User login
Over one-third report financial burden from breast cancer treatment
CHICAGO – Women who have treatment for breast cancer seldom talk about the costs of care with their medical team, but a study out of Duke University has found that more than one-third reported having a financial burden from their breast cancer treatment, even among women with health insurance, according to a report presented at the Society of Surgical Oncology Annual Cancer Symposium.
“The financial harm associated with cancer treatment is now known as ‘financial toxicity,’ ” Rachel A. Greenup, MD, MPH, said in reporting the results of an 88-item survey completed by 654 adult women who had treatment for breast cancer. The women were recruited through the Army of Women of the Dr. Susan Love Research Foundation and The Sister’s Network of North Carolina, an African-American breast cancer survivors’ organization.
Overall, 69% of survey respondents had private insurance and 26% had Medicare. Of the patients surveyed, 94% had breast cancer surgery: 40.6% lumpectomy, 23.7% mastectomy, and 29.7% bilateral mastectomy; 34% also had breast reconstruction. Among those surveyed, 43% reported considering costs in their treatment decision. Of these, 29% considered costs when making surgical treatment decisions, including 14% who reported that costs were “extremely” important.
Despite the high levels of insurance coverage, 35% of the study participants reported a financial burden resulting from cancer treatment, ranging from “somewhat” burdensome to “catastrophic.” The median out-of-pocket cost for the study participants was $4,000, and 5% exceeded $40,000 in such costs, Dr. Greenup said. “The risk of financial harm and increased out-of-pocket costs to patients differed by surgery type,” with higher financial burdens seen in women who underwent bilateral mastectomy.
Cost was one of many factors survey participants reported considering when making surgical treatment decisions, but the most important factors were the opinions and advice of the medical team and the individual patient’s fear of recurrence. However, in lower-income women, cost factored more significantly in decision making. “In a subset of women who reported an annual income of $45,000 a year or less, cost of treatment gained importance and, interestingly, became more important than many variables we routinely discuss – for example, appearance of the breast, sexuality, avoiding radiation, and breast preservation,” Dr. Greenup said. “An income of $74,000 a year was the tipping point at which women reported incorporating costs into their cancer treatment decisions.”
She added that younger, minority women who did not have Medicare coverage were more likely to consider costs in breast cancer treatment decisions.
Most women surveyed (79%) said they preferred to know their out-of-pocket costs before they begin treatment, Dr. Greenup said, “and 40% believed that we as physicians should be considering out-of-pocket costs while making medical decisions.” However, 78% of those surveyed said they never discussed costs with their cancer team – despite American Society of Clinical Oncologists guidelines, she pointed out – and 35% said their treatment costs were higher than expected.
Dr. Greenup described the study population as “well engaged … with good insurance and strong educational background that likely does not reflect the general population.” The results may not be generalizable. “We expect that in a general cohort of women, our findings would be even more exaggerated,” she said.
The study points out the need to better understand how cost transparency may affect breast cancer treatment decisions, Dr. Greenup said. “As eligible women with breast cancer choose between surgical options, it’s important that we consider the potential risk of financial harm as we guide them through these difficult treatment decisions,” she said.
Dr. Greenup and her study coauthors reported having no financial disclosures.
SOURCE: Greenup RA. SSO 2018, Abstract No. 24.
CHICAGO – Women who have treatment for breast cancer seldom talk about the costs of care with their medical team, but a study out of Duke University has found that more than one-third reported having a financial burden from their breast cancer treatment, even among women with health insurance, according to a report presented at the Society of Surgical Oncology Annual Cancer Symposium.
“The financial harm associated with cancer treatment is now known as ‘financial toxicity,’ ” Rachel A. Greenup, MD, MPH, said in reporting the results of an 88-item survey completed by 654 adult women who had treatment for breast cancer. The women were recruited through the Army of Women of the Dr. Susan Love Research Foundation and The Sister’s Network of North Carolina, an African-American breast cancer survivors’ organization.
Overall, 69% of survey respondents had private insurance and 26% had Medicare. Of the patients surveyed, 94% had breast cancer surgery: 40.6% lumpectomy, 23.7% mastectomy, and 29.7% bilateral mastectomy; 34% also had breast reconstruction. Among those surveyed, 43% reported considering costs in their treatment decision. Of these, 29% considered costs when making surgical treatment decisions, including 14% who reported that costs were “extremely” important.
Despite the high levels of insurance coverage, 35% of the study participants reported a financial burden resulting from cancer treatment, ranging from “somewhat” burdensome to “catastrophic.” The median out-of-pocket cost for the study participants was $4,000, and 5% exceeded $40,000 in such costs, Dr. Greenup said. “The risk of financial harm and increased out-of-pocket costs to patients differed by surgery type,” with higher financial burdens seen in women who underwent bilateral mastectomy.
Cost was one of many factors survey participants reported considering when making surgical treatment decisions, but the most important factors were the opinions and advice of the medical team and the individual patient’s fear of recurrence. However, in lower-income women, cost factored more significantly in decision making. “In a subset of women who reported an annual income of $45,000 a year or less, cost of treatment gained importance and, interestingly, became more important than many variables we routinely discuss – for example, appearance of the breast, sexuality, avoiding radiation, and breast preservation,” Dr. Greenup said. “An income of $74,000 a year was the tipping point at which women reported incorporating costs into their cancer treatment decisions.”
She added that younger, minority women who did not have Medicare coverage were more likely to consider costs in breast cancer treatment decisions.
Most women surveyed (79%) said they preferred to know their out-of-pocket costs before they begin treatment, Dr. Greenup said, “and 40% believed that we as physicians should be considering out-of-pocket costs while making medical decisions.” However, 78% of those surveyed said they never discussed costs with their cancer team – despite American Society of Clinical Oncologists guidelines, she pointed out – and 35% said their treatment costs were higher than expected.
Dr. Greenup described the study population as “well engaged … with good insurance and strong educational background that likely does not reflect the general population.” The results may not be generalizable. “We expect that in a general cohort of women, our findings would be even more exaggerated,” she said.
The study points out the need to better understand how cost transparency may affect breast cancer treatment decisions, Dr. Greenup said. “As eligible women with breast cancer choose between surgical options, it’s important that we consider the potential risk of financial harm as we guide them through these difficult treatment decisions,” she said.
Dr. Greenup and her study coauthors reported having no financial disclosures.
SOURCE: Greenup RA. SSO 2018, Abstract No. 24.
CHICAGO – Women who have treatment for breast cancer seldom talk about the costs of care with their medical team, but a study out of Duke University has found that more than one-third reported having a financial burden from their breast cancer treatment, even among women with health insurance, according to a report presented at the Society of Surgical Oncology Annual Cancer Symposium.
“The financial harm associated with cancer treatment is now known as ‘financial toxicity,’ ” Rachel A. Greenup, MD, MPH, said in reporting the results of an 88-item survey completed by 654 adult women who had treatment for breast cancer. The women were recruited through the Army of Women of the Dr. Susan Love Research Foundation and The Sister’s Network of North Carolina, an African-American breast cancer survivors’ organization.
Overall, 69% of survey respondents had private insurance and 26% had Medicare. Of the patients surveyed, 94% had breast cancer surgery: 40.6% lumpectomy, 23.7% mastectomy, and 29.7% bilateral mastectomy; 34% also had breast reconstruction. Among those surveyed, 43% reported considering costs in their treatment decision. Of these, 29% considered costs when making surgical treatment decisions, including 14% who reported that costs were “extremely” important.
Despite the high levels of insurance coverage, 35% of the study participants reported a financial burden resulting from cancer treatment, ranging from “somewhat” burdensome to “catastrophic.” The median out-of-pocket cost for the study participants was $4,000, and 5% exceeded $40,000 in such costs, Dr. Greenup said. “The risk of financial harm and increased out-of-pocket costs to patients differed by surgery type,” with higher financial burdens seen in women who underwent bilateral mastectomy.
Cost was one of many factors survey participants reported considering when making surgical treatment decisions, but the most important factors were the opinions and advice of the medical team and the individual patient’s fear of recurrence. However, in lower-income women, cost factored more significantly in decision making. “In a subset of women who reported an annual income of $45,000 a year or less, cost of treatment gained importance and, interestingly, became more important than many variables we routinely discuss – for example, appearance of the breast, sexuality, avoiding radiation, and breast preservation,” Dr. Greenup said. “An income of $74,000 a year was the tipping point at which women reported incorporating costs into their cancer treatment decisions.”
She added that younger, minority women who did not have Medicare coverage were more likely to consider costs in breast cancer treatment decisions.
Most women surveyed (79%) said they preferred to know their out-of-pocket costs before they begin treatment, Dr. Greenup said, “and 40% believed that we as physicians should be considering out-of-pocket costs while making medical decisions.” However, 78% of those surveyed said they never discussed costs with their cancer team – despite American Society of Clinical Oncologists guidelines, she pointed out – and 35% said their treatment costs were higher than expected.
Dr. Greenup described the study population as “well engaged … with good insurance and strong educational background that likely does not reflect the general population.” The results may not be generalizable. “We expect that in a general cohort of women, our findings would be even more exaggerated,” she said.
The study points out the need to better understand how cost transparency may affect breast cancer treatment decisions, Dr. Greenup said. “As eligible women with breast cancer choose between surgical options, it’s important that we consider the potential risk of financial harm as we guide them through these difficult treatment decisions,” she said.
Dr. Greenup and her study coauthors reported having no financial disclosures.
SOURCE: Greenup RA. SSO 2018, Abstract No. 24.
REPORTING FROM SSO 2018
Key clinical point: Treatment costs are important to many women with breast cancer, although most report not having cost discussions with their physicians.
Major finding: Despite the high levels of insurance coverage, 35% of study participants reported a financial burden resulting from cancer treatment, ranging from “somewhat” burdensome to “catastrophic.”
Study details: An 88-item survey completed by 654 adult women who had treatment for breast cancer.
Disclosures: Dr. Greenup and her coauthors reported having no financial disclosures.
Source: Greenup RA. SSO 2018, Abstract No. 24.
HDAC inhibition may boost immune therapy efficacy in breast cancer
ORLANDO – The novel combination of entinostat and nivolumab with or without ipilimumab showed encouraging safety, tolerability, and antitumor activity in early results from an ongoing phase 1 trial of patients with advanced breast cancer.
Of 30 patients who were enrolled and treated in the dose-escalation phase of the study as of Feb. 24, 2018, 20 had evaluable responses, and of those, 3 had a partial response for an overall response rate of 15%. An additional 12 had stable disease, and 5 had disease progression, Roisin M. Connolly, MD, reported in a poster at the annual conference of the National Comprehensive Cancer Network.
Responses were seen in all 3 DL1 patients, 12 of 14 DL2 patients, 3 of 4 DL3 patients, and 2 of 9 (with 4 pending first restaging) DL4 patients. Dose-limiting toxicities included one case of pneumonitis at DL2 and an allergic reaction in one DL4 patient, said Dr. Connolly of Johns Hopkins University, Baltimore.
The most common treatment-associated adverse events occurring in 6 or more patients included anemia, fatigue, neutropenia, nausea, and rash, with each occurring in 12 to 22 patients, including grade 3 anemia in 7 patients, grade 3 fatigue in 4 patients, and grade 3 neutropenia in 5 patients. Grade 4 adverse events included lymphopenia in one patient and elevated lipase in one patient, she said.
Possible immune-related adverse events included hypothyroidism in 2 DL2 patients and 3 DL3 patients, hyperthyroidism in 1 DL3 patient, colitis in 1 DL2 and 1 DL3 patient, pneumonitis in 4 DL2 patients, rash in 10 DL2-DL4 patients, and meningoencephalitis and myasthenia gravis in 1 DL3 patient.
Study participants were adults with a mean age of 60 years with metastatic or unresectable solid tumors for which standard treatments did not exist or were no longer effective, or for which treatment with anti–programmed cell death ligand1/cytotoxic T-lymphocyte antigen 4 treatment was appropriate. All had good performance status and adequate organ and pulmonary function, less than 30% liver involvement, and any brain metastases were stable. Those with active autoimmune disease or a history of autoimmune disease that might recur were excluded, as were patients treated within 14 days of enrollment.
