User login
Using engineered T cells reduced acute, chronic GVHD
A novel T-cell engineered product, Orca-T (Orca Bio), was associated with lower incidence of both acute and chronic graft-versus-host disease (GVHD) and more than double the rate of GVHD-free and relapse-free survival, compared with the current standard of care for patients undergoing hematopoietic stem cell transplants (HSCT), investigators said.
In both a multicenter phase 1 trial (NCT04013685) and single-center phase 1/2 trial (NCT01660607) with a total of 50 patients, those who received Orca-T with single-agent GVHD prophylaxis had a 1-year GVHD-free and relapse-free survival rate of 75%, compared with 31% for patients who received standard of care with two-agent prophylaxis, reported Everett H. Meyer, MD, PhD, from the Stanford (Calif.) University.
“Orca-T has good evidence for reduced acute graft-versus-host disease, reduced chromic graft-versus-host disease, and a low nonrelapse mortality,” he said at the Transplant & Cellular Therapies Meetings.
The product can be quickly manufactured and delivered to treatment centers across the continental United States, with “vein-to-vein” time of less than 72 hours, he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Orca-T consists of highly purified, donor-derived T-regulatory (Treg) cells that are sorted and delivered on day 0 with hematopoietic stem cells, without immunosuppressants, followed 2 days later with infusion of a matching dose of conventional T cells.
“The Treg cells are allowed to expand to create the right microenvironment for the [conventional T cells],” he explained.
In preclinical studies, donor-derived, high-purity Tregs delivered prior to adoptive transfer of conventional T cells prevented GVHD while maintaining graft-versus-tumor immunity, he said.
Two T-cell infusions
He reported updated results from current studies on a total of 50 adults, with a cohort of 144 patients treated concurrently with standard of care as controls.
The Orca-T–treated patients had a median age of 47 and 52% were male. Indications for transplant included acute myeloid and acute lymphoblastic leukemia, chronic myeloid leukemia, B-cell lymphoma, myelodysplastic syndrome/myelofibrosis, and other unspecified indications.
In both the Orca-T and control cohorts, patients underwent myeloablative conditioning from 10 to 2 days prior to stem cell infusion.
As noted patients in the experimental arm received infusion of hematopoietic stem/progenitor cells and Tregs, followed 2 days later by conventional T-cell infusion, and, on the day after that, tacrolimus at a target dose of 4.6 ng/mL. The conventional T cells were reserved from donor apheresis and were otherwise unmanipulated prior to infusion into the recipient, Dr. Meyer noted.
Patients in the standard-of-care arm received tacrolimus on the day before standard infusion of the apheresis product, followed by methotrexate prophylaxis on days 1, 3, 6 and 11.
Time to neutrophil engraftment, platelet engraftment, and from day 0 to hospital discharge were all significantly shorter in the Orca-T group, at 12 versus 14 days (P < .0001), 11 vs. 17 days (P < .0001), and 15 vs. 17 days (P = .01) respectively.
At 100 days of follow-up, the rate of grade 2 or greater acute GVHD was 30% among standard-of-care patients versus 10% among Orca-T–treated patients. At 1-year follow-up, respective rates of chronic GVHD were 46% vs. 3%.
Safety
“In general, the protocol is extremely well tolerated by our patients. We’ve seen no exceptional infectious disease complications, and we’ve seen no other major complications,” Dr. Meyer said.
Cytomegalovirus prophylaxis was used variably, depending on the center and on the attending physician. Epstein-Barr virus reactivation occurred in eight patients, with one requiring therapy, but there was no biopsy or radiographic evidence of posttransplant lymphoproliferative disorder.
In all, 18% of patients had serious adverse events during the reporting period, all of which resolved. There were no treatment-related deaths in the Orca-T arm, compared with 11% of controls.
Engraftment differences explored
In the question-and-answer session following the presentation, Christopher J. Gamper, MD, PhD, from the Johns Hopkins Hospital in Baltimore, told Dr. Meyer that “your outcomes from Orca-T look excellent,” and asked about the cost differential, compared with similar, unmanipulated transplants performed with standard GVHD prophylaxis.
“Is this recovered by lower costs for treatment of GVHD?” he asked.
“I have not done an economic cost analysis of course, and I think others may be looking into this,” Dr. Meyer replied. “Graft engineering can be expensive, although it’s an engineering proposition and one could imagine that the costs will go down substantially over time.”
Session moderator Alan Hanash, MD, PhD, from Memorial Sloan Kettering Cancer Center in New York, commented on the differences in engraftment between the experimental controls arms, and asked Dr. Meyer: “Do you think this is due to the difference in prophylaxis? Absence of methotrexate? Do you think that it could be a direct impact of regulatory T cells on hematopoietic engraftment?”
“Certainly not having methotrexate is beneficial for engraftment, and may account for the differences we see, Dr. Meyer said. “However, it is possible that Tregs could be playing a facilitative role. There certainly is good preclinical literature that Tregs, particularly in the bone marrow space, can facilitate bone marrow engraftment.”
The Orca-T trials are sponsored by Orca Bio and Stanford, with support from the National Institutes of Health. Dr. Meyer receives research support from Orca and is a scientific adviser to GigaGen, Triursus, Incyte, and Indee Labs. Dr. Hanash and Dr. Gamper had no relevant disclosures.
A novel T-cell engineered product, Orca-T (Orca Bio), was associated with lower incidence of both acute and chronic graft-versus-host disease (GVHD) and more than double the rate of GVHD-free and relapse-free survival, compared with the current standard of care for patients undergoing hematopoietic stem cell transplants (HSCT), investigators said.
In both a multicenter phase 1 trial (NCT04013685) and single-center phase 1/2 trial (NCT01660607) with a total of 50 patients, those who received Orca-T with single-agent GVHD prophylaxis had a 1-year GVHD-free and relapse-free survival rate of 75%, compared with 31% for patients who received standard of care with two-agent prophylaxis, reported Everett H. Meyer, MD, PhD, from the Stanford (Calif.) University.
“Orca-T has good evidence for reduced acute graft-versus-host disease, reduced chromic graft-versus-host disease, and a low nonrelapse mortality,” he said at the Transplant & Cellular Therapies Meetings.
The product can be quickly manufactured and delivered to treatment centers across the continental United States, with “vein-to-vein” time of less than 72 hours, he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Orca-T consists of highly purified, donor-derived T-regulatory (Treg) cells that are sorted and delivered on day 0 with hematopoietic stem cells, without immunosuppressants, followed 2 days later with infusion of a matching dose of conventional T cells.
“The Treg cells are allowed to expand to create the right microenvironment for the [conventional T cells],” he explained.
In preclinical studies, donor-derived, high-purity Tregs delivered prior to adoptive transfer of conventional T cells prevented GVHD while maintaining graft-versus-tumor immunity, he said.
Two T-cell infusions
He reported updated results from current studies on a total of 50 adults, with a cohort of 144 patients treated concurrently with standard of care as controls.
The Orca-T–treated patients had a median age of 47 and 52% were male. Indications for transplant included acute myeloid and acute lymphoblastic leukemia, chronic myeloid leukemia, B-cell lymphoma, myelodysplastic syndrome/myelofibrosis, and other unspecified indications.
In both the Orca-T and control cohorts, patients underwent myeloablative conditioning from 10 to 2 days prior to stem cell infusion.
As noted patients in the experimental arm received infusion of hematopoietic stem/progenitor cells and Tregs, followed 2 days later by conventional T-cell infusion, and, on the day after that, tacrolimus at a target dose of 4.6 ng/mL. The conventional T cells were reserved from donor apheresis and were otherwise unmanipulated prior to infusion into the recipient, Dr. Meyer noted.
Patients in the standard-of-care arm received tacrolimus on the day before standard infusion of the apheresis product, followed by methotrexate prophylaxis on days 1, 3, 6 and 11.
Time to neutrophil engraftment, platelet engraftment, and from day 0 to hospital discharge were all significantly shorter in the Orca-T group, at 12 versus 14 days (P < .0001), 11 vs. 17 days (P < .0001), and 15 vs. 17 days (P = .01) respectively.
At 100 days of follow-up, the rate of grade 2 or greater acute GVHD was 30% among standard-of-care patients versus 10% among Orca-T–treated patients. At 1-year follow-up, respective rates of chronic GVHD were 46% vs. 3%.
Safety
“In general, the protocol is extremely well tolerated by our patients. We’ve seen no exceptional infectious disease complications, and we’ve seen no other major complications,” Dr. Meyer said.
Cytomegalovirus prophylaxis was used variably, depending on the center and on the attending physician. Epstein-Barr virus reactivation occurred in eight patients, with one requiring therapy, but there was no biopsy or radiographic evidence of posttransplant lymphoproliferative disorder.
In all, 18% of patients had serious adverse events during the reporting period, all of which resolved. There were no treatment-related deaths in the Orca-T arm, compared with 11% of controls.
Engraftment differences explored
In the question-and-answer session following the presentation, Christopher J. Gamper, MD, PhD, from the Johns Hopkins Hospital in Baltimore, told Dr. Meyer that “your outcomes from Orca-T look excellent,” and asked about the cost differential, compared with similar, unmanipulated transplants performed with standard GVHD prophylaxis.
“Is this recovered by lower costs for treatment of GVHD?” he asked.
“I have not done an economic cost analysis of course, and I think others may be looking into this,” Dr. Meyer replied. “Graft engineering can be expensive, although it’s an engineering proposition and one could imagine that the costs will go down substantially over time.”
Session moderator Alan Hanash, MD, PhD, from Memorial Sloan Kettering Cancer Center in New York, commented on the differences in engraftment between the experimental controls arms, and asked Dr. Meyer: “Do you think this is due to the difference in prophylaxis? Absence of methotrexate? Do you think that it could be a direct impact of regulatory T cells on hematopoietic engraftment?”
“Certainly not having methotrexate is beneficial for engraftment, and may account for the differences we see, Dr. Meyer said. “However, it is possible that Tregs could be playing a facilitative role. There certainly is good preclinical literature that Tregs, particularly in the bone marrow space, can facilitate bone marrow engraftment.”
The Orca-T trials are sponsored by Orca Bio and Stanford, with support from the National Institutes of Health. Dr. Meyer receives research support from Orca and is a scientific adviser to GigaGen, Triursus, Incyte, and Indee Labs. Dr. Hanash and Dr. Gamper had no relevant disclosures.
A novel T-cell engineered product, Orca-T (Orca Bio), was associated with lower incidence of both acute and chronic graft-versus-host disease (GVHD) and more than double the rate of GVHD-free and relapse-free survival, compared with the current standard of care for patients undergoing hematopoietic stem cell transplants (HSCT), investigators said.
In both a multicenter phase 1 trial (NCT04013685) and single-center phase 1/2 trial (NCT01660607) with a total of 50 patients, those who received Orca-T with single-agent GVHD prophylaxis had a 1-year GVHD-free and relapse-free survival rate of 75%, compared with 31% for patients who received standard of care with two-agent prophylaxis, reported Everett H. Meyer, MD, PhD, from the Stanford (Calif.) University.
“Orca-T has good evidence for reduced acute graft-versus-host disease, reduced chromic graft-versus-host disease, and a low nonrelapse mortality,” he said at the Transplant & Cellular Therapies Meetings.
The product can be quickly manufactured and delivered to treatment centers across the continental United States, with “vein-to-vein” time of less than 72 hours, he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Orca-T consists of highly purified, donor-derived T-regulatory (Treg) cells that are sorted and delivered on day 0 with hematopoietic stem cells, without immunosuppressants, followed 2 days later with infusion of a matching dose of conventional T cells.
“The Treg cells are allowed to expand to create the right microenvironment for the [conventional T cells],” he explained.
In preclinical studies, donor-derived, high-purity Tregs delivered prior to adoptive transfer of conventional T cells prevented GVHD while maintaining graft-versus-tumor immunity, he said.
Two T-cell infusions
He reported updated results from current studies on a total of 50 adults, with a cohort of 144 patients treated concurrently with standard of care as controls.
The Orca-T–treated patients had a median age of 47 and 52% were male. Indications for transplant included acute myeloid and acute lymphoblastic leukemia, chronic myeloid leukemia, B-cell lymphoma, myelodysplastic syndrome/myelofibrosis, and other unspecified indications.
In both the Orca-T and control cohorts, patients underwent myeloablative conditioning from 10 to 2 days prior to stem cell infusion.
As noted patients in the experimental arm received infusion of hematopoietic stem/progenitor cells and Tregs, followed 2 days later by conventional T-cell infusion, and, on the day after that, tacrolimus at a target dose of 4.6 ng/mL. The conventional T cells were reserved from donor apheresis and were otherwise unmanipulated prior to infusion into the recipient, Dr. Meyer noted.
Patients in the standard-of-care arm received tacrolimus on the day before standard infusion of the apheresis product, followed by methotrexate prophylaxis on days 1, 3, 6 and 11.
Time to neutrophil engraftment, platelet engraftment, and from day 0 to hospital discharge were all significantly shorter in the Orca-T group, at 12 versus 14 days (P < .0001), 11 vs. 17 days (P < .0001), and 15 vs. 17 days (P = .01) respectively.
At 100 days of follow-up, the rate of grade 2 or greater acute GVHD was 30% among standard-of-care patients versus 10% among Orca-T–treated patients. At 1-year follow-up, respective rates of chronic GVHD were 46% vs. 3%.
Safety
“In general, the protocol is extremely well tolerated by our patients. We’ve seen no exceptional infectious disease complications, and we’ve seen no other major complications,” Dr. Meyer said.
Cytomegalovirus prophylaxis was used variably, depending on the center and on the attending physician. Epstein-Barr virus reactivation occurred in eight patients, with one requiring therapy, but there was no biopsy or radiographic evidence of posttransplant lymphoproliferative disorder.
In all, 18% of patients had serious adverse events during the reporting period, all of which resolved. There were no treatment-related deaths in the Orca-T arm, compared with 11% of controls.
Engraftment differences explored
In the question-and-answer session following the presentation, Christopher J. Gamper, MD, PhD, from the Johns Hopkins Hospital in Baltimore, told Dr. Meyer that “your outcomes from Orca-T look excellent,” and asked about the cost differential, compared with similar, unmanipulated transplants performed with standard GVHD prophylaxis.
“Is this recovered by lower costs for treatment of GVHD?” he asked.
“I have not done an economic cost analysis of course, and I think others may be looking into this,” Dr. Meyer replied. “Graft engineering can be expensive, although it’s an engineering proposition and one could imagine that the costs will go down substantially over time.”
Session moderator Alan Hanash, MD, PhD, from Memorial Sloan Kettering Cancer Center in New York, commented on the differences in engraftment between the experimental controls arms, and asked Dr. Meyer: “Do you think this is due to the difference in prophylaxis? Absence of methotrexate? Do you think that it could be a direct impact of regulatory T cells on hematopoietic engraftment?”
“Certainly not having methotrexate is beneficial for engraftment, and may account for the differences we see, Dr. Meyer said. “However, it is possible that Tregs could be playing a facilitative role. There certainly is good preclinical literature that Tregs, particularly in the bone marrow space, can facilitate bone marrow engraftment.”
The Orca-T trials are sponsored by Orca Bio and Stanford, with support from the National Institutes of Health. Dr. Meyer receives research support from Orca and is a scientific adviser to GigaGen, Triursus, Incyte, and Indee Labs. Dr. Hanash and Dr. Gamper had no relevant disclosures.
FROM TCT 2021
Transplant-related mortality higher with CD34 selection
In a clinical trial comparing three graft-versus-host disease (GVHD)–prevention regimens in patients undergoing hematopoietic stem cell transplants, a calcineurin inhibitor (CNI)–free strategy using CD34-selected peripheral blood stem cells (PBSCs) was associated with a nearly twofold increase in transplant-related mortality, compared with either a different CNI-free regimen or tacrolimus plus methotrexate, investigators reported.
In the phase 3 Progress II trial, patients who received CD34-selected PBSCs without post-transplant immune suppression had a hazard ratio for death of 1.74 compared with patients who received T-cell depletion with posttransplant cyclophosphamide, and a HR of 1.78, compared with patients who received tacrolimus and methotrexate after a bone marrow graft, Miguel-Angel Perales , MD, from Memorial Sloan Kettering Cancer Center, New York, reported at the Transplant & Cellular Therapies Meetings.
“CD34 selection was associated with worse overall survival, which offset any benefit from lower rates of moderate to severe chronic GVHD,” he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Neither of the two CNI-free interventions were superior to tacrolimus/methotrexate with bone marrow–derived stem cells for preventing chronic GVHD, and there were no differences in the primary endpoint of chronic GVHD/relapse-free survival, Dr. Perales said.
T-cell depletion vs. CNI
The Progress II trial was designed to see whether either of two CNI-free, T-cell depletion approaches could improve chronic GVHD rates post transplant over a CNI-based regimen.
The investigators enrolled patients aged 65 years or younger with acute leukemia or myelodysplasia with fewer than 5% blasts and a HLA-matched related or unrelated donor.
The patients were randomly assigned to either bone marrow grafts with tacrolimus/methotrexate (118 patients), bone marrow with in vivo posttransplant cyclophosphamide (114), or PBSCs with ex vivo CD34-selected cells (114).
The primary endpoint of chronic GVHD/relapse-free survival (CRFS) was a time-to-event outcome defined as moderate to severe chronic GVHD according to National Institutes of Health consensus criteria, disease relapse or progression, or death from any cause.
As noted before, there were no between-arm differences in the primary CRFS endpoint, and in multivariate analysis controlling for donor type, patient characteristics, disease category and disease risk index, the only factor significantly predictive for CRFS was being aged 50 years or older.
The 2-year posttransplant survival rates were 61.6% in the CD34-selected arm, 76.7% in the posttransplant cyclophosphamide arm, and 74.2% in the tacrolimus/methotrexate arm.
As noted before, the HR for CRFS with CD34 versus tacrolimus/methotrexate was 1.74, and for CD34 versus cyclophosphamide was 1.78 (P = .02 for both comparisons). In contrast, there was no difference in CRFS between posttransplant cyclophosphamide and tacrolimus/methotrexate.
Both relapse-free survival and transplant-related mortality were worse with the CD34-selected group, compared with the other two groups, but there were no significant differences among the arms in disease relapse.
Hematologic recovery was faster in the CD34 arm, but there were no significant differences in graft failure.
In addition, the incidence of grade II-IV acute GVHD was increased in the posttransplant cyclophosphamide group, compared with the other two, while chronic GVHD and moderate to severe chronic GVHD were reduced in the CD34 group.
There were no differences in quality of life measures among the groups, Dr. Perales said.
Practice changing?
In the question-and answer-session following the presentation, comoderator Sarah Nikiforow , MD, PhD, from the Dana-Farber Cancer Institute in Boston, who was not involved in the study, asked whether the trial results could be considered as practice changing for any centers that historically have done CD34 selection, or whether CD34 selection is still a viable approach to GVHD prophylaxis.
“That’s obviously a key question from the study, and a question that we’re asking ourselves,” Dr. Perales said. “I think the lesson that we took from this study as it pertains to CD34 selection is obviously the increased mortality, likely related to regimen toxicity, and I think the use of high-dose radiation is something that we have to reexamine.”
He said that his center is also considering whether to reduce antithymocyte globulin dosing, move it earlier in the process, and to use pharmokinetic-directed ATG as a possible means of decreasing nonrelapse mortality.
