) scintigraphy. A retrospective study comparing V/Q scan and multidetector CT scan revealed that V/Q scanning had a sensitivity and specificity of 97% and 95% for CTEPH, while CTPA had good specificity at 99% but only 51% sensitivity (Tunariu N, et al. J Nuc Med. 2007;48(5):680). If these are abnormal, then right-sided heart catheterization and invasive biplane digital subtraction pulmonary angiography are recommended. These studies confirm the diagnosis, grade its severity, and allow an evaluation for surgically accessible vs distal disease. Some CTEPH centers utilize additional imaging techniques, such as magnetic resonance angiography, optical resonance imaging, spectral CT scanning with iodine perfusion images, and intravascular ultrasound. These modalities and their place in the diagnostic algorithm are under investigation.
The goal of the initial evaluation process is to determine if the patient can undergo surgical pulmonary thromboendarterectomy (PTE), because in experienced hands, this procedure ensures the best long-term outcome for the patient. The first pulmonary thromboendarterectomy was performed at the University of California San Diego in 1970. Because the disease involves the intimal layer of the pulmonary artery, the surgery had to involve not just removal of the intravascular obstruction but also a pulmonary artery intimectomy. Surgical mortality rates were high in the initial experience. In 1984, a review of 85 worldwide cases reported an average mortality rate of 22%, and as high as 40% in some centers (Chitwood WR, Jr, et al. Clin Chest Med. 1984;5(3):507).
Over the ensuing years, refinements in surgical technique, the utilization of deep hypothermia and cardiac arrest during the procedure, development of new surgical instruments, and standardization of surgical selection and postoperative care have improved surgical mortality to <5% in experienced centers. Long-term outcomes of successful PTE surgery remain good, with 90% 3-year survival vs 70% for those who do not undergo surgery and are medically treated. Importantly, 90% of postoperative patients report functional class I or II symptoms at 1 year (Condliffe R, et al. Am J Reslpir Crit Care Med. 2008:177(10);1122). Because of this difference in early mortality and symptoms, PTE surgery remains the treatment of choice for CTEPH.
Despite the advances in PTE surgery, some patients are not operative candidates either due to surgically inaccessible disease or due to comorbidities. In 2001, Feinstein and colleagues described a series of 18 CTEPH cases treated with balloon pulmonary angioplasty (BPA). Promising hemodynamics effects were reported; however, the procedure had an unacceptable complication rate in which 11 patients developed reperfusion lung injury, 3 patients required mechanical ventilation, and 1 patient died. In the ensuing years, Japanese and Norwegian groups have independently developed and improved techniques for BPA. The procedure is done in a series of sessions (average four to six), 1 to 4 weeks apart, where small (2-3 mm) balloons are directed toward distal, diseased pulmonary vessels. Common complications include reperfusion injury, vessel injury, hemoptysis, and, more rarely, respiratory failure. Still, early experience suggests this procedure decreases pulmonary vascular resistance over time, improves right ventricular function, and improves patients’ symptoms (Andreassen A, et al. Heart. 2013;99(19):1415). The experience with this procedure is limited but growing in the United States, with only a handful of centers currently performing BPAs and collecting data.
Lifelong anticoagulation, oxygen, and diuretics for right-sided heart failure are recommended for patients with CTEPH. The first successful large phase III medication study for CTEPH was the CHEST-1 trial published in 2013. This was a multicenter, randomized, placebo-controlled trial of the soluble guanylate cyclase stimulator riociguat. The study enrolled 261 patients with inoperable CTEPH or persistent pulmonary hypertension after surgery. The primary end point was 6-minute walk distance at 12 weeks. The treatment group showed a 46 m improvement (P<.001). Secondary end points of pulmonary vascular resistance, NT-proBNP level, and functional class also improved. This pivotal trial led to the FDA approval of riociguat for inoperable or persistent postoperative CTEPH.
MERIT-1, a phase II, randomized placebo-controlled double trial of macitentan (an oral endothelin receptor antagonist) was recently completed. It enrolled 80 patients with inoperable CTEPH. The primary endpoint was pulmonary vascular resistance at week 16, expressed as a percentage of baseline. At week 16, the patients in the treatment arm had a PVR 73% of baseline vs 87.2% in the treatment group. This medication is not yet FDA-approved for the treatment of inoperable CTEPH (Ghofrani H, et al. Lancet Respir Med. 2017;5(10):785-794).
Pulmonary hypertension medication has been postulated as a possible way to “pretreat” patients before pulmonary thromboendarterectomy surgery, perhaps lowering preoperative pulmonary vascular resistance and surgical risk. However, there are currently no convincing data to support this practice, and medical treatment has been associated with a possible counterproductive delay in surgery. A phase II study including CTEPH patients with high PVR for preoperative treatment with riociguat vs placebo is currently enrolling to determine if “induction” treatment with medication prior to surgery reduces risk or delays definitive surgery. Occasionally, patients are found who have persistent thrombus but not pulmonary hypertension. Chronic thromboembolic disease (CTED) is a recently coined term describing patients who have chronic thromboembolism on imaging but have normal resting hemodynamics. Whether CTED represents simply unresolved clot that will never progress to CTEPH or is an early point on the continuum of disease not well-defined and a controversial topic among experts. At many centers, patients with CTED and symptoms will undergo exercise testing to look for exercise -induced pulmonary hypertension or an increase in dead space ventilation as a cause of their symptoms. A retrospective series of carefully chosen CTED patients who underwent PTE surgery reported improvements in symptoms and overall quality of life, without increased complications (Taboada D, et al. Eur Respir J. 2014 44(6):1635). The operation carries risk, however, and further work into the epidemiology and prognosis of CTED is required before operative intervention can be recommended.
In conclusion, CTEPH is a disease that rarely occurs after an acute PE but when undiagnosed and untreated portends a poor prognosis. The definitive treatment for this disease is surgical PTE, but to achieve the best outcomes, this procedure needs to be performed at expert centers with multidisciplinary team experience. Patients who are poor operative candidates or with surgically inaccessible disease may be considered for balloon pulmonary angioplasty. For patients without more curative options, medication improves exercise tolerance. The field of CTEPH has been rapidly expanding over the last decade, leading to better patient outcomes and more treatment options.
Dr. Bartolome is Associate Professor, Pulmonary and Critical Care Medicine; Director, CTEPH Program; and Associate Director, PH Program; UT Southwestern Medical Center, Dallas, Texas.
The “fixable” form of PH that you don’t want to miss
The “fixable” form of PH that you don’t want to miss
Chronic thromboembolic pulmonary hypertension (CTEPH) is an elevation in pulmonary vascular resistance (PVR) resulting from chronic, “scarred-in” thromboembolic material partially occluding the pulmonary arteries. This vascular obstruction, over time, results in failure of the right ventricle and early mortality.
CTEPH was first characterized in an autopsy series from the Massachusetts General Hospital in 1931. On these postmortem examinations, it was noted that the affected patients had large pulmonary artery vascular obstruction, but also normal pulmonary parenchyma distal to this vascular obstruction and extensive bronchial collateral blood flow (Means J. Ann Intern Med. 1931;5:417). Although this observation set the groundwork for the theory that surgically removing the vascular obstruction to this preserved lung tissue could improve the condition of these patients, it would take until the mid-20th century until imaging and cardiac catheterization techniques allowed the recognition of the disease in real time.
CTEPH is thought to begin with an acute pulmonary embolus, but in approximately 3.4% of patients, rather than resolving over time, the thrombus will organize and incorporate into the pulmonary artery intimal layer (Simonneau G, et al. Eur Respir Rev. 2017;26:160112) A history of venous thromboembolism in a patient with persistent dyspnea should spur a screening evaluation for CTEPH; 75% of patients with CTEPH have a history of prior known acute pulmonary embolus and 56% of patients report a prior diagnosis of deep venous thrombosis. An acute pulmonary embolus will fibrinolyse early with the vast majority of the vascular obstruction resolving by the third month. Therefore, if the patient continues to report a significant exercise limitation after 3 months of therapeutic anticoagulation therapy, or has concerning physical exam signs, a workup should be pursued. The initial evaluation for CTEPH begins with a transthoracic echocardiogram (TTE) and ventilation/perfusion (V/Q) scintigraphy. A retrospective study comparing V/Q scan and multidetector CT scan revealed that V/Q scanning had a sensitivity and specificity of 97% and 95% for CTEPH, while CTPA had good specificity at 99% but only 51% sensitivity (Tunariu N, et al. J Nuc Med. 2007;48(5):680). If these are abnormal, then right-sided heart catheterization and invasive biplane digital subtraction pulmonary angiography are recommended. These studies confirm the diagnosis, grade its severity, and allow an evaluation for surgically accessible vs distal disease. Some CTEPH centers utilize additional imaging techniques, such as magnetic resonance angiography, optical resonance imaging, spectral CT scanning with iodine perfusion images, and intravascular ultrasound. These modalities and their place in the diagnostic algorithm are under investigation.
The goal of the initial evaluation process is to determine if the patient can undergo surgical pulmonary thromboendarterectomy (PTE), because in experienced hands, this procedure ensures the best long-term outcome for the patient. The first pulmonary thromboendarterectomy was performed at the University of California San Diego in 1970. Because the disease involves the intimal layer of the pulmonary artery, the surgery had to involve not just removal of the intravascular obstruction but also a pulmonary artery intimectomy. Surgical mortality rates were high in the initial experience. In 1984, a review of 85 worldwide cases reported an average mortality rate of 22%, and as high as 40% in some centers (Chitwood WR, Jr, et al. Clin Chest Med. 1984;5(3):507).
Over the ensuing years, refinements in surgical technique, the utilization of deep hypothermia and cardiac arrest during the procedure, development of new surgical instruments, and standardization of surgical selection and postoperative care have improved surgical mortality to <5% in experienced centers. Long-term outcomes of successful PTE surgery remain good, with 90% 3-year survival vs 70% for those who do not undergo surgery and are medically treated. Importantly, 90% of postoperative patients report functional class I or II symptoms at 1 year (Condliffe R, et al. Am J Reslpir Crit Care Med. 2008:177(10);1122). Because of this difference in early mortality and symptoms, PTE surgery remains the treatment of choice for CTEPH.
