User login
Use of Fluorodeoxyglucose-Positron Emission Tomography in the Diagnosis of Intravascular Diffuse Large B-Cell Lymphoma
Patient 1
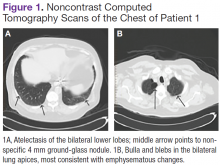
A white man aged 67 years, with diastolic heart failure and chronic obstructive pulmonary disease, presented to the emergency department (ED) with shortness of breath. The initial laboratory results were significant for a newly elevated creatinine level of 2.06 mg/dL and a brain natriuretic peptide level of 648 pg/mL.
Imaging studies included a chest radiograph, a ventilation/perfusion scan, and an echocardiogram, as well as a right heart catheterization. All were nondiagnostic.
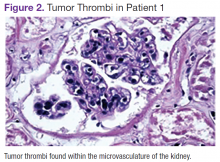
The patient's shortness of breath persisted despite treatment with diuretics, antibiotics, and steroids. Further laboratory workup revealed an elevated lactate dehydrogenase (LDH) level of 1,338 IU/L. A bone marrow biopsy performed because of concern about malignancy was unremarkable. Flow cytometry of the bone marrow aspirate did not reveal clonal B- or T-cell populations. Immunohistochemical staining was not performed. During this hospitalization for shortness of breath, the patient's mental staus began to decline, and his oxygen requirements increased. The patient was intubated but expired 48 hours after mechanical ventilation was initiated.
Patient 2
A white woman aged 67 years presented to the ED with generalized weakness, fatigue, and nausea. The patient’s medical history was significant for a diagnosis of stage IIIa ovarian cancer. She was treated with surgical resection and completed 6 cycles of adjuvant carboplatin and paclitaxel 3 months prior to this presentation. She had good response to treatment with normalization of CA-125.
After completion of chemotherapy, the patient was found to have persistent anemia and thrombocytopenia. Admission laboratory results were significant for a hemoglobin level of 8.4 g/dL, a platelet count of 20,000/μL, and an LDH level of 1,220 IU/L.
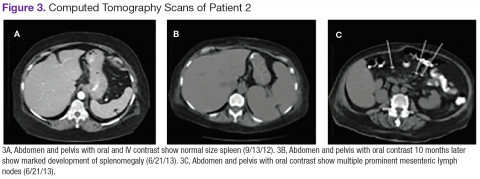
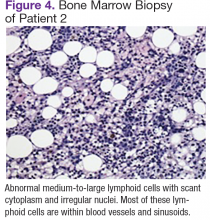
Chest, abdomen, and pelvis CT scans showed mesenteric adenopathy and splenomegaly (Figures 3A, 3B, and 3C) compared with prior imaging. Bone marrow biopsy revealed large lymphoid cells with scant cytoplasm and irregular nuclei, primarily within blood vessels and sinusoids consistent with IVLBCL (Figure 4). Flow cytometry of the bone marrow specimen showed an abnormal B-cell population with expression CD20, CD19, FMC-7, and dim κ light chain restriction. The cells were negative for CD5 and CD10. Immunohistochemical staining was positive for CD20, CD79a, PAX5, BCL-2 , and MUM1.
The patient was treated with 4 cycles of cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab, plus intrathecal methotrexate. The chemotherapy dose was reduced in the final cycle because of neuropathy in the hands and feet. The patient had undergone autologous stem-cell transplantation to allow high-dose chemotherapy. She was doing well more than 5 months after her transplant without evidence of recurrent disease.
Patient 3
A white man aged 76 years presented to the ED with cutaneous nodules, weight loss, fatigue, fevers, and epigastric pain. The patient’s medical history was significant for asymptomatic lymphoplasmacytic lymphoma diagnosed 2 months earlier, which had not required treatment. Laboratory results on admission revealed transaminitis, mild anemia with a hemoglobin level of 11 g/dL, and LDH level of 497 IU/L.
Chest, abdomen, and pelvis CT scans showed a 1.7cm hepatic lesion and mesenteric adenopathy. A bone marrow biopsy was unchanged from prior studies and showed minimal involvement (5%) of marrow space by low grade B-cell lymphoma.
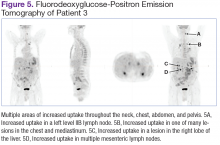
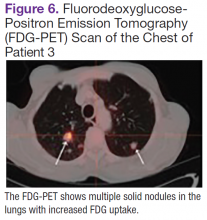
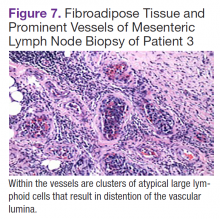
Fluorodeoxyglucose-positron emission tomography (FDG-PET) scans showed multiple areas of uptake in the neck, chest, abdomen, and pelvis (Figures 5 and 6). No increased uptake in the subcutaneous nodules was noted on examination. Laparoscopic biopsy of FDG-avid mesenteric nodes showed clusters of atypical large lymphoid cells resulting in distention of the vascular lumina, resulting in the diagnosis of IVLBCL (Figure 7).
Immunohistochemical stains showed that the intravascular lymphocytes were strongly positive for CD20 and BCL-2 and negative for CD5 and CD10. Flow cytometry on the sample was limited by a low cell count and could not be assessed for clonality. The patient completed 6 cycles of rituximab as well as intrathecal methotrexate. Restaging studies showed a complete remission.
Two months later, the patient developed a skin nodule on the right shoulder. A repeat FDG-PET scan showed increased uptake, and fine-needle biopsy confirmed recurrent disease. The patient is undergoing treatment with ifosfamide, carboplatin, etoposide, and rituximab, as well as workup for autologous stem-cell transplant.
Discussion
Intravascular large B-cell lymphoma, a subtype of diffuse large B-cell lymphoma, is unique because it is primarily extranodal and typically without significant tumor burden.1-4 Standard imaging modalities, therefore, are often nonspecific and do not aid clinicians in establishing a diagnosis. Fluorodeoxyglucose-positron emission tomography has a known role in the assessment of diffuse large B-cell lymphoma, both at time of diagnosis and in monitoring response to treatment.5 However, the use of FDGPET in the diagnosis and management of IVLBCL has not been clearly established.
In a review of the literature, 26 English-language case reports and small case series reporting individual centers’ experience with the use of this imaging modality in the diagnosis of IVLBCL were identified. Two cases were eliminated from review because they did not discuss the use of FDG-PET in relationship to diagnosis. Of the remaining 24 cases, 21 underwent initial imaging with 1 or more of the following imaging modalities: CT, magnetic resonance, ultrasound, bone scan, and gallium scintigraphy, all of which were nonspecific and did not lead to a definitive diagnosis.3,6-25 Each of the 21 cases was followed up by FDG-PET; in 19, the FDG-PET scan was positive and resulted in a diagnosis of IVLBCL. In 2 cases, the FDG-PET scan was nonrevealing and was not considered helpful in diagnosis.11,18 In 3 of the 21 cases, the FDG-PET scan was the primary imaging modality.6,14,25
In this review, all 3 patients had initial imaging with CT scans of anatomic locations that were largely unrevealing, although later histologic examination showed them to be locations of active disease either by biopsy or on autopsy. One patient who underwent early FDG-PET was found to have increased uptake in the mesenteric lymph nodes, which were later biopsied, as well as uptake in the bilateral adrenal glands, lungs, and bone.
Several characteristic FDG-PET findings that have been described in the literature have been identified in patients with IVLBCL, including diffuse accumulation in bilateral lung fields, accumulation in the renal cortex or adrenal glands, diffuse bony involvement, and hypometabolism in the brain.7,10,12,13,17,23,25 These findings show that organs with the richest blood supply, specifically the lungs and kidneys, often are affected. The brain, an obligate glucose metabolizer, would be expected to have high uptake; however, with tumor thrombi occluding small intracranial vessels, micro infarcts ensue and are evidenced by areas of low uptake on FDG-PET scans in patients with IVLBCL.7 These characteristic patterns seen on FDG-PET scans can help to support a diagnosis of IVLBCL when clinical suspicion is high. Further, clinicians may be able to use imaging results to guide an appropriate site for biopsy to confirm diagnosis.
Conclusion
Intravascular large B-cell lymphoma remains a diagnostic challenge for clinicians. Prognosis is generally poor and likely related to frequent delays in diagnosis.1 Clinicians continue to work toward improving their ability to diagnose this disease in its early stages. New diagnostic algorithms and the use of random skin biopsies have shown some promise in improving diagnostic efficiency.26-28 Based on the authors’ experience and review of the literature, FDG-PET may be another promising tool to aid early diagnosis. Characteristic FDG-PET findings have been well described and may help to support the diagnosis of IVLBCL and guide an appropriate biopsy site when clinical suspicion for IVLBCL exists.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
Patient 1
A white man aged 67 years, with diastolic heart failure and chronic obstructive pulmonary disease, presented to the emergency department (ED) with shortness of breath. The initial laboratory results were significant for a newly elevated creatinine level of 2.06 mg/dL and a brain natriuretic peptide level of 648 pg/mL.
Imaging studies included a chest radiograph, a ventilation/perfusion scan, and an echocardiogram, as well as a right heart catheterization. All were nondiagnostic.
The patient's shortness of breath persisted despite treatment with diuretics, antibiotics, and steroids. Further laboratory workup revealed an elevated lactate dehydrogenase (LDH) level of 1,338 IU/L. A bone marrow biopsy performed because of concern about malignancy was unremarkable. Flow cytometry of the bone marrow aspirate did not reveal clonal B- or T-cell populations. Immunohistochemical staining was not performed. During this hospitalization for shortness of breath, the patient's mental staus began to decline, and his oxygen requirements increased. The patient was intubated but expired 48 hours after mechanical ventilation was initiated.
Patient 2
A white woman aged 67 years presented to the ED with generalized weakness, fatigue, and nausea. The patient’s medical history was significant for a diagnosis of stage IIIa ovarian cancer. She was treated with surgical resection and completed 6 cycles of adjuvant carboplatin and paclitaxel 3 months prior to this presentation. She had good response to treatment with normalization of CA-125.
After completion of chemotherapy, the patient was found to have persistent anemia and thrombocytopenia. Admission laboratory results were significant for a hemoglobin level of 8.4 g/dL, a platelet count of 20,000/μL, and an LDH level of 1,220 IU/L.
Chest, abdomen, and pelvis CT scans showed mesenteric adenopathy and splenomegaly (Figures 3A, 3B, and 3C) compared with prior imaging. Bone marrow biopsy revealed large lymphoid cells with scant cytoplasm and irregular nuclei, primarily within blood vessels and sinusoids consistent with IVLBCL (Figure 4). Flow cytometry of the bone marrow specimen showed an abnormal B-cell population with expression CD20, CD19, FMC-7, and dim κ light chain restriction. The cells were negative for CD5 and CD10. Immunohistochemical staining was positive for CD20, CD79a, PAX5, BCL-2 , and MUM1.
The patient was treated with 4 cycles of cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab, plus intrathecal methotrexate. The chemotherapy dose was reduced in the final cycle because of neuropathy in the hands and feet. The patient had undergone autologous stem-cell transplantation to allow high-dose chemotherapy. She was doing well more than 5 months after her transplant without evidence of recurrent disease.
Patient 3
A white man aged 76 years presented to the ED with cutaneous nodules, weight loss, fatigue, fevers, and epigastric pain. The patient’s medical history was significant for asymptomatic lymphoplasmacytic lymphoma diagnosed 2 months earlier, which had not required treatment. Laboratory results on admission revealed transaminitis, mild anemia with a hemoglobin level of 11 g/dL, and LDH level of 497 IU/L.
Chest, abdomen, and pelvis CT scans showed a 1.7cm hepatic lesion and mesenteric adenopathy. A bone marrow biopsy was unchanged from prior studies and showed minimal involvement (5%) of marrow space by low grade B-cell lymphoma.
Fluorodeoxyglucose-positron emission tomography (FDG-PET) scans showed multiple areas of uptake in the neck, chest, abdomen, and pelvis (Figures 5 and 6). No increased uptake in the subcutaneous nodules was noted on examination. Laparoscopic biopsy of FDG-avid mesenteric nodes showed clusters of atypical large lymphoid cells resulting in distention of the vascular lumina, resulting in the diagnosis of IVLBCL (Figure 7).
Immunohistochemical stains showed that the intravascular lymphocytes were strongly positive for CD20 and BCL-2 and negative for CD5 and CD10. Flow cytometry on the sample was limited by a low cell count and could not be assessed for clonality. The patient completed 6 cycles of rituximab as well as intrathecal methotrexate. Restaging studies showed a complete remission.
Two months later, the patient developed a skin nodule on the right shoulder. A repeat FDG-PET scan showed increased uptake, and fine-needle biopsy confirmed recurrent disease. The patient is undergoing treatment with ifosfamide, carboplatin, etoposide, and rituximab, as well as workup for autologous stem-cell transplant.
Discussion
Intravascular large B-cell lymphoma, a subtype of diffuse large B-cell lymphoma, is unique because it is primarily extranodal and typically without significant tumor burden.1-4 Standard imaging modalities, therefore, are often nonspecific and do not aid clinicians in establishing a diagnosis. Fluorodeoxyglucose-positron emission tomography has a known role in the assessment of diffuse large B-cell lymphoma, both at time of diagnosis and in monitoring response to treatment.5 However, the use of FDGPET in the diagnosis and management of IVLBCL has not been clearly established.
In a review of the literature, 26 English-language case reports and small case series reporting individual centers’ experience with the use of this imaging modality in the diagnosis of IVLBCL were identified. Two cases were eliminated from review because they did not discuss the use of FDG-PET in relationship to diagnosis. Of the remaining 24 cases, 21 underwent initial imaging with 1 or more of the following imaging modalities: CT, magnetic resonance, ultrasound, bone scan, and gallium scintigraphy, all of which were nonspecific and did not lead to a definitive diagnosis.3,6-25 Each of the 21 cases was followed up by FDG-PET; in 19, the FDG-PET scan was positive and resulted in a diagnosis of IVLBCL. In 2 cases, the FDG-PET scan was nonrevealing and was not considered helpful in diagnosis.11,18 In 3 of the 21 cases, the FDG-PET scan was the primary imaging modality.6,14,25
In this review, all 3 patients had initial imaging with CT scans of anatomic locations that were largely unrevealing, although later histologic examination showed them to be locations of active disease either by biopsy or on autopsy. One patient who underwent early FDG-PET was found to have increased uptake in the mesenteric lymph nodes, which were later biopsied, as well as uptake in the bilateral adrenal glands, lungs, and bone.
Several characteristic FDG-PET findings that have been described in the literature have been identified in patients with IVLBCL, including diffuse accumulation in bilateral lung fields, accumulation in the renal cortex or adrenal glands, diffuse bony involvement, and hypometabolism in the brain.7,10,12,13,17,23,25 These findings show that organs with the richest blood supply, specifically the lungs and kidneys, often are affected. The brain, an obligate glucose metabolizer, would be expected to have high uptake; however, with tumor thrombi occluding small intracranial vessels, micro infarcts ensue and are evidenced by areas of low uptake on FDG-PET scans in patients with IVLBCL.7 These characteristic patterns seen on FDG-PET scans can help to support a diagnosis of IVLBCL when clinical suspicion is high. Further, clinicians may be able to use imaging results to guide an appropriate site for biopsy to confirm diagnosis.
Conclusion
Intravascular large B-cell lymphoma remains a diagnostic challenge for clinicians. Prognosis is generally poor and likely related to frequent delays in diagnosis.1 Clinicians continue to work toward improving their ability to diagnose this disease in its early stages. New diagnostic algorithms and the use of random skin biopsies have shown some promise in improving diagnostic efficiency.26-28 Based on the authors’ experience and review of the literature, FDG-PET may be another promising tool to aid early diagnosis. Characteristic FDG-PET findings have been well described and may help to support the diagnosis of IVLBCL and guide an appropriate biopsy site when clinical suspicion for IVLBCL exists.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
Patient 1
A white man aged 67 years, with diastolic heart failure and chronic obstructive pulmonary disease, presented to the emergency department (ED) with shortness of breath. The initial laboratory results were significant for a newly elevated creatinine level of 2.06 mg/dL and a brain natriuretic peptide level of 648 pg/mL.
Imaging studies included a chest radiograph, a ventilation/perfusion scan, and an echocardiogram, as well as a right heart catheterization. All were nondiagnostic.
