User login
Dose-adjusted EPOCH and rituximab beneficial for selected older patients with high-risk DLBCL
Key clinical point: Sufficiently fit older patients with high-risk diffuse large B-cell lymphoma (DLBCL) achieve favorable outcomes with dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (DA-EPOCH-R); patients with a poor performance status (PS) develop unacceptable toxicity and require less intensive therapy.
Major finding: At 3 years, the progression-free survival (PFS) and overall survival (OS) rates were 53% and 58%, respectively, and the treatment-related mortality (TRM) rate was 13%. The 3-year PFS (58% vs 32%; P < .001) and OS (64% vs 33%; P = .007) rates were significantly higher and TRM rates were significantly lower (6% vs 43%; P < .001) among patients with PS 0-2 vs 3-4.
Study details: This multicenter retrospective real-life study included 120 patients aged ≥60 years with newly diagnosed high-risk DLBCL treated with a median of 6 DA-EPOCH-R cycles per patient.
Disclosures: No information on the source of funding or conflicts of interest was provided.
Source: Mitrovic Z et al. Dose-adjusted EPOCH and rituximab (DA-EPOCH-R) in older patients with high-risk aggressive diffuse large B-cell lymphoma: A real-life multicenter study by the Croatian Cooperative Group for Hematologic diseases (KroHem). Eur J Haematol. 2023 (Mar 20). Doi: 10.1111/ejh.13957
Key clinical point: Sufficiently fit older patients with high-risk diffuse large B-cell lymphoma (DLBCL) achieve favorable outcomes with dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (DA-EPOCH-R); patients with a poor performance status (PS) develop unacceptable toxicity and require less intensive therapy.
Major finding: At 3 years, the progression-free survival (PFS) and overall survival (OS) rates were 53% and 58%, respectively, and the treatment-related mortality (TRM) rate was 13%. The 3-year PFS (58% vs 32%; P < .001) and OS (64% vs 33%; P = .007) rates were significantly higher and TRM rates were significantly lower (6% vs 43%; P < .001) among patients with PS 0-2 vs 3-4.
Study details: This multicenter retrospective real-life study included 120 patients aged ≥60 years with newly diagnosed high-risk DLBCL treated with a median of 6 DA-EPOCH-R cycles per patient.
Disclosures: No information on the source of funding or conflicts of interest was provided.
Source: Mitrovic Z et al. Dose-adjusted EPOCH and rituximab (DA-EPOCH-R) in older patients with high-risk aggressive diffuse large B-cell lymphoma: A real-life multicenter study by the Croatian Cooperative Group for Hematologic diseases (KroHem). Eur J Haematol. 2023 (Mar 20). Doi: 10.1111/ejh.13957
Key clinical point: Sufficiently fit older patients with high-risk diffuse large B-cell lymphoma (DLBCL) achieve favorable outcomes with dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab (DA-EPOCH-R); patients with a poor performance status (PS) develop unacceptable toxicity and require less intensive therapy.
Major finding: At 3 years, the progression-free survival (PFS) and overall survival (OS) rates were 53% and 58%, respectively, and the treatment-related mortality (TRM) rate was 13%. The 3-year PFS (58% vs 32%; P < .001) and OS (64% vs 33%; P = .007) rates were significantly higher and TRM rates were significantly lower (6% vs 43%; P < .001) among patients with PS 0-2 vs 3-4.
Study details: This multicenter retrospective real-life study included 120 patients aged ≥60 years with newly diagnosed high-risk DLBCL treated with a median of 6 DA-EPOCH-R cycles per patient.
Disclosures: No information on the source of funding or conflicts of interest was provided.
Source: Mitrovic Z et al. Dose-adjusted EPOCH and rituximab (DA-EPOCH-R) in older patients with high-risk aggressive diffuse large B-cell lymphoma: A real-life multicenter study by the Croatian Cooperative Group for Hematologic diseases (KroHem). Eur J Haematol. 2023 (Mar 20). Doi: 10.1111/ejh.13957
Axi-cel a promising second-line treatment option for older patients with relapsed or refractory LBCL
Key clinical point: Axicabtagene ciloleucel (axi-cel) is an effective second-line curative-intent therapy with a manageable safety profile for patients aged ≥65 years with relapsed or refractory large B-cell lymphoma (LBCL).
Major finding: At a median follow-up of 24.3 months, the median event-free survival was significantly longer with axi-cel vs standard of care (SOC; 21.5 vs 2.5 months; hazard ratio 0.276; descriptive P < .0001). The grade ≥3 treatment-emergent adverse event rates were 94% and 82% with axi-cel and SOC, respectively.
Study details: Findings are from a preplanned analysis of 109 patients aged ≥65 years with relapsed or refractory LBCL from the ZUMA-7 trial who were randomly assigned to receive second-line axi-cel (n = 51) or SOC (n = 58; 2-3 cycles of chemoimmunotherapy followed by high-dose chemotherapy with autologous stem-cell transplantation).
Disclosures: This study was supported by Kite, a Gilead Company. Some authors reported ties with various organizations, including Kite and Gilead.
Source: Westin JR et al. Safety and efficacy of axicabtagene ciloleucel versus standard of care in patients 65 years of age or older with relapsed/refractory large B-cell lymphoma. Clin Cancer Res. 2023 (Mar 31). Doi: 10.1158/1078-0432.CCR-22-3136
Key clinical point: Axicabtagene ciloleucel (axi-cel) is an effective second-line curative-intent therapy with a manageable safety profile for patients aged ≥65 years with relapsed or refractory large B-cell lymphoma (LBCL).
Major finding: At a median follow-up of 24.3 months, the median event-free survival was significantly longer with axi-cel vs standard of care (SOC; 21.5 vs 2.5 months; hazard ratio 0.276; descriptive P < .0001). The grade ≥3 treatment-emergent adverse event rates were 94% and 82% with axi-cel and SOC, respectively.
Study details: Findings are from a preplanned analysis of 109 patients aged ≥65 years with relapsed or refractory LBCL from the ZUMA-7 trial who were randomly assigned to receive second-line axi-cel (n = 51) or SOC (n = 58; 2-3 cycles of chemoimmunotherapy followed by high-dose chemotherapy with autologous stem-cell transplantation).
Disclosures: This study was supported by Kite, a Gilead Company. Some authors reported ties with various organizations, including Kite and Gilead.
Source: Westin JR et al. Safety and efficacy of axicabtagene ciloleucel versus standard of care in patients 65 years of age or older with relapsed/refractory large B-cell lymphoma. Clin Cancer Res. 2023 (Mar 31). Doi: 10.1158/1078-0432.CCR-22-3136
Key clinical point: Axicabtagene ciloleucel (axi-cel) is an effective second-line curative-intent therapy with a manageable safety profile for patients aged ≥65 years with relapsed or refractory large B-cell lymphoma (LBCL).
Major finding: At a median follow-up of 24.3 months, the median event-free survival was significantly longer with axi-cel vs standard of care (SOC; 21.5 vs 2.5 months; hazard ratio 0.276; descriptive P < .0001). The grade ≥3 treatment-emergent adverse event rates were 94% and 82% with axi-cel and SOC, respectively.
Study details: Findings are from a preplanned analysis of 109 patients aged ≥65 years with relapsed or refractory LBCL from the ZUMA-7 trial who were randomly assigned to receive second-line axi-cel (n = 51) or SOC (n = 58; 2-3 cycles of chemoimmunotherapy followed by high-dose chemotherapy with autologous stem-cell transplantation).
Disclosures: This study was supported by Kite, a Gilead Company. Some authors reported ties with various organizations, including Kite and Gilead.
Source: Westin JR et al. Safety and efficacy of axicabtagene ciloleucel versus standard of care in patients 65 years of age or older with relapsed/refractory large B-cell lymphoma. Clin Cancer Res. 2023 (Mar 31). Doi: 10.1158/1078-0432.CCR-22-3136
Lenalidomide+rituximab+venetoclax a potential therapy option for untreated MCL
Key clinical point: The addition of venetoclax to lenalidomide plus rituximab therapy may provide an effective and safe combination for the treatment of unselected patients with untreated mantle cell lymphoma (MCL).
Major finding: All patients were escalated to the maximum tolerated dose of venetoclax (400 mg daily). The overall response and complete remission rates were 96% and 86%, respectively. After a median follow-up of 27.5 months, the median overall survival and progression-free survival were not reached. No dose-limiting toxicity event was observed.
Study details: This multicenter phase 1 study included 28 unselected adult patients with untreated MCL who received induction therapy with lenalidomide (daily on days 1-21 of each cycle), rituximab (weekly during cycle 1 until cycle 2 day 1), and venetoclax (escalated weekly up to 400 mg daily) for 6-12 cycles followed by maintenance therapy.
Disclosures: This study was funded by AbbVie and the University of Michigan Rogel Cancer Center. Some authors reported ties with various organizations, including AbbVie.
