User login
Worldwide Bipolar Disorder Prevalence Estimated at 2.4%
Worldwide, the prevalence of bipolar disorder type I is estimated to be 0.6%, that of type II is 0.4%, and that of subthreshold bipolar disorder is 1.4%, yielding a total bipolar disorder spectrum prevalence of 2.4%, according to a new report in the March issue of the Archives of General Psychiatry.
These estimates were derived from what the investigators described as the first international data ever collected using Diagnostic and Statistical Manual–IV definitions for the full spectrum of bipolar disorders and standardized measures to survey nationally representative samples in 11 low-income, middle-income, and high-income countries.
Even though prevalence varied from one country to the next, disease severity, impact on daily life, and patterns of comorbidity remained strikingly similar, said Kathleen R. Merikangas, Ph.D., of the Intramural Research Program of the National Institute of Mental Health (NIMH), and her associates in the World Mental Health Survey Initiative.
This World Health Organization (WHO) project "aims to obtain accurate cross-national information on the prevalence, correlates, and service patterns of mental disorders." The investigators conducted in-person, population-based surveys of 61,392 adults in Brazil, Colombia, Mexico, the United States, Bulgaria, Romania, China, India, Japan, Lebanon, and New Zealand.
In addition to the overall prevalences, they found that the 1-year prevalence of bipolar disorder type I was 0.4%, that of bipolar disorder type II was 0.3%, and that of subthreshold bipolar disorder was 0.8%, for a total bipolar spectrum disorder annual prevalence of 1.5%.
In general, high-income countries had the highest prevalences of bipolar disease and low-income countries had the lowest. The United States had the highest prevalence of overall (4.4%) and annual (2.8%) disease, while India had the lowest (0.1% for both). Two exceptions to this rule were Japan, a high-income country with very low overall (0.7%) and annual (0.2%) prevalences, and Colombia, a low-income country with a high overall prevalence (2.6%).
The mean ages at onset were 18 years for bipolar disorder type I, 20 years for bipolar disorder type II, and 22 years for subthreshold bipolar disorder.
Patterns of comorbidity were remarkably consistent among the different countries. Three-fourths of patients with bipolar spectrum disorders also met criteria for other psychiatric disorders, and the majority had three or more of them. "Anxiety disorders, particularly panic attacks, were the most common comorbid conditions (63%), followed by behavior disorders (45%) and substance use disorders (37%)," Dr. Merikangas and her colleagues said (Arch. Gen. Psychiatry 2011;68:241-51).
The association between bipolar disorders and substance use disorders across the globe was particularly striking in light of the large differences between countries in rates of substance use and abuse. "This suggests that [bipolar disorder] can be considered a risk factor for the development of substance use disorders ... [and supports] the need for careful probing of a history of bipolarity among those with substance use disorders," they said.
The proportion of patients with suicidal behaviors rose with increasing severity of bipolar disease. Approximately 10% of those with subthreshold bipolar disorder, 20% of those with bipolar disorder type II, and 25% of those with bipolar disorder type I said that they had attempted suicide over the last 12 months.
This "striking" finding of suicidality, together with the early age at onset and the strong association with other mental health disorders, provides "further documentation of the individual and societal disability associated with this disorder," they noted.
A substantially higher proportion of patients in high-income countries, compared with middle- or low-income countries, reported having used mental health services. Lifetime use of such services was 50% in high-income countries, 34% in middle-income countries, and 25% in low-income countries.
"Because [bipolar disorder] has an average age at onset at one of the most critical periods of educational, occupational, and social development, its consequences often lead to lifelong disability," the investigators said, and this lack of mental health treatment, especially in low-income countries, is therefore "alarming."
"These data also provide the first aggregate international evidence, to our knowledge, that supports the validity of the spectrum concept of bipolarity," they added. The proportion of mood episodes rated as clinically severe, as well as the proportion in which patients reported severe impairment of work, home management, social life, and close relationships, were directly associated with increasingly severe forms of bipolar disease.
This study was supported by the NIMH’s Intramural Research Program, the U.S. Public Health Service, numerous organizations in the participating countries, and numerous drug companies. Dr. Merikangas reported her affiliation with the Intramural Research Program, and one of her associates, Ronald Kessler, Ph.D., reported ties to numerous pharmaceutical companies.
Worldwide, the prevalence of bipolar disorder type I is estimated to be 0.6%, that of type II is 0.4%, and that of subthreshold bipolar disorder is 1.4%, yielding a total bipolar disorder spectrum prevalence of 2.4%, according to a new report in the March issue of the Archives of General Psychiatry.
These estimates were derived from what the investigators described as the first international data ever collected using Diagnostic and Statistical Manual–IV definitions for the full spectrum of bipolar disorders and standardized measures to survey nationally representative samples in 11 low-income, middle-income, and high-income countries.
Even though prevalence varied from one country to the next, disease severity, impact on daily life, and patterns of comorbidity remained strikingly similar, said Kathleen R. Merikangas, Ph.D., of the Intramural Research Program of the National Institute of Mental Health (NIMH), and her associates in the World Mental Health Survey Initiative.
This World Health Organization (WHO) project "aims to obtain accurate cross-national information on the prevalence, correlates, and service patterns of mental disorders." The investigators conducted in-person, population-based surveys of 61,392 adults in Brazil, Colombia, Mexico, the United States, Bulgaria, Romania, China, India, Japan, Lebanon, and New Zealand.
In addition to the overall prevalences, they found that the 1-year prevalence of bipolar disorder type I was 0.4%, that of bipolar disorder type II was 0.3%, and that of subthreshold bipolar disorder was 0.8%, for a total bipolar spectrum disorder annual prevalence of 1.5%.
In general, high-income countries had the highest prevalences of bipolar disease and low-income countries had the lowest. The United States had the highest prevalence of overall (4.4%) and annual (2.8%) disease, while India had the lowest (0.1% for both). Two exceptions to this rule were Japan, a high-income country with very low overall (0.7%) and annual (0.2%) prevalences, and Colombia, a low-income country with a high overall prevalence (2.6%).
The mean ages at onset were 18 years for bipolar disorder type I, 20 years for bipolar disorder type II, and 22 years for subthreshold bipolar disorder.
Patterns of comorbidity were remarkably consistent among the different countries. Three-fourths of patients with bipolar spectrum disorders also met criteria for other psychiatric disorders, and the majority had three or more of them. "Anxiety disorders, particularly panic attacks, were the most common comorbid conditions (63%), followed by behavior disorders (45%) and substance use disorders (37%)," Dr. Merikangas and her colleagues said (Arch. Gen. Psychiatry 2011;68:241-51).
The association between bipolar disorders and substance use disorders across the globe was particularly striking in light of the large differences between countries in rates of substance use and abuse. "This suggests that [bipolar disorder] can be considered a risk factor for the development of substance use disorders ... [and supports] the need for careful probing of a history of bipolarity among those with substance use disorders," they said.
The proportion of patients with suicidal behaviors rose with increasing severity of bipolar disease. Approximately 10% of those with subthreshold bipolar disorder, 20% of those with bipolar disorder type II, and 25% of those with bipolar disorder type I said that they had attempted suicide over the last 12 months.
This "striking" finding of suicidality, together with the early age at onset and the strong association with other mental health disorders, provides "further documentation of the individual and societal disability associated with this disorder," they noted.
A substantially higher proportion of patients in high-income countries, compared with middle- or low-income countries, reported having used mental health services. Lifetime use of such services was 50% in high-income countries, 34% in middle-income countries, and 25% in low-income countries.
"Because [bipolar disorder] has an average age at onset at one of the most critical periods of educational, occupational, and social development, its consequences often lead to lifelong disability," the investigators said, and this lack of mental health treatment, especially in low-income countries, is therefore "alarming."
"These data also provide the first aggregate international evidence, to our knowledge, that supports the validity of the spectrum concept of bipolarity," they added. The proportion of mood episodes rated as clinically severe, as well as the proportion in which patients reported severe impairment of work, home management, social life, and close relationships, were directly associated with increasingly severe forms of bipolar disease.
This study was supported by the NIMH’s Intramural Research Program, the U.S. Public Health Service, numerous organizations in the participating countries, and numerous drug companies. Dr. Merikangas reported her affiliation with the Intramural Research Program, and one of her associates, Ronald Kessler, Ph.D., reported ties to numerous pharmaceutical companies.
Worldwide, the prevalence of bipolar disorder type I is estimated to be 0.6%, that of type II is 0.4%, and that of subthreshold bipolar disorder is 1.4%, yielding a total bipolar disorder spectrum prevalence of 2.4%, according to a new report in the March issue of the Archives of General Psychiatry.
These estimates were derived from what the investigators described as the first international data ever collected using Diagnostic and Statistical Manual–IV definitions for the full spectrum of bipolar disorders and standardized measures to survey nationally representative samples in 11 low-income, middle-income, and high-income countries.
Even though prevalence varied from one country to the next, disease severity, impact on daily life, and patterns of comorbidity remained strikingly similar, said Kathleen R. Merikangas, Ph.D., of the Intramural Research Program of the National Institute of Mental Health (NIMH), and her associates in the World Mental Health Survey Initiative.
This World Health Organization (WHO) project "aims to obtain accurate cross-national information on the prevalence, correlates, and service patterns of mental disorders." The investigators conducted in-person, population-based surveys of 61,392 adults in Brazil, Colombia, Mexico, the United States, Bulgaria, Romania, China, India, Japan, Lebanon, and New Zealand.
In addition to the overall prevalences, they found that the 1-year prevalence of bipolar disorder type I was 0.4%, that of bipolar disorder type II was 0.3%, and that of subthreshold bipolar disorder was 0.8%, for a total bipolar spectrum disorder annual prevalence of 1.5%.
In general, high-income countries had the highest prevalences of bipolar disease and low-income countries had the lowest. The United States had the highest prevalence of overall (4.4%) and annual (2.8%) disease, while India had the lowest (0.1% for both). Two exceptions to this rule were Japan, a high-income country with very low overall (0.7%) and annual (0.2%) prevalences, and Colombia, a low-income country with a high overall prevalence (2.6%).
The mean ages at onset were 18 years for bipolar disorder type I, 20 years for bipolar disorder type II, and 22 years for subthreshold bipolar disorder.
Patterns of comorbidity were remarkably consistent among the different countries. Three-fourths of patients with bipolar spectrum disorders also met criteria for other psychiatric disorders, and the majority had three or more of them. "Anxiety disorders, particularly panic attacks, were the most common comorbid conditions (63%), followed by behavior disorders (45%) and substance use disorders (37%)," Dr. Merikangas and her colleagues said (Arch. Gen. Psychiatry 2011;68:241-51).
The association between bipolar disorders and substance use disorders across the globe was particularly striking in light of the large differences between countries in rates of substance use and abuse. "This suggests that [bipolar disorder] can be considered a risk factor for the development of substance use disorders ... [and supports] the need for careful probing of a history of bipolarity among those with substance use disorders," they said.
The proportion of patients with suicidal behaviors rose with increasing severity of bipolar disease. Approximately 10% of those with subthreshold bipolar disorder, 20% of those with bipolar disorder type II, and 25% of those with bipolar disorder type I said that they had attempted suicide over the last 12 months.
This "striking" finding of suicidality, together with the early age at onset and the strong association with other mental health disorders, provides "further documentation of the individual and societal disability associated with this disorder," they noted.
A substantially higher proportion of patients in high-income countries, compared with middle- or low-income countries, reported having used mental health services. Lifetime use of such services was 50% in high-income countries, 34% in middle-income countries, and 25% in low-income countries.
"Because [bipolar disorder] has an average age at onset at one of the most critical periods of educational, occupational, and social development, its consequences often lead to lifelong disability," the investigators said, and this lack of mental health treatment, especially in low-income countries, is therefore "alarming."
"These data also provide the first aggregate international evidence, to our knowledge, that supports the validity of the spectrum concept of bipolarity," they added. The proportion of mood episodes rated as clinically severe, as well as the proportion in which patients reported severe impairment of work, home management, social life, and close relationships, were directly associated with increasingly severe forms of bipolar disease.
This study was supported by the NIMH’s Intramural Research Program, the U.S. Public Health Service, numerous organizations in the participating countries, and numerous drug companies. Dr. Merikangas reported her affiliation with the Intramural Research Program, and one of her associates, Ronald Kessler, Ph.D., reported ties to numerous pharmaceutical companies.
FROM THE ARCHIVES OF GENERAL PSYCHIATRY
Major Finding: The worldwide prevalence of bipolar spectrum disorder is 2.4%.
Data Source: Cross-sectional analysis of data collected in 11 international, population-based surveys of bipolar spectrum disorders.
Disclosures: This study was supported by the National Institute of Mental Health’s Intramural Research Program, the U.S. Public Health Service, numerous organizations in the participating countries, and numerous drug companies. Dr. Merikangas reported her affiliation with the Intramural Research Program, and one of her associates, Ronald Kessler, Ph.D., reported ties to numerous pharmaceutical companies.
Adjunctive Use of Aripiprazole Approved for Bipolar I Disorder
The atypical antipsychotic aripiprazole has been approved by the Food and Drug Administration for the maintenance treatment of people with bipolar I disorder as an adjunct to lithium or valproate, the manufacturers announced Feb. 17.
Aripiprazole, marketed as Abilify by the Bristol-Myers Squibb Co. and Otsuka Pharmaceutical Co., was approved in May 2008 as an adjunct to lithium or valproate for the acute treatment of manic and mixed episodes associated with bipolar I disorder.
The approval of the expanded adjunctive indication was based on a 52-week randomized, double-blind, placebo-controlled maintenance study of adults who met the DSM-IV criteria for bipolar I disorder and who had had a recent manic or mixed episode. The subjects also had a history of one or more manic or mixed episodes that had been severe enough to require hospitalization and/or treatment with a mood stabilizer or antipsychotic. All patients started treatment with lithium or valproate; after 2 weeks, those who had an inadequate response to one of the mood stabilizers alone began treatment with aripiprazole (15 mg/day, with the option to increase the dose to 30 mg/day or reduce the dose to 10 mg/day as early as the fourth day of treatment). After being stabilized for 12 consecutive weeks, 337 patients were randomized to continue treatment with the same aripiprazole dose with lithium or valproate or were switched to placebo with lithium or valproate.
During a period of up to 52 weeks, those who remained on aripiprazole experienced fewer manic episodes compared with those on placebo (7 observed episodes vs. 19). The number of depressive episodes was similar in the two groups (14 among those on aripiprazole vs. 18 among those on placebo), according to the statement and revised label. In the study, the most common adverse event associated with adjunctive aripiprazole was tremor, affecting 6%, compared with 2.4% of those on placebo, according to the company statement.
"An examination of population subgroups did not reveal any clear evidence of differential responsiveness on the basis of age and gender," but there were not enough patients in each of the ethnic groups "to adequately assess inter-group differences," according to a statement in the revised label.
Aripiprazole, an oral dopamine partial agonist, also is approved:
• For the acute treatment of manic or mixed episodes associated with bipolar I disorder as monotherapy in adults and pediatric patients aged 10-17 years.
• As monotherapy for the maintenance treatment of bipolar I disorder in adults and adolescents aged 13-17 years.
• For the treatment of schizophrenia in adults and adolescents aged 13-17 years.
• As an adjunctive treatment for adults with major depressive disorder who have an inadequate response to antidepressant therapy.
For the treatment of irritability associated with autistic disorder in pediatric patients aged 6 to 17 years.
It initially was approved by the FDA in 2002 for the treatment of schizophrenia.
The atypical antipsychotic aripiprazole has been approved by the Food and Drug Administration for the maintenance treatment of people with bipolar I disorder as an adjunct to lithium or valproate, the manufacturers announced Feb. 17.
Aripiprazole, marketed as Abilify by the Bristol-Myers Squibb Co. and Otsuka Pharmaceutical Co., was approved in May 2008 as an adjunct to lithium or valproate for the acute treatment of manic and mixed episodes associated with bipolar I disorder.
The approval of the expanded adjunctive indication was based on a 52-week randomized, double-blind, placebo-controlled maintenance study of adults who met the DSM-IV criteria for bipolar I disorder and who had had a recent manic or mixed episode. The subjects also had a history of one or more manic or mixed episodes that had been severe enough to require hospitalization and/or treatment with a mood stabilizer or antipsychotic. All patients started treatment with lithium or valproate; after 2 weeks, those who had an inadequate response to one of the mood stabilizers alone began treatment with aripiprazole (15 mg/day, with the option to increase the dose to 30 mg/day or reduce the dose to 10 mg/day as early as the fourth day of treatment). After being stabilized for 12 consecutive weeks, 337 patients were randomized to continue treatment with the same aripiprazole dose with lithium or valproate or were switched to placebo with lithium or valproate.
During a period of up to 52 weeks, those who remained on aripiprazole experienced fewer manic episodes compared with those on placebo (7 observed episodes vs. 19). The number of depressive episodes was similar in the two groups (14 among those on aripiprazole vs. 18 among those on placebo), according to the statement and revised label. In the study, the most common adverse event associated with adjunctive aripiprazole was tremor, affecting 6%, compared with 2.4% of those on placebo, according to the company statement.
"An examination of population subgroups did not reveal any clear evidence of differential responsiveness on the basis of age and gender," but there were not enough patients in each of the ethnic groups "to adequately assess inter-group differences," according to a statement in the revised label.
Aripiprazole, an oral dopamine partial agonist, also is approved:
• For the acute treatment of manic or mixed episodes associated with bipolar I disorder as monotherapy in adults and pediatric patients aged 10-17 years.
• As monotherapy for the maintenance treatment of bipolar I disorder in adults and adolescents aged 13-17 years.
• For the treatment of schizophrenia in adults and adolescents aged 13-17 years.
• As an adjunctive treatment for adults with major depressive disorder who have an inadequate response to antidepressant therapy.
For the treatment of irritability associated with autistic disorder in pediatric patients aged 6 to 17 years.
It initially was approved by the FDA in 2002 for the treatment of schizophrenia.
The atypical antipsychotic aripiprazole has been approved by the Food and Drug Administration for the maintenance treatment of people with bipolar I disorder as an adjunct to lithium or valproate, the manufacturers announced Feb. 17.