The patient's shortness of breath persisted despite treatment with diuretics, antibiotics, and steroids. Further laboratory workup revealed an elevated lactate dehydrogenase (LDH) level of 1,338 IU/L. A bone marrow biopsy performed because of concern about malignancy was unremarkable. Flow cytometry of the bone marrow aspirate did not reveal clonal B- or T-cell populations. Immunohistochemical staining was not performed. During this hospitalization for shortness of breath, the patient's mental staus began to decline, and his oxygen requirements increased. The patient was intubated but expired 48 hours after mechanical ventilation was initiated.
Patient 2
A white woman aged 67 years presented to the ED with generalized weakness, fatigue, and nausea. The patient’s medical history was significant for a diagnosis of stage IIIa ovarian cancer. She was treated with surgical resection and completed 6 cycles of adjuvant carboplatin and paclitaxel 3 months prior to this presentation. She had good response to treatment with normalization of CA-125.
After completion of chemotherapy, the patient was found to have persistent anemia and thrombocytopenia. Admission laboratory results were significant for a hemoglobin level of 8.4 g/dL, a platelet count of 20,000/μL, and an LDH level of 1,220 IU/L.
Chest, abdomen, and pelvis CT scans showed mesenteric adenopathy and splenomegaly (Figures 3A, 3B, and 3C) compared with prior imaging. Bone marrow biopsy revealed large lymphoid cells with scant cytoplasm and irregular nuclei, primarily within blood vessels and sinusoids consistent with IVLBCL (Figure 4). Flow cytometry of the bone marrow specimen showed an abnormal B-cell population with expression CD20, CD19, FMC-7, and dim κ light chain restriction. The cells were negative for CD5 and CD10. Immunohistochemical staining was positive for CD20, CD79a, PAX5, BCL-2 , and MUM1.
The patient was treated with 4 cycles of cyclophosphamide, doxorubicin, vincristine, prednisone, and rituximab, plus intrathecal methotrexate. The chemotherapy dose was reduced in the final cycle because of neuropathy in the hands and feet. The patient had undergone autologous stem-cell transplantation to allow high-dose chemotherapy. She was doing well more than 5 months after her transplant without evidence of recurrent disease.
Patient 3
A white man aged 76 years presented to the ED with cutaneous nodules, weight loss, fatigue, fevers, and epigastric pain. The patient’s medical history was significant for asymptomatic lymphoplasmacytic lymphoma diagnosed 2 months earlier, which had not required treatment. Laboratory results on admission revealed transaminitis, mild anemia with a hemoglobin level of 11 g/dL, and LDH level of 497 IU/L.
Chest, abdomen, and pelvis CT scans showed a 1.7cm hepatic lesion and mesenteric adenopathy. A bone marrow biopsy was unchanged from prior studies and showed minimal involvement (5%) of marrow space by low grade B-cell lymphoma.
Fluorodeoxyglucose-positron emission tomography (FDG-PET) scans showed multiple areas of uptake in the neck, chest, abdomen, and pelvis (Figures 5 and 6). No increased uptake in the subcutaneous nodules was noted on examination. Laparoscopic biopsy of FDG-avid mesenteric nodes showed clusters of atypical large lymphoid cells resulting in distention of the vascular lumina, resulting in the diagnosis of IVLBCL (Figure 7).
Immunohistochemical stains showed that the intravascular lymphocytes were strongly positive for CD20 and BCL-2 and negative for CD5 and CD10. Flow cytometry on the sample was limited by a low cell count and could not be assessed for clonality. The patient completed 6 cycles of rituximab as well as intrathecal methotrexate. Restaging studies showed a complete remission.
Two months later, the patient developed a skin nodule on the right shoulder. A repeat FDG-PET scan showed increased uptake, and fine-needle biopsy confirmed recurrent disease. The patient is undergoing treatment with ifosfamide, carboplatin, etoposide, and rituximab, as well as workup for autologous stem-cell transplant.
Discussion
Intravascular large B-cell lymphoma, a subtype of diffuse large B-cell lymphoma, is unique because it is primarily extranodal and typically without significant tumor burden.1-4 Standard imaging modalities, therefore, are often nonspecific and do not aid clinicians in establishing a diagnosis. Fluorodeoxyglucose-positron emission tomography has a known role in the assessment of diffuse large B-cell lymphoma, both at time of diagnosis and in monitoring response to treatment.5 However, the use of FDGPET in the diagnosis and management of IVLBCL has not been clearly established.
In a review of the literature, 26 English-language case reports and small case series reporting individual centers’ experience with the use of this imaging modality in the diagnosis of IVLBCL were identified. Two cases were eliminated from review because they did not discuss the use of FDG-PET in relationship to diagnosis. Of the remaining 24 cases, 21 underwent initial imaging with 1 or more of the following imaging modalities: CT, magnetic resonance, ultrasound, bone scan, and gallium scintigraphy, all of which were nonspecific and did not lead to a definitive diagnosis.3,6-25 Each of the 21 cases was followed up by FDG-PET; in 19, the FDG-PET scan was positive and resulted in a diagnosis of IVLBCL. In 2 cases, the FDG-PET scan was nonrevealing and was not considered helpful in diagnosis.11,18 In 3 of the 21 cases, the FDG-PET scan was the primary imaging modality.6,14,25
In this review, all 3 patients had initial imaging with CT scans of anatomic locations that were largely unrevealing, although later histologic examination showed them to be locations of active disease either by biopsy or on autopsy. One patient who underwent early FDG-PET was found to have increased uptake in the mesenteric lymph nodes, which were later biopsied, as well as uptake in the bilateral adrenal glands, lungs, and bone.
Several characteristic FDG-PET findings that have been described in the literature have been identified in patients with IVLBCL, including diffuse accumulation in bilateral lung fields, accumulation in the renal cortex or adrenal glands, diffuse bony involvement, and hypometabolism in the brain.7,10,12,13,17,23,25 These findings show that organs with the richest blood supply, specifically the lungs and kidneys, often are affected. The brain, an obligate glucose metabolizer, would be expected to have high uptake; however, with tumor thrombi occluding small intracranial vessels, micro infarcts ensue and are evidenced by areas of low uptake on FDG-PET scans in patients with IVLBCL.7 These characteristic patterns seen on FDG-PET scans can help to support a diagnosis of IVLBCL when clinical suspicion is high. Further, clinicians may be able to use imaging results to guide an appropriate site for biopsy to confirm diagnosis.
Conclusion
Intravascular large B-cell lymphoma remains a diagnostic challenge for clinicians. Prognosis is generally poor and likely related to frequent delays in diagnosis.1 Clinicians continue to work toward improving their ability to diagnose this disease in its early stages. New diagnostic algorithms and the use of random skin biopsies have shown some promise in improving diagnostic efficiency.26-28 Based on the authors’ experience and review of the literature, FDG-PET may be another promising tool to aid early diagnosis. Characteristic FDG-PET findings have been well described and may help to support the diagnosis of IVLBCL and guide an appropriate biopsy site when clinical suspicion for IVLBCL exists.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
γ-δ T-Cell Lymphoma With Disseminated Intravascular Coagulation and Autoimmune Hemolytic Anemia
Gamma-delta (γ-δ) T-cell lymphomas (GDTCL) are rare and aggressive cancers with specific morphologic, phenotypic, and functional properties. When discovered in 1984, the T-cell receptor (TCR) was characterized as an alpha-beta (α-β) heterodimer. The γ-δ heterodimer was discovered later, when a third rearranging gene was recognized.1
Gaulard and colleagues described the first case of peripheral neoplasm with the γ-δ TCR.2 Now the present authors report the case of a patient with an autoimmune hemolytic anemia (AIHA) with both cold and warm antibodies—an atypical presentation of this rare form of TCL. Such a case has not been previously reported.
Clinical History
A 77-year-old woman with a past medical history of osteoarthritis, gout, mitral stenosis, bioprosthetic aortic valve replacement, and obesity presented to the emergency department (ED) reporting progressive weakness, confusion, and jaundice. She had been recently discharged from
another hospital after an 18-day stay for gangrenous cholecystitis and shingles. Her home medications were metronidazole and acyclovir. In the ED, she was febrile at 100.5°. Laboratory test results revealed anemia with a hemoglobin level of 50 g/L (83 g/L in clinic 2 weeks earlier) and neutropenia with an absolute neutrophilic count of 500 cells/μL (normal range 1,520-6,370 cells/μL). She also was thrombocytopenic with a platelet count of 71x109/L (normal range 150-450×109/L).
On admission, the hematology service was consulted for pancytopenia. The pertinent workup included a lactate dehydrogenase level of 31.16 μkat/L (normal range 1.7-3.4 μkat/L), a haptoglobin level of < 1,500 mg/L (normal range 260-1,850 mg/L), and a direct bilirubin level of 13.68 μmol/L (normal range 1.7-5.1 μmol/L). A peripheral blood smear was negative for schistocytes. Fibrin split products were 40 mg/L (normal < 10 mg/L), fibrinogen level was 6.94 μmol/L (normal range 5.8-11.8 μmol/L), prothrombin time was 14.6 seconds (normal range 10-14 sec), and international normalized ratio was 1.3 (normal < 1). The concomitant decrease in fibrinogen level and increase in fibrin split product titers were consistent with the diagnosis of acute disseminated intravascular coagulation. Iron studies were consistent with anemia of chronic disease (low reticulocyte count of 0.4%) and vitamin B12 deficiency (level 195). Coombs test results were positive for both cold and warm antibodies, with cold being more prominent. Abdominal ultrasonography revealed hepatosplenomegaly (HSM).
The patient was diagnosed with AIHA with no initial obvious underlying etiology. The differential diagnosis included autoimmune disorder, lymphoproliferative disease, and drug-induced process. She also was diagnosed with sepsis, which was thought to be contributing to the pancytopenia.
Broad-spectrum antibiotics (cefepime, metronidazole) and vitamin B12 supplements were started. After a blood transfusion, the patient developed fever and hypoxia, which required transfer to the medical intensive care unit. The differentials at this time included a transfusion reaction and/or transfusion-associated circulatory overload. Intravenous immunoglobulin was started at 1 g/kg to help with cold agglutinins. Prednisone 1 mg/kg was started as well. Peripheral blood flow cytometry results were positive for an abnormal T-cell population likely consistent with T-cell lineage lymphoma. Bone marrow biopsy results were consistent with GDTCL. Computed tomography (CT) of chest/abdomen/pelvis showed bilateral lung nodules < 1 cm, HSM with multiple spleen infarcts, and a 4.7-cm right adnexal soft-tissue lesion. Liver biopsy results were consistent with GDTCL. Results of a workup for cytomegalovirus and Epstein-Barr virus were negative, as was a mycoplasma screen. The patient was diagnosed with GDTCL with hepatic involvement, and CHOP (cyclophosphamide, hydroxydaunorubicin [doxorubicin], Oncovin [vincristine], prednisone) therapy was started.
Discussion
Peripheral TCL (PTCL) are a rare, typically extranodal group of malignancies. They are aggressive and generally have a poor outcome, with most patients dying of lymphoma within 2 years.3 T-cell lymphomas most commonly express the γ-δ TCR. About 2% to 4% of TCLs express the γ-δ TCR.4 In 2008, the World Health Organization recognized 2 distinct GDTCL subgroups: hepatosplenic GDTCL (HSGDTCL) and primary cutaneous GDTCL.5 As the patient presented with hepatic involvement, this discussion focused on HSGDTCL.
Hepatosplenic GDTCL are rare types of PTCL. First described as a separate TCL subgroup in the 1990 REAL (Revised European-American Lymphoma) classification,6 they are estimated to represent about 1.4% of all TCL, with about 100 cases reported in the literature.4
The GDTCL cells tend to live in mucosa, lymphoid tissue, epithelial-rich tissues (skin, gastrointestinal tract), and red pulp of spleen.7 They develop from thymic precursors in bone marrow and are CD4-/CD8- and thus known as double negative cells.8 They mimic natural killer cells, behave as cytotoxic cells, and are capable of TCR rearrangement as well as phagocytosis.9
Hepatosplenic GDTCL are usually phenotypically CD2+, CD3+, CD4-, CD5-, CD7+, CD8-, and TCR γ-δ+.10 They are rarely associated with Epstein-Barr virus infection; reported cases seem more common in Asia.11 Peak incidence is in young men (median age 20-25 years; male:female ratio 10:1). At-risk populations include the chronically immunosuppressed, including solid organ transplanted patients and patients under prolonged antigenic stimulation.12
The most common clinical features of HSGDTCL include B symptoms (fever of unknown origin, night sweats, loss of > 10% of body weight), marked HSM, and lack of lymphadenopathy. Patients often present with fever, weakness, and abdominal pain. Laboratory test results
typically show abnormal liver function and abnormal lactate dehydrogenase levels. Bone marrow is almost always involved, with possible trilineage cytopenia. Anemia and thrombocytopenia are reported in 75% and 85% of cases, respectively.13
Warm (70%) and cold auto-antibodies are the 2 classifications of AIHA.14 The AIHA can be primary, idiopathic, or a manifestation of underlying disease conditions, including non-Hodgkin lymphomas, systemic autoimmune diseases, chronic infections, postorgan transplantation, and solid tumors. It has also been reported as a complication of treatment with nucleoside analogues.15
Lacking specific symptoms, HSGDTCL is usually diagnosed late. The diagnosis should be suspected in young men who present with the aforementioned symptoms. However, not everyone with HSGDTCL falls in that group—the present patient was a 77-year-old woman.
Hepatosplenic GDTCL staging is similar to staging of other non-Hodgkin lymphomas. Total-body CT with contrast, bone marrow aspiration/biopsy, and direct lesion biopsy are required. Although positron emission tomography is generally thought to be as useful in TCL as in B-cell lymphomas, there is not enough evidence to support its use specifically in HSGDTCL.16 The staging classification follows the Ann Arbor system, with the majority of cases classified as stage IV.
Hepatosplenic GDTCL are aggressive tumors with a strong tendency to rapidly progress, and they are highly resistant to primary chemotherapy agents. Remission is rarely complete with use of conventional chemotherapy agents. Most patients die of the disease within 2 years of
diagnosis.12 Although the rarity of HSGDTCL has made it difficult to identify any clear prognostic factors, a correlation between thrombocytopenia severity and disease progression has been found in many studies.17 There is no standard treatment regimen. Proposed therapies
include splenectomy (for diagnosis or thrombocytopenia management), corticosteroids, alkylating agents, purine analogue, anthracycline-containing regimens, and cytarabine/cisplatin combinations. The anthracycline-based regimen most commonly used as first-line therapy is CHOP, or CHOP derivatives, with complete remission rates between 30% and 45%. However, long-term results remain disappointing (median relapse time 4 months).10 In 3 reviews, median survival was 16 months, 11 months, and 9.5 months.10,17,18 In the International T-Cell Lymphoma Project study, the 5-year failure-free survival rate was 0%, and the overall survival rate was 7%.4 In these studies, the majority of patients received some variation of CHOP-based therapy, and although positive responses were appreciated in many of the cases, they were generally short-lived.
These results have been disappointing, and other modalities have been tried—including high-dose cytarabine regimens, 2'-deoxycoformycin (pentostatin), and anti-CD52 monoclonal antibodies (alemtuzumab).19 In an HSGDTCL study, 2 of 21 patients treated with platinum/cytarabine-based induction regimens were still in remission at 42 and 52 months.17 Another study examined a variety of induction regimens used to treat HSGDTCL in 15 patients.18 Responses tended to be more durable in patients who received a dose-intense Hyper-CVIDDoxil regimen (fractionated cyclophosphamide, liposomal doxorubicin, vincristine, dexamethasone) alternated with methotrexate and cytarabine. Complete response was 50%, and median duration of complete response was 8 months. Over the past 10 years, a few case reports have described successful treatment with autologous or allogeneic stem cell transplantation.20
Conclusion
The present case represents a unique HSGDTCL presentation. To the authors’ knowledge, this is the first report of HSGDTCL presenting with acute disseminated intravascular coagulation and AIHA with both cold and warm antibodies.
Hepatosplenic GDTCL is a rare, novel disease. To understand more about this pathology, investigators need to better characterize the disease process and the manifestations. The hope is that more information will contribute to the development of more effective therapies. The unique presentation reported here may help in further characterizing and understanding this uncommon disease.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
1. Saito H, Kranz DM, Takagaki Y, Hayday AC, Eisen HN, Tonegawa S. A third rearranged and expressed gene in a clone of cytotoxic T lymphocytes. Nature. 1984;312(5989):36-40.
