User login
Non-Hodgkin Lymphoma Death Rates Continue to Fall
The 5-year relative survival rate for non-Hodgkin lymphoma (NHL) climbed to 72.7% and is as high as 82.6% for localized NHL, according to the most recent SEER data. The number of new cases remains high at 19.1 per 100,000 people (all races) per year; however the number of deaths is relatively low at 5.7 deaths per 100,000 people (all races) per year. Death rates have been falling on average 2.4% each year from 2004 to 2013.
While the new cases represent 4.3% of all new cancer diagnoses, NHL deaths represent 3.4% of all cancer deaths. Based on 2011-2013 SEER data, about 2.1% of men and women will receive a NHL diagnosis at some point during their lifetime.
Patient diagnoses by stage:
- 28% are diagnosed at the local stage
- 15% are diagnosed with spread to regional lymph nodes
- 50% are diagnosed after distant cancer has metastasized
- 8% unknown/unstaged
As of 2013, there were an estimated 569,536 people living with NHL in the U.S.
Using statistical models for analysis, rates for new non-Hodgkin lymphoma cases have not changed significantly over the past 10 years.
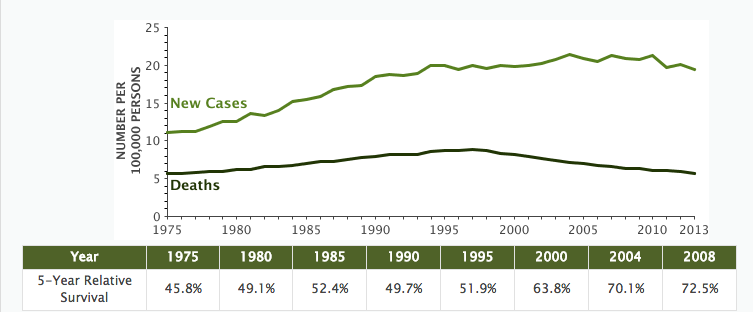
The 5-year relative survival rate for non-Hodgkin lymphoma (NHL) climbed to 72.7% and is as high as 82.6% for localized NHL, according to the most recent SEER data. The number of new cases remains high at 19.1 per 100,000 people (all races) per year; however the number of deaths is relatively low at 5.7 deaths per 100,000 people (all races) per year. Death rates have been falling on average 2.4% each year from 2004 to 2013.
While the new cases represent 4.3% of all new cancer diagnoses, NHL deaths represent 3.4% of all cancer deaths. Based on 2011-2013 SEER data, about 2.1% of men and women will receive a NHL diagnosis at some point during their lifetime.
Patient diagnoses by stage:
- 28% are diagnosed at the local stage
- 15% are diagnosed with spread to regional lymph nodes
- 50% are diagnosed after distant cancer has metastasized
- 8% unknown/unstaged
As of 2013, there were an estimated 569,536 people living with NHL in the U.S.
Using statistical models for analysis, rates for new non-Hodgkin lymphoma cases have not changed significantly over the past 10 years.

The 5-year relative survival rate for non-Hodgkin lymphoma (NHL) climbed to 72.7% and is as high as 82.6% for localized NHL, according to the most recent SEER data. The number of new cases remains high at 19.1 per 100,000 people (all races) per year; however the number of deaths is relatively low at 5.7 deaths per 100,000 people (all races) per year. Death rates have been falling on average 2.4% each year from 2004 to 2013.
While the new cases represent 4.3% of all new cancer diagnoses, NHL deaths represent 3.4% of all cancer deaths. Based on 2011-2013 SEER data, about 2.1% of men and women will receive a NHL diagnosis at some point during their lifetime.
Patient diagnoses by stage:
- 28% are diagnosed at the local stage
- 15% are diagnosed with spread to regional lymph nodes
- 50% are diagnosed after distant cancer has metastasized
- 8% unknown/unstaged
As of 2013, there were an estimated 569,536 people living with NHL in the U.S.
Using statistical models for analysis, rates for new non-Hodgkin lymphoma cases have not changed significantly over the past 10 years.

Hashimoto’s Thyroiditis and Lymphoma
A “heightened index of suspicion” is called for when a patient with Hashimoto’s thyroiditis (HT) presents with an enlarging neck mass, say researchers from Tan Tock Seng Hospital, Singapore, in a case report. According to the researchers, because the complication of thyroid lymphoma is rare, physicians commonly forgotten it. But primary thyroid lymphomas (PTLs) have a 60-fold risk in patients with HT.
Related: Study Points to Risk Factors for Lymphoma
Hashimoto’s thyroiditis typically is treated successfully with thyroxine. The study patient, however, began to lose weight and developed a mass in her neck that was diagnosed as diffuse large B-cell lymphoma. Previously, research suggested that having HT for ≥ 20 years increased the risk of thyroid lymphoma, but small studies have found that the interval between diagnosis of HT and diagnosis of thyroid lymphoma might be shorter—4 to 9 years, the researchers note. They cite another study that found the median interval was 18 months, as with their patient. Symptoms usually last from a few days to 36 months before diagnosis; in the study patient, symptoms of compression occurred over 2 to 3 weeks.
That shorter time frame may indicate that ultrasonography surveillance should be started early and done periodically, the researchers say, to detect lymphoma development as soon as possible. Radiologic imaging is helpful but “only serves as an adjunct to the diagnosis.” Histologic diagnosis is still needed for definitive diagnosis.
Timely diagnosis and early treatment mean the prognosis can be good for PTL, with relatively high survival rates after chemotherapy and radiotherapy. In this case, the patient underwent 6 cycles of chemotherapy with adjuvant radiotherapy. She then was maintained on thyroxine 75 µg daily. She remains euthyroid and disease free 1 year after completing her cancer treatment.
Source:
Chiang B, Cheng S, Seow CJ. BMJ Case Rep. 2016;pii:bcr2016217568.
doi: 10.1136/bcr-2016-217568.
A “heightened index of suspicion” is called for when a patient with Hashimoto’s thyroiditis (HT) presents with an enlarging neck mass, say researchers from Tan Tock Seng Hospital, Singapore, in a case report. According to the researchers, because the complication of thyroid lymphoma is rare, physicians commonly forgotten it. But primary thyroid lymphomas (PTLs) have a 60-fold risk in patients with HT.
Related: Study Points to Risk Factors for Lymphoma
Hashimoto’s thyroiditis typically is treated successfully with thyroxine. The study patient, however, began to lose weight and developed a mass in her neck that was diagnosed as diffuse large B-cell lymphoma. Previously, research suggested that having HT for ≥ 20 years increased the risk of thyroid lymphoma, but small studies have found that the interval between diagnosis of HT and diagnosis of thyroid lymphoma might be shorter—4 to 9 years, the researchers note. They cite another study that found the median interval was 18 months, as with their patient. Symptoms usually last from a few days to 36 months before diagnosis; in the study patient, symptoms of compression occurred over 2 to 3 weeks.
That shorter time frame may indicate that ultrasonography surveillance should be started early and done periodically, the researchers say, to detect lymphoma development as soon as possible. Radiologic imaging is helpful but “only serves as an adjunct to the diagnosis.” Histologic diagnosis is still needed for definitive diagnosis.
Timely diagnosis and early treatment mean the prognosis can be good for PTL, with relatively high survival rates after chemotherapy and radiotherapy. In this case, the patient underwent 6 cycles of chemotherapy with adjuvant radiotherapy. She then was maintained on thyroxine 75 µg daily. She remains euthyroid and disease free 1 year after completing her cancer treatment.
Source:
Chiang B, Cheng S, Seow CJ. BMJ Case Rep. 2016;pii:bcr2016217568.
doi: 10.1136/bcr-2016-217568.
