User login
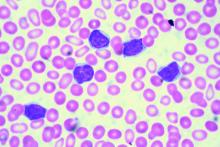
Low-dose radiation therapy looks effective in hard-to-treat MCL
Low-dose radiation therapy – with or without concurrent chemotherapy – appears promising as a treatment for patients with relapsed or refractory mantle cell lymphoma (MCL) or at least a bridge to subsequent therapy, according to findings published in Blood Advances.
Matthew S. Ning, MD, of the department of radiation oncology at the University of Texas MD Anderson Cancer Center, Houston, and colleagues, said this is the first study to evaluate low-dose radiation therapy (LDRT) with chemotherapy as a treatment modality outside of palliative care for relapsed, multiple refractory MCL patients.
“Our findings indicate that LDRT imparts excellent [local control], minimal toxicity, and favorable outcomes in this setting,” the researchers said.
The study included 19 patients with a total of 98 sites of relapsed, refractory MCL who were treated from 2014 to 2018. The median follow-up was 51.3 months from initial diagnosis and 15.4 months from initial treatment with low-dose radiation therapy, given at a dose of 4 Gy.
These were hard-to-treat patients who had received multiple prior therapies since diagnosis, including carfilzomib, ibrutinib, bortezomib, anthracycline, and rituximab. In total, 8 of the patients had previously undergone autologous stem cell transplant and 11 were refractory to ibrutinib by the time of initial radiation therapy.
Median age of the patients was 69 years; 15 patients had classical histology and 4 had blastoid variant. Among the 98 tumor sites treated, the median tumor size was 2.8 cm.
In all, 14 patients received initial LDRT that was concurrent with chemotherapy. The remaining 5 patients had stopped chemotherapy prior to starting LDRT.
LDRT was given in 1-2 daily fractions via 3-dimensional conformal radiation therapy or electron beam.
Of the 98 tumor sites treated, complete response was achieved for 79 sites (81%) and the median time to complete response was 2.7 months after the start of LDRT. The researchers removed one patient who was an outlier with 27 tumor sites treated, and that dropped the complete response rate down to 76%. The overall response rate, which include an additional five sites with partial response, was 86%.
The researchers found links between complete response and soft tissue site versus non–soft tissue site (hazard ratio, 1.80; 1.12-2.90, P = .02). However, there were no associations between response and chemo-refractory status, ibrutinib-refractory status, prior chemotherapy courts, receipt of concurrent chemotherapy, tumor size, number of fractions, lesions treated per course, or blastoid variant.
The overall survival at 1 year after LDRT initiation was 90% and the 1-year progression-free survival was 55%. All five patients who died were refractory to ibrutinib.
The researchers reported finding no radiation therapy–related toxicities, even when patients received concurrent chemotherapy.
The use of LDRT has the potential to bridge refractory patients to subsequent therapies or to provide treatment breaks as patients recover from toxicities, the researchers said. However, they called for additional studies to confirm that this approach improves progression-free survival over chemotherapy alone.
The study was supported in part by a grant from the National Cancer Institute. The researchers reported having no competing financial interests.
SOURCE: Ning MS et al. Blood Adv. 2019. Jul 9;3(13):2035-9.
Low-dose radiation therapy – with or without concurrent chemotherapy – appears promising as a treatment for patients with relapsed or refractory mantle cell lymphoma (MCL) or at least a bridge to subsequent therapy, according to findings published in Blood Advances.
Matthew S. Ning, MD, of the department of radiation oncology at the University of Texas MD Anderson Cancer Center, Houston, and colleagues, said this is the first study to evaluate low-dose radiation therapy (LDRT) with chemotherapy as a treatment modality outside of palliative care for relapsed, multiple refractory MCL patients.
“Our findings indicate that LDRT imparts excellent [local control], minimal toxicity, and favorable outcomes in this setting,” the researchers said.
The study included 19 patients with a total of 98 sites of relapsed, refractory MCL who were treated from 2014 to 2018. The median follow-up was 51.3 months from initial diagnosis and 15.4 months from initial treatment with low-dose radiation therapy, given at a dose of 4 Gy.
These were hard-to-treat patients who had received multiple prior therapies since diagnosis, including carfilzomib, ibrutinib, bortezomib, anthracycline, and rituximab. In total, 8 of the patients had previously undergone autologous stem cell transplant and 11 were refractory to ibrutinib by the time of initial radiation therapy.
Median age of the patients was 69 years; 15 patients had classical histology and 4 had blastoid variant. Among the 98 tumor sites treated, the median tumor size was 2.8 cm.
In all, 14 patients received initial LDRT that was concurrent with chemotherapy. The remaining 5 patients had stopped chemotherapy prior to starting LDRT.
LDRT was given in 1-2 daily fractions via 3-dimensional conformal radiation therapy or electron beam.
Of the 98 tumor sites treated, complete response was achieved for 79 sites (81%) and the median time to complete response was 2.7 months after the start of LDRT. The researchers removed one patient who was an outlier with 27 tumor sites treated, and that dropped the complete response rate down to 76%. The overall response rate, which include an additional five sites with partial response, was 86%.
The researchers found links between complete response and soft tissue site versus non–soft tissue site (hazard ratio, 1.80; 1.12-2.90, P = .02). However, there were no associations between response and chemo-refractory status, ibrutinib-refractory status, prior chemotherapy courts, receipt of concurrent chemotherapy, tumor size, number of fractions, lesions treated per course, or blastoid variant.
The overall survival at 1 year after LDRT initiation was 90% and the 1-year progression-free survival was 55%. All five patients who died were refractory to ibrutinib.
The researchers reported finding no radiation therapy–related toxicities, even when patients received concurrent chemotherapy.
The use of LDRT has the potential to bridge refractory patients to subsequent therapies or to provide treatment breaks as patients recover from toxicities, the researchers said. However, they called for additional studies to confirm that this approach improves progression-free survival over chemotherapy alone.
The study was supported in part by a grant from the National Cancer Institute. The researchers reported having no competing financial interests.
SOURCE: Ning MS et al. Blood Adv. 2019. Jul 9;3(13):2035-9.
Low-dose radiation therapy – with or without concurrent chemotherapy – appears promising as a treatment for patients with relapsed or refractory mantle cell lymphoma (MCL) or at least a bridge to subsequent therapy, according to findings published in Blood Advances.
Matthew S. Ning, MD, of the department of radiation oncology at the University of Texas MD Anderson Cancer Center, Houston, and colleagues, said this is the first study to evaluate low-dose radiation therapy (LDRT) with chemotherapy as a treatment modality outside of palliative care for relapsed, multiple refractory MCL patients.
“Our findings indicate that LDRT imparts excellent [local control], minimal toxicity, and favorable outcomes in this setting,” the researchers said.
The study included 19 patients with a total of 98 sites of relapsed, refractory MCL who were treated from 2014 to 2018. The median follow-up was 51.3 months from initial diagnosis and 15.4 months from initial treatment with low-dose radiation therapy, given at a dose of 4 Gy.
These were hard-to-treat patients who had received multiple prior therapies since diagnosis, including carfilzomib, ibrutinib, bortezomib, anthracycline, and rituximab. In total, 8 of the patients had previously undergone autologous stem cell transplant and 11 were refractory to ibrutinib by the time of initial radiation therapy.
Median age of the patients was 69 years; 15 patients had classical histology and 4 had blastoid variant. Among the 98 tumor sites treated, the median tumor size was 2.8 cm.
In all, 14 patients received initial LDRT that was concurrent with chemotherapy. The remaining 5 patients had stopped chemotherapy prior to starting LDRT.
LDRT was given in 1-2 daily fractions via 3-dimensional conformal radiation therapy or electron beam.
Of the 98 tumor sites treated, complete response was achieved for 79 sites (81%) and the median time to complete response was 2.7 months after the start of LDRT. The researchers removed one patient who was an outlier with 27 tumor sites treated, and that dropped the complete response rate down to 76%. The overall response rate, which include an additional five sites with partial response, was 86%.
The researchers found links between complete response and soft tissue site versus non–soft tissue site (hazard ratio, 1.80; 1.12-2.90, P = .02). However, there were no associations between response and chemo-refractory status, ibrutinib-refractory status, prior chemotherapy courts, receipt of concurrent chemotherapy, tumor size, number of fractions, lesions treated per course, or blastoid variant.
The overall survival at 1 year after LDRT initiation was 90% and the 1-year progression-free survival was 55%. All five patients who died were refractory to ibrutinib.
The researchers reported finding no radiation therapy–related toxicities, even when patients received concurrent chemotherapy.
The use of LDRT has the potential to bridge refractory patients to subsequent therapies or to provide treatment breaks as patients recover from toxicities, the researchers said. However, they called for additional studies to confirm that this approach improves progression-free survival over chemotherapy alone.
The study was supported in part by a grant from the National Cancer Institute. The researchers reported having no competing financial interests.
SOURCE: Ning MS et al. Blood Adv. 2019. Jul 9;3(13):2035-9.
FROM BLOOD ADVANCES
Key clinical point:
Major finding: The overall survival was 90% at 1 year following the initiation of low-dose radiation therapy (4 Gy).
Study details: A study of 19 patients with relapsed, refractory mantle cell lymphoma who received low-dose radiation at doses of 4 Gy at 98 sites of disease.
Disclosures: The study was supported in part by a grant from the National Cancer Institute. The researchers reported having no competing financial interests.
Source: Ning MS et al. Blood Adv. 2019. Jul 9;3(13):2035-9.
ICYMI: Ibrutinib/rituximab combo improves CLL survival
Patients with previously untreated chronic lymphocytic leukemia (CLL) aged 70 years or younger who received ibrutinib/rituximab therapy experienced significantly greater progression-free survival, compared with those who received standard chemotherapy with fludarabine, cyclophosphamide, and rituximab (89.4% vs. 72.9% at 3 years; hazard ratio, 0.35; 95% confidence interval, 0.22-0.56; P less than .001), according to results from a randomized, phase 3 trial published in the New England Journal of Medicine (2019;381:432-43).
We first reported on the results of this trial when they were presented at the annual meeting of the American Society of Hematology. Find our coverage at the link below.
Patients with previously untreated chronic lymphocytic leukemia (CLL) aged 70 years or younger who received ibrutinib/rituximab therapy experienced significantly greater progression-free survival, compared with those who received standard chemotherapy with fludarabine, cyclophosphamide, and rituximab (89.4% vs. 72.9% at 3 years; hazard ratio, 0.35; 95% confidence interval, 0.22-0.56; P less than .001), according to results from a randomized, phase 3 trial published in the New England Journal of Medicine (2019;381:432-43).
We first reported on the results of this trial when they were presented at the annual meeting of the American Society of Hematology. Find our coverage at the link below.
