User login
VIDEO: Office-based hereditary cancer risk testing is doable
AUSTIN, TEXAS – , according to Mark S. DeFrancesco, MD, and his associates.
Few community-based ob.gyns. routinely screen their patients for hereditary cancer risks, Dr. DeFrancesco said at the annual meeting of the American College of Obstetricians and Gynecologists, despite ACOG’s position that they are fully trained and qualified to do so. He and his colleagues studied an intervention aimed at streamlining and standardizing genetic assessment in their practice.
A team of physicians, staff, genetic counselors, and process engineers analyzed how hereditary cancer risk assessment was being done at five clinical sites of two community ob.gyn. practices – Dr. DeFrancesco’s practice in Waterbury, Conn., and that of Richard Waldman, MD, in Syracuse, N.Y. – then refined workflows and added tools to create a turnkey process for assessment and screening, Dr. DeFrancesco said.
Under the new process, patients completed a family cancer history in the exam room prior to seeing their physician. Genetic testing was offered to patients who met National Comprehensive Cancer Network (NCCN) guidelines for hereditary/familial high-risk assessment for breast and ovarian cancer (J Natl Compr Canc Netw. 2017 Jan;15[1]:9-20). Those who chose to be tested were able to provide a saliva sample in the office. Counseling was provided to appropriate patients.
The number of patients tested for hereditary risk of breast and ovarian cancer increased dramatically with the new process. During the 8-week period after the intervention, 4% (165) were tested out of 4,107 total patients seen; during the 8 weeks preceding, 1% (43) of 3,882 patients were tested.
Overall, 92.8% (3,811) of patients seen after the intervention provided a family cancer history. Almost a quarter – 23.5% (906) – met NCCN criteria for genetic testing.
A total of 318 patients agreed to undergo genetic testing and 165 (51.9%) completed the process. Nine patients (5.5%) were found to carry a pathogenic gene variant associated with hereditary breast and/or ovarian cancer or Lynch syndrome, Dr. DeFrancesco and colleagues reported.
Patients and providers also were surveyed regarding their experience with the new process. Patients overwhelming noted that they understood the information provided (98.8%), and that they were satisfied with the overall process (97.6%). All 15 providers said that they would continue to use the new process in their practice and most – 13 of 15 – said they found the process thorough and felt comfortable recommending genetic counseling without referral to a genetic counselor (2 were undecided).
“I think that this study really proves the concept that in a community-based practice, we can test our patients,” Dr. DeFrancesco said in an interview.
Myriad Genetics sponsored the study. Dr. DeFrancesco reported no financial conflicts of interest. His coauthors include employees of Myriad Genetics, some with ownership interests.
SOURCE: DeFrancesco, MS et al. ACOG 2018 3K.
AUSTIN, TEXAS – , according to Mark S. DeFrancesco, MD, and his associates.
Few community-based ob.gyns. routinely screen their patients for hereditary cancer risks, Dr. DeFrancesco said at the annual meeting of the American College of Obstetricians and Gynecologists, despite ACOG’s position that they are fully trained and qualified to do so. He and his colleagues studied an intervention aimed at streamlining and standardizing genetic assessment in their practice.
A team of physicians, staff, genetic counselors, and process engineers analyzed how hereditary cancer risk assessment was being done at five clinical sites of two community ob.gyn. practices – Dr. DeFrancesco’s practice in Waterbury, Conn., and that of Richard Waldman, MD, in Syracuse, N.Y. – then refined workflows and added tools to create a turnkey process for assessment and screening, Dr. DeFrancesco said.
Under the new process, patients completed a family cancer history in the exam room prior to seeing their physician. Genetic testing was offered to patients who met National Comprehensive Cancer Network (NCCN) guidelines for hereditary/familial high-risk assessment for breast and ovarian cancer (J Natl Compr Canc Netw. 2017 Jan;15[1]:9-20). Those who chose to be tested were able to provide a saliva sample in the office. Counseling was provided to appropriate patients.
The number of patients tested for hereditary risk of breast and ovarian cancer increased dramatically with the new process. During the 8-week period after the intervention, 4% (165) were tested out of 4,107 total patients seen; during the 8 weeks preceding, 1% (43) of 3,882 patients were tested.
Overall, 92.8% (3,811) of patients seen after the intervention provided a family cancer history. Almost a quarter – 23.5% (906) – met NCCN criteria for genetic testing.
A total of 318 patients agreed to undergo genetic testing and 165 (51.9%) completed the process. Nine patients (5.5%) were found to carry a pathogenic gene variant associated with hereditary breast and/or ovarian cancer or Lynch syndrome, Dr. DeFrancesco and colleagues reported.
Patients and providers also were surveyed regarding their experience with the new process. Patients overwhelming noted that they understood the information provided (98.8%), and that they were satisfied with the overall process (97.6%). All 15 providers said that they would continue to use the new process in their practice and most – 13 of 15 – said they found the process thorough and felt comfortable recommending genetic counseling without referral to a genetic counselor (2 were undecided).
“I think that this study really proves the concept that in a community-based practice, we can test our patients,” Dr. DeFrancesco said in an interview.
Myriad Genetics sponsored the study. Dr. DeFrancesco reported no financial conflicts of interest. His coauthors include employees of Myriad Genetics, some with ownership interests.
SOURCE: DeFrancesco, MS et al. ACOG 2018 3K.
AUSTIN, TEXAS – , according to Mark S. DeFrancesco, MD, and his associates.
Few community-based ob.gyns. routinely screen their patients for hereditary cancer risks, Dr. DeFrancesco said at the annual meeting of the American College of Obstetricians and Gynecologists, despite ACOG’s position that they are fully trained and qualified to do so. He and his colleagues studied an intervention aimed at streamlining and standardizing genetic assessment in their practice.
A team of physicians, staff, genetic counselors, and process engineers analyzed how hereditary cancer risk assessment was being done at five clinical sites of two community ob.gyn. practices – Dr. DeFrancesco’s practice in Waterbury, Conn., and that of Richard Waldman, MD, in Syracuse, N.Y. – then refined workflows and added tools to create a turnkey process for assessment and screening, Dr. DeFrancesco said.
Under the new process, patients completed a family cancer history in the exam room prior to seeing their physician. Genetic testing was offered to patients who met National Comprehensive Cancer Network (NCCN) guidelines for hereditary/familial high-risk assessment for breast and ovarian cancer (J Natl Compr Canc Netw. 2017 Jan;15[1]:9-20). Those who chose to be tested were able to provide a saliva sample in the office. Counseling was provided to appropriate patients.
The number of patients tested for hereditary risk of breast and ovarian cancer increased dramatically with the new process. During the 8-week period after the intervention, 4% (165) were tested out of 4,107 total patients seen; during the 8 weeks preceding, 1% (43) of 3,882 patients were tested.
Overall, 92.8% (3,811) of patients seen after the intervention provided a family cancer history. Almost a quarter – 23.5% (906) – met NCCN criteria for genetic testing.
A total of 318 patients agreed to undergo genetic testing and 165 (51.9%) completed the process. Nine patients (5.5%) were found to carry a pathogenic gene variant associated with hereditary breast and/or ovarian cancer or Lynch syndrome, Dr. DeFrancesco and colleagues reported.
Patients and providers also were surveyed regarding their experience with the new process. Patients overwhelming noted that they understood the information provided (98.8%), and that they were satisfied with the overall process (97.6%). All 15 providers said that they would continue to use the new process in their practice and most – 13 of 15 – said they found the process thorough and felt comfortable recommending genetic counseling without referral to a genetic counselor (2 were undecided).
“I think that this study really proves the concept that in a community-based practice, we can test our patients,” Dr. DeFrancesco said in an interview.
Myriad Genetics sponsored the study. Dr. DeFrancesco reported no financial conflicts of interest. His coauthors include employees of Myriad Genetics, some with ownership interests.
SOURCE: DeFrancesco, MS et al. ACOG 2018 3K.
REPORTING FROM ACOG 2018
Key clinical point: Ob.gyns. can successfully integrate hereditary cancer risk testing into their practices.
Major finding: Office-based genetic testing increased from 1% to 4% of patients seen.
Study details: Prospective, single-arm process intervention study screening more than 4,000 women at 5 ob.gyn. practice sites.
Disclosures: Myriad Genetics sponsored the study. Dr. DeFrancesco reported no financial conflicts of interest. His coauthors included employees of Myriad Genetics, some with ownership interests.
Source: DeFrancesco, MS et al. ACOG 2018 poster 3K.
How does oral contraceptive use affect one’s risk of ovarian, endometrial, breast, and colorectal cancers?
EXPERT COMMENTARY
Hormonal contraception (HC), including OC, is a central component of women’s health care worldwide. In addition to its many potential health benefits (pregnancy prevention, menstrual symptom management), HC use modifies the risk of various cancers. As we discussed in the February 2018 issue of OBG Management, a recent large population-based study in Denmark showed a small but statistically significant increase in breast cancer risk in HC users.1,2 Conversely, HC use has a long recognized protective effect against ovarian and endometrial cancers. These risk relationships may be altered by other modifiable lifestyle characteristics, such as smoking, alcohol use, obesity, and physical activity.
Details of the study
Michels and colleagues evaluated the association between OC use and multiple cancers, stratifying these risks by duration of use and various modifiable lifestyle characteristics.3 The authors used a prospective survey-based cohort (the NIH-AARP Diet and Health Study) linked with state cancer registries to evaluate this relationship in a diverse population of 196,536 women across 6 US states and 2 metropolitan areas. Women were enrolled in 1995–1996 and followed until 2011. Cancer risks were presented as hazard ratios (HR), which indicate the risk of developing a specific cancer type in OC users compared with nonusers. HRs differ from relative risks (RR) and odds ratios because they compare the instantaneous risk difference between the 2 groups, rather than the cumulative risk difference over the entire study period.4
Duration of OC use and risk reduction
In this study population, OC use was associated with a significantly decreased risk of ovarian cancer, and this risk increased with longer duration of use (TABLE). Similarly, long-term OC use was associated with a decreased risk for endometrial cancer. These effects were true across various lifestyle characteristics, including smoking status, alcohol use, body mass index (BMI), and physical activity level.
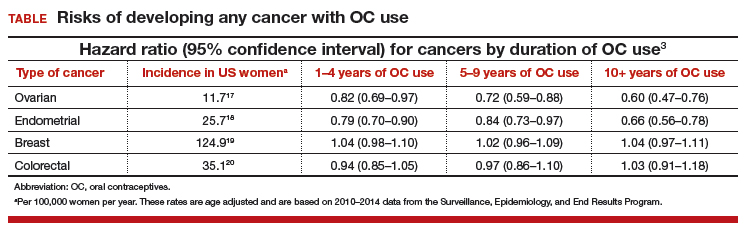
There was a nonsignificant trend toward increased risk of breast cancer among OC users. The most significant elevation in breast cancer risk was found in long-term users who were current smokers (HR, 1.21 [95% confidence interval (CI), 1.01–1.44]). OC use had a minimal effect on colorectal cancer risk.
The bottom line. US women using OCs were significantly less likely to develop ovarian and endometrial cancers compared with nonusers. This risk reduction increased with longer duration of OC use and was true regardless of lifestyle. Conversely, there was a trend toward a slightly increased risk of developing breast cancer in OC users.
Study strengths and weaknesses
The effect on breast cancer risk is less pronounced than that reported in a recent large, prospective cohort study in Denmark, which reported an RR of developing breast cancer of 1.20 (95% CI, 1.14–1.26) among all current or recent HC users.1 These differing results may be due to the US study population’s increased heterogeneity compared with the Danish cohort; potential recall bias in the US study (not present in the Danish study because pharmacy records were used); the larger size of the Danish study (that is, ability to detect very small effect sizes); and lack of information on OC formulation, recency of use, and parity in the US study.
Nevertheless, the significant protective effect against ovarian and endometrial cancers (reported previously in numerous studies) should be a part of totality of cancer risk when counseling patients on any potential increased risk of breast cancer with OC use.
According to the study by Michels and colleagues, overall, women using OCs had a decreased risk of ovarian and endometrial cancers and a trend toward a slightly increased risk of breast cancer.3 Based on this and prior estimates, the overall risk of developing any cancer appears to be lower in OC users than in nonusers.5,6
Consider discussing the points below when counseling women on OC use and cancer risk.
Cancer prevention
- OC use was associated with a significantly decreased risk of both ovarian and endometrial cancers. This effect increased with longer duration of use.
- Ovarian cancer risk reduction persisted regardless of smoking status, BMI, alcohol use, or physical activity level.
- The largest reduction in endometrial cancer was seen in current smokers and patients with a BMI greater than 30 kg/m2.
Breast cancer risk
- There was a trend toward a slightly increased risk of breast cancer with OC use of any duration.
- A Danish cohort study showed a significantly higher risk (although still an overall low risk) of breast cancer with HC use (RR, 1.20 [95% CI, 1.14-1.26]).1
- The differences in these 2 results may be related to study design and population characteristic differences.
Overall cancer risk
- The definitive and larger risk reductions in ovarian and endometrial cancer compared with the lesser risk increase in breast cancer suggest a net decrease in developing any cancer for OC users.3,5,6
Risks of pregnancy prevention failure
- OCs are an effective method for preventing unintended pregnancy. Risks of OCs should be weighed against the risks of unintended pregnancy.
- In the United States, the maternal mortality rate (2015) is 26.4 deaths for every 100,000 women.7 The risk of maternal mortality is substantially higher than even the highest published estimates of HC-attributable breast cancer rates (that is, 13 incremental breast cancers for every 100,000 women using HC; 2 incremental breast cancers for every 100,000 women 35 years of age or younger using HC).1
- Unintended pregnancy is a serious maternal-child health problem, and it has substantial health, social, and economic consequences.8-14
- Unintended pregnancies generate a significant economic burden (an estimated $21 billion in direct and indirect costs for the US health care system per year).15 Approximately 42% of unintended pregnancies end in abortion.16
-- Dana M. Scott, MD, and Mark D. Pearlman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Mørch LS, Skovlund CW, Hannaford PC, Iversen L, Fielding S, Lidegaard Ø. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377(23):2228–2239.
- Scott DM, Pearlman MD. Does hormonal contraception increase the risk of breast cancer? OBG Manag. 2018;30(2):16–17.
- Michels KA, Pfeiffer RM, Brinton LA, Trabert B. Modification of the associations between duration of oral contraceptive use and ovarian, endometrial, breast, and colorectal cancers [published online January 18, 2018]. JAMA Oncol. doi:10.1001/jamaoncol.2017.4942.
- Sedgwick P. Hazards and hazard ratios. BMJ. 2012;345:e5980.
- Bassuk SS, Manson JE. Oral contraceptives and menopausal hormone therapy: relative and attributable risks of cardiovascular disease, cancer, and other health outcomes. Ann Epidemiol. 2015;25(3):193–200.
- Hunter D. Oral contraceptives and the small increased risk of breast cancer. N Engl J Med. 2017;377(23):2276–2277.
- GBD 2015 Maternal Mortality Collaborators. Global, regional, and national levels of maternal mortality, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1775–1812.
- Brown SS, Eisenberg L, eds. The best intentions: unintended pregnancy and the well-being of children and families. Washington, DC: The National Academies Press; 1995:50–90.
- Klein JD; American Academy of Pediatrics Committee on Adolescence. Adolescent pregnancy: current trends and issues. Pediatrics. 2005;116(1):281–286.
- Logan C, Holcombe E, Manlove J, Ryan S; The National Campaign to Prevent Teen Pregnancy and Child Trends. The consequences of unintended childbearing. https://pdfs.semanticscholar.org/b353/b02ae6cad716a7f64ca48b3edae63544c03e.pdf?_ga=2.149310646.1402594583.1524236972-1233479770.1524236972&_gac=1.195699992.1524237056. Accessed April 20, 2018.
- Finer LB, Sonfield A. The evidence mounts on the benefits of preventing unintended pregnancy. Contraception. 2013;87(2):126–127.
- Trussell J, Henry N, Hassan F, Prezioso A, Law A, Filonenko A. Burden of unintended pregnancy in the United States: potential savings with increased use of long-acting reversible contraception. Contraception. 2013;87(2):154–161.
- Sonfield A, Kost K. Public costs from unintended pregnancies and the role of public insurance programs in paying for pregnancy and infant care: estimates for 2008. Guttmacher Institute. https://www.guttmacher.org/sites/default/files/report_pdf/public-costs-of-up.pdf. Published October 2013. Accessed April 20, 2018.
- Forrest JD, Singh S. Public-sector savings resulting from expenditures for contraceptive services. Fam Plann Perspect. 1990;22(1):6–15.
- Sonfield A, Kost K. Public costs from unintended pregnancies and the role of public insurance programs in paying for pregnancy-related care: national and state estimates for 2010. Guttmacher Institute. http://www.guttmacher.org/pubs/public-costs-of-UP-2010.pdf. Published February 2015. Accessed April 20, 2018.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374(9):843–852.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: ovarian cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/ovary.html. Accessed April 20, 2018.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: uterine cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/corp.html. Accessed April 20, 2018.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: female breast cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/breast.html. Accessed April 20, 2018.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: colorectal cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/colorect.html. Accessed April 20, 2018.
EXPERT COMMENTARY
Hormonal contraception (HC), including OC, is a central component of women’s health care worldwide. In addition to its many potential health benefits (pregnancy prevention, menstrual symptom management), HC use modifies the risk of various cancers. As we discussed in the February 2018 issue of OBG Management, a recent large population-based study in Denmark showed a small but statistically significant increase in breast cancer risk in HC users.1,2 Conversely, HC use has a long recognized protective effect against ovarian and endometrial cancers. These risk relationships may be altered by other modifiable lifestyle characteristics, such as smoking, alcohol use, obesity, and physical activity.
Details of the study
Michels and colleagues evaluated the association between OC use and multiple cancers, stratifying these risks by duration of use and various modifiable lifestyle characteristics.3 The authors used a prospective survey-based cohort (the NIH-AARP Diet and Health Study) linked with state cancer registries to evaluate this relationship in a diverse population of 196,536 women across 6 US states and 2 metropolitan areas. Women were enrolled in 1995–1996 and followed until 2011. Cancer risks were presented as hazard ratios (HR), which indicate the risk of developing a specific cancer type in OC users compared with nonusers. HRs differ from relative risks (RR) and odds ratios because they compare the instantaneous risk difference between the 2 groups, rather than the cumulative risk difference over the entire study period.4
Duration of OC use and risk reduction
In this study population, OC use was associated with a significantly decreased risk of ovarian cancer, and this risk increased with longer duration of use (TABLE). Similarly, long-term OC use was associated with a decreased risk for endometrial cancer. These effects were true across various lifestyle characteristics, including smoking status, alcohol use, body mass index (BMI), and physical activity level.

There was a nonsignificant trend toward increased risk of breast cancer among OC users. The most significant elevation in breast cancer risk was found in long-term users who were current smokers (HR, 1.21 [95% confidence interval (CI), 1.01–1.44]). OC use had a minimal effect on colorectal cancer risk.
The bottom line. US women using OCs were significantly less likely to develop ovarian and endometrial cancers compared with nonusers. This risk reduction increased with longer duration of OC use and was true regardless of lifestyle. Conversely, there was a trend toward a slightly increased risk of developing breast cancer in OC users.
Study strengths and weaknesses
The effect on breast cancer risk is less pronounced than that reported in a recent large, prospective cohort study in Denmark, which reported an RR of developing breast cancer of 1.20 (95% CI, 1.14–1.26) among all current or recent HC users.1 These differing results may be due to the US study population’s increased heterogeneity compared with the Danish cohort; potential recall bias in the US study (not present in the Danish study because pharmacy records were used); the larger size of the Danish study (that is, ability to detect very small effect sizes); and lack of information on OC formulation, recency of use, and parity in the US study.
Nevertheless, the significant protective effect against ovarian and endometrial cancers (reported previously in numerous studies) should be a part of totality of cancer risk when counseling patients on any potential increased risk of breast cancer with OC use.
According to the study by Michels and colleagues, overall, women using OCs had a decreased risk of ovarian and endometrial cancers and a trend toward a slightly increased risk of breast cancer.3 Based on this and prior estimates, the overall risk of developing any cancer appears to be lower in OC users than in nonusers.5,6
Consider discussing the points below when counseling women on OC use and cancer risk.
Cancer prevention
- OC use was associated with a significantly decreased risk of both ovarian and endometrial cancers. This effect increased with longer duration of use.
- Ovarian cancer risk reduction persisted regardless of smoking status, BMI, alcohol use, or physical activity level.
- The largest reduction in endometrial cancer was seen in current smokers and patients with a BMI greater than 30 kg/m2.
Breast cancer risk
- There was a trend toward a slightly increased risk of breast cancer with OC use of any duration.
- A Danish cohort study showed a significantly higher risk (although still an overall low risk) of breast cancer with HC use (RR, 1.20 [95% CI, 1.14-1.26]).1
- The differences in these 2 results may be related to study design and population characteristic differences.
Overall cancer risk
- The definitive and larger risk reductions in ovarian and endometrial cancer compared with the lesser risk increase in breast cancer suggest a net decrease in developing any cancer for OC users.3,5,6
Risks of pregnancy prevention failure
- OCs are an effective method for preventing unintended pregnancy. Risks of OCs should be weighed against the risks of unintended pregnancy.
