User login
A New View for the VA
In June 2015, David J. Shulkin, MD was sworn in as VA Under Secretary of Health—a position that had been empty for more than a year following the resignation of Robert Petzel, MD, in the wake of the Phoenix wait-time controversy. Carolyn M. Clancy, MD, had filled the position on an interim basis. Federal Practitioner recently sat down with Dr. Shulkin to discuss his plans for improving both health care quality and employee morale across the VA, which has been sapped by the recent scandals.
Building a New Health Care System
VA Under Secretary of Health David J. Shulkin, MD.I think there is an overlap between the design of a new health care system and having high levels of staff satisfaction. To me, it starts with being able to provide your employees, physicians, and staff with the types of tools and resources that they need to be able to take care of patients and a work environment that allows them to feel that they can practice to the best of their professional levels.
When I go around to visit VA facilities across the country, I see tremendous variation. I see some facilities that have invested in great programs, so their staff feels like they are practicing in world class facilities. And I see other places [where] it looks like they’re practicing 30 years ago.
The plan that we’ve put forth proposes that we invest our resources in a way that allows everyone who works in the VA to feel like they are working in a world class center. But that also means that we won’t be able to do everything for everybody. When you make investments, it means that you’re going to invest in one place and not everywhere. So there are some things that the VA may actually no longer do.
However, our plan allows us to develop the types of facilities where people will want to work and continue to want to work, by leveraging what already exists that’s being done well in the private community.
Applying Best Practices to the VA
Dr. Shulkin. Implementing best practices is not the same to me as standardizing everything. I think that all you have to do as a physician is reflect back upon medical school. Almost everything I learned in medical school is no longer relevant and none of the drugs. So there is a recognition that in order to be good at something, you need to be continually challenging the evidence that you have and the beliefs that you have and learning and improving and evolving and innovating. So implementing best practices, to me, is not stifling innovation or stifling experimentation. What it says is that if you have an organization as big as ours and somebody is doing something well, others should be learning from it and adopting it.
The example I point to is James A. Haley Veterans’ Hospital in Tampa, Florida. They’re doing same-day access primary care. They have no wait times. That’s our issue, wait times, and here’s a place that has no wait times. And the staff love it and the patients love it.
Why isn’t every VA doing that? I can’t think of a reason. It doesn’t mean they’re going to have to do it exactly the same way, and it doesn’t mean 5 years from now there won’t be a different way of having patients get appointments and access to care. But today, that’s the best practice that I can see, and I want every VA doing that.
Bringing an Outside Perspective
Dr. Shulkin. There were times when having an outside person come in to be Under Secretary would have been a challenge. But I think right now, given where the VA is, there is a recognition that having outside eyes is a good thing. Within the VA, there is a thirst for understanding how problems are solved in the private sector, and there is a real openness to new ideas. I think this comes from a recognition that it doesn’t feel good to be working as hard as I know most VA professionals are and continuing to see themselves bashed in the press
So people are open to new ideas, and they want to get beyond where we are. Having outside eyes on this is seen, today, as a good thing. But it needs to be done carefully and in a way that respects the things that have made the VA great, the good parts of the culture, and the hard work of the professionals who are caring for veterans every day. This can’t be a message of changing everything in the VA because, clearly, there are lots of things that are working really well. But there are areas where we can benefit from perspectives outside of the VA, and that’s what I’m trying to bring to the organization.
Increased Hiring Within the VA
Dr. Shulkin. A large part of the Veterans Choice Act funds that were authorized in 2014 were targeted toward new hires. The VA has hired a net increase of 14,000 employees. I think there were 1,600 net new physicians and 3,500 net new nurses. We’ve hired professionals in almost every part of the organization, including homeless coordinators, social workers, and pharmacists. You’ve seen hiring across the board. I think that you will continue to see that, but it will be more targeted toward areas we’ve identified that will impact access the most and where the shortages are the greatest.
Primary care doctors and psychiatrists will be 2 areas where there will be targeted hiring, and there are specialty areas that, depending on the geography, that will be targeted for hiring as well. Depending upon what our budget looks like, which we still don’t yet know for 2016, 2017, and 2018, we will see how much additional flexibility may be there for hiring.
Cost vs Quality of Care Debate: Lessons Learned From Hepatitis C Care
Dr. Shulkin. I think the challenge and the lessons that we’re trying to learn from the hepatitis C example was that when we put our 2015 budget together, we didn’t even know that new hepatitis C drug [cures] existed. We were looking at the older version—the interferons, which of course, weren’t curative in the same way. By the time that our budget actually hit, there was a new drug that offered new hope. Frankly, for the VA, that amounted to about a billion dollars in unfunded monies, because we didn’t know about the drug when we put that budget together. So that is a challenge for us.
Having said that, I’m very proud of the way that VA responded by moving money around to make sure that veterans got the right care. Nobody in this country has treated more veterans with hepatitis C than the VA. Nobody even comes close. More than 35,000 veterans received treatment for hepatitis C that’s curative in 2015. Nobody does it by addressing disparities in health care the way the VA does. We reach out to those that are in most need. Those with the mental health issues. Those that are essentially socially isolated. We’re calling them and bringing them in. No other health system does this. The VA has actually shown why it’s a great organization in responding this way. 2016 and beyond, we are committed to trying to find ways to do that. We will work with our ethics people, our hepatologists, our policy people, Congress, and drug makers to make sure that we can do the best we can for veterans.
Providing Long-term, Quality Care While Offering Options to Veterans
Dr. Shulkin.The VA is not a voucher program. This is not sending people out into the community to find their own care. VA health care is a well-integrated coordinated plan for people who we feel a responsibility for, for life. They are going to be VA patients as long as they want to be VA patients. But this is our responsibility, so that when they go out into the community, their care needs to be coordinated and tied back into VA health care. It can’t be seen as a separate health care system or a fragmented health care system.
That’s the problem that we’ve learned in health care, both in the VA and outside the VA. When you fragment care, when you separate care, that’s where you find quality problems and gaps in care that lead to people missing necessary testing or treatments that they need.
We deliberately designed Veterans’ Choice to address these issues, because these are our patients and our responsibility. When they go into the community, it doesn’t mean they leave VA. It means they’re getting care in the community as part of the VA health care system.
In June 2015, David J. Shulkin, MD was sworn in as VA Under Secretary of Health—a position that had been empty for more than a year following the resignation of Robert Petzel, MD, in the wake of the Phoenix wait-time controversy. Carolyn M. Clancy, MD, had filled the position on an interim basis. Federal Practitioner recently sat down with Dr. Shulkin to discuss his plans for improving both health care quality and employee morale across the VA, which has been sapped by the recent scandals.
Building a New Health Care System
VA Under Secretary of Health David J. Shulkin, MD.I think there is an overlap between the design of a new health care system and having high levels of staff satisfaction. To me, it starts with being able to provide your employees, physicians, and staff with the types of tools and resources that they need to be able to take care of patients and a work environment that allows them to feel that they can practice to the best of their professional levels.
When I go around to visit VA facilities across the country, I see tremendous variation. I see some facilities that have invested in great programs, so their staff feels like they are practicing in world class facilities. And I see other places [where] it looks like they’re practicing 30 years ago.
The plan that we’ve put forth proposes that we invest our resources in a way that allows everyone who works in the VA to feel like they are working in a world class center. But that also means that we won’t be able to do everything for everybody. When you make investments, it means that you’re going to invest in one place and not everywhere. So there are some things that the VA may actually no longer do.
However, our plan allows us to develop the types of facilities where people will want to work and continue to want to work, by leveraging what already exists that’s being done well in the private community.
Applying Best Practices to the VA
Dr. Shulkin. Implementing best practices is not the same to me as standardizing everything. I think that all you have to do as a physician is reflect back upon medical school. Almost everything I learned in medical school is no longer relevant and none of the drugs. So there is a recognition that in order to be good at something, you need to be continually challenging the evidence that you have and the beliefs that you have and learning and improving and evolving and innovating. So implementing best practices, to me, is not stifling innovation or stifling experimentation. What it says is that if you have an organization as big as ours and somebody is doing something well, others should be learning from it and adopting it.
The example I point to is James A. Haley Veterans’ Hospital in Tampa, Florida. They’re doing same-day access primary care. They have no wait times. That’s our issue, wait times, and here’s a place that has no wait times. And the staff love it and the patients love it.
Why isn’t every VA doing that? I can’t think of a reason. It doesn’t mean they’re going to have to do it exactly the same way, and it doesn’t mean 5 years from now there won’t be a different way of having patients get appointments and access to care. But today, that’s the best practice that I can see, and I want every VA doing that.
Bringing an Outside Perspective
Dr. Shulkin. There were times when having an outside person come in to be Under Secretary would have been a challenge. But I think right now, given where the VA is, there is a recognition that having outside eyes is a good thing. Within the VA, there is a thirst for understanding how problems are solved in the private sector, and there is a real openness to new ideas. I think this comes from a recognition that it doesn’t feel good to be working as hard as I know most VA professionals are and continuing to see themselves bashed in the press
So people are open to new ideas, and they want to get beyond where we are. Having outside eyes on this is seen, today, as a good thing. But it needs to be done carefully and in a way that respects the things that have made the VA great, the good parts of the culture, and the hard work of the professionals who are caring for veterans every day. This can’t be a message of changing everything in the VA because, clearly, there are lots of things that are working really well. But there are areas where we can benefit from perspectives outside of the VA, and that’s what I’m trying to bring to the organization.
Increased Hiring Within the VA
Dr. Shulkin. A large part of the Veterans Choice Act funds that were authorized in 2014 were targeted toward new hires. The VA has hired a net increase of 14,000 employees. I think there were 1,600 net new physicians and 3,500 net new nurses. We’ve hired professionals in almost every part of the organization, including homeless coordinators, social workers, and pharmacists. You’ve seen hiring across the board. I think that you will continue to see that, but it will be more targeted toward areas we’ve identified that will impact access the most and where the shortages are the greatest.
Primary care doctors and psychiatrists will be 2 areas where there will be targeted hiring, and there are specialty areas that, depending on the geography, that will be targeted for hiring as well. Depending upon what our budget looks like, which we still don’t yet know for 2016, 2017, and 2018, we will see how much additional flexibility may be there for hiring.
Cost vs Quality of Care Debate: Lessons Learned From Hepatitis C Care
Dr. Shulkin. I think the challenge and the lessons that we’re trying to learn from the hepatitis C example was that when we put our 2015 budget together, we didn’t even know that new hepatitis C drug [cures] existed. We were looking at the older version—the interferons, which of course, weren’t curative in the same way. By the time that our budget actually hit, there was a new drug that offered new hope. Frankly, for the VA, that amounted to about a billion dollars in unfunded monies, because we didn’t know about the drug when we put that budget together. So that is a challenge for us.
Having said that, I’m very proud of the way that VA responded by moving money around to make sure that veterans got the right care. Nobody in this country has treated more veterans with hepatitis C than the VA. Nobody even comes close. More than 35,000 veterans received treatment for hepatitis C that’s curative in 2015. Nobody does it by addressing disparities in health care the way the VA does. We reach out to those that are in most need. Those with the mental health issues. Those that are essentially socially isolated. We’re calling them and bringing them in. No other health system does this. The VA has actually shown why it’s a great organization in responding this way. 2016 and beyond, we are committed to trying to find ways to do that. We will work with our ethics people, our hepatologists, our policy people, Congress, and drug makers to make sure that we can do the best we can for veterans.
Providing Long-term, Quality Care While Offering Options to Veterans
Dr. Shulkin.The VA is not a voucher program. This is not sending people out into the community to find their own care. VA health care is a well-integrated coordinated plan for people who we feel a responsibility for, for life. They are going to be VA patients as long as they want to be VA patients. But this is our responsibility, so that when they go out into the community, their care needs to be coordinated and tied back into VA health care. It can’t be seen as a separate health care system or a fragmented health care system.
That’s the problem that we’ve learned in health care, both in the VA and outside the VA. When you fragment care, when you separate care, that’s where you find quality problems and gaps in care that lead to people missing necessary testing or treatments that they need.
We deliberately designed Veterans’ Choice to address these issues, because these are our patients and our responsibility. When they go into the community, it doesn’t mean they leave VA. It means they’re getting care in the community as part of the VA health care system.
In June 2015, David J. Shulkin, MD was sworn in as VA Under Secretary of Health—a position that had been empty for more than a year following the resignation of Robert Petzel, MD, in the wake of the Phoenix wait-time controversy. Carolyn M. Clancy, MD, had filled the position on an interim basis. Federal Practitioner recently sat down with Dr. Shulkin to discuss his plans for improving both health care quality and employee morale across the VA, which has been sapped by the recent scandals.
Building a New Health Care System
VA Under Secretary of Health David J. Shulkin, MD.I think there is an overlap between the design of a new health care system and having high levels of staff satisfaction. To me, it starts with being able to provide your employees, physicians, and staff with the types of tools and resources that they need to be able to take care of patients and a work environment that allows them to feel that they can practice to the best of their professional levels.
When I go around to visit VA facilities across the country, I see tremendous variation. I see some facilities that have invested in great programs, so their staff feels like they are practicing in world class facilities. And I see other places [where] it looks like they’re practicing 30 years ago.
The plan that we’ve put forth proposes that we invest our resources in a way that allows everyone who works in the VA to feel like they are working in a world class center. But that also means that we won’t be able to do everything for everybody. When you make investments, it means that you’re going to invest in one place and not everywhere. So there are some things that the VA may actually no longer do.
However, our plan allows us to develop the types of facilities where people will want to work and continue to want to work, by leveraging what already exists that’s being done well in the private community.
Applying Best Practices to the VA
Dr. Shulkin. Implementing best practices is not the same to me as standardizing everything. I think that all you have to do as a physician is reflect back upon medical school. Almost everything I learned in medical school is no longer relevant and none of the drugs. So there is a recognition that in order to be good at something, you need to be continually challenging the evidence that you have and the beliefs that you have and learning and improving and evolving and innovating. So implementing best practices, to me, is not stifling innovation or stifling experimentation. What it says is that if you have an organization as big as ours and somebody is doing something well, others should be learning from it and adopting it.
The example I point to is James A. Haley Veterans’ Hospital in Tampa, Florida. They’re doing same-day access primary care. They have no wait times. That’s our issue, wait times, and here’s a place that has no wait times. And the staff love it and the patients love it.
Why isn’t every VA doing that? I can’t think of a reason. It doesn’t mean they’re going to have to do it exactly the same way, and it doesn’t mean 5 years from now there won’t be a different way of having patients get appointments and access to care. But today, that’s the best practice that I can see, and I want every VA doing that.
Bringing an Outside Perspective
Dr. Shulkin. There were times when having an outside person come in to be Under Secretary would have been a challenge. But I think right now, given where the VA is, there is a recognition that having outside eyes is a good thing. Within the VA, there is a thirst for understanding how problems are solved in the private sector, and there is a real openness to new ideas. I think this comes from a recognition that it doesn’t feel good to be working as hard as I know most VA professionals are and continuing to see themselves bashed in the press
So people are open to new ideas, and they want to get beyond where we are. Having outside eyes on this is seen, today, as a good thing. But it needs to be done carefully and in a way that respects the things that have made the VA great, the good parts of the culture, and the hard work of the professionals who are caring for veterans every day. This can’t be a message of changing everything in the VA because, clearly, there are lots of things that are working really well. But there are areas where we can benefit from perspectives outside of the VA, and that’s what I’m trying to bring to the organization.
Increased Hiring Within the VA
Dr. Shulkin. A large part of the Veterans Choice Act funds that were authorized in 2014 were targeted toward new hires. The VA has hired a net increase of 14,000 employees. I think there were 1,600 net new physicians and 3,500 net new nurses. We’ve hired professionals in almost every part of the organization, including homeless coordinators, social workers, and pharmacists. You’ve seen hiring across the board. I think that you will continue to see that, but it will be more targeted toward areas we’ve identified that will impact access the most and where the shortages are the greatest.
Primary care doctors and psychiatrists will be 2 areas where there will be targeted hiring, and there are specialty areas that, depending on the geography, that will be targeted for hiring as well. Depending upon what our budget looks like, which we still don’t yet know for 2016, 2017, and 2018, we will see how much additional flexibility may be there for hiring.
Cost vs Quality of Care Debate: Lessons Learned From Hepatitis C Care
Dr. Shulkin. I think the challenge and the lessons that we’re trying to learn from the hepatitis C example was that when we put our 2015 budget together, we didn’t even know that new hepatitis C drug [cures] existed. We were looking at the older version—the interferons, which of course, weren’t curative in the same way. By the time that our budget actually hit, there was a new drug that offered new hope. Frankly, for the VA, that amounted to about a billion dollars in unfunded monies, because we didn’t know about the drug when we put that budget together. So that is a challenge for us.
Having said that, I’m very proud of the way that VA responded by moving money around to make sure that veterans got the right care. Nobody in this country has treated more veterans with hepatitis C than the VA. Nobody even comes close. More than 35,000 veterans received treatment for hepatitis C that’s curative in 2015. Nobody does it by addressing disparities in health care the way the VA does. We reach out to those that are in most need. Those with the mental health issues. Those that are essentially socially isolated. We’re calling them and bringing them in. No other health system does this. The VA has actually shown why it’s a great organization in responding this way. 2016 and beyond, we are committed to trying to find ways to do that. We will work with our ethics people, our hepatologists, our policy people, Congress, and drug makers to make sure that we can do the best we can for veterans.
Providing Long-term, Quality Care While Offering Options to Veterans
Dr. Shulkin.The VA is not a voucher program. This is not sending people out into the community to find their own care. VA health care is a well-integrated coordinated plan for people who we feel a responsibility for, for life. They are going to be VA patients as long as they want to be VA patients. But this is our responsibility, so that when they go out into the community, their care needs to be coordinated and tied back into VA health care. It can’t be seen as a separate health care system or a fragmented health care system.
That’s the problem that we’ve learned in health care, both in the VA and outside the VA. When you fragment care, when you separate care, that’s where you find quality problems and gaps in care that lead to people missing necessary testing or treatments that they need.
We deliberately designed Veterans’ Choice to address these issues, because these are our patients and our responsibility. When they go into the community, it doesn’t mean they leave VA. It means they’re getting care in the community as part of the VA health care system.
Managing Diabetes in Women of Childbearing Age
There were 13.4 million women (ages 20 and older) with either type 1 or type 2 diabetes in the United States in 2012, according to the CDC.1 By 2050, overall prevalence of diabetes is expected to double or triple.2 Since the number of women with diabetes will continue to increase, it is important for clinicians to familiarize themselves with management of the condition in those of childbearing age—particularly with regard to medication selection.
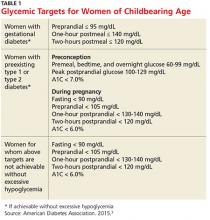
Diabetes management in women of childbearing age presents multiple complexities. First, strict glucose control from preconception through pregnancy is necessary to reduce the risk for complications in mother and fetus. The American Diabetes Association (ADA) recommends an A1C of less than 7% during the preconception period, if achievable without hypoglycemia.3 Full glycemic targets for women are outlined in Table 1.
Continue for medication classes with pregnancy category >>
Second, many medications used to manage diabetes and pregnancy-associated comorbidities can be fetotoxic. The FDA assigns all drugs to a pregnancy category, the definitions of which are available at http://chemm.nlm.nih.gov/pregnancycategories.htm.4 The ADA recommends that sexually active women of childbearing age avoid any potentially teratogenic medications (see Table 2) if they are not using reliable contraception.3
Excellent control of diabetes is necessary to decrease risk for birth defects. Infants born to mothers with preconception diabetes have been shown to have higher rates of morbidity and mortality.5 Infants born to women with diabetes are generally large for gestational age and experience hypoglycemia in the first 24 to 48 hours of life.6 Large-for-gestational-age babies are at increased risk for trauma at birth, including orthopedic injuries (eg, shoulder dislocation) and brachial plexus injuries. There is also an increased risk for fetal cardiac defects and congenital congestive heart failure.6
This article will review four cases of diabetes management in women of childbearing age. The ADA guidelines form the basis for all recommendations.
Continue for case 1 >>
Case 1 A 32-year-old obese woman with type 2 diabetes mellitus (T2DM) presents for routine follow-up. Recent lab results reveal an A1C of 6.4%; GFR > 100 mL/min/1.73 m2; and microalbuminuria (110 mg/d). She is currently taking lisinopril (2.5 mg once daily), metformin (1,000 mg bid), and glyburide (5 mg bid). She plans to become pregnant in the next six months and wants advice.
Discussion
This patient should be counseled on preconception glycemic targets and switched to pregnancy-safe medications. She should also be advised that the recommended weight gain in pregnancy for women with T2DM is 15 to 25 lb in overweight women and 10 to 20 lb in obese women.3
The ADA recommends a target A1C < 7%, in the absence of severe hypoglycemia, prior to conception in patients with type 1 diabetes mellitus (T1DM) or T2DM.3 For women with preconception diabetes who become pregnant, it is recommended that their premeal, bedtime, and overnight glucose be maintained at 60 to 99 mg/dL, their peak postprandial glucose at 100 to 129 mg/dL, and their A1C < 6% during pregnancy (all without excessive hypoglycemia), due to increases in red blood cell turnover.3 It is also recommended that they avoid statins, ACE inhibitors, angiotensin II receptor blockers (ARBs), certain beta blockers, and most noninsulin therapies.3
This patient is currently taking lisinopril, a medication with a pregnancy category of X. The ACE inhibitor class of medications is known to cause oligohydramnios, intrauterine growth retardation, structural malformation, premature birth, fetal renal dysplasia, and other congenital abnormalities, and use of these drugs should be avoided in women trying to conceive.7
Safer options for blood pressure control include clonidine, diltiazam, labetalol, methyldopa, or prazosin.3 Diuretics can reduce placental blood perfusion and should be avoided.8 An alternative for management of microalbuminuria in women of childbearing age is nifedipine.9 In multiple studies, this medication was not only safer in pregnancy, with no major teratogenic risk, but also effectively reduced urine microalbumin levels.10,11
For T2DM management, metformin (pregnancy category B) and glyburide (pregnancy category B/C, depending on manufacturer) can be used.12,13 Glyburide, the most studied sulfonylurea, is recommended as the drug of choice in its class.14-16 While insulin is the standard for managing diabetes in pregnancy—earlier research supported a switch from oral medications to insulin in women interested in becoming pregnant—recent studies have demonstrated that oral medications can be safely used.17 In addition, lifestyle changes (eg, carbohydrate counting, limited meal portions, and regular moderate exercise) prior to and during pregnancy can be beneficial for diabetes management.18,19
Also remind the patient to take regular prenatal vitamins. The US Preventive Services Task Force recommends that all women planning to become or capable of becoming pregnant take 400 to 800 µg supplements of folic acid daily.20 For women at high risk for neural tube defects or who have had a previous pregnancy with neural tube defects, 4 mg/d is recommended.21 In women with diabetes who are trying to conceive, a folic acid supplement of 5 mg/d is recommended, beginning three months prior to conception.22
Research shows that diabetic women are less likely to take folic acid supplementation during pregnancy. A study of 6,835 obese or overweight women with diabetes showed that only 35% reported daily folic acid supplementation.23 The study authors recommended all women of childbearing age, especially those who are obese or have diabetes, take folic acid daily.23 Encourage all women intending to become pregnant to start prenatal vitamin supplementation.
Continue for case 2 >>
Case 2 A 26-year-old obese patient, 28 weeks primigravida, presents for follow-up on her 3-hour glucose tolerance test. Results indicate a 3-hour glucose level of 148 mg/dL. The patient has a family history of T2DM and gestational diabetes.
Discussion
Gestational diabetes is defined by the ADA as diabetes diagnosed during the second or third trimester of pregnancy that is not T1DM or T2DM.3 The ADA recommends lifestyle management of gestational diabetes before medications are introduced. A1C should be maintained at 6% or less without hypoglycemia. In general, insulin is preferred over oral agents for treatment of gestational diabetes.3
There tends to be a spike in insulin resistance in the second or third trimester; women with preconception diabetes, for example, may require frequent increases in daily insulin dose to maintain glycemic levels, compared to the first trimester.3 A baseline ophthalmology exam should be performed in the first trimester for patients with preconception diabetes, with additional monitoring as needed.3
Following pregnancy, screening should be conducted for diabetes or prediabetes at six to 12 weeks’ postpartum and every one to three years afterward.3 The cumulative incidence of T2DM varies considerably among studies, ranging from 17% to 63% in five to 16 years postpartum.24,25 Thus, women with gestational diabetes should maintain lifestyle changes, including diet and exercise, to reduce the risk for T2DM later in life.
Continue for case 3 >>
Case 3 A 43-year-old woman with T1DM becomes pregnant while taking atorvastatin (20 mg), insulin detemir (18 units qhs), and insulin aspart with meals, as per her calculated insulin-to-carbohydrate ratio (ICR; 1 U aspart for 18 g carbohydrates) and insulin sensitivity factor (ISF; 1 U aspart for every 60 mg/dL above 130 mg/dL). Her biggest concern today is her medication list and potential adverse effects on the fetus. Her most recent A1C, two months ago, was 6.5%. She senses hypoglycemia at glucose levels of about 60 mg/dL and admits to having such measurements about twice per week.
Discussion
In this case, the patient needs to stop taking her statin and check her blood glucose regularly, as she is at increased risk for hypoglycemia. In their 2013 guidelines, the American College of Cardiology/American Heart Association stated that statins “should not be used in women of childbearing potential unless these women are using effective contraception and are not nursing.”26 This presents a major problem for many women of childbearing age with diabetes.
Statins are associated with a variety of congenital abnormalities, including fetal growth restriction and structural abnormalities in the fetus.27 It is advised that women planning for pregnancy avoid use of statins.28 If the patient has severe hypertriglyceridemia that puts her at risk for acute pancreatitis, fenofibrate (pregnancy category C) can be considered in the second and third trimesters.29,30
With T1DM in pregnancy, there is an increased risk for hypoglycemia in the first trimester.3 This risk increases as women adapt to more strict blood glucose control. Frequent recalculation of the ICR and ISF may be needed as the pregnancy progresses and weight gain occurs. Most insulin formulations are pregnancy class B, with the exception of glargine, degludec, and glulisine, which are pregnancy category C.3
Continue for case 4 >>
Case 4 A 21-year-old woman with T1DM wishes to start contraception but has concerns about long-term options. She seeks your advice in making a decision.
Discussion
For long-term pregnancy prevention, either the copper or progesterone-containing intrauterine device (IUD) is safe and effective for women with T1DM or T2DM.31 While the levonorgestrel IUD does not produce metabolic changes in T1DM, it has not yet been adequately studied in T2DM. Demographics suggest that young women with T2DM could become viable candidates for intrauterine contraception.31
The hormone-releasing “ring” has been found to be reliable and safe for women of late reproductive age with T1DM.32 Combined hormonal contraceptives and the transdermal contraceptive patch are best avoided to reduce risk for complications associated with estrogen-containing contraceptives (eg, venous thromboembolism and myocardial infarction).33
Continue for the conclusion >>
Conclusion
All women with diabetes should be counseled on glucose control prior to pregnancy. Achieving a goal A1C below 6% in the absence of hypoglycemia is recommended by the ADA.3 Long-term contraception options should be considered in women of childbearing age with diabetes to prevent pregnancy. Clinicians should carefully select medications for management of diabetes and its comorbidities in women planning to become pregnant. Healthy dietary habits and regular exercise should be encouraged in all patients with diabetes, especially prior to pregnancy.