A “heightened index of suspicion” is called for when a patient with Hashimoto’s thyroiditis (HT) presents with an enlarging neck mass, say researchers from Tan Tock Seng Hospital, Singapore, in a case report. According to the researchers, because the complication of thyroid lymphoma is rare, physicians commonly forgotten it. But primary thyroid lymphomas (PTLs) have a 60-fold risk in patients with HT.
Related: Study Points to Risk Factors for Lymphoma
Hashimoto’s thyroiditis typically is treated successfully with thyroxine. The study patient, however, began to lose weight and developed a mass in her neck that was diagnosed as diffuse large B-cell lymphoma. Previously, research suggested that having HT for ≥ 20 years increased the risk of thyroid lymphoma, but small studies have found that the interval between diagnosis of HT and diagnosis of thyroid lymphoma might be shorter—4 to 9 years, the researchers note. They cite another study that found the median interval was 18 months, as with their patient. Symptoms usually last from a few days to 36 months before diagnosis; in the study patient, symptoms of compression occurred over 2 to 3 weeks.
That shorter time frame may indicate that ultrasonography surveillance should be started early and done periodically, the researchers say, to detect lymphoma development as soon as possible. Radiologic imaging is helpful but “only serves as an adjunct to the diagnosis.” Histologic diagnosis is still needed for definitive diagnosis.
Timely diagnosis and early treatment mean the prognosis can be good for PTL, with relatively high survival rates after chemotherapy and radiotherapy. In this case, the patient underwent 6 cycles of chemotherapy with adjuvant radiotherapy. She then was maintained on thyroxine 75 µg daily. She remains euthyroid and disease free 1 year after completing her cancer treatment.
Source:
Chiang B, Cheng S, Seow CJ. BMJ Case Rep. 2016;pii:bcr2016217568.
doi: 10.1136/bcr-2016-217568.
Hepatitis infection raises non-Hodgkin lymphoma risk in HIV patients
HIV-infected individuals on antiretroviral therapy who also have chronic coinfection with hepatitis B or C virus have an increased risk of developing non-Hodgkin lymphoma, according to new research published in Annals of Internal Medicine.
Lead author Qing Wang, PhD, of the Basel Institute for Clinical Epidemiology & Biostatistics at University Hospital Basel, Switzerland, and her coauthors said there is growing evidence of an association between both chronic hepatitis B virus infection (HBV) and chronic hepatitis C virus infection (HCV), and non-Hodgkin lymphoma, with chronic immune activation and B cell proliferation suggested as potential mechanisms. However, the impact of chronic coinfection in individuals with HIV is unclear.
Researchers undertook a cohort study of 52,479 treatment-naive individuals with HIV infection, using 18 of 33 cohorts from the Collaboration of Observational HIV Epidemiological Research Europe. Of these participants, 1,336 had chronic HBV and 7,506 had chronic HCV infection, and more than three-quarters (77%) later started treatment with antiretroviral therapy.
After 13 months of follow-up in the treatment-naive group and 50 months in the antiretroviral group, there were 252 cases of non-Hodgkin lymphoma in the treatment-naive group and 310 cases in the treated group (Ann Intern Med. 2016 Oct 17. doi: 10.7326/M16-0240).
Antiretroviral-treated patients with chronic hepatitis B showed a significant 74% greater risk (95% confidence interval, 1.08-2.82) and those with hepatitis C showed a 73% greater risk (95% CI, 1.21-2.46) of non-Hodgkin lymphoma compared to treated individuals with neither coinfection. However, the differences in non-Hodgkin lymphoma rates in treatment-naive HBV and HCV coinfected individuals were not significant, which the authors suggested could be due to lower numbers of events and limited follow-up.
“The median CD4 count at the time of NHL diagnosis was less than 0.250 x 109 cells/L in both ART-naive and treated patients coinfected with HBV and HCV, indicating that coinfected patients with NHL initiate ART late or have insufficient HIV viral control and immune recovery that may be due to multiple reasons,” the authors wrote. “This unfavorable constellation is aggravated by the fact that chronic HBV infection attenuates immune recovery in ART-treated patients; whether this is also the case for chronic HCV infection is less clear.”
The authors said routine screening for chronic HBV and HCV infection, in conjunction with early diagnosis and treatment of HIV infection, was essential to reduce morbidity and mortality from non-Hodgkin lymphoma.
“Our findings provide strong evidence that HCV coinfected patients with poor immune status or restoration (CD4 count lower than 0.250 x 109 cells/L) are at high risk for NHL and death and deserve high priority for access to well-tolerated, interferon-free, direct-acting antiviral treatment programs similar to those for patients with advanced liver fibrosis or cirrhosis.”
The study was supported by the European Union Seventh Framework Programme, Schweizerische Krebsliga, Agence Nationale de Recherches sur le SIDA et les Hepatites Virales (ANRS), Paris; the HIV Monitoring Foundation, Amsterdam; and the Augustinus Foundation, Copenhagen. Eleven authors declared grants, personal fees, and other support from pharmaceutical companies including those involved in the manufacture of HIV and hepatitis drugs. No other conflicts of interest were reported.
HIV-infected individuals on antiretroviral therapy who also have chronic coinfection with hepatitis B or C virus have an increased risk of developing non-Hodgkin lymphoma, according to new research published in Annals of Internal Medicine.
Lead author Qing Wang, PhD, of the Basel Institute for Clinical Epidemiology & Biostatistics at University Hospital Basel, Switzerland, and her coauthors said there is growing evidence of an association between both chronic hepatitis B virus infection (HBV) and chronic hepatitis C virus infection (HCV), and non-Hodgkin lymphoma, with chronic immune activation and B cell proliferation suggested as potential mechanisms. However, the impact of chronic coinfection in individuals with HIV is unclear.
Researchers undertook a cohort study of 52,479 treatment-naive individuals with HIV infection, using 18 of 33 cohorts from the Collaboration of Observational HIV Epidemiological Research Europe. Of these participants, 1,336 had chronic HBV and 7,506 had chronic HCV infection, and more than three-quarters (77%) later started treatment with antiretroviral therapy.
After 13 months of follow-up in the treatment-naive group and 50 months in the antiretroviral group, there were 252 cases of non-Hodgkin lymphoma in the treatment-naive group and 310 cases in the treated group (Ann Intern Med. 2016 Oct 17. doi: 10.7326/M16-0240).
Antiretroviral-treated patients with chronic hepatitis B showed a significant 74% greater risk (95% confidence interval, 1.08-2.82) and those with hepatitis C showed a 73% greater risk (95% CI, 1.21-2.46) of non-Hodgkin lymphoma compared to treated individuals with neither coinfection. However, the differences in non-Hodgkin lymphoma rates in treatment-naive HBV and HCV coinfected individuals were not significant, which the authors suggested could be due to lower numbers of events and limited follow-up.
“The median CD4 count at the time of NHL diagnosis was less than 0.250 x 109 cells/L in both ART-naive and treated patients coinfected with HBV and HCV, indicating that coinfected patients with NHL initiate ART late or have insufficient HIV viral control and immune recovery that may be due to multiple reasons,” the authors wrote. “This unfavorable constellation is aggravated by the fact that chronic HBV infection attenuates immune recovery in ART-treated patients; whether this is also the case for chronic HCV infection is less clear.”
The authors said routine screening for chronic HBV and HCV infection, in conjunction with early diagnosis and treatment of HIV infection, was essential to reduce morbidity and mortality from non-Hodgkin lymphoma.
“Our findings provide strong evidence that HCV coinfected patients with poor immune status or restoration (CD4 count lower than 0.250 x 109 cells/L) are at high risk for NHL and death and deserve high priority for access to well-tolerated, interferon-free, direct-acting antiviral treatment programs similar to those for patients with advanced liver fibrosis or cirrhosis.”