Aripiprazole, marketed as Abilify by the Bristol-Myers Squibb Co. and Otsuka Pharmaceutical Co., was approved in May 2008 as an adjunct to lithium or valproate for the acute treatment of manic and mixed episodes associated with bipolar I disorder.
The approval of the expanded adjunctive indication was based on a 52-week randomized, double-blind, placebo-controlled maintenance study of adults who met the DSM-IV criteria for bipolar I disorder and who had had a recent manic or mixed episode. The subjects also had a history of one or more manic or mixed episodes that had been severe enough to require hospitalization and/or treatment with a mood stabilizer or antipsychotic. All patients started treatment with lithium or valproate; after 2 weeks, those who had an inadequate response to one of the mood stabilizers alone began treatment with aripiprazole (15 mg/day, with the option to increase the dose to 30 mg/day or reduce the dose to 10 mg/day as early as the fourth day of treatment). After being stabilized for 12 consecutive weeks, 337 patients were randomized to continue treatment with the same aripiprazole dose with lithium or valproate or were switched to placebo with lithium or valproate.
During a period of up to 52 weeks, those who remained on aripiprazole experienced fewer manic episodes compared with those on placebo (7 observed episodes vs. 19). The number of depressive episodes was similar in the two groups (14 among those on aripiprazole vs. 18 among those on placebo), according to the statement and revised label. In the study, the most common adverse event associated with adjunctive aripiprazole was tremor, affecting 6%, compared with 2.4% of those on placebo, according to the company statement.
"An examination of population subgroups did not reveal any clear evidence of differential responsiveness on the basis of age and gender," but there were not enough patients in each of the ethnic groups "to adequately assess inter-group differences," according to a statement in the revised label.
Aripiprazole, an oral dopamine partial agonist, also is approved:
• For the acute treatment of manic or mixed episodes associated with bipolar I disorder as monotherapy in adults and pediatric patients aged 10-17 years.
• As monotherapy for the maintenance treatment of bipolar I disorder in adults and adolescents aged 13-17 years.
• For the treatment of schizophrenia in adults and adolescents aged 13-17 years.
• As an adjunctive treatment for adults with major depressive disorder who have an inadequate response to antidepressant therapy.
For the treatment of irritability associated with autistic disorder in pediatric patients aged 6 to 17 years.
It initially was approved by the FDA in 2002 for the treatment of schizophrenia.
Expert: PCOS Risk Makes Valproate the Last Treatment Option for Women With Bipolar
LOS ANGELES – It’s reasonable to consider the anticonvulsant valproate as a last option for women with bipolar disorder, given the drug’s associations with the risk of developing isolated features of polycystic ovary syndrome, according to Dr. Harold Carlson.
"I don’t have any problem with that," he said, when an audience member suggested it and also noted the drug’s teratogenicity.
Women under age 25, and particularly adolescents in their midteens, are most at risk for valproate-induced PCOS, usually within the first year of treatment, said Dr. Carlson, professor of endocrinology at Stony Brook (N.Y.) University.
In one study, 9 of 86 women with bipolar disorder (10.5%) treated with valproate developed PCOS; 2 of 144 women with bipolar disorder (1.4%) developed PCOS when treated with other mood stabilizers (Biol. Psychiatry 2006;59:1078-86).
Valproate seems to pose a particular risk for the condition driven by something more than the weight gain caused by the drug.
After all, "the folks [who] gain all that weight on olanzapine don’t get PCOS," Dr. Carlson noted.
Valproate appears to act directly on the ovaries, altering their hormone production. Cultured ovarian cells produce more testosterone in its presence. The excess testosterone shuts off menstruation, and causes acne and hirsutism. Obesity, insulin resistance, and dyslipidemia are problems in PCOS, as well.
When valproate cannot be switched out for a mood stabilizer, prescribing birth control pills at the start of therapy might be a smart move, Dr. Carlson said at a psychopharmacology update, sponsored by the American Academy of Child and Adolescent Psychiatry. That appeared to help prevent PCOS in valproate-treated women in one study (Seizure 2003;12:323-9).
Baseline pelvic ultrasounds might seem like a good idea, too, but they’re "not worth doing," he said.
The reason is that 10%-15% of healthy women have cysts on their ovaries without having PCOS, and ovarian cysts aren’t always present in PCOS.
When women are started on the drug, ask them "every time you see them about their menstrual function. Look at them and see if they are getting acne and hirsutism. Ask them about it. Provide some counseling on diet and exercise to avoid the excessive weight gain, which only makes it worse," Dr. Carlson said.
Should PCOS develop, Metformin is the first-line symptom treatment. Clomiphene can induce ovulation if pregnancy is the goal.
Endocrinology, urology, or gynecology referrals also are in order to help with symptoms, Dr. Carlson said.
He said he is a consultant to Eli Lilly & Co. He also disclosed receiving research funding from GlaxoSmithKline.
LOS ANGELES – It’s reasonable to consider the anticonvulsant valproate as a last option for women with bipolar disorder, given the drug’s associations with the risk of developing isolated features of polycystic ovary syndrome, according to Dr. Harold Carlson.
"I don’t have any problem with that," he said, when an audience member suggested it and also noted the drug’s teratogenicity.
Women under age 25, and particularly adolescents in their midteens, are most at risk for valproate-induced PCOS, usually within the first year of treatment, said Dr. Carlson, professor of endocrinology at Stony Brook (N.Y.) University.
In one study, 9 of 86 women with bipolar disorder (10.5%) treated with valproate developed PCOS; 2 of 144 women with bipolar disorder (1.4%) developed PCOS when treated with other mood stabilizers (Biol. Psychiatry 2006;59:1078-86).
Valproate seems to pose a particular risk for the condition driven by something more than the weight gain caused by the drug.
After all, "the folks [who] gain all that weight on olanzapine don’t get PCOS," Dr. Carlson noted.
Valproate appears to act directly on the ovaries, altering their hormone production. Cultured ovarian cells produce more testosterone in its presence. The excess testosterone shuts off menstruation, and causes acne and hirsutism. Obesity, insulin resistance, and dyslipidemia are problems in PCOS, as well.
When valproate cannot be switched out for a mood stabilizer, prescribing birth control pills at the start of therapy might be a smart move, Dr. Carlson said at a psychopharmacology update, sponsored by the American Academy of Child and Adolescent Psychiatry. That appeared to help prevent PCOS in valproate-treated women in one study (Seizure 2003;12:323-9).
Baseline pelvic ultrasounds might seem like a good idea, too, but they’re "not worth doing," he said.
The reason is that 10%-15% of healthy women have cysts on their ovaries without having PCOS, and ovarian cysts aren’t always present in PCOS.
When women are started on the drug, ask them "every time you see them about their menstrual function. Look at them and see if they are getting acne and hirsutism. Ask them about it. Provide some counseling on diet and exercise to avoid the excessive weight gain, which only makes it worse," Dr. Carlson said.
Should PCOS develop, Metformin is the first-line symptom treatment. Clomiphene can induce ovulation if pregnancy is the goal.
Endocrinology, urology, or gynecology referrals also are in order to help with symptoms, Dr. Carlson said.
He said he is a consultant to Eli Lilly & Co. He also disclosed receiving research funding from GlaxoSmithKline.
LOS ANGELES – It’s reasonable to consider the anticonvulsant valproate as a last option for women with bipolar disorder, given the drug’s associations with the risk of developing isolated features of polycystic ovary syndrome, according to Dr. Harold Carlson.
"I don’t have any problem with that," he said, when an audience member suggested it and also noted the drug’s teratogenicity.
Women under age 25, and particularly adolescents in their midteens, are most at risk for valproate-induced PCOS, usually within the first year of treatment, said Dr. Carlson, professor of endocrinology at Stony Brook (N.Y.) University.
In one study, 9 of 86 women with bipolar disorder (10.5%) treated with valproate developed PCOS; 2 of 144 women with bipolar disorder (1.4%) developed PCOS when treated with other mood stabilizers (Biol. Psychiatry 2006;59:1078-86).
Valproate seems to pose a particular risk for the condition driven by something more than the weight gain caused by the drug.
After all, "the folks [who] gain all that weight on olanzapine don’t get PCOS," Dr. Carlson noted.
Valproate appears to act directly on the ovaries, altering their hormone production. Cultured ovarian cells produce more testosterone in its presence. The excess testosterone shuts off menstruation, and causes acne and hirsutism. Obesity, insulin resistance, and dyslipidemia are problems in PCOS, as well.
When valproate cannot be switched out for a mood stabilizer, prescribing birth control pills at the start of therapy might be a smart move, Dr. Carlson said at a psychopharmacology update, sponsored by the American Academy of Child and Adolescent Psychiatry. That appeared to help prevent PCOS in valproate-treated women in one study (Seizure 2003;12:323-9).
Baseline pelvic ultrasounds might seem like a good idea, too, but they’re "not worth doing," he said.
The reason is that 10%-15% of healthy women have cysts on their ovaries without having PCOS, and ovarian cysts aren’t always present in PCOS.
When women are started on the drug, ask them "every time you see them about their menstrual function. Look at them and see if they are getting acne and hirsutism. Ask them about it. Provide some counseling on diet and exercise to avoid the excessive weight gain, which only makes it worse," Dr. Carlson said.
Should PCOS develop, Metformin is the first-line symptom treatment. Clomiphene can induce ovulation if pregnancy is the goal.
Endocrinology, urology, or gynecology referrals also are in order to help with symptoms, Dr. Carlson said.
He said he is a consultant to Eli Lilly & Co. He also disclosed receiving research funding from GlaxoSmithKline.
EXPERT ANALYSIS FROM A PSYCHOPHARMACOLOGY UPDATE
What to look for when evaluating mood swings in children and adolescents
Not all mood swings are bipolar disorder
M, age 13, is referred by her pediatrician with the chief complaint of “severe mood swings, rule out bipolar disorder (BD).” In the past she was treated for attention-deficit/hyperactivity disorder (ADHD) with stimulants with mixed results. M’s parents are concerned about her “flipping out” whenever she is asked to do something she does not want to do. Her mother has a history of depression and anxiety; her father had a “drinking problem.” There is no history of BD in her first- or second-degree relatives. Are M’s rapid mood swings a sign of BD or another disorder?
The differential diagnosis of “mood swings” is important because they are a common presenting symptom of many children and adolescents with mood and behavioral disorders. Mood swings often occur in children and adolescents with ADHD, oppositional defiant disorder (ODD), developmental disorders, depressive disorders, BD, anxiety disorders, and conduct disorders. Mood swings are analogous to a fever in pediatrics—they indicate something potentially is wrong with the patient, but are not diagnostic as an isolated symptom.
Mood swings in children are common, nonspecific symptoms that more often are a sign of anxiety or behavioral disorders than BD. This article discusses the differential diagnosis of mood swings in children and adolescents and how to best screen and diagnose these patients.
What are ‘mood swings’?
Mood swings is a popular term that is nonspecific and not part of DSM-IV-TR diagnostic criteria for BD. The complaint of “mood swings” may reflect severe mood lability of pediatric patients with BD. This mood lability is best described by the Kiddie-Mania Rating Scale (K-MRS) developed by Axelson and colleagues as “rapid mood variation with several mood states within a brief period of time which appears internally driven without regard to the circumstance.”1 On K-MRS mood lability items, children with mania typically score:
- Moderate—many mood changes throughout the day, can vary from elevated mood to anger to sadness within a few hours; changes in mood are clearly out of proportion to circumstances and cause impairment in functioning
- Severe—rapid mood swings nearly all of the time, with mood intensity greatly out of proportion to circumstances
- Extreme—constant, explosive variability in mood, several mood changes occurring within minutes, difficult to identify a particular mood, changes in mood radically out of proportion to circumstances.
Patients with BD typically exhibit what is best described as a “mood cycle”—a pronounced shift in mood and energy from 1 extreme to another.2 An example of this would be a child who wakes up with extreme silliness, high energy, and intrusive behavior that persists for several hours and then later in the day becomes sad, depressed, and suicidal with no precipitant for either mood cycle. BD patients also will exhibit other symptoms of mania during these mood cycling periods.
Rapid cycling is a DSM-IV course specifier that indicates ≥4 mood episodes per year in patients with BD with a typical course of mania or hypomania followed by depression, or vice versa.3 The episodes must be demarcated by full or partial remission that lasts ≥2 months or by a switch to a mood state of opposite polarity. In the past, children with frequent mood swings were described incorrectly as “rapid cycling,” but this term has been dropped because it engenders confusion between adult and pediatric BD phenomenology.2
A more precise method of describing mood symptoms in a child or adolescent is to use the FIND criteria, which include:4
- Frequency of symptoms per week
- Intensity of mood symptoms
- Number of mood cycles per day
- Duration of symptoms per day.
Visit this article at CurrentPsychiatry.com to view a table that outlines what to look for when using the FIND criteria to evaluate common pediatric psychiatric disorders that include mood swings. Table 1
describes clinical characteristics and tools and resources used to differentiate these and other disorders.4
Table 1
Clinical characteristics of psychiatric disorders that often feature mood swings
| Disorder | Clinical description | Useful tools/resources |
|---|---|---|
| ADHD | Chronic symptoms of hyperactivity, distractibility, impulsivity, poor attentional skills, disorganization | Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L) |
| ODD | Chronic symptoms of oppositionality, negativity; short, frequent mood swings in response to being asked to do something they do not want to do | CPRS-R:L |
| Anxiety disorders | Excessive ‘worry,’ difficulty with transitions, increased mood swings during stressful periods, psychosomatic symptoms | Self-Report for Childhood Anxiety Related Disorders |
| ARND | History of exposure to alcohol in-utero; mild dysmorphia, attentional, mood, and executive functioning problems | National Organization on Fetal Alcohol Syndrome |
| Bipolar disorder | In children: clustering together of episodes or ‘mini-episodes’ (several days) of increased energy, decreased need for sleep, increased mood cycling, pressured speech, etc. In adolescents: depressive episodes with episodes of hypomania or mania | Mood Disorders Questionnaire Kiddie Schedule for Affective Disorders and Schizophrenia Mania Rating Scale |
| ADHD: attention-deficit/hyperactivity disorder; ARND: alcohol-related neurodevelopmental disorder; ODD: oppositional defiant disorder | ||
| Source: Reference 4 | ||
Mood swings: A chart review
We recently completed a retrospective chart review of 100 patients consecutively referred to our pediatric mood disorders clinic for evaluation of “mood swings, rule out BD.” These patients were self-referred, referred by a psychiatrist for a second opinion, or referred by their primary care physician. The mean age of these patients was 8±2.8 years and 68% were male.
Two experienced clinicians (RAK and EM) interviewed each patient and their caregivers and reviewed results of the Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L)5 and other outside information.
Figure 1 illustrates these patients’ diagnoses. Diagnoses for each of these disorders were made using DSM-IV-TR criteria.3
The most common diagnoses among patients with the chief complaint of mood swings were ADHD (39%); ODD with ADHD (15%); an anxiety disorder, usually generalized anxiety disorder (GAD) (15%); BD (12%); and a secondary mood disorder, usually fetal alcohol spectrum disorder (10%). We were surprised at how often ADHD, ODD, and anxiety disorders were found to be responsible for these patients’ mood swings and how frequently the referring clinician did not recognize these disorders. In the following sections, we discuss each of these disorders and how they differ from BD.
Figure 1 Underlying diagnoses of 100 children/adolescents referred for ‘mood swings’
ADHD: attention-deficit/hyperactivity disorder; BD: bipolar disorder; MDD: major depressive disorder; ODD: oppositional defiant disorder; PDD: pervasive developmental disorder
ADHD and ODD
In our sample, patients with undiagnosed ADHD made up the largest group of those with frequent mood swings. ADHD inattentive type was missed frequently in adolescent girls who still had behavioral aspects of ADHD, including impulsivity and aggression.6
The CPRS-R:L is useful for screening and diagnosing children and adolescents with ADHD and ODD. It contains 80 items, can be used in males and females and patients age 3 to 17, and has validated norms by age and sex.5 It takes parents approximately 10 minutes to fill out this questionnaire and the results can be scored by hand. The CPRS-R:L includes the following scales: oppositional; cognitive problems/inattention; hyperactivity; anxious-shy; perfectionism; social problems; psychosomatic; Connors’ global index; DSM-IV symptom subscales; and an ADHD index. Patients with mood swings and ADHD combined typically score >2 standard deviations above their age/sex mean on the CPRS-R:L hyperactivity scale, Connors’ Global Index, and ADHD index.5
A common childhood disorder, ODD has multiple etiologies.7 The first DSM-IV criteria for ODD is “often loses temper”3—essentially mood swings that often are expressed behaviorally as anger and at times as aggressive outbursts.
Dodge and Cole8 categorized aggression as reactive (impulsivity with a high affective valence) or proactive (characterized by low arousal and premeditation, ie, predatory conduct disorder). Reactive aggression typically is an angry defensive response to frustration, threat, or provocation, whereas proactive aggression is deliberate, coercive behavior often used to obtain a goal.9 Reactive aggression is common among children with ADHD and ODD and typically begins as a mood swing that escalates into reactive aggressive behavior. In a study of 268 consecutively referred children and adolescents with ADHD and 100 community controls, Connor et al10 found significantly more reactive than proactive forms of aggression in ADHD patients.
It can be difficult to differentiate the moods swings and symptoms of ODD from those of pediatric BD. Mick et al11 found that severe irritability may be a diagnostic indicator of BD in children with ADHD. Using the Kiddie Schedule for Affective Disorders and Schizophrenia (epidemiologic version) structured diagnostic interview,12 they evaluated 274 children (mean age 10.8±3.2) with ADHD; 37% had no comorbid mood disorder, 36% had ADHD with depression, and 11% had ADHD with BD. Researchers characterized 3 types of irritability in these patients:
- ODD-type irritability characterized by a low frustration tolerance that is seen in ODD
- Mad/cranky irritability found in depressive disorders
- Super-angry/grouchy/cranky irritability with frequent, prolonged, and largely unprovoked anger episodes and characteristics of mania.