References
1. CDC. National Diabetes Statistics Report, 2014. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed January 12, 2016.
2. CDC. Number of Americans with diabetes projected to double or triple by 2050. 2010. www.cdc.gov/media/pressrel/2010/r101022.html. Accessed January 12, 2016.
3. American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care. 2015;38(suppl 1):S1-S93.
4. Chemical Hazards Emergency Medical Management. FDA pregnancy categories. http://chemm.nlm.nih.gov/pregnancycategories.htm. Accessed January 12, 2016.
5. Weindling AM. Offspring of diabetic pregnancy: short-term outcomes. Semin Fetal Neonatal Med. 2009;14(2):111-118.
6. Kaneshiro NK. Infant of diabetic mother (2013). Medline Plus. www.nlm.nih.gov/medlineplus/ency/article/001597.htm. Accessed January 12, 2016.
7. Shotan A, Widerhorn J, Hurst A, Elkayam U. Risks of angiotensin-converting enzyme inhibition during pregnancy: experimental and clinical evidence, potential mechanisms, and recommendations for use. Am J Med. 1994;96(5):451-456.
8. Sibai BM. Treatment of hypertension in pregnant women. N Engl J Med. 1996;335 (4):257-265.
9. Ismail AA, Medhat I, Tawfic TA, Kholeif A. Evaluation of calcium-antagonists (nifedipine) in the treatment of pre-eclampsia. Int J Gynaecol Obstet. 1993;40:39-43.
10. Magee LA, Schick B, Donnenfeld AE, et al. The safety of calcium channel blockers in human pregnancy: a prospective, multicenter cohort study. Am J Obstet Gynecol. 1996;174(3):823-828.
11. Kattah AG, Garovic VD. The management of hypertension in pregnancy. Adv Chronic Kidney Dis. 2013;20(3):229-239.
12. Carroll DG, Kelley KW. Review of metformin and glyburide in the management of gestational diabetes. Pharm Pract (Granada). 2014;12(4):528.
13. Koren G. Glyburide and fetal safety; transplacental pharmacokinetic considerations. Reprod Toxicol. 2001;15(3):227-229.
14. Elliott BD, Langer O, Schenker S, Johnson RF. Insignificant transfer of glyburide occurs across the human placenta. Am J Obstet Gynecol. 1991;165:807-812.
15. Moore TR. Glyburide for the treatment of gestational diabetes: a critical appraisal. Diabetes Care. 2007;30(suppl 2):S209-S213.
16. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183-1197.
17. Kalra B, Gupta Y, Singla R, Kalra S. Use of oral anti-diabetic agents in pregnancy: a pragmatic approach. N Am J Med Sci. 2015; 7(1):6-12.
18. Zhang C, Ning Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: review of epidemiologic evidence. Am J Clin Nutr. 2011;94(6 suppl):1975S-1979S.
19. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260.
20. US Preventive Services Task Force. Folic acid to prevent neural tube defects: preventive medication, 2015. www.uspreventiveservices taskforce.org/Page/Document/Update SummaryFinal/folic-acid-to-prevent-neural-tube-defects-preventive-medication. Accessed January 12, 2016.
21. Cheschier N; ACOG Committee on Practice Bulletins—Obstetrics. Neural tube defects. ACOG Practice Bulletin no 44. Int J Gynaecol Obstet. 2003;83(1):123-133.
22. Blumer I, Hadar E, Hadden DR, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(11):4227-4249.
23. Case AP, Ramadhani TA, Canfield MA, et al. Folic acid supplementation among diabetic, overweight, or obese women of childbearing age. J Obstet Gynecol Neonatal Nurs. 2007;36(4):335-341.
24. Hanna FWF, Peters JR. Screening for gestational diabetes; past, present and future. Diabet Med. 2002;19:351-358.
25. Ben-haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabet Med. 2004;21(2):103-113.
26. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1-S45.
27. Patel C, Edgerton L, Flake D. What precautions should we use with statins for women of childbearing age? J Fam Pract. 2006; 55(1):75-77.
28. Kazmin A, Garcia-Bournissen F, Koren G. Risks of statin use during pregnancy: a systematic review. J Obstet Gynaecol Can. 2007;29(11):906-908.
29. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012; 97(9):2969-2989.
30. Saadi HF, Kurlander DJ, Erkins JM, Hoogwerf BJ. Severe hypertriglyceridemia and acute pancreatitis during pregnancy: treatment with gemfibrozil. Endocr Pract. 1999;5(1):33-36.
31. Goldstuck ND, Steyn PS. The intrauterine device in women with diabetes mellitus type I and II: a systematic review. ISRN Obstet Gynecol. 2013;2013:814062.
32. Grigoryan OR, Grodnitskaya EE, Andreeva EN, et al. Use of the NuvaRing hormone-releasing system in late reproductive-age women with type 1 diabetes mellitus. Gynecol Endocrinol. 2008;24(2):99-104.
33. Bonnema RA, McNamara MC, Spencer AL. Contraception choices in women with underlying medical conditions. Am Fam Physician. 2010;82(6):621-628.
There were 13.4 million women (ages 20 and older) with either type 1 or type 2 diabetes in the United States in 2012, according to the CDC.1 By 2050, overall prevalence of diabetes is expected to double or triple.2 Since the number of women with diabetes will continue to increase, it is important for clinicians to familiarize themselves with management of the condition in those of childbearing age—particularly with regard to medication selection.
Diabetes management in women of childbearing age presents multiple complexities. First, strict glucose control from preconception through pregnancy is necessary to reduce the risk for complications in mother and fetus. The American Diabetes Association (ADA) recommends an A1C of less than 7% during the preconception period, if achievable without hypoglycemia.3 Full glycemic targets for women are outlined in Table 1.
Continue for medication classes with pregnancy category >>
Second, many medications used to manage diabetes and pregnancy-associated comorbidities can be fetotoxic. The FDA assigns all drugs to a pregnancy category, the definitions of which are available at http://chemm.nlm.nih.gov/pregnancycategories.htm.4 The ADA recommends that sexually active women of childbearing age avoid any potentially teratogenic medications (see Table 2) if they are not using reliable contraception.3
Excellent control of diabetes is necessary to decrease risk for birth defects. Infants born to mothers with preconception diabetes have been shown to have higher rates of morbidity and mortality.5 Infants born to women with diabetes are generally large for gestational age and experience hypoglycemia in the first 24 to 48 hours of life.6 Large-for-gestational-age babies are at increased risk for trauma at birth, including orthopedic injuries (eg, shoulder dislocation) and brachial plexus injuries. There is also an increased risk for fetal cardiac defects and congenital congestive heart failure.6
This article will review four cases of diabetes management in women of childbearing age. The ADA guidelines form the basis for all recommendations.
Continue for case 1 >>
Case 1 A 32-year-old obese woman with type 2 diabetes mellitus (T2DM) presents for routine follow-up. Recent lab results reveal an A1C of 6.4%; GFR > 100 mL/min/1.73 m2; and microalbuminuria (110 mg/d). She is currently taking lisinopril (2.5 mg once daily), metformin (1,000 mg bid), and glyburide (5 mg bid). She plans to become pregnant in the next six months and wants advice.
Discussion
This patient should be counseled on preconception glycemic targets and switched to pregnancy-safe medications. She should also be advised that the recommended weight gain in pregnancy for women with T2DM is 15 to 25 lb in overweight women and 10 to 20 lb in obese women.3
The ADA recommends a target A1C < 7%, in the absence of severe hypoglycemia, prior to conception in patients with type 1 diabetes mellitus (T1DM) or T2DM.3 For women with preconception diabetes who become pregnant, it is recommended that their premeal, bedtime, and overnight glucose be maintained at 60 to 99 mg/dL, their peak postprandial glucose at 100 to 129 mg/dL, and their A1C < 6% during pregnancy (all without excessive hypoglycemia), due to increases in red blood cell turnover.3 It is also recommended that they avoid statins, ACE inhibitors, angiotensin II receptor blockers (ARBs), certain beta blockers, and most noninsulin therapies.3
This patient is currently taking lisinopril, a medication with a pregnancy category of X. The ACE inhibitor class of medications is known to cause oligohydramnios, intrauterine growth retardation, structural malformation, premature birth, fetal renal dysplasia, and other congenital abnormalities, and use of these drugs should be avoided in women trying to conceive.7
Safer options for blood pressure control include clonidine, diltiazam, labetalol, methyldopa, or prazosin.3 Diuretics can reduce placental blood perfusion and should be avoided.8 An alternative for management of microalbuminuria in women of childbearing age is nifedipine.9 In multiple studies, this medication was not only safer in pregnancy, with no major teratogenic risk, but also effectively reduced urine microalbumin levels.10,11
For T2DM management, metformin (pregnancy category B) and glyburide (pregnancy category B/C, depending on manufacturer) can be used.12,13 Glyburide, the most studied sulfonylurea, is recommended as the drug of choice in its class.14-16 While insulin is the standard for managing diabetes in pregnancy—earlier research supported a switch from oral medications to insulin in women interested in becoming pregnant—recent studies have demonstrated that oral medications can be safely used.17 In addition, lifestyle changes (eg, carbohydrate counting, limited meal portions, and regular moderate exercise) prior to and during pregnancy can be beneficial for diabetes management.18,19
Also remind the patient to take regular prenatal vitamins. The US Preventive Services Task Force recommends that all women planning to become or capable of becoming pregnant take 400 to 800 µg supplements of folic acid daily.20 For women at high risk for neural tube defects or who have had a previous pregnancy with neural tube defects, 4 mg/d is recommended.21 In women with diabetes who are trying to conceive, a folic acid supplement of 5 mg/d is recommended, beginning three months prior to conception.22
Research shows that diabetic women are less likely to take folic acid supplementation during pregnancy. A study of 6,835 obese or overweight women with diabetes showed that only 35% reported daily folic acid supplementation.23 The study authors recommended all women of childbearing age, especially those who are obese or have diabetes, take folic acid daily.23 Encourage all women intending to become pregnant to start prenatal vitamin supplementation.
Continue for case 2 >>
Case 2 A 26-year-old obese patient, 28 weeks primigravida, presents for follow-up on her 3-hour glucose tolerance test. Results indicate a 3-hour glucose level of 148 mg/dL. The patient has a family history of T2DM and gestational diabetes.
Discussion
Gestational diabetes is defined by the ADA as diabetes diagnosed during the second or third trimester of pregnancy that is not T1DM or T2DM.3 The ADA recommends lifestyle management of gestational diabetes before medications are introduced. A1C should be maintained at 6% or less without hypoglycemia. In general, insulin is preferred over oral agents for treatment of gestational diabetes.3
There tends to be a spike in insulin resistance in the second or third trimester; women with preconception diabetes, for example, may require frequent increases in daily insulin dose to maintain glycemic levels, compared to the first trimester.3 A baseline ophthalmology exam should be performed in the first trimester for patients with preconception diabetes, with additional monitoring as needed.3
Following pregnancy, screening should be conducted for diabetes or prediabetes at six to 12 weeks’ postpartum and every one to three years afterward.3 The cumulative incidence of T2DM varies considerably among studies, ranging from 17% to 63% in five to 16 years postpartum.24,25 Thus, women with gestational diabetes should maintain lifestyle changes, including diet and exercise, to reduce the risk for T2DM later in life.
Continue for case 3 >>
Case 3 A 43-year-old woman with T1DM becomes pregnant while taking atorvastatin (20 mg), insulin detemir (18 units qhs), and insulin aspart with meals, as per her calculated insulin-to-carbohydrate ratio (ICR; 1 U aspart for 18 g carbohydrates) and insulin sensitivity factor (ISF; 1 U aspart for every 60 mg/dL above 130 mg/dL). Her biggest concern today is her medication list and potential adverse effects on the fetus. Her most recent A1C, two months ago, was 6.5%. She senses hypoglycemia at glucose levels of about 60 mg/dL and admits to having such measurements about twice per week.
Discussion
In this case, the patient needs to stop taking her statin and check her blood glucose regularly, as she is at increased risk for hypoglycemia. In their 2013 guidelines, the American College of Cardiology/American Heart Association stated that statins “should not be used in women of childbearing potential unless these women are using effective contraception and are not nursing.”26 This presents a major problem for many women of childbearing age with diabetes.
Statins are associated with a variety of congenital abnormalities, including fetal growth restriction and structural abnormalities in the fetus.27 It is advised that women planning for pregnancy avoid use of statins.28 If the patient has severe hypertriglyceridemia that puts her at risk for acute pancreatitis, fenofibrate (pregnancy category C) can be considered in the second and third trimesters.29,30
With T1DM in pregnancy, there is an increased risk for hypoglycemia in the first trimester.3 This risk increases as women adapt to more strict blood glucose control. Frequent recalculation of the ICR and ISF may be needed as the pregnancy progresses and weight gain occurs. Most insulin formulations are pregnancy class B, with the exception of glargine, degludec, and glulisine, which are pregnancy category C.3
Continue for case 4 >>
Case 4 A 21-year-old woman with T1DM wishes to start contraception but has concerns about long-term options. She seeks your advice in making a decision.
Discussion
For long-term pregnancy prevention, either the copper or progesterone-containing intrauterine device (IUD) is safe and effective for women with T1DM or T2DM.31 While the levonorgestrel IUD does not produce metabolic changes in T1DM, it has not yet been adequately studied in T2DM. Demographics suggest that young women with T2DM could become viable candidates for intrauterine contraception.31
The hormone-releasing “ring” has been found to be reliable and safe for women of late reproductive age with T1DM.32 Combined hormonal contraceptives and the transdermal contraceptive patch are best avoided to reduce risk for complications associated with estrogen-containing contraceptives (eg, venous thromboembolism and myocardial infarction).33
Continue for the conclusion >>
Conclusion
All women with diabetes should be counseled on glucose control prior to pregnancy. Achieving a goal A1C below 6% in the absence of hypoglycemia is recommended by the ADA.3 Long-term contraception options should be considered in women of childbearing age with diabetes to prevent pregnancy. Clinicians should carefully select medications for management of diabetes and its comorbidities in women planning to become pregnant. Healthy dietary habits and regular exercise should be encouraged in all patients with diabetes, especially prior to pregnancy.
References
1. CDC. National Diabetes Statistics Report, 2014. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed January 12, 2016.
2. CDC. Number of Americans with diabetes projected to double or triple by 2050. 2010. www.cdc.gov/media/pressrel/2010/r101022.html. Accessed January 12, 2016.
3. American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care. 2015;38(suppl 1):S1-S93.
4. Chemical Hazards Emergency Medical Management. FDA pregnancy categories. http://chemm.nlm.nih.gov/pregnancycategories.htm. Accessed January 12, 2016.
5. Weindling AM. Offspring of diabetic pregnancy: short-term outcomes. Semin Fetal Neonatal Med. 2009;14(2):111-118.
6. Kaneshiro NK. Infant of diabetic mother (2013). Medline Plus. www.nlm.nih.gov/medlineplus/ency/article/001597.htm. Accessed January 12, 2016.
7. Shotan A, Widerhorn J, Hurst A, Elkayam U. Risks of angiotensin-converting enzyme inhibition during pregnancy: experimental and clinical evidence, potential mechanisms, and recommendations for use. Am J Med. 1994;96(5):451-456.
8. Sibai BM. Treatment of hypertension in pregnant women. N Engl J Med. 1996;335 (4):257-265.
9. Ismail AA, Medhat I, Tawfic TA, Kholeif A. Evaluation of calcium-antagonists (nifedipine) in the treatment of pre-eclampsia. Int J Gynaecol Obstet. 1993;40:39-43.
10. Magee LA, Schick B, Donnenfeld AE, et al. The safety of calcium channel blockers in human pregnancy: a prospective, multicenter cohort study. Am J Obstet Gynecol. 1996;174(3):823-828.
11. Kattah AG, Garovic VD. The management of hypertension in pregnancy. Adv Chronic Kidney Dis. 2013;20(3):229-239.
12. Carroll DG, Kelley KW. Review of metformin and glyburide in the management of gestational diabetes. Pharm Pract (Granada). 2014;12(4):528.
13. Koren G. Glyburide and fetal safety; transplacental pharmacokinetic considerations. Reprod Toxicol. 2001;15(3):227-229.
14. Elliott BD, Langer O, Schenker S, Johnson RF. Insignificant transfer of glyburide occurs across the human placenta. Am J Obstet Gynecol. 1991;165:807-812.
15. Moore TR. Glyburide for the treatment of gestational diabetes: a critical appraisal. Diabetes Care. 2007;30(suppl 2):S209-S213.
16. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183-1197.
17. Kalra B, Gupta Y, Singla R, Kalra S. Use of oral anti-diabetic agents in pregnancy: a pragmatic approach. N Am J Med Sci. 2015; 7(1):6-12.
18. Zhang C, Ning Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: review of epidemiologic evidence. Am J Clin Nutr. 2011;94(6 suppl):1975S-1979S.
19. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260.
20. US Preventive Services Task Force. Folic acid to prevent neural tube defects: preventive medication, 2015. www.uspreventiveservices taskforce.org/Page/Document/Update SummaryFinal/folic-acid-to-prevent-neural-tube-defects-preventive-medication. Accessed January 12, 2016.
21. Cheschier N; ACOG Committee on Practice Bulletins—Obstetrics. Neural tube defects. ACOG Practice Bulletin no 44. Int J Gynaecol Obstet. 2003;83(1):123-133.
22. Blumer I, Hadar E, Hadden DR, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(11):4227-4249.
23. Case AP, Ramadhani TA, Canfield MA, et al. Folic acid supplementation among diabetic, overweight, or obese women of childbearing age. J Obstet Gynecol Neonatal Nurs. 2007;36(4):335-341.
24. Hanna FWF, Peters JR. Screening for gestational diabetes; past, present and future. Diabet Med. 2002;19:351-358.
25. Ben-haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabet Med. 2004;21(2):103-113.
26. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1-S45.
27. Patel C, Edgerton L, Flake D. What precautions should we use with statins for women of childbearing age? J Fam Pract. 2006; 55(1):75-77.
28. Kazmin A, Garcia-Bournissen F, Koren G. Risks of statin use during pregnancy: a systematic review. J Obstet Gynaecol Can. 2007;29(11):906-908.
29. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012; 97(9):2969-2989.
30. Saadi HF, Kurlander DJ, Erkins JM, Hoogwerf BJ. Severe hypertriglyceridemia and acute pancreatitis during pregnancy: treatment with gemfibrozil. Endocr Pract. 1999;5(1):33-36.
31. Goldstuck ND, Steyn PS. The intrauterine device in women with diabetes mellitus type I and II: a systematic review. ISRN Obstet Gynecol. 2013;2013:814062.
32. Grigoryan OR, Grodnitskaya EE, Andreeva EN, et al. Use of the NuvaRing hormone-releasing system in late reproductive-age women with type 1 diabetes mellitus. Gynecol Endocrinol. 2008;24(2):99-104.
33. Bonnema RA, McNamara MC, Spencer AL. Contraception choices in women with underlying medical conditions. Am Fam Physician. 2010;82(6):621-628.
There were 13.4 million women (ages 20 and older) with either type 1 or type 2 diabetes in the United States in 2012, according to the CDC.1 By 2050, overall prevalence of diabetes is expected to double or triple.2 Since the number of women with diabetes will continue to increase, it is important for clinicians to familiarize themselves with management of the condition in those of childbearing age—particularly with regard to medication selection.
Diabetes management in women of childbearing age presents multiple complexities. First, strict glucose control from preconception through pregnancy is necessary to reduce the risk for complications in mother and fetus. The American Diabetes Association (ADA) recommends an A1C of less than 7% during the preconception period, if achievable without hypoglycemia.3 Full glycemic targets for women are outlined in Table 1.
Continue for medication classes with pregnancy category >>
Second, many medications used to manage diabetes and pregnancy-associated comorbidities can be fetotoxic. The FDA assigns all drugs to a pregnancy category, the definitions of which are available at http://chemm.nlm.nih.gov/pregnancycategories.htm.4 The ADA recommends that sexually active women of childbearing age avoid any potentially teratogenic medications (see Table 2) if they are not using reliable contraception.3
Excellent control of diabetes is necessary to decrease risk for birth defects. Infants born to mothers with preconception diabetes have been shown to have higher rates of morbidity and mortality.5 Infants born to women with diabetes are generally large for gestational age and experience hypoglycemia in the first 24 to 48 hours of life.6 Large-for-gestational-age babies are at increased risk for trauma at birth, including orthopedic injuries (eg, shoulder dislocation) and brachial plexus injuries. There is also an increased risk for fetal cardiac defects and congenital congestive heart failure.6
This article will review four cases of diabetes management in women of childbearing age. The ADA guidelines form the basis for all recommendations.
Continue for case 1 >>
Case 1 A 32-year-old obese woman with type 2 diabetes mellitus (T2DM) presents for routine follow-up. Recent lab results reveal an A1C of 6.4%; GFR > 100 mL/min/1.73 m2; and microalbuminuria (110 mg/d). She is currently taking lisinopril (2.5 mg once daily), metformin (1,000 mg bid), and glyburide (5 mg bid). She plans to become pregnant in the next six months and wants advice.
Discussion
This patient should be counseled on preconception glycemic targets and switched to pregnancy-safe medications. She should also be advised that the recommended weight gain in pregnancy for women with T2DM is 15 to 25 lb in overweight women and 10 to 20 lb in obese women.3
The ADA recommends a target A1C < 7%, in the absence of severe hypoglycemia, prior to conception in patients with type 1 diabetes mellitus (T1DM) or T2DM.3 For women with preconception diabetes who become pregnant, it is recommended that their premeal, bedtime, and overnight glucose be maintained at 60 to 99 mg/dL, their peak postprandial glucose at 100 to 129 mg/dL, and their A1C < 6% during pregnancy (all without excessive hypoglycemia), due to increases in red blood cell turnover.3 It is also recommended that they avoid statins, ACE inhibitors, angiotensin II receptor blockers (ARBs), certain beta blockers, and most noninsulin therapies.3
This patient is currently taking lisinopril, a medication with a pregnancy category of X. The ACE inhibitor class of medications is known to cause oligohydramnios, intrauterine growth retardation, structural malformation, premature birth, fetal renal dysplasia, and other congenital abnormalities, and use of these drugs should be avoided in women trying to conceive.7
Safer options for blood pressure control include clonidine, diltiazam, labetalol, methyldopa, or prazosin.3 Diuretics can reduce placental blood perfusion and should be avoided.8 An alternative for management of microalbuminuria in women of childbearing age is nifedipine.9 In multiple studies, this medication was not only safer in pregnancy, with no major teratogenic risk, but also effectively reduced urine microalbumin levels.10,11
For T2DM management, metformin (pregnancy category B) and glyburide (pregnancy category B/C, depending on manufacturer) can be used.12,13 Glyburide, the most studied sulfonylurea, is recommended as the drug of choice in its class.14-16 While insulin is the standard for managing diabetes in pregnancy—earlier research supported a switch from oral medications to insulin in women interested in becoming pregnant—recent studies have demonstrated that oral medications can be safely used.17 In addition, lifestyle changes (eg, carbohydrate counting, limited meal portions, and regular moderate exercise) prior to and during pregnancy can be beneficial for diabetes management.18,19
Also remind the patient to take regular prenatal vitamins. The US Preventive Services Task Force recommends that all women planning to become or capable of becoming pregnant take 400 to 800 µg supplements of folic acid daily.20 For women at high risk for neural tube defects or who have had a previous pregnancy with neural tube defects, 4 mg/d is recommended.21 In women with diabetes who are trying to conceive, a folic acid supplement of 5 mg/d is recommended, beginning three months prior to conception.22
Research shows that diabetic women are less likely to take folic acid supplementation during pregnancy. A study of 6,835 obese or overweight women with diabetes showed that only 35% reported daily folic acid supplementation.23 The study authors recommended all women of childbearing age, especially those who are obese or have diabetes, take folic acid daily.23 Encourage all women intending to become pregnant to start prenatal vitamin supplementation.
Continue for case 2 >>
Case 2 A 26-year-old obese patient, 28 weeks primigravida, presents for follow-up on her 3-hour glucose tolerance test. Results indicate a 3-hour glucose level of 148 mg/dL. The patient has a family history of T2DM and gestational diabetes.
Discussion
Gestational diabetes is defined by the ADA as diabetes diagnosed during the second or third trimester of pregnancy that is not T1DM or T2DM.3 The ADA recommends lifestyle management of gestational diabetes before medications are introduced. A1C should be maintained at 6% or less without hypoglycemia. In general, insulin is preferred over oral agents for treatment of gestational diabetes.3
There tends to be a spike in insulin resistance in the second or third trimester; women with preconception diabetes, for example, may require frequent increases in daily insulin dose to maintain glycemic levels, compared to the first trimester.3 A baseline ophthalmology exam should be performed in the first trimester for patients with preconception diabetes, with additional monitoring as needed.3
Following pregnancy, screening should be conducted for diabetes or prediabetes at six to 12 weeks’ postpartum and every one to three years afterward.3 The cumulative incidence of T2DM varies considerably among studies, ranging from 17% to 63% in five to 16 years postpartum.24,25 Thus, women with gestational diabetes should maintain lifestyle changes, including diet and exercise, to reduce the risk for T2DM later in life.
Continue for case 3 >>
Case 3 A 43-year-old woman with T1DM becomes pregnant while taking atorvastatin (20 mg), insulin detemir (18 units qhs), and insulin aspart with meals, as per her calculated insulin-to-carbohydrate ratio (ICR; 1 U aspart for 18 g carbohydrates) and insulin sensitivity factor (ISF; 1 U aspart for every 60 mg/dL above 130 mg/dL). Her biggest concern today is her medication list and potential adverse effects on the fetus. Her most recent A1C, two months ago, was 6.5%. She senses hypoglycemia at glucose levels of about 60 mg/dL and admits to having such measurements about twice per week.
Discussion
In this case, the patient needs to stop taking her statin and check her blood glucose regularly, as she is at increased risk for hypoglycemia. In their 2013 guidelines, the American College of Cardiology/American Heart Association stated that statins “should not be used in women of childbearing potential unless these women are using effective contraception and are not nursing.”26 This presents a major problem for many women of childbearing age with diabetes.
Statins are associated with a variety of congenital abnormalities, including fetal growth restriction and structural abnormalities in the fetus.27 It is advised that women planning for pregnancy avoid use of statins.28 If the patient has severe hypertriglyceridemia that puts her at risk for acute pancreatitis, fenofibrate (pregnancy category C) can be considered in the second and third trimesters.29,30
With T1DM in pregnancy, there is an increased risk for hypoglycemia in the first trimester.3 This risk increases as women adapt to more strict blood glucose control. Frequent recalculation of the ICR and ISF may be needed as the pregnancy progresses and weight gain occurs. Most insulin formulations are pregnancy class B, with the exception of glargine, degludec, and glulisine, which are pregnancy category C.3
Continue for case 4 >>
Case 4 A 21-year-old woman with T1DM wishes to start contraception but has concerns about long-term options. She seeks your advice in making a decision.
