User login
Aspirin, hydrochlorothiazide okay in gout
It’s okay in most cases for gout patients to be on thiazide diuretics or low-dose aspirin, so long as hypouricemic therapy is adjusted as needed, according to rheumatologist and Cleveland Clinic professor Dr. Brian F. Mandell.
Coronary artery disease is common in gout, so patients benefit from inexpensive, well-tolerated, and effective treatments like thiazides for hypertension and 81 mg aspirin daily for cardioprotection, Dr. Mandell said at the annual Perspectives in Rheumatic Diseases conference held by Global Academy for Medical Education.
The concern, however, is that both can elevate serum uric acid. Observational and survey data suggest that the drugs may be associated with an increase in attacks, so some shy away from them in gout.
To make sense of the issue, the first thing to remember is that the drugs cause only “minimal elevations in serum uric acid. Serum urate can still be lowered to less than 6 mg/dL with appropriate therapy without stopping them,” Dr. Mandell said.
Low-dose aspirin raises serum urate by about 0.3 mg/dL. At 12.5 or 25 mg once a day – common hypertension doses – hydrochlorothiazide increases serum urate by 0.8 mg/dL or less in patients with normal renal function (Arthritis Rheum. 2012 Jan;64[1]:121-9).
“In patients with chronic gout treated with a xanthine oxidase inhibitor (allopurinol or febuxostat) to lower the serum urate” to recommended target levels below 6.0 mg/dL, “the small elevation in serum urate is unlikely to negate the clinical efficacy of these drugs when dosing is optimized,” Dr. Mandell wrote in an article that expands upon his presentation points (Cleve Clin J Med. 2014 Feb;81[2]:83-6).
Based on those considerations, “my practice in most patients is to use a thiazide if it helps to control the blood pressure and to adjust the dose of the hypouricemic therapy as needed to reduce the serum urate to the desired level. ... Continuing thiazide therapy and, if necessary, adjusting hypouricemic therapy will not worsen the control of the serum urate level or gouty arthritis, and in most patients will not complicate the management of gout.” In general, “when I add a thiazide to a patient’s regimen, I do not usually need to increase the dose of allopurinol significantly to keep the serum urate level below the desired target,” he said.
The approach is similar with low-dose aspirin. Because of its negligible effect on serum uric acid levels, it does not need to be stopped in hyperuricemia or gout. “Since patients with gout have a higher risk of having cardiovascular disease, metabolic syndrome, and chronic kidney disease, many will benefit from low-dose aspirin therapy,” he said.
Occasionally, it makes sense to switch from a thiazide to another hypertensive, such as losartan, in chronic, hypertensive gout patients with serum urate levels marginally above the precipitation threshold of 6.7 mg/dL. “Losartan is a weak uricosuric and can lower the serum urate level slightly, possibly making the addition of another hypouricemic agent unnecessary, while still controlling the blood pressure with a single pill,” Dr. Mandell said.
The decision “must be individualized, taking into consideration the efficacy and cost of the alternative antihypertensive drug, as well as the potential but as yet unproven cardiovascular and renal benefits of lowering the serum urate with a more potent hypouricemic to a degree not likely to be attained with losartan alone,” he said.
Dr. Mandell has been a consultant for Savient/Crealta, AstraZeneca, Regeneron, and Novartis. Global Academy for Medical Education and this news organization are owned by the same parent company.
It’s okay in most cases for gout patients to be on thiazide diuretics or low-dose aspirin, so long as hypouricemic therapy is adjusted as needed, according to rheumatologist and Cleveland Clinic professor Dr. Brian F. Mandell.
Coronary artery disease is common in gout, so patients benefit from inexpensive, well-tolerated, and effective treatments like thiazides for hypertension and 81 mg aspirin daily for cardioprotection, Dr. Mandell said at the annual Perspectives in Rheumatic Diseases conference held by Global Academy for Medical Education.
The concern, however, is that both can elevate serum uric acid. Observational and survey data suggest that the drugs may be associated with an increase in attacks, so some shy away from them in gout.
To make sense of the issue, the first thing to remember is that the drugs cause only “minimal elevations in serum uric acid. Serum urate can still be lowered to less than 6 mg/dL with appropriate therapy without stopping them,” Dr. Mandell said.
Low-dose aspirin raises serum urate by about 0.3 mg/dL. At 12.5 or 25 mg once a day – common hypertension doses – hydrochlorothiazide increases serum urate by 0.8 mg/dL or less in patients with normal renal function (Arthritis Rheum. 2012 Jan;64[1]:121-9).
“In patients with chronic gout treated with a xanthine oxidase inhibitor (allopurinol or febuxostat) to lower the serum urate” to recommended target levels below 6.0 mg/dL, “the small elevation in serum urate is unlikely to negate the clinical efficacy of these drugs when dosing is optimized,” Dr. Mandell wrote in an article that expands upon his presentation points (Cleve Clin J Med. 2014 Feb;81[2]:83-6).
Based on those considerations, “my practice in most patients is to use a thiazide if it helps to control the blood pressure and to adjust the dose of the hypouricemic therapy as needed to reduce the serum urate to the desired level. ... Continuing thiazide therapy and, if necessary, adjusting hypouricemic therapy will not worsen the control of the serum urate level or gouty arthritis, and in most patients will not complicate the management of gout.” In general, “when I add a thiazide to a patient’s regimen, I do not usually need to increase the dose of allopurinol significantly to keep the serum urate level below the desired target,” he said.
The approach is similar with low-dose aspirin. Because of its negligible effect on serum uric acid levels, it does not need to be stopped in hyperuricemia or gout. “Since patients with gout have a higher risk of having cardiovascular disease, metabolic syndrome, and chronic kidney disease, many will benefit from low-dose aspirin therapy,” he said.
Occasionally, it makes sense to switch from a thiazide to another hypertensive, such as losartan, in chronic, hypertensive gout patients with serum urate levels marginally above the precipitation threshold of 6.7 mg/dL. “Losartan is a weak uricosuric and can lower the serum urate level slightly, possibly making the addition of another hypouricemic agent unnecessary, while still controlling the blood pressure with a single pill,” Dr. Mandell said.
The decision “must be individualized, taking into consideration the efficacy and cost of the alternative antihypertensive drug, as well as the potential but as yet unproven cardiovascular and renal benefits of lowering the serum urate with a more potent hypouricemic to a degree not likely to be attained with losartan alone,” he said.
Dr. Mandell has been a consultant for Savient/Crealta, AstraZeneca, Regeneron, and Novartis. Global Academy for Medical Education and this news organization are owned by the same parent company.
It’s okay in most cases for gout patients to be on thiazide diuretics or low-dose aspirin, so long as hypouricemic therapy is adjusted as needed, according to rheumatologist and Cleveland Clinic professor Dr. Brian F. Mandell.
Coronary artery disease is common in gout, so patients benefit from inexpensive, well-tolerated, and effective treatments like thiazides for hypertension and 81 mg aspirin daily for cardioprotection, Dr. Mandell said at the annual Perspectives in Rheumatic Diseases conference held by Global Academy for Medical Education.
The concern, however, is that both can elevate serum uric acid. Observational and survey data suggest that the drugs may be associated with an increase in attacks, so some shy away from them in gout.
To make sense of the issue, the first thing to remember is that the drugs cause only “minimal elevations in serum uric acid. Serum urate can still be lowered to less than 6 mg/dL with appropriate therapy without stopping them,” Dr. Mandell said.
Low-dose aspirin raises serum urate by about 0.3 mg/dL. At 12.5 or 25 mg once a day – common hypertension doses – hydrochlorothiazide increases serum urate by 0.8 mg/dL or less in patients with normal renal function (Arthritis Rheum. 2012 Jan;64[1]:121-9).
“In patients with chronic gout treated with a xanthine oxidase inhibitor (allopurinol or febuxostat) to lower the serum urate” to recommended target levels below 6.0 mg/dL, “the small elevation in serum urate is unlikely to negate the clinical efficacy of these drugs when dosing is optimized,” Dr. Mandell wrote in an article that expands upon his presentation points (Cleve Clin J Med. 2014 Feb;81[2]:83-6).
Based on those considerations, “my practice in most patients is to use a thiazide if it helps to control the blood pressure and to adjust the dose of the hypouricemic therapy as needed to reduce the serum urate to the desired level. ... Continuing thiazide therapy and, if necessary, adjusting hypouricemic therapy will not worsen the control of the serum urate level or gouty arthritis, and in most patients will not complicate the management of gout.” In general, “when I add a thiazide to a patient’s regimen, I do not usually need to increase the dose of allopurinol significantly to keep the serum urate level below the desired target,” he said.
The approach is similar with low-dose aspirin. Because of its negligible effect on serum uric acid levels, it does not need to be stopped in hyperuricemia or gout. “Since patients with gout have a higher risk of having cardiovascular disease, metabolic syndrome, and chronic kidney disease, many will benefit from low-dose aspirin therapy,” he said.
Occasionally, it makes sense to switch from a thiazide to another hypertensive, such as losartan, in chronic, hypertensive gout patients with serum urate levels marginally above the precipitation threshold of 6.7 mg/dL. “Losartan is a weak uricosuric and can lower the serum urate level slightly, possibly making the addition of another hypouricemic agent unnecessary, while still controlling the blood pressure with a single pill,” Dr. Mandell said.
The decision “must be individualized, taking into consideration the efficacy and cost of the alternative antihypertensive drug, as well as the potential but as yet unproven cardiovascular and renal benefits of lowering the serum urate with a more potent hypouricemic to a degree not likely to be attained with losartan alone,” he said.
Dr. Mandell has been a consultant for Savient/Crealta, AstraZeneca, Regeneron, and Novartis. Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM THE ANNUAL PERSPECTIVES IN RHEUMATIC DISEASES
New ACR/EULAR gout classification criteria offer better sensitivity, specificity
The presence of monosodium urate monohydrate crystals in a symptomatic joint, bursa, or tophus is sufficient to diagnose gout, according to new gout classification criteria from the American College of Rheumatology and the European League Against Rheumatism.
When symptomatic urate crystals are missing, other signs and symptoms are considered and scored; a score of 8 or more constitutes gout. “The threshold chosen for this classification criteria set yielded the best combination of sensitivity and specificity,” at 92% and 89%, respectively, and outperformed previous classification schemes, said the authors, led by Dr. Tuhina Neogi of Boston University.
To qualify for gout, patients must first have at least one episode of swelling, pain, or tenderness in a peripheral joint or bursa. They get a score of 1 if that happens in the ankle or midfoot, and a score of 2 if it involves a metatarsophalangeal joint. If the affected joint is red and too painful to touch and use, patients get an additional score of 3. Chalklike drainage from a subcutaneous nodule in a gout-prone area, and serum urate at or above 10 mg/dL, both get a score of 4. Imaging of one or more gout erosions in the hands or feet also gets a score of 4 (Arthritis Rheumatol. 2015 Oct;67[10]:2557-68. doi: 10.1002/art.39254).
Overall, the criteria incorporate clinical, laboratory, and imaging evidence. A web-based calculator makes the scoring easy.
“Although MSU [monosodium urate monohydrate] crystal results are extremely helpful when positive, they are not a feasible universal standard, particularly because many potential study subjects are likely to be recruited from nonrheumatology settings. We aimed to develop a new set of criteria that could be flexible enough to enable accurate classification of gout regardless of MSU status,” the authors said.
“This classification criteria set will enable a standardized approach to identifying a relatively homogeneous group of individuals who have the clinical entity of gout for enrollment into studies. The criteria permit characterization of an individual as having gout regardless of whether he or she is currently experiencing an acute symptomatic episode and regardless of any comorbidities,” they said.
The hope of the work is to facilitate a better understanding of gout and speed development of new trials and treatments. The criteria will “help to ensure that patients with the same disease are being evaluated, which will enhance our ability to study the disease, including performing outcomes studies and clinical trials,” Dr. Neogi said in a written statement.
Previous gout classification criteria were developed when advanced imaging was not available. “Additionally, the increasing prevalence of gout, advances in therapeutics, and the development of international research collaborations to understand the impact, mechanisms, and optimal treatment of this condition emphasize the need for accurate and uniform classification criteria for gout,” according to the statement.
The new criteria are based on a systematic review of the literature on advanced gout imaging; a diagnostic study in which the presence of MSU crystals in synovial fluid or tophi was the gold standard; a ranking exercise of paper patient cases; and a multicriterion decision analysis exercise. The criteria were then validated in 330 patients.
