User login
Ischemic mitral regurgitation: valve repair vs. replacement
SNOWMASS, COLO. – A clear message from the first-ever randomized trial of surgical mitral valve repair versus replacement for patients with severe ischemic mitral regurgitation is that replacement should be utilized more liberally, Dr. Michael J. Mack said at the Annual Cardiovascular Conference at Snowmass.
The results of prosthetic valve implantation proved far more durable than repair. At 2 years of follow-up in this 251-patient multicenter trial conducted by the Cardiothoracic Surgical Trials Network (CSTN), the incidence of recurrent moderate or severe mitral regurgitation was just 3.8% in the valve replacement group, compared with 58.8% with repair via restrictive annuloplasty. As a result, the repair group had significantly more heart failure–related adverse events and cardiovascular hospitalizations and a lower rate of clinically meaningful improvement in quality of life scores, noted Dr. Mack, an investigator in the trial and medical director of the Baylor Health Care System in Plano, Tex.
“I think surgical mitral valve replacement has had a bad name over the years, and one of the reasons is because of the worse left ventricular function afterwards. However, that was a casualty of excising the mitral valve and the subvalvular apparatus, causing atrial-ventricular disconnection. We’ve gotten smarter about this. The techniques we now use are valve sparing,” the cardiothoracic surgeon said.
He was quick to add, however, that the CSTN study results are by no means the death knell for restrictive mitral annuloplasty. Indeed, participants in the mitral valve repair group who didn’t develop recurrent regurgitation actually experienced significant positive reverse remodeling as reflected by improvement in their left ventricular end-systolic volume index, the primary endpoint of the study (N Engl J Med. 2016;374:344-35).
The key to successful outcomes in mitral valve repair is to save the procedure for patients who are unlikely to develop recurrent regurgitation. And a substudy of the CTSN trial led by Dr. Irving L. Kron, professor of surgery at the University of Virginia, Charlottesville, provides practical guidance on that score. The investigators conducted a logistic regression analysis of the mitral valve repair group’s baseline echocardiographic and clinical characteristics and identified a collection of strong predictors of recurrent regurgitation within 2 years (J Thorac Cardiovasc Surg. 2015 Mar;149[3]:752-61).
“The bottom line is, the more tethering you have of the mitral valve leaflets, the more likely you are to have recurrent mitral regurgitation after mitral valve annuloplasty,” Dr. Mack said.
The predictors of recurrent regurgitation included a coaptation depth greater than 10 mm, a posterior leaflet angle in excess of 45 degrees, a distal anterior leaflet angle greater than 25 degrees, inferior basal aneurysm, mitral annular calcification, and a left ventricular end diastolic diameter greater than 65 mm, as well as other indices of advanced left ventricular remodeling.
No or only mild annular dilation, as occurs, for example, in patients whose mitral regurgitation is caused by atrial fibrillation, is another independent predictor of recurrent regurgitation post repair.
“Shrinking the annulus isn’t going to make a difference if the annulus wasn’t dilated to begin with,” the surgeon observed. “If surgery is performed, we now know those patients who are most likely to recur – and they should have mitral valve replacement. If those factors are not present, then repair is still a viable option,” according to Dr. Mack.
That being said, it’s still not known whether correcting severe ischemic mitral regurgitation prolongs life or improves quality of life long term, compared with guideline-directed medical therapy, he stressed.
“Secondary mitral regurgitation is a disease of the left ventricle, not the mitral valve. So it’s possible that mitral regurgitation reduction has no benefit because the regurgitation is a surrogate marker not causally related to outcome. I don’t think so, but it is a possibility,” Dr. Mack conceded.
This is a clinically important unresolved question because secondary mitral regurgitation is extremely common. In a retrospective echocardiographic study of 558 heart failure patients with a left ventricular ejection fraction of 35% or less and class III-IV symptoms, 90% of them had some degree of mitral regurgitation (J Card Fail. 2004 Aug;10[4]:285-91).
Together with Columbia University cardiologist Dr. Gregg W. Stone, Dr. Mack is coprincipal investigator of the COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation) trial, which is expected to provide an answer to this key question. The multicenter U.S. study involves a planned 420 patients with severely symptomatic secondary mitral regurgitation who are deemed at prohibitive risk for surgery. They are to be randomized to guideline-directed medical therapy with or without transcatheter mitral valve repair using the MitraClip device. Enrollment should be completed by May, with initial results available in late 2017.
Dr. Mack reported receiving research grants from Abbott Vascular, which is sponsoring the COAPT trial, as well as from Edwards Lifesciences.
SNOWMASS, COLO. – A clear message from the first-ever randomized trial of surgical mitral valve repair versus replacement for patients with severe ischemic mitral regurgitation is that replacement should be utilized more liberally, Dr. Michael J. Mack said at the Annual Cardiovascular Conference at Snowmass.
The results of prosthetic valve implantation proved far more durable than repair. At 2 years of follow-up in this 251-patient multicenter trial conducted by the Cardiothoracic Surgical Trials Network (CSTN), the incidence of recurrent moderate or severe mitral regurgitation was just 3.8% in the valve replacement group, compared with 58.8% with repair via restrictive annuloplasty. As a result, the repair group had significantly more heart failure–related adverse events and cardiovascular hospitalizations and a lower rate of clinically meaningful improvement in quality of life scores, noted Dr. Mack, an investigator in the trial and medical director of the Baylor Health Care System in Plano, Tex.
“I think surgical mitral valve replacement has had a bad name over the years, and one of the reasons is because of the worse left ventricular function afterwards. However, that was a casualty of excising the mitral valve and the subvalvular apparatus, causing atrial-ventricular disconnection. We’ve gotten smarter about this. The techniques we now use are valve sparing,” the cardiothoracic surgeon said.
He was quick to add, however, that the CSTN study results are by no means the death knell for restrictive mitral annuloplasty. Indeed, participants in the mitral valve repair group who didn’t develop recurrent regurgitation actually experienced significant positive reverse remodeling as reflected by improvement in their left ventricular end-systolic volume index, the primary endpoint of the study (N Engl J Med. 2016;374:344-35).
The key to successful outcomes in mitral valve repair is to save the procedure for patients who are unlikely to develop recurrent regurgitation. And a substudy of the CTSN trial led by Dr. Irving L. Kron, professor of surgery at the University of Virginia, Charlottesville, provides practical guidance on that score. The investigators conducted a logistic regression analysis of the mitral valve repair group’s baseline echocardiographic and clinical characteristics and identified a collection of strong predictors of recurrent regurgitation within 2 years (J Thorac Cardiovasc Surg. 2015 Mar;149[3]:752-61).
“The bottom line is, the more tethering you have of the mitral valve leaflets, the more likely you are to have recurrent mitral regurgitation after mitral valve annuloplasty,” Dr. Mack said.
The predictors of recurrent regurgitation included a coaptation depth greater than 10 mm, a posterior leaflet angle in excess of 45 degrees, a distal anterior leaflet angle greater than 25 degrees, inferior basal aneurysm, mitral annular calcification, and a left ventricular end diastolic diameter greater than 65 mm, as well as other indices of advanced left ventricular remodeling.
No or only mild annular dilation, as occurs, for example, in patients whose mitral regurgitation is caused by atrial fibrillation, is another independent predictor of recurrent regurgitation post repair.
“Shrinking the annulus isn’t going to make a difference if the annulus wasn’t dilated to begin with,” the surgeon observed. “If surgery is performed, we now know those patients who are most likely to recur – and they should have mitral valve replacement. If those factors are not present, then repair is still a viable option,” according to Dr. Mack.
That being said, it’s still not known whether correcting severe ischemic mitral regurgitation prolongs life or improves quality of life long term, compared with guideline-directed medical therapy, he stressed.
“Secondary mitral regurgitation is a disease of the left ventricle, not the mitral valve. So it’s possible that mitral regurgitation reduction has no benefit because the regurgitation is a surrogate marker not causally related to outcome. I don’t think so, but it is a possibility,” Dr. Mack conceded.
This is a clinically important unresolved question because secondary mitral regurgitation is extremely common. In a retrospective echocardiographic study of 558 heart failure patients with a left ventricular ejection fraction of 35% or less and class III-IV symptoms, 90% of them had some degree of mitral regurgitation (J Card Fail. 2004 Aug;10[4]:285-91).
Together with Columbia University cardiologist Dr. Gregg W. Stone, Dr. Mack is coprincipal investigator of the COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation) trial, which is expected to provide an answer to this key question. The multicenter U.S. study involves a planned 420 patients with severely symptomatic secondary mitral regurgitation who are deemed at prohibitive risk for surgery. They are to be randomized to guideline-directed medical therapy with or without transcatheter mitral valve repair using the MitraClip device. Enrollment should be completed by May, with initial results available in late 2017.
Dr. Mack reported receiving research grants from Abbott Vascular, which is sponsoring the COAPT trial, as well as from Edwards Lifesciences.
SNOWMASS, COLO. – A clear message from the first-ever randomized trial of surgical mitral valve repair versus replacement for patients with severe ischemic mitral regurgitation is that replacement should be utilized more liberally, Dr. Michael J. Mack said at the Annual Cardiovascular Conference at Snowmass.
The results of prosthetic valve implantation proved far more durable than repair. At 2 years of follow-up in this 251-patient multicenter trial conducted by the Cardiothoracic Surgical Trials Network (CSTN), the incidence of recurrent moderate or severe mitral regurgitation was just 3.8% in the valve replacement group, compared with 58.8% with repair via restrictive annuloplasty. As a result, the repair group had significantly more heart failure–related adverse events and cardiovascular hospitalizations and a lower rate of clinically meaningful improvement in quality of life scores, noted Dr. Mack, an investigator in the trial and medical director of the Baylor Health Care System in Plano, Tex.