“The rationale for the study was based on preclinical work suggesting that epigenetic modifiers might be able to enhance the efficacy of immune therapies, and this would be particularly important for ‘colder’ tumor types like breast cancer that might not have the same sort of responses that we see in other tumor types,” Dr. Connolly explained in an interview. “The lab work suggested, for example, that the [histone deacetylase] inhibitor entinostat might affect myeloid-derived suppressor and regulatory T cells that might prevent cytotoxic T cells from fighting the cancer.”
There may be other mechanisms for this activity as well, she noted.
The run-in period with entinostat alone allowed collection of pre- and posttreatment biopsies to examine the effects on the tissues, such as whether treatment affects T cells, myeloid-derived suppressor cells, or their pathways, she said.
“We’re seeing [the] same types of toxicities seen with combination immune-oncology strategies, and we’re seeing some tumor responses that are of interest. Now we will delve into the tissue biopsies and blood samples we’ve collected to explore the mechanisms in more detail. In the near future we will open our breast cancer expansion cohort to look in more detail at what these drugs might be doing in breast cancer,” she added.
Specifically, she and her colleagues are evaluating the effects of treatment on immune-related biomarkers, measuring tumor-specific mutations and mutant neoantigens recognized by patient T cells in tumor biopsies, evaluating changes in the frequency of T cells recognizing tumor-specific mutant neoantigens in peripheral blood lymphocytes pre- and posttherapy, and looking at epigenetic changes pre- and posttherapy.
These preliminary findings suggest that the combination of entinostat and nivolumab with or without ipilimumab is safe and tolerable, with expected rates of immune-related adverse events, Dr. Connolly said, noting that the recommended phase 2 dose to be used in the dose expansion phase of the study has yet to be determined.
The findings, should they be confirmed as the trial progresses, could have important implications because immune checkpoint inhibitors, which work best in patients with immunogenic cancers that naturally attract T-cell infiltration into their tumor microenvironment, have limited single-agent activity in tumors, such as breast cancer, that are not believed to be immunogenic, she reported. Such cancers have thus far had only modest responses to single-agent immune checkpoint inhibition in advanced triple-negative and HER2+ breast cancer, with overall response rates of 5%-20%.
However, women who do respond to immune checkpoint inhibition tend to have durable and sustainable responses, she said, explaining that suboptimal immune responsiveness is likely a result of a lack of tumor antigen expression and/or recognition, as well as multiple suppressive signals in the tumor microenvironment.
Should the novel strategy tested in this study for converting breast cancers into immune responsive tumors facilitate improved response to immune checkpoint agents, it has the potential to significantly extend survival in breast cancer patients, she concluded.
This study was funded by grants from the National Institutes of Health, Bloomberg Kimmel Institute for Immunotherapy, NCCN, and the Mary Kay Foundation, as well as a V Foundation award. Dr. Connolly reported having no disclosures
SOURCE: Connolly RM et al. NCCN, Poster 3.
ORLANDO – The novel combination of entinostat and nivolumab with or without ipilimumab showed encouraging safety, tolerability, and antitumor activity in early results from an ongoing phase 1 trial of patients with advanced breast cancer.
Of 30 patients who were enrolled and treated in the dose-escalation phase of the study as of Feb. 24, 2018, 20 had evaluable responses, and of those, 3 had a partial response for an overall response rate of 15%. An additional 12 had stable disease, and 5 had disease progression, Roisin M. Connolly, MD, reported in a poster at the annual conference of the National Comprehensive Cancer Network.
Responses were seen in all 3 DL1 patients, 12 of 14 DL2 patients, 3 of 4 DL3 patients, and 2 of 9 (with 4 pending first restaging) DL4 patients. Dose-limiting toxicities included one case of pneumonitis at DL2 and an allergic reaction in one DL4 patient, said Dr. Connolly of Johns Hopkins University, Baltimore.
The most common treatment-associated adverse events occurring in 6 or more patients included anemia, fatigue, neutropenia, nausea, and rash, with each occurring in 12 to 22 patients, including grade 3 anemia in 7 patients, grade 3 fatigue in 4 patients, and grade 3 neutropenia in 5 patients. Grade 4 adverse events included lymphopenia in one patient and elevated lipase in one patient, she said.
Possible immune-related adverse events included hypothyroidism in 2 DL2 patients and 3 DL3 patients, hyperthyroidism in 1 DL3 patient, colitis in 1 DL2 and 1 DL3 patient, pneumonitis in 4 DL2 patients, rash in 10 DL2-DL4 patients, and meningoencephalitis and myasthenia gravis in 1 DL3 patient.
Study participants were adults with a mean age of 60 years with metastatic or unresectable solid tumors for which standard treatments did not exist or were no longer effective, or for which treatment with anti–programmed cell death ligand1/cytotoxic T-lymphocyte antigen 4 treatment was appropriate. All had good performance status and adequate organ and pulmonary function, less than 30% liver involvement, and any brain metastases were stable. Those with active autoimmune disease or a history of autoimmune disease that might recur were excluded, as were patients treated within 14 days of enrollment.
“The rationale for the study was based on preclinical work suggesting that epigenetic modifiers might be able to enhance the efficacy of immune therapies, and this would be particularly important for ‘colder’ tumor types like breast cancer that might not have the same sort of responses that we see in other tumor types,” Dr. Connolly explained in an interview. “The lab work suggested, for example, that the [histone deacetylase] inhibitor entinostat might affect myeloid-derived suppressor and regulatory T cells that might prevent cytotoxic T cells from fighting the cancer.”
There may be other mechanisms for this activity as well, she noted.
The run-in period with entinostat alone allowed collection of pre- and posttreatment biopsies to examine the effects on the tissues, such as whether treatment affects T cells, myeloid-derived suppressor cells, or their pathways, she said.
“We’re seeing [the] same types of toxicities seen with combination immune-oncology strategies, and we’re seeing some tumor responses that are of interest. Now we will delve into the tissue biopsies and blood samples we’ve collected to explore the mechanisms in more detail. In the near future we will open our breast cancer expansion cohort to look in more detail at what these drugs might be doing in breast cancer,” she added.
Specifically, she and her colleagues are evaluating the effects of treatment on immune-related biomarkers, measuring tumor-specific mutations and mutant neoantigens recognized by patient T cells in tumor biopsies, evaluating changes in the frequency of T cells recognizing tumor-specific mutant neoantigens in peripheral blood lymphocytes pre- and posttherapy, and looking at epigenetic changes pre- and posttherapy.
These preliminary findings suggest that the combination of entinostat and nivolumab with or without ipilimumab is safe and tolerable, with expected rates of immune-related adverse events, Dr. Connolly said, noting that the recommended phase 2 dose to be used in the dose expansion phase of the study has yet to be determined.
The findings, should they be confirmed as the trial progresses, could have important implications because immune checkpoint inhibitors, which work best in patients with immunogenic cancers that naturally attract T-cell infiltration into their tumor microenvironment, have limited single-agent activity in tumors, such as breast cancer, that are not believed to be immunogenic, she reported. Such cancers have thus far had only modest responses to single-agent immune checkpoint inhibition in advanced triple-negative and HER2+ breast cancer, with overall response rates of 5%-20%.
However, women who do respond to immune checkpoint inhibition tend to have durable and sustainable responses, she said, explaining that suboptimal immune responsiveness is likely a result of a lack of tumor antigen expression and/or recognition, as well as multiple suppressive signals in the tumor microenvironment.
Should the novel strategy tested in this study for converting breast cancers into immune responsive tumors facilitate improved response to immune checkpoint agents, it has the potential to significantly extend survival in breast cancer patients, she concluded.
This study was funded by grants from the National Institutes of Health, Bloomberg Kimmel Institute for Immunotherapy, NCCN, and the Mary Kay Foundation, as well as a V Foundation award. Dr. Connolly reported having no disclosures
SOURCE: Connolly RM et al. NCCN, Poster 3.
ORLANDO – The novel combination of entinostat and nivolumab with or without ipilimumab showed encouraging safety, tolerability, and antitumor activity in early results from an ongoing phase 1 trial of patients with advanced breast cancer.
Of 30 patients who were enrolled and treated in the dose-escalation phase of the study as of Feb. 24, 2018, 20 had evaluable responses, and of those, 3 had a partial response for an overall response rate of 15%. An additional 12 had stable disease, and 5 had disease progression, Roisin M. Connolly, MD, reported in a poster at the annual conference of the National Comprehensive Cancer Network.
Responses were seen in all 3 DL1 patients, 12 of 14 DL2 patients, 3 of 4 DL3 patients, and 2 of 9 (with 4 pending first restaging) DL4 patients. Dose-limiting toxicities included one case of pneumonitis at DL2 and an allergic reaction in one DL4 patient, said Dr. Connolly of Johns Hopkins University, Baltimore.
The most common treatment-associated adverse events occurring in 6 or more patients included anemia, fatigue, neutropenia, nausea, and rash, with each occurring in 12 to 22 patients, including grade 3 anemia in 7 patients, grade 3 fatigue in 4 patients, and grade 3 neutropenia in 5 patients. Grade 4 adverse events included lymphopenia in one patient and elevated lipase in one patient, she said.
Possible immune-related adverse events included hypothyroidism in 2 DL2 patients and 3 DL3 patients, hyperthyroidism in 1 DL3 patient, colitis in 1 DL2 and 1 DL3 patient, pneumonitis in 4 DL2 patients, rash in 10 DL2-DL4 patients, and meningoencephalitis and myasthenia gravis in 1 DL3 patient.
Study participants were adults with a mean age of 60 years with metastatic or unresectable solid tumors for which standard treatments did not exist or were no longer effective, or for which treatment with anti–programmed cell death ligand1/cytotoxic T-lymphocyte antigen 4 treatment was appropriate. All had good performance status and adequate organ and pulmonary function, less than 30% liver involvement, and any brain metastases were stable. Those with active autoimmune disease or a history of autoimmune disease that might recur were excluded, as were patients treated within 14 days of enrollment.
“The rationale for the study was based on preclinical work suggesting that epigenetic modifiers might be able to enhance the efficacy of immune therapies, and this would be particularly important for ‘colder’ tumor types like breast cancer that might not have the same sort of responses that we see in other tumor types,” Dr. Connolly explained in an interview. “The lab work suggested, for example, that the [histone deacetylase] inhibitor entinostat might affect myeloid-derived suppressor and regulatory T cells that might prevent cytotoxic T cells from fighting the cancer.”
There may be other mechanisms for this activity as well, she noted.
The run-in period with entinostat alone allowed collection of pre- and posttreatment biopsies to examine the effects on the tissues, such as whether treatment affects T cells, myeloid-derived suppressor cells, or their pathways, she said.
“We’re seeing [the] same types of toxicities seen with combination immune-oncology strategies, and we’re seeing some tumor responses that are of interest. Now we will delve into the tissue biopsies and blood samples we’ve collected to explore the mechanisms in more detail. In the near future we will open our breast cancer expansion cohort to look in more detail at what these drugs might be doing in breast cancer,” she added.
Specifically, she and her colleagues are evaluating the effects of treatment on immune-related biomarkers, measuring tumor-specific mutations and mutant neoantigens recognized by patient T cells in tumor biopsies, evaluating changes in the frequency of T cells recognizing tumor-specific mutant neoantigens in peripheral blood lymphocytes pre- and posttherapy, and looking at epigenetic changes pre- and posttherapy.
These preliminary findings suggest that the combination of entinostat and nivolumab with or without ipilimumab is safe and tolerable, with expected rates of immune-related adverse events, Dr. Connolly said, noting that the recommended phase 2 dose to be used in the dose expansion phase of the study has yet to be determined.
The findings, should they be confirmed as the trial progresses, could have important implications because immune checkpoint inhibitors, which work best in patients with immunogenic cancers that naturally attract T-cell infiltration into their tumor microenvironment, have limited single-agent activity in tumors, such as breast cancer, that are not believed to be immunogenic, she reported. Such cancers have thus far had only modest responses to single-agent immune checkpoint inhibition in advanced triple-negative and HER2+ breast cancer, with overall response rates of 5%-20%.