“I think it remains a useful platform for adoptive cell therapy, potentially targeting relapsed disease,” he added.
The study was supported by the National Heart, Lung, and Blood Institute. Dr. Perales disclosed advisory board activities and consulting for multiple companies, and receiving research funding for clinical trials from several more. Dr. Nikiforow disclosed a consulting/advisory role for Kite Pharma, and travel accommodations and expense from Celyad Oncology.
In a clinical trial comparing three graft-versus-host disease (GVHD)–prevention regimens in patients undergoing hematopoietic stem cell transplants, a calcineurin inhibitor (CNI)–free strategy using CD34-selected peripheral blood stem cells (PBSCs) was associated with a nearly twofold increase in transplant-related mortality, compared with either a different CNI-free regimen or tacrolimus plus methotrexate, investigators reported.
In the phase 3 Progress II trial, patients who received CD34-selected PBSCs without post-transplant immune suppression had a hazard ratio for death of 1.74 compared with patients who received T-cell depletion with posttransplant cyclophosphamide, and a HR of 1.78, compared with patients who received tacrolimus and methotrexate after a bone marrow graft, Miguel-Angel Perales , MD, from Memorial Sloan Kettering Cancer Center, New York, reported at the Transplant & Cellular Therapies Meetings.
“CD34 selection was associated with worse overall survival, which offset any benefit from lower rates of moderate to severe chronic GVHD,” he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Neither of the two CNI-free interventions were superior to tacrolimus/methotrexate with bone marrow–derived stem cells for preventing chronic GVHD, and there were no differences in the primary endpoint of chronic GVHD/relapse-free survival, Dr. Perales said.
T-cell depletion vs. CNI
The Progress II trial was designed to see whether either of two CNI-free, T-cell depletion approaches could improve chronic GVHD rates post transplant over a CNI-based regimen.
The investigators enrolled patients aged 65 years or younger with acute leukemia or myelodysplasia with fewer than 5% blasts and a HLA-matched related or unrelated donor.
The patients were randomly assigned to either bone marrow grafts with tacrolimus/methotrexate (118 patients), bone marrow with in vivo posttransplant cyclophosphamide (114), or PBSCs with ex vivo CD34-selected cells (114).
The primary endpoint of chronic GVHD/relapse-free survival (CRFS) was a time-to-event outcome defined as moderate to severe chronic GVHD according to National Institutes of Health consensus criteria, disease relapse or progression, or death from any cause.
As noted before, there were no between-arm differences in the primary CRFS endpoint, and in multivariate analysis controlling for donor type, patient characteristics, disease category and disease risk index, the only factor significantly predictive for CRFS was being aged 50 years or older.
The 2-year posttransplant survival rates were 61.6% in the CD34-selected arm, 76.7% in the posttransplant cyclophosphamide arm, and 74.2% in the tacrolimus/methotrexate arm.
As noted before, the HR for CRFS with CD34 versus tacrolimus/methotrexate was 1.74, and for CD34 versus cyclophosphamide was 1.78 (P = .02 for both comparisons). In contrast, there was no difference in CRFS between posttransplant cyclophosphamide and tacrolimus/methotrexate.
Both relapse-free survival and transplant-related mortality were worse with the CD34-selected group, compared with the other two groups, but there were no significant differences among the arms in disease relapse.
Hematologic recovery was faster in the CD34 arm, but there were no significant differences in graft failure.
In addition, the incidence of grade II-IV acute GVHD was increased in the posttransplant cyclophosphamide group, compared with the other two, while chronic GVHD and moderate to severe chronic GVHD were reduced in the CD34 group.
There were no differences in quality of life measures among the groups, Dr. Perales said.
Practice changing?
In the question-and answer-session following the presentation, comoderator Sarah Nikiforow , MD, PhD, from the Dana-Farber Cancer Institute in Boston, who was not involved in the study, asked whether the trial results could be considered as practice changing for any centers that historically have done CD34 selection, or whether CD34 selection is still a viable approach to GVHD prophylaxis.
“That’s obviously a key question from the study, and a question that we’re asking ourselves,” Dr. Perales said. “I think the lesson that we took from this study as it pertains to CD34 selection is obviously the increased mortality, likely related to regimen toxicity, and I think the use of high-dose radiation is something that we have to reexamine.”
He said that his center is also considering whether to reduce antithymocyte globulin dosing, move it earlier in the process, and to use pharmokinetic-directed ATG as a possible means of decreasing nonrelapse mortality.
“I think it remains a useful platform for adoptive cell therapy, potentially targeting relapsed disease,” he added.
The study was supported by the National Heart, Lung, and Blood Institute. Dr. Perales disclosed advisory board activities and consulting for multiple companies, and receiving research funding for clinical trials from several more. Dr. Nikiforow disclosed a consulting/advisory role for Kite Pharma, and travel accommodations and expense from Celyad Oncology.
In a clinical trial comparing three graft-versus-host disease (GVHD)–prevention regimens in patients undergoing hematopoietic stem cell transplants, a calcineurin inhibitor (CNI)–free strategy using CD34-selected peripheral blood stem cells (PBSCs) was associated with a nearly twofold increase in transplant-related mortality, compared with either a different CNI-free regimen or tacrolimus plus methotrexate, investigators reported.
In the phase 3 Progress II trial, patients who received CD34-selected PBSCs without post-transplant immune suppression had a hazard ratio for death of 1.74 compared with patients who received T-cell depletion with posttransplant cyclophosphamide, and a HR of 1.78, compared with patients who received tacrolimus and methotrexate after a bone marrow graft, Miguel-Angel Perales , MD, from Memorial Sloan Kettering Cancer Center, New York, reported at the Transplant & Cellular Therapies Meetings.
“CD34 selection was associated with worse overall survival, which offset any benefit from lower rates of moderate to severe chronic GVHD,” he said at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Neither of the two CNI-free interventions were superior to tacrolimus/methotrexate with bone marrow–derived stem cells for preventing chronic GVHD, and there were no differences in the primary endpoint of chronic GVHD/relapse-free survival, Dr. Perales said.
T-cell depletion vs. CNI
The Progress II trial was designed to see whether either of two CNI-free, T-cell depletion approaches could improve chronic GVHD rates post transplant over a CNI-based regimen.
The investigators enrolled patients aged 65 years or younger with acute leukemia or myelodysplasia with fewer than 5% blasts and a HLA-matched related or unrelated donor.
The patients were randomly assigned to either bone marrow grafts with tacrolimus/methotrexate (118 patients), bone marrow with in vivo posttransplant cyclophosphamide (114), or PBSCs with ex vivo CD34-selected cells (114).
The primary endpoint of chronic GVHD/relapse-free survival (CRFS) was a time-to-event outcome defined as moderate to severe chronic GVHD according to National Institutes of Health consensus criteria, disease relapse or progression, or death from any cause.
As noted before, there were no between-arm differences in the primary CRFS endpoint, and in multivariate analysis controlling for donor type, patient characteristics, disease category and disease risk index, the only factor significantly predictive for CRFS was being aged 50 years or older.
The 2-year posttransplant survival rates were 61.6% in the CD34-selected arm, 76.7% in the posttransplant cyclophosphamide arm, and 74.2% in the tacrolimus/methotrexate arm.
As noted before, the HR for CRFS with CD34 versus tacrolimus/methotrexate was 1.74, and for CD34 versus cyclophosphamide was 1.78 (P = .02 for both comparisons). In contrast, there was no difference in CRFS between posttransplant cyclophosphamide and tacrolimus/methotrexate.
Both relapse-free survival and transplant-related mortality were worse with the CD34-selected group, compared with the other two groups, but there were no significant differences among the arms in disease relapse.
Hematologic recovery was faster in the CD34 arm, but there were no significant differences in graft failure.
In addition, the incidence of grade II-IV acute GVHD was increased in the posttransplant cyclophosphamide group, compared with the other two, while chronic GVHD and moderate to severe chronic GVHD were reduced in the CD34 group.
There were no differences in quality of life measures among the groups, Dr. Perales said.
Practice changing?
In the question-and answer-session following the presentation, comoderator Sarah Nikiforow , MD, PhD, from the Dana-Farber Cancer Institute in Boston, who was not involved in the study, asked whether the trial results could be considered as practice changing for any centers that historically have done CD34 selection, or whether CD34 selection is still a viable approach to GVHD prophylaxis.
“That’s obviously a key question from the study, and a question that we’re asking ourselves,” Dr. Perales said. “I think the lesson that we took from this study as it pertains to CD34 selection is obviously the increased mortality, likely related to regimen toxicity, and I think the use of high-dose radiation is something that we have to reexamine.”
He said that his center is also considering whether to reduce antithymocyte globulin dosing, move it earlier in the process, and to use pharmokinetic-directed ATG as a possible means of decreasing nonrelapse mortality.
“I think it remains a useful platform for adoptive cell therapy, potentially targeting relapsed disease,” he added.
The study was supported by the National Heart, Lung, and Blood Institute. Dr. Perales disclosed advisory board activities and consulting for multiple companies, and receiving research funding for clinical trials from several more. Dr. Nikiforow disclosed a consulting/advisory role for Kite Pharma, and travel accommodations and expense from Celyad Oncology.
FROM TCT 2021
Chronic GVHD therapies offer hope for treating refractory disease
Despite improvements in prevention of graft-versus-host disease, chronic GVHD still occurs in 10%-50% of patients who undergo an allogeneic hematopoietic stem cell transplant, and these patients may require prolonged treatment with multiple lines of therapy, said a hematologist and transplant researcher.
“More effective, less toxic therapies for chronic GVHD are needed,” Stephanie Lee, MD, MPH, from the Fred Hutchinson Cancer Research Center in Seattle said at the Transplant & Cellular Therapies Meetings.
Dr. Lee reviewed clinical trials for chronic GVHD at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Although the incidence of chronic GVHD has gradually declined over the last 40 years and both relapse-free and overall survival following a chronic GVHD diagnosis have improved, “for patients who are diagnosed with chronic GVHD, they still will see many lines of therapy and many years of therapy,” she said.
Among 148 patients with chronic GVHD treated at her center, for example, 66% went on to two lines of therapy, 50% went on to three lines, 37% required four lines of therapy, and 20% needed five lines or more.
Salvage therapies for patients with chronic GVHD have evolved away from immunomodulators and immunosuppressants in the early 1990s, toward monoclonal antibodies such as rituximab in the early 2000s, to interleukin-2 and to tyrosine kinase inhibitors such as ruxolitinib (Jakafi) and ibrutinib (Imbruvica).
There are currently 36 agents that are FDA approved for at least one indication and can also be prescribed for the treatment of chronic GVHD, Dr. Lee noted.
Treatment goals
Dr. Lee laid out six goals for treating patients with chronic GVHD. They include:
- Controlling current signs and symptoms, measured by response rates and patient-reported outcomes
- Preventing further tissue and organ damage
- Minimizing toxicity
- Maintaining graft-versus-tumor effect
- Achieving graft tolerance and stopping immunosuppression
- Decreasing nonrelapse mortality and improving survival
Active trials
Dr. Lee identified 33 trials with chronic GVHD as an indication that are currently recruiting, and an additional 13 trials that are active but closed to recruiting. The trials can be generally grouped by mechanism of action, and involve agents targeting T-regulatory cells, B cells and/or B-cell receptor (BCR) signaling, monocytes/macrophages, costimulatory blockage, a proteasome inhibition, Janus kinase (JAK) 1/2 inhibitors, ROCK2 inhibitors, hedgehog pathway inhibition, cellular therapy, and organ-targeted therapy.
Most of the trials have overall response rate as the primary endpoint, and all but five are currently in phase 1 or 2. The currently active phase 3 trials include two with ibrutinib, one with the investigational agent itacitinib, one with ruxolitinib, and one with mesenchymal stem cells.
“I’ll note that, when results are reported, the denominator really matters for the overall response rate, especially if you’re talking about small trials, because if you require the patient to be treated with an agent for a certain period of time, and you take out all the people who didn’t make it to that time point, then your overall response rate looks better,” she said.
BTK inhibitors
The first-in-class Bruton tyrosine kinase (BTK) inhibitor ibrutinib was the first and thus far only agent approved by the Food and Drug Administration for chronic GVHD. The approval was based on a single-arm, multicenter trial with 42 patients.
The ORR in this trial was 69%, consisting of 31% complete responses and 38% partial responses, with a duration of response longer than 10 months in slightly more than half of all patients. In all, 24% of patients had improvement of symptoms in two consecutive visits, and 29% continued on ibrutinib at the time of the primary analysis in 2017.
Based on these promising results, acalabrutinib, which is more potent and selective for BTK than ibrutinib, with no effect on either platelets or natural killer cells, is currently under investigation in a phase 2 trial in 50 patients at a dose of 100 mg orally twice daily.
JAK1/2 inhibition
The JAK1 inhibitor itacitinib failed to meet its primary ORR endpoint in the phase 3 GRAVITAS-301 study, according to a press release, but the manufacturer (Incyte) said that it is continuing its commitment to JAK inhibitors with ruxolitinib, which has shown activity against acute, steroid-refractory GVHD, and is being explored for prevention of chronic GVHD in the randomized, phase 3 REACH3 study.
The trial met its primary endpoint for a higher ORR at week 24 with ruxolitinib versus best available therapy, at 49.7% versus 25.6%, respectively, which translated into an odds ratio for response with the JAK inhibitor of 2.99 (P < .0001).
Selective T-cell expansion
Efavaleukin alfa is an IL-2-mutated protein (mutein), with a mutation in the IL-2RB-binding portion of IL-2 causing increased selectivity for regulatory T-cell expansion. It is bound to an IgG-Fc domain that is itself mutated, with reduced Fc receptor binding and IgG effector function to give it a longer half life. This agent is being studied in a phase 1/2 trial in a subcutaneous formulation delivered every 1 or 2 weeks to 68 patients.
Monocyte/macrophage depletion
Axatilimab is a high-affinity antibody targeting colony stimulating factor–1 receptor (CSF-1R) expressed on monocytes and macrophages. By blocking CSF-1R, it depletes circulation of nonclassical monocytes and prevents the differentiation and survival of M2 macrophages in tissue.
It is currently being investigated 30 patients in a phase 1/2 study in an intravenous formulation delivered over 30 minutes every 2-4 weeks.
Hedgehog pathway inhibition
There is evidence suggesting that hedgehog pathway inhibition can lessen fibrosis. Glasdegib (Daurismo) a potent selective oral inhibitor of the hedgehog signaling pathway, is approved for use with low-dose cytarabine for patients with newly diagnosed acute myeloid leukemia aged older than 75 years or have comorbidities precluding intensive chemotherapy.
This agent is associated with drug intolerance because of muscle spasms, dysgeusia, and alopecia, however.
The drug is currently in phase 1/2 at a dose of 50 mg orally per day in 20 patients.
ROCK2 inhibition
Belumosudil (formerly KD025) “appears to rebalance the immune system,” Dr. Lee said. Investigators think that the drug dampens an autoaggressive inflammatory response by selective inhibition of ROCK2.
This drug has been studied in a dose-escalation study and a phase 2 trial, in which 132 participants were randomized to receive belumosudil 200 mg either once or twice daily.
At a median follow-up of 8 months, the ORR with belumosudil 200 mg once and twice daily was 73% and 74%, respectively. Similar results were seen in patients who had previously received either ruxolitinib or ibrutinib. High response rates were seen in patients with severe chronic GVHD, involvement of four or more organs and a refractory response to their last line of therapy.
Hard-to-manage patients
“We’re very hopeful for many of these agents, but we have to acknowledge that there are still many management dilemmas, patients that we just don’t really know what to do with,” Dr. Lee said. “These include patients who have bad sclerosis and fasciitis, nonhealing skin ulcers, bronchiolitis obliterans, serositis that can be very difficult to manage, severe keratoconjunctivitis that can be eyesight threatening, nonhealing mouth ulcers, esophageal structures, and always patients who have frequent infections.
“We are hopeful that some these agents will be useful for our patients who have severe manifestations, but often the number of patients with these manifestations in the trials is too low to say something specific about them,” she added.
‘Exciting time’
“It’s an exciting time because there are a lot of different drugs that are being studied for chronic GVHD,” commented Betty Hamilton, MD, a hematologist/oncologist at the Cleveland Clinic.
“I think that where the field is going in terms of treatment is recognizing that chronic GVHD is a pretty heterogeneous disease, and we have to learn even more about the underlying biologic pathways to be able to determine which class of drugs to use and when,” she said in an interview.
She agreed with Dr. Lee that the goals of treating patients with chronic GVHD include improving symptoms and quality, preventing progression, ideally tapering patients off immunosuppression, and achieving a balance between preventing negative consequences of GVHD while maintain the benefits of a graft-versus-leukemia effect.
“In our center, drug choice is based on physician preference and comfort with how often they’ve used the drug, patients’ comorbidities, toxicities of the drug, and logistical considerations,” Dr. Hamilton said.
Dr. Lee disclosed consulting activities for Pfizer and Kadmon, travel and lodging from Amgen, and research funding from those companies and others. Dr. Hamilton disclosed consulting for Syndax and Incyte.
Despite improvements in prevention of graft-versus-host disease, chronic GVHD still occurs in 10%-50% of patients who undergo an allogeneic hematopoietic stem cell transplant, and these patients may require prolonged treatment with multiple lines of therapy, said a hematologist and transplant researcher.
“More effective, less toxic therapies for chronic GVHD are needed,” Stephanie Lee, MD, MPH, from the Fred Hutchinson Cancer Research Center in Seattle said at the Transplant & Cellular Therapies Meetings.
Dr. Lee reviewed clinical trials for chronic GVHD at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Although the incidence of chronic GVHD has gradually declined over the last 40 years and both relapse-free and overall survival following a chronic GVHD diagnosis have improved, “for patients who are diagnosed with chronic GVHD, they still will see many lines of therapy and many years of therapy,” she said.
Among 148 patients with chronic GVHD treated at her center, for example, 66% went on to two lines of therapy, 50% went on to three lines, 37% required four lines of therapy, and 20% needed five lines or more.
Salvage therapies for patients with chronic GVHD have evolved away from immunomodulators and immunosuppressants in the early 1990s, toward monoclonal antibodies such as rituximab in the early 2000s, to interleukin-2 and to tyrosine kinase inhibitors such as ruxolitinib (Jakafi) and ibrutinib (Imbruvica).
There are currently 36 agents that are FDA approved for at least one indication and can also be prescribed for the treatment of chronic GVHD, Dr. Lee noted.
Treatment goals
Dr. Lee laid out six goals for treating patients with chronic GVHD. They include:
- Controlling current signs and symptoms, measured by response rates and patient-reported outcomes
- Preventing further tissue and organ damage
- Minimizing toxicity
- Maintaining graft-versus-tumor effect
- Achieving graft tolerance and stopping immunosuppression
- Decreasing nonrelapse mortality and improving survival
Active trials
Dr. Lee identified 33 trials with chronic GVHD as an indication that are currently recruiting, and an additional 13 trials that are active but closed to recruiting. The trials can be generally grouped by mechanism of action, and involve agents targeting T-regulatory cells, B cells and/or B-cell receptor (BCR) signaling, monocytes/macrophages, costimulatory blockage, a proteasome inhibition, Janus kinase (JAK) 1/2 inhibitors, ROCK2 inhibitors, hedgehog pathway inhibition, cellular therapy, and organ-targeted therapy.
