User login
Early or delayed AFib ablation after heart failure hospitalization?
TOPLINE:
Among patients with atrial fibrillation (AFib) hospitalized for worsening heart failure (HF), catheter (cath) ablation within 90 days of admission, compared with other times, is associated with reduced risk for all-cause mortality and HF-related mortality.
METHODOLOGY:
Cath ablation has become technically safer for patients with both AFib and HF, but the best timing for the ablation procedure after HF hospitalization has been unclear.
The study included 2,786 patients with HF who underwent cath ablation for AFib at 128 centers in the nationwide Japanese Registry of Acute Decompensated Heart Failure, were hospitalized with worsening HF, and survived at least 90 days after discharge.
The population included 103 individuals who underwent cath ablation within 90 days after admission; the remaining 2,683 participants served as the control group.
The researchers also looked at all-cause mortality 90 days after admission for HF in analysis of 83 early-ablation cases vs. 83 propensity-matched controls.
TAKEAWAY:
The early–cath ablation group was younger, predominantly male, had less history of prior HF hospitalizations, and greater incidence of paroxysmal AF, compared with the control group.
All-cause mortality was significantly lower in the early–cath ablation group than in the control group (hazard ratio, 0.38; 95% confidence interval, 0.24-0.60; P < .001) over a median of 4.1 years.
Risk reductions were similarly significant for secondary endpoints, including cardiovascular (CV) mortality and HF mortality.
In the matched cohort analysis (83 in both groups) all-cause mortality was significantly reduced for those in the early–cath ablation group, compared with the matched controls (HR, 0.47; 95% CI, 0.25-0.88; P = .014), with similarly significant risk reductions for CV mortality and HF mortality.
IN PRACTICE:
the report states. Early catheter ablation, as early as during the hospitalization for HF, “might be a way to stabilize HF and solve the problems associated with long hospitalization periods and polypharmacy.”
SOURCE:
The study was conducted by Kazuo Sakamoto, MD, PhD, Kyushu University, Fukuoka, Japan, and colleagues. It was published online July 19, 2023 in JACC: Clinical Electrophysiology.
LIMITATIONS:
The early-ablation cohort was much smaller than the control group, and the analysis could not adjust for any variation in institutional characteristics, such as location and available equipment. Other unmeasured potential confounders include duration of AFib and patient lifestyle characteristics and success or failure of ablation.
DISCLOSURES:
The study was funded by Johnson & Johnson, the Japan Agency for Medical Research and Development, and Ministry of Health and Labor. Dr. Sakamoto reports no relevant conflicts.
A version of this article first appeared on Medscape.com.
TOPLINE:
Among patients with atrial fibrillation (AFib) hospitalized for worsening heart failure (HF), catheter (cath) ablation within 90 days of admission, compared with other times, is associated with reduced risk for all-cause mortality and HF-related mortality.
METHODOLOGY:
Cath ablation has become technically safer for patients with both AFib and HF, but the best timing for the ablation procedure after HF hospitalization has been unclear.
The study included 2,786 patients with HF who underwent cath ablation for AFib at 128 centers in the nationwide Japanese Registry of Acute Decompensated Heart Failure, were hospitalized with worsening HF, and survived at least 90 days after discharge.
The population included 103 individuals who underwent cath ablation within 90 days after admission; the remaining 2,683 participants served as the control group.
The researchers also looked at all-cause mortality 90 days after admission for HF in analysis of 83 early-ablation cases vs. 83 propensity-matched controls.
TAKEAWAY:
The early–cath ablation group was younger, predominantly male, had less history of prior HF hospitalizations, and greater incidence of paroxysmal AF, compared with the control group.
All-cause mortality was significantly lower in the early–cath ablation group than in the control group (hazard ratio, 0.38; 95% confidence interval, 0.24-0.60; P < .001) over a median of 4.1 years.
Risk reductions were similarly significant for secondary endpoints, including cardiovascular (CV) mortality and HF mortality.
In the matched cohort analysis (83 in both groups) all-cause mortality was significantly reduced for those in the early–cath ablation group, compared with the matched controls (HR, 0.47; 95% CI, 0.25-0.88; P = .014), with similarly significant risk reductions for CV mortality and HF mortality.
IN PRACTICE:
the report states. Early catheter ablation, as early as during the hospitalization for HF, “might be a way to stabilize HF and solve the problems associated with long hospitalization periods and polypharmacy.”
SOURCE:
The study was conducted by Kazuo Sakamoto, MD, PhD, Kyushu University, Fukuoka, Japan, and colleagues. It was published online July 19, 2023 in JACC: Clinical Electrophysiology.
LIMITATIONS:
The early-ablation cohort was much smaller than the control group, and the analysis could not adjust for any variation in institutional characteristics, such as location and available equipment. Other unmeasured potential confounders include duration of AFib and patient lifestyle characteristics and success or failure of ablation.
DISCLOSURES:
The study was funded by Johnson & Johnson, the Japan Agency for Medical Research and Development, and Ministry of Health and Labor. Dr. Sakamoto reports no relevant conflicts.
A version of this article first appeared on Medscape.com.
TOPLINE:
Among patients with atrial fibrillation (AFib) hospitalized for worsening heart failure (HF), catheter (cath) ablation within 90 days of admission, compared with other times, is associated with reduced risk for all-cause mortality and HF-related mortality.
METHODOLOGY:
Cath ablation has become technically safer for patients with both AFib and HF, but the best timing for the ablation procedure after HF hospitalization has been unclear.
The study included 2,786 patients with HF who underwent cath ablation for AFib at 128 centers in the nationwide Japanese Registry of Acute Decompensated Heart Failure, were hospitalized with worsening HF, and survived at least 90 days after discharge.
The population included 103 individuals who underwent cath ablation within 90 days after admission; the remaining 2,683 participants served as the control group.
The researchers also looked at all-cause mortality 90 days after admission for HF in analysis of 83 early-ablation cases vs. 83 propensity-matched controls.
TAKEAWAY:
The early–cath ablation group was younger, predominantly male, had less history of prior HF hospitalizations, and greater incidence of paroxysmal AF, compared with the control group.
All-cause mortality was significantly lower in the early–cath ablation group than in the control group (hazard ratio, 0.38; 95% confidence interval, 0.24-0.60; P < .001) over a median of 4.1 years.
Risk reductions were similarly significant for secondary endpoints, including cardiovascular (CV) mortality and HF mortality.
In the matched cohort analysis (83 in both groups) all-cause mortality was significantly reduced for those in the early–cath ablation group, compared with the matched controls (HR, 0.47; 95% CI, 0.25-0.88; P = .014), with similarly significant risk reductions for CV mortality and HF mortality.
IN PRACTICE:
the report states. Early catheter ablation, as early as during the hospitalization for HF, “might be a way to stabilize HF and solve the problems associated with long hospitalization periods and polypharmacy.”
SOURCE:
The study was conducted by Kazuo Sakamoto, MD, PhD, Kyushu University, Fukuoka, Japan, and colleagues. It was published online July 19, 2023 in JACC: Clinical Electrophysiology.
LIMITATIONS:
The early-ablation cohort was much smaller than the control group, and the analysis could not adjust for any variation in institutional characteristics, such as location and available equipment. Other unmeasured potential confounders include duration of AFib and patient lifestyle characteristics and success or failure of ablation.
DISCLOSURES:
The study was funded by Johnson & Johnson, the Japan Agency for Medical Research and Development, and Ministry of Health and Labor. Dr. Sakamoto reports no relevant conflicts.
A version of this article first appeared on Medscape.com.
FROM JACC: CLINICAL ELECTROPHYSIOLOGY
Global burden of brain disorders surpasses cardiovascular disease and cancer
– at huge cost to health care systems and society, an analysis of data from the most recent Global Burden of Disease (GBD) study shows.
“The burden of brain conditions will increase as populations continue to grow and age,” said study presenter Shayla Smith, MPH, an epidemiologist at the Institute for Health Metrics and Evaluation, the University of Washington, Seattle, in a press release.
“By 2050, more than 50 million people will be aged 65-79,” she explained, adding that the COVID-19 pandemic “has also influenced the prevalence of mental disorders globally, as people were forced to isolate and social networks broke down.”
Other factors related to brain disorders, she noted, include education level, obesity, and smoking.
“There’s still research to be done on what is the most effective way to maintain brain health, but some literature suggests a healthy brain can be achieved through a healthy lifestyle of managing conditions such as high blood pressure and diabetes, limiting alcohol consumption and smoking, prioritizing sleep, eating healthy, and staying physically and mentally active,” said Ms. Smith.
The findings were presented at the annual meeting of the Congress of the European Academy of Neurology.
An ‘ambitious exercise’
Coinvestigator Xaviera Steele, also from the IHME, told press conference attendees that the institute was established at the University of Washington in 2007 with the aim of “standardizing the measurement of health outcomes around the world and for all health conditions.”
A central part of that is the GBD study, “which is a very ambitious exercise in descriptive epidemiology in an effort to systematically quantify health loss” due to disease, injury, and risk factors over time, stratified by country, region, age, and sex. In addition, researchers are mapping and projecting trends over the next century and are estimating disease expenditure by country, by type of expense, and by condition “to derive a health care access and quality score for each health system in the world,” Ms. Steele said.
They are also estimating exposure to risk factors, how those risk factors contribute to health burden, and associated health outcomes by race and ethnicity to reflect the “disparities that we know are very prevalent in countries such as the United States.” From that work, Ms. Steele said that brain health and related conditions “do emerge as one of the more pressing challenges of the 21st century.”
Increase in dementia, mental health conditions
The data, which were gathered from 200,000 sources by the IHME, indicate that the number of individuals aged 65 years or older will increase by 350% by 2100. Ms. Steele underlined that “policy action will be needed to help families, who will struggle to provide high-quality care for their loved ones with dementia at a reasonable cost.”
The IHME calculates that in Europe health care spending on Alzheimer’s disease will increase by 226% between 2015 and 2040.
Turning to other conditions, Ms. Steele showed that since 1990, the number of individuals living with anxiety in the European region has increased by 14%, while the number living with depressive disorders has gone up by 13%.
Worldwide, the figures are even starker. Depression is estimated to affect 300 million people across the globe, which represents a 71% increase since 1990. The number of strokes increased by 95% over the same period.
Nevertheless, the “impact of brain conditions such as stroke has decreased since the 1990s due to improved treatments available,” Ms. Smith noted in the press release.
To estimate the toll caused by brain conditions, including neurologic disorders, mental disorders, cerebrovascular disease, brain cancer, brain injuries, and select infectious conditions, the researchers calculated disability-adjusted life years (DALYs).
This, Ms. Smith explained in her presentation, “captures the morbidity and mortality associated with brain conditions” and is adjusted for patient location, age, and sex.
The investigators found that, globally, brain conditions accounted for more than 15% of all health loss in 2021, at 406 DALYs – more than the 206 million DALYs that were associated with cancer, and the 402 million that were linked to cardiovascular disease.
This health loss is associated with a $1.22 trillion loss in income for people living with health disorders worldwide and accounts for $1.14 trillion in direct health care costs.
The burden of mental disorders, neurologic conditions, and stroke is expected to increase dramatically between now and 2050, said Ms. Smith, who noted that health loss linked to brain conditions is higher in younger patients. This will create “new challenges for health systems, employers, patients, and families,” she said in the press release.
“Our goal is to see an improved prevention and treatment landscape for other brain conditions and reverse the growing health loss that we are currently forecasting.”
Worrying increase in stroke
Jurgita Valaikiene, MD, PhD, center of neurology, clinic of neurology and neurosurgery, Vilnius (Lithuania) University Faculty of Medicine, who chaired the session, was taken aback by the findings, particularly by the worldwide increase in stroke cases.
“I work in stroke,” she said, and “we spend a lot of time on the diagnosis of stroke” and its prevention. “We try to be faster, to catch asymptomatic stenosis in the neck or head, and to apply the best medical treatment to avoid a stroke. But despite that, the numbers are increasing. I understand the population is getting older ... but still it’s a huge number.”
Dr. Valaikiene pointed out that stroke is not necessarily a condition of aging, insofar as increasing age “is not related directly to stenosis in the neck. “For example, we can have healthier vessels in older age and unhealthy vessels, with high-grade stenosis, in someone aged 30 or 40 years.”
“There are a lot of risk factors, such as smoking, physical activity, and so on. It depends on the individual,” she added.
The study was funded by the Institute for Health Metrics and Evaluation at the University of Washington. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
– at huge cost to health care systems and society, an analysis of data from the most recent Global Burden of Disease (GBD) study shows.
“The burden of brain conditions will increase as populations continue to grow and age,” said study presenter Shayla Smith, MPH, an epidemiologist at the Institute for Health Metrics and Evaluation, the University of Washington, Seattle, in a press release.
“By 2050, more than 50 million people will be aged 65-79,” she explained, adding that the COVID-19 pandemic “has also influenced the prevalence of mental disorders globally, as people were forced to isolate and social networks broke down.”
Other factors related to brain disorders, she noted, include education level, obesity, and smoking.
“There’s still research to be done on what is the most effective way to maintain brain health, but some literature suggests a healthy brain can be achieved through a healthy lifestyle of managing conditions such as high blood pressure and diabetes, limiting alcohol consumption and smoking, prioritizing sleep, eating healthy, and staying physically and mentally active,” said Ms. Smith.
The findings were presented at the annual meeting of the Congress of the European Academy of Neurology.
An ‘ambitious exercise’
Coinvestigator Xaviera Steele, also from the IHME, told press conference attendees that the institute was established at the University of Washington in 2007 with the aim of “standardizing the measurement of health outcomes around the world and for all health conditions.”
A central part of that is the GBD study, “which is a very ambitious exercise in descriptive epidemiology in an effort to systematically quantify health loss” due to disease, injury, and risk factors over time, stratified by country, region, age, and sex. In addition, researchers are mapping and projecting trends over the next century and are estimating disease expenditure by country, by type of expense, and by condition “to derive a health care access and quality score for each health system in the world,” Ms. Steele said.
They are also estimating exposure to risk factors, how those risk factors contribute to health burden, and associated health outcomes by race and ethnicity to reflect the “disparities that we know are very prevalent in countries such as the United States.” From that work, Ms. Steele said that brain health and related conditions “do emerge as one of the more pressing challenges of the 21st century.”
Increase in dementia, mental health conditions
The data, which were gathered from 200,000 sources by the IHME, indicate that the number of individuals aged 65 years or older will increase by 350% by 2100. Ms. Steele underlined that “policy action will be needed to help families, who will struggle to provide high-quality care for their loved ones with dementia at a reasonable cost.”
The IHME calculates that in Europe health care spending on Alzheimer’s disease will increase by 226% between 2015 and 2040.
Turning to other conditions, Ms. Steele showed that since 1990, the number of individuals living with anxiety in the European region has increased by 14%, while the number living with depressive disorders has gone up by 13%.
Worldwide, the figures are even starker. Depression is estimated to affect 300 million people across the globe, which represents a 71% increase since 1990. The number of strokes increased by 95% over the same period.
Nevertheless, the “impact of brain conditions such as stroke has decreased since the 1990s due to improved treatments available,” Ms. Smith noted in the press release.
To estimate the toll caused by brain conditions, including neurologic disorders, mental disorders, cerebrovascular disease, brain cancer, brain injuries, and select infectious conditions, the researchers calculated disability-adjusted life years (DALYs).
This, Ms. Smith explained in her presentation, “captures the morbidity and mortality associated with brain conditions” and is adjusted for patient location, age, and sex.
The investigators found that, globally, brain conditions accounted for more than 15% of all health loss in 2021, at 406 DALYs – more than the 206 million DALYs that were associated with cancer, and the 402 million that were linked to cardiovascular disease.
This health loss is associated with a $1.22 trillion loss in income for people living with health disorders worldwide and accounts for $1.14 trillion in direct health care costs.
The burden of mental disorders, neurologic conditions, and stroke is expected to increase dramatically between now and 2050, said Ms. Smith, who noted that health loss linked to brain conditions is higher in younger patients. This will create “new challenges for health systems, employers, patients, and families,” she said in the press release.
“Our goal is to see an improved prevention and treatment landscape for other brain conditions and reverse the growing health loss that we are currently forecasting.”
Worrying increase in stroke
Jurgita Valaikiene, MD, PhD, center of neurology, clinic of neurology and neurosurgery, Vilnius (Lithuania) University Faculty of Medicine, who chaired the session, was taken aback by the findings, particularly by the worldwide increase in stroke cases.
“I work in stroke,” she said, and “we spend a lot of time on the diagnosis of stroke” and its prevention. “We try to be faster, to catch asymptomatic stenosis in the neck or head, and to apply the best medical treatment to avoid a stroke. But despite that, the numbers are increasing. I understand the population is getting older ... but still it’s a huge number.”
Dr. Valaikiene pointed out that stroke is not necessarily a condition of aging, insofar as increasing age “is not related directly to stenosis in the neck. “For example, we can have healthier vessels in older age and unhealthy vessels, with high-grade stenosis, in someone aged 30 or 40 years.”
“There are a lot of risk factors, such as smoking, physical activity, and so on. It depends on the individual,” she added.
The study was funded by the Institute for Health Metrics and Evaluation at the University of Washington. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
– at huge cost to health care systems and society, an analysis of data from the most recent Global Burden of Disease (GBD) study shows.
“The burden of brain conditions will increase as populations continue to grow and age,” said study presenter Shayla Smith, MPH, an epidemiologist at the Institute for Health Metrics and Evaluation, the University of Washington, Seattle, in a press release.
“By 2050, more than 50 million people will be aged 65-79,” she explained, adding that the COVID-19 pandemic “has also influenced the prevalence of mental disorders globally, as people were forced to isolate and social networks broke down.”
Other factors related to brain disorders, she noted, include education level, obesity, and smoking.
“There’s still research to be done on what is the most effective way to maintain brain health, but some literature suggests a healthy brain can be achieved through a healthy lifestyle of managing conditions such as high blood pressure and diabetes, limiting alcohol consumption and smoking, prioritizing sleep, eating healthy, and staying physically and mentally active,” said Ms. Smith.
The findings were presented at the annual meeting of the Congress of the European Academy of Neurology.
An ‘ambitious exercise’
Coinvestigator Xaviera Steele, also from the IHME, told press conference attendees that the institute was established at the University of Washington in 2007 with the aim of “standardizing the measurement of health outcomes around the world and for all health conditions.”
A central part of that is the GBD study, “which is a very ambitious exercise in descriptive epidemiology in an effort to systematically quantify health loss” due to disease, injury, and risk factors over time, stratified by country, region, age, and sex. In addition, researchers are mapping and projecting trends over the next century and are estimating disease expenditure by country, by type of expense, and by condition “to derive a health care access and quality score for each health system in the world,” Ms. Steele said.
They are also estimating exposure to risk factors, how those risk factors contribute to health burden, and associated health outcomes by race and ethnicity to reflect the “disparities that we know are very prevalent in countries such as the United States.” From that work, Ms. Steele said that brain health and related conditions “do emerge as one of the more pressing challenges of the 21st century.”
Increase in dementia, mental health conditions
The data, which were gathered from 200,000 sources by the IHME, indicate that the number of individuals aged 65 years or older will increase by 350% by 2100. Ms. Steele underlined that “policy action will be needed to help families, who will struggle to provide high-quality care for their loved ones with dementia at a reasonable cost.”
The IHME calculates that in Europe health care spending on Alzheimer’s disease will increase by 226% between 2015 and 2040.
Turning to other conditions, Ms. Steele showed that since 1990, the number of individuals living with anxiety in the European region has increased by 14%, while the number living with depressive disorders has gone up by 13%.
Worldwide, the figures are even starker. Depression is estimated to affect 300 million people across the globe, which represents a 71% increase since 1990. The number of strokes increased by 95% over the same period.
Nevertheless, the “impact of brain conditions such as stroke has decreased since the 1990s due to improved treatments available,” Ms. Smith noted in the press release.
To estimate the toll caused by brain conditions, including neurologic disorders, mental disorders, cerebrovascular disease, brain cancer, brain injuries, and select infectious conditions, the researchers calculated disability-adjusted life years (DALYs).
This, Ms. Smith explained in her presentation, “captures the morbidity and mortality associated with brain conditions” and is adjusted for patient location, age, and sex.
The investigators found that, globally, brain conditions accounted for more than 15% of all health loss in 2021, at 406 DALYs – more than the 206 million DALYs that were associated with cancer, and the 402 million that were linked to cardiovascular disease.
This health loss is associated with a $1.22 trillion loss in income for people living with health disorders worldwide and accounts for $1.14 trillion in direct health care costs.
The burden of mental disorders, neurologic conditions, and stroke is expected to increase dramatically between now and 2050, said Ms. Smith, who noted that health loss linked to brain conditions is higher in younger patients. This will create “new challenges for health systems, employers, patients, and families,” she said in the press release.
“Our goal is to see an improved prevention and treatment landscape for other brain conditions and reverse the growing health loss that we are currently forecasting.”
Worrying increase in stroke
Jurgita Valaikiene, MD, PhD, center of neurology, clinic of neurology and neurosurgery, Vilnius (Lithuania) University Faculty of Medicine, who chaired the session, was taken aback by the findings, particularly by the worldwide increase in stroke cases.