“I think surgical mitral valve replacement has had a bad name over the years, and one of the reasons is because of the worse left ventricular function afterwards. However, that was a casualty of excising the mitral valve and the subvalvular apparatus, causing atrial-ventricular disconnection. We’ve gotten smarter about this. The techniques we now use are valve sparing,” the cardiothoracic surgeon said.
He was quick to add, however, that the CSTN study results are by no means the death knell for restrictive mitral annuloplasty. Indeed, participants in the mitral valve repair group who didn’t develop recurrent regurgitation actually experienced significant positive reverse remodeling as reflected by improvement in their left ventricular end-systolic volume index, the primary endpoint of the study (N Engl J Med. 2016;374:344-35).
The key to successful outcomes in mitral valve repair is to save the procedure for patients who are unlikely to develop recurrent regurgitation. And a substudy of the CTSN trial led by Dr. Irving L. Kron, professor of surgery at the University of Virginia, Charlottesville, provides practical guidance on that score. The investigators conducted a logistic regression analysis of the mitral valve repair group’s baseline echocardiographic and clinical characteristics and identified a collection of strong predictors of recurrent regurgitation within 2 years (J Thorac Cardiovasc Surg. 2015 Mar;149[3]:752-61).
“The bottom line is, the more tethering you have of the mitral valve leaflets, the more likely you are to have recurrent mitral regurgitation after mitral valve annuloplasty,” Dr. Mack said.
The predictors of recurrent regurgitation included a coaptation depth greater than 10 mm, a posterior leaflet angle in excess of 45 degrees, a distal anterior leaflet angle greater than 25 degrees, inferior basal aneurysm, mitral annular calcification, and a left ventricular end diastolic diameter greater than 65 mm, as well as other indices of advanced left ventricular remodeling.
No or only mild annular dilation, as occurs, for example, in patients whose mitral regurgitation is caused by atrial fibrillation, is another independent predictor of recurrent regurgitation post repair.
“Shrinking the annulus isn’t going to make a difference if the annulus wasn’t dilated to begin with,” the surgeon observed. “If surgery is performed, we now know those patients who are most likely to recur – and they should have mitral valve replacement. If those factors are not present, then repair is still a viable option,” according to Dr. Mack.
That being said, it’s still not known whether correcting severe ischemic mitral regurgitation prolongs life or improves quality of life long term, compared with guideline-directed medical therapy, he stressed.
“Secondary mitral regurgitation is a disease of the left ventricle, not the mitral valve. So it’s possible that mitral regurgitation reduction has no benefit because the regurgitation is a surrogate marker not causally related to outcome. I don’t think so, but it is a possibility,” Dr. Mack conceded.
This is a clinically important unresolved question because secondary mitral regurgitation is extremely common. In a retrospective echocardiographic study of 558 heart failure patients with a left ventricular ejection fraction of 35% or less and class III-IV symptoms, 90% of them had some degree of mitral regurgitation (J Card Fail. 2004 Aug;10[4]:285-91).
Together with Columbia University cardiologist Dr. Gregg W. Stone, Dr. Mack is coprincipal investigator of the COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation) trial, which is expected to provide an answer to this key question. The multicenter U.S. study involves a planned 420 patients with severely symptomatic secondary mitral regurgitation who are deemed at prohibitive risk for surgery. They are to be randomized to guideline-directed medical therapy with or without transcatheter mitral valve repair using the MitraClip device. Enrollment should be completed by May, with initial results available in late 2017.
Dr. Mack reported receiving research grants from Abbott Vascular, which is sponsoring the COAPT trial, as well as from Edwards Lifesciences.
EXPERT ANALYSIS FROM THE CARDIOVASCULAR CONFERENCE AT SNOWMASS
VIDEO: U.S. TAVR growth continues, mostly among octogenarians
PHOENIX – Use of transcatheter aortic valve replacement continued to expand through the first half of 2015, but the procedure remained primarily targeted to patients at least 80 years old, according to data collected in a U.S. postmarketing registry.
When the Food and Drug Administration first approved a transcatheter aortic valve replacement (TAVR) system for routine U.S. use in late 2011, the patients who underwent TAVR “were either at very high risk or inoperable, and we’ve seen that move into high-risk patients – and I’m sure we’ll see more introduction of this into patients who are at medium risk,” said Dr. Frederick L. Grover in a video interview at the annual meeting of the Society of Thoracic Surgeons.
Despite this downward trend in risk level, the median and average ages of TAVR patients remain above 80 years.
In 2015, U.S. TAVR recipients had a median age of 83 years and a mean age of 81 years, virtually unchanged from the 84-year median and 82-year mean during routine U.S. practice in 2012, the first year for data collection by the STS and American College of Cardiology Transcatheter Valve Therapy (TVT) Registry. Dr. Grover reported the latest data from the registry at the meeting, through roughly the first half of 2015.
“There has been some movement downward” from 2012 to 2014 in the predicted 30-day mortality rate of patients as measured by their preprocedural STS risk score. The rate declined from an average predicted mortality rate of 7.05% in 2012 to an average of 6.69% among patients treated during 2014.
Despite this shift, TAVR patients remain highly vulnerable to surgical complications because of their advanced age and frailty, said Dr. Grover, a professor of cardiothoracic surgery at the University of Colorado in Aurora and vice chairman of the registry steering committee.
STS encourages surgeons and cardiologists who collaborate on the heart teams that judge patient suitability for TAVR to measure frailty with the 5-meter walk test, run sequentially three times. Patients who take an average of 6 seconds or more to complete the test are deemed frail and eligible for TAVR. Registry data show that during 2012-2014, 81% of TAVR patients met this frailty criterion.
Perhaps the most notable statistics in the registry are the snowballing numbers of procedures performed, which have come close to doubling each year.
In the first full year of commercial use, 2012, 4,601 patients underwent TAVR, which jumped to 9,128 patients in 2013, 16,314 patients in 2014, and 23,002 patients during just the first part of 2015, Dr. Grover reported.
Dr. Grover had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
PHOENIX – Use of transcatheter aortic valve replacement continued to expand through the first half of 2015, but the procedure remained primarily targeted to patients at least 80 years old, according to data collected in a U.S. postmarketing registry.
When the Food and Drug Administration first approved a transcatheter aortic valve replacement (TAVR) system for routine U.S. use in late 2011, the patients who underwent TAVR “were either at very high risk or inoperable, and we’ve seen that move into high-risk patients – and I’m sure we’ll see more introduction of this into patients who are at medium risk,” said Dr. Frederick L. Grover in a video interview at the annual meeting of the Society of Thoracic Surgeons.
Despite this downward trend in risk level, the median and average ages of TAVR patients remain above 80 years.
In 2015, U.S. TAVR recipients had a median age of 83 years and a mean age of 81 years, virtually unchanged from the 84-year median and 82-year mean during routine U.S. practice in 2012, the first year for data collection by the STS and American College of Cardiology Transcatheter Valve Therapy (TVT) Registry. Dr. Grover reported the latest data from the registry at the meeting, through roughly the first half of 2015.
“There has been some movement downward” from 2012 to 2014 in the predicted 30-day mortality rate of patients as measured by their preprocedural STS risk score. The rate declined from an average predicted mortality rate of 7.05% in 2012 to an average of 6.69% among patients treated during 2014.
Despite this shift, TAVR patients remain highly vulnerable to surgical complications because of their advanced age and frailty, said Dr. Grover, a professor of cardiothoracic surgery at the University of Colorado in Aurora and vice chairman of the registry steering committee.
STS encourages surgeons and cardiologists who collaborate on the heart teams that judge patient suitability for TAVR to measure frailty with the 5-meter walk test, run sequentially three times. Patients who take an average of 6 seconds or more to complete the test are deemed frail and eligible for TAVR. Registry data show that during 2012-2014, 81% of TAVR patients met this frailty criterion.
Perhaps the most notable statistics in the registry are the snowballing numbers of procedures performed, which have come close to doubling each year.
In the first full year of commercial use, 2012, 4,601 patients underwent TAVR, which jumped to 9,128 patients in 2013, 16,314 patients in 2014, and 23,002 patients during just the first part of 2015, Dr. Grover reported.
Dr. Grover had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
PHOENIX – Use of transcatheter aortic valve replacement continued to expand through the first half of 2015, but the procedure remained primarily targeted to patients at least 80 years old, according to data collected in a U.S. postmarketing registry.
When the Food and Drug Administration first approved a transcatheter aortic valve replacement (TAVR) system for routine U.S. use in late 2011, the patients who underwent TAVR “were either at very high risk or inoperable, and we’ve seen that move into high-risk patients – and I’m sure we’ll see more introduction of this into patients who are at medium risk,” said Dr. Frederick L. Grover in a video interview at the annual meeting of the Society of Thoracic Surgeons.
Despite this downward trend in risk level, the median and average ages of TAVR patients remain above 80 years.
In 2015, U.S. TAVR recipients had a median age of 83 years and a mean age of 81 years, virtually unchanged from the 84-year median and 82-year mean during routine U.S. practice in 2012, the first year for data collection by the STS and American College of Cardiology Transcatheter Valve Therapy (TVT) Registry. Dr. Grover reported the latest data from the registry at the meeting, through roughly the first half of 2015.
“There has been some movement downward” from 2012 to 2014 in the predicted 30-day mortality rate of patients as measured by their preprocedural STS risk score. The rate declined from an average predicted mortality rate of 7.05% in 2012 to an average of 6.69% among patients treated during 2014.
