User login
CPI-613 receives orphan designation for BL
The US Food and Drug Administration (FDA) has granted orphan drug designation to CPI-613 for the treatment of Burkitt lymphoma (BL).
CPI-613 is a novel lipoic acid analogue that inhibits multiple enzyme targets within the tricarboxylic acid cycle.
The drug is in development as a treatment for hematologic malignancies and solid tumors.
In a phase 1 trial of patients with advanced hematologic malignancies, CPI-613 produced a response in a patient with relapsed BL.
Now, Rafael Pharmaceuticals, Inc., the company developing CPI-613, is launching a phase 2 trial of the drug in patients with relapsed or refractory BL and high-grade B-cell lymphoma with rearrangements of MYC and BCL2 and/or BCL6.
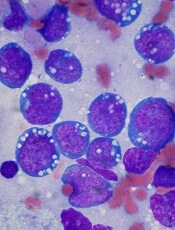
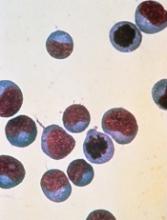
In the phase 1 trial, the patient with relapsed BL achieved a partial response to CPI-613 monotherapy and was ultimately cleared of disease after surgery.
The patient, a 19-year-old female, began taking CPI-613 (2940 mg/m2) after her second relapse. She achieved a radiographic partial response after the third cycle of CPI-613.
The patient completed 17 cycles of CPI-613 over 51 weeks. She decided to stop treatment after the 17th cycle to pursue a surgical resection of residual tumor. The pathology of the surgical specimen revealed BL with extensive necrosis.
Clinical follow-up on the patient showed no evidence of disease more than 36 months later. And CPI-613 was considered well tolerated in this patient.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The US Food and Drug Administration (FDA) has granted orphan drug designation to CPI-613 for the treatment of Burkitt lymphoma (BL).
CPI-613 is a novel lipoic acid analogue that inhibits multiple enzyme targets within the tricarboxylic acid cycle.
The drug is in development as a treatment for hematologic malignancies and solid tumors.
In a phase 1 trial of patients with advanced hematologic malignancies, CPI-613 produced a response in a patient with relapsed BL.
Now, Rafael Pharmaceuticals, Inc., the company developing CPI-613, is launching a phase 2 trial of the drug in patients with relapsed or refractory BL and high-grade B-cell lymphoma with rearrangements of MYC and BCL2 and/or BCL6.
In the phase 1 trial, the patient with relapsed BL achieved a partial response to CPI-613 monotherapy and was ultimately cleared of disease after surgery.
The patient, a 19-year-old female, began taking CPI-613 (2940 mg/m2) after her second relapse. She achieved a radiographic partial response after the third cycle of CPI-613.
The patient completed 17 cycles of CPI-613 over 51 weeks. She decided to stop treatment after the 17th cycle to pursue a surgical resection of residual tumor. The pathology of the surgical specimen revealed BL with extensive necrosis.
Clinical follow-up on the patient showed no evidence of disease more than 36 months later. And CPI-613 was considered well tolerated in this patient.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
The US Food and Drug Administration (FDA) has granted orphan drug designation to CPI-613 for the treatment of Burkitt lymphoma (BL).
CPI-613 is a novel lipoic acid analogue that inhibits multiple enzyme targets within the tricarboxylic acid cycle.
The drug is in development as a treatment for hematologic malignancies and solid tumors.
In a phase 1 trial of patients with advanced hematologic malignancies, CPI-613 produced a response in a patient with relapsed BL.
Now, Rafael Pharmaceuticals, Inc., the company developing CPI-613, is launching a phase 2 trial of the drug in patients with relapsed or refractory BL and high-grade B-cell lymphoma with rearrangements of MYC and BCL2 and/or BCL6.
In the phase 1 trial, the patient with relapsed BL achieved a partial response to CPI-613 monotherapy and was ultimately cleared of disease after surgery.
The patient, a 19-year-old female, began taking CPI-613 (2940 mg/m2) after her second relapse. She achieved a radiographic partial response after the third cycle of CPI-613.
The patient completed 17 cycles of CPI-613 over 51 weeks. She decided to stop treatment after the 17th cycle to pursue a surgical resection of residual tumor. The pathology of the surgical specimen revealed BL with extensive necrosis.
Clinical follow-up on the patient showed no evidence of disease more than 36 months later. And CPI-613 was considered well tolerated in this patient.
About orphan designation
The FDA grants orphan designation to products intended to treat, diagnose, or prevent diseases/disorders that affect fewer than 200,000 people in the US.
The designation provides incentives for sponsors to develop products for rare diseases. This may include tax credits toward the cost of clinical trials, prescription drug user fee waivers, and 7 years of market exclusivity if the product is approved.
CAR T in DLBCL: Liso-cel has ‘remarkable’ efficacy in cohort
CHICAGO – The CD19–directed chimeric antigen receptor (CAR) T-cell product lisocabtagene maraleucel (liso-cel, JCAR017) produced durable responses in poor-prognosis patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL), follow-up results of a phase 1 trial show.
Nearly 90% of DLBCL patients who achieved complete response as their best response on liso-cel were alive at 1 year in the study, according to investigator Jeremy S. Abramson, MD, of Massachusetts General Hospital Cancer Center in Boston.
That result is “far superior to what we would have anticipated with conventional therapies in a largely chemorefractory DLBCL population,” Dr. Abramson said at the annual meeting of the American Society of Clinical Oncology.
The new data on liso-cel come on the heels of a second approval of a CAR T-cell therapy for DLBCL, noted Caron Jacobson, MD, of Dana-Farber Cancer Institute, Boston.
Axicabtagene ciloleucel (Yescarta) was approved in October 2017 by the U.S. Food and Drug Administration for relapsed or refractory large B-cell lymphomas, including DLBCL. In May 2018, tisagenlecleucel (Kymriah) received its second FDA approval to treat relapsed or refractory large B-cell lymphomas, including DLBCL.
CAR T-cell therapy “really has transformed outcomes for a group of patients who previously had no other standard of care and who… have a relatively short overall survival,” Dr. Jacobson said.
At the meeting, Dr. Abramson presented findings on DLBCL patients in TRANSCEND NHL 001, a phase 1, multicenter, open-label study of the CD-19 targeted CAR T-cell therapy in relapsed and refractory B-cell non-Hodgkin lymphoma.
About 90% of treated DLBCL patients had one or more poor-risk disease features, such as ECOG performance status 2 and primary refractory disease, which predict poor overall survival, according to Dr. Abramson.
Dr. Abramson’s presentation focused on 102 evaluable DLBCL patients in the dose-finding and dose-expansion cohorts of the TRANSCEND study, including a subset analysis of a core group of 73 patients who met the criteria for pivotal dose cohort of the study (1 x 108 cells given as a single dose).
For the full set of 102 DLBCL patients, the best overall response rate was 75%, including a best complete remission rate of 55%, according to presented data. In the core group of 73 DLBCL patients, best overall response and complete remission rates were 80% and 59%, respectively.
Investigators saw “encouraging” durable response rates at 6 months and beyond in the core DLBCL population, according to Dr. Abramson. Of patients with a complete remission at 3 months, 88% remained in complete remission at the 6-month follow-up, and 93% of those in remission at the 6-month time point were in ongoing response at a median follow-up of 8 months.
Median overall survival had not been reached in either the full or core DLBCL cohorts with a median of 12 months follow-up, he added, noting that 90% of patients who achieved complete remission as their best response remained alive at 1 year.
In terms of adverse effects, liso-cel is showing a low and manageable toxicity profile, with very low rates of severe cytokine release syndrome (CRS) and neurotoxicity at 1% and 13%, respectively, Dr. Abramson reported.
“This anti-CD19 CAR T cell has remarkable efficacy in a group of highly refractory aggressive B-cell non-Hodgkin lymphoma patients,” said Dr. Jacobson, commenting on results of the DLBCL subset.
Based on the data presented, liso-cel is “clearly competitive” with the approved CAR T-cell therapies, though she advised caution in comparing across studies. “I don’t think that there will be a randomized study of all three agents, but I do think that we’ll start to get comparative data from single institution experiences that are using all three products,” she said.
The pivotal DLBCL cohort of TRANSCEND NHL 001 has completed accrual and results will be presented at a future meeting, Dr. Abramson said.
Dr. Abramson reported disclosures related to Celgene, Genentech/Roche, Gilead Sciences, Novartis, Seattle Genetics, and Millennium.
SOURCE: Abramson JS et al. ASCO 2018. Abstract 7505.
CHICAGO – The CD19–directed chimeric antigen receptor (CAR) T-cell product lisocabtagene maraleucel (liso-cel, JCAR017) produced durable responses in poor-prognosis patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL), follow-up results of a phase 1 trial show.
Nearly 90% of DLBCL patients who achieved complete response as their best response on liso-cel were alive at 1 year in the study, according to investigator Jeremy S. Abramson, MD, of Massachusetts General Hospital Cancer Center in Boston.
That result is “far superior to what we would have anticipated with conventional therapies in a largely chemorefractory DLBCL population,” Dr. Abramson said at the annual meeting of the American Society of Clinical Oncology.
The new data on liso-cel come on the heels of a second approval of a CAR T-cell therapy for DLBCL, noted Caron Jacobson, MD, of Dana-Farber Cancer Institute, Boston.
Axicabtagene ciloleucel (Yescarta) was approved in October 2017 by the U.S. Food and Drug Administration for relapsed or refractory large B-cell lymphomas, including DLBCL. In May 2018, tisagenlecleucel (Kymriah) received its second FDA approval to treat relapsed or refractory large B-cell lymphomas, including DLBCL.
CAR T-cell therapy “really has transformed outcomes for a group of patients who previously had no other standard of care and who… have a relatively short overall survival,” Dr. Jacobson said.
At the meeting, Dr. Abramson presented findings on DLBCL patients in TRANSCEND NHL 001, a phase 1, multicenter, open-label study of the CD-19 targeted CAR T-cell therapy in relapsed and refractory B-cell non-Hodgkin lymphoma.
About 90% of treated DLBCL patients had one or more poor-risk disease features, such as ECOG performance status 2 and primary refractory disease, which predict poor overall survival, according to Dr. Abramson.
Dr. Abramson’s presentation focused on 102 evaluable DLBCL patients in the dose-finding and dose-expansion cohorts of the TRANSCEND study, including a subset analysis of a core group of 73 patients who met the criteria for pivotal dose cohort of the study (1 x 108 cells given as a single dose).
For the full set of 102 DLBCL patients, the best overall response rate was 75%, including a best complete remission rate of 55%, according to presented data. In the core group of 73 DLBCL patients, best overall response and complete remission rates were 80% and 59%, respectively.
Investigators saw “encouraging” durable response rates at 6 months and beyond in the core DLBCL population, according to Dr. Abramson. Of patients with a complete remission at 3 months, 88% remained in complete remission at the 6-month follow-up, and 93% of those in remission at the 6-month time point were in ongoing response at a median follow-up of 8 months.
Median overall survival had not been reached in either the full or core DLBCL cohorts with a median of 12 months follow-up, he added, noting that 90% of patients who achieved complete remission as their best response remained alive at 1 year.
In terms of adverse effects, liso-cel is showing a low and manageable toxicity profile, with very low rates of severe cytokine release syndrome (CRS) and neurotoxicity at 1% and 13%, respectively, Dr. Abramson reported.
“This anti-CD19 CAR T cell has remarkable efficacy in a group of highly refractory aggressive B-cell non-Hodgkin lymphoma patients,” said Dr. Jacobson, commenting on results of the DLBCL subset.
Based on the data presented, liso-cel is “clearly competitive” with the approved CAR T-cell therapies, though she advised caution in comparing across studies. “I don’t think that there will be a randomized study of all three agents, but I do think that we’ll start to get comparative data from single institution experiences that are using all three products,” she said.
The pivotal DLBCL cohort of TRANSCEND NHL 001 has completed accrual and results will be presented at a future meeting, Dr. Abramson said.
Dr. Abramson reported disclosures related to Celgene, Genentech/Roche, Gilead Sciences, Novartis, Seattle Genetics, and Millennium.
SOURCE: Abramson JS et al. ASCO 2018. Abstract 7505.