Despite the advances in PTE surgery, some patients are not operative candidates either due to surgically inaccessible disease or due to comorbidities. In 2001, Feinstein and colleagues described a series of 18 CTEPH cases treated with balloon pulmonary angioplasty (BPA). Promising hemodynamics effects were reported; however, the procedure had an unacceptable complication rate in which 11 patients developed reperfusion lung injury, 3 patients required mechanical ventilation, and 1 patient died. In the ensuing years, Japanese and Norwegian groups have independently developed and improved techniques for BPA. The procedure is done in a series of sessions (average four to six), 1 to 4 weeks apart, where small (2-3 mm) balloons are directed toward distal, diseased pulmonary vessels. Common complications include reperfusion injury, vessel injury, hemoptysis, and, more rarely, respiratory failure. Still, early experience suggests this procedure decreases pulmonary vascular resistance over time, improves right ventricular function, and improves patients’ symptoms (Andreassen A, et al. Heart. 2013;99(19):1415). The experience with this procedure is limited but growing in the United States, with only a handful of centers currently performing BPAs and collecting data.
Lifelong anticoagulation, oxygen, and diuretics for right-sided heart failure are recommended for patients with CTEPH. The first successful large phase III medication study for CTEPH was the CHEST-1 trial published in 2013. This was a multicenter, randomized, placebo-controlled trial of the soluble guanylate cyclase stimulator riociguat. The study enrolled 261 patients with inoperable CTEPH or persistent pulmonary hypertension after surgery. The primary end point was 6-minute walk distance at 12 weeks. The treatment group showed a 46 m improvement (P<.001). Secondary end points of pulmonary vascular resistance, NT-proBNP level, and functional class also improved. This pivotal trial led to the FDA approval of riociguat for inoperable or persistent postoperative CTEPH.
MERIT-1, a phase II, randomized placebo-controlled double trial of macitentan (an oral endothelin receptor antagonist) was recently completed. It enrolled 80 patients with inoperable CTEPH. The primary endpoint was pulmonary vascular resistance at week 16, expressed as a percentage of baseline. At week 16, the patients in the treatment arm had a PVR 73% of baseline vs 87.2% in the treatment group. This medication is not yet FDA-approved for the treatment of inoperable CTEPH (Ghofrani H, et al. Lancet Respir Med. 2017;5(10):785-794).
Pulmonary hypertension medication has been postulated as a possible way to “pretreat” patients before pulmonary thromboendarterectomy surgery, perhaps lowering preoperative pulmonary vascular resistance and surgical risk. However, there are currently no convincing data to support this practice, and medical treatment has been associated with a possible counterproductive delay in surgery. A phase II study including CTEPH patients with high PVR for preoperative treatment with riociguat vs placebo is currently enrolling to determine if “induction” treatment with medication prior to surgery reduces risk or delays definitive surgery. Occasionally, patients are found who have persistent thrombus but not pulmonary hypertension. Chronic thromboembolic disease (CTED) is a recently coined term describing patients who have chronic thromboembolism on imaging but have normal resting hemodynamics. Whether CTED represents simply unresolved clot that will never progress to CTEPH or is an early point on the continuum of disease not well-defined and a controversial topic among experts. At many centers, patients with CTED and symptoms will undergo exercise testing to look for exercise -induced pulmonary hypertension or an increase in dead space ventilation as a cause of their symptoms. A retrospective series of carefully chosen CTED patients who underwent PTE surgery reported improvements in symptoms and overall quality of life, without increased complications (Taboada D, et al. Eur Respir J. 2014 44(6):1635). The operation carries risk, however, and further work into the epidemiology and prognosis of CTED is required before operative intervention can be recommended.
In conclusion, CTEPH is a disease that rarely occurs after an acute PE but when undiagnosed and untreated portends a poor prognosis. The definitive treatment for this disease is surgical PTE, but to achieve the best outcomes, this procedure needs to be performed at expert centers with multidisciplinary team experience. Patients who are poor operative candidates or with surgically inaccessible disease may be considered for balloon pulmonary angioplasty. For patients without more curative options, medication improves exercise tolerance. The field of CTEPH has been rapidly expanding over the last decade, leading to better patient outcomes and more treatment options.
Dr. Bartolome is Associate Professor, Pulmonary and Critical Care Medicine; Director, CTEPH Program; and Associate Director, PH Program; UT Southwestern Medical Center, Dallas, Texas.
Chronic thromboembolic pulmonary hypertension (CTEPH) is an elevation in pulmonary vascular resistance (PVR) resulting from chronic, “scarred-in” thromboembolic material partially occluding the pulmonary arteries. This vascular obstruction, over time, results in failure of the right ventricle and early mortality.
CTEPH was first characterized in an autopsy series from the Massachusetts General Hospital in 1931. On these postmortem examinations, it was noted that the affected patients had large pulmonary artery vascular obstruction, but also normal pulmonary parenchyma distal to this vascular obstruction and extensive bronchial collateral blood flow (Means J. Ann Intern Med. 1931;5:417). Although this observation set the groundwork for the theory that surgically removing the vascular obstruction to this preserved lung tissue could improve the condition of these patients, it would take until the mid-20th century until imaging and cardiac catheterization techniques allowed the recognition of the disease in real time.
CTEPH is thought to begin with an acute pulmonary embolus, but in approximately 3.4% of patients, rather than resolving over time, the thrombus will organize and incorporate into the pulmonary artery intimal layer (Simonneau G, et al. Eur Respir Rev. 2017;26:160112) A history of venous thromboembolism in a patient with persistent dyspnea should spur a screening evaluation for CTEPH; 75% of patients with CTEPH have a history of prior known acute pulmonary embolus and 56% of patients report a prior diagnosis of deep venous thrombosis. An acute pulmonary embolus will fibrinolyse early with the vast majority of the vascular obstruction resolving by the third month. Therefore, if the patient continues to report a significant exercise limitation after 3 months of therapeutic anticoagulation therapy, or has concerning physical exam signs, a workup should be pursued. The initial evaluation for CTEPH begins with a transthoracic echocardiogram (TTE) and ventilation/perfusion (V/Q) scintigraphy. A retrospective study comparing V/Q scan and multidetector CT scan revealed that V/Q scanning had a sensitivity and specificity of 97% and 95% for CTEPH, while CTPA had good specificity at 99% but only 51% sensitivity (Tunariu N, et al. J Nuc Med. 2007;48(5):680). If these are abnormal, then right-sided heart catheterization and invasive biplane digital subtraction pulmonary angiography are recommended. These studies confirm the diagnosis, grade its severity, and allow an evaluation for surgically accessible vs distal disease. Some CTEPH centers utilize additional imaging techniques, such as magnetic resonance angiography, optical resonance imaging, spectral CT scanning with iodine perfusion images, and intravascular ultrasound. These modalities and their place in the diagnostic algorithm are under investigation.
The goal of the initial evaluation process is to determine if the patient can undergo surgical pulmonary thromboendarterectomy (PTE), because in experienced hands, this procedure ensures the best long-term outcome for the patient. The first pulmonary thromboendarterectomy was performed at the University of California San Diego in 1970. Because the disease involves the intimal layer of the pulmonary artery, the surgery had to involve not just removal of the intravascular obstruction but also a pulmonary artery intimectomy. Surgical mortality rates were high in the initial experience. In 1984, a review of 85 worldwide cases reported an average mortality rate of 22%, and as high as 40% in some centers (Chitwood WR, Jr, et al. Clin Chest Med. 1984;5(3):507).
Over the ensuing years, refinements in surgical technique, the utilization of deep hypothermia and cardiac arrest during the procedure, development of new surgical instruments, and standardization of surgical selection and postoperative care have improved surgical mortality to <5% in experienced centers. Long-term outcomes of successful PTE surgery remain good, with 90% 3-year survival vs 70% for those who do not undergo surgery and are medically treated. Importantly, 90% of postoperative patients report functional class I or II symptoms at 1 year (Condliffe R, et al. Am J Reslpir Crit Care Med. 2008:177(10);1122). Because of this difference in early mortality and symptoms, PTE surgery remains the treatment of choice for CTEPH.
Despite the advances in PTE surgery, some patients are not operative candidates either due to surgically inaccessible disease or due to comorbidities. In 2001, Feinstein and colleagues described a series of 18 CTEPH cases treated with balloon pulmonary angioplasty (BPA). Promising hemodynamics effects were reported; however, the procedure had an unacceptable complication rate in which 11 patients developed reperfusion lung injury, 3 patients required mechanical ventilation, and 1 patient died. In the ensuing years, Japanese and Norwegian groups have independently developed and improved techniques for BPA. The procedure is done in a series of sessions (average four to six), 1 to 4 weeks apart, where small (2-3 mm) balloons are directed toward distal, diseased pulmonary vessels. Common complications include reperfusion injury, vessel injury, hemoptysis, and, more rarely, respiratory failure. Still, early experience suggests this procedure decreases pulmonary vascular resistance over time, improves right ventricular function, and improves patients’ symptoms (Andreassen A, et al. Heart. 2013;99(19):1415). The experience with this procedure is limited but growing in the United States, with only a handful of centers currently performing BPAs and collecting data.
Lifelong anticoagulation, oxygen, and diuretics for right-sided heart failure are recommended for patients with CTEPH. The first successful large phase III medication study for CTEPH was the CHEST-1 trial published in 2013. This was a multicenter, randomized, placebo-controlled trial of the soluble guanylate cyclase stimulator riociguat. The study enrolled 261 patients with inoperable CTEPH or persistent pulmonary hypertension after surgery. The primary end point was 6-minute walk distance at 12 weeks. The treatment group showed a 46 m improvement (P<.001). Secondary end points of pulmonary vascular resistance, NT-proBNP level, and functional class also improved. This pivotal trial led to the FDA approval of riociguat for inoperable or persistent postoperative CTEPH.
MERIT-1, a phase II, randomized placebo-controlled double trial of macitentan (an oral endothelin receptor antagonist) was recently completed. It enrolled 80 patients with inoperable CTEPH. The primary endpoint was pulmonary vascular resistance at week 16, expressed as a percentage of baseline. At week 16, the patients in the treatment arm had a PVR 73% of baseline vs 87.2% in the treatment group. This medication is not yet FDA-approved for the treatment of inoperable CTEPH (Ghofrani H, et al. Lancet Respir Med. 2017;5(10):785-794).