Despite this shift, TAVR patients remain highly vulnerable to surgical complications because of their advanced age and frailty, said Dr. Grover, a professor of cardiothoracic surgery at the University of Colorado in Aurora and vice chairman of the registry steering committee.
STS encourages surgeons and cardiologists who collaborate on the heart teams that judge patient suitability for TAVR to measure frailty with the 5-meter walk test, run sequentially three times. Patients who take an average of 6 seconds or more to complete the test are deemed frail and eligible for TAVR. Registry data show that during 2012-2014, 81% of TAVR patients met this frailty criterion.
Perhaps the most notable statistics in the registry are the snowballing numbers of procedures performed, which have come close to doubling each year.
In the first full year of commercial use, 2012, 4,601 patients underwent TAVR, which jumped to 9,128 patients in 2013, 16,314 patients in 2014, and 23,002 patients during just the first part of 2015, Dr. Grover reported.
Dr. Grover had no relevant disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
AT THE STS ANNUAL MEETING
Key clinical point: The U.S. postmarketing database for TAVR showed steadily increasing growth in use from 2012 to 2015, with an ongoing focus on treating octogenarian patients.
Major finding: U.S. TAVR use jumped from 4,600 procedures in 2012 to 23,000 procedures in roughly the first half of 2015.
Data source: The STS/ACC TVT registry, which included 53,045 U.S. TAVR patients through mid 2015.
Disclosures: Dr. Grover had no relevant disclosures.
VIDEO: Shorter gap from heart attack to CABG shown safe
PHOENIX – Patients who are stable following a myocardial infarction and need isolated coronary artery bypass surgery (CABG) don’t need to wait 5 or so days for their surgery, a delay that many surgeons and cardiologists often impose.
The operation can safely occur after just a 1- or 2-day gap following either an ST-elevation MI or a non–ST-elevation MI, based on real-world outcomes seen in more than 3,000 patients treated at any of seven U.S. medical centers.
“Waiting an arbitrary 5 days is not important,” Elizabeth L. Nichols said during a video interview and during her report at the annual meeting of the Society of Thoracic Surgeons.
Ms. Nichols and her associates analyzed the in-hospital mortality rates among 3,060 patients who underwent isolated CABG during 2008-2014 at any of the seven medical centers that participate in the Northern New England Cardiovascular Disease Study Group and offer CABG. They included patients who had their surgery within 21 days of their MI, and excluded patients who had their CABG within 6 hours of their MI, had emergency surgery, or those with shock or incomplete data. The study group included 529 patients who had a ST-elevation MI and 2,531 patients with a non-ST-elevation MI.
The analysis divided patients into four groups based on timing of their CABG: 99 patients (3%) had surgery within the first 24 hours, 369 patients (12%) had their surgery 1-2 days after their MI, 1,966 (64%) had their operation 3-7 days following their MI, and 626 (21%) had their surgery 8-21 days after the MI.
The unadjusted mortality rates for these four subgroups were 5.1%, 1.6%, 1.6%, and 2.7%, respectively, reported Ms. Nichols, a health services researcher at the Dartmouth Institute for Health Policy & Clinical Practice, Lebanon, N.H.
After researchers adjusted for several demographic and clinical variables, the mortality rates remained identical for patients who underwent CABG 1 or 2 days following their MI, compared with patients whose surgery was deferred until 3-7 days after the MI. Patients with surgery 8-21 days following the MI had a small but not statistically significant higher rate of in-hospital death.
Patients who had their surgery 7-23 hours following an MI had a statistically significant increased hospital mortality following surgery that ran more than threefold greater than patients who underwent CABG 3-7 days after their MI.
The main message from the analysis is that for the typical, stable MI patient who requires CABG to treat multivessel coronary disease, no need exists to wait several days following an MI to do the surgery, Ms. Nichols explained. A delay of just 1 or 2 days is safe and sufficient, as long as it provides adequate time for any acutely administered antiplatelet or antithrombotic drugs to clear.
The findings “provide a degree of comfort for not waiting the 3-5 days that had previously been thought necessary,” said Dr. Jock N. McCullough, chief of cardiac surgery at Dartmouth-Hitchcock Medical Center in Lebanon and a collaborator on the study.
The findings are not meant to supersede clinical judgment, both Dr. McCullough and Ms. Nichols emphasized. Individual patients might have good reasons to either undergo faster surgery or to wait at least 8 days following their MI.
“The patients who waited 8-21 days had a lot of comorbidities and were sicker patients, and their delay is often warranted” to make sure the patient is stable enough for surgery, Ms. Nichols explained. Other patients might be worsening following their MI and need to undergo their surgery within 24 hours of their MI.
“Clinical judgment is always the trump card,” Ms. Nichols said.
Ms. Nichols and Dr. McCullough had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
PHOENIX – Patients who are stable following a myocardial infarction and need isolated coronary artery bypass surgery (CABG) don’t need to wait 5 or so days for their surgery, a delay that many surgeons and cardiologists often impose.
The operation can safely occur after just a 1- or 2-day gap following either an ST-elevation MI or a non–ST-elevation MI, based on real-world outcomes seen in more than 3,000 patients treated at any of seven U.S. medical centers.
“Waiting an arbitrary 5 days is not important,” Elizabeth L. Nichols said during a video interview and during her report at the annual meeting of the Society of Thoracic Surgeons.
Ms. Nichols and her associates analyzed the in-hospital mortality rates among 3,060 patients who underwent isolated CABG during 2008-2014 at any of the seven medical centers that participate in the Northern New England Cardiovascular Disease Study Group and offer CABG. They included patients who had their surgery within 21 days of their MI, and excluded patients who had their CABG within 6 hours of their MI, had emergency surgery, or those with shock or incomplete data. The study group included 529 patients who had a ST-elevation MI and 2,531 patients with a non-ST-elevation MI.
The analysis divided patients into four groups based on timing of their CABG: 99 patients (3%) had surgery within the first 24 hours, 369 patients (12%) had their surgery 1-2 days after their MI, 1,966 (64%) had their operation 3-7 days following their MI, and 626 (21%) had their surgery 8-21 days after the MI.
The unadjusted mortality rates for these four subgroups were 5.1%, 1.6%, 1.6%, and 2.7%, respectively, reported Ms. Nichols, a health services researcher at the Dartmouth Institute for Health Policy & Clinical Practice, Lebanon, N.H.
After researchers adjusted for several demographic and clinical variables, the mortality rates remained identical for patients who underwent CABG 1 or 2 days following their MI, compared with patients whose surgery was deferred until 3-7 days after the MI. Patients with surgery 8-21 days following the MI had a small but not statistically significant higher rate of in-hospital death.
Patients who had their surgery 7-23 hours following an MI had a statistically significant increased hospital mortality following surgery that ran more than threefold greater than patients who underwent CABG 3-7 days after their MI.
The main message from the analysis is that for the typical, stable MI patient who requires CABG to treat multivessel coronary disease, no need exists to wait several days following an MI to do the surgery, Ms. Nichols explained. A delay of just 1 or 2 days is safe and sufficient, as long as it provides adequate time for any acutely administered antiplatelet or antithrombotic drugs to clear.
The findings “provide a degree of comfort for not waiting the 3-5 days that had previously been thought necessary,” said Dr. Jock N. McCullough, chief of cardiac surgery at Dartmouth-Hitchcock Medical Center in Lebanon and a collaborator on the study.
The findings are not meant to supersede clinical judgment, both Dr. McCullough and Ms. Nichols emphasized. Individual patients might have good reasons to either undergo faster surgery or to wait at least 8 days following their MI.
“The patients who waited 8-21 days had a lot of comorbidities and were sicker patients, and their delay is often warranted” to make sure the patient is stable enough for surgery, Ms. Nichols explained. Other patients might be worsening following their MI and need to undergo their surgery within 24 hours of their MI.
“Clinical judgment is always the trump card,” Ms. Nichols said.
Ms. Nichols and Dr. McCullough had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
PHOENIX – Patients who are stable following a myocardial infarction and need isolated coronary artery bypass surgery (CABG) don’t need to wait 5 or so days for their surgery, a delay that many surgeons and cardiologists often impose.
The operation can safely occur after just a 1- or 2-day gap following either an ST-elevation MI or a non–ST-elevation MI, based on real-world outcomes seen in more than 3,000 patients treated at any of seven U.S. medical centers.
“Waiting an arbitrary 5 days is not important,” Elizabeth L. Nichols said during a video interview and during her report at the annual meeting of the Society of Thoracic Surgeons.
Ms. Nichols and her associates analyzed the in-hospital mortality rates among 3,060 patients who underwent isolated CABG during 2008-2014 at any of the seven medical centers that participate in the Northern New England Cardiovascular Disease Study Group and offer CABG. They included patients who had their surgery within 21 days of their MI, and excluded patients who had their CABG within 6 hours of their MI, had emergency surgery, or those with shock or incomplete data. The study group included 529 patients who had a ST-elevation MI and 2,531 patients with a non-ST-elevation MI.
The analysis divided patients into four groups based on timing of their CABG: 99 patients (3%) had surgery within the first 24 hours, 369 patients (12%) had their surgery 1-2 days after their MI, 1,966 (64%) had their operation 3-7 days following their MI, and 626 (21%) had their surgery 8-21 days after the MI.
The unadjusted mortality rates for these four subgroups were 5.1%, 1.6%, 1.6%, and 2.7%, respectively, reported Ms. Nichols, a health services researcher at the Dartmouth Institute for Health Policy & Clinical Practice, Lebanon, N.H.
