User login
FDA approves first leadless pacemaker
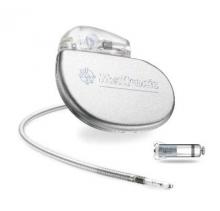
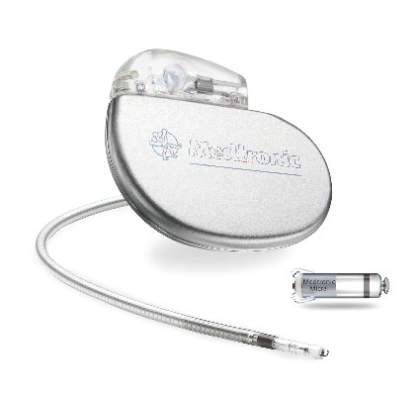
The Food and Drug Administration has approved the first leadless pacemaker, the Micra transcatheter pacing system, the FDA stated in a release accompanying its approval.
The Micra pacemaker eliminates the need for wired leads and the risk of associated complications. The single-chamber ventricular pacemaker is 93% smaller than traditional pacemakers, according to a summary document submitted to the FDA by Medtronic, which makes the device. Like other ventricular pacemakers, Micra provides rate-adaptive pacing, with automated pacing capture threshold management to maximize battery life, which the company estimates at about 10 years.
The pacemaker is inserted directly into the right ventricle through the femoral vein by means of a steerable catheter. Pressing a button on the distal end of the catheter releases four flexible, electrically inactive nitinol tines that hook into the myocardium to secure the device. Engagement by two tines exerts 15 times the amount of force needed to secure the device in place, according to Medtronic.
The device’s approval was based on a pivotal prospective, nonrandomized uncontrolled study of 719 patients at 56 investigational sites in North America, Europe, Asia, Australia, and Africa. The primary efficacy endpoint, low and stable pacing capture thresholds at 6 months (up to 2.0 V at a pulse width of 0.24 milliseconds and an increase of up to 1.5 V from the time of implantation) was achieved for 98% of patients (95% confidence interval, 96%-99.5%), reported Dr. Dwight Reynolds of the University of Oklahoma Health Sciences Center in Oklahoma City and his associates (N Engl J Med. 2016 Feb. 11. doi: 10.1056/NEJMoa1511643).
The researchers also compared safety outcomes among Micra recipients and 2,667 historical controls from six previously published studies. The Micra pacemaker was associated with significantly lower hospitalization and system revision rates, with “no systemic infections, no pneumothoraxes, and no radiographically visible dislodgements or device emboli,” they said. In all, 4% of Micra recipients had complications leading to death or requiring invasive revision, treatment cessation, or hospitalization, which resembled recent reports of transvenous systems and was significantly lower than the rate for historical controls, according to the investigators. However, the rate of cardiac perforation or effusion was 1.6%, slightly higher than the rate of 1.1% for historical controls. Other major complications included cardiac failure (0.9% of study patients), atrioventricular fistula or pseudoaneurysm at the groin puncture site (0.7%), and deep vein thrombosis or pulmonary thromboembolism (0.3%).
Medtronic funded the pivotal study on which approval of the Micra pacemaker was based. Dr. Reynolds had no relevant financial disclosures. Several coinvestigators reported financial relationships with Medtronic and several other cardiac device manufacturers.
The Food and Drug Administration has approved the first leadless pacemaker, the Micra transcatheter pacing system, the FDA stated in a release accompanying its approval.
The Micra pacemaker eliminates the need for wired leads and the risk of associated complications. The single-chamber ventricular pacemaker is 93% smaller than traditional pacemakers, according to a summary document submitted to the FDA by Medtronic, which makes the device. Like other ventricular pacemakers, Micra provides rate-adaptive pacing, with automated pacing capture threshold management to maximize battery life, which the company estimates at about 10 years.
The pacemaker is inserted directly into the right ventricle through the femoral vein by means of a steerable catheter. Pressing a button on the distal end of the catheter releases four flexible, electrically inactive nitinol tines that hook into the myocardium to secure the device. Engagement by two tines exerts 15 times the amount of force needed to secure the device in place, according to Medtronic.
The device’s approval was based on a pivotal prospective, nonrandomized uncontrolled study of 719 patients at 56 investigational sites in North America, Europe, Asia, Australia, and Africa. The primary efficacy endpoint, low and stable pacing capture thresholds at 6 months (up to 2.0 V at a pulse width of 0.24 milliseconds and an increase of up to 1.5 V from the time of implantation) was achieved for 98% of patients (95% confidence interval, 96%-99.5%), reported Dr. Dwight Reynolds of the University of Oklahoma Health Sciences Center in Oklahoma City and his associates (N Engl J Med. 2016 Feb. 11. doi: 10.1056/NEJMoa1511643).
The researchers also compared safety outcomes among Micra recipients and 2,667 historical controls from six previously published studies. The Micra pacemaker was associated with significantly lower hospitalization and system revision rates, with “no systemic infections, no pneumothoraxes, and no radiographically visible dislodgements or device emboli,” they said. In all, 4% of Micra recipients had complications leading to death or requiring invasive revision, treatment cessation, or hospitalization, which resembled recent reports of transvenous systems and was significantly lower than the rate for historical controls, according to the investigators. However, the rate of cardiac perforation or effusion was 1.6%, slightly higher than the rate of 1.1% for historical controls. Other major complications included cardiac failure (0.9% of study patients), atrioventricular fistula or pseudoaneurysm at the groin puncture site (0.7%), and deep vein thrombosis or pulmonary thromboembolism (0.3%).
Medtronic funded the pivotal study on which approval of the Micra pacemaker was based. Dr. Reynolds had no relevant financial disclosures. Several coinvestigators reported financial relationships with Medtronic and several other cardiac device manufacturers.
The Food and Drug Administration has approved the first leadless pacemaker, the Micra transcatheter pacing system, the FDA stated in a release accompanying its approval.
The Micra pacemaker eliminates the need for wired leads and the risk of associated complications. The single-chamber ventricular pacemaker is 93% smaller than traditional pacemakers, according to a summary document submitted to the FDA by Medtronic, which makes the device. Like other ventricular pacemakers, Micra provides rate-adaptive pacing, with automated pacing capture threshold management to maximize battery life, which the company estimates at about 10 years.
The pacemaker is inserted directly into the right ventricle through the femoral vein by means of a steerable catheter. Pressing a button on the distal end of the catheter releases four flexible, electrically inactive nitinol tines that hook into the myocardium to secure the device. Engagement by two tines exerts 15 times the amount of force needed to secure the device in place, according to Medtronic.
The device’s approval was based on a pivotal prospective, nonrandomized uncontrolled study of 719 patients at 56 investigational sites in North America, Europe, Asia, Australia, and Africa. The primary efficacy endpoint, low and stable pacing capture thresholds at 6 months (up to 2.0 V at a pulse width of 0.24 milliseconds and an increase of up to 1.5 V from the time of implantation) was achieved for 98% of patients (95% confidence interval, 96%-99.5%), reported Dr. Dwight Reynolds of the University of Oklahoma Health Sciences Center in Oklahoma City and his associates (N Engl J Med. 2016 Feb. 11. doi: 10.1056/NEJMoa1511643).
The researchers also compared safety outcomes among Micra recipients and 2,667 historical controls from six previously published studies. The Micra pacemaker was associated with significantly lower hospitalization and system revision rates, with “no systemic infections, no pneumothoraxes, and no radiographically visible dislodgements or device emboli,” they said. In all, 4% of Micra recipients had complications leading to death or requiring invasive revision, treatment cessation, or hospitalization, which resembled recent reports of transvenous systems and was significantly lower than the rate for historical controls, according to the investigators. However, the rate of cardiac perforation or effusion was 1.6%, slightly higher than the rate of 1.1% for historical controls. Other major complications included cardiac failure (0.9% of study patients), atrioventricular fistula or pseudoaneurysm at the groin puncture site (0.7%), and deep vein thrombosis or pulmonary thromboembolism (0.3%).
Medtronic funded the pivotal study on which approval of the Micra pacemaker was based. Dr. Reynolds had no relevant financial disclosures. Several coinvestigators reported financial relationships with Medtronic and several other cardiac device manufacturers.
FIRE AND ICE trial called a win for cryoablation of AF
CHICAGO – The largest-ever randomized trial of catheter ablation of atrial fibrillation has ended in a draw between radiofrequency and cryoballoon ablation in safety and efficacy – and that actually represents a win for cryoablation, a simpler and far more easily mastered procedure, Dr. Karl-Heinz Kuck said at the annual meeting of the American College of Cardiology.
“We can teach physicians how to do cryoablation much more easily. That will allow more patients with atrial fibrillation to get access to catheter ablation, which is what we really need,” according to Dr. Kuck, principal investigator in the poetically named FIRE AND ICE trial and head of cardiology at St. Georg Hospital in Hamburg (Germany).
FIRE AND ICE included 769 patients in eight European countries. The participants, all of whom had antiarrhythmic drug–refractory paroxysmal atrial fibrillation (AF), were randomized to radiofrequency ablation – the long-time standard – or to cryoablation, a newer technology. Radiofrequency ablation was guided by three-dimensional electroanatomic mapping, while cryoablation utilized fluoroscopic guidance.
The primary efficacy endpoint was the 1-year rate of clinical failure, defined as an occurrence of AF, atrial flutter, or atrial tachycardia lasting for at least 30 seconds, or repeat ablation or the use of antiarrhythmic drugs following a 90-day postprocedural blanking period. The clinical failure rate was 34.6% in the cryoballoon group and similar at 35.9% in the radiofrequency group.
Serious treatment-related adverse events occurred in 10.2% of the cryoballoon group and 12.8% of the radiofrequency group, a nonsignificant difference. No procedural deaths occurred in the study.
There were, however, several significant procedural differences. Procedure time averaged 124 minutes in the cryoablation group, nearly 20 minutes less than the 142 minutes for radiofrequency ablation. However, the 17-minute fluoroscopy time in the radiofrequency group was 5 minutes shorter than for cryoablation.
Dr. Kuck said the study underestimates the true procedural differences because FIRE AND ICE was carried out by extremely experienced operators. In routine clinical practice involving non-elite operators, it’s not unusual for radiofrequency ablation fluoroscopy times to be two or even three times longer than the 17 minutes seen in the study. Plus, FIRE AND ICE was conducted when the procedure entailed two applications of the cryoballoon. Now only one application is recommended, cutting an additional 12 minutes off the total procedure time, he added.
Radiofrequency ablation takes longer because it entails creating a series of point-to-point lesions in a circle to isolate the pulmonary veins. With cryoablation, the balloon is moved into position, inflated, and a 3-minute-freeze is administered to create a circle of necrotic tissue in a single-step procedure.
Discussant Dr. Hugh G. Calkins praised the FIRE AND ICE investigators’ use of a rigorous definition of recurrence that required as little as a 30-second episode of atrial arrhythmia.
“That’s a very high bar, so I think the results are very impressive,” said Dr. Calkins, professor of medicine and of pediatrics and director of the cardiac arrhythmia service at Johns Hopkins University, Baltimore.
He commented that “this study is a clear reminder that 90% success rates just don’t happen in this field,” despite what some practitioners have claimed.
Asked how he predicts the study results will influence the field of AF ablation, Dr. Kuck replied that he foresees much wider adoption of cryoablation and a stronger endorsement of the technology in updated guideline recommendations.
“I personally believe this will be the most important development in our field in the next several years,” he added.
The electrophysiologist noted that even though current guidelines give a class Ia recommendation to catheter ablation of paroxysmal AF that’s refractory to at least one antiarrhythmic drug, at present only 4% of such patients actually undergo the procedure.
“Having just 4% of patients with AF undergo catheter ablation cannot be what we are looking for as physicians,” Dr. Kuck said. “I believe if we want to roll out catheter ablation for AF, we need simple and safe tools. This trial elegantly shows that with a simpler device that allows single-shot isolation of the pulmonary veins, we can get the same safety and efficacy as with radiofrequency ablation. I often tell people that radiofrequency ablation of atrial fibrillation is the most challenging procedure in all cardiology. We do this procedure from the groin in a moving heart. It’s a very complex technology.”
His dream, he continued, is that cryoablation will eventually enable patients with atrial fibrillation to be managed the same way electrophysiologists treat patients with Wolff-Parkinson-White syndrome; with the first episode, the patient goes to the electrophysiology catheterization lab for an ablation procedure.
“I think there’s a great message here: The cryoballoon will move catheter ablation from a niche procedure performed in specialized centers by the few guys in the world who can do it really well out into the broader world. To do that you need a tool that is safe, simple, and can be handled by the average doctor,” Dr. Kuck said.
Discussant Dr. Anthony DeMaria commented that it would be premature at this point to start thinking about cryoablation as a first approach to new-onset AF, given the roughly 35% clinical failure rate at 1 year seen in FIRE AND ICE. That rate doubtless would have been even higher had patients been equipped with implantable loop recorders, added Dr. DeMaria, professor of medicine at the University of California, San Diego.
Dr. Kuck conceded that the high recurrence rate is one of the great unsolved limitations of catheter ablation of AF.
“We don’t know how to get the pulmonary veins permanently isolated,” he said. “We can create acute lesions, but over time what we’ve seen is recovery of tissue and then reconduction by the pulmonary veins. I believe that 20% of the 40% recurrence rate is due to reconduction from the pulmonary veins, and the rest is probably due to triggers coming from other sites.”
The FIRE AND ICE trial was funded in part by Medtronic, which markets the Arctic Front Advance cryoablation catheter used in the study. Dr. Kuck reported serving on a speakers’ bureau for Medtronic and acting as a consultant to Biosense Webster, Edwards, and St. Jude.
Simultaneous with Dr. Kuck’s presentation at ACC 16, the results of FIRE AND ICE were published online (N Engl J Med. 2016 Apr 4. doi: 10.1056/NEJMoa1602014).
CHICAGO – The largest-ever randomized trial of catheter ablation of atrial fibrillation has ended in a draw between radiofrequency and cryoballoon ablation in safety and efficacy – and that actually represents a win for cryoablation, a simpler and far more easily mastered procedure, Dr. Karl-Heinz Kuck said at the annual meeting of the American College of Cardiology.
“We can teach physicians how to do cryoablation much more easily. That will allow more patients with atrial fibrillation to get access to catheter ablation, which is what we really need,” according to Dr. Kuck, principal investigator in the poetically named FIRE AND ICE trial and head of cardiology at St. Georg Hospital in Hamburg (Germany).
FIRE AND ICE included 769 patients in eight European countries. The participants, all of whom had antiarrhythmic drug–refractory paroxysmal atrial fibrillation (AF), were randomized to radiofrequency ablation – the long-time standard – or to cryoablation, a newer technology. Radiofrequency ablation was guided by three-dimensional electroanatomic mapping, while cryoablation utilized fluoroscopic guidance.