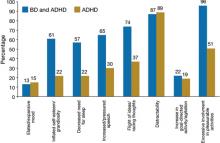
ODD-type irritability was common among all ADHD patients, was the least impairing type of irritability, and did not increase the risk of a mood disorder. Mad/cranky irritability was common only in children with ADHD and a mood disorder (depression or BD), was more impairing than ODD-type irritability, and was most predictive of unipolar depression. Super-angry/grouchy/cranky irritability was common only among children with ADHD and BD (77%), was the most impairing, and was predictive of both unipolar depression and BD. The type of irritability and clustering of DSM-IV manic symptoms best differentiated ADHD subjects from those with ADHD and BD. Figure 2 illustrates symptoms that differentiated patients with ADHD from those with ADHD and comorbid BD.11
A review of pharmacotherapy for aggression in children found the largest effects for methylphenidate for aggression in ADHD (mean effect size=0.9, combined N=844).13 Our clinical experience has been that pediatric patients with ADHD or ODD with ADHD often have high levels of reactive aggression that presents as mood swings, and aggressively treating ADHD often results in improved mood and other ADHD symptoms.
Figure 2 Symptoms that differentiate BD from BD with comorbid ADHD
ADHD: attention-deficit/hyperactivity disorder; BD: bipolar disorder
Source: Reference 11
Anxiety disorders
The estimated prevalence of child and adolescent anxiety disorders is 10% to 20%14; in our sample the prevalence was 15%. These disorders include GAD, separation anxiety disorder, social phobias, posttraumatic stress disorder (PTSD), and obsessive-compulsive disorder. Often, children with GAD worry excessively and become upset during transitions when things don’t proceed as they expect, with resultant angry outbursts and mood swings. Mood swings and difficulty sleeping are common in children with anxiety disorders or BD. Anxiety disorders often will be missed unless specific triggers of the mood swings or angry outbursts—as well as differentiating symptoms such as excessive fear, worry, and psychosomatic symptoms—are assessed.
In our clinical experience, simply asking a child if he or she is anxious is not sufficient to uncover an anxiety disorder. Although the CPRS-L:R will screen for anxiety disorders, we have found that the Self-Report for Childhood Anxiety Related Disorders (SCARED) developed by Birmaher et al15 is more specific. This tool can be used in patients age ≥8. The parent and child versions of the SCARED contain 41 items that measure 5 factors:
- general anxiety
- separation anxiety
- social phobia
- school phobia
- physical symptoms of anxiety.
The SCARED takes 5 minutes to fill out and is available in parent and child versions.
Secondary mood disorders
Many patients in our sample had a mood disorder secondary to the neurologic effects of alcohol on the developing brain. For more about maternal alcohol use, fetal alcohol spectrum disorders, and mood swings, visit this article at CurrentPsychiatry.com.
What BD looks like in children
In our sample, 12% of patients referred for mood swings were diagnosed with bipolar I disorder (BDI), bipolar II disorder (BDII), or bipolar disorder, not otherwise specified (BD-NOS). In the United States, lifetime prevalence of BDI and BDII in adolescents age 13 to 17 is 2.9%.16 No large epidemiologic studies have looked at the lifetime prevalence of BD in children age <13.
How often a clinician sees BD in children and adolescents largely depends on the type of setting in which he or she practices. Although in the general population BD is relatively rare compared with other childhood psychiatric disorders, on child/adolescent inpatient units it is common to find that 30% to 40% of patients have BD.17
The best longitudinal study to date of the phenomenology, comorbidity, and outcome of BD in children and adolescents is the National Institute of Mental Health-funded Course and Outcome of Bipolar Youth study (COBY).18 In this ongoing, longitudinal study, 413 youths (age 7 to 17) with BDI (N=244), BDII (N=28), or BD-NOS (N=141) were rigorously diagnosed using state-of-the-art measures, including the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present version19 and re-evaluated every 9.4 months for 4 years. When organizing this study, investigators found that DSM-IV criteria for BD-NOS were too vague to be useful and developed their own criteria (Table 2).18
For BDI patients in the COBY study, the mean age of onset for bipolar symptoms was 9.0±4.1 years and the mean duration of illness was 4.4±3.1 years. Researchers reported that at the 4-year assessment approximately 70% of patients with BD recovered from their index episode, and 50% had at least 1 syndromal recurrence, particularly depressive episodes.20 Analyses of these patients’ weekly mood symptoms showed that they had syndromal or subsyndromal symptoms with numerous changes in symptoms and shifts of mood polarity 60% of the time, and psychosis 3% of the time. During this study, 20% of BDII patients progressed to BDI, and 25% of BD-NOS patients converted to BDI or BDII.
Further analysis of the COBY data revealed that onset of mood symptoms preceded onset of clear bipolar episodes by an average of 1.0±1.7 years. Depression was the most common initial and most frequent episode for adolescents; mood lability was seen more often in childhood-onset and adolescents with early-onset BD. Depressed children had more severe irritability than depressed adolescents, and older age was associated with more severe and typical mood symptomatology.21
The clinical picture of a child with BD that emerges from the COBY study is:
- a fairly young child with the onset of mood symptoms between age 5 to 12
- subsyndromal and less frequently clear syndromal episodes
- primarily mixed and depressed symptoms with rapid mood cycles during these episodes.22
It is clear that there is a spectrum of bipolar disorders in children and adolescents with varying degrees of symptom expression and children differ from adolescents and adults in their initial presentation of BD.
Table 2
COBY criteria for bipolar disorder, not otherwise specified
| Presence of clinically relevant bipolar symptoms that do not fulfill DSM-IV criteria for BDI or BDII |
| In addition, patients are required to have elevated mood plus 2 associated DSM-IV symptoms or irritable mood plus 3 DSM-IV associated symptoms, along with a change in level of functioning |
| Duration of a minimum of 4 hours within a 24-hour period |
| At least 4 cumulative lifetime days meeting the criteria |
| BDI: bipolar I disorder; BDII: bipolar II disorder; COBY: Course and Outcome of Bipolar Youth study |
| Source: Reference 18 |
Related Resources
- Kowatch RA, Fristad MA, Findling RL, et al. Clinical manual for management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2009.
- Goodwin FK, Jamison KR. Manic-depressive illness. 2nd ed. Oxford, United Kingdom: Oxford University Press; 2007.
- Miklowitz DJ, Cicchetti D, eds. Understanding bipolar disorder: a developmental perspective. New York, NY: Guilford Press; 2010.
Drug Brand Name
- Methylphenidate • Ritalin, Concerta, others
Disclosures
Dr. Kowatch receives grant/research support from the National Institute of Child Health and Human Development and the National Institute of Mental Health and is a consultant to AstraZeneca, Forest Pharmaceuticals, Merck, and the REACH Foundation.
Dr. Delgado and Ms. Monroe report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Table
FIND criteria of disorders found to cause mood swings
| Criteria | BDI | BP-NOS | ODD | GAD | ARND |
|---|---|---|---|---|---|
| Frequency of symptoms/week | 7 days (more days than not in an average week) | 2 to 3 days/week | Daily (chronic) irritability and mood swings precipitated by ‘not getting their way’ | Greatest during times of change/stress | Daily |
| Intensity of symptoms | Severe—parents often are afraid to take the child out in public because of mood symptoms | Moderate | Mild/moderate | Mild/moderate when stressed | Mild/moderate |
| Number of mood cycles/day | Daily cycles of euphoria and depression | 3 to 4 | 5 to 10 | 2 to 3 | 8 to 10 |
| Duration of symptoms/day | Euphoria: 30 to 60 minutes Depression: 30 minutes to 6 hours | 4 hours total/day of mood symptoms | Short; 5 to 10 minutes | Short; 5 to 10 minutes | Short; 5 to 10 minutes |
| ARND: alcohol-related neurodevelopmental disorder; BDI: bipolar I disorder; BD-NOS: bipolar disorder, not otherwise specified; GAD: generalized anxiety disorder; ODD: oppositional defiant disorder | |||||
| Source: Kowatch RA, Fristad MA, Findling RL, et al. Clinical manual for the management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2008 | |||||
Even small amounts of alcohol use by a pregnant woman can impact her child’s development. In a controlled study examining drinking behavior of 12,678 pregnant women and the effect this had on their children, Sayel et ala found that <1 drink per week during the first trimester was clinically significant for mental health problems in girls, measured at age 4 and 8, when using parent or teacher report.
Fetal alcohol spectrum disorder describes the range of effects that can occur in an individual whose mother drank alcohol during pregnancy. These disorders include fetal alcohol syndrome (FAS), alcohol-related neurodevelopmental disorder (ARND), and alcohol-related birth defects (ARBD).
FAS. Individuals with FAS have a distinct pattern of facial abnormalities, growth deficiency, and evidence of CNS dysfunction. Characteristic facial abnormalities may include a smooth philtrum, thin upper lip, upturned nose, flat nasal bridge and midface, epicanthal folds, small palpebral fissures, and small head circumference. Growth deficiency begins in-utero and continues throughout childhood and into adulthood. CNS abnormalities can include impaired brain growth or abnormal structure, manifested differently depending on age.
ARND. Many individuals affected by alcohol exposure before birth do not have the characteristic facial abnormalities and growth retardation identified with full FAS, yet have significant brain and behavioral impairments. Individuals with ARND have either the facial anomalies, growth retardation, and other physical abnormalities, or a complex pattern of behavioral or cognitive abnormalities inconsistent with developmental level and unexplained by genetic background or environmental conditions (ie, poor impulse control, language deficits, problems with abstraction, mathematical and social perception deficits, learning problems, and impairment in attention, memory, or judgment).b
ARBD. Persons with ARBD have malformations of the skeletal and major organ systems, such as cardiac or renal abnormalities.
Comorbid psychiatric conditions in children with prenatal alcohol exposure are 5 to 16 times more prevalent than in the general population; these children are 38% more likely to have an anger disorder.c O’Connor and Paleyd found that “…mood disorder symptoms were significantly higher for children with parental alcohol exposure compared to children without exposure.” Children with ARND are treated symptomatically depending upon which deficits and behaviors they exhibit.e
References
a. Sayal K, Heron J, Golding J, et al. Binge pattern of alcohol consumption during pregnancy and childhood mental health outcomes: longitudinal population-based study. Pediatrics. 2009;123(2):e289-296.
b. Warren KR, Foudin LL. Alcohol-related birth defects—the past, present, and future. Alcohol Res Health. 2001;25(3):153-158.
c. Burd L, Klug MG, Martsolf JT, et al. Fetal alcohol syndrome: neuropsychiatric phenomics. Neurotoxicol Teratol. 2003;25(6):697-705.
d. O’Connor MJ, Paley B. Psychiatric conditions associated with prenatal alcohol exposure. Dev Disabil Res Rev. 2009;15(3):225-234.
e. Paley B, O’Connor MJ. Intervention for individuals with fetal alcohol spectrum disorders: treatment approaches and case management. Dev Disabil Res Rev. 2009;15(3):258-267.
1. Axelson D, Birmaher BJ, Brent D, et al. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. J Child Adolesc Psychopharmacol. 2003;13(4):463-470.
2. Youngstrom EA, Birmaher B, Findling RL. Pediatric bipolar disorder: validity, phenomenology, and recommendations for diagnosis. Bipolar Disord. 2008;10(1 Pt 2):194-214.
3. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
4. Kowatch RA, Fristad MA, Findling RL, et al. Clinical manual for the management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2008.
5. Conners CK. Conners’ Parent Rating Scale Long Form (CPRS-R:L) North Tonawanda, NY: Multi-Health Systems, Inc.; 1997.
6. Martel MM. Research review: a new perspective on attention-deficit/hyperactivity disorder: emotion dysregulation and trait models. J Child Psychol Psychiatry. 2009;50(9):1042-1051.
7. Steiner H, Remsing L. and the Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(1):126-141.
8. Dodge KA, Cole JD. Social-information-processing factors in reactive and proactive aggression in children’s peer groups. J Pers Soc Psychol. 1987;53(6):1146-1158.
9. Connor DF, Steingard RJ, Cunningham JA, et al. Proactive and reactive aggression in referred children and adolescents. Am J Orthopsychiatry. 2004;74(2):129-136.
10. Connor DF, Chartier KG, Preen EC, et al. Impulsive aggression in attention-deficit/hyperactivity disorder: symptom severity, co-morbidity, and attention-deficit/hyperactivity disorder subtype. J Child Adolesc Psychopharmacol. 2010;20(2):119-126.
11. Mick E, Spencer T, Wozniak J, et al. Heterogeneity of irritability in attention-deficit/hyperactivity disorder subjects with and without mood disorders. Biol Psychiatry. 2005;58(7):576-582.
12. Orvaschel H. Schizophrenia and Affective Disorders Schedule for children—Epidemiological Version (KSADS-E). Fort Lauderdale, FL: Nova Southeastern University; 1995.
13. Pappadopulos E, Woolston S, Chait A, et al. Pharmacotherapy of aggression in children and adolescents: efficacy and effect size. J Can Acad Child Adolesc Psychiatry. 2006;15(1):27-39.
14. Achenbach TM, Howell CT, McConaughy SH, et al. Six-year predictors of problems in a national sample: IV. Young adult signs of disturbance. J Am Acad Child Adolesc Psychiatry. 1998;37(7):718-727.
15. Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36:545-553.
16. Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication—Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980-989.
17. Youngstrom EA, Duax J. Evidence-based assessment of pediatric bipolar disorder, part I: base rate and family history. J Am Acad Child Adolesc Psychiatry. 2005;44(7):712-717.
18. Birmaher B, Axelson D, Strober M, et al. Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63(2):175-183.
19. Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980-988.
20. Birmaher B, Axelson D. Course and outcome of bipolar spectrum disorder in children and adolescents: a review of the existing literature. Dev Psychopathol. 2006;18(4):1023-1035.
21. Birmaher B, Axelson D, Strober M, et al. Comparison of manic and depressive symptoms between children and adolescents with bipolar spectrum disorders. Bipolar Disord. 2009;11(1):52-62.
22. Birmaher B, Axelson D, Goldstein B, et al. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the Course and Outcome of Bipolar Youth (COBY) study. Am J Psychiatry. 2009;166(7):795-804.
M, age 13, is referred by her pediatrician with the chief complaint of “severe mood swings, rule out bipolar disorder (BD).” In the past she was treated for attention-deficit/hyperactivity disorder (ADHD) with stimulants with mixed results. M’s parents are concerned about her “flipping out” whenever she is asked to do something she does not want to do. Her mother has a history of depression and anxiety; her father had a “drinking problem.” There is no history of BD in her first- or second-degree relatives. Are M’s rapid mood swings a sign of BD or another disorder?
The differential diagnosis of “mood swings” is important because they are a common presenting symptom of many children and adolescents with mood and behavioral disorders. Mood swings often occur in children and adolescents with ADHD, oppositional defiant disorder (ODD), developmental disorders, depressive disorders, BD, anxiety disorders, and conduct disorders. Mood swings are analogous to a fever in pediatrics—they indicate something potentially is wrong with the patient, but are not diagnostic as an isolated symptom.
Mood swings in children are common, nonspecific symptoms that more often are a sign of anxiety or behavioral disorders than BD. This article discusses the differential diagnosis of mood swings in children and adolescents and how to best screen and diagnose these patients.
What are ‘mood swings’?
Mood swings is a popular term that is nonspecific and not part of DSM-IV-TR diagnostic criteria for BD. The complaint of “mood swings” may reflect severe mood lability of pediatric patients with BD. This mood lability is best described by the Kiddie-Mania Rating Scale (K-MRS) developed by Axelson and colleagues as “rapid mood variation with several mood states within a brief period of time which appears internally driven without regard to the circumstance.”1 On K-MRS mood lability items, children with mania typically score:
- Moderate—many mood changes throughout the day, can vary from elevated mood to anger to sadness within a few hours; changes in mood are clearly out of proportion to circumstances and cause impairment in functioning
- Severe—rapid mood swings nearly all of the time, with mood intensity greatly out of proportion to circumstances
- Extreme—constant, explosive variability in mood, several mood changes occurring within minutes, difficult to identify a particular mood, changes in mood radically out of proportion to circumstances.
Patients with BD typically exhibit what is best described as a “mood cycle”—a pronounced shift in mood and energy from 1 extreme to another.2 An example of this would be a child who wakes up with extreme silliness, high energy, and intrusive behavior that persists for several hours and then later in the day becomes sad, depressed, and suicidal with no precipitant for either mood cycle. BD patients also will exhibit other symptoms of mania during these mood cycling periods.
Rapid cycling is a DSM-IV course specifier that indicates ≥4 mood episodes per year in patients with BD with a typical course of mania or hypomania followed by depression, or vice versa.3 The episodes must be demarcated by full or partial remission that lasts ≥2 months or by a switch to a mood state of opposite polarity. In the past, children with frequent mood swings were described incorrectly as “rapid cycling,” but this term has been dropped because it engenders confusion between adult and pediatric BD phenomenology.2
A more precise method of describing mood symptoms in a child or adolescent is to use the FIND criteria, which include:4
- Frequency of symptoms per week
- Intensity of mood symptoms
- Number of mood cycles per day
- Duration of symptoms per day.
Visit this article at CurrentPsychiatry.com to view a table that outlines what to look for when using the FIND criteria to evaluate common pediatric psychiatric disorders that include mood swings. Table 1
describes clinical characteristics and tools and resources used to differentiate these and other disorders.4
Table 1
Clinical characteristics of psychiatric disorders that often feature mood swings
| Disorder | Clinical description | Useful tools/resources |
|---|---|---|
| ADHD | Chronic symptoms of hyperactivity, distractibility, impulsivity, poor attentional skills, disorganization | Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L) |
| ODD | Chronic symptoms of oppositionality, negativity; short, frequent mood swings in response to being asked to do something they do not want to do | CPRS-R:L |
| Anxiety disorders | Excessive ‘worry,’ difficulty with transitions, increased mood swings during stressful periods, psychosomatic symptoms | Self-Report for Childhood Anxiety Related Disorders |
| ARND | History of exposure to alcohol in-utero; mild dysmorphia, attentional, mood, and executive functioning problems | National Organization on Fetal Alcohol Syndrome |
| Bipolar disorder | In children: clustering together of episodes or ‘mini-episodes’ (several days) of increased energy, decreased need for sleep, increased mood cycling, pressured speech, etc. In adolescents: depressive episodes with episodes of hypomania or mania | Mood Disorders Questionnaire Kiddie Schedule for Affective Disorders and Schizophrenia Mania Rating Scale |
| ADHD: attention-deficit/hyperactivity disorder; ARND: alcohol-related neurodevelopmental disorder; ODD: oppositional defiant disorder | ||
| Source: Reference 4 | ||
Mood swings: A chart review
We recently completed a retrospective chart review of 100 patients consecutively referred to our pediatric mood disorders clinic for evaluation of “mood swings, rule out BD.” These patients were self-referred, referred by a psychiatrist for a second opinion, or referred by their primary care physician. The mean age of these patients was 8±2.8 years and 68% were male.