Discussion
For long-term pregnancy prevention, either the copper or progesterone-containing intrauterine device (IUD) is safe and effective for women with T1DM or T2DM.31 While the levonorgestrel IUD does not produce metabolic changes in T1DM, it has not yet been adequately studied in T2DM. Demographics suggest that young women with T2DM could become viable candidates for intrauterine contraception.31
The hormone-releasing “ring” has been found to be reliable and safe for women of late reproductive age with T1DM.32 Combined hormonal contraceptives and the transdermal contraceptive patch are best avoided to reduce risk for complications associated with estrogen-containing contraceptives (eg, venous thromboembolism and myocardial infarction).33
Continue for the conclusion >>
Conclusion
All women with diabetes should be counseled on glucose control prior to pregnancy. Achieving a goal A1C below 6% in the absence of hypoglycemia is recommended by the ADA.3 Long-term contraception options should be considered in women of childbearing age with diabetes to prevent pregnancy. Clinicians should carefully select medications for management of diabetes and its comorbidities in women planning to become pregnant. Healthy dietary habits and regular exercise should be encouraged in all patients with diabetes, especially prior to pregnancy.
References
1. CDC. National Diabetes Statistics Report, 2014. www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed January 12, 2016.
2. CDC. Number of Americans with diabetes projected to double or triple by 2050. 2010. www.cdc.gov/media/pressrel/2010/r101022.html. Accessed January 12, 2016.
3. American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care. 2015;38(suppl 1):S1-S93.
4. Chemical Hazards Emergency Medical Management. FDA pregnancy categories. http://chemm.nlm.nih.gov/pregnancycategories.htm. Accessed January 12, 2016.
5. Weindling AM. Offspring of diabetic pregnancy: short-term outcomes. Semin Fetal Neonatal Med. 2009;14(2):111-118.
6. Kaneshiro NK. Infant of diabetic mother (2013). Medline Plus. www.nlm.nih.gov/medlineplus/ency/article/001597.htm. Accessed January 12, 2016.
7. Shotan A, Widerhorn J, Hurst A, Elkayam U. Risks of angiotensin-converting enzyme inhibition during pregnancy: experimental and clinical evidence, potential mechanisms, and recommendations for use. Am J Med. 1994;96(5):451-456.
8. Sibai BM. Treatment of hypertension in pregnant women. N Engl J Med. 1996;335 (4):257-265.
9. Ismail AA, Medhat I, Tawfic TA, Kholeif A. Evaluation of calcium-antagonists (nifedipine) in the treatment of pre-eclampsia. Int J Gynaecol Obstet. 1993;40:39-43.
10. Magee LA, Schick B, Donnenfeld AE, et al. The safety of calcium channel blockers in human pregnancy: a prospective, multicenter cohort study. Am J Obstet Gynecol. 1996;174(3):823-828.
11. Kattah AG, Garovic VD. The management of hypertension in pregnancy. Adv Chronic Kidney Dis. 2013;20(3):229-239.
12. Carroll DG, Kelley KW. Review of metformin and glyburide in the management of gestational diabetes. Pharm Pract (Granada). 2014;12(4):528.
13. Koren G. Glyburide and fetal safety; transplacental pharmacokinetic considerations. Reprod Toxicol. 2001;15(3):227-229.
14. Elliott BD, Langer O, Schenker S, Johnson RF. Insignificant transfer of glyburide occurs across the human placenta. Am J Obstet Gynecol. 1991;165:807-812.
15. Moore TR. Glyburide for the treatment of gestational diabetes: a critical appraisal. Diabetes Care. 2007;30(suppl 2):S209-S213.
16. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183-1197.
17. Kalra B, Gupta Y, Singla R, Kalra S. Use of oral anti-diabetic agents in pregnancy: a pragmatic approach. N Am J Med Sci. 2015; 7(1):6-12.
18. Zhang C, Ning Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: review of epidemiologic evidence. Am J Clin Nutr. 2011;94(6 suppl):1975S-1979S.
19. Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(suppl 2):S251-S260.
20. US Preventive Services Task Force. Folic acid to prevent neural tube defects: preventive medication, 2015. www.uspreventiveservices taskforce.org/Page/Document/Update SummaryFinal/folic-acid-to-prevent-neural-tube-defects-preventive-medication. Accessed January 12, 2016.
21. Cheschier N; ACOG Committee on Practice Bulletins—Obstetrics. Neural tube defects. ACOG Practice Bulletin no 44. Int J Gynaecol Obstet. 2003;83(1):123-133.
22. Blumer I, Hadar E, Hadden DR, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(11):4227-4249.
23. Case AP, Ramadhani TA, Canfield MA, et al. Folic acid supplementation among diabetic, overweight, or obese women of childbearing age. J Obstet Gynecol Neonatal Nurs. 2007;36(4):335-341.
24. Hanna FWF, Peters JR. Screening for gestational diabetes; past, present and future. Diabet Med. 2002;19:351-358.
25. Ben-haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabet Med. 2004;21(2):103-113.
26. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1-S45.
27. Patel C, Edgerton L, Flake D. What precautions should we use with statins for women of childbearing age? J Fam Pract. 2006; 55(1):75-77.
28. Kazmin A, Garcia-Bournissen F, Koren G. Risks of statin use during pregnancy: a systematic review. J Obstet Gynaecol Can. 2007;29(11):906-908.
29. Berglund L, Brunzell JD, Goldberg AC, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012; 97(9):2969-2989.
30. Saadi HF, Kurlander DJ, Erkins JM, Hoogwerf BJ. Severe hypertriglyceridemia and acute pancreatitis during pregnancy: treatment with gemfibrozil. Endocr Pract. 1999;5(1):33-36.
31. Goldstuck ND, Steyn PS. The intrauterine device in women with diabetes mellitus type I and II: a systematic review. ISRN Obstet Gynecol. 2013;2013:814062.
32. Grigoryan OR, Grodnitskaya EE, Andreeva EN, et al. Use of the NuvaRing hormone-releasing system in late reproductive-age women with type 1 diabetes mellitus. Gynecol Endocrinol. 2008;24(2):99-104.
33. Bonnema RA, McNamara MC, Spencer AL. Contraception choices in women with underlying medical conditions. Am Fam Physician. 2010;82(6):621-628.
Disease Education
Q) The billing consultant who came to our office said we can increase our reimbursements if we also provide education to our patients with chronic kidney disease (CKD). Is she right?
In 2010, under an omnibus bill, kidney disease education (KDE) classes were added as a Medicare benefit. These are for patients with stage 4 CKD (glomerular filtration rate, 15-30 mL/min) and are to be taught by a qualified instructor (MD, PA, NP, or CNS).
The classes can be taught on the same day as an evaluation/management visit (ie, a regular office visit) and are compensated by the hour. (Side note: Medicare defines an hour as 31 minutes—yes, 31 minutes; Medicare takes for granted that you will also need time to chart!) You can teach two classes in the same day. Thus, if you wanted to, you could have a patient arrive for an office visit, then teach two 31-minute classes, and bill all three for the same day. The entire visit could be 75 minutes (although this may be exhausting for this population).
You can conduct the classes in a number of settings, including nursing homes, hospitals, skilled nursing facilities, the office, or even the patient’s home. Many PAs and NPs have taught these classes to hospitalized patients who have lost kidney function due to an acute insult (ie, medications, dehydration, contrast).
Each Medicare recipient has a lifetime benefit of six KDE classes. The CPT billing code is G0420 for an individual class and G0421 for a group class. You must make sure you also code for the stage 4 CKD diagnosis (code: 585.4).
Congress stipulated KDE classes must include information on causes, symptoms, and treatments and comprise a posttest at a specific health literacy level. To make it simple, the National Kidney Foundation Council of Advanced Practitioners (NKF-CAP) has developed two free Power-Point slide decks for clinicians to use in KDE classes (available at www.kidney.org/professionals/CAP/sub_resources#kde). References and updated peer-reviewed guidelines are included. You can print the slides for your patients and/or share the program with your colleagues.
Many nephrology practitioners teach the two slide sets over and over, because patients only retain one-third of the info we provide them on a given day. So if you teach each slide set three times, you have six lifetime classes—and hopefully the patient will have retained everything.
One caveat: Before you initiate KDE classes for a specific patient, check with the patient’s nephrology group (we hope at stage 4 the patient has a nephrologist) to see if they are providing the education. —KZ and JD
Kim Zuber, PA-C, MSPS, DFAAPA
American Academy of Nephrology PAs
Jane S. Davis, CRNP, DNP
Division of Nephrology at the University of Alabama
National Kidney Foundation's Council of Advanced Practitioners
Q) The billing consultant who came to our office said we can increase our reimbursements if we also provide education to our patients with chronic kidney disease (CKD). Is she right?
In 2010, under an omnibus bill, kidney disease education (KDE) classes were added as a Medicare benefit. These are for patients with stage 4 CKD (glomerular filtration rate, 15-30 mL/min) and are to be taught by a qualified instructor (MD, PA, NP, or CNS).
The classes can be taught on the same day as an evaluation/management visit (ie, a regular office visit) and are compensated by the hour. (Side note: Medicare defines an hour as 31 minutes—yes, 31 minutes; Medicare takes for granted that you will also need time to chart!) You can teach two classes in the same day. Thus, if you wanted to, you could have a patient arrive for an office visit, then teach two 31-minute classes, and bill all three for the same day. The entire visit could be 75 minutes (although this may be exhausting for this population).
You can conduct the classes in a number of settings, including nursing homes, hospitals, skilled nursing facilities, the office, or even the patient’s home. Many PAs and NPs have taught these classes to hospitalized patients who have lost kidney function due to an acute insult (ie, medications, dehydration, contrast).
Each Medicare recipient has a lifetime benefit of six KDE classes. The CPT billing code is G0420 for an individual class and G0421 for a group class. You must make sure you also code for the stage 4 CKD diagnosis (code: 585.4).
Congress stipulated KDE classes must include information on causes, symptoms, and treatments and comprise a posttest at a specific health literacy level. To make it simple, the National Kidney Foundation Council of Advanced Practitioners (NKF-CAP) has developed two free Power-Point slide decks for clinicians to use in KDE classes (available at www.kidney.org/professionals/CAP/sub_resources#kde). References and updated peer-reviewed guidelines are included. You can print the slides for your patients and/or share the program with your colleagues.
Many nephrology practitioners teach the two slide sets over and over, because patients only retain one-third of the info we provide them on a given day. So if you teach each slide set three times, you have six lifetime classes—and hopefully the patient will have retained everything.
One caveat: Before you initiate KDE classes for a specific patient, check with the patient’s nephrology group (we hope at stage 4 the patient has a nephrologist) to see if they are providing the education. —KZ and JD
Kim Zuber, PA-C, MSPS, DFAAPA
American Academy of Nephrology PAs
Jane S. Davis, CRNP, DNP
Division of Nephrology at the University of Alabama
National Kidney Foundation's Council of Advanced Practitioners
Q) The billing consultant who came to our office said we can increase our reimbursements if we also provide education to our patients with chronic kidney disease (CKD). Is she right?
In 2010, under an omnibus bill, kidney disease education (KDE) classes were added as a Medicare benefit. These are for patients with stage 4 CKD (glomerular filtration rate, 15-30 mL/min) and are to be taught by a qualified instructor (MD, PA, NP, or CNS).
The classes can be taught on the same day as an evaluation/management visit (ie, a regular office visit) and are compensated by the hour. (Side note: Medicare defines an hour as 31 minutes—yes, 31 minutes; Medicare takes for granted that you will also need time to chart!) You can teach two classes in the same day. Thus, if you wanted to, you could have a patient arrive for an office visit, then teach two 31-minute classes, and bill all three for the same day. The entire visit could be 75 minutes (although this may be exhausting for this population).
You can conduct the classes in a number of settings, including nursing homes, hospitals, skilled nursing facilities, the office, or even the patient’s home. Many PAs and NPs have taught these classes to hospitalized patients who have lost kidney function due to an acute insult (ie, medications, dehydration, contrast).
Each Medicare recipient has a lifetime benefit of six KDE classes. The CPT billing code is G0420 for an individual class and G0421 for a group class. You must make sure you also code for the stage 4 CKD diagnosis (code: 585.4).
Congress stipulated KDE classes must include information on causes, symptoms, and treatments and comprise a posttest at a specific health literacy level. To make it simple, the National Kidney Foundation Council of Advanced Practitioners (NKF-CAP) has developed two free Power-Point slide decks for clinicians to use in KDE classes (available at www.kidney.org/professionals/CAP/sub_resources#kde). References and updated peer-reviewed guidelines are included. You can print the slides for your patients and/or share the program with your colleagues.
Many nephrology practitioners teach the two slide sets over and over, because patients only retain one-third of the info we provide them on a given day. So if you teach each slide set three times, you have six lifetime classes—and hopefully the patient will have retained everything.
One caveat: Before you initiate KDE classes for a specific patient, check with the patient’s nephrology group (we hope at stage 4 the patient has a nephrologist) to see if they are providing the education. —KZ and JD
Kim Zuber, PA-C, MSPS, DFAAPA
American Academy of Nephrology PAs
Jane S. Davis, CRNP, DNP
Division of Nephrology at the University of Alabama
National Kidney Foundation's Council of Advanced Practitioners
Kidney Transplants
Q) All I hear about is the shortage of kidneys for transplantation. A friend of mine is on the local transplant list, and it is eight years long! Are there any ideas out there to grow your own kidneys?
Eight years is a long time for people dealing with the physical and emotional effects of kidney disease coupled with hemodialysis or peritoneal dialysis. Your friend is one of 110,000 patients (as of January 2015) in the United States on the United Network for Organ Sharing (UNOS) kidney transplant waiting list.1 The UNOS/Organ Procurement and Transplant Network (OPTN) implemented new polices in 2014 to shorten the wait.
Among them: For pediatric patients (those younger than 18), the wait list time starts when the glomerular filtration rate (GFR) is ≤ 20 mL/min or the child starts dialysis. UNOS also has attempted to match posttransplant survival time of the graft with posttransplant survival time of the recipient through use of calculations that classify kidneys on the basis of how long they are likely to function once transplanted. Priority is now given to candidates with high immune system sensitivity or uncommon blood types, as they are less likely to obtain a kidney otherwise.2
The million-dollar question is how to obtain a kidney transplant in a timely fashion. Grave robbing, in case you are wondering, is not a viable option! Nor is transplant tourism (traveling outside the US to obtain an organ transplant), which confers a higher risk for severe infectious complications and acute rejection, possibly related to less extensive donor screening.3
There are other possibilities: Living donors can donate one kidney. Or, as is becoming increasingly common, paired organ transplants can be arranged. These occur when a patient in need of a kidney has a willing but incompatible donor; if a different match can be found, a “swap” is orchestrated, in which Donor A’s kidney is transplanted into Recipient B and Donor B’s kidney is given to Recipient A. This can be and has been done with multiple donors and recipients—in some cases, dozens—allowing the gift of donation to go on and on. (See “Trading Kidneys: Innovative Program Could Save Thousands of Lives” for an overview of how this concept started.)
Some exciting research is going on with regard to 3D printing of kidneys; they are miniature for now but showing survival of the printed cells. Another area of exploration is regenerative medicine; researchers in the field are investigating the bioengineering of organs by taking the “scaffolding” of an organ and implanting a patient’s own cells to “grow” a new organ (which, if successful, would also eliminate the need for immunosuppressants). Other signs of progress include recent news that scientists are getting lab-grown kidneys to work in animals.
It will be several years before any of these options will be viable. In the meantime, it never hurts to ask loved ones if they are willing to donate a kidney. Best wishes to your friend. —DC
Della Connor, PhD, RN, FNP-BC
East Texas Nephrology Associates, Lufkin, Texas
REFERENCES
1. Organ Procurement and Transplantation Network. http://optn.transplant.hrsa.gov. Accessed December 10, 2015.
2. Organ Procurement and Transplantation Network. New OPTN requirements and resources for the living donor kidney transplant programs. Prog Transplant. 2013;23(2):117.
3. Gill J, Madhira BR, Gjertson D, et al. Transplant tourism in the United States: a single-center experience. Clin J Am Soc Nephrol. 2008;3(6):1820-1828.
Q) All I hear about is the shortage of kidneys for transplantation. A friend of mine is on the local transplant list, and it is eight years long! Are there any ideas out there to grow your own kidneys?
Eight years is a long time for people dealing with the physical and emotional effects of kidney disease coupled with hemodialysis or peritoneal dialysis. Your friend is one of 110,000 patients (as of January 2015) in the United States on the United Network for Organ Sharing (UNOS) kidney transplant waiting list.1 The UNOS/Organ Procurement and Transplant Network (OPTN) implemented new polices in 2014 to shorten the wait.
Among them: For pediatric patients (those younger than 18), the wait list time starts when the glomerular filtration rate (GFR) is ≤ 20 mL/min or the child starts dialysis. UNOS also has attempted to match posttransplant survival time of the graft with posttransplant survival time of the recipient through use of calculations that classify kidneys on the basis of how long they are likely to function once transplanted. Priority is now given to candidates with high immune system sensitivity or uncommon blood types, as they are less likely to obtain a kidney otherwise.2
The million-dollar question is how to obtain a kidney transplant in a timely fashion. Grave robbing, in case you are wondering, is not a viable option! Nor is transplant tourism (traveling outside the US to obtain an organ transplant), which confers a higher risk for severe infectious complications and acute rejection, possibly related to less extensive donor screening.3
There are other possibilities: Living donors can donate one kidney. Or, as is becoming increasingly common, paired organ transplants can be arranged. These occur when a patient in need of a kidney has a willing but incompatible donor; if a different match can be found, a “swap” is orchestrated, in which Donor A’s kidney is transplanted into Recipient B and Donor B’s kidney is given to Recipient A. This can be and has been done with multiple donors and recipients—in some cases, dozens—allowing the gift of donation to go on and on. (See “Trading Kidneys: Innovative Program Could Save Thousands of Lives” for an overview of how this concept started.)
Some exciting research is going on with regard to 3D printing of kidneys; they are miniature for now but showing survival of the printed cells. Another area of exploration is regenerative medicine; researchers in the field are investigating the bioengineering of organs by taking the “scaffolding” of an organ and implanting a patient’s own cells to “grow” a new organ (which, if successful, would also eliminate the need for immunosuppressants). Other signs of progress include recent news that scientists are getting lab-grown kidneys to work in animals.
It will be several years before any of these options will be viable. In the meantime, it never hurts to ask loved ones if they are willing to donate a kidney. Best wishes to your friend. —DC
Della Connor, PhD, RN, FNP-BC
East Texas Nephrology Associates, Lufkin, Texas
REFERENCES
1. Organ Procurement and Transplantation Network. http://optn.transplant.hrsa.gov. Accessed December 10, 2015.
2. Organ Procurement and Transplantation Network. New OPTN requirements and resources for the living donor kidney transplant programs. Prog Transplant. 2013;23(2):117.
3. Gill J, Madhira BR, Gjertson D, et al. Transplant tourism in the United States: a single-center experience. Clin J Am Soc Nephrol. 2008;3(6):1820-1828.
Q) All I hear about is the shortage of kidneys for transplantation. A friend of mine is on the local transplant list, and it is eight years long! Are there any ideas out there to grow your own kidneys?
Eight years is a long time for people dealing with the physical and emotional effects of kidney disease coupled with hemodialysis or peritoneal dialysis. Your friend is one of 110,000 patients (as of January 2015) in the United States on the United Network for Organ Sharing (UNOS) kidney transplant waiting list.1 The UNOS/Organ Procurement and Transplant Network (OPTN) implemented new polices in 2014 to shorten the wait.
Among them: For pediatric patients (those younger than 18), the wait list time starts when the glomerular filtration rate (GFR) is ≤ 20 mL/min or the child starts dialysis. UNOS also has attempted to match posttransplant survival time of the graft with posttransplant survival time of the recipient through use of calculations that classify kidneys on the basis of how long they are likely to function once transplanted. Priority is now given to candidates with high immune system sensitivity or uncommon blood types, as they are less likely to obtain a kidney otherwise.2
The million-dollar question is how to obtain a kidney transplant in a timely fashion. Grave robbing, in case you are wondering, is not a viable option! Nor is transplant tourism (traveling outside the US to obtain an organ transplant), which confers a higher risk for severe infectious complications and acute rejection, possibly related to less extensive donor screening.3
There are other possibilities: Living donors can donate one kidney. Or, as is becoming increasingly common, paired organ transplants can be arranged. These occur when a patient in need of a kidney has a willing but incompatible donor; if a different match can be found, a “swap” is orchestrated, in which Donor A’s kidney is transplanted into Recipient B and Donor B’s kidney is given to Recipient A. This can be and has been done with multiple donors and recipients—in some cases, dozens—allowing the gift of donation to go on and on. (See “Trading Kidneys: Innovative Program Could Save Thousands of Lives” for an overview of how this concept started.)
Some exciting research is going on with regard to 3D printing of kidneys; they are miniature for now but showing survival of the printed cells. Another area of exploration is regenerative medicine; researchers in the field are investigating the bioengineering of organs by taking the “scaffolding” of an organ and implanting a patient’s own cells to “grow” a new organ (which, if successful, would also eliminate the need for immunosuppressants). Other signs of progress include recent news that scientists are getting lab-grown kidneys to work in animals.
It will be several years before any of these options will be viable. In the meantime, it never hurts to ask loved ones if they are willing to donate a kidney. Best wishes to your friend. —DC
Della Connor, PhD, RN, FNP-BC
East Texas Nephrology Associates, Lufkin, Texas
REFERENCES
1. Organ Procurement and Transplantation Network. http://optn.transplant.hrsa.gov. Accessed December 10, 2015.
2. Organ Procurement and Transplantation Network. New OPTN requirements and resources for the living donor kidney transplant programs. Prog Transplant. 2013;23(2):117.
3. Gill J, Madhira BR, Gjertson D, et al. Transplant tourism in the United States: a single-center experience. Clin J Am Soc Nephrol. 2008;3(6):1820-1828.
Are Those Glucometer Results Accurate?
CLINICAL CASE FROM 2009
JF, a 64-year-old man with a 30-year history of type 2 diabetes managed with basal and rapid-acting prandial insulin, started peritoneal dialysis using icodextrin dialysis solution. Since starting dialysis, JF has experienced persistently elevated blood glucose readings (in the high 200 mg/dL to high 300 mg/dL range) using his Accu-Chek Compact glucometer purchased in 2008. In response, JF has been taking higher doses of rapid-acting insulin with meals and for correction, with two-to-three-hour postprandial blood glucose readings persistently elevated (in the high 200s). JF has no fevers, chills, abdominal pain, or other signs/symptoms of infection. Urine ketone testing is negative.
Yesterday, JF’s pre-lunch blood glucose registered at 380 mg/dL on his glucometer, and he took a dose of rapid-acting insulin that was double what he would have taken prior to starting dialysis. About 90 minutes after lunch, JF felt weak and diaphoretic and became unresponsive, with seizure-like activity. His wife called the paramedics; when they arrived, JF’s fingerstick glucose level was 28 mg/dL (using a One Touch Ultra glucometer).
JF was treated acutely with IV dextrose and then transported to a nearby hospital. During his hospitalization, his blood glucose level was maintained in the mid-100 to high-200 mg/dL range, with approximately 50% lower doses of rapid-acting insulin with meals. Hospital work-up revealed no evidence of secondary causes of hyperglycemia. EEG was negative.
Further investigation determined that JF’s Accu-Chek Compact glucometer used GDH-PQQ methodology, which is unable to distinguish between the blood glucose level and the maltose metabolite of icodextrin contained in the peritoneal dialysis solution—leading to falsely elevated glucose results. JF switched to a different glucometer that did not use test strips containing the GDH-PQQ method, allowing for more accurate blood glucose readings and no recurrent episodes of severe hypoglycemia.