The work was supported in part by the National Institutes of Health, the Agency for Healthcare Research and Quality, and Arthritis New Zealand. Numerous authors reported receiving consulting fees, speaking fees, and/or honoraria from companies that market drugs or specialty foods for gout.
The presence of monosodium urate monohydrate crystals in a symptomatic joint, bursa, or tophus is sufficient to diagnose gout, according to new gout classification criteria from the American College of Rheumatology and the European League Against Rheumatism.
When symptomatic urate crystals are missing, other signs and symptoms are considered and scored; a score of 8 or more constitutes gout. “The threshold chosen for this classification criteria set yielded the best combination of sensitivity and specificity,” at 92% and 89%, respectively, and outperformed previous classification schemes, said the authors, led by Dr. Tuhina Neogi of Boston University.
To qualify for gout, patients must first have at least one episode of swelling, pain, or tenderness in a peripheral joint or bursa. They get a score of 1 if that happens in the ankle or midfoot, and a score of 2 if it involves a metatarsophalangeal joint. If the affected joint is red and too painful to touch and use, patients get an additional score of 3. Chalklike drainage from a subcutaneous nodule in a gout-prone area, and serum urate at or above 10 mg/dL, both get a score of 4. Imaging of one or more gout erosions in the hands or feet also gets a score of 4 (Arthritis Rheumatol. 2015 Oct;67[10]:2557-68. doi: 10.1002/art.39254).
Overall, the criteria incorporate clinical, laboratory, and imaging evidence. A web-based calculator makes the scoring easy.
“Although MSU [monosodium urate monohydrate] crystal results are extremely helpful when positive, they are not a feasible universal standard, particularly because many potential study subjects are likely to be recruited from nonrheumatology settings. We aimed to develop a new set of criteria that could be flexible enough to enable accurate classification of gout regardless of MSU status,” the authors said.
“This classification criteria set will enable a standardized approach to identifying a relatively homogeneous group of individuals who have the clinical entity of gout for enrollment into studies. The criteria permit characterization of an individual as having gout regardless of whether he or she is currently experiencing an acute symptomatic episode and regardless of any comorbidities,” they said.
The hope of the work is to facilitate a better understanding of gout and speed development of new trials and treatments. The criteria will “help to ensure that patients with the same disease are being evaluated, which will enhance our ability to study the disease, including performing outcomes studies and clinical trials,” Dr. Neogi said in a written statement.
Previous gout classification criteria were developed when advanced imaging was not available. “Additionally, the increasing prevalence of gout, advances in therapeutics, and the development of international research collaborations to understand the impact, mechanisms, and optimal treatment of this condition emphasize the need for accurate and uniform classification criteria for gout,” according to the statement.
The new criteria are based on a systematic review of the literature on advanced gout imaging; a diagnostic study in which the presence of MSU crystals in synovial fluid or tophi was the gold standard; a ranking exercise of paper patient cases; and a multicriterion decision analysis exercise. The criteria were then validated in 330 patients.
The work was supported in part by the National Institutes of Health, the Agency for Healthcare Research and Quality, and Arthritis New Zealand. Numerous authors reported receiving consulting fees, speaking fees, and/or honoraria from companies that market drugs or specialty foods for gout.
The presence of monosodium urate monohydrate crystals in a symptomatic joint, bursa, or tophus is sufficient to diagnose gout, according to new gout classification criteria from the American College of Rheumatology and the European League Against Rheumatism.
When symptomatic urate crystals are missing, other signs and symptoms are considered and scored; a score of 8 or more constitutes gout. “The threshold chosen for this classification criteria set yielded the best combination of sensitivity and specificity,” at 92% and 89%, respectively, and outperformed previous classification schemes, said the authors, led by Dr. Tuhina Neogi of Boston University.
To qualify for gout, patients must first have at least one episode of swelling, pain, or tenderness in a peripheral joint or bursa. They get a score of 1 if that happens in the ankle or midfoot, and a score of 2 if it involves a metatarsophalangeal joint. If the affected joint is red and too painful to touch and use, patients get an additional score of 3. Chalklike drainage from a subcutaneous nodule in a gout-prone area, and serum urate at or above 10 mg/dL, both get a score of 4. Imaging of one or more gout erosions in the hands or feet also gets a score of 4 (Arthritis Rheumatol. 2015 Oct;67[10]:2557-68. doi: 10.1002/art.39254).
Overall, the criteria incorporate clinical, laboratory, and imaging evidence. A web-based calculator makes the scoring easy.
“Although MSU [monosodium urate monohydrate] crystal results are extremely helpful when positive, they are not a feasible universal standard, particularly because many potential study subjects are likely to be recruited from nonrheumatology settings. We aimed to develop a new set of criteria that could be flexible enough to enable accurate classification of gout regardless of MSU status,” the authors said.
“This classification criteria set will enable a standardized approach to identifying a relatively homogeneous group of individuals who have the clinical entity of gout for enrollment into studies. The criteria permit characterization of an individual as having gout regardless of whether he or she is currently experiencing an acute symptomatic episode and regardless of any comorbidities,” they said.
The hope of the work is to facilitate a better understanding of gout and speed development of new trials and treatments. The criteria will “help to ensure that patients with the same disease are being evaluated, which will enhance our ability to study the disease, including performing outcomes studies and clinical trials,” Dr. Neogi said in a written statement.
Previous gout classification criteria were developed when advanced imaging was not available. “Additionally, the increasing prevalence of gout, advances in therapeutics, and the development of international research collaborations to understand the impact, mechanisms, and optimal treatment of this condition emphasize the need for accurate and uniform classification criteria for gout,” according to the statement.
The new criteria are based on a systematic review of the literature on advanced gout imaging; a diagnostic study in which the presence of MSU crystals in synovial fluid or tophi was the gold standard; a ranking exercise of paper patient cases; and a multicriterion decision analysis exercise. The criteria were then validated in 330 patients.
The work was supported in part by the National Institutes of Health, the Agency for Healthcare Research and Quality, and Arthritis New Zealand. Numerous authors reported receiving consulting fees, speaking fees, and/or honoraria from companies that market drugs or specialty foods for gout.
FROM ARTHRITIS & RHEUMATOLOGY
EULAR: Panel targets six rheumatic disease comorbidities
ROME – Clinicians who care for patients with chronic inflammatory rheumatic diseases should consider regularly assessing six potential comorbidities these patients might develop, according to a set of “points to consider” developed by a task force of the European League Against Rheumatism.
The six comorbidities the working group’s report cites are ischemic cardiovascular disease, malignancies, infections, peptic ulcer, osteoporosis, and depression, Dr. Maxime Dougados said at the European Congress of Rheumatology.
This is the “minimum list of comorbidities to systematically check” for patients with inflammatory rheumatic diseases, said Dr. Dougados, professor and chief of rheumatology at Cochin Hospital in Paris.
The task force he heads plans to soon make available on the EULAR Website screening questionnaires for assessing the status of each of these six comorbidities. “We hope you will consider this initiative and implement these points to consider in your practice,” he said.
A seventh comorbidity to potentially add to the list for regular assessment is hypertension, said Dr. Deborah P.M. Symmons, professor of rheumatology and musculoskeletal epidemiology at the University of Manchester (England), in a separate talk at the meeting. Roughly 80% of patients with rheumatoid arthritis (RA) have at least one comorbidity, she noted.
Recent study results have documented the prevalence of comorbidities in patients with RA, Dr. Symmons said. For example, an analysis of data collected during 2011 and 2012 from 3,920 RA patients in 17 countries, including 400 U.S. patients, showed that depression was the most common comorbidity, affecting 15% of patients; other comorbidities included ischemic cardiovascular disease in 6%, malignancy in 5%, and hypertension in 11% (Ann. Rheum. Dis. 2014;73:62-8). A separate survey of 9,874 RA patients from 34 countries also published last year found patients had a median of two comorbidities each. The most common were hypertension in 32% of patients, osteoporosis in 18%, and osteoarthritis in 16% (Clin. Exp. Rheum. 2014;32:869-77).
“Chronic diseases cluster together, more than you would expect by chance, perhaps because of shared risk factors such as genetic or environmental, the direct impact of inflammation, and because of treatment” patients receive for their rheumatic disease, Dr. Symmons said.
The consequence is that clinicians who manage patients with RA or other rheumatic disease must be on the lookout for comorbidities and take them into consideration when planning management strategies. A rheumatologist might be most concerned about how comobidities will affect the rheumatic disease, but for patients the overriding concern is how all their chronic diseases, not just their rheumatic disease, will affect their quality of life and physical function, she noted. “We must constantly ask ourselves whether treatment of the RA will worsen the comorbidities, or will treatment of the comorbidities worsen the RA?”
Knowledge of how RA treatments will affect comorbidities is often lacking because patients with comorbidities are usually not enrolled in clinical trials, Dr. Symmons said.
She recommended that rheumatologists systematically screen patients annually for comorbidities and discuss with each patient and with clinicians from other relevant specialties appropriate treatment based on the patient’s global status. Steroid treatment should be minimized because of the risk it poses for causing or exacerbating hypertension, hyperlipidemia, diabetes, osteoporosis, and infection.
The rheumatologist does not necessarily need to be the clinician who manages all of a patient’s comorbidities, which might be better done by a primary care physician, but the rheumatologist should know that a patient’s comorbidities are being managed by someone, and this fact should be documented in the rheumatologist’s records for each patient, she said.
Dr. Dougados and Dr. Symmons said they had no relevant financial disclosures.
On Twitter@mitchelzoler
ROME – Clinicians who care for patients with chronic inflammatory rheumatic diseases should consider regularly assessing six potential comorbidities these patients might develop, according to a set of “points to consider” developed by a task force of the European League Against Rheumatism.
The six comorbidities the working group’s report cites are ischemic cardiovascular disease, malignancies, infections, peptic ulcer, osteoporosis, and depression, Dr. Maxime Dougados said at the European Congress of Rheumatology.
This is the “minimum list of comorbidities to systematically check” for patients with inflammatory rheumatic diseases, said Dr. Dougados, professor and chief of rheumatology at Cochin Hospital in Paris.
The task force he heads plans to soon make available on the EULAR Website screening questionnaires for assessing the status of each of these six comorbidities. “We hope you will consider this initiative and implement these points to consider in your practice,” he said.
A seventh comorbidity to potentially add to the list for regular assessment is hypertension, said Dr. Deborah P.M. Symmons, professor of rheumatology and musculoskeletal epidemiology at the University of Manchester (England), in a separate talk at the meeting. Roughly 80% of patients with rheumatoid arthritis (RA) have at least one comorbidity, she noted.
Recent study results have documented the prevalence of comorbidities in patients with RA, Dr. Symmons said. For example, an analysis of data collected during 2011 and 2012 from 3,920 RA patients in 17 countries, including 400 U.S. patients, showed that depression was the most common comorbidity, affecting 15% of patients; other comorbidities included ischemic cardiovascular disease in 6%, malignancy in 5%, and hypertension in 11% (Ann. Rheum. Dis. 2014;73:62-8). A separate survey of 9,874 RA patients from 34 countries also published last year found patients had a median of two comorbidities each. The most common were hypertension in 32% of patients, osteoporosis in 18%, and osteoarthritis in 16% (Clin. Exp. Rheum. 2014;32:869-77).
“Chronic diseases cluster together, more than you would expect by chance, perhaps because of shared risk factors such as genetic or environmental, the direct impact of inflammation, and because of treatment” patients receive for their rheumatic disease, Dr. Symmons said.
The consequence is that clinicians who manage patients with RA or other rheumatic disease must be on the lookout for comorbidities and take them into consideration when planning management strategies. A rheumatologist might be most concerned about how comobidities will affect the rheumatic disease, but for patients the overriding concern is how all their chronic diseases, not just their rheumatic disease, will affect their quality of life and physical function, she noted. “We must constantly ask ourselves whether treatment of the RA will worsen the comorbidities, or will treatment of the comorbidities worsen the RA?”
Knowledge of how RA treatments will affect comorbidities is often lacking because patients with comorbidities are usually not enrolled in clinical trials, Dr. Symmons said.
She recommended that rheumatologists systematically screen patients annually for comorbidities and discuss with each patient and with clinicians from other relevant specialties appropriate treatment based on the patient’s global status. Steroid treatment should be minimized because of the risk it poses for causing or exacerbating hypertension, hyperlipidemia, diabetes, osteoporosis, and infection.