Source: Phillips TJ et al. Adding venetoclax to lenalidomide and rituximab is safe and effective in patients with untreated mantle cell lymphoma. Blood Adv. 2023 (Apr 4). Doi: 10.1182/bloodadvances.2023009992
Key clinical point: The addition of venetoclax to lenalidomide plus rituximab therapy may provide an effective and safe combination for the treatment of unselected patients with untreated mantle cell lymphoma (MCL).
Major finding: All patients were escalated to the maximum tolerated dose of venetoclax (400 mg daily). The overall response and complete remission rates were 96% and 86%, respectively. After a median follow-up of 27.5 months, the median overall survival and progression-free survival were not reached. No dose-limiting toxicity event was observed.
Study details: This multicenter phase 1 study included 28 unselected adult patients with untreated MCL who received induction therapy with lenalidomide (daily on days 1-21 of each cycle), rituximab (weekly during cycle 1 until cycle 2 day 1), and venetoclax (escalated weekly up to 400 mg daily) for 6-12 cycles followed by maintenance therapy.
Disclosures: This study was funded by AbbVie and the University of Michigan Rogel Cancer Center. Some authors reported ties with various organizations, including AbbVie.
Source: Phillips TJ et al. Adding venetoclax to lenalidomide and rituximab is safe and effective in patients with untreated mantle cell lymphoma. Blood Adv. 2023 (Apr 4). Doi: 10.1182/bloodadvances.2023009992
Key clinical point: The addition of venetoclax to lenalidomide plus rituximab therapy may provide an effective and safe combination for the treatment of unselected patients with untreated mantle cell lymphoma (MCL).
Major finding: All patients were escalated to the maximum tolerated dose of venetoclax (400 mg daily). The overall response and complete remission rates were 96% and 86%, respectively. After a median follow-up of 27.5 months, the median overall survival and progression-free survival were not reached. No dose-limiting toxicity event was observed.
Study details: This multicenter phase 1 study included 28 unselected adult patients with untreated MCL who received induction therapy with lenalidomide (daily on days 1-21 of each cycle), rituximab (weekly during cycle 1 until cycle 2 day 1), and venetoclax (escalated weekly up to 400 mg daily) for 6-12 cycles followed by maintenance therapy.
Disclosures: This study was funded by AbbVie and the University of Michigan Rogel Cancer Center. Some authors reported ties with various organizations, including AbbVie.
Source: Phillips TJ et al. Adding venetoclax to lenalidomide and rituximab is safe and effective in patients with untreated mantle cell lymphoma. Blood Adv. 2023 (Apr 4). Doi: 10.1182/bloodadvances.2023009992
Higher rates of hospitalization, blood disorders, and infections among patients with MCL
Key clinical point: Compared with matched control individuals, patients with mantle cell lymphoma (MCL) treated with or without high-dose chemotherapy with autologous stem cell transplantation (HD-ASCT) had higher hospitalization rates and relative risks for blood disorders and infections.
Major finding: Patients with MCL vs control individuals had a significantly increased incidence rate of outpatient (incidence rate ratio [IRR] 2.0; 95% CI 1.8-2.2) and inpatient (IRR 7.2; 95% CI 6.3-8.3) visits and relative risks for blood disorders (non-HD-ASCT: hazard ratio [HR] 9.84; 95% CI 6.91-14.00; HD-ASCT: HR 5.80; 95% CI 3.42-9.84) and infections (non-HD-ASCT: HR 4.66; 95% CI 3.62-5.99; HD-ASCT: HR 5.62; 95% CI 4.20-7.52).
Study details: Findings are from a population-based study including 620 adult patients with MCL who did (n = 247) or did not (n = 373) receive HD-ASCT and 6200 matched control individuals without MCL.
Disclosures: This study was supported by the Swedish Cancer Society. The authors reported ties with various organizations.
Source: Ekberg S et al. Late effects in patients with mantle cell lymphoma treated with or without autologous stem cell transplantation. Blood Adv. 2023;7(5):866-874 (Mar 14). Doi: 10.1182/bloodadvances.2022007241
Key clinical point: Compared with matched control individuals, patients with mantle cell lymphoma (MCL) treated with or without high-dose chemotherapy with autologous stem cell transplantation (HD-ASCT) had higher hospitalization rates and relative risks for blood disorders and infections.
Major finding: Patients with MCL vs control individuals had a significantly increased incidence rate of outpatient (incidence rate ratio [IRR] 2.0; 95% CI 1.8-2.2) and inpatient (IRR 7.2; 95% CI 6.3-8.3) visits and relative risks for blood disorders (non-HD-ASCT: hazard ratio [HR] 9.84; 95% CI 6.91-14.00; HD-ASCT: HR 5.80; 95% CI 3.42-9.84) and infections (non-HD-ASCT: HR 4.66; 95% CI 3.62-5.99; HD-ASCT: HR 5.62; 95% CI 4.20-7.52).
Study details: Findings are from a population-based study including 620 adult patients with MCL who did (n = 247) or did not (n = 373) receive HD-ASCT and 6200 matched control individuals without MCL.
Disclosures: This study was supported by the Swedish Cancer Society. The authors reported ties with various organizations.
Source: Ekberg S et al. Late effects in patients with mantle cell lymphoma treated with or without autologous stem cell transplantation. Blood Adv. 2023;7(5):866-874 (Mar 14). Doi: 10.1182/bloodadvances.2022007241
Key clinical point: Compared with matched control individuals, patients with mantle cell lymphoma (MCL) treated with or without high-dose chemotherapy with autologous stem cell transplantation (HD-ASCT) had higher hospitalization rates and relative risks for blood disorders and infections.
Major finding: Patients with MCL vs control individuals had a significantly increased incidence rate of outpatient (incidence rate ratio [IRR] 2.0; 95% CI 1.8-2.2) and inpatient (IRR 7.2; 95% CI 6.3-8.3) visits and relative risks for blood disorders (non-HD-ASCT: hazard ratio [HR] 9.84; 95% CI 6.91-14.00; HD-ASCT: HR 5.80; 95% CI 3.42-9.84) and infections (non-HD-ASCT: HR 4.66; 95% CI 3.62-5.99; HD-ASCT: HR 5.62; 95% CI 4.20-7.52).
Study details: Findings are from a population-based study including 620 adult patients with MCL who did (n = 247) or did not (n = 373) receive HD-ASCT and 6200 matched control individuals without MCL.
Disclosures: This study was supported by the Swedish Cancer Society. The authors reported ties with various organizations.
Source: Ekberg S et al. Late effects in patients with mantle cell lymphoma treated with or without autologous stem cell transplantation. Blood Adv. 2023;7(5):866-874 (Mar 14). Doi: 10.1182/bloodadvances.2022007241
Ibrutinib+BR a promising treatment option for newly diagnosed MCL ineligible for intensive therapy
Key clinical point: Compared with bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) and rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), ibrutinib plus bendamustine and rituximab (Ibru+BR) prolongs progression-free survival (PFS) in patients with newly diagnosed mantle cell lymphoma (MCL) who are ineligible for intensive therapy.
Major finding: Ibru+BR significantly improved PFS compared with VR-CAP (hazard ratio [HR] 0.55; P = .03) and R-CHOP (HR 0.35; P < .001). Adverse event risks were not significantly different in the Ibru+BR, VR-CAP, R-CHOP, and BR treatment arms.
Study details: The data come from a network meta-analysis of 3 studies involving 1459 patients with newly diagnosed MCL who were ineligible for intensive therapy and received first-line Ibru+BR, VR-CAP, R-CHOP, or BR.
Disclosures: This study did not report the source of funding. The authors declared no conflicts of interest.
Source: Sheng Z and Wang L. Superiority of ibrutinib plus bendamustine and rituximab in newly diagnosed patients with mantle-cell lymphoma ineligible for intensive therapy: A network meta-analysis. Eur J Haematol. 2023 (Mar 14). Doi: 10.1111/ejh.13953
Key clinical point: Compared with bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) and rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), ibrutinib plus bendamustine and rituximab (Ibru+BR) prolongs progression-free survival (PFS) in patients with newly diagnosed mantle cell lymphoma (MCL) who are ineligible for intensive therapy.
Major finding: Ibru+BR significantly improved PFS compared with VR-CAP (hazard ratio [HR] 0.55; P = .03) and R-CHOP (HR 0.35; P < .001). Adverse event risks were not significantly different in the Ibru+BR, VR-CAP, R-CHOP, and BR treatment arms.
Study details: The data come from a network meta-analysis of 3 studies involving 1459 patients with newly diagnosed MCL who were ineligible for intensive therapy and received first-line Ibru+BR, VR-CAP, R-CHOP, or BR.
Disclosures: This study did not report the source of funding. The authors declared no conflicts of interest.
