User login
Oncology 2015: new therapies and new transitions toward value-based cancer care
The past year has been an exciting one for new oncology and hematology drug approvals and the continued evolution of our oncology delivery system toward high quality and value. In all, at press time in mid-November, the US Food and Drug Administration (FDA) had approved or granted expanded indications for 24 drugs, compared with 19 in the 2 preceding years. Of those 24 approvals, 7 were accelerated and 6 were expanded approvals, and 3 alone were for the immunotherapeutic drug, nivolumab – 2 for non-small-cell lung cancer (NSCLC) and 1 for metastatic melanoma.
Click on the PDF icon at the top of this introduction to read the full article.
The past year has been an exciting one for new oncology and hematology drug approvals and the continued evolution of our oncology delivery system toward high quality and value. In all, at press time in mid-November, the US Food and Drug Administration (FDA) had approved or granted expanded indications for 24 drugs, compared with 19 in the 2 preceding years. Of those 24 approvals, 7 were accelerated and 6 were expanded approvals, and 3 alone were for the immunotherapeutic drug, nivolumab – 2 for non-small-cell lung cancer (NSCLC) and 1 for metastatic melanoma.
Click on the PDF icon at the top of this introduction to read the full article.
The past year has been an exciting one for new oncology and hematology drug approvals and the continued evolution of our oncology delivery system toward high quality and value. In all, at press time in mid-November, the US Food and Drug Administration (FDA) had approved or granted expanded indications for 24 drugs, compared with 19 in the 2 preceding years. Of those 24 approvals, 7 were accelerated and 6 were expanded approvals, and 3 alone were for the immunotherapeutic drug, nivolumab – 2 for non-small-cell lung cancer (NSCLC) and 1 for metastatic melanoma.
Click on the PDF icon at the top of this introduction to read the full article.
Treatment delay linked with worse outcome for head and neck cancer
An analysis of more than 50,000 patients with head and neck squamous cell carcinoma (HNSCC) found that prolonged time to treatment initiation (TTI) was an independent predictor of worse mortality.
Median overall survival for TTI of 67 days or fewer was 71 months compared with 49 months for TTI less than 67 days (P less than .001). For TTI of 46 to 52 days, median overall survival (OS) was 72 months; for TTI of 53 to 67 days, 61 months, and for TTI of greater than 67 days, 47 months (P less than .001).
The results provide strong evidence that “TTI greater than 67 days is too long and should be considered unacceptable,” wrote Dr. Colin Murphy, a radiation oncologist at Fox Chase Cancer Center, Philadelphia, and his colleagues.
“The current analysis suggests that increasing TTI beyond the threshold established in this monograph alters HNSCC survival and represents a public health issue,” the researchers stated (J Clin Onc. 2015 Dec 2. doi: 10.1200/JCO.2015.5906).
Data from the National Cancer Data Base pertained to 51,655 patients with head and neck squamous cell carcinoma, including oral tongue, oropharynx, larynx, and hypopharynx, during 1998-2011; median follow-up time was 84 months.
Academic institutions had significantly higher median TTI (28 days), compared with community programs (22-23 days), probably because of patients transitioning care, which was an independently associated factor in higher TTI. Despite higher median TTI, academic institutions were associated with improved overall survival, compared with community hospitals, as were care transitions.
Despite rapid tumor proliferation in HNSCC that can result in stage progression, 9.6% of all patients in 2011 had TTI of greater than 67 days, and 25% (29% at academic institutions) had TTI of greater than 46 days, another benchmark level identified in the study.
Mortality risk, according to TTI, was greater for patients with stage I or II disease, compared with stage III or IV disease, a finding that may be because of lymph node involvement. Development of nodal disease at stage III is a significant risk factor for mortality.
The investigators note that health systems elsewhere, in Denmark for example, have addressed the problem of prolonged TTI. Such efforts require coordination among providers and mandate expedited appointments for a patients with a new cancer diagnosis.
“Recently piloted programs offering next-day appointments with cancer specialists address this reversible predictor of mortality and may partially alleviate increasing TTI. Without such reforms, it is conceivable that outcomes will continue to worsen because of prolonged TTI,” they wrote.
An analysis of more than 50,000 patients with head and neck squamous cell carcinoma (HNSCC) found that prolonged time to treatment initiation (TTI) was an independent predictor of worse mortality.
Median overall survival for TTI of 67 days or fewer was 71 months compared with 49 months for TTI less than 67 days (P less than .001). For TTI of 46 to 52 days, median overall survival (OS) was 72 months; for TTI of 53 to 67 days, 61 months, and for TTI of greater than 67 days, 47 months (P less than .001).
The results provide strong evidence that “TTI greater than 67 days is too long and should be considered unacceptable,” wrote Dr. Colin Murphy, a radiation oncologist at Fox Chase Cancer Center, Philadelphia, and his colleagues.
“The current analysis suggests that increasing TTI beyond the threshold established in this monograph alters HNSCC survival and represents a public health issue,” the researchers stated (J Clin Onc. 2015 Dec 2. doi: 10.1200/JCO.2015.5906).
Data from the National Cancer Data Base pertained to 51,655 patients with head and neck squamous cell carcinoma, including oral tongue, oropharynx, larynx, and hypopharynx, during 1998-2011; median follow-up time was 84 months.
Academic institutions had significantly higher median TTI (28 days), compared with community programs (22-23 days), probably because of patients transitioning care, which was an independently associated factor in higher TTI. Despite higher median TTI, academic institutions were associated with improved overall survival, compared with community hospitals, as were care transitions.
Despite rapid tumor proliferation in HNSCC that can result in stage progression, 9.6% of all patients in 2011 had TTI of greater than 67 days, and 25% (29% at academic institutions) had TTI of greater than 46 days, another benchmark level identified in the study.
Mortality risk, according to TTI, was greater for patients with stage I or II disease, compared with stage III or IV disease, a finding that may be because of lymph node involvement. Development of nodal disease at stage III is a significant risk factor for mortality.
The investigators note that health systems elsewhere, in Denmark for example, have addressed the problem of prolonged TTI. Such efforts require coordination among providers and mandate expedited appointments for a patients with a new cancer diagnosis.
“Recently piloted programs offering next-day appointments with cancer specialists address this reversible predictor of mortality and may partially alleviate increasing TTI. Without such reforms, it is conceivable that outcomes will continue to worsen because of prolonged TTI,” they wrote.
An analysis of more than 50,000 patients with head and neck squamous cell carcinoma (HNSCC) found that prolonged time to treatment initiation (TTI) was an independent predictor of worse mortality.
Median overall survival for TTI of 67 days or fewer was 71 months compared with 49 months for TTI less than 67 days (P less than .001). For TTI of 46 to 52 days, median overall survival (OS) was 72 months; for TTI of 53 to 67 days, 61 months, and for TTI of greater than 67 days, 47 months (P less than .001).
The results provide strong evidence that “TTI greater than 67 days is too long and should be considered unacceptable,” wrote Dr. Colin Murphy, a radiation oncologist at Fox Chase Cancer Center, Philadelphia, and his colleagues.
“The current analysis suggests that increasing TTI beyond the threshold established in this monograph alters HNSCC survival and represents a public health issue,” the researchers stated (J Clin Onc. 2015 Dec 2. doi: 10.1200/JCO.2015.5906).
Data from the National Cancer Data Base pertained to 51,655 patients with head and neck squamous cell carcinoma, including oral tongue, oropharynx, larynx, and hypopharynx, during 1998-2011; median follow-up time was 84 months.
Academic institutions had significantly higher median TTI (28 days), compared with community programs (22-23 days), probably because of patients transitioning care, which was an independently associated factor in higher TTI. Despite higher median TTI, academic institutions were associated with improved overall survival, compared with community hospitals, as were care transitions.
Despite rapid tumor proliferation in HNSCC that can result in stage progression, 9.6% of all patients in 2011 had TTI of greater than 67 days, and 25% (29% at academic institutions) had TTI of greater than 46 days, another benchmark level identified in the study.
Mortality risk, according to TTI, was greater for patients with stage I or II disease, compared with stage III or IV disease, a finding that may be because of lymph node involvement. Development of nodal disease at stage III is a significant risk factor for mortality.
The investigators note that health systems elsewhere, in Denmark for example, have addressed the problem of prolonged TTI. Such efforts require coordination among providers and mandate expedited appointments for a patients with a new cancer diagnosis.
“Recently piloted programs offering next-day appointments with cancer specialists address this reversible predictor of mortality and may partially alleviate increasing TTI. Without such reforms, it is conceivable that outcomes will continue to worsen because of prolonged TTI,” they wrote.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Patients with time to treatment initiation (TTI) greater than 46-52 days had increased risk of mortality, with greatest risk increases for early-stage disease.
Major finding: Median overall survival was 72 months for TTI of 46-52 days, 61 months for 53-67 days, and 47 months for greater than 67 days (P less than .001).
Data source: Data from the National Cancer Data Base pertained to 51,655 patients with had and neck squamous cell carcinoma, including oral tongue, oropharynx, larynx, and hypopharynx, from 1998 to 2011; median follow-up time was 84 months.
Disclosures: Dr. Murphy reported having no disclosures. Several of his coauthors reported ties to industry.
Balancing clinical and supportive care at every step of the disease continuum
It seems it was just yesterday that we did our first “mutation analysis” to help guide us in our treatment of patients with a drug that was more likely to work than not. Of course, I am referring to estrogen-receptor/progesterone receptor (ER/PR) blocking therapy, and “yesterday” actually goes all the way back to the 1970s! When tamoxifen was first given to unselected metastatic breast cancer patients, the response rate was low, but when the study population was “enriched” with breast cancer patients who were ER/ PR-positive, the response rates improved and the outcomes were more favorable. So began the era of tumor markers and enriching patient populations, and the process now referred to as mutation analysis, which is becoming more broadly applicable to other tumors as well.
Click on the PDF icon at the top of this introduction to read the full article.
It seems it was just yesterday that we did our first “mutation analysis” to help guide us in our treatment of patients with a drug that was more likely to work than not. Of course, I am referring to estrogen-receptor/progesterone receptor (ER/PR) blocking therapy, and “yesterday” actually goes all the way back to the 1970s! When tamoxifen was first given to unselected metastatic breast cancer patients, the response rate was low, but when the study population was “enriched” with breast cancer patients who were ER/ PR-positive, the response rates improved and the outcomes were more favorable. So began the era of tumor markers and enriching patient populations, and the process now referred to as mutation analysis, which is becoming more broadly applicable to other tumors as well.