- In the United States, the maternal mortality rate (2015) is 26.4 deaths for every 100,000 women.7 The risk of maternal mortality is substantially higher than even the highest published estimates of HC-attributable breast cancer rates (that is, 13 incremental breast cancers for every 100,000 women using HC; 2 incremental breast cancers for every 100,000 women 35 years of age or younger using HC).1
- Unintended pregnancy is a serious maternal-child health problem, and it has substantial health, social, and economic consequences.8-14
- Unintended pregnancies generate a significant economic burden (an estimated $21 billion in direct and indirect costs for the US health care system per year).15 Approximately 42% of unintended pregnancies end in abortion.16
-- Dana M. Scott, MD, and Mark D. Pearlman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
EXPERT COMMENTARY
Hormonal contraception (HC), including OC, is a central component of women’s health care worldwide. In addition to its many potential health benefits (pregnancy prevention, menstrual symptom management), HC use modifies the risk of various cancers. As we discussed in the February 2018 issue of OBG Management, a recent large population-based study in Denmark showed a small but statistically significant increase in breast cancer risk in HC users.1,2 Conversely, HC use has a long recognized protective effect against ovarian and endometrial cancers. These risk relationships may be altered by other modifiable lifestyle characteristics, such as smoking, alcohol use, obesity, and physical activity.
Details of the study
Michels and colleagues evaluated the association between OC use and multiple cancers, stratifying these risks by duration of use and various modifiable lifestyle characteristics.3 The authors used a prospective survey-based cohort (the NIH-AARP Diet and Health Study) linked with state cancer registries to evaluate this relationship in a diverse population of 196,536 women across 6 US states and 2 metropolitan areas. Women were enrolled in 1995–1996 and followed until 2011. Cancer risks were presented as hazard ratios (HR), which indicate the risk of developing a specific cancer type in OC users compared with nonusers. HRs differ from relative risks (RR) and odds ratios because they compare the instantaneous risk difference between the 2 groups, rather than the cumulative risk difference over the entire study period.4
Duration of OC use and risk reduction
In this study population, OC use was associated with a significantly decreased risk of ovarian cancer, and this risk increased with longer duration of use (TABLE). Similarly, long-term OC use was associated with a decreased risk for endometrial cancer. These effects were true across various lifestyle characteristics, including smoking status, alcohol use, body mass index (BMI), and physical activity level.

There was a nonsignificant trend toward increased risk of breast cancer among OC users. The most significant elevation in breast cancer risk was found in long-term users who were current smokers (HR, 1.21 [95% confidence interval (CI), 1.01–1.44]). OC use had a minimal effect on colorectal cancer risk.
The bottom line. US women using OCs were significantly less likely to develop ovarian and endometrial cancers compared with nonusers. This risk reduction increased with longer duration of OC use and was true regardless of lifestyle. Conversely, there was a trend toward a slightly increased risk of developing breast cancer in OC users.
Study strengths and weaknesses
The effect on breast cancer risk is less pronounced than that reported in a recent large, prospective cohort study in Denmark, which reported an RR of developing breast cancer of 1.20 (95% CI, 1.14–1.26) among all current or recent HC users.1 These differing results may be due to the US study population’s increased heterogeneity compared with the Danish cohort; potential recall bias in the US study (not present in the Danish study because pharmacy records were used); the larger size of the Danish study (that is, ability to detect very small effect sizes); and lack of information on OC formulation, recency of use, and parity in the US study.
Nevertheless, the significant protective effect against ovarian and endometrial cancers (reported previously in numerous studies) should be a part of totality of cancer risk when counseling patients on any potential increased risk of breast cancer with OC use.
According to the study by Michels and colleagues, overall, women using OCs had a decreased risk of ovarian and endometrial cancers and a trend toward a slightly increased risk of breast cancer.3 Based on this and prior estimates, the overall risk of developing any cancer appears to be lower in OC users than in nonusers.5,6
Consider discussing the points below when counseling women on OC use and cancer risk.
Cancer prevention
- OC use was associated with a significantly decreased risk of both ovarian and endometrial cancers. This effect increased with longer duration of use.
- Ovarian cancer risk reduction persisted regardless of smoking status, BMI, alcohol use, or physical activity level.
- The largest reduction in endometrial cancer was seen in current smokers and patients with a BMI greater than 30 kg/m2.
Breast cancer risk
- There was a trend toward a slightly increased risk of breast cancer with OC use of any duration.
- A Danish cohort study showed a significantly higher risk (although still an overall low risk) of breast cancer with HC use (RR, 1.20 [95% CI, 1.14-1.26]).1
- The differences in these 2 results may be related to study design and population characteristic differences.
Overall cancer risk
- The definitive and larger risk reductions in ovarian and endometrial cancer compared with the lesser risk increase in breast cancer suggest a net decrease in developing any cancer for OC users.3,5,6
Risks of pregnancy prevention failure
- OCs are an effective method for preventing unintended pregnancy. Risks of OCs should be weighed against the risks of unintended pregnancy.
- In the United States, the maternal mortality rate (2015) is 26.4 deaths for every 100,000 women.7 The risk of maternal mortality is substantially higher than even the highest published estimates of HC-attributable breast cancer rates (that is, 13 incremental breast cancers for every 100,000 women using HC; 2 incremental breast cancers for every 100,000 women 35 years of age or younger using HC).1
- Unintended pregnancy is a serious maternal-child health problem, and it has substantial health, social, and economic consequences.8-14
- Unintended pregnancies generate a significant economic burden (an estimated $21 billion in direct and indirect costs for the US health care system per year).15 Approximately 42% of unintended pregnancies end in abortion.16
-- Dana M. Scott, MD, and Mark D. Pearlman, MD
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Mørch LS, Skovlund CW, Hannaford PC, Iversen L, Fielding S, Lidegaard Ø. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377(23):2228–2239.
- Scott DM, Pearlman MD. Does hormonal contraception increase the risk of breast cancer? OBG Manag. 2018;30(2):16–17.
- Michels KA, Pfeiffer RM, Brinton LA, Trabert B. Modification of the associations between duration of oral contraceptive use and ovarian, endometrial, breast, and colorectal cancers [published online January 18, 2018]. JAMA Oncol. doi:10.1001/jamaoncol.2017.4942.
- Sedgwick P. Hazards and hazard ratios. BMJ. 2012;345:e5980.
- Bassuk SS, Manson JE. Oral contraceptives and menopausal hormone therapy: relative and attributable risks of cardiovascular disease, cancer, and other health outcomes. Ann Epidemiol. 2015;25(3):193–200.
- Hunter D. Oral contraceptives and the small increased risk of breast cancer. N Engl J Med. 2017;377(23):2276–2277.
- GBD 2015 Maternal Mortality Collaborators. Global, regional, and national levels of maternal mortality, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1775–1812.
- Brown SS, Eisenberg L, eds. The best intentions: unintended pregnancy and the well-being of children and families. Washington, DC: The National Academies Press; 1995:50–90.
- Klein JD; American Academy of Pediatrics Committee on Adolescence. Adolescent pregnancy: current trends and issues. Pediatrics. 2005;116(1):281–286.
- Logan C, Holcombe E, Manlove J, Ryan S; The National Campaign to Prevent Teen Pregnancy and Child Trends. The consequences of unintended childbearing. https://pdfs.semanticscholar.org/b353/b02ae6cad716a7f64ca48b3edae63544c03e.pdf?_ga=2.149310646.1402594583.1524236972-1233479770.1524236972&_gac=1.195699992.1524237056. Accessed April 20, 2018.
- Finer LB, Sonfield A. The evidence mounts on the benefits of preventing unintended pregnancy. Contraception. 2013;87(2):126–127.
- Trussell J, Henry N, Hassan F, Prezioso A, Law A, Filonenko A. Burden of unintended pregnancy in the United States: potential savings with increased use of long-acting reversible contraception. Contraception. 2013;87(2):154–161.
- Sonfield A, Kost K. Public costs from unintended pregnancies and the role of public insurance programs in paying for pregnancy and infant care: estimates for 2008. Guttmacher Institute. https://www.guttmacher.org/sites/default/files/report_pdf/public-costs-of-up.pdf. Published October 2013. Accessed April 20, 2018.
- Forrest JD, Singh S. Public-sector savings resulting from expenditures for contraceptive services. Fam Plann Perspect. 1990;22(1):6–15.
- Sonfield A, Kost K. Public costs from unintended pregnancies and the role of public insurance programs in paying for pregnancy-related care: national and state estimates for 2010. Guttmacher Institute. http://www.guttmacher.org/pubs/public-costs-of-UP-2010.pdf. Published February 2015. Accessed April 20, 2018.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374(9):843–852.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: ovarian cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/ovary.html. Accessed April 20, 2018.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: uterine cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/corp.html. Accessed April 20, 2018.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: female breast cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/breast.html. Accessed April 20, 2018.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: colorectal cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/colorect.html. Accessed April 20, 2018.
- Mørch LS, Skovlund CW, Hannaford PC, Iversen L, Fielding S, Lidegaard Ø. Contemporary hormonal contraception and the risk of breast cancer. N Engl J Med. 2017;377(23):2228–2239.
- Scott DM, Pearlman MD. Does hormonal contraception increase the risk of breast cancer? OBG Manag. 2018;30(2):16–17.
- Michels KA, Pfeiffer RM, Brinton LA, Trabert B. Modification of the associations between duration of oral contraceptive use and ovarian, endometrial, breast, and colorectal cancers [published online January 18, 2018]. JAMA Oncol. doi:10.1001/jamaoncol.2017.4942.
- Sedgwick P. Hazards and hazard ratios. BMJ. 2012;345:e5980.
- Bassuk SS, Manson JE. Oral contraceptives and menopausal hormone therapy: relative and attributable risks of cardiovascular disease, cancer, and other health outcomes. Ann Epidemiol. 2015;25(3):193–200.
- Hunter D. Oral contraceptives and the small increased risk of breast cancer. N Engl J Med. 2017;377(23):2276–2277.
- GBD 2015 Maternal Mortality Collaborators. Global, regional, and national levels of maternal mortality, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1775–1812.
- Brown SS, Eisenberg L, eds. The best intentions: unintended pregnancy and the well-being of children and families. Washington, DC: The National Academies Press; 1995:50–90.
- Klein JD; American Academy of Pediatrics Committee on Adolescence. Adolescent pregnancy: current trends and issues. Pediatrics. 2005;116(1):281–286.
- Logan C, Holcombe E, Manlove J, Ryan S; The National Campaign to Prevent Teen Pregnancy and Child Trends. The consequences of unintended childbearing. https://pdfs.semanticscholar.org/b353/b02ae6cad716a7f64ca48b3edae63544c03e.pdf?_ga=2.149310646.1402594583.1524236972-1233479770.1524236972&_gac=1.195699992.1524237056. Accessed April 20, 2018.
- Finer LB, Sonfield A. The evidence mounts on the benefits of preventing unintended pregnancy. Contraception. 2013;87(2):126–127.
- Trussell J, Henry N, Hassan F, Prezioso A, Law A, Filonenko A. Burden of unintended pregnancy in the United States: potential savings with increased use of long-acting reversible contraception. Contraception. 2013;87(2):154–161.
- Sonfield A, Kost K. Public costs from unintended pregnancies and the role of public insurance programs in paying for pregnancy and infant care: estimates for 2008. Guttmacher Institute. https://www.guttmacher.org/sites/default/files/report_pdf/public-costs-of-up.pdf. Published October 2013. Accessed April 20, 2018.
- Forrest JD, Singh S. Public-sector savings resulting from expenditures for contraceptive services. Fam Plann Perspect. 1990;22(1):6–15.
- Sonfield A, Kost K. Public costs from unintended pregnancies and the role of public insurance programs in paying for pregnancy-related care: national and state estimates for 2010. Guttmacher Institute. http://www.guttmacher.org/pubs/public-costs-of-UP-2010.pdf. Published February 2015. Accessed April 20, 2018.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374(9):843–852.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: ovarian cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/ovary.html. Accessed April 20, 2018.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: uterine cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/corp.html. Accessed April 20, 2018.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: female breast cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/breast.html. Accessed April 20, 2018.
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: colorectal cancer. Bethesda, MD; National Cancer Institute. http://seer.cancer.gov/statfacts/html/colorect.html. Accessed April 20, 2018.
Early breast cancer: Patients report favorable quality of life after partial breast irradiation
In women with breast cancer undergoing breast-conserving surgery, accelerated partial breast irradiation (APBI) using multicatheter brachytherapy does not negatively affect quality of life, compared with standard whole breast irradiation, investigators have reported.
Patients reported similar quality of life scores for multicatheter brachytherapy–based APBI and whole breast irradiation in the study by the Groupe Européen de Curiethérapie of European Society for Radiotherapy and Oncology (GEC-ESTRO).
Moreover, breast symptom scores were significantly worse for whole-breast radiation, Rebekka Schäfer, MD, of the department of radiation oncology at the University Hospital Würzburg (Germany) and colleagues reported in Lancet Oncology.
“This trial provides further clinical evidence that APBI with interstitial brachytherapy can be considered as an alternative treatment option after breast-conserving surgery for patients with low-risk breast cancer,” Dr. Schäfer and coauthors wrote.
In several previous studies, APBI has been shown to have clinical outcomes equivalent to those of whole breast irradiation in terms of disease recurrence, survival, and treatment side effects, they added.
The quality of life findings in the present report come from long-term follow-up of a randomized, controlled, phase 3 trial conducted at 16 European centers. This study included 1,184 women with early breast cancer randomly who, after receiving breast-conserving surgery, were assigned either to APBI that used multicatheter brachytherapy or to whole breast irradiation.
Women in the study completed validated quality of life questionnaires right before and right after radiotherapy, as well as during follow-up.
A little more than half of the women in each group completed the quality of life questionnaires after the treatment and again at follow-up, investigators said.
Global health status was stable over time in both groups, investigators reported. In the APBI group, global health status score on a scale of 0-100 was 65.6 right after the procedure and 66.2 at 5 years; similarly, scores in the whole breast irradiation group were 64.6 after radiotherapy and 66.0 at 5 years.
The only quality of life difference between arms that investigators characterized as moderately clinically relevant was in breast symptom scores, which were significantly worse in the whole breast radiation group right after radiotherapy (difference of means, 13.6; 95% CI, 9.7-17.5; P less than .0001) and at 3-month follow-up (difference of means, 12.7; 95% CI, 9.8-15.6; P less than .0001).
Emotional functioning, fatigue, and financial difficulty scores in the APBI group were “slightly better” than in the whole breast radiation group right after radiotherapy and at a 3-month follow-up, investigators reported; however, at 5 year follow-up, there were no significant differences between arms in those measures.
“Our findings show that APBI using multicatheter interstitial brachytherapy does not result in clinically significant deterioration of overall quality of life and that the different domains of quality of life after APBI were not worse in comparison with whole breast irradiation in terms of clinically relevant differences,” Dr. Schäfer and colleagues concluded in their report.
Dr. Schäfer reported no conflicts of interest. Coauthors reported disclosures outside of the submitted work including Nucletron Operations BV, Elekta Company, Merck Serono, Novocure, AstraZeneca, and Bristol-Myers Squibb, among others.
SOURCE: Schäfer R et al. Lancet Oncol. 2018 Apr 22. doi: 10.1016/S1470-2045(18)30195-5.
This study by Schäfer and colleagues supports results of earlier and smaller studies showing promising quality of life results following accelerated partial breast irradiation (APBI) using multicatheter brachytherapy, according to Reshma Jagsi, MD.
“The results suggest that for quality of life, multicatheter brachytherapy-based APBI does not adversely affect outcomes, compared with whole breast irradiation,” Dr. Jagsi wrote in an editorial accompanying the article.
In previous trials, APBI using external radiation beam techniques has likewise shown favorable and promising quality of life outcomes.
There are now eagerly anticipated studies of APBI delivered primarily using external beam techniques that have included rigorous collection of quality of life outcomes, Dr. Jagsi added.
Those trials, which include RAPID and RTOG 0413/NSABP B39, will provide additional evidence to consider alongside those of the trial reported by Schäfer and colleagues on behalf of the Groupe Européen de Curiethérapie of European Society for Radiotherapy and Oncology (GEC-ESTRO).
“Together with the results from the GEC-ESTRO trial, results from these trials will be meaningful to the many tens of thousands of women who undergo breast-conserving surgery and adjuvant radiotherapy each year,” Dr. Jagsi wrote.
Reshma Jagsi, MD, is with the department of radiation oncology at the University of Michigan, Ann Arbor. These comments are derived from editorial in Lancet Oncology . Dr. Jagsi reported receiving personal fees from Amgen.
This study by Schäfer and colleagues supports results of earlier and smaller studies showing promising quality of life results following accelerated partial breast irradiation (APBI) using multicatheter brachytherapy, according to Reshma Jagsi, MD.
“The results suggest that for quality of life, multicatheter brachytherapy-based APBI does not adversely affect outcomes, compared with whole breast irradiation,” Dr. Jagsi wrote in an editorial accompanying the article.
In previous trials, APBI using external radiation beam techniques has likewise shown favorable and promising quality of life outcomes.
There are now eagerly anticipated studies of APBI delivered primarily using external beam techniques that have included rigorous collection of quality of life outcomes, Dr. Jagsi added.
Those trials, which include RAPID and RTOG 0413/NSABP B39, will provide additional evidence to consider alongside those of the trial reported by Schäfer and colleagues on behalf of the Groupe Européen de Curiethérapie of European Society for Radiotherapy and Oncology (GEC-ESTRO).
“Together with the results from the GEC-ESTRO trial, results from these trials will be meaningful to the many tens of thousands of women who undergo breast-conserving surgery and adjuvant radiotherapy each year,” Dr. Jagsi wrote.
Reshma Jagsi, MD, is with the department of radiation oncology at the University of Michigan, Ann Arbor. These comments are derived from editorial in Lancet Oncology . Dr. Jagsi reported receiving personal fees from Amgen.
This study by Schäfer and colleagues supports results of earlier and smaller studies showing promising quality of life results following accelerated partial breast irradiation (APBI) using multicatheter brachytherapy, according to Reshma Jagsi, MD.
“The results suggest that for quality of life, multicatheter brachytherapy-based APBI does not adversely affect outcomes, compared with whole breast irradiation,” Dr. Jagsi wrote in an editorial accompanying the article.
In previous trials, APBI using external radiation beam techniques has likewise shown favorable and promising quality of life outcomes.
There are now eagerly anticipated studies of APBI delivered primarily using external beam techniques that have included rigorous collection of quality of life outcomes, Dr. Jagsi added.
Those trials, which include RAPID and RTOG 0413/NSABP B39, will provide additional evidence to consider alongside those of the trial reported by Schäfer and colleagues on behalf of the Groupe Européen de Curiethérapie of European Society for Radiotherapy and Oncology (GEC-ESTRO).
“Together with the results from the GEC-ESTRO trial, results from these trials will be meaningful to the many tens of thousands of women who undergo breast-conserving surgery and adjuvant radiotherapy each year,” Dr. Jagsi wrote.
Reshma Jagsi, MD, is with the department of radiation oncology at the University of Michigan, Ann Arbor. These comments are derived from editorial in Lancet Oncology . Dr. Jagsi reported receiving personal fees from Amgen.
In women with breast cancer undergoing breast-conserving surgery, accelerated partial breast irradiation (APBI) using multicatheter brachytherapy does not negatively affect quality of life, compared with standard whole breast irradiation, investigators have reported.
Patients reported similar quality of life scores for multicatheter brachytherapy–based APBI and whole breast irradiation in the study by the Groupe Européen de Curiethérapie of European Society for Radiotherapy and Oncology (GEC-ESTRO).
Moreover, breast symptom scores were significantly worse for whole-breast radiation, Rebekka Schäfer, MD, of the department of radiation oncology at the University Hospital Würzburg (Germany) and colleagues reported in Lancet Oncology.
“This trial provides further clinical evidence that APBI with interstitial brachytherapy can be considered as an alternative treatment option after breast-conserving surgery for patients with low-risk breast cancer,” Dr. Schäfer and coauthors wrote.
In several previous studies, APBI has been shown to have clinical outcomes equivalent to those of whole breast irradiation in terms of disease recurrence, survival, and treatment side effects, they added.
The quality of life findings in the present report come from long-term follow-up of a randomized, controlled, phase 3 trial conducted at 16 European centers. This study included 1,184 women with early breast cancer randomly who, after receiving breast-conserving surgery, were assigned either to APBI that used multicatheter brachytherapy or to whole breast irradiation.
Women in the study completed validated quality of life questionnaires right before and right after radiotherapy, as well as during follow-up.
A little more than half of the women in each group completed the quality of life questionnaires after the treatment and again at follow-up, investigators said.
Global health status was stable over time in both groups, investigators reported. In the APBI group, global health status score on a scale of 0-100 was 65.6 right after the procedure and 66.2 at 5 years; similarly, scores in the whole breast irradiation group were 64.6 after radiotherapy and 66.0 at 5 years.
The only quality of life difference between arms that investigators characterized as moderately clinically relevant was in breast symptom scores, which were significantly worse in the whole breast radiation group right after radiotherapy (difference of means, 13.6; 95% CI, 9.7-17.5; P less than .0001) and at 3-month follow-up (difference of means, 12.7; 95% CI, 9.8-15.6; P less than .0001).
Emotional functioning, fatigue, and financial difficulty scores in the APBI group were “slightly better” than in the whole breast radiation group right after radiotherapy and at a 3-month follow-up, investigators reported; however, at 5 year follow-up, there were no significant differences between arms in those measures.
