User login
VIDEO: PARTNER 2 results extend TAVR to intermediate-risk patients
CHICAGO – The “groundbreaking” results from the PARTNER 2 trial of transcatheter aortic valve replacement in patients at intermediate risk for death or complications from surgical replacement of a stenotic aortic valve provide substantially increased certainty that the transcatheter approach is at least as good as surgical valve replacement, commented Dr. Roxana Mehran in a video interview at the annual meeting of the American College of Cardiology.
In addition to providing important new data on the equivalence of surgical and transcatheter aortic valve replacement (TAVR) in a new target population--intermediate-risk patients, the findings from the randomized, multicenter PARTNER trial, which enrolled 2,032 patients, may also lead to expansion of the Food and Drug Administration labeling of the balloon-expandable TAVR device used in the study, the Sapien XT, to intermediate-risk patients. This expanded indication may also apply to the newer, smaller and more advanced iteration of this device, the Sapien 3, which has largely replaced the XT in U.S. practice, Dr. Mehran suggested.
Although the results came from the XT TAVR system, “these results have big relevance” to current U.S. practice said Dr. Mehran, professor and director of interventional cardiovascular research at Mount Sinai Hospital in New York.
The primary outcome of the Placement of Aortic Transcatheter Valves (PARTNER) 2 trial showed that all-cause mortality or disabling stroke after 2 years follow-up occurred in 21.2% (695) of the surgery patients and 19.3% (775) of the TAVR patients, meeting the prespecified noninferiority endpoint, with a P value of .001. Full results from the study were published online concurrent with their presentation at the meeting (New Engl J Med 2016;doi:10.1056/NEJMoa1514616).
Dr. Mehran has received consultant fees and honoraria from Abbott Vascular, AstraZeneca, and Bayer.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
CHICAGO – The “groundbreaking” results from the PARTNER 2 trial of transcatheter aortic valve replacement in patients at intermediate risk for death or complications from surgical replacement of a stenotic aortic valve provide substantially increased certainty that the transcatheter approach is at least as good as surgical valve replacement, commented Dr. Roxana Mehran in a video interview at the annual meeting of the American College of Cardiology.
In addition to providing important new data on the equivalence of surgical and transcatheter aortic valve replacement (TAVR) in a new target population--intermediate-risk patients, the findings from the randomized, multicenter PARTNER trial, which enrolled 2,032 patients, may also lead to expansion of the Food and Drug Administration labeling of the balloon-expandable TAVR device used in the study, the Sapien XT, to intermediate-risk patients. This expanded indication may also apply to the newer, smaller and more advanced iteration of this device, the Sapien 3, which has largely replaced the XT in U.S. practice, Dr. Mehran suggested.
Although the results came from the XT TAVR system, “these results have big relevance” to current U.S. practice said Dr. Mehran, professor and director of interventional cardiovascular research at Mount Sinai Hospital in New York.
The primary outcome of the Placement of Aortic Transcatheter Valves (PARTNER) 2 trial showed that all-cause mortality or disabling stroke after 2 years follow-up occurred in 21.2% (695) of the surgery patients and 19.3% (775) of the TAVR patients, meeting the prespecified noninferiority endpoint, with a P value of .001. Full results from the study were published online concurrent with their presentation at the meeting (New Engl J Med 2016;doi:10.1056/NEJMoa1514616).
Dr. Mehran has received consultant fees and honoraria from Abbott Vascular, AstraZeneca, and Bayer.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
CHICAGO – The “groundbreaking” results from the PARTNER 2 trial of transcatheter aortic valve replacement in patients at intermediate risk for death or complications from surgical replacement of a stenotic aortic valve provide substantially increased certainty that the transcatheter approach is at least as good as surgical valve replacement, commented Dr. Roxana Mehran in a video interview at the annual meeting of the American College of Cardiology.
In addition to providing important new data on the equivalence of surgical and transcatheter aortic valve replacement (TAVR) in a new target population--intermediate-risk patients, the findings from the randomized, multicenter PARTNER trial, which enrolled 2,032 patients, may also lead to expansion of the Food and Drug Administration labeling of the balloon-expandable TAVR device used in the study, the Sapien XT, to intermediate-risk patients. This expanded indication may also apply to the newer, smaller and more advanced iteration of this device, the Sapien 3, which has largely replaced the XT in U.S. practice, Dr. Mehran suggested.
Although the results came from the XT TAVR system, “these results have big relevance” to current U.S. practice said Dr. Mehran, professor and director of interventional cardiovascular research at Mount Sinai Hospital in New York.
The primary outcome of the Placement of Aortic Transcatheter Valves (PARTNER) 2 trial showed that all-cause mortality or disabling stroke after 2 years follow-up occurred in 21.2% (695) of the surgery patients and 19.3% (775) of the TAVR patients, meeting the prespecified noninferiority endpoint, with a P value of .001. Full results from the study were published online concurrent with their presentation at the meeting (New Engl J Med 2016;doi:10.1056/NEJMoa1514616).
Dr. Mehran has received consultant fees and honoraria from Abbott Vascular, AstraZeneca, and Bayer.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
AT ACC 16
CHEST issues guidelines on EBUS-TBNA
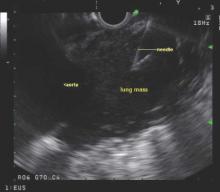
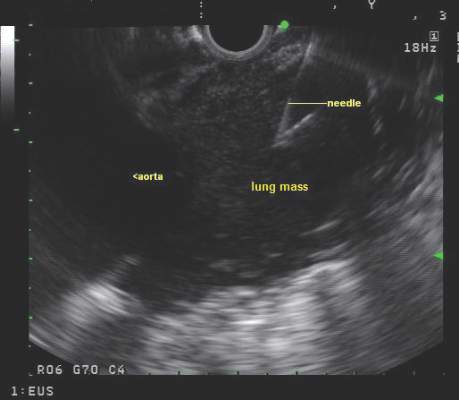
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) has been recommended for diagnosis of suspected sarcoidosis or suspected tuberculosis with adenopathy and may be used as an initial diagnostic test for suspected lymphoma, according to guidelines issued by CHEST (the American College of Chest Physicians).
The guidelines, which are primarily focused on technical aspects of EBUS-TBNA, also advise obtaining additional samples for the purpose of molecular analysis in patients who undergo the procedure for the diagnosis or staging of non–small cell lung cancer.
The guidelines are based on a systematic review and critical analysis of the literature by an expert panel chaired by Dr. Momen M. Wahidi of Duke University Medical Center, Durham, N.C. Of the 12 guideline statements by the panel, 7 were graded evidence-based recommendations and 5 were ungraded consensus-based statements.
The guideline (Chest. 2016 Mar;149[3]:816-35) has been endorsed by the American Association of Bronchology and Interventional Pulmonology, American Association for Thoracic Surgery, Canadian Thoracic Society, European Association for Bronchology and Interventional Pulmonology, and Society of Thoracic Surgeons.
Use of EBUS-TBNA for diagnosis in patients with suspected sarcoidosis with mediastinal and/or hilar adenopathy is ranked Grade 1C (strong recommendation, low-quality evidence, benefits outweigh risks). The guideline writers concluded that EBUS-TBNA provides safe and minimally invasive access to the mediastinal and hilar lymph nodes with a pooled diagnostic accuracy of 79.1%. They qualified, however, that it may be difficult to use EBUS-TBNA to obtain adequate tissue from fibrotic lymph nodes and conventional bronchoscopic techniques such as transbronchial lung biopsy and endobronchial biopsy may be needed in selected patients.
One systematic review and meta-analysis study included 15 studies with a total of 553 patients with sarcoidosis. The diagnostic yield of EBUS-TBNA ranged from 54% to 93%, with the pooled diagnostic accuracy of 79% (95% confidence interval, 71-86). Ten additional studies including 573 combined patients were identified through updated searches of the systematic review, and led to a pooled diagnostic accuracy of 78.2%.
Similarly, a Grade 1C recommendation was made for using EBUS-TBNA for diagnosis in when other modalities are not diagnostic in patients with suspected tuberculosis with mediastinal and/or hilar adenopathy who require lymph node sampling. However, “it must be noted that no single study assessed the role of EBUS-TBNA for the diagnosis of TB [tuberculosis] as the primary outcome measure,” they wrote. Various techniques are available for the diagnosis of TB and should be incorporated during the diagnostic evaluation.
In patients with suspected lymphoma, EBUS-TBNA is an acceptable initial, minimally invasive diagnostic test, the guideline writers said in an Ungraded Consensus-Based Statement.
In some conditions, minimally invasive EBUS-TBNA may be preferred over surgical intervention. Repeat mediastinoscopy or surgical biopsy after treatment for relapsed lymphoma can be challenging, for example, with a lower diagnostic yield and higher complication rate.
Because treatment regimens for both non-Hodgkin and Hodgkin lymphoma depend on the specific subtype and histologic grade, a definitive diagnosis of lymphoma requires the evaluation of cell morphology, immunophenotype, and the overall architecture of the tissue. Reed-Sternberg cells, diagnostic of Hodgkin lymphoma, are usually scarce in cytologic aspirates, and it often is impossible to evaluate the overall background architecture. Currently available EBUS-TBNA needles provide only cytologic specimens, with reported high discordance between cytologic specimens and histologic specimens.
In five retrospective studies with a total of 212 patients undergoing EBUS-TBNA for suspected lymphoma, the pooled diagnostic accuracy was 68.7%, and there was heterogeneity across studies in the proportion of patients with de novo lymphoma and relapsed lymphoma. Higher diagnostic yield was noted for relapsed lymphoma, compared with de novo lymphoma. Also, the two studies with the highest yield included cases as diagnostic, even when additional tissue sampling was necessary to subclassify the lymphoma for clinical management.
The panel gave a weak recommendation (Grade 2C) based on low-quality evidence to their conclusion that moderate or deep sedation is acceptable for EBUS-TBNA, based on three studies. Moderate sedation allows patients to respond purposefully to verbal commands while maintaining a functional airway, spontaneous ventilation, and cardiovascular function. In deep sedation, patients cannot be easily aroused but respond purposefully to repeated or painful stimulation and may have compromised airway function and spontaneous ventilation; cardiovascular function usually is maintained.
In one retrospective multivariable analysis of 309 patients at two centers, deep sedation had a statistically significant benefit on diagnostic yield. In a prospective randomized, controlled study of 149 patients at a single center with a single operator, there was no difference in diagnostic yield for moderate and deep sedation. However, fewer patients in the moderate sedation group were able to complete the procedure, compared with the deep-sedation group. Patient comfort and satisfaction were similar for the two sedation groups, and no patients had major complications or needed escalation of care.
In terms of diagnostic yield, there was insufficient evidence to recommend for or against using an artificial airway when inserting the EBUS bronchoscope, the authors said. Reported practice is scattered and is largely based on expert opinion, operator comfort, sedation type, and institutional standards.
The placement of the endotracheal tube may block the ultrasonographic view of the higher paratracheal lymph nodes (lymph node stations 1, 2R, 2L, and 3P) and should be avoided if one of these lymph nodes is the sampling target of the procedure, they advised.
If using a transoral artificial airway, a bite block should be considered to protect the bronchoscope from bite damage; this approach is recommended independent of the depth of sedation. A minimum size of 8.0 should be used if placing an endotracheal tube for EBUS-TBNA to accommodate the scope diameter and leave room for gas exchange.
In an Ungraded Consensus-Based Statement, the guideline authors said that ultrasonographic features, such as size, shape, border, heterogeneity, central hilar structure, and necrosis can be used to predict malignant and benign diagnoses, but tissue samples still should be obtained to confirm a diagnosis.
Nine studies provided analysis of the characteristics of lymph nodes that predict malignancy during EBUS; however, the ultrasonographic features assessed were not the same in each study or they had varying definitions of what constituted “abnormal.” As a result, the ultrasonographic predictors of malignancy in lymph nodes are not reliable enough to forgo biopsy to obtain a definitive tissue diagnosis. However, the ultrasound features can be useful to guide sampling from lymph nodes most likely to be malignant.
A round shape, distinct margins, heterogeneous echogenicity, and a central necrosis sign were independently predictive of malignancy in one multivariate analysis that included more than 1,000 lymph nodes in nearly 500 patients. Furthermore, when all four factors were absent, 96% of the lymph nodes were benign.
In three additional studies, size criteria had conflicting results; one found size was not a reliable indicator, two others found that larger lymph nodes are more likely to harbor metastases. These studies also confirmed that round-shaped lymph nodes were more likely malignant than were triangular or draping lymph nodes. The measures used to define size may have caused the inconsistencies.
In a study that examined vascular image patterns within lymph nodes as a way to predict malignancy, nodes were considered malignant if vessel involvement increased in the node to rich flow with more than four vessels (grades 2 and 3) with a sensitivity of 87.7% and a specificity of 69.6%, suggesting that increased vascularity assessed by using power/color Doppler mode ultrasound is useful in predicting malignancy.
Two studies have assessed ultrasound features of lymph nodes in patients with sarcoidosis. In the first, lymph nodes with homogeneous echogenicity and a germinal center were more likely to indicate sarcoidosis than lung cancer. In the second, coagulation necrosis and heterogeneous echogenicity within lymph nodes were more likely to be present in tuberculosis as opposed to sarcoidosis.
In another Ungraded Consensus-Based Statement, the guideline authors said tissue sampling may be performed either with or without suction. In cases in which EBUS-TBNA is being performed with suction and the samples obtained are bloody, operators should consider switching to the use of no suction at the same sampling site. If intranodal blood vessels are visualized on EBUS imaging with or without Doppler imaging, operators should also consider obtaining samples without suction.
Needle choice should be determined by the operator, and the use of either a 21- or 22-gauge needle are acceptable options based on five trials comparing needle sizes, the authors said in a Grade 1C recommendation. No data are available on the use of 25-gauge needles.
“Future studies should investigate if ... smaller or more flexible needles would improve sampling at station 4L (known for its slightly angulated location) or if smaller needles would result in less blood contamination when sampling more vascular nodes. Studies should also examine if a particular needle size should be used depending on how the specimens are being processed (histopathology vs. cytopathology) and if needle size can affect the diagnosis of diseases that are more difficult to diagnose by EBUS-TBNA, such as lymphoma,” the authors wrote.
In the absence of rapid on-site evaluation (ROSE), the authors advised a minimum of three separate needle passes per sampling site in patients suspected of having lung cancer. The recommendation is an Ungraded Consensus-Based Statement.
Just one study of 102 patients with potentially operable non–small cell lung cancer and mediastinal adenopathy has examined the number of needle passes per sampling site. The results indicated optimal diagnostic values are reached after three passes. Each pass typically includes 5-15 agitations of the needle within the target site.
Sample adequacy was 90.1% after the first pass, 98.1% after two passes, and reached 100% after three passes. The sensitivity for differentiating malignant from benign lymph node stations was 69.8%, 83.7%, 95.3%, and 95.3% for one, two, three, and four passes, respectively.
No data exist regarding the number of needle passes required to obtain a sufficient diagnostic yield for lymphoma or nonmalignant diseases of the mediastinum.
In a Grade 1C recommendation, the authors said that tissue sampling can be performed with or without rapid on-site evaluation. ROSE does not affect the diagnostic yield in EBUS-TBNA procedures, but it may decrease the number of punctures and reduce the need for additional staging and diagnostic procedures. ROSE may be beneficial in judging the quantity of available malignant cells when testing for molecular markers is planned in patients with advanced adenocarcinoma of the lung.
In another Grade 1C recommendation, the authors said that patients undergoing EBUS-TBNA for the diagnosis or staging of suspected or known non–small cell lung cancer should have additional samples obtained for molecular analysis.
Molecular marker testing is necessary for tailoring chemotherapy to the cancer characteristics of each individual patient. The current data are insufficient to identify the number of passes needed to obtain adequate tissue for molecular marker testing, but it is strongly suggested that additional samples, over the proposed diagnostic threshold of three passes are recommended.
The guideline authors found insufficient quality of evidence to support any route of bronchoscope insertion for EBUS-TBNA over another. Translating the experience and literature from conventional flexible bronchoscopy given the size and rigidity of the EBUS bronchoscope distal tip, as well as the limited bronchoscopic view, is difficult, according to the guideline writers.
They noted that no studies were found that addressed the use of saline-filled balloons to overcome poor contact between the ultrasound probe and the bronchial wall. Although the saline-filled balloon can enhance image acquisition, it is unclear whether that translates into a better diagnostic yield, thus, no recommendations or suggestions can be made.
In a Grade 2C recommendation, they advised that low-fidelity inanimate mechanical airway models and high-fidelity computer-based electronic simulation be incorporated into training. In the three studies that compared conventional EBUS-TBNA training and simulation-based training incorporating either a low- or high-fidelity simulation tool, the same level of training could be acquired via conventional or simulation-based training; however, simulation-based training minimizes novice operators’ practice on patients.
In an Ungraded Consensus-Based Statement, the guideline authors advised that validated EBUS skills assessment tests be used to objectively assess skill level, but added that “none of the included simulation studies examined whether the skills demonstrated on a simulation assessment are transferred to an improvement in clinical skills as performed in patients.”
On Twitter @maryjodales
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) has been recommended for diagnosis of suspected sarcoidosis or suspected tuberculosis with adenopathy and may be used as an initial diagnostic test for suspected lymphoma, according to guidelines issued by CHEST (the American College of Chest Physicians).
The guidelines, which are primarily focused on technical aspects of EBUS-TBNA, also advise obtaining additional samples for the purpose of molecular analysis in patients who undergo the procedure for the diagnosis or staging of non–small cell lung cancer.
The guidelines are based on a systematic review and critical analysis of the literature by an expert panel chaired by Dr. Momen M. Wahidi of Duke University Medical Center, Durham, N.C. Of the 12 guideline statements by the panel, 7 were graded evidence-based recommendations and 5 were ungraded consensus-based statements.