However, women who do respond to immune checkpoint inhibition tend to have durable and sustainable responses, she said, explaining that suboptimal immune responsiveness is likely a result of a lack of tumor antigen expression and/or recognition, as well as multiple suppressive signals in the tumor microenvironment.
Should the novel strategy tested in this study for converting breast cancers into immune responsive tumors facilitate improved response to immune checkpoint agents, it has the potential to significantly extend survival in breast cancer patients, she concluded.
This study was funded by grants from the National Institutes of Health, Bloomberg Kimmel Institute for Immunotherapy, NCCN, and the Mary Kay Foundation, as well as a V Foundation award. Dr. Connolly reported having no disclosures
SOURCE: Connolly RM et al. NCCN, Poster 3.
REPORTING FROM THE NCCN ANNUAL CONFERENCE
Key clinical point:
Major finding: Three patients had a partial response, 12 had stable disease, 5 progressed.
Study details: A phase 1 dose-expansion study involving 30 patients.
Disclosures: This study was funded by grants from the National Institutes of Health, Bloomberg Kimmel Institute for Immunotherapy, NCCN, and the Mary Kay Foundation, and by a V Foundation award. Dr. Connolly reported having no disclosures.
Source: Connolly RM et al. NCCN, Poster 3.
Late toxicities with PARP inhibitor plus RT in inflammatory breast cancer
Using the PARP inhibitor veliparib as a radiosensitizer for chest wall radiation in women with inflammatory or locally recurrent breast cancer was associated with a high rate of late grade 3 adverse events, results of a phase 1 study show.
Although the trial’s upper limit of dose-limiting toxicities during 6 weeks of treatment and 4 weeks of follow-up was not met, 46.7% of 30 patients treated with veliparib and radiation after complete surgical resection had at least one grade 3 adverse event by 3 years of follow-up, reported Reshma Jagsi, MD, of the University of Michigan, Ann Arbor.
“In this multicenter phase 1 trial, severe acute toxicity did not exceed the prespecified target of 30%, even at the highest tested dose of veliparib (200 mg twice a day), and we observed no grade 4 or 5 events. However, given observations of grade 3 late toxicity in nearly one-half of all patients evaluated at 3 years, we recommend a phase 2 dose of 50 mg twice a day if veliparib is investigated further for radiosensitization in patients with breast cancer at high risk of locoregional recurrence and in need of treatment intensification,” they wrote in the Journal of Clinical Oncology.
In preclinical studies, PARP (poly [ADP-ribose] polymerase) inhibitors have been shown to enhance radiosensitivty of breast malignancies when given concurrently with radiation.
In a phase 1 dosing and safety study, 30 women with inflammatory or locally recurrent breast cancer of the chest wall underwent complete surgical resection and were then assigned to radiation consisting of 50 Gy to the chest wall and regional lymph nodes, plus a 10 Gy boost. The patients also received oral veliparib at a dose of either 50, 100, 150, or 200 mg taken twice daily during the 6-week course of radiotherapy.
During the 6 weeks of therapy and 4 weeks of follow-up, there were five dose-limiting toxicities, including two cases each of confluent moist desquamation greater than 100 cm2 in the 100- and 150-mg dose groups, and one case of neutropenia in a patient at the 200-mg dose level.
The respective rates of any grade 3 toxicity, treatment related or otherwise, at 1, 2, and 3 years of follow-up were 10%, 16.7%, and 46.7%.
The investigators noted that, at year 3, severe fibrosis in the treatment field was seen in 6 of the 15 surviving patients. Of the six patients, two also had grade 3 skin induration, and two had grade 3 lymphedema.
“Although some of the late adverse events we observed might have occurred even in the absence of the investigational agent and with standard therapy, severe late toxicity is relatively uncommon with standard therapy alone, so we believe that a cautious approach is prudent,” Dr. Jagsi and associates wrote.
The study was supported by the Translational Breast Cancer Research Consortium, Breast Cancer Research Foundation, University of Michigan Comprehensive Cancer Center, and Michigan Institute for Clinical and Health Research. Dr. Jagsi reported institutional research support from AbbVie, which donated the veliparib used in the study.
SOURCE: Jagsi R et al. J Clin Oncol. 2018 Mar 20. doi: 10.1200/JCO.2017.77.2665
Using the PARP inhibitor veliparib as a radiosensitizer for chest wall radiation in women with inflammatory or locally recurrent breast cancer was associated with a high rate of late grade 3 adverse events, results of a phase 1 study show.
Although the trial’s upper limit of dose-limiting toxicities during 6 weeks of treatment and 4 weeks of follow-up was not met, 46.7% of 30 patients treated with veliparib and radiation after complete surgical resection had at least one grade 3 adverse event by 3 years of follow-up, reported Reshma Jagsi, MD, of the University of Michigan, Ann Arbor.
“In this multicenter phase 1 trial, severe acute toxicity did not exceed the prespecified target of 30%, even at the highest tested dose of veliparib (200 mg twice a day), and we observed no grade 4 or 5 events. However, given observations of grade 3 late toxicity in nearly one-half of all patients evaluated at 3 years, we recommend a phase 2 dose of 50 mg twice a day if veliparib is investigated further for radiosensitization in patients with breast cancer at high risk of locoregional recurrence and in need of treatment intensification,” they wrote in the Journal of Clinical Oncology.
In preclinical studies, PARP (poly [ADP-ribose] polymerase) inhibitors have been shown to enhance radiosensitivty of breast malignancies when given concurrently with radiation.
In a phase 1 dosing and safety study, 30 women with inflammatory or locally recurrent breast cancer of the chest wall underwent complete surgical resection and were then assigned to radiation consisting of 50 Gy to the chest wall and regional lymph nodes, plus a 10 Gy boost. The patients also received oral veliparib at a dose of either 50, 100, 150, or 200 mg taken twice daily during the 6-week course of radiotherapy.
During the 6 weeks of therapy and 4 weeks of follow-up, there were five dose-limiting toxicities, including two cases each of confluent moist desquamation greater than 100 cm2 in the 100- and 150-mg dose groups, and one case of neutropenia in a patient at the 200-mg dose level.
The respective rates of any grade 3 toxicity, treatment related or otherwise, at 1, 2, and 3 years of follow-up were 10%, 16.7%, and 46.7%.
The investigators noted that, at year 3, severe fibrosis in the treatment field was seen in 6 of the 15 surviving patients. Of the six patients, two also had grade 3 skin induration, and two had grade 3 lymphedema.
“Although some of the late adverse events we observed might have occurred even in the absence of the investigational agent and with standard therapy, severe late toxicity is relatively uncommon with standard therapy alone, so we believe that a cautious approach is prudent,” Dr. Jagsi and associates wrote.
The study was supported by the Translational Breast Cancer Research Consortium, Breast Cancer Research Foundation, University of Michigan Comprehensive Cancer Center, and Michigan Institute for Clinical and Health Research. Dr. Jagsi reported institutional research support from AbbVie, which donated the veliparib used in the study.
SOURCE: Jagsi R et al. J Clin Oncol. 2018 Mar 20. doi: 10.1200/JCO.2017.77.2665
Using the PARP inhibitor veliparib as a radiosensitizer for chest wall radiation in women with inflammatory or locally recurrent breast cancer was associated with a high rate of late grade 3 adverse events, results of a phase 1 study show.
Although the trial’s upper limit of dose-limiting toxicities during 6 weeks of treatment and 4 weeks of follow-up was not met, 46.7% of 30 patients treated with veliparib and radiation after complete surgical resection had at least one grade 3 adverse event by 3 years of follow-up, reported Reshma Jagsi, MD, of the University of Michigan, Ann Arbor.
“In this multicenter phase 1 trial, severe acute toxicity did not exceed the prespecified target of 30%, even at the highest tested dose of veliparib (200 mg twice a day), and we observed no grade 4 or 5 events. However, given observations of grade 3 late toxicity in nearly one-half of all patients evaluated at 3 years, we recommend a phase 2 dose of 50 mg twice a day if veliparib is investigated further for radiosensitization in patients with breast cancer at high risk of locoregional recurrence and in need of treatment intensification,” they wrote in the Journal of Clinical Oncology.
In preclinical studies, PARP (poly [ADP-ribose] polymerase) inhibitors have been shown to enhance radiosensitivty of breast malignancies when given concurrently with radiation.
In a phase 1 dosing and safety study, 30 women with inflammatory or locally recurrent breast cancer of the chest wall underwent complete surgical resection and were then assigned to radiation consisting of 50 Gy to the chest wall and regional lymph nodes, plus a 10 Gy boost. The patients also received oral veliparib at a dose of either 50, 100, 150, or 200 mg taken twice daily during the 6-week course of radiotherapy.
During the 6 weeks of therapy and 4 weeks of follow-up, there were five dose-limiting toxicities, including two cases each of confluent moist desquamation greater than 100 cm2 in the 100- and 150-mg dose groups, and one case of neutropenia in a patient at the 200-mg dose level.
The respective rates of any grade 3 toxicity, treatment related or otherwise, at 1, 2, and 3 years of follow-up were 10%, 16.7%, and 46.7%.
The investigators noted that, at year 3, severe fibrosis in the treatment field was seen in 6 of the 15 surviving patients. Of the six patients, two also had grade 3 skin induration, and two had grade 3 lymphedema.
“Although some of the late adverse events we observed might have occurred even in the absence of the investigational agent and with standard therapy, severe late toxicity is relatively uncommon with standard therapy alone, so we believe that a cautious approach is prudent,” Dr. Jagsi and associates wrote.
The study was supported by the Translational Breast Cancer Research Consortium, Breast Cancer Research Foundation, University of Michigan Comprehensive Cancer Center, and Michigan Institute for Clinical and Health Research. Dr. Jagsi reported institutional research support from AbbVie, which donated the veliparib used in the study.
SOURCE: Jagsi R et al. J Clin Oncol. 2018 Mar 20. doi: 10.1200/JCO.2017.77.2665
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: PARP inhibitors have a radiosensitizing effect when used in treatment of inflammatory breast cancer but are associated with late grade 3 adverse events.
Major finding: At 3 years, 46.7% of patients had a grade 3 adverse event of any kind.
Study details: Phase 1 dose-finding and safety study in 30 women treated with radiation and veliparib after complete surgical resection of inflammatory or recurrent breast cancer of the chest wall and regional lymph nodes.
Disclosures: The study was supported by the Translational Breast Cancer Research Consortium, Breast Cancer Research Foundation, University of Michigan Comprehensive Cancer Center, and Michigan Institute for Clinical and Health Research. Dr. Jagsi reported institutional research support from AbbVie, which donated the veliparib used in the study.
Source: Jagsi R et al. J Clin Oncol. 2018 Mar 20. doi: 10.1200/JCO.2017.77.2665.
Accelerated breast irradiation advocated by ASTRO guideline
Hypofractionation is the preferred means of giving whole breast irradiation to women with invasive breast cancer, according to updated guidance from the American Society for Radiation Oncology.
A dose of 4,000 cGy given in 15 fractions or 4,250 cGy in 16 fractions is recommended, with or without inclusion of the low axilla, and regardless of a variety of factors such as tumor grade, prior chemotherapy, and patient age.
“Previously, accelerated treatment was recommended only for certain patients, including older patients and those with less advanced disease,” Benjamin Smith, MD, one of the cochairs of the guideline task force, said in an ASTRO news release.
Dr. Smith, of the University of Texas MD Anderson Cancer Center, Houston, added that recent long-term data from several large trials “strongly support the safety and efficacy of accelerated treatment for most breast cancer patients.”
Treatment decisions and plans still need to be individualized, but the updated ASTRO guidance notes that whole breast irradiation (WBI) can be offered to most women with invasive breast cancer independent of breast size and whether or not the cancer is in the left or right breast, provided that homogeneous dosing can be achieved. Hormone receptor, HER2 status, and postsurgical margin status also appear not to matter.