Two experienced clinicians (RAK and EM) interviewed each patient and their caregivers and reviewed results of the Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L)5 and other outside information.
Figure 1 illustrates these patients’ diagnoses. Diagnoses for each of these disorders were made using DSM-IV-TR criteria.3
The most common diagnoses among patients with the chief complaint of mood swings were ADHD (39%); ODD with ADHD (15%); an anxiety disorder, usually generalized anxiety disorder (GAD) (15%); BD (12%); and a secondary mood disorder, usually fetal alcohol spectrum disorder (10%). We were surprised at how often ADHD, ODD, and anxiety disorders were found to be responsible for these patients’ mood swings and how frequently the referring clinician did not recognize these disorders. In the following sections, we discuss each of these disorders and how they differ from BD.
Figure 1 Underlying diagnoses of 100 children/adolescents referred for ‘mood swings’
ADHD: attention-deficit/hyperactivity disorder; BD: bipolar disorder; MDD: major depressive disorder; ODD: oppositional defiant disorder; PDD: pervasive developmental disorder
ADHD and ODD
In our sample, patients with undiagnosed ADHD made up the largest group of those with frequent mood swings. ADHD inattentive type was missed frequently in adolescent girls who still had behavioral aspects of ADHD, including impulsivity and aggression.6
The CPRS-R:L is useful for screening and diagnosing children and adolescents with ADHD and ODD. It contains 80 items, can be used in males and females and patients age 3 to 17, and has validated norms by age and sex.5 It takes parents approximately 10 minutes to fill out this questionnaire and the results can be scored by hand. The CPRS-R:L includes the following scales: oppositional; cognitive problems/inattention; hyperactivity; anxious-shy; perfectionism; social problems; psychosomatic; Connors’ global index; DSM-IV symptom subscales; and an ADHD index. Patients with mood swings and ADHD combined typically score >2 standard deviations above their age/sex mean on the CPRS-R:L hyperactivity scale, Connors’ Global Index, and ADHD index.5
A common childhood disorder, ODD has multiple etiologies.7 The first DSM-IV criteria for ODD is “often loses temper”3—essentially mood swings that often are expressed behaviorally as anger and at times as aggressive outbursts.
Dodge and Cole8 categorized aggression as reactive (impulsivity with a high affective valence) or proactive (characterized by low arousal and premeditation, ie, predatory conduct disorder). Reactive aggression typically is an angry defensive response to frustration, threat, or provocation, whereas proactive aggression is deliberate, coercive behavior often used to obtain a goal.9 Reactive aggression is common among children with ADHD and ODD and typically begins as a mood swing that escalates into reactive aggressive behavior. In a study of 268 consecutively referred children and adolescents with ADHD and 100 community controls, Connor et al10 found significantly more reactive than proactive forms of aggression in ADHD patients.
It can be difficult to differentiate the moods swings and symptoms of ODD from those of pediatric BD. Mick et al11 found that severe irritability may be a diagnostic indicator of BD in children with ADHD. Using the Kiddie Schedule for Affective Disorders and Schizophrenia (epidemiologic version) structured diagnostic interview,12 they evaluated 274 children (mean age 10.8±3.2) with ADHD; 37% had no comorbid mood disorder, 36% had ADHD with depression, and 11% had ADHD with BD. Researchers characterized 3 types of irritability in these patients:
- ODD-type irritability characterized by a low frustration tolerance that is seen in ODD
- Mad/cranky irritability found in depressive disorders
- Super-angry/grouchy/cranky irritability with frequent, prolonged, and largely unprovoked anger episodes and characteristics of mania.
ODD-type irritability was common among all ADHD patients, was the least impairing type of irritability, and did not increase the risk of a mood disorder. Mad/cranky irritability was common only in children with ADHD and a mood disorder (depression or BD), was more impairing than ODD-type irritability, and was most predictive of unipolar depression. Super-angry/grouchy/cranky irritability was common only among children with ADHD and BD (77%), was the most impairing, and was predictive of both unipolar depression and BD. The type of irritability and clustering of DSM-IV manic symptoms best differentiated ADHD subjects from those with ADHD and BD. Figure 2 illustrates symptoms that differentiated patients with ADHD from those with ADHD and comorbid BD.11
A review of pharmacotherapy for aggression in children found the largest effects for methylphenidate for aggression in ADHD (mean effect size=0.9, combined N=844).13 Our clinical experience has been that pediatric patients with ADHD or ODD with ADHD often have high levels of reactive aggression that presents as mood swings, and aggressively treating ADHD often results in improved mood and other ADHD symptoms.
Figure 2 Symptoms that differentiate BD from BD with comorbid ADHD
ADHD: attention-deficit/hyperactivity disorder; BD: bipolar disorder
Source: Reference 11
Anxiety disorders
The estimated prevalence of child and adolescent anxiety disorders is 10% to 20%14; in our sample the prevalence was 15%. These disorders include GAD, separation anxiety disorder, social phobias, posttraumatic stress disorder (PTSD), and obsessive-compulsive disorder. Often, children with GAD worry excessively and become upset during transitions when things don’t proceed as they expect, with resultant angry outbursts and mood swings. Mood swings and difficulty sleeping are common in children with anxiety disorders or BD. Anxiety disorders often will be missed unless specific triggers of the mood swings or angry outbursts—as well as differentiating symptoms such as excessive fear, worry, and psychosomatic symptoms—are assessed.
In our clinical experience, simply asking a child if he or she is anxious is not sufficient to uncover an anxiety disorder. Although the CPRS-L:R will screen for anxiety disorders, we have found that the Self-Report for Childhood Anxiety Related Disorders (SCARED) developed by Birmaher et al15 is more specific. This tool can be used in patients age ≥8. The parent and child versions of the SCARED contain 41 items that measure 5 factors:
- general anxiety
- separation anxiety
- social phobia
- school phobia
- physical symptoms of anxiety.
The SCARED takes 5 minutes to fill out and is available in parent and child versions.
Secondary mood disorders
Many patients in our sample had a mood disorder secondary to the neurologic effects of alcohol on the developing brain. For more about maternal alcohol use, fetal alcohol spectrum disorders, and mood swings, visit this article at CurrentPsychiatry.com.
What BD looks like in children
In our sample, 12% of patients referred for mood swings were diagnosed with bipolar I disorder (BDI), bipolar II disorder (BDII), or bipolar disorder, not otherwise specified (BD-NOS). In the United States, lifetime prevalence of BDI and BDII in adolescents age 13 to 17 is 2.9%.16 No large epidemiologic studies have looked at the lifetime prevalence of BD in children age <13.
How often a clinician sees BD in children and adolescents largely depends on the type of setting in which he or she practices. Although in the general population BD is relatively rare compared with other childhood psychiatric disorders, on child/adolescent inpatient units it is common to find that 30% to 40% of patients have BD.17
The best longitudinal study to date of the phenomenology, comorbidity, and outcome of BD in children and adolescents is the National Institute of Mental Health-funded Course and Outcome of Bipolar Youth study (COBY).18 In this ongoing, longitudinal study, 413 youths (age 7 to 17) with BDI (N=244), BDII (N=28), or BD-NOS (N=141) were rigorously diagnosed using state-of-the-art measures, including the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present version19 and re-evaluated every 9.4 months for 4 years. When organizing this study, investigators found that DSM-IV criteria for BD-NOS were too vague to be useful and developed their own criteria (Table 2).18
For BDI patients in the COBY study, the mean age of onset for bipolar symptoms was 9.0±4.1 years and the mean duration of illness was 4.4±3.1 years. Researchers reported that at the 4-year assessment approximately 70% of patients with BD recovered from their index episode, and 50% had at least 1 syndromal recurrence, particularly depressive episodes.20 Analyses of these patients’ weekly mood symptoms showed that they had syndromal or subsyndromal symptoms with numerous changes in symptoms and shifts of mood polarity 60% of the time, and psychosis 3% of the time. During this study, 20% of BDII patients progressed to BDI, and 25% of BD-NOS patients converted to BDI or BDII.
Further analysis of the COBY data revealed that onset of mood symptoms preceded onset of clear bipolar episodes by an average of 1.0±1.7 years. Depression was the most common initial and most frequent episode for adolescents; mood lability was seen more often in childhood-onset and adolescents with early-onset BD. Depressed children had more severe irritability than depressed adolescents, and older age was associated with more severe and typical mood symptomatology.21
The clinical picture of a child with BD that emerges from the COBY study is:
- a fairly young child with the onset of mood symptoms between age 5 to 12
- subsyndromal and less frequently clear syndromal episodes
- primarily mixed and depressed symptoms with rapid mood cycles during these episodes.22
It is clear that there is a spectrum of bipolar disorders in children and adolescents with varying degrees of symptom expression and children differ from adolescents and adults in their initial presentation of BD.
Table 2
COBY criteria for bipolar disorder, not otherwise specified
| Presence of clinically relevant bipolar symptoms that do not fulfill DSM-IV criteria for BDI or BDII |
| In addition, patients are required to have elevated mood plus 2 associated DSM-IV symptoms or irritable mood plus 3 DSM-IV associated symptoms, along with a change in level of functioning |
| Duration of a minimum of 4 hours within a 24-hour period |
| At least 4 cumulative lifetime days meeting the criteria |
| BDI: bipolar I disorder; BDII: bipolar II disorder; COBY: Course and Outcome of Bipolar Youth study |
| Source: Reference 18 |
Related Resources
- Kowatch RA, Fristad MA, Findling RL, et al. Clinical manual for management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2009.
- Goodwin FK, Jamison KR. Manic-depressive illness. 2nd ed. Oxford, United Kingdom: Oxford University Press; 2007.
- Miklowitz DJ, Cicchetti D, eds. Understanding bipolar disorder: a developmental perspective. New York, NY: Guilford Press; 2010.
Drug Brand Name
- Methylphenidate • Ritalin, Concerta, others
Disclosures
Dr. Kowatch receives grant/research support from the National Institute of Child Health and Human Development and the National Institute of Mental Health and is a consultant to AstraZeneca, Forest Pharmaceuticals, Merck, and the REACH Foundation.
Dr. Delgado and Ms. Monroe report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Table
FIND criteria of disorders found to cause mood swings
| Criteria | BDI | BP-NOS | ODD | GAD | ARND |
|---|---|---|---|---|---|
| Frequency of symptoms/week | 7 days (more days than not in an average week) | 2 to 3 days/week | Daily (chronic) irritability and mood swings precipitated by ‘not getting their way’ | Greatest during times of change/stress | Daily |
| Intensity of symptoms | Severe—parents often are afraid to take the child out in public because of mood symptoms | Moderate | Mild/moderate | Mild/moderate when stressed | Mild/moderate |
| Number of mood cycles/day | Daily cycles of euphoria and depression | 3 to 4 | 5 to 10 | 2 to 3 | 8 to 10 |
| Duration of symptoms/day | Euphoria: 30 to 60 minutes Depression: 30 minutes to 6 hours | 4 hours total/day of mood symptoms | Short; 5 to 10 minutes | Short; 5 to 10 minutes | Short; 5 to 10 minutes |
| ARND: alcohol-related neurodevelopmental disorder; BDI: bipolar I disorder; BD-NOS: bipolar disorder, not otherwise specified; GAD: generalized anxiety disorder; ODD: oppositional defiant disorder | |||||
| Source: Kowatch RA, Fristad MA, Findling RL, et al. Clinical manual for the management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2008 | |||||
Even small amounts of alcohol use by a pregnant woman can impact her child’s development. In a controlled study examining drinking behavior of 12,678 pregnant women and the effect this had on their children, Sayel et ala found that <1 drink per week during the first trimester was clinically significant for mental health problems in girls, measured at age 4 and 8, when using parent or teacher report.
Fetal alcohol spectrum disorder describes the range of effects that can occur in an individual whose mother drank alcohol during pregnancy. These disorders include fetal alcohol syndrome (FAS), alcohol-related neurodevelopmental disorder (ARND), and alcohol-related birth defects (ARBD).
FAS. Individuals with FAS have a distinct pattern of facial abnormalities, growth deficiency, and evidence of CNS dysfunction. Characteristic facial abnormalities may include a smooth philtrum, thin upper lip, upturned nose, flat nasal bridge and midface, epicanthal folds, small palpebral fissures, and small head circumference. Growth deficiency begins in-utero and continues throughout childhood and into adulthood. CNS abnormalities can include impaired brain growth or abnormal structure, manifested differently depending on age.
ARND. Many individuals affected by alcohol exposure before birth do not have the characteristic facial abnormalities and growth retardation identified with full FAS, yet have significant brain and behavioral impairments. Individuals with ARND have either the facial anomalies, growth retardation, and other physical abnormalities, or a complex pattern of behavioral or cognitive abnormalities inconsistent with developmental level and unexplained by genetic background or environmental conditions (ie, poor impulse control, language deficits, problems with abstraction, mathematical and social perception deficits, learning problems, and impairment in attention, memory, or judgment).b
ARBD. Persons with ARBD have malformations of the skeletal and major organ systems, such as cardiac or renal abnormalities.
Comorbid psychiatric conditions in children with prenatal alcohol exposure are 5 to 16 times more prevalent than in the general population; these children are 38% more likely to have an anger disorder.c O’Connor and Paleyd found that “…mood disorder symptoms were significantly higher for children with parental alcohol exposure compared to children without exposure.” Children with ARND are treated symptomatically depending upon which deficits and behaviors they exhibit.e
References
a. Sayal K, Heron J, Golding J, et al. Binge pattern of alcohol consumption during pregnancy and childhood mental health outcomes: longitudinal population-based study. Pediatrics. 2009;123(2):e289-296.
b. Warren KR, Foudin LL. Alcohol-related birth defects—the past, present, and future. Alcohol Res Health. 2001;25(3):153-158.
c. Burd L, Klug MG, Martsolf JT, et al. Fetal alcohol syndrome: neuropsychiatric phenomics. Neurotoxicol Teratol. 2003;25(6):697-705.
d. O’Connor MJ, Paley B. Psychiatric conditions associated with prenatal alcohol exposure. Dev Disabil Res Rev. 2009;15(3):225-234.
e. Paley B, O’Connor MJ. Intervention for individuals with fetal alcohol spectrum disorders: treatment approaches and case management. Dev Disabil Res Rev. 2009;15(3):258-267.
M, age 13, is referred by her pediatrician with the chief complaint of “severe mood swings, rule out bipolar disorder (BD).” In the past she was treated for attention-deficit/hyperactivity disorder (ADHD) with stimulants with mixed results. M’s parents are concerned about her “flipping out” whenever she is asked to do something she does not want to do. Her mother has a history of depression and anxiety; her father had a “drinking problem.” There is no history of BD in her first- or second-degree relatives. Are M’s rapid mood swings a sign of BD or another disorder?
The differential diagnosis of “mood swings” is important because they are a common presenting symptom of many children and adolescents with mood and behavioral disorders. Mood swings often occur in children and adolescents with ADHD, oppositional defiant disorder (ODD), developmental disorders, depressive disorders, BD, anxiety disorders, and conduct disorders. Mood swings are analogous to a fever in pediatrics—they indicate something potentially is wrong with the patient, but are not diagnostic as an isolated symptom.
Mood swings in children are common, nonspecific symptoms that more often are a sign of anxiety or behavioral disorders than BD. This article discusses the differential diagnosis of mood swings in children and adolescents and how to best screen and diagnose these patients.
What are ‘mood swings’?
Mood swings is a popular term that is nonspecific and not part of DSM-IV-TR diagnostic criteria for BD. The complaint of “mood swings” may reflect severe mood lability of pediatric patients with BD. This mood lability is best described by the Kiddie-Mania Rating Scale (K-MRS) developed by Axelson and colleagues as “rapid mood variation with several mood states within a brief period of time which appears internally driven without regard to the circumstance.”1 On K-MRS mood lability items, children with mania typically score:
- Moderate—many mood changes throughout the day, can vary from elevated mood to anger to sadness within a few hours; changes in mood are clearly out of proportion to circumstances and cause impairment in functioning
- Severe—rapid mood swings nearly all of the time, with mood intensity greatly out of proportion to circumstances
- Extreme—constant, explosive variability in mood, several mood changes occurring within minutes, difficult to identify a particular mood, changes in mood radically out of proportion to circumstances.
Patients with BD typically exhibit what is best described as a “mood cycle”—a pronounced shift in mood and energy from 1 extreme to another.2 An example of this would be a child who wakes up with extreme silliness, high energy, and intrusive behavior that persists for several hours and then later in the day becomes sad, depressed, and suicidal with no precipitant for either mood cycle. BD patients also will exhibit other symptoms of mania during these mood cycling periods.
Rapid cycling is a DSM-IV course specifier that indicates ≥4 mood episodes per year in patients with BD with a typical course of mania or hypomania followed by depression, or vice versa.3 The episodes must be demarcated by full or partial remission that lasts ≥2 months or by a switch to a mood state of opposite polarity. In the past, children with frequent mood swings were described incorrectly as “rapid cycling,” but this term has been dropped because it engenders confusion between adult and pediatric BD phenomenology.2
A more precise method of describing mood symptoms in a child or adolescent is to use the FIND criteria, which include:4
- Frequency of symptoms per week
- Intensity of mood symptoms
- Number of mood cycles per day
- Duration of symptoms per day.
