User login
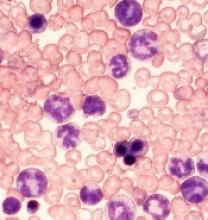
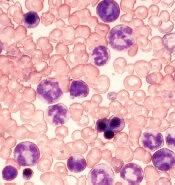
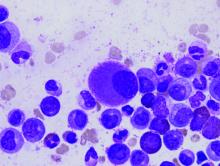
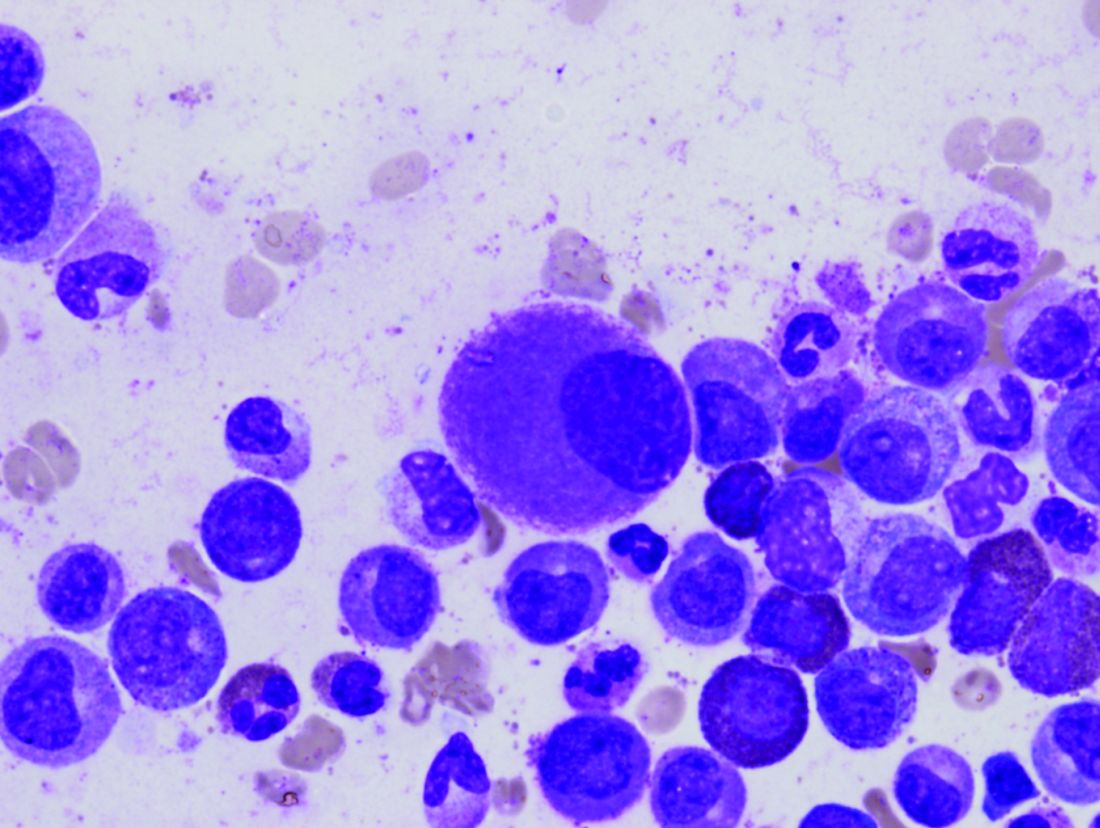
Novel inhibitor proves ‘potent’ in hematologic malignancies
BOSTON—A pair of preclinical studies suggest the FLT3/BTK inhibitor CG’806 is active in a range of hematologic malignancies.
In one of the studies, CG’806 proved particularly effective against acute myeloid leukemia (AML) cells harboring mutant forms of FLT3, and the compound was able to eradicate AML in mice.
In another study, researchers found CG’806 exhibited “broad potency” against leukemias, lymphomas, myelodysplastic syndromes (MDS), and myeloproliferative neoplasms (MPNs).
Both studies were presented as posters at Hematologic Malignancies: Translating Discoveries to Novel Therapies (poster 25 and poster 44).
Both studies involved researchers from Aptose Biosciences, the company developing CG’806.
Poster 25
Weiguo Zhang, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston, and his colleagues presented poster 25, “CG’806, a first-in-class FLT3/BTK inhibitor, exerts superior potency against AML cells harboring ITD, TKD and gatekeeper mutated FLT3 or wild-type FLT3.”
The researchers tested CG’806 and other FLT3 inhibitors in human or murine leukemia cell lines with wild-type (WT) FLT3, FLT3-ITD mutations, FLT3 TKD domain mutations, or ITD plus TKD mutations.
Compared to second-generation FLT3 inhibitors (quizartinib, gilteritinib, or crenolanib), CG’806 showed more pronounced anti-proliferative effects in leukemia cells with ITD mutations, D835 mutations, ITD plus F691I/Y842D/D835 mutations, or in FLT3 WT cells.
With CG’086, the IC50s in human AML cell lines were 0.17 nM for MV4-11 (FLT3-ITD) and 0.82 nM for MOLM13 (FLT3-ITD).
The IC50s in the murine leukemia cell lines were 9.49 nM for Ba/F3 (FLT3-WT), 0.30 nM for Ba/F3 (FLT3-ITD), 8.26 nM for Ba/F3 (FLT3-D835Y), 9.72 nM for Ba/F3 (FLT3-ITD+D835Y), and 0.43 nM for Ba/F3 (FLT3-ITD+F691L).
The researchers also found that CG’806 “triggers marked apoptosis” in FLT3-ITD-mutated primary AML samples but minimal apoptosis in normal bone marrow cells.
Another finding was that once-daily oral dosing of CG’806 in a murine model of AML (MV4-11) resulted in sustained micromolar plasma concentration over a 24-hour period.
This was accompanied by complete elimination of AML FLT3-ITD tumors without toxicity, the researchers said.
Poster 44
Stephen E. Kurtz, PhD, of Oregon Health & Science University in Portland, and his colleagues presented poster 44, “CG’806, a First-in-Class FLT3/BTK Inhibitor, Exhibits Potent Activity against AML Patient Samples with Mutant or Wild-Type FLT3, as well as Other Hematologic Malignancy Subtypes.”
The researchers tested CG’806 in samples from patients with AML (n=82), MDS/MPNs (n=15), acute lymphoblastic leukemia (ALL, n=17), chronic lymphocytic leukemia (CLL, n=58), and chronic myeloid leukemia (CML, n=4).
The team observed “broad sensitivity” to CG’806, with 59% (48/82) of AML, 53% (8/15) of MDS/MPN, 40% (23/58) of CLL, 29% (5/17) of ALL, and 25% (1/4) of CML cases exhibiting an IC50 of less than 100 nM.
Among the 38 tested AML samples with known FLT3 mutational status, the FLT3-ITD+ AML samples tended to have enhanced sensitivity to CG’806 (median IC50 = 20 nM, n=8) relative to the FLT3-WT samples (median IC50 = 120 nM, n=30).
The researchers also found that CG’806 exerted potent anti-proliferative activity against human AML, B-ALL, mantle cell lymphoma, Burkitt lymphoma, and diffuse large B-cell lymphoma cell lines.
“The analyses of CG’806 against primary hematologic malignancy patient samples and cultured cell lines show evidence of potent and broad drug activity in AML and other disease subtypes and support further development of this agent for hematologic malignancies,” Dr Kurtz said. ![]()
BOSTON—A pair of preclinical studies suggest the FLT3/BTK inhibitor CG’806 is active in a range of hematologic malignancies.
In one of the studies, CG’806 proved particularly effective against acute myeloid leukemia (AML) cells harboring mutant forms of FLT3, and the compound was able to eradicate AML in mice.
In another study, researchers found CG’806 exhibited “broad potency” against leukemias, lymphomas, myelodysplastic syndromes (MDS), and myeloproliferative neoplasms (MPNs).
Both studies were presented as posters at Hematologic Malignancies: Translating Discoveries to Novel Therapies (poster 25 and poster 44).
Both studies involved researchers from Aptose Biosciences, the company developing CG’806.
Poster 25
Weiguo Zhang, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston, and his colleagues presented poster 25, “CG’806, a first-in-class FLT3/BTK inhibitor, exerts superior potency against AML cells harboring ITD, TKD and gatekeeper mutated FLT3 or wild-type FLT3.”
The researchers tested CG’806 and other FLT3 inhibitors in human or murine leukemia cell lines with wild-type (WT) FLT3, FLT3-ITD mutations, FLT3 TKD domain mutations, or ITD plus TKD mutations.
Compared to second-generation FLT3 inhibitors (quizartinib, gilteritinib, or crenolanib), CG’806 showed more pronounced anti-proliferative effects in leukemia cells with ITD mutations, D835 mutations, ITD plus F691I/Y842D/D835 mutations, or in FLT3 WT cells.
With CG’086, the IC50s in human AML cell lines were 0.17 nM for MV4-11 (FLT3-ITD) and 0.82 nM for MOLM13 (FLT3-ITD).
The IC50s in the murine leukemia cell lines were 9.49 nM for Ba/F3 (FLT3-WT), 0.30 nM for Ba/F3 (FLT3-ITD), 8.26 nM for Ba/F3 (FLT3-D835Y), 9.72 nM for Ba/F3 (FLT3-ITD+D835Y), and 0.43 nM for Ba/F3 (FLT3-ITD+F691L).
The researchers also found that CG’806 “triggers marked apoptosis” in FLT3-ITD-mutated primary AML samples but minimal apoptosis in normal bone marrow cells.
Another finding was that once-daily oral dosing of CG’806 in a murine model of AML (MV4-11) resulted in sustained micromolar plasma concentration over a 24-hour period.
This was accompanied by complete elimination of AML FLT3-ITD tumors without toxicity, the researchers said.
Poster 44
Stephen E. Kurtz, PhD, of Oregon Health & Science University in Portland, and his colleagues presented poster 44, “CG’806, a First-in-Class FLT3/BTK Inhibitor, Exhibits Potent Activity against AML Patient Samples with Mutant or Wild-Type FLT3, as well as Other Hematologic Malignancy Subtypes.”
The researchers tested CG’806 in samples from patients with AML (n=82), MDS/MPNs (n=15), acute lymphoblastic leukemia (ALL, n=17), chronic lymphocytic leukemia (CLL, n=58), and chronic myeloid leukemia (CML, n=4).
The team observed “broad sensitivity” to CG’806, with 59% (48/82) of AML, 53% (8/15) of MDS/MPN, 40% (23/58) of CLL, 29% (5/17) of ALL, and 25% (1/4) of CML cases exhibiting an IC50 of less than 100 nM.
Among the 38 tested AML samples with known FLT3 mutational status, the FLT3-ITD+ AML samples tended to have enhanced sensitivity to CG’806 (median IC50 = 20 nM, n=8) relative to the FLT3-WT samples (median IC50 = 120 nM, n=30).
The researchers also found that CG’806 exerted potent anti-proliferative activity against human AML, B-ALL, mantle cell lymphoma, Burkitt lymphoma, and diffuse large B-cell lymphoma cell lines.