The rheumatologist does not necessarily need to be the clinician who manages all of a patient’s comorbidities, which might be better done by a primary care physician, but the rheumatologist should know that a patient’s comorbidities are being managed by someone, and this fact should be documented in the rheumatologist’s records for each patient, she said.
Dr. Dougados and Dr. Symmons said they had no relevant financial disclosures.
On Twitter@mitchelzoler
ROME – Clinicians who care for patients with chronic inflammatory rheumatic diseases should consider regularly assessing six potential comorbidities these patients might develop, according to a set of “points to consider” developed by a task force of the European League Against Rheumatism.
The six comorbidities the working group’s report cites are ischemic cardiovascular disease, malignancies, infections, peptic ulcer, osteoporosis, and depression, Dr. Maxime Dougados said at the European Congress of Rheumatology.
This is the “minimum list of comorbidities to systematically check” for patients with inflammatory rheumatic diseases, said Dr. Dougados, professor and chief of rheumatology at Cochin Hospital in Paris.
The task force he heads plans to soon make available on the EULAR Website screening questionnaires for assessing the status of each of these six comorbidities. “We hope you will consider this initiative and implement these points to consider in your practice,” he said.
A seventh comorbidity to potentially add to the list for regular assessment is hypertension, said Dr. Deborah P.M. Symmons, professor of rheumatology and musculoskeletal epidemiology at the University of Manchester (England), in a separate talk at the meeting. Roughly 80% of patients with rheumatoid arthritis (RA) have at least one comorbidity, she noted.
Recent study results have documented the prevalence of comorbidities in patients with RA, Dr. Symmons said. For example, an analysis of data collected during 2011 and 2012 from 3,920 RA patients in 17 countries, including 400 U.S. patients, showed that depression was the most common comorbidity, affecting 15% of patients; other comorbidities included ischemic cardiovascular disease in 6%, malignancy in 5%, and hypertension in 11% (Ann. Rheum. Dis. 2014;73:62-8). A separate survey of 9,874 RA patients from 34 countries also published last year found patients had a median of two comorbidities each. The most common were hypertension in 32% of patients, osteoporosis in 18%, and osteoarthritis in 16% (Clin. Exp. Rheum. 2014;32:869-77).
“Chronic diseases cluster together, more than you would expect by chance, perhaps because of shared risk factors such as genetic or environmental, the direct impact of inflammation, and because of treatment” patients receive for their rheumatic disease, Dr. Symmons said.
The consequence is that clinicians who manage patients with RA or other rheumatic disease must be on the lookout for comorbidities and take them into consideration when planning management strategies. A rheumatologist might be most concerned about how comobidities will affect the rheumatic disease, but for patients the overriding concern is how all their chronic diseases, not just their rheumatic disease, will affect their quality of life and physical function, she noted. “We must constantly ask ourselves whether treatment of the RA will worsen the comorbidities, or will treatment of the comorbidities worsen the RA?”
Knowledge of how RA treatments will affect comorbidities is often lacking because patients with comorbidities are usually not enrolled in clinical trials, Dr. Symmons said.
She recommended that rheumatologists systematically screen patients annually for comorbidities and discuss with each patient and with clinicians from other relevant specialties appropriate treatment based on the patient’s global status. Steroid treatment should be minimized because of the risk it poses for causing or exacerbating hypertension, hyperlipidemia, diabetes, osteoporosis, and infection.
The rheumatologist does not necessarily need to be the clinician who manages all of a patient’s comorbidities, which might be better done by a primary care physician, but the rheumatologist should know that a patient’s comorbidities are being managed by someone, and this fact should be documented in the rheumatologist’s records for each patient, she said.
Dr. Dougados and Dr. Symmons said they had no relevant financial disclosures.
On Twitter@mitchelzoler
AT THE EULAR 2015 CONGRESS
No causal effect found between high uric acid concentrations and diabetes
Despite observed associations between uric acid concentrations and diabetes risk, the relationship has not been found to be causal, according to results published April 27 in the journal Diabetes.
Dr. Ivonne Sluijs of University Medical Center Utrecht (the Netherlands) and her associates performed a Mendelian randomization study in a cohort of 24,265 European patients using 24 uric acid loci.
Higher uric acid concentrations were associated with greater diabetes risk (hazard ratio, 1.20 per 59.48 μmol/L; 95% CI, 1.11-1.30), though this did not have a causal effect on diabetes, the authors reported (HR, 1.01; 95% CI, 0.87-1.16).
The findings suggest that “increased uric acid concentrations are a consequence of an adverse metabolic profile, rather than a cause of diabetes,” Dr. Sluijs and her associates wrote (Diabetes 2015 April 27 [doi:10.2337/db14-0742]). Thus, therapies that lower uric acid may not be effective in lowering diabetes risk, they said. “Uric acid has limited value as therapeutic target in preventing diabetes,” the investigators concluded.
Dr. Slujis and her associates did not disclose any conflicts of interest.
Despite observed associations between uric acid concentrations and diabetes risk, the relationship has not been found to be causal, according to results published April 27 in the journal Diabetes.
Dr. Ivonne Sluijs of University Medical Center Utrecht (the Netherlands) and her associates performed a Mendelian randomization study in a cohort of 24,265 European patients using 24 uric acid loci.
Higher uric acid concentrations were associated with greater diabetes risk (hazard ratio, 1.20 per 59.48 μmol/L; 95% CI, 1.11-1.30), though this did not have a causal effect on diabetes, the authors reported (HR, 1.01; 95% CI, 0.87-1.16).
The findings suggest that “increased uric acid concentrations are a consequence of an adverse metabolic profile, rather than a cause of diabetes,” Dr. Sluijs and her associates wrote (Diabetes 2015 April 27 [doi:10.2337/db14-0742]). Thus, therapies that lower uric acid may not be effective in lowering diabetes risk, they said. “Uric acid has limited value as therapeutic target in preventing diabetes,” the investigators concluded.
Dr. Slujis and her associates did not disclose any conflicts of interest.
Despite observed associations between uric acid concentrations and diabetes risk, the relationship has not been found to be causal, according to results published April 27 in the journal Diabetes.
Dr. Ivonne Sluijs of University Medical Center Utrecht (the Netherlands) and her associates performed a Mendelian randomization study in a cohort of 24,265 European patients using 24 uric acid loci.
Higher uric acid concentrations were associated with greater diabetes risk (hazard ratio, 1.20 per 59.48 μmol/L; 95% CI, 1.11-1.30), though this did not have a causal effect on diabetes, the authors reported (HR, 1.01; 95% CI, 0.87-1.16).
The findings suggest that “increased uric acid concentrations are a consequence of an adverse metabolic profile, rather than a cause of diabetes,” Dr. Sluijs and her associates wrote (Diabetes 2015 April 27 [doi:10.2337/db14-0742]). Thus, therapies that lower uric acid may not be effective in lowering diabetes risk, they said. “Uric acid has limited value as therapeutic target in preventing diabetes,” the investigators concluded.
Dr. Slujis and her associates did not disclose any conflicts of interest.
FROM DIABETES
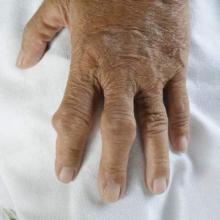
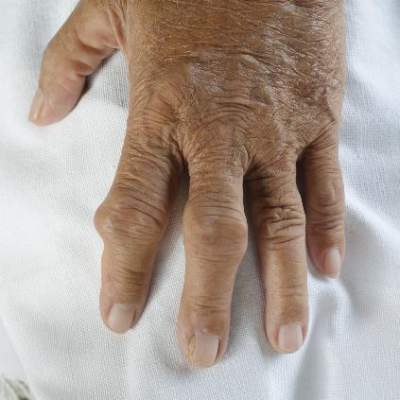
Gout increases risk of vascular disease, especially for women
Gout’s association with a host of vascular events was confirmed in a new study that explored the links between the inflammatory condition and coronary artery disease, peripheral vascular disease, and cerebrovascular events.
Though both men and women with gout were at increased risk for vascular events overall, the association appeared strongest for women. Dr. Lorna Clarson of Keele (England) University and her associates drew these conclusions from a retrospective cohort study of men and women with an incident diagnosis of gout (Ann. Rheum. Dis. 2015;74:642-7).
Gout, caused by the deposition of uric acid crystals in joints, is characterized by acute flares of intensely painful and inflamed joints. However, the state of hyperuricemia that predisposes patients to acute attacks of gout may precede the first attack by years, and may persist between flares. The proinflammatory course of the natural history of gout has increasingly been recognized as a potential contributor to vascular disease.
The precise mechanism by which gout may increase vascular risk has not been identified. Dr. Clarson and associates noted that in addition to the acute and chronic inflammation associated with gout and hyperuricemia, serum uric acid may have a more direct effect on vascular health, as urate crystal deposition on vessel walls may promote vascular damage.
To clarify gout’s impact on vascular risk, Dr. Clarson and her associates used the Clinical Practice Datalink, a large United Kingdom health database, to compare 8,366 patients with gout to 39,766 age- and sex-matched controls. None of those studied had a baseline history of vascular disease, and all were aged 50 or older.
Careful accounting for covariates was accomplished by multivariate analysis that took into account sex, age, body mass index, tobacco and alcohol consumption, statin or aspirin use, and any history of hypertension, dyslipidemia, or chronic kidney disease. In addition, the study employed the composite Charlson Comorbidity Index, which weights 19 comorbid conditions – including diabetes – to arrive at a single score that captures many risk factors. Patients in the cohort were tracked until their first vascular event, or until death or loss to follow-up. Patient data collection was censored at 10 years from baseline or at the end of study data collection, whichever came first.
To assess the incidence of vascular events, the study noted the first recording in the medical record of any events signaling vascular disease. These included angina or myocardial infarction, transient ischemic attack and stroke, and a range of diagnoses associated with peripheral vascular disease.
Final analysis after accounting for the many covariates tracked in the study showed increased risk for vascular events for those with gout, with a definite difference between the sexes. For men, gout predicted an increased risk of any vascular event (hazard ratio, 1.06; 95% confidence interval, 1.01–1.12) and of coronary heart disease and peripheral vascular disease. For women, gout predicted an increased risk of all vascular events (HR, 1.25; 95% CI, 1.15-1.35) except myocardial infarction and cerebrovascular disease overall. Further, the degree of increased risk of vascular events was greater for women than for men with gout (P< .001 for intersex difference).
Dr. Clarson and her associates proposed that the higher risk for vascular events among women with gout may arise from the longer exposure to elevated serum uric acid, since women have a longer prodrome before first gout attack, though they recommend further study to elucidate the mechanism.
Noting that “clinical management of gout in primary care is suboptimal,” Dr. Clarson and her colleagues urged greater attention to screening for vascular risk in those diagnosed with gout; these individuals comprise a significant population of over 8 million people in the United States. International guidelines recommend screening for cardiovascular risk when gout is diagnosed, but only one in four gout patients are so evaluated.
Regarding the sex differences unearthed in their study, Dr. Clarson and her associates observed that “both gout and vascular disease have historically been considered diseases of men ... [M]ore attention should be paid to prompt and reliable diagnosis of gout, followed by optimal management in female patients, including serious consideration of vascular risk reduction.”
The United Kingdom’s National School for Primary Research funded the study. The authors reported no relevant disclosures.
The significant strengths of Dr. Clarson and colleagues’ study of the association between gout and vascular disease are its large sample size, its accounting for many potentially confounding risk factors, and its exclusion of those with known antecedent heart disease. It is limited by its lack of validation of gout and cardiac diagnoses and the self-selection bias inherent in using primary care registries, rather than a true population-based cohort.
For individuals without gout, women’s overall vascular risk is lower, but the lack of a difference in cardiovascular disease risk in men and women with gout means that we do not yet understand why women’s risk rises more with the disease. It is possible that systemic inflammation induced by gout in women is more atherogenic than that in men, which necessitates studies to determine whether there are sex-based differences in the pathogenesis of gout-associated heart disease.

|
Dr. Jasvinder Singh |
Patients older than 35 or 40 with gout should be screened and followed with lipid profiles, hemoglobin A1c levels, blood pressure levels, and smoking status, and should undergo an assessment of other lifestyle factors that may impact cardiovascular risk.
Recognizing gout’s contribution to cardiac risk, and managing both the disease and associated risk factors, will be a key task for primary care doctors, rheumatologists, and cardiologists going forward.