Patients with previously untreated chronic lymphocytic leukemia (CLL) aged 70 years or younger who received ibrutinib/rituximab therapy experienced significantly greater progression-free survival, compared with those who received standard chemotherapy with fludarabine, cyclophosphamide, and rituximab (89.4% vs. 72.9% at 3 years; hazard ratio, 0.35; 95% confidence interval, 0.22-0.56; P less than .001), according to results from a randomized, phase 3 trial published in the New England Journal of Medicine (2019;381:432-43).
We first reported on the results of this trial when they were presented at the annual meeting of the American Society of Hematology. Find our coverage at the link below.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
BTK mutations linked to CLL progression on ibrutinib
Mutations in Bruton’s tyrosine kinase (BTK) are associated with progression of chronic lymphocytic leukemia (CLL) in patients taking ibrutinib, according to a new study.
Researchers analyzed a “real-life” cohort of CLL patients taking ibrutinib for about 3 years and found that patients with BTK mutations were significantly more likely to progress (P = .0005).
“Our findings support that mutational analysis should be considered in patients receiving ibrutinib who have residual clonal lymphocytosis, and that clinical trials are needed to evaluate whether patients with a BTK mutation may benefit from an early switch to another treatment,” wrote Anne Quinquenel, MD, PhD, of Hôpital Robert Debré, Université Reims (France) Champagne-Ardenne, and colleagues. Their report is in Blood.
The researchers studied 57 CLL patients who were still on ibrutinib after at least 3 years and provided fresh blood samples. The median time between the start of ibrutinib and sample collection was 3.5 years.
All 57 patients had minimal residual disease at baseline. Of the 55 patients with response data available, 48 had a partial response, and 7 had a partial response with lymphocytosis.
Mutational profiling was possible in 30 patients who had a CLL clone greater than or equal to 0.5 x 109/L.
BTK mutations were present in 17 of the 30 patients (57%). There were 20 BTK mutations in total, all were at C481, and 14 were at C481S.
The researchers also identified 15 patients with TP53 mutations and 4 patients with phospholipase Cg2 (PLCG2) mutations. All 4 patients with PLCG2 mutations also had a BTK mutation and a TP53 mutation.
However, there were no significant associations between BTK mutations and other mutations. BTK mutations were not associated with the number of previous therapies a patient received or the need for ibrutinib dose interruptions or reductions.
The researchers assessed CLL progression at median of 8.5 months from sample collection and found the presence of a BTK mutation was significantly associated with progression (P = .0005).
Of the 17 patients with a BTK mutation, 14 progressed with one case of Richter’s syndrome. Three patients who progressed were still on ibrutinib, nine patients received venetoclax, and two patients died without further treatment.
Of the 13 patients without BTK mutations, just two patients progressed. One patient died without further treatment, and the other received venetoclax.
The event-free survival was significantly shorter in patients with a BTK mutation than in those without (P = .0380), but there was no significant difference in overall survival.
This research was supported by Sunesis Pharmaceuticals and the Force Hemato (fonds de recherche clinique en hématologie) foundation. The researchers reported relationships with Janssen, Gilead, Roche, and AbbVie.
SOURCE: Quinquenel A et al. Blood. 2019 Jun 26. doi: 10.1182/blood.2019000854.
Mutations in Bruton’s tyrosine kinase (BTK) are associated with progression of chronic lymphocytic leukemia (CLL) in patients taking ibrutinib, according to a new study.
Researchers analyzed a “real-life” cohort of CLL patients taking ibrutinib for about 3 years and found that patients with BTK mutations were significantly more likely to progress (P = .0005).
“Our findings support that mutational analysis should be considered in patients receiving ibrutinib who have residual clonal lymphocytosis, and that clinical trials are needed to evaluate whether patients with a BTK mutation may benefit from an early switch to another treatment,” wrote Anne Quinquenel, MD, PhD, of Hôpital Robert Debré, Université Reims (France) Champagne-Ardenne, and colleagues. Their report is in Blood.
The researchers studied 57 CLL patients who were still on ibrutinib after at least 3 years and provided fresh blood samples. The median time between the start of ibrutinib and sample collection was 3.5 years.
All 57 patients had minimal residual disease at baseline. Of the 55 patients with response data available, 48 had a partial response, and 7 had a partial response with lymphocytosis.
Mutational profiling was possible in 30 patients who had a CLL clone greater than or equal to 0.5 x 109/L.
BTK mutations were present in 17 of the 30 patients (57%). There were 20 BTK mutations in total, all were at C481, and 14 were at C481S.
The researchers also identified 15 patients with TP53 mutations and 4 patients with phospholipase Cg2 (PLCG2) mutations. All 4 patients with PLCG2 mutations also had a BTK mutation and a TP53 mutation.
However, there were no significant associations between BTK mutations and other mutations. BTK mutations were not associated with the number of previous therapies a patient received or the need for ibrutinib dose interruptions or reductions.
The researchers assessed CLL progression at median of 8.5 months from sample collection and found the presence of a BTK mutation was significantly associated with progression (P = .0005).
Of the 17 patients with a BTK mutation, 14 progressed with one case of Richter’s syndrome. Three patients who progressed were still on ibrutinib, nine patients received venetoclax, and two patients died without further treatment.
Of the 13 patients without BTK mutations, just two patients progressed. One patient died without further treatment, and the other received venetoclax.
The event-free survival was significantly shorter in patients with a BTK mutation than in those without (P = .0380), but there was no significant difference in overall survival.
This research was supported by Sunesis Pharmaceuticals and the Force Hemato (fonds de recherche clinique en hématologie) foundation. The researchers reported relationships with Janssen, Gilead, Roche, and AbbVie.
SOURCE: Quinquenel A et al. Blood. 2019 Jun 26. doi: 10.1182/blood.2019000854.
Mutations in Bruton’s tyrosine kinase (BTK) are associated with progression of chronic lymphocytic leukemia (CLL) in patients taking ibrutinib, according to a new study.
Researchers analyzed a “real-life” cohort of CLL patients taking ibrutinib for about 3 years and found that patients with BTK mutations were significantly more likely to progress (P = .0005).
“Our findings support that mutational analysis should be considered in patients receiving ibrutinib who have residual clonal lymphocytosis, and that clinical trials are needed to evaluate whether patients with a BTK mutation may benefit from an early switch to another treatment,” wrote Anne Quinquenel, MD, PhD, of Hôpital Robert Debré, Université Reims (France) Champagne-Ardenne, and colleagues. Their report is in Blood.
The researchers studied 57 CLL patients who were still on ibrutinib after at least 3 years and provided fresh blood samples. The median time between the start of ibrutinib and sample collection was 3.5 years.
All 57 patients had minimal residual disease at baseline. Of the 55 patients with response data available, 48 had a partial response, and 7 had a partial response with lymphocytosis.
Mutational profiling was possible in 30 patients who had a CLL clone greater than or equal to 0.5 x 109/L.
BTK mutations were present in 17 of the 30 patients (57%). There were 20 BTK mutations in total, all were at C481, and 14 were at C481S.
The researchers also identified 15 patients with TP53 mutations and 4 patients with phospholipase Cg2 (PLCG2) mutations. All 4 patients with PLCG2 mutations also had a BTK mutation and a TP53 mutation.
However, there were no significant associations between BTK mutations and other mutations. BTK mutations were not associated with the number of previous therapies a patient received or the need for ibrutinib dose interruptions or reductions.
The researchers assessed CLL progression at median of 8.5 months from sample collection and found the presence of a BTK mutation was significantly associated with progression (P = .0005).
Of the 17 patients with a BTK mutation, 14 progressed with one case of Richter’s syndrome. Three patients who progressed were still on ibrutinib, nine patients received venetoclax, and two patients died without further treatment.
Of the 13 patients without BTK mutations, just two patients progressed. One patient died without further treatment, and the other received venetoclax.
The event-free survival was significantly shorter in patients with a BTK mutation than in those without (P = .0380), but there was no significant difference in overall survival.
This research was supported by Sunesis Pharmaceuticals and the Force Hemato (fonds de recherche clinique en hématologie) foundation. The researchers reported relationships with Janssen, Gilead, Roche, and AbbVie.
SOURCE: Quinquenel A et al. Blood. 2019 Jun 26. doi: 10.1182/blood.2019000854.
FROM BLOOD
FDA approves rituximab biosimilar for cancer, autoimmune disorders
The Food and Drug Administration has approved rituximab-pvvr (Ruxience) for adults with non-Hodgkin lymphoma, chronic lymphocytic leukemia (CLL), and granulomatosis with polyangiitis and microscopic polyangiitis. It is the first biosimilar approved to treat these two rare autoimmune conditions.
Specifically, the biosimilar product is approved as single-agent therapy for relapsed or refractory, low grade or follicular, CD20-positive B-cell non-Hodgkin lymphoma; in combination with chemotherapy for other types of previously untreated CD20-positive B-cell non-Hodgkin lymphoma; and as a single agent for nonprogressing, low-grade, CD20-positive B-cell non-Hodgkin lymphoma after first-line chemotherapy treatment. It is also approved for both previously untreated and previously treated CD20-positive CLL in combination with chemotherapy. And it is approved for granulomatosis with polyangiitis and microscopic polyangiitis in combination with glucocorticoids.
The approval is based on demonstration that rituximab-pvvr had no clinically meaningful differences in safety or efficacy when compared with the reference drug, rituximab (Rituxan), according to a release from the biosimilar’s developer. As with rituximab, rituximab-pvvr’s label comes with an FDA boxed warning. In the biosimilar’s case, it warns against fatal infusion-related reactions, severe mucocutaneous reactions, hepatitis B virus reactivation, and progressive multifocal leukoencephalopathy. Other adverse reactions include fever, headache, neutropenia, and lymphopenia.
The Food and Drug Administration has approved rituximab-pvvr (Ruxience) for adults with non-Hodgkin lymphoma, chronic lymphocytic leukemia (CLL), and granulomatosis with polyangiitis and microscopic polyangiitis. It is the first biosimilar approved to treat these two rare autoimmune conditions.
Specifically, the biosimilar product is approved as single-agent therapy for relapsed or refractory, low grade or follicular, CD20-positive B-cell non-Hodgkin lymphoma; in combination with chemotherapy for other types of previously untreated CD20-positive B-cell non-Hodgkin lymphoma; and as a single agent for nonprogressing, low-grade, CD20-positive B-cell non-Hodgkin lymphoma after first-line chemotherapy treatment. It is also approved for both previously untreated and previously treated CD20-positive CLL in combination with chemotherapy. And it is approved for granulomatosis with polyangiitis and microscopic polyangiitis in combination with glucocorticoids.