Continue for biochemistry of glucose measurements >>
BIOCHEMISTRY OF GLUCOSE MEASUREMENTS
In 1964, Ernie Adams invented Dextrostix, a paper strip that developed varying shades of color proportional to the glucose concentration. In 1970, Anton Clemens developed the first glucometer, the Ames Reflectance Meter (ARM), to detect reflected light from a Dextrostix. The ARM weighed 3 lb and cost $650.1
Modern glucometers analyze whole blood using both an enzymatic reaction and a detector. The enzyme is packaged in a dehydrated state contained in a disposable strip. The glucose in the patient’s blood rehydrates and reacts with enzymes in the strip to produce a detectable product.1
The gold standard for measuring glucose is isotope dilution mass spectrometry; however, this is not commonly performed in clinical laboratories. The accuracy of glucometers is most commonly assessed by comparing the glucometer result to a venous plasma sample collected at the same time and analyzed by a clinical laboratory using multi-analyte automated instrumentation.1
The two main types of commercially available glucometers are the glucose oxidase (GO) and glucose dehydrogenase (GDH) systems. The GO meters utilize the GO enzyme to catalyze the oxidation of glucose into gluconic acid. The oxidation reaction produces electrons that generate current proportional to the glucose level in the test sample.1-3
With GDH glucometers, several different enzymes can catalyze glucose oxidation, including nicotinamide adenine dinucleotide (GDH-NAD), flavin adenine dinucleotide (GDH-FAD), pyrroloquinoline quinone (GDH-PQQ), or mutant glucose dehydrogenase PQQ (Mut Q-GDH).2,4,5
Measurement of glucose using the hexokinase enzyme is considered more accurate than both the GO and GDH systems and is commonly used in clinical laboratories. However, the cost of this system is more than that of the commercially available glucometers, and thus it is not widely available.2
Continue for performance requirements for glucometer systems >>
PERFORMANCE REQUIREMENTS FOR GLUCOMETER SYSTEMS
There is no single standard for glucometer accuracy. Per Guideline 15197, issued by the International Organization for Standardization (ISO) in 2013, the minimum criteria for accuracy is at least 95% of blood glucose results within ± 15 mg/dL of the reference value at blood sugar concentrations < 100 mg/dL and within ± 15% at blood sugar concentrations ≥ 100 mg/dL.6 For OTC glucometers, the FDA has recommended that at least 95% of measurements fall within ± 15% and at least 99% of measurements fall within ± 20% of reference values across the entire claimed range of the glucometer system.7
The ISO and FDA both recommend that industry test glucometer accuracy using glucose levels ranging from ≤ 50 mg/dL to ≥ 400 mg/dL.6,7 They also recommend evaluating blood glucose accuracy at different hematocrit levels and assessing accuracy in the presence of interfering substances, such as acetaminophen, ibuprofen, salicylate, sodium, ascorbic acid, bilirubin, creatinine, dopamine, maltose, xylose, galactose, hemoglobin, heparin, L-dopa, methyldopa, triglycerides, cholesterol, sugar alcohols, and uric acid.6,7 The FDA additionally recommends testing glucometer accuracy in the presence of temperature extremes, humidity, and different altitudes.7
Currently, the premarket evaluation of glucometers is a one-time procedure that is typically conducted by the manufacturer. Not all available glucometers currently comply with the less stringent ISO accuracy standards from 2003, and most currently available glucometer systems fail to meet the more stringent accuracy criteria outlined by the ISO in 2013 and the FDA in 2014. Furthermore, there can be inconsistency in the measurement quality between different test strip lots, adding another variable to assessing glucometer accuracy.6
Continue for variables affecting glucometer accuracy >>
VARIABLES AFFECTING GLUCOMETER ACCURACY
Patient and environmental factors
Both patient and environmental factors can interfere with obtaining accurate glucometer results. These include sampling errors, improper storage of test strips, inadequate amount of blood applied to the test strip, improper meter coding, and altitude.1
Temperature extremes and humidity can denature, inactivate, or prematurely rehydrate enzymes and proteins within the test strip.1 GO meters can overestimate glucose levels at low temperatures, while GDH meters can produce unpredictable results in increased humidity.1 The detector portion of the meter is composed of electronics and should be protected from temperature extremes and excessive moisture as well.1
In high altitude, both GO and GDH meters can produce unreliable results, with a tendency to overestimate blood glucose levels.8 Another variable confounding the accuracy of glucometer readings at high altitude is the potential for secondary polycythemia, which can result in underestimation of glucose levels.8,9
Physiologic factors
Physiologic factors that can cause inaccurate glucometer results include hypoxia, abnormal pH, hyperuricemia, jaundice, polycythemia, anemia, peripheral vascular disease, and hypotension resulting in poor perfusion.1,7,9
Elevated oxygen tension in patients receiving oxygen therapy can falsely lower glucometer results for GO meters, while hypoxia can falsely elevate glucose results for these meters.1,3
Low pH (< 6.95), such as in diabetic ketoacidosis, falsely lowers glucose readings in GO meters, while a high pH falsely elevates glucose readings.1,10 Elevated serum uric acid (> 10-16 mg/dL) and elevated total bilirubin concentration (> 20 mg/dL) can cause overestimation of blood glucose levels due to electrochemical interaction at the electrode site in GDH-PQQ meters.11
Polycythemia can result in underestimation of glucose levels, and glucose levels can be overestimated in the setting of anemia.9 In anemia, the reduced red blood cell volume results in less displacement of plasma, causing more glucose molecules to be available to react with the enzyme contained in the test strip.12
Despite manufacturers’ claims that glucometers are reliable to a hematocrit range of 20% to 25%, clinically significant errors of greater than 20% were observed when the hematocrit level dropped below 34%, which can present challenges if glucometers are used in the ICU.13 Mathematical formulas to correct point-of-care glucometer measurements based on the hematocrit level have been proposed and have demonstrated effectiveness in decreasing the incidence of hypoglycemia in critically ill patients treated with insulin.12
Medications
Drugs that most commonly interfere with glucometer measurements include acetaminophen (especially at a serum concentration > 8 mg/dL), ascorbic acid, maltose, galactose, and xylose.1,11 Acetaminophen and ascorbic acid consume peroxide, resulting in falsely lowered blood glucose readings in GO meters. In GDH meters, direct oxidation can occur at the electrode site in the presence of acetaminophen and ascorbic acid, resulting in falsely elevated glucose levels.6,9,12
Maltose, galactose, and xylose are nonglucose sugars found in certain drug and biologic formulations, such as icodextrin peritoneal dialysis solution, certain immunoglobulins (Octagam 5%, WinRho SDF Liquid, Vaccinia Immune Globulin Intravenous [Human], and HepGamB), Orencia, and BEXXAR radioimmunotherapy agent.14
The GDH-PQQ meters cannot distinguish between glucose and nonglucose sugars, resulting in either undetected hypoglycemia or a falsely elevated glucose result (up to 3 to 15 times higher than corresponding laboratory results), which can lead to inappropriate medication dosing that results in potential hypoglycemia, coma, or death.14 Laboratory-based blood glucose assays, the GO, and most GDH-FAD, GDH-NAD, Mut Q-GDH, and hexokinase test strips do not have the potential for cross-reactivity from sugars other than glucose.4,14
It should be noted that in the United States, most GDH-PQQ test strips are no longer manufactured for home glucose testing. However, it is important to review the product insert contained in the test strip box for verification of the specific enzymatic methodology used in the test strip.4,5
Continue for the conclusion >>
CONCLUSION
Multiple factors affect the accuracy of currently available glucometers. Consideration of patient comorbidities, medication use, operational technique, and the conditions under which test strips are stored is important when utilizing glucometer data to make medication adjustments in diabetes management. It is important to refer to specific glucometer and test strip manufacturer device labeling to help select the appropriate glucometer for a particular patient.
The case presentation from 2009, involving falsely elevated blood glucose readings in a patient using a GDH-PQQ meter while receiving icodextrin peritoneal dialysis solution, highlights the importance of background knowledge of glucometer operational mechanisms. For a full list of test strips that are compatible with icodextrin peritoneal dialysis solution, please see the Country-Specific Glucose Monitor List at www.glucosesafety.com.5
Examples of specific GO meters include the OneTouch Ultra, iBGStar, and ReliOn meters. Although the GO meters do not cross-react with icodextrin, these meters should be avoided in patients receiving supplemental oxygen, due to the potential for falsely lowered readings.
The GDH-FAD, GDH-NAD, and Mut Q-GDH test strips may be used in patients receiving icodextrin peritoneal dialysis solution and those receiving supplemental oxygen.3,5 Examples of GDH-FAD meters include most currently available FreeStyle meters, Bayer Contour meters, and One Touch Verio meters. The Precision Xtra meter uses GDH-NAD test strips. Most Accu-Chek meters currently use Mut Q-GDH test strips.
REFERENCES
1. Tonyushkina K, Nichols JH. Glucose meters: a review of technical challenges to obtaining accurate results. J Diabetes Sci Technol. 2009;3(4):971-980.
2. Floré KMJ, Delanghe JR. Analytical interferences in point-of-care testing glucometers by icodextrin and its metabolites: an overview. Peritoneal Dial Int. 2009;29(4):377-383.
3. Tang Z, Louie RF, Lee JH, et al. Oxygen effects on glucose meter measurements with glucose dehydrogenase- and oxidase-based test strips for point-of-care testing. Crit Care Med. 2001;29(5):1062-1070.
4. Olansky L. Finger-stick glucose monitoring: issues of accuracy and specificity. Diabetes Care. 2010;33(4):948-949.
5. Baxter Healthcare Corporation. Country-specific glucose monitor list, 2015. www.glucosesafety.com/us/pdf/Glucose_Monitor_List.pdf. Accessed November 18, 2015.
6. Freckmann G, Schmid C, Baumstark A, et al. Analytical performance requirements for systems for self-monitoring of blood glucose with focus on system accuracy: relevant differences among ISO 15197:2003, ISO 15197: 2013, and current FDA recommendations. J Diabetes Sci Technol. 2015;9(4):885-894.
7. FDA. Self-Monitoring Blood Glucose Test Systems for Over-The-Counter Use: Draft Guidance for Industry and Food and Drug Administration Staff (2014). www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm380327.pdf. Accessed November 18, 2015.
8. Olateju T, Begley J, Flanagan D, Kerr D. Effects of simulated altitude on blood glucose meter performance: implications for in-flight blood glucose monitoring. J Diabetes Sci Technol. 2012;6(4):867-874.
9. Rao LV, Jakubiak F, Sidwell JS, et al. Accuracy evaluation of a new glucometer with automated hematocrit measurement and correction. Clinica Chimica Acta. 2005;356(1-2):178-183.
10. Tang Z, Du X, Louie RF, Kost GJ. Effects of pH on glucose measurements with handheld glucose meters and a portable glucose analyzer for point-of-care testing. Arch Pathol Lab Med. 2000;124:577-582.
11. Eastham JH, Mason D, Barnes DL, Kollins J. Prevalence of interfering substances with point-of-care glucose testing in a community hospital. Am J Health Syst Pharm. 2009;66: 167-170.
12 Pidcoke HF, Wade CE, Mann EA, et al. Anemia causes hypoglycemia in ICU patients due to error in single-channel glucometers: methods of reducing patient risk. Crit Care Med. 2010;38(2):471-476.
13. Mann EA, Pidcoke HF, Salinas J, et al. Accuracy of glucometers should not be assumed. Am J Crit Care. 2007;16(6):531-532.
14. FDA. FDA Public Health Notification: Potentially Fatal Errors with GDH-PQQ Glucose Monitoring Technology (2009). www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm176992.htm. Accessed November 18, 2015.
CLINICAL CASE FROM 2009
JF, a 64-year-old man with a 30-year history of type 2 diabetes managed with basal and rapid-acting prandial insulin, started peritoneal dialysis using icodextrin dialysis solution. Since starting dialysis, JF has experienced persistently elevated blood glucose readings (in the high 200 mg/dL to high 300 mg/dL range) using his Accu-Chek Compact glucometer purchased in 2008. In response, JF has been taking higher doses of rapid-acting insulin with meals and for correction, with two-to-three-hour postprandial blood glucose readings persistently elevated (in the high 200s). JF has no fevers, chills, abdominal pain, or other signs/symptoms of infection. Urine ketone testing is negative.
Yesterday, JF’s pre-lunch blood glucose registered at 380 mg/dL on his glucometer, and he took a dose of rapid-acting insulin that was double what he would have taken prior to starting dialysis. About 90 minutes after lunch, JF felt weak and diaphoretic and became unresponsive, with seizure-like activity. His wife called the paramedics; when they arrived, JF’s fingerstick glucose level was 28 mg/dL (using a One Touch Ultra glucometer).
JF was treated acutely with IV dextrose and then transported to a nearby hospital. During his hospitalization, his blood glucose level was maintained in the mid-100 to high-200 mg/dL range, with approximately 50% lower doses of rapid-acting insulin with meals. Hospital work-up revealed no evidence of secondary causes of hyperglycemia. EEG was negative.
Further investigation determined that JF’s Accu-Chek Compact glucometer used GDH-PQQ methodology, which is unable to distinguish between the blood glucose level and the maltose metabolite of icodextrin contained in the peritoneal dialysis solution—leading to falsely elevated glucose results. JF switched to a different glucometer that did not use test strips containing the GDH-PQQ method, allowing for more accurate blood glucose readings and no recurrent episodes of severe hypoglycemia.
Continue for biochemistry of glucose measurements >>
BIOCHEMISTRY OF GLUCOSE MEASUREMENTS
In 1964, Ernie Adams invented Dextrostix, a paper strip that developed varying shades of color proportional to the glucose concentration. In 1970, Anton Clemens developed the first glucometer, the Ames Reflectance Meter (ARM), to detect reflected light from a Dextrostix. The ARM weighed 3 lb and cost $650.1
Modern glucometers analyze whole blood using both an enzymatic reaction and a detector. The enzyme is packaged in a dehydrated state contained in a disposable strip. The glucose in the patient’s blood rehydrates and reacts with enzymes in the strip to produce a detectable product.1
The gold standard for measuring glucose is isotope dilution mass spectrometry; however, this is not commonly performed in clinical laboratories. The accuracy of glucometers is most commonly assessed by comparing the glucometer result to a venous plasma sample collected at the same time and analyzed by a clinical laboratory using multi-analyte automated instrumentation.1
The two main types of commercially available glucometers are the glucose oxidase (GO) and glucose dehydrogenase (GDH) systems. The GO meters utilize the GO enzyme to catalyze the oxidation of glucose into gluconic acid. The oxidation reaction produces electrons that generate current proportional to the glucose level in the test sample.1-3
With GDH glucometers, several different enzymes can catalyze glucose oxidation, including nicotinamide adenine dinucleotide (GDH-NAD), flavin adenine dinucleotide (GDH-FAD), pyrroloquinoline quinone (GDH-PQQ), or mutant glucose dehydrogenase PQQ (Mut Q-GDH).2,4,5
Measurement of glucose using the hexokinase enzyme is considered more accurate than both the GO and GDH systems and is commonly used in clinical laboratories. However, the cost of this system is more than that of the commercially available glucometers, and thus it is not widely available.2
Continue for performance requirements for glucometer systems >>
PERFORMANCE REQUIREMENTS FOR GLUCOMETER SYSTEMS
There is no single standard for glucometer accuracy. Per Guideline 15197, issued by the International Organization for Standardization (ISO) in 2013, the minimum criteria for accuracy is at least 95% of blood glucose results within ± 15 mg/dL of the reference value at blood sugar concentrations < 100 mg/dL and within ± 15% at blood sugar concentrations ≥ 100 mg/dL.6 For OTC glucometers, the FDA has recommended that at least 95% of measurements fall within ± 15% and at least 99% of measurements fall within ± 20% of reference values across the entire claimed range of the glucometer system.7
The ISO and FDA both recommend that industry test glucometer accuracy using glucose levels ranging from ≤ 50 mg/dL to ≥ 400 mg/dL.6,7 They also recommend evaluating blood glucose accuracy at different hematocrit levels and assessing accuracy in the presence of interfering substances, such as acetaminophen, ibuprofen, salicylate, sodium, ascorbic acid, bilirubin, creatinine, dopamine, maltose, xylose, galactose, hemoglobin, heparin, L-dopa, methyldopa, triglycerides, cholesterol, sugar alcohols, and uric acid.6,7 The FDA additionally recommends testing glucometer accuracy in the presence of temperature extremes, humidity, and different altitudes.7
Currently, the premarket evaluation of glucometers is a one-time procedure that is typically conducted by the manufacturer. Not all available glucometers currently comply with the less stringent ISO accuracy standards from 2003, and most currently available glucometer systems fail to meet the more stringent accuracy criteria outlined by the ISO in 2013 and the FDA in 2014. Furthermore, there can be inconsistency in the measurement quality between different test strip lots, adding another variable to assessing glucometer accuracy.6
Continue for variables affecting glucometer accuracy >>
VARIABLES AFFECTING GLUCOMETER ACCURACY
Patient and environmental factors
Both patient and environmental factors can interfere with obtaining accurate glucometer results. These include sampling errors, improper storage of test strips, inadequate amount of blood applied to the test strip, improper meter coding, and altitude.1
Temperature extremes and humidity can denature, inactivate, or prematurely rehydrate enzymes and proteins within the test strip.1 GO meters can overestimate glucose levels at low temperatures, while GDH meters can produce unpredictable results in increased humidity.1 The detector portion of the meter is composed of electronics and should be protected from temperature extremes and excessive moisture as well.1
In high altitude, both GO and GDH meters can produce unreliable results, with a tendency to overestimate blood glucose levels.8 Another variable confounding the accuracy of glucometer readings at high altitude is the potential for secondary polycythemia, which can result in underestimation of glucose levels.8,9
Physiologic factors
Physiologic factors that can cause inaccurate glucometer results include hypoxia, abnormal pH, hyperuricemia, jaundice, polycythemia, anemia, peripheral vascular disease, and hypotension resulting in poor perfusion.1,7,9
Elevated oxygen tension in patients receiving oxygen therapy can falsely lower glucometer results for GO meters, while hypoxia can falsely elevate glucose results for these meters.1,3
Low pH (< 6.95), such as in diabetic ketoacidosis, falsely lowers glucose readings in GO meters, while a high pH falsely elevates glucose readings.1,10 Elevated serum uric acid (> 10-16 mg/dL) and elevated total bilirubin concentration (> 20 mg/dL) can cause overestimation of blood glucose levels due to electrochemical interaction at the electrode site in GDH-PQQ meters.11
Polycythemia can result in underestimation of glucose levels, and glucose levels can be overestimated in the setting of anemia.9 In anemia, the reduced red blood cell volume results in less displacement of plasma, causing more glucose molecules to be available to react with the enzyme contained in the test strip.12
Despite manufacturers’ claims that glucometers are reliable to a hematocrit range of 20% to 25%, clinically significant errors of greater than 20% were observed when the hematocrit level dropped below 34%, which can present challenges if glucometers are used in the ICU.13 Mathematical formulas to correct point-of-care glucometer measurements based on the hematocrit level have been proposed and have demonstrated effectiveness in decreasing the incidence of hypoglycemia in critically ill patients treated with insulin.12
Medications
Drugs that most commonly interfere with glucometer measurements include acetaminophen (especially at a serum concentration > 8 mg/dL), ascorbic acid, maltose, galactose, and xylose.1,11 Acetaminophen and ascorbic acid consume peroxide, resulting in falsely lowered blood glucose readings in GO meters. In GDH meters, direct oxidation can occur at the electrode site in the presence of acetaminophen and ascorbic acid, resulting in falsely elevated glucose levels.6,9,12
Maltose, galactose, and xylose are nonglucose sugars found in certain drug and biologic formulations, such as icodextrin peritoneal dialysis solution, certain immunoglobulins (Octagam 5%, WinRho SDF Liquid, Vaccinia Immune Globulin Intravenous [Human], and HepGamB), Orencia, and BEXXAR radioimmunotherapy agent.14
The GDH-PQQ meters cannot distinguish between glucose and nonglucose sugars, resulting in either undetected hypoglycemia or a falsely elevated glucose result (up to 3 to 15 times higher than corresponding laboratory results), which can lead to inappropriate medication dosing that results in potential hypoglycemia, coma, or death.14 Laboratory-based blood glucose assays, the GO, and most GDH-FAD, GDH-NAD, Mut Q-GDH, and hexokinase test strips do not have the potential for cross-reactivity from sugars other than glucose.4,14
It should be noted that in the United States, most GDH-PQQ test strips are no longer manufactured for home glucose testing. However, it is important to review the product insert contained in the test strip box for verification of the specific enzymatic methodology used in the test strip.4,5
Continue for the conclusion >>
CONCLUSION
Multiple factors affect the accuracy of currently available glucometers. Consideration of patient comorbidities, medication use, operational technique, and the conditions under which test strips are stored is important when utilizing glucometer data to make medication adjustments in diabetes management. It is important to refer to specific glucometer and test strip manufacturer device labeling to help select the appropriate glucometer for a particular patient.
The case presentation from 2009, involving falsely elevated blood glucose readings in a patient using a GDH-PQQ meter while receiving icodextrin peritoneal dialysis solution, highlights the importance of background knowledge of glucometer operational mechanisms. For a full list of test strips that are compatible with icodextrin peritoneal dialysis solution, please see the Country-Specific Glucose Monitor List at www.glucosesafety.com.5
Examples of specific GO meters include the OneTouch Ultra, iBGStar, and ReliOn meters. Although the GO meters do not cross-react with icodextrin, these meters should be avoided in patients receiving supplemental oxygen, due to the potential for falsely lowered readings.
The GDH-FAD, GDH-NAD, and Mut Q-GDH test strips may be used in patients receiving icodextrin peritoneal dialysis solution and those receiving supplemental oxygen.3,5 Examples of GDH-FAD meters include most currently available FreeStyle meters, Bayer Contour meters, and One Touch Verio meters. The Precision Xtra meter uses GDH-NAD test strips. Most Accu-Chek meters currently use Mut Q-GDH test strips.
REFERENCES
1. Tonyushkina K, Nichols JH. Glucose meters: a review of technical challenges to obtaining accurate results. J Diabetes Sci Technol. 2009;3(4):971-980.
2. Floré KMJ, Delanghe JR. Analytical interferences in point-of-care testing glucometers by icodextrin and its metabolites: an overview. Peritoneal Dial Int. 2009;29(4):377-383.
3. Tang Z, Louie RF, Lee JH, et al. Oxygen effects on glucose meter measurements with glucose dehydrogenase- and oxidase-based test strips for point-of-care testing. Crit Care Med. 2001;29(5):1062-1070.
4. Olansky L. Finger-stick glucose monitoring: issues of accuracy and specificity. Diabetes Care. 2010;33(4):948-949.
5. Baxter Healthcare Corporation. Country-specific glucose monitor list, 2015. www.glucosesafety.com/us/pdf/Glucose_Monitor_List.pdf. Accessed November 18, 2015.
6. Freckmann G, Schmid C, Baumstark A, et al. Analytical performance requirements for systems for self-monitoring of blood glucose with focus on system accuracy: relevant differences among ISO 15197:2003, ISO 15197: 2013, and current FDA recommendations. J Diabetes Sci Technol. 2015;9(4):885-894.
7. FDA. Self-Monitoring Blood Glucose Test Systems for Over-The-Counter Use: Draft Guidance for Industry and Food and Drug Administration Staff (2014). www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm380327.pdf. Accessed November 18, 2015.
8. Olateju T, Begley J, Flanagan D, Kerr D. Effects of simulated altitude on blood glucose meter performance: implications for in-flight blood glucose monitoring. J Diabetes Sci Technol. 2012;6(4):867-874.
9. Rao LV, Jakubiak F, Sidwell JS, et al. Accuracy evaluation of a new glucometer with automated hematocrit measurement and correction. Clinica Chimica Acta. 2005;356(1-2):178-183.
10. Tang Z, Du X, Louie RF, Kost GJ. Effects of pH on glucose measurements with handheld glucose meters and a portable glucose analyzer for point-of-care testing. Arch Pathol Lab Med. 2000;124:577-582.
11. Eastham JH, Mason D, Barnes DL, Kollins J. Prevalence of interfering substances with point-of-care glucose testing in a community hospital. Am J Health Syst Pharm. 2009;66: 167-170.
12 Pidcoke HF, Wade CE, Mann EA, et al. Anemia causes hypoglycemia in ICU patients due to error in single-channel glucometers: methods of reducing patient risk. Crit Care Med. 2010;38(2):471-476.
13. Mann EA, Pidcoke HF, Salinas J, et al. Accuracy of glucometers should not be assumed. Am J Crit Care. 2007;16(6):531-532.
14. FDA. FDA Public Health Notification: Potentially Fatal Errors with GDH-PQQ Glucose Monitoring Technology (2009). www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm176992.htm. Accessed November 18, 2015.
CLINICAL CASE FROM 2009
JF, a 64-year-old man with a 30-year history of type 2 diabetes managed with basal and rapid-acting prandial insulin, started peritoneal dialysis using icodextrin dialysis solution. Since starting dialysis, JF has experienced persistently elevated blood glucose readings (in the high 200 mg/dL to high 300 mg/dL range) using his Accu-Chek Compact glucometer purchased in 2008. In response, JF has been taking higher doses of rapid-acting insulin with meals and for correction, with two-to-three-hour postprandial blood glucose readings persistently elevated (in the high 200s). JF has no fevers, chills, abdominal pain, or other signs/symptoms of infection. Urine ketone testing is negative.
Yesterday, JF’s pre-lunch blood glucose registered at 380 mg/dL on his glucometer, and he took a dose of rapid-acting insulin that was double what he would have taken prior to starting dialysis. About 90 minutes after lunch, JF felt weak and diaphoretic and became unresponsive, with seizure-like activity. His wife called the paramedics; when they arrived, JF’s fingerstick glucose level was 28 mg/dL (using a One Touch Ultra glucometer).
JF was treated acutely with IV dextrose and then transported to a nearby hospital. During his hospitalization, his blood glucose level was maintained in the mid-100 to high-200 mg/dL range, with approximately 50% lower doses of rapid-acting insulin with meals. Hospital work-up revealed no evidence of secondary causes of hyperglycemia. EEG was negative.
Further investigation determined that JF’s Accu-Chek Compact glucometer used GDH-PQQ methodology, which is unable to distinguish between the blood glucose level and the maltose metabolite of icodextrin contained in the peritoneal dialysis solution—leading to falsely elevated glucose results. JF switched to a different glucometer that did not use test strips containing the GDH-PQQ method, allowing for more accurate blood glucose readings and no recurrent episodes of severe hypoglycemia.
