User login
Intervention reduces CLABSIs in pediatric patients

Staphylococcus infection
Photo by Bill Branson
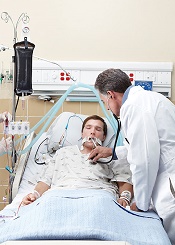
NASHVILLE—A single-center study has shown that incorporating antimicrobial cloths into an infection-prevention protocol can reduce the incidence of central line-associated bloodstream infections (CLABSIs) in pediatric patients.
After the hospital implemented daily “baths” with disposable cloths containing 2% chlorhexidine gluconate (CHG), its CLABSI incidence fell 59% over a 6-month period.
The details of this experience were presented at the APIC 2015 Annual Conference (abstract 013).
The study was conducted at Riley Hospital for Children at Indiana University Health in Indianapolis. The hospital previously used CHG for daily bathing in the hematology/oncology unit and found it successfully reduced CLABSIs there.
This prompted infection preventionists to consider implementing the practice hospital-wide, regardless of whether patients had central-line catheters.
The infection-prevention team worked with nursing staff, parents, and hospital leadership to adopt daily CHG bathing for all patients and to strengthen adherence to a bundle of prevention practices already in place for patients with central lines.
In addition to daily bathing with CHG-impregnated wipes, the strategies included daily linen changes, assessment of central-line dressings, ensuring use of the appropriate technique for giving medications, and regular tubing and cap changes on the lines.
“We took great care to ensure successful implementation of the new bathing regimen,” said Adam N. Karcz, an infection preventionist at the hospital.
“By educating everyone on the care team, including parents, and standardizing bathing procedures, we were able to dramatically reduce infections and save healthcare dollars in just 6 months.”
Bathing compliance increased from 45% to 81% during the 6-month study period. During the control period—6 months prior to implementation—the 269-bed hospital had 22 CLABSIs. During the implementation period, there were 9 CLABSIs.
The hospital also experienced a 56% drop in the number of methicillin-resistant Staphylococcus aureus (MRSA) infections during this time period.
The reduction in healthcare-associated infections during the implementation period represents a potential cost savings of $297,999. ![]()

Staphylococcus infection
Photo by Bill Branson
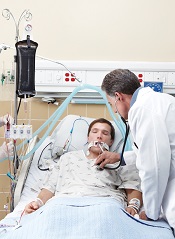
NASHVILLE—A single-center study has shown that incorporating antimicrobial cloths into an infection-prevention protocol can reduce the incidence of central line-associated bloodstream infections (CLABSIs) in pediatric patients.
After the hospital implemented daily “baths” with disposable cloths containing 2% chlorhexidine gluconate (CHG), its CLABSI incidence fell 59% over a 6-month period.
The details of this experience were presented at the APIC 2015 Annual Conference (abstract 013).
The study was conducted at Riley Hospital for Children at Indiana University Health in Indianapolis. The hospital previously used CHG for daily bathing in the hematology/oncology unit and found it successfully reduced CLABSIs there.
This prompted infection preventionists to consider implementing the practice hospital-wide, regardless of whether patients had central-line catheters.
The infection-prevention team worked with nursing staff, parents, and hospital leadership to adopt daily CHG bathing for all patients and to strengthen adherence to a bundle of prevention practices already in place for patients with central lines.
In addition to daily bathing with CHG-impregnated wipes, the strategies included daily linen changes, assessment of central-line dressings, ensuring use of the appropriate technique for giving medications, and regular tubing and cap changes on the lines.
“We took great care to ensure successful implementation of the new bathing regimen,” said Adam N. Karcz, an infection preventionist at the hospital.
“By educating everyone on the care team, including parents, and standardizing bathing procedures, we were able to dramatically reduce infections and save healthcare dollars in just 6 months.”
Bathing compliance increased from 45% to 81% during the 6-month study period. During the control period—6 months prior to implementation—the 269-bed hospital had 22 CLABSIs. During the implementation period, there were 9 CLABSIs.
The hospital also experienced a 56% drop in the number of methicillin-resistant Staphylococcus aureus (MRSA) infections during this time period.
The reduction in healthcare-associated infections during the implementation period represents a potential cost savings of $297,999. ![]()

Staphylococcus infection
Photo by Bill Branson
NASHVILLE—A single-center study has shown that incorporating antimicrobial cloths into an infection-prevention protocol can reduce the incidence of central line-associated bloodstream infections (CLABSIs) in pediatric patients.
After the hospital implemented daily “baths” with disposable cloths containing 2% chlorhexidine gluconate (CHG), its CLABSI incidence fell 59% over a 6-month period.
The details of this experience were presented at the APIC 2015 Annual Conference (abstract 013).
The study was conducted at Riley Hospital for Children at Indiana University Health in Indianapolis. The hospital previously used CHG for daily bathing in the hematology/oncology unit and found it successfully reduced CLABSIs there.
This prompted infection preventionists to consider implementing the practice hospital-wide, regardless of whether patients had central-line catheters.
The infection-prevention team worked with nursing staff, parents, and hospital leadership to adopt daily CHG bathing for all patients and to strengthen adherence to a bundle of prevention practices already in place for patients with central lines.
In addition to daily bathing with CHG-impregnated wipes, the strategies included daily linen changes, assessment of central-line dressings, ensuring use of the appropriate technique for giving medications, and regular tubing and cap changes on the lines.
“We took great care to ensure successful implementation of the new bathing regimen,” said Adam N. Karcz, an infection preventionist at the hospital.
“By educating everyone on the care team, including parents, and standardizing bathing procedures, we were able to dramatically reduce infections and save healthcare dollars in just 6 months.”
Bathing compliance increased from 45% to 81% during the 6-month study period. During the control period—6 months prior to implementation—the 269-bed hospital had 22 CLABSIs. During the implementation period, there were 9 CLABSIs.
The hospital also experienced a 56% drop in the number of methicillin-resistant Staphylococcus aureus (MRSA) infections during this time period.
The reduction in healthcare-associated infections during the implementation period represents a potential cost savings of $297,999. ![]()
Interventions reduce bloodstream infections
A bundled intervention can considerably reduce central line-associated bloodstream infections (CLABSIs), according to research published in Infection Control & Hospital Epidemiology.
The intervention focused on evidence-based infection prevention practices, safety culture and teamwork, and scheduled measurement of infection rates.
By implementing these measures, intensive care units (ICUs) in Abu Dhabi achieved an overall 38% reduction in CLABSIs.
“These hospitals were able to show significant improvements in infection rates and have been able to sustain the improvements a year after we finished the project,” said study author Asad Latif, MBBS, MD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland.
“Our results suggest that ICUs in disparate settings around the world could use this program and achieve similar results, significantly reducing the global morbidity, mortality, and excess costs associated with CLABSIs. In addition, this collaborative could serve as a model for future efforts to reduce other types of preventable medical harms in the Middle East and around the world.”
This study was a collaborative effort by the Armstrong Institute, Johns Hopkins Medicine International, and the Abu Dhabi Health Services Company (SEHA), which operates the government healthcare system in Abu Dhabi.
For the study, ICUs were instructed to assemble a comprehensive unit-based safety program (CUSP) team comprising local physician and nursing leaders, a senior executive, frontline healthcare providers, an infection control provider, and hospital quality and safety leaders.
The ICUs included 10 adult, 5 neonatal, and 3 pediatric ICUs, accounting for 77% of the adult, 74% of the neonatal, and 100% of the pediatric ICU beds in Abu Dhabi.
Starting in May 2012, the SEHA corporate quality team and ICU CUSP teams attended 14 weekly live webinars on CLABSI prevention conducted by Armstrong Institute faculty, followed by content and coaching webinars every 2 weeks for 24 months. The webinars were recorded by SEHA and posted on a local, shared computer drive, along with educational and training materials.
Armstrong faculty also conducted 4 site visits in Abu Dhabi at the beginning of the study, visiting each ICU to meet the CUSP team and tour the units. A year later, they conducted a 3-day patient safety workshop for participating hospitals.
CUSP teams implemented 3 interventions as part of the program: an effort to prevent CLABSIs that targeted clinicians’ use of evidence-based infection prevention recommendations from the Centers for Disease Control and Prevention, a CUSP process to improve safety culture and teamwork, and measurement of monthly CLABSI data and feedback to safety teams, senior leaders, and ICU staff.
The overall mean crude CLABSI rate for participating ICUs decreased from 2.56 infections per 1000 catheter days to 1.79 per 1,000 catheter days by the end of the study, corresponding to a 30% reduction.
By unit type, CLABSI rates decreased by 16% among adult ICUs, 48% among pediatric ICUs, and 47% in neonatal ICUs. The percentage of ICUs that achieved a quarterly CLABSI rate of less than 1 infection per 1000 catheter days increased from 44% to 61% after the interventions.
“Despite growing awareness, many hospitals around the world continue to struggle in their efforts to meaningfully reduce their CLABSI rates in a sustained manner,” said Sean M. Berenholtz, MD, of the Johns Hopkins University School of Medicine.
“In addition, hospitals and healthcare systems in the Middle East have unique barriers to implementing quality improvement programs, such as challenges with staff recruitment and retention, and personnel fearful of punitive repercussions from speaking up regarding patient safety concerns. In our study, bringing all stakeholders to the same table allowed everyone to share their concerns and ensure their voices were heard.” ![]()