The primary efficacy endpoint was the 1-year rate of clinical failure, defined as an occurrence of AF, atrial flutter, or atrial tachycardia lasting for at least 30 seconds, or repeat ablation or the use of antiarrhythmic drugs following a 90-day postprocedural blanking period. The clinical failure rate was 34.6% in the cryoballoon group and similar at 35.9% in the radiofrequency group.
Serious treatment-related adverse events occurred in 10.2% of the cryoballoon group and 12.8% of the radiofrequency group, a nonsignificant difference. No procedural deaths occurred in the study.
There were, however, several significant procedural differences. Procedure time averaged 124 minutes in the cryoablation group, nearly 20 minutes less than the 142 minutes for radiofrequency ablation. However, the 17-minute fluoroscopy time in the radiofrequency group was 5 minutes shorter than for cryoablation.
Dr. Kuck said the study underestimates the true procedural differences because FIRE AND ICE was carried out by extremely experienced operators. In routine clinical practice involving non-elite operators, it’s not unusual for radiofrequency ablation fluoroscopy times to be two or even three times longer than the 17 minutes seen in the study. Plus, FIRE AND ICE was conducted when the procedure entailed two applications of the cryoballoon. Now only one application is recommended, cutting an additional 12 minutes off the total procedure time, he added.
Radiofrequency ablation takes longer because it entails creating a series of point-to-point lesions in a circle to isolate the pulmonary veins. With cryoablation, the balloon is moved into position, inflated, and a 3-minute-freeze is administered to create a circle of necrotic tissue in a single-step procedure.
Discussant Dr. Hugh G. Calkins praised the FIRE AND ICE investigators’ use of a rigorous definition of recurrence that required as little as a 30-second episode of atrial arrhythmia.
“That’s a very high bar, so I think the results are very impressive,” said Dr. Calkins, professor of medicine and of pediatrics and director of the cardiac arrhythmia service at Johns Hopkins University, Baltimore.
He commented that “this study is a clear reminder that 90% success rates just don’t happen in this field,” despite what some practitioners have claimed.
Asked how he predicts the study results will influence the field of AF ablation, Dr. Kuck replied that he foresees much wider adoption of cryoablation and a stronger endorsement of the technology in updated guideline recommendations.
“I personally believe this will be the most important development in our field in the next several years,” he added.
The electrophysiologist noted that even though current guidelines give a class Ia recommendation to catheter ablation of paroxysmal AF that’s refractory to at least one antiarrhythmic drug, at present only 4% of such patients actually undergo the procedure.
“Having just 4% of patients with AF undergo catheter ablation cannot be what we are looking for as physicians,” Dr. Kuck said. “I believe if we want to roll out catheter ablation for AF, we need simple and safe tools. This trial elegantly shows that with a simpler device that allows single-shot isolation of the pulmonary veins, we can get the same safety and efficacy as with radiofrequency ablation. I often tell people that radiofrequency ablation of atrial fibrillation is the most challenging procedure in all cardiology. We do this procedure from the groin in a moving heart. It’s a very complex technology.”
His dream, he continued, is that cryoablation will eventually enable patients with atrial fibrillation to be managed the same way electrophysiologists treat patients with Wolff-Parkinson-White syndrome; with the first episode, the patient goes to the electrophysiology catheterization lab for an ablation procedure.
“I think there’s a great message here: The cryoballoon will move catheter ablation from a niche procedure performed in specialized centers by the few guys in the world who can do it really well out into the broader world. To do that you need a tool that is safe, simple, and can be handled by the average doctor,” Dr. Kuck said.
Discussant Dr. Anthony DeMaria commented that it would be premature at this point to start thinking about cryoablation as a first approach to new-onset AF, given the roughly 35% clinical failure rate at 1 year seen in FIRE AND ICE. That rate doubtless would have been even higher had patients been equipped with implantable loop recorders, added Dr. DeMaria, professor of medicine at the University of California, San Diego.
Dr. Kuck conceded that the high recurrence rate is one of the great unsolved limitations of catheter ablation of AF.
“We don’t know how to get the pulmonary veins permanently isolated,” he said. “We can create acute lesions, but over time what we’ve seen is recovery of tissue and then reconduction by the pulmonary veins. I believe that 20% of the 40% recurrence rate is due to reconduction from the pulmonary veins, and the rest is probably due to triggers coming from other sites.”
The FIRE AND ICE trial was funded in part by Medtronic, which markets the Arctic Front Advance cryoablation catheter used in the study. Dr. Kuck reported serving on a speakers’ bureau for Medtronic and acting as a consultant to Biosense Webster, Edwards, and St. Jude.
Simultaneous with Dr. Kuck’s presentation at ACC 16, the results of FIRE AND ICE were published online (N Engl J Med. 2016 Apr 4. doi: 10.1056/NEJMoa1602014).
CHICAGO – The largest-ever randomized trial of catheter ablation of atrial fibrillation has ended in a draw between radiofrequency and cryoballoon ablation in safety and efficacy – and that actually represents a win for cryoablation, a simpler and far more easily mastered procedure, Dr. Karl-Heinz Kuck said at the annual meeting of the American College of Cardiology.
“We can teach physicians how to do cryoablation much more easily. That will allow more patients with atrial fibrillation to get access to catheter ablation, which is what we really need,” according to Dr. Kuck, principal investigator in the poetically named FIRE AND ICE trial and head of cardiology at St. Georg Hospital in Hamburg (Germany).
FIRE AND ICE included 769 patients in eight European countries. The participants, all of whom had antiarrhythmic drug–refractory paroxysmal atrial fibrillation (AF), were randomized to radiofrequency ablation – the long-time standard – or to cryoablation, a newer technology. Radiofrequency ablation was guided by three-dimensional electroanatomic mapping, while cryoablation utilized fluoroscopic guidance.
The primary efficacy endpoint was the 1-year rate of clinical failure, defined as an occurrence of AF, atrial flutter, or atrial tachycardia lasting for at least 30 seconds, or repeat ablation or the use of antiarrhythmic drugs following a 90-day postprocedural blanking period. The clinical failure rate was 34.6% in the cryoballoon group and similar at 35.9% in the radiofrequency group.
Serious treatment-related adverse events occurred in 10.2% of the cryoballoon group and 12.8% of the radiofrequency group, a nonsignificant difference. No procedural deaths occurred in the study.
There were, however, several significant procedural differences. Procedure time averaged 124 minutes in the cryoablation group, nearly 20 minutes less than the 142 minutes for radiofrequency ablation. However, the 17-minute fluoroscopy time in the radiofrequency group was 5 minutes shorter than for cryoablation.
Dr. Kuck said the study underestimates the true procedural differences because FIRE AND ICE was carried out by extremely experienced operators. In routine clinical practice involving non-elite operators, it’s not unusual for radiofrequency ablation fluoroscopy times to be two or even three times longer than the 17 minutes seen in the study. Plus, FIRE AND ICE was conducted when the procedure entailed two applications of the cryoballoon. Now only one application is recommended, cutting an additional 12 minutes off the total procedure time, he added.
Radiofrequency ablation takes longer because it entails creating a series of point-to-point lesions in a circle to isolate the pulmonary veins. With cryoablation, the balloon is moved into position, inflated, and a 3-minute-freeze is administered to create a circle of necrotic tissue in a single-step procedure.
Discussant Dr. Hugh G. Calkins praised the FIRE AND ICE investigators’ use of a rigorous definition of recurrence that required as little as a 30-second episode of atrial arrhythmia.
“That’s a very high bar, so I think the results are very impressive,” said Dr. Calkins, professor of medicine and of pediatrics and director of the cardiac arrhythmia service at Johns Hopkins University, Baltimore.
He commented that “this study is a clear reminder that 90% success rates just don’t happen in this field,” despite what some practitioners have claimed.
Asked how he predicts the study results will influence the field of AF ablation, Dr. Kuck replied that he foresees much wider adoption of cryoablation and a stronger endorsement of the technology in updated guideline recommendations.
“I personally believe this will be the most important development in our field in the next several years,” he added.
The electrophysiologist noted that even though current guidelines give a class Ia recommendation to catheter ablation of paroxysmal AF that’s refractory to at least one antiarrhythmic drug, at present only 4% of such patients actually undergo the procedure.
“Having just 4% of patients with AF undergo catheter ablation cannot be what we are looking for as physicians,” Dr. Kuck said. “I believe if we want to roll out catheter ablation for AF, we need simple and safe tools. This trial elegantly shows that with a simpler device that allows single-shot isolation of the pulmonary veins, we can get the same safety and efficacy as with radiofrequency ablation. I often tell people that radiofrequency ablation of atrial fibrillation is the most challenging procedure in all cardiology. We do this procedure from the groin in a moving heart. It’s a very complex technology.”
His dream, he continued, is that cryoablation will eventually enable patients with atrial fibrillation to be managed the same way electrophysiologists treat patients with Wolff-Parkinson-White syndrome; with the first episode, the patient goes to the electrophysiology catheterization lab for an ablation procedure.
“I think there’s a great message here: The cryoballoon will move catheter ablation from a niche procedure performed in specialized centers by the few guys in the world who can do it really well out into the broader world. To do that you need a tool that is safe, simple, and can be handled by the average doctor,” Dr. Kuck said.
Discussant Dr. Anthony DeMaria commented that it would be premature at this point to start thinking about cryoablation as a first approach to new-onset AF, given the roughly 35% clinical failure rate at 1 year seen in FIRE AND ICE. That rate doubtless would have been even higher had patients been equipped with implantable loop recorders, added Dr. DeMaria, professor of medicine at the University of California, San Diego.
Dr. Kuck conceded that the high recurrence rate is one of the great unsolved limitations of catheter ablation of AF.
“We don’t know how to get the pulmonary veins permanently isolated,” he said. “We can create acute lesions, but over time what we’ve seen is recovery of tissue and then reconduction by the pulmonary veins. I believe that 20% of the 40% recurrence rate is due to reconduction from the pulmonary veins, and the rest is probably due to triggers coming from other sites.”
The FIRE AND ICE trial was funded in part by Medtronic, which markets the Arctic Front Advance cryoablation catheter used in the study. Dr. Kuck reported serving on a speakers’ bureau for Medtronic and acting as a consultant to Biosense Webster, Edwards, and St. Jude.
Simultaneous with Dr. Kuck’s presentation at ACC 16, the results of FIRE AND ICE were published online (N Engl J Med. 2016 Apr 4. doi: 10.1056/NEJMoa1602014).
AT ACC 16
Key clinical point: Cryoablation of atrial fibrillation offers significant advantages over radiofrequency ablation.
Major finding: In a rigorous randomized trial, cryoablation of atrial fibrillation had a 34.6% clinical failure rate at 1 year, similar to the 35.9% rate for radiofrequency ablation.
Data source: The FIRE AND ICE trial, which randomized 769 patients with paroxysmal atrial fibrillation in eight European countries.
Disclosures: FIRE AND ICE was funded in part by Medtronic. The presenter reported serving on a speakers’ bureau for the company.
VIDEO: Sapien 3 TAVR bests surgery in intermediate-risk patients
CHICAGO – Transcatheter aortic valve replacement (TAVR) using Sapien 3 – the latest-generation valve – is associated with low mortality, stroke, and paravalvular regurgitation rates at 1 year in intermediate-risk patients, and is superior to surgical valve replacement, according to findings from the SAPIEN 3 study.
The mortality rate at 1 year in the 1,077 patients in the observational study was 7.4% overall and 6.5% in a transfemoral access subgroup, the disabling stroke rate was 2.3%, the aortic valve reintervention rate was 0.6%, and the moderate/severe paravalvular regurgitation rate was 1.5%, Dr. Vinod H. Thourani reported on behalf of the PARTNER trial investigators at the annual meeting of the American College of Cardiology. The findings were published simultaneously in The Lancet (2016 Apr 3. doi: 10.1016/So140-6736[19]30073-3).
A prespecified propensity score analysis comparing 963 SAPIEN 3 patients with 747 similar intermediate-risk patients from the PARTNER 2A trial who underwent surgical valve replacement showed that not only was Sapien 3 TAVR noninferior to surgery for the primary composite endpoint of mortality, strokes, and moderate or severe aortic regurgitation, it was also superior to surgery (pooled weighted proportion difference, –9.2% for each). The differences were highly statistically significant.
In fact, Sapien 3 TAVR “blew it out of the water” for both noninferiority and superiority vs. surgery, Dr. Thourani of Emory University, Atlanta said.
The propensity score incorporated 22 characteristics, and the analysis was conducted by blinded investigators. Even using the most conservative strategy for the analysis as approved by the Food and Drug Administration, with the heaviest weighting against TAVR, Sapien 3 TAVR was superior to surgery for the primary composite endpoint, he noted.
Of note, while Sapien 3 TAVR was superior for the individual components of mortality and stroke from the composite endpoint, surgery was superior to Sapien 3 TAVR for the component of moderate or greater aortic regurgitation, he said.
However, the findings represent “strong evidence that in intermediate-risk patients with severe aortic stenosis, SAPIEN 3, compared to surgery, improves clinical outcomes and is the preferred therapy,” he concluded.
In a video interview, he said that if approved by the FDA, “this will become the impetus for [use in] a lower-risk population of patients. Currently we have the inoperative and high-risk patients, and this will open up the intermediate-risk patients for having transcatheter valve therapies, and I think it becomes exceedingly powerful.”
Two ongoing industry-sponsored randomized trials in low-risk patients (those with a Society of Thoracic Surgeons score of less than 4) are underway, he noted.
Sapien 3 TAVR was previously shown to improve 30-day outcomes in intermediate-risk patients with severe aortic stenosis (Eur Heart J. 2016 Mar 31. doi: 10.1093/eurheartj/ehw112), but longer-term data were lacking, and no comparisons with surgery in intermediate-risk patients were available.
For the current study, patients with a mean age of 82 years were evaluated at 51 centers in the United States and Canada during February-September 2014. Subjects had a median Society of Thoracic Surgeons score of 5.2% (range, 4-8) and 73% had New York Heart Association class III/IV heart failure. Almost 90% were treated via the transfemoral route, Dr. Thourani said.
The Sapien 3 device is a balloon expandable valve that differs from prior-generation devices in that it has improved geometry of the trileaflet bovine pericardial valve, a longer cobalt alloy frame with more open outlet cells and denser inlet cells, a polyethylene terephthalate fabric skirt that provides an external circumferential seal to reduce paravalvular leak, four valve sizes, and lower-profile delivery catheters with more precise valve positioning inserted through 14 or 16 French sheaths for increased use of transfemoral access.
Discussant Dr. David E. Kandzari, director of interventional cardiology and chief scientific officer at Piedmont Heart Institute, Atlanta, congratulated Dr. Thourani and his colleagues on “a terrific trial and impactful result.”
“There are, with regard to the Sapien 3 technology, many reasons to believe that this could be an advancement above existing predicate technologies,” he said, specifically mentioning the improvements in the device, compared with prior generations, such as the modification to reduce paravalvular leak, which has been associated with worse outcomes for patients.
“In parallel, there were changes in practice, and one of them implemented in the context of SAPIEN 3 was the use of [computed tomography] imaging to help guide and inform the procedure itself,” he said, adding that the results of the trial “really open the door for at least two very broad pathways.”
First, they expand TAVR to intermediate-risk patients.
“Secondly, they lead the way even further with greater reassurance toward two large ongoing clinical trials in patients considered at low risk, as well,” he said.
The remarkable outcomes in regard to mortality and stroke are “clinically meaningful and some of the best outcomes we’ve ever witnessed with transcatheter therapy,” he said.
This study was funded by Edwards Lifesciences. Dr. Thourani disclosed that he has received consulting fees and/or research grants from Edwards Lifesciences, St. Jude Medical, Abbott Medical, Boston Scientific, Claret Medical, DirectFlow, Medtronic, and Sorin. Dr. Kandzari has received consultant fees and honoraria from Boston Scientific, The Medicines Company, and Medtronic.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Transcatheter aortic valve replacement (TAVR) using Sapien 3 – the latest-generation valve – is associated with low mortality, stroke, and paravalvular regurgitation rates at 1 year in intermediate-risk patients, and is superior to surgical valve replacement, according to findings from the SAPIEN 3 study.
The mortality rate at 1 year in the 1,077 patients in the observational study was 7.4% overall and 6.5% in a transfemoral access subgroup, the disabling stroke rate was 2.3%, the aortic valve reintervention rate was 0.6%, and the moderate/severe paravalvular regurgitation rate was 1.5%, Dr. Vinod H. Thourani reported on behalf of the PARTNER trial investigators at the annual meeting of the American College of Cardiology. The findings were published simultaneously in The Lancet (2016 Apr 3. doi: 10.1016/So140-6736[19]30073-3).
A prespecified propensity score analysis comparing 963 SAPIEN 3 patients with 747 similar intermediate-risk patients from the PARTNER 2A trial who underwent surgical valve replacement showed that not only was Sapien 3 TAVR noninferior to surgery for the primary composite endpoint of mortality, strokes, and moderate or severe aortic regurgitation, it was also superior to surgery (pooled weighted proportion difference, –9.2% for each). The differences were highly statistically significant.
In fact, Sapien 3 TAVR “blew it out of the water” for both noninferiority and superiority vs. surgery, Dr. Thourani of Emory University, Atlanta said.
The propensity score incorporated 22 characteristics, and the analysis was conducted by blinded investigators. Even using the most conservative strategy for the analysis as approved by the Food and Drug Administration, with the heaviest weighting against TAVR, Sapien 3 TAVR was superior to surgery for the primary composite endpoint, he noted.
Of note, while Sapien 3 TAVR was superior for the individual components of mortality and stroke from the composite endpoint, surgery was superior to Sapien 3 TAVR for the component of moderate or greater aortic regurgitation, he said.
However, the findings represent “strong evidence that in intermediate-risk patients with severe aortic stenosis, SAPIEN 3, compared to surgery, improves clinical outcomes and is the preferred therapy,” he concluded.
In a video interview, he said that if approved by the FDA, “this will become the impetus for [use in] a lower-risk population of patients. Currently we have the inoperative and high-risk patients, and this will open up the intermediate-risk patients for having transcatheter valve therapies, and I think it becomes exceedingly powerful.”
Two ongoing industry-sponsored randomized trials in low-risk patients (those with a Society of Thoracic Surgeons score of less than 4) are underway, he noted.
Sapien 3 TAVR was previously shown to improve 30-day outcomes in intermediate-risk patients with severe aortic stenosis (Eur Heart J. 2016 Mar 31. doi: 10.1093/eurheartj/ehw112), but longer-term data were lacking, and no comparisons with surgery in intermediate-risk patients were available.
For the current study, patients with a mean age of 82 years were evaluated at 51 centers in the United States and Canada during February-September 2014. Subjects had a median Society of Thoracic Surgeons score of 5.2% (range, 4-8) and 73% had New York Heart Association class III/IV heart failure. Almost 90% were treated via the transfemoral route, Dr. Thourani said.
The Sapien 3 device is a balloon expandable valve that differs from prior-generation devices in that it has improved geometry of the trileaflet bovine pericardial valve, a longer cobalt alloy frame with more open outlet cells and denser inlet cells, a polyethylene terephthalate fabric skirt that provides an external circumferential seal to reduce paravalvular leak, four valve sizes, and lower-profile delivery catheters with more precise valve positioning inserted through 14 or 16 French sheaths for increased use of transfemoral access.
Discussant Dr. David E. Kandzari, director of interventional cardiology and chief scientific officer at Piedmont Heart Institute, Atlanta, congratulated Dr. Thourani and his colleagues on “a terrific trial and impactful result.”
“There are, with regard to the Sapien 3 technology, many reasons to believe that this could be an advancement above existing predicate technologies,” he said, specifically mentioning the improvements in the device, compared with prior generations, such as the modification to reduce paravalvular leak, which has been associated with worse outcomes for patients.
“In parallel, there were changes in practice, and one of them implemented in the context of SAPIEN 3 was the use of [computed tomography] imaging to help guide and inform the procedure itself,” he said, adding that the results of the trial “really open the door for at least two very broad pathways.”
First, they expand TAVR to intermediate-risk patients.
“Secondly, they lead the way even further with greater reassurance toward two large ongoing clinical trials in patients considered at low risk, as well,” he said.
The remarkable outcomes in regard to mortality and stroke are “clinically meaningful and some of the best outcomes we’ve ever witnessed with transcatheter therapy,” he said.
This study was funded by Edwards Lifesciences. Dr. Thourani disclosed that he has received consulting fees and/or research grants from Edwards Lifesciences, St. Jude Medical, Abbott Medical, Boston Scientific, Claret Medical, DirectFlow, Medtronic, and Sorin. Dr. Kandzari has received consultant fees and honoraria from Boston Scientific, The Medicines Company, and Medtronic.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Transcatheter aortic valve replacement (TAVR) using Sapien 3 – the latest-generation valve – is associated with low mortality, stroke, and paravalvular regurgitation rates at 1 year in intermediate-risk patients, and is superior to surgical valve replacement, according to findings from the SAPIEN 3 study.
The mortality rate at 1 year in the 1,077 patients in the observational study was 7.4% overall and 6.5% in a transfemoral access subgroup, the disabling stroke rate was 2.3%, the aortic valve reintervention rate was 0.6%, and the moderate/severe paravalvular regurgitation rate was 1.5%, Dr. Vinod H. Thourani reported on behalf of the PARTNER trial investigators at the annual meeting of the American College of Cardiology. The findings were published simultaneously in The Lancet (2016 Apr 3. doi: 10.1016/So140-6736[19]30073-3).
A prespecified propensity score analysis comparing 963 SAPIEN 3 patients with 747 similar intermediate-risk patients from the PARTNER 2A trial who underwent surgical valve replacement showed that not only was Sapien 3 TAVR noninferior to surgery for the primary composite endpoint of mortality, strokes, and moderate or severe aortic regurgitation, it was also superior to surgery (pooled weighted proportion difference, –9.2% for each). The differences were highly statistically significant.
In fact, Sapien 3 TAVR “blew it out of the water” for both noninferiority and superiority vs. surgery, Dr. Thourani of Emory University, Atlanta said.
The propensity score incorporated 22 characteristics, and the analysis was conducted by blinded investigators. Even using the most conservative strategy for the analysis as approved by the Food and Drug Administration, with the heaviest weighting against TAVR, Sapien 3 TAVR was superior to surgery for the primary composite endpoint, he noted.
Of note, while Sapien 3 TAVR was superior for the individual components of mortality and stroke from the composite endpoint, surgery was superior to Sapien 3 TAVR for the component of moderate or greater aortic regurgitation, he said.
However, the findings represent “strong evidence that in intermediate-risk patients with severe aortic stenosis, SAPIEN 3, compared to surgery, improves clinical outcomes and is the preferred therapy,” he concluded.
In a video interview, he said that if approved by the FDA, “this will become the impetus for [use in] a lower-risk population of patients. Currently we have the inoperative and high-risk patients, and this will open up the intermediate-risk patients for having transcatheter valve therapies, and I think it becomes exceedingly powerful.”
Two ongoing industry-sponsored randomized trials in low-risk patients (those with a Society of Thoracic Surgeons score of less than 4) are underway, he noted.
Sapien 3 TAVR was previously shown to improve 30-day outcomes in intermediate-risk patients with severe aortic stenosis (Eur Heart J. 2016 Mar 31. doi: 10.1093/eurheartj/ehw112), but longer-term data were lacking, and no comparisons with surgery in intermediate-risk patients were available.
For the current study, patients with a mean age of 82 years were evaluated at 51 centers in the United States and Canada during February-September 2014. Subjects had a median Society of Thoracic Surgeons score of 5.2% (range, 4-8) and 73% had New York Heart Association class III/IV heart failure. Almost 90% were treated via the transfemoral route, Dr. Thourani said.
The Sapien 3 device is a balloon expandable valve that differs from prior-generation devices in that it has improved geometry of the trileaflet bovine pericardial valve, a longer cobalt alloy frame with more open outlet cells and denser inlet cells, a polyethylene terephthalate fabric skirt that provides an external circumferential seal to reduce paravalvular leak, four valve sizes, and lower-profile delivery catheters with more precise valve positioning inserted through 14 or 16 French sheaths for increased use of transfemoral access.
Discussant Dr. David E. Kandzari, director of interventional cardiology and chief scientific officer at Piedmont Heart Institute, Atlanta, congratulated Dr. Thourani and his colleagues on “a terrific trial and impactful result.”
“There are, with regard to the Sapien 3 technology, many reasons to believe that this could be an advancement above existing predicate technologies,” he said, specifically mentioning the improvements in the device, compared with prior generations, such as the modification to reduce paravalvular leak, which has been associated with worse outcomes for patients.
“In parallel, there were changes in practice, and one of them implemented in the context of SAPIEN 3 was the use of [computed tomography] imaging to help guide and inform the procedure itself,” he said, adding that the results of the trial “really open the door for at least two very broad pathways.”
First, they expand TAVR to intermediate-risk patients.
“Secondly, they lead the way even further with greater reassurance toward two large ongoing clinical trials in patients considered at low risk, as well,” he said.
The remarkable outcomes in regard to mortality and stroke are “clinically meaningful and some of the best outcomes we’ve ever witnessed with transcatheter therapy,” he said.
This study was funded by Edwards Lifesciences. Dr. Thourani disclosed that he has received consulting fees and/or research grants from Edwards Lifesciences, St. Jude Medical, Abbott Medical, Boston Scientific, Claret Medical, DirectFlow, Medtronic, and Sorin. Dr. Kandzari has received consultant fees and honoraria from Boston Scientific, The Medicines Company, and Medtronic.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ACC 16
Key clinical point: Transcatheter aortic valve replacement using Sapien 3 is associated with low mortality, stroke, and paravalvular regurgitation rates at 1 year in intermediate-risk patients, and is superior to surgical valve replacement.
Major finding: A propensity score analysis showed that Sapien 3 TAVR was superior to surgical valve replacement (pooled weighted proportion difference, –9.2%).
Data source: An observational study of 1,077 SAPIEN 3 patients, and a propensity score analysis comparing 963 SAPIEN 3 patients and 747 surgical valve replacement patients.
Disclosures: SAPIEN 3 was funded by Edwards Lifesciences. Dr. Thourani disclosed that he has received consulting fees from Edwards Lifesciences and St. Jude Medical, and research grants from Abbott Medical, Boston Scientific, Claret Medical, DirectFlow, Medtronic, and Sorin.
Similarities seen in rate and rhythm control for postsurgical AF
CHICAGO – Rate and rhythm control proved equally effective for treatment of new-onset post–cardiac surgery atrial fibrillation in a randomized trial that was far and away the largest ever to examine the best way to address this common and costly arrhythmia, Dr. A. Marc Gillinov said at the annual meeting of the American College of Cardiology.
Thus, either strategy is acceptable. That being said, rate control gets the edge as the initial treatment strategy because it avoids the considerable toxicities accompanying amiodarone for rhythm control, most of which arise only after patients have been discharged from the hospital. In contrast, when rate control doesn’t work, it becomes evident while the patient is still in the hospital, according to Dr. Gillinov, a cardiothoracic surgeon at the Cleveland Clinic .
Atrial fibrillation (AF) is the most common complication of cardiac surgery, with an incidence variously reported at 20%-50%. It results in lengthier hospital stays, greater cost of care, and increased risks of mortality, stroke, heart failure, and infection. Postoperative AF adds an estimated $1 billion per year to health care costs in the United States.
While current ACC/AHA/Heart Rhythm Society joint guidelines recommend rate control with a beta-blocker as first-line therapy for patients with this postoperative complication, with a class I, level-of-evidence A rating, upon closer inspection the evidence cited mainly involves extrapolation from studies looking at how to prevent postoperative AF. Because no persuasive evidence existed as to how best to treat this common and economically and medically costly condition, Dr. Gillinov and his coinvestigators in the National Institutes of Health–funded Cardiothoracic Surgical Trials Network carried out a randomized trial 10-fold larger than anything prior.
The 23-site study included 2,109 patients enrolled prior to cardiac surgery, of whom 40% underwent isolated coronary artery bypass grafting (CABG) while the other 60% had valve surgery, either alone or with CABG. These proportions reflect current cardiac surgery treatment patterns nationally. Overall, 33% of the cardiac surgery patients experienced postoperative AF. The incidence was 28% in patients who underwent isolated CABG but rose with increasing surgical complexity to nearly 50% in patients who had combined CABG and valve operations. The average time to onset of postoperative AF was 2.4 days.
A total of 523 patients with postoperative AF were randomized to rate or rhythm control. Rate control most often entailed use of a beta-blocker, while amiodarone was prescribed for rhythm control.
The primary endpoint in the trial was a measure of health care resource utilization: total days in hospital during a 60-day period starting from the time of randomization. This endpoint was a draw: a median of 5.1 days with rate control and 5.0 days with rhythm control.
At hospital discharge, 89.9% of patients in the rate control group and 93.5% in the rhythm control group had a stable heart rhythm without AF. From discharge to 60 days, 84.2% of patients in the rate control group and a similar 86.9% of the rhythm control group remained free of AF.
Rates of serious adverse events were similar in the two groups: 24.8 per 100 patient-months in the rate control arm and 26.4 per 100 patient-months in the rhythm control arm. Three patients in the rate control arm died during the 60-day study period, and two died in the rhythm control group.
Of note, roughly one-quarter of patients in each study arm crossed over to the other arm. In the rate control group, this was typically due to drug ineffectiveness, while in the rhythm control arm the switch was most often made in response to amiodarone side effects.
Roughly 43% of patients in each group were placed on anticoagulation with warfarin for 60 days according to study protocol, which called for such action if a patient remained in AF 48 hours after randomization.
There were five strokes, one case of transient ischemic attack, and four noncerebral thromboembolisms. Also, 21 bleeding events occurred, 17 of which were classified as serious; 90% of the bleeding events happened in patients on warfarin.
“I found the results very striking and very reassuring,” said discussant Hugh G. Calkins. “To me, the clinical message is clearly that rate control is the preference.”
It was troubling, however, to see that 10 thromboembolic events occurred in 523 patients over the course of just 60 days. “Should we be anticoagulating these postsurgical atrial fibrillation patients a lot more frequently?” asked Dr. Calkins, professor of medicine and of pediatrics and director of the cardiac arrhythmia service at Johns Hopkins University, Baltimore.
Dr. Gillinov replied that he and his colleagues in the Cardiothoracic Surgical Trials Network consider that to be the key remaining question regarding postoperative AF. They are now planning a clinical trial aimed at finding the optimal balance between stroke protection via anticoagulation and bleeding risk.
The National Institutes of Health and the Canadian Institutes of Health Research funded the work. Dr. Gillinov reported serving as a consultant to five surgical device companies, none of which played any role in the study.
Simultaneously with Dr. Gillinov’s presentation at ACC 16, the study results were published in the New England Journal of Medicine (doi: 10.1056/NEJMoa1602002).
CHICAGO – Rate and rhythm control proved equally effective for treatment of new-onset post–cardiac surgery atrial fibrillation in a randomized trial that was far and away the largest ever to examine the best way to address this common and costly arrhythmia, Dr. A. Marc Gillinov said at the annual meeting of the American College of Cardiology.
Thus, either strategy is acceptable. That being said, rate control gets the edge as the initial treatment strategy because it avoids the considerable toxicities accompanying amiodarone for rhythm control, most of which arise only after patients have been discharged from the hospital. In contrast, when rate control doesn’t work, it becomes evident while the patient is still in the hospital, according to Dr. Gillinov, a cardiothoracic surgeon at the Cleveland Clinic .
Atrial fibrillation (AF) is the most common complication of cardiac surgery, with an incidence variously reported at 20%-50%. It results in lengthier hospital stays, greater cost of care, and increased risks of mortality, stroke, heart failure, and infection. Postoperative AF adds an estimated $1 billion per year to health care costs in the United States.
While current ACC/AHA/Heart Rhythm Society joint guidelines recommend rate control with a beta-blocker as first-line therapy for patients with this postoperative complication, with a class I, level-of-evidence A rating, upon closer inspection the evidence cited mainly involves extrapolation from studies looking at how to prevent postoperative AF. Because no persuasive evidence existed as to how best to treat this common and economically and medically costly condition, Dr. Gillinov and his coinvestigators in the National Institutes of Health–funded Cardiothoracic Surgical Trials Network carried out a randomized trial 10-fold larger than anything prior.
The 23-site study included 2,109 patients enrolled prior to cardiac surgery, of whom 40% underwent isolated coronary artery bypass grafting (CABG) while the other 60% had valve surgery, either alone or with CABG. These proportions reflect current cardiac surgery treatment patterns nationally. Overall, 33% of the cardiac surgery patients experienced postoperative AF. The incidence was 28% in patients who underwent isolated CABG but rose with increasing surgical complexity to nearly 50% in patients who had combined CABG and valve operations. The average time to onset of postoperative AF was 2.4 days.
A total of 523 patients with postoperative AF were randomized to rate or rhythm control. Rate control most often entailed use of a beta-blocker, while amiodarone was prescribed for rhythm control.
The primary endpoint in the trial was a measure of health care resource utilization: total days in hospital during a 60-day period starting from the time of randomization. This endpoint was a draw: a median of 5.1 days with rate control and 5.0 days with rhythm control.
At hospital discharge, 89.9% of patients in the rate control group and 93.5% in the rhythm control group had a stable heart rhythm without AF. From discharge to 60 days, 84.2% of patients in the rate control group and a similar 86.9% of the rhythm control group remained free of AF.
Rates of serious adverse events were similar in the two groups: 24.8 per 100 patient-months in the rate control arm and 26.4 per 100 patient-months in the rhythm control arm. Three patients in the rate control arm died during the 60-day study period, and two died in the rhythm control group.
Of note, roughly one-quarter of patients in each study arm crossed over to the other arm. In the rate control group, this was typically due to drug ineffectiveness, while in the rhythm control arm the switch was most often made in response to amiodarone side effects.
Roughly 43% of patients in each group were placed on anticoagulation with warfarin for 60 days according to study protocol, which called for such action if a patient remained in AF 48 hours after randomization.
There were five strokes, one case of transient ischemic attack, and four noncerebral thromboembolisms. Also, 21 bleeding events occurred, 17 of which were classified as serious; 90% of the bleeding events happened in patients on warfarin.
“I found the results very striking and very reassuring,” said discussant Hugh G. Calkins. “To me, the clinical message is clearly that rate control is the preference.”
It was troubling, however, to see that 10 thromboembolic events occurred in 523 patients over the course of just 60 days. “Should we be anticoagulating these postsurgical atrial fibrillation patients a lot more frequently?” asked Dr. Calkins, professor of medicine and of pediatrics and director of the cardiac arrhythmia service at Johns Hopkins University, Baltimore.
Dr. Gillinov replied that he and his colleagues in the Cardiothoracic Surgical Trials Network consider that to be the key remaining question regarding postoperative AF. They are now planning a clinical trial aimed at finding the optimal balance between stroke protection via anticoagulation and bleeding risk.
The National Institutes of Health and the Canadian Institutes of Health Research funded the work. Dr. Gillinov reported serving as a consultant to five surgical device companies, none of which played any role in the study.
Simultaneously with Dr. Gillinov’s presentation at ACC 16, the study results were published in the New England Journal of Medicine (doi: 10.1056/NEJMoa1602002).
CHICAGO – Rate and rhythm control proved equally effective for treatment of new-onset post–cardiac surgery atrial fibrillation in a randomized trial that was far and away the largest ever to examine the best way to address this common and costly arrhythmia, Dr. A. Marc Gillinov said at the annual meeting of the American College of Cardiology.
Thus, either strategy is acceptable. That being said, rate control gets the edge as the initial treatment strategy because it avoids the considerable toxicities accompanying amiodarone for rhythm control, most of which arise only after patients have been discharged from the hospital. In contrast, when rate control doesn’t work, it becomes evident while the patient is still in the hospital, according to Dr. Gillinov, a cardiothoracic surgeon at the Cleveland Clinic .
Atrial fibrillation (AF) is the most common complication of cardiac surgery, with an incidence variously reported at 20%-50%. It results in lengthier hospital stays, greater cost of care, and increased risks of mortality, stroke, heart failure, and infection. Postoperative AF adds an estimated $1 billion per year to health care costs in the United States.
While current ACC/AHA/Heart Rhythm Society joint guidelines recommend rate control with a beta-blocker as first-line therapy for patients with this postoperative complication, with a class I, level-of-evidence A rating, upon closer inspection the evidence cited mainly involves extrapolation from studies looking at how to prevent postoperative AF. Because no persuasive evidence existed as to how best to treat this common and economically and medically costly condition, Dr. Gillinov and his coinvestigators in the National Institutes of Health–funded Cardiothoracic Surgical Trials Network carried out a randomized trial 10-fold larger than anything prior.
The 23-site study included 2,109 patients enrolled prior to cardiac surgery, of whom 40% underwent isolated coronary artery bypass grafting (CABG) while the other 60% had valve surgery, either alone or with CABG. These proportions reflect current cardiac surgery treatment patterns nationally. Overall, 33% of the cardiac surgery patients experienced postoperative AF. The incidence was 28% in patients who underwent isolated CABG but rose with increasing surgical complexity to nearly 50% in patients who had combined CABG and valve operations. The average time to onset of postoperative AF was 2.4 days.
A total of 523 patients with postoperative AF were randomized to rate or rhythm control. Rate control most often entailed use of a beta-blocker, while amiodarone was prescribed for rhythm control.
The primary endpoint in the trial was a measure of health care resource utilization: total days in hospital during a 60-day period starting from the time of randomization. This endpoint was a draw: a median of 5.1 days with rate control and 5.0 days with rhythm control.
At hospital discharge, 89.9% of patients in the rate control group and 93.5% in the rhythm control group had a stable heart rhythm without AF. From discharge to 60 days, 84.2% of patients in the rate control group and a similar 86.9% of the rhythm control group remained free of AF.
Rates of serious adverse events were similar in the two groups: 24.8 per 100 patient-months in the rate control arm and 26.4 per 100 patient-months in the rhythm control arm. Three patients in the rate control arm died during the 60-day study period, and two died in the rhythm control group.
Of note, roughly one-quarter of patients in each study arm crossed over to the other arm. In the rate control group, this was typically due to drug ineffectiveness, while in the rhythm control arm the switch was most often made in response to amiodarone side effects.
Roughly 43% of patients in each group were placed on anticoagulation with warfarin for 60 days according to study protocol, which called for such action if a patient remained in AF 48 hours after randomization.
There were five strokes, one case of transient ischemic attack, and four noncerebral thromboembolisms. Also, 21 bleeding events occurred, 17 of which were classified as serious; 90% of the bleeding events happened in patients on warfarin.
“I found the results very striking and very reassuring,” said discussant Hugh G. Calkins. “To me, the clinical message is clearly that rate control is the preference.”
It was troubling, however, to see that 10 thromboembolic events occurred in 523 patients over the course of just 60 days. “Should we be anticoagulating these postsurgical atrial fibrillation patients a lot more frequently?” asked Dr. Calkins, professor of medicine and of pediatrics and director of the cardiac arrhythmia service at Johns Hopkins University, Baltimore.
Dr. Gillinov replied that he and his colleagues in the Cardiothoracic Surgical Trials Network consider that to be the key remaining question regarding postoperative AF. They are now planning a clinical trial aimed at finding the optimal balance between stroke protection via anticoagulation and bleeding risk.
The National Institutes of Health and the Canadian Institutes of Health Research funded the work. Dr. Gillinov reported serving as a consultant to five surgical device companies, none of which played any role in the study.
Simultaneously with Dr. Gillinov’s presentation at ACC 16, the study results were published in the New England Journal of Medicine (doi: 10.1056/NEJMoa1602002).
AT ACC 16
Key clinical point: Rate control offers the advantage of simplicity over a rhythm control strategy in new-onset atrial fibrillation after cardiac surgery.
Major finding: Rate and rhythm control strategies for treatment of new-onset atrial fibrillation after cardiac surgery resulted in equal numbers of hospital days, similar serious complication rates, and low rates of persistent atrial fibrillation at 60 days of follow-up.
Data source: A randomized clinical trial of 523 patients with new-onset atrial fibrillation following cardiac surgery at 23 U.S. and Canadian academic medical centers.
Disclosures: The study was funded by the National Institutes of Health and the Canadian Institutes of Health Research and carried out through the Cardiothoracic Surgical Trials Network. The presenter reported having no relevant financial interests.
Coronary bypass shows compelling advantages in ischemic cardiomyopathy
CHICAGO – Coronary artery bypass grafting plus guideline-directed medical therapy resulted in significantly lower all-cause mortality than did optimal medical therapy alone at 10 years of follow-up in the Surgical Treatment for Ischemic Heart Failure Extension Study (STICHES), Dr. Eric J. Velazquez reported at the annual meeting of the American College of Cardiology.
“We believe these results have the immediate clinical implications that the presence of severe left ventricular dysfunction should prompt an evaluation for the extent and severity of angiographic CAD, and that among patients with ischemic cardiomyopathy, CABG should be strongly considered in order to improve long-term survival,” declared Dr. Velazquez, professor of medicine in the division of cardiology at Duke University, Durham, N.C.
STICHES included 1,212 patients in 22 countries, all with heart failure and an ejection fraction of 35% or less along with CAD deemed suitable for surgical revascularization. They were randomized to CABG plus guideline-directed medical therapy or to the medical therapy alone. The 98% successful follow-up rate over the course of 10 years in this trial drew audience praise as a herculean effort.
At a median 9.8 years of follow-up, all-cause mortality – the primary study endpoint – had occurred in 58.9% of the CABG group and 66.1% of medically managed patients. That translates to a 16% relative risk reduction and an absolute 8% difference in favor of CABG. The median survival extension conferred by CABG was 1.4 years. The number of patients needed to treat with CABG in order to prevent one death from any cause was 14.
The CABG group also did significantly better in terms of secondary endpoints. The cardiovascular mortality rate was 40.5% in the CABG group versus 49.3% with medical therapy, for a 21% relative risk reduction favoring CABG and a number needed to treat of 11. The composite endpoint of all-cause mortality or cardiovascular hospitalization occurred in 76.6% of the CABG group and 87% of the medically treated patients.
In an earlier analysis based upon 56 months of follow-up, there was a trend favoring CABG in terms of all-cause mortality, but it didn’t reach statistical significance (N Engl J Med. 2011;364:1607-16). With an additional 5 years of prospective follow-up, however, the divergence in outcome between the two study arms increased sufficiently that the difference achieved statistical significance. But the more impressive study finding, in Dr. Velazquez’s view, was the durability of the CABG benefits out to 10 years.
Discussant Dr. Jeroean J. Bax of Leiden (the Netherlands) University commented that while the solid advantage in outcomes displayed by the CABG group was noteworthy, he finds it sobering that even though the STICHES participants averaged only 60 years of age at entry, the majority were dead at 10 years’ follow-up. What, he asked, is the likely mechanism for the very high mortality seen in this population?
“My take-home after many years working with our team is that I believe these patients have very low reserve, and they are at risk any time they take a hit. I don’t believe just one mechanism is involved. In our previous analysis of the 5-year follow-up data, we showed the results can’t be explained solely by viability, ischemia, or functional recovery. I think the issue of arrhythmia reduction and substrate reduction is important. But for me, it’s a combination of many factors. Any additional hit for this high-risk population is not well tolerated; that’s what leads to death,” Dr. Velazquez replied.
Asked how he thinks multivessel percutaneous coronary intervention would perform as an alternative to CABG in patients with ischemic cardiomyopathy, Dr. Velazquez responded that he has no idea because it hasn’t been studied.
“I can picture reasons for and against PCI providing benefits similar to CABG,” he added.
Simultaneous with Dr. Velazquez’s presentation at ACC 16, the STICHES results were published online (N Engl J Med. 2016 April 3. doi:10.1056/NEJMoa1602001).
In an accompanying editorial, Dr. Robert A. Guyton and Dr. Andrew L. Smith of Emory University in Atlanta asserted that these strong results from STICHES make a compelling case that CABG for patients with ischemic cardiomyopathy should be upgraded in the ACC/AHA heart failure management guidelines from its current status as a class IIb recommendation that “might be considered” to class IIa, indicating it is “probably beneficial” (N Engl J Med. 2016 April 3. doi:10.1056/NEJMe1603615).
STICHES was funded by the National Institutes of Health. The study presenter reported having no financial conflicts regarding the study.
CHICAGO – Coronary artery bypass grafting plus guideline-directed medical therapy resulted in significantly lower all-cause mortality than did optimal medical therapy alone at 10 years of follow-up in the Surgical Treatment for Ischemic Heart Failure Extension Study (STICHES), Dr. Eric J. Velazquez reported at the annual meeting of the American College of Cardiology.
“We believe these results have the immediate clinical implications that the presence of severe left ventricular dysfunction should prompt an evaluation for the extent and severity of angiographic CAD, and that among patients with ischemic cardiomyopathy, CABG should be strongly considered in order to improve long-term survival,” declared Dr. Velazquez, professor of medicine in the division of cardiology at Duke University, Durham, N.C.
STICHES included 1,212 patients in 22 countries, all with heart failure and an ejection fraction of 35% or less along with CAD deemed suitable for surgical revascularization. They were randomized to CABG plus guideline-directed medical therapy or to the medical therapy alone. The 98% successful follow-up rate over the course of 10 years in this trial drew audience praise as a herculean effort.
At a median 9.8 years of follow-up, all-cause mortality – the primary study endpoint – had occurred in 58.9% of the CABG group and 66.1% of medically managed patients. That translates to a 16% relative risk reduction and an absolute 8% difference in favor of CABG. The median survival extension conferred by CABG was 1.4 years. The number of patients needed to treat with CABG in order to prevent one death from any cause was 14.
The CABG group also did significantly better in terms of secondary endpoints. The cardiovascular mortality rate was 40.5% in the CABG group versus 49.3% with medical therapy, for a 21% relative risk reduction favoring CABG and a number needed to treat of 11. The composite endpoint of all-cause mortality or cardiovascular hospitalization occurred in 76.6% of the CABG group and 87% of the medically treated patients.
In an earlier analysis based upon 56 months of follow-up, there was a trend favoring CABG in terms of all-cause mortality, but it didn’t reach statistical significance (N Engl J Med. 2011;364:1607-16). With an additional 5 years of prospective follow-up, however, the divergence in outcome between the two study arms increased sufficiently that the difference achieved statistical significance. But the more impressive study finding, in Dr. Velazquez’s view, was the durability of the CABG benefits out to 10 years.
Discussant Dr. Jeroean J. Bax of Leiden (the Netherlands) University commented that while the solid advantage in outcomes displayed by the CABG group was noteworthy, he finds it sobering that even though the STICHES participants averaged only 60 years of age at entry, the majority were dead at 10 years’ follow-up. What, he asked, is the likely mechanism for the very high mortality seen in this population?
“My take-home after many years working with our team is that I believe these patients have very low reserve, and they are at risk any time they take a hit. I don’t believe just one mechanism is involved. In our previous analysis of the 5-year follow-up data, we showed the results can’t be explained solely by viability, ischemia, or functional recovery. I think the issue of arrhythmia reduction and substrate reduction is important. But for me, it’s a combination of many factors. Any additional hit for this high-risk population is not well tolerated; that’s what leads to death,” Dr. Velazquez replied.
Asked how he thinks multivessel percutaneous coronary intervention would perform as an alternative to CABG in patients with ischemic cardiomyopathy, Dr. Velazquez responded that he has no idea because it hasn’t been studied.
“I can picture reasons for and against PCI providing benefits similar to CABG,” he added.
Simultaneous with Dr. Velazquez’s presentation at ACC 16, the STICHES results were published online (N Engl J Med. 2016 April 3. doi:10.1056/NEJMoa1602001).
In an accompanying editorial, Dr. Robert A. Guyton and Dr. Andrew L. Smith of Emory University in Atlanta asserted that these strong results from STICHES make a compelling case that CABG for patients with ischemic cardiomyopathy should be upgraded in the ACC/AHA heart failure management guidelines from its current status as a class IIb recommendation that “might be considered” to class IIa, indicating it is “probably beneficial” (N Engl J Med. 2016 April 3. doi:10.1056/NEJMe1603615).
STICHES was funded by the National Institutes of Health. The study presenter reported having no financial conflicts regarding the study.
CHICAGO – Coronary artery bypass grafting plus guideline-directed medical therapy resulted in significantly lower all-cause mortality than did optimal medical therapy alone at 10 years of follow-up in the Surgical Treatment for Ischemic Heart Failure Extension Study (STICHES), Dr. Eric J. Velazquez reported at the annual meeting of the American College of Cardiology.
“We believe these results have the immediate clinical implications that the presence of severe left ventricular dysfunction should prompt an evaluation for the extent and severity of angiographic CAD, and that among patients with ischemic cardiomyopathy, CABG should be strongly considered in order to improve long-term survival,” declared Dr. Velazquez, professor of medicine in the division of cardiology at Duke University, Durham, N.C.
STICHES included 1,212 patients in 22 countries, all with heart failure and an ejection fraction of 35% or less along with CAD deemed suitable for surgical revascularization. They were randomized to CABG plus guideline-directed medical therapy or to the medical therapy alone. The 98% successful follow-up rate over the course of 10 years in this trial drew audience praise as a herculean effort.
At a median 9.8 years of follow-up, all-cause mortality – the primary study endpoint – had occurred in 58.9% of the CABG group and 66.1% of medically managed patients. That translates to a 16% relative risk reduction and an absolute 8% difference in favor of CABG. The median survival extension conferred by CABG was 1.4 years. The number of patients needed to treat with CABG in order to prevent one death from any cause was 14.
The CABG group also did significantly better in terms of secondary endpoints. The cardiovascular mortality rate was 40.5% in the CABG group versus 49.3% with medical therapy, for a 21% relative risk reduction favoring CABG and a number needed to treat of 11. The composite endpoint of all-cause mortality or cardiovascular hospitalization occurred in 76.6% of the CABG group and 87% of the medically treated patients.
In an earlier analysis based upon 56 months of follow-up, there was a trend favoring CABG in terms of all-cause mortality, but it didn’t reach statistical significance (N Engl J Med. 2011;364:1607-16). With an additional 5 years of prospective follow-up, however, the divergence in outcome between the two study arms increased sufficiently that the difference achieved statistical significance. But the more impressive study finding, in Dr. Velazquez’s view, was the durability of the CABG benefits out to 10 years.
Discussant Dr. Jeroean J. Bax of Leiden (the Netherlands) University commented that while the solid advantage in outcomes displayed by the CABG group was noteworthy, he finds it sobering that even though the STICHES participants averaged only 60 years of age at entry, the majority were dead at 10 years’ follow-up. What, he asked, is the likely mechanism for the very high mortality seen in this population?
“My take-home after many years working with our team is that I believe these patients have very low reserve, and they are at risk any time they take a hit. I don’t believe just one mechanism is involved. In our previous analysis of the 5-year follow-up data, we showed the results can’t be explained solely by viability, ischemia, or functional recovery. I think the issue of arrhythmia reduction and substrate reduction is important. But for me, it’s a combination of many factors. Any additional hit for this high-risk population is not well tolerated; that’s what leads to death,” Dr. Velazquez replied.
Asked how he thinks multivessel percutaneous coronary intervention would perform as an alternative to CABG in patients with ischemic cardiomyopathy, Dr. Velazquez responded that he has no idea because it hasn’t been studied.
“I can picture reasons for and against PCI providing benefits similar to CABG,” he added.
Simultaneous with Dr. Velazquez’s presentation at ACC 16, the STICHES results were published online (N Engl J Med. 2016 April 3. doi:10.1056/NEJMoa1602001).
In an accompanying editorial, Dr. Robert A. Guyton and Dr. Andrew L. Smith of Emory University in Atlanta asserted that these strong results from STICHES make a compelling case that CABG for patients with ischemic cardiomyopathy should be upgraded in the ACC/AHA heart failure management guidelines from its current status as a class IIb recommendation that “might be considered” to class IIa, indicating it is “probably beneficial” (N Engl J Med. 2016 April 3. doi:10.1056/NEJMe1603615).
STICHES was funded by the National Institutes of Health. The study presenter reported having no financial conflicts regarding the study.
AT ACC 16
Key clinical point: CABG plus optimal medical therapy is the treatment of choice in patients with ischemic cardiomyopathy.
Major finding: The number of patients with ischemic cardiomyopathy who need to be treated with CABG plus optimal medical therapy instead of medical therapy alone in order to prevent one additional death due to any cause is 14.
Data source: This was a randomized, unblinded clinical trial involving 1,212 patients with ischemic cardiomyopathy in 22 countries.
Disclosures: STICHES was funded by the National Institutes of Health. The study presenter reported having no financial conflicts regarding the study.
VIDEO: STICHES trial update boosts CABG in ischemic cardiomyopathy
CHICAGO – The results of the Surgical Treatment for Ischemic Heart Failure Extension Study (STICHES) presented at the annual meeting of the American College of Cardiology ought to change the clinical management of patients with coronary artery disease and heart failure with severe left ventricular dysfunction, according to Dr. Robert O. Bonow.
STICHES is the 10-year follow-up of 1,212 such patients who were randomized to coronary artery bypass graft surgery plus optimal guideline-directed medical therapy or to the medical therapy alone. At 10 years, the CABG group showed a significant survival advantage: an all-cause mortality rate of 58.9%, a significant 16% relative risk reduction compared with the 66.1% rate in the medically managed group. Secondary endpoints were also strongly in favor of the CABG group.
These findings indicate CABG is beneficial in patients with ischemic cardiomyopathy, and patients deserve to be so informed, according to Dr. Bonow, a member of the STICHES publication committee and professor of cardiology and director of the Center for Cardiovascular Innovation at Northwestern University in Chicago, who discussed the findings in this video interview.
He reported having no financial conflicts regarding this National Institutes of Health–funded study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – The results of the Surgical Treatment for Ischemic Heart Failure Extension Study (STICHES) presented at the annual meeting of the American College of Cardiology ought to change the clinical management of patients with coronary artery disease and heart failure with severe left ventricular dysfunction, according to Dr. Robert O. Bonow.
STICHES is the 10-year follow-up of 1,212 such patients who were randomized to coronary artery bypass graft surgery plus optimal guideline-directed medical therapy or to the medical therapy alone. At 10 years, the CABG group showed a significant survival advantage: an all-cause mortality rate of 58.9%, a significant 16% relative risk reduction compared with the 66.1% rate in the medically managed group. Secondary endpoints were also strongly in favor of the CABG group.
These findings indicate CABG is beneficial in patients with ischemic cardiomyopathy, and patients deserve to be so informed, according to Dr. Bonow, a member of the STICHES publication committee and professor of cardiology and director of the Center for Cardiovascular Innovation at Northwestern University in Chicago, who discussed the findings in this video interview.
He reported having no financial conflicts regarding this National Institutes of Health–funded study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – The results of the Surgical Treatment for Ischemic Heart Failure Extension Study (STICHES) presented at the annual meeting of the American College of Cardiology ought to change the clinical management of patients with coronary artery disease and heart failure with severe left ventricular dysfunction, according to Dr. Robert O. Bonow.
STICHES is the 10-year follow-up of 1,212 such patients who were randomized to coronary artery bypass graft surgery plus optimal guideline-directed medical therapy or to the medical therapy alone. At 10 years, the CABG group showed a significant survival advantage: an all-cause mortality rate of 58.9%, a significant 16% relative risk reduction compared with the 66.1% rate in the medically managed group. Secondary endpoints were also strongly in favor of the CABG group.
These findings indicate CABG is beneficial in patients with ischemic cardiomyopathy, and patients deserve to be so informed, according to Dr. Bonow, a member of the STICHES publication committee and professor of cardiology and director of the Center for Cardiovascular Innovation at Northwestern University in Chicago, who discussed the findings in this video interview.
He reported having no financial conflicts regarding this National Institutes of Health–funded study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ACC 16
VIDEO: STICHES trial update boosts CABG in ischemic cardiomyopathy
CHICAGO – The results of the Surgical Treatment for Ischemic Heart Failure Extension Study (STICHES) presented at the annual meeting of the American College of Cardiology ought to change the clinical management of patients with coronary artery disease and heart failure with severe left ventricular dysfunction, according to Dr. Robert O. Bonow.
STICHES is the 10-year follow-up of 1,212 such patients who were randomized to coronary artery bypass graft surgery plus optimal guideline-directed medical therapy or to the medical therapy alone. At 10 years, the CABG group showed a significant survival advantage: an all-cause mortality rate of 58.9%, a significant 16% relative risk reduction compared with the 66.1% rate in the medically managed group. Secondary endpoints were also strongly in favor of the CABG group.
These findings indicate CABG is beneficial in patients with ischemic cardiomyopathy, and patients deserve to be so informed, according to Dr. Bonow, a member of the STICHES publication committee and professor of cardiology and director of the Center for Cardiovascular Innovation at Northwestern University in Chicago, who discussed the findings in this video interview.
He reported having no financial conflicts regarding this National Institutes of Health–funded study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – The results of the Surgical Treatment for Ischemic Heart Failure Extension Study (STICHES) presented at the annual meeting of the American College of Cardiology ought to change the clinical management of patients with coronary artery disease and heart failure with severe left ventricular dysfunction, according to Dr. Robert O. Bonow.
STICHES is the 10-year follow-up of 1,212 such patients who were randomized to coronary artery bypass graft surgery plus optimal guideline-directed medical therapy or to the medical therapy alone. At 10 years, the CABG group showed a significant survival advantage: an all-cause mortality rate of 58.9%, a significant 16% relative risk reduction compared with the 66.1% rate in the medically managed group. Secondary endpoints were also strongly in favor of the CABG group.
These findings indicate CABG is beneficial in patients with ischemic cardiomyopathy, and patients deserve to be so informed, according to Dr. Bonow, a member of the STICHES publication committee and professor of cardiology and director of the Center for Cardiovascular Innovation at Northwestern University in Chicago, who discussed the findings in this video interview.
He reported having no financial conflicts regarding this National Institutes of Health–funded study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – The results of the Surgical Treatment for Ischemic Heart Failure Extension Study (STICHES) presented at the annual meeting of the American College of Cardiology ought to change the clinical management of patients with coronary artery disease and heart failure with severe left ventricular dysfunction, according to Dr. Robert O. Bonow.
STICHES is the 10-year follow-up of 1,212 such patients who were randomized to coronary artery bypass graft surgery plus optimal guideline-directed medical therapy or to the medical therapy alone. At 10 years, the CABG group showed a significant survival advantage: an all-cause mortality rate of 58.9%, a significant 16% relative risk reduction compared with the 66.1% rate in the medically managed group. Secondary endpoints were also strongly in favor of the CABG group.
These findings indicate CABG is beneficial in patients with ischemic cardiomyopathy, and patients deserve to be so informed, according to Dr. Bonow, a member of the STICHES publication committee and professor of cardiology and director of the Center for Cardiovascular Innovation at Northwestern University in Chicago, who discussed the findings in this video interview.
He reported having no financial conflicts regarding this National Institutes of Health–funded study.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ACC 16
Adding mitral valve repair to CABG found risky, with limited benefits
In patients with moderate ischemic mitral regurgitation, adding mitral valve repair to coronary artery bypass graft did not significantly affect the extent of left ventricular remodeling at 2 years, according to a randomized multicenter trial.
Compared with revascularization alone, the combined procedure was associated with a two-thirds lower rate of moderate to severe mitral regurgitation at the end of year 2, reported Dr. Robert Michler at Albert Einstein College of Medicine, New York, together with his associates. But improved durability did not translate to better survival or lower rates of major adverse cardiac and cerebrovascular events, heart failure, or hospital readmissions, they said. Furthermore, adding mitral valve repair to CABG was linked to higher rates of postoperative neurologic events and supraventricular arrhythmias than for CABG alone, the investigators reported at the annual meeting of the American College of Cardiology and simultaneously online April 3 in the New England Journal of Medicine.
Surgeons have debated whether to add restrictive mitral annuloplasty to CABG in patients with moderate ischemic mitral regurgitation. The combined approach requires open-heart exposure and longer aortic cross-clamping and cardiopulmonary bypass time, all of which increase perioperative risk, the researchers noted. To explore the risk-benefit balance of these approaches, they randomly assigned 301 patients to either revascularization alone or to the combined procedure, and assessed clinical and echocardiographic outcomes over 2 years (New Engl. J. Med. 2016 April 3 doi: 10.1056/NEJMoa1602003).
At year 1, the arms resembled each other in terms of survival, rates of major adverse cardiac or cerebrovascular events (MACCEs), and left ventricular reverse remodeling, as measured by left ventricular end-systolic volume index (LVESVI), said the investigators. At year 2, the extent of remodeling remained similar between the arms, with mean LVESVIs of 41.2 mL (standard deviation, 20.0 mL) per square meter of body surface area in the CABG-only group and 43.2 mL (SD, 20.6 mL) in the combined procedure group, for a mean decrease from baseline of 14.1 and 14.6 mL, respectively. About two-thirds of improvements in LVESI occurred during year 1 regardless of procedure type, the researchers noted.
About a third of CABG-only patients had moderate to severe mitral regurgitation at 2 years, compared with only 11% of patients who underwent the combined procedure (P less than .001). Patients who had mild or no mitral regurgitation had more than twice the percentage improvement in the global wall motion index and in the inferior-posterior-lateral regional wall motion score, compared with patients with moderate to severe regurgitation. Although both groups improved on several quality-of-life indices, the combined-procedure group scored more than 5 points higher on the Duke Activity Status Index, which measures self-reported exercise capacity. That difference was not only clinically meaningful, but resembled the increase in peak myocardial oxygen consumption in a similar, smaller trial (New Engl. J. Med. 2016 Jan. 28 doi: 10.1056/NEJMoa151291), the investigators noted.
However, mortality rates were similar between the arms at 2 years – 10.6% for CABG only and 10% for the combined procedure, for a non-significant hazard ratio of 0.9. Likewise, rates of heart failure and other serious adverse events were similar between the arms, while the combined-procedure arm had more than three times more postoperative neurologic events and more than twice as many occurrences of supraventricular arrhythmia. Therefore, treatment choice requires “balancing the risks of adverse perioperative events against the uncertain benefits of a lower incidence of postoperative moderate or severe mitral regurgitation,” said the researchers. “Effective revascularization, as reflected in improved regional and global left ventricular function, plays an important role independent of mitral valve repair.”
The National Institutes of Health and the Canadian Institutes of Health Research funded the study. Disclosures were reported separately at nejm.org.
In patients with moderate ischemic mitral regurgitation, adding mitral valve repair to coronary artery bypass graft did not significantly affect the extent of left ventricular remodeling at 2 years, according to a randomized multicenter trial.
Compared with revascularization alone, the combined procedure was associated with a two-thirds lower rate of moderate to severe mitral regurgitation at the end of year 2, reported Dr. Robert Michler at Albert Einstein College of Medicine, New York, together with his associates. But improved durability did not translate to better survival or lower rates of major adverse cardiac and cerebrovascular events, heart failure, or hospital readmissions, they said. Furthermore, adding mitral valve repair to CABG was linked to higher rates of postoperative neurologic events and supraventricular arrhythmias than for CABG alone, the investigators reported at the annual meeting of the American College of Cardiology and simultaneously online April 3 in the New England Journal of Medicine.
Surgeons have debated whether to add restrictive mitral annuloplasty to CABG in patients with moderate ischemic mitral regurgitation. The combined approach requires open-heart exposure and longer aortic cross-clamping and cardiopulmonary bypass time, all of which increase perioperative risk, the researchers noted. To explore the risk-benefit balance of these approaches, they randomly assigned 301 patients to either revascularization alone or to the combined procedure, and assessed clinical and echocardiographic outcomes over 2 years (New Engl. J. Med. 2016 April 3 doi: 10.1056/NEJMoa1602003).
At year 1, the arms resembled each other in terms of survival, rates of major adverse cardiac or cerebrovascular events (MACCEs), and left ventricular reverse remodeling, as measured by left ventricular end-systolic volume index (LVESVI), said the investigators. At year 2, the extent of remodeling remained similar between the arms, with mean LVESVIs of 41.2 mL (standard deviation, 20.0 mL) per square meter of body surface area in the CABG-only group and 43.2 mL (SD, 20.6 mL) in the combined procedure group, for a mean decrease from baseline of 14.1 and 14.6 mL, respectively. About two-thirds of improvements in LVESI occurred during year 1 regardless of procedure type, the researchers noted.
About a third of CABG-only patients had moderate to severe mitral regurgitation at 2 years, compared with only 11% of patients who underwent the combined procedure (P less than .001). Patients who had mild or no mitral regurgitation had more than twice the percentage improvement in the global wall motion index and in the inferior-posterior-lateral regional wall motion score, compared with patients with moderate to severe regurgitation. Although both groups improved on several quality-of-life indices, the combined-procedure group scored more than 5 points higher on the Duke Activity Status Index, which measures self-reported exercise capacity. That difference was not only clinically meaningful, but resembled the increase in peak myocardial oxygen consumption in a similar, smaller trial (New Engl. J. Med. 2016 Jan. 28 doi: 10.1056/NEJMoa151291), the investigators noted.
However, mortality rates were similar between the arms at 2 years – 10.6% for CABG only and 10% for the combined procedure, for a non-significant hazard ratio of 0.9. Likewise, rates of heart failure and other serious adverse events were similar between the arms, while the combined-procedure arm had more than three times more postoperative neurologic events and more than twice as many occurrences of supraventricular arrhythmia. Therefore, treatment choice requires “balancing the risks of adverse perioperative events against the uncertain benefits of a lower incidence of postoperative moderate or severe mitral regurgitation,” said the researchers. “Effective revascularization, as reflected in improved regional and global left ventricular function, plays an important role independent of mitral valve repair.”
The National Institutes of Health and the Canadian Institutes of Health Research funded the study. Disclosures were reported separately at nejm.org.
In patients with moderate ischemic mitral regurgitation, adding mitral valve repair to coronary artery bypass graft did not significantly affect the extent of left ventricular remodeling at 2 years, according to a randomized multicenter trial.
Compared with revascularization alone, the combined procedure was associated with a two-thirds lower rate of moderate to severe mitral regurgitation at the end of year 2, reported Dr. Robert Michler at Albert Einstein College of Medicine, New York, together with his associates. But improved durability did not translate to better survival or lower rates of major adverse cardiac and cerebrovascular events, heart failure, or hospital readmissions, they said. Furthermore, adding mitral valve repair to CABG was linked to higher rates of postoperative neurologic events and supraventricular arrhythmias than for CABG alone, the investigators reported at the annual meeting of the American College of Cardiology and simultaneously online April 3 in the New England Journal of Medicine.
Surgeons have debated whether to add restrictive mitral annuloplasty to CABG in patients with moderate ischemic mitral regurgitation. The combined approach requires open-heart exposure and longer aortic cross-clamping and cardiopulmonary bypass time, all of which increase perioperative risk, the researchers noted. To explore the risk-benefit balance of these approaches, they randomly assigned 301 patients to either revascularization alone or to the combined procedure, and assessed clinical and echocardiographic outcomes over 2 years (New Engl. J. Med. 2016 April 3 doi: 10.1056/NEJMoa1602003).
At year 1, the arms resembled each other in terms of survival, rates of major adverse cardiac or cerebrovascular events (MACCEs), and left ventricular reverse remodeling, as measured by left ventricular end-systolic volume index (LVESVI), said the investigators. At year 2, the extent of remodeling remained similar between the arms, with mean LVESVIs of 41.2 mL (standard deviation, 20.0 mL) per square meter of body surface area in the CABG-only group and 43.2 mL (SD, 20.6 mL) in the combined procedure group, for a mean decrease from baseline of 14.1 and 14.6 mL, respectively. About two-thirds of improvements in LVESI occurred during year 1 regardless of procedure type, the researchers noted.
About a third of CABG-only patients had moderate to severe mitral regurgitation at 2 years, compared with only 11% of patients who underwent the combined procedure (P less than .001). Patients who had mild or no mitral regurgitation had more than twice the percentage improvement in the global wall motion index and in the inferior-posterior-lateral regional wall motion score, compared with patients with moderate to severe regurgitation. Although both groups improved on several quality-of-life indices, the combined-procedure group scored more than 5 points higher on the Duke Activity Status Index, which measures self-reported exercise capacity. That difference was not only clinically meaningful, but resembled the increase in peak myocardial oxygen consumption in a similar, smaller trial (New Engl. J. Med. 2016 Jan. 28 doi: 10.1056/NEJMoa151291), the investigators noted.
However, mortality rates were similar between the arms at 2 years – 10.6% for CABG only and 10% for the combined procedure, for a non-significant hazard ratio of 0.9. Likewise, rates of heart failure and other serious adverse events were similar between the arms, while the combined-procedure arm had more than three times more postoperative neurologic events and more than twice as many occurrences of supraventricular arrhythmia. Therefore, treatment choice requires “balancing the risks of adverse perioperative events against the uncertain benefits of a lower incidence of postoperative moderate or severe mitral regurgitation,” said the researchers. “Effective revascularization, as reflected in improved regional and global left ventricular function, plays an important role independent of mitral valve repair.”
The National Institutes of Health and the Canadian Institutes of Health Research funded the study. Disclosures were reported separately at nejm.org.
FROM ACC 16
Key clinical point: In patients with moderate ischemic mitral regurgitation, adding mitral valve repair to coronary artery bypass graft did not significantly affect left ventricular remodeling at two years.
Major finding: Mean decreases in left ventricular end systolic volume index were 14.1 mL and 14.6 mL, respectively.
Data source: A multicenter randomized trial of 301 patients with moderate to severe baseline mitral regurgitation.
Disclosures: The National Institutes of Health and the Canadian Institutes of Health Research funded the study. Disclosures were reported separately at nejm.org.
Self-expanding TAVR bests surgery based on 3-year stroke and death risks
Patients with severe aortic stenosis that puts them at increased risk for surgery continue to do better at 3 years after receiving a self-expanding transcatheter aortic valve replacement than do similar patients who have an open surgical valve replacement, according to new results from a randomized trial presented at the annual meeting of the American College of Cardiology.
Two-year follow-up results from the same trial cohort, the CoreValve U.S. Pivotal High Risk Trial, showed superior survival and stroke outcomes for TAVR compared with open surgery (J Am Coll Cardiol. 2015;66[2]:113-21). The difference in outcomes was thought to stem mainly from fewer postprocedural complications and faster recovery in the TAVR group.
The new study, presented at the meeting and simultaneously published online April 3 in the Journal of the American College of Cardiology (doi: 10.1016/j.jacc.2016.03.506) aimed to determine whether the previously seen benefits extended into the third year and whether these were accompanied by differences in valve hemodynamics.
Dr. G. Michael Deeb, Herbert Sloan Collegiate Professor of Cardiac Surgery at the University of Michigan, Ann Arbor, and his colleagues evaluated three-year clinical and echocardiographic outcomes from the 391 patients who underwent TAVR and 359 who had SAVR. At baseline all patients had severe aortic stenosis and were considered to be at increased risk for SAVR, with an estimated 30-day mortality risk 15% or greater and a combined 30-day surgical mortality and major morbidity risk less than 50%.
At 3 years follow-up in the treated groups, combined all-cause mortality or stroke was significantly lower at 37% in TAVR patients as compared to nearly 47% in SAVR patients. All-cause mortality was 33% with TAVR and 39% with SAVR, a difference that did not reach statistical significance. Stroke rates were nearly 13% with TAVR and 19% with SAVR; major adverse cardiovascular or cerebrovascular events were 40% with TAVR and 48% for SAVR. Both were significant differences.
While mean aortic valve gradient measures were more favorable – 7.62 ± 3.57 mm Hg with TAVR and 11.40 ± 6.81 mm Hg with SAVR – regurgitation was significantly higher at nearly 7% with TAVR and no regurgitation with SAVR. Valve thrombosis and valve structural deterioration were not observed in either group.
While the findings show sustained 3-year clinical benefit of self-expanding TAVR over SAVR in patients with aortic stenosis at increased risk for surgery, longer studies are needed to determine whether the crimping and re-crimping of the transcatheter valve would have an impact on long-term bioprosthesis durability.
The study was funded by the device manufacturer Medtronic, and 21 of its 28 authors disclosed financial relationships with Medtronic and/or other manufacturers; one is a Medtronic employee. Dr. Deeb disclosed serving as an unpaid advisor to Medtronic.
Patients with severe aortic stenosis that puts them at increased risk for surgery continue to do better at 3 years after receiving a self-expanding transcatheter aortic valve replacement than do similar patients who have an open surgical valve replacement, according to new results from a randomized trial presented at the annual meeting of the American College of Cardiology.
Two-year follow-up results from the same trial cohort, the CoreValve U.S. Pivotal High Risk Trial, showed superior survival and stroke outcomes for TAVR compared with open surgery (J Am Coll Cardiol. 2015;66[2]:113-21). The difference in outcomes was thought to stem mainly from fewer postprocedural complications and faster recovery in the TAVR group.
The new study, presented at the meeting and simultaneously published online April 3 in the Journal of the American College of Cardiology (doi: 10.1016/j.jacc.2016.03.506) aimed to determine whether the previously seen benefits extended into the third year and whether these were accompanied by differences in valve hemodynamics.
Dr. G. Michael Deeb, Herbert Sloan Collegiate Professor of Cardiac Surgery at the University of Michigan, Ann Arbor, and his colleagues evaluated three-year clinical and echocardiographic outcomes from the 391 patients who underwent TAVR and 359 who had SAVR. At baseline all patients had severe aortic stenosis and were considered to be at increased risk for SAVR, with an estimated 30-day mortality risk 15% or greater and a combined 30-day surgical mortality and major morbidity risk less than 50%.
At 3 years follow-up in the treated groups, combined all-cause mortality or stroke was significantly lower at 37% in TAVR patients as compared to nearly 47% in SAVR patients. All-cause mortality was 33% with TAVR and 39% with SAVR, a difference that did not reach statistical significance. Stroke rates were nearly 13% with TAVR and 19% with SAVR; major adverse cardiovascular or cerebrovascular events were 40% with TAVR and 48% for SAVR. Both were significant differences.
While mean aortic valve gradient measures were more favorable – 7.62 ± 3.57 mm Hg with TAVR and 11.40 ± 6.81 mm Hg with SAVR – regurgitation was significantly higher at nearly 7% with TAVR and no regurgitation with SAVR. Valve thrombosis and valve structural deterioration were not observed in either group.
While the findings show sustained 3-year clinical benefit of self-expanding TAVR over SAVR in patients with aortic stenosis at increased risk for surgery, longer studies are needed to determine whether the crimping and re-crimping of the transcatheter valve would have an impact on long-term bioprosthesis durability.
The study was funded by the device manufacturer Medtronic, and 21 of its 28 authors disclosed financial relationships with Medtronic and/or other manufacturers; one is a Medtronic employee. Dr. Deeb disclosed serving as an unpaid advisor to Medtronic.
Patients with severe aortic stenosis that puts them at increased risk for surgery continue to do better at 3 years after receiving a self-expanding transcatheter aortic valve replacement than do similar patients who have an open surgical valve replacement, according to new results from a randomized trial presented at the annual meeting of the American College of Cardiology.
Two-year follow-up results from the same trial cohort, the CoreValve U.S. Pivotal High Risk Trial, showed superior survival and stroke outcomes for TAVR compared with open surgery (J Am Coll Cardiol. 2015;66[2]:113-21). The difference in outcomes was thought to stem mainly from fewer postprocedural complications and faster recovery in the TAVR group.
The new study, presented at the meeting and simultaneously published online April 3 in the Journal of the American College of Cardiology (doi: 10.1016/j.jacc.2016.03.506) aimed to determine whether the previously seen benefits extended into the third year and whether these were accompanied by differences in valve hemodynamics.
Dr. G. Michael Deeb, Herbert Sloan Collegiate Professor of Cardiac Surgery at the University of Michigan, Ann Arbor, and his colleagues evaluated three-year clinical and echocardiographic outcomes from the 391 patients who underwent TAVR and 359 who had SAVR. At baseline all patients had severe aortic stenosis and were considered to be at increased risk for SAVR, with an estimated 30-day mortality risk 15% or greater and a combined 30-day surgical mortality and major morbidity risk less than 50%.
At 3 years follow-up in the treated groups, combined all-cause mortality or stroke was significantly lower at 37% in TAVR patients as compared to nearly 47% in SAVR patients. All-cause mortality was 33% with TAVR and 39% with SAVR, a difference that did not reach statistical significance. Stroke rates were nearly 13% with TAVR and 19% with SAVR; major adverse cardiovascular or cerebrovascular events were 40% with TAVR and 48% for SAVR. Both were significant differences.
While mean aortic valve gradient measures were more favorable – 7.62 ± 3.57 mm Hg with TAVR and 11.40 ± 6.81 mm Hg with SAVR – regurgitation was significantly higher at nearly 7% with TAVR and no regurgitation with SAVR. Valve thrombosis and valve structural deterioration were not observed in either group.
While the findings show sustained 3-year clinical benefit of self-expanding TAVR over SAVR in patients with aortic stenosis at increased risk for surgery, longer studies are needed to determine whether the crimping and re-crimping of the transcatheter valve would have an impact on long-term bioprosthesis durability.
The study was funded by the device manufacturer Medtronic, and 21 of its 28 authors disclosed financial relationships with Medtronic and/or other manufacturers; one is a Medtronic employee. Dr. Deeb disclosed serving as an unpaid advisor to Medtronic.
FROM ACC 2016
Key clinical point: Patients randomized to self-expanding TAVR or open surgical aortic valve replacement were less likely to have died or had a stroke at 3 years post-procedure
Major finding:. Three-year all-cause mortality and stroke rate was significantly lower in TAVR patients – 37% versus nearly 47% in SAVR patients.
Data source: A cohort of 750 patients deemed high risk who underwent open aortic valve replacement or TAVR after randomization; procedures performed at 45 sites
Disclosures: The study was sponsored by Medtronic, maker of the self-expanding transcatheter technology. Most study authors, though not the lead author, had direct financial involvement.
TAVR matches surgery in intermediate-risk patients
CHICAGO – Transcatheter aortic-valve replacement performed as well as surgical-valve replacement in patients with an intermediate mortality risk in a prospective, randomized trial with more than 2,000 patients followed for 2 years, the first randomized trial to compare the efficacy and safety of transcatheter aortic-valve replacement against surgical replacement in patients who did not have a high mortality risk.
The results “support TAVR [transcatheter aortic-valve replacement] as an alternative to surgery in intermediate-risk patients similar to those included in this trial,” said Dr. Craig R. Smith at the annual meeting of the American College of Cardiology. The findings from the Placement of Aortic Transcatheter Valves (PARTNER) 2 cohort A trial “will increase use of TAVR,” predicted Dr. Smith, professor and chairman of surgery at New York–Presbyterian Hospital/Columbia University in New York.
Until now, TAVR had been compared with surgical aortic-valve replacement in two prospective, randomized trials that both enrolled either high-risk or inoperable patients with severe aortic stenosis, the PARTNER 1 trial that tested the original Sapien TAVR system, and the U.S. CoreValve High-Risk Study that tested the original CoreValve system (often now called CoreValve classic). The average Society of Thoracic Surgeons (STS) operative risk score of high-risk patients enrolled in PARTNER 1 was 11.8%, and the average risk score in patients enrolled in the CoreValve study was 7.3%. In contrast, the design of PARTNER 2A specified that enrolled patients have a STS risk score of 4%-8%, a criterion actually met by 81% of the enrolled patients, and the average STS risk score of all patients enrolled in PARTNER 2A was 5.8%.
Although U.S. labeling for both the Sapien valve and its later iterations, Sapien XT and S3, and for CoreValve and its later iteration, Evolut R, specify that treated patients should have a STS risk score of at least 8%, the labeling also gives the heart teams that perform TAVR the latitude to treat patients with risk scores below 8% when the heart teams identify other patient factors that confer high risk such as frailty or comorbidities. U.S. and European TAVR registries have documented that many patients with STS risk scores below 8% have undergone TAVR since these systems received regulatory approval. The new results from PARTNER 2A may change that by leading to revised labeling that cuts the STS risk-score threshold.
“These findings might lead to a labeling change that would avoid a lot of the patient-evaluation gymnastics that have been used to justify” TAVR treatment, noted Dr. Smith. New labeling like this “would sanction what is already going on” in terms of which patients undergo TAVR.
Others who heard these results at the meeting agreed they were an important milestone in TAVR development and its expanding use.
The new results “make a huge difference,” commented Dr. David R. Holmes Jr., an interventional cardiologist and professor at the Mayo Clinic in Rochester, Minn. “We base many of our guidelines on the results from randomized, controlled trials. It’s true that there are reports of lower-risk patients undergoing TAVR, but we now have results from a well-designed trial with well-controlled and adjudicated endpoints that documents the safety and efficacy of TAVR in intermediate-risk patients,” Dr. Holmes said in an interview.
“The results will have a very important influence on the choice between TAVR and surgery,” commented Dr. Duane S. Pinto, an interventional cardiologist at Beth Israel Deaconess Medical Center in Boston. “It validates the strategy” of using TAVR in patients with a risk score of 4%-8%. “TAVR has already been used in these patients, but these results validate this, especially when used in a transfemoral approach,” Dr. Pinto said in an interview.
One aspect of PARTNER 2A that received a lot of discussion at the meeting was whether enrolled patients could appropriately be characterized as “intermediate” in their risk level. Although their average STS risk score of 5.8% fell squarely within the target range specified for the study, they averaged 82 years old, and other clinical features at baseline suggested a higher risk population. The published report of the PARTNER 2A results that appeared online concurrent with Dr. Smith’s report at the meeting (New Engl J Med 2016;doi:10.1056/NEJMoa1514616) acknowledged that STS risk scores of 4%-8% place the enrolled patients into the upper 20% for risk of all U.S. patients who undergo surgical aortic-valve replacement
“I would characterize the enrolled patients as ‘less high risk’ rather than intermediate risk,” said Dr. Pinto.
But as Dr. Smith explained “even if the enrolled patients are not ‘intermediate’ risk they are at a different risk level” than were the patients enrolled in the prior TAVR randomized trials.
In the PARTNER 1 high-risk trial, the overall 1-year rate of all-cause mortality was 24% and 27% in the TAVR and surgical arms of the study, respectively. In the CoreValve trial these rates were 14% with TAVR and 19% with surgery. In PARTNER 2A 1-year all-cause mortality was 12% with TAVR and 13% with surgery.
Two other notable findings of PARTNER 2A were the superior outcomes of patients who underwent TAVR using a transfemoral approach, and the improved outcomes that all TAVR patients had compared with surgical valve replacement for several secondary outcomes.
The rate of the study’s primary outcome, all-cause death or disabling stroke after 2 years, was cut by a relative 21% in the 77% of TAVR patients who underwent a transfemoral procedure, compared with the surgery patients, a difference that was of borderline statistical significance. In contrast, the entire group of TAVR patients, including those treated via nontransfemoral routes, had an 11% relative reduction of the primary endpoint, compared with surgery, a difference that was not statistically significant but did easily meet the study’s prespecified definition of noninferority. Dr. Smith and others were especially encouraged by these findings as PARTNER 2A used the older Sapien XT TAVR system that is not often used today in U.S. practice. When U.S. patients undergo TAVR with a balloon-expandable valve they most often receive treatment with the S3 system, much smaller than XT and hence much more likely to be used with a transfemoral approach.
Other secondary outcomes included life-threatening or disabling bleeding events, which after 2 years had occurred in 17% of the TAVR patients and 47% of those who underwent surgery; atrial fibrillation, which occurred in 11% of the TAVR patients and 27% of those undergoing surgery; and acute kidney injury which occurred in 4% of TAVR patients and 6% of the surgery patients. With 2-year follow-up, the rate of disabling strokes was 6% in both arms of the study.
PARTNER 2A was sponsored by Edwards Lifesciences, the company that markets the Sapien TAVR systems. Dr. Smith has received travel grants from Edwards. Dr. Holmes had no disclosures, Dr. Pinto has been a consultant to Medtronic.
On Twitter @mitchelzoler
Registries of patients who have undergone transcatheter aortic-valve replacement in Europe and the United States show that this procedure has already been frequently used in selected patients with Society of Thoracic Surgeons operative-risk scores of 4%-8%. Even though regulatory approval specifies using the procedure in high-risk patients with risk scores of at least 8%, the labeling leaves the decision of which patients are at high risk up to local heart teams, and factors other than the risk score play into a patient’s overall risk assessment including frailty and comorbidities.
Despite the prior experience using TAVR in patients with STS risk scores of 4%-8% the results of PARTNER 2A are a game changer because they come from a prospective, randomized, controlled trial.
The PARTNER 2A results are also notable because this is the second randomized trial (in addition to the CoreValve high-risk trial) with results that show or suggest that transcatheter aortic-valve replacement (TAVR) produces better outcomes than surgery, especially in patients who undergo TAVR via a transfemoral approach. Other notable advantages of TAVR over surgery seen in PARTNER 2A include substantial reductions in disabling or life-threatening bleeding events and in new-onset atrial fibrillation, a statistically significant reduction in acute kidney injury, and no significant difference in the incidence of disabling strokes. In the past, we expected stroke rates to be higher with TAVR, but in PARTNER 2A, with neurologists adjudicating the strokes, we saw no difference in the TAVR and surgical stroke rates, a finding that was probably unexpected for many people.

|
Dr. Ajay J. Kirtane |
The patients enrolled in PARTNER 2A were clearly at lower risk for all-cause mortality than the patients enrolled in the earlier TAVR trials. The operative risk score is just one of several ways to estimate patient risk. The data collected in PARTNER 2A provide a robust resource for finding new, additional ways to assess patients who are at intermediate risk and to match patients seen during routine practice to those who entered this trial.
Dr. Ajay J. Kirtane is an interventional cardiologist and director of the coronary catheterization laboratory at New York–Presbyterian/Columbia University in New York. He was a coinvestigator on prior Sapien TAVR studies but did not participate in PARTNER 2. His institution has received research support from Edwards and from Boston Scientific. He made these comments in an interview.
Registries of patients who have undergone transcatheter aortic-valve replacement in Europe and the United States show that this procedure has already been frequently used in selected patients with Society of Thoracic Surgeons operative-risk scores of 4%-8%. Even though regulatory approval specifies using the procedure in high-risk patients with risk scores of at least 8%, the labeling leaves the decision of which patients are at high risk up to local heart teams, and factors other than the risk score play into a patient’s overall risk assessment including frailty and comorbidities.
Despite the prior experience using TAVR in patients with STS risk scores of 4%-8% the results of PARTNER 2A are a game changer because they come from a prospective, randomized, controlled trial.
The PARTNER 2A results are also notable because this is the second randomized trial (in addition to the CoreValve high-risk trial) with results that show or suggest that transcatheter aortic-valve replacement (TAVR) produces better outcomes than surgery, especially in patients who undergo TAVR via a transfemoral approach. Other notable advantages of TAVR over surgery seen in PARTNER 2A include substantial reductions in disabling or life-threatening bleeding events and in new-onset atrial fibrillation, a statistically significant reduction in acute kidney injury, and no significant difference in the incidence of disabling strokes. In the past, we expected stroke rates to be higher with TAVR, but in PARTNER 2A, with neurologists adjudicating the strokes, we saw no difference in the TAVR and surgical stroke rates, a finding that was probably unexpected for many people.

|
Dr. Ajay J. Kirtane |
The patients enrolled in PARTNER 2A were clearly at lower risk for all-cause mortality than the patients enrolled in the earlier TAVR trials. The operative risk score is just one of several ways to estimate patient risk. The data collected in PARTNER 2A provide a robust resource for finding new, additional ways to assess patients who are at intermediate risk and to match patients seen during routine practice to those who entered this trial.
Dr. Ajay J. Kirtane is an interventional cardiologist and director of the coronary catheterization laboratory at New York–Presbyterian/Columbia University in New York. He was a coinvestigator on prior Sapien TAVR studies but did not participate in PARTNER 2. His institution has received research support from Edwards and from Boston Scientific. He made these comments in an interview.
Registries of patients who have undergone transcatheter aortic-valve replacement in Europe and the United States show that this procedure has already been frequently used in selected patients with Society of Thoracic Surgeons operative-risk scores of 4%-8%. Even though regulatory approval specifies using the procedure in high-risk patients with risk scores of at least 8%, the labeling leaves the decision of which patients are at high risk up to local heart teams, and factors other than the risk score play into a patient’s overall risk assessment including frailty and comorbidities.
Despite the prior experience using TAVR in patients with STS risk scores of 4%-8% the results of PARTNER 2A are a game changer because they come from a prospective, randomized, controlled trial.
The PARTNER 2A results are also notable because this is the second randomized trial (in addition to the CoreValve high-risk trial) with results that show or suggest that transcatheter aortic-valve replacement (TAVR) produces better outcomes than surgery, especially in patients who undergo TAVR via a transfemoral approach. Other notable advantages of TAVR over surgery seen in PARTNER 2A include substantial reductions in disabling or life-threatening bleeding events and in new-onset atrial fibrillation, a statistically significant reduction in acute kidney injury, and no significant difference in the incidence of disabling strokes. In the past, we expected stroke rates to be higher with TAVR, but in PARTNER 2A, with neurologists adjudicating the strokes, we saw no difference in the TAVR and surgical stroke rates, a finding that was probably unexpected for many people.

|
Dr. Ajay J. Kirtane |
The patients enrolled in PARTNER 2A were clearly at lower risk for all-cause mortality than the patients enrolled in the earlier TAVR trials. The operative risk score is just one of several ways to estimate patient risk. The data collected in PARTNER 2A provide a robust resource for finding new, additional ways to assess patients who are at intermediate risk and to match patients seen during routine practice to those who entered this trial.
Dr. Ajay J. Kirtane is an interventional cardiologist and director of the coronary catheterization laboratory at New York–Presbyterian/Columbia University in New York. He was a coinvestigator on prior Sapien TAVR studies but did not participate in PARTNER 2. His institution has received research support from Edwards and from Boston Scientific. He made these comments in an interview.
CHICAGO – Transcatheter aortic-valve replacement performed as well as surgical-valve replacement in patients with an intermediate mortality risk in a prospective, randomized trial with more than 2,000 patients followed for 2 years, the first randomized trial to compare the efficacy and safety of transcatheter aortic-valve replacement against surgical replacement in patients who did not have a high mortality risk.
The results “support TAVR [transcatheter aortic-valve replacement] as an alternative to surgery in intermediate-risk patients similar to those included in this trial,” said Dr. Craig R. Smith at the annual meeting of the American College of Cardiology. The findings from the Placement of Aortic Transcatheter Valves (PARTNER) 2 cohort A trial “will increase use of TAVR,” predicted Dr. Smith, professor and chairman of surgery at New York–Presbyterian Hospital/Columbia University in New York.
Until now, TAVR had been compared with surgical aortic-valve replacement in two prospective, randomized trials that both enrolled either high-risk or inoperable patients with severe aortic stenosis, the PARTNER 1 trial that tested the original Sapien TAVR system, and the U.S. CoreValve High-Risk Study that tested the original CoreValve system (often now called CoreValve classic). The average Society of Thoracic Surgeons (STS) operative risk score of high-risk patients enrolled in PARTNER 1 was 11.8%, and the average risk score in patients enrolled in the CoreValve study was 7.3%. In contrast, the design of PARTNER 2A specified that enrolled patients have a STS risk score of 4%-8%, a criterion actually met by 81% of the enrolled patients, and the average STS risk score of all patients enrolled in PARTNER 2A was 5.8%.
Although U.S. labeling for both the Sapien valve and its later iterations, Sapien XT and S3, and for CoreValve and its later iteration, Evolut R, specify that treated patients should have a STS risk score of at least 8%, the labeling also gives the heart teams that perform TAVR the latitude to treat patients with risk scores below 8% when the heart teams identify other patient factors that confer high risk such as frailty or comorbidities. U.S. and European TAVR registries have documented that many patients with STS risk scores below 8% have undergone TAVR since these systems received regulatory approval. The new results from PARTNER 2A may change that by leading to revised labeling that cuts the STS risk-score threshold.
“These findings might lead to a labeling change that would avoid a lot of the patient-evaluation gymnastics that have been used to justify” TAVR treatment, noted Dr. Smith. New labeling like this “would sanction what is already going on” in terms of which patients undergo TAVR.
Others who heard these results at the meeting agreed they were an important milestone in TAVR development and its expanding use.
The new results “make a huge difference,” commented Dr. David R. Holmes Jr., an interventional cardiologist and professor at the Mayo Clinic in Rochester, Minn. “We base many of our guidelines on the results from randomized, controlled trials. It’s true that there are reports of lower-risk patients undergoing TAVR, but we now have results from a well-designed trial with well-controlled and adjudicated endpoints that documents the safety and efficacy of TAVR in intermediate-risk patients,” Dr. Holmes said in an interview.
“The results will have a very important influence on the choice between TAVR and surgery,” commented Dr. Duane S. Pinto, an interventional cardiologist at Beth Israel Deaconess Medical Center in Boston. “It validates the strategy” of using TAVR in patients with a risk score of 4%-8%. “TAVR has already been used in these patients, but these results validate this, especially when used in a transfemoral approach,” Dr. Pinto said in an interview.
One aspect of PARTNER 2A that received a lot of discussion at the meeting was whether enrolled patients could appropriately be characterized as “intermediate” in their risk level. Although their average STS risk score of 5.8% fell squarely within the target range specified for the study, they averaged 82 years old, and other clinical features at baseline suggested a higher risk population. The published report of the PARTNER 2A results that appeared online concurrent with Dr. Smith’s report at the meeting (New Engl J Med 2016;doi:10.1056/NEJMoa1514616) acknowledged that STS risk scores of 4%-8% place the enrolled patients into the upper 20% for risk of all U.S. patients who undergo surgical aortic-valve replacement
“I would characterize the enrolled patients as ‘less high risk’ rather than intermediate risk,” said Dr. Pinto.
But as Dr. Smith explained “even if the enrolled patients are not ‘intermediate’ risk they are at a different risk level” than were the patients enrolled in the prior TAVR randomized trials.
In the PARTNER 1 high-risk trial, the overall 1-year rate of all-cause mortality was 24% and 27% in the TAVR and surgical arms of the study, respectively. In the CoreValve trial these rates were 14% with TAVR and 19% with surgery. In PARTNER 2A 1-year all-cause mortality was 12% with TAVR and 13% with surgery.
Two other notable findings of PARTNER 2A were the superior outcomes of patients who underwent TAVR using a transfemoral approach, and the improved outcomes that all TAVR patients had compared with surgical valve replacement for several secondary outcomes.
The rate of the study’s primary outcome, all-cause death or disabling stroke after 2 years, was cut by a relative 21% in the 77% of TAVR patients who underwent a transfemoral procedure, compared with the surgery patients, a difference that was of borderline statistical significance. In contrast, the entire group of TAVR patients, including those treated via nontransfemoral routes, had an 11% relative reduction of the primary endpoint, compared with surgery, a difference that was not statistically significant but did easily meet the study’s prespecified definition of noninferority. Dr. Smith and others were especially encouraged by these findings as PARTNER 2A used the older Sapien XT TAVR system that is not often used today in U.S. practice. When U.S. patients undergo TAVR with a balloon-expandable valve they most often receive treatment with the S3 system, much smaller than XT and hence much more likely to be used with a transfemoral approach.
Other secondary outcomes included life-threatening or disabling bleeding events, which after 2 years had occurred in 17% of the TAVR patients and 47% of those who underwent surgery; atrial fibrillation, which occurred in 11% of the TAVR patients and 27% of those undergoing surgery; and acute kidney injury which occurred in 4% of TAVR patients and 6% of the surgery patients. With 2-year follow-up, the rate of disabling strokes was 6% in both arms of the study.
PARTNER 2A was sponsored by Edwards Lifesciences, the company that markets the Sapien TAVR systems. Dr. Smith has received travel grants from Edwards. Dr. Holmes had no disclosures, Dr. Pinto has been a consultant to Medtronic.
On Twitter @mitchelzoler
CHICAGO – Transcatheter aortic-valve replacement performed as well as surgical-valve replacement in patients with an intermediate mortality risk in a prospective, randomized trial with more than 2,000 patients followed for 2 years, the first randomized trial to compare the efficacy and safety of transcatheter aortic-valve replacement against surgical replacement in patients who did not have a high mortality risk.
The results “support TAVR [transcatheter aortic-valve replacement] as an alternative to surgery in intermediate-risk patients similar to those included in this trial,” said Dr. Craig R. Smith at the annual meeting of the American College of Cardiology. The findings from the Placement of Aortic Transcatheter Valves (PARTNER) 2 cohort A trial “will increase use of TAVR,” predicted Dr. Smith, professor and chairman of surgery at New York–Presbyterian Hospital/Columbia University in New York.
Until now, TAVR had been compared with surgical aortic-valve replacement in two prospective, randomized trials that both enrolled either high-risk or inoperable patients with severe aortic stenosis, the PARTNER 1 trial that tested the original Sapien TAVR system, and the U.S. CoreValve High-Risk Study that tested the original CoreValve system (often now called CoreValve classic). The average Society of Thoracic Surgeons (STS) operative risk score of high-risk patients enrolled in PARTNER 1 was 11.8%, and the average risk score in patients enrolled in the CoreValve study was 7.3%. In contrast, the design of PARTNER 2A specified that enrolled patients have a STS risk score of 4%-8%, a criterion actually met by 81% of the enrolled patients, and the average STS risk score of all patients enrolled in PARTNER 2A was 5.8%.
Although U.S. labeling for both the Sapien valve and its later iterations, Sapien XT and S3, and for CoreValve and its later iteration, Evolut R, specify that treated patients should have a STS risk score of at least 8%, the labeling also gives the heart teams that perform TAVR the latitude to treat patients with risk scores below 8% when the heart teams identify other patient factors that confer high risk such as frailty or comorbidities. U.S. and European TAVR registries have documented that many patients with STS risk scores below 8% have undergone TAVR since these systems received regulatory approval. The new results from PARTNER 2A may change that by leading to revised labeling that cuts the STS risk-score threshold.
“These findings might lead to a labeling change that would avoid a lot of the patient-evaluation gymnastics that have been used to justify” TAVR treatment, noted Dr. Smith. New labeling like this “would sanction what is already going on” in terms of which patients undergo TAVR.
Others who heard these results at the meeting agreed they were an important milestone in TAVR development and its expanding use.
The new results “make a huge difference,” commented Dr. David R. Holmes Jr., an interventional cardiologist and professor at the Mayo Clinic in Rochester, Minn. “We base many of our guidelines on the results from randomized, controlled trials. It’s true that there are reports of lower-risk patients undergoing TAVR, but we now have results from a well-designed trial with well-controlled and adjudicated endpoints that documents the safety and efficacy of TAVR in intermediate-risk patients,” Dr. Holmes said in an interview.
“The results will have a very important influence on the choice between TAVR and surgery,” commented Dr. Duane S. Pinto, an interventional cardiologist at Beth Israel Deaconess Medical Center in Boston. “It validates the strategy” of using TAVR in patients with a risk score of 4%-8%. “TAVR has already been used in these patients, but these results validate this, especially when used in a transfemoral approach,” Dr. Pinto said in an interview.
One aspect of PARTNER 2A that received a lot of discussion at the meeting was whether enrolled patients could appropriately be characterized as “intermediate” in their risk level. Although their average STS risk score of 5.8% fell squarely within the target range specified for the study, they averaged 82 years old, and other clinical features at baseline suggested a higher risk population. The published report of the PARTNER 2A results that appeared online concurrent with Dr. Smith’s report at the meeting (New Engl J Med 2016;doi:10.1056/NEJMoa1514616) acknowledged that STS risk scores of 4%-8% place the enrolled patients into the upper 20% for risk of all U.S. patients who undergo surgical aortic-valve replacement
“I would characterize the enrolled patients as ‘less high risk’ rather than intermediate risk,” said Dr. Pinto.
But as Dr. Smith explained “even if the enrolled patients are not ‘intermediate’ risk they are at a different risk level” than were the patients enrolled in the prior TAVR randomized trials.
In the PARTNER 1 high-risk trial, the overall 1-year rate of all-cause mortality was 24% and 27% in the TAVR and surgical arms of the study, respectively. In the CoreValve trial these rates were 14% with TAVR and 19% with surgery. In PARTNER 2A 1-year all-cause mortality was 12% with TAVR and 13% with surgery.
Two other notable findings of PARTNER 2A were the superior outcomes of patients who underwent TAVR using a transfemoral approach, and the improved outcomes that all TAVR patients had compared with surgical valve replacement for several secondary outcomes.
The rate of the study’s primary outcome, all-cause death or disabling stroke after 2 years, was cut by a relative 21% in the 77% of TAVR patients who underwent a transfemoral procedure, compared with the surgery patients, a difference that was of borderline statistical significance. In contrast, the entire group of TAVR patients, including those treated via nontransfemoral routes, had an 11% relative reduction of the primary endpoint, compared with surgery, a difference that was not statistically significant but did easily meet the study’s prespecified definition of noninferority. Dr. Smith and others were especially encouraged by these findings as PARTNER 2A used the older Sapien XT TAVR system that is not often used today in U.S. practice. When U.S. patients undergo TAVR with a balloon-expandable valve they most often receive treatment with the S3 system, much smaller than XT and hence much more likely to be used with a transfemoral approach.
Other secondary outcomes included life-threatening or disabling bleeding events, which after 2 years had occurred in 17% of the TAVR patients and 47% of those who underwent surgery; atrial fibrillation, which occurred in 11% of the TAVR patients and 27% of those undergoing surgery; and acute kidney injury which occurred in 4% of TAVR patients and 6% of the surgery patients. With 2-year follow-up, the rate of disabling strokes was 6% in both arms of the study.
PARTNER 2A was sponsored by Edwards Lifesciences, the company that markets the Sapien TAVR systems. Dr. Smith has received travel grants from Edwards. Dr. Holmes had no disclosures, Dr. Pinto has been a consultant to Medtronic.
On Twitter @mitchelzoler
AT ACC 16
Key clinical point: Intermediate-risk patients who had transcatheter aortic valve replacement had a 2-year death or disabling stroke rate that was no different from patients who had surgical valve replacement.
Major finding: The 2-year rate of death or disabling stroke was 19% with transcatheter valve replacement and 21% with surgical replacement.
Data source: PARTNER 2A, a prospective, multicenter, North American trial with 2,032 enrolled patients with severe aortic-valve stenosis.
Disclosures: PARTNER 2A was sponsored by Edwards Lifesciences, the company that markets the Sapien TAVR systems. Dr. Smith has received travel grants from Edwards. Dr. Holmes had no disclosures, Dr. Pinto has been a consultant to Medtronic.