Pulmonary hypertension medication has been postulated as a possible way to “pretreat” patients before pulmonary thromboendarterectomy surgery, perhaps lowering preoperative pulmonary vascular resistance and surgical risk. However, there are currently no convincing data to support this practice, and medical treatment has been associated with a possible counterproductive delay in surgery. A phase II study including CTEPH patients with high PVR for preoperative treatment with riociguat vs placebo is currently enrolling to determine if “induction” treatment with medication prior to surgery reduces risk or delays definitive surgery. Occasionally, patients are found who have persistent thrombus but not pulmonary hypertension. Chronic thromboembolic disease (CTED) is a recently coined term describing patients who have chronic thromboembolism on imaging but have normal resting hemodynamics. Whether CTED represents simply unresolved clot that will never progress to CTEPH or is an early point on the continuum of disease not well-defined and a controversial topic among experts. At many centers, patients with CTED and symptoms will undergo exercise testing to look for exercise -induced pulmonary hypertension or an increase in dead space ventilation as a cause of their symptoms. A retrospective series of carefully chosen CTED patients who underwent PTE surgery reported improvements in symptoms and overall quality of life, without increased complications (Taboada D, et al. Eur Respir J. 2014 44(6):1635). The operation carries risk, however, and further work into the epidemiology and prognosis of CTED is required before operative intervention can be recommended.
In conclusion, CTEPH is a disease that rarely occurs after an acute PE but when undiagnosed and untreated portends a poor prognosis. The definitive treatment for this disease is surgical PTE, but to achieve the best outcomes, this procedure needs to be performed at expert centers with multidisciplinary team experience. Patients who are poor operative candidates or with surgically inaccessible disease may be considered for balloon pulmonary angioplasty. For patients without more curative options, medication improves exercise tolerance. The field of CTEPH has been rapidly expanding over the last decade, leading to better patient outcomes and more treatment options.
Dr. Bartolome is Associate Professor, Pulmonary and Critical Care Medicine; Director, CTEPH Program; and Associate Director, PH Program; UT Southwestern Medical Center, Dallas, Texas.
New Section Editor for Pulmonary Perspectives®
We are pleased to announce Corey Kershaw, MD, as the new Section Editor for Pulmonary Perspectives. Dr. Kershaw is the Medical Director of the Medical Intensive Care Unit at Clements University Hospital and an Associate Professor, Division of Pulmonary and Critical Care Medicine, University of Texas Southwestern Medical Center, in Dallas, Texas. He currently serves on the American College of Chest Physicians Interstitial and Diffuse Lung Disease NetWork. Dr. Kershaw’s research interests revolve around clinical trials for the treatment of idiopathic pulmonary fibrosis and other fibrosing interstitial lung diseases.
We thank Nitin Puri, MD, FCCP, for his outstanding service as the Pulmonary Perspectives Section Editor for the previous 3
We are pleased to announce Corey Kershaw, MD, as the new Section Editor for Pulmonary Perspectives. Dr. Kershaw is the Medical Director of the Medical Intensive Care Unit at Clements University Hospital and an Associate Professor, Division of Pulmonary and Critical Care Medicine, University of Texas Southwestern Medical Center, in Dallas, Texas. He currently serves on the American College of Chest Physicians Interstitial and Diffuse Lung Disease NetWork. Dr. Kershaw’s research interests revolve around clinical trials for the treatment of idiopathic pulmonary fibrosis and other fibrosing interstitial lung diseases.
We thank Nitin Puri, MD, FCCP, for his outstanding service as the Pulmonary Perspectives Section Editor for the previous 3
We are pleased to announce Corey Kershaw, MD, as the new Section Editor for Pulmonary Perspectives. Dr. Kershaw is the Medical Director of the Medical Intensive Care Unit at Clements University Hospital and an Associate Professor, Division of Pulmonary and Critical Care Medicine, University of Texas Southwestern Medical Center, in Dallas, Texas. He currently serves on the American College of Chest Physicians Interstitial and Diffuse Lung Disease NetWork. Dr. Kershaw’s research interests revolve around clinical trials for the treatment of idiopathic pulmonary fibrosis and other fibrosing interstitial lung diseases.
We thank Nitin Puri, MD, FCCP, for his outstanding service as the Pulmonary Perspectives Section Editor for the previous 3
Use of ECMO in the management of influenza-associated ARDS
Now that we are in the midst of flu season, many discussions regarding the management of patients with influenza virus infections are ensuing. While prevention is always preferable, and we encourage everyone to get vaccinated, influenza remains a rapidly widespread infection. In the United States during last year’s flu season (2017-18), there was an estimated 49 million cases of influenza, 960,000 hospitalizations, and 79,000 deaths. Approximately 86% of all deaths were estimated to occur in those aged 65 and older (Centers for Disease Control and Prevention webpage on Burden of Influenza).
Despite our best efforts, there are inevitable times when some patients become ill enough to require hospitalization. Patients aged 65 and older make up the overwhelming majority of patients with influenza who eventually require hospitalization (Fig 1) (The Centers for Disease Control and Prevention FluView Database). Comorbidities also confer higher risk for more severe illness and potential hospitalization irrespective of age (Fig 2). In children with known medical conditions, asthma confers highest risk of hospitalization, as 27% of those with asthma were hospitalized after developing the flu. In adults, 52% of those with cardiovascular disease and 30% of adult patients with chronic lung disease who were confirmed to have influenza required hospitalization for treatment (Fig 2, The Centers for Disease Control and Prevention FluView Database).
The most severe cases of influenza can require ICU care and advanced management of respiratory failure as a result of the acute respiratory distress syndrome (ARDS). The lungs suffer significant injury due to the viral infection, and they lose their ability to effectively oxygenate the blood. Secondary bacterial infections can also occur as a complication, which compounds the injury. Given the fact that so many patients have significant comorbidities and are of advanced age, it is reasonable to expect that a fair proportion of those with influenza would develop respiratory failure as a consequence. For some of these patients, the hypoxemia that develops as a result of the lung injury can be exceptionally challenging to manage. In extreme cases, conventional ventilator management is insufficient, and the need for additional, advanced therapies arise.
Studies of VV ECMO in severe influenza
ECMO (extracorporeal membrane oxygenation) is a treatment that has been employed to help support patients with severe hypoxemic respiratory failure while their lungs recover from acute injury. Venovenous (VV) ECMO requires peripheral insertion of large cannulae into the venous system to take deoxygenated blood, deliver it through the membrane oxygenator and return the oxygenated blood back to the venous system. In simplest terms, the membrane of ECMO circuit serves as a substitute for the gas exchange function of the lungs and provides the oxygenation that the injured alveoli of the lung are unable to provide. The overall intent is to have the external ECMO circuit do all of the gas exchange work while the lungs heal.
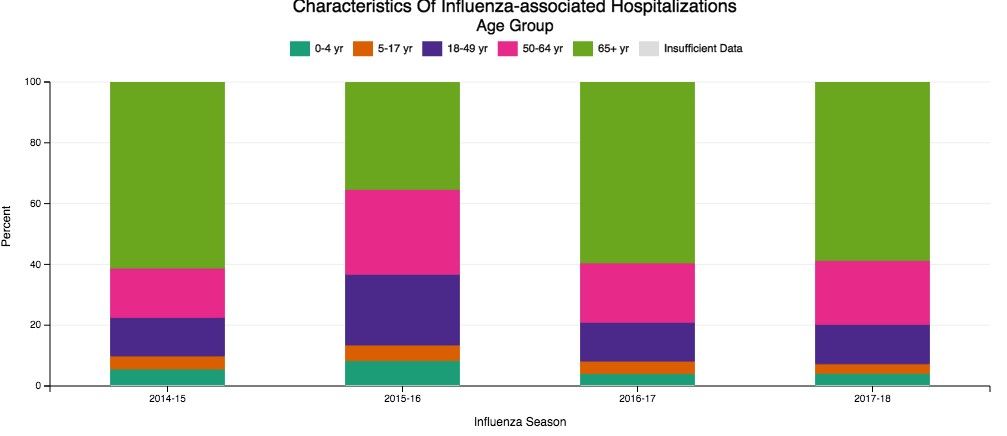
Much research has been done on VV ECMO as an adjunct or salvage therapy in patients with refractory hypoxemic respiratory failure due to ARDS. Historical and recent studies have shown that approximately 60% of patients with ARDS have viral (approximately 20%) or bacterial (approximately 40%) pneumonia as the underlying cause (Zapol, et al. JAMA. 1979; 242[20]:2193; Combes A, et al. N Engl J Med. 2018;378:1965). Naturally, given the frequency of infection as a cause for ARDS, and the severity of illness that can develop with influenza infection in particular, an interest has arisen in the applicability of ECMO in cases of severe influenza-related ARDS.
In 2009, during the H1N1 influenza pandemic, the ANZ ECMO investigators in Australia and New Zealand described a 78% survival rate for their patients with severe H1N1 associated ARDS treated with VV ECMO between June and August of that year (Davies A, et al. JAMA. 2009;302[17]:1888). The eagerly awaited results of the randomized, controlled CESAR trial (Peek G, et al. Lancet. 2009;374:1351) that studied patients aged 18 to 64 with severe, refractory respiratory failure transferred to a specialized center for ECMO care had additional impact in catalyzing interest in ECMO use. This trial showed improved survival with ECMO (63% in ECMO vs 47% control, RR 0.69; 95% CI 0.05-0.97 P=.03) with a gain of 0.03 QALY (quality-adjusted life years) with additional cost of 40,000 pounds sterling. However, a major critique is that 24% of patients transferred to the specialized center never were treated with ECMO. Significantly, there was incomplete follow-up data on nearly half of the patients, as well. Many conclude that the survival benefit seen in this study may be more reflective of the expertise in respiratory failure management (especially as it relates to lung protective ventilation) at this center than therapy with ECMO itself.
Additional cohort studies in the United Kingdom (Noah MA, et al. JAMA. 2011;306[15]:1659) and Italy (Pappalardo F, et al. Intensive Care Med. 2013;39[2]:275) showed approximately 70% in-hospital survival rates for patients with H1N1 influenza transferred to a specialized ECMO center and treated with ECMO.
Nonetheless, the information gained from the observational data from ANZ ECMO, along with data published in European cohort studies and the randomized controlled CESAR trial after the 2009 H1N1 influenza pandemic, greatly contributed to the rise in use of ECMO for refractory ARDS due to influenza. Subsequently, there has been a rapid establishment and expansion of ECMO centers over the past decade, primarily to meet the anticipated demands of treating severe influenza-related ARDS.