A bundled intervention can considerably reduce central line-associated bloodstream infections (CLABSIs), according to research published in Infection Control & Hospital Epidemiology.
The intervention focused on evidence-based infection prevention practices, safety culture and teamwork, and scheduled measurement of infection rates.
By implementing these measures, intensive care units (ICUs) in Abu Dhabi achieved an overall 38% reduction in CLABSIs.
“These hospitals were able to show significant improvements in infection rates and have been able to sustain the improvements a year after we finished the project,” said study author Asad Latif, MBBS, MD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland.
“Our results suggest that ICUs in disparate settings around the world could use this program and achieve similar results, significantly reducing the global morbidity, mortality, and excess costs associated with CLABSIs. In addition, this collaborative could serve as a model for future efforts to reduce other types of preventable medical harms in the Middle East and around the world.”
This study was a collaborative effort by the Armstrong Institute, Johns Hopkins Medicine International, and the Abu Dhabi Health Services Company (SEHA), which operates the government healthcare system in Abu Dhabi.
For the study, ICUs were instructed to assemble a comprehensive unit-based safety program (CUSP) team comprising local physician and nursing leaders, a senior executive, frontline healthcare providers, an infection control provider, and hospital quality and safety leaders.
The ICUs included 10 adult, 5 neonatal, and 3 pediatric ICUs, accounting for 77% of the adult, 74% of the neonatal, and 100% of the pediatric ICU beds in Abu Dhabi.
Starting in May 2012, the SEHA corporate quality team and ICU CUSP teams attended 14 weekly live webinars on CLABSI prevention conducted by Armstrong Institute faculty, followed by content and coaching webinars every 2 weeks for 24 months. The webinars were recorded by SEHA and posted on a local, shared computer drive, along with educational and training materials.
Armstrong faculty also conducted 4 site visits in Abu Dhabi at the beginning of the study, visiting each ICU to meet the CUSP team and tour the units. A year later, they conducted a 3-day patient safety workshop for participating hospitals.
CUSP teams implemented 3 interventions as part of the program: an effort to prevent CLABSIs that targeted clinicians’ use of evidence-based infection prevention recommendations from the Centers for Disease Control and Prevention, a CUSP process to improve safety culture and teamwork, and measurement of monthly CLABSI data and feedback to safety teams, senior leaders, and ICU staff.
The overall mean crude CLABSI rate for participating ICUs decreased from 2.56 infections per 1000 catheter days to 1.79 per 1,000 catheter days by the end of the study, corresponding to a 30% reduction.
By unit type, CLABSI rates decreased by 16% among adult ICUs, 48% among pediatric ICUs, and 47% in neonatal ICUs. The percentage of ICUs that achieved a quarterly CLABSI rate of less than 1 infection per 1000 catheter days increased from 44% to 61% after the interventions.
“Despite growing awareness, many hospitals around the world continue to struggle in their efforts to meaningfully reduce their CLABSI rates in a sustained manner,” said Sean M. Berenholtz, MD, of the Johns Hopkins University School of Medicine.
“In addition, hospitals and healthcare systems in the Middle East have unique barriers to implementing quality improvement programs, such as challenges with staff recruitment and retention, and personnel fearful of punitive repercussions from speaking up regarding patient safety concerns. In our study, bringing all stakeholders to the same table allowed everyone to share their concerns and ensure their voices were heard.” ![]()

A bundled intervention can considerably reduce central line-associated bloodstream infections (CLABSIs), according to research published in Infection Control & Hospital Epidemiology.
The intervention focused on evidence-based infection prevention practices, safety culture and teamwork, and scheduled measurement of infection rates.
By implementing these measures, intensive care units (ICUs) in Abu Dhabi achieved an overall 38% reduction in CLABSIs.
“These hospitals were able to show significant improvements in infection rates and have been able to sustain the improvements a year after we finished the project,” said study author Asad Latif, MBBS, MD, of the Johns Hopkins University School of Medicine in Baltimore, Maryland.
“Our results suggest that ICUs in disparate settings around the world could use this program and achieve similar results, significantly reducing the global morbidity, mortality, and excess costs associated with CLABSIs. In addition, this collaborative could serve as a model for future efforts to reduce other types of preventable medical harms in the Middle East and around the world.”
This study was a collaborative effort by the Armstrong Institute, Johns Hopkins Medicine International, and the Abu Dhabi Health Services Company (SEHA), which operates the government healthcare system in Abu Dhabi.
For the study, ICUs were instructed to assemble a comprehensive unit-based safety program (CUSP) team comprising local physician and nursing leaders, a senior executive, frontline healthcare providers, an infection control provider, and hospital quality and safety leaders.
The ICUs included 10 adult, 5 neonatal, and 3 pediatric ICUs, accounting for 77% of the adult, 74% of the neonatal, and 100% of the pediatric ICU beds in Abu Dhabi.
Starting in May 2012, the SEHA corporate quality team and ICU CUSP teams attended 14 weekly live webinars on CLABSI prevention conducted by Armstrong Institute faculty, followed by content and coaching webinars every 2 weeks for 24 months. The webinars were recorded by SEHA and posted on a local, shared computer drive, along with educational and training materials.
Armstrong faculty also conducted 4 site visits in Abu Dhabi at the beginning of the study, visiting each ICU to meet the CUSP team and tour the units. A year later, they conducted a 3-day patient safety workshop for participating hospitals.
CUSP teams implemented 3 interventions as part of the program: an effort to prevent CLABSIs that targeted clinicians’ use of evidence-based infection prevention recommendations from the Centers for Disease Control and Prevention, a CUSP process to improve safety culture and teamwork, and measurement of monthly CLABSI data and feedback to safety teams, senior leaders, and ICU staff.
The overall mean crude CLABSI rate for participating ICUs decreased from 2.56 infections per 1000 catheter days to 1.79 per 1,000 catheter days by the end of the study, corresponding to a 30% reduction.
By unit type, CLABSI rates decreased by 16% among adult ICUs, 48% among pediatric ICUs, and 47% in neonatal ICUs. The percentage of ICUs that achieved a quarterly CLABSI rate of less than 1 infection per 1000 catheter days increased from 44% to 61% after the interventions.
“Despite growing awareness, many hospitals around the world continue to struggle in their efforts to meaningfully reduce their CLABSI rates in a sustained manner,” said Sean M. Berenholtz, MD, of the Johns Hopkins University School of Medicine.
“In addition, hospitals and healthcare systems in the Middle East have unique barriers to implementing quality improvement programs, such as challenges with staff recruitment and retention, and personnel fearful of punitive repercussions from speaking up regarding patient safety concerns. In our study, bringing all stakeholders to the same table allowed everyone to share their concerns and ensure their voices were heard.” ![]()
Environmental factors affect bloodstream infections

New research suggests environmental factors can affect the development of catheter-related bloodstream infections (CRBSIs) in patients who
receive parenteral nutrition therapy at home.
Using a peripherally inserted central venous catheter (PICC) for 1 additional infusion day per week significantly reduced the amount of time before a first CRBSI, and using a tunneled vascular access device managed by a home care nurse increased the mean incidence of CRBSIs.
Laura Fuglsang Bech, of Aalborg University in Denmark, and her colleagues reported these results in the Journal of Parenteral and Enteral Nutrition.
The researchers set out to determine if environmental factors play a role in the development of bloodstream infections among patients receiving parenteral nutrition therapy via vascular access devices or PICCs, the 2 most commonly used catheters.
The team looked at factors such as smoking, catheter management by a home care nurse, colectomy with stoma, number of infusion days per week, and C-reactive protein values at catheter insertion day.
Adult patients suffering from intestinal failure and receiving home parenteral nutrition were included in the study. There were 295 catheters—169 tunneled vascular access devices and 126 PICCs—used in 136 patients.
The researchers found that using a PICC for 1 additional infusion day per week significantly reduced the amount of time before a first bloodstream infection. The time to first CRBSI decreased by a factor of 2.47 with 1 additional infusion day per week (P=0.04).
The team also found that using a tunneled vascular access device managed by a home care nurse increased the mean incidence of bloodstream infections. The mean CRBSI incidence per 1000 catheter days was 1.45± 0.68 for catheters managed by a home care nurse and 0.56 ± 0.24 for catheters that were not (P<0.001).
None of the other factors the researchers analyzed had any significant impact on the timing or incidence of CRBSIs.
Based on these results, the researchers recommended revisions to current home care guidelines. They advised using PICCs only for short-term home therapy and when few infusion days per week are needed. And they said management of tunneled vascular access devices by home care nurses should be further specialized. ![]()

New research suggests environmental factors can affect the development of catheter-related bloodstream infections (CRBSIs) in patients who
receive parenteral nutrition therapy at home.
Using a peripherally inserted central venous catheter (PICC) for 1 additional infusion day per week significantly reduced the amount of time before a first CRBSI, and using a tunneled vascular access device managed by a home care nurse increased the mean incidence of CRBSIs.
Laura Fuglsang Bech, of Aalborg University in Denmark, and her colleagues reported these results in the Journal of Parenteral and Enteral Nutrition.
The researchers set out to determine if environmental factors play a role in the development of bloodstream infections among patients receiving parenteral nutrition therapy via vascular access devices or PICCs, the 2 most commonly used catheters.
The team looked at factors such as smoking, catheter management by a home care nurse, colectomy with stoma, number of infusion days per week, and C-reactive protein values at catheter insertion day.
Adult patients suffering from intestinal failure and receiving home parenteral nutrition were included in the study. There were 295 catheters—169 tunneled vascular access devices and 126 PICCs—used in 136 patients.
The researchers found that using a PICC for 1 additional infusion day per week significantly reduced the amount of time before a first bloodstream infection. The time to first CRBSI decreased by a factor of 2.47 with 1 additional infusion day per week (P=0.04).
The team also found that using a tunneled vascular access device managed by a home care nurse increased the mean incidence of bloodstream infections. The mean CRBSI incidence per 1000 catheter days was 1.45± 0.68 for catheters managed by a home care nurse and 0.56 ± 0.24 for catheters that were not (P<0.001).
None of the other factors the researchers analyzed had any significant impact on the timing or incidence of CRBSIs.
Based on these results, the researchers recommended revisions to current home care guidelines. They advised using PICCs only for short-term home therapy and when few infusion days per week are needed. And they said management of tunneled vascular access devices by home care nurses should be further specialized. ![]()