2. Gaulard P, Zafrani ES, Mavier P, et al. Peripheral T-cell lymphoma presenting as predominant liver disease: a report of three cases. Hepatology. 1986;6(5):864-868.
3. Gaulard P, de Leval L. Pathology of peripheral T-cell lymphomas: where do we stand? Semin Hematol. 2014;51(1):5-16.
4. Vose J, Armitage J, Weisenburger D; International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124-4130.
5. The International Agency for Research on Cancer. In: Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Vol 2. 4th ed. Lyon, France: IARC Press; 2008.
6. Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasm: a proposal from the International Lymphoma Study Group. Blood. 1994;84(5):1361-1392.
7. Farcet JP, Gaulard P, Marolleau JP, et al. Hepatosplenic T-cell lymphoma: sinusal/sinusoidal localization of malignant cells expressing the T-cell receptor gamma delta. Blood. 1990;75(11):2213-2219.
8. Bluestone JA, Khattri R, Sciammas R, Sperling AI. TCR gamma delta cells: a specialized T-cell subset in the immune system. Annu Rev Cell Dev Biol. 1995;11:307-353.
9. Holtmeier W, Kabelitz D. Gamma delta T cells link innate and adaptive immune responses. Chem Immunol Allergy. 2005;86:151-183.
10. Weidmann E. Hepatosplenic T cell lymphoma. A review on 45 cases since the first report describing the disease as a distinct lymphoma entity in 1990. Leukemia. 2000;14(6):991-997.
11. Yu WW, Hsieh PP, Chuang SS. Cutaneous EBV-positive γδ T-cell lymphoma vs. extranodal NK/T-cell lymphoma: a case report and literature review. J Cutan Pathol. 2013;40(3):310-316.
12. Tripodo C, Iannitto E, Florena AM, et al. Gamma-delta T-cell lymphomas. Nat Rev Clin Oncol. 2009;6(12):707-717.
13. Foppoli M, Ferreri AJM. Gamma-delta T-cell lymphomas. Eur J Haematol. 2015;94(3):206-218.
14. Hoffbrand AV, Catovsky D, Tuddenham EGD, Green AR, eds. Postgraduate Haematology. 6th ed. Oxford, England: Wiley-Blackwell; 2011.
15. Valent P, Lechner K. Diagnosis and treatment of autoimmune haemolytic anaemias in adults: a clinical review. Wien Klin Wochenschr. 2008;120(5-6):136-151.
16. Khong PL, Pang CB, Liang R, Kwong YL, Au WY. Fluorine-18 fluorodeoxyglucose positron emission tomography in mature T-cell and natural killer cell malignancies. Ann Hematol. 2008;87(8):613-621.
17. Belhadj K, Reyes F, Farcet JP, et al. Hepatosplenic gammadelta T-cell lymphoma is a rare clinicopathologic entity with poor outcome: report on a series of 21 patients. Blood. 2003;102(13):4261-4269.
18. Falchook GS, Vega F, Dang NH, et al. Hepatosplenic gamma-delta T-cell lymphoma: clinicopathological features and treatment. Ann Oncol. 2009;20(6):1080-1085.
19. Konuma T, Ooi J, Takahashi S, et al. Allogeneic stem cell transplantation for hepatosplenic
gammadelta T-cell lymphoma. Leuk Lymphoma. 2007;48(3):630-632.
20. Ferreri AJ, Govi S, Pileri SA. Hepatosplenic gamma-delta T-cell lymphoma. Crit
Rev Oncol Hematol. 2012;83(2):283-292.
Gamma-delta (γ-δ) T-cell lymphomas (GDTCL) are rare and aggressive cancers with specific morphologic, phenotypic, and functional properties. When discovered in 1984, the T-cell receptor (TCR) was characterized as an alpha-beta (α-β) heterodimer. The γ-δ heterodimer was discovered later, when a third rearranging gene was recognized.1
Gaulard and colleagues described the first case of peripheral neoplasm with the γ-δ TCR.2 Now the present authors report the case of a patient with an autoimmune hemolytic anemia (AIHA) with both cold and warm antibodies—an atypical presentation of this rare form of TCL. Such a case has not been previously reported.
Clinical History
A 77-year-old woman with a past medical history of osteoarthritis, gout, mitral stenosis, bioprosthetic aortic valve replacement, and obesity presented to the emergency department (ED) reporting progressive weakness, confusion, and jaundice. She had been recently discharged from
another hospital after an 18-day stay for gangrenous cholecystitis and shingles. Her home medications were metronidazole and acyclovir. In the ED, she was febrile at 100.5°. Laboratory test results revealed anemia with a hemoglobin level of 50 g/L (83 g/L in clinic 2 weeks earlier) and neutropenia with an absolute neutrophilic count of 500 cells/μL (normal range 1,520-6,370 cells/μL). She also was thrombocytopenic with a platelet count of 71x109/L (normal range 150-450×109/L).
On admission, the hematology service was consulted for pancytopenia. The pertinent workup included a lactate dehydrogenase level of 31.16 μkat/L (normal range 1.7-3.4 μkat/L), a haptoglobin level of < 1,500 mg/L (normal range 260-1,850 mg/L), and a direct bilirubin level of 13.68 μmol/L (normal range 1.7-5.1 μmol/L). A peripheral blood smear was negative for schistocytes. Fibrin split products were 40 mg/L (normal < 10 mg/L), fibrinogen level was 6.94 μmol/L (normal range 5.8-11.8 μmol/L), prothrombin time was 14.6 seconds (normal range 10-14 sec), and international normalized ratio was 1.3 (normal < 1). The concomitant decrease in fibrinogen level and increase in fibrin split product titers were consistent with the diagnosis of acute disseminated intravascular coagulation. Iron studies were consistent with anemia of chronic disease (low reticulocyte count of 0.4%) and vitamin B12 deficiency (level 195). Coombs test results were positive for both cold and warm antibodies, with cold being more prominent. Abdominal ultrasonography revealed hepatosplenomegaly (HSM).
The patient was diagnosed with AIHA with no initial obvious underlying etiology. The differential diagnosis included autoimmune disorder, lymphoproliferative disease, and drug-induced process. She also was diagnosed with sepsis, which was thought to be contributing to the pancytopenia.
Broad-spectrum antibiotics (cefepime, metronidazole) and vitamin B12 supplements were started. After a blood transfusion, the patient developed fever and hypoxia, which required transfer to the medical intensive care unit. The differentials at this time included a transfusion reaction and/or transfusion-associated circulatory overload. Intravenous immunoglobulin was started at 1 g/kg to help with cold agglutinins. Prednisone 1 mg/kg was started as well. Peripheral blood flow cytometry results were positive for an abnormal T-cell population likely consistent with T-cell lineage lymphoma. Bone marrow biopsy results were consistent with GDTCL. Computed tomography (CT) of chest/abdomen/pelvis showed bilateral lung nodules < 1 cm, HSM with multiple spleen infarcts, and a 4.7-cm right adnexal soft-tissue lesion. Liver biopsy results were consistent with GDTCL. Results of a workup for cytomegalovirus and Epstein-Barr virus were negative, as was a mycoplasma screen. The patient was diagnosed with GDTCL with hepatic involvement, and CHOP (cyclophosphamide, hydroxydaunorubicin [doxorubicin], Oncovin [vincristine], prednisone) therapy was started.
Discussion
Peripheral TCL (PTCL) are a rare, typically extranodal group of malignancies. They are aggressive and generally have a poor outcome, with most patients dying of lymphoma within 2 years.3 T-cell lymphomas most commonly express the γ-δ TCR. About 2% to 4% of TCLs express the γ-δ TCR.4 In 2008, the World Health Organization recognized 2 distinct GDTCL subgroups: hepatosplenic GDTCL (HSGDTCL) and primary cutaneous GDTCL.5 As the patient presented with hepatic involvement, this discussion focused on HSGDTCL.
Hepatosplenic GDTCL are rare types of PTCL. First described as a separate TCL subgroup in the 1990 REAL (Revised European-American Lymphoma) classification,6 they are estimated to represent about 1.4% of all TCL, with about 100 cases reported in the literature.4
The GDTCL cells tend to live in mucosa, lymphoid tissue, epithelial-rich tissues (skin, gastrointestinal tract), and red pulp of spleen.7 They develop from thymic precursors in bone marrow and are CD4-/CD8- and thus known as double negative cells.8 They mimic natural killer cells, behave as cytotoxic cells, and are capable of TCR rearrangement as well as phagocytosis.9
Hepatosplenic GDTCL are usually phenotypically CD2+, CD3+, CD4-, CD5-, CD7+, CD8-, and TCR γ-δ+.10 They are rarely associated with Epstein-Barr virus infection; reported cases seem more common in Asia.11 Peak incidence is in young men (median age 20-25 years; male:female ratio 10:1). At-risk populations include the chronically immunosuppressed, including solid organ transplanted patients and patients under prolonged antigenic stimulation.12
The most common clinical features of HSGDTCL include B symptoms (fever of unknown origin, night sweats, loss of > 10% of body weight), marked HSM, and lack of lymphadenopathy. Patients often present with fever, weakness, and abdominal pain. Laboratory test results
typically show abnormal liver function and abnormal lactate dehydrogenase levels. Bone marrow is almost always involved, with possible trilineage cytopenia. Anemia and thrombocytopenia are reported in 75% and 85% of cases, respectively.13
Warm (70%) and cold auto-antibodies are the 2 classifications of AIHA.14 The AIHA can be primary, idiopathic, or a manifestation of underlying disease conditions, including non-Hodgkin lymphomas, systemic autoimmune diseases, chronic infections, postorgan transplantation, and solid tumors. It has also been reported as a complication of treatment with nucleoside analogues.15
Lacking specific symptoms, HSGDTCL is usually diagnosed late. The diagnosis should be suspected in young men who present with the aforementioned symptoms. However, not everyone with HSGDTCL falls in that group—the present patient was a 77-year-old woman.
Hepatosplenic GDTCL staging is similar to staging of other non-Hodgkin lymphomas. Total-body CT with contrast, bone marrow aspiration/biopsy, and direct lesion biopsy are required. Although positron emission tomography is generally thought to be as useful in TCL as in B-cell lymphomas, there is not enough evidence to support its use specifically in HSGDTCL.16 The staging classification follows the Ann Arbor system, with the majority of cases classified as stage IV.
Hepatosplenic GDTCL are aggressive tumors with a strong tendency to rapidly progress, and they are highly resistant to primary chemotherapy agents. Remission is rarely complete with use of conventional chemotherapy agents. Most patients die of the disease within 2 years of
diagnosis.12 Although the rarity of HSGDTCL has made it difficult to identify any clear prognostic factors, a correlation between thrombocytopenia severity and disease progression has been found in many studies.17 There is no standard treatment regimen. Proposed therapies
include splenectomy (for diagnosis or thrombocytopenia management), corticosteroids, alkylating agents, purine analogue, anthracycline-containing regimens, and cytarabine/cisplatin combinations. The anthracycline-based regimen most commonly used as first-line therapy is CHOP, or CHOP derivatives, with complete remission rates between 30% and 45%. However, long-term results remain disappointing (median relapse time 4 months).10 In 3 reviews, median survival was 16 months, 11 months, and 9.5 months.10,17,18 In the International T-Cell Lymphoma Project study, the 5-year failure-free survival rate was 0%, and the overall survival rate was 7%.4 In these studies, the majority of patients received some variation of CHOP-based therapy, and although positive responses were appreciated in many of the cases, they were generally short-lived.
These results have been disappointing, and other modalities have been tried—including high-dose cytarabine regimens, 2'-deoxycoformycin (pentostatin), and anti-CD52 monoclonal antibodies (alemtuzumab).19 In an HSGDTCL study, 2 of 21 patients treated with platinum/cytarabine-based induction regimens were still in remission at 42 and 52 months.17 Another study examined a variety of induction regimens used to treat HSGDTCL in 15 patients.18 Responses tended to be more durable in patients who received a dose-intense Hyper-CVIDDoxil regimen (fractionated cyclophosphamide, liposomal doxorubicin, vincristine, dexamethasone) alternated with methotrexate and cytarabine. Complete response was 50%, and median duration of complete response was 8 months. Over the past 10 years, a few case reports have described successful treatment with autologous or allogeneic stem cell transplantation.20
Conclusion
The present case represents a unique HSGDTCL presentation. To the authors’ knowledge, this is the first report of HSGDTCL presenting with acute disseminated intravascular coagulation and AIHA with both cold and warm antibodies.
Hepatosplenic GDTCL is a rare, novel disease. To understand more about this pathology, investigators need to better characterize the disease process and the manifestations. The hope is that more information will contribute to the development of more effective therapies. The unique presentation reported here may help in further characterizing and understanding this uncommon disease.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
Gamma-delta (γ-δ) T-cell lymphomas (GDTCL) are rare and aggressive cancers with specific morphologic, phenotypic, and functional properties. When discovered in 1984, the T-cell receptor (TCR) was characterized as an alpha-beta (α-β) heterodimer. The γ-δ heterodimer was discovered later, when a third rearranging gene was recognized.1
Gaulard and colleagues described the first case of peripheral neoplasm with the γ-δ TCR.2 Now the present authors report the case of a patient with an autoimmune hemolytic anemia (AIHA) with both cold and warm antibodies—an atypical presentation of this rare form of TCL. Such a case has not been previously reported.
Clinical History
A 77-year-old woman with a past medical history of osteoarthritis, gout, mitral stenosis, bioprosthetic aortic valve replacement, and obesity presented to the emergency department (ED) reporting progressive weakness, confusion, and jaundice. She had been recently discharged from
another hospital after an 18-day stay for gangrenous cholecystitis and shingles. Her home medications were metronidazole and acyclovir. In the ED, she was febrile at 100.5°. Laboratory test results revealed anemia with a hemoglobin level of 50 g/L (83 g/L in clinic 2 weeks earlier) and neutropenia with an absolute neutrophilic count of 500 cells/μL (normal range 1,520-6,370 cells/μL). She also was thrombocytopenic with a platelet count of 71x109/L (normal range 150-450×109/L).
On admission, the hematology service was consulted for pancytopenia. The pertinent workup included a lactate dehydrogenase level of 31.16 μkat/L (normal range 1.7-3.4 μkat/L), a haptoglobin level of < 1,500 mg/L (normal range 260-1,850 mg/L), and a direct bilirubin level of 13.68 μmol/L (normal range 1.7-5.1 μmol/L). A peripheral blood smear was negative for schistocytes. Fibrin split products were 40 mg/L (normal < 10 mg/L), fibrinogen level was 6.94 μmol/L (normal range 5.8-11.8 μmol/L), prothrombin time was 14.6 seconds (normal range 10-14 sec), and international normalized ratio was 1.3 (normal < 1). The concomitant decrease in fibrinogen level and increase in fibrin split product titers were consistent with the diagnosis of acute disseminated intravascular coagulation. Iron studies were consistent with anemia of chronic disease (low reticulocyte count of 0.4%) and vitamin B12 deficiency (level 195). Coombs test results were positive for both cold and warm antibodies, with cold being more prominent. Abdominal ultrasonography revealed hepatosplenomegaly (HSM).
The patient was diagnosed with AIHA with no initial obvious underlying etiology. The differential diagnosis included autoimmune disorder, lymphoproliferative disease, and drug-induced process. She also was diagnosed with sepsis, which was thought to be contributing to the pancytopenia.
Broad-spectrum antibiotics (cefepime, metronidazole) and vitamin B12 supplements were started. After a blood transfusion, the patient developed fever and hypoxia, which required transfer to the medical intensive care unit. The differentials at this time included a transfusion reaction and/or transfusion-associated circulatory overload. Intravenous immunoglobulin was started at 1 g/kg to help with cold agglutinins. Prednisone 1 mg/kg was started as well. Peripheral blood flow cytometry results were positive for an abnormal T-cell population likely consistent with T-cell lineage lymphoma. Bone marrow biopsy results were consistent with GDTCL. Computed tomography (CT) of chest/abdomen/pelvis showed bilateral lung nodules < 1 cm, HSM with multiple spleen infarcts, and a 4.7-cm right adnexal soft-tissue lesion. Liver biopsy results were consistent with GDTCL. Results of a workup for cytomegalovirus and Epstein-Barr virus were negative, as was a mycoplasma screen. The patient was diagnosed with GDTCL with hepatic involvement, and CHOP (cyclophosphamide, hydroxydaunorubicin [doxorubicin], Oncovin [vincristine], prednisone) therapy was started.