Source: Sheng Z and Wang L. Superiority of ibrutinib plus bendamustine and rituximab in newly diagnosed patients with mantle-cell lymphoma ineligible for intensive therapy: A network meta-analysis. Eur J Haematol. 2023 (Mar 14). Doi: 10.1111/ejh.13953
Key clinical point: Compared with bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) and rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), ibrutinib plus bendamustine and rituximab (Ibru+BR) prolongs progression-free survival (PFS) in patients with newly diagnosed mantle cell lymphoma (MCL) who are ineligible for intensive therapy.
Major finding: Ibru+BR significantly improved PFS compared with VR-CAP (hazard ratio [HR] 0.55; P = .03) and R-CHOP (HR 0.35; P < .001). Adverse event risks were not significantly different in the Ibru+BR, VR-CAP, R-CHOP, and BR treatment arms.
Study details: The data come from a network meta-analysis of 3 studies involving 1459 patients with newly diagnosed MCL who were ineligible for intensive therapy and received first-line Ibru+BR, VR-CAP, R-CHOP, or BR.
Disclosures: This study did not report the source of funding. The authors declared no conflicts of interest.
Source: Sheng Z and Wang L. Superiority of ibrutinib plus bendamustine and rituximab in newly diagnosed patients with mantle-cell lymphoma ineligible for intensive therapy: A network meta-analysis. Eur J Haematol. 2023 (Mar 14). Doi: 10.1111/ejh.13953
Real-world data support the continued use of second-line targeted therapies in CLL
Key clinical point: Compared with chemoimmunotherapy, second-line targeted therapies improved treatment-free survival (TFS) and tended to improve overall survival (OS) in patients with chronic lymphocytic leukemia (CLL), including those who were frail and had comorbidities.
Major finding: The 3-year TFS and estimated OS rates were higher in patients receiving targeted therapies (63%, 95% CI 50%-76%; and 79%, 95% CI 68%-91%, respectively) vs fludarabine, cyclophosphamide, and rituximab or bendamustine and rituximab (FCR/BR; 37%,; 95% CI 26%-48%; and 70%, 95% CI 60%-81%, respectively) or chlorambucil+/−CD20-antibody (CD20Clb/Clb; 22%, 95% CI 10%-33%; and 60%, 95% CI 47%-74%, respectively). The grade ≥3 adverse event rate was similar with targeted treatment and FCR/BR.
Study details: This retrospective population-based real-world study included 286 patients with CLL who relapsed or were refractory to first-line treatment and received second-line targeted treatment (ibrutinib+venetoclax, venetoclax, venetoclax+rituximab/obinutuzumab, idelalisib, or idelalisib+rituximab), FCR/BR, or CD20Clb/Clb.
Disclosures: This study was partly supported by grants from Rigshospitalets Foundation. Some authors reported ties with various sources.
Source: Vainer N et al. Real-world outcomes upon second-line treatment in patients with chronic lymphocytic leukaemia. Br J Haematol. 2023 (Mar 10). Doi: 10.1111/bjh.18715
Key clinical point: Compared with chemoimmunotherapy, second-line targeted therapies improved treatment-free survival (TFS) and tended to improve overall survival (OS) in patients with chronic lymphocytic leukemia (CLL), including those who were frail and had comorbidities.
Major finding: The 3-year TFS and estimated OS rates were higher in patients receiving targeted therapies (63%, 95% CI 50%-76%; and 79%, 95% CI 68%-91%, respectively) vs fludarabine, cyclophosphamide, and rituximab or bendamustine and rituximab (FCR/BR; 37%,; 95% CI 26%-48%; and 70%, 95% CI 60%-81%, respectively) or chlorambucil+/−CD20-antibody (CD20Clb/Clb; 22%, 95% CI 10%-33%; and 60%, 95% CI 47%-74%, respectively). The grade ≥3 adverse event rate was similar with targeted treatment and FCR/BR.
Study details: This retrospective population-based real-world study included 286 patients with CLL who relapsed or were refractory to first-line treatment and received second-line targeted treatment (ibrutinib+venetoclax, venetoclax, venetoclax+rituximab/obinutuzumab, idelalisib, or idelalisib+rituximab), FCR/BR, or CD20Clb/Clb.
Disclosures: This study was partly supported by grants from Rigshospitalets Foundation. Some authors reported ties with various sources.
Source: Vainer N et al. Real-world outcomes upon second-line treatment in patients with chronic lymphocytic leukaemia. Br J Haematol. 2023 (Mar 10). Doi: 10.1111/bjh.18715
Key clinical point: Compared with chemoimmunotherapy, second-line targeted therapies improved treatment-free survival (TFS) and tended to improve overall survival (OS) in patients with chronic lymphocytic leukemia (CLL), including those who were frail and had comorbidities.
Major finding: The 3-year TFS and estimated OS rates were higher in patients receiving targeted therapies (63%, 95% CI 50%-76%; and 79%, 95% CI 68%-91%, respectively) vs fludarabine, cyclophosphamide, and rituximab or bendamustine and rituximab (FCR/BR; 37%,; 95% CI 26%-48%; and 70%, 95% CI 60%-81%, respectively) or chlorambucil+/−CD20-antibody (CD20Clb/Clb; 22%, 95% CI 10%-33%; and 60%, 95% CI 47%-74%, respectively). The grade ≥3 adverse event rate was similar with targeted treatment and FCR/BR.
Study details: This retrospective population-based real-world study included 286 patients with CLL who relapsed or were refractory to first-line treatment and received second-line targeted treatment (ibrutinib+venetoclax, venetoclax, venetoclax+rituximab/obinutuzumab, idelalisib, or idelalisib+rituximab), FCR/BR, or CD20Clb/Clb.
Disclosures: This study was partly supported by grants from Rigshospitalets Foundation. Some authors reported ties with various sources.
Source: Vainer N et al. Real-world outcomes upon second-line treatment in patients with chronic lymphocytic leukaemia. Br J Haematol. 2023 (Mar 10). Doi: 10.1111/bjh.18715
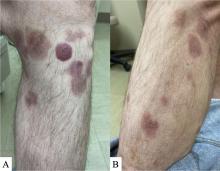
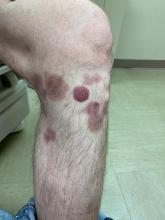
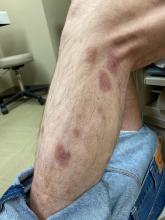
A 50-year-old White male presented with a 4- to 5-year history of progressively growing violaceous lesions on his left lower extremity
with scarce T-cells, classically presenting as rapidly progressive, plum-colored lesions on the lower extremities.1,2 CBCLs, with PCDLBCL-LT accounting for 4%, make up the minority of cutaneous lymphomas in the Western world.1-3 The leg type variant, typically demonstrating a female predominance and median age of onset in the 70s, is clinically aggressive and associated with a poorer prognosis, increased recurrence rate, and 40%-60% 5-year survival rate.1-5
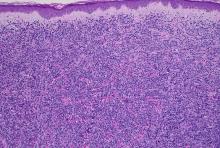
Histologically, this variant demonstrates a diffuse sheet-like growth of enlarged atypical B-cells distinctively separated from the epidermis by a prominent grenz zone. Classic PCDLBCL-LT immunophenotype includes B-cell markers CD20 and IgM; triple expressor phenotype indicating c-MYC, BCL-2, and BCL-6 positivity; as well as CD10 negativity, lack of BCL-2 rearrangement, and presence of a positive MYD-88 molecular result.
Other characteristic histopathological findings include positivity for post-germinal markers IRF4/MUM-1 and FOXP-1, positivity for additional B-cell markers, including CD79 and PAX5, and negativity of t(14;18) (q32;21).1,3-5
This case is of significant interest as it falls within the approximately 10% of PCDLBCL-LT cases demonstrating weak to negative MUM-1 staining, in addition to its presentation in a younger male individual.
While MUM-1 positivity is common in this subtype, its presence, or lack thereof, should not be looked at in isolation when evaluating diagnostic criteria, nor has it been shown to have a statistically significant effect on survival rate – in contrast to factors like lesion location on the leg versus non-leg lesions, multiple lesions at diagnosis, and dissemination to other sites.2,6
PCDLBCL-LT can uncommonly present in non-leg locations and only 10% depict associated B-symptoms, such as fatigue, night sweats, weight loss, or lymphadenopathy.2,6 First-line treatment is with the R-CHOP chemotherapy regimen – consisting of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone – although radiotherapy is sometimes considered in patients with a single small lesion.1,2
Because of possible cutaneous involvement beyond the legs, common lack of systemic symptoms, and variable immunophenotypes, this case of MUM-1 negative PCDLBCL-LT highlights the importance of a clinicopathological approach to differentiate the subtypes of CBCLs, allowing for proper and individualized stratification of risk, prognosis, and treatment.
This case was submitted and written by Marlee Hill, BS, Michael Franzetti, MD, Jeffrey McBride, MD, and Allison Hood, MD, of the University of Oklahoma, Oklahoma City. They also provided the photos. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Willemze R et al. Blood. 2019;133(16):1703-14.