Click on the PDF icon at the top of this introduction to read the full article.
It seems it was just yesterday that we did our first “mutation analysis” to help guide us in our treatment of patients with a drug that was more likely to work than not. Of course, I am referring to estrogen-receptor/progesterone receptor (ER/PR) blocking therapy, and “yesterday” actually goes all the way back to the 1970s! When tamoxifen was first given to unselected metastatic breast cancer patients, the response rate was low, but when the study population was “enriched” with breast cancer patients who were ER/ PR-positive, the response rates improved and the outcomes were more favorable. So began the era of tumor markers and enriching patient populations, and the process now referred to as mutation analysis, which is becoming more broadly applicable to other tumors as well.
Click on the PDF icon at the top of this introduction to read the full article.
Suicide rate high in patients with head and neck cancer
Patients with head and neck cancer had a threefold higher risk of suicide, compared with the general population, with higher rates among male patients and those with later-stage disease, according to an analysis of SEER data.
Patients with cancers of the hypopharynx had the highest rates (standardized mortality ratio, compared with the general population, 13.91), followed by cancer of the larynx (5.48) and cancer of the oral cavity and oropharynx (5.23).
“Routine screening for suicide risk may not be needed in every patient,” said David Kam, a medical student at Rutgers New Jersey Medical School, Newark, and his colleagues, “but we have identified a certain subset of patients often seen by otolaryngologists as being at increased risk (those who are older, male, with cancers of the hypopharynx, or with history of radiation therapy)” (JAMA Otolaryngol Head Neck Surg. 2015 Nov 12. doi: 10.1001/jamaoto.2015.2480).
Patients who underwent radiation therapy without surgery had about twice the suicide risk as those who underwent surgery alone (5.12 vs. 2.57). The researchers noted a potential selection bias among those treated with radiation alone, as patients with unresectable disease or significant comorbidities may undergo radiation instead of surgery.
Radiation therapy is integral to treating many head and neck cancers but is associated with a lower quality of life because of related morbidity. Despite improvements in quality of life measures associated with intensity-modulated radiation therapy (IMRT), the analysis showed no improvement in suicide rates after 2005, when IMRT was widely commercially available and a large fraction of patients would presumably have received the newer treatment.
An analysis of SEER (Surveillance, Epidemiology, and End Results) data from 1973 to 2011 showed 857 suicides among 350,413 individuals with head and neck cancer. Among all patients, the greatest increase in suicide rates occurred in the first 5 years after diagnosis.
Because of the significantly increased suicide risk among patients with head and neck cancers, research on survival outcomes should expand to include the psychological toll that the cancer, treatments, and resulting morbidity have on patients, the researchers said.
Patients with head and neck cancer had a threefold higher risk of suicide, compared with the general population, with higher rates among male patients and those with later-stage disease, according to an analysis of SEER data.
Patients with cancers of the hypopharynx had the highest rates (standardized mortality ratio, compared with the general population, 13.91), followed by cancer of the larynx (5.48) and cancer of the oral cavity and oropharynx (5.23).
“Routine screening for suicide risk may not be needed in every patient,” said David Kam, a medical student at Rutgers New Jersey Medical School, Newark, and his colleagues, “but we have identified a certain subset of patients often seen by otolaryngologists as being at increased risk (those who are older, male, with cancers of the hypopharynx, or with history of radiation therapy)” (JAMA Otolaryngol Head Neck Surg. 2015 Nov 12. doi: 10.1001/jamaoto.2015.2480).
Patients who underwent radiation therapy without surgery had about twice the suicide risk as those who underwent surgery alone (5.12 vs. 2.57). The researchers noted a potential selection bias among those treated with radiation alone, as patients with unresectable disease or significant comorbidities may undergo radiation instead of surgery.
Radiation therapy is integral to treating many head and neck cancers but is associated with a lower quality of life because of related morbidity. Despite improvements in quality of life measures associated with intensity-modulated radiation therapy (IMRT), the analysis showed no improvement in suicide rates after 2005, when IMRT was widely commercially available and a large fraction of patients would presumably have received the newer treatment.
An analysis of SEER (Surveillance, Epidemiology, and End Results) data from 1973 to 2011 showed 857 suicides among 350,413 individuals with head and neck cancer. Among all patients, the greatest increase in suicide rates occurred in the first 5 years after diagnosis.
Because of the significantly increased suicide risk among patients with head and neck cancers, research on survival outcomes should expand to include the psychological toll that the cancer, treatments, and resulting morbidity have on patients, the researchers said.
Patients with head and neck cancer had a threefold higher risk of suicide, compared with the general population, with higher rates among male patients and those with later-stage disease, according to an analysis of SEER data.
Patients with cancers of the hypopharynx had the highest rates (standardized mortality ratio, compared with the general population, 13.91), followed by cancer of the larynx (5.48) and cancer of the oral cavity and oropharynx (5.23).
“Routine screening for suicide risk may not be needed in every patient,” said David Kam, a medical student at Rutgers New Jersey Medical School, Newark, and his colleagues, “but we have identified a certain subset of patients often seen by otolaryngologists as being at increased risk (those who are older, male, with cancers of the hypopharynx, or with history of radiation therapy)” (JAMA Otolaryngol Head Neck Surg. 2015 Nov 12. doi: 10.1001/jamaoto.2015.2480).
Patients who underwent radiation therapy without surgery had about twice the suicide risk as those who underwent surgery alone (5.12 vs. 2.57). The researchers noted a potential selection bias among those treated with radiation alone, as patients with unresectable disease or significant comorbidities may undergo radiation instead of surgery.
Radiation therapy is integral to treating many head and neck cancers but is associated with a lower quality of life because of related morbidity. Despite improvements in quality of life measures associated with intensity-modulated radiation therapy (IMRT), the analysis showed no improvement in suicide rates after 2005, when IMRT was widely commercially available and a large fraction of patients would presumably have received the newer treatment.
An analysis of SEER (Surveillance, Epidemiology, and End Results) data from 1973 to 2011 showed 857 suicides among 350,413 individuals with head and neck cancer. Among all patients, the greatest increase in suicide rates occurred in the first 5 years after diagnosis.
Because of the significantly increased suicide risk among patients with head and neck cancers, research on survival outcomes should expand to include the psychological toll that the cancer, treatments, and resulting morbidity have on patients, the researchers said.
Key clinical point: Patients with head and neck cancer have a significantly higher suicide rate than the general population.
Major finding: The standardized suicide ratio for patients with head and neck cancer, compared with the general population, was 3.21.
Data source: An analysis of SEER data from 1973 to 2011 showed 857 suicides among 350,413 individuals with head and neck cancer.
Disclosures: David Kam and his coauthors reported having no relevant financial disclosures.
Experimental LOXO-101 induces regression in several hard-to-treat cancers
BOSTON – An experimental agent that targets the byproducts of gene fusions has shown surprising clinical activity against notoriously treatment-refractory cancers in early results from a phase I trial.
“I think that’s what remarkable about this is that we have all these different histologies and many different fusions, but all seem to be having some kind of [response] to this molecule,” said lead investigator Dr. David S. Hong, deputy chair and associate professor in the department of investigational cancer therapeutics at the University of Texas MD Anderson Cancer Center, Houston.
The molecule, LOXO-101 is a selective inhibitor of abnormal TRKA, TRKAB, and TRKC kinases that arise from gene fusions. TRK fusions have been implicated in tumor development in preclinical studies, he reported at the AACR–NCI–EORTC International Conference on Molecular Targets and Cancer Therapeutics.
One patient, a 41-year-old woman with undifferentiated soft tissue sarcoma of the groin that had metastasized to her lungs had rapid and “substantial” tumor and lung-nodule regression that radiographically fell just shy of a complete response, Dr. Hong said.
The drug also induced robust partial responses in a 55-year-old man with a gastrointestinal stromal tumor (GIST) for whom five prior targeted agents had failed, and a 33-year-old man with mammary analogue secretory carcinoma of the salivary gland (MASC) that had progressed on chemotherapy with docetaxel, carboplatin, and 5-fluorauracil.
A fourth patient, a man (age unspecified) who had papillary thyroid cancer with palpable lymphadenopathy, had no palpable nodes at 1-month follow-up, and appears to be responding to the drug, Dr. Hong added.
“This is really a dramatic example, I think, of a targeted therapy that is not histology specific, and this is another area in oncology where it’s new, and we’re just feeling our way,” commented Dr. Lee C. Helman, a sarcoma specialist at the National Cancer Institute in Bethesda, Md., who moderated the briefing where Dr. Hong presented the data.
Tumor promoters
The TRK family of proteins consists of separate tyrosine kinases that, when functioning normally, are important for the development of peripheral central nervous system processes such as pain and thermoregulation, Dr. Hong explained.
“The NTRK 1, 2, and 3 genes which encode for the TRK proteins are subject to these gene rearrangements and fusions, and the resulting TRK gene fusions may become erroneously expressed, and the kinase domain constitutively activated,” he said.
Gene fusions resulting in constitutively active TRKA, B, and C kinases can occur in the range of about 2%-25% in a wide variety of tumors, including adenocarcinomas of the lung, gliomas, thyroid tumors, head and neck cancers, sarcomas, and other types. These fusions have also been detected in more than 75% of MASC tumors, secretory breast carcinomas, and infantile (congenital) fibrosarcoma.
LOXO-101 is an orally delivered small molecule that was rationally designed to target the TRK fusions while largely leaving other kinases alone. In preclinical studies, the compound showed potent tumor growth inhibition and regression in mice bearing NTRK fusions.
The phase I trial is a dose-finding study including a total of 24 patients with various treatment-refractory advanced or metastatic solid tumors. Patients were enrolled regardless of fusional status.
Well tolerated
At the selected dose of 100 mg twice daily, patients appeared to tolerate the drug very well, with few off-target adverse events, Dr. Hong said. The most common side effect was mild dizziness. Grade 3 or 4 adverse events occurring at all doses levels include fatigue (one patient), anemia (two), abdominal pain (one), increased alkaline phosphatase (one), increased aspartate aminotransferase, delirium, and syncope (two each).
A total of six of the 24 patients had TRK fusions in their tumors, and three of these patients were available for assessment as of Oct. 20, 2015.