The study was supported by the European Union Seventh Framework Programme, Schweizerische Krebsliga, Agence Nationale de Recherches sur le SIDA et les Hepatites Virales (ANRS), Paris; the HIV Monitoring Foundation, Amsterdam; and the Augustinus Foundation, Copenhagen. Eleven authors declared grants, personal fees, and other support from pharmaceutical companies including those involved in the manufacture of HIV and hepatitis drugs. No other conflicts of interest were reported.
HIV-infected individuals on antiretroviral therapy who also have chronic coinfection with hepatitis B or C virus have an increased risk of developing non-Hodgkin lymphoma, according to new research published in Annals of Internal Medicine.
Lead author Qing Wang, PhD, of the Basel Institute for Clinical Epidemiology & Biostatistics at University Hospital Basel, Switzerland, and her coauthors said there is growing evidence of an association between both chronic hepatitis B virus infection (HBV) and chronic hepatitis C virus infection (HCV), and non-Hodgkin lymphoma, with chronic immune activation and B cell proliferation suggested as potential mechanisms. However, the impact of chronic coinfection in individuals with HIV is unclear.
Researchers undertook a cohort study of 52,479 treatment-naive individuals with HIV infection, using 18 of 33 cohorts from the Collaboration of Observational HIV Epidemiological Research Europe. Of these participants, 1,336 had chronic HBV and 7,506 had chronic HCV infection, and more than three-quarters (77%) later started treatment with antiretroviral therapy.
After 13 months of follow-up in the treatment-naive group and 50 months in the antiretroviral group, there were 252 cases of non-Hodgkin lymphoma in the treatment-naive group and 310 cases in the treated group (Ann Intern Med. 2016 Oct 17. doi: 10.7326/M16-0240).
Antiretroviral-treated patients with chronic hepatitis B showed a significant 74% greater risk (95% confidence interval, 1.08-2.82) and those with hepatitis C showed a 73% greater risk (95% CI, 1.21-2.46) of non-Hodgkin lymphoma compared to treated individuals with neither coinfection. However, the differences in non-Hodgkin lymphoma rates in treatment-naive HBV and HCV coinfected individuals were not significant, which the authors suggested could be due to lower numbers of events and limited follow-up.
“The median CD4 count at the time of NHL diagnosis was less than 0.250 x 109 cells/L in both ART-naive and treated patients coinfected with HBV and HCV, indicating that coinfected patients with NHL initiate ART late or have insufficient HIV viral control and immune recovery that may be due to multiple reasons,” the authors wrote. “This unfavorable constellation is aggravated by the fact that chronic HBV infection attenuates immune recovery in ART-treated patients; whether this is also the case for chronic HCV infection is less clear.”
The authors said routine screening for chronic HBV and HCV infection, in conjunction with early diagnosis and treatment of HIV infection, was essential to reduce morbidity and mortality from non-Hodgkin lymphoma.
“Our findings provide strong evidence that HCV coinfected patients with poor immune status or restoration (CD4 count lower than 0.250 x 109 cells/L) are at high risk for NHL and death and deserve high priority for access to well-tolerated, interferon-free, direct-acting antiviral treatment programs similar to those for patients with advanced liver fibrosis or cirrhosis.”
The study was supported by the European Union Seventh Framework Programme, Schweizerische Krebsliga, Agence Nationale de Recherches sur le SIDA et les Hepatites Virales (ANRS), Paris; the HIV Monitoring Foundation, Amsterdam; and the Augustinus Foundation, Copenhagen. Eleven authors declared grants, personal fees, and other support from pharmaceutical companies including those involved in the manufacture of HIV and hepatitis drugs. No other conflicts of interest were reported.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: HIV-infected individuals on antiretroviral therapy who also have chronic coinfection with hepatitis B or C virus have an increased risk of developing non-Hodgkin lymphoma.
Major finding: Antiretroviral-treated patients with chronic hepatitis B showed a significant 74% greater risk and those with hepatitis C showed a 73% greater risk of non-Hodgkin lymphoma, compared to treated individuals with neither coinfection.
Data source: A cohort study of 52,479 treatment-naive individuals with HIV infection, using 18 of 33 cohorts from the Collaboration of Observational HIV Epidemiological Research Europe.
Disclosures: The study was supported by the European Union Seventh Framework Programme, Schweizerische Krebsliga, Agence Nationale de Recherches sur le SIDA et les Hepatites Virales (ANRS), Paris; the HIV Monitoring Foundation, Amsterdam; and the Augustinus Foundation, Copenhagen. Eleven authors declared grants, personal fees, and other support from pharmaceutical companies including those involved in the manufacture of HIV and hepatitis drugs. No other conflicts of interest were reported.
Study Points to Risk Factors for Lymphoma
Certain lifestyle, dietary, environmental, serologic, and genetic factors may raise the risk of non-Hodgkin lymphoma (NHL), according to researchers who reviewed 40 years of follow-up data from the Nurses’ Health Study (NHS).
Related: Exercise Lowers Risk of Some Cancers
The researchers, from Brigham and Women’s Hospital, Harvard, and Boston University, all in Massachusetts, aimed to highlight the NHS’s contributions to epidemiologic knowledge of endometrial, ovarian, pancreatic, and hematologic cancers. They focused on findings that identified novel risk factors or markers of early detection or helped clarify discrepant literature.
Because the researchers say severe immune compromise is the “strongest, best-established risk factor” for NHL, they studied factors that might lead to subclinical immune dysregulation, such as diet, body mass index (BMI), and supplement use. They found several risk factors and biomarkers for NHL and more than 35 distinct tumors in that category, including chronic lymphocytic leukemia. Trans fats and red meat, for instance, doubled the risk of NHL. The researchers also found a higher risk for women who reported long-term multivitamin use. However, they found no risk associated with diet or sugar-sweetened soda or aspartame or with dietary intake of vitamin D.
Related: IBD and the Risk of Oral Cancer
Greater adiposity during childhood and adolescence was significantly associated with NHL. The researchers also observed a 19% increased risk of all NHL per 5 kg/m2 increase in BMI in young adulthood. Interestingly, taller women also had a higher risk of NHL.
The researchers conducted one of the first prospective studies to evaluate a putative inverse association of NHL risk with exposure to ambient ultraviolet radiation. They found, “contrary to expectation,” a 10% to 20% increased risk of NHL among women with the highest (vs lowest) ultraviolet-B exposure at baseline and birth, 15 years, and 30 years.
In investigating biomarkers, the researchers noted a “suggestive increase” in chronic lymphocytic leukemia risk associated with an Epstein-Barr virus antibody profile indicative of poor host immune control of the virus.
Related: Sexual Orientation and Cancer Risk
The researchers have established several working groups to study cancers, such as NHL and multiple myeloma. They also are collecting archival tissue specimens for NHL, multiple myeloma, and Hodgkin lymphoma, for better evaluation of factors related to the unique molecular subsets of hematologic tumors.
Source:
Birmann BM, Barnard ME, Bertrand KA, et al. Am J Public Health. 2016;106(9):1608-1615.
doi: 10.2105/AJPH.2016.303337.
Certain lifestyle, dietary, environmental, serologic, and genetic factors may raise the risk of non-Hodgkin lymphoma (NHL), according to researchers who reviewed 40 years of follow-up data from the Nurses’ Health Study (NHS).
Related: Exercise Lowers Risk of Some Cancers
The researchers, from Brigham and Women’s Hospital, Harvard, and Boston University, all in Massachusetts, aimed to highlight the NHS’s contributions to epidemiologic knowledge of endometrial, ovarian, pancreatic, and hematologic cancers. They focused on findings that identified novel risk factors or markers of early detection or helped clarify discrepant literature.