Historically, conventional fractionation (CF) with or without a tumor bed boost was used for WBI, Dr. Smith and associates wrote in the guidelines, which were published online in Practical Radiation Oncology. This consisted of daily doses of 180-200 cGy for a total dose of 4,500-5,000 cGy.
“Recognizing the limitations of CF for convenience and cost, randomized trials in the 1990s and 2000s investigated if moderate hypofractionation [HF], defined as daily doses of 265-330 cGy, could yield oncologic and functional/cosmetic outcomes similar to CF-WBI,” they said.
Initial results of these trials “supported the safety and effectiveness of HF-WBI” and were then used to form ASTRO’s 2011 guideline on dose fractionation for WBI. With longer term data from these trials now available, it was time to review the evidence again. A systematic literature review was thus conducted to identify all relevant studies published during 2009-2016, and 100 articles met the task force criteria and were used to create the updated guideline.
Aside from the delivery and dosing of WBI, other key recommendations look at the use of a radiation boost to the tumor bed, and preferred techniques for treatment planning.
With regards to a radiation boost, this needs to be considered on an individual basis but can be independent of any previous WBI. A radiation boost is recommended if patients have any grade invasive cancer and are aged 50 years or younger, have a high-grade tumor and are aged 51-70 years, or if there is a positive margin following surgery. A radiation boost also is recommended in women with ductal carcinoma in situ if they are aged 50 years or younger, have a high-grade tumor, and positive or close postsurgical margins.
As for treatment planning, 3-dimensional conformal treatment planning with a “field-in-field” technique is recommended as the initial approach. This is to minimize the volume of breast tissue that receives more than 105% of the radiation dose. The guideline also covers optimal patient positioning and how to avoid nearby tissues and organs, such as the heart, lungs and contralateral breast.
ASTRO hopes that the updated guideline will increase the use of hypofractionation, which has been reportedly low in recent years, with as few as 35% of eligible patients received hypofractionation in one study (JAMA. 2014;312[23]:2542-50).
“We hope that this guideline encourages providers to counsel their patients on options including hypofractionation,” said Reshma Jagsi, MD, DPhil, professor of radiation oncology at the University of Michigan, Ann Arbor, who cochaired the guideline task force with Dr. Smith.
“Hypofractionated radiation therapy offers patients a more convenient and lower cost option for their treatment without compromising the likelihood that their cancer will return or increasing their risk of side effects,” Dr. Jagsi noted. Furthermore, “a shorter course of radiation equates to more time with family, less time away from work and lower treatment costs.”
SOURCE: Smith BD et al. Pract Radiat Oncol. 2018 March 12. doi: 10.1016/j.prro.2018.01.012.
Hypofractionation is the preferred means of giving whole breast irradiation to women with invasive breast cancer, according to updated guidance from the American Society for Radiation Oncology.
A dose of 4,000 cGy given in 15 fractions or 4,250 cGy in 16 fractions is recommended, with or without inclusion of the low axilla, and regardless of a variety of factors such as tumor grade, prior chemotherapy, and patient age.
“Previously, accelerated treatment was recommended only for certain patients, including older patients and those with less advanced disease,” Benjamin Smith, MD, one of the cochairs of the guideline task force, said in an ASTRO news release.
Dr. Smith, of the University of Texas MD Anderson Cancer Center, Houston, added that recent long-term data from several large trials “strongly support the safety and efficacy of accelerated treatment for most breast cancer patients.”
Treatment decisions and plans still need to be individualized, but the updated ASTRO guidance notes that whole breast irradiation (WBI) can be offered to most women with invasive breast cancer independent of breast size and whether or not the cancer is in the left or right breast, provided that homogeneous dosing can be achieved. Hormone receptor, HER2 status, and postsurgical margin status also appear not to matter.
Historically, conventional fractionation (CF) with or without a tumor bed boost was used for WBI, Dr. Smith and associates wrote in the guidelines, which were published online in Practical Radiation Oncology. This consisted of daily doses of 180-200 cGy for a total dose of 4,500-5,000 cGy.
“Recognizing the limitations of CF for convenience and cost, randomized trials in the 1990s and 2000s investigated if moderate hypofractionation [HF], defined as daily doses of 265-330 cGy, could yield oncologic and functional/cosmetic outcomes similar to CF-WBI,” they said.
Initial results of these trials “supported the safety and effectiveness of HF-WBI” and were then used to form ASTRO’s 2011 guideline on dose fractionation for WBI. With longer term data from these trials now available, it was time to review the evidence again. A systematic literature review was thus conducted to identify all relevant studies published during 2009-2016, and 100 articles met the task force criteria and were used to create the updated guideline.
Aside from the delivery and dosing of WBI, other key recommendations look at the use of a radiation boost to the tumor bed, and preferred techniques for treatment planning.
With regards to a radiation boost, this needs to be considered on an individual basis but can be independent of any previous WBI. A radiation boost is recommended if patients have any grade invasive cancer and are aged 50 years or younger, have a high-grade tumor and are aged 51-70 years, or if there is a positive margin following surgery. A radiation boost also is recommended in women with ductal carcinoma in situ if they are aged 50 years or younger, have a high-grade tumor, and positive or close postsurgical margins.
As for treatment planning, 3-dimensional conformal treatment planning with a “field-in-field” technique is recommended as the initial approach. This is to minimize the volume of breast tissue that receives more than 105% of the radiation dose. The guideline also covers optimal patient positioning and how to avoid nearby tissues and organs, such as the heart, lungs and contralateral breast.
ASTRO hopes that the updated guideline will increase the use of hypofractionation, which has been reportedly low in recent years, with as few as 35% of eligible patients received hypofractionation in one study (JAMA. 2014;312[23]:2542-50).
“We hope that this guideline encourages providers to counsel their patients on options including hypofractionation,” said Reshma Jagsi, MD, DPhil, professor of radiation oncology at the University of Michigan, Ann Arbor, who cochaired the guideline task force with Dr. Smith.
“Hypofractionated radiation therapy offers patients a more convenient and lower cost option for their treatment without compromising the likelihood that their cancer will return or increasing their risk of side effects,” Dr. Jagsi noted. Furthermore, “a shorter course of radiation equates to more time with family, less time away from work and lower treatment costs.”
SOURCE: Smith BD et al. Pract Radiat Oncol. 2018 March 12. doi: 10.1016/j.prro.2018.01.012.
Hypofractionation is the preferred means of giving whole breast irradiation to women with invasive breast cancer, according to updated guidance from the American Society for Radiation Oncology.
A dose of 4,000 cGy given in 15 fractions or 4,250 cGy in 16 fractions is recommended, with or without inclusion of the low axilla, and regardless of a variety of factors such as tumor grade, prior chemotherapy, and patient age.
“Previously, accelerated treatment was recommended only for certain patients, including older patients and those with less advanced disease,” Benjamin Smith, MD, one of the cochairs of the guideline task force, said in an ASTRO news release.
Dr. Smith, of the University of Texas MD Anderson Cancer Center, Houston, added that recent long-term data from several large trials “strongly support the safety and efficacy of accelerated treatment for most breast cancer patients.”
Treatment decisions and plans still need to be individualized, but the updated ASTRO guidance notes that whole breast irradiation (WBI) can be offered to most women with invasive breast cancer independent of breast size and whether or not the cancer is in the left or right breast, provided that homogeneous dosing can be achieved. Hormone receptor, HER2 status, and postsurgical margin status also appear not to matter.
Historically, conventional fractionation (CF) with or without a tumor bed boost was used for WBI, Dr. Smith and associates wrote in the guidelines, which were published online in Practical Radiation Oncology. This consisted of daily doses of 180-200 cGy for a total dose of 4,500-5,000 cGy.
“Recognizing the limitations of CF for convenience and cost, randomized trials in the 1990s and 2000s investigated if moderate hypofractionation [HF], defined as daily doses of 265-330 cGy, could yield oncologic and functional/cosmetic outcomes similar to CF-WBI,” they said.
Initial results of these trials “supported the safety and effectiveness of HF-WBI” and were then used to form ASTRO’s 2011 guideline on dose fractionation for WBI. With longer term data from these trials now available, it was time to review the evidence again. A systematic literature review was thus conducted to identify all relevant studies published during 2009-2016, and 100 articles met the task force criteria and were used to create the updated guideline.
Aside from the delivery and dosing of WBI, other key recommendations look at the use of a radiation boost to the tumor bed, and preferred techniques for treatment planning.
With regards to a radiation boost, this needs to be considered on an individual basis but can be independent of any previous WBI. A radiation boost is recommended if patients have any grade invasive cancer and are aged 50 years or younger, have a high-grade tumor and are aged 51-70 years, or if there is a positive margin following surgery. A radiation boost also is recommended in women with ductal carcinoma in situ if they are aged 50 years or younger, have a high-grade tumor, and positive or close postsurgical margins.
As for treatment planning, 3-dimensional conformal treatment planning with a “field-in-field” technique is recommended as the initial approach. This is to minimize the volume of breast tissue that receives more than 105% of the radiation dose. The guideline also covers optimal patient positioning and how to avoid nearby tissues and organs, such as the heart, lungs and contralateral breast.
ASTRO hopes that the updated guideline will increase the use of hypofractionation, which has been reportedly low in recent years, with as few as 35% of eligible patients received hypofractionation in one study (JAMA. 2014;312[23]:2542-50).
“We hope that this guideline encourages providers to counsel their patients on options including hypofractionation,” said Reshma Jagsi, MD, DPhil, professor of radiation oncology at the University of Michigan, Ann Arbor, who cochaired the guideline task force with Dr. Smith.
“Hypofractionated radiation therapy offers patients a more convenient and lower cost option for their treatment without compromising the likelihood that their cancer will return or increasing their risk of side effects,” Dr. Jagsi noted. Furthermore, “a shorter course of radiation equates to more time with family, less time away from work and lower treatment costs.”
SOURCE: Smith BD et al. Pract Radiat Oncol. 2018 March 12. doi: 10.1016/j.prro.2018.01.012.
FROM PRACTICAL RADIATION ONCOLOGY
Key clinical point: For invasive cancer, the preferred scheme is hypofractionated whole breast irradiation (HF-WBI).
Major finding: HF-WBI should be given to a total dose of 4,000 cGy in 15 fractions or 4,250 cGy in 16 fractions.
Study details: A systematic literature review of all relevant studies published during 2009-2016.
Disclosures: The guidelines were sponsored by the American Society for Radiation Oncology.
Source: Smith BD et al. Pract Radiat Oncol. 2018 March 12. doi: 10.1016/j.prro.2018.01.012.
Fulvestrant plus neratinib reversed treatment-acquired HER2 mutations in metastatic ER+ breast cancer
Dual therapy with fulvestrant and the irreversible HER2 kinase inhibitor neratinib reversed treatment-acquired hormone resistance in metastatic estrogen receptor (ER)–positive breast cancer cells.
Elaine Mardis, PhD, a spokesperson for the American Association of Cancer Research, hailed the research by Utthara Nayar, PhD, and colleagues as “groundbreaking and unexpected” during a briefing held in advance of the annual meeting of the American Association for Cancer Research. The lab experiments were part of a whole-exome sequencing study of metastatic ER-positive tumor biopsies from 168 patients, 12 of whom had acquired the HER2 mutations, said Dr. Nayar of the Dana-Farber Cancer Institute, Boston.
The findings have prompted a phase 2 trial of the combination, which is now recruiting patients, Dr. Nayar said. The 5-year study seeks 152 women with inoperable locally advanced or metastatic ER-positive breast cancer with a confirmed HER2-positive mutation. Patients will be randomized to the combination of neratinib and fulvestrant or to neratinib alone. The primary outcome is progression-free survival.
“We also hope to be able to develop upfront combinations to preempt the resistance and lead to more durable responses,” Dr. Nayar said.
All of the 168 patients who contributed metastatic tumor biopsy samples to the study had developed resistance to estrogen receptor treatments, including aromatase inhibitors, tamoxifen, and fulvestrant. Of these biopsies, 12 had HER2 mutations, 8 of which had been previously characterized as activating.