Dr. Hong and colleagues previously reportedon the case of the 41-year old woman with sarcoma. She was found to have a fusion of LMNA (a gene that encodes for nuclear membrane proteins) with NTRK1. She was started on the 100-mg b.i.d. dose of LOX-101 and had rapid resolution of dyspnea and hypoxemia. She had a confirmed partial response, and a CT scan showed that her multiple pulmonary nodules had regressed, with just a single, small disease site remaining at most recent follow-up. This patient continues on treatment and has been followed for more than 8 months.
The man with GIST had experienced disease progression while on therapy with imatinib, sunitinib, sorafenib, nilotinib, and regorafenib. He was treated in the 150-mg b.i.d. dose cohort, had a confirmed partial response, and remains on study after 4-plus months.
The third patient, the 33-year-old man with MASC, was assigned to the 100-mg b.i.d. dose. He too had a partial response, with radiologic evidence of substantial tumor shrinkage, and a persistent, tumor-related cough that disappeared after about 10 days of treatment, and remains on study after more than 3 months of follow-up.
The investigators have begun accruing patients for a phase II “basket” trial in adult patients with advanced or metastatic solid tumors displaying TRK fusions, including non–small cell lung cancers, thyroid tumors, sarcomas, colorectal cancer, salivary gland cancers, primary central nervous system cancers, and all other solid tumors.
Dr. Hong said that the results indicate the importance of tumor profiling for the majority of patients, especially those whose disease is refractory to standard therapies.
The study was funded by Loxo Oncology. Dr. Hong disclosed receiving travel expenses from the company. Three coauthors are employees and shareholders of the company.
BOSTON – An experimental agent that targets the byproducts of gene fusions has shown surprising clinical activity against notoriously treatment-refractory cancers in early results from a phase I trial.
“I think that’s what remarkable about this is that we have all these different histologies and many different fusions, but all seem to be having some kind of [response] to this molecule,” said lead investigator Dr. David S. Hong, deputy chair and associate professor in the department of investigational cancer therapeutics at the University of Texas MD Anderson Cancer Center, Houston.
The molecule, LOXO-101 is a selective inhibitor of abnormal TRKA, TRKAB, and TRKC kinases that arise from gene fusions. TRK fusions have been implicated in tumor development in preclinical studies, he reported at the AACR–NCI–EORTC International Conference on Molecular Targets and Cancer Therapeutics.
One patient, a 41-year-old woman with undifferentiated soft tissue sarcoma of the groin that had metastasized to her lungs had rapid and “substantial” tumor and lung-nodule regression that radiographically fell just shy of a complete response, Dr. Hong said.
The drug also induced robust partial responses in a 55-year-old man with a gastrointestinal stromal tumor (GIST) for whom five prior targeted agents had failed, and a 33-year-old man with mammary analogue secretory carcinoma of the salivary gland (MASC) that had progressed on chemotherapy with docetaxel, carboplatin, and 5-fluorauracil.
A fourth patient, a man (age unspecified) who had papillary thyroid cancer with palpable lymphadenopathy, had no palpable nodes at 1-month follow-up, and appears to be responding to the drug, Dr. Hong added.
“This is really a dramatic example, I think, of a targeted therapy that is not histology specific, and this is another area in oncology where it’s new, and we’re just feeling our way,” commented Dr. Lee C. Helman, a sarcoma specialist at the National Cancer Institute in Bethesda, Md., who moderated the briefing where Dr. Hong presented the data.
Tumor promoters
The TRK family of proteins consists of separate tyrosine kinases that, when functioning normally, are important for the development of peripheral central nervous system processes such as pain and thermoregulation, Dr. Hong explained.
“The NTRK 1, 2, and 3 genes which encode for the TRK proteins are subject to these gene rearrangements and fusions, and the resulting TRK gene fusions may become erroneously expressed, and the kinase domain constitutively activated,” he said.
Gene fusions resulting in constitutively active TRKA, B, and C kinases can occur in the range of about 2%-25% in a wide variety of tumors, including adenocarcinomas of the lung, gliomas, thyroid tumors, head and neck cancers, sarcomas, and other types. These fusions have also been detected in more than 75% of MASC tumors, secretory breast carcinomas, and infantile (congenital) fibrosarcoma.
LOXO-101 is an orally delivered small molecule that was rationally designed to target the TRK fusions while largely leaving other kinases alone. In preclinical studies, the compound showed potent tumor growth inhibition and regression in mice bearing NTRK fusions.
The phase I trial is a dose-finding study including a total of 24 patients with various treatment-refractory advanced or metastatic solid tumors. Patients were enrolled regardless of fusional status.
Well tolerated
At the selected dose of 100 mg twice daily, patients appeared to tolerate the drug very well, with few off-target adverse events, Dr. Hong said. The most common side effect was mild dizziness. Grade 3 or 4 adverse events occurring at all doses levels include fatigue (one patient), anemia (two), abdominal pain (one), increased alkaline phosphatase (one), increased aspartate aminotransferase, delirium, and syncope (two each).
A total of six of the 24 patients had TRK fusions in their tumors, and three of these patients were available for assessment as of Oct. 20, 2015.
Dr. Hong and colleagues previously reportedon the case of the 41-year old woman with sarcoma. She was found to have a fusion of LMNA (a gene that encodes for nuclear membrane proteins) with NTRK1. She was started on the 100-mg b.i.d. dose of LOX-101 and had rapid resolution of dyspnea and hypoxemia. She had a confirmed partial response, and a CT scan showed that her multiple pulmonary nodules had regressed, with just a single, small disease site remaining at most recent follow-up. This patient continues on treatment and has been followed for more than 8 months.
The man with GIST had experienced disease progression while on therapy with imatinib, sunitinib, sorafenib, nilotinib, and regorafenib. He was treated in the 150-mg b.i.d. dose cohort, had a confirmed partial response, and remains on study after 4-plus months.
The third patient, the 33-year-old man with MASC, was assigned to the 100-mg b.i.d. dose. He too had a partial response, with radiologic evidence of substantial tumor shrinkage, and a persistent, tumor-related cough that disappeared after about 10 days of treatment, and remains on study after more than 3 months of follow-up.
The investigators have begun accruing patients for a phase II “basket” trial in adult patients with advanced or metastatic solid tumors displaying TRK fusions, including non–small cell lung cancers, thyroid tumors, sarcomas, colorectal cancer, salivary gland cancers, primary central nervous system cancers, and all other solid tumors.
Dr. Hong said that the results indicate the importance of tumor profiling for the majority of patients, especially those whose disease is refractory to standard therapies.
The study was funded by Loxo Oncology. Dr. Hong disclosed receiving travel expenses from the company. Three coauthors are employees and shareholders of the company.
BOSTON – An experimental agent that targets the byproducts of gene fusions has shown surprising clinical activity against notoriously treatment-refractory cancers in early results from a phase I trial.
“I think that’s what remarkable about this is that we have all these different histologies and many different fusions, but all seem to be having some kind of [response] to this molecule,” said lead investigator Dr. David S. Hong, deputy chair and associate professor in the department of investigational cancer therapeutics at the University of Texas MD Anderson Cancer Center, Houston.
The molecule, LOXO-101 is a selective inhibitor of abnormal TRKA, TRKAB, and TRKC kinases that arise from gene fusions. TRK fusions have been implicated in tumor development in preclinical studies, he reported at the AACR–NCI–EORTC International Conference on Molecular Targets and Cancer Therapeutics.
One patient, a 41-year-old woman with undifferentiated soft tissue sarcoma of the groin that had metastasized to her lungs had rapid and “substantial” tumor and lung-nodule regression that radiographically fell just shy of a complete response, Dr. Hong said.
The drug also induced robust partial responses in a 55-year-old man with a gastrointestinal stromal tumor (GIST) for whom five prior targeted agents had failed, and a 33-year-old man with mammary analogue secretory carcinoma of the salivary gland (MASC) that had progressed on chemotherapy with docetaxel, carboplatin, and 5-fluorauracil.
A fourth patient, a man (age unspecified) who had papillary thyroid cancer with palpable lymphadenopathy, had no palpable nodes at 1-month follow-up, and appears to be responding to the drug, Dr. Hong added.
“This is really a dramatic example, I think, of a targeted therapy that is not histology specific, and this is another area in oncology where it’s new, and we’re just feeling our way,” commented Dr. Lee C. Helman, a sarcoma specialist at the National Cancer Institute in Bethesda, Md., who moderated the briefing where Dr. Hong presented the data.
Tumor promoters
The TRK family of proteins consists of separate tyrosine kinases that, when functioning normally, are important for the development of peripheral central nervous system processes such as pain and thermoregulation, Dr. Hong explained.
“The NTRK 1, 2, and 3 genes which encode for the TRK proteins are subject to these gene rearrangements and fusions, and the resulting TRK gene fusions may become erroneously expressed, and the kinase domain constitutively activated,” he said.
Gene fusions resulting in constitutively active TRKA, B, and C kinases can occur in the range of about 2%-25% in a wide variety of tumors, including adenocarcinomas of the lung, gliomas, thyroid tumors, head and neck cancers, sarcomas, and other types. These fusions have also been detected in more than 75% of MASC tumors, secretory breast carcinomas, and infantile (congenital) fibrosarcoma.
LOXO-101 is an orally delivered small molecule that was rationally designed to target the TRK fusions while largely leaving other kinases alone. In preclinical studies, the compound showed potent tumor growth inhibition and regression in mice bearing NTRK fusions.
The phase I trial is a dose-finding study including a total of 24 patients with various treatment-refractory advanced or metastatic solid tumors. Patients were enrolled regardless of fusional status.
Well tolerated
At the selected dose of 100 mg twice daily, patients appeared to tolerate the drug very well, with few off-target adverse events, Dr. Hong said. The most common side effect was mild dizziness. Grade 3 or 4 adverse events occurring at all doses levels include fatigue (one patient), anemia (two), abdominal pain (one), increased alkaline phosphatase (one), increased aspartate aminotransferase, delirium, and syncope (two each).
A total of six of the 24 patients had TRK fusions in their tumors, and three of these patients were available for assessment as of Oct. 20, 2015.
Dr. Hong and colleagues previously reportedon the case of the 41-year old woman with sarcoma. She was found to have a fusion of LMNA (a gene that encodes for nuclear membrane proteins) with NTRK1. She was started on the 100-mg b.i.d. dose of LOX-101 and had rapid resolution of dyspnea and hypoxemia. She had a confirmed partial response, and a CT scan showed that her multiple pulmonary nodules had regressed, with just a single, small disease site remaining at most recent follow-up. This patient continues on treatment and has been followed for more than 8 months.