The recently published EOLIA trial (Combes A, et al. N Engl J Med. 2018;378:1965) was designed to study the benefit of VV ECMO vs conventional mechanical ventilation in ARDS and demonstrated an 11% absolute reduction in 60-day mortality, which did not reach statistical significance. Like the CESAR trial, there are critiques of the outcome, especially as it relates to stopping the trial early due to the inability to show a significant benefit of VV ECMO over mechanical ventilation.
All of the aforementioned studies evaluated adults under age 65. Interestingly, there are no specific age contraindications for the use of ECMO (ELSO Guidelines for Cardiopulmonary Extracorporeal Life Support, Extracorporeal Life Support Organization, Version 1.4 August 2017), but many consider older age as a risk for poor outcome. Approximately 2,300 adult patients in the United States have been treated with ECMO for respiratory failure each year, and only 10% of those are over age 65 (CMS Changes in ECMO Reimbursements – ELSO Report). The outcome benefit of ECMO for a relatively healthy patient over age 65 is not known, as those patients have not been evaluated in studies thus far. When comparison to data from decades ago is made, one must keep in mind that populations worldwide are living longer, and a continued increase in number of adults over the age 65 is expected.
While the overall interpretation of the outcomes of studies of ECMO may be fraught with controversy, there is little debate that providing care for patients with refractory respiratory failure in centers that provide high-level skill and expertise in management of respiratory failure has a clear benefit, irrespective of whether the patient eventually receives therapy with ECMO. What is also clear is that ECMO is costly, with per-patient costs demonstrated to be at least double that of those receiving mechanical ventilation alone (Peek G, et al. Lancet. 2009;374:1351). This substantial cost associated with ECMO cannot be ignored in today’s era of value-based care.
Fortuitously, CMS recently released new DRG reimbursement scales for the use of ECMO effective Oct 1, 2018. VV ECMO could have as much as a 70% reduction in reimbursement, and many insurance companies are expected to follow suit (CMS Changes in ECMO Reimbursements –ELSO Report). Only time will tell what impact this, along with the current evidence, will have on long-term provision of ECMO care for our sickest of patients with influenza and associated respiratory illnesses.
Dr. Tatem is with the Division of Pulmonary and Critical Care Medicine, Department of Medicine, Henry Ford Hospital, Detroit, Michigan.
Now that we are in the midst of flu season, many discussions regarding the management of patients with influenza virus infections are ensuing. While prevention is always preferable, and we encourage everyone to get vaccinated, influenza remains a rapidly widespread infection. In the United States during last year’s flu season (2017-18), there was an estimated 49 million cases of influenza, 960,000 hospitalizations, and 79,000 deaths. Approximately 86% of all deaths were estimated to occur in those aged 65 and older (Centers for Disease Control and Prevention webpage on Burden of Influenza).
Despite our best efforts, there are inevitable times when some patients become ill enough to require hospitalization. Patients aged 65 and older make up the overwhelming majority of patients with influenza who eventually require hospitalization (Fig 1) (The Centers for Disease Control and Prevention FluView Database). Comorbidities also confer higher risk for more severe illness and potential hospitalization irrespective of age (Fig 2). In children with known medical conditions, asthma confers highest risk of hospitalization, as 27% of those with asthma were hospitalized after developing the flu. In adults, 52% of those with cardiovascular disease and 30% of adult patients with chronic lung disease who were confirmed to have influenza required hospitalization for treatment (Fig 2, The Centers for Disease Control and Prevention FluView Database).
The most severe cases of influenza can require ICU care and advanced management of respiratory failure as a result of the acute respiratory distress syndrome (ARDS). The lungs suffer significant injury due to the viral infection, and they lose their ability to effectively oxygenate the blood. Secondary bacterial infections can also occur as a complication, which compounds the injury. Given the fact that so many patients have significant comorbidities and are of advanced age, it is reasonable to expect that a fair proportion of those with influenza would develop respiratory failure as a consequence. For some of these patients, the hypoxemia that develops as a result of the lung injury can be exceptionally challenging to manage. In extreme cases, conventional ventilator management is insufficient, and the need for additional, advanced therapies arise.
Studies of VV ECMO in severe influenza
ECMO (extracorporeal membrane oxygenation) is a treatment that has been employed to help support patients with severe hypoxemic respiratory failure while their lungs recover from acute injury. Venovenous (VV) ECMO requires peripheral insertion of large cannulae into the venous system to take deoxygenated blood, deliver it through the membrane oxygenator and return the oxygenated blood back to the venous system. In simplest terms, the membrane of ECMO circuit serves as a substitute for the gas exchange function of the lungs and provides the oxygenation that the injured alveoli of the lung are unable to provide. The overall intent is to have the external ECMO circuit do all of the gas exchange work while the lungs heal.

Much research has been done on VV ECMO as an adjunct or salvage therapy in patients with refractory hypoxemic respiratory failure due to ARDS. Historical and recent studies have shown that approximately 60% of patients with ARDS have viral (approximately 20%) or bacterial (approximately 40%) pneumonia as the underlying cause (Zapol, et al. JAMA. 1979; 242[20]:2193; Combes A, et al. N Engl J Med. 2018;378:1965). Naturally, given the frequency of infection as a cause for ARDS, and the severity of illness that can develop with influenza infection in particular, an interest has arisen in the applicability of ECMO in cases of severe influenza-related ARDS.
In 2009, during the H1N1 influenza pandemic, the ANZ ECMO investigators in Australia and New Zealand described a 78% survival rate for their patients with severe H1N1 associated ARDS treated with VV ECMO between June and August of that year (Davies A, et al. JAMA. 2009;302[17]:1888). The eagerly awaited results of the randomized, controlled CESAR trial (Peek G, et al. Lancet. 2009;374:1351) that studied patients aged 18 to 64 with severe, refractory respiratory failure transferred to a specialized center for ECMO care had additional impact in catalyzing interest in ECMO use. This trial showed improved survival with ECMO (63% in ECMO vs 47% control, RR 0.69; 95% CI 0.05-0.97 P=.03) with a gain of 0.03 QALY (quality-adjusted life years) with additional cost of 40,000 pounds sterling. However, a major critique is that 24% of patients transferred to the specialized center never were treated with ECMO. Significantly, there was incomplete follow-up data on nearly half of the patients, as well. Many conclude that the survival benefit seen in this study may be more reflective of the expertise in respiratory failure management (especially as it relates to lung protective ventilation) at this center than therapy with ECMO itself.
Additional cohort studies in the United Kingdom (Noah MA, et al. JAMA. 2011;306[15]:1659) and Italy (Pappalardo F, et al. Intensive Care Med. 2013;39[2]:275) showed approximately 70% in-hospital survival rates for patients with H1N1 influenza transferred to a specialized ECMO center and treated with ECMO.
Nonetheless, the information gained from the observational data from ANZ ECMO, along with data published in European cohort studies and the randomized controlled CESAR trial after the 2009 H1N1 influenza pandemic, greatly contributed to the rise in use of ECMO for refractory ARDS due to influenza. Subsequently, there has been a rapid establishment and expansion of ECMO centers over the past decade, primarily to meet the anticipated demands of treating severe influenza-related ARDS.

The recently published EOLIA trial (Combes A, et al. N Engl J Med. 2018;378:1965) was designed to study the benefit of VV ECMO vs conventional mechanical ventilation in ARDS and demonstrated an 11% absolute reduction in 60-day mortality, which did not reach statistical significance. Like the CESAR trial, there are critiques of the outcome, especially as it relates to stopping the trial early due to the inability to show a significant benefit of VV ECMO over mechanical ventilation.
All of the aforementioned studies evaluated adults under age 65. Interestingly, there are no specific age contraindications for the use of ECMO (ELSO Guidelines for Cardiopulmonary Extracorporeal Life Support, Extracorporeal Life Support Organization, Version 1.4 August 2017), but many consider older age as a risk for poor outcome. Approximately 2,300 adult patients in the United States have been treated with ECMO for respiratory failure each year, and only 10% of those are over age 65 (CMS Changes in ECMO Reimbursements – ELSO Report). The outcome benefit of ECMO for a relatively healthy patient over age 65 is not known, as those patients have not been evaluated in studies thus far. When comparison to data from decades ago is made, one must keep in mind that populations worldwide are living longer, and a continued increase in number of adults over the age 65 is expected.
While the overall interpretation of the outcomes of studies of ECMO may be fraught with controversy, there is little debate that providing care for patients with refractory respiratory failure in centers that provide high-level skill and expertise in management of respiratory failure has a clear benefit, irrespective of whether the patient eventually receives therapy with ECMO. What is also clear is that ECMO is costly, with per-patient costs demonstrated to be at least double that of those receiving mechanical ventilation alone (Peek G, et al. Lancet. 2009;374:1351). This substantial cost associated with ECMO cannot be ignored in today’s era of value-based care.
Fortuitously, CMS recently released new DRG reimbursement scales for the use of ECMO effective Oct 1, 2018. VV ECMO could have as much as a 70% reduction in reimbursement, and many insurance companies are expected to follow suit (CMS Changes in ECMO Reimbursements –ELSO Report). Only time will tell what impact this, along with the current evidence, will have on long-term provision of ECMO care for our sickest of patients with influenza and associated respiratory illnesses.
Dr. Tatem is with the Division of Pulmonary and Critical Care Medicine, Department of Medicine, Henry Ford Hospital, Detroit, Michigan.
Now that we are in the midst of flu season, many discussions regarding the management of patients with influenza virus infections are ensuing. While prevention is always preferable, and we encourage everyone to get vaccinated, influenza remains a rapidly widespread infection. In the United States during last year’s flu season (2017-18), there was an estimated 49 million cases of influenza, 960,000 hospitalizations, and 79,000 deaths. Approximately 86% of all deaths were estimated to occur in those aged 65 and older (Centers for Disease Control and Prevention webpage on Burden of Influenza).
