User login
Does Fish Oil During Pregnancy Help Prevent Asthma in Kids?

A 24-year-old G2P1 at 24 weeks’ gestation presents to your clinic for a routine prenatal visit. Her older daughter has asthma, and she wants to know if there is anything she can do to reduce her second child’s risk for it. What do you recommend?
Asthma is the most common chronic disease in children in resource-rich countries such as the United States.2 According to the CDC, 8.4% of children were diagnosed with asthma in 2015.3
Omega-3 fatty acids, found naturally in fish oil, are thought to confer anti-inflammatory properties that offer protection against asthma. Clinical trials have shown that fish oil supplementation in pregnancy results in higher levels of omega-3 fatty acids, along with anti-inflammatory changes, in offspring.4 Previous epidemiologic studies have also found that consumption of omega-3 fatty acids decreases the risk for atopy and asthma in offspring.5,6
A Cochrane review published in 2015, however, concluded that omega-3 supplementation during pregnancy had no benefit on wheeze or asthma in offspring.7 Five RCTs were included in the analysis. The largest trial, by Palmer et al, which included 706 women, showed no benefit for supplementation.8 The second largest, by Olsen et al, which included 533 women, did show a benefit (hazard ratio [HR], 0.37; number needed to treat [NNT], 19.6).9
These results, however, were limited by heterogeneity in the amount of fish oil supplemented and duration of follow-up. For example, the children in the Palmer study were followed only until age 3, which is around the time that asthma can be formally diagnosed—potentially leading to underreporting.8 In addition, the diagnosis of asthma was based on parent report of three episodes of wheezing, use of daily asthma medication, or use of a national registry—all of which can underestimate the incidence of asthma. The reported rate of childhood asthma with IgE-sensitization (rate without sensitization was not reported) was 1.8% in both study groups—much lower than the CDC’s rate of 8.4%, suggesting underdiagnosis.3,8 Due to these biases and other potential confounders, no firm conclusions can be drawn from the Cochrane review.
STUDY SUMMARY
Maternal fish oil supplementation reduces asthma in children
This single-center, double-blind RCT of 736 pregnant women evaluated the effect of 2.4 g/d of n-3 long-chain polyunsaturated fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) or placebo (olive oil), starting at an estimated gestational age of 24 to 26 weeks, on wheeze or asthma incidence in their offspring.1
Eligible women were between 22 and 26 weeks’ pregnant at the time of recruitment. Exclusion criteria included supplementation of 600 IU/d or more of vitamin D, or having any endocrine, cardiac, or renal disorders. The investigators randomized the women in a 1:1 ratio to either fish oil or placebo. Maternal EPA and DHA blood levels were tested at the time of randomization and one week after birth.
The primary outcome was persistent wheeze or asthma (after age 3, persistent wheeze was termed asthma), determined based on daily diary recordings of five episodes of troublesome lung symptoms within the past six months (each lasting for at least three consecutive days); rescue use of inhaled ß2-agonists; and/or relapse after a three-month course of inhaled glucocorticoids. Secondary outcomes included reduced incidence of respiratory tract infections, asthma exacerbations, eczema, and allergic sensitization.
In total, 695 offspring were included in the study, with 95.5% follow-up at three years and 93.1% at five. The children had scheduled pediatric visits at 1 week; at one, three, six, 12, 18, 24, 30, and 36 months; and at 4 and 5 years. They also had acute visits for any pulmonary, allergic, or dermatologic symptoms that arose.
Results. The investigators found that the children of mothers who took fish oil had a lower risk for persistent wheeze or asthma at ages 3 to 5, compared to those who received placebo (16.9% vs 23.7%; HR, 0.69; NNT, 14.7). But this effect was significant only in the children whose mothers had baseline EPA and DHA levels in the lowest third (17.5% vs 34.1%; HR, 0.46; NNT, 5.6). Similarly, fish oil supplementation had a greater benefit in children whose mothers had consumed the least EPA and DHA before the start of the study (18.5% vs 32.4%; HR, 0.55; NNT, 7.2).
As for the secondary outcomes, only a reduction in lower respiratory infections was associated with fish oil supplementation compared with placebo (38.8% vs 45.5%; HR, 0.77; NNT, 14.9). There was no reduction in asthma exacerbations, eczema, or risk for sensitization in the fish oil group.
WHAT’S NEW?
Study adds fuel to the fire
This study strengthens the case for fish oil supplementation during pregnancy to reduce the risk for asthma in offspring, despite the recent Cochrane review that showed no benefit.1,7 The Palmer study used a much lower amount of omega-3s (900 mg/d fish oil vs 2,400 mg/d in the current trial).1,8 Olsen et al supplemented with a greater amount of omega-3s (2,700 mg/d) and did find a benefit.9 The NNT from the Olsen study (19.6) is consistent with that of the current investigation, suggesting that a higher dosage may be necessary to prevent the onset of asthma.
Additionally, this study followed children for a longer period than did the Palmer study, which may have led to more accurate diagnoses of asthma.1,8 Lastly, the diagnosis of asthma in the Palmer study was based on parent survey data and use of daily asthma medicine rather than on daily diary cards, which are often more accurate.
Consider fish consumption. Both this study and the Olsen trial were performed in Denmark.1,9 While Denmark and the United States have had a relatively similar level of fish consumption since the 1990s, women in Denmark may eat a higher proportion of oily fish than women in the United States, given the more common inclusion of mackerel and herring in their diet.10 Thus, the effect of supplementation may be more pronounced in women in the US.
CAVEATS
Ideal dose? Which women to treat?
The FDA currently recommends 8 to 12 oz of fish per week for pregnant women, but there are no guidelines on the ideal amount of fish oil to be consumed.11 The Palmer study, using 900 mg/d of fish oil, did not show a benefit, whereas there did appear to be a benefit in this study (2,400 mg/d) and the Olsen study (2,700 mg/d).1,8,9 Further research is needed to determine the optimal dosage.
The decreased risk for persistent wheeze or asthma was seen only in the children of women whose EPA and DHA blood levels were in the lowest third of the study population. Thus, only women whose blood levels are low to begin with will likely benefit from this intervention. Currently, EPA and DHA levels are not routinely checked, but there may be some benefit to doing so.
One proxy for blood levels is maternal intake of fish at baseline. The investigators found that there was an association between dietary intake of fish and blood levels of EPA and DHA (r, 0.32).1 Therefore, additional screening questions to gauge fish consumption would be useful to identify women most likely to benefit from supplementation.
CHALLENGES TO IMPLEMENTATION
Multiple pills, additional cost
Since omega-3 fatty acids are relatively safe and the NNT in the general population is low, it may be worth supplementing all pregnant women, even without a commercially available blood test for EPA or DHA. Nevertheless, some women may find it challenging to take up to four additional pills per day for 13 or more weeks. Also, there is an associated cost with these supplements, although it is low.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67[2]: 100-102).
1. Bisgaard H, Stokholm J, Chawes BL, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375(26):2530-2539.
2. Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dissemination Committee Report. Allergy. 2004;59(5):469-478.
3. CDC . Asthma. www.cdc.gov/asthma/most_recent_data.htm. Accessed February 1, 2018.
4. Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol Int. 2015;64(1):27-34.
5. Salam MT, Li YF, Langholz B, et al. Maternal fish consumption during pregnancy and risk of early childhood asthma. J Asthma. 2005;42(6):513-518.
6. Calvani M, Alessandri C, Sopo SM, et al. Consumption of fish, butter and margarine during pregnancy and development of allergic sensitizations in the offspring: role of maternal atopy. Pediatr Allergy Immunol. 2006;17(2):94-102.
7. Gunaratne AW, Makrides M, Collins CT. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev. 2015;22(7): CD010085.
8. Palmer D, Sullivan T, Gold M, et al. Randomized controlled trial of fish oil supplementation in pregnancy on childhood allergies. Allergy. 2013;68:1370-1376.
9. Olsen SF, Østerdal ML, Salvig JD, et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am J Clin Nutr. 2008;88(1): 167-175.
10. Helgi Library. Fish consumption per capita by country. www.helgilibrary.com/indicators/fish-consumption-per-capita/. Accessed February 1, 2018.
11. FDA Advice About Eating Fish, From the Environmental Protection Agency and Food and Drug Administration; Revised Fish Advice; Availability. Fed Regist. 2017;82:6571-6574.

A 24-year-old G2P1 at 24 weeks’ gestation presents to your clinic for a routine prenatal visit. Her older daughter has asthma, and she wants to know if there is anything she can do to reduce her second child’s risk for it. What do you recommend?
Asthma is the most common chronic disease in children in resource-rich countries such as the United States.2 According to the CDC, 8.4% of children were diagnosed with asthma in 2015.3
Omega-3 fatty acids, found naturally in fish oil, are thought to confer anti-inflammatory properties that offer protection against asthma. Clinical trials have shown that fish oil supplementation in pregnancy results in higher levels of omega-3 fatty acids, along with anti-inflammatory changes, in offspring.4 Previous epidemiologic studies have also found that consumption of omega-3 fatty acids decreases the risk for atopy and asthma in offspring.5,6
A Cochrane review published in 2015, however, concluded that omega-3 supplementation during pregnancy had no benefit on wheeze or asthma in offspring.7 Five RCTs were included in the analysis. The largest trial, by Palmer et al, which included 706 women, showed no benefit for supplementation.8 The second largest, by Olsen et al, which included 533 women, did show a benefit (hazard ratio [HR], 0.37; number needed to treat [NNT], 19.6).9
These results, however, were limited by heterogeneity in the amount of fish oil supplemented and duration of follow-up. For example, the children in the Palmer study were followed only until age 3, which is around the time that asthma can be formally diagnosed—potentially leading to underreporting.8 In addition, the diagnosis of asthma was based on parent report of three episodes of wheezing, use of daily asthma medication, or use of a national registry—all of which can underestimate the incidence of asthma. The reported rate of childhood asthma with IgE-sensitization (rate without sensitization was not reported) was 1.8% in both study groups—much lower than the CDC’s rate of 8.4%, suggesting underdiagnosis.3,8 Due to these biases and other potential confounders, no firm conclusions can be drawn from the Cochrane review.
STUDY SUMMARY
Maternal fish oil supplementation reduces asthma in children
This single-center, double-blind RCT of 736 pregnant women evaluated the effect of 2.4 g/d of n-3 long-chain polyunsaturated fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) or placebo (olive oil), starting at an estimated gestational age of 24 to 26 weeks, on wheeze or asthma incidence in their offspring.1
Eligible women were between 22 and 26 weeks’ pregnant at the time of recruitment. Exclusion criteria included supplementation of 600 IU/d or more of vitamin D, or having any endocrine, cardiac, or renal disorders. The investigators randomized the women in a 1:1 ratio to either fish oil or placebo. Maternal EPA and DHA blood levels were tested at the time of randomization and one week after birth.
The primary outcome was persistent wheeze or asthma (after age 3, persistent wheeze was termed asthma), determined based on daily diary recordings of five episodes of troublesome lung symptoms within the past six months (each lasting for at least three consecutive days); rescue use of inhaled ß2-agonists; and/or relapse after a three-month course of inhaled glucocorticoids. Secondary outcomes included reduced incidence of respiratory tract infections, asthma exacerbations, eczema, and allergic sensitization.
In total, 695 offspring were included in the study, with 95.5% follow-up at three years and 93.1% at five. The children had scheduled pediatric visits at 1 week; at one, three, six, 12, 18, 24, 30, and 36 months; and at 4 and 5 years. They also had acute visits for any pulmonary, allergic, or dermatologic symptoms that arose.
Results. The investigators found that the children of mothers who took fish oil had a lower risk for persistent wheeze or asthma at ages 3 to 5, compared to those who received placebo (16.9% vs 23.7%; HR, 0.69; NNT, 14.7). But this effect was significant only in the children whose mothers had baseline EPA and DHA levels in the lowest third (17.5% vs 34.1%; HR, 0.46; NNT, 5.6). Similarly, fish oil supplementation had a greater benefit in children whose mothers had consumed the least EPA and DHA before the start of the study (18.5% vs 32.4%; HR, 0.55; NNT, 7.2).
As for the secondary outcomes, only a reduction in lower respiratory infections was associated with fish oil supplementation compared with placebo (38.8% vs 45.5%; HR, 0.77; NNT, 14.9). There was no reduction in asthma exacerbations, eczema, or risk for sensitization in the fish oil group.
WHAT’S NEW?
Study adds fuel to the fire
This study strengthens the case for fish oil supplementation during pregnancy to reduce the risk for asthma in offspring, despite the recent Cochrane review that showed no benefit.1,7 The Palmer study used a much lower amount of omega-3s (900 mg/d fish oil vs 2,400 mg/d in the current trial).1,8 Olsen et al supplemented with a greater amount of omega-3s (2,700 mg/d) and did find a benefit.9 The NNT from the Olsen study (19.6) is consistent with that of the current investigation, suggesting that a higher dosage may be necessary to prevent the onset of asthma.
Additionally, this study followed children for a longer period than did the Palmer study, which may have led to more accurate diagnoses of asthma.1,8 Lastly, the diagnosis of asthma in the Palmer study was based on parent survey data and use of daily asthma medicine rather than on daily diary cards, which are often more accurate.
Consider fish consumption. Both this study and the Olsen trial were performed in Denmark.1,9 While Denmark and the United States have had a relatively similar level of fish consumption since the 1990s, women in Denmark may eat a higher proportion of oily fish than women in the United States, given the more common inclusion of mackerel and herring in their diet.10 Thus, the effect of supplementation may be more pronounced in women in the US.
CAVEATS
Ideal dose? Which women to treat?
The FDA currently recommends 8 to 12 oz of fish per week for pregnant women, but there are no guidelines on the ideal amount of fish oil to be consumed.11 The Palmer study, using 900 mg/d of fish oil, did not show a benefit, whereas there did appear to be a benefit in this study (2,400 mg/d) and the Olsen study (2,700 mg/d).1,8,9 Further research is needed to determine the optimal dosage.
The decreased risk for persistent wheeze or asthma was seen only in the children of women whose EPA and DHA blood levels were in the lowest third of the study population. Thus, only women whose blood levels are low to begin with will likely benefit from this intervention. Currently, EPA and DHA levels are not routinely checked, but there may be some benefit to doing so.
One proxy for blood levels is maternal intake of fish at baseline. The investigators found that there was an association between dietary intake of fish and blood levels of EPA and DHA (r, 0.32).1 Therefore, additional screening questions to gauge fish consumption would be useful to identify women most likely to benefit from supplementation.
CHALLENGES TO IMPLEMENTATION
Multiple pills, additional cost
Since omega-3 fatty acids are relatively safe and the NNT in the general population is low, it may be worth supplementing all pregnant women, even without a commercially available blood test for EPA or DHA. Nevertheless, some women may find it challenging to take up to four additional pills per day for 13 or more weeks. Also, there is an associated cost with these supplements, although it is low.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67[2]: 100-102).

A 24-year-old G2P1 at 24 weeks’ gestation presents to your clinic for a routine prenatal visit. Her older daughter has asthma, and she wants to know if there is anything she can do to reduce her second child’s risk for it. What do you recommend?
Asthma is the most common chronic disease in children in resource-rich countries such as the United States.2 According to the CDC, 8.4% of children were diagnosed with asthma in 2015.3
Omega-3 fatty acids, found naturally in fish oil, are thought to confer anti-inflammatory properties that offer protection against asthma. Clinical trials have shown that fish oil supplementation in pregnancy results in higher levels of omega-3 fatty acids, along with anti-inflammatory changes, in offspring.4 Previous epidemiologic studies have also found that consumption of omega-3 fatty acids decreases the risk for atopy and asthma in offspring.5,6
A Cochrane review published in 2015, however, concluded that omega-3 supplementation during pregnancy had no benefit on wheeze or asthma in offspring.7 Five RCTs were included in the analysis. The largest trial, by Palmer et al, which included 706 women, showed no benefit for supplementation.8 The second largest, by Olsen et al, which included 533 women, did show a benefit (hazard ratio [HR], 0.37; number needed to treat [NNT], 19.6).9
These results, however, were limited by heterogeneity in the amount of fish oil supplemented and duration of follow-up. For example, the children in the Palmer study were followed only until age 3, which is around the time that asthma can be formally diagnosed—potentially leading to underreporting.8 In addition, the diagnosis of asthma was based on parent report of three episodes of wheezing, use of daily asthma medication, or use of a national registry—all of which can underestimate the incidence of asthma. The reported rate of childhood asthma with IgE-sensitization (rate without sensitization was not reported) was 1.8% in both study groups—much lower than the CDC’s rate of 8.4%, suggesting underdiagnosis.3,8 Due to these biases and other potential confounders, no firm conclusions can be drawn from the Cochrane review.
STUDY SUMMARY
Maternal fish oil supplementation reduces asthma in children
This single-center, double-blind RCT of 736 pregnant women evaluated the effect of 2.4 g/d of n-3 long-chain polyunsaturated fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) or placebo (olive oil), starting at an estimated gestational age of 24 to 26 weeks, on wheeze or asthma incidence in their offspring.1
Eligible women were between 22 and 26 weeks’ pregnant at the time of recruitment. Exclusion criteria included supplementation of 600 IU/d or more of vitamin D, or having any endocrine, cardiac, or renal disorders. The investigators randomized the women in a 1:1 ratio to either fish oil or placebo. Maternal EPA and DHA blood levels were tested at the time of randomization and one week after birth.
The primary outcome was persistent wheeze or asthma (after age 3, persistent wheeze was termed asthma), determined based on daily diary recordings of five episodes of troublesome lung symptoms within the past six months (each lasting for at least three consecutive days); rescue use of inhaled ß2-agonists; and/or relapse after a three-month course of inhaled glucocorticoids. Secondary outcomes included reduced incidence of respiratory tract infections, asthma exacerbations, eczema, and allergic sensitization.
In total, 695 offspring were included in the study, with 95.5% follow-up at three years and 93.1% at five. The children had scheduled pediatric visits at 1 week; at one, three, six, 12, 18, 24, 30, and 36 months; and at 4 and 5 years. They also had acute visits for any pulmonary, allergic, or dermatologic symptoms that arose.