“Our findings show that APBI using multicatheter interstitial brachytherapy does not result in clinically significant deterioration of overall quality of life and that the different domains of quality of life after APBI were not worse in comparison with whole breast irradiation in terms of clinically relevant differences,” Dr. Schäfer and colleagues concluded in their report.
Dr. Schäfer reported no conflicts of interest. Coauthors reported disclosures outside of the submitted work including Nucletron Operations BV, Elekta Company, Merck Serono, Novocure, AstraZeneca, and Bristol-Myers Squibb, among others.
SOURCE: Schäfer R et al. Lancet Oncol. 2018 Apr 22. doi: 10.1016/S1470-2045(18)30195-5.
In women with breast cancer undergoing breast-conserving surgery, accelerated partial breast irradiation (APBI) using multicatheter brachytherapy does not negatively affect quality of life, compared with standard whole breast irradiation, investigators have reported.
Patients reported similar quality of life scores for multicatheter brachytherapy–based APBI and whole breast irradiation in the study by the Groupe Européen de Curiethérapie of European Society for Radiotherapy and Oncology (GEC-ESTRO).
Moreover, breast symptom scores were significantly worse for whole-breast radiation, Rebekka Schäfer, MD, of the department of radiation oncology at the University Hospital Würzburg (Germany) and colleagues reported in Lancet Oncology.
“This trial provides further clinical evidence that APBI with interstitial brachytherapy can be considered as an alternative treatment option after breast-conserving surgery for patients with low-risk breast cancer,” Dr. Schäfer and coauthors wrote.
In several previous studies, APBI has been shown to have clinical outcomes equivalent to those of whole breast irradiation in terms of disease recurrence, survival, and treatment side effects, they added.
The quality of life findings in the present report come from long-term follow-up of a randomized, controlled, phase 3 trial conducted at 16 European centers. This study included 1,184 women with early breast cancer randomly who, after receiving breast-conserving surgery, were assigned either to APBI that used multicatheter brachytherapy or to whole breast irradiation.
Women in the study completed validated quality of life questionnaires right before and right after radiotherapy, as well as during follow-up.
A little more than half of the women in each group completed the quality of life questionnaires after the treatment and again at follow-up, investigators said.
Global health status was stable over time in both groups, investigators reported. In the APBI group, global health status score on a scale of 0-100 was 65.6 right after the procedure and 66.2 at 5 years; similarly, scores in the whole breast irradiation group were 64.6 after radiotherapy and 66.0 at 5 years.
The only quality of life difference between arms that investigators characterized as moderately clinically relevant was in breast symptom scores, which were significantly worse in the whole breast radiation group right after radiotherapy (difference of means, 13.6; 95% CI, 9.7-17.5; P less than .0001) and at 3-month follow-up (difference of means, 12.7; 95% CI, 9.8-15.6; P less than .0001).
Emotional functioning, fatigue, and financial difficulty scores in the APBI group were “slightly better” than in the whole breast radiation group right after radiotherapy and at a 3-month follow-up, investigators reported; however, at 5 year follow-up, there were no significant differences between arms in those measures.
“Our findings show that APBI using multicatheter interstitial brachytherapy does not result in clinically significant deterioration of overall quality of life and that the different domains of quality of life after APBI were not worse in comparison with whole breast irradiation in terms of clinically relevant differences,” Dr. Schäfer and colleagues concluded in their report.
Dr. Schäfer reported no conflicts of interest. Coauthors reported disclosures outside of the submitted work including Nucletron Operations BV, Elekta Company, Merck Serono, Novocure, AstraZeneca, and Bristol-Myers Squibb, among others.
SOURCE: Schäfer R et al. Lancet Oncol. 2018 Apr 22. doi: 10.1016/S1470-2045(18)30195-5.
FROM LANCET ONCOLOGY
Key clinical point: Quality of life results support the use of accelerated partial breast irradiation (APBI) using multicatheter brachytherapy as an alternative to whole breast radiation after breast-conserving surgery.
Major finding: Patients reported similar quality of life scores for the two modalities, while breast symptom scores for whole breast radiation were significantly worse right after radiotherapy (difference of means, 13.6; 95% confidence interval, 9.7-17.5; P less than .0001) and at 3-month follow-up (difference of means, 12.7; 95% CI, 9.8-15.6; P less than .0001), compared with those for APBI.
Study details: 5-year quality of life results from a European phase 3 trial including 1,184 women with early breast cancer who, after undergoing breast-conserving surgery, received either whole breast irradiation or APBI using multicatheter brachytherapy.
Disclosures: Authors reported disclosures outside of the submitted work including Nucletron Operations BV, Elekta Company, Merck Serono, Novocure, AstraZeneca, and Bristol-Myers Squibb, among others.
Source: Schäfer R et al. Lancet Oncol. 2018 Apr 22. doi: 10.1016/S1470-2045(18)30195-5.
Hormone therapy raises diabetes risk in breast cancer survivors
Hormone therapy for breast cancer more than doubles a woman’s risk for developing type 2 diabetes, results of a case-cohort study suggest.
Hormone therapy with tamoxifen was associated with a more than twofold increase in risk of diabetes, and aromatase inhibitors were associated with a more than fourfold increase, reported Hatem Hamood, MD, of Leumit Health Services in Karmiel, Israel, and colleagues.
Among 2,246 women with breast cancer and no diabetes at baseline, followed for a mean of 5.9 years (longest follow-up 13 years), the crude cumulative lifetime incidence rate of diabetes was 20.9%, the investigators wrote. The report was published in the Journal of Clinical Oncology.
“[Hormone therapy] is a significant risk factor of diabetes among breast cancer survivors. The underlying mechanism is unclear, and additional research is warranted. Although cessation of treatment is not recommended and progression of breast cancer often is inevitable, devised strategies aimed at lifestyle modifications in patients at high risk of diabetes could at least preserve the natural history of breast cancer,” they wrote.
Diabetes has previously been identified as a possible risk factor for breast cancer, but the potential for breast cancer therapy as a precipitating factor for diabetes is uncertain, the authors said.
“Given the detrimental impact of diabetes on breast cancer survival, additional exploration of the role of breast cancer treatment in the development of diabetes is important not only because it would add valuable information on the etiology of diabetes but also because it would help to identify high-risk patients in need of accentuated clinical care,” they wrote.
To explore the possible association between hormone therapy and diabetes risk, the investigators performed a retrospective case-cohort study of 2,246 women who had been diagnosed with primary nonmetastatic breast cancer treated with hormone therapy from 2002 through 2012.
They examined data on a randomly selected cohort of 448 breast cancer survivors and all patients in the parent (no diabetes at baseline) cohort who developed diabetes during the study period (324 patients).
They found that the prevalence of diabetes among their source population of 2,644 breast cancer survivors (including those with baseline diabetes) increased “drastically” from 6% in 2002 to 28% in 2015. The prevalence exceeded Israeli national norms from 2010 through 2013, with standardized prevalence ratios of 1.61 to 1.81 (P less than .001).
As noted, in the population without baseline diabetes, the crude cumulative incidence rate of diabetes in the presence of death as a competing risk factor was 20.9%.
In multivariate analyses controlling for demographic and socioeconomic factors, and for chemotherapy type, hypertension, outpatient visits, use of corticosteroids, thiazide diuretics, beta-blockers, statins, and year of breast cancer diagnosis, factors significantly associated with diabetes risk were use of hormone therapy (adjusted hazard ratio [HR] 2.40, P = .008), tamoxifen (aHR 2.25, P = .013), aromatase inhibitors (aHR 4.27, P = .013), therapy duration more than 1 year (aHR 2.36, P = .009), and 1 year or less (aHR 6.48, P = .004).
The investigators noted that although other reports have found no association between aromatase inhibitors and diabetes risk, those studies had small samples or offered no explanation of the lack of association.
In contrast, a 2016 joint ACS/ASCO breast cancer survivorship-care guideline notes that aromatase inhibitors may raise the risk of diabetes, the investigators noted.
The study was supported by grants from the Israeli Council for Higher Education. The investigators reported no conflicts of interest.
SOURCE: Hamood H et al. J Clin Oncol. 2018 Apr 24. doi: 10.1200/JCO.2017.76.3524.
Hormone therapy for breast cancer more than doubles a woman’s risk for developing type 2 diabetes, results of a case-cohort study suggest.
Hormone therapy with tamoxifen was associated with a more than twofold increase in risk of diabetes, and aromatase inhibitors were associated with a more than fourfold increase, reported Hatem Hamood, MD, of Leumit Health Services in Karmiel, Israel, and colleagues.
Among 2,246 women with breast cancer and no diabetes at baseline, followed for a mean of 5.9 years (longest follow-up 13 years), the crude cumulative lifetime incidence rate of diabetes was 20.9%, the investigators wrote. The report was published in the Journal of Clinical Oncology.
“[Hormone therapy] is a significant risk factor of diabetes among breast cancer survivors. The underlying mechanism is unclear, and additional research is warranted. Although cessation of treatment is not recommended and progression of breast cancer often is inevitable, devised strategies aimed at lifestyle modifications in patients at high risk of diabetes could at least preserve the natural history of breast cancer,” they wrote.
Diabetes has previously been identified as a possible risk factor for breast cancer, but the potential for breast cancer therapy as a precipitating factor for diabetes is uncertain, the authors said.
“Given the detrimental impact of diabetes on breast cancer survival, additional exploration of the role of breast cancer treatment in the development of diabetes is important not only because it would add valuable information on the etiology of diabetes but also because it would help to identify high-risk patients in need of accentuated clinical care,” they wrote.
To explore the possible association between hormone therapy and diabetes risk, the investigators performed a retrospective case-cohort study of 2,246 women who had been diagnosed with primary nonmetastatic breast cancer treated with hormone therapy from 2002 through 2012.
They examined data on a randomly selected cohort of 448 breast cancer survivors and all patients in the parent (no diabetes at baseline) cohort who developed diabetes during the study period (324 patients).
They found that the prevalence of diabetes among their source population of 2,644 breast cancer survivors (including those with baseline diabetes) increased “drastically” from 6% in 2002 to 28% in 2015. The prevalence exceeded Israeli national norms from 2010 through 2013, with standardized prevalence ratios of 1.61 to 1.81 (P less than .001).
As noted, in the population without baseline diabetes, the crude cumulative incidence rate of diabetes in the presence of death as a competing risk factor was 20.9%.
In multivariate analyses controlling for demographic and socioeconomic factors, and for chemotherapy type, hypertension, outpatient visits, use of corticosteroids, thiazide diuretics, beta-blockers, statins, and year of breast cancer diagnosis, factors significantly associated with diabetes risk were use of hormone therapy (adjusted hazard ratio [HR] 2.40, P = .008), tamoxifen (aHR 2.25, P = .013), aromatase inhibitors (aHR 4.27, P = .013), therapy duration more than 1 year (aHR 2.36, P = .009), and 1 year or less (aHR 6.48, P = .004).
The investigators noted that although other reports have found no association between aromatase inhibitors and diabetes risk, those studies had small samples or offered no explanation of the lack of association.
In contrast, a 2016 joint ACS/ASCO breast cancer survivorship-care guideline notes that aromatase inhibitors may raise the risk of diabetes, the investigators noted.
The study was supported by grants from the Israeli Council for Higher Education. The investigators reported no conflicts of interest.
SOURCE: Hamood H et al. J Clin Oncol. 2018 Apr 24. doi: 10.1200/JCO.2017.76.3524.
Hormone therapy for breast cancer more than doubles a woman’s risk for developing type 2 diabetes, results of a case-cohort study suggest.
Hormone therapy with tamoxifen was associated with a more than twofold increase in risk of diabetes, and aromatase inhibitors were associated with a more than fourfold increase, reported Hatem Hamood, MD, of Leumit Health Services in Karmiel, Israel, and colleagues.
Among 2,246 women with breast cancer and no diabetes at baseline, followed for a mean of 5.9 years (longest follow-up 13 years), the crude cumulative lifetime incidence rate of diabetes was 20.9%, the investigators wrote. The report was published in the Journal of Clinical Oncology.
“[Hormone therapy] is a significant risk factor of diabetes among breast cancer survivors. The underlying mechanism is unclear, and additional research is warranted. Although cessation of treatment is not recommended and progression of breast cancer often is inevitable, devised strategies aimed at lifestyle modifications in patients at high risk of diabetes could at least preserve the natural history of breast cancer,” they wrote.
Diabetes has previously been identified as a possible risk factor for breast cancer, but the potential for breast cancer therapy as a precipitating factor for diabetes is uncertain, the authors said.
“Given the detrimental impact of diabetes on breast cancer survival, additional exploration of the role of breast cancer treatment in the development of diabetes is important not only because it would add valuable information on the etiology of diabetes but also because it would help to identify high-risk patients in need of accentuated clinical care,” they wrote.
To explore the possible association between hormone therapy and diabetes risk, the investigators performed a retrospective case-cohort study of 2,246 women who had been diagnosed with primary nonmetastatic breast cancer treated with hormone therapy from 2002 through 2012.
They examined data on a randomly selected cohort of 448 breast cancer survivors and all patients in the parent (no diabetes at baseline) cohort who developed diabetes during the study period (324 patients).
They found that the prevalence of diabetes among their source population of 2,644 breast cancer survivors (including those with baseline diabetes) increased “drastically” from 6% in 2002 to 28% in 2015. The prevalence exceeded Israeli national norms from 2010 through 2013, with standardized prevalence ratios of 1.61 to 1.81 (P less than .001).
As noted, in the population without baseline diabetes, the crude cumulative incidence rate of diabetes in the presence of death as a competing risk factor was 20.9%.
In multivariate analyses controlling for demographic and socioeconomic factors, and for chemotherapy type, hypertension, outpatient visits, use of corticosteroids, thiazide diuretics, beta-blockers, statins, and year of breast cancer diagnosis, factors significantly associated with diabetes risk were use of hormone therapy (adjusted hazard ratio [HR] 2.40, P = .008), tamoxifen (aHR 2.25, P = .013), aromatase inhibitors (aHR 4.27, P = .013), therapy duration more than 1 year (aHR 2.36, P = .009), and 1 year or less (aHR 6.48, P = .004).
The investigators noted that although other reports have found no association between aromatase inhibitors and diabetes risk, those studies had small samples or offered no explanation of the lack of association.
In contrast, a 2016 joint ACS/ASCO breast cancer survivorship-care guideline notes that aromatase inhibitors may raise the risk of diabetes, the investigators noted.
The study was supported by grants from the Israeli Council for Higher Education. The investigators reported no conflicts of interest.
SOURCE: Hamood H et al. J Clin Oncol. 2018 Apr 24. doi: 10.1200/JCO.2017.76.3524.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Consider screening survivors of nonmetastatic breast cancer for diabetes.
Major finding: The crude lifetime incidence of diabetes following hormone therapy for breast cancer was 20.9%.
Study details: Case-cohort study of 2,246 women with nonmetastatic breast cancer and no baseline diabetes treated with hormone therapy.
Disclosures: The study was supported by grants from the Israeli Council for Higher Education. The investigators reported no conflicts of interest.
Source: Hamood H et al. J Clin Oncol. 2018 Apr 24. doi: 10.1200/JCO.2017.76.3524.
OlympiAD: No statistically significant boost in OS with olaparib in HER2-negative mBC
CHICAGO – Median overall survival (OS) in patients with HER2-negative metastatic breast cancer (mBC) and germline BRCA mutation (gBRCAm), although not statistically significant, was 2.2 months longer with olaparib versus physician’s choice chemotherapy (TPC), according to the final analysis of the OlympiAD study.
The results suggested the possibility of greater benefit among chemotherapy naive patients for metastatic breast cancer, with no cumulative toxicity reported with extended exposure, Mark E. Robson, MD, said at the annual meeting of the American Association for Cancer Research.
OlympiAD was a randomized, controlled, open-label, multicenter, phase 3 study of olaparib tablet monotherapy (300 mg, twice daily) compared with predeclared TPC monotherapy (capecitabine, vinorelbine, or eribulin). Patients were stratified by prior chemotherapy, prior platinum, and receptor status (ER+ and/or PR+ vs. TNBC). Of 302 randomized patients, 205 received olaparib and 91 received TPC (6 TPC patients declined treatment). Eligible patients had HER2-negative mBC and a germline BRCA mutation. In addition, patients should have received less than or equal to two chemotherapy lines in the metastatic setting, with prior anthracycline and taxane treatment either as (neo)adjuvant therapy or in the metastatic setting.
The data presented at AACR was a follow-up on the primary progression-free survival (PFS) analysis, which demonstrated significant benefit in olaparib over standard chemotherapy TPC (7.0 vs 4.2 months, HR 0.58, 95% confidence interval, 0.43-0.80, P less than .001). Overall response rate (ORR) in the olaparib arm was double of that observed on the TPC arm in measurable disease patients (59.9% vs. 28.8%).
At the final OS analysis with 192 deaths, HR for OS in the olaparib vs TPC group was 0.90 (95% CI, 0.66-1.23; P = .513), reported Dr. Robson of Memorial Sloan Kettering Cancer Center, New York.
“The preplanned subgroup analyses according to the stratification factors were not powered to detect survival advantages, and were considered only hypothesis generating,” he said.
In patients who had not received chemotherapy in the metastatic setting, there was a median difference in OS of 7.9 months with olaparib (HR 0.51, 95% CI, 0.29-0.90; nominal P = .02; median 22.6 vs. 14.7 months).
Median follow-up for OS was 18.9 months for olaparib vs. 15.5 months in the TPC group.
No differences were observed between patients that were ER and/or PgR positive vs. TNBC, or whether patients received prior platinum, Dr. Robson said.
Grade 3 adverse events were similar to those in the primary analysis with no cumulative toxicity with extended exposure, he said.
SOURCE: Robson ME et al. AACR Annual Meeting Abstract CT038.
CHICAGO – Median overall survival (OS) in patients with HER2-negative metastatic breast cancer (mBC) and germline BRCA mutation (gBRCAm), although not statistically significant, was 2.2 months longer with olaparib versus physician’s choice chemotherapy (TPC), according to the final analysis of the OlympiAD study.
The results suggested the possibility of greater benefit among chemotherapy naive patients for metastatic breast cancer, with no cumulative toxicity reported with extended exposure, Mark E. Robson, MD, said at the annual meeting of the American Association for Cancer Research.
OlympiAD was a randomized, controlled, open-label, multicenter, phase 3 study of olaparib tablet monotherapy (300 mg, twice daily) compared with predeclared TPC monotherapy (capecitabine, vinorelbine, or eribulin). Patients were stratified by prior chemotherapy, prior platinum, and receptor status (ER+ and/or PR+ vs. TNBC). Of 302 randomized patients, 205 received olaparib and 91 received TPC (6 TPC patients declined treatment). Eligible patients had HER2-negative mBC and a germline BRCA mutation. In addition, patients should have received less than or equal to two chemotherapy lines in the metastatic setting, with prior anthracycline and taxane treatment either as (neo)adjuvant therapy or in the metastatic setting.
The data presented at AACR was a follow-up on the primary progression-free survival (PFS) analysis, which demonstrated significant benefit in olaparib over standard chemotherapy TPC (7.0 vs 4.2 months, HR 0.58, 95% confidence interval, 0.43-0.80, P less than .001). Overall response rate (ORR) in the olaparib arm was double of that observed on the TPC arm in measurable disease patients (59.9% vs. 28.8%).
At the final OS analysis with 192 deaths, HR for OS in the olaparib vs TPC group was 0.90 (95% CI, 0.66-1.23; P = .513), reported Dr. Robson of Memorial Sloan Kettering Cancer Center, New York.
“The preplanned subgroup analyses according to the stratification factors were not powered to detect survival advantages, and were considered only hypothesis generating,” he said.
In patients who had not received chemotherapy in the metastatic setting, there was a median difference in OS of 7.9 months with olaparib (HR 0.51, 95% CI, 0.29-0.90; nominal P = .02; median 22.6 vs. 14.7 months).
Median follow-up for OS was 18.9 months for olaparib vs. 15.5 months in the TPC group.
No differences were observed between patients that were ER and/or PgR positive vs. TNBC, or whether patients received prior platinum, Dr. Robson said.
Grade 3 adverse events were similar to those in the primary analysis with no cumulative toxicity with extended exposure, he said.
SOURCE: Robson ME et al. AACR Annual Meeting Abstract CT038.
CHICAGO – Median overall survival (OS) in patients with HER2-negative metastatic breast cancer (mBC) and germline BRCA mutation (gBRCAm), although not statistically significant, was 2.2 months longer with olaparib versus physician’s choice chemotherapy (TPC), according to the final analysis of the OlympiAD study.
The results suggested the possibility of greater benefit among chemotherapy naive patients for metastatic breast cancer, with no cumulative toxicity reported with extended exposure, Mark E. Robson, MD, said at the annual meeting of the American Association for Cancer Research.
OlympiAD was a randomized, controlled, open-label, multicenter, phase 3 study of olaparib tablet monotherapy (300 mg, twice daily) compared with predeclared TPC monotherapy (capecitabine, vinorelbine, or eribulin). Patients were stratified by prior chemotherapy, prior platinum, and receptor status (ER+ and/or PR+ vs. TNBC). Of 302 randomized patients, 205 received olaparib and 91 received TPC (6 TPC patients declined treatment). Eligible patients had HER2-negative mBC and a germline BRCA mutation. In addition, patients should have received less than or equal to two chemotherapy lines in the metastatic setting, with prior anthracycline and taxane treatment either as (neo)adjuvant therapy or in the metastatic setting.