The approval is based on demonstration that rituximab-pvvr had no clinically meaningful differences in safety or efficacy when compared with the reference drug, rituximab (Rituxan), according to a release from the biosimilar’s developer. As with rituximab, rituximab-pvvr’s label comes with an FDA boxed warning. In the biosimilar’s case, it warns against fatal infusion-related reactions, severe mucocutaneous reactions, hepatitis B virus reactivation, and progressive multifocal leukoencephalopathy. Other adverse reactions include fever, headache, neutropenia, and lymphopenia.
The Food and Drug Administration has approved rituximab-pvvr (Ruxience) for adults with non-Hodgkin lymphoma, chronic lymphocytic leukemia (CLL), and granulomatosis with polyangiitis and microscopic polyangiitis. It is the first biosimilar approved to treat these two rare autoimmune conditions.
Specifically, the biosimilar product is approved as single-agent therapy for relapsed or refractory, low grade or follicular, CD20-positive B-cell non-Hodgkin lymphoma; in combination with chemotherapy for other types of previously untreated CD20-positive B-cell non-Hodgkin lymphoma; and as a single agent for nonprogressing, low-grade, CD20-positive B-cell non-Hodgkin lymphoma after first-line chemotherapy treatment. It is also approved for both previously untreated and previously treated CD20-positive CLL in combination with chemotherapy. And it is approved for granulomatosis with polyangiitis and microscopic polyangiitis in combination with glucocorticoids.
The approval is based on demonstration that rituximab-pvvr had no clinically meaningful differences in safety or efficacy when compared with the reference drug, rituximab (Rituxan), according to a release from the biosimilar’s developer. As with rituximab, rituximab-pvvr’s label comes with an FDA boxed warning. In the biosimilar’s case, it warns against fatal infusion-related reactions, severe mucocutaneous reactions, hepatitis B virus reactivation, and progressive multifocal leukoencephalopathy. Other adverse reactions include fever, headache, neutropenia, and lymphopenia.
Chemo-free combo gets high response rate in relapsed or refractory DLBCL
LUGANO, Switzerland – A chemotherapy-free combination of lenalidomide (Revlimid) and the novel anti-CD19 antibody tafasitamab (MOR208) continues to show encouraging clinical activity against relapsed/refractory diffuse large B cell lymphoma, with durable responses and promising progression-free and overall survival, investigators in the phase 2 L-MIND study reported.
After a median follow-up of 17.3 months, the overall response rate (ORR) – the primary endpoint in the single arm trial – was 60%, consisting of 42.5% complete responses (CR) and 17.5% partial responses (PR), reported Giles Salles, MD, PhD, of Claude Bernard University in Lyon, France.
“We see consistently high activity in transplant-ineligible subgroups, patients who have limited treatment options and who have really poor prognosis,” he said at the International Conference on Malignant Lymphoma (15-ICML).
In a preclinical study, a combination of MOR208 and lenalidomide showed synergistic antileukemic and antilymphoma activity both in vivo and in vitro. In addition, both lenalidomide and MOR208 have shown significant activity against relapsed, refractory B-cell non-Hodgkin lymphomas.
At the previous ICML meeting in 2017, Dr. Salles reported early interim results from the study, which showed that among 34 patients evaluable for response, the ORR was 56%, including complete responses in 32% of patients.
The L-MIND investigators enrolled transplant-ineligible patients 18 years and older with relapsed/refractory DLBCL, Eastern Cooperative Oncology Group performance status 0-2, and adequate organ function who had disease progression after 1-3 prior lines of therapy.
Patients with primary refractory DLBCL, double-hit or triple-hit DLBCL (i.e., mutations in Myc, BCL2, and/or BCL6), other non-Hodgkin lymphoma histological subtypes, or central nervous system lymphoma involvement were excluded.
Patients received tafasitamab 12 mg/kg intravenously on days 1, 8, 15, and 22 for cycles 1-3 and on days 1 and 15 of cycles 4-12. Lenalidomide 25 mg orally was delivered on days 1-21 of each cycle. Patients with stable disease or better at the end of 12 cycles could be maintained on tafasitamab at the same dose on days 1 and 15.
As noted, the combination was associated with an ORR among 80 patients of 60%, consisting of 34 CR (42.5%) complete responses and 14 (17.5%) PR. An additional 11 patients (13.75%) had stable disease, 13 (16.25%) had disease progression, and eight (10%) were not evaluable because of missing post-baseline tumor assessments.
The median duration of response in the entire cohort was 21.7 months. For patients with a CR, the median duration of response had not been reached at the time of data cutoff. For patients with a PR, the median duration of response was 4.4 months.
Hematologic treatment-emergent toxicities occurring in 10% or more of patients included (in descending order of frequency) neutropenia, anemia, thrombocytopenia, leukopenia, and febrile neutropenia.
Nonhematologic treatment-emergent events occurring in at least 10% of patients included diarrhea, asthenia, peripheral edema, pyrexia, rash, decreased appetite, hypokalemia, fatigue, and similar events, the majority of which were grade 1 or 2 in severity.
“The durable responses and favorable overall survival I would say represent a remarkable outcome, and this combination of lenalidomide with tafasitamab results in a new chemo-free immunotherapy for patients with relapsed/refractory DLBCL,” Dr. Salles said.
The L-MIND study is funded by MorphoSys Ag. Dr. Salles reported receiving fees for advisory board/consulting activities and educational activities from MorphoSys and other companies.
SOURCE: Salles G et al. 15-ICML, Abstract 124.
LUGANO, Switzerland – A chemotherapy-free combination of lenalidomide (Revlimid) and the novel anti-CD19 antibody tafasitamab (MOR208) continues to show encouraging clinical activity against relapsed/refractory diffuse large B cell lymphoma, with durable responses and promising progression-free and overall survival, investigators in the phase 2 L-MIND study reported.
After a median follow-up of 17.3 months, the overall response rate (ORR) – the primary endpoint in the single arm trial – was 60%, consisting of 42.5% complete responses (CR) and 17.5% partial responses (PR), reported Giles Salles, MD, PhD, of Claude Bernard University in Lyon, France.
“We see consistently high activity in transplant-ineligible subgroups, patients who have limited treatment options and who have really poor prognosis,” he said at the International Conference on Malignant Lymphoma (15-ICML).
In a preclinical study, a combination of MOR208 and lenalidomide showed synergistic antileukemic and antilymphoma activity both in vivo and in vitro. In addition, both lenalidomide and MOR208 have shown significant activity against relapsed, refractory B-cell non-Hodgkin lymphomas.
At the previous ICML meeting in 2017, Dr. Salles reported early interim results from the study, which showed that among 34 patients evaluable for response, the ORR was 56%, including complete responses in 32% of patients.
The L-MIND investigators enrolled transplant-ineligible patients 18 years and older with relapsed/refractory DLBCL, Eastern Cooperative Oncology Group performance status 0-2, and adequate organ function who had disease progression after 1-3 prior lines of therapy.
Patients with primary refractory DLBCL, double-hit or triple-hit DLBCL (i.e., mutations in Myc, BCL2, and/or BCL6), other non-Hodgkin lymphoma histological subtypes, or central nervous system lymphoma involvement were excluded.
Patients received tafasitamab 12 mg/kg intravenously on days 1, 8, 15, and 22 for cycles 1-3 and on days 1 and 15 of cycles 4-12. Lenalidomide 25 mg orally was delivered on days 1-21 of each cycle. Patients with stable disease or better at the end of 12 cycles could be maintained on tafasitamab at the same dose on days 1 and 15.
As noted, the combination was associated with an ORR among 80 patients of 60%, consisting of 34 CR (42.5%) complete responses and 14 (17.5%) PR. An additional 11 patients (13.75%) had stable disease, 13 (16.25%) had disease progression, and eight (10%) were not evaluable because of missing post-baseline tumor assessments.
The median duration of response in the entire cohort was 21.7 months. For patients with a CR, the median duration of response had not been reached at the time of data cutoff. For patients with a PR, the median duration of response was 4.4 months.
Hematologic treatment-emergent toxicities occurring in 10% or more of patients included (in descending order of frequency) neutropenia, anemia, thrombocytopenia, leukopenia, and febrile neutropenia.
Nonhematologic treatment-emergent events occurring in at least 10% of patients included diarrhea, asthenia, peripheral edema, pyrexia, rash, decreased appetite, hypokalemia, fatigue, and similar events, the majority of which were grade 1 or 2 in severity.
“The durable responses and favorable overall survival I would say represent a remarkable outcome, and this combination of lenalidomide with tafasitamab results in a new chemo-free immunotherapy for patients with relapsed/refractory DLBCL,” Dr. Salles said.
The L-MIND study is funded by MorphoSys Ag. Dr. Salles reported receiving fees for advisory board/consulting activities and educational activities from MorphoSys and other companies.
SOURCE: Salles G et al. 15-ICML, Abstract 124.
LUGANO, Switzerland – A chemotherapy-free combination of lenalidomide (Revlimid) and the novel anti-CD19 antibody tafasitamab (MOR208) continues to show encouraging clinical activity against relapsed/refractory diffuse large B cell lymphoma, with durable responses and promising progression-free and overall survival, investigators in the phase 2 L-MIND study reported.
After a median follow-up of 17.3 months, the overall response rate (ORR) – the primary endpoint in the single arm trial – was 60%, consisting of 42.5% complete responses (CR) and 17.5% partial responses (PR), reported Giles Salles, MD, PhD, of Claude Bernard University in Lyon, France.
“We see consistently high activity in transplant-ineligible subgroups, patients who have limited treatment options and who have really poor prognosis,” he said at the International Conference on Malignant Lymphoma (15-ICML).
In a preclinical study, a combination of MOR208 and lenalidomide showed synergistic antileukemic and antilymphoma activity both in vivo and in vitro. In addition, both lenalidomide and MOR208 have shown significant activity against relapsed, refractory B-cell non-Hodgkin lymphomas.
At the previous ICML meeting in 2017, Dr. Salles reported early interim results from the study, which showed that among 34 patients evaluable for response, the ORR was 56%, including complete responses in 32% of patients.
The L-MIND investigators enrolled transplant-ineligible patients 18 years and older with relapsed/refractory DLBCL, Eastern Cooperative Oncology Group performance status 0-2, and adequate organ function who had disease progression after 1-3 prior lines of therapy.
Patients with primary refractory DLBCL, double-hit or triple-hit DLBCL (i.e., mutations in Myc, BCL2, and/or BCL6), other non-Hodgkin lymphoma histological subtypes, or central nervous system lymphoma involvement were excluded.
Patients received tafasitamab 12 mg/kg intravenously on days 1, 8, 15, and 22 for cycles 1-3 and on days 1 and 15 of cycles 4-12. Lenalidomide 25 mg orally was delivered on days 1-21 of each cycle. Patients with stable disease or better at the end of 12 cycles could be maintained on tafasitamab at the same dose on days 1 and 15.