“The analyses of CG’806 against primary hematologic malignancy patient samples and cultured cell lines show evidence of potent and broad drug activity in AML and other disease subtypes and support further development of this agent for hematologic malignancies,” Dr Kurtz said. ![]()
BOSTON—A pair of preclinical studies suggest the FLT3/BTK inhibitor CG’806 is active in a range of hematologic malignancies.
In one of the studies, CG’806 proved particularly effective against acute myeloid leukemia (AML) cells harboring mutant forms of FLT3, and the compound was able to eradicate AML in mice.
In another study, researchers found CG’806 exhibited “broad potency” against leukemias, lymphomas, myelodysplastic syndromes (MDS), and myeloproliferative neoplasms (MPNs).
Both studies were presented as posters at Hematologic Malignancies: Translating Discoveries to Novel Therapies (poster 25 and poster 44).
Both studies involved researchers from Aptose Biosciences, the company developing CG’806.
Poster 25
Weiguo Zhang, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston, and his colleagues presented poster 25, “CG’806, a first-in-class FLT3/BTK inhibitor, exerts superior potency against AML cells harboring ITD, TKD and gatekeeper mutated FLT3 or wild-type FLT3.”
The researchers tested CG’806 and other FLT3 inhibitors in human or murine leukemia cell lines with wild-type (WT) FLT3, FLT3-ITD mutations, FLT3 TKD domain mutations, or ITD plus TKD mutations.
Compared to second-generation FLT3 inhibitors (quizartinib, gilteritinib, or crenolanib), CG’806 showed more pronounced anti-proliferative effects in leukemia cells with ITD mutations, D835 mutations, ITD plus F691I/Y842D/D835 mutations, or in FLT3 WT cells.
With CG’086, the IC50s in human AML cell lines were 0.17 nM for MV4-11 (FLT3-ITD) and 0.82 nM for MOLM13 (FLT3-ITD).
The IC50s in the murine leukemia cell lines were 9.49 nM for Ba/F3 (FLT3-WT), 0.30 nM for Ba/F3 (FLT3-ITD), 8.26 nM for Ba/F3 (FLT3-D835Y), 9.72 nM for Ba/F3 (FLT3-ITD+D835Y), and 0.43 nM for Ba/F3 (FLT3-ITD+F691L).
The researchers also found that CG’806 “triggers marked apoptosis” in FLT3-ITD-mutated primary AML samples but minimal apoptosis in normal bone marrow cells.
Another finding was that once-daily oral dosing of CG’806 in a murine model of AML (MV4-11) resulted in sustained micromolar plasma concentration over a 24-hour period.
This was accompanied by complete elimination of AML FLT3-ITD tumors without toxicity, the researchers said.
Poster 44
Stephen E. Kurtz, PhD, of Oregon Health & Science University in Portland, and his colleagues presented poster 44, “CG’806, a First-in-Class FLT3/BTK Inhibitor, Exhibits Potent Activity against AML Patient Samples with Mutant or Wild-Type FLT3, as well as Other Hematologic Malignancy Subtypes.”
The researchers tested CG’806 in samples from patients with AML (n=82), MDS/MPNs (n=15), acute lymphoblastic leukemia (ALL, n=17), chronic lymphocytic leukemia (CLL, n=58), and chronic myeloid leukemia (CML, n=4).
The team observed “broad sensitivity” to CG’806, with 59% (48/82) of AML, 53% (8/15) of MDS/MPN, 40% (23/58) of CLL, 29% (5/17) of ALL, and 25% (1/4) of CML cases exhibiting an IC50 of less than 100 nM.
Among the 38 tested AML samples with known FLT3 mutational status, the FLT3-ITD+ AML samples tended to have enhanced sensitivity to CG’806 (median IC50 = 20 nM, n=8) relative to the FLT3-WT samples (median IC50 = 120 nM, n=30).
The researchers also found that CG’806 exerted potent anti-proliferative activity against human AML, B-ALL, mantle cell lymphoma, Burkitt lymphoma, and diffuse large B-cell lymphoma cell lines.
“The analyses of CG’806 against primary hematologic malignancy patient samples and cultured cell lines show evidence of potent and broad drug activity in AML and other disease subtypes and support further development of this agent for hematologic malignancies,” Dr Kurtz said. ![]()
High CD86+pDC counts may predict CML relapses
Patients with chronic myeloid leukemia (CML) with high CD86+pDC counts had a higher risk of relapse after discontinuing tyrosine kinase inhibitor (TKI) therapy, according to new findings published in Leukemia.
Of patients who achieve a deep molecular remission, only a minority are able to sustain it and remain off therapy. Even when deep remission is achieved, TKI therapy fails to eradicate CML stem cells, which can perpetuate disease.
“This is clinically reflected by the long-term persistence of BCR-ABL messenger RNA (mRNA) in the majority of patients,” wrote C. Schütz, MD, of the University Hospital Marburg (Germany) and colleagues (Leukemia. 2017 Apr;31[4]:829-36). “Even with undetectable BCR-ABL mRNA levels, patients frequently relapse after TKI cessation.”
The researchers investigated whether the expression of the T-cell inhibitory receptor (CTLA-4)-ligand CD86 (B7.2) on plasmacytoid dendritic cells (pDC) could have an effect on the risk of relapse in CML patients who discontinue TKI therapy after achieving remission.
The frequency of CD86+pDC was analyzed in 14 CML patients who were in treatment-free remission, in 130 patients in molecular remission who were part of the CML-V study, and prospectively in 122 EURO-SKI patients right before they discontinued TKI therapy.
The authors found that CML patients in molecular remission had a significantly higher frequency of CD86+pDC expression, compared with normal donors (P less than .0024). In contrast, those who were in treatment-free remission had consistently low CD86+pDC.
These results suggest that low CD86+pDC could be predictive of treatment-free remission.
To test the hypothesis that low CD86+pDC frequencies during TKI-induced molecular remission were associated with a lower risk of molecular relapse after stopping TKI therapy, the study authors measured CD86+pDC levels in the 122 EURO-SKI patients before they stopped therapy, and then prospectively monitored them for relapse.
Findings showed that the 122 EURO-SKI patients had a significantly higher CD86+pDC frequency than did 8 healthy donors (median, 20.8% vs. 7.3%; P = .0024).
When matched with the treatment-free remission patients, the 73 patients in the EURO-SKI group who did not relapse within the first 12 months after stopping therapy had a significantly lower median frequency of CD86+pDC at baseline, compared with the 49 patients who did relapse (P = .014).
Patients who relapsed also demonstrated higher absolute CD86+pDC counts (CD86+pDC per 105 lymphocytes) at baseline (median, 86.1 vs. 50.6; P = .0147).
Based on the findings, the authors noted that they provided “for the first time evidence that relapse biology after TKI discontinuation depends on the quantity of activated pDC and a T-cell exhaustion phenotype, rather than TKI pretreatment duration per se.”
The Clinical Research Group of the German Research Foundation and the German José Carreras Leukaemia Foundation funded the study. Several of the authors report relationships with Ariad, Bristol-Myers Squibb, Novartis, and Pfizer.
Patients with chronic myeloid leukemia (CML) with high CD86+pDC counts had a higher risk of relapse after discontinuing tyrosine kinase inhibitor (TKI) therapy, according to new findings published in Leukemia.
Of patients who achieve a deep molecular remission, only a minority are able to sustain it and remain off therapy. Even when deep remission is achieved, TKI therapy fails to eradicate CML stem cells, which can perpetuate disease.
“This is clinically reflected by the long-term persistence of BCR-ABL messenger RNA (mRNA) in the majority of patients,” wrote C. Schütz, MD, of the University Hospital Marburg (Germany) and colleagues (Leukemia. 2017 Apr;31[4]:829-36). “Even with undetectable BCR-ABL mRNA levels, patients frequently relapse after TKI cessation.”
The researchers investigated whether the expression of the T-cell inhibitory receptor (CTLA-4)-ligand CD86 (B7.2) on plasmacytoid dendritic cells (pDC) could have an effect on the risk of relapse in CML patients who discontinue TKI therapy after achieving remission.
The frequency of CD86+pDC was analyzed in 14 CML patients who were in treatment-free remission, in 130 patients in molecular remission who were part of the CML-V study, and prospectively in 122 EURO-SKI patients right before they discontinued TKI therapy.
The authors found that CML patients in molecular remission had a significantly higher frequency of CD86+pDC expression, compared with normal donors (P less than .0024). In contrast, those who were in treatment-free remission had consistently low CD86+pDC.
These results suggest that low CD86+pDC could be predictive of treatment-free remission.
To test the hypothesis that low CD86+pDC frequencies during TKI-induced molecular remission were associated with a lower risk of molecular relapse after stopping TKI therapy, the study authors measured CD86+pDC levels in the 122 EURO-SKI patients before they stopped therapy, and then prospectively monitored them for relapse.
Findings showed that the 122 EURO-SKI patients had a significantly higher CD86+pDC frequency than did 8 healthy donors (median, 20.8% vs. 7.3%; P = .0024).
When matched with the treatment-free remission patients, the 73 patients in the EURO-SKI group who did not relapse within the first 12 months after stopping therapy had a significantly lower median frequency of CD86+pDC at baseline, compared with the 49 patients who did relapse (P = .014).
Patients who relapsed also demonstrated higher absolute CD86+pDC counts (CD86+pDC per 105 lymphocytes) at baseline (median, 86.1 vs. 50.6; P = .0147).
Based on the findings, the authors noted that they provided “for the first time evidence that relapse biology after TKI discontinuation depends on the quantity of activated pDC and a T-cell exhaustion phenotype, rather than TKI pretreatment duration per se.”
The Clinical Research Group of the German Research Foundation and the German José Carreras Leukaemia Foundation funded the study. Several of the authors report relationships with Ariad, Bristol-Myers Squibb, Novartis, and Pfizer.
Patients with chronic myeloid leukemia (CML) with high CD86+pDC counts had a higher risk of relapse after discontinuing tyrosine kinase inhibitor (TKI) therapy, according to new findings published in Leukemia.
Of patients who achieve a deep molecular remission, only a minority are able to sustain it and remain off therapy. Even when deep remission is achieved, TKI therapy fails to eradicate CML stem cells, which can perpetuate disease.
“This is clinically reflected by the long-term persistence of BCR-ABL messenger RNA (mRNA) in the majority of patients,” wrote C. Schütz, MD, of the University Hospital Marburg (Germany) and colleagues (Leukemia. 2017 Apr;31[4]:829-36). “Even with undetectable BCR-ABL mRNA levels, patients frequently relapse after TKI cessation.”
The researchers investigated whether the expression of the T-cell inhibitory receptor (CTLA-4)-ligand CD86 (B7.2) on plasmacytoid dendritic cells (pDC) could have an effect on the risk of relapse in CML patients who discontinue TKI therapy after achieving remission.
The frequency of CD86+pDC was analyzed in 14 CML patients who were in treatment-free remission, in 130 patients in molecular remission who were part of the CML-V study, and prospectively in 122 EURO-SKI patients right before they discontinued TKI therapy.