Continue for biochemistry of glucose measurements >>
BIOCHEMISTRY OF GLUCOSE MEASUREMENTS
In 1964, Ernie Adams invented Dextrostix, a paper strip that developed varying shades of color proportional to the glucose concentration. In 1970, Anton Clemens developed the first glucometer, the Ames Reflectance Meter (ARM), to detect reflected light from a Dextrostix. The ARM weighed 3 lb and cost $650.1
Modern glucometers analyze whole blood using both an enzymatic reaction and a detector. The enzyme is packaged in a dehydrated state contained in a disposable strip. The glucose in the patient’s blood rehydrates and reacts with enzymes in the strip to produce a detectable product.1
The gold standard for measuring glucose is isotope dilution mass spectrometry; however, this is not commonly performed in clinical laboratories. The accuracy of glucometers is most commonly assessed by comparing the glucometer result to a venous plasma sample collected at the same time and analyzed by a clinical laboratory using multi-analyte automated instrumentation.1
The two main types of commercially available glucometers are the glucose oxidase (GO) and glucose dehydrogenase (GDH) systems. The GO meters utilize the GO enzyme to catalyze the oxidation of glucose into gluconic acid. The oxidation reaction produces electrons that generate current proportional to the glucose level in the test sample.1-3
With GDH glucometers, several different enzymes can catalyze glucose oxidation, including nicotinamide adenine dinucleotide (GDH-NAD), flavin adenine dinucleotide (GDH-FAD), pyrroloquinoline quinone (GDH-PQQ), or mutant glucose dehydrogenase PQQ (Mut Q-GDH).2,4,5
Measurement of glucose using the hexokinase enzyme is considered more accurate than both the GO and GDH systems and is commonly used in clinical laboratories. However, the cost of this system is more than that of the commercially available glucometers, and thus it is not widely available.2
Continue for performance requirements for glucometer systems >>
PERFORMANCE REQUIREMENTS FOR GLUCOMETER SYSTEMS
There is no single standard for glucometer accuracy. Per Guideline 15197, issued by the International Organization for Standardization (ISO) in 2013, the minimum criteria for accuracy is at least 95% of blood glucose results within ± 15 mg/dL of the reference value at blood sugar concentrations < 100 mg/dL and within ± 15% at blood sugar concentrations ≥ 100 mg/dL.6 For OTC glucometers, the FDA has recommended that at least 95% of measurements fall within ± 15% and at least 99% of measurements fall within ± 20% of reference values across the entire claimed range of the glucometer system.7
The ISO and FDA both recommend that industry test glucometer accuracy using glucose levels ranging from ≤ 50 mg/dL to ≥ 400 mg/dL.6,7 They also recommend evaluating blood glucose accuracy at different hematocrit levels and assessing accuracy in the presence of interfering substances, such as acetaminophen, ibuprofen, salicylate, sodium, ascorbic acid, bilirubin, creatinine, dopamine, maltose, xylose, galactose, hemoglobin, heparin, L-dopa, methyldopa, triglycerides, cholesterol, sugar alcohols, and uric acid.6,7 The FDA additionally recommends testing glucometer accuracy in the presence of temperature extremes, humidity, and different altitudes.7
Currently, the premarket evaluation of glucometers is a one-time procedure that is typically conducted by the manufacturer. Not all available glucometers currently comply with the less stringent ISO accuracy standards from 2003, and most currently available glucometer systems fail to meet the more stringent accuracy criteria outlined by the ISO in 2013 and the FDA in 2014. Furthermore, there can be inconsistency in the measurement quality between different test strip lots, adding another variable to assessing glucometer accuracy.6
Continue for variables affecting glucometer accuracy >>
VARIABLES AFFECTING GLUCOMETER ACCURACY
Patient and environmental factors
Both patient and environmental factors can interfere with obtaining accurate glucometer results. These include sampling errors, improper storage of test strips, inadequate amount of blood applied to the test strip, improper meter coding, and altitude.1
Temperature extremes and humidity can denature, inactivate, or prematurely rehydrate enzymes and proteins within the test strip.1 GO meters can overestimate glucose levels at low temperatures, while GDH meters can produce unpredictable results in increased humidity.1 The detector portion of the meter is composed of electronics and should be protected from temperature extremes and excessive moisture as well.1
In high altitude, both GO and GDH meters can produce unreliable results, with a tendency to overestimate blood glucose levels.8 Another variable confounding the accuracy of glucometer readings at high altitude is the potential for secondary polycythemia, which can result in underestimation of glucose levels.8,9
Physiologic factors
Physiologic factors that can cause inaccurate glucometer results include hypoxia, abnormal pH, hyperuricemia, jaundice, polycythemia, anemia, peripheral vascular disease, and hypotension resulting in poor perfusion.1,7,9
Elevated oxygen tension in patients receiving oxygen therapy can falsely lower glucometer results for GO meters, while hypoxia can falsely elevate glucose results for these meters.1,3
Low pH (< 6.95), such as in diabetic ketoacidosis, falsely lowers glucose readings in GO meters, while a high pH falsely elevates glucose readings.1,10 Elevated serum uric acid (> 10-16 mg/dL) and elevated total bilirubin concentration (> 20 mg/dL) can cause overestimation of blood glucose levels due to electrochemical interaction at the electrode site in GDH-PQQ meters.11
Polycythemia can result in underestimation of glucose levels, and glucose levels can be overestimated in the setting of anemia.9 In anemia, the reduced red blood cell volume results in less displacement of plasma, causing more glucose molecules to be available to react with the enzyme contained in the test strip.12
Despite manufacturers’ claims that glucometers are reliable to a hematocrit range of 20% to 25%, clinically significant errors of greater than 20% were observed when the hematocrit level dropped below 34%, which can present challenges if glucometers are used in the ICU.13 Mathematical formulas to correct point-of-care glucometer measurements based on the hematocrit level have been proposed and have demonstrated effectiveness in decreasing the incidence of hypoglycemia in critically ill patients treated with insulin.12
Medications
Drugs that most commonly interfere with glucometer measurements include acetaminophen (especially at a serum concentration > 8 mg/dL), ascorbic acid, maltose, galactose, and xylose.1,11 Acetaminophen and ascorbic acid consume peroxide, resulting in falsely lowered blood glucose readings in GO meters. In GDH meters, direct oxidation can occur at the electrode site in the presence of acetaminophen and ascorbic acid, resulting in falsely elevated glucose levels.6,9,12
Maltose, galactose, and xylose are nonglucose sugars found in certain drug and biologic formulations, such as icodextrin peritoneal dialysis solution, certain immunoglobulins (Octagam 5%, WinRho SDF Liquid, Vaccinia Immune Globulin Intravenous [Human], and HepGamB), Orencia, and BEXXAR radioimmunotherapy agent.14
The GDH-PQQ meters cannot distinguish between glucose and nonglucose sugars, resulting in either undetected hypoglycemia or a falsely elevated glucose result (up to 3 to 15 times higher than corresponding laboratory results), which can lead to inappropriate medication dosing that results in potential hypoglycemia, coma, or death.14 Laboratory-based blood glucose assays, the GO, and most GDH-FAD, GDH-NAD, Mut Q-GDH, and hexokinase test strips do not have the potential for cross-reactivity from sugars other than glucose.4,14
It should be noted that in the United States, most GDH-PQQ test strips are no longer manufactured for home glucose testing. However, it is important to review the product insert contained in the test strip box for verification of the specific enzymatic methodology used in the test strip.4,5
Continue for the conclusion >>
CONCLUSION
Multiple factors affect the accuracy of currently available glucometers. Consideration of patient comorbidities, medication use, operational technique, and the conditions under which test strips are stored is important when utilizing glucometer data to make medication adjustments in diabetes management. It is important to refer to specific glucometer and test strip manufacturer device labeling to help select the appropriate glucometer for a particular patient.
The case presentation from 2009, involving falsely elevated blood glucose readings in a patient using a GDH-PQQ meter while receiving icodextrin peritoneal dialysis solution, highlights the importance of background knowledge of glucometer operational mechanisms. For a full list of test strips that are compatible with icodextrin peritoneal dialysis solution, please see the Country-Specific Glucose Monitor List at www.glucosesafety.com.5
Examples of specific GO meters include the OneTouch Ultra, iBGStar, and ReliOn meters. Although the GO meters do not cross-react with icodextrin, these meters should be avoided in patients receiving supplemental oxygen, due to the potential for falsely lowered readings.
The GDH-FAD, GDH-NAD, and Mut Q-GDH test strips may be used in patients receiving icodextrin peritoneal dialysis solution and those receiving supplemental oxygen.3,5 Examples of GDH-FAD meters include most currently available FreeStyle meters, Bayer Contour meters, and One Touch Verio meters. The Precision Xtra meter uses GDH-NAD test strips. Most Accu-Chek meters currently use Mut Q-GDH test strips.
REFERENCES
1. Tonyushkina K, Nichols JH. Glucose meters: a review of technical challenges to obtaining accurate results. J Diabetes Sci Technol. 2009;3(4):971-980.
2. Floré KMJ, Delanghe JR. Analytical interferences in point-of-care testing glucometers by icodextrin and its metabolites: an overview. Peritoneal Dial Int. 2009;29(4):377-383.
3. Tang Z, Louie RF, Lee JH, et al. Oxygen effects on glucose meter measurements with glucose dehydrogenase- and oxidase-based test strips for point-of-care testing. Crit Care Med. 2001;29(5):1062-1070.
4. Olansky L. Finger-stick glucose monitoring: issues of accuracy and specificity. Diabetes Care. 2010;33(4):948-949.
5. Baxter Healthcare Corporation. Country-specific glucose monitor list, 2015. www.glucosesafety.com/us/pdf/Glucose_Monitor_List.pdf. Accessed November 18, 2015.
6. Freckmann G, Schmid C, Baumstark A, et al. Analytical performance requirements for systems for self-monitoring of blood glucose with focus on system accuracy: relevant differences among ISO 15197:2003, ISO 15197: 2013, and current FDA recommendations. J Diabetes Sci Technol. 2015;9(4):885-894.
7. FDA. Self-Monitoring Blood Glucose Test Systems for Over-The-Counter Use: Draft Guidance for Industry and Food and Drug Administration Staff (2014). www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm380327.pdf. Accessed November 18, 2015.
8. Olateju T, Begley J, Flanagan D, Kerr D. Effects of simulated altitude on blood glucose meter performance: implications for in-flight blood glucose monitoring. J Diabetes Sci Technol. 2012;6(4):867-874.
9. Rao LV, Jakubiak F, Sidwell JS, et al. Accuracy evaluation of a new glucometer with automated hematocrit measurement and correction. Clinica Chimica Acta. 2005;356(1-2):178-183.
10. Tang Z, Du X, Louie RF, Kost GJ. Effects of pH on glucose measurements with handheld glucose meters and a portable glucose analyzer for point-of-care testing. Arch Pathol Lab Med. 2000;124:577-582.
11. Eastham JH, Mason D, Barnes DL, Kollins J. Prevalence of interfering substances with point-of-care glucose testing in a community hospital. Am J Health Syst Pharm. 2009;66: 167-170.
12 Pidcoke HF, Wade CE, Mann EA, et al. Anemia causes hypoglycemia in ICU patients due to error in single-channel glucometers: methods of reducing patient risk. Crit Care Med. 2010;38(2):471-476.
13. Mann EA, Pidcoke HF, Salinas J, et al. Accuracy of glucometers should not be assumed. Am J Crit Care. 2007;16(6):531-532.
14. FDA. FDA Public Health Notification: Potentially Fatal Errors with GDH-PQQ Glucose Monitoring Technology (2009). www.fda.gov/MedicalDevices/Safety/AlertsandNotices/PublicHealthNotifications/ucm176992.htm. Accessed November 18, 2015.
Renal Denervation
Q) I’ve heard a lot of references to “renal denervation” and its use for resistant hypertension. What is it? Does it work? Is it common in the US?
Renal denervation is a minimally invasive endovascular procedure that ablates (or disrupts) the renal nerves in and around the renal arteries with radiofrequency energy.5 Renal denervation has been approved in the US and other countries and is being used clinically in Europe, Canada, and Australia.6
It is thought that renal denervation interrupts the efferent and afferent signals that stimulate the renin-angiotensin-aldosterone system (RAAS) and regulate whole-body sympathetic nervous system activity.5 Similar to surgical sympathectomy, renal denervation should theoretically lower blood pressure. However, Ezzahti et al found that renin levels did not decrease in patients following renal denervation.7
Drug-resistant hypertension is defined as blood pressure that remains greater than 140/90 mm Hg despite treatment with three or more antihypertensive medications, including a diuretic.8 Patients with resistant hypertension have increased cardiovascular risk.9 Clinical trials of renal denervation have focused on treatment of resistant hypertension, in the hope of reducing the associated morbidity and mortality.
Results of the Symplicity HTN-3 trial, which assessed the safety and efficacy of renal denervation, were anxiously awaited, since prior trials yielded mixed results. Although the Symplicity HTN-1 and Symplicity HTN-2 studies demonstrated a possible benefit of renal denervation to lower office measured blood pressure, other studies did not show a decrease in BP in patients who had undergone renal denervation.6,7 These early trials, however, were small and did not randomize patients to a sham procedure.10
The Symplicity HTN-3 trial included 535 patients at 88 centers in the US. Patients were randomly assigned to receive either renal denervation plus baseline antihypertensive medications or a sham procedure plus baseline antihypertensive medications.
The researchers found that the sham procedure was just as effective as the “true” renal denervation in decreasing systolic blood pressure in patients with resistant hypertension.10 In other words, renal denervation did not demonstrate efficacy for this purpose.
In response to the results of this well-designed trial, the FDA has halted approval to perform renal denervation in patients with resistant hypertension in the US. However, clinical investigation will continue among subgroups of hypertensive patients or separate populations.
Despite a lack of efficacy, renal denervation does appear to be well tolerated, as evidenced by safety data from Symplicity HTN-3. —JK
Jessica Knight, ACNP
University of New Mexico Hospital, Albuquerque
REFERENCES
5. Esler MD, Krum H, Schlaich M, et al. Renal sympathetic denervation for the treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation. 2012;126(25):2976-2982.
6. Thukkani AK, Bhatt LD. Renal denervation therapy for hypertension. Circulation. 2013;128:2251-2254.
7. Ezzahti M, Moelker A, Friesema E, et al. Blood pressure and neurohormonal responses to renal nerve ablation in treatment-resistant hypertension. J Hypertens. 2014;32(1):135-141.
8. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: Diagnosis, evaluation, and treatment: A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51(6):1403-1419.
9. Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125(13):1635-1642.
10. Bhatt DL, Kandzari DE, O’Neill WW, et al; Symplicity HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370(15):1393-1401.
The author would like to thank Eric Judd, MD, of the University of Alabama at Birmingham, for his advice on the preparation of this response.
Q) I’ve heard a lot of references to “renal denervation” and its use for resistant hypertension. What is it? Does it work? Is it common in the US?
Renal denervation is a minimally invasive endovascular procedure that ablates (or disrupts) the renal nerves in and around the renal arteries with radiofrequency energy.5 Renal denervation has been approved in the US and other countries and is being used clinically in Europe, Canada, and Australia.6
It is thought that renal denervation interrupts the efferent and afferent signals that stimulate the renin-angiotensin-aldosterone system (RAAS) and regulate whole-body sympathetic nervous system activity.5 Similar to surgical sympathectomy, renal denervation should theoretically lower blood pressure. However, Ezzahti et al found that renin levels did not decrease in patients following renal denervation.7
Drug-resistant hypertension is defined as blood pressure that remains greater than 140/90 mm Hg despite treatment with three or more antihypertensive medications, including a diuretic.8 Patients with resistant hypertension have increased cardiovascular risk.9 Clinical trials of renal denervation have focused on treatment of resistant hypertension, in the hope of reducing the associated morbidity and mortality.
Results of the Symplicity HTN-3 trial, which assessed the safety and efficacy of renal denervation, were anxiously awaited, since prior trials yielded mixed results. Although the Symplicity HTN-1 and Symplicity HTN-2 studies demonstrated a possible benefit of renal denervation to lower office measured blood pressure, other studies did not show a decrease in BP in patients who had undergone renal denervation.6,7 These early trials, however, were small and did not randomize patients to a sham procedure.10
The Symplicity HTN-3 trial included 535 patients at 88 centers in the US. Patients were randomly assigned to receive either renal denervation plus baseline antihypertensive medications or a sham procedure plus baseline antihypertensive medications.
The researchers found that the sham procedure was just as effective as the “true” renal denervation in decreasing systolic blood pressure in patients with resistant hypertension.10 In other words, renal denervation did not demonstrate efficacy for this purpose.
In response to the results of this well-designed trial, the FDA has halted approval to perform renal denervation in patients with resistant hypertension in the US. However, clinical investigation will continue among subgroups of hypertensive patients or separate populations.
Despite a lack of efficacy, renal denervation does appear to be well tolerated, as evidenced by safety data from Symplicity HTN-3. —JK
Jessica Knight, ACNP
University of New Mexico Hospital, Albuquerque
REFERENCES
5. Esler MD, Krum H, Schlaich M, et al. Renal sympathetic denervation for the treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation. 2012;126(25):2976-2982.
6. Thukkani AK, Bhatt LD. Renal denervation therapy for hypertension. Circulation. 2013;128:2251-2254.
7. Ezzahti M, Moelker A, Friesema E, et al. Blood pressure and neurohormonal responses to renal nerve ablation in treatment-resistant hypertension. J Hypertens. 2014;32(1):135-141.
8. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: Diagnosis, evaluation, and treatment: A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51(6):1403-1419.
9. Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125(13):1635-1642.
10. Bhatt DL, Kandzari DE, O’Neill WW, et al; Symplicity HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370(15):1393-1401.
The author would like to thank Eric Judd, MD, of the University of Alabama at Birmingham, for his advice on the preparation of this response.
Q) I’ve heard a lot of references to “renal denervation” and its use for resistant hypertension. What is it? Does it work? Is it common in the US?
Renal denervation is a minimally invasive endovascular procedure that ablates (or disrupts) the renal nerves in and around the renal arteries with radiofrequency energy.5 Renal denervation has been approved in the US and other countries and is being used clinically in Europe, Canada, and Australia.6
It is thought that renal denervation interrupts the efferent and afferent signals that stimulate the renin-angiotensin-aldosterone system (RAAS) and regulate whole-body sympathetic nervous system activity.5 Similar to surgical sympathectomy, renal denervation should theoretically lower blood pressure. However, Ezzahti et al found that renin levels did not decrease in patients following renal denervation.7
Drug-resistant hypertension is defined as blood pressure that remains greater than 140/90 mm Hg despite treatment with three or more antihypertensive medications, including a diuretic.8 Patients with resistant hypertension have increased cardiovascular risk.9 Clinical trials of renal denervation have focused on treatment of resistant hypertension, in the hope of reducing the associated morbidity and mortality.
Results of the Symplicity HTN-3 trial, which assessed the safety and efficacy of renal denervation, were anxiously awaited, since prior trials yielded mixed results. Although the Symplicity HTN-1 and Symplicity HTN-2 studies demonstrated a possible benefit of renal denervation to lower office measured blood pressure, other studies did not show a decrease in BP in patients who had undergone renal denervation.6,7 These early trials, however, were small and did not randomize patients to a sham procedure.10
The Symplicity HTN-3 trial included 535 patients at 88 centers in the US. Patients were randomly assigned to receive either renal denervation plus baseline antihypertensive medications or a sham procedure plus baseline antihypertensive medications.
The researchers found that the sham procedure was just as effective as the “true” renal denervation in decreasing systolic blood pressure in patients with resistant hypertension.10 In other words, renal denervation did not demonstrate efficacy for this purpose.
In response to the results of this well-designed trial, the FDA has halted approval to perform renal denervation in patients with resistant hypertension in the US. However, clinical investigation will continue among subgroups of hypertensive patients or separate populations.
Despite a lack of efficacy, renal denervation does appear to be well tolerated, as evidenced by safety data from Symplicity HTN-3. —JK
Jessica Knight, ACNP
University of New Mexico Hospital, Albuquerque
REFERENCES
5. Esler MD, Krum H, Schlaich M, et al. Renal sympathetic denervation for the treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation. 2012;126(25):2976-2982.
6. Thukkani AK, Bhatt LD. Renal denervation therapy for hypertension. Circulation. 2013;128:2251-2254.
7. Ezzahti M, Moelker A, Friesema E, et al. Blood pressure and neurohormonal responses to renal nerve ablation in treatment-resistant hypertension. J Hypertens. 2014;32(1):135-141.
8. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: Diagnosis, evaluation, and treatment: A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51(6):1403-1419.
9. Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125(13):1635-1642.
10. Bhatt DL, Kandzari DE, O’Neill WW, et al; Symplicity HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370(15):1393-1401.
The author would like to thank Eric Judd, MD, of the University of Alabama at Birmingham, for his advice on the preparation of this response.
Predictive Factors for CKD
Q) Quite a few of my teenage patients are overweight. I know they are at risk for diabetes, but does their weight also affect their kidneys? Isn’t diabetes the main cause of kidney failure?
The number one cause of chronic kidney disease (CKD) in the United States and worldwide is diabetes, but it is certainly not the only risk factor. Studies have shown a link between obesity and CKD; even in the absence of kidney disease, obesity may cause glomerular dysfunction and an increase in glomerular size.1
Obesity during adolescence has been identified as a strong predictor of CKD in adulthood. Other diseases and conditions that, if present in adolescence, indicate future risk for kidney disease include diabetes, hypertension, inflammation, and proteinuria.
A recent Swedish study followed patients from adolescence to adulthood to identify markers that would predict later kidney disease. In this study, the most predictive factor of kidney failure in adulthood was proteinuria in adolescence (odds ratio, 7.72). These results may be limited by the homogeneity of the predominantly white, male study population, but the extensive follow-up period, which “highlights the long natural history” of kidney disease, is one strength of this study.2
Based on these and other findings, you know that if your teenage patients have proteinuria, they are much more likely to develop kidney failure as an adult. Yet, in the US, the American Academy of Pediatrics and the US Preventive Services Task Force do not recommend urine screening for asymptomatic children.3
Interestingly, however, a survey of pediatric practices revealed that 58% of pediatricians screen adolescents with urinalysis, even if they are asymptomatic.4 In other words, they ignore the guidelines. If they did not, we would likely miss what is possibly the most important predictive factor for kidney failure in adults. —TAH
Tia Austin Hayes, FNP-C
UMMC/JMM Outpatient Dialysis/Renal Clinic, Jackson, Mississippi
REFERENCES
1. Rocchini A. Childhood obesity and a diabetes epidemic. N Engl J Med. 2002;346(11):854-855.
2. Sundin PO, Udumyan R, Sjöström P, Montgomery S. Predictors in adolescence of ESRD in middle-aged men. Am J Kidney Dis. 2014;64(5):723-729.
3. Kaplan RE, Springate JE, Feld LG. Screening dipstick urinalysis: a time to change. Pediatrics. 1997;100(6):919-921.
4. Sox CM, Christakis DA. Pediatricians’ screening urinalysis practices. J Pediatr. 2005; 147(3):362-365.
The author would like to thank Eric Judd, MD, of the University of Alabama at Birmingham, for his advice on the preparation of this response.
Q) Quite a few of my teenage patients are overweight. I know they are at risk for diabetes, but does their weight also affect their kidneys? Isn’t diabetes the main cause of kidney failure?
The number one cause of chronic kidney disease (CKD) in the United States and worldwide is diabetes, but it is certainly not the only risk factor. Studies have shown a link between obesity and CKD; even in the absence of kidney disease, obesity may cause glomerular dysfunction and an increase in glomerular size.1
Obesity during adolescence has been identified as a strong predictor of CKD in adulthood. Other diseases and conditions that, if present in adolescence, indicate future risk for kidney disease include diabetes, hypertension, inflammation, and proteinuria.
A recent Swedish study followed patients from adolescence to adulthood to identify markers that would predict later kidney disease. In this study, the most predictive factor of kidney failure in adulthood was proteinuria in adolescence (odds ratio, 7.72). These results may be limited by the homogeneity of the predominantly white, male study population, but the extensive follow-up period, which “highlights the long natural history” of kidney disease, is one strength of this study.2
Based on these and other findings, you know that if your teenage patients have proteinuria, they are much more likely to develop kidney failure as an adult. Yet, in the US, the American Academy of Pediatrics and the US Preventive Services Task Force do not recommend urine screening for asymptomatic children.3
Interestingly, however, a survey of pediatric practices revealed that 58% of pediatricians screen adolescents with urinalysis, even if they are asymptomatic.4 In other words, they ignore the guidelines. If they did not, we would likely miss what is possibly the most important predictive factor for kidney failure in adults. —TAH
Tia Austin Hayes, FNP-C
UMMC/JMM Outpatient Dialysis/Renal Clinic, Jackson, Mississippi
REFERENCES
1. Rocchini A. Childhood obesity and a diabetes epidemic. N Engl J Med. 2002;346(11):854-855.
2. Sundin PO, Udumyan R, Sjöström P, Montgomery S. Predictors in adolescence of ESRD in middle-aged men. Am J Kidney Dis. 2014;64(5):723-729.
3. Kaplan RE, Springate JE, Feld LG. Screening dipstick urinalysis: a time to change. Pediatrics. 1997;100(6):919-921.
4. Sox CM, Christakis DA. Pediatricians’ screening urinalysis practices. J Pediatr. 2005; 147(3):362-365.
The author would like to thank Eric Judd, MD, of the University of Alabama at Birmingham, for his advice on the preparation of this response.
Q) Quite a few of my teenage patients are overweight. I know they are at risk for diabetes, but does their weight also affect their kidneys? Isn’t diabetes the main cause of kidney failure?
The number one cause of chronic kidney disease (CKD) in the United States and worldwide is diabetes, but it is certainly not the only risk factor. Studies have shown a link between obesity and CKD; even in the absence of kidney disease, obesity may cause glomerular dysfunction and an increase in glomerular size.1
Obesity during adolescence has been identified as a strong predictor of CKD in adulthood. Other diseases and conditions that, if present in adolescence, indicate future risk for kidney disease include diabetes, hypertension, inflammation, and proteinuria.
A recent Swedish study followed patients from adolescence to adulthood to identify markers that would predict later kidney disease. In this study, the most predictive factor of kidney failure in adulthood was proteinuria in adolescence (odds ratio, 7.72). These results may be limited by the homogeneity of the predominantly white, male study population, but the extensive follow-up period, which “highlights the long natural history” of kidney disease, is one strength of this study.2
Based on these and other findings, you know that if your teenage patients have proteinuria, they are much more likely to develop kidney failure as an adult. Yet, in the US, the American Academy of Pediatrics and the US Preventive Services Task Force do not recommend urine screening for asymptomatic children.3
Interestingly, however, a survey of pediatric practices revealed that 58% of pediatricians screen adolescents with urinalysis, even if they are asymptomatic.4 In other words, they ignore the guidelines. If they did not, we would likely miss what is possibly the most important predictive factor for kidney failure in adults. —TAH
Tia Austin Hayes, FNP-C
UMMC/JMM Outpatient Dialysis/Renal Clinic, Jackson, Mississippi
REFERENCES
1. Rocchini A. Childhood obesity and a diabetes epidemic. N Engl J Med. 2002;346(11):854-855.
2. Sundin PO, Udumyan R, Sjöström P, Montgomery S. Predictors in adolescence of ESRD in middle-aged men. Am J Kidney Dis. 2014;64(5):723-729.
3. Kaplan RE, Springate JE, Feld LG. Screening dipstick urinalysis: a time to change. Pediatrics. 1997;100(6):919-921.
4. Sox CM, Christakis DA. Pediatricians’ screening urinalysis practices. J Pediatr. 2005; 147(3):362-365.
The author would like to thank Eric Judd, MD, of the University of Alabama at Birmingham, for his advice on the preparation of this response.
Investigating Unstable Thyroid Function
A 43-year-old man presents for his thyroid checkup. He has known hypothyroidism secondary to Hashimoto thyroiditis, also known as chronic lymphocytic thyroiditis. He is taking levothyroxine (LT4) 250 μg (two 125-μg tablets once per day). Review of his prior lab results and notes (see Table 1) reveals frequent dose changes (about every three to six months) and a high dosage of LT4, considering his weight (185 lb).
Patients with little or no residual thyroid function require replacement doses of LT4 at approximately 1.6 μg/kg/d, based on lean body weight.1 Since the case patient weighs 84 kg, the expected LT4 dosage would be around 125 to 150 μg/d.
This patient requires a significantly higher dose than expected, and his thyroid levels are fluctuating. These facts should trigger further investigation.
Important historical questions I consider when patients have frequent or significant fluctuations in TSH include
• Are you consistent in taking your medication?
• How do you take your thyroid medication?
• Are you taking any iron supplements, vitamins with iron, or contraceptive pills containing iron?
• Has there been any change in your other medication regimen(s) or medical condition(s)?
• Did you change pharmacies, or did the shape or color of your pill change?
• Have you experienced significant weight changes?
• Do you have any gastrointestinal complaints (nausea/vomiting/diarrhea/bloating)?
MEDICATION ADHERENCE
It is well known but still puzzling to hear that, overall, patients’ medication adherence is merely 50%.2 It is very important that you verify whether your patient is taking his/her medication consistently. Rather than asking “Are you taking your medications?” (to which they are more likely to answer “yes”), I ask “How many pills do you miss in a given week or month?”
For those who have a hard time remembering to take their medication on a regular basis, I recommend setting up a routine: Keep the medication at their bedside and take it first thing upon awakening, or place it beside the toothpaste so they see it every time they brush their teeth in the morning. Another option is of course to set up an alarm as a reminder.
Continue for rules for taking hypothyroid >>
RULES FOR TAKING HYPOTHYROID MEDICATIONS
Thyroid hormone replacement has a narrow therapeutic index, and a subtle change in dosage can significantly alter the therapeutic target. Hypothyroid medications are absorbed in the jejunum/ileum, and an acidic pH in the stomach is optimal for thyroid absorption.3 Therefore, taking the medication on an empty stomach (fasting) with a full glass of water and waiting at least one hour before breakfast is recommended, if possible. An alternate option is to take it at bedtime, at least three hours after the last meal. Taking medication along with food, especially high-fiber and soy products, can decrease absorption of thyroid hormone, which may result in an unstable thyroid function test.