Most of the trials have overall response rate as the primary endpoint, and all but five are currently in phase 1 or 2. The currently active phase 3 trials include two with ibrutinib, one with the investigational agent itacitinib, one with ruxolitinib, and one with mesenchymal stem cells.
“I’ll note that, when results are reported, the denominator really matters for the overall response rate, especially if you’re talking about small trials, because if you require the patient to be treated with an agent for a certain period of time, and you take out all the people who didn’t make it to that time point, then your overall response rate looks better,” she said.
BTK inhibitors
The first-in-class Bruton tyrosine kinase (BTK) inhibitor ibrutinib was the first and thus far only agent approved by the Food and Drug Administration for chronic GVHD. The approval was based on a single-arm, multicenter trial with 42 patients.
The ORR in this trial was 69%, consisting of 31% complete responses and 38% partial responses, with a duration of response longer than 10 months in slightly more than half of all patients. In all, 24% of patients had improvement of symptoms in two consecutive visits, and 29% continued on ibrutinib at the time of the primary analysis in 2017.
Based on these promising results, acalabrutinib, which is more potent and selective for BTK than ibrutinib, with no effect on either platelets or natural killer cells, is currently under investigation in a phase 2 trial in 50 patients at a dose of 100 mg orally twice daily.
JAK1/2 inhibition
The JAK1 inhibitor itacitinib failed to meet its primary ORR endpoint in the phase 3 GRAVITAS-301 study, according to a press release, but the manufacturer (Incyte) said that it is continuing its commitment to JAK inhibitors with ruxolitinib, which has shown activity against acute, steroid-refractory GVHD, and is being explored for prevention of chronic GVHD in the randomized, phase 3 REACH3 study.
The trial met its primary endpoint for a higher ORR at week 24 with ruxolitinib versus best available therapy, at 49.7% versus 25.6%, respectively, which translated into an odds ratio for response with the JAK inhibitor of 2.99 (P < .0001).
Selective T-cell expansion
Efavaleukin alfa is an IL-2-mutated protein (mutein), with a mutation in the IL-2RB-binding portion of IL-2 causing increased selectivity for regulatory T-cell expansion. It is bound to an IgG-Fc domain that is itself mutated, with reduced Fc receptor binding and IgG effector function to give it a longer half life. This agent is being studied in a phase 1/2 trial in a subcutaneous formulation delivered every 1 or 2 weeks to 68 patients.
Monocyte/macrophage depletion
Axatilimab is a high-affinity antibody targeting colony stimulating factor–1 receptor (CSF-1R) expressed on monocytes and macrophages. By blocking CSF-1R, it depletes circulation of nonclassical monocytes and prevents the differentiation and survival of M2 macrophages in tissue.
It is currently being investigated 30 patients in a phase 1/2 study in an intravenous formulation delivered over 30 minutes every 2-4 weeks.
Hedgehog pathway inhibition
There is evidence suggesting that hedgehog pathway inhibition can lessen fibrosis. Glasdegib (Daurismo) a potent selective oral inhibitor of the hedgehog signaling pathway, is approved for use with low-dose cytarabine for patients with newly diagnosed acute myeloid leukemia aged older than 75 years or have comorbidities precluding intensive chemotherapy.
This agent is associated with drug intolerance because of muscle spasms, dysgeusia, and alopecia, however.
The drug is currently in phase 1/2 at a dose of 50 mg orally per day in 20 patients.
ROCK2 inhibition
Belumosudil (formerly KD025) “appears to rebalance the immune system,” Dr. Lee said. Investigators think that the drug dampens an autoaggressive inflammatory response by selective inhibition of ROCK2.
This drug has been studied in a dose-escalation study and a phase 2 trial, in which 132 participants were randomized to receive belumosudil 200 mg either once or twice daily.
At a median follow-up of 8 months, the ORR with belumosudil 200 mg once and twice daily was 73% and 74%, respectively. Similar results were seen in patients who had previously received either ruxolitinib or ibrutinib. High response rates were seen in patients with severe chronic GVHD, involvement of four or more organs and a refractory response to their last line of therapy.
Hard-to-manage patients
“We’re very hopeful for many of these agents, but we have to acknowledge that there are still many management dilemmas, patients that we just don’t really know what to do with,” Dr. Lee said. “These include patients who have bad sclerosis and fasciitis, nonhealing skin ulcers, bronchiolitis obliterans, serositis that can be very difficult to manage, severe keratoconjunctivitis that can be eyesight threatening, nonhealing mouth ulcers, esophageal structures, and always patients who have frequent infections.
“We are hopeful that some these agents will be useful for our patients who have severe manifestations, but often the number of patients with these manifestations in the trials is too low to say something specific about them,” she added.
‘Exciting time’
“It’s an exciting time because there are a lot of different drugs that are being studied for chronic GVHD,” commented Betty Hamilton, MD, a hematologist/oncologist at the Cleveland Clinic.
“I think that where the field is going in terms of treatment is recognizing that chronic GVHD is a pretty heterogeneous disease, and we have to learn even more about the underlying biologic pathways to be able to determine which class of drugs to use and when,” she said in an interview.
She agreed with Dr. Lee that the goals of treating patients with chronic GVHD include improving symptoms and quality, preventing progression, ideally tapering patients off immunosuppression, and achieving a balance between preventing negative consequences of GVHD while maintain the benefits of a graft-versus-leukemia effect.
“In our center, drug choice is based on physician preference and comfort with how often they’ve used the drug, patients’ comorbidities, toxicities of the drug, and logistical considerations,” Dr. Hamilton said.
Dr. Lee disclosed consulting activities for Pfizer and Kadmon, travel and lodging from Amgen, and research funding from those companies and others. Dr. Hamilton disclosed consulting for Syndax and Incyte.
Despite improvements in prevention of graft-versus-host disease, chronic GVHD still occurs in 10%-50% of patients who undergo an allogeneic hematopoietic stem cell transplant, and these patients may require prolonged treatment with multiple lines of therapy, said a hematologist and transplant researcher.
“More effective, less toxic therapies for chronic GVHD are needed,” Stephanie Lee, MD, MPH, from the Fred Hutchinson Cancer Research Center in Seattle said at the Transplant & Cellular Therapies Meetings.
Dr. Lee reviewed clinical trials for chronic GVHD at the meeting held by the American Society for Blood and Marrow Transplantation and the Center for International Blood and Marrow Transplant Research.
Although the incidence of chronic GVHD has gradually declined over the last 40 years and both relapse-free and overall survival following a chronic GVHD diagnosis have improved, “for patients who are diagnosed with chronic GVHD, they still will see many lines of therapy and many years of therapy,” she said.
Among 148 patients with chronic GVHD treated at her center, for example, 66% went on to two lines of therapy, 50% went on to three lines, 37% required four lines of therapy, and 20% needed five lines or more.
Salvage therapies for patients with chronic GVHD have evolved away from immunomodulators and immunosuppressants in the early 1990s, toward monoclonal antibodies such as rituximab in the early 2000s, to interleukin-2 and to tyrosine kinase inhibitors such as ruxolitinib (Jakafi) and ibrutinib (Imbruvica).
There are currently 36 agents that are FDA approved for at least one indication and can also be prescribed for the treatment of chronic GVHD, Dr. Lee noted.
Treatment goals
Dr. Lee laid out six goals for treating patients with chronic GVHD. They include:
- Controlling current signs and symptoms, measured by response rates and patient-reported outcomes
- Preventing further tissue and organ damage
- Minimizing toxicity
- Maintaining graft-versus-tumor effect
- Achieving graft tolerance and stopping immunosuppression
- Decreasing nonrelapse mortality and improving survival
Active trials
Dr. Lee identified 33 trials with chronic GVHD as an indication that are currently recruiting, and an additional 13 trials that are active but closed to recruiting. The trials can be generally grouped by mechanism of action, and involve agents targeting T-regulatory cells, B cells and/or B-cell receptor (BCR) signaling, monocytes/macrophages, costimulatory blockage, a proteasome inhibition, Janus kinase (JAK) 1/2 inhibitors, ROCK2 inhibitors, hedgehog pathway inhibition, cellular therapy, and organ-targeted therapy.
Most of the trials have overall response rate as the primary endpoint, and all but five are currently in phase 1 or 2. The currently active phase 3 trials include two with ibrutinib, one with the investigational agent itacitinib, one with ruxolitinib, and one with mesenchymal stem cells.
“I’ll note that, when results are reported, the denominator really matters for the overall response rate, especially if you’re talking about small trials, because if you require the patient to be treated with an agent for a certain period of time, and you take out all the people who didn’t make it to that time point, then your overall response rate looks better,” she said.
BTK inhibitors
The first-in-class Bruton tyrosine kinase (BTK) inhibitor ibrutinib was the first and thus far only agent approved by the Food and Drug Administration for chronic GVHD. The approval was based on a single-arm, multicenter trial with 42 patients.
The ORR in this trial was 69%, consisting of 31% complete responses and 38% partial responses, with a duration of response longer than 10 months in slightly more than half of all patients. In all, 24% of patients had improvement of symptoms in two consecutive visits, and 29% continued on ibrutinib at the time of the primary analysis in 2017.
Based on these promising results, acalabrutinib, which is more potent and selective for BTK than ibrutinib, with no effect on either platelets or natural killer cells, is currently under investigation in a phase 2 trial in 50 patients at a dose of 100 mg orally twice daily.
JAK1/2 inhibition
The JAK1 inhibitor itacitinib failed to meet its primary ORR endpoint in the phase 3 GRAVITAS-301 study, according to a press release, but the manufacturer (Incyte) said that it is continuing its commitment to JAK inhibitors with ruxolitinib, which has shown activity against acute, steroid-refractory GVHD, and is being explored for prevention of chronic GVHD in the randomized, phase 3 REACH3 study.
The trial met its primary endpoint for a higher ORR at week 24 with ruxolitinib versus best available therapy, at 49.7% versus 25.6%, respectively, which translated into an odds ratio for response with the JAK inhibitor of 2.99 (P < .0001).
Selective T-cell expansion
Efavaleukin alfa is an IL-2-mutated protein (mutein), with a mutation in the IL-2RB-binding portion of IL-2 causing increased selectivity for regulatory T-cell expansion. It is bound to an IgG-Fc domain that is itself mutated, with reduced Fc receptor binding and IgG effector function to give it a longer half life. This agent is being studied in a phase 1/2 trial in a subcutaneous formulation delivered every 1 or 2 weeks to 68 patients.
Monocyte/macrophage depletion
Axatilimab is a high-affinity antibody targeting colony stimulating factor–1 receptor (CSF-1R) expressed on monocytes and macrophages. By blocking CSF-1R, it depletes circulation of nonclassical monocytes and prevents the differentiation and survival of M2 macrophages in tissue.
It is currently being investigated 30 patients in a phase 1/2 study in an intravenous formulation delivered over 30 minutes every 2-4 weeks.
Hedgehog pathway inhibition
There is evidence suggesting that hedgehog pathway inhibition can lessen fibrosis. Glasdegib (Daurismo) a potent selective oral inhibitor of the hedgehog signaling pathway, is approved for use with low-dose cytarabine for patients with newly diagnosed acute myeloid leukemia aged older than 75 years or have comorbidities precluding intensive chemotherapy.
This agent is associated with drug intolerance because of muscle spasms, dysgeusia, and alopecia, however.
The drug is currently in phase 1/2 at a dose of 50 mg orally per day in 20 patients.
ROCK2 inhibition
Belumosudil (formerly KD025) “appears to rebalance the immune system,” Dr. Lee said. Investigators think that the drug dampens an autoaggressive inflammatory response by selective inhibition of ROCK2.
This drug has been studied in a dose-escalation study and a phase 2 trial, in which 132 participants were randomized to receive belumosudil 200 mg either once or twice daily.
At a median follow-up of 8 months, the ORR with belumosudil 200 mg once and twice daily was 73% and 74%, respectively. Similar results were seen in patients who had previously received either ruxolitinib or ibrutinib. High response rates were seen in patients with severe chronic GVHD, involvement of four or more organs and a refractory response to their last line of therapy.
Hard-to-manage patients
“We’re very hopeful for many of these agents, but we have to acknowledge that there are still many management dilemmas, patients that we just don’t really know what to do with,” Dr. Lee said. “These include patients who have bad sclerosis and fasciitis, nonhealing skin ulcers, bronchiolitis obliterans, serositis that can be very difficult to manage, severe keratoconjunctivitis that can be eyesight threatening, nonhealing mouth ulcers, esophageal structures, and always patients who have frequent infections.
“We are hopeful that some these agents will be useful for our patients who have severe manifestations, but often the number of patients with these manifestations in the trials is too low to say something specific about them,” she added.
‘Exciting time’
“It’s an exciting time because there are a lot of different drugs that are being studied for chronic GVHD,” commented Betty Hamilton, MD, a hematologist/oncologist at the Cleveland Clinic.
“I think that where the field is going in terms of treatment is recognizing that chronic GVHD is a pretty heterogeneous disease, and we have to learn even more about the underlying biologic pathways to be able to determine which class of drugs to use and when,” she said in an interview.
She agreed with Dr. Lee that the goals of treating patients with chronic GVHD include improving symptoms and quality, preventing progression, ideally tapering patients off immunosuppression, and achieving a balance between preventing negative consequences of GVHD while maintain the benefits of a graft-versus-leukemia effect.
“In our center, drug choice is based on physician preference and comfort with how often they’ve used the drug, patients’ comorbidities, toxicities of the drug, and logistical considerations,” Dr. Hamilton said.
Dr. Lee disclosed consulting activities for Pfizer and Kadmon, travel and lodging from Amgen, and research funding from those companies and others. Dr. Hamilton disclosed consulting for Syndax and Incyte.
FROM TCT 2021
Sequential Targeted Treatment for a Geriatric Patient with Acute Myeloid Leukemia with Concurrent FLT3-TKD and IDH1 Mutations
Nearly 20,000 patients are diagnosed with acute myeloid leukemia (AML) in the US annually.1 Despite the use of aggressive chemotherapeutic agents, the prognosis remains poor, with a mean 5-year survival of 28.3%.2 Fortunately, with the refinement of next-generation sequencing (NGS) hematology panels and development of systemic targeted therapies, the treatment landscape for eligible patients has improved, both in frontline and relapsed or refractory (R/R) patients.
Specifically, investigations into alterations within the FMS-like tyrosine kinase (FLT3) and isocitrate dehydrogenase (IDH) genes have led to the discovery of a number of targeted treatments. Midostaurin is US Food and Drug Administration (FDA)-approved for use in combination with induction chemotherapy for patients with internal tandem duplication of the FLT3 (FLT3-ITD) gene or mutations within the tyrosine kinase domain (FLT3-TKD).3 Ivosidenib is indicated for frontline treatment for those who are poor candidates for induction chemotherapy, and R/R patients who have an R132H mutation in IDH1.4,5 Enasidenib is FDA-approved for R/R patients with R140Q, R172S, and R172K mutations in IDH2.6
The optimal treatment for patients with AML with ≥ 2 clinically actionable mutations has not been established. In this article we describe a geriatric patient who initially was diagnosed with AML with concurrent FLT3-TKD and IDH1 mutations and received targeted, sequential management. We detail changes in disease phenotype and mutational status by repeating an NGS hematology panel and cytogenetic studies after each stage of therapy. Lastly, we discuss the clonal evolution apparent within leukemic cells with use of ≥ 1 or more targeted agents.
Case Presentation
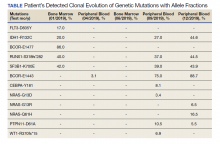
A 68-year-old man presented to the Emergency Department at The Durham Veterans Affairs Medical Center in North Carolina with fatigue and light-headedness. Because of his symptoms and pancytopenia, a bone marrow aspiration and trephine biopsy were performed, which showed 57% myeloblasts, 12% promyelocytes/myelocytes, and 2% metamyelocytes in 20 to 30% cellular bone marrow. Flow cytometry confirmed a blast population consistent with AML. A LeukoVantage (Quest Diagnostics) hematologic NGS panel revealed the presence of FLT3-TKD, IDH1, RUNX1, BCOR-E1477, and SF3B1 mutations (Table). Initial fluorescence in situ hybridization (FISH) results showed a normal pattern of hybridization with no translocations. His disease was deemed to be intermediate-high risk because of the presence of FLT3-TKD and RUNX1 mutations, despite the normal cytogenetic profile and absence of additional clinical features.
Induction chemotherapy was started with idarubicin, 12 mg/m2, on days 1 to 3 and cytarabine, 200 mg/m2, on days 1 to 7. Because of the presence of a FLT3-TKD mutation, midostaurin was planned for days 8 to 21. After induction chemotherapy, a bone marrow biopsy on day 14 revealed an acellular marrow with no observed myeloblasts. A bone marrow biopsy conducted before initiating consolidation therapy, revealed 30% cellularity with morphologic remission. However, flow cytometry found 5% myeloblasts expressing CD34, CD117, CD13, CD38, and HLA-DR, consistent with measurable residual disease. He received 2 cycles of consolidation therapy with high-dose cytarabine combined with midostaurin. After the patient's second cycle of consolidation, he continued to experience transfusion-dependent cytopenias. Another bone marrow evaluation demonstrated 10% cellularity with nearly all cells appearing to be myeloblasts. A repeat LeukoVantage NGS panel demonstrated undetectable FLT3-TKD mutation and persistent IDH1-R123C mutation. FISH studies revealed a complex karyotype with monosomy of chromosomes 5 and 7 and trisomy of chromosome 8.
We discussed with the patient and his family the options available, which included initiating targeted therapy for his IDH1 mutation, administering hypomethylation therapy with or without venetoclax, or pursuing palliative measures. We collectively decided to pursue therapy with single-agent oral ivosidenib, 500 mg daily. After 1 month of treatment, our patient developed worsening fatigue. His white blood cell count had increased to > 43 k/cm2, raising concern for differentiation syndrome.
A review of the peripheral smear showed a wide-spectrum of maturing granulocytes, with a large percentage of blasts. Peripheral flow cytometry confirmed a blast population of 15%. After a short period of symptom improvement with steroids, the patient developed worsening confusion. Brain imaging identified 2 subdural hemorrhages. Because of a significant peripheral blast population and the development of these hemorrhages, palliative measures were pursued, and the patient was discharged to an inpatient hospice facility. A final NGS panel performed from peripheral blood detected mutations in IDH1, RUNX1, PTPN11, NRAS, BCOR-E1443, and SF3B1 genes.
Discussion
To our knowledge, this is the first reported case of a patient who sequentially received targeted treatments directed against both FLT3 and IDH1 mutations. Initial management with midostaurin and cytarabine resulted in sustained remission of his FLT3-TKD mutation. However, despite receiving prompt standard of care with combination induction chemotherapy and targeted therapy, the patient experienced unfavorable clonal evolution based upon his molecular and cytogenetic testing. Addition of ivosidenib as a second targeting agent for his IDH1 mutation did not achieve a second remission.