New research suggests environmental factors can affect the development of catheter-related bloodstream infections (CRBSIs) in patients who
receive parenteral nutrition therapy at home.
Using a peripherally inserted central venous catheter (PICC) for 1 additional infusion day per week significantly reduced the amount of time before a first CRBSI, and using a tunneled vascular access device managed by a home care nurse increased the mean incidence of CRBSIs.
Laura Fuglsang Bech, of Aalborg University in Denmark, and her colleagues reported these results in the Journal of Parenteral and Enteral Nutrition.
The researchers set out to determine if environmental factors play a role in the development of bloodstream infections among patients receiving parenteral nutrition therapy via vascular access devices or PICCs, the 2 most commonly used catheters.
The team looked at factors such as smoking, catheter management by a home care nurse, colectomy with stoma, number of infusion days per week, and C-reactive protein values at catheter insertion day.
Adult patients suffering from intestinal failure and receiving home parenteral nutrition were included in the study. There were 295 catheters—169 tunneled vascular access devices and 126 PICCs—used in 136 patients.
The researchers found that using a PICC for 1 additional infusion day per week significantly reduced the amount of time before a first bloodstream infection. The time to first CRBSI decreased by a factor of 2.47 with 1 additional infusion day per week (P=0.04).
The team also found that using a tunneled vascular access device managed by a home care nurse increased the mean incidence of bloodstream infections. The mean CRBSI incidence per 1000 catheter days was 1.45± 0.68 for catheters managed by a home care nurse and 0.56 ± 0.24 for catheters that were not (P<0.001).
None of the other factors the researchers analyzed had any significant impact on the timing or incidence of CRBSIs.
Based on these results, the researchers recommended revisions to current home care guidelines. They advised using PICCs only for short-term home therapy and when few infusion days per week are needed. And they said management of tunneled vascular access devices by home care nurses should be further specialized. ![]()
Collaboration may help prevent CLABSIs
Collaborative relationships between nurses and physicians may help reduce the rates of healthcare-associated infections in critical care, according to research published in Critical Care Nurse.
Study investigators found lower rates of central line-associated bloodstream infections (CLABSIs) and ventilator-associated pneumonia (VAP) in critical care units in which nurses reported a more favorable perception of nurse-physician collaboration.
“Our findings suggest that raising the quality of collaboration and communication among nurses and physicians has the potential to improve patient safety,” said study author Christine Boev, RN, PhD, CCRN, of Wegmans School of Nursing at St John Fisher College in Rochester, New York.
Dr Boev and her colleagues analyzed 5 years of data from 671 surveys of nurses in 4 specialized intensive care units (ICUs) at a 750-bed New York hospital.
The investigators also collected patient outcome data from those units for the same period, focusing on patients with CLABSIs or VAP. And the team analyzed unit-level variables such as nurses’ skill mix, nursing hours per patient day, and voluntary turnover.
Results revealed a significant association between nurse-physician collaboration and both CLABSIs and VAP. For every 0.5 unit increase in collaboration, the rate of CLABSIs decreased by 2.98 (P=0.005), and the rate of VAP decreased by 1.13 (P=0.005).
In addition, ICUs with a higher proportion of certified nurses had significantly lower incidences of both CLABSIs and VAP—0.43 (P=0.02) and 0.17 (P=0.01), respectively. And ICUs with higher numbers of nursing hours per patient day had significantly lower rates of CLABSIs—0.42 (P=0.05).
However, there was no significant difference in VAP rates according to nursing hours. And there was no significant difference in the rate of either type of infection according to nurses’ skill mix or voluntary turnover.
Dr Boev said these results suggest that efforts to prevent healthcare-associated infections should include interventions to improve nurse-physician collaboration. Such interventions might include multidisciplinary daily patient rounds and interprofessional educational programs, such as shared simulation training. ![]()

Collaborative relationships between nurses and physicians may help reduce the rates of healthcare-associated infections in critical care, according to research published in Critical Care Nurse.
Study investigators found lower rates of central line-associated bloodstream infections (CLABSIs) and ventilator-associated pneumonia (VAP) in critical care units in which nurses reported a more favorable perception of nurse-physician collaboration.
“Our findings suggest that raising the quality of collaboration and communication among nurses and physicians has the potential to improve patient safety,” said study author Christine Boev, RN, PhD, CCRN, of Wegmans School of Nursing at St John Fisher College in Rochester, New York.
Dr Boev and her colleagues analyzed 5 years of data from 671 surveys of nurses in 4 specialized intensive care units (ICUs) at a 750-bed New York hospital.
The investigators also collected patient outcome data from those units for the same period, focusing on patients with CLABSIs or VAP. And the team analyzed unit-level variables such as nurses’ skill mix, nursing hours per patient day, and voluntary turnover.
Results revealed a significant association between nurse-physician collaboration and both CLABSIs and VAP. For every 0.5 unit increase in collaboration, the rate of CLABSIs decreased by 2.98 (P=0.005), and the rate of VAP decreased by 1.13 (P=0.005).
In addition, ICUs with a higher proportion of certified nurses had significantly lower incidences of both CLABSIs and VAP—0.43 (P=0.02) and 0.17 (P=0.01), respectively. And ICUs with higher numbers of nursing hours per patient day had significantly lower rates of CLABSIs—0.42 (P=0.05).
However, there was no significant difference in VAP rates according to nursing hours. And there was no significant difference in the rate of either type of infection according to nurses’ skill mix or voluntary turnover.
Dr Boev said these results suggest that efforts to prevent healthcare-associated infections should include interventions to improve nurse-physician collaboration. Such interventions might include multidisciplinary daily patient rounds and interprofessional educational programs, such as shared simulation training. ![]()

Collaborative relationships between nurses and physicians may help reduce the rates of healthcare-associated infections in critical care, according to research published in Critical Care Nurse.
Study investigators found lower rates of central line-associated bloodstream infections (CLABSIs) and ventilator-associated pneumonia (VAP) in critical care units in which nurses reported a more favorable perception of nurse-physician collaboration.
“Our findings suggest that raising the quality of collaboration and communication among nurses and physicians has the potential to improve patient safety,” said study author Christine Boev, RN, PhD, CCRN, of Wegmans School of Nursing at St John Fisher College in Rochester, New York.
Dr Boev and her colleagues analyzed 5 years of data from 671 surveys of nurses in 4 specialized intensive care units (ICUs) at a 750-bed New York hospital.
The investigators also collected patient outcome data from those units for the same period, focusing on patients with CLABSIs or VAP. And the team analyzed unit-level variables such as nurses’ skill mix, nursing hours per patient day, and voluntary turnover.
Results revealed a significant association between nurse-physician collaboration and both CLABSIs and VAP. For every 0.5 unit increase in collaboration, the rate of CLABSIs decreased by 2.98 (P=0.005), and the rate of VAP decreased by 1.13 (P=0.005).
In addition, ICUs with a higher proportion of certified nurses had significantly lower incidences of both CLABSIs and VAP—0.43 (P=0.02) and 0.17 (P=0.01), respectively. And ICUs with higher numbers of nursing hours per patient day had significantly lower rates of CLABSIs—0.42 (P=0.05).
However, there was no significant difference in VAP rates according to nursing hours. And there was no significant difference in the rate of either type of infection according to nurses’ skill mix or voluntary turnover.
Dr Boev said these results suggest that efforts to prevent healthcare-associated infections should include interventions to improve nurse-physician collaboration. Such interventions might include multidisciplinary daily patient rounds and interprofessional educational programs, such as shared simulation training. ![]()
Studies investigate risk of DVT, infection with PICCs
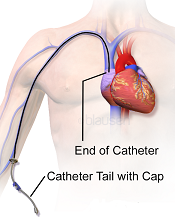
Image courtesy of Blausen
Medical Communications, Inc.
Three recently published papers may help ensure the appropriate use of peripherally inserted central catheters (PICCs).
A case-control study uncovered several factors that appear to affect the risk of deep vein thrombosis (DVT) associated with PICCs.
A retrospective study revealed the “prevalence, patterns, and predictors” of PICC-associated bloodstream infections.
And a literature review informed the creation of a guide to help hospitalists choose the right intravenous device.
“[PICCs] are very popular, but in an under-the-radar way, because they make care more convenient and can be placed relatively easily,” said Vineet Chopra, MD, an author on all 3 articles and a hospitalist at the University of Michigan Health System in Ann Arbor.
“But our new results, and review of research on the topic, show it’s important for physicians to think hard about both the risks and the benefits.”
Case-control study
In the study of PICC-DVT, published in Thrombosis Research, the investigators looked at records from 909 patients who received PICCs at the University of Michigan Health System in 2012 and 2013.
Patients had PICCs placed so they could receive long-term intravenous antibiotic therapy (n=447; 49.1%) or total parenteral nutrition (n=120; 6.7%). They also required PICCs for in-hospital venous access for blood draws or infusion of medications (n=342; 44.2%).
In all, 268 patients developed a DVT associated with their PICC. The median time to DVT development was 12.4 days from PICC placement.
Patients who were already taking aspirin and statins before their PICC was placed had a significantly lower risk of DVT-PICC (odds ratio [OR]=0.31). Patients who received pharmacological DVT prophylaxis had a lower risk of DVT-PICC than patients who were not on prophylaxis, although the difference was not statistically significant (OR=0.72).
Patients had a significantly greater risk of PICC-DVT if they underwent surgery during their hospital stay (OR=2.17) or had a prior history of venous thromboembolism (OR=1.70). And, compared to patients who received 4-Fr PICCs, those who had 5-Fr or 6-Fr PICCs had a significantly greater risk of DVT (ORs=2.74 and 7.40, respectively).
Retrospective study
In The American Journal of Medicine, Dr Chopra and his colleagues described a retrospective study of bloodstream infections associated with PICCs.
The investigators analyzed data from 747 patients who received 966 PICCs for a total of 26,887 catheter days. Patients had PICCs for long-term antibiotic administration (n=503; 52%), venous access (n=201; 21%), total parenteral nutrition (n=155; 16%), and chemotherapy (n=107; 11%).
There were 58 cases (6%) of PICC-bloodstream infection over 1156 catheter days, for an infection rate of 2.16 per 1000 catheter days. The median time to infection was 10 days.
Bivariate analysis revealed several factors associated with PICC-bloodstream infections. These included intensive care unit status (ICU; OR=3.23), mechanical ventilation (OR=4.39), length of stay (hospital, OR=1.04; ICU, OR=1.03), PowerPICCs (OR=2.58), devices placed by interventional radiology (OR=2.57), double lumens (OR=5.21), and triple lumens (OR=10.84).
In multivariable analysis, only some of these factors remained significantly associated with PICC-bloodstream infection. These included hospital length of stay (OR=1.04), ICU status (OR=1.02), double lumens (OR=3.99), and triple lumens (OR=6.34).
Dr Chopra said the results of these 2 studies suggest hospitalists should tread carefully when considering PICCs.
“These are not innocuous devices,” he said. “The time has come to stop thinking of them as a device of convenience and rather one with clear risks and benefits.”
Review and guide
In the Journal of Hospital Medicine, Dr Chopra and his colleagues offered advice on choosing vascular access devices. After reviewing the literature, the investigators designed a flow chart that can help hospitalists decide which device is appropriate for each patient.
For instance, different devices will work best depending on whether access to the bloodstream is needed urgently or less urgently, whether the patient will likely need the device for more or less than a week, what kind of drug or nutrition the doctor will order, and whether the patient’s kidneys are functioning normally. ![]()