After researchers adjusted for several demographic and clinical variables, the mortality rates remained identical for patients who underwent CABG 1 or 2 days following their MI, compared with patients whose surgery was deferred until 3-7 days after the MI. Patients with surgery 8-21 days following the MI had a small but not statistically significant higher rate of in-hospital death.
Patients who had their surgery 7-23 hours following an MI had a statistically significant increased hospital mortality following surgery that ran more than threefold greater than patients who underwent CABG 3-7 days after their MI.
The main message from the analysis is that for the typical, stable MI patient who requires CABG to treat multivessel coronary disease, no need exists to wait several days following an MI to do the surgery, Ms. Nichols explained. A delay of just 1 or 2 days is safe and sufficient, as long as it provides adequate time for any acutely administered antiplatelet or antithrombotic drugs to clear.
The findings “provide a degree of comfort for not waiting the 3-5 days that had previously been thought necessary,” said Dr. Jock N. McCullough, chief of cardiac surgery at Dartmouth-Hitchcock Medical Center in Lebanon and a collaborator on the study.
The findings are not meant to supersede clinical judgment, both Dr. McCullough and Ms. Nichols emphasized. Individual patients might have good reasons to either undergo faster surgery or to wait at least 8 days following their MI.
“The patients who waited 8-21 days had a lot of comorbidities and were sicker patients, and their delay is often warranted” to make sure the patient is stable enough for surgery, Ms. Nichols explained. Other patients might be worsening following their MI and need to undergo their surgery within 24 hours of their MI.
“Clinical judgment is always the trump card,” Ms. Nichols said.
Ms. Nichols and Dr. McCullough had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
AT THE STS ANNUAL MEETING
Key clinical point: Performing coronary artery bypass grafting 1-2 days following an MI was as safe as when surgery was delayed 3-7 days.
Major finding: In-hospital mortality after CABG was identical in patients operated on 1-2 days or 3-7 days following an MI.
Data source: Retrospective analysis of 3,060 patients who underwent CABG within 21 days following an MI at any of seven U.S. centers.
Disclosures: Ms. Nichols and Dr. McCullough had no disclosures.
Poor adherence to quality indicators found for NSCLC surgery
PHOENIX – National adherence to quality indicators for surgery in stage I non–small cell lung cancer is suboptimal, results from a large analysis of national data suggest.
“Compliance with such guidelines is a strong predictor of long-term survival, and vigorous efforts should be instituted at the level of national societies to improve such adherence,” researchers led by Dr. Pamela P. Samson wrote in an abstract presented at the annual meeting of the Society of Thoracic Surgeons. “National organizations, including American College of Chest Physicians, the National Comprehensive Cancer Network, and the American College of Surgeons Commission on Cancer, have recommended quality standards for surgery in early-stage non–small cell lung cancer (NSCLC). The determinants and outcomes of adherence to these guidelines for early-stage lung cancer patients are largely unknown.”
Dr. Samson, a general surgery resident at Washington University in St. Louis, and her associates used the National Cancer Data Base to evaluate data from 146,908 patients undergoing surgery for clinical stage I NSCLC between 2004 and 2013. They selected the following four quality measures for evaluation: performing an anatomical pulmonary resection, surgery within 8 weeks of diagnosis, R0 resection, and evaluation of 10 or more lymph nodes. Next, the researchers fitted multivariate models to identify variables independently associated with adherence to quality measures, and created a Cox multivariate model to evaluate long-term overall survival.
Dr. Varun Puri, senior author of the study, presented the findings at the STS meeting on behalf of Dr. Samson, and discussed the findings in a video interview. The researchers found that between 2004 and 2013, nearly 100% of patients met at least one of the four recommended criteria, 95% met two, 69% met three, and 22% met all four. Sampling of 10 or more lymph nodes was the least frequently met measure, occurring in only 31% of surgical patients. Patient factors associated with a greater likelihood of receiving all four quality measures included average income in ZIP code of at least $38,000 (odds ratio, 1.20), private insurance (OR, 1.22), or having Medicare (OR, 1.16). Institutional factors associated with a greater likelihood of meeting all four quality measures included higher-volume centers, defined as treating at least 38 cases per year (OR, 1.18), or being an academic institution (OR, 1.31).
At the same time, factors associated with a lower likelihood of recommended surgical care included increasing age (per year increase, OR, 0.99) and a higher Charlson/Deyo comorbidity score (OR, 0.90 for a score of 1 and OR, 0.82 for a score of 2 or more). The strongest determinant of long-term overall survival included pathologic upstaging (HR 1.84) and meeting all four quality indicators (HR 0.39). Every additional quality met was associated with a significant reduction in overall mortality.
“We believe this study can be a starting point to draw attention to institution- and surgeon-specific practice patterns that may vary widely,” Dr. Samson said in an interview prior to the meeting. “At our own institution, we are working to decrease time to surgery, as well as implementing quality improvement measures to increase nodal sampling rates. Improving these trends nationally must start at the local level, with a tailored approach.”
Dr. Samson is currently supported by a T32 NIH training grant for research fellows in cardiothoracic surgery. Study coauthor Dr. Bryan Meyers, has received honoraria from Varian Medical Systems and is a consultant/advisory board member of Ethicon. Senior author Dr. Varun Puri is supported by NIH career awards.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PHOENIX – National adherence to quality indicators for surgery in stage I non–small cell lung cancer is suboptimal, results from a large analysis of national data suggest.
“Compliance with such guidelines is a strong predictor of long-term survival, and vigorous efforts should be instituted at the level of national societies to improve such adherence,” researchers led by Dr. Pamela P. Samson wrote in an abstract presented at the annual meeting of the Society of Thoracic Surgeons. “National organizations, including American College of Chest Physicians, the National Comprehensive Cancer Network, and the American College of Surgeons Commission on Cancer, have recommended quality standards for surgery in early-stage non–small cell lung cancer (NSCLC). The determinants and outcomes of adherence to these guidelines for early-stage lung cancer patients are largely unknown.”
Dr. Samson, a general surgery resident at Washington University in St. Louis, and her associates used the National Cancer Data Base to evaluate data from 146,908 patients undergoing surgery for clinical stage I NSCLC between 2004 and 2013. They selected the following four quality measures for evaluation: performing an anatomical pulmonary resection, surgery within 8 weeks of diagnosis, R0 resection, and evaluation of 10 or more lymph nodes. Next, the researchers fitted multivariate models to identify variables independently associated with adherence to quality measures, and created a Cox multivariate model to evaluate long-term overall survival.
Dr. Varun Puri, senior author of the study, presented the findings at the STS meeting on behalf of Dr. Samson, and discussed the findings in a video interview. The researchers found that between 2004 and 2013, nearly 100% of patients met at least one of the four recommended criteria, 95% met two, 69% met three, and 22% met all four. Sampling of 10 or more lymph nodes was the least frequently met measure, occurring in only 31% of surgical patients. Patient factors associated with a greater likelihood of receiving all four quality measures included average income in ZIP code of at least $38,000 (odds ratio, 1.20), private insurance (OR, 1.22), or having Medicare (OR, 1.16). Institutional factors associated with a greater likelihood of meeting all four quality measures included higher-volume centers, defined as treating at least 38 cases per year (OR, 1.18), or being an academic institution (OR, 1.31).
At the same time, factors associated with a lower likelihood of recommended surgical care included increasing age (per year increase, OR, 0.99) and a higher Charlson/Deyo comorbidity score (OR, 0.90 for a score of 1 and OR, 0.82 for a score of 2 or more). The strongest determinant of long-term overall survival included pathologic upstaging (HR 1.84) and meeting all four quality indicators (HR 0.39). Every additional quality met was associated with a significant reduction in overall mortality.
“We believe this study can be a starting point to draw attention to institution- and surgeon-specific practice patterns that may vary widely,” Dr. Samson said in an interview prior to the meeting. “At our own institution, we are working to decrease time to surgery, as well as implementing quality improvement measures to increase nodal sampling rates. Improving these trends nationally must start at the local level, with a tailored approach.”
Dr. Samson is currently supported by a T32 NIH training grant for research fellows in cardiothoracic surgery. Study coauthor Dr. Bryan Meyers, has received honoraria from Varian Medical Systems and is a consultant/advisory board member of Ethicon. Senior author Dr. Varun Puri is supported by NIH career awards.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PHOENIX – National adherence to quality indicators for surgery in stage I non–small cell lung cancer is suboptimal, results from a large analysis of national data suggest.
“Compliance with such guidelines is a strong predictor of long-term survival, and vigorous efforts should be instituted at the level of national societies to improve such adherence,” researchers led by Dr. Pamela P. Samson wrote in an abstract presented at the annual meeting of the Society of Thoracic Surgeons. “National organizations, including American College of Chest Physicians, the National Comprehensive Cancer Network, and the American College of Surgeons Commission on Cancer, have recommended quality standards for surgery in early-stage non–small cell lung cancer (NSCLC). The determinants and outcomes of adherence to these guidelines for early-stage lung cancer patients are largely unknown.”
Dr. Samson, a general surgery resident at Washington University in St. Louis, and her associates used the National Cancer Data Base to evaluate data from 146,908 patients undergoing surgery for clinical stage I NSCLC between 2004 and 2013. They selected the following four quality measures for evaluation: performing an anatomical pulmonary resection, surgery within 8 weeks of diagnosis, R0 resection, and evaluation of 10 or more lymph nodes. Next, the researchers fitted multivariate models to identify variables independently associated with adherence to quality measures, and created a Cox multivariate model to evaluate long-term overall survival.