The authors found that CML patients in molecular remission had a significantly higher frequency of CD86+pDC expression, compared with normal donors (P less than .0024). In contrast, those who were in treatment-free remission had consistently low CD86+pDC.
These results suggest that low CD86+pDC could be predictive of treatment-free remission.
To test the hypothesis that low CD86+pDC frequencies during TKI-induced molecular remission were associated with a lower risk of molecular relapse after stopping TKI therapy, the study authors measured CD86+pDC levels in the 122 EURO-SKI patients before they stopped therapy, and then prospectively monitored them for relapse.
Findings showed that the 122 EURO-SKI patients had a significantly higher CD86+pDC frequency than did 8 healthy donors (median, 20.8% vs. 7.3%; P = .0024).
When matched with the treatment-free remission patients, the 73 patients in the EURO-SKI group who did not relapse within the first 12 months after stopping therapy had a significantly lower median frequency of CD86+pDC at baseline, compared with the 49 patients who did relapse (P = .014).
Patients who relapsed also demonstrated higher absolute CD86+pDC counts (CD86+pDC per 105 lymphocytes) at baseline (median, 86.1 vs. 50.6; P = .0147).
Based on the findings, the authors noted that they provided “for the first time evidence that relapse biology after TKI discontinuation depends on the quantity of activated pDC and a T-cell exhaustion phenotype, rather than TKI pretreatment duration per se.”
The Clinical Research Group of the German Research Foundation and the German José Carreras Leukaemia Foundation funded the study. Several of the authors report relationships with Ariad, Bristol-Myers Squibb, Novartis, and Pfizer.
FROM LEUKEMIA
Key clinical point: High CD86+pDC counts predicted relapses in CML patients who stopped TKI therapy.
Major finding: CML patients in molecular remission had significantly higher CD86+pDC frequencies, while patients in treatment-free remission had consistently low CD86+pDC.
Data source: A study that used patient cohorts (n = 14, n = 130, n = 122) at different stages of TKI discontinuation and remission.
Disclosures: The Clinical Research Group of the German Research Foundation and the German José Carreras Leukaemia Foundation funded the study. Several of the authors report relationships with Ariad, Bristol-Myers Squibb, Novartis, and Pfizer.
Scientists create online database to aid CML research
A newly launched online database provides researchers with access to data on gene expression in chronic myeloid leukemia (CML).
The LEUKomics database includes datasets relating to clinical parameters in CML, normal and leukemic stem cells, CML disease stages, treatments for the disease, and mouse models of CML.
The database is free for researchers to use and share.
The scientists who developed the database hope it will increase our understanding of CML and lead to new treatments for the disease.
“LEUKomics is a very valuable resource and could help us to reveal new underlying mechanisms that drive CML,” said Jeff Evans, Director of the Institute of Cancer Sciences at the University of Glasgow in Scotland.
“It has the potential to transform CML research on a global level, as the findings can be downloaded and shared with other researchers across the world. We also hope it inspires new research ideas and ultimately fuels a global search into finding cures for CML.”
The LEUKomics database includes datasets with information on gene expression related to:
- Clinical parameters in CML, such as disease aggressiveness and response to treatment
- Stem and progenitor cells from CML patients and healthy individuals
- Chronic, accelerated, and blast phases of CML
- CML treatment (currently only tyrosine kinase inhibitors)
- Stem and progenitor cells from mouse models of CML.
The LEUKomics database was launched by scientists at the University of Glasgow and the University of Melbourne.
The website has been built as part of the stem cell database Stemformatics, with funding from the Scottish Cancer Foundation and Bloodwise.
“Thanks to research, most patients with CML will now live a normal life by taking a single pill,” said Alasdair Rankin, Director of Research at Bloodwise in London, England.
“But treatment is life-long, and not everyone can tolerate the side effects from their treatment or may not respond and see their CML return. There remains a need to develop a permanent cure for all people with this blood cancer. LEUKomics is a highly innovative way to speed up this search for a cure and should be a valuable asset for the global blood cancer research community. We look forward to seeing its impact in the months to come.”
The LEUKomics database can be accessed at: www.stemformatics.org/leukomics. ![]()
A newly launched online database provides researchers with access to data on gene expression in chronic myeloid leukemia (CML).
The LEUKomics database includes datasets relating to clinical parameters in CML, normal and leukemic stem cells, CML disease stages, treatments for the disease, and mouse models of CML.
The database is free for researchers to use and share.
The scientists who developed the database hope it will increase our understanding of CML and lead to new treatments for the disease.
“LEUKomics is a very valuable resource and could help us to reveal new underlying mechanisms that drive CML,” said Jeff Evans, Director of the Institute of Cancer Sciences at the University of Glasgow in Scotland.
“It has the potential to transform CML research on a global level, as the findings can be downloaded and shared with other researchers across the world. We also hope it inspires new research ideas and ultimately fuels a global search into finding cures for CML.”
The LEUKomics database includes datasets with information on gene expression related to:
- Clinical parameters in CML, such as disease aggressiveness and response to treatment
- Stem and progenitor cells from CML patients and healthy individuals
- Chronic, accelerated, and blast phases of CML
- CML treatment (currently only tyrosine kinase inhibitors)
- Stem and progenitor cells from mouse models of CML.
The LEUKomics database was launched by scientists at the University of Glasgow and the University of Melbourne.
The website has been built as part of the stem cell database Stemformatics, with funding from the Scottish Cancer Foundation and Bloodwise.
“Thanks to research, most patients with CML will now live a normal life by taking a single pill,” said Alasdair Rankin, Director of Research at Bloodwise in London, England.
“But treatment is life-long, and not everyone can tolerate the side effects from their treatment or may not respond and see their CML return. There remains a need to develop a permanent cure for all people with this blood cancer. LEUKomics is a highly innovative way to speed up this search for a cure and should be a valuable asset for the global blood cancer research community. We look forward to seeing its impact in the months to come.”
The LEUKomics database can be accessed at: www.stemformatics.org/leukomics. ![]()
A newly launched online database provides researchers with access to data on gene expression in chronic myeloid leukemia (CML).
The LEUKomics database includes datasets relating to clinical parameters in CML, normal and leukemic stem cells, CML disease stages, treatments for the disease, and mouse models of CML.
The database is free for researchers to use and share.
The scientists who developed the database hope it will increase our understanding of CML and lead to new treatments for the disease.
“LEUKomics is a very valuable resource and could help us to reveal new underlying mechanisms that drive CML,” said Jeff Evans, Director of the Institute of Cancer Sciences at the University of Glasgow in Scotland.
“It has the potential to transform CML research on a global level, as the findings can be downloaded and shared with other researchers across the world. We also hope it inspires new research ideas and ultimately fuels a global search into finding cures for CML.”
The LEUKomics database includes datasets with information on gene expression related to:
- Clinical parameters in CML, such as disease aggressiveness and response to treatment
- Stem and progenitor cells from CML patients and healthy individuals
- Chronic, accelerated, and blast phases of CML
- CML treatment (currently only tyrosine kinase inhibitors)
- Stem and progenitor cells from mouse models of CML.
The LEUKomics database was launched by scientists at the University of Glasgow and the University of Melbourne.
The website has been built as part of the stem cell database Stemformatics, with funding from the Scottish Cancer Foundation and Bloodwise.
“Thanks to research, most patients with CML will now live a normal life by taking a single pill,” said Alasdair Rankin, Director of Research at Bloodwise in London, England.
“But treatment is life-long, and not everyone can tolerate the side effects from their treatment or may not respond and see their CML return. There remains a need to develop a permanent cure for all people with this blood cancer. LEUKomics is a highly innovative way to speed up this search for a cure and should be a valuable asset for the global blood cancer research community. We look forward to seeing its impact in the months to come.”
The LEUKomics database can be accessed at: www.stemformatics.org/leukomics. ![]()
VIDEO: Careful TKI hiatus makes CML pregnancy possible
NEW YORK – The success that tyrosine kinase inhibitors have had in prolonging life and producing deep hematologic and molecular remissions in patients with chronic myeloid leukemia has led to an unexpected bonus for young women living with the disease: an opportunity to safely become pregnant and mother a child.
The approach is not yet routine and poses a level of risk to both the mother and fetus, especially because tyrosine kinase inhibitors (TKIs) are teratogenic. But with careful planning, close gestational monitoring, and with support from skilled obstetricians, the scenario of a successful pregnancy in women with chronic myeloid leukemia (CML) has now played out several dozen times at a handful of U.S. centers, Mrinal S. Patnaik, MD, said in a talk at the conference held by Imedex.
“We make it clear that this is experimental and is associated with risk, and we share the data [from case reports]; but if the woman wants to go forward,” a protocol now exists “to successfully get them to pregnancy,” said Dr. Patnaik, a hematologist oncologist at the Mayo Clinic in Rochester, Minn.
At Mayo alone, upwards of 20 women with CML have been successfully shepherded through pregnancy, he said in a video interview.
The prospect for a planned pregnancy is reserved for women with their CML well controlled for at least 2 years using a TKI, most often imatinib (Gleevec). In addition to being under complete hematologic control, the candidate patient must also show a deep molecular response, which means a blood level of the BRC-ABL tyrosine kinase that drives CML at least 4 or 4.5 logs (10,000-50,000-fold) below pretreatment levels or molecularly undetectable.
The patient then monitors her ovulatory cycle and stops her medication at the time of ovulation, attempts conception, and then monitors whether pregnancy has actually started. If it has, she needs to stay off her TKI regimen through at least the first 18 weeks of gestation, although an even longer drug holiday is preferred. If not, she resumes the medication and repeats the process later if she wants.
Once the women is pregnant and remains off her TKI regimen Dr. Patnaik and his associates closely follow the woman for signs of a molecular or hematologic relapse, although the latter are unusual. If a resurgence of CML stem cells occurs, the woman receives treatment with pegylated interferon-alpha, which is safe during pregnancy. When possible, TKI treatment remains on hold into the breast-feeding period.
During pregnancy and delivery, the patient requires careful and regular follow-up by a maternal-fetal medicine specialist and has an ongoing risk for high platelet counts causing placental blood clots, fetuses that are small for gestational age, preterm labor, premature rupture of membranes, and other complications.
“These are manageable with good obstetrical care,” Dr. Patnaik said. “We have developed a good system to work out the obstetrical complications.
“By and large we can be successful, but it requires a lot of monitoring and a lot of patient compliance with regular follow-ups,” he stressed.
In a video interview at the meeting, Dr. Patnaik discussed the approach he takes with his patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
NEW YORK – The success that tyrosine kinase inhibitors have had in prolonging life and producing deep hematologic and molecular remissions in patients with chronic myeloid leukemia has led to an unexpected bonus for young women living with the disease: an opportunity to safely become pregnant and mother a child.
The approach is not yet routine and poses a level of risk to both the mother and fetus, especially because tyrosine kinase inhibitors (TKIs) are teratogenic. But with careful planning, close gestational monitoring, and with support from skilled obstetricians, the scenario of a successful pregnancy in women with chronic myeloid leukemia (CML) has now played out several dozen times at a handful of U.S. centers, Mrinal S. Patnaik, MD, said in a talk at the conference held by Imedex.