Discussion
Peripheral TCL (PTCL) are a rare, typically extranodal group of malignancies. They are aggressive and generally have a poor outcome, with most patients dying of lymphoma within 2 years.3 T-cell lymphomas most commonly express the γ-δ TCR. About 2% to 4% of TCLs express the γ-δ TCR.4 In 2008, the World Health Organization recognized 2 distinct GDTCL subgroups: hepatosplenic GDTCL (HSGDTCL) and primary cutaneous GDTCL.5 As the patient presented with hepatic involvement, this discussion focused on HSGDTCL.
Hepatosplenic GDTCL are rare types of PTCL. First described as a separate TCL subgroup in the 1990 REAL (Revised European-American Lymphoma) classification,6 they are estimated to represent about 1.4% of all TCL, with about 100 cases reported in the literature.4
The GDTCL cells tend to live in mucosa, lymphoid tissue, epithelial-rich tissues (skin, gastrointestinal tract), and red pulp of spleen.7 They develop from thymic precursors in bone marrow and are CD4-/CD8- and thus known as double negative cells.8 They mimic natural killer cells, behave as cytotoxic cells, and are capable of TCR rearrangement as well as phagocytosis.9
Hepatosplenic GDTCL are usually phenotypically CD2+, CD3+, CD4-, CD5-, CD7+, CD8-, and TCR γ-δ+.10 They are rarely associated with Epstein-Barr virus infection; reported cases seem more common in Asia.11 Peak incidence is in young men (median age 20-25 years; male:female ratio 10:1). At-risk populations include the chronically immunosuppressed, including solid organ transplanted patients and patients under prolonged antigenic stimulation.12
The most common clinical features of HSGDTCL include B symptoms (fever of unknown origin, night sweats, loss of > 10% of body weight), marked HSM, and lack of lymphadenopathy. Patients often present with fever, weakness, and abdominal pain. Laboratory test results
typically show abnormal liver function and abnormal lactate dehydrogenase levels. Bone marrow is almost always involved, with possible trilineage cytopenia. Anemia and thrombocytopenia are reported in 75% and 85% of cases, respectively.13
Warm (70%) and cold auto-antibodies are the 2 classifications of AIHA.14 The AIHA can be primary, idiopathic, or a manifestation of underlying disease conditions, including non-Hodgkin lymphomas, systemic autoimmune diseases, chronic infections, postorgan transplantation, and solid tumors. It has also been reported as a complication of treatment with nucleoside analogues.15
Lacking specific symptoms, HSGDTCL is usually diagnosed late. The diagnosis should be suspected in young men who present with the aforementioned symptoms. However, not everyone with HSGDTCL falls in that group—the present patient was a 77-year-old woman.
Hepatosplenic GDTCL staging is similar to staging of other non-Hodgkin lymphomas. Total-body CT with contrast, bone marrow aspiration/biopsy, and direct lesion biopsy are required. Although positron emission tomography is generally thought to be as useful in TCL as in B-cell lymphomas, there is not enough evidence to support its use specifically in HSGDTCL.16 The staging classification follows the Ann Arbor system, with the majority of cases classified as stage IV.
Hepatosplenic GDTCL are aggressive tumors with a strong tendency to rapidly progress, and they are highly resistant to primary chemotherapy agents. Remission is rarely complete with use of conventional chemotherapy agents. Most patients die of the disease within 2 years of
diagnosis.12 Although the rarity of HSGDTCL has made it difficult to identify any clear prognostic factors, a correlation between thrombocytopenia severity and disease progression has been found in many studies.17 There is no standard treatment regimen. Proposed therapies
include splenectomy (for diagnosis or thrombocytopenia management), corticosteroids, alkylating agents, purine analogue, anthracycline-containing regimens, and cytarabine/cisplatin combinations. The anthracycline-based regimen most commonly used as first-line therapy is CHOP, or CHOP derivatives, with complete remission rates between 30% and 45%. However, long-term results remain disappointing (median relapse time 4 months).10 In 3 reviews, median survival was 16 months, 11 months, and 9.5 months.10,17,18 In the International T-Cell Lymphoma Project study, the 5-year failure-free survival rate was 0%, and the overall survival rate was 7%.4 In these studies, the majority of patients received some variation of CHOP-based therapy, and although positive responses were appreciated in many of the cases, they were generally short-lived.
These results have been disappointing, and other modalities have been tried—including high-dose cytarabine regimens, 2'-deoxycoformycin (pentostatin), and anti-CD52 monoclonal antibodies (alemtuzumab).19 In an HSGDTCL study, 2 of 21 patients treated with platinum/cytarabine-based induction regimens were still in remission at 42 and 52 months.17 Another study examined a variety of induction regimens used to treat HSGDTCL in 15 patients.18 Responses tended to be more durable in patients who received a dose-intense Hyper-CVIDDoxil regimen (fractionated cyclophosphamide, liposomal doxorubicin, vincristine, dexamethasone) alternated with methotrexate and cytarabine. Complete response was 50%, and median duration of complete response was 8 months. Over the past 10 years, a few case reports have described successful treatment with autologous or allogeneic stem cell transplantation.20
Conclusion
The present case represents a unique HSGDTCL presentation. To the authors’ knowledge, this is the first report of HSGDTCL presenting with acute disseminated intravascular coagulation and AIHA with both cold and warm antibodies.
Hepatosplenic GDTCL is a rare, novel disease. To understand more about this pathology, investigators need to better characterize the disease process and the manifestations. The hope is that more information will contribute to the development of more effective therapies. The unique presentation reported here may help in further characterizing and understanding this uncommon disease.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
1. Saito H, Kranz DM, Takagaki Y, Hayday AC, Eisen HN, Tonegawa S. A third rearranged and expressed gene in a clone of cytotoxic T lymphocytes. Nature. 1984;312(5989):36-40.
2. Gaulard P, Zafrani ES, Mavier P, et al. Peripheral T-cell lymphoma presenting as predominant liver disease: a report of three cases. Hepatology. 1986;6(5):864-868.
3. Gaulard P, de Leval L. Pathology of peripheral T-cell lymphomas: where do we stand? Semin Hematol. 2014;51(1):5-16.
4. Vose J, Armitage J, Weisenburger D; International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124-4130.
5. The International Agency for Research on Cancer. In: Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Vol 2. 4th ed. Lyon, France: IARC Press; 2008.
6. Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasm: a proposal from the International Lymphoma Study Group. Blood. 1994;84(5):1361-1392.
7. Farcet JP, Gaulard P, Marolleau JP, et al. Hepatosplenic T-cell lymphoma: sinusal/sinusoidal localization of malignant cells expressing the T-cell receptor gamma delta. Blood. 1990;75(11):2213-2219.
8. Bluestone JA, Khattri R, Sciammas R, Sperling AI. TCR gamma delta cells: a specialized T-cell subset in the immune system. Annu Rev Cell Dev Biol. 1995;11:307-353.
9. Holtmeier W, Kabelitz D. Gamma delta T cells link innate and adaptive immune responses. Chem Immunol Allergy. 2005;86:151-183.
10. Weidmann E. Hepatosplenic T cell lymphoma. A review on 45 cases since the first report describing the disease as a distinct lymphoma entity in 1990. Leukemia. 2000;14(6):991-997.
11. Yu WW, Hsieh PP, Chuang SS. Cutaneous EBV-positive γδ T-cell lymphoma vs. extranodal NK/T-cell lymphoma: a case report and literature review. J Cutan Pathol. 2013;40(3):310-316.
12. Tripodo C, Iannitto E, Florena AM, et al. Gamma-delta T-cell lymphomas. Nat Rev Clin Oncol. 2009;6(12):707-717.
13. Foppoli M, Ferreri AJM. Gamma-delta T-cell lymphomas. Eur J Haematol. 2015;94(3):206-218.
14. Hoffbrand AV, Catovsky D, Tuddenham EGD, Green AR, eds. Postgraduate Haematology. 6th ed. Oxford, England: Wiley-Blackwell; 2011.
15. Valent P, Lechner K. Diagnosis and treatment of autoimmune haemolytic anaemias in adults: a clinical review. Wien Klin Wochenschr. 2008;120(5-6):136-151.
16. Khong PL, Pang CB, Liang R, Kwong YL, Au WY. Fluorine-18 fluorodeoxyglucose positron emission tomography in mature T-cell and natural killer cell malignancies. Ann Hematol. 2008;87(8):613-621.
17. Belhadj K, Reyes F, Farcet JP, et al. Hepatosplenic gammadelta T-cell lymphoma is a rare clinicopathologic entity with poor outcome: report on a series of 21 patients. Blood. 2003;102(13):4261-4269.
18. Falchook GS, Vega F, Dang NH, et al. Hepatosplenic gamma-delta T-cell lymphoma: clinicopathological features and treatment. Ann Oncol. 2009;20(6):1080-1085.
19. Konuma T, Ooi J, Takahashi S, et al. Allogeneic stem cell transplantation for hepatosplenic
gammadelta T-cell lymphoma. Leuk Lymphoma. 2007;48(3):630-632.
20. Ferreri AJ, Govi S, Pileri SA. Hepatosplenic gamma-delta T-cell lymphoma. Crit
Rev Oncol Hematol. 2012;83(2):283-292.
1. Saito H, Kranz DM, Takagaki Y, Hayday AC, Eisen HN, Tonegawa S. A third rearranged and expressed gene in a clone of cytotoxic T lymphocytes. Nature. 1984;312(5989):36-40.
2. Gaulard P, Zafrani ES, Mavier P, et al. Peripheral T-cell lymphoma presenting as predominant liver disease: a report of three cases. Hepatology. 1986;6(5):864-868.
3. Gaulard P, de Leval L. Pathology of peripheral T-cell lymphomas: where do we stand? Semin Hematol. 2014;51(1):5-16.
4. Vose J, Armitage J, Weisenburger D; International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124-4130.
5. The International Agency for Research on Cancer. In: Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Vol 2. 4th ed. Lyon, France: IARC Press; 2008.
6. Harris NL, Jaffe ES, Stein H, et al. A revised European-American classification of lymphoid neoplasm: a proposal from the International Lymphoma Study Group. Blood. 1994;84(5):1361-1392.
7. Farcet JP, Gaulard P, Marolleau JP, et al. Hepatosplenic T-cell lymphoma: sinusal/sinusoidal localization of malignant cells expressing the T-cell receptor gamma delta. Blood. 1990;75(11):2213-2219.
8. Bluestone JA, Khattri R, Sciammas R, Sperling AI. TCR gamma delta cells: a specialized T-cell subset in the immune system. Annu Rev Cell Dev Biol. 1995;11:307-353.
9. Holtmeier W, Kabelitz D. Gamma delta T cells link innate and adaptive immune responses. Chem Immunol Allergy. 2005;86:151-183.
10. Weidmann E. Hepatosplenic T cell lymphoma. A review on 45 cases since the first report describing the disease as a distinct lymphoma entity in 1990. Leukemia. 2000;14(6):991-997.
11. Yu WW, Hsieh PP, Chuang SS. Cutaneous EBV-positive γδ T-cell lymphoma vs. extranodal NK/T-cell lymphoma: a case report and literature review. J Cutan Pathol. 2013;40(3):310-316.
12. Tripodo C, Iannitto E, Florena AM, et al. Gamma-delta T-cell lymphomas. Nat Rev Clin Oncol. 2009;6(12):707-717.
13. Foppoli M, Ferreri AJM. Gamma-delta T-cell lymphomas. Eur J Haematol. 2015;94(3):206-218.
14. Hoffbrand AV, Catovsky D, Tuddenham EGD, Green AR, eds. Postgraduate Haematology. 6th ed. Oxford, England: Wiley-Blackwell; 2011.
15. Valent P, Lechner K. Diagnosis and treatment of autoimmune haemolytic anaemias in adults: a clinical review. Wien Klin Wochenschr. 2008;120(5-6):136-151.
16. Khong PL, Pang CB, Liang R, Kwong YL, Au WY. Fluorine-18 fluorodeoxyglucose positron emission tomography in mature T-cell and natural killer cell malignancies. Ann Hematol. 2008;87(8):613-621.
17. Belhadj K, Reyes F, Farcet JP, et al. Hepatosplenic gammadelta T-cell lymphoma is a rare clinicopathologic entity with poor outcome: report on a series of 21 patients. Blood. 2003;102(13):4261-4269.
18. Falchook GS, Vega F, Dang NH, et al. Hepatosplenic gamma-delta T-cell lymphoma: clinicopathological features and treatment. Ann Oncol. 2009;20(6):1080-1085.
19. Konuma T, Ooi J, Takahashi S, et al. Allogeneic stem cell transplantation for hepatosplenic
gammadelta T-cell lymphoma. Leuk Lymphoma. 2007;48(3):630-632.
20. Ferreri AJ, Govi S, Pileri SA. Hepatosplenic gamma-delta T-cell lymphoma. Crit
Rev Oncol Hematol. 2012;83(2):283-292.
Consensus Statement Supporting the Recommendation for Single-Fraction Palliative Radiotherapy for Uncomplicated, Painful Bone Metastases
The authors would like to acknowledge Tony Quang, MD, JD, for the advice given on this project.
Palliative radiotherapy for bone metastases is typically delivered either as a short course of 1 to 5 fractions or protracted over longer courses of up to 20 treatments. These longer courses can be burdensome and discourage its utilization, despite a 50% to 80% likelihood of meaningful pain relief from only a single fraction of radiation therapy. Meanwhile, there are multiple randomized studies that have demonstrated that shorter course(s) are equivalent for pain control.
Although the VHA currently has 143 medical facilities that have cancer diagnostic and treatment capabilities, only 40 have radiation oncology services on-site.1 Thus, access to palliative radiotherapy may be limited for veterans who do not live close by, and many may seek care outside the VHA. At VHA radiation oncology centers, single-fraction radiation therapy (SFRT) is routinely offered by the majority of radiation oncologists.2,3 However, the longer course is commonly preferred outside the VA, and a recent SEER-Medicare analysis of more than 3,000 patients demonstrated that the majority of patients treated outside the VA actually receive more than 10 treatments.4 For this reason, the VA National Palliative Radiotherapy Task Force prepared this document to provide guidance for clinicians within and outside the VA to increase awareness of the appropriateness, effectiveness, and convenience of SFRT as opposed to longer courses of treatment that increase the burden of care at the end of life and often are unnecessary.
Veterans, Cancer, and Metastases
Within the VA, an estimated 40,000 new cancer cases are diagnosed each year, and 175,000 veterans undergo cancer care within the VHA annually.1 Unfortunately, the majority will develop bone metastases with postmortem examinations, suggesting that the rate can be as high as 90% at the end of life.5-7 For many, including veterans with cancer, pain control can be difficult, and access to palliative radiotherapy is critical.8
Single-Fraction Palliatiev Radiation Therapy
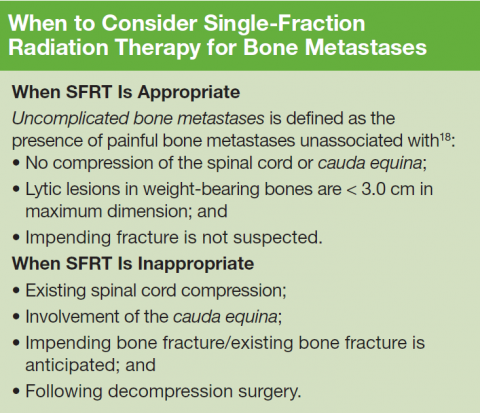
Historically, patients with painful bone metastases have been treated with courses of palliative radiotherapy ranging between 2 and 4 weeks of daily treatments. However, several large randomized clinical trials comparing a single treatment with multiple treatments have established that SFRT provides equivalent rates of pain relief even when it may be required for a second time.9-12 Recommendations based on these trials have been incorporated into various treatment guidelines that widely acknowledge the efficacy of SFRT.13-15
For this reason, SFRT is often preferred at many centers because it is substantially more convenient for patients with cancer. It reduces travel time for daily radiation clinic visits, which allows for more time with loved ones outside the medical establishment. Furthermore, SFRT improves patient access to radiotherapy and reduces costs. The benefits can be direct as well as indirect to those who have to take time for numerous visits.