Visit this article at CurrentPsychiatry.com to view a table that outlines what to look for when using the FIND criteria to evaluate common pediatric psychiatric disorders that include mood swings. Table 1
describes clinical characteristics and tools and resources used to differentiate these and other disorders.4
Table 1
Clinical characteristics of psychiatric disorders that often feature mood swings
| Disorder | Clinical description | Useful tools/resources |
|---|---|---|
| ADHD | Chronic symptoms of hyperactivity, distractibility, impulsivity, poor attentional skills, disorganization | Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L) |
| ODD | Chronic symptoms of oppositionality, negativity; short, frequent mood swings in response to being asked to do something they do not want to do | CPRS-R:L |
| Anxiety disorders | Excessive ‘worry,’ difficulty with transitions, increased mood swings during stressful periods, psychosomatic symptoms | Self-Report for Childhood Anxiety Related Disorders |
| ARND | History of exposure to alcohol in-utero; mild dysmorphia, attentional, mood, and executive functioning problems | National Organization on Fetal Alcohol Syndrome |
| Bipolar disorder | In children: clustering together of episodes or ‘mini-episodes’ (several days) of increased energy, decreased need for sleep, increased mood cycling, pressured speech, etc. In adolescents: depressive episodes with episodes of hypomania or mania | Mood Disorders Questionnaire Kiddie Schedule for Affective Disorders and Schizophrenia Mania Rating Scale |
| ADHD: attention-deficit/hyperactivity disorder; ARND: alcohol-related neurodevelopmental disorder; ODD: oppositional defiant disorder | ||
| Source: Reference 4 | ||
Mood swings: A chart review
We recently completed a retrospective chart review of 100 patients consecutively referred to our pediatric mood disorders clinic for evaluation of “mood swings, rule out BD.” These patients were self-referred, referred by a psychiatrist for a second opinion, or referred by their primary care physician. The mean age of these patients was 8±2.8 years and 68% were male.
Two experienced clinicians (RAK and EM) interviewed each patient and their caregivers and reviewed results of the Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L)5 and other outside information.
Figure 1 illustrates these patients’ diagnoses. Diagnoses for each of these disorders were made using DSM-IV-TR criteria.3
The most common diagnoses among patients with the chief complaint of mood swings were ADHD (39%); ODD with ADHD (15%); an anxiety disorder, usually generalized anxiety disorder (GAD) (15%); BD (12%); and a secondary mood disorder, usually fetal alcohol spectrum disorder (10%). We were surprised at how often ADHD, ODD, and anxiety disorders were found to be responsible for these patients’ mood swings and how frequently the referring clinician did not recognize these disorders. In the following sections, we discuss each of these disorders and how they differ from BD.
Figure 1 Underlying diagnoses of 100 children/adolescents referred for ‘mood swings’
ADHD: attention-deficit/hyperactivity disorder; BD: bipolar disorder; MDD: major depressive disorder; ODD: oppositional defiant disorder; PDD: pervasive developmental disorder
ADHD and ODD
In our sample, patients with undiagnosed ADHD made up the largest group of those with frequent mood swings. ADHD inattentive type was missed frequently in adolescent girls who still had behavioral aspects of ADHD, including impulsivity and aggression.6
The CPRS-R:L is useful for screening and diagnosing children and adolescents with ADHD and ODD. It contains 80 items, can be used in males and females and patients age 3 to 17, and has validated norms by age and sex.5 It takes parents approximately 10 minutes to fill out this questionnaire and the results can be scored by hand. The CPRS-R:L includes the following scales: oppositional; cognitive problems/inattention; hyperactivity; anxious-shy; perfectionism; social problems; psychosomatic; Connors’ global index; DSM-IV symptom subscales; and an ADHD index. Patients with mood swings and ADHD combined typically score >2 standard deviations above their age/sex mean on the CPRS-R:L hyperactivity scale, Connors’ Global Index, and ADHD index.5
A common childhood disorder, ODD has multiple etiologies.7 The first DSM-IV criteria for ODD is “often loses temper”3—essentially mood swings that often are expressed behaviorally as anger and at times as aggressive outbursts.
Dodge and Cole8 categorized aggression as reactive (impulsivity with a high affective valence) or proactive (characterized by low arousal and premeditation, ie, predatory conduct disorder). Reactive aggression typically is an angry defensive response to frustration, threat, or provocation, whereas proactive aggression is deliberate, coercive behavior often used to obtain a goal.9 Reactive aggression is common among children with ADHD and ODD and typically begins as a mood swing that escalates into reactive aggressive behavior. In a study of 268 consecutively referred children and adolescents with ADHD and 100 community controls, Connor et al10 found significantly more reactive than proactive forms of aggression in ADHD patients.
It can be difficult to differentiate the moods swings and symptoms of ODD from those of pediatric BD. Mick et al11 found that severe irritability may be a diagnostic indicator of BD in children with ADHD. Using the Kiddie Schedule for Affective Disorders and Schizophrenia (epidemiologic version) structured diagnostic interview,12 they evaluated 274 children (mean age 10.8±3.2) with ADHD; 37% had no comorbid mood disorder, 36% had ADHD with depression, and 11% had ADHD with BD. Researchers characterized 3 types of irritability in these patients:
- ODD-type irritability characterized by a low frustration tolerance that is seen in ODD
- Mad/cranky irritability found in depressive disorders
- Super-angry/grouchy/cranky irritability with frequent, prolonged, and largely unprovoked anger episodes and characteristics of mania.
ODD-type irritability was common among all ADHD patients, was the least impairing type of irritability, and did not increase the risk of a mood disorder. Mad/cranky irritability was common only in children with ADHD and a mood disorder (depression or BD), was more impairing than ODD-type irritability, and was most predictive of unipolar depression. Super-angry/grouchy/cranky irritability was common only among children with ADHD and BD (77%), was the most impairing, and was predictive of both unipolar depression and BD. The type of irritability and clustering of DSM-IV manic symptoms best differentiated ADHD subjects from those with ADHD and BD. Figure 2 illustrates symptoms that differentiated patients with ADHD from those with ADHD and comorbid BD.11
A review of pharmacotherapy for aggression in children found the largest effects for methylphenidate for aggression in ADHD (mean effect size=0.9, combined N=844).13 Our clinical experience has been that pediatric patients with ADHD or ODD with ADHD often have high levels of reactive aggression that presents as mood swings, and aggressively treating ADHD often results in improved mood and other ADHD symptoms.
Figure 2 Symptoms that differentiate BD from BD with comorbid ADHD
ADHD: attention-deficit/hyperactivity disorder; BD: bipolar disorder
Source: Reference 11
Anxiety disorders
The estimated prevalence of child and adolescent anxiety disorders is 10% to 20%14; in our sample the prevalence was 15%. These disorders include GAD, separation anxiety disorder, social phobias, posttraumatic stress disorder (PTSD), and obsessive-compulsive disorder. Often, children with GAD worry excessively and become upset during transitions when things don’t proceed as they expect, with resultant angry outbursts and mood swings. Mood swings and difficulty sleeping are common in children with anxiety disorders or BD. Anxiety disorders often will be missed unless specific triggers of the mood swings or angry outbursts—as well as differentiating symptoms such as excessive fear, worry, and psychosomatic symptoms—are assessed.
In our clinical experience, simply asking a child if he or she is anxious is not sufficient to uncover an anxiety disorder. Although the CPRS-L:R will screen for anxiety disorders, we have found that the Self-Report for Childhood Anxiety Related Disorders (SCARED) developed by Birmaher et al15 is more specific. This tool can be used in patients age ≥8. The parent and child versions of the SCARED contain 41 items that measure 5 factors:
- general anxiety
- separation anxiety
- social phobia
- school phobia
- physical symptoms of anxiety.
The SCARED takes 5 minutes to fill out and is available in parent and child versions.
Secondary mood disorders
Many patients in our sample had a mood disorder secondary to the neurologic effects of alcohol on the developing brain. For more about maternal alcohol use, fetal alcohol spectrum disorders, and mood swings, visit this article at CurrentPsychiatry.com.
What BD looks like in children
In our sample, 12% of patients referred for mood swings were diagnosed with bipolar I disorder (BDI), bipolar II disorder (BDII), or bipolar disorder, not otherwise specified (BD-NOS). In the United States, lifetime prevalence of BDI and BDII in adolescents age 13 to 17 is 2.9%.16 No large epidemiologic studies have looked at the lifetime prevalence of BD in children age <13.
How often a clinician sees BD in children and adolescents largely depends on the type of setting in which he or she practices. Although in the general population BD is relatively rare compared with other childhood psychiatric disorders, on child/adolescent inpatient units it is common to find that 30% to 40% of patients have BD.17
The best longitudinal study to date of the phenomenology, comorbidity, and outcome of BD in children and adolescents is the National Institute of Mental Health-funded Course and Outcome of Bipolar Youth study (COBY).18 In this ongoing, longitudinal study, 413 youths (age 7 to 17) with BDI (N=244), BDII (N=28), or BD-NOS (N=141) were rigorously diagnosed using state-of-the-art measures, including the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present version19 and re-evaluated every 9.4 months for 4 years. When organizing this study, investigators found that DSM-IV criteria for BD-NOS were too vague to be useful and developed their own criteria (Table 2).18
For BDI patients in the COBY study, the mean age of onset for bipolar symptoms was 9.0±4.1 years and the mean duration of illness was 4.4±3.1 years. Researchers reported that at the 4-year assessment approximately 70% of patients with BD recovered from their index episode, and 50% had at least 1 syndromal recurrence, particularly depressive episodes.20 Analyses of these patients’ weekly mood symptoms showed that they had syndromal or subsyndromal symptoms with numerous changes in symptoms and shifts of mood polarity 60% of the time, and psychosis 3% of the time. During this study, 20% of BDII patients progressed to BDI, and 25% of BD-NOS patients converted to BDI or BDII.
Further analysis of the COBY data revealed that onset of mood symptoms preceded onset of clear bipolar episodes by an average of 1.0±1.7 years. Depression was the most common initial and most frequent episode for adolescents; mood lability was seen more often in childhood-onset and adolescents with early-onset BD. Depressed children had more severe irritability than depressed adolescents, and older age was associated with more severe and typical mood symptomatology.21
The clinical picture of a child with BD that emerges from the COBY study is:
- a fairly young child with the onset of mood symptoms between age 5 to 12
- subsyndromal and less frequently clear syndromal episodes
- primarily mixed and depressed symptoms with rapid mood cycles during these episodes.22
It is clear that there is a spectrum of bipolar disorders in children and adolescents with varying degrees of symptom expression and children differ from adolescents and adults in their initial presentation of BD.
Table 2
COBY criteria for bipolar disorder, not otherwise specified
| Presence of clinically relevant bipolar symptoms that do not fulfill DSM-IV criteria for BDI or BDII |
| In addition, patients are required to have elevated mood plus 2 associated DSM-IV symptoms or irritable mood plus 3 DSM-IV associated symptoms, along with a change in level of functioning |
| Duration of a minimum of 4 hours within a 24-hour period |
| At least 4 cumulative lifetime days meeting the criteria |
| BDI: bipolar I disorder; BDII: bipolar II disorder; COBY: Course and Outcome of Bipolar Youth study |
| Source: Reference 18 |
Related Resources
- Kowatch RA, Fristad MA, Findling RL, et al. Clinical manual for management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2009.
- Goodwin FK, Jamison KR. Manic-depressive illness. 2nd ed. Oxford, United Kingdom: Oxford University Press; 2007.
- Miklowitz DJ, Cicchetti D, eds. Understanding bipolar disorder: a developmental perspective. New York, NY: Guilford Press; 2010.
Drug Brand Name
- Methylphenidate • Ritalin, Concerta, others
Disclosures
Dr. Kowatch receives grant/research support from the National Institute of Child Health and Human Development and the National Institute of Mental Health and is a consultant to AstraZeneca, Forest Pharmaceuticals, Merck, and the REACH Foundation.
Dr. Delgado and Ms. Monroe report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Table
FIND criteria of disorders found to cause mood swings
| Criteria | BDI | BP-NOS | ODD | GAD | ARND |
|---|---|---|---|---|---|
| Frequency of symptoms/week | 7 days (more days than not in an average week) | 2 to 3 days/week | Daily (chronic) irritability and mood swings precipitated by ‘not getting their way’ | Greatest during times of change/stress | Daily |
| Intensity of symptoms | Severe—parents often are afraid to take the child out in public because of mood symptoms | Moderate | Mild/moderate | Mild/moderate when stressed | Mild/moderate |
| Number of mood cycles/day | Daily cycles of euphoria and depression | 3 to 4 | 5 to 10 | 2 to 3 | 8 to 10 |
| Duration of symptoms/day | Euphoria: 30 to 60 minutes Depression: 30 minutes to 6 hours | 4 hours total/day of mood symptoms | Short; 5 to 10 minutes | Short; 5 to 10 minutes | Short; 5 to 10 minutes |
| ARND: alcohol-related neurodevelopmental disorder; BDI: bipolar I disorder; BD-NOS: bipolar disorder, not otherwise specified; GAD: generalized anxiety disorder; ODD: oppositional defiant disorder | |||||
| Source: Kowatch RA, Fristad MA, Findling RL, et al. Clinical manual for the management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2008 | |||||
Even small amounts of alcohol use by a pregnant woman can impact her child’s development. In a controlled study examining drinking behavior of 12,678 pregnant women and the effect this had on their children, Sayel et ala found that <1 drink per week during the first trimester was clinically significant for mental health problems in girls, measured at age 4 and 8, when using parent or teacher report.
Fetal alcohol spectrum disorder describes the range of effects that can occur in an individual whose mother drank alcohol during pregnancy. These disorders include fetal alcohol syndrome (FAS), alcohol-related neurodevelopmental disorder (ARND), and alcohol-related birth defects (ARBD).
FAS. Individuals with FAS have a distinct pattern of facial abnormalities, growth deficiency, and evidence of CNS dysfunction. Characteristic facial abnormalities may include a smooth philtrum, thin upper lip, upturned nose, flat nasal bridge and midface, epicanthal folds, small palpebral fissures, and small head circumference. Growth deficiency begins in-utero and continues throughout childhood and into adulthood. CNS abnormalities can include impaired brain growth or abnormal structure, manifested differently depending on age.
ARND. Many individuals affected by alcohol exposure before birth do not have the characteristic facial abnormalities and growth retardation identified with full FAS, yet have significant brain and behavioral impairments. Individuals with ARND have either the facial anomalies, growth retardation, and other physical abnormalities, or a complex pattern of behavioral or cognitive abnormalities inconsistent with developmental level and unexplained by genetic background or environmental conditions (ie, poor impulse control, language deficits, problems with abstraction, mathematical and social perception deficits, learning problems, and impairment in attention, memory, or judgment).b
ARBD. Persons with ARBD have malformations of the skeletal and major organ systems, such as cardiac or renal abnormalities.
Comorbid psychiatric conditions in children with prenatal alcohol exposure are 5 to 16 times more prevalent than in the general population; these children are 38% more likely to have an anger disorder.c O’Connor and Paleyd found that “…mood disorder symptoms were significantly higher for children with parental alcohol exposure compared to children without exposure.” Children with ARND are treated symptomatically depending upon which deficits and behaviors they exhibit.e
References
a. Sayal K, Heron J, Golding J, et al. Binge pattern of alcohol consumption during pregnancy and childhood mental health outcomes: longitudinal population-based study. Pediatrics. 2009;123(2):e289-296.
b. Warren KR, Foudin LL. Alcohol-related birth defects—the past, present, and future. Alcohol Res Health. 2001;25(3):153-158.
c. Burd L, Klug MG, Martsolf JT, et al. Fetal alcohol syndrome: neuropsychiatric phenomics. Neurotoxicol Teratol. 2003;25(6):697-705.
d. O’Connor MJ, Paley B. Psychiatric conditions associated with prenatal alcohol exposure. Dev Disabil Res Rev. 2009;15(3):225-234.
e. Paley B, O’Connor MJ. Intervention for individuals with fetal alcohol spectrum disorders: treatment approaches and case management. Dev Disabil Res Rev. 2009;15(3):258-267.
1. Axelson D, Birmaher BJ, Brent D, et al. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. J Child Adolesc Psychopharmacol. 2003;13(4):463-470.
2. Youngstrom EA, Birmaher B, Findling RL. Pediatric bipolar disorder: validity, phenomenology, and recommendations for diagnosis. Bipolar Disord. 2008;10(1 Pt 2):194-214.
3. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
4. Kowatch RA, Fristad MA, Findling RL, et al. Clinical manual for the management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2008.
5. Conners CK. Conners’ Parent Rating Scale Long Form (CPRS-R:L) North Tonawanda, NY: Multi-Health Systems, Inc.; 1997.
6. Martel MM. Research review: a new perspective on attention-deficit/hyperactivity disorder: emotion dysregulation and trait models. J Child Psychol Psychiatry. 2009;50(9):1042-1051.
7. Steiner H, Remsing L. and the Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(1):126-141.
8. Dodge KA, Cole JD. Social-information-processing factors in reactive and proactive aggression in children’s peer groups. J Pers Soc Psychol. 1987;53(6):1146-1158.
9. Connor DF, Steingard RJ, Cunningham JA, et al. Proactive and reactive aggression in referred children and adolescents. Am J Orthopsychiatry. 2004;74(2):129-136.
10. Connor DF, Chartier KG, Preen EC, et al. Impulsive aggression in attention-deficit/hyperactivity disorder: symptom severity, co-morbidity, and attention-deficit/hyperactivity disorder subtype. J Child Adolesc Psychopharmacol. 2010;20(2):119-126.
11. Mick E, Spencer T, Wozniak J, et al. Heterogeneity of irritability in attention-deficit/hyperactivity disorder subjects with and without mood disorders. Biol Psychiatry. 2005;58(7):576-582.
12. Orvaschel H. Schizophrenia and Affective Disorders Schedule for children—Epidemiological Version (KSADS-E). Fort Lauderdale, FL: Nova Southeastern University; 1995.