Results. The investigators found that the children of mothers who took fish oil had a lower risk for persistent wheeze or asthma at ages 3 to 5, compared to those who received placebo (16.9% vs 23.7%; HR, 0.69; NNT, 14.7). But this effect was significant only in the children whose mothers had baseline EPA and DHA levels in the lowest third (17.5% vs 34.1%; HR, 0.46; NNT, 5.6). Similarly, fish oil supplementation had a greater benefit in children whose mothers had consumed the least EPA and DHA before the start of the study (18.5% vs 32.4%; HR, 0.55; NNT, 7.2).
As for the secondary outcomes, only a reduction in lower respiratory infections was associated with fish oil supplementation compared with placebo (38.8% vs 45.5%; HR, 0.77; NNT, 14.9). There was no reduction in asthma exacerbations, eczema, or risk for sensitization in the fish oil group.
WHAT’S NEW?
Study adds fuel to the fire
This study strengthens the case for fish oil supplementation during pregnancy to reduce the risk for asthma in offspring, despite the recent Cochrane review that showed no benefit.1,7 The Palmer study used a much lower amount of omega-3s (900 mg/d fish oil vs 2,400 mg/d in the current trial).1,8 Olsen et al supplemented with a greater amount of omega-3s (2,700 mg/d) and did find a benefit.9 The NNT from the Olsen study (19.6) is consistent with that of the current investigation, suggesting that a higher dosage may be necessary to prevent the onset of asthma.
Additionally, this study followed children for a longer period than did the Palmer study, which may have led to more accurate diagnoses of asthma.1,8 Lastly, the diagnosis of asthma in the Palmer study was based on parent survey data and use of daily asthma medicine rather than on daily diary cards, which are often more accurate.
Consider fish consumption. Both this study and the Olsen trial were performed in Denmark.1,9 While Denmark and the United States have had a relatively similar level of fish consumption since the 1990s, women in Denmark may eat a higher proportion of oily fish than women in the United States, given the more common inclusion of mackerel and herring in their diet.10 Thus, the effect of supplementation may be more pronounced in women in the US.
CAVEATS
Ideal dose? Which women to treat?
The FDA currently recommends 8 to 12 oz of fish per week for pregnant women, but there are no guidelines on the ideal amount of fish oil to be consumed.11 The Palmer study, using 900 mg/d of fish oil, did not show a benefit, whereas there did appear to be a benefit in this study (2,400 mg/d) and the Olsen study (2,700 mg/d).1,8,9 Further research is needed to determine the optimal dosage.
The decreased risk for persistent wheeze or asthma was seen only in the children of women whose EPA and DHA blood levels were in the lowest third of the study population. Thus, only women whose blood levels are low to begin with will likely benefit from this intervention. Currently, EPA and DHA levels are not routinely checked, but there may be some benefit to doing so.
One proxy for blood levels is maternal intake of fish at baseline. The investigators found that there was an association between dietary intake of fish and blood levels of EPA and DHA (r, 0.32).1 Therefore, additional screening questions to gauge fish consumption would be useful to identify women most likely to benefit from supplementation.
CHALLENGES TO IMPLEMENTATION
Multiple pills, additional cost
Since omega-3 fatty acids are relatively safe and the NNT in the general population is low, it may be worth supplementing all pregnant women, even without a commercially available blood test for EPA or DHA. Nevertheless, some women may find it challenging to take up to four additional pills per day for 13 or more weeks. Also, there is an associated cost with these supplements, although it is low.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67[2]: 100-102).
1. Bisgaard H, Stokholm J, Chawes BL, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375(26):2530-2539.
2. Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dissemination Committee Report. Allergy. 2004;59(5):469-478.
3. CDC . Asthma. www.cdc.gov/asthma/most_recent_data.htm. Accessed February 1, 2018.
4. Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol Int. 2015;64(1):27-34.
5. Salam MT, Li YF, Langholz B, et al. Maternal fish consumption during pregnancy and risk of early childhood asthma. J Asthma. 2005;42(6):513-518.
6. Calvani M, Alessandri C, Sopo SM, et al. Consumption of fish, butter and margarine during pregnancy and development of allergic sensitizations in the offspring: role of maternal atopy. Pediatr Allergy Immunol. 2006;17(2):94-102.
7. Gunaratne AW, Makrides M, Collins CT. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev. 2015;22(7): CD010085.
8. Palmer D, Sullivan T, Gold M, et al. Randomized controlled trial of fish oil supplementation in pregnancy on childhood allergies. Allergy. 2013;68:1370-1376.
9. Olsen SF, Østerdal ML, Salvig JD, et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am J Clin Nutr. 2008;88(1): 167-175.
10. Helgi Library. Fish consumption per capita by country. www.helgilibrary.com/indicators/fish-consumption-per-capita/. Accessed February 1, 2018.
11. FDA Advice About Eating Fish, From the Environmental Protection Agency and Food and Drug Administration; Revised Fish Advice; Availability. Fed Regist. 2017;82:6571-6574.
1. Bisgaard H, Stokholm J, Chawes BL, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375(26):2530-2539.
2. Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dissemination Committee Report. Allergy. 2004;59(5):469-478.
3. CDC . Asthma. www.cdc.gov/asthma/most_recent_data.htm. Accessed February 1, 2018.
4. Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol Int. 2015;64(1):27-34.
5. Salam MT, Li YF, Langholz B, et al. Maternal fish consumption during pregnancy and risk of early childhood asthma. J Asthma. 2005;42(6):513-518.
6. Calvani M, Alessandri C, Sopo SM, et al. Consumption of fish, butter and margarine during pregnancy and development of allergic sensitizations in the offspring: role of maternal atopy. Pediatr Allergy Immunol. 2006;17(2):94-102.
7. Gunaratne AW, Makrides M, Collins CT. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev. 2015;22(7): CD010085.
8. Palmer D, Sullivan T, Gold M, et al. Randomized controlled trial of fish oil supplementation in pregnancy on childhood allergies. Allergy. 2013;68:1370-1376.
9. Olsen SF, Østerdal ML, Salvig JD, et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am J Clin Nutr. 2008;88(1): 167-175.
10. Helgi Library. Fish consumption per capita by country. www.helgilibrary.com/indicators/fish-consumption-per-capita/. Accessed February 1, 2018.
11. FDA Advice About Eating Fish, From the Environmental Protection Agency and Food and Drug Administration; Revised Fish Advice; Availability. Fed Regist. 2017;82:6571-6574.
An easy approach to obtaining clean-catch urine from infants
ILLUSTRATIVE CASE
A fussy 6-month-old infant is brought into the emergency department (ED) with a rectal temperature of 101.5° F. She is consolable, breathing normally, and appears well hydrated. You find no clear etiology for her fever and suspect that a urinary tract infection (UTI) may be the source of her illness. How do you proceed with obtaining a urine sample?
A febrile infant in the family physician’s office or ED is a familiar clinical situation that may require an invasive diagnostic work-up. Up to 7% of infants ages 2 to 24 months with fever of unknown origin may have a UTI.2 Collecting a urine sample from pre-toilet-trained children can be time consuming. In fact, obtaining a clean-catch urine sample in this age group took an average of more than one hour in one randomized controlled trial (RCT).3 More convenient methods of urine collection, such as placing a cotton ball in the diaper or using a perineal collection bag, have contamination rates of up to 63%.4
The American Academy of Pediatrics (AAP) guidelines for evaluating possible UTI in a febrile child <2 years of age recommend obtaining a sample for urinalysis “through the most convenient means.”5 If urinalysis is positive, only urine obtained by catheterization or suprapubic aspiration should be cultured. Guidelines from the National Institute for Health and Care Excellence in the United Kingdom are similar, but allow for culture of clean-catch urine samples.6
A recent prospective cohort study examined a noninvasive alternating lumbar-bladder tapping method to stimulate voiding in infants ages 0 to 6 months.7 Within 5 minutes, 49% of the infants provided a clean-catch sample, with contamination rates similar to those of samples obtained using invasive methods.7 Younger infants were more likely to void within the time allotted. Another trial of bladder tapping conducted in hospitalized infants <30 days old showed similar results.8
There are, however, no previously reported randomized trials demonstrating the efficacy of a noninvasive urine collection technique in the outpatient setting.
Use of invasive collection methods requires skilled personnel and may cause significant discomfort for patients (and parents). Noninvasive methods, such as bag urine collection, have unacceptable contamination rates. In addition, waiting to catch a potentially cleaner urine sample is time-consuming, so better strategies to collect urine from infants are needed. This RCT is the first to examine the efficacy of a unique stimulation technique to obtain a clean-catch urine sample from infants ages 1 to 12 months.
STUDY SUMMARY
Noninvasive stimulation method triggers faster clean urine samples
A nonblinded, single-center RCT conducted in Australia compared 2 methods for obtaining a clean-catch urine sample within 5 minutes: the Quick-Wee method (suprapubic stimulation with gauze soaked in cold fluid) or usual care (waiting for spontaneous voiding with no stimulation).1 Three hundred fifty-four infants (ages 1-12 months) who required urine sample collection were randomized in a 1:1 ratio; allocation was concealed. Infants with anatomic or neurologic abnormalities and those needing immediate antibiotic therapy were excluded.
The most common reasons for obtaining the urine sample were fever of unknown origin and “unsettled baby,” followed by poor feeding and suspected UTI. The primary outcome was voiding within 5 minutes; secondary outcomes included time to void, whether urine was successfully caught, contamination rate, and parent/clinician satisfaction.
Study personnel removed the diaper, then cleaned the genitals of all patients with room temperature sterile water. A caregiver or clinician was ready and waiting to catch urine when the patient voided. In the Quick-Wee group, a clinician rubbed the patient’s suprapubic area in a circular fashion with gauze soaked in refrigerated saline (2.8° C). At 5 minutes, clinicians recorded the voiding status and decided how to proceed.
Using intention-to-treat analysis, 31% of the patients in the Quick-Wee group voided within 5 minutes, compared with 12% of the usual-care patients. Similarly, 30% of patients in the Quick-Wee group provided a successful clean-catch sample within 5 minutes compared with 9% in the usual-care group (P<.001; number needed to treat=4.7; 95% CI, 3.4-7.7). Contamination rates were no different between the Quick-Wee and usual-care samples. Both parents and clinicians were more satisfied with the Quick-Wee method than with usual care (median score of 2 vs 3 on a 5-point Likert scale, in which 1 is most satisfied; P<.001). There was no difference when results were adjusted for age or sex. No adverse events occurred.
WHAT’S NEW
New method could reduce the need for invasive sampling
A simple suprapubic stimulation technique increased the number of infants who provided a clean-catch voided urine sample within 5 minutes—a clinically relevant and satisfying outcome. In appropriate patients, use of the Quick-Wee method to obtain a clean-catch voided sample for initial urinalysis, rather than attempting methods with known high contamination rates, may potentially reduce the need for invasive sampling using catheterization or suprapubic aspiration.
CAVEATS
Complete age range and ideal storage temperature are unknown
Neonates and pre-continent children older than 12 months were not included in this trial, so these conclusions do not apply to those groups of patients. The intervention period lasted only 5 minutes, but other published studies suggest that this amount of time is adequate for voiding to occur.6,7 Although this study used soaking fluid stored at 2.8° C, the ideal storage temperature is unknown.
CHALLENGES TO IMPLEMENTATION
AAP doesn’t endorse clean-catch urine samples for culture
The Quick-Wee method is simple and easy to implement, and requires no specialized training or equipment. AAP guidelines do not endorse the use of clean-catch voided urine for culture, which may be a barrier to changing urine collection practices in some settings.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Kaufman J, Fitzpatrick P, Tosif S, et al. Faster clean catch urine collection (Quick-Wee method) from infants: randomised controlled trial. BMJ. 2017;357:j1341.
2. Shaikh N, Morone NE, Bost JE, et al. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J. 2008;27:302-308.
3. Davies P, Greenwood R, Benger J. Randomised trial of a vibrating bladder stimulator—the time to pee study. Arch Dis Child. 2008;93:423-424.
4. Al-Orifi F, McGillivray D, Tange S, et al. Urine culture from bag specimens in young children: are the risks too high? J Pediatr. 2000;137:221-226.
5. Reaffirmation of AAP clinical practice guideline: the diagnosis and management of the initial urinary tract infection in febrile infants and young children 2-24 months of age. Pediatrics. 2016;138:e20163026.
6. National Institute for Health and Care Excellence. Urinary tract infection in under 16s: diagnosis and management. Clinical guideline CG54. Published August 2007. Available at: https://www.nice.org.uk/guidance/cg54/chapter/1-guidance. Accessed May 30, 2017.
7. Labrosse M, Levy A, Autmizguine J, et al. Evaluation of a new strategy for clean-catch urine in infants. Pediatrics. 2016;138:e20160573.
8. Herreros Fernández ML, González Merino N, Tagarro García A, et al. A new technique for fast and safe collection of urine in newborns. Arch Dis Child. 2013;98:27-29.
ILLUSTRATIVE CASE
A fussy 6-month-old infant is brought into the emergency department (ED) with a rectal temperature of 101.5° F. She is consolable, breathing normally, and appears well hydrated. You find no clear etiology for her fever and suspect that a urinary tract infection (UTI) may be the source of her illness. How do you proceed with obtaining a urine sample?
A febrile infant in the family physician’s office or ED is a familiar clinical situation that may require an invasive diagnostic work-up. Up to 7% of infants ages 2 to 24 months with fever of unknown origin may have a UTI.2 Collecting a urine sample from pre-toilet-trained children can be time consuming. In fact, obtaining a clean-catch urine sample in this age group took an average of more than one hour in one randomized controlled trial (RCT).3 More convenient methods of urine collection, such as placing a cotton ball in the diaper or using a perineal collection bag, have contamination rates of up to 63%.4
The American Academy of Pediatrics (AAP) guidelines for evaluating possible UTI in a febrile child <2 years of age recommend obtaining a sample for urinalysis “through the most convenient means.”5 If urinalysis is positive, only urine obtained by catheterization or suprapubic aspiration should be cultured. Guidelines from the National Institute for Health and Care Excellence in the United Kingdom are similar, but allow for culture of clean-catch urine samples.6
A recent prospective cohort study examined a noninvasive alternating lumbar-bladder tapping method to stimulate voiding in infants ages 0 to 6 months.7 Within 5 minutes, 49% of the infants provided a clean-catch sample, with contamination rates similar to those of samples obtained using invasive methods.7 Younger infants were more likely to void within the time allotted. Another trial of bladder tapping conducted in hospitalized infants <30 days old showed similar results.8
There are, however, no previously reported randomized trials demonstrating the efficacy of a noninvasive urine collection technique in the outpatient setting.
Use of invasive collection methods requires skilled personnel and may cause significant discomfort for patients (and parents). Noninvasive methods, such as bag urine collection, have unacceptable contamination rates. In addition, waiting to catch a potentially cleaner urine sample is time-consuming, so better strategies to collect urine from infants are needed. This RCT is the first to examine the efficacy of a unique stimulation technique to obtain a clean-catch urine sample from infants ages 1 to 12 months.
STUDY SUMMARY
Noninvasive stimulation method triggers faster clean urine samples
A nonblinded, single-center RCT conducted in Australia compared 2 methods for obtaining a clean-catch urine sample within 5 minutes: the Quick-Wee method (suprapubic stimulation with gauze soaked in cold fluid) or usual care (waiting for spontaneous voiding with no stimulation).1 Three hundred fifty-four infants (ages 1-12 months) who required urine sample collection were randomized in a 1:1 ratio; allocation was concealed. Infants with anatomic or neurologic abnormalities and those needing immediate antibiotic therapy were excluded.
The most common reasons for obtaining the urine sample were fever of unknown origin and “unsettled baby,” followed by poor feeding and suspected UTI. The primary outcome was voiding within 5 minutes; secondary outcomes included time to void, whether urine was successfully caught, contamination rate, and parent/clinician satisfaction.
Study personnel removed the diaper, then cleaned the genitals of all patients with room temperature sterile water. A caregiver or clinician was ready and waiting to catch urine when the patient voided. In the Quick-Wee group, a clinician rubbed the patient’s suprapubic area in a circular fashion with gauze soaked in refrigerated saline (2.8° C). At 5 minutes, clinicians recorded the voiding status and decided how to proceed.
Using intention-to-treat analysis, 31% of the patients in the Quick-Wee group voided within 5 minutes, compared with 12% of the usual-care patients. Similarly, 30% of patients in the Quick-Wee group provided a successful clean-catch sample within 5 minutes compared with 9% in the usual-care group (P<.001; number needed to treat=4.7; 95% CI, 3.4-7.7). Contamination rates were no different between the Quick-Wee and usual-care samples. Both parents and clinicians were more satisfied with the Quick-Wee method than with usual care (median score of 2 vs 3 on a 5-point Likert scale, in which 1 is most satisfied; P<.001). There was no difference when results were adjusted for age or sex. No adverse events occurred.
WHAT’S NEW
New method could reduce the need for invasive sampling
A simple suprapubic stimulation technique increased the number of infants who provided a clean-catch voided urine sample within 5 minutes—a clinically relevant and satisfying outcome. In appropriate patients, use of the Quick-Wee method to obtain a clean-catch voided sample for initial urinalysis, rather than attempting methods with known high contamination rates, may potentially reduce the need for invasive sampling using catheterization or suprapubic aspiration.
CAVEATS
Complete age range and ideal storage temperature are unknown
Neonates and pre-continent children older than 12 months were not included in this trial, so these conclusions do not apply to those groups of patients. The intervention period lasted only 5 minutes, but other published studies suggest that this amount of time is adequate for voiding to occur.6,7 Although this study used soaking fluid stored at 2.8° C, the ideal storage temperature is unknown.
CHALLENGES TO IMPLEMENTATION
AAP doesn’t endorse clean-catch urine samples for culture
The Quick-Wee method is simple and easy to implement, and requires no specialized training or equipment. AAP guidelines do not endorse the use of clean-catch voided urine for culture, which may be a barrier to changing urine collection practices in some settings.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A fussy 6-month-old infant is brought into the emergency department (ED) with a rectal temperature of 101.5° F. She is consolable, breathing normally, and appears well hydrated. You find no clear etiology for her fever and suspect that a urinary tract infection (UTI) may be the source of her illness. How do you proceed with obtaining a urine sample?