Longer courses of palliative radiotherapy can be burdensome for patients and primary care providers. Unnecessarily protracted courses of palliative radiotherapy also delay the receipt of systemic therapies because they are typically considered unsafe to administer concurrently. Moreover, when SFRT is unavailable, the burden of long-course palliation is known to discourage health care providers from referring patients since opioid therapy is more convenient, even though it exchanges lucidity for analgesia.16,17
For this reason, the authors believe that it is in the best interest for veterans with terminal cancers and their providers to be aware of the shorter SFRT for effective, convenient pain relief. This treatment option is particularly relevant for patients with a poor performance status, patients already in hospice care, or patient who travel long distances.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
2. Moghanaki D, Cheuk AV, Fosmire H, et al; U.S. Veterans Healthcare Administration National Palliative Radiotherapy Taskforce. Availability of single fraction palliative radiotherapy for cancer patients receiving end-of-life care within the Veterans Healthcare Administration. J Palliat Med. 2014;17(11):1221-1225.
3. Dawson GA, Glushko I, Hagan MP. A cross-sectional view of radiation dose fractionation schemes used for painful bone metastases cases within Veterans Health Administration Radiation Oncology Centers. J Clin Oncol. 2015;33(29 suppl):abstract 177.
4. Bekelman JE, Epstein AJ, Emanuel EJ. Single- vs multiple-fraction radiotherapy for bone metastases from prostate cancer. JAMA. 2013;310(14):1501-1502.
5. Galasko CSB. The anatomy and pathways of skeletal metastases. In: Weiss L, Gilbert AH, eds. Bone Metastasis. Boston, MA: GK Hall; 1981:49-63.
6. Bubendorf L, Schöpfer A, Wagner U, et al. Metastatic patterns in prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31(5):578-583.
7. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20, pt 2):6243s-6249s.
8. Geriatrics and Extended Care Strategic Healthcare Group, National Pain Management Coordinating Committee, Veterans Health Administration. Pain as the 5th Vital Sign Toolkit. Rev. ed. Washington, DC: National Pain Management Coordinating Committee; 2000.
9. Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97(11):798-804.
10. Chow E, Hoskins PJ, Wu J, et al. A phase III international randomised trial comparing single with multiple fractions for re-irradiation of painful bone metastases: National Cancer Institute of Canada Clinical Trials Group (NCTC CTG) SC 20. Clin Oncol (R Coll Radiol). 2006;18(2):125-128.
11. Fairchild A, Barnes E, Ghosh S, et al. International patterns of practice in palliative radiotherapy for painful bone metastases: evidence-based practice? Int J Radiat Oncol Biol Phys. 2009;75(5):1501-1510.
12. Chow E, van der Linden YM, Roos D, et al. Single fraction versus multiple fractions of repeat radiation for painful bone metastases: a randomised, controlled, non-inferiority trial. Lancet Oncol. 2014;15(2):164-171.
13. Lutz ST, Berk L, Chang E, et al; American Society for Radiation Oncology (ASTRO). Palliative radiotherapy for bone metastases: an ASTRO evidencebased guideline. Int J Radiat Oncol, Biol, Phys. 2011;79(4):965-976.
14. Expert Panel on Radiation Oncology-Bone Metastases, Lo SS, Lutz ST, Chang EL, et al. ACR Appropriateness Criteria® spinal bone metastases. J Palliat Med. 2013;16(1):9-19.
15. Expert Panel on Radiation Oncology-Bone Metastases, Lutz ST, Lo SS, Chang EL, et al. ACR Appropriateness Criteria® non-spinal bone metastases. J Palliative Med. 2012;15(5):521-526.
16. Guadagnolo BA, Liao KP, Elting L, Giordano S, Buchholz TA, Shih YC. Use of radiation therapy in the last 30 days of life among a large population-based cohort of elderly patients in the United States. J Clin Oncol. 2013;31(1):80-87.
17. Schuster J, Han T, Anscher M, Moghanaki D. Hospice providers awareness of the benefits and availability of single-fraction palliative radiotherapy. J Hospice Palliat Care Nurs. 2014;16(2):67-72.
18. Cheon PM, Wong E, Thavarajah N, et al. A definition of “uncomplicated bone metastases” based on previous bone metastases trials comparing single-fraction and multi-fraction radiation therapy. J Bone Oncol. 2015;4(1):13-17.
The authors would like to acknowledge Tony Quang, MD, JD, for the advice given on this project.
Palliative radiotherapy for bone metastases is typically delivered either as a short course of 1 to 5 fractions or protracted over longer courses of up to 20 treatments. These longer courses can be burdensome and discourage its utilization, despite a 50% to 80% likelihood of meaningful pain relief from only a single fraction of radiation therapy. Meanwhile, there are multiple randomized studies that have demonstrated that shorter course(s) are equivalent for pain control.
Although the VHA currently has 143 medical facilities that have cancer diagnostic and treatment capabilities, only 40 have radiation oncology services on-site.1 Thus, access to palliative radiotherapy may be limited for veterans who do not live close by, and many may seek care outside the VHA. At VHA radiation oncology centers, single-fraction radiation therapy (SFRT) is routinely offered by the majority of radiation oncologists.2,3 However, the longer course is commonly preferred outside the VA, and a recent SEER-Medicare analysis of more than 3,000 patients demonstrated that the majority of patients treated outside the VA actually receive more than 10 treatments.4 For this reason, the VA National Palliative Radiotherapy Task Force prepared this document to provide guidance for clinicians within and outside the VA to increase awareness of the appropriateness, effectiveness, and convenience of SFRT as opposed to longer courses of treatment that increase the burden of care at the end of life and often are unnecessary.
Veterans, Cancer, and Metastases
Within the VA, an estimated 40,000 new cancer cases are diagnosed each year, and 175,000 veterans undergo cancer care within the VHA annually.1 Unfortunately, the majority will develop bone metastases with postmortem examinations, suggesting that the rate can be as high as 90% at the end of life.5-7 For many, including veterans with cancer, pain control can be difficult, and access to palliative radiotherapy is critical.8
Single-Fraction Palliatiev Radiation Therapy
Historically, patients with painful bone metastases have been treated with courses of palliative radiotherapy ranging between 2 and 4 weeks of daily treatments. However, several large randomized clinical trials comparing a single treatment with multiple treatments have established that SFRT provides equivalent rates of pain relief even when it may be required for a second time.9-12 Recommendations based on these trials have been incorporated into various treatment guidelines that widely acknowledge the efficacy of SFRT.13-15
For this reason, SFRT is often preferred at many centers because it is substantially more convenient for patients with cancer. It reduces travel time for daily radiation clinic visits, which allows for more time with loved ones outside the medical establishment. Furthermore, SFRT improves patient access to radiotherapy and reduces costs. The benefits can be direct as well as indirect to those who have to take time for numerous visits.
Longer courses of palliative radiotherapy can be burdensome for patients and primary care providers. Unnecessarily protracted courses of palliative radiotherapy also delay the receipt of systemic therapies because they are typically considered unsafe to administer concurrently. Moreover, when SFRT is unavailable, the burden of long-course palliation is known to discourage health care providers from referring patients since opioid therapy is more convenient, even though it exchanges lucidity for analgesia.16,17
For this reason, the authors believe that it is in the best interest for veterans with terminal cancers and their providers to be aware of the shorter SFRT for effective, convenient pain relief. This treatment option is particularly relevant for patients with a poor performance status, patients already in hospice care, or patient who travel long distances.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
The authors would like to acknowledge Tony Quang, MD, JD, for the advice given on this project.
Palliative radiotherapy for bone metastases is typically delivered either as a short course of 1 to 5 fractions or protracted over longer courses of up to 20 treatments. These longer courses can be burdensome and discourage its utilization, despite a 50% to 80% likelihood of meaningful pain relief from only a single fraction of radiation therapy. Meanwhile, there are multiple randomized studies that have demonstrated that shorter course(s) are equivalent for pain control.
Although the VHA currently has 143 medical facilities that have cancer diagnostic and treatment capabilities, only 40 have radiation oncology services on-site.1 Thus, access to palliative radiotherapy may be limited for veterans who do not live close by, and many may seek care outside the VHA. At VHA radiation oncology centers, single-fraction radiation therapy (SFRT) is routinely offered by the majority of radiation oncologists.2,3 However, the longer course is commonly preferred outside the VA, and a recent SEER-Medicare analysis of more than 3,000 patients demonstrated that the majority of patients treated outside the VA actually receive more than 10 treatments.4 For this reason, the VA National Palliative Radiotherapy Task Force prepared this document to provide guidance for clinicians within and outside the VA to increase awareness of the appropriateness, effectiveness, and convenience of SFRT as opposed to longer courses of treatment that increase the burden of care at the end of life and often are unnecessary.
Veterans, Cancer, and Metastases
Within the VA, an estimated 40,000 new cancer cases are diagnosed each year, and 175,000 veterans undergo cancer care within the VHA annually.1 Unfortunately, the majority will develop bone metastases with postmortem examinations, suggesting that the rate can be as high as 90% at the end of life.5-7 For many, including veterans with cancer, pain control can be difficult, and access to palliative radiotherapy is critical.8
Single-Fraction Palliatiev Radiation Therapy
Historically, patients with painful bone metastases have been treated with courses of palliative radiotherapy ranging between 2 and 4 weeks of daily treatments. However, several large randomized clinical trials comparing a single treatment with multiple treatments have established that SFRT provides equivalent rates of pain relief even when it may be required for a second time.9-12 Recommendations based on these trials have been incorporated into various treatment guidelines that widely acknowledge the efficacy of SFRT.13-15
For this reason, SFRT is often preferred at many centers because it is substantially more convenient for patients with cancer. It reduces travel time for daily radiation clinic visits, which allows for more time with loved ones outside the medical establishment. Furthermore, SFRT improves patient access to radiotherapy and reduces costs. The benefits can be direct as well as indirect to those who have to take time for numerous visits.
Longer courses of palliative radiotherapy can be burdensome for patients and primary care providers. Unnecessarily protracted courses of palliative radiotherapy also delay the receipt of systemic therapies because they are typically considered unsafe to administer concurrently. Moreover, when SFRT is unavailable, the burden of long-course palliation is known to discourage health care providers from referring patients since opioid therapy is more convenient, even though it exchanges lucidity for analgesia.16,17
For this reason, the authors believe that it is in the best interest for veterans with terminal cancers and their providers to be aware of the shorter SFRT for effective, convenient pain relief. This treatment option is particularly relevant for patients with a poor performance status, patients already in hospice care, or patient who travel long distances.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Click here to read the digital edition.
1. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
2. Moghanaki D, Cheuk AV, Fosmire H, et al; U.S. Veterans Healthcare Administration National Palliative Radiotherapy Taskforce. Availability of single fraction palliative radiotherapy for cancer patients receiving end-of-life care within the Veterans Healthcare Administration. J Palliat Med. 2014;17(11):1221-1225.
3. Dawson GA, Glushko I, Hagan MP. A cross-sectional view of radiation dose fractionation schemes used for painful bone metastases cases within Veterans Health Administration Radiation Oncology Centers. J Clin Oncol. 2015;33(29 suppl):abstract 177.
4. Bekelman JE, Epstein AJ, Emanuel EJ. Single- vs multiple-fraction radiotherapy for bone metastases from prostate cancer. JAMA. 2013;310(14):1501-1502.
5. Galasko CSB. The anatomy and pathways of skeletal metastases. In: Weiss L, Gilbert AH, eds. Bone Metastasis. Boston, MA: GK Hall; 1981:49-63.
6. Bubendorf L, Schöpfer A, Wagner U, et al. Metastatic patterns in prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31(5):578-583.
7. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20, pt 2):6243s-6249s.
8. Geriatrics and Extended Care Strategic Healthcare Group, National Pain Management Coordinating Committee, Veterans Health Administration. Pain as the 5th Vital Sign Toolkit. Rev. ed. Washington, DC: National Pain Management Coordinating Committee; 2000.
9. Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97(11):798-804.
10. Chow E, Hoskins PJ, Wu J, et al. A phase III international randomised trial comparing single with multiple fractions for re-irradiation of painful bone metastases: National Cancer Institute of Canada Clinical Trials Group (NCTC CTG) SC 20. Clin Oncol (R Coll Radiol). 2006;18(2):125-128.
11. Fairchild A, Barnes E, Ghosh S, et al. International patterns of practice in palliative radiotherapy for painful bone metastases: evidence-based practice? Int J Radiat Oncol Biol Phys. 2009;75(5):1501-1510.
12. Chow E, van der Linden YM, Roos D, et al. Single fraction versus multiple fractions of repeat radiation for painful bone metastases: a randomised, controlled, non-inferiority trial. Lancet Oncol. 2014;15(2):164-171.
13. Lutz ST, Berk L, Chang E, et al; American Society for Radiation Oncology (ASTRO). Palliative radiotherapy for bone metastases: an ASTRO evidencebased guideline. Int J Radiat Oncol, Biol, Phys. 2011;79(4):965-976.
14. Expert Panel on Radiation Oncology-Bone Metastases, Lo SS, Lutz ST, Chang EL, et al. ACR Appropriateness Criteria® spinal bone metastases. J Palliat Med. 2013;16(1):9-19.
15. Expert Panel on Radiation Oncology-Bone Metastases, Lutz ST, Lo SS, Chang EL, et al. ACR Appropriateness Criteria® non-spinal bone metastases. J Palliative Med. 2012;15(5):521-526.
16. Guadagnolo BA, Liao KP, Elting L, Giordano S, Buchholz TA, Shih YC. Use of radiation therapy in the last 30 days of life among a large population-based cohort of elderly patients in the United States. J Clin Oncol. 2013;31(1):80-87.
17. Schuster J, Han T, Anscher M, Moghanaki D. Hospice providers awareness of the benefits and availability of single-fraction palliative radiotherapy. J Hospice Palliat Care Nurs. 2014;16(2):67-72.
18. Cheon PM, Wong E, Thavarajah N, et al. A definition of “uncomplicated bone metastases” based on previous bone metastases trials comparing single-fraction and multi-fraction radiation therapy. J Bone Oncol. 2015;4(1):13-17.
1. Zullig LL, Jackson GL, Dorn RA, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System. Mil Med. 2012;177(6):693-701.
2. Moghanaki D, Cheuk AV, Fosmire H, et al; U.S. Veterans Healthcare Administration National Palliative Radiotherapy Taskforce. Availability of single fraction palliative radiotherapy for cancer patients receiving end-of-life care within the Veterans Healthcare Administration. J Palliat Med. 2014;17(11):1221-1225.
3. Dawson GA, Glushko I, Hagan MP. A cross-sectional view of radiation dose fractionation schemes used for painful bone metastases cases within Veterans Health Administration Radiation Oncology Centers. J Clin Oncol. 2015;33(29 suppl):abstract 177.
4. Bekelman JE, Epstein AJ, Emanuel EJ. Single- vs multiple-fraction radiotherapy for bone metastases from prostate cancer. JAMA. 2013;310(14):1501-1502.
5. Galasko CSB. The anatomy and pathways of skeletal metastases. In: Weiss L, Gilbert AH, eds. Bone Metastasis. Boston, MA: GK Hall; 1981:49-63.
6. Bubendorf L, Schöpfer A, Wagner U, et al. Metastatic patterns in prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31(5):578-583.
7. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20, pt 2):6243s-6249s.
8. Geriatrics and Extended Care Strategic Healthcare Group, National Pain Management Coordinating Committee, Veterans Health Administration. Pain as the 5th Vital Sign Toolkit. Rev. ed. Washington, DC: National Pain Management Coordinating Committee; 2000.
9. Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97(11):798-804.
10. Chow E, Hoskins PJ, Wu J, et al. A phase III international randomised trial comparing single with multiple fractions for re-irradiation of painful bone metastases: National Cancer Institute of Canada Clinical Trials Group (NCTC CTG) SC 20. Clin Oncol (R Coll Radiol). 2006;18(2):125-128.
11. Fairchild A, Barnes E, Ghosh S, et al. International patterns of practice in palliative radiotherapy for painful bone metastases: evidence-based practice? Int J Radiat Oncol Biol Phys. 2009;75(5):1501-1510.