Dr. Nayar and colleagues examined the untreated primary tumors in five of these patients; there was no mutation in four, suggesting that the mutations were a response to treatment. “In these 80%, the mutations were acquired as tumors were exposed to treatment and not present in the original tumor,” Dr. Nayar said.
These acquired HER2 mutations were mutually exclusive with ER mutations, which suggested a different mechanism of resistance to ER-directed therapies, she noted in her abstract. The mutations conferred resistance to tamoxifen, fulvestrant, and palbociclib.
However, the combination of fulvestrant and neratinib, an irreversible HER2 kinase inhibitor, overcame resistance in these cells.
In addition to pioneering a potentially important therapy for treatment-resistant metastatic breast cancer, the study highlights the importance of gene sequencing metastatic tumors, said Nikhil Wagle, MD, Dr. Nayar’s colleague and deputy director of the Center for Cancer Precision Medicine at Dana-Farber.
“Our study highlights how important it is to profile resistant metastatic tumors since these tumors may harbor targetable mechanisms of resistance that were not present in the original tumor biopsy,” Dr. Wagle noted in a press statement. “Repeated sequencing of tumors can pinpoint new genetic changes that cause resistance to therapies. This in turn can enable physicians to personalize therapy depending on the specific genetic changes in a patient’s tumor over time.”
The study was supported by the Department of Defense, the National Cancer Institute, the Susan G. Komen Foundation, the Dana-Farber Cancer Center, and a number of other private funders. Dr. Wagle is a stockholder in Foundation Medicine. Dr. Nayar had no financial disclosure.
SOURCE: Nayer U et al. AACR 2018, Abstract 4952
Dual therapy with fulvestrant and the irreversible HER2 kinase inhibitor neratinib reversed treatment-acquired hormone resistance in metastatic estrogen receptor (ER)–positive breast cancer cells.
Elaine Mardis, PhD, a spokesperson for the American Association of Cancer Research, hailed the research by Utthara Nayar, PhD, and colleagues as “groundbreaking and unexpected” during a briefing held in advance of the annual meeting of the American Association for Cancer Research. The lab experiments were part of a whole-exome sequencing study of metastatic ER-positive tumor biopsies from 168 patients, 12 of whom had acquired the HER2 mutations, said Dr. Nayar of the Dana-Farber Cancer Institute, Boston.
The findings have prompted a phase 2 trial of the combination, which is now recruiting patients, Dr. Nayar said. The 5-year study seeks 152 women with inoperable locally advanced or metastatic ER-positive breast cancer with a confirmed HER2-positive mutation. Patients will be randomized to the combination of neratinib and fulvestrant or to neratinib alone. The primary outcome is progression-free survival.
“We also hope to be able to develop upfront combinations to preempt the resistance and lead to more durable responses,” Dr. Nayar said.
All of the 168 patients who contributed metastatic tumor biopsy samples to the study had developed resistance to estrogen receptor treatments, including aromatase inhibitors, tamoxifen, and fulvestrant. Of these biopsies, 12 had HER2 mutations, 8 of which had been previously characterized as activating.
Dr. Nayar and colleagues examined the untreated primary tumors in five of these patients; there was no mutation in four, suggesting that the mutations were a response to treatment. “In these 80%, the mutations were acquired as tumors were exposed to treatment and not present in the original tumor,” Dr. Nayar said.
These acquired HER2 mutations were mutually exclusive with ER mutations, which suggested a different mechanism of resistance to ER-directed therapies, she noted in her abstract. The mutations conferred resistance to tamoxifen, fulvestrant, and palbociclib.
However, the combination of fulvestrant and neratinib, an irreversible HER2 kinase inhibitor, overcame resistance in these cells.
In addition to pioneering a potentially important therapy for treatment-resistant metastatic breast cancer, the study highlights the importance of gene sequencing metastatic tumors, said Nikhil Wagle, MD, Dr. Nayar’s colleague and deputy director of the Center for Cancer Precision Medicine at Dana-Farber.
“Our study highlights how important it is to profile resistant metastatic tumors since these tumors may harbor targetable mechanisms of resistance that were not present in the original tumor biopsy,” Dr. Wagle noted in a press statement. “Repeated sequencing of tumors can pinpoint new genetic changes that cause resistance to therapies. This in turn can enable physicians to personalize therapy depending on the specific genetic changes in a patient’s tumor over time.”
The study was supported by the Department of Defense, the National Cancer Institute, the Susan G. Komen Foundation, the Dana-Farber Cancer Center, and a number of other private funders. Dr. Wagle is a stockholder in Foundation Medicine. Dr. Nayar had no financial disclosure.
SOURCE: Nayer U et al. AACR 2018, Abstract 4952
Dual therapy with fulvestrant and the irreversible HER2 kinase inhibitor neratinib reversed treatment-acquired hormone resistance in metastatic estrogen receptor (ER)–positive breast cancer cells.
Elaine Mardis, PhD, a spokesperson for the American Association of Cancer Research, hailed the research by Utthara Nayar, PhD, and colleagues as “groundbreaking and unexpected” during a briefing held in advance of the annual meeting of the American Association for Cancer Research. The lab experiments were part of a whole-exome sequencing study of metastatic ER-positive tumor biopsies from 168 patients, 12 of whom had acquired the HER2 mutations, said Dr. Nayar of the Dana-Farber Cancer Institute, Boston.
The findings have prompted a phase 2 trial of the combination, which is now recruiting patients, Dr. Nayar said. The 5-year study seeks 152 women with inoperable locally advanced or metastatic ER-positive breast cancer with a confirmed HER2-positive mutation. Patients will be randomized to the combination of neratinib and fulvestrant or to neratinib alone. The primary outcome is progression-free survival.
“We also hope to be able to develop upfront combinations to preempt the resistance and lead to more durable responses,” Dr. Nayar said.
All of the 168 patients who contributed metastatic tumor biopsy samples to the study had developed resistance to estrogen receptor treatments, including aromatase inhibitors, tamoxifen, and fulvestrant. Of these biopsies, 12 had HER2 mutations, 8 of which had been previously characterized as activating.
Dr. Nayar and colleagues examined the untreated primary tumors in five of these patients; there was no mutation in four, suggesting that the mutations were a response to treatment. “In these 80%, the mutations were acquired as tumors were exposed to treatment and not present in the original tumor,” Dr. Nayar said.
These acquired HER2 mutations were mutually exclusive with ER mutations, which suggested a different mechanism of resistance to ER-directed therapies, she noted in her abstract. The mutations conferred resistance to tamoxifen, fulvestrant, and palbociclib.
However, the combination of fulvestrant and neratinib, an irreversible HER2 kinase inhibitor, overcame resistance in these cells.
In addition to pioneering a potentially important therapy for treatment-resistant metastatic breast cancer, the study highlights the importance of gene sequencing metastatic tumors, said Nikhil Wagle, MD, Dr. Nayar’s colleague and deputy director of the Center for Cancer Precision Medicine at Dana-Farber.
“Our study highlights how important it is to profile resistant metastatic tumors since these tumors may harbor targetable mechanisms of resistance that were not present in the original tumor biopsy,” Dr. Wagle noted in a press statement. “Repeated sequencing of tumors can pinpoint new genetic changes that cause resistance to therapies. This in turn can enable physicians to personalize therapy depending on the specific genetic changes in a patient’s tumor over time.”
The study was supported by the Department of Defense, the National Cancer Institute, the Susan G. Komen Foundation, the Dana-Farber Cancer Center, and a number of other private funders. Dr. Wagle is a stockholder in Foundation Medicine. Dr. Nayar had no financial disclosure.
SOURCE: Nayer U et al. AACR 2018, Abstract 4952
FROM THE AACR 2018 ANNUAL MEETING
Key clinical point: The combination of fulvestrant and neratinib reversed acquired HER2 mutations in ER+ metastatic breast cancer cells.
Major finding: Of 168 biopsies, 12 had acquired HER2 mutations after hormone treatment; these mutations were reversed with the dual therapy.
Study details: The exome sequencing study comprised 168 biopsies, and the in vitro study comprised 12.
Disclosures: The study was supported by the Department of Defense, the National Cancer Institute, the Susan G. Komen Foundation, the Dana-Farber Cancer Institute, and other private funders. Dr. Wagle is a stockholder in Foundation Medicine. Dr. Nayar had no financial disclosure.
Source: Nayar U et al. AACR 2018, Abstract 4952
Possible increased breast cancer risk found in women with schizophrenia
A meta-analysis has found an increased risk of breast cancer in women with schizophrenia, but its authors noted significant diversity of results across the included studies.
In the meta-analysis, Chuanjun Zhuo, MD, PhD, and Patrick Todd Triplett, MD, presented the results of 12 cohort studies involving 125,760 women that showed the risk of breast cancer in women with schizophrenia, compared with the general population.
They found that women with schizophrenia had a 31% higher standardized incidence ratio of breast cancer (95% confidence interval, 1.14-1.50; P less than .001). However, significant heterogeneity was found between studies, with the prediction interval ranging from 0.81 to 2.10. The report was published in JAMA Psychiatry.
“Accordingly, it is possible that a future study will show a decreased breast cancer risk in women with schizophrenia compared with the general population,” said Dr. Zhuo of Tianjin Medical University, China, and Dr. Triplett, of Johns Hopkins University, Baltimore.
As it turns out, one of the subgroup analyses showed that the association between schizophrenia and breast cancer was significant only in studies that excluded women who were diagnosed with breast cancer before they were diagnosed with schizophrenia (standardized incidence ratio, 1.34; 95% CI, 1.20-1.51; P less than .001).
The same was seen in studies where there were more than 100 cases of breast cancer (SIR, 1.31; 95% CI, 1.18-1.46; P less than .001), while the association was not significant in studies with fewer than 100 cases.
The authors said their findings contradict a hypothesis that schizophrenia might be protective against cancer.
“These results, together with our recent meta-analysis results showing no association with lung cancer risk but a reduced hepatic cancer risk in schizophrenia, indicated that the association between schizophrenia and cancer risk may be complicated and depend on the cancer site,” wrote Dr. Zhuo and Dr. Triplett.
In terms of possible mechanisms underlying the increased risk of breast cancer seen in this study, the authors suggested that people with schizophrenia could experience other clinical conditions such as obesity that might increase their risk of breast cancer.
“As breast cancer may be a hormone-dependent cancer, a significant positive association between plasma prolactin levels and the risk of breast cancer has been observed; in addition, increased prolactin levels have been documented in women with schizophrenia, particularly for those receiving certain antipsychotics,” they wrote.
While the incidence of cancer in people with schizophrenia might not necessarily differ from that of the general population, the authors said studies have found that people with schizophrenia have higher cancer mortality. Because “breast cancer prevention and treatment options are less optimal in women with schizophrenia, our results highlight that women with schizophrenia deserve focused care for breast cancer screening and treatment,” they wrote.
The Tianjin Health Bureau Foundation and the Natural Science Foundation of Tianjin, China, supported the study. No conflicts of interest were declared.
SOURCE: Zhuo C et al. JAMA Psychiatry. 2018 Mar 7. doi: 10.1001/jamapsychiatry.2017.4748.
A meta-analysis has found an increased risk of breast cancer in women with schizophrenia, but its authors noted significant diversity of results across the included studies.
In the meta-analysis, Chuanjun Zhuo, MD, PhD, and Patrick Todd Triplett, MD, presented the results of 12 cohort studies involving 125,760 women that showed the risk of breast cancer in women with schizophrenia, compared with the general population.
They found that women with schizophrenia had a 31% higher standardized incidence ratio of breast cancer (95% confidence interval, 1.14-1.50; P less than .001). However, significant heterogeneity was found between studies, with the prediction interval ranging from 0.81 to 2.10. The report was published in JAMA Psychiatry.
“Accordingly, it is possible that a future study will show a decreased breast cancer risk in women with schizophrenia compared with the general population,” said Dr. Zhuo of Tianjin Medical University, China, and Dr. Triplett, of Johns Hopkins University, Baltimore.