Image courtesy of Blausen
Medical Communications, Inc.
Three recently published papers may help ensure the appropriate use of peripherally inserted central catheters (PICCs).
A case-control study uncovered several factors that appear to affect the risk of deep vein thrombosis (DVT) associated with PICCs.
A retrospective study revealed the “prevalence, patterns, and predictors” of PICC-associated bloodstream infections.
And a literature review informed the creation of a guide to help hospitalists choose the right intravenous device.
“[PICCs] are very popular, but in an under-the-radar way, because they make care more convenient and can be placed relatively easily,” said Vineet Chopra, MD, an author on all 3 articles and a hospitalist at the University of Michigan Health System in Ann Arbor.
“But our new results, and review of research on the topic, show it’s important for physicians to think hard about both the risks and the benefits.”
Case-control study
In the study of PICC-DVT, published in Thrombosis Research, the investigators looked at records from 909 patients who received PICCs at the University of Michigan Health System in 2012 and 2013.
Patients had PICCs placed so they could receive long-term intravenous antibiotic therapy (n=447; 49.1%) or total parenteral nutrition (n=120; 6.7%). They also required PICCs for in-hospital venous access for blood draws or infusion of medications (n=342; 44.2%).
In all, 268 patients developed a DVT associated with their PICC. The median time to DVT development was 12.4 days from PICC placement.
Patients who were already taking aspirin and statins before their PICC was placed had a significantly lower risk of DVT-PICC (odds ratio [OR]=0.31). Patients who received pharmacological DVT prophylaxis had a lower risk of DVT-PICC than patients who were not on prophylaxis, although the difference was not statistically significant (OR=0.72).
Patients had a significantly greater risk of PICC-DVT if they underwent surgery during their hospital stay (OR=2.17) or had a prior history of venous thromboembolism (OR=1.70). And, compared to patients who received 4-Fr PICCs, those who had 5-Fr or 6-Fr PICCs had a significantly greater risk of DVT (ORs=2.74 and 7.40, respectively).
Retrospective study
In The American Journal of Medicine, Dr Chopra and his colleagues described a retrospective study of bloodstream infections associated with PICCs.
The investigators analyzed data from 747 patients who received 966 PICCs for a total of 26,887 catheter days. Patients had PICCs for long-term antibiotic administration (n=503; 52%), venous access (n=201; 21%), total parenteral nutrition (n=155; 16%), and chemotherapy (n=107; 11%).
There were 58 cases (6%) of PICC-bloodstream infection over 1156 catheter days, for an infection rate of 2.16 per 1000 catheter days. The median time to infection was 10 days.
Bivariate analysis revealed several factors associated with PICC-bloodstream infections. These included intensive care unit status (ICU; OR=3.23), mechanical ventilation (OR=4.39), length of stay (hospital, OR=1.04; ICU, OR=1.03), PowerPICCs (OR=2.58), devices placed by interventional radiology (OR=2.57), double lumens (OR=5.21), and triple lumens (OR=10.84).
In multivariable analysis, only some of these factors remained significantly associated with PICC-bloodstream infection. These included hospital length of stay (OR=1.04), ICU status (OR=1.02), double lumens (OR=3.99), and triple lumens (OR=6.34).
Dr Chopra said the results of these 2 studies suggest hospitalists should tread carefully when considering PICCs.
“These are not innocuous devices,” he said. “The time has come to stop thinking of them as a device of convenience and rather one with clear risks and benefits.”
Review and guide
In the Journal of Hospital Medicine, Dr Chopra and his colleagues offered advice on choosing vascular access devices. After reviewing the literature, the investigators designed a flow chart that can help hospitalists decide which device is appropriate for each patient.
For instance, different devices will work best depending on whether access to the bloodstream is needed urgently or less urgently, whether the patient will likely need the device for more or less than a week, what kind of drug or nutrition the doctor will order, and whether the patient’s kidneys are functioning normally. ![]()

Image courtesy of Blausen
Medical Communications, Inc.
Three recently published papers may help ensure the appropriate use of peripherally inserted central catheters (PICCs).
A case-control study uncovered several factors that appear to affect the risk of deep vein thrombosis (DVT) associated with PICCs.
A retrospective study revealed the “prevalence, patterns, and predictors” of PICC-associated bloodstream infections.
And a literature review informed the creation of a guide to help hospitalists choose the right intravenous device.
“[PICCs] are very popular, but in an under-the-radar way, because they make care more convenient and can be placed relatively easily,” said Vineet Chopra, MD, an author on all 3 articles and a hospitalist at the University of Michigan Health System in Ann Arbor.
“But our new results, and review of research on the topic, show it’s important for physicians to think hard about both the risks and the benefits.”
Case-control study
In the study of PICC-DVT, published in Thrombosis Research, the investigators looked at records from 909 patients who received PICCs at the University of Michigan Health System in 2012 and 2013.
Patients had PICCs placed so they could receive long-term intravenous antibiotic therapy (n=447; 49.1%) or total parenteral nutrition (n=120; 6.7%). They also required PICCs for in-hospital venous access for blood draws or infusion of medications (n=342; 44.2%).
In all, 268 patients developed a DVT associated with their PICC. The median time to DVT development was 12.4 days from PICC placement.
Patients who were already taking aspirin and statins before their PICC was placed had a significantly lower risk of DVT-PICC (odds ratio [OR]=0.31). Patients who received pharmacological DVT prophylaxis had a lower risk of DVT-PICC than patients who were not on prophylaxis, although the difference was not statistically significant (OR=0.72).
Patients had a significantly greater risk of PICC-DVT if they underwent surgery during their hospital stay (OR=2.17) or had a prior history of venous thromboembolism (OR=1.70). And, compared to patients who received 4-Fr PICCs, those who had 5-Fr or 6-Fr PICCs had a significantly greater risk of DVT (ORs=2.74 and 7.40, respectively).
Retrospective study
In The American Journal of Medicine, Dr Chopra and his colleagues described a retrospective study of bloodstream infections associated with PICCs.
The investigators analyzed data from 747 patients who received 966 PICCs for a total of 26,887 catheter days. Patients had PICCs for long-term antibiotic administration (n=503; 52%), venous access (n=201; 21%), total parenteral nutrition (n=155; 16%), and chemotherapy (n=107; 11%).
There were 58 cases (6%) of PICC-bloodstream infection over 1156 catheter days, for an infection rate of 2.16 per 1000 catheter days. The median time to infection was 10 days.
Bivariate analysis revealed several factors associated with PICC-bloodstream infections. These included intensive care unit status (ICU; OR=3.23), mechanical ventilation (OR=4.39), length of stay (hospital, OR=1.04; ICU, OR=1.03), PowerPICCs (OR=2.58), devices placed by interventional radiology (OR=2.57), double lumens (OR=5.21), and triple lumens (OR=10.84).
In multivariable analysis, only some of these factors remained significantly associated with PICC-bloodstream infection. These included hospital length of stay (OR=1.04), ICU status (OR=1.02), double lumens (OR=3.99), and triple lumens (OR=6.34).
Dr Chopra said the results of these 2 studies suggest hospitalists should tread carefully when considering PICCs.
“These are not innocuous devices,” he said. “The time has come to stop thinking of them as a device of convenience and rather one with clear risks and benefits.”
Review and guide
In the Journal of Hospital Medicine, Dr Chopra and his colleagues offered advice on choosing vascular access devices. After reviewing the literature, the investigators designed a flow chart that can help hospitalists decide which device is appropriate for each patient.
For instance, different devices will work best depending on whether access to the bloodstream is needed urgently or less urgently, whether the patient will likely need the device for more or less than a week, what kind of drug or nutrition the doctor will order, and whether the patient’s kidneys are functioning normally. ![]()
Lowering the cost of cancer drugs in the US

Photo by Petr Kratochvil
Increasingly high prices for cancer drugs are affecting patient care and the overall healthcare system in the US, according to authors of an article in Mayo Clinic Proceedings.
The authors noted that the average price of cancer drugs for about a year of therapy increased from $5000 to $10,000 before 2000, and to more than $100,000 by 2012.
Over nearly the same period, the average household income in the US decreased by about 8%.
“Americans with cancer pay 50% to 100% more for the same patented drug than patients in other countries,” said author S. Vincent Rajkumar, MD, of the Mayo Clinic in Rochester, Minnesota.
“As oncologists, we have a moral obligation to advocate for affordable cancer drugs for our patients.”
Dr Rajkumar and co-author Hagop Kantarjian, MD, of MD Anderson Cancer Center in Houston, Texas, rebutted the major arguments the pharmaceutical industry uses to justify the high price of cancer drugs; namely, the expense of conducting research and drug development, the comparative benefits to patients, that market forces will settle prices to reasonable levels, and that price controls on cancer drugs will stifle innovation.
“One of the facts that people do not realize is that cancer drugs, for the most part, are not operating under a free market economy,” Dr Rajkumar said. “The fact that there are 5 approved drugs to treat an incurable cancer does not mean there is competition.”
“Typically, the standard of care is that each drug is used sequentially or in combination, so that each new drug represents a monopoly with exclusivity granted by patent protection for many years.”
Drs Rajkumar and Kantarjian said other reasons for the high cost of cancer drugs include legislation that prevents Medicare from being able to negotiate drug prices and a lack of value-based pricing, which ties the cost of a drug to its relative effectiveness compared to other drugs.
The authors recommended a set of potential solutions to help control and reduce the high cost of cancer drugs in the US. Some of their recommendations are already in practice in other developed countries. Their recommendations include:
- Allow Medicare to negotiate drug prices
- Develop cancer treatment pathways/guidelines that incorporate the cost and benefit of cancer drugs
- Allow the Food and Drug Administration or physician panels to recommend target prices based on a drug’s magnitude of benefit (value-based pricing)
- Eliminate “pay-for-delay” strategies in which a pharmaceutical company with a brand name drug shares profits on that drug with a generic drug manufacturer for the remainder of a patent period, effectively eliminating a patent challenge and competition
- Allow the importation of drugs from abroad for personal use
- Allow the Patient-Centered Outcomes Research Institute and other cancer advocacy groups to consider cost in their recommendations
- Create patient-driven grassroots movements and organizations to advocate effectively for the interests of patients with cancer to balance advocacy efforts of pharmaceutical companies, insurance companies, pharmacy outlets, and hospitals.
Dr Kantarjian has organized a petition, which is available on change.org, asking the federal government to implement these changes. ![]()