“We make it clear that this is experimental and is associated with risk, and we share the data [from case reports]; but if the woman wants to go forward,” a protocol now exists “to successfully get them to pregnancy,” said Dr. Patnaik, a hematologist oncologist at the Mayo Clinic in Rochester, Minn.
At Mayo alone, upwards of 20 women with CML have been successfully shepherded through pregnancy, he said in a video interview.
The prospect for a planned pregnancy is reserved for women with their CML well controlled for at least 2 years using a TKI, most often imatinib (Gleevec). In addition to being under complete hematologic control, the candidate patient must also show a deep molecular response, which means a blood level of the BRC-ABL tyrosine kinase that drives CML at least 4 or 4.5 logs (10,000-50,000-fold) below pretreatment levels or molecularly undetectable.
The patient then monitors her ovulatory cycle and stops her medication at the time of ovulation, attempts conception, and then monitors whether pregnancy has actually started. If it has, she needs to stay off her TKI regimen through at least the first 18 weeks of gestation, although an even longer drug holiday is preferred. If not, she resumes the medication and repeats the process later if she wants.
Once the women is pregnant and remains off her TKI regimen Dr. Patnaik and his associates closely follow the woman for signs of a molecular or hematologic relapse, although the latter are unusual. If a resurgence of CML stem cells occurs, the woman receives treatment with pegylated interferon-alpha, which is safe during pregnancy. When possible, TKI treatment remains on hold into the breast-feeding period.
During pregnancy and delivery, the patient requires careful and regular follow-up by a maternal-fetal medicine specialist and has an ongoing risk for high platelet counts causing placental blood clots, fetuses that are small for gestational age, preterm labor, premature rupture of membranes, and other complications.
“These are manageable with good obstetrical care,” Dr. Patnaik said. “We have developed a good system to work out the obstetrical complications.
“By and large we can be successful, but it requires a lot of monitoring and a lot of patient compliance with regular follow-ups,” he stressed.
In a video interview at the meeting, Dr. Patnaik discussed the approach he takes with his patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
NEW YORK – The success that tyrosine kinase inhibitors have had in prolonging life and producing deep hematologic and molecular remissions in patients with chronic myeloid leukemia has led to an unexpected bonus for young women living with the disease: an opportunity to safely become pregnant and mother a child.
The approach is not yet routine and poses a level of risk to both the mother and fetus, especially because tyrosine kinase inhibitors (TKIs) are teratogenic. But with careful planning, close gestational monitoring, and with support from skilled obstetricians, the scenario of a successful pregnancy in women with chronic myeloid leukemia (CML) has now played out several dozen times at a handful of U.S. centers, Mrinal S. Patnaik, MD, said in a talk at the conference held by Imedex.
“We make it clear that this is experimental and is associated with risk, and we share the data [from case reports]; but if the woman wants to go forward,” a protocol now exists “to successfully get them to pregnancy,” said Dr. Patnaik, a hematologist oncologist at the Mayo Clinic in Rochester, Minn.
At Mayo alone, upwards of 20 women with CML have been successfully shepherded through pregnancy, he said in a video interview.
The prospect for a planned pregnancy is reserved for women with their CML well controlled for at least 2 years using a TKI, most often imatinib (Gleevec). In addition to being under complete hematologic control, the candidate patient must also show a deep molecular response, which means a blood level of the BRC-ABL tyrosine kinase that drives CML at least 4 or 4.5 logs (10,000-50,000-fold) below pretreatment levels or molecularly undetectable.
The patient then monitors her ovulatory cycle and stops her medication at the time of ovulation, attempts conception, and then monitors whether pregnancy has actually started. If it has, she needs to stay off her TKI regimen through at least the first 18 weeks of gestation, although an even longer drug holiday is preferred. If not, she resumes the medication and repeats the process later if she wants.
Once the women is pregnant and remains off her TKI regimen Dr. Patnaik and his associates closely follow the woman for signs of a molecular or hematologic relapse, although the latter are unusual. If a resurgence of CML stem cells occurs, the woman receives treatment with pegylated interferon-alpha, which is safe during pregnancy. When possible, TKI treatment remains on hold into the breast-feeding period.
During pregnancy and delivery, the patient requires careful and regular follow-up by a maternal-fetal medicine specialist and has an ongoing risk for high platelet counts causing placental blood clots, fetuses that are small for gestational age, preterm labor, premature rupture of membranes, and other complications.
“These are manageable with good obstetrical care,” Dr. Patnaik said. “We have developed a good system to work out the obstetrical complications.
“By and large we can be successful, but it requires a lot of monitoring and a lot of patient compliance with regular follow-ups,” he stressed.
In a video interview at the meeting, Dr. Patnaik discussed the approach he takes with his patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM A MEETING ON HEMATOLOGIC MALIGNANCIES
Proteins may be therapeutic targets for TKI-resistant CML, ALL
Researchers say they have identified 2 signaling proteins that enable resistance to tyrosine kinase inhibitors (TKIs) and could be therapeutic targets for acute lymphoblastic leukemia (ALL) and chronic myeloid leukemia (CML).
The team found that by deleting these proteins—c-Fos and Dusp1—they could eradicate BCR-ABL-induced B-cell ALL in mice.
And treatment combining c-Fos and Dusp1 inhibitors with the TKI imatinib was able to cure mice with BCR-ABL-driven CML.
The researchers reported these findings in Nature Medicine.
“We think that, within the next 5 years, our data will change the way people think about cancer development and targeted therapy,” said study author Mohammad Azam, PhD, of Cincinnati Children’s Hospital Medical Center in Ohio.
“This study identifies a potential Achilles’ heel of kinase-driven cancers, and what we propose is intended to be curative, not just treatment.”
The potential Achilles’ heel is a common point of passage in cells—a signaling node—that appears to be required to generate cancer cells. The node is formed by the signaling proteins c-Fos and Dusp1, according to the researchers.
The team identified c-Fos and Dusp1 by conducting global gene-expression analysis of mouse leukemia cells and human CML cells. Analysis of the human cells revealed extremely high levels of c-FOS and DUSP1 in BCR-ABL-positive, TKI-resistant cells.
Dr Azam and his colleagues found that signaling from tyrosine kinase and growth factor proteins that support cell expansion (such as IL-3 and IL-6) converge to elevate c-Fos and Dusp1 levels in leukemia cells.
Working together, these molecules maintain the survival of leukemia stem cells (LSCs), which translates to minimal residual disease (MRD) after treatment.
Dr Azam said Dusp1 and c-Fos support the survival of LSCs by increasing the toxic threshold needed to kill them. This means imatinib and other TKIs cannot eliminate the residual LSCs.
After describing the roles of c-Fos and Dusp1, Dr Azam and his colleagues put their ideas to the test in mouse models of CML.
The team tested several treatments in these mice, including:
- monotherapy with imatinib
- inhibitors of c-Fos and Dusp1
- treatment with imatinib and inhibitors of c-Fos and Dusp1.
As suspected, treatment with imatinib alone initially stopped CML progression, but mice ultimately relapsed.
Treatment with c-Fos and Dusp1 inhibitors significantly slowed CML progression and prolonged survival in a majority of mice, but this treatment wasn’t curative.
However, a month of treatment with c-Fos and Dusp1 inhibitors as well as imatinib cured about 90% of mice with CML, and there were no signs of MRD.
The researchers also found that simply deleting c-Fos and Dusp1 was sufficient to block the development of B-cell ALL in mice.
The team said they are following up this study by testing c-Fos and Dusp1 as treatment targets for different kinase-fueled cancers. ![]()
Researchers say they have identified 2 signaling proteins that enable resistance to tyrosine kinase inhibitors (TKIs) and could be therapeutic targets for acute lymphoblastic leukemia (ALL) and chronic myeloid leukemia (CML).
The team found that by deleting these proteins—c-Fos and Dusp1—they could eradicate BCR-ABL-induced B-cell ALL in mice.
And treatment combining c-Fos and Dusp1 inhibitors with the TKI imatinib was able to cure mice with BCR-ABL-driven CML.
The researchers reported these findings in Nature Medicine.
“We think that, within the next 5 years, our data will change the way people think about cancer development and targeted therapy,” said study author Mohammad Azam, PhD, of Cincinnati Children’s Hospital Medical Center in Ohio.
“This study identifies a potential Achilles’ heel of kinase-driven cancers, and what we propose is intended to be curative, not just treatment.”
The potential Achilles’ heel is a common point of passage in cells—a signaling node—that appears to be required to generate cancer cells. The node is formed by the signaling proteins c-Fos and Dusp1, according to the researchers.
The team identified c-Fos and Dusp1 by conducting global gene-expression analysis of mouse leukemia cells and human CML cells. Analysis of the human cells revealed extremely high levels of c-FOS and DUSP1 in BCR-ABL-positive, TKI-resistant cells.
Dr Azam and his colleagues found that signaling from tyrosine kinase and growth factor proteins that support cell expansion (such as IL-3 and IL-6) converge to elevate c-Fos and Dusp1 levels in leukemia cells.
Working together, these molecules maintain the survival of leukemia stem cells (LSCs), which translates to minimal residual disease (MRD) after treatment.
Dr Azam said Dusp1 and c-Fos support the survival of LSCs by increasing the toxic threshold needed to kill them. This means imatinib and other TKIs cannot eliminate the residual LSCs.
After describing the roles of c-Fos and Dusp1, Dr Azam and his colleagues put their ideas to the test in mouse models of CML.
The team tested several treatments in these mice, including:
- monotherapy with imatinib
- inhibitors of c-Fos and Dusp1
- treatment with imatinib and inhibitors of c-Fos and Dusp1.
As suspected, treatment with imatinib alone initially stopped CML progression, but mice ultimately relapsed.
Treatment with c-Fos and Dusp1 inhibitors significantly slowed CML progression and prolonged survival in a majority of mice, but this treatment wasn’t curative.
However, a month of treatment with c-Fos and Dusp1 inhibitors as well as imatinib cured about 90% of mice with CML, and there were no signs of MRD.
The researchers also found that simply deleting c-Fos and Dusp1 was sufficient to block the development of B-cell ALL in mice.
The team said they are following up this study by testing c-Fos and Dusp1 as treatment targets for different kinase-fueled cancers. ![]()
Researchers say they have identified 2 signaling proteins that enable resistance to tyrosine kinase inhibitors (TKIs) and could be therapeutic targets for acute lymphoblastic leukemia (ALL) and chronic myeloid leukemia (CML).
The team found that by deleting these proteins—c-Fos and Dusp1—they could eradicate BCR-ABL-induced B-cell ALL in mice.
And treatment combining c-Fos and Dusp1 inhibitors with the TKI imatinib was able to cure mice with BCR-ABL-driven CML.
The researchers reported these findings in Nature Medicine.
“We think that, within the next 5 years, our data will change the way people think about cancer development and targeted therapy,” said study author Mohammad Azam, PhD, of Cincinnati Children’s Hospital Medical Center in Ohio.