The data presented at AACR was a follow-up on the primary progression-free survival (PFS) analysis, which demonstrated significant benefit in olaparib over standard chemotherapy TPC (7.0 vs 4.2 months, HR 0.58, 95% confidence interval, 0.43-0.80, P less than .001). Overall response rate (ORR) in the olaparib arm was double of that observed on the TPC arm in measurable disease patients (59.9% vs. 28.8%).
At the final OS analysis with 192 deaths, HR for OS in the olaparib vs TPC group was 0.90 (95% CI, 0.66-1.23; P = .513), reported Dr. Robson of Memorial Sloan Kettering Cancer Center, New York.
“The preplanned subgroup analyses according to the stratification factors were not powered to detect survival advantages, and were considered only hypothesis generating,” he said.
In patients who had not received chemotherapy in the metastatic setting, there was a median difference in OS of 7.9 months with olaparib (HR 0.51, 95% CI, 0.29-0.90; nominal P = .02; median 22.6 vs. 14.7 months).
Median follow-up for OS was 18.9 months for olaparib vs. 15.5 months in the TPC group.
No differences were observed between patients that were ER and/or PgR positive vs. TNBC, or whether patients received prior platinum, Dr. Robson said.
Grade 3 adverse events were similar to those in the primary analysis with no cumulative toxicity with extended exposure, he said.
SOURCE: Robson ME et al. AACR Annual Meeting Abstract CT038.
FROM THE AACR ANNUAL MEETING
Key clinical point: Median overall survival was not significantly different with olaparib versus chemotherapy in patients with BRCA-mutated, HER2-negative metastatic breast cancer.
Major finding: Median overall survival in patients with HER2-negative metastatic breast cancer and a germline BRCA mutation was 19.3 months versus 17.1 months for olaparib versus chemotherapy (HR 0.90 95% CI 0.66, 1.23; P = .513).
Study details: Randomized, controlled, open-label, phase 3 trial (OlympiAD) of olaparib tablet monotherapy (300 mg, twice daily) compared with predeclared physician’s choice chemotherapy (capecitabine, vinorelbine, or eribulin).
Disclosures: Dr. Robson disclosed relationships with AstraZeneca, AbbVie, McKesson, Myriad Genetics, and Medivation.
Source: Robson ME et al. AACR Annual Meeting Abstract CT038.
Can cN0 and pCR limit axillary surgery in some breast cancer patients?
CHICAGO – Patients with clinically node-negative HER2-positive or triple-negative breast cancer (TNBC) who achieve a pathological complete response in the breast after neoadjuvant chemotherapy could benefit from clinical trials to evaluate the option of omitting axillary node surgery in this population, according to a retrospective analysis of more than 22,000 cases in the National Cancer Database reported at the Society of Surgical Oncology Annual Cancer Symposium.
Alison U. Barron, MD, breast surgery oncology fellow at Mayo, presented the results. “In patients with HER2+ breast cancer and TNBC who are clinically node negative (cN0) and achieve a breast pathological complete response, this data supports omitting axillary surgery in clinical trials assessing no surgery after neoadjuvant chemotherapy (NAC),” she said. “In patients who present with clinically positive node [cN1] disease with a breast pathological complete response, surgical staging of the axilla is still recommended.”
“Response rates to NAC have increased,” Dr. Barron said. She cited previous reports that showed response rates ranging from 9%-13% for anthracyclines to 19%-26% with the addition of taxanes, and to 60%-70% with the addition of trastuzumab and pertuzumab in HER2+ disease. “Furthermore, we know that tumor biology affects response rates, with TNBC and HER2+ disease having the highest rates of pathologic complete response,” she said.
“In the current era when we frequently operate on patients, we find no residual cancer in the tissue at the time of surgery,” Dr. Barron said. “The question arises as to whether we can limit surgery in patients with a pathological complete response.” While imaging has limited ability to reliably detect pCR with 100% specificity, she noted that recent trials have shown the potential of tumor-bed biopsy to identify pCR in patients after NAC (Ann Surg. Published online Oct. 23, 2017. doi: 10.1097/SLA.0000000000002573; JAMA Surg. 2017;152(7):665-70).
The National Cancer Database data the Mayo researchers analyzed yielded an overall breast pCR of 29%. “When broken down by tumor subtype, we saw significantly higher rates of breast pCR in patients with HER2+ disease (42%, n = 3,107) and TNBC (35%, n = 2,469), compared with patients with hormone-receptor positive (HR+)/HER2-negative disease (12%, n = 1,020),” she said.
When the analysis looked specifically at patients who were clinically node negative at presentation and had a pCR, the rates of positive lymph nodes at the time of surgery were 1.6% in HER2+ patients and 1.7% in TNBC disease, Dr. Barron said. “If there was residual disease in the breast, the nodal positivity rate was significantly higher, at 18% in HER2+ and 12% in TNBC,” she added. In those who were clinical N1, the breast pCR rates were similar – 41% in HER2+ and 35% in TNBC – but nodal positivity rates were significantly higher, at 13% and 14%, respectively.
The HR+/HER2- group had significantly lower rates of pCR: 12% in the cN0 and 13% in the cN1 subgroups. This subgroup also had higher nodal positivity rates – in the cN0 subgroup, 4% in those with a breast pCR and 34% in those with residual disease in the breast, and in the cN1 subgroup, 30% and 83%, respectively.
When the investigators looked at the extent of nodal burden in cN0 patients with breast pCR, they found the rate of N2 and N3 disease was near zero across all biologic subtypes. “In patients who were cN1 at presentation and achieved a breast pCR but had residual axillary disease, the majority had N1 disease with only 1.5%-4% having four or more positive lymph nodes,” Dr. Barron said.
In the discussion, session moderator Carla Fisher, MD, of Indiana University, Indianapolis, said, “While we might not be ready for prime time to not evaluate the lymph nodes of these patients, this study does speak to the importance of establishing N0 and N1 prior to NAC.” In reply to her question about how Mayo routinely evaluates node status prior to NAC, Dr. Barron noted that Mayo performs routine axillary ultrasound. However, the NCDB data does not specify what imaging was done. This is thought to vary across the centers in the NCDB, Dr. Barron said.
Noted Dr. Boughey, “The findings from this study provide data that can be used moving forward for planning future clinical trials.” She also said that these findings do not alter the current standard of care; that still calls for breast and nodal surgery after NAC. However, the ongoing NRG-BR005 phase II clinical trial is assessing the accuracy of tumor-bed biopsy in these situations (ClinicalTrials.gov Identifier: NCT03188393). “The results from that trial will help inform future trials evaluating eliminating breast surgery in patients with an excellent response to NAC,” Dr. Boughey said. “Those patients could also potentially avoid axillary surgery based on the data we have now.”
Dr. Barron and Dr. Boughey and coauthors reported having no financial disclosures.
SOURCE: Barron AU et al. Society of Surgical Oncology Annual Cancer Symposium, Abstract 48.
CHICAGO – Patients with clinically node-negative HER2-positive or triple-negative breast cancer (TNBC) who achieve a pathological complete response in the breast after neoadjuvant chemotherapy could benefit from clinical trials to evaluate the option of omitting axillary node surgery in this population, according to a retrospective analysis of more than 22,000 cases in the National Cancer Database reported at the Society of Surgical Oncology Annual Cancer Symposium.
Alison U. Barron, MD, breast surgery oncology fellow at Mayo, presented the results. “In patients with HER2+ breast cancer and TNBC who are clinically node negative (cN0) and achieve a breast pathological complete response, this data supports omitting axillary surgery in clinical trials assessing no surgery after neoadjuvant chemotherapy (NAC),” she said. “In patients who present with clinically positive node [cN1] disease with a breast pathological complete response, surgical staging of the axilla is still recommended.”
“Response rates to NAC have increased,” Dr. Barron said. She cited previous reports that showed response rates ranging from 9%-13% for anthracyclines to 19%-26% with the addition of taxanes, and to 60%-70% with the addition of trastuzumab and pertuzumab in HER2+ disease. “Furthermore, we know that tumor biology affects response rates, with TNBC and HER2+ disease having the highest rates of pathologic complete response,” she said.
“In the current era when we frequently operate on patients, we find no residual cancer in the tissue at the time of surgery,” Dr. Barron said. “The question arises as to whether we can limit surgery in patients with a pathological complete response.” While imaging has limited ability to reliably detect pCR with 100% specificity, she noted that recent trials have shown the potential of tumor-bed biopsy to identify pCR in patients after NAC (Ann Surg. Published online Oct. 23, 2017. doi: 10.1097/SLA.0000000000002573; JAMA Surg. 2017;152(7):665-70).
The National Cancer Database data the Mayo researchers analyzed yielded an overall breast pCR of 29%. “When broken down by tumor subtype, we saw significantly higher rates of breast pCR in patients with HER2+ disease (42%, n = 3,107) and TNBC (35%, n = 2,469), compared with patients with hormone-receptor positive (HR+)/HER2-negative disease (12%, n = 1,020),” she said.
When the analysis looked specifically at patients who were clinically node negative at presentation and had a pCR, the rates of positive lymph nodes at the time of surgery were 1.6% in HER2+ patients and 1.7% in TNBC disease, Dr. Barron said. “If there was residual disease in the breast, the nodal positivity rate was significantly higher, at 18% in HER2+ and 12% in TNBC,” she added. In those who were clinical N1, the breast pCR rates were similar – 41% in HER2+ and 35% in TNBC – but nodal positivity rates were significantly higher, at 13% and 14%, respectively.
The HR+/HER2- group had significantly lower rates of pCR: 12% in the cN0 and 13% in the cN1 subgroups. This subgroup also had higher nodal positivity rates – in the cN0 subgroup, 4% in those with a breast pCR and 34% in those with residual disease in the breast, and in the cN1 subgroup, 30% and 83%, respectively.
When the investigators looked at the extent of nodal burden in cN0 patients with breast pCR, they found the rate of N2 and N3 disease was near zero across all biologic subtypes. “In patients who were cN1 at presentation and achieved a breast pCR but had residual axillary disease, the majority had N1 disease with only 1.5%-4% having four or more positive lymph nodes,” Dr. Barron said.
In the discussion, session moderator Carla Fisher, MD, of Indiana University, Indianapolis, said, “While we might not be ready for prime time to not evaluate the lymph nodes of these patients, this study does speak to the importance of establishing N0 and N1 prior to NAC.” In reply to her question about how Mayo routinely evaluates node status prior to NAC, Dr. Barron noted that Mayo performs routine axillary ultrasound. However, the NCDB data does not specify what imaging was done. This is thought to vary across the centers in the NCDB, Dr. Barron said.
Noted Dr. Boughey, “The findings from this study provide data that can be used moving forward for planning future clinical trials.” She also said that these findings do not alter the current standard of care; that still calls for breast and nodal surgery after NAC. However, the ongoing NRG-BR005 phase II clinical trial is assessing the accuracy of tumor-bed biopsy in these situations (ClinicalTrials.gov Identifier: NCT03188393). “The results from that trial will help inform future trials evaluating eliminating breast surgery in patients with an excellent response to NAC,” Dr. Boughey said. “Those patients could also potentially avoid axillary surgery based on the data we have now.”
Dr. Barron and Dr. Boughey and coauthors reported having no financial disclosures.
SOURCE: Barron AU et al. Society of Surgical Oncology Annual Cancer Symposium, Abstract 48.
CHICAGO – Patients with clinically node-negative HER2-positive or triple-negative breast cancer (TNBC) who achieve a pathological complete response in the breast after neoadjuvant chemotherapy could benefit from clinical trials to evaluate the option of omitting axillary node surgery in this population, according to a retrospective analysis of more than 22,000 cases in the National Cancer Database reported at the Society of Surgical Oncology Annual Cancer Symposium.
Alison U. Barron, MD, breast surgery oncology fellow at Mayo, presented the results. “In patients with HER2+ breast cancer and TNBC who are clinically node negative (cN0) and achieve a breast pathological complete response, this data supports omitting axillary surgery in clinical trials assessing no surgery after neoadjuvant chemotherapy (NAC),” she said. “In patients who present with clinically positive node [cN1] disease with a breast pathological complete response, surgical staging of the axilla is still recommended.”
“Response rates to NAC have increased,” Dr. Barron said. She cited previous reports that showed response rates ranging from 9%-13% for anthracyclines to 19%-26% with the addition of taxanes, and to 60%-70% with the addition of trastuzumab and pertuzumab in HER2+ disease. “Furthermore, we know that tumor biology affects response rates, with TNBC and HER2+ disease having the highest rates of pathologic complete response,” she said.
“In the current era when we frequently operate on patients, we find no residual cancer in the tissue at the time of surgery,” Dr. Barron said. “The question arises as to whether we can limit surgery in patients with a pathological complete response.” While imaging has limited ability to reliably detect pCR with 100% specificity, she noted that recent trials have shown the potential of tumor-bed biopsy to identify pCR in patients after NAC (Ann Surg. Published online Oct. 23, 2017. doi: 10.1097/SLA.0000000000002573; JAMA Surg. 2017;152(7):665-70).
The National Cancer Database data the Mayo researchers analyzed yielded an overall breast pCR of 29%. “When broken down by tumor subtype, we saw significantly higher rates of breast pCR in patients with HER2+ disease (42%, n = 3,107) and TNBC (35%, n = 2,469), compared with patients with hormone-receptor positive (HR+)/HER2-negative disease (12%, n = 1,020),” she said.
When the analysis looked specifically at patients who were clinically node negative at presentation and had a pCR, the rates of positive lymph nodes at the time of surgery were 1.6% in HER2+ patients and 1.7% in TNBC disease, Dr. Barron said. “If there was residual disease in the breast, the nodal positivity rate was significantly higher, at 18% in HER2+ and 12% in TNBC,” she added. In those who were clinical N1, the breast pCR rates were similar – 41% in HER2+ and 35% in TNBC – but nodal positivity rates were significantly higher, at 13% and 14%, respectively.
The HR+/HER2- group had significantly lower rates of pCR: 12% in the cN0 and 13% in the cN1 subgroups. This subgroup also had higher nodal positivity rates – in the cN0 subgroup, 4% in those with a breast pCR and 34% in those with residual disease in the breast, and in the cN1 subgroup, 30% and 83%, respectively.
When the investigators looked at the extent of nodal burden in cN0 patients with breast pCR, they found the rate of N2 and N3 disease was near zero across all biologic subtypes. “In patients who were cN1 at presentation and achieved a breast pCR but had residual axillary disease, the majority had N1 disease with only 1.5%-4% having four or more positive lymph nodes,” Dr. Barron said.
In the discussion, session moderator Carla Fisher, MD, of Indiana University, Indianapolis, said, “While we might not be ready for prime time to not evaluate the lymph nodes of these patients, this study does speak to the importance of establishing N0 and N1 prior to NAC.” In reply to her question about how Mayo routinely evaluates node status prior to NAC, Dr. Barron noted that Mayo performs routine axillary ultrasound. However, the NCDB data does not specify what imaging was done. This is thought to vary across the centers in the NCDB, Dr. Barron said.
Noted Dr. Boughey, “The findings from this study provide data that can be used moving forward for planning future clinical trials.” She also said that these findings do not alter the current standard of care; that still calls for breast and nodal surgery after NAC. However, the ongoing NRG-BR005 phase II clinical trial is assessing the accuracy of tumor-bed biopsy in these situations (ClinicalTrials.gov Identifier: NCT03188393). “The results from that trial will help inform future trials evaluating eliminating breast surgery in patients with an excellent response to NAC,” Dr. Boughey said. “Those patients could also potentially avoid axillary surgery based on the data we have now.”
Dr. Barron and Dr. Boughey and coauthors reported having no financial disclosures.
SOURCE: Barron AU et al. Society of Surgical Oncology Annual Cancer Symposium, Abstract 48.
REPORTING FROM SSO 2018
Key clinical point: Neoadjuvant chemotherapy for certain breast cancers achieves low rates of nodal positivity.
Major finding: In clinically node-negative HER2+ and triple-negative disease, nodal positivity after NAC in patients that had breast pathological complete response was less than 2%.
Study details: Review of 22,695 patients in NCDB with clinical T1 or T2 disease from 2010 to 2014.
Disclosure: Dr. Barron and coauthors reported having no financial disclosures.
Source: Barron AU et al. Society of Surgical Oncology Annual Cancer Symposium, Abstract 48.
Analgesic management in radiation oncology for painful bone metastases
Bone metastases are a common cause of pain in patients with advanced cancer, with about three-quarters of patients with bone metastases experiencing pain as the dominant symptom.1 Inadequately treated cancer pain impairs patient quality of life, and is associated with higher rates of depression, anxiety, and fatigue. Palliative radiotherapy (RT) is effective in alleviating pain from bone metastases.4 Local field external beam radiotherapy can provide some pain relief at the site of treated metastasis in 80%-90% of cases, with complete pain relief in 50%-60% of cases.5,6 However, maximal pain relief from RT is delayed, in some cases taking days to up to multiple weeks to attain.7,8 Therefore, optimal management of bone metastases pain may require the use of analgesics until RT takes adequate effect.
National Comprehensive Cancer Network (NCCN) Guidelines for Adult Cancer Pain (v. 2.2015) recommend that pain intensity rating (PIR; range, 0-10, where 0 denotes no pain and 10, worst pain imaginable) be used to quantify pain for all symptomatic patients. These guidelines also recommend the pain medication regimen be assessed for all symptomatic patients. For patients with moderate or severe pain (PIR of ≥4), NCCN guidelines recommend that analgesic regimen be intervened upon by alteration of the analgesic regimen (initiating, rotating, or titrating analgesic) or consideration of referral to pain/symptom management specialty.
Previous findings have demonstrated inadequate analgesic management for cancer pain,2,9 including within the radiation oncology (RO) clinic, suggesting that patients seen in consultation for palliative RT may experience uncontrolled pain for days to weeks before the onset of relief from RT. Possible reasons for inadequate acute pain intervention in the RO clinic may be provider discomfort with analgesic management and infrequent formal integration of palliative care within RO.10
Limited single-institution data from the few institutions with dedicated palliative RO services have suggested that these services improve the quality of palliative care delivery, as demonstrated by providers perceptions’ of the clinical impact of a dedicated service11 and the implementation of expedited palliative RT delivery for acute cancer pain.12,13 To our knowledge, the impact of a dedicated palliative RO service on analgesic management for cancer pain has not been assessed.
Here, we report how often patients with symptomatic bone metastases had assessments of existing analgesic regimens and interventions at RO consultation at 2 cancer centers. Center 1 had implemented a dedicated palliative RO service in 2011, consisting of rotating attending physicians and residents as well as dedicated palliative care trained nurse practitioners and a fellow, with the service structured around daily rounds,11 whereas Center 2 had not yet implemented a dedicated service. Using data from both centers, we assessed the impact of a palliative RO service on analgesic assessment and management in patients with bone metastases.
Methods
We searched our institutional databases for patients seen in RO consultation for bone metastases using ICD-9 code 198.5, and retrospectively reviewed consultation notes for those patients during June-July 2008, January-February 2010, January-February 2013, and June-July 2014. Those time periods were chosen as evenly spaced representative samples before and after implementation of a dedicated palliative RO service in 2011 at Center 1. Center 2 did not implement a dedicated palliative RO service in these time periods.
Within consultation notes, we recorded the following data from the History of the Present Illness section: symptoms from bone metastases (symptomatic was defined as any pain present); PIR (range, 0-10); and whether or not the preconsultation analgesic regimen was reported for symptomatic patients (including analgesic type, dosing, effectiveness, and adherence).
Documentation of the analgesic regimen in the history section of the notes was considered the proxy for analgesic regimen assessment at time of RO consultation. Analgesics within the Medications list, which were autopopulated in the consultation note by the electronic medical record, were recorded.
Whether or not pain was addressed with initiation or titration of analgesics for patients with a PIR of ≥4 was recorded from the Assessment and Plan portion of the notes, and that metric was considered the proxy for pain intervention. In addition, the case was coded as having had pain intervention if there was documentation of the patient declining recommended analgesic intervention, or the patient had been referred to a symptom management service for intervention (eg, referral to a specialty palliative care clinic), or there was recommendation for the patient to discuss uncontrolled pain with the original prescriber. A PIR of 4 was chosen as the threshold for analgesic intervention because at that level, NCCN guidelines for cancer pain state that the analgesic regimen should be titrated, whereas for a PIR of 3 or less, the guidelines recommend only consideration of titrating the analgesic. Only patients with a documented PIR were included in the pain intervention analysis.
Frequencies of analgesic assessment and analgesic intervention were compared using t tests (Wizard Pro, v1.8.5; Evan Miller, Chicago IL).
Results
A total of 271 patients with RO consultation notes were identified at the 2 centers within the 4 time periods (Table 1).
Among symptomatic patients, any component of the preconsultation analgesic regimen (including analgesic type, dosing, pain response, and adherence) was documented for 37.9% of the entire cohort at RO consultation (Table 3). At Centers 1 and 2, the frequencies of analgesic regimen assessment were documented for 41.3% and 28.1%, respectively (P = .06). Among symptomatic patients, 81.5% had an opioid or nonopioid analgesic listed in the Medications section in the electronic medical record at time of consultation.
Patients seen on the dedicated palliative RO service at Center 1 had an analgesic assessment documentation rate of 59.5%, whereas the patients not seen on a palliative RO service (ie, patients seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had an assessment documentation rate of 33.5% (P = .002; Figure 1). There was no significant difference between rates of analgesic regimen assessment between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (28.1% vs 35.9%, respectively; P = .27).
