User login
MD-IQ only
Video roundtable–Endometriosis: Expert perspectives on medical and surgical management

Read the article: Endometriosis: Expert perspectives on medical and surgical management

Read the article: Endometriosis: Expert perspectives on medical and surgical management

Read the article: Endometriosis: Expert perspectives on medical and surgical management
Endometriosis surgery on a young woman: $483,351 award
Endometriosis surgery on a young woman: $483,351 award
A 17-year-old woman reported cramping and heavy bleeding during her menses. Her gynecologist suspected that the patient had endometriosis and recommended laparoscopic surgery with cauterization.
During surgery, the gynecologist found 2 metal staples in the patient’s pelvic region from a prior appendectomy. He continued with the surgery as planned, using monopolar cauterization to excise the endometriosis.
The following day, the patient sought emergency treatment for pain. Physicians discovered 2 perforations in her anterior rectum and performed an emergency colectomy. She spent 18 days in the hospital. When the colectomy was reversed 3 months later, she was hospitalized for 8 days and later developed a postoperative surgical site infection requiring IV antibiotics and weeks of wound care.
The patient was in the middle of her senior year of high school when she had the colectomy and could not return to normal activities. She was unable to graduate with her class and had to relinquish a college scholarship. As a result, she completed her senior year via homeschooling and graduated a year later.
PATIENT'S CLAIM: The gynecologist’s negligent use of the cautery device within millimeters of the staples caused the bowel injury and necessitated the colectomy. The electric current from the cautery device heated the staples, injuring the rectum, which became necrotic. While she had no long-term physical limitations, wearing the colostomy bag, missing her senior year, not being able to graduate with her class, and not being able to participate in typical senior year activities left her emotionally distressed.
PHYSICIAN'S DEFENSE: The staples were not found near the rectal injury. The injury was a known complication of cauterization, not a result of negligence.
VERDICT: A $588,351 California verdict was returned but was reduced to $483,351 because of the state cap on pain and suffering.
RELATED
Surgical excision of the most severe form of endometriosis
Sigmoid colon injury during hysterectomy
A 42-year-old woman had uterine fibroids that caused such heavy bleeding that she became anemic and required a transfusion. On June 26, she underwent laparoscopic-assisted supracervical hysterectomy performed by her primary ObGyn and an assisting ObGyn.
The next day, the patient developed pain and fever and her vital signs were unstable. The primary ObGyn called in a general surgeon. A CT scan showed a tear on the underside of the sigmoid colon. The general surgeon performed a laparotomy, resected the colon, and created a temporary colostomy. The colostomy reversal took place on September 25.
PATIENT'S CLAIM: The patient sued both ObGyns, alleging that they should have found the colon injury during surgery. The primary ObGyn settled before trial and the case continued against the assisting ObGyn. It was undisputed that one or both of the physicians caused the tear, but that was not the patient’s claim. The patient alleged that negligence occurred when the injury was not intraoperatively detected. Had the injury been found during surgery, a general surgeon could have performed a primary repair, saving the patient from further surgery and colostomy. The patient claimed mental anguish and embarrassment from the colostomy. Her abdomen is still tender and she has significant scarring.
PHYSICIAN'S CLAIM: There was no negligence. Nothing was unusual about the nature of the procedure, and nothing unusual was seen intraoperatively that would have led them to search for an injury. They performed adequate and appropriate exploration before closing. The linear tear on the underside of the sigmoid colon was very inconspicuous in size, shape, and location, and was away from the operative area. The injury likely occurred during manipulation of the sigmoid colon, which generally has to be retracted before the uterus can be removed. Even if the injury had been found intraoperatively, a general surgeon would have had to convert to laparoscopy to repair the colon.
VERDICT: After a settlement was reached with the primary gynecologist, a Texas defense verdict was returned for the assisting gynecologist.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Endometriosis surgery on a young woman: $483,351 award
A 17-year-old woman reported cramping and heavy bleeding during her menses. Her gynecologist suspected that the patient had endometriosis and recommended laparoscopic surgery with cauterization.
During surgery, the gynecologist found 2 metal staples in the patient’s pelvic region from a prior appendectomy. He continued with the surgery as planned, using monopolar cauterization to excise the endometriosis.
The following day, the patient sought emergency treatment for pain. Physicians discovered 2 perforations in her anterior rectum and performed an emergency colectomy. She spent 18 days in the hospital. When the colectomy was reversed 3 months later, she was hospitalized for 8 days and later developed a postoperative surgical site infection requiring IV antibiotics and weeks of wound care.
The patient was in the middle of her senior year of high school when she had the colectomy and could not return to normal activities. She was unable to graduate with her class and had to relinquish a college scholarship. As a result, she completed her senior year via homeschooling and graduated a year later.
PATIENT'S CLAIM: The gynecologist’s negligent use of the cautery device within millimeters of the staples caused the bowel injury and necessitated the colectomy. The electric current from the cautery device heated the staples, injuring the rectum, which became necrotic. While she had no long-term physical limitations, wearing the colostomy bag, missing her senior year, not being able to graduate with her class, and not being able to participate in typical senior year activities left her emotionally distressed.
PHYSICIAN'S DEFENSE: The staples were not found near the rectal injury. The injury was a known complication of cauterization, not a result of negligence.
VERDICT: A $588,351 California verdict was returned but was reduced to $483,351 because of the state cap on pain and suffering.
RELATED
Surgical excision of the most severe form of endometriosis
Sigmoid colon injury during hysterectomy
A 42-year-old woman had uterine fibroids that caused such heavy bleeding that she became anemic and required a transfusion. On June 26, she underwent laparoscopic-assisted supracervical hysterectomy performed by her primary ObGyn and an assisting ObGyn.
The next day, the patient developed pain and fever and her vital signs were unstable. The primary ObGyn called in a general surgeon. A CT scan showed a tear on the underside of the sigmoid colon. The general surgeon performed a laparotomy, resected the colon, and created a temporary colostomy. The colostomy reversal took place on September 25.
PATIENT'S CLAIM: The patient sued both ObGyns, alleging that they should have found the colon injury during surgery. The primary ObGyn settled before trial and the case continued against the assisting ObGyn. It was undisputed that one or both of the physicians caused the tear, but that was not the patient’s claim. The patient alleged that negligence occurred when the injury was not intraoperatively detected. Had the injury been found during surgery, a general surgeon could have performed a primary repair, saving the patient from further surgery and colostomy. The patient claimed mental anguish and embarrassment from the colostomy. Her abdomen is still tender and she has significant scarring.
PHYSICIAN'S CLAIM: There was no negligence. Nothing was unusual about the nature of the procedure, and nothing unusual was seen intraoperatively that would have led them to search for an injury. They performed adequate and appropriate exploration before closing. The linear tear on the underside of the sigmoid colon was very inconspicuous in size, shape, and location, and was away from the operative area. The injury likely occurred during manipulation of the sigmoid colon, which generally has to be retracted before the uterus can be removed. Even if the injury had been found intraoperatively, a general surgeon would have had to convert to laparoscopy to repair the colon.
VERDICT: After a settlement was reached with the primary gynecologist, a Texas defense verdict was returned for the assisting gynecologist.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Endometriosis surgery on a young woman: $483,351 award
A 17-year-old woman reported cramping and heavy bleeding during her menses. Her gynecologist suspected that the patient had endometriosis and recommended laparoscopic surgery with cauterization.
During surgery, the gynecologist found 2 metal staples in the patient’s pelvic region from a prior appendectomy. He continued with the surgery as planned, using monopolar cauterization to excise the endometriosis.
The following day, the patient sought emergency treatment for pain. Physicians discovered 2 perforations in her anterior rectum and performed an emergency colectomy. She spent 18 days in the hospital. When the colectomy was reversed 3 months later, she was hospitalized for 8 days and later developed a postoperative surgical site infection requiring IV antibiotics and weeks of wound care.
The patient was in the middle of her senior year of high school when she had the colectomy and could not return to normal activities. She was unable to graduate with her class and had to relinquish a college scholarship. As a result, she completed her senior year via homeschooling and graduated a year later.
PATIENT'S CLAIM: The gynecologist’s negligent use of the cautery device within millimeters of the staples caused the bowel injury and necessitated the colectomy. The electric current from the cautery device heated the staples, injuring the rectum, which became necrotic. While she had no long-term physical limitations, wearing the colostomy bag, missing her senior year, not being able to graduate with her class, and not being able to participate in typical senior year activities left her emotionally distressed.
PHYSICIAN'S DEFENSE: The staples were not found near the rectal injury. The injury was a known complication of cauterization, not a result of negligence.
VERDICT: A $588,351 California verdict was returned but was reduced to $483,351 because of the state cap on pain and suffering.
RELATED
Surgical excision of the most severe form of endometriosis
Sigmoid colon injury during hysterectomy
A 42-year-old woman had uterine fibroids that caused such heavy bleeding that she became anemic and required a transfusion. On June 26, she underwent laparoscopic-assisted supracervical hysterectomy performed by her primary ObGyn and an assisting ObGyn.
The next day, the patient developed pain and fever and her vital signs were unstable. The primary ObGyn called in a general surgeon. A CT scan showed a tear on the underside of the sigmoid colon. The general surgeon performed a laparotomy, resected the colon, and created a temporary colostomy. The colostomy reversal took place on September 25.
PATIENT'S CLAIM: The patient sued both ObGyns, alleging that they should have found the colon injury during surgery. The primary ObGyn settled before trial and the case continued against the assisting ObGyn. It was undisputed that one or both of the physicians caused the tear, but that was not the patient’s claim. The patient alleged that negligence occurred when the injury was not intraoperatively detected. Had the injury been found during surgery, a general surgeon could have performed a primary repair, saving the patient from further surgery and colostomy. The patient claimed mental anguish and embarrassment from the colostomy. Her abdomen is still tender and she has significant scarring.
PHYSICIAN'S CLAIM: There was no negligence. Nothing was unusual about the nature of the procedure, and nothing unusual was seen intraoperatively that would have led them to search for an injury. They performed adequate and appropriate exploration before closing. The linear tear on the underside of the sigmoid colon was very inconspicuous in size, shape, and location, and was away from the operative area. The injury likely occurred during manipulation of the sigmoid colon, which generally has to be retracted before the uterus can be removed. Even if the injury had been found intraoperatively, a general surgeon would have had to convert to laparoscopy to repair the colon.
VERDICT: After a settlement was reached with the primary gynecologist, a Texas defense verdict was returned for the assisting gynecologist.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Endometrial Evaluation: Are You Still Relying on a Blind Biopsy?
One-third of patients who visit a gynecologist are there because of abnormal uterine bleeding (AUB), which is believed to account for more than 70% of gyneco¬logic consults in perimenopausal and postmenopausal women. The American College of Obstetricians and Gynecologists Practice Bulle¬tin on AUB now states that a negative blind endometrial biopsy is not a stopping point in persistent bleeding.
In this supplement, learn about the advantages of diagnosing AUB using a hysteroscope.
- Authors:
- Steven R. Goldstein, MD, CCD, NCMP, FACOG
- New York University Medical Center New York, New York
- Ted L. Anderson, MD, PhD
- Vanderbilt University Medical School Nashville, Tennessee
- Click here to read this supplement.
To access the posttest and evaluation, visit
www.worldclasscme.com/endometrialevaluation
One-third of patients who visit a gynecologist are there because of abnormal uterine bleeding (AUB), which is believed to account for more than 70% of gyneco¬logic consults in perimenopausal and postmenopausal women. The American College of Obstetricians and Gynecologists Practice Bulle¬tin on AUB now states that a negative blind endometrial biopsy is not a stopping point in persistent bleeding.
In this supplement, learn about the advantages of diagnosing AUB using a hysteroscope.
- Authors:
- Steven R. Goldstein, MD, CCD, NCMP, FACOG
- New York University Medical Center New York, New York
- Ted L. Anderson, MD, PhD
- Vanderbilt University Medical School Nashville, Tennessee
- Click here to read this supplement.
To access the posttest and evaluation, visit
www.worldclasscme.com/endometrialevaluation
One-third of patients who visit a gynecologist are there because of abnormal uterine bleeding (AUB), which is believed to account for more than 70% of gyneco¬logic consults in perimenopausal and postmenopausal women. The American College of Obstetricians and Gynecologists Practice Bulle¬tin on AUB now states that a negative blind endometrial biopsy is not a stopping point in persistent bleeding.
In this supplement, learn about the advantages of diagnosing AUB using a hysteroscope.
- Authors:
- Steven R. Goldstein, MD, CCD, NCMP, FACOG
- New York University Medical Center New York, New York
- Ted L. Anderson, MD, PhD
- Vanderbilt University Medical School Nashville, Tennessee
- Click here to read this supplement.
To access the posttest and evaluation, visit
www.worldclasscme.com/endometrialevaluation
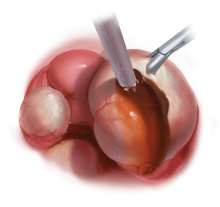
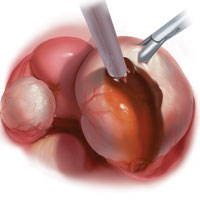
Endometriomas: Classification and surgical management
Related article:
Endometriosis: Expert answers to 7 crucial questions on diagnosis
Etiology
Endometriomas are extensively described in the literature, and their origin is the subject of several theories. In 1921, Sampson noted luteal membrane and ovarian epithelial tissues within endometriomas and was the first to indicate that endometriomas may result from the invasion of functional cysts by endometrial tissue.2,4,5 In 1979, Czernobilsky and Morris6 found endometrial and oviduct-like epithelium in ovarian endometriosis and concluded that ovarian tissue may be a common histologic precursor. Several other authors subsequently have reported finding different types of tissue within ovarian endometriomas, and not all of these chocolate cysts showed histologic evidence of endometriosis.4,7,8
Read about the classification of endometriomas
Disease classification
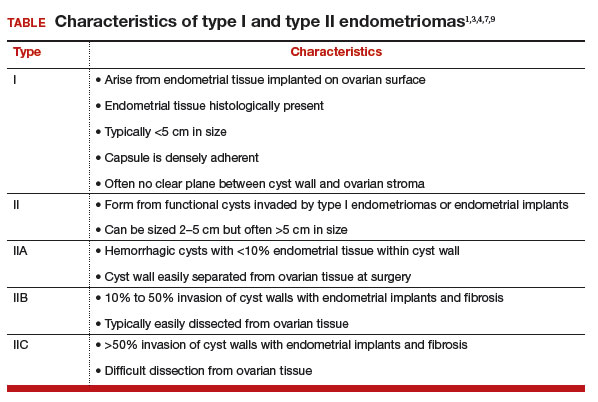
Our classification system identifies 2 types of endometriomas on the basis of their etiologies and characteristics. Type I, which arise from endometrial tissue implanted on the ovarian surface, are also called true endometriomas. Invagination of cortex and subsequent hemorrhage from endometrial tissue result in cyst formation. Endometrial tissue (endometrial stroma and glands) is histologically present in all type I endometriomas.1,4,9 These endometriomas usually are small (<5 cm in diameter) and have a densely adherent fibrous capsule.4 Often, there is no clear plane between cyst wall and ovarian stroma.3
Type II endometriomas arise from functional cysts involved in or invaded by cortical or pelvic side-wall endometrial implants or by type I endometriomas. Type II endometriomas are subclassified by the extent of endometrial implant involvement in the cyst wall. Type IIA endometriomas are hemorrhagic cysts with less than 10% of endometrial tissue within the cyst wall. Similar to the functional cysts from which they originate, type IIA endometriomas have a cyst wall that is separated easily from ovarian tissue during surgery.4,7,9 Although type II endometriomas tend to be larger than their type I counterparts, in some cases they are identified at an early stage of 2 to 5 cm. Endometriomas larger than 5 cm are almost always type II.4
Type IIB and IIC endometriomas have endometrial implants and fibrosis within their cyst walls, with progressively more endometrial invasion in type IIC endometriomas (>50%) than in type IIB (10% to 50%). Consequently, type IIB cysts are relatively easy to dissect from ovarian tissue, except adjacent to an endometriotic area where the cyst densely adheres to the ovarian stroma. In type IIC, endometrial tissue more extensively penetrates the capsule, making dissection of diseased tissue from the ovarian stroma more difficult; in fact, separating type IIC cyst wall from ovarian stroma can be as challenging as excising a type I endometrioma.7 In most cases, a type IIC cyst is attached by adhesions and fibrosis to the pelvic side wall or uterus and ruptures during mobilization (TABLE).