As it turns out, one of the subgroup analyses showed that the association between schizophrenia and breast cancer was significant only in studies that excluded women who were diagnosed with breast cancer before they were diagnosed with schizophrenia (standardized incidence ratio, 1.34; 95% CI, 1.20-1.51; P less than .001).
The same was seen in studies where there were more than 100 cases of breast cancer (SIR, 1.31; 95% CI, 1.18-1.46; P less than .001), while the association was not significant in studies with fewer than 100 cases.
The authors said their findings contradict a hypothesis that schizophrenia might be protective against cancer.
“These results, together with our recent meta-analysis results showing no association with lung cancer risk but a reduced hepatic cancer risk in schizophrenia, indicated that the association between schizophrenia and cancer risk may be complicated and depend on the cancer site,” wrote Dr. Zhuo and Dr. Triplett.
In terms of possible mechanisms underlying the increased risk of breast cancer seen in this study, the authors suggested that people with schizophrenia could experience other clinical conditions such as obesity that might increase their risk of breast cancer.
“As breast cancer may be a hormone-dependent cancer, a significant positive association between plasma prolactin levels and the risk of breast cancer has been observed; in addition, increased prolactin levels have been documented in women with schizophrenia, particularly for those receiving certain antipsychotics,” they wrote.
While the incidence of cancer in people with schizophrenia might not necessarily differ from that of the general population, the authors said studies have found that people with schizophrenia have higher cancer mortality. Because “breast cancer prevention and treatment options are less optimal in women with schizophrenia, our results highlight that women with schizophrenia deserve focused care for breast cancer screening and treatment,” they wrote.
The Tianjin Health Bureau Foundation and the Natural Science Foundation of Tianjin, China, supported the study. No conflicts of interest were declared.
SOURCE: Zhuo C et al. JAMA Psychiatry. 2018 Mar 7. doi: 10.1001/jamapsychiatry.2017.4748.
A meta-analysis has found an increased risk of breast cancer in women with schizophrenia, but its authors noted significant diversity of results across the included studies.
In the meta-analysis, Chuanjun Zhuo, MD, PhD, and Patrick Todd Triplett, MD, presented the results of 12 cohort studies involving 125,760 women that showed the risk of breast cancer in women with schizophrenia, compared with the general population.
They found that women with schizophrenia had a 31% higher standardized incidence ratio of breast cancer (95% confidence interval, 1.14-1.50; P less than .001). However, significant heterogeneity was found between studies, with the prediction interval ranging from 0.81 to 2.10. The report was published in JAMA Psychiatry.
“Accordingly, it is possible that a future study will show a decreased breast cancer risk in women with schizophrenia compared with the general population,” said Dr. Zhuo of Tianjin Medical University, China, and Dr. Triplett, of Johns Hopkins University, Baltimore.
As it turns out, one of the subgroup analyses showed that the association between schizophrenia and breast cancer was significant only in studies that excluded women who were diagnosed with breast cancer before they were diagnosed with schizophrenia (standardized incidence ratio, 1.34; 95% CI, 1.20-1.51; P less than .001).
The same was seen in studies where there were more than 100 cases of breast cancer (SIR, 1.31; 95% CI, 1.18-1.46; P less than .001), while the association was not significant in studies with fewer than 100 cases.
The authors said their findings contradict a hypothesis that schizophrenia might be protective against cancer.
“These results, together with our recent meta-analysis results showing no association with lung cancer risk but a reduced hepatic cancer risk in schizophrenia, indicated that the association between schizophrenia and cancer risk may be complicated and depend on the cancer site,” wrote Dr. Zhuo and Dr. Triplett.
In terms of possible mechanisms underlying the increased risk of breast cancer seen in this study, the authors suggested that people with schizophrenia could experience other clinical conditions such as obesity that might increase their risk of breast cancer.
“As breast cancer may be a hormone-dependent cancer, a significant positive association between plasma prolactin levels and the risk of breast cancer has been observed; in addition, increased prolactin levels have been documented in women with schizophrenia, particularly for those receiving certain antipsychotics,” they wrote.
While the incidence of cancer in people with schizophrenia might not necessarily differ from that of the general population, the authors said studies have found that people with schizophrenia have higher cancer mortality. Because “breast cancer prevention and treatment options are less optimal in women with schizophrenia, our results highlight that women with schizophrenia deserve focused care for breast cancer screening and treatment,” they wrote.
The Tianjin Health Bureau Foundation and the Natural Science Foundation of Tianjin, China, supported the study. No conflicts of interest were declared.
SOURCE: Zhuo C et al. JAMA Psychiatry. 2018 Mar 7. doi: 10.1001/jamapsychiatry.2017.4748.
FROM JAMA PSYCHIATRY
Key clinical point: Women diagnosed with schizophrenia should receive intensive screening and treatment for breast cancer.
Major finding: Women with schizophrenia showed a 31% higher standardized incidence ratio of breast cancer than that of the general population.
Data source: Meta-analysis of 12 cohort studies involving 125,760 women.
Disclosures: The Tianjin Health Bureau Foundation and the Natural Science Foundation of Tianjin, China, supported the work. No conflicts of interest were declared.
Source: Zhuo C et al. JAMA Psychiatry. 2018 Mar 7. doi: 10.1001/jamapsychiatry.2017.4748.
Gaps exist in receipt of clinically indicated genetic counseling after breast cancer diagnosis
, according to an analysis of NCI Surveillance, Epidemiology, and End Results (SEER) data published in Journal of Clinical Oncology.
More expertise is required in genetic counseling, either formal counseling given by an expert, or by a cancer physician (physician-directed), wrote Steven J. Katz and his colleagues at the University of Michigan, Ann Arbor. With BRCA1/2-only testing, being replaced by multi-gene panel testing, further consideration and/or discussion of results and formulation of a management plan is required, they said.
Of those, 47.4% did not get tested, 40.7% tested negative, 7.4% had a variant of uncertain significance only, and 4.5% had a pathogenic mutation. Three quarters (74.6%) received some form of genetic counseling (43.5%, formal counseling and 31.1%, physician-directed discussion). Almost all tested patients (96.1%) reported some form of genetic discussion. One half (50.6%) of those not tested received any discussion about genetics, reported the authors.
In addition, younger women more often reported some type of counseling (odds ratio, 4.5;95% confidence interval, 2.6-8.0; 1.9;95% CI, 1.1-3.3; and 1.5;95% CI, 1.0-2.3 for women younger than 50 years of age, 50-59 years of age, and 60-69 years of age, respectively, versus those 70 years of age and older).
Patients’ assessment of the amount of information they received about whether to have testing was high, “whether they received formal genetic counseling or a physician-directed discussion only (80.8% v 79.4% stated information was ‘just right’; P = .58),” the researchers noted.
As high-throughput molecular testing becomes increasingly complex, personalizing and tailoring the information to a individual patients’ need is crucial, the authors said. They further suggest a multipronged strategy that will train oncologists to integrate genetic testing into clinical decision making; including timely testing of patients at an elevated risk.
The study was supported by Grant P01 CA163233 to the University of Michigan from the National Cancer Institute. Potential conflict of interests were reported by Lauren P. Wallner, PhD (GlaxoSmithKline); Monica Morrow, MD (Genomic Health); Reshma Jagsi, MD (Amgen and AbbVie); and Allison W. Kurian, MD (Myriad Genetics, Invitae, Ambry Genetics, Genomic Health, GeneDx/BioReference, Genentech (a member of the Roche Group).
SOURCE: Katz SJ et al. J Clin Oncol. 2018 Mar 12. doi: 10.1200/JCO.2017.76.2369.
, according to an analysis of NCI Surveillance, Epidemiology, and End Results (SEER) data published in Journal of Clinical Oncology.
More expertise is required in genetic counseling, either formal counseling given by an expert, or by a cancer physician (physician-directed), wrote Steven J. Katz and his colleagues at the University of Michigan, Ann Arbor. With BRCA1/2-only testing, being replaced by multi-gene panel testing, further consideration and/or discussion of results and formulation of a management plan is required, they said.
Of those, 47.4% did not get tested, 40.7% tested negative, 7.4% had a variant of uncertain significance only, and 4.5% had a pathogenic mutation. Three quarters (74.6%) received some form of genetic counseling (43.5%, formal counseling and 31.1%, physician-directed discussion). Almost all tested patients (96.1%) reported some form of genetic discussion. One half (50.6%) of those not tested received any discussion about genetics, reported the authors.
In addition, younger women more often reported some type of counseling (odds ratio, 4.5;95% confidence interval, 2.6-8.0; 1.9;95% CI, 1.1-3.3; and 1.5;95% CI, 1.0-2.3 for women younger than 50 years of age, 50-59 years of age, and 60-69 years of age, respectively, versus those 70 years of age and older).
Patients’ assessment of the amount of information they received about whether to have testing was high, “whether they received formal genetic counseling or a physician-directed discussion only (80.8% v 79.4% stated information was ‘just right’; P = .58),” the researchers noted.
As high-throughput molecular testing becomes increasingly complex, personalizing and tailoring the information to a individual patients’ need is crucial, the authors said. They further suggest a multipronged strategy that will train oncologists to integrate genetic testing into clinical decision making; including timely testing of patients at an elevated risk.
The study was supported by Grant P01 CA163233 to the University of Michigan from the National Cancer Institute. Potential conflict of interests were reported by Lauren P. Wallner, PhD (GlaxoSmithKline); Monica Morrow, MD (Genomic Health); Reshma Jagsi, MD (Amgen and AbbVie); and Allison W. Kurian, MD (Myriad Genetics, Invitae, Ambry Genetics, Genomic Health, GeneDx/BioReference, Genentech (a member of the Roche Group).
SOURCE: Katz SJ et al. J Clin Oncol. 2018 Mar 12. doi: 10.1200/JCO.2017.76.2369.
, according to an analysis of NCI Surveillance, Epidemiology, and End Results (SEER) data published in Journal of Clinical Oncology.
More expertise is required in genetic counseling, either formal counseling given by an expert, or by a cancer physician (physician-directed), wrote Steven J. Katz and his colleagues at the University of Michigan, Ann Arbor. With BRCA1/2-only testing, being replaced by multi-gene panel testing, further consideration and/or discussion of results and formulation of a management plan is required, they said.
Of those, 47.4% did not get tested, 40.7% tested negative, 7.4% had a variant of uncertain significance only, and 4.5% had a pathogenic mutation. Three quarters (74.6%) received some form of genetic counseling (43.5%, formal counseling and 31.1%, physician-directed discussion). Almost all tested patients (96.1%) reported some form of genetic discussion. One half (50.6%) of those not tested received any discussion about genetics, reported the authors.
In addition, younger women more often reported some type of counseling (odds ratio, 4.5;95% confidence interval, 2.6-8.0; 1.9;95% CI, 1.1-3.3; and 1.5;95% CI, 1.0-2.3 for women younger than 50 years of age, 50-59 years of age, and 60-69 years of age, respectively, versus those 70 years of age and older).
Patients’ assessment of the amount of information they received about whether to have testing was high, “whether they received formal genetic counseling or a physician-directed discussion only (80.8% v 79.4% stated information was ‘just right’; P = .58),” the researchers noted.
As high-throughput molecular testing becomes increasingly complex, personalizing and tailoring the information to a individual patients’ need is crucial, the authors said. They further suggest a multipronged strategy that will train oncologists to integrate genetic testing into clinical decision making; including timely testing of patients at an elevated risk.
The study was supported by Grant P01 CA163233 to the University of Michigan from the National Cancer Institute. Potential conflict of interests were reported by Lauren P. Wallner, PhD (GlaxoSmithKline); Monica Morrow, MD (Genomic Health); Reshma Jagsi, MD (Amgen and AbbVie); and Allison W. Kurian, MD (Myriad Genetics, Invitae, Ambry Genetics, Genomic Health, GeneDx/BioReference, Genentech (a member of the Roche Group).