Because the researchers say severe immune compromise is the “strongest, best-established risk factor” for NHL, they studied factors that might lead to subclinical immune dysregulation, such as diet, body mass index (BMI), and supplement use. They found several risk factors and biomarkers for NHL and more than 35 distinct tumors in that category, including chronic lymphocytic leukemia. Trans fats and red meat, for instance, doubled the risk of NHL. The researchers also found a higher risk for women who reported long-term multivitamin use. However, they found no risk associated with diet or sugar-sweetened soda or aspartame or with dietary intake of vitamin D.
Related: IBD and the Risk of Oral Cancer
Greater adiposity during childhood and adolescence was significantly associated with NHL. The researchers also observed a 19% increased risk of all NHL per 5 kg/m2 increase in BMI in young adulthood. Interestingly, taller women also had a higher risk of NHL.
The researchers conducted one of the first prospective studies to evaluate a putative inverse association of NHL risk with exposure to ambient ultraviolet radiation. They found, “contrary to expectation,” a 10% to 20% increased risk of NHL among women with the highest (vs lowest) ultraviolet-B exposure at baseline and birth, 15 years, and 30 years.
In investigating biomarkers, the researchers noted a “suggestive increase” in chronic lymphocytic leukemia risk associated with an Epstein-Barr virus antibody profile indicative of poor host immune control of the virus.
Related: Sexual Orientation and Cancer Risk
The researchers have established several working groups to study cancers, such as NHL and multiple myeloma. They also are collecting archival tissue specimens for NHL, multiple myeloma, and Hodgkin lymphoma, for better evaluation of factors related to the unique molecular subsets of hematologic tumors.
Source:
Birmann BM, Barnard ME, Bertrand KA, et al. Am J Public Health. 2016;106(9):1608-1615.
doi: 10.2105/AJPH.2016.303337.
Certain lifestyle, dietary, environmental, serologic, and genetic factors may raise the risk of non-Hodgkin lymphoma (NHL), according to researchers who reviewed 40 years of follow-up data from the Nurses’ Health Study (NHS).
Related: Exercise Lowers Risk of Some Cancers
The researchers, from Brigham and Women’s Hospital, Harvard, and Boston University, all in Massachusetts, aimed to highlight the NHS’s contributions to epidemiologic knowledge of endometrial, ovarian, pancreatic, and hematologic cancers. They focused on findings that identified novel risk factors or markers of early detection or helped clarify discrepant literature.
Because the researchers say severe immune compromise is the “strongest, best-established risk factor” for NHL, they studied factors that might lead to subclinical immune dysregulation, such as diet, body mass index (BMI), and supplement use. They found several risk factors and biomarkers for NHL and more than 35 distinct tumors in that category, including chronic lymphocytic leukemia. Trans fats and red meat, for instance, doubled the risk of NHL. The researchers also found a higher risk for women who reported long-term multivitamin use. However, they found no risk associated with diet or sugar-sweetened soda or aspartame or with dietary intake of vitamin D.
Related: IBD and the Risk of Oral Cancer
Greater adiposity during childhood and adolescence was significantly associated with NHL. The researchers also observed a 19% increased risk of all NHL per 5 kg/m2 increase in BMI in young adulthood. Interestingly, taller women also had a higher risk of NHL.
The researchers conducted one of the first prospective studies to evaluate a putative inverse association of NHL risk with exposure to ambient ultraviolet radiation. They found, “contrary to expectation,” a 10% to 20% increased risk of NHL among women with the highest (vs lowest) ultraviolet-B exposure at baseline and birth, 15 years, and 30 years.
In investigating biomarkers, the researchers noted a “suggestive increase” in chronic lymphocytic leukemia risk associated with an Epstein-Barr virus antibody profile indicative of poor host immune control of the virus.
Related: Sexual Orientation and Cancer Risk
The researchers have established several working groups to study cancers, such as NHL and multiple myeloma. They also are collecting archival tissue specimens for NHL, multiple myeloma, and Hodgkin lymphoma, for better evaluation of factors related to the unique molecular subsets of hematologic tumors.
Source:
Birmann BM, Barnard ME, Bertrand KA, et al. Am J Public Health. 2016;106(9):1608-1615.
doi: 10.2105/AJPH.2016.303337.
Managing MGUS Consultations Electronically—A Single Center Experience
Non-visit electronic consultations (NVCs) are an important component of care for VA patients requiring sub-specialty consultation but not requiring urgent face to face evaluation. Here we specifically analyzed referrals for monoclonal gammopathy of undetermined significance (MGUS), since this is a diagnosis that requires ongoing surveillance once identified. It is an ideal model to study utilization and effectiveness of NVCs over time.
We identified 615 electronic hematology consultation encounters from 1/1/11-12/31/11 at our institution. Of these, 37 (6%) were consults for MGUS. Patient records were evaluated up to 5 years following the original consultation. We found that 16% (6/37) of MGUS patients subsequently had a face to face evaluation. 4 of these were due to onset of malignancy (3 multiple myeloma and 1 non-Hodgkin lymphoma). Over the 5 year study period, 51% (19/37) have been one-time consults while 32% (12/37) have utilized multiple NVCs. Typical recommendations at our institution for MGUS include yearly SPEP, serum free light chain assessment, and a baseline skeletal survey. Bone marrow biopsy is not routinely recommended for low-risk patients. Surveillance and re-consultation by a specialist is at the discretion of the referring provider.
22 of 37 MGUS patients had a documented skeletal survey. Of the 15 without evaluation, 7 had M-protein values that were only positive by immunofixation, while 4 were IgM cases. We do not routinely promote DEXA, though 14% of MGUS patients also had a DEXA (for any reason). In addition, the majority of MGUS patients (28/37) were found to have documented 25-OH vitamin D levels. Among these, only 4 had a mean vitamin D level that would be considered deficient (under 20 ng/mL).
Overall, we describe a cohort of MGUS patients initially identified via electronic consultation at our institution. With an average study follow-up of only 4-5 years, we identified 4 cases of malignancy (10.8% of MGUS NVCs), demonstrating the importance of careful evaluation of MGUS cases referred for specialty consultation. Areas of uncertainty that require further attention include: 1) skeletal surveys in patients with scant monoclonal protein, 2) routine promotion of DEXA and 3) targeted vitamin D supplementation practices among MGUS patients.
Non-visit electronic consultations (NVCs) are an important component of care for VA patients requiring sub-specialty consultation but not requiring urgent face to face evaluation. Here we specifically analyzed referrals for monoclonal gammopathy of undetermined significance (MGUS), since this is a diagnosis that requires ongoing surveillance once identified. It is an ideal model to study utilization and effectiveness of NVCs over time.
We identified 615 electronic hematology consultation encounters from 1/1/11-12/31/11 at our institution. Of these, 37 (6%) were consults for MGUS. Patient records were evaluated up to 5 years following the original consultation. We found that 16% (6/37) of MGUS patients subsequently had a face to face evaluation. 4 of these were due to onset of malignancy (3 multiple myeloma and 1 non-Hodgkin lymphoma). Over the 5 year study period, 51% (19/37) have been one-time consults while 32% (12/37) have utilized multiple NVCs. Typical recommendations at our institution for MGUS include yearly SPEP, serum free light chain assessment, and a baseline skeletal survey. Bone marrow biopsy is not routinely recommended for low-risk patients. Surveillance and re-consultation by a specialist is at the discretion of the referring provider.
22 of 37 MGUS patients had a documented skeletal survey. Of the 15 without evaluation, 7 had M-protein values that were only positive by immunofixation, while 4 were IgM cases. We do not routinely promote DEXA, though 14% of MGUS patients also had a DEXA (for any reason). In addition, the majority of MGUS patients (28/37) were found to have documented 25-OH vitamin D levels. Among these, only 4 had a mean vitamin D level that would be considered deficient (under 20 ng/mL).