“I work in stroke,” she said, and “we spend a lot of time on the diagnosis of stroke” and its prevention. “We try to be faster, to catch asymptomatic stenosis in the neck or head, and to apply the best medical treatment to avoid a stroke. But despite that, the numbers are increasing. I understand the population is getting older ... but still it’s a huge number.”
Dr. Valaikiene pointed out that stroke is not necessarily a condition of aging, insofar as increasing age “is not related directly to stenosis in the neck. “For example, we can have healthier vessels in older age and unhealthy vessels, with high-grade stenosis, in someone aged 30 or 40 years.”
“There are a lot of risk factors, such as smoking, physical activity, and so on. It depends on the individual,” she added.
The study was funded by the Institute for Health Metrics and Evaluation at the University of Washington. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PCPs key to heart failure care after discharge
Madeline Sterling, MD, knew something was wrong when she heard her patient’s voice on the phone. The patient was breathing too fast and sounded fatigued. Like many people with heart failure, this patient had several comorbidities: diabetes, high blood pressure, and cancer, which was in remission.
The patient had been in and out of the hospital several times and was afraid of going back, but Dr. Sterling, a primary care physician, advised her that it was the safe thing to do.
During the woman’s stay, the inpatient cardiology team called Dr. Sterling to provide status updates and ask for input. When the patient was discharged, Dr. Sterling received information on what medicines had been changed and scheduled follow-up care within 10 days. Dr. Sterling, who’d cared for the woman for many years, called her family, her home health aide, and another caregiver to discuss the plan.
“When you know these patients really well, it’s helpful,” Dr. Sterling, a professor of medicine at Weill Cornell Medicine, New York, said. Primary care clinicians have “an appreciation for how all these conditions fit together, how the medicines fit together, and how to put that patient’s priorities at the front of the equation.”
Research has shown that follow-up care within 7-10 days after discharge, especially for patients with heart failure, can prevent hospital readmissions. Patients’ health can change rapidly following discharge: They may start retaining fluid or may not know how to maintain a low-sodium diet, or they might have trouble obtaining medication. Primary care clinicians spot these early warning signs in follow-up visits.
Heart failure affects more than 6 million adults in the United States, according to the Centers for Disease Control and Prevention. The condition is a common cause of hospital readmissions within 30 days of discharge, according to research published by the American Heart Association.
Patients with heart failure are particularly challenging to care for because of comorbidities.
“They’re a very, very sick group of patients that are very difficult to manage,” said Noah Moss, MD, an advanced heart failure and transplant cardiologist at Mount Sinai Hospital, New York.
But patients do not always receive the follow-up care they need, some studies have found.
Right drugs at the right time
Kelly Axsom, MD, a cardiologist at the Columbia University Medical Center, New York, and director of the centralized heart failure management program at the New York–Presbyterian Hospital System, called the primary care clinician the “captain of the ship,” ensuring that medications are reconciled and providing education about what to eat after discharge.
“It’s actually pretty complicated to go from being in the hospital to being at home,” Dr. Axsom said. “There are often many medication changes, there are lots of instructions that are told to you as a patient that are hard to remember.”
A patient’s weight might fluctuate in the days following discharge because the dose of diuretics might be too low or too high and need to be adjusted, according to Ishani Ganguli, MD, MPH, an assistant professor of medicine and a general internist in the Division of General Internal Medicine and Primary Care at Harvard Medical School and Brigham and Women’s Hospital, Boston.
K. Melissa Hayes, DNP, ANP-BC, CHFN, an assistant professor in the adult gerontology primary care program at the Vanderbilt University School of Nursing, Nashville, Tenn., recalled one patient who was given a months’ worth of medications following his discharge from the hospital.
“He was given expensive medications he couldn’t afford and not any refills or how to get those medications,” Dr. Hayes said.
Sometimes patients have no way to get to the pharmacy, or their pharmacy doesn’t have the medication they need, or their insurance doesn’t cover the drugs.
“The average patient is on at least six medications for heart failure, maybe even seven, and then that’s not including all their other medications,” Dr. Hayes said. “That can be a lot for people to keep up with.”
Dr. Hayes talks to her patients with heart failure about what drugs they have been prescribed and what medications they require more of, and she deprescribes any that are duplicative.
Helping patients understand why they are taking each drug encourages them to stick to the regimen. Diuretics, for example, can lead to frequent urination. If patients are unable to take regular bathroom breaks, they may be tempted to stop using the medication – a potentially catastrophic mistake.
“Often I have patients say, ‘Nobody ever explained it to me that way,’ ” Dr. Hayes said. “Someone can have a PhD but not understand their medications.”
Clinicians also can alert patients to commonly used medications that can worsen heart failure, such as diabetes drugs and over-the-counter medications such as ibuprofen.
Patients should be prescribed a combination of four recommended medications. But several studies have found that clinicians often fail to achieve the target doses for those medications. The use of guideline-directed medications reduces mortality and hospitalization rates, according to multiple clinical trials.
Eyes and ears on the patient
Once home, patients must stick to the right diet, weigh themselves every day, and monitor their blood pressure. But changing behaviors can be a struggle.
“Being seen quickly within a couple of days of discharge, you can catch things,” said Dr. Hayes, who has edited a book on managing patients with heart failure in primary care.
“It’s an opportunity to see how they’re doing at home, make sure they have their medications, make sure there’s been no misunderstanding or miscommunication about what they’re supposed to be doing at home,” says Marc Itskowitz, MD, a primary care physician affiliated with Allegheny General Hospital, Pittsburgh.
Ideally, a record that readily integrates information from wearables – such as blood pressure and weight – would make it easier to spot abnormalities, Dr. Itskowtiz said. “I think we’re still in the infancy of the electronic health record,” he said.
Ensuring that follow-up visits are as accessible as possible for patients is also important. Telehealth makes it easier for patients after they return home from the hospital, Dr. Itskowitz said.
More infrastructure
Another challenge of providing follow-up care for patients with heart failure is completing all the tasks a clinician must do within a 20-minute visit: an examination; education on the condition and medications; counseling on diet and exercise; coordination of medical equipment, such as a blood pressure cuff for home use; and making appointments with specialists.
“In the current system, additional support for primary care is needed so we can do all this,” Dr. Sterling said.
Staff at primary care clinics should be trained to answer calls from patients when they experience changes in their weight or are worried about other potential problems. “A lot of primary care practices are bare bones,” Dr. Hayes said, meaning they might not have the staff to field those calls. Educating patients as to when they should call their physician, especially after experiencing worsening symptoms, is also important.
Dr. Hayes suggests setting aside time in the schedule each week to see patients who have been recently discharged from the hospital. In the Cardiology and Vascular Clinic at Nashville General Hospital, Tenn., where she spends half a day each week, Dr. Hayes requests 30 minutes to see patients who have recently been discharged from hospital.
Even when the process goes smoothly, some patients will return to the hospital because of the progressive nature of heart failure, according to Dr. Hayes. Improving care following their hospitalization can keep these people from rapidly declining.
“Most patients with heart failure want to be taking care of the grandchildren or be able to enjoy family dinners together,” Dr. Axsom said. “I think anything we can do to help improve their quality of life is really important.”
Take-home
- See heart failure patients early after their discharge from hospital, ideally within 7-10 days.
- Make sure patients have access to the right medications at the right dosages and that they know why they’re taking them.
- Educate patients about the diet they should be following.
- Have a system to monitor patients’ symptoms and let them know when they should call.
A version of this article first appeared on Medscape.com.
Madeline Sterling, MD, knew something was wrong when she heard her patient’s voice on the phone. The patient was breathing too fast and sounded fatigued. Like many people with heart failure, this patient had several comorbidities: diabetes, high blood pressure, and cancer, which was in remission.
The patient had been in and out of the hospital several times and was afraid of going back, but Dr. Sterling, a primary care physician, advised her that it was the safe thing to do.
During the woman’s stay, the inpatient cardiology team called Dr. Sterling to provide status updates and ask for input. When the patient was discharged, Dr. Sterling received information on what medicines had been changed and scheduled follow-up care within 10 days. Dr. Sterling, who’d cared for the woman for many years, called her family, her home health aide, and another caregiver to discuss the plan.
“When you know these patients really well, it’s helpful,” Dr. Sterling, a professor of medicine at Weill Cornell Medicine, New York, said. Primary care clinicians have “an appreciation for how all these conditions fit together, how the medicines fit together, and how to put that patient’s priorities at the front of the equation.”
Research has shown that follow-up care within 7-10 days after discharge, especially for patients with heart failure, can prevent hospital readmissions. Patients’ health can change rapidly following discharge: They may start retaining fluid or may not know how to maintain a low-sodium diet, or they might have trouble obtaining medication. Primary care clinicians spot these early warning signs in follow-up visits.
Heart failure affects more than 6 million adults in the United States, according to the Centers for Disease Control and Prevention. The condition is a common cause of hospital readmissions within 30 days of discharge, according to research published by the American Heart Association.
Patients with heart failure are particularly challenging to care for because of comorbidities.
“They’re a very, very sick group of patients that are very difficult to manage,” said Noah Moss, MD, an advanced heart failure and transplant cardiologist at Mount Sinai Hospital, New York.
But patients do not always receive the follow-up care they need, some studies have found.
Right drugs at the right time
Kelly Axsom, MD, a cardiologist at the Columbia University Medical Center, New York, and director of the centralized heart failure management program at the New York–Presbyterian Hospital System, called the primary care clinician the “captain of the ship,” ensuring that medications are reconciled and providing education about what to eat after discharge.
“It’s actually pretty complicated to go from being in the hospital to being at home,” Dr. Axsom said. “There are often many medication changes, there are lots of instructions that are told to you as a patient that are hard to remember.”
A patient’s weight might fluctuate in the days following discharge because the dose of diuretics might be too low or too high and need to be adjusted, according to Ishani Ganguli, MD, MPH, an assistant professor of medicine and a general internist in the Division of General Internal Medicine and Primary Care at Harvard Medical School and Brigham and Women’s Hospital, Boston.
K. Melissa Hayes, DNP, ANP-BC, CHFN, an assistant professor in the adult gerontology primary care program at the Vanderbilt University School of Nursing, Nashville, Tenn., recalled one patient who was given a months’ worth of medications following his discharge from the hospital.
“He was given expensive medications he couldn’t afford and not any refills or how to get those medications,” Dr. Hayes said.
Sometimes patients have no way to get to the pharmacy, or their pharmacy doesn’t have the medication they need, or their insurance doesn’t cover the drugs.
“The average patient is on at least six medications for heart failure, maybe even seven, and then that’s not including all their other medications,” Dr. Hayes said. “That can be a lot for people to keep up with.”
Dr. Hayes talks to her patients with heart failure about what drugs they have been prescribed and what medications they require more of, and she deprescribes any that are duplicative.
Helping patients understand why they are taking each drug encourages them to stick to the regimen. Diuretics, for example, can lead to frequent urination. If patients are unable to take regular bathroom breaks, they may be tempted to stop using the medication – a potentially catastrophic mistake.
“Often I have patients say, ‘Nobody ever explained it to me that way,’ ” Dr. Hayes said. “Someone can have a PhD but not understand their medications.”
Clinicians also can alert patients to commonly used medications that can worsen heart failure, such as diabetes drugs and over-the-counter medications such as ibuprofen.
Patients should be prescribed a combination of four recommended medications. But several studies have found that clinicians often fail to achieve the target doses for those medications. The use of guideline-directed medications reduces mortality and hospitalization rates, according to multiple clinical trials.
Eyes and ears on the patient
Once home, patients must stick to the right diet, weigh themselves every day, and monitor their blood pressure. But changing behaviors can be a struggle.
“Being seen quickly within a couple of days of discharge, you can catch things,” said Dr. Hayes, who has edited a book on managing patients with heart failure in primary care.
“It’s an opportunity to see how they’re doing at home, make sure they have their medications, make sure there’s been no misunderstanding or miscommunication about what they’re supposed to be doing at home,” says Marc Itskowitz, MD, a primary care physician affiliated with Allegheny General Hospital, Pittsburgh.
Ideally, a record that readily integrates information from wearables – such as blood pressure and weight – would make it easier to spot abnormalities, Dr. Itskowtiz said. “I think we’re still in the infancy of the electronic health record,” he said.
Ensuring that follow-up visits are as accessible as possible for patients is also important. Telehealth makes it easier for patients after they return home from the hospital, Dr. Itskowitz said.
More infrastructure
Another challenge of providing follow-up care for patients with heart failure is completing all the tasks a clinician must do within a 20-minute visit: an examination; education on the condition and medications; counseling on diet and exercise; coordination of medical equipment, such as a blood pressure cuff for home use; and making appointments with specialists.
“In the current system, additional support for primary care is needed so we can do all this,” Dr. Sterling said.
Staff at primary care clinics should be trained to answer calls from patients when they experience changes in their weight or are worried about other potential problems. “A lot of primary care practices are bare bones,” Dr. Hayes said, meaning they might not have the staff to field those calls. Educating patients as to when they should call their physician, especially after experiencing worsening symptoms, is also important.
Dr. Hayes suggests setting aside time in the schedule each week to see patients who have been recently discharged from the hospital. In the Cardiology and Vascular Clinic at Nashville General Hospital, Tenn., where she spends half a day each week, Dr. Hayes requests 30 minutes to see patients who have recently been discharged from hospital.
Even when the process goes smoothly, some patients will return to the hospital because of the progressive nature of heart failure, according to Dr. Hayes. Improving care following their hospitalization can keep these people from rapidly declining.
“Most patients with heart failure want to be taking care of the grandchildren or be able to enjoy family dinners together,” Dr. Axsom said. “I think anything we can do to help improve their quality of life is really important.”
Take-home
- See heart failure patients early after their discharge from hospital, ideally within 7-10 days.
- Make sure patients have access to the right medications at the right dosages and that they know why they’re taking them.
- Educate patients about the diet they should be following.
- Have a system to monitor patients’ symptoms and let them know when they should call.
A version of this article first appeared on Medscape.com.
Madeline Sterling, MD, knew something was wrong when she heard her patient’s voice on the phone. The patient was breathing too fast and sounded fatigued. Like many people with heart failure, this patient had several comorbidities: diabetes, high blood pressure, and cancer, which was in remission.
The patient had been in and out of the hospital several times and was afraid of going back, but Dr. Sterling, a primary care physician, advised her that it was the safe thing to do.
During the woman’s stay, the inpatient cardiology team called Dr. Sterling to provide status updates and ask for input. When the patient was discharged, Dr. Sterling received information on what medicines had been changed and scheduled follow-up care within 10 days. Dr. Sterling, who’d cared for the woman for many years, called her family, her home health aide, and another caregiver to discuss the plan.
“When you know these patients really well, it’s helpful,” Dr. Sterling, a professor of medicine at Weill Cornell Medicine, New York, said. Primary care clinicians have “an appreciation for how all these conditions fit together, how the medicines fit together, and how to put that patient’s priorities at the front of the equation.”
Research has shown that follow-up care within 7-10 days after discharge, especially for patients with heart failure, can prevent hospital readmissions. Patients’ health can change rapidly following discharge: They may start retaining fluid or may not know how to maintain a low-sodium diet, or they might have trouble obtaining medication. Primary care clinicians spot these early warning signs in follow-up visits.
Heart failure affects more than 6 million adults in the United States, according to the Centers for Disease Control and Prevention. The condition is a common cause of hospital readmissions within 30 days of discharge, according to research published by the American Heart Association.
Patients with heart failure are particularly challenging to care for because of comorbidities.
“They’re a very, very sick group of patients that are very difficult to manage,” said Noah Moss, MD, an advanced heart failure and transplant cardiologist at Mount Sinai Hospital, New York.
But patients do not always receive the follow-up care they need, some studies have found.
Right drugs at the right time
Kelly Axsom, MD, a cardiologist at the Columbia University Medical Center, New York, and director of the centralized heart failure management program at the New York–Presbyterian Hospital System, called the primary care clinician the “captain of the ship,” ensuring that medications are reconciled and providing education about what to eat after discharge.
“It’s actually pretty complicated to go from being in the hospital to being at home,” Dr. Axsom said. “There are often many medication changes, there are lots of instructions that are told to you as a patient that are hard to remember.”
A patient’s weight might fluctuate in the days following discharge because the dose of diuretics might be too low or too high and need to be adjusted, according to Ishani Ganguli, MD, MPH, an assistant professor of medicine and a general internist in the Division of General Internal Medicine and Primary Care at Harvard Medical School and Brigham and Women’s Hospital, Boston.
K. Melissa Hayes, DNP, ANP-BC, CHFN, an assistant professor in the adult gerontology primary care program at the Vanderbilt University School of Nursing, Nashville, Tenn., recalled one patient who was given a months’ worth of medications following his discharge from the hospital.
“He was given expensive medications he couldn’t afford and not any refills or how to get those medications,” Dr. Hayes said.
Sometimes patients have no way to get to the pharmacy, or their pharmacy doesn’t have the medication they need, or their insurance doesn’t cover the drugs.
“The average patient is on at least six medications for heart failure, maybe even seven, and then that’s not including all their other medications,” Dr. Hayes said. “That can be a lot for people to keep up with.”
Dr. Hayes talks to her patients with heart failure about what drugs they have been prescribed and what medications they require more of, and she deprescribes any that are duplicative.
Helping patients understand why they are taking each drug encourages them to stick to the regimen. Diuretics, for example, can lead to frequent urination. If patients are unable to take regular bathroom breaks, they may be tempted to stop using the medication – a potentially catastrophic mistake.
“Often I have patients say, ‘Nobody ever explained it to me that way,’ ” Dr. Hayes said. “Someone can have a PhD but not understand their medications.”
Clinicians also can alert patients to commonly used medications that can worsen heart failure, such as diabetes drugs and over-the-counter medications such as ibuprofen.
Patients should be prescribed a combination of four recommended medications. But several studies have found that clinicians often fail to achieve the target doses for those medications. The use of guideline-directed medications reduces mortality and hospitalization rates, according to multiple clinical trials.
Eyes and ears on the patient
Once home, patients must stick to the right diet, weigh themselves every day, and monitor their blood pressure. But changing behaviors can be a struggle.
“Being seen quickly within a couple of days of discharge, you can catch things,” said Dr. Hayes, who has edited a book on managing patients with heart failure in primary care.
“It’s an opportunity to see how they’re doing at home, make sure they have their medications, make sure there’s been no misunderstanding or miscommunication about what they’re supposed to be doing at home,” says Marc Itskowitz, MD, a primary care physician affiliated with Allegheny General Hospital, Pittsburgh.
Ideally, a record that readily integrates information from wearables – such as blood pressure and weight – would make it easier to spot abnormalities, Dr. Itskowtiz said. “I think we’re still in the infancy of the electronic health record,” he said.
Ensuring that follow-up visits are as accessible as possible for patients is also important. Telehealth makes it easier for patients after they return home from the hospital, Dr. Itskowitz said.
More infrastructure
Another challenge of providing follow-up care for patients with heart failure is completing all the tasks a clinician must do within a 20-minute visit: an examination; education on the condition and medications; counseling on diet and exercise; coordination of medical equipment, such as a blood pressure cuff for home use; and making appointments with specialists.
“In the current system, additional support for primary care is needed so we can do all this,” Dr. Sterling said.
Staff at primary care clinics should be trained to answer calls from patients when they experience changes in their weight or are worried about other potential problems. “A lot of primary care practices are bare bones,” Dr. Hayes said, meaning they might not have the staff to field those calls. Educating patients as to when they should call their physician, especially after experiencing worsening symptoms, is also important.
Dr. Hayes suggests setting aside time in the schedule each week to see patients who have been recently discharged from the hospital. In the Cardiology and Vascular Clinic at Nashville General Hospital, Tenn., where she spends half a day each week, Dr. Hayes requests 30 minutes to see patients who have recently been discharged from hospital.
Even when the process goes smoothly, some patients will return to the hospital because of the progressive nature of heart failure, according to Dr. Hayes. Improving care following their hospitalization can keep these people from rapidly declining.
“Most patients with heart failure want to be taking care of the grandchildren or be able to enjoy family dinners together,” Dr. Axsom said. “I think anything we can do to help improve their quality of life is really important.”
Take-home
- See heart failure patients early after their discharge from hospital, ideally within 7-10 days.
- Make sure patients have access to the right medications at the right dosages and that they know why they’re taking them.
- Educate patients about the diet they should be following.
- Have a system to monitor patients’ symptoms and let them know when they should call.
A version of this article first appeared on Medscape.com.
New definition for iron deficiency in CV disease proposed
with implications that may extend to cardiovascular disease in general.
In the study involving more than 900 patients with PH, investigators at seven U.S. centers determined the prevalence of iron deficiency by two separate definitions and assessed its associations with functional measures and quality of life (QoL) scores.
An iron deficiency definition used conventionally in heart failure (HF) – ferritin less than 100 g/mL or 100-299 ng/mL with transferrin saturation (TSAT) less than 20% – failed to discriminate patients with reduced peak oxygen consumption (peakVO2), 6-minute walk test (6MWT) results, and QoL scores on the 36-item Short Form Survey (SF-36).