Despite our best efforts, there are inevitable times when some patients become ill enough to require hospitalization. Patients aged 65 and older make up the overwhelming majority of patients with influenza who eventually require hospitalization (Fig 1) (The Centers for Disease Control and Prevention FluView Database). Comorbidities also confer higher risk for more severe illness and potential hospitalization irrespective of age (Fig 2). In children with known medical conditions, asthma confers highest risk of hospitalization, as 27% of those with asthma were hospitalized after developing the flu. In adults, 52% of those with cardiovascular disease and 30% of adult patients with chronic lung disease who were confirmed to have influenza required hospitalization for treatment (Fig 2, The Centers for Disease Control and Prevention FluView Database).
The most severe cases of influenza can require ICU care and advanced management of respiratory failure as a result of the acute respiratory distress syndrome (ARDS). The lungs suffer significant injury due to the viral infection, and they lose their ability to effectively oxygenate the blood. Secondary bacterial infections can also occur as a complication, which compounds the injury. Given the fact that so many patients have significant comorbidities and are of advanced age, it is reasonable to expect that a fair proportion of those with influenza would develop respiratory failure as a consequence. For some of these patients, the hypoxemia that develops as a result of the lung injury can be exceptionally challenging to manage. In extreme cases, conventional ventilator management is insufficient, and the need for additional, advanced therapies arise.
Studies of VV ECMO in severe influenza
ECMO (extracorporeal membrane oxygenation) is a treatment that has been employed to help support patients with severe hypoxemic respiratory failure while their lungs recover from acute injury. Venovenous (VV) ECMO requires peripheral insertion of large cannulae into the venous system to take deoxygenated blood, deliver it through the membrane oxygenator and return the oxygenated blood back to the venous system. In simplest terms, the membrane of ECMO circuit serves as a substitute for the gas exchange function of the lungs and provides the oxygenation that the injured alveoli of the lung are unable to provide. The overall intent is to have the external ECMO circuit do all of the gas exchange work while the lungs heal.

Much research has been done on VV ECMO as an adjunct or salvage therapy in patients with refractory hypoxemic respiratory failure due to ARDS. Historical and recent studies have shown that approximately 60% of patients with ARDS have viral (approximately 20%) or bacterial (approximately 40%) pneumonia as the underlying cause (Zapol, et al. JAMA. 1979; 242[20]:2193; Combes A, et al. N Engl J Med. 2018;378:1965). Naturally, given the frequency of infection as a cause for ARDS, and the severity of illness that can develop with influenza infection in particular, an interest has arisen in the applicability of ECMO in cases of severe influenza-related ARDS.
In 2009, during the H1N1 influenza pandemic, the ANZ ECMO investigators in Australia and New Zealand described a 78% survival rate for their patients with severe H1N1 associated ARDS treated with VV ECMO between June and August of that year (Davies A, et al. JAMA. 2009;302[17]:1888). The eagerly awaited results of the randomized, controlled CESAR trial (Peek G, et al. Lancet. 2009;374:1351) that studied patients aged 18 to 64 with severe, refractory respiratory failure transferred to a specialized center for ECMO care had additional impact in catalyzing interest in ECMO use. This trial showed improved survival with ECMO (63% in ECMO vs 47% control, RR 0.69; 95% CI 0.05-0.97 P=.03) with a gain of 0.03 QALY (quality-adjusted life years) with additional cost of 40,000 pounds sterling. However, a major critique is that 24% of patients transferred to the specialized center never were treated with ECMO. Significantly, there was incomplete follow-up data on nearly half of the patients, as well. Many conclude that the survival benefit seen in this study may be more reflective of the expertise in respiratory failure management (especially as it relates to lung protective ventilation) at this center than therapy with ECMO itself.
Additional cohort studies in the United Kingdom (Noah MA, et al. JAMA. 2011;306[15]:1659) and Italy (Pappalardo F, et al. Intensive Care Med. 2013;39[2]:275) showed approximately 70% in-hospital survival rates for patients with H1N1 influenza transferred to a specialized ECMO center and treated with ECMO.
Nonetheless, the information gained from the observational data from ANZ ECMO, along with data published in European cohort studies and the randomized controlled CESAR trial after the 2009 H1N1 influenza pandemic, greatly contributed to the rise in use of ECMO for refractory ARDS due to influenza. Subsequently, there has been a rapid establishment and expansion of ECMO centers over the past decade, primarily to meet the anticipated demands of treating severe influenza-related ARDS.

The recently published EOLIA trial (Combes A, et al. N Engl J Med. 2018;378:1965) was designed to study the benefit of VV ECMO vs conventional mechanical ventilation in ARDS and demonstrated an 11% absolute reduction in 60-day mortality, which did not reach statistical significance. Like the CESAR trial, there are critiques of the outcome, especially as it relates to stopping the trial early due to the inability to show a significant benefit of VV ECMO over mechanical ventilation.
All of the aforementioned studies evaluated adults under age 65. Interestingly, there are no specific age contraindications for the use of ECMO (ELSO Guidelines for Cardiopulmonary Extracorporeal Life Support, Extracorporeal Life Support Organization, Version 1.4 August 2017), but many consider older age as a risk for poor outcome. Approximately 2,300 adult patients in the United States have been treated with ECMO for respiratory failure each year, and only 10% of those are over age 65 (CMS Changes in ECMO Reimbursements – ELSO Report). The outcome benefit of ECMO for a relatively healthy patient over age 65 is not known, as those patients have not been evaluated in studies thus far. When comparison to data from decades ago is made, one must keep in mind that populations worldwide are living longer, and a continued increase in number of adults over the age 65 is expected.
While the overall interpretation of the outcomes of studies of ECMO may be fraught with controversy, there is little debate that providing care for patients with refractory respiratory failure in centers that provide high-level skill and expertise in management of respiratory failure has a clear benefit, irrespective of whether the patient eventually receives therapy with ECMO. What is also clear is that ECMO is costly, with per-patient costs demonstrated to be at least double that of those receiving mechanical ventilation alone (Peek G, et al. Lancet. 2009;374:1351). This substantial cost associated with ECMO cannot be ignored in today’s era of value-based care.
Fortuitously, CMS recently released new DRG reimbursement scales for the use of ECMO effective Oct 1, 2018. VV ECMO could have as much as a 70% reduction in reimbursement, and many insurance companies are expected to follow suit (CMS Changes in ECMO Reimbursements –ELSO Report). Only time will tell what impact this, along with the current evidence, will have on long-term provision of ECMO care for our sickest of patients with influenza and associated respiratory illnesses.
Dr. Tatem is with the Division of Pulmonary and Critical Care Medicine, Department of Medicine, Henry Ford Hospital, Detroit, Michigan.
The importance of diversity and inclusion in medicine
Diversity
There is growing appreciation for diversity and inclusion (DI) as drivers of excellence in medicine. CHEST also promotes excellence in medicine. Therefore, it is intuitive that CHEST promote DI. Diversity encompasses differences in gender, race/ethnicity, vocational training, age, sexual orientation, thought processes, etc.
Academic medicine is rich with examples of how diversity is critical to the health of our nation:
– Diverse student populations have been shown to improve our learners’ satisfaction with their educational experience.
– Diverse teams have been shown to be more capable of solving complex problems than homogenous teams.
– Health care is moving toward a team-based, interprofessional model that values the contributions of a range of providers’ perspectives in improving patient outcomes.
– In biomedical research, investigators ask different research questions based on their own background and experiences. This implies that finding solutions to diseases that affect specific populations will require a diverse pool of biomedical researchers.
– Faculty diversity as a key component of excellence for medical education and research has been documented.
Diversity alone doesn’t drive inclusion. Noted diversity advocate, Verna Myers, stated, “Diversity is being invited to the party. Inclusion is being asked to dance.” In my opinion, diversity is the commencement of work, but inclusion helps complete the task.
Inclusion
An inclusive environment values the unique contributions all members bring. Teams with diversity of thought are more innovative as individual members with different backgrounds and points of view bring an extensive range of ideas and creativity to scientific discovery and decision-making processes. Inclusion leverages the power of our unique differences to accomplish our mutual goals. By valuing everyone’s perspective, we demonstrate excellence.
I recommend an article from the Harvard Business Review (HBR Feb 2017). The authors suggest several ways to promote inclusiveness: (1) ensuring team members speak up and are heard; (2) making it safe to propose novel ideas; (3) empowering team members to make decisions; (4) taking advice and implementing feedback; (5) giving actionable feedback; and ( 6) sharing credit for team success. If the team leader possesses at least three of these traits, 87% of team members say they feel welcome and included in their team; 87% say they feel free to express their views and opinions; and 74% say they feel that their ideas are heard and recognized. If the team leader possessed none of these traits, those percentages dropped to 51%, 46%, and 37%, respectively. I believe this concept is applicable in medicine also.
Sponsors
What can we do to advance diversity and inclusion individually and in our individual institutions? A sponsor is a senior level leader who advocates for key assignments, promotes for and puts his or her reputation on the line for the protégé’s advancement. This invigorates and drives engagement. One key to rising above the playing field for women and people of color is sponsorship. Being a sponsor does not mean one would recommend someone who is not qualified. It means one recommends or supports those who are capable of doing the job but would not otherwise be given the opportunity.
Ask yourself: Have I served as a sponsor? What would prevent me from being a sponsor? Do I believe in this concept?
Cause for Alarm
Numerous publications have recently discussed the crisis of the decline of black men entering medicine. In 1978, there were 1,410 black male applicants to medical school, and in 2014, there were 1,337. Additionally, the number of black male matriculants to medical school over more than 35 years has not surpassed the 1978 numbers. In 1978, there were 542 black male matriculants, and in 2014, there were 515 (J of Racial and Ethnic Health Disparities. 2017, 4:317-321). This report is thorough and insightful and illustrates the work that we must do to help improve this situation.
Dr. Marc Nivet, Association of American Medical Colleges (AAMC) Chief Diversity Officer, stated “No other minority group has experienced such declines. The inability to find, engage, and develop candidates for careers in medicine from all members of our society limits our ability to improve health care for all.” I recommend you read the 2015 AAMC publication entitled: Altering the Course: Black Males in Medicine.
Health-care Disparities
Research suggests that the overall health of Americans has improved; however, disparities continue to persist among many populations within the United States. Racial and ethnic minority populations have poorer access to care and worse outcomes than their white counterparts. Approximately 20% of the nation living in rural areas is less likely than those living in urban areas to receive preventive care and more likely to experience language barriers.