13. Pappadopulos E, Woolston S, Chait A, et al. Pharmacotherapy of aggression in children and adolescents: efficacy and effect size. J Can Acad Child Adolesc Psychiatry. 2006;15(1):27-39.
14. Achenbach TM, Howell CT, McConaughy SH, et al. Six-year predictors of problems in a national sample: IV. Young adult signs of disturbance. J Am Acad Child Adolesc Psychiatry. 1998;37(7):718-727.
15. Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36:545-553.
16. Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication—Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980-989.
17. Youngstrom EA, Duax J. Evidence-based assessment of pediatric bipolar disorder, part I: base rate and family history. J Am Acad Child Adolesc Psychiatry. 2005;44(7):712-717.
18. Birmaher B, Axelson D, Strober M, et al. Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63(2):175-183.
19. Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980-988.
20. Birmaher B, Axelson D. Course and outcome of bipolar spectrum disorder in children and adolescents: a review of the existing literature. Dev Psychopathol. 2006;18(4):1023-1035.
21. Birmaher B, Axelson D, Strober M, et al. Comparison of manic and depressive symptoms between children and adolescents with bipolar spectrum disorders. Bipolar Disord. 2009;11(1):52-62.
22. Birmaher B, Axelson D, Goldstein B, et al. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the Course and Outcome of Bipolar Youth (COBY) study. Am J Psychiatry. 2009;166(7):795-804.
1. Axelson D, Birmaher BJ, Brent D, et al. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. J Child Adolesc Psychopharmacol. 2003;13(4):463-470.
2. Youngstrom EA, Birmaher B, Findling RL. Pediatric bipolar disorder: validity, phenomenology, and recommendations for diagnosis. Bipolar Disord. 2008;10(1 Pt 2):194-214.
3. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
4. Kowatch RA, Fristad MA, Findling RL, et al. Clinical manual for the management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2008.
5. Conners CK. Conners’ Parent Rating Scale Long Form (CPRS-R:L) North Tonawanda, NY: Multi-Health Systems, Inc.; 1997.
6. Martel MM. Research review: a new perspective on attention-deficit/hyperactivity disorder: emotion dysregulation and trait models. J Child Psychol Psychiatry. 2009;50(9):1042-1051.
7. Steiner H, Remsing L. and the Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(1):126-141.
8. Dodge KA, Cole JD. Social-information-processing factors in reactive and proactive aggression in children’s peer groups. J Pers Soc Psychol. 1987;53(6):1146-1158.
9. Connor DF, Steingard RJ, Cunningham JA, et al. Proactive and reactive aggression in referred children and adolescents. Am J Orthopsychiatry. 2004;74(2):129-136.
10. Connor DF, Chartier KG, Preen EC, et al. Impulsive aggression in attention-deficit/hyperactivity disorder: symptom severity, co-morbidity, and attention-deficit/hyperactivity disorder subtype. J Child Adolesc Psychopharmacol. 2010;20(2):119-126.
11. Mick E, Spencer T, Wozniak J, et al. Heterogeneity of irritability in attention-deficit/hyperactivity disorder subjects with and without mood disorders. Biol Psychiatry. 2005;58(7):576-582.
12. Orvaschel H. Schizophrenia and Affective Disorders Schedule for children—Epidemiological Version (KSADS-E). Fort Lauderdale, FL: Nova Southeastern University; 1995.
13. Pappadopulos E, Woolston S, Chait A, et al. Pharmacotherapy of aggression in children and adolescents: efficacy and effect size. J Can Acad Child Adolesc Psychiatry. 2006;15(1):27-39.
14. Achenbach TM, Howell CT, McConaughy SH, et al. Six-year predictors of problems in a national sample: IV. Young adult signs of disturbance. J Am Acad Child Adolesc Psychiatry. 1998;37(7):718-727.
15. Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36:545-553.
16. Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication—Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980-989.
17. Youngstrom EA, Duax J. Evidence-based assessment of pediatric bipolar disorder, part I: base rate and family history. J Am Acad Child Adolesc Psychiatry. 2005;44(7):712-717.
18. Birmaher B, Axelson D, Strober M, et al. Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63(2):175-183.
19. Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980-988.
20. Birmaher B, Axelson D. Course and outcome of bipolar spectrum disorder in children and adolescents: a review of the existing literature. Dev Psychopathol. 2006;18(4):1023-1035.
21. Birmaher B, Axelson D, Strober M, et al. Comparison of manic and depressive symptoms between children and adolescents with bipolar spectrum disorders. Bipolar Disord. 2009;11(1):52-62.
22. Birmaher B, Axelson D, Goldstein B, et al. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the Course and Outcome of Bipolar Youth (COBY) study. Am J Psychiatry. 2009;166(7):795-804.
Blog: Psychotherapy on the Great White Way
That show, Next to Normal, didn’t disappoint as entertainment. It had an enjoyable and involving rock score, a talented cast of six actors, and inventive staging and lighting. It also had a plot that dealt seriously, often movingly, about something that’s not a natural for a musical’s premise: mental illness. The show’s book received enough attention after the opening in April 2009 that earlier this year it won the Pulitzer Prize for drama, following a Tony the score received as 2009′s best.
Despite that, it seemed to me that the show’s book and lyric author, Brian Yorkey, flubbed the challenge of building a satisfying dramatic arc on this subject while setting it to music and coming in under 2 hours. Whether or not that’s the explanation, the show’s story of a middle-aged mother and wife haunted by a powerful, delusional fantasy of the son she lost when he was 8 months old, slips up with its simplistically negative and clichéd portrayal of cand psychotherapy. At the end of Next to Normal, the central character, Diana, prevails by facing down her mental demons drug- and treatment-free, finally talking with her daughter and husband about their troubled past. Familial love and open communication allow Diana to overcome her delusions and manic depression. The bad guys in this feel-good ending are Diana’s two psychiatrists and their ineffectual treatments.
What’s remarkable is that Mr. Yorkey denies making the psychiatrists the villains. Quoted in an article by Patricia Cohen in The New York Times soon after Next to Normal opened last year, Mr. Yorkey says: “Some people have walked away thinking we were against psychologists or psychotropic drugs and nothing could be further from the truth.” That assertion makes me wonder which theater he’s been visiting and whether it was the same one I sat in the other evening.
The musical I saw sets the tone with an early, first act song in which Diana reviews her pharmacologic history in the song ”My Psychopharmacologist and I,” which catalogs the “Zoloft and Paxil and Buspar and Xanex, Depacon, Chronaphin, Ambien, Prozac, Ativan calms me when I see the bills. These are a few of my favorite pills.” Diana later croons about the adverse effects she develops, and that her regimen has left her mentally numb. “I don’t feel anything,” she says, to which her psychiatrist sagely replies: “Patient stable.” Soon after, Diana decides to go drug free, happily disposing of her “Risperdal, Valium, and Xanax.”
When she then has an unsuccessful suicide attempt, a new psychiatrist (described in the show as a “rock star”–an infallible expert) recommends electroconvulsive therapy, a treatment depicted as menacing and extreme and, once again, ultimately ineffectual. All the ECT achieves for Diana is substantial memory loss, and then a return of her powerful and incapacitating delusions. As the second act winds down, she abandons most psychiatric treatments, accepting only talk therapy, and at the show’s end Diana achieves transcendence fueled by her family’s love and new-found openness.
Of course these can be powerfully therapeutic, but the show casts it as a simple black and white situation, with a facile Forty-second street philosophy working while mainstream psychiatry completely failed.
—Mitchel Zoler (on Twitter @mitchelzoler)
That show, Next to Normal, didn’t disappoint as entertainment. It had an enjoyable and involving rock score, a talented cast of six actors, and inventive staging and lighting. It also had a plot that dealt seriously, often movingly, about something that’s not a natural for a musical’s premise: mental illness. The show’s book received enough attention after the opening in April 2009 that earlier this year it won the Pulitzer Prize for drama, following a Tony the score received as 2009′s best.
Despite that, it seemed to me that the show’s book and lyric author, Brian Yorkey, flubbed the challenge of building a satisfying dramatic arc on this subject while setting it to music and coming in under 2 hours. Whether or not that’s the explanation, the show’s story of a middle-aged mother and wife haunted by a powerful, delusional fantasy of the son she lost when he was 8 months old, slips up with its simplistically negative and clichéd portrayal of cand psychotherapy. At the end of Next to Normal, the central character, Diana, prevails by facing down her mental demons drug- and treatment-free, finally talking with her daughter and husband about their troubled past. Familial love and open communication allow Diana to overcome her delusions and manic depression. The bad guys in this feel-good ending are Diana’s two psychiatrists and their ineffectual treatments.
What’s remarkable is that Mr. Yorkey denies making the psychiatrists the villains. Quoted in an article by Patricia Cohen in The New York Times soon after Next to Normal opened last year, Mr. Yorkey says: “Some people have walked away thinking we were against psychologists or psychotropic drugs and nothing could be further from the truth.” That assertion makes me wonder which theater he’s been visiting and whether it was the same one I sat in the other evening.
The musical I saw sets the tone with an early, first act song in which Diana reviews her pharmacologic history in the song ”My Psychopharmacologist and I,” which catalogs the “Zoloft and Paxil and Buspar and Xanex, Depacon, Chronaphin, Ambien, Prozac, Ativan calms me when I see the bills. These are a few of my favorite pills.” Diana later croons about the adverse effects she develops, and that her regimen has left her mentally numb. “I don’t feel anything,” she says, to which her psychiatrist sagely replies: “Patient stable.” Soon after, Diana decides to go drug free, happily disposing of her “Risperdal, Valium, and Xanax.”
When she then has an unsuccessful suicide attempt, a new psychiatrist (described in the show as a “rock star”–an infallible expert) recommends electroconvulsive therapy, a treatment depicted as menacing and extreme and, once again, ultimately ineffectual. All the ECT achieves for Diana is substantial memory loss, and then a return of her powerful and incapacitating delusions. As the second act winds down, she abandons most psychiatric treatments, accepting only talk therapy, and at the show’s end Diana achieves transcendence fueled by her family’s love and new-found openness.
Of course these can be powerfully therapeutic, but the show casts it as a simple black and white situation, with a facile Forty-second street philosophy working while mainstream psychiatry completely failed.
—Mitchel Zoler (on Twitter @mitchelzoler)
That show, Next to Normal, didn’t disappoint as entertainment. It had an enjoyable and involving rock score, a talented cast of six actors, and inventive staging and lighting. It also had a plot that dealt seriously, often movingly, about something that’s not a natural for a musical’s premise: mental illness. The show’s book received enough attention after the opening in April 2009 that earlier this year it won the Pulitzer Prize for drama, following a Tony the score received as 2009′s best.
Despite that, it seemed to me that the show’s book and lyric author, Brian Yorkey, flubbed the challenge of building a satisfying dramatic arc on this subject while setting it to music and coming in under 2 hours. Whether or not that’s the explanation, the show’s story of a middle-aged mother and wife haunted by a powerful, delusional fantasy of the son she lost when he was 8 months old, slips up with its simplistically negative and clichéd portrayal of cand psychotherapy. At the end of Next to Normal, the central character, Diana, prevails by facing down her mental demons drug- and treatment-free, finally talking with her daughter and husband about their troubled past. Familial love and open communication allow Diana to overcome her delusions and manic depression. The bad guys in this feel-good ending are Diana’s two psychiatrists and their ineffectual treatments.
What’s remarkable is that Mr. Yorkey denies making the psychiatrists the villains. Quoted in an article by Patricia Cohen in The New York Times soon after Next to Normal opened last year, Mr. Yorkey says: “Some people have walked away thinking we were against psychologists or psychotropic drugs and nothing could be further from the truth.” That assertion makes me wonder which theater he’s been visiting and whether it was the same one I sat in the other evening.
The musical I saw sets the tone with an early, first act song in which Diana reviews her pharmacologic history in the song ”My Psychopharmacologist and I,” which catalogs the “Zoloft and Paxil and Buspar and Xanex, Depacon, Chronaphin, Ambien, Prozac, Ativan calms me when I see the bills. These are a few of my favorite pills.” Diana later croons about the adverse effects she develops, and that her regimen has left her mentally numb. “I don’t feel anything,” she says, to which her psychiatrist sagely replies: “Patient stable.” Soon after, Diana decides to go drug free, happily disposing of her “Risperdal, Valium, and Xanax.”
When she then has an unsuccessful suicide attempt, a new psychiatrist (described in the show as a “rock star”–an infallible expert) recommends electroconvulsive therapy, a treatment depicted as menacing and extreme and, once again, ultimately ineffectual. All the ECT achieves for Diana is substantial memory loss, and then a return of her powerful and incapacitating delusions. As the second act winds down, she abandons most psychiatric treatments, accepting only talk therapy, and at the show’s end Diana achieves transcendence fueled by her family’s love and new-found openness.
Of course these can be powerfully therapeutic, but the show casts it as a simple black and white situation, with a facile Forty-second street philosophy working while mainstream psychiatry completely failed.
—Mitchel Zoler (on Twitter @mitchelzoler)
Combo Therapy Best at Averting Bipolar Relapse
EDINBURGH – Long-term combination therapy with lithium plus valproate in patients with bipolar I disorder proved to be markedly more effective than valproate monotherapy in the randomized BALANCE trial.
BALANCE (Bipolar Affective Disorder: Lithium Anticonvulsant Evaluation) will be a practice-changing study, both in the United States and in the United Kingdom, where it originated, study coordinator and chief investigator Dr. John R. Geddes predicted at the congress.
“As valproate monotherapy is substantially the most commonly used treatment in the United States, BALANCE should probably lead to some change in practice over there. …. I think BALANCE would suggest that for a majority of patients, combination therapy would be a better bet than valproate monotherapy. And even lithium might be better as a first-line therapy,” commented Dr. Geddes, professor of epidemiological psychiatry and a senior clinical research fellow at the University of Oxford (England).
BALANCE included 330 patients aged 16 and older with bipolar I disorder at 41 sites in the United Kingdom, France, the United States, and Italy who were randomized to open-label lithium monotherapy at a target dose of 0.4-1.0 mmol/L, valproate monotherapy at 750-1,250 mg/day, or both agents in combination.
The primary outcome was emergence of a new mood episode requiring further intervention (defined as either another medication or hospitalization) during 2 years of follow-up. This occurred in 69% of the valproate group, 59% of those taking lithium, and 54% on combination therapy (Lancet 2010;375:385-95).
Thus, combination therapy resulted in a 41% relative risk reduction in the primary end point compared with valproate monotherapy, and an 18% reduction compared with lithium. Lithium monotherapy achieved a 39% relative risk reduction compared with valproate.
The time to a 10% hospitalization rate averaged 4.7 months in the valproate group, 7.7 months with lithium, and 11.3 months with combination therapy.
“You may well want to put patients on combination therapy before they've had the chance to fail on monotherapy, in the same way that in cancer therapy we often use combination therapy right from day 1. It really gives you the best chance of controlling the condition,” he said.
In terms of numbers needed to treat (NNT), BALANCE showed that seven bipolar patients would need to receive combination therapy for 2 years instead of valproate in order for there to be one fewer relapse; that's a very favorable NNT, Dr. Geddes observed. The NNT was 10 for lithium vs. valproate, and 20 for combination therapy vs. lithium monotherapy.
“We can neither refute nor confirm an added benefit for combination therapy over lithium alone,” Dr. Geddes said. “The trial would have to be quite a lot larger to pick up any added benefit.”
No baseline predictors of individual treatment response could be identified. Genotype data are now being analyzed. “The results so far aren't overly impressive, so don't hold your breath on that one,” he advised.
Lithium proved to be better than valproate for prevention of depressive as well as manic relapses in BALANCE, contrary to the conventional wisdom that holds that valproate is the more effective agent against depressive symptoms. However, this finding is consistent with the results of a recent meta-analysis by Dr. Geddes and his colleagues, which he said showed “quite convincingly” that lithium was more effective than valproate in preventing depressive symptoms.
The BALANCE trial was funded by the Stanley Medical Research Institute, with donation of drugs by Sanofi-Aventis. Dr. Geddes disclosed having received research funds from the Medical Research Council, the Economic and Social Research Council, and the National Institute for Health Research, as well as the Stanley Medical Research Institute.
EDINBURGH – Long-term combination therapy with lithium plus valproate in patients with bipolar I disorder proved to be markedly more effective than valproate monotherapy in the randomized BALANCE trial.
BALANCE (Bipolar Affective Disorder: Lithium Anticonvulsant Evaluation) will be a practice-changing study, both in the United States and in the United Kingdom, where it originated, study coordinator and chief investigator Dr. John R. Geddes predicted at the congress.
“As valproate monotherapy is substantially the most commonly used treatment in the United States, BALANCE should probably lead to some change in practice over there. …. I think BALANCE would suggest that for a majority of patients, combination therapy would be a better bet than valproate monotherapy. And even lithium might be better as a first-line therapy,” commented Dr. Geddes, professor of epidemiological psychiatry and a senior clinical research fellow at the University of Oxford (England).
BALANCE included 330 patients aged 16 and older with bipolar I disorder at 41 sites in the United Kingdom, France, the United States, and Italy who were randomized to open-label lithium monotherapy at a target dose of 0.4-1.0 mmol/L, valproate monotherapy at 750-1,250 mg/day, or both agents in combination.
The primary outcome was emergence of a new mood episode requiring further intervention (defined as either another medication or hospitalization) during 2 years of follow-up. This occurred in 69% of the valproate group, 59% of those taking lithium, and 54% on combination therapy (Lancet 2010;375:385-95).
Thus, combination therapy resulted in a 41% relative risk reduction in the primary end point compared with valproate monotherapy, and an 18% reduction compared with lithium. Lithium monotherapy achieved a 39% relative risk reduction compared with valproate.
The time to a 10% hospitalization rate averaged 4.7 months in the valproate group, 7.7 months with lithium, and 11.3 months with combination therapy.
“You may well want to put patients on combination therapy before they've had the chance to fail on monotherapy, in the same way that in cancer therapy we often use combination therapy right from day 1. It really gives you the best chance of controlling the condition,” he said.