Clonal evolution is a well-described phenomenon in hematology. Indolent conditions, such as clonal hematopoiesis of intermediate potential, or malignancies, such as myelodysplastic syndromes and myeloproliferative neoplasms, could transform into acute leukemia through the accumulation of driver mutations and/or cytogenetic abnormalities. Clonal evolution often is viewed as the culprit in patients with AML whose disease relapses after remission with initial chemotherapy.7-10 With the increasing availability of commercial NGS panels designed to assess mutations among patients experiencing hematologic malignancies, patterns of relapse, and, models of clonal evolution could be observed closely in patients with AML.
We were able to monitor molecular changes within our patient’s predominant clonal populations by repeating peripheral comprehensive NGS panels after lines of targeted therapies. The repeated sequencing revealed that clones with FLT3-TKD mutations responded to midostaurin with first-line chemotherapy whereas it was unclear whether clones with IDH1 mutation responded to ivosidenib. Development of complex cytogenetic findings along with the clonal expansion of BCOR mutation-harboring cells likely contributed to our patient’s acutely worsening condition. Several studies have found that the presence of a BCOR mutation in adults with AML leads to lower overall survival and relapse-free survival.11,12 As of now, there are no treatments specifically targeting BCOR mutations.
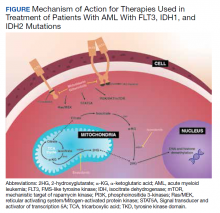
Although there are novel targeting agents with proven efficacy for both FLT3 and IDH1 mutations (Figure), it is difficult to determine which pathogenic mutation drives disease onset. No evidence suggests that these drugs could be administered in tandem. At the present time, interest is directed towards targeting all AML subclones simultaneously, which could reduce the likelihood of evolution among founder clones.7,10,13 In their comparison between molecular profiles and outcomes of patients with AML, Papaemmanuil and colleagues observed that > 80% of patients with AML harbor ≥ 2 driver mutations concurrently.14 Moreover, FLT3-ITD and IDH1 mutations tend to co-occur in approximately 9 to 27% of AML cases.15-18 Available targeted agents for AML are relatively new and hematologists’ familiarity with these drugs is continuing to grow. As the number of novel agents increases, investigations directed toward assessing the safety profile and efficacy of combining targeted agents will be beneficial for patients with AML with ≥ 1 driver mutation.
Conclusions
For our patient with AML, sequential targeted management of FLT3-TKD and IDH1 mutations was not beneficial. Higher-risk disease features, such as the development of a complex karyotype, likely contributed to our patient’s poor response to second-line ivosidenib. The sequential NGS malignant hematology panels allowed us to closely monitor changes to the molecular structure of our patient’s AML after each line of targeted therapy. Future investigations of combining targeted agents for patients with AML with concurrent actionable mutations would provide insight into outcomes of treating multiple clonal populations simultaneously.
1. De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6(7):e441. doi:10.1038/bcj.2016.50.
2. National Cancer Institute. Cancer Stat Facts: Leukemia — acute myeloid leukemia (AML). Accessed November 4, 2020. https://seer.cancer.gov/statfacts/html/amyl.html
3. Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454-464. doi:10.1056/NEJMoa1614359.
4. DiNardo CD, Stein EM, de Botton S, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378(25):2386-2398. doi:10.1056/NEJMoa1716984.
5. Roboz, GJ, DiNardo, CD, Stein, EM, et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood. 2019;135(7), 463-471. doi: 10.1182/blood.2019002140
6. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722-731. doi:10.1182/blood-2017-04-779405.
7. Jan M, Majeti R. Clonal evolution of acute leukemia genomes. Oncogene. 2013;32(2):135-140. doi:10.1038/onc.2012.48.
8. Grove CS, Vassiliou GS. Acute myeloid leukaemia: a paradigm for the clonal evolution of cancer? Dis Model Mech. 2014;7(8):941-951. doi:10.1242/dmm.015974.
9. Anderson K, Lutz C, van Delft FW, et al. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature. 2011;469(7330):356-561. doi: 10.1038/nature09650.
10. Ding L, Ley TJ, Larson DE, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481(7382):506-510. doi:10.1038/nature10738.
11. Terada K, Yamaguchi H, Ueki T, et al. Usefulness of BCOR gene mutation as a prognostic factor in acute myeloid leukemia with intermediate cytogenetic prognosis. Genes Chromosomes Cancer. 2018;57(8):401-408. doi:10.1002/gcc.22542.
12. Grossmann V, Tiacci E, Holmes AB, et al. Whole-exome sequencing identifies somatic mutations of BCOR in acute myeloid leukemia with normal karyotype. Blood. 2011;118(23):6153-6163. doi:10.1182/blood-2011-07-365320.
13. Parkin B, Ouillette P, Li Y, et al. Clonal evolution and devolution after chemotherapy in adult acute myelogenous leukemia. Blood. 2013;121(2):369-377. doi:10.1182/blood-2012-04-427039.
14. Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209-2221. doi:10.1056/NEJMoa1516192.
15. DiNardo CD, Ravandi F, Agresta S, et al. Characteristics, clinical outcome, and prognostic significance of IDH mutations in AML. Am J Hematol. 2015;90(8):732-736. doi:10.1002/ajh.24072.
16. Rakheja D, Konoplev S, Medeiros LJ, Chen W. IDH mutations in acute myeloid leukemia. Hum Pathol. 2012;43 (10):1541-1551. doi:10.1016/j.humpath.2012.05.003.
17. Lai C, Doucette K, Norsworthy K. Recent drug approvals for acute myeloid leukemia. J H Oncol. 2019;12(1):100. doi:10.1186/s13045-019-0774-x.
18. Boddu P, Takahashi K, Pemmaraju N, et al. Influence of IDH on FLT3-ITD status in newly diagnosed AML. Leukemia. 2017;31(11):2526-2529. doi:10.1038/leu.2017.244.
Nearly 20,000 patients are diagnosed with acute myeloid leukemia (AML) in the US annually.1 Despite the use of aggressive chemotherapeutic agents, the prognosis remains poor, with a mean 5-year survival of 28.3%.2 Fortunately, with the refinement of next-generation sequencing (NGS) hematology panels and development of systemic targeted therapies, the treatment landscape for eligible patients has improved, both in frontline and relapsed or refractory (R/R) patients.
Specifically, investigations into alterations within the FMS-like tyrosine kinase (FLT3) and isocitrate dehydrogenase (IDH) genes have led to the discovery of a number of targeted treatments. Midostaurin is US Food and Drug Administration (FDA)-approved for use in combination with induction chemotherapy for patients with internal tandem duplication of the FLT3 (FLT3-ITD) gene or mutations within the tyrosine kinase domain (FLT3-TKD).3 Ivosidenib is indicated for frontline treatment for those who are poor candidates for induction chemotherapy, and R/R patients who have an R132H mutation in IDH1.4,5 Enasidenib is FDA-approved for R/R patients with R140Q, R172S, and R172K mutations in IDH2.6
The optimal treatment for patients with AML with ≥ 2 clinically actionable mutations has not been established. In this article we describe a geriatric patient who initially was diagnosed with AML with concurrent FLT3-TKD and IDH1 mutations and received targeted, sequential management. We detail changes in disease phenotype and mutational status by repeating an NGS hematology panel and cytogenetic studies after each stage of therapy. Lastly, we discuss the clonal evolution apparent within leukemic cells with use of ≥ 1 or more targeted agents.
Case Presentation
A 68-year-old man presented to the Emergency Department at The Durham Veterans Affairs Medical Center in North Carolina with fatigue and light-headedness. Because of his symptoms and pancytopenia, a bone marrow aspiration and trephine biopsy were performed, which showed 57% myeloblasts, 12% promyelocytes/myelocytes, and 2% metamyelocytes in 20 to 30% cellular bone marrow. Flow cytometry confirmed a blast population consistent with AML. A LeukoVantage (Quest Diagnostics) hematologic NGS panel revealed the presence of FLT3-TKD, IDH1, RUNX1, BCOR-E1477, and SF3B1 mutations (Table). Initial fluorescence in situ hybridization (FISH) results showed a normal pattern of hybridization with no translocations. His disease was deemed to be intermediate-high risk because of the presence of FLT3-TKD and RUNX1 mutations, despite the normal cytogenetic profile and absence of additional clinical features.
Induction chemotherapy was started with idarubicin, 12 mg/m2, on days 1 to 3 and cytarabine, 200 mg/m2, on days 1 to 7. Because of the presence of a FLT3-TKD mutation, midostaurin was planned for days 8 to 21. After induction chemotherapy, a bone marrow biopsy on day 14 revealed an acellular marrow with no observed myeloblasts. A bone marrow biopsy conducted before initiating consolidation therapy, revealed 30% cellularity with morphologic remission. However, flow cytometry found 5% myeloblasts expressing CD34, CD117, CD13, CD38, and HLA-DR, consistent with measurable residual disease. He received 2 cycles of consolidation therapy with high-dose cytarabine combined with midostaurin. After the patient's second cycle of consolidation, he continued to experience transfusion-dependent cytopenias. Another bone marrow evaluation demonstrated 10% cellularity with nearly all cells appearing to be myeloblasts. A repeat LeukoVantage NGS panel demonstrated undetectable FLT3-TKD mutation and persistent IDH1-R123C mutation. FISH studies revealed a complex karyotype with monosomy of chromosomes 5 and 7 and trisomy of chromosome 8.
We discussed with the patient and his family the options available, which included initiating targeted therapy for his IDH1 mutation, administering hypomethylation therapy with or without venetoclax, or pursuing palliative measures. We collectively decided to pursue therapy with single-agent oral ivosidenib, 500 mg daily. After 1 month of treatment, our patient developed worsening fatigue. His white blood cell count had increased to > 43 k/cm2, raising concern for differentiation syndrome.
A review of the peripheral smear showed a wide-spectrum of maturing granulocytes, with a large percentage of blasts. Peripheral flow cytometry confirmed a blast population of 15%. After a short period of symptom improvement with steroids, the patient developed worsening confusion. Brain imaging identified 2 subdural hemorrhages. Because of a significant peripheral blast population and the development of these hemorrhages, palliative measures were pursued, and the patient was discharged to an inpatient hospice facility. A final NGS panel performed from peripheral blood detected mutations in IDH1, RUNX1, PTPN11, NRAS, BCOR-E1443, and SF3B1 genes.
Discussion
To our knowledge, this is the first reported case of a patient who sequentially received targeted treatments directed against both FLT3 and IDH1 mutations. Initial management with midostaurin and cytarabine resulted in sustained remission of his FLT3-TKD mutation. However, despite receiving prompt standard of care with combination induction chemotherapy and targeted therapy, the patient experienced unfavorable clonal evolution based upon his molecular and cytogenetic testing. Addition of ivosidenib as a second targeting agent for his IDH1 mutation did not achieve a second remission.
Clonal evolution is a well-described phenomenon in hematology. Indolent conditions, such as clonal hematopoiesis of intermediate potential, or malignancies, such as myelodysplastic syndromes and myeloproliferative neoplasms, could transform into acute leukemia through the accumulation of driver mutations and/or cytogenetic abnormalities. Clonal evolution often is viewed as the culprit in patients with AML whose disease relapses after remission with initial chemotherapy.7-10 With the increasing availability of commercial NGS panels designed to assess mutations among patients experiencing hematologic malignancies, patterns of relapse, and, models of clonal evolution could be observed closely in patients with AML.
We were able to monitor molecular changes within our patient’s predominant clonal populations by repeating peripheral comprehensive NGS panels after lines of targeted therapies. The repeated sequencing revealed that clones with FLT3-TKD mutations responded to midostaurin with first-line chemotherapy whereas it was unclear whether clones with IDH1 mutation responded to ivosidenib. Development of complex cytogenetic findings along with the clonal expansion of BCOR mutation-harboring cells likely contributed to our patient’s acutely worsening condition. Several studies have found that the presence of a BCOR mutation in adults with AML leads to lower overall survival and relapse-free survival.11,12 As of now, there are no treatments specifically targeting BCOR mutations.
Although there are novel targeting agents with proven efficacy for both FLT3 and IDH1 mutations (Figure), it is difficult to determine which pathogenic mutation drives disease onset. No evidence suggests that these drugs could be administered in tandem. At the present time, interest is directed towards targeting all AML subclones simultaneously, which could reduce the likelihood of evolution among founder clones.7,10,13 In their comparison between molecular profiles and outcomes of patients with AML, Papaemmanuil and colleagues observed that > 80% of patients with AML harbor ≥ 2 driver mutations concurrently.14 Moreover, FLT3-ITD and IDH1 mutations tend to co-occur in approximately 9 to 27% of AML cases.15-18 Available targeted agents for AML are relatively new and hematologists’ familiarity with these drugs is continuing to grow. As the number of novel agents increases, investigations directed toward assessing the safety profile and efficacy of combining targeted agents will be beneficial for patients with AML with ≥ 1 driver mutation.
Conclusions
For our patient with AML, sequential targeted management of FLT3-TKD and IDH1 mutations was not beneficial. Higher-risk disease features, such as the development of a complex karyotype, likely contributed to our patient’s poor response to second-line ivosidenib. The sequential NGS malignant hematology panels allowed us to closely monitor changes to the molecular structure of our patient’s AML after each line of targeted therapy. Future investigations of combining targeted agents for patients with AML with concurrent actionable mutations would provide insight into outcomes of treating multiple clonal populations simultaneously.
Nearly 20,000 patients are diagnosed with acute myeloid leukemia (AML) in the US annually.1 Despite the use of aggressive chemotherapeutic agents, the prognosis remains poor, with a mean 5-year survival of 28.3%.2 Fortunately, with the refinement of next-generation sequencing (NGS) hematology panels and development of systemic targeted therapies, the treatment landscape for eligible patients has improved, both in frontline and relapsed or refractory (R/R) patients.
Specifically, investigations into alterations within the FMS-like tyrosine kinase (FLT3) and isocitrate dehydrogenase (IDH) genes have led to the discovery of a number of targeted treatments. Midostaurin is US Food and Drug Administration (FDA)-approved for use in combination with induction chemotherapy for patients with internal tandem duplication of the FLT3 (FLT3-ITD) gene or mutations within the tyrosine kinase domain (FLT3-TKD).3 Ivosidenib is indicated for frontline treatment for those who are poor candidates for induction chemotherapy, and R/R patients who have an R132H mutation in IDH1.4,5 Enasidenib is FDA-approved for R/R patients with R140Q, R172S, and R172K mutations in IDH2.6
The optimal treatment for patients with AML with ≥ 2 clinically actionable mutations has not been established. In this article we describe a geriatric patient who initially was diagnosed with AML with concurrent FLT3-TKD and IDH1 mutations and received targeted, sequential management. We detail changes in disease phenotype and mutational status by repeating an NGS hematology panel and cytogenetic studies after each stage of therapy. Lastly, we discuss the clonal evolution apparent within leukemic cells with use of ≥ 1 or more targeted agents.
Case Presentation
A 68-year-old man presented to the Emergency Department at The Durham Veterans Affairs Medical Center in North Carolina with fatigue and light-headedness. Because of his symptoms and pancytopenia, a bone marrow aspiration and trephine biopsy were performed, which showed 57% myeloblasts, 12% promyelocytes/myelocytes, and 2% metamyelocytes in 20 to 30% cellular bone marrow. Flow cytometry confirmed a blast population consistent with AML. A LeukoVantage (Quest Diagnostics) hematologic NGS panel revealed the presence of FLT3-TKD, IDH1, RUNX1, BCOR-E1477, and SF3B1 mutations (Table). Initial fluorescence in situ hybridization (FISH) results showed a normal pattern of hybridization with no translocations. His disease was deemed to be intermediate-high risk because of the presence of FLT3-TKD and RUNX1 mutations, despite the normal cytogenetic profile and absence of additional clinical features.
Induction chemotherapy was started with idarubicin, 12 mg/m2, on days 1 to 3 and cytarabine, 200 mg/m2, on days 1 to 7. Because of the presence of a FLT3-TKD mutation, midostaurin was planned for days 8 to 21. After induction chemotherapy, a bone marrow biopsy on day 14 revealed an acellular marrow with no observed myeloblasts. A bone marrow biopsy conducted before initiating consolidation therapy, revealed 30% cellularity with morphologic remission. However, flow cytometry found 5% myeloblasts expressing CD34, CD117, CD13, CD38, and HLA-DR, consistent with measurable residual disease. He received 2 cycles of consolidation therapy with high-dose cytarabine combined with midostaurin. After the patient's second cycle of consolidation, he continued to experience transfusion-dependent cytopenias. Another bone marrow evaluation demonstrated 10% cellularity with nearly all cells appearing to be myeloblasts. A repeat LeukoVantage NGS panel demonstrated undetectable FLT3-TKD mutation and persistent IDH1-R123C mutation. FISH studies revealed a complex karyotype with monosomy of chromosomes 5 and 7 and trisomy of chromosome 8.
We discussed with the patient and his family the options available, which included initiating targeted therapy for his IDH1 mutation, administering hypomethylation therapy with or without venetoclax, or pursuing palliative measures. We collectively decided to pursue therapy with single-agent oral ivosidenib, 500 mg daily. After 1 month of treatment, our patient developed worsening fatigue. His white blood cell count had increased to > 43 k/cm2, raising concern for differentiation syndrome.
A review of the peripheral smear showed a wide-spectrum of maturing granulocytes, with a large percentage of blasts. Peripheral flow cytometry confirmed a blast population of 15%. After a short period of symptom improvement with steroids, the patient developed worsening confusion. Brain imaging identified 2 subdural hemorrhages. Because of a significant peripheral blast population and the development of these hemorrhages, palliative measures were pursued, and the patient was discharged to an inpatient hospice facility. A final NGS panel performed from peripheral blood detected mutations in IDH1, RUNX1, PTPN11, NRAS, BCOR-E1443, and SF3B1 genes.
Discussion
To our knowledge, this is the first reported case of a patient who sequentially received targeted treatments directed against both FLT3 and IDH1 mutations. Initial management with midostaurin and cytarabine resulted in sustained remission of his FLT3-TKD mutation. However, despite receiving prompt standard of care with combination induction chemotherapy and targeted therapy, the patient experienced unfavorable clonal evolution based upon his molecular and cytogenetic testing. Addition of ivosidenib as a second targeting agent for his IDH1 mutation did not achieve a second remission.
Clonal evolution is a well-described phenomenon in hematology. Indolent conditions, such as clonal hematopoiesis of intermediate potential, or malignancies, such as myelodysplastic syndromes and myeloproliferative neoplasms, could transform into acute leukemia through the accumulation of driver mutations and/or cytogenetic abnormalities. Clonal evolution often is viewed as the culprit in patients with AML whose disease relapses after remission with initial chemotherapy.7-10 With the increasing availability of commercial NGS panels designed to assess mutations among patients experiencing hematologic malignancies, patterns of relapse, and, models of clonal evolution could be observed closely in patients with AML.
We were able to monitor molecular changes within our patient’s predominant clonal populations by repeating peripheral comprehensive NGS panels after lines of targeted therapies. The repeated sequencing revealed that clones with FLT3-TKD mutations responded to midostaurin with first-line chemotherapy whereas it was unclear whether clones with IDH1 mutation responded to ivosidenib. Development of complex cytogenetic findings along with the clonal expansion of BCOR mutation-harboring cells likely contributed to our patient’s acutely worsening condition. Several studies have found that the presence of a BCOR mutation in adults with AML leads to lower overall survival and relapse-free survival.11,12 As of now, there are no treatments specifically targeting BCOR mutations.