Photo by Petr Kratochvil
Increasingly high prices for cancer drugs are affecting patient care and the overall healthcare system in the US, according to authors of an article in Mayo Clinic Proceedings.
The authors noted that the average price of cancer drugs for about a year of therapy increased from $5000 to $10,000 before 2000, and to more than $100,000 by 2012.
Over nearly the same period, the average household income in the US decreased by about 8%.
“Americans with cancer pay 50% to 100% more for the same patented drug than patients in other countries,” said author S. Vincent Rajkumar, MD, of the Mayo Clinic in Rochester, Minnesota.
“As oncologists, we have a moral obligation to advocate for affordable cancer drugs for our patients.”
Dr Rajkumar and co-author Hagop Kantarjian, MD, of MD Anderson Cancer Center in Houston, Texas, rebutted the major arguments the pharmaceutical industry uses to justify the high price of cancer drugs; namely, the expense of conducting research and drug development, the comparative benefits to patients, that market forces will settle prices to reasonable levels, and that price controls on cancer drugs will stifle innovation.
“One of the facts that people do not realize is that cancer drugs, for the most part, are not operating under a free market economy,” Dr Rajkumar said. “The fact that there are 5 approved drugs to treat an incurable cancer does not mean there is competition.”
“Typically, the standard of care is that each drug is used sequentially or in combination, so that each new drug represents a monopoly with exclusivity granted by patent protection for many years.”
Drs Rajkumar and Kantarjian said other reasons for the high cost of cancer drugs include legislation that prevents Medicare from being able to negotiate drug prices and a lack of value-based pricing, which ties the cost of a drug to its relative effectiveness compared to other drugs.
The authors recommended a set of potential solutions to help control and reduce the high cost of cancer drugs in the US. Some of their recommendations are already in practice in other developed countries. Their recommendations include:
- Allow Medicare to negotiate drug prices
- Develop cancer treatment pathways/guidelines that incorporate the cost and benefit of cancer drugs
- Allow the Food and Drug Administration or physician panels to recommend target prices based on a drug’s magnitude of benefit (value-based pricing)
- Eliminate “pay-for-delay” strategies in which a pharmaceutical company with a brand name drug shares profits on that drug with a generic drug manufacturer for the remainder of a patent period, effectively eliminating a patent challenge and competition
- Allow the importation of drugs from abroad for personal use
- Allow the Patient-Centered Outcomes Research Institute and other cancer advocacy groups to consider cost in their recommendations
- Create patient-driven grassroots movements and organizations to advocate effectively for the interests of patients with cancer to balance advocacy efforts of pharmaceutical companies, insurance companies, pharmacy outlets, and hospitals.
Dr Kantarjian has organized a petition, which is available on change.org, asking the federal government to implement these changes. ![]()

Photo by Petr Kratochvil
Increasingly high prices for cancer drugs are affecting patient care and the overall healthcare system in the US, according to authors of an article in Mayo Clinic Proceedings.
The authors noted that the average price of cancer drugs for about a year of therapy increased from $5000 to $10,000 before 2000, and to more than $100,000 by 2012.
Over nearly the same period, the average household income in the US decreased by about 8%.
“Americans with cancer pay 50% to 100% more for the same patented drug than patients in other countries,” said author S. Vincent Rajkumar, MD, of the Mayo Clinic in Rochester, Minnesota.
“As oncologists, we have a moral obligation to advocate for affordable cancer drugs for our patients.”
Dr Rajkumar and co-author Hagop Kantarjian, MD, of MD Anderson Cancer Center in Houston, Texas, rebutted the major arguments the pharmaceutical industry uses to justify the high price of cancer drugs; namely, the expense of conducting research and drug development, the comparative benefits to patients, that market forces will settle prices to reasonable levels, and that price controls on cancer drugs will stifle innovation.
“One of the facts that people do not realize is that cancer drugs, for the most part, are not operating under a free market economy,” Dr Rajkumar said. “The fact that there are 5 approved drugs to treat an incurable cancer does not mean there is competition.”
“Typically, the standard of care is that each drug is used sequentially or in combination, so that each new drug represents a monopoly with exclusivity granted by patent protection for many years.”
Drs Rajkumar and Kantarjian said other reasons for the high cost of cancer drugs include legislation that prevents Medicare from being able to negotiate drug prices and a lack of value-based pricing, which ties the cost of a drug to its relative effectiveness compared to other drugs.
The authors recommended a set of potential solutions to help control and reduce the high cost of cancer drugs in the US. Some of their recommendations are already in practice in other developed countries. Their recommendations include:
- Allow Medicare to negotiate drug prices
- Develop cancer treatment pathways/guidelines that incorporate the cost and benefit of cancer drugs
- Allow the Food and Drug Administration or physician panels to recommend target prices based on a drug’s magnitude of benefit (value-based pricing)
- Eliminate “pay-for-delay” strategies in which a pharmaceutical company with a brand name drug shares profits on that drug with a generic drug manufacturer for the remainder of a patent period, effectively eliminating a patent challenge and competition
- Allow the importation of drugs from abroad for personal use
- Allow the Patient-Centered Outcomes Research Institute and other cancer advocacy groups to consider cost in their recommendations
- Create patient-driven grassroots movements and organizations to advocate effectively for the interests of patients with cancer to balance advocacy efforts of pharmaceutical companies, insurance companies, pharmacy outlets, and hospitals.
Dr Kantarjian has organized a petition, which is available on change.org, asking the federal government to implement these changes. ![]()
Quick antibiotic delivery reduces intensive care needs

Photo by Logan Tuttle
Time is of the essence when delivering antibiotics to pediatric cancer patients who present with fever and neutropenia, a new study suggests.
Patients who received antibiotics within 60 minutes of hospital admission were significantly less likely to require intensive care than patients who received antibiotics outside of an hour.
Children who received antibiotics faster also had a lower mortality rate, but the difference between the 2 groups was not statistically significant.
Joanne Hilden, MD, of Children’s Hospital Colorado in Aurora, and her colleagues detailed these results in Pediatric Blood & Cancer.
Dr Hilden noted that administering antibiotics within 60 minutes of a patient’s admission can be difficult, but she and her colleagues were able to adopt policies that sped up the process at their institution.
“We’re talking about kids who have gone home after chemotherapy and then a parent calls the hospital reporting a fever,” Dr Hilden said. “The question is, can we get the patient back to the hospital, then get a white cell count, and get antibiotics on board when needed all within an hour of their arrival?”
“It’s a huge challenge. This study shows that it’s important we make it happen. There’s less intensive care and fewer fatalities for kids who get antibiotics sooner.”
To determine the impact of timely antibiotic administration, Dr Hilden and her colleagues initially analyzed 116 children with hematologic and solid tumor malignancies who developed fever and neutropenia.
But the team found no significant differences in outcomes whether patients received antibiotics within or outside of the 60-minute window.
So the researchers extended the time period of their study and expanded the cohort to 220 patients.
This time, only the need for intensive care unit (ICU)-level care was significantly different between the 2 groups, with 12.6% of patients who received antibiotics within 60 minutes requiring ICU-level care, compared to 29.9% of patients who received antibiotics outside of an hour (P=0.003).
The researchers also found differences between the 2 groups with regard to the mean length of hospital stay (6.9 days vs 5.7 days), the mean duration of fever (3 days vs 2 days), the need for imaging workup (5.2% vs 9.1%), the incidence of bacteremia (13% vs 15.4%), and mortality rate (3.9% vs 0.7%). But none of these differences were statistically significant.
Still, Dr Hilden and her colleagues said it was important to reduce the time to antibiotic delivery at their institution, which took an average of 150 minutes when this study began. By instituting new policies, the team found they could deliver antibiotics in less than 60 minutes nearly 100% of the time.
To do this, hospital staff began prescribing antibiotics upon a pediatric cancer patient’s arrival, holding that order, and then allowing antibiotics to be delivered immediately after learning the results of neutrophil count testing. This eliminated the need to find a prescriber once the patient’s white blood cell count was known.
The researchers also found they could cut the time needed to determine a patient’s neutrophil count. Traditionally, determining neutropenia requires a full white blood cell count, followed by a differential by a human technician. But human verification reverses the machine results in less than 0.5% of cases.
The team discovered that the benefit of speed obtained by eliminating human verification outweighed the risk of administering unneeded antibiotics in very few cases. Depending on preliminary rather than technician-verified results of white cell counts reduced the time of testing from 45 minutes to 20.
The researchers also instituted changes to clinic flow procedures, such as notifying the full care team as soon as a family was advised to come into the hospital.
“Another thing we show is that just increasing the awareness of how important it is to get antibiotics on board quickly in these cases speeds delivery,” Dr Hilden said.
This knowledge and the aforementioned interventions allowed the researchers to reduce the time to antibiotic delivery to a median of 46 minutes.
“Only 11% of pediatric cancer patients with fever and neutropenia have serious complications,” Dr Hilden noted. “That’s low, but we can make it 0%, and this study shows that getting antibiotics onboard quickly goes a long way toward that goal.” ![]()