In terms of numbers needed to treat (NNT), BALANCE showed that seven bipolar patients would need to receive combination therapy for 2 years instead of valproate in order for there to be one fewer relapse; that's a very favorable NNT, Dr. Geddes observed. The NNT was 10 for lithium vs. valproate, and 20 for combination therapy vs. lithium monotherapy.
“We can neither refute nor confirm an added benefit for combination therapy over lithium alone,” Dr. Geddes said. “The trial would have to be quite a lot larger to pick up any added benefit.”
No baseline predictors of individual treatment response could be identified. Genotype data are now being analyzed. “The results so far aren't overly impressive, so don't hold your breath on that one,” he advised.
Lithium proved to be better than valproate for prevention of depressive as well as manic relapses in BALANCE, contrary to the conventional wisdom that holds that valproate is the more effective agent against depressive symptoms. However, this finding is consistent with the results of a recent meta-analysis by Dr. Geddes and his colleagues, which he said showed “quite convincingly” that lithium was more effective than valproate in preventing depressive symptoms.
The BALANCE trial was funded by the Stanley Medical Research Institute, with donation of drugs by Sanofi-Aventis. Dr. Geddes disclosed having received research funds from the Medical Research Council, the Economic and Social Research Council, and the National Institute for Health Research, as well as the Stanley Medical Research Institute.
EDINBURGH – Long-term combination therapy with lithium plus valproate in patients with bipolar I disorder proved to be markedly more effective than valproate monotherapy in the randomized BALANCE trial.
BALANCE (Bipolar Affective Disorder: Lithium Anticonvulsant Evaluation) will be a practice-changing study, both in the United States and in the United Kingdom, where it originated, study coordinator and chief investigator Dr. John R. Geddes predicted at the congress.
“As valproate monotherapy is substantially the most commonly used treatment in the United States, BALANCE should probably lead to some change in practice over there. …. I think BALANCE would suggest that for a majority of patients, combination therapy would be a better bet than valproate monotherapy. And even lithium might be better as a first-line therapy,” commented Dr. Geddes, professor of epidemiological psychiatry and a senior clinical research fellow at the University of Oxford (England).
BALANCE included 330 patients aged 16 and older with bipolar I disorder at 41 sites in the United Kingdom, France, the United States, and Italy who were randomized to open-label lithium monotherapy at a target dose of 0.4-1.0 mmol/L, valproate monotherapy at 750-1,250 mg/day, or both agents in combination.
The primary outcome was emergence of a new mood episode requiring further intervention (defined as either another medication or hospitalization) during 2 years of follow-up. This occurred in 69% of the valproate group, 59% of those taking lithium, and 54% on combination therapy (Lancet 2010;375:385-95).
Thus, combination therapy resulted in a 41% relative risk reduction in the primary end point compared with valproate monotherapy, and an 18% reduction compared with lithium. Lithium monotherapy achieved a 39% relative risk reduction compared with valproate.
The time to a 10% hospitalization rate averaged 4.7 months in the valproate group, 7.7 months with lithium, and 11.3 months with combination therapy.
“You may well want to put patients on combination therapy before they've had the chance to fail on monotherapy, in the same way that in cancer therapy we often use combination therapy right from day 1. It really gives you the best chance of controlling the condition,” he said.
In terms of numbers needed to treat (NNT), BALANCE showed that seven bipolar patients would need to receive combination therapy for 2 years instead of valproate in order for there to be one fewer relapse; that's a very favorable NNT, Dr. Geddes observed. The NNT was 10 for lithium vs. valproate, and 20 for combination therapy vs. lithium monotherapy.
“We can neither refute nor confirm an added benefit for combination therapy over lithium alone,” Dr. Geddes said. “The trial would have to be quite a lot larger to pick up any added benefit.”
No baseline predictors of individual treatment response could be identified. Genotype data are now being analyzed. “The results so far aren't overly impressive, so don't hold your breath on that one,” he advised.
Lithium proved to be better than valproate for prevention of depressive as well as manic relapses in BALANCE, contrary to the conventional wisdom that holds that valproate is the more effective agent against depressive symptoms. However, this finding is consistent with the results of a recent meta-analysis by Dr. Geddes and his colleagues, which he said showed “quite convincingly” that lithium was more effective than valproate in preventing depressive symptoms.
The BALANCE trial was funded by the Stanley Medical Research Institute, with donation of drugs by Sanofi-Aventis. Dr. Geddes disclosed having received research funds from the Medical Research Council, the Economic and Social Research Council, and the National Institute for Health Research, as well as the Stanley Medical Research Institute.
When is lamotrigine a good choice?
FDA-approved for maintenance treatment of bipolar I disorder, lamotrigine is more effective than lithium for preventing depressive relapses. Lamotrigine combined with lithium, carbamazepine, or valproate provides good protection against recurrences of mania and depression.
Unlike selective serotonin reuptake inhibitors and other antidepressants, lamotrigine does not appear to increase risk of hypomania or mania in bipolar patients.1 Unlike valproate and lithium, it is weight-neutral and requires no serum level monitoring.2 Although lamotrigine’s slow titration and prolonged period until reaching therapeutic effect limits its efficacy as monotherapy in an inpatient setting, the drug can be initiated along with quicker acting agents in the hospital and then titrated after discharge. This strategy allows close monitoring during initial exposure.
Consider lamotrigine as an adjunct for treatment-resistant major depression.3 It is useful for treating aggression and agitation in patients with traumatic brain injury4 or dementia.5 Borderline personality disorder patients treated with lamotrigine may show less affective lability, impulsivity, or aggression.6,7 Lamotrigine can act synergistically with clozapine in some patients with refractory schizophrenia.8
Metabolism and drug interactions
Lamotrigine is metabolized via glucuronidation and eliminated renally. Other drugs metabolized by glucuronidation could interact with lamotrigine (Table).9
Table
Drug interactions associated with lamotrigine
| Interacting drug | Effect on lamotrigine | Management |
|---|---|---|
| Carbamazepine Phenytoin Phenobarbital Primidone Rifampin | Increased clearance | Double dose of lamotrigine when used concomitantly |
| Oral contraceptives containing estrogen | Increased clearance | Lamotrigine dose may need to be increased. Efficacy of oral contraceptives may be decreased; dose modification of oral contraceptive also may be required |
| Valproic acid | Decreased clearance | Reduce dose by at least half, even if your patient is on a medication with the potential to increase clearance |
| Source: Reference 9 | ||
Adverse reactions
Lamotrigine is well tolerated chronically, with fewer adverse effects than other mood stabilizers. Serious rashes, including Stevens-Johnson syndrome and toxic epidermal necrolysis, have been reported in 0.08% to 0.13% of patients treated with lamotrigine for bipolar disorder or other mood disorders.9 The risk of developing a skin rash within 2 to 8 weeks of therapy necessitates starting with a low dose, usually 25 mg/d, and gradually titrating.2,9
The FDA added a warning about increased risk of suicidality to the labeling of all anticonvulsants, regardless of indication.10 In a meta-analysis of 199 trials, for every 530 patients treated with anticonvulsants there was 1 additional case of suicidality—not completed suicide.10 Inform patients and their families about the potential risk for increased suicidality and document this discussion of risk vs benefit. All patients should be monitored for worsening depression or suicidal thoughts or behavior throughout treatment.
Other potential side effects occurring in at least 5% of patients receiving lamotrigine include somnolence, headache, rash, and the dose-related side effects of nausea, vomiting, dizziness, ataxia, blurred vision, and diplopia.9
1. Bowden CL. Lamotrigine in the treatment of bipolar disorder. Expert Opin Pharmacother. 2002;3(10):1513-1519.
2. Goldsmith DR, Wagstaff AJ, Ibbotson T, et al. Lamotrigine: a review of its use in bipolar disorder. Drugs. 2003;63(19):2029-2050.
3. Gabriel A. Lamotrigine adjunctive treatment in resistant unipolar depression: an open descriptive study. Depress Anxiety. 2006;23:485-488.
4. Pachet A, Friesen S, Winkelaar D, et al. Beneficial behavioural effects of lamotrigine in traumatic brain injury. Brain Inj. 2003;17(8):715-722.
5. Sajatovic M, Ramsay E, Nanry K, et al. Lamotrigine therapy in elderly patients with epilepsy, bipolar disorder or dementia. Int J Geriatr Psychiatry. 2007;22:945-950.
6. Pinto OC, Akiskal HS. Lamotrigine as a promising approach to borderline personality: an open case series without concurrent DSM-IV major mood disorder. J Affect Disord. 1998;51:333-343.
7. Bellino S, Paradiso E, Bogetto F. Efficacy and tolerability of pharmacotherapies for borderline personality disorder. CNS Drugs. 2008;22(8):671-692.
8. Dursun SM, Deakin JF. Augmenting antipsychotic treatment with lamotrigine or topiramate in patients with treatment-resistant schizophrenia: a naturalistic case-series outcome study. J Psychopharmacol. 2001;15:297-301.
9. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2007.
10. Food and Drug Administration Statistical review and evaluation: antiepileptic drugs and suicidality. Available at: http://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugsSafetyInformationforpatientsand%20providers/ucm192556.pdf. Accessed August 23, 2010.
FDA-approved for maintenance treatment of bipolar I disorder, lamotrigine is more effective than lithium for preventing depressive relapses. Lamotrigine combined with lithium, carbamazepine, or valproate provides good protection against recurrences of mania and depression.
Unlike selective serotonin reuptake inhibitors and other antidepressants, lamotrigine does not appear to increase risk of hypomania or mania in bipolar patients.1 Unlike valproate and lithium, it is weight-neutral and requires no serum level monitoring.2 Although lamotrigine’s slow titration and prolonged period until reaching therapeutic effect limits its efficacy as monotherapy in an inpatient setting, the drug can be initiated along with quicker acting agents in the hospital and then titrated after discharge. This strategy allows close monitoring during initial exposure.
Consider lamotrigine as an adjunct for treatment-resistant major depression.3 It is useful for treating aggression and agitation in patients with traumatic brain injury4 or dementia.5 Borderline personality disorder patients treated with lamotrigine may show less affective lability, impulsivity, or aggression.6,7 Lamotrigine can act synergistically with clozapine in some patients with refractory schizophrenia.8
Metabolism and drug interactions
Lamotrigine is metabolized via glucuronidation and eliminated renally. Other drugs metabolized by glucuronidation could interact with lamotrigine (Table).9
Table
Drug interactions associated with lamotrigine
| Interacting drug | Effect on lamotrigine | Management |
|---|---|---|
| Carbamazepine Phenytoin Phenobarbital Primidone Rifampin | Increased clearance | Double dose of lamotrigine when used concomitantly |
| Oral contraceptives containing estrogen | Increased clearance | Lamotrigine dose may need to be increased. Efficacy of oral contraceptives may be decreased; dose modification of oral contraceptive also may be required |
| Valproic acid | Decreased clearance | Reduce dose by at least half, even if your patient is on a medication with the potential to increase clearance |
| Source: Reference 9 | ||
Adverse reactions
Lamotrigine is well tolerated chronically, with fewer adverse effects than other mood stabilizers. Serious rashes, including Stevens-Johnson syndrome and toxic epidermal necrolysis, have been reported in 0.08% to 0.13% of patients treated with lamotrigine for bipolar disorder or other mood disorders.9 The risk of developing a skin rash within 2 to 8 weeks of therapy necessitates starting with a low dose, usually 25 mg/d, and gradually titrating.2,9
The FDA added a warning about increased risk of suicidality to the labeling of all anticonvulsants, regardless of indication.10 In a meta-analysis of 199 trials, for every 530 patients treated with anticonvulsants there was 1 additional case of suicidality—not completed suicide.10 Inform patients and their families about the potential risk for increased suicidality and document this discussion of risk vs benefit. All patients should be monitored for worsening depression or suicidal thoughts or behavior throughout treatment.
Other potential side effects occurring in at least 5% of patients receiving lamotrigine include somnolence, headache, rash, and the dose-related side effects of nausea, vomiting, dizziness, ataxia, blurred vision, and diplopia.9
FDA-approved for maintenance treatment of bipolar I disorder, lamotrigine is more effective than lithium for preventing depressive relapses. Lamotrigine combined with lithium, carbamazepine, or valproate provides good protection against recurrences of mania and depression.
Unlike selective serotonin reuptake inhibitors and other antidepressants, lamotrigine does not appear to increase risk of hypomania or mania in bipolar patients.1 Unlike valproate and lithium, it is weight-neutral and requires no serum level monitoring.2 Although lamotrigine’s slow titration and prolonged period until reaching therapeutic effect limits its efficacy as monotherapy in an inpatient setting, the drug can be initiated along with quicker acting agents in the hospital and then titrated after discharge. This strategy allows close monitoring during initial exposure.
Consider lamotrigine as an adjunct for treatment-resistant major depression.3 It is useful for treating aggression and agitation in patients with traumatic brain injury4 or dementia.5 Borderline personality disorder patients treated with lamotrigine may show less affective lability, impulsivity, or aggression.6,7 Lamotrigine can act synergistically with clozapine in some patients with refractory schizophrenia.8
Metabolism and drug interactions
Lamotrigine is metabolized via glucuronidation and eliminated renally. Other drugs metabolized by glucuronidation could interact with lamotrigine (Table).9
Table
Drug interactions associated with lamotrigine
| Interacting drug | Effect on lamotrigine | Management |
|---|---|---|
| Carbamazepine Phenytoin Phenobarbital Primidone Rifampin | Increased clearance | Double dose of lamotrigine when used concomitantly |
| Oral contraceptives containing estrogen | Increased clearance | Lamotrigine dose may need to be increased. Efficacy of oral contraceptives may be decreased; dose modification of oral contraceptive also may be required |
| Valproic acid | Decreased clearance | Reduce dose by at least half, even if your patient is on a medication with the potential to increase clearance |
| Source: Reference 9 | ||
Adverse reactions
Lamotrigine is well tolerated chronically, with fewer adverse effects than other mood stabilizers. Serious rashes, including Stevens-Johnson syndrome and toxic epidermal necrolysis, have been reported in 0.08% to 0.13% of patients treated with lamotrigine for bipolar disorder or other mood disorders.9 The risk of developing a skin rash within 2 to 8 weeks of therapy necessitates starting with a low dose, usually 25 mg/d, and gradually titrating.2,9
The FDA added a warning about increased risk of suicidality to the labeling of all anticonvulsants, regardless of indication.10 In a meta-analysis of 199 trials, for every 530 patients treated with anticonvulsants there was 1 additional case of suicidality—not completed suicide.10 Inform patients and their families about the potential risk for increased suicidality and document this discussion of risk vs benefit. All patients should be monitored for worsening depression or suicidal thoughts or behavior throughout treatment.
Other potential side effects occurring in at least 5% of patients receiving lamotrigine include somnolence, headache, rash, and the dose-related side effects of nausea, vomiting, dizziness, ataxia, blurred vision, and diplopia.9
1. Bowden CL. Lamotrigine in the treatment of bipolar disorder. Expert Opin Pharmacother. 2002;3(10):1513-1519.
2. Goldsmith DR, Wagstaff AJ, Ibbotson T, et al. Lamotrigine: a review of its use in bipolar disorder. Drugs. 2003;63(19):2029-2050.
3. Gabriel A. Lamotrigine adjunctive treatment in resistant unipolar depression: an open descriptive study. Depress Anxiety. 2006;23:485-488.
4. Pachet A, Friesen S, Winkelaar D, et al. Beneficial behavioural effects of lamotrigine in traumatic brain injury. Brain Inj. 2003;17(8):715-722.
5. Sajatovic M, Ramsay E, Nanry K, et al. Lamotrigine therapy in elderly patients with epilepsy, bipolar disorder or dementia. Int J Geriatr Psychiatry. 2007;22:945-950.
6. Pinto OC, Akiskal HS. Lamotrigine as a promising approach to borderline personality: an open case series without concurrent DSM-IV major mood disorder. J Affect Disord. 1998;51:333-343.
7. Bellino S, Paradiso E, Bogetto F. Efficacy and tolerability of pharmacotherapies for borderline personality disorder. CNS Drugs. 2008;22(8):671-692.
8. Dursun SM, Deakin JF. Augmenting antipsychotic treatment with lamotrigine or topiramate in patients with treatment-resistant schizophrenia: a naturalistic case-series outcome study. J Psychopharmacol. 2001;15:297-301.
9. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2007.
10. Food and Drug Administration Statistical review and evaluation: antiepileptic drugs and suicidality. Available at: http://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugsSafetyInformationforpatientsand%20providers/ucm192556.pdf. Accessed August 23, 2010.
1. Bowden CL. Lamotrigine in the treatment of bipolar disorder. Expert Opin Pharmacother. 2002;3(10):1513-1519.
2. Goldsmith DR, Wagstaff AJ, Ibbotson T, et al. Lamotrigine: a review of its use in bipolar disorder. Drugs. 2003;63(19):2029-2050.
3. Gabriel A. Lamotrigine adjunctive treatment in resistant unipolar depression: an open descriptive study. Depress Anxiety. 2006;23:485-488.
4. Pachet A, Friesen S, Winkelaar D, et al. Beneficial behavioural effects of lamotrigine in traumatic brain injury. Brain Inj. 2003;17(8):715-722.
5. Sajatovic M, Ramsay E, Nanry K, et al. Lamotrigine therapy in elderly patients with epilepsy, bipolar disorder or dementia. Int J Geriatr Psychiatry. 2007;22:945-950.
6. Pinto OC, Akiskal HS. Lamotrigine as a promising approach to borderline personality: an open case series without concurrent DSM-IV major mood disorder. J Affect Disord. 1998;51:333-343.
7. Bellino S, Paradiso E, Bogetto F. Efficacy and tolerability of pharmacotherapies for borderline personality disorder. CNS Drugs. 2008;22(8):671-692.
8. Dursun SM, Deakin JF. Augmenting antipsychotic treatment with lamotrigine or topiramate in patients with treatment-resistant schizophrenia: a naturalistic case-series outcome study. J Psychopharmacol. 2001;15:297-301.
9. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2007.