Dr. Jasvinder Singh is a professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama, Birmingham. These comments are summarized from his editorial accompanying Dr. Clarson’s report (Ann. Rheum. Dis. 2015;74:631-4). He reported receiving research and travel grants from Takeda and Savient, and consultant fees from Takeda, Savient, Regeneron, and Allergan.
The significant strengths of Dr. Clarson and colleagues’ study of the association between gout and vascular disease are its large sample size, its accounting for many potentially confounding risk factors, and its exclusion of those with known antecedent heart disease. It is limited by its lack of validation of gout and cardiac diagnoses and the self-selection bias inherent in using primary care registries, rather than a true population-based cohort.
For individuals without gout, women’s overall vascular risk is lower, but the lack of a difference in cardiovascular disease risk in men and women with gout means that we do not yet understand why women’s risk rises more with the disease. It is possible that systemic inflammation induced by gout in women is more atherogenic than that in men, which necessitates studies to determine whether there are sex-based differences in the pathogenesis of gout-associated heart disease.

|
Dr. Jasvinder Singh |
Patients older than 35 or 40 with gout should be screened and followed with lipid profiles, hemoglobin A1c levels, blood pressure levels, and smoking status, and should undergo an assessment of other lifestyle factors that may impact cardiovascular risk.
Recognizing gout’s contribution to cardiac risk, and managing both the disease and associated risk factors, will be a key task for primary care doctors, rheumatologists, and cardiologists going forward.
Dr. Jasvinder Singh is a professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama, Birmingham. These comments are summarized from his editorial accompanying Dr. Clarson’s report (Ann. Rheum. Dis. 2015;74:631-4). He reported receiving research and travel grants from Takeda and Savient, and consultant fees from Takeda, Savient, Regeneron, and Allergan.
The significant strengths of Dr. Clarson and colleagues’ study of the association between gout and vascular disease are its large sample size, its accounting for many potentially confounding risk factors, and its exclusion of those with known antecedent heart disease. It is limited by its lack of validation of gout and cardiac diagnoses and the self-selection bias inherent in using primary care registries, rather than a true population-based cohort.
For individuals without gout, women’s overall vascular risk is lower, but the lack of a difference in cardiovascular disease risk in men and women with gout means that we do not yet understand why women’s risk rises more with the disease. It is possible that systemic inflammation induced by gout in women is more atherogenic than that in men, which necessitates studies to determine whether there are sex-based differences in the pathogenesis of gout-associated heart disease.

|
Dr. Jasvinder Singh |
Patients older than 35 or 40 with gout should be screened and followed with lipid profiles, hemoglobin A1c levels, blood pressure levels, and smoking status, and should undergo an assessment of other lifestyle factors that may impact cardiovascular risk.
Recognizing gout’s contribution to cardiac risk, and managing both the disease and associated risk factors, will be a key task for primary care doctors, rheumatologists, and cardiologists going forward.
Dr. Jasvinder Singh is a professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama, Birmingham. These comments are summarized from his editorial accompanying Dr. Clarson’s report (Ann. Rheum. Dis. 2015;74:631-4). He reported receiving research and travel grants from Takeda and Savient, and consultant fees from Takeda, Savient, Regeneron, and Allergan.
Gout’s association with a host of vascular events was confirmed in a new study that explored the links between the inflammatory condition and coronary artery disease, peripheral vascular disease, and cerebrovascular events.
Though both men and women with gout were at increased risk for vascular events overall, the association appeared strongest for women. Dr. Lorna Clarson of Keele (England) University and her associates drew these conclusions from a retrospective cohort study of men and women with an incident diagnosis of gout (Ann. Rheum. Dis. 2015;74:642-7).
Gout, caused by the deposition of uric acid crystals in joints, is characterized by acute flares of intensely painful and inflamed joints. However, the state of hyperuricemia that predisposes patients to acute attacks of gout may precede the first attack by years, and may persist between flares. The proinflammatory course of the natural history of gout has increasingly been recognized as a potential contributor to vascular disease.
The precise mechanism by which gout may increase vascular risk has not been identified. Dr. Clarson and associates noted that in addition to the acute and chronic inflammation associated with gout and hyperuricemia, serum uric acid may have a more direct effect on vascular health, as urate crystal deposition on vessel walls may promote vascular damage.
To clarify gout’s impact on vascular risk, Dr. Clarson and her associates used the Clinical Practice Datalink, a large United Kingdom health database, to compare 8,366 patients with gout to 39,766 age- and sex-matched controls. None of those studied had a baseline history of vascular disease, and all were aged 50 or older.
Careful accounting for covariates was accomplished by multivariate analysis that took into account sex, age, body mass index, tobacco and alcohol consumption, statin or aspirin use, and any history of hypertension, dyslipidemia, or chronic kidney disease. In addition, the study employed the composite Charlson Comorbidity Index, which weights 19 comorbid conditions – including diabetes – to arrive at a single score that captures many risk factors. Patients in the cohort were tracked until their first vascular event, or until death or loss to follow-up. Patient data collection was censored at 10 years from baseline or at the end of study data collection, whichever came first.
To assess the incidence of vascular events, the study noted the first recording in the medical record of any events signaling vascular disease. These included angina or myocardial infarction, transient ischemic attack and stroke, and a range of diagnoses associated with peripheral vascular disease.
Final analysis after accounting for the many covariates tracked in the study showed increased risk for vascular events for those with gout, with a definite difference between the sexes. For men, gout predicted an increased risk of any vascular event (hazard ratio, 1.06; 95% confidence interval, 1.01–1.12) and of coronary heart disease and peripheral vascular disease. For women, gout predicted an increased risk of all vascular events (HR, 1.25; 95% CI, 1.15-1.35) except myocardial infarction and cerebrovascular disease overall. Further, the degree of increased risk of vascular events was greater for women than for men with gout (P< .001 for intersex difference).
Dr. Clarson and her associates proposed that the higher risk for vascular events among women with gout may arise from the longer exposure to elevated serum uric acid, since women have a longer prodrome before first gout attack, though they recommend further study to elucidate the mechanism.
Noting that “clinical management of gout in primary care is suboptimal,” Dr. Clarson and her colleagues urged greater attention to screening for vascular risk in those diagnosed with gout; these individuals comprise a significant population of over 8 million people in the United States. International guidelines recommend screening for cardiovascular risk when gout is diagnosed, but only one in four gout patients are so evaluated.
Regarding the sex differences unearthed in their study, Dr. Clarson and her associates observed that “both gout and vascular disease have historically been considered diseases of men ... [M]ore attention should be paid to prompt and reliable diagnosis of gout, followed by optimal management in female patients, including serious consideration of vascular risk reduction.”
The United Kingdom’s National School for Primary Research funded the study. The authors reported no relevant disclosures.
Gout’s association with a host of vascular events was confirmed in a new study that explored the links between the inflammatory condition and coronary artery disease, peripheral vascular disease, and cerebrovascular events.
Though both men and women with gout were at increased risk for vascular events overall, the association appeared strongest for women. Dr. Lorna Clarson of Keele (England) University and her associates drew these conclusions from a retrospective cohort study of men and women with an incident diagnosis of gout (Ann. Rheum. Dis. 2015;74:642-7).
Gout, caused by the deposition of uric acid crystals in joints, is characterized by acute flares of intensely painful and inflamed joints. However, the state of hyperuricemia that predisposes patients to acute attacks of gout may precede the first attack by years, and may persist between flares. The proinflammatory course of the natural history of gout has increasingly been recognized as a potential contributor to vascular disease.
The precise mechanism by which gout may increase vascular risk has not been identified. Dr. Clarson and associates noted that in addition to the acute and chronic inflammation associated with gout and hyperuricemia, serum uric acid may have a more direct effect on vascular health, as urate crystal deposition on vessel walls may promote vascular damage.
To clarify gout’s impact on vascular risk, Dr. Clarson and her associates used the Clinical Practice Datalink, a large United Kingdom health database, to compare 8,366 patients with gout to 39,766 age- and sex-matched controls. None of those studied had a baseline history of vascular disease, and all were aged 50 or older.
Careful accounting for covariates was accomplished by multivariate analysis that took into account sex, age, body mass index, tobacco and alcohol consumption, statin or aspirin use, and any history of hypertension, dyslipidemia, or chronic kidney disease. In addition, the study employed the composite Charlson Comorbidity Index, which weights 19 comorbid conditions – including diabetes – to arrive at a single score that captures many risk factors. Patients in the cohort were tracked until their first vascular event, or until death or loss to follow-up. Patient data collection was censored at 10 years from baseline or at the end of study data collection, whichever came first.
To assess the incidence of vascular events, the study noted the first recording in the medical record of any events signaling vascular disease. These included angina or myocardial infarction, transient ischemic attack and stroke, and a range of diagnoses associated with peripheral vascular disease.
Final analysis after accounting for the many covariates tracked in the study showed increased risk for vascular events for those with gout, with a definite difference between the sexes. For men, gout predicted an increased risk of any vascular event (hazard ratio, 1.06; 95% confidence interval, 1.01–1.12) and of coronary heart disease and peripheral vascular disease. For women, gout predicted an increased risk of all vascular events (HR, 1.25; 95% CI, 1.15-1.35) except myocardial infarction and cerebrovascular disease overall. Further, the degree of increased risk of vascular events was greater for women than for men with gout (P< .001 for intersex difference).
Dr. Clarson and her associates proposed that the higher risk for vascular events among women with gout may arise from the longer exposure to elevated serum uric acid, since women have a longer prodrome before first gout attack, though they recommend further study to elucidate the mechanism.
Noting that “clinical management of gout in primary care is suboptimal,” Dr. Clarson and her colleagues urged greater attention to screening for vascular risk in those diagnosed with gout; these individuals comprise a significant population of over 8 million people in the United States. International guidelines recommend screening for cardiovascular risk when gout is diagnosed, but only one in four gout patients are so evaluated.
Regarding the sex differences unearthed in their study, Dr. Clarson and her associates observed that “both gout and vascular disease have historically been considered diseases of men ... [M]ore attention should be paid to prompt and reliable diagnosis of gout, followed by optimal management in female patients, including serious consideration of vascular risk reduction.”
The United Kingdom’s National School for Primary Research funded the study. The authors reported no relevant disclosures.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point: Gout increased the risk of a variety of vascular events, with women at greatest risk.
Major finding: Men with gout were at increased risk of vascular events with an adjusted hazard ratio (HR) of 1.06 overall; women’s risk was greater, with an overall adjusted HR of 1.25.
Data source: Comparison of 8,836 patients with gout to 39,766 matched controls without gout, from the United Kingdom’s Clinical Practice Research Datalink.
Disclosures: The United Kingdom’s National School for Primary Research funded the study. The authors reported no relevant disclosures.
Gout may lower Alzheimer’s risk
Patients with a history of gout may be at a lower risk for developing Alzheimer’s disease, reported Na Lu of Boston University and coauthors.
The large cohort study used electronic medical records from general practices in the United Kingdom’s The Health Improvement Network (THIN) to find 309 new cases of Alzheimer’s in 55,224 patients with gout and 1,942 cases in a comparison group of 238,805 patients during a median follow-up of 5 years. Gout patients had a multivariate-adjusted 24% lower risk of developing Alzheimer’s, the investigators found (hazard ratio, 0.76; 95% confidence interval, 0.66-0.87).
This difference may be due to the potential neuroprotective effects of uric acid, Ms. Lu and colleagues said in the report. “Overall, these findings support the proposed hypothesis that supplementary use of the metabolic precursor to uric acid, like inosine or hypoxanthine, could prevent and attenuate the progression of AD,” they wrote.
Read the full paper in Annals of the Rheumatic Diseases (2015 March 4 [doi:10.1136/annrheumdis-2014-206917]).
Patients with a history of gout may be at a lower risk for developing Alzheimer’s disease, reported Na Lu of Boston University and coauthors.
The large cohort study used electronic medical records from general practices in the United Kingdom’s The Health Improvement Network (THIN) to find 309 new cases of Alzheimer’s in 55,224 patients with gout and 1,942 cases in a comparison group of 238,805 patients during a median follow-up of 5 years. Gout patients had a multivariate-adjusted 24% lower risk of developing Alzheimer’s, the investigators found (hazard ratio, 0.76; 95% confidence interval, 0.66-0.87).