As noted, the combination was associated with an ORR among 80 patients of 60%, consisting of 34 CR (42.5%) complete responses and 14 (17.5%) PR. An additional 11 patients (13.75%) had stable disease, 13 (16.25%) had disease progression, and eight (10%) were not evaluable because of missing post-baseline tumor assessments.
The median duration of response in the entire cohort was 21.7 months. For patients with a CR, the median duration of response had not been reached at the time of data cutoff. For patients with a PR, the median duration of response was 4.4 months.
Hematologic treatment-emergent toxicities occurring in 10% or more of patients included (in descending order of frequency) neutropenia, anemia, thrombocytopenia, leukopenia, and febrile neutropenia.
Nonhematologic treatment-emergent events occurring in at least 10% of patients included diarrhea, asthenia, peripheral edema, pyrexia, rash, decreased appetite, hypokalemia, fatigue, and similar events, the majority of which were grade 1 or 2 in severity.
“The durable responses and favorable overall survival I would say represent a remarkable outcome, and this combination of lenalidomide with tafasitamab results in a new chemo-free immunotherapy for patients with relapsed/refractory DLBCL,” Dr. Salles said.
The L-MIND study is funded by MorphoSys Ag. Dr. Salles reported receiving fees for advisory board/consulting activities and educational activities from MorphoSys and other companies.
SOURCE: Salles G et al. 15-ICML, Abstract 124.
REPORTING FROM 15-ICML
Ibrutinib-venetoclax found highly active in hard-to-treat CLL
The strategy of simultaneously inhibiting proliferation and reactivating apoptosis can eradicate chronic lymphocytic leukemia (CLL) in a large share of patients, suggest results from the phase 2 CLARITY trial.
“Both ibrutinib and venetoclax are active in CLL with improved survival; however, as monotherapies, both currently are given until disease progression,” wrote Peter Hillmen, MBChB, PhD, St. James’s University Hospital, Leeds, England, and his colleagues.
In the single-arm, open-label trial, the investigators treated 53 patients with relapsed or refractory CLL with combination ibrutinib (Imbruvica), a small-molecule inhibitor of Bruton’s tyrosine kinase, and venetoclax (Venclexta), a small molecule inhibitor of the anti-apoptotic protein Bcl-2. The primary endpoint was MRD negativity, defined as presence of fewer than one CLL cell in 10,000 leukocytes, after 12 months of combination therapy.
Results reported in the Journal of Clinical Oncology showed that the combination was highly active, with 53% of patients achieving MRD negativity in the blood and 36% achieving MRD negativity in the marrow.
Most patients, 89%, had a treatment response, and slightly more than half, 51%, achieved a complete remission. With a median 21.1-month follow-up, only a single patient experienced progression and all were still alive.
Adverse effects were generally manageable. Grade 3-4 adverse events of special interest included 34 cases of neutropenia and 1 case of biochemical tumor lysis syndrome that was managed by delaying venetoclax.
“We have demonstrated promising efficacy that indicates potent synergy between ibrutinib and venetoclax for inducing MRD-negative responses with manageable adverse effects,” the investigators wrote. “The observation that a significant proportion of patients experience MRD-negative remission indicates that this combination can be given for a limited period and then stopped after patients achieve a deep remission.”
Whether the combination leads to permanent disease eradication in certain patients is still unclear, the investigators added.
The trial was supported by Bloodwise under the Trials Acceleration Programme, by the National Institute for Health Research Leeds Clinical Research Facility, and by an unrestricted educational grant from Janssen-Cilag and AbbVie. Ibrutinib was provided free of charge by Janssen-Cilag, and venetoclax was provided free of charge by AbbVie. Dr. Hillman reported financial relationships with Janssen, AbbVie, Roche, Pharmacyclics, and Gilead Sciences.
SOURCE: Hillmen P et al. J Clin Oncol. 2019 Jul 11. doi: 10.1200/JCO.19.00894.
The strategy of simultaneously inhibiting proliferation and reactivating apoptosis can eradicate chronic lymphocytic leukemia (CLL) in a large share of patients, suggest results from the phase 2 CLARITY trial.
“Both ibrutinib and venetoclax are active in CLL with improved survival; however, as monotherapies, both currently are given until disease progression,” wrote Peter Hillmen, MBChB, PhD, St. James’s University Hospital, Leeds, England, and his colleagues.
In the single-arm, open-label trial, the investigators treated 53 patients with relapsed or refractory CLL with combination ibrutinib (Imbruvica), a small-molecule inhibitor of Bruton’s tyrosine kinase, and venetoclax (Venclexta), a small molecule inhibitor of the anti-apoptotic protein Bcl-2. The primary endpoint was MRD negativity, defined as presence of fewer than one CLL cell in 10,000 leukocytes, after 12 months of combination therapy.
Results reported in the Journal of Clinical Oncology showed that the combination was highly active, with 53% of patients achieving MRD negativity in the blood and 36% achieving MRD negativity in the marrow.
Most patients, 89%, had a treatment response, and slightly more than half, 51%, achieved a complete remission. With a median 21.1-month follow-up, only a single patient experienced progression and all were still alive.
Adverse effects were generally manageable. Grade 3-4 adverse events of special interest included 34 cases of neutropenia and 1 case of biochemical tumor lysis syndrome that was managed by delaying venetoclax.
“We have demonstrated promising efficacy that indicates potent synergy between ibrutinib and venetoclax for inducing MRD-negative responses with manageable adverse effects,” the investigators wrote. “The observation that a significant proportion of patients experience MRD-negative remission indicates that this combination can be given for a limited period and then stopped after patients achieve a deep remission.”
Whether the combination leads to permanent disease eradication in certain patients is still unclear, the investigators added.
The trial was supported by Bloodwise under the Trials Acceleration Programme, by the National Institute for Health Research Leeds Clinical Research Facility, and by an unrestricted educational grant from Janssen-Cilag and AbbVie. Ibrutinib was provided free of charge by Janssen-Cilag, and venetoclax was provided free of charge by AbbVie. Dr. Hillman reported financial relationships with Janssen, AbbVie, Roche, Pharmacyclics, and Gilead Sciences.
SOURCE: Hillmen P et al. J Clin Oncol. 2019 Jul 11. doi: 10.1200/JCO.19.00894.
The strategy of simultaneously inhibiting proliferation and reactivating apoptosis can eradicate chronic lymphocytic leukemia (CLL) in a large share of patients, suggest results from the phase 2 CLARITY trial.
“Both ibrutinib and venetoclax are active in CLL with improved survival; however, as monotherapies, both currently are given until disease progression,” wrote Peter Hillmen, MBChB, PhD, St. James’s University Hospital, Leeds, England, and his colleagues.
In the single-arm, open-label trial, the investigators treated 53 patients with relapsed or refractory CLL with combination ibrutinib (Imbruvica), a small-molecule inhibitor of Bruton’s tyrosine kinase, and venetoclax (Venclexta), a small molecule inhibitor of the anti-apoptotic protein Bcl-2. The primary endpoint was MRD negativity, defined as presence of fewer than one CLL cell in 10,000 leukocytes, after 12 months of combination therapy.
Results reported in the Journal of Clinical Oncology showed that the combination was highly active, with 53% of patients achieving MRD negativity in the blood and 36% achieving MRD negativity in the marrow.
Most patients, 89%, had a treatment response, and slightly more than half, 51%, achieved a complete remission. With a median 21.1-month follow-up, only a single patient experienced progression and all were still alive.
Adverse effects were generally manageable. Grade 3-4 adverse events of special interest included 34 cases of neutropenia and 1 case of biochemical tumor lysis syndrome that was managed by delaying venetoclax.
“We have demonstrated promising efficacy that indicates potent synergy between ibrutinib and venetoclax for inducing MRD-negative responses with manageable adverse effects,” the investigators wrote. “The observation that a significant proportion of patients experience MRD-negative remission indicates that this combination can be given for a limited period and then stopped after patients achieve a deep remission.”
Whether the combination leads to permanent disease eradication in certain patients is still unclear, the investigators added.
The trial was supported by Bloodwise under the Trials Acceleration Programme, by the National Institute for Health Research Leeds Clinical Research Facility, and by an unrestricted educational grant from Janssen-Cilag and AbbVie. Ibrutinib was provided free of charge by Janssen-Cilag, and venetoclax was provided free of charge by AbbVie. Dr. Hillman reported financial relationships with Janssen, AbbVie, Roche, Pharmacyclics, and Gilead Sciences.
SOURCE: Hillmen P et al. J Clin Oncol. 2019 Jul 11. doi: 10.1200/JCO.19.00894.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
CAR T-cell therapy less effective in transformed follicular lymphoma
All complete responders with FL were still in remission at a median follow-up of 24 months, but the median duration of response was 10.2 months for patients with tFL.
Alexandre V. Hirayama, MD, of the Fred Hutchinson Cancer Research Center in Seattle, and colleagues reported these results in Blood.
The trial enrolled 21 adults with relapsed/refractory CD19+ B-cell malignancies, including 8 patients with FL and 13 with tFL. At baseline, the FL/tFL patients had a median age of 56 years (range, 51-62), and 67% were male. Most patients (n = 19) had stage III/IV disease, 17 had extranodal disease, 8 had bulky disease, and 6 had bone marrow involvement. The patients had received a median of 5 prior therapies (range, 2-8), and 13 had received a transplant.
In this study, patients received a lymphodepleting regimen of cyclophosphamide and fludarabine, followed by 2 x 106 CD19 CAR T cells/kg. Five patients (one with FL and four with tFL) also received bridging chemotherapy between leukapheresis and lymphodepletion.
Grade 1-2 cytokine release syndrome occurred in 50% of FL patients and 39% of tFL patients (P = .35). Grade 1-2 neurotoxicity occurred in 50% and 23%, respectively (P = .67). There were no cases of grade 3 or higher cytokine release syndrome or neurotoxicity.
Most FL patients (7 of 8; 88%) achieved a complete response (CR) to treatment, and all of these patients were still in CR at a median follow-up of 24 months (range, 5-37 months). One FL patient received a transplant while in CR.
Six of 13 tFL patients (46%) achieved a CR. At a median follow-up of 38 months (range, 3-39 months), the median duration of response was 10.2 months. The median progression-free survival was 11.2 months in patients who achieved a CR and 1.4 months in all tFL patients.
The researchers noted that peak CAR T-cell counts and the duration of CAR T-cell detection were similar between FL and tFL patients. However, tFL patients had higher serum interleukin-8 concentrations and higher lactate dehydrogenase levels before treatment.