Overall, we describe a cohort of MGUS patients initially identified via electronic consultation at our institution. With an average study follow-up of only 4-5 years, we identified 4 cases of malignancy (10.8% of MGUS NVCs), demonstrating the importance of careful evaluation of MGUS cases referred for specialty consultation. Areas of uncertainty that require further attention include: 1) skeletal surveys in patients with scant monoclonal protein, 2) routine promotion of DEXA and 3) targeted vitamin D supplementation practices among MGUS patients.
Non-visit electronic consultations (NVCs) are an important component of care for VA patients requiring sub-specialty consultation but not requiring urgent face to face evaluation. Here we specifically analyzed referrals for monoclonal gammopathy of undetermined significance (MGUS), since this is a diagnosis that requires ongoing surveillance once identified. It is an ideal model to study utilization and effectiveness of NVCs over time.
We identified 615 electronic hematology consultation encounters from 1/1/11-12/31/11 at our institution. Of these, 37 (6%) were consults for MGUS. Patient records were evaluated up to 5 years following the original consultation. We found that 16% (6/37) of MGUS patients subsequently had a face to face evaluation. 4 of these were due to onset of malignancy (3 multiple myeloma and 1 non-Hodgkin lymphoma). Over the 5 year study period, 51% (19/37) have been one-time consults while 32% (12/37) have utilized multiple NVCs. Typical recommendations at our institution for MGUS include yearly SPEP, serum free light chain assessment, and a baseline skeletal survey. Bone marrow biopsy is not routinely recommended for low-risk patients. Surveillance and re-consultation by a specialist is at the discretion of the referring provider.
22 of 37 MGUS patients had a documented skeletal survey. Of the 15 without evaluation, 7 had M-protein values that were only positive by immunofixation, while 4 were IgM cases. We do not routinely promote DEXA, though 14% of MGUS patients also had a DEXA (for any reason). In addition, the majority of MGUS patients (28/37) were found to have documented 25-OH vitamin D levels. Among these, only 4 had a mean vitamin D level that would be considered deficient (under 20 ng/mL).
Overall, we describe a cohort of MGUS patients initially identified via electronic consultation at our institution. With an average study follow-up of only 4-5 years, we identified 4 cases of malignancy (10.8% of MGUS NVCs), demonstrating the importance of careful evaluation of MGUS cases referred for specialty consultation. Areas of uncertainty that require further attention include: 1) skeletal surveys in patients with scant monoclonal protein, 2) routine promotion of DEXA and 3) targeted vitamin D supplementation practices among MGUS patients.
CT-Guided Bone Marrow Aspiration and Biopsy Is a Safe and Feasible Option to Decompress Busy Hematology/Oncology Clinics
Purpose: To disseminate information regarding the Louis Stokes Cleveland VAMC process for CT guided bone marrow aspiration and biopsies (BMAB).
Relevant Background/Problem: With timely access to quality care at the forefront of many VA-based initiatives we sought to decrease wait times for new patients with hematology concerns. Upon review of clinic utilization we recognized that many established patients requiring BMAB were scheduled into a new patient slot to allow enough time for the procedure. At the same time, our colleagues in Interventional Radiology (IR) approached us regarding the feasibility of performing BMAB using CT guidance.
Methods: We performed a retrospective review of all BMAB done between September 2014 and August 2015 before the IR guided procedure was offered to determine number of procedures performed. We then examined those cases performed from September 2015 to June 2016 after rollout of IR guided BMAB to determine numbers of cases, location of procedure (IR versus Hematology/Oncology), operator (IR versus staff versus fellow), and complications.
Data Analysis: From September 2014 to August 2015, 211 BMAB were performed, averaging 17 per month. From September 2015 to June 2016, 207 BMAB were performed with an average of 20 per month. During the latter time period, 50% of BMAB were performed using IR guidance with the other 50% performed by either Hematology/Oncology staff or fellows. There were no complications reported regardless of location and operator. Exposure to radiation dose was extremely low.
Results: IR guided BMAB is a safe and feasible option for patients and Hematology/Oncology providers.
Implications: IR guided BMAB can be one option to decompress already overbooked Hematology/Oncology clinics and to provide quicker access to care for patients with newly diagnosed hematologic and oncologic conditions.
Purpose: To disseminate information regarding the Louis Stokes Cleveland VAMC process for CT guided bone marrow aspiration and biopsies (BMAB).
Relevant Background/Problem: With timely access to quality care at the forefront of many VA-based initiatives we sought to decrease wait times for new patients with hematology concerns. Upon review of clinic utilization we recognized that many established patients requiring BMAB were scheduled into a new patient slot to allow enough time for the procedure. At the same time, our colleagues in Interventional Radiology (IR) approached us regarding the feasibility of performing BMAB using CT guidance.
Methods: We performed a retrospective review of all BMAB done between September 2014 and August 2015 before the IR guided procedure was offered to determine number of procedures performed. We then examined those cases performed from September 2015 to June 2016 after rollout of IR guided BMAB to determine numbers of cases, location of procedure (IR versus Hematology/Oncology), operator (IR versus staff versus fellow), and complications.
Data Analysis: From September 2014 to August 2015, 211 BMAB were performed, averaging 17 per month. From September 2015 to June 2016, 207 BMAB were performed with an average of 20 per month. During the latter time period, 50% of BMAB were performed using IR guidance with the other 50% performed by either Hematology/Oncology staff or fellows. There were no complications reported regardless of location and operator. Exposure to radiation dose was extremely low.
Results: IR guided BMAB is a safe and feasible option for patients and Hematology/Oncology providers.
Implications: IR guided BMAB can be one option to decompress already overbooked Hematology/Oncology clinics and to provide quicker access to care for patients with newly diagnosed hematologic and oncologic conditions.
Purpose: To disseminate information regarding the Louis Stokes Cleveland VAMC process for CT guided bone marrow aspiration and biopsies (BMAB).
Relevant Background/Problem: With timely access to quality care at the forefront of many VA-based initiatives we sought to decrease wait times for new patients with hematology concerns. Upon review of clinic utilization we recognized that many established patients requiring BMAB were scheduled into a new patient slot to allow enough time for the procedure. At the same time, our colleagues in Interventional Radiology (IR) approached us regarding the feasibility of performing BMAB using CT guidance.
Methods: We performed a retrospective review of all BMAB done between September 2014 and August 2015 before the IR guided procedure was offered to determine number of procedures performed. We then examined those cases performed from September 2015 to June 2016 after rollout of IR guided BMAB to determine numbers of cases, location of procedure (IR versus Hematology/Oncology), operator (IR versus staff versus fellow), and complications.
Data Analysis: From September 2014 to August 2015, 211 BMAB were performed, averaging 17 per month. From September 2015 to June 2016, 207 BMAB were performed with an average of 20 per month. During the latter time period, 50% of BMAB were performed using IR guidance with the other 50% performed by either Hematology/Oncology staff or fellows. There were no complications reported regardless of location and operator. Exposure to radiation dose was extremely low.
Results: IR guided BMAB is a safe and feasible option for patients and Hematology/Oncology providers.
Implications: IR guided BMAB can be one option to decompress already overbooked Hematology/Oncology clinics and to provide quicker access to care for patients with newly diagnosed hematologic and oncologic conditions.
Double-Expressor Lymphoma (DEL) in Veterans at DC VAMC
Purpose: To identify DEL amongst veteran patients with diffuse large B cell lymphoma (DLBCL) and its outcome.
Background: Molecular profile determines prognosis in DLBCL. Activated B-cell (ABC), a subtype of DLBCL, is associated with poor outcome compared to germinal center Bcell (GCB). Poor response to standard chemotherapy is seen with double-hit lymphomas as detected by FISH (5% -10% of DLBCL) and DELs that express both MYC and BCL-2 as detected by immunohistochemistry (IHC) (cutoffs—30% MYC, 40% BCL-2), with a median overall survival of <12 months.