In patients seen at Center 1 only, those seen on the palliative RO service had a higher documentation rate of analgesic assessment compared with those seen by other services after implementation of the dedicated service (59.5% vs 38%, respectively; P = .018). Time period (after versus before 2011) was not significantly associated with the rate of documentation of analgesic assessment at either Center 1 (after vs before 2011: 44.4% vs 31%, P = .23) or Center 2 (31.4% vs 24.1%, P = .60).
Among patients with a PIR of ≥4, analgesic intervention was reported for 17.2% of patients within the entire cohort (20.8% at Center 1 and 0% at Center 2, P = .05). Among those with a PIR of ≥4, documentation of analgesic assessment noted in the History of the Present Illness section was associated with increased documentation of an analgesic intervention in the Assessment and Plan section (25% vs 7.3%; odds ratio [OR], 4.22; 95% confidence interval [CI], 1.1-16.0; P = .03).
Patients seen on the dedicated palliative RO service at Center 1 had a documented analgesic intervention rate of 31.6%, whereas the patients not seen on a palliative RO service (ie, those seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had a documented analgesic intervention rate of 9.2% (P = .01; Figure 2). There was no statistically significant difference between rates of documentation of an analgesic regimen intervention between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (0% vs 17.2%, respectively; P = .07).
Looking at only patients seen at Center 1, patients with a PIR of ≥4 seen on the dedicated palliative RO service had a nearly significant higher rate of documented analgesic interventions in the time period after implementation of the dedicate service (31.6% if seen on the dedicated service vs 12% if seen on a nondedicated service, P = .06).
Discussion
Multiple studies demonstrate the undertreatment of cancer pain in the outpatient setting.2,9,14,15 At 2 cancer centers, we found that about half of patients who present for consideration of palliative RT for bone metastases had a PIR of ≥4, yet only 17% of them had documentation of analgesic intervention as recommended by NCCN guidelines for cancer pain. Underlying this low rate of appropriate intervention may be the assumption of rapid pain relief by RT. However, RT often does not begin at time of consultation,16 and maximal pain relief may take days to weeks after commencement of RT.17 It is estimated that a quarter of all patients with cancer develop bone metastases during the course of their disease,12 and most of those patients suffer from pain. Thus, inherent delay in pain relief before, during, and after RT results in significant morbidity for the cancer patient population if adequate analgesic management is not provided.
The low rate of appropriate analgesic intervention at the time of RO consultation may also be related to the low incidence of proper analgesic assessment. In our cohort, 80% of symptomatic patients had an opioid or nonopioid analgesic listed in their medications within the electronic medical record at time of consultation, but only 38% had the analgesic regimen and/or its effectiveness described in the History of the Present Illness section of the record. Inattentiveness to analgesic type, dosing, and effectiveness during consultation may result in any inadequacies of the analgesic regimen going unnoticed. Consistent with this notion, we found that the rate of appropriate intervention for patients with a PIR of ≥4 was higher among patients who had analgesic regimen reported in the consultation note. Thus, interventions to implement routine review and documentation of the analgesic regimen, for example within the electronic medical record, may be one way to improve pain management.
Another possible reason for low rates of acute pain management within the RO clinic is low provider confidence in regard to analgesic management. In a recent national survey, 96% of radiation oncologists stated they were at least moderately confident with assessment of pain, yet only 77% were at least moderately confident with titrating opioids, and just 56% were at least moderately confident with rotating opioids.10 Educational interventions that improve providers’ facility with analgesic management may increase the frequency of pain management in the RO clinic.
Patients seen on the dedicated palliative RO service had significantly higher rates of documented analgesic regimen assessment and appropriate intervention during RO consultation, compared with patients seen at Center 2 and those not seen on the dedicated palliative RO service at Center 1. The improvements we observed in analgesic assessment and intervention at Center 1 for patients seen on the palliative RO service are likely owing to involvement of palliative RO and not to secular trends, because there were not similar improvements for patients at Center 1 who were not seen by the palliative RO service and those at Center 2, where there was no service.
At Center 1, the dedicated palliative RO service was created to provide specialized care to patients with metastatic disease undergoing palliative radiation. Within its structure, topics within palliative RO, such as technical aspects of palliative RT, symptom management, and communication are taught and reinforced in a case-based approach. Such palliative care awareness, integration, and education within RO achieved by the palliative RO service likely contribute to the improved rates of analgesic management we found in our study. We do note that rate of analgesic intervention in the palliative RO cohort, though higher than in the nonpalliative RO group, was still low, with only a third of patients receiving proper analgesic management. These findings highlight the importance of continued effort in increasing providers’ awareness of the need to assess pain and raise comfort with analgesic initiation and titration and of having dedicated palliative care clinicians embedded within the RO setting.
Since the data for this study was acquired, Center 2 has implemented a short palliative RO didactic course for residents, which improved their comfort levels in assessing analgesic effectiveness and intervening for uncontrolled pain.18 The impact of this intervention on clinical care will need to be evaluated, but the improved provider comfort levels may translate into better-quality care.
Limitations
An important limitation of this retrospective study is the reliance on the documentation provided in the consultation note for determining frequencies of analgesic regimen assessment and intervention. The actual rates of analgesic management that occurred in clinic may have been higher than reported in the documentation. However, such discrepancy in documentation of analgesic management would also be an area for quality improvement. Inadequate documentation limits the ability for proper follow-up of cancer pain as recommended by a joint guidance statement from the American Society of Clinical Oncology and the American Academy of Hospice and Palliative Medicine.19,20 The results of our study may also partly reflect a positive impact in documentation of analgesic management by a dedicated palliative RO service.
Given the multi-institutional nature of this study, it may be that general practice differences confound the impact of the dedicated palliative RO service at Center 1. However, with excluding Center 2, the dedicated service was still strongly associated with a higher rate of analgesic assessment within Center 1 and was almost significantly associated with appropriate analgesic intervention within Center 1.
We used a PIR of ≥4 as a threshold for appropriate analgesic regimen intervention because it is what is recommended by the NCCN guidelines. However, close attention should be paid to the impact that any amount of pain has on an individual patient. The functional, spiritual, and existential impact of pain is unique to each patient’s experience, and optimal symptom management should take those elements into account.
Conclusion
In conclusion, this study indicates that advanced cancer patient pain assessment and intervention according to NCCN cancer pain management guidelines is not common in the RO setting, and it is an area that should be targeted for quality improvement because of the positive implications for patient well-being. Pain assessment and intervention were greater in the setting of a dedicated structure for palliative care within RO, suggesting that the integration of palliative care within RO is a promising means of improving quality of pain management.
This work was presented at the 2016 ASCO Palliative Care in Oncology Symposium (September 9-10, 2016), where this work received a Conquer Cancer Foundation Merit Award.
1. Amichetti M, Orrù P, Madeddu A, et al. Comparative evaluation of two hypofractionated radiotherapy regimens for painful bone metastases. Tumori. 2004;90(1):91-95.
2. Vuong S, Pulenzas N, DeAngelis C, et al. Inadequate pain management in cancer patients attending an outpatient palliative radiotherapy clinic. Support Care Cancer. 2016;24(2):887-892.
3. Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: characteristics and impact in patients with cancer pain. Pain. 1999;81(1-2):129-134.
4. Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy - a systematic review of the randomised trials. Sze WM, ed. Cochrane Database Syst Rev. 2004;(2):CD004721-CD004721.
5. Ratanatharathorn V, Powers WE, Moss WT, Perez CA. Bone metastasis: review and critical analysis of random allocation trials of local field treatment. Int J Radiat Oncol Biol Phys. 1999;44(1):1-18.
6. Kirou-Mauro A, Hird A, Wong J, et al. Is response to radiotherapy in patients related to the severity of pretreatment pain? Int J Radiat Oncol Biol Phys. 2008;71(4):1208-1212.
7. Frassica DA. General principles of external beam radiation therapy for skeletal metastases. Clin Orthop Relat Res. 2003;(415 Suppl):S158-S164.
8. McDonald R, Ding K, Brundage M, et al. Effect of radiotherapy on painful bone metastases: a secondary analysis of the NCIC Clinical Trials Group Symptom Control Trial SC.23. JAMA Oncol. 2017 Jul 1;3(7):953-959.
9. Greco MT, Roberto A, Corli O, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32(36):4149-4154.
10. Wei RL, Mattes MD, Yu J, et al. Attitudes of radiation oncologists toward palliative and supportive care in the united states: report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract Radiat Oncol. 2017;7(2):113-119.
11. Tseng YD, Krishnan MS, Jones JA, et al. Supportive and palliative radiation oncology service: impact of a dedicated service on palliative cancer care. Pract Radiat Oncol. 2014;4(4):247-253.
12. Fairchild A, Pituskin E, Rose B, et al. The rapid access palliative radiotherapy program: blueprint for initiation of a one-stop multidisciplinary bone metastases clinic. Support Care Cancer. 2009;17(2):163-170.
13. de Sa E, Sinclair E, Mitera G, et al. Continued success of the rapid response radiotherapy program: a review of 2004-2008. Support Care Cancer. 2009;17(7):757-762.
14. Deandrea S, Montanari M, Moja L, Apolone G. Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol. 2008;19(12):1985-1991.
15. Mitera G, Zeiadin N, Kirou-Mauro A, et al. Retrospective assessment of cancer pain management in an outpatient palliative radiotherapy clinic using the Pain Management Index. J Pain Symptom Manage. 2010;39(2):259-267.
16. Danjoux C, Chow E, Drossos A, et al. An innovative rapid response radiotherapy program to reduce waiting time for palliative radiotherapy. Support Care Cancer. 2006;14(1):38-43.
17. Feyer PC, Steingraeber M. Radiotherapy of bone metastasis in breast cancer patients – current approaches. Breast Care (Basel). 2012;7(2):108-112.
18. Garcia MA, Braunstein SE, Anderson WG. Palliative Care Didactic Course for Radiation Oncology Residents. Int J Radiat Oncol Biol Phys. 2017;97(5):884-885.
19. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96-112.
20. Bickel KE, McNiff K, Buss MK, et al. Defining high-quality palliative care in oncology practice: an American Society of Clinical Oncology/American Academy of Hospice and Palliative Medicine guidance statement. J Oncol Pract. 2016;12(9):e828-e838.
Bone metastases are a common cause of pain in patients with advanced cancer, with about three-quarters of patients with bone metastases experiencing pain as the dominant symptom.1 Inadequately treated cancer pain impairs patient quality of life, and is associated with higher rates of depression, anxiety, and fatigue. Palliative radiotherapy (RT) is effective in alleviating pain from bone metastases.4 Local field external beam radiotherapy can provide some pain relief at the site of treated metastasis in 80%-90% of cases, with complete pain relief in 50%-60% of cases.5,6 However, maximal pain relief from RT is delayed, in some cases taking days to up to multiple weeks to attain.7,8 Therefore, optimal management of bone metastases pain may require the use of analgesics until RT takes adequate effect.
National Comprehensive Cancer Network (NCCN) Guidelines for Adult Cancer Pain (v. 2.2015) recommend that pain intensity rating (PIR; range, 0-10, where 0 denotes no pain and 10, worst pain imaginable) be used to quantify pain for all symptomatic patients. These guidelines also recommend the pain medication regimen be assessed for all symptomatic patients. For patients with moderate or severe pain (PIR of ≥4), NCCN guidelines recommend that analgesic regimen be intervened upon by alteration of the analgesic regimen (initiating, rotating, or titrating analgesic) or consideration of referral to pain/symptom management specialty.
Previous findings have demonstrated inadequate analgesic management for cancer pain,2,9 including within the radiation oncology (RO) clinic, suggesting that patients seen in consultation for palliative RT may experience uncontrolled pain for days to weeks before the onset of relief from RT. Possible reasons for inadequate acute pain intervention in the RO clinic may be provider discomfort with analgesic management and infrequent formal integration of palliative care within RO.10
Limited single-institution data from the few institutions with dedicated palliative RO services have suggested that these services improve the quality of palliative care delivery, as demonstrated by providers perceptions’ of the clinical impact of a dedicated service11 and the implementation of expedited palliative RT delivery for acute cancer pain.12,13 To our knowledge, the impact of a dedicated palliative RO service on analgesic management for cancer pain has not been assessed.
Here, we report how often patients with symptomatic bone metastases had assessments of existing analgesic regimens and interventions at RO consultation at 2 cancer centers. Center 1 had implemented a dedicated palliative RO service in 2011, consisting of rotating attending physicians and residents as well as dedicated palliative care trained nurse practitioners and a fellow, with the service structured around daily rounds,11 whereas Center 2 had not yet implemented a dedicated service. Using data from both centers, we assessed the impact of a palliative RO service on analgesic assessment and management in patients with bone metastases.
Methods
We searched our institutional databases for patients seen in RO consultation for bone metastases using ICD-9 code 198.5, and retrospectively reviewed consultation notes for those patients during June-July 2008, January-February 2010, January-February 2013, and June-July 2014. Those time periods were chosen as evenly spaced representative samples before and after implementation of a dedicated palliative RO service in 2011 at Center 1. Center 2 did not implement a dedicated palliative RO service in these time periods.
Within consultation notes, we recorded the following data from the History of the Present Illness section: symptoms from bone metastases (symptomatic was defined as any pain present); PIR (range, 0-10); and whether or not the preconsultation analgesic regimen was reported for symptomatic patients (including analgesic type, dosing, effectiveness, and adherence).
Documentation of the analgesic regimen in the history section of the notes was considered the proxy for analgesic regimen assessment at time of RO consultation. Analgesics within the Medications list, which were autopopulated in the consultation note by the electronic medical record, were recorded.
Whether or not pain was addressed with initiation or titration of analgesics for patients with a PIR of ≥4 was recorded from the Assessment and Plan portion of the notes, and that metric was considered the proxy for pain intervention. In addition, the case was coded as having had pain intervention if there was documentation of the patient declining recommended analgesic intervention, or the patient had been referred to a symptom management service for intervention (eg, referral to a specialty palliative care clinic), or there was recommendation for the patient to discuss uncontrolled pain with the original prescriber. A PIR of 4 was chosen as the threshold for analgesic intervention because at that level, NCCN guidelines for cancer pain state that the analgesic regimen should be titrated, whereas for a PIR of 3 or less, the guidelines recommend only consideration of titrating the analgesic. Only patients with a documented PIR were included in the pain intervention analysis.
Frequencies of analgesic assessment and analgesic intervention were compared using t tests (Wizard Pro, v1.8.5; Evan Miller, Chicago IL).
Results
A total of 271 patients with RO consultation notes were identified at the 2 centers within the 4 time periods (Table 1).
Among symptomatic patients, any component of the preconsultation analgesic regimen (including analgesic type, dosing, pain response, and adherence) was documented for 37.9% of the entire cohort at RO consultation (Table 3). At Centers 1 and 2, the frequencies of analgesic regimen assessment were documented for 41.3% and 28.1%, respectively (P = .06). Among symptomatic patients, 81.5% had an opioid or nonopioid analgesic listed in the Medications section in the electronic medical record at time of consultation.
Patients seen on the dedicated palliative RO service at Center 1 had an analgesic assessment documentation rate of 59.5%, whereas the patients not seen on a palliative RO service (ie, patients seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had an assessment documentation rate of 33.5% (P = .002; Figure 1). There was no significant difference between rates of analgesic regimen assessment between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (28.1% vs 35.9%, respectively; P = .27).
In patients seen at Center 1 only, those seen on the palliative RO service had a higher documentation rate of analgesic assessment compared with those seen by other services after implementation of the dedicated service (59.5% vs 38%, respectively; P = .018). Time period (after versus before 2011) was not significantly associated with the rate of documentation of analgesic assessment at either Center 1 (after vs before 2011: 44.4% vs 31%, P = .23) or Center 2 (31.4% vs 24.1%, P = .60).
Among patients with a PIR of ≥4, analgesic intervention was reported for 17.2% of patients within the entire cohort (20.8% at Center 1 and 0% at Center 2, P = .05). Among those with a PIR of ≥4, documentation of analgesic assessment noted in the History of the Present Illness section was associated with increased documentation of an analgesic intervention in the Assessment and Plan section (25% vs 7.3%; odds ratio [OR], 4.22; 95% confidence interval [CI], 1.1-16.0; P = .03).
Patients seen on the dedicated palliative RO service at Center 1 had a documented analgesic intervention rate of 31.6%, whereas the patients not seen on a palliative RO service (ie, those seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had a documented analgesic intervention rate of 9.2% (P = .01; Figure 2). There was no statistically significant difference between rates of documentation of an analgesic regimen intervention between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (0% vs 17.2%, respectively; P = .07).
Looking at only patients seen at Center 1, patients with a PIR of ≥4 seen on the dedicated palliative RO service had a nearly significant higher rate of documented analgesic interventions in the time period after implementation of the dedicate service (31.6% if seen on the dedicated service vs 12% if seen on a nondedicated service, P = .06).
Discussion
Multiple studies demonstrate the undertreatment of cancer pain in the outpatient setting.2,9,14,15 At 2 cancer centers, we found that about half of patients who present for consideration of palliative RT for bone metastases had a PIR of ≥4, yet only 17% of them had documentation of analgesic intervention as recommended by NCCN guidelines for cancer pain. Underlying this low rate of appropriate intervention may be the assumption of rapid pain relief by RT. However, RT often does not begin at time of consultation,16 and maximal pain relief may take days to weeks after commencement of RT.17 It is estimated that a quarter of all patients with cancer develop bone metastases during the course of their disease,12 and most of those patients suffer from pain. Thus, inherent delay in pain relief before, during, and after RT results in significant morbidity for the cancer patient population if adequate analgesic management is not provided.
The low rate of appropriate analgesic intervention at the time of RO consultation may also be related to the low incidence of proper analgesic assessment. In our cohort, 80% of symptomatic patients had an opioid or nonopioid analgesic listed in their medications within the electronic medical record at time of consultation, but only 38% had the analgesic regimen and/or its effectiveness described in the History of the Present Illness section of the record. Inattentiveness to analgesic type, dosing, and effectiveness during consultation may result in any inadequacies of the analgesic regimen going unnoticed. Consistent with this notion, we found that the rate of appropriate intervention for patients with a PIR of ≥4 was higher among patients who had analgesic regimen reported in the consultation note. Thus, interventions to implement routine review and documentation of the analgesic regimen, for example within the electronic medical record, may be one way to improve pain management.
Another possible reason for low rates of acute pain management within the RO clinic is low provider confidence in regard to analgesic management. In a recent national survey, 96% of radiation oncologists stated they were at least moderately confident with assessment of pain, yet only 77% were at least moderately confident with titrating opioids, and just 56% were at least moderately confident with rotating opioids.10 Educational interventions that improve providers’ facility with analgesic management may increase the frequency of pain management in the RO clinic.
Patients seen on the dedicated palliative RO service had significantly higher rates of documented analgesic regimen assessment and appropriate intervention during RO consultation, compared with patients seen at Center 2 and those not seen on the dedicated palliative RO service at Center 1. The improvements we observed in analgesic assessment and intervention at Center 1 for patients seen on the palliative RO service are likely owing to involvement of palliative RO and not to secular trends, because there were not similar improvements for patients at Center 1 who were not seen by the palliative RO service and those at Center 2, where there was no service.
At Center 1, the dedicated palliative RO service was created to provide specialized care to patients with metastatic disease undergoing palliative radiation. Within its structure, topics within palliative RO, such as technical aspects of palliative RT, symptom management, and communication are taught and reinforced in a case-based approach. Such palliative care awareness, integration, and education within RO achieved by the palliative RO service likely contribute to the improved rates of analgesic management we found in our study. We do note that rate of analgesic intervention in the palliative RO cohort, though higher than in the nonpalliative RO group, was still low, with only a third of patients receiving proper analgesic management. These findings highlight the importance of continued effort in increasing providers’ awareness of the need to assess pain and raise comfort with analgesic initiation and titration and of having dedicated palliative care clinicians embedded within the RO setting.
Since the data for this study was acquired, Center 2 has implemented a short palliative RO didactic course for residents, which improved their comfort levels in assessing analgesic effectiveness and intervening for uncontrolled pain.18 The impact of this intervention on clinical care will need to be evaluated, but the improved provider comfort levels may translate into better-quality care.
Limitations
An important limitation of this retrospective study is the reliance on the documentation provided in the consultation note for determining frequencies of analgesic regimen assessment and intervention. The actual rates of analgesic management that occurred in clinic may have been higher than reported in the documentation. However, such discrepancy in documentation of analgesic management would also be an area for quality improvement. Inadequate documentation limits the ability for proper follow-up of cancer pain as recommended by a joint guidance statement from the American Society of Clinical Oncology and the American Academy of Hospice and Palliative Medicine.19,20 The results of our study may also partly reflect a positive impact in documentation of analgesic management by a dedicated palliative RO service.
Given the multi-institutional nature of this study, it may be that general practice differences confound the impact of the dedicated palliative RO service at Center 1. However, with excluding Center 2, the dedicated service was still strongly associated with a higher rate of analgesic assessment within Center 1 and was almost significantly associated with appropriate analgesic intervention within Center 1.
We used a PIR of ≥4 as a threshold for appropriate analgesic regimen intervention because it is what is recommended by the NCCN guidelines. However, close attention should be paid to the impact that any amount of pain has on an individual patient. The functional, spiritual, and existential impact of pain is unique to each patient’s experience, and optimal symptom management should take those elements into account.