Photo by Logan Tuttle
Time is of the essence when delivering antibiotics to pediatric cancer patients who present with fever and neutropenia, a new study suggests.
Patients who received antibiotics within 60 minutes of hospital admission were significantly less likely to require intensive care than patients who received antibiotics outside of an hour.
Children who received antibiotics faster also had a lower mortality rate, but the difference between the 2 groups was not statistically significant.
Joanne Hilden, MD, of Children’s Hospital Colorado in Aurora, and her colleagues detailed these results in Pediatric Blood & Cancer.
Dr Hilden noted that administering antibiotics within 60 minutes of a patient’s admission can be difficult, but she and her colleagues were able to adopt policies that sped up the process at their institution.
“We’re talking about kids who have gone home after chemotherapy and then a parent calls the hospital reporting a fever,” Dr Hilden said. “The question is, can we get the patient back to the hospital, then get a white cell count, and get antibiotics on board when needed all within an hour of their arrival?”
“It’s a huge challenge. This study shows that it’s important we make it happen. There’s less intensive care and fewer fatalities for kids who get antibiotics sooner.”
To determine the impact of timely antibiotic administration, Dr Hilden and her colleagues initially analyzed 116 children with hematologic and solid tumor malignancies who developed fever and neutropenia.
But the team found no significant differences in outcomes whether patients received antibiotics within or outside of the 60-minute window.
So the researchers extended the time period of their study and expanded the cohort to 220 patients.
This time, only the need for intensive care unit (ICU)-level care was significantly different between the 2 groups, with 12.6% of patients who received antibiotics within 60 minutes requiring ICU-level care, compared to 29.9% of patients who received antibiotics outside of an hour (P=0.003).
The researchers also found differences between the 2 groups with regard to the mean length of hospital stay (6.9 days vs 5.7 days), the mean duration of fever (3 days vs 2 days), the need for imaging workup (5.2% vs 9.1%), the incidence of bacteremia (13% vs 15.4%), and mortality rate (3.9% vs 0.7%). But none of these differences were statistically significant.
Still, Dr Hilden and her colleagues said it was important to reduce the time to antibiotic delivery at their institution, which took an average of 150 minutes when this study began. By instituting new policies, the team found they could deliver antibiotics in less than 60 minutes nearly 100% of the time.
To do this, hospital staff began prescribing antibiotics upon a pediatric cancer patient’s arrival, holding that order, and then allowing antibiotics to be delivered immediately after learning the results of neutrophil count testing. This eliminated the need to find a prescriber once the patient’s white blood cell count was known.
The researchers also found they could cut the time needed to determine a patient’s neutrophil count. Traditionally, determining neutropenia requires a full white blood cell count, followed by a differential by a human technician. But human verification reverses the machine results in less than 0.5% of cases.
The team discovered that the benefit of speed obtained by eliminating human verification outweighed the risk of administering unneeded antibiotics in very few cases. Depending on preliminary rather than technician-verified results of white cell counts reduced the time of testing from 45 minutes to 20.
The researchers also instituted changes to clinic flow procedures, such as notifying the full care team as soon as a family was advised to come into the hospital.
“Another thing we show is that just increasing the awareness of how important it is to get antibiotics on board quickly in these cases speeds delivery,” Dr Hilden said.
This knowledge and the aforementioned interventions allowed the researchers to reduce the time to antibiotic delivery to a median of 46 minutes.
“Only 11% of pediatric cancer patients with fever and neutropenia have serious complications,” Dr Hilden noted. “That’s low, but we can make it 0%, and this study shows that getting antibiotics onboard quickly goes a long way toward that goal.” ![]()

Photo by Logan Tuttle
Time is of the essence when delivering antibiotics to pediatric cancer patients who present with fever and neutropenia, a new study suggests.
Patients who received antibiotics within 60 minutes of hospital admission were significantly less likely to require intensive care than patients who received antibiotics outside of an hour.
Children who received antibiotics faster also had a lower mortality rate, but the difference between the 2 groups was not statistically significant.
Joanne Hilden, MD, of Children’s Hospital Colorado in Aurora, and her colleagues detailed these results in Pediatric Blood & Cancer.
Dr Hilden noted that administering antibiotics within 60 minutes of a patient’s admission can be difficult, but she and her colleagues were able to adopt policies that sped up the process at their institution.
“We’re talking about kids who have gone home after chemotherapy and then a parent calls the hospital reporting a fever,” Dr Hilden said. “The question is, can we get the patient back to the hospital, then get a white cell count, and get antibiotics on board when needed all within an hour of their arrival?”
“It’s a huge challenge. This study shows that it’s important we make it happen. There’s less intensive care and fewer fatalities for kids who get antibiotics sooner.”
To determine the impact of timely antibiotic administration, Dr Hilden and her colleagues initially analyzed 116 children with hematologic and solid tumor malignancies who developed fever and neutropenia.
But the team found no significant differences in outcomes whether patients received antibiotics within or outside of the 60-minute window.
So the researchers extended the time period of their study and expanded the cohort to 220 patients.
This time, only the need for intensive care unit (ICU)-level care was significantly different between the 2 groups, with 12.6% of patients who received antibiotics within 60 minutes requiring ICU-level care, compared to 29.9% of patients who received antibiotics outside of an hour (P=0.003).
The researchers also found differences between the 2 groups with regard to the mean length of hospital stay (6.9 days vs 5.7 days), the mean duration of fever (3 days vs 2 days), the need for imaging workup (5.2% vs 9.1%), the incidence of bacteremia (13% vs 15.4%), and mortality rate (3.9% vs 0.7%). But none of these differences were statistically significant.
Still, Dr Hilden and her colleagues said it was important to reduce the time to antibiotic delivery at their institution, which took an average of 150 minutes when this study began. By instituting new policies, the team found they could deliver antibiotics in less than 60 minutes nearly 100% of the time.
To do this, hospital staff began prescribing antibiotics upon a pediatric cancer patient’s arrival, holding that order, and then allowing antibiotics to be delivered immediately after learning the results of neutrophil count testing. This eliminated the need to find a prescriber once the patient’s white blood cell count was known.
The researchers also found they could cut the time needed to determine a patient’s neutrophil count. Traditionally, determining neutropenia requires a full white blood cell count, followed by a differential by a human technician. But human verification reverses the machine results in less than 0.5% of cases.
The team discovered that the benefit of speed obtained by eliminating human verification outweighed the risk of administering unneeded antibiotics in very few cases. Depending on preliminary rather than technician-verified results of white cell counts reduced the time of testing from 45 minutes to 20.
The researchers also instituted changes to clinic flow procedures, such as notifying the full care team as soon as a family was advised to come into the hospital.
“Another thing we show is that just increasing the awareness of how important it is to get antibiotics on board quickly in these cases speeds delivery,” Dr Hilden said.
This knowledge and the aforementioned interventions allowed the researchers to reduce the time to antibiotic delivery to a median of 46 minutes.
“Only 11% of pediatric cancer patients with fever and neutropenia have serious complications,” Dr Hilden noted. “That’s low, but we can make it 0%, and this study shows that getting antibiotics onboard quickly goes a long way toward that goal.”
FDA clears new blood-draw device
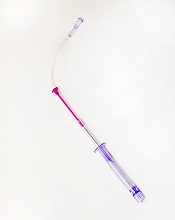
Photo courtesy of
Velano Vascular
The US Food and Drug Administration (FDA) has cleared for marketing a device that reduces the need for venipunctures for in-hospital blood draws.
Velano Vascular’s blood-draw device resembles a common syringe.
It allows peripheral intravenous catheters to be repurposed to draw blood from patients, thereby reducing the need for additional needle sticks among patients receiving medications and hydration via intravenous delivery.
The single-use device will soon be used for clinical evaluation in select hospitals, including the University of Pennsylvania in Philadelphia and University Hospitals Case Medical Center of Cleveland in Ohio.
“A fundamental benefit of this technology is reducing the ‘pin cushion effect,’ in which hospitalized patients are ‘stuck’ several times daily to obtain blood tests,” said Eric M. Stone, co-founder and chief executive officer of Velano Vascular, the company developing the blood-draw device.
“Oftentimes, the draw procedure is plagued by multiple failed attempts. The FDA’s clearance of this novel technology validates the existing clinical need and will allow us to expedite our efforts to bring this innovation to patients, healthcare providers, and hospitals around the world.”
According to research conducted by Velano Vascular, 1 of every 3 hospital patients is stuck 2 or more times daily for blood draws, with a significant subset of these patients receiving 3 or more blood draws, along with numerous needle sticks.
Twenty-eight percent of adult venipunctures and 44% of pediatric venipunctures require more than one stick to successfully draw blood.
“Traditional blood draws are one of the most common and most problematic healthcare procedures,” said Karen Daley, PhD, RN, past president of the American Nurses Association and a healthcare worker safety advocate.
“It is an antiquated technology that creates pain and anxiety for many patients, a significant safety risk for healthcare professionals, and a real inefficiency in our healthcare system. Velano Vascular has developed a common-sense solution to this pervasive, long-standing problem.”

Photo courtesy of
Velano Vascular
The US Food and Drug Administration (FDA) has cleared for marketing a device that reduces the need for venipunctures for in-hospital blood draws.
Velano Vascular’s blood-draw device resembles a common syringe.
It allows peripheral intravenous catheters to be repurposed to draw blood from patients, thereby reducing the need for additional needle sticks among patients receiving medications and hydration via intravenous delivery.
The single-use device will soon be used for clinical evaluation in select hospitals, including the University of Pennsylvania in Philadelphia and University Hospitals Case Medical Center of Cleveland in Ohio.
“A fundamental benefit of this technology is reducing the ‘pin cushion effect,’ in which hospitalized patients are ‘stuck’ several times daily to obtain blood tests,” said Eric M. Stone, co-founder and chief executive officer of Velano Vascular, the company developing the blood-draw device.
“Oftentimes, the draw procedure is plagued by multiple failed attempts. The FDA’s clearance of this novel technology validates the existing clinical need and will allow us to expedite our efforts to bring this innovation to patients, healthcare providers, and hospitals around the world.”
According to research conducted by Velano Vascular, 1 of every 3 hospital patients is stuck 2 or more times daily for blood draws, with a significant subset of these patients receiving 3 or more blood draws, along with numerous needle sticks.
Twenty-eight percent of adult venipunctures and 44% of pediatric venipunctures require more than one stick to successfully draw blood.
“Traditional blood draws are one of the most common and most problematic healthcare procedures,” said Karen Daley, PhD, RN, past president of the American Nurses Association and a healthcare worker safety advocate.
“It is an antiquated technology that creates pain and anxiety for many patients, a significant safety risk for healthcare professionals, and a real inefficiency in our healthcare system. Velano Vascular has developed a common-sense solution to this pervasive, long-standing problem.”