But an alternative definition for iron deficiency, simply a TSAT less than 21%, did predict such patients with reduced peakVO2, 6MWT, and QoL. It was also associated with an increased mortality risk. The study was published in the European Heart Journal.
“A low TSAT, less than 21%, is key in the pathophysiology of iron deficiency in pulmonary hypertension” and is associated with those important clinical and functional characteristics, lead author Pieter Martens MD, PhD, said in an interview. The study “underscores the importance of these criteria in future intervention studies in the field of pulmonary hypertension testing iron therapies.”
A broader implication is that “we should revise how we define iron deficiency in heart failure and cardiovascular disease in general and how we select patients for iron therapies,” said Dr. Martens, of the Heart, Vascular & Thoracic Institute of the Cleveland Clinic.
Iron’s role in pulmonary vascular disease
“Iron deficiency is associated with an energetic deficit, especially in high energy–demanding tissue, leading to early skeletal muscle acidification and diminished left and right ventricular (RV) contractile reserve during exercise,” the published report states. It can lead to “maladaptive RV remodeling,” which is a “hallmark feature” predictive of morbidity and mortality in patients with pulmonary vascular disease (PVD).
Some studies have suggested that iron deficiency is a common comorbidity in patients with PVD, their estimates of its prevalence ranging widely due in part to the “absence of a uniform definition,” write the authors.
Dr. Martens said the current study was conducted partly in response to the increasingly common observation that the HF-associated definition of iron deficiency “has limitations.” Yet, “without validation in the field of pulmonary hypertension, the 2022 pulmonary hypertension guidelines endorse this definition.”
As iron deficiency is a causal risk factor for HF progression, Dr. Martens added, the HF field has “taught us the importance of using validated definitions for iron deficiency when selecting patients for iron treatment in randomized controlled trials.”
Moreover, some evidence suggests that iron deficiency by some definitions may be associated with diminished exercise capacity and QoL in patients with PVD, which are associations that have not been confirmed in large studies, the report notes.
Therefore, it continues, the study sought to “determine and validate” the optimal definition of iron deficiency in patients with PVD; document its prevalence; and explore associations between iron deficiency and exercise capacity, QoL, and cardiac and pulmonary vascular remodeling.
Evaluating definitions of iron deficiency
The prospective study, called PVDOMICS, entered 1,195 subjects with available iron levels. After exclusion of 38 patients with sarcoidosis, myeloproliferative disease, or hemoglobinopathy, there remained 693 patients with “overt” PH, 225 with a milder form of PH who served as PVD comparators, and 90 age-, sex-, race/ethnicity- matched “healthy” adults who served as controls.
According to the conventional HF definition of iron deficiency – that is, ferritin 100-299 ng/mL and TSAT less than 20% – the prevalences were 74% in patients with overt PH and 72% of those “across the PVD spectrum.”
But by that definition, iron deficient and non-iron deficient patients didn’t differ significantly in peakVO2, 6MWT distance, or SF-36 physical component scores.
In contrast, patients meeting the alternative definition of iron deficiency of TSAT less than 21% showed significantly reduced functional and QoL measures, compared with those with TSAT greater than or equal to 21%.
The group with TSAT less than 21% also showed significantly more RV remodeling at cardiac MRI, compared with those who had TSAT greater than or equal to 21%, but their invasively measured pulmonary vascular resistance was comparable.
Of note, those with TSAT less than 21% also showed significantly increased all-cause mortality (hazard ratio, 1.63; 95% confidence interval, 1.13-2.34; P = .009) after adjustment for age, sex, hemoglobin, and natriuretic peptide levels.
“Proper validation of the definition of iron deficiency is important for prognostication,” the published report states, “but also for providing a working definition that can be used to identify suitable patients for inclusion in randomized controlled trials” of drugs for iron deficiency.
Additionally, the finding that TSAT less than 21% points to patients with diminished functional and exercise capacity is “consistent with more recent studies in the field of heart failure” that suggest “functional abnormalities and adverse cardiac remodeling are worse in patients with a low TSAT.” Indeed, the report states, such treatment effects have been “the most convincing” in HF trials.
Broader implications
An accompanying editorial agrees that the study’s implications apply well beyond PH. It highlights that iron deficiency is common in PH, while such PH is “not substantially different from the problem in patients with heart failure, chronic kidney disease, and cardiovascular disease in general,” lead editorialist John G.F. Cleland, MD, PhD, University of Glasgow, said in an interview. “It’s also common as people get older, even in those without these diseases.”
Dr. Cleland said the anemia definition currently used in cardiovascular research and practice is based on a hemoglobin concentration below the 5th percentile of age and sex in primarily young, healthy people, and not on its association with clinical outcomes.
“We recently analyzed data on a large population in the United Kingdom with a broad range of cardiovascular diseases and found that unless anemia is severe, [other] markers of iron deficiency are usually not measured,” he said. A low hemoglobin and TSAT, but not low ferritin levels, are associated with worse prognosis.
Dr. Cleland agreed that the HF-oriented definition is “poor,” with profound implications for the conduct of clinical trials. “If the definition of iron deficiency lacks specificity, then clinical trials will include many patients without iron deficiency who are unlikely to benefit from and might be harmed by IV iron.” Inclusion of such patients may also “dilute” any benefit that might emerge and render the outcome inaccurate.
But if the definition of iron deficiency lacks sensitivity, “then in clinical practice, many patients with iron deficiency may be denied a simple and effective treatment.”
Measuring serum iron could potentially be useful, but it’s usually not done in randomized trials “especially since taking an iron tablet can give a temporary ‘blip’ in serum iron,” Dr. Cleland said. “So TSAT is a reasonable compromise.” He said he “looks forward” to any further data on serum iron as a way of assessing iron deficiency and anemia.
Half full vs. half empty
Dr. Cleland likened the question of whom to treat with iron supplementation as a “glass half full versus half empty” clinical dilemma. “One approach is to give iron to everyone unless there’s evidence that they’re overloaded,” he said, “while the other is to withhold iron from everyone unless there’s evidence that they’re iron depleted.”
Recent evidence from the IRONMAN trial suggested that its patients with HF who received intravenous iron were less likely to be hospitalized for infections, particularly COVID-19, than a usual-care group. The treatment may also help reduce frailty.
“So should we be offering IV iron specifically to people considered iron deficient, or should we be ensuring that everyone over age 70 get iron supplements?” Dr. Cleland mused rhetorically. On a cautionary note, he added, perhaps iron supplementation will be harmful if it’s not necessary.
Dr. Cleland proposed “focusing for the moment on people who are iron deficient but investigating the possibility that we are being overly restrictive and should be giving iron to a much broader population.” That course, however, would require large population-based studies.
“We need more experience,” Dr. Cleland said, “to make sure that the benefits outweigh any risks before we can just give iron to everyone.”
Dr. Martens has received consultancy fees from AstraZeneca, Abbott, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Novartis, Novo Nordisk, and Vifor Pharma. Dr. Cleland declares grant support, support for travel, and personal honoraria from Pharmacosmos and Vifor. Disclosures for other authors are in the published report and editorial.
A version of this article first appeared on Medscape.com.
with implications that may extend to cardiovascular disease in general.
In the study involving more than 900 patients with PH, investigators at seven U.S. centers determined the prevalence of iron deficiency by two separate definitions and assessed its associations with functional measures and quality of life (QoL) scores.
An iron deficiency definition used conventionally in heart failure (HF) – ferritin less than 100 g/mL or 100-299 ng/mL with transferrin saturation (TSAT) less than 20% – failed to discriminate patients with reduced peak oxygen consumption (peakVO2), 6-minute walk test (6MWT) results, and QoL scores on the 36-item Short Form Survey (SF-36).
But an alternative definition for iron deficiency, simply a TSAT less than 21%, did predict such patients with reduced peakVO2, 6MWT, and QoL. It was also associated with an increased mortality risk. The study was published in the European Heart Journal.
“A low TSAT, less than 21%, is key in the pathophysiology of iron deficiency in pulmonary hypertension” and is associated with those important clinical and functional characteristics, lead author Pieter Martens MD, PhD, said in an interview. The study “underscores the importance of these criteria in future intervention studies in the field of pulmonary hypertension testing iron therapies.”
A broader implication is that “we should revise how we define iron deficiency in heart failure and cardiovascular disease in general and how we select patients for iron therapies,” said Dr. Martens, of the Heart, Vascular & Thoracic Institute of the Cleveland Clinic.
Iron’s role in pulmonary vascular disease
“Iron deficiency is associated with an energetic deficit, especially in high energy–demanding tissue, leading to early skeletal muscle acidification and diminished left and right ventricular (RV) contractile reserve during exercise,” the published report states. It can lead to “maladaptive RV remodeling,” which is a “hallmark feature” predictive of morbidity and mortality in patients with pulmonary vascular disease (PVD).
Some studies have suggested that iron deficiency is a common comorbidity in patients with PVD, their estimates of its prevalence ranging widely due in part to the “absence of a uniform definition,” write the authors.
Dr. Martens said the current study was conducted partly in response to the increasingly common observation that the HF-associated definition of iron deficiency “has limitations.” Yet, “without validation in the field of pulmonary hypertension, the 2022 pulmonary hypertension guidelines endorse this definition.”
As iron deficiency is a causal risk factor for HF progression, Dr. Martens added, the HF field has “taught us the importance of using validated definitions for iron deficiency when selecting patients for iron treatment in randomized controlled trials.”
Moreover, some evidence suggests that iron deficiency by some definitions may be associated with diminished exercise capacity and QoL in patients with PVD, which are associations that have not been confirmed in large studies, the report notes.
Therefore, it continues, the study sought to “determine and validate” the optimal definition of iron deficiency in patients with PVD; document its prevalence; and explore associations between iron deficiency and exercise capacity, QoL, and cardiac and pulmonary vascular remodeling.
Evaluating definitions of iron deficiency
The prospective study, called PVDOMICS, entered 1,195 subjects with available iron levels. After exclusion of 38 patients with sarcoidosis, myeloproliferative disease, or hemoglobinopathy, there remained 693 patients with “overt” PH, 225 with a milder form of PH who served as PVD comparators, and 90 age-, sex-, race/ethnicity- matched “healthy” adults who served as controls.
According to the conventional HF definition of iron deficiency – that is, ferritin 100-299 ng/mL and TSAT less than 20% – the prevalences were 74% in patients with overt PH and 72% of those “across the PVD spectrum.”
But by that definition, iron deficient and non-iron deficient patients didn’t differ significantly in peakVO2, 6MWT distance, or SF-36 physical component scores.
In contrast, patients meeting the alternative definition of iron deficiency of TSAT less than 21% showed significantly reduced functional and QoL measures, compared with those with TSAT greater than or equal to 21%.
The group with TSAT less than 21% also showed significantly more RV remodeling at cardiac MRI, compared with those who had TSAT greater than or equal to 21%, but their invasively measured pulmonary vascular resistance was comparable.
Of note, those with TSAT less than 21% also showed significantly increased all-cause mortality (hazard ratio, 1.63; 95% confidence interval, 1.13-2.34; P = .009) after adjustment for age, sex, hemoglobin, and natriuretic peptide levels.
“Proper validation of the definition of iron deficiency is important for prognostication,” the published report states, “but also for providing a working definition that can be used to identify suitable patients for inclusion in randomized controlled trials” of drugs for iron deficiency.
Additionally, the finding that TSAT less than 21% points to patients with diminished functional and exercise capacity is “consistent with more recent studies in the field of heart failure” that suggest “functional abnormalities and adverse cardiac remodeling are worse in patients with a low TSAT.” Indeed, the report states, such treatment effects have been “the most convincing” in HF trials.
Broader implications
An accompanying editorial agrees that the study’s implications apply well beyond PH. It highlights that iron deficiency is common in PH, while such PH is “not substantially different from the problem in patients with heart failure, chronic kidney disease, and cardiovascular disease in general,” lead editorialist John G.F. Cleland, MD, PhD, University of Glasgow, said in an interview. “It’s also common as people get older, even in those without these diseases.”
Dr. Cleland said the anemia definition currently used in cardiovascular research and practice is based on a hemoglobin concentration below the 5th percentile of age and sex in primarily young, healthy people, and not on its association with clinical outcomes.
“We recently analyzed data on a large population in the United Kingdom with a broad range of cardiovascular diseases and found that unless anemia is severe, [other] markers of iron deficiency are usually not measured,” he said. A low hemoglobin and TSAT, but not low ferritin levels, are associated with worse prognosis.
Dr. Cleland agreed that the HF-oriented definition is “poor,” with profound implications for the conduct of clinical trials. “If the definition of iron deficiency lacks specificity, then clinical trials will include many patients without iron deficiency who are unlikely to benefit from and might be harmed by IV iron.” Inclusion of such patients may also “dilute” any benefit that might emerge and render the outcome inaccurate.
But if the definition of iron deficiency lacks sensitivity, “then in clinical practice, many patients with iron deficiency may be denied a simple and effective treatment.”
Measuring serum iron could potentially be useful, but it’s usually not done in randomized trials “especially since taking an iron tablet can give a temporary ‘blip’ in serum iron,” Dr. Cleland said. “So TSAT is a reasonable compromise.” He said he “looks forward” to any further data on serum iron as a way of assessing iron deficiency and anemia.
Half full vs. half empty
Dr. Cleland likened the question of whom to treat with iron supplementation as a “glass half full versus half empty” clinical dilemma. “One approach is to give iron to everyone unless there’s evidence that they’re overloaded,” he said, “while the other is to withhold iron from everyone unless there’s evidence that they’re iron depleted.”
Recent evidence from the IRONMAN trial suggested that its patients with HF who received intravenous iron were less likely to be hospitalized for infections, particularly COVID-19, than a usual-care group. The treatment may also help reduce frailty.
“So should we be offering IV iron specifically to people considered iron deficient, or should we be ensuring that everyone over age 70 get iron supplements?” Dr. Cleland mused rhetorically. On a cautionary note, he added, perhaps iron supplementation will be harmful if it’s not necessary.
Dr. Cleland proposed “focusing for the moment on people who are iron deficient but investigating the possibility that we are being overly restrictive and should be giving iron to a much broader population.” That course, however, would require large population-based studies.
“We need more experience,” Dr. Cleland said, “to make sure that the benefits outweigh any risks before we can just give iron to everyone.”
Dr. Martens has received consultancy fees from AstraZeneca, Abbott, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Novartis, Novo Nordisk, and Vifor Pharma. Dr. Cleland declares grant support, support for travel, and personal honoraria from Pharmacosmos and Vifor. Disclosures for other authors are in the published report and editorial.
A version of this article first appeared on Medscape.com.
with implications that may extend to cardiovascular disease in general.
In the study involving more than 900 patients with PH, investigators at seven U.S. centers determined the prevalence of iron deficiency by two separate definitions and assessed its associations with functional measures and quality of life (QoL) scores.
An iron deficiency definition used conventionally in heart failure (HF) – ferritin less than 100 g/mL or 100-299 ng/mL with transferrin saturation (TSAT) less than 20% – failed to discriminate patients with reduced peak oxygen consumption (peakVO2), 6-minute walk test (6MWT) results, and QoL scores on the 36-item Short Form Survey (SF-36).
But an alternative definition for iron deficiency, simply a TSAT less than 21%, did predict such patients with reduced peakVO2, 6MWT, and QoL. It was also associated with an increased mortality risk. The study was published in the European Heart Journal.
“A low TSAT, less than 21%, is key in the pathophysiology of iron deficiency in pulmonary hypertension” and is associated with those important clinical and functional characteristics, lead author Pieter Martens MD, PhD, said in an interview. The study “underscores the importance of these criteria in future intervention studies in the field of pulmonary hypertension testing iron therapies.”
A broader implication is that “we should revise how we define iron deficiency in heart failure and cardiovascular disease in general and how we select patients for iron therapies,” said Dr. Martens, of the Heart, Vascular & Thoracic Institute of the Cleveland Clinic.
Iron’s role in pulmonary vascular disease
“Iron deficiency is associated with an energetic deficit, especially in high energy–demanding tissue, leading to early skeletal muscle acidification and diminished left and right ventricular (RV) contractile reserve during exercise,” the published report states. It can lead to “maladaptive RV remodeling,” which is a “hallmark feature” predictive of morbidity and mortality in patients with pulmonary vascular disease (PVD).
Some studies have suggested that iron deficiency is a common comorbidity in patients with PVD, their estimates of its prevalence ranging widely due in part to the “absence of a uniform definition,” write the authors.
Dr. Martens said the current study was conducted partly in response to the increasingly common observation that the HF-associated definition of iron deficiency “has limitations.” Yet, “without validation in the field of pulmonary hypertension, the 2022 pulmonary hypertension guidelines endorse this definition.”
As iron deficiency is a causal risk factor for HF progression, Dr. Martens added, the HF field has “taught us the importance of using validated definitions for iron deficiency when selecting patients for iron treatment in randomized controlled trials.”
Moreover, some evidence suggests that iron deficiency by some definitions may be associated with diminished exercise capacity and QoL in patients with PVD, which are associations that have not been confirmed in large studies, the report notes.
Therefore, it continues, the study sought to “determine and validate” the optimal definition of iron deficiency in patients with PVD; document its prevalence; and explore associations between iron deficiency and exercise capacity, QoL, and cardiac and pulmonary vascular remodeling.
Evaluating definitions of iron deficiency
The prospective study, called PVDOMICS, entered 1,195 subjects with available iron levels. After exclusion of 38 patients with sarcoidosis, myeloproliferative disease, or hemoglobinopathy, there remained 693 patients with “overt” PH, 225 with a milder form of PH who served as PVD comparators, and 90 age-, sex-, race/ethnicity- matched “healthy” adults who served as controls.
According to the conventional HF definition of iron deficiency – that is, ferritin 100-299 ng/mL and TSAT less than 20% – the prevalences were 74% in patients with overt PH and 72% of those “across the PVD spectrum.”
But by that definition, iron deficient and non-iron deficient patients didn’t differ significantly in peakVO2, 6MWT distance, or SF-36 physical component scores.
In contrast, patients meeting the alternative definition of iron deficiency of TSAT less than 21% showed significantly reduced functional and QoL measures, compared with those with TSAT greater than or equal to 21%.
The group with TSAT less than 21% also showed significantly more RV remodeling at cardiac MRI, compared with those who had TSAT greater than or equal to 21%, but their invasively measured pulmonary vascular resistance was comparable.
Of note, those with TSAT less than 21% also showed significantly increased all-cause mortality (hazard ratio, 1.63; 95% confidence interval, 1.13-2.34; P = .009) after adjustment for age, sex, hemoglobin, and natriuretic peptide levels.
“Proper validation of the definition of iron deficiency is important for prognostication,” the published report states, “but also for providing a working definition that can be used to identify suitable patients for inclusion in randomized controlled trials” of drugs for iron deficiency.
Additionally, the finding that TSAT less than 21% points to patients with diminished functional and exercise capacity is “consistent with more recent studies in the field of heart failure” that suggest “functional abnormalities and adverse cardiac remodeling are worse in patients with a low TSAT.” Indeed, the report states, such treatment effects have been “the most convincing” in HF trials.
Broader implications
An accompanying editorial agrees that the study’s implications apply well beyond PH. It highlights that iron deficiency is common in PH, while such PH is “not substantially different from the problem in patients with heart failure, chronic kidney disease, and cardiovascular disease in general,” lead editorialist John G.F. Cleland, MD, PhD, University of Glasgow, said in an interview. “It’s also common as people get older, even in those without these diseases.”
Dr. Cleland said the anemia definition currently used in cardiovascular research and practice is based on a hemoglobin concentration below the 5th percentile of age and sex in primarily young, healthy people, and not on its association with clinical outcomes.
“We recently analyzed data on a large population in the United Kingdom with a broad range of cardiovascular diseases and found that unless anemia is severe, [other] markers of iron deficiency are usually not measured,” he said. A low hemoglobin and TSAT, but not low ferritin levels, are associated with worse prognosis.
Dr. Cleland agreed that the HF-oriented definition is “poor,” with profound implications for the conduct of clinical trials. “If the definition of iron deficiency lacks specificity, then clinical trials will include many patients without iron deficiency who are unlikely to benefit from and might be harmed by IV iron.” Inclusion of such patients may also “dilute” any benefit that might emerge and render the outcome inaccurate.
But if the definition of iron deficiency lacks sensitivity, “then in clinical practice, many patients with iron deficiency may be denied a simple and effective treatment.”
Measuring serum iron could potentially be useful, but it’s usually not done in randomized trials “especially since taking an iron tablet can give a temporary ‘blip’ in serum iron,” Dr. Cleland said. “So TSAT is a reasonable compromise.” He said he “looks forward” to any further data on serum iron as a way of assessing iron deficiency and anemia.
Half full vs. half empty
Dr. Cleland likened the question of whom to treat with iron supplementation as a “glass half full versus half empty” clinical dilemma. “One approach is to give iron to everyone unless there’s evidence that they’re overloaded,” he said, “while the other is to withhold iron from everyone unless there’s evidence that they’re iron depleted.”
Recent evidence from the IRONMAN trial suggested that its patients with HF who received intravenous iron were less likely to be hospitalized for infections, particularly COVID-19, than a usual-care group. The treatment may also help reduce frailty.