12. Chow E, van der Linden YM, Roos D, et al. Single fraction versus multiple fractions of repeat radiation for painful bone metastases: a randomised, controlled, non-inferiority trial. Lancet Oncol. 2014;15(2):164-171.
13. Lutz ST, Berk L, Chang E, et al; American Society for Radiation Oncology (ASTRO). Palliative radiotherapy for bone metastases: an ASTRO evidencebased guideline. Int J Radiat Oncol, Biol, Phys. 2011;79(4):965-976.
14. Expert Panel on Radiation Oncology-Bone Metastases, Lo SS, Lutz ST, Chang EL, et al. ACR Appropriateness Criteria® spinal bone metastases. J Palliat Med. 2013;16(1):9-19.
15. Expert Panel on Radiation Oncology-Bone Metastases, Lutz ST, Lo SS, Chang EL, et al. ACR Appropriateness Criteria® non-spinal bone metastases. J Palliative Med. 2012;15(5):521-526.
16. Guadagnolo BA, Liao KP, Elting L, Giordano S, Buchholz TA, Shih YC. Use of radiation therapy in the last 30 days of life among a large population-based cohort of elderly patients in the United States. J Clin Oncol. 2013;31(1):80-87.
17. Schuster J, Han T, Anscher M, Moghanaki D. Hospice providers awareness of the benefits and availability of single-fraction palliative radiotherapy. J Hospice Palliat Care Nurs. 2014;16(2):67-72.
18. Cheon PM, Wong E, Thavarajah N, et al. A definition of “uncomplicated bone metastases” based on previous bone metastases trials comparing single-fraction and multi-fraction radiation therapy. J Bone Oncol. 2015;4(1):13-17.
‘Strong evidence’ links obesity to cancers
There is strong evidence linking adiposity to esophageal adenocarcinoma, multiple myeloma, and cancer of the colon, rectum, biliary tract, pancreas, endometrium, kidney, and postmenopausal breast, according to the authors of an umbrella review published in the Feb. 28 edition of the BMJ.
“Several meta-analyses support the link between obesity and cancer, but substantial heterogeneity exists between studies,” wrote Maria Kyrgiou, MD, of Imperial College London and her coauthors. “The reported associations may be causal, but they may also be flawed, as inherent study biases such as residual confounding and selective reporting of positive results may exaggerate the effect of obesity on cancer.”
In this umbrella review, researchers analyzed 49 papers that included a total of 204 meta-analyses, which in turn summarized 2,179 individual study estimates from 507 unique cohort or case-control studies.
When researchers applied a threshold for significance of P less than .000001, the summary random effects were significant in 35 meta-analyses; 31 of these found increased risk with adiposity of esophageal adenocarcinoma, multiple myeloma, and cancers of the colon, rectum, liver, biliary tract system (cancers of gallbladder, extrahepatic bile duct, and ampulla of Vater), pancreas, postmenopausal breast, endometrium, and kidney.
“The effect of obesity on the incidence and mortality of cancer is well recognized and was evident in our umbrella review, with approximately 77% of the included meta-analyses reporting a nominally statistically significant summary random effects estimate,” the authors reported.
Overall, the summary estimates were similar between men and women for esophageal adenocarcinoma, esophageal squamous cell carcinoma, multiple myeloma, leukemia, and gastric, lung, kidney, and thyroid cancers.
However, men had a 30% higher risk of colon cancer per 5-kg/m2 increase of body mass index, compared with a 9% increase in risk in women for the same rise in BMI. Men also showed an increased risk of melanoma with increasing BMI, whereas women did not.
Women who had never used hormone therapy showed an 11% increase in the risk of postmenopausal breast cancer with each 5 kg of weight gained. Similarly, each 0.1 increase in waist-to-hip ratio in these women was associated with a 21% increase in the risk of endometrial cancer.
The analysis also revealed an inverse relationship in four meta-analyses for esophageal squamous cell carcinoma and lung cancer.
The authors said their findings agree with those of the World Cancer Research Fund, which currently states there is a convincing causal relationship with obesity for esophageal adenocarcinoma and cancers of the pancreas, colorectum, postmenopausal breast, endometrium, kidney, and liver.
While this umbrella analysis did not find strong evidence for an association with liver cancer, the authors said the evidence was “highly suggestive” but suffered from small study effects, excess significance bias, and substantial heterogeneity between studies.
“To draw firmer conclusions, we need prospective studies and large consortiums with better assessment of the changing nature of body fatness and with comprehensive standardized reporting of analyses,” they wrote. “As obesity becomes one of the greatest public health problems worldwide, evidence of the strength of the associations between obesity and cancer may allow finer selection of people at high risk, who could be selected for personalized primary and secondary prevention strategies.”
The study was supported by the Genesis Research Trust, Sigrid Jusélius Fellowship, the World Cancer Research Fund International Regular Grant Programme, Ovarian Cancer Action, the Imperial Experimental Cancer Medicine Centre, the Cancer Research UK Imperial Centre, Imperial Healthcare NHS Trust NIHR BRC. No relevant conflicts of interest were declared.
There is strong evidence linking adiposity to esophageal adenocarcinoma, multiple myeloma, and cancer of the colon, rectum, biliary tract, pancreas, endometrium, kidney, and postmenopausal breast, according to the authors of an umbrella review published in the Feb. 28 edition of the BMJ.
“Several meta-analyses support the link between obesity and cancer, but substantial heterogeneity exists between studies,” wrote Maria Kyrgiou, MD, of Imperial College London and her coauthors. “The reported associations may be causal, but they may also be flawed, as inherent study biases such as residual confounding and selective reporting of positive results may exaggerate the effect of obesity on cancer.”
In this umbrella review, researchers analyzed 49 papers that included a total of 204 meta-analyses, which in turn summarized 2,179 individual study estimates from 507 unique cohort or case-control studies.
When researchers applied a threshold for significance of P less than .000001, the summary random effects were significant in 35 meta-analyses; 31 of these found increased risk with adiposity of esophageal adenocarcinoma, multiple myeloma, and cancers of the colon, rectum, liver, biliary tract system (cancers of gallbladder, extrahepatic bile duct, and ampulla of Vater), pancreas, postmenopausal breast, endometrium, and kidney.
“The effect of obesity on the incidence and mortality of cancer is well recognized and was evident in our umbrella review, with approximately 77% of the included meta-analyses reporting a nominally statistically significant summary random effects estimate,” the authors reported.
Overall, the summary estimates were similar between men and women for esophageal adenocarcinoma, esophageal squamous cell carcinoma, multiple myeloma, leukemia, and gastric, lung, kidney, and thyroid cancers.
However, men had a 30% higher risk of colon cancer per 5-kg/m2 increase of body mass index, compared with a 9% increase in risk in women for the same rise in BMI. Men also showed an increased risk of melanoma with increasing BMI, whereas women did not.
Women who had never used hormone therapy showed an 11% increase in the risk of postmenopausal breast cancer with each 5 kg of weight gained. Similarly, each 0.1 increase in waist-to-hip ratio in these women was associated with a 21% increase in the risk of endometrial cancer.
The analysis also revealed an inverse relationship in four meta-analyses for esophageal squamous cell carcinoma and lung cancer.
The authors said their findings agree with those of the World Cancer Research Fund, which currently states there is a convincing causal relationship with obesity for esophageal adenocarcinoma and cancers of the pancreas, colorectum, postmenopausal breast, endometrium, kidney, and liver.
While this umbrella analysis did not find strong evidence for an association with liver cancer, the authors said the evidence was “highly suggestive” but suffered from small study effects, excess significance bias, and substantial heterogeneity between studies.
“To draw firmer conclusions, we need prospective studies and large consortiums with better assessment of the changing nature of body fatness and with comprehensive standardized reporting of analyses,” they wrote. “As obesity becomes one of the greatest public health problems worldwide, evidence of the strength of the associations between obesity and cancer may allow finer selection of people at high risk, who could be selected for personalized primary and secondary prevention strategies.”
The study was supported by the Genesis Research Trust, Sigrid Jusélius Fellowship, the World Cancer Research Fund International Regular Grant Programme, Ovarian Cancer Action, the Imperial Experimental Cancer Medicine Centre, the Cancer Research UK Imperial Centre, Imperial Healthcare NHS Trust NIHR BRC. No relevant conflicts of interest were declared.
There is strong evidence linking adiposity to esophageal adenocarcinoma, multiple myeloma, and cancer of the colon, rectum, biliary tract, pancreas, endometrium, kidney, and postmenopausal breast, according to the authors of an umbrella review published in the Feb. 28 edition of the BMJ.
“Several meta-analyses support the link between obesity and cancer, but substantial heterogeneity exists between studies,” wrote Maria Kyrgiou, MD, of Imperial College London and her coauthors. “The reported associations may be causal, but they may also be flawed, as inherent study biases such as residual confounding and selective reporting of positive results may exaggerate the effect of obesity on cancer.”
In this umbrella review, researchers analyzed 49 papers that included a total of 204 meta-analyses, which in turn summarized 2,179 individual study estimates from 507 unique cohort or case-control studies.
When researchers applied a threshold for significance of P less than .000001, the summary random effects were significant in 35 meta-analyses; 31 of these found increased risk with adiposity of esophageal adenocarcinoma, multiple myeloma, and cancers of the colon, rectum, liver, biliary tract system (cancers of gallbladder, extrahepatic bile duct, and ampulla of Vater), pancreas, postmenopausal breast, endometrium, and kidney.
“The effect of obesity on the incidence and mortality of cancer is well recognized and was evident in our umbrella review, with approximately 77% of the included meta-analyses reporting a nominally statistically significant summary random effects estimate,” the authors reported.
Overall, the summary estimates were similar between men and women for esophageal adenocarcinoma, esophageal squamous cell carcinoma, multiple myeloma, leukemia, and gastric, lung, kidney, and thyroid cancers.
However, men had a 30% higher risk of colon cancer per 5-kg/m2 increase of body mass index, compared with a 9% increase in risk in women for the same rise in BMI. Men also showed an increased risk of melanoma with increasing BMI, whereas women did not.
Women who had never used hormone therapy showed an 11% increase in the risk of postmenopausal breast cancer with each 5 kg of weight gained. Similarly, each 0.1 increase in waist-to-hip ratio in these women was associated with a 21% increase in the risk of endometrial cancer.
The analysis also revealed an inverse relationship in four meta-analyses for esophageal squamous cell carcinoma and lung cancer.
The authors said their findings agree with those of the World Cancer Research Fund, which currently states there is a convincing causal relationship with obesity for esophageal adenocarcinoma and cancers of the pancreas, colorectum, postmenopausal breast, endometrium, kidney, and liver.
While this umbrella analysis did not find strong evidence for an association with liver cancer, the authors said the evidence was “highly suggestive” but suffered from small study effects, excess significance bias, and substantial heterogeneity between studies.
“To draw firmer conclusions, we need prospective studies and large consortiums with better assessment of the changing nature of body fatness and with comprehensive standardized reporting of analyses,” they wrote. “As obesity becomes one of the greatest public health problems worldwide, evidence of the strength of the associations between obesity and cancer may allow finer selection of people at high risk, who could be selected for personalized primary and secondary prevention strategies.”
The study was supported by the Genesis Research Trust, Sigrid Jusélius Fellowship, the World Cancer Research Fund International Regular Grant Programme, Ovarian Cancer Action, the Imperial Experimental Cancer Medicine Centre, the Cancer Research UK Imperial Centre, Imperial Healthcare NHS Trust NIHR BRC. No relevant conflicts of interest were declared.
FROM BMJ
Key clinical point: An umbrella analysis of systematic reviews has found strong evidence linking adiposity to a range of cancers including esophageal adenocarcinoma, and cancer of the colon, kidney, and pancreas.
Major finding: Adiposity is significantly associated with cancers of the esophagus, colon, rectum, biliary tract, pancreas, endometrium, kidney, postmenopausal breast, and to multiple myeloma.
Data source: An umbrella review of 204 meta-analyses.
Disclosures: The study was supported by the Genesis Research Trust, Sigrid Jusélius Fellowship, the World Cancer Research Fund International Regular Grant Programme, Ovarian Cancer Action, the Imperial Experimental Cancer Medicine Centre, the Cancer Research UK Imperial Centre, Imperial Healthcare NHS Trust NIHR BRC. No relevant conflicts of interest were declared.
Phase III trial: VZV protects auto-HCT patients
ORLANDO – An inactivated varicella zoster virus vaccine currently in development for adult patients undergoing autologous hematopoietic stem cell transplantation is efficacious and well tolerated, according to findings from a randomized, placebo-controlled, phase III trial.
During the course of the 2 1/2-year pivotal multicenter trial, confirmed herpes zoster infections occurred in 42 of 560 patients who were randomized to receive inactivated varicella zoster virus vaccine (ZVIN) consistency lot (overall incidence of 32.8 cases/1,000 patient-years), compared with 113 of 564 patients who received placebo (overall incidence of 91.8/1,000 patient-years). The estimated vaccine efficacy was 63.8% after adjusting for age and duration of antiviral prophylaxis, Drew J. Winston, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The vaccine also was effective for reducing moderate and severe herpes zoster pain (estimated vaccine efficacy, 69.5%), for preventing postherpetic neuralgia (estimated vaccine efficacy, 83.7%), and for prevention of herpes zoster–related complications (estimated vaccine efficacy, 73.5%), he noted.
Study subjects were adults aged 18 years or older who were undergoing autologous hematopoietic stem cell transplantation (auto-HCT) for a malignancy or other indication. The most common underlying diseases were lymphoma and multiple myeloma. All patients had a history of varicella infection or were seropositive for varicella zoster virus (VZV) antibody, and had no history of VZV vaccine or herpes zoster infection within the prior year.
They were randomized to receive a four-dose regimen of either ZVIN consistency lot, ZVIN high-antigen lot, or placebo. A group of 106 patients who received the ZVIN high-antigen lot were included in the safety analysis only. The first ZVIN dose was administered about a month before transplantation, and doses two through four were administered about 30, 60, and 90 days after transplantation. About 90% in each group received antiviral agents after transplantation, and the duration of the use of antivirals also was similar in the groups. All patients were followed for the duration of the study, and those who developed herpes zoster were followed for 6 months after onset.
Herpes zoster cases were confirmed by polymerase chain reaction or by blinded endpoint committee adjudication.
Serious adverse events and vaccine-related serious adverse events occurred in a similar proportion of patients in the treatment and placebo groups (32.9% and 32.7%, and 0.8% and 0.9%, respectively). Vaccine-related events were primarily injection-site reactions. Systemic adverse events that occurred up to 28 days after vaccination were mainly gastrointestinal side effects, such as diarrhea, nausea, and vomiting. Pyrexia, oral mucositis, thrombocytopenia, and febrile neutropenia also were reported.
The most common serious adverse events were infectious complications, such as febrile neutropenia and relapse of underlying disease.
The findings are notable, as patients undergoing auto-HCT have an increased risk of developing herpes zoster infection and its complications, including postherpetic neuralgia, secondary bacterial infections, and disseminated VZV infection, as well as an increased risk of hospitalization and mortality, Dr. Winston explained.
Herpes zoster infections are associated primarily with cell-mediated immunity, and in older studies done prior to the routine use of antiviral prophylaxis, the reported incidence in auto-HCT patients was between 16% and 25%. Because of this high risk, current guidelines call for antiviral prophylaxis during auto-HCT, but even in this current era of acyclovir or valacyclovir prophylaxis, infections occur at relatively high rates after auto-HCT, he noted.
“Now another approach to prevention of herpes zoster infection is vaccination,” he said.
The live attenuated vaccine currently on the market is generally contraindicated in immunocompromised patients – at least in early period after transplantation, but ZVIN showed promise with respect to safety in earlier studies, which led to the current trial.
“This study demonstrated that the inactivated varicella vaccine is very effective for preventing herpes zoster after autologous stem cell transplantation,” Dr. Winston said, noting that efficacy was observed both in those younger than age 50 years and in those aged 50 and older, and also in those who received prophylaxis for less than 3 months and for 3-6 months.
“Finally!” said one audience member, who noted during a discussion of the findings that there has long been a need for a vaccine to prevent herpes zoster in auto-HCT patients.
Dr. Winston reported receiving research funding from Oxford, and serving as a consultant to Merck and Chimerix.