SOURCE: Katz SJ et al. J Clin Oncol. 2018 Mar 12. doi: 10.1200/JCO.2017.76.2369.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: There exists a large gap between mandates for timely pretest formal genetic counseling of higher-risk, breast cancer patients and its implementation in clinical practice.
Major finding: Almost half (47.4%) of patients diagnosed with early breast cancer with an indication for genetic risk evaluation did not get genetic tests. Of those who got genetic testing, 43.5% received formal counseling and 31.1% received physician-directed discussion.
Study details: Data on 5,080 women aged 20-79 years diagnosed with early stage breast during 2013-2015, reported to National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) registries of Georgia and Los Angeles County.
Disclosures: Potential conflict of interests were reported by Lauren P. Wallner, PhD (GlaxoSmithKline); Monica Morrow, MD (Genomic Health); Reshma Jagsi, MD (Amgen and AbbVie); and Allison W. Kurian, MD (Myriad Genetics, Invitae, Ambry Genetics, Genomic Health, GeneDx/BioReference, Genentech (a member of the Roche Group).
Source: Katz SJ et al. J Clin Oncol. 2018 Mar 12. doi: 10.1200/JCO.2017.76.2369.
CECCY: Carvedilol didn’t curb cardiotoxicity in breast cancer patients
ORLANDO – Anthracycline chemotherapy was associated with a cardiotoxicity incidence of roughly 14% of breast cancer patients regardless of treatment with carvedilol, based on data from a randomized trial of 200 patients.
“Cardio-oncology has been neglected,” Monica Samuel Avila, MD, of Hospital das Clínicas da Faculdade de Medicina da Universidade in São Paulo, Brazil, said in a video interview at the annual meeting of the American College of Cardiology. “We have seen improvement of survival in patients with cancer, but with that comes complications related to treatment. I think that the interactions between cardiologists and oncologists are increasing in a more important way,” she said.
In the Carvedilol for Prevention of Chemotherapy-Induced Cardiotoxicity (CECCY) Trial, Dr. Avila and colleagues evaluated primary prevention of cardiotoxicity in women with normal hearts who were undergoing chemotherapy for breast cancer.
Patients in the treatment group received a median carvedilol dose of 18.4 mg/day. The primary endpoint of cardiotoxicity, defined as a decrease in left ventricular ejection fraction (LVEF) of at least 10% at 6 months, occurred in 15% of carvedilol patients and 14% placebo patients, a nonsignificant difference. No significant differences occurred in diastolic dysfunction or in B-type natriuretic peptide (BNP) levels at 6 weeks, 12 weeks, or 24 weeks between the groups.
However, carvedilol patients showed significantly reduced troponin 1 levels compared with placebo, which suggests protection against myocardial injury, Dr. Avila said.
“In short follow up, we can see cardiotoxicity appearing, and we know we have to treat it promptly to prevent cardiac events,” she said.
Dr. Avila and colleagues identified 200 women older than 18 years with HER2-negative breast cancer tumor status and normal left ventricular ejection fraction. The patients were undergoing chemotherapy with 240 mg/m2 of anthracycline and were randomized to treatment with carvedilol or a placebo. Baseline characteristics were similar between the two groups.
Adverse effects were not significantly different between the groups, and the most common events in each group included dizziness, dry mouth, symptomatic hypertension, stomachache, and nausea. Although the results suggest that carvedilol can reduce the risk of myocardial injury, more research is needed to address the question of the increase in troponin without change in the LVEF, Dr. Avila noted. The study is ongoing and the research team intends to follow the low-risk patient population for a total of 2 years. “For high-risk patients, I am already giving carvedilol,” she said. “We believe if we find a difference in LVEF or clinical events, we could encourage cardiologists to give carvedilol in a low-risk population,” she said.
“This study highlights that there is no safe dose of anthracycline,” commented Bonnie Ky, MD of the University of Pennsylvania, Philadelphia, at a press briefing. She emphasized the value of carvedilol for a high-risk population, and stressed the importance of following long-term changes in heart injury markers after 1-2 years for low-risk patients.
Dr. Avila had no financial conflicts to disclose. Dr. Ky disclosed relationships with multiple companies including Bioinvent and Bristol Myers.
The findings were published simultaneously in the Journal of the American College of Cardiology.
SOURCE: Avila, M. ACC 18
ORLANDO – Anthracycline chemotherapy was associated with a cardiotoxicity incidence of roughly 14% of breast cancer patients regardless of treatment with carvedilol, based on data from a randomized trial of 200 patients.
“Cardio-oncology has been neglected,” Monica Samuel Avila, MD, of Hospital das Clínicas da Faculdade de Medicina da Universidade in São Paulo, Brazil, said in a video interview at the annual meeting of the American College of Cardiology. “We have seen improvement of survival in patients with cancer, but with that comes complications related to treatment. I think that the interactions between cardiologists and oncologists are increasing in a more important way,” she said.
In the Carvedilol for Prevention of Chemotherapy-Induced Cardiotoxicity (CECCY) Trial, Dr. Avila and colleagues evaluated primary prevention of cardiotoxicity in women with normal hearts who were undergoing chemotherapy for breast cancer.
Patients in the treatment group received a median carvedilol dose of 18.4 mg/day. The primary endpoint of cardiotoxicity, defined as a decrease in left ventricular ejection fraction (LVEF) of at least 10% at 6 months, occurred in 15% of carvedilol patients and 14% placebo patients, a nonsignificant difference. No significant differences occurred in diastolic dysfunction or in B-type natriuretic peptide (BNP) levels at 6 weeks, 12 weeks, or 24 weeks between the groups.
However, carvedilol patients showed significantly reduced troponin 1 levels compared with placebo, which suggests protection against myocardial injury, Dr. Avila said.
“In short follow up, we can see cardiotoxicity appearing, and we know we have to treat it promptly to prevent cardiac events,” she said.
Dr. Avila and colleagues identified 200 women older than 18 years with HER2-negative breast cancer tumor status and normal left ventricular ejection fraction. The patients were undergoing chemotherapy with 240 mg/m2 of anthracycline and were randomized to treatment with carvedilol or a placebo. Baseline characteristics were similar between the two groups.
Adverse effects were not significantly different between the groups, and the most common events in each group included dizziness, dry mouth, symptomatic hypertension, stomachache, and nausea. Although the results suggest that carvedilol can reduce the risk of myocardial injury, more research is needed to address the question of the increase in troponin without change in the LVEF, Dr. Avila noted. The study is ongoing and the research team intends to follow the low-risk patient population for a total of 2 years. “For high-risk patients, I am already giving carvedilol,” she said. “We believe if we find a difference in LVEF or clinical events, we could encourage cardiologists to give carvedilol in a low-risk population,” she said.
“This study highlights that there is no safe dose of anthracycline,” commented Bonnie Ky, MD of the University of Pennsylvania, Philadelphia, at a press briefing. She emphasized the value of carvedilol for a high-risk population, and stressed the importance of following long-term changes in heart injury markers after 1-2 years for low-risk patients.
Dr. Avila had no financial conflicts to disclose. Dr. Ky disclosed relationships with multiple companies including Bioinvent and Bristol Myers.
The findings were published simultaneously in the Journal of the American College of Cardiology.
SOURCE: Avila, M. ACC 18
ORLANDO – Anthracycline chemotherapy was associated with a cardiotoxicity incidence of roughly 14% of breast cancer patients regardless of treatment with carvedilol, based on data from a randomized trial of 200 patients.
“Cardio-oncology has been neglected,” Monica Samuel Avila, MD, of Hospital das Clínicas da Faculdade de Medicina da Universidade in São Paulo, Brazil, said in a video interview at the annual meeting of the American College of Cardiology. “We have seen improvement of survival in patients with cancer, but with that comes complications related to treatment. I think that the interactions between cardiologists and oncologists are increasing in a more important way,” she said.
In the Carvedilol for Prevention of Chemotherapy-Induced Cardiotoxicity (CECCY) Trial, Dr. Avila and colleagues evaluated primary prevention of cardiotoxicity in women with normal hearts who were undergoing chemotherapy for breast cancer.
Patients in the treatment group received a median carvedilol dose of 18.4 mg/day. The primary endpoint of cardiotoxicity, defined as a decrease in left ventricular ejection fraction (LVEF) of at least 10% at 6 months, occurred in 15% of carvedilol patients and 14% placebo patients, a nonsignificant difference. No significant differences occurred in diastolic dysfunction or in B-type natriuretic peptide (BNP) levels at 6 weeks, 12 weeks, or 24 weeks between the groups.
However, carvedilol patients showed significantly reduced troponin 1 levels compared with placebo, which suggests protection against myocardial injury, Dr. Avila said.
“In short follow up, we can see cardiotoxicity appearing, and we know we have to treat it promptly to prevent cardiac events,” she said.
Dr. Avila and colleagues identified 200 women older than 18 years with HER2-negative breast cancer tumor status and normal left ventricular ejection fraction. The patients were undergoing chemotherapy with 240 mg/m2 of anthracycline and were randomized to treatment with carvedilol or a placebo. Baseline characteristics were similar between the two groups.
Adverse effects were not significantly different between the groups, and the most common events in each group included dizziness, dry mouth, symptomatic hypertension, stomachache, and nausea. Although the results suggest that carvedilol can reduce the risk of myocardial injury, more research is needed to address the question of the increase in troponin without change in the LVEF, Dr. Avila noted. The study is ongoing and the research team intends to follow the low-risk patient population for a total of 2 years. “For high-risk patients, I am already giving carvedilol,” she said. “We believe if we find a difference in LVEF or clinical events, we could encourage cardiologists to give carvedilol in a low-risk population,” she said.
“This study highlights that there is no safe dose of anthracycline,” commented Bonnie Ky, MD of the University of Pennsylvania, Philadelphia, at a press briefing. She emphasized the value of carvedilol for a high-risk population, and stressed the importance of following long-term changes in heart injury markers after 1-2 years for low-risk patients.
Dr. Avila had no financial conflicts to disclose. Dr. Ky disclosed relationships with multiple companies including Bioinvent and Bristol Myers.
The findings were published simultaneously in the Journal of the American College of Cardiology.
SOURCE: Avila, M. ACC 18
REPORTING FROM ACC 18
Key clinical point:
Major finding: Cardiotoxicity was roughly 14% in breast cancer patients treated with anthracycline whether they received carvedilol or placebo.
Study details: CECCY was a randomized, placebo-controlled trial of 200 patients with HER2-negative breast cancer tumor status.
Disclosures: Dr. Avila had no financial conflicts to disclose.
Source: Avila M. ACC 2018.
Age at time of breast cancer diagnosis differs by race/ethnicity
according to an analysis of Surveillance, Epidemiology, and End Results (SEER) Program data for almost 750,000 women.
“Our finding challenges established norms with regard to screening practices and provides empirical evidence that race-based screening should be considered,” Sahael M. Stapleton, MD, and his associates at Massachusetts General Hospital, Boston, wrote in a research letter published online March 7 by JAMA Surgery.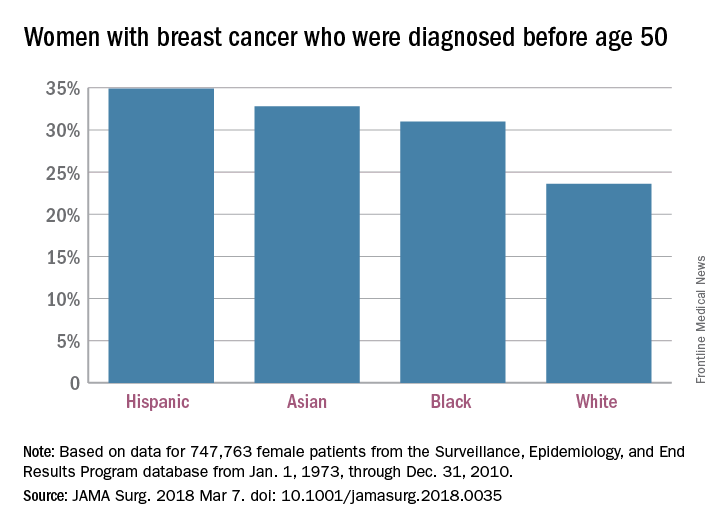
The researchers charted age at diagnosis and race/ethnicity for the 747,763 women in the SEER database from Jan. 1, 1973, through Dec. 31, 2010; this revealed “two distinct distribution patterns of age at diagnosis for female breast cancers: White patients peak in their 60s, whereas nonwhite patients peak in their 40s,” the investigators wrote.