The man with GIST had experienced disease progression while on therapy with imatinib, sunitinib, sorafenib, nilotinib, and regorafenib. He was treated in the 150-mg b.i.d. dose cohort, had a confirmed partial response, and remains on study after 4-plus months.
The third patient, the 33-year-old man with MASC, was assigned to the 100-mg b.i.d. dose. He too had a partial response, with radiologic evidence of substantial tumor shrinkage, and a persistent, tumor-related cough that disappeared after about 10 days of treatment, and remains on study after more than 3 months of follow-up.
The investigators have begun accruing patients for a phase II “basket” trial in adult patients with advanced or metastatic solid tumors displaying TRK fusions, including non–small cell lung cancers, thyroid tumors, sarcomas, colorectal cancer, salivary gland cancers, primary central nervous system cancers, and all other solid tumors.
Dr. Hong said that the results indicate the importance of tumor profiling for the majority of patients, especially those whose disease is refractory to standard therapies.
The study was funded by Loxo Oncology. Dr. Hong disclosed receiving travel expenses from the company. Three coauthors are employees and shareholders of the company.
Key clinical point:Inhibition of TRK gene fusion products is a novel strategy for treating cancer.
Major finding: Three of three patients evaluable for response had near-complete responses.
Data source: Phase I dose-finding study in 24 patients, with and without TRK gene fusions.
Disclosures: The study was funded by Loxo Oncology. Dr. Hong disclosed receiving travel expenses from the company. Three coauthors are employees and shareholders of the company.
Adjuvant lapatinib added no benefit against head and neck squamous cell carcinoma
Lapatinib in combination with platinum-based chemoradiotherapy and as long-term maintenance therapy showed no benefit in patients with surgically treated high-risk squamous cell carcinoma of the head and neck (SCCHN).
No difference was observed between treatment arms in the primary endpoint of disease-free survival (DFS) or the secondary endpoints of investigator-assessed DFS and overall survival.
“Certainly, these findings should serve as a note of caution on the risks of initiating large phase III studies (of this and other drugs) with insufficient evidence of single-agent activity,” wrote Dr. Kevin Harrington of the division of radiotherapy and imaging at the Institute of Cancer Research and Royal Marsden Hospital, London, and his colleagues (Journ Clin Onc. 2015 Nov. 2. doi: 10.1200/JCO.2015.61.4370).
The placebo-controlled phase III trial from 84 sites in 21 countries randomized 688 patients with resected SCCHN to receive placebo or lapatinib. The study was halted early because of the apparent plateauing of DFS events at the median follow-up time of 35.3 months, the investigators reported.
DFS events (disease recurrence or death) occurred in 32% of patients who received placebo versus 35% of patients who received lapatinib (HR, 1.10; 95% CI, 0.85-1.43; P = .45). No significant differences in DFS were observed between treatment arms by human papillomavirus or EGFR status.
Lapatinib is a small-molecule inhibitor of epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 2 (HER2) and was postulated to be active in squamous cell carcinoma of the head and neck tumors because many overexpress EGFR. Lapatinib has shown efficacy in HER2-positive metastatic breast cancer, but not in other EGFR-driven cancers.
Compliance was high in both placebo and lapatinib arms, with 83% and 76%, respectively, achieving greater than 80% compliance. Adverse events of grade 3 or higher were observed in 67% of the placebo arm and 75% of the lapatinib arm. The most common grade 3 or 4 adverse events were lymphopenia and mucosal inflammation.
Lapatinib in combination with platinum-based chemoradiotherapy and as long-term maintenance therapy showed no benefit in patients with surgically treated high-risk squamous cell carcinoma of the head and neck (SCCHN).
No difference was observed between treatment arms in the primary endpoint of disease-free survival (DFS) or the secondary endpoints of investigator-assessed DFS and overall survival.
“Certainly, these findings should serve as a note of caution on the risks of initiating large phase III studies (of this and other drugs) with insufficient evidence of single-agent activity,” wrote Dr. Kevin Harrington of the division of radiotherapy and imaging at the Institute of Cancer Research and Royal Marsden Hospital, London, and his colleagues (Journ Clin Onc. 2015 Nov. 2. doi: 10.1200/JCO.2015.61.4370).
The placebo-controlled phase III trial from 84 sites in 21 countries randomized 688 patients with resected SCCHN to receive placebo or lapatinib. The study was halted early because of the apparent plateauing of DFS events at the median follow-up time of 35.3 months, the investigators reported.
DFS events (disease recurrence or death) occurred in 32% of patients who received placebo versus 35% of patients who received lapatinib (HR, 1.10; 95% CI, 0.85-1.43; P = .45). No significant differences in DFS were observed between treatment arms by human papillomavirus or EGFR status.
Lapatinib is a small-molecule inhibitor of epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 2 (HER2) and was postulated to be active in squamous cell carcinoma of the head and neck tumors because many overexpress EGFR. Lapatinib has shown efficacy in HER2-positive metastatic breast cancer, but not in other EGFR-driven cancers.
Compliance was high in both placebo and lapatinib arms, with 83% and 76%, respectively, achieving greater than 80% compliance. Adverse events of grade 3 or higher were observed in 67% of the placebo arm and 75% of the lapatinib arm. The most common grade 3 or 4 adverse events were lymphopenia and mucosal inflammation.
Lapatinib in combination with platinum-based chemoradiotherapy and as long-term maintenance therapy showed no benefit in patients with surgically treated high-risk squamous cell carcinoma of the head and neck (SCCHN).
No difference was observed between treatment arms in the primary endpoint of disease-free survival (DFS) or the secondary endpoints of investigator-assessed DFS and overall survival.
“Certainly, these findings should serve as a note of caution on the risks of initiating large phase III studies (of this and other drugs) with insufficient evidence of single-agent activity,” wrote Dr. Kevin Harrington of the division of radiotherapy and imaging at the Institute of Cancer Research and Royal Marsden Hospital, London, and his colleagues (Journ Clin Onc. 2015 Nov. 2. doi: 10.1200/JCO.2015.61.4370).
The placebo-controlled phase III trial from 84 sites in 21 countries randomized 688 patients with resected SCCHN to receive placebo or lapatinib. The study was halted early because of the apparent plateauing of DFS events at the median follow-up time of 35.3 months, the investigators reported.
DFS events (disease recurrence or death) occurred in 32% of patients who received placebo versus 35% of patients who received lapatinib (HR, 1.10; 95% CI, 0.85-1.43; P = .45). No significant differences in DFS were observed between treatment arms by human papillomavirus or EGFR status.
Lapatinib is a small-molecule inhibitor of epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 2 (HER2) and was postulated to be active in squamous cell carcinoma of the head and neck tumors because many overexpress EGFR. Lapatinib has shown efficacy in HER2-positive metastatic breast cancer, but not in other EGFR-driven cancers.
Compliance was high in both placebo and lapatinib arms, with 83% and 76%, respectively, achieving greater than 80% compliance. Adverse events of grade 3 or higher were observed in 67% of the placebo arm and 75% of the lapatinib arm. The most common grade 3 or 4 adverse events were lymphopenia and mucosal inflammation.
Key clinical point: After surgery for high-risk squamous cell carcinoma of the head and neck, the addition of lapatinib to chemoradiotherapy and as long-term maintenance therapy offered no benefit.
Major finding: Disease-free survival events occurred in 32% of patients who received placebo versus 35% of patients who received lapatinib (HR, 1.10; 95% CI, 0.85-1.43; P = .45).
Data source: A phase III trial from 84 sites in 21 countries randomizing 688 patients with resected SCCHN to receive placebo or lapatinib.
Disclosures: Dr. Harrington reported financial ties to Merck Sharp & Dohme, Oncos Therapeutics, Cellgene, Viralytics, Lytix, Oncolytics Biotech, Genelux, and AstraZeneca. Several of his coauthors reported ties to industry sources.
ITC: Study provides first evidence of paclitaxel benefit for anaplastic thyroid cancer
LAKE BUENA VISTA, FLA. – Weekly infusions of paclitaxel delayed progression in some patients with the very aggressive anaplastic thyroid cancer, a small prospective study determined.
The drug was most effective as adjuvant therapy for patients who had already undergone chemotherapy plus resection of the primary tumor, Dr. Naoyoshi Onoda reported in a poster session at the International Thyroid Conference. They survived for a median of 1 year (112-788 days) – an impressive feat considering that most patients with anaplastic thyroid cancer die within 6 months of diagnosis.
This finding suggests a place for paclitaxel as a standardized therapy for such patients, said Dr. Onoda of Osaka (Japan) City University. “We have objective data supporting standardized chemotherapy for the first time in the world.”
Anaplastic thyroid cancer is a very rare – but very aggressive – disease; there is no standardized treatment option. Dr. Onoda and his colleagues conducted a national prospective open-label study of weekly paclitaxel infusions in 56 patients with the malignancy.
The cohort was a median of 71 years old. All had stage IV disease: 10 were grade A, 18 grade B, 24 grade C, and four grade X. They received 80 mg/m2 infusions once a week. The median number of cycles was 2, although it ranged from 0-23 cycles.
Almost everyone (98%) experienced adverse events; the most common was anemia (77%). About a quarter (28%) experienced adverse events of at least grade 3, but there were no serious events and no deaths related to the study drug.
The objective response rate and the clinical benefit rate were 23% and 79%. The agent was not curative; at the last follow-up, no patient had achieved a complete response, and 43 of 56 in the study had died of their disease. “Overall, the median time to progression was only 47 days, and median overall survival just 227 days. That is so very short. But it’s a little bit longer than we had been seeing rates from reported cases,” he said at the meeting held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society.
The study was sponsored by the Prospective Clinical Study Committee of the Anaplastic Thyroid Carcinoma Research Consortium of Japan (ATCCJ). Dr. Onoda had no financial disclosures.
LAKE BUENA VISTA, FLA. – Weekly infusions of paclitaxel delayed progression in some patients with the very aggressive anaplastic thyroid cancer, a small prospective study determined.
The drug was most effective as adjuvant therapy for patients who had already undergone chemotherapy plus resection of the primary tumor, Dr. Naoyoshi Onoda reported in a poster session at the International Thyroid Conference. They survived for a median of 1 year (112-788 days) – an impressive feat considering that most patients with anaplastic thyroid cancer die within 6 months of diagnosis.