2. Willemze R et al. Blood. 2005;105(10):3768-85.
3. Sukswai N et al. Pathology. 2020;52(1):53-67.
4. Hristov AC. Arch Pathol Lab Med. 2012;136(8):876-81.
5. Sokol L et al. Cancer Control. 2012;19(3):236-44.
6. Grange F et al. Arch Dermatol. 2007;143(9):1144-50.
with scarce T-cells, classically presenting as rapidly progressive, plum-colored lesions on the lower extremities.1,2 CBCLs, with PCDLBCL-LT accounting for 4%, make up the minority of cutaneous lymphomas in the Western world.1-3 The leg type variant, typically demonstrating a female predominance and median age of onset in the 70s, is clinically aggressive and associated with a poorer prognosis, increased recurrence rate, and 40%-60% 5-year survival rate.1-5
Histologically, this variant demonstrates a diffuse sheet-like growth of enlarged atypical B-cells distinctively separated from the epidermis by a prominent grenz zone. Classic PCDLBCL-LT immunophenotype includes B-cell markers CD20 and IgM; triple expressor phenotype indicating c-MYC, BCL-2, and BCL-6 positivity; as well as CD10 negativity, lack of BCL-2 rearrangement, and presence of a positive MYD-88 molecular result.
Other characteristic histopathological findings include positivity for post-germinal markers IRF4/MUM-1 and FOXP-1, positivity for additional B-cell markers, including CD79 and PAX5, and negativity of t(14;18) (q32;21).1,3-5
This case is of significant interest as it falls within the approximately 10% of PCDLBCL-LT cases demonstrating weak to negative MUM-1 staining, in addition to its presentation in a younger male individual.
While MUM-1 positivity is common in this subtype, its presence, or lack thereof, should not be looked at in isolation when evaluating diagnostic criteria, nor has it been shown to have a statistically significant effect on survival rate – in contrast to factors like lesion location on the leg versus non-leg lesions, multiple lesions at diagnosis, and dissemination to other sites.2,6
PCDLBCL-LT can uncommonly present in non-leg locations and only 10% depict associated B-symptoms, such as fatigue, night sweats, weight loss, or lymphadenopathy.2,6 First-line treatment is with the R-CHOP chemotherapy regimen – consisting of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone – although radiotherapy is sometimes considered in patients with a single small lesion.1,2
Because of possible cutaneous involvement beyond the legs, common lack of systemic symptoms, and variable immunophenotypes, this case of MUM-1 negative PCDLBCL-LT highlights the importance of a clinicopathological approach to differentiate the subtypes of CBCLs, allowing for proper and individualized stratification of risk, prognosis, and treatment.
This case was submitted and written by Marlee Hill, BS, Michael Franzetti, MD, Jeffrey McBride, MD, and Allison Hood, MD, of the University of Oklahoma, Oklahoma City. They also provided the photos. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Willemze R et al. Blood. 2019;133(16):1703-14.
2. Willemze R et al. Blood. 2005;105(10):3768-85.
3. Sukswai N et al. Pathology. 2020;52(1):53-67.
4. Hristov AC. Arch Pathol Lab Med. 2012;136(8):876-81.
5. Sokol L et al. Cancer Control. 2012;19(3):236-44.
6. Grange F et al. Arch Dermatol. 2007;143(9):1144-50.
with scarce T-cells, classically presenting as rapidly progressive, plum-colored lesions on the lower extremities.1,2 CBCLs, with PCDLBCL-LT accounting for 4%, make up the minority of cutaneous lymphomas in the Western world.1-3 The leg type variant, typically demonstrating a female predominance and median age of onset in the 70s, is clinically aggressive and associated with a poorer prognosis, increased recurrence rate, and 40%-60% 5-year survival rate.1-5
Histologically, this variant demonstrates a diffuse sheet-like growth of enlarged atypical B-cells distinctively separated from the epidermis by a prominent grenz zone. Classic PCDLBCL-LT immunophenotype includes B-cell markers CD20 and IgM; triple expressor phenotype indicating c-MYC, BCL-2, and BCL-6 positivity; as well as CD10 negativity, lack of BCL-2 rearrangement, and presence of a positive MYD-88 molecular result.
Other characteristic histopathological findings include positivity for post-germinal markers IRF4/MUM-1 and FOXP-1, positivity for additional B-cell markers, including CD79 and PAX5, and negativity of t(14;18) (q32;21).1,3-5
This case is of significant interest as it falls within the approximately 10% of PCDLBCL-LT cases demonstrating weak to negative MUM-1 staining, in addition to its presentation in a younger male individual.
While MUM-1 positivity is common in this subtype, its presence, or lack thereof, should not be looked at in isolation when evaluating diagnostic criteria, nor has it been shown to have a statistically significant effect on survival rate – in contrast to factors like lesion location on the leg versus non-leg lesions, multiple lesions at diagnosis, and dissemination to other sites.2,6
PCDLBCL-LT can uncommonly present in non-leg locations and only 10% depict associated B-symptoms, such as fatigue, night sweats, weight loss, or lymphadenopathy.2,6 First-line treatment is with the R-CHOP chemotherapy regimen – consisting of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone – although radiotherapy is sometimes considered in patients with a single small lesion.1,2
Because of possible cutaneous involvement beyond the legs, common lack of systemic symptoms, and variable immunophenotypes, this case of MUM-1 negative PCDLBCL-LT highlights the importance of a clinicopathological approach to differentiate the subtypes of CBCLs, allowing for proper and individualized stratification of risk, prognosis, and treatment.
This case was submitted and written by Marlee Hill, BS, Michael Franzetti, MD, Jeffrey McBride, MD, and Allison Hood, MD, of the University of Oklahoma, Oklahoma City. They also provided the photos. Donna Bilu Martin, MD, edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Willemze R et al. Blood. 2019;133(16):1703-14.
2. Willemze R et al. Blood. 2005;105(10):3768-85.
3. Sukswai N et al. Pathology. 2020;52(1):53-67.
4. Hristov AC. Arch Pathol Lab Med. 2012;136(8):876-81.
5. Sokol L et al. Cancer Control. 2012;19(3):236-44.
6. Grange F et al. Arch Dermatol. 2007;143(9):1144-50.
There was no cervical, axillary, or inguinal lymphadenopathy.
Commentary: Updates on the Treatment of Mantle Cell Lymphoma, April 2023
Mantle cell lymphoma (MCL) is an uncommon subtype of non-Hodgkin lymphoma (NHL) that is clinically heterogeneous, ranging from indolent to aggressive in nature. As with other subtypes of NHL, the treatment landscape is rapidly evolving.
Chemoimmunotherapy remains the standard first-line therapy for younger, fit patients. Although multiple induction regimens are used in this setting, it is typical to use a cytarabine-containing approach. Recently, the long-term analysis of the MCL Younger trial continued to demonstrate improved outcomes with this strategy.1 This phase 3 study included 497 patients aged ≥ 18 to < 66 years with previously untreated MCL who were randomly assigned to R-CHOP (cyclophosphamide, doxorubicin, prednisone, rituximab, and vincristine; n = 249) or R-DHAP (rituximab, dexamethasone, cytarabine, cisplatin; n = 248). After a median follow-up of 10.6 years, the R-DHAP vs R-CHOP arm continued to have a significantly longer time to treatment failure (hazard ratio [HR] 0.59; P = .038) and overall survival (Mantle Cell Lymphoma International Prognostic Index + Ki-67–adjusted HR 0.60; P = .0066).
Following chemoimmunotherapy, treatment for this patient population typically consists of consolidation with autologous stem cell transplantation (ASCT) and maintenance rituximab.2 Recently, the role of ASCT has been called into question.3 Preliminary data from the phase 3 TRIANGLE study demonstrated improvement in outcomes when the Bruton tyrosine kinase (BTK) inhibitor ibrutinib was added to chemoimmunotherapy, regardless of whether patients received ASCT.4 Additional studies evaluating the role of transplantation, particularly among patients who are minimal residual disease negative after chemoimmunotherapy, are ongoing (NCT03267433).
Options continue to expand in the relapsed/refractory setting. The chimeric antigen receptor (CAR) T-cell therapy, brexucabtagene autoleucel (brexu-cel), was approved by the US Food and Drug Administration for relapsed/refractory MCL on the basis of the results of the ZUMA-2 study.5 Recently, a multicenter, retrospective study demonstrated promising efficacy in the real world as well (Wang et al). This study was performed across 16 medical centers and included 189 patients with relapsed/refractory MCL who underwent leukapheresis for commercial manufacturing of brexu-cel, of which 168 received brexu-cel infusion. Of all patients receiving leukapheresis, 149 (79%) would not have met the eligibility criteria for ZUMA-2. At a median follow-up of 14.3 months after infusion, the best overall and complete response rates were 90% and 82%, respectively. The 6- and 12-month progression-free survival (PFS) rates were 69% (95% CI 61%-75%) and 59% (95% CI 51%-66%), respectively. This approach, however, was associated with significant toxicity, with a nonrelapse mortality rate of 9.1% at 1 year, primarily because of infections. The grade ≥ 3 cytokine release syndrome and neurotoxicity rates were 8% and 32%, respectively. Despite risks, this study confirms the role of CAR T-cell therapy for patients with relapsed/refractory MCL.