“This study identifies a potential Achilles’ heel of kinase-driven cancers, and what we propose is intended to be curative, not just treatment.”
The potential Achilles’ heel is a common point of passage in cells—a signaling node—that appears to be required to generate cancer cells. The node is formed by the signaling proteins c-Fos and Dusp1, according to the researchers.
The team identified c-Fos and Dusp1 by conducting global gene-expression analysis of mouse leukemia cells and human CML cells. Analysis of the human cells revealed extremely high levels of c-FOS and DUSP1 in BCR-ABL-positive, TKI-resistant cells.
Dr Azam and his colleagues found that signaling from tyrosine kinase and growth factor proteins that support cell expansion (such as IL-3 and IL-6) converge to elevate c-Fos and Dusp1 levels in leukemia cells.
Working together, these molecules maintain the survival of leukemia stem cells (LSCs), which translates to minimal residual disease (MRD) after treatment.
Dr Azam said Dusp1 and c-Fos support the survival of LSCs by increasing the toxic threshold needed to kill them. This means imatinib and other TKIs cannot eliminate the residual LSCs.
After describing the roles of c-Fos and Dusp1, Dr Azam and his colleagues put their ideas to the test in mouse models of CML.
The team tested several treatments in these mice, including:
- monotherapy with imatinib
- inhibitors of c-Fos and Dusp1
- treatment with imatinib and inhibitors of c-Fos and Dusp1.
As suspected, treatment with imatinib alone initially stopped CML progression, but mice ultimately relapsed.
Treatment with c-Fos and Dusp1 inhibitors significantly slowed CML progression and prolonged survival in a majority of mice, but this treatment wasn’t curative.
However, a month of treatment with c-Fos and Dusp1 inhibitors as well as imatinib cured about 90% of mice with CML, and there were no signs of MRD.
The researchers also found that simply deleting c-Fos and Dusp1 was sufficient to block the development of B-cell ALL in mice.
The team said they are following up this study by testing c-Fos and Dusp1 as treatment targets for different kinase-fueled cancers. ![]()
Imatinib is safe, effective long-term, team says
Long-term follow-up of patients treated with imatinib suggests the drug can remain effective beyond 10 years and does not confer “unacceptable” cumulative toxicity, according to researchers.
The group evaluated data on patients who had newly diagnosed, chronic-phase chronic myeloid leukemia (CML) when they began treatment with imatinib.
The median treatment duration was 8.9 years, and the estimated 10-year survival rate ranged from 64.4% to 84.4%.
The researchers said serious adverse events (AEs) thought to be related to imatinib were uncommon and typically occurred early, within the first year of treatment.
These results were reported in NEJM. The research was funded by Novartis Pharmaceuticals, which markets imatinib as Gleevec.
“The long-term success of this treatment confirms the remarkable success we’ve seen since the very first Gleevec trials,” said study author Brian Druker, MD, a physician-scientist at Oregon Health & Science University in Portland, Oregon, who led the original clinical development of Gleevec.
“This study reinforces the notion that we can create effective and non-toxic therapies.”
The study enrolled 1106 newly diagnosed, chronic-phase CML patients at 177 cancer centers in more than 16 countries. Half were assigned to treatment with imatinib (n=533) and the other half to interferon alfa plus cytarabine.
This study allowed for cross-over between the treatment arms, and 65.6% of patients in the cytarabine/interferon alfa arm ultimately crossed over to the imatinib arm.
However, when assessing the effects of imatinib, the researchers focused only on the patients who were first randomized to receive imatinib.
The median follow-up was 10.9 years (range, 0 to 11.7, which included follow-up after patients discontinued study treatment).
Of the patients randomized to imatinib, 48.3% (n=267) completed treatment with the drug. The median duration of first-line imatinib was 8.9 years (range, <0.1 to 11.7).
For patients who did not complete imatinib treatment, reasons for discontinuation included a lack of efficacy (15.9%), withdrawn consent (10.3%), AEs (6.9%), because they proceeded to transplant (3.8%), death (3.4%), protocol violation (3.1%), loss to follow-up (2.7%), cross over to the interferon arm (2.5%), administrative problems (2.2%), abnormal laboratory values (0.5%), or abnormal procedure (0.4%).
Safety
The incidence of serious AEs considered related to imatinib was 9.3% (51/551).
Drug-related serious AEs occurring in at least 2 patients included abdominal pain (n=4), anemia (n=3), congestive cardiac failure (n=3), gastrointestinal hemorrhage (n=3), vomiting (n=3), alanine aminotransferase increase (n=2), cardiac arrest (n=2), conjunctival hemorrhage (n=2), and melana (n=2).
Six patients had a second neoplasm (benign, malignant, or unspecified).
Response
The cumulative rate of complete cytogenetic response (CCR) at the end of the trial was 82.8%.
In the intent-to-treat population, the rate of CCR went from 52.8% in the first year to 22.2% at year 10.
Among evaluable patients, the rate of CCR went from 70.9% (292/412) in the first year to 91.8% (123/134) in year 10.
In the intent-to-treat population, the rate of major molecular response went from 27.7% in the first year to 34.4% at year 10.
Among evaluable patients, the rate of major molecular response went from 50.2% (153/305) in the first year to 93.1% (190/204) in year 10.
Progression and survival
The rate of progression was 6.9% (38/553) in the intent-to-treat population. Most of these patients (n=34) progressed during the first 4 years.
There were 260 patients who were still alive and receiving imatinib at 10 years and 96 patients who were alive but not receiving imatinib.
The researchers did not know the survival status of 111 patients, and there were 86 known deaths at 10 years (89 by the end of the study).
The estimated 10-year survival rate ranged from 64.4% (assuming all 111 patients with unknown status had died) to 84.4% (assuming all 111 were alive).
The cause of death was CML in 50 patients, a secondary malignant condition in 11, a cardiac disorder/cardiovascular disease in 7, infectious disease in 5, and “other” causes in 16 patients. ![]()
Long-term follow-up of patients treated with imatinib suggests the drug can remain effective beyond 10 years and does not confer “unacceptable” cumulative toxicity, according to researchers.
The group evaluated data on patients who had newly diagnosed, chronic-phase chronic myeloid leukemia (CML) when they began treatment with imatinib.
The median treatment duration was 8.9 years, and the estimated 10-year survival rate ranged from 64.4% to 84.4%.
The researchers said serious adverse events (AEs) thought to be related to imatinib were uncommon and typically occurred early, within the first year of treatment.
These results were reported in NEJM. The research was funded by Novartis Pharmaceuticals, which markets imatinib as Gleevec.
“The long-term success of this treatment confirms the remarkable success we’ve seen since the very first Gleevec trials,” said study author Brian Druker, MD, a physician-scientist at Oregon Health & Science University in Portland, Oregon, who led the original clinical development of Gleevec.
“This study reinforces the notion that we can create effective and non-toxic therapies.”
The study enrolled 1106 newly diagnosed, chronic-phase CML patients at 177 cancer centers in more than 16 countries. Half were assigned to treatment with imatinib (n=533) and the other half to interferon alfa plus cytarabine.
This study allowed for cross-over between the treatment arms, and 65.6% of patients in the cytarabine/interferon alfa arm ultimately crossed over to the imatinib arm.
However, when assessing the effects of imatinib, the researchers focused only on the patients who were first randomized to receive imatinib.
The median follow-up was 10.9 years (range, 0 to 11.7, which included follow-up after patients discontinued study treatment).
Of the patients randomized to imatinib, 48.3% (n=267) completed treatment with the drug. The median duration of first-line imatinib was 8.9 years (range, <0.1 to 11.7).
For patients who did not complete imatinib treatment, reasons for discontinuation included a lack of efficacy (15.9%), withdrawn consent (10.3%), AEs (6.9%), because they proceeded to transplant (3.8%), death (3.4%), protocol violation (3.1%), loss to follow-up (2.7%), cross over to the interferon arm (2.5%), administrative problems (2.2%), abnormal laboratory values (0.5%), or abnormal procedure (0.4%).
Safety
The incidence of serious AEs considered related to imatinib was 9.3% (51/551).
Drug-related serious AEs occurring in at least 2 patients included abdominal pain (n=4), anemia (n=3), congestive cardiac failure (n=3), gastrointestinal hemorrhage (n=3), vomiting (n=3), alanine aminotransferase increase (n=2), cardiac arrest (n=2), conjunctival hemorrhage (n=2), and melana (n=2).
Six patients had a second neoplasm (benign, malignant, or unspecified).
Response
The cumulative rate of complete cytogenetic response (CCR) at the end of the trial was 82.8%.
In the intent-to-treat population, the rate of CCR went from 52.8% in the first year to 22.2% at year 10.
Among evaluable patients, the rate of CCR went from 70.9% (292/412) in the first year to 91.8% (123/134) in year 10.
In the intent-to-treat population, the rate of major molecular response went from 27.7% in the first year to 34.4% at year 10.
Among evaluable patients, the rate of major molecular response went from 50.2% (153/305) in the first year to 93.1% (190/204) in year 10.
Progression and survival
The rate of progression was 6.9% (38/553) in the intent-to-treat population. Most of these patients (n=34) progressed during the first 4 years.
There were 260 patients who were still alive and receiving imatinib at 10 years and 96 patients who were alive but not receiving imatinib.
The researchers did not know the survival status of 111 patients, and there were 86 known deaths at 10 years (89 by the end of the study).
The estimated 10-year survival rate ranged from 64.4% (assuming all 111 patients with unknown status had died) to 84.4% (assuming all 111 were alive).
The cause of death was CML in 50 patients, a secondary malignant condition in 11, a cardiac disorder/cardiovascular disease in 7, infectious disease in 5, and “other” causes in 16 patients. ![]()
Long-term follow-up of patients treated with imatinib suggests the drug can remain effective beyond 10 years and does not confer “unacceptable” cumulative toxicity, according to researchers.
The group evaluated data on patients who had newly diagnosed, chronic-phase chronic myeloid leukemia (CML) when they began treatment with imatinib.
The median treatment duration was 8.9 years, and the estimated 10-year survival rate ranged from 64.4% to 84.4%.
The researchers said serious adverse events (AEs) thought to be related to imatinib were uncommon and typically occurred early, within the first year of treatment.
These results were reported in NEJM. The research was funded by Novartis Pharmaceuticals, which markets imatinib as Gleevec.
“The long-term success of this treatment confirms the remarkable success we’ve seen since the very first Gleevec trials,” said study author Brian Druker, MD, a physician-scientist at Oregon Health & Science University in Portland, Oregon, who led the original clinical development of Gleevec.
“This study reinforces the notion that we can create effective and non-toxic therapies.”
The study enrolled 1106 newly diagnosed, chronic-phase CML patients at 177 cancer centers in more than 16 countries. Half were assigned to treatment with imatinib (n=533) and the other half to interferon alfa plus cytarabine.
This study allowed for cross-over between the treatment arms, and 65.6% of patients in the cytarabine/interferon alfa arm ultimately crossed over to the imatinib arm.