This finding suggests a place for paclitaxel as a standardized therapy for such patients, said Dr. Onoda of Osaka (Japan) City University. “We have objective data supporting standardized chemotherapy for the first time in the world.”
Anaplastic thyroid cancer is a very rare – but very aggressive – disease; there is no standardized treatment option. Dr. Onoda and his colleagues conducted a national prospective open-label study of weekly paclitaxel infusions in 56 patients with the malignancy.
The cohort was a median of 71 years old. All had stage IV disease: 10 were grade A, 18 grade B, 24 grade C, and four grade X. They received 80 mg/m2 infusions once a week. The median number of cycles was 2, although it ranged from 0-23 cycles.
Almost everyone (98%) experienced adverse events; the most common was anemia (77%). About a quarter (28%) experienced adverse events of at least grade 3, but there were no serious events and no deaths related to the study drug.
The objective response rate and the clinical benefit rate were 23% and 79%. The agent was not curative; at the last follow-up, no patient had achieved a complete response, and 43 of 56 in the study had died of their disease. “Overall, the median time to progression was only 47 days, and median overall survival just 227 days. That is so very short. But it’s a little bit longer than we had been seeing rates from reported cases,” he said at the meeting held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society.
The study was sponsored by the Prospective Clinical Study Committee of the Anaplastic Thyroid Carcinoma Research Consortium of Japan (ATCCJ). Dr. Onoda had no financial disclosures.
LAKE BUENA VISTA, FLA. – Weekly infusions of paclitaxel delayed progression in some patients with the very aggressive anaplastic thyroid cancer, a small prospective study determined.
The drug was most effective as adjuvant therapy for patients who had already undergone chemotherapy plus resection of the primary tumor, Dr. Naoyoshi Onoda reported in a poster session at the International Thyroid Conference. They survived for a median of 1 year (112-788 days) – an impressive feat considering that most patients with anaplastic thyroid cancer die within 6 months of diagnosis.
This finding suggests a place for paclitaxel as a standardized therapy for such patients, said Dr. Onoda of Osaka (Japan) City University. “We have objective data supporting standardized chemotherapy for the first time in the world.”
Anaplastic thyroid cancer is a very rare – but very aggressive – disease; there is no standardized treatment option. Dr. Onoda and his colleagues conducted a national prospective open-label study of weekly paclitaxel infusions in 56 patients with the malignancy.
The cohort was a median of 71 years old. All had stage IV disease: 10 were grade A, 18 grade B, 24 grade C, and four grade X. They received 80 mg/m2 infusions once a week. The median number of cycles was 2, although it ranged from 0-23 cycles.
Almost everyone (98%) experienced adverse events; the most common was anemia (77%). About a quarter (28%) experienced adverse events of at least grade 3, but there were no serious events and no deaths related to the study drug.
The objective response rate and the clinical benefit rate were 23% and 79%. The agent was not curative; at the last follow-up, no patient had achieved a complete response, and 43 of 56 in the study had died of their disease. “Overall, the median time to progression was only 47 days, and median overall survival just 227 days. That is so very short. But it’s a little bit longer than we had been seeing rates from reported cases,” he said at the meeting held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society.
The study was sponsored by the Prospective Clinical Study Committee of the Anaplastic Thyroid Carcinoma Research Consortium of Japan (ATCCJ). Dr. Onoda had no financial disclosures.
AT ITC 2015
Key clinical point: Paclitaxel may have use as a standardized treatment in anaplastic thyroid cancer.
Major finding: Weekly paclitaxel extended survival to a median of 1 year as adjuvant therapy for patients with resected anaplastic thyroid cancer.
Data source: The prospective open-label study involved 56 patients.
Disclosures: The study was sponsored by the Prospective Clinical Study Committee of the Anaplastic Thyroid Carcinoma Research Consortium of Japan (ATCCJ). Dr. Onoda had no financial disclosures.
ASTRO: Less intense chemoradiation may be possible for HPV-related oropharyngeal cancers
SAN ANTONIO – Patients who have low-risk, human papillomavirus (HPV)–associated oropharyngeal cancers may be effectively and safely treated with a reduced intensity chemoradiotherapy regimen, according to research presented at the annual meeting of the American Society for Radiation Oncology.
In the prospective, multi-institutional, phase II trial, complete pathologic responses (pCR) were seen in 86% of the 43 patients treated. The six cases that did not show a pCR were limited to microscopic areas of residual cancer.
The study provides strong preliminary evidence that reduced intensity chemoradiotherapy may be as effective as standard-dose chemoradiotherapy, Dr. Bhishamjit Chera of the University of North Carolina at Chapel Hill said at a press briefing. While it is too early to use outside of a clinical trial at present, he said, there is the potential for less intensive treatment to be given, and it could be the standard practice in years to come.
“At most institutions, the standard treatment for oropharynx cancer is definite chemoradiation,” Dr. Chera explained. This typically involves delivery of 70 Gy of radiation, given in 2-Gy fractions over 7 weeks for a total 35 days of treatment, and administration of three doses of cisplatin 100 mg/m2 concurrently.
“This treatment provides the best chances of sparing the tonsil, the throat, and the tongue, basically preserving organ function,” he added. Surgery is not usually performed unless there are signs on imaging that the cancer has not completely resolved 12 weeks after treatment.
“We know that many patients are cured with oropharyngeal carcinoma, but many patients live with significant long-term side effects such as dry mouth and difficulty swallowing,” Dr. Chera observed. Thus the aim of the present trial was to see if reducing the intensity of the chemoradiotherapy might avoid some of the side effects seen.
The deintensified regimen used in the study consisted of a 10-Gy reduction in the total dose of radiation delivered to 60 Gy, which was given in 2-Gy fractions once daily over a period of 6 weeks. The dose of cisplatin also was reduced by approximately 40% to 30 mg/m2 given in 6 weekly doses
Patients were eligible for inclusion if they had stage 0-3 squamous cell carcinoma of the oropharynx, with limited nodal (N0-N2c) and no metastatic involvement. Patients had to have a minimal smoking history and be HPV or p16 positive.
After the chemoradiation, all patients underwent a planned biopsy and limited neck dissection to remove any lymph nodes that had cancer in them prior to treatment. Thus the primary endpoint was the pCR rather a radiographically measured tumor response rate, Dr. Chera observed.
Considering just the primary site of the cancer (the base of tongue and tonsil), there were 41 patients who could be evaluated and all but one of these (98%) achieved a pCR. Looking at patients with neck involvement at baseline (n = 39), 84% achieved a pCR.
“All of these 43 patients are alive with no evidence of cancer recurrence with a follow-up of 21 months,” Dr. Chera reported.
Throughout the study, patient-reported outcomes were captured using the National Cancer Institute’s Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) and the European Organization for Cancer Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ).
While patients did report an increase in adverse effects 6-8 weeks after the chemoradiation, the intensity of these side effects decreased to baseline levels over time. Not surprisingly, the two most common symptoms were dry mouth and problems with swallowing, with 75% and 55% of patients, respectively, experiencing severe or very severe xerostomia and dysphagia. Other grade 3-4 adverse events included mucositis in 45%, pain in 48%, nausea in 52%, and vomiting in 34%.
Results of the quality of life analyses show that patients’ quality of life is returning to baseline levels after 1 year.
“There is a lot of interest in reducing the intensity of treatment in these patients,” Dr. Chera said, adding that patients are likely being overtreated at present. In the current clinical environment, patients are very savvy, he said, so performing a large randomized phase III trial may not be possible if patients learn that deintensified therapy may be an option. So the results of larger and longer phase II trials may be the best evidence that will be obtained.
The NRG Oncology group is going to evaluate the same deintensified chemotherapy regimen in another, larger phase II trial, the NRG-HN002 trial, Dr. Chera said. This trial aims to accrue almost 300 patients and will compare the University of North Carolina regimen versus radiation alone, which will be given at a total dose of 50 Gy 5 days a week for 5 weeks.
And, as for his group’s further research plans, Dr. Chera noted in an interview that further follow-up would be required to determine if the regimen used was efficacious and safe. A second phase II trial with the same deintensified regimen in which surgery or biopsies were not mandated is about to close soon, and there is a third study that will look at using genetic information to determine if it is safe to deintensifty patients’ treatment.
Dr. Chera had no conflicts of interest to disclose.
SAN ANTONIO – Patients who have low-risk, human papillomavirus (HPV)–associated oropharyngeal cancers may be effectively and safely treated with a reduced intensity chemoradiotherapy regimen, according to research presented at the annual meeting of the American Society for Radiation Oncology.
In the prospective, multi-institutional, phase II trial, complete pathologic responses (pCR) were seen in 86% of the 43 patients treated. The six cases that did not show a pCR were limited to microscopic areas of residual cancer.
The study provides strong preliminary evidence that reduced intensity chemoradiotherapy may be as effective as standard-dose chemoradiotherapy, Dr. Bhishamjit Chera of the University of North Carolina at Chapel Hill said at a press briefing. While it is too early to use outside of a clinical trial at present, he said, there is the potential for less intensive treatment to be given, and it could be the standard practice in years to come.
“At most institutions, the standard treatment for oropharynx cancer is definite chemoradiation,” Dr. Chera explained. This typically involves delivery of 70 Gy of radiation, given in 2-Gy fractions over 7 weeks for a total 35 days of treatment, and administration of three doses of cisplatin 100 mg/m2 concurrently.
“This treatment provides the best chances of sparing the tonsil, the throat, and the tongue, basically preserving organ function,” he added. Surgery is not usually performed unless there are signs on imaging that the cancer has not completely resolved 12 weeks after treatment.
“We know that many patients are cured with oropharyngeal carcinoma, but many patients live with significant long-term side effects such as dry mouth and difficulty swallowing,” Dr. Chera observed. Thus the aim of the present trial was to see if reducing the intensity of the chemoradiotherapy might avoid some of the side effects seen.
The deintensified regimen used in the study consisted of a 10-Gy reduction in the total dose of radiation delivered to 60 Gy, which was given in 2-Gy fractions once daily over a period of 6 weeks. The dose of cisplatin also was reduced by approximately 40% to 30 mg/m2 given in 6 weekly doses
Patients were eligible for inclusion if they had stage 0-3 squamous cell carcinoma of the oropharynx, with limited nodal (N0-N2c) and no metastatic involvement. Patients had to have a minimal smoking history and be HPV or p16 positive.