There are supplements and medications that can decrease hypothyroid medication absorption; it is recommended that patients separate these medications by four hours or more in order to minimize this interference. A full list is available in Table 2, but the most commonly encountered are iron supplements, calcium supplements, and proton pump inhibitors.2
In many patients—especially the elderly and those with multiple comorbidities that require polypharmacy—it can be very challenging, if not impossible, to isolate thyroid medication. For these patients, recommend that they be “consistent” with their routine to ensure they achieve a similar absorption rate each time. For example, a patient’s hypothyroid medication absorption might be reduced by 50% by taking it with omeprazole, but as long as the patient consistently takes the medication that way, she can have stable thyroid function.
NEW MEDICATION REGIMEN OR MEDICAL CONDITION
In addition to medications that can interfere with the absorption of thyroid hormone replacement, there are those that affect levels of thyroxine-binding globulin. This affects the bioavailability of thyroid hormones and alters thyroid status.
Thyroid hormones such as thyroxine (T4) and triiodothyronine (T3) are predominantly bound to carrier proteins, and < 1% is unbound (so-called free hormones). Changes in thyroid-binding proteins can alter free hormone levels and thereby change TSH levels. In disease-free euthyroid subjects, the body can compensate by adjusting hormone production for changes in binding proteins to keep the free hormone levels within normal ranges. However, patients who are at or near full replacement doses of hypothyroid medication cannot adjust to the changes.
In patients with hypothyroidism who are taking thyroid hormone replacement, medications or conditions that increase binding proteins will decrease free hormones (by increasing bound hormones) and thereby raise TSH (hypothyroid state). Vice versa, medications and conditions that decrease binding protein will increase free hormones (by decreasing bound hormones) and thereby lower TSH (thyrotoxic state). Table 3 lists commonly encountered medications and conditions associated with altered thyroid-binding proteins.1
It is important to consider pregnancy in women of childbearing age whose TSH has risen for no apparent reason, as their thyroid levels should be maintained in a narrow therapeutic range to prevent fetal complications. Details on thyroid disease during pregnancy can be found in the April 2015 Endocrine Consult, “Managing Thyroid Disease in Pregnancy.”
In women treated for hypothyroidism, starting or discontinuing estrogen-containing medications (birth control pills or hormone replacement therapy) often results in changes in thyroid status. It is a good practice to inform the patient about these changes and to recheck her thyroid labs four to eight weeks after she starts or discontinues estrogen, adjusting the dose if needed.
Continue for changes in manufacturer/brand >>
CHANGES IN MANUFACTURER/BRAND
There are currently multiple brands and generic manufacturers supplying hypothyroid medications and reports that absorption rates and bioavailability vary among them.2 Switching products can result in changes in thyroid status and in TSH levels.
Once a patient has reached euthyroid status, it is imperative to stay on the same dose from the same manufacturer. This may be challenging, as it can be affected by the patient’s insurance carrier, policy changes, or even a change in the pharmacy’s medication supplier. Although patients are supposed to be informed by the pharmacy when the manufacturer is being changed, you may want to educate them to check the shape, color, and dose of their pills and also verify that the manufacturer listed on the bottle is consistent each time they refill their hypothyroid medications. This is especially important for those who require a very narrow TSH target, such as young children, thyroid cancer patients, pregnant women, and frail patients.3
WEIGHT CHANGES
As mentioned, thyroid medications are weight-based, and big changes in weight can lead to changes in thyroid function studies. It is the lean body mass, rather than total body weight, that will affect the thyroid requirement.3 A quick review of the patient’s weight history needs to be done when thyroid function test results have changed.
GASTROINTESTINAL DISTURBANCES
Hypothyroid medications are absorbed in the small intestine, and gastric acidity levels have an impact on absorption. Any acute or chronic conditions that affect these areas can alter medication absorption quite significantly. Commonly encountered diseases and conditions are H pylori–related gastritis, atrophic gastritis, celiac disease, and lactose intolerance. Treating these diseases and conditions can improve medication absorption.
I went through the list with the patient, but there was no applicable scenario. I adjusted his medication but went ahead and tested for tissue transglutaminase antibody IgA to rule out celiac disease; results came back mildly positive. The patient was referred to a gastroenterologist, who performed a small intestine biopsy for definitive diagnosis. This revealed “severe” celiac disease. A strict gluten-free diet was started, and the patient’s LT4 dose was adjusted, with regular monitoring, down to 150 μg/d.
Common symptoms of celiac disease include bloating, abdominal pain, and loose stool after consumption of gluten-containing meals. It should be noted that this patient denied all these symptoms, even though he was asked specifically about them. After he started a gluten-free diet, he reported that he actually felt “very calm” in his abdomen and realized he did have symptoms of celiac disease—but he’d had them for so long that he considered it normal. As is often the case, presence of symptoms would raise suspicion ... but lack of symptoms (or report thereof) does not rule out the disease.
CONCLUSION
Most patients with hypothyroidism are fairly well managed with relatively stable medication dosages, but there are subsets of patients who struggle to maintain euthyroid range. The latter require frequent office visits and dosage changes. Carefully reviewing the list of possible reasons for thyroid level changes can improve stability and patient quality of life, prevent complications of fluctuating thyroid levels, and reduce medical costs, such as repeated labs and frequent clinic visits.
REFERENCES
1. Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults [published correction appears in Endocr Pract. 2013;19(1):175]. Endocr Pract. 2012;18(6):988-1028.
2. Sabate E. Adherence to Long-Term Therapies: Evidence for Action. Geneva, Switzerland: World Health Organization; 2003.
3. Jonklaas J, Bianco AC, Bauer AJ, et al; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism. Thyroid. 2014;24(12):1670-1751.
A 43-year-old man presents for his thyroid checkup. He has known hypothyroidism secondary to Hashimoto thyroiditis, also known as chronic lymphocytic thyroiditis. He is taking levothyroxine (LT4) 250 μg (two 125-μg tablets once per day). Review of his prior lab results and notes (see Table 1) reveals frequent dose changes (about every three to six months) and a high dosage of LT4, considering his weight (185 lb).
Patients with little or no residual thyroid function require replacement doses of LT4 at approximately 1.6 μg/kg/d, based on lean body weight.1 Since the case patient weighs 84 kg, the expected LT4 dosage would be around 125 to 150 μg/d.
This patient requires a significantly higher dose than expected, and his thyroid levels are fluctuating. These facts should trigger further investigation.
Important historical questions I consider when patients have frequent or significant fluctuations in TSH include
• Are you consistent in taking your medication?
• How do you take your thyroid medication?
• Are you taking any iron supplements, vitamins with iron, or contraceptive pills containing iron?
• Has there been any change in your other medication regimen(s) or medical condition(s)?
• Did you change pharmacies, or did the shape or color of your pill change?
• Have you experienced significant weight changes?
• Do you have any gastrointestinal complaints (nausea/vomiting/diarrhea/bloating)?
MEDICATION ADHERENCE
It is well known but still puzzling to hear that, overall, patients’ medication adherence is merely 50%.2 It is very important that you verify whether your patient is taking his/her medication consistently. Rather than asking “Are you taking your medications?” (to which they are more likely to answer “yes”), I ask “How many pills do you miss in a given week or month?”
For those who have a hard time remembering to take their medication on a regular basis, I recommend setting up a routine: Keep the medication at their bedside and take it first thing upon awakening, or place it beside the toothpaste so they see it every time they brush their teeth in the morning. Another option is of course to set up an alarm as a reminder.
Continue for rules for taking hypothyroid >>
RULES FOR TAKING HYPOTHYROID MEDICATIONS
Thyroid hormone replacement has a narrow therapeutic index, and a subtle change in dosage can significantly alter the therapeutic target. Hypothyroid medications are absorbed in the jejunum/ileum, and an acidic pH in the stomach is optimal for thyroid absorption.3 Therefore, taking the medication on an empty stomach (fasting) with a full glass of water and waiting at least one hour before breakfast is recommended, if possible. An alternate option is to take it at bedtime, at least three hours after the last meal. Taking medication along with food, especially high-fiber and soy products, can decrease absorption of thyroid hormone, which may result in an unstable thyroid function test.
There are supplements and medications that can decrease hypothyroid medication absorption; it is recommended that patients separate these medications by four hours or more in order to minimize this interference. A full list is available in Table 2, but the most commonly encountered are iron supplements, calcium supplements, and proton pump inhibitors.2
In many patients—especially the elderly and those with multiple comorbidities that require polypharmacy—it can be very challenging, if not impossible, to isolate thyroid medication. For these patients, recommend that they be “consistent” with their routine to ensure they achieve a similar absorption rate each time. For example, a patient’s hypothyroid medication absorption might be reduced by 50% by taking it with omeprazole, but as long as the patient consistently takes the medication that way, she can have stable thyroid function.
NEW MEDICATION REGIMEN OR MEDICAL CONDITION
In addition to medications that can interfere with the absorption of thyroid hormone replacement, there are those that affect levels of thyroxine-binding globulin. This affects the bioavailability of thyroid hormones and alters thyroid status.
Thyroid hormones such as thyroxine (T4) and triiodothyronine (T3) are predominantly bound to carrier proteins, and < 1% is unbound (so-called free hormones). Changes in thyroid-binding proteins can alter free hormone levels and thereby change TSH levels. In disease-free euthyroid subjects, the body can compensate by adjusting hormone production for changes in binding proteins to keep the free hormone levels within normal ranges. However, patients who are at or near full replacement doses of hypothyroid medication cannot adjust to the changes.
In patients with hypothyroidism who are taking thyroid hormone replacement, medications or conditions that increase binding proteins will decrease free hormones (by increasing bound hormones) and thereby raise TSH (hypothyroid state). Vice versa, medications and conditions that decrease binding protein will increase free hormones (by decreasing bound hormones) and thereby lower TSH (thyrotoxic state). Table 3 lists commonly encountered medications and conditions associated with altered thyroid-binding proteins.1
It is important to consider pregnancy in women of childbearing age whose TSH has risen for no apparent reason, as their thyroid levels should be maintained in a narrow therapeutic range to prevent fetal complications. Details on thyroid disease during pregnancy can be found in the April 2015 Endocrine Consult, “Managing Thyroid Disease in Pregnancy.”
In women treated for hypothyroidism, starting or discontinuing estrogen-containing medications (birth control pills or hormone replacement therapy) often results in changes in thyroid status. It is a good practice to inform the patient about these changes and to recheck her thyroid labs four to eight weeks after she starts or discontinues estrogen, adjusting the dose if needed.
Continue for changes in manufacturer/brand >>
CHANGES IN MANUFACTURER/BRAND
There are currently multiple brands and generic manufacturers supplying hypothyroid medications and reports that absorption rates and bioavailability vary among them.2 Switching products can result in changes in thyroid status and in TSH levels.
Once a patient has reached euthyroid status, it is imperative to stay on the same dose from the same manufacturer. This may be challenging, as it can be affected by the patient’s insurance carrier, policy changes, or even a change in the pharmacy’s medication supplier. Although patients are supposed to be informed by the pharmacy when the manufacturer is being changed, you may want to educate them to check the shape, color, and dose of their pills and also verify that the manufacturer listed on the bottle is consistent each time they refill their hypothyroid medications. This is especially important for those who require a very narrow TSH target, such as young children, thyroid cancer patients, pregnant women, and frail patients.3
WEIGHT CHANGES
As mentioned, thyroid medications are weight-based, and big changes in weight can lead to changes in thyroid function studies. It is the lean body mass, rather than total body weight, that will affect the thyroid requirement.3 A quick review of the patient’s weight history needs to be done when thyroid function test results have changed.
GASTROINTESTINAL DISTURBANCES
Hypothyroid medications are absorbed in the small intestine, and gastric acidity levels have an impact on absorption. Any acute or chronic conditions that affect these areas can alter medication absorption quite significantly. Commonly encountered diseases and conditions are H pylori–related gastritis, atrophic gastritis, celiac disease, and lactose intolerance. Treating these diseases and conditions can improve medication absorption.
I went through the list with the patient, but there was no applicable scenario. I adjusted his medication but went ahead and tested for tissue transglutaminase antibody IgA to rule out celiac disease; results came back mildly positive. The patient was referred to a gastroenterologist, who performed a small intestine biopsy for definitive diagnosis. This revealed “severe” celiac disease. A strict gluten-free diet was started, and the patient’s LT4 dose was adjusted, with regular monitoring, down to 150 μg/d.
Common symptoms of celiac disease include bloating, abdominal pain, and loose stool after consumption of gluten-containing meals. It should be noted that this patient denied all these symptoms, even though he was asked specifically about them. After he started a gluten-free diet, he reported that he actually felt “very calm” in his abdomen and realized he did have symptoms of celiac disease—but he’d had them for so long that he considered it normal. As is often the case, presence of symptoms would raise suspicion ... but lack of symptoms (or report thereof) does not rule out the disease.
CONCLUSION
Most patients with hypothyroidism are fairly well managed with relatively stable medication dosages, but there are subsets of patients who struggle to maintain euthyroid range. The latter require frequent office visits and dosage changes. Carefully reviewing the list of possible reasons for thyroid level changes can improve stability and patient quality of life, prevent complications of fluctuating thyroid levels, and reduce medical costs, such as repeated labs and frequent clinic visits.
REFERENCES
1. Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults [published correction appears in Endocr Pract. 2013;19(1):175]. Endocr Pract. 2012;18(6):988-1028.
2. Sabate E. Adherence to Long-Term Therapies: Evidence for Action. Geneva, Switzerland: World Health Organization; 2003.
3. Jonklaas J, Bianco AC, Bauer AJ, et al; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism. Thyroid. 2014;24(12):1670-1751.
A 43-year-old man presents for his thyroid checkup. He has known hypothyroidism secondary to Hashimoto thyroiditis, also known as chronic lymphocytic thyroiditis. He is taking levothyroxine (LT4) 250 μg (two 125-μg tablets once per day). Review of his prior lab results and notes (see Table 1) reveals frequent dose changes (about every three to six months) and a high dosage of LT4, considering his weight (185 lb).
Patients with little or no residual thyroid function require replacement doses of LT4 at approximately 1.6 μg/kg/d, based on lean body weight.1 Since the case patient weighs 84 kg, the expected LT4 dosage would be around 125 to 150 μg/d.
This patient requires a significantly higher dose than expected, and his thyroid levels are fluctuating. These facts should trigger further investigation.
Important historical questions I consider when patients have frequent or significant fluctuations in TSH include
• Are you consistent in taking your medication?
• How do you take your thyroid medication?
• Are you taking any iron supplements, vitamins with iron, or contraceptive pills containing iron?
• Has there been any change in your other medication regimen(s) or medical condition(s)?
• Did you change pharmacies, or did the shape or color of your pill change?
• Have you experienced significant weight changes?
• Do you have any gastrointestinal complaints (nausea/vomiting/diarrhea/bloating)?
MEDICATION ADHERENCE
It is well known but still puzzling to hear that, overall, patients’ medication adherence is merely 50%.2 It is very important that you verify whether your patient is taking his/her medication consistently. Rather than asking “Are you taking your medications?” (to which they are more likely to answer “yes”), I ask “How many pills do you miss in a given week or month?”
For those who have a hard time remembering to take their medication on a regular basis, I recommend setting up a routine: Keep the medication at their bedside and take it first thing upon awakening, or place it beside the toothpaste so they see it every time they brush their teeth in the morning. Another option is of course to set up an alarm as a reminder.
Continue for rules for taking hypothyroid >>
RULES FOR TAKING HYPOTHYROID MEDICATIONS
Thyroid hormone replacement has a narrow therapeutic index, and a subtle change in dosage can significantly alter the therapeutic target. Hypothyroid medications are absorbed in the jejunum/ileum, and an acidic pH in the stomach is optimal for thyroid absorption.3 Therefore, taking the medication on an empty stomach (fasting) with a full glass of water and waiting at least one hour before breakfast is recommended, if possible. An alternate option is to take it at bedtime, at least three hours after the last meal. Taking medication along with food, especially high-fiber and soy products, can decrease absorption of thyroid hormone, which may result in an unstable thyroid function test.
There are supplements and medications that can decrease hypothyroid medication absorption; it is recommended that patients separate these medications by four hours or more in order to minimize this interference. A full list is available in Table 2, but the most commonly encountered are iron supplements, calcium supplements, and proton pump inhibitors.2
In many patients—especially the elderly and those with multiple comorbidities that require polypharmacy—it can be very challenging, if not impossible, to isolate thyroid medication. For these patients, recommend that they be “consistent” with their routine to ensure they achieve a similar absorption rate each time. For example, a patient’s hypothyroid medication absorption might be reduced by 50% by taking it with omeprazole, but as long as the patient consistently takes the medication that way, she can have stable thyroid function.
NEW MEDICATION REGIMEN OR MEDICAL CONDITION
In addition to medications that can interfere with the absorption of thyroid hormone replacement, there are those that affect levels of thyroxine-binding globulin. This affects the bioavailability of thyroid hormones and alters thyroid status.
Thyroid hormones such as thyroxine (T4) and triiodothyronine (T3) are predominantly bound to carrier proteins, and < 1% is unbound (so-called free hormones). Changes in thyroid-binding proteins can alter free hormone levels and thereby change TSH levels. In disease-free euthyroid subjects, the body can compensate by adjusting hormone production for changes in binding proteins to keep the free hormone levels within normal ranges. However, patients who are at or near full replacement doses of hypothyroid medication cannot adjust to the changes.
In patients with hypothyroidism who are taking thyroid hormone replacement, medications or conditions that increase binding proteins will decrease free hormones (by increasing bound hormones) and thereby raise TSH (hypothyroid state). Vice versa, medications and conditions that decrease binding protein will increase free hormones (by decreasing bound hormones) and thereby lower TSH (thyrotoxic state). Table 3 lists commonly encountered medications and conditions associated with altered thyroid-binding proteins.1
It is important to consider pregnancy in women of childbearing age whose TSH has risen for no apparent reason, as their thyroid levels should be maintained in a narrow therapeutic range to prevent fetal complications. Details on thyroid disease during pregnancy can be found in the April 2015 Endocrine Consult, “Managing Thyroid Disease in Pregnancy.”
In women treated for hypothyroidism, starting or discontinuing estrogen-containing medications (birth control pills or hormone replacement therapy) often results in changes in thyroid status. It is a good practice to inform the patient about these changes and to recheck her thyroid labs four to eight weeks after she starts or discontinues estrogen, adjusting the dose if needed.
Continue for changes in manufacturer/brand >>
CHANGES IN MANUFACTURER/BRAND
There are currently multiple brands and generic manufacturers supplying hypothyroid medications and reports that absorption rates and bioavailability vary among them.2 Switching products can result in changes in thyroid status and in TSH levels.
Once a patient has reached euthyroid status, it is imperative to stay on the same dose from the same manufacturer. This may be challenging, as it can be affected by the patient’s insurance carrier, policy changes, or even a change in the pharmacy’s medication supplier. Although patients are supposed to be informed by the pharmacy when the manufacturer is being changed, you may want to educate them to check the shape, color, and dose of their pills and also verify that the manufacturer listed on the bottle is consistent each time they refill their hypothyroid medications. This is especially important for those who require a very narrow TSH target, such as young children, thyroid cancer patients, pregnant women, and frail patients.3
WEIGHT CHANGES
As mentioned, thyroid medications are weight-based, and big changes in weight can lead to changes in thyroid function studies. It is the lean body mass, rather than total body weight, that will affect the thyroid requirement.3 A quick review of the patient’s weight history needs to be done when thyroid function test results have changed.
GASTROINTESTINAL DISTURBANCES
Hypothyroid medications are absorbed in the small intestine, and gastric acidity levels have an impact on absorption. Any acute or chronic conditions that affect these areas can alter medication absorption quite significantly. Commonly encountered diseases and conditions are H pylori–related gastritis, atrophic gastritis, celiac disease, and lactose intolerance. Treating these diseases and conditions can improve medication absorption.
I went through the list with the patient, but there was no applicable scenario. I adjusted his medication but went ahead and tested for tissue transglutaminase antibody IgA to rule out celiac disease; results came back mildly positive. The patient was referred to a gastroenterologist, who performed a small intestine biopsy for definitive diagnosis. This revealed “severe” celiac disease. A strict gluten-free diet was started, and the patient’s LT4 dose was adjusted, with regular monitoring, down to 150 μg/d.
Common symptoms of celiac disease include bloating, abdominal pain, and loose stool after consumption of gluten-containing meals. It should be noted that this patient denied all these symptoms, even though he was asked specifically about them. After he started a gluten-free diet, he reported that he actually felt “very calm” in his abdomen and realized he did have symptoms of celiac disease—but he’d had them for so long that he considered it normal. As is often the case, presence of symptoms would raise suspicion ... but lack of symptoms (or report thereof) does not rule out the disease.
CONCLUSION
Most patients with hypothyroidism are fairly well managed with relatively stable medication dosages, but there are subsets of patients who struggle to maintain euthyroid range. The latter require frequent office visits and dosage changes. Carefully reviewing the list of possible reasons for thyroid level changes can improve stability and patient quality of life, prevent complications of fluctuating thyroid levels, and reduce medical costs, such as repeated labs and frequent clinic visits.
REFERENCES
1. Garber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults [published correction appears in Endocr Pract. 2013;19(1):175]. Endocr Pract. 2012;18(6):988-1028.
2. Sabate E. Adherence to Long-Term Therapies: Evidence for Action. Geneva, Switzerland: World Health Organization; 2003.
3. Jonklaas J, Bianco AC, Bauer AJ, et al; American Thyroid Association Task Force on Thyroid Hormone Replacement. Guidelines for the treatment of hypothyroidism. Thyroid. 2014;24(12):1670-1751.
The Defense Health Agency Stands Up
For the past 2 years, military health has been undergoing one of the largest transformations in its history. In the midst of an active war in Afghanistan, the wind down to another in Iraq, and a humanitarian mission to Liberia, the transformation has been ongoing. “We were building the airplane as we flew it,” Lt Gen Douglas J. Robb, DO, admitted.
The Defense Health Agency (DHA) brings together the previously independent health care operations of the Army, Navy, and Air Force, with unique cultures, procedures, and technologies. The underlying DHA goals have been to improve interoperability, efficiency, and cost reduction by sharing services.
The operation is massive. The DHA cares for a TRICARE-eligible population of 9.5 million, including 1.4 million service members on active duty, with more than 1 million inpatient admissions and 95.6 million outpatient visits in 2014.
That transformation formally ends on October 1, 2015, as the DHA becomes fully operational and the organization moves into its next phase. Building such a large system has been a daunting challenge, but it has been “exhilarating… to watch what our people can do if you give them the opportunity,” Lt Gen Robb explained.
Establishing the Defense Health Agency
Lt Gen Douglas J. Robb, DO. You have to go back to look at where the seeds were planted on the journey that we have been on since June 2011. Back in 2011, then Deputy Secretary of Defense William Lynn established an internal task force to take a look at whether there is a better way to conduct a military health system governance.
How do we ensure the incredible medical support for our current and future military operations in an environment that was becoming fiscally constrained? We needed to look at how we could transform ourselves to make us better, stronger, more relevant, and, ultimately, viable. One of the other things that we had going for us at the time was broad congressional support that also supported a need for change.
We had a task force that assemmbled. I think this is key—it was a very broad based and a very representative task force. We had military departments, the Joint Staff, and the Office of the Secretary of Defense [OSD] who were all part of this task force…. Individuals that had a vested interest in the way we would organize a new entity that would, hopefully, and I would argue will, change the way we practice medicine....
Out of that task force came some recommendations. And one of those recommendations had to do with the overall governance of the military health system. People may be aware there are several models out there. In fact, there were 5 models that we looked at. One was a unified medical command, one was a defense health agency, one was a single-service model, another was a hybrid model, and then the status quo.
And what the task force recommendation that was put forth came down to was the recommendation of a defense health agency. And with that, the DEPSECDEF [Deputy Secretary of Defense] said, “Plan for it.” In November 2012, we had a planning work group report that went to the DEPSECDEF. And then, finally, in March 2013, the DEPSECDEF said, “Go forth and create and stand up the Defense Health Agency,” in what was then known as the Nine Commandments Memo.
The bottom line was no matter what model we chose, whatever organizational construct, the bottom line was we needed to ensure a medically ready force and a ready medical force…. One of the things that I think is key is that through these 10 years of conflict—actually, now going on 13—we have witnessed the ability for our medical services (the Army, Navy, the Air Force, and the Marine Corps) to come together in a joint environment, in the deployed setting, to essentially produce the lowest lethality rate in the history of recorded conflict. And it is amazing what our people have been able to do in saving the lives of our soldiers, sailors, and marines coalition forces and our civilians.
At the same time, we have also come together in avery joint manner to also achieve, what we call, the lowest disease nonbattle injury rate in the history of recorded conflict. That is a tribute to the services ensuring that all our forces are ready and deployable.
Shared Services
Lt Gen Robb. Essentially, we were running, in many cases, 3 parallel health care systems, 3 separate health information and technology systems. Three separate facilities divisions…. There was a lot of duplication, and there was a lot of redundancy. And so if you look at the challenge of the fiscal environment coupled with how to continue to provide high-quality health care in a deployed environment and in garrison, that was really the driving force behind the Defense Health Agency.
How could we find significant cost savings? How do we reduce the duplication? How do we reduce the variation? That’s what our models looked at. How do you create a dispute resolution process with clear decision authority and clear accountability as you move toward joint solutions where they make sense?
One of the other issues that we had was: Is it doable? Is whatever we propose doable in the environments and acceptable not only to the services, but to the Office of the Secretary of Defense? And so all those came into play as we proposed what then became the Defense Health Agency proposal for a new wave of doing governance.
When we built the Defense Health Agency, we looked at the 10 shared services… where we could see savings either in efficiencies or quality or dollars. Those 10 shared services were facilities, medical logistics, health information and technology, TRICARE, pharmacy operations, budget and resource management, contracting, research, development, acquisition, medical education and training, and public health.… We felt that there was opportunity there.
Now, as we moved forward, and people need to remember this, the Defense Health Agency and the future governance model was not created in a vacuum. It was created by the services’ participation—Army, Navy, and Air Force medicine. Each of those shared services had subject matter experts from all 3 services participating in shaping the future joint force solutions, where it makes sense. That is key. It wasn’t a bunch of headquarters officials or OSD or joint staff sitting in a dark room creating this in a vacuum and then bringing it out and saying, “Hey, this is what we’re going to do.” It was transparent, it was open, and then it actually ended up running through what we would then create the new governance system as we moved forward.
Each of those shared services underwent, what I call, a rigorous—and I’m going to repeat that word, rigorous—reproducible and transparent business case analysis. And after that, then you say, “Hey is there opportunity here?” Then part 2 was a rigorous, transparent, and reproducible business process re-engineering. And so we went through each of those shared services. And it just so happened that there was opportunity. In other words, there was opportunity for increased efficiencies, increased effectiveness, dollar savings, or resource savings, some of the above or all the above in all of these 10 shared services.
We put $3.5 billion on the table as potential shared services cost savings for the fiscal years [FY] 2015 to 2019. That’s not an insignificant number. Now folks say, “That’s a lot of money to put on the table. Are you going to deliver?” And the answer is yes, we will deliver. I’m going to be honest with you, they took that right off the topline of our Defense Health budget right off the bat, so we had no choice but to deliver now. But I’m confident that we will because of the very rigorous work and dedication of those who did that.