A febrile infant in the family physician’s office or ED is a familiar clinical situation that may require an invasive diagnostic work-up. Up to 7% of infants ages 2 to 24 months with fever of unknown origin may have a UTI.2 Collecting a urine sample from pre-toilet-trained children can be time consuming. In fact, obtaining a clean-catch urine sample in this age group took an average of more than one hour in one randomized controlled trial (RCT).3 More convenient methods of urine collection, such as placing a cotton ball in the diaper or using a perineal collection bag, have contamination rates of up to 63%.4
The American Academy of Pediatrics (AAP) guidelines for evaluating possible UTI in a febrile child <2 years of age recommend obtaining a sample for urinalysis “through the most convenient means.”5 If urinalysis is positive, only urine obtained by catheterization or suprapubic aspiration should be cultured. Guidelines from the National Institute for Health and Care Excellence in the United Kingdom are similar, but allow for culture of clean-catch urine samples.6
A recent prospective cohort study examined a noninvasive alternating lumbar-bladder tapping method to stimulate voiding in infants ages 0 to 6 months.7 Within 5 minutes, 49% of the infants provided a clean-catch sample, with contamination rates similar to those of samples obtained using invasive methods.7 Younger infants were more likely to void within the time allotted. Another trial of bladder tapping conducted in hospitalized infants <30 days old showed similar results.8
There are, however, no previously reported randomized trials demonstrating the efficacy of a noninvasive urine collection technique in the outpatient setting.
Use of invasive collection methods requires skilled personnel and may cause significant discomfort for patients (and parents). Noninvasive methods, such as bag urine collection, have unacceptable contamination rates. In addition, waiting to catch a potentially cleaner urine sample is time-consuming, so better strategies to collect urine from infants are needed. This RCT is the first to examine the efficacy of a unique stimulation technique to obtain a clean-catch urine sample from infants ages 1 to 12 months.
STUDY SUMMARY
Noninvasive stimulation method triggers faster clean urine samples
A nonblinded, single-center RCT conducted in Australia compared 2 methods for obtaining a clean-catch urine sample within 5 minutes: the Quick-Wee method (suprapubic stimulation with gauze soaked in cold fluid) or usual care (waiting for spontaneous voiding with no stimulation).1 Three hundred fifty-four infants (ages 1-12 months) who required urine sample collection were randomized in a 1:1 ratio; allocation was concealed. Infants with anatomic or neurologic abnormalities and those needing immediate antibiotic therapy were excluded.
The most common reasons for obtaining the urine sample were fever of unknown origin and “unsettled baby,” followed by poor feeding and suspected UTI. The primary outcome was voiding within 5 minutes; secondary outcomes included time to void, whether urine was successfully caught, contamination rate, and parent/clinician satisfaction.
Study personnel removed the diaper, then cleaned the genitals of all patients with room temperature sterile water. A caregiver or clinician was ready and waiting to catch urine when the patient voided. In the Quick-Wee group, a clinician rubbed the patient’s suprapubic area in a circular fashion with gauze soaked in refrigerated saline (2.8° C). At 5 minutes, clinicians recorded the voiding status and decided how to proceed.
Using intention-to-treat analysis, 31% of the patients in the Quick-Wee group voided within 5 minutes, compared with 12% of the usual-care patients. Similarly, 30% of patients in the Quick-Wee group provided a successful clean-catch sample within 5 minutes compared with 9% in the usual-care group (P<.001; number needed to treat=4.7; 95% CI, 3.4-7.7). Contamination rates were no different between the Quick-Wee and usual-care samples. Both parents and clinicians were more satisfied with the Quick-Wee method than with usual care (median score of 2 vs 3 on a 5-point Likert scale, in which 1 is most satisfied; P<.001). There was no difference when results were adjusted for age or sex. No adverse events occurred.
WHAT’S NEW
New method could reduce the need for invasive sampling
A simple suprapubic stimulation technique increased the number of infants who provided a clean-catch voided urine sample within 5 minutes—a clinically relevant and satisfying outcome. In appropriate patients, use of the Quick-Wee method to obtain a clean-catch voided sample for initial urinalysis, rather than attempting methods with known high contamination rates, may potentially reduce the need for invasive sampling using catheterization or suprapubic aspiration.
CAVEATS
Complete age range and ideal storage temperature are unknown
Neonates and pre-continent children older than 12 months were not included in this trial, so these conclusions do not apply to those groups of patients. The intervention period lasted only 5 minutes, but other published studies suggest that this amount of time is adequate for voiding to occur.6,7 Although this study used soaking fluid stored at 2.8° C, the ideal storage temperature is unknown.
CHALLENGES TO IMPLEMENTATION
AAP doesn’t endorse clean-catch urine samples for culture
The Quick-Wee method is simple and easy to implement, and requires no specialized training or equipment. AAP guidelines do not endorse the use of clean-catch voided urine for culture, which may be a barrier to changing urine collection practices in some settings.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Kaufman J, Fitzpatrick P, Tosif S, et al. Faster clean catch urine collection (Quick-Wee method) from infants: randomised controlled trial. BMJ. 2017;357:j1341.
2. Shaikh N, Morone NE, Bost JE, et al. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J. 2008;27:302-308.
3. Davies P, Greenwood R, Benger J. Randomised trial of a vibrating bladder stimulator—the time to pee study. Arch Dis Child. 2008;93:423-424.
4. Al-Orifi F, McGillivray D, Tange S, et al. Urine culture from bag specimens in young children: are the risks too high? J Pediatr. 2000;137:221-226.
5. Reaffirmation of AAP clinical practice guideline: the diagnosis and management of the initial urinary tract infection in febrile infants and young children 2-24 months of age. Pediatrics. 2016;138:e20163026.
6. National Institute for Health and Care Excellence. Urinary tract infection in under 16s: diagnosis and management. Clinical guideline CG54. Published August 2007. Available at: https://www.nice.org.uk/guidance/cg54/chapter/1-guidance. Accessed May 30, 2017.
7. Labrosse M, Levy A, Autmizguine J, et al. Evaluation of a new strategy for clean-catch urine in infants. Pediatrics. 2016;138:e20160573.
8. Herreros Fernández ML, González Merino N, Tagarro García A, et al. A new technique for fast and safe collection of urine in newborns. Arch Dis Child. 2013;98:27-29.
1. Kaufman J, Fitzpatrick P, Tosif S, et al. Faster clean catch urine collection (Quick-Wee method) from infants: randomised controlled trial. BMJ. 2017;357:j1341.
2. Shaikh N, Morone NE, Bost JE, et al. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J. 2008;27:302-308.
3. Davies P, Greenwood R, Benger J. Randomised trial of a vibrating bladder stimulator—the time to pee study. Arch Dis Child. 2008;93:423-424.
4. Al-Orifi F, McGillivray D, Tange S, et al. Urine culture from bag specimens in young children: are the risks too high? J Pediatr. 2000;137:221-226.
5. Reaffirmation of AAP clinical practice guideline: the diagnosis and management of the initial urinary tract infection in febrile infants and young children 2-24 months of age. Pediatrics. 2016;138:e20163026.
6. National Institute for Health and Care Excellence. Urinary tract infection in under 16s: diagnosis and management. Clinical guideline CG54. Published August 2007. Available at: https://www.nice.org.uk/guidance/cg54/chapter/1-guidance. Accessed May 30, 2017.
7. Labrosse M, Levy A, Autmizguine J, et al. Evaluation of a new strategy for clean-catch urine in infants. Pediatrics. 2016;138:e20160573.
8. Herreros Fernández ML, González Merino N, Tagarro García A, et al. A new technique for fast and safe collection of urine in newborns. Arch Dis Child. 2013;98:27-29.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Apply gauze soaked in cold sterile saline to the suprapubic area to stimulate infants ages 1 to 12 months to provide a clean-catch urine sample. Doing so produces significantly more clean-catch urine samples within 5 minutes than simply waiting for the patient to void, with no difference in contamination and with increased parental and provider satisfaction.1
STRENGTH OF RECOMMENDATION
B: Based on a single good-quality, randomized controlled trial.
Kaufman J, Fitzpatrick P, Tosif S, et al. Faster clean catch urine collection (Quick-Wee method) from infants: randomised controlled trial. BMJ. 2017;357:j1341.
Tamsulosin for Patients With Ureteral Stones?
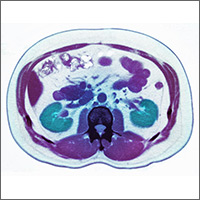
A 54-year-old man presents to the emergency department (ED) with acute-onset left flank pain that radiates to the groin. CT of the abdomen/pelvis without contrast reveals a 7-mm distal ureteral stone. He is deemed an appropriate candidate for outpatient management. In addition to pain medications, should you prescribe tamsulosin?
According to the most recent National Health and Nutrition Examination Survey, the population prevalence of kidney stones is 8.8%, with a self-reported prevalence of 10.6% in men and 7.1% in women.2 Most ureteral stones can be treated in the outpatient setting with oral hydration, antiemetics, and pain control with NSAIDs as firstline treatment and opioids as a second-line option.3
In addition, α-blockers are used for medical expulsive therapy (MET). In fact, the European Association of Urology guideline on urolithiasis states that MET may accelerate passage of ureteral stones.3
Recently, however, uncertainty has surrounded the effectiveness of the α-blocker tamsulosin. Two systematic reviews (limited by heterogeneity because some of the studies lacked a placebo control and blinding) concluded that α-blockers increased stone passage within one to six weeks when compared with placebo or no additional therapy.4,5 However, a recent large, multicenter RCT revealed no difference between tamsulosin and nifedipine, or either one compared with placebo, at decreasing the need for further treatment to achieve stone passage within four weeks.6
STUDY SUMMARY
Results broken down by stone size
This meta-analysis, comprising eight double-blind RCTs, examined the effect of oral tamsulosin (0.4 mg/d; average course, 28 d) on distal ureteral stone passage in adult patients (N = 1,384).1 A subgroup analysis comparing stone size (< 5 mm and 5-10 mm) was also conducted to determine whether size modified the effect of tamsulosin.
The eight selected studies were published between 2009 and 2015; the trials were conducted in multiple countries, in ED and outpatient urology settings. The main outcome measure was the risk difference (RD) in stone passage between the tamsulosin group and placebo group after follow-up imaging at three weeks with CT or plain film radiographs.
Tamsulosin helps some, but not all. The pooled risk for stone passage was higher in the tamsulosin group than in the placebo group (85% vs 66%; RD, 17%), but significant heterogeneity existed across the trials (I2, 80.2%). Subgroup analysis by stone size (< 5 mm vs 5-10 mm) revealed that, compared to placebo, tamsulosin was beneficial for larger stones (6 trials, N = 514; RD, 22%; number needed to treat, 5) but not for smaller stones (4 trials, N = 533; RD, –0.3%). The 5-to-10–mm subgroup had a less heterogeneous population of studies than did the < 5-mm subgroup (I2, 33% and 0% respectively).
In terms of adverse events, tamsulosin did not increase the risk for dizziness (RD, 0.2%) or postural hypotension (RD, 0.1%), compared with placebo.
WHAT’S NEW
Increased passage of larger stones
This meta-analysis included only double-blind RCTs; prior meta-analyses did not. Also, this review included the SUSPEND (Spontaneous Urinary Stone Passage Enabled by Drugs) trial, an RCT discussed in a previous PURL (Clinician Reviews. 2016;26[4]:20,44), which recommended against the use of α-blockers tamsulosin and nifedipine for ureteral stones measuring < 10 mm.6,7
But the subgroup analysis in this review went one step further by examining passage rates by stone size (< 5 mm vs 5-10 mm) and revealing that passage of larger stones increased with tamsulosin use. The different results based on stone size may explain the recent uncertainty as to whether tamsulosin improves the rate of stone passage.
CAVEATS
What about proximal or XL stones?
Only distal stones were included in seven of the eight trials in this analysis. Thus, this meta-analysis was unable to determine the effect on more proximal stones. Also, it’s unclear if the drug provides any benefit with stones > 10 mm in size.
CHALLENGES TO IMPLEMENTATION
None worth mentioning
We see no challenges to implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[1]:37-38).
1. Wang RC, Smith-Bindman R, Whitaker E, et al. Effect of tamsulosin on stone passage for ureteral stones: a systematic review and meta-analysis. Ann Emerg Med. 2017;69(3):353-361.
2. Scales CD Jr, Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62(1): 160-165.
3. Türk C, Petrik A, Sarica K, et al. EAU guidelines on diagnosis and conservative management of urolithiasis. Eur Urol. 2016;69(3):468-474.
4. Hollingsworth JM, Canales BK, Rogers MAM, et al. Alpha blockers for treatment of ureteric stones: systematic review and meta-analysis. BMJ. 2016;355:i6112.
5. Campschroer T, Zhu Y, Duijvesz D, et al. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014:CD008509.
6. Pickard R, Starr K, MacLennan G, et al. Medical expulsion therapy in adults with ureteric colic: a multicentre, randomized, placebo-controlled trial. Lancet. 2015; 386(9991):341-349.
7. Slattengren AH, Prasad S, Jarrett JB. Kidney stones? It’s time to rethink those meds. J Fam Pract. 2016;65(2): 118-120.

A 54-year-old man presents to the emergency department (ED) with acute-onset left flank pain that radiates to the groin. CT of the abdomen/pelvis without contrast reveals a 7-mm distal ureteral stone. He is deemed an appropriate candidate for outpatient management. In addition to pain medications, should you prescribe tamsulosin?
According to the most recent National Health and Nutrition Examination Survey, the population prevalence of kidney stones is 8.8%, with a self-reported prevalence of 10.6% in men and 7.1% in women.2 Most ureteral stones can be treated in the outpatient setting with oral hydration, antiemetics, and pain control with NSAIDs as firstline treatment and opioids as a second-line option.3
In addition, α-blockers are used for medical expulsive therapy (MET). In fact, the European Association of Urology guideline on urolithiasis states that MET may accelerate passage of ureteral stones.3
Recently, however, uncertainty has surrounded the effectiveness of the α-blocker tamsulosin. Two systematic reviews (limited by heterogeneity because some of the studies lacked a placebo control and blinding) concluded that α-blockers increased stone passage within one to six weeks when compared with placebo or no additional therapy.4,5 However, a recent large, multicenter RCT revealed no difference between tamsulosin and nifedipine, or either one compared with placebo, at decreasing the need for further treatment to achieve stone passage within four weeks.6
STUDY SUMMARY
Results broken down by stone size
This meta-analysis, comprising eight double-blind RCTs, examined the effect of oral tamsulosin (0.4 mg/d; average course, 28 d) on distal ureteral stone passage in adult patients (N = 1,384).1 A subgroup analysis comparing stone size (< 5 mm and 5-10 mm) was also conducted to determine whether size modified the effect of tamsulosin.
The eight selected studies were published between 2009 and 2015; the trials were conducted in multiple countries, in ED and outpatient urology settings. The main outcome measure was the risk difference (RD) in stone passage between the tamsulosin group and placebo group after follow-up imaging at three weeks with CT or plain film radiographs.
Tamsulosin helps some, but not all. The pooled risk for stone passage was higher in the tamsulosin group than in the placebo group (85% vs 66%; RD, 17%), but significant heterogeneity existed across the trials (I2, 80.2%). Subgroup analysis by stone size (< 5 mm vs 5-10 mm) revealed that, compared to placebo, tamsulosin was beneficial for larger stones (6 trials, N = 514; RD, 22%; number needed to treat, 5) but not for smaller stones (4 trials, N = 533; RD, –0.3%). The 5-to-10–mm subgroup had a less heterogeneous population of studies than did the < 5-mm subgroup (I2, 33% and 0% respectively).
In terms of adverse events, tamsulosin did not increase the risk for dizziness (RD, 0.2%) or postural hypotension (RD, 0.1%), compared with placebo.
WHAT’S NEW
Increased passage of larger stones
This meta-analysis included only double-blind RCTs; prior meta-analyses did not. Also, this review included the SUSPEND (Spontaneous Urinary Stone Passage Enabled by Drugs) trial, an RCT discussed in a previous PURL (Clinician Reviews. 2016;26[4]:20,44), which recommended against the use of α-blockers tamsulosin and nifedipine for ureteral stones measuring < 10 mm.6,7
But the subgroup analysis in this review went one step further by examining passage rates by stone size (< 5 mm vs 5-10 mm) and revealing that passage of larger stones increased with tamsulosin use. The different results based on stone size may explain the recent uncertainty as to whether tamsulosin improves the rate of stone passage.
CAVEATS
What about proximal or XL stones?
Only distal stones were included in seven of the eight trials in this analysis. Thus, this meta-analysis was unable to determine the effect on more proximal stones. Also, it’s unclear if the drug provides any benefit with stones > 10 mm in size.
CHALLENGES TO IMPLEMENTATION
None worth mentioning
We see no challenges to implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[1]:37-38).

A 54-year-old man presents to the emergency department (ED) with acute-onset left flank pain that radiates to the groin. CT of the abdomen/pelvis without contrast reveals a 7-mm distal ureteral stone. He is deemed an appropriate candidate for outpatient management. In addition to pain medications, should you prescribe tamsulosin?
According to the most recent National Health and Nutrition Examination Survey, the population prevalence of kidney stones is 8.8%, with a self-reported prevalence of 10.6% in men and 7.1% in women.2 Most ureteral stones can be treated in the outpatient setting with oral hydration, antiemetics, and pain control with NSAIDs as firstline treatment and opioids as a second-line option.3
In addition, α-blockers are used for medical expulsive therapy (MET). In fact, the European Association of Urology guideline on urolithiasis states that MET may accelerate passage of ureteral stones.3
Recently, however, uncertainty has surrounded the effectiveness of the α-blocker tamsulosin. Two systematic reviews (limited by heterogeneity because some of the studies lacked a placebo control and blinding) concluded that α-blockers increased stone passage within one to six weeks when compared with placebo or no additional therapy.4,5 However, a recent large, multicenter RCT revealed no difference between tamsulosin and nifedipine, or either one compared with placebo, at decreasing the need for further treatment to achieve stone passage within four weeks.6
STUDY SUMMARY
Results broken down by stone size
This meta-analysis, comprising eight double-blind RCTs, examined the effect of oral tamsulosin (0.4 mg/d; average course, 28 d) on distal ureteral stone passage in adult patients (N = 1,384).1 A subgroup analysis comparing stone size (< 5 mm and 5-10 mm) was also conducted to determine whether size modified the effect of tamsulosin.