Related article:
Imaging the endometrioma and mature cystic teratoma
Presentation and diagnosis
Almost all patients with an endometrioma concurrently have peritoneal endometriosis, which is characterized by dysmenorrhea, dyspareunia, chronic pelvic pain, infertility, and, in some cases, gastrointestinal or genitourinary dysfunction.1 Pelvic examination may reveal an adnexal mass that is an endometrioma, or an endometrioma may appear on imaging obtained in a pelvic pain or infertility work-up. Given its 73% sensitivity, 94% specificity, safety, and low cost, transvaginal ultrasonography is the preferred imaging modality for endometrioma.3 The characteristic ultrasonographic appearance is that of a round, homogeneous, fluid-filled mass with low-level echoes.1 Magnetic resonance imaging is appropriate when a more sensitive imaging modality is indicated, as for a patient with risk factors for malignancy.3,10–12
Read about the surgical management of endometriomas
Surgical management
Indications for surgical excision of endometriomas include pelvic pain, infertility, and prevention and diagnosis of malignancy. Endometriomas may be excised prior to use of assisted reproductive technology.13–15 Medical therapy, such as oral contraceptives, can be used to reduce the size of endometriomas but does not improve fertility.3 Certain ovarian cancers are more common in women with endometriosis, and ovarian tumors are thought to develop in about 1% of ovarian endometriosis cases.1,12 Therefore, endometrioma excision may reduce the risk of malignancy. As with other ovarian cysts, large endometriomas may be excised to reduce the risks of rupture and torsion.
Approach
Laparoscopy is the preferred approach for endometrioma excision. Controversy exists regarding the ideal procedure: complete excision (with stripping of the cyst capsule) or drainage and ablation of the cyst wall. Compared with drainage and ablation, excision reduces recurrence of endometriomas; relieves dysmenorrhea, dyspareunia, pelvic pain, and other symptoms; and improves fertility.13,16 The recurrence rate may be as low as 5.8% with complete excision but is 90% with simple transvaginal aspiration.17,18 If not performed properly, however, cyst capsule stripping may damage nearby ovarian stroma and decrease the ovarian reserve.14 Some authors have advocated combining excision and ablation—performing cystectomy until there is no longer a clear plane between capsule and ovarian stroma and then ablating any remaining endometrial tissue.8
With type I and IIC endometriomas, we have seen the endometrial cyst wall infiltrating the ovarian stroma so deeply there is not always a definable plane. By contrast, type IIA and IIB endometriomas typically have a plane between the cyst wall and the ovarian cortex. In type II endometriomas, endometrial implants on the ovarian cortex infiltrate the plane of the cyst wall such that the juxtaposing lipomatous follicular cyst detaches with minimal intraoperative traction. Portions of type II endometriomas containing fibrosis and adhesions may become more difficult to peel off the cyst wall. For most endometriomas, at least 1 spot is difficult to peel off the ovary, and extra care must be taken at the hilum of ovary to avoid excising healthy ovarian cortex.4,5,7,8
Our surgical approach accounts for the described variations in type I and II endometriomas. Endometrial contents often spill as the endometrioma is dissected off neighboring structures. When possible, endometriomas should be aspirated and irrigated prior to cystectomy to avoid seeding the pelvis and abdomen with spilled endometriotic contents. We use hydrodissection, the injection of dilute vasopressin with a laparoscopic needle, to create a plane between cyst wall and ovarian stroma and strip the cyst capsule with laparoscopic graspers. Type I endometriomas adhere densely to the ovary. Given the presence of fibrosis and adhesions, the cyst is excised in a piecemeal fashion. Care is taken to remove any endometrial implants from the ovary while preserving as much of the ovarian tissue as possible.1
Type II endometriomas are larger cysts originating from the invasion of endometrial implants or type I endometrioma into functional cysts. The difficulty of capsule excision varies according to the extent of endometrial invasion. Type IIA endometriomas contain less than 10% endometrial tissue within the cyst capsule. Thus, the standard ovarian cystectomy stripping technique is successful in removing more than 90% of the cyst capsule. Special care is taken in stripping the residual small portion that involves the endometrial glands and stroma and adheres densely to the ovary.
The larger proportion of endometrial tissue present in type IIB and IIC endometriomas degrades the plane between the cyst capsule and the ovarian stroma, making excision more difficult. Similar to the type I excision, a piecemeal approach is often necessary. If complete stripping of the cyst capsule would result in extensive loss of healthy ovarian tissue, then electrocautery, plasma energy, or laser ablation can be selectively used to destroy focal areas of endometrial invasion. Complete ablation may be difficult, as the endometrioma wall can be up to 5 mm thick.19 For these thick-walled endometriomas, we recommend excision (vs ablation), which lowers the risk of endometrioma recurrence.
Related article:
Endometriosis and pain: Expert answers to 6 questions targeting your management options
- Endometriomas are common adnexal masses in women affected by endometriosis and may exacerbate pelvic pain and impair fertility. Classification of endometriomas into type I and type II,depending on their etiology and characteristics, can guide minimally invasive surgical management.
- Type I endometriomas arise from invagination of endometrial implants on the ovarian cortex, resulting in dense fibrosis and adhesions. These lesions typically require piecemeal excision in order to completely remove the cyst capsule.
- Type II endometriomas result from invasion of endometrial tissue into preexisting functional cysts and are further subclassified by the proportion of cyst capsule containing endometrial tissue (IIA <10%, IIB 10% to 50%, IIC >50%).
- The difficulty of excising type II endometriomas correlates with the degree of endometrial invasion, with type IIA being relatively straightforward and type IIC being as challenging and piecemeal as type I.
- We generally favor complete excision rather than ablation of the cyst capsule, except for when excision would result in an unacceptable loss of healthy ovarian tissue.
Conclusion
Endometriomas, common adnexal masses in women affected by endometriosis, may exacerbate pelvic pain and impair fertility. Gynecologists should be prepared to excise endometriomas completely and exercise care in preserving as much of the ovarian stroma as possible. We classify endometriomas into 2 types: type I, which develop from invagination of endometrial implants in the ovarian cortex, and type II, which stem from invasion of functional cysts by endometrial implants or type I endometrioma. This distinction guides surgical management. We hope this article and its accompanying video will be helpful in guiding laparoscopic excision of type I and II endometriomas.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Nezhat C, Buescher E, Paka C, et al. Video-assisted laparoscopic treatment of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat’s Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013:265–302.
- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98(3):511–519.
- Keyhan S, Hughes C, Price T, Muasher S. An update on surgical versus expectant management of ovarian endometriomas in infertile women. Biomed Res Int. 2015;2015:204792.
- Nezhat F, Nezhat C, Allan CJ, Metzger DA, Sears DL. Clinical and histologic classification of endometriomas. Implications for a mechanism of pathogenesis. J Reprod Med. 1992;37(9):771–776.
- Burney RO, Giudice LC. The pathogenesis of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat’s Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013:252–258.
- Czernobilsky B, Morris WJ. A histologic study of ovarian endometriosis with emphasis on hyperplastic and atypical changes. Obstet Gynecol. 1979;53(3):318–323.
- Nezhat F, Nezhat C, Nezhat C, Admon D. A fresh look at ovarian endometriomas. Contemp Ob Gyn. 1994;39(11):81–94.
- Donnez J, Lousse JC, Jadoul P, Donnez O, Squifflet J. Laparoscopic management of endometriomas using a combined technique of excisional (cystectomy) and ablative surgery. Fertil Steril. 2010;94(1):28–32.
- Nezhat C, Nezhat F, Nezhat C, Seidman DS. Classification of endometriosis. Improving the classification of endometriotic ovarian cysts. Hum Reprod. 1994;9(12):2212–2213.
- Nezhat FR, Pejovic T, Reis FM, Guo SW. The link between endometriosis and ovarian cancer: clinical implications. Int J Gynecol Cancer. 2014;24(4):623–628.
- Nezhat F, Apostol R, Mahmoud M, el Daouk M. Malignant transformation of endometriosis and its clinical significance. Fertil Steril. 2014;102(2):342–344.
- Nezhat FR, Apostal R, Nezhat C, Pejovic T. New insights in the pathophysiology of ovarian cancer and implications for screening and prevention. Am J Obstet Gynecol. 2015;213(3):262–267.
- Hart RJ, Hickey M, Maouris P, Buckett W. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2008;(2):CD004992.
- Yates J. Endometriosis and infertility: expert answers to 6 questions to help pinpoint the best route to pregnancy. OBG Manag. 2015;27(6):30–35.
- Littman E, Giudice L, Lathi R, Berker B, Milki A, Nezhat C. Role of laparoscopic treatment of endometriosis in patients with failed in vitro fertilization cycles. Fertil Steril. 2005;84(6):1574–1578.
- Exacoustos C, Zupi E, Amadio A, et al. Laparoscopic removal of endometriomas: sonographic evaluation of residual functioning ovarian tissue. Am J Obstet Gynecol. 2004;191(1):68–72.
- Gonçalves FC, Andres MP, Passman LJ, Gonçalves MO, Podgaec S. A systematic review of ultrasonography-guided transvaginal aspiration of recurrent ovarian endometrioma. Int J Gynaecol Obstet. 2016;134(1):3–7.
- Alborzi S, Momtahan M, Parsanezhad ME, Dehbashi S, Zolghadri J, Alborzi S. A prospective, randomized study comparing laparoscopic ovarian cystectomy versus fenestration and coagulation in patients with endometriomas. Fertil Steril. 2004;82(6):1633–1637.
- Nezhat C, Crowgey SR, Garrison CP. Surgical treatment of endometriosis via laser laparoscopy. Fertil Steril. 1986;45(6):778–783.
Related article:
Endometriosis: Expert answers to 7 crucial questions on diagnosis
Etiology
Endometriomas are extensively described in the literature, and their origin is the subject of several theories. In 1921, Sampson noted luteal membrane and ovarian epithelial tissues within endometriomas and was the first to indicate that endometriomas may result from the invasion of functional cysts by endometrial tissue.2,4,5 In 1979, Czernobilsky and Morris6 found endometrial and oviduct-like epithelium in ovarian endometriosis and concluded that ovarian tissue may be a common histologic precursor. Several other authors subsequently have reported finding different types of tissue within ovarian endometriomas, and not all of these chocolate cysts showed histologic evidence of endometriosis.4,7,8
Read about the classification of endometriomas
Disease classification
Our classification system identifies 2 types of endometriomas on the basis of their etiologies and characteristics. Type I, which arise from endometrial tissue implanted on the ovarian surface, are also called true endometriomas. Invagination of cortex and subsequent hemorrhage from endometrial tissue result in cyst formation. Endometrial tissue (endometrial stroma and glands) is histologically present in all type I endometriomas.1,4,9 These endometriomas usually are small (<5 cm in diameter) and have a densely adherent fibrous capsule.4 Often, there is no clear plane between cyst wall and ovarian stroma.3
Type II endometriomas arise from functional cysts involved in or invaded by cortical or pelvic side-wall endometrial implants or by type I endometriomas. Type II endometriomas are subclassified by the extent of endometrial implant involvement in the cyst wall. Type IIA endometriomas are hemorrhagic cysts with less than 10% of endometrial tissue within the cyst wall. Similar to the functional cysts from which they originate, type IIA endometriomas have a cyst wall that is separated easily from ovarian tissue during surgery.4,7,9 Although type II endometriomas tend to be larger than their type I counterparts, in some cases they are identified at an early stage of 2 to 5 cm. Endometriomas larger than 5 cm are almost always type II.4
Type IIB and IIC endometriomas have endometrial implants and fibrosis within their cyst walls, with progressively more endometrial invasion in type IIC endometriomas (>50%) than in type IIB (10% to 50%). Consequently, type IIB cysts are relatively easy to dissect from ovarian tissue, except adjacent to an endometriotic area where the cyst densely adheres to the ovarian stroma. In type IIC, endometrial tissue more extensively penetrates the capsule, making dissection of diseased tissue from the ovarian stroma more difficult; in fact, separating type IIC cyst wall from ovarian stroma can be as challenging as excising a type I endometrioma.7 In most cases, a type IIC cyst is attached by adhesions and fibrosis to the pelvic side wall or uterus and ruptures during mobilization (TABLE).

Related article:
Imaging the endometrioma and mature cystic teratoma
Presentation and diagnosis
Almost all patients with an endometrioma concurrently have peritoneal endometriosis, which is characterized by dysmenorrhea, dyspareunia, chronic pelvic pain, infertility, and, in some cases, gastrointestinal or genitourinary dysfunction.1 Pelvic examination may reveal an adnexal mass that is an endometrioma, or an endometrioma may appear on imaging obtained in a pelvic pain or infertility work-up. Given its 73% sensitivity, 94% specificity, safety, and low cost, transvaginal ultrasonography is the preferred imaging modality for endometrioma.3 The characteristic ultrasonographic appearance is that of a round, homogeneous, fluid-filled mass with low-level echoes.1 Magnetic resonance imaging is appropriate when a more sensitive imaging modality is indicated, as for a patient with risk factors for malignancy.3,10–12
Read about the surgical management of endometriomas
Surgical management
Indications for surgical excision of endometriomas include pelvic pain, infertility, and prevention and diagnosis of malignancy. Endometriomas may be excised prior to use of assisted reproductive technology.13–15 Medical therapy, such as oral contraceptives, can be used to reduce the size of endometriomas but does not improve fertility.3 Certain ovarian cancers are more common in women with endometriosis, and ovarian tumors are thought to develop in about 1% of ovarian endometriosis cases.1,12 Therefore, endometrioma excision may reduce the risk of malignancy. As with other ovarian cysts, large endometriomas may be excised to reduce the risks of rupture and torsion.