Past research suggested that IL-8 mediates the recruitment of tumor-associated neutrophils, promotes diffuse large B-cell lymphoma progression, and can contribute to local immune suppression. Other studies have linked elevated lactate dehydrogenase to aggressive disease and a more immunosuppressive tumor microenvironment.
“Although these data raise the possibility that differences in the tumor microenvironment may, in part, contribute to differences in outcomes after CAR T-cell immunotherapy in FL and tFL patients, additional studies are required,” the researchers wrote.
This research was supported by the National Institutes of Health, the Life Science Discovery Fund, the Bezos family, the University of British Columbia Clinician Investigator Program, the Fred Hutchinson Cancer Research Center’s Immunotherapy Integrated Research Center, and Juno Therapeutics/Celgene.
The researchers disclosed relationships with Celgene, Juno Therapeutics, Lyell Immunopharma, Adaptive Biotechnologies, Nohla, Kite Pharma, Gilead, Genentech, Novartis, Eureka Therapeutics, Nektar Therapeutics, Caribou Biosciences, Precision Biosciences, Aptevo, Humanigen, and Allogene.
SOURCE: Hirayama AV et al. Blood. 2019 Jun 26. doi: 10.1182/blood.2019000905
All complete responders with FL were still in remission at a median follow-up of 24 months, but the median duration of response was 10.2 months for patients with tFL.
Alexandre V. Hirayama, MD, of the Fred Hutchinson Cancer Research Center in Seattle, and colleagues reported these results in Blood.
The trial enrolled 21 adults with relapsed/refractory CD19+ B-cell malignancies, including 8 patients with FL and 13 with tFL. At baseline, the FL/tFL patients had a median age of 56 years (range, 51-62), and 67% were male. Most patients (n = 19) had stage III/IV disease, 17 had extranodal disease, 8 had bulky disease, and 6 had bone marrow involvement. The patients had received a median of 5 prior therapies (range, 2-8), and 13 had received a transplant.
In this study, patients received a lymphodepleting regimen of cyclophosphamide and fludarabine, followed by 2 x 106 CD19 CAR T cells/kg. Five patients (one with FL and four with tFL) also received bridging chemotherapy between leukapheresis and lymphodepletion.
Grade 1-2 cytokine release syndrome occurred in 50% of FL patients and 39% of tFL patients (P = .35). Grade 1-2 neurotoxicity occurred in 50% and 23%, respectively (P = .67). There were no cases of grade 3 or higher cytokine release syndrome or neurotoxicity.
Most FL patients (7 of 8; 88%) achieved a complete response (CR) to treatment, and all of these patients were still in CR at a median follow-up of 24 months (range, 5-37 months). One FL patient received a transplant while in CR.
Six of 13 tFL patients (46%) achieved a CR. At a median follow-up of 38 months (range, 3-39 months), the median duration of response was 10.2 months. The median progression-free survival was 11.2 months in patients who achieved a CR and 1.4 months in all tFL patients.
The researchers noted that peak CAR T-cell counts and the duration of CAR T-cell detection were similar between FL and tFL patients. However, tFL patients had higher serum interleukin-8 concentrations and higher lactate dehydrogenase levels before treatment.
Past research suggested that IL-8 mediates the recruitment of tumor-associated neutrophils, promotes diffuse large B-cell lymphoma progression, and can contribute to local immune suppression. Other studies have linked elevated lactate dehydrogenase to aggressive disease and a more immunosuppressive tumor microenvironment.
“Although these data raise the possibility that differences in the tumor microenvironment may, in part, contribute to differences in outcomes after CAR T-cell immunotherapy in FL and tFL patients, additional studies are required,” the researchers wrote.
This research was supported by the National Institutes of Health, the Life Science Discovery Fund, the Bezos family, the University of British Columbia Clinician Investigator Program, the Fred Hutchinson Cancer Research Center’s Immunotherapy Integrated Research Center, and Juno Therapeutics/Celgene.
The researchers disclosed relationships with Celgene, Juno Therapeutics, Lyell Immunopharma, Adaptive Biotechnologies, Nohla, Kite Pharma, Gilead, Genentech, Novartis, Eureka Therapeutics, Nektar Therapeutics, Caribou Biosciences, Precision Biosciences, Aptevo, Humanigen, and Allogene.
SOURCE: Hirayama AV et al. Blood. 2019 Jun 26. doi: 10.1182/blood.2019000905
All complete responders with FL were still in remission at a median follow-up of 24 months, but the median duration of response was 10.2 months for patients with tFL.
Alexandre V. Hirayama, MD, of the Fred Hutchinson Cancer Research Center in Seattle, and colleagues reported these results in Blood.
The trial enrolled 21 adults with relapsed/refractory CD19+ B-cell malignancies, including 8 patients with FL and 13 with tFL. At baseline, the FL/tFL patients had a median age of 56 years (range, 51-62), and 67% were male. Most patients (n = 19) had stage III/IV disease, 17 had extranodal disease, 8 had bulky disease, and 6 had bone marrow involvement. The patients had received a median of 5 prior therapies (range, 2-8), and 13 had received a transplant.
In this study, patients received a lymphodepleting regimen of cyclophosphamide and fludarabine, followed by 2 x 106 CD19 CAR T cells/kg. Five patients (one with FL and four with tFL) also received bridging chemotherapy between leukapheresis and lymphodepletion.
Grade 1-2 cytokine release syndrome occurred in 50% of FL patients and 39% of tFL patients (P = .35). Grade 1-2 neurotoxicity occurred in 50% and 23%, respectively (P = .67). There were no cases of grade 3 or higher cytokine release syndrome or neurotoxicity.
Most FL patients (7 of 8; 88%) achieved a complete response (CR) to treatment, and all of these patients were still in CR at a median follow-up of 24 months (range, 5-37 months). One FL patient received a transplant while in CR.
Six of 13 tFL patients (46%) achieved a CR. At a median follow-up of 38 months (range, 3-39 months), the median duration of response was 10.2 months. The median progression-free survival was 11.2 months in patients who achieved a CR and 1.4 months in all tFL patients.
The researchers noted that peak CAR T-cell counts and the duration of CAR T-cell detection were similar between FL and tFL patients. However, tFL patients had higher serum interleukin-8 concentrations and higher lactate dehydrogenase levels before treatment.
Past research suggested that IL-8 mediates the recruitment of tumor-associated neutrophils, promotes diffuse large B-cell lymphoma progression, and can contribute to local immune suppression. Other studies have linked elevated lactate dehydrogenase to aggressive disease and a more immunosuppressive tumor microenvironment.
“Although these data raise the possibility that differences in the tumor microenvironment may, in part, contribute to differences in outcomes after CAR T-cell immunotherapy in FL and tFL patients, additional studies are required,” the researchers wrote.
This research was supported by the National Institutes of Health, the Life Science Discovery Fund, the Bezos family, the University of British Columbia Clinician Investigator Program, the Fred Hutchinson Cancer Research Center’s Immunotherapy Integrated Research Center, and Juno Therapeutics/Celgene.
The researchers disclosed relationships with Celgene, Juno Therapeutics, Lyell Immunopharma, Adaptive Biotechnologies, Nohla, Kite Pharma, Gilead, Genentech, Novartis, Eureka Therapeutics, Nektar Therapeutics, Caribou Biosciences, Precision Biosciences, Aptevo, Humanigen, and Allogene.
SOURCE: Hirayama AV et al. Blood. 2019 Jun 26. doi: 10.1182/blood.2019000905
FROM BLOOD
Flavopiridol elicits poor response in mantle cell lymphoma, DLBCL
Flavopiridol – also known as alvocidib – showed minimal clinical response in patients with relapsed or refractory mantle cell lymphoma (MCL), diffuse large B-cell lymphoma (DLBCL), and other B-cell lymphomas, according to results from a single-center, phase 1/2 trial.
“Promising preclinical data in cell lines derived from MCL and activated DLBCL led to a series of clinical trials of flavopiridol in various hematological malignancies,” wrote Milos D. Miljković, MD, and colleagues in the lymphoid malignancies branch of the National Cancer Institute in Bethesda, Md. The findings were published in a letter to the editor in Leukemia & Lymphoma.
The study included 28 patients with relapsed/refractory MCL, DLBCL, transformed follicular lymphoma, and primary mediastinal B-cell lymphoma who received a hybrid dosing regimen of the novel CDK inhibitor. Flavopiridol was administered as a 30-minute bolus, followed by a 4-hour infusion.
The researchers used an intrapatient dose escalation between the first and successive cycles, in addition to a three-plus-three interpatient escalation, to lessen the risk of tumor lysis syndrome (TLS).
The primary outcomes were the clinical response rate, maximum tolerated dose, dose-limiting toxicities, and toxicity profile of the hybrid dosing regimen.
Of 26 evaluable patients, one patient with DLBCL maintained a partial response for 84 days (overall response rate, 3.8%). One patient with MCL had a 50% decrease in the size of target lesions at 2 months, but this was not sustained at 4 months. In total, nine patients had stable disease for a disease control rate of 38.4%.
“[Flavopiridol] had minimal efficacy in patients with relapsed/refractory non-Hodgkin B-cell lymphoma, casting doubt on the utility of CDK inhibition in this disease,” the researchers wrote.
With respect to safety, there were eight dose-limiting toxicities reported in three patients. These included grade 3 TLS, elevated transaminase levels, hypoalbuminemia, hyperkalemia, non-neutropenic infection, and grade 4 metabolic acidosis and gastrointestinal perforation.
The most common treatment-related toxicities were hematologic, including neutropenia, anemia, thrombocytopenia, leukocytosis, and lymphopenia.
Dr. Miljković and colleagues noted that CDK inhibitor therapy may elicit better responses when used in combination with other agents.
“Ongoing trials of more specific CDK inhibitors in combination with other agents will help elucidate their role in lymphoma treatment,” they wrote.
The trial is sponsored by the National Cancer Institute and the study authors are employees of the National Cancer Institute.
SOURCE: Miljkovic MD et al. Leuk Lymphoma. 2019 Jun 17. doi: 10.1080/10428194.2019.1627540.
Flavopiridol – also known as alvocidib – showed minimal clinical response in patients with relapsed or refractory mantle cell lymphoma (MCL), diffuse large B-cell lymphoma (DLBCL), and other B-cell lymphomas, according to results from a single-center, phase 1/2 trial.
“Promising preclinical data in cell lines derived from MCL and activated DLBCL led to a series of clinical trials of flavopiridol in various hematological malignancies,” wrote Milos D. Miljković, MD, and colleagues in the lymphoid malignancies branch of the National Cancer Institute in Bethesda, Md. The findings were published in a letter to the editor in Leukemia & Lymphoma.
The study included 28 patients with relapsed/refractory MCL, DLBCL, transformed follicular lymphoma, and primary mediastinal B-cell lymphoma who received a hybrid dosing regimen of the novel CDK inhibitor. Flavopiridol was administered as a 30-minute bolus, followed by a 4-hour infusion.