“So should we be offering IV iron specifically to people considered iron deficient, or should we be ensuring that everyone over age 70 get iron supplements?” Dr. Cleland mused rhetorically. On a cautionary note, he added, perhaps iron supplementation will be harmful if it’s not necessary.
Dr. Cleland proposed “focusing for the moment on people who are iron deficient but investigating the possibility that we are being overly restrictive and should be giving iron to a much broader population.” That course, however, would require large population-based studies.
“We need more experience,” Dr. Cleland said, “to make sure that the benefits outweigh any risks before we can just give iron to everyone.”
Dr. Martens has received consultancy fees from AstraZeneca, Abbott, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Novartis, Novo Nordisk, and Vifor Pharma. Dr. Cleland declares grant support, support for travel, and personal honoraria from Pharmacosmos and Vifor. Disclosures for other authors are in the published report and editorial.
A version of this article first appeared on Medscape.com.
FROM EUROPEAN HEART JOURNAL
Starting indicated heart failure meds in-hospital: Progress, opportunities
Most patients aren’t receiving all the medications they should based on guidelines, nor are they getting them at the most effective time in their disease course, suggests a registry study of patients in the United States hospitalized with heart failure with reduced ejection fraction (HFrEF).
On average, one such medication was initiated per patient for every 6 days in the hospital.
Shortfalls in predischarge GDMT initiation disproportionately landed on women, patients at rural centers, and those with renal failure or other comorbidities. But they didn’t seem related to patient race or ethnicity in the study reported in JACC: Heart Failure.
The analysis covers the 3 years preceding the May 2020 first-time approval of a sodium-glucose cotransporter 2 (SGLT2) inhibitor for nondiabetic patients with HFrEF, and therefore doesn’t cover such drugs for that indication. The SGLT2 inhibitors would later join beta-blockers, renin-angiotensin system (RAS) inhibitors, and mineralocorticoid receptor antagonists (MRAs) in the quartet of core GDMT medications broadly indicated for HFrEF.
In-hospital initiation of GDMT for HFrEF is considered a predictor of being on those medications after discharge and is itself guideline recommended. There’s clear evidence that treatment with the four core medications boosts survival and cuts rehospitalization risk, and that “getting those on board as soon as possible will eventually benefit many patients,” Paul L. Hess, MD, MHS, said in an interview.
Dr. Hess, University of Colorado Anschutz Medical Campus, Aurora, is senior author on the report from the Get With The Guidelines–Heart Failure (GWTG-HF) quality improvement program of the American Heart Association.
Broad uptake of new medical therapies into practice may sometimes take 15 or more years from first publication, Dr. Hess said, so, “I find it encouraging in the study that over a shorter time period, 2017-2020, there was improvement.”
Indeed, the odds of in-hospital initiation of an indicated med during that period on average climbed a significant 8% every 3 months, the report states.
The finding suggests that “heart failure hospitalization is, in and of itself, an important intervention for getting folks on the appropriate medications,” Dr. Hess said. It also means “we’re getting better at it,” at least at the study’s 160 GWTG-HF participating hospitals nationwide.
Those centers, the report acknowledges, varied in size, geography, and teaching status but were not necessarily representative of all U.S. hospitals. In another potential limitation, the study couldn’t account for patients who weren’t prescribed all indicated medications for clinically valid reasons. It excluded patients with “clear contraindications,” Dr. Hess said. But there could have been “legitimate reasons” some indicated medications weren’t always prescribed, including patient frailty, hemodynamic intolerance, renal dysfunction, or polypharmacy concerns.
“Positive takeaways” from the analysis, notes an accompanying editorial, include improved prescription rates for key GDMT categories across more than 3 years of data, and evidence that in-hospital initiation “was feasible and, at least for some medications, reliably undertaken.”
Of note, new GDMT prescriptions from admission to discharge went from 70% to almost 98% for beta-blockers, from 59% to about 91% for RAS inhibitors, from about 26% to 56% for MRAs, and from 15.5% to 27.4% for hydralazine/nitrates, wrote Karen E. Joynt Maddox, MD, MPH, and Daniel K. Fox, MD, PhD, both of Washington University in St. Louis.
“Key areas for improvement,” they noted, include prescriptions for women, who were 12% less likely than men to have appropriate GDMT initiated during hospitalization (P < .001); and practice at rural hospitals, which were 40% less likely than urban centers to have patients on full GDMT by discharge (P = .017).
Although only 2.6% of the GWTG-HF centers were in rural locations, “rural hospitals make up approximately one-third of general acute care hospitals in this country,” the editorial states. They therefore “represent a key source of health disparity” in the United States in need of further study.
The analysis of 50,170 patients hospitalized with HFrEF compared the number of GDMT medications for which they were eligible, on at-hospital admission, and by discharge.
The drug categories included “evidence based beta blockers,” that is, bisoprolol, carvedilol, or sustained-release metoprolol; RAS inhibitors, specifically ACE inhibitors, angiotensin receptor blockers, or sacubitril/valsartan (Entresto); MRAs; SGLT2 inhibitors in patients with diabetes; diuretics for congestion; oral anticoagulants for atrial fibrillation; and hydralazine/nitrates in African Americans.
About 15% of the patients at hospital admission were on all indicated HFrEF medications for which they were eligible. The proportion more than doubled to 32.8% by discharge.
Factors significantly associated with reduced odds for in-hospital GDMT initiation include older age (odds ratio, 0.94 per 5-year increment), being female versus male (OR, 0.88), rural location (OR, 0.60), Medicaid versus Medicare or private insurance (OR, 0.93), stroke history (OR, 0.91), peripheral artery disease (OR, 0.93), chronic obstructive pulmonary disease or asthma (OR, 0.86), and renal insufficiency (OR, 0.77).
The findings suggest that there has been at least some progress in getting hospitalized patients “on the right meds” by discharge, Dr. Hess observed. To help address shortfalls in some patient groups, “there is interest in engaging pharmacists in helping us encourage providers on the front lines to initiate and titrate medications.”
The GWTG-HF program is sponsored, in part, by Novartis, Boehringer Ingelheim, Novo Nordisk, AstraZeneca, Bayer, Tylenol, and Alnylam Pharmaceuticals. Dr. Hess disclosed no relevant financial relationships. Dr. Maddox disclosed serving on the Health Policy Advisory Council for Centene. Dr. Fox reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most patients aren’t receiving all the medications they should based on guidelines, nor are they getting them at the most effective time in their disease course, suggests a registry study of patients in the United States hospitalized with heart failure with reduced ejection fraction (HFrEF).
On average, one such medication was initiated per patient for every 6 days in the hospital.
Shortfalls in predischarge GDMT initiation disproportionately landed on women, patients at rural centers, and those with renal failure or other comorbidities. But they didn’t seem related to patient race or ethnicity in the study reported in JACC: Heart Failure.
The analysis covers the 3 years preceding the May 2020 first-time approval of a sodium-glucose cotransporter 2 (SGLT2) inhibitor for nondiabetic patients with HFrEF, and therefore doesn’t cover such drugs for that indication. The SGLT2 inhibitors would later join beta-blockers, renin-angiotensin system (RAS) inhibitors, and mineralocorticoid receptor antagonists (MRAs) in the quartet of core GDMT medications broadly indicated for HFrEF.
In-hospital initiation of GDMT for HFrEF is considered a predictor of being on those medications after discharge and is itself guideline recommended. There’s clear evidence that treatment with the four core medications boosts survival and cuts rehospitalization risk, and that “getting those on board as soon as possible will eventually benefit many patients,” Paul L. Hess, MD, MHS, said in an interview.
Dr. Hess, University of Colorado Anschutz Medical Campus, Aurora, is senior author on the report from the Get With The Guidelines–Heart Failure (GWTG-HF) quality improvement program of the American Heart Association.
Broad uptake of new medical therapies into practice may sometimes take 15 or more years from first publication, Dr. Hess said, so, “I find it encouraging in the study that over a shorter time period, 2017-2020, there was improvement.”
Indeed, the odds of in-hospital initiation of an indicated med during that period on average climbed a significant 8% every 3 months, the report states.
The finding suggests that “heart failure hospitalization is, in and of itself, an important intervention for getting folks on the appropriate medications,” Dr. Hess said. It also means “we’re getting better at it,” at least at the study’s 160 GWTG-HF participating hospitals nationwide.
Those centers, the report acknowledges, varied in size, geography, and teaching status but were not necessarily representative of all U.S. hospitals. In another potential limitation, the study couldn’t account for patients who weren’t prescribed all indicated medications for clinically valid reasons. It excluded patients with “clear contraindications,” Dr. Hess said. But there could have been “legitimate reasons” some indicated medications weren’t always prescribed, including patient frailty, hemodynamic intolerance, renal dysfunction, or polypharmacy concerns.
“Positive takeaways” from the analysis, notes an accompanying editorial, include improved prescription rates for key GDMT categories across more than 3 years of data, and evidence that in-hospital initiation “was feasible and, at least for some medications, reliably undertaken.”
Of note, new GDMT prescriptions from admission to discharge went from 70% to almost 98% for beta-blockers, from 59% to about 91% for RAS inhibitors, from about 26% to 56% for MRAs, and from 15.5% to 27.4% for hydralazine/nitrates, wrote Karen E. Joynt Maddox, MD, MPH, and Daniel K. Fox, MD, PhD, both of Washington University in St. Louis.
“Key areas for improvement,” they noted, include prescriptions for women, who were 12% less likely than men to have appropriate GDMT initiated during hospitalization (P < .001); and practice at rural hospitals, which were 40% less likely than urban centers to have patients on full GDMT by discharge (P = .017).
Although only 2.6% of the GWTG-HF centers were in rural locations, “rural hospitals make up approximately one-third of general acute care hospitals in this country,” the editorial states. They therefore “represent a key source of health disparity” in the United States in need of further study.
The analysis of 50,170 patients hospitalized with HFrEF compared the number of GDMT medications for which they were eligible, on at-hospital admission, and by discharge.
The drug categories included “evidence based beta blockers,” that is, bisoprolol, carvedilol, or sustained-release metoprolol; RAS inhibitors, specifically ACE inhibitors, angiotensin receptor blockers, or sacubitril/valsartan (Entresto); MRAs; SGLT2 inhibitors in patients with diabetes; diuretics for congestion; oral anticoagulants for atrial fibrillation; and hydralazine/nitrates in African Americans.
About 15% of the patients at hospital admission were on all indicated HFrEF medications for which they were eligible. The proportion more than doubled to 32.8% by discharge.
Factors significantly associated with reduced odds for in-hospital GDMT initiation include older age (odds ratio, 0.94 per 5-year increment), being female versus male (OR, 0.88), rural location (OR, 0.60), Medicaid versus Medicare or private insurance (OR, 0.93), stroke history (OR, 0.91), peripheral artery disease (OR, 0.93), chronic obstructive pulmonary disease or asthma (OR, 0.86), and renal insufficiency (OR, 0.77).
The findings suggest that there has been at least some progress in getting hospitalized patients “on the right meds” by discharge, Dr. Hess observed. To help address shortfalls in some patient groups, “there is interest in engaging pharmacists in helping us encourage providers on the front lines to initiate and titrate medications.”
The GWTG-HF program is sponsored, in part, by Novartis, Boehringer Ingelheim, Novo Nordisk, AstraZeneca, Bayer, Tylenol, and Alnylam Pharmaceuticals. Dr. Hess disclosed no relevant financial relationships. Dr. Maddox disclosed serving on the Health Policy Advisory Council for Centene. Dr. Fox reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most patients aren’t receiving all the medications they should based on guidelines, nor are they getting them at the most effective time in their disease course, suggests a registry study of patients in the United States hospitalized with heart failure with reduced ejection fraction (HFrEF).
On average, one such medication was initiated per patient for every 6 days in the hospital.
Shortfalls in predischarge GDMT initiation disproportionately landed on women, patients at rural centers, and those with renal failure or other comorbidities. But they didn’t seem related to patient race or ethnicity in the study reported in JACC: Heart Failure.
The analysis covers the 3 years preceding the May 2020 first-time approval of a sodium-glucose cotransporter 2 (SGLT2) inhibitor for nondiabetic patients with HFrEF, and therefore doesn’t cover such drugs for that indication. The SGLT2 inhibitors would later join beta-blockers, renin-angiotensin system (RAS) inhibitors, and mineralocorticoid receptor antagonists (MRAs) in the quartet of core GDMT medications broadly indicated for HFrEF.
In-hospital initiation of GDMT for HFrEF is considered a predictor of being on those medications after discharge and is itself guideline recommended. There’s clear evidence that treatment with the four core medications boosts survival and cuts rehospitalization risk, and that “getting those on board as soon as possible will eventually benefit many patients,” Paul L. Hess, MD, MHS, said in an interview.
Dr. Hess, University of Colorado Anschutz Medical Campus, Aurora, is senior author on the report from the Get With The Guidelines–Heart Failure (GWTG-HF) quality improvement program of the American Heart Association.
Broad uptake of new medical therapies into practice may sometimes take 15 or more years from first publication, Dr. Hess said, so, “I find it encouraging in the study that over a shorter time period, 2017-2020, there was improvement.”
Indeed, the odds of in-hospital initiation of an indicated med during that period on average climbed a significant 8% every 3 months, the report states.
The finding suggests that “heart failure hospitalization is, in and of itself, an important intervention for getting folks on the appropriate medications,” Dr. Hess said. It also means “we’re getting better at it,” at least at the study’s 160 GWTG-HF participating hospitals nationwide.
Those centers, the report acknowledges, varied in size, geography, and teaching status but were not necessarily representative of all U.S. hospitals. In another potential limitation, the study couldn’t account for patients who weren’t prescribed all indicated medications for clinically valid reasons. It excluded patients with “clear contraindications,” Dr. Hess said. But there could have been “legitimate reasons” some indicated medications weren’t always prescribed, including patient frailty, hemodynamic intolerance, renal dysfunction, or polypharmacy concerns.
“Positive takeaways” from the analysis, notes an accompanying editorial, include improved prescription rates for key GDMT categories across more than 3 years of data, and evidence that in-hospital initiation “was feasible and, at least for some medications, reliably undertaken.”
Of note, new GDMT prescriptions from admission to discharge went from 70% to almost 98% for beta-blockers, from 59% to about 91% for RAS inhibitors, from about 26% to 56% for MRAs, and from 15.5% to 27.4% for hydralazine/nitrates, wrote Karen E. Joynt Maddox, MD, MPH, and Daniel K. Fox, MD, PhD, both of Washington University in St. Louis.
“Key areas for improvement,” they noted, include prescriptions for women, who were 12% less likely than men to have appropriate GDMT initiated during hospitalization (P < .001); and practice at rural hospitals, which were 40% less likely than urban centers to have patients on full GDMT by discharge (P = .017).
Although only 2.6% of the GWTG-HF centers were in rural locations, “rural hospitals make up approximately one-third of general acute care hospitals in this country,” the editorial states. They therefore “represent a key source of health disparity” in the United States in need of further study.
The analysis of 50,170 patients hospitalized with HFrEF compared the number of GDMT medications for which they were eligible, on at-hospital admission, and by discharge.
The drug categories included “evidence based beta blockers,” that is, bisoprolol, carvedilol, or sustained-release metoprolol; RAS inhibitors, specifically ACE inhibitors, angiotensin receptor blockers, or sacubitril/valsartan (Entresto); MRAs; SGLT2 inhibitors in patients with diabetes; diuretics for congestion; oral anticoagulants for atrial fibrillation; and hydralazine/nitrates in African Americans.
About 15% of the patients at hospital admission were on all indicated HFrEF medications for which they were eligible. The proportion more than doubled to 32.8% by discharge.
Factors significantly associated with reduced odds for in-hospital GDMT initiation include older age (odds ratio, 0.94 per 5-year increment), being female versus male (OR, 0.88), rural location (OR, 0.60), Medicaid versus Medicare or private insurance (OR, 0.93), stroke history (OR, 0.91), peripheral artery disease (OR, 0.93), chronic obstructive pulmonary disease or asthma (OR, 0.86), and renal insufficiency (OR, 0.77).
The findings suggest that there has been at least some progress in getting hospitalized patients “on the right meds” by discharge, Dr. Hess observed. To help address shortfalls in some patient groups, “there is interest in engaging pharmacists in helping us encourage providers on the front lines to initiate and titrate medications.”
The GWTG-HF program is sponsored, in part, by Novartis, Boehringer Ingelheim, Novo Nordisk, AstraZeneca, Bayer, Tylenol, and Alnylam Pharmaceuticals. Dr. Hess disclosed no relevant financial relationships. Dr. Maddox disclosed serving on the Health Policy Advisory Council for Centene. Dr. Fox reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JACC: HEART FAILURE
Antibody linked to spontaneous reversal of ATTR-CM
Cardiac transthyretin amyloidosis (also called ATTR amyloidosis cardiomyopathy or ATTR-CM) is a progressive disease and a cause of heart failure resulting from accumulation of the protein transthyretin, which misfolds and forms amyloid deposits on the walls of the heart, causing both systolic and diastolic dysfunction.
The condition is progressive and normally fatal within a few years of diagnosis. Treatment options are limited and aimed at slowing progression; nothing has been shown to reverse the course of the disease.
However, an international team of researchers is now reporting the discovery of three patients with ATTR-CM–associated heart failure in whom the condition resolved spontaneously, with reversion to near normal cardiac structure and function. On further investigation, it was found that these three patients had developed circulating polyclonal IgG antibodies to human ATTR amyloid.
They are hopeful that a monoclonal form of these antibodies could be developed and may represent a novel treatment, or even a cure, for the condition.
The researchers report their findings in a letter to the New England Journal of Medicine.
“We are very optimistic about this discovery of these antibodies. They could become the first treatment to clear the amyloid that causes this horribly progressive and fatal condition,” senior author Julian Gillmore, MD, head of the University College London Centre for Amyloidosis, based at the Royal Free Hospital, said in an interview.
“Obviously, there is a lot of work to do before we can say this is the case, but it is very exciting,” he added.
Dr. Gillmore explained how the antibodies were discovered. “This disease has a universally progressive course, but we had one patient who on a repeat appointment said he felt better and on detailed cardiac MRI imaging, we found that the amyloid in his heart had reduced. That is totally unheard of,” he said.
“We then looked back at our cohort of 1,663 patients with ATTR-cardiomyopathy, and we discovered two others who had also improved both on imaging and clinically,” Dr. Gillmore said.
Each of these three patients reported a reduction in symptoms, although they had not received any new or potentially disease-modifying treatments. None of the patients had had recent vaccinations, notable infections, or any clinical suggestion of myocarditis.
Clinical recovery was corroborated by substantial improvement or normalization of findings on echocardiography, serum biomarker levels, and results of cardiopulmonary exercise tests and scintigraphy.
Serial cardiac MRI scans confirmed near-complete regression of myocardial extracellular volume, coupled with remodeling to near-normal cardiac structure and function without scarring.
The researchers wondered whether the changes in these patients may have been brought about by an antibody response. On further investigation, they found antibodies in the three patients that bound specifically to ATTR amyloid deposits in a transgenic mouse model of the condition, and to synthetic ATTR amyloid. No such antibodies were present in the other 350 patients in the cohort with a typical clinical course.
“The cause and clinical significance of the anti-ATTR amyloid antibodies are intriguing and presently unclear. However, the clinical recovery of these patients establishes the unanticipated potential for reversibility of ATTR-CM and raises expectations for its treatment,” the researchers conclude.
Dr. Gillmore said they didn’t know why these three patients had these antibodies, while all the other patients did not. “There must be something different about these patients. We don’t know what that is at present, but we are looking hard.”
The researchers are hoping that after this publication, other centers caring for patients with ATTR-cardiomyopathy will look in their cohorts and see if they can identify other cases where there has been improvement.
“It is very plausible that they do have such cases, but they will be rare, as we all think of this disease as universally progressive and fatal,” Dr. Gillmore noted.
“We haven’t absolutely proven that the antibodies have caused the clearance of amyloid in these patients, but we strongly suspect this to be the case,” Dr. Gillmore said. The researchers are planning to try to confirm this by isolating the antibodies and treating the transgenic mice.
Dr. Gillmore attributed the current discovery to the development of novel imaging cardiac MRI techniques. “This allowed us to monitor closely the amyloid burden in the heart. The observation that this had diminished in these three patients was the breakthrough that led us to look for antibodies.”
Another antibody product directed against ATTR cardiomyopathy is also in development by Neurimmune, a Swiss biopharmaceutical company. A phase 1 study of this agent was recently published, suggesting that it appeared to reduce the amount of amyloid protein deposited in the heart.
Dr. Gillmore said the antibody they have detected is different from the Neurimmune product.
The research was supported by a British Heart Foundation Intermediate Clinical Research Fellowship, a Medical Research Council Career Development Award, and a project grant from the British Heart Foundation. Dr. Gillmore reports being a consultant or expert advisory board member for Alnylam Pharmaceuticals, AstraZeneca, ATTRalus, Eidos Therapeutics, Intellia Therapeutics, Ionis Pharmaceuticals, and Pfizer.
A version of this article originally appeared on Medscape.com.