CHICAGO – The CD19–directed chimeric antigen receptor (CAR) T-cell product lisocabtagene maraleucel (liso-cel, JCAR017) produced durable responses in poor-prognosis patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL), follow-up results of a phase 1 trial show.
Nearly 90% of DLBCL patients who achieved complete response as their best response on liso-cel were alive at 1 year in the study, according to investigator Jeremy S. Abramson, MD, of Massachusetts General Hospital Cancer Center in Boston.
That result is “far superior to what we would have anticipated with conventional therapies in a largely chemorefractory DLBCL population,” Dr. Abramson said at the annual meeting of the American Society of Clinical Oncology.
The new data on liso-cel come on the heels of a second approval of a CAR T-cell therapy for DLBCL, noted Caron Jacobson, MD, of Dana-Farber Cancer Institute, Boston.
Axicabtagene ciloleucel (Yescarta) was approved in October 2017 by the U.S. Food and Drug Administration for relapsed or refractory large B-cell lymphomas, including DLBCL. In May 2018, tisagenlecleucel (Kymriah) received its second FDA approval to treat relapsed or refractory large B-cell lymphomas, including DLBCL.
CAR T-cell therapy “really has transformed outcomes for a group of patients who previously had no other standard of care and who… have a relatively short overall survival,” Dr. Jacobson said.
At the meeting, Dr. Abramson presented findings on DLBCL patients in TRANSCEND NHL 001, a phase 1, multicenter, open-label study of the CD-19 targeted CAR T-cell therapy in relapsed and refractory B-cell non-Hodgkin lymphoma.
About 90% of treated DLBCL patients had one or more poor-risk disease features, such as ECOG performance status 2 and primary refractory disease, which predict poor overall survival, according to Dr. Abramson.
Dr. Abramson’s presentation focused on 102 evaluable DLBCL patients in the dose-finding and dose-expansion cohorts of the TRANSCEND study, including a subset analysis of a core group of 73 patients who met the criteria for pivotal dose cohort of the study (1 x 108 cells given as a single dose).
For the full set of 102 DLBCL patients, the best overall response rate was 75%, including a best complete remission rate of 55%, according to presented data. In the core group of 73 DLBCL patients, best overall response and complete remission rates were 80% and 59%, respectively.
Investigators saw “encouraging” durable response rates at 6 months and beyond in the core DLBCL population, according to Dr. Abramson. Of patients with a complete remission at 3 months, 88% remained in complete remission at the 6-month follow-up, and 93% of those in remission at the 6-month time point were in ongoing response at a median follow-up of 8 months.
Median overall survival had not been reached in either the full or core DLBCL cohorts with a median of 12 months follow-up, he added, noting that 90% of patients who achieved complete remission as their best response remained alive at 1 year.
In terms of adverse effects, liso-cel is showing a low and manageable toxicity profile, with very low rates of severe cytokine release syndrome (CRS) and neurotoxicity at 1% and 13%, respectively, Dr. Abramson reported.
“This anti-CD19 CAR T cell has remarkable efficacy in a group of highly refractory aggressive B-cell non-Hodgkin lymphoma patients,” said Dr. Jacobson, commenting on results of the DLBCL subset.
Based on the data presented, liso-cel is “clearly competitive” with the approved CAR T-cell therapies, though she advised caution in comparing across studies. “I don’t think that there will be a randomized study of all three agents, but I do think that we’ll start to get comparative data from single institution experiences that are using all three products,” she said.
The pivotal DLBCL cohort of TRANSCEND NHL 001 has completed accrual and results will be presented at a future meeting, Dr. Abramson said.
Dr. Abramson reported disclosures related to Celgene, Genentech/Roche, Gilead Sciences, Novartis, Seattle Genetics, and Millennium.
SOURCE: Abramson JS et al. ASCO 2018. Abstract 7505.
REPORTING FROM ASCO 2018
Key clinical point: (DLBCL).
Major finding: Among DLBCL patients treated with the pivotal dose of liso-cel, 88% who were in complete remission at 3 months remained in complete remission at the 6 month follow-up.
Study details: Follow-up report on a cohort of DLBCL patients from TRANSCEND NHL 001, a phase 1 trial of liso-cel in relapsed and refractory B-cell NHL.
Disclosures: Dr. Abramson reported disclosures related to Celgene, Genentech/Roche, Gilead Sciences, Novartis, Seattle Genetics, and Millennium.
Source: Abramson JS et al. ASCO 2018. Abstract 7505.
Ibrutinib sNDA receives priority review
The US Food and Drug Administration (FDA) has accepted for priority review a supplemental new drug application (sNDA) for ibrutinib (Imbruvica®) to be used in combination with rituximab in patients with Waldenström’s macroglobulinemia (WM).
The FDA intends to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
Ibrutinib is a first-in-class Bruton’s tyrosine kinase inhibitor jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and Janssen Biotech, Inc.
Ibrutinib is already FDA-approved to treat chronic lymphocytic leukemia/small lymphocytic lymphoma, previously treated mantle cell lymphoma, previously treated marginal zone lymphoma, previously treated chronic graft-versus-host disease, and WM.
If the new sNDA is approved, the use of ibrutinib in WM will extend beyond its current approved use as a single agent.
Phase 3 trial
The sNDA for ibrutinib and rituximab in WM is supported by data from the phase 3 iNNOVATE study. Results from this trial were presented at the 2018 ASCO Annual Meeting (abstract 8003) and were simultaneously published in NEJM.
iNNOVATE is a placebo-controlled, double-blind, phase 3 study that enrolled 150 patients with relapsed/refractory and treatment-naïve WM.
All patients received rituximab at 375 mg/m2 with weekly infusions at weeks 1 to 4 and 17 to 20. They also received either ibrutinib (420 mg) or placebo once daily continuously until criteria for permanent discontinuation were met.
Overall response rates were significantly higher in the ibrutinib arm than the placebo arm—92% and 47%, respectively (P<0.0001). Complete response rates were 3% and 1%, respectively.
The median time to next treatment was not reached for the ibrutinib arm and was 18 months for the placebo arm (hazard ratio=0.096; P<0.0001). Of the patients randomized to ibrutinib plus rituximab, 75% continued on treatment at last follow-up.
The 30-month progression-free survival rates were 82% in the ibrutinib arm and 28% in the placebo arm. The median progression-free survival was not reached in the ibrutinib arm and was 20.3 months in the placebo arm (hazard ratio=0.20; P<0.0001).
The 30-month overall survival rates were 94% in the ibrutinib arm and 92% in the placebo arm.
Grade 3 or higher treatment-emergent adverse events (AEs) occurred in 60% of patients in the ibrutinib arm and 61% in the placebo arm.
Serious AEs occurred in 43% and 33% of patients, respectively. There were no fatal AEs in the ibrutinib arm and 3 in the rituximab arm.
Grade 3 or higher AEs that occurred more frequently in the ibrutinib arm than the placebo arm included atrial fibrillation (12% vs 1%) and hypertension (13% vs 4%).
AEs that occurred less frequently in the ibrutinib arm than the placebo arm included grade 3 or higher infusion reactions (1% vs 16%) and any-grade IgM flare (8% vs 47%).
The US Food and Drug Administration (FDA) has accepted for priority review a supplemental new drug application (sNDA) for ibrutinib (Imbruvica®) to be used in combination with rituximab in patients with Waldenström’s macroglobulinemia (WM).
The FDA intends to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
Ibrutinib is a first-in-class Bruton’s tyrosine kinase inhibitor jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and Janssen Biotech, Inc.
Ibrutinib is already FDA-approved to treat chronic lymphocytic leukemia/small lymphocytic lymphoma, previously treated mantle cell lymphoma, previously treated marginal zone lymphoma, previously treated chronic graft-versus-host disease, and WM.
If the new sNDA is approved, the use of ibrutinib in WM will extend beyond its current approved use as a single agent.
Phase 3 trial
The sNDA for ibrutinib and rituximab in WM is supported by data from the phase 3 iNNOVATE study. Results from this trial were presented at the 2018 ASCO Annual Meeting (abstract 8003) and were simultaneously published in NEJM.
iNNOVATE is a placebo-controlled, double-blind, phase 3 study that enrolled 150 patients with relapsed/refractory and treatment-naïve WM.
All patients received rituximab at 375 mg/m2 with weekly infusions at weeks 1 to 4 and 17 to 20. They also received either ibrutinib (420 mg) or placebo once daily continuously until criteria for permanent discontinuation were met.
Overall response rates were significantly higher in the ibrutinib arm than the placebo arm—92% and 47%, respectively (P<0.0001). Complete response rates were 3% and 1%, respectively.
The median time to next treatment was not reached for the ibrutinib arm and was 18 months for the placebo arm (hazard ratio=0.096; P<0.0001). Of the patients randomized to ibrutinib plus rituximab, 75% continued on treatment at last follow-up.
The 30-month progression-free survival rates were 82% in the ibrutinib arm and 28% in the placebo arm. The median progression-free survival was not reached in the ibrutinib arm and was 20.3 months in the placebo arm (hazard ratio=0.20; P<0.0001).
The 30-month overall survival rates were 94% in the ibrutinib arm and 92% in the placebo arm.
Grade 3 or higher treatment-emergent adverse events (AEs) occurred in 60% of patients in the ibrutinib arm and 61% in the placebo arm.
Serious AEs occurred in 43% and 33% of patients, respectively. There were no fatal AEs in the ibrutinib arm and 3 in the rituximab arm.
Grade 3 or higher AEs that occurred more frequently in the ibrutinib arm than the placebo arm included atrial fibrillation (12% vs 1%) and hypertension (13% vs 4%).
AEs that occurred less frequently in the ibrutinib arm than the placebo arm included grade 3 or higher infusion reactions (1% vs 16%) and any-grade IgM flare (8% vs 47%).
The US Food and Drug Administration (FDA) has accepted for priority review a supplemental new drug application (sNDA) for ibrutinib (Imbruvica®) to be used in combination with rituximab in patients with Waldenström’s macroglobulinemia (WM).
The FDA intends to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
Ibrutinib is a first-in-class Bruton’s tyrosine kinase inhibitor jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and Janssen Biotech, Inc.
Ibrutinib is already FDA-approved to treat chronic lymphocytic leukemia/small lymphocytic lymphoma, previously treated mantle cell lymphoma, previously treated marginal zone lymphoma, previously treated chronic graft-versus-host disease, and WM.
If the new sNDA is approved, the use of ibrutinib in WM will extend beyond its current approved use as a single agent.
Phase 3 trial
The sNDA for ibrutinib and rituximab in WM is supported by data from the phase 3 iNNOVATE study. Results from this trial were presented at the 2018 ASCO Annual Meeting (abstract 8003) and were simultaneously published in NEJM.
iNNOVATE is a placebo-controlled, double-blind, phase 3 study that enrolled 150 patients with relapsed/refractory and treatment-naïve WM.
All patients received rituximab at 375 mg/m2 with weekly infusions at weeks 1 to 4 and 17 to 20. They also received either ibrutinib (420 mg) or placebo once daily continuously until criteria for permanent discontinuation were met.
Overall response rates were significantly higher in the ibrutinib arm than the placebo arm—92% and 47%, respectively (P<0.0001). Complete response rates were 3% and 1%, respectively.
The median time to next treatment was not reached for the ibrutinib arm and was 18 months for the placebo arm (hazard ratio=0.096; P<0.0001). Of the patients randomized to ibrutinib plus rituximab, 75% continued on treatment at last follow-up.
The 30-month progression-free survival rates were 82% in the ibrutinib arm and 28% in the placebo arm. The median progression-free survival was not reached in the ibrutinib arm and was 20.3 months in the placebo arm (hazard ratio=0.20; P<0.0001).
The 30-month overall survival rates were 94% in the ibrutinib arm and 92% in the placebo arm.
Grade 3 or higher treatment-emergent adverse events (AEs) occurred in 60% of patients in the ibrutinib arm and 61% in the placebo arm.
Serious AEs occurred in 43% and 33% of patients, respectively. There were no fatal AEs in the ibrutinib arm and 3 in the rituximab arm.
Grade 3 or higher AEs that occurred more frequently in the ibrutinib arm than the placebo arm included atrial fibrillation (12% vs 1%) and hypertension (13% vs 4%).
AEs that occurred less frequently in the ibrutinib arm than the placebo arm included grade 3 or higher infusion reactions (1% vs 16%) and any-grade IgM flare (8% vs 47%).