ORLANDO – An inactivated varicella zoster virus vaccine currently in development for adult patients undergoing autologous hematopoietic stem cell transplantation is efficacious and well tolerated, according to findings from a randomized, placebo-controlled, phase III trial.
During the course of the 2 1/2-year pivotal multicenter trial, confirmed herpes zoster infections occurred in 42 of 560 patients who were randomized to receive inactivated varicella zoster virus vaccine (ZVIN) consistency lot (overall incidence of 32.8 cases/1,000 patient-years), compared with 113 of 564 patients who received placebo (overall incidence of 91.8/1,000 patient-years). The estimated vaccine efficacy was 63.8% after adjusting for age and duration of antiviral prophylaxis, Drew J. Winston, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The vaccine also was effective for reducing moderate and severe herpes zoster pain (estimated vaccine efficacy, 69.5%), for preventing postherpetic neuralgia (estimated vaccine efficacy, 83.7%), and for prevention of herpes zoster–related complications (estimated vaccine efficacy, 73.5%), he noted.
Study subjects were adults aged 18 years or older who were undergoing autologous hematopoietic stem cell transplantation (auto-HCT) for a malignancy or other indication. The most common underlying diseases were lymphoma and multiple myeloma. All patients had a history of varicella infection or were seropositive for varicella zoster virus (VZV) antibody, and had no history of VZV vaccine or herpes zoster infection within the prior year.
They were randomized to receive a four-dose regimen of either ZVIN consistency lot, ZVIN high-antigen lot, or placebo. A group of 106 patients who received the ZVIN high-antigen lot were included in the safety analysis only. The first ZVIN dose was administered about a month before transplantation, and doses two through four were administered about 30, 60, and 90 days after transplantation. About 90% in each group received antiviral agents after transplantation, and the duration of the use of antivirals also was similar in the groups. All patients were followed for the duration of the study, and those who developed herpes zoster were followed for 6 months after onset.
Herpes zoster cases were confirmed by polymerase chain reaction or by blinded endpoint committee adjudication.
Serious adverse events and vaccine-related serious adverse events occurred in a similar proportion of patients in the treatment and placebo groups (32.9% and 32.7%, and 0.8% and 0.9%, respectively). Vaccine-related events were primarily injection-site reactions. Systemic adverse events that occurred up to 28 days after vaccination were mainly gastrointestinal side effects, such as diarrhea, nausea, and vomiting. Pyrexia, oral mucositis, thrombocytopenia, and febrile neutropenia also were reported.
The most common serious adverse events were infectious complications, such as febrile neutropenia and relapse of underlying disease.
The findings are notable, as patients undergoing auto-HCT have an increased risk of developing herpes zoster infection and its complications, including postherpetic neuralgia, secondary bacterial infections, and disseminated VZV infection, as well as an increased risk of hospitalization and mortality, Dr. Winston explained.
Herpes zoster infections are associated primarily with cell-mediated immunity, and in older studies done prior to the routine use of antiviral prophylaxis, the reported incidence in auto-HCT patients was between 16% and 25%. Because of this high risk, current guidelines call for antiviral prophylaxis during auto-HCT, but even in this current era of acyclovir or valacyclovir prophylaxis, infections occur at relatively high rates after auto-HCT, he noted.
“Now another approach to prevention of herpes zoster infection is vaccination,” he said.
The live attenuated vaccine currently on the market is generally contraindicated in immunocompromised patients – at least in early period after transplantation, but ZVIN showed promise with respect to safety in earlier studies, which led to the current trial.
“This study demonstrated that the inactivated varicella vaccine is very effective for preventing herpes zoster after autologous stem cell transplantation,” Dr. Winston said, noting that efficacy was observed both in those younger than age 50 years and in those aged 50 and older, and also in those who received prophylaxis for less than 3 months and for 3-6 months.
“Finally!” said one audience member, who noted during a discussion of the findings that there has long been a need for a vaccine to prevent herpes zoster in auto-HCT patients.
Dr. Winston reported receiving research funding from Oxford, and serving as a consultant to Merck and Chimerix.
ORLANDO – An inactivated varicella zoster virus vaccine currently in development for adult patients undergoing autologous hematopoietic stem cell transplantation is efficacious and well tolerated, according to findings from a randomized, placebo-controlled, phase III trial.
During the course of the 2 1/2-year pivotal multicenter trial, confirmed herpes zoster infections occurred in 42 of 560 patients who were randomized to receive inactivated varicella zoster virus vaccine (ZVIN) consistency lot (overall incidence of 32.8 cases/1,000 patient-years), compared with 113 of 564 patients who received placebo (overall incidence of 91.8/1,000 patient-years). The estimated vaccine efficacy was 63.8% after adjusting for age and duration of antiviral prophylaxis, Drew J. Winston, MD, reported at the combined annual meetings of the Center for International Blood & Marrow Transplant Research and the American Society for Blood and Marrow Transplantation.
The vaccine also was effective for reducing moderate and severe herpes zoster pain (estimated vaccine efficacy, 69.5%), for preventing postherpetic neuralgia (estimated vaccine efficacy, 83.7%), and for prevention of herpes zoster–related complications (estimated vaccine efficacy, 73.5%), he noted.
Study subjects were adults aged 18 years or older who were undergoing autologous hematopoietic stem cell transplantation (auto-HCT) for a malignancy or other indication. The most common underlying diseases were lymphoma and multiple myeloma. All patients had a history of varicella infection or were seropositive for varicella zoster virus (VZV) antibody, and had no history of VZV vaccine or herpes zoster infection within the prior year.
They were randomized to receive a four-dose regimen of either ZVIN consistency lot, ZVIN high-antigen lot, or placebo. A group of 106 patients who received the ZVIN high-antigen lot were included in the safety analysis only. The first ZVIN dose was administered about a month before transplantation, and doses two through four were administered about 30, 60, and 90 days after transplantation. About 90% in each group received antiviral agents after transplantation, and the duration of the use of antivirals also was similar in the groups. All patients were followed for the duration of the study, and those who developed herpes zoster were followed for 6 months after onset.
Herpes zoster cases were confirmed by polymerase chain reaction or by blinded endpoint committee adjudication.
Serious adverse events and vaccine-related serious adverse events occurred in a similar proportion of patients in the treatment and placebo groups (32.9% and 32.7%, and 0.8% and 0.9%, respectively). Vaccine-related events were primarily injection-site reactions. Systemic adverse events that occurred up to 28 days after vaccination were mainly gastrointestinal side effects, such as diarrhea, nausea, and vomiting. Pyrexia, oral mucositis, thrombocytopenia, and febrile neutropenia also were reported.
The most common serious adverse events were infectious complications, such as febrile neutropenia and relapse of underlying disease.
The findings are notable, as patients undergoing auto-HCT have an increased risk of developing herpes zoster infection and its complications, including postherpetic neuralgia, secondary bacterial infections, and disseminated VZV infection, as well as an increased risk of hospitalization and mortality, Dr. Winston explained.
Herpes zoster infections are associated primarily with cell-mediated immunity, and in older studies done prior to the routine use of antiviral prophylaxis, the reported incidence in auto-HCT patients was between 16% and 25%. Because of this high risk, current guidelines call for antiviral prophylaxis during auto-HCT, but even in this current era of acyclovir or valacyclovir prophylaxis, infections occur at relatively high rates after auto-HCT, he noted.
“Now another approach to prevention of herpes zoster infection is vaccination,” he said.
The live attenuated vaccine currently on the market is generally contraindicated in immunocompromised patients – at least in early period after transplantation, but ZVIN showed promise with respect to safety in earlier studies, which led to the current trial.
“This study demonstrated that the inactivated varicella vaccine is very effective for preventing herpes zoster after autologous stem cell transplantation,” Dr. Winston said, noting that efficacy was observed both in those younger than age 50 years and in those aged 50 and older, and also in those who received prophylaxis for less than 3 months and for 3-6 months.
“Finally!” said one audience member, who noted during a discussion of the findings that there has long been a need for a vaccine to prevent herpes zoster in auto-HCT patients.
Dr. Winston reported receiving research funding from Oxford, and serving as a consultant to Merck and Chimerix.
AT THE 2017 BMT TANDEM MEETINGS
Key clinical point:
Major finding: Overall incidence of herpes zoster was 32.8 cases/1,000 patient-years vs. 91.8/1,000 patient-years in patients in the vaccine and placebo groups, respectively.
Data source: A randomized, placebo-controlled phase III trial involving 1,230 patients.
Disclosures: Dr. Winston reported receiving research funding from Oxford, and serving as a consultant to Merck and Chimerix.
Novel Treatment Shows Promise for Acute Lymphoblastic Leukemia
Thanks to gene-editing treatment, 2 babies who had leukemia have a longer lease on life. Researchers from University College London, Great Ormond Street Hospital National Health Service Trust, King’s College, and Sheffield Children’s Hospital, in the United Kingdom used genetically engineered white blood cells from healthy individuals to target the cancer cells.
Related: New Treatments for Chronic Lymphocytic Leukemia
One infant was 11 months old, and the second was 16 months old. Both had refractory relapsed B-cell acute lymphoblastic leukemia and had undergone multidrug treatments. Although the potential of the method has been demonstrated in autologous and human-leukocyte-antigen–matched allogeneic settings, the researchers say, “the infrastructure and expertise required to produce personalized cell products present challenges, and low T cell counts in heavily treated individuals may preclude autologous approaches.”
The treatment involves lymphodepleting chemotherapy and anti-CD52 serotherapy, followed by a single-dose infusion of universal CAR19 cells (autologous T cells engineered to express chimeric antigen receptor against the B-cell antigen CD19). This “bridge-to-transplantation strategy” demonstrates the therapeutic potential of gene-editing technology, the researchers say.
Related: Six Open Clinical Trials That Are Expanding Our Understanding of Immunotherapies
Just 28 days after the treatment, molecular markers showed remission in both infants. The treatments were well tolerated. The first infant had an immune reaction (cytopenia and graft-vs-host disease [GVHD] in skin and marrow) in the 2 months after the infusion and was treated with steroids and bone marrow transplantation. The other baby had no adverse reactions apart from mild skin GVHD that reversed “promptly” with topical steroids and an episode of “unexplained irritability” in the 3 weeks after infusion.
The first and second infant remained cancer free 18 and 12 months later.
Source:
Qasim W, Zhan H, Samarasinghe S, et al. Sci Transl Med. 2017;9(374):pii: eaaj2013.
doi: 10.1126/scitranslmed.aaj2013.
Thanks to gene-editing treatment, 2 babies who had leukemia have a longer lease on life. Researchers from University College London, Great Ormond Street Hospital National Health Service Trust, King’s College, and Sheffield Children’s Hospital, in the United Kingdom used genetically engineered white blood cells from healthy individuals to target the cancer cells.
Related: New Treatments for Chronic Lymphocytic Leukemia
One infant was 11 months old, and the second was 16 months old. Both had refractory relapsed B-cell acute lymphoblastic leukemia and had undergone multidrug treatments. Although the potential of the method has been demonstrated in autologous and human-leukocyte-antigen–matched allogeneic settings, the researchers say, “the infrastructure and expertise required to produce personalized cell products present challenges, and low T cell counts in heavily treated individuals may preclude autologous approaches.”
The treatment involves lymphodepleting chemotherapy and anti-CD52 serotherapy, followed by a single-dose infusion of universal CAR19 cells (autologous T cells engineered to express chimeric antigen receptor against the B-cell antigen CD19). This “bridge-to-transplantation strategy” demonstrates the therapeutic potential of gene-editing technology, the researchers say.
Related: Six Open Clinical Trials That Are Expanding Our Understanding of Immunotherapies
Just 28 days after the treatment, molecular markers showed remission in both infants. The treatments were well tolerated. The first infant had an immune reaction (cytopenia and graft-vs-host disease [GVHD] in skin and marrow) in the 2 months after the infusion and was treated with steroids and bone marrow transplantation. The other baby had no adverse reactions apart from mild skin GVHD that reversed “promptly” with topical steroids and an episode of “unexplained irritability” in the 3 weeks after infusion.
The first and second infant remained cancer free 18 and 12 months later.
Source:
Qasim W, Zhan H, Samarasinghe S, et al. Sci Transl Med. 2017;9(374):pii: eaaj2013.
doi: 10.1126/scitranslmed.aaj2013.
Thanks to gene-editing treatment, 2 babies who had leukemia have a longer lease on life. Researchers from University College London, Great Ormond Street Hospital National Health Service Trust, King’s College, and Sheffield Children’s Hospital, in the United Kingdom used genetically engineered white blood cells from healthy individuals to target the cancer cells.
Related: New Treatments for Chronic Lymphocytic Leukemia
One infant was 11 months old, and the second was 16 months old. Both had refractory relapsed B-cell acute lymphoblastic leukemia and had undergone multidrug treatments. Although the potential of the method has been demonstrated in autologous and human-leukocyte-antigen–matched allogeneic settings, the researchers say, “the infrastructure and expertise required to produce personalized cell products present challenges, and low T cell counts in heavily treated individuals may preclude autologous approaches.”
The treatment involves lymphodepleting chemotherapy and anti-CD52 serotherapy, followed by a single-dose infusion of universal CAR19 cells (autologous T cells engineered to express chimeric antigen receptor against the B-cell antigen CD19). This “bridge-to-transplantation strategy” demonstrates the therapeutic potential of gene-editing technology, the researchers say.
Related: Six Open Clinical Trials That Are Expanding Our Understanding of Immunotherapies
Just 28 days after the treatment, molecular markers showed remission in both infants. The treatments were well tolerated. The first infant had an immune reaction (cytopenia and graft-vs-host disease [GVHD] in skin and marrow) in the 2 months after the infusion and was treated with steroids and bone marrow transplantation. The other baby had no adverse reactions apart from mild skin GVHD that reversed “promptly” with topical steroids and an episode of “unexplained irritability” in the 3 weeks after infusion.
The first and second infant remained cancer free 18 and 12 months later.
Source:
Qasim W, Zhan H, Samarasinghe S, et al. Sci Transl Med. 2017;9(374):pii: eaaj2013.
doi: 10.1126/scitranslmed.aaj2013.
Long view shows doubling of survival in non-Hodgkin lymphoma
Five-year survival for patients with non-Hodgkin lymphoma has more than doubled since the early 1950s, according to Ali H. Mokdad, PhD, and his associates.
Data from the Surveillance, Epidemiology, and End Results Program show that the 5-year relative survival rate for non-Hodgkin lymphoma in the United States went from 33% in 1950-1954 to 71.2% in 2008-2013, an increase of 116%, Dr. Mokdad and his associates reported (JAMA 2017;317[4]:388-406).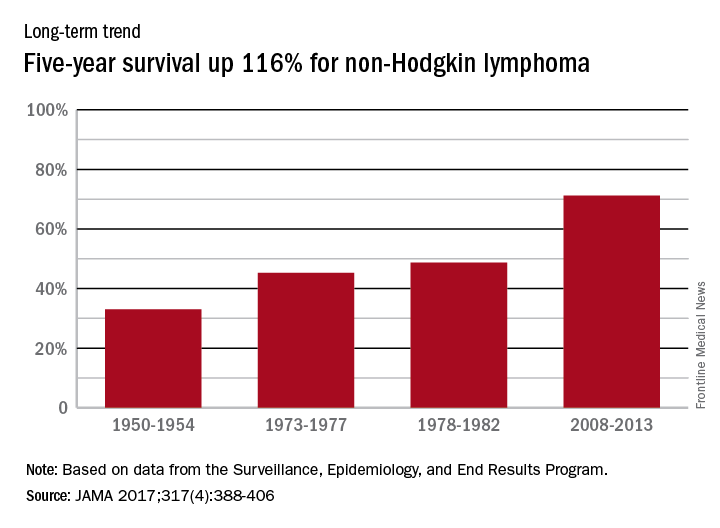
In 2014, mortality for non-Hodgkin lymphoma was the 7th highest among the 29 cancers included in the study, and more than 487,000 years of life were lost, which put it 6th among the 29 cancers, said Dr. Mokdad and his associates from the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.
Five-year survival for patients with non-Hodgkin lymphoma has more than doubled since the early 1950s, according to Ali H. Mokdad, PhD, and his associates.
Data from the Surveillance, Epidemiology, and End Results Program show that the 5-year relative survival rate for non-Hodgkin lymphoma in the United States went from 33% in 1950-1954 to 71.2% in 2008-2013, an increase of 116%, Dr. Mokdad and his associates reported (JAMA 2017;317[4]:388-406).