The eight selected studies were published between 2009 and 2015; the trials were conducted in multiple countries, in ED and outpatient urology settings. The main outcome measure was the risk difference (RD) in stone passage between the tamsulosin group and placebo group after follow-up imaging at three weeks with CT or plain film radiographs.
Tamsulosin helps some, but not all. The pooled risk for stone passage was higher in the tamsulosin group than in the placebo group (85% vs 66%; RD, 17%), but significant heterogeneity existed across the trials (I2, 80.2%). Subgroup analysis by stone size (< 5 mm vs 5-10 mm) revealed that, compared to placebo, tamsulosin was beneficial for larger stones (6 trials, N = 514; RD, 22%; number needed to treat, 5) but not for smaller stones (4 trials, N = 533; RD, –0.3%). The 5-to-10–mm subgroup had a less heterogeneous population of studies than did the < 5-mm subgroup (I2, 33% and 0% respectively).
In terms of adverse events, tamsulosin did not increase the risk for dizziness (RD, 0.2%) or postural hypotension (RD, 0.1%), compared with placebo.
WHAT’S NEW
Increased passage of larger stones
This meta-analysis included only double-blind RCTs; prior meta-analyses did not. Also, this review included the SUSPEND (Spontaneous Urinary Stone Passage Enabled by Drugs) trial, an RCT discussed in a previous PURL (Clinician Reviews. 2016;26[4]:20,44), which recommended against the use of α-blockers tamsulosin and nifedipine for ureteral stones measuring < 10 mm.6,7
But the subgroup analysis in this review went one step further by examining passage rates by stone size (< 5 mm vs 5-10 mm) and revealing that passage of larger stones increased with tamsulosin use. The different results based on stone size may explain the recent uncertainty as to whether tamsulosin improves the rate of stone passage.
CAVEATS
What about proximal or XL stones?
Only distal stones were included in seven of the eight trials in this analysis. Thus, this meta-analysis was unable to determine the effect on more proximal stones. Also, it’s unclear if the drug provides any benefit with stones > 10 mm in size.
CHALLENGES TO IMPLEMENTATION
None worth mentioning
We see no challenges to implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018; 67[1]:37-38).
1. Wang RC, Smith-Bindman R, Whitaker E, et al. Effect of tamsulosin on stone passage for ureteral stones: a systematic review and meta-analysis. Ann Emerg Med. 2017;69(3):353-361.
2. Scales CD Jr, Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62(1): 160-165.
3. Türk C, Petrik A, Sarica K, et al. EAU guidelines on diagnosis and conservative management of urolithiasis. Eur Urol. 2016;69(3):468-474.
4. Hollingsworth JM, Canales BK, Rogers MAM, et al. Alpha blockers for treatment of ureteric stones: systematic review and meta-analysis. BMJ. 2016;355:i6112.
5. Campschroer T, Zhu Y, Duijvesz D, et al. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014:CD008509.
6. Pickard R, Starr K, MacLennan G, et al. Medical expulsion therapy in adults with ureteric colic: a multicentre, randomized, placebo-controlled trial. Lancet. 2015; 386(9991):341-349.
7. Slattengren AH, Prasad S, Jarrett JB. Kidney stones? It’s time to rethink those meds. J Fam Pract. 2016;65(2): 118-120.
1. Wang RC, Smith-Bindman R, Whitaker E, et al. Effect of tamsulosin on stone passage for ureteral stones: a systematic review and meta-analysis. Ann Emerg Med. 2017;69(3):353-361.
2. Scales CD Jr, Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62(1): 160-165.
3. Türk C, Petrik A, Sarica K, et al. EAU guidelines on diagnosis and conservative management of urolithiasis. Eur Urol. 2016;69(3):468-474.
4. Hollingsworth JM, Canales BK, Rogers MAM, et al. Alpha blockers for treatment of ureteric stones: systematic review and meta-analysis. BMJ. 2016;355:i6112.
5. Campschroer T, Zhu Y, Duijvesz D, et al. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014:CD008509.
6. Pickard R, Starr K, MacLennan G, et al. Medical expulsion therapy in adults with ureteric colic: a multicentre, randomized, placebo-controlled trial. Lancet. 2015; 386(9991):341-349.
7. Slattengren AH, Prasad S, Jarrett JB. Kidney stones? It’s time to rethink those meds. J Fam Pract. 2016;65(2): 118-120.
Does fish oil during pregnancy help prevent asthma in kids?
ILLUSTRATIVE CASE
A 24-year-old G2P1 at 24 weeks’ gestation presents to your clinic for a routine prenatal visit. Her older daughter has asthma and she is inquiring as to whether there is anything she can do to lower the risk of her second child developing asthma in the future. What do you recommend?
Asthma is the most common chronic disease in children in resource-rich countries such as the United States.2 The Centers for Disease Control and Prevention (CDC) reported that 8.4% of children were diagnosed with asthma in 2015.3
Omega-3 fatty acids, found naturally in fish oil, are thought to confer anti-inflammatory properties that offer protection against asthma. Clinical trials have shown that fish oil supplementation in pregnancy results in higher levels of omega-3 fatty acids, along with anti-inflammatory changes, in offspring.4 Previous epidemiologic studies have also found that consumption of omega-3 fatty acids decreased the risk of atopy and asthma in offspring.5,6
A Cochrane review published in 2015, however, concluded that omega-3 supplementation during pregnancy had no benefit on wheeze or asthma in offspring.7 Five RCTs were included in the analysis. The largest trial by Palmer et al, which included 706 women, showed no benefit for omega-3 supplementation.8 The second largest by Olsen et al, which included 533 women, did show a benefit (hazard ratio [HR]=0.37; 95% confidence interval [CI], 0.15-0.92; number needed to treat [NNT]=19.6).9
These results, however, were limited by heterogeneity in the amount of fish oil supplemented and duration of follow-up. For example, the children in the Palmer study were followed only until 3 years of age, which is around the time that asthma can be formally diagnosed, potentially leading to under-reporting.8 In addition, the diagnosis of asthma was based on parent report of 3 episodes of wheezing, use of daily asthma medication, or use of a national registry—all of which can underestimate the incidence of asthma. The reported rate of childhood asthma with IgE-sensitization (they did not report the rate without sensitization) was 1.8% in both arms, which is much lower than the CDC’s rate of 8.4%, suggesting underdiagnosis.3,8 Due to these biases and other potential confounders, no firm conclusions can be drawn from the Cochrane review.
STUDY SUMMARY
Maternal fish oil supplementation reduces incidence of asthma in children
This single-center, double-blinded RCT of 736 pregnant women evaluated the effect of 2.4 g/d of n-3 long-chain polyunsaturated fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) or placebo (olive oil), starting at an estimated gestational age of 24 to 26 weeks, on wheeze or asthma incidence in their offspring.1
Eligible women were between 22 and 26 weeks’ pregnant at the time of recruitment. Exclusion criteria included supplementation of 600 IU/d or more of vitamin D, or having any endocrine, cardiac, or renal disorders. The investigators randomized the women in a 1:1 ratio to either fish oil or placebo. Maternal EPA and DHA blood levels were tested at the time of randomization and one week after birth.
The primary outcome was persistent wheeze or asthma (after 3 years of age, the diagnosis of persistent wheeze was termed asthma) based on daily diary recordings of 5 episodes of troublesome lung symptoms within the last 6 months (each lasting for at least 3 consecutive days), rescue use of inhaled beta2-agonists, and/or relapse after a 3-month course of inhaled glucocorticoids. Secondary outcomes included lower respiratory tract infections, asthma exacerbations, eczema, and allergic sensitization.
In total, 695 offspring were included in the study with 95.5% follow-up at 3 years and 93.1% follow-up at 5 years. The children had scheduled pediatric visits at 1 week; 1, 3, 6, 12, 18, 24, 30, and 36 months; and at 4 and 5 years, and acute visits for any pulmonary, allergic, or dermatologic symptoms that arose.
Results. The investigators found that the children of the mothers who received the fish oil had a lower risk of persistent wheeze or asthma at ages 3 to 5 years compared to those who received placebo (16.9% vs 23.7%; HR=0.69; 95% CI, 0.49-0.97; P=.035; NNT=14.7). But the effect of the fish oil supplementation was significant only in the children of the mothers with baseline EPA and DHA levels in the lowest third (17.5% vs 34.1%; HR=0.46; 95% CI, 0.25-0.83; P=.011; NNT=5.6). Similarly, in mothers who consumed the least EPA and DHA before the start of the study, fish oil supplementation had a greater benefit in terms of decreased wheeze and asthma (18.5% vs 32.4%; HR=0.55; 95% CI, 0.30-0.98; P=.043; NNT=7.2).
As for the secondary outcomes, only a reduction in lower respiratory tract infections was associated with the fish oil supplementation vs the control (38.8% vs 45.5%; HR=0.77; 95% CI, 0.61-0.99; P=.041; NNT=14.9). There was no reduction in asthma exacerbations, eczema, or risk of sensitization in the fish oil group.
WHAT'S NEW?
Study adds fuel to the fire
This study strengthens the case for fish oil supplementation during pregnancy to reduce the risk of asthma in offspring, despite the recent Cochrane review that showed no benefit.1,7 The Palmer study used a much lower amount of omega-3s (900 mg/d fish oil vs 2400 mg/d in the current trial).1,8 Olsen et al supplemented with a greater amount of omega-3s (2700 mg/d) and did find a benefit.9 The NNT from the Olsen study (19.6) is consistent with that of the current investigation, suggesting that a higher dosage may be necessary to prevent the onset of asthma.
Additionally, this study followed children for a longer period than did the Palmer study, which may have led to more accurate diagnoses of asthma.1,8 Lastly, the diagnosis of asthma in the Palmer study was based on parent survey data and use of daily asthma medicine rather than on daily diary cards, which are often more accurate.
Consider fish consumption. Both this study and the Olsen trial were performed in Denmark.1,9 While Denmark and the United States have had a relatively similar level of fish consumption since the 1990s, women in Denmark may eat a higher proportion of oily fish than women in the United States, given the more common inclusion of mackerel and herring in their diet.10 Thus, the effect of supplementation may be more pronounced in women in the United States.
CAVEATS
Questions remain: Ideal dose and which women to treat?
The US Food and Drug Administration currently recommends 8 to 12 ounces of fish per week for pregnant women, but there are no guidelines on the ideal amount of fish oil to be consumed.11 The Palmer study,8 using 900 mg/d fish oil, did not show a benefit, whereas there did appear to be benefit in this study (2400 mg/d)1 and the Olsen study (2700 mg/d).9 Further research is needed to determine the optimal dosage.
The decreased risk of persistent wheeze or asthma was seen only in the children of the women whose EPA and DHA blood levels were in the lowest third of the study population. Thus, only women whose blood levels are low to begin with will likely benefit from this intervention. Currently, EPA and DHA levels are not routinely checked, but there may be some benefit to doing so.
One proxy for blood levels is maternal intake of fish at baseline. The investigators found that there was an association between dietary intake of fish and blood levels of EPA and DHA (r=0.32; P<.001).1 Therefore, additional screening questions to determine fish consumption would be useful for identifying women most likely to benefit from supplementation.
CHALLENGES TO IMPLEMENTATION
Multiple pills and additional cost
Since omega-3 fatty acids are relatively safe and the NNT in the general population is low, it may be worth supplementing all pregnant women, even without a commercially-available blood test for EPA or DHA. Nevertheless, some women may find it challenging to take up to an additional 4 pills/d for 13 or more weeks. Also, there is an associated cost with these supplements, although it is low.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Bisgaard H, Stokholm J, Chawes BL, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375:2530-2539.
2. Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dessemination Committee Report. Allergy. 2004;59:469-478.
3. Centers for Disease Control and Prevention. Asthma. Available at: https://www.cdc.gov/asthma/most_recent_data.htm. Accessed October 9, 2017.
4. Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol Int. 2015;64:27-34.
5. Salam MT, Li YF, Langholz B, et al. Maternal fish consumption during pregnancy and risk of early childhood asthma. J Asthma. 2005;42:513-518.
6. Calvani M, Alessandri C, Sopo SM, et al. Consumption of fish, butter and margarine during pregnancy and development of allergic sensitizations in the offspring: role of maternal atopy. Pediatr Allergy Immunol. 2006;17:94-102.
7. Gunaratne AW, Makrides M, Collins CT. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev. 2015;22:CD010085.
8. Palmer D, Sullivan T, Gold M, et al. Randomized controlled trial of fish oil supplementation in pregnancy on childhood allergies. Allergy. 2013;68:1370-1376.
9. Olsen SF, Østerdal ML, Salvig JD, et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am J Clin Nutr. 2008;88:167-175.
10. Helgi Library. Fish consumption per capita by country. Available at: http://www.helgilibrary.com/indicators/fish-consumption-per-capita/. Accessed September 27, 2017.
11. FDA Advice About Eating Fish, From the Environmental Protection Agency and Food and Drug Administration; Revised Fish Advice; Availability. Federal Register.2017;82:6571-6574.
ILLUSTRATIVE CASE
A 24-year-old G2P1 at 24 weeks’ gestation presents to your clinic for a routine prenatal visit. Her older daughter has asthma and she is inquiring as to whether there is anything she can do to lower the risk of her second child developing asthma in the future. What do you recommend?
Asthma is the most common chronic disease in children in resource-rich countries such as the United States.2 The Centers for Disease Control and Prevention (CDC) reported that 8.4% of children were diagnosed with asthma in 2015.3
Omega-3 fatty acids, found naturally in fish oil, are thought to confer anti-inflammatory properties that offer protection against asthma. Clinical trials have shown that fish oil supplementation in pregnancy results in higher levels of omega-3 fatty acids, along with anti-inflammatory changes, in offspring.4 Previous epidemiologic studies have also found that consumption of omega-3 fatty acids decreased the risk of atopy and asthma in offspring.5,6
A Cochrane review published in 2015, however, concluded that omega-3 supplementation during pregnancy had no benefit on wheeze or asthma in offspring.7 Five RCTs were included in the analysis. The largest trial by Palmer et al, which included 706 women, showed no benefit for omega-3 supplementation.8 The second largest by Olsen et al, which included 533 women, did show a benefit (hazard ratio [HR]=0.37; 95% confidence interval [CI], 0.15-0.92; number needed to treat [NNT]=19.6).9
These results, however, were limited by heterogeneity in the amount of fish oil supplemented and duration of follow-up. For example, the children in the Palmer study were followed only until 3 years of age, which is around the time that asthma can be formally diagnosed, potentially leading to under-reporting.8 In addition, the diagnosis of asthma was based on parent report of 3 episodes of wheezing, use of daily asthma medication, or use of a national registry—all of which can underestimate the incidence of asthma. The reported rate of childhood asthma with IgE-sensitization (they did not report the rate without sensitization) was 1.8% in both arms, which is much lower than the CDC’s rate of 8.4%, suggesting underdiagnosis.3,8 Due to these biases and other potential confounders, no firm conclusions can be drawn from the Cochrane review.
STUDY SUMMARY
Maternal fish oil supplementation reduces incidence of asthma in children
This single-center, double-blinded RCT of 736 pregnant women evaluated the effect of 2.4 g/d of n-3 long-chain polyunsaturated fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) or placebo (olive oil), starting at an estimated gestational age of 24 to 26 weeks, on wheeze or asthma incidence in their offspring.1
Eligible women were between 22 and 26 weeks’ pregnant at the time of recruitment. Exclusion criteria included supplementation of 600 IU/d or more of vitamin D, or having any endocrine, cardiac, or renal disorders. The investigators randomized the women in a 1:1 ratio to either fish oil or placebo. Maternal EPA and DHA blood levels were tested at the time of randomization and one week after birth.
The primary outcome was persistent wheeze or asthma (after 3 years of age, the diagnosis of persistent wheeze was termed asthma) based on daily diary recordings of 5 episodes of troublesome lung symptoms within the last 6 months (each lasting for at least 3 consecutive days), rescue use of inhaled beta2-agonists, and/or relapse after a 3-month course of inhaled glucocorticoids. Secondary outcomes included lower respiratory tract infections, asthma exacerbations, eczema, and allergic sensitization.
In total, 695 offspring were included in the study with 95.5% follow-up at 3 years and 93.1% follow-up at 5 years. The children had scheduled pediatric visits at 1 week; 1, 3, 6, 12, 18, 24, 30, and 36 months; and at 4 and 5 years, and acute visits for any pulmonary, allergic, or dermatologic symptoms that arose.
Results. The investigators found that the children of the mothers who received the fish oil had a lower risk of persistent wheeze or asthma at ages 3 to 5 years compared to those who received placebo (16.9% vs 23.7%; HR=0.69; 95% CI, 0.49-0.97; P=.035; NNT=14.7). But the effect of the fish oil supplementation was significant only in the children of the mothers with baseline EPA and DHA levels in the lowest third (17.5% vs 34.1%; HR=0.46; 95% CI, 0.25-0.83; P=.011; NNT=5.6). Similarly, in mothers who consumed the least EPA and DHA before the start of the study, fish oil supplementation had a greater benefit in terms of decreased wheeze and asthma (18.5% vs 32.4%; HR=0.55; 95% CI, 0.30-0.98; P=.043; NNT=7.2).
As for the secondary outcomes, only a reduction in lower respiratory tract infections was associated with the fish oil supplementation vs the control (38.8% vs 45.5%; HR=0.77; 95% CI, 0.61-0.99; P=.041; NNT=14.9). There was no reduction in asthma exacerbations, eczema, or risk of sensitization in the fish oil group.
WHAT'S NEW?