Individuals identifying as lesbian, gay, bisexual, or transgender are likely to experience discrimination in health-care settings. These individuals often face insurance-based barriers and are less likely to have a usual source of care than patients who identify as straight.
A 2002 report by the Institute of Medicine entitled: Unequal Treatment: What Healthcare Providers Need to Know about Racial and Ethnic Disparities in Healthcare is revealing. Salient information reported is: It is generally accepted that a diverse workforce is a key component in the delivery of quality, competent care throughout the nation. Physicians from racial and ethnic backgrounds typically underrepresented in medicine are significantly more likely to practice primary care than white physicians and are more likely to practice in impoverished and medically underserved areas. Diversity in the physician workforce impacts the quality of care received by patients. Race concordance between patient and physician results in longer visits and increased patient satisfaction, and language concordance is positively associated with adherence to treatment among certain racial or ethnic groups.
Improving the patient experience or quality of care received also requires attention to education and training on cultural competence. By weaving together a diverse and culturally responsive pool of physicians working collaboratively with other health-care professionals, access and quality of care can improve throughout the nation.
CHEST cannot attain more racial diversity in our organization if we don’t have this diversity in medical education and training. This is why CHEST must be actively involved in addressing these issues.
Unconscious Bias
Despite many examples of how diversity enriches the quality of health care and health research, there is still much work to be done to address the human biases that impede our ability to benefit from diversity in medicine. While academic medicine has made progress toward addressing overt discrimination, unconscious bias (implicit bias) represents another threat. Unconscious bias describes the prejudices we don’t know we have. While unconscious biases vary from person to person, we all possess them. The existence of unconscious bias in academic medicine, while uncomfortable and unsettling, is a reality. The AAMC developed an unconscious bias learning lab for the health professions and produced an oft-cited video about addressing unconscious bias in the faculty advancement, promotion, and tenure process. We must consider this and other ways in which we can help promote the acknowledgment of unconscious bias. The CHEST staff have undergone unconscious bias training, and I recommend it for all faculty in academic medicine.
Summary
Diversity and inclusion in medicine is of paramount importance. It leads to better patient care and better trainee education and will decrease health-care disparities. Progress has been made, but there is more work to be done.
CHEST is supportive of these efforts and has worked on this previously and with a renewed push in the past 2 years with the DI Task Force initially and, now, the DI Roundtable, which has representatives from each of the standing committees, including the Board of Regents. This roundtable group will help advance the DI initiatives of the organization. I ask that each person reading this article consider what we as individuals can do in helping make DI in medicine a priority.
Dr. Haynes is Professor of Medicine at The University of Mississippi Medical Center in Jackson, MS. He is also the Executive Vice Chair of the Department of Medicine. At CHEST, he is a member of the training and transitions committee, executive scientific program committee, former chair of the diversity and inclusion task force, and is the current chair of the diversity and inclusion roundtable.
Diversity
There is growing appreciation for diversity and inclusion (DI) as drivers of excellence in medicine. CHEST also promotes excellence in medicine. Therefore, it is intuitive that CHEST promote DI. Diversity encompasses differences in gender, race/ethnicity, vocational training, age, sexual orientation, thought processes, etc.
Academic medicine is rich with examples of how diversity is critical to the health of our nation:
– Diverse student populations have been shown to improve our learners’ satisfaction with their educational experience.
– Diverse teams have been shown to be more capable of solving complex problems than homogenous teams.
– Health care is moving toward a team-based, interprofessional model that values the contributions of a range of providers’ perspectives in improving patient outcomes.
– In biomedical research, investigators ask different research questions based on their own background and experiences. This implies that finding solutions to diseases that affect specific populations will require a diverse pool of biomedical researchers.
– Faculty diversity as a key component of excellence for medical education and research has been documented.
Diversity alone doesn’t drive inclusion. Noted diversity advocate, Verna Myers, stated, “Diversity is being invited to the party. Inclusion is being asked to dance.” In my opinion, diversity is the commencement of work, but inclusion helps complete the task.
Inclusion
An inclusive environment values the unique contributions all members bring. Teams with diversity of thought are more innovative as individual members with different backgrounds and points of view bring an extensive range of ideas and creativity to scientific discovery and decision-making processes. Inclusion leverages the power of our unique differences to accomplish our mutual goals. By valuing everyone’s perspective, we demonstrate excellence.
I recommend an article from the Harvard Business Review (HBR Feb 2017). The authors suggest several ways to promote inclusiveness: (1) ensuring team members speak up and are heard; (2) making it safe to propose novel ideas; (3) empowering team members to make decisions; (4) taking advice and implementing feedback; (5) giving actionable feedback; and ( 6) sharing credit for team success. If the team leader possesses at least three of these traits, 87% of team members say they feel welcome and included in their team; 87% say they feel free to express their views and opinions; and 74% say they feel that their ideas are heard and recognized. If the team leader possessed none of these traits, those percentages dropped to 51%, 46%, and 37%, respectively. I believe this concept is applicable in medicine also.
Sponsors
What can we do to advance diversity and inclusion individually and in our individual institutions? A sponsor is a senior level leader who advocates for key assignments, promotes for and puts his or her reputation on the line for the protégé’s advancement. This invigorates and drives engagement. One key to rising above the playing field for women and people of color is sponsorship. Being a sponsor does not mean one would recommend someone who is not qualified. It means one recommends or supports those who are capable of doing the job but would not otherwise be given the opportunity.
Ask yourself: Have I served as a sponsor? What would prevent me from being a sponsor? Do I believe in this concept?
Cause for Alarm
Numerous publications have recently discussed the crisis of the decline of black men entering medicine. In 1978, there were 1,410 black male applicants to medical school, and in 2014, there were 1,337. Additionally, the number of black male matriculants to medical school over more than 35 years has not surpassed the 1978 numbers. In 1978, there were 542 black male matriculants, and in 2014, there were 515 (J of Racial and Ethnic Health Disparities. 2017, 4:317-321). This report is thorough and insightful and illustrates the work that we must do to help improve this situation.
Dr. Marc Nivet, Association of American Medical Colleges (AAMC) Chief Diversity Officer, stated “No other minority group has experienced such declines. The inability to find, engage, and develop candidates for careers in medicine from all members of our society limits our ability to improve health care for all.” I recommend you read the 2015 AAMC publication entitled: Altering the Course: Black Males in Medicine.
Health-care Disparities
Research suggests that the overall health of Americans has improved; however, disparities continue to persist among many populations within the United States. Racial and ethnic minority populations have poorer access to care and worse outcomes than their white counterparts. Approximately 20% of the nation living in rural areas is less likely than those living in urban areas to receive preventive care and more likely to experience language barriers.
Individuals identifying as lesbian, gay, bisexual, or transgender are likely to experience discrimination in health-care settings. These individuals often face insurance-based barriers and are less likely to have a usual source of care than patients who identify as straight.
A 2002 report by the Institute of Medicine entitled: Unequal Treatment: What Healthcare Providers Need to Know about Racial and Ethnic Disparities in Healthcare is revealing. Salient information reported is: It is generally accepted that a diverse workforce is a key component in the delivery of quality, competent care throughout the nation. Physicians from racial and ethnic backgrounds typically underrepresented in medicine are significantly more likely to practice primary care than white physicians and are more likely to practice in impoverished and medically underserved areas. Diversity in the physician workforce impacts the quality of care received by patients. Race concordance between patient and physician results in longer visits and increased patient satisfaction, and language concordance is positively associated with adherence to treatment among certain racial or ethnic groups.
Improving the patient experience or quality of care received also requires attention to education and training on cultural competence. By weaving together a diverse and culturally responsive pool of physicians working collaboratively with other health-care professionals, access and quality of care can improve throughout the nation.
CHEST cannot attain more racial diversity in our organization if we don’t have this diversity in medical education and training. This is why CHEST must be actively involved in addressing these issues.
Unconscious Bias
Despite many examples of how diversity enriches the quality of health care and health research, there is still much work to be done to address the human biases that impede our ability to benefit from diversity in medicine. While academic medicine has made progress toward addressing overt discrimination, unconscious bias (implicit bias) represents another threat. Unconscious bias describes the prejudices we don’t know we have. While unconscious biases vary from person to person, we all possess them. The existence of unconscious bias in academic medicine, while uncomfortable and unsettling, is a reality. The AAMC developed an unconscious bias learning lab for the health professions and produced an oft-cited video about addressing unconscious bias in the faculty advancement, promotion, and tenure process. We must consider this and other ways in which we can help promote the acknowledgment of unconscious bias. The CHEST staff have undergone unconscious bias training, and I recommend it for all faculty in academic medicine.
Summary
Diversity and inclusion in medicine is of paramount importance. It leads to better patient care and better trainee education and will decrease health-care disparities. Progress has been made, but there is more work to be done.
CHEST is supportive of these efforts and has worked on this previously and with a renewed push in the past 2 years with the DI Task Force initially and, now, the DI Roundtable, which has representatives from each of the standing committees, including the Board of Regents. This roundtable group will help advance the DI initiatives of the organization. I ask that each person reading this article consider what we as individuals can do in helping make DI in medicine a priority.
Dr. Haynes is Professor of Medicine at The University of Mississippi Medical Center in Jackson, MS. He is also the Executive Vice Chair of the Department of Medicine. At CHEST, he is a member of the training and transitions committee, executive scientific program committee, former chair of the diversity and inclusion task force, and is the current chair of the diversity and inclusion roundtable.
Diversity
There is growing appreciation for diversity and inclusion (DI) as drivers of excellence in medicine. CHEST also promotes excellence in medicine. Therefore, it is intuitive that CHEST promote DI. Diversity encompasses differences in gender, race/ethnicity, vocational training, age, sexual orientation, thought processes, etc.
Academic medicine is rich with examples of how diversity is critical to the health of our nation:
– Diverse student populations have been shown to improve our learners’ satisfaction with their educational experience.
– Diverse teams have been shown to be more capable of solving complex problems than homogenous teams.
– Health care is moving toward a team-based, interprofessional model that values the contributions of a range of providers’ perspectives in improving patient outcomes.
– In biomedical research, investigators ask different research questions based on their own background and experiences. This implies that finding solutions to diseases that affect specific populations will require a diverse pool of biomedical researchers.
– Faculty diversity as a key component of excellence for medical education and research has been documented.