Approach
Laparoscopy is the preferred approach for endometrioma excision. Controversy exists regarding the ideal procedure: complete excision (with stripping of the cyst capsule) or drainage and ablation of the cyst wall. Compared with drainage and ablation, excision reduces recurrence of endometriomas; relieves dysmenorrhea, dyspareunia, pelvic pain, and other symptoms; and improves fertility.13,16 The recurrence rate may be as low as 5.8% with complete excision but is 90% with simple transvaginal aspiration.17,18 If not performed properly, however, cyst capsule stripping may damage nearby ovarian stroma and decrease the ovarian reserve.14 Some authors have advocated combining excision and ablation—performing cystectomy until there is no longer a clear plane between capsule and ovarian stroma and then ablating any remaining endometrial tissue.8
With type I and IIC endometriomas, we have seen the endometrial cyst wall infiltrating the ovarian stroma so deeply there is not always a definable plane. By contrast, type IIA and IIB endometriomas typically have a plane between the cyst wall and the ovarian cortex. In type II endometriomas, endometrial implants on the ovarian cortex infiltrate the plane of the cyst wall such that the juxtaposing lipomatous follicular cyst detaches with minimal intraoperative traction. Portions of type II endometriomas containing fibrosis and adhesions may become more difficult to peel off the cyst wall. For most endometriomas, at least 1 spot is difficult to peel off the ovary, and extra care must be taken at the hilum of ovary to avoid excising healthy ovarian cortex.4,5,7,8
Our surgical approach accounts for the described variations in type I and II endometriomas. Endometrial contents often spill as the endometrioma is dissected off neighboring structures. When possible, endometriomas should be aspirated and irrigated prior to cystectomy to avoid seeding the pelvis and abdomen with spilled endometriotic contents. We use hydrodissection, the injection of dilute vasopressin with a laparoscopic needle, to create a plane between cyst wall and ovarian stroma and strip the cyst capsule with laparoscopic graspers. Type I endometriomas adhere densely to the ovary. Given the presence of fibrosis and adhesions, the cyst is excised in a piecemeal fashion. Care is taken to remove any endometrial implants from the ovary while preserving as much of the ovarian tissue as possible.1
Type II endometriomas are larger cysts originating from the invasion of endometrial implants or type I endometrioma into functional cysts. The difficulty of capsule excision varies according to the extent of endometrial invasion. Type IIA endometriomas contain less than 10% endometrial tissue within the cyst capsule. Thus, the standard ovarian cystectomy stripping technique is successful in removing more than 90% of the cyst capsule. Special care is taken in stripping the residual small portion that involves the endometrial glands and stroma and adheres densely to the ovary.
The larger proportion of endometrial tissue present in type IIB and IIC endometriomas degrades the plane between the cyst capsule and the ovarian stroma, making excision more difficult. Similar to the type I excision, a piecemeal approach is often necessary. If complete stripping of the cyst capsule would result in extensive loss of healthy ovarian tissue, then electrocautery, plasma energy, or laser ablation can be selectively used to destroy focal areas of endometrial invasion. Complete ablation may be difficult, as the endometrioma wall can be up to 5 mm thick.19 For these thick-walled endometriomas, we recommend excision (vs ablation), which lowers the risk of endometrioma recurrence.
Related article:
Endometriosis and pain: Expert answers to 6 questions targeting your management options
- Endometriomas are common adnexal masses in women affected by endometriosis and may exacerbate pelvic pain and impair fertility. Classification of endometriomas into type I and type II,depending on their etiology and characteristics, can guide minimally invasive surgical management.
- Type I endometriomas arise from invagination of endometrial implants on the ovarian cortex, resulting in dense fibrosis and adhesions. These lesions typically require piecemeal excision in order to completely remove the cyst capsule.
- Type II endometriomas result from invasion of endometrial tissue into preexisting functional cysts and are further subclassified by the proportion of cyst capsule containing endometrial tissue (IIA <10%, IIB 10% to 50%, IIC >50%).
- The difficulty of excising type II endometriomas correlates with the degree of endometrial invasion, with type IIA being relatively straightforward and type IIC being as challenging and piecemeal as type I.
- We generally favor complete excision rather than ablation of the cyst capsule, except for when excision would result in an unacceptable loss of healthy ovarian tissue.
Conclusion
Endometriomas, common adnexal masses in women affected by endometriosis, may exacerbate pelvic pain and impair fertility. Gynecologists should be prepared to excise endometriomas completely and exercise care in preserving as much of the ovarian stroma as possible. We classify endometriomas into 2 types: type I, which develop from invagination of endometrial implants in the ovarian cortex, and type II, which stem from invasion of functional cysts by endometrial implants or type I endometrioma. This distinction guides surgical management. We hope this article and its accompanying video will be helpful in guiding laparoscopic excision of type I and II endometriomas.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Related article:
Endometriosis: Expert answers to 7 crucial questions on diagnosis
Etiology
Endometriomas are extensively described in the literature, and their origin is the subject of several theories. In 1921, Sampson noted luteal membrane and ovarian epithelial tissues within endometriomas and was the first to indicate that endometriomas may result from the invasion of functional cysts by endometrial tissue.2,4,5 In 1979, Czernobilsky and Morris6 found endometrial and oviduct-like epithelium in ovarian endometriosis and concluded that ovarian tissue may be a common histologic precursor. Several other authors subsequently have reported finding different types of tissue within ovarian endometriomas, and not all of these chocolate cysts showed histologic evidence of endometriosis.4,7,8
Read about the classification of endometriomas
Disease classification
Our classification system identifies 2 types of endometriomas on the basis of their etiologies and characteristics. Type I, which arise from endometrial tissue implanted on the ovarian surface, are also called true endometriomas. Invagination of cortex and subsequent hemorrhage from endometrial tissue result in cyst formation. Endometrial tissue (endometrial stroma and glands) is histologically present in all type I endometriomas.1,4,9 These endometriomas usually are small (<5 cm in diameter) and have a densely adherent fibrous capsule.4 Often, there is no clear plane between cyst wall and ovarian stroma.3
Type II endometriomas arise from functional cysts involved in or invaded by cortical or pelvic side-wall endometrial implants or by type I endometriomas. Type II endometriomas are subclassified by the extent of endometrial implant involvement in the cyst wall. Type IIA endometriomas are hemorrhagic cysts with less than 10% of endometrial tissue within the cyst wall. Similar to the functional cysts from which they originate, type IIA endometriomas have a cyst wall that is separated easily from ovarian tissue during surgery.4,7,9 Although type II endometriomas tend to be larger than their type I counterparts, in some cases they are identified at an early stage of 2 to 5 cm. Endometriomas larger than 5 cm are almost always type II.4
Type IIB and IIC endometriomas have endometrial implants and fibrosis within their cyst walls, with progressively more endometrial invasion in type IIC endometriomas (>50%) than in type IIB (10% to 50%). Consequently, type IIB cysts are relatively easy to dissect from ovarian tissue, except adjacent to an endometriotic area where the cyst densely adheres to the ovarian stroma. In type IIC, endometrial tissue more extensively penetrates the capsule, making dissection of diseased tissue from the ovarian stroma more difficult; in fact, separating type IIC cyst wall from ovarian stroma can be as challenging as excising a type I endometrioma.7 In most cases, a type IIC cyst is attached by adhesions and fibrosis to the pelvic side wall or uterus and ruptures during mobilization (TABLE).

Related article:
Imaging the endometrioma and mature cystic teratoma
Presentation and diagnosis
Almost all patients with an endometrioma concurrently have peritoneal endometriosis, which is characterized by dysmenorrhea, dyspareunia, chronic pelvic pain, infertility, and, in some cases, gastrointestinal or genitourinary dysfunction.1 Pelvic examination may reveal an adnexal mass that is an endometrioma, or an endometrioma may appear on imaging obtained in a pelvic pain or infertility work-up. Given its 73% sensitivity, 94% specificity, safety, and low cost, transvaginal ultrasonography is the preferred imaging modality for endometrioma.3 The characteristic ultrasonographic appearance is that of a round, homogeneous, fluid-filled mass with low-level echoes.1 Magnetic resonance imaging is appropriate when a more sensitive imaging modality is indicated, as for a patient with risk factors for malignancy.3,10–12
Read about the surgical management of endometriomas
Surgical management
Indications for surgical excision of endometriomas include pelvic pain, infertility, and prevention and diagnosis of malignancy. Endometriomas may be excised prior to use of assisted reproductive technology.13–15 Medical therapy, such as oral contraceptives, can be used to reduce the size of endometriomas but does not improve fertility.3 Certain ovarian cancers are more common in women with endometriosis, and ovarian tumors are thought to develop in about 1% of ovarian endometriosis cases.1,12 Therefore, endometrioma excision may reduce the risk of malignancy. As with other ovarian cysts, large endometriomas may be excised to reduce the risks of rupture and torsion.
Approach
Laparoscopy is the preferred approach for endometrioma excision. Controversy exists regarding the ideal procedure: complete excision (with stripping of the cyst capsule) or drainage and ablation of the cyst wall. Compared with drainage and ablation, excision reduces recurrence of endometriomas; relieves dysmenorrhea, dyspareunia, pelvic pain, and other symptoms; and improves fertility.13,16 The recurrence rate may be as low as 5.8% with complete excision but is 90% with simple transvaginal aspiration.17,18 If not performed properly, however, cyst capsule stripping may damage nearby ovarian stroma and decrease the ovarian reserve.14 Some authors have advocated combining excision and ablation—performing cystectomy until there is no longer a clear plane between capsule and ovarian stroma and then ablating any remaining endometrial tissue.8
With type I and IIC endometriomas, we have seen the endometrial cyst wall infiltrating the ovarian stroma so deeply there is not always a definable plane. By contrast, type IIA and IIB endometriomas typically have a plane between the cyst wall and the ovarian cortex. In type II endometriomas, endometrial implants on the ovarian cortex infiltrate the plane of the cyst wall such that the juxtaposing lipomatous follicular cyst detaches with minimal intraoperative traction. Portions of type II endometriomas containing fibrosis and adhesions may become more difficult to peel off the cyst wall. For most endometriomas, at least 1 spot is difficult to peel off the ovary, and extra care must be taken at the hilum of ovary to avoid excising healthy ovarian cortex.4,5,7,8
Our surgical approach accounts for the described variations in type I and II endometriomas. Endometrial contents often spill as the endometrioma is dissected off neighboring structures. When possible, endometriomas should be aspirated and irrigated prior to cystectomy to avoid seeding the pelvis and abdomen with spilled endometriotic contents. We use hydrodissection, the injection of dilute vasopressin with a laparoscopic needle, to create a plane between cyst wall and ovarian stroma and strip the cyst capsule with laparoscopic graspers. Type I endometriomas adhere densely to the ovary. Given the presence of fibrosis and adhesions, the cyst is excised in a piecemeal fashion. Care is taken to remove any endometrial implants from the ovary while preserving as much of the ovarian tissue as possible.1
Type II endometriomas are larger cysts originating from the invasion of endometrial implants or type I endometrioma into functional cysts. The difficulty of capsule excision varies according to the extent of endometrial invasion. Type IIA endometriomas contain less than 10% endometrial tissue within the cyst capsule. Thus, the standard ovarian cystectomy stripping technique is successful in removing more than 90% of the cyst capsule. Special care is taken in stripping the residual small portion that involves the endometrial glands and stroma and adheres densely to the ovary.
The larger proportion of endometrial tissue present in type IIB and IIC endometriomas degrades the plane between the cyst capsule and the ovarian stroma, making excision more difficult. Similar to the type I excision, a piecemeal approach is often necessary. If complete stripping of the cyst capsule would result in extensive loss of healthy ovarian tissue, then electrocautery, plasma energy, or laser ablation can be selectively used to destroy focal areas of endometrial invasion. Complete ablation may be difficult, as the endometrioma wall can be up to 5 mm thick.19 For these thick-walled endometriomas, we recommend excision (vs ablation), which lowers the risk of endometrioma recurrence.
Related article:
Endometriosis and pain: Expert answers to 6 questions targeting your management options
- Endometriomas are common adnexal masses in women affected by endometriosis and may exacerbate pelvic pain and impair fertility. Classification of endometriomas into type I and type II,depending on their etiology and characteristics, can guide minimally invasive surgical management.
- Type I endometriomas arise from invagination of endometrial implants on the ovarian cortex, resulting in dense fibrosis and adhesions. These lesions typically require piecemeal excision in order to completely remove the cyst capsule.
- Type II endometriomas result from invasion of endometrial tissue into preexisting functional cysts and are further subclassified by the proportion of cyst capsule containing endometrial tissue (IIA <10%, IIB 10% to 50%, IIC >50%).
- The difficulty of excising type II endometriomas correlates with the degree of endometrial invasion, with type IIA being relatively straightforward and type IIC being as challenging and piecemeal as type I.
- We generally favor complete excision rather than ablation of the cyst capsule, except for when excision would result in an unacceptable loss of healthy ovarian tissue.
Conclusion
Endometriomas, common adnexal masses in women affected by endometriosis, may exacerbate pelvic pain and impair fertility. Gynecologists should be prepared to excise endometriomas completely and exercise care in preserving as much of the ovarian stroma as possible. We classify endometriomas into 2 types: type I, which develop from invagination of endometrial implants in the ovarian cortex, and type II, which stem from invasion of functional cysts by endometrial implants or type I endometrioma. This distinction guides surgical management. We hope this article and its accompanying video will be helpful in guiding laparoscopic excision of type I and II endometriomas.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Nezhat C, Buescher E, Paka C, et al. Video-assisted laparoscopic treatment of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat’s Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013:265–302.
- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98(3):511–519.
- Keyhan S, Hughes C, Price T, Muasher S. An update on surgical versus expectant management of ovarian endometriomas in infertile women. Biomed Res Int. 2015;2015:204792.
- Nezhat F, Nezhat C, Allan CJ, Metzger DA, Sears DL. Clinical and histologic classification of endometriomas. Implications for a mechanism of pathogenesis. J Reprod Med. 1992;37(9):771–776.
- Burney RO, Giudice LC. The pathogenesis of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat’s Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013:252–258.
- Czernobilsky B, Morris WJ. A histologic study of ovarian endometriosis with emphasis on hyperplastic and atypical changes. Obstet Gynecol. 1979;53(3):318–323.
- Nezhat F, Nezhat C, Nezhat C, Admon D. A fresh look at ovarian endometriomas. Contemp Ob Gyn. 1994;39(11):81–94.
- Donnez J, Lousse JC, Jadoul P, Donnez O, Squifflet J. Laparoscopic management of endometriomas using a combined technique of excisional (cystectomy) and ablative surgery. Fertil Steril. 2010;94(1):28–32.
- Nezhat C, Nezhat F, Nezhat C, Seidman DS. Classification of endometriosis. Improving the classification of endometriotic ovarian cysts. Hum Reprod. 1994;9(12):2212–2213.
- Nezhat FR, Pejovic T, Reis FM, Guo SW. The link between endometriosis and ovarian cancer: clinical implications. Int J Gynecol Cancer. 2014;24(4):623–628.
- Nezhat F, Apostol R, Mahmoud M, el Daouk M. Malignant transformation of endometriosis and its clinical significance. Fertil Steril. 2014;102(2):342–344.
- Nezhat FR, Apostal R, Nezhat C, Pejovic T. New insights in the pathophysiology of ovarian cancer and implications for screening and prevention. Am J Obstet Gynecol. 2015;213(3):262–267.
- Hart RJ, Hickey M, Maouris P, Buckett W. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2008;(2):CD004992.
- Yates J. Endometriosis and infertility: expert answers to 6 questions to help pinpoint the best route to pregnancy. OBG Manag. 2015;27(6):30–35.
- Littman E, Giudice L, Lathi R, Berker B, Milki A, Nezhat C. Role of laparoscopic treatment of endometriosis in patients with failed in vitro fertilization cycles. Fertil Steril. 2005;84(6):1574–1578.
- Exacoustos C, Zupi E, Amadio A, et al. Laparoscopic removal of endometriomas: sonographic evaluation of residual functioning ovarian tissue. Am J Obstet Gynecol. 2004;191(1):68–72.
- Gonçalves FC, Andres MP, Passman LJ, Gonçalves MO, Podgaec S. A systematic review of ultrasonography-guided transvaginal aspiration of recurrent ovarian endometrioma. Int J Gynaecol Obstet. 2016;134(1):3–7.
- Alborzi S, Momtahan M, Parsanezhad ME, Dehbashi S, Zolghadri J, Alborzi S. A prospective, randomized study comparing laparoscopic ovarian cystectomy versus fenestration and coagulation in patients with endometriomas. Fertil Steril. 2004;82(6):1633–1637.
- Nezhat C, Crowgey SR, Garrison CP. Surgical treatment of endometriosis via laser laparoscopy. Fertil Steril. 1986;45(6):778–783.
- Nezhat C, Buescher E, Paka C, et al. Video-assisted laparoscopic treatment of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat’s Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013:265–302.
- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98(3):511–519.
- Keyhan S, Hughes C, Price T, Muasher S. An update on surgical versus expectant management of ovarian endometriomas in infertile women. Biomed Res Int. 2015;2015:204792.
- Nezhat F, Nezhat C, Allan CJ, Metzger DA, Sears DL. Clinical and histologic classification of endometriomas. Implications for a mechanism of pathogenesis. J Reprod Med. 1992;37(9):771–776.
- Burney RO, Giudice LC. The pathogenesis of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat’s Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013:252–258.
- Czernobilsky B, Morris WJ. A histologic study of ovarian endometriosis with emphasis on hyperplastic and atypical changes. Obstet Gynecol. 1979;53(3):318–323.
- Nezhat F, Nezhat C, Nezhat C, Admon D. A fresh look at ovarian endometriomas. Contemp Ob Gyn. 1994;39(11):81–94.
- Donnez J, Lousse JC, Jadoul P, Donnez O, Squifflet J. Laparoscopic management of endometriomas using a combined technique of excisional (cystectomy) and ablative surgery. Fertil Steril. 2010;94(1):28–32.
- Nezhat C, Nezhat F, Nezhat C, Seidman DS. Classification of endometriosis. Improving the classification of endometriotic ovarian cysts. Hum Reprod. 1994;9(12):2212–2213.
- Nezhat FR, Pejovic T, Reis FM, Guo SW. The link between endometriosis and ovarian cancer: clinical implications. Int J Gynecol Cancer. 2014;24(4):623–628.
- Nezhat F, Apostol R, Mahmoud M, el Daouk M. Malignant transformation of endometriosis and its clinical significance. Fertil Steril. 2014;102(2):342–344.
- Nezhat FR, Apostal R, Nezhat C, Pejovic T. New insights in the pathophysiology of ovarian cancer and implications for screening and prevention. Am J Obstet Gynecol. 2015;213(3):262–267.
- Hart RJ, Hickey M, Maouris P, Buckett W. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2008;(2):CD004992.
- Yates J. Endometriosis and infertility: expert answers to 6 questions to help pinpoint the best route to pregnancy. OBG Manag. 2015;27(6):30–35.
- Littman E, Giudice L, Lathi R, Berker B, Milki A, Nezhat C. Role of laparoscopic treatment of endometriosis in patients with failed in vitro fertilization cycles. Fertil Steril. 2005;84(6):1574–1578.
- Exacoustos C, Zupi E, Amadio A, et al. Laparoscopic removal of endometriomas: sonographic evaluation of residual functioning ovarian tissue. Am J Obstet Gynecol. 2004;191(1):68–72.
- Gonçalves FC, Andres MP, Passman LJ, Gonçalves MO, Podgaec S. A systematic review of ultrasonography-guided transvaginal aspiration of recurrent ovarian endometrioma. Int J Gynaecol Obstet. 2016;134(1):3–7.
- Alborzi S, Momtahan M, Parsanezhad ME, Dehbashi S, Zolghadri J, Alborzi S. A prospective, randomized study comparing laparoscopic ovarian cystectomy versus fenestration and coagulation in patients with endometriomas. Fertil Steril. 2004;82(6):1633–1637.
- Nezhat C, Crowgey SR, Garrison CP. Surgical treatment of endometriosis via laser laparoscopy. Fertil Steril. 1986;45(6):778–783.
IN THIS ARTICLE
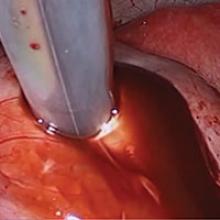
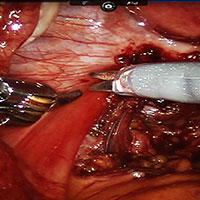
Robot-assisted laparoscopic excision of a rectovaginal endometriotic nodule
A rectovaginal endometriosis (RVE) is the most severe form of endometriosis. The gold standard for diagnosis is laparoscopy with histologic confirmation. A review of the literature suggests that surgery improves up to 70% of symptoms with generally favorable outcomes.
In this video, we provide a general introduction to endometriosis and a discussion of disease treatment options, ranging from hormonal suppression to radical bowel resections. We also illustrate the steps in robot-assisted laparoscopic excision of an RVE nodule:
- identify the borders of the rectosigmoid
- dissect the pararectal spaces
- release the rectosigmoid from its attachment to the RVE nodule
- identify and isolate the ureter(s)
- determine the margins of the nodule
- ensure complete resection.
Excision of an RVE nodule is a technically challenging surgical procedure. Use of the robot for resection is safe and feasible when performed by a trained and experienced surgeon.
I am pleased to bring you this video, and I hope that it is helpful to your practice.
>> Arnold P. Advincula, MD

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
A rectovaginal endometriosis (RVE) is the most severe form of endometriosis. The gold standard for diagnosis is laparoscopy with histologic confirmation. A review of the literature suggests that surgery improves up to 70% of symptoms with generally favorable outcomes.
In this video, we provide a general introduction to endometriosis and a discussion of disease treatment options, ranging from hormonal suppression to radical bowel resections. We also illustrate the steps in robot-assisted laparoscopic excision of an RVE nodule:
- identify the borders of the rectosigmoid
- dissect the pararectal spaces
- release the rectosigmoid from its attachment to the RVE nodule
- identify and isolate the ureter(s)
- determine the margins of the nodule
- ensure complete resection.
Excision of an RVE nodule is a technically challenging surgical procedure. Use of the robot for resection is safe and feasible when performed by a trained and experienced surgeon.
I am pleased to bring you this video, and I hope that it is helpful to your practice.
>> Arnold P. Advincula, MD

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
A rectovaginal endometriosis (RVE) is the most severe form of endometriosis. The gold standard for diagnosis is laparoscopy with histologic confirmation. A review of the literature suggests that surgery improves up to 70% of symptoms with generally favorable outcomes.
In this video, we provide a general introduction to endometriosis and a discussion of disease treatment options, ranging from hormonal suppression to radical bowel resections. We also illustrate the steps in robot-assisted laparoscopic excision of an RVE nodule:
- identify the borders of the rectosigmoid
- dissect the pararectal spaces
- release the rectosigmoid from its attachment to the RVE nodule
- identify and isolate the ureter(s)
- determine the margins of the nodule
- ensure complete resection.
Excision of an RVE nodule is a technically challenging surgical procedure. Use of the robot for resection is safe and feasible when performed by a trained and experienced surgeon.
I am pleased to bring you this video, and I hope that it is helpful to your practice.
>> Arnold P. Advincula, MD

Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Why are there delays in the diagnosis of endometriosis?
Endometriosis is a common gynecologic problem in adolescents and women. It often presents with pelvic pain, an ovarian endometrioma, and/or subfertility. In a prospective study of 116,678 nurses, the incidence of a new surgical diagnosis of endometriosis was greatest among women aged 25 to 29 years and lowest among women older than age 44.1 Using the incidence data from this study, the calculated prevalence of endometriosis in this large cohort of women of reproductive age was approximately 8%.
Although endometriosis is known to be a very common gynecologic problem, many studies report that there can be long delays between onset of pelvic pain symptoms and the diagnosis of endometriosis (Figure 1).2−6 Combining the results from 5 studies, involving 1,187 women, the mean age of onset of pelvic pain symptoms was 22.1 years, and the mean age at the diagnosis of endometriosis was 30.7 years. This is a difference of 8.6 years between the age of symptom onset and age at diagnosis.2−6

What factors contribute to the diagnosis delay?
Both patient and physician factors contribute to the reported lengthy delay between symptom onset and endometriosis diagnosis.7,8 Differentiating dysmenorrhea due to primary and secondary causes is difficult for both patients and physicians. Women may conceal the severity of menstrual pain to avoid both the embarrassment of drawing attention to themselves and being stigmatized as unable to cope. Most disappointing is that many women with endometriosis reported that they asked their clinician if endometriosis could be the cause of their severe dysmenorrhea and were told, “No.”7,8
Of interest, the reported delay in the diagnosis of endometriosis is much shorter for women who pre-sent with infertility than for women who present with pelvic pain. In one study from the United States, the delay to diagnosis was 3.13 years for women who presented with infertility and 6.35 years for women who presented with severe pelvic pain.3 This suggests that clinicians and patients more rapidly pursue the diagnosis of endometriosis in women with infertility, but not pelvic pain.
Related article:
Endometriosis: Expert answers to 7 crucial questions on diagnosis
Initial treatment of pelvic pain with NSAIDs and estrogen—progestin contraceptives
Many women with undiagnosed endometriosis present with pelvic pain symptoms including moderate to severe dysmenorrhea. These women are often empirically treated with nonsteroidal anti-inflammatory drugs (NSAIDs) and combination estrogen−progestin contraceptives in either a cyclic or continuous manner.9,10 Since many women with endometriosis will have a reduction in their pelvic pain with NSAID and contraceptive treatment, diagnosis of their endometriosis may be delayed until their disease progresses years after their initial presentation. It is important to gently alert these women to the possibility that they have undiagnosed endometriosis as the cause of their pain symptoms and encourage them to report any worsening pain symptoms in a timely manner.
Sometimes women with pelvic pain are treated with NSAIDs and contraceptives but no significant reduction in pain symptoms occurs. For these women, speedy consideration should be given to offering a laparoscopy to determine the cause of their pain.
Related article:
Avoiding “shotgun” treatment: New thoughts on endometriosis-associated pelvic pain
Diagnosing endometriosis relies on identifying flags in the patient’s history
The gold standard for endometriosis diagnosis is surgical visualization of endometriosis lesions, most often with laparoscopy, plus histologic confirmation of endometriosis on a tissue biopsy.9,10 A key to reducing the time between onset of symptoms and diagnosis of endometriosis is identifying adolescents and women who are at high risk for having the disease. These women should be offered a laparoscopy procedure. In women with moderate to severe pelvic pain of at least 6 months duration, medical history, physical examination, and imaging studies can be helpful in identifying those at increased risk for endometriosis.
Items from the patient history that might raise the likelihood of endometriosis include:
- abdominopelvic pain, dysmenorrhea, menorrhagia, subfertility, dyspareunia and/or postcoital bleeding11
- symptoms of dysmenorrhea and/or dyspareunia that are not responsive to NSAIDs or estrogen−progestin contraceptives12
- symptoms of dysmenorrhea and/or dyspareunia associated with absenteeism from school or work13
- multiple visits to the emergency department for severe dysmenorrhea
- endometriosis in the patient’s mother or sister
- subfertility with regular ovulation, patent fallopian tubes, and a partner with a normal semen analysis
- urinary frequency, urgency, and/or pain on urination
- diarrhea, constipation, nausea, dyschezia, bowel cramping, abdominal distention, and early satiety.
A daunting clinical challenge is that symptoms of endometriosis overlap with other gynecologic and nongynecologic problems including pelvic infection, adhesions, ovarian cysts, fibroids, irritable bowel syndrome, inflammatory bowel disease, interstitial cystitis, myofascial pain, depression, and history of sexual abuse.
Diagnosing endometriosis relies on identifying flags on physical exam
Physical examination findings that raise the likelihood that the patient has endometriosis include:
- fixed and retroverted uterus
- adnexal mass
- lesions of the cervix or posterior fornix that visually appear to be endometriosis
- uterosacral ligament abnormalities, including tenderness, thickening, and/or nodularity14,15
- lateral displacement of the cervix (FIGURE 2)16,17
- severe cervical stenosis.
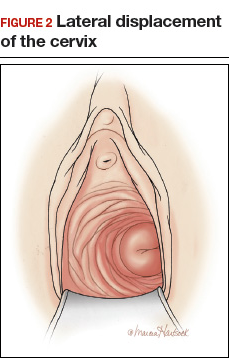
In one study of 57 women with a surgical diagnosis of endometriosis, uterosacral ligament abnormalities, lateral displacement of the cervix, and cervical stenosis were observed in 47%, 28%, and 19% of the women, respectively.17 In this same study 22 women had none of these findings, but 8 had a complex ovarian mass consistent with endometriosis.
The possibility of endometriosis increases as the number of history and physical examination findings suggestive of endometriosis increase.
Related article:
Endometriosis and pain: Expert answers to 6 questions targeting your management options
When transvaginal ultrasound can aid diagnosis
Most women with endometriosis have normal transvaginal ultrasonography (TVUS) results because ultrasound cannot detect small isolated peritoneal lesions of endometriosis present in Stage I disease, the most common stage of endometriosis. However, ultrasound is useful in detecting both ovarian endometriomas and nodules of deep infiltrating endometriosis (DIE).18 TVUS has excellent sensitivity (>90%) and specificity (>90%) for the detection of ovarian endometriomas because these cysts have characteristic, homogenous, low-level internal echoes.19,20 For the diagnosis of DIE of the uterosacral ligaments and rectovaginal septum, TVUS has fair sensitivity (>50%) and excellent specificity (>90%).21 In most studies, magnetic resonance imaging performs no better than TVUS for imaging ovarian endometriomas and DIE. Hence, TVUS is the preferred imaging modality for detecting endometriosis.22
- Endometriosis is a common gynecologic disease. Approximately 8% of women of reproductive age have the condition.
- Many patients report lengthy delays between the onset of symptoms of pelvic pain and the diagnosis of endometriosis.
- Both patients and clinicians contribute to the delay in the diagnosis of endometriosis: Women are often reluctant to report the severity of their pelvic pain symptoms, and clinicians often under-respond to a patient's report of severe pelvic pain symptoms.
- First-line therapy for the treatment of moderate to severe dysmenorrhea is nonsteroidal anti-inflammatory drugs and estrogen−progestin contraceptives.
- Increasing vigilance for endometriosis will shorten the time between onset of symptoms and definitive diagnosis.
- Reducing the time between the onset of symptoms and diagnosis of endometriosis will improve the quality of life of women with the disease because they will receive timely treatment.
This is a practice gap we can close
Clinicians take great pride in accurately solving patient problems in a timely and efficient manner. Substantial research indicates that we can improve the timeliness of our diagnosis of endometriosis. By acknowledging patients’ pain symptoms and recognizing the myriad symptoms and physical examination and imaging findings that are associated with endometriosis, we will close the gap and make this diagnosis with greater speed.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160(8):784−796.
- Hadfield R, Mardon H, Barlow D, Kennedy S. Delay in the diagnosis of endometriosis: a survey of women from the USA and UK. Hum Reprod. 1996;11(4):878−880.
- Dmowski WP, Lesniewicz R, Rana N, Pepping P, Noursalehi M. Changing trends in the diagnosis of endometriosis: a comparative study of women with pelvic endometriosis presenting with chronic pelvic pain or infertility. Fertil Steril. 1997;67(2):238−243.
- Arruda MS, Petta CA, Abrão MS, Benetti-Pinto CL. Time elapsed from onset of symptoms to diagnosis of endometriosis in a cohort study of Brazilian women. Hum Reprod. 2003;18(4):756−759.
- Husby GK, Haugen RS, Moen MH. Diagnostic delay in women with pain and endometriosis. Acta Obstet Gynecol Scand. 2003;82(7):649−653.
- Hudelist G, Fritzer N, Thomas A, et al. Diagnostic delay for endometriosis in Austria and Germany: causes and possible consequences. Hum Reprod. 2012;27(12):3412−3416.
- Ballard K, Lowton K, Wright J. What's the delay? A qualitative study of women's experiences of reaching a diagnosis of endometriosis. Fertil Steril. 2006;86(5):1296−1301.
- Seear K. The etiquette of endometriosis: stigmatisation, menstrual concealment and the diagnostic delay. Soc Sci Med. 2009;69(8):1220−1227.
- Falcone T, Lebovic DI. Clinical management of endometriosis. Obstet Gynecol. 2011;118(3):691−705.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 114: Management of endometriosis. Obstet Gynecol. 2010;116(1):223−236.
- Ballard KD, Seaman HE, de Vries CS, Wright JT. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study--Part 1. BJOG. 2008;115(11):1382−1391.
- Steenberg CK, Tanbo TG, Qvigstad E. Endometriosis in adolescence: predictive markers and management. Acta Obstet Gynecol Scand. 2013;92(5):491−495.
- Zannoni L, Giorgi M, Spagnolo E, Montanari G, Villa G, Seracchioli R. Dysmenorrhea, absenteeism from school, and symptoms suspicious for endometriosis in adolescents. J Pediatr Adolesc Gynecol. 2014;27(5):258−265.
- Cheewadhanaraks S, Peeyananjarassri K, Dhanaworavibul K, Liabsuetrakul T. Positive predictive value of clinical diagnosis of endometriosis. J Med Assoc Thai. 2004;87(7):740−744.
- Guerriero S, Ajossa S, Gerada M, Virgilio B, Angioni S, Melis GB. Diagnostic value of transvaginal 'tenderness-guided' ultrasonography for the prediction of location of deep endometriosis. Hum Reprod. 2008;23(11):2452−2457.
- Propst AM, Storti K, Barbieri RL. Lateral cervical displacement is associated with endometriosis. Fertil Steril. 1998;70(3):568−570.
- Barbieri RL, Propst AM. Physical examination findings in women with endometriosis: uterosacral ligament abnormalities, lateral cervical displacement and cervical stenosis. J Gynecol Techniques. 1999;5:157−159.
- Guerriero S, Condous G, van den Bosch T, et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol. 2016;48(3):318−332.
- Nisenblat V, Bossuyt PM, Farquhar C, Johnson N, Hull ML. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Somigliana E, Vercellini P, Vigano P, Benaglia L, Crosignani PG, Fedele L. Non-invasive diagnosis of endometriosis: the goal or own goal? Hum Reprod. 2010;25(8):1863−1868.
- Guerriero S, Ajossa S, Minguez JA, et al. Accuracy of transvaginal ultrasound for diagnosis of deep endometriosis in uterosacral ligaments, rectovaginal septum, vagina and bladder: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2015;46(5):534−545.
- Benacerraf BR, Groszmann Y. Sonography should be the first imaging examination done to evaluate patients with suspected endometriosis. J Ultrasound Med. 2012;31(4):651−653.
Endometriosis is a common gynecologic problem in adolescents and women. It often presents with pelvic pain, an ovarian endometrioma, and/or subfertility. In a prospective study of 116,678 nurses, the incidence of a new surgical diagnosis of endometriosis was greatest among women aged 25 to 29 years and lowest among women older than age 44.1 Using the incidence data from this study, the calculated prevalence of endometriosis in this large cohort of women of reproductive age was approximately 8%.
Although endometriosis is known to be a very common gynecologic problem, many studies report that there can be long delays between onset of pelvic pain symptoms and the diagnosis of endometriosis (Figure 1).2−6 Combining the results from 5 studies, involving 1,187 women, the mean age of onset of pelvic pain symptoms was 22.1 years, and the mean age at the diagnosis of endometriosis was 30.7 years. This is a difference of 8.6 years between the age of symptom onset and age at diagnosis.2−6

What factors contribute to the diagnosis delay?
Both patient and physician factors contribute to the reported lengthy delay between symptom onset and endometriosis diagnosis.7,8 Differentiating dysmenorrhea due to primary and secondary causes is difficult for both patients and physicians. Women may conceal the severity of menstrual pain to avoid both the embarrassment of drawing attention to themselves and being stigmatized as unable to cope. Most disappointing is that many women with endometriosis reported that they asked their clinician if endometriosis could be the cause of their severe dysmenorrhea and were told, “No.”7,8
Of interest, the reported delay in the diagnosis of endometriosis is much shorter for women who pre-sent with infertility than for women who present with pelvic pain. In one study from the United States, the delay to diagnosis was 3.13 years for women who presented with infertility and 6.35 years for women who presented with severe pelvic pain.3 This suggests that clinicians and patients more rapidly pursue the diagnosis of endometriosis in women with infertility, but not pelvic pain.
Related article:
Endometriosis: Expert answers to 7 crucial questions on diagnosis
Initial treatment of pelvic pain with NSAIDs and estrogen—progestin contraceptives
Many women with undiagnosed endometriosis present with pelvic pain symptoms including moderate to severe dysmenorrhea. These women are often empirically treated with nonsteroidal anti-inflammatory drugs (NSAIDs) and combination estrogen−progestin contraceptives in either a cyclic or continuous manner.9,10 Since many women with endometriosis will have a reduction in their pelvic pain with NSAID and contraceptive treatment, diagnosis of their endometriosis may be delayed until their disease progresses years after their initial presentation. It is important to gently alert these women to the possibility that they have undiagnosed endometriosis as the cause of their pain symptoms and encourage them to report any worsening pain symptoms in a timely manner.
Sometimes women with pelvic pain are treated with NSAIDs and contraceptives but no significant reduction in pain symptoms occurs. For these women, speedy consideration should be given to offering a laparoscopy to determine the cause of their pain.
Related article:
Avoiding “shotgun” treatment: New thoughts on endometriosis-associated pelvic pain
Diagnosing endometriosis relies on identifying flags in the patient’s history
The gold standard for endometriosis diagnosis is surgical visualization of endometriosis lesions, most often with laparoscopy, plus histologic confirmation of endometriosis on a tissue biopsy.9,10 A key to reducing the time between onset of symptoms and diagnosis of endometriosis is identifying adolescents and women who are at high risk for having the disease. These women should be offered a laparoscopy procedure. In women with moderate to severe pelvic pain of at least 6 months duration, medical history, physical examination, and imaging studies can be helpful in identifying those at increased risk for endometriosis.
Items from the patient history that might raise the likelihood of endometriosis include:
- abdominopelvic pain, dysmenorrhea, menorrhagia, subfertility, dyspareunia and/or postcoital bleeding11
- symptoms of dysmenorrhea and/or dyspareunia that are not responsive to NSAIDs or estrogen−progestin contraceptives12
- symptoms of dysmenorrhea and/or dyspareunia associated with absenteeism from school or work13
- multiple visits to the emergency department for severe dysmenorrhea
- endometriosis in the patient’s mother or sister
- subfertility with regular ovulation, patent fallopian tubes, and a partner with a normal semen analysis
- urinary frequency, urgency, and/or pain on urination
- diarrhea, constipation, nausea, dyschezia, bowel cramping, abdominal distention, and early satiety.
A daunting clinical challenge is that symptoms of endometriosis overlap with other gynecologic and nongynecologic problems including pelvic infection, adhesions, ovarian cysts, fibroids, irritable bowel syndrome, inflammatory bowel disease, interstitial cystitis, myofascial pain, depression, and history of sexual abuse.
Diagnosing endometriosis relies on identifying flags on physical exam
Physical examination findings that raise the likelihood that the patient has endometriosis include:
- fixed and retroverted uterus
- adnexal mass
- lesions of the cervix or posterior fornix that visually appear to be endometriosis
- uterosacral ligament abnormalities, including tenderness, thickening, and/or nodularity14,15
- lateral displacement of the cervix (FIGURE 2)16,17
- severe cervical stenosis.

In one study of 57 women with a surgical diagnosis of endometriosis, uterosacral ligament abnormalities, lateral displacement of the cervix, and cervical stenosis were observed in 47%, 28%, and 19% of the women, respectively.17 In this same study 22 women had none of these findings, but 8 had a complex ovarian mass consistent with endometriosis.
The possibility of endometriosis increases as the number of history and physical examination findings suggestive of endometriosis increase.
Related article:
Endometriosis and pain: Expert answers to 6 questions targeting your management options
When transvaginal ultrasound can aid diagnosis
Most women with endometriosis have normal transvaginal ultrasonography (TVUS) results because ultrasound cannot detect small isolated peritoneal lesions of endometriosis present in Stage I disease, the most common stage of endometriosis. However, ultrasound is useful in detecting both ovarian endometriomas and nodules of deep infiltrating endometriosis (DIE).18 TVUS has excellent sensitivity (>90%) and specificity (>90%) for the detection of ovarian endometriomas because these cysts have characteristic, homogenous, low-level internal echoes.19,20 For the diagnosis of DIE of the uterosacral ligaments and rectovaginal septum, TVUS has fair sensitivity (>50%) and excellent specificity (>90%).21 In most studies, magnetic resonance imaging performs no better than TVUS for imaging ovarian endometriomas and DIE. Hence, TVUS is the preferred imaging modality for detecting endometriosis.22
- Endometriosis is a common gynecologic disease. Approximately 8% of women of reproductive age have the condition.
- Many patients report lengthy delays between the onset of symptoms of pelvic pain and the diagnosis of endometriosis.
- Both patients and clinicians contribute to the delay in the diagnosis of endometriosis: Women are often reluctant to report the severity of their pelvic pain symptoms, and clinicians often under-respond to a patient's report of severe pelvic pain symptoms.
- First-line therapy for the treatment of moderate to severe dysmenorrhea is nonsteroidal anti-inflammatory drugs and estrogen−progestin contraceptives.
- Increasing vigilance for endometriosis will shorten the time between onset of symptoms and definitive diagnosis.
- Reducing the time between the onset of symptoms and diagnosis of endometriosis will improve the quality of life of women with the disease because they will receive timely treatment.
This is a practice gap we can close
Clinicians take great pride in accurately solving patient problems in a timely and efficient manner. Substantial research indicates that we can improve the timeliness of our diagnosis of endometriosis. By acknowledging patients’ pain symptoms and recognizing the myriad symptoms and physical examination and imaging findings that are associated with endometriosis, we will close the gap and make this diagnosis with greater speed.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Endometriosis is a common gynecologic problem in adolescents and women. It often presents with pelvic pain, an ovarian endometrioma, and/or subfertility. In a prospective study of 116,678 nurses, the incidence of a new surgical diagnosis of endometriosis was greatest among women aged 25 to 29 years and lowest among women older than age 44.1 Using the incidence data from this study, the calculated prevalence of endometriosis in this large cohort of women of reproductive age was approximately 8%.
Although endometriosis is known to be a very common gynecologic problem, many studies report that there can be long delays between onset of pelvic pain symptoms and the diagnosis of endometriosis (Figure 1).2−6 Combining the results from 5 studies, involving 1,187 women, the mean age of onset of pelvic pain symptoms was 22.1 years, and the mean age at the diagnosis of endometriosis was 30.7 years. This is a difference of 8.6 years between the age of symptom onset and age at diagnosis.2−6

What factors contribute to the diagnosis delay?
Both patient and physician factors contribute to the reported lengthy delay between symptom onset and endometriosis diagnosis.7,8 Differentiating dysmenorrhea due to primary and secondary causes is difficult for both patients and physicians. Women may conceal the severity of menstrual pain to avoid both the embarrassment of drawing attention to themselves and being stigmatized as unable to cope. Most disappointing is that many women with endometriosis reported that they asked their clinician if endometriosis could be the cause of their severe dysmenorrhea and were told, “No.”7,8
Of interest, the reported delay in the diagnosis of endometriosis is much shorter for women who pre-sent with infertility than for women who present with pelvic pain. In one study from the United States, the delay to diagnosis was 3.13 years for women who presented with infertility and 6.35 years for women who presented with severe pelvic pain.3 This suggests that clinicians and patients more rapidly pursue the diagnosis of endometriosis in women with infertility, but not pelvic pain.
Related article:
Endometriosis: Expert answers to 7 crucial questions on diagnosis
Initial treatment of pelvic pain with NSAIDs and estrogen—progestin contraceptives
Many women with undiagnosed endometriosis present with pelvic pain symptoms including moderate to severe dysmenorrhea. These women are often empirically treated with nonsteroidal anti-inflammatory drugs (NSAIDs) and combination estrogen−progestin contraceptives in either a cyclic or continuous manner.9,10 Since many women with endometriosis will have a reduction in their pelvic pain with NSAID and contraceptive treatment, diagnosis of their endometriosis may be delayed until their disease progresses years after their initial presentation. It is important to gently alert these women to the possibility that they have undiagnosed endometriosis as the cause of their pain symptoms and encourage them to report any worsening pain symptoms in a timely manner.
Sometimes women with pelvic pain are treated with NSAIDs and contraceptives but no significant reduction in pain symptoms occurs. For these women, speedy consideration should be given to offering a laparoscopy to determine the cause of their pain.
Related article:
Avoiding “shotgun” treatment: New thoughts on endometriosis-associated pelvic pain
Diagnosing endometriosis relies on identifying flags in the patient’s history
The gold standard for endometriosis diagnosis is surgical visualization of endometriosis lesions, most often with laparoscopy, plus histologic confirmation of endometriosis on a tissue biopsy.9,10 A key to reducing the time between onset of symptoms and diagnosis of endometriosis is identifying adolescents and women who are at high risk for having the disease. These women should be offered a laparoscopy procedure. In women with moderate to severe pelvic pain of at least 6 months duration, medical history, physical examination, and imaging studies can be helpful in identifying those at increased risk for endometriosis.
Items from the patient history that might raise the likelihood of endometriosis include:
- abdominopelvic pain, dysmenorrhea, menorrhagia, subfertility, dyspareunia and/or postcoital bleeding11
- symptoms of dysmenorrhea and/or dyspareunia that are not responsive to NSAIDs or estrogen−progestin contraceptives12
- symptoms of dysmenorrhea and/or dyspareunia associated with absenteeism from school or work13
- multiple visits to the emergency department for severe dysmenorrhea
- endometriosis in the patient’s mother or sister
- subfertility with regular ovulation, patent fallopian tubes, and a partner with a normal semen analysis
- urinary frequency, urgency, and/or pain on urination
- diarrhea, constipation, nausea, dyschezia, bowel cramping, abdominal distention, and early satiety.
A daunting clinical challenge is that symptoms of endometriosis overlap with other gynecologic and nongynecologic problems including pelvic infection, adhesions, ovarian cysts, fibroids, irritable bowel syndrome, inflammatory bowel disease, interstitial cystitis, myofascial pain, depression, and history of sexual abuse.
Diagnosing endometriosis relies on identifying flags on physical exam
Physical examination findings that raise the likelihood that the patient has endometriosis include:
- fixed and retroverted uterus
- adnexal mass
- lesions of the cervix or posterior fornix that visually appear to be endometriosis
- uterosacral ligament abnormalities, including tenderness, thickening, and/or nodularity14,15
- lateral displacement of the cervix (FIGURE 2)16,17
- severe cervical stenosis.

In one study of 57 women with a surgical diagnosis of endometriosis, uterosacral ligament abnormalities, lateral displacement of the cervix, and cervical stenosis were observed in 47%, 28%, and 19% of the women, respectively.17 In this same study 22 women had none of these findings, but 8 had a complex ovarian mass consistent with endometriosis.
The possibility of endometriosis increases as the number of history and physical examination findings suggestive of endometriosis increase.
Related article:
Endometriosis and pain: Expert answers to 6 questions targeting your management options
When transvaginal ultrasound can aid diagnosis
Most women with endometriosis have normal transvaginal ultrasonography (TVUS) results because ultrasound cannot detect small isolated peritoneal lesions of endometriosis present in Stage I disease, the most common stage of endometriosis. However, ultrasound is useful in detecting both ovarian endometriomas and nodules of deep infiltrating endometriosis (DIE).18 TVUS has excellent sensitivity (>90%) and specificity (>90%) for the detection of ovarian endometriomas because these cysts have characteristic, homogenous, low-level internal echoes.19,20 For the diagnosis of DIE of the uterosacral ligaments and rectovaginal septum, TVUS has fair sensitivity (>50%) and excellent specificity (>90%).21 In most studies, magnetic resonance imaging performs no better than TVUS for imaging ovarian endometriomas and DIE. Hence, TVUS is the preferred imaging modality for detecting endometriosis.22
- Endometriosis is a common gynecologic disease. Approximately 8% of women of reproductive age have the condition.
- Many patients report lengthy delays between the onset of symptoms of pelvic pain and the diagnosis of endometriosis.
- Both patients and clinicians contribute to the delay in the diagnosis of endometriosis: Women are often reluctant to report the severity of their pelvic pain symptoms, and clinicians often under-respond to a patient's report of severe pelvic pain symptoms.
- First-line therapy for the treatment of moderate to severe dysmenorrhea is nonsteroidal anti-inflammatory drugs and estrogen−progestin contraceptives.
- Increasing vigilance for endometriosis will shorten the time between onset of symptoms and definitive diagnosis.
- Reducing the time between the onset of symptoms and diagnosis of endometriosis will improve the quality of life of women with the disease because they will receive timely treatment.
This is a practice gap we can close
Clinicians take great pride in accurately solving patient problems in a timely and efficient manner. Substantial research indicates that we can improve the timeliness of our diagnosis of endometriosis. By acknowledging patients’ pain symptoms and recognizing the myriad symptoms and physical examination and imaging findings that are associated with endometriosis, we will close the gap and make this diagnosis with greater speed.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160(8):784−796.
- Hadfield R, Mardon H, Barlow D, Kennedy S. Delay in the diagnosis of endometriosis: a survey of women from the USA and UK. Hum Reprod. 1996;11(4):878−880.
- Dmowski WP, Lesniewicz R, Rana N, Pepping P, Noursalehi M. Changing trends in the diagnosis of endometriosis: a comparative study of women with pelvic endometriosis presenting with chronic pelvic pain or infertility. Fertil Steril. 1997;67(2):238−243.
- Arruda MS, Petta CA, Abrão MS, Benetti-Pinto CL. Time elapsed from onset of symptoms to diagnosis of endometriosis in a cohort study of Brazilian women. Hum Reprod. 2003;18(4):756−759.
- Husby GK, Haugen RS, Moen MH. Diagnostic delay in women with pain and endometriosis. Acta Obstet Gynecol Scand. 2003;82(7):649−653.
- Hudelist G, Fritzer N, Thomas A, et al. Diagnostic delay for endometriosis in Austria and Germany: causes and possible consequences. Hum Reprod. 2012;27(12):3412−3416.
- Ballard K, Lowton K, Wright J. What's the delay? A qualitative study of women's experiences of reaching a diagnosis of endometriosis. Fertil Steril. 2006;86(5):1296−1301.
- Seear K. The etiquette of endometriosis: stigmatisation, menstrual concealment and the diagnostic delay. Soc Sci Med. 2009;69(8):1220−1227.
- Falcone T, Lebovic DI. Clinical management of endometriosis. Obstet Gynecol. 2011;118(3):691−705.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 114: Management of endometriosis. Obstet Gynecol. 2010;116(1):223−236.
- Ballard KD, Seaman HE, de Vries CS, Wright JT. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study--Part 1. BJOG. 2008;115(11):1382−1391.
- Steenberg CK, Tanbo TG, Qvigstad E. Endometriosis in adolescence: predictive markers and management. Acta Obstet Gynecol Scand. 2013;92(5):491−495.
- Zannoni L, Giorgi M, Spagnolo E, Montanari G, Villa G, Seracchioli R. Dysmenorrhea, absenteeism from school, and symptoms suspicious for endometriosis in adolescents. J Pediatr Adolesc Gynecol. 2014;27(5):258−265.
- Cheewadhanaraks S, Peeyananjarassri K, Dhanaworavibul K, Liabsuetrakul T. Positive predictive value of clinical diagnosis of endometriosis. J Med Assoc Thai. 2004;87(7):740−744.
- Guerriero S, Ajossa S, Gerada M, Virgilio B, Angioni S, Melis GB. Diagnostic value of transvaginal 'tenderness-guided' ultrasonography for the prediction of location of deep endometriosis. Hum Reprod. 2008;23(11):2452−2457.
- Propst AM, Storti K, Barbieri RL. Lateral cervical displacement is associated with endometriosis. Fertil Steril. 1998;70(3):568−570.
- Barbieri RL, Propst AM. Physical examination findings in women with endometriosis: uterosacral ligament abnormalities, lateral cervical displacement and cervical stenosis. J Gynecol Techniques. 1999;5:157−159.
- Guerriero S, Condous G, van den Bosch T, et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol. 2016;48(3):318−332.
- Nisenblat V, Bossuyt PM, Farquhar C, Johnson N, Hull ML. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Somigliana E, Vercellini P, Vigano P, Benaglia L, Crosignani PG, Fedele L. Non-invasive diagnosis of endometriosis: the goal or own goal? Hum Reprod. 2010;25(8):1863−1868.
- Guerriero S, Ajossa S, Minguez JA, et al. Accuracy of transvaginal ultrasound for diagnosis of deep endometriosis in uterosacral ligaments, rectovaginal septum, vagina and bladder: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2015;46(5):534−545.
- Benacerraf BR, Groszmann Y. Sonography should be the first imaging examination done to evaluate patients with suspected endometriosis. J Ultrasound Med. 2012;31(4):651−653.
- Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160(8):784−796.
- Hadfield R, Mardon H, Barlow D, Kennedy S. Delay in the diagnosis of endometriosis: a survey of women from the USA and UK. Hum Reprod. 1996;11(4):878−880.
- Dmowski WP, Lesniewicz R, Rana N, Pepping P, Noursalehi M. Changing trends in the diagnosis of endometriosis: a comparative study of women with pelvic endometriosis presenting with chronic pelvic pain or infertility. Fertil Steril. 1997;67(2):238−243.
- Arruda MS, Petta CA, Abrão MS, Benetti-Pinto CL. Time elapsed from onset of symptoms to diagnosis of endometriosis in a cohort study of Brazilian women. Hum Reprod. 2003;18(4):756−759.
- Husby GK, Haugen RS, Moen MH. Diagnostic delay in women with pain and endometriosis. Acta Obstet Gynecol Scand. 2003;82(7):649−653.
- Hudelist G, Fritzer N, Thomas A, et al. Diagnostic delay for endometriosis in Austria and Germany: causes and possible consequences. Hum Reprod. 2012;27(12):3412−3416.
- Ballard K, Lowton K, Wright J. What's the delay? A qualitative study of women's experiences of reaching a diagnosis of endometriosis. Fertil Steril. 2006;86(5):1296−1301.
- Seear K. The etiquette of endometriosis: stigmatisation, menstrual concealment and the diagnostic delay. Soc Sci Med. 2009;69(8):1220−1227.
- Falcone T, Lebovic DI. Clinical management of endometriosis. Obstet Gynecol. 2011;118(3):691−705.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 114: Management of endometriosis. Obstet Gynecol. 2010;116(1):223−236.
- Ballard KD, Seaman HE, de Vries CS, Wright JT. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study--Part 1. BJOG. 2008;115(11):1382−1391.
- Steenberg CK, Tanbo TG, Qvigstad E. Endometriosis in adolescence: predictive markers and management. Acta Obstet Gynecol Scand. 2013;92(5):491−495.
- Zannoni L, Giorgi M, Spagnolo E, Montanari G, Villa G, Seracchioli R. Dysmenorrhea, absenteeism from school, and symptoms suspicious for endometriosis in adolescents. J Pediatr Adolesc Gynecol. 2014;27(5):258−265.
- Cheewadhanaraks S, Peeyananjarassri K, Dhanaworavibul K, Liabsuetrakul T. Positive predictive value of clinical diagnosis of endometriosis. J Med Assoc Thai. 2004;87(7):740−744.
- Guerriero S, Ajossa S, Gerada M, Virgilio B, Angioni S, Melis GB. Diagnostic value of transvaginal 'tenderness-guided' ultrasonography for the prediction of location of deep endometriosis. Hum Reprod. 2008;23(11):2452−2457.
- Propst AM, Storti K, Barbieri RL. Lateral cervical displacement is associated with endometriosis. Fertil Steril. 1998;70(3):568−570.
- Barbieri RL, Propst AM. Physical examination findings in women with endometriosis: uterosacral ligament abnormalities, lateral cervical displacement and cervical stenosis. J Gynecol Techniques. 1999;5:157−159.
- Guerriero S, Condous G, van den Bosch T, et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol. 2016;48(3):318−332.
- Nisenblat V, Bossuyt PM, Farquhar C, Johnson N, Hull ML. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Somigliana E, Vercellini P, Vigano P, Benaglia L, Crosignani PG, Fedele L. Non-invasive diagnosis of endometriosis: the goal or own goal? Hum Reprod. 2010;25(8):1863−1868.
- Guerriero S, Ajossa S, Minguez JA, et al. Accuracy of transvaginal ultrasound for diagnosis of deep endometriosis in uterosacral ligaments, rectovaginal septum, vagina and bladder: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2015;46(5):534−545.
- Benacerraf BR, Groszmann Y. Sonography should be the first imaging examination done to evaluate patients with suspected endometriosis. J Ultrasound Med. 2012;31(4):651−653.
Sonovaginography bests negative ‘sliding sign’ in predicting deep infiltrating endometriosis
ORLANDO – Direct visualization with sonovaginography had greater success in predicting rectal/rectosigmoid deep infiltrating endometriosis than did negative transvaginal ultrasound uterine “sliding sign,” according to the findings of a prospective study of 189 women.
“Both performed quite well,” but sonovaginography was superior for predicting rectal deep infiltrating endometriosis on all measures, including accuracy – 92% vs. 88%, said Bassem Gerges, MBBS, an ob.gyn. at the University of Sydney, Kingswood.
Dr. Gerges and his colleagues evaluated 189 women of reproductive age who were scheduled for operative laparoscopy at a tertiary referral center for women. The patients had a history of chronic pelvic pain and/or endometriosis and presented between 2009 and 2013.
The women first had transvaginal ultrasound to determine if their uterine sliding sign was positive or negative, followed by sonovaginography to assess the posterior pelvic compartment for rectal or rectosigmoid deep infiltrating endometriosis. All patients then underwent laparoscopic surgery for endometriosis.
Laparoscopy revealed pouch of Douglas obliteration in 47 of the 189 women and rectal and/or rectosigmoid deep infiltrating endometriosis in 43 women.
The sensitivity of sonovaginography to predict deep infiltrating endometriosis was 88%, compared with 74% for the sliding-sign approach. Specificity was the same with the two methods at 93%. The positive predictive value was 79% vs. 74%, respectively, and the negative predictive value was 97% vs. 93%.
“These findings can help clinicians with preoperative planning,” Dr. Gerges said at the meeting, which was sponsored by AAGL.
Dr. Gerges and his colleagues also identified 11 false-negative cases in which the sliding sign was positive but laparoscopy confirmed rectal deep infiltrating endometriosis.
Previous research suggests that, in women with suspected endometriosis, a negative transvaginal ultrasound uterine sliding sign can predict rectal or rectosigmoid deep infiltrating endometriosis (Ultrasound Obstet Gynecol. 2013;41[6]:692-5, J Ultrasound Med. 2014;33:315-21). A negative sliding sign indicates the presence of uterorectal adhesions and whether the pouch of Douglas might be obliterated. The current study, however, suggested that sonovaginography might be the better method.
Dr. Gerges reported having no relevant financial disclosures.
ORLANDO – Direct visualization with sonovaginography had greater success in predicting rectal/rectosigmoid deep infiltrating endometriosis than did negative transvaginal ultrasound uterine “sliding sign,” according to the findings of a prospective study of 189 women.
“Both performed quite well,” but sonovaginography was superior for predicting rectal deep infiltrating endometriosis on all measures, including accuracy – 92% vs. 88%, said Bassem Gerges, MBBS, an ob.gyn. at the University of Sydney, Kingswood.
Dr. Gerges and his colleagues evaluated 189 women of reproductive age who were scheduled for operative laparoscopy at a tertiary referral center for women. The patients had a history of chronic pelvic pain and/or endometriosis and presented between 2009 and 2013.
The women first had transvaginal ultrasound to determine if their uterine sliding sign was positive or negative, followed by sonovaginography to assess the posterior pelvic compartment for rectal or rectosigmoid deep infiltrating endometriosis. All patients then underwent laparoscopic surgery for endometriosis.
Laparoscopy revealed pouch of Douglas obliteration in 47 of the 189 women and rectal and/or rectosigmoid deep infiltrating endometriosis in 43 women.
The sensitivity of sonovaginography to predict deep infiltrating endometriosis was 88%, compared with 74% for the sliding-sign approach. Specificity was the same with the two methods at 93%. The positive predictive value was 79% vs. 74%, respectively, and the negative predictive value was 97% vs. 93%.
“These findings can help clinicians with preoperative planning,” Dr. Gerges said at the meeting, which was sponsored by AAGL.
Dr. Gerges and his colleagues also identified 11 false-negative cases in which the sliding sign was positive but laparoscopy confirmed rectal deep infiltrating endometriosis.
Previous research suggests that, in women with suspected endometriosis, a negative transvaginal ultrasound uterine sliding sign can predict rectal or rectosigmoid deep infiltrating endometriosis (Ultrasound Obstet Gynecol. 2013;41[6]:692-5, J Ultrasound Med. 2014;33:315-21). A negative sliding sign indicates the presence of uterorectal adhesions and whether the pouch of Douglas might be obliterated. The current study, however, suggested that sonovaginography might be the better method.
Dr. Gerges reported having no relevant financial disclosures.
ORLANDO – Direct visualization with sonovaginography had greater success in predicting rectal/rectosigmoid deep infiltrating endometriosis than did negative transvaginal ultrasound uterine “sliding sign,” according to the findings of a prospective study of 189 women.
“Both performed quite well,” but sonovaginography was superior for predicting rectal deep infiltrating endometriosis on all measures, including accuracy – 92% vs. 88%, said Bassem Gerges, MBBS, an ob.gyn. at the University of Sydney, Kingswood.
Dr. Gerges and his colleagues evaluated 189 women of reproductive age who were scheduled for operative laparoscopy at a tertiary referral center for women. The patients had a history of chronic pelvic pain and/or endometriosis and presented between 2009 and 2013.
The women first had transvaginal ultrasound to determine if their uterine sliding sign was positive or negative, followed by sonovaginography to assess the posterior pelvic compartment for rectal or rectosigmoid deep infiltrating endometriosis. All patients then underwent laparoscopic surgery for endometriosis.
Laparoscopy revealed pouch of Douglas obliteration in 47 of the 189 women and rectal and/or rectosigmoid deep infiltrating endometriosis in 43 women.
The sensitivity of sonovaginography to predict deep infiltrating endometriosis was 88%, compared with 74% for the sliding-sign approach. Specificity was the same with the two methods at 93%. The positive predictive value was 79% vs. 74%, respectively, and the negative predictive value was 97% vs. 93%.
“These findings can help clinicians with preoperative planning,” Dr. Gerges said at the meeting, which was sponsored by AAGL.
Dr. Gerges and his colleagues also identified 11 false-negative cases in which the sliding sign was positive but laparoscopy confirmed rectal deep infiltrating endometriosis.
Previous research suggests that, in women with suspected endometriosis, a negative transvaginal ultrasound uterine sliding sign can predict rectal or rectosigmoid deep infiltrating endometriosis (Ultrasound Obstet Gynecol. 2013;41[6]:692-5, J Ultrasound Med. 2014;33:315-21). A negative sliding sign indicates the presence of uterorectal adhesions and whether the pouch of Douglas might be obliterated. The current study, however, suggested that sonovaginography might be the better method.
Dr. Gerges reported having no relevant financial disclosures.
AT THE AAGL GLOBAL CONGRESS
Plasma energy ablation yields pregnancy rates similar to cystectomy
ORLANDO – The first study to directly compare plasma energy treatment of ovarian endometriomas to cystectomy demonstrates similar postintervention pregnancy rates, suggesting that plasma energy ablation may be a comparable treatment option.
Researchers evaluated 104 women seeking pregnancy after 1 year or more of infertility. Women presented with unilateral or bilateral ovarian endometriomas larger than 3 cm between January 2009 and June 2014. Clinicians treated 64 patients with plasma energy ablation and another 40 with cystectomy and followed them to compare pregnancy rates.
After at least 1 year of follow-up, pregnancy rates were 68% following plasma energy ablation, compared with 80% after cystectomy. Of the 76 pregnancies, 24 were due to spontaneous conception, including 40% of pregnancies in the plasma energy group and 18% in the cystectomy group. Even after adjustment for multiple factors, the type of intervention had no statistically significant impact on achieving a subsequent pregnancy.
“Ablation using plasma energy may be considered a valuable tool that allows a high pregnancy rate,” Basma Darwish, MD, an ob.gyn. at Rouen (France) University Hospital, said at the meeting, which was sponsored by AAGL.
These similar outcomes were observed despite a higher prevalence of risk predictors for infertility in the plasma energy group at baseline. For instance, women in this cohort were significantly older and had significantly higher revised American Fertility Society (rAFS) classification scores, as well as higher rates of pouch of Douglas obliteration, deep endometriosis, and colorectal localizations.
“Endometrial ablation using plasma energy allows good postoperative [pregnancy] rates, that is well known,” Dr. Darwish said, but the technique has not been directly compared with cystectomy outcomes.
Pregnancy rates remained similar in both groups at 24 and 36 months. The probability of pregnancy was 61% in the plasma energy group versus 69% in the cystectomy group at 24 months. At 36 months, these rates changed to 84% and 78%, respectively.
A unique property of plasma energy ablation is “very limited thermal spread, both in depth and laterally,” Dr. Darwish said.
A lack of randomization is a potential limitation of the study. “Each surgeon chose the technique he or she was best at, which may have explained our good outcomes.” Dr. Darwish said. Strengths of the study include a prospective design and follow-up to 5 years. In addition, the six centers involved in the study included both private and public hospitals, “so it’s a good reflection of what happens in real life.”
The investigators plan to conduct a randomized controlled trial to confirm these findings.
ORLANDO – The first study to directly compare plasma energy treatment of ovarian endometriomas to cystectomy demonstrates similar postintervention pregnancy rates, suggesting that plasma energy ablation may be a comparable treatment option.
Researchers evaluated 104 women seeking pregnancy after 1 year or more of infertility. Women presented with unilateral or bilateral ovarian endometriomas larger than 3 cm between January 2009 and June 2014. Clinicians treated 64 patients with plasma energy ablation and another 40 with cystectomy and followed them to compare pregnancy rates.
After at least 1 year of follow-up, pregnancy rates were 68% following plasma energy ablation, compared with 80% after cystectomy. Of the 76 pregnancies, 24 were due to spontaneous conception, including 40% of pregnancies in the plasma energy group and 18% in the cystectomy group. Even after adjustment for multiple factors, the type of intervention had no statistically significant impact on achieving a subsequent pregnancy.
“Ablation using plasma energy may be considered a valuable tool that allows a high pregnancy rate,” Basma Darwish, MD, an ob.gyn. at Rouen (France) University Hospital, said at the meeting, which was sponsored by AAGL.
These similar outcomes were observed despite a higher prevalence of risk predictors for infertility in the plasma energy group at baseline. For instance, women in this cohort were significantly older and had significantly higher revised American Fertility Society (rAFS) classification scores, as well as higher rates of pouch of Douglas obliteration, deep endometriosis, and colorectal localizations.
“Endometrial ablation using plasma energy allows good postoperative [pregnancy] rates, that is well known,” Dr. Darwish said, but the technique has not been directly compared with cystectomy outcomes.
Pregnancy rates remained similar in both groups at 24 and 36 months. The probability of pregnancy was 61% in the plasma energy group versus 69% in the cystectomy group at 24 months. At 36 months, these rates changed to 84% and 78%, respectively.
A unique property of plasma energy ablation is “very limited thermal spread, both in depth and laterally,” Dr. Darwish said.
A lack of randomization is a potential limitation of the study. “Each surgeon chose the technique he or she was best at, which may have explained our good outcomes.” Dr. Darwish said. Strengths of the study include a prospective design and follow-up to 5 years. In addition, the six centers involved in the study included both private and public hospitals, “so it’s a good reflection of what happens in real life.”
The investigators plan to conduct a randomized controlled trial to confirm these findings.
ORLANDO – The first study to directly compare plasma energy treatment of ovarian endometriomas to cystectomy demonstrates similar postintervention pregnancy rates, suggesting that plasma energy ablation may be a comparable treatment option.
Researchers evaluated 104 women seeking pregnancy after 1 year or more of infertility. Women presented with unilateral or bilateral ovarian endometriomas larger than 3 cm between January 2009 and June 2014. Clinicians treated 64 patients with plasma energy ablation and another 40 with cystectomy and followed them to compare pregnancy rates.
After at least 1 year of follow-up, pregnancy rates were 68% following plasma energy ablation, compared with 80% after cystectomy. Of the 76 pregnancies, 24 were due to spontaneous conception, including 40% of pregnancies in the plasma energy group and 18% in the cystectomy group. Even after adjustment for multiple factors, the type of intervention had no statistically significant impact on achieving a subsequent pregnancy.
“Ablation using plasma energy may be considered a valuable tool that allows a high pregnancy rate,” Basma Darwish, MD, an ob.gyn. at Rouen (France) University Hospital, said at the meeting, which was sponsored by AAGL.
These similar outcomes were observed despite a higher prevalence of risk predictors for infertility in the plasma energy group at baseline. For instance, women in this cohort were significantly older and had significantly higher revised American Fertility Society (rAFS) classification scores, as well as higher rates of pouch of Douglas obliteration, deep endometriosis, and colorectal localizations.
“Endometrial ablation using plasma energy allows good postoperative [pregnancy] rates, that is well known,” Dr. Darwish said, but the technique has not been directly compared with cystectomy outcomes.
Pregnancy rates remained similar in both groups at 24 and 36 months. The probability of pregnancy was 61% in the plasma energy group versus 69% in the cystectomy group at 24 months. At 36 months, these rates changed to 84% and 78%, respectively.
A unique property of plasma energy ablation is “very limited thermal spread, both in depth and laterally,” Dr. Darwish said.
A lack of randomization is a potential limitation of the study. “Each surgeon chose the technique he or she was best at, which may have explained our good outcomes.” Dr. Darwish said. Strengths of the study include a prospective design and follow-up to 5 years. In addition, the six centers involved in the study included both private and public hospitals, “so it’s a good reflection of what happens in real life.”
The investigators plan to conduct a randomized controlled trial to confirm these findings.
AT THE AAGL GLOBAL CONGRESS
Narrow band imaging could expand endometriosis detection
ORLANDO – Narrow band imaging detects neovascularization associated with endometriosis and can be a useful adjunct to laparoscopic white light evaluation, a prospective cohort trial of 53 women with pelvic pain suggested.
The women in the study had no deep infiltrating endometriosis on preoperative ultrasound. Investigators then conducted a standard laparoscopic survey of the pelvis with white light to identify areas of suspected superficial endometriosis, followed by secondary analysis with narrow band imaging.
In the group of 32 patients, follow-up biopsy results confirmed endometriosis in 24 women, including 7 who also had lesions detected by narrow band imaging. Six of these seven were positive for endometriosis on histology. The women were enrolled in the study from September 2014 to October 2015.
“We found that the [narrow band imaging] is useful in detecting additional areas in patients who had histopathology-proven endometriosis,” said Tony J. Ma, MBBS, a fellow at Mercy Hospital for Women in Melbourne.
In the group of 21 women with no white light lesions, narrow band imaging detected four suspicious lesions. However, these four were not positive for endometriosis.
Narrow band imaging is a mixture of blue and green light, opposite of red color. “It causes blood vessels to be more visually prominent,” Dr. Ma said at the meeting sponsored by AAGL. White light laparoscopy for detection of endometriosis “is the gold standard … but depends on experience of [the] surgeon and severity of the disease.”
These findings support those of a 2008 study that reported a high detection rate of lesions with narrow band imaging, Dr. Ma said. In this earlier prospective cohort study of 20 women, 7 patients with endometriosis who had been ruled out by white light evaluation had a positive histologic finding with narrow band imaging (J Minim Invasive Gynecol. 2008 Sep-Oct;15[5]:636-9).
“Narrow band imaging is a simple, noninvasive adjunct that can assist in the identification of additional sites of endometriosis at laparoscopy,” Dr. Ma said. “The shortened depth of field with narrow band imaging requires the operator to inspect the surface quite closely.”
The study topic is an important one because endometriosis is such a challenge to diagnose and treat, said study discussant Sawsan As-Sanie, MD, a minimally invasive gynecologic surgeon at the University of Michigan, Ann Arbor. She estimated that with the current approach – visualization by white light followed by histopathology – “about 50%-70% of patients we think have endometriosis ultimately do.”
But several unanswered questions remain, Dr. As-Sanie said, such as whether the narrow band imaging findings are clinically relevant and whether they will lead to improved patient outcomes.
“We don’t know the answer yet,” she said. “The end game is really only relevant if we improve patient outcomes.”
Dr. Ma reported having no relevant financial disclosures.
ORLANDO – Narrow band imaging detects neovascularization associated with endometriosis and can be a useful adjunct to laparoscopic white light evaluation, a prospective cohort trial of 53 women with pelvic pain suggested.
The women in the study had no deep infiltrating endometriosis on preoperative ultrasound. Investigators then conducted a standard laparoscopic survey of the pelvis with white light to identify areas of suspected superficial endometriosis, followed by secondary analysis with narrow band imaging.
In the group of 32 patients, follow-up biopsy results confirmed endometriosis in 24 women, including 7 who also had lesions detected by narrow band imaging. Six of these seven were positive for endometriosis on histology. The women were enrolled in the study from September 2014 to October 2015.