Study adds fuel to the fire
This study strengthens the case for fish oil supplementation during pregnancy to reduce the risk of asthma in offspring, despite the recent Cochrane review that showed no benefit.1,7 The Palmer study used a much lower amount of omega-3s (900 mg/d fish oil vs 2400 mg/d in the current trial).1,8 Olsen et al supplemented with a greater amount of omega-3s (2700 mg/d) and did find a benefit.9 The NNT from the Olsen study (19.6) is consistent with that of the current investigation, suggesting that a higher dosage may be necessary to prevent the onset of asthma.
Additionally, this study followed children for a longer period than did the Palmer study, which may have led to more accurate diagnoses of asthma.1,8 Lastly, the diagnosis of asthma in the Palmer study was based on parent survey data and use of daily asthma medicine rather than on daily diary cards, which are often more accurate.
Consider fish consumption. Both this study and the Olsen trial were performed in Denmark.1,9 While Denmark and the United States have had a relatively similar level of fish consumption since the 1990s, women in Denmark may eat a higher proportion of oily fish than women in the United States, given the more common inclusion of mackerel and herring in their diet.10 Thus, the effect of supplementation may be more pronounced in women in the United States.
CAVEATS
Questions remain: Ideal dose and which women to treat?
The US Food and Drug Administration currently recommends 8 to 12 ounces of fish per week for pregnant women, but there are no guidelines on the ideal amount of fish oil to be consumed.11 The Palmer study,8 using 900 mg/d fish oil, did not show a benefit, whereas there did appear to be benefit in this study (2400 mg/d)1 and the Olsen study (2700 mg/d).9 Further research is needed to determine the optimal dosage.
The decreased risk of persistent wheeze or asthma was seen only in the children of the women whose EPA and DHA blood levels were in the lowest third of the study population. Thus, only women whose blood levels are low to begin with will likely benefit from this intervention. Currently, EPA and DHA levels are not routinely checked, but there may be some benefit to doing so.
One proxy for blood levels is maternal intake of fish at baseline. The investigators found that there was an association between dietary intake of fish and blood levels of EPA and DHA (r=0.32; P<.001).1 Therefore, additional screening questions to determine fish consumption would be useful for identifying women most likely to benefit from supplementation.
CHALLENGES TO IMPLEMENTATION
Multiple pills and additional cost
Since omega-3 fatty acids are relatively safe and the NNT in the general population is low, it may be worth supplementing all pregnant women, even without a commercially-available blood test for EPA or DHA. Nevertheless, some women may find it challenging to take up to an additional 4 pills/d for 13 or more weeks. Also, there is an associated cost with these supplements, although it is low.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 24-year-old G2P1 at 24 weeks’ gestation presents to your clinic for a routine prenatal visit. Her older daughter has asthma and she is inquiring as to whether there is anything she can do to lower the risk of her second child developing asthma in the future. What do you recommend?
Asthma is the most common chronic disease in children in resource-rich countries such as the United States.2 The Centers for Disease Control and Prevention (CDC) reported that 8.4% of children were diagnosed with asthma in 2015.3
Omega-3 fatty acids, found naturally in fish oil, are thought to confer anti-inflammatory properties that offer protection against asthma. Clinical trials have shown that fish oil supplementation in pregnancy results in higher levels of omega-3 fatty acids, along with anti-inflammatory changes, in offspring.4 Previous epidemiologic studies have also found that consumption of omega-3 fatty acids decreased the risk of atopy and asthma in offspring.5,6
A Cochrane review published in 2015, however, concluded that omega-3 supplementation during pregnancy had no benefit on wheeze or asthma in offspring.7 Five RCTs were included in the analysis. The largest trial by Palmer et al, which included 706 women, showed no benefit for omega-3 supplementation.8 The second largest by Olsen et al, which included 533 women, did show a benefit (hazard ratio [HR]=0.37; 95% confidence interval [CI], 0.15-0.92; number needed to treat [NNT]=19.6).9
These results, however, were limited by heterogeneity in the amount of fish oil supplemented and duration of follow-up. For example, the children in the Palmer study were followed only until 3 years of age, which is around the time that asthma can be formally diagnosed, potentially leading to under-reporting.8 In addition, the diagnosis of asthma was based on parent report of 3 episodes of wheezing, use of daily asthma medication, or use of a national registry—all of which can underestimate the incidence of asthma. The reported rate of childhood asthma with IgE-sensitization (they did not report the rate without sensitization) was 1.8% in both arms, which is much lower than the CDC’s rate of 8.4%, suggesting underdiagnosis.3,8 Due to these biases and other potential confounders, no firm conclusions can be drawn from the Cochrane review.
STUDY SUMMARY
Maternal fish oil supplementation reduces incidence of asthma in children
This single-center, double-blinded RCT of 736 pregnant women evaluated the effect of 2.4 g/d of n-3 long-chain polyunsaturated fatty acids (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) or placebo (olive oil), starting at an estimated gestational age of 24 to 26 weeks, on wheeze or asthma incidence in their offspring.1
Eligible women were between 22 and 26 weeks’ pregnant at the time of recruitment. Exclusion criteria included supplementation of 600 IU/d or more of vitamin D, or having any endocrine, cardiac, or renal disorders. The investigators randomized the women in a 1:1 ratio to either fish oil or placebo. Maternal EPA and DHA blood levels were tested at the time of randomization and one week after birth.
The primary outcome was persistent wheeze or asthma (after 3 years of age, the diagnosis of persistent wheeze was termed asthma) based on daily diary recordings of 5 episodes of troublesome lung symptoms within the last 6 months (each lasting for at least 3 consecutive days), rescue use of inhaled beta2-agonists, and/or relapse after a 3-month course of inhaled glucocorticoids. Secondary outcomes included lower respiratory tract infections, asthma exacerbations, eczema, and allergic sensitization.
In total, 695 offspring were included in the study with 95.5% follow-up at 3 years and 93.1% follow-up at 5 years. The children had scheduled pediatric visits at 1 week; 1, 3, 6, 12, 18, 24, 30, and 36 months; and at 4 and 5 years, and acute visits for any pulmonary, allergic, or dermatologic symptoms that arose.
Results. The investigators found that the children of the mothers who received the fish oil had a lower risk of persistent wheeze or asthma at ages 3 to 5 years compared to those who received placebo (16.9% vs 23.7%; HR=0.69; 95% CI, 0.49-0.97; P=.035; NNT=14.7). But the effect of the fish oil supplementation was significant only in the children of the mothers with baseline EPA and DHA levels in the lowest third (17.5% vs 34.1%; HR=0.46; 95% CI, 0.25-0.83; P=.011; NNT=5.6). Similarly, in mothers who consumed the least EPA and DHA before the start of the study, fish oil supplementation had a greater benefit in terms of decreased wheeze and asthma (18.5% vs 32.4%; HR=0.55; 95% CI, 0.30-0.98; P=.043; NNT=7.2).
As for the secondary outcomes, only a reduction in lower respiratory tract infections was associated with the fish oil supplementation vs the control (38.8% vs 45.5%; HR=0.77; 95% CI, 0.61-0.99; P=.041; NNT=14.9). There was no reduction in asthma exacerbations, eczema, or risk of sensitization in the fish oil group.
WHAT'S NEW?
Study adds fuel to the fire
This study strengthens the case for fish oil supplementation during pregnancy to reduce the risk of asthma in offspring, despite the recent Cochrane review that showed no benefit.1,7 The Palmer study used a much lower amount of omega-3s (900 mg/d fish oil vs 2400 mg/d in the current trial).1,8 Olsen et al supplemented with a greater amount of omega-3s (2700 mg/d) and did find a benefit.9 The NNT from the Olsen study (19.6) is consistent with that of the current investigation, suggesting that a higher dosage may be necessary to prevent the onset of asthma.
Additionally, this study followed children for a longer period than did the Palmer study, which may have led to more accurate diagnoses of asthma.1,8 Lastly, the diagnosis of asthma in the Palmer study was based on parent survey data and use of daily asthma medicine rather than on daily diary cards, which are often more accurate.
Consider fish consumption. Both this study and the Olsen trial were performed in Denmark.1,9 While Denmark and the United States have had a relatively similar level of fish consumption since the 1990s, women in Denmark may eat a higher proportion of oily fish than women in the United States, given the more common inclusion of mackerel and herring in their diet.10 Thus, the effect of supplementation may be more pronounced in women in the United States.
CAVEATS
Questions remain: Ideal dose and which women to treat?
The US Food and Drug Administration currently recommends 8 to 12 ounces of fish per week for pregnant women, but there are no guidelines on the ideal amount of fish oil to be consumed.11 The Palmer study,8 using 900 mg/d fish oil, did not show a benefit, whereas there did appear to be benefit in this study (2400 mg/d)1 and the Olsen study (2700 mg/d).9 Further research is needed to determine the optimal dosage.
The decreased risk of persistent wheeze or asthma was seen only in the children of the women whose EPA and DHA blood levels were in the lowest third of the study population. Thus, only women whose blood levels are low to begin with will likely benefit from this intervention. Currently, EPA and DHA levels are not routinely checked, but there may be some benefit to doing so.
One proxy for blood levels is maternal intake of fish at baseline. The investigators found that there was an association between dietary intake of fish and blood levels of EPA and DHA (r=0.32; P<.001).1 Therefore, additional screening questions to determine fish consumption would be useful for identifying women most likely to benefit from supplementation.
CHALLENGES TO IMPLEMENTATION
Multiple pills and additional cost
Since omega-3 fatty acids are relatively safe and the NNT in the general population is low, it may be worth supplementing all pregnant women, even without a commercially-available blood test for EPA or DHA. Nevertheless, some women may find it challenging to take up to an additional 4 pills/d for 13 or more weeks. Also, there is an associated cost with these supplements, although it is low.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Bisgaard H, Stokholm J, Chawes BL, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375:2530-2539.
2. Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dessemination Committee Report. Allergy. 2004;59:469-478.
3. Centers for Disease Control and Prevention. Asthma. Available at: https://www.cdc.gov/asthma/most_recent_data.htm. Accessed October 9, 2017.
4. Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol Int. 2015;64:27-34.
5. Salam MT, Li YF, Langholz B, et al. Maternal fish consumption during pregnancy and risk of early childhood asthma. J Asthma. 2005;42:513-518.
6. Calvani M, Alessandri C, Sopo SM, et al. Consumption of fish, butter and margarine during pregnancy and development of allergic sensitizations in the offspring: role of maternal atopy. Pediatr Allergy Immunol. 2006;17:94-102.
7. Gunaratne AW, Makrides M, Collins CT. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev. 2015;22:CD010085.
8. Palmer D, Sullivan T, Gold M, et al. Randomized controlled trial of fish oil supplementation in pregnancy on childhood allergies. Allergy. 2013;68:1370-1376.
9. Olsen SF, Østerdal ML, Salvig JD, et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am J Clin Nutr. 2008;88:167-175.
10. Helgi Library. Fish consumption per capita by country. Available at: http://www.helgilibrary.com/indicators/fish-consumption-per-capita/. Accessed September 27, 2017.
11. FDA Advice About Eating Fish, From the Environmental Protection Agency and Food and Drug Administration; Revised Fish Advice; Availability. Federal Register.2017;82:6571-6574.
1. Bisgaard H, Stokholm J, Chawes BL, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375:2530-2539.
2. Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dessemination Committee Report. Allergy. 2004;59:469-478.
3. Centers for Disease Control and Prevention. Asthma. Available at: https://www.cdc.gov/asthma/most_recent_data.htm. Accessed October 9, 2017.
4. Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol Int. 2015;64:27-34.
5. Salam MT, Li YF, Langholz B, et al. Maternal fish consumption during pregnancy and risk of early childhood asthma. J Asthma. 2005;42:513-518.
6. Calvani M, Alessandri C, Sopo SM, et al. Consumption of fish, butter and margarine during pregnancy and development of allergic sensitizations in the offspring: role of maternal atopy. Pediatr Allergy Immunol. 2006;17:94-102.
7. Gunaratne AW, Makrides M, Collins CT. Maternal prenatal and/or postnatal n-3 long chain polyunsaturated fatty acids (LCPUFA) supplementation for preventing allergies in early childhood. Cochrane Database Syst Rev. 2015;22:CD010085.
8. Palmer D, Sullivan T, Gold M, et al. Randomized controlled trial of fish oil supplementation in pregnancy on childhood allergies. Allergy. 2013;68:1370-1376.
9. Olsen SF, Østerdal ML, Salvig JD, et al. Fish oil intake compared with olive oil intake in late pregnancy and asthma in the offspring: 16 y of registry-based follow-up from a randomized controlled trial. Am J Clin Nutr. 2008;88:167-175.
10. Helgi Library. Fish consumption per capita by country. Available at: http://www.helgilibrary.com/indicators/fish-consumption-per-capita/. Accessed September 27, 2017.
11. FDA Advice About Eating Fish, From the Environmental Protection Agency and Food and Drug Administration; Revised Fish Advice; Availability. Federal Register.2017;82:6571-6574.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Fish oil supplementation taken by women in the third trimester of pregnancy can reduce the risk of persistent wheeze, asthma, and infections of the lower respiratory tract in their children.1
STRENGTH OF RECOMMENDATION
B: Based on 2 double-blinded randomized controlled trials (RCTs).
Bisgaard H, Stokholm J, Chawes BL, et al. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N Engl J Med. 2016;375:2530-2539.1
Does Azithromycin Have a Role in Cesarean Sections?

A 26-year-old G1P0 at 40w1d presents in spontaneous labor and is dilated to 4 cm. The patient reached complete cervical dilation after artificial rupture of membranes and oxytocin augmentation. After four hours of pushing, there has been minimal descent of the fetal vertex beyond +1 station with significant caput succedaneum. Her physician decides to proceed with cesarean delivery.2,3 What antibiotics should be administered prior to incision to reduce postoperative infection?
The CDC reports that nearly 1.3 million cesarean deliveries were performed in the United States in 2015, which represents about a third of all births.4 C-section is the most common major surgical procedure performed in this country and is associated with an infection rate five to 10 times that of vaginal delivery.5,6 Pregnancy-associated infection, particularly during delivery, is a significant risk and the fourth most common cause of maternal death in the United States.5
The current standard of care in cesarean delivery is antibiotic prophylaxis (often a first-generation cephalosporin) prior to skin incision.7 The majority of c-sections performed are nonelective, and of these, postoperative infections occur in 12% of women who receive standard prophylaxis.8,9 A small, single-center design trial suggested that azithromycin adjunctive therapy expands antibiotic coverage to Ureaplasma species, resulting in a lower risk for postoperative infection.10
This study evaluated the use of azithromycin adjunctive therapy, in addition to standard antibiotic prophylaxis, to reduce the risk for postoperative infections in women receiving nonelective c-sections.

STUDY SUMMARY
Reduced infections up to 6 weeks post–c-section
A multicenter, randomized, double-blind trial conducted in 14 hospitals in the United States evaluated the effect of a one-time dose of azithromycin (500 mg IV) on postcesarean infections. Women with a singleton pregnancy of at least 24 weeks’ gestation were eligible for inclusion if they required nonelective cesarean delivery during labor or at least four hours after membrane rupture. Patients were excluded if they had a known azithromycin allergy, subsequent vaginal delivery, azithromycin use within the week prior to randomization, extensive hepatic or renal dysfunction, a known history of prolonged QT interval, or substantial electrolyte abnormalities. Patients were eligible even if they were receiving other antibiotics for a positive group B Streptococcus screening.1
All patients (N = 2,013) were treated with standard antibiotic prophylaxis (most often cefazolin), according to individual institution protocols. The women were randomized to receive either an azithromycin 500 mg/250 mL IV infusion (n = 1,019) or an identical placebo IV infusion (n = 994) within one hour of the procedure. The primary outcome was a composite endpoint of endometritis, wound infection, or other infections occurring up to six weeks after the c-section. Secondary outcomes included neonatal death, sepsis, and other neonatal and maternal complications.1
Patients in the placebo group had a higher rate of smoking during pregnancy; the researchers found no other significant differences.1
Results. The primary composite outcome occurred less frequently in the azithromycin group than in the placebo group (6.1% vs 12.0%; relative risk [RR], 0.51; number needed to treat [NNT], 17). When the researchers looked at the individual elements of the primary composite outcome, two had significant reductions versus placebo. Endometritis (3.8% vs 6.1%; RR, 0.62; NNT, 43) and wound infections (2.4% vs 6.6%; RR, 0.35; NNT, 24) occurred significantly less frequently, but there was no difference for other infections (0.3% vs 0.6%; RR, 0.49).
Serious maternal adverse events were also lower in the treatment group than in the control group (1.5% vs 2.9%; RR, 0.5; NNT, 71). There was no difference in composite secondary neonatal outcomes, including death and serious complications (14.3% vs 13.6%; RR, 1.05).1
WHAT’S NEW
Fewer infections without more adverse events
This study showed that adding azithromycin to standard antibiotic prophylaxis within one hour of a c-section reduces post–cesarean delivery infection rates without increasing the risk for maternal or neonatal adverse events.
CAVEATS
Caution with prolonged QT
While azithromycin was efficacious and well tolerated in the study, not every patient can take it. Patients with a previous drug reaction or allergy should avoid it, and experts advise prescribing it with caution for patients who have (or are at increased risk for) a prolonged QT interval, including those on other QT-prolonging medications.
Of note, women with scheduled c-sections and those with chorioamnionitis or another infection requiring postpartum antibiotics were excluded from this study. Thus, it is unknown if azithromycin use decreases complications in these patients.
CHALLENGES TO IMPLEMENTATION
Drug availability is key
Nonelective c-sections are performed based on many factors, including a nonreassuring fetal heart rate. In many of these cases, speed of cesarean delivery may mean the difference between positive and negative outcomes. Availability of azithromycin on labor and delivery floors for timely administration within one hour of the procedure is important.
Additionally, azithromycin has known QT prolongation risks.11 While the baseline QT interval is not known for many healthy young women, this should be considered when azithromycin is utilized in combination with other medications that may prolong the QT interval.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[12]:762-764).
1. Tita AT, Szychowski JM, Boggess K, et al. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016; 375:1231-1241.
2. American College of Obstetricians and Gynecologists. Safe prevention of the primary cesarean delivery. Obstetric Care Consensus No. 1. Obstet Gynecol. 2014;123:693-711.