However, when assessing the effects of imatinib, the researchers focused only on the patients who were first randomized to receive imatinib.
The median follow-up was 10.9 years (range, 0 to 11.7, which included follow-up after patients discontinued study treatment).
Of the patients randomized to imatinib, 48.3% (n=267) completed treatment with the drug. The median duration of first-line imatinib was 8.9 years (range, <0.1 to 11.7).
For patients who did not complete imatinib treatment, reasons for discontinuation included a lack of efficacy (15.9%), withdrawn consent (10.3%), AEs (6.9%), because they proceeded to transplant (3.8%), death (3.4%), protocol violation (3.1%), loss to follow-up (2.7%), cross over to the interferon arm (2.5%), administrative problems (2.2%), abnormal laboratory values (0.5%), or abnormal procedure (0.4%).
Safety
The incidence of serious AEs considered related to imatinib was 9.3% (51/551).
Drug-related serious AEs occurring in at least 2 patients included abdominal pain (n=4), anemia (n=3), congestive cardiac failure (n=3), gastrointestinal hemorrhage (n=3), vomiting (n=3), alanine aminotransferase increase (n=2), cardiac arrest (n=2), conjunctival hemorrhage (n=2), and melana (n=2).
Six patients had a second neoplasm (benign, malignant, or unspecified).
Response
The cumulative rate of complete cytogenetic response (CCR) at the end of the trial was 82.8%.
In the intent-to-treat population, the rate of CCR went from 52.8% in the first year to 22.2% at year 10.
Among evaluable patients, the rate of CCR went from 70.9% (292/412) in the first year to 91.8% (123/134) in year 10.
In the intent-to-treat population, the rate of major molecular response went from 27.7% in the first year to 34.4% at year 10.
Among evaluable patients, the rate of major molecular response went from 50.2% (153/305) in the first year to 93.1% (190/204) in year 10.
Progression and survival
The rate of progression was 6.9% (38/553) in the intent-to-treat population. Most of these patients (n=34) progressed during the first 4 years.
There were 260 patients who were still alive and receiving imatinib at 10 years and 96 patients who were alive but not receiving imatinib.
The researchers did not know the survival status of 111 patients, and there were 86 known deaths at 10 years (89 by the end of the study).
The estimated 10-year survival rate ranged from 64.4% (assuming all 111 patients with unknown status had died) to 84.4% (assuming all 111 were alive).
The cause of death was CML in 50 patients, a secondary malignant condition in 11, a cardiac disorder/cardiovascular disease in 7, infectious disease in 5, and “other” causes in 16 patients. ![]()
In CML, imatinib benefits persist at 10 years
In patients with chronic myeloid leukemia (CML), the safety and benefits of imatinib persisted over the course of 10 years in a follow-up study reported online March 9 in the New England Journal of Medicine.
The initial results of the phase III International Randomized Study of Interferon and ST1571 (IRIS) trial “fundamentally changed CML treatment and led to marked improvements in prognosis for patients” when imatinib was shown more effective than interferon plus cytarabine among newly diagnosed patients in the chronic phase of the disease, said Andreas Hochhaus, MD, of the Klinik für Innere Medizin II, Universitätsklinikum Jena (Germany), and his associates. The researchers are now reporting the final follow-up results after a median of 10.9 years for the 553 patients who had been randomly assigned to receive daily oral imatinib in the IRIS trial.
The high rate of crossover to the imatinib group among IRIS study participants precluded a direct comparison of overall survival between the two study groups at 10 years. However, the estimated overall 10-year survival in the imatinib group alone was 83.3%. “A total of 260 patients (47.0%) were alive and still receiving study treatment at 10 years, 96 patients (17.4%) were alive and not receiving study treatment, 86 known deaths (15.6% of patients) had occurred, and 11 patients (20.1%) had unknown survival status,” Dr. Hochhaus and his associates reported (N Engl J Med. 2017 Mar 9. doi: 10.1056/NEJMoa1609324).
Approximately 9% of those in the imatinib group had a serious adverse event during follow-up, including 4 patients (0.7%) in whom the event was considered to be related to the drug. Most occurred during the first year of treatment and declined over time. No new safety signals were observed after the 5-year follow-up.
“These results highlight the safety and efficacy of imatinib therapy, with a clear improvement over the outcomes that were expected n patients who received a diagnosis of CML before the introduction of tyrosine kinase inhibitor therapy, when interferon alfa and hematopoietic stem-cell transplantation were the standard therapies,” the investigators said.
Second-generation tyrosine kinase inhibitors have been developed since the IRIS trial was begun, and “it remains to be seen whether they will have similarly favorable long-term safety. Given the long-term safety and efficacy results with imatinib and the increasing availability of generic imatinib, comparative analyses evaluating the available tyrosine kinase inhibitors for first-line therapy are likely to be forthcoming,” they noted.
In patients with chronic myeloid leukemia (CML), the safety and benefits of imatinib persisted over the course of 10 years in a follow-up study reported online March 9 in the New England Journal of Medicine.
The initial results of the phase III International Randomized Study of Interferon and ST1571 (IRIS) trial “fundamentally changed CML treatment and led to marked improvements in prognosis for patients” when imatinib was shown more effective than interferon plus cytarabine among newly diagnosed patients in the chronic phase of the disease, said Andreas Hochhaus, MD, of the Klinik für Innere Medizin II, Universitätsklinikum Jena (Germany), and his associates. The researchers are now reporting the final follow-up results after a median of 10.9 years for the 553 patients who had been randomly assigned to receive daily oral imatinib in the IRIS trial.
The high rate of crossover to the imatinib group among IRIS study participants precluded a direct comparison of overall survival between the two study groups at 10 years. However, the estimated overall 10-year survival in the imatinib group alone was 83.3%. “A total of 260 patients (47.0%) were alive and still receiving study treatment at 10 years, 96 patients (17.4%) were alive and not receiving study treatment, 86 known deaths (15.6% of patients) had occurred, and 11 patients (20.1%) had unknown survival status,” Dr. Hochhaus and his associates reported (N Engl J Med. 2017 Mar 9. doi: 10.1056/NEJMoa1609324).
Approximately 9% of those in the imatinib group had a serious adverse event during follow-up, including 4 patients (0.7%) in whom the event was considered to be related to the drug. Most occurred during the first year of treatment and declined over time. No new safety signals were observed after the 5-year follow-up.
“These results highlight the safety and efficacy of imatinib therapy, with a clear improvement over the outcomes that were expected n patients who received a diagnosis of CML before the introduction of tyrosine kinase inhibitor therapy, when interferon alfa and hematopoietic stem-cell transplantation were the standard therapies,” the investigators said.
Second-generation tyrosine kinase inhibitors have been developed since the IRIS trial was begun, and “it remains to be seen whether they will have similarly favorable long-term safety. Given the long-term safety and efficacy results with imatinib and the increasing availability of generic imatinib, comparative analyses evaluating the available tyrosine kinase inhibitors for first-line therapy are likely to be forthcoming,” they noted.
In patients with chronic myeloid leukemia (CML), the safety and benefits of imatinib persisted over the course of 10 years in a follow-up study reported online March 9 in the New England Journal of Medicine.
The initial results of the phase III International Randomized Study of Interferon and ST1571 (IRIS) trial “fundamentally changed CML treatment and led to marked improvements in prognosis for patients” when imatinib was shown more effective than interferon plus cytarabine among newly diagnosed patients in the chronic phase of the disease, said Andreas Hochhaus, MD, of the Klinik für Innere Medizin II, Universitätsklinikum Jena (Germany), and his associates. The researchers are now reporting the final follow-up results after a median of 10.9 years for the 553 patients who had been randomly assigned to receive daily oral imatinib in the IRIS trial.
The high rate of crossover to the imatinib group among IRIS study participants precluded a direct comparison of overall survival between the two study groups at 10 years. However, the estimated overall 10-year survival in the imatinib group alone was 83.3%. “A total of 260 patients (47.0%) were alive and still receiving study treatment at 10 years, 96 patients (17.4%) were alive and not receiving study treatment, 86 known deaths (15.6% of patients) had occurred, and 11 patients (20.1%) had unknown survival status,” Dr. Hochhaus and his associates reported (N Engl J Med. 2017 Mar 9. doi: 10.1056/NEJMoa1609324).
Approximately 9% of those in the imatinib group had a serious adverse event during follow-up, including 4 patients (0.7%) in whom the event was considered to be related to the drug. Most occurred during the first year of treatment and declined over time. No new safety signals were observed after the 5-year follow-up.
“These results highlight the safety and efficacy of imatinib therapy, with a clear improvement over the outcomes that were expected n patients who received a diagnosis of CML before the introduction of tyrosine kinase inhibitor therapy, when interferon alfa and hematopoietic stem-cell transplantation were the standard therapies,” the investigators said.
Second-generation tyrosine kinase inhibitors have been developed since the IRIS trial was begun, and “it remains to be seen whether they will have similarly favorable long-term safety. Given the long-term safety and efficacy results with imatinib and the increasing availability of generic imatinib, comparative analyses evaluating the available tyrosine kinase inhibitors for first-line therapy are likely to be forthcoming,” they noted.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Imatinib’s benefits in chronic myeloid leukemia and its safety profile persisted over the long term in a 10-year follow-up study.
Major finding: The estimated overall 10-year survival was 83.3%, and no new safety signals were observed after 5-year follow-up.
Data source: Extended follow-up of an open-label international randomized trial involving 553 adults with CML.
Disclosures: This study was funded by Novartis. Dr. Hochhaus reported ties to Novartis and other drug companies.
Acquired mutations may compromise assay
A newly discovered issue with the Ba/F3 transformation assay could “jeopardize attempts to characterize the signaling mechanisms and drug sensitivities of leukemic oncogenes,” researchers reported in Oncotarget.
The researchers said the assay “remains an invaluable tool” for validating activating mutations in primary leukemias.
However, the assay is prone to a previously unreported flaw, where Ba/F3 cells can acquire additional mutations.
This issue was discovered by Kevin Watanabe-Smith, PhD, of Oregon Health & Science University in Portland.
He identified the problem while studying a growth-activating mutation in a patient with T-cell leukemia. (The results of that research were published in Leukemia in April 2016).
“When I was sequencing the patient’s DNA to make sure the original, known mutation is there, I was finding additional, unexpected mutations in the gene that I didn’t put there,” Dr Watanabe-Smith said. “And I was getting different mutations every time.”
“After we saw this in several cases, we knew it was worth further study,” added Cristina Tognon, PhD, of Oregon Health & Science University.
So the researchers decided to study Ba/F3 cell lines with and without 4 mutations (found in 3 cytokine receptors) that have “known transformative capacity,” including:
- CSF2RB R461C, a germline mutation found in a patient with T-cell acute lymphoblastic leukemia (ALL)
- CSF3R T618I, a mutation found in chronic neutrophilic leukemia (CNL) and atypical chronic myelogenous leukemia (aCML)
- CSF3R W791X, also found in CNL and aCML
- IL7R 243InsPPCL, which was found in a patient with B-cell ALL.