Other options in the relapsed setting include BTK and anti-apoptotic protein B-cell lymphoma (BCL-2) inhibitors. Although venetoclax, a BCL-2 inhibitor, has demonstrated activity in MCL in early-phase clinical trials, the role of this drug in clinical practice remains unclear.6,7 A recent multicenter, retrospective study evaluated the use of venetoclax in 81 adult patients with relapsed/refractory MCL, most of whom were heavily pretreated (median of three prior treatments) and had high-risk features, including high Ki-67 and TP53 alterations, who received venetoclax without (n = 50) or with (n = 31) other agents (Sawalha et al). In this study, venetoclax resulted in a good overall response rate (ORR) but short PFS. At a median follow-up of 16.4 months, patients had a median PFS and overall survival of 3.7 months (95% CI 2.3-5.6) and 12.5 months (95% CI 6.2-28.2), respectively, and an ORR of 40%. Studies of venetoclax in earlier lines of therapy and in combination with other agents are ongoing. There may also be a role for this treatment as a bridge to more definitive therapies, including CAR T-cell therapy or allogeneic stem cell transplantation. Other studies that are evaluating the role of bispecific antibodies and antibody drug conjugates are also underway, suggesting the potential for additional options in this patient population.
Additional References
1. Hermine O, Jiang L, Walewski J, et al. High-dose cytarabine and autologous stem-cell transplantation in mantle cell lymphoma: Long-term follow-up of the randomized Mantle Cell Lymphoma Younger Trial of the European Mantle Cell Lymphoma Network. J Clin Oncol. 2023;41:479-484. doi: 10.1200/JCO.22.01780
2. Le Gouill S, Thieblemont C, Oberic L, et al. Rituximab after autologous stem-cell transplantation in mantle-cell lymphoma. N Engl J Med. 2017;377:1250-1260. doi: 10.1056/NEJMoa1701769
3. Martin P, Cohen JB, Wang M, et al. Treatment outcomes and roles of transplantation and maintenance rituximab in patients with previously untreated mantle cell lymphoma: Results from large real-world cohorts. J Clin Oncol. 2023;41:541-554. doi: 10.1200/JCO.21.02698
4. Dreyling M, Doorduijn JK, Gine E, et al. Efficacy and safety of ibrutinib combined with standard first-line treatment or as substitute for autologous stem cell transplantation in younger patients with mantle cell lymphoma: Results from the randomized Triangle Trial by the European MCL Network. Blood. 2022;140(Suppl 1):1-3. doi: 10.1182/blood-2022-163018
5. Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-Cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382:1331-1342. doi: 10.1056/NEJMoa1914347
6. Davids MS, Roberts AW, Seymour JF, et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol. 2017;35:826-833. doi: 10.1200/JCO.2016.70.4320
7. Tam CS, Anderson MA, Pott C, et al. Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N Engl J Med. 2018;378:1211-1223. doi: 10.1056/NEJMoa1715519
Mantle cell lymphoma (MCL) is an uncommon subtype of non-Hodgkin lymphoma (NHL) that is clinically heterogeneous, ranging from indolent to aggressive in nature. As with other subtypes of NHL, the treatment landscape is rapidly evolving.
Chemoimmunotherapy remains the standard first-line therapy for younger, fit patients. Although multiple induction regimens are used in this setting, it is typical to use a cytarabine-containing approach. Recently, the long-term analysis of the MCL Younger trial continued to demonstrate improved outcomes with this strategy.1 This phase 3 study included 497 patients aged ≥ 18 to < 66 years with previously untreated MCL who were randomly assigned to R-CHOP (cyclophosphamide, doxorubicin, prednisone, rituximab, and vincristine; n = 249) or R-DHAP (rituximab, dexamethasone, cytarabine, cisplatin; n = 248). After a median follow-up of 10.6 years, the R-DHAP vs R-CHOP arm continued to have a significantly longer time to treatment failure (hazard ratio [HR] 0.59; P = .038) and overall survival (Mantle Cell Lymphoma International Prognostic Index + Ki-67–adjusted HR 0.60; P = .0066).
Following chemoimmunotherapy, treatment for this patient population typically consists of consolidation with autologous stem cell transplantation (ASCT) and maintenance rituximab.2 Recently, the role of ASCT has been called into question.3 Preliminary data from the phase 3 TRIANGLE study demonstrated improvement in outcomes when the Bruton tyrosine kinase (BTK) inhibitor ibrutinib was added to chemoimmunotherapy, regardless of whether patients received ASCT.4 Additional studies evaluating the role of transplantation, particularly among patients who are minimal residual disease negative after chemoimmunotherapy, are ongoing (NCT03267433).
Options continue to expand in the relapsed/refractory setting. The chimeric antigen receptor (CAR) T-cell therapy, brexucabtagene autoleucel (brexu-cel), was approved by the US Food and Drug Administration for relapsed/refractory MCL on the basis of the results of the ZUMA-2 study.5 Recently, a multicenter, retrospective study demonstrated promising efficacy in the real world as well (Wang et al). This study was performed across 16 medical centers and included 189 patients with relapsed/refractory MCL who underwent leukapheresis for commercial manufacturing of brexu-cel, of which 168 received brexu-cel infusion. Of all patients receiving leukapheresis, 149 (79%) would not have met the eligibility criteria for ZUMA-2. At a median follow-up of 14.3 months after infusion, the best overall and complete response rates were 90% and 82%, respectively. The 6- and 12-month progression-free survival (PFS) rates were 69% (95% CI 61%-75%) and 59% (95% CI 51%-66%), respectively. This approach, however, was associated with significant toxicity, with a nonrelapse mortality rate of 9.1% at 1 year, primarily because of infections. The grade ≥ 3 cytokine release syndrome and neurotoxicity rates were 8% and 32%, respectively. Despite risks, this study confirms the role of CAR T-cell therapy for patients with relapsed/refractory MCL.
Other options in the relapsed setting include BTK and anti-apoptotic protein B-cell lymphoma (BCL-2) inhibitors. Although venetoclax, a BCL-2 inhibitor, has demonstrated activity in MCL in early-phase clinical trials, the role of this drug in clinical practice remains unclear.6,7 A recent multicenter, retrospective study evaluated the use of venetoclax in 81 adult patients with relapsed/refractory MCL, most of whom were heavily pretreated (median of three prior treatments) and had high-risk features, including high Ki-67 and TP53 alterations, who received venetoclax without (n = 50) or with (n = 31) other agents (Sawalha et al). In this study, venetoclax resulted in a good overall response rate (ORR) but short PFS. At a median follow-up of 16.4 months, patients had a median PFS and overall survival of 3.7 months (95% CI 2.3-5.6) and 12.5 months (95% CI 6.2-28.2), respectively, and an ORR of 40%. Studies of venetoclax in earlier lines of therapy and in combination with other agents are ongoing. There may also be a role for this treatment as a bridge to more definitive therapies, including CAR T-cell therapy or allogeneic stem cell transplantation. Other studies that are evaluating the role of bispecific antibodies and antibody drug conjugates are also underway, suggesting the potential for additional options in this patient population.
Additional References
1. Hermine O, Jiang L, Walewski J, et al. High-dose cytarabine and autologous stem-cell transplantation in mantle cell lymphoma: Long-term follow-up of the randomized Mantle Cell Lymphoma Younger Trial of the European Mantle Cell Lymphoma Network. J Clin Oncol. 2023;41:479-484. doi: 10.1200/JCO.22.01780
2. Le Gouill S, Thieblemont C, Oberic L, et al. Rituximab after autologous stem-cell transplantation in mantle-cell lymphoma. N Engl J Med. 2017;377:1250-1260. doi: 10.1056/NEJMoa1701769
3. Martin P, Cohen JB, Wang M, et al. Treatment outcomes and roles of transplantation and maintenance rituximab in patients with previously untreated mantle cell lymphoma: Results from large real-world cohorts. J Clin Oncol. 2023;41:541-554. doi: 10.1200/JCO.21.02698
4. Dreyling M, Doorduijn JK, Gine E, et al. Efficacy and safety of ibrutinib combined with standard first-line treatment or as substitute for autologous stem cell transplantation in younger patients with mantle cell lymphoma: Results from the randomized Triangle Trial by the European MCL Network. Blood. 2022;140(Suppl 1):1-3. doi: 10.1182/blood-2022-163018
5. Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-Cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382:1331-1342. doi: 10.1056/NEJMoa1914347
6. Davids MS, Roberts AW, Seymour JF, et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol. 2017;35:826-833. doi: 10.1200/JCO.2016.70.4320
7. Tam CS, Anderson MA, Pott C, et al. Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N Engl J Med. 2018;378:1211-1223. doi: 10.1056/NEJMoa1715519
Mantle cell lymphoma (MCL) is an uncommon subtype of non-Hodgkin lymphoma (NHL) that is clinically heterogeneous, ranging from indolent to aggressive in nature. As with other subtypes of NHL, the treatment landscape is rapidly evolving.