Cardiac transthyretin amyloidosis (also called ATTR amyloidosis cardiomyopathy or ATTR-CM) is a progressive disease and a cause of heart failure resulting from accumulation of the protein transthyretin, which misfolds and forms amyloid deposits on the walls of the heart, causing both systolic and diastolic dysfunction.
The condition is progressive and normally fatal within a few years of diagnosis. Treatment options are limited and aimed at slowing progression; nothing has been shown to reverse the course of the disease.
However, an international team of researchers is now reporting the discovery of three patients with ATTR-CM–associated heart failure in whom the condition resolved spontaneously, with reversion to near normal cardiac structure and function. On further investigation, it was found that these three patients had developed circulating polyclonal IgG antibodies to human ATTR amyloid.
They are hopeful that a monoclonal form of these antibodies could be developed and may represent a novel treatment, or even a cure, for the condition.
The researchers report their findings in a letter to the New England Journal of Medicine.
“We are very optimistic about this discovery of these antibodies. They could become the first treatment to clear the amyloid that causes this horribly progressive and fatal condition,” senior author Julian Gillmore, MD, head of the University College London Centre for Amyloidosis, based at the Royal Free Hospital, said in an interview.
“Obviously, there is a lot of work to do before we can say this is the case, but it is very exciting,” he added.
Dr. Gillmore explained how the antibodies were discovered. “This disease has a universally progressive course, but we had one patient who on a repeat appointment said he felt better and on detailed cardiac MRI imaging, we found that the amyloid in his heart had reduced. That is totally unheard of,” he said.
“We then looked back at our cohort of 1,663 patients with ATTR-cardiomyopathy, and we discovered two others who had also improved both on imaging and clinically,” Dr. Gillmore said.
Each of these three patients reported a reduction in symptoms, although they had not received any new or potentially disease-modifying treatments. None of the patients had had recent vaccinations, notable infections, or any clinical suggestion of myocarditis.
Clinical recovery was corroborated by substantial improvement or normalization of findings on echocardiography, serum biomarker levels, and results of cardiopulmonary exercise tests and scintigraphy.
Serial cardiac MRI scans confirmed near-complete regression of myocardial extracellular volume, coupled with remodeling to near-normal cardiac structure and function without scarring.
The researchers wondered whether the changes in these patients may have been brought about by an antibody response. On further investigation, they found antibodies in the three patients that bound specifically to ATTR amyloid deposits in a transgenic mouse model of the condition, and to synthetic ATTR amyloid. No such antibodies were present in the other 350 patients in the cohort with a typical clinical course.
“The cause and clinical significance of the anti-ATTR amyloid antibodies are intriguing and presently unclear. However, the clinical recovery of these patients establishes the unanticipated potential for reversibility of ATTR-CM and raises expectations for its treatment,” the researchers conclude.
Dr. Gillmore said they didn’t know why these three patients had these antibodies, while all the other patients did not. “There must be something different about these patients. We don’t know what that is at present, but we are looking hard.”
The researchers are hoping that after this publication, other centers caring for patients with ATTR-cardiomyopathy will look in their cohorts and see if they can identify other cases where there has been improvement.
“It is very plausible that they do have such cases, but they will be rare, as we all think of this disease as universally progressive and fatal,” Dr. Gillmore noted.
“We haven’t absolutely proven that the antibodies have caused the clearance of amyloid in these patients, but we strongly suspect this to be the case,” Dr. Gillmore said. The researchers are planning to try to confirm this by isolating the antibodies and treating the transgenic mice.
Dr. Gillmore attributed the current discovery to the development of novel imaging cardiac MRI techniques. “This allowed us to monitor closely the amyloid burden in the heart. The observation that this had diminished in these three patients was the breakthrough that led us to look for antibodies.”
Another antibody product directed against ATTR cardiomyopathy is also in development by Neurimmune, a Swiss biopharmaceutical company. A phase 1 study of this agent was recently published, suggesting that it appeared to reduce the amount of amyloid protein deposited in the heart.
Dr. Gillmore said the antibody they have detected is different from the Neurimmune product.
The research was supported by a British Heart Foundation Intermediate Clinical Research Fellowship, a Medical Research Council Career Development Award, and a project grant from the British Heart Foundation. Dr. Gillmore reports being a consultant or expert advisory board member for Alnylam Pharmaceuticals, AstraZeneca, ATTRalus, Eidos Therapeutics, Intellia Therapeutics, Ionis Pharmaceuticals, and Pfizer.
A version of this article originally appeared on Medscape.com.
Cardiac transthyretin amyloidosis (also called ATTR amyloidosis cardiomyopathy or ATTR-CM) is a progressive disease and a cause of heart failure resulting from accumulation of the protein transthyretin, which misfolds and forms amyloid deposits on the walls of the heart, causing both systolic and diastolic dysfunction.
The condition is progressive and normally fatal within a few years of diagnosis. Treatment options are limited and aimed at slowing progression; nothing has been shown to reverse the course of the disease.
However, an international team of researchers is now reporting the discovery of three patients with ATTR-CM–associated heart failure in whom the condition resolved spontaneously, with reversion to near normal cardiac structure and function. On further investigation, it was found that these three patients had developed circulating polyclonal IgG antibodies to human ATTR amyloid.
They are hopeful that a monoclonal form of these antibodies could be developed and may represent a novel treatment, or even a cure, for the condition.
The researchers report their findings in a letter to the New England Journal of Medicine.
“We are very optimistic about this discovery of these antibodies. They could become the first treatment to clear the amyloid that causes this horribly progressive and fatal condition,” senior author Julian Gillmore, MD, head of the University College London Centre for Amyloidosis, based at the Royal Free Hospital, said in an interview.
“Obviously, there is a lot of work to do before we can say this is the case, but it is very exciting,” he added.
Dr. Gillmore explained how the antibodies were discovered. “This disease has a universally progressive course, but we had one patient who on a repeat appointment said he felt better and on detailed cardiac MRI imaging, we found that the amyloid in his heart had reduced. That is totally unheard of,” he said.
“We then looked back at our cohort of 1,663 patients with ATTR-cardiomyopathy, and we discovered two others who had also improved both on imaging and clinically,” Dr. Gillmore said.
Each of these three patients reported a reduction in symptoms, although they had not received any new or potentially disease-modifying treatments. None of the patients had had recent vaccinations, notable infections, or any clinical suggestion of myocarditis.
Clinical recovery was corroborated by substantial improvement or normalization of findings on echocardiography, serum biomarker levels, and results of cardiopulmonary exercise tests and scintigraphy.
Serial cardiac MRI scans confirmed near-complete regression of myocardial extracellular volume, coupled with remodeling to near-normal cardiac structure and function without scarring.
The researchers wondered whether the changes in these patients may have been brought about by an antibody response. On further investigation, they found antibodies in the three patients that bound specifically to ATTR amyloid deposits in a transgenic mouse model of the condition, and to synthetic ATTR amyloid. No such antibodies were present in the other 350 patients in the cohort with a typical clinical course.
“The cause and clinical significance of the anti-ATTR amyloid antibodies are intriguing and presently unclear. However, the clinical recovery of these patients establishes the unanticipated potential for reversibility of ATTR-CM and raises expectations for its treatment,” the researchers conclude.
Dr. Gillmore said they didn’t know why these three patients had these antibodies, while all the other patients did not. “There must be something different about these patients. We don’t know what that is at present, but we are looking hard.”
The researchers are hoping that after this publication, other centers caring for patients with ATTR-cardiomyopathy will look in their cohorts and see if they can identify other cases where there has been improvement.
“It is very plausible that they do have such cases, but they will be rare, as we all think of this disease as universally progressive and fatal,” Dr. Gillmore noted.
“We haven’t absolutely proven that the antibodies have caused the clearance of amyloid in these patients, but we strongly suspect this to be the case,” Dr. Gillmore said. The researchers are planning to try to confirm this by isolating the antibodies and treating the transgenic mice.
Dr. Gillmore attributed the current discovery to the development of novel imaging cardiac MRI techniques. “This allowed us to monitor closely the amyloid burden in the heart. The observation that this had diminished in these three patients was the breakthrough that led us to look for antibodies.”
Another antibody product directed against ATTR cardiomyopathy is also in development by Neurimmune, a Swiss biopharmaceutical company. A phase 1 study of this agent was recently published, suggesting that it appeared to reduce the amount of amyloid protein deposited in the heart.
Dr. Gillmore said the antibody they have detected is different from the Neurimmune product.
The research was supported by a British Heart Foundation Intermediate Clinical Research Fellowship, a Medical Research Council Career Development Award, and a project grant from the British Heart Foundation. Dr. Gillmore reports being a consultant or expert advisory board member for Alnylam Pharmaceuticals, AstraZeneca, ATTRalus, Eidos Therapeutics, Intellia Therapeutics, Ionis Pharmaceuticals, and Pfizer.
A version of this article originally appeared on Medscape.com.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Big boost in sodium excretion with HF diuretic protocol
In patients with acute heart failure, a urine sodium-guided diuretic protocol, currently recommended in guidelines from the Heart Failure Association of the European Society of Cardiology (HFA-ESC), led to significant increases in natriuresis and diuresis over 2 days in the prospective ENACT-HF clinical trial.
The guideline protocol was based on a 2019 HFA position paper with expert consensus, but it had not been tested prospectively, Jeroen Dauw, MD, of AZ Sint-Lucas Ghent (Belgium), explained in a presentation at HFA-ESC 2023.
“We had 282 millimoles of sodium excretion after one day, which is an increase of 64%, compared with standard of care,” Dr. Dauw told meeting attendees. “We wanted to power for 15%, so we’re way above it, with a P value of lower than 0.001.”
The effect was consistent across predefined subgroups, he said. “In addition, there’s an even higher benefit in patients with a lower eGFR [estimated glomerular filtration rate] and a higher home dose of loop diuretics, which might signal more diuretic resistance and more benefit of the protocol.”
After 2 days, the investigators saw 52% higher natriuresis and 33% higher diuresis, compared with usual care.
In an interview, Dr. Dauw said, “The protocol is feasible, safe, and very effective. Cardiologists might consider how to implement a similar protocol in their center to improve the care of their acute heart failure patients.”
Twice the oral home dose
The investigators conducted a multicenter, open-label, nonrandomized pragmatic trial at 29 centers in 18 countries globally. “We aimed to recruit 500 to detect a 15% difference in natriuresis,” Dr. Dauw said in his presentation, “but because we were a really low-budget trial, we had to stop after 3 years of recruitment.”
Therefore, 401 patients participated, 254 in the SOC arm and 147 in the protocol arm, because of the sequential nature of the study; that is, patients in the SOC arm of the two-phase study were recruited first.
Patients’ mean age was 70 years, 38% were women, and they all had at least one sign of volume overload. They were on a maintenance daily diuretic dose of 40 mg of furosemide for a month or more, and the NT-proBNP was above 1,000.
In phase 1 of the study, all centers treated 10 consecutive patients according to the local standard of care, at the discretion of the physician. In phase 2, the centers again recruited and treated at least 10 consecutive patients, this time according to the standardized diuretic protocol.
In the protocol phase, patients were treated with twice the oral home dose as an IV bolus. “This meant if, for example, you have 40 mg of furosemide at home, then you receive 80 mg as a first bolus,” Dr. Dauw told attendees. A spot urine sample was taken after 2 hours, and the response was evaluated after 6 hours. A urine sodium above 50 millimoles per liter was considered a good response.
On the second day, patients were reevaluated in the morning using urine output as a measure of diuretic response. If it was above 3 L, then the same bolus was repeated again twice daily, with 6-12 hours between administrations.
As noted, after one day, natriuresis was 174 millimoles in the SOC arm versus 282 millimoles in the protocol group – an increase of 64%. The effect was consistent across subgroups, and those with a lower eGFR and a higher home dose of loop diuretics benefited more.
Furthermore, Dr. Dauw said, there was no interaction on the endpoints with SGLT2 inhibitor use at baseline.
After two days, natriuresis was 52% higher in the protocol group and diuresis was 33% higher.
However, there was no significant difference in weight loss and no difference in the congestion score.
“We did expect to see a difference in weight loss between the study groups, as higher natriuresis and diuresis would normally be associated with higher weight loss in the protocol group,” Dr. Dauw told this news organization. “However, looking back at the study design, weight was collected from the electronic health records and not rigorously collected by study nurses. Previous studies have shown discrepancies between fluid loss and weight loss, so this is an ‘explainable’ finding.”
Participants also had a relatively high congestion score at baseline, with edema above the knee and also some pleural effusion, he told meeting attendees. Therefore, it might take more time to see a change in congestion score in those patients.
The protocol also led to a shorter length of stay – one day less in the hospital – and was very safe on renal endpoints, Dr. Dauw concluded.
A session chair asked why only patients already on diuretics were included in the study, noting that in his clinic, about half of the admissions are de novo.
Dr. Dauw said that patients already taking diuretics chronically would benefit most from the protocol. “If patients are diuretic-naive, they probably will respond well to whatever you do; if you just give a higher dose, they will respond well,” he said. “We expected that the largest benefit would be in patients already taking diuretics because they have a higher chance of not responding well.”
“There also was a big difference in the starting dose,” he added. “In the SOC arm, the baseline dose was about 60 mg, whereas we gave 120 mg, and we could already see a high difference in the effect. So, in those patients, I think the gain is bigger if you follow the protocol.”
More data coming
Looking ahead, “we only showed efficacy in the first 2 days of treatment and a shorter length of stay, probably reflecting a faster decongestion, but we don’t know for sure,” Dr. Dauw told this news organization.
“It would be important to have a study where the protocol is followed until full decongestion is reached,” he said. “That way, we can directly prove that decongestion is better and/or faster with the protocol.”
“A good decongestive strategy is one that is fast, safe and effective in decreasing signs and symptoms that patients suffer from,” he added. “We believe our protocol can achieve that, but our study is only one piece of the puzzle.”
More data on natriuresis-guided decongestion is coming this year, he said, with the PUSH-AHF study from Groningen, the European DECONGEST study, and the U.S. ESCALATE study.
The study had no funding. Dr. Dauw declared no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In patients with acute heart failure, a urine sodium-guided diuretic protocol, currently recommended in guidelines from the Heart Failure Association of the European Society of Cardiology (HFA-ESC), led to significant increases in natriuresis and diuresis over 2 days in the prospective ENACT-HF clinical trial.
The guideline protocol was based on a 2019 HFA position paper with expert consensus, but it had not been tested prospectively, Jeroen Dauw, MD, of AZ Sint-Lucas Ghent (Belgium), explained in a presentation at HFA-ESC 2023.
“We had 282 millimoles of sodium excretion after one day, which is an increase of 64%, compared with standard of care,” Dr. Dauw told meeting attendees. “We wanted to power for 15%, so we’re way above it, with a P value of lower than 0.001.”
The effect was consistent across predefined subgroups, he said. “In addition, there’s an even higher benefit in patients with a lower eGFR [estimated glomerular filtration rate] and a higher home dose of loop diuretics, which might signal more diuretic resistance and more benefit of the protocol.”
After 2 days, the investigators saw 52% higher natriuresis and 33% higher diuresis, compared with usual care.
In an interview, Dr. Dauw said, “The protocol is feasible, safe, and very effective. Cardiologists might consider how to implement a similar protocol in their center to improve the care of their acute heart failure patients.”
Twice the oral home dose
The investigators conducted a multicenter, open-label, nonrandomized pragmatic trial at 29 centers in 18 countries globally. “We aimed to recruit 500 to detect a 15% difference in natriuresis,” Dr. Dauw said in his presentation, “but because we were a really low-budget trial, we had to stop after 3 years of recruitment.”
Therefore, 401 patients participated, 254 in the SOC arm and 147 in the protocol arm, because of the sequential nature of the study; that is, patients in the SOC arm of the two-phase study were recruited first.
Patients’ mean age was 70 years, 38% were women, and they all had at least one sign of volume overload. They were on a maintenance daily diuretic dose of 40 mg of furosemide for a month or more, and the NT-proBNP was above 1,000.
In phase 1 of the study, all centers treated 10 consecutive patients according to the local standard of care, at the discretion of the physician. In phase 2, the centers again recruited and treated at least 10 consecutive patients, this time according to the standardized diuretic protocol.
In the protocol phase, patients were treated with twice the oral home dose as an IV bolus. “This meant if, for example, you have 40 mg of furosemide at home, then you receive 80 mg as a first bolus,” Dr. Dauw told attendees. A spot urine sample was taken after 2 hours, and the response was evaluated after 6 hours. A urine sodium above 50 millimoles per liter was considered a good response.
On the second day, patients were reevaluated in the morning using urine output as a measure of diuretic response. If it was above 3 L, then the same bolus was repeated again twice daily, with 6-12 hours between administrations.
As noted, after one day, natriuresis was 174 millimoles in the SOC arm versus 282 millimoles in the protocol group – an increase of 64%. The effect was consistent across subgroups, and those with a lower eGFR and a higher home dose of loop diuretics benefited more.
Furthermore, Dr. Dauw said, there was no interaction on the endpoints with SGLT2 inhibitor use at baseline.
After two days, natriuresis was 52% higher in the protocol group and diuresis was 33% higher.
However, there was no significant difference in weight loss and no difference in the congestion score.
“We did expect to see a difference in weight loss between the study groups, as higher natriuresis and diuresis would normally be associated with higher weight loss in the protocol group,” Dr. Dauw told this news organization. “However, looking back at the study design, weight was collected from the electronic health records and not rigorously collected by study nurses. Previous studies have shown discrepancies between fluid loss and weight loss, so this is an ‘explainable’ finding.”
Participants also had a relatively high congestion score at baseline, with edema above the knee and also some pleural effusion, he told meeting attendees. Therefore, it might take more time to see a change in congestion score in those patients.
The protocol also led to a shorter length of stay – one day less in the hospital – and was very safe on renal endpoints, Dr. Dauw concluded.
A session chair asked why only patients already on diuretics were included in the study, noting that in his clinic, about half of the admissions are de novo.
Dr. Dauw said that patients already taking diuretics chronically would benefit most from the protocol. “If patients are diuretic-naive, they probably will respond well to whatever you do; if you just give a higher dose, they will respond well,” he said. “We expected that the largest benefit would be in patients already taking diuretics because they have a higher chance of not responding well.”
“There also was a big difference in the starting dose,” he added. “In the SOC arm, the baseline dose was about 60 mg, whereas we gave 120 mg, and we could already see a high difference in the effect. So, in those patients, I think the gain is bigger if you follow the protocol.”
More data coming
Looking ahead, “we only showed efficacy in the first 2 days of treatment and a shorter length of stay, probably reflecting a faster decongestion, but we don’t know for sure,” Dr. Dauw told this news organization.
“It would be important to have a study where the protocol is followed until full decongestion is reached,” he said. “That way, we can directly prove that decongestion is better and/or faster with the protocol.”
“A good decongestive strategy is one that is fast, safe and effective in decreasing signs and symptoms that patients suffer from,” he added. “We believe our protocol can achieve that, but our study is only one piece of the puzzle.”
More data on natriuresis-guided decongestion is coming this year, he said, with the PUSH-AHF study from Groningen, the European DECONGEST study, and the U.S. ESCALATE study.
The study had no funding. Dr. Dauw declared no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In patients with acute heart failure, a urine sodium-guided diuretic protocol, currently recommended in guidelines from the Heart Failure Association of the European Society of Cardiology (HFA-ESC), led to significant increases in natriuresis and diuresis over 2 days in the prospective ENACT-HF clinical trial.
The guideline protocol was based on a 2019 HFA position paper with expert consensus, but it had not been tested prospectively, Jeroen Dauw, MD, of AZ Sint-Lucas Ghent (Belgium), explained in a presentation at HFA-ESC 2023.
“We had 282 millimoles of sodium excretion after one day, which is an increase of 64%, compared with standard of care,” Dr. Dauw told meeting attendees. “We wanted to power for 15%, so we’re way above it, with a P value of lower than 0.001.”
The effect was consistent across predefined subgroups, he said. “In addition, there’s an even higher benefit in patients with a lower eGFR [estimated glomerular filtration rate] and a higher home dose of loop diuretics, which might signal more diuretic resistance and more benefit of the protocol.”
After 2 days, the investigators saw 52% higher natriuresis and 33% higher diuresis, compared with usual care.
In an interview, Dr. Dauw said, “The protocol is feasible, safe, and very effective. Cardiologists might consider how to implement a similar protocol in their center to improve the care of their acute heart failure patients.”
Twice the oral home dose
The investigators conducted a multicenter, open-label, nonrandomized pragmatic trial at 29 centers in 18 countries globally. “We aimed to recruit 500 to detect a 15% difference in natriuresis,” Dr. Dauw said in his presentation, “but because we were a really low-budget trial, we had to stop after 3 years of recruitment.”
Therefore, 401 patients participated, 254 in the SOC arm and 147 in the protocol arm, because of the sequential nature of the study; that is, patients in the SOC arm of the two-phase study were recruited first.
Patients’ mean age was 70 years, 38% were women, and they all had at least one sign of volume overload. They were on a maintenance daily diuretic dose of 40 mg of furosemide for a month or more, and the NT-proBNP was above 1,000.
In phase 1 of the study, all centers treated 10 consecutive patients according to the local standard of care, at the discretion of the physician. In phase 2, the centers again recruited and treated at least 10 consecutive patients, this time according to the standardized diuretic protocol.