Conclusion
In conclusion, this study indicates that advanced cancer patient pain assessment and intervention according to NCCN cancer pain management guidelines is not common in the RO setting, and it is an area that should be targeted for quality improvement because of the positive implications for patient well-being. Pain assessment and intervention were greater in the setting of a dedicated structure for palliative care within RO, suggesting that the integration of palliative care within RO is a promising means of improving quality of pain management.
This work was presented at the 2016 ASCO Palliative Care in Oncology Symposium (September 9-10, 2016), where this work received a Conquer Cancer Foundation Merit Award.
Bone metastases are a common cause of pain in patients with advanced cancer, with about three-quarters of patients with bone metastases experiencing pain as the dominant symptom.1 Inadequately treated cancer pain impairs patient quality of life, and is associated with higher rates of depression, anxiety, and fatigue. Palliative radiotherapy (RT) is effective in alleviating pain from bone metastases.4 Local field external beam radiotherapy can provide some pain relief at the site of treated metastasis in 80%-90% of cases, with complete pain relief in 50%-60% of cases.5,6 However, maximal pain relief from RT is delayed, in some cases taking days to up to multiple weeks to attain.7,8 Therefore, optimal management of bone metastases pain may require the use of analgesics until RT takes adequate effect.
National Comprehensive Cancer Network (NCCN) Guidelines for Adult Cancer Pain (v. 2.2015) recommend that pain intensity rating (PIR; range, 0-10, where 0 denotes no pain and 10, worst pain imaginable) be used to quantify pain for all symptomatic patients. These guidelines also recommend the pain medication regimen be assessed for all symptomatic patients. For patients with moderate or severe pain (PIR of ≥4), NCCN guidelines recommend that analgesic regimen be intervened upon by alteration of the analgesic regimen (initiating, rotating, or titrating analgesic) or consideration of referral to pain/symptom management specialty.
Previous findings have demonstrated inadequate analgesic management for cancer pain,2,9 including within the radiation oncology (RO) clinic, suggesting that patients seen in consultation for palliative RT may experience uncontrolled pain for days to weeks before the onset of relief from RT. Possible reasons for inadequate acute pain intervention in the RO clinic may be provider discomfort with analgesic management and infrequent formal integration of palliative care within RO.10
Limited single-institution data from the few institutions with dedicated palliative RO services have suggested that these services improve the quality of palliative care delivery, as demonstrated by providers perceptions’ of the clinical impact of a dedicated service11 and the implementation of expedited palliative RT delivery for acute cancer pain.12,13 To our knowledge, the impact of a dedicated palliative RO service on analgesic management for cancer pain has not been assessed.
Here, we report how often patients with symptomatic bone metastases had assessments of existing analgesic regimens and interventions at RO consultation at 2 cancer centers. Center 1 had implemented a dedicated palliative RO service in 2011, consisting of rotating attending physicians and residents as well as dedicated palliative care trained nurse practitioners and a fellow, with the service structured around daily rounds,11 whereas Center 2 had not yet implemented a dedicated service. Using data from both centers, we assessed the impact of a palliative RO service on analgesic assessment and management in patients with bone metastases.
Methods
We searched our institutional databases for patients seen in RO consultation for bone metastases using ICD-9 code 198.5, and retrospectively reviewed consultation notes for those patients during June-July 2008, January-February 2010, January-February 2013, and June-July 2014. Those time periods were chosen as evenly spaced representative samples before and after implementation of a dedicated palliative RO service in 2011 at Center 1. Center 2 did not implement a dedicated palliative RO service in these time periods.
Within consultation notes, we recorded the following data from the History of the Present Illness section: symptoms from bone metastases (symptomatic was defined as any pain present); PIR (range, 0-10); and whether or not the preconsultation analgesic regimen was reported for symptomatic patients (including analgesic type, dosing, effectiveness, and adherence).
Documentation of the analgesic regimen in the history section of the notes was considered the proxy for analgesic regimen assessment at time of RO consultation. Analgesics within the Medications list, which were autopopulated in the consultation note by the electronic medical record, were recorded.
Whether or not pain was addressed with initiation or titration of analgesics for patients with a PIR of ≥4 was recorded from the Assessment and Plan portion of the notes, and that metric was considered the proxy for pain intervention. In addition, the case was coded as having had pain intervention if there was documentation of the patient declining recommended analgesic intervention, or the patient had been referred to a symptom management service for intervention (eg, referral to a specialty palliative care clinic), or there was recommendation for the patient to discuss uncontrolled pain with the original prescriber. A PIR of 4 was chosen as the threshold for analgesic intervention because at that level, NCCN guidelines for cancer pain state that the analgesic regimen should be titrated, whereas for a PIR of 3 or less, the guidelines recommend only consideration of titrating the analgesic. Only patients with a documented PIR were included in the pain intervention analysis.
Frequencies of analgesic assessment and analgesic intervention were compared using t tests (Wizard Pro, v1.8.5; Evan Miller, Chicago IL).
Results
A total of 271 patients with RO consultation notes were identified at the 2 centers within the 4 time periods (Table 1).
Among symptomatic patients, any component of the preconsultation analgesic regimen (including analgesic type, dosing, pain response, and adherence) was documented for 37.9% of the entire cohort at RO consultation (Table 3). At Centers 1 and 2, the frequencies of analgesic regimen assessment were documented for 41.3% and 28.1%, respectively (P = .06). Among symptomatic patients, 81.5% had an opioid or nonopioid analgesic listed in the Medications section in the electronic medical record at time of consultation.
Patients seen on the dedicated palliative RO service at Center 1 had an analgesic assessment documentation rate of 59.5%, whereas the patients not seen on a palliative RO service (ie, patients seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had an assessment documentation rate of 33.5% (P = .002; Figure 1). There was no significant difference between rates of analgesic regimen assessment between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (28.1% vs 35.9%, respectively; P = .27).
In patients seen at Center 1 only, those seen on the palliative RO service had a higher documentation rate of analgesic assessment compared with those seen by other services after implementation of the dedicated service (59.5% vs 38%, respectively; P = .018). Time period (after versus before 2011) was not significantly associated with the rate of documentation of analgesic assessment at either Center 1 (after vs before 2011: 44.4% vs 31%, P = .23) or Center 2 (31.4% vs 24.1%, P = .60).
Among patients with a PIR of ≥4, analgesic intervention was reported for 17.2% of patients within the entire cohort (20.8% at Center 1 and 0% at Center 2, P = .05). Among those with a PIR of ≥4, documentation of analgesic assessment noted in the History of the Present Illness section was associated with increased documentation of an analgesic intervention in the Assessment and Plan section (25% vs 7.3%; odds ratio [OR], 4.22; 95% confidence interval [CI], 1.1-16.0; P = .03).
Patients seen on the dedicated palliative RO service at Center 1 had a documented analgesic intervention rate of 31.6%, whereas the patients not seen on a palliative RO service (ie, those seen on a nonpalliative RO service at Center 1 plus all patients at Center 2) had a documented analgesic intervention rate of 9.2% (P = .01; Figure 2). There was no statistically significant difference between rates of documentation of an analgesic regimen intervention between patients seen at Center 2 and patients seen within nondedicated palliative RO services at Center 1 (0% vs 17.2%, respectively; P = .07).
Looking at only patients seen at Center 1, patients with a PIR of ≥4 seen on the dedicated palliative RO service had a nearly significant higher rate of documented analgesic interventions in the time period after implementation of the dedicate service (31.6% if seen on the dedicated service vs 12% if seen on a nondedicated service, P = .06).
Discussion
Multiple studies demonstrate the undertreatment of cancer pain in the outpatient setting.2,9,14,15 At 2 cancer centers, we found that about half of patients who present for consideration of palliative RT for bone metastases had a PIR of ≥4, yet only 17% of them had documentation of analgesic intervention as recommended by NCCN guidelines for cancer pain. Underlying this low rate of appropriate intervention may be the assumption of rapid pain relief by RT. However, RT often does not begin at time of consultation,16 and maximal pain relief may take days to weeks after commencement of RT.17 It is estimated that a quarter of all patients with cancer develop bone metastases during the course of their disease,12 and most of those patients suffer from pain. Thus, inherent delay in pain relief before, during, and after RT results in significant morbidity for the cancer patient population if adequate analgesic management is not provided.
The low rate of appropriate analgesic intervention at the time of RO consultation may also be related to the low incidence of proper analgesic assessment. In our cohort, 80% of symptomatic patients had an opioid or nonopioid analgesic listed in their medications within the electronic medical record at time of consultation, but only 38% had the analgesic regimen and/or its effectiveness described in the History of the Present Illness section of the record. Inattentiveness to analgesic type, dosing, and effectiveness during consultation may result in any inadequacies of the analgesic regimen going unnoticed. Consistent with this notion, we found that the rate of appropriate intervention for patients with a PIR of ≥4 was higher among patients who had analgesic regimen reported in the consultation note. Thus, interventions to implement routine review and documentation of the analgesic regimen, for example within the electronic medical record, may be one way to improve pain management.
Another possible reason for low rates of acute pain management within the RO clinic is low provider confidence in regard to analgesic management. In a recent national survey, 96% of radiation oncologists stated they were at least moderately confident with assessment of pain, yet only 77% were at least moderately confident with titrating opioids, and just 56% were at least moderately confident with rotating opioids.10 Educational interventions that improve providers’ facility with analgesic management may increase the frequency of pain management in the RO clinic.
Patients seen on the dedicated palliative RO service had significantly higher rates of documented analgesic regimen assessment and appropriate intervention during RO consultation, compared with patients seen at Center 2 and those not seen on the dedicated palliative RO service at Center 1. The improvements we observed in analgesic assessment and intervention at Center 1 for patients seen on the palliative RO service are likely owing to involvement of palliative RO and not to secular trends, because there were not similar improvements for patients at Center 1 who were not seen by the palliative RO service and those at Center 2, where there was no service.
At Center 1, the dedicated palliative RO service was created to provide specialized care to patients with metastatic disease undergoing palliative radiation. Within its structure, topics within palliative RO, such as technical aspects of palliative RT, symptom management, and communication are taught and reinforced in a case-based approach. Such palliative care awareness, integration, and education within RO achieved by the palliative RO service likely contribute to the improved rates of analgesic management we found in our study. We do note that rate of analgesic intervention in the palliative RO cohort, though higher than in the nonpalliative RO group, was still low, with only a third of patients receiving proper analgesic management. These findings highlight the importance of continued effort in increasing providers’ awareness of the need to assess pain and raise comfort with analgesic initiation and titration and of having dedicated palliative care clinicians embedded within the RO setting.
Since the data for this study was acquired, Center 2 has implemented a short palliative RO didactic course for residents, which improved their comfort levels in assessing analgesic effectiveness and intervening for uncontrolled pain.18 The impact of this intervention on clinical care will need to be evaluated, but the improved provider comfort levels may translate into better-quality care.
Limitations
An important limitation of this retrospective study is the reliance on the documentation provided in the consultation note for determining frequencies of analgesic regimen assessment and intervention. The actual rates of analgesic management that occurred in clinic may have been higher than reported in the documentation. However, such discrepancy in documentation of analgesic management would also be an area for quality improvement. Inadequate documentation limits the ability for proper follow-up of cancer pain as recommended by a joint guidance statement from the American Society of Clinical Oncology and the American Academy of Hospice and Palliative Medicine.19,20 The results of our study may also partly reflect a positive impact in documentation of analgesic management by a dedicated palliative RO service.
Given the multi-institutional nature of this study, it may be that general practice differences confound the impact of the dedicated palliative RO service at Center 1. However, with excluding Center 2, the dedicated service was still strongly associated with a higher rate of analgesic assessment within Center 1 and was almost significantly associated with appropriate analgesic intervention within Center 1.
We used a PIR of ≥4 as a threshold for appropriate analgesic regimen intervention because it is what is recommended by the NCCN guidelines. However, close attention should be paid to the impact that any amount of pain has on an individual patient. The functional, spiritual, and existential impact of pain is unique to each patient’s experience, and optimal symptom management should take those elements into account.
Conclusion
In conclusion, this study indicates that advanced cancer patient pain assessment and intervention according to NCCN cancer pain management guidelines is not common in the RO setting, and it is an area that should be targeted for quality improvement because of the positive implications for patient well-being. Pain assessment and intervention were greater in the setting of a dedicated structure for palliative care within RO, suggesting that the integration of palliative care within RO is a promising means of improving quality of pain management.
This work was presented at the 2016 ASCO Palliative Care in Oncology Symposium (September 9-10, 2016), where this work received a Conquer Cancer Foundation Merit Award.
1. Amichetti M, Orrù P, Madeddu A, et al. Comparative evaluation of two hypofractionated radiotherapy regimens for painful bone metastases. Tumori. 2004;90(1):91-95.
2. Vuong S, Pulenzas N, DeAngelis C, et al. Inadequate pain management in cancer patients attending an outpatient palliative radiotherapy clinic. Support Care Cancer. 2016;24(2):887-892.
3. Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: characteristics and impact in patients with cancer pain. Pain. 1999;81(1-2):129-134.
4. Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy - a systematic review of the randomised trials. Sze WM, ed. Cochrane Database Syst Rev. 2004;(2):CD004721-CD004721.
5. Ratanatharathorn V, Powers WE, Moss WT, Perez CA. Bone metastasis: review and critical analysis of random allocation trials of local field treatment. Int J Radiat Oncol Biol Phys. 1999;44(1):1-18.
6. Kirou-Mauro A, Hird A, Wong J, et al. Is response to radiotherapy in patients related to the severity of pretreatment pain? Int J Radiat Oncol Biol Phys. 2008;71(4):1208-1212.
7. Frassica DA. General principles of external beam radiation therapy for skeletal metastases. Clin Orthop Relat Res. 2003;(415 Suppl):S158-S164.
8. McDonald R, Ding K, Brundage M, et al. Effect of radiotherapy on painful bone metastases: a secondary analysis of the NCIC Clinical Trials Group Symptom Control Trial SC.23. JAMA Oncol. 2017 Jul 1;3(7):953-959.
9. Greco MT, Roberto A, Corli O, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32(36):4149-4154.
10. Wei RL, Mattes MD, Yu J, et al. Attitudes of radiation oncologists toward palliative and supportive care in the united states: report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract Radiat Oncol. 2017;7(2):113-119.
11. Tseng YD, Krishnan MS, Jones JA, et al. Supportive and palliative radiation oncology service: impact of a dedicated service on palliative cancer care. Pract Radiat Oncol. 2014;4(4):247-253.
12. Fairchild A, Pituskin E, Rose B, et al. The rapid access palliative radiotherapy program: blueprint for initiation of a one-stop multidisciplinary bone metastases clinic. Support Care Cancer. 2009;17(2):163-170.
13. de Sa E, Sinclair E, Mitera G, et al. Continued success of the rapid response radiotherapy program: a review of 2004-2008. Support Care Cancer. 2009;17(7):757-762.
14. Deandrea S, Montanari M, Moja L, Apolone G. Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol. 2008;19(12):1985-1991.
15. Mitera G, Zeiadin N, Kirou-Mauro A, et al. Retrospective assessment of cancer pain management in an outpatient palliative radiotherapy clinic using the Pain Management Index. J Pain Symptom Manage. 2010;39(2):259-267.
16. Danjoux C, Chow E, Drossos A, et al. An innovative rapid response radiotherapy program to reduce waiting time for palliative radiotherapy. Support Care Cancer. 2006;14(1):38-43.
17. Feyer PC, Steingraeber M. Radiotherapy of bone metastasis in breast cancer patients – current approaches. Breast Care (Basel). 2012;7(2):108-112.
18. Garcia MA, Braunstein SE, Anderson WG. Palliative Care Didactic Course for Radiation Oncology Residents. Int J Radiat Oncol Biol Phys. 2017;97(5):884-885.
19. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96-112.
20. Bickel KE, McNiff K, Buss MK, et al. Defining high-quality palliative care in oncology practice: an American Society of Clinical Oncology/American Academy of Hospice and Palliative Medicine guidance statement. J Oncol Pract. 2016;12(9):e828-e838.
1. Amichetti M, Orrù P, Madeddu A, et al. Comparative evaluation of two hypofractionated radiotherapy regimens for painful bone metastases. Tumori. 2004;90(1):91-95.
2. Vuong S, Pulenzas N, DeAngelis C, et al. Inadequate pain management in cancer patients attending an outpatient palliative radiotherapy clinic. Support Care Cancer. 2016;24(2):887-892.
3. Portenoy RK, Payne D, Jacobsen P. Breakthrough pain: characteristics and impact in patients with cancer pain. Pain. 1999;81(1-2):129-134.
4. Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: single fraction versus multifraction radiotherapy - a systematic review of the randomised trials. Sze WM, ed. Cochrane Database Syst Rev. 2004;(2):CD004721-CD004721.
5. Ratanatharathorn V, Powers WE, Moss WT, Perez CA. Bone metastasis: review and critical analysis of random allocation trials of local field treatment. Int J Radiat Oncol Biol Phys. 1999;44(1):1-18.
6. Kirou-Mauro A, Hird A, Wong J, et al. Is response to radiotherapy in patients related to the severity of pretreatment pain? Int J Radiat Oncol Biol Phys. 2008;71(4):1208-1212.
7. Frassica DA. General principles of external beam radiation therapy for skeletal metastases. Clin Orthop Relat Res. 2003;(415 Suppl):S158-S164.
8. McDonald R, Ding K, Brundage M, et al. Effect of radiotherapy on painful bone metastases: a secondary analysis of the NCIC Clinical Trials Group Symptom Control Trial SC.23. JAMA Oncol. 2017 Jul 1;3(7):953-959.
9. Greco MT, Roberto A, Corli O, et al. Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Clin Oncol. 2014;32(36):4149-4154.
10. Wei RL, Mattes MD, Yu J, et al. Attitudes of radiation oncologists toward palliative and supportive care in the united states: report on national membership survey by the American Society for Radiation Oncology (ASTRO). Pract Radiat Oncol. 2017;7(2):113-119.
11. Tseng YD, Krishnan MS, Jones JA, et al. Supportive and palliative radiation oncology service: impact of a dedicated service on palliative cancer care. Pract Radiat Oncol. 2014;4(4):247-253.
12. Fairchild A, Pituskin E, Rose B, et al. The rapid access palliative radiotherapy program: blueprint for initiation of a one-stop multidisciplinary bone metastases clinic. Support Care Cancer. 2009;17(2):163-170.
13. de Sa E, Sinclair E, Mitera G, et al. Continued success of the rapid response radiotherapy program: a review of 2004-2008. Support Care Cancer. 2009;17(7):757-762.
14. Deandrea S, Montanari M, Moja L, Apolone G. Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol. 2008;19(12):1985-1991.
15. Mitera G, Zeiadin N, Kirou-Mauro A, et al. Retrospective assessment of cancer pain management in an outpatient palliative radiotherapy clinic using the Pain Management Index. J Pain Symptom Manage. 2010;39(2):259-267.
16. Danjoux C, Chow E, Drossos A, et al. An innovative rapid response radiotherapy program to reduce waiting time for palliative radiotherapy. Support Care Cancer. 2006;14(1):38-43.
17. Feyer PC, Steingraeber M. Radiotherapy of bone metastasis in breast cancer patients – current approaches. Breast Care (Basel). 2012;7(2):108-112.
18. Garcia MA, Braunstein SE, Anderson WG. Palliative Care Didactic Course for Radiation Oncology Residents. Int J Radiat Oncol Biol Phys. 2017;97(5):884-885.
19. Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(1):96-112.
20. Bickel KE, McNiff K, Buss MK, et al. Defining high-quality palliative care in oncology practice: an American Society of Clinical Oncology/American Academy of Hospice and Palliative Medicine guidance statement. J Oncol Pract. 2016;12(9):e828-e838.
Sarcopenia, body fat linked with mortality in nonmetastatic breast cancer
Among women with nonmetastatic breast cancer, low muscle mass and excess body fat are significantly associated with worse survival, investigators report.
An observational study of 3,241 women diagnosed with stage II or III breast cancer showed that low muscle mass (sarcopenia) was independently associated with a 41% increase in risk for overall mortality, and that total adipose tissue (TAT) measures were associated with a 35% increase in overall mortality.
Women with sarcopenia and high total TAT scores had a nearly twofold higher risk for death, reported Bette J. Caan, DrPH, of Kaiser Permanente in Oakland, Calif., and her colleagues.
Although low muscle mass was found to be a significant risk factor for death, neither poor muscle quality, measured by radiodensity, nor body mass index (BMI) were significantly associated with overall mortality, the investigators reported in a study published online in JAMA Oncology.
“Both muscle and adiposity represent modifiable risk factors in patients with breast cancer. In addition to weight loss, we should also consider interventions to improve muscle mass, such as resistance training or protein supplementation. In the era of precision medicine, the direct measurement of muscle and adiposity will help to guide treatment plans and interventions to optimize survival outcomes,” they wrote.
Although moderate to severe obesity measured by high BMI has been associated with worse outcomes for patients with breast cancer and other malignancies, the evidence is mixed for those who are merely overweight or have borderline obesity, the authors noted.
BMI is a simple ratio of height to weight, and does not measure body composition, and “low BMI can mask excess adiposity while high BMI can mask low muscularity,” they wrote.
To determine whether associations between measures of body composition could be prognostic for overall mortality, the investigators conducted a retrospective cohort study with patients from Kaiser Permanente Northern California and the Dana-Farber Cancer Institute in Boston.