Chemoimmunotherapy remains the standard first-line therapy for younger, fit patients. Although multiple induction regimens are used in this setting, it is typical to use a cytarabine-containing approach. Recently, the long-term analysis of the MCL Younger trial continued to demonstrate improved outcomes with this strategy.1 This phase 3 study included 497 patients aged ≥ 18 to < 66 years with previously untreated MCL who were randomly assigned to R-CHOP (cyclophosphamide, doxorubicin, prednisone, rituximab, and vincristine; n = 249) or R-DHAP (rituximab, dexamethasone, cytarabine, cisplatin; n = 248). After a median follow-up of 10.6 years, the R-DHAP vs R-CHOP arm continued to have a significantly longer time to treatment failure (hazard ratio [HR] 0.59; P = .038) and overall survival (Mantle Cell Lymphoma International Prognostic Index + Ki-67–adjusted HR 0.60; P = .0066).
Following chemoimmunotherapy, treatment for this patient population typically consists of consolidation with autologous stem cell transplantation (ASCT) and maintenance rituximab.2 Recently, the role of ASCT has been called into question.3 Preliminary data from the phase 3 TRIANGLE study demonstrated improvement in outcomes when the Bruton tyrosine kinase (BTK) inhibitor ibrutinib was added to chemoimmunotherapy, regardless of whether patients received ASCT.4 Additional studies evaluating the role of transplantation, particularly among patients who are minimal residual disease negative after chemoimmunotherapy, are ongoing (NCT03267433).
Options continue to expand in the relapsed/refractory setting. The chimeric antigen receptor (CAR) T-cell therapy, brexucabtagene autoleucel (brexu-cel), was approved by the US Food and Drug Administration for relapsed/refractory MCL on the basis of the results of the ZUMA-2 study.5 Recently, a multicenter, retrospective study demonstrated promising efficacy in the real world as well (Wang et al). This study was performed across 16 medical centers and included 189 patients with relapsed/refractory MCL who underwent leukapheresis for commercial manufacturing of brexu-cel, of which 168 received brexu-cel infusion. Of all patients receiving leukapheresis, 149 (79%) would not have met the eligibility criteria for ZUMA-2. At a median follow-up of 14.3 months after infusion, the best overall and complete response rates were 90% and 82%, respectively. The 6- and 12-month progression-free survival (PFS) rates were 69% (95% CI 61%-75%) and 59% (95% CI 51%-66%), respectively. This approach, however, was associated with significant toxicity, with a nonrelapse mortality rate of 9.1% at 1 year, primarily because of infections. The grade ≥ 3 cytokine release syndrome and neurotoxicity rates were 8% and 32%, respectively. Despite risks, this study confirms the role of CAR T-cell therapy for patients with relapsed/refractory MCL.
Other options in the relapsed setting include BTK and anti-apoptotic protein B-cell lymphoma (BCL-2) inhibitors. Although venetoclax, a BCL-2 inhibitor, has demonstrated activity in MCL in early-phase clinical trials, the role of this drug in clinical practice remains unclear.6,7 A recent multicenter, retrospective study evaluated the use of venetoclax in 81 adult patients with relapsed/refractory MCL, most of whom were heavily pretreated (median of three prior treatments) and had high-risk features, including high Ki-67 and TP53 alterations, who received venetoclax without (n = 50) or with (n = 31) other agents (Sawalha et al). In this study, venetoclax resulted in a good overall response rate (ORR) but short PFS. At a median follow-up of 16.4 months, patients had a median PFS and overall survival of 3.7 months (95% CI 2.3-5.6) and 12.5 months (95% CI 6.2-28.2), respectively, and an ORR of 40%. Studies of venetoclax in earlier lines of therapy and in combination with other agents are ongoing. There may also be a role for this treatment as a bridge to more definitive therapies, including CAR T-cell therapy or allogeneic stem cell transplantation. Other studies that are evaluating the role of bispecific antibodies and antibody drug conjugates are also underway, suggesting the potential for additional options in this patient population.
Additional References
1. Hermine O, Jiang L, Walewski J, et al. High-dose cytarabine and autologous stem-cell transplantation in mantle cell lymphoma: Long-term follow-up of the randomized Mantle Cell Lymphoma Younger Trial of the European Mantle Cell Lymphoma Network. J Clin Oncol. 2023;41:479-484. doi: 10.1200/JCO.22.01780
2. Le Gouill S, Thieblemont C, Oberic L, et al. Rituximab after autologous stem-cell transplantation in mantle-cell lymphoma. N Engl J Med. 2017;377:1250-1260. doi: 10.1056/NEJMoa1701769
3. Martin P, Cohen JB, Wang M, et al. Treatment outcomes and roles of transplantation and maintenance rituximab in patients with previously untreated mantle cell lymphoma: Results from large real-world cohorts. J Clin Oncol. 2023;41:541-554. doi: 10.1200/JCO.21.02698
4. Dreyling M, Doorduijn JK, Gine E, et al. Efficacy and safety of ibrutinib combined with standard first-line treatment or as substitute for autologous stem cell transplantation in younger patients with mantle cell lymphoma: Results from the randomized Triangle Trial by the European MCL Network. Blood. 2022;140(Suppl 1):1-3. doi: 10.1182/blood-2022-163018
5. Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-Cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382:1331-1342. doi: 10.1056/NEJMoa1914347
6. Davids MS, Roberts AW, Seymour JF, et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol. 2017;35:826-833. doi: 10.1200/JCO.2016.70.4320
7. Tam CS, Anderson MA, Pott C, et al. Ibrutinib plus venetoclax for the treatment of mantle-cell lymphoma. N Engl J Med. 2018;378:1211-1223. doi: 10.1056/NEJMoa1715519
B-cell cancers: Sparse insight into preventing infections
Researchers found just 22 randomized controlled studies into prophylactic strategies, with several of them conducted prior to 2000. According to the report, published in Blood Advances, the studies together only evaluated a few thousand participants.
Reliable findings are so sparse that study coauthor Zoe McQuilten, MBBS, PhD, MD, a hematologist at Monash University, Melbourne, said “we simply don’t know” which preventive strategy is most effective. This is especially worrisome because more patients will survive their cancers and “be at risk of infection or have significant cytopenias and will experience impaired quality of life as a result,” she said in an interview.
The study authors launched the analysis to better understand the evidence regarding infection prevention and to guide the development of clinical trials, study coauthor Robert Weinkove, MBBS, PhD, a hematologist at Malaghan Institute of Medical Research, Wellington, New Zealand, said in an interview.
As he explained, targeted therapies have revolutionized the treatment of some B-cell cancers. They also have boosted the number of patients who survive the diseases yet still have profound hypogammaglobulinemia.
“Indeed, we may soon reach the point at which infection, and not tumor progression, is the leading cause of death for patients with certain B-cell cancers,” he said. “The evidence base for managing hypogammaglobulinemia is largely based on randomized trials of immunoglobulin replacement conducted in the 1980s and early 1990s, before the advent of B cell–targeted therapies. Immunoglobulin replacement is a costly intervention, and many countries are facing a shortage of immunoglobulin.”
The report authors identified 22 total randomized controlled trials, including one led by Dr. McQuilten: 8 studies into prophylactic immunoglobulin (n = 370; all but 1 study published prior to 2000), 5 into prophylactic antibiotics (n = 1,587), 7 into vaccination (n = 3,996), and 1 comparing immunoglobulin versus antibiotics (n = 60).
No evidence was found to support a lowering of risk by prophylactic antibiotics, although they caused adverse events.
Prophylactic immunoglobulin also caused adverse events, but a meta-analysis found that it reduced the risk of clinically documented infection by 28% (n = 2 trials; relative risk, 0.72; 95% confidence interval, 0.54-0.96). Three trials reported adverse events and found a higher risk overall (RR, 2.23; 95% CI, 1.67-2.99).
Varicella zoster virus vaccination reduced the risk of one or more infections by 63% (n = 5 trials, RR, 0.37; 95% CI, 0.30-0.45, n = 3,515). Prophylactic antibiotics did not reduce the risk.
No intervention reduced all-cause mortality.
“Our findings should be interpreted with caution, Dr. McQuilten said, “because of the low number of patients, high risk of bias in the included studies, and lack of contemporary data applicable to the current standard of care for such patients.”
The lack of useful data is surprising, she said, especially considering “how commonly these interventions are used in current clinical practice and the cost and supply constraints for immunoglobulin. Given the variation in international guidelines, rising global demand and cost of immunoglobulin, and concerns regarding antimicrobial resistance, more evidence is needed to inform infection prevention strategies for this patient population.”