The guideline (Chest. 2016 Mar;149[3]:816-35) has been endorsed by the American Association of Bronchology and Interventional Pulmonology, American Association for Thoracic Surgery, Canadian Thoracic Society, European Association for Bronchology and Interventional Pulmonology, and Society of Thoracic Surgeons.
Use of EBUS-TBNA for diagnosis in patients with suspected sarcoidosis with mediastinal and/or hilar adenopathy is ranked Grade 1C (strong recommendation, low-quality evidence, benefits outweigh risks). The guideline writers concluded that EBUS-TBNA provides safe and minimally invasive access to the mediastinal and hilar lymph nodes with a pooled diagnostic accuracy of 79.1%. They qualified, however, that it may be difficult to use EBUS-TBNA to obtain adequate tissue from fibrotic lymph nodes and conventional bronchoscopic techniques such as transbronchial lung biopsy and endobronchial biopsy may be needed in selected patients.
One systematic review and meta-analysis study included 15 studies with a total of 553 patients with sarcoidosis. The diagnostic yield of EBUS-TBNA ranged from 54% to 93%, with the pooled diagnostic accuracy of 79% (95% confidence interval, 71-86). Ten additional studies including 573 combined patients were identified through updated searches of the systematic review, and led to a pooled diagnostic accuracy of 78.2%.
Similarly, a Grade 1C recommendation was made for using EBUS-TBNA for diagnosis in when other modalities are not diagnostic in patients with suspected tuberculosis with mediastinal and/or hilar adenopathy who require lymph node sampling. However, “it must be noted that no single study assessed the role of EBUS-TBNA for the diagnosis of TB [tuberculosis] as the primary outcome measure,” they wrote. Various techniques are available for the diagnosis of TB and should be incorporated during the diagnostic evaluation.
In patients with suspected lymphoma, EBUS-TBNA is an acceptable initial, minimally invasive diagnostic test, the guideline writers said in an Ungraded Consensus-Based Statement.
In some conditions, minimally invasive EBUS-TBNA may be preferred over surgical intervention. Repeat mediastinoscopy or surgical biopsy after treatment for relapsed lymphoma can be challenging, for example, with a lower diagnostic yield and higher complication rate.
Because treatment regimens for both non-Hodgkin and Hodgkin lymphoma depend on the specific subtype and histologic grade, a definitive diagnosis of lymphoma requires the evaluation of cell morphology, immunophenotype, and the overall architecture of the tissue. Reed-Sternberg cells, diagnostic of Hodgkin lymphoma, are usually scarce in cytologic aspirates, and it often is impossible to evaluate the overall background architecture. Currently available EBUS-TBNA needles provide only cytologic specimens, with reported high discordance between cytologic specimens and histologic specimens.
In five retrospective studies with a total of 212 patients undergoing EBUS-TBNA for suspected lymphoma, the pooled diagnostic accuracy was 68.7%, and there was heterogeneity across studies in the proportion of patients with de novo lymphoma and relapsed lymphoma. Higher diagnostic yield was noted for relapsed lymphoma, compared with de novo lymphoma. Also, the two studies with the highest yield included cases as diagnostic, even when additional tissue sampling was necessary to subclassify the lymphoma for clinical management.
The panel gave a weak recommendation (Grade 2C) based on low-quality evidence to their conclusion that moderate or deep sedation is acceptable for EBUS-TBNA, based on three studies. Moderate sedation allows patients to respond purposefully to verbal commands while maintaining a functional airway, spontaneous ventilation, and cardiovascular function. In deep sedation, patients cannot be easily aroused but respond purposefully to repeated or painful stimulation and may have compromised airway function and spontaneous ventilation; cardiovascular function usually is maintained.
In one retrospective multivariable analysis of 309 patients at two centers, deep sedation had a statistically significant benefit on diagnostic yield. In a prospective randomized, controlled study of 149 patients at a single center with a single operator, there was no difference in diagnostic yield for moderate and deep sedation. However, fewer patients in the moderate sedation group were able to complete the procedure, compared with the deep-sedation group. Patient comfort and satisfaction were similar for the two sedation groups, and no patients had major complications or needed escalation of care.
In terms of diagnostic yield, there was insufficient evidence to recommend for or against using an artificial airway when inserting the EBUS bronchoscope, the authors said. Reported practice is scattered and is largely based on expert opinion, operator comfort, sedation type, and institutional standards.
The placement of the endotracheal tube may block the ultrasonographic view of the higher paratracheal lymph nodes (lymph node stations 1, 2R, 2L, and 3P) and should be avoided if one of these lymph nodes is the sampling target of the procedure, they advised.
If using a transoral artificial airway, a bite block should be considered to protect the bronchoscope from bite damage; this approach is recommended independent of the depth of sedation. A minimum size of 8.0 should be used if placing an endotracheal tube for EBUS-TBNA to accommodate the scope diameter and leave room for gas exchange.
In an Ungraded Consensus-Based Statement, the guideline authors said that ultrasonographic features, such as size, shape, border, heterogeneity, central hilar structure, and necrosis can be used to predict malignant and benign diagnoses, but tissue samples still should be obtained to confirm a diagnosis.
Nine studies provided analysis of the characteristics of lymph nodes that predict malignancy during EBUS; however, the ultrasonographic features assessed were not the same in each study or they had varying definitions of what constituted “abnormal.” As a result, the ultrasonographic predictors of malignancy in lymph nodes are not reliable enough to forgo biopsy to obtain a definitive tissue diagnosis. However, the ultrasound features can be useful to guide sampling from lymph nodes most likely to be malignant.
A round shape, distinct margins, heterogeneous echogenicity, and a central necrosis sign were independently predictive of malignancy in one multivariate analysis that included more than 1,000 lymph nodes in nearly 500 patients. Furthermore, when all four factors were absent, 96% of the lymph nodes were benign.
In three additional studies, size criteria had conflicting results; one found size was not a reliable indicator, two others found that larger lymph nodes are more likely to harbor metastases. These studies also confirmed that round-shaped lymph nodes were more likely malignant than were triangular or draping lymph nodes. The measures used to define size may have caused the inconsistencies.
In a study that examined vascular image patterns within lymph nodes as a way to predict malignancy, nodes were considered malignant if vessel involvement increased in the node to rich flow with more than four vessels (grades 2 and 3) with a sensitivity of 87.7% and a specificity of 69.6%, suggesting that increased vascularity assessed by using power/color Doppler mode ultrasound is useful in predicting malignancy.
Two studies have assessed ultrasound features of lymph nodes in patients with sarcoidosis. In the first, lymph nodes with homogeneous echogenicity and a germinal center were more likely to indicate sarcoidosis than lung cancer. In the second, coagulation necrosis and heterogeneous echogenicity within lymph nodes were more likely to be present in tuberculosis as opposed to sarcoidosis.
In another Ungraded Consensus-Based Statement, the guideline authors said tissue sampling may be performed either with or without suction. In cases in which EBUS-TBNA is being performed with suction and the samples obtained are bloody, operators should consider switching to the use of no suction at the same sampling site. If intranodal blood vessels are visualized on EBUS imaging with or without Doppler imaging, operators should also consider obtaining samples without suction.
Needle choice should be determined by the operator, and the use of either a 21- or 22-gauge needle are acceptable options based on five trials comparing needle sizes, the authors said in a Grade 1C recommendation. No data are available on the use of 25-gauge needles.
“Future studies should investigate if ... smaller or more flexible needles would improve sampling at station 4L (known for its slightly angulated location) or if smaller needles would result in less blood contamination when sampling more vascular nodes. Studies should also examine if a particular needle size should be used depending on how the specimens are being processed (histopathology vs. cytopathology) and if needle size can affect the diagnosis of diseases that are more difficult to diagnose by EBUS-TBNA, such as lymphoma,” the authors wrote.
In the absence of rapid on-site evaluation (ROSE), the authors advised a minimum of three separate needle passes per sampling site in patients suspected of having lung cancer. The recommendation is an Ungraded Consensus-Based Statement.
Just one study of 102 patients with potentially operable non–small cell lung cancer and mediastinal adenopathy has examined the number of needle passes per sampling site. The results indicated optimal diagnostic values are reached after three passes. Each pass typically includes 5-15 agitations of the needle within the target site.
Sample adequacy was 90.1% after the first pass, 98.1% after two passes, and reached 100% after three passes. The sensitivity for differentiating malignant from benign lymph node stations was 69.8%, 83.7%, 95.3%, and 95.3% for one, two, three, and four passes, respectively.
No data exist regarding the number of needle passes required to obtain a sufficient diagnostic yield for lymphoma or nonmalignant diseases of the mediastinum.
In a Grade 1C recommendation, the authors said that tissue sampling can be performed with or without rapid on-site evaluation. ROSE does not affect the diagnostic yield in EBUS-TBNA procedures, but it may decrease the number of punctures and reduce the need for additional staging and diagnostic procedures. ROSE may be beneficial in judging the quantity of available malignant cells when testing for molecular markers is planned in patients with advanced adenocarcinoma of the lung.
In another Grade 1C recommendation, the authors said that patients undergoing EBUS-TBNA for the diagnosis or staging of suspected or known non–small cell lung cancer should have additional samples obtained for molecular analysis.
Molecular marker testing is necessary for tailoring chemotherapy to the cancer characteristics of each individual patient. The current data are insufficient to identify the number of passes needed to obtain adequate tissue for molecular marker testing, but it is strongly suggested that additional samples, over the proposed diagnostic threshold of three passes are recommended.
The guideline authors found insufficient quality of evidence to support any route of bronchoscope insertion for EBUS-TBNA over another. Translating the experience and literature from conventional flexible bronchoscopy given the size and rigidity of the EBUS bronchoscope distal tip, as well as the limited bronchoscopic view, is difficult, according to the guideline writers.
They noted that no studies were found that addressed the use of saline-filled balloons to overcome poor contact between the ultrasound probe and the bronchial wall. Although the saline-filled balloon can enhance image acquisition, it is unclear whether that translates into a better diagnostic yield, thus, no recommendations or suggestions can be made.
In a Grade 2C recommendation, they advised that low-fidelity inanimate mechanical airway models and high-fidelity computer-based electronic simulation be incorporated into training. In the three studies that compared conventional EBUS-TBNA training and simulation-based training incorporating either a low- or high-fidelity simulation tool, the same level of training could be acquired via conventional or simulation-based training; however, simulation-based training minimizes novice operators’ practice on patients.
In an Ungraded Consensus-Based Statement, the guideline authors advised that validated EBUS skills assessment tests be used to objectively assess skill level, but added that “none of the included simulation studies examined whether the skills demonstrated on a simulation assessment are transferred to an improvement in clinical skills as performed in patients.”
On Twitter @maryjodales
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) has been recommended for diagnosis of suspected sarcoidosis or suspected tuberculosis with adenopathy and may be used as an initial diagnostic test for suspected lymphoma, according to guidelines issued by CHEST (the American College of Chest Physicians).
The guidelines, which are primarily focused on technical aspects of EBUS-TBNA, also advise obtaining additional samples for the purpose of molecular analysis in patients who undergo the procedure for the diagnosis or staging of non–small cell lung cancer.
The guidelines are based on a systematic review and critical analysis of the literature by an expert panel chaired by Dr. Momen M. Wahidi of Duke University Medical Center, Durham, N.C. Of the 12 guideline statements by the panel, 7 were graded evidence-based recommendations and 5 were ungraded consensus-based statements.
The guideline (Chest. 2016 Mar;149[3]:816-35) has been endorsed by the American Association of Bronchology and Interventional Pulmonology, American Association for Thoracic Surgery, Canadian Thoracic Society, European Association for Bronchology and Interventional Pulmonology, and Society of Thoracic Surgeons.
Use of EBUS-TBNA for diagnosis in patients with suspected sarcoidosis with mediastinal and/or hilar adenopathy is ranked Grade 1C (strong recommendation, low-quality evidence, benefits outweigh risks). The guideline writers concluded that EBUS-TBNA provides safe and minimally invasive access to the mediastinal and hilar lymph nodes with a pooled diagnostic accuracy of 79.1%. They qualified, however, that it may be difficult to use EBUS-TBNA to obtain adequate tissue from fibrotic lymph nodes and conventional bronchoscopic techniques such as transbronchial lung biopsy and endobronchial biopsy may be needed in selected patients.
One systematic review and meta-analysis study included 15 studies with a total of 553 patients with sarcoidosis. The diagnostic yield of EBUS-TBNA ranged from 54% to 93%, with the pooled diagnostic accuracy of 79% (95% confidence interval, 71-86). Ten additional studies including 573 combined patients were identified through updated searches of the systematic review, and led to a pooled diagnostic accuracy of 78.2%.
Similarly, a Grade 1C recommendation was made for using EBUS-TBNA for diagnosis in when other modalities are not diagnostic in patients with suspected tuberculosis with mediastinal and/or hilar adenopathy who require lymph node sampling. However, “it must be noted that no single study assessed the role of EBUS-TBNA for the diagnosis of TB [tuberculosis] as the primary outcome measure,” they wrote. Various techniques are available for the diagnosis of TB and should be incorporated during the diagnostic evaluation.
In patients with suspected lymphoma, EBUS-TBNA is an acceptable initial, minimally invasive diagnostic test, the guideline writers said in an Ungraded Consensus-Based Statement.
In some conditions, minimally invasive EBUS-TBNA may be preferred over surgical intervention. Repeat mediastinoscopy or surgical biopsy after treatment for relapsed lymphoma can be challenging, for example, with a lower diagnostic yield and higher complication rate.
Because treatment regimens for both non-Hodgkin and Hodgkin lymphoma depend on the specific subtype and histologic grade, a definitive diagnosis of lymphoma requires the evaluation of cell morphology, immunophenotype, and the overall architecture of the tissue. Reed-Sternberg cells, diagnostic of Hodgkin lymphoma, are usually scarce in cytologic aspirates, and it often is impossible to evaluate the overall background architecture. Currently available EBUS-TBNA needles provide only cytologic specimens, with reported high discordance between cytologic specimens and histologic specimens.
In five retrospective studies with a total of 212 patients undergoing EBUS-TBNA for suspected lymphoma, the pooled diagnostic accuracy was 68.7%, and there was heterogeneity across studies in the proportion of patients with de novo lymphoma and relapsed lymphoma. Higher diagnostic yield was noted for relapsed lymphoma, compared with de novo lymphoma. Also, the two studies with the highest yield included cases as diagnostic, even when additional tissue sampling was necessary to subclassify the lymphoma for clinical management.
The panel gave a weak recommendation (Grade 2C) based on low-quality evidence to their conclusion that moderate or deep sedation is acceptable for EBUS-TBNA, based on three studies. Moderate sedation allows patients to respond purposefully to verbal commands while maintaining a functional airway, spontaneous ventilation, and cardiovascular function. In deep sedation, patients cannot be easily aroused but respond purposefully to repeated or painful stimulation and may have compromised airway function and spontaneous ventilation; cardiovascular function usually is maintained.
In one retrospective multivariable analysis of 309 patients at two centers, deep sedation had a statistically significant benefit on diagnostic yield. In a prospective randomized, controlled study of 149 patients at a single center with a single operator, there was no difference in diagnostic yield for moderate and deep sedation. However, fewer patients in the moderate sedation group were able to complete the procedure, compared with the deep-sedation group. Patient comfort and satisfaction were similar for the two sedation groups, and no patients had major complications or needed escalation of care.
In terms of diagnostic yield, there was insufficient evidence to recommend for or against using an artificial airway when inserting the EBUS bronchoscope, the authors said. Reported practice is scattered and is largely based on expert opinion, operator comfort, sedation type, and institutional standards.
The placement of the endotracheal tube may block the ultrasonographic view of the higher paratracheal lymph nodes (lymph node stations 1, 2R, 2L, and 3P) and should be avoided if one of these lymph nodes is the sampling target of the procedure, they advised.
If using a transoral artificial airway, a bite block should be considered to protect the bronchoscope from bite damage; this approach is recommended independent of the depth of sedation. A minimum size of 8.0 should be used if placing an endotracheal tube for EBUS-TBNA to accommodate the scope diameter and leave room for gas exchange.
In an Ungraded Consensus-Based Statement, the guideline authors said that ultrasonographic features, such as size, shape, border, heterogeneity, central hilar structure, and necrosis can be used to predict malignant and benign diagnoses, but tissue samples still should be obtained to confirm a diagnosis.
Nine studies provided analysis of the characteristics of lymph nodes that predict malignancy during EBUS; however, the ultrasonographic features assessed were not the same in each study or they had varying definitions of what constituted “abnormal.” As a result, the ultrasonographic predictors of malignancy in lymph nodes are not reliable enough to forgo biopsy to obtain a definitive tissue diagnosis. However, the ultrasound features can be useful to guide sampling from lymph nodes most likely to be malignant.
A round shape, distinct margins, heterogeneous echogenicity, and a central necrosis sign were independently predictive of malignancy in one multivariate analysis that included more than 1,000 lymph nodes in nearly 500 patients. Furthermore, when all four factors were absent, 96% of the lymph nodes were benign.
In three additional studies, size criteria had conflicting results; one found size was not a reliable indicator, two others found that larger lymph nodes are more likely to harbor metastases. These studies also confirmed that round-shaped lymph nodes were more likely malignant than were triangular or draping lymph nodes. The measures used to define size may have caused the inconsistencies.
In a study that examined vascular image patterns within lymph nodes as a way to predict malignancy, nodes were considered malignant if vessel involvement increased in the node to rich flow with more than four vessels (grades 2 and 3) with a sensitivity of 87.7% and a specificity of 69.6%, suggesting that increased vascularity assessed by using power/color Doppler mode ultrasound is useful in predicting malignancy.
Two studies have assessed ultrasound features of lymph nodes in patients with sarcoidosis. In the first, lymph nodes with homogeneous echogenicity and a germinal center were more likely to indicate sarcoidosis than lung cancer. In the second, coagulation necrosis and heterogeneous echogenicity within lymph nodes were more likely to be present in tuberculosis as opposed to sarcoidosis.