T-cell therapy induced CMRs with no CRS
CHICAGO—A novel CD19-targeted T-cell therapy induced complete metabolic responses (CMRs) and no cytokine release syndrome (CRS) in patients with B-cell lymphomas in a first-in-human clinical study.
All subjects achieving CMR at the 1-month safety and efficacy assessment continued to show CMR at 3 months, investigators reported at the 2018 ASCO Annual Meeting (abstract 3049*).
The therapy is built on a novel platform, ARTEMIS, designed to match the potency of chimeric antigen receptor (CAR) T-cell therapy but trigger less cytokine release when the target is engaged, investigators explained.
That platform is “potentially a major improvement” over existing CAR-T cell therapy, said Zhi Tao Ying, MD, of Peking University Cancer Hospital & Institute in Beijing, China, and coauthors in a poster presented at ASCO.
The treatment, called ET190L1-ARTEMIS, utilizes the T-cell receptor platform and a proprietary human anti-CD19 antibody to target CD19-positive malignancies.
The investigators reported on 21 adults with CD-19 positive relapsed and refractory B-cell lymphomas who had received a median of 4 lines of previous therapy.
Patients received autologous ET190L1-ARTEMIS T cells in 1 of 3 dosing cohorts: 3 patients at 1 x 106/kg, 13 at 3 x 106/kg, and 5 at 6 x 106/kg.
Of 17 patients completing a first-month efficacy assessment, 11 (65%) responded, including 7 CMRs and 3 partial responses. One patient had stable disease.
Seven of the 11 responders completed a third-month efficacy assessment, as of this analysis. Of 5 patients with CMR at month 1, all 5 maintained CMR at month 3. Likewise, 1 patient in partial response and 1 with stable disease at month 1 had the same response status at month 3.
There were no cases of CRS or neurotoxicity in 17 patients who completed the 1-month safety and efficacy assessment reported at ASCO. Grade 3 or greater adverse events in those subjects included lymphopenia in 17 (100%) and neutropenia in 5 (29%).
Eureka Therapeutics Inc., of Emeryville, California, is developing ET190L1-ARTEMIS. Co-investigators in this trial were from Eureka, Xi-An Jiaotong University in China, and Duke University School of Medicine in Durham, North Carolina.
A phase 1 trial of ET190L1-ARTEMIS in patients with relapsed and refractory non-Hodgkin lymphoma has been initiated at Duke University, and investigators say another US phase 1 trial including relapsed and refractory pediatric acute lymphoblastic leukemia patients will begin later this year.
Data in the abstract differ from that presented in the poster.
CHICAGO—A novel CD19-targeted T-cell therapy induced complete metabolic responses (CMRs) and no cytokine release syndrome (CRS) in patients with B-cell lymphomas in a first-in-human clinical study.
All subjects achieving CMR at the 1-month safety and efficacy assessment continued to show CMR at 3 months, investigators reported at the 2018 ASCO Annual Meeting (abstract 3049*).
The therapy is built on a novel platform, ARTEMIS, designed to match the potency of chimeric antigen receptor (CAR) T-cell therapy but trigger less cytokine release when the target is engaged, investigators explained.
That platform is “potentially a major improvement” over existing CAR-T cell therapy, said Zhi Tao Ying, MD, of Peking University Cancer Hospital & Institute in Beijing, China, and coauthors in a poster presented at ASCO.
The treatment, called ET190L1-ARTEMIS, utilizes the T-cell receptor platform and a proprietary human anti-CD19 antibody to target CD19-positive malignancies.
The investigators reported on 21 adults with CD-19 positive relapsed and refractory B-cell lymphomas who had received a median of 4 lines of previous therapy.
Patients received autologous ET190L1-ARTEMIS T cells in 1 of 3 dosing cohorts: 3 patients at 1 x 106/kg, 13 at 3 x 106/kg, and 5 at 6 x 106/kg.
Of 17 patients completing a first-month efficacy assessment, 11 (65%) responded, including 7 CMRs and 3 partial responses. One patient had stable disease.
Seven of the 11 responders completed a third-month efficacy assessment, as of this analysis. Of 5 patients with CMR at month 1, all 5 maintained CMR at month 3. Likewise, 1 patient in partial response and 1 with stable disease at month 1 had the same response status at month 3.
There were no cases of CRS or neurotoxicity in 17 patients who completed the 1-month safety and efficacy assessment reported at ASCO. Grade 3 or greater adverse events in those subjects included lymphopenia in 17 (100%) and neutropenia in 5 (29%).
Eureka Therapeutics Inc., of Emeryville, California, is developing ET190L1-ARTEMIS. Co-investigators in this trial were from Eureka, Xi-An Jiaotong University in China, and Duke University School of Medicine in Durham, North Carolina.
A phase 1 trial of ET190L1-ARTEMIS in patients with relapsed and refractory non-Hodgkin lymphoma has been initiated at Duke University, and investigators say another US phase 1 trial including relapsed and refractory pediatric acute lymphoblastic leukemia patients will begin later this year.
Data in the abstract differ from that presented in the poster.
CHICAGO—A novel CD19-targeted T-cell therapy induced complete metabolic responses (CMRs) and no cytokine release syndrome (CRS) in patients with B-cell lymphomas in a first-in-human clinical study.
All subjects achieving CMR at the 1-month safety and efficacy assessment continued to show CMR at 3 months, investigators reported at the 2018 ASCO Annual Meeting (abstract 3049*).
The therapy is built on a novel platform, ARTEMIS, designed to match the potency of chimeric antigen receptor (CAR) T-cell therapy but trigger less cytokine release when the target is engaged, investigators explained.
That platform is “potentially a major improvement” over existing CAR-T cell therapy, said Zhi Tao Ying, MD, of Peking University Cancer Hospital & Institute in Beijing, China, and coauthors in a poster presented at ASCO.
The treatment, called ET190L1-ARTEMIS, utilizes the T-cell receptor platform and a proprietary human anti-CD19 antibody to target CD19-positive malignancies.
The investigators reported on 21 adults with CD-19 positive relapsed and refractory B-cell lymphomas who had received a median of 4 lines of previous therapy.
Patients received autologous ET190L1-ARTEMIS T cells in 1 of 3 dosing cohorts: 3 patients at 1 x 106/kg, 13 at 3 x 106/kg, and 5 at 6 x 106/kg.
Of 17 patients completing a first-month efficacy assessment, 11 (65%) responded, including 7 CMRs and 3 partial responses. One patient had stable disease.
Seven of the 11 responders completed a third-month efficacy assessment, as of this analysis. Of 5 patients with CMR at month 1, all 5 maintained CMR at month 3. Likewise, 1 patient in partial response and 1 with stable disease at month 1 had the same response status at month 3.
There were no cases of CRS or neurotoxicity in 17 patients who completed the 1-month safety and efficacy assessment reported at ASCO. Grade 3 or greater adverse events in those subjects included lymphopenia in 17 (100%) and neutropenia in 5 (29%).
Eureka Therapeutics Inc., of Emeryville, California, is developing ET190L1-ARTEMIS. Co-investigators in this trial were from Eureka, Xi-An Jiaotong University in China, and Duke University School of Medicine in Durham, North Carolina.
A phase 1 trial of ET190L1-ARTEMIS in patients with relapsed and refractory non-Hodgkin lymphoma has been initiated at Duke University, and investigators say another US phase 1 trial including relapsed and refractory pediatric acute lymphoblastic leukemia patients will begin later this year.
Data in the abstract differ from that presented in the poster.
Inhibitor exhibits activity in B- and T-cell NHLs
STOCKHOLM—The dual SYK/JAK inhibitor cerdulatinib has demonstrated efficacy in a phase 2 trial of patients with heavily pretreated B- and T-cell non-Hodgkin lymphomas (NHLs).
There were a few deaths due to sepsis or septic shock that were considered related to cerdulatinib, but investigators have taken steps to prevent additional deaths.
Cerdulatinib produced responses in patients with peripheral T-cell lymphoma (PTCL), cutaneous T-cell lymphoma (CTCL), chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), follicular lymphoma (FL), and other NHLs.
The 5 deaths due to sepsis or septic shock (3 concomitant with pneumonia) occurred early on in the trial, and dose reductions, monitoring, and antibiotic prophylaxis appeared to be effective in preventing additional deaths.
Results from this trial were presented in a poster (abstract PF437) at the 23rd Congress of the European Hematology Association (EHA).
The research was sponsored by Portola Pharmaceuticals, Inc.
The trial enrolled 114 patients. They had FL (grade 1-3A; n=39), PTCL (n=25), CTCL (n=5), CLL/SLL (n=28), other indolent NHLs (Waldenstrom’s macroglobulinemia and marginal zone lymphoma; n=12), or aggressive NHL (defined as diffuse large B-cell lymphoma [DLBCL], grade 3B FL, mantle cell lymphoma, and transformed NHL with relapsed disease; n=5).
The patients’ median age was 68 (range, 34-93), and 59% were male. The median number of prior treatment regimens was 3 (range, 1-13), and 37% of patients had refractory disease.
Patients received cerdulatinib at 25, 30, or 35 mg twice daily (BID). A total of 101 patients were evaluable as of May 4, 2018.
Response
The objective response rate (ORR) was 47% in the entire population. Thirteen patients achieved a complete response (CR), and 34 had a partial response (PR). Thirty-four patients remained on cerdulatinib at the data cut-off.
The ORR was 46% in the FL patients, with 3 patients achieving a CR and 13 achieving a PR. Thirteen FL patients remained on cerdulatinib.
In the CLL/SLL patients, the ORR was 61%. Two patients had a CR, and 15 had a PR. Four CLL/SLL patients remained on cerdulatinib.
In PTCL, the ORR was 35%. All 7 responders had a CR. Eleven PTCL patients remained on cerdulatinib.
Only 1 CTCL patient was evaluable, but this patient achieved a CR and remained on cerdulatinib.
The ORR was 42% for patients with other indolent NHLs, with 5 PRs and no CRs. Only 1 patient in this group remained on cerdulatinib.
For aggressive NHL, the ORR was 20%, with 1 PR and no CRs. None of the patients in this group stayed on cerdulatinib.
“Cerdulatinib continues to demonstrate promising results across a wide range of B- and T-cell malignancies, including early indications of the potential for durable responses,” said study investigator Paul Hamlin, MD, of Memorial Sloan Kettering Cancer Center in New York, New York.
“The new signals in relapsed/refractory PTCL and CTCL are particularly compelling when you consider the limited treatment options for patients that fail frontline therapy.”
Safety
Grade 3 or higher adverse events included lipase increase (18%), neutropenia (17%), pneumonia/lung infection (11%), diarrhea (8%), fatigue (6%), amylase increase (5%), sepsis/septic shock (4%), hypertension (4%), anemia (4%), thrombocytopenia (4%), and hypophosphatemia (4%).
The 5 deaths due to sepsis or septic shock (3 of which were concomitant with pneumonia) were considered related to cerdulatinib. Three of the deaths occurred in patients with CLL, 1 in a DLBCL patient, and 1 in an FL patient.
“One of the things we have seen [with CLL patients] is the background infection rate is quite a bit higher,” said John T. Curnutte, MD, PhD, interim co-president and head of research and development at Portola Pharmaceuticals.
“You see this with multiple other agents, so we were not particularly surprised to see [sepsis/septic shock in CLL].”
Dr Curnutte noted that grade 5 sepsis/septic shock tended to occur in patients who were pre-colonized and/or had high plasma levels of cerdulatinib.
So, to prevent these adverse events, the starting dose of cerdulatinib was lowered, investigators began monitoring patients’ plasma levels, and all patients began receiving antibiotic prophylaxis. (Previously, only CLL patients had received this prophylaxis.)
The investigators found that lowering the starting dose from 35 mg BID to 30 mg or even 25 mg BID reduced plasma levels.
“At the 35 mg dose, 1 out of every 4 patients showed accumulation of the drug,” Dr Curnutte said. “In general, the use of the 30 mg BID or step-down 25 mg BID did not result in accumulation of drug.”
Dr Curnutte also noted that enrollment of CLL/SLL patients is complete, and investigators would be “very careful” if the study were to be re-opened to these patients.