3. Rouse DJ, Weiner SJ, Bloom SL, et al. Second-stage labor duration in nulliparous women: relationship to maternal and perinatal outcomes. Am J Obstet Gynecol. 2009;201(4):357.e1-e7.
4. CDC National Center for Health Statistics. Births—Method of Delivery. www.cdc.gov/nchs/fastats/delivery.htm. Accessed December 1, 2017.
5. Perencevich EN, Sands KE, Cosgrove SE, et al. Health and economic impact of surgical site infections diagnosed after hospital discharge. Emerg Infect Dis. 2003;9:196-203.
6. DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13. 2007;(165):1-209.
7. American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 120: use of prophylactic antibiotics in labor and delivery. Obstet Gynecol. 2011;117:1472-1483.
8. Thigpen BD, Hood WA, Chauhan S, et al. Timing of prophylactic antibiotic administration in the uninfected laboring gravida: a randomized clinical trial. Am J Obstet Gynecol. 2005; 192:1864-1868.
9. Costantine MM, Rahman M, Ghulmiyah L, et al. Timing of perioperative antibiotics for cesarean delivery: a meta-analysis. Am J Obstet Gynecol. 2008;199:301.e1-e6.
10. Andrews WW, Hauth JC, Cliver SP, et al. Randomized clinical trial of extended spectrum antibiotic prophylaxis with coverage for Ureaplasma urealyticum to reduce post-cesarean delivery endometritis. Obstet Gynecol. 2003;101:1183-1189.
11. Howard PA. Azithromycin-induced proarrhythmia and cardiovascular death. Ann Pharmacother. 2013;47:1547-1551.

A 26-year-old G1P0 at 40w1d presents in spontaneous labor and is dilated to 4 cm. The patient reached complete cervical dilation after artificial rupture of membranes and oxytocin augmentation. After four hours of pushing, there has been minimal descent of the fetal vertex beyond +1 station with significant caput succedaneum. Her physician decides to proceed with cesarean delivery.2,3 What antibiotics should be administered prior to incision to reduce postoperative infection?
The CDC reports that nearly 1.3 million cesarean deliveries were performed in the United States in 2015, which represents about a third of all births.4 C-section is the most common major surgical procedure performed in this country and is associated with an infection rate five to 10 times that of vaginal delivery.5,6 Pregnancy-associated infection, particularly during delivery, is a significant risk and the fourth most common cause of maternal death in the United States.5
The current standard of care in cesarean delivery is antibiotic prophylaxis (often a first-generation cephalosporin) prior to skin incision.7 The majority of c-sections performed are nonelective, and of these, postoperative infections occur in 12% of women who receive standard prophylaxis.8,9 A small, single-center design trial suggested that azithromycin adjunctive therapy expands antibiotic coverage to Ureaplasma species, resulting in a lower risk for postoperative infection.10
This study evaluated the use of azithromycin adjunctive therapy, in addition to standard antibiotic prophylaxis, to reduce the risk for postoperative infections in women receiving nonelective c-sections.

STUDY SUMMARY
Reduced infections up to 6 weeks post–c-section
A multicenter, randomized, double-blind trial conducted in 14 hospitals in the United States evaluated the effect of a one-time dose of azithromycin (500 mg IV) on postcesarean infections. Women with a singleton pregnancy of at least 24 weeks’ gestation were eligible for inclusion if they required nonelective cesarean delivery during labor or at least four hours after membrane rupture. Patients were excluded if they had a known azithromycin allergy, subsequent vaginal delivery, azithromycin use within the week prior to randomization, extensive hepatic or renal dysfunction, a known history of prolonged QT interval, or substantial electrolyte abnormalities. Patients were eligible even if they were receiving other antibiotics for a positive group B Streptococcus screening.1
All patients (N = 2,013) were treated with standard antibiotic prophylaxis (most often cefazolin), according to individual institution protocols. The women were randomized to receive either an azithromycin 500 mg/250 mL IV infusion (n = 1,019) or an identical placebo IV infusion (n = 994) within one hour of the procedure. The primary outcome was a composite endpoint of endometritis, wound infection, or other infections occurring up to six weeks after the c-section. Secondary outcomes included neonatal death, sepsis, and other neonatal and maternal complications.1
Patients in the placebo group had a higher rate of smoking during pregnancy; the researchers found no other significant differences.1
Results. The primary composite outcome occurred less frequently in the azithromycin group than in the placebo group (6.1% vs 12.0%; relative risk [RR], 0.51; number needed to treat [NNT], 17). When the researchers looked at the individual elements of the primary composite outcome, two had significant reductions versus placebo. Endometritis (3.8% vs 6.1%; RR, 0.62; NNT, 43) and wound infections (2.4% vs 6.6%; RR, 0.35; NNT, 24) occurred significantly less frequently, but there was no difference for other infections (0.3% vs 0.6%; RR, 0.49).
Serious maternal adverse events were also lower in the treatment group than in the control group (1.5% vs 2.9%; RR, 0.5; NNT, 71). There was no difference in composite secondary neonatal outcomes, including death and serious complications (14.3% vs 13.6%; RR, 1.05).1
WHAT’S NEW
Fewer infections without more adverse events
This study showed that adding azithromycin to standard antibiotic prophylaxis within one hour of a c-section reduces post–cesarean delivery infection rates without increasing the risk for maternal or neonatal adverse events.
CAVEATS
Caution with prolonged QT
While azithromycin was efficacious and well tolerated in the study, not every patient can take it. Patients with a previous drug reaction or allergy should avoid it, and experts advise prescribing it with caution for patients who have (or are at increased risk for) a prolonged QT interval, including those on other QT-prolonging medications.
Of note, women with scheduled c-sections and those with chorioamnionitis or another infection requiring postpartum antibiotics were excluded from this study. Thus, it is unknown if azithromycin use decreases complications in these patients.
CHALLENGES TO IMPLEMENTATION
Drug availability is key
Nonelective c-sections are performed based on many factors, including a nonreassuring fetal heart rate. In many of these cases, speed of cesarean delivery may mean the difference between positive and negative outcomes. Availability of azithromycin on labor and delivery floors for timely administration within one hour of the procedure is important.
Additionally, azithromycin has known QT prolongation risks.11 While the baseline QT interval is not known for many healthy young women, this should be considered when azithromycin is utilized in combination with other medications that may prolong the QT interval.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[12]:762-764).

A 26-year-old G1P0 at 40w1d presents in spontaneous labor and is dilated to 4 cm. The patient reached complete cervical dilation after artificial rupture of membranes and oxytocin augmentation. After four hours of pushing, there has been minimal descent of the fetal vertex beyond +1 station with significant caput succedaneum. Her physician decides to proceed with cesarean delivery.2,3 What antibiotics should be administered prior to incision to reduce postoperative infection?
The CDC reports that nearly 1.3 million cesarean deliveries were performed in the United States in 2015, which represents about a third of all births.4 C-section is the most common major surgical procedure performed in this country and is associated with an infection rate five to 10 times that of vaginal delivery.5,6 Pregnancy-associated infection, particularly during delivery, is a significant risk and the fourth most common cause of maternal death in the United States.5
The current standard of care in cesarean delivery is antibiotic prophylaxis (often a first-generation cephalosporin) prior to skin incision.7 The majority of c-sections performed are nonelective, and of these, postoperative infections occur in 12% of women who receive standard prophylaxis.8,9 A small, single-center design trial suggested that azithromycin adjunctive therapy expands antibiotic coverage to Ureaplasma species, resulting in a lower risk for postoperative infection.10
This study evaluated the use of azithromycin adjunctive therapy, in addition to standard antibiotic prophylaxis, to reduce the risk for postoperative infections in women receiving nonelective c-sections.

STUDY SUMMARY
Reduced infections up to 6 weeks post–c-section
A multicenter, randomized, double-blind trial conducted in 14 hospitals in the United States evaluated the effect of a one-time dose of azithromycin (500 mg IV) on postcesarean infections. Women with a singleton pregnancy of at least 24 weeks’ gestation were eligible for inclusion if they required nonelective cesarean delivery during labor or at least four hours after membrane rupture. Patients were excluded if they had a known azithromycin allergy, subsequent vaginal delivery, azithromycin use within the week prior to randomization, extensive hepatic or renal dysfunction, a known history of prolonged QT interval, or substantial electrolyte abnormalities. Patients were eligible even if they were receiving other antibiotics for a positive group B Streptococcus screening.1
All patients (N = 2,013) were treated with standard antibiotic prophylaxis (most often cefazolin), according to individual institution protocols. The women were randomized to receive either an azithromycin 500 mg/250 mL IV infusion (n = 1,019) or an identical placebo IV infusion (n = 994) within one hour of the procedure. The primary outcome was a composite endpoint of endometritis, wound infection, or other infections occurring up to six weeks after the c-section. Secondary outcomes included neonatal death, sepsis, and other neonatal and maternal complications.1
Patients in the placebo group had a higher rate of smoking during pregnancy; the researchers found no other significant differences.1
Results. The primary composite outcome occurred less frequently in the azithromycin group than in the placebo group (6.1% vs 12.0%; relative risk [RR], 0.51; number needed to treat [NNT], 17). When the researchers looked at the individual elements of the primary composite outcome, two had significant reductions versus placebo. Endometritis (3.8% vs 6.1%; RR, 0.62; NNT, 43) and wound infections (2.4% vs 6.6%; RR, 0.35; NNT, 24) occurred significantly less frequently, but there was no difference for other infections (0.3% vs 0.6%; RR, 0.49).
Serious maternal adverse events were also lower in the treatment group than in the control group (1.5% vs 2.9%; RR, 0.5; NNT, 71). There was no difference in composite secondary neonatal outcomes, including death and serious complications (14.3% vs 13.6%; RR, 1.05).1
WHAT’S NEW
Fewer infections without more adverse events
This study showed that adding azithromycin to standard antibiotic prophylaxis within one hour of a c-section reduces post–cesarean delivery infection rates without increasing the risk for maternal or neonatal adverse events.
CAVEATS
Caution with prolonged QT
While azithromycin was efficacious and well tolerated in the study, not every patient can take it. Patients with a previous drug reaction or allergy should avoid it, and experts advise prescribing it with caution for patients who have (or are at increased risk for) a prolonged QT interval, including those on other QT-prolonging medications.
Of note, women with scheduled c-sections and those with chorioamnionitis or another infection requiring postpartum antibiotics were excluded from this study. Thus, it is unknown if azithromycin use decreases complications in these patients.
CHALLENGES TO IMPLEMENTATION
Drug availability is key
Nonelective c-sections are performed based on many factors, including a nonreassuring fetal heart rate. In many of these cases, speed of cesarean delivery may mean the difference between positive and negative outcomes. Availability of azithromycin on labor and delivery floors for timely administration within one hour of the procedure is important.
Additionally, azithromycin has known QT prolongation risks.11 While the baseline QT interval is not known for many healthy young women, this should be considered when azithromycin is utilized in combination with other medications that may prolong the QT interval.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[12]:762-764).
1. Tita AT, Szychowski JM, Boggess K, et al. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016; 375:1231-1241.
2. American College of Obstetricians and Gynecologists. Safe prevention of the primary cesarean delivery. Obstetric Care Consensus No. 1. Obstet Gynecol. 2014;123:693-711.
3. Rouse DJ, Weiner SJ, Bloom SL, et al. Second-stage labor duration in nulliparous women: relationship to maternal and perinatal outcomes. Am J Obstet Gynecol. 2009;201(4):357.e1-e7.
4. CDC National Center for Health Statistics. Births—Method of Delivery. www.cdc.gov/nchs/fastats/delivery.htm. Accessed December 1, 2017.
5. Perencevich EN, Sands KE, Cosgrove SE, et al. Health and economic impact of surgical site infections diagnosed after hospital discharge. Emerg Infect Dis. 2003;9:196-203.
6. DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13. 2007;(165):1-209.
7. American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 120: use of prophylactic antibiotics in labor and delivery. Obstet Gynecol. 2011;117:1472-1483.
8. Thigpen BD, Hood WA, Chauhan S, et al. Timing of prophylactic antibiotic administration in the uninfected laboring gravida: a randomized clinical trial. Am J Obstet Gynecol. 2005; 192:1864-1868.
9. Costantine MM, Rahman M, Ghulmiyah L, et al. Timing of perioperative antibiotics for cesarean delivery: a meta-analysis. Am J Obstet Gynecol. 2008;199:301.e1-e6.
10. Andrews WW, Hauth JC, Cliver SP, et al. Randomized clinical trial of extended spectrum antibiotic prophylaxis with coverage for Ureaplasma urealyticum to reduce post-cesarean delivery endometritis. Obstet Gynecol. 2003;101:1183-1189.
11. Howard PA. Azithromycin-induced proarrhythmia and cardiovascular death. Ann Pharmacother. 2013;47:1547-1551.
1. Tita AT, Szychowski JM, Boggess K, et al. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016; 375:1231-1241.
2. American College of Obstetricians and Gynecologists. Safe prevention of the primary cesarean delivery. Obstetric Care Consensus No. 1. Obstet Gynecol. 2014;123:693-711.
3. Rouse DJ, Weiner SJ, Bloom SL, et al. Second-stage labor duration in nulliparous women: relationship to maternal and perinatal outcomes. Am J Obstet Gynecol. 2009;201(4):357.e1-e7.
4. CDC National Center for Health Statistics. Births—Method of Delivery. www.cdc.gov/nchs/fastats/delivery.htm. Accessed December 1, 2017.
5. Perencevich EN, Sands KE, Cosgrove SE, et al. Health and economic impact of surgical site infections diagnosed after hospital discharge. Emerg Infect Dis. 2003;9:196-203.
6. DeFrances CJ, Cullen KA, Kozak LJ. National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data. Vital Health Stat 13. 2007;(165):1-209.
7. American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 120: use of prophylactic antibiotics in labor and delivery. Obstet Gynecol. 2011;117:1472-1483.
8. Thigpen BD, Hood WA, Chauhan S, et al. Timing of prophylactic antibiotic administration in the uninfected laboring gravida: a randomized clinical trial. Am J Obstet Gynecol. 2005; 192:1864-1868.
9. Costantine MM, Rahman M, Ghulmiyah L, et al. Timing of perioperative antibiotics for cesarean delivery: a meta-analysis. Am J Obstet Gynecol. 2008;199:301.e1-e6.
10. Andrews WW, Hauth JC, Cliver SP, et al. Randomized clinical trial of extended spectrum antibiotic prophylaxis with coverage for Ureaplasma urealyticum to reduce post-cesarean delivery endometritis. Obstet Gynecol. 2003;101:1183-1189.
11. Howard PA. Azithromycin-induced proarrhythmia and cardiovascular death. Ann Pharmacother. 2013;47:1547-1551.
Tamsulosin for patients with ureteral stones?
ILLUSTRATIVE CASE
A 54-year-old man presents to the emergency department (ED) with acute onset left flank pain that radiates to the groin. A computed tomography (CT) scan of the abdomen/pelvis without contrast reveals a 7-mm distal ureteral stone. He is deemed appropriate for outpatient management. In addition to pain medications, should you prescribe tamsulosin?
According to the most recent National Health and Nutrition Examination Survey, the population prevalence of kidney stones is 8.8% with a self-reported prevalence in men of 10.6% and a self-reported prevalence in women of 7.1%.2 Most ureteral stones can be treated in the outpatient setting with oral hydration, antiemetics, and pain control with nonsteroidal anti-inflammatory medications as first-line treatment and opioids as a second-line option.3 In addition, alpha-blockers are used for medical expulsive therapy (MET). In fact, the European Association of Urology guideline on urolithiasis states that MET may accelerate passage of ureteral stones.3
Recently, however, uncertainty has surrounded the effectiveness of the alpha-blocker tamsulosin. Two systematic reviews, limited by heterogeneity because some of the studies lacked a placebo control and blinding, concluded that alpha-blockers increased stone passage within one to 6 weeks when compared with placebo or no additional therapy.4,5 However, a recent large multicenter, randomized controlled trial (RCT) revealed no difference between tamsulosin and nifedipine or either one compared with placebo at decreasing the need for further treatment to achieve stone passage within 4 weeks.6
[polldaddy:9906038]
STUDY SUMMARY
New meta-analysis breaks down results by stone size
This meta-analysis by Wang et al, consisting of 8 randomized, double-blind, placebo-controlled trials of adult patients (N=1384), examined the effect of oral tamsulosin 0.4 mg/d (average of a 28-day course) on distal ureteral stone passage.1 A subgroup analysis comparing stone size (<5 mm and 5-10 mm) was also conducted to determine if stone size modified the effect of tamsulosin.
Although the initial search included studies published between 1966 and 2015, the 8 that were eventually analyzed were published between 2009 and 2015, were conducted in multiple countries (and included regardless of language), and were conducted in ED and outpatient urology settings. The main outcome measure was the risk difference in stone passage between the tamsulosin group and placebo group after follow-up imaging at 3 weeks with CT or plain film radiographs.
Tamsulosin helps some, but not all. The pooled risk of stone passage was higher in the tamsulosin group than in the placebo group (85% vs 66%; risk difference [RD]=17%; 95% confidence interval [CI], 6%-27%), but significant heterogeneity existed across the trials (I2=80.2%). After subgroup analysis by stone size, the researchers found that tamsulosin was beneficial for larger stones, 5 to 10 mm in size (6 trials, N=514; RD=22%; 95% CI, 12%-33%; number needed to treat=5), compared with placebo, but not for smaller stones, <5 mm in size (4 trials, N=533; RD=-0.3%; 95% CI, -4% to 3%). The measure of heterogeneity in the 5- to 10-mm subgroup demonstrated a less heterogeneous population of studies (I2=33%) than that for the <5-mm subgroup (I2=0%).
In terms of adverse events, tamsulosin did not increase the risk of dizziness (RD=.2%; 95% CI, -2.1% to 2.5%) or postural hypotension (RD=.1%; 95% CI, -0.4% to 0.5%) compared with placebo.