Although there are novel targeting agents with proven efficacy for both FLT3 and IDH1 mutations (Figure), it is difficult to determine which pathogenic mutation drives disease onset. No evidence suggests that these drugs could be administered in tandem. At the present time, interest is directed towards targeting all AML subclones simultaneously, which could reduce the likelihood of evolution among founder clones.7,10,13 In their comparison between molecular profiles and outcomes of patients with AML, Papaemmanuil and colleagues observed that > 80% of patients with AML harbor ≥ 2 driver mutations concurrently.14 Moreover, FLT3-ITD and IDH1 mutations tend to co-occur in approximately 9 to 27% of AML cases.15-18 Available targeted agents for AML are relatively new and hematologists’ familiarity with these drugs is continuing to grow. As the number of novel agents increases, investigations directed toward assessing the safety profile and efficacy of combining targeted agents will be beneficial for patients with AML with ≥ 1 driver mutation.
Conclusions
For our patient with AML, sequential targeted management of FLT3-TKD and IDH1 mutations was not beneficial. Higher-risk disease features, such as the development of a complex karyotype, likely contributed to our patient’s poor response to second-line ivosidenib. The sequential NGS malignant hematology panels allowed us to closely monitor changes to the molecular structure of our patient’s AML after each line of targeted therapy. Future investigations of combining targeted agents for patients with AML with concurrent actionable mutations would provide insight into outcomes of treating multiple clonal populations simultaneously.
1. De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6(7):e441. doi:10.1038/bcj.2016.50.
2. National Cancer Institute. Cancer Stat Facts: Leukemia — acute myeloid leukemia (AML). Accessed November 4, 2020. https://seer.cancer.gov/statfacts/html/amyl.html
3. Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454-464. doi:10.1056/NEJMoa1614359.
4. DiNardo CD, Stein EM, de Botton S, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378(25):2386-2398. doi:10.1056/NEJMoa1716984.
5. Roboz, GJ, DiNardo, CD, Stein, EM, et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood. 2019;135(7), 463-471. doi: 10.1182/blood.2019002140
6. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722-731. doi:10.1182/blood-2017-04-779405.
7. Jan M, Majeti R. Clonal evolution of acute leukemia genomes. Oncogene. 2013;32(2):135-140. doi:10.1038/onc.2012.48.
8. Grove CS, Vassiliou GS. Acute myeloid leukaemia: a paradigm for the clonal evolution of cancer? Dis Model Mech. 2014;7(8):941-951. doi:10.1242/dmm.015974.
9. Anderson K, Lutz C, van Delft FW, et al. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature. 2011;469(7330):356-561. doi: 10.1038/nature09650.
10. Ding L, Ley TJ, Larson DE, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481(7382):506-510. doi:10.1038/nature10738.
11. Terada K, Yamaguchi H, Ueki T, et al. Usefulness of BCOR gene mutation as a prognostic factor in acute myeloid leukemia with intermediate cytogenetic prognosis. Genes Chromosomes Cancer. 2018;57(8):401-408. doi:10.1002/gcc.22542.
12. Grossmann V, Tiacci E, Holmes AB, et al. Whole-exome sequencing identifies somatic mutations of BCOR in acute myeloid leukemia with normal karyotype. Blood. 2011;118(23):6153-6163. doi:10.1182/blood-2011-07-365320.
13. Parkin B, Ouillette P, Li Y, et al. Clonal evolution and devolution after chemotherapy in adult acute myelogenous leukemia. Blood. 2013;121(2):369-377. doi:10.1182/blood-2012-04-427039.
14. Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209-2221. doi:10.1056/NEJMoa1516192.
15. DiNardo CD, Ravandi F, Agresta S, et al. Characteristics, clinical outcome, and prognostic significance of IDH mutations in AML. Am J Hematol. 2015;90(8):732-736. doi:10.1002/ajh.24072.
16. Rakheja D, Konoplev S, Medeiros LJ, Chen W. IDH mutations in acute myeloid leukemia. Hum Pathol. 2012;43 (10):1541-1551. doi:10.1016/j.humpath.2012.05.003.
17. Lai C, Doucette K, Norsworthy K. Recent drug approvals for acute myeloid leukemia. J H Oncol. 2019;12(1):100. doi:10.1186/s13045-019-0774-x.
18. Boddu P, Takahashi K, Pemmaraju N, et al. Influence of IDH on FLT3-ITD status in newly diagnosed AML. Leukemia. 2017;31(11):2526-2529. doi:10.1038/leu.2017.244.
1. De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6(7):e441. doi:10.1038/bcj.2016.50.
2. National Cancer Institute. Cancer Stat Facts: Leukemia — acute myeloid leukemia (AML). Accessed November 4, 2020. https://seer.cancer.gov/statfacts/html/amyl.html
3. Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377(5):454-464. doi:10.1056/NEJMoa1614359.
4. DiNardo CD, Stein EM, de Botton S, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378(25):2386-2398. doi:10.1056/NEJMoa1716984.
5. Roboz, GJ, DiNardo, CD, Stein, EM, et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood. 2019;135(7), 463-471. doi: 10.1182/blood.2019002140
6. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130(6):722-731. doi:10.1182/blood-2017-04-779405.
7. Jan M, Majeti R. Clonal evolution of acute leukemia genomes. Oncogene. 2013;32(2):135-140. doi:10.1038/onc.2012.48.
8. Grove CS, Vassiliou GS. Acute myeloid leukaemia: a paradigm for the clonal evolution of cancer? Dis Model Mech. 2014;7(8):941-951. doi:10.1242/dmm.015974.
9. Anderson K, Lutz C, van Delft FW, et al. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature. 2011;469(7330):356-561. doi: 10.1038/nature09650.
10. Ding L, Ley TJ, Larson DE, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481(7382):506-510. doi:10.1038/nature10738.
11. Terada K, Yamaguchi H, Ueki T, et al. Usefulness of BCOR gene mutation as a prognostic factor in acute myeloid leukemia with intermediate cytogenetic prognosis. Genes Chromosomes Cancer. 2018;57(8):401-408. doi:10.1002/gcc.22542.
12. Grossmann V, Tiacci E, Holmes AB, et al. Whole-exome sequencing identifies somatic mutations of BCOR in acute myeloid leukemia with normal karyotype. Blood. 2011;118(23):6153-6163. doi:10.1182/blood-2011-07-365320.
13. Parkin B, Ouillette P, Li Y, et al. Clonal evolution and devolution after chemotherapy in adult acute myelogenous leukemia. Blood. 2013;121(2):369-377. doi:10.1182/blood-2012-04-427039.
14. Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374(23):2209-2221. doi:10.1056/NEJMoa1516192.
15. DiNardo CD, Ravandi F, Agresta S, et al. Characteristics, clinical outcome, and prognostic significance of IDH mutations in AML. Am J Hematol. 2015;90(8):732-736. doi:10.1002/ajh.24072.
16. Rakheja D, Konoplev S, Medeiros LJ, Chen W. IDH mutations in acute myeloid leukemia. Hum Pathol. 2012;43 (10):1541-1551. doi:10.1016/j.humpath.2012.05.003.
17. Lai C, Doucette K, Norsworthy K. Recent drug approvals for acute myeloid leukemia. J H Oncol. 2019;12(1):100. doi:10.1186/s13045-019-0774-x.
18. Boddu P, Takahashi K, Pemmaraju N, et al. Influence of IDH on FLT3-ITD status in newly diagnosed AML. Leukemia. 2017;31(11):2526-2529. doi:10.1038/leu.2017.244.
Black race linked to poorer survival in AML
Black race is the most important risk factor for patients with acute myeloid leukemia (AML) and is associated with poor survival, according to new findings.
Among patients with AML younger than 60 years, the rate of overall 3-year survival was significantly less among Black patients than White patients (34% vs. 43%). The risk for death was 27% higher for Black patients compared with White patients.
“Our study demonstrates the delicate interplay between a variety of factors that influence survival disparities, particularly for younger Black AML patients,” said first author Bhavana Bhatnagar, DO, of the Ohio State University’s Comprehensive Cancer Center, Columbus. “We were able to confirm the impact of socioeconomic factors while also demonstrating that being Black is, in and of itself, an independent poor prognostic variable for survival.”
She noted that the persistently poor outcomes of young Black patients that were seen despite similar treatments in clinical trials strongly suggest that additional factors have a bearing on their survival.
The findings of the study were presented during the plenary session of the annual meeting of the American Society of Hematology, which was held online this year. The study was simultaneously published in Cancer Discovery.
Racial disparities in cancer outcomes remain a challenge. The term “health disparities” describes the differences of health outcomes among different groups, said Chancellor Donald, MD, of Tulane University, New Orleans, who introduced the article at the meeting. “Racial health disparities usually result from an unequal distribution of power and resources, not genetics.
“The examination of health disparities is certainly a worthwhile endeavor,” he continued. “For generations, differences in key health outcomes have negatively impacted the quality of life and shortened the life span of countless individuals. As scientists, clinicians, and invested members of our shared society, we are obligated to obtain a profound understanding of the mechanisms and impact of this morbid reality.”
Black race a risk factor
For their study, Dr. Bhatnagar and colleagues conducted a nationwide population analysis using data from the Surveillance Epidemiology End Results (SEER) Program of the National Cancer Institute to identify 11,190 adults aged 18-60 years who were diagnosed with AML between 1986 and 2015.
To characterize molecular features, they conducted targeted sequencing of 81 genes in 1,339 patients with AML who were treated on frontline Cancer and Leukemia Group B/Alliance for Clinical Trials in Oncology (Alliance) protocols based on standard-intensity cytarabine/anthracycline induction followed by consolidation between 1986 and 2016. None of these patients received an allogeneic stem cell transplant when they achieved complete remission.
Although overall survival has improved during the past 3 decades, survival disparities between Black and White patients has widened over time (P < .001). The authors found a nonstatistically significant difference in survival between 1986 and 1995 (White patients, n = 1,365; Black patients, n = 160; P = .19). However, the difference was significant between 1996 and 2005 (White patients, n = 2,994; Black patients, n = 480; P = .004). “And it became even more noticeable in the most recent decade,” said Dr. Bhatnagar. “Furthermore, younger Black AML patients were found to have worse survival compared with younger White AML patients.”
Results from the second analysis of patients treated on Alliance protocols did not show any significant differences in early death rates (10% vs. 46%; P = .02) and complete remission rates (71% vs. 71%; P = 1.00). “While relapse rates were slightly higher in Black compared to White patients, this difference did not reach statistical significance,” said Dr. Bhatnagar. “There was also no significant difference in the number of cycles of consolidation chemotherapy administered to these patients.”
However, both disease-free and overall survival were significantly worse for Black patients, suggesting that factors other than treatment selection were likely at play in influencing the survival disparity. The median disease-free survival for Black patients was 0.8 years, vs. 1.4 years for White patients (P = .02). Overall survival was 1.2 years vs. 1.8 years (P = .02).
Relapse rates were slightly higher in Black patients than in White patients, at 71% vs. 59%, but this difference did not reach statistical significance (P = .14).
Differences in biomarkers
With regard to underlying molecular differences between Black and White patients, the investigators found that the most common mutations were in NPM1, FLT3-ITD, and DNM3TA. Mutations were detected in more than 20% of Black patients. Other commonly mutated genes were IDH2, NRAS, TET2, IDH1, and TP53, which were mutated in more than 10% of patients. “All of these genes are established commonly mutated genes in AML,” said Bhatnagar.
On univariable and multivariable outcome analyses, which were used to identify clinical or molecular features that had a bearing on outcome, FLT3-ITD and IDH2 mutations were the only mutations associated with a higher risk for death among Black patients.
“This is actually a very important finding, as both FLT3 and IDH2 are now targetable with small-molecule inhibitors,” said Dr. Bhatnagar. “In addition, it is also worth noting that other gene mutations that have known prognostic significance in AML, such as NPM1, as well as RUNX1 and TP53, did not remain in the final statistical model.
“Importantly, our study provides powerful evidence that suggests differences in underlying disease biology between young Black and White AML patients, as evidenced by differences in the frequencies of recurrent gene mutations, “ she said.
Understudied disparities
Although the study showed that Black patients had worse outcomes, “surprisingly, the authors found these outcomes hold even when the patients are participating in clinical trials,” noted Elisa Weiss, PhD, senior vice president of education, services, and health research for the Leukemia and Lymphoma Society.
“The study makes clear that the medical and science community need to do more to better understand the social, economic, environmental, and biological causes of these disparities,” she said in an interview. “In fact, the findings suggest that there are myriad complex and understudied causes of the identified disparities, and they are likely to lie at the intersection of all levels of the social ecology that impact an individual’s ability to access timely and unbiased care, maintain their mental and physical health, and receive needed social support and resources.”
She noted that the Leukemia and Lymphoma Society has an Equity in Access research program that aims to “advance study of underlying causes of inequitable access to care and identify policies, strategies, and interventions that have the potential to reduce inequities and increase access to health care, services, and programs for blood cancer patients and survivors.”
The research was supported in part by the National Cancer Institute of the National Institutes of Health, other institutions, and through several scholar awards. Dr. Bhatnagar has received advisory board honoraria from Novartis, Kite Pharma, Celgene, Astellas, and Cell Therapeutics. Dr. Weiss has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Black race is the most important risk factor for patients with acute myeloid leukemia (AML) and is associated with poor survival, according to new findings.
Among patients with AML younger than 60 years, the rate of overall 3-year survival was significantly less among Black patients than White patients (34% vs. 43%). The risk for death was 27% higher for Black patients compared with White patients.
“Our study demonstrates the delicate interplay between a variety of factors that influence survival disparities, particularly for younger Black AML patients,” said first author Bhavana Bhatnagar, DO, of the Ohio State University’s Comprehensive Cancer Center, Columbus. “We were able to confirm the impact of socioeconomic factors while also demonstrating that being Black is, in and of itself, an independent poor prognostic variable for survival.”
She noted that the persistently poor outcomes of young Black patients that were seen despite similar treatments in clinical trials strongly suggest that additional factors have a bearing on their survival.
The findings of the study were presented during the plenary session of the annual meeting of the American Society of Hematology, which was held online this year. The study was simultaneously published in Cancer Discovery.
Racial disparities in cancer outcomes remain a challenge. The term “health disparities” describes the differences of health outcomes among different groups, said Chancellor Donald, MD, of Tulane University, New Orleans, who introduced the article at the meeting. “Racial health disparities usually result from an unequal distribution of power and resources, not genetics.
“The examination of health disparities is certainly a worthwhile endeavor,” he continued. “For generations, differences in key health outcomes have negatively impacted the quality of life and shortened the life span of countless individuals. As scientists, clinicians, and invested members of our shared society, we are obligated to obtain a profound understanding of the mechanisms and impact of this morbid reality.”
Black race a risk factor
For their study, Dr. Bhatnagar and colleagues conducted a nationwide population analysis using data from the Surveillance Epidemiology End Results (SEER) Program of the National Cancer Institute to identify 11,190 adults aged 18-60 years who were diagnosed with AML between 1986 and 2015.
To characterize molecular features, they conducted targeted sequencing of 81 genes in 1,339 patients with AML who were treated on frontline Cancer and Leukemia Group B/Alliance for Clinical Trials in Oncology (Alliance) protocols based on standard-intensity cytarabine/anthracycline induction followed by consolidation between 1986 and 2016. None of these patients received an allogeneic stem cell transplant when they achieved complete remission.
Although overall survival has improved during the past 3 decades, survival disparities between Black and White patients has widened over time (P < .001). The authors found a nonstatistically significant difference in survival between 1986 and 1995 (White patients, n = 1,365; Black patients, n = 160; P = .19). However, the difference was significant between 1996 and 2005 (White patients, n = 2,994; Black patients, n = 480; P = .004). “And it became even more noticeable in the most recent decade,” said Dr. Bhatnagar. “Furthermore, younger Black AML patients were found to have worse survival compared with younger White AML patients.”
Results from the second analysis of patients treated on Alliance protocols did not show any significant differences in early death rates (10% vs. 46%; P = .02) and complete remission rates (71% vs. 71%; P = 1.00). “While relapse rates were slightly higher in Black compared to White patients, this difference did not reach statistical significance,” said Dr. Bhatnagar. “There was also no significant difference in the number of cycles of consolidation chemotherapy administered to these patients.”
However, both disease-free and overall survival were significantly worse for Black patients, suggesting that factors other than treatment selection were likely at play in influencing the survival disparity. The median disease-free survival for Black patients was 0.8 years, vs. 1.4 years for White patients (P = .02). Overall survival was 1.2 years vs. 1.8 years (P = .02).
Relapse rates were slightly higher in Black patients than in White patients, at 71% vs. 59%, but this difference did not reach statistical significance (P = .14).
Differences in biomarkers
With regard to underlying molecular differences between Black and White patients, the investigators found that the most common mutations were in NPM1, FLT3-ITD, and DNM3TA. Mutations were detected in more than 20% of Black patients. Other commonly mutated genes were IDH2, NRAS, TET2, IDH1, and TP53, which were mutated in more than 10% of patients. “All of these genes are established commonly mutated genes in AML,” said Bhatnagar.
On univariable and multivariable outcome analyses, which were used to identify clinical or molecular features that had a bearing on outcome, FLT3-ITD and IDH2 mutations were the only mutations associated with a higher risk for death among Black patients.
“This is actually a very important finding, as both FLT3 and IDH2 are now targetable with small-molecule inhibitors,” said Dr. Bhatnagar. “In addition, it is also worth noting that other gene mutations that have known prognostic significance in AML, such as NPM1, as well as RUNX1 and TP53, did not remain in the final statistical model.
“Importantly, our study provides powerful evidence that suggests differences in underlying disease biology between young Black and White AML patients, as evidenced by differences in the frequencies of recurrent gene mutations, “ she said.
Understudied disparities
Although the study showed that Black patients had worse outcomes, “surprisingly, the authors found these outcomes hold even when the patients are participating in clinical trials,” noted Elisa Weiss, PhD, senior vice president of education, services, and health research for the Leukemia and Lymphoma Society.
“The study makes clear that the medical and science community need to do more to better understand the social, economic, environmental, and biological causes of these disparities,” she said in an interview. “In fact, the findings suggest that there are myriad complex and understudied causes of the identified disparities, and they are likely to lie at the intersection of all levels of the social ecology that impact an individual’s ability to access timely and unbiased care, maintain their mental and physical health, and receive needed social support and resources.”
She noted that the Leukemia and Lymphoma Society has an Equity in Access research program that aims to “advance study of underlying causes of inequitable access to care and identify policies, strategies, and interventions that have the potential to reduce inequities and increase access to health care, services, and programs for blood cancer patients and survivors.”
The research was supported in part by the National Cancer Institute of the National Institutes of Health, other institutions, and through several scholar awards. Dr. Bhatnagar has received advisory board honoraria from Novartis, Kite Pharma, Celgene, Astellas, and Cell Therapeutics. Dr. Weiss has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Black race is the most important risk factor for patients with acute myeloid leukemia (AML) and is associated with poor survival, according to new findings.
Among patients with AML younger than 60 years, the rate of overall 3-year survival was significantly less among Black patients than White patients (34% vs. 43%). The risk for death was 27% higher for Black patients compared with White patients.