If you want to look at an early win here: In March of 2013 is when DEPSECDEF said, “Go forth and stand up the Defense Health Agency.” And then we set a target date of 1 October 2013 to be at initial operating capability when we stood up the Defense Health Agency. So that first year in FY14, the Defense Health Agency achieved—and this was not included in the FY15 to FY19 [budget]—achieved cost savings of $350 million….
Standing up 1 October 2013 in the middle of sequestration, I told my staff, “If there is any money you need for initial investment, you’re going to have to either find it yourself or make it.” And they did.… We paid our own way that first year, and I’m not so sure there are a lot of organizations out there that can say they paid their own way the first year. But I was very proud of our staff, especially when you create an organization that is supposed to lean out.
Remember, our staff in the Defense Health Agency is made of the men and women, the subject matter experts, the extreme talent that comes from the Army, Navy, and the Air Force medical services. When I talk about the Defense Health Agency, they’re not Defense Health Agency people. These are people that are in the Defense Health Agency that are providing services back and capability back to Army, Navy, Air Force, and Marine medicine. It is truly a team effort and a collaborative effort.
Standing Up
Lt Gen Robb. When I come to work each day, I think about the progress we’ve made in the journey of this military health system transformation. When you look at it, this is probably the largest military health care transformation that has occurred in decades, if not ever.
Dr. Jonathan Woods is an incredible leader, number one; but number two, he has a strategic vision and a strategic ability to make things happen. And he has a great deputy in Dr. Karen Guice. Both are incredible leaders at the right place, at the right time, coupled with congressional support. And then through the task force and the services, getting the Joint Staff and the services support as we move forward.
On 1 October 2013 we stood up and we created an organizational construct…. Those 10 shared services are embedded in an organizational construct that has 6 directorates. One is health care operations, number 2 is health information technology, number 3 is research and development, number 4 is education and training, number 5 is business support, and then, number 6 through a process that evolved [into] … the Multiservice Market National Capital Region Directorate.
Let’s look at the commitment not only by the OSD, but also from the services. So you’ve got 6 directorates and each of those directorates are led by a general officer, an admiral, or a senior executive service official…. There were no new general offices allotted to the Defense Health Agency. So those general offices came from the services. It [was] with the men and women who were part of the Army, Navy, and Air Force medicine who are now part of the Defense Health Agency.
What we’ve done in these 2 years is we’ve molded and we’ve melded and we’ve grown those teams to support those directorates and then the divisions within those directorates and the staff to support the shared services inside our organizational construct.
Joint Platforms
Lt Gen Robb. We’ve matured and there are in each of those directorates, in each of those shared services, success stories It’s one thing to stand something up. But we often say, “We were building the airplane as we flew it.” And we were producing, again, what I call, at times long overdue, joint products in support of the services.…
I’m excited about standing up again a joint platform that allows the military health system to accelerate business and operational elements to make a more effective and efficient military health system. But probably just as important, if not more important, it allows us to be a lot more agile and responsive to the challenges that come our way.
One of the positive spinoffs that I’ve had the privilege to experience is that when we stood up the Defense Health Agency, it then became a member of a group of organizations that in many ways work together.… The Defense Health Agency, Defense Information Systems Agency (DISA), and Defense Logistics Agency (DLA) exist solely to provide capability and joint capability where it makes sense to the services, and they are enablers.
The Defense Health Agency is also a designated combat support agency, which means not only are we answerable to the service surgeons general and to the service chiefs, but we are also directly responsible to the Chairman of the Joint Chiefs of Staff to provide combat support capability for our commanders.…
We are supporting and we will be responsive to the needs of the services. We will look for opportunity. We will continue to mature. We will continue to progress in our organizational construct. But at the same time… we have set up a senior level group from the services led by a general officer who will look at making sure that we are delivering on our initial 10 shared services and that we are continuing to meet what we said we were going to do. And then also for them to feed back to us where is there opportunity, where are there needs, but also that group is out there to look at where are there future opportunities.
Is there another shared service out there, or is there another shared joint first solution opportunity out there that we need to put into the queue to address to make us better, stronger, more relevant in the 21st century but at the same time, viable and in a very fiscally constrained environment?
Quality, Safety, and Access for Patients
Lt Gen Robb. The world doesn’t stop just because you’re building an organization.... Now that we’ve got this joint platform, we can aggregate the patient safety and the quality data that we have out there and look at where there is opportunity for the military health system to improve. We have bought an enterprise-wide analytic capability that will support the services as we continue to drive toward a high reliability organization, number one, and to continuously improve both quality, safety, and access. Much like DLA is to the logistics world and DISA is to the information systems world, we’re a centralized organizational construct that can bring the services together to create, what I call, an interoperable or joint force solution where it makes sense.
We have stood up the P4I initiative, which is a partnership for improvement of which the core of that will be the Defense Health Agency analytic cell, but the Defense Health Agency Healthcare Operations has become a gathering spot or the platform where the services come together. And for the first time, we have an enterprise dashboard. There [are] about 30 metrics out there where we’re looking at quality, safety, and access…. That’s just one example. And I could go through each of the shared services one by one by one and talk about where we have made a difference.
Consolidating Services
Lt Gen Robb. One of the ones that has been as exciting as anything and challenging at the same time is our health information and technology consolidation, which is being led by Mr. Dave Bowen, our chief information officer [CIO]. We had a single health care record, AHLTA, but we were basically running 3 separate health information and technology systems—Army, Navy, and Air Force. When you talk about being interoperable on the battlefield, sometimes we had some centralization on the battlefield, but as it worked its way back, you started working your way into 3 separate systems.
When you look at any major health care organization that has consolidated,… we absolutely spent time with leaders in the health care industry about how you set up an enterprise-wide health care system that’s effective and efficient. But most important, how do you drive quality and how do you drive safety? Standardization is key not only in what we would call cost and resource things, but standardization also drives—and study after study also drives—increased quality.…
What we’re doing is we’re going basically from the major data warehouse servers all the way down to the desktop, [it] is going to be managed centrally. But when I say “managed,” I’m talking about manned and managed. So the men and women that were running the health care information technology for the Army or the Navy or the Air Force are now part of a large organization called the Health Information and Technology [HIT] Directorate.
And we are standardizing. We’re standardizing the desktop, we are standardizing the infrastructure at the base level, at the service level; and with the help of the DoD CIO across the board. This is exciting. And as you can imagine, there are savings to be had there in the reduction of duplications. In fact, in 2014 just in the infrastructure consolidation, HIT came up with about $5 million [savings] and then another $12 million in savings so far in 2015. We have created a single, joint integrated infrastructure that supports our joint integrated delivered health care so it makes sense.
About 45%, almost 50% of our health care direct care systems, in other words our military treatment facilities, is delivered in 6 markets where 2 or more of the services—Army, Navy, or Air Force—exist side by side. You think of San Antonio with the Army and the Air Force; you think of the National Capital Region Army, Navy, and Air Force medicine; you think about the tidewater area where you have Army, Navy, and Air Force medicine. It makes sense that we have a single, integrated, consolidated health information and technology.
Interoperability and Interdependence
Lt Gen Robb. By nature of what we do, we’ve created an interoperability and interdependence within the Defense Health Agency.
Let’s look at education and training. The 3 services had up to 23 different online knowledge systems. It was either a library of knowledge or there was training going on. The Education and Training Directorate leadership said, “Hey, it makes sense to put all of our different learning portals on 1 portal.” So we’re consolidating from about 23 down to a single learning portal.
And you can just begin to imagine the efficiencies gained there, not to mention the savings. We’re looking at about $500,000 in savings in 2015 and probably another million [dollars] for 2016 just on consolidation of that. So these are all early deliveries by a very young but enthusiastic and aggressive organization called the Defense Health Agency.
We’re looking at a single entity for, what we call, third-party collections across all 3 services. We could never do that before, but now we can. We’re also looking at the way we account for dollars. In other words, when you want to manage your budget, and, as you know, we have different bags of money and each of them is used for certain things, but we weren’t doing that in a standardized manner. So if you want to make a system efficient, you’ve got to call things the same, you’ve got to measure things the same, you’ve got to measure them in the same bucket of money.…
Let’s think about logistic support. Those individuals form a community of practice have always been joint oriented, but it’s always been tough for them to get what was best for the enterprise, because the services wanted to do it but when they went back and they prioritized within the services, it may not have made the cut. And so not that we didn’t want to do it from an enterprise, but the services prioritized different.
But now with the logistics directorate, we prioritize as an enterprise we run it through governance, and we make a decision. So we now have very robust e-commerce. And there were different ways. Folks were using what we call the credit card method before, because it was convenient. But the problem was it’s more expensive to do it that way. So now we’ve made a more robust and more user-friendly and customer-friendly e-commerce. And so now we’re up to about 70% compliance, and we’re saving millions of dollars right there.
When you think about the Defense Logistics Agency, their job is to get the best price and product for the Department of Defense. So can you imagine before they were having to deal with the Army medicine, Navy medicine, and Air Force medicine. Now they’re dealing with the Defense Health Agency Logistics Directorate, so it’s a single point of contact. Now when we go out and do group buys, they can get a better deal for us. So what makes us look good makes them look good.…
DISA used to have to negotiate way ahead with Army, Navy, and Air Force medicine. Now they’re negotiating and looking at a joint force solution where it makes sense for the enterprise. That’s 2 examples right there, and it’s been exhilarating to watch. When you take the blinders off and you take the muzzle off, what our people can do if you give them the opportunity.
Working With the VA
Lt Gen Robb. I’m sure you’re aware that right now the Department of Defense and the VA have about 8.4 million shared records through what we would call a joint legacy viewer and enterprise. But what’s the future look like?
With the consolidation of the Health Information and Technology Directorate and then as we move forward with the acquisition of this new electronic health record, what our consolidated Health Information and Technology Directorate has done is created a single point of contact and a single entity for all things in relation to the new electronic health record.
Before, we had Army, Navy, and Air Force health information and technologies and it would have been… a lot harder to acquire something this large when you were dealing with 3 [systems]. Now we’re dealing with one entity. It is also the backbone and that’s where, what I would call, our academic center of gravity is and also our workhorses.
What is key for the interoperability between the Department of Defense and the VA as we transition the service member across is that the data flow from the Department of Defense to the Department of Veterans Affairs. We were handing over 3 different packages of data to the VA. Now we’re going to bring 1 package of data. So now the Department of Defense will have a single plug to go into the Department of Veterans Affairs.
The Department of Defense and the Department of Veterans Affairs have been working very hard the last couple of years, quietly in the background. But we are working on standardized data elements. In other words, what I call the Department of Defense and the VA will speak the same language and the same dialect when it comes to moving data. You don’t have to have the same electronic health record.… You have to have the ability to move those common data elements through your system.
The standardization of the infrastructure has allowed us to roll out the electronic health record, which will be our backbone and then we’ll move that data to the VA electronic health record of the future…. Our people inside the Defense Health Agency have been working with all the teams with these infrastructure upgrades and the new electronic health records [requirements]. It’s working the data elements, it’s working the joint requirements. All these things are all coming together to support our soldiers, sailors, airmen, and marines as they move forward in the transition from the Department of Defense to the Department of Veterans Affairs.
For the past 2 years, military health has been undergoing one of the largest transformations in its history. In the midst of an active war in Afghanistan, the wind down to another in Iraq, and a humanitarian mission to Liberia, the transformation has been ongoing. “We were building the airplane as we flew it,” Lt Gen Douglas J. Robb, DO, admitted.
The Defense Health Agency (DHA) brings together the previously independent health care operations of the Army, Navy, and Air Force, with unique cultures, procedures, and technologies. The underlying DHA goals have been to improve interoperability, efficiency, and cost reduction by sharing services.
The operation is massive. The DHA cares for a TRICARE-eligible population of 9.5 million, including 1.4 million service members on active duty, with more than 1 million inpatient admissions and 95.6 million outpatient visits in 2014.
That transformation formally ends on October 1, 2015, as the DHA becomes fully operational and the organization moves into its next phase. Building such a large system has been a daunting challenge, but it has been “exhilarating… to watch what our people can do if you give them the opportunity,” Lt Gen Robb explained.
Establishing the Defense Health Agency
Lt Gen Douglas J. Robb, DO. You have to go back to look at where the seeds were planted on the journey that we have been on since June 2011. Back in 2011, then Deputy Secretary of Defense William Lynn established an internal task force to take a look at whether there is a better way to conduct a military health system governance.
How do we ensure the incredible medical support for our current and future military operations in an environment that was becoming fiscally constrained? We needed to look at how we could transform ourselves to make us better, stronger, more relevant, and, ultimately, viable. One of the other things that we had going for us at the time was broad congressional support that also supported a need for change.
We had a task force that assemmbled. I think this is key—it was a very broad based and a very representative task force. We had military departments, the Joint Staff, and the Office of the Secretary of Defense [OSD] who were all part of this task force…. Individuals that had a vested interest in the way we would organize a new entity that would, hopefully, and I would argue will, change the way we practice medicine....
Out of that task force came some recommendations. And one of those recommendations had to do with the overall governance of the military health system. People may be aware there are several models out there. In fact, there were 5 models that we looked at. One was a unified medical command, one was a defense health agency, one was a single-service model, another was a hybrid model, and then the status quo.
And what the task force recommendation that was put forth came down to was the recommendation of a defense health agency. And with that, the DEPSECDEF [Deputy Secretary of Defense] said, “Plan for it.” In November 2012, we had a planning work group report that went to the DEPSECDEF. And then, finally, in March 2013, the DEPSECDEF said, “Go forth and create and stand up the Defense Health Agency,” in what was then known as the Nine Commandments Memo.
The bottom line was no matter what model we chose, whatever organizational construct, the bottom line was we needed to ensure a medically ready force and a ready medical force…. One of the things that I think is key is that through these 10 years of conflict—actually, now going on 13—we have witnessed the ability for our medical services (the Army, Navy, the Air Force, and the Marine Corps) to come together in a joint environment, in the deployed setting, to essentially produce the lowest lethality rate in the history of recorded conflict. And it is amazing what our people have been able to do in saving the lives of our soldiers, sailors, and marines coalition forces and our civilians.
At the same time, we have also come together in avery joint manner to also achieve, what we call, the lowest disease nonbattle injury rate in the history of recorded conflict. That is a tribute to the services ensuring that all our forces are ready and deployable.
Shared Services
Lt Gen Robb. Essentially, we were running, in many cases, 3 parallel health care systems, 3 separate health information and technology systems. Three separate facilities divisions…. There was a lot of duplication, and there was a lot of redundancy. And so if you look at the challenge of the fiscal environment coupled with how to continue to provide high-quality health care in a deployed environment and in garrison, that was really the driving force behind the Defense Health Agency.
How could we find significant cost savings? How do we reduce the duplication? How do we reduce the variation? That’s what our models looked at. How do you create a dispute resolution process with clear decision authority and clear accountability as you move toward joint solutions where they make sense?
One of the other issues that we had was: Is it doable? Is whatever we propose doable in the environments and acceptable not only to the services, but to the Office of the Secretary of Defense? And so all those came into play as we proposed what then became the Defense Health Agency proposal for a new wave of doing governance.
When we built the Defense Health Agency, we looked at the 10 shared services… where we could see savings either in efficiencies or quality or dollars. Those 10 shared services were facilities, medical logistics, health information and technology, TRICARE, pharmacy operations, budget and resource management, contracting, research, development, acquisition, medical education and training, and public health.… We felt that there was opportunity there.
Now, as we moved forward, and people need to remember this, the Defense Health Agency and the future governance model was not created in a vacuum. It was created by the services’ participation—Army, Navy, and Air Force medicine. Each of those shared services had subject matter experts from all 3 services participating in shaping the future joint force solutions, where it makes sense. That is key. It wasn’t a bunch of headquarters officials or OSD or joint staff sitting in a dark room creating this in a vacuum and then bringing it out and saying, “Hey, this is what we’re going to do.” It was transparent, it was open, and then it actually ended up running through what we would then create the new governance system as we moved forward.
Each of those shared services underwent, what I call, a rigorous—and I’m going to repeat that word, rigorous—reproducible and transparent business case analysis. And after that, then you say, “Hey is there opportunity here?” Then part 2 was a rigorous, transparent, and reproducible business process re-engineering. And so we went through each of those shared services. And it just so happened that there was opportunity. In other words, there was opportunity for increased efficiencies, increased effectiveness, dollar savings, or resource savings, some of the above or all the above in all of these 10 shared services.
We put $3.5 billion on the table as potential shared services cost savings for the fiscal years [FY] 2015 to 2019. That’s not an insignificant number. Now folks say, “That’s a lot of money to put on the table. Are you going to deliver?” And the answer is yes, we will deliver. I’m going to be honest with you, they took that right off the topline of our Defense Health budget right off the bat, so we had no choice but to deliver now. But I’m confident that we will because of the very rigorous work and dedication of those who did that.
If you want to look at an early win here: In March of 2013 is when DEPSECDEF said, “Go forth and stand up the Defense Health Agency.” And then we set a target date of 1 October 2013 to be at initial operating capability when we stood up the Defense Health Agency. So that first year in FY14, the Defense Health Agency achieved—and this was not included in the FY15 to FY19 [budget]—achieved cost savings of $350 million….
Standing up 1 October 2013 in the middle of sequestration, I told my staff, “If there is any money you need for initial investment, you’re going to have to either find it yourself or make it.” And they did.… We paid our own way that first year, and I’m not so sure there are a lot of organizations out there that can say they paid their own way the first year. But I was very proud of our staff, especially when you create an organization that is supposed to lean out.
Remember, our staff in the Defense Health Agency is made of the men and women, the subject matter experts, the extreme talent that comes from the Army, Navy, and the Air Force medical services. When I talk about the Defense Health Agency, they’re not Defense Health Agency people. These are people that are in the Defense Health Agency that are providing services back and capability back to Army, Navy, Air Force, and Marine medicine. It is truly a team effort and a collaborative effort.
Standing Up
Lt Gen Robb. When I come to work each day, I think about the progress we’ve made in the journey of this military health system transformation. When you look at it, this is probably the largest military health care transformation that has occurred in decades, if not ever.
Dr. Jonathan Woods is an incredible leader, number one; but number two, he has a strategic vision and a strategic ability to make things happen. And he has a great deputy in Dr. Karen Guice. Both are incredible leaders at the right place, at the right time, coupled with congressional support. And then through the task force and the services, getting the Joint Staff and the services support as we move forward.
On 1 October 2013 we stood up and we created an organizational construct…. Those 10 shared services are embedded in an organizational construct that has 6 directorates. One is health care operations, number 2 is health information technology, number 3 is research and development, number 4 is education and training, number 5 is business support, and then, number 6 through a process that evolved [into] … the Multiservice Market National Capital Region Directorate.
Let’s look at the commitment not only by the OSD, but also from the services. So you’ve got 6 directorates and each of those directorates are led by a general officer, an admiral, or a senior executive service official…. There were no new general offices allotted to the Defense Health Agency. So those general offices came from the services. It [was] with the men and women who were part of the Army, Navy, and Air Force medicine who are now part of the Defense Health Agency.
What we’ve done in these 2 years is we’ve molded and we’ve melded and we’ve grown those teams to support those directorates and then the divisions within those directorates and the staff to support the shared services inside our organizational construct.
Joint Platforms
Lt Gen Robb. We’ve matured and there are in each of those directorates, in each of those shared services, success stories It’s one thing to stand something up. But we often say, “We were building the airplane as we flew it.” And we were producing, again, what I call, at times long overdue, joint products in support of the services.…
I’m excited about standing up again a joint platform that allows the military health system to accelerate business and operational elements to make a more effective and efficient military health system. But probably just as important, if not more important, it allows us to be a lot more agile and responsive to the challenges that come our way.
One of the positive spinoffs that I’ve had the privilege to experience is that when we stood up the Defense Health Agency, it then became a member of a group of organizations that in many ways work together.… The Defense Health Agency, Defense Information Systems Agency (DISA), and Defense Logistics Agency (DLA) exist solely to provide capability and joint capability where it makes sense to the services, and they are enablers.
The Defense Health Agency is also a designated combat support agency, which means not only are we answerable to the service surgeons general and to the service chiefs, but we are also directly responsible to the Chairman of the Joint Chiefs of Staff to provide combat support capability for our commanders.…
We are supporting and we will be responsive to the needs of the services. We will look for opportunity. We will continue to mature. We will continue to progress in our organizational construct. But at the same time… we have set up a senior level group from the services led by a general officer who will look at making sure that we are delivering on our initial 10 shared services and that we are continuing to meet what we said we were going to do. And then also for them to feed back to us where is there opportunity, where are there needs, but also that group is out there to look at where are there future opportunities.
Is there another shared service out there, or is there another shared joint first solution opportunity out there that we need to put into the queue to address to make us better, stronger, more relevant in the 21st century but at the same time, viable and in a very fiscally constrained environment?
Quality, Safety, and Access for Patients
Lt Gen Robb. The world doesn’t stop just because you’re building an organization.... Now that we’ve got this joint platform, we can aggregate the patient safety and the quality data that we have out there and look at where there is opportunity for the military health system to improve. We have bought an enterprise-wide analytic capability that will support the services as we continue to drive toward a high reliability organization, number one, and to continuously improve both quality, safety, and access. Much like DLA is to the logistics world and DISA is to the information systems world, we’re a centralized organizational construct that can bring the services together to create, what I call, an interoperable or joint force solution where it makes sense.
We have stood up the P4I initiative, which is a partnership for improvement of which the core of that will be the Defense Health Agency analytic cell, but the Defense Health Agency Healthcare Operations has become a gathering spot or the platform where the services come together. And for the first time, we have an enterprise dashboard. There [are] about 30 metrics out there where we’re looking at quality, safety, and access…. That’s just one example. And I could go through each of the shared services one by one by one and talk about where we have made a difference.
Consolidating Services
Lt Gen Robb. One of the ones that has been as exciting as anything and challenging at the same time is our health information and technology consolidation, which is being led by Mr. Dave Bowen, our chief information officer [CIO]. We had a single health care record, AHLTA, but we were basically running 3 separate health information and technology systems—Army, Navy, and Air Force. When you talk about being interoperable on the battlefield, sometimes we had some centralization on the battlefield, but as it worked its way back, you started working your way into 3 separate systems.
When you look at any major health care organization that has consolidated,… we absolutely spent time with leaders in the health care industry about how you set up an enterprise-wide health care system that’s effective and efficient. But most important, how do you drive quality and how do you drive safety? Standardization is key not only in what we would call cost and resource things, but standardization also drives—and study after study also drives—increased quality.…
What we’re doing is we’re going basically from the major data warehouse servers all the way down to the desktop, [it] is going to be managed centrally. But when I say “managed,” I’m talking about manned and managed. So the men and women that were running the health care information technology for the Army or the Navy or the Air Force are now part of a large organization called the Health Information and Technology [HIT] Directorate.
And we are standardizing. We’re standardizing the desktop, we are standardizing the infrastructure at the base level, at the service level; and with the help of the DoD CIO across the board. This is exciting. And as you can imagine, there are savings to be had there in the reduction of duplications. In fact, in 2014 just in the infrastructure consolidation, HIT came up with about $5 million [savings] and then another $12 million in savings so far in 2015. We have created a single, joint integrated infrastructure that supports our joint integrated delivered health care so it makes sense.
About 45%, almost 50% of our health care direct care systems, in other words our military treatment facilities, is delivered in 6 markets where 2 or more of the services—Army, Navy, or Air Force—exist side by side. You think of San Antonio with the Army and the Air Force; you think of the National Capital Region Army, Navy, and Air Force medicine; you think about the tidewater area where you have Army, Navy, and Air Force medicine. It makes sense that we have a single, integrated, consolidated health information and technology.
Interoperability and Interdependence
Lt Gen Robb. By nature of what we do, we’ve created an interoperability and interdependence within the Defense Health Agency.
Let’s look at education and training. The 3 services had up to 23 different online knowledge systems. It was either a library of knowledge or there was training going on. The Education and Training Directorate leadership said, “Hey, it makes sense to put all of our different learning portals on 1 portal.” So we’re consolidating from about 23 down to a single learning portal.
And you can just begin to imagine the efficiencies gained there, not to mention the savings. We’re looking at about $500,000 in savings in 2015 and probably another million [dollars] for 2016 just on consolidation of that. So these are all early deliveries by a very young but enthusiastic and aggressive organization called the Defense Health Agency.
We’re looking at a single entity for, what we call, third-party collections across all 3 services. We could never do that before, but now we can. We’re also looking at the way we account for dollars. In other words, when you want to manage your budget, and, as you know, we have different bags of money and each of them is used for certain things, but we weren’t doing that in a standardized manner. So if you want to make a system efficient, you’ve got to call things the same, you’ve got to measure things the same, you’ve got to measure them in the same bucket of money.…
Let’s think about logistic support. Those individuals form a community of practice have always been joint oriented, but it’s always been tough for them to get what was best for the enterprise, because the services wanted to do it but when they went back and they prioritized within the services, it may not have made the cut. And so not that we didn’t want to do it from an enterprise, but the services prioritized different.
But now with the logistics directorate, we prioritize as an enterprise we run it through governance, and we make a decision. So we now have very robust e-commerce. And there were different ways. Folks were using what we call the credit card method before, because it was convenient. But the problem was it’s more expensive to do it that way. So now we’ve made a more robust and more user-friendly and customer-friendly e-commerce. And so now we’re up to about 70% compliance, and we’re saving millions of dollars right there.
When you think about the Defense Logistics Agency, their job is to get the best price and product for the Department of Defense. So can you imagine before they were having to deal with the Army medicine, Navy medicine, and Air Force medicine. Now they’re dealing with the Defense Health Agency Logistics Directorate, so it’s a single point of contact. Now when we go out and do group buys, they can get a better deal for us. So what makes us look good makes them look good.…
DISA used to have to negotiate way ahead with Army, Navy, and Air Force medicine. Now they’re negotiating and looking at a joint force solution where it makes sense for the enterprise. That’s 2 examples right there, and it’s been exhilarating to watch. When you take the blinders off and you take the muzzle off, what our people can do if you give them the opportunity.
Working With the VA
Lt Gen Robb. I’m sure you’re aware that right now the Department of Defense and the VA have about 8.4 million shared records through what we would call a joint legacy viewer and enterprise. But what’s the future look like?
With the consolidation of the Health Information and Technology Directorate and then as we move forward with the acquisition of this new electronic health record, what our consolidated Health Information and Technology Directorate has done is created a single point of contact and a single entity for all things in relation to the new electronic health record.
Before, we had Army, Navy, and Air Force health information and technologies and it would have been… a lot harder to acquire something this large when you were dealing with 3 [systems]. Now we’re dealing with one entity. It is also the backbone and that’s where, what I would call, our academic center of gravity is and also our workhorses.
What is key for the interoperability between the Department of Defense and the VA as we transition the service member across is that the data flow from the Department of Defense to the Department of Veterans Affairs. We were handing over 3 different packages of data to the VA. Now we’re going to bring 1 package of data. So now the Department of Defense will have a single plug to go into the Department of Veterans Affairs.