WHAT’S NEW
Passage of larger stones increases with tamsulosin
This meta-analysis included only randomized, double-blind, placebo-controlled trials. Prior meta-analyses did not. Also, this review included the SUSPEND (Spontaneous Urinary Stone Passage Enabled by Drugs) trial, an RCT discussed in a previous PURL (Kidney stones? It’s time to rethink those meds. J Fam Pract. 2016;65:118-120.) that recommended against the alpha-blockers tamsulosin and nifedipine for ureteral stones measuring <10 mm.6,7
But the subgroup analysis in this more recent review went one step further in the investigation of tamsulosin’s effect by examining passage rates by stone size (<5 mm vs 5-10 mm) and revealing that passage of larger stones (5-10 mm) increased with tamsulosin. The different results based on stone size may explain the recent uncertainty as to whether tamsulosin improves the rate of stone passage.
CAVEATS
Study doesn’t address proximal, or extra-large stones
Only distal stones were included in 7 of the 8 trials. Thus, this meta-analysis was unable to determine the effect on more proximal stones. Also, it’s unclear if the drug provides any benefit with stones >10 mm in size.
CHALLENGES TO IMPLEMENTATION
None worth mentioning
We see no challenges to implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Wang RC, Smith-Bindman R, Whitaker E, et al. Effect of tamsulosin on stone passage for ureteral stones: a systematic review and meta-analysis. Ann Emerg Med. 2017;69:353-361.
2. Scales CD Jr, Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160-165.
3. Türk C, Petrik A, Sarica K, et al. EAU guidelines on diagnosis and conservative management of urolithiasis. Eur Urol. 2016;69:468-474.
4. Hollingsworth JM, Canales BK, Rogers MAM, et al. Alpha blockers for treatment of ureteric stones: systematic review and meta-analysis. BMJ. 2016;355:i6112.
5. Campschroer T, Zhu Y, Duijvesz D, et al. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014:CD008509.
6. Pickard R, Starr K, MacLennan G, et al. Medical expulsion therapy in adults with ureteric colic: a multicentre, randomized, placebo-controlled trial. Lancet. 2015;386:341-349.
7. Slattengren AH, Prasad S, Jarrett JB. Kidney stones? It’s time to rethink those meds. J Fam Pract. 2016;65:118-120.
ILLUSTRATIVE CASE
A 54-year-old man presents to the emergency department (ED) with acute onset left flank pain that radiates to the groin. A computed tomography (CT) scan of the abdomen/pelvis without contrast reveals a 7-mm distal ureteral stone. He is deemed appropriate for outpatient management. In addition to pain medications, should you prescribe tamsulosin?
According to the most recent National Health and Nutrition Examination Survey, the population prevalence of kidney stones is 8.8% with a self-reported prevalence in men of 10.6% and a self-reported prevalence in women of 7.1%.2 Most ureteral stones can be treated in the outpatient setting with oral hydration, antiemetics, and pain control with nonsteroidal anti-inflammatory medications as first-line treatment and opioids as a second-line option.3 In addition, alpha-blockers are used for medical expulsive therapy (MET). In fact, the European Association of Urology guideline on urolithiasis states that MET may accelerate passage of ureteral stones.3
Recently, however, uncertainty has surrounded the effectiveness of the alpha-blocker tamsulosin. Two systematic reviews, limited by heterogeneity because some of the studies lacked a placebo control and blinding, concluded that alpha-blockers increased stone passage within one to 6 weeks when compared with placebo or no additional therapy.4,5 However, a recent large multicenter, randomized controlled trial (RCT) revealed no difference between tamsulosin and nifedipine or either one compared with placebo at decreasing the need for further treatment to achieve stone passage within 4 weeks.6
[polldaddy:9906038]
STUDY SUMMARY
New meta-analysis breaks down results by stone size
This meta-analysis by Wang et al, consisting of 8 randomized, double-blind, placebo-controlled trials of adult patients (N=1384), examined the effect of oral tamsulosin 0.4 mg/d (average of a 28-day course) on distal ureteral stone passage.1 A subgroup analysis comparing stone size (<5 mm and 5-10 mm) was also conducted to determine if stone size modified the effect of tamsulosin.
Although the initial search included studies published between 1966 and 2015, the 8 that were eventually analyzed were published between 2009 and 2015, were conducted in multiple countries (and included regardless of language), and were conducted in ED and outpatient urology settings. The main outcome measure was the risk difference in stone passage between the tamsulosin group and placebo group after follow-up imaging at 3 weeks with CT or plain film radiographs.
Tamsulosin helps some, but not all. The pooled risk of stone passage was higher in the tamsulosin group than in the placebo group (85% vs 66%; risk difference [RD]=17%; 95% confidence interval [CI], 6%-27%), but significant heterogeneity existed across the trials (I2=80.2%). After subgroup analysis by stone size, the researchers found that tamsulosin was beneficial for larger stones, 5 to 10 mm in size (6 trials, N=514; RD=22%; 95% CI, 12%-33%; number needed to treat=5), compared with placebo, but not for smaller stones, <5 mm in size (4 trials, N=533; RD=-0.3%; 95% CI, -4% to 3%). The measure of heterogeneity in the 5- to 10-mm subgroup demonstrated a less heterogeneous population of studies (I2=33%) than that for the <5-mm subgroup (I2=0%).
In terms of adverse events, tamsulosin did not increase the risk of dizziness (RD=.2%; 95% CI, -2.1% to 2.5%) or postural hypotension (RD=.1%; 95% CI, -0.4% to 0.5%) compared with placebo.
WHAT’S NEW
Passage of larger stones increases with tamsulosin
This meta-analysis included only randomized, double-blind, placebo-controlled trials. Prior meta-analyses did not. Also, this review included the SUSPEND (Spontaneous Urinary Stone Passage Enabled by Drugs) trial, an RCT discussed in a previous PURL (Kidney stones? It’s time to rethink those meds. J Fam Pract. 2016;65:118-120.) that recommended against the alpha-blockers tamsulosin and nifedipine for ureteral stones measuring <10 mm.6,7
But the subgroup analysis in this more recent review went one step further in the investigation of tamsulosin’s effect by examining passage rates by stone size (<5 mm vs 5-10 mm) and revealing that passage of larger stones (5-10 mm) increased with tamsulosin. The different results based on stone size may explain the recent uncertainty as to whether tamsulosin improves the rate of stone passage.
CAVEATS
Study doesn’t address proximal, or extra-large stones
Only distal stones were included in 7 of the 8 trials. Thus, this meta-analysis was unable to determine the effect on more proximal stones. Also, it’s unclear if the drug provides any benefit with stones >10 mm in size.
CHALLENGES TO IMPLEMENTATION
None worth mentioning
We see no challenges to implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 54-year-old man presents to the emergency department (ED) with acute onset left flank pain that radiates to the groin. A computed tomography (CT) scan of the abdomen/pelvis without contrast reveals a 7-mm distal ureteral stone. He is deemed appropriate for outpatient management. In addition to pain medications, should you prescribe tamsulosin?
According to the most recent National Health and Nutrition Examination Survey, the population prevalence of kidney stones is 8.8% with a self-reported prevalence in men of 10.6% and a self-reported prevalence in women of 7.1%.2 Most ureteral stones can be treated in the outpatient setting with oral hydration, antiemetics, and pain control with nonsteroidal anti-inflammatory medications as first-line treatment and opioids as a second-line option.3 In addition, alpha-blockers are used for medical expulsive therapy (MET). In fact, the European Association of Urology guideline on urolithiasis states that MET may accelerate passage of ureteral stones.3
Recently, however, uncertainty has surrounded the effectiveness of the alpha-blocker tamsulosin. Two systematic reviews, limited by heterogeneity because some of the studies lacked a placebo control and blinding, concluded that alpha-blockers increased stone passage within one to 6 weeks when compared with placebo or no additional therapy.4,5 However, a recent large multicenter, randomized controlled trial (RCT) revealed no difference between tamsulosin and nifedipine or either one compared with placebo at decreasing the need for further treatment to achieve stone passage within 4 weeks.6
[polldaddy:9906038]
STUDY SUMMARY
New meta-analysis breaks down results by stone size
This meta-analysis by Wang et al, consisting of 8 randomized, double-blind, placebo-controlled trials of adult patients (N=1384), examined the effect of oral tamsulosin 0.4 mg/d (average of a 28-day course) on distal ureteral stone passage.1 A subgroup analysis comparing stone size (<5 mm and 5-10 mm) was also conducted to determine if stone size modified the effect of tamsulosin.
Although the initial search included studies published between 1966 and 2015, the 8 that were eventually analyzed were published between 2009 and 2015, were conducted in multiple countries (and included regardless of language), and were conducted in ED and outpatient urology settings. The main outcome measure was the risk difference in stone passage between the tamsulosin group and placebo group after follow-up imaging at 3 weeks with CT or plain film radiographs.
Tamsulosin helps some, but not all. The pooled risk of stone passage was higher in the tamsulosin group than in the placebo group (85% vs 66%; risk difference [RD]=17%; 95% confidence interval [CI], 6%-27%), but significant heterogeneity existed across the trials (I2=80.2%). After subgroup analysis by stone size, the researchers found that tamsulosin was beneficial for larger stones, 5 to 10 mm in size (6 trials, N=514; RD=22%; 95% CI, 12%-33%; number needed to treat=5), compared with placebo, but not for smaller stones, <5 mm in size (4 trials, N=533; RD=-0.3%; 95% CI, -4% to 3%). The measure of heterogeneity in the 5- to 10-mm subgroup demonstrated a less heterogeneous population of studies (I2=33%) than that for the <5-mm subgroup (I2=0%).
In terms of adverse events, tamsulosin did not increase the risk of dizziness (RD=.2%; 95% CI, -2.1% to 2.5%) or postural hypotension (RD=.1%; 95% CI, -0.4% to 0.5%) compared with placebo.
WHAT’S NEW
Passage of larger stones increases with tamsulosin
This meta-analysis included only randomized, double-blind, placebo-controlled trials. Prior meta-analyses did not. Also, this review included the SUSPEND (Spontaneous Urinary Stone Passage Enabled by Drugs) trial, an RCT discussed in a previous PURL (Kidney stones? It’s time to rethink those meds. J Fam Pract. 2016;65:118-120.) that recommended against the alpha-blockers tamsulosin and nifedipine for ureteral stones measuring <10 mm.6,7
But the subgroup analysis in this more recent review went one step further in the investigation of tamsulosin’s effect by examining passage rates by stone size (<5 mm vs 5-10 mm) and revealing that passage of larger stones (5-10 mm) increased with tamsulosin. The different results based on stone size may explain the recent uncertainty as to whether tamsulosin improves the rate of stone passage.
CAVEATS
Study doesn’t address proximal, or extra-large stones
Only distal stones were included in 7 of the 8 trials. Thus, this meta-analysis was unable to determine the effect on more proximal stones. Also, it’s unclear if the drug provides any benefit with stones >10 mm in size.
CHALLENGES TO IMPLEMENTATION
None worth mentioning
We see no challenges to implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Wang RC, Smith-Bindman R, Whitaker E, et al. Effect of tamsulosin on stone passage for ureteral stones: a systematic review and meta-analysis. Ann Emerg Med. 2017;69:353-361.
2. Scales CD Jr, Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160-165.
3. Türk C, Petrik A, Sarica K, et al. EAU guidelines on diagnosis and conservative management of urolithiasis. Eur Urol. 2016;69:468-474.
4. Hollingsworth JM, Canales BK, Rogers MAM, et al. Alpha blockers for treatment of ureteric stones: systematic review and meta-analysis. BMJ. 2016;355:i6112.
5. Campschroer T, Zhu Y, Duijvesz D, et al. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014:CD008509.
6. Pickard R, Starr K, MacLennan G, et al. Medical expulsion therapy in adults with ureteric colic: a multicentre, randomized, placebo-controlled trial. Lancet. 2015;386:341-349.
7. Slattengren AH, Prasad S, Jarrett JB. Kidney stones? It’s time to rethink those meds. J Fam Pract. 2016;65:118-120.
1. Wang RC, Smith-Bindman R, Whitaker E, et al. Effect of tamsulosin on stone passage for ureteral stones: a systematic review and meta-analysis. Ann Emerg Med. 2017;69:353-361.
2. Scales CD Jr, Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160-165.
3. Türk C, Petrik A, Sarica K, et al. EAU guidelines on diagnosis and conservative management of urolithiasis. Eur Urol. 2016;69:468-474.
4. Hollingsworth JM, Canales BK, Rogers MAM, et al. Alpha blockers for treatment of ureteric stones: systematic review and meta-analysis. BMJ. 2016;355:i6112.
5. Campschroer T, Zhu Y, Duijvesz D, et al. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014:CD008509.
6. Pickard R, Starr K, MacLennan G, et al. Medical expulsion therapy in adults with ureteric colic: a multicentre, randomized, placebo-controlled trial. Lancet. 2015;386:341-349.
7. Slattengren AH, Prasad S, Jarrett JB. Kidney stones? It’s time to rethink those meds. J Fam Pract. 2016;65:118-120.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Prescribe tamsulosin for stone expulsion in patients with distal ureteral stones 5 to 10 mm in size.1
STRENGTH OF RECOMMENDATION
A: Based on a meta-analysis of randomized controlled trials.
Wang RC, Smith-Bindman R, Whitaker E, et al. Effect of tamsulosin on stone passage for ureteral stones: a systematic review and meta-analysis. Ann Emerg Med. 2017;69:353-361.
PPIs With Warfarin Regimens: Balancing the Perks and Pitfalls

A 60-year-old man establishes care with you. He has well-controlled osteoarthritis (as long as he takes his low-dose daily aspirin) and chronic atrial fibrillation, for which he takes warfarin. His international normalized ratio (INR) is consistently within the recommended target range of 2 to 3. He feels well and has never had GERD or a gastrointestinal (GI) bleed. Should you recommend a proton pump inhibitor (PPI) to decrease the likelihood of a future upper GI bleed?
Anticoagulation therapy creates a dilemma—the need to balance the benefit of preventing embolization with the risk for serious bleeding. Concurrent use of NSAIDs, aspirin, and other antiplatelet agents further increases the latter risk.2
Clinicians have long used PPIs to treat upper GI bleeds. They prevent acid secretion and are the most effective drugs for healing peptic ulcers.3,4 But while previous case-control studies show that PPIs reduce the risk for upper GI bleeds in patients taking antiplatelet agents or NSAIDs, they do not show a statistically significant benefit for patients taking warfarin.5,6 What’s more, while one expert consensus report recommends that patients taking dual warfarin and antiplatelet agent/NSAID therapy take a PPI to decrease the risk for upper GI bleeding, other guidelines do not address this clinical question.2,7,8
STUDY SUMMARY
Study supports PPI use in a high-risk group
This retrospective cohort study sought to answer the question: “Does PPI co-therapy decrease the rate of serious upper GI bleeds in patients taking warfarin?” Researchers examined rates of hospitalization for upper GI bleeding in Medicare and Medicaid patients taking warfarin, with and without PPI co-therapy (tracked via prescription fill dates). They also evaluated concomitant use of NSAIDs and antiplatelet agents.
The authors excluded patients with a recent history of severe bleeding or certain illnesses that predispose patients to GI bleeding (eg, esophageal varices). Patients with risk factors for an upper GI bleed (eg, abdominal pain, peptic ulcer disease, anemia) were more likely to be taking PPI co-therapy. Researchers analyzed the effect of PPI co-therapy in patients with and without these additional risk factors.
Results. The study followed more than 75,000 person-years of active warfarin therapy (Medicaid, > 52,000 person-years; Medicare, > 23,000 person-years). Hospitalizations due to upper GI bleeding occurred at a rate of 127/10,000 person-years (incidence was similar in both the Medicaid and Medicare groups).
Among all patients taking warfarin (regardless of whether they were also taking an NSAID or antiplatelet agent), PPI co-therapy reduced the risk for hospitalization for upper GI bleeding by 24% (adjusted hazard ratio [HR], 0.76), which translates into 29 fewer hospitalizations per 10,000 person-years. The number needed to treat (NNT) was 345 person-years, meaning that 345 patients taking warfarin would have to take a PPI for one year to prevent one hospitalization for an upper GI bleed. As one might expect, PPI co-therapy did not significantly reduce the risk for lower GI, other GI, or non-GI bleeding.
In patients taking both warfarin and concurrent antiplatelet agents or NSAIDs, PPI co-therapy reduced the risk for hospitalization for upper GI bleeding by about half (HR, 0.55). Hospitalizations decreased by 128/10,000 person-years (NNT, 78 person-years). For patients taking warfarin but not antiplatelet agents or NSAIDs, PPI co-therapy did not significantly reduce the risk for hospitalization due to upper GI bleeding (HR, 0.86).
Additional risk factors for GI bleeds. Researchers also looked at patients who had additional risk factors for GI bleeds (other than the exclusion criteria). For patients taking both warfarin and an antiplatelet agent/NSAID, PPI co-therapy decreased the risk for upper GI bleeding regardless of whether the patients had other bleeding risk factors. Again, for patients who had additional bleeding risk factors, but were not taking an antiplatelet agent or NSAID, PPI therapy showed no statistically significant effect.
WHAT’S NEW
PPIs offer benefits, but not to warfarin-only patients
The statistically significant results in this large observational study suggest that PPI co-therapy is beneficial in reducing the risk for upper GI bleeding in patients taking warfarin plus an antiplatelet agent/NSAID, but that PPI co-therapy provides no benefit to patients taking warfarin exclusively.
CAVEATS
Not a randomized controlled trial
This study was observational, not a randomized control trial (RCT). Therefore, unknown confounding variables may have skewed results. For example, patients could have taken OTC medications that influenced or obscured results but were not included in the data analysis (misclassification bias).
At best, we can infer a correlation between PPIs and decreased risk for upper GI bleeds. We need RCTs to determine whether PPIs cause a risk reduction.
Don’t overlook the risks of PPIs. This study assessed the ability of PPIs to prevent bleeds but did not address the risks of long-term PPI therapy. Adverse effects of PPIs include increased risk for pneumonia, infection with Clostridium difficile, hip and spinal fractures, anemia, and possibly chronic kidney disease and dementia.9-11 In addition, cost-analysis studies of PPI therapy are limited and their results are inconsistent.12 Therefore, it’s best to make decisions regarding PPIs after discussing other risks and benefits.