Diversity alone doesn’t drive inclusion. Noted diversity advocate, Verna Myers, stated, “Diversity is being invited to the party. Inclusion is being asked to dance.” In my opinion, diversity is the commencement of work, but inclusion helps complete the task.
Inclusion
An inclusive environment values the unique contributions all members bring. Teams with diversity of thought are more innovative as individual members with different backgrounds and points of view bring an extensive range of ideas and creativity to scientific discovery and decision-making processes. Inclusion leverages the power of our unique differences to accomplish our mutual goals. By valuing everyone’s perspective, we demonstrate excellence.
I recommend an article from the Harvard Business Review (HBR Feb 2017). The authors suggest several ways to promote inclusiveness: (1) ensuring team members speak up and are heard; (2) making it safe to propose novel ideas; (3) empowering team members to make decisions; (4) taking advice and implementing feedback; (5) giving actionable feedback; and ( 6) sharing credit for team success. If the team leader possesses at least three of these traits, 87% of team members say they feel welcome and included in their team; 87% say they feel free to express their views and opinions; and 74% say they feel that their ideas are heard and recognized. If the team leader possessed none of these traits, those percentages dropped to 51%, 46%, and 37%, respectively. I believe this concept is applicable in medicine also.
Sponsors
What can we do to advance diversity and inclusion individually and in our individual institutions? A sponsor is a senior level leader who advocates for key assignments, promotes for and puts his or her reputation on the line for the protégé’s advancement. This invigorates and drives engagement. One key to rising above the playing field for women and people of color is sponsorship. Being a sponsor does not mean one would recommend someone who is not qualified. It means one recommends or supports those who are capable of doing the job but would not otherwise be given the opportunity.
Ask yourself: Have I served as a sponsor? What would prevent me from being a sponsor? Do I believe in this concept?
Cause for Alarm
Numerous publications have recently discussed the crisis of the decline of black men entering medicine. In 1978, there were 1,410 black male applicants to medical school, and in 2014, there were 1,337. Additionally, the number of black male matriculants to medical school over more than 35 years has not surpassed the 1978 numbers. In 1978, there were 542 black male matriculants, and in 2014, there were 515 (J of Racial and Ethnic Health Disparities. 2017, 4:317-321). This report is thorough and insightful and illustrates the work that we must do to help improve this situation.
Dr. Marc Nivet, Association of American Medical Colleges (AAMC) Chief Diversity Officer, stated “No other minority group has experienced such declines. The inability to find, engage, and develop candidates for careers in medicine from all members of our society limits our ability to improve health care for all.” I recommend you read the 2015 AAMC publication entitled: Altering the Course: Black Males in Medicine.
Health-care Disparities
Research suggests that the overall health of Americans has improved; however, disparities continue to persist among many populations within the United States. Racial and ethnic minority populations have poorer access to care and worse outcomes than their white counterparts. Approximately 20% of the nation living in rural areas is less likely than those living in urban areas to receive preventive care and more likely to experience language barriers.
Individuals identifying as lesbian, gay, bisexual, or transgender are likely to experience discrimination in health-care settings. These individuals often face insurance-based barriers and are less likely to have a usual source of care than patients who identify as straight.
A 2002 report by the Institute of Medicine entitled: Unequal Treatment: What Healthcare Providers Need to Know about Racial and Ethnic Disparities in Healthcare is revealing. Salient information reported is: It is generally accepted that a diverse workforce is a key component in the delivery of quality, competent care throughout the nation. Physicians from racial and ethnic backgrounds typically underrepresented in medicine are significantly more likely to practice primary care than white physicians and are more likely to practice in impoverished and medically underserved areas. Diversity in the physician workforce impacts the quality of care received by patients. Race concordance between patient and physician results in longer visits and increased patient satisfaction, and language concordance is positively associated with adherence to treatment among certain racial or ethnic groups.
Improving the patient experience or quality of care received also requires attention to education and training on cultural competence. By weaving together a diverse and culturally responsive pool of physicians working collaboratively with other health-care professionals, access and quality of care can improve throughout the nation.
CHEST cannot attain more racial diversity in our organization if we don’t have this diversity in medical education and training. This is why CHEST must be actively involved in addressing these issues.
Unconscious Bias
Despite many examples of how diversity enriches the quality of health care and health research, there is still much work to be done to address the human biases that impede our ability to benefit from diversity in medicine. While academic medicine has made progress toward addressing overt discrimination, unconscious bias (implicit bias) represents another threat. Unconscious bias describes the prejudices we don’t know we have. While unconscious biases vary from person to person, we all possess them. The existence of unconscious bias in academic medicine, while uncomfortable and unsettling, is a reality. The AAMC developed an unconscious bias learning lab for the health professions and produced an oft-cited video about addressing unconscious bias in the faculty advancement, promotion, and tenure process. We must consider this and other ways in which we can help promote the acknowledgment of unconscious bias. The CHEST staff have undergone unconscious bias training, and I recommend it for all faculty in academic medicine.
Summary
Diversity and inclusion in medicine is of paramount importance. It leads to better patient care and better trainee education and will decrease health-care disparities. Progress has been made, but there is more work to be done.
CHEST is supportive of these efforts and has worked on this previously and with a renewed push in the past 2 years with the DI Task Force initially and, now, the DI Roundtable, which has representatives from each of the standing committees, including the Board of Regents. This roundtable group will help advance the DI initiatives of the organization. I ask that each person reading this article consider what we as individuals can do in helping make DI in medicine a priority.
Dr. Haynes is Professor of Medicine at The University of Mississippi Medical Center in Jackson, MS. He is also the Executive Vice Chair of the Department of Medicine. At CHEST, he is a member of the training and transitions committee, executive scientific program committee, former chair of the diversity and inclusion task force, and is the current chair of the diversity and inclusion roundtable.
Hurricane relief and patient care
In October 2017, in support of the Federal Emergency Management Agency’s response to assist the Governor and people of Puerto Rico, three Department of Defense (DOD) military hospital platforms were deployed; one each, by the US Army, Navy, and Air Force. They arrived on the island at different times with predominantly wartime surgical capabilities and augmented the Federal Emergency Management Agency (FEMA), US Public Health Service, National Guard, and Puerto Rico Department of Health efforts. My perspective is that of patient care and transport between the Centro Medico hospital complex in San Juan, the larger regional hospitals, the Veterans Administration hospital, the DOD response, FEMA Disaster Medical Assistance Teams (DMAT), and FEMA Federal Medical Shelters about 4 to 6 weeks after Hurricanes Maria and Irma struck. Based upon this experience, I would like to offer the following.
Pre-Disaster: All clinicians have a few patients that teeter “on the edge.” When basic services go away, these patients fall over that edge and become inpatients. Establish a list of patients who require oxygen and devices such as vests, cough-assist, or ventilation. If evacuation before the disaster is possible, those patients need to leave. If they refuse, or are unable to leave, they need to be able to supply their own generated power for a prolonged period of time, as batteries will run out prior to power restoration. They must be able to use oxygen concentrators, as tank re-supply may not be readily available. By law, FEMA cannot give generators to individuals, so individuals must prepare for themselves. In a hurricane-prone area where seasonal risk can be established, planning medication refills at the beginning of the season or giving a larger than normal supply may prove useful. In an area prone to sudden disaster, such as earthquake or tornado, then counseling patients to request refills at least 2 weeks early may be adequate.
Post-Disaster: The most reliable form of communication will be text. You likely already have text contacts for your staff and family members; add other providers, responders, planners, pharmacists, and oxygen suppliers to your text contacts. While you may wish to share a text point of contact with patients, understand that your ability to actually help during the initial disaster will likely be limited. Identify possible language translation needs and possible translators among your staff and/or friends as telephone services will be limited or absent following the disaster. Finally, identify your local emergency response planners on Facebook, Twitter, or other social media feeds. This will allow you to direct others to these sites for accurate information after the disaster.
Responder Recommendations: A single social media post can DESTROY your plans and hamper your efforts. Advertise a single contact point and an information resource (eg, bulletin board, webpage) early and often. Publicly and accurately declare the means by which people will access health care and health-care services, such as medications, dialysis, and oxygen. There will be nongovernment organizations (NGOs), friends, and other well-meaning individuals who will try to assist people in need through unconventional channels. Yet, by requesting assistance through nonroutine channels, those efforts tend to delay assistance, cause confusion, and/or squander resources. Continue to direct those requests through the established response channels, ie, the local 911 equivalent.
Plan to use cellular texts to communicate. While satellite telephones are great in concept, in execution, they are difficult to utilize when transmitting complex medical information. If you have an expansive budget, there are now devices available that allow for Iridium satellite-based text communications that require batteries but not intact cellular towers.
Facilities with electricity, water, oxygen, medications, laboratory testing, and CT scanners need to be identified and advertised within the responder community. If FEMA is involved, these resources will be identified and updated on a routine basis. The information will be distributed to their DMAT teams. Those DMAT teams will be distributed throughout the response area. Additionally, if the resources and budgeting are approved, then FEMA will also help re-establish medical transport, as well as Federal Medical Shelters (FMS). The FMS can temporarily house patients who can perform basic activities of daily living but require power, oxygen, or medication administration. For those patients in need of medications without insurance, FEMA may activate medication assistance through the Emergency Prescription Assistance Program. This will allow up to 30 days of medication to be distributed at no cost to the individual through participating pharmacies.
External responders will obviously need to pair with local providers/professionals who can navigate the system and, if necessary, can translate medical terms and care plans. Additionally, external responders will be targets for individuals looking to obtain resources for secondary gain or profit. Establishing a plan or consistently redirecting people to the appropriate resources for those needs may limit the inevitable damage these individuals will cause. Additionally, understand that the efficiencies of the modern society will be gone, and tasks will take much longer than expected. Even if you can communicate by text, the transporting of patients, delivering supplies, meeting with groups, and assessing sites will take far longer than you are used to when none of the stoplights are functional or if gasoline is in limited supply.
Finally, there will be patients for whom no solution, short of an intact, well-resourced medical system, exists—those with severe congenital issues, patients with advanced dementia, patients with advanced cancer, and those with multiple-antibiotic-resistant osteomyelitis are a few of the patients that this response encountered. If transport out of the area is unavailable, NGOs and other charities may be the best, and at times, the only resource for these patients. During this response, I observed NGO and charities helping individual patients and their families with their power, shelter, and medical needs that could not be legally provided by federal government response.