The Department of Defense and the Department of Veterans Affairs have been working very hard the last couple of years, quietly in the background. But we are working on standardized data elements. In other words, what I call the Department of Defense and the VA will speak the same language and the same dialect when it comes to moving data. You don’t have to have the same electronic health record.… You have to have the ability to move those common data elements through your system.
The standardization of the infrastructure has allowed us to roll out the electronic health record, which will be our backbone and then we’ll move that data to the VA electronic health record of the future…. Our people inside the Defense Health Agency have been working with all the teams with these infrastructure upgrades and the new electronic health records [requirements]. It’s working the data elements, it’s working the joint requirements. All these things are all coming together to support our soldiers, sailors, airmen, and marines as they move forward in the transition from the Department of Defense to the Department of Veterans Affairs.
For the past 2 years, military health has been undergoing one of the largest transformations in its history. In the midst of an active war in Afghanistan, the wind down to another in Iraq, and a humanitarian mission to Liberia, the transformation has been ongoing. “We were building the airplane as we flew it,” Lt Gen Douglas J. Robb, DO, admitted.
The Defense Health Agency (DHA) brings together the previously independent health care operations of the Army, Navy, and Air Force, with unique cultures, procedures, and technologies. The underlying DHA goals have been to improve interoperability, efficiency, and cost reduction by sharing services.
The operation is massive. The DHA cares for a TRICARE-eligible population of 9.5 million, including 1.4 million service members on active duty, with more than 1 million inpatient admissions and 95.6 million outpatient visits in 2014.
That transformation formally ends on October 1, 2015, as the DHA becomes fully operational and the organization moves into its next phase. Building such a large system has been a daunting challenge, but it has been “exhilarating… to watch what our people can do if you give them the opportunity,” Lt Gen Robb explained.
Establishing the Defense Health Agency
Lt Gen Douglas J. Robb, DO. You have to go back to look at where the seeds were planted on the journey that we have been on since June 2011. Back in 2011, then Deputy Secretary of Defense William Lynn established an internal task force to take a look at whether there is a better way to conduct a military health system governance.
How do we ensure the incredible medical support for our current and future military operations in an environment that was becoming fiscally constrained? We needed to look at how we could transform ourselves to make us better, stronger, more relevant, and, ultimately, viable. One of the other things that we had going for us at the time was broad congressional support that also supported a need for change.
We had a task force that assemmbled. I think this is key—it was a very broad based and a very representative task force. We had military departments, the Joint Staff, and the Office of the Secretary of Defense [OSD] who were all part of this task force…. Individuals that had a vested interest in the way we would organize a new entity that would, hopefully, and I would argue will, change the way we practice medicine....
Out of that task force came some recommendations. And one of those recommendations had to do with the overall governance of the military health system. People may be aware there are several models out there. In fact, there were 5 models that we looked at. One was a unified medical command, one was a defense health agency, one was a single-service model, another was a hybrid model, and then the status quo.
And what the task force recommendation that was put forth came down to was the recommendation of a defense health agency. And with that, the DEPSECDEF [Deputy Secretary of Defense] said, “Plan for it.” In November 2012, we had a planning work group report that went to the DEPSECDEF. And then, finally, in March 2013, the DEPSECDEF said, “Go forth and create and stand up the Defense Health Agency,” in what was then known as the Nine Commandments Memo.
The bottom line was no matter what model we chose, whatever organizational construct, the bottom line was we needed to ensure a medically ready force and a ready medical force…. One of the things that I think is key is that through these 10 years of conflict—actually, now going on 13—we have witnessed the ability for our medical services (the Army, Navy, the Air Force, and the Marine Corps) to come together in a joint environment, in the deployed setting, to essentially produce the lowest lethality rate in the history of recorded conflict. And it is amazing what our people have been able to do in saving the lives of our soldiers, sailors, and marines coalition forces and our civilians.
At the same time, we have also come together in avery joint manner to also achieve, what we call, the lowest disease nonbattle injury rate in the history of recorded conflict. That is a tribute to the services ensuring that all our forces are ready and deployable.
Shared Services
Lt Gen Robb. Essentially, we were running, in many cases, 3 parallel health care systems, 3 separate health information and technology systems. Three separate facilities divisions…. There was a lot of duplication, and there was a lot of redundancy. And so if you look at the challenge of the fiscal environment coupled with how to continue to provide high-quality health care in a deployed environment and in garrison, that was really the driving force behind the Defense Health Agency.
How could we find significant cost savings? How do we reduce the duplication? How do we reduce the variation? That’s what our models looked at. How do you create a dispute resolution process with clear decision authority and clear accountability as you move toward joint solutions where they make sense?
One of the other issues that we had was: Is it doable? Is whatever we propose doable in the environments and acceptable not only to the services, but to the Office of the Secretary of Defense? And so all those came into play as we proposed what then became the Defense Health Agency proposal for a new wave of doing governance.
When we built the Defense Health Agency, we looked at the 10 shared services… where we could see savings either in efficiencies or quality or dollars. Those 10 shared services were facilities, medical logistics, health information and technology, TRICARE, pharmacy operations, budget and resource management, contracting, research, development, acquisition, medical education and training, and public health.… We felt that there was opportunity there.
Now, as we moved forward, and people need to remember this, the Defense Health Agency and the future governance model was not created in a vacuum. It was created by the services’ participation—Army, Navy, and Air Force medicine. Each of those shared services had subject matter experts from all 3 services participating in shaping the future joint force solutions, where it makes sense. That is key. It wasn’t a bunch of headquarters officials or OSD or joint staff sitting in a dark room creating this in a vacuum and then bringing it out and saying, “Hey, this is what we’re going to do.” It was transparent, it was open, and then it actually ended up running through what we would then create the new governance system as we moved forward.
Each of those shared services underwent, what I call, a rigorous—and I’m going to repeat that word, rigorous—reproducible and transparent business case analysis. And after that, then you say, “Hey is there opportunity here?” Then part 2 was a rigorous, transparent, and reproducible business process re-engineering. And so we went through each of those shared services. And it just so happened that there was opportunity. In other words, there was opportunity for increased efficiencies, increased effectiveness, dollar savings, or resource savings, some of the above or all the above in all of these 10 shared services.
We put $3.5 billion on the table as potential shared services cost savings for the fiscal years [FY] 2015 to 2019. That’s not an insignificant number. Now folks say, “That’s a lot of money to put on the table. Are you going to deliver?” And the answer is yes, we will deliver. I’m going to be honest with you, they took that right off the topline of our Defense Health budget right off the bat, so we had no choice but to deliver now. But I’m confident that we will because of the very rigorous work and dedication of those who did that.
If you want to look at an early win here: In March of 2013 is when DEPSECDEF said, “Go forth and stand up the Defense Health Agency.” And then we set a target date of 1 October 2013 to be at initial operating capability when we stood up the Defense Health Agency. So that first year in FY14, the Defense Health Agency achieved—and this was not included in the FY15 to FY19 [budget]—achieved cost savings of $350 million….
Standing up 1 October 2013 in the middle of sequestration, I told my staff, “If there is any money you need for initial investment, you’re going to have to either find it yourself or make it.” And they did.… We paid our own way that first year, and I’m not so sure there are a lot of organizations out there that can say they paid their own way the first year. But I was very proud of our staff, especially when you create an organization that is supposed to lean out.
Remember, our staff in the Defense Health Agency is made of the men and women, the subject matter experts, the extreme talent that comes from the Army, Navy, and the Air Force medical services. When I talk about the Defense Health Agency, they’re not Defense Health Agency people. These are people that are in the Defense Health Agency that are providing services back and capability back to Army, Navy, Air Force, and Marine medicine. It is truly a team effort and a collaborative effort.
Standing Up
Lt Gen Robb. When I come to work each day, I think about the progress we’ve made in the journey of this military health system transformation. When you look at it, this is probably the largest military health care transformation that has occurred in decades, if not ever.
Dr. Jonathan Woods is an incredible leader, number one; but number two, he has a strategic vision and a strategic ability to make things happen. And he has a great deputy in Dr. Karen Guice. Both are incredible leaders at the right place, at the right time, coupled with congressional support. And then through the task force and the services, getting the Joint Staff and the services support as we move forward.
On 1 October 2013 we stood up and we created an organizational construct…. Those 10 shared services are embedded in an organizational construct that has 6 directorates. One is health care operations, number 2 is health information technology, number 3 is research and development, number 4 is education and training, number 5 is business support, and then, number 6 through a process that evolved [into] … the Multiservice Market National Capital Region Directorate.
Let’s look at the commitment not only by the OSD, but also from the services. So you’ve got 6 directorates and each of those directorates are led by a general officer, an admiral, or a senior executive service official…. There were no new general offices allotted to the Defense Health Agency. So those general offices came from the services. It [was] with the men and women who were part of the Army, Navy, and Air Force medicine who are now part of the Defense Health Agency.
What we’ve done in these 2 years is we’ve molded and we’ve melded and we’ve grown those teams to support those directorates and then the divisions within those directorates and the staff to support the shared services inside our organizational construct.
Joint Platforms
Lt Gen Robb. We’ve matured and there are in each of those directorates, in each of those shared services, success stories It’s one thing to stand something up. But we often say, “We were building the airplane as we flew it.” And we were producing, again, what I call, at times long overdue, joint products in support of the services.…
I’m excited about standing up again a joint platform that allows the military health system to accelerate business and operational elements to make a more effective and efficient military health system. But probably just as important, if not more important, it allows us to be a lot more agile and responsive to the challenges that come our way.
One of the positive spinoffs that I’ve had the privilege to experience is that when we stood up the Defense Health Agency, it then became a member of a group of organizations that in many ways work together.… The Defense Health Agency, Defense Information Systems Agency (DISA), and Defense Logistics Agency (DLA) exist solely to provide capability and joint capability where it makes sense to the services, and they are enablers.
The Defense Health Agency is also a designated combat support agency, which means not only are we answerable to the service surgeons general and to the service chiefs, but we are also directly responsible to the Chairman of the Joint Chiefs of Staff to provide combat support capability for our commanders.…
We are supporting and we will be responsive to the needs of the services. We will look for opportunity. We will continue to mature. We will continue to progress in our organizational construct. But at the same time… we have set up a senior level group from the services led by a general officer who will look at making sure that we are delivering on our initial 10 shared services and that we are continuing to meet what we said we were going to do. And then also for them to feed back to us where is there opportunity, where are there needs, but also that group is out there to look at where are there future opportunities.
Is there another shared service out there, or is there another shared joint first solution opportunity out there that we need to put into the queue to address to make us better, stronger, more relevant in the 21st century but at the same time, viable and in a very fiscally constrained environment?
Quality, Safety, and Access for Patients
Lt Gen Robb. The world doesn’t stop just because you’re building an organization.... Now that we’ve got this joint platform, we can aggregate the patient safety and the quality data that we have out there and look at where there is opportunity for the military health system to improve. We have bought an enterprise-wide analytic capability that will support the services as we continue to drive toward a high reliability organization, number one, and to continuously improve both quality, safety, and access. Much like DLA is to the logistics world and DISA is to the information systems world, we’re a centralized organizational construct that can bring the services together to create, what I call, an interoperable or joint force solution where it makes sense.
We have stood up the P4I initiative, which is a partnership for improvement of which the core of that will be the Defense Health Agency analytic cell, but the Defense Health Agency Healthcare Operations has become a gathering spot or the platform where the services come together. And for the first time, we have an enterprise dashboard. There [are] about 30 metrics out there where we’re looking at quality, safety, and access…. That’s just one example. And I could go through each of the shared services one by one by one and talk about where we have made a difference.
Consolidating Services
Lt Gen Robb. One of the ones that has been as exciting as anything and challenging at the same time is our health information and technology consolidation, which is being led by Mr. Dave Bowen, our chief information officer [CIO]. We had a single health care record, AHLTA, but we were basically running 3 separate health information and technology systems—Army, Navy, and Air Force. When you talk about being interoperable on the battlefield, sometimes we had some centralization on the battlefield, but as it worked its way back, you started working your way into 3 separate systems.
When you look at any major health care organization that has consolidated,… we absolutely spent time with leaders in the health care industry about how you set up an enterprise-wide health care system that’s effective and efficient. But most important, how do you drive quality and how do you drive safety? Standardization is key not only in what we would call cost and resource things, but standardization also drives—and study after study also drives—increased quality.…
What we’re doing is we’re going basically from the major data warehouse servers all the way down to the desktop, [it] is going to be managed centrally. But when I say “managed,” I’m talking about manned and managed. So the men and women that were running the health care information technology for the Army or the Navy or the Air Force are now part of a large organization called the Health Information and Technology [HIT] Directorate.
And we are standardizing. We’re standardizing the desktop, we are standardizing the infrastructure at the base level, at the service level; and with the help of the DoD CIO across the board. This is exciting. And as you can imagine, there are savings to be had there in the reduction of duplications. In fact, in 2014 just in the infrastructure consolidation, HIT came up with about $5 million [savings] and then another $12 million in savings so far in 2015. We have created a single, joint integrated infrastructure that supports our joint integrated delivered health care so it makes sense.
About 45%, almost 50% of our health care direct care systems, in other words our military treatment facilities, is delivered in 6 markets where 2 or more of the services—Army, Navy, or Air Force—exist side by side. You think of San Antonio with the Army and the Air Force; you think of the National Capital Region Army, Navy, and Air Force medicine; you think about the tidewater area where you have Army, Navy, and Air Force medicine. It makes sense that we have a single, integrated, consolidated health information and technology.
Interoperability and Interdependence
Lt Gen Robb. By nature of what we do, we’ve created an interoperability and interdependence within the Defense Health Agency.
Let’s look at education and training. The 3 services had up to 23 different online knowledge systems. It was either a library of knowledge or there was training going on. The Education and Training Directorate leadership said, “Hey, it makes sense to put all of our different learning portals on 1 portal.” So we’re consolidating from about 23 down to a single learning portal.
And you can just begin to imagine the efficiencies gained there, not to mention the savings. We’re looking at about $500,000 in savings in 2015 and probably another million [dollars] for 2016 just on consolidation of that. So these are all early deliveries by a very young but enthusiastic and aggressive organization called the Defense Health Agency.
We’re looking at a single entity for, what we call, third-party collections across all 3 services. We could never do that before, but now we can. We’re also looking at the way we account for dollars. In other words, when you want to manage your budget, and, as you know, we have different bags of money and each of them is used for certain things, but we weren’t doing that in a standardized manner. So if you want to make a system efficient, you’ve got to call things the same, you’ve got to measure things the same, you’ve got to measure them in the same bucket of money.…
Let’s think about logistic support. Those individuals form a community of practice have always been joint oriented, but it’s always been tough for them to get what was best for the enterprise, because the services wanted to do it but when they went back and they prioritized within the services, it may not have made the cut. And so not that we didn’t want to do it from an enterprise, but the services prioritized different.
But now with the logistics directorate, we prioritize as an enterprise we run it through governance, and we make a decision. So we now have very robust e-commerce. And there were different ways. Folks were using what we call the credit card method before, because it was convenient. But the problem was it’s more expensive to do it that way. So now we’ve made a more robust and more user-friendly and customer-friendly e-commerce. And so now we’re up to about 70% compliance, and we’re saving millions of dollars right there.
When you think about the Defense Logistics Agency, their job is to get the best price and product for the Department of Defense. So can you imagine before they were having to deal with the Army medicine, Navy medicine, and Air Force medicine. Now they’re dealing with the Defense Health Agency Logistics Directorate, so it’s a single point of contact. Now when we go out and do group buys, they can get a better deal for us. So what makes us look good makes them look good.…
DISA used to have to negotiate way ahead with Army, Navy, and Air Force medicine. Now they’re negotiating and looking at a joint force solution where it makes sense for the enterprise. That’s 2 examples right there, and it’s been exhilarating to watch. When you take the blinders off and you take the muzzle off, what our people can do if you give them the opportunity.
Working With the VA
Lt Gen Robb. I’m sure you’re aware that right now the Department of Defense and the VA have about 8.4 million shared records through what we would call a joint legacy viewer and enterprise. But what’s the future look like?
With the consolidation of the Health Information and Technology Directorate and then as we move forward with the acquisition of this new electronic health record, what our consolidated Health Information and Technology Directorate has done is created a single point of contact and a single entity for all things in relation to the new electronic health record.
Before, we had Army, Navy, and Air Force health information and technologies and it would have been… a lot harder to acquire something this large when you were dealing with 3 [systems]. Now we’re dealing with one entity. It is also the backbone and that’s where, what I would call, our academic center of gravity is and also our workhorses.
What is key for the interoperability between the Department of Defense and the VA as we transition the service member across is that the data flow from the Department of Defense to the Department of Veterans Affairs. We were handing over 3 different packages of data to the VA. Now we’re going to bring 1 package of data. So now the Department of Defense will have a single plug to go into the Department of Veterans Affairs.
The Department of Defense and the Department of Veterans Affairs have been working very hard the last couple of years, quietly in the background. But we are working on standardized data elements. In other words, what I call the Department of Defense and the VA will speak the same language and the same dialect when it comes to moving data. You don’t have to have the same electronic health record.… You have to have the ability to move those common data elements through your system.
The standardization of the infrastructure has allowed us to roll out the electronic health record, which will be our backbone and then we’ll move that data to the VA electronic health record of the future…. Our people inside the Defense Health Agency have been working with all the teams with these infrastructure upgrades and the new electronic health records [requirements]. It’s working the data elements, it’s working the joint requirements. All these things are all coming together to support our soldiers, sailors, airmen, and marines as they move forward in the transition from the Department of Defense to the Department of Veterans Affairs.
Problematic Medications: "Stomach Medicine"
Q) I am getting calls from patients saying they heard a “stomach medicine” would hurt their kidneys. What is the basis, and how should I respond?
Emerging evidence is suggestive of a causal association between proton pump inhibitor (PPI) use and acute kidney injury/interstitial nephritis. Acute kidney injury is defined as either a decrease in urine output to less than 0.5 mL/kg/h for six hours, a rise in serum creatinine of 0.3 mg/dL or more within 48 hours, or an increase in creatinine of 50% or more above baseline within a week. Acute interstitial nephritis is often definitively diagnosed by renal biopsy, with findings of acute inflammatory cells, interstitial edema, and infiltration. Medications are the most common etiology for acute interstitial nephritis and account for more than 75% of cases.5
According to results published in the American Journal of Kidney Diseases, a retrospective study of 133 biopsy-proven cases of acute interstitial nephritis found 70% were associated with medication use. Of these, 14% were linked to use of a PPI (other drug culprits included antibiotics and NSAIDs, responsible for 49% and 11% of cases, respectively). Overall, omeprazole was the top drug cause, at 12%.6
In a nested case-control study of 572,661 subjects (mean age, 65.4) taking either lansoprazole, omeprazole, or pantoprazole, 46 definite cases and 26 probable cases of first-time acute interstitial nephritis were identified. Omeprazole was the most commonly dispensed PPI in this study. The crude incidence rate per 100,000 person-years for current use of a PPI was 11.98 and for past use, 1.68.7
Another nested case-control study of 184,480 subjects (ages 18 and older) reported 854 cases of acute kidney injury, with a positive association between use of a PPI and development of renal disease, even after controlling for confounding factors (P < .0001). Of note, no significant relationship was found between acute renal injury and use of H2 blocker therapy.8—CAS
Cynthia A. Smith, DNP, APRN, FNP-BC
Renal Consultants PLLC, South Charleston, West Virginia
REFERENCES
1. Velazquez H, Perazella MA, Wright FS, Ellison DH. Renal mechanism of trimethoprim-induced hyperkalemia. Ann Intern Med. 1993;119:296-301.
2. Horn JR, Hansten PD. Trimethoprim and potassium-sparing drugs: a risk for hyperkalemia. www.pharmacytimes.com/publications/issue/2011/February2011/DrugInteractions-0211. Accessed August 24, 2015.
3. Medina I, Mills J, Leoung G, et al. Oral therapy for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome: a controlled trial of trimethoprim-sulfamethoxazole versus trimethoprim-dapsone. N Engl J Med. 1990;323:776-782.
4. Fralick M, Macdonald EM, Gomes T, et al. Co-trimoxazole and sudden death in patients receiving inhibitors of renin-angiotensin system: population based study. BMJ. 2014;349:g6196.
5. Gilbert SJ, Weiner DE, Gipson DS, et al. National Kidney Foundation’s Primer on Kidney Diseases. Philadelphia, PA: Elsevier; 2014.
6. Muriithi AK, Leung N, Valeri AM, et al. Biopsy-proven acute interstitial nephritis, 1993-2011: a case series. Am J Kidney Dis. 2014;64(4):558-566.
7. Blank ML, Parkin L, Paul C, Herbison P. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86(4):837-844.
8. Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrology. 2013;14:150.
Q) I am getting calls from patients saying they heard a “stomach medicine” would hurt their kidneys. What is the basis, and how should I respond?
Emerging evidence is suggestive of a causal association between proton pump inhibitor (PPI) use and acute kidney injury/interstitial nephritis. Acute kidney injury is defined as either a decrease in urine output to less than 0.5 mL/kg/h for six hours, a rise in serum creatinine of 0.3 mg/dL or more within 48 hours, or an increase in creatinine of 50% or more above baseline within a week. Acute interstitial nephritis is often definitively diagnosed by renal biopsy, with findings of acute inflammatory cells, interstitial edema, and infiltration. Medications are the most common etiology for acute interstitial nephritis and account for more than 75% of cases.5
According to results published in the American Journal of Kidney Diseases, a retrospective study of 133 biopsy-proven cases of acute interstitial nephritis found 70% were associated with medication use. Of these, 14% were linked to use of a PPI (other drug culprits included antibiotics and NSAIDs, responsible for 49% and 11% of cases, respectively). Overall, omeprazole was the top drug cause, at 12%.6
In a nested case-control study of 572,661 subjects (mean age, 65.4) taking either lansoprazole, omeprazole, or pantoprazole, 46 definite cases and 26 probable cases of first-time acute interstitial nephritis were identified. Omeprazole was the most commonly dispensed PPI in this study. The crude incidence rate per 100,000 person-years for current use of a PPI was 11.98 and for past use, 1.68.7
Another nested case-control study of 184,480 subjects (ages 18 and older) reported 854 cases of acute kidney injury, with a positive association between use of a PPI and development of renal disease, even after controlling for confounding factors (P < .0001). Of note, no significant relationship was found between acute renal injury and use of H2 blocker therapy.8—CAS
Cynthia A. Smith, DNP, APRN, FNP-BC
Renal Consultants PLLC, South Charleston, West Virginia
REFERENCES
1. Velazquez H, Perazella MA, Wright FS, Ellison DH. Renal mechanism of trimethoprim-induced hyperkalemia. Ann Intern Med. 1993;119:296-301.
2. Horn JR, Hansten PD. Trimethoprim and potassium-sparing drugs: a risk for hyperkalemia. www.pharmacytimes.com/publications/issue/2011/February2011/DrugInteractions-0211. Accessed August 24, 2015.
3. Medina I, Mills J, Leoung G, et al. Oral therapy for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome: a controlled trial of trimethoprim-sulfamethoxazole versus trimethoprim-dapsone. N Engl J Med. 1990;323:776-782.
4. Fralick M, Macdonald EM, Gomes T, et al. Co-trimoxazole and sudden death in patients receiving inhibitors of renin-angiotensin system: population based study. BMJ. 2014;349:g6196.
5. Gilbert SJ, Weiner DE, Gipson DS, et al. National Kidney Foundation’s Primer on Kidney Diseases. Philadelphia, PA: Elsevier; 2014.
6. Muriithi AK, Leung N, Valeri AM, et al. Biopsy-proven acute interstitial nephritis, 1993-2011: a case series. Am J Kidney Dis. 2014;64(4):558-566.
7. Blank ML, Parkin L, Paul C, Herbison P. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86(4):837-844.
8. Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrology. 2013;14:150.
Q) I am getting calls from patients saying they heard a “stomach medicine” would hurt their kidneys. What is the basis, and how should I respond?
Emerging evidence is suggestive of a causal association between proton pump inhibitor (PPI) use and acute kidney injury/interstitial nephritis. Acute kidney injury is defined as either a decrease in urine output to less than 0.5 mL/kg/h for six hours, a rise in serum creatinine of 0.3 mg/dL or more within 48 hours, or an increase in creatinine of 50% or more above baseline within a week. Acute interstitial nephritis is often definitively diagnosed by renal biopsy, with findings of acute inflammatory cells, interstitial edema, and infiltration. Medications are the most common etiology for acute interstitial nephritis and account for more than 75% of cases.5
According to results published in the American Journal of Kidney Diseases, a retrospective study of 133 biopsy-proven cases of acute interstitial nephritis found 70% were associated with medication use. Of these, 14% were linked to use of a PPI (other drug culprits included antibiotics and NSAIDs, responsible for 49% and 11% of cases, respectively). Overall, omeprazole was the top drug cause, at 12%.6
In a nested case-control study of 572,661 subjects (mean age, 65.4) taking either lansoprazole, omeprazole, or pantoprazole, 46 definite cases and 26 probable cases of first-time acute interstitial nephritis were identified. Omeprazole was the most commonly dispensed PPI in this study. The crude incidence rate per 100,000 person-years for current use of a PPI was 11.98 and for past use, 1.68.7
Another nested case-control study of 184,480 subjects (ages 18 and older) reported 854 cases of acute kidney injury, with a positive association between use of a PPI and development of renal disease, even after controlling for confounding factors (P < .0001). Of note, no significant relationship was found between acute renal injury and use of H2 blocker therapy.8—CAS
Cynthia A. Smith, DNP, APRN, FNP-BC
Renal Consultants PLLC, South Charleston, West Virginia
REFERENCES
1. Velazquez H, Perazella MA, Wright FS, Ellison DH. Renal mechanism of trimethoprim-induced hyperkalemia. Ann Intern Med. 1993;119:296-301.
2. Horn JR, Hansten PD. Trimethoprim and potassium-sparing drugs: a risk for hyperkalemia. www.pharmacytimes.com/publications/issue/2011/February2011/DrugInteractions-0211. Accessed August 24, 2015.
3. Medina I, Mills J, Leoung G, et al. Oral therapy for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome: a controlled trial of trimethoprim-sulfamethoxazole versus trimethoprim-dapsone. N Engl J Med. 1990;323:776-782.
4. Fralick M, Macdonald EM, Gomes T, et al. Co-trimoxazole and sudden death in patients receiving inhibitors of renin-angiotensin system: population based study. BMJ. 2014;349:g6196.
5. Gilbert SJ, Weiner DE, Gipson DS, et al. National Kidney Foundation’s Primer on Kidney Diseases. Philadelphia, PA: Elsevier; 2014.
6. Muriithi AK, Leung N, Valeri AM, et al. Biopsy-proven acute interstitial nephritis, 1993-2011: a case series. Am J Kidney Dis. 2014;64(4):558-566.
7. Blank ML, Parkin L, Paul C, Herbison P. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86(4):837-844.
8. Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrology. 2013;14:150.