“Our study demonstrates the delicate interplay between a variety of factors that influence survival disparities, particularly for younger Black AML patients,” said first author Bhavana Bhatnagar, DO, of the Ohio State University’s Comprehensive Cancer Center, Columbus. “We were able to confirm the impact of socioeconomic factors while also demonstrating that being Black is, in and of itself, an independent poor prognostic variable for survival.”
She noted that the persistently poor outcomes of young Black patients that were seen despite similar treatments in clinical trials strongly suggest that additional factors have a bearing on their survival.
The findings of the study were presented during the plenary session of the annual meeting of the American Society of Hematology, which was held online this year. The study was simultaneously published in Cancer Discovery.
Racial disparities in cancer outcomes remain a challenge. The term “health disparities” describes the differences of health outcomes among different groups, said Chancellor Donald, MD, of Tulane University, New Orleans, who introduced the article at the meeting. “Racial health disparities usually result from an unequal distribution of power and resources, not genetics.
“The examination of health disparities is certainly a worthwhile endeavor,” he continued. “For generations, differences in key health outcomes have negatively impacted the quality of life and shortened the life span of countless individuals. As scientists, clinicians, and invested members of our shared society, we are obligated to obtain a profound understanding of the mechanisms and impact of this morbid reality.”
Black race a risk factor
For their study, Dr. Bhatnagar and colleagues conducted a nationwide population analysis using data from the Surveillance Epidemiology End Results (SEER) Program of the National Cancer Institute to identify 11,190 adults aged 18-60 years who were diagnosed with AML between 1986 and 2015.
To characterize molecular features, they conducted targeted sequencing of 81 genes in 1,339 patients with AML who were treated on frontline Cancer and Leukemia Group B/Alliance for Clinical Trials in Oncology (Alliance) protocols based on standard-intensity cytarabine/anthracycline induction followed by consolidation between 1986 and 2016. None of these patients received an allogeneic stem cell transplant when they achieved complete remission.
Although overall survival has improved during the past 3 decades, survival disparities between Black and White patients has widened over time (P < .001). The authors found a nonstatistically significant difference in survival between 1986 and 1995 (White patients, n = 1,365; Black patients, n = 160; P = .19). However, the difference was significant between 1996 and 2005 (White patients, n = 2,994; Black patients, n = 480; P = .004). “And it became even more noticeable in the most recent decade,” said Dr. Bhatnagar. “Furthermore, younger Black AML patients were found to have worse survival compared with younger White AML patients.”
Results from the second analysis of patients treated on Alliance protocols did not show any significant differences in early death rates (10% vs. 46%; P = .02) and complete remission rates (71% vs. 71%; P = 1.00). “While relapse rates were slightly higher in Black compared to White patients, this difference did not reach statistical significance,” said Dr. Bhatnagar. “There was also no significant difference in the number of cycles of consolidation chemotherapy administered to these patients.”
However, both disease-free and overall survival were significantly worse for Black patients, suggesting that factors other than treatment selection were likely at play in influencing the survival disparity. The median disease-free survival for Black patients was 0.8 years, vs. 1.4 years for White patients (P = .02). Overall survival was 1.2 years vs. 1.8 years (P = .02).
Relapse rates were slightly higher in Black patients than in White patients, at 71% vs. 59%, but this difference did not reach statistical significance (P = .14).
Differences in biomarkers
With regard to underlying molecular differences between Black and White patients, the investigators found that the most common mutations were in NPM1, FLT3-ITD, and DNM3TA. Mutations were detected in more than 20% of Black patients. Other commonly mutated genes were IDH2, NRAS, TET2, IDH1, and TP53, which were mutated in more than 10% of patients. “All of these genes are established commonly mutated genes in AML,” said Bhatnagar.
On univariable and multivariable outcome analyses, which were used to identify clinical or molecular features that had a bearing on outcome, FLT3-ITD and IDH2 mutations were the only mutations associated with a higher risk for death among Black patients.
“This is actually a very important finding, as both FLT3 and IDH2 are now targetable with small-molecule inhibitors,” said Dr. Bhatnagar. “In addition, it is also worth noting that other gene mutations that have known prognostic significance in AML, such as NPM1, as well as RUNX1 and TP53, did not remain in the final statistical model.
“Importantly, our study provides powerful evidence that suggests differences in underlying disease biology between young Black and White AML patients, as evidenced by differences in the frequencies of recurrent gene mutations, “ she said.
Understudied disparities
Although the study showed that Black patients had worse outcomes, “surprisingly, the authors found these outcomes hold even when the patients are participating in clinical trials,” noted Elisa Weiss, PhD, senior vice president of education, services, and health research for the Leukemia and Lymphoma Society.
“The study makes clear that the medical and science community need to do more to better understand the social, economic, environmental, and biological causes of these disparities,” she said in an interview. “In fact, the findings suggest that there are myriad complex and understudied causes of the identified disparities, and they are likely to lie at the intersection of all levels of the social ecology that impact an individual’s ability to access timely and unbiased care, maintain their mental and physical health, and receive needed social support and resources.”
She noted that the Leukemia and Lymphoma Society has an Equity in Access research program that aims to “advance study of underlying causes of inequitable access to care and identify policies, strategies, and interventions that have the potential to reduce inequities and increase access to health care, services, and programs for blood cancer patients and survivors.”
The research was supported in part by the National Cancer Institute of the National Institutes of Health, other institutions, and through several scholar awards. Dr. Bhatnagar has received advisory board honoraria from Novartis, Kite Pharma, Celgene, Astellas, and Cell Therapeutics. Dr. Weiss has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Prevention of HMA failure a goal for high-risk MDS posttransplant
Prognoses remain extremely poor after hypomethylating agents (HMAs) fail in patients with higher-risk myelodysplastic syndromes (HR-MDS). But a hematologist-oncologist told colleagues that novel therapies are in the works, and some show promise.
Still, “the clinical development for drugs in this setting has been quite challenging, and we have had a lot of drugs that have died in this space over the years,” cautioned Amer Zeidan, MBBS, MHS, an associate professor at Yale University, New Haven, Conn., in a presentation at the virtual Acute Leukemia Forum of Hemedicus. For now, “the best way to manage HMA failure in MDS patients is by preventing HMA failure.”
Dr. Zeidan highlighted a 2016 study – which he led – that found the median overall survival from diagnosis was just a median of 17.0 months (95% confidence interval, 15.8-18.4) in 632 patients with HR-MDS. Another 2016 study, which he also led, reported median overall survival of 11 months (95% CI, 10-14) and 12 months (95% CI, 11-16; P = .26) for patients aged 66 or older who had HR-MDS and took azacitidine and decitabine, respectively. Median survival is even shorter after HMA failure, he said.
The most important obstacle to effective therapy is “the biologic and molecular heterogeneity of the disease,” he said. “Only a certain number of genes are altered in a significant number of patients. And then you have a very long tail, with so many alterations, but most of them are rare. That makes targeting all patients with the same mechanism quite challenging. Also, we poorly understand how hypomethylating agents work and the mechanism of primary and secondary failure. And many MDS patients are older with multiple conditions, multiple comorbidities. By the time of failure, they are generally beaten up and very difficult to enroll in clinical trials.”
Even so, he said, “the understanding of the molecular pathogenesis of MDS is starting to open the door for new drug development opportunities. What’s been changing over the last 5 years is an increased understanding of targeting some of the alterations that are specific to the patient – individualized targeting or precision medicine.”
Novel therapies
Dr. Zeidan said the novel therapies for HR-MDS after HMA failure fall into these categories: molecularly targeted agents, genetically agnostic small-molecule inhibitors, immunotherapies, and chemotherapy/epigenetic agents.
Multiple trials, for example, are examining a chemotherapy treatment CPX-351 (liposomal cytarabine-daunorubicin) in HR-MDS, and a 2018 study showed improvement in median survival in older patients with newly diagnosed secondary acute myeloid leukemia. “However, this remains an investigational treatment,” Dr. Zeidan cautioned.
Venetoclax is also being studied. Animal and cell culture data suggest there may be helpful synergistic activity between venetoclax and azacitidine in both the frontline and relapse settings. Dr. Zeidan highlighted his own 2019 report on a phase 1b study of venetoclax versus venetoclax and azacitidine in the HMA failure/HR-MDS setting. The results are “quite exciting,” he said.
The report noted that, “although the study is still ongoing, the 6-month OS [overall survival] estimate of 57% in monotherapy [patients] compares favorably to historical controls.”
Glasdegib is “another drug of interest,” although it’s mostly been studied in the frontline setting, he said, and “we don’t have much data with this drug in the refractory setting for MDS patients.” APR-246 is also intriguing, he said, but again lacks data in the refractory setting.
Dr. Zeidan noted research into other treatments – rigosertib (recent findings have been disappointing), ivosidenib for IDH1-mutated MDS, AG221-001 and enasidenib (targeting IDH2 mutations), trametinib (targeting RAS pathway mutations), and others. For now, “clinical trial participation should be the best way to manage these patients.”
Dr. Zeidan disclosed multiple disclosures, including relationships with Pfizer, Novartis, Abbvie, Pfizer, Medimmune/AstraZeneca and Boehringer Ingelheim, among others.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
Prognoses remain extremely poor after hypomethylating agents (HMAs) fail in patients with higher-risk myelodysplastic syndromes (HR-MDS). But a hematologist-oncologist told colleagues that novel therapies are in the works, and some show promise.
Still, “the clinical development for drugs in this setting has been quite challenging, and we have had a lot of drugs that have died in this space over the years,” cautioned Amer Zeidan, MBBS, MHS, an associate professor at Yale University, New Haven, Conn., in a presentation at the virtual Acute Leukemia Forum of Hemedicus. For now, “the best way to manage HMA failure in MDS patients is by preventing HMA failure.”
Dr. Zeidan highlighted a 2016 study – which he led – that found the median overall survival from diagnosis was just a median of 17.0 months (95% confidence interval, 15.8-18.4) in 632 patients with HR-MDS. Another 2016 study, which he also led, reported median overall survival of 11 months (95% CI, 10-14) and 12 months (95% CI, 11-16; P = .26) for patients aged 66 or older who had HR-MDS and took azacitidine and decitabine, respectively. Median survival is even shorter after HMA failure, he said.
The most important obstacle to effective therapy is “the biologic and molecular heterogeneity of the disease,” he said. “Only a certain number of genes are altered in a significant number of patients. And then you have a very long tail, with so many alterations, but most of them are rare. That makes targeting all patients with the same mechanism quite challenging. Also, we poorly understand how hypomethylating agents work and the mechanism of primary and secondary failure. And many MDS patients are older with multiple conditions, multiple comorbidities. By the time of failure, they are generally beaten up and very difficult to enroll in clinical trials.”
Even so, he said, “the understanding of the molecular pathogenesis of MDS is starting to open the door for new drug development opportunities. What’s been changing over the last 5 years is an increased understanding of targeting some of the alterations that are specific to the patient – individualized targeting or precision medicine.”
Novel therapies
Dr. Zeidan said the novel therapies for HR-MDS after HMA failure fall into these categories: molecularly targeted agents, genetically agnostic small-molecule inhibitors, immunotherapies, and chemotherapy/epigenetic agents.
Multiple trials, for example, are examining a chemotherapy treatment CPX-351 (liposomal cytarabine-daunorubicin) in HR-MDS, and a 2018 study showed improvement in median survival in older patients with newly diagnosed secondary acute myeloid leukemia. “However, this remains an investigational treatment,” Dr. Zeidan cautioned.
Venetoclax is also being studied. Animal and cell culture data suggest there may be helpful synergistic activity between venetoclax and azacitidine in both the frontline and relapse settings. Dr. Zeidan highlighted his own 2019 report on a phase 1b study of venetoclax versus venetoclax and azacitidine in the HMA failure/HR-MDS setting. The results are “quite exciting,” he said.
The report noted that, “although the study is still ongoing, the 6-month OS [overall survival] estimate of 57% in monotherapy [patients] compares favorably to historical controls.”
Glasdegib is “another drug of interest,” although it’s mostly been studied in the frontline setting, he said, and “we don’t have much data with this drug in the refractory setting for MDS patients.” APR-246 is also intriguing, he said, but again lacks data in the refractory setting.
Dr. Zeidan noted research into other treatments – rigosertib (recent findings have been disappointing), ivosidenib for IDH1-mutated MDS, AG221-001 and enasidenib (targeting IDH2 mutations), trametinib (targeting RAS pathway mutations), and others. For now, “clinical trial participation should be the best way to manage these patients.”
Dr. Zeidan disclosed multiple disclosures, including relationships with Pfizer, Novartis, Abbvie, Pfizer, Medimmune/AstraZeneca and Boehringer Ingelheim, among others.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
Prognoses remain extremely poor after hypomethylating agents (HMAs) fail in patients with higher-risk myelodysplastic syndromes (HR-MDS). But a hematologist-oncologist told colleagues that novel therapies are in the works, and some show promise.
Still, “the clinical development for drugs in this setting has been quite challenging, and we have had a lot of drugs that have died in this space over the years,” cautioned Amer Zeidan, MBBS, MHS, an associate professor at Yale University, New Haven, Conn., in a presentation at the virtual Acute Leukemia Forum of Hemedicus. For now, “the best way to manage HMA failure in MDS patients is by preventing HMA failure.”
Dr. Zeidan highlighted a 2016 study – which he led – that found the median overall survival from diagnosis was just a median of 17.0 months (95% confidence interval, 15.8-18.4) in 632 patients with HR-MDS. Another 2016 study, which he also led, reported median overall survival of 11 months (95% CI, 10-14) and 12 months (95% CI, 11-16; P = .26) for patients aged 66 or older who had HR-MDS and took azacitidine and decitabine, respectively. Median survival is even shorter after HMA failure, he said.
The most important obstacle to effective therapy is “the biologic and molecular heterogeneity of the disease,” he said. “Only a certain number of genes are altered in a significant number of patients. And then you have a very long tail, with so many alterations, but most of them are rare. That makes targeting all patients with the same mechanism quite challenging. Also, we poorly understand how hypomethylating agents work and the mechanism of primary and secondary failure. And many MDS patients are older with multiple conditions, multiple comorbidities. By the time of failure, they are generally beaten up and very difficult to enroll in clinical trials.”
Even so, he said, “the understanding of the molecular pathogenesis of MDS is starting to open the door for new drug development opportunities. What’s been changing over the last 5 years is an increased understanding of targeting some of the alterations that are specific to the patient – individualized targeting or precision medicine.”
Novel therapies
Dr. Zeidan said the novel therapies for HR-MDS after HMA failure fall into these categories: molecularly targeted agents, genetically agnostic small-molecule inhibitors, immunotherapies, and chemotherapy/epigenetic agents.
Multiple trials, for example, are examining a chemotherapy treatment CPX-351 (liposomal cytarabine-daunorubicin) in HR-MDS, and a 2018 study showed improvement in median survival in older patients with newly diagnosed secondary acute myeloid leukemia. “However, this remains an investigational treatment,” Dr. Zeidan cautioned.
Venetoclax is also being studied. Animal and cell culture data suggest there may be helpful synergistic activity between venetoclax and azacitidine in both the frontline and relapse settings. Dr. Zeidan highlighted his own 2019 report on a phase 1b study of venetoclax versus venetoclax and azacitidine in the HMA failure/HR-MDS setting. The results are “quite exciting,” he said.
The report noted that, “although the study is still ongoing, the 6-month OS [overall survival] estimate of 57% in monotherapy [patients] compares favorably to historical controls.”
Glasdegib is “another drug of interest,” although it’s mostly been studied in the frontline setting, he said, and “we don’t have much data with this drug in the refractory setting for MDS patients.” APR-246 is also intriguing, he said, but again lacks data in the refractory setting.
Dr. Zeidan noted research into other treatments – rigosertib (recent findings have been disappointing), ivosidenib for IDH1-mutated MDS, AG221-001 and enasidenib (targeting IDH2 mutations), trametinib (targeting RAS pathway mutations), and others. For now, “clinical trial participation should be the best way to manage these patients.”
Dr. Zeidan disclosed multiple disclosures, including relationships with Pfizer, Novartis, Abbvie, Pfizer, Medimmune/AstraZeneca and Boehringer Ingelheim, among others.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
FROM ALF 2020
Beat AML: Precision medicine strategy feasible, superior to SOC for AML
The 30-day mortality rates were 3.7% versus 20.4% in 224 patients who enrolled in the Beat AML trial precision medicine substudies within 7 days of prospective genomic profiling and 103 who elected SOC chemotherapy, respectively, Amy Burd, PhD, vice president of research strategy for the Leukemia & Lymphoma Society, Rye Brook, N.Y. and her colleagues reported online in Nature Medicine.
Overall survival (OS) at a median of 7.1 months was also significantly longer with precision medicine than with SOC chemotherapy (median, 12.8 vs. 3.9 months), the investigators found.
In an additional 28 patients who selected an investigational therapy rather than a precision medicine strategy or SOC chemotherapy, median OS was not reached, and in 38 who chose palliative care, median OS was 0.6 months, they noted. Care type was unknown in two patients.
The results were similar after controlling for demographic, clinical, and molecular variables and did not change when patients with adverse events of special interest were excluded from the analysis or when only those with survival greater than 2 weeks were included in the analysis.
AML confers an adverse outcome in older adults and therefore is typically treated rapidly after diagnosis. This has precluded consideration of patients’ mutational profile for treatment decisions.
Beat AML, however, sought to prospectively assess the feasibility of quickly ascertaining cytogenetic and mutational data for the purpose of improving outcomes through targeted treatment.
“The study shows that delaying treatment up to 7 days is feasible and safe, and that patients who opted for the precision medicine approach experienced a lower early death rate and superior overall survival, compared with patients who opted for standard of care,” lead study author John C. Byrd, MD, the D. Warren Brown Chair of Leukemia Research of the Ohio State University, Columbus, noted in a press statement from the Leukemia & Lymphoma Society, which conducted the trial. “This patient-centric study shows that we can move away from chemotherapy treatment for patients who won’t respond or can’t withstand the harsh effects of the same chemotherapies we’ve been using for 40 years and match them with a treatment better suited for their individual cases.”
The ongoing Beat AML trial was launched by LLS in 2016 to assess various novel targeted therapies in newly diagnosed AML patients aged 60 years and older. Participants underwent next-generation genomic sequencing, were matched to the appropriate targeted therapy, and were given the option of enrolling on the relevant substudy or selecting an alternate treatment strategy. There are currently 11 substudies assessing novel therapies that have emerged in the wake of “significant progress in understanding the molecular pathogenesis of AML.”
The current findings represent outcomes in patients enrolled between Nov. 2016 and Jan. 2018. The patients had a mean age of 72 years, and those selecting precision medicine vs. SOC had similar demographic and genetic features, the authors noted.
LLS president and chief executive officer Louis J. DeGennaro, PhD, said the findings are practice changing and provide a template for studying precision medicine in other cancers.
“The study is changing significantly the way we look at treating patients with AML, showing that precision medicine ... can improve short- and long-term outcomes for patients with this deadly blood cancer,” he said in the LLS statement. “Further, BEAT AML has proven to be a viable model for other cancer clinical trials to emulate.”
In fact, the model has been applied to the recently launched Beat COVID trial, which looks at acalabrutinib in patients with hematologic cancers and COVID-19 infection, and other trials, including the LLS PedAL global precision medicine trial for children with relapsed acute leukemia, are planned.