For now, Portola is focused on completing enrollment in other patient groups on this phase 2 trial. The company is also hoping to conduct a phase 3 trial of cerdulatinib in PTCL that could begin as early as the end of this year.
STOCKHOLM—The dual SYK/JAK inhibitor cerdulatinib has demonstrated efficacy in a phase 2 trial of patients with heavily pretreated B- and T-cell non-Hodgkin lymphomas (NHLs).
There were a few deaths due to sepsis or septic shock that were considered related to cerdulatinib, but investigators have taken steps to prevent additional deaths.
Cerdulatinib produced responses in patients with peripheral T-cell lymphoma (PTCL), cutaneous T-cell lymphoma (CTCL), chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), follicular lymphoma (FL), and other NHLs.
The 5 deaths due to sepsis or septic shock (3 concomitant with pneumonia) occurred early on in the trial, and dose reductions, monitoring, and antibiotic prophylaxis appeared to be effective in preventing additional deaths.
Results from this trial were presented in a poster (abstract PF437) at the 23rd Congress of the European Hematology Association (EHA).
The research was sponsored by Portola Pharmaceuticals, Inc.
The trial enrolled 114 patients. They had FL (grade 1-3A; n=39), PTCL (n=25), CTCL (n=5), CLL/SLL (n=28), other indolent NHLs (Waldenstrom’s macroglobulinemia and marginal zone lymphoma; n=12), or aggressive NHL (defined as diffuse large B-cell lymphoma [DLBCL], grade 3B FL, mantle cell lymphoma, and transformed NHL with relapsed disease; n=5).
The patients’ median age was 68 (range, 34-93), and 59% were male. The median number of prior treatment regimens was 3 (range, 1-13), and 37% of patients had refractory disease.
Patients received cerdulatinib at 25, 30, or 35 mg twice daily (BID). A total of 101 patients were evaluable as of May 4, 2018.
Response
The objective response rate (ORR) was 47% in the entire population. Thirteen patients achieved a complete response (CR), and 34 had a partial response (PR). Thirty-four patients remained on cerdulatinib at the data cut-off.
The ORR was 46% in the FL patients, with 3 patients achieving a CR and 13 achieving a PR. Thirteen FL patients remained on cerdulatinib.
In the CLL/SLL patients, the ORR was 61%. Two patients had a CR, and 15 had a PR. Four CLL/SLL patients remained on cerdulatinib.
In PTCL, the ORR was 35%. All 7 responders had a CR. Eleven PTCL patients remained on cerdulatinib.
Only 1 CTCL patient was evaluable, but this patient achieved a CR and remained on cerdulatinib.
The ORR was 42% for patients with other indolent NHLs, with 5 PRs and no CRs. Only 1 patient in this group remained on cerdulatinib.
For aggressive NHL, the ORR was 20%, with 1 PR and no CRs. None of the patients in this group stayed on cerdulatinib.
“Cerdulatinib continues to demonstrate promising results across a wide range of B- and T-cell malignancies, including early indications of the potential for durable responses,” said study investigator Paul Hamlin, MD, of Memorial Sloan Kettering Cancer Center in New York, New York.
“The new signals in relapsed/refractory PTCL and CTCL are particularly compelling when you consider the limited treatment options for patients that fail frontline therapy.”
Safety
Grade 3 or higher adverse events included lipase increase (18%), neutropenia (17%), pneumonia/lung infection (11%), diarrhea (8%), fatigue (6%), amylase increase (5%), sepsis/septic shock (4%), hypertension (4%), anemia (4%), thrombocytopenia (4%), and hypophosphatemia (4%).
The 5 deaths due to sepsis or septic shock (3 of which were concomitant with pneumonia) were considered related to cerdulatinib. Three of the deaths occurred in patients with CLL, 1 in a DLBCL patient, and 1 in an FL patient.
“One of the things we have seen [with CLL patients] is the background infection rate is quite a bit higher,” said John T. Curnutte, MD, PhD, interim co-president and head of research and development at Portola Pharmaceuticals.
“You see this with multiple other agents, so we were not particularly surprised to see [sepsis/septic shock in CLL].”
Dr Curnutte noted that grade 5 sepsis/septic shock tended to occur in patients who were pre-colonized and/or had high plasma levels of cerdulatinib.
So, to prevent these adverse events, the starting dose of cerdulatinib was lowered, investigators began monitoring patients’ plasma levels, and all patients began receiving antibiotic prophylaxis. (Previously, only CLL patients had received this prophylaxis.)
The investigators found that lowering the starting dose from 35 mg BID to 30 mg or even 25 mg BID reduced plasma levels.
“At the 35 mg dose, 1 out of every 4 patients showed accumulation of the drug,” Dr Curnutte said. “In general, the use of the 30 mg BID or step-down 25 mg BID did not result in accumulation of drug.”
Dr Curnutte also noted that enrollment of CLL/SLL patients is complete, and investigators would be “very careful” if the study were to be re-opened to these patients.
For now, Portola is focused on completing enrollment in other patient groups on this phase 2 trial. The company is also hoping to conduct a phase 3 trial of cerdulatinib in PTCL that could begin as early as the end of this year.
STOCKHOLM—The dual SYK/JAK inhibitor cerdulatinib has demonstrated efficacy in a phase 2 trial of patients with heavily pretreated B- and T-cell non-Hodgkin lymphomas (NHLs).
There were a few deaths due to sepsis or septic shock that were considered related to cerdulatinib, but investigators have taken steps to prevent additional deaths.
Cerdulatinib produced responses in patients with peripheral T-cell lymphoma (PTCL), cutaneous T-cell lymphoma (CTCL), chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL), follicular lymphoma (FL), and other NHLs.
The 5 deaths due to sepsis or septic shock (3 concomitant with pneumonia) occurred early on in the trial, and dose reductions, monitoring, and antibiotic prophylaxis appeared to be effective in preventing additional deaths.
Results from this trial were presented in a poster (abstract PF437) at the 23rd Congress of the European Hematology Association (EHA).
The research was sponsored by Portola Pharmaceuticals, Inc.
The trial enrolled 114 patients. They had FL (grade 1-3A; n=39), PTCL (n=25), CTCL (n=5), CLL/SLL (n=28), other indolent NHLs (Waldenstrom’s macroglobulinemia and marginal zone lymphoma; n=12), or aggressive NHL (defined as diffuse large B-cell lymphoma [DLBCL], grade 3B FL, mantle cell lymphoma, and transformed NHL with relapsed disease; n=5).
The patients’ median age was 68 (range, 34-93), and 59% were male. The median number of prior treatment regimens was 3 (range, 1-13), and 37% of patients had refractory disease.
Patients received cerdulatinib at 25, 30, or 35 mg twice daily (BID). A total of 101 patients were evaluable as of May 4, 2018.
Response
The objective response rate (ORR) was 47% in the entire population. Thirteen patients achieved a complete response (CR), and 34 had a partial response (PR). Thirty-four patients remained on cerdulatinib at the data cut-off.
The ORR was 46% in the FL patients, with 3 patients achieving a CR and 13 achieving a PR. Thirteen FL patients remained on cerdulatinib.
In the CLL/SLL patients, the ORR was 61%. Two patients had a CR, and 15 had a PR. Four CLL/SLL patients remained on cerdulatinib.
In PTCL, the ORR was 35%. All 7 responders had a CR. Eleven PTCL patients remained on cerdulatinib.
Only 1 CTCL patient was evaluable, but this patient achieved a CR and remained on cerdulatinib.
The ORR was 42% for patients with other indolent NHLs, with 5 PRs and no CRs. Only 1 patient in this group remained on cerdulatinib.
For aggressive NHL, the ORR was 20%, with 1 PR and no CRs. None of the patients in this group stayed on cerdulatinib.
“Cerdulatinib continues to demonstrate promising results across a wide range of B- and T-cell malignancies, including early indications of the potential for durable responses,” said study investigator Paul Hamlin, MD, of Memorial Sloan Kettering Cancer Center in New York, New York.
“The new signals in relapsed/refractory PTCL and CTCL are particularly compelling when you consider the limited treatment options for patients that fail frontline therapy.”
Safety
Grade 3 or higher adverse events included lipase increase (18%), neutropenia (17%), pneumonia/lung infection (11%), diarrhea (8%), fatigue (6%), amylase increase (5%), sepsis/septic shock (4%), hypertension (4%), anemia (4%), thrombocytopenia (4%), and hypophosphatemia (4%).
The 5 deaths due to sepsis or septic shock (3 of which were concomitant with pneumonia) were considered related to cerdulatinib. Three of the deaths occurred in patients with CLL, 1 in a DLBCL patient, and 1 in an FL patient.
“One of the things we have seen [with CLL patients] is the background infection rate is quite a bit higher,” said John T. Curnutte, MD, PhD, interim co-president and head of research and development at Portola Pharmaceuticals.
“You see this with multiple other agents, so we were not particularly surprised to see [sepsis/septic shock in CLL].”
Dr Curnutte noted that grade 5 sepsis/septic shock tended to occur in patients who were pre-colonized and/or had high plasma levels of cerdulatinib.
So, to prevent these adverse events, the starting dose of cerdulatinib was lowered, investigators began monitoring patients’ plasma levels, and all patients began receiving antibiotic prophylaxis. (Previously, only CLL patients had received this prophylaxis.)
The investigators found that lowering the starting dose from 35 mg BID to 30 mg or even 25 mg BID reduced plasma levels.
“At the 35 mg dose, 1 out of every 4 patients showed accumulation of the drug,” Dr Curnutte said. “In general, the use of the 30 mg BID or step-down 25 mg BID did not result in accumulation of drug.”
Dr Curnutte also noted that enrollment of CLL/SLL patients is complete, and investigators would be “very careful” if the study were to be re-opened to these patients.
For now, Portola is focused on completing enrollment in other patient groups on this phase 2 trial. The company is also hoping to conduct a phase 3 trial of cerdulatinib in PTCL that could begin as early as the end of this year.
Combo proves ‘beneficial’ for ‘unfit’ CLL patients
STOCKHOLM—Obinutuzumab plus chlorambucil (G-Clb) is a “valid and beneficial” frontline treatment option for “unfit” patients with chronic lymphocytic leukemia (CLL), according to a speaker at the 23rd Congress of the European Hematology Association (EHA).
Final results from the CLL11 study have revealed additional benefits of G-Clb over rituximab plus chlorambucil (R-Clb) in patients with previously untreated CLL and comorbidities.
Prior results from this study showed that G-Clb produced higher response rates and prolonged progression-fee survival (PFS) compared to R-Clb.
Now, with a median follow-up of 5 years, researchers have found that G-Clb prolongs overall survival (OS) and time to next treatment (TTNT) as well.
Valentin Goede, MD, of the University Hospital Cologne in Germany, presented these results during the Presidential Symposium of the EHA Congress (abstract S151).
The study was sponsored by Hoffmann-La Roche.
CLL11 enrolled patients with previously untreated CLL and coexisting medical conditions. They were randomized to receive six 28-day cycles of Clb alone, G-Clb, or R-Clb.
In stage 1, researchers compared G-Clb (n=238) to Clb alone (n=118) and R-Clb (n=233) to Clb alone (n=118). In stage 2, they compared G-Clb (n=333) and R-Clb (n=330).
“The treatment arms were well-balanced, not just with regard to patient [characteristics] but also with disease characteristics,” Dr Goede said.
Overall, the median age was 73 (range, 39-90), the median Cumulative Illness Rating Scale score was 8, and the median creatinine clearance was 62 mL/min.
Efficacy: G-Clb vs Clb
The median observation time for G-Clb vs Clb was 62.5 months.
The median PFS was 31.1 months in the G-Clb arm and 11.1 months in the Clb arm. The 5-year PFS rates were 25% and 2%, respectively. The hazard ratio (HR) was 0.21 (P<0.0001).
The median OS was not reached in the G-Clb arm and was 66.7 months in the Clb arm. The 5-year OS rates were 66% and 53%, respectively. The HR was 0.68 (P=0.0196).
Thirty-nine percent of the G-Clb arm died, as did 49% of the Clb arm. The main causes of death were adverse events (AEs) and disease progression.
Efficacy: G-Clb vs R-Clb
The median observation time for G-Clb vs R-Clb was 59.4 months.