Methods: Sixty-nine DLBCL patients diagnosed at DC VAMC from 1/1996-4/2016 were identified utilizing cancer registry. IHC stains were reviewed for CD3, CD10, CD20, BCL-2, BCL-6, C-MYC, MUM-1, MIB1, and p53. DLBCL were sub-classified as GCB and ABC based on CD10, BCL6 and MUM1 stains. Demographic data, diagnosis, treatment and outcome in terms of relapse and death are analyzed and will be presented at the meeting.
Results: Of the 69 DLBCL cases, only 37 met inclusion criteria; 32 were excluded due to unavailable blocks (20, mostly sent to outside institutions), tissue exhaustion with incomplete IHC data (6), T-cell rich B cell lymphoma (5) and pending (1). 20 cases are GCB and 17 ABC. All cases are CD20 positive with high mib1. MYC is positive in 17 cases (46%) and 15 of them double positive for BCL-2 (40%).
Implications/Future Directions: DLBCL veterans at the DC VAMC have a high percentage of double expressors when compared to the literature. It will be important to examine clinical data, treatment, and outcome to develop better treatment guidelines for double-expressor DLBCL. Future studies are in plan to compare double hit lymphomas to double expressors.
Purpose: To identify DEL amongst veteran patients with diffuse large B cell lymphoma (DLBCL) and its outcome.
Background: Molecular profile determines prognosis in DLBCL. Activated B-cell (ABC), a subtype of DLBCL, is associated with poor outcome compared to germinal center Bcell (GCB). Poor response to standard chemotherapy is seen with double-hit lymphomas as detected by FISH (5% -10% of DLBCL) and DELs that express both MYC and BCL-2 as detected by immunohistochemistry (IHC) (cutoffs—30% MYC, 40% BCL-2), with a median overall survival of <12 months.
Methods: Sixty-nine DLBCL patients diagnosed at DC VAMC from 1/1996-4/2016 were identified utilizing cancer registry. IHC stains were reviewed for CD3, CD10, CD20, BCL-2, BCL-6, C-MYC, MUM-1, MIB1, and p53. DLBCL were sub-classified as GCB and ABC based on CD10, BCL6 and MUM1 stains. Demographic data, diagnosis, treatment and outcome in terms of relapse and death are analyzed and will be presented at the meeting.
Results: Of the 69 DLBCL cases, only 37 met inclusion criteria; 32 were excluded due to unavailable blocks (20, mostly sent to outside institutions), tissue exhaustion with incomplete IHC data (6), T-cell rich B cell lymphoma (5) and pending (1). 20 cases are GCB and 17 ABC. All cases are CD20 positive with high mib1. MYC is positive in 17 cases (46%) and 15 of them double positive for BCL-2 (40%).
Implications/Future Directions: DLBCL veterans at the DC VAMC have a high percentage of double expressors when compared to the literature. It will be important to examine clinical data, treatment, and outcome to develop better treatment guidelines for double-expressor DLBCL. Future studies are in plan to compare double hit lymphomas to double expressors.
Purpose: To identify DEL amongst veteran patients with diffuse large B cell lymphoma (DLBCL) and its outcome.
Background: Molecular profile determines prognosis in DLBCL. Activated B-cell (ABC), a subtype of DLBCL, is associated with poor outcome compared to germinal center Bcell (GCB). Poor response to standard chemotherapy is seen with double-hit lymphomas as detected by FISH (5% -10% of DLBCL) and DELs that express both MYC and BCL-2 as detected by immunohistochemistry (IHC) (cutoffs—30% MYC, 40% BCL-2), with a median overall survival of <12 months.
Methods: Sixty-nine DLBCL patients diagnosed at DC VAMC from 1/1996-4/2016 were identified utilizing cancer registry. IHC stains were reviewed for CD3, CD10, CD20, BCL-2, BCL-6, C-MYC, MUM-1, MIB1, and p53. DLBCL were sub-classified as GCB and ABC based on CD10, BCL6 and MUM1 stains. Demographic data, diagnosis, treatment and outcome in terms of relapse and death are analyzed and will be presented at the meeting.
Results: Of the 69 DLBCL cases, only 37 met inclusion criteria; 32 were excluded due to unavailable blocks (20, mostly sent to outside institutions), tissue exhaustion with incomplete IHC data (6), T-cell rich B cell lymphoma (5) and pending (1). 20 cases are GCB and 17 ABC. All cases are CD20 positive with high mib1. MYC is positive in 17 cases (46%) and 15 of them double positive for BCL-2 (40%).
Implications/Future Directions: DLBCL veterans at the DC VAMC have a high percentage of double expressors when compared to the literature. It will be important to examine clinical data, treatment, and outcome to develop better treatment guidelines for double-expressor DLBCL. Future studies are in plan to compare double hit lymphomas to double expressors.
Demographic and Clinical Characteristics of Patients With Polycythemia Vera (PV) in the U.S. Veterans Population
Introduction: PV is associated with an increased risk of thrombosis, which contributes to morbidity and mortality of patients. Limited data exist on patients with PV among the Veterans Health Administration (VHA) population. The objective of this study is to describe the demographic and clinical characteristics of patients with PV in the VHA population.
Methods: A retrospective, observational analysis was conducted using longitudinal data from the VHA database. The analysis included adult patients who had ≥ 2 claims for PV (ICD-9 238.4) ≥ 30 days apart between 01/01/2007 and 12/31/2009 and ≥ 12 months of continuous enrollment before the first PV claim (index date). Patients were followed from the index date until the earliest date of death, disenrollment, or end of study (9/30/2012). Demographics and comorbid conditions during the pre-index period, and cytoreductive treatments, select laboratory values, thrombotic event (TE) rate, and mortality rate during the follow-up period are reported.
Results: The analysis included 7718 patients with PV; most patients were ≥ 60 years of age (70.7%), male (97.9%), and white (63.9%). The 3 most common comorbid conditions reported during the pre-index period were hypertension (71.7%), dyslipidemia (54.2%), and diabetes (24.0%). Additionally, 8.8% had arterial thrombosis, 4.5% had venous thrombosis, and 8.7% had bleeding. During the follow-up period (median 4.8 years), 23.2% of patients received cytoreductive pharmacotherapy (86.7% hydroxyurea), 32.8% had phlebotomy, and 53.0% had neither cytoreductive therapy nor phlebotomy. 86.4% and 63.3% of patients were using antihypertensive agents and anti-lipid medications, respectively. 86.7% of patients had ≥ 2 elevated HCT levels (≥ 45%) and 37.3% had ≥ 2 elevated WBC counts ( ≥ 11*109/L). 22.9% of patients had ≥ 1 TE (16.5% arterial thrombosis and 8.78% venous thrombosis). The TE rate was 60.5 per 1,000 patient years. Deaths due to any cause were reported for 23.0% of patients during follow-up.
Conclusion: The TE burden is significant among patients with PV in the VHA population. A large proportion of patients had elevated blood values, which may indicate uncontrolled PV, and may predispose patients to greater risk of clinical complications and consequences of PV.
Introduction: PV is associated with an increased risk of thrombosis, which contributes to morbidity and mortality of patients. Limited data exist on patients with PV among the Veterans Health Administration (VHA) population. The objective of this study is to describe the demographic and clinical characteristics of patients with PV in the VHA population.
Methods: A retrospective, observational analysis was conducted using longitudinal data from the VHA database. The analysis included adult patients who had ≥ 2 claims for PV (ICD-9 238.4) ≥ 30 days apart between 01/01/2007 and 12/31/2009 and ≥ 12 months of continuous enrollment before the first PV claim (index date). Patients were followed from the index date until the earliest date of death, disenrollment, or end of study (9/30/2012). Demographics and comorbid conditions during the pre-index period, and cytoreductive treatments, select laboratory values, thrombotic event (TE) rate, and mortality rate during the follow-up period are reported.