“This study sets the path to establish the safety of precision medicine in AML and sets the stage to extend this same approach to younger patients with this disease and other cancers that are urgently treated as a single disease despite recognition of multiple subtypes, the authors concluded.
Dr. Burd is an employee of LLS, which received funding from AbbVie, Agios Pharmaceuticals, Alexion Pharmaceuticals, and a variety of other pharmaceutical and biotechnology companies. Dr. Byrd has received research support from Acerta Pharma, Genentech, Janssen Pharmaceutica, and Pharmacyclics and has served on the advisory board of Syndax Pharmaceuticals.
SOURCE: Burd A et al. Nature Medicine 2020 Oct 26. doi: 10.1038/s41591-020-1089-8.
The 30-day mortality rates were 3.7% versus 20.4% in 224 patients who enrolled in the Beat AML trial precision medicine substudies within 7 days of prospective genomic profiling and 103 who elected SOC chemotherapy, respectively, Amy Burd, PhD, vice president of research strategy for the Leukemia & Lymphoma Society, Rye Brook, N.Y. and her colleagues reported online in Nature Medicine.
Overall survival (OS) at a median of 7.1 months was also significantly longer with precision medicine than with SOC chemotherapy (median, 12.8 vs. 3.9 months), the investigators found.
In an additional 28 patients who selected an investigational therapy rather than a precision medicine strategy or SOC chemotherapy, median OS was not reached, and in 38 who chose palliative care, median OS was 0.6 months, they noted. Care type was unknown in two patients.
The results were similar after controlling for demographic, clinical, and molecular variables and did not change when patients with adverse events of special interest were excluded from the analysis or when only those with survival greater than 2 weeks were included in the analysis.
AML confers an adverse outcome in older adults and therefore is typically treated rapidly after diagnosis. This has precluded consideration of patients’ mutational profile for treatment decisions.
Beat AML, however, sought to prospectively assess the feasibility of quickly ascertaining cytogenetic and mutational data for the purpose of improving outcomes through targeted treatment.
“The study shows that delaying treatment up to 7 days is feasible and safe, and that patients who opted for the precision medicine approach experienced a lower early death rate and superior overall survival, compared with patients who opted for standard of care,” lead study author John C. Byrd, MD, the D. Warren Brown Chair of Leukemia Research of the Ohio State University, Columbus, noted in a press statement from the Leukemia & Lymphoma Society, which conducted the trial. “This patient-centric study shows that we can move away from chemotherapy treatment for patients who won’t respond or can’t withstand the harsh effects of the same chemotherapies we’ve been using for 40 years and match them with a treatment better suited for their individual cases.”
The ongoing Beat AML trial was launched by LLS in 2016 to assess various novel targeted therapies in newly diagnosed AML patients aged 60 years and older. Participants underwent next-generation genomic sequencing, were matched to the appropriate targeted therapy, and were given the option of enrolling on the relevant substudy or selecting an alternate treatment strategy. There are currently 11 substudies assessing novel therapies that have emerged in the wake of “significant progress in understanding the molecular pathogenesis of AML.”
The current findings represent outcomes in patients enrolled between Nov. 2016 and Jan. 2018. The patients had a mean age of 72 years, and those selecting precision medicine vs. SOC had similar demographic and genetic features, the authors noted.
LLS president and chief executive officer Louis J. DeGennaro, PhD, said the findings are practice changing and provide a template for studying precision medicine in other cancers.
“The study is changing significantly the way we look at treating patients with AML, showing that precision medicine ... can improve short- and long-term outcomes for patients with this deadly blood cancer,” he said in the LLS statement. “Further, BEAT AML has proven to be a viable model for other cancer clinical trials to emulate.”
In fact, the model has been applied to the recently launched Beat COVID trial, which looks at acalabrutinib in patients with hematologic cancers and COVID-19 infection, and other trials, including the LLS PedAL global precision medicine trial for children with relapsed acute leukemia, are planned.
“This study sets the path to establish the safety of precision medicine in AML and sets the stage to extend this same approach to younger patients with this disease and other cancers that are urgently treated as a single disease despite recognition of multiple subtypes, the authors concluded.
Dr. Burd is an employee of LLS, which received funding from AbbVie, Agios Pharmaceuticals, Alexion Pharmaceuticals, and a variety of other pharmaceutical and biotechnology companies. Dr. Byrd has received research support from Acerta Pharma, Genentech, Janssen Pharmaceutica, and Pharmacyclics and has served on the advisory board of Syndax Pharmaceuticals.
SOURCE: Burd A et al. Nature Medicine 2020 Oct 26. doi: 10.1038/s41591-020-1089-8.
The 30-day mortality rates were 3.7% versus 20.4% in 224 patients who enrolled in the Beat AML trial precision medicine substudies within 7 days of prospective genomic profiling and 103 who elected SOC chemotherapy, respectively, Amy Burd, PhD, vice president of research strategy for the Leukemia & Lymphoma Society, Rye Brook, N.Y. and her colleagues reported online in Nature Medicine.
Overall survival (OS) at a median of 7.1 months was also significantly longer with precision medicine than with SOC chemotherapy (median, 12.8 vs. 3.9 months), the investigators found.
In an additional 28 patients who selected an investigational therapy rather than a precision medicine strategy or SOC chemotherapy, median OS was not reached, and in 38 who chose palliative care, median OS was 0.6 months, they noted. Care type was unknown in two patients.
The results were similar after controlling for demographic, clinical, and molecular variables and did not change when patients with adverse events of special interest were excluded from the analysis or when only those with survival greater than 2 weeks were included in the analysis.
AML confers an adverse outcome in older adults and therefore is typically treated rapidly after diagnosis. This has precluded consideration of patients’ mutational profile for treatment decisions.
Beat AML, however, sought to prospectively assess the feasibility of quickly ascertaining cytogenetic and mutational data for the purpose of improving outcomes through targeted treatment.
“The study shows that delaying treatment up to 7 days is feasible and safe, and that patients who opted for the precision medicine approach experienced a lower early death rate and superior overall survival, compared with patients who opted for standard of care,” lead study author John C. Byrd, MD, the D. Warren Brown Chair of Leukemia Research of the Ohio State University, Columbus, noted in a press statement from the Leukemia & Lymphoma Society, which conducted the trial. “This patient-centric study shows that we can move away from chemotherapy treatment for patients who won’t respond or can’t withstand the harsh effects of the same chemotherapies we’ve been using for 40 years and match them with a treatment better suited for their individual cases.”
The ongoing Beat AML trial was launched by LLS in 2016 to assess various novel targeted therapies in newly diagnosed AML patients aged 60 years and older. Participants underwent next-generation genomic sequencing, were matched to the appropriate targeted therapy, and were given the option of enrolling on the relevant substudy or selecting an alternate treatment strategy. There are currently 11 substudies assessing novel therapies that have emerged in the wake of “significant progress in understanding the molecular pathogenesis of AML.”
The current findings represent outcomes in patients enrolled between Nov. 2016 and Jan. 2018. The patients had a mean age of 72 years, and those selecting precision medicine vs. SOC had similar demographic and genetic features, the authors noted.
LLS president and chief executive officer Louis J. DeGennaro, PhD, said the findings are practice changing and provide a template for studying precision medicine in other cancers.
“The study is changing significantly the way we look at treating patients with AML, showing that precision medicine ... can improve short- and long-term outcomes for patients with this deadly blood cancer,” he said in the LLS statement. “Further, BEAT AML has proven to be a viable model for other cancer clinical trials to emulate.”
In fact, the model has been applied to the recently launched Beat COVID trial, which looks at acalabrutinib in patients with hematologic cancers and COVID-19 infection, and other trials, including the LLS PedAL global precision medicine trial for children with relapsed acute leukemia, are planned.
“This study sets the path to establish the safety of precision medicine in AML and sets the stage to extend this same approach to younger patients with this disease and other cancers that are urgently treated as a single disease despite recognition of multiple subtypes, the authors concluded.
Dr. Burd is an employee of LLS, which received funding from AbbVie, Agios Pharmaceuticals, Alexion Pharmaceuticals, and a variety of other pharmaceutical and biotechnology companies. Dr. Byrd has received research support from Acerta Pharma, Genentech, Janssen Pharmaceutica, and Pharmacyclics and has served on the advisory board of Syndax Pharmaceuticals.
SOURCE: Burd A et al. Nature Medicine 2020 Oct 26. doi: 10.1038/s41591-020-1089-8.
FROM NATURE MEDICINE
Are HMAS appropriate for posttransplant maintenance in acute leukemias?
Hematopoietic stem cell transplantation (HCT) is one of the most important treatment options for acute leukemias. However, posttransplant cancer recurrence remains a continuing issue. And while there are reasons to think that hypomethylating agents (HMAS) could be helpful as maintenance tools to prevent cancer recurrence after HCT in leukemia, a hematologist/oncologist told colleagues that the treatment isn’t yet ready for prime time.
“I don’t think you can prefer hypomethylating agents over anything right now. Unfortunately, there’s no data that we can hang our hat on that says they are of benefit in the posttransplant setting,” said Frederick Appelbaum, MD, executive vice president and deputy director of the Fred Hutchinson Cancer Research Center, Seattle, in a presentation at the virtual Acute Leukemia Forum of Hemedicus.
However, there’s still plenty of room for improvement for patients following HCT, he said, pointing to the findings of a 2020 study. The report, which he cowrote, found that 200-day mortality after HCT fell by a third from 2003-2007 to 2013-20017, but also noted that “relapse of cancer remains the largest obstacle to better survival outcomes.”
Dr. Appelbaum described the findings this way: “Without a doubt, the major limitation to transplants for hematologic malignancies today is disease recurrence,” he said. “In fact, if you look at patients after day 100, over 60% of the reason for failure is tumor regrowth. Thus, people are very anxious to look at any method that we can to prevent posttransplant relapse, including the use of hypomethylating agents.”
In regard to strategy, “we don’t have to get rid of every last leukemic cell. Just delaying recurrence might be enough,” he said. “If you can keep the patient from relapsing for the first 3 months, and then take the brakes off the immune suppression and allow immunity to regrow, that may be enough to allow increased numbers of patients to be cured of their disease.”
A potential role
Why might HMAS be a possible option after transplant? They do appear to play a role after chemotherapy, he said, pointing to four 2019 studies: One that examined decitabine and three that examined azacytidine: Here, here, and here.
“These four studies provide convincing evidence that hypomethylating-agent therapy after conventional chemotherapy may either prevent or delay relapse when given as maintenance,” Dr. Appelbaum said.
If HMAS work after standard chemotherapy, why might they fail to work after transplantation? “For one, by the time the disease has been able to go through chemotherapy and transplant, you’re left with highly resistant cells,” he said. “Therefore, hypomethylating agents may not be enough to get rid of the disease. Secondly, any of you who have tried to give a maintenance therapy after transplantation know how difficult it can be with CMV [cytomegalovirus] reactivation, count suppression with ganciclovir, graft-versus-host disease [GVHD] causing nausea and vomiting, diarrhea and renal dysfunction caused by calcineurin inhibitors. These are daily events during the first 3 months after transplantation, making drug administration difficult.”
In addition, he said, “even if you can give the drug, the clinical and disease variability may make it very difficult to detect an effect.”
In another study, researchers “did make a valiant attempt to study azacitidine in the posttransplant setting by randomizing 181 patients to either azacitidine or observation,” Dr. Appelbaum said. “Unfortunately, as they reported in 2018, they could not detect a difference in either disease-free or overall survival.”
The researchers reported that nearly 75% of patients in the azacitidine arm failed to complete the planned 12 cycles of treatment, he said. “The reasons for stopping the drug were pretty profound. Half of the patients stopped because they relapsed. Others had stopped because of grades three or four toxicity, death, or severe GVHD or significant infections. It is very difficult to give the drug.”
In the future, “if we truly want to optimize the benefit of using hypomethylating agents after transplantation, it’s going to be very important for us to understand how they work,” he said. “Understanding that would then help us to select which drug we should use, what the dosing and schedule might be, and also to select patients that might benefit from it. Unfortunately, right now, it’s pretty much of a black box. We don’t really understand the effects of hypomethylating agents in the posttransplant period.”
Still, he added, “without question, the results that we have seen with the use of hypomethylating agents after conventional chemotherapy – prolonging disease-free and, probably, overall survival – are going to provide a very, very strong stimulus to study hypomethylating agents after transplantation as well.”
Dr. Appelbaum reports no disclosures.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
Hematopoietic stem cell transplantation (HCT) is one of the most important treatment options for acute leukemias. However, posttransplant cancer recurrence remains a continuing issue. And while there are reasons to think that hypomethylating agents (HMAS) could be helpful as maintenance tools to prevent cancer recurrence after HCT in leukemia, a hematologist/oncologist told colleagues that the treatment isn’t yet ready for prime time.
“I don’t think you can prefer hypomethylating agents over anything right now. Unfortunately, there’s no data that we can hang our hat on that says they are of benefit in the posttransplant setting,” said Frederick Appelbaum, MD, executive vice president and deputy director of the Fred Hutchinson Cancer Research Center, Seattle, in a presentation at the virtual Acute Leukemia Forum of Hemedicus.
However, there’s still plenty of room for improvement for patients following HCT, he said, pointing to the findings of a 2020 study. The report, which he cowrote, found that 200-day mortality after HCT fell by a third from 2003-2007 to 2013-20017, but also noted that “relapse of cancer remains the largest obstacle to better survival outcomes.”
Dr. Appelbaum described the findings this way: “Without a doubt, the major limitation to transplants for hematologic malignancies today is disease recurrence,” he said. “In fact, if you look at patients after day 100, over 60% of the reason for failure is tumor regrowth. Thus, people are very anxious to look at any method that we can to prevent posttransplant relapse, including the use of hypomethylating agents.”
In regard to strategy, “we don’t have to get rid of every last leukemic cell. Just delaying recurrence might be enough,” he said. “If you can keep the patient from relapsing for the first 3 months, and then take the brakes off the immune suppression and allow immunity to regrow, that may be enough to allow increased numbers of patients to be cured of their disease.”
A potential role
Why might HMAS be a possible option after transplant? They do appear to play a role after chemotherapy, he said, pointing to four 2019 studies: One that examined decitabine and three that examined azacytidine: Here, here, and here.
“These four studies provide convincing evidence that hypomethylating-agent therapy after conventional chemotherapy may either prevent or delay relapse when given as maintenance,” Dr. Appelbaum said.
If HMAS work after standard chemotherapy, why might they fail to work after transplantation? “For one, by the time the disease has been able to go through chemotherapy and transplant, you’re left with highly resistant cells,” he said. “Therefore, hypomethylating agents may not be enough to get rid of the disease. Secondly, any of you who have tried to give a maintenance therapy after transplantation know how difficult it can be with CMV [cytomegalovirus] reactivation, count suppression with ganciclovir, graft-versus-host disease [GVHD] causing nausea and vomiting, diarrhea and renal dysfunction caused by calcineurin inhibitors. These are daily events during the first 3 months after transplantation, making drug administration difficult.”
In addition, he said, “even if you can give the drug, the clinical and disease variability may make it very difficult to detect an effect.”
In another study, researchers “did make a valiant attempt to study azacitidine in the posttransplant setting by randomizing 181 patients to either azacitidine or observation,” Dr. Appelbaum said. “Unfortunately, as they reported in 2018, they could not detect a difference in either disease-free or overall survival.”
The researchers reported that nearly 75% of patients in the azacitidine arm failed to complete the planned 12 cycles of treatment, he said. “The reasons for stopping the drug were pretty profound. Half of the patients stopped because they relapsed. Others had stopped because of grades three or four toxicity, death, or severe GVHD or significant infections. It is very difficult to give the drug.”
In the future, “if we truly want to optimize the benefit of using hypomethylating agents after transplantation, it’s going to be very important for us to understand how they work,” he said. “Understanding that would then help us to select which drug we should use, what the dosing and schedule might be, and also to select patients that might benefit from it. Unfortunately, right now, it’s pretty much of a black box. We don’t really understand the effects of hypomethylating agents in the posttransplant period.”
Still, he added, “without question, the results that we have seen with the use of hypomethylating agents after conventional chemotherapy – prolonging disease-free and, probably, overall survival – are going to provide a very, very strong stimulus to study hypomethylating agents after transplantation as well.”
Dr. Appelbaum reports no disclosures.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
Hematopoietic stem cell transplantation (HCT) is one of the most important treatment options for acute leukemias. However, posttransplant cancer recurrence remains a continuing issue. And while there are reasons to think that hypomethylating agents (HMAS) could be helpful as maintenance tools to prevent cancer recurrence after HCT in leukemia, a hematologist/oncologist told colleagues that the treatment isn’t yet ready for prime time.
“I don’t think you can prefer hypomethylating agents over anything right now. Unfortunately, there’s no data that we can hang our hat on that says they are of benefit in the posttransplant setting,” said Frederick Appelbaum, MD, executive vice president and deputy director of the Fred Hutchinson Cancer Research Center, Seattle, in a presentation at the virtual Acute Leukemia Forum of Hemedicus.
However, there’s still plenty of room for improvement for patients following HCT, he said, pointing to the findings of a 2020 study. The report, which he cowrote, found that 200-day mortality after HCT fell by a third from 2003-2007 to 2013-20017, but also noted that “relapse of cancer remains the largest obstacle to better survival outcomes.”
Dr. Appelbaum described the findings this way: “Without a doubt, the major limitation to transplants for hematologic malignancies today is disease recurrence,” he said. “In fact, if you look at patients after day 100, over 60% of the reason for failure is tumor regrowth. Thus, people are very anxious to look at any method that we can to prevent posttransplant relapse, including the use of hypomethylating agents.”
In regard to strategy, “we don’t have to get rid of every last leukemic cell. Just delaying recurrence might be enough,” he said. “If you can keep the patient from relapsing for the first 3 months, and then take the brakes off the immune suppression and allow immunity to regrow, that may be enough to allow increased numbers of patients to be cured of their disease.”
A potential role
Why might HMAS be a possible option after transplant? They do appear to play a role after chemotherapy, he said, pointing to four 2019 studies: One that examined decitabine and three that examined azacytidine: Here, here, and here.
“These four studies provide convincing evidence that hypomethylating-agent therapy after conventional chemotherapy may either prevent or delay relapse when given as maintenance,” Dr. Appelbaum said.
If HMAS work after standard chemotherapy, why might they fail to work after transplantation? “For one, by the time the disease has been able to go through chemotherapy and transplant, you’re left with highly resistant cells,” he said. “Therefore, hypomethylating agents may not be enough to get rid of the disease. Secondly, any of you who have tried to give a maintenance therapy after transplantation know how difficult it can be with CMV [cytomegalovirus] reactivation, count suppression with ganciclovir, graft-versus-host disease [GVHD] causing nausea and vomiting, diarrhea and renal dysfunction caused by calcineurin inhibitors. These are daily events during the first 3 months after transplantation, making drug administration difficult.”
In addition, he said, “even if you can give the drug, the clinical and disease variability may make it very difficult to detect an effect.”
In another study, researchers “did make a valiant attempt to study azacitidine in the posttransplant setting by randomizing 181 patients to either azacitidine or observation,” Dr. Appelbaum said. “Unfortunately, as they reported in 2018, they could not detect a difference in either disease-free or overall survival.”