In the protocol phase, patients were treated with twice the oral home dose as an IV bolus. “This meant if, for example, you have 40 mg of furosemide at home, then you receive 80 mg as a first bolus,” Dr. Dauw told attendees. A spot urine sample was taken after 2 hours, and the response was evaluated after 6 hours. A urine sodium above 50 millimoles per liter was considered a good response.
On the second day, patients were reevaluated in the morning using urine output as a measure of diuretic response. If it was above 3 L, then the same bolus was repeated again twice daily, with 6-12 hours between administrations.
As noted, after one day, natriuresis was 174 millimoles in the SOC arm versus 282 millimoles in the protocol group – an increase of 64%. The effect was consistent across subgroups, and those with a lower eGFR and a higher home dose of loop diuretics benefited more.
Furthermore, Dr. Dauw said, there was no interaction on the endpoints with SGLT2 inhibitor use at baseline.
After two days, natriuresis was 52% higher in the protocol group and diuresis was 33% higher.
However, there was no significant difference in weight loss and no difference in the congestion score.
“We did expect to see a difference in weight loss between the study groups, as higher natriuresis and diuresis would normally be associated with higher weight loss in the protocol group,” Dr. Dauw told this news organization. “However, looking back at the study design, weight was collected from the electronic health records and not rigorously collected by study nurses. Previous studies have shown discrepancies between fluid loss and weight loss, so this is an ‘explainable’ finding.”
Participants also had a relatively high congestion score at baseline, with edema above the knee and also some pleural effusion, he told meeting attendees. Therefore, it might take more time to see a change in congestion score in those patients.
The protocol also led to a shorter length of stay – one day less in the hospital – and was very safe on renal endpoints, Dr. Dauw concluded.
A session chair asked why only patients already on diuretics were included in the study, noting that in his clinic, about half of the admissions are de novo.
Dr. Dauw said that patients already taking diuretics chronically would benefit most from the protocol. “If patients are diuretic-naive, they probably will respond well to whatever you do; if you just give a higher dose, they will respond well,” he said. “We expected that the largest benefit would be in patients already taking diuretics because they have a higher chance of not responding well.”
“There also was a big difference in the starting dose,” he added. “In the SOC arm, the baseline dose was about 60 mg, whereas we gave 120 mg, and we could already see a high difference in the effect. So, in those patients, I think the gain is bigger if you follow the protocol.”
More data coming
Looking ahead, “we only showed efficacy in the first 2 days of treatment and a shorter length of stay, probably reflecting a faster decongestion, but we don’t know for sure,” Dr. Dauw told this news organization.
“It would be important to have a study where the protocol is followed until full decongestion is reached,” he said. “That way, we can directly prove that decongestion is better and/or faster with the protocol.”
“A good decongestive strategy is one that is fast, safe and effective in decreasing signs and symptoms that patients suffer from,” he added. “We believe our protocol can achieve that, but our study is only one piece of the puzzle.”
More data on natriuresis-guided decongestion is coming this year, he said, with the PUSH-AHF study from Groningen, the European DECONGEST study, and the U.S. ESCALATE study.
The study had no funding. Dr. Dauw declared no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM HFA-ESC 2023
First-line or BiV backup? Conduction system pacing for CRT in heart failure
Pacing as a device therapy for heart failure (HF) is headed for what is probably its next big advance.
After decades of biventricular (BiV) pacemaker success in resynchronizing the ventricles and improving clinical outcomes, relatively new conduction-system pacing (CSP) techniques that avoid the pitfalls of right-ventricular (RV) pacing using BiV lead systems have been supplanting traditional cardiac resynchronization therapy (CRT) in selected patients at some major centers. In fact, they are solidly ensconced in a new guideline document addressing indications for CSP and BiV pacing in HF.
But , an alternative when BiV pacing isn’t appropriate or can’t be engaged.
That’s mainly because the limited, mostly observational evidence supporting CSP in the document can’t measure up to the clinical experience and plethora of large, randomized trials behind BiV-CRT.
But that shortfall is headed for change. Several new comparative studies, including a small, randomized trial, have added significantly to evidence suggesting that CSP is at least as effective as traditional CRT for procedural, functional safety, and clinical outcomes.
The new studies “are inherently prone to bias, but their results are really good,” observed Juan C. Diaz, MD. They show improvements in left ventricular ejection fraction (LVEF) and symptoms with CSP that are “outstanding compared to what we have been doing for the last 20 years,” he said in an interview.
Dr. Diaz, Clínica Las Vegas, Medellin, Colombia, is an investigator with the observational SYNCHRONY, which is among the new CSP studies formally presented at the annual scientific sessions of the Heart Rhythm Society. He is also lead author on its same-day publication in JACC: Clinical Electrophysiology.
Dr. Diaz said that CSP, which sustains pacing via the native conduction system, makes more “physiologic sense” than BiV pacing and represents “a step forward” for HF device therapy.
SYNCHRONY compared LBB-area with BiV pacing as the initial strategy for achieving cardiac resynchronization in patients with ischemic or nonischemic cardiomyopathy.
CSP is “a long way” from replacing conventional CRT, he said. But the new studies at the HRS sessions should help extend His-bundle and LBB-area pacing to more patients, he added, given the significant long-term “drawbacks” of BiV pacing. These include inevitable RV pacing, multiple leads, and the risks associated with chronic transvenous leads.
Zachary Goldberger, MD, University of Wisconsin–Madison, went a bit further in support of CSP as invited discussant for the SYNCHRONY presentation.
Given that it improved LVEF, heart failure class, HF hospitalizations (HFH), and mortality in that study and others, Dr. Goldberger said, CSP could potentially “become the dominant mode of resynchronization going forward.”
Other experts at the meeting saw CSP’s potential more as one of several pacing techniques that could be brought to bear for patients with CRT indications.
“Conduction system pacing is going to be a huge complement to biventricular pacing,” to which about 30% of patients have a “less than optimal response,” said Pugazhendhi Vijayaraman, MD, chief of clinical electrophysiology, Geisinger Heart Institute, Danville, Pa.
“I don’t think it needs to replace biventricular pacing, because biventricular pacing is a well-established, incredibly powerful therapy,” he told this news organization. But CSP is likely to provide “a good alternative option” in patients with poor responses to BiV-CRT.
It may, however, render some current BiV-pacing alternatives “obsolete,” Dr. Vijayaraman observed. “At our center, at least for the last 5 years, no patient has needed epicardial surgical left ventricular lead placement” because CSP was a better backup option.
Dr. Vijayaraman presented two of the meeting’s CSP vs. BiV pacing comparisons. In one, the 100-patient randomized HOT-CRT trial, contractile function improved significantly on CSP, which could be either His-bundle or LBB-area pacing.
He also presented an observational study of LBB-area pacing at 15 centers in Asia, Europe, and North America and led the authors of its simultaneous publication in the Journal of the American College of Cardiology.
“I think left-bundle conduction system pacing is the future, for sure,” Jagmeet P. Singh, MD, DPhil, told this news organization. Still, it doesn’t always work and when it does, it “doesn’t work equally in all patients,” he said.
“Conduction system pacing certainly makes a lot of sense,” especially in patients with left-bundle-branch block (LBBB), and “maybe not as a primary approach but certainly as a secondary approach,” said Dr. Singh, Massachusetts General Hospital, Boston, who is not a coauthor on any of the three studies.
He acknowledged that CSP may work well as a first-line option in patients with LBBB at some experienced centers. For those without LBBB or who have an intraventricular conduction delay, who represent 45%-50% of current CRT cases, Dr. Singh observed, “there’s still more evidence” that BiV-CRT is a more appropriate initial approach.
Standard CRT may fail, however, even in some patients who otherwise meet guideline-based indications. “We don’t really understand all the mechanisms for nonresponse in conventional biventricular pacing,” observed Niraj Varma, MD, PhD, Cleveland Clinic, also not involved with any of the three studies.
In some groups, including “patients with larger ventricles,” for example, BiV-CRT doesn’t always narrow the electrocardiographic QRS complex or preexcite delayed left ventricular (LV) activation, hallmarks of successful CRT, he said in an interview.
“I think we need to understand why this occurs in both situations,” but in such cases, CSP alone or as an adjunct to direct LV pacing may be successful. “Sometimes we need both an LV lead and the conduction-system pacing lead.”
Narrower, more efficient use of CSP as a BiV-CRT alternative may also boost its chances for success, Dr. Varma added. “I think we need to refine patient selection.”
HOT-CRT: Randomized CSP vs. BiV pacing trial
Conducted at three centers in a single health system, the His-optimized cardiac resynchronization therapy study (HOT-CRT) randomly assigned 100 patients with primary or secondary CRT indications to either to CSP – by either His-bundle or LBB-area pacing – or to standard BiV-CRT as the first-line resynchronization method.
Treatment crossovers, allowed for either pacing modality in the event of implantation failure, occurred in two patients and nine patients initially assigned to CSP and BiV pacing, respectively (4% vs. 18%), Dr. Vijayaraman reported.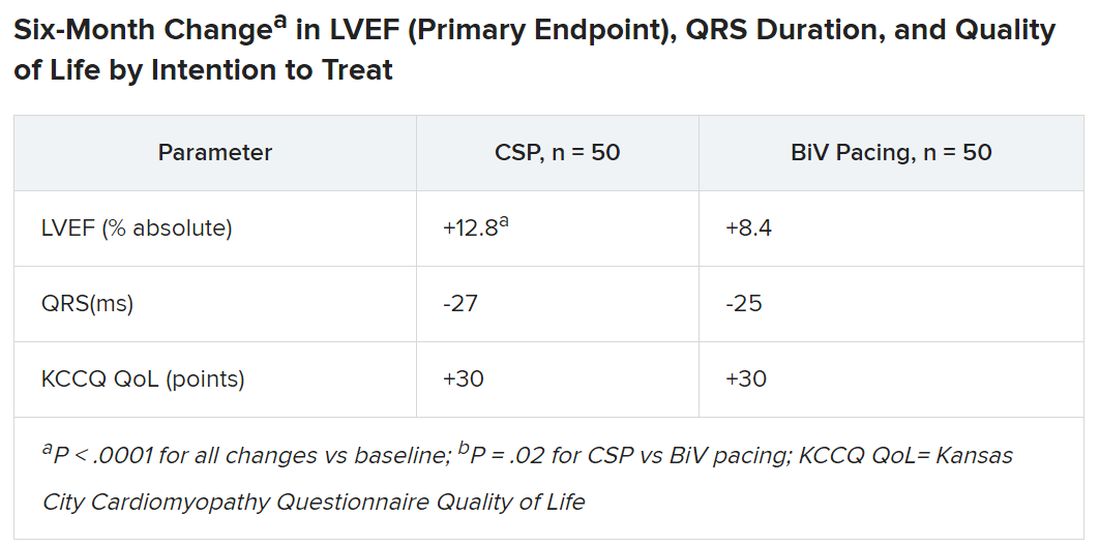
Historically in trials, BiV pacing has elevated LVEF by about 7%, he said. The mean 12-point increase observed with CSP “is huge, in that sense.” HOT-CRT enrolled a predominantly male and White population at centers highly experienced in both CSP and BiV pacing, limiting its broad relevance to practice, as pointed out by both Dr. Vijayaraman and his presentation’s invited discussant, Yong-Mei Cha, MD, Mayo Clinic, Rochester, Minn. Dr. Cha, who is director of cardiac device services at her center, also highlighted the greater rate of crossover from BiV pacing to CSP, 18% vs. 4% in the other direction. “This is a very encouraging result,” because the implant-failure rate for LBB-area pacing may drop once more operators become “familiar and skilled with conduction-system pacing.” Overall, the study supports CSP as “a very good alternative for heart failure patients when BiV pacing fails.”
International comparison of CSP and BiV pacing
In Dr. Vijayaraman’s other study, the observational comparison of LBB-area pacing and BiV-CRT, the CSP technique emerged as a “reasonable alternative to biventricular pacing, not only for improvement in LV function but also to reduce adverse clinical outcomes.”
Indeed, in the international study of 1,778 mostly male patients with primary or secondary CRT indications who received LBB-area or BiV pacing (797 and 981 patients, respectively), those on CSP saw a significant drop in risk for the primary endpoint, death or HFH.
Mean LVEF improved from 27% to 41% in the LBB-area pacing group and 27% to 37% with BiV pacing (P < .001 for both changes) over a follow-up averaging 33 months. The difference in improvement between CSP and BiV pacing was significant at P < .001.
In adjusted analysis, the risk for death or HFH was greater for BiV-pacing patients, a difference driven by HFH events.
- Death or HF: hazard ratio, 1.49 (95% confidence interval, 1.21-1.84; P < .001).
- Death: HR, 1.14 (95% CI, 0.88-1.48; P = .313).
- HFH: HR, 1.49 (95% CI, 1.16-1.92; P = .002)
The analysis has all the “inherent biases” of an observational study. The risk for patient-selection bias, however, was somewhat mitigated by consistent practice patterns at participating centers, Dr. Vijayaraman told this news organization.
For example, he said, operators at six of the institutions were most likely to use CSP as the first-line approach, and the same number of centers usually went with BiV pacing.
SYNCHRONY: First-line LBB-area pacing vs. BiV-CRT
Outcomes using the two approaches were similar in the prospective, international, observational study of 371 patients with ischemic or nonischemic cardiomyopathy and standard CRT indications. Allocation of 128 patients to LBB-area pacing and 243 to BiV-CRT was based on patient and operator preferences, reported Jorge Romero Jr, MD, Brigham and Women’s Hospital, Boston, at the HRS sessions.
Risk for the death-HFH primary endpoint dropped 38% for those initially treated with LBB-area pacing, compared with BiV pacing, primarily because of a lower HFH risk:
- Death or HFH: HR, 0.62 (95% CI, 0.41-0.93; P = .02).
- Death: HR, 0.57 (95% CI, 0.25-1.32; P = .19).
- HFH: HR, 0.61 (95% CI, 0.34-0.93; P = .02)
Patients in the CSP group were also more likely to improve by at least one NYHA (New York Heart Association) class (80.4% vs. 67.9%; P < .001), consistent with their greater absolute change in LVEF (8.0 vs. 3.9 points; P < .01).
The findings “suggest that LBBAP [left-bundle branch area pacing] is an excellent alternative to BiV pacing,” with a comparable safety profile, write Jayanthi N. Koneru, MBBS, and Kenneth A. Ellenbogen, MD, in an editorial accompanying the published SYNCHRONY report.
“The differences in improvement of LVEF are encouraging for both groups,” but were superior for LBB-area pacing, continue Dr. Koneru and Dr. Ellenbogen, both with Virginia Commonwealth University Medical Center, Richmond. “Whether these results would have regressed to the mean over a longer period of follow-up or diverge further with LBB-area pacing continuing to be superior is unknown.”
Years for an answer?
A large randomized comparison of CSP and BiV-CRT, called Left vs. Left, is currently in early stages, Sana M. Al-Khatib, MD, MHS, Duke University Medical Center, Durham, N.C., said in a media presentation on two of the presented studies. It has a planned enrollment of more than 2,100 patients on optimal meds with an LVEF of 50% or lower and either a QRS duration of at least 130 ms or an anticipated burden of RV pacing exceeding 40%.
The trial, she said, “will take years to give an answer, but it is actually designed to address the question of whether a composite endpoint of time to death or heart failure hospitalization can be improved with conduction system pacing vs. biventricular pacing.”
Dr. Al-Khatib is a coauthor on the new guideline covering both CSP and BiV-CRT in HF, as are Dr. Cha, Dr. Varma, Dr. Singh, Dr. Vijayaraman, and Dr. Goldberger; Dr. Ellenbogen is one of the reviewers.
Dr. Diaz discloses receiving honoraria or fees for speaking or teaching from Bayer Healthcare, Pfizer, AstraZeneca, Boston Scientific, and Medtronic. Dr. Vijayaraman discloses receiving honoraria or fees for speaking, teaching, or consulting for Abbott, Medtronic, Biotronik, and Boston Scientific; and receiving research grants from Medtronic. Dr. Varma discloses receiving honoraria or fees for speaking or consulting as an independent contractor for Medtronic, Boston Scientific, Biotronik, Impulse Dynamics USA, Cardiologs, Abbott, Pacemate, Implicity, and EP Solutions. Dr. Singh discloses receiving fees for consulting from EBR Systems, Merit Medical Systems, New Century Health, Biotronik, Abbott, Medtronic, MicroPort Scientific, Cardiologs, Sanofi, CVRx, Impulse Dynamics USA, Octagos, Implicity, Orchestra Biomed, Rhythm Management Group, and Biosense Webster; and receiving honoraria or fees for speaking and teaching from Medscape. Dr. Cha had no relevant financial relationships. Dr. Romero discloses receiving research grants from Biosense Webster; and speaking or receiving honoraria or fees for consulting, speaking, or teaching, or serving on a board for Sanofi, Boston Scientific, and AtriCure. Dr. Koneru discloses consulting for Medtronic and receiving honoraria from Abbott. Dr. Ellenbogen discloses consulting or lecturing for or receiving honoraria from Medtronic, Boston Scientific, and Abbott. Dr. Goldberger discloses receiving royalty income from and serving as an independent contractor for Elsevier. Dr. Al-Khatib discloses receiving research grants from Medtronic and Boston Scientific.
A version of this article first appeared on Medscape.com.
Pacing as a device therapy for heart failure (HF) is headed for what is probably its next big advance.
After decades of biventricular (BiV) pacemaker success in resynchronizing the ventricles and improving clinical outcomes, relatively new conduction-system pacing (CSP) techniques that avoid the pitfalls of right-ventricular (RV) pacing using BiV lead systems have been supplanting traditional cardiac resynchronization therapy (CRT) in selected patients at some major centers. In fact, they are solidly ensconced in a new guideline document addressing indications for CSP and BiV pacing in HF.
But , an alternative when BiV pacing isn’t appropriate or can’t be engaged.
That’s mainly because the limited, mostly observational evidence supporting CSP in the document can’t measure up to the clinical experience and plethora of large, randomized trials behind BiV-CRT.
But that shortfall is headed for change. Several new comparative studies, including a small, randomized trial, have added significantly to evidence suggesting that CSP is at least as effective as traditional CRT for procedural, functional safety, and clinical outcomes.
The new studies “are inherently prone to bias, but their results are really good,” observed Juan C. Diaz, MD. They show improvements in left ventricular ejection fraction (LVEF) and symptoms with CSP that are “outstanding compared to what we have been doing for the last 20 years,” he said in an interview.
Dr. Diaz, Clínica Las Vegas, Medellin, Colombia, is an investigator with the observational SYNCHRONY, which is among the new CSP studies formally presented at the annual scientific sessions of the Heart Rhythm Society. He is also lead author on its same-day publication in JACC: Clinical Electrophysiology.
Dr. Diaz said that CSP, which sustains pacing via the native conduction system, makes more “physiologic sense” than BiV pacing and represents “a step forward” for HF device therapy.
SYNCHRONY compared LBB-area with BiV pacing as the initial strategy for achieving cardiac resynchronization in patients with ischemic or nonischemic cardiomyopathy.
CSP is “a long way” from replacing conventional CRT, he said. But the new studies at the HRS sessions should help extend His-bundle and LBB-area pacing to more patients, he added, given the significant long-term “drawbacks” of BiV pacing. These include inevitable RV pacing, multiple leads, and the risks associated with chronic transvenous leads.
Zachary Goldberger, MD, University of Wisconsin–Madison, went a bit further in support of CSP as invited discussant for the SYNCHRONY presentation.
Given that it improved LVEF, heart failure class, HF hospitalizations (HFH), and mortality in that study and others, Dr. Goldberger said, CSP could potentially “become the dominant mode of resynchronization going forward.”
Other experts at the meeting saw CSP’s potential more as one of several pacing techniques that could be brought to bear for patients with CRT indications.
“Conduction system pacing is going to be a huge complement to biventricular pacing,” to which about 30% of patients have a “less than optimal response,” said Pugazhendhi Vijayaraman, MD, chief of clinical electrophysiology, Geisinger Heart Institute, Danville, Pa.
“I don’t think it needs to replace biventricular pacing, because biventricular pacing is a well-established, incredibly powerful therapy,” he told this news organization. But CSP is likely to provide “a good alternative option” in patients with poor responses to BiV-CRT.
It may, however, render some current BiV-pacing alternatives “obsolete,” Dr. Vijayaraman observed. “At our center, at least for the last 5 years, no patient has needed epicardial surgical left ventricular lead placement” because CSP was a better backup option.
Dr. Vijayaraman presented two of the meeting’s CSP vs. BiV pacing comparisons. In one, the 100-patient randomized HOT-CRT trial, contractile function improved significantly on CSP, which could be either His-bundle or LBB-area pacing.
He also presented an observational study of LBB-area pacing at 15 centers in Asia, Europe, and North America and led the authors of its simultaneous publication in the Journal of the American College of Cardiology.
“I think left-bundle conduction system pacing is the future, for sure,” Jagmeet P. Singh, MD, DPhil, told this news organization. Still, it doesn’t always work and when it does, it “doesn’t work equally in all patients,” he said.