After the chemoradiation, all patients underwent a planned biopsy and limited neck dissection to remove any lymph nodes that had cancer in them prior to treatment. Thus the primary endpoint was the pCR rather a radiographically measured tumor response rate, Dr. Chera observed.
Considering just the primary site of the cancer (the base of tongue and tonsil), there were 41 patients who could be evaluated and all but one of these (98%) achieved a pCR. Looking at patients with neck involvement at baseline (n = 39), 84% achieved a pCR.
“All of these 43 patients are alive with no evidence of cancer recurrence with a follow-up of 21 months,” Dr. Chera reported.
Throughout the study, patient-reported outcomes were captured using the National Cancer Institute’s Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) and the European Organization for Cancer Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ).
While patients did report an increase in adverse effects 6-8 weeks after the chemoradiation, the intensity of these side effects decreased to baseline levels over time. Not surprisingly, the two most common symptoms were dry mouth and problems with swallowing, with 75% and 55% of patients, respectively, experiencing severe or very severe xerostomia and dysphagia. Other grade 3-4 adverse events included mucositis in 45%, pain in 48%, nausea in 52%, and vomiting in 34%.
Results of the quality of life analyses show that patients’ quality of life is returning to baseline levels after 1 year.
“There is a lot of interest in reducing the intensity of treatment in these patients,” Dr. Chera said, adding that patients are likely being overtreated at present. In the current clinical environment, patients are very savvy, he said, so performing a large randomized phase III trial may not be possible if patients learn that deintensified therapy may be an option. So the results of larger and longer phase II trials may be the best evidence that will be obtained.
The NRG Oncology group is going to evaluate the same deintensified chemotherapy regimen in another, larger phase II trial, the NRG-HN002 trial, Dr. Chera said. This trial aims to accrue almost 300 patients and will compare the University of North Carolina regimen versus radiation alone, which will be given at a total dose of 50 Gy 5 days a week for 5 weeks.
And, as for his group’s further research plans, Dr. Chera noted in an interview that further follow-up would be required to determine if the regimen used was efficacious and safe. A second phase II trial with the same deintensified regimen in which surgery or biopsies were not mandated is about to close soon, and there is a third study that will look at using genetic information to determine if it is safe to deintensifty patients’ treatment.
Dr. Chera had no conflicts of interest to disclose.
SAN ANTONIO – Patients who have low-risk, human papillomavirus (HPV)–associated oropharyngeal cancers may be effectively and safely treated with a reduced intensity chemoradiotherapy regimen, according to research presented at the annual meeting of the American Society for Radiation Oncology.
In the prospective, multi-institutional, phase II trial, complete pathologic responses (pCR) were seen in 86% of the 43 patients treated. The six cases that did not show a pCR were limited to microscopic areas of residual cancer.
The study provides strong preliminary evidence that reduced intensity chemoradiotherapy may be as effective as standard-dose chemoradiotherapy, Dr. Bhishamjit Chera of the University of North Carolina at Chapel Hill said at a press briefing. While it is too early to use outside of a clinical trial at present, he said, there is the potential for less intensive treatment to be given, and it could be the standard practice in years to come.
“At most institutions, the standard treatment for oropharynx cancer is definite chemoradiation,” Dr. Chera explained. This typically involves delivery of 70 Gy of radiation, given in 2-Gy fractions over 7 weeks for a total 35 days of treatment, and administration of three doses of cisplatin 100 mg/m2 concurrently.
“This treatment provides the best chances of sparing the tonsil, the throat, and the tongue, basically preserving organ function,” he added. Surgery is not usually performed unless there are signs on imaging that the cancer has not completely resolved 12 weeks after treatment.
“We know that many patients are cured with oropharyngeal carcinoma, but many patients live with significant long-term side effects such as dry mouth and difficulty swallowing,” Dr. Chera observed. Thus the aim of the present trial was to see if reducing the intensity of the chemoradiotherapy might avoid some of the side effects seen.
The deintensified regimen used in the study consisted of a 10-Gy reduction in the total dose of radiation delivered to 60 Gy, which was given in 2-Gy fractions once daily over a period of 6 weeks. The dose of cisplatin also was reduced by approximately 40% to 30 mg/m2 given in 6 weekly doses
Patients were eligible for inclusion if they had stage 0-3 squamous cell carcinoma of the oropharynx, with limited nodal (N0-N2c) and no metastatic involvement. Patients had to have a minimal smoking history and be HPV or p16 positive.
After the chemoradiation, all patients underwent a planned biopsy and limited neck dissection to remove any lymph nodes that had cancer in them prior to treatment. Thus the primary endpoint was the pCR rather a radiographically measured tumor response rate, Dr. Chera observed.
Considering just the primary site of the cancer (the base of tongue and tonsil), there were 41 patients who could be evaluated and all but one of these (98%) achieved a pCR. Looking at patients with neck involvement at baseline (n = 39), 84% achieved a pCR.
“All of these 43 patients are alive with no evidence of cancer recurrence with a follow-up of 21 months,” Dr. Chera reported.
Throughout the study, patient-reported outcomes were captured using the National Cancer Institute’s Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) and the European Organization for Cancer Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ).
While patients did report an increase in adverse effects 6-8 weeks after the chemoradiation, the intensity of these side effects decreased to baseline levels over time. Not surprisingly, the two most common symptoms were dry mouth and problems with swallowing, with 75% and 55% of patients, respectively, experiencing severe or very severe xerostomia and dysphagia. Other grade 3-4 adverse events included mucositis in 45%, pain in 48%, nausea in 52%, and vomiting in 34%.
Results of the quality of life analyses show that patients’ quality of life is returning to baseline levels after 1 year.
“There is a lot of interest in reducing the intensity of treatment in these patients,” Dr. Chera said, adding that patients are likely being overtreated at present. In the current clinical environment, patients are very savvy, he said, so performing a large randomized phase III trial may not be possible if patients learn that deintensified therapy may be an option. So the results of larger and longer phase II trials may be the best evidence that will be obtained.
The NRG Oncology group is going to evaluate the same deintensified chemotherapy regimen in another, larger phase II trial, the NRG-HN002 trial, Dr. Chera said. This trial aims to accrue almost 300 patients and will compare the University of North Carolina regimen versus radiation alone, which will be given at a total dose of 50 Gy 5 days a week for 5 weeks.
And, as for his group’s further research plans, Dr. Chera noted in an interview that further follow-up would be required to determine if the regimen used was efficacious and safe. A second phase II trial with the same deintensified regimen in which surgery or biopsies were not mandated is about to close soon, and there is a third study that will look at using genetic information to determine if it is safe to deintensifty patients’ treatment.
Dr. Chera had no conflicts of interest to disclose.
AT THE ASTRO ANNUAL MEETING
Key clinical point: Preliminary evidence shows a reduced dose radiation and chemotherapy regimen was effective and may have lower toxicity than standard regimens.
Major finding: A complete pathologic response was seen in 37/43 (86%) of patients.
Data source: Prospective, multicenter, phase II study of 43 patients with favorable risk (T0-3, N0-N2c, M0) HPV-associated oropharyngeal squamous cell carcinoma.
Disclosures: Dr. Chera had no conflicts of interest to disclose.
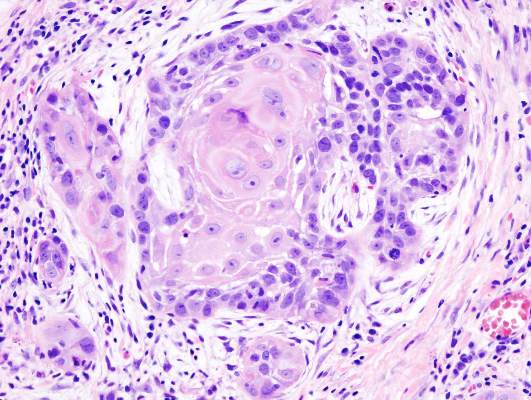
Increased surveillance may explain post-Fukushima pediatric thyroid cancers
LAKE BUENA VISTA, FLA. – More cases of thyroid cancer are being seen in Japanese youth after the Fukushima Daiichi nuclear power plant accident, but the increased incidence may be an artifact of heightened surveillance.
“The thyroid cancers appear to have already occurred prior to radiation exposure,” said Dr. Shinichi Suzuki of the department of thyroid and endocrinology at Fukushima (Japan) Medical University. Radiation-induced thyroid cancers take about 5 years to become detectable, so physicians should just now be seeing the earliest cases of thyroid cancer related to Fukushima radiation exposure, according to Dr. Suzuki. He presented interim results of Japan’s universal screening protocol for children potentially affected by the Fukushima incident at the International Thyroid Conference.
The protocol, designed to screen everyone residing in the Fukushima prefecture and aged 19 years or younger at the time of the 2011 incident, has been highly successful, with over 80% of those eligible receiving a baseline screening that included a thyroid ultrasound exam.
Screening consisted of an initial thyroid ultrasound exam performed with a portable ultrasound device. If no cyst or nodule was found, then the patient would be seen at the next scheduled thyroid ultrasound exam, 2 years later. Patients with cysts 20 mm or less in greatest diameter or nodules 5 mm or smaller also were deferred to the next scheduled examination. Patients with cysts larger than 20 mm or nodules larger than 5 mm received confirmatory examination by detailed ultrasound examination, blood work, and fine-needle aspiration.
Of the 300,476 patients who received the preliminary baseline survey, 2,294 (0.8%) had an abnormality that warranted confirmatory examination and 91.9% of patients went on to have the confirmatory exams. Of these, 113 were assessed as malignant or suspicious for malignancy. Ninety-nine patients had surgery, with findings of 98 cases of thyroid cancer and one benign tumor.
Patients examined after April 2014 were part of an expanded protocol. Under this protocol, 169,455 patients (44.7% participation) were examined and 1,223 patients (0.8%) had suspicious findings on thyroid ultrasound exam. Participation rate for confirmatory testing for this group was 62.7%, with 25 patients’ thyroids having malignant or potentially malignant findings. Six of these patients had surgery, and thyroid cancer was found in all six cases.
Pooling data from the 138 malignant or suspicious cases from the two groups, 105 patients in total have had surgery, 13 patients with small, noninvasive masses are being watched, and a further 20 are awaiting surgery, Dr. Suzuki said at the meeting held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society.