This difference may be due to the potential neuroprotective effects of uric acid, Ms. Lu and colleagues said in the report. “Overall, these findings support the proposed hypothesis that supplementary use of the metabolic precursor to uric acid, like inosine or hypoxanthine, could prevent and attenuate the progression of AD,” they wrote.
Read the full paper in Annals of the Rheumatic Diseases (2015 March 4 [doi:10.1136/annrheumdis-2014-206917]).
Patients with a history of gout may be at a lower risk for developing Alzheimer’s disease, reported Na Lu of Boston University and coauthors.
The large cohort study used electronic medical records from general practices in the United Kingdom’s The Health Improvement Network (THIN) to find 309 new cases of Alzheimer’s in 55,224 patients with gout and 1,942 cases in a comparison group of 238,805 patients during a median follow-up of 5 years. Gout patients had a multivariate-adjusted 24% lower risk of developing Alzheimer’s, the investigators found (hazard ratio, 0.76; 95% confidence interval, 0.66-0.87).
This difference may be due to the potential neuroprotective effects of uric acid, Ms. Lu and colleagues said in the report. “Overall, these findings support the proposed hypothesis that supplementary use of the metabolic precursor to uric acid, like inosine or hypoxanthine, could prevent and attenuate the progression of AD,” they wrote.
Read the full paper in Annals of the Rheumatic Diseases (2015 March 4 [doi:10.1136/annrheumdis-2014-206917]).
Gout management best practices require getting around misconceptions
MAUI, HAWAII – Urate-lowering therapy doesn’t have to be suspended while gout patients are treated for acute attacks, according to rheumatologist Orrin Troum of Santa Monica, Calif.
In fact, there are arguments against it; stopping allopurinol or other urate-lowering therapies (ULTs) during an attack doesn’t seem to help, and there’s a chance that patients might have another attack when it’s reintroduced. Still, the practice persists despite evidence and recommendations to the contrary, Dr. Troum said at the 2015 Rheumatology Winter Clinical Symposium.
Gout is a curable or at least eminently manageable condition, but it remains a tricky problem to treat. Part of that is because referring physicians might be using an out-of-date playbook before sending patients for a rheumatology consult; another issue is that optimal care requires follow-up visits, which might not always be possible.
Gout management guidelines from the European League Against Rheumatism (Ann. Rheum. Dis. 2006;65:1312-24) and the American College of Rheumatology (Arthritis Care Res. 2012;64:1431-46) now largely concur on how best to handle the condition, which might help bring uniformity to gout management if their message filters through to other branches of medicine, said rheumatologist and copresenter Martin Bergman of the department of rheumatology at Drexel University in Philadelphia.
Another persistent misconception is that ULT can’t be started during an acute attack. “There are some very good studies showing” that it can so long as antiflare drugs are on board and patients follow up to check for hypersensitivity reactions and other potential ULT problems, said Dr. Bergman, also chief of rheumatology at Taylor Hospital in Ridley Park, Pa.
Starting low and going slow, a key concept with ULT, hasn’t fully taken hold outside the rheumatology community, either. With allopurinol, that means starting at 100 mg/day – and 50 mg/day in those with chronic kidney disease – then titrating up slowly over several follow-up visits to an effective serum uric acid (SUA)–lowering dose. The idea is to lower serum uric acid slowly, to avoid precipitating an acute attack.
Even so, patients are still sometimes started on 300 mg/day, and although more than half will need more than 300 mg/day to reach SUA targets, that dose is still sometimes considered to be the maximum allowable.
Overall, the consensus on both sides of the Atlantic is that gout patients need to have serum urate levels below 6 mg/dL, and below 5 mg/dL if they have tophi.
“So the next question is, ‘How low do you go?’ ” It’s recently been found that “lifelong maintenance on very low levels of uric acid might actually increase the risk of neurodegenerative diseases, such as Parkinson’s, multiple sclerosis, and dementia.” Uric acid is a strong antioxidant that, perhaps, has protective effects in the central nervous system, Dr. Troum said.
It might be best to go below 5 mg/dL in severe gout for only 3-5 years, then loosen the target to 5-6 mg/dL (Nat. Rev. Rheumatol. 2014;10:271-83), he added.
Among other recent developments, it’s now known that psoriasis and psoriatic arthritis substantially increase the risk of gout (Ann. Rheum. Dis. 2014 March 20 [doi:10.1136/annrheumdis-2014-205212]), so it’s important to check for gout crystals when aspirating inflamed joints in those conditions. It remains unclear, however, if psoriasis or gout should take precedence when crystals are found, Dr. Bergman said.
Also, it makes sense to screen patients for their HLA-B genotype. Carriers of the variant allele HLA-B*5801 are at high risk for severe cutaneous adverse reactions with allopurinol, so another ULT is probably a better option. The variant is most common in individuals of Korean, Han Chinese, or Thai descent (Clin. Pharmacol. Ther. 2013;93:153-8).
Dr. Troum is an adviser, consultant, speaker, or grant recipient for several companies, including AbbVie, Amgen, Bristol-Myers Squibb, Centocor, Novartis, Pfizer, and Roche. He holds shares in Theralogix. Dr. Bergman is an adviser, speaker, or consultant for several companies, as well, including AbbVie, Celgene, Amgen, and Roche. He holds shares in Bristol-Myers Squibb, Pfizer, and Johnson & Johnson.
MAUI, HAWAII – Urate-lowering therapy doesn’t have to be suspended while gout patients are treated for acute attacks, according to rheumatologist Orrin Troum of Santa Monica, Calif.
In fact, there are arguments against it; stopping allopurinol or other urate-lowering therapies (ULTs) during an attack doesn’t seem to help, and there’s a chance that patients might have another attack when it’s reintroduced. Still, the practice persists despite evidence and recommendations to the contrary, Dr. Troum said at the 2015 Rheumatology Winter Clinical Symposium.
Gout is a curable or at least eminently manageable condition, but it remains a tricky problem to treat. Part of that is because referring physicians might be using an out-of-date playbook before sending patients for a rheumatology consult; another issue is that optimal care requires follow-up visits, which might not always be possible.
Gout management guidelines from the European League Against Rheumatism (Ann. Rheum. Dis. 2006;65:1312-24) and the American College of Rheumatology (Arthritis Care Res. 2012;64:1431-46) now largely concur on how best to handle the condition, which might help bring uniformity to gout management if their message filters through to other branches of medicine, said rheumatologist and copresenter Martin Bergman of the department of rheumatology at Drexel University in Philadelphia.
Another persistent misconception is that ULT can’t be started during an acute attack. “There are some very good studies showing” that it can so long as antiflare drugs are on board and patients follow up to check for hypersensitivity reactions and other potential ULT problems, said Dr. Bergman, also chief of rheumatology at Taylor Hospital in Ridley Park, Pa.
Starting low and going slow, a key concept with ULT, hasn’t fully taken hold outside the rheumatology community, either. With allopurinol, that means starting at 100 mg/day – and 50 mg/day in those with chronic kidney disease – then titrating up slowly over several follow-up visits to an effective serum uric acid (SUA)–lowering dose. The idea is to lower serum uric acid slowly, to avoid precipitating an acute attack.
Even so, patients are still sometimes started on 300 mg/day, and although more than half will need more than 300 mg/day to reach SUA targets, that dose is still sometimes considered to be the maximum allowable.
Overall, the consensus on both sides of the Atlantic is that gout patients need to have serum urate levels below 6 mg/dL, and below 5 mg/dL if they have tophi.
“So the next question is, ‘How low do you go?’ ” It’s recently been found that “lifelong maintenance on very low levels of uric acid might actually increase the risk of neurodegenerative diseases, such as Parkinson’s, multiple sclerosis, and dementia.” Uric acid is a strong antioxidant that, perhaps, has protective effects in the central nervous system, Dr. Troum said.
It might be best to go below 5 mg/dL in severe gout for only 3-5 years, then loosen the target to 5-6 mg/dL (Nat. Rev. Rheumatol. 2014;10:271-83), he added.
Among other recent developments, it’s now known that psoriasis and psoriatic arthritis substantially increase the risk of gout (Ann. Rheum. Dis. 2014 March 20 [doi:10.1136/annrheumdis-2014-205212]), so it’s important to check for gout crystals when aspirating inflamed joints in those conditions. It remains unclear, however, if psoriasis or gout should take precedence when crystals are found, Dr. Bergman said.
Also, it makes sense to screen patients for their HLA-B genotype. Carriers of the variant allele HLA-B*5801 are at high risk for severe cutaneous adverse reactions with allopurinol, so another ULT is probably a better option. The variant is most common in individuals of Korean, Han Chinese, or Thai descent (Clin. Pharmacol. Ther. 2013;93:153-8).
Dr. Troum is an adviser, consultant, speaker, or grant recipient for several companies, including AbbVie, Amgen, Bristol-Myers Squibb, Centocor, Novartis, Pfizer, and Roche. He holds shares in Theralogix. Dr. Bergman is an adviser, speaker, or consultant for several companies, as well, including AbbVie, Celgene, Amgen, and Roche. He holds shares in Bristol-Myers Squibb, Pfizer, and Johnson & Johnson.
MAUI, HAWAII – Urate-lowering therapy doesn’t have to be suspended while gout patients are treated for acute attacks, according to rheumatologist Orrin Troum of Santa Monica, Calif.
In fact, there are arguments against it; stopping allopurinol or other urate-lowering therapies (ULTs) during an attack doesn’t seem to help, and there’s a chance that patients might have another attack when it’s reintroduced. Still, the practice persists despite evidence and recommendations to the contrary, Dr. Troum said at the 2015 Rheumatology Winter Clinical Symposium.
Gout is a curable or at least eminently manageable condition, but it remains a tricky problem to treat. Part of that is because referring physicians might be using an out-of-date playbook before sending patients for a rheumatology consult; another issue is that optimal care requires follow-up visits, which might not always be possible.
Gout management guidelines from the European League Against Rheumatism (Ann. Rheum. Dis. 2006;65:1312-24) and the American College of Rheumatology (Arthritis Care Res. 2012;64:1431-46) now largely concur on how best to handle the condition, which might help bring uniformity to gout management if their message filters through to other branches of medicine, said rheumatologist and copresenter Martin Bergman of the department of rheumatology at Drexel University in Philadelphia.
Another persistent misconception is that ULT can’t be started during an acute attack. “There are some very good studies showing” that it can so long as antiflare drugs are on board and patients follow up to check for hypersensitivity reactions and other potential ULT problems, said Dr. Bergman, also chief of rheumatology at Taylor Hospital in Ridley Park, Pa.
Starting low and going slow, a key concept with ULT, hasn’t fully taken hold outside the rheumatology community, either. With allopurinol, that means starting at 100 mg/day – and 50 mg/day in those with chronic kidney disease – then titrating up slowly over several follow-up visits to an effective serum uric acid (SUA)–lowering dose. The idea is to lower serum uric acid slowly, to avoid precipitating an acute attack.
Even so, patients are still sometimes started on 300 mg/day, and although more than half will need more than 300 mg/day to reach SUA targets, that dose is still sometimes considered to be the maximum allowable.
Overall, the consensus on both sides of the Atlantic is that gout patients need to have serum urate levels below 6 mg/dL, and below 5 mg/dL if they have tophi.
“So the next question is, ‘How low do you go?’ ” It’s recently been found that “lifelong maintenance on very low levels of uric acid might actually increase the risk of neurodegenerative diseases, such as Parkinson’s, multiple sclerosis, and dementia.” Uric acid is a strong antioxidant that, perhaps, has protective effects in the central nervous system, Dr. Troum said.
It might be best to go below 5 mg/dL in severe gout for only 3-5 years, then loosen the target to 5-6 mg/dL (Nat. Rev. Rheumatol. 2014;10:271-83), he added.
Among other recent developments, it’s now known that psoriasis and psoriatic arthritis substantially increase the risk of gout (Ann. Rheum. Dis. 2014 March 20 [doi:10.1136/annrheumdis-2014-205212]), so it’s important to check for gout crystals when aspirating inflamed joints in those conditions. It remains unclear, however, if psoriasis or gout should take precedence when crystals are found, Dr. Bergman said.
Also, it makes sense to screen patients for their HLA-B genotype. Carriers of the variant allele HLA-B*5801 are at high risk for severe cutaneous adverse reactions with allopurinol, so another ULT is probably a better option. The variant is most common in individuals of Korean, Han Chinese, or Thai descent (Clin. Pharmacol. Ther. 2013;93:153-8).