While I hope you may never need to use them, preparations for evacuation, medication, power, and communications before a potential disaster occurs will prove helpful to your patients. After the disaster, consistent and simple communications to the public will be necessary to limit the damage from the social media rumor mill. Working within the organized response framework and leveraging local knowledge and targeted NGO involvement will maximize the effect of your efforts.
In October 2017, in support of the Federal Emergency Management Agency’s response to assist the Governor and people of Puerto Rico, three Department of Defense (DOD) military hospital platforms were deployed; one each, by the US Army, Navy, and Air Force. They arrived on the island at different times with predominantly wartime surgical capabilities and augmented the Federal Emergency Management Agency (FEMA), US Public Health Service, National Guard, and Puerto Rico Department of Health efforts. My perspective is that of patient care and transport between the Centro Medico hospital complex in San Juan, the larger regional hospitals, the Veterans Administration hospital, the DOD response, FEMA Disaster Medical Assistance Teams (DMAT), and FEMA Federal Medical Shelters about 4 to 6 weeks after Hurricanes Maria and Irma struck. Based upon this experience, I would like to offer the following.
Pre-Disaster: All clinicians have a few patients that teeter “on the edge.” When basic services go away, these patients fall over that edge and become inpatients. Establish a list of patients who require oxygen and devices such as vests, cough-assist, or ventilation. If evacuation before the disaster is possible, those patients need to leave. If they refuse, or are unable to leave, they need to be able to supply their own generated power for a prolonged period of time, as batteries will run out prior to power restoration. They must be able to use oxygen concentrators, as tank re-supply may not be readily available. By law, FEMA cannot give generators to individuals, so individuals must prepare for themselves. In a hurricane-prone area where seasonal risk can be established, planning medication refills at the beginning of the season or giving a larger than normal supply may prove useful. In an area prone to sudden disaster, such as earthquake or tornado, then counseling patients to request refills at least 2 weeks early may be adequate.
Post-Disaster: The most reliable form of communication will be text. You likely already have text contacts for your staff and family members; add other providers, responders, planners, pharmacists, and oxygen suppliers to your text contacts. While you may wish to share a text point of contact with patients, understand that your ability to actually help during the initial disaster will likely be limited. Identify possible language translation needs and possible translators among your staff and/or friends as telephone services will be limited or absent following the disaster. Finally, identify your local emergency response planners on Facebook, Twitter, or other social media feeds. This will allow you to direct others to these sites for accurate information after the disaster.
Responder Recommendations: A single social media post can DESTROY your plans and hamper your efforts. Advertise a single contact point and an information resource (eg, bulletin board, webpage) early and often. Publicly and accurately declare the means by which people will access health care and health-care services, such as medications, dialysis, and oxygen. There will be nongovernment organizations (NGOs), friends, and other well-meaning individuals who will try to assist people in need through unconventional channels. Yet, by requesting assistance through nonroutine channels, those efforts tend to delay assistance, cause confusion, and/or squander resources. Continue to direct those requests through the established response channels, ie, the local 911 equivalent.
Plan to use cellular texts to communicate. While satellite telephones are great in concept, in execution, they are difficult to utilize when transmitting complex medical information. If you have an expansive budget, there are now devices available that allow for Iridium satellite-based text communications that require batteries but not intact cellular towers.
Facilities with electricity, water, oxygen, medications, laboratory testing, and CT scanners need to be identified and advertised within the responder community. If FEMA is involved, these resources will be identified and updated on a routine basis. The information will be distributed to their DMAT teams. Those DMAT teams will be distributed throughout the response area. Additionally, if the resources and budgeting are approved, then FEMA will also help re-establish medical transport, as well as Federal Medical Shelters (FMS). The FMS can temporarily house patients who can perform basic activities of daily living but require power, oxygen, or medication administration. For those patients in need of medications without insurance, FEMA may activate medication assistance through the Emergency Prescription Assistance Program. This will allow up to 30 days of medication to be distributed at no cost to the individual through participating pharmacies.
External responders will obviously need to pair with local providers/professionals who can navigate the system and, if necessary, can translate medical terms and care plans. Additionally, external responders will be targets for individuals looking to obtain resources for secondary gain or profit. Establishing a plan or consistently redirecting people to the appropriate resources for those needs may limit the inevitable damage these individuals will cause. Additionally, understand that the efficiencies of the modern society will be gone, and tasks will take much longer than expected. Even if you can communicate by text, the transporting of patients, delivering supplies, meeting with groups, and assessing sites will take far longer than you are used to when none of the stoplights are functional or if gasoline is in limited supply.
Finally, there will be patients for whom no solution, short of an intact, well-resourced medical system, exists—those with severe congenital issues, patients with advanced dementia, patients with advanced cancer, and those with multiple-antibiotic-resistant osteomyelitis are a few of the patients that this response encountered. If transport out of the area is unavailable, NGOs and other charities may be the best, and at times, the only resource for these patients. During this response, I observed NGO and charities helping individual patients and their families with their power, shelter, and medical needs that could not be legally provided by federal government response.
While I hope you may never need to use them, preparations for evacuation, medication, power, and communications before a potential disaster occurs will prove helpful to your patients. After the disaster, consistent and simple communications to the public will be necessary to limit the damage from the social media rumor mill. Working within the organized response framework and leveraging local knowledge and targeted NGO involvement will maximize the effect of your efforts.
In October 2017, in support of the Federal Emergency Management Agency’s response to assist the Governor and people of Puerto Rico, three Department of Defense (DOD) military hospital platforms were deployed; one each, by the US Army, Navy, and Air Force. They arrived on the island at different times with predominantly wartime surgical capabilities and augmented the Federal Emergency Management Agency (FEMA), US Public Health Service, National Guard, and Puerto Rico Department of Health efforts. My perspective is that of patient care and transport between the Centro Medico hospital complex in San Juan, the larger regional hospitals, the Veterans Administration hospital, the DOD response, FEMA Disaster Medical Assistance Teams (DMAT), and FEMA Federal Medical Shelters about 4 to 6 weeks after Hurricanes Maria and Irma struck. Based upon this experience, I would like to offer the following.
Pre-Disaster: All clinicians have a few patients that teeter “on the edge.” When basic services go away, these patients fall over that edge and become inpatients. Establish a list of patients who require oxygen and devices such as vests, cough-assist, or ventilation. If evacuation before the disaster is possible, those patients need to leave. If they refuse, or are unable to leave, they need to be able to supply their own generated power for a prolonged period of time, as batteries will run out prior to power restoration. They must be able to use oxygen concentrators, as tank re-supply may not be readily available. By law, FEMA cannot give generators to individuals, so individuals must prepare for themselves. In a hurricane-prone area where seasonal risk can be established, planning medication refills at the beginning of the season or giving a larger than normal supply may prove useful. In an area prone to sudden disaster, such as earthquake or tornado, then counseling patients to request refills at least 2 weeks early may be adequate.
Post-Disaster: The most reliable form of communication will be text. You likely already have text contacts for your staff and family members; add other providers, responders, planners, pharmacists, and oxygen suppliers to your text contacts. While you may wish to share a text point of contact with patients, understand that your ability to actually help during the initial disaster will likely be limited. Identify possible language translation needs and possible translators among your staff and/or friends as telephone services will be limited or absent following the disaster. Finally, identify your local emergency response planners on Facebook, Twitter, or other social media feeds. This will allow you to direct others to these sites for accurate information after the disaster.
Responder Recommendations: A single social media post can DESTROY your plans and hamper your efforts. Advertise a single contact point and an information resource (eg, bulletin board, webpage) early and often. Publicly and accurately declare the means by which people will access health care and health-care services, such as medications, dialysis, and oxygen. There will be nongovernment organizations (NGOs), friends, and other well-meaning individuals who will try to assist people in need through unconventional channels. Yet, by requesting assistance through nonroutine channels, those efforts tend to delay assistance, cause confusion, and/or squander resources. Continue to direct those requests through the established response channels, ie, the local 911 equivalent.
Plan to use cellular texts to communicate. While satellite telephones are great in concept, in execution, they are difficult to utilize when transmitting complex medical information. If you have an expansive budget, there are now devices available that allow for Iridium satellite-based text communications that require batteries but not intact cellular towers.
Facilities with electricity, water, oxygen, medications, laboratory testing, and CT scanners need to be identified and advertised within the responder community. If FEMA is involved, these resources will be identified and updated on a routine basis. The information will be distributed to their DMAT teams. Those DMAT teams will be distributed throughout the response area. Additionally, if the resources and budgeting are approved, then FEMA will also help re-establish medical transport, as well as Federal Medical Shelters (FMS). The FMS can temporarily house patients who can perform basic activities of daily living but require power, oxygen, or medication administration. For those patients in need of medications without insurance, FEMA may activate medication assistance through the Emergency Prescription Assistance Program. This will allow up to 30 days of medication to be distributed at no cost to the individual through participating pharmacies.
External responders will obviously need to pair with local providers/professionals who can navigate the system and, if necessary, can translate medical terms and care plans. Additionally, external responders will be targets for individuals looking to obtain resources for secondary gain or profit. Establishing a plan or consistently redirecting people to the appropriate resources for those needs may limit the inevitable damage these individuals will cause. Additionally, understand that the efficiencies of the modern society will be gone, and tasks will take much longer than expected. Even if you can communicate by text, the transporting of patients, delivering supplies, meeting with groups, and assessing sites will take far longer than you are used to when none of the stoplights are functional or if gasoline is in limited supply.
Finally, there will be patients for whom no solution, short of an intact, well-resourced medical system, exists—those with severe congenital issues, patients with advanced dementia, patients with advanced cancer, and those with multiple-antibiotic-resistant osteomyelitis are a few of the patients that this response encountered. If transport out of the area is unavailable, NGOs and other charities may be the best, and at times, the only resource for these patients. During this response, I observed NGO and charities helping individual patients and their families with their power, shelter, and medical needs that could not be legally provided by federal government response.
While I hope you may never need to use them, preparations for evacuation, medication, power, and communications before a potential disaster occurs will prove helpful to your patients. After the disaster, consistent and simple communications to the public will be necessary to limit the damage from the social media rumor mill. Working within the organized response framework and leveraging local knowledge and targeted NGO involvement will maximize the effect of your efforts.