“We found that the [narrow band imaging] is useful in detecting additional areas in patients who had histopathology-proven endometriosis,” said Tony J. Ma, MBBS, a fellow at Mercy Hospital for Women in Melbourne.
In the group of 21 women with no white light lesions, narrow band imaging detected four suspicious lesions. However, these four were not positive for endometriosis.
Narrow band imaging is a mixture of blue and green light, opposite of red color. “It causes blood vessels to be more visually prominent,” Dr. Ma said at the meeting sponsored by AAGL. White light laparoscopy for detection of endometriosis “is the gold standard … but depends on experience of [the] surgeon and severity of the disease.”
These findings support those of a 2008 study that reported a high detection rate of lesions with narrow band imaging, Dr. Ma said. In this earlier prospective cohort study of 20 women, 7 patients with endometriosis who had been ruled out by white light evaluation had a positive histologic finding with narrow band imaging (J Minim Invasive Gynecol. 2008 Sep-Oct;15[5]:636-9).
“Narrow band imaging is a simple, noninvasive adjunct that can assist in the identification of additional sites of endometriosis at laparoscopy,” Dr. Ma said. “The shortened depth of field with narrow band imaging requires the operator to inspect the surface quite closely.”
The study topic is an important one because endometriosis is such a challenge to diagnose and treat, said study discussant Sawsan As-Sanie, MD, a minimally invasive gynecologic surgeon at the University of Michigan, Ann Arbor. She estimated that with the current approach – visualization by white light followed by histopathology – “about 50%-70% of patients we think have endometriosis ultimately do.”
But several unanswered questions remain, Dr. As-Sanie said, such as whether the narrow band imaging findings are clinically relevant and whether they will lead to improved patient outcomes.
“We don’t know the answer yet,” she said. “The end game is really only relevant if we improve patient outcomes.”
Dr. Ma reported having no relevant financial disclosures.
ORLANDO – Narrow band imaging detects neovascularization associated with endometriosis and can be a useful adjunct to laparoscopic white light evaluation, a prospective cohort trial of 53 women with pelvic pain suggested.
The women in the study had no deep infiltrating endometriosis on preoperative ultrasound. Investigators then conducted a standard laparoscopic survey of the pelvis with white light to identify areas of suspected superficial endometriosis, followed by secondary analysis with narrow band imaging.
In the group of 32 patients, follow-up biopsy results confirmed endometriosis in 24 women, including 7 who also had lesions detected by narrow band imaging. Six of these seven were positive for endometriosis on histology. The women were enrolled in the study from September 2014 to October 2015.
“We found that the [narrow band imaging] is useful in detecting additional areas in patients who had histopathology-proven endometriosis,” said Tony J. Ma, MBBS, a fellow at Mercy Hospital for Women in Melbourne.
In the group of 21 women with no white light lesions, narrow band imaging detected four suspicious lesions. However, these four were not positive for endometriosis.
Narrow band imaging is a mixture of blue and green light, opposite of red color. “It causes blood vessels to be more visually prominent,” Dr. Ma said at the meeting sponsored by AAGL. White light laparoscopy for detection of endometriosis “is the gold standard … but depends on experience of [the] surgeon and severity of the disease.”
These findings support those of a 2008 study that reported a high detection rate of lesions with narrow band imaging, Dr. Ma said. In this earlier prospective cohort study of 20 women, 7 patients with endometriosis who had been ruled out by white light evaluation had a positive histologic finding with narrow band imaging (J Minim Invasive Gynecol. 2008 Sep-Oct;15[5]:636-9).
“Narrow band imaging is a simple, noninvasive adjunct that can assist in the identification of additional sites of endometriosis at laparoscopy,” Dr. Ma said. “The shortened depth of field with narrow band imaging requires the operator to inspect the surface quite closely.”
The study topic is an important one because endometriosis is such a challenge to diagnose and treat, said study discussant Sawsan As-Sanie, MD, a minimally invasive gynecologic surgeon at the University of Michigan, Ann Arbor. She estimated that with the current approach – visualization by white light followed by histopathology – “about 50%-70% of patients we think have endometriosis ultimately do.”
But several unanswered questions remain, Dr. As-Sanie said, such as whether the narrow band imaging findings are clinically relevant and whether they will lead to improved patient outcomes.
“We don’t know the answer yet,” she said. “The end game is really only relevant if we improve patient outcomes.”
Dr. Ma reported having no relevant financial disclosures.
AT THE AAGL GLOBAL CONGRESS
Patient denies consent to endometriosis treatment
Patient denies consent to endometriosis treatment
A woman underwent laparoscopic surgery to remove an ovarian cyst. During surgery, the gynecologist found endometriosis and used electrosurgery to destroy the implants. Following extensive electrosurgery, a hemorrhage occurred. The gynecologist placed 5 large clips to control bleeding. The patient was discharged. When she returned to the hospital in pain, she was sent home again. She visited another emergency department (ED), where a clip was found to have blocked a ureter. She underwent ureteroneocystostomy to repair the damage. The patient now reports incontinence, a ligated ureter, and extensive scar tissue, which may keep her from being able to become pregnant.
Patient’s claim She only consented to ovarian cyst removal, not to any other procedures. The gynecologist was negligent in performing electrosurgery and placing the clips. A urologist should have checked for injury.
Physician’s defense When a patient agrees to laparoscopic surgery, she also agrees to exploratory abdominal surgery. The gynecologist performs electrosurgery to treat endometriosis in 30% to 60% of her surgical cases. Electrosurgery was essential to stop the patient’s pain. The clips were carefully placed when treating the hemorrhage.
Verdict A $206,886 California verdict was returned against the gynecologist.
Eclampsia, death: $6.9M settlement
A mother delivered a healthy baby on May 21. Twice in the week following delivery, she returned to the ED reporting shortness of breath, swollen legs, and elevated blood pressure. Pulmonary embolism was excluded both times. After the second visit, she was discharged with a diagnosis of shortness of breath of unknown etiology. The patient’s ObGyn was not contacted nor was urinalysis performed. On June 1, she suffered seizures and brain injury; she died on June 10.
Estate’s claim The ED physicians failed to diagnose and treat eclampsia.
Defendant’s defense The case was settled early in the trial.
Verdict A $6.9 million Illinois settlement was reached with the hospital.
Child has CP: $8M settlement
At 40 1/7 weeks’ gestation, labor was induced in an obese woman who had a heart condition. Over the next 36 hours, dinoprostone and oxytocin were administered, but her cervix only dilated to 2 cm. Two days later, the fetal heart rate reached 160 bpm. The ObGyn ordered terbutaline in anticipation of cesarean delivery, but he did not come to the hospital. The fetal heart rate continued to rise and then bradycardia occurred. When notified, the ObGyn came to the hospital for an emergency cesarean delivery. The child was severely depressed at birth with Apgar scores of 0, 1, and 2 at 1, 5, and 10 minutes, respectively. Magnetic resonance imaging at 23 days showed distinct hypoxic ischemic injury in the infant. Cerebral palsy was later diagnosed. The child is nonambulatory with significant cognitive impairment.
Parents’ claim Failure to perform cesarean delivery in a timely manner caused injury to the child.
Defendant’s defense The case was settled during the trial.
Verdict An $8 million Wisconsin settlement was reached with the medical center and physician group.
Infant has a stroke: $3M settlement
A 26-year-old diabetic woman was referred to a maternal-fetal medicine (MFM) specialist. She had been hospitalized for nausea and dehydration several times during her pregnancy, but it appeared that fetal development was normal.
As labor progressed, fetal distress was diagnosed in the setting of low blood pressure. When notified, the MFM immediately ordered an emergency cesarean delivery. A few days after birth, it was determined that the child had a stroke in utero.
Parents’ claim Emergency cesarean delivery should have been performed earlier. Hospital staff did not communicate fetal distress to the MFM in a timely manner.
Defendant’s defense The case was settled at trial.
Verdict A $3 million Connecticut settlement was reached with the hospital.
Patient denies consent to endometriosis treatment
A woman underwent laparoscopic surgery to remove an ovarian cyst. During surgery, the gynecologist found endometriosis and used electrosurgery to destroy the implants. Following extensive electrosurgery, a hemorrhage occurred. The gynecologist placed 5 large clips to control bleeding. The patient was discharged. When she returned to the hospital in pain, she was sent home again. She visited another emergency department (ED), where a clip was found to have blocked a ureter. She underwent ureteroneocystostomy to repair the damage. The patient now reports incontinence, a ligated ureter, and extensive scar tissue, which may keep her from being able to become pregnant.
Patient’s claim She only consented to ovarian cyst removal, not to any other procedures. The gynecologist was negligent in performing electrosurgery and placing the clips. A urologist should have checked for injury.
Physician’s defense When a patient agrees to laparoscopic surgery, she also agrees to exploratory abdominal surgery. The gynecologist performs electrosurgery to treat endometriosis in 30% to 60% of her surgical cases. Electrosurgery was essential to stop the patient’s pain. The clips were carefully placed when treating the hemorrhage.
Verdict A $206,886 California verdict was returned against the gynecologist.
Eclampsia, death: $6.9M settlement
A mother delivered a healthy baby on May 21. Twice in the week following delivery, she returned to the ED reporting shortness of breath, swollen legs, and elevated blood pressure. Pulmonary embolism was excluded both times. After the second visit, she was discharged with a diagnosis of shortness of breath of unknown etiology. The patient’s ObGyn was not contacted nor was urinalysis performed. On June 1, she suffered seizures and brain injury; she died on June 10.
Estate’s claim The ED physicians failed to diagnose and treat eclampsia.
Defendant’s defense The case was settled early in the trial.
Verdict A $6.9 million Illinois settlement was reached with the hospital.
Child has CP: $8M settlement
At 40 1/7 weeks’ gestation, labor was induced in an obese woman who had a heart condition. Over the next 36 hours, dinoprostone and oxytocin were administered, but her cervix only dilated to 2 cm. Two days later, the fetal heart rate reached 160 bpm. The ObGyn ordered terbutaline in anticipation of cesarean delivery, but he did not come to the hospital. The fetal heart rate continued to rise and then bradycardia occurred. When notified, the ObGyn came to the hospital for an emergency cesarean delivery. The child was severely depressed at birth with Apgar scores of 0, 1, and 2 at 1, 5, and 10 minutes, respectively. Magnetic resonance imaging at 23 days showed distinct hypoxic ischemic injury in the infant. Cerebral palsy was later diagnosed. The child is nonambulatory with significant cognitive impairment.
Parents’ claim Failure to perform cesarean delivery in a timely manner caused injury to the child.
Defendant’s defense The case was settled during the trial.
Verdict An $8 million Wisconsin settlement was reached with the medical center and physician group.
Infant has a stroke: $3M settlement
A 26-year-old diabetic woman was referred to a maternal-fetal medicine (MFM) specialist. She had been hospitalized for nausea and dehydration several times during her pregnancy, but it appeared that fetal development was normal.
As labor progressed, fetal distress was diagnosed in the setting of low blood pressure. When notified, the MFM immediately ordered an emergency cesarean delivery. A few days after birth, it was determined that the child had a stroke in utero.
Parents’ claim Emergency cesarean delivery should have been performed earlier. Hospital staff did not communicate fetal distress to the MFM in a timely manner.
Defendant’s defense The case was settled at trial.
Verdict A $3 million Connecticut settlement was reached with the hospital.
Patient denies consent to endometriosis treatment
A woman underwent laparoscopic surgery to remove an ovarian cyst. During surgery, the gynecologist found endometriosis and used electrosurgery to destroy the implants. Following extensive electrosurgery, a hemorrhage occurred. The gynecologist placed 5 large clips to control bleeding. The patient was discharged. When she returned to the hospital in pain, she was sent home again. She visited another emergency department (ED), where a clip was found to have blocked a ureter. She underwent ureteroneocystostomy to repair the damage. The patient now reports incontinence, a ligated ureter, and extensive scar tissue, which may keep her from being able to become pregnant.
Patient’s claim She only consented to ovarian cyst removal, not to any other procedures. The gynecologist was negligent in performing electrosurgery and placing the clips. A urologist should have checked for injury.
Physician’s defense When a patient agrees to laparoscopic surgery, she also agrees to exploratory abdominal surgery. The gynecologist performs electrosurgery to treat endometriosis in 30% to 60% of her surgical cases. Electrosurgery was essential to stop the patient’s pain. The clips were carefully placed when treating the hemorrhage.
Verdict A $206,886 California verdict was returned against the gynecologist.
Eclampsia, death: $6.9M settlement
A mother delivered a healthy baby on May 21. Twice in the week following delivery, she returned to the ED reporting shortness of breath, swollen legs, and elevated blood pressure. Pulmonary embolism was excluded both times. After the second visit, she was discharged with a diagnosis of shortness of breath of unknown etiology. The patient’s ObGyn was not contacted nor was urinalysis performed. On June 1, she suffered seizures and brain injury; she died on June 10.
Estate’s claim The ED physicians failed to diagnose and treat eclampsia.
Defendant’s defense The case was settled early in the trial.
Verdict A $6.9 million Illinois settlement was reached with the hospital.
Child has CP: $8M settlement
At 40 1/7 weeks’ gestation, labor was induced in an obese woman who had a heart condition. Over the next 36 hours, dinoprostone and oxytocin were administered, but her cervix only dilated to 2 cm. Two days later, the fetal heart rate reached 160 bpm. The ObGyn ordered terbutaline in anticipation of cesarean delivery, but he did not come to the hospital. The fetal heart rate continued to rise and then bradycardia occurred. When notified, the ObGyn came to the hospital for an emergency cesarean delivery. The child was severely depressed at birth with Apgar scores of 0, 1, and 2 at 1, 5, and 10 minutes, respectively. Magnetic resonance imaging at 23 days showed distinct hypoxic ischemic injury in the infant. Cerebral palsy was later diagnosed. The child is nonambulatory with significant cognitive impairment.
Parents’ claim Failure to perform cesarean delivery in a timely manner caused injury to the child.
Defendant’s defense The case was settled during the trial.
Verdict An $8 million Wisconsin settlement was reached with the medical center and physician group.
Infant has a stroke: $3M settlement
A 26-year-old diabetic woman was referred to a maternal-fetal medicine (MFM) specialist. She had been hospitalized for nausea and dehydration several times during her pregnancy, but it appeared that fetal development was normal.
As labor progressed, fetal distress was diagnosed in the setting of low blood pressure. When notified, the MFM immediately ordered an emergency cesarean delivery. A few days after birth, it was determined that the child had a stroke in utero.
Parents’ claim Emergency cesarean delivery should have been performed earlier. Hospital staff did not communicate fetal distress to the MFM in a timely manner.
Defendant’s defense The case was settled at trial.
Verdict A $3 million Connecticut settlement was reached with the hospital.
In this Article
- Eclampsia, death: $6.9M settlement
- Child has CP: $8M settlement
- Infant has a stroke: $3M settlement