Results: The analysis included 7718 patients with PV; most patients were ≥ 60 years of age (70.7%), male (97.9%), and white (63.9%). The 3 most common comorbid conditions reported during the pre-index period were hypertension (71.7%), dyslipidemia (54.2%), and diabetes (24.0%). Additionally, 8.8% had arterial thrombosis, 4.5% had venous thrombosis, and 8.7% had bleeding. During the follow-up period (median 4.8 years), 23.2% of patients received cytoreductive pharmacotherapy (86.7% hydroxyurea), 32.8% had phlebotomy, and 53.0% had neither cytoreductive therapy nor phlebotomy. 86.4% and 63.3% of patients were using antihypertensive agents and anti-lipid medications, respectively. 86.7% of patients had ≥ 2 elevated HCT levels (≥ 45%) and 37.3% had ≥ 2 elevated WBC counts ( ≥ 11*109/L). 22.9% of patients had ≥ 1 TE (16.5% arterial thrombosis and 8.78% venous thrombosis). The TE rate was 60.5 per 1,000 patient years. Deaths due to any cause were reported for 23.0% of patients during follow-up.
Conclusion: The TE burden is significant among patients with PV in the VHA population. A large proportion of patients had elevated blood values, which may indicate uncontrolled PV, and may predispose patients to greater risk of clinical complications and consequences of PV.
Introduction: PV is associated with an increased risk of thrombosis, which contributes to morbidity and mortality of patients. Limited data exist on patients with PV among the Veterans Health Administration (VHA) population. The objective of this study is to describe the demographic and clinical characteristics of patients with PV in the VHA population.
Methods: A retrospective, observational analysis was conducted using longitudinal data from the VHA database. The analysis included adult patients who had ≥ 2 claims for PV (ICD-9 238.4) ≥ 30 days apart between 01/01/2007 and 12/31/2009 and ≥ 12 months of continuous enrollment before the first PV claim (index date). Patients were followed from the index date until the earliest date of death, disenrollment, or end of study (9/30/2012). Demographics and comorbid conditions during the pre-index period, and cytoreductive treatments, select laboratory values, thrombotic event (TE) rate, and mortality rate during the follow-up period are reported.
Results: The analysis included 7718 patients with PV; most patients were ≥ 60 years of age (70.7%), male (97.9%), and white (63.9%). The 3 most common comorbid conditions reported during the pre-index period were hypertension (71.7%), dyslipidemia (54.2%), and diabetes (24.0%). Additionally, 8.8% had arterial thrombosis, 4.5% had venous thrombosis, and 8.7% had bleeding. During the follow-up period (median 4.8 years), 23.2% of patients received cytoreductive pharmacotherapy (86.7% hydroxyurea), 32.8% had phlebotomy, and 53.0% had neither cytoreductive therapy nor phlebotomy. 86.4% and 63.3% of patients were using antihypertensive agents and anti-lipid medications, respectively. 86.7% of patients had ≥ 2 elevated HCT levels (≥ 45%) and 37.3% had ≥ 2 elevated WBC counts ( ≥ 11*109/L). 22.9% of patients had ≥ 1 TE (16.5% arterial thrombosis and 8.78% venous thrombosis). The TE rate was 60.5 per 1,000 patient years. Deaths due to any cause were reported for 23.0% of patients during follow-up.
Conclusion: The TE burden is significant among patients with PV in the VHA population. A large proportion of patients had elevated blood values, which may indicate uncontrolled PV, and may predispose patients to greater risk of clinical complications and consequences of PV.
Implementing a New Protocol for Heparin Anticoagulation
Purpose: Intravenous unfractionated heparin (UFH) remains an important anticoagulation (AC) agent, particularly in the inpatient setting. Historically, the activated partial thromboplastin time (aPTT) has been the primary laboratory test used to monitor and adjust UFH. Given that several biologic factors can influence the aPTT, independent of the effects of UFH, institutions have transitioned to monitoring heparin with anti-Xa levels. Clinical data show that conversion from aPTT to anti-Xa monitoring may offer a smoother dose-response curve, such that levels remain more stable, requiring fewer blood samples and dosage adjustments.
Background/Problem: The Cleveland VA Medical Center (CVAMC) provides annual care to over 105,000 veterans. It was recently designated as a center for implantation of left ventricular assist devices (LVADs.) As part of the AC monitoring for these patients, a hematologist introduced the use of anti-Xa assay as the test of choice to monitor heparin. Favorable results in this patient cohort prompted consideration for a hospital-wide change in heparin monitoring and a new heparin dosing protocol.
Methods: A multidisciplinary group assembled in November 2015 and developed a low-intensity and high-intensity heparin protocol with anti-Xa as the test to monitor heparin. Laboratory staffing was increased to accommodate phlebotomy rounds. Alaris IV pumps were re-programmed. Physicians developed a specific order set. Nurses designed an AC nurse’s note, and pharmacists devised safe-guard strategies when dose changes are made. Clinical Nurse Specialists developed an educational program for all 228 inpatient registered nurses which will be completed on July 3rd. All stakeholders are expected to meet and confirm their readiness to fully implement the new protocol.
Data Analysis: Anti-Xa equipment was purchased and validation tests were completed. In LVAD patients, therapeutic levels within 24 hours were noted in 86% of the cases.
Results: Hospital-wide implementation of the new heparin protocol is projected for August 1, 2016.
Implications: Presently, there are only 9 VAMCs using the anti-Xa assay to manage heparin anticoagulation. The CVAMC has developed a comprehensive implementation process that consists of new order sets, templates, training programs, and tools for common references. A poster at the AVAHO meeting will illustrate the process and provide postimplementation updates.
Purpose: Intravenous unfractionated heparin (UFH) remains an important anticoagulation (AC) agent, particularly in the inpatient setting. Historically, the activated partial thromboplastin time (aPTT) has been the primary laboratory test used to monitor and adjust UFH. Given that several biologic factors can influence the aPTT, independent of the effects of UFH, institutions have transitioned to monitoring heparin with anti-Xa levels. Clinical data show that conversion from aPTT to anti-Xa monitoring may offer a smoother dose-response curve, such that levels remain more stable, requiring fewer blood samples and dosage adjustments.
Background/Problem: The Cleveland VA Medical Center (CVAMC) provides annual care to over 105,000 veterans. It was recently designated as a center for implantation of left ventricular assist devices (LVADs.) As part of the AC monitoring for these patients, a hematologist introduced the use of anti-Xa assay as the test of choice to monitor heparin. Favorable results in this patient cohort prompted consideration for a hospital-wide change in heparin monitoring and a new heparin dosing protocol.
Methods: A multidisciplinary group assembled in November 2015 and developed a low-intensity and high-intensity heparin protocol with anti-Xa as the test to monitor heparin. Laboratory staffing was increased to accommodate phlebotomy rounds. Alaris IV pumps were re-programmed. Physicians developed a specific order set. Nurses designed an AC nurse’s note, and pharmacists devised safe-guard strategies when dose changes are made. Clinical Nurse Specialists developed an educational program for all 228 inpatient registered nurses which will be completed on July 3rd. All stakeholders are expected to meet and confirm their readiness to fully implement the new protocol.
Data Analysis: Anti-Xa equipment was purchased and validation tests were completed. In LVAD patients, therapeutic levels within 24 hours were noted in 86% of the cases.
Results: Hospital-wide implementation of the new heparin protocol is projected for August 1, 2016.
Implications: Presently, there are only 9 VAMCs using the anti-Xa assay to manage heparin anticoagulation. The CVAMC has developed a comprehensive implementation process that consists of new order sets, templates, training programs, and tools for common references. A poster at the AVAHO meeting will illustrate the process and provide postimplementation updates.