Photo courtesy of
Velano Vascular
The US Food and Drug Administration (FDA) has cleared for marketing a device that reduces the need for venipunctures for in-hospital blood draws.
Velano Vascular’s blood-draw device resembles a common syringe.
It allows peripheral intravenous catheters to be repurposed to draw blood from patients, thereby reducing the need for additional needle sticks among patients receiving medications and hydration via intravenous delivery.
The single-use device will soon be used for clinical evaluation in select hospitals, including the University of Pennsylvania in Philadelphia and University Hospitals Case Medical Center of Cleveland in Ohio.
“A fundamental benefit of this technology is reducing the ‘pin cushion effect,’ in which hospitalized patients are ‘stuck’ several times daily to obtain blood tests,” said Eric M. Stone, co-founder and chief executive officer of Velano Vascular, the company developing the blood-draw device.
“Oftentimes, the draw procedure is plagued by multiple failed attempts. The FDA’s clearance of this novel technology validates the existing clinical need and will allow us to expedite our efforts to bring this innovation to patients, healthcare providers, and hospitals around the world.”
According to research conducted by Velano Vascular, 1 of every 3 hospital patients is stuck 2 or more times daily for blood draws, with a significant subset of these patients receiving 3 or more blood draws, along with numerous needle sticks.
Twenty-eight percent of adult venipunctures and 44% of pediatric venipunctures require more than one stick to successfully draw blood.
“Traditional blood draws are one of the most common and most problematic healthcare procedures,” said Karen Daley, PhD, RN, past president of the American Nurses Association and a healthcare worker safety advocate.
“It is an antiquated technology that creates pain and anxiety for many patients, a significant safety risk for healthcare professionals, and a real inefficiency in our healthcare system. Velano Vascular has developed a common-sense solution to this pervasive, long-standing problem.”
EMA wants to suspend drugs due to data manipulation

Credit: Steven Harbour
The European Medicines Agency (EMA) has recommended suspending a number of drugs that were approved in the European Union (EU) based on clinical studies conducted at GVK Biosciences in Hyderabad, India.
An inspection of the site suggested GVK Bio employees may have manipulated data from trials that took place there.
So the EMA compiled a list of drugs—which includes clopidogrel, dexamethasone, and tacrolimus, among others—that should be suspended.
However, the EMA said there is no evidence of harm or lack of effectiveness linked to the conduct of studies by GVK Bio, and patients should continue taking their medicines as prescribed.
The EMA’s opinion has been forwarded to the European Commission (EC), which will adopt a legally binding decision.
It was at the request of the EC that the EMA’s Committee for Medicinal Products for Human Use (CHMP) reviewed the drugs set to be suspended.
An inspection by the French medicines agency, Agence nationale de sécurité du médicament et des produits de santé (ANSM), in May 2014 uncovered “non-compliance with good clinical practice” at the GVK Bio site in Hyderabad.
The ANSM inspector analyzed 9 studies conducted there from 2008 to 2013 and found evidence suggesting that data from electrocardiograms (ECGs) had been manipulated. It appeared that GVK Bio employees were taking multiple ECGs of one volunteer and presenting them as ECGs of other volunteers.
The EMA said the systematic nature of these data manipulations, the extended period of time during which they took place, and the number of staff members involved casts doubt on the integrity of the way trials were performed at the Hyderabad facility and on the reliability of data generated there.
With this in mind, the CHMP looked at more than 1000 pharmaceutical forms and strengths of medicines studied at the site. For more than 300 of the medications, there was sufficient data from other sources to support the drugs’ authorization, so these will remain on the market in the EU.
For drugs that lack data from other studies, the CHMP recommended suspension unless they are of critical importance for patients because alternatives will not be able to meet patients’ needs.
Whether a medicine is critical for patients will be determined by the national authorities of EU member states, depending on the situation in their country. For drugs that are considered critical, companies developing those drugs will be given 12 months to submit additional data.
The CHMP’s recommendation will be sent to the EC for a legally binding decision that will apply to all EU member states whether or not they have taken interim measures to suspend medicines.
GVK Bio responds
GVK Bio said an internal audit conducted following the ANSM inspection suggested that standard operating procedures were followed for the 9 trials analyzed. The organization also sought the opinion of 4 independent cardiologists, who said it was difficult to determine if the ECGs belong to the same volunteer or more than one person.
GVK Bio presented this information to the ANSM, but the agency concluded that the studies did not meet good clinical practice guidelines and should be rejected.
Following subsequent meetings and analyses, the EMA came to a similar conclusion—that the overall findings cast doubt on the results of trials conducted at the Hyderabad facility from 2008 to 2014.
GVK Bio said it respects the EMA’s decision, but their recommended suspension of drugs is “unprecedented and highly disproportional” for a few reasons.
First, the ANSM said the ECGs in question were not essential to demonstrate the bioequivalence of the drugs tested, and the agency’s recommendation to reject the 9 studies was a “precautionary” measure.
The ANSM also said the observations made during the inspection should not be extrapolated to other trial-related activities at the Hyderabad site or GVK Bio’s other site in Ahmedabad.
And finally, the EMA itself said there is no evidence proving that the drugs in question are ineffective or pose a risk to human health.

Credit: Steven Harbour
The European Medicines Agency (EMA) has recommended suspending a number of drugs that were approved in the European Union (EU) based on clinical studies conducted at GVK Biosciences in Hyderabad, India.
An inspection of the site suggested GVK Bio employees may have manipulated data from trials that took place there.
So the EMA compiled a list of drugs—which includes clopidogrel, dexamethasone, and tacrolimus, among others—that should be suspended.
However, the EMA said there is no evidence of harm or lack of effectiveness linked to the conduct of studies by GVK Bio, and patients should continue taking their medicines as prescribed.
The EMA’s opinion has been forwarded to the European Commission (EC), which will adopt a legally binding decision.
It was at the request of the EC that the EMA’s Committee for Medicinal Products for Human Use (CHMP) reviewed the drugs set to be suspended.
An inspection by the French medicines agency, Agence nationale de sécurité du médicament et des produits de santé (ANSM), in May 2014 uncovered “non-compliance with good clinical practice” at the GVK Bio site in Hyderabad.
The ANSM inspector analyzed 9 studies conducted there from 2008 to 2013 and found evidence suggesting that data from electrocardiograms (ECGs) had been manipulated. It appeared that GVK Bio employees were taking multiple ECGs of one volunteer and presenting them as ECGs of other volunteers.
The EMA said the systematic nature of these data manipulations, the extended period of time during which they took place, and the number of staff members involved casts doubt on the integrity of the way trials were performed at the Hyderabad facility and on the reliability of data generated there.
With this in mind, the CHMP looked at more than 1000 pharmaceutical forms and strengths of medicines studied at the site. For more than 300 of the medications, there was sufficient data from other sources to support the drugs’ authorization, so these will remain on the market in the EU.
For drugs that lack data from other studies, the CHMP recommended suspension unless they are of critical importance for patients because alternatives will not be able to meet patients’ needs.
Whether a medicine is critical for patients will be determined by the national authorities of EU member states, depending on the situation in their country. For drugs that are considered critical, companies developing those drugs will be given 12 months to submit additional data.
The CHMP’s recommendation will be sent to the EC for a legally binding decision that will apply to all EU member states whether or not they have taken interim measures to suspend medicines.
GVK Bio responds
GVK Bio said an internal audit conducted following the ANSM inspection suggested that standard operating procedures were followed for the 9 trials analyzed. The organization also sought the opinion of 4 independent cardiologists, who said it was difficult to determine if the ECGs belong to the same volunteer or more than one person.
GVK Bio presented this information to the ANSM, but the agency concluded that the studies did not meet good clinical practice guidelines and should be rejected.
Following subsequent meetings and analyses, the EMA came to a similar conclusion—that the overall findings cast doubt on the results of trials conducted at the Hyderabad facility from 2008 to 2014.
GVK Bio said it respects the EMA’s decision, but their recommended suspension of drugs is “unprecedented and highly disproportional” for a few reasons.
First, the ANSM said the ECGs in question were not essential to demonstrate the bioequivalence of the drugs tested, and the agency’s recommendation to reject the 9 studies was a “precautionary” measure.
The ANSM also said the observations made during the inspection should not be extrapolated to other trial-related activities at the Hyderabad site or GVK Bio’s other site in Ahmedabad.
And finally, the EMA itself said there is no evidence proving that the drugs in question are ineffective or pose a risk to human health.

Credit: Steven Harbour
The European Medicines Agency (EMA) has recommended suspending a number of drugs that were approved in the European Union (EU) based on clinical studies conducted at GVK Biosciences in Hyderabad, India.
An inspection of the site suggested GVK Bio employees may have manipulated data from trials that took place there.
So the EMA compiled a list of drugs—which includes clopidogrel, dexamethasone, and tacrolimus, among others—that should be suspended.
However, the EMA said there is no evidence of harm or lack of effectiveness linked to the conduct of studies by GVK Bio, and patients should continue taking their medicines as prescribed.
The EMA’s opinion has been forwarded to the European Commission (EC), which will adopt a legally binding decision.
It was at the request of the EC that the EMA’s Committee for Medicinal Products for Human Use (CHMP) reviewed the drugs set to be suspended.
An inspection by the French medicines agency, Agence nationale de sécurité du médicament et des produits de santé (ANSM), in May 2014 uncovered “non-compliance with good clinical practice” at the GVK Bio site in Hyderabad.
The ANSM inspector analyzed 9 studies conducted there from 2008 to 2013 and found evidence suggesting that data from electrocardiograms (ECGs) had been manipulated. It appeared that GVK Bio employees were taking multiple ECGs of one volunteer and presenting them as ECGs of other volunteers.
The EMA said the systematic nature of these data manipulations, the extended period of time during which they took place, and the number of staff members involved casts doubt on the integrity of the way trials were performed at the Hyderabad facility and on the reliability of data generated there.
With this in mind, the CHMP looked at more than 1000 pharmaceutical forms and strengths of medicines studied at the site. For more than 300 of the medications, there was sufficient data from other sources to support the drugs’ authorization, so these will remain on the market in the EU.
For drugs that lack data from other studies, the CHMP recommended suspension unless they are of critical importance for patients because alternatives will not be able to meet patients’ needs.
Whether a medicine is critical for patients will be determined by the national authorities of EU member states, depending on the situation in their country. For drugs that are considered critical, companies developing those drugs will be given 12 months to submit additional data.
The CHMP’s recommendation will be sent to the EC for a legally binding decision that will apply to all EU member states whether or not they have taken interim measures to suspend medicines.
GVK Bio responds
GVK Bio said an internal audit conducted following the ANSM inspection suggested that standard operating procedures were followed for the 9 trials analyzed. The organization also sought the opinion of 4 independent cardiologists, who said it was difficult to determine if the ECGs belong to the same volunteer or more than one person.
GVK Bio presented this information to the ANSM, but the agency concluded that the studies did not meet good clinical practice guidelines and should be rejected.
Following subsequent meetings and analyses, the EMA came to a similar conclusion—that the overall findings cast doubt on the results of trials conducted at the Hyderabad facility from 2008 to 2014.
GVK Bio said it respects the EMA’s decision, but their recommended suspension of drugs is “unprecedented and highly disproportional” for a few reasons.
First, the ANSM said the ECGs in question were not essential to demonstrate the bioequivalence of the drugs tested, and the agency’s recommendation to reject the 9 studies was a “precautionary” measure.
The ANSM also said the observations made during the inspection should not be extrapolated to other trial-related activities at the Hyderabad site or GVK Bio’s other site in Ahmedabad.
And finally, the EMA itself said there is no evidence proving that the drugs in question are ineffective or pose a risk to human health.
System can detect Candida better, faster than blood culture