What about DOACs? Another option is to prescribe a direct oral anticoagulant (DOAC; eg, dabigatran, rivaroxaban, or apixaban) instead of warfarin. DOACs are at least as effective as warfarin at preventing stroke in patients with atrial fibrillation and may even be safer.13 Dabigatran 110 mg causes fewer “major bleeding” events than warfarin.13 Compared to warfarin, rivaroxaban has been shown to result in fewer fatal bleeding events due to intracranial bleeds, although it is associated with more GI bleeding.13 Apixaban is associated with fewer GI bleeds and lower bleeding rates overall, compared with warfarin.13 Further research is warranted to determine if PPI therapy is beneficial to patients who are taking DOACs.
CHALLENGES TO IMPLEMENTATION
It’s still a balancing act
When long-term anticoagulation is necessary, providers and patients must attempt to prevent thrombotic events while minimizing the risk for GI bleeds. PPIs may be beneficial in preventing upper GI bleeds in patients taking dual warfarin and antiplatelet therapy, but the long-term consequences of PPI therapy should not be ignored.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[11]:694-696).
1. Ray WA, Chung CP, Murray KT, et al. Association of proton pump inhibitors with reduced risk of warfarin-related serious upper gastrointestinal bleeding. Gastroenterology. 2016;151:1105-1112.
2. Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2008;52:1502-1517.
3. Salas M, Ward A, Caro J. Are proton pump inhibitors the first choice for acute treatment of gastric ulcers? A meta analysis of randomized clinical trials. BMC Gastroenterol. 2002;2:17.
4. Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep. 2008;10:528-534.
5. Lanas A, García-Rodríguez LA, Arroyo MT, et al. Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol. 2007;102:507-515.
6. Lin KJ, Hernández-Díaz S, García Rodríguez LA. Acid suppressants reduce risk of gastrointestinal bleeding in patients on antithrombotic or anti-inflammatory therapy. Gastroenterology. 2011;141:71-79.
7. Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 suppl):160S-198S.
8. Schulman S, Beyth RJ, Kearon C, et al. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 suppl):257S-298S.
9. Ament PW, Dicola DB, James ME. Reducing adverse effects of proton pump inhibitors. Am Fam Physician. 2012;86:66-70.
10. Gomm W, von Holt HK, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73:410-416.
11. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176:238-246.
12. Smeets HM, Hoes AW, de Wit NJ. Effectiveness and costs of implementation strategies to reduce acid suppressive drug prescriptions: a systematic review. BMC Health Serv Res. 2007;7:177.
13. Hanley CM, Kowey PR. Are the novel anticoagulants better than warfarin for patients with atrial fibrillation? J Thorac Dis. 2015;7:165-171.

A 60-year-old man establishes care with you. He has well-controlled osteoarthritis (as long as he takes his low-dose daily aspirin) and chronic atrial fibrillation, for which he takes warfarin. His international normalized ratio (INR) is consistently within the recommended target range of 2 to 3. He feels well and has never had GERD or a gastrointestinal (GI) bleed. Should you recommend a proton pump inhibitor (PPI) to decrease the likelihood of a future upper GI bleed?
Anticoagulation therapy creates a dilemma—the need to balance the benefit of preventing embolization with the risk for serious bleeding. Concurrent use of NSAIDs, aspirin, and other antiplatelet agents further increases the latter risk.2
Clinicians have long used PPIs to treat upper GI bleeds. They prevent acid secretion and are the most effective drugs for healing peptic ulcers.3,4 But while previous case-control studies show that PPIs reduce the risk for upper GI bleeds in patients taking antiplatelet agents or NSAIDs, they do not show a statistically significant benefit for patients taking warfarin.5,6 What’s more, while one expert consensus report recommends that patients taking dual warfarin and antiplatelet agent/NSAID therapy take a PPI to decrease the risk for upper GI bleeding, other guidelines do not address this clinical question.2,7,8
STUDY SUMMARY
Study supports PPI use in a high-risk group
This retrospective cohort study sought to answer the question: “Does PPI co-therapy decrease the rate of serious upper GI bleeds in patients taking warfarin?” Researchers examined rates of hospitalization for upper GI bleeding in Medicare and Medicaid patients taking warfarin, with and without PPI co-therapy (tracked via prescription fill dates). They also evaluated concomitant use of NSAIDs and antiplatelet agents.
The authors excluded patients with a recent history of severe bleeding or certain illnesses that predispose patients to GI bleeding (eg, esophageal varices). Patients with risk factors for an upper GI bleed (eg, abdominal pain, peptic ulcer disease, anemia) were more likely to be taking PPI co-therapy. Researchers analyzed the effect of PPI co-therapy in patients with and without these additional risk factors.
Results. The study followed more than 75,000 person-years of active warfarin therapy (Medicaid, > 52,000 person-years; Medicare, > 23,000 person-years). Hospitalizations due to upper GI bleeding occurred at a rate of 127/10,000 person-years (incidence was similar in both the Medicaid and Medicare groups).
Among all patients taking warfarin (regardless of whether they were also taking an NSAID or antiplatelet agent), PPI co-therapy reduced the risk for hospitalization for upper GI bleeding by 24% (adjusted hazard ratio [HR], 0.76), which translates into 29 fewer hospitalizations per 10,000 person-years. The number needed to treat (NNT) was 345 person-years, meaning that 345 patients taking warfarin would have to take a PPI for one year to prevent one hospitalization for an upper GI bleed. As one might expect, PPI co-therapy did not significantly reduce the risk for lower GI, other GI, or non-GI bleeding.
In patients taking both warfarin and concurrent antiplatelet agents or NSAIDs, PPI co-therapy reduced the risk for hospitalization for upper GI bleeding by about half (HR, 0.55). Hospitalizations decreased by 128/10,000 person-years (NNT, 78 person-years). For patients taking warfarin but not antiplatelet agents or NSAIDs, PPI co-therapy did not significantly reduce the risk for hospitalization due to upper GI bleeding (HR, 0.86).
Additional risk factors for GI bleeds. Researchers also looked at patients who had additional risk factors for GI bleeds (other than the exclusion criteria). For patients taking both warfarin and an antiplatelet agent/NSAID, PPI co-therapy decreased the risk for upper GI bleeding regardless of whether the patients had other bleeding risk factors. Again, for patients who had additional bleeding risk factors, but were not taking an antiplatelet agent or NSAID, PPI therapy showed no statistically significant effect.
WHAT’S NEW
PPIs offer benefits, but not to warfarin-only patients
The statistically significant results in this large observational study suggest that PPI co-therapy is beneficial in reducing the risk for upper GI bleeding in patients taking warfarin plus an antiplatelet agent/NSAID, but that PPI co-therapy provides no benefit to patients taking warfarin exclusively.
CAVEATS
Not a randomized controlled trial
This study was observational, not a randomized control trial (RCT). Therefore, unknown confounding variables may have skewed results. For example, patients could have taken OTC medications that influenced or obscured results but were not included in the data analysis (misclassification bias).
At best, we can infer a correlation between PPIs and decreased risk for upper GI bleeds. We need RCTs to determine whether PPIs cause a risk reduction.
Don’t overlook the risks of PPIs. This study assessed the ability of PPIs to prevent bleeds but did not address the risks of long-term PPI therapy. Adverse effects of PPIs include increased risk for pneumonia, infection with Clostridium difficile, hip and spinal fractures, anemia, and possibly chronic kidney disease and dementia.9-11 In addition, cost-analysis studies of PPI therapy are limited and their results are inconsistent.12 Therefore, it’s best to make decisions regarding PPIs after discussing other risks and benefits.
What about DOACs? Another option is to prescribe a direct oral anticoagulant (DOAC; eg, dabigatran, rivaroxaban, or apixaban) instead of warfarin. DOACs are at least as effective as warfarin at preventing stroke in patients with atrial fibrillation and may even be safer.13 Dabigatran 110 mg causes fewer “major bleeding” events than warfarin.13 Compared to warfarin, rivaroxaban has been shown to result in fewer fatal bleeding events due to intracranial bleeds, although it is associated with more GI bleeding.13 Apixaban is associated with fewer GI bleeds and lower bleeding rates overall, compared with warfarin.13 Further research is warranted to determine if PPI therapy is beneficial to patients who are taking DOACs.
CHALLENGES TO IMPLEMENTATION
It’s still a balancing act
When long-term anticoagulation is necessary, providers and patients must attempt to prevent thrombotic events while minimizing the risk for GI bleeds. PPIs may be beneficial in preventing upper GI bleeds in patients taking dual warfarin and antiplatelet therapy, but the long-term consequences of PPI therapy should not be ignored.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[11]:694-696).

A 60-year-old man establishes care with you. He has well-controlled osteoarthritis (as long as he takes his low-dose daily aspirin) and chronic atrial fibrillation, for which he takes warfarin. His international normalized ratio (INR) is consistently within the recommended target range of 2 to 3. He feels well and has never had GERD or a gastrointestinal (GI) bleed. Should you recommend a proton pump inhibitor (PPI) to decrease the likelihood of a future upper GI bleed?
Anticoagulation therapy creates a dilemma—the need to balance the benefit of preventing embolization with the risk for serious bleeding. Concurrent use of NSAIDs, aspirin, and other antiplatelet agents further increases the latter risk.2
Clinicians have long used PPIs to treat upper GI bleeds. They prevent acid secretion and are the most effective drugs for healing peptic ulcers.3,4 But while previous case-control studies show that PPIs reduce the risk for upper GI bleeds in patients taking antiplatelet agents or NSAIDs, they do not show a statistically significant benefit for patients taking warfarin.5,6 What’s more, while one expert consensus report recommends that patients taking dual warfarin and antiplatelet agent/NSAID therapy take a PPI to decrease the risk for upper GI bleeding, other guidelines do not address this clinical question.2,7,8
STUDY SUMMARY
Study supports PPI use in a high-risk group
This retrospective cohort study sought to answer the question: “Does PPI co-therapy decrease the rate of serious upper GI bleeds in patients taking warfarin?” Researchers examined rates of hospitalization for upper GI bleeding in Medicare and Medicaid patients taking warfarin, with and without PPI co-therapy (tracked via prescription fill dates). They also evaluated concomitant use of NSAIDs and antiplatelet agents.
The authors excluded patients with a recent history of severe bleeding or certain illnesses that predispose patients to GI bleeding (eg, esophageal varices). Patients with risk factors for an upper GI bleed (eg, abdominal pain, peptic ulcer disease, anemia) were more likely to be taking PPI co-therapy. Researchers analyzed the effect of PPI co-therapy in patients with and without these additional risk factors.
Results. The study followed more than 75,000 person-years of active warfarin therapy (Medicaid, > 52,000 person-years; Medicare, > 23,000 person-years). Hospitalizations due to upper GI bleeding occurred at a rate of 127/10,000 person-years (incidence was similar in both the Medicaid and Medicare groups).
Among all patients taking warfarin (regardless of whether they were also taking an NSAID or antiplatelet agent), PPI co-therapy reduced the risk for hospitalization for upper GI bleeding by 24% (adjusted hazard ratio [HR], 0.76), which translates into 29 fewer hospitalizations per 10,000 person-years. The number needed to treat (NNT) was 345 person-years, meaning that 345 patients taking warfarin would have to take a PPI for one year to prevent one hospitalization for an upper GI bleed. As one might expect, PPI co-therapy did not significantly reduce the risk for lower GI, other GI, or non-GI bleeding.
In patients taking both warfarin and concurrent antiplatelet agents or NSAIDs, PPI co-therapy reduced the risk for hospitalization for upper GI bleeding by about half (HR, 0.55). Hospitalizations decreased by 128/10,000 person-years (NNT, 78 person-years). For patients taking warfarin but not antiplatelet agents or NSAIDs, PPI co-therapy did not significantly reduce the risk for hospitalization due to upper GI bleeding (HR, 0.86).
Additional risk factors for GI bleeds. Researchers also looked at patients who had additional risk factors for GI bleeds (other than the exclusion criteria). For patients taking both warfarin and an antiplatelet agent/NSAID, PPI co-therapy decreased the risk for upper GI bleeding regardless of whether the patients had other bleeding risk factors. Again, for patients who had additional bleeding risk factors, but were not taking an antiplatelet agent or NSAID, PPI therapy showed no statistically significant effect.
WHAT’S NEW
PPIs offer benefits, but not to warfarin-only patients
The statistically significant results in this large observational study suggest that PPI co-therapy is beneficial in reducing the risk for upper GI bleeding in patients taking warfarin plus an antiplatelet agent/NSAID, but that PPI co-therapy provides no benefit to patients taking warfarin exclusively.
CAVEATS
Not a randomized controlled trial
This study was observational, not a randomized control trial (RCT). Therefore, unknown confounding variables may have skewed results. For example, patients could have taken OTC medications that influenced or obscured results but were not included in the data analysis (misclassification bias).
At best, we can infer a correlation between PPIs and decreased risk for upper GI bleeds. We need RCTs to determine whether PPIs cause a risk reduction.
Don’t overlook the risks of PPIs. This study assessed the ability of PPIs to prevent bleeds but did not address the risks of long-term PPI therapy. Adverse effects of PPIs include increased risk for pneumonia, infection with Clostridium difficile, hip and spinal fractures, anemia, and possibly chronic kidney disease and dementia.9-11 In addition, cost-analysis studies of PPI therapy are limited and their results are inconsistent.12 Therefore, it’s best to make decisions regarding PPIs after discussing other risks and benefits.
What about DOACs? Another option is to prescribe a direct oral anticoagulant (DOAC; eg, dabigatran, rivaroxaban, or apixaban) instead of warfarin. DOACs are at least as effective as warfarin at preventing stroke in patients with atrial fibrillation and may even be safer.13 Dabigatran 110 mg causes fewer “major bleeding” events than warfarin.13 Compared to warfarin, rivaroxaban has been shown to result in fewer fatal bleeding events due to intracranial bleeds, although it is associated with more GI bleeding.13 Apixaban is associated with fewer GI bleeds and lower bleeding rates overall, compared with warfarin.13 Further research is warranted to determine if PPI therapy is beneficial to patients who are taking DOACs.
CHALLENGES TO IMPLEMENTATION
It’s still a balancing act
When long-term anticoagulation is necessary, providers and patients must attempt to prevent thrombotic events while minimizing the risk for GI bleeds. PPIs may be beneficial in preventing upper GI bleeds in patients taking dual warfarin and antiplatelet therapy, but the long-term consequences of PPI therapy should not be ignored.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[11]:694-696).
1. Ray WA, Chung CP, Murray KT, et al. Association of proton pump inhibitors with reduced risk of warfarin-related serious upper gastrointestinal bleeding. Gastroenterology. 2016;151:1105-1112.
2. Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2008;52:1502-1517.
3. Salas M, Ward A, Caro J. Are proton pump inhibitors the first choice for acute treatment of gastric ulcers? A meta analysis of randomized clinical trials. BMC Gastroenterol. 2002;2:17.
4. Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep. 2008;10:528-534.
5. Lanas A, García-Rodríguez LA, Arroyo MT, et al. Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol. 2007;102:507-515.
6. Lin KJ, Hernández-Díaz S, García Rodríguez LA. Acid suppressants reduce risk of gastrointestinal bleeding in patients on antithrombotic or anti-inflammatory therapy. Gastroenterology. 2011;141:71-79.
7. Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 suppl):160S-198S.
8. Schulman S, Beyth RJ, Kearon C, et al. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 suppl):257S-298S.
9. Ament PW, Dicola DB, James ME. Reducing adverse effects of proton pump inhibitors. Am Fam Physician. 2012;86:66-70.
10. Gomm W, von Holt HK, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73:410-416.
11. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176:238-246.
12. Smeets HM, Hoes AW, de Wit NJ. Effectiveness and costs of implementation strategies to reduce acid suppressive drug prescriptions: a systematic review. BMC Health Serv Res. 2007;7:177.
13. Hanley CM, Kowey PR. Are the novel anticoagulants better than warfarin for patients with atrial fibrillation? J Thorac Dis. 2015;7:165-171.
1. Ray WA, Chung CP, Murray KT, et al. Association of proton pump inhibitors with reduced risk of warfarin-related serious upper gastrointestinal bleeding. Gastroenterology. 2016;151:1105-1112.
2. Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2008;52:1502-1517.
3. Salas M, Ward A, Caro J. Are proton pump inhibitors the first choice for acute treatment of gastric ulcers? A meta analysis of randomized clinical trials. BMC Gastroenterol. 2002;2:17.
4. Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep. 2008;10:528-534.
5. Lanas A, García-Rodríguez LA, Arroyo MT, et al. Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol. 2007;102:507-515.
6. Lin KJ, Hernández-Díaz S, García Rodríguez LA. Acid suppressants reduce risk of gastrointestinal bleeding in patients on antithrombotic or anti-inflammatory therapy. Gastroenterology. 2011;141:71-79.
7. Ansell J, Hirsh J, Hylek E, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 suppl):160S-198S.
8. Schulman S, Beyth RJ, Kearon C, et al. Hemorrhagic complications of anticoagulant and thrombolytic treatment: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6 suppl):257S-298S.
9. Ament PW, Dicola DB, James ME. Reducing adverse effects of proton pump inhibitors. Am Fam Physician. 2012;86:66-70.
10. Gomm W, von Holt HK, Thomé F, et al. Association of proton pump inhibitors with risk of dementia: a pharmacoepidemiological claims data analysis. JAMA Neurol. 2016;73:410-416.
11. Lazarus B, Chen Y, Wilson FP, et al. Proton pump inhibitor use and the risk of chronic kidney disease. JAMA Intern Med. 2016;176:238-246.
12. Smeets HM, Hoes AW, de Wit NJ. Effectiveness and costs of implementation strategies to reduce acid suppressive drug prescriptions: a systematic review. BMC Health Serv Res. 2007;7:177.
13. Hanley CM, Kowey PR. Are the novel anticoagulants better than warfarin for patients with atrial fibrillation? J Thorac Dis. 2015;7:165-171.