More data is expected soon. One ongoing study is examining intravenous immunoglobulin versus placebo in patients with CLL. It’s expected to be completed in September 2023.
What should clinicians do for now? “Given the lack of a proven survival benefit in favor of prophylactic immunoglobulin replacement, one strategy is to maximize use of vaccination and to educate both patients and clinicians regarding the need for early treatment of infections,” Dr. Weinkove said. “For people who have recurrent or severe infections despite these measures, both immunoglobulin replacement and prophylactic antibiotics are clinical options. It would be reasonable to take account of patient preference, logistical considerations, and reimbursement and availability in deciding between these options.”
He added that, “for people with severe hypogammaglobulinemia who experience recurrent or severe infections despite prophylactic antibiotics, switching to immunoglobulin replacement would be appropriate. We advocate enrollment in clinical trials, if possible.”
In an interview, Juthaporn Cowan, MD, PhD, an infectious disease physician with the University of Ottawa, said many patients with B-cell lymphomas develop acquired hypogammaglobulinemia. “Patients tend to get prolonged colds, frequent sinusitis, bronchitis, or pneumonia. Some can end up with severe infection. Many patients told me that, even though their cancer is cured or in remission, quality of life is still quite poor due to these infections and fatigue.”
Dr. Cowan said the new report is somewhat useful, although “concluding that vaccination reduces infection is misleading. Vaccination reduces the infection that patients were vaccinated against. Patients who received Shingrix will have less shingles but will continue to have bronchitis and other infections.”
As for advice for clinicians, she said preventing acquired hypogammaglobulinemia is difficult since it can be caused by the malignancies, by treatment, or both. “The other item to consider is that we do not know how long we should continue [immunoglobulin] treatment in these patients. I have a patient post CAR [chimeric antigen receptor] T therapy who still does not have B-cell 5-6 years after CAR T, while I have lymphoma patients who could safely discontinue [immunoglobulin] treatment in a few years.”
Dr. Cowan added that patients on immunoglobulin treatment can still get opportunistic infections from cytomegalovirus or herpes simplex virus “because the mechanism of host defense against these infections is different. Antimicrobial prophylaxis should still be considered as vaccination is not available for every single potential opportunistic infection.”
Australia funded the research through the National Blood Authority. Dr. McQuilten and Dr. Weinkove reported no disclosures. Other report authors disclosed ties with Aegros, CSL Behring, Janssen, AbbVie, and BeiGene. Monash University has received funding for unrelated projects from CSL Behring. Dr. Cowan reports honoraria from Takeda, CSL Behring, Octapharma, GlaxoSmithKline, Merck, and AstraZeneca.
Researchers found just 22 randomized controlled studies into prophylactic strategies, with several of them conducted prior to 2000. According to the report, published in Blood Advances, the studies together only evaluated a few thousand participants.
Reliable findings are so sparse that study coauthor Zoe McQuilten, MBBS, PhD, MD, a hematologist at Monash University, Melbourne, said “we simply don’t know” which preventive strategy is most effective. This is especially worrisome because more patients will survive their cancers and “be at risk of infection or have significant cytopenias and will experience impaired quality of life as a result,” she said in an interview.
The study authors launched the analysis to better understand the evidence regarding infection prevention and to guide the development of clinical trials, study coauthor Robert Weinkove, MBBS, PhD, a hematologist at Malaghan Institute of Medical Research, Wellington, New Zealand, said in an interview.
As he explained, targeted therapies have revolutionized the treatment of some B-cell cancers. They also have boosted the number of patients who survive the diseases yet still have profound hypogammaglobulinemia.
“Indeed, we may soon reach the point at which infection, and not tumor progression, is the leading cause of death for patients with certain B-cell cancers,” he said. “The evidence base for managing hypogammaglobulinemia is largely based on randomized trials of immunoglobulin replacement conducted in the 1980s and early 1990s, before the advent of B cell–targeted therapies. Immunoglobulin replacement is a costly intervention, and many countries are facing a shortage of immunoglobulin.”
The report authors identified 22 total randomized controlled trials, including one led by Dr. McQuilten: 8 studies into prophylactic immunoglobulin (n = 370; all but 1 study published prior to 2000), 5 into prophylactic antibiotics (n = 1,587), 7 into vaccination (n = 3,996), and 1 comparing immunoglobulin versus antibiotics (n = 60).
No evidence was found to support a lowering of risk by prophylactic antibiotics, although they caused adverse events.
Prophylactic immunoglobulin also caused adverse events, but a meta-analysis found that it reduced the risk of clinically documented infection by 28% (n = 2 trials; relative risk, 0.72; 95% confidence interval, 0.54-0.96). Three trials reported adverse events and found a higher risk overall (RR, 2.23; 95% CI, 1.67-2.99).
Varicella zoster virus vaccination reduced the risk of one or more infections by 63% (n = 5 trials, RR, 0.37; 95% CI, 0.30-0.45, n = 3,515). Prophylactic antibiotics did not reduce the risk.
No intervention reduced all-cause mortality.
“Our findings should be interpreted with caution, Dr. McQuilten said, “because of the low number of patients, high risk of bias in the included studies, and lack of contemporary data applicable to the current standard of care for such patients.”
The lack of useful data is surprising, she said, especially considering “how commonly these interventions are used in current clinical practice and the cost and supply constraints for immunoglobulin. Given the variation in international guidelines, rising global demand and cost of immunoglobulin, and concerns regarding antimicrobial resistance, more evidence is needed to inform infection prevention strategies for this patient population.”
More data is expected soon. One ongoing study is examining intravenous immunoglobulin versus placebo in patients with CLL. It’s expected to be completed in September 2023.
What should clinicians do for now? “Given the lack of a proven survival benefit in favor of prophylactic immunoglobulin replacement, one strategy is to maximize use of vaccination and to educate both patients and clinicians regarding the need for early treatment of infections,” Dr. Weinkove said. “For people who have recurrent or severe infections despite these measures, both immunoglobulin replacement and prophylactic antibiotics are clinical options. It would be reasonable to take account of patient preference, logistical considerations, and reimbursement and availability in deciding between these options.”
He added that, “for people with severe hypogammaglobulinemia who experience recurrent or severe infections despite prophylactic antibiotics, switching to immunoglobulin replacement would be appropriate. We advocate enrollment in clinical trials, if possible.”
In an interview, Juthaporn Cowan, MD, PhD, an infectious disease physician with the University of Ottawa, said many patients with B-cell lymphomas develop acquired hypogammaglobulinemia. “Patients tend to get prolonged colds, frequent sinusitis, bronchitis, or pneumonia. Some can end up with severe infection. Many patients told me that, even though their cancer is cured or in remission, quality of life is still quite poor due to these infections and fatigue.”
Dr. Cowan said the new report is somewhat useful, although “concluding that vaccination reduces infection is misleading. Vaccination reduces the infection that patients were vaccinated against. Patients who received Shingrix will have less shingles but will continue to have bronchitis and other infections.”
As for advice for clinicians, she said preventing acquired hypogammaglobulinemia is difficult since it can be caused by the malignancies, by treatment, or both. “The other item to consider is that we do not know how long we should continue [immunoglobulin] treatment in these patients. I have a patient post CAR [chimeric antigen receptor] T therapy who still does not have B-cell 5-6 years after CAR T, while I have lymphoma patients who could safely discontinue [immunoglobulin] treatment in a few years.”
Dr. Cowan added that patients on immunoglobulin treatment can still get opportunistic infections from cytomegalovirus or herpes simplex virus “because the mechanism of host defense against these infections is different. Antimicrobial prophylaxis should still be considered as vaccination is not available for every single potential opportunistic infection.”
Australia funded the research through the National Blood Authority. Dr. McQuilten and Dr. Weinkove reported no disclosures. Other report authors disclosed ties with Aegros, CSL Behring, Janssen, AbbVie, and BeiGene. Monash University has received funding for unrelated projects from CSL Behring. Dr. Cowan reports honoraria from Takeda, CSL Behring, Octapharma, GlaxoSmithKline, Merck, and AstraZeneca.
Researchers found just 22 randomized controlled studies into prophylactic strategies, with several of them conducted prior to 2000. According to the report, published in Blood Advances, the studies together only evaluated a few thousand participants.
Reliable findings are so sparse that study coauthor Zoe McQuilten, MBBS, PhD, MD, a hematologist at Monash University, Melbourne, said “we simply don’t know” which preventive strategy is most effective. This is especially worrisome because more patients will survive their cancers and “be at risk of infection or have significant cytopenias and will experience impaired quality of life as a result,” she said in an interview.
The study authors launched the analysis to better understand the evidence regarding infection prevention and to guide the development of clinical trials, study coauthor Robert Weinkove, MBBS, PhD, a hematologist at Malaghan Institute of Medical Research, Wellington, New Zealand, said in an interview.