Of the 97 patients with thyroid cancer who were treated at Fukushima University, 61 were female. The mean patient age at the time of the disaster was 14.8 ± 2.7 years (range, 6-18 years), while the mean age at diagnosis was 17.4 ± 2.8 years (range, 9-22 years). All patients were asymptomatic.
Tumors were unilateral in all but two patients. Mean tumor size was 15.1 ± 0.8 mm (range, 5-53 mm). Nearly all of the tumors (94/97) were papillary thyroid carcinoma, with 86 of those being classical-type papillary thyroid carcinoma. Three patients had poorly differentiated thyroid carcinoma. Fifty-eight patients (60%) had some intraglandular spread, while 71 (73%) had calcifications.
Dr. Suzuki and his collaborators compared these 97 cases with 37 cases of pediatric thyroid cancer in an historical Japanese cohort and to the 26 cases seen in a cohort from Belarus following the Chernobyl disaster. The Fukushima patients were significantly older than either comparison group, with mean age of 11.9 years for the historical Japanese cohort and 10.6 years for the children from Belarus. Tumor size was smaller than the historical Japanese cohort’s mean of 4.1 cm but about the same as that seen in Belarus (1.4 cm). Pulmonary metastases were more common in the historical Japanese cohort (19% vs. 4% in Belarus and 2% in Fukushima).
To have reference data that use similar techniques on a similar population, Japanese researchers are conducting thyroid ultrasound examsaccording to the Fukushima protocol concurrently in three other Japanese prefectures. This is especially important, Dr. Suzuki said, because rapid technological advances in ultrasound imaging mean that screening is much more likely to detect small abnormalities in the thyroid than would have been the case even a few years ago. For this reason, and also because much more radiation was released at the site of the Chernobyl nuclear disaster, only limited comparisons can be made between pediatric thyroid cancer rates from the two nuclear accidents.
Thyroid ultrasound exam “has the ability to detect a lot of thyroid cancers,” he said, so care must be taken to avoid overdiagnosis and overtreatment in this group of young people. Information to date from the Fukushima surveillance project does not yet “give us the clear view about the influence of radiation exposure after the accident on thyroid cancer occurrence,” he said.
Dr. Suzuki reported no relevant disclosures.
On Twitter @karioakes
LAKE BUENA VISTA, FLA. – More cases of thyroid cancer are being seen in Japanese youth after the Fukushima Daiichi nuclear power plant accident, but the increased incidence may be an artifact of heightened surveillance.
“The thyroid cancers appear to have already occurred prior to radiation exposure,” said Dr. Shinichi Suzuki of the department of thyroid and endocrinology at Fukushima (Japan) Medical University. Radiation-induced thyroid cancers take about 5 years to become detectable, so physicians should just now be seeing the earliest cases of thyroid cancer related to Fukushima radiation exposure, according to Dr. Suzuki. He presented interim results of Japan’s universal screening protocol for children potentially affected by the Fukushima incident at the International Thyroid Conference.
The protocol, designed to screen everyone residing in the Fukushima prefecture and aged 19 years or younger at the time of the 2011 incident, has been highly successful, with over 80% of those eligible receiving a baseline screening that included a thyroid ultrasound exam.
Screening consisted of an initial thyroid ultrasound exam performed with a portable ultrasound device. If no cyst or nodule was found, then the patient would be seen at the next scheduled thyroid ultrasound exam, 2 years later. Patients with cysts 20 mm or less in greatest diameter or nodules 5 mm or smaller also were deferred to the next scheduled examination. Patients with cysts larger than 20 mm or nodules larger than 5 mm received confirmatory examination by detailed ultrasound examination, blood work, and fine-needle aspiration.
Of the 300,476 patients who received the preliminary baseline survey, 2,294 (0.8%) had an abnormality that warranted confirmatory examination and 91.9% of patients went on to have the confirmatory exams. Of these, 113 were assessed as malignant or suspicious for malignancy. Ninety-nine patients had surgery, with findings of 98 cases of thyroid cancer and one benign tumor.
Patients examined after April 2014 were part of an expanded protocol. Under this protocol, 169,455 patients (44.7% participation) were examined and 1,223 patients (0.8%) had suspicious findings on thyroid ultrasound exam. Participation rate for confirmatory testing for this group was 62.7%, with 25 patients’ thyroids having malignant or potentially malignant findings. Six of these patients had surgery, and thyroid cancer was found in all six cases.
Pooling data from the 138 malignant or suspicious cases from the two groups, 105 patients in total have had surgery, 13 patients with small, noninvasive masses are being watched, and a further 20 are awaiting surgery, Dr. Suzuki said at the meeting held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society.
Of the 97 patients with thyroid cancer who were treated at Fukushima University, 61 were female. The mean patient age at the time of the disaster was 14.8 ± 2.7 years (range, 6-18 years), while the mean age at diagnosis was 17.4 ± 2.8 years (range, 9-22 years). All patients were asymptomatic.
Tumors were unilateral in all but two patients. Mean tumor size was 15.1 ± 0.8 mm (range, 5-53 mm). Nearly all of the tumors (94/97) were papillary thyroid carcinoma, with 86 of those being classical-type papillary thyroid carcinoma. Three patients had poorly differentiated thyroid carcinoma. Fifty-eight patients (60%) had some intraglandular spread, while 71 (73%) had calcifications.
Dr. Suzuki and his collaborators compared these 97 cases with 37 cases of pediatric thyroid cancer in an historical Japanese cohort and to the 26 cases seen in a cohort from Belarus following the Chernobyl disaster. The Fukushima patients were significantly older than either comparison group, with mean age of 11.9 years for the historical Japanese cohort and 10.6 years for the children from Belarus. Tumor size was smaller than the historical Japanese cohort’s mean of 4.1 cm but about the same as that seen in Belarus (1.4 cm). Pulmonary metastases were more common in the historical Japanese cohort (19% vs. 4% in Belarus and 2% in Fukushima).
To have reference data that use similar techniques on a similar population, Japanese researchers are conducting thyroid ultrasound examsaccording to the Fukushima protocol concurrently in three other Japanese prefectures. This is especially important, Dr. Suzuki said, because rapid technological advances in ultrasound imaging mean that screening is much more likely to detect small abnormalities in the thyroid than would have been the case even a few years ago. For this reason, and also because much more radiation was released at the site of the Chernobyl nuclear disaster, only limited comparisons can be made between pediatric thyroid cancer rates from the two nuclear accidents.
Thyroid ultrasound exam “has the ability to detect a lot of thyroid cancers,” he said, so care must be taken to avoid overdiagnosis and overtreatment in this group of young people. Information to date from the Fukushima surveillance project does not yet “give us the clear view about the influence of radiation exposure after the accident on thyroid cancer occurrence,” he said.
Dr. Suzuki reported no relevant disclosures.
On Twitter @karioakes
LAKE BUENA VISTA, FLA. – More cases of thyroid cancer are being seen in Japanese youth after the Fukushima Daiichi nuclear power plant accident, but the increased incidence may be an artifact of heightened surveillance.
“The thyroid cancers appear to have already occurred prior to radiation exposure,” said Dr. Shinichi Suzuki of the department of thyroid and endocrinology at Fukushima (Japan) Medical University. Radiation-induced thyroid cancers take about 5 years to become detectable, so physicians should just now be seeing the earliest cases of thyroid cancer related to Fukushima radiation exposure, according to Dr. Suzuki. He presented interim results of Japan’s universal screening protocol for children potentially affected by the Fukushima incident at the International Thyroid Conference.
The protocol, designed to screen everyone residing in the Fukushima prefecture and aged 19 years or younger at the time of the 2011 incident, has been highly successful, with over 80% of those eligible receiving a baseline screening that included a thyroid ultrasound exam.
Screening consisted of an initial thyroid ultrasound exam performed with a portable ultrasound device. If no cyst or nodule was found, then the patient would be seen at the next scheduled thyroid ultrasound exam, 2 years later. Patients with cysts 20 mm or less in greatest diameter or nodules 5 mm or smaller also were deferred to the next scheduled examination. Patients with cysts larger than 20 mm or nodules larger than 5 mm received confirmatory examination by detailed ultrasound examination, blood work, and fine-needle aspiration.
Of the 300,476 patients who received the preliminary baseline survey, 2,294 (0.8%) had an abnormality that warranted confirmatory examination and 91.9% of patients went on to have the confirmatory exams. Of these, 113 were assessed as malignant or suspicious for malignancy. Ninety-nine patients had surgery, with findings of 98 cases of thyroid cancer and one benign tumor.
Patients examined after April 2014 were part of an expanded protocol. Under this protocol, 169,455 patients (44.7% participation) were examined and 1,223 patients (0.8%) had suspicious findings on thyroid ultrasound exam. Participation rate for confirmatory testing for this group was 62.7%, with 25 patients’ thyroids having malignant or potentially malignant findings. Six of these patients had surgery, and thyroid cancer was found in all six cases.
Pooling data from the 138 malignant or suspicious cases from the two groups, 105 patients in total have had surgery, 13 patients with small, noninvasive masses are being watched, and a further 20 are awaiting surgery, Dr. Suzuki said at the meeting held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society.
Of the 97 patients with thyroid cancer who were treated at Fukushima University, 61 were female. The mean patient age at the time of the disaster was 14.8 ± 2.7 years (range, 6-18 years), while the mean age at diagnosis was 17.4 ± 2.8 years (range, 9-22 years). All patients were asymptomatic.
Tumors were unilateral in all but two patients. Mean tumor size was 15.1 ± 0.8 mm (range, 5-53 mm). Nearly all of the tumors (94/97) were papillary thyroid carcinoma, with 86 of those being classical-type papillary thyroid carcinoma. Three patients had poorly differentiated thyroid carcinoma. Fifty-eight patients (60%) had some intraglandular spread, while 71 (73%) had calcifications.
Dr. Suzuki and his collaborators compared these 97 cases with 37 cases of pediatric thyroid cancer in an historical Japanese cohort and to the 26 cases seen in a cohort from Belarus following the Chernobyl disaster. The Fukushima patients were significantly older than either comparison group, with mean age of 11.9 years for the historical Japanese cohort and 10.6 years for the children from Belarus. Tumor size was smaller than the historical Japanese cohort’s mean of 4.1 cm but about the same as that seen in Belarus (1.4 cm). Pulmonary metastases were more common in the historical Japanese cohort (19% vs. 4% in Belarus and 2% in Fukushima).