In 2014, mortality for non-Hodgkin lymphoma was the 7th highest among the 29 cancers included in the study, and more than 487,000 years of life were lost, which put it 6th among the 29 cancers, said Dr. Mokdad and his associates from the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.
Five-year survival for patients with non-Hodgkin lymphoma has more than doubled since the early 1950s, according to Ali H. Mokdad, PhD, and his associates.
Data from the Surveillance, Epidemiology, and End Results Program show that the 5-year relative survival rate for non-Hodgkin lymphoma in the United States went from 33% in 1950-1954 to 71.2% in 2008-2013, an increase of 116%, Dr. Mokdad and his associates reported (JAMA 2017;317[4]:388-406).
In 2014, mortality for non-Hodgkin lymphoma was the 7th highest among the 29 cancers included in the study, and more than 487,000 years of life were lost, which put it 6th among the 29 cancers, said Dr. Mokdad and his associates from the Institute for Health Metrics and Evaluation at the University of Washington, Seattle.
FROM JAMA
What Do Parasites Have to Do With Leukemia?
Parasites have been shown to have both pro- and antitumor effects. Malaria parasites (Plasmodium spp) are among those known to have this possible “bidirectional role” in carcinogenesis, say researchers from Aix-Marseille Université in France. They reviewed the current thinking on whether malaria—a worldwide killer—can be useful in cancer prevention and treatment.
Positive relationships between malaria and virus-associated cancers are relatively well documented, the researchers say. Evidence suggests that malaria can alter immune responses by modulating both humoral and cell-mediated immunity. Plasmodium-related cancers are primarily lymphoproliferative, vulnerable to virus reactivation. Epstein-Barr virus (EBV), for example, has been observed in lymphatic and hematologic tumors such as Hodgkin disease and T cell lymphoma, and malaria can reactivate EBV.
In animal studies, malarial infection with Plasmodium berghei (P berghei) increased the rate of spontaneous leukemia. In one study, concurrent infection with P berghei increased the incidence of malignant lymphoma in mice injected with Moloney leukemogenic virus.
On the other hand, Plasmodium spp also produces proteins that demonstrate certain anti-oncogenic effects, they note. The researchers suggest that using proteins in cancer treatment should be explored, adding that it’s a “safer approach than the inoculation of wild type Plasmodium.” Positive parasite-induced effects against cancers of the hematopoietic and lymphoid tissues are mentioned only for 2 species and those only in a decades-old study. Based on current knowledge, the researchers say, the antitumor effects observed are attributable to modifications to the host immune response. Thus, their characteristics and locations within the host can be highly diverse.
All in all, the researchers conclude, the growing evidence is opening intriguing pathways for using one ill to cure another.
Source:
Faure E. Parasitology. 2016;143(14):1811-1823.
Parasites have been shown to have both pro- and antitumor effects. Malaria parasites (Plasmodium spp) are among those known to have this possible “bidirectional role” in carcinogenesis, say researchers from Aix-Marseille Université in France. They reviewed the current thinking on whether malaria—a worldwide killer—can be useful in cancer prevention and treatment.
Positive relationships between malaria and virus-associated cancers are relatively well documented, the researchers say. Evidence suggests that malaria can alter immune responses by modulating both humoral and cell-mediated immunity. Plasmodium-related cancers are primarily lymphoproliferative, vulnerable to virus reactivation. Epstein-Barr virus (EBV), for example, has been observed in lymphatic and hematologic tumors such as Hodgkin disease and T cell lymphoma, and malaria can reactivate EBV.
In animal studies, malarial infection with Plasmodium berghei (P berghei) increased the rate of spontaneous leukemia. In one study, concurrent infection with P berghei increased the incidence of malignant lymphoma in mice injected with Moloney leukemogenic virus.
On the other hand, Plasmodium spp also produces proteins that demonstrate certain anti-oncogenic effects, they note. The researchers suggest that using proteins in cancer treatment should be explored, adding that it’s a “safer approach than the inoculation of wild type Plasmodium.” Positive parasite-induced effects against cancers of the hematopoietic and lymphoid tissues are mentioned only for 2 species and those only in a decades-old study. Based on current knowledge, the researchers say, the antitumor effects observed are attributable to modifications to the host immune response. Thus, their characteristics and locations within the host can be highly diverse.
All in all, the researchers conclude, the growing evidence is opening intriguing pathways for using one ill to cure another.
Source:
Faure E. Parasitology. 2016;143(14):1811-1823.
Parasites have been shown to have both pro- and antitumor effects. Malaria parasites (Plasmodium spp) are among those known to have this possible “bidirectional role” in carcinogenesis, say researchers from Aix-Marseille Université in France. They reviewed the current thinking on whether malaria—a worldwide killer—can be useful in cancer prevention and treatment.
Positive relationships between malaria and virus-associated cancers are relatively well documented, the researchers say. Evidence suggests that malaria can alter immune responses by modulating both humoral and cell-mediated immunity. Plasmodium-related cancers are primarily lymphoproliferative, vulnerable to virus reactivation. Epstein-Barr virus (EBV), for example, has been observed in lymphatic and hematologic tumors such as Hodgkin disease and T cell lymphoma, and malaria can reactivate EBV.
In animal studies, malarial infection with Plasmodium berghei (P berghei) increased the rate of spontaneous leukemia. In one study, concurrent infection with P berghei increased the incidence of malignant lymphoma in mice injected with Moloney leukemogenic virus.
On the other hand, Plasmodium spp also produces proteins that demonstrate certain anti-oncogenic effects, they note. The researchers suggest that using proteins in cancer treatment should be explored, adding that it’s a “safer approach than the inoculation of wild type Plasmodium.” Positive parasite-induced effects against cancers of the hematopoietic and lymphoid tissues are mentioned only for 2 species and those only in a decades-old study. Based on current knowledge, the researchers say, the antitumor effects observed are attributable to modifications to the host immune response. Thus, their characteristics and locations within the host can be highly diverse.
All in all, the researchers conclude, the growing evidence is opening intriguing pathways for using one ill to cure another.
Source:
Faure E. Parasitology. 2016;143(14):1811-1823.
Multiple Myeloma and Stroke: What’s the Risk?
Related: Treating Patients With Multiple Myeloma in the VA
Patients were enrolled in Total Therapy protocols (TT2, TT3a, and TT3b), which tested varying combinations of thalidomide, bortezomib, lenalidomide, and dexamethasone. Of 1,148 patients, 46 (4%) had strokes, usually ischemic stroke (33 patients, or 72%). Hypercoagulability, atrial fibrillation and small-vessel occlusion were common mechanisms. Whereas other research has found a higher risk of arterial thrombosis from activated prothrombotic factors, especially during the early period of chemotherapy, in this study vascular events occurred months later.
Seven patients died in the hospital (15% compared with a national average of 5%). Although 6 of those deaths were stroke related, 36 patients were discharged home or to a rehabilitation facility; 2 were discharged to a long-term nursing facility. During a median follow-up of 10 years, 6 patients had another stroke. The cumulative risk of recurrent stroke was 15% compared with 5% for the general population.
Related: Link Found Between Agent Orange Exposure and Multiple Myeloma
Stage I and II cancers and renal insufficiency independently predicted stroke. Also noteworthy, according to the researchers: Patients with MM who developed renal insufficiency had worse clinical outcomes despite improvement in their renal function or lack of significant difference in their baseline renal functions between various treatment protocols. Thus, the increased risk of stroke, recurrent stroke, and mortality could partly be due to renal disease, which may or may not have resulted from myeloma.
Use of combination chemotherapy has “markedly improved” clinical outcomes for MM patients, the researchers say, but those drugs have also been associated with an increased risk of VTE, especially during the first months of chemotherapy. Thalidomide alone did not increase the risk of VTE, nor did lenalinomide on its own. However, thalidomide combined with multiagent chemotherapy increased VTE risk as much as 34% in newly diagnosed patients, and lenalinomide with dexamethasone boosted risk as high as 75%.
The researchers found no significant relationship between mortality and use of thalidomide. Median survival was 103 months for a thalidomide-based regimen and 78 months for a regimen without thalidomide.
Related: Multiple Myeloma: Updates on Diagnosis and Management
The researchers noted that the patients developed strokes despite a trend toward coagulopathy, to the extent that half were ineligible for immediate use of antiplatelet agents. The study findings “heightened our awareness,” the researchers say, that aggressive preventive measures can help reduce the incidence of stroke in patients with renal insufficiency.
Source:
Hinduja A, Limaye K, Ravilla R, et al. PLoS One. 2016;11(11): e0166627.
doi: 10.1371/journal.pone.0166627.
Related: Treating Patients With Multiple Myeloma in the VA
Patients were enrolled in Total Therapy protocols (TT2, TT3a, and TT3b), which tested varying combinations of thalidomide, bortezomib, lenalidomide, and dexamethasone. Of 1,148 patients, 46 (4%) had strokes, usually ischemic stroke (33 patients, or 72%). Hypercoagulability, atrial fibrillation and small-vessel occlusion were common mechanisms. Whereas other research has found a higher risk of arterial thrombosis from activated prothrombotic factors, especially during the early period of chemotherapy, in this study vascular events occurred months later.
Seven patients died in the hospital (15% compared with a national average of 5%). Although 6 of those deaths were stroke related, 36 patients were discharged home or to a rehabilitation facility; 2 were discharged to a long-term nursing facility. During a median follow-up of 10 years, 6 patients had another stroke. The cumulative risk of recurrent stroke was 15% compared with 5% for the general population.
Related: Link Found Between Agent Orange Exposure and Multiple Myeloma
Stage I and II cancers and renal insufficiency independently predicted stroke. Also noteworthy, according to the researchers: Patients with MM who developed renal insufficiency had worse clinical outcomes despite improvement in their renal function or lack of significant difference in their baseline renal functions between various treatment protocols. Thus, the increased risk of stroke, recurrent stroke, and mortality could partly be due to renal disease, which may or may not have resulted from myeloma.
Use of combination chemotherapy has “markedly improved” clinical outcomes for MM patients, the researchers say, but those drugs have also been associated with an increased risk of VTE, especially during the first months of chemotherapy. Thalidomide alone did not increase the risk of VTE, nor did lenalinomide on its own. However, thalidomide combined with multiagent chemotherapy increased VTE risk as much as 34% in newly diagnosed patients, and lenalinomide with dexamethasone boosted risk as high as 75%.
The researchers found no significant relationship between mortality and use of thalidomide. Median survival was 103 months for a thalidomide-based regimen and 78 months for a regimen without thalidomide.
Related: Multiple Myeloma: Updates on Diagnosis and Management
The researchers noted that the patients developed strokes despite a trend toward coagulopathy, to the extent that half were ineligible for immediate use of antiplatelet agents. The study findings “heightened our awareness,” the researchers say, that aggressive preventive measures can help reduce the incidence of stroke in patients with renal insufficiency.
Source:
Hinduja A, Limaye K, Ravilla R, et al. PLoS One. 2016;11(11): e0166627.
doi: 10.1371/journal.pone.0166627.
Related: Treating Patients With Multiple Myeloma in the VA
Patients were enrolled in Total Therapy protocols (TT2, TT3a, and TT3b), which tested varying combinations of thalidomide, bortezomib, lenalidomide, and dexamethasone. Of 1,148 patients, 46 (4%) had strokes, usually ischemic stroke (33 patients, or 72%). Hypercoagulability, atrial fibrillation and small-vessel occlusion were common mechanisms. Whereas other research has found a higher risk of arterial thrombosis from activated prothrombotic factors, especially during the early period of chemotherapy, in this study vascular events occurred months later.
Seven patients died in the hospital (15% compared with a national average of 5%). Although 6 of those deaths were stroke related, 36 patients were discharged home or to a rehabilitation facility; 2 were discharged to a long-term nursing facility. During a median follow-up of 10 years, 6 patients had another stroke. The cumulative risk of recurrent stroke was 15% compared with 5% for the general population.
Related: Link Found Between Agent Orange Exposure and Multiple Myeloma
Stage I and II cancers and renal insufficiency independently predicted stroke. Also noteworthy, according to the researchers: Patients with MM who developed renal insufficiency had worse clinical outcomes despite improvement in their renal function or lack of significant difference in their baseline renal functions between various treatment protocols. Thus, the increased risk of stroke, recurrent stroke, and mortality could partly be due to renal disease, which may or may not have resulted from myeloma.
Use of combination chemotherapy has “markedly improved” clinical outcomes for MM patients, the researchers say, but those drugs have also been associated with an increased risk of VTE, especially during the first months of chemotherapy. Thalidomide alone did not increase the risk of VTE, nor did lenalinomide on its own. However, thalidomide combined with multiagent chemotherapy increased VTE risk as much as 34% in newly diagnosed patients, and lenalinomide with dexamethasone boosted risk as high as 75%.
The researchers found no significant relationship between mortality and use of thalidomide. Median survival was 103 months for a thalidomide-based regimen and 78 months for a regimen without thalidomide.
Related: Multiple Myeloma: Updates on Diagnosis and Management
The researchers noted that the patients developed strokes despite a trend toward coagulopathy, to the extent that half were ineligible for immediate use of antiplatelet agents. The study findings “heightened our awareness,” the researchers say, that aggressive preventive measures can help reduce the incidence of stroke in patients with renal insufficiency.
Source:
Hinduja A, Limaye K, Ravilla R, et al. PLoS One. 2016;11(11): e0166627.
doi: 10.1371/journal.pone.0166627.
HHS Buys Growth Factor Products for Emergency Use
High doses of radiation are often followed by infection. HHS is preparing for emergencies by buying 2 colony-stimulating factor (CSF) products to reduce infection and risk of death in radiologic or nuclear incidents.
Related: Emergency Test for Absorbed Radiation
HHS is purchasing Neulasta (Amgen USA, Inc) and Leukine (Sanofi-Aventis US), under agreements totaling about $37.7 million and 37.6 million, respectively. Neulasta already is FDA approved to treat cancer patients exposed to high levels of radiation that damage bone marrow. Leukine is undergoing studies needed for approval.
The Biomedical Advanced Research and Development Authority had earlier sponsored advanced development and purchase of Neupogen, another leukocyte growth factor product approved for treating adults and children exposed to radiation that damages bone marrow.
The deal for Neulasta and Leukine thus increases the number of CSF factor doses available for use in an emergency. It also increases operational capability, HHS says, since treatments with Neulasta are given once weekly, whereas treatment with Neupogen is daily.
High doses of radiation are often followed by infection. HHS is preparing for emergencies by buying 2 colony-stimulating factor (CSF) products to reduce infection and risk of death in radiologic or nuclear incidents.
Related: Emergency Test for Absorbed Radiation
HHS is purchasing Neulasta (Amgen USA, Inc) and Leukine (Sanofi-Aventis US), under agreements totaling about $37.7 million and 37.6 million, respectively. Neulasta already is FDA approved to treat cancer patients exposed to high levels of radiation that damage bone marrow. Leukine is undergoing studies needed for approval.
The Biomedical Advanced Research and Development Authority had earlier sponsored advanced development and purchase of Neupogen, another leukocyte growth factor product approved for treating adults and children exposed to radiation that damages bone marrow.
The deal for Neulasta and Leukine thus increases the number of CSF factor doses available for use in an emergency. It also increases operational capability, HHS says, since treatments with Neulasta are given once weekly, whereas treatment with Neupogen is daily.
High doses of radiation are often followed by infection. HHS is preparing for emergencies by buying 2 colony-stimulating factor (CSF) products to reduce infection and risk of death in radiologic or nuclear incidents.
Related: Emergency Test for Absorbed Radiation
HHS is purchasing Neulasta (Amgen USA, Inc) and Leukine (Sanofi-Aventis US), under agreements totaling about $37.7 million and 37.6 million, respectively. Neulasta already is FDA approved to treat cancer patients exposed to high levels of radiation that damage bone marrow. Leukine is undergoing studies needed for approval.
The Biomedical Advanced Research and Development Authority had earlier sponsored advanced development and purchase of Neupogen, another leukocyte growth factor product approved for treating adults and children exposed to radiation that damages bone marrow.
The deal for Neulasta and Leukine thus increases the number of CSF factor doses available for use in an emergency. It also increases operational capability, HHS says, since treatments with Neulasta are given once weekly, whereas treatment with Neupogen is daily.