Dr. Varun Puri, senior author of the study, presented the findings at the STS meeting on behalf of Dr. Samson, and discussed the findings in a video interview. The researchers found that between 2004 and 2013, nearly 100% of patients met at least one of the four recommended criteria, 95% met two, 69% met three, and 22% met all four. Sampling of 10 or more lymph nodes was the least frequently met measure, occurring in only 31% of surgical patients. Patient factors associated with a greater likelihood of receiving all four quality measures included average income in ZIP code of at least $38,000 (odds ratio, 1.20), private insurance (OR, 1.22), or having Medicare (OR, 1.16). Institutional factors associated with a greater likelihood of meeting all four quality measures included higher-volume centers, defined as treating at least 38 cases per year (OR, 1.18), or being an academic institution (OR, 1.31).
At the same time, factors associated with a lower likelihood of recommended surgical care included increasing age (per year increase, OR, 0.99) and a higher Charlson/Deyo comorbidity score (OR, 0.90 for a score of 1 and OR, 0.82 for a score of 2 or more). The strongest determinant of long-term overall survival included pathologic upstaging (HR 1.84) and meeting all four quality indicators (HR 0.39). Every additional quality met was associated with a significant reduction in overall mortality.
“We believe this study can be a starting point to draw attention to institution- and surgeon-specific practice patterns that may vary widely,” Dr. Samson said in an interview prior to the meeting. “At our own institution, we are working to decrease time to surgery, as well as implementing quality improvement measures to increase nodal sampling rates. Improving these trends nationally must start at the local level, with a tailored approach.”
Dr. Samson is currently supported by a T32 NIH training grant for research fellows in cardiothoracic surgery. Study coauthor Dr. Bryan Meyers, has received honoraria from Varian Medical Systems and is a consultant/advisory board member of Ethicon. Senior author Dr. Varun Puri is supported by NIH career awards.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE STS ANNUAL MEETING
Key clinical point: At the national level, compliance with core indicators for surgery in stage I NSCLC is poor.
Major finding: Between 2004 and 2013, nearly 100% of patients met at least one of four recommended criteria for evaluation of stage I NSCLC, 95% met two, 69% met three, and 22% met all four.
Data source: An analysis of 146,908 patients undergoing surgery for clinical stage I NSCLC between 2004 and 2013.
Disclosures: Dr. Samson is currently supported by a T32 NIH training grant for research fellows in cardiothoracic surgery. Study coauthor Dr. Bryan Meyers, has received honoraria from Varian Medical Systems and is a consultant/advisory board member of Ethicon. Senior author Dr. Varun Puri is supported by NIH career awards.
VIDEO: Expert discusses VATS thymectomy for myasthenia gravis
PHOENIX – In the clinical experience of Dr. Joshua R. Sonett, VATS thymectomy for myasthenia gravis is best performed in a bilateral thoracoscopic fashion.
In this approach, surgeons do about 95% of the operation on the left side to form a maximal thymectomy, “and finish taking out the specimen on the right side, making sure we can see both phrenic nerves in their entirety,” Dr. Sonett, chief of general thoracic surgery at Columbia University Medical Center, New York, said in a video interview at the annual meeting of the Society of Thoracic Surgeons.
Although there is no proof to date that thymectomy improves long-term outcomes for patients with myasthenia gravis, results from a large, international trial sponsored by the National Institutes of Health are expected to inform clinical practice about this topic, said Dr. Sonett, who is also director of the university’s high-risk lung assessment program.
Dr. Sonett reported having no relevant financial conflicts.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PHOENIX – In the clinical experience of Dr. Joshua R. Sonett, VATS thymectomy for myasthenia gravis is best performed in a bilateral thoracoscopic fashion.
In this approach, surgeons do about 95% of the operation on the left side to form a maximal thymectomy, “and finish taking out the specimen on the right side, making sure we can see both phrenic nerves in their entirety,” Dr. Sonett, chief of general thoracic surgery at Columbia University Medical Center, New York, said in a video interview at the annual meeting of the Society of Thoracic Surgeons.
Although there is no proof to date that thymectomy improves long-term outcomes for patients with myasthenia gravis, results from a large, international trial sponsored by the National Institutes of Health are expected to inform clinical practice about this topic, said Dr. Sonett, who is also director of the university’s high-risk lung assessment program.
Dr. Sonett reported having no relevant financial conflicts.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PHOENIX – In the clinical experience of Dr. Joshua R. Sonett, VATS thymectomy for myasthenia gravis is best performed in a bilateral thoracoscopic fashion.
In this approach, surgeons do about 95% of the operation on the left side to form a maximal thymectomy, “and finish taking out the specimen on the right side, making sure we can see both phrenic nerves in their entirety,” Dr. Sonett, chief of general thoracic surgery at Columbia University Medical Center, New York, said in a video interview at the annual meeting of the Society of Thoracic Surgeons.
Although there is no proof to date that thymectomy improves long-term outcomes for patients with myasthenia gravis, results from a large, international trial sponsored by the National Institutes of Health are expected to inform clinical practice about this topic, said Dr. Sonett, who is also director of the university’s high-risk lung assessment program.
Dr. Sonett reported having no relevant financial conflicts.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYIS FROM THE STS ANNUAL MEETING
STS: BMI impacts risk for complications after lung resection
PHOENIX – Being underweight is associated with a substantially increased risk of complications following lung resection for cancer, results from a large database study found.
“This is not generally known among surgeons or their patients,” Dr. Trevor Williams said in an interview before the annual meeting of the Society of Thoracic Surgeons. “Studies are conflicting about the relationship of BMI [body mass index] to surgical outcomes. Most of the previous studies simply categorize BMI as overweight or not. We’ve stratified based on World Health Organization categories to get a more precise look at BMI.”
Dr. Williams, a surgeon at the University of Chicago Medical Center, and his associates evaluated 41,446 patients in the STS General Thoracic Surgery Database who underwent elective anatomic lung resection for cancer between 2009 and 2014. Their mean age was 68 years, and 53% were female. The researchers performed multivariable analysis after adjusting for validated STS risk model covariates, including gender and spirometry.
According to WHO criteria for BMI, 3% were underweight (less than 18.5 kg/m2); 33.5% were normal weight (18.5-24.9 kg/m2); 35.4% were overweight (25-29.9 kg/m2); 18.1% were obese I (30-34.9 kg/m2); 6.4% were obese II (35-39.9 kg/m2), and 3.6% were obese III (40 kg/m2 or greater). Dr. Williams and his associates observed that women were more often underweight, compared with men (4.1% vs. 1.8%, respectively; P less than .001), and underweight patients more often had chronic obstructive pulmonary disease (51.7% vs. 35.2%; P less than .001). Pulmonary complication rates were higher among underweight and obese III patients (P less than .001), while being underweight was also associated with higher rates of infections and any surgical complications.
Multivariable analysis revealed that pulmonary and any postoperative complications were more common among underweight patients (odds ratio, 1.44 and OR, 1.41, respectively), while any major complication was more common among obese III patients (OR, 1.18). Overweight and obese I-II patients were less likely to have any postoperative and pulmonary complications, compared with patients who had a normal BMI. “The finding of underweight patients being such a high-risk patient population is suggested in the literature but not demonstrated as clearly as in this study,” Dr. Williams said. “A truly surprising finding was that obese patients actually have a lower risk of pulmonary and overall complications than ‘normal’-BMI patients.”
He concluded that according to the current analysis, “careful risk assessment is appropriate when considering operating on underweight patients. Whether there are interventions that could be instituted to improve an individual’s risk profile has not been determined. Any preconceived notions about not operating on obese patients due to elevated risk appear to be unfounded.”
Dr. Williams reported having no financial disclosures.
PHOENIX – Being underweight is associated with a substantially increased risk of complications following lung resection for cancer, results from a large database study found.
“This is not generally known among surgeons or their patients,” Dr. Trevor Williams said in an interview before the annual meeting of the Society of Thoracic Surgeons. “Studies are conflicting about the relationship of BMI [body mass index] to surgical outcomes. Most of the previous studies simply categorize BMI as overweight or not. We’ve stratified based on World Health Organization categories to get a more precise look at BMI.”
Dr. Williams, a surgeon at the University of Chicago Medical Center, and his associates evaluated 41,446 patients in the STS General Thoracic Surgery Database who underwent elective anatomic lung resection for cancer between 2009 and 2014. Their mean age was 68 years, and 53% were female. The researchers performed multivariable analysis after adjusting for validated STS risk model covariates, including gender and spirometry.
According to WHO criteria for BMI, 3% were underweight (less than 18.5 kg/m2); 33.5% were normal weight (18.5-24.9 kg/m2); 35.4% were overweight (25-29.9 kg/m2); 18.1% were obese I (30-34.9 kg/m2); 6.4% were obese II (35-39.9 kg/m2), and 3.6% were obese III (40 kg/m2 or greater). Dr. Williams and his associates observed that women were more often underweight, compared with men (4.1% vs. 1.8%, respectively; P less than .001), and underweight patients more often had chronic obstructive pulmonary disease (51.7% vs. 35.2%; P less than .001). Pulmonary complication rates were higher among underweight and obese III patients (P less than .001), while being underweight was also associated with higher rates of infections and any surgical complications.
Multivariable analysis revealed that pulmonary and any postoperative complications were more common among underweight patients (odds ratio, 1.44 and OR, 1.41, respectively), while any major complication was more common among obese III patients (OR, 1.18). Overweight and obese I-II patients were less likely to have any postoperative and pulmonary complications, compared with patients who had a normal BMI. “The finding of underweight patients being such a high-risk patient population is suggested in the literature but not demonstrated as clearly as in this study,” Dr. Williams said. “A truly surprising finding was that obese patients actually have a lower risk of pulmonary and overall complications than ‘normal’-BMI patients.”