Credit: Jeremy L. Grisham
A diagnostic system can detect sepsis pathogens with high sensitivity and specificity in 3 to 5 hours, eliminating the need for blood culture, according to a study published in Clinical Infectious Diseases.
The system consists of the T2Candida Panel and the T2Dx Instrument, and it provides direct detection of 5 yeast pathogens—Candida albicans, Candida tropicalis, Candida parapsilosis, Candida glabrata, and Candida krusei—in whole blood samples.
T2Candida and T2Dx were cleared for use by the US Food and Drug Administration in September. They are the first diagnostic products powered by T2MR, a magnetic resonance-based diagnostic technology platform that does not require blood culture and sample purification or preparation.
“The ability to determine the presence or absence of Candida within hours—compared to days [with blood culture]—is paradigm-changing for patients at risk for these infections,” said study author Eleftherios Mylonakis, MD, PhD, of Rhode Island Hospital and The Miriam Hospital in Providence.
“It will allow us to move from a ‘best-guess’ approach in treating high-risk patients, such as cancer and transplant patients and patients in the intensive care unit, to a more informed approach where we can quickly direct the best course of therapy, potentially improving patient outcomes and saving lives.”
Study findings
For this multicenter study, Dr Mylonakis and his colleagues collected blood specimens from 1801 hospitalized patients between the ages of 18 and 95 who had a blood culture ordered as part of routine care.
T2Candida and T2Dx demonstrated an overall specificity of 99.4% per assay and 98.1% per patient. The system yielded a specificity of 98.9% for C albicans/C tropicalis, 99.3% for C parapsilosis, and 99.9% for C krusei/C glabrata.
The system had an overall sensitivity of 91.1% per assay and 91.0% per patient. It yielded a sensitivity of 92.3% for C albicans/C tropicalis, 94.2% for C parapsilosis, and 88.1% for C krusei/C glabrata.
The mean time to a positive result for T2Candida and T2Dx was 4.4 hours, compared to 129 hours for blood culture and species identification. The mean time to negative result for T2Candida and T2Dx was 4.2 hours, compared to at least 120 hours for blood culture.
In one case described in the paper, T2Candida and T2Dx detected a Candida infection that blood culture missed in 12 successive tests.
Seven days after the T2Candida result was obtained, physicians performed an invasive procedure to obtain a tissue culture, which proved that the T2Candida result accurately identified a case of intra-abdominal candidiasis.
“Blood culture, the current standard of care for the diagnosis of Candida infections, is known to have poor sensitivity overall and has 38% sensitivity in proven and probable cases of invasive candidiasis,” said study author Cornelius J. Clancy, MD, of the University of Pittsburgh in Pennsylvania.
“In our case, the T2Candida Panel has shown that it can rapidly identify intra-abdominal candidiasis where 12 serial blood culture results were negative. In many patients at risk for candidiasis, the collection of tissue samples for diagnosis is not possible due to their underlying medical conditions. Achieving the level of sensitivity demonstrated in this case, without requiring an intra-abdominal sample, has the potential to positively impact the practice of medicine for these patients.”

Credit: Jeremy L. Grisham
A diagnostic system can detect sepsis pathogens with high sensitivity and specificity in 3 to 5 hours, eliminating the need for blood culture, according to a study published in Clinical Infectious Diseases.
The system consists of the T2Candida Panel and the T2Dx Instrument, and it provides direct detection of 5 yeast pathogens—Candida albicans, Candida tropicalis, Candida parapsilosis, Candida glabrata, and Candida krusei—in whole blood samples.
T2Candida and T2Dx were cleared for use by the US Food and Drug Administration in September. They are the first diagnostic products powered by T2MR, a magnetic resonance-based diagnostic technology platform that does not require blood culture and sample purification or preparation.
“The ability to determine the presence or absence of Candida within hours—compared to days [with blood culture]—is paradigm-changing for patients at risk for these infections,” said study author Eleftherios Mylonakis, MD, PhD, of Rhode Island Hospital and The Miriam Hospital in Providence.
“It will allow us to move from a ‘best-guess’ approach in treating high-risk patients, such as cancer and transplant patients and patients in the intensive care unit, to a more informed approach where we can quickly direct the best course of therapy, potentially improving patient outcomes and saving lives.”
Study findings
For this multicenter study, Dr Mylonakis and his colleagues collected blood specimens from 1801 hospitalized patients between the ages of 18 and 95 who had a blood culture ordered as part of routine care.
T2Candida and T2Dx demonstrated an overall specificity of 99.4% per assay and 98.1% per patient. The system yielded a specificity of 98.9% for C albicans/C tropicalis, 99.3% for C parapsilosis, and 99.9% for C krusei/C glabrata.
The system had an overall sensitivity of 91.1% per assay and 91.0% per patient. It yielded a sensitivity of 92.3% for C albicans/C tropicalis, 94.2% for C parapsilosis, and 88.1% for C krusei/C glabrata.
The mean time to a positive result for T2Candida and T2Dx was 4.4 hours, compared to 129 hours for blood culture and species identification. The mean time to negative result for T2Candida and T2Dx was 4.2 hours, compared to at least 120 hours for blood culture.
In one case described in the paper, T2Candida and T2Dx detected a Candida infection that blood culture missed in 12 successive tests.
Seven days after the T2Candida result was obtained, physicians performed an invasive procedure to obtain a tissue culture, which proved that the T2Candida result accurately identified a case of intra-abdominal candidiasis.
“Blood culture, the current standard of care for the diagnosis of Candida infections, is known to have poor sensitivity overall and has 38% sensitivity in proven and probable cases of invasive candidiasis,” said study author Cornelius J. Clancy, MD, of the University of Pittsburgh in Pennsylvania.
“In our case, the T2Candida Panel has shown that it can rapidly identify intra-abdominal candidiasis where 12 serial blood culture results were negative. In many patients at risk for candidiasis, the collection of tissue samples for diagnosis is not possible due to their underlying medical conditions. Achieving the level of sensitivity demonstrated in this case, without requiring an intra-abdominal sample, has the potential to positively impact the practice of medicine for these patients.”

Credit: Jeremy L. Grisham
A diagnostic system can detect sepsis pathogens with high sensitivity and specificity in 3 to 5 hours, eliminating the need for blood culture, according to a study published in Clinical Infectious Diseases.
The system consists of the T2Candida Panel and the T2Dx Instrument, and it provides direct detection of 5 yeast pathogens—Candida albicans, Candida tropicalis, Candida parapsilosis, Candida glabrata, and Candida krusei—in whole blood samples.
T2Candida and T2Dx were cleared for use by the US Food and Drug Administration in September. They are the first diagnostic products powered by T2MR, a magnetic resonance-based diagnostic technology platform that does not require blood culture and sample purification or preparation.
“The ability to determine the presence or absence of Candida within hours—compared to days [with blood culture]—is paradigm-changing for patients at risk for these infections,” said study author Eleftherios Mylonakis, MD, PhD, of Rhode Island Hospital and The Miriam Hospital in Providence.
“It will allow us to move from a ‘best-guess’ approach in treating high-risk patients, such as cancer and transplant patients and patients in the intensive care unit, to a more informed approach where we can quickly direct the best course of therapy, potentially improving patient outcomes and saving lives.”
Study findings
For this multicenter study, Dr Mylonakis and his colleagues collected blood specimens from 1801 hospitalized patients between the ages of 18 and 95 who had a blood culture ordered as part of routine care.
T2Candida and T2Dx demonstrated an overall specificity of 99.4% per assay and 98.1% per patient. The system yielded a specificity of 98.9% for C albicans/C tropicalis, 99.3% for C parapsilosis, and 99.9% for C krusei/C glabrata.
The system had an overall sensitivity of 91.1% per assay and 91.0% per patient. It yielded a sensitivity of 92.3% for C albicans/C tropicalis, 94.2% for C parapsilosis, and 88.1% for C krusei/C glabrata.
The mean time to a positive result for T2Candida and T2Dx was 4.4 hours, compared to 129 hours for blood culture and species identification. The mean time to negative result for T2Candida and T2Dx was 4.2 hours, compared to at least 120 hours for blood culture.
In one case described in the paper, T2Candida and T2Dx detected a Candida infection that blood culture missed in 12 successive tests.
Seven days after the T2Candida result was obtained, physicians performed an invasive procedure to obtain a tissue culture, which proved that the T2Candida result accurately identified a case of intra-abdominal candidiasis.
“Blood culture, the current standard of care for the diagnosis of Candida infections, is known to have poor sensitivity overall and has 38% sensitivity in proven and probable cases of invasive candidiasis,” said study author Cornelius J. Clancy, MD, of the University of Pittsburgh in Pennsylvania.
“In our case, the T2Candida Panel has shown that it can rapidly identify intra-abdominal candidiasis where 12 serial blood culture results were negative. In many patients at risk for candidiasis, the collection of tissue samples for diagnosis is not possible due to their underlying medical conditions. Achieving the level of sensitivity demonstrated in this case, without requiring an intra-abdominal sample, has the potential to positively impact the practice of medicine for these patients.”