The median PFS was 28.9 months in the G-Clb arm and 15.7 months in the R-Clb arm. The 5-year PFS rates were 23% and 9%, respectively. The HR was 0.49 (P<0.0001).
“The median PFS was nearly doubled, from approximately 15 months in the rituximab arm to almost 30 months in the obinutuzumab arm,” Dr Goede said. “And this translated into a clinically meaningful prolongation of time to next treatment.”
The median TTNT was 56.4 months in the G-Clb arm and 34.9 months in the R-Clb arm. At 5 years, TTNT rates were 49% and 32%, respectively. The HR was 0.58 (P<0.0001).
“In the rituximab arm, the median time to next treatment was a little greater than 2.5 years, and, in the obinutuzumab arm, it was almost 5 years,” Dr Goede said. “From a clinical perspective, I would consider treatment-free intervals of that duration as highly relevant and beneficial in an elderly population.”
The median OS was not reached in the G-Clb arm and was 73.1 months in the R-Clb arm. The 5-year OS rates were 66% and 57%, respectively. The HR was 0.76 (P=0.0245).
“This difference is clinically meaningful, and it is also remarkable in the context of the long follow-up, given the fact that approximately half of the patients have received at least 1 salvage treatment in the meantime,” Dr Goede said.
In all, 37% of the G-Clb arm died, as did 45% of the R-Clb arm. Again, the main causes of death were AEs and disease progression.
Safety
Dr Goede said no new safety signals or late-onset toxicities were detected.
“Adverse events of any grade, but particularly grade 3-5 and serious adverse events, were more frequent in the obinutuzumab arm compared to the other 2 arms,” he noted. “[This] was mainly driven by more infusion reactions and some greater hematological toxicity.”
“Importantly, the rate of fatal adverse events, during treatment but also during follow-up, was not higher in the obinutuzumab arm. And the most common fatal adverse events were second malignancies.”
G-Clb vs Clb
Ninety-five percent of patients in the G-Clb arm and 83% of those in the Clb arm had at least 1 AE. The rates of grade 3-5 AEs were 74% and 51%, respectively. The rates of serious AEs were 47% and 39%, respectively. The rates of fatal AEs were 8% and 11%, respectively.
Seventeen percent of patients in the G-Clb arm and 11% of those in the Clb arm had late-onset neutropenia. The rates of prolonged neutropenia were 3% and 9%, respectively.
Fourteen percent of patients in the G-Clb arm and 7% of those in the Clb arm had second malignancies (starting 6 months after treatment initiation). The most common of these were squamous cell carcinoma (2% vs 0%) and basal cell carcinoma (2% vs <1%).
G-Clb vs R-Clb
Ninety-four percent of patients in the G-Clb arm and 90% of those in the R-Clb arm had at least 1 AE. The rates of grade 3-5 AEs were 72% and 60%, respectively. The rates of serious AEs were 45% and 39%, respectively. The rates of fatal AEs were 7% and 10%, respectively.
Fifteen percent of patients in the G-Clb arm and 12% of those in the R-Clb arm had late-onset neutropenia. The rates of prolonged neutropenia were 2% and 4%, respectively.
Eleven percent of patients in the G-Clb arm and 10% of those in the Clb arm had second malignancies. Squamous cell carcinoma occurred in 2% of patients in both arms. Basal cell carcinoma occurred in 2% of G-Clb recipients and 1% of R-Clb recipients.
STOCKHOLM—Obinutuzumab plus chlorambucil (G-Clb) is a “valid and beneficial” frontline treatment option for “unfit” patients with chronic lymphocytic leukemia (CLL), according to a speaker at the 23rd Congress of the European Hematology Association (EHA).
Final results from the CLL11 study have revealed additional benefits of G-Clb over rituximab plus chlorambucil (R-Clb) in patients with previously untreated CLL and comorbidities.
Prior results from this study showed that G-Clb produced higher response rates and prolonged progression-fee survival (PFS) compared to R-Clb.
Now, with a median follow-up of 5 years, researchers have found that G-Clb prolongs overall survival (OS) and time to next treatment (TTNT) as well.
Valentin Goede, MD, of the University Hospital Cologne in Germany, presented these results during the Presidential Symposium of the EHA Congress (abstract S151).
The study was sponsored by Hoffmann-La Roche.
CLL11 enrolled patients with previously untreated CLL and coexisting medical conditions. They were randomized to receive six 28-day cycles of Clb alone, G-Clb, or R-Clb.
In stage 1, researchers compared G-Clb (n=238) to Clb alone (n=118) and R-Clb (n=233) to Clb alone (n=118). In stage 2, they compared G-Clb (n=333) and R-Clb (n=330).
“The treatment arms were well-balanced, not just with regard to patient [characteristics] but also with disease characteristics,” Dr Goede said.
Overall, the median age was 73 (range, 39-90), the median Cumulative Illness Rating Scale score was 8, and the median creatinine clearance was 62 mL/min.
Efficacy: G-Clb vs Clb
The median observation time for G-Clb vs Clb was 62.5 months.
The median PFS was 31.1 months in the G-Clb arm and 11.1 months in the Clb arm. The 5-year PFS rates were 25% and 2%, respectively. The hazard ratio (HR) was 0.21 (P<0.0001).
The median OS was not reached in the G-Clb arm and was 66.7 months in the Clb arm. The 5-year OS rates were 66% and 53%, respectively. The HR was 0.68 (P=0.0196).
Thirty-nine percent of the G-Clb arm died, as did 49% of the Clb arm. The main causes of death were adverse events (AEs) and disease progression.
Efficacy: G-Clb vs R-Clb
The median observation time for G-Clb vs R-Clb was 59.4 months.
The median PFS was 28.9 months in the G-Clb arm and 15.7 months in the R-Clb arm. The 5-year PFS rates were 23% and 9%, respectively. The HR was 0.49 (P<0.0001).
“The median PFS was nearly doubled, from approximately 15 months in the rituximab arm to almost 30 months in the obinutuzumab arm,” Dr Goede said. “And this translated into a clinically meaningful prolongation of time to next treatment.”
The median TTNT was 56.4 months in the G-Clb arm and 34.9 months in the R-Clb arm. At 5 years, TTNT rates were 49% and 32%, respectively. The HR was 0.58 (P<0.0001).
“In the rituximab arm, the median time to next treatment was a little greater than 2.5 years, and, in the obinutuzumab arm, it was almost 5 years,” Dr Goede said. “From a clinical perspective, I would consider treatment-free intervals of that duration as highly relevant and beneficial in an elderly population.”
The median OS was not reached in the G-Clb arm and was 73.1 months in the R-Clb arm. The 5-year OS rates were 66% and 57%, respectively. The HR was 0.76 (P=0.0245).
“This difference is clinically meaningful, and it is also remarkable in the context of the long follow-up, given the fact that approximately half of the patients have received at least 1 salvage treatment in the meantime,” Dr Goede said.
In all, 37% of the G-Clb arm died, as did 45% of the R-Clb arm. Again, the main causes of death were AEs and disease progression.
Safety
Dr Goede said no new safety signals or late-onset toxicities were detected.
“Adverse events of any grade, but particularly grade 3-5 and serious adverse events, were more frequent in the obinutuzumab arm compared to the other 2 arms,” he noted. “[This] was mainly driven by more infusion reactions and some greater hematological toxicity.”
“Importantly, the rate of fatal adverse events, during treatment but also during follow-up, was not higher in the obinutuzumab arm. And the most common fatal adverse events were second malignancies.”
G-Clb vs Clb
Ninety-five percent of patients in the G-Clb arm and 83% of those in the Clb arm had at least 1 AE. The rates of grade 3-5 AEs were 74% and 51%, respectively. The rates of serious AEs were 47% and 39%, respectively. The rates of fatal AEs were 8% and 11%, respectively.
Seventeen percent of patients in the G-Clb arm and 11% of those in the Clb arm had late-onset neutropenia. The rates of prolonged neutropenia were 3% and 9%, respectively.
Fourteen percent of patients in the G-Clb arm and 7% of those in the Clb arm had second malignancies (starting 6 months after treatment initiation). The most common of these were squamous cell carcinoma (2% vs 0%) and basal cell carcinoma (2% vs <1%).
G-Clb vs R-Clb
Ninety-four percent of patients in the G-Clb arm and 90% of those in the R-Clb arm had at least 1 AE. The rates of grade 3-5 AEs were 72% and 60%, respectively. The rates of serious AEs were 45% and 39%, respectively. The rates of fatal AEs were 7% and 10%, respectively.
Fifteen percent of patients in the G-Clb arm and 12% of those in the R-Clb arm had late-onset neutropenia. The rates of prolonged neutropenia were 2% and 4%, respectively.
Eleven percent of patients in the G-Clb arm and 10% of those in the Clb arm had second malignancies. Squamous cell carcinoma occurred in 2% of patients in both arms. Basal cell carcinoma occurred in 2% of G-Clb recipients and 1% of R-Clb recipients.
STOCKHOLM—Obinutuzumab plus chlorambucil (G-Clb) is a “valid and beneficial” frontline treatment option for “unfit” patients with chronic lymphocytic leukemia (CLL), according to a speaker at the 23rd Congress of the European Hematology Association (EHA).
Final results from the CLL11 study have revealed additional benefits of G-Clb over rituximab plus chlorambucil (R-Clb) in patients with previously untreated CLL and comorbidities.
Prior results from this study showed that G-Clb produced higher response rates and prolonged progression-fee survival (PFS) compared to R-Clb.
Now, with a median follow-up of 5 years, researchers have found that G-Clb prolongs overall survival (OS) and time to next treatment (TTNT) as well.
Valentin Goede, MD, of the University Hospital Cologne in Germany, presented these results during the Presidential Symposium of the EHA Congress (abstract S151).
The study was sponsored by Hoffmann-La Roche.
CLL11 enrolled patients with previously untreated CLL and coexisting medical conditions. They were randomized to receive six 28-day cycles of Clb alone, G-Clb, or R-Clb.
In stage 1, researchers compared G-Clb (n=238) to Clb alone (n=118) and R-Clb (n=233) to Clb alone (n=118). In stage 2, they compared G-Clb (n=333) and R-Clb (n=330).
“The treatment arms were well-balanced, not just with regard to patient [characteristics] but also with disease characteristics,” Dr Goede said.
Overall, the median age was 73 (range, 39-90), the median Cumulative Illness Rating Scale score was 8, and the median creatinine clearance was 62 mL/min.
Efficacy: G-Clb vs Clb
The median observation time for G-Clb vs Clb was 62.5 months.
The median PFS was 31.1 months in the G-Clb arm and 11.1 months in the Clb arm. The 5-year PFS rates were 25% and 2%, respectively. The hazard ratio (HR) was 0.21 (P<0.0001).
The median OS was not reached in the G-Clb arm and was 66.7 months in the Clb arm. The 5-year OS rates were 66% and 53%, respectively. The HR was 0.68 (P=0.0196).
Thirty-nine percent of the G-Clb arm died, as did 49% of the Clb arm. The main causes of death were adverse events (AEs) and disease progression.
Efficacy: G-Clb vs R-Clb
The median observation time for G-Clb vs R-Clb was 59.4 months.
The median PFS was 28.9 months in the G-Clb arm and 15.7 months in the R-Clb arm. The 5-year PFS rates were 23% and 9%, respectively. The HR was 0.49 (P<0.0001).
“The median PFS was nearly doubled, from approximately 15 months in the rituximab arm to almost 30 months in the obinutuzumab arm,” Dr Goede said. “And this translated into a clinically meaningful prolongation of time to next treatment.”
The median TTNT was 56.4 months in the G-Clb arm and 34.9 months in the R-Clb arm. At 5 years, TTNT rates were 49% and 32%, respectively. The HR was 0.58 (P<0.0001).
“In the rituximab arm, the median time to next treatment was a little greater than 2.5 years, and, in the obinutuzumab arm, it was almost 5 years,” Dr Goede said. “From a clinical perspective, I would consider treatment-free intervals of that duration as highly relevant and beneficial in an elderly population.”
The median OS was not reached in the G-Clb arm and was 73.1 months in the R-Clb arm. The 5-year OS rates were 66% and 57%, respectively. The HR was 0.76 (P=0.0245).
“This difference is clinically meaningful, and it is also remarkable in the context of the long follow-up, given the fact that approximately half of the patients have received at least 1 salvage treatment in the meantime,” Dr Goede said.