He concluded that according to the current analysis, “careful risk assessment is appropriate when considering operating on underweight patients. Whether there are interventions that could be instituted to improve an individual’s risk profile has not been determined. Any preconceived notions about not operating on obese patients due to elevated risk appear to be unfounded.”
Dr. Williams reported having no financial disclosures.
PHOENIX – Being underweight is associated with a substantially increased risk of complications following lung resection for cancer, results from a large database study found.
“This is not generally known among surgeons or their patients,” Dr. Trevor Williams said in an interview before the annual meeting of the Society of Thoracic Surgeons. “Studies are conflicting about the relationship of BMI [body mass index] to surgical outcomes. Most of the previous studies simply categorize BMI as overweight or not. We’ve stratified based on World Health Organization categories to get a more precise look at BMI.”
Dr. Williams, a surgeon at the University of Chicago Medical Center, and his associates evaluated 41,446 patients in the STS General Thoracic Surgery Database who underwent elective anatomic lung resection for cancer between 2009 and 2014. Their mean age was 68 years, and 53% were female. The researchers performed multivariable analysis after adjusting for validated STS risk model covariates, including gender and spirometry.
According to WHO criteria for BMI, 3% were underweight (less than 18.5 kg/m2); 33.5% were normal weight (18.5-24.9 kg/m2); 35.4% were overweight (25-29.9 kg/m2); 18.1% were obese I (30-34.9 kg/m2); 6.4% were obese II (35-39.9 kg/m2), and 3.6% were obese III (40 kg/m2 or greater). Dr. Williams and his associates observed that women were more often underweight, compared with men (4.1% vs. 1.8%, respectively; P less than .001), and underweight patients more often had chronic obstructive pulmonary disease (51.7% vs. 35.2%; P less than .001). Pulmonary complication rates were higher among underweight and obese III patients (P less than .001), while being underweight was also associated with higher rates of infections and any surgical complications.
Multivariable analysis revealed that pulmonary and any postoperative complications were more common among underweight patients (odds ratio, 1.44 and OR, 1.41, respectively), while any major complication was more common among obese III patients (OR, 1.18). Overweight and obese I-II patients were less likely to have any postoperative and pulmonary complications, compared with patients who had a normal BMI. “The finding of underweight patients being such a high-risk patient population is suggested in the literature but not demonstrated as clearly as in this study,” Dr. Williams said. “A truly surprising finding was that obese patients actually have a lower risk of pulmonary and overall complications than ‘normal’-BMI patients.”
He concluded that according to the current analysis, “careful risk assessment is appropriate when considering operating on underweight patients. Whether there are interventions that could be instituted to improve an individual’s risk profile has not been determined. Any preconceived notions about not operating on obese patients due to elevated risk appear to be unfounded.”
Dr. Williams reported having no financial disclosures.
AT THE STS ANNUAL MEETING
Key clinical point: Careful risk assessment is appropriate when considering performing lung resection on underweight patients.
Major finding: Multivariable analysis revealed that pulmonary and any postoperative complications were more common among underweight patients (OR, 1.44 and OR, 1.41, respectively), while any major complication was more common among obese III patients (OR, 1.18).
Data source: An analysis of 41,446 patients in the STS General Thoracic Surgery Database who underwent elective lung resection for cancer between 2009 and 2014.
Disclosures: Dr. Williams reported having no financial disclosures.
VIDEO: Novel imaging technique helps hunt for pulmonary lesions
PHOENIX – Each year more than 250,000 patients present with ground-glass opacities and other solitary pulmonary nodules, and they are difficult to locate.
“There’s been a need for our field to develop new technologies to find these nodules in the OR,” Dr. Sunil Singhal said in a video interview at the annual meeting of the Society of Thoracic Surgeons. “The fallback plan has always been that we can make a thoracotomy. Some studies have shown that in about one out of every two cases you end up opening a patient just to find a tiny little nodule.”
Dr. Singhal of the division of cardiothoracic surgery at the University of Pennsylvania School of Medicine, Philadelphia, discussed preoperative and intraoperative localization methods, including an investigational technology in which patients receive an intravascular dye that localizes the pulmonary tumor. “When we put our video-assisted thoracoscopic surgery camera in, the tumors are glowing,” he said. “We can then do a localized wedge excision and confirm margins of the staple line. We’ve done this [in] about 80 patients, and it’s been non-toxic, very safe, and very effective. Our biggest limitation has been our depth of penetration.”
Dr. Singhal reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PHOENIX – Each year more than 250,000 patients present with ground-glass opacities and other solitary pulmonary nodules, and they are difficult to locate.
“There’s been a need for our field to develop new technologies to find these nodules in the OR,” Dr. Sunil Singhal said in a video interview at the annual meeting of the Society of Thoracic Surgeons. “The fallback plan has always been that we can make a thoracotomy. Some studies have shown that in about one out of every two cases you end up opening a patient just to find a tiny little nodule.”
Dr. Singhal of the division of cardiothoracic surgery at the University of Pennsylvania School of Medicine, Philadelphia, discussed preoperative and intraoperative localization methods, including an investigational technology in which patients receive an intravascular dye that localizes the pulmonary tumor. “When we put our video-assisted thoracoscopic surgery camera in, the tumors are glowing,” he said. “We can then do a localized wedge excision and confirm margins of the staple line. We’ve done this [in] about 80 patients, and it’s been non-toxic, very safe, and very effective. Our biggest limitation has been our depth of penetration.”
Dr. Singhal reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PHOENIX – Each year more than 250,000 patients present with ground-glass opacities and other solitary pulmonary nodules, and they are difficult to locate.
“There’s been a need for our field to develop new technologies to find these nodules in the OR,” Dr. Sunil Singhal said in a video interview at the annual meeting of the Society of Thoracic Surgeons. “The fallback plan has always been that we can make a thoracotomy. Some studies have shown that in about one out of every two cases you end up opening a patient just to find a tiny little nodule.”
Dr. Singhal of the division of cardiothoracic surgery at the University of Pennsylvania School of Medicine, Philadelphia, discussed preoperative and intraoperative localization methods, including an investigational technology in which patients receive an intravascular dye that localizes the pulmonary tumor. “When we put our video-assisted thoracoscopic surgery camera in, the tumors are glowing,” he said. “We can then do a localized wedge excision and confirm margins of the staple line. We’ve done this [in] about 80 patients, and it’s been non-toxic, very safe, and very effective. Our biggest limitation has been our depth of penetration.”
Dr. Singhal reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM THE STS ANNUAL MEETING
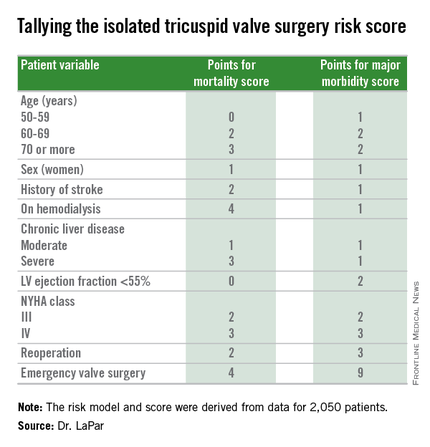
STS: Score stratifies risks for isolated tricuspid valve surgery patients
PHOENIX – A team of cardiac surgeons has developed the first clinical risk score for predicting the risk that patients face for operative mortality and postsurgical major morbidity when undergoing isolated tricuspid valve repair or replacement.
The risk score uses nine easily collected variables, and the derived model discriminates outcomes based on patients who score from 0-10 or more points on both a mortality and a morbidity risk scale, Dr. Damien J. LaPar said at the annual meeting of the Society of Thoracic Surgeons.
The risk scores allow surgeons to better describe and quantify to patients considering isolated tricuspid valve surgery the risks they face from the operation, and they have already been incorporated into practice at the University of Virginia, in Charlottesville, where Dr. LaPar practices.
“Patients love to better understand their risks. We can provide them with empirical data from a large, heterogeneous population that are better than a surgeon’s gut feeling” about the risks they face, said Dr. LaPar, a cardiothoracic surgeon at the University.
Another consequence of having the new risk model and score is that it identified certain key risk factors that are controllable, and thereby, “makes the case for early referrals” for isolated tricuspid valve surgery, Dr. LaPar said in an interview. For example, the risk score shows that patients who are older, on hemodialysis, have a reduced left ventricular ejection fraction, or require emergency intervention all contribute to worse outcomes, compared with patients who are younger, have better renal function, better cardiac output, or can be treated on a more routine basis.
Many physicians have viewed isolated tricuspid valve surgery as posing similar risks to all patients, with an overall average operative mortality rate of about 10%, he noted. The new risk score model shows that some patients who are younger and healthier have operative mortality rates below 5%, while older and sicker patients have rates that can surpass 20%.
“Our data show a spectrum of risk, and that it is better to operate sooner than later. That is the huge clinical message of these data,” Dr. LaPar said.
Designated discussant Dr. Michael A. Acker noted that the risk score for tricuspid-valve surgery “is a first of its kind and a major contribution.” Dr. Acker is professor of surgery and chief of cardiovascular surgery at the University of Pennsylvania in Philadelphia. He is a consultant to Thoratec and HeartWare.
Dr. LaPar and his associates derived the risk model and score from data collected on 2,050 patients who underwent isolated tricuspid valve repair or replacement at 49 hospitals in Virginia or Michigan during 2002-2014. The data came from databases maintained by the Virginia Cardiac Surgery Quality Initiative and by the Michigan Society of Thoracic & Cardiovascular Surgeons, and reported to the Adult Cardiac Surgery Database of the Society of Thoracic Surgeons. The model they developed showed operative mortality rates that ranged from 2%, for patients with a mortality score of zero, to 34% for patients with a score of 10 or more. It further showed major morbidity rates of 13%, for patients with a morbidity score of zero, to 71% for those with a score of 10 or more. Scoring for mortality uses a slightly different system than the scoring for morbidity, so the scores must be calculated individually, and the score totals for a patient can differ for each endpoint. The maximum score is 22 for mortality and 23 for morbidity.
Only 5%-15% of patients undergoing tricuspid valve surgery have an isolated procedure, so a relatively limited number of patients fall into this category, a fact that has in the past limited collection of data from large numbers of patients. The dataset used for this analysis, with 2,050 patients “is one of the largest series collected,” and made possible derivation of a robust risk model and scoring system. Future analysis of even more patients should further improve the model and scoring system.
“These data set the stage for looking at national-level data to further refine the model and make it even more generalizable,” Dr. LaPar said.
On Twitter @mitchelzoler
PHOENIX – A team of cardiac surgeons has developed the first clinical risk score for predicting the risk that patients face for operative mortality and postsurgical major morbidity when undergoing isolated tricuspid valve repair or replacement.
The risk score uses nine easily collected variables, and the derived model discriminates outcomes based on patients who score from 0-10 or more points on both a mortality and a morbidity risk scale, Dr. Damien J. LaPar said at the annual meeting of the Society of Thoracic Surgeons.
The risk scores allow surgeons to better describe and quantify to patients considering isolated tricuspid valve surgery the risks they face from the operation, and they have already been incorporated into practice at the University of Virginia, in Charlottesville, where Dr. LaPar practices.
“Patients love to better understand their risks. We can provide them with empirical data from a large, heterogeneous population that are better than a surgeon’s gut feeling” about the risks they face, said Dr. LaPar, a cardiothoracic surgeon at the University.
Another consequence of having the new risk model and score is that it identified certain key risk factors that are controllable, and thereby, “makes the case for early referrals” for isolated tricuspid valve surgery, Dr. LaPar said in an interview. For example, the risk score shows that patients who are older, on hemodialysis, have a reduced left ventricular ejection fraction, or require emergency intervention all contribute to worse outcomes, compared with patients who are younger, have better renal function, better cardiac output, or can be treated on a more routine basis.

Many physicians have viewed isolated tricuspid valve surgery as posing similar risks to all patients, with an overall average operative mortality rate of about 10%, he noted. The new risk score model shows that some patients who are younger and healthier have operative mortality rates below 5%, while older and sicker patients have rates that can surpass 20%.
“Our data show a spectrum of risk, and that it is better to operate sooner than later. That is the huge clinical message of these data,” Dr. LaPar said.
Designated discussant Dr. Michael A. Acker noted that the risk score for tricuspid-valve surgery “is a first of its kind and a major contribution.” Dr. Acker is professor of surgery and chief of cardiovascular surgery at the University of Pennsylvania in Philadelphia. He is a consultant to Thoratec and HeartWare.
Dr. LaPar and his associates derived the risk model and score from data collected on 2,050 patients who underwent isolated tricuspid valve repair or replacement at 49 hospitals in Virginia or Michigan during 2002-2014. The data came from databases maintained by the Virginia Cardiac Surgery Quality Initiative and by the Michigan Society of Thoracic & Cardiovascular Surgeons, and reported to the Adult Cardiac Surgery Database of the Society of Thoracic Surgeons. The model they developed showed operative mortality rates that ranged from 2%, for patients with a mortality score of zero, to 34% for patients with a score of 10 or more. It further showed major morbidity rates of 13%, for patients with a morbidity score of zero, to 71% for those with a score of 10 or more. Scoring for mortality uses a slightly different system than the scoring for morbidity, so the scores must be calculated individually, and the score totals for a patient can differ for each endpoint. The maximum score is 22 for mortality and 23 for morbidity.
Only 5%-15% of patients undergoing tricuspid valve surgery have an isolated procedure, so a relatively limited number of patients fall into this category, a fact that has in the past limited collection of data from large numbers of patients. The dataset used for this analysis, with 2,050 patients “is one of the largest series collected,” and made possible derivation of a robust risk model and scoring system. Future analysis of even more patients should further improve the model and scoring system.
“These data set the stage for looking at national-level data to further refine the model and make it even more generalizable,” Dr. LaPar said.
On Twitter @mitchelzoler
PHOENIX – A team of cardiac surgeons has developed the first clinical risk score for predicting the risk that patients face for operative mortality and postsurgical major morbidity when undergoing isolated tricuspid valve repair or replacement.
The risk score uses nine easily collected variables, and the derived model discriminates outcomes based on patients who score from 0-10 or more points on both a mortality and a morbidity risk scale, Dr. Damien J. LaPar said at the annual meeting of the Society of Thoracic Surgeons.
The risk scores allow surgeons to better describe and quantify to patients considering isolated tricuspid valve surgery the risks they face from the operation, and they have already been incorporated into practice at the University of Virginia, in Charlottesville, where Dr. LaPar practices.
“Patients love to better understand their risks. We can provide them with empirical data from a large, heterogeneous population that are better than a surgeon’s gut feeling” about the risks they face, said Dr. LaPar, a cardiothoracic surgeon at the University.
Another consequence of having the new risk model and score is that it identified certain key risk factors that are controllable, and thereby, “makes the case for early referrals” for isolated tricuspid valve surgery, Dr. LaPar said in an interview. For example, the risk score shows that patients who are older, on hemodialysis, have a reduced left ventricular ejection fraction, or require emergency intervention all contribute to worse outcomes, compared with patients who are younger, have better renal function, better cardiac output, or can be treated on a more routine basis.

Many physicians have viewed isolated tricuspid valve surgery as posing similar risks to all patients, with an overall average operative mortality rate of about 10%, he noted. The new risk score model shows that some patients who are younger and healthier have operative mortality rates below 5%, while older and sicker patients have rates that can surpass 20%.
“Our data show a spectrum of risk, and that it is better to operate sooner than later. That is the huge clinical message of these data,” Dr. LaPar said.
Designated discussant Dr. Michael A. Acker noted that the risk score for tricuspid-valve surgery “is a first of its kind and a major contribution.” Dr. Acker is professor of surgery and chief of cardiovascular surgery at the University of Pennsylvania in Philadelphia. He is a consultant to Thoratec and HeartWare.
Dr. LaPar and his associates derived the risk model and score from data collected on 2,050 patients who underwent isolated tricuspid valve repair or replacement at 49 hospitals in Virginia or Michigan during 2002-2014. The data came from databases maintained by the Virginia Cardiac Surgery Quality Initiative and by the Michigan Society of Thoracic & Cardiovascular Surgeons, and reported to the Adult Cardiac Surgery Database of the Society of Thoracic Surgeons. The model they developed showed operative mortality rates that ranged from 2%, for patients with a mortality score of zero, to 34% for patients with a score of 10 or more. It further showed major morbidity rates of 13%, for patients with a morbidity score of zero, to 71% for those with a score of 10 or more. Scoring for mortality uses a slightly different system than the scoring for morbidity, so the scores must be calculated individually, and the score totals for a patient can differ for each endpoint. The maximum score is 22 for mortality and 23 for morbidity.
Only 5%-15% of patients undergoing tricuspid valve surgery have an isolated procedure, so a relatively limited number of patients fall into this category, a fact that has in the past limited collection of data from large numbers of patients. The dataset used for this analysis, with 2,050 patients “is one of the largest series collected,” and made possible derivation of a robust risk model and scoring system. Future analysis of even more patients should further improve the model and scoring system.
“These data set the stage for looking at national-level data to further refine the model and make it even more generalizable,” Dr. LaPar said.
On Twitter @mitchelzoler
AT THE STS ANNUAL MEETING
Key clinical point: A risk-scoring system estimates a patient’s mortality and morbidity risk when undergoing isolated tricuspid valve surgery.
Major finding: The scoring system discriminated mortality risk from 2% to 34%, and major morbidity risk from 13% to 71%.
Data source: Analysis of 2,050 patients who underwent isolated tricuspid valve surgery in the STS Adult Cardiac Surgery Database.
Disclosures: Dr. LaPar had no disclosures.
VIDEO: Preventing healthcare acquired infections after CT surgery
PHOENIX – More and more attention is being paid to preventing healthcare acquired infections (HAIs) in the hospital setting, and the role of HAIs in cardiothoracic surgery is a particlularly important area of focus.
“The good news is that cardiothoracic surgeons are really good at preventing infections. There’s been a lot of pressure over the past many years to report infections after cardiothoracic surgery, and so they’ve gotten a lot of things right,” Dr. Emily Landon said in a video interview at the annual meeting of the Society of Thoracic Surgeons.
“However, patients that undergo cardiothoracic surgery are still at risk of the infections that plague everyone in hospitals ... all of these are a problem based on whatever the hospital’s current situation is.”
Dr. Landon, who is the medical director of antimicrobial stewardship and infection control at University of Chicago Medicine, Chicago, discussed how cardiothroacic surgeons can maintain their own good outcomes and how they can have a postive impact outside the OR on protecting their patients after surgery.
Dr. Landon reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PHOENIX – More and more attention is being paid to preventing healthcare acquired infections (HAIs) in the hospital setting, and the role of HAIs in cardiothoracic surgery is a particlularly important area of focus.
“The good news is that cardiothoracic surgeons are really good at preventing infections. There’s been a lot of pressure over the past many years to report infections after cardiothoracic surgery, and so they’ve gotten a lot of things right,” Dr. Emily Landon said in a video interview at the annual meeting of the Society of Thoracic Surgeons.