In another Ungraded Consensus-Based Statement, the guideline authors said tissue sampling may be performed either with or without suction. In cases in which EBUS-TBNA is being performed with suction and the samples obtained are bloody, operators should consider switching to the use of no suction at the same sampling site. If intranodal blood vessels are visualized on EBUS imaging with or without Doppler imaging, operators should also consider obtaining samples without suction.
Needle choice should be determined by the operator, and the use of either a 21- or 22-gauge needle are acceptable options based on five trials comparing needle sizes, the authors said in a Grade 1C recommendation. No data are available on the use of 25-gauge needles.
“Future studies should investigate if ... smaller or more flexible needles would improve sampling at station 4L (known for its slightly angulated location) or if smaller needles would result in less blood contamination when sampling more vascular nodes. Studies should also examine if a particular needle size should be used depending on how the specimens are being processed (histopathology vs. cytopathology) and if needle size can affect the diagnosis of diseases that are more difficult to diagnose by EBUS-TBNA, such as lymphoma,” the authors wrote.
In the absence of rapid on-site evaluation (ROSE), the authors advised a minimum of three separate needle passes per sampling site in patients suspected of having lung cancer. The recommendation is an Ungraded Consensus-Based Statement.
Just one study of 102 patients with potentially operable non–small cell lung cancer and mediastinal adenopathy has examined the number of needle passes per sampling site. The results indicated optimal diagnostic values are reached after three passes. Each pass typically includes 5-15 agitations of the needle within the target site.
Sample adequacy was 90.1% after the first pass, 98.1% after two passes, and reached 100% after three passes. The sensitivity for differentiating malignant from benign lymph node stations was 69.8%, 83.7%, 95.3%, and 95.3% for one, two, three, and four passes, respectively.
No data exist regarding the number of needle passes required to obtain a sufficient diagnostic yield for lymphoma or nonmalignant diseases of the mediastinum.
In a Grade 1C recommendation, the authors said that tissue sampling can be performed with or without rapid on-site evaluation. ROSE does not affect the diagnostic yield in EBUS-TBNA procedures, but it may decrease the number of punctures and reduce the need for additional staging and diagnostic procedures. ROSE may be beneficial in judging the quantity of available malignant cells when testing for molecular markers is planned in patients with advanced adenocarcinoma of the lung.
In another Grade 1C recommendation, the authors said that patients undergoing EBUS-TBNA for the diagnosis or staging of suspected or known non–small cell lung cancer should have additional samples obtained for molecular analysis.
Molecular marker testing is necessary for tailoring chemotherapy to the cancer characteristics of each individual patient. The current data are insufficient to identify the number of passes needed to obtain adequate tissue for molecular marker testing, but it is strongly suggested that additional samples, over the proposed diagnostic threshold of three passes are recommended.
The guideline authors found insufficient quality of evidence to support any route of bronchoscope insertion for EBUS-TBNA over another. Translating the experience and literature from conventional flexible bronchoscopy given the size and rigidity of the EBUS bronchoscope distal tip, as well as the limited bronchoscopic view, is difficult, according to the guideline writers.
They noted that no studies were found that addressed the use of saline-filled balloons to overcome poor contact between the ultrasound probe and the bronchial wall. Although the saline-filled balloon can enhance image acquisition, it is unclear whether that translates into a better diagnostic yield, thus, no recommendations or suggestions can be made.
In a Grade 2C recommendation, they advised that low-fidelity inanimate mechanical airway models and high-fidelity computer-based electronic simulation be incorporated into training. In the three studies that compared conventional EBUS-TBNA training and simulation-based training incorporating either a low- or high-fidelity simulation tool, the same level of training could be acquired via conventional or simulation-based training; however, simulation-based training minimizes novice operators’ practice on patients.
In an Ungraded Consensus-Based Statement, the guideline authors advised that validated EBUS skills assessment tests be used to objectively assess skill level, but added that “none of the included simulation studies examined whether the skills demonstrated on a simulation assessment are transferred to an improvement in clinical skills as performed in patients.”
On Twitter @maryjodales
FROM CHEST
Study: Tricuspid annuloplasty with mitral replacement appeared safe and effective
Controversy has surrounded the idea of concomitant tricuspid annuloplasty (TAP) with mitral valve surgery (MVR) as a way to prevent further progression of tricuspid regurgitation, and while several reports have suggested the procedures can be done safely and effectively, few reports have explored the idea of concomitant procedures in people with moderate or mild tricuspid regurgitation (TR) as a measure to prevent progression to more severe TR.
But investigators at Sungkyunkwan University in Seoul have found that TAP at the same time as MVR can be done without increasing surgical risks for patients, according to a report in the March issue of the Journal of Thoracic and Cardiovascular Surgery (2016;151:788-95).
“Although prophylactic TAP showed marginal clinical benefits for patients with less than moderate TR, we believe it is plausible to anticipate a long-term trend of a benefit of TAP in longer-term follow-up studies,“ Dr. Heemoon Lee and coauthors wrote. “Many other longer-term prospective randomized studies are needed to confirm our study findings and to ascertain clinical benefits of prophylactic TAP during mitral valve surgery.”
Despite existing clinical guidelines that recommend tricuspid valve repair for severe TR associated with mitral valve disease that requires MVR, prophylactic TAP for patients with less than moderate TR at the time of left-side valve surgery remains controversial because TR has been linked to multiple complex factors. They include etiology, whether degenerative or rheumatic; concomitant atrial fibrillation (AF); unreliable TR grading; or measurement of tricuspid annular diameter.
The investigators performed primary mechanical MVR on two groups of patients between November 1994 and December 2010: 151 with TAP; and 142 without TAP. All operations were performed through a standard median sternotomy and median follow-up was 107 months.
They looked at both early and late outcomes. There was no early mortality in either group; two bleeding episodes that required further surgery and one pacemaker insertion in the no-TAP group; and no bleeding complications and heart blocks requiring pacemaker insertion in the TAP group. While ICU stays were similar for both groups, the TAP group had significantly shorter hospital stays: 9 days vs. 11 days (P less than .001).
In terms of overall and cardiac-related deaths at 10 years, the investigators reported no significant differences between the two groups. “TAP did not appear to improve cardiac-related mortality,” Dr. Lee and coauthors said. Nor did freedom from tricuspid valve–related events differ appreciably between the two groups: 84.8% in TAP and 77.5% in no-TAP at year 10 (P = .087).
But the TAP group showed far lower rates of progression to late TR at 10 years – one in the TAP group; one (less than 1%) vs. nine (6.3%) in the no-TAP group; 96.8% in the TAP group were free from TR recurrence of grade 2 or greater vs. 85.6% in no-TAP.
“TAP can be performed safely without increases in early mortality and morbidities, including heart block,” Dr. Lee and coauthors said. “We also found that prophylactic TAP can prevent progression of late moderate or greater TR. TAP showed a tendency to prevent tricuspid valve–related events and was marginally significant.”
The researchers also evaluated the role of AF as a risk factor for progression of late TR; the effects of TAP on recurrence of moderate or greater TR were “prominent” in patients with sinus rhythm vs. AF at discharge. “These findings may reflect that the maze procedure is more important than prophylactic TAP in improving late outcomes in mitral valve disease with AF,” Dr. Lee and colleagues wrote.
The investigators acknowledged a number of limits of their study: its retrospective nature; how indications and techniques of MVR, TAP, and the maze procedure evolved over the study duration; and that the maze procedure and prophylactic TAP were not widely embraced in the early years of the study.
Dr. Lee and colleagues had no financial relationships to disclose.
The proposition of performing a concomitant TAP during MVR to prevent late progression of tricuspid regurgitation (TR) “raises safety concerns, Dr. J. Hunter Mehaffey and Dr. Irving L. Kron of the University of Virginia, Charlottesville, said in their invited commentary (J. Thorac. Cardiovasc. Surg. 2016;151:796-7). They cited their own study of 400 patients that reported higher death and complication rates in concomitant TAP-MVR procedures than in MVR alone (Ann. Thorac. Surg. 2012;52-8).

|
| M. Alex Otto/Frontline Medical News Dr. Hunter Mehaffey |
While Dr. Mehaffey and Dr. Kron commended Dr. Lee and colleagues for including atrial fibrillation in their risk analysis, they also questioned why the investigators did not address how to identify patients who would benefit from TAP to reduce the potential for long-term tricuspid, valve-related problems like reoperation for TR, right heart failure and pacemaker insertion. Nor do the investigators explain why they performed TAP in some patients and not others.
They called the study by Dr. Lee and colleagues an “interesting retrospective analysis” of concomitant TAP with MVR. “Unfortunately, this study raises numerous questions that cannot be adequately answered by propensity matching,” the coauthors wrote. A randomized, prospective trial would resolve many of these issues, they said.
Dr. Mehaffey and Dr. Kron had no financial relationships to disclose.
The proposition of performing a concomitant TAP during MVR to prevent late progression of tricuspid regurgitation (TR) “raises safety concerns, Dr. J. Hunter Mehaffey and Dr. Irving L. Kron of the University of Virginia, Charlottesville, said in their invited commentary (J. Thorac. Cardiovasc. Surg. 2016;151:796-7). They cited their own study of 400 patients that reported higher death and complication rates in concomitant TAP-MVR procedures than in MVR alone (Ann. Thorac. Surg. 2012;52-8).

|
| M. Alex Otto/Frontline Medical News Dr. Hunter Mehaffey |
While Dr. Mehaffey and Dr. Kron commended Dr. Lee and colleagues for including atrial fibrillation in their risk analysis, they also questioned why the investigators did not address how to identify patients who would benefit from TAP to reduce the potential for long-term tricuspid, valve-related problems like reoperation for TR, right heart failure and pacemaker insertion. Nor do the investigators explain why they performed TAP in some patients and not others.
They called the study by Dr. Lee and colleagues an “interesting retrospective analysis” of concomitant TAP with MVR. “Unfortunately, this study raises numerous questions that cannot be adequately answered by propensity matching,” the coauthors wrote. A randomized, prospective trial would resolve many of these issues, they said.
Dr. Mehaffey and Dr. Kron had no financial relationships to disclose.
The proposition of performing a concomitant TAP during MVR to prevent late progression of tricuspid regurgitation (TR) “raises safety concerns, Dr. J. Hunter Mehaffey and Dr. Irving L. Kron of the University of Virginia, Charlottesville, said in their invited commentary (J. Thorac. Cardiovasc. Surg. 2016;151:796-7). They cited their own study of 400 patients that reported higher death and complication rates in concomitant TAP-MVR procedures than in MVR alone (Ann. Thorac. Surg. 2012;52-8).

|
| M. Alex Otto/Frontline Medical News Dr. Hunter Mehaffey |
While Dr. Mehaffey and Dr. Kron commended Dr. Lee and colleagues for including atrial fibrillation in their risk analysis, they also questioned why the investigators did not address how to identify patients who would benefit from TAP to reduce the potential for long-term tricuspid, valve-related problems like reoperation for TR, right heart failure and pacemaker insertion. Nor do the investigators explain why they performed TAP in some patients and not others.
They called the study by Dr. Lee and colleagues an “interesting retrospective analysis” of concomitant TAP with MVR. “Unfortunately, this study raises numerous questions that cannot be adequately answered by propensity matching,” the coauthors wrote. A randomized, prospective trial would resolve many of these issues, they said.
Dr. Mehaffey and Dr. Kron had no financial relationships to disclose.
Controversy has surrounded the idea of concomitant tricuspid annuloplasty (TAP) with mitral valve surgery (MVR) as a way to prevent further progression of tricuspid regurgitation, and while several reports have suggested the procedures can be done safely and effectively, few reports have explored the idea of concomitant procedures in people with moderate or mild tricuspid regurgitation (TR) as a measure to prevent progression to more severe TR.
But investigators at Sungkyunkwan University in Seoul have found that TAP at the same time as MVR can be done without increasing surgical risks for patients, according to a report in the March issue of the Journal of Thoracic and Cardiovascular Surgery (2016;151:788-95).
“Although prophylactic TAP showed marginal clinical benefits for patients with less than moderate TR, we believe it is plausible to anticipate a long-term trend of a benefit of TAP in longer-term follow-up studies,“ Dr. Heemoon Lee and coauthors wrote. “Many other longer-term prospective randomized studies are needed to confirm our study findings and to ascertain clinical benefits of prophylactic TAP during mitral valve surgery.”
Despite existing clinical guidelines that recommend tricuspid valve repair for severe TR associated with mitral valve disease that requires MVR, prophylactic TAP for patients with less than moderate TR at the time of left-side valve surgery remains controversial because TR has been linked to multiple complex factors. They include etiology, whether degenerative or rheumatic; concomitant atrial fibrillation (AF); unreliable TR grading; or measurement of tricuspid annular diameter.
The investigators performed primary mechanical MVR on two groups of patients between November 1994 and December 2010: 151 with TAP; and 142 without TAP. All operations were performed through a standard median sternotomy and median follow-up was 107 months.
They looked at both early and late outcomes. There was no early mortality in either group; two bleeding episodes that required further surgery and one pacemaker insertion in the no-TAP group; and no bleeding complications and heart blocks requiring pacemaker insertion in the TAP group. While ICU stays were similar for both groups, the TAP group had significantly shorter hospital stays: 9 days vs. 11 days (P less than .001).
In terms of overall and cardiac-related deaths at 10 years, the investigators reported no significant differences between the two groups. “TAP did not appear to improve cardiac-related mortality,” Dr. Lee and coauthors said. Nor did freedom from tricuspid valve–related events differ appreciably between the two groups: 84.8% in TAP and 77.5% in no-TAP at year 10 (P = .087).
But the TAP group showed far lower rates of progression to late TR at 10 years – one in the TAP group; one (less than 1%) vs. nine (6.3%) in the no-TAP group; 96.8% in the TAP group were free from TR recurrence of grade 2 or greater vs. 85.6% in no-TAP.
“TAP can be performed safely without increases in early mortality and morbidities, including heart block,” Dr. Lee and coauthors said. “We also found that prophylactic TAP can prevent progression of late moderate or greater TR. TAP showed a tendency to prevent tricuspid valve–related events and was marginally significant.”
The researchers also evaluated the role of AF as a risk factor for progression of late TR; the effects of TAP on recurrence of moderate or greater TR were “prominent” in patients with sinus rhythm vs. AF at discharge. “These findings may reflect that the maze procedure is more important than prophylactic TAP in improving late outcomes in mitral valve disease with AF,” Dr. Lee and colleagues wrote.
The investigators acknowledged a number of limits of their study: its retrospective nature; how indications and techniques of MVR, TAP, and the maze procedure evolved over the study duration; and that the maze procedure and prophylactic TAP were not widely embraced in the early years of the study.
Dr. Lee and colleagues had no financial relationships to disclose.
Controversy has surrounded the idea of concomitant tricuspid annuloplasty (TAP) with mitral valve surgery (MVR) as a way to prevent further progression of tricuspid regurgitation, and while several reports have suggested the procedures can be done safely and effectively, few reports have explored the idea of concomitant procedures in people with moderate or mild tricuspid regurgitation (TR) as a measure to prevent progression to more severe TR.
But investigators at Sungkyunkwan University in Seoul have found that TAP at the same time as MVR can be done without increasing surgical risks for patients, according to a report in the March issue of the Journal of Thoracic and Cardiovascular Surgery (2016;151:788-95).
“Although prophylactic TAP showed marginal clinical benefits for patients with less than moderate TR, we believe it is plausible to anticipate a long-term trend of a benefit of TAP in longer-term follow-up studies,“ Dr. Heemoon Lee and coauthors wrote. “Many other longer-term prospective randomized studies are needed to confirm our study findings and to ascertain clinical benefits of prophylactic TAP during mitral valve surgery.”
Despite existing clinical guidelines that recommend tricuspid valve repair for severe TR associated with mitral valve disease that requires MVR, prophylactic TAP for patients with less than moderate TR at the time of left-side valve surgery remains controversial because TR has been linked to multiple complex factors. They include etiology, whether degenerative or rheumatic; concomitant atrial fibrillation (AF); unreliable TR grading; or measurement of tricuspid annular diameter.
The investigators performed primary mechanical MVR on two groups of patients between November 1994 and December 2010: 151 with TAP; and 142 without TAP. All operations were performed through a standard median sternotomy and median follow-up was 107 months.
They looked at both early and late outcomes. There was no early mortality in either group; two bleeding episodes that required further surgery and one pacemaker insertion in the no-TAP group; and no bleeding complications and heart blocks requiring pacemaker insertion in the TAP group. While ICU stays were similar for both groups, the TAP group had significantly shorter hospital stays: 9 days vs. 11 days (P less than .001).
In terms of overall and cardiac-related deaths at 10 years, the investigators reported no significant differences between the two groups. “TAP did not appear to improve cardiac-related mortality,” Dr. Lee and coauthors said. Nor did freedom from tricuspid valve–related events differ appreciably between the two groups: 84.8% in TAP and 77.5% in no-TAP at year 10 (P = .087).
But the TAP group showed far lower rates of progression to late TR at 10 years – one in the TAP group; one (less than 1%) vs. nine (6.3%) in the no-TAP group; 96.8% in the TAP group were free from TR recurrence of grade 2 or greater vs. 85.6% in no-TAP.
“TAP can be performed safely without increases in early mortality and morbidities, including heart block,” Dr. Lee and coauthors said. “We also found that prophylactic TAP can prevent progression of late moderate or greater TR. TAP showed a tendency to prevent tricuspid valve–related events and was marginally significant.”
The researchers also evaluated the role of AF as a risk factor for progression of late TR; the effects of TAP on recurrence of moderate or greater TR were “prominent” in patients with sinus rhythm vs. AF at discharge. “These findings may reflect that the maze procedure is more important than prophylactic TAP in improving late outcomes in mitral valve disease with AF,” Dr. Lee and colleagues wrote.
The investigators acknowledged a number of limits of their study: its retrospective nature; how indications and techniques of MVR, TAP, and the maze procedure evolved over the study duration; and that the maze procedure and prophylactic TAP were not widely embraced in the early years of the study.