Their calculations show that “screening ages would need to decrease to 47 years for black, 46 years for Hispanic, and 47 years for Asian patients ... to achieve a similar capture rate for nonwhite patients as current guidelines do for white patients” at 50 years of age.
Dr. Stapleton reported receiving support from two Massachusetts General Hospital fellowships. No other disclosures were reported.
SOURCE: Stapleton SM et al. JAMA Surg. 2018 Mar 7. doi: 10.1001/jamasurg.2018.003.
according to an analysis of Surveillance, Epidemiology, and End Results (SEER) Program data for almost 750,000 women.
“Our finding challenges established norms with regard to screening practices and provides empirical evidence that race-based screening should be considered,” Sahael M. Stapleton, MD, and his associates at Massachusetts General Hospital, Boston, wrote in a research letter published online March 7 by JAMA Surgery.
The researchers charted age at diagnosis and race/ethnicity for the 747,763 women in the SEER database from Jan. 1, 1973, through Dec. 31, 2010; this revealed “two distinct distribution patterns of age at diagnosis for female breast cancers: White patients peak in their 60s, whereas nonwhite patients peak in their 40s,” the investigators wrote.
Their calculations show that “screening ages would need to decrease to 47 years for black, 46 years for Hispanic, and 47 years for Asian patients ... to achieve a similar capture rate for nonwhite patients as current guidelines do for white patients” at 50 years of age.
Dr. Stapleton reported receiving support from two Massachusetts General Hospital fellowships. No other disclosures were reported.
SOURCE: Stapleton SM et al. JAMA Surg. 2018 Mar 7. doi: 10.1001/jamasurg.2018.003.
according to an analysis of Surveillance, Epidemiology, and End Results (SEER) Program data for almost 750,000 women.
“Our finding challenges established norms with regard to screening practices and provides empirical evidence that race-based screening should be considered,” Sahael M. Stapleton, MD, and his associates at Massachusetts General Hospital, Boston, wrote in a research letter published online March 7 by JAMA Surgery.
The researchers charted age at diagnosis and race/ethnicity for the 747,763 women in the SEER database from Jan. 1, 1973, through Dec. 31, 2010; this revealed “two distinct distribution patterns of age at diagnosis for female breast cancers: White patients peak in their 60s, whereas nonwhite patients peak in their 40s,” the investigators wrote.
Their calculations show that “screening ages would need to decrease to 47 years for black, 46 years for Hispanic, and 47 years for Asian patients ... to achieve a similar capture rate for nonwhite patients as current guidelines do for white patients” at 50 years of age.
Dr. Stapleton reported receiving support from two Massachusetts General Hospital fellowships. No other disclosures were reported.
SOURCE: Stapleton SM et al. JAMA Surg. 2018 Mar 7. doi: 10.1001/jamasurg.2018.003.
FROM JAMA SURGERY
Breast cancer care delayed when patients have high deductibles
High-deductible health insurance plans may be bad for women’s health, suggest results of a new study.
An analysis of data on women without evidence of breast cancer who were covered for at least 1 year in a low annual deductible plan and then switched by their employers to high annual deductible plans showed that when women were forced to shell out substantially more money before their insurance kicked in, they were significantly more likely to have delays in diagnostic breast imaging, breast biopsy, and initiation of chemotherapy.
“Such delays might lead to adverse long-term breast cancer outcomes. Policymakers, health insurers, and employers should consider designing or incentivizing health insurance benefits that facilitate transitions through key steps along the cancer care pathway,” wrote J. Frank Wharam, MB, and colleagues at Harvard Medical School and Harvard Pilgrim Health Care Institute in Boston. The report was published in Journal of Clinical Oncology.
The investigators conducted a controlled pre-post study to measure the occurrence of outcomes both before and after women were switched from a low-deductible health plan, defined as a maximum annual deductible of $500 or less, to a high-deductible plan, defined as an annual deductible of $1,000 or more.
The study population comprised 273,499 women aged 25-64 years who had no evidence of breast cancer before they were included in the study. The women had all been enrolled in a low-deductible plan for at least 1 year, and were then switched by employer mandate to a high-deductible plan and followed for up to 4 additional years.
Controls included 2.4 million women matched by time of inclusion whose employers continued to offer only low-deductible health plans.
Although at baseline there were no differences between the study sample and the controls in time to first diagnostic breast imaging, breast biopsy, diagnosis of early stage breast cancer, or initiation of breast cancer chemotherapy, at follow-up the women who had been switched to the high-deductible plans had significant delays in all categories.
Compared with controls, the hazard ratios (HR) for each parameter were as follows:
Time to first diagnostic breast imaging: HR = 0.96 (95% confidence interval 0.94-0.96)
Time to first breast biopsy: HR = 0.92 (0.89-0.95)
Time to early stage breast cancer diagnosis: HR = 0.83 (0.78-0.90)
Time to breast cancer chemotherapy: HR = 0.79 (0.72-0.86)
“The findings imply that the high out-of-pocket obligations under HDHPs [high-deductible health plans] might be a barrier to timely receipt of essential breast cancer services. Women in HDHPs might either delay presenting for concerning symptoms or, if proceeding along the pathway from breast cancer screening to diagnostic testing to treatment, be hesitant to undergo subsequent (and generally more expensive) care,” the authors wrote.
They noted that initially modest delays in diagnostic imaging appeared to snowball into longer delays as women proceeded through stages of care.
They recommend a strategy whereby insurers carve out exemptions to high deductibles for services such as diagnostic imaging and breast biopsy.
SOURCE: Wharam et al. J Clin Oncol. 2018 Feb 28. doi: 10.1200/JCO.2017.75.2501.
High-deductible health insurance plans may be bad for women’s health, suggest results of a new study.
An analysis of data on women without evidence of breast cancer who were covered for at least 1 year in a low annual deductible plan and then switched by their employers to high annual deductible plans showed that when women were forced to shell out substantially more money before their insurance kicked in, they were significantly more likely to have delays in diagnostic breast imaging, breast biopsy, and initiation of chemotherapy.
“Such delays might lead to adverse long-term breast cancer outcomes. Policymakers, health insurers, and employers should consider designing or incentivizing health insurance benefits that facilitate transitions through key steps along the cancer care pathway,” wrote J. Frank Wharam, MB, and colleagues at Harvard Medical School and Harvard Pilgrim Health Care Institute in Boston. The report was published in Journal of Clinical Oncology.
The investigators conducted a controlled pre-post study to measure the occurrence of outcomes both before and after women were switched from a low-deductible health plan, defined as a maximum annual deductible of $500 or less, to a high-deductible plan, defined as an annual deductible of $1,000 or more.
The study population comprised 273,499 women aged 25-64 years who had no evidence of breast cancer before they were included in the study. The women had all been enrolled in a low-deductible plan for at least 1 year, and were then switched by employer mandate to a high-deductible plan and followed for up to 4 additional years.
Controls included 2.4 million women matched by time of inclusion whose employers continued to offer only low-deductible health plans.
Although at baseline there were no differences between the study sample and the controls in time to first diagnostic breast imaging, breast biopsy, diagnosis of early stage breast cancer, or initiation of breast cancer chemotherapy, at follow-up the women who had been switched to the high-deductible plans had significant delays in all categories.
Compared with controls, the hazard ratios (HR) for each parameter were as follows:
Time to first diagnostic breast imaging: HR = 0.96 (95% confidence interval 0.94-0.96)
Time to first breast biopsy: HR = 0.92 (0.89-0.95)
Time to early stage breast cancer diagnosis: HR = 0.83 (0.78-0.90)
Time to breast cancer chemotherapy: HR = 0.79 (0.72-0.86)
“The findings imply that the high out-of-pocket obligations under HDHPs [high-deductible health plans] might be a barrier to timely receipt of essential breast cancer services. Women in HDHPs might either delay presenting for concerning symptoms or, if proceeding along the pathway from breast cancer screening to diagnostic testing to treatment, be hesitant to undergo subsequent (and generally more expensive) care,” the authors wrote.
They noted that initially modest delays in diagnostic imaging appeared to snowball into longer delays as women proceeded through stages of care.
They recommend a strategy whereby insurers carve out exemptions to high deductibles for services such as diagnostic imaging and breast biopsy.
SOURCE: Wharam et al. J Clin Oncol. 2018 Feb 28. doi: 10.1200/JCO.2017.75.2501.
High-deductible health insurance plans may be bad for women’s health, suggest results of a new study.
An analysis of data on women without evidence of breast cancer who were covered for at least 1 year in a low annual deductible plan and then switched by their employers to high annual deductible plans showed that when women were forced to shell out substantially more money before their insurance kicked in, they were significantly more likely to have delays in diagnostic breast imaging, breast biopsy, and initiation of chemotherapy.
“Such delays might lead to adverse long-term breast cancer outcomes. Policymakers, health insurers, and employers should consider designing or incentivizing health insurance benefits that facilitate transitions through key steps along the cancer care pathway,” wrote J. Frank Wharam, MB, and colleagues at Harvard Medical School and Harvard Pilgrim Health Care Institute in Boston. The report was published in Journal of Clinical Oncology.
The investigators conducted a controlled pre-post study to measure the occurrence of outcomes both before and after women were switched from a low-deductible health plan, defined as a maximum annual deductible of $500 or less, to a high-deductible plan, defined as an annual deductible of $1,000 or more.
The study population comprised 273,499 women aged 25-64 years who had no evidence of breast cancer before they were included in the study. The women had all been enrolled in a low-deductible plan for at least 1 year, and were then switched by employer mandate to a high-deductible plan and followed for up to 4 additional years.
Controls included 2.4 million women matched by time of inclusion whose employers continued to offer only low-deductible health plans.
Although at baseline there were no differences between the study sample and the controls in time to first diagnostic breast imaging, breast biopsy, diagnosis of early stage breast cancer, or initiation of breast cancer chemotherapy, at follow-up the women who had been switched to the high-deductible plans had significant delays in all categories.
Compared with controls, the hazard ratios (HR) for each parameter were as follows:
Time to first diagnostic breast imaging: HR = 0.96 (95% confidence interval 0.94-0.96)
Time to first breast biopsy: HR = 0.92 (0.89-0.95)
Time to early stage breast cancer diagnosis: HR = 0.83 (0.78-0.90)
Time to breast cancer chemotherapy: HR = 0.79 (0.72-0.86)
“The findings imply that the high out-of-pocket obligations under HDHPs [high-deductible health plans] might be a barrier to timely receipt of essential breast cancer services. Women in HDHPs might either delay presenting for concerning symptoms or, if proceeding along the pathway from breast cancer screening to diagnostic testing to treatment, be hesitant to undergo subsequent (and generally more expensive) care,” the authors wrote.
They noted that initially modest delays in diagnostic imaging appeared to snowball into longer delays as women proceeded through stages of care.
They recommend a strategy whereby insurers carve out exemptions to high deductibles for services such as diagnostic imaging and breast biopsy.
SOURCE: Wharam et al. J Clin Oncol. 2018 Feb 28. doi: 10.1200/JCO.2017.75.2501.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Many women have high-deductible health plans that may discourage them from seeking essential care when needed.
Major finding: Women with an employer-mandated switch from a low- to high-deductible health plan had significant delays in diagnostic imaging, biopsy, diagnosis, and cancer care.
Study details: Controlled pre-post study of data on 273,499 women and 2.4 million controls.
Disclosures: The study was supported by National Cancer Institute and National Institute of Health grants. Dr. Wharam and three coauthors reported no conflicts of interest. Three coauthors reported honoraria and/or consulting/advisory roles with various companies.
Source: Wharam et al. J Clin Oncol. 2018 Feb 28. doi: 10.1200/JCO.2017.75.2501.