To have reference data that use similar techniques on a similar population, Japanese researchers are conducting thyroid ultrasound examsaccording to the Fukushima protocol concurrently in three other Japanese prefectures. This is especially important, Dr. Suzuki said, because rapid technological advances in ultrasound imaging mean that screening is much more likely to detect small abnormalities in the thyroid than would have been the case even a few years ago. For this reason, and also because much more radiation was released at the site of the Chernobyl nuclear disaster, only limited comparisons can be made between pediatric thyroid cancer rates from the two nuclear accidents.
Thyroid ultrasound exam “has the ability to detect a lot of thyroid cancers,” he said, so care must be taken to avoid overdiagnosis and overtreatment in this group of young people. Information to date from the Fukushima surveillance project does not yet “give us the clear view about the influence of radiation exposure after the accident on thyroid cancer occurrence,” he said.
Dr. Suzuki reported no relevant disclosures.
On Twitter @karioakes
AT ITC 2015
Key clinical point: The increased incidence of thyroid cancers in Japanese youth after the Fukushima nuclear accident may be an artifact of increased surveillance.
Major finding: A total of 138 thyroid cancers have been found when screening 469,931 children in Fukushima after the 2011 nuclear power plant accident.
Data source: Universal screening for thyroid cancer among individuals who were aged 18 years or younger and resident in Fukushima at the time of the accident.
Disclosures: Dr. Suzuki reported no relevant disclosures.
Percutaneous ethanol effective for small papillary thyroid cancers
LAKE BUENA VISTA, FLA. – An outpatient procedure may represent an efficacious and safe alternative to surgery for those patients with small papillary thyroid cancers who prefer definitive treatment over the “wait and watch” approach. Further, at one institution, the cost-effective alternative to surgery saved almost $40,000 per patient.
Ultrasound-guided percutaneous ethanol injection (UPEA) of small (cT1N0) intrathyroidal papillary thyroid cancer (SIPC) successfully reduced tumor volume by a median of 92%, eliminated tumor blood flow, and was very well tolerated by a series of 13 patients who received UPEA at the Mayo Clinic, Rochester, Minn.
Dr. Ian D. Hay, a consultant in Mayo’s division of endocrinology, diabetes, metabolism, and nutrition, presented the findings during a poster session at the International Thyroid Congress.
Dr. Hay and his colleagues treated 13 patients with a total of 15 tumors with injections of percutaneous ethanol. The first patient received just one injection; the remaining patients received one injection to each tumor site on each of 2 consecutive days. Five of the tumor foci had less than a 50% reduction in tumor volume at the first follow-up visit, so those tumors were injected a third time.
Patients in the series ranged from 38 to 86 years old (median 45), and five patients had significant comorbidities: one had congestive heart failure and the other four had concomitant unrelated cancers. Tumors were a median 8 mm in size, with volumes ranging from 25 to 676 mm3 (median 140 mm3).
All of the injections were performed under ultrasound guidance, and a median of 0.9 cc of ethanol was injected into each tumor. Ultrasound examination was performed at each follow-up visit to evaluate tumor volume and blood flow. Dr. Hay reported that the procedure was well tolerated: Local neck tenderness resolved within a day or two, and there were no reports of hoarseness or laryngeal nerve palsy.
Patients were followed for a mean 2.0 years (range, 0.4-5.7 years), with a median tumor reduction of 92% (range 46%-100%). For the nine tumors that were still identifiable on ultrasound at the time of reporting, the mean volume had decreased by 73%. Six tumor foci had completely disappeared, and no tumor had detectable blood flow on Doppler exam. Tumor thyroglobulin levels remained stable in all patients, and no nodal metastases were identified, Dr. Hay reported at the meeting, which was held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society.
Internationally, the approach to managing SIPC varies from lobectomy to near-total thyroidectomy to active surveillance. For patients who prefer definitive management of their tumors but are reluctant to have surgery or who may have significant comorbidities, UPEA may represent a safe alternative, and at significant cost savings compared to surgery: Dr. Hay and his colleagues reported that they estimated the average cost savings at their institution to be over $38,000 per patient. “If prospective trials of observation vs. surgery for SIPC are to occur in the USA, perhaps it could be included as a ‘third arm’ in such trials,” Dr. Hay and his colleagues said.
On Twitter @karioakes
LAKE BUENA VISTA, FLA. – An outpatient procedure may represent an efficacious and safe alternative to surgery for those patients with small papillary thyroid cancers who prefer definitive treatment over the “wait and watch” approach. Further, at one institution, the cost-effective alternative to surgery saved almost $40,000 per patient.
Ultrasound-guided percutaneous ethanol injection (UPEA) of small (cT1N0) intrathyroidal papillary thyroid cancer (SIPC) successfully reduced tumor volume by a median of 92%, eliminated tumor blood flow, and was very well tolerated by a series of 13 patients who received UPEA at the Mayo Clinic, Rochester, Minn.
Dr. Ian D. Hay, a consultant in Mayo’s division of endocrinology, diabetes, metabolism, and nutrition, presented the findings during a poster session at the International Thyroid Congress.
Dr. Hay and his colleagues treated 13 patients with a total of 15 tumors with injections of percutaneous ethanol. The first patient received just one injection; the remaining patients received one injection to each tumor site on each of 2 consecutive days. Five of the tumor foci had less than a 50% reduction in tumor volume at the first follow-up visit, so those tumors were injected a third time.
Patients in the series ranged from 38 to 86 years old (median 45), and five patients had significant comorbidities: one had congestive heart failure and the other four had concomitant unrelated cancers. Tumors were a median 8 mm in size, with volumes ranging from 25 to 676 mm3 (median 140 mm3).
All of the injections were performed under ultrasound guidance, and a median of 0.9 cc of ethanol was injected into each tumor. Ultrasound examination was performed at each follow-up visit to evaluate tumor volume and blood flow. Dr. Hay reported that the procedure was well tolerated: Local neck tenderness resolved within a day or two, and there were no reports of hoarseness or laryngeal nerve palsy.
Patients were followed for a mean 2.0 years (range, 0.4-5.7 years), with a median tumor reduction of 92% (range 46%-100%). For the nine tumors that were still identifiable on ultrasound at the time of reporting, the mean volume had decreased by 73%. Six tumor foci had completely disappeared, and no tumor had detectable blood flow on Doppler exam. Tumor thyroglobulin levels remained stable in all patients, and no nodal metastases were identified, Dr. Hay reported at the meeting, which was held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society.
Internationally, the approach to managing SIPC varies from lobectomy to near-total thyroidectomy to active surveillance. For patients who prefer definitive management of their tumors but are reluctant to have surgery or who may have significant comorbidities, UPEA may represent a safe alternative, and at significant cost savings compared to surgery: Dr. Hay and his colleagues reported that they estimated the average cost savings at their institution to be over $38,000 per patient. “If prospective trials of observation vs. surgery for SIPC are to occur in the USA, perhaps it could be included as a ‘third arm’ in such trials,” Dr. Hay and his colleagues said.
On Twitter @karioakes
LAKE BUENA VISTA, FLA. – An outpatient procedure may represent an efficacious and safe alternative to surgery for those patients with small papillary thyroid cancers who prefer definitive treatment over the “wait and watch” approach. Further, at one institution, the cost-effective alternative to surgery saved almost $40,000 per patient.
Ultrasound-guided percutaneous ethanol injection (UPEA) of small (cT1N0) intrathyroidal papillary thyroid cancer (SIPC) successfully reduced tumor volume by a median of 92%, eliminated tumor blood flow, and was very well tolerated by a series of 13 patients who received UPEA at the Mayo Clinic, Rochester, Minn.
Dr. Ian D. Hay, a consultant in Mayo’s division of endocrinology, diabetes, metabolism, and nutrition, presented the findings during a poster session at the International Thyroid Congress.
Dr. Hay and his colleagues treated 13 patients with a total of 15 tumors with injections of percutaneous ethanol. The first patient received just one injection; the remaining patients received one injection to each tumor site on each of 2 consecutive days. Five of the tumor foci had less than a 50% reduction in tumor volume at the first follow-up visit, so those tumors were injected a third time.
Patients in the series ranged from 38 to 86 years old (median 45), and five patients had significant comorbidities: one had congestive heart failure and the other four had concomitant unrelated cancers. Tumors were a median 8 mm in size, with volumes ranging from 25 to 676 mm3 (median 140 mm3).
All of the injections were performed under ultrasound guidance, and a median of 0.9 cc of ethanol was injected into each tumor. Ultrasound examination was performed at each follow-up visit to evaluate tumor volume and blood flow. Dr. Hay reported that the procedure was well tolerated: Local neck tenderness resolved within a day or two, and there were no reports of hoarseness or laryngeal nerve palsy.
Patients were followed for a mean 2.0 years (range, 0.4-5.7 years), with a median tumor reduction of 92% (range 46%-100%). For the nine tumors that were still identifiable on ultrasound at the time of reporting, the mean volume had decreased by 73%. Six tumor foci had completely disappeared, and no tumor had detectable blood flow on Doppler exam. Tumor thyroglobulin levels remained stable in all patients, and no nodal metastases were identified, Dr. Hay reported at the meeting, which was held by the American Thyroid Association, Asia-Oceania Thyroid Association, European Thyroid Association, and Latin American Thyroid Society.
Internationally, the approach to managing SIPC varies from lobectomy to near-total thyroidectomy to active surveillance. For patients who prefer definitive management of their tumors but are reluctant to have surgery or who may have significant comorbidities, UPEA may represent a safe alternative, and at significant cost savings compared to surgery: Dr. Hay and his colleagues reported that they estimated the average cost savings at their institution to be over $38,000 per patient. “If prospective trials of observation vs. surgery for SIPC are to occur in the USA, perhaps it could be included as a ‘third arm’ in such trials,” Dr. Hay and his colleagues said.
On Twitter @karioakes
AT ITC 2015
Key clinical point: Ultrasound-guided percutaneous ethanol ablation (UPEA) is an efficacious, cost-effective, and noninvasive definitive treatment for small papillary thyroid cancers.
Major finding: Fifteen tumors in 13 patients were successfully treated with UPEA with a mean 92% reduction in tumor volume and no complications or metastasis at a mean 2-year follow-up.
Data source: Series of 13 patients with 15 tumors treated at the Mayo Clinic for small intrathyroidal papillary cancers.
Disclosures: No disclosures were identified.