Dr. Troum is an adviser, consultant, speaker, or grant recipient for several companies, including AbbVie, Amgen, Bristol-Myers Squibb, Centocor, Novartis, Pfizer, and Roche. He holds shares in Theralogix. Dr. Bergman is an adviser, speaker, or consultant for several companies, as well, including AbbVie, Celgene, Amgen, and Roche. He holds shares in Bristol-Myers Squibb, Pfizer, and Johnson & Johnson.
EXPERT ANALYSIS AT RWCS 2015
Court allows generic colchicine to enter market
After a 5-year monopoly on the sale of colchicine put the gout medication out of reach for many patients, a federal judge in January denied an injunction request by Takeda Pharmaceuticals U.S.A. to halt the distribution of colchicine products by Hikma Pharmaceuticals PLC.
The availability of a generic colchicine will introduce competition into the marketplace and drive down costs, said Dr. E. William St.Clair, president of the American College of Rheumatology and chief of the division of rheumatology and immunology at Duke University, Durham, N.C. The ACR issued a friend-of-the-court brief to the federal district court in support of a generic colchicine product entering the market.
“With the steep price increase in colchicine, many patients with gout were now unable to afford chronic colchicine therapy,” Dr. St.Clair said in an interview. “Improving access to colchicine by making it more affordable will increase patient compliance and reduce the suffering and disability associated with repeated gout flares.”
The debate over colchicine and the right to market the medication has a lengthy history. The drug has been prescribed to treat gout for decades, predating the law that requires drugs to be approved by the Food and Drug Administration. In 2009, the FDA approved a brand name colchicine product (Colcrys) by Mutual Pharmaceutical Company/URL Pharmacy Inc. – now Takeda Pharmaceuticals U.S.A. – after the company conducted clinical trials on dosing regimens and performed drug interaction studies. The FDA’s approval of Colcrys came with exclusive marketing rights for gout for 3 years and for familial Mediterranean fever for 7 years.
Mutual Pharmaceutical Company then sued other manufacturers of colchicine, claiming the drug makers were falsely implying that their products were FDA approved. Shortly later, the FDA ordered companies marketing single-ingredient oral colchicine to remove their unapproved products from the market. Physicians and patients meanwhile saw the price of colchicine increase from about 10 cents per tablet to $5 per tablet.
In September 2014, the FDA granted approval for Hikma to market and sell Mitigare, a colchicine capsule for the prophylactic treatment of gout. Hikma had also planned to launch an authorized generic of Mitigare. Before Mitigare could fully launch, Takeda obtained a temporary restraining order against the sale of colchicine products by Hikma, citing Takeda’s patents for acute gout treatment. Takeda simultaneously sued the FDA in a separate proceeding. Takeda said the FDA’s approval of Hikma’s colchicine product was legally impermissible.
In its brief to the federal court, the ACR argued the public interest would be severely disserved by the barring of Hikma’s colchicine product.
“The unfortunate reality is that nearly 30% of patients in the United States take risky and potentially dangerous steps to save money on prescription medicines, with many choosing to skip doses, or not fill their prescriptions altogether,” the ACR said in its brief. “Takeda’s monopoly and the associated price increase for colchicine has resulted in precisely the sorts of risky behavior described.”
A lower court denied Takeda’s preliminary injunction request and the decision was upheld by the U.S. Court of Appeals for the Federal Circuit on Jan. 9. Pending the outcome of further litigation, the federal court ruled that both Takeda and Hikma are free to immediately offer colchicine products for prophylactic use. Also on Jan. 9, the U.S. District Court for the District of Columbia denied Takeda’s request to overturn the FDA’s approval of Mitigare.
In an interview, a Takeda spokeswoman said the company will continue its patent infringement litigation against Hikma and its U.S. subsidiary, West-Ward Pharmaceuticals, along with its lawsuit against the FDA. The company offered no comment on the judge’s decision to deny the injunction.
West-Ward, meanwhile, launched its authorized generic to Mitigare following the judge’s Jan. 9 decision, and Mitigare’s entry to the market was resumed.
“We immediately sought to launch a generic of Mitigare to ensure that adult patients in need of treatment for the prophylaxis of gout flares had access to a lower-cost, alternative colchicine capsule product,” said Spiro Gavaris, vice president of sales and marketing for West-Ward Pharmaceuticals. “We understood that in recent years, some patients may have lost access to, or became frustrated with, colchicine when there was one brand product at a significantly higher price point. With our launch of the authorized generic of Mitigare capsules, doctors can now choose to prescribe Mitigare for the prophylaxis of gout flares in adults, thereby providing these patients with a lower cost generic medication.”
Days after the decision, Takeda announced that it also would be offering access to a generic colchicine. In a statement, the company said it would partner with Prasco Laboratories to distribute Colchicine Tablets, USP, an authorized generic version of Colcrys. The product is being marketed under the Prasco label and became available in U.S. pharmacies in mid-January.
“At Takeda, we remain committed to providing patients with therapies that are safe, efficacious and meet high quality standards,” Douglas Cole, Takeda Pharmaceuticals President, said in a statement. “This new partnership will help enhance patient access to an important gout medicine by supplying Prasco with Colchicine Tablets, USP, manufactured under the same rigorous standards and processes as Colcrys.”
Prices for the Mitigare authorized generic and the Colcrys authorized generic have not been publicly announced. In an interview, West-Ward said it could not comment on exact savings to patients as savings will vary between insurance plans and pharmacy distribution channels.
“Our goal is to provide the most aggressive discounts on generic colchicine in the market with the intent for those discounts to be passed on to adult patients in need of treatment for the prophylaxis of gout flares,” Mr. Gavaris said.
At this article’s deadline, Takeda and Prasco had not responded to a question about the price of the generic Colcrys.
While rheumatologists expressed relief that generic colchicine products have finally become available, they also voiced concerns about potential barriers to access.
“I am proud of what the ACR did here and would hope that it serves as a model for future efforts by professional medical organizations to get into the (often legal) trenches and truly help their patients get affordable care. … [They] put their mouth where the money is and stood up for patients,” said Dr. Christopher M. Burns, a rheumatologist at the Geisel School of Medicine at Dartmouth College, Lebanon, N.H.
“I hope that this will, in fact, reduce the pricing for colchicine, and that it will not allow payers to add an onerous out-of-pocket cost for the drug far in excess of the cost if paid without utilizing pharmacy benefits,” said Dr. Norman B. Gaylis, a rheumatologist in private practice in Aventura, Fla.
Dr. St.Clair added that the future availability and cost of generic colchicine is not certain given common supply-and-demand problems that can arise with generic drugs.
“We have observed critical shortages of several generic medications during the past several years that have drastically affected medical therapy for many common conditions,” he said in an interview. “We will need to keep a close eye on the supply of generic colchicine to ensure it keeps up with the demand. The FDA and the pharmaceutical industry has an obligation to ensure that patients have access to these critical generic drugs.”
On Twitter @legal_med
After a 5-year monopoly on the sale of colchicine put the gout medication out of reach for many patients, a federal judge in January denied an injunction request by Takeda Pharmaceuticals U.S.A. to halt the distribution of colchicine products by Hikma Pharmaceuticals PLC.
The availability of a generic colchicine will introduce competition into the marketplace and drive down costs, said Dr. E. William St.Clair, president of the American College of Rheumatology and chief of the division of rheumatology and immunology at Duke University, Durham, N.C. The ACR issued a friend-of-the-court brief to the federal district court in support of a generic colchicine product entering the market.
“With the steep price increase in colchicine, many patients with gout were now unable to afford chronic colchicine therapy,” Dr. St.Clair said in an interview. “Improving access to colchicine by making it more affordable will increase patient compliance and reduce the suffering and disability associated with repeated gout flares.”
The debate over colchicine and the right to market the medication has a lengthy history. The drug has been prescribed to treat gout for decades, predating the law that requires drugs to be approved by the Food and Drug Administration. In 2009, the FDA approved a brand name colchicine product (Colcrys) by Mutual Pharmaceutical Company/URL Pharmacy Inc. – now Takeda Pharmaceuticals U.S.A. – after the company conducted clinical trials on dosing regimens and performed drug interaction studies. The FDA’s approval of Colcrys came with exclusive marketing rights for gout for 3 years and for familial Mediterranean fever for 7 years.
Mutual Pharmaceutical Company then sued other manufacturers of colchicine, claiming the drug makers were falsely implying that their products were FDA approved. Shortly later, the FDA ordered companies marketing single-ingredient oral colchicine to remove their unapproved products from the market. Physicians and patients meanwhile saw the price of colchicine increase from about 10 cents per tablet to $5 per tablet.
In September 2014, the FDA granted approval for Hikma to market and sell Mitigare, a colchicine capsule for the prophylactic treatment of gout. Hikma had also planned to launch an authorized generic of Mitigare. Before Mitigare could fully launch, Takeda obtained a temporary restraining order against the sale of colchicine products by Hikma, citing Takeda’s patents for acute gout treatment. Takeda simultaneously sued the FDA in a separate proceeding. Takeda said the FDA’s approval of Hikma’s colchicine product was legally impermissible.
In its brief to the federal court, the ACR argued the public interest would be severely disserved by the barring of Hikma’s colchicine product.
“The unfortunate reality is that nearly 30% of patients in the United States take risky and potentially dangerous steps to save money on prescription medicines, with many choosing to skip doses, or not fill their prescriptions altogether,” the ACR said in its brief. “Takeda’s monopoly and the associated price increase for colchicine has resulted in precisely the sorts of risky behavior described.”
A lower court denied Takeda’s preliminary injunction request and the decision was upheld by the U.S. Court of Appeals for the Federal Circuit on Jan. 9. Pending the outcome of further litigation, the federal court ruled that both Takeda and Hikma are free to immediately offer colchicine products for prophylactic use. Also on Jan. 9, the U.S. District Court for the District of Columbia denied Takeda’s request to overturn the FDA’s approval of Mitigare.
In an interview, a Takeda spokeswoman said the company will continue its patent infringement litigation against Hikma and its U.S. subsidiary, West-Ward Pharmaceuticals, along with its lawsuit against the FDA. The company offered no comment on the judge’s decision to deny the injunction.
West-Ward, meanwhile, launched its authorized generic to Mitigare following the judge’s Jan. 9 decision, and Mitigare’s entry to the market was resumed.
“We immediately sought to launch a generic of Mitigare to ensure that adult patients in need of treatment for the prophylaxis of gout flares had access to a lower-cost, alternative colchicine capsule product,” said Spiro Gavaris, vice president of sales and marketing for West-Ward Pharmaceuticals. “We understood that in recent years, some patients may have lost access to, or became frustrated with, colchicine when there was one brand product at a significantly higher price point. With our launch of the authorized generic of Mitigare capsules, doctors can now choose to prescribe Mitigare for the prophylaxis of gout flares in adults, thereby providing these patients with a lower cost generic medication.”
Days after the decision, Takeda announced that it also would be offering access to a generic colchicine. In a statement, the company said it would partner with Prasco Laboratories to distribute Colchicine Tablets, USP, an authorized generic version of Colcrys. The product is being marketed under the Prasco label and became available in U.S. pharmacies in mid-January.
“At Takeda, we remain committed to providing patients with therapies that are safe, efficacious and meet high quality standards,” Douglas Cole, Takeda Pharmaceuticals President, said in a statement. “This new partnership will help enhance patient access to an important gout medicine by supplying Prasco with Colchicine Tablets, USP, manufactured under the same rigorous standards and processes as Colcrys.”
Prices for the Mitigare authorized generic and the Colcrys authorized generic have not been publicly announced. In an interview, West-Ward said it could not comment on exact savings to patients as savings will vary between insurance plans and pharmacy distribution channels.
“Our goal is to provide the most aggressive discounts on generic colchicine in the market with the intent for those discounts to be passed on to adult patients in need of treatment for the prophylaxis of gout flares,” Mr. Gavaris said.
At this article’s deadline, Takeda and Prasco had not responded to a question about the price of the generic Colcrys.
While rheumatologists expressed relief that generic colchicine products have finally become available, they also voiced concerns about potential barriers to access.
“I am proud of what the ACR did here and would hope that it serves as a model for future efforts by professional medical organizations to get into the (often legal) trenches and truly help their patients get affordable care. … [They] put their mouth where the money is and stood up for patients,” said Dr. Christopher M. Burns, a rheumatologist at the Geisel School of Medicine at Dartmouth College, Lebanon, N.H.