“Conduction system pacing certainly makes a lot of sense,” especially in patients with left-bundle-branch block (LBBB), and “maybe not as a primary approach but certainly as a secondary approach,” said Dr. Singh, Massachusetts General Hospital, Boston, who is not a coauthor on any of the three studies.
He acknowledged that CSP may work well as a first-line option in patients with LBBB at some experienced centers. For those without LBBB or who have an intraventricular conduction delay, who represent 45%-50% of current CRT cases, Dr. Singh observed, “there’s still more evidence” that BiV-CRT is a more appropriate initial approach.
Standard CRT may fail, however, even in some patients who otherwise meet guideline-based indications. “We don’t really understand all the mechanisms for nonresponse in conventional biventricular pacing,” observed Niraj Varma, MD, PhD, Cleveland Clinic, also not involved with any of the three studies.
In some groups, including “patients with larger ventricles,” for example, BiV-CRT doesn’t always narrow the electrocardiographic QRS complex or preexcite delayed left ventricular (LV) activation, hallmarks of successful CRT, he said in an interview.
“I think we need to understand why this occurs in both situations,” but in such cases, CSP alone or as an adjunct to direct LV pacing may be successful. “Sometimes we need both an LV lead and the conduction-system pacing lead.”
Narrower, more efficient use of CSP as a BiV-CRT alternative may also boost its chances for success, Dr. Varma added. “I think we need to refine patient selection.”
HOT-CRT: Randomized CSP vs. BiV pacing trial
Conducted at three centers in a single health system, the His-optimized cardiac resynchronization therapy study (HOT-CRT) randomly assigned 100 patients with primary or secondary CRT indications to either to CSP – by either His-bundle or LBB-area pacing – or to standard BiV-CRT as the first-line resynchronization method.
Treatment crossovers, allowed for either pacing modality in the event of implantation failure, occurred in two patients and nine patients initially assigned to CSP and BiV pacing, respectively (4% vs. 18%), Dr. Vijayaraman reported.
Historically in trials, BiV pacing has elevated LVEF by about 7%, he said. The mean 12-point increase observed with CSP “is huge, in that sense.” HOT-CRT enrolled a predominantly male and White population at centers highly experienced in both CSP and BiV pacing, limiting its broad relevance to practice, as pointed out by both Dr. Vijayaraman and his presentation’s invited discussant, Yong-Mei Cha, MD, Mayo Clinic, Rochester, Minn. Dr. Cha, who is director of cardiac device services at her center, also highlighted the greater rate of crossover from BiV pacing to CSP, 18% vs. 4% in the other direction. “This is a very encouraging result,” because the implant-failure rate for LBB-area pacing may drop once more operators become “familiar and skilled with conduction-system pacing.” Overall, the study supports CSP as “a very good alternative for heart failure patients when BiV pacing fails.”
International comparison of CSP and BiV pacing
In Dr. Vijayaraman’s other study, the observational comparison of LBB-area pacing and BiV-CRT, the CSP technique emerged as a “reasonable alternative to biventricular pacing, not only for improvement in LV function but also to reduce adverse clinical outcomes.”
Indeed, in the international study of 1,778 mostly male patients with primary or secondary CRT indications who received LBB-area or BiV pacing (797 and 981 patients, respectively), those on CSP saw a significant drop in risk for the primary endpoint, death or HFH.
Mean LVEF improved from 27% to 41% in the LBB-area pacing group and 27% to 37% with BiV pacing (P < .001 for both changes) over a follow-up averaging 33 months. The difference in improvement between CSP and BiV pacing was significant at P < .001.
In adjusted analysis, the risk for death or HFH was greater for BiV-pacing patients, a difference driven by HFH events.
- Death or HF: hazard ratio, 1.49 (95% confidence interval, 1.21-1.84; P < .001).
- Death: HR, 1.14 (95% CI, 0.88-1.48; P = .313).
- HFH: HR, 1.49 (95% CI, 1.16-1.92; P = .002)
The analysis has all the “inherent biases” of an observational study. The risk for patient-selection bias, however, was somewhat mitigated by consistent practice patterns at participating centers, Dr. Vijayaraman told this news organization.
For example, he said, operators at six of the institutions were most likely to use CSP as the first-line approach, and the same number of centers usually went with BiV pacing.
SYNCHRONY: First-line LBB-area pacing vs. BiV-CRT
Outcomes using the two approaches were similar in the prospective, international, observational study of 371 patients with ischemic or nonischemic cardiomyopathy and standard CRT indications. Allocation of 128 patients to LBB-area pacing and 243 to BiV-CRT was based on patient and operator preferences, reported Jorge Romero Jr, MD, Brigham and Women’s Hospital, Boston, at the HRS sessions.
Risk for the death-HFH primary endpoint dropped 38% for those initially treated with LBB-area pacing, compared with BiV pacing, primarily because of a lower HFH risk:
- Death or HFH: HR, 0.62 (95% CI, 0.41-0.93; P = .02).
- Death: HR, 0.57 (95% CI, 0.25-1.32; P = .19).
- HFH: HR, 0.61 (95% CI, 0.34-0.93; P = .02)
Patients in the CSP group were also more likely to improve by at least one NYHA (New York Heart Association) class (80.4% vs. 67.9%; P < .001), consistent with their greater absolute change in LVEF (8.0 vs. 3.9 points; P < .01).
The findings “suggest that LBBAP [left-bundle branch area pacing] is an excellent alternative to BiV pacing,” with a comparable safety profile, write Jayanthi N. Koneru, MBBS, and Kenneth A. Ellenbogen, MD, in an editorial accompanying the published SYNCHRONY report.
“The differences in improvement of LVEF are encouraging for both groups,” but were superior for LBB-area pacing, continue Dr. Koneru and Dr. Ellenbogen, both with Virginia Commonwealth University Medical Center, Richmond. “Whether these results would have regressed to the mean over a longer period of follow-up or diverge further with LBB-area pacing continuing to be superior is unknown.”
Years for an answer?
A large randomized comparison of CSP and BiV-CRT, called Left vs. Left, is currently in early stages, Sana M. Al-Khatib, MD, MHS, Duke University Medical Center, Durham, N.C., said in a media presentation on two of the presented studies. It has a planned enrollment of more than 2,100 patients on optimal meds with an LVEF of 50% or lower and either a QRS duration of at least 130 ms or an anticipated burden of RV pacing exceeding 40%.
The trial, she said, “will take years to give an answer, but it is actually designed to address the question of whether a composite endpoint of time to death or heart failure hospitalization can be improved with conduction system pacing vs. biventricular pacing.”
Dr. Al-Khatib is a coauthor on the new guideline covering both CSP and BiV-CRT in HF, as are Dr. Cha, Dr. Varma, Dr. Singh, Dr. Vijayaraman, and Dr. Goldberger; Dr. Ellenbogen is one of the reviewers.
Dr. Diaz discloses receiving honoraria or fees for speaking or teaching from Bayer Healthcare, Pfizer, AstraZeneca, Boston Scientific, and Medtronic. Dr. Vijayaraman discloses receiving honoraria or fees for speaking, teaching, or consulting for Abbott, Medtronic, Biotronik, and Boston Scientific; and receiving research grants from Medtronic. Dr. Varma discloses receiving honoraria or fees for speaking or consulting as an independent contractor for Medtronic, Boston Scientific, Biotronik, Impulse Dynamics USA, Cardiologs, Abbott, Pacemate, Implicity, and EP Solutions. Dr. Singh discloses receiving fees for consulting from EBR Systems, Merit Medical Systems, New Century Health, Biotronik, Abbott, Medtronic, MicroPort Scientific, Cardiologs, Sanofi, CVRx, Impulse Dynamics USA, Octagos, Implicity, Orchestra Biomed, Rhythm Management Group, and Biosense Webster; and receiving honoraria or fees for speaking and teaching from Medscape. Dr. Cha had no relevant financial relationships. Dr. Romero discloses receiving research grants from Biosense Webster; and speaking or receiving honoraria or fees for consulting, speaking, or teaching, or serving on a board for Sanofi, Boston Scientific, and AtriCure. Dr. Koneru discloses consulting for Medtronic and receiving honoraria from Abbott. Dr. Ellenbogen discloses consulting or lecturing for or receiving honoraria from Medtronic, Boston Scientific, and Abbott. Dr. Goldberger discloses receiving royalty income from and serving as an independent contractor for Elsevier. Dr. Al-Khatib discloses receiving research grants from Medtronic and Boston Scientific.
A version of this article first appeared on Medscape.com.
Pacing as a device therapy for heart failure (HF) is headed for what is probably its next big advance.
After decades of biventricular (BiV) pacemaker success in resynchronizing the ventricles and improving clinical outcomes, relatively new conduction-system pacing (CSP) techniques that avoid the pitfalls of right-ventricular (RV) pacing using BiV lead systems have been supplanting traditional cardiac resynchronization therapy (CRT) in selected patients at some major centers. In fact, they are solidly ensconced in a new guideline document addressing indications for CSP and BiV pacing in HF.
But , an alternative when BiV pacing isn’t appropriate or can’t be engaged.
That’s mainly because the limited, mostly observational evidence supporting CSP in the document can’t measure up to the clinical experience and plethora of large, randomized trials behind BiV-CRT.
But that shortfall is headed for change. Several new comparative studies, including a small, randomized trial, have added significantly to evidence suggesting that CSP is at least as effective as traditional CRT for procedural, functional safety, and clinical outcomes.
The new studies “are inherently prone to bias, but their results are really good,” observed Juan C. Diaz, MD. They show improvements in left ventricular ejection fraction (LVEF) and symptoms with CSP that are “outstanding compared to what we have been doing for the last 20 years,” he said in an interview.
Dr. Diaz, Clínica Las Vegas, Medellin, Colombia, is an investigator with the observational SYNCHRONY, which is among the new CSP studies formally presented at the annual scientific sessions of the Heart Rhythm Society. He is also lead author on its same-day publication in JACC: Clinical Electrophysiology.
Dr. Diaz said that CSP, which sustains pacing via the native conduction system, makes more “physiologic sense” than BiV pacing and represents “a step forward” for HF device therapy.
SYNCHRONY compared LBB-area with BiV pacing as the initial strategy for achieving cardiac resynchronization in patients with ischemic or nonischemic cardiomyopathy.
CSP is “a long way” from replacing conventional CRT, he said. But the new studies at the HRS sessions should help extend His-bundle and LBB-area pacing to more patients, he added, given the significant long-term “drawbacks” of BiV pacing. These include inevitable RV pacing, multiple leads, and the risks associated with chronic transvenous leads.
Zachary Goldberger, MD, University of Wisconsin–Madison, went a bit further in support of CSP as invited discussant for the SYNCHRONY presentation.
Given that it improved LVEF, heart failure class, HF hospitalizations (HFH), and mortality in that study and others, Dr. Goldberger said, CSP could potentially “become the dominant mode of resynchronization going forward.”
Other experts at the meeting saw CSP’s potential more as one of several pacing techniques that could be brought to bear for patients with CRT indications.
“Conduction system pacing is going to be a huge complement to biventricular pacing,” to which about 30% of patients have a “less than optimal response,” said Pugazhendhi Vijayaraman, MD, chief of clinical electrophysiology, Geisinger Heart Institute, Danville, Pa.
“I don’t think it needs to replace biventricular pacing, because biventricular pacing is a well-established, incredibly powerful therapy,” he told this news organization. But CSP is likely to provide “a good alternative option” in patients with poor responses to BiV-CRT.
It may, however, render some current BiV-pacing alternatives “obsolete,” Dr. Vijayaraman observed. “At our center, at least for the last 5 years, no patient has needed epicardial surgical left ventricular lead placement” because CSP was a better backup option.
Dr. Vijayaraman presented two of the meeting’s CSP vs. BiV pacing comparisons. In one, the 100-patient randomized HOT-CRT trial, contractile function improved significantly on CSP, which could be either His-bundle or LBB-area pacing.
He also presented an observational study of LBB-area pacing at 15 centers in Asia, Europe, and North America and led the authors of its simultaneous publication in the Journal of the American College of Cardiology.
“I think left-bundle conduction system pacing is the future, for sure,” Jagmeet P. Singh, MD, DPhil, told this news organization. Still, it doesn’t always work and when it does, it “doesn’t work equally in all patients,” he said.
“Conduction system pacing certainly makes a lot of sense,” especially in patients with left-bundle-branch block (LBBB), and “maybe not as a primary approach but certainly as a secondary approach,” said Dr. Singh, Massachusetts General Hospital, Boston, who is not a coauthor on any of the three studies.
He acknowledged that CSP may work well as a first-line option in patients with LBBB at some experienced centers. For those without LBBB or who have an intraventricular conduction delay, who represent 45%-50% of current CRT cases, Dr. Singh observed, “there’s still more evidence” that BiV-CRT is a more appropriate initial approach.
Standard CRT may fail, however, even in some patients who otherwise meet guideline-based indications. “We don’t really understand all the mechanisms for nonresponse in conventional biventricular pacing,” observed Niraj Varma, MD, PhD, Cleveland Clinic, also not involved with any of the three studies.
In some groups, including “patients with larger ventricles,” for example, BiV-CRT doesn’t always narrow the electrocardiographic QRS complex or preexcite delayed left ventricular (LV) activation, hallmarks of successful CRT, he said in an interview.
“I think we need to understand why this occurs in both situations,” but in such cases, CSP alone or as an adjunct to direct LV pacing may be successful. “Sometimes we need both an LV lead and the conduction-system pacing lead.”
Narrower, more efficient use of CSP as a BiV-CRT alternative may also boost its chances for success, Dr. Varma added. “I think we need to refine patient selection.”
HOT-CRT: Randomized CSP vs. BiV pacing trial
Conducted at three centers in a single health system, the His-optimized cardiac resynchronization therapy study (HOT-CRT) randomly assigned 100 patients with primary or secondary CRT indications to either to CSP – by either His-bundle or LBB-area pacing – or to standard BiV-CRT as the first-line resynchronization method.
Treatment crossovers, allowed for either pacing modality in the event of implantation failure, occurred in two patients and nine patients initially assigned to CSP and BiV pacing, respectively (4% vs. 18%), Dr. Vijayaraman reported.
Historically in trials, BiV pacing has elevated LVEF by about 7%, he said. The mean 12-point increase observed with CSP “is huge, in that sense.” HOT-CRT enrolled a predominantly male and White population at centers highly experienced in both CSP and BiV pacing, limiting its broad relevance to practice, as pointed out by both Dr. Vijayaraman and his presentation’s invited discussant, Yong-Mei Cha, MD, Mayo Clinic, Rochester, Minn. Dr. Cha, who is director of cardiac device services at her center, also highlighted the greater rate of crossover from BiV pacing to CSP, 18% vs. 4% in the other direction. “This is a very encouraging result,” because the implant-failure rate for LBB-area pacing may drop once more operators become “familiar and skilled with conduction-system pacing.” Overall, the study supports CSP as “a very good alternative for heart failure patients when BiV pacing fails.”
International comparison of CSP and BiV pacing
In Dr. Vijayaraman’s other study, the observational comparison of LBB-area pacing and BiV-CRT, the CSP technique emerged as a “reasonable alternative to biventricular pacing, not only for improvement in LV function but also to reduce adverse clinical outcomes.”
Indeed, in the international study of 1,778 mostly male patients with primary or secondary CRT indications who received LBB-area or BiV pacing (797 and 981 patients, respectively), those on CSP saw a significant drop in risk for the primary endpoint, death or HFH.
Mean LVEF improved from 27% to 41% in the LBB-area pacing group and 27% to 37% with BiV pacing (P < .001 for both changes) over a follow-up averaging 33 months. The difference in improvement between CSP and BiV pacing was significant at P < .001.
In adjusted analysis, the risk for death or HFH was greater for BiV-pacing patients, a difference driven by HFH events.
- Death or HF: hazard ratio, 1.49 (95% confidence interval, 1.21-1.84; P < .001).
- Death: HR, 1.14 (95% CI, 0.88-1.48; P = .313).
- HFH: HR, 1.49 (95% CI, 1.16-1.92; P = .002)
The analysis has all the “inherent biases” of an observational study. The risk for patient-selection bias, however, was somewhat mitigated by consistent practice patterns at participating centers, Dr. Vijayaraman told this news organization.
For example, he said, operators at six of the institutions were most likely to use CSP as the first-line approach, and the same number of centers usually went with BiV pacing.
SYNCHRONY: First-line LBB-area pacing vs. BiV-CRT
Outcomes using the two approaches were similar in the prospective, international, observational study of 371 patients with ischemic or nonischemic cardiomyopathy and standard CRT indications. Allocation of 128 patients to LBB-area pacing and 243 to BiV-CRT was based on patient and operator preferences, reported Jorge Romero Jr, MD, Brigham and Women’s Hospital, Boston, at the HRS sessions.
Risk for the death-HFH primary endpoint dropped 38% for those initially treated with LBB-area pacing, compared with BiV pacing, primarily because of a lower HFH risk:
- Death or HFH: HR, 0.62 (95% CI, 0.41-0.93; P = .02).
- Death: HR, 0.57 (95% CI, 0.25-1.32; P = .19).
- HFH: HR, 0.61 (95% CI, 0.34-0.93; P = .02)
Patients in the CSP group were also more likely to improve by at least one NYHA (New York Heart Association) class (80.4% vs. 67.9%; P < .001), consistent with their greater absolute change in LVEF (8.0 vs. 3.9 points; P < .01).
The findings “suggest that LBBAP [left-bundle branch area pacing] is an excellent alternative to BiV pacing,” with a comparable safety profile, write Jayanthi N. Koneru, MBBS, and Kenneth A. Ellenbogen, MD, in an editorial accompanying the published SYNCHRONY report.
“The differences in improvement of LVEF are encouraging for both groups,” but were superior for LBB-area pacing, continue Dr. Koneru and Dr. Ellenbogen, both with Virginia Commonwealth University Medical Center, Richmond. “Whether these results would have regressed to the mean over a longer period of follow-up or diverge further with LBB-area pacing continuing to be superior is unknown.”
Years for an answer?
A large randomized comparison of CSP and BiV-CRT, called Left vs. Left, is currently in early stages, Sana M. Al-Khatib, MD, MHS, Duke University Medical Center, Durham, N.C., said in a media presentation on two of the presented studies. It has a planned enrollment of more than 2,100 patients on optimal meds with an LVEF of 50% or lower and either a QRS duration of at least 130 ms or an anticipated burden of RV pacing exceeding 40%.
The trial, she said, “will take years to give an answer, but it is actually designed to address the question of whether a composite endpoint of time to death or heart failure hospitalization can be improved with conduction system pacing vs. biventricular pacing.”
Dr. Al-Khatib is a coauthor on the new guideline covering both CSP and BiV-CRT in HF, as are Dr. Cha, Dr. Varma, Dr. Singh, Dr. Vijayaraman, and Dr. Goldberger; Dr. Ellenbogen is one of the reviewers.
Dr. Diaz discloses receiving honoraria or fees for speaking or teaching from Bayer Healthcare, Pfizer, AstraZeneca, Boston Scientific, and Medtronic. Dr. Vijayaraman discloses receiving honoraria or fees for speaking, teaching, or consulting for Abbott, Medtronic, Biotronik, and Boston Scientific; and receiving research grants from Medtronic. Dr. Varma discloses receiving honoraria or fees for speaking or consulting as an independent contractor for Medtronic, Boston Scientific, Biotronik, Impulse Dynamics USA, Cardiologs, Abbott, Pacemate, Implicity, and EP Solutions. Dr. Singh discloses receiving fees for consulting from EBR Systems, Merit Medical Systems, New Century Health, Biotronik, Abbott, Medtronic, MicroPort Scientific, Cardiologs, Sanofi, CVRx, Impulse Dynamics USA, Octagos, Implicity, Orchestra Biomed, Rhythm Management Group, and Biosense Webster; and receiving honoraria or fees for speaking and teaching from Medscape. Dr. Cha had no relevant financial relationships. Dr. Romero discloses receiving research grants from Biosense Webster; and speaking or receiving honoraria or fees for consulting, speaking, or teaching, or serving on a board for Sanofi, Boston Scientific, and AtriCure. Dr. Koneru discloses consulting for Medtronic and receiving honoraria from Abbott. Dr. Ellenbogen discloses consulting or lecturing for or receiving honoraria from Medtronic, Boston Scientific, and Abbott. Dr. Goldberger discloses receiving royalty income from and serving as an independent contractor for Elsevier. Dr. Al-Khatib discloses receiving research grants from Medtronic and Boston Scientific.
A version of this article first appeared on Medscape.com.
FROM HEART RHYTHM 2023
Dapagliflozin matches non–loop diuretic for congestion in AHF: DAPA-RESIST
suggests a new randomized trial. The drugs were given to the study’s loop diuretic–resistant patients on top of furosemide.
Changes in volume status and measures of pulmonary congestion and risk for serious adverse events were similar for those assigned to take dapagliflozin, an SGLT2 inhibitor, or metolazone, a quinazoline diuretic. Those on dapagliflozin zone ultimately received a larger cumulative furosemide dose in the 61-patient trial, called DAPA-RESIST.