The researchers said they observed acquired mutations in 1 of 3 CSF2RB wild-type cell lines that transformed and all 4 CSF3R wild-type cell lines, which all transformed.
Furthermore, most CSF2RB R461C lines (4/5) and CSF3R W791X lines (3/4) had additional acquired mutations. However, the CSF3R T618I lines and the IL7R 243InsPPCL lines did not contain acquired mutations.
The researchers said acquired mutations were observed only in weakly transforming oncogenes, which were defined as mutations with a weaker ability to transform cells (less than 1 in every 200 cells) or a slower time to outgrowth (5 days or longer to reach a 5-fold increase over the initial cell number).
The team also said their findings indicate that the majority of the acquired mutations likely exist before IL-3 withdrawal but only expand to levels detectable by Sanger sequencing in the absence of IL-3.
“The potential impact [of these acquired mutations] is that a non-functional mutation could appear functional, and a researcher could publish results that would not be reproducible,” Dr Watanabe-Smith said.
To overcome this problem, Dr Watanabe-Smith and his colleagues recommended taking an additional step when using the Ba/F3 assay—sequencing outgrown cell lines. They also said additional research is needed to devise methods that reduce the incidence of acquired mutations in such assays. ![]()
A newly discovered issue with the Ba/F3 transformation assay could “jeopardize attempts to characterize the signaling mechanisms and drug sensitivities of leukemic oncogenes,” researchers reported in Oncotarget.
The researchers said the assay “remains an invaluable tool” for validating activating mutations in primary leukemias.
However, the assay is prone to a previously unreported flaw, where Ba/F3 cells can acquire additional mutations.
This issue was discovered by Kevin Watanabe-Smith, PhD, of Oregon Health & Science University in Portland.
He identified the problem while studying a growth-activating mutation in a patient with T-cell leukemia. (The results of that research were published in Leukemia in April 2016).
“When I was sequencing the patient’s DNA to make sure the original, known mutation is there, I was finding additional, unexpected mutations in the gene that I didn’t put there,” Dr Watanabe-Smith said. “And I was getting different mutations every time.”
“After we saw this in several cases, we knew it was worth further study,” added Cristina Tognon, PhD, of Oregon Health & Science University.
So the researchers decided to study Ba/F3 cell lines with and without 4 mutations (found in 3 cytokine receptors) that have “known transformative capacity,” including:
- CSF2RB R461C, a germline mutation found in a patient with T-cell acute lymphoblastic leukemia (ALL)
- CSF3R T618I, a mutation found in chronic neutrophilic leukemia (CNL) and atypical chronic myelogenous leukemia (aCML)
- CSF3R W791X, also found in CNL and aCML
- IL7R 243InsPPCL, which was found in a patient with B-cell ALL.
The researchers said they observed acquired mutations in 1 of 3 CSF2RB wild-type cell lines that transformed and all 4 CSF3R wild-type cell lines, which all transformed.
Furthermore, most CSF2RB R461C lines (4/5) and CSF3R W791X lines (3/4) had additional acquired mutations. However, the CSF3R T618I lines and the IL7R 243InsPPCL lines did not contain acquired mutations.
The researchers said acquired mutations were observed only in weakly transforming oncogenes, which were defined as mutations with a weaker ability to transform cells (less than 1 in every 200 cells) or a slower time to outgrowth (5 days or longer to reach a 5-fold increase over the initial cell number).
The team also said their findings indicate that the majority of the acquired mutations likely exist before IL-3 withdrawal but only expand to levels detectable by Sanger sequencing in the absence of IL-3.
“The potential impact [of these acquired mutations] is that a non-functional mutation could appear functional, and a researcher could publish results that would not be reproducible,” Dr Watanabe-Smith said.
To overcome this problem, Dr Watanabe-Smith and his colleagues recommended taking an additional step when using the Ba/F3 assay—sequencing outgrown cell lines. They also said additional research is needed to devise methods that reduce the incidence of acquired mutations in such assays. ![]()
A newly discovered issue with the Ba/F3 transformation assay could “jeopardize attempts to characterize the signaling mechanisms and drug sensitivities of leukemic oncogenes,” researchers reported in Oncotarget.
The researchers said the assay “remains an invaluable tool” for validating activating mutations in primary leukemias.
However, the assay is prone to a previously unreported flaw, where Ba/F3 cells can acquire additional mutations.
This issue was discovered by Kevin Watanabe-Smith, PhD, of Oregon Health & Science University in Portland.
He identified the problem while studying a growth-activating mutation in a patient with T-cell leukemia. (The results of that research were published in Leukemia in April 2016).
“When I was sequencing the patient’s DNA to make sure the original, known mutation is there, I was finding additional, unexpected mutations in the gene that I didn’t put there,” Dr Watanabe-Smith said. “And I was getting different mutations every time.”
“After we saw this in several cases, we knew it was worth further study,” added Cristina Tognon, PhD, of Oregon Health & Science University.
So the researchers decided to study Ba/F3 cell lines with and without 4 mutations (found in 3 cytokine receptors) that have “known transformative capacity,” including:
- CSF2RB R461C, a germline mutation found in a patient with T-cell acute lymphoblastic leukemia (ALL)
- CSF3R T618I, a mutation found in chronic neutrophilic leukemia (CNL) and atypical chronic myelogenous leukemia (aCML)
- CSF3R W791X, also found in CNL and aCML
- IL7R 243InsPPCL, which was found in a patient with B-cell ALL.
The researchers said they observed acquired mutations in 1 of 3 CSF2RB wild-type cell lines that transformed and all 4 CSF3R wild-type cell lines, which all transformed.
Furthermore, most CSF2RB R461C lines (4/5) and CSF3R W791X lines (3/4) had additional acquired mutations. However, the CSF3R T618I lines and the IL7R 243InsPPCL lines did not contain acquired mutations.
The researchers said acquired mutations were observed only in weakly transforming oncogenes, which were defined as mutations with a weaker ability to transform cells (less than 1 in every 200 cells) or a slower time to outgrowth (5 days or longer to reach a 5-fold increase over the initial cell number).
The team also said their findings indicate that the majority of the acquired mutations likely exist before IL-3 withdrawal but only expand to levels detectable by Sanger sequencing in the absence of IL-3.
“The potential impact [of these acquired mutations] is that a non-functional mutation could appear functional, and a researcher could publish results that would not be reproducible,” Dr Watanabe-Smith said.
To overcome this problem, Dr Watanabe-Smith and his colleagues recommended taking an additional step when using the Ba/F3 assay—sequencing outgrown cell lines. They also said additional research is needed to devise methods that reduce the incidence of acquired mutations in such assays. ![]()
Group ranks TKIs according to cardiotoxicity
Researchers say they have devised a way to rank tyrosine kinase inhibitors (TKIs) based on their likelihood of causing lasting heart damage in patients.
The team found they could accurately identify those TKIs already known to be the most dangerous.
The researchers believe that, in the future, their system may prove useful in the early stages of drug development to screen new compounds for cardiotoxicity.
“This type of study represents a critical step forward from the usual process running from initial drug discovery and clinical trials in human patients,” said study author Joseph Wu, MD, PhD, of Stanford University School of Medicine in California.
“It will help pharmaceutical companies better focus their efforts on developing safer drugs, and it will provide patients more effective drugs with fewer side effects.”
Dr Wu and his colleagues described this research in Science Translational Medicine.
The researchers began with human induced pluripotent stem cell-derived cardiomyocytes generated from the cells of 11 healthy people and 2 patients with kidney cancer.
The team grew the cardiomyocytes in a dish and tested the effects of 21 commonly used TKIs on the cells.
Treatment with drug levels equivalent to those taken by patients often caused the cells to beat irregularly and begin to die. The cells also displayed differences in the electrophysiological signaling that controls their contraction.
The researchers used these and other measurements to develop a cardiac safety index for each drug. They found that TKIs known to be particularly dangerous to heart function had the lowest safety indices, and TKIs known to be better tolerated by patients ranked higher on the safety index.
High cardiotoxicity
Seven of the 21 TKIs tested were assigned cardiac safety indices at or below 0.1—the threshold limit at which the researchers designated a drug highly cardiotoxic.
These TKIs (from lowest to highest safety score) were vemurafenib, sorafenib, doxorubicin, regorafenib, vandetanib, crizotinib, and nilotinib.
Three of the most cardiotoxic TKIs (regorafenib, sorafenib, and vandetanib) are known to inhibit the same 2 signaling pathways: VEGFR2 and PDGFR.
The researchers noticed that cells treated with these 3 drugs ramped up the activity of a cellular signaling pathway that responds to insulin or IGF1, an insulin-like growth factor.
This discovery, coupled with the fact that treatment with insulin or IGF1 is known to enhance heart function during adverse cardiac events such as heart attacks, led the researchers to experiment further.
They found that exposing the cells to insulin or IGF1 made it less likely they would die due to TKIs blocking the VEGFR2 and PDGFR pathways. Although more research is needed, these findings suggest it may be possible to alleviate some of the heart damage in patients receiving these TKIs.
“There is a critical need for a way to ‘safety test’ all drugs earlier in development before they are administered to patients,” Dr Wu said. “Our drug safety index is a step in that direction.” ![]()
Researchers say they have devised a way to rank tyrosine kinase inhibitors (TKIs) based on their likelihood of causing lasting heart damage in patients.
The team found they could accurately identify those TKIs already known to be the most dangerous.
The researchers believe that, in the future, their system may prove useful in the early stages of drug development to screen new compounds for cardiotoxicity.
“This type of study represents a critical step forward from the usual process running from initial drug discovery and clinical trials in human patients,” said study author Joseph Wu, MD, PhD, of Stanford University School of Medicine in California.
“It will help pharmaceutical companies better focus their efforts on developing safer drugs, and it will provide patients more effective drugs with fewer side effects.”
Dr Wu and his colleagues described this research in Science Translational Medicine.
The researchers began with human induced pluripotent stem cell-derived cardiomyocytes generated from the cells of 11 healthy people and 2 patients with kidney cancer.
The team grew the cardiomyocytes in a dish and tested the effects of 21 commonly used TKIs on the cells.
Treatment with drug levels equivalent to those taken by patients often caused the cells to beat irregularly and begin to die. The cells also displayed differences in the electrophysiological signaling that controls their contraction.
The researchers used these and other measurements to develop a cardiac safety index for each drug. They found that TKIs known to be particularly dangerous to heart function had the lowest safety indices, and TKIs known to be better tolerated by patients ranked higher on the safety index.
High cardiotoxicity
Seven of the 21 TKIs tested were assigned cardiac safety indices at or below 0.1—the threshold limit at which the researchers designated a drug highly cardiotoxic.
These TKIs (from lowest to highest safety score) were vemurafenib, sorafenib, doxorubicin, regorafenib, vandetanib, crizotinib, and nilotinib.
Three of the most cardiotoxic TKIs (regorafenib, sorafenib, and vandetanib) are known to inhibit the same 2 signaling pathways: VEGFR2 and PDGFR.
The researchers noticed that cells treated with these 3 drugs ramped up the activity of a cellular signaling pathway that responds to insulin or IGF1, an insulin-like growth factor.