“However, patients that undergo cardiothoracic surgery are still at risk of the infections that plague everyone in hospitals ... all of these are a problem based on whatever the hospital’s current situation is.”
Dr. Landon, who is the medical director of antimicrobial stewardship and infection control at University of Chicago Medicine, Chicago, discussed how cardiothroacic surgeons can maintain their own good outcomes and how they can have a postive impact outside the OR on protecting their patients after surgery.
Dr. Landon reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
PHOENIX – More and more attention is being paid to preventing healthcare acquired infections (HAIs) in the hospital setting, and the role of HAIs in cardiothoracic surgery is a particlularly important area of focus.
“The good news is that cardiothoracic surgeons are really good at preventing infections. There’s been a lot of pressure over the past many years to report infections after cardiothoracic surgery, and so they’ve gotten a lot of things right,” Dr. Emily Landon said in a video interview at the annual meeting of the Society of Thoracic Surgeons.
“However, patients that undergo cardiothoracic surgery are still at risk of the infections that plague everyone in hospitals ... all of these are a problem based on whatever the hospital’s current situation is.”
Dr. Landon, who is the medical director of antimicrobial stewardship and infection control at University of Chicago Medicine, Chicago, discussed how cardiothroacic surgeons can maintain their own good outcomes and how they can have a postive impact outside the OR on protecting their patients after surgery.
Dr. Landon reported having no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM THE STS ANNUAL MEETING
STS: Hybrid thoracic suite leverages CT’s imaging sensitivity
PHOENIX – Using CT imaging to detect lung cancers in people at high risk for developing it has made it possible to find small tumors with substantially increased sensitivity than is possible with radiography, However, this approach has posed a new challenge to thoracic surgeons: How to visualize these nodules – subcentimeter and nonpalpable – for biopsy or for resection?
The answer may be the hybrid thoracic operating room developed by Dr. Kazuhiro Yasufuku and his associates at Toronto General Hospital, a novel surgical suite that he described at the annual meeting of the Society of Thoracic Surgeons.
Dr. Yasufuku and his team began using the hybrid operating room on an investigational basis in 2013 and have now done about 50 cases as part of several research protocols. The trials address the feasibility of resection, biopsy, and nodule localization, as well as whether the hybrid approach reduces the amount of radiation exposure to both patients and to the surgical team, he said. They plan to report some of their initial results later this year.
The Toronto group assembled the hybrid array of equipment into a single operating room that includes both a dual-source, dual-energy CT scanner and a robotic cone-beam CT scanner, equipment for minimally invasive procedures including video-assisted thoracoscopic and robotic surgery, and advanced endoscopic technology including endobronchial ultrasound and navigational bronchoscopy. “We use innovative methods that we already know about, but bring them all together” within a single space, Dr. Yasufuku explained. “Rather than having patients go to several locations, we can do everything at the same time in one room.”
Perhaps the most novel aspect of this operating room is inclusion of a robotic cone-beam CT scanner, which uses mobile, flat CT-imaging panels that overcome the limitations of a conventional, fixed CT scanner. “They scan the patient and then we can retract them and get them out of the way” to better facilitate surgery, he said in an interview.
“We do not have a culture in thoracic surgery of using imaging during surgery,” said Dr. Yasufuku, director of the interventional thoracic surgery program at the University of Toronto. Hybrid operating rooms using noninvasive or minimally invasive equipment and procedures have become commonplace for cardiovascular surgeons and cardiac interventionalists, but this approach has generally not yet been applied to thoracic surgery for cancer, in large part because of the imaging limitations, he said. “It is difficult to perform video-assisted thorascopic surgery using fixed CT.”
Bronchoscopic technologies provide additional, important tools for minimally invasive thoracic surgery. “We use the hybrid operating room to mark small [nonpalpable] lesions.” One approach to marking is to place a microcoil within the nodule with a percutaneous needle. Another approach is to tag the nodule with a fluorescent dye using navigational bronchoscopy.
Dr. Yasufuku also emphasized that the hybrid operating room will also be valuable when new, minimally invasive, nonsurgical therapeutic options for treatment of lung cancer become available in the near future.
Dr. Yasufuku said that he had no relevant disclosures.
On Twitter @mitchelzoler
PHOENIX – Using CT imaging to detect lung cancers in people at high risk for developing it has made it possible to find small tumors with substantially increased sensitivity than is possible with radiography, However, this approach has posed a new challenge to thoracic surgeons: How to visualize these nodules – subcentimeter and nonpalpable – for biopsy or for resection?
The answer may be the hybrid thoracic operating room developed by Dr. Kazuhiro Yasufuku and his associates at Toronto General Hospital, a novel surgical suite that he described at the annual meeting of the Society of Thoracic Surgeons.
Dr. Yasufuku and his team began using the hybrid operating room on an investigational basis in 2013 and have now done about 50 cases as part of several research protocols. The trials address the feasibility of resection, biopsy, and nodule localization, as well as whether the hybrid approach reduces the amount of radiation exposure to both patients and to the surgical team, he said. They plan to report some of their initial results later this year.
The Toronto group assembled the hybrid array of equipment into a single operating room that includes both a dual-source, dual-energy CT scanner and a robotic cone-beam CT scanner, equipment for minimally invasive procedures including video-assisted thoracoscopic and robotic surgery, and advanced endoscopic technology including endobronchial ultrasound and navigational bronchoscopy. “We use innovative methods that we already know about, but bring them all together” within a single space, Dr. Yasufuku explained. “Rather than having patients go to several locations, we can do everything at the same time in one room.”
Perhaps the most novel aspect of this operating room is inclusion of a robotic cone-beam CT scanner, which uses mobile, flat CT-imaging panels that overcome the limitations of a conventional, fixed CT scanner. “They scan the patient and then we can retract them and get them out of the way” to better facilitate surgery, he said in an interview.
“We do not have a culture in thoracic surgery of using imaging during surgery,” said Dr. Yasufuku, director of the interventional thoracic surgery program at the University of Toronto. Hybrid operating rooms using noninvasive or minimally invasive equipment and procedures have become commonplace for cardiovascular surgeons and cardiac interventionalists, but this approach has generally not yet been applied to thoracic surgery for cancer, in large part because of the imaging limitations, he said. “It is difficult to perform video-assisted thorascopic surgery using fixed CT.”
Bronchoscopic technologies provide additional, important tools for minimally invasive thoracic surgery. “We use the hybrid operating room to mark small [nonpalpable] lesions.” One approach to marking is to place a microcoil within the nodule with a percutaneous needle. Another approach is to tag the nodule with a fluorescent dye using navigational bronchoscopy.
Dr. Yasufuku also emphasized that the hybrid operating room will also be valuable when new, minimally invasive, nonsurgical therapeutic options for treatment of lung cancer become available in the near future.
Dr. Yasufuku said that he had no relevant disclosures.
On Twitter @mitchelzoler
PHOENIX – Using CT imaging to detect lung cancers in people at high risk for developing it has made it possible to find small tumors with substantially increased sensitivity than is possible with radiography, However, this approach has posed a new challenge to thoracic surgeons: How to visualize these nodules – subcentimeter and nonpalpable – for biopsy or for resection?
The answer may be the hybrid thoracic operating room developed by Dr. Kazuhiro Yasufuku and his associates at Toronto General Hospital, a novel surgical suite that he described at the annual meeting of the Society of Thoracic Surgeons.
Dr. Yasufuku and his team began using the hybrid operating room on an investigational basis in 2013 and have now done about 50 cases as part of several research protocols. The trials address the feasibility of resection, biopsy, and nodule localization, as well as whether the hybrid approach reduces the amount of radiation exposure to both patients and to the surgical team, he said. They plan to report some of their initial results later this year.
The Toronto group assembled the hybrid array of equipment into a single operating room that includes both a dual-source, dual-energy CT scanner and a robotic cone-beam CT scanner, equipment for minimally invasive procedures including video-assisted thoracoscopic and robotic surgery, and advanced endoscopic technology including endobronchial ultrasound and navigational bronchoscopy. “We use innovative methods that we already know about, but bring them all together” within a single space, Dr. Yasufuku explained. “Rather than having patients go to several locations, we can do everything at the same time in one room.”
Perhaps the most novel aspect of this operating room is inclusion of a robotic cone-beam CT scanner, which uses mobile, flat CT-imaging panels that overcome the limitations of a conventional, fixed CT scanner. “They scan the patient and then we can retract them and get them out of the way” to better facilitate surgery, he said in an interview.
“We do not have a culture in thoracic surgery of using imaging during surgery,” said Dr. Yasufuku, director of the interventional thoracic surgery program at the University of Toronto. Hybrid operating rooms using noninvasive or minimally invasive equipment and procedures have become commonplace for cardiovascular surgeons and cardiac interventionalists, but this approach has generally not yet been applied to thoracic surgery for cancer, in large part because of the imaging limitations, he said. “It is difficult to perform video-assisted thorascopic surgery using fixed CT.”
Bronchoscopic technologies provide additional, important tools for minimally invasive thoracic surgery. “We use the hybrid operating room to mark small [nonpalpable] lesions.” One approach to marking is to place a microcoil within the nodule with a percutaneous needle. Another approach is to tag the nodule with a fluorescent dye using navigational bronchoscopy.
Dr. Yasufuku also emphasized that the hybrid operating room will also be valuable when new, minimally invasive, nonsurgical therapeutic options for treatment of lung cancer become available in the near future.
Dr. Yasufuku said that he had no relevant disclosures.
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE STS ANNUAL MEETING