The researchers used an intrapatient dose escalation between the first and successive cycles, in addition to a three-plus-three interpatient escalation, to lessen the risk of tumor lysis syndrome (TLS).
The primary outcomes were the clinical response rate, maximum tolerated dose, dose-limiting toxicities, and toxicity profile of the hybrid dosing regimen.
Of 26 evaluable patients, one patient with DLBCL maintained a partial response for 84 days (overall response rate, 3.8%). One patient with MCL had a 50% decrease in the size of target lesions at 2 months, but this was not sustained at 4 months. In total, nine patients had stable disease for a disease control rate of 38.4%.
“[Flavopiridol] had minimal efficacy in patients with relapsed/refractory non-Hodgkin B-cell lymphoma, casting doubt on the utility of CDK inhibition in this disease,” the researchers wrote.
With respect to safety, there were eight dose-limiting toxicities reported in three patients. These included grade 3 TLS, elevated transaminase levels, hypoalbuminemia, hyperkalemia, non-neutropenic infection, and grade 4 metabolic acidosis and gastrointestinal perforation.
The most common treatment-related toxicities were hematologic, including neutropenia, anemia, thrombocytopenia, leukocytosis, and lymphopenia.
Dr. Miljković and colleagues noted that CDK inhibitor therapy may elicit better responses when used in combination with other agents.
“Ongoing trials of more specific CDK inhibitors in combination with other agents will help elucidate their role in lymphoma treatment,” they wrote.
The trial is sponsored by the National Cancer Institute and the study authors are employees of the National Cancer Institute.
SOURCE: Miljkovic MD et al. Leuk Lymphoma. 2019 Jun 17. doi: 10.1080/10428194.2019.1627540.
Flavopiridol – also known as alvocidib – showed minimal clinical response in patients with relapsed or refractory mantle cell lymphoma (MCL), diffuse large B-cell lymphoma (DLBCL), and other B-cell lymphomas, according to results from a single-center, phase 1/2 trial.
“Promising preclinical data in cell lines derived from MCL and activated DLBCL led to a series of clinical trials of flavopiridol in various hematological malignancies,” wrote Milos D. Miljković, MD, and colleagues in the lymphoid malignancies branch of the National Cancer Institute in Bethesda, Md. The findings were published in a letter to the editor in Leukemia & Lymphoma.
The study included 28 patients with relapsed/refractory MCL, DLBCL, transformed follicular lymphoma, and primary mediastinal B-cell lymphoma who received a hybrid dosing regimen of the novel CDK inhibitor. Flavopiridol was administered as a 30-minute bolus, followed by a 4-hour infusion.
The researchers used an intrapatient dose escalation between the first and successive cycles, in addition to a three-plus-three interpatient escalation, to lessen the risk of tumor lysis syndrome (TLS).
The primary outcomes were the clinical response rate, maximum tolerated dose, dose-limiting toxicities, and toxicity profile of the hybrid dosing regimen.
Of 26 evaluable patients, one patient with DLBCL maintained a partial response for 84 days (overall response rate, 3.8%). One patient with MCL had a 50% decrease in the size of target lesions at 2 months, but this was not sustained at 4 months. In total, nine patients had stable disease for a disease control rate of 38.4%.
“[Flavopiridol] had minimal efficacy in patients with relapsed/refractory non-Hodgkin B-cell lymphoma, casting doubt on the utility of CDK inhibition in this disease,” the researchers wrote.
With respect to safety, there were eight dose-limiting toxicities reported in three patients. These included grade 3 TLS, elevated transaminase levels, hypoalbuminemia, hyperkalemia, non-neutropenic infection, and grade 4 metabolic acidosis and gastrointestinal perforation.
The most common treatment-related toxicities were hematologic, including neutropenia, anemia, thrombocytopenia, leukocytosis, and lymphopenia.
Dr. Miljković and colleagues noted that CDK inhibitor therapy may elicit better responses when used in combination with other agents.
“Ongoing trials of more specific CDK inhibitors in combination with other agents will help elucidate their role in lymphoma treatment,” they wrote.
The trial is sponsored by the National Cancer Institute and the study authors are employees of the National Cancer Institute.
SOURCE: Miljkovic MD et al. Leuk Lymphoma. 2019 Jun 17. doi: 10.1080/10428194.2019.1627540.
FROM LEUKEMIA & LYMPHOMA
Intravenous CNS chemo looks best for testicular DLBCL
For patients with testicular diffuse large B-cell lymphoma (T-DLBCL), intravenous central nervous system (CNS)–directed chemotherapy and prophylactic treatment of the contralateral testis offer the best survival outcomes, according to findings from a recent retrospective analysis.
In contrast, intrathecal chemotherapy offered no benefit, reported lead author Susanna Mannisto, MD, of Helsinki University Hospital in Finland, and her colleagues. Survival advantages gained by CNS-directed chemotherapy were generally due to control of systemic disease rather than prevention of CNS progression, they noted.
There have not been randomized trials conducted specifically in T-DLBCL and treatment guidelines are currently based on phase 2 trials. Treatment for T-DLBCL typically involves cyclophosphamide, doxorubicin, vincristine, prednisolone plus rituximab (R–CHOP), with the addition of CNS prophylaxis with either intravenous or intrathecal methotrexate or cytarabine (AraC) in eligible patients. “Thus far, however, no prospective randomised studies on the benefit of this approach have been published,” the investigators wrote. The study is in the European Journal of Cancer.
They drew data from the Danish Lymphoma Registry and three Southern Finland University Hospitals. Out of 235 patients diagnosed with T-DLBCL between 1987 and 2013, 192 (82%) were treated with curative anthracycline-based chemotherapy. As some cases dated back to 1987, about one-third (36%) were treated before rituximab was available. A slightly higher proportion received intravenous CNS-directed chemotherapy (40%). Due to data availability, 189 patients were included in the survival analysis.
Results of multivariate analyses showed that CNS-directed chemotherapy (intravenous methotrexate, high-dose AraC, or both), was associated with an approximately 58% decreased risk of death across the entire population (hazard ratio [HR] for overall survival, 0.419; 95% confidence interval, 0.256-0.686; P = .001), with elderly patients realizing the greatest benefit.
“[I]t is important to note that we did not observe reduction in the CNS recurrence rate, but rather a better control of the systemic disease, suggesting that R–CHOP alone may not be a sufficient therapy for the patients with testicular DLBCL involvement,” the investigators wrote.
Intrathecal CNS-targeted therapy did not correlate with improved survival in the study. Likewise, for the entire population, rituximab did not boost survival; however, for high risk patients (International Prognostic Index, 3-5), immunochemotherapy did provide a better rate of 5-year disease-specific survival than that of conventional chemotherapy (44% vs 14%; P = .019).
Treatment of the contralateral testis was worthwhile for the entire population, with a 46% decreased risk of death (HR for overall survival, 0.514, 95% CI, 0.338-0.782; P = .002).
Shortest overall survival times, regardless of treatment type, were reported among patients with poor performance status, elevated lactate dehydrogenase (LDH), primary T-DLBCL, more extranodal sites, higher International Prognostic Index, and patients older than 70 years.
“[O]ur results recommend aggressive chemotherapy with [intravenous high-dose methotrexate and/or high-dose AraC] for better control of systemic disease for eligible patients,” the investigators concluded. “In addition, treatment of the contralateral testis with either radiotherapy or orchiectomy should be included in the management strategy of patients with T-DLBCL.”
The study was funded by the Academy of Finland, the Finnish Cancer Foundation, the Sigrid Juselius Foundation, and others. The investigators reported additional relationships with Pfizer, Takeda, Roche, Sanofi, and others.
SOURCE: Mannisto S et al. Eur J Cancer. 2019 May 10. doi: 10.1016/j.ejca.2019.04.004.
For patients with testicular diffuse large B-cell lymphoma (T-DLBCL), intravenous central nervous system (CNS)–directed chemotherapy and prophylactic treatment of the contralateral testis offer the best survival outcomes, according to findings from a recent retrospective analysis.
In contrast, intrathecal chemotherapy offered no benefit, reported lead author Susanna Mannisto, MD, of Helsinki University Hospital in Finland, and her colleagues. Survival advantages gained by CNS-directed chemotherapy were generally due to control of systemic disease rather than prevention of CNS progression, they noted.
There have not been randomized trials conducted specifically in T-DLBCL and treatment guidelines are currently based on phase 2 trials. Treatment for T-DLBCL typically involves cyclophosphamide, doxorubicin, vincristine, prednisolone plus rituximab (R–CHOP), with the addition of CNS prophylaxis with either intravenous or intrathecal methotrexate or cytarabine (AraC) in eligible patients. “Thus far, however, no prospective randomised studies on the benefit of this approach have been published,” the investigators wrote. The study is in the European Journal of Cancer.
They drew data from the Danish Lymphoma Registry and three Southern Finland University Hospitals. Out of 235 patients diagnosed with T-DLBCL between 1987 and 2013, 192 (82%) were treated with curative anthracycline-based chemotherapy. As some cases dated back to 1987, about one-third (36%) were treated before rituximab was available. A slightly higher proportion received intravenous CNS-directed chemotherapy (40%). Due to data availability, 189 patients were included in the survival analysis.
Results of multivariate analyses showed that CNS-directed chemotherapy (intravenous methotrexate, high-dose AraC, or both), was associated with an approximately 58% decreased risk of death across the entire population (hazard ratio [HR] for overall survival, 0.419; 95% confidence interval, 0.256-0.686; P = .001), with elderly patients realizing the greatest benefit.
“[I]t is important to note that we did not observe reduction in the CNS recurrence rate, but rather a better control of the systemic disease, suggesting that R–CHOP alone may not be a sufficient therapy for the patients with testicular DLBCL involvement,” the investigators wrote.
Intrathecal CNS-targeted therapy did not correlate with improved survival in the study. Likewise, for the entire population, rituximab did not boost survival; however, for high risk patients (International Prognostic Index, 3-5), immunochemotherapy did provide a better rate of 5-year disease-specific survival than that of conventional chemotherapy (44% vs 14%; P = .019).
Treatment of the contralateral testis was worthwhile for the entire population, with a 46% decreased risk of death (HR for overall survival, 0.514, 95% CI, 0.338-0.782; P = .002).
Shortest overall survival times, regardless of treatment type, were reported among patients with poor performance status, elevated lactate dehydrogenase (LDH), primary T-DLBCL, more extranodal sites, higher International Prognostic Index, and patients older than 70 years.
“[O]ur results recommend aggressive chemotherapy with [intravenous high-dose methotrexate and/or high-dose AraC] for better control of systemic disease for eligible patients,” the investigators concluded. “In addition, treatment of the contralateral testis with either radiotherapy or orchiectomy should be included in the management strategy of patients with T-DLBCL.”