This discovery, coupled with the fact that treatment with insulin or IGF1 is known to enhance heart function during adverse cardiac events such as heart attacks, led the researchers to experiment further.
They found that exposing the cells to insulin or IGF1 made it less likely they would die due to TKIs blocking the VEGFR2 and PDGFR pathways. Although more research is needed, these findings suggest it may be possible to alleviate some of the heart damage in patients receiving these TKIs.
“There is a critical need for a way to ‘safety test’ all drugs earlier in development before they are administered to patients,” Dr Wu said. “Our drug safety index is a step in that direction.” ![]()
Researchers say they have devised a way to rank tyrosine kinase inhibitors (TKIs) based on their likelihood of causing lasting heart damage in patients.
The team found they could accurately identify those TKIs already known to be the most dangerous.
The researchers believe that, in the future, their system may prove useful in the early stages of drug development to screen new compounds for cardiotoxicity.
“This type of study represents a critical step forward from the usual process running from initial drug discovery and clinical trials in human patients,” said study author Joseph Wu, MD, PhD, of Stanford University School of Medicine in California.
“It will help pharmaceutical companies better focus their efforts on developing safer drugs, and it will provide patients more effective drugs with fewer side effects.”
Dr Wu and his colleagues described this research in Science Translational Medicine.
The researchers began with human induced pluripotent stem cell-derived cardiomyocytes generated from the cells of 11 healthy people and 2 patients with kidney cancer.
The team grew the cardiomyocytes in a dish and tested the effects of 21 commonly used TKIs on the cells.
Treatment with drug levels equivalent to those taken by patients often caused the cells to beat irregularly and begin to die. The cells also displayed differences in the electrophysiological signaling that controls their contraction.
The researchers used these and other measurements to develop a cardiac safety index for each drug. They found that TKIs known to be particularly dangerous to heart function had the lowest safety indices, and TKIs known to be better tolerated by patients ranked higher on the safety index.
High cardiotoxicity
Seven of the 21 TKIs tested were assigned cardiac safety indices at or below 0.1—the threshold limit at which the researchers designated a drug highly cardiotoxic.
These TKIs (from lowest to highest safety score) were vemurafenib, sorafenib, doxorubicin, regorafenib, vandetanib, crizotinib, and nilotinib.
Three of the most cardiotoxic TKIs (regorafenib, sorafenib, and vandetanib) are known to inhibit the same 2 signaling pathways: VEGFR2 and PDGFR.
The researchers noticed that cells treated with these 3 drugs ramped up the activity of a cellular signaling pathway that responds to insulin or IGF1, an insulin-like growth factor.
This discovery, coupled with the fact that treatment with insulin or IGF1 is known to enhance heart function during adverse cardiac events such as heart attacks, led the researchers to experiment further.
They found that exposing the cells to insulin or IGF1 made it less likely they would die due to TKIs blocking the VEGFR2 and PDGFR pathways. Although more research is needed, these findings suggest it may be possible to alleviate some of the heart damage in patients receiving these TKIs.
“There is a critical need for a way to ‘safety test’ all drugs earlier in development before they are administered to patients,” Dr Wu said. “Our drug safety index is a step in that direction.” ![]()
Styrene exposure linked to myeloid leukemia, HL
A new study links styrene—a chemical used in the manufacture of plastics, rubber, and resins—to certain cancers.
The research showed that, contrary to previous suggestions, employees who have worked with styrene do not have an increased incidence of esophageal, pancreatic, lung, kidney, or bladder cancer.
On the other hand, they may have an increased risk of nasal and paranasal cancer, as well as myeloid leukemia and Hodgkin lymphoma (HL).
The research was published in Epidemiology.
“It is important to know for present and former workers exposed to styrene that they are unlikely to have become ill by doing their job if they have developed cancer of the esophagus, pancreas, lungs, kidneys, bladder, or a wide range of other types of cancer,” said study author Henrik A. Kolstad, MD, PhD, of Aarhus University in Denmark.
“This is also new and important knowledge in the USA, where styrene was added to the list of carcinogenic substances in 2011.”
In relation to the cancers for which the study shows a possible increased risk, Dr Kolstad emphasized that additional research is needed to determine if styrene is the actual cause of the employees’ disease.
For the current study, Dr Kolstad and his colleagues analyzed data on 72,292 employees who worked for 1 of 443 small and medium-sized companies in Denmark that used styrene for the production of wind turbines, pleasure boats, and other products from 1964 to 2007.
There were 8961 incident cases of cancer in this cohort from 1968 to 2012. The standardized incidence rate ratio (SIR) for all cancers was 1.04. When the researchers included a 10-year lag period, the SIR for all cancers was still 1.04.
As for hematologic malignancies, the researchers said they observed increased rate ratios associated with increased duration of employment for HL and myeloid leukemia.
For HL, the SIRs were 1.21 with no lag and 1.22 with a 10-year lag. For myeloid leukemia, the SIRs were 1.06 and 1.13, respectively.
The SIRs for non-Hodgkin lymphoma were 0.97 with no lag and 0.94 with a 10-year lag. The SIRs for multiple myeloma were 0.79 and 0.77, respectively.
For cancers of lymphatic and hematopoietic tissue, the SIRs were 0.97 with no lag and 0.96 with a 10-year lag. For lymphatic leukemia, the SIR was 0.96 for both time points.
The SIRs for monocytic leukemia were 0.77 with no lag and 0.56 with a 10-year lag. The SIRs for other and unspecified leukemias were 1.05 and 1.26, respectively.
The researchers noted that workers first employed in the 1960s had a higher risk of HL than workers first employed in subsequent years.
The SIRs were 2.12 for those first employed in 1964-1969, 0.82 for 1970-1979, 1.07 for 1980-1989, 1.52 for 1990-1999, and 1.10 for those first employed in 2000-2007.
There were no such associations for other cancer sites. ![]()
A new study links styrene—a chemical used in the manufacture of plastics, rubber, and resins—to certain cancers.
The research showed that, contrary to previous suggestions, employees who have worked with styrene do not have an increased incidence of esophageal, pancreatic, lung, kidney, or bladder cancer.
On the other hand, they may have an increased risk of nasal and paranasal cancer, as well as myeloid leukemia and Hodgkin lymphoma (HL).
The research was published in Epidemiology.
“It is important to know for present and former workers exposed to styrene that they are unlikely to have become ill by doing their job if they have developed cancer of the esophagus, pancreas, lungs, kidneys, bladder, or a wide range of other types of cancer,” said study author Henrik A. Kolstad, MD, PhD, of Aarhus University in Denmark.
“This is also new and important knowledge in the USA, where styrene was added to the list of carcinogenic substances in 2011.”
In relation to the cancers for which the study shows a possible increased risk, Dr Kolstad emphasized that additional research is needed to determine if styrene is the actual cause of the employees’ disease.
For the current study, Dr Kolstad and his colleagues analyzed data on 72,292 employees who worked for 1 of 443 small and medium-sized companies in Denmark that used styrene for the production of wind turbines, pleasure boats, and other products from 1964 to 2007.
There were 8961 incident cases of cancer in this cohort from 1968 to 2012. The standardized incidence rate ratio (SIR) for all cancers was 1.04. When the researchers included a 10-year lag period, the SIR for all cancers was still 1.04.
As for hematologic malignancies, the researchers said they observed increased rate ratios associated with increased duration of employment for HL and myeloid leukemia.
For HL, the SIRs were 1.21 with no lag and 1.22 with a 10-year lag. For myeloid leukemia, the SIRs were 1.06 and 1.13, respectively.
The SIRs for non-Hodgkin lymphoma were 0.97 with no lag and 0.94 with a 10-year lag. The SIRs for multiple myeloma were 0.79 and 0.77, respectively.
For cancers of lymphatic and hematopoietic tissue, the SIRs were 0.97 with no lag and 0.96 with a 10-year lag. For lymphatic leukemia, the SIR was 0.96 for both time points.
The SIRs for monocytic leukemia were 0.77 with no lag and 0.56 with a 10-year lag. The SIRs for other and unspecified leukemias were 1.05 and 1.26, respectively.
The researchers noted that workers first employed in the 1960s had a higher risk of HL than workers first employed in subsequent years.
The SIRs were 2.12 for those first employed in 1964-1969, 0.82 for 1970-1979, 1.07 for 1980-1989, 1.52 for 1990-1999, and 1.10 for those first employed in 2000-2007.
There were no such associations for other cancer sites. ![]()
A new study links styrene—a chemical used in the manufacture of plastics, rubber, and resins—to certain cancers.
The research showed that, contrary to previous suggestions, employees who have worked with styrene do not have an increased incidence of esophageal, pancreatic, lung, kidney, or bladder cancer.
On the other hand, they may have an increased risk of nasal and paranasal cancer, as well as myeloid leukemia and Hodgkin lymphoma (HL).
The research was published in Epidemiology.
“It is important to know for present and former workers exposed to styrene that they are unlikely to have become ill by doing their job if they have developed cancer of the esophagus, pancreas, lungs, kidneys, bladder, or a wide range of other types of cancer,” said study author Henrik A. Kolstad, MD, PhD, of Aarhus University in Denmark.
“This is also new and important knowledge in the USA, where styrene was added to the list of carcinogenic substances in 2011.”
In relation to the cancers for which the study shows a possible increased risk, Dr Kolstad emphasized that additional research is needed to determine if styrene is the actual cause of the employees’ disease.
For the current study, Dr Kolstad and his colleagues analyzed data on 72,292 employees who worked for 1 of 443 small and medium-sized companies in Denmark that used styrene for the production of wind turbines, pleasure boats, and other products from 1964 to 2007.
There were 8961 incident cases of cancer in this cohort from 1968 to 2012. The standardized incidence rate ratio (SIR) for all cancers was 1.04. When the researchers included a 10-year lag period, the SIR for all cancers was still 1.04.
As for hematologic malignancies, the researchers said they observed increased rate ratios associated with increased duration of employment for HL and myeloid leukemia.
For HL, the SIRs were 1.21 with no lag and 1.22 with a 10-year lag. For myeloid leukemia, the SIRs were 1.06 and 1.13, respectively.
The SIRs for non-Hodgkin lymphoma were 0.97 with no lag and 0.94 with a 10-year lag. The SIRs for multiple myeloma were 0.79 and 0.77, respectively.
For cancers of lymphatic and hematopoietic tissue, the SIRs were 0.97 with no lag and 0.96 with a 10-year lag. For lymphatic leukemia, the SIR was 0.96 for both time points.
The SIRs for monocytic leukemia were 0.77 with no lag and 0.56 with a 10-year lag. The SIRs for other and unspecified leukemias were 1.05 and 1.26, respectively.
The researchers noted that workers first employed in the 1960s had a higher risk of HL than workers first employed in subsequent years.
The SIRs were 2.12 for those first employed in 1964-1969, 0.82 for 1970-1979, 1.07 for 1980-1989, 1.52 for 1990-1999, and 1.10 for those first employed in 2000-2007.
There were no such associations for other cancer sites. ![]()