In all, 37% of the G-Clb arm died, as did 45% of the R-Clb arm. Again, the main causes of death were AEs and disease progression.
Safety
Dr Goede said no new safety signals or late-onset toxicities were detected.
“Adverse events of any grade, but particularly grade 3-5 and serious adverse events, were more frequent in the obinutuzumab arm compared to the other 2 arms,” he noted. “[This] was mainly driven by more infusion reactions and some greater hematological toxicity.”
“Importantly, the rate of fatal adverse events, during treatment but also during follow-up, was not higher in the obinutuzumab arm. And the most common fatal adverse events were second malignancies.”
G-Clb vs Clb
Ninety-five percent of patients in the G-Clb arm and 83% of those in the Clb arm had at least 1 AE. The rates of grade 3-5 AEs were 74% and 51%, respectively. The rates of serious AEs were 47% and 39%, respectively. The rates of fatal AEs were 8% and 11%, respectively.
Seventeen percent of patients in the G-Clb arm and 11% of those in the Clb arm had late-onset neutropenia. The rates of prolonged neutropenia were 3% and 9%, respectively.
Fourteen percent of patients in the G-Clb arm and 7% of those in the Clb arm had second malignancies (starting 6 months after treatment initiation). The most common of these were squamous cell carcinoma (2% vs 0%) and basal cell carcinoma (2% vs <1%).
G-Clb vs R-Clb
Ninety-four percent of patients in the G-Clb arm and 90% of those in the R-Clb arm had at least 1 AE. The rates of grade 3-5 AEs were 72% and 60%, respectively. The rates of serious AEs were 45% and 39%, respectively. The rates of fatal AEs were 7% and 10%, respectively.
Fifteen percent of patients in the G-Clb arm and 12% of those in the R-Clb arm had late-onset neutropenia. The rates of prolonged neutropenia were 2% and 4%, respectively.
Eleven percent of patients in the G-Clb arm and 10% of those in the Clb arm had second malignancies. Squamous cell carcinoma occurred in 2% of patients in both arms. Basal cell carcinoma occurred in 2% of G-Clb recipients and 1% of R-Clb recipients.
FDA places SB-generated CAR T-cell therapy on clinical hold
The US Food and Drug Administrated (FDA) placed a clinical hold on the phase 1 trial of the Sleeping Beauty (SB)-generated CAR T-cell therapy in relapsed or refractory leukemia and lymphoma patients.
The Sleeping Beauty platform was designed to very rapidly manufacture CD19-specific CAR T cells at the point of care.
All SB-CAR T-cell processing is planned to take place within 2 days at the healthcare facility, thus eliminating shipping cells from hospitals to production sites and back again.
The FDA is requesting more chemistry, manufacturing, and control (CMC) information before allowing the trial to proceed.
The Sleeping Beauty technology, a non-viral transposon/transposase system, has the potential to reduce the costs and complexity associated with recombinant viral vector-based immunotherapy, according to developers.
Ziopharm Oncology, Precigen, Inc, a wholly owned subsidiary of Intrexon Corporation, and the University of Texas MD Anderson Cancer Center, are developing the Sleeping Beauty CAR T cell therapy.
“We know what is needed to address the hold issues and are looking forward to responding to the agency in a timely manner,” said Laurence Cooper, MD, PhD, chief executive officer of Ziopharm, in a corporate release.
“We are undertaking cutting-edge science and are on the verge of a paradigm shift based on our approach to very rapidly manufacture CD19-specific T cells within 2 days using our non-viral approach to CAR-T therapy based on the Sleeping Beauty platform.”
The phase 1 trial in question is a third-generation trial in which the CAR T cells are designed to co-express CD19-specific CAR, membrane-bound interleukin 15, and a safety switch.
The findings from earlier generation phase 1 trials have been previously reported in The Journal of Clinical Investigation.
The US Food and Drug Administrated (FDA) placed a clinical hold on the phase 1 trial of the Sleeping Beauty (SB)-generated CAR T-cell therapy in relapsed or refractory leukemia and lymphoma patients.
The Sleeping Beauty platform was designed to very rapidly manufacture CD19-specific CAR T cells at the point of care.
All SB-CAR T-cell processing is planned to take place within 2 days at the healthcare facility, thus eliminating shipping cells from hospitals to production sites and back again.
The FDA is requesting more chemistry, manufacturing, and control (CMC) information before allowing the trial to proceed.
The Sleeping Beauty technology, a non-viral transposon/transposase system, has the potential to reduce the costs and complexity associated with recombinant viral vector-based immunotherapy, according to developers.
Ziopharm Oncology, Precigen, Inc, a wholly owned subsidiary of Intrexon Corporation, and the University of Texas MD Anderson Cancer Center, are developing the Sleeping Beauty CAR T cell therapy.
“We know what is needed to address the hold issues and are looking forward to responding to the agency in a timely manner,” said Laurence Cooper, MD, PhD, chief executive officer of Ziopharm, in a corporate release.
“We are undertaking cutting-edge science and are on the verge of a paradigm shift based on our approach to very rapidly manufacture CD19-specific T cells within 2 days using our non-viral approach to CAR-T therapy based on the Sleeping Beauty platform.”
The phase 1 trial in question is a third-generation trial in which the CAR T cells are designed to co-express CD19-specific CAR, membrane-bound interleukin 15, and a safety switch.
The findings from earlier generation phase 1 trials have been previously reported in The Journal of Clinical Investigation.
The US Food and Drug Administrated (FDA) placed a clinical hold on the phase 1 trial of the Sleeping Beauty (SB)-generated CAR T-cell therapy in relapsed or refractory leukemia and lymphoma patients.
The Sleeping Beauty platform was designed to very rapidly manufacture CD19-specific CAR T cells at the point of care.
All SB-CAR T-cell processing is planned to take place within 2 days at the healthcare facility, thus eliminating shipping cells from hospitals to production sites and back again.
The FDA is requesting more chemistry, manufacturing, and control (CMC) information before allowing the trial to proceed.
The Sleeping Beauty technology, a non-viral transposon/transposase system, has the potential to reduce the costs and complexity associated with recombinant viral vector-based immunotherapy, according to developers.
Ziopharm Oncology, Precigen, Inc, a wholly owned subsidiary of Intrexon Corporation, and the University of Texas MD Anderson Cancer Center, are developing the Sleeping Beauty CAR T cell therapy.
“We know what is needed to address the hold issues and are looking forward to responding to the agency in a timely manner,” said Laurence Cooper, MD, PhD, chief executive officer of Ziopharm, in a corporate release.
“We are undertaking cutting-edge science and are on the verge of a paradigm shift based on our approach to very rapidly manufacture CD19-specific T cells within 2 days using our non-viral approach to CAR-T therapy based on the Sleeping Beauty platform.”
The phase 1 trial in question is a third-generation trial in which the CAR T cells are designed to co-express CD19-specific CAR, membrane-bound interleukin 15, and a safety switch.
The findings from earlier generation phase 1 trials have been previously reported in The Journal of Clinical Investigation.
Peripheral blood MRD correlates with treatment benefit in CLL
CHICAGO—Minimal residual disease (MRD) kinetics confirms the high, durable MRD-negativity with venetoclax plus rituximab in relapsed/refractory chronic lymphocytic leukemia (CLL), according to a further examination of the phase 3 MURANO study.
Undetectable MRD-negativity is associated with extended progression-free survival (PFS) and overall survival in patients receiving chemoimmunotherapy for CLL.
“Attainment of MRD-negativity in relapsed/refractory CLL is also a desired trial endpoint due to the subjectivity of complete response definition regarding pathologic lymph node size,” said Peter Hillmen, MD, of St James’s University Hospital, Leeds, United Kingdom, at the 2018 ASCO Annual Meeting.
Dr Hillmen reported new data on MRD response in cytogenetic and molecular risk groups, MRD sustainability and kinetics, and MRD conversion in the MURANO trial (abstract 7508).
MURANO trial (NCT02005471)
In the trial, venetoclax-rituximab showed superior PFS and peripheral blood and bone marrow MRD-negativity as compared to bendamustine plus rituximab (BR) in relapsed/refractory CLL patients.
Patients were randomized to venetoclax-rituximab for 6 months, followed by single-agent venetoclax for up to 1.5 years, or BR for 6 months. Peripheral blood samples were serially collected and bone marrow was collected at the end of combination treatment or at best response.
MRD findings
The new results show higher concordance in MRD-negativity between bone marrow and peripheral blood in venetoclax-rituximab (45 of 50 patients, 90%) vs BR (3 of 10 patients, 30%) in paired samples.
Focusing on peripheral blood MRD, Dr Hillmen said the best MRD-negativity rates were higher with venetoclax-rituximab (84%) than BR (23%). These results were independent of high-risk factors—such as del 17p, IGVH unmutated, and mutated TP53—only for venetoclax-rituximab treated patients.
“The superior peripheral blood MRD response with venetoclax-rituximab was consistent across subgroups at the end of completion of treatment,” Dr Hillmen said. “Most patients who achieved peripheral blood MRD-negativity on venetoclax-rituximab remained MRD-negative and were progression-free.”
Among 121 of 194 (62%) patients on venetoclax-rituximab who achieved MRD-negativity at the end of combination therapy, 100 (83%) patients maintained MRD-negativity and were progression-free at a median follow-up of 13.8 months. Two patients developed progressive disease and 2 patients died (unrelated to CLL).
Two patients developed Richter’s disease (with one MRD-positive directly before therapy) and 15 (12%) patients converted to confirmed MRD-positive at a median MRD-positive follow-up of 5.6 months.
“High peripheral blood MRD-negativity at the end of combination treatment and concordance with bone marrow MRD with venetoclax-rituximab,” Dr Hillmen said, “confirms the value of peripheral blood MRD for evaluation of treatment benefit in relapsed/refractory CLL patients. The high rate of peripheral blood MRD-negativity at end of combination treatment with venetoclax-rituximab was attained regardless of risk features.”
Some conversion to MRD-positivity occurred only in a small proportion of patients. Most cases were of intermediate level and remained progression-free, he said.
“MRD kinetics indicate that peripheral blood MRD-negativity with venetoclax-rituximab occurs early and is maintained over time with current follow-up,” Dr Hillmen added. The MRD data now provide a framework for designing response adaptive therapy.
The US Food and Drug Administration recently approved venetoclax-rituximab for CLL or small lymphocytic lymphoma for patients with or without del 17p.
Venetoclax is being developed by Genentech and Abbvie.
CHICAGO—Minimal residual disease (MRD) kinetics confirms the high, durable MRD-negativity with venetoclax plus rituximab in relapsed/refractory chronic lymphocytic leukemia (CLL), according to a further examination of the phase 3 MURANO study.
Undetectable MRD-negativity is associated with extended progression-free survival (PFS) and overall survival in patients receiving chemoimmunotherapy for CLL.
“Attainment of MRD-negativity in relapsed/refractory CLL is also a desired trial endpoint due to the subjectivity of complete response definition regarding pathologic lymph node size,” said Peter Hillmen, MD, of St James’s University Hospital, Leeds, United Kingdom, at the 2018 ASCO Annual Meeting.
Dr Hillmen reported new data on MRD response in cytogenetic and molecular risk groups, MRD sustainability and kinetics, and MRD conversion in the MURANO trial (abstract 7508).
MURANO trial (NCT02005471)
In the trial, venetoclax-rituximab showed superior PFS and peripheral blood and bone marrow MRD-negativity as compared to bendamustine plus rituximab (BR) in relapsed/refractory CLL patients.
Patients were randomized to venetoclax-rituximab for 6 months, followed by single-agent venetoclax for up to 1.5 years, or BR for 6 months. Peripheral blood samples were serially collected and bone marrow was collected at the end of combination treatment or at best response.
MRD findings
The new results show higher concordance in MRD-negativity between bone marrow and peripheral blood in venetoclax-rituximab (45 of 50 patients, 90%) vs BR (3 of 10 patients, 30%) in paired samples.
Focusing on peripheral blood MRD, Dr Hillmen said the best MRD-negativity rates were higher with venetoclax-rituximab (84%) than BR (23%). These results were independent of high-risk factors—such as del 17p, IGVH unmutated, and mutated TP53—only for venetoclax-rituximab treated patients.