The researchers reported that nearly 75% of patients in the azacitidine arm failed to complete the planned 12 cycles of treatment, he said. “The reasons for stopping the drug were pretty profound. Half of the patients stopped because they relapsed. Others had stopped because of grades three or four toxicity, death, or severe GVHD or significant infections. It is very difficult to give the drug.”
In the future, “if we truly want to optimize the benefit of using hypomethylating agents after transplantation, it’s going to be very important for us to understand how they work,” he said. “Understanding that would then help us to select which drug we should use, what the dosing and schedule might be, and also to select patients that might benefit from it. Unfortunately, right now, it’s pretty much of a black box. We don’t really understand the effects of hypomethylating agents in the posttransplant period.”
Still, he added, “without question, the results that we have seen with the use of hypomethylating agents after conventional chemotherapy – prolonging disease-free and, probably, overall survival – are going to provide a very, very strong stimulus to study hypomethylating agents after transplantation as well.”
Dr. Appelbaum reports no disclosures.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
FROM ALF 2020
Novel agents hold promise for frontline AML treatment
Novel therapies are poised to dramatically change frontline therapy for acute myeloid leukemia (AML), and they have the potential to replace chemotherapy, a hematologist/oncologist told colleagues at the virtual Acute Leukemia Forum of Hemedicus.
But more work needs to be done, noted Alexander Perl, MD, MS, associate professor at the University of Pennsylvania, Philadelphia. While advances have transformed AML treatment in the relapsed/refractory setting, “we’re just not seeing that substantive improvement” for newly diagnosed patients, he said. “We need to find the disease-modifying drugs that work in the relapsed/refractory setting and move those frontline. That’s where we’re going to see the transformations.”
Research suggests that low-intensity therapy holds tremendous promise, he said, “with the idea that we could make therapy much more tolerable for the vast majority of patients affected by AML, who, as we know, are older patients.”
Dr. Perl highlighted the 2020 VIALE-A study – venetoclax/azacitidine versus azacitidine/placebo – which reported that “in previously untreated patients who were ineligible for intensive chemotherapy, overall survival was longer and the incidence of remission was higher among patients who received azacitidine plus venetoclax than among those who received azacitidine alone.”
Venetoclax promotes apoptosis in leukemia cells, Dr. Perl said. “To a certain extent, you can think of it as putting the rubber to the road in terms of what actually chemotherapy is designed to do, which is to make leukemic blasts apoptose. It does so without DNA damage and with much less toxicity to the patient. Therefore it can be added to any number of regimens – granted, with mild suppression, but with relatively little extramedullary toxicity.”
Dr. Perl noted that the venetoclax arm “showed a higher response rate than azacitidine in pretty much every subgroup that was looked at, whether patients had de novo leukemia, secondary leukemia, multiple mutational complements, various different karyotypes. The response rates on this study are as high as what we often will see with intensive chemotherapy.” He added that “the winning arm on this trial seems to hold up against any low-intensity therapy, and I would argue against many high-intensity therapies in older patients.”
As for other targeted agents, isocitrate dehydrogenase (IDH) inhibitors “are very promising drugs in the relapsed/refractory setting, which is primarily where these drugs are given. In regard to frontline treatment, “data are coming from a very small study, but they’re very encouraging. It’s hard to entirely say that we’re ready to change practice based on this. But it’s very encouraging – the idea that earlier use of a drug-targeting IDH mutation might lead to substantially better outcomes.”
Moving forward, he said, “we could put all of our eggs in one basket and use many active drugs [at] front line. Or we can perhaps be smart about sequencing these drugs one after another, or using more intensive approaches followed by maintenance approaches followed by more intensive approaches.”
This approach is similar to strategies in myeloma patients “who less and less are relying on an autologous transplant for durable control of their disease, and more and more are using low-intensity biologically targeted drugs,” he said.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
Dr. Perl reported numerous disclosures, including relationships with Daiichi Sankyo, Abbvie, and Astellas.
Novel therapies are poised to dramatically change frontline therapy for acute myeloid leukemia (AML), and they have the potential to replace chemotherapy, a hematologist/oncologist told colleagues at the virtual Acute Leukemia Forum of Hemedicus.
But more work needs to be done, noted Alexander Perl, MD, MS, associate professor at the University of Pennsylvania, Philadelphia. While advances have transformed AML treatment in the relapsed/refractory setting, “we’re just not seeing that substantive improvement” for newly diagnosed patients, he said. “We need to find the disease-modifying drugs that work in the relapsed/refractory setting and move those frontline. That’s where we’re going to see the transformations.”
Research suggests that low-intensity therapy holds tremendous promise, he said, “with the idea that we could make therapy much more tolerable for the vast majority of patients affected by AML, who, as we know, are older patients.”
Dr. Perl highlighted the 2020 VIALE-A study – venetoclax/azacitidine versus azacitidine/placebo – which reported that “in previously untreated patients who were ineligible for intensive chemotherapy, overall survival was longer and the incidence of remission was higher among patients who received azacitidine plus venetoclax than among those who received azacitidine alone.”
Venetoclax promotes apoptosis in leukemia cells, Dr. Perl said. “To a certain extent, you can think of it as putting the rubber to the road in terms of what actually chemotherapy is designed to do, which is to make leukemic blasts apoptose. It does so without DNA damage and with much less toxicity to the patient. Therefore it can be added to any number of regimens – granted, with mild suppression, but with relatively little extramedullary toxicity.”
Dr. Perl noted that the venetoclax arm “showed a higher response rate than azacitidine in pretty much every subgroup that was looked at, whether patients had de novo leukemia, secondary leukemia, multiple mutational complements, various different karyotypes. The response rates on this study are as high as what we often will see with intensive chemotherapy.” He added that “the winning arm on this trial seems to hold up against any low-intensity therapy, and I would argue against many high-intensity therapies in older patients.”
As for other targeted agents, isocitrate dehydrogenase (IDH) inhibitors “are very promising drugs in the relapsed/refractory setting, which is primarily where these drugs are given. In regard to frontline treatment, “data are coming from a very small study, but they’re very encouraging. It’s hard to entirely say that we’re ready to change practice based on this. But it’s very encouraging – the idea that earlier use of a drug-targeting IDH mutation might lead to substantially better outcomes.”
Moving forward, he said, “we could put all of our eggs in one basket and use many active drugs [at] front line. Or we can perhaps be smart about sequencing these drugs one after another, or using more intensive approaches followed by maintenance approaches followed by more intensive approaches.”
This approach is similar to strategies in myeloma patients “who less and less are relying on an autologous transplant for durable control of their disease, and more and more are using low-intensity biologically targeted drugs,” he said.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
Dr. Perl reported numerous disclosures, including relationships with Daiichi Sankyo, Abbvie, and Astellas.
Novel therapies are poised to dramatically change frontline therapy for acute myeloid leukemia (AML), and they have the potential to replace chemotherapy, a hematologist/oncologist told colleagues at the virtual Acute Leukemia Forum of Hemedicus.
But more work needs to be done, noted Alexander Perl, MD, MS, associate professor at the University of Pennsylvania, Philadelphia. While advances have transformed AML treatment in the relapsed/refractory setting, “we’re just not seeing that substantive improvement” for newly diagnosed patients, he said. “We need to find the disease-modifying drugs that work in the relapsed/refractory setting and move those frontline. That’s where we’re going to see the transformations.”
Research suggests that low-intensity therapy holds tremendous promise, he said, “with the idea that we could make therapy much more tolerable for the vast majority of patients affected by AML, who, as we know, are older patients.”
Dr. Perl highlighted the 2020 VIALE-A study – venetoclax/azacitidine versus azacitidine/placebo – which reported that “in previously untreated patients who were ineligible for intensive chemotherapy, overall survival was longer and the incidence of remission was higher among patients who received azacitidine plus venetoclax than among those who received azacitidine alone.”
Venetoclax promotes apoptosis in leukemia cells, Dr. Perl said. “To a certain extent, you can think of it as putting the rubber to the road in terms of what actually chemotherapy is designed to do, which is to make leukemic blasts apoptose. It does so without DNA damage and with much less toxicity to the patient. Therefore it can be added to any number of regimens – granted, with mild suppression, but with relatively little extramedullary toxicity.”
Dr. Perl noted that the venetoclax arm “showed a higher response rate than azacitidine in pretty much every subgroup that was looked at, whether patients had de novo leukemia, secondary leukemia, multiple mutational complements, various different karyotypes. The response rates on this study are as high as what we often will see with intensive chemotherapy.” He added that “the winning arm on this trial seems to hold up against any low-intensity therapy, and I would argue against many high-intensity therapies in older patients.”
As for other targeted agents, isocitrate dehydrogenase (IDH) inhibitors “are very promising drugs in the relapsed/refractory setting, which is primarily where these drugs are given. In regard to frontline treatment, “data are coming from a very small study, but they’re very encouraging. It’s hard to entirely say that we’re ready to change practice based on this. But it’s very encouraging – the idea that earlier use of a drug-targeting IDH mutation might lead to substantially better outcomes.”
Moving forward, he said, “we could put all of our eggs in one basket and use many active drugs [at] front line. Or we can perhaps be smart about sequencing these drugs one after another, or using more intensive approaches followed by maintenance approaches followed by more intensive approaches.”
This approach is similar to strategies in myeloma patients “who less and less are relying on an autologous transplant for durable control of their disease, and more and more are using low-intensity biologically targeted drugs,” he said.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
Dr. Perl reported numerous disclosures, including relationships with Daiichi Sankyo, Abbvie, and Astellas.
FROM ALF 2020
Standard treatment lacking in relapsed refractory AML
Despite a variety of options, patients with relapsed/refractory acute myeloid leukemia (AML) continue to face poor prognoses, and a standard of care remains elusive, a hematologist/oncologist told colleagues.
“Clearly we have a problem with this group of patients,” Ehab Atallah, MD, professor of medicine at Medical College of Wisconsin, Milwaukee, said in a presentation at the virtual Acute Leukemia Forum of Hemedicus. In regard to treatments, he added, “we still have multiple unanswered questions.”
As Dr. Atallah noted, a 2018 study of 3,012 patients – in 9 successive ECOG‐ACRIN trials for newly diagnosed AML from 1984-2008 – showed poor outcomes for relapsed/refractory patients. At a median follow-up of 9.7 years, 59.1% reached first complete remission (CR1), and 58.9% of those relapsed. In the relapsed patients, the median overall survival from relapse was 0.5 years, and the overall survival (OS) over 5 years was 10%.
“Even among patients who relapsed with better prognostic factors – age < 40 and CR1 > 12 months – there was no significant OS difference between the studies,” the study noted. “In conclusion, this large cohort appears to confirm that the survival of AML patients post relapse continues to be dismal and has not improved during the past quarter of a century.”
There isn’t a clear standard of care, said Dr. Atallah, as shown by a 2014 phase 3 study of elacytarabine vs. investigator choice in relapsed/refractory AML patients. The investigators chose seven treatment options for the control arm.
So how can physicians make the best decisions about treatment? A 2018 report finds that some factors do offer guidance about how well relapsed patients will do, Dr. Atallah said, including worse prognoses for higher age (>50 years), time to relapse (< 1 year), number of cycles of treatment needed to achieve remission (more than 1), and unfavorable cytogenetics. And, he said, “practically no one is cured when their leukemia relapses without stem cell transplantation.”
Also keep comorbidities in mind, he said, and consider previous therapies – not just the ones implemented prior to their induction but from all treatments they received: “How much anthracycline did they get? Do they still have room to receive any more anthracycline? Do they have any pulmonary complications from GVHD [graft versus host disease]?”
Another tool may be helpful. A 2013 study found that geriatric assessment predicted survival for older adults with AML who took induction chemotherapy, he said. “I’m pretty sure that this geriatric assessment would also have significant prognostic information for patients with relapsed refractory AML.”
Molecular changes add to the complexity of treatment for relapsed/refractory AML, Dr. Atallah said, in light of new molecularly targeted drugs. He pointed to a 2019 study that showed a slight increase in median overall survival (9.3 months vs. 5.6 months) for gilteritinib vs. salvage chemotherapy in relapsed/refractory patients with FLT3-mutated AML. Other studies have shown limited effects of ID1 inhibitors, he said.
In the big picture, “there are many patient-, disease-, and prior-therapy-related variables that are involved in our decisions plus donor availability, social support, whether they have a transplant before, what kind of treatment they got before the functional assessment, and comorbidities. Even with the current choices for relapsed/refractory AML, the overall survival remains poor. Enrollment in clinical trials would be the best option for these patients.”
Dr. Atallah disclosed ties with Jazz, Abbvie, Takeda, Celgene, and Novartis.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
SOURCE: “Why Is There No Standard of Care for Relapsed AML?” Acute Leukemia Forum of Hemedicus, Oct. 15, 2020.
Despite a variety of options, patients with relapsed/refractory acute myeloid leukemia (AML) continue to face poor prognoses, and a standard of care remains elusive, a hematologist/oncologist told colleagues.
“Clearly we have a problem with this group of patients,” Ehab Atallah, MD, professor of medicine at Medical College of Wisconsin, Milwaukee, said in a presentation at the virtual Acute Leukemia Forum of Hemedicus. In regard to treatments, he added, “we still have multiple unanswered questions.”
As Dr. Atallah noted, a 2018 study of 3,012 patients – in 9 successive ECOG‐ACRIN trials for newly diagnosed AML from 1984-2008 – showed poor outcomes for relapsed/refractory patients. At a median follow-up of 9.7 years, 59.1% reached first complete remission (CR1), and 58.9% of those relapsed. In the relapsed patients, the median overall survival from relapse was 0.5 years, and the overall survival (OS) over 5 years was 10%.
“Even among patients who relapsed with better prognostic factors – age < 40 and CR1 > 12 months – there was no significant OS difference between the studies,” the study noted. “In conclusion, this large cohort appears to confirm that the survival of AML patients post relapse continues to be dismal and has not improved during the past quarter of a century.”
There isn’t a clear standard of care, said Dr. Atallah, as shown by a 2014 phase 3 study of elacytarabine vs. investigator choice in relapsed/refractory AML patients. The investigators chose seven treatment options for the control arm.
So how can physicians make the best decisions about treatment? A 2018 report finds that some factors do offer guidance about how well relapsed patients will do, Dr. Atallah said, including worse prognoses for higher age (>50 years), time to relapse (< 1 year), number of cycles of treatment needed to achieve remission (more than 1), and unfavorable cytogenetics. And, he said, “practically no one is cured when their leukemia relapses without stem cell transplantation.”
Also keep comorbidities in mind, he said, and consider previous therapies – not just the ones implemented prior to their induction but from all treatments they received: “How much anthracycline did they get? Do they still have room to receive any more anthracycline? Do they have any pulmonary complications from GVHD [graft versus host disease]?”
Another tool may be helpful. A 2013 study found that geriatric assessment predicted survival for older adults with AML who took induction chemotherapy, he said. “I’m pretty sure that this geriatric assessment would also have significant prognostic information for patients with relapsed refractory AML.”
Molecular changes add to the complexity of treatment for relapsed/refractory AML, Dr. Atallah said, in light of new molecularly targeted drugs. He pointed to a 2019 study that showed a slight increase in median overall survival (9.3 months vs. 5.6 months) for gilteritinib vs. salvage chemotherapy in relapsed/refractory patients with FLT3-mutated AML. Other studies have shown limited effects of ID1 inhibitors, he said.
In the big picture, “there are many patient-, disease-, and prior-therapy-related variables that are involved in our decisions plus donor availability, social support, whether they have a transplant before, what kind of treatment they got before the functional assessment, and comorbidities. Even with the current choices for relapsed/refractory AML, the overall survival remains poor. Enrollment in clinical trials would be the best option for these patients.”
Dr. Atallah disclosed ties with Jazz, Abbvie, Takeda, Celgene, and Novartis.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
SOURCE: “Why Is There No Standard of Care for Relapsed AML?” Acute Leukemia Forum of Hemedicus, Oct. 15, 2020.
Despite a variety of options, patients with relapsed/refractory acute myeloid leukemia (AML) continue to face poor prognoses, and a standard of care remains elusive, a hematologist/oncologist told colleagues.
“Clearly we have a problem with this group of patients,” Ehab Atallah, MD, professor of medicine at Medical College of Wisconsin, Milwaukee, said in a presentation at the virtual Acute Leukemia Forum of Hemedicus. In regard to treatments, he added, “we still have multiple unanswered questions.”
As Dr. Atallah noted, a 2018 study of 3,012 patients – in 9 successive ECOG‐ACRIN trials for newly diagnosed AML from 1984-2008 – showed poor outcomes for relapsed/refractory patients. At a median follow-up of 9.7 years, 59.1% reached first complete remission (CR1), and 58.9% of those relapsed. In the relapsed patients, the median overall survival from relapse was 0.5 years, and the overall survival (OS) over 5 years was 10%.
“Even among patients who relapsed with better prognostic factors – age < 40 and CR1 > 12 months – there was no significant OS difference between the studies,” the study noted. “In conclusion, this large cohort appears to confirm that the survival of AML patients post relapse continues to be dismal and has not improved during the past quarter of a century.”
There isn’t a clear standard of care, said Dr. Atallah, as shown by a 2014 phase 3 study of elacytarabine vs. investigator choice in relapsed/refractory AML patients. The investigators chose seven treatment options for the control arm.
So how can physicians make the best decisions about treatment? A 2018 report finds that some factors do offer guidance about how well relapsed patients will do, Dr. Atallah said, including worse prognoses for higher age (>50 years), time to relapse (< 1 year), number of cycles of treatment needed to achieve remission (more than 1), and unfavorable cytogenetics. And, he said, “practically no one is cured when their leukemia relapses without stem cell transplantation.”
Also keep comorbidities in mind, he said, and consider previous therapies – not just the ones implemented prior to their induction but from all treatments they received: “How much anthracycline did they get? Do they still have room to receive any more anthracycline? Do they have any pulmonary complications from GVHD [graft versus host disease]?”
Another tool may be helpful. A 2013 study found that geriatric assessment predicted survival for older adults with AML who took induction chemotherapy, he said. “I’m pretty sure that this geriatric assessment would also have significant prognostic information for patients with relapsed refractory AML.”
Molecular changes add to the complexity of treatment for relapsed/refractory AML, Dr. Atallah said, in light of new molecularly targeted drugs. He pointed to a 2019 study that showed a slight increase in median overall survival (9.3 months vs. 5.6 months) for gilteritinib vs. salvage chemotherapy in relapsed/refractory patients with FLT3-mutated AML. Other studies have shown limited effects of ID1 inhibitors, he said.
In the big picture, “there are many patient-, disease-, and prior-therapy-related variables that are involved in our decisions plus donor availability, social support, whether they have a transplant before, what kind of treatment they got before the functional assessment, and comorbidities. Even with the current choices for relapsed/refractory AML, the overall survival remains poor. Enrollment in clinical trials would be the best option for these patients.”
Dr. Atallah disclosed ties with Jazz, Abbvie, Takeda, Celgene, and Novartis.
The Acute Leukemia Forum is held by Hemedicus, which is owned by the same company as this news organization.
SOURCE: “Why Is There No Standard of Care for Relapsed AML?” Acute Leukemia Forum of Hemedicus, Oct. 15, 2020.
FROM ALF 2020