The cohort included 3,241 women diagnosed with stage II or III invasive breast cancer during 2005-2013 in California and during 2000-2012 in Boston. All of the patients included had either abdominal or pelvic CT scans or PET-CT scans at the time of diagnosis.
The investigators looked at the associations between sarcopenia, TAT, and low muscle radiodensity, and created hazard ratio (HR) estimates of the effects of the various interactions on overall mortality, adjusted for sociodemographics, tumor characteristics, treatment, BMI, and other body composition measures.
They found that after a median follow-up of 6 years, patients with sarcopenia had a significantly greater risk for overall mortality than did patients without sarcopenia (HR, 1.41; 95% confidence interval, 1.18-1.69).
Additionally, patients in the highest tertile of TAT also had significantly higher overall mortality, compared with patients in the lowest tertile (HR, 1.35; CI, 1.08-1.69).
As noted before, poor muscle quality was not significantly associated with overall mortality.
Looking at both sarcopenia and TAT, the authors found that the highest risk for death was in those patients with both sarcopenia and high TAT (HR, 1.88; CI, 1.30-2.73).
However, they also found that BMI was not an independent predictor of overall mortality, and did identify those patients who were at risk because of their body composition.
“We demonstrate that sarcopenia is not a condition restricted to patients with later-stage disease but rather is highly prevalent among patients with nonmetastatic disease across all levels of BMI. Our findings are likely generalizable across many other nonmetastatic cancers because the associations with muscle and improved survival for those with metastatic cancer has been observed across a variety of solid tumors,” Dr. Caan and her associates wrote in their conclusion.
The article did not report a funding source for the study. The investigators reported having no conflicts of interest to disclose.
SOURCE: Cann BJ et al. JAMA Oncol. 2018 Apr 5. doi: 10.1001/jamaoncol.2018.0137.
Obesity is highly prevalent among breast cancer survivors, and in addition to its effects on cancer development and outcomes, it also can affect treatment efficacy and adverse effects and complicate clinical management of breast cancer from obesity-related comorbidities such as hypertension and diabetes. As such, the American Society of Clinical Oncology made obesity and cancer one of their core priorities in 2013 and launched the Obesity & Cancer Initiative with activities ranging from education and awareness to clinical guidance, promotion of research, and policy and advocacy.
Despite its limitations, body mass index remains an easy tool to help health care clinicians identify patients at greater risk for poor outcomes and adverse effects and guide their recommendations, as well as to educate patients in self-assessing their weight status. Weight management and control are likely to have many benefits for breast cancer survivors but should always be tailored to individual patients’ needs. When CT imaging is available, the study by Caan et al. suggests that body composition measures can be useful in identifying women at higher risk of mortality. Their findings are an important reminder that weight loss and/or weight control programs must always incorporate physical activity with the goal of not just reducing adiposity, but also maintaining and increasing muscle mass, which would not only reduce the risk of death, but might also help improve quality of life after a cancer diagnosis.
Elisa V. Bandera, MD, PhD, is with the Rutgers Cancer Institute of New Jersey, Rutgers University, New Brunswick. Esther M. John, PhD, is with Stanford (Calif.) University. Both editorialists reported having no conflicts of interest to disclose. Their remarks are adapted from an accompanying invited commentary (JAMA Oncol. doi: 10.1001/jamaoncol.2018.0137).
Obesity is highly prevalent among breast cancer survivors, and in addition to its effects on cancer development and outcomes, it also can affect treatment efficacy and adverse effects and complicate clinical management of breast cancer from obesity-related comorbidities such as hypertension and diabetes. As such, the American Society of Clinical Oncology made obesity and cancer one of their core priorities in 2013 and launched the Obesity & Cancer Initiative with activities ranging from education and awareness to clinical guidance, promotion of research, and policy and advocacy.
Despite its limitations, body mass index remains an easy tool to help health care clinicians identify patients at greater risk for poor outcomes and adverse effects and guide their recommendations, as well as to educate patients in self-assessing their weight status. Weight management and control are likely to have many benefits for breast cancer survivors but should always be tailored to individual patients’ needs. When CT imaging is available, the study by Caan et al. suggests that body composition measures can be useful in identifying women at higher risk of mortality. Their findings are an important reminder that weight loss and/or weight control programs must always incorporate physical activity with the goal of not just reducing adiposity, but also maintaining and increasing muscle mass, which would not only reduce the risk of death, but might also help improve quality of life after a cancer diagnosis.
Elisa V. Bandera, MD, PhD, is with the Rutgers Cancer Institute of New Jersey, Rutgers University, New Brunswick. Esther M. John, PhD, is with Stanford (Calif.) University. Both editorialists reported having no conflicts of interest to disclose. Their remarks are adapted from an accompanying invited commentary (JAMA Oncol. doi: 10.1001/jamaoncol.2018.0137).
Obesity is highly prevalent among breast cancer survivors, and in addition to its effects on cancer development and outcomes, it also can affect treatment efficacy and adverse effects and complicate clinical management of breast cancer from obesity-related comorbidities such as hypertension and diabetes. As such, the American Society of Clinical Oncology made obesity and cancer one of their core priorities in 2013 and launched the Obesity & Cancer Initiative with activities ranging from education and awareness to clinical guidance, promotion of research, and policy and advocacy.
Despite its limitations, body mass index remains an easy tool to help health care clinicians identify patients at greater risk for poor outcomes and adverse effects and guide their recommendations, as well as to educate patients in self-assessing their weight status. Weight management and control are likely to have many benefits for breast cancer survivors but should always be tailored to individual patients’ needs. When CT imaging is available, the study by Caan et al. suggests that body composition measures can be useful in identifying women at higher risk of mortality. Their findings are an important reminder that weight loss and/or weight control programs must always incorporate physical activity with the goal of not just reducing adiposity, but also maintaining and increasing muscle mass, which would not only reduce the risk of death, but might also help improve quality of life after a cancer diagnosis.
Elisa V. Bandera, MD, PhD, is with the Rutgers Cancer Institute of New Jersey, Rutgers University, New Brunswick. Esther M. John, PhD, is with Stanford (Calif.) University. Both editorialists reported having no conflicts of interest to disclose. Their remarks are adapted from an accompanying invited commentary (JAMA Oncol. doi: 10.1001/jamaoncol.2018.0137).
Among women with nonmetastatic breast cancer, low muscle mass and excess body fat are significantly associated with worse survival, investigators report.
An observational study of 3,241 women diagnosed with stage II or III breast cancer showed that low muscle mass (sarcopenia) was independently associated with a 41% increase in risk for overall mortality, and that total adipose tissue (TAT) measures were associated with a 35% increase in overall mortality.
Women with sarcopenia and high total TAT scores had a nearly twofold higher risk for death, reported Bette J. Caan, DrPH, of Kaiser Permanente in Oakland, Calif., and her colleagues.
Although low muscle mass was found to be a significant risk factor for death, neither poor muscle quality, measured by radiodensity, nor body mass index (BMI) were significantly associated with overall mortality, the investigators reported in a study published online in JAMA Oncology.
“Both muscle and adiposity represent modifiable risk factors in patients with breast cancer. In addition to weight loss, we should also consider interventions to improve muscle mass, such as resistance training or protein supplementation. In the era of precision medicine, the direct measurement of muscle and adiposity will help to guide treatment plans and interventions to optimize survival outcomes,” they wrote.
Although moderate to severe obesity measured by high BMI has been associated with worse outcomes for patients with breast cancer and other malignancies, the evidence is mixed for those who are merely overweight or have borderline obesity, the authors noted.
BMI is a simple ratio of height to weight, and does not measure body composition, and “low BMI can mask excess adiposity while high BMI can mask low muscularity,” they wrote.
To determine whether associations between measures of body composition could be prognostic for overall mortality, the investigators conducted a retrospective cohort study with patients from Kaiser Permanente Northern California and the Dana-Farber Cancer Institute in Boston.
The cohort included 3,241 women diagnosed with stage II or III invasive breast cancer during 2005-2013 in California and during 2000-2012 in Boston. All of the patients included had either abdominal or pelvic CT scans or PET-CT scans at the time of diagnosis.
The investigators looked at the associations between sarcopenia, TAT, and low muscle radiodensity, and created hazard ratio (HR) estimates of the effects of the various interactions on overall mortality, adjusted for sociodemographics, tumor characteristics, treatment, BMI, and other body composition measures.
They found that after a median follow-up of 6 years, patients with sarcopenia had a significantly greater risk for overall mortality than did patients without sarcopenia (HR, 1.41; 95% confidence interval, 1.18-1.69).
Additionally, patients in the highest tertile of TAT also had significantly higher overall mortality, compared with patients in the lowest tertile (HR, 1.35; CI, 1.08-1.69).
As noted before, poor muscle quality was not significantly associated with overall mortality.
Looking at both sarcopenia and TAT, the authors found that the highest risk for death was in those patients with both sarcopenia and high TAT (HR, 1.88; CI, 1.30-2.73).
However, they also found that BMI was not an independent predictor of overall mortality, and did identify those patients who were at risk because of their body composition.
“We demonstrate that sarcopenia is not a condition restricted to patients with later-stage disease but rather is highly prevalent among patients with nonmetastatic disease across all levels of BMI. Our findings are likely generalizable across many other nonmetastatic cancers because the associations with muscle and improved survival for those with metastatic cancer has been observed across a variety of solid tumors,” Dr. Caan and her associates wrote in their conclusion.
The article did not report a funding source for the study. The investigators reported having no conflicts of interest to disclose.
SOURCE: Cann BJ et al. JAMA Oncol. 2018 Apr 5. doi: 10.1001/jamaoncol.2018.0137.
Among women with nonmetastatic breast cancer, low muscle mass and excess body fat are significantly associated with worse survival, investigators report.
An observational study of 3,241 women diagnosed with stage II or III breast cancer showed that low muscle mass (sarcopenia) was independently associated with a 41% increase in risk for overall mortality, and that total adipose tissue (TAT) measures were associated with a 35% increase in overall mortality.
Women with sarcopenia and high total TAT scores had a nearly twofold higher risk for death, reported Bette J. Caan, DrPH, of Kaiser Permanente in Oakland, Calif., and her colleagues.
Although low muscle mass was found to be a significant risk factor for death, neither poor muscle quality, measured by radiodensity, nor body mass index (BMI) were significantly associated with overall mortality, the investigators reported in a study published online in JAMA Oncology.
“Both muscle and adiposity represent modifiable risk factors in patients with breast cancer. In addition to weight loss, we should also consider interventions to improve muscle mass, such as resistance training or protein supplementation. In the era of precision medicine, the direct measurement of muscle and adiposity will help to guide treatment plans and interventions to optimize survival outcomes,” they wrote.
Although moderate to severe obesity measured by high BMI has been associated with worse outcomes for patients with breast cancer and other malignancies, the evidence is mixed for those who are merely overweight or have borderline obesity, the authors noted.
BMI is a simple ratio of height to weight, and does not measure body composition, and “low BMI can mask excess adiposity while high BMI can mask low muscularity,” they wrote.
To determine whether associations between measures of body composition could be prognostic for overall mortality, the investigators conducted a retrospective cohort study with patients from Kaiser Permanente Northern California and the Dana-Farber Cancer Institute in Boston.
The cohort included 3,241 women diagnosed with stage II or III invasive breast cancer during 2005-2013 in California and during 2000-2012 in Boston. All of the patients included had either abdominal or pelvic CT scans or PET-CT scans at the time of diagnosis.
The investigators looked at the associations between sarcopenia, TAT, and low muscle radiodensity, and created hazard ratio (HR) estimates of the effects of the various interactions on overall mortality, adjusted for sociodemographics, tumor characteristics, treatment, BMI, and other body composition measures.
They found that after a median follow-up of 6 years, patients with sarcopenia had a significantly greater risk for overall mortality than did patients without sarcopenia (HR, 1.41; 95% confidence interval, 1.18-1.69).
Additionally, patients in the highest tertile of TAT also had significantly higher overall mortality, compared with patients in the lowest tertile (HR, 1.35; CI, 1.08-1.69).
As noted before, poor muscle quality was not significantly associated with overall mortality.
Looking at both sarcopenia and TAT, the authors found that the highest risk for death was in those patients with both sarcopenia and high TAT (HR, 1.88; CI, 1.30-2.73).
However, they also found that BMI was not an independent predictor of overall mortality, and did identify those patients who were at risk because of their body composition.
“We demonstrate that sarcopenia is not a condition restricted to patients with later-stage disease but rather is highly prevalent among patients with nonmetastatic disease across all levels of BMI. Our findings are likely generalizable across many other nonmetastatic cancers because the associations with muscle and improved survival for those with metastatic cancer has been observed across a variety of solid tumors,” Dr. Caan and her associates wrote in their conclusion.
The article did not report a funding source for the study. The investigators reported having no conflicts of interest to disclose.
SOURCE: Cann BJ et al. JAMA Oncol. 2018 Apr 5. doi: 10.1001/jamaoncol.2018.0137.
FROM JAMA ONCOLOGY
Key clinical point: Helping women with nonmetastatic breast cancer control weight and improve muscle strength could lower their risk of death.
Major finding: Women with sarcopenia and high total adipose tissue had a hazard ratio of 1.89 for overall mortality.
Study details: Retrospective cohort study of 3,241 women diagnosed with stage II or III invasive breast cancer in California and Massachusetts.
Disclosures: The article did not report a funding source for the study. The investigators reported having no conflicts of interest to disclose.
Source: Cann BJ et al. JAMA Oncol. 2018 Apr 5. doi:10.1001/jamaoncol.2018.0137.
21-gene assay predicts survival in male and female breast cancer
A study of the molecular and genomic features of breast cancer in men, compared with those in women, highlights the prognostic value of a 21-gene breast recurrence score in both sexes, investigators say.
Men and women with estrogen receptor (ER)–positive breast cancer who had recurrence scores (RS) of 0 to 30 on the 21-gene assay (Oncotype DX) had excellent breast cancer–specific survival rates, which suggests that such patients could be spared from more aggressive treatments, such as chemotherapy, according to Suleiman Alfred Massarweh, MD, of Stanford (Calif.) University and his colleagues.
“Future adjuvant trials in ER-positive breast cancer may need to focus on targeting endocrine resistance in those patients with RS greater than 31 and may need to consider the weight of competing mortality risk when investigating the value of any additional treatment beyond endocrine therapy,” they wrote in the Journal of Clinical Oncology.
In 2016, an estimated 2,600 men were diagnosed with breast cancer in the United States.
“Approximately 95% of breast cancers diagnosed in men express the estrogen receptor and progesterone receptor (PR), which is a higher percentage than in women and suggests a key role for ER in the biology of breast cancer in men,” the investigators noted.
Although treatment of men with breast cancer has traditionally been extrapolated from treatment of women with breast cancer, genomic studies have suggested some key differences, the investigators noted, citing a study of the genomic landscape of male breast cancers presented at the 2014 San Antonio Breast Cancer Symposium.
In that study, investigators from the Memorial Sloan Kettering Cancer Center in New York and other institutions found that all male breast cancers in their sample of 64 patients were ER+ and human epidermal growth factor receptor 2 (HER2)–negative, predominantly of the luminal B subtype, and that the genetic alterations seen in male breast cancers frequently target DNA-repair fibroblast growth factor pathways. However, the pathways that are known to drive luminal cancers when mutated in women are seen less often among men, said Salvatore Piscuoglio, PhD, then a research fellow at MSKCC.
The current study helps to confirm and expand on the findings from that study, commented Steven J. Isakoff, MD, PhD, of the Massachusetts General Hospital Cancer Center in Boston, who was not involved in either study.
“I think it’s helpful to see in a larger dataset what the spectrum of oncotypes [Oncotype DX] looks like in men. In general, as the study described, we have a real lack of large-scale data in men and certainly no prospective data with oncotypes,” he said in an interview.
To get a better idea of the molecular characteristics of breast cancer in men and how they relate to breast cancer–specific mortality, Dr. Massarweh and his associates looked at deidentified 21-gene assay data from the Genomic Health Laboratory database on 3,806 men and 571,115 women with breast cancer with either no nodal involvement, micrometastases only, or one to three involved lymph nodes.
They also looked at survival data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) population of patients with breast cancer diagnosed during 2004-2012, which included data on 332 men and 55,842 women with ER-positive and/or PR-positive invasive breast cancer.
Among the entire 21-gene assay sample, they found that men were significantly older than women at the time of diagnosis, at a mean age of 64.2 vs. 59.1 years (P less than .001).
Both men and women had infiltrating ductal carcinoma as the most common histology; the prevalence was slightly higher among men at 87.6% versus 81.3% for women.
The average recurrence score in men was 16.8 versus 17.0 in women, a difference that was not statistically significant. A majority of both men and women had RS scores below 18 (65.8% and 58.2%, respectively), although significantly more men than women had RS scores of 31 or higher (12.4% vs. 7.4%; P less than .001).
“This relative predominance of high RS results in men was encountered across age groups but was most prominent in men younger than 40 years of age,” the investigators wrote.
At the other end of the scale, RS lower than 11, especially RS 0, were seen more frequently in men than in women, except among those younger than 40 years.
Looking at individual gene expression profiles, the authors found that mean gene expression was higher in men for genes associated with ER, proliferation, and invasion. ER expression was lowest and PR expression was highest in women younger than 50 years, but ER expression increased progressively with age.
Among men, those younger than 50 years had slightly lower ER and PR expression than did older men.
In the analysis of SEER survival data, they found that 5-year breast cancer severity score (BCSS) was 99% for men with RS below 18, 95.7% for those with RS between18 and 30, and 81% for those with RS of 31 or higher. Among women, 5-year BCSS was 99.5% for those with RS under 18, 98.6% for those with RS between 18 and 30, and 94.9% for those with RS of 31 or higher.
Five-year overall survival estimates were 92.6% for men with RS below 18, 86% for those with RS between 18 and 30, and 69% for those with RS of 31 or higher. Respective 5-year OS rates for women were 95%, 94.2%, and 89.9%.
“The 21-gene RS provided clear prognostic information in our cohort, with a significantly different 5-year BCSS determined by RS in both men and women,” the investigators wrote.
They noted that patients with low and intermediate RS have excellent prognoses regardless of nodal status, which suggests that these patients have more indolent disease and better outcomes than do patients with higher RS.
The more frequent use of adjuvant chemotherapy in the RS 31 and higher group indicates that “the prognostic utility of RS results is evident despite adjuvant chemotherapy use,” they wrote.
Dr. Isakoff pointed out, however, that the population in the study is from a registry of patients eligible for the 21-gene assay, which can only be used for patients with ER-positive and HER2-negative tumors.
“In other words, this is not a random sample. This is a sample of patients for whom the treating physician was on the fence about chemotherapy and in some way thought that getting an oncotype might be helpful,” he said.
He added that although the study findings “don’t change anything we have been doing, they provide reassurance that oncotype is a reasonable test to consider in patients with male breast cancer for whom we’re considering including or avoiding chemotherapy,” he said.
A funding source for the study was not reported. Dr. Massarweh disclosed stock or ownership in Radius Health, consulting for Novartis, and institutional research funding from multiple companies. Three coauthors are employees and stockholders of Genomic Health, maker of the Oncotype DX assay used in the study. Dr. Isakoff reported no conflicts of interest related to the study
SOURCE: Massarweh SA et al. 2018 Mar 27. doi: 10.1200/JCO.2017.76.8861.
A study of the molecular and genomic features of breast cancer in men, compared with those in women, highlights the prognostic value of a 21-gene breast recurrence score in both sexes, investigators say.
Men and women with estrogen receptor (ER)–positive breast cancer who had recurrence scores (RS) of 0 to 30 on the 21-gene assay (Oncotype DX) had excellent breast cancer–specific survival rates, which suggests that such patients could be spared from more aggressive treatments, such as chemotherapy, according to Suleiman Alfred Massarweh, MD, of Stanford (Calif.) University and his colleagues.
“Future adjuvant trials in ER-positive breast cancer may need to focus on targeting endocrine resistance in those patients with RS greater than 31 and may need to consider the weight of competing mortality risk when investigating the value of any additional treatment beyond endocrine therapy,” they wrote in the Journal of Clinical Oncology.
In 2016, an estimated 2,600 men were diagnosed with breast cancer in the United States.
“Approximately 95% of breast cancers diagnosed in men express the estrogen receptor and progesterone receptor (PR), which is a higher percentage than in women and suggests a key role for ER in the biology of breast cancer in men,” the investigators noted.
Although treatment of men with breast cancer has traditionally been extrapolated from treatment of women with breast cancer, genomic studies have suggested some key differences, the investigators noted, citing a study of the genomic landscape of male breast cancers presented at the 2014 San Antonio Breast Cancer Symposium.
In that study, investigators from the Memorial Sloan Kettering Cancer Center in New York and other institutions found that all male breast cancers in their sample of 64 patients were ER+ and human epidermal growth factor receptor 2 (HER2)–negative, predominantly of the luminal B subtype, and that the genetic alterations seen in male breast cancers frequently target DNA-repair fibroblast growth factor pathways. However, the pathways that are known to drive luminal cancers when mutated in women are seen less often among men, said Salvatore Piscuoglio, PhD, then a research fellow at MSKCC.
The current study helps to confirm and expand on the findings from that study, commented Steven J. Isakoff, MD, PhD, of the Massachusetts General Hospital Cancer Center in Boston, who was not involved in either study.
“I think it’s helpful to see in a larger dataset what the spectrum of oncotypes [Oncotype DX] looks like in men. In general, as the study described, we have a real lack of large-scale data in men and certainly no prospective data with oncotypes,” he said in an interview.