Purpose: Intravenous unfractionated heparin (UFH) remains an important anticoagulation (AC) agent, particularly in the inpatient setting. Historically, the activated partial thromboplastin time (aPTT) has been the primary laboratory test used to monitor and adjust UFH. Given that several biologic factors can influence the aPTT, independent of the effects of UFH, institutions have transitioned to monitoring heparin with anti-Xa levels. Clinical data show that conversion from aPTT to anti-Xa monitoring may offer a smoother dose-response curve, such that levels remain more stable, requiring fewer blood samples and dosage adjustments.
Background/Problem: The Cleveland VA Medical Center (CVAMC) provides annual care to over 105,000 veterans. It was recently designated as a center for implantation of left ventricular assist devices (LVADs.) As part of the AC monitoring for these patients, a hematologist introduced the use of anti-Xa assay as the test of choice to monitor heparin. Favorable results in this patient cohort prompted consideration for a hospital-wide change in heparin monitoring and a new heparin dosing protocol.
Methods: A multidisciplinary group assembled in November 2015 and developed a low-intensity and high-intensity heparin protocol with anti-Xa as the test to monitor heparin. Laboratory staffing was increased to accommodate phlebotomy rounds. Alaris IV pumps were re-programmed. Physicians developed a specific order set. Nurses designed an AC nurse’s note, and pharmacists devised safe-guard strategies when dose changes are made. Clinical Nurse Specialists developed an educational program for all 228 inpatient registered nurses which will be completed on July 3rd. All stakeholders are expected to meet and confirm their readiness to fully implement the new protocol.
Data Analysis: Anti-Xa equipment was purchased and validation tests were completed. In LVAD patients, therapeutic levels within 24 hours were noted in 86% of the cases.
Results: Hospital-wide implementation of the new heparin protocol is projected for August 1, 2016.
Implications: Presently, there are only 9 VAMCs using the anti-Xa assay to manage heparin anticoagulation. The CVAMC has developed a comprehensive implementation process that consists of new order sets, templates, training programs, and tools for common references. A poster at the AVAHO meeting will illustrate the process and provide postimplementation updates.
Influence of Tyrosine Kinase Inhibitors on Renal Function and Current Monitoring Procedures at the Cincinnati Veterans Affairs Medical Center
Purpose: Patients with chronic myeloid leukemia (CML) and gastrointestinal stromal tumors (GIST) are treated with tyrosine kinase inhibitors (TKI), namely, imatinib, nilotinib, and dasatinib. Recent studies suggest that TKI therapy may be linked to the development of an acute kidney injury (AKI) and chronic kidney disease (CKD). This review evaluates current monitoring procedures at the Cincinnati VAMC.
Methods: A retrospective chart review using the electronic medical record was used to identify patients receiving TKI therapy ≥ 1 year with a diagnosis of CML or GIST. Demographics collected include: age, gender, baseline and subsequent serum creatinine, comorbid conditions possibly confounding kidney dysfunction, and receipt of nephrotoxic agents. The average change in renal function for the duration of treatment as well as per year of therapy with TKI and average number of days between lab draws were calculated.
Results: Forty-two patients were identified with active prescriptions for a TKI between January 1, 2005 and December 31, 2014. Twenty-four patients were included, of which 22 did not receive a basic metabolic panel at the recommended interval based on VA PBM Guidance. The average time between lab draws was 114 days. Fifteen patients incurred an acute kidney injury. The average change in serum creatinine for the duration of treatment was a +0.29 mg/dL. Five patients were identified that met manufacturer renal dosing criteria. Of these patients, 2 had an appropriately adjusted dose. Two patients developed CKD during the treatment period who did not have CKD at baseline.
Conclusion: Current monitoring of renal function at the Cincinnati VAMC is not in compliance with VA PBM recommendations for patients receiving TKI therapy. However, they are in line with manufacturer recommendations. While a large portion of patients developed an AKI with therapy, direct causation cannot be established as several of these patients received nephrotoxic agents in the immediately preceding 7 days of the elevated serum creatinine value. The increase in serum creatinine does not appear to be sustained, as the average change in serum creatinine for the duration of therapy was not large. Thus, quarterly monitoring of renal function appears to be appropriate in this population.
Purpose: Patients with chronic myeloid leukemia (CML) and gastrointestinal stromal tumors (GIST) are treated with tyrosine kinase inhibitors (TKI), namely, imatinib, nilotinib, and dasatinib. Recent studies suggest that TKI therapy may be linked to the development of an acute kidney injury (AKI) and chronic kidney disease (CKD). This review evaluates current monitoring procedures at the Cincinnati VAMC.
Methods: A retrospective chart review using the electronic medical record was used to identify patients receiving TKI therapy ≥ 1 year with a diagnosis of CML or GIST. Demographics collected include: age, gender, baseline and subsequent serum creatinine, comorbid conditions possibly confounding kidney dysfunction, and receipt of nephrotoxic agents. The average change in renal function for the duration of treatment as well as per year of therapy with TKI and average number of days between lab draws were calculated.
Results: Forty-two patients were identified with active prescriptions for a TKI between January 1, 2005 and December 31, 2014. Twenty-four patients were included, of which 22 did not receive a basic metabolic panel at the recommended interval based on VA PBM Guidance. The average time between lab draws was 114 days. Fifteen patients incurred an acute kidney injury. The average change in serum creatinine for the duration of treatment was a +0.29 mg/dL. Five patients were identified that met manufacturer renal dosing criteria. Of these patients, 2 had an appropriately adjusted dose. Two patients developed CKD during the treatment period who did not have CKD at baseline.
Conclusion: Current monitoring of renal function at the Cincinnati VAMC is not in compliance with VA PBM recommendations for patients receiving TKI therapy. However, they are in line with manufacturer recommendations. While a large portion of patients developed an AKI with therapy, direct causation cannot be established as several of these patients received nephrotoxic agents in the immediately preceding 7 days of the elevated serum creatinine value. The increase in serum creatinine does not appear to be sustained, as the average change in serum creatinine for the duration of therapy was not large. Thus, quarterly monitoring of renal function appears to be appropriate in this population.
Purpose: Patients with chronic myeloid leukemia (CML) and gastrointestinal stromal tumors (GIST) are treated with tyrosine kinase inhibitors (TKI), namely, imatinib, nilotinib, and dasatinib. Recent studies suggest that TKI therapy may be linked to the development of an acute kidney injury (AKI) and chronic kidney disease (CKD). This review evaluates current monitoring procedures at the Cincinnati VAMC.
Methods: A retrospective chart review using the electronic medical record was used to identify patients receiving TKI therapy ≥ 1 year with a diagnosis of CML or GIST. Demographics collected include: age, gender, baseline and subsequent serum creatinine, comorbid conditions possibly confounding kidney dysfunction, and receipt of nephrotoxic agents. The average change in renal function for the duration of treatment as well as per year of therapy with TKI and average number of days between lab draws were calculated.
Results: Forty-two patients were identified with active prescriptions for a TKI between January 1, 2005 and December 31, 2014. Twenty-four patients were included, of which 22 did not receive a basic metabolic panel at the recommended interval based on VA PBM Guidance. The average time between lab draws was 114 days. Fifteen patients incurred an acute kidney injury. The average change in serum creatinine for the duration of treatment was a +0.29 mg/dL. Five patients were identified that met manufacturer renal dosing criteria. Of these patients, 2 had an appropriately adjusted dose. Two patients developed CKD during the treatment period who did not have CKD at baseline.
Conclusion: Current monitoring of renal function at the Cincinnati VAMC is not in compliance with VA PBM recommendations for patients receiving TKI therapy. However, they are in line with manufacturer recommendations. While a large portion of patients developed an AKI with therapy, direct causation cannot be established as several of these patients received nephrotoxic agents in the immediately preceding 7 days of the elevated serum creatinine value. The increase in serum creatinine does not appear to be sustained, as the average change in serum creatinine for the duration of therapy was not large. Thus, quarterly monitoring of renal function appears to be appropriate in this population.