As he explained, targeted therapies have revolutionized the treatment of some B-cell cancers. They also have boosted the number of patients who survive the diseases yet still have profound hypogammaglobulinemia.
“Indeed, we may soon reach the point at which infection, and not tumor progression, is the leading cause of death for patients with certain B-cell cancers,” he said. “The evidence base for managing hypogammaglobulinemia is largely based on randomized trials of immunoglobulin replacement conducted in the 1980s and early 1990s, before the advent of B cell–targeted therapies. Immunoglobulin replacement is a costly intervention, and many countries are facing a shortage of immunoglobulin.”
The report authors identified 22 total randomized controlled trials, including one led by Dr. McQuilten: 8 studies into prophylactic immunoglobulin (n = 370; all but 1 study published prior to 2000), 5 into prophylactic antibiotics (n = 1,587), 7 into vaccination (n = 3,996), and 1 comparing immunoglobulin versus antibiotics (n = 60).
No evidence was found to support a lowering of risk by prophylactic antibiotics, although they caused adverse events.
Prophylactic immunoglobulin also caused adverse events, but a meta-analysis found that it reduced the risk of clinically documented infection by 28% (n = 2 trials; relative risk, 0.72; 95% confidence interval, 0.54-0.96). Three trials reported adverse events and found a higher risk overall (RR, 2.23; 95% CI, 1.67-2.99).
Varicella zoster virus vaccination reduced the risk of one or more infections by 63% (n = 5 trials, RR, 0.37; 95% CI, 0.30-0.45, n = 3,515). Prophylactic antibiotics did not reduce the risk.
No intervention reduced all-cause mortality.
“Our findings should be interpreted with caution, Dr. McQuilten said, “because of the low number of patients, high risk of bias in the included studies, and lack of contemporary data applicable to the current standard of care for such patients.”
The lack of useful data is surprising, she said, especially considering “how commonly these interventions are used in current clinical practice and the cost and supply constraints for immunoglobulin. Given the variation in international guidelines, rising global demand and cost of immunoglobulin, and concerns regarding antimicrobial resistance, more evidence is needed to inform infection prevention strategies for this patient population.”
More data is expected soon. One ongoing study is examining intravenous immunoglobulin versus placebo in patients with CLL. It’s expected to be completed in September 2023.
What should clinicians do for now? “Given the lack of a proven survival benefit in favor of prophylactic immunoglobulin replacement, one strategy is to maximize use of vaccination and to educate both patients and clinicians regarding the need for early treatment of infections,” Dr. Weinkove said. “For people who have recurrent or severe infections despite these measures, both immunoglobulin replacement and prophylactic antibiotics are clinical options. It would be reasonable to take account of patient preference, logistical considerations, and reimbursement and availability in deciding between these options.”
He added that, “for people with severe hypogammaglobulinemia who experience recurrent or severe infections despite prophylactic antibiotics, switching to immunoglobulin replacement would be appropriate. We advocate enrollment in clinical trials, if possible.”
In an interview, Juthaporn Cowan, MD, PhD, an infectious disease physician with the University of Ottawa, said many patients with B-cell lymphomas develop acquired hypogammaglobulinemia. “Patients tend to get prolonged colds, frequent sinusitis, bronchitis, or pneumonia. Some can end up with severe infection. Many patients told me that, even though their cancer is cured or in remission, quality of life is still quite poor due to these infections and fatigue.”
Dr. Cowan said the new report is somewhat useful, although “concluding that vaccination reduces infection is misleading. Vaccination reduces the infection that patients were vaccinated against. Patients who received Shingrix will have less shingles but will continue to have bronchitis and other infections.”
As for advice for clinicians, she said preventing acquired hypogammaglobulinemia is difficult since it can be caused by the malignancies, by treatment, or both. “The other item to consider is that we do not know how long we should continue [immunoglobulin] treatment in these patients. I have a patient post CAR [chimeric antigen receptor] T therapy who still does not have B-cell 5-6 years after CAR T, while I have lymphoma patients who could safely discontinue [immunoglobulin] treatment in a few years.”
Dr. Cowan added that patients on immunoglobulin treatment can still get opportunistic infections from cytomegalovirus or herpes simplex virus “because the mechanism of host defense against these infections is different. Antimicrobial prophylaxis should still be considered as vaccination is not available for every single potential opportunistic infection.”
Australia funded the research through the National Blood Authority. Dr. McQuilten and Dr. Weinkove reported no disclosures. Other report authors disclosed ties with Aegros, CSL Behring, Janssen, AbbVie, and BeiGene. Monash University has received funding for unrelated projects from CSL Behring. Dr. Cowan reports honoraria from Takeda, CSL Behring, Octapharma, GlaxoSmithKline, Merck, and AstraZeneca.
FROM BLOOD ADVANCES
Standard first‐line chemotherapies for indolent B‐cell lymphoma impose varying risks for a second cancer
Key clinical point: The risk for a second primary malignancy (SPM) was higher in patients with indolent B‐cell lymphoma (iBCL) treated with bendamustine/rituximab (BR) vs rituximab monotherapy and rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (RCHOP) or rituximab, cyclophosphamide, vincristine, and prednisone (RCVP) or rituximab, pirarubicin, cyclophosphamide, vincristine, and prednisolone (RTHPCOP).
Major finding: The cumulative incidence of SPM was significantly higher among patients receiving BR vs rituximab monotherapy (P < .01) or RCHOP/RCVP/RTHPCOP (P < .0001). The 5‐year cumulative incidence rates with BR, rituximab monotherapy, and RCHOP/RCVP/RTHPCOP were 18.1%, 12.5%, and 12.9%, respectively.
Study details: This retrospective observational study included 5234 adult patients with iBCL who received rituximab monotherapy (n = 780), RCHOP/RCVP/RTHPCOP (n = 2298), or BR (n = 2156).
Disclosures: This study was supported by the Japan Society for the Promotion of Science. Y Muraki declared receiving a lecture honorarium from Pfizer Japan, Inc.
Source: Dote S et al. Risk of a second cancer and infection in patients with indolent B-cell lymphoma exposed to first-line bendamustine plus rituximab: A retrospective analysis of an administrative claims database. Hematol Oncol. 2023 (Feb 15). Doi: 10.1002/hon.3128.
Key clinical point: The risk for a second primary malignancy (SPM) was higher in patients with indolent B‐cell lymphoma (iBCL) treated with bendamustine/rituximab (BR) vs rituximab monotherapy and rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (RCHOP) or rituximab, cyclophosphamide, vincristine, and prednisone (RCVP) or rituximab, pirarubicin, cyclophosphamide, vincristine, and prednisolone (RTHPCOP).
Major finding: The cumulative incidence of SPM was significantly higher among patients receiving BR vs rituximab monotherapy (P < .01) or RCHOP/RCVP/RTHPCOP (P < .0001). The 5‐year cumulative incidence rates with BR, rituximab monotherapy, and RCHOP/RCVP/RTHPCOP were 18.1%, 12.5%, and 12.9%, respectively.
Study details: This retrospective observational study included 5234 adult patients with iBCL who received rituximab monotherapy (n = 780), RCHOP/RCVP/RTHPCOP (n = 2298), or BR (n = 2156).
Disclosures: This study was supported by the Japan Society for the Promotion of Science. Y Muraki declared receiving a lecture honorarium from Pfizer Japan, Inc.
Source: Dote S et al. Risk of a second cancer and infection in patients with indolent B-cell lymphoma exposed to first-line bendamustine plus rituximab: A retrospective analysis of an administrative claims database. Hematol Oncol. 2023 (Feb 15). Doi: 10.1002/hon.3128.
Key clinical point: The risk for a second primary malignancy (SPM) was higher in patients with indolent B‐cell lymphoma (iBCL) treated with bendamustine/rituximab (BR) vs rituximab monotherapy and rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (RCHOP) or rituximab, cyclophosphamide, vincristine, and prednisone (RCVP) or rituximab, pirarubicin, cyclophosphamide, vincristine, and prednisolone (RTHPCOP).
Major finding: The cumulative incidence of SPM was significantly higher among patients receiving BR vs rituximab monotherapy (P < .01) or RCHOP/RCVP/RTHPCOP (P < .0001). The 5‐year cumulative incidence rates with BR, rituximab monotherapy, and RCHOP/RCVP/RTHPCOP were 18.1%, 12.5%, and 12.9%, respectively.
Study details: This retrospective observational study included 5234 adult patients with iBCL who received rituximab monotherapy (n = 780), RCHOP/RCVP/RTHPCOP (n = 2298), or BR (n = 2156).
Disclosures: This study was supported by the Japan Society for the Promotion of Science. Y Muraki declared receiving a lecture honorarium from Pfizer Japan, Inc.
Source: Dote S et al. Risk of a second cancer and infection in patients with indolent B-cell lymphoma exposed to first-line bendamustine plus rituximab: A retrospective analysis of an administrative claims database. Hematol Oncol. 2023 (Feb 15). Doi: 10.1002/hon.3128.