10. Food and Drug Administration Statistical review and evaluation: antiepileptic drugs and suicidality. Available at: http://www.fda.gov/downloads/Drugs/DrugSafety/PostmarketDrugsSafetyInformationforpatientsand%20providers/ucm192556.pdf. Accessed August 23, 2010.
Ketamine Infusion Relieves Bipolar Depression Quickly
A single infusion of ketamine relieved bipolar depression within 40 minutes in patients with treatment-resistant bipolar disorder, according to a randomized, placebo-controlled, double-blind crossover study involving 18 patients.
The effect lasted at least 3 days, wrote Dr. Nancy Diazgranados and her colleagues from the National Institute of Mental Health. Patients in the study were an average of 48 years old, had suffered from bipolar I or bipolar II depression for an average of 28 years, and had failed an average of seven antidepressant treatments before the ketamine study. Fifty-five percent of the participants had failed to respond to electroconvulsive therapy.
Two-thirds of participants were on psychiatric disability, and all but one were unemployed (Arch. Gen. Psychiatry 2010;67:793-802).
Patients were randomly assigned to receive an infusion of 0.5 mg/kg of ketamine or placebo. Two weeks later, the patients who had been given ketamine were given placebo and vice versa. Of the 17 patients who completed the ketamine phase of the study, 12 (71%) responded to ketamine.
In contrast, of the 16 patients who completed the placebo phase of the study, only 1 (6%) responded to placebo.
Investigators assessed the patients at baseline using several rating scales, including the Montgomery-Åsberg Depression Rating Scale, the Hamilton Scale for Depression, and the Beck Depression Inventory. Patients showed statistically significant improvements in depression with ketamine, compared with placebo on all three scales beginning at 40 minutes after infusion and continuing for at least 3 days. Mean scores on the rating scales did not differ from placebo on days 7, 10, and 14.
Within 40 minutes, 9 of 16 patients receiving ketamine (56%) responded and an additional 2 (13%) experienced complete remission of their depression. One day after the infusion, 44% of the patients had responded and 31% had remitted.
None of the patients experienced serious adverse events during the study. Among the adverse events associated with ketamine and experienced by at least 10% of the patients were disassociation; feeling strange, weird, or bizarre; dry mouth; tachycardia; and increased blood pressure.
Ketamine has been used in human and veterinary medicine since 1962, most commonly for inducing and maintaining general anesthesia, sedation in intensive care, analgesia, and treatment of bronchospasm. In the late 1990s, it increasingly became known as a drug of abuse and a date-rape drug. A previous study showed that a single infusion of ketamine improved suicidal ideation within 40 minutes (“Single Ketamine Injection Reduces Suicidal Ideation,” November 2009, p. 13).
When used for general anesthesia, the initial dose of intravenous ketamine is typically 1.5-4.5 mg/kg, substantially higher than the level used in this study. Ketamine is thought to act as a noncompetitive inhibitor of the N-methyl-D-aspartate (NMDA) receptor, which is part of the glutaminergic neurotransmitter system. Several lines of evidence have implicated the glutaminergic system in bipolar disorder.
A single infusion of ketamine relieved bipolar depression within 40 minutes in patients with treatment-resistant bipolar disorder, according to a randomized, placebo-controlled, double-blind crossover study involving 18 patients.
The effect lasted at least 3 days, wrote Dr. Nancy Diazgranados and her colleagues from the National Institute of Mental Health. Patients in the study were an average of 48 years old, had suffered from bipolar I or bipolar II depression for an average of 28 years, and had failed an average of seven antidepressant treatments before the ketamine study. Fifty-five percent of the participants had failed to respond to electroconvulsive therapy.
Two-thirds of participants were on psychiatric disability, and all but one were unemployed (Arch. Gen. Psychiatry 2010;67:793-802).
Patients were randomly assigned to receive an infusion of 0.5 mg/kg of ketamine or placebo. Two weeks later, the patients who had been given ketamine were given placebo and vice versa. Of the 17 patients who completed the ketamine phase of the study, 12 (71%) responded to ketamine.
In contrast, of the 16 patients who completed the placebo phase of the study, only 1 (6%) responded to placebo.
Investigators assessed the patients at baseline using several rating scales, including the Montgomery-Åsberg Depression Rating Scale, the Hamilton Scale for Depression, and the Beck Depression Inventory. Patients showed statistically significant improvements in depression with ketamine, compared with placebo on all three scales beginning at 40 minutes after infusion and continuing for at least 3 days. Mean scores on the rating scales did not differ from placebo on days 7, 10, and 14.
Within 40 minutes, 9 of 16 patients receiving ketamine (56%) responded and an additional 2 (13%) experienced complete remission of their depression. One day after the infusion, 44% of the patients had responded and 31% had remitted.
None of the patients experienced serious adverse events during the study. Among the adverse events associated with ketamine and experienced by at least 10% of the patients were disassociation; feeling strange, weird, or bizarre; dry mouth; tachycardia; and increased blood pressure.
Ketamine has been used in human and veterinary medicine since 1962, most commonly for inducing and maintaining general anesthesia, sedation in intensive care, analgesia, and treatment of bronchospasm. In the late 1990s, it increasingly became known as a drug of abuse and a date-rape drug. A previous study showed that a single infusion of ketamine improved suicidal ideation within 40 minutes (“Single Ketamine Injection Reduces Suicidal Ideation,” November 2009, p. 13).
When used for general anesthesia, the initial dose of intravenous ketamine is typically 1.5-4.5 mg/kg, substantially higher than the level used in this study. Ketamine is thought to act as a noncompetitive inhibitor of the N-methyl-D-aspartate (NMDA) receptor, which is part of the glutaminergic neurotransmitter system. Several lines of evidence have implicated the glutaminergic system in bipolar disorder.
A single infusion of ketamine relieved bipolar depression within 40 minutes in patients with treatment-resistant bipolar disorder, according to a randomized, placebo-controlled, double-blind crossover study involving 18 patients.
The effect lasted at least 3 days, wrote Dr. Nancy Diazgranados and her colleagues from the National Institute of Mental Health. Patients in the study were an average of 48 years old, had suffered from bipolar I or bipolar II depression for an average of 28 years, and had failed an average of seven antidepressant treatments before the ketamine study. Fifty-five percent of the participants had failed to respond to electroconvulsive therapy.
Two-thirds of participants were on psychiatric disability, and all but one were unemployed (Arch. Gen. Psychiatry 2010;67:793-802).
Patients were randomly assigned to receive an infusion of 0.5 mg/kg of ketamine or placebo. Two weeks later, the patients who had been given ketamine were given placebo and vice versa. Of the 17 patients who completed the ketamine phase of the study, 12 (71%) responded to ketamine.
In contrast, of the 16 patients who completed the placebo phase of the study, only 1 (6%) responded to placebo.
Investigators assessed the patients at baseline using several rating scales, including the Montgomery-Åsberg Depression Rating Scale, the Hamilton Scale for Depression, and the Beck Depression Inventory. Patients showed statistically significant improvements in depression with ketamine, compared with placebo on all three scales beginning at 40 minutes after infusion and continuing for at least 3 days. Mean scores on the rating scales did not differ from placebo on days 7, 10, and 14.
Within 40 minutes, 9 of 16 patients receiving ketamine (56%) responded and an additional 2 (13%) experienced complete remission of their depression. One day after the infusion, 44% of the patients had responded and 31% had remitted.
None of the patients experienced serious adverse events during the study. Among the adverse events associated with ketamine and experienced by at least 10% of the patients were disassociation; feeling strange, weird, or bizarre; dry mouth; tachycardia; and increased blood pressure.
Ketamine has been used in human and veterinary medicine since 1962, most commonly for inducing and maintaining general anesthesia, sedation in intensive care, analgesia, and treatment of bronchospasm. In the late 1990s, it increasingly became known as a drug of abuse and a date-rape drug. A previous study showed that a single infusion of ketamine improved suicidal ideation within 40 minutes (“Single Ketamine Injection Reduces Suicidal Ideation,” November 2009, p. 13).
When used for general anesthesia, the initial dose of intravenous ketamine is typically 1.5-4.5 mg/kg, substantially higher than the level used in this study. Ketamine is thought to act as a noncompetitive inhibitor of the N-methyl-D-aspartate (NMDA) receptor, which is part of the glutaminergic neurotransmitter system. Several lines of evidence have implicated the glutaminergic system in bipolar disorder.
ARCHIVES OF GENERAL PSYCHIATRY
Major Finding: In patients with treatment-resistant bipolar
depression, an infusion of 0.5 mg/kg of ketamine significantly relieved
depression within 40 minutes, an effect that lasted at least 3 days.
Data Source: Randomized, placebo-controlled, double-blind, crossover study involving 18 patients.
Disclosures: The study was funded by the National Institute of
Mental Health and by the National Alliance for Research on
Schizophrenia and Depression. A patent application for the use of
ketamine for depression has been submitted, listing two of the
investigators among the inventors; they have assigned their rights on
the patent to the U.S. government.
Risperidone Efficacy Differs in Pediatric Patients
Major Finding: Relative risk of risperidone inefficacy is lower among pediatric patients (0.43), compared with adults (0.64).
Data Source: Meta-analysis of 30 studies with 872 pediatric patients and 2,655 adults.
Disclosures: Dr. Maayan said he has received research support from Eli Lilly and Pfizer. The current study had no funding source.
BOCA RATON, FLA. – The efficacy and tolerability of risperidone differ between pediatric and adult patients, according to a meta-analysis of 32 double-blind, placebo-controlled trials.
For pediatric prescribers of risperidone, there is good news, mixed news, and not-so-good news, Dr. Lawrence A. Maayan said.
“The good news is study-defined efficacy: Treatment did better than placebo for both children and adults, but significantly better in children,” said Dr. Maayan, a child and adolescent psychiatrist at the Nathan S. Kline Institute for Psychiatric Research at New York University in Orangeburg, N.Y.
Of the studies that included data on efficacy, Dr. Maayan and his colleagues compared 12 studies of 872 pediatric patients with 18 such trials of 2,655 adults. They found that a relative risk of inefficacy was lower among children and adolescents (0.43), compared with adults (0.64). The difference was most pronounced in schizophrenia, which favored pediatric patients (RR, 0.34), compared with adults (RR, 0.62).
“By this metric, risperidone works better in children than in adults. It is reassuring to a pediatric prescriber,” Dr. Maayan said in an interview at his poster. Risperidone is manufactured by Ortho-McNeil-Janssen.
The meta-analysis also included populations with bipolar disorder, subaverage intelligence/disruptive behavior disorders, posttraumatic stress disorder, autism/pervasive developmental disorder, and Alzheimer's dementia. The 32 studies comprised 1,184 children and adolescents (72% male, 65% white) and 3,909 adults (53% male, 85% white).
There was some mixed news regarding adverse events, Dr. Maayan said. For example, “children and adults did about equally” with discontinuation. A total of 26 studies assessed all-cause discontinuation and discontinuation because of inefficacy or intolerability/side effects.
There were no age-group differences in all-cause discontinuation between pediatric and adult patients, Dr. Maayan said. Although pediatric patients had a lower risk (RR, 0.60) compared with adults (RR, 0.77), the magnitude of the difference between risperidone and placebo groups was not statistically different between age groups, “which is reassuring.”
Pediatric patients also had a lower risk for discontinuation because of treatment inefficacy (RR, 0.30), compared with adults (RR, 0.52), Dr. Maayan said. “Children were able to continue [risperidone] just as well as adults.”
However, children were more likely to report at least one adverse event during risperidone treatment (RR, 1.30), compared with adults (RR, 1.01).
“The not-so-good news was raw weight gain: It was about the same in children as in adults,” Dr. Maayan said. “Two kilograms in a 40-year-old is different than a 2-kg gain in a 10-year-old.”
Dr. Maayan and his associates assessed weight gain as a percentage of baseline body weight. “The kids gained about three times as much.” Weight gain was 5.7% for children, vs. 1.5% for adults, a significant difference.
Counsel and talk to patients and families about this increased risk of weight gain with risperidone treatment as part of a full disclosure of benefits and risks, Dr. Maayan advised.
Major Finding: Relative risk of risperidone inefficacy is lower among pediatric patients (0.43), compared with adults (0.64).
Data Source: Meta-analysis of 30 studies with 872 pediatric patients and 2,655 adults.
Disclosures: Dr. Maayan said he has received research support from Eli Lilly and Pfizer. The current study had no funding source.
BOCA RATON, FLA. – The efficacy and tolerability of risperidone differ between pediatric and adult patients, according to a meta-analysis of 32 double-blind, placebo-controlled trials.
For pediatric prescribers of risperidone, there is good news, mixed news, and not-so-good news, Dr. Lawrence A. Maayan said.
“The good news is study-defined efficacy: Treatment did better than placebo for both children and adults, but significantly better in children,” said Dr. Maayan, a child and adolescent psychiatrist at the Nathan S. Kline Institute for Psychiatric Research at New York University in Orangeburg, N.Y.
Of the studies that included data on efficacy, Dr. Maayan and his colleagues compared 12 studies of 872 pediatric patients with 18 such trials of 2,655 adults. They found that a relative risk of inefficacy was lower among children and adolescents (0.43), compared with adults (0.64). The difference was most pronounced in schizophrenia, which favored pediatric patients (RR, 0.34), compared with adults (RR, 0.62).
“By this metric, risperidone works better in children than in adults. It is reassuring to a pediatric prescriber,” Dr. Maayan said in an interview at his poster. Risperidone is manufactured by Ortho-McNeil-Janssen.
The meta-analysis also included populations with bipolar disorder, subaverage intelligence/disruptive behavior disorders, posttraumatic stress disorder, autism/pervasive developmental disorder, and Alzheimer's dementia. The 32 studies comprised 1,184 children and adolescents (72% male, 65% white) and 3,909 adults (53% male, 85% white).
There was some mixed news regarding adverse events, Dr. Maayan said. For example, “children and adults did about equally” with discontinuation. A total of 26 studies assessed all-cause discontinuation and discontinuation because of inefficacy or intolerability/side effects.
There were no age-group differences in all-cause discontinuation between pediatric and adult patients, Dr. Maayan said. Although pediatric patients had a lower risk (RR, 0.60) compared with adults (RR, 0.77), the magnitude of the difference between risperidone and placebo groups was not statistically different between age groups, “which is reassuring.”
Pediatric patients also had a lower risk for discontinuation because of treatment inefficacy (RR, 0.30), compared with adults (RR, 0.52), Dr. Maayan said. “Children were able to continue [risperidone] just as well as adults.”
However, children were more likely to report at least one adverse event during risperidone treatment (RR, 1.30), compared with adults (RR, 1.01).
“The not-so-good news was raw weight gain: It was about the same in children as in adults,” Dr. Maayan said. “Two kilograms in a 40-year-old is different than a 2-kg gain in a 10-year-old.”
Dr. Maayan and his associates assessed weight gain as a percentage of baseline body weight. “The kids gained about three times as much.” Weight gain was 5.7% for children, vs. 1.5% for adults, a significant difference.
Counsel and talk to patients and families about this increased risk of weight gain with risperidone treatment as part of a full disclosure of benefits and risks, Dr. Maayan advised.
Major Finding: Relative risk of risperidone inefficacy is lower among pediatric patients (0.43), compared with adults (0.64).
Data Source: Meta-analysis of 30 studies with 872 pediatric patients and 2,655 adults.
Disclosures: Dr. Maayan said he has received research support from Eli Lilly and Pfizer. The current study had no funding source.
BOCA RATON, FLA. – The efficacy and tolerability of risperidone differ between pediatric and adult patients, according to a meta-analysis of 32 double-blind, placebo-controlled trials.
For pediatric prescribers of risperidone, there is good news, mixed news, and not-so-good news, Dr. Lawrence A. Maayan said.
“The good news is study-defined efficacy: Treatment did better than placebo for both children and adults, but significantly better in children,” said Dr. Maayan, a child and adolescent psychiatrist at the Nathan S. Kline Institute for Psychiatric Research at New York University in Orangeburg, N.Y.
Of the studies that included data on efficacy, Dr. Maayan and his colleagues compared 12 studies of 872 pediatric patients with 18 such trials of 2,655 adults. They found that a relative risk of inefficacy was lower among children and adolescents (0.43), compared with adults (0.64). The difference was most pronounced in schizophrenia, which favored pediatric patients (RR, 0.34), compared with adults (RR, 0.62).
“By this metric, risperidone works better in children than in adults. It is reassuring to a pediatric prescriber,” Dr. Maayan said in an interview at his poster. Risperidone is manufactured by Ortho-McNeil-Janssen.
The meta-analysis also included populations with bipolar disorder, subaverage intelligence/disruptive behavior disorders, posttraumatic stress disorder, autism/pervasive developmental disorder, and Alzheimer's dementia. The 32 studies comprised 1,184 children and adolescents (72% male, 65% white) and 3,909 adults (53% male, 85% white).
There was some mixed news regarding adverse events, Dr. Maayan said. For example, “children and adults did about equally” with discontinuation. A total of 26 studies assessed all-cause discontinuation and discontinuation because of inefficacy or intolerability/side effects.
There were no age-group differences in all-cause discontinuation between pediatric and adult patients, Dr. Maayan said. Although pediatric patients had a lower risk (RR, 0.60) compared with adults (RR, 0.77), the magnitude of the difference between risperidone and placebo groups was not statistically different between age groups, “which is reassuring.”
Pediatric patients also had a lower risk for discontinuation because of treatment inefficacy (RR, 0.30), compared with adults (RR, 0.52), Dr. Maayan said. “Children were able to continue [risperidone] just as well as adults.”
However, children were more likely to report at least one adverse event during risperidone treatment (RR, 1.30), compared with adults (RR, 1.01).
“The not-so-good news was raw weight gain: It was about the same in children as in adults,” Dr. Maayan said. “Two kilograms in a 40-year-old is different than a 2-kg gain in a 10-year-old.”
Dr. Maayan and his associates assessed weight gain as a percentage of baseline body weight. “The kids gained about three times as much.” Weight gain was 5.7% for children, vs. 1.5% for adults, a significant difference.
Counsel and talk to patients and families about this increased risk of weight gain with risperidone treatment as part of a full disclosure of benefits and risks, Dr. Maayan advised.