The study was funded by the Academy of Finland, the Finnish Cancer Foundation, the Sigrid Juselius Foundation, and others. The investigators reported additional relationships with Pfizer, Takeda, Roche, Sanofi, and others.
SOURCE: Mannisto S et al. Eur J Cancer. 2019 May 10. doi: 10.1016/j.ejca.2019.04.004.
For patients with testicular diffuse large B-cell lymphoma (T-DLBCL), intravenous central nervous system (CNS)–directed chemotherapy and prophylactic treatment of the contralateral testis offer the best survival outcomes, according to findings from a recent retrospective analysis.
In contrast, intrathecal chemotherapy offered no benefit, reported lead author Susanna Mannisto, MD, of Helsinki University Hospital in Finland, and her colleagues. Survival advantages gained by CNS-directed chemotherapy were generally due to control of systemic disease rather than prevention of CNS progression, they noted.
There have not been randomized trials conducted specifically in T-DLBCL and treatment guidelines are currently based on phase 2 trials. Treatment for T-DLBCL typically involves cyclophosphamide, doxorubicin, vincristine, prednisolone plus rituximab (R–CHOP), with the addition of CNS prophylaxis with either intravenous or intrathecal methotrexate or cytarabine (AraC) in eligible patients. “Thus far, however, no prospective randomised studies on the benefit of this approach have been published,” the investigators wrote. The study is in the European Journal of Cancer.
They drew data from the Danish Lymphoma Registry and three Southern Finland University Hospitals. Out of 235 patients diagnosed with T-DLBCL between 1987 and 2013, 192 (82%) were treated with curative anthracycline-based chemotherapy. As some cases dated back to 1987, about one-third (36%) were treated before rituximab was available. A slightly higher proportion received intravenous CNS-directed chemotherapy (40%). Due to data availability, 189 patients were included in the survival analysis.
Results of multivariate analyses showed that CNS-directed chemotherapy (intravenous methotrexate, high-dose AraC, or both), was associated with an approximately 58% decreased risk of death across the entire population (hazard ratio [HR] for overall survival, 0.419; 95% confidence interval, 0.256-0.686; P = .001), with elderly patients realizing the greatest benefit.
“[I]t is important to note that we did not observe reduction in the CNS recurrence rate, but rather a better control of the systemic disease, suggesting that R–CHOP alone may not be a sufficient therapy for the patients with testicular DLBCL involvement,” the investigators wrote.
Intrathecal CNS-targeted therapy did not correlate with improved survival in the study. Likewise, for the entire population, rituximab did not boost survival; however, for high risk patients (International Prognostic Index, 3-5), immunochemotherapy did provide a better rate of 5-year disease-specific survival than that of conventional chemotherapy (44% vs 14%; P = .019).
Treatment of the contralateral testis was worthwhile for the entire population, with a 46% decreased risk of death (HR for overall survival, 0.514, 95% CI, 0.338-0.782; P = .002).
Shortest overall survival times, regardless of treatment type, were reported among patients with poor performance status, elevated lactate dehydrogenase (LDH), primary T-DLBCL, more extranodal sites, higher International Prognostic Index, and patients older than 70 years.
“[O]ur results recommend aggressive chemotherapy with [intravenous high-dose methotrexate and/or high-dose AraC] for better control of systemic disease for eligible patients,” the investigators concluded. “In addition, treatment of the contralateral testis with either radiotherapy or orchiectomy should be included in the management strategy of patients with T-DLBCL.”
The study was funded by the Academy of Finland, the Finnish Cancer Foundation, the Sigrid Juselius Foundation, and others. The investigators reported additional relationships with Pfizer, Takeda, Roche, Sanofi, and others.
SOURCE: Mannisto S et al. Eur J Cancer. 2019 May 10. doi: 10.1016/j.ejca.2019.04.004.
FROM EUROPEAN JOURNAL OF CANCER
On second thought, lenalidomide does improve DLBCL outcomes
LUGANO, Switzerland – Hot on the heels of the phase 3 ROBUST study showing that adding lenalidomide to standard chemotherapy did not improve outcomes for patients with untreated diffuse large B-cell lymphoma come results of a different study showing a significant benefit with the therapy.
Although, as previously reported, adding lenalidomide (Revlimid) to standard chemotherapy for patients with newly diagnosed ABC-type diffuse large B-cell lymphoma (DLBCL) – the so-called R2-CHOP regimen – did not significantly improve either progression-free or overall survival, compared with R-CHOP alone in ROBUST, results from the randomized phase 2 ECOG-ACRIN 1412 study showed that R2-CHOP was associated with a 34% reduction in the risk of disease progression or death, compared with R-CHOP alone.
“The efficacy endpoints are consistent, with trends toward higher PET complete response rate and improved overall survival with R-squared CHOP,” Grzegorz S. Nowakowski, MD, of the Mayo Clinic, Rochester, Minn., said at the International Conference on Malignant Lymphoma.
So what’s behind the conflicting findings?
The differences between the results of the two studies may be accounted for by the higher lenalidomide dose used in ECOG-ACRIN 1412, the patient populations – all comers in ECOG-ACRIN versus only patients with activated B-cell (ABC) type DLBCL in ROBUST – and by a 10-day shorter median time to treatment in ECOG-ACRIN 1412, said invited discussant Margaret A. Shipp, MD, of the Dana-Farber Cancer Institute in Boston.
The rationale for adding lenalidomide to R-CHOP came from in vitro studies showing antiproliferative and immunomodulatory action of lenalidomide against DLBCL, as well as two proof-of-concept clinical studies (REAL07 and MC078E) indicating efficacy against non-germinal center-like B (GCB) type DLBCL.
In a subanalysis of patients enrolled in MC078E, Dr. Nowakowski and colleagues found that using classification of patients by cell of origin with the NanoString Lymphoma Subtype assay, the addition of lenalidomide to R-CHOP “appears to mitigate the negative impact of an ABC molecular subtype on the outcome.”
ECOG-ACRIN 1412 details
Goals of the ECOG-ACRIN 1412 study were to evaluate the effect of lenalidomide both in all DLBCL subtypes and in the ABC subtype, maximize the synergy of the immunomodulator with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) while maintaining R-CHOP dose intensity, and facilitate the enrollment of patients with rapidly progressive disease.
To accomplish the last goal, the study was designed to allow enrollment based on local laboratory findings, scans, and diagnostic pathology, without required identification of the cell of origin. Built in to the design was the plan for final eligibility to be based on central pathology review. In other words, the trial design took into account the likelihood that some enrolled patients would not qualify for eligibility based on later pathology review.
The investigators enrolled 349 adults aged 18 years or older with pathologically confirmed DLBCL (regardless of the cell of origin), stage II bulky to stage IV disease, International Prognostic Index (IPI) scores of 2 or greater, and Eastern Cooperative Oncology Group performance status scores of 2 or less.
The patients were stratified by age (younger than 60 years vs. 60 years and older) and by IPI score (2/3 vs. 4/5), and then randomized to receive either six cycles of R-CHOP or R2-CHOP. Lenalidomide was given in a dose of 25 mg on days 1-10 of each cycle. In contrast, the dose used in ROBUST was 15 mg given on days 1-14 of each cycle.
In ECOG-ACRIN 1412, patients assigned to lenalidomide received mandatory neutropenia prophylaxis with granulocyte-colony stimulating factor.
The time from diagnosis to treatment was a median of 21 days, with only 61 of 280 evaluable patients starting treatment more than 31 days after diagnosis. In ROBUST, the median time to start therapy was 31 days.
Dr. Nowakowski and his colleagues had previously shown that time to treatment is an important prognostic factor in DLBCL.
The efficacy evaluation included 145 patients assigned to R2-CHOP, and 135 assigned to R-CHOP. Primary reasons for exclusion were ineligibity following central pathology review or lack of diagnostic material for review.
After a median follow-up of 2.5 years, R2-CHOP was associated with a 34% improvement in progression-free survival, the primary endpoint (hazard ratio [HR] 0.66, P = .03). The 1-year progression-free survival rates were 83% with R2-CHOP and 73% with R-CHOP. Respective 2-year progression-free survival rates were 76% and 70%.
There was no significant difference, however, in the secondary overall survival endpoints with 1-year and 2-year overall survival of 93% vs. 87% and 86% vs. 80%, respectively.
Similarly, there was no difference in rates of PET-ascertained complete response, at 72% with R2-CHOP and 67% with R-CHOP.
R2-CHOP showed greater benefit across most subgroups, including patients with lower IPI score, patients with bulky disease, patients younger than 60 years, women, and patients with shorter time to treatment. There were also nonsignificant trends hinting at better outcomes with R2-CHOP, regardless of cell of origin.
Toxicities were typical for R-CHOP, although patients on R2-CHOP had significantly higher rates of grade 3 or 4 diarrhea, febrile neutropenia, and thrombocytopenia.
In addition to the trial differences mentioned previously, the differences in outcomes might be explained by the possibility that lenalidomide activity is not restricted to ABC DLBCL, Dr. Shipp said.
“One of the things that’s important to remember about lenalidomide is that it’s an immunomodulating agent and it has also has effects on tumor-infiltrating T cells,” she said.
Differences in response to therapy may also be explained by recent findings showing genetic heterogeneity in transcription-defined ABC DLBCLs, she said.
ECOG-ACRIN 1412 was supported by the National Cancer Institute and by Celgene. Dr. Nowakowski reported consulting/advising for and research funding from Celgene and others. Dr. Shipp reported consulting/advising for Bristol-Myers Squibb, honoraria from BMS and AstraZeneca, and institutional research funding from BMS and Bayer.
SOURCE: Nowakowski GS et al. 15-ICML, Abstract 006.
LUGANO, Switzerland – Hot on the heels of the phase 3 ROBUST study showing that adding lenalidomide to standard chemotherapy did not improve outcomes for patients with untreated diffuse large B-cell lymphoma come results of a different study showing a significant benefit with the therapy.
Although, as previously reported, adding lenalidomide (Revlimid) to standard chemotherapy for patients with newly diagnosed ABC-type diffuse large B-cell lymphoma (DLBCL) – the so-called R2-CHOP regimen – did not significantly improve either progression-free or overall survival, compared with R-CHOP alone in ROBUST, results from the randomized phase 2 ECOG-ACRIN 1412 study showed that R2-CHOP was associated with a 34% reduction in the risk of disease progression or death, compared with R-CHOP alone.
“The efficacy endpoints are consistent, with trends toward higher PET complete response rate and improved overall survival with R-squared CHOP,” Grzegorz S. Nowakowski, MD, of the Mayo Clinic, Rochester, Minn., said at the International Conference on Malignant Lymphoma.
So what’s behind the conflicting findings?
The differences between the results of the two studies may be accounted for by the higher lenalidomide dose used in ECOG-ACRIN 1412, the patient populations – all comers in ECOG-ACRIN versus only patients with activated B-cell (ABC) type DLBCL in ROBUST – and by a 10-day shorter median time to treatment in ECOG-ACRIN 1412, said invited discussant Margaret A. Shipp, MD, of the Dana-Farber Cancer Institute in Boston.
The rationale for adding lenalidomide to R-CHOP came from in vitro studies showing antiproliferative and immunomodulatory action of lenalidomide against DLBCL, as well as two proof-of-concept clinical studies (REAL07 and MC078E) indicating efficacy against non-germinal center-like B (GCB) type DLBCL.
In a subanalysis of patients enrolled in MC078E, Dr. Nowakowski and colleagues found that using classification of patients by cell of origin with the NanoString Lymphoma Subtype assay, the addition of lenalidomide to R-CHOP “appears to mitigate the negative impact of an ABC molecular subtype on the outcome.”
ECOG-ACRIN 1412 details
Goals of the ECOG-ACRIN 1412 study were to evaluate the effect of lenalidomide both in all DLBCL subtypes and in the ABC subtype, maximize the synergy of the immunomodulator with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) while maintaining R-CHOP dose intensity, and facilitate the enrollment of patients with rapidly progressive disease.
To accomplish the last goal, the study was designed to allow enrollment based on local laboratory findings, scans, and diagnostic pathology, without required identification of the cell of origin. Built in to the design was the plan for final eligibility to be based on central pathology review. In other words, the trial design took into account the likelihood that some enrolled patients would not qualify for eligibility based on later pathology review.
The investigators enrolled 349 adults aged 18 years or older with pathologically confirmed DLBCL (regardless of the cell of origin), stage II bulky to stage IV disease, International Prognostic Index (IPI) scores of 2 or greater, and Eastern Cooperative Oncology Group performance status scores of 2 or less.
The patients were stratified by age (younger than 60 years vs. 60 years and older) and by IPI score (2/3 vs. 4/5), and then randomized to receive either six cycles of R-CHOP or R2-CHOP. Lenalidomide was given in a dose of 25 mg on days 1-10 of each cycle. In contrast, the dose used in ROBUST was 15 mg given on days 1-14 of each cycle.
In ECOG-ACRIN 1412, patients assigned to lenalidomide received mandatory neutropenia prophylaxis with granulocyte-colony stimulating factor.
The time from diagnosis to treatment was a median of 21 days, with only 61 of 280 evaluable patients starting treatment more than 31 days after diagnosis. In ROBUST, the median time to start therapy was 31 days.
Dr. Nowakowski and his colleagues had previously shown that time to treatment is an important prognostic factor in DLBCL.
The efficacy evaluation included 145 patients assigned to R2-CHOP, and 135 assigned to R-CHOP. Primary reasons for exclusion were ineligibity following central pathology review or lack of diagnostic material for review.
After a median follow-up of 2.5 years, R2-CHOP was associated with a 34% improvement in progression-free survival, the primary endpoint (hazard ratio [HR] 0.66, P = .03). The 1-year progression-free survival rates were 83% with R2-CHOP and 73% with R-CHOP. Respective 2-year progression-free survival rates were 76% and 70%.
There was no significant difference, however, in the secondary overall survival endpoints with 1-year and 2-year overall survival of 93% vs. 87% and 86% vs. 80%, respectively.
Similarly, there was no difference in rates of PET-ascertained complete response, at 72% with R2-CHOP and 67% with R-CHOP.
R2-CHOP showed greater benefit across most subgroups, including patients with lower IPI score, patients with bulky disease, patients younger than 60 years, women, and patients with shorter time to treatment. There were also nonsignificant trends hinting at better outcomes with R2-CHOP, regardless of cell of origin.
Toxicities were typical for R-CHOP, although patients on R2-CHOP had significantly higher rates of grade 3 or 4 diarrhea, febrile neutropenia, and thrombocytopenia.
In addition to the trial differences mentioned previously, the differences in outcomes might be explained by the possibility that lenalidomide activity is not restricted to ABC DLBCL, Dr. Shipp said.
“One of the things that’s important to remember about lenalidomide is that it’s an immunomodulating agent and it has also has effects on tumor-infiltrating T cells,” she said.
Differences in response to therapy may also be explained by recent findings showing genetic heterogeneity in transcription-defined ABC DLBCLs, she said.
ECOG-ACRIN 1412 was supported by the National Cancer Institute and by Celgene. Dr. Nowakowski reported consulting/advising for and research funding from Celgene and others. Dr. Shipp reported consulting/advising for Bristol-Myers Squibb, honoraria from BMS and AstraZeneca, and institutional research funding from BMS and Bayer.
SOURCE: Nowakowski GS et al. 15-ICML, Abstract 006.
LUGANO, Switzerland – Hot on the heels of the phase 3 ROBUST study showing that adding lenalidomide to standard chemotherapy did not improve outcomes for patients with untreated diffuse large B-cell lymphoma come results of a different study showing a significant benefit with the therapy.
Although, as previously reported, adding lenalidomide (Revlimid) to standard chemotherapy for patients with newly diagnosed ABC-type diffuse large B-cell lymphoma (DLBCL) – the so-called R2-CHOP regimen – did not significantly improve either progression-free or overall survival, compared with R-CHOP alone in ROBUST, results from the randomized phase 2 ECOG-ACRIN 1412 study showed that R2-CHOP was associated with a 34% reduction in the risk of disease progression or death, compared with R-CHOP alone.
“The efficacy endpoints are consistent, with trends toward higher PET complete response rate and improved overall survival with R-squared CHOP,” Grzegorz S. Nowakowski, MD, of the Mayo Clinic, Rochester, Minn., said at the International Conference on Malignant Lymphoma.
So what’s behind the conflicting findings?
The differences between the results of the two studies may be accounted for by the higher lenalidomide dose used in ECOG-ACRIN 1412, the patient populations – all comers in ECOG-ACRIN versus only patients with activated B-cell (ABC) type DLBCL in ROBUST – and by a 10-day shorter median time to treatment in ECOG-ACRIN 1412, said invited discussant Margaret A. Shipp, MD, of the Dana-Farber Cancer Institute in Boston.
The rationale for adding lenalidomide to R-CHOP came from in vitro studies showing antiproliferative and immunomodulatory action of lenalidomide against DLBCL, as well as two proof-of-concept clinical studies (REAL07 and MC078E) indicating efficacy against non-germinal center-like B (GCB) type DLBCL.
In a subanalysis of patients enrolled in MC078E, Dr. Nowakowski and colleagues found that using classification of patients by cell of origin with the NanoString Lymphoma Subtype assay, the addition of lenalidomide to R-CHOP “appears to mitigate the negative impact of an ABC molecular subtype on the outcome.”
ECOG-ACRIN 1412 details
Goals of the ECOG-ACRIN 1412 study were to evaluate the effect of lenalidomide both in all DLBCL subtypes and in the ABC subtype, maximize the synergy of the immunomodulator with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone) while maintaining R-CHOP dose intensity, and facilitate the enrollment of patients with rapidly progressive disease.
To accomplish the last goal, the study was designed to allow enrollment based on local laboratory findings, scans, and diagnostic pathology, without required identification of the cell of origin. Built in to the design was the plan for final eligibility to be based on central pathology review. In other words, the trial design took into account the likelihood that some enrolled patients would not qualify for eligibility based on later pathology review.
The investigators enrolled 349 adults aged 18 years or older with pathologically confirmed DLBCL (regardless of the cell of origin), stage II bulky to stage IV disease, International Prognostic Index (IPI) scores of 2 or greater, and Eastern Cooperative Oncology Group performance status scores of 2 or less.
The patients were stratified by age (younger than 60 years vs. 60 years and older) and by IPI score (2/3 vs. 4/5), and then randomized to receive either six cycles of R-CHOP or R2-CHOP. Lenalidomide was given in a dose of 25 mg on days 1-10 of each cycle. In contrast, the dose used in ROBUST was 15 mg given on days 1-14 of each cycle.
In ECOG-ACRIN 1412, patients assigned to lenalidomide received mandatory neutropenia prophylaxis with granulocyte-colony stimulating factor.
The time from diagnosis to treatment was a median of 21 days, with only 61 of 280 evaluable patients starting treatment more than 31 days after diagnosis. In ROBUST, the median time to start therapy was 31 days.
Dr. Nowakowski and his colleagues had previously shown that time to treatment is an important prognostic factor in DLBCL.
The efficacy evaluation included 145 patients assigned to R2-CHOP, and 135 assigned to R-CHOP. Primary reasons for exclusion were ineligibity following central pathology review or lack of diagnostic material for review.
After a median follow-up of 2.5 years, R2-CHOP was associated with a 34% improvement in progression-free survival, the primary endpoint (hazard ratio [HR] 0.66, P = .03). The 1-year progression-free survival rates were 83% with R2-CHOP and 73% with R-CHOP. Respective 2-year progression-free survival rates were 76% and 70%.
There was no significant difference, however, in the secondary overall survival endpoints with 1-year and 2-year overall survival of 93% vs. 87% and 86% vs. 80%, respectively.
Similarly, there was no difference in rates of PET-ascertained complete response, at 72% with R2-CHOP and 67% with R-CHOP.
R2-CHOP showed greater benefit across most subgroups, including patients with lower IPI score, patients with bulky disease, patients younger than 60 years, women, and patients with shorter time to treatment. There were also nonsignificant trends hinting at better outcomes with R2-CHOP, regardless of cell of origin.
Toxicities were typical for R-CHOP, although patients on R2-CHOP had significantly higher rates of grade 3 or 4 diarrhea, febrile neutropenia, and thrombocytopenia.
In addition to the trial differences mentioned previously, the differences in outcomes might be explained by the possibility that lenalidomide activity is not restricted to ABC DLBCL, Dr. Shipp said.
“One of the things that’s important to remember about lenalidomide is that it’s an immunomodulating agent and it has also has effects on tumor-infiltrating T cells,” she said.
Differences in response to therapy may also be explained by recent findings showing genetic heterogeneity in transcription-defined ABC DLBCLs, she said.
ECOG-ACRIN 1412 was supported by the National Cancer Institute and by Celgene. Dr. Nowakowski reported consulting/advising for and research funding from Celgene and others. Dr. Shipp reported consulting/advising for Bristol-Myers Squibb, honoraria from BMS and AstraZeneca, and institutional research funding from BMS and Bayer.
SOURCE: Nowakowski GS et al. 15-ICML, Abstract 006.
REPORTING FROM 15-ICML