“The next steps are to assess whether a strategy of using SGLT2 inhibitors up front in patients with HF reduces the incidence of diuretic resistance, and to test further combinations of diuretics such as thiazide or thiazide-like diuretics, compared with acetazolamide, when used in addition to an IV loop diuretic and SGLT2 inhibitors together,” Ross T. Campbell, MBChB, PhD, University of Glasgow and Queen Elizabeth University Hospital, also in Glasgow, said in an interview.
Dr. Campbell presented the findings at the annual meeting of the Heart Failure Association of the European Society of Cardiology and is senior author on its simultaneous publication in the European Heart Journal.
The multicenter trial randomly assigned 61 patients with AHF to receive dapagliflozin at a fixed dose of 10 mg once daily or metolazone 5 mg or 10 mg (starting dosage at physician discretion) once daily for 3 days of treatment on an open-label basis.
Patients had entered the trial on furosemide at a mean daily dosage of 260 mg in the dapagliflozin group and 229 mg for those assigned metolazone; dosages for the loop diuretic in the trial weren’t prespecified.
Their median age was 79 and 54% were women; 44% had HF with reduced ejection fraction. Their mean glomerular filtration rate was below 30 mL/min per 1.73 m2 in 26%, 90% had chronic kidney disease, 98% had peripheral edema, and 46% had diabetes.
The mean cumulative furosemide dose was significantly higher among the dapagliflozin group’s 31 patients, 976 mg versus 704 mg for the 30 on acetazolamide (P < .05), 96 hours after the start of randomized therapy. However, patients on dapagliflozin experienced a lesser increase in creatinine (P < .05) and in blood urea (P < .01), a greater change in serum sodium (P < .05), and a smaller reduction in serum potassium (P < .01).
Although the trial wasn’t powered for those outcomes, Dr. Campbell said, “less biochemical upset could be associated with better outcomes in terms of less medium- to long-term renal impairment, and in the short-term length of stay.”
The mean decrease in weight at 96 hours, the primary endpoint, reached 3 kg on dapagliflozin, compared with 3.6 kg with metolazone (P = .082), a difference that fell short of significance.
Loop diuretic efficiency, that is weight change in kg per 40 mg furosemide, “was smaller with dapagliflozin than with metolazone at each time point after randomization, although the difference was only significant at 24 hours,” the published report states.
Changes in pulmonary congestion (by lung ultrasound) and fluid volume were similar between the groups.
“This trial further adds to the evidence base and safety profile for using SGLT2 inhibitors in patients with acute heart failure,” and “gives further confidence to clinicians that this class can be started in ‘sicker’ patients with HF who also have diuretic resistance,” Dr. Campbell said.
Asked during his presentation’s question and answer whether dapagliflozin might have shown a greater effect had the dosage been higher, Dr. Campbell explained that the drug was investigational when the trial started. Adding a higher-dose dapagliflozin arm, he said, would have made for an excessively complex study. But “that’s a great research question for another trial.”
DAPA-RESIST was funded by AstraZeneca. Dr. Campbell disclosed receiving honoraria from AstraZeneca for speaking and from Bayer for serving on an advisory board.
A version of this article first appeared on Medscape.com.
suggests a new randomized trial. The drugs were given to the study’s loop diuretic–resistant patients on top of furosemide.
Changes in volume status and measures of pulmonary congestion and risk for serious adverse events were similar for those assigned to take dapagliflozin, an SGLT2 inhibitor, or metolazone, a quinazoline diuretic. Those on dapagliflozin zone ultimately received a larger cumulative furosemide dose in the 61-patient trial, called DAPA-RESIST.
“The next steps are to assess whether a strategy of using SGLT2 inhibitors up front in patients with HF reduces the incidence of diuretic resistance, and to test further combinations of diuretics such as thiazide or thiazide-like diuretics, compared with acetazolamide, when used in addition to an IV loop diuretic and SGLT2 inhibitors together,” Ross T. Campbell, MBChB, PhD, University of Glasgow and Queen Elizabeth University Hospital, also in Glasgow, said in an interview.
Dr. Campbell presented the findings at the annual meeting of the Heart Failure Association of the European Society of Cardiology and is senior author on its simultaneous publication in the European Heart Journal.
The multicenter trial randomly assigned 61 patients with AHF to receive dapagliflozin at a fixed dose of 10 mg once daily or metolazone 5 mg or 10 mg (starting dosage at physician discretion) once daily for 3 days of treatment on an open-label basis.
Patients had entered the trial on furosemide at a mean daily dosage of 260 mg in the dapagliflozin group and 229 mg for those assigned metolazone; dosages for the loop diuretic in the trial weren’t prespecified.
Their median age was 79 and 54% were women; 44% had HF with reduced ejection fraction. Their mean glomerular filtration rate was below 30 mL/min per 1.73 m2 in 26%, 90% had chronic kidney disease, 98% had peripheral edema, and 46% had diabetes.
The mean cumulative furosemide dose was significantly higher among the dapagliflozin group’s 31 patients, 976 mg versus 704 mg for the 30 on acetazolamide (P < .05), 96 hours after the start of randomized therapy. However, patients on dapagliflozin experienced a lesser increase in creatinine (P < .05) and in blood urea (P < .01), a greater change in serum sodium (P < .05), and a smaller reduction in serum potassium (P < .01).
Although the trial wasn’t powered for those outcomes, Dr. Campbell said, “less biochemical upset could be associated with better outcomes in terms of less medium- to long-term renal impairment, and in the short-term length of stay.”
The mean decrease in weight at 96 hours, the primary endpoint, reached 3 kg on dapagliflozin, compared with 3.6 kg with metolazone (P = .082), a difference that fell short of significance.
Loop diuretic efficiency, that is weight change in kg per 40 mg furosemide, “was smaller with dapagliflozin than with metolazone at each time point after randomization, although the difference was only significant at 24 hours,” the published report states.
Changes in pulmonary congestion (by lung ultrasound) and fluid volume were similar between the groups.
“This trial further adds to the evidence base and safety profile for using SGLT2 inhibitors in patients with acute heart failure,” and “gives further confidence to clinicians that this class can be started in ‘sicker’ patients with HF who also have diuretic resistance,” Dr. Campbell said.
Asked during his presentation’s question and answer whether dapagliflozin might have shown a greater effect had the dosage been higher, Dr. Campbell explained that the drug was investigational when the trial started. Adding a higher-dose dapagliflozin arm, he said, would have made for an excessively complex study. But “that’s a great research question for another trial.”
DAPA-RESIST was funded by AstraZeneca. Dr. Campbell disclosed receiving honoraria from AstraZeneca for speaking and from Bayer for serving on an advisory board.
A version of this article first appeared on Medscape.com.
suggests a new randomized trial. The drugs were given to the study’s loop diuretic–resistant patients on top of furosemide.
Changes in volume status and measures of pulmonary congestion and risk for serious adverse events were similar for those assigned to take dapagliflozin, an SGLT2 inhibitor, or metolazone, a quinazoline diuretic. Those on dapagliflozin zone ultimately received a larger cumulative furosemide dose in the 61-patient trial, called DAPA-RESIST.
“The next steps are to assess whether a strategy of using SGLT2 inhibitors up front in patients with HF reduces the incidence of diuretic resistance, and to test further combinations of diuretics such as thiazide or thiazide-like diuretics, compared with acetazolamide, when used in addition to an IV loop diuretic and SGLT2 inhibitors together,” Ross T. Campbell, MBChB, PhD, University of Glasgow and Queen Elizabeth University Hospital, also in Glasgow, said in an interview.
Dr. Campbell presented the findings at the annual meeting of the Heart Failure Association of the European Society of Cardiology and is senior author on its simultaneous publication in the European Heart Journal.
The multicenter trial randomly assigned 61 patients with AHF to receive dapagliflozin at a fixed dose of 10 mg once daily or metolazone 5 mg or 10 mg (starting dosage at physician discretion) once daily for 3 days of treatment on an open-label basis.
Patients had entered the trial on furosemide at a mean daily dosage of 260 mg in the dapagliflozin group and 229 mg for those assigned metolazone; dosages for the loop diuretic in the trial weren’t prespecified.
Their median age was 79 and 54% were women; 44% had HF with reduced ejection fraction. Their mean glomerular filtration rate was below 30 mL/min per 1.73 m2 in 26%, 90% had chronic kidney disease, 98% had peripheral edema, and 46% had diabetes.
The mean cumulative furosemide dose was significantly higher among the dapagliflozin group’s 31 patients, 976 mg versus 704 mg for the 30 on acetazolamide (P < .05), 96 hours after the start of randomized therapy. However, patients on dapagliflozin experienced a lesser increase in creatinine (P < .05) and in blood urea (P < .01), a greater change in serum sodium (P < .05), and a smaller reduction in serum potassium (P < .01).
Although the trial wasn’t powered for those outcomes, Dr. Campbell said, “less biochemical upset could be associated with better outcomes in terms of less medium- to long-term renal impairment, and in the short-term length of stay.”
The mean decrease in weight at 96 hours, the primary endpoint, reached 3 kg on dapagliflozin, compared with 3.6 kg with metolazone (P = .082), a difference that fell short of significance.
Loop diuretic efficiency, that is weight change in kg per 40 mg furosemide, “was smaller with dapagliflozin than with metolazone at each time point after randomization, although the difference was only significant at 24 hours,” the published report states.
Changes in pulmonary congestion (by lung ultrasound) and fluid volume were similar between the groups.
“This trial further adds to the evidence base and safety profile for using SGLT2 inhibitors in patients with acute heart failure,” and “gives further confidence to clinicians that this class can be started in ‘sicker’ patients with HF who also have diuretic resistance,” Dr. Campbell said.
Asked during his presentation’s question and answer whether dapagliflozin might have shown a greater effect had the dosage been higher, Dr. Campbell explained that the drug was investigational when the trial started. Adding a higher-dose dapagliflozin arm, he said, would have made for an excessively complex study. But “that’s a great research question for another trial.”
DAPA-RESIST was funded by AstraZeneca. Dr. Campbell disclosed receiving honoraria from AstraZeneca for speaking and from Bayer for serving on an advisory board.
A version of this article first appeared on Medscape.com.
FROM HFA-ESC 2023
Medicaid patients with heart failure get poor follow-up after hospital discharge
Nearly 60% of Medicaid-covered adults with concurrent diabetes and heart failure did not receive guideline-concordant postdischarge care within 7-10 days of leaving the hospital, according to a large Alabama study. Moreover, affected Black and Hispanic/other Alabamians were less likely than were their White counterparts to receive recommended postdischarge care.
In comparison with White participants, Black and Hispanic adults were less likely to have any postdischarge ambulatory care visits after HF hospitalization or had a delayed visit, according to researchers led by Yulia Khodneva, MD, PhD, an internist at the University of Alabama at Birmingham. “This is likely a reflection of a structural racism and implicit bias against racial and ethnic minorities that persists in the U.S. health care system,” she and her colleagues wrote.
The findings point to the need for strategies to improve access to postdischarge care for lower-income HF patients.
Among U.S. states, Alabama is the sixth-poorest, the third in diabetes prevalence (14%), and has the highest rates of heart failure hospitalizations and cardiovascular mortality, the authors noted.
Study details
The cohort included 9,857 adults with diabetes and first hospitalizations for heart failure who were covered by Alabama Medicaid during 2010-2019. The investigators analyzed patients’ claims for ambulatory care (any, primary, cardiology, or endocrinology) within 60 days of discharge.
The mean age of participants was 53.7 years; 47.3% were Black; 41.8% non-Hispanic White; and 10.9% Hispanic/other, with other including those identifying as non-White Hispanic, American Indian, Pacific Islander, and Asian. About two-thirds (65.4%) of participants were women.
Analysis revealed low rates of follow-up care after hospital discharge; 26.7% had an ambulatory visit within 0-7 days, 15.2% within 8-14 days, 31.3% within 15-60 days, and 26.8% had no follow-up visit at all. Of those having a follow-up visit, 71% saw a primary care physician and 12% saw a cardiologist.
In contrast, a much higher proportion of heart failure patients in a Swedish registry – 63% – received ambulatory follow-up in cardiology.
Ethnic/gender/age disparities
Black and Hispanic/other adults were less likely to have any postdischarge ambulatory visit (P <.0001) or had the visit delayed by 1.8 days (P = .0006) and 2.8 days (P = .0016), respectively. They were less likely to see a primary care physician than were non-Hispanic White adults: adjusted incidence rate ratio, 0.96 (95% confidence interval [CI], 0.91-1.00) and 0.91 (95% CI, 0.89-0.98), respectively.
Men and those with longer-standing heart failure were less likely to be seen in primary care, while the presence of multiple comorbidities was associated with a higher likelihood of a postdischarge primary care visit. Men were more likely to be seen by a cardiologist, while older discharged patients were less likely to be seen by an endocrinologist within 60 days. There was a U-shaped relationship between the timing of the first postdischarge ambulatory visit and all-cause mortality among adults with diabetes and heart failure. Higher rates of 60-day all-cause mortality were observed both in those who had seen a provider within 0-7 days after discharge and in those who had not seen any provider during the 60-day study period compared with those having an ambulatory care visit within 7-14 or 15-60 days. “The group with early follow-up (0-7 days) likely represents a sicker population of patients with heart failure with more comorbidity burden and higher overall health care use, including readmissions, as was demonstrated in our analysis,” Dr. Khodneva and associates wrote. “Interventions that improve access to postdischarge ambulatory care for low-income patients with diabetes and heart failure and eliminate racial and ethnic disparities may be warranted,” they added.
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases and the University of Alabama at Birmingham Diabetes Research Center. Dr. Khodneva reported funding from the University of Alabama at Birmingham and the Forge Ahead Center as well as from the NIDDK, the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Alabama Medicaid Agency. Coauthor Emily Levitan, ScD, reported research funding from Amgen and has served on Amgen advisory boards. She has also served as a scientific consultant for a research project funded by Novartis.
Nearly 60% of Medicaid-covered adults with concurrent diabetes and heart failure did not receive guideline-concordant postdischarge care within 7-10 days of leaving the hospital, according to a large Alabama study. Moreover, affected Black and Hispanic/other Alabamians were less likely than were their White counterparts to receive recommended postdischarge care.
In comparison with White participants, Black and Hispanic adults were less likely to have any postdischarge ambulatory care visits after HF hospitalization or had a delayed visit, according to researchers led by Yulia Khodneva, MD, PhD, an internist at the University of Alabama at Birmingham. “This is likely a reflection of a structural racism and implicit bias against racial and ethnic minorities that persists in the U.S. health care system,” she and her colleagues wrote.
The findings point to the need for strategies to improve access to postdischarge care for lower-income HF patients.
Among U.S. states, Alabama is the sixth-poorest, the third in diabetes prevalence (14%), and has the highest rates of heart failure hospitalizations and cardiovascular mortality, the authors noted.
Study details
The cohort included 9,857 adults with diabetes and first hospitalizations for heart failure who were covered by Alabama Medicaid during 2010-2019. The investigators analyzed patients’ claims for ambulatory care (any, primary, cardiology, or endocrinology) within 60 days of discharge.
The mean age of participants was 53.7 years; 47.3% were Black; 41.8% non-Hispanic White; and 10.9% Hispanic/other, with other including those identifying as non-White Hispanic, American Indian, Pacific Islander, and Asian. About two-thirds (65.4%) of participants were women.
Analysis revealed low rates of follow-up care after hospital discharge; 26.7% had an ambulatory visit within 0-7 days, 15.2% within 8-14 days, 31.3% within 15-60 days, and 26.8% had no follow-up visit at all. Of those having a follow-up visit, 71% saw a primary care physician and 12% saw a cardiologist.
In contrast, a much higher proportion of heart failure patients in a Swedish registry – 63% – received ambulatory follow-up in cardiology.
Ethnic/gender/age disparities
Black and Hispanic/other adults were less likely to have any postdischarge ambulatory visit (P <.0001) or had the visit delayed by 1.8 days (P = .0006) and 2.8 days (P = .0016), respectively. They were less likely to see a primary care physician than were non-Hispanic White adults: adjusted incidence rate ratio, 0.96 (95% confidence interval [CI], 0.91-1.00) and 0.91 (95% CI, 0.89-0.98), respectively.
Men and those with longer-standing heart failure were less likely to be seen in primary care, while the presence of multiple comorbidities was associated with a higher likelihood of a postdischarge primary care visit. Men were more likely to be seen by a cardiologist, while older discharged patients were less likely to be seen by an endocrinologist within 60 days. There was a U-shaped relationship between the timing of the first postdischarge ambulatory visit and all-cause mortality among adults with diabetes and heart failure. Higher rates of 60-day all-cause mortality were observed both in those who had seen a provider within 0-7 days after discharge and in those who had not seen any provider during the 60-day study period compared with those having an ambulatory care visit within 7-14 or 15-60 days. “The group with early follow-up (0-7 days) likely represents a sicker population of patients with heart failure with more comorbidity burden and higher overall health care use, including readmissions, as was demonstrated in our analysis,” Dr. Khodneva and associates wrote. “Interventions that improve access to postdischarge ambulatory care for low-income patients with diabetes and heart failure and eliminate racial and ethnic disparities may be warranted,” they added.
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases and the University of Alabama at Birmingham Diabetes Research Center. Dr. Khodneva reported funding from the University of Alabama at Birmingham and the Forge Ahead Center as well as from the NIDDK, the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Alabama Medicaid Agency. Coauthor Emily Levitan, ScD, reported research funding from Amgen and has served on Amgen advisory boards. She has also served as a scientific consultant for a research project funded by Novartis.
Nearly 60% of Medicaid-covered adults with concurrent diabetes and heart failure did not receive guideline-concordant postdischarge care within 7-10 days of leaving the hospital, according to a large Alabama study. Moreover, affected Black and Hispanic/other Alabamians were less likely than were their White counterparts to receive recommended postdischarge care.
In comparison with White participants, Black and Hispanic adults were less likely to have any postdischarge ambulatory care visits after HF hospitalization or had a delayed visit, according to researchers led by Yulia Khodneva, MD, PhD, an internist at the University of Alabama at Birmingham. “This is likely a reflection of a structural racism and implicit bias against racial and ethnic minorities that persists in the U.S. health care system,” she and her colleagues wrote.
The findings point to the need for strategies to improve access to postdischarge care for lower-income HF patients.
Among U.S. states, Alabama is the sixth-poorest, the third in diabetes prevalence (14%), and has the highest rates of heart failure hospitalizations and cardiovascular mortality, the authors noted.
Study details
The cohort included 9,857 adults with diabetes and first hospitalizations for heart failure who were covered by Alabama Medicaid during 2010-2019. The investigators analyzed patients’ claims for ambulatory care (any, primary, cardiology, or endocrinology) within 60 days of discharge.
The mean age of participants was 53.7 years; 47.3% were Black; 41.8% non-Hispanic White; and 10.9% Hispanic/other, with other including those identifying as non-White Hispanic, American Indian, Pacific Islander, and Asian. About two-thirds (65.4%) of participants were women.
Analysis revealed low rates of follow-up care after hospital discharge; 26.7% had an ambulatory visit within 0-7 days, 15.2% within 8-14 days, 31.3% within 15-60 days, and 26.8% had no follow-up visit at all. Of those having a follow-up visit, 71% saw a primary care physician and 12% saw a cardiologist.
In contrast, a much higher proportion of heart failure patients in a Swedish registry – 63% – received ambulatory follow-up in cardiology.
Ethnic/gender/age disparities
Black and Hispanic/other adults were less likely to have any postdischarge ambulatory visit (P <.0001) or had the visit delayed by 1.8 days (P = .0006) and 2.8 days (P = .0016), respectively. They were less likely to see a primary care physician than were non-Hispanic White adults: adjusted incidence rate ratio, 0.96 (95% confidence interval [CI], 0.91-1.00) and 0.91 (95% CI, 0.89-0.98), respectively.
Men and those with longer-standing heart failure were less likely to be seen in primary care, while the presence of multiple comorbidities was associated with a higher likelihood of a postdischarge primary care visit. Men were more likely to be seen by a cardiologist, while older discharged patients were less likely to be seen by an endocrinologist within 60 days. There was a U-shaped relationship between the timing of the first postdischarge ambulatory visit and all-cause mortality among adults with diabetes and heart failure. Higher rates of 60-day all-cause mortality were observed both in those who had seen a provider within 0-7 days after discharge and in those who had not seen any provider during the 60-day study period compared with those having an ambulatory care visit within 7-14 or 15-60 days. “The group with early follow-up (0-7 days) likely represents a sicker population of patients with heart failure with more comorbidity burden and higher overall health care use, including readmissions, as was demonstrated in our analysis,” Dr. Khodneva and associates wrote. “Interventions that improve access to postdischarge ambulatory care for low-income patients with diabetes and heart failure and eliminate racial and ethnic disparities may be warranted,” they added.
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases and the University of Alabama at Birmingham Diabetes Research Center. Dr. Khodneva reported funding from the University of Alabama at Birmingham and the Forge Ahead Center as well as from the NIDDK, the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Alabama Medicaid Agency. Coauthor Emily Levitan, ScD, reported research funding from Amgen and has served on Amgen advisory boards. She has also served as a scientific consultant for a research project funded by Novartis.
FROM JOURNAL OF THE AMERICAN HEART ASSOCIATION