“The superior peripheral blood MRD response with venetoclax-rituximab was consistent across subgroups at the end of completion of treatment,” Dr Hillmen said. “Most patients who achieved peripheral blood MRD-negativity on venetoclax-rituximab remained MRD-negative and were progression-free.”
Among 121 of 194 (62%) patients on venetoclax-rituximab who achieved MRD-negativity at the end of combination therapy, 100 (83%) patients maintained MRD-negativity and were progression-free at a median follow-up of 13.8 months. Two patients developed progressive disease and 2 patients died (unrelated to CLL).
Two patients developed Richter’s disease (with one MRD-positive directly before therapy) and 15 (12%) patients converted to confirmed MRD-positive at a median MRD-positive follow-up of 5.6 months.
“High peripheral blood MRD-negativity at the end of combination treatment and concordance with bone marrow MRD with venetoclax-rituximab,” Dr Hillmen said, “confirms the value of peripheral blood MRD for evaluation of treatment benefit in relapsed/refractory CLL patients. The high rate of peripheral blood MRD-negativity at end of combination treatment with venetoclax-rituximab was attained regardless of risk features.”
Some conversion to MRD-positivity occurred only in a small proportion of patients. Most cases were of intermediate level and remained progression-free, he said.
“MRD kinetics indicate that peripheral blood MRD-negativity with venetoclax-rituximab occurs early and is maintained over time with current follow-up,” Dr Hillmen added. The MRD data now provide a framework for designing response adaptive therapy.
The US Food and Drug Administration recently approved venetoclax-rituximab for CLL or small lymphocytic lymphoma for patients with or without del 17p.
Venetoclax is being developed by Genentech and Abbvie.
CHICAGO—Minimal residual disease (MRD) kinetics confirms the high, durable MRD-negativity with venetoclax plus rituximab in relapsed/refractory chronic lymphocytic leukemia (CLL), according to a further examination of the phase 3 MURANO study.
Undetectable MRD-negativity is associated with extended progression-free survival (PFS) and overall survival in patients receiving chemoimmunotherapy for CLL.
“Attainment of MRD-negativity in relapsed/refractory CLL is also a desired trial endpoint due to the subjectivity of complete response definition regarding pathologic lymph node size,” said Peter Hillmen, MD, of St James’s University Hospital, Leeds, United Kingdom, at the 2018 ASCO Annual Meeting.
Dr Hillmen reported new data on MRD response in cytogenetic and molecular risk groups, MRD sustainability and kinetics, and MRD conversion in the MURANO trial (abstract 7508).
MURANO trial (NCT02005471)
In the trial, venetoclax-rituximab showed superior PFS and peripheral blood and bone marrow MRD-negativity as compared to bendamustine plus rituximab (BR) in relapsed/refractory CLL patients.
Patients were randomized to venetoclax-rituximab for 6 months, followed by single-agent venetoclax for up to 1.5 years, or BR for 6 months. Peripheral blood samples were serially collected and bone marrow was collected at the end of combination treatment or at best response.
MRD findings
The new results show higher concordance in MRD-negativity between bone marrow and peripheral blood in venetoclax-rituximab (45 of 50 patients, 90%) vs BR (3 of 10 patients, 30%) in paired samples.
Focusing on peripheral blood MRD, Dr Hillmen said the best MRD-negativity rates were higher with venetoclax-rituximab (84%) than BR (23%). These results were independent of high-risk factors—such as del 17p, IGVH unmutated, and mutated TP53—only for venetoclax-rituximab treated patients.
“The superior peripheral blood MRD response with venetoclax-rituximab was consistent across subgroups at the end of completion of treatment,” Dr Hillmen said. “Most patients who achieved peripheral blood MRD-negativity on venetoclax-rituximab remained MRD-negative and were progression-free.”
Among 121 of 194 (62%) patients on venetoclax-rituximab who achieved MRD-negativity at the end of combination therapy, 100 (83%) patients maintained MRD-negativity and were progression-free at a median follow-up of 13.8 months. Two patients developed progressive disease and 2 patients died (unrelated to CLL).
Two patients developed Richter’s disease (with one MRD-positive directly before therapy) and 15 (12%) patients converted to confirmed MRD-positive at a median MRD-positive follow-up of 5.6 months.
“High peripheral blood MRD-negativity at the end of combination treatment and concordance with bone marrow MRD with venetoclax-rituximab,” Dr Hillmen said, “confirms the value of peripheral blood MRD for evaluation of treatment benefit in relapsed/refractory CLL patients. The high rate of peripheral blood MRD-negativity at end of combination treatment with venetoclax-rituximab was attained regardless of risk features.”
Some conversion to MRD-positivity occurred only in a small proportion of patients. Most cases were of intermediate level and remained progression-free, he said.
“MRD kinetics indicate that peripheral blood MRD-negativity with venetoclax-rituximab occurs early and is maintained over time with current follow-up,” Dr Hillmen added. The MRD data now provide a framework for designing response adaptive therapy.
The US Food and Drug Administration recently approved venetoclax-rituximab for CLL or small lymphocytic lymphoma for patients with or without del 17p.
Venetoclax is being developed by Genentech and Abbvie.
DLBCL survival improved with novel antibody-drug conjugate
STOCKHOLM, SWEDEN – Adding an experimental antibody-drug conjugate to bendamustine and rituximab more than doubled overall survival over bendamustine/rituximab alone in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), investigators reported.
Among 80 transplant-ineligible patients with relapsed or refractory DLBCL in a phase 2 trial, the combination of the antibody-drug conjugate (ADC) polatuzumab vedotin plus bendamustine/rituximab (BR) was associated with a 40% complete response rate, compared with 15% for BR alone.
More importantly, the ADC was associated with 6.7 months median progression-free survival (PFS) versus 2 months for BR, and 11.8 months median overall survival (OS), versus 4.7 months for BR alone, reported Laurie Sehn, MD, from BC Cancer in Vancouver, British Columbia, Canada.
“This randomized phase 2 trial really is, so far, the only head-to-head comparison of a novel targeted agent against a standard therapy in this patient population that’s ineligible for transplant, and it demonstrated that the combination polatuzumab vedotin with BR significantly improved the response rates and progression-free survival, as well as overall survival,” she said at the annual congress of the European Hematology Association.
However, in a separate cohort of patients with follicular lymphoma in the same trial, there was no difference in either PFS or OS during follow-up to date, Dr. Sehn reported.
Polatuzumab vedotin consists of an antibody targeted against CD79b, an antigenic protein expressed on the surface of normal B cells, as well as DLBCL and follicular lymphoma cells.
Dr. Sehn and her colleagues enrolled 80 patients with DLBCL for whom first-line chemoimmunotherapy had failed and who were ineligible for stem cell transplant due to age and/or comorbidities.
A second cohort included 80 patients with follicular lymphoma. In this group, median PFS with polatuzumab vedotin/BR was 17 months versus 17.3 months for BR alone, and median overall survival had not been reached in either arm at the time of the data cutoff.
In the DLBCL cohort, patients were randomized to receive polatuzumab vedotin 1.8 mg/kg plus bendamustine 90mg/m2 for 2 days and rituximab 375mg/m2) or BR alone for six 21-day cycles.
The complete response rate by PET scan – the primary endpoint – was significantly higher with polatuzmab/BR at 40% versus 15% for BR alone (P = .012). Respective overall response rates were 45% versus 18% (P = .008). Also, median PFS with the polatuzmab/BR therapy was 6.7 months versus 2.0 months for BR alone, translating into a hazard ratio of 0.31 (P less than .0001).
Respective median overall survival was 11.8 versus 4.7 months, translating into a hazard ratio for the polatuzmab/BR combination of 0.35 (P = .0008).
The PET complete response rates were higher with polatuzmab/BR regardless of prior lines of therapy or refractory status, Dr. Sehn noted.
“In terms of the safety, I think importantly in the combination there were no unexpected toxicities, so typical to what we would expect with what’s known with this drug alone,” Dr. Sehn said.
Grade 3 or greater toxicities that were higher with the polatuzmab/BR combination included cytopenias, febrile neutropenia, and infections. The single serious adverse event that had a higher incidence in the polatuzumab/BR arm was febrile neutropenia (DLBCL). In total, 12% of patients in the polatuzumab-containing arm and 11% of patients in the BR-only arm died on study. Many of the deaths were due to disease progression.
Anton Hagenbeek, MD, PhD, from the Academic Medical Center at the University of Amsterdam, the Netherlands, who moderated the briefing but was not involved in the study, said that about 20%-30% of patients with relapsed/refractory DLBCL are positive for the CD33 antigen, the target of brentuximab vedotin (Adcetris), and noted that this agent is also being tested in a phase 2 trial.
Martin Hutchings, MD, PhD, from Rigshospitalet in Copenhagen, the Netherlands, who co-moderated the oral abstract session, commented that “it’s not so often that we see significant overall survival differences in a phase 2 study with 80 patients.”
Based on the results of this trial, polatuzumab has been granted breakthrough therapy designation by the U.S. Food and Drug Administration and a PRIME (priority medicine) designation from the European Medicines Agency.
SOURCE: Sehn LH et al. EHA Congress, Abstract S802.
STOCKHOLM, SWEDEN – Adding an experimental antibody-drug conjugate to bendamustine and rituximab more than doubled overall survival over bendamustine/rituximab alone in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), investigators reported.
Among 80 transplant-ineligible patients with relapsed or refractory DLBCL in a phase 2 trial, the combination of the antibody-drug conjugate (ADC) polatuzumab vedotin plus bendamustine/rituximab (BR) was associated with a 40% complete response rate, compared with 15% for BR alone.
More importantly, the ADC was associated with 6.7 months median progression-free survival (PFS) versus 2 months for BR, and 11.8 months median overall survival (OS), versus 4.7 months for BR alone, reported Laurie Sehn, MD, from BC Cancer in Vancouver, British Columbia, Canada.
“This randomized phase 2 trial really is, so far, the only head-to-head comparison of a novel targeted agent against a standard therapy in this patient population that’s ineligible for transplant, and it demonstrated that the combination polatuzumab vedotin with BR significantly improved the response rates and progression-free survival, as well as overall survival,” she said at the annual congress of the European Hematology Association.
However, in a separate cohort of patients with follicular lymphoma in the same trial, there was no difference in either PFS or OS during follow-up to date, Dr. Sehn reported.
Polatuzumab vedotin consists of an antibody targeted against CD79b, an antigenic protein expressed on the surface of normal B cells, as well as DLBCL and follicular lymphoma cells.
Dr. Sehn and her colleagues enrolled 80 patients with DLBCL for whom first-line chemoimmunotherapy had failed and who were ineligible for stem cell transplant due to age and/or comorbidities.
A second cohort included 80 patients with follicular lymphoma. In this group, median PFS with polatuzumab vedotin/BR was 17 months versus 17.3 months for BR alone, and median overall survival had not been reached in either arm at the time of the data cutoff.
In the DLBCL cohort, patients were randomized to receive polatuzumab vedotin 1.8 mg/kg plus bendamustine 90mg/m2 for 2 days and rituximab 375mg/m2) or BR alone for six 21-day cycles.
The complete response rate by PET scan – the primary endpoint – was significantly higher with polatuzmab/BR at 40% versus 15% for BR alone (P = .012). Respective overall response rates were 45% versus 18% (P = .008). Also, median PFS with the polatuzmab/BR therapy was 6.7 months versus 2.0 months for BR alone, translating into a hazard ratio of 0.31 (P less than .0001).
Respective median overall survival was 11.8 versus 4.7 months, translating into a hazard ratio for the polatuzmab/BR combination of 0.35 (P = .0008).
The PET complete response rates were higher with polatuzmab/BR regardless of prior lines of therapy or refractory status, Dr. Sehn noted.
“In terms of the safety, I think importantly in the combination there were no unexpected toxicities, so typical to what we would expect with what’s known with this drug alone,” Dr. Sehn said.
Grade 3 or greater toxicities that were higher with the polatuzmab/BR combination included cytopenias, febrile neutropenia, and infections. The single serious adverse event that had a higher incidence in the polatuzumab/BR arm was febrile neutropenia (DLBCL). In total, 12% of patients in the polatuzumab-containing arm and 11% of patients in the BR-only arm died on study. Many of the deaths were due to disease progression.