“I hope that this will, in fact, reduce the pricing for colchicine, and that it will not allow payers to add an onerous out-of-pocket cost for the drug far in excess of the cost if paid without utilizing pharmacy benefits,” said Dr. Norman B. Gaylis, a rheumatologist in private practice in Aventura, Fla.
Dr. St.Clair added that the future availability and cost of generic colchicine is not certain given common supply-and-demand problems that can arise with generic drugs.
“We have observed critical shortages of several generic medications during the past several years that have drastically affected medical therapy for many common conditions,” he said in an interview. “We will need to keep a close eye on the supply of generic colchicine to ensure it keeps up with the demand. The FDA and the pharmaceutical industry has an obligation to ensure that patients have access to these critical generic drugs.”
On Twitter @legal_med
After a 5-year monopoly on the sale of colchicine put the gout medication out of reach for many patients, a federal judge in January denied an injunction request by Takeda Pharmaceuticals U.S.A. to halt the distribution of colchicine products by Hikma Pharmaceuticals PLC.
The availability of a generic colchicine will introduce competition into the marketplace and drive down costs, said Dr. E. William St.Clair, president of the American College of Rheumatology and chief of the division of rheumatology and immunology at Duke University, Durham, N.C. The ACR issued a friend-of-the-court brief to the federal district court in support of a generic colchicine product entering the market.
“With the steep price increase in colchicine, many patients with gout were now unable to afford chronic colchicine therapy,” Dr. St.Clair said in an interview. “Improving access to colchicine by making it more affordable will increase patient compliance and reduce the suffering and disability associated with repeated gout flares.”
The debate over colchicine and the right to market the medication has a lengthy history. The drug has been prescribed to treat gout for decades, predating the law that requires drugs to be approved by the Food and Drug Administration. In 2009, the FDA approved a brand name colchicine product (Colcrys) by Mutual Pharmaceutical Company/URL Pharmacy Inc. – now Takeda Pharmaceuticals U.S.A. – after the company conducted clinical trials on dosing regimens and performed drug interaction studies. The FDA’s approval of Colcrys came with exclusive marketing rights for gout for 3 years and for familial Mediterranean fever for 7 years.
Mutual Pharmaceutical Company then sued other manufacturers of colchicine, claiming the drug makers were falsely implying that their products were FDA approved. Shortly later, the FDA ordered companies marketing single-ingredient oral colchicine to remove their unapproved products from the market. Physicians and patients meanwhile saw the price of colchicine increase from about 10 cents per tablet to $5 per tablet.
In September 2014, the FDA granted approval for Hikma to market and sell Mitigare, a colchicine capsule for the prophylactic treatment of gout. Hikma had also planned to launch an authorized generic of Mitigare. Before Mitigare could fully launch, Takeda obtained a temporary restraining order against the sale of colchicine products by Hikma, citing Takeda’s patents for acute gout treatment. Takeda simultaneously sued the FDA in a separate proceeding. Takeda said the FDA’s approval of Hikma’s colchicine product was legally impermissible.
In its brief to the federal court, the ACR argued the public interest would be severely disserved by the barring of Hikma’s colchicine product.
“The unfortunate reality is that nearly 30% of patients in the United States take risky and potentially dangerous steps to save money on prescription medicines, with many choosing to skip doses, or not fill their prescriptions altogether,” the ACR said in its brief. “Takeda’s monopoly and the associated price increase for colchicine has resulted in precisely the sorts of risky behavior described.”
A lower court denied Takeda’s preliminary injunction request and the decision was upheld by the U.S. Court of Appeals for the Federal Circuit on Jan. 9. Pending the outcome of further litigation, the federal court ruled that both Takeda and Hikma are free to immediately offer colchicine products for prophylactic use. Also on Jan. 9, the U.S. District Court for the District of Columbia denied Takeda’s request to overturn the FDA’s approval of Mitigare.
In an interview, a Takeda spokeswoman said the company will continue its patent infringement litigation against Hikma and its U.S. subsidiary, West-Ward Pharmaceuticals, along with its lawsuit against the FDA. The company offered no comment on the judge’s decision to deny the injunction.
West-Ward, meanwhile, launched its authorized generic to Mitigare following the judge’s Jan. 9 decision, and Mitigare’s entry to the market was resumed.
“We immediately sought to launch a generic of Mitigare to ensure that adult patients in need of treatment for the prophylaxis of gout flares had access to a lower-cost, alternative colchicine capsule product,” said Spiro Gavaris, vice president of sales and marketing for West-Ward Pharmaceuticals. “We understood that in recent years, some patients may have lost access to, or became frustrated with, colchicine when there was one brand product at a significantly higher price point. With our launch of the authorized generic of Mitigare capsules, doctors can now choose to prescribe Mitigare for the prophylaxis of gout flares in adults, thereby providing these patients with a lower cost generic medication.”
Days after the decision, Takeda announced that it also would be offering access to a generic colchicine. In a statement, the company said it would partner with Prasco Laboratories to distribute Colchicine Tablets, USP, an authorized generic version of Colcrys. The product is being marketed under the Prasco label and became available in U.S. pharmacies in mid-January.
“At Takeda, we remain committed to providing patients with therapies that are safe, efficacious and meet high quality standards,” Douglas Cole, Takeda Pharmaceuticals President, said in a statement. “This new partnership will help enhance patient access to an important gout medicine by supplying Prasco with Colchicine Tablets, USP, manufactured under the same rigorous standards and processes as Colcrys.”
Prices for the Mitigare authorized generic and the Colcrys authorized generic have not been publicly announced. In an interview, West-Ward said it could not comment on exact savings to patients as savings will vary between insurance plans and pharmacy distribution channels.
“Our goal is to provide the most aggressive discounts on generic colchicine in the market with the intent for those discounts to be passed on to adult patients in need of treatment for the prophylaxis of gout flares,” Mr. Gavaris said.
At this article’s deadline, Takeda and Prasco had not responded to a question about the price of the generic Colcrys.
While rheumatologists expressed relief that generic colchicine products have finally become available, they also voiced concerns about potential barriers to access.
“I am proud of what the ACR did here and would hope that it serves as a model for future efforts by professional medical organizations to get into the (often legal) trenches and truly help their patients get affordable care. … [They] put their mouth where the money is and stood up for patients,” said Dr. Christopher M. Burns, a rheumatologist at the Geisel School of Medicine at Dartmouth College, Lebanon, N.H.
“I hope that this will, in fact, reduce the pricing for colchicine, and that it will not allow payers to add an onerous out-of-pocket cost for the drug far in excess of the cost if paid without utilizing pharmacy benefits,” said Dr. Norman B. Gaylis, a rheumatologist in private practice in Aventura, Fla.
Dr. St.Clair added that the future availability and cost of generic colchicine is not certain given common supply-and-demand problems that can arise with generic drugs.
“We have observed critical shortages of several generic medications during the past several years that have drastically affected medical therapy for many common conditions,” he said in an interview. “We will need to keep a close eye on the supply of generic colchicine to ensure it keeps up with the demand. The FDA and the pharmaceutical industry has an obligation to ensure that patients have access to these critical generic drugs.”
On Twitter @legal_med
Up to 90% of gout hospitalizations might be preventable
BOSTON – Nearly all hospitalizations of people who were discharged with a primary diagnosis of gout were likely preventable, based on the results of a retrospective analysis of 79 cases at a single institution.
Because most of these patients presented to the emergency department rather than their doctor’s office, and were in pain and had other comorbidities, admission seemed the correct medical care decision, said Dr. Thomas Olenginski of the Geisinger Health System and one of the study’s lead authors. If the ED had gotten a rheumatology consult during the patients’ observation periods, however, the diagnosis could have been confirmed and most of these admissions would likely have been avoided, with a potential savings of over $200,000 in total hospitalization-related costs, Dr. Olenginski said at the annual meeting of the American College of Rheumatology.
Further, most of these patients were not adherent to their prescribed gout medications. Better clinical care and compliance with gout therapy might prevent most gout flareups that result in hospitalization, he added.
As a result of these findings, Geisinger has reassessed its approach to gout patients, especially those who present to the ED. For identified gout patients, they have ramped up efforts to make sure patients are adhering to therapy and are at goal, as well as educating them about their disease and how it can worsen without adherence. Rheumatologists are available to the ED by pager, encouraging arthrocentesis and crystal confirmation, thereby allowing the ED to focus on treating any associated skin infections and comorbidities, Dr. Olenginski said.
Of 56 gout-related admissions to their hospital, the Geisinger researchers found that 50 (89%) met the study’s definition of a preventable admission. A preventable admission was defined as one with a primary admitting diagnosis of mono- or polyarthritis subsequently diagnosed as gout and without any concomitant illness warranting admission on presentation.
The clinical diagnoses included 76% septic arthritis, 14% inflammatory polyarthritis, and 8% cellulitis.
Of the 50 preventable admissions, 33 patients underwent arthrocentesis, 24 of which were performed in the ED where the diagnosis could have been made based on crystal-confirmed diagnosis, he said.
Among the 35 patients with a prior history of gout, there were 23 patients whose serum uric acid levels were recorded within 1 year of their hospitalization, and 18 (78%) did not reach the goal of less than 6 mg/dL. Of 15 patients on long-term gout treatment, 5 (33%) were noncompliant with their treatment plans.
The total additive length of stay for the preventable gout admissions was 171 days with a mean stay of 3.42 days. Total hospitalization-related costs of these admissions were $208,000, with an average cost per admission of $4,160.
The study was performed as a quality initiative at Geisinger Health System. Dr. Olenginski had no relevant financial disclosures.
BOSTON – Nearly all hospitalizations of people who were discharged with a primary diagnosis of gout were likely preventable, based on the results of a retrospective analysis of 79 cases at a single institution.
Because most of these patients presented to the emergency department rather than their doctor’s office, and were in pain and had other comorbidities, admission seemed the correct medical care decision, said Dr. Thomas Olenginski of the Geisinger Health System and one of the study’s lead authors. If the ED had gotten a rheumatology consult during the patients’ observation periods, however, the diagnosis could have been confirmed and most of these admissions would likely have been avoided, with a potential savings of over $200,000 in total hospitalization-related costs, Dr. Olenginski said at the annual meeting of the American College of Rheumatology.
Further, most of these patients were not adherent to their prescribed gout medications. Better clinical care and compliance with gout therapy might prevent most gout flareups that result in hospitalization, he added.
As a result of these findings, Geisinger has reassessed its approach to gout patients, especially those who present to the ED. For identified gout patients, they have ramped up efforts to make sure patients are adhering to therapy and are at goal, as well as educating them about their disease and how it can worsen without adherence. Rheumatologists are available to the ED by pager, encouraging arthrocentesis and crystal confirmation, thereby allowing the ED to focus on treating any associated skin infections and comorbidities, Dr. Olenginski said.
Of 56 gout-related admissions to their hospital, the Geisinger researchers found that 50 (89%) met the study’s definition of a preventable admission. A preventable admission was defined as one with a primary admitting diagnosis of mono- or polyarthritis subsequently diagnosed as gout and without any concomitant illness warranting admission on presentation.
The clinical diagnoses included 76% septic arthritis, 14% inflammatory polyarthritis, and 8% cellulitis.
Of the 50 preventable admissions, 33 patients underwent arthrocentesis, 24 of which were performed in the ED where the diagnosis could have been made based on crystal-confirmed diagnosis, he said.
Among the 35 patients with a prior history of gout, there were 23 patients whose serum uric acid levels were recorded within 1 year of their hospitalization, and 18 (78%) did not reach the goal of less than 6 mg/dL. Of 15 patients on long-term gout treatment, 5 (33%) were noncompliant with their treatment plans.
The total additive length of stay for the preventable gout admissions was 171 days with a mean stay of 3.42 days. Total hospitalization-related costs of these admissions were $208,000, with an average cost per admission of $4,160.
The study was performed as a quality initiative at Geisinger Health System. Dr. Olenginski had no relevant financial disclosures.
BOSTON – Nearly all hospitalizations of people who were discharged with a primary diagnosis of gout were likely preventable, based on the results of a retrospective analysis of 79 cases at a single institution.
Because most of these patients presented to the emergency department rather than their doctor’s office, and were in pain and had other comorbidities, admission seemed the correct medical care decision, said Dr. Thomas Olenginski of the Geisinger Health System and one of the study’s lead authors. If the ED had gotten a rheumatology consult during the patients’ observation periods, however, the diagnosis could have been confirmed and most of these admissions would likely have been avoided, with a potential savings of over $200,000 in total hospitalization-related costs, Dr. Olenginski said at the annual meeting of the American College of Rheumatology.