Dr. Lee and colleagues had no financial relationships to disclose.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Tricuspid annuloplasty performed at the same time as mitral valve surgery in people with moderate or less tricuspid regurgitation can be performed safely and achieve its clinical objective.
Major finding: Patients who had the concomitant procedure had similar rates of surgery-related death and complications as did those who had mitral valve surgery only and lower rates of late progression of tricuspid regurgitation.
Data source: Retrospective review of data for 293 patients who underwent primary mitral valve repair with mechanical prosthesis at a single center from November 1994 to December 2010.
Disclosures: The study authors had no financial relationships to disclose.
Wanted: Better evidence on fast-track lung resection
A host of medical specialties have adopted strategies to speed recovery of surgical patients, reduce length of hospital stays, and cut costs, known as fast-track or enhanced-recovery pathways, but when it comes to elective lung resection, the medical evidence has yet to establish if patients in expedited recovery protocols fare any better than do those in a conventional recovery course, a systematic review in the March issue of the Journal of Thoracic and Cardiovascular Surgery reported (2016 Mar;151:708-15).
A team of investigators from McGill University in Montreal performed a systematic review of six studies that evaluated patient outcomes of both traditional and enhanced-recovery pathways (ERPs) in elective lung resection. They concluded that ERPs may reduce the length of hospital stays and hospital costs but that well-designed trials are needed to overcome limitations of existing studies.
“The influence of ERPs on postoperative outcomes after lung resection has not been extensively studied in comparative studies involving a control group receiving traditional care,” lead author Julio F. Fiore Jr., Ph.D., and his colleagues said. One of the six studies they reviewed was a randomized clinical trial. The six studies involved a total of 1,612 participants (821 ERP, 791 control).
The researchers also reported that the studies they analyzed shared a significant limitation. “Risk of bias favoring enhanced-recovery pathways was high,” Dr. Fiore and his colleagues wrote. The studies were unclear if patient selection may have factored into the results.
Five studies reported shorter hospital length of stay (LOS) for the ERP group. “The majority of the studies reported that LOS was significantly shorter when patients undergoing lung resection were treated within an ERP, which corroborates the results observed in other surgical populations,” Dr. Fiore and his colleagues said.
Three nonrandomized studies also evaluated costs per patient. Two reported significantly lower costs for ERP patients: $13,093 vs. $14,439 for controls; and $13,432 vs. $17,103 for controls (Jpn. J. Thorac. Cardiovasc. Surg. 2006 Sep;54:387-90; Ann. Thorac. Surg. 1998 Sep;66:914-9). The third showed what the authors said was no statistically significant cost differential between the two groups: $14,792 for ERP vs. $16,063 for controls (Ann. Thorac. Surg. 1997 Aug;64:299-302).
Three studies evaluated readmission rates, but only one showed measurably lower rates for the ERP group: 3% vs. 10% for controls (Lung Cancer. 2012 Dec;78:270-5). Three studies measured complication rates in both groups. Two reported cardiopulmonary complication rates of 18% and 17% in the ERP group vs. 16% and 14% in the control group, respectively (Eur. J. Cardiothorac. Surg. 2012 May;41:1083-7; Lung Cancer. 2012 Dec;78:270-5). One reported rates of pulmonary complications of 7% for ERP vs. 36% for controls (Eur. J. Cardiothorac. Surg. 2008 Jul;34:174-80).
Dr. Fiore and his colleagues pointed out that some of the studies they reviewed were completed before video-assisted thoracic surgery became routine for lung resection. But they acknowledged that research in other surgical specialties have validated the role of ERP, along with minimally invasive surgery, to improve outcomes. “Future research should investigate whether this holds true for patients undergoing lung resection,” they said.
The study authors had no financial relationships to disclose.
The task that Dr. Fiore and colleagues undertook to evaluate and compare disparate studies of fast-track surgery in lung resection is “akin to comparing not just apples and oranges but apples to zucchini,” Dr. Lisa M. Brown of University of California, Davis, Medical Center said in her invited analysis (J. Thorac. Cardiovasc. Surg. 2016 Mar;151:715-16). Without the authors’ “descriptive approach,” Dr. Brown said, “the results of a true meta-analysis would be uninterpretable.”

|
Dr. Lisa M. Brown |
Nonetheless, the systematic review underscores the need for a blinded, randomized trial, Dr. Brown said. “Furthermore, rather than measuring [hospital] stay, subjects should be evaluated for readiness for discharge, because this would reduce the effect of systems-based obstacles to discharge,” she said. Enhanced recovery pathways (ERPs) in colorectal surgery have been used as models for other specialties, but the novelty of these pathways versus traditional care may be difficult to replicate in thoracic surgery, she said. Strategies such as antibiotic prophylaxis and epidural analgesia in thoracic surgery “are not dissimilar enough from standard care to elicit a difference in outcome,” she said.
In thoracic surgery, ERPs must consider the challenges of pain control and chest tube management unique in these patients, Dr. Brown said. For pain control, paravertebral blockade rather than epidural analgesia could lead to earlier hospital discharges. Use of chest tubes is commonly a matter of surgeon preference, she said, but chest tubes without an air leak and with acceptable fluid output can be safely removed, and even patients with an air leak but no pneumothorax on water seal can go home with a chest tube, Dr. Brown said.
Dr. Brown had no financial relationships to disclose.
The task that Dr. Fiore and colleagues undertook to evaluate and compare disparate studies of fast-track surgery in lung resection is “akin to comparing not just apples and oranges but apples to zucchini,” Dr. Lisa M. Brown of University of California, Davis, Medical Center said in her invited analysis (J. Thorac. Cardiovasc. Surg. 2016 Mar;151:715-16). Without the authors’ “descriptive approach,” Dr. Brown said, “the results of a true meta-analysis would be uninterpretable.”

|
Dr. Lisa M. Brown |
Nonetheless, the systematic review underscores the need for a blinded, randomized trial, Dr. Brown said. “Furthermore, rather than measuring [hospital] stay, subjects should be evaluated for readiness for discharge, because this would reduce the effect of systems-based obstacles to discharge,” she said. Enhanced recovery pathways (ERPs) in colorectal surgery have been used as models for other specialties, but the novelty of these pathways versus traditional care may be difficult to replicate in thoracic surgery, she said. Strategies such as antibiotic prophylaxis and epidural analgesia in thoracic surgery “are not dissimilar enough from standard care to elicit a difference in outcome,” she said.
In thoracic surgery, ERPs must consider the challenges of pain control and chest tube management unique in these patients, Dr. Brown said. For pain control, paravertebral blockade rather than epidural analgesia could lead to earlier hospital discharges. Use of chest tubes is commonly a matter of surgeon preference, she said, but chest tubes without an air leak and with acceptable fluid output can be safely removed, and even patients with an air leak but no pneumothorax on water seal can go home with a chest tube, Dr. Brown said.
Dr. Brown had no financial relationships to disclose.
The task that Dr. Fiore and colleagues undertook to evaluate and compare disparate studies of fast-track surgery in lung resection is “akin to comparing not just apples and oranges but apples to zucchini,” Dr. Lisa M. Brown of University of California, Davis, Medical Center said in her invited analysis (J. Thorac. Cardiovasc. Surg. 2016 Mar;151:715-16). Without the authors’ “descriptive approach,” Dr. Brown said, “the results of a true meta-analysis would be uninterpretable.”

|
Dr. Lisa M. Brown |
Nonetheless, the systematic review underscores the need for a blinded, randomized trial, Dr. Brown said. “Furthermore, rather than measuring [hospital] stay, subjects should be evaluated for readiness for discharge, because this would reduce the effect of systems-based obstacles to discharge,” she said. Enhanced recovery pathways (ERPs) in colorectal surgery have been used as models for other specialties, but the novelty of these pathways versus traditional care may be difficult to replicate in thoracic surgery, she said. Strategies such as antibiotic prophylaxis and epidural analgesia in thoracic surgery “are not dissimilar enough from standard care to elicit a difference in outcome,” she said.
In thoracic surgery, ERPs must consider the challenges of pain control and chest tube management unique in these patients, Dr. Brown said. For pain control, paravertebral blockade rather than epidural analgesia could lead to earlier hospital discharges. Use of chest tubes is commonly a matter of surgeon preference, she said, but chest tubes without an air leak and with acceptable fluid output can be safely removed, and even patients with an air leak but no pneumothorax on water seal can go home with a chest tube, Dr. Brown said.
Dr. Brown had no financial relationships to disclose.
A host of medical specialties have adopted strategies to speed recovery of surgical patients, reduce length of hospital stays, and cut costs, known as fast-track or enhanced-recovery pathways, but when it comes to elective lung resection, the medical evidence has yet to establish if patients in expedited recovery protocols fare any better than do those in a conventional recovery course, a systematic review in the March issue of the Journal of Thoracic and Cardiovascular Surgery reported (2016 Mar;151:708-15).
A team of investigators from McGill University in Montreal performed a systematic review of six studies that evaluated patient outcomes of both traditional and enhanced-recovery pathways (ERPs) in elective lung resection. They concluded that ERPs may reduce the length of hospital stays and hospital costs but that well-designed trials are needed to overcome limitations of existing studies.
“The influence of ERPs on postoperative outcomes after lung resection has not been extensively studied in comparative studies involving a control group receiving traditional care,” lead author Julio F. Fiore Jr., Ph.D., and his colleagues said. One of the six studies they reviewed was a randomized clinical trial. The six studies involved a total of 1,612 participants (821 ERP, 791 control).
The researchers also reported that the studies they analyzed shared a significant limitation. “Risk of bias favoring enhanced-recovery pathways was high,” Dr. Fiore and his colleagues wrote. The studies were unclear if patient selection may have factored into the results.
Five studies reported shorter hospital length of stay (LOS) for the ERP group. “The majority of the studies reported that LOS was significantly shorter when patients undergoing lung resection were treated within an ERP, which corroborates the results observed in other surgical populations,” Dr. Fiore and his colleagues said.
Three nonrandomized studies also evaluated costs per patient. Two reported significantly lower costs for ERP patients: $13,093 vs. $14,439 for controls; and $13,432 vs. $17,103 for controls (Jpn. J. Thorac. Cardiovasc. Surg. 2006 Sep;54:387-90; Ann. Thorac. Surg. 1998 Sep;66:914-9). The third showed what the authors said was no statistically significant cost differential between the two groups: $14,792 for ERP vs. $16,063 for controls (Ann. Thorac. Surg. 1997 Aug;64:299-302).
Three studies evaluated readmission rates, but only one showed measurably lower rates for the ERP group: 3% vs. 10% for controls (Lung Cancer. 2012 Dec;78:270-5). Three studies measured complication rates in both groups. Two reported cardiopulmonary complication rates of 18% and 17% in the ERP group vs. 16% and 14% in the control group, respectively (Eur. J. Cardiothorac. Surg. 2012 May;41:1083-7; Lung Cancer. 2012 Dec;78:270-5). One reported rates of pulmonary complications of 7% for ERP vs. 36% for controls (Eur. J. Cardiothorac. Surg. 2008 Jul;34:174-80).
Dr. Fiore and his colleagues pointed out that some of the studies they reviewed were completed before video-assisted thoracic surgery became routine for lung resection. But they acknowledged that research in other surgical specialties have validated the role of ERP, along with minimally invasive surgery, to improve outcomes. “Future research should investigate whether this holds true for patients undergoing lung resection,” they said.
The study authors had no financial relationships to disclose.
A host of medical specialties have adopted strategies to speed recovery of surgical patients, reduce length of hospital stays, and cut costs, known as fast-track or enhanced-recovery pathways, but when it comes to elective lung resection, the medical evidence has yet to establish if patients in expedited recovery protocols fare any better than do those in a conventional recovery course, a systematic review in the March issue of the Journal of Thoracic and Cardiovascular Surgery reported (2016 Mar;151:708-15).
A team of investigators from McGill University in Montreal performed a systematic review of six studies that evaluated patient outcomes of both traditional and enhanced-recovery pathways (ERPs) in elective lung resection. They concluded that ERPs may reduce the length of hospital stays and hospital costs but that well-designed trials are needed to overcome limitations of existing studies.
“The influence of ERPs on postoperative outcomes after lung resection has not been extensively studied in comparative studies involving a control group receiving traditional care,” lead author Julio F. Fiore Jr., Ph.D., and his colleagues said. One of the six studies they reviewed was a randomized clinical trial. The six studies involved a total of 1,612 participants (821 ERP, 791 control).
The researchers also reported that the studies they analyzed shared a significant limitation. “Risk of bias favoring enhanced-recovery pathways was high,” Dr. Fiore and his colleagues wrote. The studies were unclear if patient selection may have factored into the results.
Five studies reported shorter hospital length of stay (LOS) for the ERP group. “The majority of the studies reported that LOS was significantly shorter when patients undergoing lung resection were treated within an ERP, which corroborates the results observed in other surgical populations,” Dr. Fiore and his colleagues said.
Three nonrandomized studies also evaluated costs per patient. Two reported significantly lower costs for ERP patients: $13,093 vs. $14,439 for controls; and $13,432 vs. $17,103 for controls (Jpn. J. Thorac. Cardiovasc. Surg. 2006 Sep;54:387-90; Ann. Thorac. Surg. 1998 Sep;66:914-9). The third showed what the authors said was no statistically significant cost differential between the two groups: $14,792 for ERP vs. $16,063 for controls (Ann. Thorac. Surg. 1997 Aug;64:299-302).
Three studies evaluated readmission rates, but only one showed measurably lower rates for the ERP group: 3% vs. 10% for controls (Lung Cancer. 2012 Dec;78:270-5). Three studies measured complication rates in both groups. Two reported cardiopulmonary complication rates of 18% and 17% in the ERP group vs. 16% and 14% in the control group, respectively (Eur. J. Cardiothorac. Surg. 2012 May;41:1083-7; Lung Cancer. 2012 Dec;78:270-5). One reported rates of pulmonary complications of 7% for ERP vs. 36% for controls (Eur. J. Cardiothorac. Surg. 2008 Jul;34:174-80).
Dr. Fiore and his colleagues pointed out that some of the studies they reviewed were completed before video-assisted thoracic surgery became routine for lung resection. But they acknowledged that research in other surgical specialties have validated the role of ERP, along with minimally invasive surgery, to improve outcomes. “Future research should investigate whether this holds true for patients undergoing lung resection,” they said.
The study authors had no financial relationships to disclose.
Key clinical point: Well-designed clinical trials are needed to determine the effectiveness of fast-track recovery pathways in lung resection.
Major finding: Fast-track lung resection patients showed no differences in readmissions, overall complication and death rates compared to patients subjected to a traditional recovery course.
Data source: Systematic review of six studies published from 1997 to 2012 that involved 1,612 individuals who had lung resection.
Disclosures: The study authors had no financial relationships to disclose.
Sutureless AVR an option for higher-risk patients
The first North American experience with a sutureless bioprosthetic aortic valve that has been available in Europe since 2005 and is well suited for minimally invasive surgery has underscored the utility of the device as an alternative to conventional aortic valve replacement (AVR) in higher-risk patients, investigators from McGill University Health Center in Montreal reported in the March issue of the Journal of Thoracic and Cardiovascular Surgery (2016;151:735-742).
The investigators, led by Dr. Benoir de Varennes, reported on their experience implanting the Enable valve (Medtronic, Minneapolis) in 63 patients between August 2012 and October 2014. “The enable bioprosthesis is an acceptable alternative to conventional aortic valve replacement in higher-risk patients,” Dr. de Varennes and colleagues said. “The early hemodynamic performance seems favorable.” Their findings were first presented at the 95th annual meeting of the American Association for Thoracic Surgery in April 2015 in Seattle. A video of the presentation is available.
The Enable valve has been the subject of four European studies with 429 patients. It received its CE Mark in Europe in 2009, but is not yet commercially approved in the United States.
In the McGill study, one patient died within 30 days of receiving the valve and two died after 30 days, but none of the deaths were valve related. Four patients (6.3%) required revision during the implantation operation, and one patient required reoperation for early migration. Peak and mean gradients after surgery were 17 mm Hg and 9 mm Hg, respectively. Three patients had reported complications: Two (3.1%) required a pacemaker and one (1.6%) had a heart attack. Mean follow-up was 10 months.
Patient ages ranged from 57 to 89 years, with an average age of 80. Before surgery, all patients had calcific aortic stenosis, 43 (68%) had some degree of associated aortic regurgitation, and 46 (73%) were in New York Heart Association (NYHA) class III or IV. At the last follow-up after surgery, 61 patients (97%) were in NYHA class I.
The investigators implanted the valve through a full sternotomy or a partial upper sternotomy into the fourth intercostal space, and they used perioperative transesophageal echocardiography in all patients. They performed high-transverse aortotomy and completely excised the native valve.
The average cross-clamp time for the 30 patients who had isolated AVR was 44 minutes and 77 minutes for the 33 patients who had combined procedures. Dr. de Varennes and colleagues acknowledged the cross-clamp time for isolated AVR is “similar” to European series but “not very different” from recent reports on sutured AVR (J. Thorac. Cardiovasc. Surg. 2015;149:451-460). “This may be explained partly by the learning period of all three surgeons and the aggressive debridement of the annulus in all cases,” they said. “We think that, as further experience is gained, the clamp time will be further reduced, and this will benefit mostly higher-risk patients or those requiring concomitant procedures.”
They noted that some patients received the Enable prosthesis because of “hostile” aortas with extensive root calcification.
Dr. de Varennes disclosed he is a consultant for Medtronic and a proctor for Enable training. The coauthors had no relationships to disclose.
One of the key advantages that advocates of sutureless valves point to is shorter bypass times than sutured valves, but in his invited commentary Dr. Thomas G. Gleason of the University of Pittsburgh questioned this rationale based on the results Dr. de Varennes and colleagues reported (J. Thorac. Cardiovasc. Surg. 2016;151:743-744). The cardiac bypass times they observed “are not appreciably different from those reported in larger series of conventional aortic valve replacement,” Dr. Gleason said.
Dr. Gleason suggested that “market forces” might be driving the push into sutureless aortic valve replacement. “The attraction, particularly to consumers, of the ministernotomy (and thus things that might facilitate it) is both cosmetic and the perception that it is less invasive,” he said. “These attractions notwithstanding, it has been difficult to demonstrate that ministernotomy or minithoracotomy yield better primary outcomes (e.g., mortality, stroke, or major complication rates) or even quality of life indicators, particularly when measured beyond the perioperative period.”

|
Dr. Thomas G. Gleason |
He alluded to the “elephant in the room” with regard to sutureless aortic valve technologies: their cost and unknown durability compared with conventional sutured bioprostheses.
“As health care costs continue to rise and large populations of patients are either underinsured or see rationed care, trimming direct costs may be a more relevant concern for the modern era than trimming cross-clamp time,” he said. Analyses have not yet evaluated the increased costs of sutureless valves in terms of shortened hospital stays or lower morbidity, particularly in the moderate-risk population with aortic stenosis, he said.
“Moving forward, there is little doubt that the current value of the sutureless valve will be dictated by the market, but in the end it will be measured by the long-term outcomes of the ‘minimally invaded,’” Dr. Gleason said.
Dr. Gleason had no financial relationships to disclose.
One of the key advantages that advocates of sutureless valves point to is shorter bypass times than sutured valves, but in his invited commentary Dr. Thomas G. Gleason of the University of Pittsburgh questioned this rationale based on the results Dr. de Varennes and colleagues reported (J. Thorac. Cardiovasc. Surg. 2016;151:743-744). The cardiac bypass times they observed “are not appreciably different from those reported in larger series of conventional aortic valve replacement,” Dr. Gleason said.
Dr. Gleason suggested that “market forces” might be driving the push into sutureless aortic valve replacement. “The attraction, particularly to consumers, of the ministernotomy (and thus things that might facilitate it) is both cosmetic and the perception that it is less invasive,” he said. “These attractions notwithstanding, it has been difficult to demonstrate that ministernotomy or minithoracotomy yield better primary outcomes (e.g., mortality, stroke, or major complication rates) or even quality of life indicators, particularly when measured beyond the perioperative period.”

|
Dr. Thomas G. Gleason |
He alluded to the “elephant in the room” with regard to sutureless aortic valve technologies: their cost and unknown durability compared with conventional sutured bioprostheses.
“As health care costs continue to rise and large populations of patients are either underinsured or see rationed care, trimming direct costs may be a more relevant concern for the modern era than trimming cross-clamp time,” he said. Analyses have not yet evaluated the increased costs of sutureless valves in terms of shortened hospital stays or lower morbidity, particularly in the moderate-risk population with aortic stenosis, he said.
“Moving forward, there is little doubt that the current value of the sutureless valve will be dictated by the market, but in the end it will be measured by the long-term outcomes of the ‘minimally invaded,’” Dr. Gleason said.
Dr. Gleason had no financial relationships to disclose.
One of the key advantages that advocates of sutureless valves point to is shorter bypass times than sutured valves, but in his invited commentary Dr. Thomas G. Gleason of the University of Pittsburgh questioned this rationale based on the results Dr. de Varennes and colleagues reported (J. Thorac. Cardiovasc. Surg. 2016;151:743-744). The cardiac bypass times they observed “are not appreciably different from those reported in larger series of conventional aortic valve replacement,” Dr. Gleason said.
Dr. Gleason suggested that “market forces” might be driving the push into sutureless aortic valve replacement. “The attraction, particularly to consumers, of the ministernotomy (and thus things that might facilitate it) is both cosmetic and the perception that it is less invasive,” he said. “These attractions notwithstanding, it has been difficult to demonstrate that ministernotomy or minithoracotomy yield better primary outcomes (e.g., mortality, stroke, or major complication rates) or even quality of life indicators, particularly when measured beyond the perioperative period.”

|
Dr. Thomas G. Gleason |
He alluded to the “elephant in the room” with regard to sutureless aortic valve technologies: their cost and unknown durability compared with conventional sutured bioprostheses.
“As health care costs continue to rise and large populations of patients are either underinsured or see rationed care, trimming direct costs may be a more relevant concern for the modern era than trimming cross-clamp time,” he said. Analyses have not yet evaluated the increased costs of sutureless valves in terms of shortened hospital stays or lower morbidity, particularly in the moderate-risk population with aortic stenosis, he said.
“Moving forward, there is little doubt that the current value of the sutureless valve will be dictated by the market, but in the end it will be measured by the long-term outcomes of the ‘minimally invaded,’” Dr. Gleason said.
Dr. Gleason had no financial relationships to disclose.
The first North American experience with a sutureless bioprosthetic aortic valve that has been available in Europe since 2005 and is well suited for minimally invasive surgery has underscored the utility of the device as an alternative to conventional aortic valve replacement (AVR) in higher-risk patients, investigators from McGill University Health Center in Montreal reported in the March issue of the Journal of Thoracic and Cardiovascular Surgery (2016;151:735-742).
The investigators, led by Dr. Benoir de Varennes, reported on their experience implanting the Enable valve (Medtronic, Minneapolis) in 63 patients between August 2012 and October 2014. “The enable bioprosthesis is an acceptable alternative to conventional aortic valve replacement in higher-risk patients,” Dr. de Varennes and colleagues said. “The early hemodynamic performance seems favorable.” Their findings were first presented at the 95th annual meeting of the American Association for Thoracic Surgery in April 2015 in Seattle. A video of the presentation is available.
The Enable valve has been the subject of four European studies with 429 patients. It received its CE Mark in Europe in 2009, but is not yet commercially approved in the United States.
In the McGill study, one patient died within 30 days of receiving the valve and two died after 30 days, but none of the deaths were valve related. Four patients (6.3%) required revision during the implantation operation, and one patient required reoperation for early migration. Peak and mean gradients after surgery were 17 mm Hg and 9 mm Hg, respectively. Three patients had reported complications: Two (3.1%) required a pacemaker and one (1.6%) had a heart attack. Mean follow-up was 10 months.
Patient ages ranged from 57 to 89 years, with an average age of 80. Before surgery, all patients had calcific aortic stenosis, 43 (68%) had some degree of associated aortic regurgitation, and 46 (73%) were in New York Heart Association (NYHA) class III or IV. At the last follow-up after surgery, 61 patients (97%) were in NYHA class I.
The investigators implanted the valve through a full sternotomy or a partial upper sternotomy into the fourth intercostal space, and they used perioperative transesophageal echocardiography in all patients. They performed high-transverse aortotomy and completely excised the native valve.
The average cross-clamp time for the 30 patients who had isolated AVR was 44 minutes and 77 minutes for the 33 patients who had combined procedures. Dr. de Varennes and colleagues acknowledged the cross-clamp time for isolated AVR is “similar” to European series but “not very different” from recent reports on sutured AVR (J. Thorac. Cardiovasc. Surg. 2015;149:451-460). “This may be explained partly by the learning period of all three surgeons and the aggressive debridement of the annulus in all cases,” they said. “We think that, as further experience is gained, the clamp time will be further reduced, and this will benefit mostly higher-risk patients or those requiring concomitant procedures.”
They noted that some patients received the Enable prosthesis because of “hostile” aortas with extensive root calcification.
Dr. de Varennes disclosed he is a consultant for Medtronic and a proctor for Enable training. The coauthors had no relationships to disclose.
The first North American experience with a sutureless bioprosthetic aortic valve that has been available in Europe since 2005 and is well suited for minimally invasive surgery has underscored the utility of the device as an alternative to conventional aortic valve replacement (AVR) in higher-risk patients, investigators from McGill University Health Center in Montreal reported in the March issue of the Journal of Thoracic and Cardiovascular Surgery (2016;151:735-742).
The investigators, led by Dr. Benoir de Varennes, reported on their experience implanting the Enable valve (Medtronic, Minneapolis) in 63 patients between August 2012 and October 2014. “The enable bioprosthesis is an acceptable alternative to conventional aortic valve replacement in higher-risk patients,” Dr. de Varennes and colleagues said. “The early hemodynamic performance seems favorable.” Their findings were first presented at the 95th annual meeting of the American Association for Thoracic Surgery in April 2015 in Seattle. A video of the presentation is available.
The Enable valve has been the subject of four European studies with 429 patients. It received its CE Mark in Europe in 2009, but is not yet commercially approved in the United States.
In the McGill study, one patient died within 30 days of receiving the valve and two died after 30 days, but none of the deaths were valve related. Four patients (6.3%) required revision during the implantation operation, and one patient required reoperation for early migration. Peak and mean gradients after surgery were 17 mm Hg and 9 mm Hg, respectively. Three patients had reported complications: Two (3.1%) required a pacemaker and one (1.6%) had a heart attack. Mean follow-up was 10 months.
Patient ages ranged from 57 to 89 years, with an average age of 80. Before surgery, all patients had calcific aortic stenosis, 43 (68%) had some degree of associated aortic regurgitation, and 46 (73%) were in New York Heart Association (NYHA) class III or IV. At the last follow-up after surgery, 61 patients (97%) were in NYHA class I.
The investigators implanted the valve through a full sternotomy or a partial upper sternotomy into the fourth intercostal space, and they used perioperative transesophageal echocardiography in all patients. They performed high-transverse aortotomy and completely excised the native valve.
The average cross-clamp time for the 30 patients who had isolated AVR was 44 minutes and 77 minutes for the 33 patients who had combined procedures. Dr. de Varennes and colleagues acknowledged the cross-clamp time for isolated AVR is “similar” to European series but “not very different” from recent reports on sutured AVR (J. Thorac. Cardiovasc. Surg. 2015;149:451-460). “This may be explained partly by the learning period of all three surgeons and the aggressive debridement of the annulus in all cases,” they said. “We think that, as further experience is gained, the clamp time will be further reduced, and this will benefit mostly higher-risk patients or those requiring concomitant procedures.”
They noted that some patients received the Enable prosthesis because of “hostile” aortas with extensive root calcification.
Dr. de Varennes disclosed he is a consultant for Medtronic and a proctor for Enable training. The coauthors had no relationships to disclose.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Sutureless aortic valves have the potential to achieve shorter procedure times and benefit increased-risk patients with aortic stenosis.
Major finding: Thirty-day mortality of patients who received the Enable aortic valve was 1.6%, and late mortality was 3.2%. No deaths were valve related.
Data source: Sixty-three patients with aortic stenosis who had Enable bioprosthetic valve implantation between August 2012 and October 2014 at McGill University Health Center.
Disclosures: Lead author Dr. Benoit de Varennes is a consultant for Medtronic and a trainer for the Enable device. The other authors had no relationships to disclose.
Flu vaccination found safe in surgical patients
Immunizing surgical patients against seasonal influenza before they are discharged from the hospital appears safe and is a sound strategy for expanding vaccine coverage, especially among people at high risk, according to a report published online March 14 in Annals of Internal Medicine.
All health care contacts, including hospitalizations, are considered excellent opportunities for influenza vaccination, and current recommendations advise that eligible inpatients receive the immunization before discharge. However, surgical patients don’t often get the flu vaccine before they leave the hospital, likely because of concerns that potential adverse effects like fever and myalgia could be falsely attributed to surgical complications. This would lead to unnecessary patient evaluations and could interfere with postsurgical care, said Sara Y. Tartof, Ph.D., and her associates in the department of research and evaluation, Kaiser Permanente Southern California, Pasadena.
“Although this concern is understandable, few clinical data support it,” they noted.
“To provide clinical evidence that would either substantiate or refute” these concerns about perioperative flu vaccination, the investigators analyzed data in the electronic health records for 81,647 surgeries. All the study participants were deemed eligible for flu vaccination. They were socioeconomically and ethnically diverse, ranged in age from 6 months to 106 years, and underwent surgery at 14 hospitals during three consecutive flu seasons. Operations included general, cardiac, eye, dermatologic, ENT, neurologic, ob.gyn., oral/maxillofacial, orthopedic, plastic, podiatric, urologic, and vascular procedures.
Patients received a flu vaccine in 6,420 hospital stays for surgery – only 15% of 42,777 eligible hospitalizations – usually on the day of discharge. (The remaining 38,870 patients either had been vaccinated before hospital admission or were vaccinated more than a week after discharge and were not included in further analyses.)
Compared with eligible patients who didn’t receive a flu vaccine during hospitalization for surgery, those who did showed no increased risk for subsequent inpatient visits, ED visits, or clinical work-ups for infection. Patients who received the flu vaccine before discharge showed a minimally increased risk for outpatient visits during the week following hospitalization, but this was considered unlikely “to translate into substantial clinical impact,” especially when balanced against the benefit of immunization, Dr. Tartof and her associates said (Ann Intern Med. 2016 Mar 14. doi: 10.7326/M15-1667).
Giving the flu vaccine during a surgical hospitalization “is an opportunity to protect a high-risk population,” because surgery patients tend to be of an age, and to have comorbid conditions, that raise their risk for flu complications. In addition, previous research has reported that 39%-46% of adults hospitalized for influenza-related disease in a given year had been hospitalized during the preceding autumn, indicating that recent hospitalization also raises the risk for flu complications, the investigators said.
“Our data support the rationale for increasing vaccination rates among surgical inpatients,” they said.
This study was funded by the U.S. Centers for Disease Control and Prevention through the Vaccine Safety Datalink program. Dr. Tartof reported receiving grants from Merck outside of this work; two of her associates reported receiving grants from Novartis and GlaxoSmithKline outside of this work.
Immunizing surgical patients against seasonal influenza before they are discharged from the hospital appears safe and is a sound strategy for expanding vaccine coverage, especially among people at high risk, according to a report published online March 14 in Annals of Internal Medicine.
All health care contacts, including hospitalizations, are considered excellent opportunities for influenza vaccination, and current recommendations advise that eligible inpatients receive the immunization before discharge. However, surgical patients don’t often get the flu vaccine before they leave the hospital, likely because of concerns that potential adverse effects like fever and myalgia could be falsely attributed to surgical complications. This would lead to unnecessary patient evaluations and could interfere with postsurgical care, said Sara Y. Tartof, Ph.D., and her associates in the department of research and evaluation, Kaiser Permanente Southern California, Pasadena.
“Although this concern is understandable, few clinical data support it,” they noted.
“To provide clinical evidence that would either substantiate or refute” these concerns about perioperative flu vaccination, the investigators analyzed data in the electronic health records for 81,647 surgeries. All the study participants were deemed eligible for flu vaccination. They were socioeconomically and ethnically diverse, ranged in age from 6 months to 106 years, and underwent surgery at 14 hospitals during three consecutive flu seasons. Operations included general, cardiac, eye, dermatologic, ENT, neurologic, ob.gyn., oral/maxillofacial, orthopedic, plastic, podiatric, urologic, and vascular procedures.
Patients received a flu vaccine in 6,420 hospital stays for surgery – only 15% of 42,777 eligible hospitalizations – usually on the day of discharge. (The remaining 38,870 patients either had been vaccinated before hospital admission or were vaccinated more than a week after discharge and were not included in further analyses.)
Compared with eligible patients who didn’t receive a flu vaccine during hospitalization for surgery, those who did showed no increased risk for subsequent inpatient visits, ED visits, or clinical work-ups for infection. Patients who received the flu vaccine before discharge showed a minimally increased risk for outpatient visits during the week following hospitalization, but this was considered unlikely “to translate into substantial clinical impact,” especially when balanced against the benefit of immunization, Dr. Tartof and her associates said (Ann Intern Med. 2016 Mar 14. doi: 10.7326/M15-1667).
Giving the flu vaccine during a surgical hospitalization “is an opportunity to protect a high-risk population,” because surgery patients tend to be of an age, and to have comorbid conditions, that raise their risk for flu complications. In addition, previous research has reported that 39%-46% of adults hospitalized for influenza-related disease in a given year had been hospitalized during the preceding autumn, indicating that recent hospitalization also raises the risk for flu complications, the investigators said.
“Our data support the rationale for increasing vaccination rates among surgical inpatients,” they said.
This study was funded by the U.S. Centers for Disease Control and Prevention through the Vaccine Safety Datalink program. Dr. Tartof reported receiving grants from Merck outside of this work; two of her associates reported receiving grants from Novartis and GlaxoSmithKline outside of this work.
Immunizing surgical patients against seasonal influenza before they are discharged from the hospital appears safe and is a sound strategy for expanding vaccine coverage, especially among people at high risk, according to a report published online March 14 in Annals of Internal Medicine.
All health care contacts, including hospitalizations, are considered excellent opportunities for influenza vaccination, and current recommendations advise that eligible inpatients receive the immunization before discharge. However, surgical patients don’t often get the flu vaccine before they leave the hospital, likely because of concerns that potential adverse effects like fever and myalgia could be falsely attributed to surgical complications. This would lead to unnecessary patient evaluations and could interfere with postsurgical care, said Sara Y. Tartof, Ph.D., and her associates in the department of research and evaluation, Kaiser Permanente Southern California, Pasadena.
“Although this concern is understandable, few clinical data support it,” they noted.
“To provide clinical evidence that would either substantiate or refute” these concerns about perioperative flu vaccination, the investigators analyzed data in the electronic health records for 81,647 surgeries. All the study participants were deemed eligible for flu vaccination. They were socioeconomically and ethnically diverse, ranged in age from 6 months to 106 years, and underwent surgery at 14 hospitals during three consecutive flu seasons. Operations included general, cardiac, eye, dermatologic, ENT, neurologic, ob.gyn., oral/maxillofacial, orthopedic, plastic, podiatric, urologic, and vascular procedures.
Patients received a flu vaccine in 6,420 hospital stays for surgery – only 15% of 42,777 eligible hospitalizations – usually on the day of discharge. (The remaining 38,870 patients either had been vaccinated before hospital admission or were vaccinated more than a week after discharge and were not included in further analyses.)
Compared with eligible patients who didn’t receive a flu vaccine during hospitalization for surgery, those who did showed no increased risk for subsequent inpatient visits, ED visits, or clinical work-ups for infection. Patients who received the flu vaccine before discharge showed a minimally increased risk for outpatient visits during the week following hospitalization, but this was considered unlikely “to translate into substantial clinical impact,” especially when balanced against the benefit of immunization, Dr. Tartof and her associates said (Ann Intern Med. 2016 Mar 14. doi: 10.7326/M15-1667).
Giving the flu vaccine during a surgical hospitalization “is an opportunity to protect a high-risk population,” because surgery patients tend to be of an age, and to have comorbid conditions, that raise their risk for flu complications. In addition, previous research has reported that 39%-46% of adults hospitalized for influenza-related disease in a given year had been hospitalized during the preceding autumn, indicating that recent hospitalization also raises the risk for flu complications, the investigators said.
“Our data support the rationale for increasing vaccination rates among surgical inpatients,” they said.
This study was funded by the U.S. Centers for Disease Control and Prevention through the Vaccine Safety Datalink program. Dr. Tartof reported receiving grants from Merck outside of this work; two of her associates reported receiving grants from Novartis and GlaxoSmithKline outside of this work.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Immunizing surgical patients against seasonal influenza before they leave the hospital appears safe.
Major finding: Patients received a flu vaccine in only 6,420 hospital stays for surgery, comprising only 15% of the patient hospitalizations that were eligible.
Data source: A retrospective cohort study involving 81,647 surgeries at 14 California hospitals during three consecutive flu seasons.
Disclosures: This study was funded by the U.S. Centers for Disease Control and Prevention through the Vaccine Safety Datalink program. Dr. Tartof reported receiving grants from Merck outside of this work; two of her associates reported receiving grants from Novartis and GlaxoSmithKline outside of this work.
Outcomes in major surgery unchanged by continuing clopidogrel
MONTREAL – Patients who stayed on antiplatelet therapy close to – or even up until – major surgery fared just as well as those who stopped their medication earlier in a retrospective, single-center study.
The study found no difference in blood product administration, adverse perioperative events, or all-cause 30-day mortality regardless of whether patients stopped clopidogrel (Plavix) the recommended 5 days before surgery.
“We believe that continuing clopidogrel in elective and emergent surgical situations appears to be safe, and may challenge the current recommendations,” said presenter Dr. David Strosberg.
The study addressed a thorny question for surgeons, Dr. Strosberg said. “As surgeons, we face a dilemma: Do we take the risk of thrombotic complications in stopping the antiplatelet drugs, or do we take the risk of increased surgical bleeding with continuing therapy?”
The package insert for clopidogrel advises discontinuation 5 days prior to surgery. However, manufacturer labeling also states that discontinuation of clopidogrel can lead to adverse cardiac events, said Dr. Strosberg, a general surgery resident at Ohio State University in Columbus.
The aim of the study, presented at the annual meeting of the Central Surgical Association, was to ascertain whether continuing antiplatelet therapy increased the rate of adverse surgical outcomes in those undergoing major emergent or elective surgery.
Dr. Strosberg and his colleagues retrospectively reviewed the record of patients over a 4-year period at a single institution and included those undergoing major general, thoracic, or vascular surgery who were taking clopidogrel at the time of presentation.
Data collected included patient characteristics, including demographic data and comorbidities, as well as transfusion requirements and perioperative events.
A total of 200 patients who had 205 qualifying procedures and were taking clopidogrel were included in the study. Of these, 116 patients (Group A) had their clopidogrel held for at least 5 days preoperatively. The remaining 89 patients (Group B) had their clopidogrel held for less than 5 days, or not at all.
Patient demographics were similar between the two groups. Patients in Group A were more likely to have emergency surgery, to have peripheral stents placed, to have COPD or peripheral vascular disease, to have a malignancy, and to have received aspirin within five days of surgery (P less than .01 for all).
Blood product administration rates and volumes did not differ significantly between the two groups, and there was no difference between the groups in the incidence of myocardial infarctions, cerebrovascular events, or acute visceral or lower extremity ischemia.
Three patients in each group died within 30 days of the procedure, a nonsignificant difference. However, in the group that had clopidogrel held, three patients had perioperative myocardial infarctions, and two of these patients died. In discussing the study, Dr. Michael Dalsing said, “I think a lot of us would accept bleeding more over myocardial infarction.”
A subgroup analysis of the group who had clopidogrel held for fewer than 5 days compared outcomes for emergent vs. non-emergent surgery. The emergent surgery subgroup had a significantly higher rate of preoperative platelet transfusions, although numbers overall were small (2/17, 11.8%, vs. 0/72; P = .03).
Dr. Strosberg noted study limitations that included the retrospective, single-center nature of the study, and the fact that one variable, estimated blood loss, is notoriously subjective and inaccurate.
Dr. Dalsing, chief of vascular surgery at Indiana University, Indianapolis, said that he “was surprised that not even one patient went back for postoperative bleeding in this high-risk group of patients,” and raised the question of potential selection bias. Dr. Strosberg replied that comorbidities were ascertained by the physician at the time of surgery planning; since no differences were seen between study groups, investigators didn’t go back and parse out details about comorbid conditions.
In discussion following the presentation, surgeons spoke to the real-world challenges of performing surgery on a patient with antiplatelet therapy on board.
“Overall, I think your data support kind of a bias I have. Since I’m a vascular surgeon, we almost always operate on clopidogrel, and I don’t know if our bleeding risk is worse or better. But it’s something we almost have to do to keep our grafts going,” Dr. Dalsing said.
Dr. Peter Henke, professor of vascular surgery at the University of Michigan, Ann Arbor, said, “I’d be a little bit cautious with this. If you’ve ever done a big aortic procedure on someone on Plavix, I’ve seen them lose up to a couple of liters of blood just with oozing.”
“Those of us who do open aortic surgery know that very few things bleed like the back wall of an aortic anastomosis of a patient on Plavix,” echoed Dr. Peter Rossi, associate professor of vascular surgery at the Medical College of Wisconsin, Milwaukee.
The study authors reported no relevant disclosures.
On Twitter @karioakes
MONTREAL – Patients who stayed on antiplatelet therapy close to – or even up until – major surgery fared just as well as those who stopped their medication earlier in a retrospective, single-center study.
The study found no difference in blood product administration, adverse perioperative events, or all-cause 30-day mortality regardless of whether patients stopped clopidogrel (Plavix) the recommended 5 days before surgery.
“We believe that continuing clopidogrel in elective and emergent surgical situations appears to be safe, and may challenge the current recommendations,” said presenter Dr. David Strosberg.
The study addressed a thorny question for surgeons, Dr. Strosberg said. “As surgeons, we face a dilemma: Do we take the risk of thrombotic complications in stopping the antiplatelet drugs, or do we take the risk of increased surgical bleeding with continuing therapy?”
The package insert for clopidogrel advises discontinuation 5 days prior to surgery. However, manufacturer labeling also states that discontinuation of clopidogrel can lead to adverse cardiac events, said Dr. Strosberg, a general surgery resident at Ohio State University in Columbus.
The aim of the study, presented at the annual meeting of the Central Surgical Association, was to ascertain whether continuing antiplatelet therapy increased the rate of adverse surgical outcomes in those undergoing major emergent or elective surgery.
Dr. Strosberg and his colleagues retrospectively reviewed the record of patients over a 4-year period at a single institution and included those undergoing major general, thoracic, or vascular surgery who were taking clopidogrel at the time of presentation.
Data collected included patient characteristics, including demographic data and comorbidities, as well as transfusion requirements and perioperative events.
A total of 200 patients who had 205 qualifying procedures and were taking clopidogrel were included in the study. Of these, 116 patients (Group A) had their clopidogrel held for at least 5 days preoperatively. The remaining 89 patients (Group B) had their clopidogrel held for less than 5 days, or not at all.
Patient demographics were similar between the two groups. Patients in Group A were more likely to have emergency surgery, to have peripheral stents placed, to have COPD or peripheral vascular disease, to have a malignancy, and to have received aspirin within five days of surgery (P less than .01 for all).
Blood product administration rates and volumes did not differ significantly between the two groups, and there was no difference between the groups in the incidence of myocardial infarctions, cerebrovascular events, or acute visceral or lower extremity ischemia.
Three patients in each group died within 30 days of the procedure, a nonsignificant difference. However, in the group that had clopidogrel held, three patients had perioperative myocardial infarctions, and two of these patients died. In discussing the study, Dr. Michael Dalsing said, “I think a lot of us would accept bleeding more over myocardial infarction.”
A subgroup analysis of the group who had clopidogrel held for fewer than 5 days compared outcomes for emergent vs. non-emergent surgery. The emergent surgery subgroup had a significantly higher rate of preoperative platelet transfusions, although numbers overall were small (2/17, 11.8%, vs. 0/72; P = .03).
Dr. Strosberg noted study limitations that included the retrospective, single-center nature of the study, and the fact that one variable, estimated blood loss, is notoriously subjective and inaccurate.
Dr. Dalsing, chief of vascular surgery at Indiana University, Indianapolis, said that he “was surprised that not even one patient went back for postoperative bleeding in this high-risk group of patients,” and raised the question of potential selection bias. Dr. Strosberg replied that comorbidities were ascertained by the physician at the time of surgery planning; since no differences were seen between study groups, investigators didn’t go back and parse out details about comorbid conditions.
In discussion following the presentation, surgeons spoke to the real-world challenges of performing surgery on a patient with antiplatelet therapy on board.
“Overall, I think your data support kind of a bias I have. Since I’m a vascular surgeon, we almost always operate on clopidogrel, and I don’t know if our bleeding risk is worse or better. But it’s something we almost have to do to keep our grafts going,” Dr. Dalsing said.
Dr. Peter Henke, professor of vascular surgery at the University of Michigan, Ann Arbor, said, “I’d be a little bit cautious with this. If you’ve ever done a big aortic procedure on someone on Plavix, I’ve seen them lose up to a couple of liters of blood just with oozing.”
“Those of us who do open aortic surgery know that very few things bleed like the back wall of an aortic anastomosis of a patient on Plavix,” echoed Dr. Peter Rossi, associate professor of vascular surgery at the Medical College of Wisconsin, Milwaukee.
The study authors reported no relevant disclosures.
On Twitter @karioakes
MONTREAL – Patients who stayed on antiplatelet therapy close to – or even up until – major surgery fared just as well as those who stopped their medication earlier in a retrospective, single-center study.
The study found no difference in blood product administration, adverse perioperative events, or all-cause 30-day mortality regardless of whether patients stopped clopidogrel (Plavix) the recommended 5 days before surgery.
“We believe that continuing clopidogrel in elective and emergent surgical situations appears to be safe, and may challenge the current recommendations,” said presenter Dr. David Strosberg.
The study addressed a thorny question for surgeons, Dr. Strosberg said. “As surgeons, we face a dilemma: Do we take the risk of thrombotic complications in stopping the antiplatelet drugs, or do we take the risk of increased surgical bleeding with continuing therapy?”
The package insert for clopidogrel advises discontinuation 5 days prior to surgery. However, manufacturer labeling also states that discontinuation of clopidogrel can lead to adverse cardiac events, said Dr. Strosberg, a general surgery resident at Ohio State University in Columbus.
The aim of the study, presented at the annual meeting of the Central Surgical Association, was to ascertain whether continuing antiplatelet therapy increased the rate of adverse surgical outcomes in those undergoing major emergent or elective surgery.
Dr. Strosberg and his colleagues retrospectively reviewed the record of patients over a 4-year period at a single institution and included those undergoing major general, thoracic, or vascular surgery who were taking clopidogrel at the time of presentation.
Data collected included patient characteristics, including demographic data and comorbidities, as well as transfusion requirements and perioperative events.
A total of 200 patients who had 205 qualifying procedures and were taking clopidogrel were included in the study. Of these, 116 patients (Group A) had their clopidogrel held for at least 5 days preoperatively. The remaining 89 patients (Group B) had their clopidogrel held for less than 5 days, or not at all.
Patient demographics were similar between the two groups. Patients in Group A were more likely to have emergency surgery, to have peripheral stents placed, to have COPD or peripheral vascular disease, to have a malignancy, and to have received aspirin within five days of surgery (P less than .01 for all).
Blood product administration rates and volumes did not differ significantly between the two groups, and there was no difference between the groups in the incidence of myocardial infarctions, cerebrovascular events, or acute visceral or lower extremity ischemia.
Three patients in each group died within 30 days of the procedure, a nonsignificant difference. However, in the group that had clopidogrel held, three patients had perioperative myocardial infarctions, and two of these patients died. In discussing the study, Dr. Michael Dalsing said, “I think a lot of us would accept bleeding more over myocardial infarction.”
A subgroup analysis of the group who had clopidogrel held for fewer than 5 days compared outcomes for emergent vs. non-emergent surgery. The emergent surgery subgroup had a significantly higher rate of preoperative platelet transfusions, although numbers overall were small (2/17, 11.8%, vs. 0/72; P = .03).
Dr. Strosberg noted study limitations that included the retrospective, single-center nature of the study, and the fact that one variable, estimated blood loss, is notoriously subjective and inaccurate.
Dr. Dalsing, chief of vascular surgery at Indiana University, Indianapolis, said that he “was surprised that not even one patient went back for postoperative bleeding in this high-risk group of patients,” and raised the question of potential selection bias. Dr. Strosberg replied that comorbidities were ascertained by the physician at the time of surgery planning; since no differences were seen between study groups, investigators didn’t go back and parse out details about comorbid conditions.
In discussion following the presentation, surgeons spoke to the real-world challenges of performing surgery on a patient with antiplatelet therapy on board.
“Overall, I think your data support kind of a bias I have. Since I’m a vascular surgeon, we almost always operate on clopidogrel, and I don’t know if our bleeding risk is worse or better. But it’s something we almost have to do to keep our grafts going,” Dr. Dalsing said.
Dr. Peter Henke, professor of vascular surgery at the University of Michigan, Ann Arbor, said, “I’d be a little bit cautious with this. If you’ve ever done a big aortic procedure on someone on Plavix, I’ve seen them lose up to a couple of liters of blood just with oozing.”
“Those of us who do open aortic surgery know that very few things bleed like the back wall of an aortic anastomosis of a patient on Plavix,” echoed Dr. Peter Rossi, associate professor of vascular surgery at the Medical College of Wisconsin, Milwaukee.
The study authors reported no relevant disclosures.
On Twitter @karioakes
AT THE ANNUAL MEETING OF THE CENTRAL SURGICAL ASSOCIATION
Key clinical point: Outcomes were similar whether patients did or didn’t stop clopidogrel before major surgery.
Major finding: No significant differences in blood product use, adverse events, or death were seen with continuing clopidogrel.
Data source: Retrospective, single-center review of 200 patients undergoing major elective or emergent surgery and taking clopidogrel.
Disclosures: The study authors reported no relevant disclosures.
Risk-prediction tool for early TAVR mortality
Experts in the Society for Thoracic Surgeons and the American College of Cardiology used data from more than 13,000 consecutive transcatheter aortic valve replacement procedures to develop a new tool for predicting the risk of in-hospital mortality in patients undergoing TAVR, according to a report published online March 9 in JAMA Cardiology.
Their risk-prediction model was only “modestly” accurate but performed better than any existing methods for assessing risk in this patient population. It should be considered the first iteration of this tool and will be modified as the procedure itself evolves and as more data concerning TAVR are collected and analyzed. Ongoing analysis “may well define clinical subsets of patients who accrue particular benefit from the procedure or, conversely, reveal subsets not well served by TAVR,” said Dr. Fred H. Edwards and his associates on the steering committee of the STS/ACC Transcatheter Valve Therapy Registry.
They noted that more models soon will be developed to predict 30-day and 1-year mortality after TAVR. Models to predict the risk of neurologic deficit following TAVR are currently being developed, and models for other nonfatal outcomes will be developed soon.
This tool predicting in-hospital mortality is expected to become “a valuable adjunct for patient counseling, performance assessment, local quality improvement, and national monitoring of the appropriateness of patient selection for TAVR,” said Dr. Edwards, who is also in the department of surgery, University of Florida, Jacksonville, and his associates.
They began by analyzing the registry data for virtually every commercial TAVR performed at 265 participating sites in the United States during a 27-month period. In general, patients were selected for TAVR because they were considered unsuitable candidates for surgical aortic valve replacement. The mean patient age was 82.1 years. A total of 730 patients died before leaving the hospital, for an in-hospital mortality of 5.3%.
Working from an initial list of 39 possible patient variables to include in their statistical prediction model, the researchers narrowed it down to the 7 most predictive factors available in the registry data: older age, poorer glomerular filtration rate, the need for hemodialysis, NYHA class IV status, the presence of severe chronic lung disease, a category 2 or 4 critical hemodynamic state (i.e., preprocedural acuity status), and need for a nonfemoral approach during the procedure.
The model was then tested in a separate validation cohort of 6,868 patients (52% men) treated at 314 sites during a 7-month period. It performed better at predicting in-hospital mortality than did either the EuroSCORE (European System for Cardiac Operative Risk Evaluation) or the FRANCE 2 (French Aortic National Corevalve and Edwards 2) models.
This STS/ACC model should assist clinicians in patient selection for TAVR, not by dictating which patients are candidates for TAVR, but by being used as “one element in the selection process, to be considered in concert with history, physical examination, laboratory information, and clinical judgment. The model may also provide useful information for patient counseling,” the investigators said (JAMA Cardiol. 2016 Mar 9. doi: 10.1001/jamacardiol.2015.0326). One factor that is generally recognized as an important risk predictor but isn’t yet incorporated into this tool is a measure of patient frailty. Data on frailty are not yet collected consistently in the STS/ACC registry. As more complete data become available, frailty likely will be included as a predictive factor in this tool.
Another important issue that eventually should be considered alongside survival prediction is the effect TAVR has on quality of life. The STS/ACC registry “is one of the few clinical registries to collect quality-of-life data,” and it could prove to be a critical adjunct to patient selection. A given patient might have a favorable outlook regarding mortality after the procedure, but would still be a poor candidate if he or she wouldn’t derive significant benefit from it, Dr. Edwards and his associates said.
It is encouraging that Edwards et al. plan to refine this predictive model further, with the goal of developing a tool that provides a fuller picture of anticipated survival and functional outcomes for the TAVR population, because the demographics of this patient population are likely to change considerably in the coming years.

|
Dr. Laura Mauri |
The experience in Europe shows that TAVR is no longer reserved for high-risk patients there but is disseminating into the population at intermediate surgical risk. A similar trend is widely expected to occur in the United States after publication of favorable results from randomized clinical trials.
A reliable tool for predicting risk might eventually give providers and treatment centers a way to benchmark their current outcomes against those in the past and against those of other sites. Thus, it could serve as an instrument for continuous quality improvement for local heart care teams.
Dr. Laura Mauri and Dr. Patrick T. O’Gara are in the cardiovascular division at Brigham and Women’s Hospital and Harvard Medical School, Boston. They reported that their institution receives grants from Abbott, Boston Scientific, and Medtronic. Dr. Mauri and Dr. O’Gara made these remarks in an invited commentary accompanying Dr. Edwards’ report (JAMA Cardiol. 2016 Mar 9. doi: 10.1001/jamacardiol.2016.0006).
It is encouraging that Edwards et al. plan to refine this predictive model further, with the goal of developing a tool that provides a fuller picture of anticipated survival and functional outcomes for the TAVR population, because the demographics of this patient population are likely to change considerably in the coming years.

|
Dr. Laura Mauri |
The experience in Europe shows that TAVR is no longer reserved for high-risk patients there but is disseminating into the population at intermediate surgical risk. A similar trend is widely expected to occur in the United States after publication of favorable results from randomized clinical trials.
A reliable tool for predicting risk might eventually give providers and treatment centers a way to benchmark their current outcomes against those in the past and against those of other sites. Thus, it could serve as an instrument for continuous quality improvement for local heart care teams.
Dr. Laura Mauri and Dr. Patrick T. O’Gara are in the cardiovascular division at Brigham and Women’s Hospital and Harvard Medical School, Boston. They reported that their institution receives grants from Abbott, Boston Scientific, and Medtronic. Dr. Mauri and Dr. O’Gara made these remarks in an invited commentary accompanying Dr. Edwards’ report (JAMA Cardiol. 2016 Mar 9. doi: 10.1001/jamacardiol.2016.0006).
It is encouraging that Edwards et al. plan to refine this predictive model further, with the goal of developing a tool that provides a fuller picture of anticipated survival and functional outcomes for the TAVR population, because the demographics of this patient population are likely to change considerably in the coming years.

|
Dr. Laura Mauri |
The experience in Europe shows that TAVR is no longer reserved for high-risk patients there but is disseminating into the population at intermediate surgical risk. A similar trend is widely expected to occur in the United States after publication of favorable results from randomized clinical trials.
A reliable tool for predicting risk might eventually give providers and treatment centers a way to benchmark their current outcomes against those in the past and against those of other sites. Thus, it could serve as an instrument for continuous quality improvement for local heart care teams.
Dr. Laura Mauri and Dr. Patrick T. O’Gara are in the cardiovascular division at Brigham and Women’s Hospital and Harvard Medical School, Boston. They reported that their institution receives grants from Abbott, Boston Scientific, and Medtronic. Dr. Mauri and Dr. O’Gara made these remarks in an invited commentary accompanying Dr. Edwards’ report (JAMA Cardiol. 2016 Mar 9. doi: 10.1001/jamacardiol.2016.0006).
Experts in the Society for Thoracic Surgeons and the American College of Cardiology used data from more than 13,000 consecutive transcatheter aortic valve replacement procedures to develop a new tool for predicting the risk of in-hospital mortality in patients undergoing TAVR, according to a report published online March 9 in JAMA Cardiology.
Their risk-prediction model was only “modestly” accurate but performed better than any existing methods for assessing risk in this patient population. It should be considered the first iteration of this tool and will be modified as the procedure itself evolves and as more data concerning TAVR are collected and analyzed. Ongoing analysis “may well define clinical subsets of patients who accrue particular benefit from the procedure or, conversely, reveal subsets not well served by TAVR,” said Dr. Fred H. Edwards and his associates on the steering committee of the STS/ACC Transcatheter Valve Therapy Registry.
They noted that more models soon will be developed to predict 30-day and 1-year mortality after TAVR. Models to predict the risk of neurologic deficit following TAVR are currently being developed, and models for other nonfatal outcomes will be developed soon.
This tool predicting in-hospital mortality is expected to become “a valuable adjunct for patient counseling, performance assessment, local quality improvement, and national monitoring of the appropriateness of patient selection for TAVR,” said Dr. Edwards, who is also in the department of surgery, University of Florida, Jacksonville, and his associates.
They began by analyzing the registry data for virtually every commercial TAVR performed at 265 participating sites in the United States during a 27-month period. In general, patients were selected for TAVR because they were considered unsuitable candidates for surgical aortic valve replacement. The mean patient age was 82.1 years. A total of 730 patients died before leaving the hospital, for an in-hospital mortality of 5.3%.
Working from an initial list of 39 possible patient variables to include in their statistical prediction model, the researchers narrowed it down to the 7 most predictive factors available in the registry data: older age, poorer glomerular filtration rate, the need for hemodialysis, NYHA class IV status, the presence of severe chronic lung disease, a category 2 or 4 critical hemodynamic state (i.e., preprocedural acuity status), and need for a nonfemoral approach during the procedure.
The model was then tested in a separate validation cohort of 6,868 patients (52% men) treated at 314 sites during a 7-month period. It performed better at predicting in-hospital mortality than did either the EuroSCORE (European System for Cardiac Operative Risk Evaluation) or the FRANCE 2 (French Aortic National Corevalve and Edwards 2) models.
This STS/ACC model should assist clinicians in patient selection for TAVR, not by dictating which patients are candidates for TAVR, but by being used as “one element in the selection process, to be considered in concert with history, physical examination, laboratory information, and clinical judgment. The model may also provide useful information for patient counseling,” the investigators said (JAMA Cardiol. 2016 Mar 9. doi: 10.1001/jamacardiol.2015.0326). One factor that is generally recognized as an important risk predictor but isn’t yet incorporated into this tool is a measure of patient frailty. Data on frailty are not yet collected consistently in the STS/ACC registry. As more complete data become available, frailty likely will be included as a predictive factor in this tool.
Another important issue that eventually should be considered alongside survival prediction is the effect TAVR has on quality of life. The STS/ACC registry “is one of the few clinical registries to collect quality-of-life data,” and it could prove to be a critical adjunct to patient selection. A given patient might have a favorable outlook regarding mortality after the procedure, but would still be a poor candidate if he or she wouldn’t derive significant benefit from it, Dr. Edwards and his associates said.
Experts in the Society for Thoracic Surgeons and the American College of Cardiology used data from more than 13,000 consecutive transcatheter aortic valve replacement procedures to develop a new tool for predicting the risk of in-hospital mortality in patients undergoing TAVR, according to a report published online March 9 in JAMA Cardiology.
Their risk-prediction model was only “modestly” accurate but performed better than any existing methods for assessing risk in this patient population. It should be considered the first iteration of this tool and will be modified as the procedure itself evolves and as more data concerning TAVR are collected and analyzed. Ongoing analysis “may well define clinical subsets of patients who accrue particular benefit from the procedure or, conversely, reveal subsets not well served by TAVR,” said Dr. Fred H. Edwards and his associates on the steering committee of the STS/ACC Transcatheter Valve Therapy Registry.
They noted that more models soon will be developed to predict 30-day and 1-year mortality after TAVR. Models to predict the risk of neurologic deficit following TAVR are currently being developed, and models for other nonfatal outcomes will be developed soon.
This tool predicting in-hospital mortality is expected to become “a valuable adjunct for patient counseling, performance assessment, local quality improvement, and national monitoring of the appropriateness of patient selection for TAVR,” said Dr. Edwards, who is also in the department of surgery, University of Florida, Jacksonville, and his associates.
They began by analyzing the registry data for virtually every commercial TAVR performed at 265 participating sites in the United States during a 27-month period. In general, patients were selected for TAVR because they were considered unsuitable candidates for surgical aortic valve replacement. The mean patient age was 82.1 years. A total of 730 patients died before leaving the hospital, for an in-hospital mortality of 5.3%.
Working from an initial list of 39 possible patient variables to include in their statistical prediction model, the researchers narrowed it down to the 7 most predictive factors available in the registry data: older age, poorer glomerular filtration rate, the need for hemodialysis, NYHA class IV status, the presence of severe chronic lung disease, a category 2 or 4 critical hemodynamic state (i.e., preprocedural acuity status), and need for a nonfemoral approach during the procedure.
The model was then tested in a separate validation cohort of 6,868 patients (52% men) treated at 314 sites during a 7-month period. It performed better at predicting in-hospital mortality than did either the EuroSCORE (European System for Cardiac Operative Risk Evaluation) or the FRANCE 2 (French Aortic National Corevalve and Edwards 2) models.
This STS/ACC model should assist clinicians in patient selection for TAVR, not by dictating which patients are candidates for TAVR, but by being used as “one element in the selection process, to be considered in concert with history, physical examination, laboratory information, and clinical judgment. The model may also provide useful information for patient counseling,” the investigators said (JAMA Cardiol. 2016 Mar 9. doi: 10.1001/jamacardiol.2015.0326). One factor that is generally recognized as an important risk predictor but isn’t yet incorporated into this tool is a measure of patient frailty. Data on frailty are not yet collected consistently in the STS/ACC registry. As more complete data become available, frailty likely will be included as a predictive factor in this tool.
Another important issue that eventually should be considered alongside survival prediction is the effect TAVR has on quality of life. The STS/ACC registry “is one of the few clinical registries to collect quality-of-life data,” and it could prove to be a critical adjunct to patient selection. A given patient might have a favorable outlook regarding mortality after the procedure, but would still be a poor candidate if he or she wouldn’t derive significant benefit from it, Dr. Edwards and his associates said.
FROM JAMA CARDIOLOGY
Key clinical point: The STS and ACC developed a new tool for predicting the risk of in-hospital mortality after transcatheter aortic valve replacement.
Major finding: The new model includes the seven most predictive patient variables available in the registry data: older age, poorer glomerular filtration rate, the need for hemodialysis, NYHA class IV status, the presence of severe chronic lung disease, a category 2 or 4 critical hemodynamic state, and need for a nonfemoral approach.
Data source: An analysis of data for 13,718 consecutive TAVR patients to develop a predictive risk model, and a validation study involving 6,868 patients to test the performance of that model.
Disclosures: This study was supported by the American College of Cardiology’s National Cardiovascular Data Registry and the Society of Thoracic Surgeons. Dr. Edwards reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
Safety of bioresorbable stents does not match that of metal stents
Bioresorbable vascular scaffold stents are improving rapidly but they are still associated with a higher risk of complications compared with drug-eluting metal stents, according to a meta-analysis of published studies presented at Cardiovascular Research Technologies 2016.
“Bioresorbable stents are clearly an attractive strategy, but our data suggest that physicians and patients should remain aware of the risks,” reported Dr. Alok Saurav of Creighton University Medical Center, Omaha, Neb.
The first bioresorbable vascular scaffold (BVS) device, Synergy, was approved this past October, but this stent, despite bioresorbable struts, still has body parts that are not fully bioresorbable. However, several fully bioresorbable devices have reached late stages of testing and may receive regulatory approval this year.
In the meta-analysis, eight studies – five randomized trials, two studies with propensity matching, and an observational study –the primary goal was to compare BVS to drug eluting metal (DEM) stents for definite stent thrombosis. Secondary outcomes included subacute stent thrombosis within 30 days and within 1 year and cardiac death, all-cause death, MI, and ischemia-driven target vessel revascularization (TVR).
Despite the fact that the mean age and gender distribution was the same when the 2,760 patients receiving BVS stents were compared to the 2,212 receiving DEM stents, and both received comparable antiplatelet regimens after the stent was placed, there was an 80% greater relative risk for definite stent thrombosis in the BVS group. Although this difference fell short of statistical significance (P = .06), Dr. Saurav called it a “strong trend.”
Several of the adverse events that were analyzed as secondary outcomes in this study were less frequent with the BVS, such as cardiac death (relative risk, 0.83) and all-cause death (RR, 0.74), but the statistics did not suggest a trend, so Dr. Saurav characterized these outcomes as similar. MI was an exception. This was more frequent in those received a BVS stent (RR, 1.35; P = .049), and this reached significance.
Most of the studies included in this analysis were conducted with the everolimus-eluting Absorb BVS device, which many are predicting will be the first fully bioresorbable stent to receive regulatory approval.
It is notable that another meta-analysis including some of the same studies and published just weeks prior to the CRT meeting drew the same conclusion about the increased risk of stent thrombosis with BVS relative to DEM stents (Lancet 2016;387:537-44). This meta-analysis was restricted to six trials with 3,738 randomized patients. Unlike the meta-analysis presented at CRT, this study compared the two types of stents for both definite and probable stent thrombosis. For BVS relative to DEM stents, the relative risk for this outcome was 1.99 (P = .05).
“We think our restriction to definite stent thrombosis provides a stricter endpoint, but it’s notable that the results were relatively consistent,” Dr. Saurav reported.
Acknowledging that the increased risk of stent thrombosis appears to be modest for BVS relative to DEM stents, Dr. Saurav emphasized that these data should not discourage further development of bioresorbable stents, which are conceptually attractive.
“We cannot take these bioresorbable devices off the table,” he said. “But we do need more data to evaluate their risks relative to the conventional devices that are now available.”
The meeting was sponsored by the Cardiovascular Research Institute at Washington Hospital Center. Dr. Saurav reported no conflicts of interest.
Bioresorbable vascular scaffold stents are improving rapidly but they are still associated with a higher risk of complications compared with drug-eluting metal stents, according to a meta-analysis of published studies presented at Cardiovascular Research Technologies 2016.
“Bioresorbable stents are clearly an attractive strategy, but our data suggest that physicians and patients should remain aware of the risks,” reported Dr. Alok Saurav of Creighton University Medical Center, Omaha, Neb.
The first bioresorbable vascular scaffold (BVS) device, Synergy, was approved this past October, but this stent, despite bioresorbable struts, still has body parts that are not fully bioresorbable. However, several fully bioresorbable devices have reached late stages of testing and may receive regulatory approval this year.
In the meta-analysis, eight studies – five randomized trials, two studies with propensity matching, and an observational study –the primary goal was to compare BVS to drug eluting metal (DEM) stents for definite stent thrombosis. Secondary outcomes included subacute stent thrombosis within 30 days and within 1 year and cardiac death, all-cause death, MI, and ischemia-driven target vessel revascularization (TVR).
Despite the fact that the mean age and gender distribution was the same when the 2,760 patients receiving BVS stents were compared to the 2,212 receiving DEM stents, and both received comparable antiplatelet regimens after the stent was placed, there was an 80% greater relative risk for definite stent thrombosis in the BVS group. Although this difference fell short of statistical significance (P = .06), Dr. Saurav called it a “strong trend.”
Several of the adverse events that were analyzed as secondary outcomes in this study were less frequent with the BVS, such as cardiac death (relative risk, 0.83) and all-cause death (RR, 0.74), but the statistics did not suggest a trend, so Dr. Saurav characterized these outcomes as similar. MI was an exception. This was more frequent in those received a BVS stent (RR, 1.35; P = .049), and this reached significance.
Most of the studies included in this analysis were conducted with the everolimus-eluting Absorb BVS device, which many are predicting will be the first fully bioresorbable stent to receive regulatory approval.
It is notable that another meta-analysis including some of the same studies and published just weeks prior to the CRT meeting drew the same conclusion about the increased risk of stent thrombosis with BVS relative to DEM stents (Lancet 2016;387:537-44). This meta-analysis was restricted to six trials with 3,738 randomized patients. Unlike the meta-analysis presented at CRT, this study compared the two types of stents for both definite and probable stent thrombosis. For BVS relative to DEM stents, the relative risk for this outcome was 1.99 (P = .05).
“We think our restriction to definite stent thrombosis provides a stricter endpoint, but it’s notable that the results were relatively consistent,” Dr. Saurav reported.
Acknowledging that the increased risk of stent thrombosis appears to be modest for BVS relative to DEM stents, Dr. Saurav emphasized that these data should not discourage further development of bioresorbable stents, which are conceptually attractive.
“We cannot take these bioresorbable devices off the table,” he said. “But we do need more data to evaluate their risks relative to the conventional devices that are now available.”
The meeting was sponsored by the Cardiovascular Research Institute at Washington Hospital Center. Dr. Saurav reported no conflicts of interest.
Bioresorbable vascular scaffold stents are improving rapidly but they are still associated with a higher risk of complications compared with drug-eluting metal stents, according to a meta-analysis of published studies presented at Cardiovascular Research Technologies 2016.
“Bioresorbable stents are clearly an attractive strategy, but our data suggest that physicians and patients should remain aware of the risks,” reported Dr. Alok Saurav of Creighton University Medical Center, Omaha, Neb.
The first bioresorbable vascular scaffold (BVS) device, Synergy, was approved this past October, but this stent, despite bioresorbable struts, still has body parts that are not fully bioresorbable. However, several fully bioresorbable devices have reached late stages of testing and may receive regulatory approval this year.
In the meta-analysis, eight studies – five randomized trials, two studies with propensity matching, and an observational study –the primary goal was to compare BVS to drug eluting metal (DEM) stents for definite stent thrombosis. Secondary outcomes included subacute stent thrombosis within 30 days and within 1 year and cardiac death, all-cause death, MI, and ischemia-driven target vessel revascularization (TVR).
Despite the fact that the mean age and gender distribution was the same when the 2,760 patients receiving BVS stents were compared to the 2,212 receiving DEM stents, and both received comparable antiplatelet regimens after the stent was placed, there was an 80% greater relative risk for definite stent thrombosis in the BVS group. Although this difference fell short of statistical significance (P = .06), Dr. Saurav called it a “strong trend.”
Several of the adverse events that were analyzed as secondary outcomes in this study were less frequent with the BVS, such as cardiac death (relative risk, 0.83) and all-cause death (RR, 0.74), but the statistics did not suggest a trend, so Dr. Saurav characterized these outcomes as similar. MI was an exception. This was more frequent in those received a BVS stent (RR, 1.35; P = .049), and this reached significance.
Most of the studies included in this analysis were conducted with the everolimus-eluting Absorb BVS device, which many are predicting will be the first fully bioresorbable stent to receive regulatory approval.
It is notable that another meta-analysis including some of the same studies and published just weeks prior to the CRT meeting drew the same conclusion about the increased risk of stent thrombosis with BVS relative to DEM stents (Lancet 2016;387:537-44). This meta-analysis was restricted to six trials with 3,738 randomized patients. Unlike the meta-analysis presented at CRT, this study compared the two types of stents for both definite and probable stent thrombosis. For BVS relative to DEM stents, the relative risk for this outcome was 1.99 (P = .05).
“We think our restriction to definite stent thrombosis provides a stricter endpoint, but it’s notable that the results were relatively consistent,” Dr. Saurav reported.
Acknowledging that the increased risk of stent thrombosis appears to be modest for BVS relative to DEM stents, Dr. Saurav emphasized that these data should not discourage further development of bioresorbable stents, which are conceptually attractive.
“We cannot take these bioresorbable devices off the table,” he said. “But we do need more data to evaluate their risks relative to the conventional devices that are now available.”
The meeting was sponsored by the Cardiovascular Research Institute at Washington Hospital Center. Dr. Saurav reported no conflicts of interest.
AT CARDIOVASCULAR RESEARCH TECHNOLOGIES 2016
Key clinical point: Trial data suggest the risk of thrombosis and other adverse events remains higher with bioresorbable stents than with conventional drug-eluting metal stents.
Major finding: In a meta-analysis, the 80% increased risk of definite stent thrombosis for bioresorbable relative to metal stents fell just short of significance (P = .06) but the 35% increased risk of subsequent MI was significant (P = .049).
Data source: Meta-analysis of eight studies.
Disclosures: Dr. Saurav reported no conflicts of interest.
Periop statins don’t prevent acute kidney injury after cardiac surgery
ORLANDO – Statins administered perioperatively offered no protection against acute kidney injury following cardiac surgery, according to new results of a 5-year randomized clinical trial.
The findings held true whether or not patients were naive to statins; serum creatinine levels actually increased significantly more for statin-naive patients given atorvastatin than those given placebo.
The study was stopped early for patients naive to statins because increased acute kidney injury was seen in those patients who had chronic kidney disease (eGFR less than 60 mL/min/1.73 m2), and was subsequently stopped early for futility for all patients.
“De novo initiation of daily perioperative atorvastatin treatment did not reduce the incidence of AKI or reduce the increase in serum creatinine concentration associated with cardiac surgery,” wrote Dr. Frederic T. Billings IV, professor of medicine at Vanderbilt University, Nashville, Tenn., and his collaborators. The findings (JAMA 2016 Feb 23. doi: 10.1001/jama.2016.0548) were published concurrently with his presentation at the Critical Care Congress, sponsored by the Society for Critical Care Medicine.
In what Dr. Phil B. Fontanarosa, executive editor of JAMA and comoderator of the late-breaking trials session at the meeting, described as “really an elegant clinical trial,” Dr. Billings and his collaborators enrolled 615 patients over 5 years at Vanderbilt University Medical Center.
Patients undergoing elective coronary artery bypass grafting, valvular heart surgery, or ascending aortic surgery were eligible. Patients were excluded if they had prior statin intolerance, acute coronary syndrome, or liver dysfunction; were taking potent CYP3A4 inhibitors or cyclosporine; were receiving renal replacement therapy or had a kidney transplant; or were pregnant.
Both patients currently on a statin and patients naive to statins were recruited. Statin-naive patients received 80 mg atorvastatin the day before surgery, and then 40 mg of atorvastatin on the day of surgery and daily following surgery, or a matched placebo regimen.
Patients who were already on a statin received the study drug only on days that they would not have received a statin if treated according to the current standard of care. It was deemed unethical to allow those patients to receive placebo during and after surgery, since observational studies suggested that doing so might increase their potential for AKI.
For those patients already on a statin, this meant that they stayed on their usual regimen until the day of surgery, and then were randomized to receive either 80 mg of atorvastatin on the day of surgery and 40 mg of atorvastatin the day after surgery, or a matching placebo regimen.
For both groups, the study drug was given at least 3 hours before surgery on the day of surgery.
Randomization was stratified for prior statin use, for chronic kidney disease, and by history of diabetes. The 199 patients naive to statins and the 416 already on a statin were similar in demographic and health characteristics. Median age was 67 years, 188 (30.6%) were women; 202 participants (32.8%) had diabetes.
The primary outcome measure was diagnosis of AKI, defined as an increase of 0.3 mg/dL in serum creatinine, or beginning renal replacement therapy within 48 hours of surgery. Baseline serum creatinine was measured no more than 7 days prior to surgery.
AKI occurred in 64 of 308 patients (20.8%) in the atorvastatin group, and in 60 of 307 patients (19.5%) receiving placebo overall (P = .75). For those naive to statins, 21.6% of the atorvastatin group and 13.4% of the placebo group developed AKI (P = .15). Overall, 179 enrolled patients had CKD, and the incidence of AKI did not significantly differ in the atorvastatin and the placebo arms of this subgroup.
The subpopulation of participants with CKD who were statin naive (n = 36), however, saw an increased incidence of AKI with atorvastatin compared to placebo. AKI occurred in 9 of 17 patients (52.9%) given atorvastatin, and in 3 of 19 (15.8%) given placebo group (RR, 3.35[95% confidence interval 0.12 to 10.05]; P = .03). “It should be noted that the number of patients in this subgroup was particularly small, leading to a wide confidence interval and an increased chance of type 1 error,” said Dr. Billings.
Secondary outcome measures were maximum increase in creatinine concentration from baseline through postop day 2, delirium in the ICU, degree of myocardial injury, and incidence of postoperative pneumonia, atrial fibrillation, or stroke. Perioperative atorvastatin administration did not affect any of these endpoints.
The safety analysis showed no indications of increased risk of skeletal muscle or liver injury with perioperative atorvastatin use.
In the real world, “Most patients presenting for cardiac surgery … are already taking statins, and in the current study there was little evidence that continuation or withdrawal from statin treatment on the day of surgery and postoperative day 1 affects AKI,” wrote Dr. Billings and his coauthors.
Study limitations included its single-center design, and the use of AKI criteria that may not be sensitive to late-developing AKI. Also, for enrolled patients who were already on statins, statin exposure was not reduced in comparison with usual care.
After the presentation, Dr. Billings reported that the researchers also collected information about other biomarkers that may signal AKI, including IgM. He and his collaborators plan later publication of those data after a full analysis.
The National Institutes of Health and the Vanderbilt University Medical Center department of anesthesiology funded the study. Dr. Brown reported receiving grants from Shire Pharmaceuticals and New Haven Pharmaceuticals, and personal fees from Novartis Pharmaceuticals and Alnylam Pharmaceuticals. The other authors reported no conflicts of interest.
On Twitter @karioakes
ORLANDO – Statins administered perioperatively offered no protection against acute kidney injury following cardiac surgery, according to new results of a 5-year randomized clinical trial.
The findings held true whether or not patients were naive to statins; serum creatinine levels actually increased significantly more for statin-naive patients given atorvastatin than those given placebo.
The study was stopped early for patients naive to statins because increased acute kidney injury was seen in those patients who had chronic kidney disease (eGFR less than 60 mL/min/1.73 m2), and was subsequently stopped early for futility for all patients.
“De novo initiation of daily perioperative atorvastatin treatment did not reduce the incidence of AKI or reduce the increase in serum creatinine concentration associated with cardiac surgery,” wrote Dr. Frederic T. Billings IV, professor of medicine at Vanderbilt University, Nashville, Tenn., and his collaborators. The findings (JAMA 2016 Feb 23. doi: 10.1001/jama.2016.0548) were published concurrently with his presentation at the Critical Care Congress, sponsored by the Society for Critical Care Medicine.
In what Dr. Phil B. Fontanarosa, executive editor of JAMA and comoderator of the late-breaking trials session at the meeting, described as “really an elegant clinical trial,” Dr. Billings and his collaborators enrolled 615 patients over 5 years at Vanderbilt University Medical Center.
Patients undergoing elective coronary artery bypass grafting, valvular heart surgery, or ascending aortic surgery were eligible. Patients were excluded if they had prior statin intolerance, acute coronary syndrome, or liver dysfunction; were taking potent CYP3A4 inhibitors or cyclosporine; were receiving renal replacement therapy or had a kidney transplant; or were pregnant.
Both patients currently on a statin and patients naive to statins were recruited. Statin-naive patients received 80 mg atorvastatin the day before surgery, and then 40 mg of atorvastatin on the day of surgery and daily following surgery, or a matched placebo regimen.
Patients who were already on a statin received the study drug only on days that they would not have received a statin if treated according to the current standard of care. It was deemed unethical to allow those patients to receive placebo during and after surgery, since observational studies suggested that doing so might increase their potential for AKI.
For those patients already on a statin, this meant that they stayed on their usual regimen until the day of surgery, and then were randomized to receive either 80 mg of atorvastatin on the day of surgery and 40 mg of atorvastatin the day after surgery, or a matching placebo regimen.
For both groups, the study drug was given at least 3 hours before surgery on the day of surgery.
Randomization was stratified for prior statin use, for chronic kidney disease, and by history of diabetes. The 199 patients naive to statins and the 416 already on a statin were similar in demographic and health characteristics. Median age was 67 years, 188 (30.6%) were women; 202 participants (32.8%) had diabetes.
The primary outcome measure was diagnosis of AKI, defined as an increase of 0.3 mg/dL in serum creatinine, or beginning renal replacement therapy within 48 hours of surgery. Baseline serum creatinine was measured no more than 7 days prior to surgery.
AKI occurred in 64 of 308 patients (20.8%) in the atorvastatin group, and in 60 of 307 patients (19.5%) receiving placebo overall (P = .75). For those naive to statins, 21.6% of the atorvastatin group and 13.4% of the placebo group developed AKI (P = .15). Overall, 179 enrolled patients had CKD, and the incidence of AKI did not significantly differ in the atorvastatin and the placebo arms of this subgroup.
The subpopulation of participants with CKD who were statin naive (n = 36), however, saw an increased incidence of AKI with atorvastatin compared to placebo. AKI occurred in 9 of 17 patients (52.9%) given atorvastatin, and in 3 of 19 (15.8%) given placebo group (RR, 3.35[95% confidence interval 0.12 to 10.05]; P = .03). “It should be noted that the number of patients in this subgroup was particularly small, leading to a wide confidence interval and an increased chance of type 1 error,” said Dr. Billings.
Secondary outcome measures were maximum increase in creatinine concentration from baseline through postop day 2, delirium in the ICU, degree of myocardial injury, and incidence of postoperative pneumonia, atrial fibrillation, or stroke. Perioperative atorvastatin administration did not affect any of these endpoints.
The safety analysis showed no indications of increased risk of skeletal muscle or liver injury with perioperative atorvastatin use.
In the real world, “Most patients presenting for cardiac surgery … are already taking statins, and in the current study there was little evidence that continuation or withdrawal from statin treatment on the day of surgery and postoperative day 1 affects AKI,” wrote Dr. Billings and his coauthors.
Study limitations included its single-center design, and the use of AKI criteria that may not be sensitive to late-developing AKI. Also, for enrolled patients who were already on statins, statin exposure was not reduced in comparison with usual care.
After the presentation, Dr. Billings reported that the researchers also collected information about other biomarkers that may signal AKI, including IgM. He and his collaborators plan later publication of those data after a full analysis.
The National Institutes of Health and the Vanderbilt University Medical Center department of anesthesiology funded the study. Dr. Brown reported receiving grants from Shire Pharmaceuticals and New Haven Pharmaceuticals, and personal fees from Novartis Pharmaceuticals and Alnylam Pharmaceuticals. The other authors reported no conflicts of interest.
On Twitter @karioakes
ORLANDO – Statins administered perioperatively offered no protection against acute kidney injury following cardiac surgery, according to new results of a 5-year randomized clinical trial.
The findings held true whether or not patients were naive to statins; serum creatinine levels actually increased significantly more for statin-naive patients given atorvastatin than those given placebo.
The study was stopped early for patients naive to statins because increased acute kidney injury was seen in those patients who had chronic kidney disease (eGFR less than 60 mL/min/1.73 m2), and was subsequently stopped early for futility for all patients.
“De novo initiation of daily perioperative atorvastatin treatment did not reduce the incidence of AKI or reduce the increase in serum creatinine concentration associated with cardiac surgery,” wrote Dr. Frederic T. Billings IV, professor of medicine at Vanderbilt University, Nashville, Tenn., and his collaborators. The findings (JAMA 2016 Feb 23. doi: 10.1001/jama.2016.0548) were published concurrently with his presentation at the Critical Care Congress, sponsored by the Society for Critical Care Medicine.
In what Dr. Phil B. Fontanarosa, executive editor of JAMA and comoderator of the late-breaking trials session at the meeting, described as “really an elegant clinical trial,” Dr. Billings and his collaborators enrolled 615 patients over 5 years at Vanderbilt University Medical Center.
Patients undergoing elective coronary artery bypass grafting, valvular heart surgery, or ascending aortic surgery were eligible. Patients were excluded if they had prior statin intolerance, acute coronary syndrome, or liver dysfunction; were taking potent CYP3A4 inhibitors or cyclosporine; were receiving renal replacement therapy or had a kidney transplant; or were pregnant.
Both patients currently on a statin and patients naive to statins were recruited. Statin-naive patients received 80 mg atorvastatin the day before surgery, and then 40 mg of atorvastatin on the day of surgery and daily following surgery, or a matched placebo regimen.
Patients who were already on a statin received the study drug only on days that they would not have received a statin if treated according to the current standard of care. It was deemed unethical to allow those patients to receive placebo during and after surgery, since observational studies suggested that doing so might increase their potential for AKI.
For those patients already on a statin, this meant that they stayed on their usual regimen until the day of surgery, and then were randomized to receive either 80 mg of atorvastatin on the day of surgery and 40 mg of atorvastatin the day after surgery, or a matching placebo regimen.
For both groups, the study drug was given at least 3 hours before surgery on the day of surgery.
Randomization was stratified for prior statin use, for chronic kidney disease, and by history of diabetes. The 199 patients naive to statins and the 416 already on a statin were similar in demographic and health characteristics. Median age was 67 years, 188 (30.6%) were women; 202 participants (32.8%) had diabetes.
The primary outcome measure was diagnosis of AKI, defined as an increase of 0.3 mg/dL in serum creatinine, or beginning renal replacement therapy within 48 hours of surgery. Baseline serum creatinine was measured no more than 7 days prior to surgery.
AKI occurred in 64 of 308 patients (20.8%) in the atorvastatin group, and in 60 of 307 patients (19.5%) receiving placebo overall (P = .75). For those naive to statins, 21.6% of the atorvastatin group and 13.4% of the placebo group developed AKI (P = .15). Overall, 179 enrolled patients had CKD, and the incidence of AKI did not significantly differ in the atorvastatin and the placebo arms of this subgroup.
The subpopulation of participants with CKD who were statin naive (n = 36), however, saw an increased incidence of AKI with atorvastatin compared to placebo. AKI occurred in 9 of 17 patients (52.9%) given atorvastatin, and in 3 of 19 (15.8%) given placebo group (RR, 3.35[95% confidence interval 0.12 to 10.05]; P = .03). “It should be noted that the number of patients in this subgroup was particularly small, leading to a wide confidence interval and an increased chance of type 1 error,” said Dr. Billings.
Secondary outcome measures were maximum increase in creatinine concentration from baseline through postop day 2, delirium in the ICU, degree of myocardial injury, and incidence of postoperative pneumonia, atrial fibrillation, or stroke. Perioperative atorvastatin administration did not affect any of these endpoints.
The safety analysis showed no indications of increased risk of skeletal muscle or liver injury with perioperative atorvastatin use.
In the real world, “Most patients presenting for cardiac surgery … are already taking statins, and in the current study there was little evidence that continuation or withdrawal from statin treatment on the day of surgery and postoperative day 1 affects AKI,” wrote Dr. Billings and his coauthors.
Study limitations included its single-center design, and the use of AKI criteria that may not be sensitive to late-developing AKI. Also, for enrolled patients who were already on statins, statin exposure was not reduced in comparison with usual care.
After the presentation, Dr. Billings reported that the researchers also collected information about other biomarkers that may signal AKI, including IgM. He and his collaborators plan later publication of those data after a full analysis.
The National Institutes of Health and the Vanderbilt University Medical Center department of anesthesiology funded the study. Dr. Brown reported receiving grants from Shire Pharmaceuticals and New Haven Pharmaceuticals, and personal fees from Novartis Pharmaceuticals and Alnylam Pharmaceuticals. The other authors reported no conflicts of interest.
On Twitter @karioakes
AT THE CRITICAL CARE CONGRESS
Key clinical point: Perioperative atorvastatin did not protect against acute kidney injury after cardiac surgery.
Major finding: Acute kidney injury occurred in 64 of 308 patients (20.8%) in the atorvastatin group, and in 60 of 307 patients (19.5%) receiving placebo overall, a nonsignificant difference (P = .75).
Data source: Randomized, double-blinded, placebo-controlled trial of 615 adults who underwent cardiac surgery.
Disclosures: The National Institutes of Health and the Vanderbilt University Medical Center department of anesthesiology funded the study. Dr. Brown reported receiving grants from Shire Pharmaceuticals and New Haven Pharmaceuticals, and personal fees from Novartis Pharmaceuticals and Alnylam Pharmaceuticals. The other authors reported no conflicts of interest.