Anton Hagenbeek, MD, PhD, from the Academic Medical Center at the University of Amsterdam, the Netherlands, who moderated the briefing but was not involved in the study, said that about 20%-30% of patients with relapsed/refractory DLBCL are positive for the CD33 antigen, the target of brentuximab vedotin (Adcetris), and noted that this agent is also being tested in a phase 2 trial.
Martin Hutchings, MD, PhD, from Rigshospitalet in Copenhagen, the Netherlands, who co-moderated the oral abstract session, commented that “it’s not so often that we see significant overall survival differences in a phase 2 study with 80 patients.”
Based on the results of this trial, polatuzumab has been granted breakthrough therapy designation by the U.S. Food and Drug Administration and a PRIME (priority medicine) designation from the European Medicines Agency.
SOURCE: Sehn LH et al. EHA Congress, Abstract S802.
STOCKHOLM, SWEDEN – Adding an experimental antibody-drug conjugate to bendamustine and rituximab more than doubled overall survival over bendamustine/rituximab alone in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), investigators reported.
Among 80 transplant-ineligible patients with relapsed or refractory DLBCL in a phase 2 trial, the combination of the antibody-drug conjugate (ADC) polatuzumab vedotin plus bendamustine/rituximab (BR) was associated with a 40% complete response rate, compared with 15% for BR alone.
More importantly, the ADC was associated with 6.7 months median progression-free survival (PFS) versus 2 months for BR, and 11.8 months median overall survival (OS), versus 4.7 months for BR alone, reported Laurie Sehn, MD, from BC Cancer in Vancouver, British Columbia, Canada.
“This randomized phase 2 trial really is, so far, the only head-to-head comparison of a novel targeted agent against a standard therapy in this patient population that’s ineligible for transplant, and it demonstrated that the combination polatuzumab vedotin with BR significantly improved the response rates and progression-free survival, as well as overall survival,” she said at the annual congress of the European Hematology Association.
However, in a separate cohort of patients with follicular lymphoma in the same trial, there was no difference in either PFS or OS during follow-up to date, Dr. Sehn reported.
Polatuzumab vedotin consists of an antibody targeted against CD79b, an antigenic protein expressed on the surface of normal B cells, as well as DLBCL and follicular lymphoma cells.
Dr. Sehn and her colleagues enrolled 80 patients with DLBCL for whom first-line chemoimmunotherapy had failed and who were ineligible for stem cell transplant due to age and/or comorbidities.
A second cohort included 80 patients with follicular lymphoma. In this group, median PFS with polatuzumab vedotin/BR was 17 months versus 17.3 months for BR alone, and median overall survival had not been reached in either arm at the time of the data cutoff.
In the DLBCL cohort, patients were randomized to receive polatuzumab vedotin 1.8 mg/kg plus bendamustine 90mg/m2 for 2 days and rituximab 375mg/m2) or BR alone for six 21-day cycles.
The complete response rate by PET scan – the primary endpoint – was significantly higher with polatuzmab/BR at 40% versus 15% for BR alone (P = .012). Respective overall response rates were 45% versus 18% (P = .008). Also, median PFS with the polatuzmab/BR therapy was 6.7 months versus 2.0 months for BR alone, translating into a hazard ratio of 0.31 (P less than .0001).
Respective median overall survival was 11.8 versus 4.7 months, translating into a hazard ratio for the polatuzmab/BR combination of 0.35 (P = .0008).
The PET complete response rates were higher with polatuzmab/BR regardless of prior lines of therapy or refractory status, Dr. Sehn noted.
“In terms of the safety, I think importantly in the combination there were no unexpected toxicities, so typical to what we would expect with what’s known with this drug alone,” Dr. Sehn said.
Grade 3 or greater toxicities that were higher with the polatuzmab/BR combination included cytopenias, febrile neutropenia, and infections. The single serious adverse event that had a higher incidence in the polatuzumab/BR arm was febrile neutropenia (DLBCL). In total, 12% of patients in the polatuzumab-containing arm and 11% of patients in the BR-only arm died on study. Many of the deaths were due to disease progression.
Anton Hagenbeek, MD, PhD, from the Academic Medical Center at the University of Amsterdam, the Netherlands, who moderated the briefing but was not involved in the study, said that about 20%-30% of patients with relapsed/refractory DLBCL are positive for the CD33 antigen, the target of brentuximab vedotin (Adcetris), and noted that this agent is also being tested in a phase 2 trial.
Martin Hutchings, MD, PhD, from Rigshospitalet in Copenhagen, the Netherlands, who co-moderated the oral abstract session, commented that “it’s not so often that we see significant overall survival differences in a phase 2 study with 80 patients.”
Based on the results of this trial, polatuzumab has been granted breakthrough therapy designation by the U.S. Food and Drug Administration and a PRIME (priority medicine) designation from the European Medicines Agency.
SOURCE: Sehn LH et al. EHA Congress, Abstract S802.
REPORTING FROM THE EHA CONGRESS
Key clinical point:
Major finding: The complete response rate with polatuzumab vedotin plus bendamustine/rituximab (BR) was 40%, compared with 15% for BR alone.
Study details: Randomized controlled phase 2 trial in 80 patients with relapsed/refractory DLBCL.
Disclosures: The study was funded by Hoffman-La Roche. Dr. Sehn reported ties to Roche/Genentech and others.
Source: Sehn LH et al. EHA Congress, Abstract S802.
‘Excellent’ survival with HCT despite early treatment failure in FL
Autologous and allogeneic hematopoietic stem cell transplantation (HCT) both offer excellent long-term survival in follicular lymphoma (FL) patients who experience early treatment failure, an analysis of a large transplant registry suggests.
Five-year survival rates exceeded 70% for patients who received autologous or matched sibling donor (MSD) transplants, according to the analysis of the Center for International Blood and Marrow Transplant Research (CIBMTR) database. The database included 440 patients who underwent a procedure between 2002 and 2014.
“Until better risk-stratification tools are available for FL, auto-HCT and MSD allo-HCT should be considered as effective treatment options with excellent long-term survival for high-risk patients as defined by early treatment failure,” Sonali M. Smith, MD, of the University of Chicago, and co-investigators wrote in the journal Cancer.
Early treatment failure in FL is associated with worse overall survival. In the National LymphoCare Study (NLCS), patients who received upfront R-CHOP therapy and progressed within 24 months had a 5-year overall survival of 50%, versus 90% for patients without early progression.
By contrast, survival figures in the present study are “provocatively higher” than those in the NLCS, in which only 8 out of 110 patients underwent HCT, Dr Smith and co-authors said.
Dr Smith’s study showed that with a median follow-up of 69 to 73 months, adjusted probability of 5-year overall survival was 70% for autologous and 73% for MSD HCT, versus 49% for matched unrelated donor HCT (P=0.0008).
Ryan C. Lynch, MD, and Ajay K. Gopal, MD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington, said that the finding “convincingly demonstrates” the benefit of transplant in the setting of early treatment failure.
“Select patients (particularly younger patients) with chemoresponsive disease who understand the risk-benefit ratio in comparison with currently approved and experimental therapies still remain good candidates for autologous HCT,” Drs Lynch and Gopal said in an editorial.
“For older patients or patients with comorbidities, we would continue to recommend clinical trials or treatment with an approved PI3K inhibitor,” they added.
The study by Dr Smith and colleagues is not the first to show a benefit of HCT in this clinical scenario. In a recent NLCS/CIBMTR analysis of FL patients, 5-year overall survival was 73% for those undergoing autologous HCT done within a year of early treatment failure, versus 60% for those who did not (P=0.05).
The two studies “collectively suggest that transplantation should be considered in this high-risk group of patients with early relapse,” Dr Smith and co-authors wrote.
Autologous and allogeneic hematopoietic stem cell transplantation (HCT) both offer excellent long-term survival in follicular lymphoma (FL) patients who experience early treatment failure, an analysis of a large transplant registry suggests.
Five-year survival rates exceeded 70% for patients who received autologous or matched sibling donor (MSD) transplants, according to the analysis of the Center for International Blood and Marrow Transplant Research (CIBMTR) database. The database included 440 patients who underwent a procedure between 2002 and 2014.
“Until better risk-stratification tools are available for FL, auto-HCT and MSD allo-HCT should be considered as effective treatment options with excellent long-term survival for high-risk patients as defined by early treatment failure,” Sonali M. Smith, MD, of the University of Chicago, and co-investigators wrote in the journal Cancer.
Early treatment failure in FL is associated with worse overall survival. In the National LymphoCare Study (NLCS), patients who received upfront R-CHOP therapy and progressed within 24 months had a 5-year overall survival of 50%, versus 90% for patients without early progression.
By contrast, survival figures in the present study are “provocatively higher” than those in the NLCS, in which only 8 out of 110 patients underwent HCT, Dr Smith and co-authors said.
Dr Smith’s study showed that with a median follow-up of 69 to 73 months, adjusted probability of 5-year overall survival was 70% for autologous and 73% for MSD HCT, versus 49% for matched unrelated donor HCT (P=0.0008).
Ryan C. Lynch, MD, and Ajay K. Gopal, MD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington, said that the finding “convincingly demonstrates” the benefit of transplant in the setting of early treatment failure.
“Select patients (particularly younger patients) with chemoresponsive disease who understand the risk-benefit ratio in comparison with currently approved and experimental therapies still remain good candidates for autologous HCT,” Drs Lynch and Gopal said in an editorial.
“For older patients or patients with comorbidities, we would continue to recommend clinical trials or treatment with an approved PI3K inhibitor,” they added.
The study by Dr Smith and colleagues is not the first to show a benefit of HCT in this clinical scenario. In a recent NLCS/CIBMTR analysis of FL patients, 5-year overall survival was 73% for those undergoing autologous HCT done within a year of early treatment failure, versus 60% for those who did not (P=0.05).
The two studies “collectively suggest that transplantation should be considered in this high-risk group of patients with early relapse,” Dr Smith and co-authors wrote.
Autologous and allogeneic hematopoietic stem cell transplantation (HCT) both offer excellent long-term survival in follicular lymphoma (FL) patients who experience early treatment failure, an analysis of a large transplant registry suggests.
Five-year survival rates exceeded 70% for patients who received autologous or matched sibling donor (MSD) transplants, according to the analysis of the Center for International Blood and Marrow Transplant Research (CIBMTR) database. The database included 440 patients who underwent a procedure between 2002 and 2014.
“Until better risk-stratification tools are available for FL, auto-HCT and MSD allo-HCT should be considered as effective treatment options with excellent long-term survival for high-risk patients as defined by early treatment failure,” Sonali M. Smith, MD, of the University of Chicago, and co-investigators wrote in the journal Cancer.
Early treatment failure in FL is associated with worse overall survival. In the National LymphoCare Study (NLCS), patients who received upfront R-CHOP therapy and progressed within 24 months had a 5-year overall survival of 50%, versus 90% for patients without early progression.
By contrast, survival figures in the present study are “provocatively higher” than those in the NLCS, in which only 8 out of 110 patients underwent HCT, Dr Smith and co-authors said.
Dr Smith’s study showed that with a median follow-up of 69 to 73 months, adjusted probability of 5-year overall survival was 70% for autologous and 73% for MSD HCT, versus 49% for matched unrelated donor HCT (P=0.0008).
Ryan C. Lynch, MD, and Ajay K. Gopal, MD, of the Fred Hutchinson Cancer Research Center in Seattle, Washington, said that the finding “convincingly demonstrates” the benefit of transplant in the setting of early treatment failure.
“Select patients (particularly younger patients) with chemoresponsive disease who understand the risk-benefit ratio in comparison with currently approved and experimental therapies still remain good candidates for autologous HCT,” Drs Lynch and Gopal said in an editorial.
“For older patients or patients with comorbidities, we would continue to recommend clinical trials or treatment with an approved PI3K inhibitor,” they added.
The study by Dr Smith and colleagues is not the first to show a benefit of HCT in this clinical scenario. In a recent NLCS/CIBMTR analysis of FL patients, 5-year overall survival was 73% for those undergoing autologous HCT done within a year of early treatment failure, versus 60% for those who did not (P=0.05).
The two studies “collectively suggest that transplantation should be considered in this high-risk group of patients with early relapse,” Dr Smith and co-authors wrote.