To get a better idea of the molecular characteristics of breast cancer in men and how they relate to breast cancer–specific mortality, Dr. Massarweh and his associates looked at deidentified 21-gene assay data from the Genomic Health Laboratory database on 3,806 men and 571,115 women with breast cancer with either no nodal involvement, micrometastases only, or one to three involved lymph nodes.
They also looked at survival data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) population of patients with breast cancer diagnosed during 2004-2012, which included data on 332 men and 55,842 women with ER-positive and/or PR-positive invasive breast cancer.
Among the entire 21-gene assay sample, they found that men were significantly older than women at the time of diagnosis, at a mean age of 64.2 vs. 59.1 years (P less than .001).
Both men and women had infiltrating ductal carcinoma as the most common histology; the prevalence was slightly higher among men at 87.6% versus 81.3% for women.
The average recurrence score in men was 16.8 versus 17.0 in women, a difference that was not statistically significant. A majority of both men and women had RS scores below 18 (65.8% and 58.2%, respectively), although significantly more men than women had RS scores of 31 or higher (12.4% vs. 7.4%; P less than .001).
“This relative predominance of high RS results in men was encountered across age groups but was most prominent in men younger than 40 years of age,” the investigators wrote.
At the other end of the scale, RS lower than 11, especially RS 0, were seen more frequently in men than in women, except among those younger than 40 years.
Looking at individual gene expression profiles, the authors found that mean gene expression was higher in men for genes associated with ER, proliferation, and invasion. ER expression was lowest and PR expression was highest in women younger than 50 years, but ER expression increased progressively with age.
Among men, those younger than 50 years had slightly lower ER and PR expression than did older men.
In the analysis of SEER survival data, they found that 5-year breast cancer severity score (BCSS) was 99% for men with RS below 18, 95.7% for those with RS between18 and 30, and 81% for those with RS of 31 or higher. Among women, 5-year BCSS was 99.5% for those with RS under 18, 98.6% for those with RS between 18 and 30, and 94.9% for those with RS of 31 or higher.
Five-year overall survival estimates were 92.6% for men with RS below 18, 86% for those with RS between 18 and 30, and 69% for those with RS of 31 or higher. Respective 5-year OS rates for women were 95%, 94.2%, and 89.9%.
“The 21-gene RS provided clear prognostic information in our cohort, with a significantly different 5-year BCSS determined by RS in both men and women,” the investigators wrote.
They noted that patients with low and intermediate RS have excellent prognoses regardless of nodal status, which suggests that these patients have more indolent disease and better outcomes than do patients with higher RS.
The more frequent use of adjuvant chemotherapy in the RS 31 and higher group indicates that “the prognostic utility of RS results is evident despite adjuvant chemotherapy use,” they wrote.
Dr. Isakoff pointed out, however, that the population in the study is from a registry of patients eligible for the 21-gene assay, which can only be used for patients with ER-positive and HER2-negative tumors.
“In other words, this is not a random sample. This is a sample of patients for whom the treating physician was on the fence about chemotherapy and in some way thought that getting an oncotype might be helpful,” he said.
He added that although the study findings “don’t change anything we have been doing, they provide reassurance that oncotype is a reasonable test to consider in patients with male breast cancer for whom we’re considering including or avoiding chemotherapy,” he said.
A funding source for the study was not reported. Dr. Massarweh disclosed stock or ownership in Radius Health, consulting for Novartis, and institutional research funding from multiple companies. Three coauthors are employees and stockholders of Genomic Health, maker of the Oncotype DX assay used in the study. Dr. Isakoff reported no conflicts of interest related to the study
SOURCE: Massarweh SA et al. 2018 Mar 27. doi: 10.1200/JCO.2017.76.8861.
A study of the molecular and genomic features of breast cancer in men, compared with those in women, highlights the prognostic value of a 21-gene breast recurrence score in both sexes, investigators say.
Men and women with estrogen receptor (ER)–positive breast cancer who had recurrence scores (RS) of 0 to 30 on the 21-gene assay (Oncotype DX) had excellent breast cancer–specific survival rates, which suggests that such patients could be spared from more aggressive treatments, such as chemotherapy, according to Suleiman Alfred Massarweh, MD, of Stanford (Calif.) University and his colleagues.
“Future adjuvant trials in ER-positive breast cancer may need to focus on targeting endocrine resistance in those patients with RS greater than 31 and may need to consider the weight of competing mortality risk when investigating the value of any additional treatment beyond endocrine therapy,” they wrote in the Journal of Clinical Oncology.
In 2016, an estimated 2,600 men were diagnosed with breast cancer in the United States.
“Approximately 95% of breast cancers diagnosed in men express the estrogen receptor and progesterone receptor (PR), which is a higher percentage than in women and suggests a key role for ER in the biology of breast cancer in men,” the investigators noted.
Although treatment of men with breast cancer has traditionally been extrapolated from treatment of women with breast cancer, genomic studies have suggested some key differences, the investigators noted, citing a study of the genomic landscape of male breast cancers presented at the 2014 San Antonio Breast Cancer Symposium.
In that study, investigators from the Memorial Sloan Kettering Cancer Center in New York and other institutions found that all male breast cancers in their sample of 64 patients were ER+ and human epidermal growth factor receptor 2 (HER2)–negative, predominantly of the luminal B subtype, and that the genetic alterations seen in male breast cancers frequently target DNA-repair fibroblast growth factor pathways. However, the pathways that are known to drive luminal cancers when mutated in women are seen less often among men, said Salvatore Piscuoglio, PhD, then a research fellow at MSKCC.
The current study helps to confirm and expand on the findings from that study, commented Steven J. Isakoff, MD, PhD, of the Massachusetts General Hospital Cancer Center in Boston, who was not involved in either study.
“I think it’s helpful to see in a larger dataset what the spectrum of oncotypes [Oncotype DX] looks like in men. In general, as the study described, we have a real lack of large-scale data in men and certainly no prospective data with oncotypes,” he said in an interview.
To get a better idea of the molecular characteristics of breast cancer in men and how they relate to breast cancer–specific mortality, Dr. Massarweh and his associates looked at deidentified 21-gene assay data from the Genomic Health Laboratory database on 3,806 men and 571,115 women with breast cancer with either no nodal involvement, micrometastases only, or one to three involved lymph nodes.
They also looked at survival data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) population of patients with breast cancer diagnosed during 2004-2012, which included data on 332 men and 55,842 women with ER-positive and/or PR-positive invasive breast cancer.
Among the entire 21-gene assay sample, they found that men were significantly older than women at the time of diagnosis, at a mean age of 64.2 vs. 59.1 years (P less than .001).
Both men and women had infiltrating ductal carcinoma as the most common histology; the prevalence was slightly higher among men at 87.6% versus 81.3% for women.
The average recurrence score in men was 16.8 versus 17.0 in women, a difference that was not statistically significant. A majority of both men and women had RS scores below 18 (65.8% and 58.2%, respectively), although significantly more men than women had RS scores of 31 or higher (12.4% vs. 7.4%; P less than .001).
“This relative predominance of high RS results in men was encountered across age groups but was most prominent in men younger than 40 years of age,” the investigators wrote.
At the other end of the scale, RS lower than 11, especially RS 0, were seen more frequently in men than in women, except among those younger than 40 years.
Looking at individual gene expression profiles, the authors found that mean gene expression was higher in men for genes associated with ER, proliferation, and invasion. ER expression was lowest and PR expression was highest in women younger than 50 years, but ER expression increased progressively with age.
Among men, those younger than 50 years had slightly lower ER and PR expression than did older men.
In the analysis of SEER survival data, they found that 5-year breast cancer severity score (BCSS) was 99% for men with RS below 18, 95.7% for those with RS between18 and 30, and 81% for those with RS of 31 or higher. Among women, 5-year BCSS was 99.5% for those with RS under 18, 98.6% for those with RS between 18 and 30, and 94.9% for those with RS of 31 or higher.
Five-year overall survival estimates were 92.6% for men with RS below 18, 86% for those with RS between 18 and 30, and 69% for those with RS of 31 or higher. Respective 5-year OS rates for women were 95%, 94.2%, and 89.9%.
“The 21-gene RS provided clear prognostic information in our cohort, with a significantly different 5-year BCSS determined by RS in both men and women,” the investigators wrote.
They noted that patients with low and intermediate RS have excellent prognoses regardless of nodal status, which suggests that these patients have more indolent disease and better outcomes than do patients with higher RS.
The more frequent use of adjuvant chemotherapy in the RS 31 and higher group indicates that “the prognostic utility of RS results is evident despite adjuvant chemotherapy use,” they wrote.
Dr. Isakoff pointed out, however, that the population in the study is from a registry of patients eligible for the 21-gene assay, which can only be used for patients with ER-positive and HER2-negative tumors.
“In other words, this is not a random sample. This is a sample of patients for whom the treating physician was on the fence about chemotherapy and in some way thought that getting an oncotype might be helpful,” he said.
He added that although the study findings “don’t change anything we have been doing, they provide reassurance that oncotype is a reasonable test to consider in patients with male breast cancer for whom we’re considering including or avoiding chemotherapy,” he said.
A funding source for the study was not reported. Dr. Massarweh disclosed stock or ownership in Radius Health, consulting for Novartis, and institutional research funding from multiple companies. Three coauthors are employees and stockholders of Genomic Health, maker of the Oncotype DX assay used in the study. Dr. Isakoff reported no conflicts of interest related to the study
SOURCE: Massarweh SA et al. 2018 Mar 27. doi: 10.1200/JCO.2017.76.8861.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: A 21-gene assay provides useful information about survival odds for men and women with breast cancer.
Major finding: A recurrence score of 31 or greater was associated with worse survival, particularly in men.
Study details: Retrospective review of genomic and surveillance data on 3,806 men and 571,115 women with breast cancer.
Disclosures: A funding source for the study was not reported. Dr. Massarweh disclosed stock or ownership in Radius Health, consulting for Novartis, and institutional research funding from multiple companies. Three coauthors are employees and stockholders of Genomic Health, maker of the Oncotype DX assay used in the study. Dr. Isakoff reported no conflicts of interest related to the study.
Source: Massarweh SA et al. 2018 Mar 27. doi: 10.1200/JCO.2017.76.8861.
Think about breast cancer surveillance for transgender patients
CHICAGO – , said Christel de Blok, MD, sharing results of a Dutch national study.
The study included 3,078 transgender people (2,064 transgender women) who began hormone therapy (HT) at age 18 years or older. The mean age at which transgender women began HT was 33 years; for transgender men, the mean age was 25 years. In all, transgender women in the study had a total of 30,699 person-years of exposure to HT; for transgender men, the figure was 13,155 person-years.
Overall, there were 16 observed cases of breast cancer in transgender women and four in transgender men. After gender-affirming surgery, the transgender women were followed for a median of 146 months, and experienced a median of 193 months of HT. Transgender men who had mastectomies were followed for a median 93 months, and those who had a hysterectomy-oophorectomy were followed for a median 144 months. Transgender men received a median 176 months of HT.
“Breast cancer can still occur after mastectomy in [transgender] men,” Dr. de Blok said at the annual meeting of the Endocrine Society. “What is interesting is that three out of the four cases of breast cancer in [transgender] men happened after mastectomy.”
In the Netherlands, one in eight women and one in 1,000 men will develop cancer at some point during their lives. In patients who have had a subtotal mastectomy and who are BRCA-1/2 carriers, there is still an approximate 5% residual risk of breast cancer, said Dr. de Blok.
A literature review conducted by Dr. de Blok and her colleagues revealed 19 cases of breast cancer in transgender women and 13 in transgender men. However, a more general study of incidence and characteristics of breast cancer in transgender people receiving hormone treatment had not been done, said Dr. de Blok, of the VU University Medical Center, Amsterdam.
The investigators examined data for adult transgender people seen at their center from 1991 to 2017 and started on hormone treatment. This clinic, said Dr. de Blok, sees about 95% of the transgender individuals in the Netherlands.
The study was able to capitalize on comprehensive information from national databases and registries. Investigators drew from a national histopathology and cytopathology registry as well as from a national vital statistics database. A comprehensive cancer database was used to establish both reference incidence values for males and females and the number of expected cases within the study group.
In both transgender men and women, exactly 50% of cases were ductal carcinoma, compared to 85% in the group of reference women.
An additional 31% of the breast cancers in transgender women were lobular, 6% were ductal carcinoma in situ (DCIS), and the remainder were of other types. Of the cancers in transgender women, 82% were estrogen receptor positive, 64% were progesterone receptor positive, and 9% were Her2/neu positive.
For transgender men, there were no lobular carcinomas; 25% were DCIS, and 25% were of other types. Half of the cancers were estrogen receptor positive, and half were progesterone receptor positive; 25% were Her2/neu positive, and there was one case of androgen receptor positive breast cancer.
Dr. de Blok explained that their analysis compared the observed cases in both transgender men and women to the expected number of cases for the same number of males and females, yielding two standardized incidence ratios (SIRs) for each transgender group.
For transgender women, the SIR for breast cancer compared with males was 50.9 (95% confidence interval, 30.1-80.9). The SIR compared to females was 0.3 (95% CI, 0.2-0.4). This reflected the expected case number of 0.3 for males and the 58 expected cases for a matched group of females.
For transgender men, the SIR for breast cancer compared with males was 59.8 (95% CI, 19-144.3), while the SIR compared to females was 0.2 (95% CI, 0.1-0.5). The expected cases for a similar group of males would be 0.1, and for females, 18.
In many cases, whether a transgender person receives standardized screening mammogram reminders will depend on which sex is assigned to that individual in insurance and other administrative databases, Mr. de Blok noted. When electronic health records and other databases have a binary system, at-risk individuals may fall through the cracks.
Dr. de Blok reported no conflicts of interest.
SOURCE: de Blok C, et al. ENDO 2018, abstract OR 25-6.
CHICAGO – , said Christel de Blok, MD, sharing results of a Dutch national study.
The study included 3,078 transgender people (2,064 transgender women) who began hormone therapy (HT) at age 18 years or older. The mean age at which transgender women began HT was 33 years; for transgender men, the mean age was 25 years. In all, transgender women in the study had a total of 30,699 person-years of exposure to HT; for transgender men, the figure was 13,155 person-years.
Overall, there were 16 observed cases of breast cancer in transgender women and four in transgender men. After gender-affirming surgery, the transgender women were followed for a median of 146 months, and experienced a median of 193 months of HT. Transgender men who had mastectomies were followed for a median 93 months, and those who had a hysterectomy-oophorectomy were followed for a median 144 months. Transgender men received a median 176 months of HT.
“Breast cancer can still occur after mastectomy in [transgender] men,” Dr. de Blok said at the annual meeting of the Endocrine Society. “What is interesting is that three out of the four cases of breast cancer in [transgender] men happened after mastectomy.”
In the Netherlands, one in eight women and one in 1,000 men will develop cancer at some point during their lives. In patients who have had a subtotal mastectomy and who are BRCA-1/2 carriers, there is still an approximate 5% residual risk of breast cancer, said Dr. de Blok.
A literature review conducted by Dr. de Blok and her colleagues revealed 19 cases of breast cancer in transgender women and 13 in transgender men. However, a more general study of incidence and characteristics of breast cancer in transgender people receiving hormone treatment had not been done, said Dr. de Blok, of the VU University Medical Center, Amsterdam.
The investigators examined data for adult transgender people seen at their center from 1991 to 2017 and started on hormone treatment. This clinic, said Dr. de Blok, sees about 95% of the transgender individuals in the Netherlands.
The study was able to capitalize on comprehensive information from national databases and registries. Investigators drew from a national histopathology and cytopathology registry as well as from a national vital statistics database. A comprehensive cancer database was used to establish both reference incidence values for males and females and the number of expected cases within the study group.
In both transgender men and women, exactly 50% of cases were ductal carcinoma, compared to 85% in the group of reference women.
An additional 31% of the breast cancers in transgender women were lobular, 6% were ductal carcinoma in situ (DCIS), and the remainder were of other types. Of the cancers in transgender women, 82% were estrogen receptor positive, 64% were progesterone receptor positive, and 9% were Her2/neu positive.
For transgender men, there were no lobular carcinomas; 25% were DCIS, and 25% were of other types. Half of the cancers were estrogen receptor positive, and half were progesterone receptor positive; 25% were Her2/neu positive, and there was one case of androgen receptor positive breast cancer.
Dr. de Blok explained that their analysis compared the observed cases in both transgender men and women to the expected number of cases for the same number of males and females, yielding two standardized incidence ratios (SIRs) for each transgender group.
For transgender women, the SIR for breast cancer compared with males was 50.9 (95% confidence interval, 30.1-80.9). The SIR compared to females was 0.3 (95% CI, 0.2-0.4). This reflected the expected case number of 0.3 for males and the 58 expected cases for a matched group of females.
For transgender men, the SIR for breast cancer compared with males was 59.8 (95% CI, 19-144.3), while the SIR compared to females was 0.2 (95% CI, 0.1-0.5). The expected cases for a similar group of males would be 0.1, and for females, 18.
In many cases, whether a transgender person receives standardized screening mammogram reminders will depend on which sex is assigned to that individual in insurance and other administrative databases, Mr. de Blok noted. When electronic health records and other databases have a binary system, at-risk individuals may fall through the cracks.
Dr. de Blok reported no conflicts of interest.
SOURCE: de Blok C, et al. ENDO 2018, abstract OR 25-6.
CHICAGO – , said Christel de Blok, MD, sharing results of a Dutch national study.
The study included 3,078 transgender people (2,064 transgender women) who began hormone therapy (HT) at age 18 years or older. The mean age at which transgender women began HT was 33 years; for transgender men, the mean age was 25 years. In all, transgender women in the study had a total of 30,699 person-years of exposure to HT; for transgender men, the figure was 13,155 person-years.
Overall, there were 16 observed cases of breast cancer in transgender women and four in transgender men. After gender-affirming surgery, the transgender women were followed for a median of 146 months, and experienced a median of 193 months of HT. Transgender men who had mastectomies were followed for a median 93 months, and those who had a hysterectomy-oophorectomy were followed for a median 144 months. Transgender men received a median 176 months of HT.
“Breast cancer can still occur after mastectomy in [transgender] men,” Dr. de Blok said at the annual meeting of the Endocrine Society. “What is interesting is that three out of the four cases of breast cancer in [transgender] men happened after mastectomy.”
In the Netherlands, one in eight women and one in 1,000 men will develop cancer at some point during their lives. In patients who have had a subtotal mastectomy and who are BRCA-1/2 carriers, there is still an approximate 5% residual risk of breast cancer, said Dr. de Blok.
A literature review conducted by Dr. de Blok and her colleagues revealed 19 cases of breast cancer in transgender women and 13 in transgender men. However, a more general study of incidence and characteristics of breast cancer in transgender people receiving hormone treatment had not been done, said Dr. de Blok, of the VU University Medical Center, Amsterdam.
The investigators examined data for adult transgender people seen at their center from 1991 to 2017 and started on hormone treatment. This clinic, said Dr. de Blok, sees about 95% of the transgender individuals in the Netherlands.
The study was able to capitalize on comprehensive information from national databases and registries. Investigators drew from a national histopathology and cytopathology registry as well as from a national vital statistics database. A comprehensive cancer database was used to establish both reference incidence values for males and females and the number of expected cases within the study group.
In both transgender men and women, exactly 50% of cases were ductal carcinoma, compared to 85% in the group of reference women.
An additional 31% of the breast cancers in transgender women were lobular, 6% were ductal carcinoma in situ (DCIS), and the remainder were of other types. Of the cancers in transgender women, 82% were estrogen receptor positive, 64% were progesterone receptor positive, and 9% were Her2/neu positive.
For transgender men, there were no lobular carcinomas; 25% were DCIS, and 25% were of other types. Half of the cancers were estrogen receptor positive, and half were progesterone receptor positive; 25% were Her2/neu positive, and there was one case of androgen receptor positive breast cancer.
Dr. de Blok explained that their analysis compared the observed cases in both transgender men and women to the expected number of cases for the same number of males and females, yielding two standardized incidence ratios (SIRs) for each transgender group.
For transgender women, the SIR for breast cancer compared with males was 50.9 (95% confidence interval, 30.1-80.9). The SIR compared to females was 0.3 (95% CI, 0.2-0.4). This reflected the expected case number of 0.3 for males and the 58 expected cases for a matched group of females.
For transgender men, the SIR for breast cancer compared with males was 59.8 (95% CI, 19-144.3), while the SIR compared to females was 0.2 (95% CI, 0.1-0.5). The expected cases for a similar group of males would be 0.1, and for females, 18.
In many cases, whether a transgender person receives standardized screening mammogram reminders will depend on which sex is assigned to that individual in insurance and other administrative databases, Mr. de Blok noted. When electronic health records and other databases have a binary system, at-risk individuals may fall through the cracks.
Dr. de Blok reported no conflicts of interest.
SOURCE: de Blok C, et al. ENDO 2018, abstract OR 25-6.
REPORTING FROM ENDO 2018
Key clinical point: Transgender individuals had increased risk of breast cancer similar to a female reference population.
Major finding: Transgender men had a standardized incidence ratio of 59.8 compared to a male reference population.
Study details: Study of 3,078 transgender adults receiving hormone therapy.
Disclosures: Dr. de Blok reported no conflicts of interest.
Source: de Blok C, et al. ENDO 2018, abstract OR 25-6.