Further, most of these patients were not adherent to their prescribed gout medications. Better clinical care and compliance with gout therapy might prevent most gout flareups that result in hospitalization, he added.
As a result of these findings, Geisinger has reassessed its approach to gout patients, especially those who present to the ED. For identified gout patients, they have ramped up efforts to make sure patients are adhering to therapy and are at goal, as well as educating them about their disease and how it can worsen without adherence. Rheumatologists are available to the ED by pager, encouraging arthrocentesis and crystal confirmation, thereby allowing the ED to focus on treating any associated skin infections and comorbidities, Dr. Olenginski said.
Of 56 gout-related admissions to their hospital, the Geisinger researchers found that 50 (89%) met the study’s definition of a preventable admission. A preventable admission was defined as one with a primary admitting diagnosis of mono- or polyarthritis subsequently diagnosed as gout and without any concomitant illness warranting admission on presentation.
The clinical diagnoses included 76% septic arthritis, 14% inflammatory polyarthritis, and 8% cellulitis.
Of the 50 preventable admissions, 33 patients underwent arthrocentesis, 24 of which were performed in the ED where the diagnosis could have been made based on crystal-confirmed diagnosis, he said.
Among the 35 patients with a prior history of gout, there were 23 patients whose serum uric acid levels were recorded within 1 year of their hospitalization, and 18 (78%) did not reach the goal of less than 6 mg/dL. Of 15 patients on long-term gout treatment, 5 (33%) were noncompliant with their treatment plans.
The total additive length of stay for the preventable gout admissions was 171 days with a mean stay of 3.42 days. Total hospitalization-related costs of these admissions were $208,000, with an average cost per admission of $4,160.
The study was performed as a quality initiative at Geisinger Health System. Dr. Olenginski had no relevant financial disclosures.
AT THE ACR ANNUAL MEETING
Key clinical point: Gout patients are often admitted to the hospital via the emergency department; most of these admissions could be avoidable.
Major finding: Of 56 gout-related admissions to their hospital, the Geisinger researchers found that 50 (89%) met the study’s definition of a preventable admission.
Data source: A retrospective analysis of 79 cases of gout at a single institution.
Disclosures: The study was performed as a quality initiative at Geisinger Health System. Dr. Olenginski had no relevant financial disclosures.
Gout may predispose people, particularly women, to diabetes
Screen for diabetes and aggressively manage risk factors in patients with gout, especially women, investigators concluded from a retrospective, matched cohort study published Oct. 2 in Annals of the Rheumatic Diseases.
They found that women have a 71% greater risk of developing diabetes if they have gout (hazard ratio, 1.71; 95% confidence interval, 1.51-1.93; P less than .001), and men with gout have a 22% greater risk (HR, 1.22; 95% CI, 1.13-1.31; P less than .001), compared with the general population.
The study “suggests that gout may be independently associated with an increased risk of diabetes. These findings were independent of BMI [body mass index], lifestyle factors, and other known risk factors. The magnitude of excess diabetes risk in gout was significantly larger among women than men, both in risk difference and relative risk, and these findings persisted across all age categories. These findings support aggressive management of risk factors of diabetes in patients with gout,” concluded senior investigator Dr. Hyon K. Choi and his colleagues at Boston University (Ann. Rheum. Dis. 2014 Oct. 2 [doi: 10.1136/annrheumdis-2014-205827]).
Using 15 years’ worth of data from the U.K. Health Improvement Network, which contains the records of about 7.3 million patients, the investigators matched 35,339 patients with newly diagnosed gout with up to 5 control subjects for age, sex, and BMI; they then looked to see who subsequently developed diabetes.
Among patients with gout, there were 10.1 cases of new-onset diabetes in women and 9.5 cases in men per 1,000 person-years. Among the 137,056 controls without gout, there were 5.6 cases of new-onset diabetes in women and 7.2 cases in men per 1,000 person-years.
After adjustment for smoking, alcohol consumption, physician visits, comorbidities, medication use, and BMI as a continuous variable, gout increased the risk of diabetes by 48% in women (HR, 1.48; 95% CI, 1.29-1.68; P less than .001) and by 15% in men (HR, 1.15; 95% CI, 1.06-1.24; P less than .001). The sex difference persisted across age groups.
Gout patients consumed more alcohol, visited their doctor more often, had more health problems, and took steroids and diuretics more frequently than did those who did not have gout. Overall, 72.4% of the gout cases were in men with a mean age of 62.7 years; the rest were in women, but women with gout tended to be a bit older, with a mean age of 67.9 years.
Perhaps, “low-grade inflammation among patients with gout promote[s] the diabetogenic process,” the investigators wrote. “Alternatively, the link may stem from the shared metabolic factors of the two conditions, such as the correlates of the metabolic syndrome or shared genes. Furthermore, the link between hyperuricemia and the risk of type 2 diabetes may originate at the renal level, as insulin resistance and higher insulin levels are known to reduce renal excretion of urate,” they noted.
It’s unclear why women seem to be more affected. “SUA [serum uric acid] levels in men are about 1 mg/dL higher than in women during adulthood, although levels in women increase around natural menopause. Thus, the physiological impact of uric acid levels, which are high enough to cause gout, could be stronger among women than men. Furthermore, female gout patients may have higher SUA levels on average than male gout patients, which could also contribute to a larger association with the risk of diabetes among women,” they suggested.
Dr. Choi previously linked gout to the development of diabetes, but only in men with high cardiovascular risk profiles (Rheumatology 2008;47:1567-70).
The investigators had no disclosures. The work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Screen for diabetes and aggressively manage risk factors in patients with gout, especially women, investigators concluded from a retrospective, matched cohort study published Oct. 2 in Annals of the Rheumatic Diseases.
They found that women have a 71% greater risk of developing diabetes if they have gout (hazard ratio, 1.71; 95% confidence interval, 1.51-1.93; P less than .001), and men with gout have a 22% greater risk (HR, 1.22; 95% CI, 1.13-1.31; P less than .001), compared with the general population.
The study “suggests that gout may be independently associated with an increased risk of diabetes. These findings were independent of BMI [body mass index], lifestyle factors, and other known risk factors. The magnitude of excess diabetes risk in gout was significantly larger among women than men, both in risk difference and relative risk, and these findings persisted across all age categories. These findings support aggressive management of risk factors of diabetes in patients with gout,” concluded senior investigator Dr. Hyon K. Choi and his colleagues at Boston University (Ann. Rheum. Dis. 2014 Oct. 2 [doi: 10.1136/annrheumdis-2014-205827]).
Using 15 years’ worth of data from the U.K. Health Improvement Network, which contains the records of about 7.3 million patients, the investigators matched 35,339 patients with newly diagnosed gout with up to 5 control subjects for age, sex, and BMI; they then looked to see who subsequently developed diabetes.
Among patients with gout, there were 10.1 cases of new-onset diabetes in women and 9.5 cases in men per 1,000 person-years. Among the 137,056 controls without gout, there were 5.6 cases of new-onset diabetes in women and 7.2 cases in men per 1,000 person-years.
After adjustment for smoking, alcohol consumption, physician visits, comorbidities, medication use, and BMI as a continuous variable, gout increased the risk of diabetes by 48% in women (HR, 1.48; 95% CI, 1.29-1.68; P less than .001) and by 15% in men (HR, 1.15; 95% CI, 1.06-1.24; P less than .001). The sex difference persisted across age groups.
Gout patients consumed more alcohol, visited their doctor more often, had more health problems, and took steroids and diuretics more frequently than did those who did not have gout. Overall, 72.4% of the gout cases were in men with a mean age of 62.7 years; the rest were in women, but women with gout tended to be a bit older, with a mean age of 67.9 years.
Perhaps, “low-grade inflammation among patients with gout promote[s] the diabetogenic process,” the investigators wrote. “Alternatively, the link may stem from the shared metabolic factors of the two conditions, such as the correlates of the metabolic syndrome or shared genes. Furthermore, the link between hyperuricemia and the risk of type 2 diabetes may originate at the renal level, as insulin resistance and higher insulin levels are known to reduce renal excretion of urate,” they noted.
It’s unclear why women seem to be more affected. “SUA [serum uric acid] levels in men are about 1 mg/dL higher than in women during adulthood, although levels in women increase around natural menopause. Thus, the physiological impact of uric acid levels, which are high enough to cause gout, could be stronger among women than men. Furthermore, female gout patients may have higher SUA levels on average than male gout patients, which could also contribute to a larger association with the risk of diabetes among women,” they suggested.
Dr. Choi previously linked gout to the development of diabetes, but only in men with high cardiovascular risk profiles (Rheumatology 2008;47:1567-70).
The investigators had no disclosures. The work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Screen for diabetes and aggressively manage risk factors in patients with gout, especially women, investigators concluded from a retrospective, matched cohort study published Oct. 2 in Annals of the Rheumatic Diseases.
They found that women have a 71% greater risk of developing diabetes if they have gout (hazard ratio, 1.71; 95% confidence interval, 1.51-1.93; P less than .001), and men with gout have a 22% greater risk (HR, 1.22; 95% CI, 1.13-1.31; P less than .001), compared with the general population.
The study “suggests that gout may be independently associated with an increased risk of diabetes. These findings were independent of BMI [body mass index], lifestyle factors, and other known risk factors. The magnitude of excess diabetes risk in gout was significantly larger among women than men, both in risk difference and relative risk, and these findings persisted across all age categories. These findings support aggressive management of risk factors of diabetes in patients with gout,” concluded senior investigator Dr. Hyon K. Choi and his colleagues at Boston University (Ann. Rheum. Dis. 2014 Oct. 2 [doi: 10.1136/annrheumdis-2014-205827]).
Using 15 years’ worth of data from the U.K. Health Improvement Network, which contains the records of about 7.3 million patients, the investigators matched 35,339 patients with newly diagnosed gout with up to 5 control subjects for age, sex, and BMI; they then looked to see who subsequently developed diabetes.
Among patients with gout, there were 10.1 cases of new-onset diabetes in women and 9.5 cases in men per 1,000 person-years. Among the 137,056 controls without gout, there were 5.6 cases of new-onset diabetes in women and 7.2 cases in men per 1,000 person-years.
After adjustment for smoking, alcohol consumption, physician visits, comorbidities, medication use, and BMI as a continuous variable, gout increased the risk of diabetes by 48% in women (HR, 1.48; 95% CI, 1.29-1.68; P less than .001) and by 15% in men (HR, 1.15; 95% CI, 1.06-1.24; P less than .001). The sex difference persisted across age groups.
Gout patients consumed more alcohol, visited their doctor more often, had more health problems, and took steroids and diuretics more frequently than did those who did not have gout. Overall, 72.4% of the gout cases were in men with a mean age of 62.7 years; the rest were in women, but women with gout tended to be a bit older, with a mean age of 67.9 years.
Perhaps, “low-grade inflammation among patients with gout promote[s] the diabetogenic process,” the investigators wrote. “Alternatively, the link may stem from the shared metabolic factors of the two conditions, such as the correlates of the metabolic syndrome or shared genes. Furthermore, the link between hyperuricemia and the risk of type 2 diabetes may originate at the renal level, as insulin resistance and higher insulin levels are known to reduce renal excretion of urate,” they noted.
It’s unclear why women seem to be more affected. “SUA [serum uric acid] levels in men are about 1 mg/dL higher than in women during adulthood, although levels in women increase around natural menopause. Thus, the physiological impact of uric acid levels, which are high enough to cause gout, could be stronger among women than men. Furthermore, female gout patients may have higher SUA levels on average than male gout patients, which could also contribute to a larger association with the risk of diabetes among women,” they suggested.
Dr. Choi previously linked gout to the development of diabetes, but only in men with high cardiovascular risk profiles (Rheumatology 2008;47:1567-70).
The investigators had no disclosures. The work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
FROM ANNALS OF THE RHEUMATIC DISEASES
Key clinical point: Be on the lookout for diabetes in your gout patients.
Major finding: Women have a 71% greater risk of developing diabetes if they have gout (HR, 1.71; 95% CI, 1.51-1.93; P less than .001), and men with gout have a 22% greater risk (HR, 1.22; 95% CI, 1.13-1.31; P less than .001), compared with the general population.
Data source: Retrospective database study of more than 170,000 patients.
Disclosures: The investigators had no disclosures. The work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases.